User login
Training Lifeguards to Assist in Skin Cancer Prevention
Training Lifeguards to Assist in Skin Cancer Prevention
Lifeguards play a crucial role in ensuring water safety, but they also are uniquely positioned to promote skin cancer prevention and proper sunscreen use.1,2 There are several benefits and challenges to offering skin cancer prevention training for lifeguards.3 We examine the advantages of training, highlight the role lifeguards can play in larger public skin cancer prevention efforts, and address practical techniques for developing lifeguardfocused skin cancer education programs. By providing this knowledge to lifeguards, we can improve community health outcomes and encourage sun-safe behaviors in high-risk outdoor locations.
Benefits of Skin Cancer Prevention Training for Lifeguards
Research has shown that lifeguards are at an elevated risk for basal cell carcinoma, squamous cell carcinoma, and melanoma due to frequent prolonged occupational sun exposure.1,2,4-6 Therefore, comprehensive education on skin cancer prevention—including instruction on proper sunscreen application techniques and the importance of regular reapplication as well as how to recognize suspicious skin lesions—should be incorporated into lifeguard certification programs. One study evaluating the effectiveness of a skin cancer prevention program for lifeguards found that many of the participants lacked a thorough understanding of the different types of skin cancer.5 Another study found that lifeguards at pools in areas where societal norms supporting sun safety are stronger exhibited noticeably more sun protection practices, with regression estimates of 0.22 (95% CI, 0.17-0.26).7 Empowering lifeguards with valuable health knowledge during their regular training could potentially reduce their risk for skin cancer,4 as they may be more inclined to use sunscreen appropriately and reach out to a dermatologist for regular skin checks and evaluation of suspicious lesions.
Role of Lifeguards in Public Skin Cancer Prevention Efforts
Once trained on skin cancer prevention, lifeguards also can play a pivotal role in promoting sunscreen use among the public. Despite the widespread availability of high-quality sunscreens, many swimmers and beachgoers neglect to regularly apply or reapply sunscreen, especially on commonly exposed areas such as the back, shoulders, and face.8 Educating lifeguards on skin cancer prevention could enhance health outcomes by increasing early detection rates and promoting sun-safe behaviors among the general public.9 However, additional training requirements might increase the cost and time commitment for lifeguard certification, potentially leading to staffing shortages.3,7 There also is a risk of lifeguards overstepping their role and providing inaccurate medical advice, which could cause distress or even lead to liability issues.7 Balancing these factors will be crucial in developing effective and sustainable skin cancer prevention programs for lifeguards.
Implementing Lifeguard Skin Cancer Training
Implementing skin cancer prevention training programs for lifeguards requires strategic collaboration between dermatologists, and lifeguard training organizations to ensure that the participants receive consistent and comprehensive training.10 Additionally, public health campaigns can support these efforts by raising awareness about the importance of sun safety and regular skin checks.6 Tailored training modules/materials, ongoing technical assistance, and active, multicomponent approaches that account for both individual and environmental factors can increase program implementation in a variety of community settings.
Final Thoughts
Through effective education, lifeguards can potentially have a substantial impact on skin cancer prevention, both among lifeguards themselves and the general public. By promoting proper sunscreen use, lifeguards can help reduce the incidence and mortality associated with skin cancers. Future studies should focus on developing and implementing targeted education initiatives for lifeguards, fostering collaboration between relevant stakeholders, and raising public awareness about the importance of sun safety and early skin cancer detection. These efforts ultimately could lead to improved public health outcomes and reduced skin cancer rates, particularly in high-risk populations that frequently are exposed to UV radiation.
- Enos CW, Rey S, Slocum J, et al. Sun-protection behaviors among active members of the United States Lifesaving Association. J Clin Aesthet Dermatol. 2021;14:14-20.
- Verma K, Lewis DJ, Siddiqui FS, et al. Mohs micrographic surgery management of melanoma and melanoma in situ. StatPearls. Updated August 28, 2024. Accessed April 15, 2025. https://www.ncbi.nlm.nih.gov/books/NBK606123/
- Verma KK, Joshi TP, Lewis DJ, et al. Nail technicians as partners in early melanoma detection: bridging the knowledge gap. Arch Dermatol Res. 2024;316:586. doi:10.1007/s00403-024-03342-0
- Geller AC, Glanz K, Shigaki D, et al. Impact of skin cancer prevention on outdoor aquatics staff: the Pool Cool program in Hawaii and Massachusetts. Prev Med. 2001;33:155-161. doi:10.1006/pmed.2001.0870
- Hiemstra M, Glanz K, Nehl E. Changes in sunburn and tanning attitudes among lifeguards over a summer season. J Am Acad Dermatol. 2012;66:430-437. doi:10.1016/j.jaad.2010.11.050
- Verma KK, Ahmad N, Friedmann DP, et al. Melanoma in tattooed skin: diagnostic challenges and the potential for tattoo artists in early detection. Arch Dermatol Res. 2024;316:690. doi:10.1007/s00403-024-03415-0
- Hall DM, McCarty F, Elliott T, et al. Lifeguards’ sun protection habits and sunburns: association with sun-safe environments and skin cancer prevention program participation. Arch Dermatol. 2009;145:139-144. doi:10.1001/archdermatol.2008.553
- Emmons KM, Geller AC, Puleo E, et al. Skin cancer education and early detection at the beach: a randomized trial of dermatologist examination and biometric feedback. J Am Acad Dermatol. 2011;64:282-289. doi:10.1016/j.jaad.2010.01.040
- Rabin BA, Nehl E, Elliott T, et al. Individual and setting level predictors of the implementation of a skin cancer prevention program: a multilevel analysis. Implement Sci. 2010;5:40. doi:10.1186/1748-5908-5-40
- Walkosz BJ, Buller D, Buller M, et al. Sun safe workplaces: effect of an occupational skin cancer prevention program on employee sun safety practices. J Occup Environ Med. 2018;60:900-997. doi:10.1097 /JOM.0000000000001427
Lifeguards play a crucial role in ensuring water safety, but they also are uniquely positioned to promote skin cancer prevention and proper sunscreen use.1,2 There are several benefits and challenges to offering skin cancer prevention training for lifeguards.3 We examine the advantages of training, highlight the role lifeguards can play in larger public skin cancer prevention efforts, and address practical techniques for developing lifeguardfocused skin cancer education programs. By providing this knowledge to lifeguards, we can improve community health outcomes and encourage sun-safe behaviors in high-risk outdoor locations.
Benefits of Skin Cancer Prevention Training for Lifeguards
Research has shown that lifeguards are at an elevated risk for basal cell carcinoma, squamous cell carcinoma, and melanoma due to frequent prolonged occupational sun exposure.1,2,4-6 Therefore, comprehensive education on skin cancer prevention—including instruction on proper sunscreen application techniques and the importance of regular reapplication as well as how to recognize suspicious skin lesions—should be incorporated into lifeguard certification programs. One study evaluating the effectiveness of a skin cancer prevention program for lifeguards found that many of the participants lacked a thorough understanding of the different types of skin cancer.5 Another study found that lifeguards at pools in areas where societal norms supporting sun safety are stronger exhibited noticeably more sun protection practices, with regression estimates of 0.22 (95% CI, 0.17-0.26).7 Empowering lifeguards with valuable health knowledge during their regular training could potentially reduce their risk for skin cancer,4 as they may be more inclined to use sunscreen appropriately and reach out to a dermatologist for regular skin checks and evaluation of suspicious lesions.
Role of Lifeguards in Public Skin Cancer Prevention Efforts
Once trained on skin cancer prevention, lifeguards also can play a pivotal role in promoting sunscreen use among the public. Despite the widespread availability of high-quality sunscreens, many swimmers and beachgoers neglect to regularly apply or reapply sunscreen, especially on commonly exposed areas such as the back, shoulders, and face.8 Educating lifeguards on skin cancer prevention could enhance health outcomes by increasing early detection rates and promoting sun-safe behaviors among the general public.9 However, additional training requirements might increase the cost and time commitment for lifeguard certification, potentially leading to staffing shortages.3,7 There also is a risk of lifeguards overstepping their role and providing inaccurate medical advice, which could cause distress or even lead to liability issues.7 Balancing these factors will be crucial in developing effective and sustainable skin cancer prevention programs for lifeguards.
Implementing Lifeguard Skin Cancer Training
Implementing skin cancer prevention training programs for lifeguards requires strategic collaboration between dermatologists, and lifeguard training organizations to ensure that the participants receive consistent and comprehensive training.10 Additionally, public health campaigns can support these efforts by raising awareness about the importance of sun safety and regular skin checks.6 Tailored training modules/materials, ongoing technical assistance, and active, multicomponent approaches that account for both individual and environmental factors can increase program implementation in a variety of community settings.
Final Thoughts
Through effective education, lifeguards can potentially have a substantial impact on skin cancer prevention, both among lifeguards themselves and the general public. By promoting proper sunscreen use, lifeguards can help reduce the incidence and mortality associated with skin cancers. Future studies should focus on developing and implementing targeted education initiatives for lifeguards, fostering collaboration between relevant stakeholders, and raising public awareness about the importance of sun safety and early skin cancer detection. These efforts ultimately could lead to improved public health outcomes and reduced skin cancer rates, particularly in high-risk populations that frequently are exposed to UV radiation.
Lifeguards play a crucial role in ensuring water safety, but they also are uniquely positioned to promote skin cancer prevention and proper sunscreen use.1,2 There are several benefits and challenges to offering skin cancer prevention training for lifeguards.3 We examine the advantages of training, highlight the role lifeguards can play in larger public skin cancer prevention efforts, and address practical techniques for developing lifeguardfocused skin cancer education programs. By providing this knowledge to lifeguards, we can improve community health outcomes and encourage sun-safe behaviors in high-risk outdoor locations.
Benefits of Skin Cancer Prevention Training for Lifeguards
Research has shown that lifeguards are at an elevated risk for basal cell carcinoma, squamous cell carcinoma, and melanoma due to frequent prolonged occupational sun exposure.1,2,4-6 Therefore, comprehensive education on skin cancer prevention—including instruction on proper sunscreen application techniques and the importance of regular reapplication as well as how to recognize suspicious skin lesions—should be incorporated into lifeguard certification programs. One study evaluating the effectiveness of a skin cancer prevention program for lifeguards found that many of the participants lacked a thorough understanding of the different types of skin cancer.5 Another study found that lifeguards at pools in areas where societal norms supporting sun safety are stronger exhibited noticeably more sun protection practices, with regression estimates of 0.22 (95% CI, 0.17-0.26).7 Empowering lifeguards with valuable health knowledge during their regular training could potentially reduce their risk for skin cancer,4 as they may be more inclined to use sunscreen appropriately and reach out to a dermatologist for regular skin checks and evaluation of suspicious lesions.
Role of Lifeguards in Public Skin Cancer Prevention Efforts
Once trained on skin cancer prevention, lifeguards also can play a pivotal role in promoting sunscreen use among the public. Despite the widespread availability of high-quality sunscreens, many swimmers and beachgoers neglect to regularly apply or reapply sunscreen, especially on commonly exposed areas such as the back, shoulders, and face.8 Educating lifeguards on skin cancer prevention could enhance health outcomes by increasing early detection rates and promoting sun-safe behaviors among the general public.9 However, additional training requirements might increase the cost and time commitment for lifeguard certification, potentially leading to staffing shortages.3,7 There also is a risk of lifeguards overstepping their role and providing inaccurate medical advice, which could cause distress or even lead to liability issues.7 Balancing these factors will be crucial in developing effective and sustainable skin cancer prevention programs for lifeguards.
Implementing Lifeguard Skin Cancer Training
Implementing skin cancer prevention training programs for lifeguards requires strategic collaboration between dermatologists, and lifeguard training organizations to ensure that the participants receive consistent and comprehensive training.10 Additionally, public health campaigns can support these efforts by raising awareness about the importance of sun safety and regular skin checks.6 Tailored training modules/materials, ongoing technical assistance, and active, multicomponent approaches that account for both individual and environmental factors can increase program implementation in a variety of community settings.
Final Thoughts
Through effective education, lifeguards can potentially have a substantial impact on skin cancer prevention, both among lifeguards themselves and the general public. By promoting proper sunscreen use, lifeguards can help reduce the incidence and mortality associated with skin cancers. Future studies should focus on developing and implementing targeted education initiatives for lifeguards, fostering collaboration between relevant stakeholders, and raising public awareness about the importance of sun safety and early skin cancer detection. These efforts ultimately could lead to improved public health outcomes and reduced skin cancer rates, particularly in high-risk populations that frequently are exposed to UV radiation.
- Enos CW, Rey S, Slocum J, et al. Sun-protection behaviors among active members of the United States Lifesaving Association. J Clin Aesthet Dermatol. 2021;14:14-20.
- Verma K, Lewis DJ, Siddiqui FS, et al. Mohs micrographic surgery management of melanoma and melanoma in situ. StatPearls. Updated August 28, 2024. Accessed April 15, 2025. https://www.ncbi.nlm.nih.gov/books/NBK606123/
- Verma KK, Joshi TP, Lewis DJ, et al. Nail technicians as partners in early melanoma detection: bridging the knowledge gap. Arch Dermatol Res. 2024;316:586. doi:10.1007/s00403-024-03342-0
- Geller AC, Glanz K, Shigaki D, et al. Impact of skin cancer prevention on outdoor aquatics staff: the Pool Cool program in Hawaii and Massachusetts. Prev Med. 2001;33:155-161. doi:10.1006/pmed.2001.0870
- Hiemstra M, Glanz K, Nehl E. Changes in sunburn and tanning attitudes among lifeguards over a summer season. J Am Acad Dermatol. 2012;66:430-437. doi:10.1016/j.jaad.2010.11.050
- Verma KK, Ahmad N, Friedmann DP, et al. Melanoma in tattooed skin: diagnostic challenges and the potential for tattoo artists in early detection. Arch Dermatol Res. 2024;316:690. doi:10.1007/s00403-024-03415-0
- Hall DM, McCarty F, Elliott T, et al. Lifeguards’ sun protection habits and sunburns: association with sun-safe environments and skin cancer prevention program participation. Arch Dermatol. 2009;145:139-144. doi:10.1001/archdermatol.2008.553
- Emmons KM, Geller AC, Puleo E, et al. Skin cancer education and early detection at the beach: a randomized trial of dermatologist examination and biometric feedback. J Am Acad Dermatol. 2011;64:282-289. doi:10.1016/j.jaad.2010.01.040
- Rabin BA, Nehl E, Elliott T, et al. Individual and setting level predictors of the implementation of a skin cancer prevention program: a multilevel analysis. Implement Sci. 2010;5:40. doi:10.1186/1748-5908-5-40
- Walkosz BJ, Buller D, Buller M, et al. Sun safe workplaces: effect of an occupational skin cancer prevention program on employee sun safety practices. J Occup Environ Med. 2018;60:900-997. doi:10.1097 /JOM.0000000000001427
- Enos CW, Rey S, Slocum J, et al. Sun-protection behaviors among active members of the United States Lifesaving Association. J Clin Aesthet Dermatol. 2021;14:14-20.
- Verma K, Lewis DJ, Siddiqui FS, et al. Mohs micrographic surgery management of melanoma and melanoma in situ. StatPearls. Updated August 28, 2024. Accessed April 15, 2025. https://www.ncbi.nlm.nih.gov/books/NBK606123/
- Verma KK, Joshi TP, Lewis DJ, et al. Nail technicians as partners in early melanoma detection: bridging the knowledge gap. Arch Dermatol Res. 2024;316:586. doi:10.1007/s00403-024-03342-0
- Geller AC, Glanz K, Shigaki D, et al. Impact of skin cancer prevention on outdoor aquatics staff: the Pool Cool program in Hawaii and Massachusetts. Prev Med. 2001;33:155-161. doi:10.1006/pmed.2001.0870
- Hiemstra M, Glanz K, Nehl E. Changes in sunburn and tanning attitudes among lifeguards over a summer season. J Am Acad Dermatol. 2012;66:430-437. doi:10.1016/j.jaad.2010.11.050
- Verma KK, Ahmad N, Friedmann DP, et al. Melanoma in tattooed skin: diagnostic challenges and the potential for tattoo artists in early detection. Arch Dermatol Res. 2024;316:690. doi:10.1007/s00403-024-03415-0
- Hall DM, McCarty F, Elliott T, et al. Lifeguards’ sun protection habits and sunburns: association with sun-safe environments and skin cancer prevention program participation. Arch Dermatol. 2009;145:139-144. doi:10.1001/archdermatol.2008.553
- Emmons KM, Geller AC, Puleo E, et al. Skin cancer education and early detection at the beach: a randomized trial of dermatologist examination and biometric feedback. J Am Acad Dermatol. 2011;64:282-289. doi:10.1016/j.jaad.2010.01.040
- Rabin BA, Nehl E, Elliott T, et al. Individual and setting level predictors of the implementation of a skin cancer prevention program: a multilevel analysis. Implement Sci. 2010;5:40. doi:10.1186/1748-5908-5-40
- Walkosz BJ, Buller D, Buller M, et al. Sun safe workplaces: effect of an occupational skin cancer prevention program on employee sun safety practices. J Occup Environ Med. 2018;60:900-997. doi:10.1097 /JOM.0000000000001427
Training Lifeguards to Assist in Skin Cancer Prevention
Training Lifeguards to Assist in Skin Cancer Prevention
Treating Barrett’s Esophagus: Comparing EMR and ESD
Dear colleagues,
Many of us diagnose and treat patients with Barrett’s esophagus, estimated to affect up to 5.6% of the US adult population. There has been an expanding array of tools to help diagnose and effectively treat Barrett’s esophagus with dysplasia and malignancy. In particular, endoscopic submucosal dissection (ESD) has emerged as an important method for treating early cancer in the gastrointestinal tract.
But how do we incorporate ESD into our algorithm for management, especially with the popularity and effectiveness of endoscopic mucosal resection (EMR)? In this issue of Perspectives we aim to provide context for the use of ESD, as compared with EMR. Dr. Silvio de Melo discusses his preferred EMR technique and its many advantages in the management of BE, including for residual or refractory areas. In contrast, Dr. Mohamed Othman reviews the power of ESD and when we should consider this approach over EMR. We hope these discussions will facilitate your care for patients with Barrett’s esophagus.
We also welcome your thoughts on this topic — join the conversation on X at @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, and chief of endoscopy at West Haven VA Medical Center, both in Connecticut. He is an associate editor for GI & Hepatology News.
Endoscopic Mucosal Resection: The ‘Workhorse’ for Patient Care
BY SILVIO W. DE MELO JR, MD, AGAF
Barrett’s esophagus (BE) remains an important clinical problem, being one of the modifiable risk factors for esophageal adenocarcinoma. The care for BE is complex and requires several steps to correctly formulate a therapeutic plan. It starts with a proper endoscopic examination. It is recommended to spend at least 1 minute inspecting and evaluating every centimeter of the salmon-colored epithelium, looking for change in vascular pattern, erosions/ulcers, nodules, and/or masses. After the inspection, random biopsies every 1-2 cm plus targeted biopsies will guide you. It is still controversial if the addition of other sampling strategies, such as brushings or confocal endomicroscopy, is needed.
The introduction of radiofrequency ablation (RFA) was paramount in popularizing the treatment options for BE and sunsetting the previous dominant modality, photodynamic therapy (PDT). RFA proved to have a superior clinical efficacy in replacing the intestinal metaplasia/BE with neosquamous epithelium while boosting a much better safety profile, compared with PDT. However, RFA is most efficacious for “flat BE” and it is not an effective, nor recommended, method to treat nodular BE or early cancer, such as carcinoma in situ or nodular high-grade dysplasia. Endoscopic mucosal resection (EMR) is utilized to overcome those limitations.
There are several techniques utilized for EMR:
- The lift and snare technique.
- The snare-in-cap technique.
- The Band-snare technique.
The free-hand submucosal lift and snare is not frequently used in the esophagus. It is difficult to maintain visualization while being confident that one has the whole lesion inside the snare and that the distal (anal side) part of the lesion is free of any unwanted tissue (to minimize complications such as perforations or unwelcomed gastric resections). It is difficult after the first resection to lift an adjacent area, as the fluid easily leaks from the first resected spot, thus removing larger lesions in piece-meal fashion is challenging. This technique can be used in small (in my personal experience, less than 5 mm) lesions, but, given that there are better and safer alternatives, I almost never use this technique for my esophageal EMR cases. I prefer to use the band-snare technique even for lesions under 5 mm.
The snare-in-cap technique has been utilized in the esophagus. In this technique, a cap is attached to the distal end of the scope and the size of the resection is determined by the size of the cap, usually under 1.5 cm. Because of the risk of perforation without previous lifting, it is required that the lesion is lifted with a submucosal fluid, saline or any Food and Drug Administration–approved EMR solution. The lesion is then suctioned inside the cap where the snare had been previously opened inside the cap, the snare is closed, and the tissue is resected. The same limitations regarding the inability to remove larger lesions (greater than 1.5 cm) because of the challenge in lifting the adjacent area applies here. However, the perforation risk for this technique is higher than the traditional lift and the band and snare techniques. Thus, this technique has fallen out of favor for most endoscopists.
The third technique (band-snare EMR) is the one that most endoscopists use for endoscopic mucosal resection. It is a small variation of the already time-tested and very familiar procedure of esophageal variceal band ligation (EVL). There are multiple commercially available kits for esophageal EMR. The kit contains the chamber with the bands and a proprietary hexagonal snare used to resect the specimen.
The advantages of this technique are:
- It is widely commercially available.
- It builds on a familiar procedure, EVL, therefore the learning curve is short.
- The set-up is quick and the procedure can be completed safely and effectively.
- There is no need for injecting the submucosal with a lifting solution.
- Despite the band having a size limitation of 1 cm, one can remove larger lesions by repeating the band and resect process, using the rosette technique.
Band-snare EMR also has limitations:
- There are only six bands on each chamber. Depending on the size of the lesion, one may need to use multiple kits.
- It is not suitable for en bloc resection of lesions greater than 1 cm.
My experience with band EMR is that we can complete the procedure in under 1 hour. The dreaded complication of perforation occurs in under 1% of cases, most bleeding episodes can easily be controlled endoscopically, and the risk of post-EMR stricture is minimal. Therefore, band EMR is the most used technique for esophageal endoscopic resections.
Esophageal EMR is also effective for other indications in BE therapy, such as residual and recurrent BE. Band-snare EMR can be used for an en bloc resection or rosette technique for the areas resistant to ablation therapies with great success and safety.
From a financial standpoint, comparing EMR with endoscopic submucosal dissection (ESD), EMR is the superior strategy given that EMR is widely available, has a much shorter learning curve, has a greater safety profile, is applicable to a wider variety of indications, and has a more favorable return on investment. EMR should be the workhorse for the care of patients with BE, reserving ESD for specific indications.
In summary, there is no “one-size-fits-all” endoscopic therapy in the care of BE. Most Barrett’s patients can be successfully treated with a combination of ablation plus EMR, reserving ESD for select cases.
Dr. de Melo is section chief of gastroenterology at the Orlando VA Healthcare System, Orlando, Florida. He declares no conflicts of interest.
ESD Over EMR for Resecting Esophageal Lesions
BY MOHAMED O. OTHMAN, MD, AGAF
Although endoscopic submucosal dissection (ESD) is the preferred endoscopic resection method in the East, the adoption of this technique in the West, particularly in the United States, has faced many hurdles. Many endoscopists who routinely perform piecemeal endoscopic mucosal resection (EMR) question the utility of ESD, arguing that EMR is just as effective. While this may hold true in certain situations, the global trend in the endoscopic treatment of early esophageal squamous cell carcinoma, nodular Barrett’s esophagus (BE), and early esophageal adenocarcinoma (EAC) has clearly shifted toward ESD. In this perspective, I will summarize why ESD is preferred over EMR for these indications and explore why ESD has yet to gain widespread adoption in the United States.
The superiority of ESD over EMR has been well established in multiple publications from both Eastern and Western literature. Mejia-Perez et al, in a multicenter cohort study from eight centers in North America, compared outcomes of ESD vs EMR for BE with high-grade dysplasia (HGD) or T1a adenocarcinoma in 243 patients. ESD achieved significantly higher en bloc resection rates (89% vs 43%) and R0 resection rates (73% vs 56%), compared with EMR, along with a substantially lower recurrence/residual disease rate on follow-up (3.5% in the ESD group vs 31.4% in EMR group). Additionally, more patients required repeat endoscopic resection after EMR to treat residual or recurrent disease (EMR, 24.2% vs ESD, 3.5%; P < .001).
Han et al conducted a meta-analysis of 22 studies comparing ESD and EMR for early esophageal neoplasia, including both squamous cell carcinoma (SCC) and BE-associated lesions. ESD was associated with significantly higher curative resection rates than EMR (OR, 9.74; 95% CI, 4.83-19.62; P < .0001). Of note, lesion size was a critical factor in determining the advantage of ESD. For lesions ≤ 10 mm, curative resection rates were comparable between ESD and EMR. However, for lesions > 10 mm, ESD achieved significantly higher curative resection rates. This size-based recommendation has been adopted by the American Society of Gastrointestinal Endoscopy (ASGE) in their recent guidelines on ESD indications for esophageal lesions. ASGE guidelines favors ESD over EMR for SCC lesions > 15 mm and for nodular BE with dysplasia or early EAC > 20 mm.
ESD is particularly beneficial in patients who develop early adenocarcinoma after RFA or EMR. Mesureur et al evaluated the efficacy of salvage ESD for Barrett’s recurrence or residual BE following RFA. In their multicenter retrospective study of 56 patients, salvage ESD achieved an en bloc resection rate of 89.3%, despite significant fibrosis, with an R0 resection rate of 66%. At a median follow-up of 14 months, most patients remained in endoscopic remission without the need for esophagectomy.
Combining ESD with RFA has also been shown to accelerate the eradication of BE with dysplasia while reducing the number of required sessions. Our group demonstrated the high efficacy of ESD followed by RFA in 18 patients, most of whom had long-segment BE with HGD or EAC. On average, patients required only one to two RFA sessions after ESD to achieve complete eradication of intestinal metaplasia (CE-IM). Over a median follow-up of 42.5 months (IQR, 28-59.25), complete eradication of early esophageal cancer was achieved in 13 patients (100%), eradication of dysplasia in 15 patients (100%), and CE-IM in 14 patients (77.8%).
Despite the overwhelming evidence supporting ESD and the strong endorsement from professional societies, adoption in the West continues to lag. Several factors contribute to this gap. First, ESD has a steep learning curve. Our data showed that, on average, an untutored practitioner achieved competency after 150-250 procedures, a finding corroborated by other US groups.
Second, there is no specific CPT code for ESD in the United States. Physicians are forced to bill the procedure as EMR or use an unlisted code, resulting in reimbursement that does not reflect the time and complexity of the procedure. Our group showed that physician reimbursement for ESD is highly variable, ranging from $50 to $800 per case, depending on insurance type.
Third, the increasing emphasis on productivity and RVU generation in academic settings has hindered the growth of ESD training in many institutions. Still, the outlook for ESD in the United States remains encouraging. Multiple industry-sponsored training courses are held annually, and professional societies are investing heavily in expanding access to structured education in ESD. Industry is also innovating devices that improve procedural efficiency and safety. Adopting novel approaches, such as traction-assisted ESD, has made the technique more appealing to endoscopists concerned about long procedure times. For example, our group proposed a standardized esophageal ESD technique that incorporates specimen self-retraction. This method improves both safety and speed and has helped address several procedural challenges. We’ve demonstrated that consistency in technique can substantially expedite esophageal ESD.
Fast forward 5 years: We anticipate a dedicated CPT code for ESD, broader access to advanced resection tools, and an expanding number of fellowships offering structured ESD training. These developments are poised to eliminate many of the current barriers. In summary, with robust data supporting the efficacy of ESD in early esophageal cancer, the focus in the United States should shift toward mastering and integrating the technique, rather than dismissing it in favor of piecemeal EMR.
Dr. Othman is chief of the gastroenterology and hepatology section at Baylor College of Medicine and Medicine Subspecialities Service Line Chief at Baylor St Luke’s Medical Center, both in Houston. He declares no conflicts of interest.
Dear colleagues,
Many of us diagnose and treat patients with Barrett’s esophagus, estimated to affect up to 5.6% of the US adult population. There has been an expanding array of tools to help diagnose and effectively treat Barrett’s esophagus with dysplasia and malignancy. In particular, endoscopic submucosal dissection (ESD) has emerged as an important method for treating early cancer in the gastrointestinal tract.
But how do we incorporate ESD into our algorithm for management, especially with the popularity and effectiveness of endoscopic mucosal resection (EMR)? In this issue of Perspectives we aim to provide context for the use of ESD, as compared with EMR. Dr. Silvio de Melo discusses his preferred EMR technique and its many advantages in the management of BE, including for residual or refractory areas. In contrast, Dr. Mohamed Othman reviews the power of ESD and when we should consider this approach over EMR. We hope these discussions will facilitate your care for patients with Barrett’s esophagus.
We also welcome your thoughts on this topic — join the conversation on X at @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, and chief of endoscopy at West Haven VA Medical Center, both in Connecticut. He is an associate editor for GI & Hepatology News.
Endoscopic Mucosal Resection: The ‘Workhorse’ for Patient Care
BY SILVIO W. DE MELO JR, MD, AGAF
Barrett’s esophagus (BE) remains an important clinical problem, being one of the modifiable risk factors for esophageal adenocarcinoma. The care for BE is complex and requires several steps to correctly formulate a therapeutic plan. It starts with a proper endoscopic examination. It is recommended to spend at least 1 minute inspecting and evaluating every centimeter of the salmon-colored epithelium, looking for change in vascular pattern, erosions/ulcers, nodules, and/or masses. After the inspection, random biopsies every 1-2 cm plus targeted biopsies will guide you. It is still controversial if the addition of other sampling strategies, such as brushings or confocal endomicroscopy, is needed.
The introduction of radiofrequency ablation (RFA) was paramount in popularizing the treatment options for BE and sunsetting the previous dominant modality, photodynamic therapy (PDT). RFA proved to have a superior clinical efficacy in replacing the intestinal metaplasia/BE with neosquamous epithelium while boosting a much better safety profile, compared with PDT. However, RFA is most efficacious for “flat BE” and it is not an effective, nor recommended, method to treat nodular BE or early cancer, such as carcinoma in situ or nodular high-grade dysplasia. Endoscopic mucosal resection (EMR) is utilized to overcome those limitations.
There are several techniques utilized for EMR:
- The lift and snare technique.
- The snare-in-cap technique.
- The Band-snare technique.
The free-hand submucosal lift and snare is not frequently used in the esophagus. It is difficult to maintain visualization while being confident that one has the whole lesion inside the snare and that the distal (anal side) part of the lesion is free of any unwanted tissue (to minimize complications such as perforations or unwelcomed gastric resections). It is difficult after the first resection to lift an adjacent area, as the fluid easily leaks from the first resected spot, thus removing larger lesions in piece-meal fashion is challenging. This technique can be used in small (in my personal experience, less than 5 mm) lesions, but, given that there are better and safer alternatives, I almost never use this technique for my esophageal EMR cases. I prefer to use the band-snare technique even for lesions under 5 mm.
The snare-in-cap technique has been utilized in the esophagus. In this technique, a cap is attached to the distal end of the scope and the size of the resection is determined by the size of the cap, usually under 1.5 cm. Because of the risk of perforation without previous lifting, it is required that the lesion is lifted with a submucosal fluid, saline or any Food and Drug Administration–approved EMR solution. The lesion is then suctioned inside the cap where the snare had been previously opened inside the cap, the snare is closed, and the tissue is resected. The same limitations regarding the inability to remove larger lesions (greater than 1.5 cm) because of the challenge in lifting the adjacent area applies here. However, the perforation risk for this technique is higher than the traditional lift and the band and snare techniques. Thus, this technique has fallen out of favor for most endoscopists.
The third technique (band-snare EMR) is the one that most endoscopists use for endoscopic mucosal resection. It is a small variation of the already time-tested and very familiar procedure of esophageal variceal band ligation (EVL). There are multiple commercially available kits for esophageal EMR. The kit contains the chamber with the bands and a proprietary hexagonal snare used to resect the specimen.
The advantages of this technique are:
- It is widely commercially available.
- It builds on a familiar procedure, EVL, therefore the learning curve is short.
- The set-up is quick and the procedure can be completed safely and effectively.
- There is no need for injecting the submucosal with a lifting solution.
- Despite the band having a size limitation of 1 cm, one can remove larger lesions by repeating the band and resect process, using the rosette technique.
Band-snare EMR also has limitations:
- There are only six bands on each chamber. Depending on the size of the lesion, one may need to use multiple kits.
- It is not suitable for en bloc resection of lesions greater than 1 cm.
My experience with band EMR is that we can complete the procedure in under 1 hour. The dreaded complication of perforation occurs in under 1% of cases, most bleeding episodes can easily be controlled endoscopically, and the risk of post-EMR stricture is minimal. Therefore, band EMR is the most used technique for esophageal endoscopic resections.
Esophageal EMR is also effective for other indications in BE therapy, such as residual and recurrent BE. Band-snare EMR can be used for an en bloc resection or rosette technique for the areas resistant to ablation therapies with great success and safety.
From a financial standpoint, comparing EMR with endoscopic submucosal dissection (ESD), EMR is the superior strategy given that EMR is widely available, has a much shorter learning curve, has a greater safety profile, is applicable to a wider variety of indications, and has a more favorable return on investment. EMR should be the workhorse for the care of patients with BE, reserving ESD for specific indications.
In summary, there is no “one-size-fits-all” endoscopic therapy in the care of BE. Most Barrett’s patients can be successfully treated with a combination of ablation plus EMR, reserving ESD for select cases.
Dr. de Melo is section chief of gastroenterology at the Orlando VA Healthcare System, Orlando, Florida. He declares no conflicts of interest.
ESD Over EMR for Resecting Esophageal Lesions
BY MOHAMED O. OTHMAN, MD, AGAF
Although endoscopic submucosal dissection (ESD) is the preferred endoscopic resection method in the East, the adoption of this technique in the West, particularly in the United States, has faced many hurdles. Many endoscopists who routinely perform piecemeal endoscopic mucosal resection (EMR) question the utility of ESD, arguing that EMR is just as effective. While this may hold true in certain situations, the global trend in the endoscopic treatment of early esophageal squamous cell carcinoma, nodular Barrett’s esophagus (BE), and early esophageal adenocarcinoma (EAC) has clearly shifted toward ESD. In this perspective, I will summarize why ESD is preferred over EMR for these indications and explore why ESD has yet to gain widespread adoption in the United States.
The superiority of ESD over EMR has been well established in multiple publications from both Eastern and Western literature. Mejia-Perez et al, in a multicenter cohort study from eight centers in North America, compared outcomes of ESD vs EMR for BE with high-grade dysplasia (HGD) or T1a adenocarcinoma in 243 patients. ESD achieved significantly higher en bloc resection rates (89% vs 43%) and R0 resection rates (73% vs 56%), compared with EMR, along with a substantially lower recurrence/residual disease rate on follow-up (3.5% in the ESD group vs 31.4% in EMR group). Additionally, more patients required repeat endoscopic resection after EMR to treat residual or recurrent disease (EMR, 24.2% vs ESD, 3.5%; P < .001).
Han et al conducted a meta-analysis of 22 studies comparing ESD and EMR for early esophageal neoplasia, including both squamous cell carcinoma (SCC) and BE-associated lesions. ESD was associated with significantly higher curative resection rates than EMR (OR, 9.74; 95% CI, 4.83-19.62; P < .0001). Of note, lesion size was a critical factor in determining the advantage of ESD. For lesions ≤ 10 mm, curative resection rates were comparable between ESD and EMR. However, for lesions > 10 mm, ESD achieved significantly higher curative resection rates. This size-based recommendation has been adopted by the American Society of Gastrointestinal Endoscopy (ASGE) in their recent guidelines on ESD indications for esophageal lesions. ASGE guidelines favors ESD over EMR for SCC lesions > 15 mm and for nodular BE with dysplasia or early EAC > 20 mm.
ESD is particularly beneficial in patients who develop early adenocarcinoma after RFA or EMR. Mesureur et al evaluated the efficacy of salvage ESD for Barrett’s recurrence or residual BE following RFA. In their multicenter retrospective study of 56 patients, salvage ESD achieved an en bloc resection rate of 89.3%, despite significant fibrosis, with an R0 resection rate of 66%. At a median follow-up of 14 months, most patients remained in endoscopic remission without the need for esophagectomy.
Combining ESD with RFA has also been shown to accelerate the eradication of BE with dysplasia while reducing the number of required sessions. Our group demonstrated the high efficacy of ESD followed by RFA in 18 patients, most of whom had long-segment BE with HGD or EAC. On average, patients required only one to two RFA sessions after ESD to achieve complete eradication of intestinal metaplasia (CE-IM). Over a median follow-up of 42.5 months (IQR, 28-59.25), complete eradication of early esophageal cancer was achieved in 13 patients (100%), eradication of dysplasia in 15 patients (100%), and CE-IM in 14 patients (77.8%).
Despite the overwhelming evidence supporting ESD and the strong endorsement from professional societies, adoption in the West continues to lag. Several factors contribute to this gap. First, ESD has a steep learning curve. Our data showed that, on average, an untutored practitioner achieved competency after 150-250 procedures, a finding corroborated by other US groups.
Second, there is no specific CPT code for ESD in the United States. Physicians are forced to bill the procedure as EMR or use an unlisted code, resulting in reimbursement that does not reflect the time and complexity of the procedure. Our group showed that physician reimbursement for ESD is highly variable, ranging from $50 to $800 per case, depending on insurance type.
Third, the increasing emphasis on productivity and RVU generation in academic settings has hindered the growth of ESD training in many institutions. Still, the outlook for ESD in the United States remains encouraging. Multiple industry-sponsored training courses are held annually, and professional societies are investing heavily in expanding access to structured education in ESD. Industry is also innovating devices that improve procedural efficiency and safety. Adopting novel approaches, such as traction-assisted ESD, has made the technique more appealing to endoscopists concerned about long procedure times. For example, our group proposed a standardized esophageal ESD technique that incorporates specimen self-retraction. This method improves both safety and speed and has helped address several procedural challenges. We’ve demonstrated that consistency in technique can substantially expedite esophageal ESD.
Fast forward 5 years: We anticipate a dedicated CPT code for ESD, broader access to advanced resection tools, and an expanding number of fellowships offering structured ESD training. These developments are poised to eliminate many of the current barriers. In summary, with robust data supporting the efficacy of ESD in early esophageal cancer, the focus in the United States should shift toward mastering and integrating the technique, rather than dismissing it in favor of piecemeal EMR.
Dr. Othman is chief of the gastroenterology and hepatology section at Baylor College of Medicine and Medicine Subspecialities Service Line Chief at Baylor St Luke’s Medical Center, both in Houston. He declares no conflicts of interest.
Dear colleagues,
Many of us diagnose and treat patients with Barrett’s esophagus, estimated to affect up to 5.6% of the US adult population. There has been an expanding array of tools to help diagnose and effectively treat Barrett’s esophagus with dysplasia and malignancy. In particular, endoscopic submucosal dissection (ESD) has emerged as an important method for treating early cancer in the gastrointestinal tract.
But how do we incorporate ESD into our algorithm for management, especially with the popularity and effectiveness of endoscopic mucosal resection (EMR)? In this issue of Perspectives we aim to provide context for the use of ESD, as compared with EMR. Dr. Silvio de Melo discusses his preferred EMR technique and its many advantages in the management of BE, including for residual or refractory areas. In contrast, Dr. Mohamed Othman reviews the power of ESD and when we should consider this approach over EMR. We hope these discussions will facilitate your care for patients with Barrett’s esophagus.
We also welcome your thoughts on this topic — join the conversation on X at @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, and chief of endoscopy at West Haven VA Medical Center, both in Connecticut. He is an associate editor for GI & Hepatology News.
Endoscopic Mucosal Resection: The ‘Workhorse’ for Patient Care
BY SILVIO W. DE MELO JR, MD, AGAF
Barrett’s esophagus (BE) remains an important clinical problem, being one of the modifiable risk factors for esophageal adenocarcinoma. The care for BE is complex and requires several steps to correctly formulate a therapeutic plan. It starts with a proper endoscopic examination. It is recommended to spend at least 1 minute inspecting and evaluating every centimeter of the salmon-colored epithelium, looking for change in vascular pattern, erosions/ulcers, nodules, and/or masses. After the inspection, random biopsies every 1-2 cm plus targeted biopsies will guide you. It is still controversial if the addition of other sampling strategies, such as brushings or confocal endomicroscopy, is needed.
The introduction of radiofrequency ablation (RFA) was paramount in popularizing the treatment options for BE and sunsetting the previous dominant modality, photodynamic therapy (PDT). RFA proved to have a superior clinical efficacy in replacing the intestinal metaplasia/BE with neosquamous epithelium while boosting a much better safety profile, compared with PDT. However, RFA is most efficacious for “flat BE” and it is not an effective, nor recommended, method to treat nodular BE or early cancer, such as carcinoma in situ or nodular high-grade dysplasia. Endoscopic mucosal resection (EMR) is utilized to overcome those limitations.
There are several techniques utilized for EMR:
- The lift and snare technique.
- The snare-in-cap technique.
- The Band-snare technique.
The free-hand submucosal lift and snare is not frequently used in the esophagus. It is difficult to maintain visualization while being confident that one has the whole lesion inside the snare and that the distal (anal side) part of the lesion is free of any unwanted tissue (to minimize complications such as perforations or unwelcomed gastric resections). It is difficult after the first resection to lift an adjacent area, as the fluid easily leaks from the first resected spot, thus removing larger lesions in piece-meal fashion is challenging. This technique can be used in small (in my personal experience, less than 5 mm) lesions, but, given that there are better and safer alternatives, I almost never use this technique for my esophageal EMR cases. I prefer to use the band-snare technique even for lesions under 5 mm.
The snare-in-cap technique has been utilized in the esophagus. In this technique, a cap is attached to the distal end of the scope and the size of the resection is determined by the size of the cap, usually under 1.5 cm. Because of the risk of perforation without previous lifting, it is required that the lesion is lifted with a submucosal fluid, saline or any Food and Drug Administration–approved EMR solution. The lesion is then suctioned inside the cap where the snare had been previously opened inside the cap, the snare is closed, and the tissue is resected. The same limitations regarding the inability to remove larger lesions (greater than 1.5 cm) because of the challenge in lifting the adjacent area applies here. However, the perforation risk for this technique is higher than the traditional lift and the band and snare techniques. Thus, this technique has fallen out of favor for most endoscopists.
The third technique (band-snare EMR) is the one that most endoscopists use for endoscopic mucosal resection. It is a small variation of the already time-tested and very familiar procedure of esophageal variceal band ligation (EVL). There are multiple commercially available kits for esophageal EMR. The kit contains the chamber with the bands and a proprietary hexagonal snare used to resect the specimen.
The advantages of this technique are:
- It is widely commercially available.
- It builds on a familiar procedure, EVL, therefore the learning curve is short.
- The set-up is quick and the procedure can be completed safely and effectively.
- There is no need for injecting the submucosal with a lifting solution.
- Despite the band having a size limitation of 1 cm, one can remove larger lesions by repeating the band and resect process, using the rosette technique.
Band-snare EMR also has limitations:
- There are only six bands on each chamber. Depending on the size of the lesion, one may need to use multiple kits.
- It is not suitable for en bloc resection of lesions greater than 1 cm.
My experience with band EMR is that we can complete the procedure in under 1 hour. The dreaded complication of perforation occurs in under 1% of cases, most bleeding episodes can easily be controlled endoscopically, and the risk of post-EMR stricture is minimal. Therefore, band EMR is the most used technique for esophageal endoscopic resections.
Esophageal EMR is also effective for other indications in BE therapy, such as residual and recurrent BE. Band-snare EMR can be used for an en bloc resection or rosette technique for the areas resistant to ablation therapies with great success and safety.
From a financial standpoint, comparing EMR with endoscopic submucosal dissection (ESD), EMR is the superior strategy given that EMR is widely available, has a much shorter learning curve, has a greater safety profile, is applicable to a wider variety of indications, and has a more favorable return on investment. EMR should be the workhorse for the care of patients with BE, reserving ESD for specific indications.
In summary, there is no “one-size-fits-all” endoscopic therapy in the care of BE. Most Barrett’s patients can be successfully treated with a combination of ablation plus EMR, reserving ESD for select cases.
Dr. de Melo is section chief of gastroenterology at the Orlando VA Healthcare System, Orlando, Florida. He declares no conflicts of interest.
ESD Over EMR for Resecting Esophageal Lesions
BY MOHAMED O. OTHMAN, MD, AGAF
Although endoscopic submucosal dissection (ESD) is the preferred endoscopic resection method in the East, the adoption of this technique in the West, particularly in the United States, has faced many hurdles. Many endoscopists who routinely perform piecemeal endoscopic mucosal resection (EMR) question the utility of ESD, arguing that EMR is just as effective. While this may hold true in certain situations, the global trend in the endoscopic treatment of early esophageal squamous cell carcinoma, nodular Barrett’s esophagus (BE), and early esophageal adenocarcinoma (EAC) has clearly shifted toward ESD. In this perspective, I will summarize why ESD is preferred over EMR for these indications and explore why ESD has yet to gain widespread adoption in the United States.
The superiority of ESD over EMR has been well established in multiple publications from both Eastern and Western literature. Mejia-Perez et al, in a multicenter cohort study from eight centers in North America, compared outcomes of ESD vs EMR for BE with high-grade dysplasia (HGD) or T1a adenocarcinoma in 243 patients. ESD achieved significantly higher en bloc resection rates (89% vs 43%) and R0 resection rates (73% vs 56%), compared with EMR, along with a substantially lower recurrence/residual disease rate on follow-up (3.5% in the ESD group vs 31.4% in EMR group). Additionally, more patients required repeat endoscopic resection after EMR to treat residual or recurrent disease (EMR, 24.2% vs ESD, 3.5%; P < .001).
Han et al conducted a meta-analysis of 22 studies comparing ESD and EMR for early esophageal neoplasia, including both squamous cell carcinoma (SCC) and BE-associated lesions. ESD was associated with significantly higher curative resection rates than EMR (OR, 9.74; 95% CI, 4.83-19.62; P < .0001). Of note, lesion size was a critical factor in determining the advantage of ESD. For lesions ≤ 10 mm, curative resection rates were comparable between ESD and EMR. However, for lesions > 10 mm, ESD achieved significantly higher curative resection rates. This size-based recommendation has been adopted by the American Society of Gastrointestinal Endoscopy (ASGE) in their recent guidelines on ESD indications for esophageal lesions. ASGE guidelines favors ESD over EMR for SCC lesions > 15 mm and for nodular BE with dysplasia or early EAC > 20 mm.
ESD is particularly beneficial in patients who develop early adenocarcinoma after RFA or EMR. Mesureur et al evaluated the efficacy of salvage ESD for Barrett’s recurrence or residual BE following RFA. In their multicenter retrospective study of 56 patients, salvage ESD achieved an en bloc resection rate of 89.3%, despite significant fibrosis, with an R0 resection rate of 66%. At a median follow-up of 14 months, most patients remained in endoscopic remission without the need for esophagectomy.
Combining ESD with RFA has also been shown to accelerate the eradication of BE with dysplasia while reducing the number of required sessions. Our group demonstrated the high efficacy of ESD followed by RFA in 18 patients, most of whom had long-segment BE with HGD or EAC. On average, patients required only one to two RFA sessions after ESD to achieve complete eradication of intestinal metaplasia (CE-IM). Over a median follow-up of 42.5 months (IQR, 28-59.25), complete eradication of early esophageal cancer was achieved in 13 patients (100%), eradication of dysplasia in 15 patients (100%), and CE-IM in 14 patients (77.8%).
Despite the overwhelming evidence supporting ESD and the strong endorsement from professional societies, adoption in the West continues to lag. Several factors contribute to this gap. First, ESD has a steep learning curve. Our data showed that, on average, an untutored practitioner achieved competency after 150-250 procedures, a finding corroborated by other US groups.
Second, there is no specific CPT code for ESD in the United States. Physicians are forced to bill the procedure as EMR or use an unlisted code, resulting in reimbursement that does not reflect the time and complexity of the procedure. Our group showed that physician reimbursement for ESD is highly variable, ranging from $50 to $800 per case, depending on insurance type.
Third, the increasing emphasis on productivity and RVU generation in academic settings has hindered the growth of ESD training in many institutions. Still, the outlook for ESD in the United States remains encouraging. Multiple industry-sponsored training courses are held annually, and professional societies are investing heavily in expanding access to structured education in ESD. Industry is also innovating devices that improve procedural efficiency and safety. Adopting novel approaches, such as traction-assisted ESD, has made the technique more appealing to endoscopists concerned about long procedure times. For example, our group proposed a standardized esophageal ESD technique that incorporates specimen self-retraction. This method improves both safety and speed and has helped address several procedural challenges. We’ve demonstrated that consistency in technique can substantially expedite esophageal ESD.
Fast forward 5 years: We anticipate a dedicated CPT code for ESD, broader access to advanced resection tools, and an expanding number of fellowships offering structured ESD training. These developments are poised to eliminate many of the current barriers. In summary, with robust data supporting the efficacy of ESD in early esophageal cancer, the focus in the United States should shift toward mastering and integrating the technique, rather than dismissing it in favor of piecemeal EMR.
Dr. Othman is chief of the gastroenterology and hepatology section at Baylor College of Medicine and Medicine Subspecialities Service Line Chief at Baylor St Luke’s Medical Center, both in Houston. He declares no conflicts of interest.
A Practical Approach to Diagnosis and Management of Eosinophilic Esophagitis
Eosinophilic esophagitis (EoE) can be considered a “young” disease, with initial case series reported only about 30 years ago. Since that time, it has become a commonly encountered condition in both emergency and clinic settings. The most recent prevalence study estimates that 1 in 700 people in the U.S. have EoE,1 the volume of EoE-associated ED visits tripped between 2009 and 2019 and is projected to double again by 2030,2 and “new” gastroenterologists undoubtedly have learned about and seen this condition. As a chronic disease, EoE necessitates longitudinal follow-up and optimization of care to prevent complications. With increasing diagnostic delay, EoE progresses in most, but not all, patients from an inflammatory- to fibrostenotic-predominant condition.3
Diagnosis of EoE
The most likely area that you will encounter EoE is during an emergent middle-of-the-night endoscopy for food impaction. If called in for this, EoE will be the cause in more than 50% of patients.4 However, the diagnosis can only be made if esophageal biopsies are obtained at the time of the procedure. This is a critical time to decrease diagnostic delay, as half of patients are lost to follow-up after a food impaction.5 Unfortunately, although taking biopsies during index food impaction is guideline-recommended, a quality metric, and safe to obtain after the food bolus is cleared, this is infrequently done in practice.6, 7
The next most likely area for EoE detection is in the endoscopy suite where 15-23% of patients with dysphagia and 5-7% of patients undergoing upper endoscopy for any indication will have EoE.4 Sometimes EoE will be detected “incidentally” during an open-access case (for example, in a patient with diarrhea undergoing evaluation for celiac). In these cases, it is important to perform a careful history (as noted below) as subtle EoE symptoms can frequently be identified. Finally, when patients are seen in clinic for solid food dysphagia, EoE is clearly on the differential. A few percent of patients with refractory heartburn or chest pain will have EoE causing the symptoms rather than reflux,4 and all patients under consideration for antireflux surgery should have an endoscopy to assess for EoE.
When talking to patients with known or suspected EoE, the history must go beyond general questions about dysphagia or trouble swallowing. Many patients with EoE have overtly or subconsciously modified their eating behaviors over many years to minimize symptoms, may have adapted to chronic dysphagia, and will answer “no” when asked if they have trouble swallowing. Instead, use the acronym “IMPACT” to delve deeper into possible symptoms.8 Do they “Imbibe” fluids or liquids between each bite to help get food down? Do they “Modify” the way they eat (cut food into small bites; puree foods)? Do they “Prolong” mealtimes? Do they “Avoid” certain foods that stick? Do they “Chew’ until their food is a mush to get it down? And do they “Turn away” tablets or pills? Pill dysphagia is often a subtle symptom, and sometimes the only symptom elicited.
Additionally, it may be important to ask a partner or family member (if present) about their observations. They may provide insight (e.g. “yes – he chokes with every bite but never says it bothers him”) that the patient might not otherwise provide. The suspicion for EoE should also be increased in patients with concomitant atopic diseases and in those with a family history of dysphagia or who have family members needing esophageal dilation. It is important to remember that EoE can be seen across all ages, sexes, and races/ethnicities.
Diagnosis of EoE is based on the AGREE consensus,9 which is also echoed in the recently updated American College of Gastroenterology (ACG) guidelines.10 Diagnosis requires three steps. First, symptoms of esophageal dysfunction must be present. This will most typically be dysphagia in adolescents and adults, but symptoms are non-specific in children (e.g. poor growth and feeding, abdominal pain, vomiting, regurgitation, heartburn).
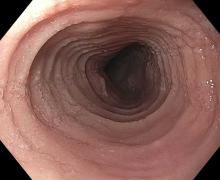
Second, at least 15 eosinophils per high-power field (eos/hpf) are required on esophageal biopsy, which implies that an endoscopy be performed. A high-quality endoscopic exam in EoE is of the utmost importance. The approach has been described elsewhere,11 but enough time on insertion should be taken to fully insufflate and examine the esophagus, including the areas of the gastroesophageal junction and upper esophageal sphincter where strictures can be missed, to gently wash debris, and to assess the endoscopic findings of EoE. Endoscopic findings should be reported using the validated EoE Endoscopy Reference Score (EREFS),12 which grades five key features. EREFS is reproducible, is responsive to treatment, and is guideline-recommended (see Figure 1).6, 10 The features are edema (present=1), rings (mild=1; moderate=2; severe=3), exudates (mild=1; severe=2), furrows (mild=1; severe=2), and stricture (present=1; also estimate diameter in mm) and are incorporated into many endoscopic reporting programs. Additionally, diffuse luminal narrowing and mucosal fragility (“crepe-paper” mucosa) should be assessed.
After this, biopsies should be obtained with at least 6 biopsy fragments from different locations in the esophagus. Any visible endoscopic abnormalities should be targeted (the highest yield is in exudates and furrows). The rationale is that EoE is patchy and at least 6 biopsies will maximize diagnostic yield.10 Ideally the initial endoscopy for EoE should be done off of treatments (like PPI or diet restriction) as these could mask the diagnosis. If a patient with suspected EoE has an endoscopy while on PPI, and the endoscopy is normal, a diagnosis of EoE cannot be made. In this case, consideration should be given as to stopping the PPI, allowing a wash out period (at least 1-2 months), and then repeating the endoscopy to confirm the diagnosis. This is important as EoE is a chronic condition necessitating life-long treatment and monitoring, so a definitive diagnosis is critical.
The third and final step in diagnosis is assessing for other conditions that could cause esophageal eosinophilia.9 The most common differential diagnosis is gastroesophageal reflux disease (GERD). In some cases, EoE and GERD overlap or can have a complex relationship.13 Unfortunately the location of the eosinophilia (i.e. distal only) and the level of the eosinophil counts are not useful in making this distinction, so all clinical features (symptoms, presence of erosive esophagitis, or a hiatal hernia endoscopically), and ancillary reflex testing when indicated may be required prior to a formal EoE diagnosis. After the diagnosis is established, there should be direct communication with the patient to review the diagnosis and select treatments. While it is possible to convey results electronically in a messaging portal or with a letter, a more formal interaction, such as a clinic visit, is recommended because this is a new diagnosis of a chronic condition. Similarly, a new diagnosis of inflammatory bowel disease would never be made in a pathology follow-up letter alone.
Treatment of EoE
When it comes to treatment, the new guidelines emphasize several points.10 First, there is the concept that anti-inflammatory treatment should be paired with assessment of fibrostenosis and esophageal dilation; to do either in isolation is incomplete treatment. It is safe to perform dilation both prior to anti-inflammatory treatment (for example, with a critical stricture in a patient with dysphagia) and after anti-inflammatory treatment has been prescribed (for example, during an endoscopy to assess treatment response).
Second, proton pump inhibitors (PPIs), swallowed topical corticosteroids (tCS), or dietary elimination are all acceptable first-line treatment options for EoE. A shared decision-making framework should be used for this discussion. If dietary elimination is selected,14 based on new clinical trial data, guidelines recommend using empiric elimination and starting with a less restrictive diet (either a one-food elimination diet with dairy alone or a two-food elimination with dairy and wheat elimination). If PPIs are selected, the dose should be double the standard reflux dose. Data are mixed as to whether to use twice daily dosing (i.e., omeprazole 20 mg twice daily) or once a day dosing (i.e., omeprazole 40 mg daily), but total dose and adherence may be more important than frequency.10
For tCS use, either budesonide or fluticasone can be selected, but budesonide oral suspension is the only FDA-approved tCS for EoE.15 Initial treatment length is usually 6-8 weeks for diet elimination and, 12 weeks for PPI and tCS. In general, it is best to pick a single treatment to start, and reserve combining therapies for patients who do not have a complete response to a single modality as there are few data to support combination therapy.
After initial treatment, it is critical to assess for treatment response.16 Goals of EoE treatment include improvement in symptoms, but also improvement in endoscopic and histologic features to prevent complications. Symptoms in EoE do not always correlate with underlying biologic disease activity: patients can minimize symptoms with careful eating; they may perceive no difference in symptoms despite histologic improvement if a stricture persists; and they may have minimal symptoms after esophageal dilation despite ongoing inflammation. Because of this, performing a follow-up endoscopy after initial treatment is guideline-recommended.10, 17 This allows assessing for endoscopic improvement, re-assessing for fibrostenosis and performing dilation if indicated, and obtaining esophageal biopsies. If there is non-response, options include switching between other first line treatments or considering “stepping-up” to dupilumab which is also an FDA-approved option for EoE that is recommended in the guidelines.10, 18 In some cases where patients have multiple severe atopic conditions such as asthma or eczema that would warrant dupilumab use, or if patients are intolerant to PPIs or tCS, dupilumab could be considered as an earlier treatment for EoE.
Long-Term Maintenance
If a patient has a good response (for example, improved symptoms, improved endoscopic features, and <15 eos/hpf on biopsy), treatment can be maintained long-term. In almost all cases, if treatment is stopped, EoE disease activity recurs.19 Patients could be seen back in clinic in 6-12 months, and then a discussion can be conducted about a follow-up endoscopy, with timing to be determined based on their individual disease features and severity.17
Patients with more severe strictures, however, may have to be seen in endoscopy for serial dilations. Continued follow-up is essential for optimal care. Just as patients can progress in their disease course with diagnostic delay, there are data that show they can also progress after diagnosis when there are gaps in care without regular follow-up.20 Unlike other chronic esophageal disorders such as GERD and Barrett’s esophagus and other chronic GI inflammatory conditions like inflammatory bowel disease, however, EoE is not associated with an increased risk of esophageal cancer.21, 22
Given its increasing frequency, EoE will be commonly encountered by gastroenterologists both new and established. Having a systematic approach for diagnosis, understanding how to elicit subtle symptoms, implementing a shared decision-making framework for treatment with a structured algorithm for assessing response, performing follow-up, maintaining treatment, and monitoring patients long-term will allow the large majority of EoE patients to be successfully managed.
Dr. Dellon is based at the Center for Esophageal Diseases and Swallowing, Center for Gastrointestinal Biology and Disease, Division of Gastroenterology and Hepatology, University of North Carolina School of Medicine, Chapel Hill. He disclosed research funding, consultant fees, and educational grants from multiple companies.
References
1. Thel HL, et al. Prevalence and Costs of Eosinophilic Esophagitis in the United States. Clin Gastroenterol Hepatol. 2025 Feb. doi: 10.1016/j.cgh.2024.09.031.
2. Lam AY, et al. Epidemiologic Burden and Projections for Eosinophilic Esophagitis-Associated Emergency Department Visits in the United States: 2009-2030. Clin Gastroenterol Hepatol. 2023 Nov. doi: 10.1016/j.cgh.2023.04.028.
3. Schoepfer AM, et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology. 2013 Dec. doi: 10.1053/j.gastro.2013.08.015.
4. Dellon ES, Hirano I. Epidemiology and Natural History of Eosinophilic Esophagitis. Gastroenterology. 2018 Jan. doi: 10.1053/j.gastro.2017.06.067.
5. Chang JW, et al. Loss to follow-up after food impaction among patients with and without eosinophilic esophagitis. Dis Esophagus. 2019 Dec. doi: 10.1093/dote/doz056.
6. Aceves SS, et al. Endoscopic approach to eosinophilic esophagitis: American Society for Gastrointestinal Endoscopy Consensus Conference. Gastrointest Endosc. 2022 Aug. doi: 10.1016/j.gie.2022.05.013.
7. Leiman DA, et al. Quality Indicators for the Diagnosis and Management of Eosinophilic Esophagitis. Am J Gastroenterol. 2023 Jun. doi: 10.14309/ajg.0000000000002138.
8. Hirano I, Furuta GT. Approaches and Challenges to Management of Pediatric and Adult Patients With Eosinophilic Esophagitis. Gastroenterology. 2020 Mar. doi: 10.1053/j.gastro.2019.09.052.
9. Dellon ES, et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis: Proceedings of the AGREE conference. Gastroenterology. 2018 Oct. doi: 10.1053/j.gastro.2018.07.009.
10. Dellon ES, et al. ACG Clinical Guideline: Diagnosis and Management of Eosinophilic Esophagitis. Am J Gastroenterol. 2025 Jan. doi: 10.14309/ajg.0000000000003194.
11. Dellon ES. Optimizing the Endoscopic Examination in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2021 Dec. doi: 10.1016/j.cgh.2021.07.011.
12. Hirano I, et al. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut. 2012 May. doi: 10.1136/gutjnl-2011-301817.
13. Spechler SJ, et al. Thoughts on the complex relationship between gastroesophageal reflux disease and eosinophilic esophagitis. Am J Gastroenterol. 2007 Jun. doi: 10.1111/j.1572-0241.2007.01179.x.
14. Chang JW, et al. Development of a Practical Guide to Implement and Monitor Diet Therapy for Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2023 Jul. doi: 10.1016/j.cgh.2023.03.006.
15. Hirano I, et al. Budesonide Oral Suspension Improves Outcomes in Patients With Eosinophilic Esophagitis: Results from a Phase 3 Trial. Clin Gastroenterol Hepatol. 2022 Mar. doi: 10.1016/j.cgh.2021.04.022.
16. Dellon ES, Gupta SK. A conceptual approach to understanding treatment response in eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2019 Oct. doi: 10.1016/j.cgh.2019.01.030.
17. von Arnim U, et al. Monitoring Patients With Eosinophilic Esophagitis in Routine Clinical Practice - International Expert Recommendations. Clin Gastroenterol Hepatol. 2023 Sep. doi: 10.1016/j.cgh.2022.12.018.
18. Dellon ES, et al. Dupilumab in Adults and Adolescents with Eosinophilic Esophagitis. N Engl J Med. 2022 Dec. doi: 10.1056/NEJMoa220598.
19. Dellon ES, et al. Rapid Recurrence of Eosinophilic Esophagitis Activity After Successful Treatment in the Observation Phase of a Randomized, Double-Blind, Double-Dummy Trial. Clin Gastroenterol Hepatol. 2020 Jun. doi: 10.1016/j.cgh.2019.08.050.
20. Chang NC, et al. A Gap in Care Leads to Progression of Fibrosis in Eosinophilic Esophagitis Patients. Clin Gastroenterol Hepatol. 2022 Aug. doi: 10.1016/j.cgh.2021.10.028.
21. Syed A, et al. The relationship between eosinophilic esophagitis and esophageal cancer. Dis Esophagus. 2017 Jul. doi: 10.1093/dote/dox050.
22. Albaneze N, et al. No Association Between Eosinophilic Oesophagitis and Oesophageal Cancer in US Adults: A Case-Control Study. Aliment Pharmacol Ther. 2025 Jan. doi: 10.1111/apt.18431.
Eosinophilic esophagitis (EoE) can be considered a “young” disease, with initial case series reported only about 30 years ago. Since that time, it has become a commonly encountered condition in both emergency and clinic settings. The most recent prevalence study estimates that 1 in 700 people in the U.S. have EoE,1 the volume of EoE-associated ED visits tripped between 2009 and 2019 and is projected to double again by 2030,2 and “new” gastroenterologists undoubtedly have learned about and seen this condition. As a chronic disease, EoE necessitates longitudinal follow-up and optimization of care to prevent complications. With increasing diagnostic delay, EoE progresses in most, but not all, patients from an inflammatory- to fibrostenotic-predominant condition.3
Diagnosis of EoE
The most likely area that you will encounter EoE is during an emergent middle-of-the-night endoscopy for food impaction. If called in for this, EoE will be the cause in more than 50% of patients.4 However, the diagnosis can only be made if esophageal biopsies are obtained at the time of the procedure. This is a critical time to decrease diagnostic delay, as half of patients are lost to follow-up after a food impaction.5 Unfortunately, although taking biopsies during index food impaction is guideline-recommended, a quality metric, and safe to obtain after the food bolus is cleared, this is infrequently done in practice.6, 7
The next most likely area for EoE detection is in the endoscopy suite where 15-23% of patients with dysphagia and 5-7% of patients undergoing upper endoscopy for any indication will have EoE.4 Sometimes EoE will be detected “incidentally” during an open-access case (for example, in a patient with diarrhea undergoing evaluation for celiac). In these cases, it is important to perform a careful history (as noted below) as subtle EoE symptoms can frequently be identified. Finally, when patients are seen in clinic for solid food dysphagia, EoE is clearly on the differential. A few percent of patients with refractory heartburn or chest pain will have EoE causing the symptoms rather than reflux,4 and all patients under consideration for antireflux surgery should have an endoscopy to assess for EoE.
When talking to patients with known or suspected EoE, the history must go beyond general questions about dysphagia or trouble swallowing. Many patients with EoE have overtly or subconsciously modified their eating behaviors over many years to minimize symptoms, may have adapted to chronic dysphagia, and will answer “no” when asked if they have trouble swallowing. Instead, use the acronym “IMPACT” to delve deeper into possible symptoms.8 Do they “Imbibe” fluids or liquids between each bite to help get food down? Do they “Modify” the way they eat (cut food into small bites; puree foods)? Do they “Prolong” mealtimes? Do they “Avoid” certain foods that stick? Do they “Chew’ until their food is a mush to get it down? And do they “Turn away” tablets or pills? Pill dysphagia is often a subtle symptom, and sometimes the only symptom elicited.
Additionally, it may be important to ask a partner or family member (if present) about their observations. They may provide insight (e.g. “yes – he chokes with every bite but never says it bothers him”) that the patient might not otherwise provide. The suspicion for EoE should also be increased in patients with concomitant atopic diseases and in those with a family history of dysphagia or who have family members needing esophageal dilation. It is important to remember that EoE can be seen across all ages, sexes, and races/ethnicities.
Diagnosis of EoE is based on the AGREE consensus,9 which is also echoed in the recently updated American College of Gastroenterology (ACG) guidelines.10 Diagnosis requires three steps. First, symptoms of esophageal dysfunction must be present. This will most typically be dysphagia in adolescents and adults, but symptoms are non-specific in children (e.g. poor growth and feeding, abdominal pain, vomiting, regurgitation, heartburn).
Second, at least 15 eosinophils per high-power field (eos/hpf) are required on esophageal biopsy, which implies that an endoscopy be performed. A high-quality endoscopic exam in EoE is of the utmost importance. The approach has been described elsewhere,11 but enough time on insertion should be taken to fully insufflate and examine the esophagus, including the areas of the gastroesophageal junction and upper esophageal sphincter where strictures can be missed, to gently wash debris, and to assess the endoscopic findings of EoE. Endoscopic findings should be reported using the validated EoE Endoscopy Reference Score (EREFS),12 which grades five key features. EREFS is reproducible, is responsive to treatment, and is guideline-recommended (see Figure 1).6, 10 The features are edema (present=1), rings (mild=1; moderate=2; severe=3), exudates (mild=1; severe=2), furrows (mild=1; severe=2), and stricture (present=1; also estimate diameter in mm) and are incorporated into many endoscopic reporting programs. Additionally, diffuse luminal narrowing and mucosal fragility (“crepe-paper” mucosa) should be assessed.
After this, biopsies should be obtained with at least 6 biopsy fragments from different locations in the esophagus. Any visible endoscopic abnormalities should be targeted (the highest yield is in exudates and furrows). The rationale is that EoE is patchy and at least 6 biopsies will maximize diagnostic yield.10 Ideally the initial endoscopy for EoE should be done off of treatments (like PPI or diet restriction) as these could mask the diagnosis. If a patient with suspected EoE has an endoscopy while on PPI, and the endoscopy is normal, a diagnosis of EoE cannot be made. In this case, consideration should be given as to stopping the PPI, allowing a wash out period (at least 1-2 months), and then repeating the endoscopy to confirm the diagnosis. This is important as EoE is a chronic condition necessitating life-long treatment and monitoring, so a definitive diagnosis is critical.
The third and final step in diagnosis is assessing for other conditions that could cause esophageal eosinophilia.9 The most common differential diagnosis is gastroesophageal reflux disease (GERD). In some cases, EoE and GERD overlap or can have a complex relationship.13 Unfortunately the location of the eosinophilia (i.e. distal only) and the level of the eosinophil counts are not useful in making this distinction, so all clinical features (symptoms, presence of erosive esophagitis, or a hiatal hernia endoscopically), and ancillary reflex testing when indicated may be required prior to a formal EoE diagnosis. After the diagnosis is established, there should be direct communication with the patient to review the diagnosis and select treatments. While it is possible to convey results electronically in a messaging portal or with a letter, a more formal interaction, such as a clinic visit, is recommended because this is a new diagnosis of a chronic condition. Similarly, a new diagnosis of inflammatory bowel disease would never be made in a pathology follow-up letter alone.
Treatment of EoE
When it comes to treatment, the new guidelines emphasize several points.10 First, there is the concept that anti-inflammatory treatment should be paired with assessment of fibrostenosis and esophageal dilation; to do either in isolation is incomplete treatment. It is safe to perform dilation both prior to anti-inflammatory treatment (for example, with a critical stricture in a patient with dysphagia) and after anti-inflammatory treatment has been prescribed (for example, during an endoscopy to assess treatment response).
Second, proton pump inhibitors (PPIs), swallowed topical corticosteroids (tCS), or dietary elimination are all acceptable first-line treatment options for EoE. A shared decision-making framework should be used for this discussion. If dietary elimination is selected,14 based on new clinical trial data, guidelines recommend using empiric elimination and starting with a less restrictive diet (either a one-food elimination diet with dairy alone or a two-food elimination with dairy and wheat elimination). If PPIs are selected, the dose should be double the standard reflux dose. Data are mixed as to whether to use twice daily dosing (i.e., omeprazole 20 mg twice daily) or once a day dosing (i.e., omeprazole 40 mg daily), but total dose and adherence may be more important than frequency.10
For tCS use, either budesonide or fluticasone can be selected, but budesonide oral suspension is the only FDA-approved tCS for EoE.15 Initial treatment length is usually 6-8 weeks for diet elimination and, 12 weeks for PPI and tCS. In general, it is best to pick a single treatment to start, and reserve combining therapies for patients who do not have a complete response to a single modality as there are few data to support combination therapy.
After initial treatment, it is critical to assess for treatment response.16 Goals of EoE treatment include improvement in symptoms, but also improvement in endoscopic and histologic features to prevent complications. Symptoms in EoE do not always correlate with underlying biologic disease activity: patients can minimize symptoms with careful eating; they may perceive no difference in symptoms despite histologic improvement if a stricture persists; and they may have minimal symptoms after esophageal dilation despite ongoing inflammation. Because of this, performing a follow-up endoscopy after initial treatment is guideline-recommended.10, 17 This allows assessing for endoscopic improvement, re-assessing for fibrostenosis and performing dilation if indicated, and obtaining esophageal biopsies. If there is non-response, options include switching between other first line treatments or considering “stepping-up” to dupilumab which is also an FDA-approved option for EoE that is recommended in the guidelines.10, 18 In some cases where patients have multiple severe atopic conditions such as asthma or eczema that would warrant dupilumab use, or if patients are intolerant to PPIs or tCS, dupilumab could be considered as an earlier treatment for EoE.
Long-Term Maintenance
If a patient has a good response (for example, improved symptoms, improved endoscopic features, and <15 eos/hpf on biopsy), treatment can be maintained long-term. In almost all cases, if treatment is stopped, EoE disease activity recurs.19 Patients could be seen back in clinic in 6-12 months, and then a discussion can be conducted about a follow-up endoscopy, with timing to be determined based on their individual disease features and severity.17
Patients with more severe strictures, however, may have to be seen in endoscopy for serial dilations. Continued follow-up is essential for optimal care. Just as patients can progress in their disease course with diagnostic delay, there are data that show they can also progress after diagnosis when there are gaps in care without regular follow-up.20 Unlike other chronic esophageal disorders such as GERD and Barrett’s esophagus and other chronic GI inflammatory conditions like inflammatory bowel disease, however, EoE is not associated with an increased risk of esophageal cancer.21, 22
Given its increasing frequency, EoE will be commonly encountered by gastroenterologists both new and established. Having a systematic approach for diagnosis, understanding how to elicit subtle symptoms, implementing a shared decision-making framework for treatment with a structured algorithm for assessing response, performing follow-up, maintaining treatment, and monitoring patients long-term will allow the large majority of EoE patients to be successfully managed.
Dr. Dellon is based at the Center for Esophageal Diseases and Swallowing, Center for Gastrointestinal Biology and Disease, Division of Gastroenterology and Hepatology, University of North Carolina School of Medicine, Chapel Hill. He disclosed research funding, consultant fees, and educational grants from multiple companies.
References
1. Thel HL, et al. Prevalence and Costs of Eosinophilic Esophagitis in the United States. Clin Gastroenterol Hepatol. 2025 Feb. doi: 10.1016/j.cgh.2024.09.031.
2. Lam AY, et al. Epidemiologic Burden and Projections for Eosinophilic Esophagitis-Associated Emergency Department Visits in the United States: 2009-2030. Clin Gastroenterol Hepatol. 2023 Nov. doi: 10.1016/j.cgh.2023.04.028.
3. Schoepfer AM, et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology. 2013 Dec. doi: 10.1053/j.gastro.2013.08.015.
4. Dellon ES, Hirano I. Epidemiology and Natural History of Eosinophilic Esophagitis. Gastroenterology. 2018 Jan. doi: 10.1053/j.gastro.2017.06.067.
5. Chang JW, et al. Loss to follow-up after food impaction among patients with and without eosinophilic esophagitis. Dis Esophagus. 2019 Dec. doi: 10.1093/dote/doz056.
6. Aceves SS, et al. Endoscopic approach to eosinophilic esophagitis: American Society for Gastrointestinal Endoscopy Consensus Conference. Gastrointest Endosc. 2022 Aug. doi: 10.1016/j.gie.2022.05.013.
7. Leiman DA, et al. Quality Indicators for the Diagnosis and Management of Eosinophilic Esophagitis. Am J Gastroenterol. 2023 Jun. doi: 10.14309/ajg.0000000000002138.
8. Hirano I, Furuta GT. Approaches and Challenges to Management of Pediatric and Adult Patients With Eosinophilic Esophagitis. Gastroenterology. 2020 Mar. doi: 10.1053/j.gastro.2019.09.052.
9. Dellon ES, et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis: Proceedings of the AGREE conference. Gastroenterology. 2018 Oct. doi: 10.1053/j.gastro.2018.07.009.
10. Dellon ES, et al. ACG Clinical Guideline: Diagnosis and Management of Eosinophilic Esophagitis. Am J Gastroenterol. 2025 Jan. doi: 10.14309/ajg.0000000000003194.
11. Dellon ES. Optimizing the Endoscopic Examination in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2021 Dec. doi: 10.1016/j.cgh.2021.07.011.
12. Hirano I, et al. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut. 2012 May. doi: 10.1136/gutjnl-2011-301817.
13. Spechler SJ, et al. Thoughts on the complex relationship between gastroesophageal reflux disease and eosinophilic esophagitis. Am J Gastroenterol. 2007 Jun. doi: 10.1111/j.1572-0241.2007.01179.x.
14. Chang JW, et al. Development of a Practical Guide to Implement and Monitor Diet Therapy for Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2023 Jul. doi: 10.1016/j.cgh.2023.03.006.
15. Hirano I, et al. Budesonide Oral Suspension Improves Outcomes in Patients With Eosinophilic Esophagitis: Results from a Phase 3 Trial. Clin Gastroenterol Hepatol. 2022 Mar. doi: 10.1016/j.cgh.2021.04.022.
16. Dellon ES, Gupta SK. A conceptual approach to understanding treatment response in eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2019 Oct. doi: 10.1016/j.cgh.2019.01.030.
17. von Arnim U, et al. Monitoring Patients With Eosinophilic Esophagitis in Routine Clinical Practice - International Expert Recommendations. Clin Gastroenterol Hepatol. 2023 Sep. doi: 10.1016/j.cgh.2022.12.018.
18. Dellon ES, et al. Dupilumab in Adults and Adolescents with Eosinophilic Esophagitis. N Engl J Med. 2022 Dec. doi: 10.1056/NEJMoa220598.
19. Dellon ES, et al. Rapid Recurrence of Eosinophilic Esophagitis Activity After Successful Treatment in the Observation Phase of a Randomized, Double-Blind, Double-Dummy Trial. Clin Gastroenterol Hepatol. 2020 Jun. doi: 10.1016/j.cgh.2019.08.050.
20. Chang NC, et al. A Gap in Care Leads to Progression of Fibrosis in Eosinophilic Esophagitis Patients. Clin Gastroenterol Hepatol. 2022 Aug. doi: 10.1016/j.cgh.2021.10.028.
21. Syed A, et al. The relationship between eosinophilic esophagitis and esophageal cancer. Dis Esophagus. 2017 Jul. doi: 10.1093/dote/dox050.
22. Albaneze N, et al. No Association Between Eosinophilic Oesophagitis and Oesophageal Cancer in US Adults: A Case-Control Study. Aliment Pharmacol Ther. 2025 Jan. doi: 10.1111/apt.18431.
Eosinophilic esophagitis (EoE) can be considered a “young” disease, with initial case series reported only about 30 years ago. Since that time, it has become a commonly encountered condition in both emergency and clinic settings. The most recent prevalence study estimates that 1 in 700 people in the U.S. have EoE,1 the volume of EoE-associated ED visits tripped between 2009 and 2019 and is projected to double again by 2030,2 and “new” gastroenterologists undoubtedly have learned about and seen this condition. As a chronic disease, EoE necessitates longitudinal follow-up and optimization of care to prevent complications. With increasing diagnostic delay, EoE progresses in most, but not all, patients from an inflammatory- to fibrostenotic-predominant condition.3
Diagnosis of EoE
The most likely area that you will encounter EoE is during an emergent middle-of-the-night endoscopy for food impaction. If called in for this, EoE will be the cause in more than 50% of patients.4 However, the diagnosis can only be made if esophageal biopsies are obtained at the time of the procedure. This is a critical time to decrease diagnostic delay, as half of patients are lost to follow-up after a food impaction.5 Unfortunately, although taking biopsies during index food impaction is guideline-recommended, a quality metric, and safe to obtain after the food bolus is cleared, this is infrequently done in practice.6, 7
The next most likely area for EoE detection is in the endoscopy suite where 15-23% of patients with dysphagia and 5-7% of patients undergoing upper endoscopy for any indication will have EoE.4 Sometimes EoE will be detected “incidentally” during an open-access case (for example, in a patient with diarrhea undergoing evaluation for celiac). In these cases, it is important to perform a careful history (as noted below) as subtle EoE symptoms can frequently be identified. Finally, when patients are seen in clinic for solid food dysphagia, EoE is clearly on the differential. A few percent of patients with refractory heartburn or chest pain will have EoE causing the symptoms rather than reflux,4 and all patients under consideration for antireflux surgery should have an endoscopy to assess for EoE.
When talking to patients with known or suspected EoE, the history must go beyond general questions about dysphagia or trouble swallowing. Many patients with EoE have overtly or subconsciously modified their eating behaviors over many years to minimize symptoms, may have adapted to chronic dysphagia, and will answer “no” when asked if they have trouble swallowing. Instead, use the acronym “IMPACT” to delve deeper into possible symptoms.8 Do they “Imbibe” fluids or liquids between each bite to help get food down? Do they “Modify” the way they eat (cut food into small bites; puree foods)? Do they “Prolong” mealtimes? Do they “Avoid” certain foods that stick? Do they “Chew’ until their food is a mush to get it down? And do they “Turn away” tablets or pills? Pill dysphagia is often a subtle symptom, and sometimes the only symptom elicited.
Additionally, it may be important to ask a partner or family member (if present) about their observations. They may provide insight (e.g. “yes – he chokes with every bite but never says it bothers him”) that the patient might not otherwise provide. The suspicion for EoE should also be increased in patients with concomitant atopic diseases and in those with a family history of dysphagia or who have family members needing esophageal dilation. It is important to remember that EoE can be seen across all ages, sexes, and races/ethnicities.
Diagnosis of EoE is based on the AGREE consensus,9 which is also echoed in the recently updated American College of Gastroenterology (ACG) guidelines.10 Diagnosis requires three steps. First, symptoms of esophageal dysfunction must be present. This will most typically be dysphagia in adolescents and adults, but symptoms are non-specific in children (e.g. poor growth and feeding, abdominal pain, vomiting, regurgitation, heartburn).
Second, at least 15 eosinophils per high-power field (eos/hpf) are required on esophageal biopsy, which implies that an endoscopy be performed. A high-quality endoscopic exam in EoE is of the utmost importance. The approach has been described elsewhere,11 but enough time on insertion should be taken to fully insufflate and examine the esophagus, including the areas of the gastroesophageal junction and upper esophageal sphincter where strictures can be missed, to gently wash debris, and to assess the endoscopic findings of EoE. Endoscopic findings should be reported using the validated EoE Endoscopy Reference Score (EREFS),12 which grades five key features. EREFS is reproducible, is responsive to treatment, and is guideline-recommended (see Figure 1).6, 10 The features are edema (present=1), rings (mild=1; moderate=2; severe=3), exudates (mild=1; severe=2), furrows (mild=1; severe=2), and stricture (present=1; also estimate diameter in mm) and are incorporated into many endoscopic reporting programs. Additionally, diffuse luminal narrowing and mucosal fragility (“crepe-paper” mucosa) should be assessed.
After this, biopsies should be obtained with at least 6 biopsy fragments from different locations in the esophagus. Any visible endoscopic abnormalities should be targeted (the highest yield is in exudates and furrows). The rationale is that EoE is patchy and at least 6 biopsies will maximize diagnostic yield.10 Ideally the initial endoscopy for EoE should be done off of treatments (like PPI or diet restriction) as these could mask the diagnosis. If a patient with suspected EoE has an endoscopy while on PPI, and the endoscopy is normal, a diagnosis of EoE cannot be made. In this case, consideration should be given as to stopping the PPI, allowing a wash out period (at least 1-2 months), and then repeating the endoscopy to confirm the diagnosis. This is important as EoE is a chronic condition necessitating life-long treatment and monitoring, so a definitive diagnosis is critical.
The third and final step in diagnosis is assessing for other conditions that could cause esophageal eosinophilia.9 The most common differential diagnosis is gastroesophageal reflux disease (GERD). In some cases, EoE and GERD overlap or can have a complex relationship.13 Unfortunately the location of the eosinophilia (i.e. distal only) and the level of the eosinophil counts are not useful in making this distinction, so all clinical features (symptoms, presence of erosive esophagitis, or a hiatal hernia endoscopically), and ancillary reflex testing when indicated may be required prior to a formal EoE diagnosis. After the diagnosis is established, there should be direct communication with the patient to review the diagnosis and select treatments. While it is possible to convey results electronically in a messaging portal or with a letter, a more formal interaction, such as a clinic visit, is recommended because this is a new diagnosis of a chronic condition. Similarly, a new diagnosis of inflammatory bowel disease would never be made in a pathology follow-up letter alone.
Treatment of EoE
When it comes to treatment, the new guidelines emphasize several points.10 First, there is the concept that anti-inflammatory treatment should be paired with assessment of fibrostenosis and esophageal dilation; to do either in isolation is incomplete treatment. It is safe to perform dilation both prior to anti-inflammatory treatment (for example, with a critical stricture in a patient with dysphagia) and after anti-inflammatory treatment has been prescribed (for example, during an endoscopy to assess treatment response).
Second, proton pump inhibitors (PPIs), swallowed topical corticosteroids (tCS), or dietary elimination are all acceptable first-line treatment options for EoE. A shared decision-making framework should be used for this discussion. If dietary elimination is selected,14 based on new clinical trial data, guidelines recommend using empiric elimination and starting with a less restrictive diet (either a one-food elimination diet with dairy alone or a two-food elimination with dairy and wheat elimination). If PPIs are selected, the dose should be double the standard reflux dose. Data are mixed as to whether to use twice daily dosing (i.e., omeprazole 20 mg twice daily) or once a day dosing (i.e., omeprazole 40 mg daily), but total dose and adherence may be more important than frequency.10
For tCS use, either budesonide or fluticasone can be selected, but budesonide oral suspension is the only FDA-approved tCS for EoE.15 Initial treatment length is usually 6-8 weeks for diet elimination and, 12 weeks for PPI and tCS. In general, it is best to pick a single treatment to start, and reserve combining therapies for patients who do not have a complete response to a single modality as there are few data to support combination therapy.
After initial treatment, it is critical to assess for treatment response.16 Goals of EoE treatment include improvement in symptoms, but also improvement in endoscopic and histologic features to prevent complications. Symptoms in EoE do not always correlate with underlying biologic disease activity: patients can minimize symptoms with careful eating; they may perceive no difference in symptoms despite histologic improvement if a stricture persists; and they may have minimal symptoms after esophageal dilation despite ongoing inflammation. Because of this, performing a follow-up endoscopy after initial treatment is guideline-recommended.10, 17 This allows assessing for endoscopic improvement, re-assessing for fibrostenosis and performing dilation if indicated, and obtaining esophageal biopsies. If there is non-response, options include switching between other first line treatments or considering “stepping-up” to dupilumab which is also an FDA-approved option for EoE that is recommended in the guidelines.10, 18 In some cases where patients have multiple severe atopic conditions such as asthma or eczema that would warrant dupilumab use, or if patients are intolerant to PPIs or tCS, dupilumab could be considered as an earlier treatment for EoE.
Long-Term Maintenance
If a patient has a good response (for example, improved symptoms, improved endoscopic features, and <15 eos/hpf on biopsy), treatment can be maintained long-term. In almost all cases, if treatment is stopped, EoE disease activity recurs.19 Patients could be seen back in clinic in 6-12 months, and then a discussion can be conducted about a follow-up endoscopy, with timing to be determined based on their individual disease features and severity.17
Patients with more severe strictures, however, may have to be seen in endoscopy for serial dilations. Continued follow-up is essential for optimal care. Just as patients can progress in their disease course with diagnostic delay, there are data that show they can also progress after diagnosis when there are gaps in care without regular follow-up.20 Unlike other chronic esophageal disorders such as GERD and Barrett’s esophagus and other chronic GI inflammatory conditions like inflammatory bowel disease, however, EoE is not associated with an increased risk of esophageal cancer.21, 22
Given its increasing frequency, EoE will be commonly encountered by gastroenterologists both new and established. Having a systematic approach for diagnosis, understanding how to elicit subtle symptoms, implementing a shared decision-making framework for treatment with a structured algorithm for assessing response, performing follow-up, maintaining treatment, and monitoring patients long-term will allow the large majority of EoE patients to be successfully managed.
Dr. Dellon is based at the Center for Esophageal Diseases and Swallowing, Center for Gastrointestinal Biology and Disease, Division of Gastroenterology and Hepatology, University of North Carolina School of Medicine, Chapel Hill. He disclosed research funding, consultant fees, and educational grants from multiple companies.
References
1. Thel HL, et al. Prevalence and Costs of Eosinophilic Esophagitis in the United States. Clin Gastroenterol Hepatol. 2025 Feb. doi: 10.1016/j.cgh.2024.09.031.
2. Lam AY, et al. Epidemiologic Burden and Projections for Eosinophilic Esophagitis-Associated Emergency Department Visits in the United States: 2009-2030. Clin Gastroenterol Hepatol. 2023 Nov. doi: 10.1016/j.cgh.2023.04.028.
3. Schoepfer AM, et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology. 2013 Dec. doi: 10.1053/j.gastro.2013.08.015.
4. Dellon ES, Hirano I. Epidemiology and Natural History of Eosinophilic Esophagitis. Gastroenterology. 2018 Jan. doi: 10.1053/j.gastro.2017.06.067.
5. Chang JW, et al. Loss to follow-up after food impaction among patients with and without eosinophilic esophagitis. Dis Esophagus. 2019 Dec. doi: 10.1093/dote/doz056.
6. Aceves SS, et al. Endoscopic approach to eosinophilic esophagitis: American Society for Gastrointestinal Endoscopy Consensus Conference. Gastrointest Endosc. 2022 Aug. doi: 10.1016/j.gie.2022.05.013.
7. Leiman DA, et al. Quality Indicators for the Diagnosis and Management of Eosinophilic Esophagitis. Am J Gastroenterol. 2023 Jun. doi: 10.14309/ajg.0000000000002138.
8. Hirano I, Furuta GT. Approaches and Challenges to Management of Pediatric and Adult Patients With Eosinophilic Esophagitis. Gastroenterology. 2020 Mar. doi: 10.1053/j.gastro.2019.09.052.
9. Dellon ES, et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis: Proceedings of the AGREE conference. Gastroenterology. 2018 Oct. doi: 10.1053/j.gastro.2018.07.009.
10. Dellon ES, et al. ACG Clinical Guideline: Diagnosis and Management of Eosinophilic Esophagitis. Am J Gastroenterol. 2025 Jan. doi: 10.14309/ajg.0000000000003194.
11. Dellon ES. Optimizing the Endoscopic Examination in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2021 Dec. doi: 10.1016/j.cgh.2021.07.011.
12. Hirano I, et al. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut. 2012 May. doi: 10.1136/gutjnl-2011-301817.
13. Spechler SJ, et al. Thoughts on the complex relationship between gastroesophageal reflux disease and eosinophilic esophagitis. Am J Gastroenterol. 2007 Jun. doi: 10.1111/j.1572-0241.2007.01179.x.
14. Chang JW, et al. Development of a Practical Guide to Implement and Monitor Diet Therapy for Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2023 Jul. doi: 10.1016/j.cgh.2023.03.006.
15. Hirano I, et al. Budesonide Oral Suspension Improves Outcomes in Patients With Eosinophilic Esophagitis: Results from a Phase 3 Trial. Clin Gastroenterol Hepatol. 2022 Mar. doi: 10.1016/j.cgh.2021.04.022.
16. Dellon ES, Gupta SK. A conceptual approach to understanding treatment response in eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2019 Oct. doi: 10.1016/j.cgh.2019.01.030.
17. von Arnim U, et al. Monitoring Patients With Eosinophilic Esophagitis in Routine Clinical Practice - International Expert Recommendations. Clin Gastroenterol Hepatol. 2023 Sep. doi: 10.1016/j.cgh.2022.12.018.
18. Dellon ES, et al. Dupilumab in Adults and Adolescents with Eosinophilic Esophagitis. N Engl J Med. 2022 Dec. doi: 10.1056/NEJMoa220598.
19. Dellon ES, et al. Rapid Recurrence of Eosinophilic Esophagitis Activity After Successful Treatment in the Observation Phase of a Randomized, Double-Blind, Double-Dummy Trial. Clin Gastroenterol Hepatol. 2020 Jun. doi: 10.1016/j.cgh.2019.08.050.
20. Chang NC, et al. A Gap in Care Leads to Progression of Fibrosis in Eosinophilic Esophagitis Patients. Clin Gastroenterol Hepatol. 2022 Aug. doi: 10.1016/j.cgh.2021.10.028.
21. Syed A, et al. The relationship between eosinophilic esophagitis and esophageal cancer. Dis Esophagus. 2017 Jul. doi: 10.1093/dote/dox050.
22. Albaneze N, et al. No Association Between Eosinophilic Oesophagitis and Oesophageal Cancer in US Adults: A Case-Control Study. Aliment Pharmacol Ther. 2025 Jan. doi: 10.1111/apt.18431.
Building Your Referral Base

In this video, Lisa Mathew, MD, of South Denver Gastroenterology in Denver, Colorado, and Raja Taunk, MD, of Anne Arundel Gastroenterology Associates, in Annapolis, Maryland, share insights on private practice gastroenterology and offer tips on building your practice – specifically improving your referral base.

In this video, Lisa Mathew, MD, of South Denver Gastroenterology in Denver, Colorado, and Raja Taunk, MD, of Anne Arundel Gastroenterology Associates, in Annapolis, Maryland, share insights on private practice gastroenterology and offer tips on building your practice – specifically improving your referral base.

In this video, Lisa Mathew, MD, of South Denver Gastroenterology in Denver, Colorado, and Raja Taunk, MD, of Anne Arundel Gastroenterology Associates, in Annapolis, Maryland, share insights on private practice gastroenterology and offer tips on building your practice – specifically improving your referral base.
Gastroenterology Knows No Country
The United States boasts one of the premier health care systems for medical education in the world. Indeed, institutions such as Johns Hopkins, Harvard, and the Mayo Clinic have storied reputations and are recognized names the world over. The United States also stands as a country of remarkable discovery in medicine with an abundance of enormously talented and productive medical scientists. This reputation draws physicians from every corner of the world who dream of studying medicine in our country.
Unfortunately, many US medical institutions, particularly the most prestigious medical centers, lean heavily toward preferential acceptance of US medical school graduates as an indicator of the highest-quality trainees. This historical bias is being further compounded by our current government’s pejorative view of immigrants in general. Will this affect the pool of tomorrow’s stars who will change the course of American medicine?
A glance at the list of recent AGA Presidents may yield some insight; over the past 10 years, three of our presidents trained internationally at universities in Malta, Libya, and Germany. This is a small snapshot of the multitude of international graduates in gastroenterology and hepatology who have served as division chiefs, AGA award winners, and journal editors, all now US citizens. This is not to mention the influence of varied insights and talents native to international study and culture that enhance our practice of medicine and biomedical research.
We live in time when “immigrant” has been assigned a negative and almost subhuman connotation, and diversity has become something to be demonized rather than celebrated. Yet, intuitively, should a top US medical graduate be any more intelligent or driven than a top graduate from the United Kingdom, India, China, or Syria? As American medical physicians, we place the utmost value on our traditions and high standards. We boast an unmatched depth of medical talent spread across our GI divisions and practices and take pride in the way we teach medicine, like no other nation. American medicine benefits from their talent and they inspire us to remember and care for diseases in our field that affect the world’s population, not just ours.
Over 100 years ago, Dr. William Mayo stated “American practice is too broad to be national. It had the scientific spirit, and science knows no country.” Dr. Mayo also said, “Democracy is safe only so long as culture is in the ascendancy.” These lessons apply more than ever today.
David Katzka, MD
Associate Editor
The United States boasts one of the premier health care systems for medical education in the world. Indeed, institutions such as Johns Hopkins, Harvard, and the Mayo Clinic have storied reputations and are recognized names the world over. The United States also stands as a country of remarkable discovery in medicine with an abundance of enormously talented and productive medical scientists. This reputation draws physicians from every corner of the world who dream of studying medicine in our country.
Unfortunately, many US medical institutions, particularly the most prestigious medical centers, lean heavily toward preferential acceptance of US medical school graduates as an indicator of the highest-quality trainees. This historical bias is being further compounded by our current government’s pejorative view of immigrants in general. Will this affect the pool of tomorrow’s stars who will change the course of American medicine?
A glance at the list of recent AGA Presidents may yield some insight; over the past 10 years, three of our presidents trained internationally at universities in Malta, Libya, and Germany. This is a small snapshot of the multitude of international graduates in gastroenterology and hepatology who have served as division chiefs, AGA award winners, and journal editors, all now US citizens. This is not to mention the influence of varied insights and talents native to international study and culture that enhance our practice of medicine and biomedical research.
We live in time when “immigrant” has been assigned a negative and almost subhuman connotation, and diversity has become something to be demonized rather than celebrated. Yet, intuitively, should a top US medical graduate be any more intelligent or driven than a top graduate from the United Kingdom, India, China, or Syria? As American medical physicians, we place the utmost value on our traditions and high standards. We boast an unmatched depth of medical talent spread across our GI divisions and practices and take pride in the way we teach medicine, like no other nation. American medicine benefits from their talent and they inspire us to remember and care for diseases in our field that affect the world’s population, not just ours.
Over 100 years ago, Dr. William Mayo stated “American practice is too broad to be national. It had the scientific spirit, and science knows no country.” Dr. Mayo also said, “Democracy is safe only so long as culture is in the ascendancy.” These lessons apply more than ever today.
David Katzka, MD
Associate Editor
The United States boasts one of the premier health care systems for medical education in the world. Indeed, institutions such as Johns Hopkins, Harvard, and the Mayo Clinic have storied reputations and are recognized names the world over. The United States also stands as a country of remarkable discovery in medicine with an abundance of enormously talented and productive medical scientists. This reputation draws physicians from every corner of the world who dream of studying medicine in our country.
Unfortunately, many US medical institutions, particularly the most prestigious medical centers, lean heavily toward preferential acceptance of US medical school graduates as an indicator of the highest-quality trainees. This historical bias is being further compounded by our current government’s pejorative view of immigrants in general. Will this affect the pool of tomorrow’s stars who will change the course of American medicine?
A glance at the list of recent AGA Presidents may yield some insight; over the past 10 years, three of our presidents trained internationally at universities in Malta, Libya, and Germany. This is a small snapshot of the multitude of international graduates in gastroenterology and hepatology who have served as division chiefs, AGA award winners, and journal editors, all now US citizens. This is not to mention the influence of varied insights and talents native to international study and culture that enhance our practice of medicine and biomedical research.
We live in time when “immigrant” has been assigned a negative and almost subhuman connotation, and diversity has become something to be demonized rather than celebrated. Yet, intuitively, should a top US medical graduate be any more intelligent or driven than a top graduate from the United Kingdom, India, China, or Syria? As American medical physicians, we place the utmost value on our traditions and high standards. We boast an unmatched depth of medical talent spread across our GI divisions and practices and take pride in the way we teach medicine, like no other nation. American medicine benefits from their talent and they inspire us to remember and care for diseases in our field that affect the world’s population, not just ours.
Over 100 years ago, Dr. William Mayo stated “American practice is too broad to be national. It had the scientific spirit, and science knows no country.” Dr. Mayo also said, “Democracy is safe only so long as culture is in the ascendancy.” These lessons apply more than ever today.
David Katzka, MD
Associate Editor
Achieving Psychological Safety in High Reliability Organizations
Achieving Psychological Safety in High Reliability Organizations
Worldwide, health care is becoming increasingly complex as a result of greater clinical workforce demands, expanded roles and responsibilities, health care system mergers, stakeholder calls for new capabilities, and digital transformation. 1,2These increasing demands has prompted many health care institutions to place greater focus on the psychological safety of their workforce, particularly in high reliability organizations (HROs). Building a robust foundation for high reliability in health care requires the presence of psychological safety—that is, staff members at all levels of the organization must feel comfortable speaking up when they have questions or concerns.3,4 Psychological safety can improve the safety and quality of patient care but has not reached its full potential in health care.5,6 However, there are strategies that promote the widespread implementation of psychological safety in health care organizations.3-6
PSYCHOLOGICAL SAFETY
The concept of psychological safety in organizational behavior originated in 1965 when Edgar Schein and Warren Bennis, leaders in organizational psychology and management, published their reflections on the importance of psychological safety in helping individuals feel secure in the work environment.5-7 Psychological safety in the workplace is foundational to staff members feeling comfortable asking questions or expressing concerns without fear of negative consequences.8,9 It supports both individual and team efforts to raise safety concerns and report near misses and adverse events so that similar events can be averted in the future.9 Patients aren’t the only ones who benefit; psychological safety has also been found to promote job satisfaction and employee well-being.10
THE VETERANS HEALTH ADMINISTRATION JOURNEY
Achieving psychological safety is by no means an easy or comfortable process. As with any organizational change, a multipronged approach offers the best chance of success.6,9 When the Veterans Health Administration (VHA) began its incremental, enterprise-wide journey to high reliability in 2019, 3 cohorts were identified. In February 2019, 18 US Department of Veterans Affairs (VA) medical centers (VAMCs) (cohort 1) began the process of becoming HROs. Cohort 2 followed in October 2020 and included 54 VAMC. Finally, in October 2021, 67 additional VAMCs (cohort 3) started the process.2 During cohort 2, the VA Providence Healthcare System (VAPHCS) decided to emphasize psychological safety at the start of the journey to becoming an HRO. This system is part of the VA New England Healthcare System (VISN 1), which includes VAMCs and clinics in Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, and Vermont.11 Soon thereafter, the VA Bedford Healthcare System and the VA Connecticut Healthcare System adopted similar strategies. Since then, other VAMCs have also adopted this approach. These collective experiences identified 4 useful strategies for achieving psychological safety: leadership engagement, open communication, education and training, and accountability.
Leadership Engagement
Health care organization leaders play a critical role in making psychological safety happen—especially in complex and constantly changing environments, such as HROs.4 Leaders behaviors are consistently linked to the perception of psychological safety at the individual, team, and organizational levels.8 It is especially important to have leaders who recognize the views of individuals and team members and encourage staff participation in discussions to gain additional perspectives.7,8,12 Psychological safety can also be facilitated when leaders are visible, approachable, and communicative.4,7-9
Organizational practices, policies, and processes (eg, reporting adverse events without the fear of negative consequences) are also important ways that leaders can establish and sustain psychological safety. On a more granular level, leaders can enhance psychological safety by promoting and acknowledging individuals who speak up, regularly asking staff about safety concerns, highlighting “good catches” when harm is avoided, and using staff feedback to initiate improvements.4,7,13Finally, in the authors’ experience, psychological safety requires clear commitment from leaders at all levels of an organization. Communication should be bidirectional, and leaders should close the proverbial “loop” with feedback and timely follow-up. This encourages and reinforces staff engagement and speaking up behaviors.2,4,7,13
Open Communication
Promoting an environment of open communication, where all individuals and teams feel empowered to speak up with questions, concerns, and recommendations—regardless of position within the organization—is critical to psychological safety.4,6,9 Open communication is especially critical when processes and systems are constantly changing and advancing as a result of new information and technology.9 Promoting open, bidirectional communication during the delivery of patient care can be accomplished with huddles, tiered safety huddles, leader rounding for high reliability, and time-outs.2,4,6 These opportunities allow team members to discuss concerns, identify resources that support safe, high-quality care; reflect on successes and opportunities for improvement; and circle back on concerns.2,6 Open communication in psychologically safe environments empowers staff to raise patient care concerns and is instrumental for improving patient safety, increasing staff job satisfaction, and decreasing turnover.6,14
Education and Training
Education and training for all staff—from the frontline to the executive level—are essential to successfully implementing the principles and practices of psychological safety.5-7 VHA training covers many topics, including the origins, benefits, and implementation strategies of psychological safety (Table). Role-playing simulation is an effective teaching format, providing staff with opportunities to practice techniques for raising concerns or share feedback in a controlled environment.6 In addition, education should be ongoing; it helps leaders and staff members feel competent and confident when implementing psychological safety across the health care organization.6,10

Accountability
The final critical strategy for achieving psychological safety is accountability. It is the responsibility of all leadership—from senior leaders to clinical and nonclinical managers—to create a culture of shared accountability.5 But first, expectations must be set. Leadership must establish well-defined behavioral expectations that align with the organization’s values. Understanding behavioral expectations will help to ensure that employees know what achievement looks like, as well as how they are being held accountable for their individual actions.4,5,7 In practical terms, this means ensuring that staff members have the skills and resources to achieve goals and expectations, providing performance feedback in a timely manner, and including expectations in annual performance evaluations (as they are in the VHA).
Consistency is key. Accountability should be the expectation across all levels and services of the health care organization. No staff member should be exempt from promoting a psychologically safe work environment. Compliance with behavioral expectations should be monitored and if a person’s actions are not consistent with expectations, the situation will need to be addressed. Interventions will depend on the type, severity, and frequency of the problematic behaviors. Depending on an organization’s policies and practices, courses of action can range from feedback counseling to employment termination.5
A practical matter in ensuring accountability is implementing a psychologically safe process for reporting concerns. Staff members must feel comfortable reporting behavioral concerns without fear of retaliation, negative judgment, or consequences from peers and supervisors. One method for doing this is to create a confidential, centralized process for reporting concerns.5
First-Hand Results
VAPHCS has seen the results of implementing the strategies outlined here. For example, VAPHCS has observed a 45% increase in the use of the patient safety reporting system that logs medical errors and near-misses. In addition, there have been improvements in levels of psychological safety and patient safety reported in the annual VHA All Employee Survey, which is conducted annually to gauge workplace satisfaction, culture, climate, turnover, supervisory behaviors, and general workplace perceptions. VAPHCS has shown consistent improvements in 12 patient safety elements scored on a 5-point scale (1, very dissatisfied; 5, very satisfied) (Figure). Notably, employee ratings of error prevention discussed increased from 4.0 in 2022 to 4.3 in 2024. Data collection and analysis are ongoing; more comprehensive findings will be published in the future.
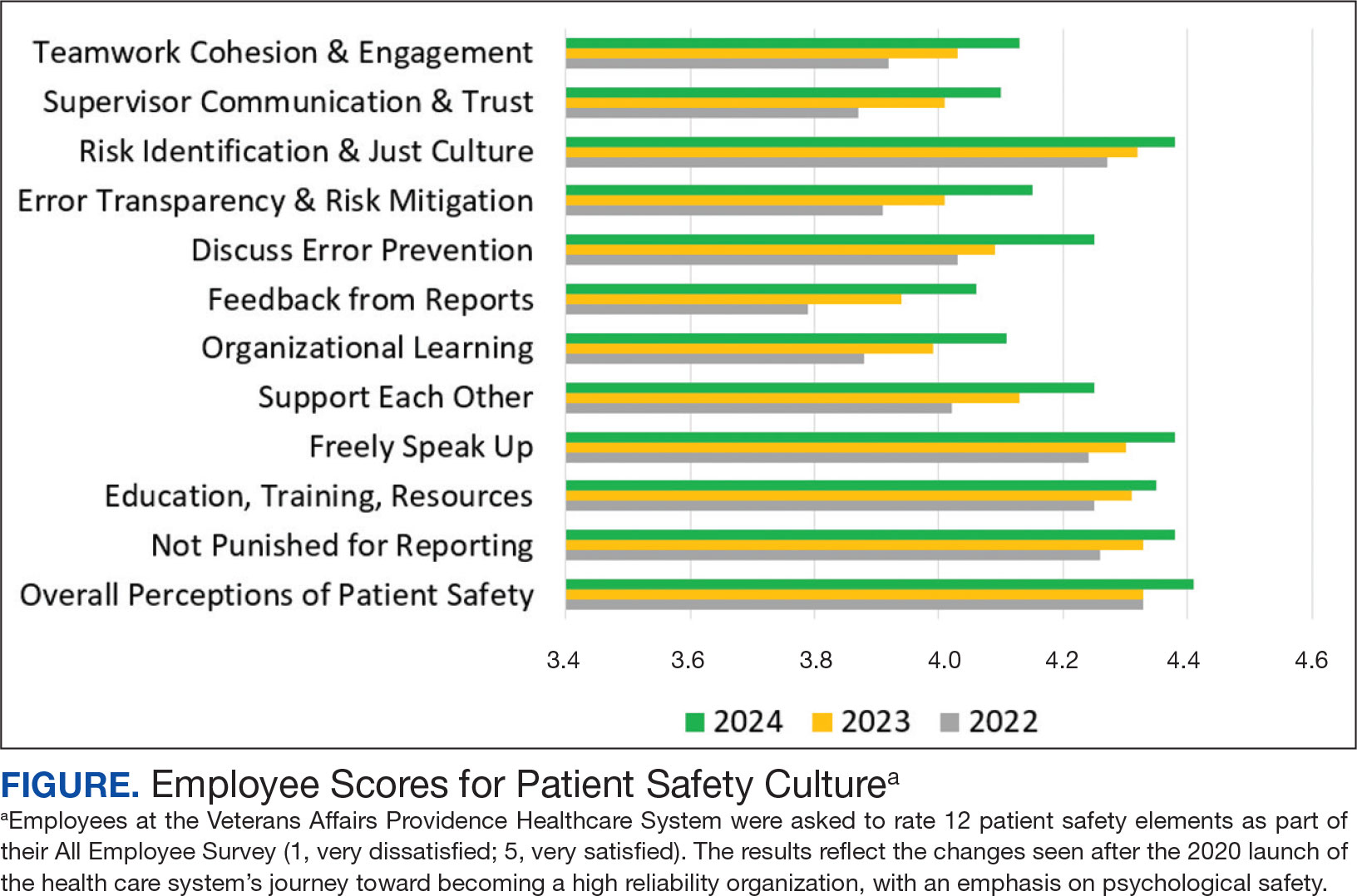
CONCLUSIONS
Health care organizations are increasingly recognizing the importance of psychologically safe workplaces in order to provide safe, high-quality patient care. Psychological safety is a critical tool for empowering staff to raise concerns, ask tough questions, challenge the status quo, and share new ideas for providing health care services. While psychological safety has been slowly adopted in health care, it’s clear that evidence-based strategies can make psychological safety a reality.
- Spanos S, Leask E, Patel R, Datyner M, Loh E, Braithwaite J. Healthcare leaders navigating complexity: A scoping review of key trends in future roles and competencies. BMC Med Educ. 2024;24(1):720. doi:10.1186/s12909-024-05689-4
- Murray JS, Baghdadi A, Dannenberg W, Crews P, Walsh ND. The role of high reliability organization foundational practices in building a culture of safety. Fed Pract. 2024;41(7):214-221. doi:10.12788/fp.0486
- Bransby DP, Kerrissey M, Edmondson AC. Paradise lost (and restored?): a study of psychological safety over time. Acad Manag Discov. Published online March 14, 2024. doi:10.5465/amd.2023.0084
- Murray JS, Kelly S, Hanover C. Promoting psychological safety in healthcare organizations. Mil Med. 2022;187(7-8):808-810. doi:10.1093/milmed/usac041
- Jamal N, Young VN, Shapiro J, Brenner MJ, Schmalbach CE. Patient safety/quality improvement primer, part IV: Psychological safety-drivers to outcomes and well-being. Otolaryngol Head Neck Surg. 2023;168(4):881-888. doi:10.1177/01945998221126966
- Sarofim M. Psychological safety in medicine: What is it, and who cares? Med J Aust. 2024;220(8):398-399. doi:10.5694/mja2.52263
- Edmondson AC, Bransby DP. Psychological safety comes of age: Observed themes in an established literature. Annu Rev Organ Psychol Organ Behav. 2023;10:55-78. doi.org/10.1146/annurev-orgpsych-120920-055217
- Kumar S. Psychological safety: What it is, why teams need it, and how to make it flourish. Chest. 2024; 165(4):942-949. doi:10.1016/j.chest.2023.11.016
- Hallam KT, Popovic N, Karimi L. Identifying the key elements of psychologically safe workplaces in healthcare settings. Brain Sci. 2023;13(10):1450. doi:10.3390/brainsci13101450
- Grailey KE, Murray E, Reader T, Brett SJ. The presence and potential impact of psychological safety in the healthcare setting: an evidence synthesis. BMC Health Serv Res. 2021;21(1):773. doi:10.1186/s12913-021-06740-6
- US Department of Veterans Affairs. VISN 1: VA New England Healthcare System. Accessed March 25, 2025. https://department.va.gov/integrated-service-networks/visn-01
- Brimhall KC, Tsai CY, Eckardt R, Dionne S, Yang B, Sharp A. The effects of leadership for self-worth, inclusion, trust, and psychological safety on medical error reporting. Health Care Manage Rev. 2023;48(2):120-129. doi:10.1097/HMR.0000000000000358
- Adair KC, Heath A, Frye MA, et al. The Psychological Safety Scale of the Safety, Communication, Operational, Reliability, and Engagement (SCORE) Survey: a brief, diagnostic, and actionable metric for the ability to speak up in healthcare settings. J Patient Saf. 2022;18(6):513-520. doi:10.1097/PTS.0000000000001048
- Cho H, Steege LM, Arsenault Knudsen ÉN. Psychological safety, communication openness, nurse job outcomes, and patient safety in hospital nurses. Res Nurs Health. 2023;46(4):445-453.
- Practical Tool 2: 5 minute psychological safety audit. Accessed March 25, 2025. https://www.educationsupport.org.uk/media/jlnf3cju/practical-tool-2-psychological-safety-audit.pdf
Worldwide, health care is becoming increasingly complex as a result of greater clinical workforce demands, expanded roles and responsibilities, health care system mergers, stakeholder calls for new capabilities, and digital transformation. 1,2These increasing demands has prompted many health care institutions to place greater focus on the psychological safety of their workforce, particularly in high reliability organizations (HROs). Building a robust foundation for high reliability in health care requires the presence of psychological safety—that is, staff members at all levels of the organization must feel comfortable speaking up when they have questions or concerns.3,4 Psychological safety can improve the safety and quality of patient care but has not reached its full potential in health care.5,6 However, there are strategies that promote the widespread implementation of psychological safety in health care organizations.3-6
PSYCHOLOGICAL SAFETY
The concept of psychological safety in organizational behavior originated in 1965 when Edgar Schein and Warren Bennis, leaders in organizational psychology and management, published their reflections on the importance of psychological safety in helping individuals feel secure in the work environment.5-7 Psychological safety in the workplace is foundational to staff members feeling comfortable asking questions or expressing concerns without fear of negative consequences.8,9 It supports both individual and team efforts to raise safety concerns and report near misses and adverse events so that similar events can be averted in the future.9 Patients aren’t the only ones who benefit; psychological safety has also been found to promote job satisfaction and employee well-being.10
THE VETERANS HEALTH ADMINISTRATION JOURNEY
Achieving psychological safety is by no means an easy or comfortable process. As with any organizational change, a multipronged approach offers the best chance of success.6,9 When the Veterans Health Administration (VHA) began its incremental, enterprise-wide journey to high reliability in 2019, 3 cohorts were identified. In February 2019, 18 US Department of Veterans Affairs (VA) medical centers (VAMCs) (cohort 1) began the process of becoming HROs. Cohort 2 followed in October 2020 and included 54 VAMC. Finally, in October 2021, 67 additional VAMCs (cohort 3) started the process.2 During cohort 2, the VA Providence Healthcare System (VAPHCS) decided to emphasize psychological safety at the start of the journey to becoming an HRO. This system is part of the VA New England Healthcare System (VISN 1), which includes VAMCs and clinics in Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, and Vermont.11 Soon thereafter, the VA Bedford Healthcare System and the VA Connecticut Healthcare System adopted similar strategies. Since then, other VAMCs have also adopted this approach. These collective experiences identified 4 useful strategies for achieving psychological safety: leadership engagement, open communication, education and training, and accountability.
Leadership Engagement
Health care organization leaders play a critical role in making psychological safety happen—especially in complex and constantly changing environments, such as HROs.4 Leaders behaviors are consistently linked to the perception of psychological safety at the individual, team, and organizational levels.8 It is especially important to have leaders who recognize the views of individuals and team members and encourage staff participation in discussions to gain additional perspectives.7,8,12 Psychological safety can also be facilitated when leaders are visible, approachable, and communicative.4,7-9
Organizational practices, policies, and processes (eg, reporting adverse events without the fear of negative consequences) are also important ways that leaders can establish and sustain psychological safety. On a more granular level, leaders can enhance psychological safety by promoting and acknowledging individuals who speak up, regularly asking staff about safety concerns, highlighting “good catches” when harm is avoided, and using staff feedback to initiate improvements.4,7,13Finally, in the authors’ experience, psychological safety requires clear commitment from leaders at all levels of an organization. Communication should be bidirectional, and leaders should close the proverbial “loop” with feedback and timely follow-up. This encourages and reinforces staff engagement and speaking up behaviors.2,4,7,13
Open Communication
Promoting an environment of open communication, where all individuals and teams feel empowered to speak up with questions, concerns, and recommendations—regardless of position within the organization—is critical to psychological safety.4,6,9 Open communication is especially critical when processes and systems are constantly changing and advancing as a result of new information and technology.9 Promoting open, bidirectional communication during the delivery of patient care can be accomplished with huddles, tiered safety huddles, leader rounding for high reliability, and time-outs.2,4,6 These opportunities allow team members to discuss concerns, identify resources that support safe, high-quality care; reflect on successes and opportunities for improvement; and circle back on concerns.2,6 Open communication in psychologically safe environments empowers staff to raise patient care concerns and is instrumental for improving patient safety, increasing staff job satisfaction, and decreasing turnover.6,14
Education and Training
Education and training for all staff—from the frontline to the executive level—are essential to successfully implementing the principles and practices of psychological safety.5-7 VHA training covers many topics, including the origins, benefits, and implementation strategies of psychological safety (Table). Role-playing simulation is an effective teaching format, providing staff with opportunities to practice techniques for raising concerns or share feedback in a controlled environment.6 In addition, education should be ongoing; it helps leaders and staff members feel competent and confident when implementing psychological safety across the health care organization.6,10

Accountability
The final critical strategy for achieving psychological safety is accountability. It is the responsibility of all leadership—from senior leaders to clinical and nonclinical managers—to create a culture of shared accountability.5 But first, expectations must be set. Leadership must establish well-defined behavioral expectations that align with the organization’s values. Understanding behavioral expectations will help to ensure that employees know what achievement looks like, as well as how they are being held accountable for their individual actions.4,5,7 In practical terms, this means ensuring that staff members have the skills and resources to achieve goals and expectations, providing performance feedback in a timely manner, and including expectations in annual performance evaluations (as they are in the VHA).
Consistency is key. Accountability should be the expectation across all levels and services of the health care organization. No staff member should be exempt from promoting a psychologically safe work environment. Compliance with behavioral expectations should be monitored and if a person’s actions are not consistent with expectations, the situation will need to be addressed. Interventions will depend on the type, severity, and frequency of the problematic behaviors. Depending on an organization’s policies and practices, courses of action can range from feedback counseling to employment termination.5
A practical matter in ensuring accountability is implementing a psychologically safe process for reporting concerns. Staff members must feel comfortable reporting behavioral concerns without fear of retaliation, negative judgment, or consequences from peers and supervisors. One method for doing this is to create a confidential, centralized process for reporting concerns.5
First-Hand Results
VAPHCS has seen the results of implementing the strategies outlined here. For example, VAPHCS has observed a 45% increase in the use of the patient safety reporting system that logs medical errors and near-misses. In addition, there have been improvements in levels of psychological safety and patient safety reported in the annual VHA All Employee Survey, which is conducted annually to gauge workplace satisfaction, culture, climate, turnover, supervisory behaviors, and general workplace perceptions. VAPHCS has shown consistent improvements in 12 patient safety elements scored on a 5-point scale (1, very dissatisfied; 5, very satisfied) (Figure). Notably, employee ratings of error prevention discussed increased from 4.0 in 2022 to 4.3 in 2024. Data collection and analysis are ongoing; more comprehensive findings will be published in the future.

CONCLUSIONS
Health care organizations are increasingly recognizing the importance of psychologically safe workplaces in order to provide safe, high-quality patient care. Psychological safety is a critical tool for empowering staff to raise concerns, ask tough questions, challenge the status quo, and share new ideas for providing health care services. While psychological safety has been slowly adopted in health care, it’s clear that evidence-based strategies can make psychological safety a reality.
Worldwide, health care is becoming increasingly complex as a result of greater clinical workforce demands, expanded roles and responsibilities, health care system mergers, stakeholder calls for new capabilities, and digital transformation. 1,2These increasing demands has prompted many health care institutions to place greater focus on the psychological safety of their workforce, particularly in high reliability organizations (HROs). Building a robust foundation for high reliability in health care requires the presence of psychological safety—that is, staff members at all levels of the organization must feel comfortable speaking up when they have questions or concerns.3,4 Psychological safety can improve the safety and quality of patient care but has not reached its full potential in health care.5,6 However, there are strategies that promote the widespread implementation of psychological safety in health care organizations.3-6
PSYCHOLOGICAL SAFETY
The concept of psychological safety in organizational behavior originated in 1965 when Edgar Schein and Warren Bennis, leaders in organizational psychology and management, published their reflections on the importance of psychological safety in helping individuals feel secure in the work environment.5-7 Psychological safety in the workplace is foundational to staff members feeling comfortable asking questions or expressing concerns without fear of negative consequences.8,9 It supports both individual and team efforts to raise safety concerns and report near misses and adverse events so that similar events can be averted in the future.9 Patients aren’t the only ones who benefit; psychological safety has also been found to promote job satisfaction and employee well-being.10
THE VETERANS HEALTH ADMINISTRATION JOURNEY
Achieving psychological safety is by no means an easy or comfortable process. As with any organizational change, a multipronged approach offers the best chance of success.6,9 When the Veterans Health Administration (VHA) began its incremental, enterprise-wide journey to high reliability in 2019, 3 cohorts were identified. In February 2019, 18 US Department of Veterans Affairs (VA) medical centers (VAMCs) (cohort 1) began the process of becoming HROs. Cohort 2 followed in October 2020 and included 54 VAMC. Finally, in October 2021, 67 additional VAMCs (cohort 3) started the process.2 During cohort 2, the VA Providence Healthcare System (VAPHCS) decided to emphasize psychological safety at the start of the journey to becoming an HRO. This system is part of the VA New England Healthcare System (VISN 1), which includes VAMCs and clinics in Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, and Vermont.11 Soon thereafter, the VA Bedford Healthcare System and the VA Connecticut Healthcare System adopted similar strategies. Since then, other VAMCs have also adopted this approach. These collective experiences identified 4 useful strategies for achieving psychological safety: leadership engagement, open communication, education and training, and accountability.
Leadership Engagement
Health care organization leaders play a critical role in making psychological safety happen—especially in complex and constantly changing environments, such as HROs.4 Leaders behaviors are consistently linked to the perception of psychological safety at the individual, team, and organizational levels.8 It is especially important to have leaders who recognize the views of individuals and team members and encourage staff participation in discussions to gain additional perspectives.7,8,12 Psychological safety can also be facilitated when leaders are visible, approachable, and communicative.4,7-9
Organizational practices, policies, and processes (eg, reporting adverse events without the fear of negative consequences) are also important ways that leaders can establish and sustain psychological safety. On a more granular level, leaders can enhance psychological safety by promoting and acknowledging individuals who speak up, regularly asking staff about safety concerns, highlighting “good catches” when harm is avoided, and using staff feedback to initiate improvements.4,7,13Finally, in the authors’ experience, psychological safety requires clear commitment from leaders at all levels of an organization. Communication should be bidirectional, and leaders should close the proverbial “loop” with feedback and timely follow-up. This encourages and reinforces staff engagement and speaking up behaviors.2,4,7,13
Open Communication
Promoting an environment of open communication, where all individuals and teams feel empowered to speak up with questions, concerns, and recommendations—regardless of position within the organization—is critical to psychological safety.4,6,9 Open communication is especially critical when processes and systems are constantly changing and advancing as a result of new information and technology.9 Promoting open, bidirectional communication during the delivery of patient care can be accomplished with huddles, tiered safety huddles, leader rounding for high reliability, and time-outs.2,4,6 These opportunities allow team members to discuss concerns, identify resources that support safe, high-quality care; reflect on successes and opportunities for improvement; and circle back on concerns.2,6 Open communication in psychologically safe environments empowers staff to raise patient care concerns and is instrumental for improving patient safety, increasing staff job satisfaction, and decreasing turnover.6,14
Education and Training
Education and training for all staff—from the frontline to the executive level—are essential to successfully implementing the principles and practices of psychological safety.5-7 VHA training covers many topics, including the origins, benefits, and implementation strategies of psychological safety (Table). Role-playing simulation is an effective teaching format, providing staff with opportunities to practice techniques for raising concerns or share feedback in a controlled environment.6 In addition, education should be ongoing; it helps leaders and staff members feel competent and confident when implementing psychological safety across the health care organization.6,10

Accountability
The final critical strategy for achieving psychological safety is accountability. It is the responsibility of all leadership—from senior leaders to clinical and nonclinical managers—to create a culture of shared accountability.5 But first, expectations must be set. Leadership must establish well-defined behavioral expectations that align with the organization’s values. Understanding behavioral expectations will help to ensure that employees know what achievement looks like, as well as how they are being held accountable for their individual actions.4,5,7 In practical terms, this means ensuring that staff members have the skills and resources to achieve goals and expectations, providing performance feedback in a timely manner, and including expectations in annual performance evaluations (as they are in the VHA).
Consistency is key. Accountability should be the expectation across all levels and services of the health care organization. No staff member should be exempt from promoting a psychologically safe work environment. Compliance with behavioral expectations should be monitored and if a person’s actions are not consistent with expectations, the situation will need to be addressed. Interventions will depend on the type, severity, and frequency of the problematic behaviors. Depending on an organization’s policies and practices, courses of action can range from feedback counseling to employment termination.5
A practical matter in ensuring accountability is implementing a psychologically safe process for reporting concerns. Staff members must feel comfortable reporting behavioral concerns without fear of retaliation, negative judgment, or consequences from peers and supervisors. One method for doing this is to create a confidential, centralized process for reporting concerns.5
First-Hand Results
VAPHCS has seen the results of implementing the strategies outlined here. For example, VAPHCS has observed a 45% increase in the use of the patient safety reporting system that logs medical errors and near-misses. In addition, there have been improvements in levels of psychological safety and patient safety reported in the annual VHA All Employee Survey, which is conducted annually to gauge workplace satisfaction, culture, climate, turnover, supervisory behaviors, and general workplace perceptions. VAPHCS has shown consistent improvements in 12 patient safety elements scored on a 5-point scale (1, very dissatisfied; 5, very satisfied) (Figure). Notably, employee ratings of error prevention discussed increased from 4.0 in 2022 to 4.3 in 2024. Data collection and analysis are ongoing; more comprehensive findings will be published in the future.

CONCLUSIONS
Health care organizations are increasingly recognizing the importance of psychologically safe workplaces in order to provide safe, high-quality patient care. Psychological safety is a critical tool for empowering staff to raise concerns, ask tough questions, challenge the status quo, and share new ideas for providing health care services. While psychological safety has been slowly adopted in health care, it’s clear that evidence-based strategies can make psychological safety a reality.
- Spanos S, Leask E, Patel R, Datyner M, Loh E, Braithwaite J. Healthcare leaders navigating complexity: A scoping review of key trends in future roles and competencies. BMC Med Educ. 2024;24(1):720. doi:10.1186/s12909-024-05689-4
- Murray JS, Baghdadi A, Dannenberg W, Crews P, Walsh ND. The role of high reliability organization foundational practices in building a culture of safety. Fed Pract. 2024;41(7):214-221. doi:10.12788/fp.0486
- Bransby DP, Kerrissey M, Edmondson AC. Paradise lost (and restored?): a study of psychological safety over time. Acad Manag Discov. Published online March 14, 2024. doi:10.5465/amd.2023.0084
- Murray JS, Kelly S, Hanover C. Promoting psychological safety in healthcare organizations. Mil Med. 2022;187(7-8):808-810. doi:10.1093/milmed/usac041
- Jamal N, Young VN, Shapiro J, Brenner MJ, Schmalbach CE. Patient safety/quality improvement primer, part IV: Psychological safety-drivers to outcomes and well-being. Otolaryngol Head Neck Surg. 2023;168(4):881-888. doi:10.1177/01945998221126966
- Sarofim M. Psychological safety in medicine: What is it, and who cares? Med J Aust. 2024;220(8):398-399. doi:10.5694/mja2.52263
- Edmondson AC, Bransby DP. Psychological safety comes of age: Observed themes in an established literature. Annu Rev Organ Psychol Organ Behav. 2023;10:55-78. doi.org/10.1146/annurev-orgpsych-120920-055217
- Kumar S. Psychological safety: What it is, why teams need it, and how to make it flourish. Chest. 2024; 165(4):942-949. doi:10.1016/j.chest.2023.11.016
- Hallam KT, Popovic N, Karimi L. Identifying the key elements of psychologically safe workplaces in healthcare settings. Brain Sci. 2023;13(10):1450. doi:10.3390/brainsci13101450
- Grailey KE, Murray E, Reader T, Brett SJ. The presence and potential impact of psychological safety in the healthcare setting: an evidence synthesis. BMC Health Serv Res. 2021;21(1):773. doi:10.1186/s12913-021-06740-6
- US Department of Veterans Affairs. VISN 1: VA New England Healthcare System. Accessed March 25, 2025. https://department.va.gov/integrated-service-networks/visn-01
- Brimhall KC, Tsai CY, Eckardt R, Dionne S, Yang B, Sharp A. The effects of leadership for self-worth, inclusion, trust, and psychological safety on medical error reporting. Health Care Manage Rev. 2023;48(2):120-129. doi:10.1097/HMR.0000000000000358
- Adair KC, Heath A, Frye MA, et al. The Psychological Safety Scale of the Safety, Communication, Operational, Reliability, and Engagement (SCORE) Survey: a brief, diagnostic, and actionable metric for the ability to speak up in healthcare settings. J Patient Saf. 2022;18(6):513-520. doi:10.1097/PTS.0000000000001048
- Cho H, Steege LM, Arsenault Knudsen ÉN. Psychological safety, communication openness, nurse job outcomes, and patient safety in hospital nurses. Res Nurs Health. 2023;46(4):445-453.
- Practical Tool 2: 5 minute psychological safety audit. Accessed March 25, 2025. https://www.educationsupport.org.uk/media/jlnf3cju/practical-tool-2-psychological-safety-audit.pdf
- Spanos S, Leask E, Patel R, Datyner M, Loh E, Braithwaite J. Healthcare leaders navigating complexity: A scoping review of key trends in future roles and competencies. BMC Med Educ. 2024;24(1):720. doi:10.1186/s12909-024-05689-4
- Murray JS, Baghdadi A, Dannenberg W, Crews P, Walsh ND. The role of high reliability organization foundational practices in building a culture of safety. Fed Pract. 2024;41(7):214-221. doi:10.12788/fp.0486
- Bransby DP, Kerrissey M, Edmondson AC. Paradise lost (and restored?): a study of psychological safety over time. Acad Manag Discov. Published online March 14, 2024. doi:10.5465/amd.2023.0084
- Murray JS, Kelly S, Hanover C. Promoting psychological safety in healthcare organizations. Mil Med. 2022;187(7-8):808-810. doi:10.1093/milmed/usac041
- Jamal N, Young VN, Shapiro J, Brenner MJ, Schmalbach CE. Patient safety/quality improvement primer, part IV: Psychological safety-drivers to outcomes and well-being. Otolaryngol Head Neck Surg. 2023;168(4):881-888. doi:10.1177/01945998221126966
- Sarofim M. Psychological safety in medicine: What is it, and who cares? Med J Aust. 2024;220(8):398-399. doi:10.5694/mja2.52263
- Edmondson AC, Bransby DP. Psychological safety comes of age: Observed themes in an established literature. Annu Rev Organ Psychol Organ Behav. 2023;10:55-78. doi.org/10.1146/annurev-orgpsych-120920-055217
- Kumar S. Psychological safety: What it is, why teams need it, and how to make it flourish. Chest. 2024; 165(4):942-949. doi:10.1016/j.chest.2023.11.016
- Hallam KT, Popovic N, Karimi L. Identifying the key elements of psychologically safe workplaces in healthcare settings. Brain Sci. 2023;13(10):1450. doi:10.3390/brainsci13101450
- Grailey KE, Murray E, Reader T, Brett SJ. The presence and potential impact of psychological safety in the healthcare setting: an evidence synthesis. BMC Health Serv Res. 2021;21(1):773. doi:10.1186/s12913-021-06740-6
- US Department of Veterans Affairs. VISN 1: VA New England Healthcare System. Accessed March 25, 2025. https://department.va.gov/integrated-service-networks/visn-01
- Brimhall KC, Tsai CY, Eckardt R, Dionne S, Yang B, Sharp A. The effects of leadership for self-worth, inclusion, trust, and psychological safety on medical error reporting. Health Care Manage Rev. 2023;48(2):120-129. doi:10.1097/HMR.0000000000000358
- Adair KC, Heath A, Frye MA, et al. The Psychological Safety Scale of the Safety, Communication, Operational, Reliability, and Engagement (SCORE) Survey: a brief, diagnostic, and actionable metric for the ability to speak up in healthcare settings. J Patient Saf. 2022;18(6):513-520. doi:10.1097/PTS.0000000000001048
- Cho H, Steege LM, Arsenault Knudsen ÉN. Psychological safety, communication openness, nurse job outcomes, and patient safety in hospital nurses. Res Nurs Health. 2023;46(4):445-453.
- Practical Tool 2: 5 minute psychological safety audit. Accessed March 25, 2025. https://www.educationsupport.org.uk/media/jlnf3cju/practical-tool-2-psychological-safety-audit.pdf
Achieving Psychological Safety in High Reliability Organizations
Achieving Psychological Safety in High Reliability Organizations
The Cruelty of April: Suicide in Spring
The Cruelty of April: Suicide in Spring
April is the cruellest month, breeding
Lilacs out of the dead land, mixing
Memory and desire, stirring
Dull roots with spring rain.
T.S. Eliot1
The epigraph for this column is from The Waste Land, T.S. Eliot’s postmodern poem that, in part, reflects his experience of the destruction of an entire way of living and a generation of young men in the wake of the First World War. The terrible contemporary toll suicide has taken on veterans and the active-duty military makes it easy to forget that suicide is an inveterate and disturbing aftermath of all wars.2
There is a profound and elemental connection in the human mind between Spring and renewal. In almost every culture and religion, across nearly every historical epoch and location, Spring is associated with themes of growth, returning life, light, and hope. On a more prosaic modern level, almost all of us—us—especially those in Northern climates—look forward to warmer weather, more time spent outdoors, and the simple joys of seeing perennials return in the garden and birds nest in blooming trees.
It is a paradox of human life that suicide is more common in the season of rebirth than in the season of decline. The bare trees, freezing temperatures, and icy darkness that accompany winter in much of the world inherently lead us to contemplate our mortality. The counterintuitive finding that individuals, many of them veterans, take their own lives more often in Spring creates a cognitive dissonance to be explored in this editorial.
As a layperson, I too assumed there were more suicides in winter, especially around the holidays when the expectation of belonging, privilege, and pleasure painfully reminds the alienated, lonely, homeless, and ailing of all they lack and all they have lost. As a psychiatric intern, I anticipated that the inpatient US Department of Veterans Affairs (VA) ward where I was training would empty with the arrival of nicer weather. Instead, I was mystified when the opposite occurred and the unit was overflowing with manic and suicidal patients.
The Centers for Disease Control and Prevention National Center for Health Statistics ranked suicide by month from 1999 to 2010. Contrary to popular belief, more suicides occurred in late Spring and Summer than any other season.3 A 2023 study of systematic reviews of seasonal variation in mood disorders, suicide risk, and health care utilization found that suicide was 11% to 23% higher, suicide attempts resulting in emergency department visits showed an increase of 1.2% to 1.7%, and hospital admissions for mania rose 7.4% to 16.0% in Spring and Summer, compared with Fall and Winter.4 This general population finding is also seen in veteran and military cohorts. A recent study analyzed VA and US Department of Defense (DoD) data from 133,867 veteran suicides from 2001 to 2021. Results showed that veteran suicides were highest in Summer.5
The rise of suicide in the Spring was first observed in the 17th century and has been the object of scientific study for at least 3 decades. That research has produced several different hypotheses from a variety of disciplines, none of which are conclusive as of this writing. Cho and Lee note that the phrase “Spring fever” is a much more serious illness for those with a predisposition or diagnosis of unipolar or bipolar disorder than the quotidian irritant that afflicts those without affective disorders.6 In residency, I learned that longer exposure to light in Spring led to an imbalance in neurotransmitters that triggered manias. This is a simplistic version of the complex circadian interactions of temperature, climate, light, and other environmental variables causing dysregulation or misalignment of our natural biological cycles and those of nature proposed by chronobiologists.7
Sociological and criminal justice scholars underscore that an increase in temperature may exacerbate violent tendencies, especially in older males—a demographic profile more frequently found in veterans—and those already prone to acting out their frustrations with firearms.8 Psychologists have hypothesized that individuals with depressions persevere through Winter by telling themselves they will feel relief in the Spring. Too often the coming of Spring brings not reprieve but a deadly combination of deeper mental desperation coupled with the release from winter lassitude that energizes the now hopeless person to put ideation into action.4,9 The elevation of suicide rates in Spring is likely multidetermined with all these putative causes contributing in different variations to every individual who tragically dies by suicide.
Yet despite decades of public education, this dangerous fiction stubbornly persists in the educated public and even among many health care professionals, in part due to misguided media. For years, the Annenberg Public Policy Center (APPC) has made busting this myth of holiday suicides in the media an organizational initiative. A 2023 APPC survey found that 4 of 5 Americans picked December as the month when suicide rates were highest. The organization has been analyzing holiday—related media reports for decades; those results show some improvement, with the most recent analysis of media reports somewhat better and 40% communicating erroneous information. 10
APPC believes the opinion that suicide is more common around the holidays will persuade those struggling with an exacerbation of a mental health condition or an acute crisis to attempt or die by suicide, believing it to be a reasonable social response. While recognizing there is a real risk of such contagion behavior, I believe the reverse problem is more concerning. As I observed during my internship, the acceptance of the fiction that everyone is happy in Spring may even blind health care professionals from detecting clues that patients and even our loved ones are contemplating suicide. Our relief that Winter has passed and enjoyment of Spring activities can fool us into believing everyone else is also feeling fine and doing well and miss an opportunity to intervene and treat mania or depression to save a life—the medical manifestation of renewal.
- Elliot TS, North M. The Waste Land and Other Poems: A Norton Critical Edition. W.W. Norton & Company; 2022.
- Lester D. Suicide rates before, during, and after the world wars. Eur Psychiatry. 1994;9(5):262-264. doi:10.1017/S092493380000362X
- Centers for Disease Control Center and Prevention. National Center for Health Statistics. Fact or fiction: suicides increase during the holiday season and winter months. January 10, 2014. Accessed March 27, 2025. https://blogs.cdc.gov/nchs/2014/01/10/1121/
- Della DF, Allison S, Bidargaddi N, Wa SK, Bastiampillai T. An umbrella systematic review of seasonality in mood disorders and suicide risk: the impact on demand for primary behavioral health care and acute psychiatric services. Prim Care Companion CNS Disord. 2023;25(3):22r03395. doi:10.4088/PCC.22r03395
- Gold SA, Goodrich M, Morley SW, Stephens B, McCarthy JF. Temporal patterns of veteran suicide: variation by season, day of the week, and holidays. Suicide Life Threat Behav. 2025;55(2):e13148. doi:10.1111/sltb.13148
- Cho CH, Lee HJ. Why do mania and suicide occur most often in the Spring? Psychiatry Investig. 2018;15(3):232-234. doi:10.30773/pi.2017.12.20
- Postolache TT, Mortensen PB, Tonelli LH, et al. Seasonal spring peaks of suicide in victims with and without prior history of hospitalization for mood disorders. J Affect Disord. 2010;121(1-2):88-93. doi:10.1016/j.jad.2009.05.015
- Christodoulou C, Efstathiou V, Bouras G, Korkoliakou P, Lykouras L. Seasonal variation of suicide: a brief review. Encephalos. 2012;49:73-79.
- Shapiro M. Suicide rates spike in spring, not winter. Dome. May/June 2019. Accessed March 28, 2025. https://www.hopkinsmedicine.org/news/articles/2019/05/suicide-rates-spike-in-spring-not-winter
- Annenberg Public Policy Center. Suicides don’t spike around the holiday season, but Americans think they do. December 6, 2023. Accessed March 27, 2025. https:// www.asc.upenn.edu/news-events/news/suicides-dont-spike-around-holiday-season-americans-think-they-do
April is the cruellest month, breeding
Lilacs out of the dead land, mixing
Memory and desire, stirring
Dull roots with spring rain.
T.S. Eliot1
The epigraph for this column is from The Waste Land, T.S. Eliot’s postmodern poem that, in part, reflects his experience of the destruction of an entire way of living and a generation of young men in the wake of the First World War. The terrible contemporary toll suicide has taken on veterans and the active-duty military makes it easy to forget that suicide is an inveterate and disturbing aftermath of all wars.2
There is a profound and elemental connection in the human mind between Spring and renewal. In almost every culture and religion, across nearly every historical epoch and location, Spring is associated with themes of growth, returning life, light, and hope. On a more prosaic modern level, almost all of us—us—especially those in Northern climates—look forward to warmer weather, more time spent outdoors, and the simple joys of seeing perennials return in the garden and birds nest in blooming trees.
It is a paradox of human life that suicide is more common in the season of rebirth than in the season of decline. The bare trees, freezing temperatures, and icy darkness that accompany winter in much of the world inherently lead us to contemplate our mortality. The counterintuitive finding that individuals, many of them veterans, take their own lives more often in Spring creates a cognitive dissonance to be explored in this editorial.
As a layperson, I too assumed there were more suicides in winter, especially around the holidays when the expectation of belonging, privilege, and pleasure painfully reminds the alienated, lonely, homeless, and ailing of all they lack and all they have lost. As a psychiatric intern, I anticipated that the inpatient US Department of Veterans Affairs (VA) ward where I was training would empty with the arrival of nicer weather. Instead, I was mystified when the opposite occurred and the unit was overflowing with manic and suicidal patients.
The Centers for Disease Control and Prevention National Center for Health Statistics ranked suicide by month from 1999 to 2010. Contrary to popular belief, more suicides occurred in late Spring and Summer than any other season.3 A 2023 study of systematic reviews of seasonal variation in mood disorders, suicide risk, and health care utilization found that suicide was 11% to 23% higher, suicide attempts resulting in emergency department visits showed an increase of 1.2% to 1.7%, and hospital admissions for mania rose 7.4% to 16.0% in Spring and Summer, compared with Fall and Winter.4 This general population finding is also seen in veteran and military cohorts. A recent study analyzed VA and US Department of Defense (DoD) data from 133,867 veteran suicides from 2001 to 2021. Results showed that veteran suicides were highest in Summer.5
The rise of suicide in the Spring was first observed in the 17th century and has been the object of scientific study for at least 3 decades. That research has produced several different hypotheses from a variety of disciplines, none of which are conclusive as of this writing. Cho and Lee note that the phrase “Spring fever” is a much more serious illness for those with a predisposition or diagnosis of unipolar or bipolar disorder than the quotidian irritant that afflicts those without affective disorders.6 In residency, I learned that longer exposure to light in Spring led to an imbalance in neurotransmitters that triggered manias. This is a simplistic version of the complex circadian interactions of temperature, climate, light, and other environmental variables causing dysregulation or misalignment of our natural biological cycles and those of nature proposed by chronobiologists.7
Sociological and criminal justice scholars underscore that an increase in temperature may exacerbate violent tendencies, especially in older males—a demographic profile more frequently found in veterans—and those already prone to acting out their frustrations with firearms.8 Psychologists have hypothesized that individuals with depressions persevere through Winter by telling themselves they will feel relief in the Spring. Too often the coming of Spring brings not reprieve but a deadly combination of deeper mental desperation coupled with the release from winter lassitude that energizes the now hopeless person to put ideation into action.4,9 The elevation of suicide rates in Spring is likely multidetermined with all these putative causes contributing in different variations to every individual who tragically dies by suicide.
Yet despite decades of public education, this dangerous fiction stubbornly persists in the educated public and even among many health care professionals, in part due to misguided media. For years, the Annenberg Public Policy Center (APPC) has made busting this myth of holiday suicides in the media an organizational initiative. A 2023 APPC survey found that 4 of 5 Americans picked December as the month when suicide rates were highest. The organization has been analyzing holiday—related media reports for decades; those results show some improvement, with the most recent analysis of media reports somewhat better and 40% communicating erroneous information. 10
APPC believes the opinion that suicide is more common around the holidays will persuade those struggling with an exacerbation of a mental health condition or an acute crisis to attempt or die by suicide, believing it to be a reasonable social response. While recognizing there is a real risk of such contagion behavior, I believe the reverse problem is more concerning. As I observed during my internship, the acceptance of the fiction that everyone is happy in Spring may even blind health care professionals from detecting clues that patients and even our loved ones are contemplating suicide. Our relief that Winter has passed and enjoyment of Spring activities can fool us into believing everyone else is also feeling fine and doing well and miss an opportunity to intervene and treat mania or depression to save a life—the medical manifestation of renewal.
April is the cruellest month, breeding
Lilacs out of the dead land, mixing
Memory and desire, stirring
Dull roots with spring rain.
T.S. Eliot1
The epigraph for this column is from The Waste Land, T.S. Eliot’s postmodern poem that, in part, reflects his experience of the destruction of an entire way of living and a generation of young men in the wake of the First World War. The terrible contemporary toll suicide has taken on veterans and the active-duty military makes it easy to forget that suicide is an inveterate and disturbing aftermath of all wars.2
There is a profound and elemental connection in the human mind between Spring and renewal. In almost every culture and religion, across nearly every historical epoch and location, Spring is associated with themes of growth, returning life, light, and hope. On a more prosaic modern level, almost all of us—us—especially those in Northern climates—look forward to warmer weather, more time spent outdoors, and the simple joys of seeing perennials return in the garden and birds nest in blooming trees.
It is a paradox of human life that suicide is more common in the season of rebirth than in the season of decline. The bare trees, freezing temperatures, and icy darkness that accompany winter in much of the world inherently lead us to contemplate our mortality. The counterintuitive finding that individuals, many of them veterans, take their own lives more often in Spring creates a cognitive dissonance to be explored in this editorial.
As a layperson, I too assumed there were more suicides in winter, especially around the holidays when the expectation of belonging, privilege, and pleasure painfully reminds the alienated, lonely, homeless, and ailing of all they lack and all they have lost. As a psychiatric intern, I anticipated that the inpatient US Department of Veterans Affairs (VA) ward where I was training would empty with the arrival of nicer weather. Instead, I was mystified when the opposite occurred and the unit was overflowing with manic and suicidal patients.
The Centers for Disease Control and Prevention National Center for Health Statistics ranked suicide by month from 1999 to 2010. Contrary to popular belief, more suicides occurred in late Spring and Summer than any other season.3 A 2023 study of systematic reviews of seasonal variation in mood disorders, suicide risk, and health care utilization found that suicide was 11% to 23% higher, suicide attempts resulting in emergency department visits showed an increase of 1.2% to 1.7%, and hospital admissions for mania rose 7.4% to 16.0% in Spring and Summer, compared with Fall and Winter.4 This general population finding is also seen in veteran and military cohorts. A recent study analyzed VA and US Department of Defense (DoD) data from 133,867 veteran suicides from 2001 to 2021. Results showed that veteran suicides were highest in Summer.5
The rise of suicide in the Spring was first observed in the 17th century and has been the object of scientific study for at least 3 decades. That research has produced several different hypotheses from a variety of disciplines, none of which are conclusive as of this writing. Cho and Lee note that the phrase “Spring fever” is a much more serious illness for those with a predisposition or diagnosis of unipolar or bipolar disorder than the quotidian irritant that afflicts those without affective disorders.6 In residency, I learned that longer exposure to light in Spring led to an imbalance in neurotransmitters that triggered manias. This is a simplistic version of the complex circadian interactions of temperature, climate, light, and other environmental variables causing dysregulation or misalignment of our natural biological cycles and those of nature proposed by chronobiologists.7
Sociological and criminal justice scholars underscore that an increase in temperature may exacerbate violent tendencies, especially in older males—a demographic profile more frequently found in veterans—and those already prone to acting out their frustrations with firearms.8 Psychologists have hypothesized that individuals with depressions persevere through Winter by telling themselves they will feel relief in the Spring. Too often the coming of Spring brings not reprieve but a deadly combination of deeper mental desperation coupled with the release from winter lassitude that energizes the now hopeless person to put ideation into action.4,9 The elevation of suicide rates in Spring is likely multidetermined with all these putative causes contributing in different variations to every individual who tragically dies by suicide.
Yet despite decades of public education, this dangerous fiction stubbornly persists in the educated public and even among many health care professionals, in part due to misguided media. For years, the Annenberg Public Policy Center (APPC) has made busting this myth of holiday suicides in the media an organizational initiative. A 2023 APPC survey found that 4 of 5 Americans picked December as the month when suicide rates were highest. The organization has been analyzing holiday—related media reports for decades; those results show some improvement, with the most recent analysis of media reports somewhat better and 40% communicating erroneous information. 10
APPC believes the opinion that suicide is more common around the holidays will persuade those struggling with an exacerbation of a mental health condition or an acute crisis to attempt or die by suicide, believing it to be a reasonable social response. While recognizing there is a real risk of such contagion behavior, I believe the reverse problem is more concerning. As I observed during my internship, the acceptance of the fiction that everyone is happy in Spring may even blind health care professionals from detecting clues that patients and even our loved ones are contemplating suicide. Our relief that Winter has passed and enjoyment of Spring activities can fool us into believing everyone else is also feeling fine and doing well and miss an opportunity to intervene and treat mania or depression to save a life—the medical manifestation of renewal.
- Elliot TS, North M. The Waste Land and Other Poems: A Norton Critical Edition. W.W. Norton & Company; 2022.
- Lester D. Suicide rates before, during, and after the world wars. Eur Psychiatry. 1994;9(5):262-264. doi:10.1017/S092493380000362X
- Centers for Disease Control Center and Prevention. National Center for Health Statistics. Fact or fiction: suicides increase during the holiday season and winter months. January 10, 2014. Accessed March 27, 2025. https://blogs.cdc.gov/nchs/2014/01/10/1121/
- Della DF, Allison S, Bidargaddi N, Wa SK, Bastiampillai T. An umbrella systematic review of seasonality in mood disorders and suicide risk: the impact on demand for primary behavioral health care and acute psychiatric services. Prim Care Companion CNS Disord. 2023;25(3):22r03395. doi:10.4088/PCC.22r03395
- Gold SA, Goodrich M, Morley SW, Stephens B, McCarthy JF. Temporal patterns of veteran suicide: variation by season, day of the week, and holidays. Suicide Life Threat Behav. 2025;55(2):e13148. doi:10.1111/sltb.13148
- Cho CH, Lee HJ. Why do mania and suicide occur most often in the Spring? Psychiatry Investig. 2018;15(3):232-234. doi:10.30773/pi.2017.12.20
- Postolache TT, Mortensen PB, Tonelli LH, et al. Seasonal spring peaks of suicide in victims with and without prior history of hospitalization for mood disorders. J Affect Disord. 2010;121(1-2):88-93. doi:10.1016/j.jad.2009.05.015
- Christodoulou C, Efstathiou V, Bouras G, Korkoliakou P, Lykouras L. Seasonal variation of suicide: a brief review. Encephalos. 2012;49:73-79.
- Shapiro M. Suicide rates spike in spring, not winter. Dome. May/June 2019. Accessed March 28, 2025. https://www.hopkinsmedicine.org/news/articles/2019/05/suicide-rates-spike-in-spring-not-winter
- Annenberg Public Policy Center. Suicides don’t spike around the holiday season, but Americans think they do. December 6, 2023. Accessed March 27, 2025. https:// www.asc.upenn.edu/news-events/news/suicides-dont-spike-around-holiday-season-americans-think-they-do
- Elliot TS, North M. The Waste Land and Other Poems: A Norton Critical Edition. W.W. Norton & Company; 2022.
- Lester D. Suicide rates before, during, and after the world wars. Eur Psychiatry. 1994;9(5):262-264. doi:10.1017/S092493380000362X
- Centers for Disease Control Center and Prevention. National Center for Health Statistics. Fact or fiction: suicides increase during the holiday season and winter months. January 10, 2014. Accessed March 27, 2025. https://blogs.cdc.gov/nchs/2014/01/10/1121/
- Della DF, Allison S, Bidargaddi N, Wa SK, Bastiampillai T. An umbrella systematic review of seasonality in mood disorders and suicide risk: the impact on demand for primary behavioral health care and acute psychiatric services. Prim Care Companion CNS Disord. 2023;25(3):22r03395. doi:10.4088/PCC.22r03395
- Gold SA, Goodrich M, Morley SW, Stephens B, McCarthy JF. Temporal patterns of veteran suicide: variation by season, day of the week, and holidays. Suicide Life Threat Behav. 2025;55(2):e13148. doi:10.1111/sltb.13148
- Cho CH, Lee HJ. Why do mania and suicide occur most often in the Spring? Psychiatry Investig. 2018;15(3):232-234. doi:10.30773/pi.2017.12.20
- Postolache TT, Mortensen PB, Tonelli LH, et al. Seasonal spring peaks of suicide in victims with and without prior history of hospitalization for mood disorders. J Affect Disord. 2010;121(1-2):88-93. doi:10.1016/j.jad.2009.05.015
- Christodoulou C, Efstathiou V, Bouras G, Korkoliakou P, Lykouras L. Seasonal variation of suicide: a brief review. Encephalos. 2012;49:73-79.
- Shapiro M. Suicide rates spike in spring, not winter. Dome. May/June 2019. Accessed March 28, 2025. https://www.hopkinsmedicine.org/news/articles/2019/05/suicide-rates-spike-in-spring-not-winter
- Annenberg Public Policy Center. Suicides don’t spike around the holiday season, but Americans think they do. December 6, 2023. Accessed March 27, 2025. https:// www.asc.upenn.edu/news-events/news/suicides-dont-spike-around-holiday-season-americans-think-they-do
The Cruelty of April: Suicide in Spring
The Cruelty of April: Suicide in Spring
Efficacy and Safety of Spironolactone in Acne Management
Efficacy and Safety of Spironolactone in Acne Management
Spironolactone is an aldosterone antagonist that first was used as a potassium-sparing diuretic to treat heart failure and hypertension. It also possesses antiandrogenic mechanisms including competitively inhibiting androgen receptors, increasing steroid hormone–binding globulin production, and decreasing 5α-reductase activity.1 These properties have been leveraged in off-label use for dermatologic conditions including acne, hidradenitis suppurativa, androgenic alopecia, and hirsutism.1,2 Despite being used off-label to treat acne for more than 40 years, spironolactone has not received US Food and Drug Administration approval for this indication.3 Herein, we review the current evidence for use of spironolactone in acne management.
Spironolactone Efficacy
Spironolactone is efficacious for facial and truncal acne in adult females; it cannot be used in males given its anti-androgenic effects.4,5 In 2 large studies, spironolactone completely or partially cleared facial acne in 75.5% to 85.1% of patients.4,5 In the first study, which included 395 patients on a median dose of 100 mg/d (range, 25-200 mg/d), clearance of comedonal, papulopustular, and nodulocystic acne was observed.4 The second study included 403 patients, most of whom started on spironolactone at 100 mg/d (range, 25-200 mg/d). In addition to facial clearance, patients in this study demonstrated similar rates of partial or complete clearance of acne on the chest (84.0%) and back (80.2%) assessed via a comprehensive acne severity scale.5 In both studies, doses of 100 mg/d or higher were most effective, and the median time to initial acne improvement was 3 months, with peak effects occurring after 4 to 6 months of treatment.4,5 Most patients were using spironolactone monotherapy or spironolactone in combination with topical therapies; however, a minority used it concurrently with oral antibiotics and/or combined oral contraceptives.
Spironolactone has demonstrated comparable efficacy to tetracycline antibiotics. A study comparing the rate of switching to another systemic therapy within 1 year of treatment initiation identified similar rates in patients started on spironolactone (n=962) and those started on tetracyclines (n=4236)(14.4% vs 13.4%, respectively). As switching may indicate treatment failure due to insufficient efficacy, adverse effects, or other causes, these findings may suggest similar effectiveness for spironolactone and tetracyclines.6 These treatments also were compared in a randomized controlled trial of 133 patients receiving topical benzoyl peroxide 5% for 6 months and either spironolactone 150 mg/d for 6 months or doxycycline 100 mg/d for 3 months followed by oral placebo for 3 months. At 4 months, spironolactone performed better than doxycycline as assessed using the Adult Female Acne Scoring Tool.3 Although doxycycline was stopped after 3 months and only topical therapy was continued, this finding is notable because guidelines from the American Academy of Dermatology recommend limiting tetracycline use to 3 to 4 months, whereas spironolactone may be continued for prolonged durations.1,4
While most studies have evaluated the efficacy of spironolactone in adult females, it is increasingly being prescribed in adolescents.7 In a study that included 80 females aged 14 to 20 years, 80% (64/80) experienced acne improvement on a median dose of 100 mg/d.8 Additionally, in the study evaluating treatment switching rates, more than 80% of 1139 adolescents who were started on spironolactone were not switched to a different systemic therapy within the first year of treatment, demonstrating the efficacy of spironolactone in this demographic.6 However, treatment switching was more common among adolescents started on spironolactone compared with those who started on tetracyclines. As noted for adults, the treatment switching rates were the same for spironolactone and tetracycline users; the difference in adolescents may be due to lower influence of hormonal factors or higher therapeutic expectations in this population.6
Spironolactone Safety
Spironolactone is well tolerated at doses of 25 to 200 mg/d for acne management. Common adverse effects include diuresis (29% [26/90]), menstrual irregularities (22% [20/90]), fatigue (17% [15/90]), headache (14% [13/90]), and dizziness (12% [11/90]), but they infrequently lead to treatment discontinuation.4,9 Rates of adverse effects are lower in adolescents compared to adults, although the effects of spironolactone on early endocrine development in adolescents are unknown.7 Spironolactone should not be used during pregnancy, and concurrent contraception use is advised because spironolactone has caused feminization of male fetuses in animal studies.1,10-11
While concerns about potentially severe adverse effects including hypotension, hyperkalemia, and tumorigenicity have been raised, their occurrence in the literature is rare.5,12-18 In a study evaluating hypotension in 2084 patients taking spironolactone 50 to 200 mg/day for acne, hair loss, and/or hirsutism, 3.1% experienced absolute hypotension, and only 0.26% required dose reduction or discontinuation.12 Another study of 403 patients taking spironolactone for acne reported a statistically significant but clinically insignificant mean reduction in systolic blood pressure of 3.5 mm Hg.5 While clinically relevant hypotension is unlikely to occur, some authors still recommend measuring baseline blood pressure before spironolactone initiation.12
Many large studies have demonstrated that hyperkalemia with spironolactone use is rare in young healthy women.13-15 In one study of patients aged 18 to 45 years treated with spironolactone for acne, only 0.72% of 1802 serum potassium measurements fell within the range of mild hyperkalemia.13 Another study found a significantly greater incidence of hyperkalemia in healthy women aged 46 to 65 years compared with women younger than 45 years (16.7% vs <1%; P=.0245).14 Additionally, among 27 patients taking spironolactone and oral contraceptives containing drospirenone (a spironolactone analog), none had elevated potassium levels.15 Given these findings, American Academy of Dermatology guidelines suggest that monitoring potassium in young healthy women has low utility but should be considered in those with risk factors including older age; renal and cardiovascular disease; and concurrent medications that interfere with renal, adrenal, and hepatic function.1 If performed, monitoring should be done within the first few weeks of initiating spironolactone for early detection of hyperkalemia.16
Spironolactone has a US Food and Drug Administration warning for tumorigenicity based on studies in rats that were given up to 150 times the amount for human therapeutic doses and subsequently developed thyroid, hepatic, testicular, and breast adenomas.1 However, several large studies in humans have not found an association between spironolactone and breast cancer (BC) development.1,17,18 Furthermore, a large retrospective study found no increased risk for recurrence in BC survivors treated with spironolactone.2 Most carcinogenicity studies include older women, which may limit generalizability of the findings to younger women, who comprise the majority of patients being treated for acne. Recently, however, a retrospective study evaluating healthy females aged 9 to 40 years with acne identified no significant increased risk for BC in patients treated with spironolactone.17 When compared to tetracyclines, there was a slightly decreased BC risk with spironolactone, providing further support for the latter’s safety. Finally, a large systematic review identified no association between spironolactone and ovarian, bladder, kidney, gastric, or esophageal cancers.18
Final Thoughts
Over the past several years, an ever-expanding body of literature supporting the efficacy and safety of spironolactone has emerged. While spironolactone has been used off label for decades to treat acne in healthy adult females, there are now strong data to support its efficacy in adolescent females. Notably, spironolactone consistently demonstrates similar effectiveness to first-line tetracycline antibiotics. Additionally, data suggest that spironolactone is safe in patients with a history of BC. Overall, spironolactone is a safe, comparable, and promising alternative to antibiotics for acne management in adult and adolescent females.
- Reynolds RV, Yeung H, Cheng CE, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2024;90:1006. e1-1006.e30. doi:10.1016/j.jaad.2023.12.017
- Wei C, Bovonratwet P, Gu A, et al. Spironolactone use does not increase the risk of female breast cancer recurrence: a retrospective analysis. J Am Acad Dermatol. 2020;83:1021-1027. doi:10.1016/j.jaad.2020.05.081
- Dréno B, Nguyen JM, Hainaut E, et al. Efficacy of spironolactone compared with doxycycline in moderate acne in adult females: results of the multicentre, controlled, randomized, double-blind prospective and parallel Female Acne Spironolactone vs doxyCycline Efficacy (FASCE) study. Acta Derm Venereol. 2024;104:adv26002. doi:10.2340/actadv.v104.26002
- Roberts EE, Nowsheen S, Davis MDP, et al. Treatment of acne with spironolactone: a retrospective review of 395 adult patients at Mayo Clinic, 2007-2017. J Eur Acad Dermatol Venereol. 2020;34:2106-2110. doi:10.1111/jdv.16302
- Garg V, Choi JK, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355. doi:10.1016/j.jaad.2020.12.071
- Barbieri JS, Choi JK, Mitra N, et al. Frequency of treatment switching for spironolactone compared to oral tetracycline-class antibiotics for women with acne: a retrospective cohort study 2010-2016. J Drugs Dermatol. 2018;17:632-638.
- Horissian M, Maczuga S, Barbieri JS, et al. Trends in the prescribing pattern of spironolactone for acne and hidradenitis suppurativa in adolescents. J Am Acad Dermatol. 2022;87:684-686. doi:10.1016/j.jaad.2021.12.005
- Roberts EE, Nowsheen S, Davis DMR, et al. Use of spironolactone to treat acne in adolescent females. Pediatr Dermatol. 2021;38:72-76. doi:10.1111/pde.14391
- Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow-up study. J Cutan Med Surg. 2002;6:541-545. doi:10.1007/s10227-001-0152-4
- Hecker A, Hasan SH, Neumann F. Disturbances in sexual differentiation of rat foetuses following spironolactone treatment. Acta Endocrinol (Copenh). 1980;95:540-545. doi:10.1530/acta.0.0950540
- Jaussan V, Lemarchand-Béraud T, Gómez F. Modifications of the gonadal function in the adult rat after fetal exposure to spironolactone. Biol Reprod. 1985;32:1051-1061. doi:10.1095 /biolreprod32.5.1051
- Hill RC, Wang Y, Shaikh B, et al. Spironolactone treatment for dermatologic indications is not associated with hypotension in a single-center retrospective study. J Am Acad Dermatol. 2024;90: 1245-1247. doi:10.1016/j.jaad.2024.01.057
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. ,em>JAMA Dermatol. 2015;151:941-944. doi:10.1001 /jamadermatol.2015.34
- Thiede RM, Rastogi S, Nardone B, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (Research on Adverse Drug Events and Reports) program. Int J Womens Dermatol. 2019;5:155-157. doi:10.1016/j.ijwd.2019.04.024
- Krunic A, Ciurea A, Scheman A. Efficacy and tolerance of acne treatment using both spironolactone and a combined contraceptive containing drospirenone. J Am Acad Dermatol. 2008;58:60-62. doi:10.1016/j.jaad.2007.09.024
- Lai J, Zaenglein AL, Barbieri JS. Timing of potassium monitoring in females treated for acne with spironolactone is not optimal: a retrospective cohort study. J Am Acad Dermatol. 2024;91:982-984. doi:10.1016/j.jaad.2024.07.1446
- Garate D, Thang CJ, Golovko G, et al. A matched cohort study evaluating whether spironolactone or tetracycline-class antibiotic use among female acne patients is associated with breast cancer development risk. Arch Dermatol Res. 2024;316:196. doi:10.1007 /s00403-024-02936-y
- Bommareddy K, Hamade H, Lopez-Olivo MA, et al. Association of spironolactone use with risk of cancer: a systematic review and meta-analysis. JAMA Dermatol. 2022;158:275-282. doi:10.1001 /jamadermatol.2021.5866
Spironolactone is an aldosterone antagonist that first was used as a potassium-sparing diuretic to treat heart failure and hypertension. It also possesses antiandrogenic mechanisms including competitively inhibiting androgen receptors, increasing steroid hormone–binding globulin production, and decreasing 5α-reductase activity.1 These properties have been leveraged in off-label use for dermatologic conditions including acne, hidradenitis suppurativa, androgenic alopecia, and hirsutism.1,2 Despite being used off-label to treat acne for more than 40 years, spironolactone has not received US Food and Drug Administration approval for this indication.3 Herein, we review the current evidence for use of spironolactone in acne management.
Spironolactone Efficacy
Spironolactone is efficacious for facial and truncal acne in adult females; it cannot be used in males given its anti-androgenic effects.4,5 In 2 large studies, spironolactone completely or partially cleared facial acne in 75.5% to 85.1% of patients.4,5 In the first study, which included 395 patients on a median dose of 100 mg/d (range, 25-200 mg/d), clearance of comedonal, papulopustular, and nodulocystic acne was observed.4 The second study included 403 patients, most of whom started on spironolactone at 100 mg/d (range, 25-200 mg/d). In addition to facial clearance, patients in this study demonstrated similar rates of partial or complete clearance of acne on the chest (84.0%) and back (80.2%) assessed via a comprehensive acne severity scale.5 In both studies, doses of 100 mg/d or higher were most effective, and the median time to initial acne improvement was 3 months, with peak effects occurring after 4 to 6 months of treatment.4,5 Most patients were using spironolactone monotherapy or spironolactone in combination with topical therapies; however, a minority used it concurrently with oral antibiotics and/or combined oral contraceptives.
Spironolactone has demonstrated comparable efficacy to tetracycline antibiotics. A study comparing the rate of switching to another systemic therapy within 1 year of treatment initiation identified similar rates in patients started on spironolactone (n=962) and those started on tetracyclines (n=4236)(14.4% vs 13.4%, respectively). As switching may indicate treatment failure due to insufficient efficacy, adverse effects, or other causes, these findings may suggest similar effectiveness for spironolactone and tetracyclines.6 These treatments also were compared in a randomized controlled trial of 133 patients receiving topical benzoyl peroxide 5% for 6 months and either spironolactone 150 mg/d for 6 months or doxycycline 100 mg/d for 3 months followed by oral placebo for 3 months. At 4 months, spironolactone performed better than doxycycline as assessed using the Adult Female Acne Scoring Tool.3 Although doxycycline was stopped after 3 months and only topical therapy was continued, this finding is notable because guidelines from the American Academy of Dermatology recommend limiting tetracycline use to 3 to 4 months, whereas spironolactone may be continued for prolonged durations.1,4
While most studies have evaluated the efficacy of spironolactone in adult females, it is increasingly being prescribed in adolescents.7 In a study that included 80 females aged 14 to 20 years, 80% (64/80) experienced acne improvement on a median dose of 100 mg/d.8 Additionally, in the study evaluating treatment switching rates, more than 80% of 1139 adolescents who were started on spironolactone were not switched to a different systemic therapy within the first year of treatment, demonstrating the efficacy of spironolactone in this demographic.6 However, treatment switching was more common among adolescents started on spironolactone compared with those who started on tetracyclines. As noted for adults, the treatment switching rates were the same for spironolactone and tetracycline users; the difference in adolescents may be due to lower influence of hormonal factors or higher therapeutic expectations in this population.6
Spironolactone Safety
Spironolactone is well tolerated at doses of 25 to 200 mg/d for acne management. Common adverse effects include diuresis (29% [26/90]), menstrual irregularities (22% [20/90]), fatigue (17% [15/90]), headache (14% [13/90]), and dizziness (12% [11/90]), but they infrequently lead to treatment discontinuation.4,9 Rates of adverse effects are lower in adolescents compared to adults, although the effects of spironolactone on early endocrine development in adolescents are unknown.7 Spironolactone should not be used during pregnancy, and concurrent contraception use is advised because spironolactone has caused feminization of male fetuses in animal studies.1,10-11
While concerns about potentially severe adverse effects including hypotension, hyperkalemia, and tumorigenicity have been raised, their occurrence in the literature is rare.5,12-18 In a study evaluating hypotension in 2084 patients taking spironolactone 50 to 200 mg/day for acne, hair loss, and/or hirsutism, 3.1% experienced absolute hypotension, and only 0.26% required dose reduction or discontinuation.12 Another study of 403 patients taking spironolactone for acne reported a statistically significant but clinically insignificant mean reduction in systolic blood pressure of 3.5 mm Hg.5 While clinically relevant hypotension is unlikely to occur, some authors still recommend measuring baseline blood pressure before spironolactone initiation.12
Many large studies have demonstrated that hyperkalemia with spironolactone use is rare in young healthy women.13-15 In one study of patients aged 18 to 45 years treated with spironolactone for acne, only 0.72% of 1802 serum potassium measurements fell within the range of mild hyperkalemia.13 Another study found a significantly greater incidence of hyperkalemia in healthy women aged 46 to 65 years compared with women younger than 45 years (16.7% vs <1%; P=.0245).14 Additionally, among 27 patients taking spironolactone and oral contraceptives containing drospirenone (a spironolactone analog), none had elevated potassium levels.15 Given these findings, American Academy of Dermatology guidelines suggest that monitoring potassium in young healthy women has low utility but should be considered in those with risk factors including older age; renal and cardiovascular disease; and concurrent medications that interfere with renal, adrenal, and hepatic function.1 If performed, monitoring should be done within the first few weeks of initiating spironolactone for early detection of hyperkalemia.16
Spironolactone has a US Food and Drug Administration warning for tumorigenicity based on studies in rats that were given up to 150 times the amount for human therapeutic doses and subsequently developed thyroid, hepatic, testicular, and breast adenomas.1 However, several large studies in humans have not found an association between spironolactone and breast cancer (BC) development.1,17,18 Furthermore, a large retrospective study found no increased risk for recurrence in BC survivors treated with spironolactone.2 Most carcinogenicity studies include older women, which may limit generalizability of the findings to younger women, who comprise the majority of patients being treated for acne. Recently, however, a retrospective study evaluating healthy females aged 9 to 40 years with acne identified no significant increased risk for BC in patients treated with spironolactone.17 When compared to tetracyclines, there was a slightly decreased BC risk with spironolactone, providing further support for the latter’s safety. Finally, a large systematic review identified no association between spironolactone and ovarian, bladder, kidney, gastric, or esophageal cancers.18
Final Thoughts
Over the past several years, an ever-expanding body of literature supporting the efficacy and safety of spironolactone has emerged. While spironolactone has been used off label for decades to treat acne in healthy adult females, there are now strong data to support its efficacy in adolescent females. Notably, spironolactone consistently demonstrates similar effectiveness to first-line tetracycline antibiotics. Additionally, data suggest that spironolactone is safe in patients with a history of BC. Overall, spironolactone is a safe, comparable, and promising alternative to antibiotics for acne management in adult and adolescent females.
Spironolactone is an aldosterone antagonist that first was used as a potassium-sparing diuretic to treat heart failure and hypertension. It also possesses antiandrogenic mechanisms including competitively inhibiting androgen receptors, increasing steroid hormone–binding globulin production, and decreasing 5α-reductase activity.1 These properties have been leveraged in off-label use for dermatologic conditions including acne, hidradenitis suppurativa, androgenic alopecia, and hirsutism.1,2 Despite being used off-label to treat acne for more than 40 years, spironolactone has not received US Food and Drug Administration approval for this indication.3 Herein, we review the current evidence for use of spironolactone in acne management.
Spironolactone Efficacy
Spironolactone is efficacious for facial and truncal acne in adult females; it cannot be used in males given its anti-androgenic effects.4,5 In 2 large studies, spironolactone completely or partially cleared facial acne in 75.5% to 85.1% of patients.4,5 In the first study, which included 395 patients on a median dose of 100 mg/d (range, 25-200 mg/d), clearance of comedonal, papulopustular, and nodulocystic acne was observed.4 The second study included 403 patients, most of whom started on spironolactone at 100 mg/d (range, 25-200 mg/d). In addition to facial clearance, patients in this study demonstrated similar rates of partial or complete clearance of acne on the chest (84.0%) and back (80.2%) assessed via a comprehensive acne severity scale.5 In both studies, doses of 100 mg/d or higher were most effective, and the median time to initial acne improvement was 3 months, with peak effects occurring after 4 to 6 months of treatment.4,5 Most patients were using spironolactone monotherapy or spironolactone in combination with topical therapies; however, a minority used it concurrently with oral antibiotics and/or combined oral contraceptives.
Spironolactone has demonstrated comparable efficacy to tetracycline antibiotics. A study comparing the rate of switching to another systemic therapy within 1 year of treatment initiation identified similar rates in patients started on spironolactone (n=962) and those started on tetracyclines (n=4236)(14.4% vs 13.4%, respectively). As switching may indicate treatment failure due to insufficient efficacy, adverse effects, or other causes, these findings may suggest similar effectiveness for spironolactone and tetracyclines.6 These treatments also were compared in a randomized controlled trial of 133 patients receiving topical benzoyl peroxide 5% for 6 months and either spironolactone 150 mg/d for 6 months or doxycycline 100 mg/d for 3 months followed by oral placebo for 3 months. At 4 months, spironolactone performed better than doxycycline as assessed using the Adult Female Acne Scoring Tool.3 Although doxycycline was stopped after 3 months and only topical therapy was continued, this finding is notable because guidelines from the American Academy of Dermatology recommend limiting tetracycline use to 3 to 4 months, whereas spironolactone may be continued for prolonged durations.1,4
While most studies have evaluated the efficacy of spironolactone in adult females, it is increasingly being prescribed in adolescents.7 In a study that included 80 females aged 14 to 20 years, 80% (64/80) experienced acne improvement on a median dose of 100 mg/d.8 Additionally, in the study evaluating treatment switching rates, more than 80% of 1139 adolescents who were started on spironolactone were not switched to a different systemic therapy within the first year of treatment, demonstrating the efficacy of spironolactone in this demographic.6 However, treatment switching was more common among adolescents started on spironolactone compared with those who started on tetracyclines. As noted for adults, the treatment switching rates were the same for spironolactone and tetracycline users; the difference in adolescents may be due to lower influence of hormonal factors or higher therapeutic expectations in this population.6
Spironolactone Safety
Spironolactone is well tolerated at doses of 25 to 200 mg/d for acne management. Common adverse effects include diuresis (29% [26/90]), menstrual irregularities (22% [20/90]), fatigue (17% [15/90]), headache (14% [13/90]), and dizziness (12% [11/90]), but they infrequently lead to treatment discontinuation.4,9 Rates of adverse effects are lower in adolescents compared to adults, although the effects of spironolactone on early endocrine development in adolescents are unknown.7 Spironolactone should not be used during pregnancy, and concurrent contraception use is advised because spironolactone has caused feminization of male fetuses in animal studies.1,10-11
While concerns about potentially severe adverse effects including hypotension, hyperkalemia, and tumorigenicity have been raised, their occurrence in the literature is rare.5,12-18 In a study evaluating hypotension in 2084 patients taking spironolactone 50 to 200 mg/day for acne, hair loss, and/or hirsutism, 3.1% experienced absolute hypotension, and only 0.26% required dose reduction or discontinuation.12 Another study of 403 patients taking spironolactone for acne reported a statistically significant but clinically insignificant mean reduction in systolic blood pressure of 3.5 mm Hg.5 While clinically relevant hypotension is unlikely to occur, some authors still recommend measuring baseline blood pressure before spironolactone initiation.12
Many large studies have demonstrated that hyperkalemia with spironolactone use is rare in young healthy women.13-15 In one study of patients aged 18 to 45 years treated with spironolactone for acne, only 0.72% of 1802 serum potassium measurements fell within the range of mild hyperkalemia.13 Another study found a significantly greater incidence of hyperkalemia in healthy women aged 46 to 65 years compared with women younger than 45 years (16.7% vs <1%; P=.0245).14 Additionally, among 27 patients taking spironolactone and oral contraceptives containing drospirenone (a spironolactone analog), none had elevated potassium levels.15 Given these findings, American Academy of Dermatology guidelines suggest that monitoring potassium in young healthy women has low utility but should be considered in those with risk factors including older age; renal and cardiovascular disease; and concurrent medications that interfere with renal, adrenal, and hepatic function.1 If performed, monitoring should be done within the first few weeks of initiating spironolactone for early detection of hyperkalemia.16
Spironolactone has a US Food and Drug Administration warning for tumorigenicity based on studies in rats that were given up to 150 times the amount for human therapeutic doses and subsequently developed thyroid, hepatic, testicular, and breast adenomas.1 However, several large studies in humans have not found an association between spironolactone and breast cancer (BC) development.1,17,18 Furthermore, a large retrospective study found no increased risk for recurrence in BC survivors treated with spironolactone.2 Most carcinogenicity studies include older women, which may limit generalizability of the findings to younger women, who comprise the majority of patients being treated for acne. Recently, however, a retrospective study evaluating healthy females aged 9 to 40 years with acne identified no significant increased risk for BC in patients treated with spironolactone.17 When compared to tetracyclines, there was a slightly decreased BC risk with spironolactone, providing further support for the latter’s safety. Finally, a large systematic review identified no association between spironolactone and ovarian, bladder, kidney, gastric, or esophageal cancers.18
Final Thoughts
Over the past several years, an ever-expanding body of literature supporting the efficacy and safety of spironolactone has emerged. While spironolactone has been used off label for decades to treat acne in healthy adult females, there are now strong data to support its efficacy in adolescent females. Notably, spironolactone consistently demonstrates similar effectiveness to first-line tetracycline antibiotics. Additionally, data suggest that spironolactone is safe in patients with a history of BC. Overall, spironolactone is a safe, comparable, and promising alternative to antibiotics for acne management in adult and adolescent females.
- Reynolds RV, Yeung H, Cheng CE, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2024;90:1006. e1-1006.e30. doi:10.1016/j.jaad.2023.12.017
- Wei C, Bovonratwet P, Gu A, et al. Spironolactone use does not increase the risk of female breast cancer recurrence: a retrospective analysis. J Am Acad Dermatol. 2020;83:1021-1027. doi:10.1016/j.jaad.2020.05.081
- Dréno B, Nguyen JM, Hainaut E, et al. Efficacy of spironolactone compared with doxycycline in moderate acne in adult females: results of the multicentre, controlled, randomized, double-blind prospective and parallel Female Acne Spironolactone vs doxyCycline Efficacy (FASCE) study. Acta Derm Venereol. 2024;104:adv26002. doi:10.2340/actadv.v104.26002
- Roberts EE, Nowsheen S, Davis MDP, et al. Treatment of acne with spironolactone: a retrospective review of 395 adult patients at Mayo Clinic, 2007-2017. J Eur Acad Dermatol Venereol. 2020;34:2106-2110. doi:10.1111/jdv.16302
- Garg V, Choi JK, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355. doi:10.1016/j.jaad.2020.12.071
- Barbieri JS, Choi JK, Mitra N, et al. Frequency of treatment switching for spironolactone compared to oral tetracycline-class antibiotics for women with acne: a retrospective cohort study 2010-2016. J Drugs Dermatol. 2018;17:632-638.
- Horissian M, Maczuga S, Barbieri JS, et al. Trends in the prescribing pattern of spironolactone for acne and hidradenitis suppurativa in adolescents. J Am Acad Dermatol. 2022;87:684-686. doi:10.1016/j.jaad.2021.12.005
- Roberts EE, Nowsheen S, Davis DMR, et al. Use of spironolactone to treat acne in adolescent females. Pediatr Dermatol. 2021;38:72-76. doi:10.1111/pde.14391
- Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow-up study. J Cutan Med Surg. 2002;6:541-545. doi:10.1007/s10227-001-0152-4
- Hecker A, Hasan SH, Neumann F. Disturbances in sexual differentiation of rat foetuses following spironolactone treatment. Acta Endocrinol (Copenh). 1980;95:540-545. doi:10.1530/acta.0.0950540
- Jaussan V, Lemarchand-Béraud T, Gómez F. Modifications of the gonadal function in the adult rat after fetal exposure to spironolactone. Biol Reprod. 1985;32:1051-1061. doi:10.1095 /biolreprod32.5.1051
- Hill RC, Wang Y, Shaikh B, et al. Spironolactone treatment for dermatologic indications is not associated with hypotension in a single-center retrospective study. J Am Acad Dermatol. 2024;90: 1245-1247. doi:10.1016/j.jaad.2024.01.057
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. ,em>JAMA Dermatol. 2015;151:941-944. doi:10.1001 /jamadermatol.2015.34
- Thiede RM, Rastogi S, Nardone B, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (Research on Adverse Drug Events and Reports) program. Int J Womens Dermatol. 2019;5:155-157. doi:10.1016/j.ijwd.2019.04.024
- Krunic A, Ciurea A, Scheman A. Efficacy and tolerance of acne treatment using both spironolactone and a combined contraceptive containing drospirenone. J Am Acad Dermatol. 2008;58:60-62. doi:10.1016/j.jaad.2007.09.024
- Lai J, Zaenglein AL, Barbieri JS. Timing of potassium monitoring in females treated for acne with spironolactone is not optimal: a retrospective cohort study. J Am Acad Dermatol. 2024;91:982-984. doi:10.1016/j.jaad.2024.07.1446
- Garate D, Thang CJ, Golovko G, et al. A matched cohort study evaluating whether spironolactone or tetracycline-class antibiotic use among female acne patients is associated with breast cancer development risk. Arch Dermatol Res. 2024;316:196. doi:10.1007 /s00403-024-02936-y
- Bommareddy K, Hamade H, Lopez-Olivo MA, et al. Association of spironolactone use with risk of cancer: a systematic review and meta-analysis. JAMA Dermatol. 2022;158:275-282. doi:10.1001 /jamadermatol.2021.5866
- Reynolds RV, Yeung H, Cheng CE, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2024;90:1006. e1-1006.e30. doi:10.1016/j.jaad.2023.12.017
- Wei C, Bovonratwet P, Gu A, et al. Spironolactone use does not increase the risk of female breast cancer recurrence: a retrospective analysis. J Am Acad Dermatol. 2020;83:1021-1027. doi:10.1016/j.jaad.2020.05.081
- Dréno B, Nguyen JM, Hainaut E, et al. Efficacy of spironolactone compared with doxycycline in moderate acne in adult females: results of the multicentre, controlled, randomized, double-blind prospective and parallel Female Acne Spironolactone vs doxyCycline Efficacy (FASCE) study. Acta Derm Venereol. 2024;104:adv26002. doi:10.2340/actadv.v104.26002
- Roberts EE, Nowsheen S, Davis MDP, et al. Treatment of acne with spironolactone: a retrospective review of 395 adult patients at Mayo Clinic, 2007-2017. J Eur Acad Dermatol Venereol. 2020;34:2106-2110. doi:10.1111/jdv.16302
- Garg V, Choi JK, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355. doi:10.1016/j.jaad.2020.12.071
- Barbieri JS, Choi JK, Mitra N, et al. Frequency of treatment switching for spironolactone compared to oral tetracycline-class antibiotics for women with acne: a retrospective cohort study 2010-2016. J Drugs Dermatol. 2018;17:632-638.
- Horissian M, Maczuga S, Barbieri JS, et al. Trends in the prescribing pattern of spironolactone for acne and hidradenitis suppurativa in adolescents. J Am Acad Dermatol. 2022;87:684-686. doi:10.1016/j.jaad.2021.12.005
- Roberts EE, Nowsheen S, Davis DMR, et al. Use of spironolactone to treat acne in adolescent females. Pediatr Dermatol. 2021;38:72-76. doi:10.1111/pde.14391
- Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow-up study. J Cutan Med Surg. 2002;6:541-545. doi:10.1007/s10227-001-0152-4
- Hecker A, Hasan SH, Neumann F. Disturbances in sexual differentiation of rat foetuses following spironolactone treatment. Acta Endocrinol (Copenh). 1980;95:540-545. doi:10.1530/acta.0.0950540
- Jaussan V, Lemarchand-Béraud T, Gómez F. Modifications of the gonadal function in the adult rat after fetal exposure to spironolactone. Biol Reprod. 1985;32:1051-1061. doi:10.1095 /biolreprod32.5.1051
- Hill RC, Wang Y, Shaikh B, et al. Spironolactone treatment for dermatologic indications is not associated with hypotension in a single-center retrospective study. J Am Acad Dermatol. 2024;90: 1245-1247. doi:10.1016/j.jaad.2024.01.057
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. ,em>JAMA Dermatol. 2015;151:941-944. doi:10.1001 /jamadermatol.2015.34
- Thiede RM, Rastogi S, Nardone B, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (Research on Adverse Drug Events and Reports) program. Int J Womens Dermatol. 2019;5:155-157. doi:10.1016/j.ijwd.2019.04.024
- Krunic A, Ciurea A, Scheman A. Efficacy and tolerance of acne treatment using both spironolactone and a combined contraceptive containing drospirenone. J Am Acad Dermatol. 2008;58:60-62. doi:10.1016/j.jaad.2007.09.024
- Lai J, Zaenglein AL, Barbieri JS. Timing of potassium monitoring in females treated for acne with spironolactone is not optimal: a retrospective cohort study. J Am Acad Dermatol. 2024;91:982-984. doi:10.1016/j.jaad.2024.07.1446
- Garate D, Thang CJ, Golovko G, et al. A matched cohort study evaluating whether spironolactone or tetracycline-class antibiotic use among female acne patients is associated with breast cancer development risk. Arch Dermatol Res. 2024;316:196. doi:10.1007 /s00403-024-02936-y
- Bommareddy K, Hamade H, Lopez-Olivo MA, et al. Association of spironolactone use with risk of cancer: a systematic review and meta-analysis. JAMA Dermatol. 2022;158:275-282. doi:10.1001 /jamadermatol.2021.5866
Efficacy and Safety of Spironolactone in Acne Management
Efficacy and Safety of Spironolactone in Acne Management
VA is a Leader in Mental Health and Social Service Research and Operations
VA is a Leader in Mental Health and Social Service Research and Operations
The US Department of Veterans Affairs (VA) mission is defined by President Abraham Lincoln’s promise “to care for him who shall have borne the battle, and for his widow, and his orphan.” Critically, the biopsychosocial needs of veterans differ from the needs of civilians due to the nature of military service.1 Veterans commonly experience traumatic brain injury (TBI) due to combat- or training-related injuries.2 Psychologically, veterans are disproportionately likely to be diagnosed with mental health conditions, such as posttraumatic stress disorder (PTSD), often linked to military exposures.3 Spiritually, veterans frequently express moral injury after living through circumstances when they perpetrate, fail to prevent, or witness events that contradict moral beliefs/ expectations.4 Veterans also have significant social challenges, including high rates of homelessness. 5 A critical strength of the VA mission is its awareness of these complex sequelae and its ability to provide well-informed treatment and social services to meet veterans’ unique needs.
Foundational to a well-informed health care system is a robust research and operational quality improvement infrastructure. The VA Office of Research and Development (ORD) has worked tirelessly to understand and address the unique, idiographic needs of veterans. In 2024 the ORD had a budget of $2.4 billion, excluding quality improvement initiatives enhancing VA operations.6
The integrated VA health care system is a major strength for providing state-of-the-science to inform veterans’ treatment and social service needs. The VA features medical centers and clinics capable of synergistically leveraging extant infrastructure to facilitate collaborations and centralized procedures across sites. The VA also has dedicated research centers, such as the National Center for PTSD, Centers of Excellence, Centers of Innovation, and Mental Illness, Research, Education and Clinical Centers that focus on PTSD, suicide prevention, TBI, and other high-priority areas. These centers recruit, train, and invest in experts dedicated to improving veterans’ lives. The VA Corporate Data Warehouse provides a national, system-wide repository for patient-level data, allowing for advanced analysis of large datasets.7
This special issue is a showcase of the strengths of VA mental health and social service research, aligning with the current strategic priorities of VA research. Topics focus on the unique needs of veterans, including sequelae (eg, PTSD, homelessness, moral injury), with particular attention to veterans. Manuscripts highlight the strengths of collaborations, including those between specialized research centers and national VA operational partners. Analyses highlight the VA research approach, leveraging data and perspectives from inside and outside the VA, and studying new and established approaches to care. This issue highlights the distinct advantages that VA research provides: experts with the tools, experience, and dedication to addressing the unique needs of veterans. Given the passion for veteran care among VA researchers, including those featured in this issue, we strongly believe the VA will continue to be a leader in this research.
- Oster C, Morello A, Venning A, Redpath P, Lawn S. The health and wellbeing needs of veterans: a rapid review. BMC Psychiatry. 2017;17(1):414. doi:10.1186/s12888-017-1547-0
- Cypel YS, Vogt D, Maguen S, et al. Physical health of Post- 9/11 U.S. military veterans in the context of Healthy People 2020 targeted topic areas: results from the Comparative Health Assessment Interview Research Study. Prev Med Rep. 2023;32:102122. doi:10.1016/j.pmedr.2023.102122
- Lehavot K, Katon JG, Chen JA, Fortney JC, Simpson TL. Post-traumatic stress disorder by gender and veteran Status. Am J Prev Med. 2018;54(1):e1-e9. doi:10.1016/j.amepre.2017.09.008
- Griffin BJ, Purcell N, Burkman K, et al. Moral injury: an integrative review. J Trauma Stress. 2019;32(3):350-362. doi:10.1002/jts.22362
- Tsai J, Pietrzak RH, Szymkowiak D. The problem of veteran homelessness: an update for the new decade. Am J Prev Med. 2021;60(6):774-780. doi:10.1016/j.amepre.2020.12.012
- US Department of Veterans Affairs, Office of Research and Development. About the office of research & development. Updated January 22, 2025. Accessed March 18, 2025. https://www.research.va.gov/about/default.cfm
- Fihn SD, Francis J, Clancy C, et al. Insights from advanced analytics at the Veterans Health Administration. Health Aff (Millwood). 2014;33(7):1203-1211. doi:10.1377/hlthaff.2014.0054
The US Department of Veterans Affairs (VA) mission is defined by President Abraham Lincoln’s promise “to care for him who shall have borne the battle, and for his widow, and his orphan.” Critically, the biopsychosocial needs of veterans differ from the needs of civilians due to the nature of military service.1 Veterans commonly experience traumatic brain injury (TBI) due to combat- or training-related injuries.2 Psychologically, veterans are disproportionately likely to be diagnosed with mental health conditions, such as posttraumatic stress disorder (PTSD), often linked to military exposures.3 Spiritually, veterans frequently express moral injury after living through circumstances when they perpetrate, fail to prevent, or witness events that contradict moral beliefs/ expectations.4 Veterans also have significant social challenges, including high rates of homelessness. 5 A critical strength of the VA mission is its awareness of these complex sequelae and its ability to provide well-informed treatment and social services to meet veterans’ unique needs.
Foundational to a well-informed health care system is a robust research and operational quality improvement infrastructure. The VA Office of Research and Development (ORD) has worked tirelessly to understand and address the unique, idiographic needs of veterans. In 2024 the ORD had a budget of $2.4 billion, excluding quality improvement initiatives enhancing VA operations.6
The integrated VA health care system is a major strength for providing state-of-the-science to inform veterans’ treatment and social service needs. The VA features medical centers and clinics capable of synergistically leveraging extant infrastructure to facilitate collaborations and centralized procedures across sites. The VA also has dedicated research centers, such as the National Center for PTSD, Centers of Excellence, Centers of Innovation, and Mental Illness, Research, Education and Clinical Centers that focus on PTSD, suicide prevention, TBI, and other high-priority areas. These centers recruit, train, and invest in experts dedicated to improving veterans’ lives. The VA Corporate Data Warehouse provides a national, system-wide repository for patient-level data, allowing for advanced analysis of large datasets.7
This special issue is a showcase of the strengths of VA mental health and social service research, aligning with the current strategic priorities of VA research. Topics focus on the unique needs of veterans, including sequelae (eg, PTSD, homelessness, moral injury), with particular attention to veterans. Manuscripts highlight the strengths of collaborations, including those between specialized research centers and national VA operational partners. Analyses highlight the VA research approach, leveraging data and perspectives from inside and outside the VA, and studying new and established approaches to care. This issue highlights the distinct advantages that VA research provides: experts with the tools, experience, and dedication to addressing the unique needs of veterans. Given the passion for veteran care among VA researchers, including those featured in this issue, we strongly believe the VA will continue to be a leader in this research.
The US Department of Veterans Affairs (VA) mission is defined by President Abraham Lincoln’s promise “to care for him who shall have borne the battle, and for his widow, and his orphan.” Critically, the biopsychosocial needs of veterans differ from the needs of civilians due to the nature of military service.1 Veterans commonly experience traumatic brain injury (TBI) due to combat- or training-related injuries.2 Psychologically, veterans are disproportionately likely to be diagnosed with mental health conditions, such as posttraumatic stress disorder (PTSD), often linked to military exposures.3 Spiritually, veterans frequently express moral injury after living through circumstances when they perpetrate, fail to prevent, or witness events that contradict moral beliefs/ expectations.4 Veterans also have significant social challenges, including high rates of homelessness. 5 A critical strength of the VA mission is its awareness of these complex sequelae and its ability to provide well-informed treatment and social services to meet veterans’ unique needs.
Foundational to a well-informed health care system is a robust research and operational quality improvement infrastructure. The VA Office of Research and Development (ORD) has worked tirelessly to understand and address the unique, idiographic needs of veterans. In 2024 the ORD had a budget of $2.4 billion, excluding quality improvement initiatives enhancing VA operations.6
The integrated VA health care system is a major strength for providing state-of-the-science to inform veterans’ treatment and social service needs. The VA features medical centers and clinics capable of synergistically leveraging extant infrastructure to facilitate collaborations and centralized procedures across sites. The VA also has dedicated research centers, such as the National Center for PTSD, Centers of Excellence, Centers of Innovation, and Mental Illness, Research, Education and Clinical Centers that focus on PTSD, suicide prevention, TBI, and other high-priority areas. These centers recruit, train, and invest in experts dedicated to improving veterans’ lives. The VA Corporate Data Warehouse provides a national, system-wide repository for patient-level data, allowing for advanced analysis of large datasets.7
This special issue is a showcase of the strengths of VA mental health and social service research, aligning with the current strategic priorities of VA research. Topics focus on the unique needs of veterans, including sequelae (eg, PTSD, homelessness, moral injury), with particular attention to veterans. Manuscripts highlight the strengths of collaborations, including those between specialized research centers and national VA operational partners. Analyses highlight the VA research approach, leveraging data and perspectives from inside and outside the VA, and studying new and established approaches to care. This issue highlights the distinct advantages that VA research provides: experts with the tools, experience, and dedication to addressing the unique needs of veterans. Given the passion for veteran care among VA researchers, including those featured in this issue, we strongly believe the VA will continue to be a leader in this research.
- Oster C, Morello A, Venning A, Redpath P, Lawn S. The health and wellbeing needs of veterans: a rapid review. BMC Psychiatry. 2017;17(1):414. doi:10.1186/s12888-017-1547-0
- Cypel YS, Vogt D, Maguen S, et al. Physical health of Post- 9/11 U.S. military veterans in the context of Healthy People 2020 targeted topic areas: results from the Comparative Health Assessment Interview Research Study. Prev Med Rep. 2023;32:102122. doi:10.1016/j.pmedr.2023.102122
- Lehavot K, Katon JG, Chen JA, Fortney JC, Simpson TL. Post-traumatic stress disorder by gender and veteran Status. Am J Prev Med. 2018;54(1):e1-e9. doi:10.1016/j.amepre.2017.09.008
- Griffin BJ, Purcell N, Burkman K, et al. Moral injury: an integrative review. J Trauma Stress. 2019;32(3):350-362. doi:10.1002/jts.22362
- Tsai J, Pietrzak RH, Szymkowiak D. The problem of veteran homelessness: an update for the new decade. Am J Prev Med. 2021;60(6):774-780. doi:10.1016/j.amepre.2020.12.012
- US Department of Veterans Affairs, Office of Research and Development. About the office of research & development. Updated January 22, 2025. Accessed March 18, 2025. https://www.research.va.gov/about/default.cfm
- Fihn SD, Francis J, Clancy C, et al. Insights from advanced analytics at the Veterans Health Administration. Health Aff (Millwood). 2014;33(7):1203-1211. doi:10.1377/hlthaff.2014.0054
- Oster C, Morello A, Venning A, Redpath P, Lawn S. The health and wellbeing needs of veterans: a rapid review. BMC Psychiatry. 2017;17(1):414. doi:10.1186/s12888-017-1547-0
- Cypel YS, Vogt D, Maguen S, et al. Physical health of Post- 9/11 U.S. military veterans in the context of Healthy People 2020 targeted topic areas: results from the Comparative Health Assessment Interview Research Study. Prev Med Rep. 2023;32:102122. doi:10.1016/j.pmedr.2023.102122
- Lehavot K, Katon JG, Chen JA, Fortney JC, Simpson TL. Post-traumatic stress disorder by gender and veteran Status. Am J Prev Med. 2018;54(1):e1-e9. doi:10.1016/j.amepre.2017.09.008
- Griffin BJ, Purcell N, Burkman K, et al. Moral injury: an integrative review. J Trauma Stress. 2019;32(3):350-362. doi:10.1002/jts.22362
- Tsai J, Pietrzak RH, Szymkowiak D. The problem of veteran homelessness: an update for the new decade. Am J Prev Med. 2021;60(6):774-780. doi:10.1016/j.amepre.2020.12.012
- US Department of Veterans Affairs, Office of Research and Development. About the office of research & development. Updated January 22, 2025. Accessed March 18, 2025. https://www.research.va.gov/about/default.cfm
- Fihn SD, Francis J, Clancy C, et al. Insights from advanced analytics at the Veterans Health Administration. Health Aff (Millwood). 2014;33(7):1203-1211. doi:10.1377/hlthaff.2014.0054
VA is a Leader in Mental Health and Social Service Research and Operations
VA is a Leader in Mental Health and Social Service Research and Operations
A Threat to Scientific Progress
The United States has long been recognized as a global leader in biomedical research and scientific discovery, with federal research and development (R&D) funding serving as the bedrock of national innovation. Substantial federal investment in biomedical research has stemmed from a recognition of its importance in fueling critical discoveries that improve patient care and the health of our communities.
In the United States, academic institutions play a key role in conducting research in the national interest and collaborating with industry, with most of the federal research funding distributed by the National Institutes of Health, National Science Foundation, and other agencies awarded to university-based academic investigators. In a 2014 report, the National Academies of Sciences, Engineering and Medicine identified three pillars of a highly productive research system: a talented and interconnected workforce, adequate and dependable resources, and world-class basic research in all major areas of science.
A series of recent, short-sighted federal policy decisions threaten the future of scientific discovery by eroding these pillars. Decisions to freeze previously awarded federal grant funding, delay grant review panels, fire federal scientists, and propose crippling cuts to indirect cost rates (among others) have sent shock waves through the research community and already have led some prominent research institutions to cut staff and divert resources away from groundbreaking research. While the acute effects of these changes are just beginning to be felt, it is the long-term effects of these decisions on future medical and scientific discovery that will be most devastating to society.
In our April issue, we highlight important research advancements in inflammatory bowel disease presented at February’s Congress of the European Crohn’s and Colitis Organisation (ECCO) in Berlin. In this month’s Member Spotlight, Abigail Meyers, MPAS, PA-C, outlines her impactful work as a member of AGA’s newly formed Nurse Practitioner and Physician Assistant Task Force and shares how her personal journey as a patient with inflammatory bowel disease allows her to be a more powerful advocate for important issues impacting other patients with this condition.
Megan A. Adams, MD, JD, MSc
Editor in Chief
The United States has long been recognized as a global leader in biomedical research and scientific discovery, with federal research and development (R&D) funding serving as the bedrock of national innovation. Substantial federal investment in biomedical research has stemmed from a recognition of its importance in fueling critical discoveries that improve patient care and the health of our communities.
In the United States, academic institutions play a key role in conducting research in the national interest and collaborating with industry, with most of the federal research funding distributed by the National Institutes of Health, National Science Foundation, and other agencies awarded to university-based academic investigators. In a 2014 report, the National Academies of Sciences, Engineering and Medicine identified three pillars of a highly productive research system: a talented and interconnected workforce, adequate and dependable resources, and world-class basic research in all major areas of science.
A series of recent, short-sighted federal policy decisions threaten the future of scientific discovery by eroding these pillars. Decisions to freeze previously awarded federal grant funding, delay grant review panels, fire federal scientists, and propose crippling cuts to indirect cost rates (among others) have sent shock waves through the research community and already have led some prominent research institutions to cut staff and divert resources away from groundbreaking research. While the acute effects of these changes are just beginning to be felt, it is the long-term effects of these decisions on future medical and scientific discovery that will be most devastating to society.
In our April issue, we highlight important research advancements in inflammatory bowel disease presented at February’s Congress of the European Crohn’s and Colitis Organisation (ECCO) in Berlin. In this month’s Member Spotlight, Abigail Meyers, MPAS, PA-C, outlines her impactful work as a member of AGA’s newly formed Nurse Practitioner and Physician Assistant Task Force and shares how her personal journey as a patient with inflammatory bowel disease allows her to be a more powerful advocate for important issues impacting other patients with this condition.
Megan A. Adams, MD, JD, MSc
Editor in Chief
The United States has long been recognized as a global leader in biomedical research and scientific discovery, with federal research and development (R&D) funding serving as the bedrock of national innovation. Substantial federal investment in biomedical research has stemmed from a recognition of its importance in fueling critical discoveries that improve patient care and the health of our communities.
In the United States, academic institutions play a key role in conducting research in the national interest and collaborating with industry, with most of the federal research funding distributed by the National Institutes of Health, National Science Foundation, and other agencies awarded to university-based academic investigators. In a 2014 report, the National Academies of Sciences, Engineering and Medicine identified three pillars of a highly productive research system: a talented and interconnected workforce, adequate and dependable resources, and world-class basic research in all major areas of science.
A series of recent, short-sighted federal policy decisions threaten the future of scientific discovery by eroding these pillars. Decisions to freeze previously awarded federal grant funding, delay grant review panels, fire federal scientists, and propose crippling cuts to indirect cost rates (among others) have sent shock waves through the research community and already have led some prominent research institutions to cut staff and divert resources away from groundbreaking research. While the acute effects of these changes are just beginning to be felt, it is the long-term effects of these decisions on future medical and scientific discovery that will be most devastating to society.
In our April issue, we highlight important research advancements in inflammatory bowel disease presented at February’s Congress of the European Crohn’s and Colitis Organisation (ECCO) in Berlin. In this month’s Member Spotlight, Abigail Meyers, MPAS, PA-C, outlines her impactful work as a member of AGA’s newly formed Nurse Practitioner and Physician Assistant Task Force and shares how her personal journey as a patient with inflammatory bowel disease allows her to be a more powerful advocate for important issues impacting other patients with this condition.
Megan A. Adams, MD, JD, MSc
Editor in Chief