User login
What to tell your patients about anti-amyloids for Alzheimer’s disease
Recorded October 13, 2023. This transcript has been edited for clarity.
Kathrin LaFaver, MD: I’ll be talking today with Dr. Meredith Wicklund, senior associate consultant and behavioral neurologist specialist at Mayo Clinic in Arizona. Welcome, Meredith.
Meredith Wicklund, MD: Thank you.
Lecanemab data
Dr. LaFaver: I’m very excited about our topic. Could you give us a brief overview of why there has been so much research interest in this topic of anti-amyloid antibodies?
Dr. Wicklund: The pathologic component of what defines something as Alzheimer’s disease is, by definition, presence of amyloid plaques and tau tangles. When it was first discovered in the 1980s that the component of the plaques was actually the amyloid protein – beta amyloid specifically – interest went right from there to developing therapies to directly target the pathology that is Alzheimer’s disease.
Dr. LaFaver: Lecanemab is the first FDA-approved disease-modifying antibody in that realm. Could you review the study data, especially as it applies to both of us in daily neurology clinic?
Dr. Wicklund: The study data from a phase 3 trial did show, for the primary outcome, that there was a 27% slowing of decline compared with individuals on placebo. It’s important to point out that this was slowing of decline. It was not stabilizing decline. It was not improving decline.
I think it’s important that we inform our patients that really, even with this therapy, there’s no prospect of stabilizing or restoring cognition or function. We do progress at a slower rate compared with individuals not on this treatment, which, given that this medication is for individuals in mild disease who have relatively preserved functional status, that can be potentially very meaningful to families.
The overall benefit was small. It essentially amounts to half a point on an 18-point scale, which is statistically significant. How much clinical meaningfulness that actually leads to is unclear. Finding clinical meaningfulness cannot be defined by a particular test. It really can only be defined on the individual level, what is meaningful to them.
Recommended tests
Dr. LaFaver: It is my understanding that, to qualify for lecanemab use, one needs to have a biomarker-supported diagnosis of Alzheimer’s disease, either via an amyloid PET scan or CSF biomarkers. What would your recommendation be for a neurologist in practice to go about these requirements?
Dr. Wicklund: Since this medication is directly targeting the amyloid pathology, and it does convey a potential risk, we want to make sure that the actual pathology is present in the individuals before we treat them and potentially expose them to risk. The best way of doing that is through either an amyloid PET scan or spinal fluid testing of beta amyloid and tau.
There are several plasma-based biomarkers in development. However, I would avoid using those currently. There are still many unknowns in terms of what exactly is the right species of tau that we should be looking at, the right mechanism of the lab test, how minority status may influence it, and how different comorbidities may influence it.
I would recommend, at this time, sticking with amyloid PET or CSF testing. Given that amyloid PET is not widely available in many community practices, generally only available at academic centers, and is quite costly, many insurances do not cover it – although Medicare has a proposal to potentially start covering it – I generally go with spinal fluid testing, which is more widely available. There are several labs across the country that can process that testing in a reliable way.
Amyloid-related imaging abnormalities
Dr. LaFaver: That’s very helpful to know. There’s been a large amount of buzz just these past couple of weeks about the blood biomarker coming up. I think, as you point out, this wasn’t the marker used in the clinical studies and there are still unknowns. Maybe it’s not quite time for clinical use, unfortunately.
We also have learned that there are significant potential risks involved. One issue that’s really been a focus is ARIA – amyloid-related imaging abnormalities. Could you speak a bit about that and requirements for monitoring?
Dr. Wicklund: ARIA essentially amounts to either vasogenic edema, microhemorrhages, or superficial siderosis that develops as a result of treatment. It relates to activation of the immune system with these passive monoclonal antibodies that’s going to occur with targeting against the plaques. In the parenchyma, it will cause edema. If you have amyloid in the walls of the blood vessels, it can cause microhemorrhages.
While the term “ARIA” implies an imaging-related abnormality, and it largely is purely an imaging finding, it’s not solely an imaging-related finding. It can cause symptoms, including very serious symptoms.
Overall, with lecanemab, the incidence of ARIA within the treatment group in the phase 3 study, combined between both ARIA-E (edema/effusion) and ARIA-H (hemorrhage), was 21.5%, with about 17% being ARIA-H and about 12.5% being ARIA-E. Of course, they can occur at the same time.
Overall, in terms of people in the clinical trials, for most it was purely an imaging-related finding. About 3% developed symptomatic ARIA. Some of those were very serious symptoms, including things like seizures and need to be hospitalized. A couple of deaths have been attributed to ARIA as well.
Patients on anticoagulation
Dr. LaFaver: Along those lines, any additional words to say for people who might be on anticoagulation or might require medications for a stroke, for example?
Dr. Wicklund: While individuals on anticoagulation were allowed in the clinical trials, the current, published appropriate-use guideline is recommending against its use, as several of the serious adverse effects, including the deaths, were for the most part attributed to anticoagulation use.
When it comes to acute stroke treatment, one must carefully consider use of tPA, as two of the three deaths were tPA associated in the clinical trials. It shouldn’t necessarily be an absolute contraindication, but it can make the clinical picture very muddy. If an individual is on lecanemab and comes to the ER with acute stroke-like symptoms, it’s more likely that they’re going to be having an ARIA side effect rather than an acute stroke.
A general recommendation would be to obtain an acute head CT with a CTA, and if there is a large vessel occlusion, proceed to thrombectomy. However, if there isn’t a large vessel occlusion, if you have the ability to get a rapid MRI with diffusion-weighted imaging to screen for acute stroke changes or tissue flair with acute edema changes suggestive of ARIA, that would be preferred before proceeding with thrombolysis. These are all relative contraindications and are going to depend on what’s available near you.
Donanemab approval pending
Dr. LaFaver: This will be an issue because the population we’re talking about is definitely at risk for stroke as well as Alzheimer’s disease. Where do you see this field going as far as amyloid antibody therapy is concerned, with another agent, donanemab, possibly getting FDA approval later this year as well?
Dr. Wicklund: We’re anticipating that donanemab will get FDA approval in the next coming months. Donanemab also targets the amyloid in the brain, although lecanemab and donanemab target different aspects of the production of the amyloid plaque. They were both shown to have roughly equal efficacy in their phase 3 clinical trials. Donanemab has the benefit of being a once-monthly infusion as opposed to twice-monthly infusions with lecanemab. It does have a slightly higher risk for ARIA compared with lecanemab.
Those are just some things to take into consideration when talking with your patients. In terms of where we’re going from here, we’re moving even earlier in terms of disease state. The lecanemab and donanemab phase 3 trials were done in individuals with mild cognitive impairment or mild dementia due to Alzheimer’s disease. They should not be used in individuals with moderate or more advanced Alzheimer’s disease.
There are ongoing, large, national, multicenter clinical trials of both lecanemab and donanemab in a preclinical state of Alzheimer’s disease. These individuals have evidence of amyloidosis, either through PET imaging or through CSF, but are clinically asymptomatic and do not yet have any signs of cognitive impairment or functional decline. We look forward to those results in the next few years. Hopefully, they’ll be able to show even greater benefit when moving into these early disease states in terms of delaying or even preventing cognitive decline.
Dr. LaFaver: That’s definitely very interesting to hear about. Where can people go for more information?
Dr. Wicklund: There’s a guideline on the use of lecanemab through the American Academy of Neurology. I encourage you to look at that. Also, look at the appropriate-use recommendations that were published this year in The Journal of Prevention of Alzheimer’s Disease.
Dr. LaFaver: Wonderful. With that being said, thank you so much for talking to me. I learned a lot. Thanks, everyone, for listening.
Dr. LaFaver is a neurologist at Saratoga Hospital Medical Group, Saratoga Springs, N.Y. She disclosed having no relevant financial relationships. Dr. Wicklund is senior associate consultant in the department of Neurology at Mayo Clinic, Phoenix, Ariz. She disclosed having no relevant financial relationships.
A version of this article appeared on Medscape.com.
Recorded October 13, 2023. This transcript has been edited for clarity.
Kathrin LaFaver, MD: I’ll be talking today with Dr. Meredith Wicklund, senior associate consultant and behavioral neurologist specialist at Mayo Clinic in Arizona. Welcome, Meredith.
Meredith Wicklund, MD: Thank you.
Lecanemab data
Dr. LaFaver: I’m very excited about our topic. Could you give us a brief overview of why there has been so much research interest in this topic of anti-amyloid antibodies?
Dr. Wicklund: The pathologic component of what defines something as Alzheimer’s disease is, by definition, presence of amyloid plaques and tau tangles. When it was first discovered in the 1980s that the component of the plaques was actually the amyloid protein – beta amyloid specifically – interest went right from there to developing therapies to directly target the pathology that is Alzheimer’s disease.
Dr. LaFaver: Lecanemab is the first FDA-approved disease-modifying antibody in that realm. Could you review the study data, especially as it applies to both of us in daily neurology clinic?
Dr. Wicklund: The study data from a phase 3 trial did show, for the primary outcome, that there was a 27% slowing of decline compared with individuals on placebo. It’s important to point out that this was slowing of decline. It was not stabilizing decline. It was not improving decline.
I think it’s important that we inform our patients that really, even with this therapy, there’s no prospect of stabilizing or restoring cognition or function. We do progress at a slower rate compared with individuals not on this treatment, which, given that this medication is for individuals in mild disease who have relatively preserved functional status, that can be potentially very meaningful to families.
The overall benefit was small. It essentially amounts to half a point on an 18-point scale, which is statistically significant. How much clinical meaningfulness that actually leads to is unclear. Finding clinical meaningfulness cannot be defined by a particular test. It really can only be defined on the individual level, what is meaningful to them.
Recommended tests
Dr. LaFaver: It is my understanding that, to qualify for lecanemab use, one needs to have a biomarker-supported diagnosis of Alzheimer’s disease, either via an amyloid PET scan or CSF biomarkers. What would your recommendation be for a neurologist in practice to go about these requirements?
Dr. Wicklund: Since this medication is directly targeting the amyloid pathology, and it does convey a potential risk, we want to make sure that the actual pathology is present in the individuals before we treat them and potentially expose them to risk. The best way of doing that is through either an amyloid PET scan or spinal fluid testing of beta amyloid and tau.
There are several plasma-based biomarkers in development. However, I would avoid using those currently. There are still many unknowns in terms of what exactly is the right species of tau that we should be looking at, the right mechanism of the lab test, how minority status may influence it, and how different comorbidities may influence it.
I would recommend, at this time, sticking with amyloid PET or CSF testing. Given that amyloid PET is not widely available in many community practices, generally only available at academic centers, and is quite costly, many insurances do not cover it – although Medicare has a proposal to potentially start covering it – I generally go with spinal fluid testing, which is more widely available. There are several labs across the country that can process that testing in a reliable way.
Amyloid-related imaging abnormalities
Dr. LaFaver: That’s very helpful to know. There’s been a large amount of buzz just these past couple of weeks about the blood biomarker coming up. I think, as you point out, this wasn’t the marker used in the clinical studies and there are still unknowns. Maybe it’s not quite time for clinical use, unfortunately.
We also have learned that there are significant potential risks involved. One issue that’s really been a focus is ARIA – amyloid-related imaging abnormalities. Could you speak a bit about that and requirements for monitoring?
Dr. Wicklund: ARIA essentially amounts to either vasogenic edema, microhemorrhages, or superficial siderosis that develops as a result of treatment. It relates to activation of the immune system with these passive monoclonal antibodies that’s going to occur with targeting against the plaques. In the parenchyma, it will cause edema. If you have amyloid in the walls of the blood vessels, it can cause microhemorrhages.
While the term “ARIA” implies an imaging-related abnormality, and it largely is purely an imaging finding, it’s not solely an imaging-related finding. It can cause symptoms, including very serious symptoms.
Overall, with lecanemab, the incidence of ARIA within the treatment group in the phase 3 study, combined between both ARIA-E (edema/effusion) and ARIA-H (hemorrhage), was 21.5%, with about 17% being ARIA-H and about 12.5% being ARIA-E. Of course, they can occur at the same time.
Overall, in terms of people in the clinical trials, for most it was purely an imaging-related finding. About 3% developed symptomatic ARIA. Some of those were very serious symptoms, including things like seizures and need to be hospitalized. A couple of deaths have been attributed to ARIA as well.
Patients on anticoagulation
Dr. LaFaver: Along those lines, any additional words to say for people who might be on anticoagulation or might require medications for a stroke, for example?
Dr. Wicklund: While individuals on anticoagulation were allowed in the clinical trials, the current, published appropriate-use guideline is recommending against its use, as several of the serious adverse effects, including the deaths, were for the most part attributed to anticoagulation use.
When it comes to acute stroke treatment, one must carefully consider use of tPA, as two of the three deaths were tPA associated in the clinical trials. It shouldn’t necessarily be an absolute contraindication, but it can make the clinical picture very muddy. If an individual is on lecanemab and comes to the ER with acute stroke-like symptoms, it’s more likely that they’re going to be having an ARIA side effect rather than an acute stroke.
A general recommendation would be to obtain an acute head CT with a CTA, and if there is a large vessel occlusion, proceed to thrombectomy. However, if there isn’t a large vessel occlusion, if you have the ability to get a rapid MRI with diffusion-weighted imaging to screen for acute stroke changes or tissue flair with acute edema changes suggestive of ARIA, that would be preferred before proceeding with thrombolysis. These are all relative contraindications and are going to depend on what’s available near you.
Donanemab approval pending
Dr. LaFaver: This will be an issue because the population we’re talking about is definitely at risk for stroke as well as Alzheimer’s disease. Where do you see this field going as far as amyloid antibody therapy is concerned, with another agent, donanemab, possibly getting FDA approval later this year as well?
Dr. Wicklund: We’re anticipating that donanemab will get FDA approval in the next coming months. Donanemab also targets the amyloid in the brain, although lecanemab and donanemab target different aspects of the production of the amyloid plaque. They were both shown to have roughly equal efficacy in their phase 3 clinical trials. Donanemab has the benefit of being a once-monthly infusion as opposed to twice-monthly infusions with lecanemab. It does have a slightly higher risk for ARIA compared with lecanemab.
Those are just some things to take into consideration when talking with your patients. In terms of where we’re going from here, we’re moving even earlier in terms of disease state. The lecanemab and donanemab phase 3 trials were done in individuals with mild cognitive impairment or mild dementia due to Alzheimer’s disease. They should not be used in individuals with moderate or more advanced Alzheimer’s disease.
There are ongoing, large, national, multicenter clinical trials of both lecanemab and donanemab in a preclinical state of Alzheimer’s disease. These individuals have evidence of amyloidosis, either through PET imaging or through CSF, but are clinically asymptomatic and do not yet have any signs of cognitive impairment or functional decline. We look forward to those results in the next few years. Hopefully, they’ll be able to show even greater benefit when moving into these early disease states in terms of delaying or even preventing cognitive decline.
Dr. LaFaver: That’s definitely very interesting to hear about. Where can people go for more information?
Dr. Wicklund: There’s a guideline on the use of lecanemab through the American Academy of Neurology. I encourage you to look at that. Also, look at the appropriate-use recommendations that were published this year in The Journal of Prevention of Alzheimer’s Disease.
Dr. LaFaver: Wonderful. With that being said, thank you so much for talking to me. I learned a lot. Thanks, everyone, for listening.
Dr. LaFaver is a neurologist at Saratoga Hospital Medical Group, Saratoga Springs, N.Y. She disclosed having no relevant financial relationships. Dr. Wicklund is senior associate consultant in the department of Neurology at Mayo Clinic, Phoenix, Ariz. She disclosed having no relevant financial relationships.
A version of this article appeared on Medscape.com.
Recorded October 13, 2023. This transcript has been edited for clarity.
Kathrin LaFaver, MD: I’ll be talking today with Dr. Meredith Wicklund, senior associate consultant and behavioral neurologist specialist at Mayo Clinic in Arizona. Welcome, Meredith.
Meredith Wicklund, MD: Thank you.
Lecanemab data
Dr. LaFaver: I’m very excited about our topic. Could you give us a brief overview of why there has been so much research interest in this topic of anti-amyloid antibodies?
Dr. Wicklund: The pathologic component of what defines something as Alzheimer’s disease is, by definition, presence of amyloid plaques and tau tangles. When it was first discovered in the 1980s that the component of the plaques was actually the amyloid protein – beta amyloid specifically – interest went right from there to developing therapies to directly target the pathology that is Alzheimer’s disease.
Dr. LaFaver: Lecanemab is the first FDA-approved disease-modifying antibody in that realm. Could you review the study data, especially as it applies to both of us in daily neurology clinic?
Dr. Wicklund: The study data from a phase 3 trial did show, for the primary outcome, that there was a 27% slowing of decline compared with individuals on placebo. It’s important to point out that this was slowing of decline. It was not stabilizing decline. It was not improving decline.
I think it’s important that we inform our patients that really, even with this therapy, there’s no prospect of stabilizing or restoring cognition or function. We do progress at a slower rate compared with individuals not on this treatment, which, given that this medication is for individuals in mild disease who have relatively preserved functional status, that can be potentially very meaningful to families.
The overall benefit was small. It essentially amounts to half a point on an 18-point scale, which is statistically significant. How much clinical meaningfulness that actually leads to is unclear. Finding clinical meaningfulness cannot be defined by a particular test. It really can only be defined on the individual level, what is meaningful to them.
Recommended tests
Dr. LaFaver: It is my understanding that, to qualify for lecanemab use, one needs to have a biomarker-supported diagnosis of Alzheimer’s disease, either via an amyloid PET scan or CSF biomarkers. What would your recommendation be for a neurologist in practice to go about these requirements?
Dr. Wicklund: Since this medication is directly targeting the amyloid pathology, and it does convey a potential risk, we want to make sure that the actual pathology is present in the individuals before we treat them and potentially expose them to risk. The best way of doing that is through either an amyloid PET scan or spinal fluid testing of beta amyloid and tau.
There are several plasma-based biomarkers in development. However, I would avoid using those currently. There are still many unknowns in terms of what exactly is the right species of tau that we should be looking at, the right mechanism of the lab test, how minority status may influence it, and how different comorbidities may influence it.
I would recommend, at this time, sticking with amyloid PET or CSF testing. Given that amyloid PET is not widely available in many community practices, generally only available at academic centers, and is quite costly, many insurances do not cover it – although Medicare has a proposal to potentially start covering it – I generally go with spinal fluid testing, which is more widely available. There are several labs across the country that can process that testing in a reliable way.
Amyloid-related imaging abnormalities
Dr. LaFaver: That’s very helpful to know. There’s been a large amount of buzz just these past couple of weeks about the blood biomarker coming up. I think, as you point out, this wasn’t the marker used in the clinical studies and there are still unknowns. Maybe it’s not quite time for clinical use, unfortunately.
We also have learned that there are significant potential risks involved. One issue that’s really been a focus is ARIA – amyloid-related imaging abnormalities. Could you speak a bit about that and requirements for monitoring?
Dr. Wicklund: ARIA essentially amounts to either vasogenic edema, microhemorrhages, or superficial siderosis that develops as a result of treatment. It relates to activation of the immune system with these passive monoclonal antibodies that’s going to occur with targeting against the plaques. In the parenchyma, it will cause edema. If you have amyloid in the walls of the blood vessels, it can cause microhemorrhages.
While the term “ARIA” implies an imaging-related abnormality, and it largely is purely an imaging finding, it’s not solely an imaging-related finding. It can cause symptoms, including very serious symptoms.
Overall, with lecanemab, the incidence of ARIA within the treatment group in the phase 3 study, combined between both ARIA-E (edema/effusion) and ARIA-H (hemorrhage), was 21.5%, with about 17% being ARIA-H and about 12.5% being ARIA-E. Of course, they can occur at the same time.
Overall, in terms of people in the clinical trials, for most it was purely an imaging-related finding. About 3% developed symptomatic ARIA. Some of those were very serious symptoms, including things like seizures and need to be hospitalized. A couple of deaths have been attributed to ARIA as well.
Patients on anticoagulation
Dr. LaFaver: Along those lines, any additional words to say for people who might be on anticoagulation or might require medications for a stroke, for example?
Dr. Wicklund: While individuals on anticoagulation were allowed in the clinical trials, the current, published appropriate-use guideline is recommending against its use, as several of the serious adverse effects, including the deaths, were for the most part attributed to anticoagulation use.
When it comes to acute stroke treatment, one must carefully consider use of tPA, as two of the three deaths were tPA associated in the clinical trials. It shouldn’t necessarily be an absolute contraindication, but it can make the clinical picture very muddy. If an individual is on lecanemab and comes to the ER with acute stroke-like symptoms, it’s more likely that they’re going to be having an ARIA side effect rather than an acute stroke.
A general recommendation would be to obtain an acute head CT with a CTA, and if there is a large vessel occlusion, proceed to thrombectomy. However, if there isn’t a large vessel occlusion, if you have the ability to get a rapid MRI with diffusion-weighted imaging to screen for acute stroke changes or tissue flair with acute edema changes suggestive of ARIA, that would be preferred before proceeding with thrombolysis. These are all relative contraindications and are going to depend on what’s available near you.
Donanemab approval pending
Dr. LaFaver: This will be an issue because the population we’re talking about is definitely at risk for stroke as well as Alzheimer’s disease. Where do you see this field going as far as amyloid antibody therapy is concerned, with another agent, donanemab, possibly getting FDA approval later this year as well?
Dr. Wicklund: We’re anticipating that donanemab will get FDA approval in the next coming months. Donanemab also targets the amyloid in the brain, although lecanemab and donanemab target different aspects of the production of the amyloid plaque. They were both shown to have roughly equal efficacy in their phase 3 clinical trials. Donanemab has the benefit of being a once-monthly infusion as opposed to twice-monthly infusions with lecanemab. It does have a slightly higher risk for ARIA compared with lecanemab.
Those are just some things to take into consideration when talking with your patients. In terms of where we’re going from here, we’re moving even earlier in terms of disease state. The lecanemab and donanemab phase 3 trials were done in individuals with mild cognitive impairment or mild dementia due to Alzheimer’s disease. They should not be used in individuals with moderate or more advanced Alzheimer’s disease.
There are ongoing, large, national, multicenter clinical trials of both lecanemab and donanemab in a preclinical state of Alzheimer’s disease. These individuals have evidence of amyloidosis, either through PET imaging or through CSF, but are clinically asymptomatic and do not yet have any signs of cognitive impairment or functional decline. We look forward to those results in the next few years. Hopefully, they’ll be able to show even greater benefit when moving into these early disease states in terms of delaying or even preventing cognitive decline.
Dr. LaFaver: That’s definitely very interesting to hear about. Where can people go for more information?
Dr. Wicklund: There’s a guideline on the use of lecanemab through the American Academy of Neurology. I encourage you to look at that. Also, look at the appropriate-use recommendations that were published this year in The Journal of Prevention of Alzheimer’s Disease.
Dr. LaFaver: Wonderful. With that being said, thank you so much for talking to me. I learned a lot. Thanks, everyone, for listening.
Dr. LaFaver is a neurologist at Saratoga Hospital Medical Group, Saratoga Springs, N.Y. She disclosed having no relevant financial relationships. Dr. Wicklund is senior associate consultant in the department of Neurology at Mayo Clinic, Phoenix, Ariz. She disclosed having no relevant financial relationships.
A version of this article appeared on Medscape.com.
Headache after drinking red wine? This could be why
This transcript has been edited for clarity.
Robert Louis Stevenson famously said, “Wine is bottled poetry.” And I think it works quite well. I’ve had wines that are simple, elegant, and unpretentious like Emily Dickinson, and passionate and mysterious like Pablo Neruda. And I’ve had wines that are more analogous to the limerick you might read scrawled on a rest-stop bathroom wall. Those ones give me headaches.
– and apparently it’s not just the alcohol.
Headaches are common, and headaches after drinking alcohol are particularly common. An interesting epidemiologic phenomenon, not yet adequately explained, is why red wine is associated with more headache than other forms of alcohol. There have been many studies fingering many suspects, from sulfites to tannins to various phenolic compounds, but none have really provided a concrete explanation for what might be going on.
A new hypothesis came to the fore on Nov. 20 in the journal Scientific Reports:
To understand the idea, first a reminder of what happens when you drink alcohol, physiologically.
Alcohol is metabolized by the enzyme alcohol dehydrogenase in the gut and then in the liver. That turns it into acetaldehyde, a toxic metabolite. In most of us, aldehyde dehydrogenase (ALDH) quickly metabolizes acetaldehyde to the inert acetate, which can be safely excreted.
I say “most of us” because some populations, particularly those with East Asian ancestry, have a mutation in the ALDH gene which can lead to accumulation of toxic acetaldehyde with alcohol consumption – leading to facial flushing, nausea, and headache.
We can also inhibit the enzyme medically. That’s what the drug disulfiram, also known as Antabuse, does. It doesn’t prevent you from wanting to drink; it makes the consequences of drinking incredibly aversive.
The researchers focused in on the aldehyde dehydrogenase enzyme and conducted a screening study. Are there any compounds in red wine that naturally inhibit ALDH?
The results pointed squarely at quercetin, and particularly its metabolite quercetin glucuronide, which, at 20 micromolar concentrations, inhibited about 80% of ALDH activity.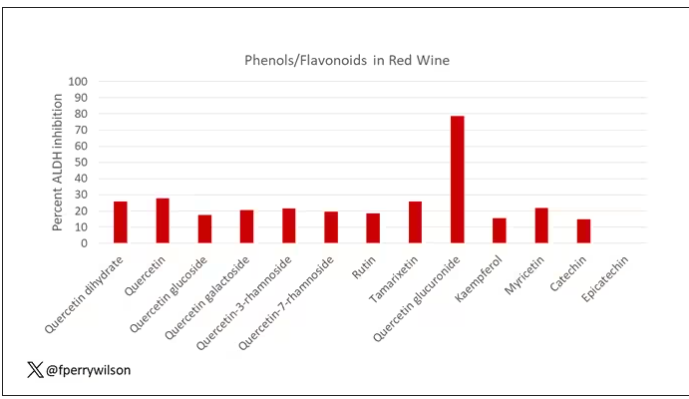
Quercetin is a flavonoid – a compound that gives color to a variety of vegetables and fruits, including grapes. In a test tube, it is an antioxidant, which is enough evidence to spawn a small quercetin-as-supplement industry, but there is no convincing evidence that it is medically useful. The authors then examined the concentration of quercetin glucuronide to achieve various inhibitions of ALDH, as you can see in this graph here.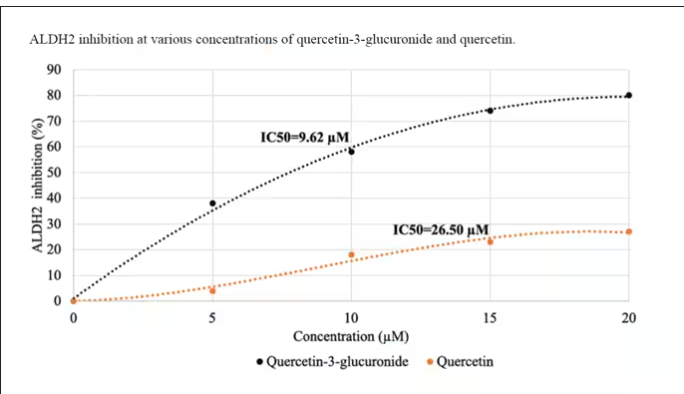
By about 10 micromolar, we see a decent amount of inhibition. Disulfiram is about 10 times more potent than that, but then again, you don’t drink three glasses of disulfiram with Thanksgiving dinner.
This is where this study stops. But it obviously tells us very little about what might be happening in the human body. For that, we need to ask the question: Can we get our quercetin levels to 10 micromolar? Is that remotely achievable?
Let’s start with how much quercetin there is in red wine. Like all things wine, it varies, but this study examining Australian wines found mean concentrations of 11 mg/L. The highest value I saw was close to 50 mg/L.
So let’s do some math. To make the numbers easy, let’s say you drank a liter of Australian wine, taking in 50 mg of quercetin glucuronide.
How much of that gets into your bloodstream? Some studies suggest a bioavailability of less than 1%, which basically means none and should probably put the quercetin hypothesis to bed. But there is some variation here too; it seems to depend on the form of quercetin you ingest.
Let’s say all 50 mg gets into your bloodstream. What blood concentration would that lead to? Well, I’ll keep the stoichiometry in the graphics and just say that if we assume that the volume of distribution of the compound is restricted to plasma alone, then you could achieve similar concentrations to what was done in petri dishes during this study.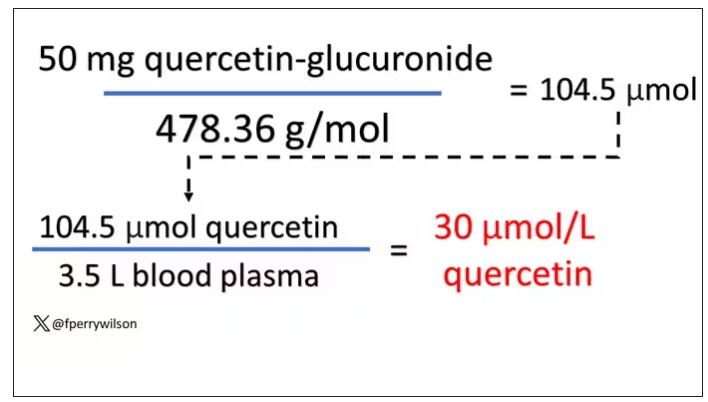
Of course, if quercetin is really the culprit behind red wine headache, I have some questions: Why aren’t the Amazon reviews of quercetin supplements chock full of warnings not to take them with alcohol? And other foods have way higher quercetin concentration than wine, but you don’t hear people warning not to take your red onions with alcohol, or your capers, or lingonberries.
There’s some more work to be done here – most importantly, some human studies. Let’s give people wine with different amounts of quercetin and see what happens. Sign me up. Seriously.
As for Thanksgiving, it’s worth noting that cranberries have a lot of quercetin in them. So between the cranberry sauce, the Beaujolais, and your uncle ranting about the contrails again, the probability of headache is pretty darn high. Stay safe out there, and Happy Thanksgiving.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Robert Louis Stevenson famously said, “Wine is bottled poetry.” And I think it works quite well. I’ve had wines that are simple, elegant, and unpretentious like Emily Dickinson, and passionate and mysterious like Pablo Neruda. And I’ve had wines that are more analogous to the limerick you might read scrawled on a rest-stop bathroom wall. Those ones give me headaches.
– and apparently it’s not just the alcohol.
Headaches are common, and headaches after drinking alcohol are particularly common. An interesting epidemiologic phenomenon, not yet adequately explained, is why red wine is associated with more headache than other forms of alcohol. There have been many studies fingering many suspects, from sulfites to tannins to various phenolic compounds, but none have really provided a concrete explanation for what might be going on.
A new hypothesis came to the fore on Nov. 20 in the journal Scientific Reports:
To understand the idea, first a reminder of what happens when you drink alcohol, physiologically.
Alcohol is metabolized by the enzyme alcohol dehydrogenase in the gut and then in the liver. That turns it into acetaldehyde, a toxic metabolite. In most of us, aldehyde dehydrogenase (ALDH) quickly metabolizes acetaldehyde to the inert acetate, which can be safely excreted.
I say “most of us” because some populations, particularly those with East Asian ancestry, have a mutation in the ALDH gene which can lead to accumulation of toxic acetaldehyde with alcohol consumption – leading to facial flushing, nausea, and headache.
We can also inhibit the enzyme medically. That’s what the drug disulfiram, also known as Antabuse, does. It doesn’t prevent you from wanting to drink; it makes the consequences of drinking incredibly aversive.
The researchers focused in on the aldehyde dehydrogenase enzyme and conducted a screening study. Are there any compounds in red wine that naturally inhibit ALDH?
The results pointed squarely at quercetin, and particularly its metabolite quercetin glucuronide, which, at 20 micromolar concentrations, inhibited about 80% of ALDH activity.
Quercetin is a flavonoid – a compound that gives color to a variety of vegetables and fruits, including grapes. In a test tube, it is an antioxidant, which is enough evidence to spawn a small quercetin-as-supplement industry, but there is no convincing evidence that it is medically useful. The authors then examined the concentration of quercetin glucuronide to achieve various inhibitions of ALDH, as you can see in this graph here.
By about 10 micromolar, we see a decent amount of inhibition. Disulfiram is about 10 times more potent than that, but then again, you don’t drink three glasses of disulfiram with Thanksgiving dinner.
This is where this study stops. But it obviously tells us very little about what might be happening in the human body. For that, we need to ask the question: Can we get our quercetin levels to 10 micromolar? Is that remotely achievable?
Let’s start with how much quercetin there is in red wine. Like all things wine, it varies, but this study examining Australian wines found mean concentrations of 11 mg/L. The highest value I saw was close to 50 mg/L.
So let’s do some math. To make the numbers easy, let’s say you drank a liter of Australian wine, taking in 50 mg of quercetin glucuronide.
How much of that gets into your bloodstream? Some studies suggest a bioavailability of less than 1%, which basically means none and should probably put the quercetin hypothesis to bed. But there is some variation here too; it seems to depend on the form of quercetin you ingest.
Let’s say all 50 mg gets into your bloodstream. What blood concentration would that lead to? Well, I’ll keep the stoichiometry in the graphics and just say that if we assume that the volume of distribution of the compound is restricted to plasma alone, then you could achieve similar concentrations to what was done in petri dishes during this study.
Of course, if quercetin is really the culprit behind red wine headache, I have some questions: Why aren’t the Amazon reviews of quercetin supplements chock full of warnings not to take them with alcohol? And other foods have way higher quercetin concentration than wine, but you don’t hear people warning not to take your red onions with alcohol, or your capers, or lingonberries.
There’s some more work to be done here – most importantly, some human studies. Let’s give people wine with different amounts of quercetin and see what happens. Sign me up. Seriously.
As for Thanksgiving, it’s worth noting that cranberries have a lot of quercetin in them. So between the cranberry sauce, the Beaujolais, and your uncle ranting about the contrails again, the probability of headache is pretty darn high. Stay safe out there, and Happy Thanksgiving.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Robert Louis Stevenson famously said, “Wine is bottled poetry.” And I think it works quite well. I’ve had wines that are simple, elegant, and unpretentious like Emily Dickinson, and passionate and mysterious like Pablo Neruda. And I’ve had wines that are more analogous to the limerick you might read scrawled on a rest-stop bathroom wall. Those ones give me headaches.
– and apparently it’s not just the alcohol.
Headaches are common, and headaches after drinking alcohol are particularly common. An interesting epidemiologic phenomenon, not yet adequately explained, is why red wine is associated with more headache than other forms of alcohol. There have been many studies fingering many suspects, from sulfites to tannins to various phenolic compounds, but none have really provided a concrete explanation for what might be going on.
A new hypothesis came to the fore on Nov. 20 in the journal Scientific Reports:
To understand the idea, first a reminder of what happens when you drink alcohol, physiologically.
Alcohol is metabolized by the enzyme alcohol dehydrogenase in the gut and then in the liver. That turns it into acetaldehyde, a toxic metabolite. In most of us, aldehyde dehydrogenase (ALDH) quickly metabolizes acetaldehyde to the inert acetate, which can be safely excreted.
I say “most of us” because some populations, particularly those with East Asian ancestry, have a mutation in the ALDH gene which can lead to accumulation of toxic acetaldehyde with alcohol consumption – leading to facial flushing, nausea, and headache.
We can also inhibit the enzyme medically. That’s what the drug disulfiram, also known as Antabuse, does. It doesn’t prevent you from wanting to drink; it makes the consequences of drinking incredibly aversive.
The researchers focused in on the aldehyde dehydrogenase enzyme and conducted a screening study. Are there any compounds in red wine that naturally inhibit ALDH?
The results pointed squarely at quercetin, and particularly its metabolite quercetin glucuronide, which, at 20 micromolar concentrations, inhibited about 80% of ALDH activity.
Quercetin is a flavonoid – a compound that gives color to a variety of vegetables and fruits, including grapes. In a test tube, it is an antioxidant, which is enough evidence to spawn a small quercetin-as-supplement industry, but there is no convincing evidence that it is medically useful. The authors then examined the concentration of quercetin glucuronide to achieve various inhibitions of ALDH, as you can see in this graph here.
By about 10 micromolar, we see a decent amount of inhibition. Disulfiram is about 10 times more potent than that, but then again, you don’t drink three glasses of disulfiram with Thanksgiving dinner.
This is where this study stops. But it obviously tells us very little about what might be happening in the human body. For that, we need to ask the question: Can we get our quercetin levels to 10 micromolar? Is that remotely achievable?
Let’s start with how much quercetin there is in red wine. Like all things wine, it varies, but this study examining Australian wines found mean concentrations of 11 mg/L. The highest value I saw was close to 50 mg/L.
So let’s do some math. To make the numbers easy, let’s say you drank a liter of Australian wine, taking in 50 mg of quercetin glucuronide.
How much of that gets into your bloodstream? Some studies suggest a bioavailability of less than 1%, which basically means none and should probably put the quercetin hypothesis to bed. But there is some variation here too; it seems to depend on the form of quercetin you ingest.
Let’s say all 50 mg gets into your bloodstream. What blood concentration would that lead to? Well, I’ll keep the stoichiometry in the graphics and just say that if we assume that the volume of distribution of the compound is restricted to plasma alone, then you could achieve similar concentrations to what was done in petri dishes during this study.
Of course, if quercetin is really the culprit behind red wine headache, I have some questions: Why aren’t the Amazon reviews of quercetin supplements chock full of warnings not to take them with alcohol? And other foods have way higher quercetin concentration than wine, but you don’t hear people warning not to take your red onions with alcohol, or your capers, or lingonberries.
There’s some more work to be done here – most importantly, some human studies. Let’s give people wine with different amounts of quercetin and see what happens. Sign me up. Seriously.
As for Thanksgiving, it’s worth noting that cranberries have a lot of quercetin in them. So between the cranberry sauce, the Beaujolais, and your uncle ranting about the contrails again, the probability of headache is pretty darn high. Stay safe out there, and Happy Thanksgiving.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
A better way to control blood pressure
My Bing AI engine, when prompted, tells me that there are about 87 journals, 45 conferences, and 53 workshops presently dedicated exclusively to hypertension. All of that attention, and yet ...
What is going on?
The top killers of Americans remain coronary artery heart disease (26%), cancer (22%), and stroke (6%). The precursors and attributable risk factors for coronary artery heart disease include hypertension (40%), obesity (20%), diabetes (15%), and combustible tobacco use (15%). The key precursors and attributable risk factors for stroke are hypertension (53%), obesity (37%), diabetes (9%), and combustible tobacco use (11%). Obviously, these are estimates, with substantial overlap.
It’s pretty obvious that
We have addressed improving tobacco control and preventing obesity and diabetes on these pages many times, and lamented the medical, public health, and societal failings. Today we turn our attention to the control of hypertension. That is much easier and far less expensive.
All physicians and medical organizations know that hypertension is a major attributable cause of many serious, expensive, and fatal illnesses. As many as 119 million (48%) of American adults have hypertension. The American Heart Association (AHA), American Medical Association (AMA), American College of Cardiology (ACC), and hundreds of other organizations have set a new target of 130/80 (revised from 140/90) for blood pressure control and have launched a major initiative, Target: BP, to reach it.
That is just great. We all wish this massive effort to succeed where few others have. But do AHA, AMA, ACC, and others understand why most efforts to this point have failed? The blame is typically aimed at patients failing to adhere to their instructions. Maybe, but why? And how does Target: BP intend to convert chronic failure into success if it just continues to do everything they have been trying to do that doesn’t work?
At this point, the Centers for Disease Control and Prevention reports that fewer than 48% of American patients with hypertension meet even the less stringent historical 140/90 goal.
A group practice in Ohio, PriMed Physicians, has consistently exceeded 90% or even 95% blood pressure control for its patients with hypertension for more than 10 years. Exemplary. How do they do it? This video of the 13th annual Lundberg Institute lecture describes this unique and successful program.
PriMed’s clinicians use the MedsEngine AI tool from MediSync and the NICaS (noninvasive cardiac system with impedance cardiography) to determine each patient’s unique blood pressure pathophysiology. Clinicians and patients understand that the simplest explanation of this pathophysiology encompasses three factors: (1) the volume of “water” (blood) in the system; (2) the strength of the pumping (pulsatile) process; and (3) the tightness (resistance) of the tubes that carry the blood. Patients “get it” when it is explained this way, and they cooperate.
At the first patient encounter, the Food and Drug Administration–approved PhysioFlow is employed to assess those three vital hemodynamic factors. The individual patient’s data are loaded into a tightly programed EHR-based algorithm with 37 clinical factors and five classes of drugs, providing multiple ways to influence the three key pathophysiologic processes. In this way, they arrive at the precise drug(s) and dosages for that patient. During the second visit, most patients are already showing improvement. By the third visit, the blood pressures of most patients have reached target control. After that, it is maintenance and tweaking.
These factors summarize why it works:
- Senior management belief, commitment, and leadership
- Informed buy-in from clinicians and patients
- A test that determines root causes of too much fluid, too strong pump action, or too tight pipes, and their proportionality
- An AI tool that matches those three pathophysiologic factors and 35 other clinical factors with the best drug or drugs (of many, not just a few) and dosages
- Persistent clinician-patient follow-up
- Refusal to accept failure
Since this approach is so successful, why is its use not everywhere?
It is not as if nobody noticed, even if you and many organizations have not. The American Medical Group Association recognized the program’s success by giving its top award to PriMed in 2015.
Klepper and Rodis wrote about this approach for managing multiple chronic conditions in 2021. Here’s a background article and an explainer, Clinical use of impedance cardiography for hemodynamic assessment of early cardiovascular disease and management of hypertension.
I found one pragmatic controlled clinical trial of impedance cardiography with a decision-support system from Beijing that did demonstrate clinical and statistical significance.
Frankly, we do need more rigorous, unbiased, large, controlled clinical trials assessing the MedsEngine and NICaS approach to managing blood pressure to facilitate a massive switch from the old and established (but failing) approach to a starkly better way.
Almost no one ever “completes a database.” All decision makers must act based upon the best data to which they have access. Data are often incomplete. The difference between success and mediocrity is often the ability of an individual or system to decide when enough information is enough and act accordingly.
Cost-effectiveness studies in three countries (United Kingdom, United States, and China) confirm sharply lower lifelong costs when blood pressure is well controlled. Of course.
For the American medical-industrial complex, lowered costs for managing common serious diseases may be an undesired rather than a good thing. In money-driven medicine, lower costs to the payer and purchaser translate to less revenue for the providers. Imagine all of those invasive and noninvasive diagnostic and therapeutic procedures forgone by prevention of hypertension. Is it possible that such an underlying truth is the real reason why American medicine is habitually unsuccessful at controlling blood pressure?
Right now, if my blood pressure were not well controlled (it is), I would find my way to Cincinnati, to give PriMed physicians, MediSync, and MedsEngine a crack at prolonging my useful life.
Dr. Lundberg is editor in chief of Cancer Commons. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
My Bing AI engine, when prompted, tells me that there are about 87 journals, 45 conferences, and 53 workshops presently dedicated exclusively to hypertension. All of that attention, and yet ...
What is going on?
The top killers of Americans remain coronary artery heart disease (26%), cancer (22%), and stroke (6%). The precursors and attributable risk factors for coronary artery heart disease include hypertension (40%), obesity (20%), diabetes (15%), and combustible tobacco use (15%). The key precursors and attributable risk factors for stroke are hypertension (53%), obesity (37%), diabetes (9%), and combustible tobacco use (11%). Obviously, these are estimates, with substantial overlap.
It’s pretty obvious that
We have addressed improving tobacco control and preventing obesity and diabetes on these pages many times, and lamented the medical, public health, and societal failings. Today we turn our attention to the control of hypertension. That is much easier and far less expensive.
All physicians and medical organizations know that hypertension is a major attributable cause of many serious, expensive, and fatal illnesses. As many as 119 million (48%) of American adults have hypertension. The American Heart Association (AHA), American Medical Association (AMA), American College of Cardiology (ACC), and hundreds of other organizations have set a new target of 130/80 (revised from 140/90) for blood pressure control and have launched a major initiative, Target: BP, to reach it.
That is just great. We all wish this massive effort to succeed where few others have. But do AHA, AMA, ACC, and others understand why most efforts to this point have failed? The blame is typically aimed at patients failing to adhere to their instructions. Maybe, but why? And how does Target: BP intend to convert chronic failure into success if it just continues to do everything they have been trying to do that doesn’t work?
At this point, the Centers for Disease Control and Prevention reports that fewer than 48% of American patients with hypertension meet even the less stringent historical 140/90 goal.
A group practice in Ohio, PriMed Physicians, has consistently exceeded 90% or even 95% blood pressure control for its patients with hypertension for more than 10 years. Exemplary. How do they do it? This video of the 13th annual Lundberg Institute lecture describes this unique and successful program.
PriMed’s clinicians use the MedsEngine AI tool from MediSync and the NICaS (noninvasive cardiac system with impedance cardiography) to determine each patient’s unique blood pressure pathophysiology. Clinicians and patients understand that the simplest explanation of this pathophysiology encompasses three factors: (1) the volume of “water” (blood) in the system; (2) the strength of the pumping (pulsatile) process; and (3) the tightness (resistance) of the tubes that carry the blood. Patients “get it” when it is explained this way, and they cooperate.
At the first patient encounter, the Food and Drug Administration–approved PhysioFlow is employed to assess those three vital hemodynamic factors. The individual patient’s data are loaded into a tightly programed EHR-based algorithm with 37 clinical factors and five classes of drugs, providing multiple ways to influence the three key pathophysiologic processes. In this way, they arrive at the precise drug(s) and dosages for that patient. During the second visit, most patients are already showing improvement. By the third visit, the blood pressures of most patients have reached target control. After that, it is maintenance and tweaking.
These factors summarize why it works:
- Senior management belief, commitment, and leadership
- Informed buy-in from clinicians and patients
- A test that determines root causes of too much fluid, too strong pump action, or too tight pipes, and their proportionality
- An AI tool that matches those three pathophysiologic factors and 35 other clinical factors with the best drug or drugs (of many, not just a few) and dosages
- Persistent clinician-patient follow-up
- Refusal to accept failure
Since this approach is so successful, why is its use not everywhere?
It is not as if nobody noticed, even if you and many organizations have not. The American Medical Group Association recognized the program’s success by giving its top award to PriMed in 2015.
Klepper and Rodis wrote about this approach for managing multiple chronic conditions in 2021. Here’s a background article and an explainer, Clinical use of impedance cardiography for hemodynamic assessment of early cardiovascular disease and management of hypertension.
I found one pragmatic controlled clinical trial of impedance cardiography with a decision-support system from Beijing that did demonstrate clinical and statistical significance.
Frankly, we do need more rigorous, unbiased, large, controlled clinical trials assessing the MedsEngine and NICaS approach to managing blood pressure to facilitate a massive switch from the old and established (but failing) approach to a starkly better way.
Almost no one ever “completes a database.” All decision makers must act based upon the best data to which they have access. Data are often incomplete. The difference between success and mediocrity is often the ability of an individual or system to decide when enough information is enough and act accordingly.
Cost-effectiveness studies in three countries (United Kingdom, United States, and China) confirm sharply lower lifelong costs when blood pressure is well controlled. Of course.
For the American medical-industrial complex, lowered costs for managing common serious diseases may be an undesired rather than a good thing. In money-driven medicine, lower costs to the payer and purchaser translate to less revenue for the providers. Imagine all of those invasive and noninvasive diagnostic and therapeutic procedures forgone by prevention of hypertension. Is it possible that such an underlying truth is the real reason why American medicine is habitually unsuccessful at controlling blood pressure?
Right now, if my blood pressure were not well controlled (it is), I would find my way to Cincinnati, to give PriMed physicians, MediSync, and MedsEngine a crack at prolonging my useful life.
Dr. Lundberg is editor in chief of Cancer Commons. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
My Bing AI engine, when prompted, tells me that there are about 87 journals, 45 conferences, and 53 workshops presently dedicated exclusively to hypertension. All of that attention, and yet ...
What is going on?
The top killers of Americans remain coronary artery heart disease (26%), cancer (22%), and stroke (6%). The precursors and attributable risk factors for coronary artery heart disease include hypertension (40%), obesity (20%), diabetes (15%), and combustible tobacco use (15%). The key precursors and attributable risk factors for stroke are hypertension (53%), obesity (37%), diabetes (9%), and combustible tobacco use (11%). Obviously, these are estimates, with substantial overlap.
It’s pretty obvious that
We have addressed improving tobacco control and preventing obesity and diabetes on these pages many times, and lamented the medical, public health, and societal failings. Today we turn our attention to the control of hypertension. That is much easier and far less expensive.
All physicians and medical organizations know that hypertension is a major attributable cause of many serious, expensive, and fatal illnesses. As many as 119 million (48%) of American adults have hypertension. The American Heart Association (AHA), American Medical Association (AMA), American College of Cardiology (ACC), and hundreds of other organizations have set a new target of 130/80 (revised from 140/90) for blood pressure control and have launched a major initiative, Target: BP, to reach it.
That is just great. We all wish this massive effort to succeed where few others have. But do AHA, AMA, ACC, and others understand why most efforts to this point have failed? The blame is typically aimed at patients failing to adhere to their instructions. Maybe, but why? And how does Target: BP intend to convert chronic failure into success if it just continues to do everything they have been trying to do that doesn’t work?
At this point, the Centers for Disease Control and Prevention reports that fewer than 48% of American patients with hypertension meet even the less stringent historical 140/90 goal.
A group practice in Ohio, PriMed Physicians, has consistently exceeded 90% or even 95% blood pressure control for its patients with hypertension for more than 10 years. Exemplary. How do they do it? This video of the 13th annual Lundberg Institute lecture describes this unique and successful program.
PriMed’s clinicians use the MedsEngine AI tool from MediSync and the NICaS (noninvasive cardiac system with impedance cardiography) to determine each patient’s unique blood pressure pathophysiology. Clinicians and patients understand that the simplest explanation of this pathophysiology encompasses three factors: (1) the volume of “water” (blood) in the system; (2) the strength of the pumping (pulsatile) process; and (3) the tightness (resistance) of the tubes that carry the blood. Patients “get it” when it is explained this way, and they cooperate.
At the first patient encounter, the Food and Drug Administration–approved PhysioFlow is employed to assess those three vital hemodynamic factors. The individual patient’s data are loaded into a tightly programed EHR-based algorithm with 37 clinical factors and five classes of drugs, providing multiple ways to influence the three key pathophysiologic processes. In this way, they arrive at the precise drug(s) and dosages for that patient. During the second visit, most patients are already showing improvement. By the third visit, the blood pressures of most patients have reached target control. After that, it is maintenance and tweaking.
These factors summarize why it works:
- Senior management belief, commitment, and leadership
- Informed buy-in from clinicians and patients
- A test that determines root causes of too much fluid, too strong pump action, or too tight pipes, and their proportionality
- An AI tool that matches those three pathophysiologic factors and 35 other clinical factors with the best drug or drugs (of many, not just a few) and dosages
- Persistent clinician-patient follow-up
- Refusal to accept failure
Since this approach is so successful, why is its use not everywhere?
It is not as if nobody noticed, even if you and many organizations have not. The American Medical Group Association recognized the program’s success by giving its top award to PriMed in 2015.
Klepper and Rodis wrote about this approach for managing multiple chronic conditions in 2021. Here’s a background article and an explainer, Clinical use of impedance cardiography for hemodynamic assessment of early cardiovascular disease and management of hypertension.
I found one pragmatic controlled clinical trial of impedance cardiography with a decision-support system from Beijing that did demonstrate clinical and statistical significance.
Frankly, we do need more rigorous, unbiased, large, controlled clinical trials assessing the MedsEngine and NICaS approach to managing blood pressure to facilitate a massive switch from the old and established (but failing) approach to a starkly better way.
Almost no one ever “completes a database.” All decision makers must act based upon the best data to which they have access. Data are often incomplete. The difference between success and mediocrity is often the ability of an individual or system to decide when enough information is enough and act accordingly.
Cost-effectiveness studies in three countries (United Kingdom, United States, and China) confirm sharply lower lifelong costs when blood pressure is well controlled. Of course.
For the American medical-industrial complex, lowered costs for managing common serious diseases may be an undesired rather than a good thing. In money-driven medicine, lower costs to the payer and purchaser translate to less revenue for the providers. Imagine all of those invasive and noninvasive diagnostic and therapeutic procedures forgone by prevention of hypertension. Is it possible that such an underlying truth is the real reason why American medicine is habitually unsuccessful at controlling blood pressure?
Right now, if my blood pressure were not well controlled (it is), I would find my way to Cincinnati, to give PriMed physicians, MediSync, and MedsEngine a crack at prolonging my useful life.
Dr. Lundberg is editor in chief of Cancer Commons. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
A mid-marathon cardiac arrest, an MD’s crisis of confidence
I was running my 25th New York City Marathon. It was 2018, and I almost pulled out of running that year. I wasn’t myself, and maybe that’s an understatement.
A month earlier, I had been involved in a malpractice case. I was found liable for $10 million. My colleagues didn’t think I had done anything wrong, but the jury did. And the local newspapers made me look like a villain.
I was devastated. But my priest, my friends, and my family all told me, “You can’t quit.” So, I decided to run for them.
I started on the Verrazzano-Narrows Bridge that morning with some friends from work. I usually listen to music as I’m running, but I didn’t that year. I was just in my zone, enjoying the crowds. They’re huge. Millions of people on the streets.
I was running well. I did half the race in an hour and 57 minutes. My family always meets me at mile 17, and I was almost there. I had reached 59th Street and was about to make the turn onto First Avenue.
That’s one of the noisiest places in the marathon. There’s a kind of tunnel, and with the crowd and the throng of runners, it’s incredibly loud. But somehow, I heard somebody yell, “Help!”
Now, how I heard that, I don’t know. And if I’d been listening to music like I always do, no way I would’ve heard it. I could swear it was an angel on my shoulder that said, “Turn around, dummy. You’ve got a person that needs your help to your left.”
I turned around and about 30 feet behind me, I saw a woman waving her hands and a runner on the ground. I thought, Somebody fainted. I pushed through the crowd to get to them. The woman was crying, saying, “My friend went down to tie her shoe and she fell back. I think she’s seizing or something.”
I got down and tried to wake the other woman up. I lifted her legs up. But I quickly realized there was more to the story.
Some volunteers and police started coming toward us. The police officers looked at me like, What’s this guy doing? I explained that I was a physician, and one of them began helping me with the CPR. As we did that, someone brought a defibrillator.
Meanwhile, runners were going past, almost over us. The police officers were trying to create a barrier.
The machine gave the woman a shock, but we didn’t get a response, so we resumed CPR. At that point, my legs began to cramp so badly I couldn’t go on. So the police officer took over, and I yelled, “I need an ambu bag!” Somebody brought one, and I started giving her oxygen.
At that point, a paramedic team arrived with a bigger defibrillator. We shocked her again. And again. That time we got results, but she quickly went out again. The fourth time, we got her heart back and she started breathing on her own.
We finally got her into an ambulance. I wanted to go with them, but the woman’s friend needed to get in, so there wasn’t enough room.
And then they were gone, and I was just standing there.
A police officer put his arm around me. He said, “Doc, you’re amazing. What do you need? Where can I take you?”
I said, “Take me? My wife is waiting for me at mile 17.”
I took off and ran. When I got to my wife and kids, they were so worried. We all wear tracking devices, and they could see that I had stopped for more than 20 minutes.
I fell into my wife’s arms and told her what had happened. I was crying. “I don’t know what to do. I need to get to the hospital.”
And she said, “No, you need to go finish the race.”
So, I did. It was painful because of the cramps, but I was numb at that point. I was thinking about the woman the whole way. My time was 5 hours and 20 minutes.
As soon as I finished, I went to every police officer I could find, but nobody knew anything. Suddenly, I remembered my cousin. He had previously been the head of EMS for New York City. I called him. “Abdo, it’s Ted, you’ve got to do me a favor.”
“What?” he said. “Are you delirious from running the marathon?”
I told him what I needed. He called me back 5 minutes later and said, “Ted, what’d you do? Everybody wants to know who you are and where you are! The woman just went out again at New York Cornell. But they got her back, and they’re bringing her up to the cath lab.”
After every marathon that I run, we host a big party at our house. My family and friends and neighbors all celebrate while I’m dying on the couch. That night, my daughter told everyone the story of what happened.
But I was still not right. Still thinking about the malpractice suit.
Yes, I just did something great. But I’d recently been called the worst physician in the world. The distraction of the marathon was gone, and I was back to thinking, What am I going to do with my life? Who’s ever going to want to see me again? I’m a pariah.
Everybody said, “Ted, what happened a month ago isn’t you. What happened today was you.”
I told them to leave it alone, but my daughter and my neighbor started calling people anyway. The next day I got a call from the local newspaper. It was the same journalist who had written about me from the trial. I told him I didn’t want to talk. I was actually pretty nasty.
But my wife said, “Ted, what are you doing? That guy was trying to help you.” So, I called back and apologized.
“Dr. Strange, we knew that story wasn’t right,” he said. “We have to write this story.”
After the article came out, I started getting more calls from the media. Channel 7 News and CBS News did segments. The New York Knicks invited us to a game and presented me with a watch. It was incredible. But I was also really embarrassed by it.
People started calling me a hero. I’m not a hero. I just did what I’m supposed to do, what I’m trained to do. Shame on me if I don’t do that. Good guy and hopefully good physician, sure, but not a hero.
I also give credit to the City of New York Police Department, the FDNY, and the volunteers. Without them, I couldn’t have done what I did. It was a true team effort.
A few weeks later, the woman went home to Minnesota. She’ll never run a marathon again, but she’s still alive to this day. It turned out she had a single lesion called the “widow-maker” lesion. She was in perfect health and had just completed an ultramarathon a few months before; but she had a genetic predisposition. She still calls me every December to thank me for another Christmas.
There’s more.
One year after this whole thing, almost to the date, I got a call from my attorney. “The court just threw out the malpractice verdict,” he said. “You didn’t do anything wrong.”
I’m a man of faith. And I believe all this happened for a reason. Maybe God was sending me a message, and that’s why I heard a call for help on 59th Street in my 25th marathon among millions of people in a crowd.
I ran the marathon the next year. And when I got to that spot, I stopped and reflected. Nobody knew why I was standing there, but I knew. To this day, I could take you to that spot.
I turn 65 next July, and I plan to keep on running the race.
Dr. Strange is chair of medicine at Staten Island University Hospital, associate ambulatory physician executive of the Staten Island Region, and an internal medicine and geriatric medicine physician with Northwell Health.
A version of this article first appeared on Medscape.com.
I was running my 25th New York City Marathon. It was 2018, and I almost pulled out of running that year. I wasn’t myself, and maybe that’s an understatement.
A month earlier, I had been involved in a malpractice case. I was found liable for $10 million. My colleagues didn’t think I had done anything wrong, but the jury did. And the local newspapers made me look like a villain.
I was devastated. But my priest, my friends, and my family all told me, “You can’t quit.” So, I decided to run for them.
I started on the Verrazzano-Narrows Bridge that morning with some friends from work. I usually listen to music as I’m running, but I didn’t that year. I was just in my zone, enjoying the crowds. They’re huge. Millions of people on the streets.
I was running well. I did half the race in an hour and 57 minutes. My family always meets me at mile 17, and I was almost there. I had reached 59th Street and was about to make the turn onto First Avenue.
That’s one of the noisiest places in the marathon. There’s a kind of tunnel, and with the crowd and the throng of runners, it’s incredibly loud. But somehow, I heard somebody yell, “Help!”
Now, how I heard that, I don’t know. And if I’d been listening to music like I always do, no way I would’ve heard it. I could swear it was an angel on my shoulder that said, “Turn around, dummy. You’ve got a person that needs your help to your left.”
I turned around and about 30 feet behind me, I saw a woman waving her hands and a runner on the ground. I thought, Somebody fainted. I pushed through the crowd to get to them. The woman was crying, saying, “My friend went down to tie her shoe and she fell back. I think she’s seizing or something.”
I got down and tried to wake the other woman up. I lifted her legs up. But I quickly realized there was more to the story.
Some volunteers and police started coming toward us. The police officers looked at me like, What’s this guy doing? I explained that I was a physician, and one of them began helping me with the CPR. As we did that, someone brought a defibrillator.
Meanwhile, runners were going past, almost over us. The police officers were trying to create a barrier.
The machine gave the woman a shock, but we didn’t get a response, so we resumed CPR. At that point, my legs began to cramp so badly I couldn’t go on. So the police officer took over, and I yelled, “I need an ambu bag!” Somebody brought one, and I started giving her oxygen.
At that point, a paramedic team arrived with a bigger defibrillator. We shocked her again. And again. That time we got results, but she quickly went out again. The fourth time, we got her heart back and she started breathing on her own.
We finally got her into an ambulance. I wanted to go with them, but the woman’s friend needed to get in, so there wasn’t enough room.
And then they were gone, and I was just standing there.
A police officer put his arm around me. He said, “Doc, you’re amazing. What do you need? Where can I take you?”
I said, “Take me? My wife is waiting for me at mile 17.”
I took off and ran. When I got to my wife and kids, they were so worried. We all wear tracking devices, and they could see that I had stopped for more than 20 minutes.
I fell into my wife’s arms and told her what had happened. I was crying. “I don’t know what to do. I need to get to the hospital.”
And she said, “No, you need to go finish the race.”
So, I did. It was painful because of the cramps, but I was numb at that point. I was thinking about the woman the whole way. My time was 5 hours and 20 minutes.
As soon as I finished, I went to every police officer I could find, but nobody knew anything. Suddenly, I remembered my cousin. He had previously been the head of EMS for New York City. I called him. “Abdo, it’s Ted, you’ve got to do me a favor.”
“What?” he said. “Are you delirious from running the marathon?”
I told him what I needed. He called me back 5 minutes later and said, “Ted, what’d you do? Everybody wants to know who you are and where you are! The woman just went out again at New York Cornell. But they got her back, and they’re bringing her up to the cath lab.”
After every marathon that I run, we host a big party at our house. My family and friends and neighbors all celebrate while I’m dying on the couch. That night, my daughter told everyone the story of what happened.
But I was still not right. Still thinking about the malpractice suit.
Yes, I just did something great. But I’d recently been called the worst physician in the world. The distraction of the marathon was gone, and I was back to thinking, What am I going to do with my life? Who’s ever going to want to see me again? I’m a pariah.
Everybody said, “Ted, what happened a month ago isn’t you. What happened today was you.”
I told them to leave it alone, but my daughter and my neighbor started calling people anyway. The next day I got a call from the local newspaper. It was the same journalist who had written about me from the trial. I told him I didn’t want to talk. I was actually pretty nasty.
But my wife said, “Ted, what are you doing? That guy was trying to help you.” So, I called back and apologized.
“Dr. Strange, we knew that story wasn’t right,” he said. “We have to write this story.”
After the article came out, I started getting more calls from the media. Channel 7 News and CBS News did segments. The New York Knicks invited us to a game and presented me with a watch. It was incredible. But I was also really embarrassed by it.
People started calling me a hero. I’m not a hero. I just did what I’m supposed to do, what I’m trained to do. Shame on me if I don’t do that. Good guy and hopefully good physician, sure, but not a hero.
I also give credit to the City of New York Police Department, the FDNY, and the volunteers. Without them, I couldn’t have done what I did. It was a true team effort.
A few weeks later, the woman went home to Minnesota. She’ll never run a marathon again, but she’s still alive to this day. It turned out she had a single lesion called the “widow-maker” lesion. She was in perfect health and had just completed an ultramarathon a few months before; but she had a genetic predisposition. She still calls me every December to thank me for another Christmas.
There’s more.
One year after this whole thing, almost to the date, I got a call from my attorney. “The court just threw out the malpractice verdict,” he said. “You didn’t do anything wrong.”
I’m a man of faith. And I believe all this happened for a reason. Maybe God was sending me a message, and that’s why I heard a call for help on 59th Street in my 25th marathon among millions of people in a crowd.
I ran the marathon the next year. And when I got to that spot, I stopped and reflected. Nobody knew why I was standing there, but I knew. To this day, I could take you to that spot.
I turn 65 next July, and I plan to keep on running the race.
Dr. Strange is chair of medicine at Staten Island University Hospital, associate ambulatory physician executive of the Staten Island Region, and an internal medicine and geriatric medicine physician with Northwell Health.
A version of this article first appeared on Medscape.com.
I was running my 25th New York City Marathon. It was 2018, and I almost pulled out of running that year. I wasn’t myself, and maybe that’s an understatement.
A month earlier, I had been involved in a malpractice case. I was found liable for $10 million. My colleagues didn’t think I had done anything wrong, but the jury did. And the local newspapers made me look like a villain.
I was devastated. But my priest, my friends, and my family all told me, “You can’t quit.” So, I decided to run for them.
I started on the Verrazzano-Narrows Bridge that morning with some friends from work. I usually listen to music as I’m running, but I didn’t that year. I was just in my zone, enjoying the crowds. They’re huge. Millions of people on the streets.
I was running well. I did half the race in an hour and 57 minutes. My family always meets me at mile 17, and I was almost there. I had reached 59th Street and was about to make the turn onto First Avenue.
That’s one of the noisiest places in the marathon. There’s a kind of tunnel, and with the crowd and the throng of runners, it’s incredibly loud. But somehow, I heard somebody yell, “Help!”
Now, how I heard that, I don’t know. And if I’d been listening to music like I always do, no way I would’ve heard it. I could swear it was an angel on my shoulder that said, “Turn around, dummy. You’ve got a person that needs your help to your left.”
I turned around and about 30 feet behind me, I saw a woman waving her hands and a runner on the ground. I thought, Somebody fainted. I pushed through the crowd to get to them. The woman was crying, saying, “My friend went down to tie her shoe and she fell back. I think she’s seizing or something.”
I got down and tried to wake the other woman up. I lifted her legs up. But I quickly realized there was more to the story.
Some volunteers and police started coming toward us. The police officers looked at me like, What’s this guy doing? I explained that I was a physician, and one of them began helping me with the CPR. As we did that, someone brought a defibrillator.
Meanwhile, runners were going past, almost over us. The police officers were trying to create a barrier.
The machine gave the woman a shock, but we didn’t get a response, so we resumed CPR. At that point, my legs began to cramp so badly I couldn’t go on. So the police officer took over, and I yelled, “I need an ambu bag!” Somebody brought one, and I started giving her oxygen.
At that point, a paramedic team arrived with a bigger defibrillator. We shocked her again. And again. That time we got results, but she quickly went out again. The fourth time, we got her heart back and she started breathing on her own.
We finally got her into an ambulance. I wanted to go with them, but the woman’s friend needed to get in, so there wasn’t enough room.
And then they were gone, and I was just standing there.
A police officer put his arm around me. He said, “Doc, you’re amazing. What do you need? Where can I take you?”
I said, “Take me? My wife is waiting for me at mile 17.”
I took off and ran. When I got to my wife and kids, they were so worried. We all wear tracking devices, and they could see that I had stopped for more than 20 minutes.
I fell into my wife’s arms and told her what had happened. I was crying. “I don’t know what to do. I need to get to the hospital.”
And she said, “No, you need to go finish the race.”
So, I did. It was painful because of the cramps, but I was numb at that point. I was thinking about the woman the whole way. My time was 5 hours and 20 minutes.
As soon as I finished, I went to every police officer I could find, but nobody knew anything. Suddenly, I remembered my cousin. He had previously been the head of EMS for New York City. I called him. “Abdo, it’s Ted, you’ve got to do me a favor.”
“What?” he said. “Are you delirious from running the marathon?”
I told him what I needed. He called me back 5 minutes later and said, “Ted, what’d you do? Everybody wants to know who you are and where you are! The woman just went out again at New York Cornell. But they got her back, and they’re bringing her up to the cath lab.”
After every marathon that I run, we host a big party at our house. My family and friends and neighbors all celebrate while I’m dying on the couch. That night, my daughter told everyone the story of what happened.
But I was still not right. Still thinking about the malpractice suit.
Yes, I just did something great. But I’d recently been called the worst physician in the world. The distraction of the marathon was gone, and I was back to thinking, What am I going to do with my life? Who’s ever going to want to see me again? I’m a pariah.
Everybody said, “Ted, what happened a month ago isn’t you. What happened today was you.”
I told them to leave it alone, but my daughter and my neighbor started calling people anyway. The next day I got a call from the local newspaper. It was the same journalist who had written about me from the trial. I told him I didn’t want to talk. I was actually pretty nasty.
But my wife said, “Ted, what are you doing? That guy was trying to help you.” So, I called back and apologized.
“Dr. Strange, we knew that story wasn’t right,” he said. “We have to write this story.”
After the article came out, I started getting more calls from the media. Channel 7 News and CBS News did segments. The New York Knicks invited us to a game and presented me with a watch. It was incredible. But I was also really embarrassed by it.
People started calling me a hero. I’m not a hero. I just did what I’m supposed to do, what I’m trained to do. Shame on me if I don’t do that. Good guy and hopefully good physician, sure, but not a hero.
I also give credit to the City of New York Police Department, the FDNY, and the volunteers. Without them, I couldn’t have done what I did. It was a true team effort.
A few weeks later, the woman went home to Minnesota. She’ll never run a marathon again, but she’s still alive to this day. It turned out she had a single lesion called the “widow-maker” lesion. She was in perfect health and had just completed an ultramarathon a few months before; but she had a genetic predisposition. She still calls me every December to thank me for another Christmas.
There’s more.
One year after this whole thing, almost to the date, I got a call from my attorney. “The court just threw out the malpractice verdict,” he said. “You didn’t do anything wrong.”
I’m a man of faith. And I believe all this happened for a reason. Maybe God was sending me a message, and that’s why I heard a call for help on 59th Street in my 25th marathon among millions of people in a crowd.
I ran the marathon the next year. And when I got to that spot, I stopped and reflected. Nobody knew why I was standing there, but I knew. To this day, I could take you to that spot.
I turn 65 next July, and I plan to keep on running the race.
Dr. Strange is chair of medicine at Staten Island University Hospital, associate ambulatory physician executive of the Staten Island Region, and an internal medicine and geriatric medicine physician with Northwell Health.
A version of this article first appeared on Medscape.com.
Prescription drug affordability boards: Another quick fix with unintended consequences?
Making medications more accessible to those who need them is the focus of attention in the media and in all levels of government. For a drug to be accessible, it must be affordable and available. Something may be affordable, but if it isn’t available, no one will have access to it. Think of toilet paper in the first year of the COVID pandemic. The opposite is also true. An item may be available, but if it isn’t affordable, access is lost. While medication affordability is viewed as the major problem for patients, lack of availability has begun to creep into our drug supply chain. We are now experiencing drug shortages for medications that are very affordable. The perverse incentives, inherent in formulary construction, favor higher-priced medications, which decreases the availability of lower-priced – yet still expensive – drugs, thus increasing patient cost share. Formulary placement and patient cost share, important determinants of accessibility, are controlled by health plans and differ considerably even from the same payer. And yet, the price of drugs remains the target of most approaches to increasing patients’ access. And now price negotiations and drug affordability boards enter into the picture.
What are prescription drug affordability boards?
Both state and federal legislatures have placed the affordability of medications front and center on their agendas. However, neither are considering how formulary construction affects patient’s access to medications. The Inflation Reduction Act is Congress’s foray into price setting/negotiation of expensive drugs. Over the last few years, states are also attempting to make drugs more affordable by creating prescription drug affordability boards (PDABs). Governors (or other state leaders) appoint PDAB members who are charged with the task of evaluating the affordability of certain drugs for both the state and its residents. How to do it, and what the limitations are, vary from state to state. In 2019, Maryland was first state to establish a PDAB, charging its members to study commercial insurance and drug pricing and make recommendations on how to make drugs more affordable for Maryland residents. Other states that have passed PDAB legislation are Colorado, Maine, Minnesota, New Hampshire, Ohio, Oregon, and Washington.
Colorado, Minnesota, and Washington – and soon Maryland and Oregon – hope to make drugs more affordable for patients by allowing their PDABs to set an upper payment limit (UPL). A UPL serves as a cap on the sales price and reimbursement for a drug. The Michigan legislature is actively debating legislation that would establish a PDAB and allow it to set UPLs. On the surface, this may appear to be a potential solution to the affordability issue. However, as always, there are many questions as to how this will work and what are the unintended consequences of price setting and establishing UPLs for medications. UPLs have the potential to harm access to provider-administered drugs. With the help of advocacy from the Coalition of State Rheumatology Organizations (CSRO), Washington’s PDAB statute potentially has a carve-out for provider-administered drugs.
Possible unintended consequences for provider-administered drugs
CSRO asked for a meeting with the Colorado PDAB after they announced their list of drugs for which UPLs would be set. We spoke with the PDAB in October, hoping to point out some of the unintended consequences that needed to be considered. One of the big questions we have revolves around the “buy and bill” provider-administered drugs. According to the language of the Colorado statute, providers would not be paid any more than the UPL for a drug administered in their office. CSRO is concerned that this would leave providers uncompensated for the service of administering the drug and associated overhead. This is not to mention that providers may not be able to find a group purchasing organization that would even sell the drug at the UPL, much less a lower price than the UPL. And even if a provider could buy it at the UPL, that would mean there would be no margin to cover the overhead for their infusion suite. Interestingly, while Colorado’s rules for the UPL state that pharmacies can be paid an additional reasonable dispensing fee beyond the UPL, no such allowance is made for providers administering one of these medications. In fact, the Colorado PDAB specifically indicated that the goal of the state’s UPL methodology was to ensure that there was no “delta” between what is paid for the drug by the provider and what is reimbursed to a provider for the drug by the payer. This may cause some providers to be unable to “afford” to administer those drugs with UPLs, which ultimately reduces access for residents of Colorado to that particular medication. This is the exact opposite of what the PDAB is supposed to accomplish.
There are still many questions. What impact will UPLs have on a medication’s placement on a formulary? As we know, preferred formulary placement is often given to drugs with the highest price concession from the manufacturers. Will setting a UPL on payment for specialty pharmacy drugs to pharmacy benefit manager-owned specialty pharmacies affect that drug’s ability to be on the formulary? And again, how will the PDAB resolve the issue of compensating the provider for overhead costs associated with administering the medication?
Even more confusing questions remain. How will the UPL be enforced when a “purchase” or “sale” of the drug is made by an out-of-state entity somewhere along the supply chain? When ultimately the drug is purchased and delivered to a Colorado consumer by a Colorado provider/pharmacy, there are multiple points of the supply chain that may be outside of the jurisdiction of Colorado to enforce the UPL. This would create a misalignment in pricing among various supply chain entities.
While the sentiment behind creating PDABs is noble, it may end up having the unintended consequence of patients losing access to these drugs because of the perverse incentives involved in formulary construction or providers’ inability to afford to offer provider-administered drugs with UPLs.
Remember, expensive specialty pharmacy medications are already discounted greatly by manufacturers, often more than 50% to pharmacy benefit managers; and yet those cost savings are not passed on to the patients. Also, there is no oversight of 340B hospital contracted pharmacies to make sure that they pass those savings on to needy patients. Perhaps PDABs should address those issues, as well, if patient access to expensive medications is the goal.
Clearly, there are no easy answers. But with so many variables in the drug supply chain affecting patient access, concentrating only on one aspect may end up causing more harm than good. If your state is thinking of passing a PDAB, please let your legislators know that there are issues with this type of legislation that perhaps should be worked out before the bill is passed.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
Making medications more accessible to those who need them is the focus of attention in the media and in all levels of government. For a drug to be accessible, it must be affordable and available. Something may be affordable, but if it isn’t available, no one will have access to it. Think of toilet paper in the first year of the COVID pandemic. The opposite is also true. An item may be available, but if it isn’t affordable, access is lost. While medication affordability is viewed as the major problem for patients, lack of availability has begun to creep into our drug supply chain. We are now experiencing drug shortages for medications that are very affordable. The perverse incentives, inherent in formulary construction, favor higher-priced medications, which decreases the availability of lower-priced – yet still expensive – drugs, thus increasing patient cost share. Formulary placement and patient cost share, important determinants of accessibility, are controlled by health plans and differ considerably even from the same payer. And yet, the price of drugs remains the target of most approaches to increasing patients’ access. And now price negotiations and drug affordability boards enter into the picture.
What are prescription drug affordability boards?
Both state and federal legislatures have placed the affordability of medications front and center on their agendas. However, neither are considering how formulary construction affects patient’s access to medications. The Inflation Reduction Act is Congress’s foray into price setting/negotiation of expensive drugs. Over the last few years, states are also attempting to make drugs more affordable by creating prescription drug affordability boards (PDABs). Governors (or other state leaders) appoint PDAB members who are charged with the task of evaluating the affordability of certain drugs for both the state and its residents. How to do it, and what the limitations are, vary from state to state. In 2019, Maryland was first state to establish a PDAB, charging its members to study commercial insurance and drug pricing and make recommendations on how to make drugs more affordable for Maryland residents. Other states that have passed PDAB legislation are Colorado, Maine, Minnesota, New Hampshire, Ohio, Oregon, and Washington.
Colorado, Minnesota, and Washington – and soon Maryland and Oregon – hope to make drugs more affordable for patients by allowing their PDABs to set an upper payment limit (UPL). A UPL serves as a cap on the sales price and reimbursement for a drug. The Michigan legislature is actively debating legislation that would establish a PDAB and allow it to set UPLs. On the surface, this may appear to be a potential solution to the affordability issue. However, as always, there are many questions as to how this will work and what are the unintended consequences of price setting and establishing UPLs for medications. UPLs have the potential to harm access to provider-administered drugs. With the help of advocacy from the Coalition of State Rheumatology Organizations (CSRO), Washington’s PDAB statute potentially has a carve-out for provider-administered drugs.
Possible unintended consequences for provider-administered drugs
CSRO asked for a meeting with the Colorado PDAB after they announced their list of drugs for which UPLs would be set. We spoke with the PDAB in October, hoping to point out some of the unintended consequences that needed to be considered. One of the big questions we have revolves around the “buy and bill” provider-administered drugs. According to the language of the Colorado statute, providers would not be paid any more than the UPL for a drug administered in their office. CSRO is concerned that this would leave providers uncompensated for the service of administering the drug and associated overhead. This is not to mention that providers may not be able to find a group purchasing organization that would even sell the drug at the UPL, much less a lower price than the UPL. And even if a provider could buy it at the UPL, that would mean there would be no margin to cover the overhead for their infusion suite. Interestingly, while Colorado’s rules for the UPL state that pharmacies can be paid an additional reasonable dispensing fee beyond the UPL, no such allowance is made for providers administering one of these medications. In fact, the Colorado PDAB specifically indicated that the goal of the state’s UPL methodology was to ensure that there was no “delta” between what is paid for the drug by the provider and what is reimbursed to a provider for the drug by the payer. This may cause some providers to be unable to “afford” to administer those drugs with UPLs, which ultimately reduces access for residents of Colorado to that particular medication. This is the exact opposite of what the PDAB is supposed to accomplish.
There are still many questions. What impact will UPLs have on a medication’s placement on a formulary? As we know, preferred formulary placement is often given to drugs with the highest price concession from the manufacturers. Will setting a UPL on payment for specialty pharmacy drugs to pharmacy benefit manager-owned specialty pharmacies affect that drug’s ability to be on the formulary? And again, how will the PDAB resolve the issue of compensating the provider for overhead costs associated with administering the medication?
Even more confusing questions remain. How will the UPL be enforced when a “purchase” or “sale” of the drug is made by an out-of-state entity somewhere along the supply chain? When ultimately the drug is purchased and delivered to a Colorado consumer by a Colorado provider/pharmacy, there are multiple points of the supply chain that may be outside of the jurisdiction of Colorado to enforce the UPL. This would create a misalignment in pricing among various supply chain entities.
While the sentiment behind creating PDABs is noble, it may end up having the unintended consequence of patients losing access to these drugs because of the perverse incentives involved in formulary construction or providers’ inability to afford to offer provider-administered drugs with UPLs.
Remember, expensive specialty pharmacy medications are already discounted greatly by manufacturers, often more than 50% to pharmacy benefit managers; and yet those cost savings are not passed on to the patients. Also, there is no oversight of 340B hospital contracted pharmacies to make sure that they pass those savings on to needy patients. Perhaps PDABs should address those issues, as well, if patient access to expensive medications is the goal.
Clearly, there are no easy answers. But with so many variables in the drug supply chain affecting patient access, concentrating only on one aspect may end up causing more harm than good. If your state is thinking of passing a PDAB, please let your legislators know that there are issues with this type of legislation that perhaps should be worked out before the bill is passed.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
Making medications more accessible to those who need them is the focus of attention in the media and in all levels of government. For a drug to be accessible, it must be affordable and available. Something may be affordable, but if it isn’t available, no one will have access to it. Think of toilet paper in the first year of the COVID pandemic. The opposite is also true. An item may be available, but if it isn’t affordable, access is lost. While medication affordability is viewed as the major problem for patients, lack of availability has begun to creep into our drug supply chain. We are now experiencing drug shortages for medications that are very affordable. The perverse incentives, inherent in formulary construction, favor higher-priced medications, which decreases the availability of lower-priced – yet still expensive – drugs, thus increasing patient cost share. Formulary placement and patient cost share, important determinants of accessibility, are controlled by health plans and differ considerably even from the same payer. And yet, the price of drugs remains the target of most approaches to increasing patients’ access. And now price negotiations and drug affordability boards enter into the picture.
What are prescription drug affordability boards?
Both state and federal legislatures have placed the affordability of medications front and center on their agendas. However, neither are considering how formulary construction affects patient’s access to medications. The Inflation Reduction Act is Congress’s foray into price setting/negotiation of expensive drugs. Over the last few years, states are also attempting to make drugs more affordable by creating prescription drug affordability boards (PDABs). Governors (or other state leaders) appoint PDAB members who are charged with the task of evaluating the affordability of certain drugs for both the state and its residents. How to do it, and what the limitations are, vary from state to state. In 2019, Maryland was first state to establish a PDAB, charging its members to study commercial insurance and drug pricing and make recommendations on how to make drugs more affordable for Maryland residents. Other states that have passed PDAB legislation are Colorado, Maine, Minnesota, New Hampshire, Ohio, Oregon, and Washington.
Colorado, Minnesota, and Washington – and soon Maryland and Oregon – hope to make drugs more affordable for patients by allowing their PDABs to set an upper payment limit (UPL). A UPL serves as a cap on the sales price and reimbursement for a drug. The Michigan legislature is actively debating legislation that would establish a PDAB and allow it to set UPLs. On the surface, this may appear to be a potential solution to the affordability issue. However, as always, there are many questions as to how this will work and what are the unintended consequences of price setting and establishing UPLs for medications. UPLs have the potential to harm access to provider-administered drugs. With the help of advocacy from the Coalition of State Rheumatology Organizations (CSRO), Washington’s PDAB statute potentially has a carve-out for provider-administered drugs.
Possible unintended consequences for provider-administered drugs
CSRO asked for a meeting with the Colorado PDAB after they announced their list of drugs for which UPLs would be set. We spoke with the PDAB in October, hoping to point out some of the unintended consequences that needed to be considered. One of the big questions we have revolves around the “buy and bill” provider-administered drugs. According to the language of the Colorado statute, providers would not be paid any more than the UPL for a drug administered in their office. CSRO is concerned that this would leave providers uncompensated for the service of administering the drug and associated overhead. This is not to mention that providers may not be able to find a group purchasing organization that would even sell the drug at the UPL, much less a lower price than the UPL. And even if a provider could buy it at the UPL, that would mean there would be no margin to cover the overhead for their infusion suite. Interestingly, while Colorado’s rules for the UPL state that pharmacies can be paid an additional reasonable dispensing fee beyond the UPL, no such allowance is made for providers administering one of these medications. In fact, the Colorado PDAB specifically indicated that the goal of the state’s UPL methodology was to ensure that there was no “delta” between what is paid for the drug by the provider and what is reimbursed to a provider for the drug by the payer. This may cause some providers to be unable to “afford” to administer those drugs with UPLs, which ultimately reduces access for residents of Colorado to that particular medication. This is the exact opposite of what the PDAB is supposed to accomplish.
There are still many questions. What impact will UPLs have on a medication’s placement on a formulary? As we know, preferred formulary placement is often given to drugs with the highest price concession from the manufacturers. Will setting a UPL on payment for specialty pharmacy drugs to pharmacy benefit manager-owned specialty pharmacies affect that drug’s ability to be on the formulary? And again, how will the PDAB resolve the issue of compensating the provider for overhead costs associated with administering the medication?
Even more confusing questions remain. How will the UPL be enforced when a “purchase” or “sale” of the drug is made by an out-of-state entity somewhere along the supply chain? When ultimately the drug is purchased and delivered to a Colorado consumer by a Colorado provider/pharmacy, there are multiple points of the supply chain that may be outside of the jurisdiction of Colorado to enforce the UPL. This would create a misalignment in pricing among various supply chain entities.
While the sentiment behind creating PDABs is noble, it may end up having the unintended consequence of patients losing access to these drugs because of the perverse incentives involved in formulary construction or providers’ inability to afford to offer provider-administered drugs with UPLs.
Remember, expensive specialty pharmacy medications are already discounted greatly by manufacturers, often more than 50% to pharmacy benefit managers; and yet those cost savings are not passed on to the patients. Also, there is no oversight of 340B hospital contracted pharmacies to make sure that they pass those savings on to needy patients. Perhaps PDABs should address those issues, as well, if patient access to expensive medications is the goal.
Clearly, there are no easy answers. But with so many variables in the drug supply chain affecting patient access, concentrating only on one aspect may end up causing more harm than good. If your state is thinking of passing a PDAB, please let your legislators know that there are issues with this type of legislation that perhaps should be worked out before the bill is passed.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
Life in the woods
“I went to the woods because I wished to live deliberately, to front only the essential facts of life, and see if I could not learn what it had to teach.” – Henry David Thoreau
I have many patients like Maxine. Tall, with a shock of white hair. Old, but still in charge. When you try to make eye contact, she looks right through you. First with her left eye. Then her right. Her face is inscrutable. What’s she thinking? Unlike many of my patients, however, this Maxine was a llama. Every morning my daughter and I tried to coax her into moving as we leaned on the cold steel gate that kept her in her pasture. We were visiting family in October and chose to stay on a working New England farm. The kids will love the animals, we thought, and we’ll appreciate the extra bedrooms.
Airbnb helped us find this charming fiber-farm in Rhode Island where they raise Leicester Longwool sheep, a historic breed that once roamed George Washington’s pastures, along with a few goats, ducks, chickens, and Maxine. It’s situated deep in the woods, which were yellow, orange, and red that week. As it happens, we were just a short drive due south of Walden Pond where Henry David Thoreau spent 2 years, 2 months and 2 days escaping “overcivilization” nearly 175 years ago. Hoisting our overweight bags over the uneven granite stone steps when we arrived, I realized this was going to be more like the Thoreau experiment than I intended. The farmhouse dated to the 1790s. There were wide, creaky floorboards, low ceilings, one staircase to the bedrooms (which could have aptly been called a ladder) and loads of book-laden shelves. Instructions posted in the kitchen warned that the heat is tricky to regulate – a redundant admonition as we watched our 3-year-old putting on her socks and shoes as she got into bed.
Now, if you’ve ever been on vacation with little kids, you know that it’s basically just childcare in a novel location. After barricading the staircase with luggage and unplugging lamps from their dicey outlets we set out to feed the chickens and try to pet a sheep. Walking the perimeter of the farm we saw stone walls that needed mending and stumbled across two ancient cemeteries, one had been for family, the other for slaves. I wondered how many farmers and weavers and menders had walked this trail with their kids over the generations.
The next morning, we learned that roosters do not in fact crow at dawn, they crow before dawn (which could also aptly be called nighttime). There were no commutes or late patients here. But there was work to be done. Chickens don’t care that it’s Sunday. It downpoured. Watching the sheep from the kitchen as I sipped my coffee, they didn’t seem to mind. Nor did our farmer hosts who trudged past them in tall boots, just as they had every other day of their farmer lives.
By the fifth day, we had fallen into the rhythms of the homestead. We cracked the blue, green, and brown eggs that our hosts placed outside our door in the early hours and made omelets that were as orange as the foliage. We finally learned to adjust the heat so we neither got chilblains nor had to open the windows and strip naked to cool down. The sky was a brilliant blue that last morning and Sloan ran around trying to catch leaves as they blew off the trees. She had no objective. No counting. No contest. Just chasing leaves as they fell. It was the ultimate atelic activity, done just for doing it. I joined her and found I was no better at this than a 3-year-old.
We might all benefit from a little time in the woods.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
“I went to the woods because I wished to live deliberately, to front only the essential facts of life, and see if I could not learn what it had to teach.” – Henry David Thoreau
I have many patients like Maxine. Tall, with a shock of white hair. Old, but still in charge. When you try to make eye contact, she looks right through you. First with her left eye. Then her right. Her face is inscrutable. What’s she thinking? Unlike many of my patients, however, this Maxine was a llama. Every morning my daughter and I tried to coax her into moving as we leaned on the cold steel gate that kept her in her pasture. We were visiting family in October and chose to stay on a working New England farm. The kids will love the animals, we thought, and we’ll appreciate the extra bedrooms.
Airbnb helped us find this charming fiber-farm in Rhode Island where they raise Leicester Longwool sheep, a historic breed that once roamed George Washington’s pastures, along with a few goats, ducks, chickens, and Maxine. It’s situated deep in the woods, which were yellow, orange, and red that week. As it happens, we were just a short drive due south of Walden Pond where Henry David Thoreau spent 2 years, 2 months and 2 days escaping “overcivilization” nearly 175 years ago. Hoisting our overweight bags over the uneven granite stone steps when we arrived, I realized this was going to be more like the Thoreau experiment than I intended. The farmhouse dated to the 1790s. There were wide, creaky floorboards, low ceilings, one staircase to the bedrooms (which could have aptly been called a ladder) and loads of book-laden shelves. Instructions posted in the kitchen warned that the heat is tricky to regulate – a redundant admonition as we watched our 3-year-old putting on her socks and shoes as she got into bed.
Now, if you’ve ever been on vacation with little kids, you know that it’s basically just childcare in a novel location. After barricading the staircase with luggage and unplugging lamps from their dicey outlets we set out to feed the chickens and try to pet a sheep. Walking the perimeter of the farm we saw stone walls that needed mending and stumbled across two ancient cemeteries, one had been for family, the other for slaves. I wondered how many farmers and weavers and menders had walked this trail with their kids over the generations.
The next morning, we learned that roosters do not in fact crow at dawn, they crow before dawn (which could also aptly be called nighttime). There were no commutes or late patients here. But there was work to be done. Chickens don’t care that it’s Sunday. It downpoured. Watching the sheep from the kitchen as I sipped my coffee, they didn’t seem to mind. Nor did our farmer hosts who trudged past them in tall boots, just as they had every other day of their farmer lives.
By the fifth day, we had fallen into the rhythms of the homestead. We cracked the blue, green, and brown eggs that our hosts placed outside our door in the early hours and made omelets that were as orange as the foliage. We finally learned to adjust the heat so we neither got chilblains nor had to open the windows and strip naked to cool down. The sky was a brilliant blue that last morning and Sloan ran around trying to catch leaves as they blew off the trees. She had no objective. No counting. No contest. Just chasing leaves as they fell. It was the ultimate atelic activity, done just for doing it. I joined her and found I was no better at this than a 3-year-old.
We might all benefit from a little time in the woods.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
“I went to the woods because I wished to live deliberately, to front only the essential facts of life, and see if I could not learn what it had to teach.” – Henry David Thoreau
I have many patients like Maxine. Tall, with a shock of white hair. Old, but still in charge. When you try to make eye contact, she looks right through you. First with her left eye. Then her right. Her face is inscrutable. What’s she thinking? Unlike many of my patients, however, this Maxine was a llama. Every morning my daughter and I tried to coax her into moving as we leaned on the cold steel gate that kept her in her pasture. We were visiting family in October and chose to stay on a working New England farm. The kids will love the animals, we thought, and we’ll appreciate the extra bedrooms.
Airbnb helped us find this charming fiber-farm in Rhode Island where they raise Leicester Longwool sheep, a historic breed that once roamed George Washington’s pastures, along with a few goats, ducks, chickens, and Maxine. It’s situated deep in the woods, which were yellow, orange, and red that week. As it happens, we were just a short drive due south of Walden Pond where Henry David Thoreau spent 2 years, 2 months and 2 days escaping “overcivilization” nearly 175 years ago. Hoisting our overweight bags over the uneven granite stone steps when we arrived, I realized this was going to be more like the Thoreau experiment than I intended. The farmhouse dated to the 1790s. There were wide, creaky floorboards, low ceilings, one staircase to the bedrooms (which could have aptly been called a ladder) and loads of book-laden shelves. Instructions posted in the kitchen warned that the heat is tricky to regulate – a redundant admonition as we watched our 3-year-old putting on her socks and shoes as she got into bed.
Now, if you’ve ever been on vacation with little kids, you know that it’s basically just childcare in a novel location. After barricading the staircase with luggage and unplugging lamps from their dicey outlets we set out to feed the chickens and try to pet a sheep. Walking the perimeter of the farm we saw stone walls that needed mending and stumbled across two ancient cemeteries, one had been for family, the other for slaves. I wondered how many farmers and weavers and menders had walked this trail with their kids over the generations.
The next morning, we learned that roosters do not in fact crow at dawn, they crow before dawn (which could also aptly be called nighttime). There were no commutes or late patients here. But there was work to be done. Chickens don’t care that it’s Sunday. It downpoured. Watching the sheep from the kitchen as I sipped my coffee, they didn’t seem to mind. Nor did our farmer hosts who trudged past them in tall boots, just as they had every other day of their farmer lives.
By the fifth day, we had fallen into the rhythms of the homestead. We cracked the blue, green, and brown eggs that our hosts placed outside our door in the early hours and made omelets that were as orange as the foliage. We finally learned to adjust the heat so we neither got chilblains nor had to open the windows and strip naked to cool down. The sky was a brilliant blue that last morning and Sloan ran around trying to catch leaves as they blew off the trees. She had no objective. No counting. No contest. Just chasing leaves as they fell. It was the ultimate atelic activity, done just for doing it. I joined her and found I was no better at this than a 3-year-old.
We might all benefit from a little time in the woods.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
How to develop a patient referral program
Here is how old I am: When I graduated from medical school in 1977, marketing was prohibited. It was the legal profession that challenged the ban on advertising by professionals, leading to a landmark Supreme Court decision (Bates v State Bar of Arizona, 1977), which opened the door to marketing in the legal and medical professions.
Since then, Strategies range from the basic Internet website through postings on the major social media sites, and occasionally to larger-budget campaigns involving local radio, television, or billboard advertising.
All these methods are effective, to varying degrees; but nothing provides as much benefit – relative to its comparatively low cost – as the original marketing tool, word-of-mouth patient referrals. According to one survey, a clear majority of Americans still consider word-of-mouth recommendations to be the most influential element driving purchase decisions. Of course, some of your new patients already come from such referrals; but you can get a lot more by actively encouraging your existing patients to sing your praises, rather than waiting for them to do it on their own.
Soliciting current patients for referrals does take a little planning, structure, and a basic understanding of exactly how patient referral programs work. When executed correctly, a patient referral program can add substantial growth to your practice at minimal cost.
Your first step, as with any new project, should be to identify your goals: Clearly define what kind of patients you are looking to attract. Do you want more patients for cosmetic procedures, medical treatment, skin cancer screenings, a specific diagnosis (such as psoriasis), or a general mix? Design your announcements, brochures, and other literature (more on that in a minute) with those goals in mind.
Next, identify any applicable federal or state laws that dictate what you can and cannot legally do to encourage such referrals. It might be tempting, for example, to offer discounts on future services for successful referrals; but some medical groups frown on it, some states prohibit it, and the Federal Anti-Kickback Statute makes it illegal to pay anyone to refer Medicare or Medicaid patients to you if you file a claim for your services. In my experience, most patients are happy to recommend someone whom they believe provides excellent care to a friend or relative without any sort of monetary incentive; but if you plan to offer a material reward of any kind, run it by your attorney first.
Once your legal ducks are in order, make patients aware that you are accepting new patients and would welcome referrals by posting notices to that effect around your office and on your website and social media pages. Outline exactly what sort of patients (based on your goals, above) you are looking for, how to refer someone, whom to contact, and what kind of information is needed. Make it clear why existing patients should refer someone to your practice. Remind them of your specialized training, advanced technology, and patient-focused approach to health care. Highlight the benefits of the program and encourage your patients to participate.
Before implementation, you will need to educate your employees about the referral program and its benefits. All staff members should understand the program and be able to answer basic questions about it from patients or referring professionals. Encourage staffers to actively promote the program during patient interactions.
Then, start making some decisions. How, specifically, will you be requesting referrals in the office? Many physicians are not comfortable asking patients themselves. If you are going to let your assistants or receptionists do it, you will need to write a script for them to follow. An example of a basic script might be, “If you are happy with the care you are receiving here, we would love for you to tell your friends and family about us.” Your staff can then hand out cards, brochures, or both to reinforce the message, and perhaps send a follow-up email to remind them.
A referral system isn’t worth the effort if you don’t know whether it is working. Establish a system to track and monitor referrals. This could be as simple as a spreadsheet or purchasing a more sophisticated software program. Ensure that you can accurately identify and credit the referring patients for their referrals.
Make sure to thank referring patients with a thank-you note or email. Expressing gratitude will encourage continued participation in the program.
A successful referral program does not happen overnight. It relies on providing exceptional patient care and building strong relationships with your existing patients. By implementing such a program, you can leverage the satisfaction and loyalty of your patients to attract new patients and grow your private practice.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Here is how old I am: When I graduated from medical school in 1977, marketing was prohibited. It was the legal profession that challenged the ban on advertising by professionals, leading to a landmark Supreme Court decision (Bates v State Bar of Arizona, 1977), which opened the door to marketing in the legal and medical professions.
Since then, Strategies range from the basic Internet website through postings on the major social media sites, and occasionally to larger-budget campaigns involving local radio, television, or billboard advertising.
All these methods are effective, to varying degrees; but nothing provides as much benefit – relative to its comparatively low cost – as the original marketing tool, word-of-mouth patient referrals. According to one survey, a clear majority of Americans still consider word-of-mouth recommendations to be the most influential element driving purchase decisions. Of course, some of your new patients already come from such referrals; but you can get a lot more by actively encouraging your existing patients to sing your praises, rather than waiting for them to do it on their own.
Soliciting current patients for referrals does take a little planning, structure, and a basic understanding of exactly how patient referral programs work. When executed correctly, a patient referral program can add substantial growth to your practice at minimal cost.
Your first step, as with any new project, should be to identify your goals: Clearly define what kind of patients you are looking to attract. Do you want more patients for cosmetic procedures, medical treatment, skin cancer screenings, a specific diagnosis (such as psoriasis), or a general mix? Design your announcements, brochures, and other literature (more on that in a minute) with those goals in mind.
Next, identify any applicable federal or state laws that dictate what you can and cannot legally do to encourage such referrals. It might be tempting, for example, to offer discounts on future services for successful referrals; but some medical groups frown on it, some states prohibit it, and the Federal Anti-Kickback Statute makes it illegal to pay anyone to refer Medicare or Medicaid patients to you if you file a claim for your services. In my experience, most patients are happy to recommend someone whom they believe provides excellent care to a friend or relative without any sort of monetary incentive; but if you plan to offer a material reward of any kind, run it by your attorney first.
Once your legal ducks are in order, make patients aware that you are accepting new patients and would welcome referrals by posting notices to that effect around your office and on your website and social media pages. Outline exactly what sort of patients (based on your goals, above) you are looking for, how to refer someone, whom to contact, and what kind of information is needed. Make it clear why existing patients should refer someone to your practice. Remind them of your specialized training, advanced technology, and patient-focused approach to health care. Highlight the benefits of the program and encourage your patients to participate.
Before implementation, you will need to educate your employees about the referral program and its benefits. All staff members should understand the program and be able to answer basic questions about it from patients or referring professionals. Encourage staffers to actively promote the program during patient interactions.
Then, start making some decisions. How, specifically, will you be requesting referrals in the office? Many physicians are not comfortable asking patients themselves. If you are going to let your assistants or receptionists do it, you will need to write a script for them to follow. An example of a basic script might be, “If you are happy with the care you are receiving here, we would love for you to tell your friends and family about us.” Your staff can then hand out cards, brochures, or both to reinforce the message, and perhaps send a follow-up email to remind them.
A referral system isn’t worth the effort if you don’t know whether it is working. Establish a system to track and monitor referrals. This could be as simple as a spreadsheet or purchasing a more sophisticated software program. Ensure that you can accurately identify and credit the referring patients for their referrals.
Make sure to thank referring patients with a thank-you note or email. Expressing gratitude will encourage continued participation in the program.
A successful referral program does not happen overnight. It relies on providing exceptional patient care and building strong relationships with your existing patients. By implementing such a program, you can leverage the satisfaction and loyalty of your patients to attract new patients and grow your private practice.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Here is how old I am: When I graduated from medical school in 1977, marketing was prohibited. It was the legal profession that challenged the ban on advertising by professionals, leading to a landmark Supreme Court decision (Bates v State Bar of Arizona, 1977), which opened the door to marketing in the legal and medical professions.
Since then, Strategies range from the basic Internet website through postings on the major social media sites, and occasionally to larger-budget campaigns involving local radio, television, or billboard advertising.
All these methods are effective, to varying degrees; but nothing provides as much benefit – relative to its comparatively low cost – as the original marketing tool, word-of-mouth patient referrals. According to one survey, a clear majority of Americans still consider word-of-mouth recommendations to be the most influential element driving purchase decisions. Of course, some of your new patients already come from such referrals; but you can get a lot more by actively encouraging your existing patients to sing your praises, rather than waiting for them to do it on their own.
Soliciting current patients for referrals does take a little planning, structure, and a basic understanding of exactly how patient referral programs work. When executed correctly, a patient referral program can add substantial growth to your practice at minimal cost.
Your first step, as with any new project, should be to identify your goals: Clearly define what kind of patients you are looking to attract. Do you want more patients for cosmetic procedures, medical treatment, skin cancer screenings, a specific diagnosis (such as psoriasis), or a general mix? Design your announcements, brochures, and other literature (more on that in a minute) with those goals in mind.
Next, identify any applicable federal or state laws that dictate what you can and cannot legally do to encourage such referrals. It might be tempting, for example, to offer discounts on future services for successful referrals; but some medical groups frown on it, some states prohibit it, and the Federal Anti-Kickback Statute makes it illegal to pay anyone to refer Medicare or Medicaid patients to you if you file a claim for your services. In my experience, most patients are happy to recommend someone whom they believe provides excellent care to a friend or relative without any sort of monetary incentive; but if you plan to offer a material reward of any kind, run it by your attorney first.
Once your legal ducks are in order, make patients aware that you are accepting new patients and would welcome referrals by posting notices to that effect around your office and on your website and social media pages. Outline exactly what sort of patients (based on your goals, above) you are looking for, how to refer someone, whom to contact, and what kind of information is needed. Make it clear why existing patients should refer someone to your practice. Remind them of your specialized training, advanced technology, and patient-focused approach to health care. Highlight the benefits of the program and encourage your patients to participate.
Before implementation, you will need to educate your employees about the referral program and its benefits. All staff members should understand the program and be able to answer basic questions about it from patients or referring professionals. Encourage staffers to actively promote the program during patient interactions.
Then, start making some decisions. How, specifically, will you be requesting referrals in the office? Many physicians are not comfortable asking patients themselves. If you are going to let your assistants or receptionists do it, you will need to write a script for them to follow. An example of a basic script might be, “If you are happy with the care you are receiving here, we would love for you to tell your friends and family about us.” Your staff can then hand out cards, brochures, or both to reinforce the message, and perhaps send a follow-up email to remind them.
A referral system isn’t worth the effort if you don’t know whether it is working. Establish a system to track and monitor referrals. This could be as simple as a spreadsheet or purchasing a more sophisticated software program. Ensure that you can accurately identify and credit the referring patients for their referrals.
Make sure to thank referring patients with a thank-you note or email. Expressing gratitude will encourage continued participation in the program.
A successful referral program does not happen overnight. It relies on providing exceptional patient care and building strong relationships with your existing patients. By implementing such a program, you can leverage the satisfaction and loyalty of your patients to attract new patients and grow your private practice.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
The future of medicine is RNA
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
Every once in a while, medicine changes in a fundamental way, and we may not realize it while it’s happening. I wasn’t around in 1928 when Fleming discovered penicillin; or in 1953 when Watson, Crick, and Franklin characterized the double-helical structure of DNA.
But looking at medicine today, there are essentially two places where I think we will see, in retrospect, that we were at a fundamental turning point. One is artificial intelligence, which gets so much attention and hype that I will simply say yes, this will change things, stay tuned.
The other is a bit more obscure, but I suspect it may be just as impactful. That other thing is
I want to start with the idea that many diseases are, fundamentally, a problem of proteins. In some cases, like hypercholesterolemia, the body produces too much protein; in others, like hemophilia, too little.
When you think about disease this way, you realize that our current medications take effect late in the disease game. We have these molecules that try to block a protein from its receptor, prevent a protein from cleaving another protein, or increase the rate that a protein is broken down. It’s all distal to the fundamental problem: the production of the bad protein in the first place.
Enter small inhibitory RNAs, or siRNAs for short, discovered in 1998 by Andrew Fire and Craig Mello at UMass Worcester. The two won the Nobel prize in medicine just 8 years later; that’s a really short time, highlighting just how important this discovery was. In contrast, Karikó and Weissman won the Nobel for mRNA vaccines this year, after inventing them 18 years ago.
siRNAs are the body’s way of targeting proteins for destruction before they are ever created. About 20 base pairs long, siRNAs seek out a complementary target mRNA, attach to it, and call in a group of proteins to destroy it. With the target mRNA gone, no protein can be created.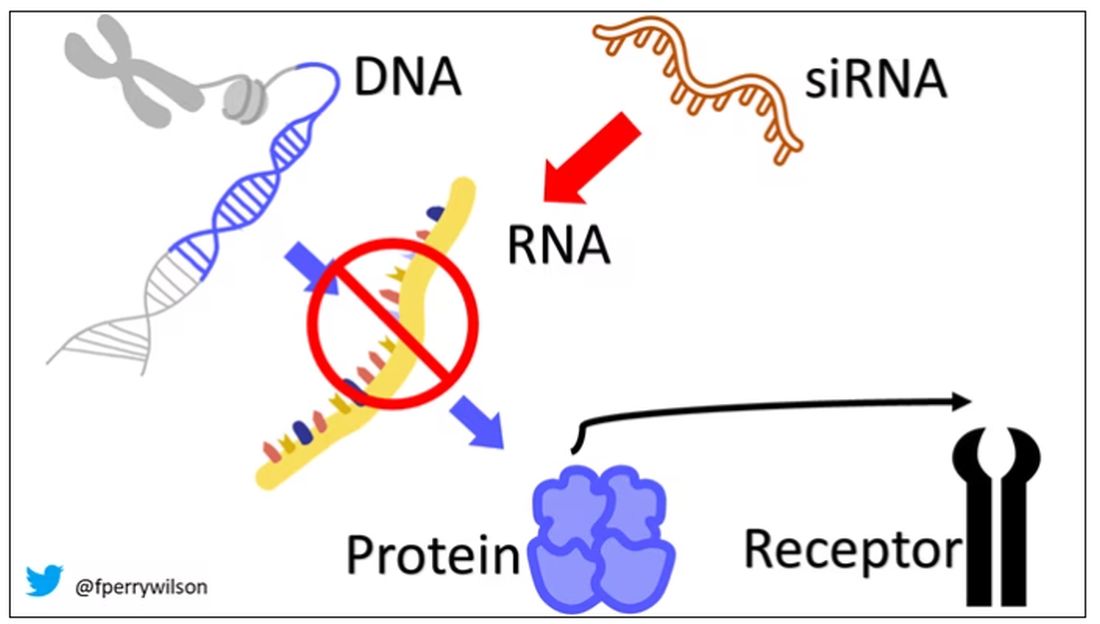
You see where this is going, right? How does high cholesterol kill you? Proteins. How does Staphylococcus aureus kill you? Proteins. Even viruses can’t replicate if their RNA is prevented from being turned into proteins.
So, how do we use siRNAs? A new paper appearing in JAMA describes a fairly impressive use case.
The background here is that higher levels of lipoprotein(a), an LDL-like protein, are associated with cardiovascular disease, heart attack, and stroke. But unfortunately, statins really don’t have any effect on lipoprotein(a) levels. Neither does diet. Your lipoprotein(a) level seems to be more or less hard-coded genetically.
So, what if we stop the genetic machinery from working? Enter lepodisiran, a drug from Eli Lilly. Unlike so many other medications, which are usually found in nature, purified, and synthesized, lepodisiran was created from scratch. It’s not hard. Thanks to the Human Genome Project, we know the genetic code for lipoprotein(a), so inventing an siRNA to target it specifically is trivial. That’s one of the key features of siRNA – you don’t have to find a chemical that binds strongly to some protein receptor, and worry about the off-target effects and all that nonsense. You just pick a protein you want to suppress and you suppress it.
Okay, it’s not that simple. siRNA is broken down very quickly by the body, so it needs to be targeted to the organ of interest – in this case, the liver, since that is where lipoprotein(a) is synthesized. Lepodisiran is targeted to the liver by this special targeting label here.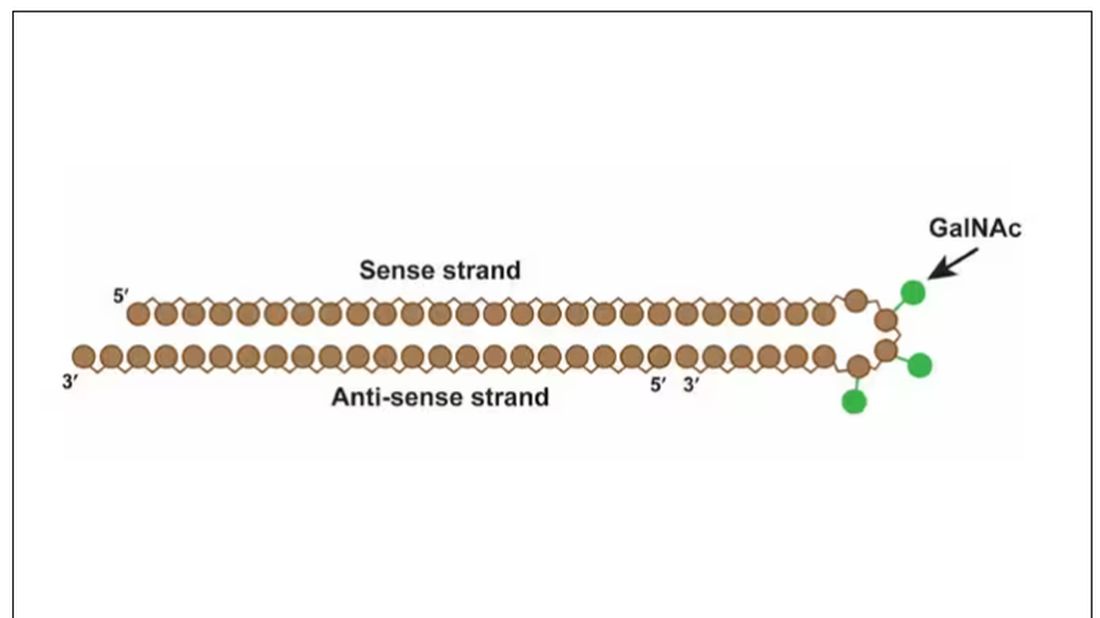
The report is a standard dose-escalation trial. Six patients, all with elevated lipoprotein(a) levels, were started with a 4-mg dose (two additional individuals got placebo). They were intensely monitored, spending 3 days in a research unit for multiple blood draws followed by weekly, and then biweekly outpatient visits. Once they had done well, the next group of six people received a higher dose (two more got placebo), and the process was repeated – six times total – until the highest dose, 608 mg, was reached.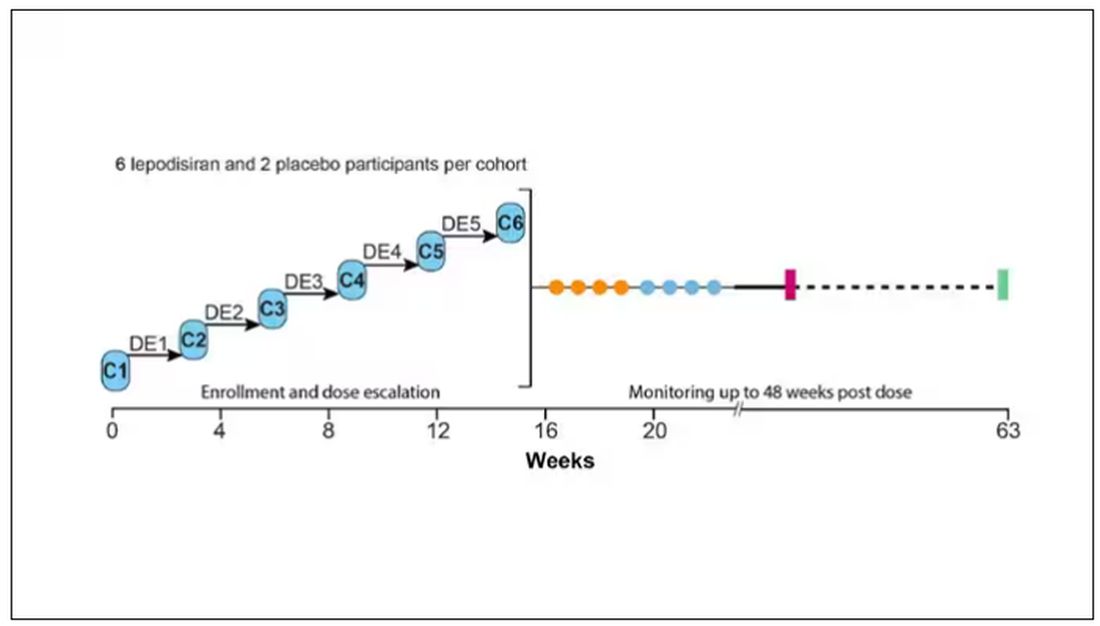
This is an injection, of course; siRNA wouldn’t withstand the harshness of the digestive system. And it’s only one injection. You can see from the blood concentration curves that within about 48 hours, circulating lepodisiran was not detectable.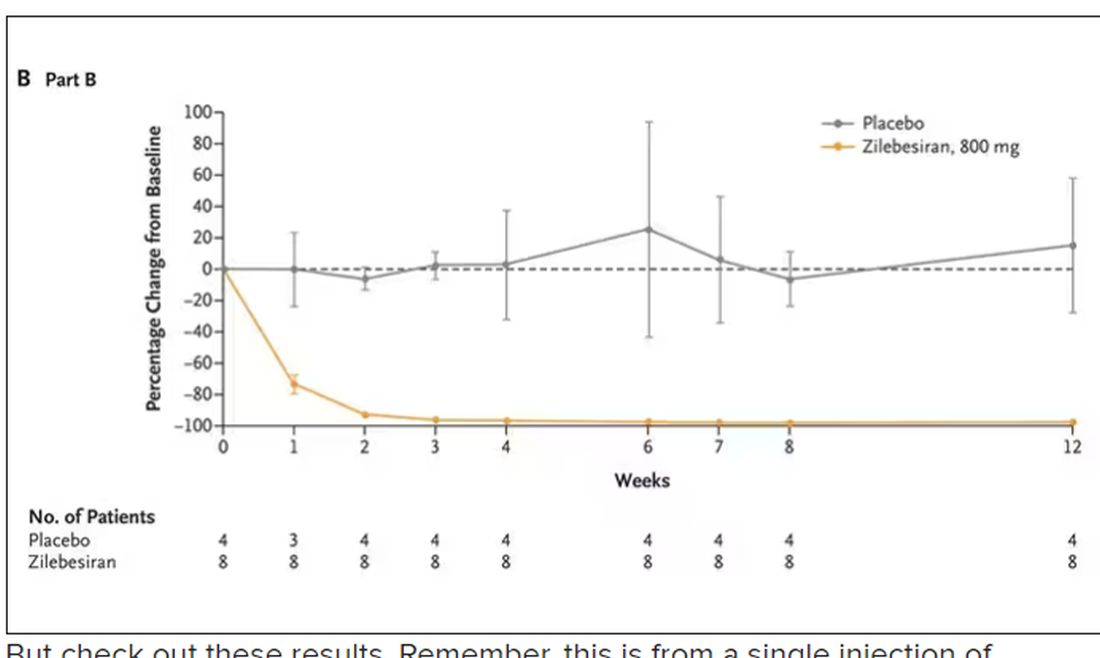
But check out these results. Remember, this is from a single injection of lepodisiran.
Lipoprotein(a) levels start to drop within a week of administration, and they stay down. In the higher-dose groups, levels are nearly undetectable a year after that injection.
It was this graph that made me sit back and think that there might be something new under the sun. A single injection that can suppress protein synthesis for an entire year? If it really works, it changes the game.
Of course, this study wasn’t powered to look at important outcomes like heart attacks and strokes. It was primarily designed to assess safety, and the drug was pretty well tolerated, with similar rates of adverse events in the drug and placebo groups.
As crazy as it sounds, the real concern here might be that this drug is too good; is it safe to drop your lipoprotein(a) levels to zero for a year? I don’t know. But lower doses don’t have quite as strong an effect.
Trust me, these drugs are going to change things. They already are. In July, The New England Journal of Medicine published a study of zilebesiran, an siRNA that inhibits the production of angiotensinogen, to control blood pressure. Similar story: One injection led to a basically complete suppression of angiotensinogen and a sustained decrease in blood pressure.
I’m not exaggerating when I say that there may come a time when you go to your doctor once a year, get your RNA shots, and don’t have to take any other medication from that point on. And that time may be, like, 5 years from now. It’s wild.
Seems to me that that rapid Nobel Prize was very well deserved.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships. This transcript has been edited for clarity.
A version of this article appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
Every once in a while, medicine changes in a fundamental way, and we may not realize it while it’s happening. I wasn’t around in 1928 when Fleming discovered penicillin; or in 1953 when Watson, Crick, and Franklin characterized the double-helical structure of DNA.
But looking at medicine today, there are essentially two places where I think we will see, in retrospect, that we were at a fundamental turning point. One is artificial intelligence, which gets so much attention and hype that I will simply say yes, this will change things, stay tuned.
The other is a bit more obscure, but I suspect it may be just as impactful. That other thing is
I want to start with the idea that many diseases are, fundamentally, a problem of proteins. In some cases, like hypercholesterolemia, the body produces too much protein; in others, like hemophilia, too little.
When you think about disease this way, you realize that our current medications take effect late in the disease game. We have these molecules that try to block a protein from its receptor, prevent a protein from cleaving another protein, or increase the rate that a protein is broken down. It’s all distal to the fundamental problem: the production of the bad protein in the first place.
Enter small inhibitory RNAs, or siRNAs for short, discovered in 1998 by Andrew Fire and Craig Mello at UMass Worcester. The two won the Nobel prize in medicine just 8 years later; that’s a really short time, highlighting just how important this discovery was. In contrast, Karikó and Weissman won the Nobel for mRNA vaccines this year, after inventing them 18 years ago.
siRNAs are the body’s way of targeting proteins for destruction before they are ever created. About 20 base pairs long, siRNAs seek out a complementary target mRNA, attach to it, and call in a group of proteins to destroy it. With the target mRNA gone, no protein can be created.
You see where this is going, right? How does high cholesterol kill you? Proteins. How does Staphylococcus aureus kill you? Proteins. Even viruses can’t replicate if their RNA is prevented from being turned into proteins.
So, how do we use siRNAs? A new paper appearing in JAMA describes a fairly impressive use case.
The background here is that higher levels of lipoprotein(a), an LDL-like protein, are associated with cardiovascular disease, heart attack, and stroke. But unfortunately, statins really don’t have any effect on lipoprotein(a) levels. Neither does diet. Your lipoprotein(a) level seems to be more or less hard-coded genetically.
So, what if we stop the genetic machinery from working? Enter lepodisiran, a drug from Eli Lilly. Unlike so many other medications, which are usually found in nature, purified, and synthesized, lepodisiran was created from scratch. It’s not hard. Thanks to the Human Genome Project, we know the genetic code for lipoprotein(a), so inventing an siRNA to target it specifically is trivial. That’s one of the key features of siRNA – you don’t have to find a chemical that binds strongly to some protein receptor, and worry about the off-target effects and all that nonsense. You just pick a protein you want to suppress and you suppress it.
Okay, it’s not that simple. siRNA is broken down very quickly by the body, so it needs to be targeted to the organ of interest – in this case, the liver, since that is where lipoprotein(a) is synthesized. Lepodisiran is targeted to the liver by this special targeting label here.
The report is a standard dose-escalation trial. Six patients, all with elevated lipoprotein(a) levels, were started with a 4-mg dose (two additional individuals got placebo). They were intensely monitored, spending 3 days in a research unit for multiple blood draws followed by weekly, and then biweekly outpatient visits. Once they had done well, the next group of six people received a higher dose (two more got placebo), and the process was repeated – six times total – until the highest dose, 608 mg, was reached.
This is an injection, of course; siRNA wouldn’t withstand the harshness of the digestive system. And it’s only one injection. You can see from the blood concentration curves that within about 48 hours, circulating lepodisiran was not detectable.
But check out these results. Remember, this is from a single injection of lepodisiran.
Lipoprotein(a) levels start to drop within a week of administration, and they stay down. In the higher-dose groups, levels are nearly undetectable a year after that injection.
It was this graph that made me sit back and think that there might be something new under the sun. A single injection that can suppress protein synthesis for an entire year? If it really works, it changes the game.
Of course, this study wasn’t powered to look at important outcomes like heart attacks and strokes. It was primarily designed to assess safety, and the drug was pretty well tolerated, with similar rates of adverse events in the drug and placebo groups.
As crazy as it sounds, the real concern here might be that this drug is too good; is it safe to drop your lipoprotein(a) levels to zero for a year? I don’t know. But lower doses don’t have quite as strong an effect.
Trust me, these drugs are going to change things. They already are. In July, The New England Journal of Medicine published a study of zilebesiran, an siRNA that inhibits the production of angiotensinogen, to control blood pressure. Similar story: One injection led to a basically complete suppression of angiotensinogen and a sustained decrease in blood pressure.
I’m not exaggerating when I say that there may come a time when you go to your doctor once a year, get your RNA shots, and don’t have to take any other medication from that point on. And that time may be, like, 5 years from now. It’s wild.
Seems to me that that rapid Nobel Prize was very well deserved.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships. This transcript has been edited for clarity.
A version of this article appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
Every once in a while, medicine changes in a fundamental way, and we may not realize it while it’s happening. I wasn’t around in 1928 when Fleming discovered penicillin; or in 1953 when Watson, Crick, and Franklin characterized the double-helical structure of DNA.
But looking at medicine today, there are essentially two places where I think we will see, in retrospect, that we were at a fundamental turning point. One is artificial intelligence, which gets so much attention and hype that I will simply say yes, this will change things, stay tuned.
The other is a bit more obscure, but I suspect it may be just as impactful. That other thing is
I want to start with the idea that many diseases are, fundamentally, a problem of proteins. In some cases, like hypercholesterolemia, the body produces too much protein; in others, like hemophilia, too little.
When you think about disease this way, you realize that our current medications take effect late in the disease game. We have these molecules that try to block a protein from its receptor, prevent a protein from cleaving another protein, or increase the rate that a protein is broken down. It’s all distal to the fundamental problem: the production of the bad protein in the first place.
Enter small inhibitory RNAs, or siRNAs for short, discovered in 1998 by Andrew Fire and Craig Mello at UMass Worcester. The two won the Nobel prize in medicine just 8 years later; that’s a really short time, highlighting just how important this discovery was. In contrast, Karikó and Weissman won the Nobel for mRNA vaccines this year, after inventing them 18 years ago.
siRNAs are the body’s way of targeting proteins for destruction before they are ever created. About 20 base pairs long, siRNAs seek out a complementary target mRNA, attach to it, and call in a group of proteins to destroy it. With the target mRNA gone, no protein can be created.
You see where this is going, right? How does high cholesterol kill you? Proteins. How does Staphylococcus aureus kill you? Proteins. Even viruses can’t replicate if their RNA is prevented from being turned into proteins.
So, how do we use siRNAs? A new paper appearing in JAMA describes a fairly impressive use case.
The background here is that higher levels of lipoprotein(a), an LDL-like protein, are associated with cardiovascular disease, heart attack, and stroke. But unfortunately, statins really don’t have any effect on lipoprotein(a) levels. Neither does diet. Your lipoprotein(a) level seems to be more or less hard-coded genetically.
So, what if we stop the genetic machinery from working? Enter lepodisiran, a drug from Eli Lilly. Unlike so many other medications, which are usually found in nature, purified, and synthesized, lepodisiran was created from scratch. It’s not hard. Thanks to the Human Genome Project, we know the genetic code for lipoprotein(a), so inventing an siRNA to target it specifically is trivial. That’s one of the key features of siRNA – you don’t have to find a chemical that binds strongly to some protein receptor, and worry about the off-target effects and all that nonsense. You just pick a protein you want to suppress and you suppress it.
Okay, it’s not that simple. siRNA is broken down very quickly by the body, so it needs to be targeted to the organ of interest – in this case, the liver, since that is where lipoprotein(a) is synthesized. Lepodisiran is targeted to the liver by this special targeting label here.
The report is a standard dose-escalation trial. Six patients, all with elevated lipoprotein(a) levels, were started with a 4-mg dose (two additional individuals got placebo). They were intensely monitored, spending 3 days in a research unit for multiple blood draws followed by weekly, and then biweekly outpatient visits. Once they had done well, the next group of six people received a higher dose (two more got placebo), and the process was repeated – six times total – until the highest dose, 608 mg, was reached.
This is an injection, of course; siRNA wouldn’t withstand the harshness of the digestive system. And it’s only one injection. You can see from the blood concentration curves that within about 48 hours, circulating lepodisiran was not detectable.
But check out these results. Remember, this is from a single injection of lepodisiran.
Lipoprotein(a) levels start to drop within a week of administration, and they stay down. In the higher-dose groups, levels are nearly undetectable a year after that injection.
It was this graph that made me sit back and think that there might be something new under the sun. A single injection that can suppress protein synthesis for an entire year? If it really works, it changes the game.
Of course, this study wasn’t powered to look at important outcomes like heart attacks and strokes. It was primarily designed to assess safety, and the drug was pretty well tolerated, with similar rates of adverse events in the drug and placebo groups.
As crazy as it sounds, the real concern here might be that this drug is too good; is it safe to drop your lipoprotein(a) levels to zero for a year? I don’t know. But lower doses don’t have quite as strong an effect.
Trust me, these drugs are going to change things. They already are. In July, The New England Journal of Medicine published a study of zilebesiran, an siRNA that inhibits the production of angiotensinogen, to control blood pressure. Similar story: One injection led to a basically complete suppression of angiotensinogen and a sustained decrease in blood pressure.
I’m not exaggerating when I say that there may come a time when you go to your doctor once a year, get your RNA shots, and don’t have to take any other medication from that point on. And that time may be, like, 5 years from now. It’s wild.
Seems to me that that rapid Nobel Prize was very well deserved.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships. This transcript has been edited for clarity.
A version of this article appeared on Medscape.com.
Mental health characteristics of refugee children
Since 1983, when I was a child and fled as a boat refugee from Vietnam with my mother, the international plight of displaced people has only worsened. From 1997 to 2022, the number of forcibly displaced people has more than tripled, growing from 34 million to more than 108 million.1
Displaced people are designated as refugees only when they cross international borders and meet the United Nations High Commissioner for Refugees’ (UNHCR) definition as “persons outside their countries of origin who are in need of international protection because of a serious threat to their life, physical integrity, or freedom in their country of origin as a result of persecution, armed conflict, violence, or serious public disorder.”2 There is a separate mandate by the United Nations for the aid of Palestinian refugees under the United Nations General Assembly’s United Nations Relief and Works Agency for Palestinian Refugees in the Near East (UNRWA).3 Of the displaced in 2022, more than 36 million were recognized as refugees under UNHCR and UNRWA mandates.1 Of these, almost 50% were children, at 17.5 million.4 To make matters worse, worldwide children represent less than one-third of the population.4 Since 2022, the increase in refugeeism is mostly driven by Ukraine and Syria, though also significantly Afghanistan, Venezuela, Sudan, Myanmar, Congo, Somalia, and Central African Republic.4 Refugeeism is a growing problem that disproportionately impacts children through sheer number, and one suspects, given their greater overall vulnerabilities compared with adults, physical and mental health consequences.
Traumas of refugees compared with non-refugee immigrants
In terms of mental health, refugees are distinct from non-refugee immigrants in that they likely experience more severe psychosocial adversities from greater poverty, greater risk of family separation, and uncertainty of the asylum process.5-8
From my own experience, this stems from the urgent nature of the refugee’s displacement, where they are often fleeing an immediate danger. My family had fled persecution from Communist forces and the social economic collapse that rendered Vietnam, for a time, one of the poorest in the world.9 Or, as my mother observed, “We had to leave because even doctors were starving.”
Refugees often have little preparation, have little legal protection since they are often criminalized, and are forced to endure dangerous conditions where they are vulnerable to smugglers and criminals who exploit their unprotected status. Once they arrive in their new country, they often do not have other family as social supports or resources. They themselves become the anchor for future legal and orderly immigration of their remaining family, given that they can extend their refugee status to those left behind.10 These non-refugee immigrants, unlike their refugee counterparts, are often flown to their new homes with more preparation, protecting them from dangerous conditions, and have the benefit of family who provide them with resources. As such, refugees tend to experience more traumatic life events than non-refugee immigrants. This was true in my family where those of us who initially escaped became the anchors to legally, and more safely, immigrate most of our family in Vietnam. We became their resources, likely making their acclimation smoother.
The mental health of refugee children and their caregivers
It is important to understand the stressors affecting the caregivers of children, since effective treatment of their mental health conditions can also benefit the children as well.11 In fact, among the greatest protective factors for refugee children is the presence of an adult caregiver, suggesting that the child’s mental health is dependent on the caregivers.
Those children who are separated show much worse mental health sequalae.12 As such, an understanding of the caregiver’s stressors is important. For example, when we were escaping Vietnam, my mom would protect me from our hardships by talking about our goals in America, minimizing our dangers by saying that we would be rewarded with things like a hamburger with its seemingly impossible amount of meat. Physically, my mother would always sleep with her arms around me and a knife hidden in order to ward off any attackers at night. When I was starving in the hull of a boat, having not eaten for days, my mother begged for food and gave me what she could get. And post-escape, my family focused on work and applied for aid for shelter and food, while encouraging us to invest in education, likely preventing involvement in criminal activities or gangs. Though overall, my family shielded me from the worst consequences, they also passed on their fears. One of my uncles had been killed by the police when he tried to escape, and so my family passed down a deep suspicion of authorities, whether they were the police or school principals. My mother had vivid memories of Communist re-education camps, which likely gave her a lasting fear that a Communist would find out our identities in America and re-capture us.
The mental health risk of refugees
Given that refugees tend to experience greater amounts of traumatic life events and a vast array of stressors sustained across years and even decades before, during, and after migration, it is no wonder they have much higher rates of mental health conditions, most predominantly PTSD and affective disorders.13,14 They are at particular risk of developing psychoses because they are more likely to experience a range of physical, psychological, and psychosocial problems associated with adversities such as violence, discrimination, economic stress, and social isolation.13 For example, the period leading up to my escape consisted of decades of prolonged war: the French-Indochina from 1945 to 1954, then the Vietnam War from 1955 to 1975) as well as the persecution and re-education camps afterward. What my family had to endure created a period of fear and loss into which I was born into in 1976. That year, my family had lost its fortune due to the Communist government seizing of our home and business, plunging us from a comfortable middle- to upper-class life to poverty. There was also widespread fear of systematic rape by the Communist victors. So my family endured great stress and the loss of a way of life leading up to our escape.
For the refugees, the escape itself is often a dangerous journey where, given its emergent nature, they are often exposed to the elements. We know about the current situation in Ukraine and Gaza, where children are fleeing from bombs and bullets. In my situation, we endured weeks of starvation crammed in the hull of boat as we forged through the Indian Ocean to the Philippines. One of my aunts, on a separate trip, perished because her boat had capsized, like so many others. Though impossible to verify, it has been estimated that up to 70% of Vietnamese refugees died during their escape.15 After the boat, my mother and I still had to brave Malaysian jungles and prisons, and then refugee camps for a year before we reached safety at an American Embassy in the Philippines. After we gained sponsorship to America, the traumas did not abate, but were only replaced by those of culture shock, poverty, and alienation. Taken by themselves, significant traumas exist in each phase of a refugee child’s escape, whether before, during, or after. These traumas are likely compounded since they are continuously layered and sustained across years, even decades. They affect not only the children, but their parents, and sometimes even a whole nation of people.
Summary
Refugee children and their families experience a variety of traumas, often sustained across years and even decades, because of armed conflict, persecution, or social upheavals. It is known that refugees are at greater risk for PTSD and affective and psychotic disorders, presumably due to increased traumatic life events before, during, and after their migration. The writer uses his own experience as a child refugee from Vietnam to elucidate the stressors evident in various phases of forced displacement.
Dr. Nguyen is a second year resident at UCSF Fresno Psychiatry Residency. He was a public high school English teacher for 15 years previously.
References
1. UNHCR. Global Trends. Forced displacement in 2016. Geneva, Switzerland: The UN Refugee Agency, 2022. https://www.unhcr.org/global-trends.
2. Office of the United Nations High Commissioner for Refugees. The refugee concept under international law. Global compact for safe, orderly and regular migration. https://www.unhcr.org/sites/
3. United Nations. (2023, November 11). The Question of Palestine. Un.org. https://www.un.org/unispal/document/un-general-assembly-renews-unrwa-mandate-press-release/
4. UNICEF. (2023, November 11). Child displacement. Data.unicef.org. https://data.unicef.org/topic/child-migration-and-displacement/displacement
5. Kinzie JD. Immigrants and refugees: The psychiatric perspective. Transcult Psychiatry. 2006 Dec;43(4):577-91. doi: 10.1177/1363461506070782.
6. Eaton W and Harrison G. Ethnic disadvantage and schizophrenia. Acta Psychiatr Scand Suppl. 2000:(407):38-43. doi: 10.1034/j.1600-0447.2000.00007.x.
7. Gilliver SC et al. Recent research on the mental health of immigrants to Sweden: a literature review. Eur J Public Health. 2014 Aug:24 Suppl 1:72-9. doi: 10.1093/eurpub/cku101.
8. Rapp MA et al. When local poverty is more important than your income: Mental health in minorities in inner cities. World Psychiatry. 2015 Jun;14(2):249-50. doi: 10.1002/wps.20221.
9. Cima, Ronald, ed. Vietnam: A Country Study. Washington: GPO for the Library of Congress, 1987.
10. United States Citizenship & Immigration Services (2023, November 12). Refugees. Uscis.gov. https://www.uscis.gov/humanitarian/refugees-and-asylum/refugees
11. Fazel M and Betancourt TS. (2018). Preventive mental health interventions for refugee children and adolescents in high-income settings. Lancet Child Adolesc Health. 2018 Feb;2(2):121-32. doi: 10.1016/S2352-4642(17)30147-5.
12. Fazel M et al. Mental health of displaced and refugee children resettled in high-income countries: risk and protective factors. Lancet. 2012 Jan 21;379(9812):266-82. doi: 10.1016/S0140-6736(11)60051-2.
13. Dapunt J et al. Refugees and psychosis: A review of the literature. Transl Psychiatry. 2017 Jun 13;7(6):e1149. doi: 10.1038/tp.2017.119.
14. Fazel M et al. Prevalence of serious mental disorder in 7,000 refugees resettled in western countries: a systematic review. Lancet. 2005 Apr;365(9467):1309-14. doi: 10.1016/S0140-6736(05)61027-6.
15. Rummel R. Statistics of Vietnamese Democide, in his Statistics of Democide. 1997. Table 6.1B,lines 730, 749-51.
Since 1983, when I was a child and fled as a boat refugee from Vietnam with my mother, the international plight of displaced people has only worsened. From 1997 to 2022, the number of forcibly displaced people has more than tripled, growing from 34 million to more than 108 million.1
Displaced people are designated as refugees only when they cross international borders and meet the United Nations High Commissioner for Refugees’ (UNHCR) definition as “persons outside their countries of origin who are in need of international protection because of a serious threat to their life, physical integrity, or freedom in their country of origin as a result of persecution, armed conflict, violence, or serious public disorder.”2 There is a separate mandate by the United Nations for the aid of Palestinian refugees under the United Nations General Assembly’s United Nations Relief and Works Agency for Palestinian Refugees in the Near East (UNRWA).3 Of the displaced in 2022, more than 36 million were recognized as refugees under UNHCR and UNRWA mandates.1 Of these, almost 50% were children, at 17.5 million.4 To make matters worse, worldwide children represent less than one-third of the population.4 Since 2022, the increase in refugeeism is mostly driven by Ukraine and Syria, though also significantly Afghanistan, Venezuela, Sudan, Myanmar, Congo, Somalia, and Central African Republic.4 Refugeeism is a growing problem that disproportionately impacts children through sheer number, and one suspects, given their greater overall vulnerabilities compared with adults, physical and mental health consequences.
Traumas of refugees compared with non-refugee immigrants
In terms of mental health, refugees are distinct from non-refugee immigrants in that they likely experience more severe psychosocial adversities from greater poverty, greater risk of family separation, and uncertainty of the asylum process.5-8
From my own experience, this stems from the urgent nature of the refugee’s displacement, where they are often fleeing an immediate danger. My family had fled persecution from Communist forces and the social economic collapse that rendered Vietnam, for a time, one of the poorest in the world.9 Or, as my mother observed, “We had to leave because even doctors were starving.”
Refugees often have little preparation, have little legal protection since they are often criminalized, and are forced to endure dangerous conditions where they are vulnerable to smugglers and criminals who exploit their unprotected status. Once they arrive in their new country, they often do not have other family as social supports or resources. They themselves become the anchor for future legal and orderly immigration of their remaining family, given that they can extend their refugee status to those left behind.10 These non-refugee immigrants, unlike their refugee counterparts, are often flown to their new homes with more preparation, protecting them from dangerous conditions, and have the benefit of family who provide them with resources. As such, refugees tend to experience more traumatic life events than non-refugee immigrants. This was true in my family where those of us who initially escaped became the anchors to legally, and more safely, immigrate most of our family in Vietnam. We became their resources, likely making their acclimation smoother.
The mental health of refugee children and their caregivers
It is important to understand the stressors affecting the caregivers of children, since effective treatment of their mental health conditions can also benefit the children as well.11 In fact, among the greatest protective factors for refugee children is the presence of an adult caregiver, suggesting that the child’s mental health is dependent on the caregivers.
Those children who are separated show much worse mental health sequalae.12 As such, an understanding of the caregiver’s stressors is important. For example, when we were escaping Vietnam, my mom would protect me from our hardships by talking about our goals in America, minimizing our dangers by saying that we would be rewarded with things like a hamburger with its seemingly impossible amount of meat. Physically, my mother would always sleep with her arms around me and a knife hidden in order to ward off any attackers at night. When I was starving in the hull of a boat, having not eaten for days, my mother begged for food and gave me what she could get. And post-escape, my family focused on work and applied for aid for shelter and food, while encouraging us to invest in education, likely preventing involvement in criminal activities or gangs. Though overall, my family shielded me from the worst consequences, they also passed on their fears. One of my uncles had been killed by the police when he tried to escape, and so my family passed down a deep suspicion of authorities, whether they were the police or school principals. My mother had vivid memories of Communist re-education camps, which likely gave her a lasting fear that a Communist would find out our identities in America and re-capture us.
The mental health risk of refugees
Given that refugees tend to experience greater amounts of traumatic life events and a vast array of stressors sustained across years and even decades before, during, and after migration, it is no wonder they have much higher rates of mental health conditions, most predominantly PTSD and affective disorders.13,14 They are at particular risk of developing psychoses because they are more likely to experience a range of physical, psychological, and psychosocial problems associated with adversities such as violence, discrimination, economic stress, and social isolation.13 For example, the period leading up to my escape consisted of decades of prolonged war: the French-Indochina from 1945 to 1954, then the Vietnam War from 1955 to 1975) as well as the persecution and re-education camps afterward. What my family had to endure created a period of fear and loss into which I was born into in 1976. That year, my family had lost its fortune due to the Communist government seizing of our home and business, plunging us from a comfortable middle- to upper-class life to poverty. There was also widespread fear of systematic rape by the Communist victors. So my family endured great stress and the loss of a way of life leading up to our escape.
For the refugees, the escape itself is often a dangerous journey where, given its emergent nature, they are often exposed to the elements. We know about the current situation in Ukraine and Gaza, where children are fleeing from bombs and bullets. In my situation, we endured weeks of starvation crammed in the hull of boat as we forged through the Indian Ocean to the Philippines. One of my aunts, on a separate trip, perished because her boat had capsized, like so many others. Though impossible to verify, it has been estimated that up to 70% of Vietnamese refugees died during their escape.15 After the boat, my mother and I still had to brave Malaysian jungles and prisons, and then refugee camps for a year before we reached safety at an American Embassy in the Philippines. After we gained sponsorship to America, the traumas did not abate, but were only replaced by those of culture shock, poverty, and alienation. Taken by themselves, significant traumas exist in each phase of a refugee child’s escape, whether before, during, or after. These traumas are likely compounded since they are continuously layered and sustained across years, even decades. They affect not only the children, but their parents, and sometimes even a whole nation of people.
Summary
Refugee children and their families experience a variety of traumas, often sustained across years and even decades, because of armed conflict, persecution, or social upheavals. It is known that refugees are at greater risk for PTSD and affective and psychotic disorders, presumably due to increased traumatic life events before, during, and after their migration. The writer uses his own experience as a child refugee from Vietnam to elucidate the stressors evident in various phases of forced displacement.
Dr. Nguyen is a second year resident at UCSF Fresno Psychiatry Residency. He was a public high school English teacher for 15 years previously.
References
1. UNHCR. Global Trends. Forced displacement in 2016. Geneva, Switzerland: The UN Refugee Agency, 2022. https://www.unhcr.org/global-trends.
2. Office of the United Nations High Commissioner for Refugees. The refugee concept under international law. Global compact for safe, orderly and regular migration. https://www.unhcr.org/sites/
3. United Nations. (2023, November 11). The Question of Palestine. Un.org. https://www.un.org/unispal/document/un-general-assembly-renews-unrwa-mandate-press-release/
4. UNICEF. (2023, November 11). Child displacement. Data.unicef.org. https://data.unicef.org/topic/child-migration-and-displacement/displacement
5. Kinzie JD. Immigrants and refugees: The psychiatric perspective. Transcult Psychiatry. 2006 Dec;43(4):577-91. doi: 10.1177/1363461506070782.
6. Eaton W and Harrison G. Ethnic disadvantage and schizophrenia. Acta Psychiatr Scand Suppl. 2000:(407):38-43. doi: 10.1034/j.1600-0447.2000.00007.x.
7. Gilliver SC et al. Recent research on the mental health of immigrants to Sweden: a literature review. Eur J Public Health. 2014 Aug:24 Suppl 1:72-9. doi: 10.1093/eurpub/cku101.
8. Rapp MA et al. When local poverty is more important than your income: Mental health in minorities in inner cities. World Psychiatry. 2015 Jun;14(2):249-50. doi: 10.1002/wps.20221.
9. Cima, Ronald, ed. Vietnam: A Country Study. Washington: GPO for the Library of Congress, 1987.
10. United States Citizenship & Immigration Services (2023, November 12). Refugees. Uscis.gov. https://www.uscis.gov/humanitarian/refugees-and-asylum/refugees
11. Fazel M and Betancourt TS. (2018). Preventive mental health interventions for refugee children and adolescents in high-income settings. Lancet Child Adolesc Health. 2018 Feb;2(2):121-32. doi: 10.1016/S2352-4642(17)30147-5.
12. Fazel M et al. Mental health of displaced and refugee children resettled in high-income countries: risk and protective factors. Lancet. 2012 Jan 21;379(9812):266-82. doi: 10.1016/S0140-6736(11)60051-2.
13. Dapunt J et al. Refugees and psychosis: A review of the literature. Transl Psychiatry. 2017 Jun 13;7(6):e1149. doi: 10.1038/tp.2017.119.
14. Fazel M et al. Prevalence of serious mental disorder in 7,000 refugees resettled in western countries: a systematic review. Lancet. 2005 Apr;365(9467):1309-14. doi: 10.1016/S0140-6736(05)61027-6.
15. Rummel R. Statistics of Vietnamese Democide, in his Statistics of Democide. 1997. Table 6.1B,lines 730, 749-51.
Since 1983, when I was a child and fled as a boat refugee from Vietnam with my mother, the international plight of displaced people has only worsened. From 1997 to 2022, the number of forcibly displaced people has more than tripled, growing from 34 million to more than 108 million.1
Displaced people are designated as refugees only when they cross international borders and meet the United Nations High Commissioner for Refugees’ (UNHCR) definition as “persons outside their countries of origin who are in need of international protection because of a serious threat to their life, physical integrity, or freedom in their country of origin as a result of persecution, armed conflict, violence, or serious public disorder.”2 There is a separate mandate by the United Nations for the aid of Palestinian refugees under the United Nations General Assembly’s United Nations Relief and Works Agency for Palestinian Refugees in the Near East (UNRWA).3 Of the displaced in 2022, more than 36 million were recognized as refugees under UNHCR and UNRWA mandates.1 Of these, almost 50% were children, at 17.5 million.4 To make matters worse, worldwide children represent less than one-third of the population.4 Since 2022, the increase in refugeeism is mostly driven by Ukraine and Syria, though also significantly Afghanistan, Venezuela, Sudan, Myanmar, Congo, Somalia, and Central African Republic.4 Refugeeism is a growing problem that disproportionately impacts children through sheer number, and one suspects, given their greater overall vulnerabilities compared with adults, physical and mental health consequences.
Traumas of refugees compared with non-refugee immigrants
In terms of mental health, refugees are distinct from non-refugee immigrants in that they likely experience more severe psychosocial adversities from greater poverty, greater risk of family separation, and uncertainty of the asylum process.5-8
From my own experience, this stems from the urgent nature of the refugee’s displacement, where they are often fleeing an immediate danger. My family had fled persecution from Communist forces and the social economic collapse that rendered Vietnam, for a time, one of the poorest in the world.9 Or, as my mother observed, “We had to leave because even doctors were starving.”
Refugees often have little preparation, have little legal protection since they are often criminalized, and are forced to endure dangerous conditions where they are vulnerable to smugglers and criminals who exploit their unprotected status. Once they arrive in their new country, they often do not have other family as social supports or resources. They themselves become the anchor for future legal and orderly immigration of their remaining family, given that they can extend their refugee status to those left behind.10 These non-refugee immigrants, unlike their refugee counterparts, are often flown to their new homes with more preparation, protecting them from dangerous conditions, and have the benefit of family who provide them with resources. As such, refugees tend to experience more traumatic life events than non-refugee immigrants. This was true in my family where those of us who initially escaped became the anchors to legally, and more safely, immigrate most of our family in Vietnam. We became their resources, likely making their acclimation smoother.
The mental health of refugee children and their caregivers
It is important to understand the stressors affecting the caregivers of children, since effective treatment of their mental health conditions can also benefit the children as well.11 In fact, among the greatest protective factors for refugee children is the presence of an adult caregiver, suggesting that the child’s mental health is dependent on the caregivers.
Those children who are separated show much worse mental health sequalae.12 As such, an understanding of the caregiver’s stressors is important. For example, when we were escaping Vietnam, my mom would protect me from our hardships by talking about our goals in America, minimizing our dangers by saying that we would be rewarded with things like a hamburger with its seemingly impossible amount of meat. Physically, my mother would always sleep with her arms around me and a knife hidden in order to ward off any attackers at night. When I was starving in the hull of a boat, having not eaten for days, my mother begged for food and gave me what she could get. And post-escape, my family focused on work and applied for aid for shelter and food, while encouraging us to invest in education, likely preventing involvement in criminal activities or gangs. Though overall, my family shielded me from the worst consequences, they also passed on their fears. One of my uncles had been killed by the police when he tried to escape, and so my family passed down a deep suspicion of authorities, whether they were the police or school principals. My mother had vivid memories of Communist re-education camps, which likely gave her a lasting fear that a Communist would find out our identities in America and re-capture us.
The mental health risk of refugees
Given that refugees tend to experience greater amounts of traumatic life events and a vast array of stressors sustained across years and even decades before, during, and after migration, it is no wonder they have much higher rates of mental health conditions, most predominantly PTSD and affective disorders.13,14 They are at particular risk of developing psychoses because they are more likely to experience a range of physical, psychological, and psychosocial problems associated with adversities such as violence, discrimination, economic stress, and social isolation.13 For example, the period leading up to my escape consisted of decades of prolonged war: the French-Indochina from 1945 to 1954, then the Vietnam War from 1955 to 1975) as well as the persecution and re-education camps afterward. What my family had to endure created a period of fear and loss into which I was born into in 1976. That year, my family had lost its fortune due to the Communist government seizing of our home and business, plunging us from a comfortable middle- to upper-class life to poverty. There was also widespread fear of systematic rape by the Communist victors. So my family endured great stress and the loss of a way of life leading up to our escape.
For the refugees, the escape itself is often a dangerous journey where, given its emergent nature, they are often exposed to the elements. We know about the current situation in Ukraine and Gaza, where children are fleeing from bombs and bullets. In my situation, we endured weeks of starvation crammed in the hull of boat as we forged through the Indian Ocean to the Philippines. One of my aunts, on a separate trip, perished because her boat had capsized, like so many others. Though impossible to verify, it has been estimated that up to 70% of Vietnamese refugees died during their escape.15 After the boat, my mother and I still had to brave Malaysian jungles and prisons, and then refugee camps for a year before we reached safety at an American Embassy in the Philippines. After we gained sponsorship to America, the traumas did not abate, but were only replaced by those of culture shock, poverty, and alienation. Taken by themselves, significant traumas exist in each phase of a refugee child’s escape, whether before, during, or after. These traumas are likely compounded since they are continuously layered and sustained across years, even decades. They affect not only the children, but their parents, and sometimes even a whole nation of people.
Summary
Refugee children and their families experience a variety of traumas, often sustained across years and even decades, because of armed conflict, persecution, or social upheavals. It is known that refugees are at greater risk for PTSD and affective and psychotic disorders, presumably due to increased traumatic life events before, during, and after their migration. The writer uses his own experience as a child refugee from Vietnam to elucidate the stressors evident in various phases of forced displacement.
Dr. Nguyen is a second year resident at UCSF Fresno Psychiatry Residency. He was a public high school English teacher for 15 years previously.
References
1. UNHCR. Global Trends. Forced displacement in 2016. Geneva, Switzerland: The UN Refugee Agency, 2022. https://www.unhcr.org/global-trends.
2. Office of the United Nations High Commissioner for Refugees. The refugee concept under international law. Global compact for safe, orderly and regular migration. https://www.unhcr.org/sites/
3. United Nations. (2023, November 11). The Question of Palestine. Un.org. https://www.un.org/unispal/document/un-general-assembly-renews-unrwa-mandate-press-release/
4. UNICEF. (2023, November 11). Child displacement. Data.unicef.org. https://data.unicef.org/topic/child-migration-and-displacement/displacement
5. Kinzie JD. Immigrants and refugees: The psychiatric perspective. Transcult Psychiatry. 2006 Dec;43(4):577-91. doi: 10.1177/1363461506070782.
6. Eaton W and Harrison G. Ethnic disadvantage and schizophrenia. Acta Psychiatr Scand Suppl. 2000:(407):38-43. doi: 10.1034/j.1600-0447.2000.00007.x.
7. Gilliver SC et al. Recent research on the mental health of immigrants to Sweden: a literature review. Eur J Public Health. 2014 Aug:24 Suppl 1:72-9. doi: 10.1093/eurpub/cku101.
8. Rapp MA et al. When local poverty is more important than your income: Mental health in minorities in inner cities. World Psychiatry. 2015 Jun;14(2):249-50. doi: 10.1002/wps.20221.
9. Cima, Ronald, ed. Vietnam: A Country Study. Washington: GPO for the Library of Congress, 1987.
10. United States Citizenship & Immigration Services (2023, November 12). Refugees. Uscis.gov. https://www.uscis.gov/humanitarian/refugees-and-asylum/refugees
11. Fazel M and Betancourt TS. (2018). Preventive mental health interventions for refugee children and adolescents in high-income settings. Lancet Child Adolesc Health. 2018 Feb;2(2):121-32. doi: 10.1016/S2352-4642(17)30147-5.
12. Fazel M et al. Mental health of displaced and refugee children resettled in high-income countries: risk and protective factors. Lancet. 2012 Jan 21;379(9812):266-82. doi: 10.1016/S0140-6736(11)60051-2.
13. Dapunt J et al. Refugees and psychosis: A review of the literature. Transl Psychiatry. 2017 Jun 13;7(6):e1149. doi: 10.1038/tp.2017.119.
14. Fazel M et al. Prevalence of serious mental disorder in 7,000 refugees resettled in western countries: a systematic review. Lancet. 2005 Apr;365(9467):1309-14. doi: 10.1016/S0140-6736(05)61027-6.
15. Rummel R. Statistics of Vietnamese Democide, in his Statistics of Democide. 1997. Table 6.1B,lines 730, 749-51.
Can vitamin and mineral supplementation prevent cancer or cardiovascular disease?
Patients often come to us with questions about vitamin and mineral supplements. Sometimes they come to us with bags full of the things they are taking. The Internet is full of the wonders these nutritional supplements can do, from turmeric curing cancer to vitamin D curing COVID. It is hard to keep up with medicine itself without learning a whole new field of nutritional supplements.
However, for cardiovascular disease (CVD) and cancer prevention, the answer is pretty easy according to USPSTF (United States Preventative Services Task Force) guidelines. They evaluated 17,459 unique citations as well as 379 full-text articles that included randomized clinical trials and observational cohort studies. The conclusions of their research showed that there was little to no benefit in taking vitamin or mineral supplements to prevent CVD, cancer, or death. In fact, beta-carotene supplementation was associated with increased risk of lung cancer and other adverse outcomes in patients at increased risk of lung cancer.
Although they are often marketed like drugs, nutritional supplements are regulated as foods, with less stringent standards.* Our current medical culture pushes us to practice evidence-based medicine. Without evidence, we simply cannot counsel patients about supplements because there is little evidence to support their use.
Additionally, many patients assume that they are safe. While this may be true for many of them, some of them can be harmful in several ways. They can interact with medications the patient may be taking for medical conditions. Some of them have been shown to cause liver and other organ damage. When they are used to replace traditional medicine, they can also lead to harm by delaying appropriate medical care. For example, a patient who believes a supplement can treat cancer when it does nothing is delaying care that might save their life. By the time they realize it is not working, the cancer may have advanced too far to be treatable.
While there may be a few studies that do show some efficacy for vitamins and minerals in certain diseases, these guidelines are looking only at use in terms of preventing cancer and CVD. As primary care physicians, we all know the screening guidelines for cancer prevention. We are better off recommending screening mammograms and colon cancer screening tests. And we all know the risk factors for CVD and how to mitigate these risks.
What can we do when patients come to us with false claims regarding supplements?
- Hear what they are saying. They don’t know who to trust. We will never become their trusted source of medical information if we don’t listen to their concerns.
- Answer their questions, no matter how ridiculous they may seem to us. Many people who sell supplements sound convincing. That is how they sell their products. Our advice may seem just as ridiculous to them. We need to explain the facts clearly and be sure the patient understands.
- Give the patient resources. Know what websites to direct them to so that they can get accurate information.
- Know what’s out there. I was once surprised when a patient told me she was going to try turmeric as a treatment for uterine cancer. We cannot combat misinformation when we don’t know what’s being said.
- Become a voice for medical information. There is so much misinformation being spread. We need more doctors to speak up about the right medical information.
Currently, patients often look for medical information online. We do them a disservice when we brush aside their questions regarding supplements, no matter how trivial they seem. We need to take a firm stand and tell them the evidence regarding these supplements: They are neither FDA approved nor studied for safety and efficacy. Anyone can sell a supplement and make any claim regarding it that they want. It is much better to eat a healthy, balanced diet to get the vitamins and minerals that you need. Not only do we need to show them the evidence, we need to convince them that it is true.
*Correction, 12/4: An earlier version of this article misstated the regulatory requirements for nutritional supplements.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. She was paid by Pfizer as a consultant on Paxlovid and is the editor in chief of Physician’s Weekly.
Patients often come to us with questions about vitamin and mineral supplements. Sometimes they come to us with bags full of the things they are taking. The Internet is full of the wonders these nutritional supplements can do, from turmeric curing cancer to vitamin D curing COVID. It is hard to keep up with medicine itself without learning a whole new field of nutritional supplements.
However, for cardiovascular disease (CVD) and cancer prevention, the answer is pretty easy according to USPSTF (United States Preventative Services Task Force) guidelines. They evaluated 17,459 unique citations as well as 379 full-text articles that included randomized clinical trials and observational cohort studies. The conclusions of their research showed that there was little to no benefit in taking vitamin or mineral supplements to prevent CVD, cancer, or death. In fact, beta-carotene supplementation was associated with increased risk of lung cancer and other adverse outcomes in patients at increased risk of lung cancer.
Although they are often marketed like drugs, nutritional supplements are regulated as foods, with less stringent standards.* Our current medical culture pushes us to practice evidence-based medicine. Without evidence, we simply cannot counsel patients about supplements because there is little evidence to support their use.
Additionally, many patients assume that they are safe. While this may be true for many of them, some of them can be harmful in several ways. They can interact with medications the patient may be taking for medical conditions. Some of them have been shown to cause liver and other organ damage. When they are used to replace traditional medicine, they can also lead to harm by delaying appropriate medical care. For example, a patient who believes a supplement can treat cancer when it does nothing is delaying care that might save their life. By the time they realize it is not working, the cancer may have advanced too far to be treatable.
While there may be a few studies that do show some efficacy for vitamins and minerals in certain diseases, these guidelines are looking only at use in terms of preventing cancer and CVD. As primary care physicians, we all know the screening guidelines for cancer prevention. We are better off recommending screening mammograms and colon cancer screening tests. And we all know the risk factors for CVD and how to mitigate these risks.
What can we do when patients come to us with false claims regarding supplements?
- Hear what they are saying. They don’t know who to trust. We will never become their trusted source of medical information if we don’t listen to their concerns.
- Answer their questions, no matter how ridiculous they may seem to us. Many people who sell supplements sound convincing. That is how they sell their products. Our advice may seem just as ridiculous to them. We need to explain the facts clearly and be sure the patient understands.
- Give the patient resources. Know what websites to direct them to so that they can get accurate information.
- Know what’s out there. I was once surprised when a patient told me she was going to try turmeric as a treatment for uterine cancer. We cannot combat misinformation when we don’t know what’s being said.
- Become a voice for medical information. There is so much misinformation being spread. We need more doctors to speak up about the right medical information.
Currently, patients often look for medical information online. We do them a disservice when we brush aside their questions regarding supplements, no matter how trivial they seem. We need to take a firm stand and tell them the evidence regarding these supplements: They are neither FDA approved nor studied for safety and efficacy. Anyone can sell a supplement and make any claim regarding it that they want. It is much better to eat a healthy, balanced diet to get the vitamins and minerals that you need. Not only do we need to show them the evidence, we need to convince them that it is true.
*Correction, 12/4: An earlier version of this article misstated the regulatory requirements for nutritional supplements.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. She was paid by Pfizer as a consultant on Paxlovid and is the editor in chief of Physician’s Weekly.
Patients often come to us with questions about vitamin and mineral supplements. Sometimes they come to us with bags full of the things they are taking. The Internet is full of the wonders these nutritional supplements can do, from turmeric curing cancer to vitamin D curing COVID. It is hard to keep up with medicine itself without learning a whole new field of nutritional supplements.
However, for cardiovascular disease (CVD) and cancer prevention, the answer is pretty easy according to USPSTF (United States Preventative Services Task Force) guidelines. They evaluated 17,459 unique citations as well as 379 full-text articles that included randomized clinical trials and observational cohort studies. The conclusions of their research showed that there was little to no benefit in taking vitamin or mineral supplements to prevent CVD, cancer, or death. In fact, beta-carotene supplementation was associated with increased risk of lung cancer and other adverse outcomes in patients at increased risk of lung cancer.
Although they are often marketed like drugs, nutritional supplements are regulated as foods, with less stringent standards.* Our current medical culture pushes us to practice evidence-based medicine. Without evidence, we simply cannot counsel patients about supplements because there is little evidence to support their use.
Additionally, many patients assume that they are safe. While this may be true for many of them, some of them can be harmful in several ways. They can interact with medications the patient may be taking for medical conditions. Some of them have been shown to cause liver and other organ damage. When they are used to replace traditional medicine, they can also lead to harm by delaying appropriate medical care. For example, a patient who believes a supplement can treat cancer when it does nothing is delaying care that might save their life. By the time they realize it is not working, the cancer may have advanced too far to be treatable.
While there may be a few studies that do show some efficacy for vitamins and minerals in certain diseases, these guidelines are looking only at use in terms of preventing cancer and CVD. As primary care physicians, we all know the screening guidelines for cancer prevention. We are better off recommending screening mammograms and colon cancer screening tests. And we all know the risk factors for CVD and how to mitigate these risks.
What can we do when patients come to us with false claims regarding supplements?
- Hear what they are saying. They don’t know who to trust. We will never become their trusted source of medical information if we don’t listen to their concerns.
- Answer their questions, no matter how ridiculous they may seem to us. Many people who sell supplements sound convincing. That is how they sell their products. Our advice may seem just as ridiculous to them. We need to explain the facts clearly and be sure the patient understands.
- Give the patient resources. Know what websites to direct them to so that they can get accurate information.
- Know what’s out there. I was once surprised when a patient told me she was going to try turmeric as a treatment for uterine cancer. We cannot combat misinformation when we don’t know what’s being said.
- Become a voice for medical information. There is so much misinformation being spread. We need more doctors to speak up about the right medical information.
Currently, patients often look for medical information online. We do them a disservice when we brush aside their questions regarding supplements, no matter how trivial they seem. We need to take a firm stand and tell them the evidence regarding these supplements: They are neither FDA approved nor studied for safety and efficacy. Anyone can sell a supplement and make any claim regarding it that they want. It is much better to eat a healthy, balanced diet to get the vitamins and minerals that you need. Not only do we need to show them the evidence, we need to convince them that it is true.
*Correction, 12/4: An earlier version of this article misstated the regulatory requirements for nutritional supplements.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. She was paid by Pfizer as a consultant on Paxlovid and is the editor in chief of Physician’s Weekly.












