User login
A Game for All Seasons
Sports and sports figures provide both a welcome relief from the stress of dealing with life and death in the ED and memorable ways of characterizing serious health care issues. When the Institute of Medicine issued its 2006 report “Hospital-Based Emergency Care: At the Breaking Point,” we thought that a quote about a popular restaurant by the late, great New York Yankees catcher Yogi Berra better described the severely overcrowded EDs and ambulance diversion: “Nobody goes there anymore—it’s too crowded.”
In late winter and early spring of this year, after vigorous attempts to repeal/revise the 2010 Affordable Care Act (ACA), a replacement bill was withdrawn immediately prior to a Congressional vote on March 24 due to a lack of support. Seven years earlier, when President Obama also seemed to have little chance of getting the ACA through Congress, we thought that there would be “many more balks before the president and Congress finally pitched a viable health care package to the nation.” Only a month later, however, with the ACA now the law, we suggested that our erroneous prediction was similar to that of “a father who convinces his son to leave for the parking lot during the bottom of the ninth inning of a 3-0 game only to hear the roar of the crowd from the exit ramp as the rookie batter hits a grand-slam home run to win the game.”
In June 2012, when the Supreme Court ruled on the constitutionality of the ACA, many reporters quickly read the Court’s rejection of the first two arguments defending the ACA, and rushed to report that it was dead, without considering that the government had “one more out to go…[the one] casting ACA as a tax—considered to be the weakest player in the lineup—[which] managed to score the winning run to uphold ACA. Game over. Final score: ACA wins 5 to 4.”
But with the 2017 baseball season finally underway, it is a recent football game that provides the perfect paradigm for emergency medicine (EM) and emergency physicians (EPs). The New England Patriots were slight favorites to win Super Bowl 51 over the Atlanta Falcons on February 5,and the first quarter ended with no score. But by halftime, Atlanta was leading 21-3. In 50 years of Super Bowls, no team had ever overcome more than a 10-point deficit to win the game, and with a little over 8 minutes left in the third quarter, the deficit had widened even further to 28-3. Then the Patriots began to turn things around. Though the Patriots never led during regulation play, and no Super Bowl had ever gone into overtime, the fourth quarter ended in a 28-28 tie, and the Patriots went on to win 34-28 in overtime.
Coming out of the locker room to play the second half of that game in front of over 111 million viewers must have been a daunting experience for the Patriots, but no more so than the experience depicted in EP/cinematographer Ryan McGarry’s award-winning documentary “Code Black,” in which he shows young EM residents walking through a packed waiting room to begin their shift, realizing that in the next 12 hours, they could never treat all of the ill patients waiting to be seen. But the young residents proceeded to treat one patient after another without ever giving up or losing their idealism, until in the end, they, too, had won the game against all odds.
Many patients arrive in EDs so ill that there is no reasonable expectation any intervention can save them, but we nevertheless try and sometimes succeed in doing the seemingly impossible. It is the type of medicine we have chosen to devote our careers to, and we are no less heroes than were the Patriots on February 5, 2017. Each time we go out to “play ball” in our overcrowded EDs, it is worth remembering another famous Yogi Berra quote: “It ain’t over till it’s over.”
Sports and sports figures provide both a welcome relief from the stress of dealing with life and death in the ED and memorable ways of characterizing serious health care issues. When the Institute of Medicine issued its 2006 report “Hospital-Based Emergency Care: At the Breaking Point,” we thought that a quote about a popular restaurant by the late, great New York Yankees catcher Yogi Berra better described the severely overcrowded EDs and ambulance diversion: “Nobody goes there anymore—it’s too crowded.”
In late winter and early spring of this year, after vigorous attempts to repeal/revise the 2010 Affordable Care Act (ACA), a replacement bill was withdrawn immediately prior to a Congressional vote on March 24 due to a lack of support. Seven years earlier, when President Obama also seemed to have little chance of getting the ACA through Congress, we thought that there would be “many more balks before the president and Congress finally pitched a viable health care package to the nation.” Only a month later, however, with the ACA now the law, we suggested that our erroneous prediction was similar to that of “a father who convinces his son to leave for the parking lot during the bottom of the ninth inning of a 3-0 game only to hear the roar of the crowd from the exit ramp as the rookie batter hits a grand-slam home run to win the game.”
In June 2012, when the Supreme Court ruled on the constitutionality of the ACA, many reporters quickly read the Court’s rejection of the first two arguments defending the ACA, and rushed to report that it was dead, without considering that the government had “one more out to go…[the one] casting ACA as a tax—considered to be the weakest player in the lineup—[which] managed to score the winning run to uphold ACA. Game over. Final score: ACA wins 5 to 4.”
But with the 2017 baseball season finally underway, it is a recent football game that provides the perfect paradigm for emergency medicine (EM) and emergency physicians (EPs). The New England Patriots were slight favorites to win Super Bowl 51 over the Atlanta Falcons on February 5,and the first quarter ended with no score. But by halftime, Atlanta was leading 21-3. In 50 years of Super Bowls, no team had ever overcome more than a 10-point deficit to win the game, and with a little over 8 minutes left in the third quarter, the deficit had widened even further to 28-3. Then the Patriots began to turn things around. Though the Patriots never led during regulation play, and no Super Bowl had ever gone into overtime, the fourth quarter ended in a 28-28 tie, and the Patriots went on to win 34-28 in overtime.
Coming out of the locker room to play the second half of that game in front of over 111 million viewers must have been a daunting experience for the Patriots, but no more so than the experience depicted in EP/cinematographer Ryan McGarry’s award-winning documentary “Code Black,” in which he shows young EM residents walking through a packed waiting room to begin their shift, realizing that in the next 12 hours, they could never treat all of the ill patients waiting to be seen. But the young residents proceeded to treat one patient after another without ever giving up or losing their idealism, until in the end, they, too, had won the game against all odds.
Many patients arrive in EDs so ill that there is no reasonable expectation any intervention can save them, but we nevertheless try and sometimes succeed in doing the seemingly impossible. It is the type of medicine we have chosen to devote our careers to, and we are no less heroes than were the Patriots on February 5, 2017. Each time we go out to “play ball” in our overcrowded EDs, it is worth remembering another famous Yogi Berra quote: “It ain’t over till it’s over.”
Sports and sports figures provide both a welcome relief from the stress of dealing with life and death in the ED and memorable ways of characterizing serious health care issues. When the Institute of Medicine issued its 2006 report “Hospital-Based Emergency Care: At the Breaking Point,” we thought that a quote about a popular restaurant by the late, great New York Yankees catcher Yogi Berra better described the severely overcrowded EDs and ambulance diversion: “Nobody goes there anymore—it’s too crowded.”
In late winter and early spring of this year, after vigorous attempts to repeal/revise the 2010 Affordable Care Act (ACA), a replacement bill was withdrawn immediately prior to a Congressional vote on March 24 due to a lack of support. Seven years earlier, when President Obama also seemed to have little chance of getting the ACA through Congress, we thought that there would be “many more balks before the president and Congress finally pitched a viable health care package to the nation.” Only a month later, however, with the ACA now the law, we suggested that our erroneous prediction was similar to that of “a father who convinces his son to leave for the parking lot during the bottom of the ninth inning of a 3-0 game only to hear the roar of the crowd from the exit ramp as the rookie batter hits a grand-slam home run to win the game.”
In June 2012, when the Supreme Court ruled on the constitutionality of the ACA, many reporters quickly read the Court’s rejection of the first two arguments defending the ACA, and rushed to report that it was dead, without considering that the government had “one more out to go…[the one] casting ACA as a tax—considered to be the weakest player in the lineup—[which] managed to score the winning run to uphold ACA. Game over. Final score: ACA wins 5 to 4.”
But with the 2017 baseball season finally underway, it is a recent football game that provides the perfect paradigm for emergency medicine (EM) and emergency physicians (EPs). The New England Patriots were slight favorites to win Super Bowl 51 over the Atlanta Falcons on February 5,and the first quarter ended with no score. But by halftime, Atlanta was leading 21-3. In 50 years of Super Bowls, no team had ever overcome more than a 10-point deficit to win the game, and with a little over 8 minutes left in the third quarter, the deficit had widened even further to 28-3. Then the Patriots began to turn things around. Though the Patriots never led during regulation play, and no Super Bowl had ever gone into overtime, the fourth quarter ended in a 28-28 tie, and the Patriots went on to win 34-28 in overtime.
Coming out of the locker room to play the second half of that game in front of over 111 million viewers must have been a daunting experience for the Patriots, but no more so than the experience depicted in EP/cinematographer Ryan McGarry’s award-winning documentary “Code Black,” in which he shows young EM residents walking through a packed waiting room to begin their shift, realizing that in the next 12 hours, they could never treat all of the ill patients waiting to be seen. But the young residents proceeded to treat one patient after another without ever giving up or losing their idealism, until in the end, they, too, had won the game against all odds.
Many patients arrive in EDs so ill that there is no reasonable expectation any intervention can save them, but we nevertheless try and sometimes succeed in doing the seemingly impossible. It is the type of medicine we have chosen to devote our careers to, and we are no less heroes than were the Patriots on February 5, 2017. Each time we go out to “play ball” in our overcrowded EDs, it is worth remembering another famous Yogi Berra quote: “It ain’t over till it’s over.”
How would repeal of the Affordable Care Act affect mental health care?
With the changing political landscape in Washington, there has been much talk about health care in the United States. The Affordable Care Act (ACA) is at risk for repeal or, at least, substantial change. As the debate heats up, many psychiatric clinicians wonder what repeal could mean for mental health care and treatment of substance use disorders.
To examine this issue, we need to understand what the ACA has accomplished so far. The Patient Protection and Affordable Care Act—known as “Obamacare”—was enacted on March 23, 2010. From 2010 to 2014, various provisions were implemented; more provisions are slated for completion by 2017 if the law remains in place. These provisions are at the heart of how those with mental illness or substance use disorders could be affected by repeal of the ACA.
Since the ACA’s implementation, an estimated 20 million Americans have gained health insurance.1 The ACA includes several provisions that made this number possible, such as the expansion of Medicaid in some states. In addition to plans offered through the Health Insurance Marketplace, private insurers are required to provide insurance to some who previously fell into non-coverage gaps.1 Young adults can remain on a parent’s plan until age 26, which is significant to mental health care because many psychiatric disorders emerge in young adulthood, and this age group is vulnerable to developing substance use disorders.
The ACA also requires private insurance plans to cover those with preexisting health conditions. This has been crucial for persons with mental illness because before the ACA, mental health disorders were the second most common preexisting condition that precipitated either an increase in the cost of a plan or coverage denial.2
These provisions have helped ensure coverage for the approximately 20% of adults in the United States who have a mental illness.3 Before the ACA, 18% of individuals who purchased their own insurance did not have mental health coverage, and more than one-third of insurers did not cover substance use disorders.4 According to the CDC, the uninsured rate for those with serious mental health disorders fell from 28.1% in 2012 to 19.5% in 2015.5 Likewise, the number of adults with mental illness who could not afford needed care decreased during the same years.5 A University of Minnesota study found that persons with mental illness are disproportionately represented among the uninsured.6 Before the ACA, 18% of individual health plans did not cover prescriptions, including those indicated for psychiatric illness.7 Simply put, the ACA has allowed people to seek assessment and treatment for mental health, whereas it would not have been as accessible before the legislation.
What does the ACA cover?
The ACA required health plans to cover Essential Health Benefits starting January 1, 2014. These include:
- medical services such as doctor visits
- emergency and urgent care services
- hospital physician and facility services
- prenatal, delivery, and postnatal care
- evaluation and treatment of mental health conditions
- services to address substance use including behavioral health treatment
- coverage of prescription medications
- rehabilitation services
- diagnostic tests and imaging
- preventive and wellness care and management of chronic diseases
- pediatric care.
As of March 2013, only 2% of existing health plans in the United States provided all of these benefits required by the ACA.7
Required coverage of mental health care and substance use disorders increases patient access to those services. Including preventive care extends the reach of mental health screening to primary care providers, who can screen for mood disorders and substance use in adults and adolescents and for autism and behavioral issues in children.8
The ACA provides further expansion and enforcement of mental health parity. In 2008, the Mental Health Parity and Addiction Equity Act was passed with the intent of providing behavioral health benefits at the same level as medical care. Although this law was beneficial in theory, it did not require insurers to cover behavioral health treatment. Rather, it only required parity if large group plans already provided behavioral health coverage; parity laws did not apply to individual or small group plans. The Essential Health Benefits of the ACA specify that insurers must provide mental health and substance use treatment. Essentially, the ACA gave the parity law teeth. The law would matter very little if low-income patients, who often suffer from mental health symptoms, have no insurance coverage.
Perhaps more concerning are the implications for those battling substance use disorders. If the ACA is repealed without appropriate replacement measures, it is unclear how those with limited income or preexisting substance use disorders would access evidence-based treatment.
Opioid use disorder affects >2 million individuals in the United States and caused 33,000 overdose deaths in 2015.9 The Opioid Initiative, established in 2015 by the U.S. Department of Health and Human Services (HHS), has worked to improve prescribing practices, increase use of naloxone to treat overdose, and expand access to medication-assisted treatment and psychosocial support. The success of this initiative relies on accessible health insurance coverage. Medication-assisted treatment and psychosocial support services would be threatened most by repeal of the ACA.9 In 2016, the HHS provided $94 million in grants, through the ACA, for free clinics to screen and treat patients for substance use disorders.10 Continued funding for these programs would be jeopardized if the ACA was repealed without replacement.
Repair rather than replace
The ACA is not without its flaws, but perhaps the best approach is to build on its successes while repairing its weaknesses. Researcher Peter Phalen, MA, looked at changes in rates, usage, affordability, and satisfaction with services for those with moderate and severe mental illness after implementation of the ACA.11 Using a nationally representative sample (N = 35,602), he discovered that those with moderate mental illness, as measured by psychological distress scales, experienced greater gains in finding affordable coverage than those without mental illness.11 However, individuals with severe mental illness showed no improvement on these measures, with the exception of increased satisfaction with current coverage and care. There were no reported increases in health care use or affordability for either group.11
Although the ACA requires prescription coverage, there is no regulation of what insurers choose to include in their formularies, and often brand name drugs, particularly antipsychotics, are not covered. The National Alliance on Mental Illness released a report in 2015 noting that, even with the ACA, individuals continue to experience difficulty accessing behavioral health providers in a timely manner, especially in rural areas. The report also described a lack of parity enforcement for behavioral health coverage.12
What if?
If the ACA is repealed, other legislative acts could continue, in some way, to address the needs of those with mental illness. The 21st Century Cures Act, which has bipartisan support, was passed in 2016 in the hope of reforming national mental health care. The American Psychiatric Association (APA) president, Maria Oquendo, MD, PhD, indicated that the bill enhances parity laws and provides better coordination for national agencies involved in treating psychiatric illness.13 The APA applauded this effort and highlighted these provisions:
- reauthorizing grants to support integrated care models
- reauthorizing grants to train school staff to identify students who need mental health care
- requiring the HHS to develop a plan to enforce parity laws
- providing $1 billion in state grants to address the opioid epidemic.13
The APA has voiced its concern about repealing the ACA without replacement. The APA issued a letter to Congressional leadership stating the organization’s concerns, emphasizing that current law has eased the burden for Americans to access “appropriate and evidence-based mental health care.”14 The APA requested that, in considering reforms to health care law, Congress does not “undo the gains which have been made over the past several years for individuals with mental illness.”14 The APA noted that the proposed ACA replacement bill, released on March 3, 2017, would “negatively impact care for people with mental illness and substance use disorders.”15
Since the ACA was implemented, we have taken for granted many provisions as permanent fixtures of our nation’s health care system. Who now can imagine a denial of coverage for a preexisting condition? How many young adults are ready to purchase their own insurance plans immediately after high school or college if employment is not readily available? Is it reasonable that an insurance plan does not provide prescription coverage or behavioral health services? How will those with mental illness or substance use disorders have reliable access to assessment and treatment?
Repealing, replacing, or enhancing the ACA is a complicated balancing act. We must be vigilant and vocal in asking Congress to continue considering the needs of those with mental illness and substance use disorders.
1. U.S. Department of Health and Human Services. 20 million people have gained health insurance coverage because of the Affordable Care Act, new estimates show. http://wayback.archive-it.org/3926/20170127190440/https://www.hhs.gov/about/news/2016/03/03/20-million-people-have-gained-health-insurance-coverage-because-affordable-care-act-new-estimates. Published March 16, 2016. Accessed February 15, 2017.
2. U.S. Department of Health and Human Services. Health insurance coverage for Americans with pre-existing conditions: the impact of the Affordable Care Act. https://aspe.hhs.gov/pdf-report/health-insurance-coverage-americans-pre-existing-conditions-impact-affordable-care-act. Published January 5, 2017. Accessed February 20, 2017.
3. National Alliance on Mental Illness. Mental health by the numbers. http://www.nami.org/Learn-More/Mental-Health-By-the-Numbers. Accessed February 20, 2017.
4. U.S. Department of Health and Human Services. Essential health benefits: individual market place. https://aspe.hhs.gov/pdf-report/essential-health-benefits-individual-market-coverage. Published December 16, 2011. Accessed February 18, 2017.
5. Cohen R, Zammitti EP. Access to care among adults aged 18-64 with serious psychological distress: early release of estimates from the National Health Interview Survey, 2012-September 2015. https://www.cdc.gov/nchs/data/nhis/earlyrelease/er_spd_access_2015_f_auer.pdf. Published May 2016. Accessed February 1, 2017.
6. Rowan K, McAlpine DD, Blewett LA. Access and cost barriers to mental health care by insurance status, 1999-2010. Health Aff (Millwood). 2013;32(10):1723-1730.
7. Health Pocket. Almost no existing health plans meet new ACA essential health benefit standards. https://www.healthpocket.com/healthcare-research/infostat/few-existing-health-plans-meet-new-aca-essential-health-benefit-standards/#.WLSSdqPMxmC. Published March 7, 2013. Accessed February 20, 2017.
8. U.S. Department of Health and Human Services. Health benefits and coverage: preventive health services. https://www.healthcare.gov/coverage/preventive-care-benefits. Accessed February 26, 2017.
9. U.S. Department of Health and Human Services. Continuing progress on the opioid epidemic: the role of the Affordable Care Act. https://aspe.hhs.gov/pdf-report/continuing-progress-opioid-epidemic-role-affordable-care-act. Published January 11, 2017. Accessed February 15, 2017.
10. U.S. Department of Health and Human Services. HHS awards 94 million to health centers help treat the prescription opioid abuse and heroin epidemic in America. http://wayback.archive-it.org/3926/20170127185615/https://www.hhs.gov/about/news/2016/03/11/hhs-awards-94-million-to-health-centers.html. Published March 11, 2016. Accessed February 1, 2017.
11. Phalen P. Psychological distress and rates of health insurance coverage and use and affordability of mental health services, 2013-2014 [published online December 15, 2016]. Psychiatr Serv. http://dx.doi.org/10.1176/appi.ps.201500544.
12. National Alliance on Mental Illness. A long road ahead: achieving true parity in mental health and substance use care. https://www.nami.org/About-NAMI/Publications-Reports/Public-Policy-Reports/A-Long-Road-Ahead/2015-ALongRoadAhead.pdf. Published April 2015. Accessed February 15, 2017.
13. American Psychiatric Association. APA commends house for approving mental health reform bill. https://www.psychiatry.org/newsroom/news-releases/apa-commends-house-for-approving-mental-health-reform-bill?_ga=1.239819267.1833283241.1466442827. Published November 30, 2016. Accessed February 26, 2017.
14. American Psychiatric Association. APA calls on Congress to protect patient access to healthcare. https://www.psychiatry.org/newsroom/news-releases/apa-calls-on-congress-to-protect-patient-access-to-health-care?_ga=1.240843011.1833283241.1466442827. Published January 5, 2017. Accessed February 15, 2017.
15. American Psychiatric Association. APA concerned about proposed ACA replacement bill. https://www.psychiatry.org/newsroom/news-releases/apa-concerned-about-proposed-aca-replacement-bill. Published March 7, 2017. Accessed March 17, 2017.
With the changing political landscape in Washington, there has been much talk about health care in the United States. The Affordable Care Act (ACA) is at risk for repeal or, at least, substantial change. As the debate heats up, many psychiatric clinicians wonder what repeal could mean for mental health care and treatment of substance use disorders.
To examine this issue, we need to understand what the ACA has accomplished so far. The Patient Protection and Affordable Care Act—known as “Obamacare”—was enacted on March 23, 2010. From 2010 to 2014, various provisions were implemented; more provisions are slated for completion by 2017 if the law remains in place. These provisions are at the heart of how those with mental illness or substance use disorders could be affected by repeal of the ACA.
Since the ACA’s implementation, an estimated 20 million Americans have gained health insurance.1 The ACA includes several provisions that made this number possible, such as the expansion of Medicaid in some states. In addition to plans offered through the Health Insurance Marketplace, private insurers are required to provide insurance to some who previously fell into non-coverage gaps.1 Young adults can remain on a parent’s plan until age 26, which is significant to mental health care because many psychiatric disorders emerge in young adulthood, and this age group is vulnerable to developing substance use disorders.
The ACA also requires private insurance plans to cover those with preexisting health conditions. This has been crucial for persons with mental illness because before the ACA, mental health disorders were the second most common preexisting condition that precipitated either an increase in the cost of a plan or coverage denial.2
These provisions have helped ensure coverage for the approximately 20% of adults in the United States who have a mental illness.3 Before the ACA, 18% of individuals who purchased their own insurance did not have mental health coverage, and more than one-third of insurers did not cover substance use disorders.4 According to the CDC, the uninsured rate for those with serious mental health disorders fell from 28.1% in 2012 to 19.5% in 2015.5 Likewise, the number of adults with mental illness who could not afford needed care decreased during the same years.5 A University of Minnesota study found that persons with mental illness are disproportionately represented among the uninsured.6 Before the ACA, 18% of individual health plans did not cover prescriptions, including those indicated for psychiatric illness.7 Simply put, the ACA has allowed people to seek assessment and treatment for mental health, whereas it would not have been as accessible before the legislation.
What does the ACA cover?
The ACA required health plans to cover Essential Health Benefits starting January 1, 2014. These include:
- medical services such as doctor visits
- emergency and urgent care services
- hospital physician and facility services
- prenatal, delivery, and postnatal care
- evaluation and treatment of mental health conditions
- services to address substance use including behavioral health treatment
- coverage of prescription medications
- rehabilitation services
- diagnostic tests and imaging
- preventive and wellness care and management of chronic diseases
- pediatric care.
As of March 2013, only 2% of existing health plans in the United States provided all of these benefits required by the ACA.7
Required coverage of mental health care and substance use disorders increases patient access to those services. Including preventive care extends the reach of mental health screening to primary care providers, who can screen for mood disorders and substance use in adults and adolescents and for autism and behavioral issues in children.8
The ACA provides further expansion and enforcement of mental health parity. In 2008, the Mental Health Parity and Addiction Equity Act was passed with the intent of providing behavioral health benefits at the same level as medical care. Although this law was beneficial in theory, it did not require insurers to cover behavioral health treatment. Rather, it only required parity if large group plans already provided behavioral health coverage; parity laws did not apply to individual or small group plans. The Essential Health Benefits of the ACA specify that insurers must provide mental health and substance use treatment. Essentially, the ACA gave the parity law teeth. The law would matter very little if low-income patients, who often suffer from mental health symptoms, have no insurance coverage.
Perhaps more concerning are the implications for those battling substance use disorders. If the ACA is repealed without appropriate replacement measures, it is unclear how those with limited income or preexisting substance use disorders would access evidence-based treatment.
Opioid use disorder affects >2 million individuals in the United States and caused 33,000 overdose deaths in 2015.9 The Opioid Initiative, established in 2015 by the U.S. Department of Health and Human Services (HHS), has worked to improve prescribing practices, increase use of naloxone to treat overdose, and expand access to medication-assisted treatment and psychosocial support. The success of this initiative relies on accessible health insurance coverage. Medication-assisted treatment and psychosocial support services would be threatened most by repeal of the ACA.9 In 2016, the HHS provided $94 million in grants, through the ACA, for free clinics to screen and treat patients for substance use disorders.10 Continued funding for these programs would be jeopardized if the ACA was repealed without replacement.
Repair rather than replace
The ACA is not without its flaws, but perhaps the best approach is to build on its successes while repairing its weaknesses. Researcher Peter Phalen, MA, looked at changes in rates, usage, affordability, and satisfaction with services for those with moderate and severe mental illness after implementation of the ACA.11 Using a nationally representative sample (N = 35,602), he discovered that those with moderate mental illness, as measured by psychological distress scales, experienced greater gains in finding affordable coverage than those without mental illness.11 However, individuals with severe mental illness showed no improvement on these measures, with the exception of increased satisfaction with current coverage and care. There were no reported increases in health care use or affordability for either group.11
Although the ACA requires prescription coverage, there is no regulation of what insurers choose to include in their formularies, and often brand name drugs, particularly antipsychotics, are not covered. The National Alliance on Mental Illness released a report in 2015 noting that, even with the ACA, individuals continue to experience difficulty accessing behavioral health providers in a timely manner, especially in rural areas. The report also described a lack of parity enforcement for behavioral health coverage.12
What if?
If the ACA is repealed, other legislative acts could continue, in some way, to address the needs of those with mental illness. The 21st Century Cures Act, which has bipartisan support, was passed in 2016 in the hope of reforming national mental health care. The American Psychiatric Association (APA) president, Maria Oquendo, MD, PhD, indicated that the bill enhances parity laws and provides better coordination for national agencies involved in treating psychiatric illness.13 The APA applauded this effort and highlighted these provisions:
- reauthorizing grants to support integrated care models
- reauthorizing grants to train school staff to identify students who need mental health care
- requiring the HHS to develop a plan to enforce parity laws
- providing $1 billion in state grants to address the opioid epidemic.13
The APA has voiced its concern about repealing the ACA without replacement. The APA issued a letter to Congressional leadership stating the organization’s concerns, emphasizing that current law has eased the burden for Americans to access “appropriate and evidence-based mental health care.”14 The APA requested that, in considering reforms to health care law, Congress does not “undo the gains which have been made over the past several years for individuals with mental illness.”14 The APA noted that the proposed ACA replacement bill, released on March 3, 2017, would “negatively impact care for people with mental illness and substance use disorders.”15
Since the ACA was implemented, we have taken for granted many provisions as permanent fixtures of our nation’s health care system. Who now can imagine a denial of coverage for a preexisting condition? How many young adults are ready to purchase their own insurance plans immediately after high school or college if employment is not readily available? Is it reasonable that an insurance plan does not provide prescription coverage or behavioral health services? How will those with mental illness or substance use disorders have reliable access to assessment and treatment?
Repealing, replacing, or enhancing the ACA is a complicated balancing act. We must be vigilant and vocal in asking Congress to continue considering the needs of those with mental illness and substance use disorders.
With the changing political landscape in Washington, there has been much talk about health care in the United States. The Affordable Care Act (ACA) is at risk for repeal or, at least, substantial change. As the debate heats up, many psychiatric clinicians wonder what repeal could mean for mental health care and treatment of substance use disorders.
To examine this issue, we need to understand what the ACA has accomplished so far. The Patient Protection and Affordable Care Act—known as “Obamacare”—was enacted on March 23, 2010. From 2010 to 2014, various provisions were implemented; more provisions are slated for completion by 2017 if the law remains in place. These provisions are at the heart of how those with mental illness or substance use disorders could be affected by repeal of the ACA.
Since the ACA’s implementation, an estimated 20 million Americans have gained health insurance.1 The ACA includes several provisions that made this number possible, such as the expansion of Medicaid in some states. In addition to plans offered through the Health Insurance Marketplace, private insurers are required to provide insurance to some who previously fell into non-coverage gaps.1 Young adults can remain on a parent’s plan until age 26, which is significant to mental health care because many psychiatric disorders emerge in young adulthood, and this age group is vulnerable to developing substance use disorders.
The ACA also requires private insurance plans to cover those with preexisting health conditions. This has been crucial for persons with mental illness because before the ACA, mental health disorders were the second most common preexisting condition that precipitated either an increase in the cost of a plan or coverage denial.2
These provisions have helped ensure coverage for the approximately 20% of adults in the United States who have a mental illness.3 Before the ACA, 18% of individuals who purchased their own insurance did not have mental health coverage, and more than one-third of insurers did not cover substance use disorders.4 According to the CDC, the uninsured rate for those with serious mental health disorders fell from 28.1% in 2012 to 19.5% in 2015.5 Likewise, the number of adults with mental illness who could not afford needed care decreased during the same years.5 A University of Minnesota study found that persons with mental illness are disproportionately represented among the uninsured.6 Before the ACA, 18% of individual health plans did not cover prescriptions, including those indicated for psychiatric illness.7 Simply put, the ACA has allowed people to seek assessment and treatment for mental health, whereas it would not have been as accessible before the legislation.
What does the ACA cover?
The ACA required health plans to cover Essential Health Benefits starting January 1, 2014. These include:
- medical services such as doctor visits
- emergency and urgent care services
- hospital physician and facility services
- prenatal, delivery, and postnatal care
- evaluation and treatment of mental health conditions
- services to address substance use including behavioral health treatment
- coverage of prescription medications
- rehabilitation services
- diagnostic tests and imaging
- preventive and wellness care and management of chronic diseases
- pediatric care.
As of March 2013, only 2% of existing health plans in the United States provided all of these benefits required by the ACA.7
Required coverage of mental health care and substance use disorders increases patient access to those services. Including preventive care extends the reach of mental health screening to primary care providers, who can screen for mood disorders and substance use in adults and adolescents and for autism and behavioral issues in children.8
The ACA provides further expansion and enforcement of mental health parity. In 2008, the Mental Health Parity and Addiction Equity Act was passed with the intent of providing behavioral health benefits at the same level as medical care. Although this law was beneficial in theory, it did not require insurers to cover behavioral health treatment. Rather, it only required parity if large group plans already provided behavioral health coverage; parity laws did not apply to individual or small group plans. The Essential Health Benefits of the ACA specify that insurers must provide mental health and substance use treatment. Essentially, the ACA gave the parity law teeth. The law would matter very little if low-income patients, who often suffer from mental health symptoms, have no insurance coverage.
Perhaps more concerning are the implications for those battling substance use disorders. If the ACA is repealed without appropriate replacement measures, it is unclear how those with limited income or preexisting substance use disorders would access evidence-based treatment.
Opioid use disorder affects >2 million individuals in the United States and caused 33,000 overdose deaths in 2015.9 The Opioid Initiative, established in 2015 by the U.S. Department of Health and Human Services (HHS), has worked to improve prescribing practices, increase use of naloxone to treat overdose, and expand access to medication-assisted treatment and psychosocial support. The success of this initiative relies on accessible health insurance coverage. Medication-assisted treatment and psychosocial support services would be threatened most by repeal of the ACA.9 In 2016, the HHS provided $94 million in grants, through the ACA, for free clinics to screen and treat patients for substance use disorders.10 Continued funding for these programs would be jeopardized if the ACA was repealed without replacement.
Repair rather than replace
The ACA is not without its flaws, but perhaps the best approach is to build on its successes while repairing its weaknesses. Researcher Peter Phalen, MA, looked at changes in rates, usage, affordability, and satisfaction with services for those with moderate and severe mental illness after implementation of the ACA.11 Using a nationally representative sample (N = 35,602), he discovered that those with moderate mental illness, as measured by psychological distress scales, experienced greater gains in finding affordable coverage than those without mental illness.11 However, individuals with severe mental illness showed no improvement on these measures, with the exception of increased satisfaction with current coverage and care. There were no reported increases in health care use or affordability for either group.11
Although the ACA requires prescription coverage, there is no regulation of what insurers choose to include in their formularies, and often brand name drugs, particularly antipsychotics, are not covered. The National Alliance on Mental Illness released a report in 2015 noting that, even with the ACA, individuals continue to experience difficulty accessing behavioral health providers in a timely manner, especially in rural areas. The report also described a lack of parity enforcement for behavioral health coverage.12
What if?
If the ACA is repealed, other legislative acts could continue, in some way, to address the needs of those with mental illness. The 21st Century Cures Act, which has bipartisan support, was passed in 2016 in the hope of reforming national mental health care. The American Psychiatric Association (APA) president, Maria Oquendo, MD, PhD, indicated that the bill enhances parity laws and provides better coordination for national agencies involved in treating psychiatric illness.13 The APA applauded this effort and highlighted these provisions:
- reauthorizing grants to support integrated care models
- reauthorizing grants to train school staff to identify students who need mental health care
- requiring the HHS to develop a plan to enforce parity laws
- providing $1 billion in state grants to address the opioid epidemic.13
The APA has voiced its concern about repealing the ACA without replacement. The APA issued a letter to Congressional leadership stating the organization’s concerns, emphasizing that current law has eased the burden for Americans to access “appropriate and evidence-based mental health care.”14 The APA requested that, in considering reforms to health care law, Congress does not “undo the gains which have been made over the past several years for individuals with mental illness.”14 The APA noted that the proposed ACA replacement bill, released on March 3, 2017, would “negatively impact care for people with mental illness and substance use disorders.”15
Since the ACA was implemented, we have taken for granted many provisions as permanent fixtures of our nation’s health care system. Who now can imagine a denial of coverage for a preexisting condition? How many young adults are ready to purchase their own insurance plans immediately after high school or college if employment is not readily available? Is it reasonable that an insurance plan does not provide prescription coverage or behavioral health services? How will those with mental illness or substance use disorders have reliable access to assessment and treatment?
Repealing, replacing, or enhancing the ACA is a complicated balancing act. We must be vigilant and vocal in asking Congress to continue considering the needs of those with mental illness and substance use disorders.
1. U.S. Department of Health and Human Services. 20 million people have gained health insurance coverage because of the Affordable Care Act, new estimates show. http://wayback.archive-it.org/3926/20170127190440/https://www.hhs.gov/about/news/2016/03/03/20-million-people-have-gained-health-insurance-coverage-because-affordable-care-act-new-estimates. Published March 16, 2016. Accessed February 15, 2017.
2. U.S. Department of Health and Human Services. Health insurance coverage for Americans with pre-existing conditions: the impact of the Affordable Care Act. https://aspe.hhs.gov/pdf-report/health-insurance-coverage-americans-pre-existing-conditions-impact-affordable-care-act. Published January 5, 2017. Accessed February 20, 2017.
3. National Alliance on Mental Illness. Mental health by the numbers. http://www.nami.org/Learn-More/Mental-Health-By-the-Numbers. Accessed February 20, 2017.
4. U.S. Department of Health and Human Services. Essential health benefits: individual market place. https://aspe.hhs.gov/pdf-report/essential-health-benefits-individual-market-coverage. Published December 16, 2011. Accessed February 18, 2017.
5. Cohen R, Zammitti EP. Access to care among adults aged 18-64 with serious psychological distress: early release of estimates from the National Health Interview Survey, 2012-September 2015. https://www.cdc.gov/nchs/data/nhis/earlyrelease/er_spd_access_2015_f_auer.pdf. Published May 2016. Accessed February 1, 2017.
6. Rowan K, McAlpine DD, Blewett LA. Access and cost barriers to mental health care by insurance status, 1999-2010. Health Aff (Millwood). 2013;32(10):1723-1730.
7. Health Pocket. Almost no existing health plans meet new ACA essential health benefit standards. https://www.healthpocket.com/healthcare-research/infostat/few-existing-health-plans-meet-new-aca-essential-health-benefit-standards/#.WLSSdqPMxmC. Published March 7, 2013. Accessed February 20, 2017.
8. U.S. Department of Health and Human Services. Health benefits and coverage: preventive health services. https://www.healthcare.gov/coverage/preventive-care-benefits. Accessed February 26, 2017.
9. U.S. Department of Health and Human Services. Continuing progress on the opioid epidemic: the role of the Affordable Care Act. https://aspe.hhs.gov/pdf-report/continuing-progress-opioid-epidemic-role-affordable-care-act. Published January 11, 2017. Accessed February 15, 2017.
10. U.S. Department of Health and Human Services. HHS awards 94 million to health centers help treat the prescription opioid abuse and heroin epidemic in America. http://wayback.archive-it.org/3926/20170127185615/https://www.hhs.gov/about/news/2016/03/11/hhs-awards-94-million-to-health-centers.html. Published March 11, 2016. Accessed February 1, 2017.
11. Phalen P. Psychological distress and rates of health insurance coverage and use and affordability of mental health services, 2013-2014 [published online December 15, 2016]. Psychiatr Serv. http://dx.doi.org/10.1176/appi.ps.201500544.
12. National Alliance on Mental Illness. A long road ahead: achieving true parity in mental health and substance use care. https://www.nami.org/About-NAMI/Publications-Reports/Public-Policy-Reports/A-Long-Road-Ahead/2015-ALongRoadAhead.pdf. Published April 2015. Accessed February 15, 2017.
13. American Psychiatric Association. APA commends house for approving mental health reform bill. https://www.psychiatry.org/newsroom/news-releases/apa-commends-house-for-approving-mental-health-reform-bill?_ga=1.239819267.1833283241.1466442827. Published November 30, 2016. Accessed February 26, 2017.
14. American Psychiatric Association. APA calls on Congress to protect patient access to healthcare. https://www.psychiatry.org/newsroom/news-releases/apa-calls-on-congress-to-protect-patient-access-to-health-care?_ga=1.240843011.1833283241.1466442827. Published January 5, 2017. Accessed February 15, 2017.
15. American Psychiatric Association. APA concerned about proposed ACA replacement bill. https://www.psychiatry.org/newsroom/news-releases/apa-concerned-about-proposed-aca-replacement-bill. Published March 7, 2017. Accessed March 17, 2017.
1. U.S. Department of Health and Human Services. 20 million people have gained health insurance coverage because of the Affordable Care Act, new estimates show. http://wayback.archive-it.org/3926/20170127190440/https://www.hhs.gov/about/news/2016/03/03/20-million-people-have-gained-health-insurance-coverage-because-affordable-care-act-new-estimates. Published March 16, 2016. Accessed February 15, 2017.
2. U.S. Department of Health and Human Services. Health insurance coverage for Americans with pre-existing conditions: the impact of the Affordable Care Act. https://aspe.hhs.gov/pdf-report/health-insurance-coverage-americans-pre-existing-conditions-impact-affordable-care-act. Published January 5, 2017. Accessed February 20, 2017.
3. National Alliance on Mental Illness. Mental health by the numbers. http://www.nami.org/Learn-More/Mental-Health-By-the-Numbers. Accessed February 20, 2017.
4. U.S. Department of Health and Human Services. Essential health benefits: individual market place. https://aspe.hhs.gov/pdf-report/essential-health-benefits-individual-market-coverage. Published December 16, 2011. Accessed February 18, 2017.
5. Cohen R, Zammitti EP. Access to care among adults aged 18-64 with serious psychological distress: early release of estimates from the National Health Interview Survey, 2012-September 2015. https://www.cdc.gov/nchs/data/nhis/earlyrelease/er_spd_access_2015_f_auer.pdf. Published May 2016. Accessed February 1, 2017.
6. Rowan K, McAlpine DD, Blewett LA. Access and cost barriers to mental health care by insurance status, 1999-2010. Health Aff (Millwood). 2013;32(10):1723-1730.
7. Health Pocket. Almost no existing health plans meet new ACA essential health benefit standards. https://www.healthpocket.com/healthcare-research/infostat/few-existing-health-plans-meet-new-aca-essential-health-benefit-standards/#.WLSSdqPMxmC. Published March 7, 2013. Accessed February 20, 2017.
8. U.S. Department of Health and Human Services. Health benefits and coverage: preventive health services. https://www.healthcare.gov/coverage/preventive-care-benefits. Accessed February 26, 2017.
9. U.S. Department of Health and Human Services. Continuing progress on the opioid epidemic: the role of the Affordable Care Act. https://aspe.hhs.gov/pdf-report/continuing-progress-opioid-epidemic-role-affordable-care-act. Published January 11, 2017. Accessed February 15, 2017.
10. U.S. Department of Health and Human Services. HHS awards 94 million to health centers help treat the prescription opioid abuse and heroin epidemic in America. http://wayback.archive-it.org/3926/20170127185615/https://www.hhs.gov/about/news/2016/03/11/hhs-awards-94-million-to-health-centers.html. Published March 11, 2016. Accessed February 1, 2017.
11. Phalen P. Psychological distress and rates of health insurance coverage and use and affordability of mental health services, 2013-2014 [published online December 15, 2016]. Psychiatr Serv. http://dx.doi.org/10.1176/appi.ps.201500544.
12. National Alliance on Mental Illness. A long road ahead: achieving true parity in mental health and substance use care. https://www.nami.org/About-NAMI/Publications-Reports/Public-Policy-Reports/A-Long-Road-Ahead/2015-ALongRoadAhead.pdf. Published April 2015. Accessed February 15, 2017.
13. American Psychiatric Association. APA commends house for approving mental health reform bill. https://www.psychiatry.org/newsroom/news-releases/apa-commends-house-for-approving-mental-health-reform-bill?_ga=1.239819267.1833283241.1466442827. Published November 30, 2016. Accessed February 26, 2017.
14. American Psychiatric Association. APA calls on Congress to protect patient access to healthcare. https://www.psychiatry.org/newsroom/news-releases/apa-calls-on-congress-to-protect-patient-access-to-health-care?_ga=1.240843011.1833283241.1466442827. Published January 5, 2017. Accessed February 15, 2017.
15. American Psychiatric Association. APA concerned about proposed ACA replacement bill. https://www.psychiatry.org/newsroom/news-releases/apa-concerned-about-proposed-aca-replacement-bill. Published March 7, 2017. Accessed March 17, 2017.
Advancing the role of advanced practice psychiatric nurses in today’s psychiatric workforce
The number of psychiatric prescribers per capita is at one of the lowest levels in history.1 Approximately 43.4 million persons (17.9%) in the United States have a diagnosable mental illness2; 9.8 million (4%) are diagnosed with a serious and persistent mental illness, such as schizophrenia, bipolar disorder, and major depressive disorder (these figures do not include substance use disorders).3
Of the 45,000 licensed psychiatrists, approximately 25,000 are in active practice.4 By comparison, there are approximately 19,000 practicing licensed psychiatric advanced practice registered nurses (APRNs).5 Annually, approximately 1,300 physicians graduate from psychiatric residency programs6 and 700 APRNs from master’s or Doctor of Nursing Practice programs.7 Combining the 2 prescribing workforces (44,000) yields a ratio of 986 patients per licensed prescriber. Seeing each patient only once every 2 months would equate to 25 patients daily considering a 5-day work week. Recognizing that some patients need much more frequent follow-up, this is an impossible task even if these providers and patients were dispersed uniformly across the United States. Currently, ratios are calculated based on the number of psychiatrists per 100,000 individuals, which in the United States is 16.8 Most psychiatrists practice in urban areas,9 whereas psychiatric nurse practitioners are found primarily in rural and less populated urban areas.10
Who can provide care?
Although the growing number of psychiatric APRNs is encouraging for the mental health workforce, their limited role and function remain a battle in the 27 states that do not grant full practice authority. This dispute has become so contentious that the Federal Trade Commission (FTC) has stated that the debate over scope of practice represents federal restraint of trade,11 while patients and their families suffer from lack of access to care.
Recognizing that 9 million patients age <65 who were enrolled in Medicaid in 2011 and treated for a mental health disorder (20% of enrollees) accounted for 50% of all Medicaid expenditures prompts the question, “Who is treating these patients?” According to the American Academy of Nurse Practitioners, 75% of nurse practitioners accept and treat both Medicaid and Medicare patients compared with 43% of psychiatrists who accepted Medicaid and 54% who accepted Medicare in 2011 (these numbers do not include potential overlap).12
Who are APRNs?
The first master’s degree in nursing was created by Hildegard Peplau, EdD, at Rutgers University in 1954, using the title Clinical Specialist in Psychiatric Mental Health Nursing (PMH-CNS). As a master’s prepared clinician, the PMH-CNS could function independently, and many chose to open private practices. Other universities began to create clinical specialty programs in a variety of disciplines. In 1996, 41 states granted prescriptive authority to the PMH-CNS. Psychiatric nurse practitioners were first certified in 2000 to meet the statutory requirements for prescriptive authority of the other 9 states. However this created 4 PMH-APRN roles: Adult and Child/Adolescent CNS and Adult and Family PMHNPs.
Clinical specialists in most areas of health care—except for psychiatry—were primarily working in institutional settings, whereas nurse practitioners were hired principally in primary care community-based settings. The public grew familiar with the term “nurse practitioner,” but these professionals functioned primarily under institutional protocols, while the PMH-CNS had the ability to practice independently. In the mid-1990s, the 4 advanced practice nursing roles of nurse midwife, nurse anesthetist, nurse practitioner, and clinical nurse specialist were encompassed under 1 title: APRN. In 2010 the American Psychiatric Nurses Association endorsed one title for the psychiatric mental health advanced practice registered nurse (PMH-APRN), the psychiatric nurse practitioner, to be educated across the lifespan.
Today, the title PMH-APRN encompasses both the PMHNP and PMH-CNS; the majority specialize in the adult population.
Licensure, accreditation, certification, and education
In 2008, after several years of heated debate among members of >70 nursing organizations, a consensus model governing advanced practice nursing was ratified. This document outlined requirements for licensure, accreditation, certification, and education of the 4 primary advanced practice nursing roles.13 According to the model, the 4 nursing roles would address 1 of 6 major patient populations: neonatal, pediatric, adult-geriatric, family, women’s health/gender-related, and psychiatric. Licensure in each state would be converted to APRN from the existing 26 titles. Each student would have to graduate from a nationally accredited program. In addition to health promotion and advanced roles, educational programs would be required to include advanced courses in pathophysiology, pharmacotherapeutics, and physicalassessment as well as population-specific courses in these same categories. In addition, supervised clinical hour minimums were established for the various population-specific programs.
Concomitantly, graduate educational programs were wrestling with the 2005 statement from the American Association of Colleges of Nursing (AACN) that all advanced practice nursing education should be at the doctoral level by 2015. Because of the knowledge explosion, nurses needed more than what could be achieved in a master’s program to meet practice requirements as well as leadership, systems evaluation, quality improvement, research, and program development. Currently, there are 264 Doctor of Nursing Practice programs in the United States with less than one-half having a PMHNP program.14
Nursing education at the collegiate level has been evolving, which is fostered and supported by the 2010 Institute of Medicine (IOM) Report on the Future of Nursing that identified 4 key recommendations to promote a workforce at capacity to help care for our nation’s growing population:
- Remove scope of practice barriers
- Expand opportunities for nurses to lead and diffuse collaborative improvement efforts
- Implement nurse residency programs
- Increase the proportion of nurses with a baccalaureate degree to 80% by 2020.
The current status of advanced practice nursing
Each of the 50 states is in varying levels of compliance with the 2015 mandates from the consensus model and the AACN. From the psychiatric workforce perspective, many state boards of nursing are concerned because titles often are linked to legislative statute or rules. Despite the 2010 IOM recommendations and the FTC, the American Medical Association (AMA) has stationed AMA lobbyists in the legislatures that are poised to open the nurse practice act to comply with the consensus model. The sole purpose of these lobbyists is to block independent practice for APRNs in the 26 states that are seeking this status and to remove independent practice from the states where it already exists. For example, in Washington the title is ARNP but to change it to APRN will require opening the state’s legislative action. The AMA is eager to remove the autonomy that has existed in that state since 1978. One of the reasons is because where the APRN is required to be in a collaborative or supervisory relationship with a physician, the physician can charge the APRN to be compliant with state regulations. (In some states, the APRN cannot see patients or be on call if the collaborator is on vacation).
This has turned into a cottage industry for many physicians. However, there are many who do not charge because they are able to add additional patients to the practice by adding an APRN and generate more revenue. Others do not charge because they are supportive and committed to the APRN role.
Some thoughts about our mutual field
Can we move past the guild issue and come together to respect our given scopes of practice? I see psychiatry far ahead of the curve compared with APRNs in other specialties. The PMH-APRN is a highly educated nurse with a specific scope of practice that provides skilled psychiatric care (assessment, diagnosis, prescribing, psychotherapy) from a nursing perspective. Independent practice certainly does not imply that we do not collaborate with one another in a professional manner.
Mental Health Professional Shortage Areas
As of January 1, 2017, there are 4,627 Mental Health Professional Shortage Areas (MHPSA) in the United States and Territories (Table), which translates to only 44.2% of the need for psychiatric practitioners being met.15 To eliminate the designation of a MHSPA there must be a population to psychiatric provider ratio of at least 30,000 to 1 (20,000 to 1 if there are unusually high needs in the community). Currently 3,397 practitioners are needed to remove the designation across the United States. The state in most need of providers is Texas with 271 clinicians required to meet the need.
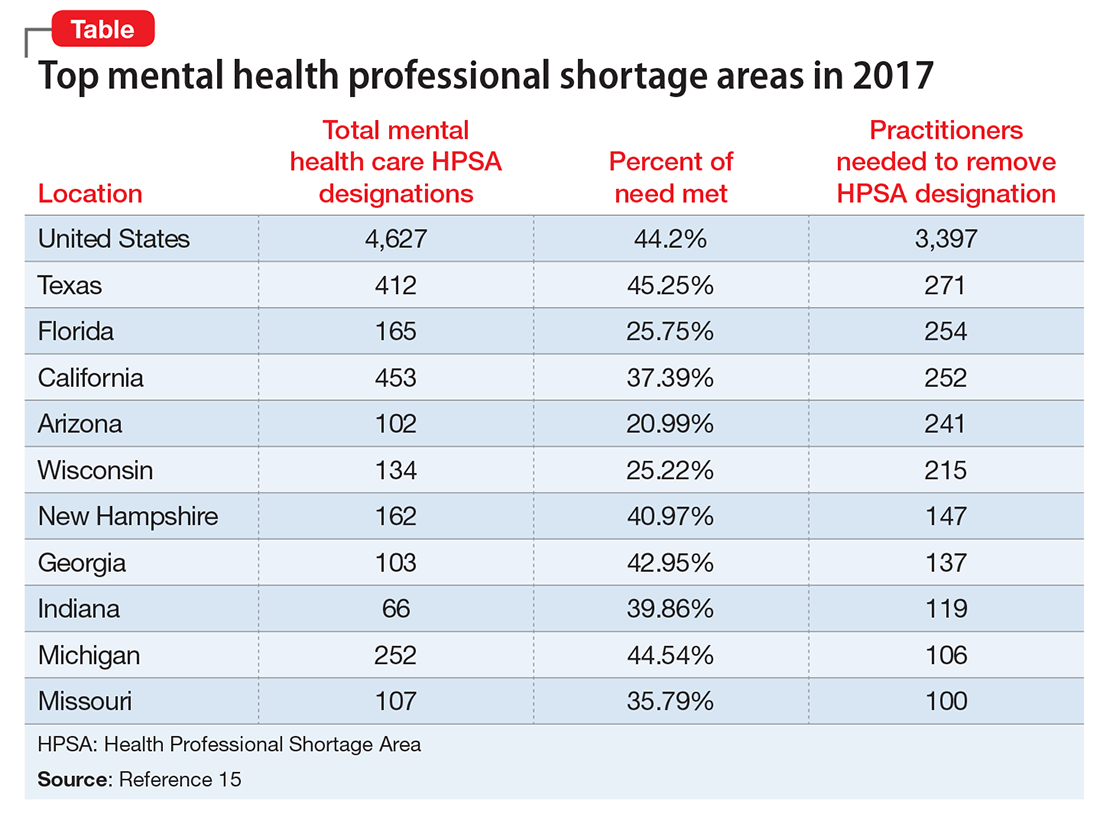
Considering that approximately 700 PMH-APRNs graduate each year16 and 1,317 psychiatry residents17 entered PGY-1 residency in 2016, it will be decades—or longer—before there are enough new providers to eliminate MHPSAs, particularly because the current workforce is aging (average age of the PMH-APRN is 55).
Because there are more than enough patients to go around, I encourage the APA to take a stand against the AMA and unite with the psychiatric APRNs to remove unnecessary barriers to practice and promote a unified and collegial workforce. This will transmit a strong message to the most underserved of our communities that psychiatrists and psychiatric nurse practitioners can emulate the therapeutic relationship by virtue of presenting a unified force. Imagine psychiatrists and psychiatric nurse practitioners going arm in arm to lobby county commissioners, state legislators, and Congressional Representatives and Senators. Together we could be a true force to be reckoned with.
1. Heisler EJ, Bagalman E. The mental health workforce: a primer. http://digitalcommons.ilr.cornell.edu/key_workplace/1410. Published April 16, 2015. Accessed March 13, 2017.
2. National Institute of Mental Health. Any mental illness (AMI) among U.S. adults. https://www.nimh.nih.gov/health/statistics/prevalence/any-mental-illness-ami-among-us-adults.shtml. Accessed March 13, 2017.
3. National Institute of Mental Health. Serious mental illness (SMI) among U.S. adults. https://www.nimh.nih.gov/health/statistics/prevalence/serious-mental-illness-smi-among-us-adults.shtml. Accessed March 13, 2017.
4. Bureau of Labor Statistics. Occupational employment and wages, May 2015. https://www.bls.gov/oes/current/oes291066.htm. Updated March 30, 2016. Accessed March 13, 2017.
5. American Association of Colleges of Nursing. Program directory. http://www.aacn.nche.edu/dnp/program-directory. Accessed March 13, 2017.
7. Fang D, Li Y, Stauffer DC, et al. 2015-2016 Enrollment and graduations in baccalaureate and graduate programs in nursing. http://www.nonpf.org/resource/resmgr/docs/NPTables15-16.pdf. Published 2016. Accessed March 13, 2017.
8. Tasman A. Too few psychiatrists for too many. Psychiatric Times. http://www.psychiatrictimes.com/cultural-psychiatry/too-few-psychiatrists-too-many. Published April 16, 2015. Accessed March 15, 2017.
9. U.S. Department of Health and Human Services. National projections of supply and demand for selected behavioral health practitioners: 2013-2025. https://bhw.hrsa.gov/sites/default/files/bhw/health-workforce-analysis/research/projections/behavioral-health2013-2025.pdf. Published November 2016. Accessed March 13, 2017.
10. Hanrahan NP, Hartley D. Employment of advanced-practice psychiatric nurses to stem rural mental health workforce shortages. Psychiatr Serv. 2008;59(1):109-111.
11. Koslov T. The doctor (or nurse practitioner) will see you now: competition and the regulation of advanced practice nurses. https://www.ftc.gov/news-events/blogs/competition-matters/2014/03/doctor-or-nurse-practitioner-will-see-you-now. Published March 7, 2014. Accessed March 14, 2017.
12. Bishop TF, Press MJ, Keyhani S, et al. Acceptance of insurance by psychiatrists and the implications for access to mental health care. JAMA Psychiatry. 2014;71(2):176-181.
13. National Council of State Boards of Nursing. APRN consensus model. The consensus model for APRN regulation, licensure, accreditation, certification and education. https://www.ncsbn.org/736.htm. Accessed March 13, 2017.
14. National Council of State Boards of Nursing. APRN title map. NCSBN’s APRN campaign for consensus: State progress toward uniformity. https://www.ncsbn.org/5398.htm. Accessed March 13, 2017.
15. Kaiser Family Foundation. Mental health care health professional shortage areas (HPSAs). http://kff.org/other/state-indicator/mental-health-care-health-professional-shortage-areas-hpsas/?activeTab=map¤tTimeframe=0&selectedDistributions=total-mental-health-care-hpsa-designations&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Accessed March 13, 2017.
16. Commission on Collegiate Nursing Education. CCNE-Accredited Doctor of Nursing Practice (DNP) Programs. http://directory.ccnecommunity.org/reports/rptAccreditedPrograms_New.asp?sort=state&sProgramType=3. Accessed March 15, 2017.
17. National Residency Match Program. 2016 match results by state, specialty, and applicant type. http://www.nrmp.org/wp-content/uploads/2016/04/Main-Match-Results-by-State-and-Specialty-2016.pdf. Accessed March 13, 2017.
The number of psychiatric prescribers per capita is at one of the lowest levels in history.1 Approximately 43.4 million persons (17.9%) in the United States have a diagnosable mental illness2; 9.8 million (4%) are diagnosed with a serious and persistent mental illness, such as schizophrenia, bipolar disorder, and major depressive disorder (these figures do not include substance use disorders).3
Of the 45,000 licensed psychiatrists, approximately 25,000 are in active practice.4 By comparison, there are approximately 19,000 practicing licensed psychiatric advanced practice registered nurses (APRNs).5 Annually, approximately 1,300 physicians graduate from psychiatric residency programs6 and 700 APRNs from master’s or Doctor of Nursing Practice programs.7 Combining the 2 prescribing workforces (44,000) yields a ratio of 986 patients per licensed prescriber. Seeing each patient only once every 2 months would equate to 25 patients daily considering a 5-day work week. Recognizing that some patients need much more frequent follow-up, this is an impossible task even if these providers and patients were dispersed uniformly across the United States. Currently, ratios are calculated based on the number of psychiatrists per 100,000 individuals, which in the United States is 16.8 Most psychiatrists practice in urban areas,9 whereas psychiatric nurse practitioners are found primarily in rural and less populated urban areas.10
Who can provide care?
Although the growing number of psychiatric APRNs is encouraging for the mental health workforce, their limited role and function remain a battle in the 27 states that do not grant full practice authority. This dispute has become so contentious that the Federal Trade Commission (FTC) has stated that the debate over scope of practice represents federal restraint of trade,11 while patients and their families suffer from lack of access to care.
Recognizing that 9 million patients age <65 who were enrolled in Medicaid in 2011 and treated for a mental health disorder (20% of enrollees) accounted for 50% of all Medicaid expenditures prompts the question, “Who is treating these patients?” According to the American Academy of Nurse Practitioners, 75% of nurse practitioners accept and treat both Medicaid and Medicare patients compared with 43% of psychiatrists who accepted Medicaid and 54% who accepted Medicare in 2011 (these numbers do not include potential overlap).12
Who are APRNs?
The first master’s degree in nursing was created by Hildegard Peplau, EdD, at Rutgers University in 1954, using the title Clinical Specialist in Psychiatric Mental Health Nursing (PMH-CNS). As a master’s prepared clinician, the PMH-CNS could function independently, and many chose to open private practices. Other universities began to create clinical specialty programs in a variety of disciplines. In 1996, 41 states granted prescriptive authority to the PMH-CNS. Psychiatric nurse practitioners were first certified in 2000 to meet the statutory requirements for prescriptive authority of the other 9 states. However this created 4 PMH-APRN roles: Adult and Child/Adolescent CNS and Adult and Family PMHNPs.
Clinical specialists in most areas of health care—except for psychiatry—were primarily working in institutional settings, whereas nurse practitioners were hired principally in primary care community-based settings. The public grew familiar with the term “nurse practitioner,” but these professionals functioned primarily under institutional protocols, while the PMH-CNS had the ability to practice independently. In the mid-1990s, the 4 advanced practice nursing roles of nurse midwife, nurse anesthetist, nurse practitioner, and clinical nurse specialist were encompassed under 1 title: APRN. In 2010 the American Psychiatric Nurses Association endorsed one title for the psychiatric mental health advanced practice registered nurse (PMH-APRN), the psychiatric nurse practitioner, to be educated across the lifespan.
Today, the title PMH-APRN encompasses both the PMHNP and PMH-CNS; the majority specialize in the adult population.
Licensure, accreditation, certification, and education
In 2008, after several years of heated debate among members of >70 nursing organizations, a consensus model governing advanced practice nursing was ratified. This document outlined requirements for licensure, accreditation, certification, and education of the 4 primary advanced practice nursing roles.13 According to the model, the 4 nursing roles would address 1 of 6 major patient populations: neonatal, pediatric, adult-geriatric, family, women’s health/gender-related, and psychiatric. Licensure in each state would be converted to APRN from the existing 26 titles. Each student would have to graduate from a nationally accredited program. In addition to health promotion and advanced roles, educational programs would be required to include advanced courses in pathophysiology, pharmacotherapeutics, and physicalassessment as well as population-specific courses in these same categories. In addition, supervised clinical hour minimums were established for the various population-specific programs.
Concomitantly, graduate educational programs were wrestling with the 2005 statement from the American Association of Colleges of Nursing (AACN) that all advanced practice nursing education should be at the doctoral level by 2015. Because of the knowledge explosion, nurses needed more than what could be achieved in a master’s program to meet practice requirements as well as leadership, systems evaluation, quality improvement, research, and program development. Currently, there are 264 Doctor of Nursing Practice programs in the United States with less than one-half having a PMHNP program.14
Nursing education at the collegiate level has been evolving, which is fostered and supported by the 2010 Institute of Medicine (IOM) Report on the Future of Nursing that identified 4 key recommendations to promote a workforce at capacity to help care for our nation’s growing population:
- Remove scope of practice barriers
- Expand opportunities for nurses to lead and diffuse collaborative improvement efforts
- Implement nurse residency programs
- Increase the proportion of nurses with a baccalaureate degree to 80% by 2020.
The current status of advanced practice nursing
Each of the 50 states is in varying levels of compliance with the 2015 mandates from the consensus model and the AACN. From the psychiatric workforce perspective, many state boards of nursing are concerned because titles often are linked to legislative statute or rules. Despite the 2010 IOM recommendations and the FTC, the American Medical Association (AMA) has stationed AMA lobbyists in the legislatures that are poised to open the nurse practice act to comply with the consensus model. The sole purpose of these lobbyists is to block independent practice for APRNs in the 26 states that are seeking this status and to remove independent practice from the states where it already exists. For example, in Washington the title is ARNP but to change it to APRN will require opening the state’s legislative action. The AMA is eager to remove the autonomy that has existed in that state since 1978. One of the reasons is because where the APRN is required to be in a collaborative or supervisory relationship with a physician, the physician can charge the APRN to be compliant with state regulations. (In some states, the APRN cannot see patients or be on call if the collaborator is on vacation).
This has turned into a cottage industry for many physicians. However, there are many who do not charge because they are able to add additional patients to the practice by adding an APRN and generate more revenue. Others do not charge because they are supportive and committed to the APRN role.
Some thoughts about our mutual field
Can we move past the guild issue and come together to respect our given scopes of practice? I see psychiatry far ahead of the curve compared with APRNs in other specialties. The PMH-APRN is a highly educated nurse with a specific scope of practice that provides skilled psychiatric care (assessment, diagnosis, prescribing, psychotherapy) from a nursing perspective. Independent practice certainly does not imply that we do not collaborate with one another in a professional manner.
Mental Health Professional Shortage Areas
As of January 1, 2017, there are 4,627 Mental Health Professional Shortage Areas (MHPSA) in the United States and Territories (Table), which translates to only 44.2% of the need for psychiatric practitioners being met.15 To eliminate the designation of a MHSPA there must be a population to psychiatric provider ratio of at least 30,000 to 1 (20,000 to 1 if there are unusually high needs in the community). Currently 3,397 practitioners are needed to remove the designation across the United States. The state in most need of providers is Texas with 271 clinicians required to meet the need.

Considering that approximately 700 PMH-APRNs graduate each year16 and 1,317 psychiatry residents17 entered PGY-1 residency in 2016, it will be decades—or longer—before there are enough new providers to eliminate MHPSAs, particularly because the current workforce is aging (average age of the PMH-APRN is 55).
Because there are more than enough patients to go around, I encourage the APA to take a stand against the AMA and unite with the psychiatric APRNs to remove unnecessary barriers to practice and promote a unified and collegial workforce. This will transmit a strong message to the most underserved of our communities that psychiatrists and psychiatric nurse practitioners can emulate the therapeutic relationship by virtue of presenting a unified force. Imagine psychiatrists and psychiatric nurse practitioners going arm in arm to lobby county commissioners, state legislators, and Congressional Representatives and Senators. Together we could be a true force to be reckoned with.
The number of psychiatric prescribers per capita is at one of the lowest levels in history.1 Approximately 43.4 million persons (17.9%) in the United States have a diagnosable mental illness2; 9.8 million (4%) are diagnosed with a serious and persistent mental illness, such as schizophrenia, bipolar disorder, and major depressive disorder (these figures do not include substance use disorders).3
Of the 45,000 licensed psychiatrists, approximately 25,000 are in active practice.4 By comparison, there are approximately 19,000 practicing licensed psychiatric advanced practice registered nurses (APRNs).5 Annually, approximately 1,300 physicians graduate from psychiatric residency programs6 and 700 APRNs from master’s or Doctor of Nursing Practice programs.7 Combining the 2 prescribing workforces (44,000) yields a ratio of 986 patients per licensed prescriber. Seeing each patient only once every 2 months would equate to 25 patients daily considering a 5-day work week. Recognizing that some patients need much more frequent follow-up, this is an impossible task even if these providers and patients were dispersed uniformly across the United States. Currently, ratios are calculated based on the number of psychiatrists per 100,000 individuals, which in the United States is 16.8 Most psychiatrists practice in urban areas,9 whereas psychiatric nurse practitioners are found primarily in rural and less populated urban areas.10
Who can provide care?
Although the growing number of psychiatric APRNs is encouraging for the mental health workforce, their limited role and function remain a battle in the 27 states that do not grant full practice authority. This dispute has become so contentious that the Federal Trade Commission (FTC) has stated that the debate over scope of practice represents federal restraint of trade,11 while patients and their families suffer from lack of access to care.
Recognizing that 9 million patients age <65 who were enrolled in Medicaid in 2011 and treated for a mental health disorder (20% of enrollees) accounted for 50% of all Medicaid expenditures prompts the question, “Who is treating these patients?” According to the American Academy of Nurse Practitioners, 75% of nurse practitioners accept and treat both Medicaid and Medicare patients compared with 43% of psychiatrists who accepted Medicaid and 54% who accepted Medicare in 2011 (these numbers do not include potential overlap).12
Who are APRNs?
The first master’s degree in nursing was created by Hildegard Peplau, EdD, at Rutgers University in 1954, using the title Clinical Specialist in Psychiatric Mental Health Nursing (PMH-CNS). As a master’s prepared clinician, the PMH-CNS could function independently, and many chose to open private practices. Other universities began to create clinical specialty programs in a variety of disciplines. In 1996, 41 states granted prescriptive authority to the PMH-CNS. Psychiatric nurse practitioners were first certified in 2000 to meet the statutory requirements for prescriptive authority of the other 9 states. However this created 4 PMH-APRN roles: Adult and Child/Adolescent CNS and Adult and Family PMHNPs.
Clinical specialists in most areas of health care—except for psychiatry—were primarily working in institutional settings, whereas nurse practitioners were hired principally in primary care community-based settings. The public grew familiar with the term “nurse practitioner,” but these professionals functioned primarily under institutional protocols, while the PMH-CNS had the ability to practice independently. In the mid-1990s, the 4 advanced practice nursing roles of nurse midwife, nurse anesthetist, nurse practitioner, and clinical nurse specialist were encompassed under 1 title: APRN. In 2010 the American Psychiatric Nurses Association endorsed one title for the psychiatric mental health advanced practice registered nurse (PMH-APRN), the psychiatric nurse practitioner, to be educated across the lifespan.
Today, the title PMH-APRN encompasses both the PMHNP and PMH-CNS; the majority specialize in the adult population.
Licensure, accreditation, certification, and education
In 2008, after several years of heated debate among members of >70 nursing organizations, a consensus model governing advanced practice nursing was ratified. This document outlined requirements for licensure, accreditation, certification, and education of the 4 primary advanced practice nursing roles.13 According to the model, the 4 nursing roles would address 1 of 6 major patient populations: neonatal, pediatric, adult-geriatric, family, women’s health/gender-related, and psychiatric. Licensure in each state would be converted to APRN from the existing 26 titles. Each student would have to graduate from a nationally accredited program. In addition to health promotion and advanced roles, educational programs would be required to include advanced courses in pathophysiology, pharmacotherapeutics, and physicalassessment as well as population-specific courses in these same categories. In addition, supervised clinical hour minimums were established for the various population-specific programs.
Concomitantly, graduate educational programs were wrestling with the 2005 statement from the American Association of Colleges of Nursing (AACN) that all advanced practice nursing education should be at the doctoral level by 2015. Because of the knowledge explosion, nurses needed more than what could be achieved in a master’s program to meet practice requirements as well as leadership, systems evaluation, quality improvement, research, and program development. Currently, there are 264 Doctor of Nursing Practice programs in the United States with less than one-half having a PMHNP program.14
Nursing education at the collegiate level has been evolving, which is fostered and supported by the 2010 Institute of Medicine (IOM) Report on the Future of Nursing that identified 4 key recommendations to promote a workforce at capacity to help care for our nation’s growing population:
- Remove scope of practice barriers
- Expand opportunities for nurses to lead and diffuse collaborative improvement efforts
- Implement nurse residency programs
- Increase the proportion of nurses with a baccalaureate degree to 80% by 2020.
The current status of advanced practice nursing
Each of the 50 states is in varying levels of compliance with the 2015 mandates from the consensus model and the AACN. From the psychiatric workforce perspective, many state boards of nursing are concerned because titles often are linked to legislative statute or rules. Despite the 2010 IOM recommendations and the FTC, the American Medical Association (AMA) has stationed AMA lobbyists in the legislatures that are poised to open the nurse practice act to comply with the consensus model. The sole purpose of these lobbyists is to block independent practice for APRNs in the 26 states that are seeking this status and to remove independent practice from the states where it already exists. For example, in Washington the title is ARNP but to change it to APRN will require opening the state’s legislative action. The AMA is eager to remove the autonomy that has existed in that state since 1978. One of the reasons is because where the APRN is required to be in a collaborative or supervisory relationship with a physician, the physician can charge the APRN to be compliant with state regulations. (In some states, the APRN cannot see patients or be on call if the collaborator is on vacation).
This has turned into a cottage industry for many physicians. However, there are many who do not charge because they are able to add additional patients to the practice by adding an APRN and generate more revenue. Others do not charge because they are supportive and committed to the APRN role.
Some thoughts about our mutual field
Can we move past the guild issue and come together to respect our given scopes of practice? I see psychiatry far ahead of the curve compared with APRNs in other specialties. The PMH-APRN is a highly educated nurse with a specific scope of practice that provides skilled psychiatric care (assessment, diagnosis, prescribing, psychotherapy) from a nursing perspective. Independent practice certainly does not imply that we do not collaborate with one another in a professional manner.
Mental Health Professional Shortage Areas
As of January 1, 2017, there are 4,627 Mental Health Professional Shortage Areas (MHPSA) in the United States and Territories (Table), which translates to only 44.2% of the need for psychiatric practitioners being met.15 To eliminate the designation of a MHSPA there must be a population to psychiatric provider ratio of at least 30,000 to 1 (20,000 to 1 if there are unusually high needs in the community). Currently 3,397 practitioners are needed to remove the designation across the United States. The state in most need of providers is Texas with 271 clinicians required to meet the need.

Considering that approximately 700 PMH-APRNs graduate each year16 and 1,317 psychiatry residents17 entered PGY-1 residency in 2016, it will be decades—or longer—before there are enough new providers to eliminate MHPSAs, particularly because the current workforce is aging (average age of the PMH-APRN is 55).
Because there are more than enough patients to go around, I encourage the APA to take a stand against the AMA and unite with the psychiatric APRNs to remove unnecessary barriers to practice and promote a unified and collegial workforce. This will transmit a strong message to the most underserved of our communities that psychiatrists and psychiatric nurse practitioners can emulate the therapeutic relationship by virtue of presenting a unified force. Imagine psychiatrists and psychiatric nurse practitioners going arm in arm to lobby county commissioners, state legislators, and Congressional Representatives and Senators. Together we could be a true force to be reckoned with.
1. Heisler EJ, Bagalman E. The mental health workforce: a primer. http://digitalcommons.ilr.cornell.edu/key_workplace/1410. Published April 16, 2015. Accessed March 13, 2017.
2. National Institute of Mental Health. Any mental illness (AMI) among U.S. adults. https://www.nimh.nih.gov/health/statistics/prevalence/any-mental-illness-ami-among-us-adults.shtml. Accessed March 13, 2017.
3. National Institute of Mental Health. Serious mental illness (SMI) among U.S. adults. https://www.nimh.nih.gov/health/statistics/prevalence/serious-mental-illness-smi-among-us-adults.shtml. Accessed March 13, 2017.
4. Bureau of Labor Statistics. Occupational employment and wages, May 2015. https://www.bls.gov/oes/current/oes291066.htm. Updated March 30, 2016. Accessed March 13, 2017.
5. American Association of Colleges of Nursing. Program directory. http://www.aacn.nche.edu/dnp/program-directory. Accessed March 13, 2017.
7. Fang D, Li Y, Stauffer DC, et al. 2015-2016 Enrollment and graduations in baccalaureate and graduate programs in nursing. http://www.nonpf.org/resource/resmgr/docs/NPTables15-16.pdf. Published 2016. Accessed March 13, 2017.
8. Tasman A. Too few psychiatrists for too many. Psychiatric Times. http://www.psychiatrictimes.com/cultural-psychiatry/too-few-psychiatrists-too-many. Published April 16, 2015. Accessed March 15, 2017.
9. U.S. Department of Health and Human Services. National projections of supply and demand for selected behavioral health practitioners: 2013-2025. https://bhw.hrsa.gov/sites/default/files/bhw/health-workforce-analysis/research/projections/behavioral-health2013-2025.pdf. Published November 2016. Accessed March 13, 2017.
10. Hanrahan NP, Hartley D. Employment of advanced-practice psychiatric nurses to stem rural mental health workforce shortages. Psychiatr Serv. 2008;59(1):109-111.
11. Koslov T. The doctor (or nurse practitioner) will see you now: competition and the regulation of advanced practice nurses. https://www.ftc.gov/news-events/blogs/competition-matters/2014/03/doctor-or-nurse-practitioner-will-see-you-now. Published March 7, 2014. Accessed March 14, 2017.
12. Bishop TF, Press MJ, Keyhani S, et al. Acceptance of insurance by psychiatrists and the implications for access to mental health care. JAMA Psychiatry. 2014;71(2):176-181.
13. National Council of State Boards of Nursing. APRN consensus model. The consensus model for APRN regulation, licensure, accreditation, certification and education. https://www.ncsbn.org/736.htm. Accessed March 13, 2017.
14. National Council of State Boards of Nursing. APRN title map. NCSBN’s APRN campaign for consensus: State progress toward uniformity. https://www.ncsbn.org/5398.htm. Accessed March 13, 2017.
15. Kaiser Family Foundation. Mental health care health professional shortage areas (HPSAs). http://kff.org/other/state-indicator/mental-health-care-health-professional-shortage-areas-hpsas/?activeTab=map¤tTimeframe=0&selectedDistributions=total-mental-health-care-hpsa-designations&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Accessed March 13, 2017.
16. Commission on Collegiate Nursing Education. CCNE-Accredited Doctor of Nursing Practice (DNP) Programs. http://directory.ccnecommunity.org/reports/rptAccreditedPrograms_New.asp?sort=state&sProgramType=3. Accessed March 15, 2017.
17. National Residency Match Program. 2016 match results by state, specialty, and applicant type. http://www.nrmp.org/wp-content/uploads/2016/04/Main-Match-Results-by-State-and-Specialty-2016.pdf. Accessed March 13, 2017.
1. Heisler EJ, Bagalman E. The mental health workforce: a primer. http://digitalcommons.ilr.cornell.edu/key_workplace/1410. Published April 16, 2015. Accessed March 13, 2017.
2. National Institute of Mental Health. Any mental illness (AMI) among U.S. adults. https://www.nimh.nih.gov/health/statistics/prevalence/any-mental-illness-ami-among-us-adults.shtml. Accessed March 13, 2017.
3. National Institute of Mental Health. Serious mental illness (SMI) among U.S. adults. https://www.nimh.nih.gov/health/statistics/prevalence/serious-mental-illness-smi-among-us-adults.shtml. Accessed March 13, 2017.
4. Bureau of Labor Statistics. Occupational employment and wages, May 2015. https://www.bls.gov/oes/current/oes291066.htm. Updated March 30, 2016. Accessed March 13, 2017.
5. American Association of Colleges of Nursing. Program directory. http://www.aacn.nche.edu/dnp/program-directory. Accessed March 13, 2017.
7. Fang D, Li Y, Stauffer DC, et al. 2015-2016 Enrollment and graduations in baccalaureate and graduate programs in nursing. http://www.nonpf.org/resource/resmgr/docs/NPTables15-16.pdf. Published 2016. Accessed March 13, 2017.
8. Tasman A. Too few psychiatrists for too many. Psychiatric Times. http://www.psychiatrictimes.com/cultural-psychiatry/too-few-psychiatrists-too-many. Published April 16, 2015. Accessed March 15, 2017.
9. U.S. Department of Health and Human Services. National projections of supply and demand for selected behavioral health practitioners: 2013-2025. https://bhw.hrsa.gov/sites/default/files/bhw/health-workforce-analysis/research/projections/behavioral-health2013-2025.pdf. Published November 2016. Accessed March 13, 2017.
10. Hanrahan NP, Hartley D. Employment of advanced-practice psychiatric nurses to stem rural mental health workforce shortages. Psychiatr Serv. 2008;59(1):109-111.
11. Koslov T. The doctor (or nurse practitioner) will see you now: competition and the regulation of advanced practice nurses. https://www.ftc.gov/news-events/blogs/competition-matters/2014/03/doctor-or-nurse-practitioner-will-see-you-now. Published March 7, 2014. Accessed March 14, 2017.
12. Bishop TF, Press MJ, Keyhani S, et al. Acceptance of insurance by psychiatrists and the implications for access to mental health care. JAMA Psychiatry. 2014;71(2):176-181.
13. National Council of State Boards of Nursing. APRN consensus model. The consensus model for APRN regulation, licensure, accreditation, certification and education. https://www.ncsbn.org/736.htm. Accessed March 13, 2017.
14. National Council of State Boards of Nursing. APRN title map. NCSBN’s APRN campaign for consensus: State progress toward uniformity. https://www.ncsbn.org/5398.htm. Accessed March 13, 2017.
15. Kaiser Family Foundation. Mental health care health professional shortage areas (HPSAs). http://kff.org/other/state-indicator/mental-health-care-health-professional-shortage-areas-hpsas/?activeTab=map¤tTimeframe=0&selectedDistributions=total-mental-health-care-hpsa-designations&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Accessed March 13, 2017.
16. Commission on Collegiate Nursing Education. CCNE-Accredited Doctor of Nursing Practice (DNP) Programs. http://directory.ccnecommunity.org/reports/rptAccreditedPrograms_New.asp?sort=state&sProgramType=3. Accessed March 15, 2017.
17. National Residency Match Program. 2016 match results by state, specialty, and applicant type. http://www.nrmp.org/wp-content/uploads/2016/04/Main-Match-Results-by-State-and-Specialty-2016.pdf. Accessed March 13, 2017.
Do you attend a patient’s funeral?
I’ve never been to a patient’s funeral, though I know plenty of other doctors who have.
I suppose this is a highly personal decision. Some feel they should go out of respect to the patient, or if they had a particularly strong or longstanding relationship with them.
Part of it is feeling like an outsider. To me, funerals are a chance for loved ones and close friends to say their goodbyes. I generally try to keep a professional distance. It makes the job easier.
Another is simply a reluctance to take time off from the office. Even though someone I cared for is gone, that person is not the only one that I see. I have to continue caring for the patients who still need me.
There’s also an aspect of fear. Family members who don’t know you well may see your presence as a sign of guilt that you did something wrong. Or, in the irrational nature of grief and anger, become belligerent, accusing you of incompetence. These sorts of confrontations can never end well for either side.
All of us are facing death sooner or later. As physicians, our job is to prolong and improve quality of life as best we can, knowing that inevitably we’ll lose. When that happens, the most we can ever ask is that we did our best. And that we continue to care for those who still depend on us.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
[polldaddy:9711658]
I’ve never been to a patient’s funeral, though I know plenty of other doctors who have.
I suppose this is a highly personal decision. Some feel they should go out of respect to the patient, or if they had a particularly strong or longstanding relationship with them.
Part of it is feeling like an outsider. To me, funerals are a chance for loved ones and close friends to say their goodbyes. I generally try to keep a professional distance. It makes the job easier.
Another is simply a reluctance to take time off from the office. Even though someone I cared for is gone, that person is not the only one that I see. I have to continue caring for the patients who still need me.
There’s also an aspect of fear. Family members who don’t know you well may see your presence as a sign of guilt that you did something wrong. Or, in the irrational nature of grief and anger, become belligerent, accusing you of incompetence. These sorts of confrontations can never end well for either side.
All of us are facing death sooner or later. As physicians, our job is to prolong and improve quality of life as best we can, knowing that inevitably we’ll lose. When that happens, the most we can ever ask is that we did our best. And that we continue to care for those who still depend on us.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
[polldaddy:9711658]
I’ve never been to a patient’s funeral, though I know plenty of other doctors who have.
I suppose this is a highly personal decision. Some feel they should go out of respect to the patient, or if they had a particularly strong or longstanding relationship with them.
Part of it is feeling like an outsider. To me, funerals are a chance for loved ones and close friends to say their goodbyes. I generally try to keep a professional distance. It makes the job easier.
Another is simply a reluctance to take time off from the office. Even though someone I cared for is gone, that person is not the only one that I see. I have to continue caring for the patients who still need me.
There’s also an aspect of fear. Family members who don’t know you well may see your presence as a sign of guilt that you did something wrong. Or, in the irrational nature of grief and anger, become belligerent, accusing you of incompetence. These sorts of confrontations can never end well for either side.
All of us are facing death sooner or later. As physicians, our job is to prolong and improve quality of life as best we can, knowing that inevitably we’ll lose. When that happens, the most we can ever ask is that we did our best. And that we continue to care for those who still depend on us.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
[polldaddy:9711658]
What about the ‘B’ in LGBTQ?
Lesbian, gay, bisexual, transgender, and questioning or queer (LGBTQ) youth face bias and discrimination within the health care setting and experience disparities in health, compared with their heterosexual cisgender peers. This is an area that is receiving increasing attention and study as health care providers and researchers work to achieve health equity within these populations.
Studies focusing specifically on the health of bisexual youth and adults are lacking. The few that do exist suggest that the experiences of people who identify as bisexual may be different from those who identify as lesbian or gay. Myths and misconceptions about bisexual, pansexual, queer, and fluid identities may in some cases put these populations at increased risks. Common myths include that bisexuality is just a phase or that youth who identify as bisexual are just confused. Studies suggest that bisexual youth account for almost half of youth who identify as LGBTQ. Understanding more about some of the challenges bisexual youth and adults may face can help us better care for all of our patients and families.
• Bisexual adults are more likely to engage in self-harming behaviors, attempt suicide, or think about suicide than heterosexual adults, lesbian women, or gay men.1
• Bisexual women have higher rates of high blood pressure, compared with heterosexual and lesbian women.2
• Bisexual women have higher rates of alcohol-related disorders than lesbian and heterosexual women.1
Some disparities appear to be related to lack of preventive care. A survey by the Williams Institute found that 39% of bisexual men and 33% of bisexual women did not disclose their sexual orientation, compared with 13% of gay men and 10% of lesbian women.1 The effect of intersecting identities also must be considered when discussing these health disparities. More than 40% of LGBTQ people of color identify as bisexual, and almost half of transgender people describe their sexual orientation as bisexual or queer.1 These individuals may be especially vulnerable to health disparities as they may experience a combination of racism, transphobia, and biphobia.
Risk factors for these disparities may develop early in life. A 2012 survey of LGBTQ youth found that:3
• Bisexual youth were less likely than lesbian and gay youth to report having supportive adults who they could turn to if they were sad.
• Only 5% of bisexual youth reported being very happy, compared with 8% of gay and lesbian youth and 21% of non-LGBT youth.
• Bisexual youth reported higher rates of experimentation with drugs and alcohol, compared with their lesbian, gay, and heterosexual peers.
• Bisexual youth reported lower levels of family acceptance and knowledge of social support systems in their communities than lesbian and gay youth. Both family acceptance and knowledge of social support systems have been identified as protective factors in the development of youth.
• Bisexual youth are less likely to be out to their friends, families, and communities.
As health care providers, recognizing, respecting, and supporting the identities of our bisexual patients is important. A few simple things we can do in practice are as follows:
• Don’t mislabel patients as lesbian, gay, or straight when they have disclosed a bisexual identity.
• Don’t assume that bisexuality is just a phase or that youths are confused when they disclose their identity.
• Don’t assume you know a patient’s sexual orientation or behaviors on the basis of the sex of the current partner or current behaviors.
• Do ask open and nonjudgmental questions about sexual attraction and behaviors, and be familiar with the terms bisexual, queer, questioning, and pansexual in addition to lesbian, gay, and straight.
• Do use inclusive terms like LGBT when referring to the community rather than gay rights.
• Do recognize potential biases and assumptions regarding sexuality and bisexuality and work to change them.
Dr. Chelvakumar is an attending physician in the division of adolescent medicine at Nationwide Children’s Hospital and an assistant professor of clinical pediatrics at the Ohio State University, both in Columbus.
Terms and definitions:
Bisexual – A person who can be attracted to more than one sex, gender, or gender identity. “Bi” is often used as an abbreviation.
Biphobia – Prejudice, fear, or hatred directed toward bisexual people.
Queer – A term people often use to express fluid identities and orientations. Historically considered a pejorative term, but used by many youth to describe their identity.
Pansexual – A person who can be attracted to any sex, gender, or gender identity.
References:
1. ”Health Disparities Among Bisexual People,” brief by the Human Rights Campaign Foundation.
2. “New Mexico’s Progress in Collecting Lesbian, Gay, Bisexual, and Transgender Health Data and Its Implications for Addressing Health Disparities,” New Mexico Department of Health, April 2010.
3. “Supporting and Caring for Our Bisexual Youth,” the Human Rights Campaign Foundation, 2014.
Lesbian, gay, bisexual, transgender, and questioning or queer (LGBTQ) youth face bias and discrimination within the health care setting and experience disparities in health, compared with their heterosexual cisgender peers. This is an area that is receiving increasing attention and study as health care providers and researchers work to achieve health equity within these populations.
Studies focusing specifically on the health of bisexual youth and adults are lacking. The few that do exist suggest that the experiences of people who identify as bisexual may be different from those who identify as lesbian or gay. Myths and misconceptions about bisexual, pansexual, queer, and fluid identities may in some cases put these populations at increased risks. Common myths include that bisexuality is just a phase or that youth who identify as bisexual are just confused. Studies suggest that bisexual youth account for almost half of youth who identify as LGBTQ. Understanding more about some of the challenges bisexual youth and adults may face can help us better care for all of our patients and families.
• Bisexual adults are more likely to engage in self-harming behaviors, attempt suicide, or think about suicide than heterosexual adults, lesbian women, or gay men.1
• Bisexual women have higher rates of high blood pressure, compared with heterosexual and lesbian women.2
• Bisexual women have higher rates of alcohol-related disorders than lesbian and heterosexual women.1
Some disparities appear to be related to lack of preventive care. A survey by the Williams Institute found that 39% of bisexual men and 33% of bisexual women did not disclose their sexual orientation, compared with 13% of gay men and 10% of lesbian women.1 The effect of intersecting identities also must be considered when discussing these health disparities. More than 40% of LGBTQ people of color identify as bisexual, and almost half of transgender people describe their sexual orientation as bisexual or queer.1 These individuals may be especially vulnerable to health disparities as they may experience a combination of racism, transphobia, and biphobia.
Risk factors for these disparities may develop early in life. A 2012 survey of LGBTQ youth found that:3
• Bisexual youth were less likely than lesbian and gay youth to report having supportive adults who they could turn to if they were sad.
• Only 5% of bisexual youth reported being very happy, compared with 8% of gay and lesbian youth and 21% of non-LGBT youth.
• Bisexual youth reported higher rates of experimentation with drugs and alcohol, compared with their lesbian, gay, and heterosexual peers.
• Bisexual youth reported lower levels of family acceptance and knowledge of social support systems in their communities than lesbian and gay youth. Both family acceptance and knowledge of social support systems have been identified as protective factors in the development of youth.
• Bisexual youth are less likely to be out to their friends, families, and communities.
As health care providers, recognizing, respecting, and supporting the identities of our bisexual patients is important. A few simple things we can do in practice are as follows:
• Don’t mislabel patients as lesbian, gay, or straight when they have disclosed a bisexual identity.
• Don’t assume that bisexuality is just a phase or that youths are confused when they disclose their identity.
• Don’t assume you know a patient’s sexual orientation or behaviors on the basis of the sex of the current partner or current behaviors.
• Do ask open and nonjudgmental questions about sexual attraction and behaviors, and be familiar with the terms bisexual, queer, questioning, and pansexual in addition to lesbian, gay, and straight.
• Do use inclusive terms like LGBT when referring to the community rather than gay rights.
• Do recognize potential biases and assumptions regarding sexuality and bisexuality and work to change them.
Dr. Chelvakumar is an attending physician in the division of adolescent medicine at Nationwide Children’s Hospital and an assistant professor of clinical pediatrics at the Ohio State University, both in Columbus.
Terms and definitions:
Bisexual – A person who can be attracted to more than one sex, gender, or gender identity. “Bi” is often used as an abbreviation.
Biphobia – Prejudice, fear, or hatred directed toward bisexual people.
Queer – A term people often use to express fluid identities and orientations. Historically considered a pejorative term, but used by many youth to describe their identity.
Pansexual – A person who can be attracted to any sex, gender, or gender identity.
References:
1. ”Health Disparities Among Bisexual People,” brief by the Human Rights Campaign Foundation.
2. “New Mexico’s Progress in Collecting Lesbian, Gay, Bisexual, and Transgender Health Data and Its Implications for Addressing Health Disparities,” New Mexico Department of Health, April 2010.
3. “Supporting and Caring for Our Bisexual Youth,” the Human Rights Campaign Foundation, 2014.
Lesbian, gay, bisexual, transgender, and questioning or queer (LGBTQ) youth face bias and discrimination within the health care setting and experience disparities in health, compared with their heterosexual cisgender peers. This is an area that is receiving increasing attention and study as health care providers and researchers work to achieve health equity within these populations.
Studies focusing specifically on the health of bisexual youth and adults are lacking. The few that do exist suggest that the experiences of people who identify as bisexual may be different from those who identify as lesbian or gay. Myths and misconceptions about bisexual, pansexual, queer, and fluid identities may in some cases put these populations at increased risks. Common myths include that bisexuality is just a phase or that youth who identify as bisexual are just confused. Studies suggest that bisexual youth account for almost half of youth who identify as LGBTQ. Understanding more about some of the challenges bisexual youth and adults may face can help us better care for all of our patients and families.
• Bisexual adults are more likely to engage in self-harming behaviors, attempt suicide, or think about suicide than heterosexual adults, lesbian women, or gay men.1
• Bisexual women have higher rates of high blood pressure, compared with heterosexual and lesbian women.2
• Bisexual women have higher rates of alcohol-related disorders than lesbian and heterosexual women.1
Some disparities appear to be related to lack of preventive care. A survey by the Williams Institute found that 39% of bisexual men and 33% of bisexual women did not disclose their sexual orientation, compared with 13% of gay men and 10% of lesbian women.1 The effect of intersecting identities also must be considered when discussing these health disparities. More than 40% of LGBTQ people of color identify as bisexual, and almost half of transgender people describe their sexual orientation as bisexual or queer.1 These individuals may be especially vulnerable to health disparities as they may experience a combination of racism, transphobia, and biphobia.
Risk factors for these disparities may develop early in life. A 2012 survey of LGBTQ youth found that:3
• Bisexual youth were less likely than lesbian and gay youth to report having supportive adults who they could turn to if they were sad.
• Only 5% of bisexual youth reported being very happy, compared with 8% of gay and lesbian youth and 21% of non-LGBT youth.
• Bisexual youth reported higher rates of experimentation with drugs and alcohol, compared with their lesbian, gay, and heterosexual peers.
• Bisexual youth reported lower levels of family acceptance and knowledge of social support systems in their communities than lesbian and gay youth. Both family acceptance and knowledge of social support systems have been identified as protective factors in the development of youth.
• Bisexual youth are less likely to be out to their friends, families, and communities.
As health care providers, recognizing, respecting, and supporting the identities of our bisexual patients is important. A few simple things we can do in practice are as follows:
• Don’t mislabel patients as lesbian, gay, or straight when they have disclosed a bisexual identity.
• Don’t assume that bisexuality is just a phase or that youths are confused when they disclose their identity.
• Don’t assume you know a patient’s sexual orientation or behaviors on the basis of the sex of the current partner or current behaviors.
• Do ask open and nonjudgmental questions about sexual attraction and behaviors, and be familiar with the terms bisexual, queer, questioning, and pansexual in addition to lesbian, gay, and straight.
• Do use inclusive terms like LGBT when referring to the community rather than gay rights.
• Do recognize potential biases and assumptions regarding sexuality and bisexuality and work to change them.
Dr. Chelvakumar is an attending physician in the division of adolescent medicine at Nationwide Children’s Hospital and an assistant professor of clinical pediatrics at the Ohio State University, both in Columbus.
Terms and definitions:
Bisexual – A person who can be attracted to more than one sex, gender, or gender identity. “Bi” is often used as an abbreviation.
Biphobia – Prejudice, fear, or hatred directed toward bisexual people.
Queer – A term people often use to express fluid identities and orientations. Historically considered a pejorative term, but used by many youth to describe their identity.
Pansexual – A person who can be attracted to any sex, gender, or gender identity.
References:
1. ”Health Disparities Among Bisexual People,” brief by the Human Rights Campaign Foundation.
2. “New Mexico’s Progress in Collecting Lesbian, Gay, Bisexual, and Transgender Health Data and Its Implications for Addressing Health Disparities,” New Mexico Department of Health, April 2010.
3. “Supporting and Caring for Our Bisexual Youth,” the Human Rights Campaign Foundation, 2014.
Self-injury
Whether you have heard about “cutting” from breathless gossip reports about young starlets or anxious parents of adolescent girls, it seems to be a phenomenon that is on the rise.
As a pediatrician, you may be the first (or only) adult in a young person’s life who notices evidence of self-injury or who asks about it. Self-injurious behaviors may signal significant underlying psychiatric issues or something more benign and brief. Being alert to self-injury is not an easy task. The thought of teenagers cutting themselves on a regular basis and acknowledging their inner distress in your office requires a pediatrician’s self-awareness and emotional preparation.
Self-injury, or nonsuicidal self-injury (NSSI) as it is known in the psychiatric literature, is indeed a relatively common phenomenon. In the United States, it affects approximately 10% of adolescents in a community sample, and as many as 35% of adolescents in treatment for any psychiatric illness. It begins most commonly between the ages of 13 and 15 years, and grows in prevalence through adolescence, dropping off in early adulthood. While adolescent girls are likely to start this behavior earlier than adolescent boys, the gender difference attenuates with age. Some studies have shown adolescent boys are more likely to engage in this behavior than girls by late adolescence.
NSSI typically takes the form of cutting oneself with a sharp object, but it also could involve scratching at the skin until it bleeds, hitting or burning oneself, or interfering with the healing of wounds. It classically was thought of as a symptom of borderline personality disorder, but is a behavior that also may occur with eating disorders, substance use disorders, and anxiety and depressive disorders in adolescents. Clinicians have conceptualized it as a maladaptive way to relieve intense emotional distress, signal distress to others, or inflict self-punishment. It usually starts as an impulsive behavior, and the combination of the intense emotions and high impulsivity of adolescence is why it is so common among this age group. For some adolescents, the impulse will be primarily one of curiosity, perhaps in the setting of some stress, and is more likely to occur if the behavior is common among a teenager’s peers. For those in intense emotional distress, it typically brings a fleeting sense of calm or numbing and an easing of tension. But this relief is usually followed by guilt and shame, and a return, sometimes compounded, of those uncomfortable emotions. Thus what starts as an impulse can become a repetitive, almost compulsive behavior.
If the self-injury happens regularly, it is very important that you show both concern and compassion. You might offer that whatever emotional pain they are experiencing, they deserve more support than a sharp object offers. You could ask about those illnesses that are frequently comorbid with self-injury: substance use, eating disorders, and anxiety and depressive disorders.
But it is essential that you ask about suicidal ideation and suicide attempts. If they are acutely suicidal or describe a history of previously hidden attempts, you will need to help them access care quickly, possibly recommending a visit to the emergency department unless they already have an outpatient treatment team. In these cases, you will need to share your concerns with their parents and help them find their way into the complex mental health system to get a comprehensive psychiatric evaluation and treatment.
Identifying and referring adolescents with NSSI is emotionally demanding work. Learn more from your patients, talk to those who evaluate them, and discuss the issues with colleagues – both to gain skills and to have support as you worry about these patients and help guide them through a complex system of care.
Dr. Swick is an attending psychiatrist in the division of child psychiatry at Massachusetts General Hospital, Boston, and director of the Parenting at a Challenging Time (PACT) Program at the Vernon Cancer Center at Newton Wellesley Hospital, also in Boston. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston.
Whether you have heard about “cutting” from breathless gossip reports about young starlets or anxious parents of adolescent girls, it seems to be a phenomenon that is on the rise.
As a pediatrician, you may be the first (or only) adult in a young person’s life who notices evidence of self-injury or who asks about it. Self-injurious behaviors may signal significant underlying psychiatric issues or something more benign and brief. Being alert to self-injury is not an easy task. The thought of teenagers cutting themselves on a regular basis and acknowledging their inner distress in your office requires a pediatrician’s self-awareness and emotional preparation.
Self-injury, or nonsuicidal self-injury (NSSI) as it is known in the psychiatric literature, is indeed a relatively common phenomenon. In the United States, it affects approximately 10% of adolescents in a community sample, and as many as 35% of adolescents in treatment for any psychiatric illness. It begins most commonly between the ages of 13 and 15 years, and grows in prevalence through adolescence, dropping off in early adulthood. While adolescent girls are likely to start this behavior earlier than adolescent boys, the gender difference attenuates with age. Some studies have shown adolescent boys are more likely to engage in this behavior than girls by late adolescence.
NSSI typically takes the form of cutting oneself with a sharp object, but it also could involve scratching at the skin until it bleeds, hitting or burning oneself, or interfering with the healing of wounds. It classically was thought of as a symptom of borderline personality disorder, but is a behavior that also may occur with eating disorders, substance use disorders, and anxiety and depressive disorders in adolescents. Clinicians have conceptualized it as a maladaptive way to relieve intense emotional distress, signal distress to others, or inflict self-punishment. It usually starts as an impulsive behavior, and the combination of the intense emotions and high impulsivity of adolescence is why it is so common among this age group. For some adolescents, the impulse will be primarily one of curiosity, perhaps in the setting of some stress, and is more likely to occur if the behavior is common among a teenager’s peers. For those in intense emotional distress, it typically brings a fleeting sense of calm or numbing and an easing of tension. But this relief is usually followed by guilt and shame, and a return, sometimes compounded, of those uncomfortable emotions. Thus what starts as an impulse can become a repetitive, almost compulsive behavior.
If the self-injury happens regularly, it is very important that you show both concern and compassion. You might offer that whatever emotional pain they are experiencing, they deserve more support than a sharp object offers. You could ask about those illnesses that are frequently comorbid with self-injury: substance use, eating disorders, and anxiety and depressive disorders.
But it is essential that you ask about suicidal ideation and suicide attempts. If they are acutely suicidal or describe a history of previously hidden attempts, you will need to help them access care quickly, possibly recommending a visit to the emergency department unless they already have an outpatient treatment team. In these cases, you will need to share your concerns with their parents and help them find their way into the complex mental health system to get a comprehensive psychiatric evaluation and treatment.
Identifying and referring adolescents with NSSI is emotionally demanding work. Learn more from your patients, talk to those who evaluate them, and discuss the issues with colleagues – both to gain skills and to have support as you worry about these patients and help guide them through a complex system of care.
Dr. Swick is an attending psychiatrist in the division of child psychiatry at Massachusetts General Hospital, Boston, and director of the Parenting at a Challenging Time (PACT) Program at the Vernon Cancer Center at Newton Wellesley Hospital, also in Boston. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston.
Whether you have heard about “cutting” from breathless gossip reports about young starlets or anxious parents of adolescent girls, it seems to be a phenomenon that is on the rise.
As a pediatrician, you may be the first (or only) adult in a young person’s life who notices evidence of self-injury or who asks about it. Self-injurious behaviors may signal significant underlying psychiatric issues or something more benign and brief. Being alert to self-injury is not an easy task. The thought of teenagers cutting themselves on a regular basis and acknowledging their inner distress in your office requires a pediatrician’s self-awareness and emotional preparation.
Self-injury, or nonsuicidal self-injury (NSSI) as it is known in the psychiatric literature, is indeed a relatively common phenomenon. In the United States, it affects approximately 10% of adolescents in a community sample, and as many as 35% of adolescents in treatment for any psychiatric illness. It begins most commonly between the ages of 13 and 15 years, and grows in prevalence through adolescence, dropping off in early adulthood. While adolescent girls are likely to start this behavior earlier than adolescent boys, the gender difference attenuates with age. Some studies have shown adolescent boys are more likely to engage in this behavior than girls by late adolescence.
NSSI typically takes the form of cutting oneself with a sharp object, but it also could involve scratching at the skin until it bleeds, hitting or burning oneself, or interfering with the healing of wounds. It classically was thought of as a symptom of borderline personality disorder, but is a behavior that also may occur with eating disorders, substance use disorders, and anxiety and depressive disorders in adolescents. Clinicians have conceptualized it as a maladaptive way to relieve intense emotional distress, signal distress to others, or inflict self-punishment. It usually starts as an impulsive behavior, and the combination of the intense emotions and high impulsivity of adolescence is why it is so common among this age group. For some adolescents, the impulse will be primarily one of curiosity, perhaps in the setting of some stress, and is more likely to occur if the behavior is common among a teenager’s peers. For those in intense emotional distress, it typically brings a fleeting sense of calm or numbing and an easing of tension. But this relief is usually followed by guilt and shame, and a return, sometimes compounded, of those uncomfortable emotions. Thus what starts as an impulse can become a repetitive, almost compulsive behavior.
If the self-injury happens regularly, it is very important that you show both concern and compassion. You might offer that whatever emotional pain they are experiencing, they deserve more support than a sharp object offers. You could ask about those illnesses that are frequently comorbid with self-injury: substance use, eating disorders, and anxiety and depressive disorders.
But it is essential that you ask about suicidal ideation and suicide attempts. If they are acutely suicidal or describe a history of previously hidden attempts, you will need to help them access care quickly, possibly recommending a visit to the emergency department unless they already have an outpatient treatment team. In these cases, you will need to share your concerns with their parents and help them find their way into the complex mental health system to get a comprehensive psychiatric evaluation and treatment.
Identifying and referring adolescents with NSSI is emotionally demanding work. Learn more from your patients, talk to those who evaluate them, and discuss the issues with colleagues – both to gain skills and to have support as you worry about these patients and help guide them through a complex system of care.
Dr. Swick is an attending psychiatrist in the division of child psychiatry at Massachusetts General Hospital, Boston, and director of the Parenting at a Challenging Time (PACT) Program at the Vernon Cancer Center at Newton Wellesley Hospital, also in Boston. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston.
Down syndrome in adolescents
Teen years, no doubt, come with expected challenges. For teens with Down syndrome (DS), the challenges are exponentially greater. Although DS is associated with mental retardation, the vast majority of people with DS have only mild to moderate intellectual disability. Still, this can lead to a great deal of internal stress for the adolescent and anxiety and stress for the parents.
Many issues must be considered for the adolescent with DS. Annual surveillance changes as the child ages and the need for intervention do as well. For example, 50% of children with DS are born with congenital heart defect. During infancy, it is important to do echocardiograms to identify and follow specific lesions. Approximately 57% of adolescents with DS will develop mitral valve prolapse and 10% will develop aortic regurgitation.1 Therefore, close evaluation should be done, listening for new onset murmurs, clicks, and unexplained fatigue, and, if these are identified, an echocardiogram should be repeated.
Dysmorphic features such as midfacial hypoplasia, tonsillar hypertrophy, and narrow ear canals also lead to issues that develop in the adolescent years. Chronic otitis, conductive hearing loss because of chronic middle-ear effusion or impacted cerumen, and enlarged tonsils can result in obstructive sleep apnea. As individuals gain weight, these issues are further affected, and a sleep study may be required. Annual hearing screens are recommended.1
Musculoskeletal disorders such as ligamentous laxity and atlantoaxial instability can also present with complications in the adolescent years. Pes planus is a very common finding that can further lead to hip and knee pain. Obesity also further adds to its occurrence (14%-67%).3
Now, the most pressing and, likely, most overlooked issue is the issue of sexuality. We spend a great deal of time educating teens without DS about the risks of unprotected sex and exposures to STIs, but many assume that these issues do not affect teens with DS. Secondary sexual characteristics develop in the same manner and at the same age that they do in children without DS. Therefore, it is a safe assumption that sexual curiosity and arousal do as well. Given that there can be varying levels of mental impairment, the approach to sex education needs to be developmentally appropriate. Normalizing the feelings and having discussions on appropriate and inappropriate expression are important.
As with all teens, acceptance and inclusion are of utmost importance. Physical and learning disabilities set them apart despite shared sexual and emotional development. This can lead to anxiety, depression, and behavior issues. Understanding that these are real issues for the adolescent with DS is important when providing the appropriate resources and support.
Management of the menstrual cycle can add new challenges for both the adolescent and the parent and, thus, should be investigated during health maintenance visits. Amenorrhea can result from introduction of hormonal therapy and should be considered. Sexual abuse also is increased in this age group, so close supervision and awareness of this issue are important.
Assisting parents of children with DS through the teen years is imperative. Making them aware of local support groups and national organizations for children with disabilities will help them navigate these years. The American Academy of Pediatrics has guidelines for health maintenance for DS.2
Dr. Pearce is a pediatrician in Frankfort, Ill. She reported no relevant financial disclosures. Email her at [email protected]
References
1. J Pediatr Health Care. 2006 May-Jun;20(3):198-205.
2. Pediatrics. 2011. doi: 10.1542/peds.2011-1605.
3. Clin Obes. 2015;5(2):52-9.
Correction, 7/26/17: An earlier version of this article misstated a recommendation about radiologic evaluation of the cervical spine in asymptomatic children
Teen years, no doubt, come with expected challenges. For teens with Down syndrome (DS), the challenges are exponentially greater. Although DS is associated with mental retardation, the vast majority of people with DS have only mild to moderate intellectual disability. Still, this can lead to a great deal of internal stress for the adolescent and anxiety and stress for the parents.
Many issues must be considered for the adolescent with DS. Annual surveillance changes as the child ages and the need for intervention do as well. For example, 50% of children with DS are born with congenital heart defect. During infancy, it is important to do echocardiograms to identify and follow specific lesions. Approximately 57% of adolescents with DS will develop mitral valve prolapse and 10% will develop aortic regurgitation.1 Therefore, close evaluation should be done, listening for new onset murmurs, clicks, and unexplained fatigue, and, if these are identified, an echocardiogram should be repeated.
Dysmorphic features such as midfacial hypoplasia, tonsillar hypertrophy, and narrow ear canals also lead to issues that develop in the adolescent years. Chronic otitis, conductive hearing loss because of chronic middle-ear effusion or impacted cerumen, and enlarged tonsils can result in obstructive sleep apnea. As individuals gain weight, these issues are further affected, and a sleep study may be required. Annual hearing screens are recommended.1
Musculoskeletal disorders such as ligamentous laxity and atlantoaxial instability can also present with complications in the adolescent years. Pes planus is a very common finding that can further lead to hip and knee pain. Obesity also further adds to its occurrence (14%-67%).3
Now, the most pressing and, likely, most overlooked issue is the issue of sexuality. We spend a great deal of time educating teens without DS about the risks of unprotected sex and exposures to STIs, but many assume that these issues do not affect teens with DS. Secondary sexual characteristics develop in the same manner and at the same age that they do in children without DS. Therefore, it is a safe assumption that sexual curiosity and arousal do as well. Given that there can be varying levels of mental impairment, the approach to sex education needs to be developmentally appropriate. Normalizing the feelings and having discussions on appropriate and inappropriate expression are important.
As with all teens, acceptance and inclusion are of utmost importance. Physical and learning disabilities set them apart despite shared sexual and emotional development. This can lead to anxiety, depression, and behavior issues. Understanding that these are real issues for the adolescent with DS is important when providing the appropriate resources and support.
Management of the menstrual cycle can add new challenges for both the adolescent and the parent and, thus, should be investigated during health maintenance visits. Amenorrhea can result from introduction of hormonal therapy and should be considered. Sexual abuse also is increased in this age group, so close supervision and awareness of this issue are important.
Assisting parents of children with DS through the teen years is imperative. Making them aware of local support groups and national organizations for children with disabilities will help them navigate these years. The American Academy of Pediatrics has guidelines for health maintenance for DS.2
Dr. Pearce is a pediatrician in Frankfort, Ill. She reported no relevant financial disclosures. Email her at [email protected]
References
1. J Pediatr Health Care. 2006 May-Jun;20(3):198-205.
2. Pediatrics. 2011. doi: 10.1542/peds.2011-1605.
3. Clin Obes. 2015;5(2):52-9.
Correction, 7/26/17: An earlier version of this article misstated a recommendation about radiologic evaluation of the cervical spine in asymptomatic children
Teen years, no doubt, come with expected challenges. For teens with Down syndrome (DS), the challenges are exponentially greater. Although DS is associated with mental retardation, the vast majority of people with DS have only mild to moderate intellectual disability. Still, this can lead to a great deal of internal stress for the adolescent and anxiety and stress for the parents.
Many issues must be considered for the adolescent with DS. Annual surveillance changes as the child ages and the need for intervention do as well. For example, 50% of children with DS are born with congenital heart defect. During infancy, it is important to do echocardiograms to identify and follow specific lesions. Approximately 57% of adolescents with DS will develop mitral valve prolapse and 10% will develop aortic regurgitation.1 Therefore, close evaluation should be done, listening for new onset murmurs, clicks, and unexplained fatigue, and, if these are identified, an echocardiogram should be repeated.
Dysmorphic features such as midfacial hypoplasia, tonsillar hypertrophy, and narrow ear canals also lead to issues that develop in the adolescent years. Chronic otitis, conductive hearing loss because of chronic middle-ear effusion or impacted cerumen, and enlarged tonsils can result in obstructive sleep apnea. As individuals gain weight, these issues are further affected, and a sleep study may be required. Annual hearing screens are recommended.1
Musculoskeletal disorders such as ligamentous laxity and atlantoaxial instability can also present with complications in the adolescent years. Pes planus is a very common finding that can further lead to hip and knee pain. Obesity also further adds to its occurrence (14%-67%).3
Now, the most pressing and, likely, most overlooked issue is the issue of sexuality. We spend a great deal of time educating teens without DS about the risks of unprotected sex and exposures to STIs, but many assume that these issues do not affect teens with DS. Secondary sexual characteristics develop in the same manner and at the same age that they do in children without DS. Therefore, it is a safe assumption that sexual curiosity and arousal do as well. Given that there can be varying levels of mental impairment, the approach to sex education needs to be developmentally appropriate. Normalizing the feelings and having discussions on appropriate and inappropriate expression are important.
As with all teens, acceptance and inclusion are of utmost importance. Physical and learning disabilities set them apart despite shared sexual and emotional development. This can lead to anxiety, depression, and behavior issues. Understanding that these are real issues for the adolescent with DS is important when providing the appropriate resources and support.
Management of the menstrual cycle can add new challenges for both the adolescent and the parent and, thus, should be investigated during health maintenance visits. Amenorrhea can result from introduction of hormonal therapy and should be considered. Sexual abuse also is increased in this age group, so close supervision and awareness of this issue are important.
Assisting parents of children with DS through the teen years is imperative. Making them aware of local support groups and national organizations for children with disabilities will help them navigate these years. The American Academy of Pediatrics has guidelines for health maintenance for DS.2
Dr. Pearce is a pediatrician in Frankfort, Ill. She reported no relevant financial disclosures. Email her at [email protected]
References
1. J Pediatr Health Care. 2006 May-Jun;20(3):198-205.
2. Pediatrics. 2011. doi: 10.1542/peds.2011-1605.
3. Clin Obes. 2015;5(2):52-9.
Correction, 7/26/17: An earlier version of this article misstated a recommendation about radiologic evaluation of the cervical spine in asymptomatic children
Anxiety in children during a new administration; Why medical psychiatry is vital for my patients; And more
Anxiety in children during a new administration
Since the current administration took office, many children continue to grapple with the initial shock of the election results and the uncertainty of what the next 4 years will bring. In the days after the election, several patients sat in my office and spoke of intense feelings of sadness, anger, and worry. Their stress levels were elevated, and they searched desperately for refuge from the unknown. On the other side of the hospital, patients expressing suicidal ideation filed into the emergency room. A similar scene played out nationally when suicide prevention hotlines experienced a sharp increase in calls.
During this emotional time, it is critical to support our children. Some will be more affected than others. Children from immigrant backgrounds might be particularly fearful of what this means for them and their families. In the days after the election, a video surfaced from a middle school in Michigan featuring kids at lunch chanting, “Build the wall!”
Bullying also is a concern. Despite being a third-generation American, an 8-year-old boy woke up the day after the election confused and scared. One mother told me that a student confronted her 11-year-old son at school, yelling that the election outcome was a “good thing” and he should “go back to his country.” Like his mother, the 11-year-old was born in the United States.
Kids get their cues from the adults in their lives. Parents and teachers play an important role in modeling behavior and providing comfort. Adults need to support children and to do that properly they need make sure they have processed their own feelings. They do not need to be unrealistic or overly positive, but should offer hope and trust in our democratic system. With discussion, children should have ample opportunity to express how they feel. Psychiatrists can evaluate a child’s symptoms and presentation. Are current medications helping enough with the recent changes? Does a child need a medication adjustment or to be seen more often? Does he (she) need to be admitted to the hospital for evaluation of suicidal ideation? As a psychiatrist, do you need to revisit the list of resources in the community and give children a crisis hotline number? Also consider referring a child to a psychotherapist if needed. Some schools offered counseling after the election. It is worthwhile to contact school officials if a student is struggling or could benefit from additional support.
Although many unknowns remain, 1 thing is certain: children will have more questions and we must be ready to answer.
Balkozar S. Adam, MD
Associate Professor of Clinical Psychiatry Child and Adolescent Psychiatry
University of Missouri
Columbia, Missouri
Co-Editor
Missouri Psychiatry Newsletter
Jefferson City, Missouri
Two additional adjunctive therapies for mental health
I was excited to read Dr. Nasrallah’s editorial about adjunctive therapies for mental health disorders (Are you neuroprotecting your patients? 10 Adjunctive therapies to consider,
The article missed 2 important vitamins that play a crucial role in positive mental health treatment outcomes: folic acid and vitamin B12. In my practice, I have found up to 50% of my patients with depression have a vitamin B12 deficiency. After supplementation, these patients’ symptoms improve to the point that we often can reduce or eliminate medication. Folic acid deficiency has been found among individuals with depression and linked to poor response to treatment.1 Higher serum levels of homocysteine—a consequence of low folic acid levels—are linked to increased risk of developing depression later in life, as well as higher risk of cardiovascular disease.2,3 Folate also can be used for enhancing treatment response to antidepressants by increasing production of neurotransmitters.2
Another factor to consider is methylenetetrahydrofolate reductase (MTHFR) variants. Approximately 20% of the population cannot methylate B vitamins because of a variation on the MTHFR gene.4,5 These patients are at increased risk for depression because they are unable to use B vitamins, which are essential in the synthesis of serotonin and dopamine. These patients do not respond to B12 and folate supplements. For these individuals, I recommend methylated products, which can be purchased online.
I have found these practices, as well as many of those listed in the editorial, are effective in treating depression and anxiety.
Lara Kain, PA-C, MPAS
Psychiatric Physician Assistant
Tidewater Psychotherapy Services
Virginia Beach, Virginia
References
1. Kaner G, Soylu M, Yüksel N, et al. Evaluation of nutritional status of patients with depression. Biomed Res Int. 2015;2015:521481. doi: 10.1155/2015/521481.
2. Seppälä J, Koponen H, Kautiainen H, et al. Association between vitamin B12 and melancholic depressive symptoms: a Finnish population-based study. BMC Psychiatry. 2013;13:145. doi: 10.1186/1471-244X-13-145.
3. Petridou ET, Kousoulis AA, Michelakos T, et al. Folate and B12 serum levels in association with depression in the aged: a systemic review and meta-analysis. Aging Ment Health. 2016;20(9):965-973.
4. Lynch B. MTHFR mutations and the conditions they cause. MTHFR.Net. http://mthfr.net/mthfr-mutations-and-the-conditions-they-cause/2011/09/07. Accessed February 16, 2017.
5. Eszlari N, Kovacs D, Petschner P, et al. Distinct effects of folate pathway genes MTHFR and MTHFD1L on ruminative response style: a potential risk mechanism for depression. Transl Psychiatry. 2016;6(3):e745. doi: 10.1038/tp.2016.19.
An honest perspective on Cannabis in therapy
I enjoyed Dr. Nasrallah’s editorial “Maddening therapies: How hallucinogens morphed into novel treatments” (From the
As a psychiatrist specializing in bipolar and psychotic disorders—as well as the founder and Board President of Doctors for Cannabis Regulation—I appreciate his reservations about the potential of Cannabis to trigger psychosis in vulnerable individuals. My reading of the literature is there is good evidence for marijuana as a trigger—not as a cause—of the disease. However, what is the evidence for hallucinogens?
Cannabis can have adverse effects on brain development, but it is not clear whether those effects are worse than those caused by alcohol. In the absence of any head-to-head studies, how can we proceed?
David L. Nathan, MD, DFAPA
Clinical Associate Professor
Rutgers Robert Wood Johnson Medical School
Director of Continuing Medical Education
Princeton HealthCare System
Princeton, New Jersey
Dr. Nasrallah responds
LSD can cause psychosis, paranoid delusions, and altered thinking in addition to vivid visual hallucinations in some individuals but not all, because vulnerability occurs on a spectrum. I postulate that the recently discovered inverse agonist of the serotonin 5-HT2A receptor, pimavanserin (FDA-approved for visual hallucinations and delusions of Parkinson’s disease psychosis), might be effective for LSD psychosis because this hallucinogen has a strong binding affinity to the serotonin 5-HT2A receptors.
Studies show that marijuana can induce apoptosis, which would adversely affect brain development. Patients with schizophrenia who abuse marijuana have a lower gray matter volume than those who do not abuse the drug, and both groups have lower gray matter volume than matched healthy controls. I strongly advise a pregnant woman against smoking marijuana because it could impair the fetus’s brain development.
Henry A. Nasrallah, MD
Professor and Chair
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
Self-administering LSD: Solution or abuse
Dr. Nasrallah’s editorial (From the Editor,
H. Steven Moffic, MD
Retired Tenured Professor of Psychiatry
Medical College of Wisconsin
Milwaukee, Wisconsin
Dr. Nasrallah responds
I am not aware of any systematic data about self-prescribed use of microdoses of LSD to reduce anxiety and depression. Among persons with anxiety and depression who have not had access to psychiatric care, self-medicating with agents such as alcohol, stimulants, ketamine, or LSD is regarded as substance abuse. It also is questionable whether people can determine which microdose of LSD to use. Finally, most drugs of abuse are not “pure,” and many are laced with potentially harmful contaminants.
Henry A. Nasrallah, MD
Professor and Chair
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
Why medical psychiatry is vital for my patients
Dr. Paul Summergrad’s guest editorial “Medical psychiatry: The skill of integrating medical and psychiatric care” (
For me, the most inspiring sentence in Dr. Summergrad’s editorial was, “It is incumbent on us to pursue the medical differential of patients when we think it is needed, even if other physicians disagree.” I believe that this describes our job as physicians who specialize in psychiatry. To have a clinician of Dr. Summergrad’s stature write this was inspiring because it goes to the core of what more of us should do.
Ruth Myers, MD
Psychiatrist
The Community Circle PLLC
Burnsville, Minnesota
Anxiety in children during a new administration
Since the current administration took office, many children continue to grapple with the initial shock of the election results and the uncertainty of what the next 4 years will bring. In the days after the election, several patients sat in my office and spoke of intense feelings of sadness, anger, and worry. Their stress levels were elevated, and they searched desperately for refuge from the unknown. On the other side of the hospital, patients expressing suicidal ideation filed into the emergency room. A similar scene played out nationally when suicide prevention hotlines experienced a sharp increase in calls.
During this emotional time, it is critical to support our children. Some will be more affected than others. Children from immigrant backgrounds might be particularly fearful of what this means for them and their families. In the days after the election, a video surfaced from a middle school in Michigan featuring kids at lunch chanting, “Build the wall!”
Bullying also is a concern. Despite being a third-generation American, an 8-year-old boy woke up the day after the election confused and scared. One mother told me that a student confronted her 11-year-old son at school, yelling that the election outcome was a “good thing” and he should “go back to his country.” Like his mother, the 11-year-old was born in the United States.
Kids get their cues from the adults in their lives. Parents and teachers play an important role in modeling behavior and providing comfort. Adults need to support children and to do that properly they need make sure they have processed their own feelings. They do not need to be unrealistic or overly positive, but should offer hope and trust in our democratic system. With discussion, children should have ample opportunity to express how they feel. Psychiatrists can evaluate a child’s symptoms and presentation. Are current medications helping enough with the recent changes? Does a child need a medication adjustment or to be seen more often? Does he (she) need to be admitted to the hospital for evaluation of suicidal ideation? As a psychiatrist, do you need to revisit the list of resources in the community and give children a crisis hotline number? Also consider referring a child to a psychotherapist if needed. Some schools offered counseling after the election. It is worthwhile to contact school officials if a student is struggling or could benefit from additional support.
Although many unknowns remain, 1 thing is certain: children will have more questions and we must be ready to answer.
Balkozar S. Adam, MD
Associate Professor of Clinical Psychiatry Child and Adolescent Psychiatry
University of Missouri
Columbia, Missouri
Co-Editor
Missouri Psychiatry Newsletter
Jefferson City, Missouri
Two additional adjunctive therapies for mental health
I was excited to read Dr. Nasrallah’s editorial about adjunctive therapies for mental health disorders (Are you neuroprotecting your patients? 10 Adjunctive therapies to consider,
The article missed 2 important vitamins that play a crucial role in positive mental health treatment outcomes: folic acid and vitamin B12. In my practice, I have found up to 50% of my patients with depression have a vitamin B12 deficiency. After supplementation, these patients’ symptoms improve to the point that we often can reduce or eliminate medication. Folic acid deficiency has been found among individuals with depression and linked to poor response to treatment.1 Higher serum levels of homocysteine—a consequence of low folic acid levels—are linked to increased risk of developing depression later in life, as well as higher risk of cardiovascular disease.2,3 Folate also can be used for enhancing treatment response to antidepressants by increasing production of neurotransmitters.2
Another factor to consider is methylenetetrahydrofolate reductase (MTHFR) variants. Approximately 20% of the population cannot methylate B vitamins because of a variation on the MTHFR gene.4,5 These patients are at increased risk for depression because they are unable to use B vitamins, which are essential in the synthesis of serotonin and dopamine. These patients do not respond to B12 and folate supplements. For these individuals, I recommend methylated products, which can be purchased online.
I have found these practices, as well as many of those listed in the editorial, are effective in treating depression and anxiety.
Lara Kain, PA-C, MPAS
Psychiatric Physician Assistant
Tidewater Psychotherapy Services
Virginia Beach, Virginia
References
1. Kaner G, Soylu M, Yüksel N, et al. Evaluation of nutritional status of patients with depression. Biomed Res Int. 2015;2015:521481. doi: 10.1155/2015/521481.
2. Seppälä J, Koponen H, Kautiainen H, et al. Association between vitamin B12 and melancholic depressive symptoms: a Finnish population-based study. BMC Psychiatry. 2013;13:145. doi: 10.1186/1471-244X-13-145.
3. Petridou ET, Kousoulis AA, Michelakos T, et al. Folate and B12 serum levels in association with depression in the aged: a systemic review and meta-analysis. Aging Ment Health. 2016;20(9):965-973.
4. Lynch B. MTHFR mutations and the conditions they cause. MTHFR.Net. http://mthfr.net/mthfr-mutations-and-the-conditions-they-cause/2011/09/07. Accessed February 16, 2017.
5. Eszlari N, Kovacs D, Petschner P, et al. Distinct effects of folate pathway genes MTHFR and MTHFD1L on ruminative response style: a potential risk mechanism for depression. Transl Psychiatry. 2016;6(3):e745. doi: 10.1038/tp.2016.19.
An honest perspective on Cannabis in therapy
I enjoyed Dr. Nasrallah’s editorial “Maddening therapies: How hallucinogens morphed into novel treatments” (From the
As a psychiatrist specializing in bipolar and psychotic disorders—as well as the founder and Board President of Doctors for Cannabis Regulation—I appreciate his reservations about the potential of Cannabis to trigger psychosis in vulnerable individuals. My reading of the literature is there is good evidence for marijuana as a trigger—not as a cause—of the disease. However, what is the evidence for hallucinogens?
Cannabis can have adverse effects on brain development, but it is not clear whether those effects are worse than those caused by alcohol. In the absence of any head-to-head studies, how can we proceed?
David L. Nathan, MD, DFAPA
Clinical Associate Professor
Rutgers Robert Wood Johnson Medical School
Director of Continuing Medical Education
Princeton HealthCare System
Princeton, New Jersey
Dr. Nasrallah responds
LSD can cause psychosis, paranoid delusions, and altered thinking in addition to vivid visual hallucinations in some individuals but not all, because vulnerability occurs on a spectrum. I postulate that the recently discovered inverse agonist of the serotonin 5-HT2A receptor, pimavanserin (FDA-approved for visual hallucinations and delusions of Parkinson’s disease psychosis), might be effective for LSD psychosis because this hallucinogen has a strong binding affinity to the serotonin 5-HT2A receptors.
Studies show that marijuana can induce apoptosis, which would adversely affect brain development. Patients with schizophrenia who abuse marijuana have a lower gray matter volume than those who do not abuse the drug, and both groups have lower gray matter volume than matched healthy controls. I strongly advise a pregnant woman against smoking marijuana because it could impair the fetus’s brain development.
Henry A. Nasrallah, MD
Professor and Chair
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
Self-administering LSD: Solution or abuse
Dr. Nasrallah’s editorial (From the Editor,
H. Steven Moffic, MD
Retired Tenured Professor of Psychiatry
Medical College of Wisconsin
Milwaukee, Wisconsin
Dr. Nasrallah responds
I am not aware of any systematic data about self-prescribed use of microdoses of LSD to reduce anxiety and depression. Among persons with anxiety and depression who have not had access to psychiatric care, self-medicating with agents such as alcohol, stimulants, ketamine, or LSD is regarded as substance abuse. It also is questionable whether people can determine which microdose of LSD to use. Finally, most drugs of abuse are not “pure,” and many are laced with potentially harmful contaminants.
Henry A. Nasrallah, MD
Professor and Chair
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
Why medical psychiatry is vital for my patients
Dr. Paul Summergrad’s guest editorial “Medical psychiatry: The skill of integrating medical and psychiatric care” (
For me, the most inspiring sentence in Dr. Summergrad’s editorial was, “It is incumbent on us to pursue the medical differential of patients when we think it is needed, even if other physicians disagree.” I believe that this describes our job as physicians who specialize in psychiatry. To have a clinician of Dr. Summergrad’s stature write this was inspiring because it goes to the core of what more of us should do.
Ruth Myers, MD
Psychiatrist
The Community Circle PLLC
Burnsville, Minnesota
Anxiety in children during a new administration
Since the current administration took office, many children continue to grapple with the initial shock of the election results and the uncertainty of what the next 4 years will bring. In the days after the election, several patients sat in my office and spoke of intense feelings of sadness, anger, and worry. Their stress levels were elevated, and they searched desperately for refuge from the unknown. On the other side of the hospital, patients expressing suicidal ideation filed into the emergency room. A similar scene played out nationally when suicide prevention hotlines experienced a sharp increase in calls.
During this emotional time, it is critical to support our children. Some will be more affected than others. Children from immigrant backgrounds might be particularly fearful of what this means for them and their families. In the days after the election, a video surfaced from a middle school in Michigan featuring kids at lunch chanting, “Build the wall!”
Bullying also is a concern. Despite being a third-generation American, an 8-year-old boy woke up the day after the election confused and scared. One mother told me that a student confronted her 11-year-old son at school, yelling that the election outcome was a “good thing” and he should “go back to his country.” Like his mother, the 11-year-old was born in the United States.
Kids get their cues from the adults in their lives. Parents and teachers play an important role in modeling behavior and providing comfort. Adults need to support children and to do that properly they need make sure they have processed their own feelings. They do not need to be unrealistic or overly positive, but should offer hope and trust in our democratic system. With discussion, children should have ample opportunity to express how they feel. Psychiatrists can evaluate a child’s symptoms and presentation. Are current medications helping enough with the recent changes? Does a child need a medication adjustment or to be seen more often? Does he (she) need to be admitted to the hospital for evaluation of suicidal ideation? As a psychiatrist, do you need to revisit the list of resources in the community and give children a crisis hotline number? Also consider referring a child to a psychotherapist if needed. Some schools offered counseling after the election. It is worthwhile to contact school officials if a student is struggling or could benefit from additional support.
Although many unknowns remain, 1 thing is certain: children will have more questions and we must be ready to answer.
Balkozar S. Adam, MD
Associate Professor of Clinical Psychiatry Child and Adolescent Psychiatry
University of Missouri
Columbia, Missouri
Co-Editor
Missouri Psychiatry Newsletter
Jefferson City, Missouri
Two additional adjunctive therapies for mental health
I was excited to read Dr. Nasrallah’s editorial about adjunctive therapies for mental health disorders (Are you neuroprotecting your patients? 10 Adjunctive therapies to consider,
The article missed 2 important vitamins that play a crucial role in positive mental health treatment outcomes: folic acid and vitamin B12. In my practice, I have found up to 50% of my patients with depression have a vitamin B12 deficiency. After supplementation, these patients’ symptoms improve to the point that we often can reduce or eliminate medication. Folic acid deficiency has been found among individuals with depression and linked to poor response to treatment.1 Higher serum levels of homocysteine—a consequence of low folic acid levels—are linked to increased risk of developing depression later in life, as well as higher risk of cardiovascular disease.2,3 Folate also can be used for enhancing treatment response to antidepressants by increasing production of neurotransmitters.2
Another factor to consider is methylenetetrahydrofolate reductase (MTHFR) variants. Approximately 20% of the population cannot methylate B vitamins because of a variation on the MTHFR gene.4,5 These patients are at increased risk for depression because they are unable to use B vitamins, which are essential in the synthesis of serotonin and dopamine. These patients do not respond to B12 and folate supplements. For these individuals, I recommend methylated products, which can be purchased online.
I have found these practices, as well as many of those listed in the editorial, are effective in treating depression and anxiety.
Lara Kain, PA-C, MPAS
Psychiatric Physician Assistant
Tidewater Psychotherapy Services
Virginia Beach, Virginia
References
1. Kaner G, Soylu M, Yüksel N, et al. Evaluation of nutritional status of patients with depression. Biomed Res Int. 2015;2015:521481. doi: 10.1155/2015/521481.
2. Seppälä J, Koponen H, Kautiainen H, et al. Association between vitamin B12 and melancholic depressive symptoms: a Finnish population-based study. BMC Psychiatry. 2013;13:145. doi: 10.1186/1471-244X-13-145.
3. Petridou ET, Kousoulis AA, Michelakos T, et al. Folate and B12 serum levels in association with depression in the aged: a systemic review and meta-analysis. Aging Ment Health. 2016;20(9):965-973.
4. Lynch B. MTHFR mutations and the conditions they cause. MTHFR.Net. http://mthfr.net/mthfr-mutations-and-the-conditions-they-cause/2011/09/07. Accessed February 16, 2017.
5. Eszlari N, Kovacs D, Petschner P, et al. Distinct effects of folate pathway genes MTHFR and MTHFD1L on ruminative response style: a potential risk mechanism for depression. Transl Psychiatry. 2016;6(3):e745. doi: 10.1038/tp.2016.19.
An honest perspective on Cannabis in therapy
I enjoyed Dr. Nasrallah’s editorial “Maddening therapies: How hallucinogens morphed into novel treatments” (From the
As a psychiatrist specializing in bipolar and psychotic disorders—as well as the founder and Board President of Doctors for Cannabis Regulation—I appreciate his reservations about the potential of Cannabis to trigger psychosis in vulnerable individuals. My reading of the literature is there is good evidence for marijuana as a trigger—not as a cause—of the disease. However, what is the evidence for hallucinogens?
Cannabis can have adverse effects on brain development, but it is not clear whether those effects are worse than those caused by alcohol. In the absence of any head-to-head studies, how can we proceed?
David L. Nathan, MD, DFAPA
Clinical Associate Professor
Rutgers Robert Wood Johnson Medical School
Director of Continuing Medical Education
Princeton HealthCare System
Princeton, New Jersey
Dr. Nasrallah responds
LSD can cause psychosis, paranoid delusions, and altered thinking in addition to vivid visual hallucinations in some individuals but not all, because vulnerability occurs on a spectrum. I postulate that the recently discovered inverse agonist of the serotonin 5-HT2A receptor, pimavanserin (FDA-approved for visual hallucinations and delusions of Parkinson’s disease psychosis), might be effective for LSD psychosis because this hallucinogen has a strong binding affinity to the serotonin 5-HT2A receptors.
Studies show that marijuana can induce apoptosis, which would adversely affect brain development. Patients with schizophrenia who abuse marijuana have a lower gray matter volume than those who do not abuse the drug, and both groups have lower gray matter volume than matched healthy controls. I strongly advise a pregnant woman against smoking marijuana because it could impair the fetus’s brain development.
Henry A. Nasrallah, MD
Professor and Chair
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
Self-administering LSD: Solution or abuse
Dr. Nasrallah’s editorial (From the Editor,
H. Steven Moffic, MD
Retired Tenured Professor of Psychiatry
Medical College of Wisconsin
Milwaukee, Wisconsin
Dr. Nasrallah responds
I am not aware of any systematic data about self-prescribed use of microdoses of LSD to reduce anxiety and depression. Among persons with anxiety and depression who have not had access to psychiatric care, self-medicating with agents such as alcohol, stimulants, ketamine, or LSD is regarded as substance abuse. It also is questionable whether people can determine which microdose of LSD to use. Finally, most drugs of abuse are not “pure,” and many are laced with potentially harmful contaminants.
Henry A. Nasrallah, MD
Professor and Chair
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
Why medical psychiatry is vital for my patients
Dr. Paul Summergrad’s guest editorial “Medical psychiatry: The skill of integrating medical and psychiatric care” (
For me, the most inspiring sentence in Dr. Summergrad’s editorial was, “It is incumbent on us to pursue the medical differential of patients when we think it is needed, even if other physicians disagree.” I believe that this describes our job as physicians who specialize in psychiatry. To have a clinician of Dr. Summergrad’s stature write this was inspiring because it goes to the core of what more of us should do.
Ruth Myers, MD
Psychiatrist
The Community Circle PLLC
Burnsville, Minnesota













