User login
Workforce Shortage of Pediatric Dermatologists: A Medical Student’s Perspective
Workforce Shortage of Pediatric Dermatologists: A Medical Student’s Perspective
There is a shortage of pediatric dermatologists in the United States, with fewer than 2% of practicing dermatologists specializing in pediatrics.1 Pediatric dermatology has the third highest referral rate by pediatricians but also is the third most challenging specialty to access, with an average appointment wait time of 92 days.2,3 Another factor leading to increased appointment wait times is the specificity of care required for pediatric patients. Frequently, pediatric patients evaluated by a general dermatologist will be referred to their pediatric dermatology colleagues. As medical students, we were introduced to the field of pediatric dermatology through different avenues—personal experience, research mentorship, or a clinical rotation in medical school. We found ourselves curious about the discrepancy between the supply of and demand for pediatric dermatologists and wondered what could be done to increase awareness of this subspecialty among medical students. We believe this workforce shortage can be ameliorated by improving early exposure to pediatric dermatology. In this article, we explore the existing framework surrounding pediatric dermatology in medical education and offer feasible recommendations and solutions to realistically combat this problem.
Pediatric dermatologists are essential to the greater dermatology community. Pediatric dermatologists receive advanced training in complex pediatric skin conditions that often is lacking in general dermatology residency. A large percentage of pediatric dermatology patients seen in academic medical centers have already been seen by general dermatologists who subsequently referred them to specialty care. In one study, 9.6% (10/108) of practicing pediatric dermatologists noted that their referrals were from general dermatologists.4 In another study, 42% (19/45) of referrals to a multidisciplinary pediatric dermatology-genetics were from general dermatologists.5 Given the shortage of pediatric dermatologists, these referrals undoubtedly overwhelm the system, and the results of these studies underscore the reality that general dermatologists do not necessarily feel adequately trained in complex pediatric conditions, creating an intrinsic need for pediatric dermatologists.
Admani et al6 reported that early mentorship was the single most important factor to 84% (91/109) of survey respondents who pursued pediatric dermatology. Forty percent (40/100) of survey respondents chose their specialty of pediatric dermatology during pediatrics residency, 34% (34/100) during medical school, 17% (17/100) during dermatology residency, and 5% (5/100) during internship, indicating that medical school is a crucial time for recruitment.6 It has been noted in the literature that more medical students matched to dermatology residency from schools with dermatology clerkships built into the curriculum than from schools without dedicated dermatology rotations, suggesting that early clinical exposure to dermatology fields has a predictable influence in matching.7 Currently, only about 10% (15/155) of allopathic medical schools in the United States offer a formal elective in pediatric dermatology via the Association of American Medical College’s Visiting Student Learning Opportunities program.8 When this information was cross-referenced with the most recently matched pediatric dermatology fellowship class (2023-2024), provided by the Fellowship Directors Chair of the Society for Pediatric Dermatology, we found that 17% (4/24) of the matched fellows attended one of these 15 medical schools. We also found that the 2023-2024 pediatric dermatology fellowship class had 12 unmatched spots out of 36 total positions nationwide (33%), highlighting a gap in pediatric dermatology care and placing further strain on an already underserved subspecialty. These data suggest that, while dermatologists may decide to pursue pediatric dermatology fellowships during residency, there is an opportunity to foster interest during medical school training and improve the fellowship match rate.
Several medical schools in the United States incorporate pediatric dermatology into their curricula, including lectures in preclinical courses and career panels to pediatric dermatology electives in the third and fourth years. These institutions can serve as models for other medical schools. Within preclinical content, we recommend creating a designated dermatology unit that can incorporate common pediatric dermatology pathologies also seen by general practitioners, such as common childhood rashes, atopic dermatitis, alopecia areata, seborrheic dermatitis, and acne. Rare pediatric diseases such as epidermolysis bullosa, tuberous sclerosis, and Ehlers-Danlos syndrome also may be included in the unit. If schools are not able to offer a stand-alone dermatology preclinical course, this content can be added to the immunology, musculoskeletal, infectious diseases, or genetics courses to account for the multisystemic effects of some of these conditions. Ideally, schools would offer elective exposure to pediatric dermatology during the clinical years of medical school to increase knowledge of the field; for example, pediatric dermatology materials could be included in core clerkships, as much of this content is applicable to the general pediatrics rotation. In particular, a lecture on common rashes in pediatric patients could be given before starting the core pediatric rotation. Additionally, problem-based pediatric dermatology cases could be implemented during the core pediatrics rotation. If students are offered an independent dermatology clinical elective, the already formatted 2- and 4-week basic dermatology courses designed by the American Academy of Dermatology could serve as suggested teaching guides or as self-teaching resources that could complement the dermatology rotation.9,10 Pediatric topics (eg, pediatric cutaneous fungal infections) are included within the American Academy of Dermatology basic dermatology curriculum.8,9
Increasing access to pediatric dermatology resources such as lecture series and mentorship opportunities could further broaden the pediatric dermatology knowledge base of medical students. Within medical school dermatology interest groups, there is an opportunity to have a pediatric dermatology lead to help coordinate lecture series and journal club sessions for interested students. The Society for Pediatric Dermatology and the Pediatric Dermatology Research Alliance have created programs to support students, and we encourage schools to raise awareness of these organizations as well as conference and grant opportunities. These initiatives foster meaningful mentor-mentee relationships, and more medical students may be interested if they are aware of these support networks.
There also may be opportunities to create residency tracks that increase the number of dermatology residency applicants. Programs such as the newly implemented pediatric dermatology track at the University of Pennsylvania and New York University allow medical students who are interested in pursuing pediatric dermatology to have a more focused and linear training path.11,12 Due to the inherent competition in matching into dermatology, we surmise that many students with interest in pediatric dermatology are lost to pediatric residencies. Given the large percentage of pediatric residents who ultimately develop an interest in pediatric dermatology, holding a spot for pediatric dermatology applicants—akin to the combined medical-dermatology spots—may be an avenue to increase the pool of pediatric dermatology fellows.1,6 Another avenue is to encourage the development of first-year pediatric internship tracks that lead directly into dermatology residency, such as newly established programs at the University of Pennsylvania and New York University.11,12
As a group of both aspiring and practicing pediatric dermatologists, we have identified opportunities for formalized education in and early exposure to this subspecialty during medical training instead of leaving the discovery of the field to chance. The gaps in medical education that we have identified have already led us to present potential curricular changes to the medical education committee at our home institution. We hope to inspire the development of strong pediatric dermatology education at the medical school level.
While the solution to the pediatric dermatology workforce shortage is complex and multifaceted, there is a unique opportunity to target medical students through mentorship, access to education, and clinical experiences. We recommend that medical schools implement these educational methods and track the efficacy of these interventions to quantify the predicted association between an increased workforce and early exposure to pediatric dermatology. Addressing a lack of exposure to the field and increasing support of students pursuing pediatric dermatology can help to alleviate the shortage at the earliest point in training.
- Prindaville B, Antaya RJ, Siegfried EC. Pediatric dermatology: past, present, and future. Pediatr Dermatol. 2015;32:1-12. doi:10.1111/pde.12362
- Wright TS. Update on the pediatric dermatology workforce shortage. Cutis. 2021;108:237-238. doi:10.12788/cutis.0379
- Stephens MR, Murthy AS, McMahon PJ. Wait times, health care touchpoints, and nonattendance in an academic pediatric dermatology clinic. ediatr Dermatol. 2019;36:893-897. doi:10.1111/pde.13943
- Fogel AL, Teng JM. A survey to assess perceived differences in referral pathways to board-certified pediatric dermatologists. Pediatr Dermatol. 2015;32:e314-e315. doi:10.1111/pde.12703
- Parker JC, Rangu S, Grand KL, et al. Genetic skin disorders: the value of a multidisciplinary clinic. Am J Med Genet A. 2021;185:1159-1167. doi:10.1002/ajmg.a.62095
- Admani S, Caufield M, Kim SS, et al. Understanding the pediatric dermatology workforce shortage: mentoring matters. J Pediatr. 2014;164:372-5.e1. doi:10.1016/j.jpeds.2013.10.004
- Ogidi P, Ahmed F, Cahn BA, et al. Medical schools as gatekeepers: a survey and analysis of factors predicting dermatology residency placement. J Am Acad Dermatol. 2022;86:490-492. doi:10.1016 /j.jaad.2021.09.027
- Visiting Student Learning Opportunities (VSLO). Accessed May 30, 2025. https://students-residents.aamc.org/visiting-student-learning-opportunities/visiting-student-learning-opportunities-vslo
- American Academy of Dermatology Association. AAD Learning Center. Basic dermatology curriculum (2-week rotation). Accessed May 12, 2025. https://learning.aad.org/Listing/Basic-Dermatology-Curriculum-2-Week-Rotation-5395
- American Academy of Dermatology Association. AAD Learning Center. Basic dermatology curriculum (4-week rotation). Accessed May 12, 2025. https://learning.aad.org/Public/Catalog/Details.aspx?id=YPssTVIbBO3Zb%2bOuf%2fM7Kg%3d%3d&returnurl=%2fUsers%2fUserOnlineCourse.aspx%3fLearningActivityID%3dYPssTVIbBO3Zb%252bOuf%252fM7Kg%253d%253d
- Penn Medicine Dermatology Residency Training Program. Residency tracks. Accessed May 12, 2025. https://dermatology.upenn.edu/residents/residency-tracks/
- Pediatric Dermatology Residency Track at NYU Grossman School of Medicine. Pediatric Track. Accessed May 30, 2025. https://med.nyu.edu/departments-institutes/dermatology/education/residency/pediatric-track
There is a shortage of pediatric dermatologists in the United States, with fewer than 2% of practicing dermatologists specializing in pediatrics.1 Pediatric dermatology has the third highest referral rate by pediatricians but also is the third most challenging specialty to access, with an average appointment wait time of 92 days.2,3 Another factor leading to increased appointment wait times is the specificity of care required for pediatric patients. Frequently, pediatric patients evaluated by a general dermatologist will be referred to their pediatric dermatology colleagues. As medical students, we were introduced to the field of pediatric dermatology through different avenues—personal experience, research mentorship, or a clinical rotation in medical school. We found ourselves curious about the discrepancy between the supply of and demand for pediatric dermatologists and wondered what could be done to increase awareness of this subspecialty among medical students. We believe this workforce shortage can be ameliorated by improving early exposure to pediatric dermatology. In this article, we explore the existing framework surrounding pediatric dermatology in medical education and offer feasible recommendations and solutions to realistically combat this problem.
Pediatric dermatologists are essential to the greater dermatology community. Pediatric dermatologists receive advanced training in complex pediatric skin conditions that often is lacking in general dermatology residency. A large percentage of pediatric dermatology patients seen in academic medical centers have already been seen by general dermatologists who subsequently referred them to specialty care. In one study, 9.6% (10/108) of practicing pediatric dermatologists noted that their referrals were from general dermatologists.4 In another study, 42% (19/45) of referrals to a multidisciplinary pediatric dermatology-genetics were from general dermatologists.5 Given the shortage of pediatric dermatologists, these referrals undoubtedly overwhelm the system, and the results of these studies underscore the reality that general dermatologists do not necessarily feel adequately trained in complex pediatric conditions, creating an intrinsic need for pediatric dermatologists.
Admani et al6 reported that early mentorship was the single most important factor to 84% (91/109) of survey respondents who pursued pediatric dermatology. Forty percent (40/100) of survey respondents chose their specialty of pediatric dermatology during pediatrics residency, 34% (34/100) during medical school, 17% (17/100) during dermatology residency, and 5% (5/100) during internship, indicating that medical school is a crucial time for recruitment.6 It has been noted in the literature that more medical students matched to dermatology residency from schools with dermatology clerkships built into the curriculum than from schools without dedicated dermatology rotations, suggesting that early clinical exposure to dermatology fields has a predictable influence in matching.7 Currently, only about 10% (15/155) of allopathic medical schools in the United States offer a formal elective in pediatric dermatology via the Association of American Medical College’s Visiting Student Learning Opportunities program.8 When this information was cross-referenced with the most recently matched pediatric dermatology fellowship class (2023-2024), provided by the Fellowship Directors Chair of the Society for Pediatric Dermatology, we found that 17% (4/24) of the matched fellows attended one of these 15 medical schools. We also found that the 2023-2024 pediatric dermatology fellowship class had 12 unmatched spots out of 36 total positions nationwide (33%), highlighting a gap in pediatric dermatology care and placing further strain on an already underserved subspecialty. These data suggest that, while dermatologists may decide to pursue pediatric dermatology fellowships during residency, there is an opportunity to foster interest during medical school training and improve the fellowship match rate.
Several medical schools in the United States incorporate pediatric dermatology into their curricula, including lectures in preclinical courses and career panels to pediatric dermatology electives in the third and fourth years. These institutions can serve as models for other medical schools. Within preclinical content, we recommend creating a designated dermatology unit that can incorporate common pediatric dermatology pathologies also seen by general practitioners, such as common childhood rashes, atopic dermatitis, alopecia areata, seborrheic dermatitis, and acne. Rare pediatric diseases such as epidermolysis bullosa, tuberous sclerosis, and Ehlers-Danlos syndrome also may be included in the unit. If schools are not able to offer a stand-alone dermatology preclinical course, this content can be added to the immunology, musculoskeletal, infectious diseases, or genetics courses to account for the multisystemic effects of some of these conditions. Ideally, schools would offer elective exposure to pediatric dermatology during the clinical years of medical school to increase knowledge of the field; for example, pediatric dermatology materials could be included in core clerkships, as much of this content is applicable to the general pediatrics rotation. In particular, a lecture on common rashes in pediatric patients could be given before starting the core pediatric rotation. Additionally, problem-based pediatric dermatology cases could be implemented during the core pediatrics rotation. If students are offered an independent dermatology clinical elective, the already formatted 2- and 4-week basic dermatology courses designed by the American Academy of Dermatology could serve as suggested teaching guides or as self-teaching resources that could complement the dermatology rotation.9,10 Pediatric topics (eg, pediatric cutaneous fungal infections) are included within the American Academy of Dermatology basic dermatology curriculum.8,9
Increasing access to pediatric dermatology resources such as lecture series and mentorship opportunities could further broaden the pediatric dermatology knowledge base of medical students. Within medical school dermatology interest groups, there is an opportunity to have a pediatric dermatology lead to help coordinate lecture series and journal club sessions for interested students. The Society for Pediatric Dermatology and the Pediatric Dermatology Research Alliance have created programs to support students, and we encourage schools to raise awareness of these organizations as well as conference and grant opportunities. These initiatives foster meaningful mentor-mentee relationships, and more medical students may be interested if they are aware of these support networks.
There also may be opportunities to create residency tracks that increase the number of dermatology residency applicants. Programs such as the newly implemented pediatric dermatology track at the University of Pennsylvania and New York University allow medical students who are interested in pursuing pediatric dermatology to have a more focused and linear training path.11,12 Due to the inherent competition in matching into dermatology, we surmise that many students with interest in pediatric dermatology are lost to pediatric residencies. Given the large percentage of pediatric residents who ultimately develop an interest in pediatric dermatology, holding a spot for pediatric dermatology applicants—akin to the combined medical-dermatology spots—may be an avenue to increase the pool of pediatric dermatology fellows.1,6 Another avenue is to encourage the development of first-year pediatric internship tracks that lead directly into dermatology residency, such as newly established programs at the University of Pennsylvania and New York University.11,12
As a group of both aspiring and practicing pediatric dermatologists, we have identified opportunities for formalized education in and early exposure to this subspecialty during medical training instead of leaving the discovery of the field to chance. The gaps in medical education that we have identified have already led us to present potential curricular changes to the medical education committee at our home institution. We hope to inspire the development of strong pediatric dermatology education at the medical school level.
While the solution to the pediatric dermatology workforce shortage is complex and multifaceted, there is a unique opportunity to target medical students through mentorship, access to education, and clinical experiences. We recommend that medical schools implement these educational methods and track the efficacy of these interventions to quantify the predicted association between an increased workforce and early exposure to pediatric dermatology. Addressing a lack of exposure to the field and increasing support of students pursuing pediatric dermatology can help to alleviate the shortage at the earliest point in training.
There is a shortage of pediatric dermatologists in the United States, with fewer than 2% of practicing dermatologists specializing in pediatrics.1 Pediatric dermatology has the third highest referral rate by pediatricians but also is the third most challenging specialty to access, with an average appointment wait time of 92 days.2,3 Another factor leading to increased appointment wait times is the specificity of care required for pediatric patients. Frequently, pediatric patients evaluated by a general dermatologist will be referred to their pediatric dermatology colleagues. As medical students, we were introduced to the field of pediatric dermatology through different avenues—personal experience, research mentorship, or a clinical rotation in medical school. We found ourselves curious about the discrepancy between the supply of and demand for pediatric dermatologists and wondered what could be done to increase awareness of this subspecialty among medical students. We believe this workforce shortage can be ameliorated by improving early exposure to pediatric dermatology. In this article, we explore the existing framework surrounding pediatric dermatology in medical education and offer feasible recommendations and solutions to realistically combat this problem.
Pediatric dermatologists are essential to the greater dermatology community. Pediatric dermatologists receive advanced training in complex pediatric skin conditions that often is lacking in general dermatology residency. A large percentage of pediatric dermatology patients seen in academic medical centers have already been seen by general dermatologists who subsequently referred them to specialty care. In one study, 9.6% (10/108) of practicing pediatric dermatologists noted that their referrals were from general dermatologists.4 In another study, 42% (19/45) of referrals to a multidisciplinary pediatric dermatology-genetics were from general dermatologists.5 Given the shortage of pediatric dermatologists, these referrals undoubtedly overwhelm the system, and the results of these studies underscore the reality that general dermatologists do not necessarily feel adequately trained in complex pediatric conditions, creating an intrinsic need for pediatric dermatologists.
Admani et al6 reported that early mentorship was the single most important factor to 84% (91/109) of survey respondents who pursued pediatric dermatology. Forty percent (40/100) of survey respondents chose their specialty of pediatric dermatology during pediatrics residency, 34% (34/100) during medical school, 17% (17/100) during dermatology residency, and 5% (5/100) during internship, indicating that medical school is a crucial time for recruitment.6 It has been noted in the literature that more medical students matched to dermatology residency from schools with dermatology clerkships built into the curriculum than from schools without dedicated dermatology rotations, suggesting that early clinical exposure to dermatology fields has a predictable influence in matching.7 Currently, only about 10% (15/155) of allopathic medical schools in the United States offer a formal elective in pediatric dermatology via the Association of American Medical College’s Visiting Student Learning Opportunities program.8 When this information was cross-referenced with the most recently matched pediatric dermatology fellowship class (2023-2024), provided by the Fellowship Directors Chair of the Society for Pediatric Dermatology, we found that 17% (4/24) of the matched fellows attended one of these 15 medical schools. We also found that the 2023-2024 pediatric dermatology fellowship class had 12 unmatched spots out of 36 total positions nationwide (33%), highlighting a gap in pediatric dermatology care and placing further strain on an already underserved subspecialty. These data suggest that, while dermatologists may decide to pursue pediatric dermatology fellowships during residency, there is an opportunity to foster interest during medical school training and improve the fellowship match rate.
Several medical schools in the United States incorporate pediatric dermatology into their curricula, including lectures in preclinical courses and career panels to pediatric dermatology electives in the third and fourth years. These institutions can serve as models for other medical schools. Within preclinical content, we recommend creating a designated dermatology unit that can incorporate common pediatric dermatology pathologies also seen by general practitioners, such as common childhood rashes, atopic dermatitis, alopecia areata, seborrheic dermatitis, and acne. Rare pediatric diseases such as epidermolysis bullosa, tuberous sclerosis, and Ehlers-Danlos syndrome also may be included in the unit. If schools are not able to offer a stand-alone dermatology preclinical course, this content can be added to the immunology, musculoskeletal, infectious diseases, or genetics courses to account for the multisystemic effects of some of these conditions. Ideally, schools would offer elective exposure to pediatric dermatology during the clinical years of medical school to increase knowledge of the field; for example, pediatric dermatology materials could be included in core clerkships, as much of this content is applicable to the general pediatrics rotation. In particular, a lecture on common rashes in pediatric patients could be given before starting the core pediatric rotation. Additionally, problem-based pediatric dermatology cases could be implemented during the core pediatrics rotation. If students are offered an independent dermatology clinical elective, the already formatted 2- and 4-week basic dermatology courses designed by the American Academy of Dermatology could serve as suggested teaching guides or as self-teaching resources that could complement the dermatology rotation.9,10 Pediatric topics (eg, pediatric cutaneous fungal infections) are included within the American Academy of Dermatology basic dermatology curriculum.8,9
Increasing access to pediatric dermatology resources such as lecture series and mentorship opportunities could further broaden the pediatric dermatology knowledge base of medical students. Within medical school dermatology interest groups, there is an opportunity to have a pediatric dermatology lead to help coordinate lecture series and journal club sessions for interested students. The Society for Pediatric Dermatology and the Pediatric Dermatology Research Alliance have created programs to support students, and we encourage schools to raise awareness of these organizations as well as conference and grant opportunities. These initiatives foster meaningful mentor-mentee relationships, and more medical students may be interested if they are aware of these support networks.
There also may be opportunities to create residency tracks that increase the number of dermatology residency applicants. Programs such as the newly implemented pediatric dermatology track at the University of Pennsylvania and New York University allow medical students who are interested in pursuing pediatric dermatology to have a more focused and linear training path.11,12 Due to the inherent competition in matching into dermatology, we surmise that many students with interest in pediatric dermatology are lost to pediatric residencies. Given the large percentage of pediatric residents who ultimately develop an interest in pediatric dermatology, holding a spot for pediatric dermatology applicants—akin to the combined medical-dermatology spots—may be an avenue to increase the pool of pediatric dermatology fellows.1,6 Another avenue is to encourage the development of first-year pediatric internship tracks that lead directly into dermatology residency, such as newly established programs at the University of Pennsylvania and New York University.11,12
As a group of both aspiring and practicing pediatric dermatologists, we have identified opportunities for formalized education in and early exposure to this subspecialty during medical training instead of leaving the discovery of the field to chance. The gaps in medical education that we have identified have already led us to present potential curricular changes to the medical education committee at our home institution. We hope to inspire the development of strong pediatric dermatology education at the medical school level.
While the solution to the pediatric dermatology workforce shortage is complex and multifaceted, there is a unique opportunity to target medical students through mentorship, access to education, and clinical experiences. We recommend that medical schools implement these educational methods and track the efficacy of these interventions to quantify the predicted association between an increased workforce and early exposure to pediatric dermatology. Addressing a lack of exposure to the field and increasing support of students pursuing pediatric dermatology can help to alleviate the shortage at the earliest point in training.
- Prindaville B, Antaya RJ, Siegfried EC. Pediatric dermatology: past, present, and future. Pediatr Dermatol. 2015;32:1-12. doi:10.1111/pde.12362
- Wright TS. Update on the pediatric dermatology workforce shortage. Cutis. 2021;108:237-238. doi:10.12788/cutis.0379
- Stephens MR, Murthy AS, McMahon PJ. Wait times, health care touchpoints, and nonattendance in an academic pediatric dermatology clinic. ediatr Dermatol. 2019;36:893-897. doi:10.1111/pde.13943
- Fogel AL, Teng JM. A survey to assess perceived differences in referral pathways to board-certified pediatric dermatologists. Pediatr Dermatol. 2015;32:e314-e315. doi:10.1111/pde.12703
- Parker JC, Rangu S, Grand KL, et al. Genetic skin disorders: the value of a multidisciplinary clinic. Am J Med Genet A. 2021;185:1159-1167. doi:10.1002/ajmg.a.62095
- Admani S, Caufield M, Kim SS, et al. Understanding the pediatric dermatology workforce shortage: mentoring matters. J Pediatr. 2014;164:372-5.e1. doi:10.1016/j.jpeds.2013.10.004
- Ogidi P, Ahmed F, Cahn BA, et al. Medical schools as gatekeepers: a survey and analysis of factors predicting dermatology residency placement. J Am Acad Dermatol. 2022;86:490-492. doi:10.1016 /j.jaad.2021.09.027
- Visiting Student Learning Opportunities (VSLO). Accessed May 30, 2025. https://students-residents.aamc.org/visiting-student-learning-opportunities/visiting-student-learning-opportunities-vslo
- American Academy of Dermatology Association. AAD Learning Center. Basic dermatology curriculum (2-week rotation). Accessed May 12, 2025. https://learning.aad.org/Listing/Basic-Dermatology-Curriculum-2-Week-Rotation-5395
- American Academy of Dermatology Association. AAD Learning Center. Basic dermatology curriculum (4-week rotation). Accessed May 12, 2025. https://learning.aad.org/Public/Catalog/Details.aspx?id=YPssTVIbBO3Zb%2bOuf%2fM7Kg%3d%3d&returnurl=%2fUsers%2fUserOnlineCourse.aspx%3fLearningActivityID%3dYPssTVIbBO3Zb%252bOuf%252fM7Kg%253d%253d
- Penn Medicine Dermatology Residency Training Program. Residency tracks. Accessed May 12, 2025. https://dermatology.upenn.edu/residents/residency-tracks/
- Pediatric Dermatology Residency Track at NYU Grossman School of Medicine. Pediatric Track. Accessed May 30, 2025. https://med.nyu.edu/departments-institutes/dermatology/education/residency/pediatric-track
- Prindaville B, Antaya RJ, Siegfried EC. Pediatric dermatology: past, present, and future. Pediatr Dermatol. 2015;32:1-12. doi:10.1111/pde.12362
- Wright TS. Update on the pediatric dermatology workforce shortage. Cutis. 2021;108:237-238. doi:10.12788/cutis.0379
- Stephens MR, Murthy AS, McMahon PJ. Wait times, health care touchpoints, and nonattendance in an academic pediatric dermatology clinic. ediatr Dermatol. 2019;36:893-897. doi:10.1111/pde.13943
- Fogel AL, Teng JM. A survey to assess perceived differences in referral pathways to board-certified pediatric dermatologists. Pediatr Dermatol. 2015;32:e314-e315. doi:10.1111/pde.12703
- Parker JC, Rangu S, Grand KL, et al. Genetic skin disorders: the value of a multidisciplinary clinic. Am J Med Genet A. 2021;185:1159-1167. doi:10.1002/ajmg.a.62095
- Admani S, Caufield M, Kim SS, et al. Understanding the pediatric dermatology workforce shortage: mentoring matters. J Pediatr. 2014;164:372-5.e1. doi:10.1016/j.jpeds.2013.10.004
- Ogidi P, Ahmed F, Cahn BA, et al. Medical schools as gatekeepers: a survey and analysis of factors predicting dermatology residency placement. J Am Acad Dermatol. 2022;86:490-492. doi:10.1016 /j.jaad.2021.09.027
- Visiting Student Learning Opportunities (VSLO). Accessed May 30, 2025. https://students-residents.aamc.org/visiting-student-learning-opportunities/visiting-student-learning-opportunities-vslo
- American Academy of Dermatology Association. AAD Learning Center. Basic dermatology curriculum (2-week rotation). Accessed May 12, 2025. https://learning.aad.org/Listing/Basic-Dermatology-Curriculum-2-Week-Rotation-5395
- American Academy of Dermatology Association. AAD Learning Center. Basic dermatology curriculum (4-week rotation). Accessed May 12, 2025. https://learning.aad.org/Public/Catalog/Details.aspx?id=YPssTVIbBO3Zb%2bOuf%2fM7Kg%3d%3d&returnurl=%2fUsers%2fUserOnlineCourse.aspx%3fLearningActivityID%3dYPssTVIbBO3Zb%252bOuf%252fM7Kg%253d%253d
- Penn Medicine Dermatology Residency Training Program. Residency tracks. Accessed May 12, 2025. https://dermatology.upenn.edu/residents/residency-tracks/
- Pediatric Dermatology Residency Track at NYU Grossman School of Medicine. Pediatric Track. Accessed May 30, 2025. https://med.nyu.edu/departments-institutes/dermatology/education/residency/pediatric-track
Workforce Shortage of Pediatric Dermatologists: A Medical Student’s Perspective
Workforce Shortage of Pediatric Dermatologists: A Medical Student’s Perspective
PRACTICE POINTS
- Addressing a lack of exposure to pediatric dermatology in medical school and increasing support for students who are interested in the field can help alleviate the shortage of physicians at the earliest point in training.
- Increasing access to pediatric dermatology resources, such as lecture series and mentorship opportunities, could further broaden the medical student knowledge base.
- There is an opportunity to create residency tracks that increase the number of dermatology residency applicants who are medical students interested in pursuing pediatric dermatology.
What About Stolen Valor is Actually Illegal?
What About Stolen Valor is Actually Illegal?
Memorial Day is the most solemn of all American military commemorations. It is the day when we honor those who sacrificed their lives so that their fellow citizens could flourish in freedom. At 3 PM, a grateful nation is called to observe 2 minutes of silence in remembrance of the heroes who died in battle or of the wounds they sustained in combat. Communities across the country will carry out ceremonies, lining national cemeteries with flags, holding patriotic parades, and conducting spiritual observances.1
Sadly, almost as long as there has been a United States, there has been a parallel practice dishonoring the uniform and deceiving veterans and the public alike known as stolen valor. Stolen valor is a persistent, yet strange, psychological behavior: individuals who never served in the US Armed Forces claim they have done heroic deeds for which they often sustained serious injuries in the line of duty and almost always won medals for their heroism.2 This editorial will trace the US legal history of stolen valor cases to provide the background for next month’s editorial examining its clinical and ethical aspects.
While many cases of stolen valor do not receive media attention, the experience of Sarah Cavanaugh, a former VA social worker who claimed to be a marine veteran who served in Iraq and Afghanistan, was the subject of the Deep Cover podcast series.3 Cavanaugh had claimed that an improvised explosive device blew up her Humvee, crushing her hip. Still she somehow was able to help her fellow Marines and earned the Bronze Star among other decorations for her heroism. That was not the only lie Cavanaugh told: she also told her friends and wife that she had advanced lung cancer due to burn pit exposure. In line with the best-worst of those who have stolen valor, her mastery of manipulation enabled her to become the commander of a local Veterans of Foreign Wars post. Using stolen identities and fraudulent documents, Cavanaugh was able to purloin veteran benefits, donated leave from other VA employees and money, and stole goods and services from various charitable organizations whose mission was to help wounded veterans and those struggling to adjust to civilian life. Before law enforcement unraveled her sordid tale, she misappropriated hundreds of thousands of dollars in VA benefits and donations and exploited dozens of generous veterans and compassionate civilians.4
Cavanaugh’s story was so sordidly compelling that I kept saying out loud to myself (and my spouse), “This has to be illegal.” The truth about stolen valor law is far more ambivalent and frustrating than I had anticipated or wanted. The first insult to my sense of justice was that lying about military service is not in itself illegal: you can pad your military resume with unearned decorations or impress a future partner or employer with your combat exploits without much fear of legal repercussions. The legal history of attempting to make stealing valor a crime has almost as many twists and turns as the fallacious narratives of military imposters and illustrates the uniquely American experiment in balancing freedom and fairness.
The Stolen Valor Act of 2005 made it a federal misdemeanor to wear, manufacture, or sell military decorations, or medals (Cavanaugh bought her medals online) without legal authorization. It also made it a crime to falsely represent oneself as having been the recipient of a decoration, medical, or service badge that Congress or the Armed Forces authorized. There were even stiffer penalties if the medal was a Silver Star, Distinguished Service Cross, US Air Force or US Navy Cross, or Purple Heart. Punishments include fines and imprisonment. The stated legislative purpose was to prohibit fraud that devalued military awards and the dignity of those who legitimately earned them.5
Next comes a distinctly American reaction to the initial Congressional attempt to protect the legacy of those who served—a lawsuit. Xavier Alvarez was an official on a California district water board claimed to be a 25-year veteran of the US Marine Corps wounded in combat and received the Congressional Medal of Honor. The Federal Bureau of Investigation exposed the lie and instead of the nation’s highest honor, Alvarez was the first to be convicted under the Stolen Valor Act of 2005. Alvarez appealed the decision, ironically claiming the law violated his free speech rights. The case landed in the Supreme Court, which ruled that the Stolen Valor Act did indeed violate the Free Speech Clause of the First Amendment. The majority opinion found the Act as passed was too encompassing of all speech and needed to target only cases in which false statements resulted in actual harm.6
The Stolen Valor Act of 2013 amends the criminal code regarding fraudulent claims about military service to include those who don’t only lie but also profit from it, as Cavanaugh did. The revised act specifically focuses on individuals who claim to have earned military honors for the intended purpose of obtaining money, property, or any other tangible benefit.7
Despite the complicated nature of Stolen Valor Law, it did prevail in Cavanaugh’s case. A US District Court Judge in Rhode Island found her guilty of stolen valor in all its permutations, along with identity theft of other veterans’ military and medical records and fraud in obtaining benefits and services intended for real veterans. Cavanaugh was sentenced to 70 months in federal prison, 3 years of supervised release, ordered to pay $284,796.82 in restitution, and to restore 261 hours of donated leave to the federal government, charitable organizations, and good Samaritans she duped and swindled.8
The revised law under which Cavanaugh was punished lasted 10 years until another classically American ethical concern—privacy—motivated additional legislative effort. A 2023/2024 US House of Representatives proposal to amend the Stolen Valor Act would have strengthened the privacy protections afforded military records. It would have required the information to only be accessed with the permission of the individual who served or their family or through a Freedom of Information Act request. This would make the kind of journalistic and law enforcement investigations that eventually caught Cavanaugh in her lies far more laborious for false valor hunters while at the same time preventing unscrupulous inquiries into service members’ personal information. Advocates for free speech and defenders of military honor are both lobbying Congress; as of this writing the legislation has not been passed.9
As we close part 1 of this review of stolen valor, we return to Memorial Day. This day provides the somber recognition that without the brave men and women of integrity who died in defense of a democracy that promotes the political activity of its citizens, we would not even be able to have this debate over justice, freedom, and truth.
- US Department of Veterans Affairs. The difference between Veterans Day and Memorial Day. October 30, 2023. Accessed May 27, 2025. https://news.va.gov/125549/difference-between-veterans-day-memorial-day/
- Home of Heroes. Stolen valor. Accessed May 27, 2025. https://homeofheroes.com/stolen-valor
- Halpern J. Deep cover: the truth about Sarah. May 2025. Accessed May 27, 2025. https://www.pushkin.fm/podcasts/deep-cover
- Stillwell B. The latest season of the ‘deep cover’ podcast dives into one of the biggest stolen valor cases ever. Military. com. May 22, 2025. Accessed May 27, 2025. https:// www.military.com/off-duty/2025/05/22/latest-season-of-deep-cover-podcast-dives-one-of-biggest-stolen-valor-cases-ever.html
- The Stolen Valor Act of 2005. Pub L No: 109-437. 120 Stat 3266
- Alvarez v United States. 567 US 2012.
- The Stolen Valor Act of 2013. 18 USC § 704(b)
- US Attorney’s Office, District of Rhode Island. Rhode Island woman sentenced to federal prison for falsifying military service; false use of military medals; identify theft, and fraudulently collecting more than $250,000, in veteran benefits and charitable contributions. March 14, 2023. Accessed May 27, 2025. https://www.justice.gov/usao-ri/pr/rhode-island-woman-sentenced-federal-prison-falsifying-military-service-false-use
- Armed Forces Benefit Association. Stolen Valor Act: all you need to know. February 21, 2024. Accessed May 27, 2025. https://www.afba.com/military-life/active-duty-and-veterans/stolen-valor-act-all-you-need-to-know/
Memorial Day is the most solemn of all American military commemorations. It is the day when we honor those who sacrificed their lives so that their fellow citizens could flourish in freedom. At 3 PM, a grateful nation is called to observe 2 minutes of silence in remembrance of the heroes who died in battle or of the wounds they sustained in combat. Communities across the country will carry out ceremonies, lining national cemeteries with flags, holding patriotic parades, and conducting spiritual observances.1
Sadly, almost as long as there has been a United States, there has been a parallel practice dishonoring the uniform and deceiving veterans and the public alike known as stolen valor. Stolen valor is a persistent, yet strange, psychological behavior: individuals who never served in the US Armed Forces claim they have done heroic deeds for which they often sustained serious injuries in the line of duty and almost always won medals for their heroism.2 This editorial will trace the US legal history of stolen valor cases to provide the background for next month’s editorial examining its clinical and ethical aspects.
While many cases of stolen valor do not receive media attention, the experience of Sarah Cavanaugh, a former VA social worker who claimed to be a marine veteran who served in Iraq and Afghanistan, was the subject of the Deep Cover podcast series.3 Cavanaugh had claimed that an improvised explosive device blew up her Humvee, crushing her hip. Still she somehow was able to help her fellow Marines and earned the Bronze Star among other decorations for her heroism. That was not the only lie Cavanaugh told: she also told her friends and wife that she had advanced lung cancer due to burn pit exposure. In line with the best-worst of those who have stolen valor, her mastery of manipulation enabled her to become the commander of a local Veterans of Foreign Wars post. Using stolen identities and fraudulent documents, Cavanaugh was able to purloin veteran benefits, donated leave from other VA employees and money, and stole goods and services from various charitable organizations whose mission was to help wounded veterans and those struggling to adjust to civilian life. Before law enforcement unraveled her sordid tale, she misappropriated hundreds of thousands of dollars in VA benefits and donations and exploited dozens of generous veterans and compassionate civilians.4
Cavanaugh’s story was so sordidly compelling that I kept saying out loud to myself (and my spouse), “This has to be illegal.” The truth about stolen valor law is far more ambivalent and frustrating than I had anticipated or wanted. The first insult to my sense of justice was that lying about military service is not in itself illegal: you can pad your military resume with unearned decorations or impress a future partner or employer with your combat exploits without much fear of legal repercussions. The legal history of attempting to make stealing valor a crime has almost as many twists and turns as the fallacious narratives of military imposters and illustrates the uniquely American experiment in balancing freedom and fairness.
The Stolen Valor Act of 2005 made it a federal misdemeanor to wear, manufacture, or sell military decorations, or medals (Cavanaugh bought her medals online) without legal authorization. It also made it a crime to falsely represent oneself as having been the recipient of a decoration, medical, or service badge that Congress or the Armed Forces authorized. There were even stiffer penalties if the medal was a Silver Star, Distinguished Service Cross, US Air Force or US Navy Cross, or Purple Heart. Punishments include fines and imprisonment. The stated legislative purpose was to prohibit fraud that devalued military awards and the dignity of those who legitimately earned them.5
Next comes a distinctly American reaction to the initial Congressional attempt to protect the legacy of those who served—a lawsuit. Xavier Alvarez was an official on a California district water board claimed to be a 25-year veteran of the US Marine Corps wounded in combat and received the Congressional Medal of Honor. The Federal Bureau of Investigation exposed the lie and instead of the nation’s highest honor, Alvarez was the first to be convicted under the Stolen Valor Act of 2005. Alvarez appealed the decision, ironically claiming the law violated his free speech rights. The case landed in the Supreme Court, which ruled that the Stolen Valor Act did indeed violate the Free Speech Clause of the First Amendment. The majority opinion found the Act as passed was too encompassing of all speech and needed to target only cases in which false statements resulted in actual harm.6
The Stolen Valor Act of 2013 amends the criminal code regarding fraudulent claims about military service to include those who don’t only lie but also profit from it, as Cavanaugh did. The revised act specifically focuses on individuals who claim to have earned military honors for the intended purpose of obtaining money, property, or any other tangible benefit.7
Despite the complicated nature of Stolen Valor Law, it did prevail in Cavanaugh’s case. A US District Court Judge in Rhode Island found her guilty of stolen valor in all its permutations, along with identity theft of other veterans’ military and medical records and fraud in obtaining benefits and services intended for real veterans. Cavanaugh was sentenced to 70 months in federal prison, 3 years of supervised release, ordered to pay $284,796.82 in restitution, and to restore 261 hours of donated leave to the federal government, charitable organizations, and good Samaritans she duped and swindled.8
The revised law under which Cavanaugh was punished lasted 10 years until another classically American ethical concern—privacy—motivated additional legislative effort. A 2023/2024 US House of Representatives proposal to amend the Stolen Valor Act would have strengthened the privacy protections afforded military records. It would have required the information to only be accessed with the permission of the individual who served or their family or through a Freedom of Information Act request. This would make the kind of journalistic and law enforcement investigations that eventually caught Cavanaugh in her lies far more laborious for false valor hunters while at the same time preventing unscrupulous inquiries into service members’ personal information. Advocates for free speech and defenders of military honor are both lobbying Congress; as of this writing the legislation has not been passed.9
As we close part 1 of this review of stolen valor, we return to Memorial Day. This day provides the somber recognition that without the brave men and women of integrity who died in defense of a democracy that promotes the political activity of its citizens, we would not even be able to have this debate over justice, freedom, and truth.
Memorial Day is the most solemn of all American military commemorations. It is the day when we honor those who sacrificed their lives so that their fellow citizens could flourish in freedom. At 3 PM, a grateful nation is called to observe 2 minutes of silence in remembrance of the heroes who died in battle or of the wounds they sustained in combat. Communities across the country will carry out ceremonies, lining national cemeteries with flags, holding patriotic parades, and conducting spiritual observances.1
Sadly, almost as long as there has been a United States, there has been a parallel practice dishonoring the uniform and deceiving veterans and the public alike known as stolen valor. Stolen valor is a persistent, yet strange, psychological behavior: individuals who never served in the US Armed Forces claim they have done heroic deeds for which they often sustained serious injuries in the line of duty and almost always won medals for their heroism.2 This editorial will trace the US legal history of stolen valor cases to provide the background for next month’s editorial examining its clinical and ethical aspects.
While many cases of stolen valor do not receive media attention, the experience of Sarah Cavanaugh, a former VA social worker who claimed to be a marine veteran who served in Iraq and Afghanistan, was the subject of the Deep Cover podcast series.3 Cavanaugh had claimed that an improvised explosive device blew up her Humvee, crushing her hip. Still she somehow was able to help her fellow Marines and earned the Bronze Star among other decorations for her heroism. That was not the only lie Cavanaugh told: she also told her friends and wife that she had advanced lung cancer due to burn pit exposure. In line with the best-worst of those who have stolen valor, her mastery of manipulation enabled her to become the commander of a local Veterans of Foreign Wars post. Using stolen identities and fraudulent documents, Cavanaugh was able to purloin veteran benefits, donated leave from other VA employees and money, and stole goods and services from various charitable organizations whose mission was to help wounded veterans and those struggling to adjust to civilian life. Before law enforcement unraveled her sordid tale, she misappropriated hundreds of thousands of dollars in VA benefits and donations and exploited dozens of generous veterans and compassionate civilians.4
Cavanaugh’s story was so sordidly compelling that I kept saying out loud to myself (and my spouse), “This has to be illegal.” The truth about stolen valor law is far more ambivalent and frustrating than I had anticipated or wanted. The first insult to my sense of justice was that lying about military service is not in itself illegal: you can pad your military resume with unearned decorations or impress a future partner or employer with your combat exploits without much fear of legal repercussions. The legal history of attempting to make stealing valor a crime has almost as many twists and turns as the fallacious narratives of military imposters and illustrates the uniquely American experiment in balancing freedom and fairness.
The Stolen Valor Act of 2005 made it a federal misdemeanor to wear, manufacture, or sell military decorations, or medals (Cavanaugh bought her medals online) without legal authorization. It also made it a crime to falsely represent oneself as having been the recipient of a decoration, medical, or service badge that Congress or the Armed Forces authorized. There were even stiffer penalties if the medal was a Silver Star, Distinguished Service Cross, US Air Force or US Navy Cross, or Purple Heart. Punishments include fines and imprisonment. The stated legislative purpose was to prohibit fraud that devalued military awards and the dignity of those who legitimately earned them.5
Next comes a distinctly American reaction to the initial Congressional attempt to protect the legacy of those who served—a lawsuit. Xavier Alvarez was an official on a California district water board claimed to be a 25-year veteran of the US Marine Corps wounded in combat and received the Congressional Medal of Honor. The Federal Bureau of Investigation exposed the lie and instead of the nation’s highest honor, Alvarez was the first to be convicted under the Stolen Valor Act of 2005. Alvarez appealed the decision, ironically claiming the law violated his free speech rights. The case landed in the Supreme Court, which ruled that the Stolen Valor Act did indeed violate the Free Speech Clause of the First Amendment. The majority opinion found the Act as passed was too encompassing of all speech and needed to target only cases in which false statements resulted in actual harm.6
The Stolen Valor Act of 2013 amends the criminal code regarding fraudulent claims about military service to include those who don’t only lie but also profit from it, as Cavanaugh did. The revised act specifically focuses on individuals who claim to have earned military honors for the intended purpose of obtaining money, property, or any other tangible benefit.7
Despite the complicated nature of Stolen Valor Law, it did prevail in Cavanaugh’s case. A US District Court Judge in Rhode Island found her guilty of stolen valor in all its permutations, along with identity theft of other veterans’ military and medical records and fraud in obtaining benefits and services intended for real veterans. Cavanaugh was sentenced to 70 months in federal prison, 3 years of supervised release, ordered to pay $284,796.82 in restitution, and to restore 261 hours of donated leave to the federal government, charitable organizations, and good Samaritans she duped and swindled.8
The revised law under which Cavanaugh was punished lasted 10 years until another classically American ethical concern—privacy—motivated additional legislative effort. A 2023/2024 US House of Representatives proposal to amend the Stolen Valor Act would have strengthened the privacy protections afforded military records. It would have required the information to only be accessed with the permission of the individual who served or their family or through a Freedom of Information Act request. This would make the kind of journalistic and law enforcement investigations that eventually caught Cavanaugh in her lies far more laborious for false valor hunters while at the same time preventing unscrupulous inquiries into service members’ personal information. Advocates for free speech and defenders of military honor are both lobbying Congress; as of this writing the legislation has not been passed.9
As we close part 1 of this review of stolen valor, we return to Memorial Day. This day provides the somber recognition that without the brave men and women of integrity who died in defense of a democracy that promotes the political activity of its citizens, we would not even be able to have this debate over justice, freedom, and truth.
- US Department of Veterans Affairs. The difference between Veterans Day and Memorial Day. October 30, 2023. Accessed May 27, 2025. https://news.va.gov/125549/difference-between-veterans-day-memorial-day/
- Home of Heroes. Stolen valor. Accessed May 27, 2025. https://homeofheroes.com/stolen-valor
- Halpern J. Deep cover: the truth about Sarah. May 2025. Accessed May 27, 2025. https://www.pushkin.fm/podcasts/deep-cover
- Stillwell B. The latest season of the ‘deep cover’ podcast dives into one of the biggest stolen valor cases ever. Military. com. May 22, 2025. Accessed May 27, 2025. https:// www.military.com/off-duty/2025/05/22/latest-season-of-deep-cover-podcast-dives-one-of-biggest-stolen-valor-cases-ever.html
- The Stolen Valor Act of 2005. Pub L No: 109-437. 120 Stat 3266
- Alvarez v United States. 567 US 2012.
- The Stolen Valor Act of 2013. 18 USC § 704(b)
- US Attorney’s Office, District of Rhode Island. Rhode Island woman sentenced to federal prison for falsifying military service; false use of military medals; identify theft, and fraudulently collecting more than $250,000, in veteran benefits and charitable contributions. March 14, 2023. Accessed May 27, 2025. https://www.justice.gov/usao-ri/pr/rhode-island-woman-sentenced-federal-prison-falsifying-military-service-false-use
- Armed Forces Benefit Association. Stolen Valor Act: all you need to know. February 21, 2024. Accessed May 27, 2025. https://www.afba.com/military-life/active-duty-and-veterans/stolen-valor-act-all-you-need-to-know/
- US Department of Veterans Affairs. The difference between Veterans Day and Memorial Day. October 30, 2023. Accessed May 27, 2025. https://news.va.gov/125549/difference-between-veterans-day-memorial-day/
- Home of Heroes. Stolen valor. Accessed May 27, 2025. https://homeofheroes.com/stolen-valor
- Halpern J. Deep cover: the truth about Sarah. May 2025. Accessed May 27, 2025. https://www.pushkin.fm/podcasts/deep-cover
- Stillwell B. The latest season of the ‘deep cover’ podcast dives into one of the biggest stolen valor cases ever. Military. com. May 22, 2025. Accessed May 27, 2025. https:// www.military.com/off-duty/2025/05/22/latest-season-of-deep-cover-podcast-dives-one-of-biggest-stolen-valor-cases-ever.html
- The Stolen Valor Act of 2005. Pub L No: 109-437. 120 Stat 3266
- Alvarez v United States. 567 US 2012.
- The Stolen Valor Act of 2013. 18 USC § 704(b)
- US Attorney’s Office, District of Rhode Island. Rhode Island woman sentenced to federal prison for falsifying military service; false use of military medals; identify theft, and fraudulently collecting more than $250,000, in veteran benefits and charitable contributions. March 14, 2023. Accessed May 27, 2025. https://www.justice.gov/usao-ri/pr/rhode-island-woman-sentenced-federal-prison-falsifying-military-service-false-use
- Armed Forces Benefit Association. Stolen Valor Act: all you need to know. February 21, 2024. Accessed May 27, 2025. https://www.afba.com/military-life/active-duty-and-veterans/stolen-valor-act-all-you-need-to-know/
What About Stolen Valor is Actually Illegal?
What About Stolen Valor is Actually Illegal?
The Use of Lung Cancer Screening to Increase Chronic Obstructive Pulmonary Disease Diagnosis in Veterans Affairs Primary Care
The Use of Lung Cancer Screening to Increase Chronic Obstructive Pulmonary Disease Diagnosis in Veterans Affairs Primary Care
Primary care practitioners (PCPs) in the US Department of Veterans Affairs (VA) provide care for patients with higher rates of many diseases—diabetes, heart disease, cancer, chronic obstructive pulmonary disease (COPD), and stroke—compared to the nonveteran population. 1 Due to the medical complexities of these diseases, they are often misdiagnosed or not diagnosed at all.
COPD is hiding in plain sight, impacting quality of life and burdening US health care systems.2 Research has yielded new treatments and evidence-based guidelines; however, COPD remains underdiagnosed. Only 13 million of the estimated 79 million US adults with COPD aged 20 to 79 years have been formally diagnosed.3 By the time patients are diagnosed, the disease is often advanced, and therapies are less effective. In 2 large studies of patients with COPD symptoms, later diagnosis was associated with worse outcomes.4,5
Veterans have a higher prevalence of COPD (8%-19%) than nonveterans (6%), likely due to higher rates of smoking and service-related exposures, especially among veterans of post-9/11 conflicts.6,7 Veterans do not always report symptoms and PCPs may not ask about symptoms, leading to underdiagnosis.8 The combination of high likelihood and underdetection of COPD presents a challenge and a target for VA quality improvement (QI).
The US Preventive Services Task Force (USPSTF) recommends against screening asymptomatic patients for COPD. However, both the USPSTF and the Global Initiative for Chronic Obstructive Lung Disease Report advocate for active case finding in primary care clinics to determine whether high-risk patients, such as smokers, experience COPD symptoms and warrant spirometry. 9,10 To make early COPD diagnoses, clinicians may use questionnaires alone or in combination with handheld peak expiratory flow rate measurements.11,12 Formal spirometry, considered the gold standard for COPD diagnosis, is ordered for patients who report COPD symptoms (ie, shortness of breath with exertion) or who have both COPD symptoms and reduced peak flow rates.
A systematic review and meta-analysis found that while the combination of questionnaires and peak flows was the more effective strategy overall, questionnaires alone were also valuable for identifying patients with possible COPD.13 Implementation of either screening method in primary care practices would be challenging. In a simulation study that applied chronic disease and preventive care guidelines to hypothetical patient panels, the time required for PCPs to provide guideline-recommended chronic and preventive care in addition to acute care far exceeded 8 hours per day, even in team-based settings.14 Overburdened PCPs are therefore unlikely to accept additional tasks like COPD case finding.
Why don’t patients report their pulmonary symptoms? Patients may not recognize the symptoms as evidence of COPD. Others may be afraid of a COPD diagnosis or the stigma that is associated with it.15 Perhaps they believe COPD treatment is ineffective because of lung damage from smoking. Some patients may not want to know if they have COPD, while others reduce activity levels to avoid symptoms.16
QUALITY IMPROVEMENT PROJECT
Given the high prevalence of COPD among veterans and the potential for underdiagnosis, VA Northeast Ohio Healthcare System (VANEOHS) internal medicine residents and faculty assessed the state of COPD diagnosis in its primary care clinic with a QI project in 2022. Patients in the clinic between August 1, 2015, and November 30, 2022, with an International Classification of Diseases-10 (ICD-10) COPD diagnosis code (J44) in the electronic health record were included. Of 157 included patients, 105 patients who had prior spirometry testing were excluded. Of the 52 patients with diagnosed COPD and no spirometry testing, 30 patients had computed tomography (CT) findings consistent with COPD (ie, airway thickening, emphysema, air trapping) that was performed for CT lung cancer screening (LCS).17 Twenty-three of these 30 patients were contacted by phone. All 23 were ever smokers and 13 reported COPD symptoms. The PCPs of the symptomatic patients were then contacted. Spirometry was ordered for all 13 patients and completed by 7. Three spirometry tests confirmed the COPD diagnosis. One PCP initiated inhaler therapy for a patient with newly diagnosed COPD.
All 11 PCPs of symptomatic patients were interviewed (many had > 1 symptomatic patient). They reported being unaware of patients’ COPD symptoms because the patients did not mention them, noting that screening for COPD was not a priority.
Role of Lung Cancer Screening
VA PCPs use electronic health record clinical reminders to track tests, consults, chronic disease education, cancer screenings, and routine health maintenance. A clinical reminder already exists (based on USPSTF recommendations) for LCS for patients aged 50 to 80 years who have a smoking history of 20 pack years. Patients who meet these criteria would also be considered high risk for COPD.
The VANEOHS QI project suggests that previously undiagnosed patients with findings of COPD on LCS may also have symptoms of COPD. Therefore, we wondered whether the LCS clinical reminder could serve a second purpose by prompting PCPs to ask veterans who meet LCS criteria about their COPD symptoms.
In 2022, about 13 million patients were eligible for LCS.18 Patients who qualify for LCS are at high risk for other cardiopulmonary disorders, such as COPD and coronary artery disease. Lung cancer is detected in only 1% of patients screened with CT at baseline. However, more often LCS yields evidence of additional cardiopulmonary disorders, such as emphysema or coronary artery calcifications. The International Early Lung Cancer Program (I-ELCAP) and the National Lung Cancer Screening Trial (NLST), which included > 79,000 patients, found evidence of emphysema on CT imaging in 24% and 31% of cases, respectively.19,20 In both cohorts, > 80% of patients with emphysema on CT imaging had no prior history of COPD.
In a 2022 article summarizing the potential impact of CT LCS on COPD diagnosis, Mulshine et al suggest that detection of emphysema on CT LCS provides “earlier recognition for PCPs to identify patients who would benefit from detailed symptom screening to prompt spirometry for COPD detection” and additional motivation for tobacco cessation.21 The VANEOHS QI project was developed and implemented prior to I-ELCAP or NLST reporting results but reinforces the value of CT LCS for COPD diagnosis.
Early diagnosis of COPD remains challenging because PCPs do not ask, patients do not tell, and symptoms can easily be dismissed. However, earlier diagnosis of COPD in symptomatic patients improves outcomes.3,4 To bridge this gap, VA PCPs and primary care patient aligned care teams (PACTs) need to commit to probing high-risk patients for COPD symptoms and ordering spirometry for those who are symptomatic. To accomplish this task, primary care teams need help.
The VANEOHS QI project confirmed that some patients with evidence of COPD on CT have symptoms of COPD that they did not share with their PCPs and suggests that LCS can be used as a dual action case finding method to screen both for lung cancer and COPD. We propose that patients who are eligible for LCS should also be probed for COPD symptoms at their clinic visits; for symptomatic patients, spirometry should be ordered, and COPD evidence-based management should be initiated when spirometry results are consistent with COPD. Annual probing for COPD symptoms could be considered in asymptomatic patients with ongoing tobacco use or emphysema on CT, since they may develop symptoms in the future. This new case-finding method bypasses the need for time-prohibitive questionnaires or peak flow measurements.
Future Opportunities
VA PCPs juggle many priorities and despite the simplicity of this new case finding COPD method, it may be unintentionally overlooked. PCPs often run out of time or may forget to ask patients about COPD symptoms when ordering LCS.
Future innovations to increase COPD diagnosis could include the creation of a yearly VA clinical reminder linked to the tobacco use reminder that has check boxes asking about symptoms of COPD in current and prior smokers. If patients have COPD symptoms, the reminder can prompt the ordering of spirometry. Similar reminders could be implemented to identify veterans with exposures to burn pits or other military environmental exposures who may have COPD symptoms. Another possible way to increase COPD diagnosis would be a partnership between primary care and the VA LCS program where patients receiving screening are asked about COPD symptoms during their LCS interviews and PACTs are alerted to order spirometry for symptomatic patients.
Elusive no longer! We can pull the veil back on COPD diagnosis and identify patients with possible COPD earlier in their course using their eligibility for LCS as a yearly reminder to probe them for symptoms. While not all patients who undergo LCS—even those with evidence of COPD on CT—will have COPD symptoms, symptoms may develop over time. LCS provides the possibility of 2 diagnoses from 1 test. This is an opportunity we cannot afford to miss.
- Betancourt JA, Granados PS, Pacheco GJ, et al. Exploring health outcomes for U.S. veterans compared to non-veterans from 2003 to 2019. Healthcare (Basel). 2021;9(5):604. doi:10.3390/healthcare90506064
- Bamonti PM, Fischer I, Moye J, Poghosyan H, Pietrzak RH. Obstructive respiratory disease in U.S. veterans: prevalence, characteristics, and health burden. J Psychiatr Res. 2024;176:140-147. doi:10.1016/j.jpsychires.2024.05.053
- Criner RN, Han MK. COPD care in the 21st century: a public health priority. Respir Care. 2018;63(5):591-600. doi:10.4187/respcare.06276
- Larsson K, Janson C, Ställberg B, et al. Impact of COPD diagnosis timing on clinical and economic outcomes: the ARCTIC observational cohort study. Int J Chron Obstruct Pulmon Dis. 2019;14:995-1008. doi:10.2147/COPD.S195382
- Kostikas K, Price D, Gutzwiller FS, et al. Clinical impact and healthcare resource utilization associated with early versus late COPD diagnosis in patients from UK CPRD Database. Int J Chron Obstruct Pulmon Dis. 2020;15:1729- 1738. doi:10.2147/COPD.S255414
- Bamonti PM, Robinson SA, Wan ES, Moy ML. Improving physiological, physical, and psychological health outcomes: a narrative review in US veterans with COPD. Int J Chron Obstruct Pulmon Dis. 2022;17:1269-1283. doi:10.2147/COPD.S339323
- Savitz DA, Woskie SR, Bello A, et al. Deployment to military bases with open burn pits and respiratory and cardiovascular disease. JAMA Netw Open. 2024;7(4):e247629. doi:10.1001/jamanetworkopen.2024.7629
- Murphy DE, Chaudhry Z, Almoosa KF, Panos RJ. High prevalence of chronic obstructive pulmonary disease among veterans in the urban midwest. Mil Med. 2011;176(5):552-560. doi:10.7205/milmed-d-10-00377
- Guirguis-Blake JM, Senger CA, Webber EM, Mularski RA, Whitlock EP. Screening for chronic obstructive pulmonary disease: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315(13):1378-1393. doi:10.1001/jama.2016.2654
- Capriotti T, Tomy R, Morales M. COPD updates: 2023 GOLD Report for primary care providers. Clinical Advisor. May 9, 2023. Accessed May 14, 2025. https://www.clinicaladvisor.com/features/copd-updates-2023-gold-report-primary-care/
- Leidy NK, Martinez FJ, Malley KG, et al. Can CAPTURE be used to identify undiagnosed patients with mild- to- moderate COPD likely to benefit from treatment? Int J Chron Obstruct Pulmon Dis. 2018;13:1901-1912. doi:10.2147/COPD.S152226
- Jithoo A, Enright PL, Burney P, et al. Case-finding options for COPD: results from the burden of obstructive lung disease study. Eur Respir J. 2013;41(3):548-555. doi:10.1183/09031936.00132011
- Haroon SM, Jordan RE, O’Beirne-Elliman J, Adab P. Effectiveness of case finding strategies for COPD in primary care: a systematic review and meta-analysis. NPJ Prim Care Respir Med. 2015;25:15056. doi:10.1038/npjpcrm.2015.56
- Porter J, Boyd C, Skandari MR, Laiteerapong N. Revisiting the time needed to provide adult primary care. J Gen Intern Med. 2023;38(1)147-155. doi:10.1007/s11606-022-07707-x
- Woo S, Zhou W, Larson JL. Stigma experiences in people with chronic obstructive pulmonary disease: an integrative review. Int J Chron Obstruct Pulmon Dis. 2021;16:1647- 1659. doi:10.2147/COPD.S306874
- Aaron SD, Montes de Oca M, Celli B, et al. Early diagnosis and treatment of COPD: the costs and benefits of case finding. Am J Respir Crit Care Med. 2024;209(8):928-937. doi:10.1164/rccm.202311-2120PP
- Kwon A, Lee C, Arafah A, Klein M, Namboodiri S, Lee C. Increasing chronic obstructive pulmonary disease (COPD) diagnosis with pulmonary function testing for patients with chest imaging evidence of COPD. Poster presented at: Society of General Internal Medicine Midwest Regional Meeting; October 19-20, 2023; Chicago, IL.
- Henderson LM, Su I, Rivera MP, et al. Prevalence of lung cancer screening in the US, 2022. JAMA Netw Open. 2024;7(3):e243190. doi:10.1001/jamanetworkopen.2024.3190
- Steiger D, Siddiqi MF, Yip R, Yankelevitz DF, Henschke CI; I-ELCAP investigators. The importance of low-dose CT screening to identify emphysema in asymptomatic participants with and without a prior diagnosis of COPD. Clin Imaging. 2021;78:136-141. doi:10.1016/j.clinimag.2021.03.012
- Pinsky PF, Lynch DA, Gierada DS. Incidental findings on low-dose CT scan lung cancer screenings and deaths from respiratory diseases. Chest. 2022;161(4):1092-1100. doi:10.1016/j.chest.2021.11.015
- Mulshine JL, Aldigé CR, Ambrose LF, et al. Emphysema detection in the course of lung cancer screening: optimizing a rare opportunity to impact population health. Ann Am Thorac Soc. 2023;20(4):499- 503. doi:10.1513/AnnalsATS.202207-631PS
Primary care practitioners (PCPs) in the US Department of Veterans Affairs (VA) provide care for patients with higher rates of many diseases—diabetes, heart disease, cancer, chronic obstructive pulmonary disease (COPD), and stroke—compared to the nonveteran population. 1 Due to the medical complexities of these diseases, they are often misdiagnosed or not diagnosed at all.
COPD is hiding in plain sight, impacting quality of life and burdening US health care systems.2 Research has yielded new treatments and evidence-based guidelines; however, COPD remains underdiagnosed. Only 13 million of the estimated 79 million US adults with COPD aged 20 to 79 years have been formally diagnosed.3 By the time patients are diagnosed, the disease is often advanced, and therapies are less effective. In 2 large studies of patients with COPD symptoms, later diagnosis was associated with worse outcomes.4,5
Veterans have a higher prevalence of COPD (8%-19%) than nonveterans (6%), likely due to higher rates of smoking and service-related exposures, especially among veterans of post-9/11 conflicts.6,7 Veterans do not always report symptoms and PCPs may not ask about symptoms, leading to underdiagnosis.8 The combination of high likelihood and underdetection of COPD presents a challenge and a target for VA quality improvement (QI).
The US Preventive Services Task Force (USPSTF) recommends against screening asymptomatic patients for COPD. However, both the USPSTF and the Global Initiative for Chronic Obstructive Lung Disease Report advocate for active case finding in primary care clinics to determine whether high-risk patients, such as smokers, experience COPD symptoms and warrant spirometry. 9,10 To make early COPD diagnoses, clinicians may use questionnaires alone or in combination with handheld peak expiratory flow rate measurements.11,12 Formal spirometry, considered the gold standard for COPD diagnosis, is ordered for patients who report COPD symptoms (ie, shortness of breath with exertion) or who have both COPD symptoms and reduced peak flow rates.
A systematic review and meta-analysis found that while the combination of questionnaires and peak flows was the more effective strategy overall, questionnaires alone were also valuable for identifying patients with possible COPD.13 Implementation of either screening method in primary care practices would be challenging. In a simulation study that applied chronic disease and preventive care guidelines to hypothetical patient panels, the time required for PCPs to provide guideline-recommended chronic and preventive care in addition to acute care far exceeded 8 hours per day, even in team-based settings.14 Overburdened PCPs are therefore unlikely to accept additional tasks like COPD case finding.
Why don’t patients report their pulmonary symptoms? Patients may not recognize the symptoms as evidence of COPD. Others may be afraid of a COPD diagnosis or the stigma that is associated with it.15 Perhaps they believe COPD treatment is ineffective because of lung damage from smoking. Some patients may not want to know if they have COPD, while others reduce activity levels to avoid symptoms.16
QUALITY IMPROVEMENT PROJECT
Given the high prevalence of COPD among veterans and the potential for underdiagnosis, VA Northeast Ohio Healthcare System (VANEOHS) internal medicine residents and faculty assessed the state of COPD diagnosis in its primary care clinic with a QI project in 2022. Patients in the clinic between August 1, 2015, and November 30, 2022, with an International Classification of Diseases-10 (ICD-10) COPD diagnosis code (J44) in the electronic health record were included. Of 157 included patients, 105 patients who had prior spirometry testing were excluded. Of the 52 patients with diagnosed COPD and no spirometry testing, 30 patients had computed tomography (CT) findings consistent with COPD (ie, airway thickening, emphysema, air trapping) that was performed for CT lung cancer screening (LCS).17 Twenty-three of these 30 patients were contacted by phone. All 23 were ever smokers and 13 reported COPD symptoms. The PCPs of the symptomatic patients were then contacted. Spirometry was ordered for all 13 patients and completed by 7. Three spirometry tests confirmed the COPD diagnosis. One PCP initiated inhaler therapy for a patient with newly diagnosed COPD.
All 11 PCPs of symptomatic patients were interviewed (many had > 1 symptomatic patient). They reported being unaware of patients’ COPD symptoms because the patients did not mention them, noting that screening for COPD was not a priority.
Role of Lung Cancer Screening
VA PCPs use electronic health record clinical reminders to track tests, consults, chronic disease education, cancer screenings, and routine health maintenance. A clinical reminder already exists (based on USPSTF recommendations) for LCS for patients aged 50 to 80 years who have a smoking history of 20 pack years. Patients who meet these criteria would also be considered high risk for COPD.
The VANEOHS QI project suggests that previously undiagnosed patients with findings of COPD on LCS may also have symptoms of COPD. Therefore, we wondered whether the LCS clinical reminder could serve a second purpose by prompting PCPs to ask veterans who meet LCS criteria about their COPD symptoms.
In 2022, about 13 million patients were eligible for LCS.18 Patients who qualify for LCS are at high risk for other cardiopulmonary disorders, such as COPD and coronary artery disease. Lung cancer is detected in only 1% of patients screened with CT at baseline. However, more often LCS yields evidence of additional cardiopulmonary disorders, such as emphysema or coronary artery calcifications. The International Early Lung Cancer Program (I-ELCAP) and the National Lung Cancer Screening Trial (NLST), which included > 79,000 patients, found evidence of emphysema on CT imaging in 24% and 31% of cases, respectively.19,20 In both cohorts, > 80% of patients with emphysema on CT imaging had no prior history of COPD.
In a 2022 article summarizing the potential impact of CT LCS on COPD diagnosis, Mulshine et al suggest that detection of emphysema on CT LCS provides “earlier recognition for PCPs to identify patients who would benefit from detailed symptom screening to prompt spirometry for COPD detection” and additional motivation for tobacco cessation.21 The VANEOHS QI project was developed and implemented prior to I-ELCAP or NLST reporting results but reinforces the value of CT LCS for COPD diagnosis.
Early diagnosis of COPD remains challenging because PCPs do not ask, patients do not tell, and symptoms can easily be dismissed. However, earlier diagnosis of COPD in symptomatic patients improves outcomes.3,4 To bridge this gap, VA PCPs and primary care patient aligned care teams (PACTs) need to commit to probing high-risk patients for COPD symptoms and ordering spirometry for those who are symptomatic. To accomplish this task, primary care teams need help.
The VANEOHS QI project confirmed that some patients with evidence of COPD on CT have symptoms of COPD that they did not share with their PCPs and suggests that LCS can be used as a dual action case finding method to screen both for lung cancer and COPD. We propose that patients who are eligible for LCS should also be probed for COPD symptoms at their clinic visits; for symptomatic patients, spirometry should be ordered, and COPD evidence-based management should be initiated when spirometry results are consistent with COPD. Annual probing for COPD symptoms could be considered in asymptomatic patients with ongoing tobacco use or emphysema on CT, since they may develop symptoms in the future. This new case-finding method bypasses the need for time-prohibitive questionnaires or peak flow measurements.
Future Opportunities
VA PCPs juggle many priorities and despite the simplicity of this new case finding COPD method, it may be unintentionally overlooked. PCPs often run out of time or may forget to ask patients about COPD symptoms when ordering LCS.
Future innovations to increase COPD diagnosis could include the creation of a yearly VA clinical reminder linked to the tobacco use reminder that has check boxes asking about symptoms of COPD in current and prior smokers. If patients have COPD symptoms, the reminder can prompt the ordering of spirometry. Similar reminders could be implemented to identify veterans with exposures to burn pits or other military environmental exposures who may have COPD symptoms. Another possible way to increase COPD diagnosis would be a partnership between primary care and the VA LCS program where patients receiving screening are asked about COPD symptoms during their LCS interviews and PACTs are alerted to order spirometry for symptomatic patients.
Elusive no longer! We can pull the veil back on COPD diagnosis and identify patients with possible COPD earlier in their course using their eligibility for LCS as a yearly reminder to probe them for symptoms. While not all patients who undergo LCS—even those with evidence of COPD on CT—will have COPD symptoms, symptoms may develop over time. LCS provides the possibility of 2 diagnoses from 1 test. This is an opportunity we cannot afford to miss.
Primary care practitioners (PCPs) in the US Department of Veterans Affairs (VA) provide care for patients with higher rates of many diseases—diabetes, heart disease, cancer, chronic obstructive pulmonary disease (COPD), and stroke—compared to the nonveteran population. 1 Due to the medical complexities of these diseases, they are often misdiagnosed or not diagnosed at all.
COPD is hiding in plain sight, impacting quality of life and burdening US health care systems.2 Research has yielded new treatments and evidence-based guidelines; however, COPD remains underdiagnosed. Only 13 million of the estimated 79 million US adults with COPD aged 20 to 79 years have been formally diagnosed.3 By the time patients are diagnosed, the disease is often advanced, and therapies are less effective. In 2 large studies of patients with COPD symptoms, later diagnosis was associated with worse outcomes.4,5
Veterans have a higher prevalence of COPD (8%-19%) than nonveterans (6%), likely due to higher rates of smoking and service-related exposures, especially among veterans of post-9/11 conflicts.6,7 Veterans do not always report symptoms and PCPs may not ask about symptoms, leading to underdiagnosis.8 The combination of high likelihood and underdetection of COPD presents a challenge and a target for VA quality improvement (QI).
The US Preventive Services Task Force (USPSTF) recommends against screening asymptomatic patients for COPD. However, both the USPSTF and the Global Initiative for Chronic Obstructive Lung Disease Report advocate for active case finding in primary care clinics to determine whether high-risk patients, such as smokers, experience COPD symptoms and warrant spirometry. 9,10 To make early COPD diagnoses, clinicians may use questionnaires alone or in combination with handheld peak expiratory flow rate measurements.11,12 Formal spirometry, considered the gold standard for COPD diagnosis, is ordered for patients who report COPD symptoms (ie, shortness of breath with exertion) or who have both COPD symptoms and reduced peak flow rates.
A systematic review and meta-analysis found that while the combination of questionnaires and peak flows was the more effective strategy overall, questionnaires alone were also valuable for identifying patients with possible COPD.13 Implementation of either screening method in primary care practices would be challenging. In a simulation study that applied chronic disease and preventive care guidelines to hypothetical patient panels, the time required for PCPs to provide guideline-recommended chronic and preventive care in addition to acute care far exceeded 8 hours per day, even in team-based settings.14 Overburdened PCPs are therefore unlikely to accept additional tasks like COPD case finding.
Why don’t patients report their pulmonary symptoms? Patients may not recognize the symptoms as evidence of COPD. Others may be afraid of a COPD diagnosis or the stigma that is associated with it.15 Perhaps they believe COPD treatment is ineffective because of lung damage from smoking. Some patients may not want to know if they have COPD, while others reduce activity levels to avoid symptoms.16
QUALITY IMPROVEMENT PROJECT
Given the high prevalence of COPD among veterans and the potential for underdiagnosis, VA Northeast Ohio Healthcare System (VANEOHS) internal medicine residents and faculty assessed the state of COPD diagnosis in its primary care clinic with a QI project in 2022. Patients in the clinic between August 1, 2015, and November 30, 2022, with an International Classification of Diseases-10 (ICD-10) COPD diagnosis code (J44) in the electronic health record were included. Of 157 included patients, 105 patients who had prior spirometry testing were excluded. Of the 52 patients with diagnosed COPD and no spirometry testing, 30 patients had computed tomography (CT) findings consistent with COPD (ie, airway thickening, emphysema, air trapping) that was performed for CT lung cancer screening (LCS).17 Twenty-three of these 30 patients were contacted by phone. All 23 were ever smokers and 13 reported COPD symptoms. The PCPs of the symptomatic patients were then contacted. Spirometry was ordered for all 13 patients and completed by 7. Three spirometry tests confirmed the COPD diagnosis. One PCP initiated inhaler therapy for a patient with newly diagnosed COPD.
All 11 PCPs of symptomatic patients were interviewed (many had > 1 symptomatic patient). They reported being unaware of patients’ COPD symptoms because the patients did not mention them, noting that screening for COPD was not a priority.
Role of Lung Cancer Screening
VA PCPs use electronic health record clinical reminders to track tests, consults, chronic disease education, cancer screenings, and routine health maintenance. A clinical reminder already exists (based on USPSTF recommendations) for LCS for patients aged 50 to 80 years who have a smoking history of 20 pack years. Patients who meet these criteria would also be considered high risk for COPD.
The VANEOHS QI project suggests that previously undiagnosed patients with findings of COPD on LCS may also have symptoms of COPD. Therefore, we wondered whether the LCS clinical reminder could serve a second purpose by prompting PCPs to ask veterans who meet LCS criteria about their COPD symptoms.
In 2022, about 13 million patients were eligible for LCS.18 Patients who qualify for LCS are at high risk for other cardiopulmonary disorders, such as COPD and coronary artery disease. Lung cancer is detected in only 1% of patients screened with CT at baseline. However, more often LCS yields evidence of additional cardiopulmonary disorders, such as emphysema or coronary artery calcifications. The International Early Lung Cancer Program (I-ELCAP) and the National Lung Cancer Screening Trial (NLST), which included > 79,000 patients, found evidence of emphysema on CT imaging in 24% and 31% of cases, respectively.19,20 In both cohorts, > 80% of patients with emphysema on CT imaging had no prior history of COPD.
In a 2022 article summarizing the potential impact of CT LCS on COPD diagnosis, Mulshine et al suggest that detection of emphysema on CT LCS provides “earlier recognition for PCPs to identify patients who would benefit from detailed symptom screening to prompt spirometry for COPD detection” and additional motivation for tobacco cessation.21 The VANEOHS QI project was developed and implemented prior to I-ELCAP or NLST reporting results but reinforces the value of CT LCS for COPD diagnosis.
Early diagnosis of COPD remains challenging because PCPs do not ask, patients do not tell, and symptoms can easily be dismissed. However, earlier diagnosis of COPD in symptomatic patients improves outcomes.3,4 To bridge this gap, VA PCPs and primary care patient aligned care teams (PACTs) need to commit to probing high-risk patients for COPD symptoms and ordering spirometry for those who are symptomatic. To accomplish this task, primary care teams need help.
The VANEOHS QI project confirmed that some patients with evidence of COPD on CT have symptoms of COPD that they did not share with their PCPs and suggests that LCS can be used as a dual action case finding method to screen both for lung cancer and COPD. We propose that patients who are eligible for LCS should also be probed for COPD symptoms at their clinic visits; for symptomatic patients, spirometry should be ordered, and COPD evidence-based management should be initiated when spirometry results are consistent with COPD. Annual probing for COPD symptoms could be considered in asymptomatic patients with ongoing tobacco use or emphysema on CT, since they may develop symptoms in the future. This new case-finding method bypasses the need for time-prohibitive questionnaires or peak flow measurements.
Future Opportunities
VA PCPs juggle many priorities and despite the simplicity of this new case finding COPD method, it may be unintentionally overlooked. PCPs often run out of time or may forget to ask patients about COPD symptoms when ordering LCS.
Future innovations to increase COPD diagnosis could include the creation of a yearly VA clinical reminder linked to the tobacco use reminder that has check boxes asking about symptoms of COPD in current and prior smokers. If patients have COPD symptoms, the reminder can prompt the ordering of spirometry. Similar reminders could be implemented to identify veterans with exposures to burn pits or other military environmental exposures who may have COPD symptoms. Another possible way to increase COPD diagnosis would be a partnership between primary care and the VA LCS program where patients receiving screening are asked about COPD symptoms during their LCS interviews and PACTs are alerted to order spirometry for symptomatic patients.
Elusive no longer! We can pull the veil back on COPD diagnosis and identify patients with possible COPD earlier in their course using their eligibility for LCS as a yearly reminder to probe them for symptoms. While not all patients who undergo LCS—even those with evidence of COPD on CT—will have COPD symptoms, symptoms may develop over time. LCS provides the possibility of 2 diagnoses from 1 test. This is an opportunity we cannot afford to miss.
- Betancourt JA, Granados PS, Pacheco GJ, et al. Exploring health outcomes for U.S. veterans compared to non-veterans from 2003 to 2019. Healthcare (Basel). 2021;9(5):604. doi:10.3390/healthcare90506064
- Bamonti PM, Fischer I, Moye J, Poghosyan H, Pietrzak RH. Obstructive respiratory disease in U.S. veterans: prevalence, characteristics, and health burden. J Psychiatr Res. 2024;176:140-147. doi:10.1016/j.jpsychires.2024.05.053
- Criner RN, Han MK. COPD care in the 21st century: a public health priority. Respir Care. 2018;63(5):591-600. doi:10.4187/respcare.06276
- Larsson K, Janson C, Ställberg B, et al. Impact of COPD diagnosis timing on clinical and economic outcomes: the ARCTIC observational cohort study. Int J Chron Obstruct Pulmon Dis. 2019;14:995-1008. doi:10.2147/COPD.S195382
- Kostikas K, Price D, Gutzwiller FS, et al. Clinical impact and healthcare resource utilization associated with early versus late COPD diagnosis in patients from UK CPRD Database. Int J Chron Obstruct Pulmon Dis. 2020;15:1729- 1738. doi:10.2147/COPD.S255414
- Bamonti PM, Robinson SA, Wan ES, Moy ML. Improving physiological, physical, and psychological health outcomes: a narrative review in US veterans with COPD. Int J Chron Obstruct Pulmon Dis. 2022;17:1269-1283. doi:10.2147/COPD.S339323
- Savitz DA, Woskie SR, Bello A, et al. Deployment to military bases with open burn pits and respiratory and cardiovascular disease. JAMA Netw Open. 2024;7(4):e247629. doi:10.1001/jamanetworkopen.2024.7629
- Murphy DE, Chaudhry Z, Almoosa KF, Panos RJ. High prevalence of chronic obstructive pulmonary disease among veterans in the urban midwest. Mil Med. 2011;176(5):552-560. doi:10.7205/milmed-d-10-00377
- Guirguis-Blake JM, Senger CA, Webber EM, Mularski RA, Whitlock EP. Screening for chronic obstructive pulmonary disease: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315(13):1378-1393. doi:10.1001/jama.2016.2654
- Capriotti T, Tomy R, Morales M. COPD updates: 2023 GOLD Report for primary care providers. Clinical Advisor. May 9, 2023. Accessed May 14, 2025. https://www.clinicaladvisor.com/features/copd-updates-2023-gold-report-primary-care/
- Leidy NK, Martinez FJ, Malley KG, et al. Can CAPTURE be used to identify undiagnosed patients with mild- to- moderate COPD likely to benefit from treatment? Int J Chron Obstruct Pulmon Dis. 2018;13:1901-1912. doi:10.2147/COPD.S152226
- Jithoo A, Enright PL, Burney P, et al. Case-finding options for COPD: results from the burden of obstructive lung disease study. Eur Respir J. 2013;41(3):548-555. doi:10.1183/09031936.00132011
- Haroon SM, Jordan RE, O’Beirne-Elliman J, Adab P. Effectiveness of case finding strategies for COPD in primary care: a systematic review and meta-analysis. NPJ Prim Care Respir Med. 2015;25:15056. doi:10.1038/npjpcrm.2015.56
- Porter J, Boyd C, Skandari MR, Laiteerapong N. Revisiting the time needed to provide adult primary care. J Gen Intern Med. 2023;38(1)147-155. doi:10.1007/s11606-022-07707-x
- Woo S, Zhou W, Larson JL. Stigma experiences in people with chronic obstructive pulmonary disease: an integrative review. Int J Chron Obstruct Pulmon Dis. 2021;16:1647- 1659. doi:10.2147/COPD.S306874
- Aaron SD, Montes de Oca M, Celli B, et al. Early diagnosis and treatment of COPD: the costs and benefits of case finding. Am J Respir Crit Care Med. 2024;209(8):928-937. doi:10.1164/rccm.202311-2120PP
- Kwon A, Lee C, Arafah A, Klein M, Namboodiri S, Lee C. Increasing chronic obstructive pulmonary disease (COPD) diagnosis with pulmonary function testing for patients with chest imaging evidence of COPD. Poster presented at: Society of General Internal Medicine Midwest Regional Meeting; October 19-20, 2023; Chicago, IL.
- Henderson LM, Su I, Rivera MP, et al. Prevalence of lung cancer screening in the US, 2022. JAMA Netw Open. 2024;7(3):e243190. doi:10.1001/jamanetworkopen.2024.3190
- Steiger D, Siddiqi MF, Yip R, Yankelevitz DF, Henschke CI; I-ELCAP investigators. The importance of low-dose CT screening to identify emphysema in asymptomatic participants with and without a prior diagnosis of COPD. Clin Imaging. 2021;78:136-141. doi:10.1016/j.clinimag.2021.03.012
- Pinsky PF, Lynch DA, Gierada DS. Incidental findings on low-dose CT scan lung cancer screenings and deaths from respiratory diseases. Chest. 2022;161(4):1092-1100. doi:10.1016/j.chest.2021.11.015
- Mulshine JL, Aldigé CR, Ambrose LF, et al. Emphysema detection in the course of lung cancer screening: optimizing a rare opportunity to impact population health. Ann Am Thorac Soc. 2023;20(4):499- 503. doi:10.1513/AnnalsATS.202207-631PS
- Betancourt JA, Granados PS, Pacheco GJ, et al. Exploring health outcomes for U.S. veterans compared to non-veterans from 2003 to 2019. Healthcare (Basel). 2021;9(5):604. doi:10.3390/healthcare90506064
- Bamonti PM, Fischer I, Moye J, Poghosyan H, Pietrzak RH. Obstructive respiratory disease in U.S. veterans: prevalence, characteristics, and health burden. J Psychiatr Res. 2024;176:140-147. doi:10.1016/j.jpsychires.2024.05.053
- Criner RN, Han MK. COPD care in the 21st century: a public health priority. Respir Care. 2018;63(5):591-600. doi:10.4187/respcare.06276
- Larsson K, Janson C, Ställberg B, et al. Impact of COPD diagnosis timing on clinical and economic outcomes: the ARCTIC observational cohort study. Int J Chron Obstruct Pulmon Dis. 2019;14:995-1008. doi:10.2147/COPD.S195382
- Kostikas K, Price D, Gutzwiller FS, et al. Clinical impact and healthcare resource utilization associated with early versus late COPD diagnosis in patients from UK CPRD Database. Int J Chron Obstruct Pulmon Dis. 2020;15:1729- 1738. doi:10.2147/COPD.S255414
- Bamonti PM, Robinson SA, Wan ES, Moy ML. Improving physiological, physical, and psychological health outcomes: a narrative review in US veterans with COPD. Int J Chron Obstruct Pulmon Dis. 2022;17:1269-1283. doi:10.2147/COPD.S339323
- Savitz DA, Woskie SR, Bello A, et al. Deployment to military bases with open burn pits and respiratory and cardiovascular disease. JAMA Netw Open. 2024;7(4):e247629. doi:10.1001/jamanetworkopen.2024.7629
- Murphy DE, Chaudhry Z, Almoosa KF, Panos RJ. High prevalence of chronic obstructive pulmonary disease among veterans in the urban midwest. Mil Med. 2011;176(5):552-560. doi:10.7205/milmed-d-10-00377
- Guirguis-Blake JM, Senger CA, Webber EM, Mularski RA, Whitlock EP. Screening for chronic obstructive pulmonary disease: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315(13):1378-1393. doi:10.1001/jama.2016.2654
- Capriotti T, Tomy R, Morales M. COPD updates: 2023 GOLD Report for primary care providers. Clinical Advisor. May 9, 2023. Accessed May 14, 2025. https://www.clinicaladvisor.com/features/copd-updates-2023-gold-report-primary-care/
- Leidy NK, Martinez FJ, Malley KG, et al. Can CAPTURE be used to identify undiagnosed patients with mild- to- moderate COPD likely to benefit from treatment? Int J Chron Obstruct Pulmon Dis. 2018;13:1901-1912. doi:10.2147/COPD.S152226
- Jithoo A, Enright PL, Burney P, et al. Case-finding options for COPD: results from the burden of obstructive lung disease study. Eur Respir J. 2013;41(3):548-555. doi:10.1183/09031936.00132011
- Haroon SM, Jordan RE, O’Beirne-Elliman J, Adab P. Effectiveness of case finding strategies for COPD in primary care: a systematic review and meta-analysis. NPJ Prim Care Respir Med. 2015;25:15056. doi:10.1038/npjpcrm.2015.56
- Porter J, Boyd C, Skandari MR, Laiteerapong N. Revisiting the time needed to provide adult primary care. J Gen Intern Med. 2023;38(1)147-155. doi:10.1007/s11606-022-07707-x
- Woo S, Zhou W, Larson JL. Stigma experiences in people with chronic obstructive pulmonary disease: an integrative review. Int J Chron Obstruct Pulmon Dis. 2021;16:1647- 1659. doi:10.2147/COPD.S306874
- Aaron SD, Montes de Oca M, Celli B, et al. Early diagnosis and treatment of COPD: the costs and benefits of case finding. Am J Respir Crit Care Med. 2024;209(8):928-937. doi:10.1164/rccm.202311-2120PP
- Kwon A, Lee C, Arafah A, Klein M, Namboodiri S, Lee C. Increasing chronic obstructive pulmonary disease (COPD) diagnosis with pulmonary function testing for patients with chest imaging evidence of COPD. Poster presented at: Society of General Internal Medicine Midwest Regional Meeting; October 19-20, 2023; Chicago, IL.
- Henderson LM, Su I, Rivera MP, et al. Prevalence of lung cancer screening in the US, 2022. JAMA Netw Open. 2024;7(3):e243190. doi:10.1001/jamanetworkopen.2024.3190
- Steiger D, Siddiqi MF, Yip R, Yankelevitz DF, Henschke CI; I-ELCAP investigators. The importance of low-dose CT screening to identify emphysema in asymptomatic participants with and without a prior diagnosis of COPD. Clin Imaging. 2021;78:136-141. doi:10.1016/j.clinimag.2021.03.012
- Pinsky PF, Lynch DA, Gierada DS. Incidental findings on low-dose CT scan lung cancer screenings and deaths from respiratory diseases. Chest. 2022;161(4):1092-1100. doi:10.1016/j.chest.2021.11.015
- Mulshine JL, Aldigé CR, Ambrose LF, et al. Emphysema detection in the course of lung cancer screening: optimizing a rare opportunity to impact population health. Ann Am Thorac Soc. 2023;20(4):499- 503. doi:10.1513/AnnalsATS.202207-631PS
The Use of Lung Cancer Screening to Increase Chronic Obstructive Pulmonary Disease Diagnosis in Veterans Affairs Primary Care
The Use of Lung Cancer Screening to Increase Chronic Obstructive Pulmonary Disease Diagnosis in Veterans Affairs Primary Care
When Patient-Centered Care Initiatives Align: Integrating VA Whole Health and Shared Decision-Making for Lung Cancer Screening
When Patient-Centered Care Initiatives Align: Integrating VA Whole Health and Shared Decision-Making for Lung Cancer Screening
The landmark Crossing the Quality Chasm report from the National Academy of Medicine identified patient- centered care as essential to health care quality. The report defines patientcentered care as “respectful of and responsive to individual patient preferences, needs, and values.”1 Many health care systems, including the Veterans Health Administration, are transforming to a patient-centered model of care.2 The US Department of Veterans Affairs (VA) Whole Health System of Care initiative is a system-wide, cultural transformation. Within whole health, what matters most to the patient—including their preferences, needs, and values—is foundational to health care and meant to be essential in every clinical encounter. Whole health implementation includes a progressive rollout with health care practitioner (HCP) trainings across the VA.2
Shared decision-making (SDM) is a different but aligned patient-centered care concept. SDM is a process through which a decision or care plan, based on patients’ preferences, needs, and values, is made or developed.3-5 SDM is ideal in situations with equipoise (decisions with equivalent choices), individualized risks, and/or greater uncertainty of the net benefit, such as with lung cancer screening (LCS).3 SDM for LCS is required by the US Centers for Medicare and Medicaid Services and has been adopted by many US health care systems, including the VA.6,7 Early detection of lung cancer can reduce death by 20% at the population level.8 However, at the patient level there is wide variation in the risk of developing lung cancer and a range of potential harms.8 LCS follow-up procedures may be more invasive than with other cancer screenings. Thus, there is concern about the risk of false-positive results leading to unnecessary care or complications.8 Given this balance between benefit and harm and the differing patient value on the trade-offs of LCS, an individualized, patient-centered approach is essential when deciding whether LCS is the right choice for a specific patient.
Despite the importance of LCS SDM, observational studies have shown poor implementation in clinical encounters.9,10 HCP barriers include competing demands, limited time, lack of familiarity with and training in SDM, and beliefs biasing screening over no screening.11-13 Additionally, HCPs may assume that patients want them to make the decision. However, research has shown that patients actually want to be more involved in their health care decisions.14 One suggested strategy to overcome these barriers is aligning SDM for LCS within an organization’s broader patient-centered initiatives.15
This project sought to align the need for SDM for LCS and the broader VA whole health initiative as part of a multilevel strategy to implement SDM for LCS across Veterans Integrated Service Network (VISN) 1.16
This article addresses HCP-level barriers. HCPs targeted are those typically involved in LCS. The VA utilizes LCS coordinators (LCSCs) in both centralized or consult models (in which LCSCs are involved in all aspects of screening) and hybrid models (in which primary care practitioners and LCSCs are both engaged in LCS tasks). The goal of this program was to generate areas of conceptual alignment between SDM and whole health as a first step in integrating these VA initiatives. This work was conducted as a foundation for an SDM for lung cancer HCP training and consultation initiative.
ALIGNMENT PROCESS
We reviewed relevant literature and resources for SDM and whole health. In reviewing the SDM literature, we included a sample of the most widely cited literature on the topic, and focused primarily on the systematic review by Bomhof-Roordink et al.4,5,17,18 This review provided a synthesis of SDM elements across SDM models and identified 53 different elements clustered into 24 components.4 The most common components were present in at least half of all SDM published models, including: make the decision, patient preferences, tailor information, deliberate, create choice awareness, and learn about the patient. Bomhof-Roordink et al provided the guiding framework for this conceptualization of SDM because that study included the available recent published SDM models.4
Second, published literature on VA whole health along with supplemental promotional and training materials were reviewed. The whole health materials included 2 sets of training slides developed for VA HCPs (available to VA employees): Implementing Whole Health in Clinical Care, which is focused on HCPs’ work with patients, and Whole Health for You and Me, which is about HCPs’ personal well-being.19 We also reviewed a publication describing the history of whole health and patient-facing online whole health tools.2,19
Each document was reviewed for key elements related to SDM, patient-centered care, and whole health. Using the 53 elements identified by Bomhof-Roordink et al, we reviewed and compared each element to the whole health materials to create the integrated model of SDM and whole health. We iteratively discussed and organized the elements until we reached consensus.
SDM and Whole Health Alignment
We created an integrated model of SDM for LCS within the context of the VA whole health initiative. This integrated model is directed at HCPs who would likely engage patients in discussions of LCS, including primary care practitioners and nurse coordinators. The model includes 3 steps for HCPs to follow that align SDM within whole health: (1) frame the conversation and partner with the patient; (2) share clinical perspective and elicit patient values; and (3) deliberate and decide together. For each step, the SDM elements, whole health elements, and integration of SDM and whole health are provided. Table 1 provides an overview of the similarities and differences between SDM and whole health. Example phrases that merge SDM and whole health for HCPs to use in patient conversations about LCS are included in Table 2.
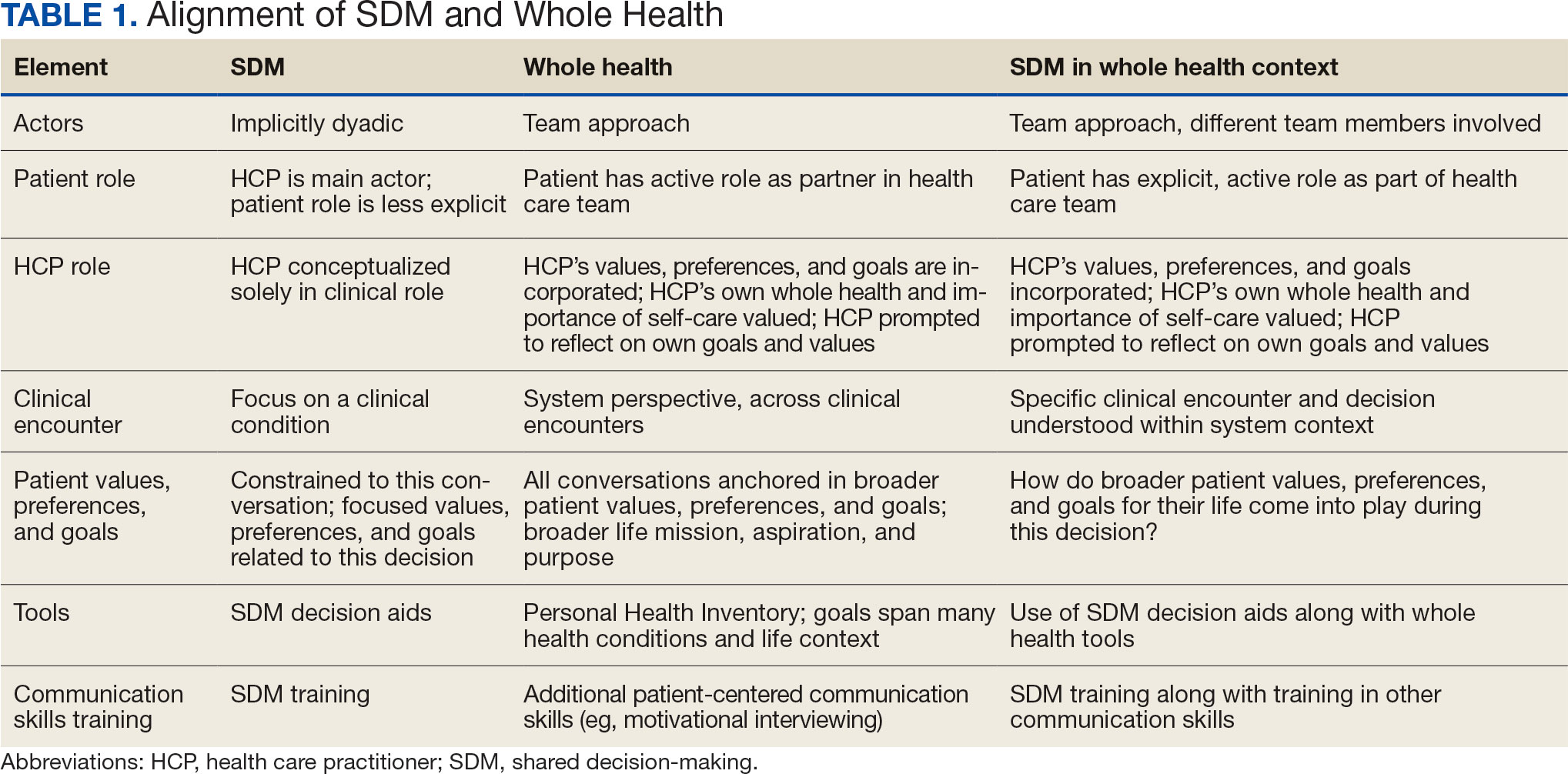

STEP 1. FRAME THE CONVERSATION AND PARTNER WITH THE PATIENT
Shared decision-making. Traditional SDM literature includes an initial step of letting patients know that there is a choice to be made between ≥ 2 clinical options.4 Ancillary elements of this first step include asking patients their preferences about the degree to which they want to be involved in SDM and about how they like to receive information (eg, verbal, written, video). These steps open the SDM conversation and ensure the patient and HCP are on the same page before moving forward. For example, the US Agency for Healthcare Research and Quality SHARE model’s first step is for HCPs to communicate that choices exist and to invite the patient to be involved in decisions.20 Similarly, Elwyn’s 3-step SDM model begins with establishing that a choice exists and inviting patient input on making that choice.17
Whole health. Patients are encouraged to play an active role in their health care. Through whole health programs such as Taking Charge of My Life and Health, patients explore their values and set self-care goals.21 HCP whole health trainings teach and reinforce communication skills, including SDM, listening skills, and motivational interviewing.19
Shared decision-making/whole health integration. SDM and whole health both prioritize respect, compassion, and patients’ expertise. They focus on the patient-HCP relationship with an emphasis on fostering egalitarian interactions. HCPs frame the SDM conversation and partner with the patient so they know what to expect and who will be involved. This conversation is framed from the outset as a collaborative discussion. HCPs empower the patient to play an active role in decision-making and help them understand why their engagement is critical.
STEP 2. SHARE CLINICAL PERSPECTIVE AND ELICIT PATIENT VALUES
Shared decision-making. HCPs share clinical perspective on LCS tailored to individual patients while explicitly inviting the patient to share their preferences and values when thinking about whether to undergo LCS. HCPs give a balanced description of LCS, including the benefits and harms, tailored to the patient’s unique information needs and questions. Sharing clinical perspective also includes describing treatment options, the most common element across SDM models.4 Decision aids, which provide unbiased information and include a values clarification exercise, may be helpful in sharing clinical perspectives and clarifying patient values related to the trade-offs of LCS.22 For example, the VA National Center for Health Promotion and Disease Prevention developed a LCS decision aid to be used for SDM for LCS.
Whole health. The conversation shifts from “What is the matter with you?” to “What matters to you?” starting with the patient’s goals and priorities rather than disease prevention, diagnosis, and treatment.2 Several whole health tools exist, including the Personal Health Inventory, used to identify what matters most to patients and understand their current well-being and self-care.23 Using the inventory, the patient and their health care team develop the patient’s personal health plan.24 Additionally, whole health trains HCPs to reflect on their own attitudes and biases when providing clinical care.
Shared decision-making/whole health integration. The LCS conversation can build on other whole health-related conversations with a HCP or other team members. HCPs can reference the patient’s personal health plan for documentation of the patient’s preferences, values, and goals in the electronic medical record. During this process, HCPs can give space for patients to discuss factors in their life and experiences that impact their perspective and decision-making. For example, patient concerns could be explored here, including fear of a cancer diagnosis, stigma around smoking, and fears around the screening and/or treatment process. HCPs may ask, “What matters most to you when making this decision?” Finally, by sharing clinical information, HCPs will focus on patient values to help overcome their own biases toward a desire for LCS. HCPs, similar to the rest of the US public, tend to hold highly favorable attitudes toward cancer screening as well as misconceptions about the magnitude of benefits from screening.13
STEP 3. DELIBERATE AND DECIDE TOGETHER
Shared decision-making. Decision-making is almost always considered the last SDM step.4 In the final step, the patient and HCP discuss the options (ie, to screen or not to screen) considering the patient’s values and preferences, and patients decide with their HCP whether they will undergo LCS. Patients may decide they need more time to think about these options. As part of deliberation, HCPs assess what other information patients may need to arrive at a decision. Family members, friends, or peers may be included in making the final decision.
Whole health. In Whole health, decisions also may include the entire health care team and other individuals important to the patient (eg, family, friends). Integration across different health care settings is also considered a key whole health element. Finally, whole health focuses on long-term relationships with patients; thus, the LCS SDM process is situated within longer term relationship building and patient empowerment, both of which will facilitate partnering with the patient in future conversations about other decisions.
Shared decision-making/whole health integration. Both SDM and whole health emphasize partnership with the patient in making a final decision. There is also focus on decision-making as an ongoing process. Deciding whether LCS is the best choice might include naming and addressing emotions, voicing questions not raised, and exploring whether screening fits the patient’s goals, values, and life context. HCPs may give guidance, but patients retain the authority to make decisions. The goal is to empower patients to know that the only right decision is the one right for them and they will be supported.
Limitations
This article describes a VA practice program and was not a formal research study. Further work is needed to evaluate the presented strategies. Additionally, we did not conduct a systematic literature review and thus elements of SDM and whole health may not be exhaustive.
CONCLUSIONS
This article describes the alignment of 2 distinct VA initiatives, whole health and SDM for LCS. The goal was to reduce known barriers to SDM, such as competing demands, limited time, and lack of familiarity with and training in SDM.11-13 These concepts are well aligned. This integrated model is the first step in informing the development of a HCP training program and materials as part of a multilevel strategy that our team is using to implement SDM for LCS in VISN 1.16 The final training and materials resulting from this work were delivered to LCSCs in 3 ways: (1) a series of 3 interactive group training sessions, including didactic elements, role play, and time for open discussion; (2) 1-on-1 academic detailing; and (3) educational handouts. In academic detailing, a member of the research team trained in academic detailing met virtually with each nurse coordinator, identified that individual’s barriers to SDM, and used the training materials to highlight messages to overcome those barriers; follow-up calls provided a forum for discussing progress and overcoming additional challenges. Although this article focused specifically on whole health and SDM, the conceptual alignment process strategy can be applied to other implementations of multiple initiatives.
- Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. The National Academies Press; 2001. doi:10.17226/10027
- Bokhour BG, Haun JN, Hyde J, Charns M, Kligler B. Transforming the Veterans Affairs to a whole health system of care: time for action and research. Med Care. 2020;58:295- 300. doi:10.1097/MLR.0000000000001316
- Elwyn G, Frosch D, Rollnick S. Dual equipoise shared decision making: definitions for decision and behaviour support interventions. Implement Sci. 2009;4:75. doi:7510.1186/1748-5908-4-75
- Bomhof-Roordink H, Gärtner FR, Stiggelbout AM, Pieterse AH. Key components of shared decision making models: a systematic review. BMJ Open. 2019;9:e031763. doi:10.1136/bmjopen-2019-031763
- Charles C, Gafni A, Whelan T. Decision-making in the physician- patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med. 1999;49:651-661. doi:10.1016/s0277-9536(99)00145-8
- Moyer VA; US Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:330- 338. doi:10.7326/m13-2771
- Centers for Medicare & Medicaid Services. Screening for lung cancer with low dose computed tomography (LDCT). February 10, 2022. Accessed February 7, 2025. https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=304
- Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. doi:10.1056/NEJMoa1102873
- Slatore CG, Wiener RS. Pulmonary nodules: a small problem for many, severe distress for some, and how to communicate about it. Chest. 2018;153:1004-1015. doi:10.1016/j.chest.2017.10.013
- Nishi SPE, Lowenstein LM, Mendoza TR, et al. Shared decision-making for lung cancer screening: how well are we “sharing”? Chest. 2021;160:330-340. doi:10.1016/j.chest.2021.01.041
- Wiener RS, Koppelman E, Bolton R, et al. Patient and clinician perspectives on shared decision-making in early adopting lung cancer screening programs: a qualitative study. J Gen Intern Med. 2018;33:1035-1042. doi:10.1007/s11606-018-4350-9
- Melzer AC, Golden SE, Ono SS, Datta S, Triplette M, Slatore CG. “We just never have enough time”: clinician views of lung cancer screening processes and implementation. Ann Am Thorac Soc. 2020. doi:10.1513/AnnalsATS.202003-262OC
- Schwartz LM, Woloshin S, Fowler FJ Jr, Welch HG. Enthusiasm for cancer screening in the United States. JAMA. 2004;291:71-78. doi:10.1001/jama.291.1.71
- Lown BA, Rosen J, Marttila J. An agenda for improving compassionate care: a survey shows about half of patients say such care is missing. Health Aff (Millwood). 2011;30:1772-1778. doi:10.1377/hlthaff.2011.0539
- Scholl I, LaRussa A, Hahlweg P, Kobrin S, Elwyn G. Organizational- and system-level characteristics that influence implementation of shared decision-making and strategies to address them - a scoping review. Implement Sci. 2018;13:40. doi:10.1186/s13012-018-0731-z
- Khanna A, Fix GM, Anderson E, et al. Towards a framework for patient-centred care coordination: a scoping review protocol. BMJ Open. 2022;12:e066808. doi:10.1136/bmjopen-2022-066808
- Elwyn G, Durand MA, Song J, et al. A three-talk model for shared decision making: multistage consultation process. BMJ. 2017;359:j4891. doi:10.1136/bmj.j4891
- Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Educ Couns. 2006;60:301-312. doi:10.1016/j.pec.2005.06.010
- Whole Health. US Department of Veterans Affairs. Accessed April 14, 2025. https://www.va.gov/wholehealth/
- Agency for Healthcare Research and Quality. The SHARE approach. Accessed April 14, 2025. https://www.ahrq.gov/health-literacy/professional-training/shared-decision/index.html
- Abadi MH, Barker AM, Rao SR, Orner M, Rychener D, Bokhour BG. Examining the impact of a peer-led group program for veteran engagement and well-being. J Altern Complement Med. 2021;27:S37-S44. doi:10.1089/acm.2020.0124
- Stacey D, Lewis KB, Smith M, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2024;1:CD001431. doi:10.1002/14651858.CD001431.pub6
- US Department of Veterans Affairs, Veterans Health Administration, Office of Patient Centered Care and Cultural Transformation. Personal health inventory. Revised April 2019. Accessed April 14, 2025. https://www.va.gov/wholehealth/docs/10-773_PHI_July2019_508.pdf
- US Department of Veterans Affairs. Build your personal health plan. Updated July 24, 2024. Accessed April 14, 2025. https://www.va.gov/wholehealth/phi.asp
The landmark Crossing the Quality Chasm report from the National Academy of Medicine identified patient- centered care as essential to health care quality. The report defines patientcentered care as “respectful of and responsive to individual patient preferences, needs, and values.”1 Many health care systems, including the Veterans Health Administration, are transforming to a patient-centered model of care.2 The US Department of Veterans Affairs (VA) Whole Health System of Care initiative is a system-wide, cultural transformation. Within whole health, what matters most to the patient—including their preferences, needs, and values—is foundational to health care and meant to be essential in every clinical encounter. Whole health implementation includes a progressive rollout with health care practitioner (HCP) trainings across the VA.2
Shared decision-making (SDM) is a different but aligned patient-centered care concept. SDM is a process through which a decision or care plan, based on patients’ preferences, needs, and values, is made or developed.3-5 SDM is ideal in situations with equipoise (decisions with equivalent choices), individualized risks, and/or greater uncertainty of the net benefit, such as with lung cancer screening (LCS).3 SDM for LCS is required by the US Centers for Medicare and Medicaid Services and has been adopted by many US health care systems, including the VA.6,7 Early detection of lung cancer can reduce death by 20% at the population level.8 However, at the patient level there is wide variation in the risk of developing lung cancer and a range of potential harms.8 LCS follow-up procedures may be more invasive than with other cancer screenings. Thus, there is concern about the risk of false-positive results leading to unnecessary care or complications.8 Given this balance between benefit and harm and the differing patient value on the trade-offs of LCS, an individualized, patient-centered approach is essential when deciding whether LCS is the right choice for a specific patient.
Despite the importance of LCS SDM, observational studies have shown poor implementation in clinical encounters.9,10 HCP barriers include competing demands, limited time, lack of familiarity with and training in SDM, and beliefs biasing screening over no screening.11-13 Additionally, HCPs may assume that patients want them to make the decision. However, research has shown that patients actually want to be more involved in their health care decisions.14 One suggested strategy to overcome these barriers is aligning SDM for LCS within an organization’s broader patient-centered initiatives.15
This project sought to align the need for SDM for LCS and the broader VA whole health initiative as part of a multilevel strategy to implement SDM for LCS across Veterans Integrated Service Network (VISN) 1.16
This article addresses HCP-level barriers. HCPs targeted are those typically involved in LCS. The VA utilizes LCS coordinators (LCSCs) in both centralized or consult models (in which LCSCs are involved in all aspects of screening) and hybrid models (in which primary care practitioners and LCSCs are both engaged in LCS tasks). The goal of this program was to generate areas of conceptual alignment between SDM and whole health as a first step in integrating these VA initiatives. This work was conducted as a foundation for an SDM for lung cancer HCP training and consultation initiative.
ALIGNMENT PROCESS
We reviewed relevant literature and resources for SDM and whole health. In reviewing the SDM literature, we included a sample of the most widely cited literature on the topic, and focused primarily on the systematic review by Bomhof-Roordink et al.4,5,17,18 This review provided a synthesis of SDM elements across SDM models and identified 53 different elements clustered into 24 components.4 The most common components were present in at least half of all SDM published models, including: make the decision, patient preferences, tailor information, deliberate, create choice awareness, and learn about the patient. Bomhof-Roordink et al provided the guiding framework for this conceptualization of SDM because that study included the available recent published SDM models.4
Second, published literature on VA whole health along with supplemental promotional and training materials were reviewed. The whole health materials included 2 sets of training slides developed for VA HCPs (available to VA employees): Implementing Whole Health in Clinical Care, which is focused on HCPs’ work with patients, and Whole Health for You and Me, which is about HCPs’ personal well-being.19 We also reviewed a publication describing the history of whole health and patient-facing online whole health tools.2,19
Each document was reviewed for key elements related to SDM, patient-centered care, and whole health. Using the 53 elements identified by Bomhof-Roordink et al, we reviewed and compared each element to the whole health materials to create the integrated model of SDM and whole health. We iteratively discussed and organized the elements until we reached consensus.
SDM and Whole Health Alignment
We created an integrated model of SDM for LCS within the context of the VA whole health initiative. This integrated model is directed at HCPs who would likely engage patients in discussions of LCS, including primary care practitioners and nurse coordinators. The model includes 3 steps for HCPs to follow that align SDM within whole health: (1) frame the conversation and partner with the patient; (2) share clinical perspective and elicit patient values; and (3) deliberate and decide together. For each step, the SDM elements, whole health elements, and integration of SDM and whole health are provided. Table 1 provides an overview of the similarities and differences between SDM and whole health. Example phrases that merge SDM and whole health for HCPs to use in patient conversations about LCS are included in Table 2.


STEP 1. FRAME THE CONVERSATION AND PARTNER WITH THE PATIENT
Shared decision-making. Traditional SDM literature includes an initial step of letting patients know that there is a choice to be made between ≥ 2 clinical options.4 Ancillary elements of this first step include asking patients their preferences about the degree to which they want to be involved in SDM and about how they like to receive information (eg, verbal, written, video). These steps open the SDM conversation and ensure the patient and HCP are on the same page before moving forward. For example, the US Agency for Healthcare Research and Quality SHARE model’s first step is for HCPs to communicate that choices exist and to invite the patient to be involved in decisions.20 Similarly, Elwyn’s 3-step SDM model begins with establishing that a choice exists and inviting patient input on making that choice.17
Whole health. Patients are encouraged to play an active role in their health care. Through whole health programs such as Taking Charge of My Life and Health, patients explore their values and set self-care goals.21 HCP whole health trainings teach and reinforce communication skills, including SDM, listening skills, and motivational interviewing.19
Shared decision-making/whole health integration. SDM and whole health both prioritize respect, compassion, and patients’ expertise. They focus on the patient-HCP relationship with an emphasis on fostering egalitarian interactions. HCPs frame the SDM conversation and partner with the patient so they know what to expect and who will be involved. This conversation is framed from the outset as a collaborative discussion. HCPs empower the patient to play an active role in decision-making and help them understand why their engagement is critical.
STEP 2. SHARE CLINICAL PERSPECTIVE AND ELICIT PATIENT VALUES
Shared decision-making. HCPs share clinical perspective on LCS tailored to individual patients while explicitly inviting the patient to share their preferences and values when thinking about whether to undergo LCS. HCPs give a balanced description of LCS, including the benefits and harms, tailored to the patient’s unique information needs and questions. Sharing clinical perspective also includes describing treatment options, the most common element across SDM models.4 Decision aids, which provide unbiased information and include a values clarification exercise, may be helpful in sharing clinical perspectives and clarifying patient values related to the trade-offs of LCS.22 For example, the VA National Center for Health Promotion and Disease Prevention developed a LCS decision aid to be used for SDM for LCS.
Whole health. The conversation shifts from “What is the matter with you?” to “What matters to you?” starting with the patient’s goals and priorities rather than disease prevention, diagnosis, and treatment.2 Several whole health tools exist, including the Personal Health Inventory, used to identify what matters most to patients and understand their current well-being and self-care.23 Using the inventory, the patient and their health care team develop the patient’s personal health plan.24 Additionally, whole health trains HCPs to reflect on their own attitudes and biases when providing clinical care.
Shared decision-making/whole health integration. The LCS conversation can build on other whole health-related conversations with a HCP or other team members. HCPs can reference the patient’s personal health plan for documentation of the patient’s preferences, values, and goals in the electronic medical record. During this process, HCPs can give space for patients to discuss factors in their life and experiences that impact their perspective and decision-making. For example, patient concerns could be explored here, including fear of a cancer diagnosis, stigma around smoking, and fears around the screening and/or treatment process. HCPs may ask, “What matters most to you when making this decision?” Finally, by sharing clinical information, HCPs will focus on patient values to help overcome their own biases toward a desire for LCS. HCPs, similar to the rest of the US public, tend to hold highly favorable attitudes toward cancer screening as well as misconceptions about the magnitude of benefits from screening.13
STEP 3. DELIBERATE AND DECIDE TOGETHER
Shared decision-making. Decision-making is almost always considered the last SDM step.4 In the final step, the patient and HCP discuss the options (ie, to screen or not to screen) considering the patient’s values and preferences, and patients decide with their HCP whether they will undergo LCS. Patients may decide they need more time to think about these options. As part of deliberation, HCPs assess what other information patients may need to arrive at a decision. Family members, friends, or peers may be included in making the final decision.
Whole health. In Whole health, decisions also may include the entire health care team and other individuals important to the patient (eg, family, friends). Integration across different health care settings is also considered a key whole health element. Finally, whole health focuses on long-term relationships with patients; thus, the LCS SDM process is situated within longer term relationship building and patient empowerment, both of which will facilitate partnering with the patient in future conversations about other decisions.
Shared decision-making/whole health integration. Both SDM and whole health emphasize partnership with the patient in making a final decision. There is also focus on decision-making as an ongoing process. Deciding whether LCS is the best choice might include naming and addressing emotions, voicing questions not raised, and exploring whether screening fits the patient’s goals, values, and life context. HCPs may give guidance, but patients retain the authority to make decisions. The goal is to empower patients to know that the only right decision is the one right for them and they will be supported.
Limitations
This article describes a VA practice program and was not a formal research study. Further work is needed to evaluate the presented strategies. Additionally, we did not conduct a systematic literature review and thus elements of SDM and whole health may not be exhaustive.
CONCLUSIONS
This article describes the alignment of 2 distinct VA initiatives, whole health and SDM for LCS. The goal was to reduce known barriers to SDM, such as competing demands, limited time, and lack of familiarity with and training in SDM.11-13 These concepts are well aligned. This integrated model is the first step in informing the development of a HCP training program and materials as part of a multilevel strategy that our team is using to implement SDM for LCS in VISN 1.16 The final training and materials resulting from this work were delivered to LCSCs in 3 ways: (1) a series of 3 interactive group training sessions, including didactic elements, role play, and time for open discussion; (2) 1-on-1 academic detailing; and (3) educational handouts. In academic detailing, a member of the research team trained in academic detailing met virtually with each nurse coordinator, identified that individual’s barriers to SDM, and used the training materials to highlight messages to overcome those barriers; follow-up calls provided a forum for discussing progress and overcoming additional challenges. Although this article focused specifically on whole health and SDM, the conceptual alignment process strategy can be applied to other implementations of multiple initiatives.
The landmark Crossing the Quality Chasm report from the National Academy of Medicine identified patient- centered care as essential to health care quality. The report defines patientcentered care as “respectful of and responsive to individual patient preferences, needs, and values.”1 Many health care systems, including the Veterans Health Administration, are transforming to a patient-centered model of care.2 The US Department of Veterans Affairs (VA) Whole Health System of Care initiative is a system-wide, cultural transformation. Within whole health, what matters most to the patient—including their preferences, needs, and values—is foundational to health care and meant to be essential in every clinical encounter. Whole health implementation includes a progressive rollout with health care practitioner (HCP) trainings across the VA.2
Shared decision-making (SDM) is a different but aligned patient-centered care concept. SDM is a process through which a decision or care plan, based on patients’ preferences, needs, and values, is made or developed.3-5 SDM is ideal in situations with equipoise (decisions with equivalent choices), individualized risks, and/or greater uncertainty of the net benefit, such as with lung cancer screening (LCS).3 SDM for LCS is required by the US Centers for Medicare and Medicaid Services and has been adopted by many US health care systems, including the VA.6,7 Early detection of lung cancer can reduce death by 20% at the population level.8 However, at the patient level there is wide variation in the risk of developing lung cancer and a range of potential harms.8 LCS follow-up procedures may be more invasive than with other cancer screenings. Thus, there is concern about the risk of false-positive results leading to unnecessary care or complications.8 Given this balance between benefit and harm and the differing patient value on the trade-offs of LCS, an individualized, patient-centered approach is essential when deciding whether LCS is the right choice for a specific patient.
Despite the importance of LCS SDM, observational studies have shown poor implementation in clinical encounters.9,10 HCP barriers include competing demands, limited time, lack of familiarity with and training in SDM, and beliefs biasing screening over no screening.11-13 Additionally, HCPs may assume that patients want them to make the decision. However, research has shown that patients actually want to be more involved in their health care decisions.14 One suggested strategy to overcome these barriers is aligning SDM for LCS within an organization’s broader patient-centered initiatives.15
This project sought to align the need for SDM for LCS and the broader VA whole health initiative as part of a multilevel strategy to implement SDM for LCS across Veterans Integrated Service Network (VISN) 1.16
This article addresses HCP-level barriers. HCPs targeted are those typically involved in LCS. The VA utilizes LCS coordinators (LCSCs) in both centralized or consult models (in which LCSCs are involved in all aspects of screening) and hybrid models (in which primary care practitioners and LCSCs are both engaged in LCS tasks). The goal of this program was to generate areas of conceptual alignment between SDM and whole health as a first step in integrating these VA initiatives. This work was conducted as a foundation for an SDM for lung cancer HCP training and consultation initiative.
ALIGNMENT PROCESS
We reviewed relevant literature and resources for SDM and whole health. In reviewing the SDM literature, we included a sample of the most widely cited literature on the topic, and focused primarily on the systematic review by Bomhof-Roordink et al.4,5,17,18 This review provided a synthesis of SDM elements across SDM models and identified 53 different elements clustered into 24 components.4 The most common components were present in at least half of all SDM published models, including: make the decision, patient preferences, tailor information, deliberate, create choice awareness, and learn about the patient. Bomhof-Roordink et al provided the guiding framework for this conceptualization of SDM because that study included the available recent published SDM models.4
Second, published literature on VA whole health along with supplemental promotional and training materials were reviewed. The whole health materials included 2 sets of training slides developed for VA HCPs (available to VA employees): Implementing Whole Health in Clinical Care, which is focused on HCPs’ work with patients, and Whole Health for You and Me, which is about HCPs’ personal well-being.19 We also reviewed a publication describing the history of whole health and patient-facing online whole health tools.2,19
Each document was reviewed for key elements related to SDM, patient-centered care, and whole health. Using the 53 elements identified by Bomhof-Roordink et al, we reviewed and compared each element to the whole health materials to create the integrated model of SDM and whole health. We iteratively discussed and organized the elements until we reached consensus.
SDM and Whole Health Alignment
We created an integrated model of SDM for LCS within the context of the VA whole health initiative. This integrated model is directed at HCPs who would likely engage patients in discussions of LCS, including primary care practitioners and nurse coordinators. The model includes 3 steps for HCPs to follow that align SDM within whole health: (1) frame the conversation and partner with the patient; (2) share clinical perspective and elicit patient values; and (3) deliberate and decide together. For each step, the SDM elements, whole health elements, and integration of SDM and whole health are provided. Table 1 provides an overview of the similarities and differences between SDM and whole health. Example phrases that merge SDM and whole health for HCPs to use in patient conversations about LCS are included in Table 2.


STEP 1. FRAME THE CONVERSATION AND PARTNER WITH THE PATIENT
Shared decision-making. Traditional SDM literature includes an initial step of letting patients know that there is a choice to be made between ≥ 2 clinical options.4 Ancillary elements of this first step include asking patients their preferences about the degree to which they want to be involved in SDM and about how they like to receive information (eg, verbal, written, video). These steps open the SDM conversation and ensure the patient and HCP are on the same page before moving forward. For example, the US Agency for Healthcare Research and Quality SHARE model’s first step is for HCPs to communicate that choices exist and to invite the patient to be involved in decisions.20 Similarly, Elwyn’s 3-step SDM model begins with establishing that a choice exists and inviting patient input on making that choice.17
Whole health. Patients are encouraged to play an active role in their health care. Through whole health programs such as Taking Charge of My Life and Health, patients explore their values and set self-care goals.21 HCP whole health trainings teach and reinforce communication skills, including SDM, listening skills, and motivational interviewing.19
Shared decision-making/whole health integration. SDM and whole health both prioritize respect, compassion, and patients’ expertise. They focus on the patient-HCP relationship with an emphasis on fostering egalitarian interactions. HCPs frame the SDM conversation and partner with the patient so they know what to expect and who will be involved. This conversation is framed from the outset as a collaborative discussion. HCPs empower the patient to play an active role in decision-making and help them understand why their engagement is critical.
STEP 2. SHARE CLINICAL PERSPECTIVE AND ELICIT PATIENT VALUES
Shared decision-making. HCPs share clinical perspective on LCS tailored to individual patients while explicitly inviting the patient to share their preferences and values when thinking about whether to undergo LCS. HCPs give a balanced description of LCS, including the benefits and harms, tailored to the patient’s unique information needs and questions. Sharing clinical perspective also includes describing treatment options, the most common element across SDM models.4 Decision aids, which provide unbiased information and include a values clarification exercise, may be helpful in sharing clinical perspectives and clarifying patient values related to the trade-offs of LCS.22 For example, the VA National Center for Health Promotion and Disease Prevention developed a LCS decision aid to be used for SDM for LCS.
Whole health. The conversation shifts from “What is the matter with you?” to “What matters to you?” starting with the patient’s goals and priorities rather than disease prevention, diagnosis, and treatment.2 Several whole health tools exist, including the Personal Health Inventory, used to identify what matters most to patients and understand their current well-being and self-care.23 Using the inventory, the patient and their health care team develop the patient’s personal health plan.24 Additionally, whole health trains HCPs to reflect on their own attitudes and biases when providing clinical care.
Shared decision-making/whole health integration. The LCS conversation can build on other whole health-related conversations with a HCP or other team members. HCPs can reference the patient’s personal health plan for documentation of the patient’s preferences, values, and goals in the electronic medical record. During this process, HCPs can give space for patients to discuss factors in their life and experiences that impact their perspective and decision-making. For example, patient concerns could be explored here, including fear of a cancer diagnosis, stigma around smoking, and fears around the screening and/or treatment process. HCPs may ask, “What matters most to you when making this decision?” Finally, by sharing clinical information, HCPs will focus on patient values to help overcome their own biases toward a desire for LCS. HCPs, similar to the rest of the US public, tend to hold highly favorable attitudes toward cancer screening as well as misconceptions about the magnitude of benefits from screening.13
STEP 3. DELIBERATE AND DECIDE TOGETHER
Shared decision-making. Decision-making is almost always considered the last SDM step.4 In the final step, the patient and HCP discuss the options (ie, to screen or not to screen) considering the patient’s values and preferences, and patients decide with their HCP whether they will undergo LCS. Patients may decide they need more time to think about these options. As part of deliberation, HCPs assess what other information patients may need to arrive at a decision. Family members, friends, or peers may be included in making the final decision.
Whole health. In Whole health, decisions also may include the entire health care team and other individuals important to the patient (eg, family, friends). Integration across different health care settings is also considered a key whole health element. Finally, whole health focuses on long-term relationships with patients; thus, the LCS SDM process is situated within longer term relationship building and patient empowerment, both of which will facilitate partnering with the patient in future conversations about other decisions.
Shared decision-making/whole health integration. Both SDM and whole health emphasize partnership with the patient in making a final decision. There is also focus on decision-making as an ongoing process. Deciding whether LCS is the best choice might include naming and addressing emotions, voicing questions not raised, and exploring whether screening fits the patient’s goals, values, and life context. HCPs may give guidance, but patients retain the authority to make decisions. The goal is to empower patients to know that the only right decision is the one right for them and they will be supported.
Limitations
This article describes a VA practice program and was not a formal research study. Further work is needed to evaluate the presented strategies. Additionally, we did not conduct a systematic literature review and thus elements of SDM and whole health may not be exhaustive.
CONCLUSIONS
This article describes the alignment of 2 distinct VA initiatives, whole health and SDM for LCS. The goal was to reduce known barriers to SDM, such as competing demands, limited time, and lack of familiarity with and training in SDM.11-13 These concepts are well aligned. This integrated model is the first step in informing the development of a HCP training program and materials as part of a multilevel strategy that our team is using to implement SDM for LCS in VISN 1.16 The final training and materials resulting from this work were delivered to LCSCs in 3 ways: (1) a series of 3 interactive group training sessions, including didactic elements, role play, and time for open discussion; (2) 1-on-1 academic detailing; and (3) educational handouts. In academic detailing, a member of the research team trained in academic detailing met virtually with each nurse coordinator, identified that individual’s barriers to SDM, and used the training materials to highlight messages to overcome those barriers; follow-up calls provided a forum for discussing progress and overcoming additional challenges. Although this article focused specifically on whole health and SDM, the conceptual alignment process strategy can be applied to other implementations of multiple initiatives.
- Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. The National Academies Press; 2001. doi:10.17226/10027
- Bokhour BG, Haun JN, Hyde J, Charns M, Kligler B. Transforming the Veterans Affairs to a whole health system of care: time for action and research. Med Care. 2020;58:295- 300. doi:10.1097/MLR.0000000000001316
- Elwyn G, Frosch D, Rollnick S. Dual equipoise shared decision making: definitions for decision and behaviour support interventions. Implement Sci. 2009;4:75. doi:7510.1186/1748-5908-4-75
- Bomhof-Roordink H, Gärtner FR, Stiggelbout AM, Pieterse AH. Key components of shared decision making models: a systematic review. BMJ Open. 2019;9:e031763. doi:10.1136/bmjopen-2019-031763
- Charles C, Gafni A, Whelan T. Decision-making in the physician- patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med. 1999;49:651-661. doi:10.1016/s0277-9536(99)00145-8
- Moyer VA; US Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:330- 338. doi:10.7326/m13-2771
- Centers for Medicare & Medicaid Services. Screening for lung cancer with low dose computed tomography (LDCT). February 10, 2022. Accessed February 7, 2025. https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=304
- Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. doi:10.1056/NEJMoa1102873
- Slatore CG, Wiener RS. Pulmonary nodules: a small problem for many, severe distress for some, and how to communicate about it. Chest. 2018;153:1004-1015. doi:10.1016/j.chest.2017.10.013
- Nishi SPE, Lowenstein LM, Mendoza TR, et al. Shared decision-making for lung cancer screening: how well are we “sharing”? Chest. 2021;160:330-340. doi:10.1016/j.chest.2021.01.041
- Wiener RS, Koppelman E, Bolton R, et al. Patient and clinician perspectives on shared decision-making in early adopting lung cancer screening programs: a qualitative study. J Gen Intern Med. 2018;33:1035-1042. doi:10.1007/s11606-018-4350-9
- Melzer AC, Golden SE, Ono SS, Datta S, Triplette M, Slatore CG. “We just never have enough time”: clinician views of lung cancer screening processes and implementation. Ann Am Thorac Soc. 2020. doi:10.1513/AnnalsATS.202003-262OC
- Schwartz LM, Woloshin S, Fowler FJ Jr, Welch HG. Enthusiasm for cancer screening in the United States. JAMA. 2004;291:71-78. doi:10.1001/jama.291.1.71
- Lown BA, Rosen J, Marttila J. An agenda for improving compassionate care: a survey shows about half of patients say such care is missing. Health Aff (Millwood). 2011;30:1772-1778. doi:10.1377/hlthaff.2011.0539
- Scholl I, LaRussa A, Hahlweg P, Kobrin S, Elwyn G. Organizational- and system-level characteristics that influence implementation of shared decision-making and strategies to address them - a scoping review. Implement Sci. 2018;13:40. doi:10.1186/s13012-018-0731-z
- Khanna A, Fix GM, Anderson E, et al. Towards a framework for patient-centred care coordination: a scoping review protocol. BMJ Open. 2022;12:e066808. doi:10.1136/bmjopen-2022-066808
- Elwyn G, Durand MA, Song J, et al. A three-talk model for shared decision making: multistage consultation process. BMJ. 2017;359:j4891. doi:10.1136/bmj.j4891
- Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Educ Couns. 2006;60:301-312. doi:10.1016/j.pec.2005.06.010
- Whole Health. US Department of Veterans Affairs. Accessed April 14, 2025. https://www.va.gov/wholehealth/
- Agency for Healthcare Research and Quality. The SHARE approach. Accessed April 14, 2025. https://www.ahrq.gov/health-literacy/professional-training/shared-decision/index.html
- Abadi MH, Barker AM, Rao SR, Orner M, Rychener D, Bokhour BG. Examining the impact of a peer-led group program for veteran engagement and well-being. J Altern Complement Med. 2021;27:S37-S44. doi:10.1089/acm.2020.0124
- Stacey D, Lewis KB, Smith M, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2024;1:CD001431. doi:10.1002/14651858.CD001431.pub6
- US Department of Veterans Affairs, Veterans Health Administration, Office of Patient Centered Care and Cultural Transformation. Personal health inventory. Revised April 2019. Accessed April 14, 2025. https://www.va.gov/wholehealth/docs/10-773_PHI_July2019_508.pdf
- US Department of Veterans Affairs. Build your personal health plan. Updated July 24, 2024. Accessed April 14, 2025. https://www.va.gov/wholehealth/phi.asp
- Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. The National Academies Press; 2001. doi:10.17226/10027
- Bokhour BG, Haun JN, Hyde J, Charns M, Kligler B. Transforming the Veterans Affairs to a whole health system of care: time for action and research. Med Care. 2020;58:295- 300. doi:10.1097/MLR.0000000000001316
- Elwyn G, Frosch D, Rollnick S. Dual equipoise shared decision making: definitions for decision and behaviour support interventions. Implement Sci. 2009;4:75. doi:7510.1186/1748-5908-4-75
- Bomhof-Roordink H, Gärtner FR, Stiggelbout AM, Pieterse AH. Key components of shared decision making models: a systematic review. BMJ Open. 2019;9:e031763. doi:10.1136/bmjopen-2019-031763
- Charles C, Gafni A, Whelan T. Decision-making in the physician- patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med. 1999;49:651-661. doi:10.1016/s0277-9536(99)00145-8
- Moyer VA; US Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:330- 338. doi:10.7326/m13-2771
- Centers for Medicare & Medicaid Services. Screening for lung cancer with low dose computed tomography (LDCT). February 10, 2022. Accessed February 7, 2025. https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=304
- Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. doi:10.1056/NEJMoa1102873
- Slatore CG, Wiener RS. Pulmonary nodules: a small problem for many, severe distress for some, and how to communicate about it. Chest. 2018;153:1004-1015. doi:10.1016/j.chest.2017.10.013
- Nishi SPE, Lowenstein LM, Mendoza TR, et al. Shared decision-making for lung cancer screening: how well are we “sharing”? Chest. 2021;160:330-340. doi:10.1016/j.chest.2021.01.041
- Wiener RS, Koppelman E, Bolton R, et al. Patient and clinician perspectives on shared decision-making in early adopting lung cancer screening programs: a qualitative study. J Gen Intern Med. 2018;33:1035-1042. doi:10.1007/s11606-018-4350-9
- Melzer AC, Golden SE, Ono SS, Datta S, Triplette M, Slatore CG. “We just never have enough time”: clinician views of lung cancer screening processes and implementation. Ann Am Thorac Soc. 2020. doi:10.1513/AnnalsATS.202003-262OC
- Schwartz LM, Woloshin S, Fowler FJ Jr, Welch HG. Enthusiasm for cancer screening in the United States. JAMA. 2004;291:71-78. doi:10.1001/jama.291.1.71
- Lown BA, Rosen J, Marttila J. An agenda for improving compassionate care: a survey shows about half of patients say such care is missing. Health Aff (Millwood). 2011;30:1772-1778. doi:10.1377/hlthaff.2011.0539
- Scholl I, LaRussa A, Hahlweg P, Kobrin S, Elwyn G. Organizational- and system-level characteristics that influence implementation of shared decision-making and strategies to address them - a scoping review. Implement Sci. 2018;13:40. doi:10.1186/s13012-018-0731-z
- Khanna A, Fix GM, Anderson E, et al. Towards a framework for patient-centred care coordination: a scoping review protocol. BMJ Open. 2022;12:e066808. doi:10.1136/bmjopen-2022-066808
- Elwyn G, Durand MA, Song J, et al. A three-talk model for shared decision making: multistage consultation process. BMJ. 2017;359:j4891. doi:10.1136/bmj.j4891
- Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Educ Couns. 2006;60:301-312. doi:10.1016/j.pec.2005.06.010
- Whole Health. US Department of Veterans Affairs. Accessed April 14, 2025. https://www.va.gov/wholehealth/
- Agency for Healthcare Research and Quality. The SHARE approach. Accessed April 14, 2025. https://www.ahrq.gov/health-literacy/professional-training/shared-decision/index.html
- Abadi MH, Barker AM, Rao SR, Orner M, Rychener D, Bokhour BG. Examining the impact of a peer-led group program for veteran engagement and well-being. J Altern Complement Med. 2021;27:S37-S44. doi:10.1089/acm.2020.0124
- Stacey D, Lewis KB, Smith M, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2024;1:CD001431. doi:10.1002/14651858.CD001431.pub6
- US Department of Veterans Affairs, Veterans Health Administration, Office of Patient Centered Care and Cultural Transformation. Personal health inventory. Revised April 2019. Accessed April 14, 2025. https://www.va.gov/wholehealth/docs/10-773_PHI_July2019_508.pdf
- US Department of Veterans Affairs. Build your personal health plan. Updated July 24, 2024. Accessed April 14, 2025. https://www.va.gov/wholehealth/phi.asp
When Patient-Centered Care Initiatives Align: Integrating VA Whole Health and Shared Decision-Making for Lung Cancer Screening
When Patient-Centered Care Initiatives Align: Integrating VA Whole Health and Shared Decision-Making for Lung Cancer Screening
Measles Resurgence: A Dermatologist’s Guide
Measles Resurgence: A Dermatologist’s Guide
Measles, also known as rubeola, is a highly contagious paramyxovirus that has neared elimination in the United States since 2000 due to widespread adoption of the measles vaccine; however, measles recently has made a comeback, with outbreaks reported in more than 60 countries. In the United States, vaccine hesitancy coupled with decreasing vaccination rates, international travel to endemic areas, and decreased funding and resources for monitoring and immunization programs likely led to a re-emergence of measles cases.1,2 The resurgence of measles is troubling given its infectiousness and potential severity in at-risk populations. Since measles has a basic reproduction number of 12 to 18 (ie, 1 infected individual will on average infect 12 to 18 others3), it has the capacity to spread quickly. This is why, prior to the development of the measles vaccine in the 1960s, it was responsible for millions of deaths across the globe.
Prior to the introduction of the measles vaccine, both physicians and the public generally were aware of the signs and symptoms of measles due to its prevalence; however, since there have been so few cases in recent decades, images and descriptions of patients presenting with measles can be found only in textbooks, and many physicians are ill-prepared to diagnose the disease.4 In response to the recent surge in measles cases, dermatologists—who often are among the first medical professionals to encounter febrile patients with rashes—must be prepared to bridge this divide. Herein, we review the clinical signs, diagnostic approach, operational precautions, and public health responsibilities that dermatologists must relearn amid the current measles outbreak.
Background
Measles is primarily transmitted via respiratory droplets and may remain airborne for up to 2 hours.5 It also can be transmitted through direct contact with secretions such as mucus. Indirect transmission via fomites, while certainly plausible, is thought to be the least effective mechanism of transmission.6 Following exposure, the incubation period ranges from 7 to 21 days, during which the virus replicates asymptomatically before causing clinical disease.7 Herd immunity for measles requires 93% immunity in the population; public health agencies typically target greater than 95% immunity.8 Humans are the only reservoir for the measles virus, making eradication possible.
The road to eradication began with the introduction of the measles vaccine in 1963 and subsequent development of the combined measles-mumps-rubella (MMR) vaccine in 1971. As MMR is a live vaccine, 2 doses confer approximately 97% protection.9 The first dose is given at 12 to 15 months of age, and the second dose is given at 4 to 6 years of age. Immunity is considered lifelong, and the Centers for Disease Control and Prevention and the World Health Organization do not recommend routine measles boosters for individuals who have completed the primary 2-dose series.10,11
Widespread vaccination led to a dramatic reduction in incidence, with many countries eliminating measles infections.7 The United States declared measles eliminated in 2000, with confirmed cases between 2000 and 2020 ranging from 37 to 1282.12 Vaccination progress stalled in the late 1990s due to vaccine hesitancy resulting from (subsequently debunked) reports of an association between the MMR vaccine and autism.13 Despite efforts to correct this misinformation, many patients continue to espouse these concerns.
Recognizing Measles: Clinical Presentation
Measles, which most often manifests in childhood but also can occur in adults, follows a distinctive clinical course. The prodromal phase is characterized by high fever, cough, coryza (nasal congestion), and conjunctivitis— conjunctivitis—the 3 “Cs” that serve as early warning signs of the disease. Patients may develop small white macules on the buccal mucosa known as Koplik spots (phonetically the fourth “C”), which appear just before the rash. Three to 5 days after the onset of systemic symptoms, patients will develop a classic morbilliform exanthem. In some cases, the exanthem manifests on the head and neck (Figure 1)—first behind the ears and along the hairline, then spreading caudally to the trunk and extremities. The lesions may become confluent, with patients presenting with diffuse erythema. The exanthem fades over several days to weeks, often accompanied by superficial desquamation.14
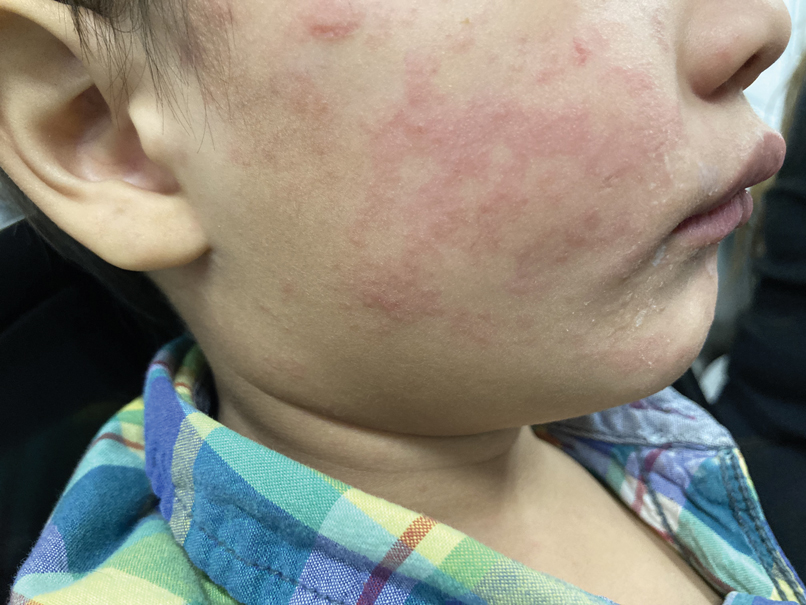
Given the nonspecificity of the early symptoms of measles, a high index of suspicion is needed for patients presenting with a febrile illness and a morbilliform eruption (Figure 2). Consideration of MMR vaccination status, exposure history, and local outbreak patterns can help guide risk stratification and the need for testing. Immunocompromised individuals, including those receiving immunosuppressive therapies for dermatologic conditions, may present atypically, lacking the prototypical exanthem or displaying milder signs and further complicating the diagnosis.15 The differential diagnosis for measles includes a drug reaction or other viral exanthem, and a detailed history may help elucidate the culprit.
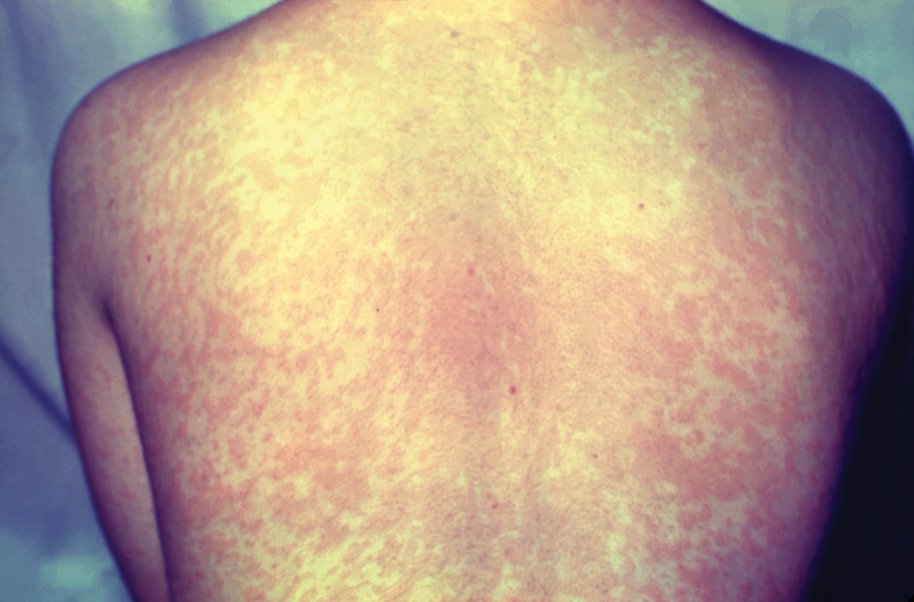
Evaluation and Diagnosis
Definitive diagnosis of measles relies on both molecular and serologic testing. Nasopharyngeal swabs for measles polymerase chain reaction testing are obtained using synthetic (noncotton) swabs placed in a viral transport medium. Serum samples also should be collected for measles IgM and IgG antibody testing. Importantly, measles is a reportable illness, and testing may be coordinated with local departments of health.
Determining a patient’s immune status may be important for certain populations. Patients with documented 2-dose MMR vaccination, positive measles IgG serology, or a prior confirmed measles infection are considered immune. While a positive measles IgG indicates immunity, a negative result in an exposed patient should prompt consideration of postexposure prophylaxis with intravenous immunoglobulin.
Many patients, specifically those presenting to dermatology, are taking immunomodulatory or immunosuppressive medications—a contraindication for vaccination with the live MMR vaccine. At the time of publication, there was a single reported case of a patient taking a tumor necrosis factor α inhibitor for rheumatoid arthritis who had acquired measles.16 While the benefits of titer assessment in patients who are starting or continuing immunomodulatory therapy are not known and currently it is not recommended by the Centers for Disease Control and Prevention, dermatologists might consider checking MMR titers and vaccinating (or referring for vaccination) nonimmune patients.17
Infection Control
Early identification of a suspected measles case is paramount. Patients in whom measles is a possibility should be isolated as quickly as possible, and the patient and accompanying caregivers should be masked. Clinical staff should don appropriate personal protective equipment, including an N95 mask. Coordination with the local department of health must occur as soon as measles is suspected.
If testing is an option in the outpatient setting, a nasopharyngeal viral swab and serologic titers can be obtained. If testing is not available on site, patients should be sent to appropriate care facilities; prenotification is critical to prevent nosocomial outbreaks. Patients should be encouraged to isolate and avoid public spaces and/or public transport for 4 days following development of an exanthem.18 Offices should develop clinical protocols for suspected measles cases with training for clinical and office staff.
Final Thoughts
As measles outbreaks become more prevalent, it is incumbent upon physicians to remind ourselves of the signs and symptoms of this largely eliminated disease so that we may pursue early detection and intervention strategies. The primary cutaneous manifestations of measles make dermatologists critical to early recognition and containment efforts. Dermatologists should prepare for the arrival of patients with measles by maintaining vigilance for the classic signs of the disease, implementing stringent isolation protocols, verifying patient immunity when appropriate, and partnering closely with public health authorities.
More broadly, efforts to contain and re-establish a paradigm for eliminating measles outbreaks must be pursued. Encouraging vaccination and developing programs to help combat misinformation surrounding vaccines are critical to this effort. In an era of vaccine hesitancy, measles is a multidisciplinary public health emergency. Dermatologists must remain ready.
- Bedford H, Elliman D. Measles rates are rising again. BMJ. 2024;384.
- Harris E. Measles outbreaks grow amid declining vaccination rates. JAMA. 2023;330:2242.
- Guerra FM, Bolotin S, Lim G, et al. The basic reproduction number (R0) of measles: a systematic review. Lancet Infect Dis. 2017;17:E420-E428.
- Swartz MK. Measles: public and professional education. J Pediatr Health Care. 2019;33:367-368.
- Centers for Disease Control and Prevention. Interim infection prevention and control recommendations for measles in healthcare settings. Accessed April 27, 2025. https://www.cdc.gov/infection-control/hcp/measles/
- Moss WJ, Griffin DE, Feinstone WH. Measles. In: Vaccines for Biodefense and Emerging and Neglected Diseases. Elsevier; 2009: 551-565.
- Moss WJ. Measles. Lancet. 2017;390:2490-2502.
- Maintain the vaccination coverage level of 2 doses of the MMR vaccine for children in kindergarten— IID04. Healthy People 2030 website. Accessed May 6, 2025. https://odphp.health.gov/healthypeople/objectives-and-data/browse-objectives/vaccination/maintain-vaccination-coverage-level-2-doses-mmr-vaccine-children-kindergarten-iid-04
- Franconeri L, Antona D, Cauchemez S, et al. Two-dose measles vaccine effectiveness remains high over time: a French observational study, 2017–2019. Vaccine. 2023;41:5797-5804.
- World Health Organization. Measles. Accessed May 8, 2025. https:// www.who.int/news-room/fact-sheets/detail/measles
- Centers for Disease Control and Prevention. Measles vaccine recommendations. Accessed May 8, 2025. https://www.cdc.gov/measles/hcp/vaccine-considerations/index.html
- Centers for Disease Control and Prevention. Measles cases and outbreaks. Accessed May 6, 2025. https://www.cdc.gov/measles/cases-outbreaks.html
- Dyer C. Lancet retracts Wakefield’s MMR paper. BMJ. 2010;340.
- Alves Graber EM, Andrade FJ, Bost W, et al. An update and review of measles for emergency physicians. J Emerg Med. 2020;58:610-615.
- Kaplan LJ, Daum RS, Smaron M, et al. Severe measles in immunocompromised patients. JAMA. 1992;267:1237-1241.
- Takahashi E, Kurosaka D, Yoshida K, et al. Onset of modified measles after etanercept treatment in rheumatoid arthritis. Japanese J Clin Immunol. 2010;33:37-41.
- Worth A, Waldman RA, Dieckhaus K, et al. Art of prevention: our approach to the measles-mumps-rubella vaccine in adult patients vaccinated against measles before 1968 on biologic therapy for the treatment of psoriasis. Int J Womens Dermatol. 2019;6:94.
- Centers for Disease Control and Prevention. Clinical overview of measles (rubeola). Accessed May 8, 2025. https://www.cdc.gov/measles/hcp/clinical-overview/index.html
Measles, also known as rubeola, is a highly contagious paramyxovirus that has neared elimination in the United States since 2000 due to widespread adoption of the measles vaccine; however, measles recently has made a comeback, with outbreaks reported in more than 60 countries. In the United States, vaccine hesitancy coupled with decreasing vaccination rates, international travel to endemic areas, and decreased funding and resources for monitoring and immunization programs likely led to a re-emergence of measles cases.1,2 The resurgence of measles is troubling given its infectiousness and potential severity in at-risk populations. Since measles has a basic reproduction number of 12 to 18 (ie, 1 infected individual will on average infect 12 to 18 others3), it has the capacity to spread quickly. This is why, prior to the development of the measles vaccine in the 1960s, it was responsible for millions of deaths across the globe.
Prior to the introduction of the measles vaccine, both physicians and the public generally were aware of the signs and symptoms of measles due to its prevalence; however, since there have been so few cases in recent decades, images and descriptions of patients presenting with measles can be found only in textbooks, and many physicians are ill-prepared to diagnose the disease.4 In response to the recent surge in measles cases, dermatologists—who often are among the first medical professionals to encounter febrile patients with rashes—must be prepared to bridge this divide. Herein, we review the clinical signs, diagnostic approach, operational precautions, and public health responsibilities that dermatologists must relearn amid the current measles outbreak.
Background
Measles is primarily transmitted via respiratory droplets and may remain airborne for up to 2 hours.5 It also can be transmitted through direct contact with secretions such as mucus. Indirect transmission via fomites, while certainly plausible, is thought to be the least effective mechanism of transmission.6 Following exposure, the incubation period ranges from 7 to 21 days, during which the virus replicates asymptomatically before causing clinical disease.7 Herd immunity for measles requires 93% immunity in the population; public health agencies typically target greater than 95% immunity.8 Humans are the only reservoir for the measles virus, making eradication possible.
The road to eradication began with the introduction of the measles vaccine in 1963 and subsequent development of the combined measles-mumps-rubella (MMR) vaccine in 1971. As MMR is a live vaccine, 2 doses confer approximately 97% protection.9 The first dose is given at 12 to 15 months of age, and the second dose is given at 4 to 6 years of age. Immunity is considered lifelong, and the Centers for Disease Control and Prevention and the World Health Organization do not recommend routine measles boosters for individuals who have completed the primary 2-dose series.10,11
Widespread vaccination led to a dramatic reduction in incidence, with many countries eliminating measles infections.7 The United States declared measles eliminated in 2000, with confirmed cases between 2000 and 2020 ranging from 37 to 1282.12 Vaccination progress stalled in the late 1990s due to vaccine hesitancy resulting from (subsequently debunked) reports of an association between the MMR vaccine and autism.13 Despite efforts to correct this misinformation, many patients continue to espouse these concerns.
Recognizing Measles: Clinical Presentation
Measles, which most often manifests in childhood but also can occur in adults, follows a distinctive clinical course. The prodromal phase is characterized by high fever, cough, coryza (nasal congestion), and conjunctivitis— conjunctivitis—the 3 “Cs” that serve as early warning signs of the disease. Patients may develop small white macules on the buccal mucosa known as Koplik spots (phonetically the fourth “C”), which appear just before the rash. Three to 5 days after the onset of systemic symptoms, patients will develop a classic morbilliform exanthem. In some cases, the exanthem manifests on the head and neck (Figure 1)—first behind the ears and along the hairline, then spreading caudally to the trunk and extremities. The lesions may become confluent, with patients presenting with diffuse erythema. The exanthem fades over several days to weeks, often accompanied by superficial desquamation.14

Given the nonspecificity of the early symptoms of measles, a high index of suspicion is needed for patients presenting with a febrile illness and a morbilliform eruption (Figure 2). Consideration of MMR vaccination status, exposure history, and local outbreak patterns can help guide risk stratification and the need for testing. Immunocompromised individuals, including those receiving immunosuppressive therapies for dermatologic conditions, may present atypically, lacking the prototypical exanthem or displaying milder signs and further complicating the diagnosis.15 The differential diagnosis for measles includes a drug reaction or other viral exanthem, and a detailed history may help elucidate the culprit.

Evaluation and Diagnosis
Definitive diagnosis of measles relies on both molecular and serologic testing. Nasopharyngeal swabs for measles polymerase chain reaction testing are obtained using synthetic (noncotton) swabs placed in a viral transport medium. Serum samples also should be collected for measles IgM and IgG antibody testing. Importantly, measles is a reportable illness, and testing may be coordinated with local departments of health.
Determining a patient’s immune status may be important for certain populations. Patients with documented 2-dose MMR vaccination, positive measles IgG serology, or a prior confirmed measles infection are considered immune. While a positive measles IgG indicates immunity, a negative result in an exposed patient should prompt consideration of postexposure prophylaxis with intravenous immunoglobulin.
Many patients, specifically those presenting to dermatology, are taking immunomodulatory or immunosuppressive medications—a contraindication for vaccination with the live MMR vaccine. At the time of publication, there was a single reported case of a patient taking a tumor necrosis factor α inhibitor for rheumatoid arthritis who had acquired measles.16 While the benefits of titer assessment in patients who are starting or continuing immunomodulatory therapy are not known and currently it is not recommended by the Centers for Disease Control and Prevention, dermatologists might consider checking MMR titers and vaccinating (or referring for vaccination) nonimmune patients.17
Infection Control
Early identification of a suspected measles case is paramount. Patients in whom measles is a possibility should be isolated as quickly as possible, and the patient and accompanying caregivers should be masked. Clinical staff should don appropriate personal protective equipment, including an N95 mask. Coordination with the local department of health must occur as soon as measles is suspected.
If testing is an option in the outpatient setting, a nasopharyngeal viral swab and serologic titers can be obtained. If testing is not available on site, patients should be sent to appropriate care facilities; prenotification is critical to prevent nosocomial outbreaks. Patients should be encouraged to isolate and avoid public spaces and/or public transport for 4 days following development of an exanthem.18 Offices should develop clinical protocols for suspected measles cases with training for clinical and office staff.
Final Thoughts
As measles outbreaks become more prevalent, it is incumbent upon physicians to remind ourselves of the signs and symptoms of this largely eliminated disease so that we may pursue early detection and intervention strategies. The primary cutaneous manifestations of measles make dermatologists critical to early recognition and containment efforts. Dermatologists should prepare for the arrival of patients with measles by maintaining vigilance for the classic signs of the disease, implementing stringent isolation protocols, verifying patient immunity when appropriate, and partnering closely with public health authorities.
More broadly, efforts to contain and re-establish a paradigm for eliminating measles outbreaks must be pursued. Encouraging vaccination and developing programs to help combat misinformation surrounding vaccines are critical to this effort. In an era of vaccine hesitancy, measles is a multidisciplinary public health emergency. Dermatologists must remain ready.
Measles, also known as rubeola, is a highly contagious paramyxovirus that has neared elimination in the United States since 2000 due to widespread adoption of the measles vaccine; however, measles recently has made a comeback, with outbreaks reported in more than 60 countries. In the United States, vaccine hesitancy coupled with decreasing vaccination rates, international travel to endemic areas, and decreased funding and resources for monitoring and immunization programs likely led to a re-emergence of measles cases.1,2 The resurgence of measles is troubling given its infectiousness and potential severity in at-risk populations. Since measles has a basic reproduction number of 12 to 18 (ie, 1 infected individual will on average infect 12 to 18 others3), it has the capacity to spread quickly. This is why, prior to the development of the measles vaccine in the 1960s, it was responsible for millions of deaths across the globe.
Prior to the introduction of the measles vaccine, both physicians and the public generally were aware of the signs and symptoms of measles due to its prevalence; however, since there have been so few cases in recent decades, images and descriptions of patients presenting with measles can be found only in textbooks, and many physicians are ill-prepared to diagnose the disease.4 In response to the recent surge in measles cases, dermatologists—who often are among the first medical professionals to encounter febrile patients with rashes—must be prepared to bridge this divide. Herein, we review the clinical signs, diagnostic approach, operational precautions, and public health responsibilities that dermatologists must relearn amid the current measles outbreak.
Background
Measles is primarily transmitted via respiratory droplets and may remain airborne for up to 2 hours.5 It also can be transmitted through direct contact with secretions such as mucus. Indirect transmission via fomites, while certainly plausible, is thought to be the least effective mechanism of transmission.6 Following exposure, the incubation period ranges from 7 to 21 days, during which the virus replicates asymptomatically before causing clinical disease.7 Herd immunity for measles requires 93% immunity in the population; public health agencies typically target greater than 95% immunity.8 Humans are the only reservoir for the measles virus, making eradication possible.
The road to eradication began with the introduction of the measles vaccine in 1963 and subsequent development of the combined measles-mumps-rubella (MMR) vaccine in 1971. As MMR is a live vaccine, 2 doses confer approximately 97% protection.9 The first dose is given at 12 to 15 months of age, and the second dose is given at 4 to 6 years of age. Immunity is considered lifelong, and the Centers for Disease Control and Prevention and the World Health Organization do not recommend routine measles boosters for individuals who have completed the primary 2-dose series.10,11
Widespread vaccination led to a dramatic reduction in incidence, with many countries eliminating measles infections.7 The United States declared measles eliminated in 2000, with confirmed cases between 2000 and 2020 ranging from 37 to 1282.12 Vaccination progress stalled in the late 1990s due to vaccine hesitancy resulting from (subsequently debunked) reports of an association between the MMR vaccine and autism.13 Despite efforts to correct this misinformation, many patients continue to espouse these concerns.
Recognizing Measles: Clinical Presentation
Measles, which most often manifests in childhood but also can occur in adults, follows a distinctive clinical course. The prodromal phase is characterized by high fever, cough, coryza (nasal congestion), and conjunctivitis— conjunctivitis—the 3 “Cs” that serve as early warning signs of the disease. Patients may develop small white macules on the buccal mucosa known as Koplik spots (phonetically the fourth “C”), which appear just before the rash. Three to 5 days after the onset of systemic symptoms, patients will develop a classic morbilliform exanthem. In some cases, the exanthem manifests on the head and neck (Figure 1)—first behind the ears and along the hairline, then spreading caudally to the trunk and extremities. The lesions may become confluent, with patients presenting with diffuse erythema. The exanthem fades over several days to weeks, often accompanied by superficial desquamation.14

Given the nonspecificity of the early symptoms of measles, a high index of suspicion is needed for patients presenting with a febrile illness and a morbilliform eruption (Figure 2). Consideration of MMR vaccination status, exposure history, and local outbreak patterns can help guide risk stratification and the need for testing. Immunocompromised individuals, including those receiving immunosuppressive therapies for dermatologic conditions, may present atypically, lacking the prototypical exanthem or displaying milder signs and further complicating the diagnosis.15 The differential diagnosis for measles includes a drug reaction or other viral exanthem, and a detailed history may help elucidate the culprit.

Evaluation and Diagnosis
Definitive diagnosis of measles relies on both molecular and serologic testing. Nasopharyngeal swabs for measles polymerase chain reaction testing are obtained using synthetic (noncotton) swabs placed in a viral transport medium. Serum samples also should be collected for measles IgM and IgG antibody testing. Importantly, measles is a reportable illness, and testing may be coordinated with local departments of health.
Determining a patient’s immune status may be important for certain populations. Patients with documented 2-dose MMR vaccination, positive measles IgG serology, or a prior confirmed measles infection are considered immune. While a positive measles IgG indicates immunity, a negative result in an exposed patient should prompt consideration of postexposure prophylaxis with intravenous immunoglobulin.
Many patients, specifically those presenting to dermatology, are taking immunomodulatory or immunosuppressive medications—a contraindication for vaccination with the live MMR vaccine. At the time of publication, there was a single reported case of a patient taking a tumor necrosis factor α inhibitor for rheumatoid arthritis who had acquired measles.16 While the benefits of titer assessment in patients who are starting or continuing immunomodulatory therapy are not known and currently it is not recommended by the Centers for Disease Control and Prevention, dermatologists might consider checking MMR titers and vaccinating (or referring for vaccination) nonimmune patients.17
Infection Control
Early identification of a suspected measles case is paramount. Patients in whom measles is a possibility should be isolated as quickly as possible, and the patient and accompanying caregivers should be masked. Clinical staff should don appropriate personal protective equipment, including an N95 mask. Coordination with the local department of health must occur as soon as measles is suspected.
If testing is an option in the outpatient setting, a nasopharyngeal viral swab and serologic titers can be obtained. If testing is not available on site, patients should be sent to appropriate care facilities; prenotification is critical to prevent nosocomial outbreaks. Patients should be encouraged to isolate and avoid public spaces and/or public transport for 4 days following development of an exanthem.18 Offices should develop clinical protocols for suspected measles cases with training for clinical and office staff.
Final Thoughts
As measles outbreaks become more prevalent, it is incumbent upon physicians to remind ourselves of the signs and symptoms of this largely eliminated disease so that we may pursue early detection and intervention strategies. The primary cutaneous manifestations of measles make dermatologists critical to early recognition and containment efforts. Dermatologists should prepare for the arrival of patients with measles by maintaining vigilance for the classic signs of the disease, implementing stringent isolation protocols, verifying patient immunity when appropriate, and partnering closely with public health authorities.
More broadly, efforts to contain and re-establish a paradigm for eliminating measles outbreaks must be pursued. Encouraging vaccination and developing programs to help combat misinformation surrounding vaccines are critical to this effort. In an era of vaccine hesitancy, measles is a multidisciplinary public health emergency. Dermatologists must remain ready.
- Bedford H, Elliman D. Measles rates are rising again. BMJ. 2024;384.
- Harris E. Measles outbreaks grow amid declining vaccination rates. JAMA. 2023;330:2242.
- Guerra FM, Bolotin S, Lim G, et al. The basic reproduction number (R0) of measles: a systematic review. Lancet Infect Dis. 2017;17:E420-E428.
- Swartz MK. Measles: public and professional education. J Pediatr Health Care. 2019;33:367-368.
- Centers for Disease Control and Prevention. Interim infection prevention and control recommendations for measles in healthcare settings. Accessed April 27, 2025. https://www.cdc.gov/infection-control/hcp/measles/
- Moss WJ, Griffin DE, Feinstone WH. Measles. In: Vaccines for Biodefense and Emerging and Neglected Diseases. Elsevier; 2009: 551-565.
- Moss WJ. Measles. Lancet. 2017;390:2490-2502.
- Maintain the vaccination coverage level of 2 doses of the MMR vaccine for children in kindergarten— IID04. Healthy People 2030 website. Accessed May 6, 2025. https://odphp.health.gov/healthypeople/objectives-and-data/browse-objectives/vaccination/maintain-vaccination-coverage-level-2-doses-mmr-vaccine-children-kindergarten-iid-04
- Franconeri L, Antona D, Cauchemez S, et al. Two-dose measles vaccine effectiveness remains high over time: a French observational study, 2017–2019. Vaccine. 2023;41:5797-5804.
- World Health Organization. Measles. Accessed May 8, 2025. https:// www.who.int/news-room/fact-sheets/detail/measles
- Centers for Disease Control and Prevention. Measles vaccine recommendations. Accessed May 8, 2025. https://www.cdc.gov/measles/hcp/vaccine-considerations/index.html
- Centers for Disease Control and Prevention. Measles cases and outbreaks. Accessed May 6, 2025. https://www.cdc.gov/measles/cases-outbreaks.html
- Dyer C. Lancet retracts Wakefield’s MMR paper. BMJ. 2010;340.
- Alves Graber EM, Andrade FJ, Bost W, et al. An update and review of measles for emergency physicians. J Emerg Med. 2020;58:610-615.
- Kaplan LJ, Daum RS, Smaron M, et al. Severe measles in immunocompromised patients. JAMA. 1992;267:1237-1241.
- Takahashi E, Kurosaka D, Yoshida K, et al. Onset of modified measles after etanercept treatment in rheumatoid arthritis. Japanese J Clin Immunol. 2010;33:37-41.
- Worth A, Waldman RA, Dieckhaus K, et al. Art of prevention: our approach to the measles-mumps-rubella vaccine in adult patients vaccinated against measles before 1968 on biologic therapy for the treatment of psoriasis. Int J Womens Dermatol. 2019;6:94.
- Centers for Disease Control and Prevention. Clinical overview of measles (rubeola). Accessed May 8, 2025. https://www.cdc.gov/measles/hcp/clinical-overview/index.html
- Bedford H, Elliman D. Measles rates are rising again. BMJ. 2024;384.
- Harris E. Measles outbreaks grow amid declining vaccination rates. JAMA. 2023;330:2242.
- Guerra FM, Bolotin S, Lim G, et al. The basic reproduction number (R0) of measles: a systematic review. Lancet Infect Dis. 2017;17:E420-E428.
- Swartz MK. Measles: public and professional education. J Pediatr Health Care. 2019;33:367-368.
- Centers for Disease Control and Prevention. Interim infection prevention and control recommendations for measles in healthcare settings. Accessed April 27, 2025. https://www.cdc.gov/infection-control/hcp/measles/
- Moss WJ, Griffin DE, Feinstone WH. Measles. In: Vaccines for Biodefense and Emerging and Neglected Diseases. Elsevier; 2009: 551-565.
- Moss WJ. Measles. Lancet. 2017;390:2490-2502.
- Maintain the vaccination coverage level of 2 doses of the MMR vaccine for children in kindergarten— IID04. Healthy People 2030 website. Accessed May 6, 2025. https://odphp.health.gov/healthypeople/objectives-and-data/browse-objectives/vaccination/maintain-vaccination-coverage-level-2-doses-mmr-vaccine-children-kindergarten-iid-04
- Franconeri L, Antona D, Cauchemez S, et al. Two-dose measles vaccine effectiveness remains high over time: a French observational study, 2017–2019. Vaccine. 2023;41:5797-5804.
- World Health Organization. Measles. Accessed May 8, 2025. https:// www.who.int/news-room/fact-sheets/detail/measles
- Centers for Disease Control and Prevention. Measles vaccine recommendations. Accessed May 8, 2025. https://www.cdc.gov/measles/hcp/vaccine-considerations/index.html
- Centers for Disease Control and Prevention. Measles cases and outbreaks. Accessed May 6, 2025. https://www.cdc.gov/measles/cases-outbreaks.html
- Dyer C. Lancet retracts Wakefield’s MMR paper. BMJ. 2010;340.
- Alves Graber EM, Andrade FJ, Bost W, et al. An update and review of measles for emergency physicians. J Emerg Med. 2020;58:610-615.
- Kaplan LJ, Daum RS, Smaron M, et al. Severe measles in immunocompromised patients. JAMA. 1992;267:1237-1241.
- Takahashi E, Kurosaka D, Yoshida K, et al. Onset of modified measles after etanercept treatment in rheumatoid arthritis. Japanese J Clin Immunol. 2010;33:37-41.
- Worth A, Waldman RA, Dieckhaus K, et al. Art of prevention: our approach to the measles-mumps-rubella vaccine in adult patients vaccinated against measles before 1968 on biologic therapy for the treatment of psoriasis. Int J Womens Dermatol. 2019;6:94.
- Centers for Disease Control and Prevention. Clinical overview of measles (rubeola). Accessed May 8, 2025. https://www.cdc.gov/measles/hcp/clinical-overview/index.html
Measles Resurgence: A Dermatologist’s Guide
Measles Resurgence: A Dermatologist’s Guide
An Uncertain Future for No-Cost Preventive Care
Later this month, the U.S. Supreme Court is anticipated to announce its decision in Kennedy vs. Braidwood Management, a case that could significantly impact the no-cost coverage of preventive healthcare services under the Patient Protection and Affordable Care Act (ACA). At the center of the case is whether the structure of the U.S. Preventive Services Task Force (USPSTF) – an independent body convened by the federal government that makes recommendations for preventive services that nearly all private insurances must cover without cost sharing under provisions of the ACA (specifically, Grade A and B recommendations) – violates the Appointments Clause of the U.S. Constitution. This clause states that “officers of the United States” may only be appointed by the president with the Senate’s approval.
The case, initiated in 2022 by a self-insured, Christian-owned business, specifically targeted the coverage of pre-exposure prophylaxis (PrEP) for preventing HIV in high-risk individuals. However, the decision could broadly affect the coverage of other preventive services, including colorectal cancer screening tests. In June 2024, the 5th Circuit Court of Appeals upheld a district court’s ruling that the ACA’s requirement to cover without cost-sharing services recommended by USPSTF is unconstitutional, paving the way for the current Supreme Court showdown.
The consequences of this ruling could be significant. This would likely reverse the progress we have made in increasing colorectal cancer screening rates by reducing financial barriers to care. Interestingly, despite a new administration, the federal government continues to advocate for upholding the law, asserting that USPSTF members are “inferior officers” such that the Secretary of Health and Human Services can dismiss individual members and oversee or veto the Task Force’s recommendations at will, potentially threatening scientific independence. Though it’s often challenging to predict the Supreme Court’s final decision, the tone of questioning during oral arguments in April hinted at a possible win for the ACA and preventive care. Stay tuned, as the decision to be released later this month has seismic clinical implications.
Megan A. Adams, MD, JD, MSc
Editor in Chief
Later this month, the U.S. Supreme Court is anticipated to announce its decision in Kennedy vs. Braidwood Management, a case that could significantly impact the no-cost coverage of preventive healthcare services under the Patient Protection and Affordable Care Act (ACA). At the center of the case is whether the structure of the U.S. Preventive Services Task Force (USPSTF) – an independent body convened by the federal government that makes recommendations for preventive services that nearly all private insurances must cover without cost sharing under provisions of the ACA (specifically, Grade A and B recommendations) – violates the Appointments Clause of the U.S. Constitution. This clause states that “officers of the United States” may only be appointed by the president with the Senate’s approval.
The case, initiated in 2022 by a self-insured, Christian-owned business, specifically targeted the coverage of pre-exposure prophylaxis (PrEP) for preventing HIV in high-risk individuals. However, the decision could broadly affect the coverage of other preventive services, including colorectal cancer screening tests. In June 2024, the 5th Circuit Court of Appeals upheld a district court’s ruling that the ACA’s requirement to cover without cost-sharing services recommended by USPSTF is unconstitutional, paving the way for the current Supreme Court showdown.
The consequences of this ruling could be significant. This would likely reverse the progress we have made in increasing colorectal cancer screening rates by reducing financial barriers to care. Interestingly, despite a new administration, the federal government continues to advocate for upholding the law, asserting that USPSTF members are “inferior officers” such that the Secretary of Health and Human Services can dismiss individual members and oversee or veto the Task Force’s recommendations at will, potentially threatening scientific independence. Though it’s often challenging to predict the Supreme Court’s final decision, the tone of questioning during oral arguments in April hinted at a possible win for the ACA and preventive care. Stay tuned, as the decision to be released later this month has seismic clinical implications.
Megan A. Adams, MD, JD, MSc
Editor in Chief
Later this month, the U.S. Supreme Court is anticipated to announce its decision in Kennedy vs. Braidwood Management, a case that could significantly impact the no-cost coverage of preventive healthcare services under the Patient Protection and Affordable Care Act (ACA). At the center of the case is whether the structure of the U.S. Preventive Services Task Force (USPSTF) – an independent body convened by the federal government that makes recommendations for preventive services that nearly all private insurances must cover without cost sharing under provisions of the ACA (specifically, Grade A and B recommendations) – violates the Appointments Clause of the U.S. Constitution. This clause states that “officers of the United States” may only be appointed by the president with the Senate’s approval.
The case, initiated in 2022 by a self-insured, Christian-owned business, specifically targeted the coverage of pre-exposure prophylaxis (PrEP) for preventing HIV in high-risk individuals. However, the decision could broadly affect the coverage of other preventive services, including colorectal cancer screening tests. In June 2024, the 5th Circuit Court of Appeals upheld a district court’s ruling that the ACA’s requirement to cover without cost-sharing services recommended by USPSTF is unconstitutional, paving the way for the current Supreme Court showdown.
The consequences of this ruling could be significant. This would likely reverse the progress we have made in increasing colorectal cancer screening rates by reducing financial barriers to care. Interestingly, despite a new administration, the federal government continues to advocate for upholding the law, asserting that USPSTF members are “inferior officers” such that the Secretary of Health and Human Services can dismiss individual members and oversee or veto the Task Force’s recommendations at will, potentially threatening scientific independence. Though it’s often challenging to predict the Supreme Court’s final decision, the tone of questioning during oral arguments in April hinted at a possible win for the ACA and preventive care. Stay tuned, as the decision to be released later this month has seismic clinical implications.
Megan A. Adams, MD, JD, MSc
Editor in Chief
Multiagent AI Systems in Health Care: Envisioning Next-Generation Intelligence
Artificial intelligence (AI) is rapidly evolving, with large language models (LLMs) marking a significant milestone in processing and generating human-like responses to natural language prompts. However, this advancement only signals the beginning of a more profound transformation in AI capabilities. The development of AI agents represents a new paradigm at the forefront of this evolution.
BACKGROUND
AI agents represent a leap forward from traditional LLM applications. While definitions may vary slightly among technology developers, the core concept remains: these agents are autonomous software entities designed to interact with their environment, make independent decisions, and execute tasks based on predefined goals.1-3 What sets AI agents apart is their combination of sophisticated components within structured architectures. At their core, AI agents incorporate an LLM for response generation, which is augmented by a suite of tools to optimize workflow and complete tasks, memory capabilities for personalized interactions, and autonomous reasoning. This combination allows AI agents to plan, create subtasks, gather information, and learn iteratively from their own experiences or other AI agents.
The true potential of this technology becomes apparent when multiple AI agents collaborate within multiagent AI systems. This concept introduces a new level of flexibility and capability in tackling complex tasks. Autogen, CrewAI, and LangChain offer various agent network configurations, including hierarchical, sequential, conditional, or even parallel task execution.4-6 This adaptability opens up a world of possibilities across various industries, but perhaps nowhere is the potential impact more exciting and profound than in health care.
AI agents in health care present an opportunity to revolutionize patient care, streamline administrative processes, and support complex clinical decision-making. This review examines 3 scenarios that illustrate the impact of AI agents in health care: a hypothetical sepsis management system, chronic disease management, and hospital patient flow optimization. This article will provide a detailed look at the technical implementation challenges, including the integration with existing health care IT systems, data privacy considerations, and the crucial role of explainable AI in maintaining trust and transparency.
It is challenging to implement AI agents in health care. Concerns include ensuring data quality and mitigating bias, seamlessly integrating these systems into existing clinical workflows, and navigating the complex ethical considerations that arise when deploying autonomous systems in health care. The integration with Internet of Things (IoT) devices for real-time patient data monitoring and the development of more sophisticated natural language interfaces to enhance future human-AI collaboration.
The adoption of AI agents in health care is only beginning, and it promises to be transformative. As AI continues to evolve, a comprehensive understanding of its applications, limitations, and ethical considerations is essential. This report provides a comprehensive overview of the current state, potential applications, and future directions of AI agents in health care, offering insights valuable to researchers, clinicians, and policymakers.
MultiAgent AI architecture
Sepsis Management
Despite advancements in broad-spectrum antibiotics, imaging, and life support systems, mortality rates associated with sepsis remain high. The complexity of optimizing care in clinical settings has hindered progress in managing sepsis. Previous attempts to develop predictive sepsis models have proven challenging.7 This report proposes a multiagent AI system designed to enhance comprehensive patient monitoring and care through coordinated AI-driven interventions.
Data Collection and Integration Agent. Powered by a controlled vocabulary to specify all data, the primary function for the data collection and integration agent is to clean, transform, and organize patient data from structured and unstructured sources. This agent prepares succinct summaries of consultant notes and formats data for human and machine consumption. All numerical data are presented graphically, including relevant historical data trends. The agent also digitally captures all orders in a structured format using a specified controlled vocabulary. This structured data feed supports the output of other agents, including documentation, treatment planning, and risk stratification, while also supplying the data structures for future training.
Diagnostic Agent. Critical illness is characterized by multiple abnormalities across a wide array of tests, ranging from plain chest X-ray, computed tomography (CT), blood cell composition, plasma chemistry, and microscopic evaluation of specimens. Additionally, life support parameters provide insights into disease severity and can inform management recommendations. These data offer a wide array of visual and numerical data to be used as input for computation, recommendation, and further training. For example, to evaluate fluid overload on chest X-rays or tissue histopathology slides, an AI agent can leverage deep learning models such as convolutional neural networks and vision transformers to analyze images like radiographs and histopathology slides.8,9 Recurrent neural networks or transformer models process sequential data like time-series vital signs. The agent also implements ensemble methods that combine multiple machine learning algorithms to enhance diagnostic accuracy.
Risk Stratification Agent. This assesses severity and predicts potential outcomes. Morbidity and mortality risks are calculated using an established scoring system and individualized based on the history of other agents’ conditional patients. These are presented graphically, with major risk factors highlighted for explainability.
Treatment Recommendation Agent. Using a reinforcement learning framework supplemented by up-to-date clinical guidelines, this system leverages historical data structured with standardized vocabulary to analyze patients with similar clinical features. Training is also conducted on the patient’s physiological data. All recommendations are presented via a dedicated user interface in a readable format, along with recommendations for editable, orderable items, references, and full-text snippets from previous research. Stop rules end computing if confidence in recommendations is too broad or no clear pathway can be computed with certainty, prompting human mitigation.
Resource Management Agent. This agent coordinates hospital resources using constraint programming techniques for optimal resource allocation, uses queueing theory models to predict and manage patient flow, and implements genetic algorithms for complex scheduling problems.10,11
Monitoring and Alert Agent. By tracking patients’ progress and alerting staff to changes, this agent uses anomaly detection algorithms to identify unusual patterns in patient data and implement time-series forecasting models, such as autoregressive integrated moving average and prophet, to predict future patient states. The agent also uses stream-processing techniques for real-time data analysis.12,13
Documentation and Reporting Agent. This agent maintains comprehensive medical records and generates reports. It employs advanced natural language processing techniques for automated report generation, uses advanced LLMs fine-tuned on medical corpora for narrative creation, and implements information-retrieval techniques to efficiently query patient records.
CLINICAL CASE STUDIES
To illustrate the functionality of a multiagent system, this report examines its application for managing sepsis. The data collection and integration agent continuously aggregates patient data from various sources, normalizing and timestamping it for consistent processing. The diagnostic agent analyzes this integrated data in real time, applying sepsis criteria and utilizing a deep learning model trained on a large sepsis dataset to detect subtle patterns.
The risk stratification agent calculates severity scores, such as the Sepsis-related Organ Failure Assessment (SOFA), quick SOFA (qSOFA), and Acute Physiology and Chronic Health Evaluation II, upon detecting a possible sepsis case.14 It predicts the likelihood of specific outcomes and estimates the potential trajectory of the patient’s condition for the next 24 to 48 hours. Based on this assessment, the treatment recommendation agent suggests an initial treatment plan, including appropriate antibiotics, fluid resuscitation protocols, and vasopressor recommendations, recommendations when indicated.
Concurrently, the resource management agent checks the availability of necessary resources and prioritizes allocation based on the severity. The monitoring agent tracks the patient’s response to interventions in real time, alerting the care team to any concerning changes or lack of expected improvement. Throughout this process, the documentation agent ensures that all actions, responses, and outcomes are meticulously recorded in a structured format and generates real-time updates for the patient’s electronic health record (EHR) and preparing summary reports for handoffs between care teams.
Administrative Workflow Support
Modern health care operations are resource-intensive, requiring coordination of advanced imaging, procedures, laboratory testing, and professional consultations.15 AI-powered health care administrative workflow systems are revolutionizing how medical facilities coordinate patient care. For patients with chronic cough, these systems seamlessly integrate scheduling, imaging, diagnostics, and follow-up care into a cohesive process that reduces administrative burden while improving patient outcomes. Through an intuitive interface and automated assistance, health care practitioners (HCPs) can track patient progress from initial consultation through diagnosis and treatment.
The process begins when an HCP enters a patient into the system, which triggers an automated CT scan scheduling system. The system considers factors like urgency, facility availability, and patient preferences to suggest optimal appointment times. Once imaging is complete, AI agents analyze the radiology reports, extract key findings, and generate structured summaries that highlight critical information such as “mild bronchial wall thickening with patchy ground-glass opacities” or “findings consistent with chronic bronchitis.”
Based on these findings, the system automatically generates evidence-based recommendations for follow-up care, such as pulmonology consultations or follow-up imaging in 3 months. These recommendations are presented to the ordering clinician, along with suggested appointment slots for specialist consultations. The system then manages the coordination of multiple appointments, ensuring each step in the patient’s care plan is properly sequenced and scheduled.
The entire process is monitored through a comprehensive dashboard that provides real-time updates on patient status, appointment schedules, and clinical recommendations. HCPs can track which patients require immediate attention, view upcoming appointments, and monitor the progress of ongoing care plans.
Multiagent AI Operation Optimization
Hospitals are complex entities that must function at different scales and respond in an agile, timely manner at all hours, deploying staff at various positions.16 A system of AI agents can receive signals from sensors monitoring foot traffic in the emergency department and trauma unit, as well as the availability of operating room staff, equipment, and intensive care unit beds. Smart sensors enable this monitoring through IoT networks. These networks benefit from advances in adaptive and consensus networking algorithms, along with recent advances in bioengineering and biocomputing.17
For example, in the case of imaging for suspected abdominal obstruction, an AI agent tasked with scheduling CTs could time the patient’s arrival based on acuity. Another AI agent could alert staff transporting the patient to the CT appointment, with the next location contingent on a clinical decision to proceed to the operating room. Yet another AI agent could summarize radiology interpretations and alert the surgery and anesthesia teams to a potential case, while others could notify operating room staff of equipment needs or reserve a bed. In this paradigm, AI agents facilitate more precise and timely communication between multiple staff members.
TECHNICAL IMPLEMENTATION
Large Language Models
Each agent uses a different LLM optimized for its specific task. For example, the diagnostic agent uses an LLM pretrained on a large corpus of biomedical literature and fine-tuned on a dataset of confirmed sepsis cases and their presentations.18 It implements few-shot learning techniques to adapt to rare or atypical presentations. The treatment recommendation agent also uses an LLM, employing a retrieval-augmented generation approach to access the latest clinical guidelines during inference. The documentation agent uses another advanced language model, fine-tuned on a large corpus of high-quality medical documentation, implementing controlled text generation techniques and utilizing a separate smaller model for real-time error checking and correction.
Interagent Quality Control
Agents learn from their own experience and the experience of other agents. They are equipped with user-defined rule-based and model-based systems for quality assurance, with clear stopping rules for human involvement and mitigation.
Sophisticated quality control measures bolster the system’s reliability, including ensemble techniques for result comparison, redundancy for critical tasks, and automatic human review for disagreements above a certain threshold. Each agent provides a calibrated confidence score with its output, used to weigh inputs in downstream tasks and trigger additional checks for low-confidence outputs.
A dedicated quality control agent monitors output from all agents, employing both supervised and unsupervised anomaly detection techniques. Feedback loops allow agents to evaluate the quality and utility of information received from other agents. The system implements a multiarmed bandit approach to dynamically adjust the influence of different agents based on their performance and periodically retrains agent models using federated learning techniques.19
Electronic Health Record Integration
Seamless EHR integration is crucial for practical implementation. The system has secure application programming interface access to various EHR platforms, implements OAuth 2.0 for authentication, and use HTTPS with perfect forward secrecy for all communications.20 It works with HL7 FHIR to ensure interoperability and uses SNOMED CT for clinical terminology to ensure semantic interoperability across different EHRs.21,22
The system implements a multilevel approval system for write-backs to EHRs, with different thresholds based on the information’s criticality. It uses digital signatures to ensure the integrity and nonrepudiation of AI-generated entries and implements blockchain technology to create an immutable and distributed ledger of all AI system actions.23
Decision Transparency
To ensure transparency in decision-making processes, the system applies techniques (eg, local interpretable model-agnostic explanations and Shapley additive explanations) to provide insights into agent decision-making processes.24-26 It provides customized visualizations for different stakeholders and allows users to explore alternative decision paths through what-if scenario modeling.27
The system provides calibrated confidence indicators for each recommendation or decision, implementing a novel confidence calibration agent that continuously monitors and adjusts confidence scores based on observed outcomes.
Continuous Learning and Adaptation
The system employs several techniques to remain current with evolving medical knowledge. Federated learning includes information from diverse datasets across multiple institutions without compromising patient privacy.28 A/B testing is used to safely deploy and compare new agent versions in controlled settings, implementing multiarmed bandit algorithms to efficiently explore new models while minimizing potential negative impacts. Human-in-the-loop learning and active learning techniques are used to incorporate feedback from HCPs and efficiently solicit expert input on the most informative data.29
CLINICAL IMPLICATIONS
The implementation of multiagent AI systems in health care has several potential benefits: enhanced diagnostic accuracy, personalized treatment, improved efficiency, continuous monitoring, and resource optimization. A recent review of AI sepsis predictive models exhibited superior results to standard clinical scoring methods like qSOFA.30 In oncology, such systems can result in more tailored treatments, enhancing outcomes.31 The implementation of an ambient dictation system can improve workflow and prevent HCP burnout.32
ETHICAL CONSIDERATIONS AND AI OVERSIGHT
Integrating AI agents into health care raises significant ethical considerations that must be carefully addressed to ensure equitable and effective care delivery. One primary concern involves cultural and linguistic competency, as AI systems may struggle with cultural nuances, idioms, and context-specific communication patterns. This becomes particularly challenging in regions with diverse ethnic populations or immigrant communities, where medical terminology may not have direct translations and cultural beliefs significantly influence health care decisions. AI systems also may inherit and amplify existing biases in health care delivery, whether through HCP bias reflected in training data, patient bias affecting acceptance of AI-assisted care, or demographic underrepresentation during system development.
AI agents present unique opportunities for improving health care access and outcomes through community engagement, though such initiatives require thoughtful implementation. Predictive analytics can identify high-risk individuals within communities who may benefit from preventive care, while analysis of social determinants of health can enable more targeted interventions. However, these capabilities must be balanced with privacy concerns and the risk of surveillance, particularly in communities that distrust health care institutions. The potential for AI to bridge health care gaps must be weighed against the need to maintain cultural sensitivity and community trust.
The governance and oversight of health care AI systems requires a multistakeholder approach with clear lines of responsibility and accountability. This includes involvement from government health care agencies, professional medical associations, ethics boards, and independent auditors, all working together to establish and enforce standards while monitoring system performance and addressing potential biases. Health care organizations must maintain transparent policies about AI use, implement regular monitoring and evaluation protocols, and establish precise mechanisms for patient feedback and grievance resolution. Ongoing assessment and adjustment of these systems, informed by community feedback and outcomes data, will be crucial for their ethical implementation, ensuring that AI agents complement, rather than replace, human judgment and cultural sensitivity.
FUTURE DIRECTIONS
Despite the potential benefits, implementing multiagent AI systems in health care faces significant challenges that require careful consideration. Beyond the fundamental issues such as data quality and bias mitigation, health care organizations struggle with fragmented systems, inconsistent data formats, and varying quality. Technical infrastructure requirements are substantial, particularly in rural or underserved areas that lack robust networks and cybersecurity. HCPs already face significant cognitive load and time pressures, making integrating AI agents into existing workflows particularly challenging. There is also the critical issue of transparency and interpretability, as health care decisions require clear reasoning and accountability that many black-box AI systems struggle to provide.
The legal landscape introduces another layer of complexity, particularly regarding liability, consent, and privacy questions. When AI agents contribute to medical decisions, establishing clear lines of responsibility becomes crucial. There are also serious concerns about algorithmic fairness and the potential for AI systems to perpetuate or amplify existing inequities. The cost of implementation remains a significant barrier, requiring substantial investment in technology, training, and ongoing maintenance while ensuring resources are not diverted from direct patient care. Moreover, HCPs may resist adoption due to concerns about job security, loss of autonomy, or skepticism about AI capabilities while paradoxically facing risks of overreliance on AI systems that could lead to the degradation of human clinical skills.
Addressing these challenges requires a multifaceted approach that combines technical solutions with organizational and policy changes. Health care organizations must implement rigorous data validation processes and interoperability standards while developing hybrid models that balance sophisticated AI capabilities with interpretable techniques. Extensive research and iterative design processes, with direct input from HCPs, are essential for successful integration. Establishing independent ethics boards to oversee system development and deployment, conducting multicenter randomized controlled trials, and creating clear regulatory frameworks will ensure safe and effective implementation. Success will ultimately depend on ongoing collaboration between technology developers, HCPs, policymakers, and patients, maintaining a steady focus on improving patient care and outcomes while carefully navigating the complex challenges of AI integration in health care.33-35
As multiagent AI systems in health care evolve, several exciting directions emerge. These include the integration of IoT and wearable devices, the development of more sophisticated natural language interfaces, and applying these systems to predictive maintenance of medical equipment.
CONCLUSIONS
The advent of multiagent AI systems in health care represents a paradigm shift in the approach to patient care, clinical decision making, and health care management. While these systems offer immense potential to transform health care delivery, their development and implementation must be guided by rigorous scientific validation, ethical considerations, and a patient-centered approach. The ultimate goal remains clear: harnessing the power of AI to improve patient outcomes, enhance the efficiency of health care delivery, and ultimately advance the health and well-being of patients.
Amazon Web Services, Inc. What are AI agents? Agents in artificial intelligence explained. Accessed April 7, 2025. https://aws.amazon.com/what-is/ai-agents/
Gutowska A. What are AI agents? IBM. Accessed April 7, 2025. https://www.ibm.com/think/topics/ai-agents
Agent AI. Microsoft Research. Accessed April 7, 2025. https://www.microsoft.com/en-us/research/project/agent-ai
Microsoft. AutoGen. Accessed April 7, 2025. https://microsoft.github.io/autogen/
Crew AI. The Leading Multi-Agent Platform. CrewAI. Accessed April 7, 2025. https://www.crewai.com/
LangChain. Accessed April 7, 2025. https://www.langchain.com/
Wong A, Otles E, Donnelly JP, et al. External validation of a widely implemented proprietary sepsis prediction model in hospitalized patients. JAMA Intern Med. 2021;181(8):1065-1070. doi:10.1001/jamainternmed.2021.2626
Willemink MJ, Roth HR, Sandfort V. Toward foundational deep learning models for medical imaging in the new era of transformer networks. Radiol Artif Intell. 2022;4(6):e210284. doi:10.1148/ryai.210284
Waqas A, Bui MM, Glassy EF, et al. Revolutionizing digital pathology with the power of generative artificial intelligence and foundation models. Lab Invest. 2023;103(11):100255. doi:10.1016/j.labinv.2023.100255
Moreno-Carrillo A, Arenas LMÁ, Fonseca JA, Caicedo CA, Tovar SV, Muñoz-Velandia OM. Application of queuing theory to optimize the triage process in a tertiary emergency care (“ER”) department. J Emerg Trauma Shock. 2019;12(4):268-273. doi:10.4103/JETS.JETS_42_19
Pongcharoen P, Hicks C, Braiden PM, Stewardson DJ. Determining optimum genetic algorithm parameters for scheduling the manufacturing and assembly of complex products. Int J Prod Econ. 2002;78(3):311-322. doi:10.1016/S0925-5273(02)00104-4
Sardar I, Akbar MA, Leiva V, Alsanad A, Mishra P. Machine learning and automatic ARIMA/Prophet models-based forecasting of COVID-19: methodology, evaluation, and case study in SAARC countries. Stoch Environ Res Risk Assess. 2023;37(1):345-359. doi:10.1007/s00477-022-02307-x
Samosir J, Indrawan-Santiago M, Haghighi PD. An evaluation of data stream processing systems for data driven applications. Procedia Comput Sci. 2016;80:439-449. doi:10.1016/j.procs.2016.05.322
Asmarawati TP, Suryantoro SD, Rosyid AN, et al. Predictive value of sequential organ failure assessment, quick sequential organ failure assessment, acute physiology and chronic health evaluation II, and new early warning signs scores estimate mortality of COVID-19 patients requiring intensive care unit. Indian J Crit Care Med. 2022;26(4):466-473. doi:10.5005/jp-journals-10071-24170
Khan S, Vandermorris A, Shepherd J, et al. Embracing uncertainty, managing complexity: applying complexity thinking principles to transformation efforts in healthcare systems. BMC Health Serv Res. 2018;18(1):192. doi:10.1186/s12913-018-2994-0
Plsek PE, Greenhalgh T. The challenge of complexity in health care. BMJ. 2001;323(7313):625-628. doi:10.1136/bmj.323.7313.625
Kouchaki S, Ding X, Sanei S. AI- and IoT-enabled solutions for healthcare. Sensors. 2024;24(8):2607. doi:10.3390/s24082607
Saab K, Tu T, Weng WH, et al. Capabilities of Gemini Models in Medicine. arXiv. doi:10.48550/arXiv.2404.18416
Villar SS, Bowden J, Wason J. Multi-armed bandit models for the optimal design of clinical trials: benefits and challenges. Stat Sci. 2015;30(2):199-215. doi:10.1214/14-STS504
Auth0. What is OAuth 2.0. Accessed April 7, 2025. https://auth0.com/intro-to-iam/what-is-oauth-2
HL7. Welcome to FHIR. Updated March 26, 2025. Accessed April 7, 2025. https://www.hl7.org/fhir/
SNOMED International. Accessed April 7, 2025. https://www.snomed.org
Hasselgren A, Kralevska K, Gligoroski D, Pedersen SA, Faxvaag A. Blockchain in healthcare and health sciences—a scoping review. Int J Med Inf. 2020;134:104040. doi:10.1016/j.ijmedinf.2019.104040
Ribeiro MT, Singh S, Guestrin C. “Why Should I Trust You?”: Explaining the predictions of any classifier. In: Proceedings of the 22nd ACM SIGKDD international conference on knowledge discovery and data mining. 2016:1135-1144. doi:10.1145/2939672.2939778
Ekanayake IU, Meddage DPP, Rathnayake U. A novel approach to explain the black-box nature of machine learning in compressive strength predictions of concrete using Shapley additive explanations (SHAP). Case Stud Constr Mater. 2022;16:e01059. doi:10.1016/j.cscm.2022.e01059
Alabi RO, Elmusrati M, Leivo I, Almangush A, Mäkitie AA. Machine learning explainability in nasopharyngeal cancer survival using LIME and SHAP. Sci Rep. 2023;13(1):8984. doi:10.1038/s41598-023-35795-0
Otto E, Culakova E, Meng S, et al. Overview of sankey flow diagrams: focusing on symptom trajectories in older adults with advanced cancer. J Geriatr Oncol. 2022;13(5):742-746. doi:10.1016/j.jgo.2021.12.017
Fereidooni H, Marchal S, Miettinen M, et al. SAFELearn: secure aggregation for private federated learning. In: 2021 IEEE security and privacy workshops (SPW). 2021:56-62. doi:10.1109/SPW53761.2021.00017
Linton DL, Pangle WM, Wyatt KH, Powell KN, Sherwood RE. Identifying key features of effective active learning: the effects of writing and peer discussion. Life Sci Educ. 2014;13(3):469-477. doi:10.1187/cbe.13-12-0242
Yang HS. Machine learning for sepsis prediction: prospects and challenges. Clin Chem. 2024;70(3):465-467. doi:10.1093/clinchem/hvae006
Liao J, Li X, Gan Y, et al. Artificial intelligence assists precision medicine in cancer treatment. Front Oncol. 2023;12. doi:10.3389/fonc.2022.998222
Tierney AA, Gayre G, Hoberman B, et al. Ambient artificial intelligence scribes to alleviate the burden of clinical documentation. NEJM Catal. 2024;5(3):CAT.23.0404. doi:10.1056/CAT.23.0404
Borkowski AA, Jakey CE, Thomas LB, Viswanadhan N, Mastorides SM. Establishing a hospital artificial intelligence committee to improve patient care. Fed Pract. 2022;39(8):334-336. doi:10.12788/fp.0299
Isaacks DB, Borkowski AA. Implementing trustworthy AI in VA high reliability health care organizations. Fed Pract.2024;41(2):40-43. doi:10.12788/fp.0454
Han R, Acosta JN, Shakeri Z, Ioannidis JPA, Topol EJ, Rajpurkar P. Randomized controlled trials evaluating artificial intelligence in clinical practice: a scoping review. Lancet Digit Health. 2024;6(5):e367-e373. doi:10.1016/S2589-7500(24)00047-5
Artificial intelligence (AI) is rapidly evolving, with large language models (LLMs) marking a significant milestone in processing and generating human-like responses to natural language prompts. However, this advancement only signals the beginning of a more profound transformation in AI capabilities. The development of AI agents represents a new paradigm at the forefront of this evolution.
BACKGROUND
AI agents represent a leap forward from traditional LLM applications. While definitions may vary slightly among technology developers, the core concept remains: these agents are autonomous software entities designed to interact with their environment, make independent decisions, and execute tasks based on predefined goals.1-3 What sets AI agents apart is their combination of sophisticated components within structured architectures. At their core, AI agents incorporate an LLM for response generation, which is augmented by a suite of tools to optimize workflow and complete tasks, memory capabilities for personalized interactions, and autonomous reasoning. This combination allows AI agents to plan, create subtasks, gather information, and learn iteratively from their own experiences or other AI agents.
The true potential of this technology becomes apparent when multiple AI agents collaborate within multiagent AI systems. This concept introduces a new level of flexibility and capability in tackling complex tasks. Autogen, CrewAI, and LangChain offer various agent network configurations, including hierarchical, sequential, conditional, or even parallel task execution.4-6 This adaptability opens up a world of possibilities across various industries, but perhaps nowhere is the potential impact more exciting and profound than in health care.
AI agents in health care present an opportunity to revolutionize patient care, streamline administrative processes, and support complex clinical decision-making. This review examines 3 scenarios that illustrate the impact of AI agents in health care: a hypothetical sepsis management system, chronic disease management, and hospital patient flow optimization. This article will provide a detailed look at the technical implementation challenges, including the integration with existing health care IT systems, data privacy considerations, and the crucial role of explainable AI in maintaining trust and transparency.
It is challenging to implement AI agents in health care. Concerns include ensuring data quality and mitigating bias, seamlessly integrating these systems into existing clinical workflows, and navigating the complex ethical considerations that arise when deploying autonomous systems in health care. The integration with Internet of Things (IoT) devices for real-time patient data monitoring and the development of more sophisticated natural language interfaces to enhance future human-AI collaboration.
The adoption of AI agents in health care is only beginning, and it promises to be transformative. As AI continues to evolve, a comprehensive understanding of its applications, limitations, and ethical considerations is essential. This report provides a comprehensive overview of the current state, potential applications, and future directions of AI agents in health care, offering insights valuable to researchers, clinicians, and policymakers.
MultiAgent AI architecture
Sepsis Management
Despite advancements in broad-spectrum antibiotics, imaging, and life support systems, mortality rates associated with sepsis remain high. The complexity of optimizing care in clinical settings has hindered progress in managing sepsis. Previous attempts to develop predictive sepsis models have proven challenging.7 This report proposes a multiagent AI system designed to enhance comprehensive patient monitoring and care through coordinated AI-driven interventions.
Data Collection and Integration Agent. Powered by a controlled vocabulary to specify all data, the primary function for the data collection and integration agent is to clean, transform, and organize patient data from structured and unstructured sources. This agent prepares succinct summaries of consultant notes and formats data for human and machine consumption. All numerical data are presented graphically, including relevant historical data trends. The agent also digitally captures all orders in a structured format using a specified controlled vocabulary. This structured data feed supports the output of other agents, including documentation, treatment planning, and risk stratification, while also supplying the data structures for future training.
Diagnostic Agent. Critical illness is characterized by multiple abnormalities across a wide array of tests, ranging from plain chest X-ray, computed tomography (CT), blood cell composition, plasma chemistry, and microscopic evaluation of specimens. Additionally, life support parameters provide insights into disease severity and can inform management recommendations. These data offer a wide array of visual and numerical data to be used as input for computation, recommendation, and further training. For example, to evaluate fluid overload on chest X-rays or tissue histopathology slides, an AI agent can leverage deep learning models such as convolutional neural networks and vision transformers to analyze images like radiographs and histopathology slides.8,9 Recurrent neural networks or transformer models process sequential data like time-series vital signs. The agent also implements ensemble methods that combine multiple machine learning algorithms to enhance diagnostic accuracy.
Risk Stratification Agent. This assesses severity and predicts potential outcomes. Morbidity and mortality risks are calculated using an established scoring system and individualized based on the history of other agents’ conditional patients. These are presented graphically, with major risk factors highlighted for explainability.
Treatment Recommendation Agent. Using a reinforcement learning framework supplemented by up-to-date clinical guidelines, this system leverages historical data structured with standardized vocabulary to analyze patients with similar clinical features. Training is also conducted on the patient’s physiological data. All recommendations are presented via a dedicated user interface in a readable format, along with recommendations for editable, orderable items, references, and full-text snippets from previous research. Stop rules end computing if confidence in recommendations is too broad or no clear pathway can be computed with certainty, prompting human mitigation.
Resource Management Agent. This agent coordinates hospital resources using constraint programming techniques for optimal resource allocation, uses queueing theory models to predict and manage patient flow, and implements genetic algorithms for complex scheduling problems.10,11
Monitoring and Alert Agent. By tracking patients’ progress and alerting staff to changes, this agent uses anomaly detection algorithms to identify unusual patterns in patient data and implement time-series forecasting models, such as autoregressive integrated moving average and prophet, to predict future patient states. The agent also uses stream-processing techniques for real-time data analysis.12,13
Documentation and Reporting Agent. This agent maintains comprehensive medical records and generates reports. It employs advanced natural language processing techniques for automated report generation, uses advanced LLMs fine-tuned on medical corpora for narrative creation, and implements information-retrieval techniques to efficiently query patient records.
CLINICAL CASE STUDIES
To illustrate the functionality of a multiagent system, this report examines its application for managing sepsis. The data collection and integration agent continuously aggregates patient data from various sources, normalizing and timestamping it for consistent processing. The diagnostic agent analyzes this integrated data in real time, applying sepsis criteria and utilizing a deep learning model trained on a large sepsis dataset to detect subtle patterns.
The risk stratification agent calculates severity scores, such as the Sepsis-related Organ Failure Assessment (SOFA), quick SOFA (qSOFA), and Acute Physiology and Chronic Health Evaluation II, upon detecting a possible sepsis case.14 It predicts the likelihood of specific outcomes and estimates the potential trajectory of the patient’s condition for the next 24 to 48 hours. Based on this assessment, the treatment recommendation agent suggests an initial treatment plan, including appropriate antibiotics, fluid resuscitation protocols, and vasopressor recommendations, recommendations when indicated.
Concurrently, the resource management agent checks the availability of necessary resources and prioritizes allocation based on the severity. The monitoring agent tracks the patient’s response to interventions in real time, alerting the care team to any concerning changes or lack of expected improvement. Throughout this process, the documentation agent ensures that all actions, responses, and outcomes are meticulously recorded in a structured format and generates real-time updates for the patient’s electronic health record (EHR) and preparing summary reports for handoffs between care teams.
Administrative Workflow Support
Modern health care operations are resource-intensive, requiring coordination of advanced imaging, procedures, laboratory testing, and professional consultations.15 AI-powered health care administrative workflow systems are revolutionizing how medical facilities coordinate patient care. For patients with chronic cough, these systems seamlessly integrate scheduling, imaging, diagnostics, and follow-up care into a cohesive process that reduces administrative burden while improving patient outcomes. Through an intuitive interface and automated assistance, health care practitioners (HCPs) can track patient progress from initial consultation through diagnosis and treatment.
The process begins when an HCP enters a patient into the system, which triggers an automated CT scan scheduling system. The system considers factors like urgency, facility availability, and patient preferences to suggest optimal appointment times. Once imaging is complete, AI agents analyze the radiology reports, extract key findings, and generate structured summaries that highlight critical information such as “mild bronchial wall thickening with patchy ground-glass opacities” or “findings consistent with chronic bronchitis.”
Based on these findings, the system automatically generates evidence-based recommendations for follow-up care, such as pulmonology consultations or follow-up imaging in 3 months. These recommendations are presented to the ordering clinician, along with suggested appointment slots for specialist consultations. The system then manages the coordination of multiple appointments, ensuring each step in the patient’s care plan is properly sequenced and scheduled.
The entire process is monitored through a comprehensive dashboard that provides real-time updates on patient status, appointment schedules, and clinical recommendations. HCPs can track which patients require immediate attention, view upcoming appointments, and monitor the progress of ongoing care plans.
Multiagent AI Operation Optimization
Hospitals are complex entities that must function at different scales and respond in an agile, timely manner at all hours, deploying staff at various positions.16 A system of AI agents can receive signals from sensors monitoring foot traffic in the emergency department and trauma unit, as well as the availability of operating room staff, equipment, and intensive care unit beds. Smart sensors enable this monitoring through IoT networks. These networks benefit from advances in adaptive and consensus networking algorithms, along with recent advances in bioengineering and biocomputing.17
For example, in the case of imaging for suspected abdominal obstruction, an AI agent tasked with scheduling CTs could time the patient’s arrival based on acuity. Another AI agent could alert staff transporting the patient to the CT appointment, with the next location contingent on a clinical decision to proceed to the operating room. Yet another AI agent could summarize radiology interpretations and alert the surgery and anesthesia teams to a potential case, while others could notify operating room staff of equipment needs or reserve a bed. In this paradigm, AI agents facilitate more precise and timely communication between multiple staff members.
TECHNICAL IMPLEMENTATION
Large Language Models
Each agent uses a different LLM optimized for its specific task. For example, the diagnostic agent uses an LLM pretrained on a large corpus of biomedical literature and fine-tuned on a dataset of confirmed sepsis cases and their presentations.18 It implements few-shot learning techniques to adapt to rare or atypical presentations. The treatment recommendation agent also uses an LLM, employing a retrieval-augmented generation approach to access the latest clinical guidelines during inference. The documentation agent uses another advanced language model, fine-tuned on a large corpus of high-quality medical documentation, implementing controlled text generation techniques and utilizing a separate smaller model for real-time error checking and correction.
Interagent Quality Control
Agents learn from their own experience and the experience of other agents. They are equipped with user-defined rule-based and model-based systems for quality assurance, with clear stopping rules for human involvement and mitigation.
Sophisticated quality control measures bolster the system’s reliability, including ensemble techniques for result comparison, redundancy for critical tasks, and automatic human review for disagreements above a certain threshold. Each agent provides a calibrated confidence score with its output, used to weigh inputs in downstream tasks and trigger additional checks for low-confidence outputs.
A dedicated quality control agent monitors output from all agents, employing both supervised and unsupervised anomaly detection techniques. Feedback loops allow agents to evaluate the quality and utility of information received from other agents. The system implements a multiarmed bandit approach to dynamically adjust the influence of different agents based on their performance and periodically retrains agent models using federated learning techniques.19
Electronic Health Record Integration
Seamless EHR integration is crucial for practical implementation. The system has secure application programming interface access to various EHR platforms, implements OAuth 2.0 for authentication, and use HTTPS with perfect forward secrecy for all communications.20 It works with HL7 FHIR to ensure interoperability and uses SNOMED CT for clinical terminology to ensure semantic interoperability across different EHRs.21,22
The system implements a multilevel approval system for write-backs to EHRs, with different thresholds based on the information’s criticality. It uses digital signatures to ensure the integrity and nonrepudiation of AI-generated entries and implements blockchain technology to create an immutable and distributed ledger of all AI system actions.23
Decision Transparency
To ensure transparency in decision-making processes, the system applies techniques (eg, local interpretable model-agnostic explanations and Shapley additive explanations) to provide insights into agent decision-making processes.24-26 It provides customized visualizations for different stakeholders and allows users to explore alternative decision paths through what-if scenario modeling.27
The system provides calibrated confidence indicators for each recommendation or decision, implementing a novel confidence calibration agent that continuously monitors and adjusts confidence scores based on observed outcomes.
Continuous Learning and Adaptation
The system employs several techniques to remain current with evolving medical knowledge. Federated learning includes information from diverse datasets across multiple institutions without compromising patient privacy.28 A/B testing is used to safely deploy and compare new agent versions in controlled settings, implementing multiarmed bandit algorithms to efficiently explore new models while minimizing potential negative impacts. Human-in-the-loop learning and active learning techniques are used to incorporate feedback from HCPs and efficiently solicit expert input on the most informative data.29
CLINICAL IMPLICATIONS
The implementation of multiagent AI systems in health care has several potential benefits: enhanced diagnostic accuracy, personalized treatment, improved efficiency, continuous monitoring, and resource optimization. A recent review of AI sepsis predictive models exhibited superior results to standard clinical scoring methods like qSOFA.30 In oncology, such systems can result in more tailored treatments, enhancing outcomes.31 The implementation of an ambient dictation system can improve workflow and prevent HCP burnout.32
ETHICAL CONSIDERATIONS AND AI OVERSIGHT
Integrating AI agents into health care raises significant ethical considerations that must be carefully addressed to ensure equitable and effective care delivery. One primary concern involves cultural and linguistic competency, as AI systems may struggle with cultural nuances, idioms, and context-specific communication patterns. This becomes particularly challenging in regions with diverse ethnic populations or immigrant communities, where medical terminology may not have direct translations and cultural beliefs significantly influence health care decisions. AI systems also may inherit and amplify existing biases in health care delivery, whether through HCP bias reflected in training data, patient bias affecting acceptance of AI-assisted care, or demographic underrepresentation during system development.
AI agents present unique opportunities for improving health care access and outcomes through community engagement, though such initiatives require thoughtful implementation. Predictive analytics can identify high-risk individuals within communities who may benefit from preventive care, while analysis of social determinants of health can enable more targeted interventions. However, these capabilities must be balanced with privacy concerns and the risk of surveillance, particularly in communities that distrust health care institutions. The potential for AI to bridge health care gaps must be weighed against the need to maintain cultural sensitivity and community trust.
The governance and oversight of health care AI systems requires a multistakeholder approach with clear lines of responsibility and accountability. This includes involvement from government health care agencies, professional medical associations, ethics boards, and independent auditors, all working together to establish and enforce standards while monitoring system performance and addressing potential biases. Health care organizations must maintain transparent policies about AI use, implement regular monitoring and evaluation protocols, and establish precise mechanisms for patient feedback and grievance resolution. Ongoing assessment and adjustment of these systems, informed by community feedback and outcomes data, will be crucial for their ethical implementation, ensuring that AI agents complement, rather than replace, human judgment and cultural sensitivity.
FUTURE DIRECTIONS
Despite the potential benefits, implementing multiagent AI systems in health care faces significant challenges that require careful consideration. Beyond the fundamental issues such as data quality and bias mitigation, health care organizations struggle with fragmented systems, inconsistent data formats, and varying quality. Technical infrastructure requirements are substantial, particularly in rural or underserved areas that lack robust networks and cybersecurity. HCPs already face significant cognitive load and time pressures, making integrating AI agents into existing workflows particularly challenging. There is also the critical issue of transparency and interpretability, as health care decisions require clear reasoning and accountability that many black-box AI systems struggle to provide.
The legal landscape introduces another layer of complexity, particularly regarding liability, consent, and privacy questions. When AI agents contribute to medical decisions, establishing clear lines of responsibility becomes crucial. There are also serious concerns about algorithmic fairness and the potential for AI systems to perpetuate or amplify existing inequities. The cost of implementation remains a significant barrier, requiring substantial investment in technology, training, and ongoing maintenance while ensuring resources are not diverted from direct patient care. Moreover, HCPs may resist adoption due to concerns about job security, loss of autonomy, or skepticism about AI capabilities while paradoxically facing risks of overreliance on AI systems that could lead to the degradation of human clinical skills.
Addressing these challenges requires a multifaceted approach that combines technical solutions with organizational and policy changes. Health care organizations must implement rigorous data validation processes and interoperability standards while developing hybrid models that balance sophisticated AI capabilities with interpretable techniques. Extensive research and iterative design processes, with direct input from HCPs, are essential for successful integration. Establishing independent ethics boards to oversee system development and deployment, conducting multicenter randomized controlled trials, and creating clear regulatory frameworks will ensure safe and effective implementation. Success will ultimately depend on ongoing collaboration between technology developers, HCPs, policymakers, and patients, maintaining a steady focus on improving patient care and outcomes while carefully navigating the complex challenges of AI integration in health care.33-35
As multiagent AI systems in health care evolve, several exciting directions emerge. These include the integration of IoT and wearable devices, the development of more sophisticated natural language interfaces, and applying these systems to predictive maintenance of medical equipment.
CONCLUSIONS
The advent of multiagent AI systems in health care represents a paradigm shift in the approach to patient care, clinical decision making, and health care management. While these systems offer immense potential to transform health care delivery, their development and implementation must be guided by rigorous scientific validation, ethical considerations, and a patient-centered approach. The ultimate goal remains clear: harnessing the power of AI to improve patient outcomes, enhance the efficiency of health care delivery, and ultimately advance the health and well-being of patients.
Artificial intelligence (AI) is rapidly evolving, with large language models (LLMs) marking a significant milestone in processing and generating human-like responses to natural language prompts. However, this advancement only signals the beginning of a more profound transformation in AI capabilities. The development of AI agents represents a new paradigm at the forefront of this evolution.
BACKGROUND
AI agents represent a leap forward from traditional LLM applications. While definitions may vary slightly among technology developers, the core concept remains: these agents are autonomous software entities designed to interact with their environment, make independent decisions, and execute tasks based on predefined goals.1-3 What sets AI agents apart is their combination of sophisticated components within structured architectures. At their core, AI agents incorporate an LLM for response generation, which is augmented by a suite of tools to optimize workflow and complete tasks, memory capabilities for personalized interactions, and autonomous reasoning. This combination allows AI agents to plan, create subtasks, gather information, and learn iteratively from their own experiences or other AI agents.
The true potential of this technology becomes apparent when multiple AI agents collaborate within multiagent AI systems. This concept introduces a new level of flexibility and capability in tackling complex tasks. Autogen, CrewAI, and LangChain offer various agent network configurations, including hierarchical, sequential, conditional, or even parallel task execution.4-6 This adaptability opens up a world of possibilities across various industries, but perhaps nowhere is the potential impact more exciting and profound than in health care.
AI agents in health care present an opportunity to revolutionize patient care, streamline administrative processes, and support complex clinical decision-making. This review examines 3 scenarios that illustrate the impact of AI agents in health care: a hypothetical sepsis management system, chronic disease management, and hospital patient flow optimization. This article will provide a detailed look at the technical implementation challenges, including the integration with existing health care IT systems, data privacy considerations, and the crucial role of explainable AI in maintaining trust and transparency.
It is challenging to implement AI agents in health care. Concerns include ensuring data quality and mitigating bias, seamlessly integrating these systems into existing clinical workflows, and navigating the complex ethical considerations that arise when deploying autonomous systems in health care. The integration with Internet of Things (IoT) devices for real-time patient data monitoring and the development of more sophisticated natural language interfaces to enhance future human-AI collaboration.
The adoption of AI agents in health care is only beginning, and it promises to be transformative. As AI continues to evolve, a comprehensive understanding of its applications, limitations, and ethical considerations is essential. This report provides a comprehensive overview of the current state, potential applications, and future directions of AI agents in health care, offering insights valuable to researchers, clinicians, and policymakers.
MultiAgent AI architecture
Sepsis Management
Despite advancements in broad-spectrum antibiotics, imaging, and life support systems, mortality rates associated with sepsis remain high. The complexity of optimizing care in clinical settings has hindered progress in managing sepsis. Previous attempts to develop predictive sepsis models have proven challenging.7 This report proposes a multiagent AI system designed to enhance comprehensive patient monitoring and care through coordinated AI-driven interventions.
Data Collection and Integration Agent. Powered by a controlled vocabulary to specify all data, the primary function for the data collection and integration agent is to clean, transform, and organize patient data from structured and unstructured sources. This agent prepares succinct summaries of consultant notes and formats data for human and machine consumption. All numerical data are presented graphically, including relevant historical data trends. The agent also digitally captures all orders in a structured format using a specified controlled vocabulary. This structured data feed supports the output of other agents, including documentation, treatment planning, and risk stratification, while also supplying the data structures for future training.
Diagnostic Agent. Critical illness is characterized by multiple abnormalities across a wide array of tests, ranging from plain chest X-ray, computed tomography (CT), blood cell composition, plasma chemistry, and microscopic evaluation of specimens. Additionally, life support parameters provide insights into disease severity and can inform management recommendations. These data offer a wide array of visual and numerical data to be used as input for computation, recommendation, and further training. For example, to evaluate fluid overload on chest X-rays or tissue histopathology slides, an AI agent can leverage deep learning models such as convolutional neural networks and vision transformers to analyze images like radiographs and histopathology slides.8,9 Recurrent neural networks or transformer models process sequential data like time-series vital signs. The agent also implements ensemble methods that combine multiple machine learning algorithms to enhance diagnostic accuracy.
Risk Stratification Agent. This assesses severity and predicts potential outcomes. Morbidity and mortality risks are calculated using an established scoring system and individualized based on the history of other agents’ conditional patients. These are presented graphically, with major risk factors highlighted for explainability.
Treatment Recommendation Agent. Using a reinforcement learning framework supplemented by up-to-date clinical guidelines, this system leverages historical data structured with standardized vocabulary to analyze patients with similar clinical features. Training is also conducted on the patient’s physiological data. All recommendations are presented via a dedicated user interface in a readable format, along with recommendations for editable, orderable items, references, and full-text snippets from previous research. Stop rules end computing if confidence in recommendations is too broad or no clear pathway can be computed with certainty, prompting human mitigation.
Resource Management Agent. This agent coordinates hospital resources using constraint programming techniques for optimal resource allocation, uses queueing theory models to predict and manage patient flow, and implements genetic algorithms for complex scheduling problems.10,11
Monitoring and Alert Agent. By tracking patients’ progress and alerting staff to changes, this agent uses anomaly detection algorithms to identify unusual patterns in patient data and implement time-series forecasting models, such as autoregressive integrated moving average and prophet, to predict future patient states. The agent also uses stream-processing techniques for real-time data analysis.12,13
Documentation and Reporting Agent. This agent maintains comprehensive medical records and generates reports. It employs advanced natural language processing techniques for automated report generation, uses advanced LLMs fine-tuned on medical corpora for narrative creation, and implements information-retrieval techniques to efficiently query patient records.
CLINICAL CASE STUDIES
To illustrate the functionality of a multiagent system, this report examines its application for managing sepsis. The data collection and integration agent continuously aggregates patient data from various sources, normalizing and timestamping it for consistent processing. The diagnostic agent analyzes this integrated data in real time, applying sepsis criteria and utilizing a deep learning model trained on a large sepsis dataset to detect subtle patterns.
The risk stratification agent calculates severity scores, such as the Sepsis-related Organ Failure Assessment (SOFA), quick SOFA (qSOFA), and Acute Physiology and Chronic Health Evaluation II, upon detecting a possible sepsis case.14 It predicts the likelihood of specific outcomes and estimates the potential trajectory of the patient’s condition for the next 24 to 48 hours. Based on this assessment, the treatment recommendation agent suggests an initial treatment plan, including appropriate antibiotics, fluid resuscitation protocols, and vasopressor recommendations, recommendations when indicated.
Concurrently, the resource management agent checks the availability of necessary resources and prioritizes allocation based on the severity. The monitoring agent tracks the patient’s response to interventions in real time, alerting the care team to any concerning changes or lack of expected improvement. Throughout this process, the documentation agent ensures that all actions, responses, and outcomes are meticulously recorded in a structured format and generates real-time updates for the patient’s electronic health record (EHR) and preparing summary reports for handoffs between care teams.
Administrative Workflow Support
Modern health care operations are resource-intensive, requiring coordination of advanced imaging, procedures, laboratory testing, and professional consultations.15 AI-powered health care administrative workflow systems are revolutionizing how medical facilities coordinate patient care. For patients with chronic cough, these systems seamlessly integrate scheduling, imaging, diagnostics, and follow-up care into a cohesive process that reduces administrative burden while improving patient outcomes. Through an intuitive interface and automated assistance, health care practitioners (HCPs) can track patient progress from initial consultation through diagnosis and treatment.
The process begins when an HCP enters a patient into the system, which triggers an automated CT scan scheduling system. The system considers factors like urgency, facility availability, and patient preferences to suggest optimal appointment times. Once imaging is complete, AI agents analyze the radiology reports, extract key findings, and generate structured summaries that highlight critical information such as “mild bronchial wall thickening with patchy ground-glass opacities” or “findings consistent with chronic bronchitis.”
Based on these findings, the system automatically generates evidence-based recommendations for follow-up care, such as pulmonology consultations or follow-up imaging in 3 months. These recommendations are presented to the ordering clinician, along with suggested appointment slots for specialist consultations. The system then manages the coordination of multiple appointments, ensuring each step in the patient’s care plan is properly sequenced and scheduled.
The entire process is monitored through a comprehensive dashboard that provides real-time updates on patient status, appointment schedules, and clinical recommendations. HCPs can track which patients require immediate attention, view upcoming appointments, and monitor the progress of ongoing care plans.
Multiagent AI Operation Optimization
Hospitals are complex entities that must function at different scales and respond in an agile, timely manner at all hours, deploying staff at various positions.16 A system of AI agents can receive signals from sensors monitoring foot traffic in the emergency department and trauma unit, as well as the availability of operating room staff, equipment, and intensive care unit beds. Smart sensors enable this monitoring through IoT networks. These networks benefit from advances in adaptive and consensus networking algorithms, along with recent advances in bioengineering and biocomputing.17
For example, in the case of imaging for suspected abdominal obstruction, an AI agent tasked with scheduling CTs could time the patient’s arrival based on acuity. Another AI agent could alert staff transporting the patient to the CT appointment, with the next location contingent on a clinical decision to proceed to the operating room. Yet another AI agent could summarize radiology interpretations and alert the surgery and anesthesia teams to a potential case, while others could notify operating room staff of equipment needs or reserve a bed. In this paradigm, AI agents facilitate more precise and timely communication between multiple staff members.
TECHNICAL IMPLEMENTATION
Large Language Models
Each agent uses a different LLM optimized for its specific task. For example, the diagnostic agent uses an LLM pretrained on a large corpus of biomedical literature and fine-tuned on a dataset of confirmed sepsis cases and their presentations.18 It implements few-shot learning techniques to adapt to rare or atypical presentations. The treatment recommendation agent also uses an LLM, employing a retrieval-augmented generation approach to access the latest clinical guidelines during inference. The documentation agent uses another advanced language model, fine-tuned on a large corpus of high-quality medical documentation, implementing controlled text generation techniques and utilizing a separate smaller model for real-time error checking and correction.
Interagent Quality Control
Agents learn from their own experience and the experience of other agents. They are equipped with user-defined rule-based and model-based systems for quality assurance, with clear stopping rules for human involvement and mitigation.
Sophisticated quality control measures bolster the system’s reliability, including ensemble techniques for result comparison, redundancy for critical tasks, and automatic human review for disagreements above a certain threshold. Each agent provides a calibrated confidence score with its output, used to weigh inputs in downstream tasks and trigger additional checks for low-confidence outputs.
A dedicated quality control agent monitors output from all agents, employing both supervised and unsupervised anomaly detection techniques. Feedback loops allow agents to evaluate the quality and utility of information received from other agents. The system implements a multiarmed bandit approach to dynamically adjust the influence of different agents based on their performance and periodically retrains agent models using federated learning techniques.19
Electronic Health Record Integration
Seamless EHR integration is crucial for practical implementation. The system has secure application programming interface access to various EHR platforms, implements OAuth 2.0 for authentication, and use HTTPS with perfect forward secrecy for all communications.20 It works with HL7 FHIR to ensure interoperability and uses SNOMED CT for clinical terminology to ensure semantic interoperability across different EHRs.21,22
The system implements a multilevel approval system for write-backs to EHRs, with different thresholds based on the information’s criticality. It uses digital signatures to ensure the integrity and nonrepudiation of AI-generated entries and implements blockchain technology to create an immutable and distributed ledger of all AI system actions.23
Decision Transparency
To ensure transparency in decision-making processes, the system applies techniques (eg, local interpretable model-agnostic explanations and Shapley additive explanations) to provide insights into agent decision-making processes.24-26 It provides customized visualizations for different stakeholders and allows users to explore alternative decision paths through what-if scenario modeling.27
The system provides calibrated confidence indicators for each recommendation or decision, implementing a novel confidence calibration agent that continuously monitors and adjusts confidence scores based on observed outcomes.
Continuous Learning and Adaptation
The system employs several techniques to remain current with evolving medical knowledge. Federated learning includes information from diverse datasets across multiple institutions without compromising patient privacy.28 A/B testing is used to safely deploy and compare new agent versions in controlled settings, implementing multiarmed bandit algorithms to efficiently explore new models while minimizing potential negative impacts. Human-in-the-loop learning and active learning techniques are used to incorporate feedback from HCPs and efficiently solicit expert input on the most informative data.29
CLINICAL IMPLICATIONS
The implementation of multiagent AI systems in health care has several potential benefits: enhanced diagnostic accuracy, personalized treatment, improved efficiency, continuous monitoring, and resource optimization. A recent review of AI sepsis predictive models exhibited superior results to standard clinical scoring methods like qSOFA.30 In oncology, such systems can result in more tailored treatments, enhancing outcomes.31 The implementation of an ambient dictation system can improve workflow and prevent HCP burnout.32
ETHICAL CONSIDERATIONS AND AI OVERSIGHT
Integrating AI agents into health care raises significant ethical considerations that must be carefully addressed to ensure equitable and effective care delivery. One primary concern involves cultural and linguistic competency, as AI systems may struggle with cultural nuances, idioms, and context-specific communication patterns. This becomes particularly challenging in regions with diverse ethnic populations or immigrant communities, where medical terminology may not have direct translations and cultural beliefs significantly influence health care decisions. AI systems also may inherit and amplify existing biases in health care delivery, whether through HCP bias reflected in training data, patient bias affecting acceptance of AI-assisted care, or demographic underrepresentation during system development.
AI agents present unique opportunities for improving health care access and outcomes through community engagement, though such initiatives require thoughtful implementation. Predictive analytics can identify high-risk individuals within communities who may benefit from preventive care, while analysis of social determinants of health can enable more targeted interventions. However, these capabilities must be balanced with privacy concerns and the risk of surveillance, particularly in communities that distrust health care institutions. The potential for AI to bridge health care gaps must be weighed against the need to maintain cultural sensitivity and community trust.
The governance and oversight of health care AI systems requires a multistakeholder approach with clear lines of responsibility and accountability. This includes involvement from government health care agencies, professional medical associations, ethics boards, and independent auditors, all working together to establish and enforce standards while monitoring system performance and addressing potential biases. Health care organizations must maintain transparent policies about AI use, implement regular monitoring and evaluation protocols, and establish precise mechanisms for patient feedback and grievance resolution. Ongoing assessment and adjustment of these systems, informed by community feedback and outcomes data, will be crucial for their ethical implementation, ensuring that AI agents complement, rather than replace, human judgment and cultural sensitivity.
FUTURE DIRECTIONS
Despite the potential benefits, implementing multiagent AI systems in health care faces significant challenges that require careful consideration. Beyond the fundamental issues such as data quality and bias mitigation, health care organizations struggle with fragmented systems, inconsistent data formats, and varying quality. Technical infrastructure requirements are substantial, particularly in rural or underserved areas that lack robust networks and cybersecurity. HCPs already face significant cognitive load and time pressures, making integrating AI agents into existing workflows particularly challenging. There is also the critical issue of transparency and interpretability, as health care decisions require clear reasoning and accountability that many black-box AI systems struggle to provide.
The legal landscape introduces another layer of complexity, particularly regarding liability, consent, and privacy questions. When AI agents contribute to medical decisions, establishing clear lines of responsibility becomes crucial. There are also serious concerns about algorithmic fairness and the potential for AI systems to perpetuate or amplify existing inequities. The cost of implementation remains a significant barrier, requiring substantial investment in technology, training, and ongoing maintenance while ensuring resources are not diverted from direct patient care. Moreover, HCPs may resist adoption due to concerns about job security, loss of autonomy, or skepticism about AI capabilities while paradoxically facing risks of overreliance on AI systems that could lead to the degradation of human clinical skills.
Addressing these challenges requires a multifaceted approach that combines technical solutions with organizational and policy changes. Health care organizations must implement rigorous data validation processes and interoperability standards while developing hybrid models that balance sophisticated AI capabilities with interpretable techniques. Extensive research and iterative design processes, with direct input from HCPs, are essential for successful integration. Establishing independent ethics boards to oversee system development and deployment, conducting multicenter randomized controlled trials, and creating clear regulatory frameworks will ensure safe and effective implementation. Success will ultimately depend on ongoing collaboration between technology developers, HCPs, policymakers, and patients, maintaining a steady focus on improving patient care and outcomes while carefully navigating the complex challenges of AI integration in health care.33-35
As multiagent AI systems in health care evolve, several exciting directions emerge. These include the integration of IoT and wearable devices, the development of more sophisticated natural language interfaces, and applying these systems to predictive maintenance of medical equipment.
CONCLUSIONS
The advent of multiagent AI systems in health care represents a paradigm shift in the approach to patient care, clinical decision making, and health care management. While these systems offer immense potential to transform health care delivery, their development and implementation must be guided by rigorous scientific validation, ethical considerations, and a patient-centered approach. The ultimate goal remains clear: harnessing the power of AI to improve patient outcomes, enhance the efficiency of health care delivery, and ultimately advance the health and well-being of patients.
Amazon Web Services, Inc. What are AI agents? Agents in artificial intelligence explained. Accessed April 7, 2025. https://aws.amazon.com/what-is/ai-agents/
Gutowska A. What are AI agents? IBM. Accessed April 7, 2025. https://www.ibm.com/think/topics/ai-agents
Agent AI. Microsoft Research. Accessed April 7, 2025. https://www.microsoft.com/en-us/research/project/agent-ai
Microsoft. AutoGen. Accessed April 7, 2025. https://microsoft.github.io/autogen/
Crew AI. The Leading Multi-Agent Platform. CrewAI. Accessed April 7, 2025. https://www.crewai.com/
LangChain. Accessed April 7, 2025. https://www.langchain.com/
Wong A, Otles E, Donnelly JP, et al. External validation of a widely implemented proprietary sepsis prediction model in hospitalized patients. JAMA Intern Med. 2021;181(8):1065-1070. doi:10.1001/jamainternmed.2021.2626
Willemink MJ, Roth HR, Sandfort V. Toward foundational deep learning models for medical imaging in the new era of transformer networks. Radiol Artif Intell. 2022;4(6):e210284. doi:10.1148/ryai.210284
Waqas A, Bui MM, Glassy EF, et al. Revolutionizing digital pathology with the power of generative artificial intelligence and foundation models. Lab Invest. 2023;103(11):100255. doi:10.1016/j.labinv.2023.100255
Moreno-Carrillo A, Arenas LMÁ, Fonseca JA, Caicedo CA, Tovar SV, Muñoz-Velandia OM. Application of queuing theory to optimize the triage process in a tertiary emergency care (“ER”) department. J Emerg Trauma Shock. 2019;12(4):268-273. doi:10.4103/JETS.JETS_42_19
Pongcharoen P, Hicks C, Braiden PM, Stewardson DJ. Determining optimum genetic algorithm parameters for scheduling the manufacturing and assembly of complex products. Int J Prod Econ. 2002;78(3):311-322. doi:10.1016/S0925-5273(02)00104-4
Sardar I, Akbar MA, Leiva V, Alsanad A, Mishra P. Machine learning and automatic ARIMA/Prophet models-based forecasting of COVID-19: methodology, evaluation, and case study in SAARC countries. Stoch Environ Res Risk Assess. 2023;37(1):345-359. doi:10.1007/s00477-022-02307-x
Samosir J, Indrawan-Santiago M, Haghighi PD. An evaluation of data stream processing systems for data driven applications. Procedia Comput Sci. 2016;80:439-449. doi:10.1016/j.procs.2016.05.322
Asmarawati TP, Suryantoro SD, Rosyid AN, et al. Predictive value of sequential organ failure assessment, quick sequential organ failure assessment, acute physiology and chronic health evaluation II, and new early warning signs scores estimate mortality of COVID-19 patients requiring intensive care unit. Indian J Crit Care Med. 2022;26(4):466-473. doi:10.5005/jp-journals-10071-24170
Khan S, Vandermorris A, Shepherd J, et al. Embracing uncertainty, managing complexity: applying complexity thinking principles to transformation efforts in healthcare systems. BMC Health Serv Res. 2018;18(1):192. doi:10.1186/s12913-018-2994-0
Plsek PE, Greenhalgh T. The challenge of complexity in health care. BMJ. 2001;323(7313):625-628. doi:10.1136/bmj.323.7313.625
Kouchaki S, Ding X, Sanei S. AI- and IoT-enabled solutions for healthcare. Sensors. 2024;24(8):2607. doi:10.3390/s24082607
Saab K, Tu T, Weng WH, et al. Capabilities of Gemini Models in Medicine. arXiv. doi:10.48550/arXiv.2404.18416
Villar SS, Bowden J, Wason J. Multi-armed bandit models for the optimal design of clinical trials: benefits and challenges. Stat Sci. 2015;30(2):199-215. doi:10.1214/14-STS504
Auth0. What is OAuth 2.0. Accessed April 7, 2025. https://auth0.com/intro-to-iam/what-is-oauth-2
HL7. Welcome to FHIR. Updated March 26, 2025. Accessed April 7, 2025. https://www.hl7.org/fhir/
SNOMED International. Accessed April 7, 2025. https://www.snomed.org
Hasselgren A, Kralevska K, Gligoroski D, Pedersen SA, Faxvaag A. Blockchain in healthcare and health sciences—a scoping review. Int J Med Inf. 2020;134:104040. doi:10.1016/j.ijmedinf.2019.104040
Ribeiro MT, Singh S, Guestrin C. “Why Should I Trust You?”: Explaining the predictions of any classifier. In: Proceedings of the 22nd ACM SIGKDD international conference on knowledge discovery and data mining. 2016:1135-1144. doi:10.1145/2939672.2939778
Ekanayake IU, Meddage DPP, Rathnayake U. A novel approach to explain the black-box nature of machine learning in compressive strength predictions of concrete using Shapley additive explanations (SHAP). Case Stud Constr Mater. 2022;16:e01059. doi:10.1016/j.cscm.2022.e01059
Alabi RO, Elmusrati M, Leivo I, Almangush A, Mäkitie AA. Machine learning explainability in nasopharyngeal cancer survival using LIME and SHAP. Sci Rep. 2023;13(1):8984. doi:10.1038/s41598-023-35795-0
Otto E, Culakova E, Meng S, et al. Overview of sankey flow diagrams: focusing on symptom trajectories in older adults with advanced cancer. J Geriatr Oncol. 2022;13(5):742-746. doi:10.1016/j.jgo.2021.12.017
Fereidooni H, Marchal S, Miettinen M, et al. SAFELearn: secure aggregation for private federated learning. In: 2021 IEEE security and privacy workshops (SPW). 2021:56-62. doi:10.1109/SPW53761.2021.00017
Linton DL, Pangle WM, Wyatt KH, Powell KN, Sherwood RE. Identifying key features of effective active learning: the effects of writing and peer discussion. Life Sci Educ. 2014;13(3):469-477. doi:10.1187/cbe.13-12-0242
Yang HS. Machine learning for sepsis prediction: prospects and challenges. Clin Chem. 2024;70(3):465-467. doi:10.1093/clinchem/hvae006
Liao J, Li X, Gan Y, et al. Artificial intelligence assists precision medicine in cancer treatment. Front Oncol. 2023;12. doi:10.3389/fonc.2022.998222
Tierney AA, Gayre G, Hoberman B, et al. Ambient artificial intelligence scribes to alleviate the burden of clinical documentation. NEJM Catal. 2024;5(3):CAT.23.0404. doi:10.1056/CAT.23.0404
Borkowski AA, Jakey CE, Thomas LB, Viswanadhan N, Mastorides SM. Establishing a hospital artificial intelligence committee to improve patient care. Fed Pract. 2022;39(8):334-336. doi:10.12788/fp.0299
Isaacks DB, Borkowski AA. Implementing trustworthy AI in VA high reliability health care organizations. Fed Pract.2024;41(2):40-43. doi:10.12788/fp.0454
Han R, Acosta JN, Shakeri Z, Ioannidis JPA, Topol EJ, Rajpurkar P. Randomized controlled trials evaluating artificial intelligence in clinical practice: a scoping review. Lancet Digit Health. 2024;6(5):e367-e373. doi:10.1016/S2589-7500(24)00047-5
Amazon Web Services, Inc. What are AI agents? Agents in artificial intelligence explained. Accessed April 7, 2025. https://aws.amazon.com/what-is/ai-agents/
Gutowska A. What are AI agents? IBM. Accessed April 7, 2025. https://www.ibm.com/think/topics/ai-agents
Agent AI. Microsoft Research. Accessed April 7, 2025. https://www.microsoft.com/en-us/research/project/agent-ai
Microsoft. AutoGen. Accessed April 7, 2025. https://microsoft.github.io/autogen/
Crew AI. The Leading Multi-Agent Platform. CrewAI. Accessed April 7, 2025. https://www.crewai.com/
LangChain. Accessed April 7, 2025. https://www.langchain.com/
Wong A, Otles E, Donnelly JP, et al. External validation of a widely implemented proprietary sepsis prediction model in hospitalized patients. JAMA Intern Med. 2021;181(8):1065-1070. doi:10.1001/jamainternmed.2021.2626
Willemink MJ, Roth HR, Sandfort V. Toward foundational deep learning models for medical imaging in the new era of transformer networks. Radiol Artif Intell. 2022;4(6):e210284. doi:10.1148/ryai.210284
Waqas A, Bui MM, Glassy EF, et al. Revolutionizing digital pathology with the power of generative artificial intelligence and foundation models. Lab Invest. 2023;103(11):100255. doi:10.1016/j.labinv.2023.100255
Moreno-Carrillo A, Arenas LMÁ, Fonseca JA, Caicedo CA, Tovar SV, Muñoz-Velandia OM. Application of queuing theory to optimize the triage process in a tertiary emergency care (“ER”) department. J Emerg Trauma Shock. 2019;12(4):268-273. doi:10.4103/JETS.JETS_42_19
Pongcharoen P, Hicks C, Braiden PM, Stewardson DJ. Determining optimum genetic algorithm parameters for scheduling the manufacturing and assembly of complex products. Int J Prod Econ. 2002;78(3):311-322. doi:10.1016/S0925-5273(02)00104-4
Sardar I, Akbar MA, Leiva V, Alsanad A, Mishra P. Machine learning and automatic ARIMA/Prophet models-based forecasting of COVID-19: methodology, evaluation, and case study in SAARC countries. Stoch Environ Res Risk Assess. 2023;37(1):345-359. doi:10.1007/s00477-022-02307-x
Samosir J, Indrawan-Santiago M, Haghighi PD. An evaluation of data stream processing systems for data driven applications. Procedia Comput Sci. 2016;80:439-449. doi:10.1016/j.procs.2016.05.322
Asmarawati TP, Suryantoro SD, Rosyid AN, et al. Predictive value of sequential organ failure assessment, quick sequential organ failure assessment, acute physiology and chronic health evaluation II, and new early warning signs scores estimate mortality of COVID-19 patients requiring intensive care unit. Indian J Crit Care Med. 2022;26(4):466-473. doi:10.5005/jp-journals-10071-24170
Khan S, Vandermorris A, Shepherd J, et al. Embracing uncertainty, managing complexity: applying complexity thinking principles to transformation efforts in healthcare systems. BMC Health Serv Res. 2018;18(1):192. doi:10.1186/s12913-018-2994-0
Plsek PE, Greenhalgh T. The challenge of complexity in health care. BMJ. 2001;323(7313):625-628. doi:10.1136/bmj.323.7313.625
Kouchaki S, Ding X, Sanei S. AI- and IoT-enabled solutions for healthcare. Sensors. 2024;24(8):2607. doi:10.3390/s24082607
Saab K, Tu T, Weng WH, et al. Capabilities of Gemini Models in Medicine. arXiv. doi:10.48550/arXiv.2404.18416
Villar SS, Bowden J, Wason J. Multi-armed bandit models for the optimal design of clinical trials: benefits and challenges. Stat Sci. 2015;30(2):199-215. doi:10.1214/14-STS504
Auth0. What is OAuth 2.0. Accessed April 7, 2025. https://auth0.com/intro-to-iam/what-is-oauth-2
HL7. Welcome to FHIR. Updated March 26, 2025. Accessed April 7, 2025. https://www.hl7.org/fhir/
SNOMED International. Accessed April 7, 2025. https://www.snomed.org
Hasselgren A, Kralevska K, Gligoroski D, Pedersen SA, Faxvaag A. Blockchain in healthcare and health sciences—a scoping review. Int J Med Inf. 2020;134:104040. doi:10.1016/j.ijmedinf.2019.104040
Ribeiro MT, Singh S, Guestrin C. “Why Should I Trust You?”: Explaining the predictions of any classifier. In: Proceedings of the 22nd ACM SIGKDD international conference on knowledge discovery and data mining. 2016:1135-1144. doi:10.1145/2939672.2939778
Ekanayake IU, Meddage DPP, Rathnayake U. A novel approach to explain the black-box nature of machine learning in compressive strength predictions of concrete using Shapley additive explanations (SHAP). Case Stud Constr Mater. 2022;16:e01059. doi:10.1016/j.cscm.2022.e01059
Alabi RO, Elmusrati M, Leivo I, Almangush A, Mäkitie AA. Machine learning explainability in nasopharyngeal cancer survival using LIME and SHAP. Sci Rep. 2023;13(1):8984. doi:10.1038/s41598-023-35795-0
Otto E, Culakova E, Meng S, et al. Overview of sankey flow diagrams: focusing on symptom trajectories in older adults with advanced cancer. J Geriatr Oncol. 2022;13(5):742-746. doi:10.1016/j.jgo.2021.12.017
Fereidooni H, Marchal S, Miettinen M, et al. SAFELearn: secure aggregation for private federated learning. In: 2021 IEEE security and privacy workshops (SPW). 2021:56-62. doi:10.1109/SPW53761.2021.00017
Linton DL, Pangle WM, Wyatt KH, Powell KN, Sherwood RE. Identifying key features of effective active learning: the effects of writing and peer discussion. Life Sci Educ. 2014;13(3):469-477. doi:10.1187/cbe.13-12-0242
Yang HS. Machine learning for sepsis prediction: prospects and challenges. Clin Chem. 2024;70(3):465-467. doi:10.1093/clinchem/hvae006
Liao J, Li X, Gan Y, et al. Artificial intelligence assists precision medicine in cancer treatment. Front Oncol. 2023;12. doi:10.3389/fonc.2022.998222
Tierney AA, Gayre G, Hoberman B, et al. Ambient artificial intelligence scribes to alleviate the burden of clinical documentation. NEJM Catal. 2024;5(3):CAT.23.0404. doi:10.1056/CAT.23.0404
Borkowski AA, Jakey CE, Thomas LB, Viswanadhan N, Mastorides SM. Establishing a hospital artificial intelligence committee to improve patient care. Fed Pract. 2022;39(8):334-336. doi:10.12788/fp.0299
Isaacks DB, Borkowski AA. Implementing trustworthy AI in VA high reliability health care organizations. Fed Pract.2024;41(2):40-43. doi:10.12788/fp.0454
Han R, Acosta JN, Shakeri Z, Ioannidis JPA, Topol EJ, Rajpurkar P. Randomized controlled trials evaluating artificial intelligence in clinical practice: a scoping review. Lancet Digit Health. 2024;6(5):e367-e373. doi:10.1016/S2589-7500(24)00047-5
Should I Stay or Should I Go? Federal Health Care Professional Retirement Dilemmas
Should I Stay or Should I Go? Federal Health Care Professional Retirement Dilemmas
The uselessness of men above sixty years of age and the incalculable benefit it would be in commercial, in political, and in professional life, if as a matter of course, men stopped working at this age.
Sir William Osler1
The first time I remember hearing the word retirement was when I was 5 or 6 years old. My mother told me that my father had been given new orders: either be promoted to general and move to oversee a hospital somewhere far away, or retire from the Army. He was a scholar, teacher, and physician with no interest or aptitude for military politics and health care administration. Reluctantly, he resigned himself to retirement before he had planned. I recall being angry with him, because in my solipsistic child mind he was depriving me of the opportunity to live in a big house across from the parade field, where the generals lived or having a reserved parking spot in front of the post exchange. As a psychiatrist, I suspect that the anger was a primitive defense against the fear of leaving the only home I had ever known on an Army base.
I recently finished reading Michael Bliss’s seminal biography of Sir William Osler (1848-1919), the great Anglo-American physician and medical educator.2 Bliss found few blemishes on Osler’s character or missteps in his stellar career, but one of the few may be his views on retirement. The epigraph is from an address Osler gave before leaving Johns Hopkins for semiretirement in Oxford, England. The farewell speech caused a media controversy with his comments reflecting attitudes that seem ageist today, when many people are active, productive, and happy long past the age of 60 years.3 I do not endorse Osler’s philosophy of aging, nor his exclusion of women (if I did, I would not be around to write this editorial). Not even Osler himself followed his advice: he was active in medicine almost until his death at 70 years old.2
Yet like many of my fellow federal health care practitioners (HCPs), I have been thinking about and planning for retirement earlier than expected, given the memos and directives about voluntary early retirement, deferred resignation, and reductions in force.4,5 The COVID-19 pandemic sadly compelled many burned-out and traumatized HCPs to cross the retirement Rubicon far sooner than they imagined.6
A Google search for information about HCP retirement, particularly among physicians, produces a cascade of advisory articles. They primarily focus on finances, with many pushing their own commercial agenda for retirement planning.7 Although money is a necessary piece of the retirement puzzle, for HCPs it may not be sufficient to ensure a healthy and satisfying retirement. Two other considerations may be even more important to weigh in making the retirement decision, namely timing and meaning.8
For earlier generations of HCPs, work was almost their sole identity. Although younger practitioners are more likely to embrace a better work-life balance, it is still a driving factor for many in the decision to retire.9 It is not just about the cliché of being a workaholic, rather many clinicians continue to enjoy lifelong learning, the rewards of helping people in need, and professional satisfaction. HCPs also spend a longer time training than many other professions; perhaps since we waited so long to practice, we want to stay a little longer.10 For those whose motivation for federal practice was a commitment to service, these may be even more powerful incentives to continue working.
When a nurse, physician, pharmacist, or social worker no longer finds the same gratification and stimulation in their work, whether due to unwelcome changes in the clinical setting or the profession at large, declining health or emotional exhaustion, or the very human need to move onto another phase of life (what Osler likely really meant), then that may be a signal to think hard about retiring. Of course, there have always been—and will continue to be—professionals of all stripes who, even in the most agreeable situation, just cannot wait to retire. Simply because there are so many other ways they want to spend their remaining energy and time: travel, grandchildren, hobbies, even a second career. Because none of us knows how far out our life extends, it is prudent to periodically ask what is the optimal path that combines both purpose and well-being.
All of us as HCPs, and even more as human beings with desires and duties far beyond our respective professions, face a dilemma: a choice between 2 goods that cannot both be fulfilled simultaneously. This is likely why HCPs frequently do what is technically called a phased retirement, a fancy name for working part-time, or retiring from 1 position and taking up another. This temporizes the decision and tempers the bittersweet emotional experience of leaving the profession in one way, and in another, it delays the inevitable.
Over the last few years, I have learned 2 important lessons while watching many of my closest friends retire. First, for those who are still working and those who are retired may seem to inhabit a separate country; hence, special efforts must be made to both appreciate them while they are in our immediate circle of concern and to make efforts to stay in contact once they are emeriti. It is almost as if after being a daily integral aspect of the workplace they have passed into a different dimension of existence. In terms of priorities and mindsets, many of them have. Second, what makes retirement a reality with peace and growth rather than regret and stagnation is owning the decision to retire. There are always constraints: financial, medical, and familial. However, those who retire on their own terms and not primarily in response to fear or uncertainty appear to fare better than those feeling the same pressures who give away their power.11 Having read about retirement in the last months, the best advice I have seen is from Harry Emerson Fosdick, a Protestant minister in the early 20th century: “Don’t simply retire from something; have something to retire to.”12
I have not yet decided about my retirement. Whatever decision you make, remember it is solely yours. After a lifetime of caring for others, retirement is all about caring for yourself.
- Osler W. The Fixed Period. In: Osler W, ed. Aequanimitas With Other Addresses to Medical Students, Nurses and Practitioners of Medicine. 3rd ed. The Blakiston Company; 1932:373-393.
- Bliss M. William Osler: A Life in Medicine. Oxford University Press; 1999.
- Anderson M, Scofield RH. The “Fixed period,” the wildfire news, and an unpublished manuscript: Osler’s farewell speech revisited in geographical breadth and emotional depth. Am J Med Sci. Published online February 11, 2025. doi:10.1016/j.amjms.2025.02.005
- Obis A. What federal workers should consider before accepting deferred resignation. Federal News Network. April 8, 2025. Accessed April 25, 2025. https://federalnewsnetwork.com/workforce/2025/04/what-federal-workers-should-consider-before-accepting-deferred-resignation/
- Dyer J. VA exempts clinical staff from OPM deferred resignation program. Federal Practitioner. February 11, 2025. Accessed April 28, 2025. https://www.mdedge.com/content/va-exempts-clinical-staff-opm-deferred-resignation-program
- Shyrock T. Retirement planning secrets for physicians. Medical Economics. 2024;101(8). Accessed April 28, 2025. https:// www.medicaleconomics.com/view/retirement-planningsecrets-for-physicians
- Sinsky CA, Brown RL, Stillman MJ, Linzer M. COVID-related stress and work intentions in a sample of US health care workers. Mayo Clin Proc Innov Qual Outcomes. 2021;5(6):1165-1173. doi:10.1016/j.mayocpiqo.2021.08.007
- Tabloski PA. Life after retirement. American Nurse. March 3, 2022. Accessed April 25, 2025. https://www.myamericannurse.com/life-after-retirement/
- Chen T-P. Young doctors want work-life balance. Older doctors say that’s not the job. The Wall Street Journal. November 3, 2024. Accessed April 25, 2025. https://www.wsj.com/lifestyle/careers/young-doctors-want-work-life-balance-older-doctors-say-thats-not-the-job-6cb37d48
- Sweeny JF. Physician retirement: Why it’s hard for doctors to retire. Medical Economics. 2019;96(4). Accessed April 25, 2025. https://www.medicaleconomics.com/view/physician-retirement-why-its-hard-doctors-retire
- Nelson J. Wisdom for Our Time. W.W. Norton; 1961.
- Silver MP, Hamilton AD, Biswas A, Williams SA. Life after medicine: a systematic review of studies physician’s adjustment to retirement. Arch Community Med Public Health. 2016;2(1):001-007. doi:10.17352/2455-5479.000006
The uselessness of men above sixty years of age and the incalculable benefit it would be in commercial, in political, and in professional life, if as a matter of course, men stopped working at this age.
Sir William Osler1
The first time I remember hearing the word retirement was when I was 5 or 6 years old. My mother told me that my father had been given new orders: either be promoted to general and move to oversee a hospital somewhere far away, or retire from the Army. He was a scholar, teacher, and physician with no interest or aptitude for military politics and health care administration. Reluctantly, he resigned himself to retirement before he had planned. I recall being angry with him, because in my solipsistic child mind he was depriving me of the opportunity to live in a big house across from the parade field, where the generals lived or having a reserved parking spot in front of the post exchange. As a psychiatrist, I suspect that the anger was a primitive defense against the fear of leaving the only home I had ever known on an Army base.
I recently finished reading Michael Bliss’s seminal biography of Sir William Osler (1848-1919), the great Anglo-American physician and medical educator.2 Bliss found few blemishes on Osler’s character or missteps in his stellar career, but one of the few may be his views on retirement. The epigraph is from an address Osler gave before leaving Johns Hopkins for semiretirement in Oxford, England. The farewell speech caused a media controversy with his comments reflecting attitudes that seem ageist today, when many people are active, productive, and happy long past the age of 60 years.3 I do not endorse Osler’s philosophy of aging, nor his exclusion of women (if I did, I would not be around to write this editorial). Not even Osler himself followed his advice: he was active in medicine almost until his death at 70 years old.2
Yet like many of my fellow federal health care practitioners (HCPs), I have been thinking about and planning for retirement earlier than expected, given the memos and directives about voluntary early retirement, deferred resignation, and reductions in force.4,5 The COVID-19 pandemic sadly compelled many burned-out and traumatized HCPs to cross the retirement Rubicon far sooner than they imagined.6
A Google search for information about HCP retirement, particularly among physicians, produces a cascade of advisory articles. They primarily focus on finances, with many pushing their own commercial agenda for retirement planning.7 Although money is a necessary piece of the retirement puzzle, for HCPs it may not be sufficient to ensure a healthy and satisfying retirement. Two other considerations may be even more important to weigh in making the retirement decision, namely timing and meaning.8
For earlier generations of HCPs, work was almost their sole identity. Although younger practitioners are more likely to embrace a better work-life balance, it is still a driving factor for many in the decision to retire.9 It is not just about the cliché of being a workaholic, rather many clinicians continue to enjoy lifelong learning, the rewards of helping people in need, and professional satisfaction. HCPs also spend a longer time training than many other professions; perhaps since we waited so long to practice, we want to stay a little longer.10 For those whose motivation for federal practice was a commitment to service, these may be even more powerful incentives to continue working.
When a nurse, physician, pharmacist, or social worker no longer finds the same gratification and stimulation in their work, whether due to unwelcome changes in the clinical setting or the profession at large, declining health or emotional exhaustion, or the very human need to move onto another phase of life (what Osler likely really meant), then that may be a signal to think hard about retiring. Of course, there have always been—and will continue to be—professionals of all stripes who, even in the most agreeable situation, just cannot wait to retire. Simply because there are so many other ways they want to spend their remaining energy and time: travel, grandchildren, hobbies, even a second career. Because none of us knows how far out our life extends, it is prudent to periodically ask what is the optimal path that combines both purpose and well-being.
All of us as HCPs, and even more as human beings with desires and duties far beyond our respective professions, face a dilemma: a choice between 2 goods that cannot both be fulfilled simultaneously. This is likely why HCPs frequently do what is technically called a phased retirement, a fancy name for working part-time, or retiring from 1 position and taking up another. This temporizes the decision and tempers the bittersweet emotional experience of leaving the profession in one way, and in another, it delays the inevitable.
Over the last few years, I have learned 2 important lessons while watching many of my closest friends retire. First, for those who are still working and those who are retired may seem to inhabit a separate country; hence, special efforts must be made to both appreciate them while they are in our immediate circle of concern and to make efforts to stay in contact once they are emeriti. It is almost as if after being a daily integral aspect of the workplace they have passed into a different dimension of existence. In terms of priorities and mindsets, many of them have. Second, what makes retirement a reality with peace and growth rather than regret and stagnation is owning the decision to retire. There are always constraints: financial, medical, and familial. However, those who retire on their own terms and not primarily in response to fear or uncertainty appear to fare better than those feeling the same pressures who give away their power.11 Having read about retirement in the last months, the best advice I have seen is from Harry Emerson Fosdick, a Protestant minister in the early 20th century: “Don’t simply retire from something; have something to retire to.”12
I have not yet decided about my retirement. Whatever decision you make, remember it is solely yours. After a lifetime of caring for others, retirement is all about caring for yourself.
The uselessness of men above sixty years of age and the incalculable benefit it would be in commercial, in political, and in professional life, if as a matter of course, men stopped working at this age.
Sir William Osler1
The first time I remember hearing the word retirement was when I was 5 or 6 years old. My mother told me that my father had been given new orders: either be promoted to general and move to oversee a hospital somewhere far away, or retire from the Army. He was a scholar, teacher, and physician with no interest or aptitude for military politics and health care administration. Reluctantly, he resigned himself to retirement before he had planned. I recall being angry with him, because in my solipsistic child mind he was depriving me of the opportunity to live in a big house across from the parade field, where the generals lived or having a reserved parking spot in front of the post exchange. As a psychiatrist, I suspect that the anger was a primitive defense against the fear of leaving the only home I had ever known on an Army base.
I recently finished reading Michael Bliss’s seminal biography of Sir William Osler (1848-1919), the great Anglo-American physician and medical educator.2 Bliss found few blemishes on Osler’s character or missteps in his stellar career, but one of the few may be his views on retirement. The epigraph is from an address Osler gave before leaving Johns Hopkins for semiretirement in Oxford, England. The farewell speech caused a media controversy with his comments reflecting attitudes that seem ageist today, when many people are active, productive, and happy long past the age of 60 years.3 I do not endorse Osler’s philosophy of aging, nor his exclusion of women (if I did, I would not be around to write this editorial). Not even Osler himself followed his advice: he was active in medicine almost until his death at 70 years old.2
Yet like many of my fellow federal health care practitioners (HCPs), I have been thinking about and planning for retirement earlier than expected, given the memos and directives about voluntary early retirement, deferred resignation, and reductions in force.4,5 The COVID-19 pandemic sadly compelled many burned-out and traumatized HCPs to cross the retirement Rubicon far sooner than they imagined.6
A Google search for information about HCP retirement, particularly among physicians, produces a cascade of advisory articles. They primarily focus on finances, with many pushing their own commercial agenda for retirement planning.7 Although money is a necessary piece of the retirement puzzle, for HCPs it may not be sufficient to ensure a healthy and satisfying retirement. Two other considerations may be even more important to weigh in making the retirement decision, namely timing and meaning.8
For earlier generations of HCPs, work was almost their sole identity. Although younger practitioners are more likely to embrace a better work-life balance, it is still a driving factor for many in the decision to retire.9 It is not just about the cliché of being a workaholic, rather many clinicians continue to enjoy lifelong learning, the rewards of helping people in need, and professional satisfaction. HCPs also spend a longer time training than many other professions; perhaps since we waited so long to practice, we want to stay a little longer.10 For those whose motivation for federal practice was a commitment to service, these may be even more powerful incentives to continue working.
When a nurse, physician, pharmacist, or social worker no longer finds the same gratification and stimulation in their work, whether due to unwelcome changes in the clinical setting or the profession at large, declining health or emotional exhaustion, or the very human need to move onto another phase of life (what Osler likely really meant), then that may be a signal to think hard about retiring. Of course, there have always been—and will continue to be—professionals of all stripes who, even in the most agreeable situation, just cannot wait to retire. Simply because there are so many other ways they want to spend their remaining energy and time: travel, grandchildren, hobbies, even a second career. Because none of us knows how far out our life extends, it is prudent to periodically ask what is the optimal path that combines both purpose and well-being.
All of us as HCPs, and even more as human beings with desires and duties far beyond our respective professions, face a dilemma: a choice between 2 goods that cannot both be fulfilled simultaneously. This is likely why HCPs frequently do what is technically called a phased retirement, a fancy name for working part-time, or retiring from 1 position and taking up another. This temporizes the decision and tempers the bittersweet emotional experience of leaving the profession in one way, and in another, it delays the inevitable.
Over the last few years, I have learned 2 important lessons while watching many of my closest friends retire. First, for those who are still working and those who are retired may seem to inhabit a separate country; hence, special efforts must be made to both appreciate them while they are in our immediate circle of concern and to make efforts to stay in contact once they are emeriti. It is almost as if after being a daily integral aspect of the workplace they have passed into a different dimension of existence. In terms of priorities and mindsets, many of them have. Second, what makes retirement a reality with peace and growth rather than regret and stagnation is owning the decision to retire. There are always constraints: financial, medical, and familial. However, those who retire on their own terms and not primarily in response to fear or uncertainty appear to fare better than those feeling the same pressures who give away their power.11 Having read about retirement in the last months, the best advice I have seen is from Harry Emerson Fosdick, a Protestant minister in the early 20th century: “Don’t simply retire from something; have something to retire to.”12
I have not yet decided about my retirement. Whatever decision you make, remember it is solely yours. After a lifetime of caring for others, retirement is all about caring for yourself.
- Osler W. The Fixed Period. In: Osler W, ed. Aequanimitas With Other Addresses to Medical Students, Nurses and Practitioners of Medicine. 3rd ed. The Blakiston Company; 1932:373-393.
- Bliss M. William Osler: A Life in Medicine. Oxford University Press; 1999.
- Anderson M, Scofield RH. The “Fixed period,” the wildfire news, and an unpublished manuscript: Osler’s farewell speech revisited in geographical breadth and emotional depth. Am J Med Sci. Published online February 11, 2025. doi:10.1016/j.amjms.2025.02.005
- Obis A. What federal workers should consider before accepting deferred resignation. Federal News Network. April 8, 2025. Accessed April 25, 2025. https://federalnewsnetwork.com/workforce/2025/04/what-federal-workers-should-consider-before-accepting-deferred-resignation/
- Dyer J. VA exempts clinical staff from OPM deferred resignation program. Federal Practitioner. February 11, 2025. Accessed April 28, 2025. https://www.mdedge.com/content/va-exempts-clinical-staff-opm-deferred-resignation-program
- Shyrock T. Retirement planning secrets for physicians. Medical Economics. 2024;101(8). Accessed April 28, 2025. https:// www.medicaleconomics.com/view/retirement-planningsecrets-for-physicians
- Sinsky CA, Brown RL, Stillman MJ, Linzer M. COVID-related stress and work intentions in a sample of US health care workers. Mayo Clin Proc Innov Qual Outcomes. 2021;5(6):1165-1173. doi:10.1016/j.mayocpiqo.2021.08.007
- Tabloski PA. Life after retirement. American Nurse. March 3, 2022. Accessed April 25, 2025. https://www.myamericannurse.com/life-after-retirement/
- Chen T-P. Young doctors want work-life balance. Older doctors say that’s not the job. The Wall Street Journal. November 3, 2024. Accessed April 25, 2025. https://www.wsj.com/lifestyle/careers/young-doctors-want-work-life-balance-older-doctors-say-thats-not-the-job-6cb37d48
- Sweeny JF. Physician retirement: Why it’s hard for doctors to retire. Medical Economics. 2019;96(4). Accessed April 25, 2025. https://www.medicaleconomics.com/view/physician-retirement-why-its-hard-doctors-retire
- Nelson J. Wisdom for Our Time. W.W. Norton; 1961.
- Silver MP, Hamilton AD, Biswas A, Williams SA. Life after medicine: a systematic review of studies physician’s adjustment to retirement. Arch Community Med Public Health. 2016;2(1):001-007. doi:10.17352/2455-5479.000006
- Osler W. The Fixed Period. In: Osler W, ed. Aequanimitas With Other Addresses to Medical Students, Nurses and Practitioners of Medicine. 3rd ed. The Blakiston Company; 1932:373-393.
- Bliss M. William Osler: A Life in Medicine. Oxford University Press; 1999.
- Anderson M, Scofield RH. The “Fixed period,” the wildfire news, and an unpublished manuscript: Osler’s farewell speech revisited in geographical breadth and emotional depth. Am J Med Sci. Published online February 11, 2025. doi:10.1016/j.amjms.2025.02.005
- Obis A. What federal workers should consider before accepting deferred resignation. Federal News Network. April 8, 2025. Accessed April 25, 2025. https://federalnewsnetwork.com/workforce/2025/04/what-federal-workers-should-consider-before-accepting-deferred-resignation/
- Dyer J. VA exempts clinical staff from OPM deferred resignation program. Federal Practitioner. February 11, 2025. Accessed April 28, 2025. https://www.mdedge.com/content/va-exempts-clinical-staff-opm-deferred-resignation-program
- Shyrock T. Retirement planning secrets for physicians. Medical Economics. 2024;101(8). Accessed April 28, 2025. https:// www.medicaleconomics.com/view/retirement-planningsecrets-for-physicians
- Sinsky CA, Brown RL, Stillman MJ, Linzer M. COVID-related stress and work intentions in a sample of US health care workers. Mayo Clin Proc Innov Qual Outcomes. 2021;5(6):1165-1173. doi:10.1016/j.mayocpiqo.2021.08.007
- Tabloski PA. Life after retirement. American Nurse. March 3, 2022. Accessed April 25, 2025. https://www.myamericannurse.com/life-after-retirement/
- Chen T-P. Young doctors want work-life balance. Older doctors say that’s not the job. The Wall Street Journal. November 3, 2024. Accessed April 25, 2025. https://www.wsj.com/lifestyle/careers/young-doctors-want-work-life-balance-older-doctors-say-thats-not-the-job-6cb37d48
- Sweeny JF. Physician retirement: Why it’s hard for doctors to retire. Medical Economics. 2019;96(4). Accessed April 25, 2025. https://www.medicaleconomics.com/view/physician-retirement-why-its-hard-doctors-retire
- Nelson J. Wisdom for Our Time. W.W. Norton; 1961.
- Silver MP, Hamilton AD, Biswas A, Williams SA. Life after medicine: a systematic review of studies physician’s adjustment to retirement. Arch Community Med Public Health. 2016;2(1):001-007. doi:10.17352/2455-5479.000006
Should I Stay or Should I Go? Federal Health Care Professional Retirement Dilemmas
Should I Stay or Should I Go? Federal Health Care Professional Retirement Dilemmas
The Rise of Antifungal-Resistant Dermatophyte Infections: What Dermatologists Need to Know
The Rise of Antifungal-Resistant Dermatophyte Infections: What Dermatologists Need to Know
Worldwide, it is estimated that up to 1 in 5 individuals will experience a dermatophyte infection (commonly called ringworm or tinea infection) in their lifetime.1 Historically, dermatophyte infections have been considered relatively minor conditions usually treated with short courses of topical antifungals.2 Oral antifungals historically were needed only for patients with nail or hair shaft infections or extensive cutaneous fungal infections, which typically occurred in immunosuppressed patients.2 However, the landscape is changing rapidly due to the global emergence of severe dermatophyte infections that frequently are resistant to first-line antifungal medications.3-5 In this article, we aimed to review the epidemiology of emerging dermatophyte infections and provide dermatologists with information needed for effective diagnosis and management.
Emergence of Trichophyton indotineae
In recent decades, public health officials and dermatologists have noted with concern the spread of the recently emerged dermatophyte species Trichophyton indotineae in South Asia.3,6 This species (previously known as Trichophyton mentagrophytes genotype VIII) usually is transmitted from person to person, either through direct skin-to-skin contact or by fomites.4,6 Potential sexual transmission of T indotineae infections also has been reported,7 and it is possible that animals may serve as reservoirs for this pathogen, although there are no known reports of direct spread from animals to humans.8,9 Major outbreaks of T indotineae are ongoing in South Asia, and cases have been documented in 6 continents.10-12 In the United States, most but not all cases have occurred in immigrants from or recently returned travelers to South Asia.6,13 The emergence and spread of T indotineae is hypothesized to be promoted by the misuse and overuse of topical antifungal products, particularly those containing combinations of potent corticosteroids with other antimicrobial drugs.14,15
Cutaneous manifestations of T indotineae infections tend to cover large body surface areas, recur frequently, and pose substantial treatment challenges.6,13,16 Several clinical presentations have been documented, including erythematous, scaly concentric plaques; papulosquamous lesions; pustular forms; and corticosteroid-modified disease (Figure 1).6,16 Affected patients seldom are immunocompromised and often have a history of multiple failed courses of topical or oral antifungals, including oral terbinafine.13 Many also have been prescribed topical corticosteroids or have used over-the-counter topical corticosteroids, which worsen the rash.17
Direct microscopy with potassium hydroxide could be used to confirm the diagnosis of dermatophyte infection, but it does not distinguish T indotineae from other dermatophyte species.2,6 Importantly, culture-based testing usually will misidentify T indotineae as other Trichophyton species such as the more common T mentagrophytes or Trichophyton interdigitale. Definitive identification of T indotineae requires advanced molecular techniques that are available only at select laboratories.6 Unfortunately, availability of such testing is limited (Table), and results may take several weeks; therefore, it is suggested that dermatologists who suspect T indotineae infections based on the patient’s history and clinical presentation begin antifungal treatment after confirmation of dermatophyte infection but not wait for definitive confirmation of the causative organism.16
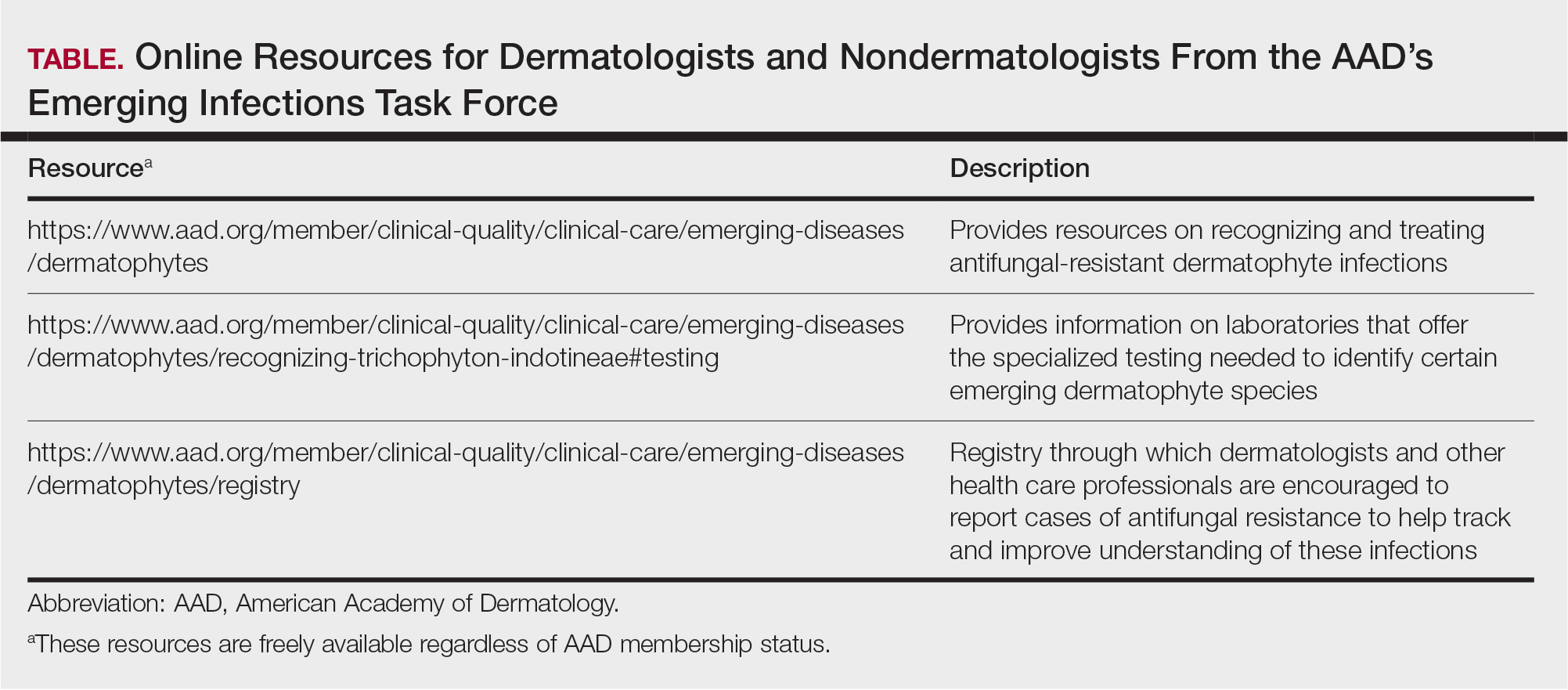
Itraconazole is considered the first-line therapy for T indotineae infection, as terbinafine usually is ineffective due to mutations in the squalene epoxidase gene.16 Dermatologists should be aware that itraconazole is available in different formulations that can affect absorption. The oral solution has greater bioavailability and should be taken on an empty stomach, whereas the capsules are required to be taken with food for effective absorption; the capsules also should be taken with an acidic beverage such as orange juice. Dermatologists should carefully assess for drug-drug interactions when prescribing itraconazole, given its extensive interaction profile with numerous other medications. Patients may require treatment with itraconazole (100 mg/d or 200 mg/d) for a minimum of 6 to 8 weeks until complete clearance has been achieved and ideally a negative potassium hydroxide preparation of skin scrapings has been obtained. A longer treatment period (eg, ≥3 months) frequently is needed, and relapses are common.6,16,18 Regular follow-up is needed to monitor for infection clearance and recurrences. It is important to note that cases of itraconazole resistance have been reported, although this currently appears to be uncommon.19,20
Other Emerging Dermatophytes to Watch
Trichophyton rubrum is the most common cause of dermatophyte infections among humans,21 and cases of terbinafine-resistant T rubrum infections have been reported increasingly in the United States and Canada.5,22-24 Onychomycosis caused by terbinafine-resistant T rubrum has been documented, and patients may have infections that do not respond to terbinafine given at the standard dose and duration.22,23 Case reports have indicated successful treatment using itraconazole 200 mg/d and posaconazole 300 mg/d.5,23
Trichophyton mentagrophytes genotype VII (TMVII) is an emerging dermatophyte that recently has been reported as a cause of sexually transmitted dermatophyte infections in Europe and the United States primarily affecting men who have sex with men.25-27 Patients may present with pruritic, annular, scaly patches and plaques involving the trunk, groin, genital region, or face (Figure 2). Although closely related to T indotineae, TMVII differs in that it more often affects the genital region, generally is susceptible to terbinafine, and in the United States and Europe usually is not related to travel or immigration involving South Asia.26 Although TMVII has not been associated with antifungal resistance, awareness among dermatologists is important because patients may experience inflamed, painful, and persistent rashes that can lead to secondary bacterial infection or scarring, and physicians might mistake it for mimics including eczema or psoriasis.25,26
Importance of Judicious Antifungal Use
Optimizing the use of antifungals is critical to improving patient outcomes and preserving available treatment options.28,29 A retrospective analysis of commercial health insurance data estimated that topical antifungal prescriptions were potentially unnecessary for more than half of the more than 560,000 patients who were prescribed these medications in 2023. In this study, it also was observed that only 16% of patients prescribed a topical antifungal had received diagnostic testing, with low rates across specialties.30 This is concerning because even among board-certified dermatologists, incorrect diagnosis of suspected fungal skin infections can occur; in one survey-based study of board-certified dermatologists who were presented with dermatomycosis images, respondents categorized cases with greater than 75% accuracy in only 31% (4/13) of instances.31 Clotrimazole-betamethasone is among the most commonly prescribed topical antifungals in the United States,14,32 and 2 recent retrospective analyses highlighted that the majority of patients prescribed this medication did not receive any fungal diagnostic testing.33,34
Final Thoughts
In an era of emerging antifungal-resistant dermatophyte infections, it is important for dermatologists to educate nondermatologists about the importance of using diagnostic testing for suspected dermatophyte infections.14,28 Dermatologists also can educate nondermatologist colleagues on the importance of avoiding the use of topical combination antifungal/corticosteroid medications and referring for dermatologic evaluation when diagnoses are uncertain.33,34 Strategies for education by dermatologists could include giving workshops, creating educational materials, and fostering open communication about optimal treatment practices and referral parameters for suspected dermatophyte infections.
- Noble SL, Forbes RC, Stamm PL. Diagnosis and management of common tinea infections. Am Fam Physician. 1998;58:163-174, 177-168.
- Ely JW, Rosenfeld S, Seabury Stone M. Diagnosis and management of tinea infections. Am Fam Physician. 2014;90:702-710.
- Uhrlaß S, Verma SB, Gräser Y, et al. Trichophyton indotineae—an emerging pathogen causing recalcitrant dermatophytoses in India and worldwide—a multidimensional perspective. J Fungi (Basel). 2022;8:757. doi:10.3390/jof8070757
- Verma SB, Panda S, Nenoff P, et al. The unprecedented epidemic-like scenario of dermatophytosis in India: I. epidemiology, risk factors and clinical features. Indian J Dermatol Venereol Leprol. 2021;87:154-175.
- Chen E, Ghannoum M, Elewski BE. Treatment]resistant tinea corporis, a potential public health issue. Br J Dermatol. 2021;184:164-165.
- Caplan AS. Notes from the field: first reported US cases of tinea caused by Trichophyton indotineae—New York City, December 2021–March 2023. MMWR Morbidity and Mortality Weekly Report. 2023;72:536-537. doi:10.15585/mmwr.mm7219a4
- Spivack S, Gold JA, Lockhart SR, et al. Potential sexual transmission of antifungal-resistant Trichophyton indotineae. Emerg Infect Dis. 2024;30:807.
- Jabet A, Brun S, Normand AC, et al. Extensive dermatophytosis caused by terbinafine-resistant Trichophyton indotineae, France. Emerg Infect Dis. 2022;28:229-233.
- Thakur S, Spruijtenburg B, Abhishek, et al. Whole genome sequence analysis of terbinafine resistant and susceptible Trichophyton isolates from human and animal origin. Mycopathologia. 2025;190:13.
- Lockhart SR, Chowdhary A, Gold JA. The rapid emergence of antifungal-resistant human-pathogenic fungi. Nat Rev Microbiol. 2023;21:818-832.
- Mosam A, Shuping L, Naicker S, et al. A case of antifungal-resistant ringworm infection in KwaZulu-Natal Province, South Africa, caused by Trichophyton indotineae. Public Health Bulletin South Africa. Accessed April 4, 2025. https://www.phbsa.ac.za/wp-content/uploads/2023/12PHBSA-Ringworm-Article-2023.pdf
- Cañete-Gibas CF, Mele J, Patterson HP, et al. Terbinafine-resistant dermatophytes and the presence of Trichophyton indotineae in North America. J Clin Microbiol. 2023;61:E0056223
- Caplan AS, Todd GC, Zhu Y, et al. Clinical course, antifungal susceptibility, and genomic sequencing of Trichophyton indotineae. JAMA Dermatol. 2024;160:701-709. doi:10.1001/jamadermatol.2024.1126
- Benedict K. Topical antifungal prescribing for Medicare Part D beneficiaries—United States, 2021. MMWR Morb Mortal Wkly Rep. 2024;73:1-5.
- Verma SB. Emergence of recalcitrant dermatophytosis in India. Lancet Infect Dis. 2018;18:718-719.
- Khurana A, Sharath S, Sardana K, et al. Clinico-mycological and therapeutic updates on cutaneous dermatophytic infections in the era of Trichophyton indotineae. J Am Acad Dermatol. 2024;91:315-323. doi:10.1016/j.jaad.2024.03.024
- Verma S. Steroid modified tinea. BMJ. 2017;356:j973.
- Khurana A, Agarwal A, Agrawal D, et al. Effect of different itraconazole dosing regimens on cure rates, treatment duration, safety, and relapse rates in adult patients with tinea corporis/cruris: a randomized clinical trial. JAMA Dermatol. 2022;158:1269-1278.
- Burmester A, Hipler UC, Uhrlaß S, et al. Indian Trichophyton mentagrophytes squalene epoxidase erg1 double mutants show high proportion of combined fluconazole and terbinafine resistance. Mycoses. 2020;63:1175-1180.
- Bhuiyan MSI, Verma SB, Illigner GM, et al. Trichophyton mentagrophytes ITS genotype VIII/Trichophyton indotineae infection and antifungal resistance in Bangladesh. J Fungi (Basel). 2024;10:768. doi:10.3390 /jof10110768
- Hay RJ. Chapter 82: superficial mycoses. In: Ryan ET, Hill DR, Solomon T, et al, eds. Hunter’s Tropical Medicine and Emerging Infectious Diseases. 10th ed. Elsevier; 2020:648-652.
- Gupta AK, Cooper EA, Wang T, et al. Detection of squalene epoxidase mutations in United States patients with onychomycosis: implications for management. J Invest Dermatol. 2023;143:2476-2483.E2477.
- Hwang JK, Bakotic WL, Gold JA, et al. Isolation of terbinafine-resistant Trichophyton rubrum from onychomycosis patients who failed treatment at an academic center in New York, United States. J Fungi. 2023;9:710.
- Gu D, Hatch M, Ghannoum M, et al. Treatment-resistant dermatophytosis: a representative case highlighting an emerging public health threat. JAAD Case Rep. 2020;6:1153-1155.
- Jabet A, Dellière S, Seang S, et al. Sexually transmitted Trichophyton mentagrophytes genotype VII infection among men who have sex with men. Emerg Infect Dis. 2023;29:1411-1414.
- Zucker J, Caplan AS, Gunaratne SH, et al. Notes from the field: Trichophyton mentagrophytes genotype VII—New York City, April-July 2024. MMWR Morb Mortal Wkly Rep. 2024;73:985-988.
- Jabet A, Bérot V, Chiarabini T, et al. Trichophyton mentagrophytes ITS genotype VII infections among men who have sex with men in France: an ongoing phenomenon. J Eur Acad Dermatol Venereol. 2025;39:407-415.
- Caplan AS, Gold JA, Smith DJ, et al. Improving antifungal stewardship in dermatology in an era of emerging dermatophyte resistance. JAAD International. 2024;15:168-169.
- Elewski B. A call for antifungal stewardship. Br J Dermatol. 2020; 183:798-799.
- Gold JAW, Benedict K, Caplan AS, et al. High rates of potentially unnecessary topical antifungal prescribing in a large commercial health insurance claims database, United States. J Am Acad Dermatol. 2025:S0190-9622(25)00098-2. doi:10.1016/j.jaad.2025.01.022
- Yadgar RJ, Bhatia N, Friedman A. Cutaneous fungal infections are commonly misdiagnosed: a survey-based study. J Am Acad Dermatol. 2017;76:562-563.
- Flint ND, Rhoads JLW, Carlisle R, et al. The continued inappropriate use and overuse of combination topical clotrimazole-betamethasone. Dermatol Online J. 2021;27. doi:10.5070/D327854686
- Currie DW, Caplan AS, Benedict K, et al. Prescribing of clotrimazolebetamethasone dipropionate, a topical combination corticosteroidantifungal product, for Medicare part D beneficiaries, United States, 2016–2022. Antimicrob Steward Healthc Epidemiol. 2024;4:E174.
- Gold JA, Caplan AS, Benedict K, et al. Clotrimazole-betamethasone dipropionate prescribing for nonfungal skin conditions. JAMA Network Open. 2024;7:E2411721-E2411721.
Worldwide, it is estimated that up to 1 in 5 individuals will experience a dermatophyte infection (commonly called ringworm or tinea infection) in their lifetime.1 Historically, dermatophyte infections have been considered relatively minor conditions usually treated with short courses of topical antifungals.2 Oral antifungals historically were needed only for patients with nail or hair shaft infections or extensive cutaneous fungal infections, which typically occurred in immunosuppressed patients.2 However, the landscape is changing rapidly due to the global emergence of severe dermatophyte infections that frequently are resistant to first-line antifungal medications.3-5 In this article, we aimed to review the epidemiology of emerging dermatophyte infections and provide dermatologists with information needed for effective diagnosis and management.
Emergence of Trichophyton indotineae
In recent decades, public health officials and dermatologists have noted with concern the spread of the recently emerged dermatophyte species Trichophyton indotineae in South Asia.3,6 This species (previously known as Trichophyton mentagrophytes genotype VIII) usually is transmitted from person to person, either through direct skin-to-skin contact or by fomites.4,6 Potential sexual transmission of T indotineae infections also has been reported,7 and it is possible that animals may serve as reservoirs for this pathogen, although there are no known reports of direct spread from animals to humans.8,9 Major outbreaks of T indotineae are ongoing in South Asia, and cases have been documented in 6 continents.10-12 In the United States, most but not all cases have occurred in immigrants from or recently returned travelers to South Asia.6,13 The emergence and spread of T indotineae is hypothesized to be promoted by the misuse and overuse of topical antifungal products, particularly those containing combinations of potent corticosteroids with other antimicrobial drugs.14,15
Cutaneous manifestations of T indotineae infections tend to cover large body surface areas, recur frequently, and pose substantial treatment challenges.6,13,16 Several clinical presentations have been documented, including erythematous, scaly concentric plaques; papulosquamous lesions; pustular forms; and corticosteroid-modified disease (Figure 1).6,16 Affected patients seldom are immunocompromised and often have a history of multiple failed courses of topical or oral antifungals, including oral terbinafine.13 Many also have been prescribed topical corticosteroids or have used over-the-counter topical corticosteroids, which worsen the rash.17

Direct microscopy with potassium hydroxide could be used to confirm the diagnosis of dermatophyte infection, but it does not distinguish T indotineae from other dermatophyte species.2,6 Importantly, culture-based testing usually will misidentify T indotineae as other Trichophyton species such as the more common T mentagrophytes or Trichophyton interdigitale. Definitive identification of T indotineae requires advanced molecular techniques that are available only at select laboratories.6 Unfortunately, availability of such testing is limited (Table), and results may take several weeks; therefore, it is suggested that dermatologists who suspect T indotineae infections based on the patient’s history and clinical presentation begin antifungal treatment after confirmation of dermatophyte infection but not wait for definitive confirmation of the causative organism.16

Itraconazole is considered the first-line therapy for T indotineae infection, as terbinafine usually is ineffective due to mutations in the squalene epoxidase gene.16 Dermatologists should be aware that itraconazole is available in different formulations that can affect absorption. The oral solution has greater bioavailability and should be taken on an empty stomach, whereas the capsules are required to be taken with food for effective absorption; the capsules also should be taken with an acidic beverage such as orange juice. Dermatologists should carefully assess for drug-drug interactions when prescribing itraconazole, given its extensive interaction profile with numerous other medications. Patients may require treatment with itraconazole (100 mg/d or 200 mg/d) for a minimum of 6 to 8 weeks until complete clearance has been achieved and ideally a negative potassium hydroxide preparation of skin scrapings has been obtained. A longer treatment period (eg, ≥3 months) frequently is needed, and relapses are common.6,16,18 Regular follow-up is needed to monitor for infection clearance and recurrences. It is important to note that cases of itraconazole resistance have been reported, although this currently appears to be uncommon.19,20
Other Emerging Dermatophytes to Watch
Trichophyton rubrum is the most common cause of dermatophyte infections among humans,21 and cases of terbinafine-resistant T rubrum infections have been reported increasingly in the United States and Canada.5,22-24 Onychomycosis caused by terbinafine-resistant T rubrum has been documented, and patients may have infections that do not respond to terbinafine given at the standard dose and duration.22,23 Case reports have indicated successful treatment using itraconazole 200 mg/d and posaconazole 300 mg/d.5,23
Trichophyton mentagrophytes genotype VII (TMVII) is an emerging dermatophyte that recently has been reported as a cause of sexually transmitted dermatophyte infections in Europe and the United States primarily affecting men who have sex with men.25-27 Patients may present with pruritic, annular, scaly patches and plaques involving the trunk, groin, genital region, or face (Figure 2). Although closely related to T indotineae, TMVII differs in that it more often affects the genital region, generally is susceptible to terbinafine, and in the United States and Europe usually is not related to travel or immigration involving South Asia.26 Although TMVII has not been associated with antifungal resistance, awareness among dermatologists is important because patients may experience inflamed, painful, and persistent rashes that can lead to secondary bacterial infection or scarring, and physicians might mistake it for mimics including eczema or psoriasis.25,26

Importance of Judicious Antifungal Use
Optimizing the use of antifungals is critical to improving patient outcomes and preserving available treatment options.28,29 A retrospective analysis of commercial health insurance data estimated that topical antifungal prescriptions were potentially unnecessary for more than half of the more than 560,000 patients who were prescribed these medications in 2023. In this study, it also was observed that only 16% of patients prescribed a topical antifungal had received diagnostic testing, with low rates across specialties.30 This is concerning because even among board-certified dermatologists, incorrect diagnosis of suspected fungal skin infections can occur; in one survey-based study of board-certified dermatologists who were presented with dermatomycosis images, respondents categorized cases with greater than 75% accuracy in only 31% (4/13) of instances.31 Clotrimazole-betamethasone is among the most commonly prescribed topical antifungals in the United States,14,32 and 2 recent retrospective analyses highlighted that the majority of patients prescribed this medication did not receive any fungal diagnostic testing.33,34
Final Thoughts
In an era of emerging antifungal-resistant dermatophyte infections, it is important for dermatologists to educate nondermatologists about the importance of using diagnostic testing for suspected dermatophyte infections.14,28 Dermatologists also can educate nondermatologist colleagues on the importance of avoiding the use of topical combination antifungal/corticosteroid medications and referring for dermatologic evaluation when diagnoses are uncertain.33,34 Strategies for education by dermatologists could include giving workshops, creating educational materials, and fostering open communication about optimal treatment practices and referral parameters for suspected dermatophyte infections.
Worldwide, it is estimated that up to 1 in 5 individuals will experience a dermatophyte infection (commonly called ringworm or tinea infection) in their lifetime.1 Historically, dermatophyte infections have been considered relatively minor conditions usually treated with short courses of topical antifungals.2 Oral antifungals historically were needed only for patients with nail or hair shaft infections or extensive cutaneous fungal infections, which typically occurred in immunosuppressed patients.2 However, the landscape is changing rapidly due to the global emergence of severe dermatophyte infections that frequently are resistant to first-line antifungal medications.3-5 In this article, we aimed to review the epidemiology of emerging dermatophyte infections and provide dermatologists with information needed for effective diagnosis and management.
Emergence of Trichophyton indotineae
In recent decades, public health officials and dermatologists have noted with concern the spread of the recently emerged dermatophyte species Trichophyton indotineae in South Asia.3,6 This species (previously known as Trichophyton mentagrophytes genotype VIII) usually is transmitted from person to person, either through direct skin-to-skin contact or by fomites.4,6 Potential sexual transmission of T indotineae infections also has been reported,7 and it is possible that animals may serve as reservoirs for this pathogen, although there are no known reports of direct spread from animals to humans.8,9 Major outbreaks of T indotineae are ongoing in South Asia, and cases have been documented in 6 continents.10-12 In the United States, most but not all cases have occurred in immigrants from or recently returned travelers to South Asia.6,13 The emergence and spread of T indotineae is hypothesized to be promoted by the misuse and overuse of topical antifungal products, particularly those containing combinations of potent corticosteroids with other antimicrobial drugs.14,15
Cutaneous manifestations of T indotineae infections tend to cover large body surface areas, recur frequently, and pose substantial treatment challenges.6,13,16 Several clinical presentations have been documented, including erythematous, scaly concentric plaques; papulosquamous lesions; pustular forms; and corticosteroid-modified disease (Figure 1).6,16 Affected patients seldom are immunocompromised and often have a history of multiple failed courses of topical or oral antifungals, including oral terbinafine.13 Many also have been prescribed topical corticosteroids or have used over-the-counter topical corticosteroids, which worsen the rash.17

Direct microscopy with potassium hydroxide could be used to confirm the diagnosis of dermatophyte infection, but it does not distinguish T indotineae from other dermatophyte species.2,6 Importantly, culture-based testing usually will misidentify T indotineae as other Trichophyton species such as the more common T mentagrophytes or Trichophyton interdigitale. Definitive identification of T indotineae requires advanced molecular techniques that are available only at select laboratories.6 Unfortunately, availability of such testing is limited (Table), and results may take several weeks; therefore, it is suggested that dermatologists who suspect T indotineae infections based on the patient’s history and clinical presentation begin antifungal treatment after confirmation of dermatophyte infection but not wait for definitive confirmation of the causative organism.16

Itraconazole is considered the first-line therapy for T indotineae infection, as terbinafine usually is ineffective due to mutations in the squalene epoxidase gene.16 Dermatologists should be aware that itraconazole is available in different formulations that can affect absorption. The oral solution has greater bioavailability and should be taken on an empty stomach, whereas the capsules are required to be taken with food for effective absorption; the capsules also should be taken with an acidic beverage such as orange juice. Dermatologists should carefully assess for drug-drug interactions when prescribing itraconazole, given its extensive interaction profile with numerous other medications. Patients may require treatment with itraconazole (100 mg/d or 200 mg/d) for a minimum of 6 to 8 weeks until complete clearance has been achieved and ideally a negative potassium hydroxide preparation of skin scrapings has been obtained. A longer treatment period (eg, ≥3 months) frequently is needed, and relapses are common.6,16,18 Regular follow-up is needed to monitor for infection clearance and recurrences. It is important to note that cases of itraconazole resistance have been reported, although this currently appears to be uncommon.19,20
Other Emerging Dermatophytes to Watch
Trichophyton rubrum is the most common cause of dermatophyte infections among humans,21 and cases of terbinafine-resistant T rubrum infections have been reported increasingly in the United States and Canada.5,22-24 Onychomycosis caused by terbinafine-resistant T rubrum has been documented, and patients may have infections that do not respond to terbinafine given at the standard dose and duration.22,23 Case reports have indicated successful treatment using itraconazole 200 mg/d and posaconazole 300 mg/d.5,23
Trichophyton mentagrophytes genotype VII (TMVII) is an emerging dermatophyte that recently has been reported as a cause of sexually transmitted dermatophyte infections in Europe and the United States primarily affecting men who have sex with men.25-27 Patients may present with pruritic, annular, scaly patches and plaques involving the trunk, groin, genital region, or face (Figure 2). Although closely related to T indotineae, TMVII differs in that it more often affects the genital region, generally is susceptible to terbinafine, and in the United States and Europe usually is not related to travel or immigration involving South Asia.26 Although TMVII has not been associated with antifungal resistance, awareness among dermatologists is important because patients may experience inflamed, painful, and persistent rashes that can lead to secondary bacterial infection or scarring, and physicians might mistake it for mimics including eczema or psoriasis.25,26

Importance of Judicious Antifungal Use
Optimizing the use of antifungals is critical to improving patient outcomes and preserving available treatment options.28,29 A retrospective analysis of commercial health insurance data estimated that topical antifungal prescriptions were potentially unnecessary for more than half of the more than 560,000 patients who were prescribed these medications in 2023. In this study, it also was observed that only 16% of patients prescribed a topical antifungal had received diagnostic testing, with low rates across specialties.30 This is concerning because even among board-certified dermatologists, incorrect diagnosis of suspected fungal skin infections can occur; in one survey-based study of board-certified dermatologists who were presented with dermatomycosis images, respondents categorized cases with greater than 75% accuracy in only 31% (4/13) of instances.31 Clotrimazole-betamethasone is among the most commonly prescribed topical antifungals in the United States,14,32 and 2 recent retrospective analyses highlighted that the majority of patients prescribed this medication did not receive any fungal diagnostic testing.33,34
Final Thoughts
In an era of emerging antifungal-resistant dermatophyte infections, it is important for dermatologists to educate nondermatologists about the importance of using diagnostic testing for suspected dermatophyte infections.14,28 Dermatologists also can educate nondermatologist colleagues on the importance of avoiding the use of topical combination antifungal/corticosteroid medications and referring for dermatologic evaluation when diagnoses are uncertain.33,34 Strategies for education by dermatologists could include giving workshops, creating educational materials, and fostering open communication about optimal treatment practices and referral parameters for suspected dermatophyte infections.
- Noble SL, Forbes RC, Stamm PL. Diagnosis and management of common tinea infections. Am Fam Physician. 1998;58:163-174, 177-168.
- Ely JW, Rosenfeld S, Seabury Stone M. Diagnosis and management of tinea infections. Am Fam Physician. 2014;90:702-710.
- Uhrlaß S, Verma SB, Gräser Y, et al. Trichophyton indotineae—an emerging pathogen causing recalcitrant dermatophytoses in India and worldwide—a multidimensional perspective. J Fungi (Basel). 2022;8:757. doi:10.3390/jof8070757
- Verma SB, Panda S, Nenoff P, et al. The unprecedented epidemic-like scenario of dermatophytosis in India: I. epidemiology, risk factors and clinical features. Indian J Dermatol Venereol Leprol. 2021;87:154-175.
- Chen E, Ghannoum M, Elewski BE. Treatment]resistant tinea corporis, a potential public health issue. Br J Dermatol. 2021;184:164-165.
- Caplan AS. Notes from the field: first reported US cases of tinea caused by Trichophyton indotineae—New York City, December 2021–March 2023. MMWR Morbidity and Mortality Weekly Report. 2023;72:536-537. doi:10.15585/mmwr.mm7219a4
- Spivack S, Gold JA, Lockhart SR, et al. Potential sexual transmission of antifungal-resistant Trichophyton indotineae. Emerg Infect Dis. 2024;30:807.
- Jabet A, Brun S, Normand AC, et al. Extensive dermatophytosis caused by terbinafine-resistant Trichophyton indotineae, France. Emerg Infect Dis. 2022;28:229-233.
- Thakur S, Spruijtenburg B, Abhishek, et al. Whole genome sequence analysis of terbinafine resistant and susceptible Trichophyton isolates from human and animal origin. Mycopathologia. 2025;190:13.
- Lockhart SR, Chowdhary A, Gold JA. The rapid emergence of antifungal-resistant human-pathogenic fungi. Nat Rev Microbiol. 2023;21:818-832.
- Mosam A, Shuping L, Naicker S, et al. A case of antifungal-resistant ringworm infection in KwaZulu-Natal Province, South Africa, caused by Trichophyton indotineae. Public Health Bulletin South Africa. Accessed April 4, 2025. https://www.phbsa.ac.za/wp-content/uploads/2023/12PHBSA-Ringworm-Article-2023.pdf
- Cañete-Gibas CF, Mele J, Patterson HP, et al. Terbinafine-resistant dermatophytes and the presence of Trichophyton indotineae in North America. J Clin Microbiol. 2023;61:E0056223
- Caplan AS, Todd GC, Zhu Y, et al. Clinical course, antifungal susceptibility, and genomic sequencing of Trichophyton indotineae. JAMA Dermatol. 2024;160:701-709. doi:10.1001/jamadermatol.2024.1126
- Benedict K. Topical antifungal prescribing for Medicare Part D beneficiaries—United States, 2021. MMWR Morb Mortal Wkly Rep. 2024;73:1-5.
- Verma SB. Emergence of recalcitrant dermatophytosis in India. Lancet Infect Dis. 2018;18:718-719.
- Khurana A, Sharath S, Sardana K, et al. Clinico-mycological and therapeutic updates on cutaneous dermatophytic infections in the era of Trichophyton indotineae. J Am Acad Dermatol. 2024;91:315-323. doi:10.1016/j.jaad.2024.03.024
- Verma S. Steroid modified tinea. BMJ. 2017;356:j973.
- Khurana A, Agarwal A, Agrawal D, et al. Effect of different itraconazole dosing regimens on cure rates, treatment duration, safety, and relapse rates in adult patients with tinea corporis/cruris: a randomized clinical trial. JAMA Dermatol. 2022;158:1269-1278.
- Burmester A, Hipler UC, Uhrlaß S, et al. Indian Trichophyton mentagrophytes squalene epoxidase erg1 double mutants show high proportion of combined fluconazole and terbinafine resistance. Mycoses. 2020;63:1175-1180.
- Bhuiyan MSI, Verma SB, Illigner GM, et al. Trichophyton mentagrophytes ITS genotype VIII/Trichophyton indotineae infection and antifungal resistance in Bangladesh. J Fungi (Basel). 2024;10:768. doi:10.3390 /jof10110768
- Hay RJ. Chapter 82: superficial mycoses. In: Ryan ET, Hill DR, Solomon T, et al, eds. Hunter’s Tropical Medicine and Emerging Infectious Diseases. 10th ed. Elsevier; 2020:648-652.
- Gupta AK, Cooper EA, Wang T, et al. Detection of squalene epoxidase mutations in United States patients with onychomycosis: implications for management. J Invest Dermatol. 2023;143:2476-2483.E2477.
- Hwang JK, Bakotic WL, Gold JA, et al. Isolation of terbinafine-resistant Trichophyton rubrum from onychomycosis patients who failed treatment at an academic center in New York, United States. J Fungi. 2023;9:710.
- Gu D, Hatch M, Ghannoum M, et al. Treatment-resistant dermatophytosis: a representative case highlighting an emerging public health threat. JAAD Case Rep. 2020;6:1153-1155.
- Jabet A, Dellière S, Seang S, et al. Sexually transmitted Trichophyton mentagrophytes genotype VII infection among men who have sex with men. Emerg Infect Dis. 2023;29:1411-1414.
- Zucker J, Caplan AS, Gunaratne SH, et al. Notes from the field: Trichophyton mentagrophytes genotype VII—New York City, April-July 2024. MMWR Morb Mortal Wkly Rep. 2024;73:985-988.
- Jabet A, Bérot V, Chiarabini T, et al. Trichophyton mentagrophytes ITS genotype VII infections among men who have sex with men in France: an ongoing phenomenon. J Eur Acad Dermatol Venereol. 2025;39:407-415.
- Caplan AS, Gold JA, Smith DJ, et al. Improving antifungal stewardship in dermatology in an era of emerging dermatophyte resistance. JAAD International. 2024;15:168-169.
- Elewski B. A call for antifungal stewardship. Br J Dermatol. 2020; 183:798-799.
- Gold JAW, Benedict K, Caplan AS, et al. High rates of potentially unnecessary topical antifungal prescribing in a large commercial health insurance claims database, United States. J Am Acad Dermatol. 2025:S0190-9622(25)00098-2. doi:10.1016/j.jaad.2025.01.022
- Yadgar RJ, Bhatia N, Friedman A. Cutaneous fungal infections are commonly misdiagnosed: a survey-based study. J Am Acad Dermatol. 2017;76:562-563.
- Flint ND, Rhoads JLW, Carlisle R, et al. The continued inappropriate use and overuse of combination topical clotrimazole-betamethasone. Dermatol Online J. 2021;27. doi:10.5070/D327854686
- Currie DW, Caplan AS, Benedict K, et al. Prescribing of clotrimazolebetamethasone dipropionate, a topical combination corticosteroidantifungal product, for Medicare part D beneficiaries, United States, 2016–2022. Antimicrob Steward Healthc Epidemiol. 2024;4:E174.
- Gold JA, Caplan AS, Benedict K, et al. Clotrimazole-betamethasone dipropionate prescribing for nonfungal skin conditions. JAMA Network Open. 2024;7:E2411721-E2411721.
- Noble SL, Forbes RC, Stamm PL. Diagnosis and management of common tinea infections. Am Fam Physician. 1998;58:163-174, 177-168.
- Ely JW, Rosenfeld S, Seabury Stone M. Diagnosis and management of tinea infections. Am Fam Physician. 2014;90:702-710.
- Uhrlaß S, Verma SB, Gräser Y, et al. Trichophyton indotineae—an emerging pathogen causing recalcitrant dermatophytoses in India and worldwide—a multidimensional perspective. J Fungi (Basel). 2022;8:757. doi:10.3390/jof8070757
- Verma SB, Panda S, Nenoff P, et al. The unprecedented epidemic-like scenario of dermatophytosis in India: I. epidemiology, risk factors and clinical features. Indian J Dermatol Venereol Leprol. 2021;87:154-175.
- Chen E, Ghannoum M, Elewski BE. Treatment]resistant tinea corporis, a potential public health issue. Br J Dermatol. 2021;184:164-165.
- Caplan AS. Notes from the field: first reported US cases of tinea caused by Trichophyton indotineae—New York City, December 2021–March 2023. MMWR Morbidity and Mortality Weekly Report. 2023;72:536-537. doi:10.15585/mmwr.mm7219a4
- Spivack S, Gold JA, Lockhart SR, et al. Potential sexual transmission of antifungal-resistant Trichophyton indotineae. Emerg Infect Dis. 2024;30:807.
- Jabet A, Brun S, Normand AC, et al. Extensive dermatophytosis caused by terbinafine-resistant Trichophyton indotineae, France. Emerg Infect Dis. 2022;28:229-233.
- Thakur S, Spruijtenburg B, Abhishek, et al. Whole genome sequence analysis of terbinafine resistant and susceptible Trichophyton isolates from human and animal origin. Mycopathologia. 2025;190:13.
- Lockhart SR, Chowdhary A, Gold JA. The rapid emergence of antifungal-resistant human-pathogenic fungi. Nat Rev Microbiol. 2023;21:818-832.
- Mosam A, Shuping L, Naicker S, et al. A case of antifungal-resistant ringworm infection in KwaZulu-Natal Province, South Africa, caused by Trichophyton indotineae. Public Health Bulletin South Africa. Accessed April 4, 2025. https://www.phbsa.ac.za/wp-content/uploads/2023/12PHBSA-Ringworm-Article-2023.pdf
- Cañete-Gibas CF, Mele J, Patterson HP, et al. Terbinafine-resistant dermatophytes and the presence of Trichophyton indotineae in North America. J Clin Microbiol. 2023;61:E0056223
- Caplan AS, Todd GC, Zhu Y, et al. Clinical course, antifungal susceptibility, and genomic sequencing of Trichophyton indotineae. JAMA Dermatol. 2024;160:701-709. doi:10.1001/jamadermatol.2024.1126
- Benedict K. Topical antifungal prescribing for Medicare Part D beneficiaries—United States, 2021. MMWR Morb Mortal Wkly Rep. 2024;73:1-5.
- Verma SB. Emergence of recalcitrant dermatophytosis in India. Lancet Infect Dis. 2018;18:718-719.
- Khurana A, Sharath S, Sardana K, et al. Clinico-mycological and therapeutic updates on cutaneous dermatophytic infections in the era of Trichophyton indotineae. J Am Acad Dermatol. 2024;91:315-323. doi:10.1016/j.jaad.2024.03.024
- Verma S. Steroid modified tinea. BMJ. 2017;356:j973.
- Khurana A, Agarwal A, Agrawal D, et al. Effect of different itraconazole dosing regimens on cure rates, treatment duration, safety, and relapse rates in adult patients with tinea corporis/cruris: a randomized clinical trial. JAMA Dermatol. 2022;158:1269-1278.
- Burmester A, Hipler UC, Uhrlaß S, et al. Indian Trichophyton mentagrophytes squalene epoxidase erg1 double mutants show high proportion of combined fluconazole and terbinafine resistance. Mycoses. 2020;63:1175-1180.
- Bhuiyan MSI, Verma SB, Illigner GM, et al. Trichophyton mentagrophytes ITS genotype VIII/Trichophyton indotineae infection and antifungal resistance in Bangladesh. J Fungi (Basel). 2024;10:768. doi:10.3390 /jof10110768
- Hay RJ. Chapter 82: superficial mycoses. In: Ryan ET, Hill DR, Solomon T, et al, eds. Hunter’s Tropical Medicine and Emerging Infectious Diseases. 10th ed. Elsevier; 2020:648-652.
- Gupta AK, Cooper EA, Wang T, et al. Detection of squalene epoxidase mutations in United States patients with onychomycosis: implications for management. J Invest Dermatol. 2023;143:2476-2483.E2477.
- Hwang JK, Bakotic WL, Gold JA, et al. Isolation of terbinafine-resistant Trichophyton rubrum from onychomycosis patients who failed treatment at an academic center in New York, United States. J Fungi. 2023;9:710.
- Gu D, Hatch M, Ghannoum M, et al. Treatment-resistant dermatophytosis: a representative case highlighting an emerging public health threat. JAAD Case Rep. 2020;6:1153-1155.
- Jabet A, Dellière S, Seang S, et al. Sexually transmitted Trichophyton mentagrophytes genotype VII infection among men who have sex with men. Emerg Infect Dis. 2023;29:1411-1414.
- Zucker J, Caplan AS, Gunaratne SH, et al. Notes from the field: Trichophyton mentagrophytes genotype VII—New York City, April-July 2024. MMWR Morb Mortal Wkly Rep. 2024;73:985-988.
- Jabet A, Bérot V, Chiarabini T, et al. Trichophyton mentagrophytes ITS genotype VII infections among men who have sex with men in France: an ongoing phenomenon. J Eur Acad Dermatol Venereol. 2025;39:407-415.
- Caplan AS, Gold JA, Smith DJ, et al. Improving antifungal stewardship in dermatology in an era of emerging dermatophyte resistance. JAAD International. 2024;15:168-169.
- Elewski B. A call for antifungal stewardship. Br J Dermatol. 2020; 183:798-799.
- Gold JAW, Benedict K, Caplan AS, et al. High rates of potentially unnecessary topical antifungal prescribing in a large commercial health insurance claims database, United States. J Am Acad Dermatol. 2025:S0190-9622(25)00098-2. doi:10.1016/j.jaad.2025.01.022
- Yadgar RJ, Bhatia N, Friedman A. Cutaneous fungal infections are commonly misdiagnosed: a survey-based study. J Am Acad Dermatol. 2017;76:562-563.
- Flint ND, Rhoads JLW, Carlisle R, et al. The continued inappropriate use and overuse of combination topical clotrimazole-betamethasone. Dermatol Online J. 2021;27. doi:10.5070/D327854686
- Currie DW, Caplan AS, Benedict K, et al. Prescribing of clotrimazolebetamethasone dipropionate, a topical combination corticosteroidantifungal product, for Medicare part D beneficiaries, United States, 2016–2022. Antimicrob Steward Healthc Epidemiol. 2024;4:E174.
- Gold JA, Caplan AS, Benedict K, et al. Clotrimazole-betamethasone dipropionate prescribing for nonfungal skin conditions. JAMA Network Open. 2024;7:E2411721-E2411721.
The Rise of Antifungal-Resistant Dermatophyte Infections: What Dermatologists Need to Know
The Rise of Antifungal-Resistant Dermatophyte Infections: What Dermatologists Need to Know
PRACTICE POINTS
- Recently emerged dermatophyte species pose a global public health concern because of infection severity, frequent resistance to terbinafine, and easy person-to-person transmission.
- Prolonged itraconazole therapy is considered the firstline treatment for infections caused by Trichophyton indotineae, a globally emerging and frequently terbinafine-resistant dermatophyte.
- Dermatologists can educate nondermatologists on the importance of mycologic confirmation and avoidance of the use of topical antifungal/ corticosteroid products, which are hypothesized to contribute to emergence and spread of resistance.
Training Lifeguards to Assist in Skin Cancer Prevention
Training Lifeguards to Assist in Skin Cancer Prevention
Lifeguards play a crucial role in ensuring water safety, but they also are uniquely positioned to promote skin cancer prevention and proper sunscreen use.1,2 There are several benefits and challenges to offering skin cancer prevention training for lifeguards.3 We examine the advantages of training, highlight the role lifeguards can play in larger public skin cancer prevention efforts, and address practical techniques for developing lifeguardfocused skin cancer education programs. By providing this knowledge to lifeguards, we can improve community health outcomes and encourage sun-safe behaviors in high-risk outdoor locations.
Benefits of Skin Cancer Prevention Training for Lifeguards
Research has shown that lifeguards are at an elevated risk for basal cell carcinoma, squamous cell carcinoma, and melanoma due to frequent prolonged occupational sun exposure.1,2,4-6 Therefore, comprehensive education on skin cancer prevention—including instruction on proper sunscreen application techniques and the importance of regular reapplication as well as how to recognize suspicious skin lesions—should be incorporated into lifeguard certification programs. One study evaluating the effectiveness of a skin cancer prevention program for lifeguards found that many of the participants lacked a thorough understanding of the different types of skin cancer.5 Another study found that lifeguards at pools in areas where societal norms supporting sun safety are stronger exhibited noticeably more sun protection practices, with regression estimates of 0.22 (95% CI, 0.17-0.26).7 Empowering lifeguards with valuable health knowledge during their regular training could potentially reduce their risk for skin cancer,4 as they may be more inclined to use sunscreen appropriately and reach out to a dermatologist for regular skin checks and evaluation of suspicious lesions.
Role of Lifeguards in Public Skin Cancer Prevention Efforts
Once trained on skin cancer prevention, lifeguards also can play a pivotal role in promoting sunscreen use among the public. Despite the widespread availability of high-quality sunscreens, many swimmers and beachgoers neglect to regularly apply or reapply sunscreen, especially on commonly exposed areas such as the back, shoulders, and face.8 Educating lifeguards on skin cancer prevention could enhance health outcomes by increasing early detection rates and promoting sun-safe behaviors among the general public.9 However, additional training requirements might increase the cost and time commitment for lifeguard certification, potentially leading to staffing shortages.3,7 There also is a risk of lifeguards overstepping their role and providing inaccurate medical advice, which could cause distress or even lead to liability issues.7 Balancing these factors will be crucial in developing effective and sustainable skin cancer prevention programs for lifeguards.
Implementing Lifeguard Skin Cancer Training
Implementing skin cancer prevention training programs for lifeguards requires strategic collaboration between dermatologists, and lifeguard training organizations to ensure that the participants receive consistent and comprehensive training.10 Additionally, public health campaigns can support these efforts by raising awareness about the importance of sun safety and regular skin checks.6 Tailored training modules/materials, ongoing technical assistance, and active, multicomponent approaches that account for both individual and environmental factors can increase program implementation in a variety of community settings.
Final Thoughts
Through effective education, lifeguards can potentially have a substantial impact on skin cancer prevention, both among lifeguards themselves and the general public. By promoting proper sunscreen use, lifeguards can help reduce the incidence and mortality associated with skin cancers. Future studies should focus on developing and implementing targeted education initiatives for lifeguards, fostering collaboration between relevant stakeholders, and raising public awareness about the importance of sun safety and early skin cancer detection. These efforts ultimately could lead to improved public health outcomes and reduced skin cancer rates, particularly in high-risk populations that frequently are exposed to UV radiation.
- Enos CW, Rey S, Slocum J, et al. Sun-protection behaviors among active members of the United States Lifesaving Association. J Clin Aesthet Dermatol. 2021;14:14-20.
- Verma K, Lewis DJ, Siddiqui FS, et al. Mohs micrographic surgery management of melanoma and melanoma in situ. StatPearls. Updated August 28, 2024. Accessed April 15, 2025. https://www.ncbi.nlm.nih.gov/books/NBK606123/
- Verma KK, Joshi TP, Lewis DJ, et al. Nail technicians as partners in early melanoma detection: bridging the knowledge gap. Arch Dermatol Res. 2024;316:586. doi:10.1007/s00403-024-03342-0
- Geller AC, Glanz K, Shigaki D, et al. Impact of skin cancer prevention on outdoor aquatics staff: the Pool Cool program in Hawaii and Massachusetts. Prev Med. 2001;33:155-161. doi:10.1006/pmed.2001.0870
- Hiemstra M, Glanz K, Nehl E. Changes in sunburn and tanning attitudes among lifeguards over a summer season. J Am Acad Dermatol. 2012;66:430-437. doi:10.1016/j.jaad.2010.11.050
- Verma KK, Ahmad N, Friedmann DP, et al. Melanoma in tattooed skin: diagnostic challenges and the potential for tattoo artists in early detection. Arch Dermatol Res. 2024;316:690. doi:10.1007/s00403-024-03415-0
- Hall DM, McCarty F, Elliott T, et al. Lifeguards’ sun protection habits and sunburns: association with sun-safe environments and skin cancer prevention program participation. Arch Dermatol. 2009;145:139-144. doi:10.1001/archdermatol.2008.553
- Emmons KM, Geller AC, Puleo E, et al. Skin cancer education and early detection at the beach: a randomized trial of dermatologist examination and biometric feedback. J Am Acad Dermatol. 2011;64:282-289. doi:10.1016/j.jaad.2010.01.040
- Rabin BA, Nehl E, Elliott T, et al. Individual and setting level predictors of the implementation of a skin cancer prevention program: a multilevel analysis. Implement Sci. 2010;5:40. doi:10.1186/1748-5908-5-40
- Walkosz BJ, Buller D, Buller M, et al. Sun safe workplaces: effect of an occupational skin cancer prevention program on employee sun safety practices. J Occup Environ Med. 2018;60:900-997. doi:10.1097 /JOM.0000000000001427
Lifeguards play a crucial role in ensuring water safety, but they also are uniquely positioned to promote skin cancer prevention and proper sunscreen use.1,2 There are several benefits and challenges to offering skin cancer prevention training for lifeguards.3 We examine the advantages of training, highlight the role lifeguards can play in larger public skin cancer prevention efforts, and address practical techniques for developing lifeguardfocused skin cancer education programs. By providing this knowledge to lifeguards, we can improve community health outcomes and encourage sun-safe behaviors in high-risk outdoor locations.
Benefits of Skin Cancer Prevention Training for Lifeguards
Research has shown that lifeguards are at an elevated risk for basal cell carcinoma, squamous cell carcinoma, and melanoma due to frequent prolonged occupational sun exposure.1,2,4-6 Therefore, comprehensive education on skin cancer prevention—including instruction on proper sunscreen application techniques and the importance of regular reapplication as well as how to recognize suspicious skin lesions—should be incorporated into lifeguard certification programs. One study evaluating the effectiveness of a skin cancer prevention program for lifeguards found that many of the participants lacked a thorough understanding of the different types of skin cancer.5 Another study found that lifeguards at pools in areas where societal norms supporting sun safety are stronger exhibited noticeably more sun protection practices, with regression estimates of 0.22 (95% CI, 0.17-0.26).7 Empowering lifeguards with valuable health knowledge during their regular training could potentially reduce their risk for skin cancer,4 as they may be more inclined to use sunscreen appropriately and reach out to a dermatologist for regular skin checks and evaluation of suspicious lesions.
Role of Lifeguards in Public Skin Cancer Prevention Efforts
Once trained on skin cancer prevention, lifeguards also can play a pivotal role in promoting sunscreen use among the public. Despite the widespread availability of high-quality sunscreens, many swimmers and beachgoers neglect to regularly apply or reapply sunscreen, especially on commonly exposed areas such as the back, shoulders, and face.8 Educating lifeguards on skin cancer prevention could enhance health outcomes by increasing early detection rates and promoting sun-safe behaviors among the general public.9 However, additional training requirements might increase the cost and time commitment for lifeguard certification, potentially leading to staffing shortages.3,7 There also is a risk of lifeguards overstepping their role and providing inaccurate medical advice, which could cause distress or even lead to liability issues.7 Balancing these factors will be crucial in developing effective and sustainable skin cancer prevention programs for lifeguards.
Implementing Lifeguard Skin Cancer Training
Implementing skin cancer prevention training programs for lifeguards requires strategic collaboration between dermatologists, and lifeguard training organizations to ensure that the participants receive consistent and comprehensive training.10 Additionally, public health campaigns can support these efforts by raising awareness about the importance of sun safety and regular skin checks.6 Tailored training modules/materials, ongoing technical assistance, and active, multicomponent approaches that account for both individual and environmental factors can increase program implementation in a variety of community settings.
Final Thoughts
Through effective education, lifeguards can potentially have a substantial impact on skin cancer prevention, both among lifeguards themselves and the general public. By promoting proper sunscreen use, lifeguards can help reduce the incidence and mortality associated with skin cancers. Future studies should focus on developing and implementing targeted education initiatives for lifeguards, fostering collaboration between relevant stakeholders, and raising public awareness about the importance of sun safety and early skin cancer detection. These efforts ultimately could lead to improved public health outcomes and reduced skin cancer rates, particularly in high-risk populations that frequently are exposed to UV radiation.
Lifeguards play a crucial role in ensuring water safety, but they also are uniquely positioned to promote skin cancer prevention and proper sunscreen use.1,2 There are several benefits and challenges to offering skin cancer prevention training for lifeguards.3 We examine the advantages of training, highlight the role lifeguards can play in larger public skin cancer prevention efforts, and address practical techniques for developing lifeguardfocused skin cancer education programs. By providing this knowledge to lifeguards, we can improve community health outcomes and encourage sun-safe behaviors in high-risk outdoor locations.
Benefits of Skin Cancer Prevention Training for Lifeguards
Research has shown that lifeguards are at an elevated risk for basal cell carcinoma, squamous cell carcinoma, and melanoma due to frequent prolonged occupational sun exposure.1,2,4-6 Therefore, comprehensive education on skin cancer prevention—including instruction on proper sunscreen application techniques and the importance of regular reapplication as well as how to recognize suspicious skin lesions—should be incorporated into lifeguard certification programs. One study evaluating the effectiveness of a skin cancer prevention program for lifeguards found that many of the participants lacked a thorough understanding of the different types of skin cancer.5 Another study found that lifeguards at pools in areas where societal norms supporting sun safety are stronger exhibited noticeably more sun protection practices, with regression estimates of 0.22 (95% CI, 0.17-0.26).7 Empowering lifeguards with valuable health knowledge during their regular training could potentially reduce their risk for skin cancer,4 as they may be more inclined to use sunscreen appropriately and reach out to a dermatologist for regular skin checks and evaluation of suspicious lesions.
Role of Lifeguards in Public Skin Cancer Prevention Efforts
Once trained on skin cancer prevention, lifeguards also can play a pivotal role in promoting sunscreen use among the public. Despite the widespread availability of high-quality sunscreens, many swimmers and beachgoers neglect to regularly apply or reapply sunscreen, especially on commonly exposed areas such as the back, shoulders, and face.8 Educating lifeguards on skin cancer prevention could enhance health outcomes by increasing early detection rates and promoting sun-safe behaviors among the general public.9 However, additional training requirements might increase the cost and time commitment for lifeguard certification, potentially leading to staffing shortages.3,7 There also is a risk of lifeguards overstepping their role and providing inaccurate medical advice, which could cause distress or even lead to liability issues.7 Balancing these factors will be crucial in developing effective and sustainable skin cancer prevention programs for lifeguards.
Implementing Lifeguard Skin Cancer Training
Implementing skin cancer prevention training programs for lifeguards requires strategic collaboration between dermatologists, and lifeguard training organizations to ensure that the participants receive consistent and comprehensive training.10 Additionally, public health campaigns can support these efforts by raising awareness about the importance of sun safety and regular skin checks.6 Tailored training modules/materials, ongoing technical assistance, and active, multicomponent approaches that account for both individual and environmental factors can increase program implementation in a variety of community settings.
Final Thoughts
Through effective education, lifeguards can potentially have a substantial impact on skin cancer prevention, both among lifeguards themselves and the general public. By promoting proper sunscreen use, lifeguards can help reduce the incidence and mortality associated with skin cancers. Future studies should focus on developing and implementing targeted education initiatives for lifeguards, fostering collaboration between relevant stakeholders, and raising public awareness about the importance of sun safety and early skin cancer detection. These efforts ultimately could lead to improved public health outcomes and reduced skin cancer rates, particularly in high-risk populations that frequently are exposed to UV radiation.
- Enos CW, Rey S, Slocum J, et al. Sun-protection behaviors among active members of the United States Lifesaving Association. J Clin Aesthet Dermatol. 2021;14:14-20.
- Verma K, Lewis DJ, Siddiqui FS, et al. Mohs micrographic surgery management of melanoma and melanoma in situ. StatPearls. Updated August 28, 2024. Accessed April 15, 2025. https://www.ncbi.nlm.nih.gov/books/NBK606123/
- Verma KK, Joshi TP, Lewis DJ, et al. Nail technicians as partners in early melanoma detection: bridging the knowledge gap. Arch Dermatol Res. 2024;316:586. doi:10.1007/s00403-024-03342-0
- Geller AC, Glanz K, Shigaki D, et al. Impact of skin cancer prevention on outdoor aquatics staff: the Pool Cool program in Hawaii and Massachusetts. Prev Med. 2001;33:155-161. doi:10.1006/pmed.2001.0870
- Hiemstra M, Glanz K, Nehl E. Changes in sunburn and tanning attitudes among lifeguards over a summer season. J Am Acad Dermatol. 2012;66:430-437. doi:10.1016/j.jaad.2010.11.050
- Verma KK, Ahmad N, Friedmann DP, et al. Melanoma in tattooed skin: diagnostic challenges and the potential for tattoo artists in early detection. Arch Dermatol Res. 2024;316:690. doi:10.1007/s00403-024-03415-0
- Hall DM, McCarty F, Elliott T, et al. Lifeguards’ sun protection habits and sunburns: association with sun-safe environments and skin cancer prevention program participation. Arch Dermatol. 2009;145:139-144. doi:10.1001/archdermatol.2008.553
- Emmons KM, Geller AC, Puleo E, et al. Skin cancer education and early detection at the beach: a randomized trial of dermatologist examination and biometric feedback. J Am Acad Dermatol. 2011;64:282-289. doi:10.1016/j.jaad.2010.01.040
- Rabin BA, Nehl E, Elliott T, et al. Individual and setting level predictors of the implementation of a skin cancer prevention program: a multilevel analysis. Implement Sci. 2010;5:40. doi:10.1186/1748-5908-5-40
- Walkosz BJ, Buller D, Buller M, et al. Sun safe workplaces: effect of an occupational skin cancer prevention program on employee sun safety practices. J Occup Environ Med. 2018;60:900-997. doi:10.1097 /JOM.0000000000001427
- Enos CW, Rey S, Slocum J, et al. Sun-protection behaviors among active members of the United States Lifesaving Association. J Clin Aesthet Dermatol. 2021;14:14-20.
- Verma K, Lewis DJ, Siddiqui FS, et al. Mohs micrographic surgery management of melanoma and melanoma in situ. StatPearls. Updated August 28, 2024. Accessed April 15, 2025. https://www.ncbi.nlm.nih.gov/books/NBK606123/
- Verma KK, Joshi TP, Lewis DJ, et al. Nail technicians as partners in early melanoma detection: bridging the knowledge gap. Arch Dermatol Res. 2024;316:586. doi:10.1007/s00403-024-03342-0
- Geller AC, Glanz K, Shigaki D, et al. Impact of skin cancer prevention on outdoor aquatics staff: the Pool Cool program in Hawaii and Massachusetts. Prev Med. 2001;33:155-161. doi:10.1006/pmed.2001.0870
- Hiemstra M, Glanz K, Nehl E. Changes in sunburn and tanning attitudes among lifeguards over a summer season. J Am Acad Dermatol. 2012;66:430-437. doi:10.1016/j.jaad.2010.11.050
- Verma KK, Ahmad N, Friedmann DP, et al. Melanoma in tattooed skin: diagnostic challenges and the potential for tattoo artists in early detection. Arch Dermatol Res. 2024;316:690. doi:10.1007/s00403-024-03415-0
- Hall DM, McCarty F, Elliott T, et al. Lifeguards’ sun protection habits and sunburns: association with sun-safe environments and skin cancer prevention program participation. Arch Dermatol. 2009;145:139-144. doi:10.1001/archdermatol.2008.553
- Emmons KM, Geller AC, Puleo E, et al. Skin cancer education and early detection at the beach: a randomized trial of dermatologist examination and biometric feedback. J Am Acad Dermatol. 2011;64:282-289. doi:10.1016/j.jaad.2010.01.040
- Rabin BA, Nehl E, Elliott T, et al. Individual and setting level predictors of the implementation of a skin cancer prevention program: a multilevel analysis. Implement Sci. 2010;5:40. doi:10.1186/1748-5908-5-40
- Walkosz BJ, Buller D, Buller M, et al. Sun safe workplaces: effect of an occupational skin cancer prevention program on employee sun safety practices. J Occup Environ Med. 2018;60:900-997. doi:10.1097 /JOM.0000000000001427
Training Lifeguards to Assist in Skin Cancer Prevention
Training Lifeguards to Assist in Skin Cancer Prevention