User login
Sparing the rod
In the wake of the allegations that a star NFL running back abused his 4-year-old son by hitting him with a switch, the debate about spanking and other forms of corporal punishment has reignited. It’s not much of a debate. It’s really just a cacophony of “experts” condemning the act. There are a few dissenting voices who find fault with this particular high-profile event, but still hold the opinion that there are certain situations in which spanking may be an acceptable option. My mother taught me to never say never. But, the occasions in which an open-handed spank on a well-padded bottom are so rare that for all practical purposes, striking a child should not appear on any list of discipline strategies.
However, I’m not sure that spanking should automatically be equated with child abuse. It is seldom effective and should raise a red flag that we are dealing with a parent who needs help in managing his or her child’s behavior, but it’s generally not abuse.
In this recent case, the father has talked about the long lineage of corporal punishment that runs through his family. However, I think that most parents in this country instinctively know that hitting their child is not the best option. They may have learned from experience that it is ineffective and has a very narrow safety margin. But, parents aren’t sure what they should have done.
They may have read magazine articles or heard talking heads on television encouraging parents to engage their misbehaving children in a dialogue to explore their motives. Or, how to condemn the misdeeds without damaging the child’s self-image. To many parents, this kind of advice fells like just so much talk. They have already discovered that one can’t have a meaningful discussion with a child in the throes of a tantrum.
In many cases, the failure of words alone is the natural result of an uncountable number of threats that have never been followed by a consistent consequence. It’s not surprising that parents often fail to follow up on their threats because they lack even the smallest arsenal of safe and effective consequences. They know that corporal punishment is wrong. But, does that mean that discipline must be completely hands off? Is any physical restraint such as a bear hug of a toddler or preschooler in the throes of a tantrum so close to spanking that it could be interpreted as child abuse? Unfortunately, I suspect that there are a few child behavior experts who might say that it is.
What about putting a child in his room for time-out? If he won’t go willingly and has to be carried, is that corporal punishment? If he won’t stay in his room for even 30 seconds unless the door is held shut or latched, is that same as a penal institution’s use of solitary confinement? Although they have a physical component, these restrictions – if done sensibly – are far safer and more effective than hitting a child.
Of course, prevention should be the keystone of any behavior-management strategy. Does the parent understand the spectrum of age-appropriate behavior for his child? Does he accept that his child’s temperament may force him to modify his expectations? Have family dynamics and schedules created situations in which the child feels underappreciated? Is the parent himself in good physical and mental health?
As pediatricians, we must make it clear that we are prepared to help parents to deal with the challenges inherent in setting limits for their children and assist them in creating a strategies of safe consequences to assure that these limits are effective.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” E-mail him at [email protected].
In the wake of the allegations that a star NFL running back abused his 4-year-old son by hitting him with a switch, the debate about spanking and other forms of corporal punishment has reignited. It’s not much of a debate. It’s really just a cacophony of “experts” condemning the act. There are a few dissenting voices who find fault with this particular high-profile event, but still hold the opinion that there are certain situations in which spanking may be an acceptable option. My mother taught me to never say never. But, the occasions in which an open-handed spank on a well-padded bottom are so rare that for all practical purposes, striking a child should not appear on any list of discipline strategies.
However, I’m not sure that spanking should automatically be equated with child abuse. It is seldom effective and should raise a red flag that we are dealing with a parent who needs help in managing his or her child’s behavior, but it’s generally not abuse.
In this recent case, the father has talked about the long lineage of corporal punishment that runs through his family. However, I think that most parents in this country instinctively know that hitting their child is not the best option. They may have learned from experience that it is ineffective and has a very narrow safety margin. But, parents aren’t sure what they should have done.
They may have read magazine articles or heard talking heads on television encouraging parents to engage their misbehaving children in a dialogue to explore their motives. Or, how to condemn the misdeeds without damaging the child’s self-image. To many parents, this kind of advice fells like just so much talk. They have already discovered that one can’t have a meaningful discussion with a child in the throes of a tantrum.
In many cases, the failure of words alone is the natural result of an uncountable number of threats that have never been followed by a consistent consequence. It’s not surprising that parents often fail to follow up on their threats because they lack even the smallest arsenal of safe and effective consequences. They know that corporal punishment is wrong. But, does that mean that discipline must be completely hands off? Is any physical restraint such as a bear hug of a toddler or preschooler in the throes of a tantrum so close to spanking that it could be interpreted as child abuse? Unfortunately, I suspect that there are a few child behavior experts who might say that it is.
What about putting a child in his room for time-out? If he won’t go willingly and has to be carried, is that corporal punishment? If he won’t stay in his room for even 30 seconds unless the door is held shut or latched, is that same as a penal institution’s use of solitary confinement? Although they have a physical component, these restrictions – if done sensibly – are far safer and more effective than hitting a child.
Of course, prevention should be the keystone of any behavior-management strategy. Does the parent understand the spectrum of age-appropriate behavior for his child? Does he accept that his child’s temperament may force him to modify his expectations? Have family dynamics and schedules created situations in which the child feels underappreciated? Is the parent himself in good physical and mental health?
As pediatricians, we must make it clear that we are prepared to help parents to deal with the challenges inherent in setting limits for their children and assist them in creating a strategies of safe consequences to assure that these limits are effective.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” E-mail him at [email protected].
In the wake of the allegations that a star NFL running back abused his 4-year-old son by hitting him with a switch, the debate about spanking and other forms of corporal punishment has reignited. It’s not much of a debate. It’s really just a cacophony of “experts” condemning the act. There are a few dissenting voices who find fault with this particular high-profile event, but still hold the opinion that there are certain situations in which spanking may be an acceptable option. My mother taught me to never say never. But, the occasions in which an open-handed spank on a well-padded bottom are so rare that for all practical purposes, striking a child should not appear on any list of discipline strategies.
However, I’m not sure that spanking should automatically be equated with child abuse. It is seldom effective and should raise a red flag that we are dealing with a parent who needs help in managing his or her child’s behavior, but it’s generally not abuse.
In this recent case, the father has talked about the long lineage of corporal punishment that runs through his family. However, I think that most parents in this country instinctively know that hitting their child is not the best option. They may have learned from experience that it is ineffective and has a very narrow safety margin. But, parents aren’t sure what they should have done.
They may have read magazine articles or heard talking heads on television encouraging parents to engage their misbehaving children in a dialogue to explore their motives. Or, how to condemn the misdeeds without damaging the child’s self-image. To many parents, this kind of advice fells like just so much talk. They have already discovered that one can’t have a meaningful discussion with a child in the throes of a tantrum.
In many cases, the failure of words alone is the natural result of an uncountable number of threats that have never been followed by a consistent consequence. It’s not surprising that parents often fail to follow up on their threats because they lack even the smallest arsenal of safe and effective consequences. They know that corporal punishment is wrong. But, does that mean that discipline must be completely hands off? Is any physical restraint such as a bear hug of a toddler or preschooler in the throes of a tantrum so close to spanking that it could be interpreted as child abuse? Unfortunately, I suspect that there are a few child behavior experts who might say that it is.
What about putting a child in his room for time-out? If he won’t go willingly and has to be carried, is that corporal punishment? If he won’t stay in his room for even 30 seconds unless the door is held shut or latched, is that same as a penal institution’s use of solitary confinement? Although they have a physical component, these restrictions – if done sensibly – are far safer and more effective than hitting a child.
Of course, prevention should be the keystone of any behavior-management strategy. Does the parent understand the spectrum of age-appropriate behavior for his child? Does he accept that his child’s temperament may force him to modify his expectations? Have family dynamics and schedules created situations in which the child feels underappreciated? Is the parent himself in good physical and mental health?
As pediatricians, we must make it clear that we are prepared to help parents to deal with the challenges inherent in setting limits for their children and assist them in creating a strategies of safe consequences to assure that these limits are effective.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” E-mail him at [email protected].
Weighing self-determination against blissful ignorance at death’s door
I had a young cousin with thalassemia major. She had the textbook chipmunk facies, but you otherwise would not have known she was ill. Despite requiring blood transfusions every 3 weeks, she led a fairly normal life, graduating from college and holding down a good job.
In 2012, we found out that she had hepatitis C. She received treatment, but it did not succeed. By this point, she’d already developed cardiomyopathy, and she would later develop atrial fibrillation and heart failure. Earlier this year, Gilead was kind enough to give her its new drug sofosbuvir for free, but given her comorbidities, she could not tolerate it.
Recently, she was discharged after a protracted admission for heart failure. At that point, her hematologist, cardiologist, and hepatologist held a family meeting with her parents and siblings and pointed out the futility of her situation. Her family brought her home. They did what Filipino families in desperation do. They prayed and reached out to a “faith healer” – someone who uses poultices made from taro leaves, mutters incantations, and provides homemade remedies for any ailment. From a patient’s perspective, they provide hope where no one else will; from an outsider’s perspective, they are simply preying on the vulnerable.
Despite her family’s efforts, my cousin died about a month after coming home. She was only 27 years old. She woke up one morning feeling short of breath, weighed down by anasarca. She was brought to the hospital. Surrounded by her family, she asked her brother why he was crying. She asked her family not to bother calling her boyfriend; she’d talk to him when he came around. Then she fell asleep for the last time.
She did not know that she was dying. Her family had chosen to keep this from her.
When I learned about the circumstances of her passing I was angry and indignant at first. Why wouldn’t they tell her? What about patient self-determination and letting her be the judge of whether she wanted to be taken back to the hospital? Why would they deprive her of the opportunity to say goodbye? How is it that this sort of paternalistic, “I know what’s best for you” attitude still exists?
But I tried to put myself in her shoes, and it didn’t take long for me to question my certitude.
We romanticize the last moments of our lives. We imagine it to be filled with equanimity, a dignified acceptance of the inevitable. But that cannot always be the case. I can just as easily imagine myself to be angry, bitter, and, worst of all, fearful. Overwhelmed with sadness that it makes my last moments joyless rather than joyful.
Dying is intensely personal. Billions of people have led lives and reached endings unique to them. We may make noise about patient self-determination, but really, what is that if not just another manifestation of our arrogance that we know best? Is not insisting on patient self-determination just the other side of the same protect-the-patient-by-withholding-information coin?
I was humbled by my own ambivalence toward how her family handled her death, and a bit ashamed that I would be so quick to judge them. They did what they thought was best; who am I to question that? I may understand the science of life and death, but I cannot claim to understand living and dying.
Dr. Chan practices rheumatology in Pawtucket, R.I.
I had a young cousin with thalassemia major. She had the textbook chipmunk facies, but you otherwise would not have known she was ill. Despite requiring blood transfusions every 3 weeks, she led a fairly normal life, graduating from college and holding down a good job.
In 2012, we found out that she had hepatitis C. She received treatment, but it did not succeed. By this point, she’d already developed cardiomyopathy, and she would later develop atrial fibrillation and heart failure. Earlier this year, Gilead was kind enough to give her its new drug sofosbuvir for free, but given her comorbidities, she could not tolerate it.
Recently, she was discharged after a protracted admission for heart failure. At that point, her hematologist, cardiologist, and hepatologist held a family meeting with her parents and siblings and pointed out the futility of her situation. Her family brought her home. They did what Filipino families in desperation do. They prayed and reached out to a “faith healer” – someone who uses poultices made from taro leaves, mutters incantations, and provides homemade remedies for any ailment. From a patient’s perspective, they provide hope where no one else will; from an outsider’s perspective, they are simply preying on the vulnerable.
Despite her family’s efforts, my cousin died about a month after coming home. She was only 27 years old. She woke up one morning feeling short of breath, weighed down by anasarca. She was brought to the hospital. Surrounded by her family, she asked her brother why he was crying. She asked her family not to bother calling her boyfriend; she’d talk to him when he came around. Then she fell asleep for the last time.
She did not know that she was dying. Her family had chosen to keep this from her.
When I learned about the circumstances of her passing I was angry and indignant at first. Why wouldn’t they tell her? What about patient self-determination and letting her be the judge of whether she wanted to be taken back to the hospital? Why would they deprive her of the opportunity to say goodbye? How is it that this sort of paternalistic, “I know what’s best for you” attitude still exists?
But I tried to put myself in her shoes, and it didn’t take long for me to question my certitude.
We romanticize the last moments of our lives. We imagine it to be filled with equanimity, a dignified acceptance of the inevitable. But that cannot always be the case. I can just as easily imagine myself to be angry, bitter, and, worst of all, fearful. Overwhelmed with sadness that it makes my last moments joyless rather than joyful.
Dying is intensely personal. Billions of people have led lives and reached endings unique to them. We may make noise about patient self-determination, but really, what is that if not just another manifestation of our arrogance that we know best? Is not insisting on patient self-determination just the other side of the same protect-the-patient-by-withholding-information coin?
I was humbled by my own ambivalence toward how her family handled her death, and a bit ashamed that I would be so quick to judge them. They did what they thought was best; who am I to question that? I may understand the science of life and death, but I cannot claim to understand living and dying.
Dr. Chan practices rheumatology in Pawtucket, R.I.
I had a young cousin with thalassemia major. She had the textbook chipmunk facies, but you otherwise would not have known she was ill. Despite requiring blood transfusions every 3 weeks, she led a fairly normal life, graduating from college and holding down a good job.
In 2012, we found out that she had hepatitis C. She received treatment, but it did not succeed. By this point, she’d already developed cardiomyopathy, and she would later develop atrial fibrillation and heart failure. Earlier this year, Gilead was kind enough to give her its new drug sofosbuvir for free, but given her comorbidities, she could not tolerate it.
Recently, she was discharged after a protracted admission for heart failure. At that point, her hematologist, cardiologist, and hepatologist held a family meeting with her parents and siblings and pointed out the futility of her situation. Her family brought her home. They did what Filipino families in desperation do. They prayed and reached out to a “faith healer” – someone who uses poultices made from taro leaves, mutters incantations, and provides homemade remedies for any ailment. From a patient’s perspective, they provide hope where no one else will; from an outsider’s perspective, they are simply preying on the vulnerable.
Despite her family’s efforts, my cousin died about a month after coming home. She was only 27 years old. She woke up one morning feeling short of breath, weighed down by anasarca. She was brought to the hospital. Surrounded by her family, she asked her brother why he was crying. She asked her family not to bother calling her boyfriend; she’d talk to him when he came around. Then she fell asleep for the last time.
She did not know that she was dying. Her family had chosen to keep this from her.
When I learned about the circumstances of her passing I was angry and indignant at first. Why wouldn’t they tell her? What about patient self-determination and letting her be the judge of whether she wanted to be taken back to the hospital? Why would they deprive her of the opportunity to say goodbye? How is it that this sort of paternalistic, “I know what’s best for you” attitude still exists?
But I tried to put myself in her shoes, and it didn’t take long for me to question my certitude.
We romanticize the last moments of our lives. We imagine it to be filled with equanimity, a dignified acceptance of the inevitable. But that cannot always be the case. I can just as easily imagine myself to be angry, bitter, and, worst of all, fearful. Overwhelmed with sadness that it makes my last moments joyless rather than joyful.
Dying is intensely personal. Billions of people have led lives and reached endings unique to them. We may make noise about patient self-determination, but really, what is that if not just another manifestation of our arrogance that we know best? Is not insisting on patient self-determination just the other side of the same protect-the-patient-by-withholding-information coin?
I was humbled by my own ambivalence toward how her family handled her death, and a bit ashamed that I would be so quick to judge them. They did what they thought was best; who am I to question that? I may understand the science of life and death, but I cannot claim to understand living and dying.
Dr. Chan practices rheumatology in Pawtucket, R.I.
Meaningful Use – Stage 2 (Part 2 of 2)
In last month’s column, we began our discussion of Stage 2 of meaningful use. As a reminder, we noted that clinicians must meet or exceed the thresholds for the 17 core objectives and three of six menu objectives, as well as report on defined Clinical Quality Measures. We reviewed in detail the rationale for the program, as well as details of the core and menu measures.
For Stage 2 of meaningful use, the menu items and quality measures are aimed at enhancing actionable decision support to improve the quality of medical care and enable population management for patients who come into our office (and even for those who don’t). Stage 2 is also meant to facilitate physician-patient communication.

As a point of reminder and clarification, on August 29th the U.S. Department of Health and Human Services published a final rule allowing certain eligible providers the flexibility to continue using the Stage 1 criteria for the 2014 attestation year, even if they were due to start Stage 2. Unfortunately, this only applies to those who have been unable to obtain the 2014-certified software in time because of vendor delays. The reprieve does not extend to those who can’t meet Stage 2 due to measure difficulty or procrastination in purchasing software or adopting new work flow. As always, we recommend speaking with a meaningful use expert or consultant before attempting to take advantage of this flexibility. Either way, you’ll need to proceed with the 2014 Clinical Quality Measures, as these new definitions are now required by both the Stage 1 and Stage 2 goals. In this month’s EHR Report, we will highlight the most noteworthy 2014 Clinical Quality Measures.
Clinical Quality Measures are meant to measure and track the quality of health care services that are provided by the practitioner. Clinical Quality Measures are constructed to measure these aspects of care:
• Health outcomes
• Cinical processes
• Patient safety
• Efficient use of health care resources
• Care coordination
• Patient engagements
• Population and public health
• Adherence to clinical guidelines
Beginning in 2014, practitioners must select and report on 9 out of a list of 64 approved Clinical Quality Measures for the EHR Incentive Programs.
Clinical Quality Measures may be reported electronically through the EHR if this function is available through your EHR software. It can also be done through CMS’s Physician Quality Reporting System Portal. In order for a practice to report through the portal, the practice needs to sign up through CMS, which can be done through the CMS website. In addition, reporting can be done through a number of group reporting options if a practice is part of a large group of practices or an ACO, or via attestation as before. While the details go of how to report go beyond what we can cover in this column, your IT support person or consultant should be well acquainted with the process.
The Clinical Quality Measures are divided into six different domains of care, and providers must report on Clinical Quality Measures from at least three different domains (Table 1).
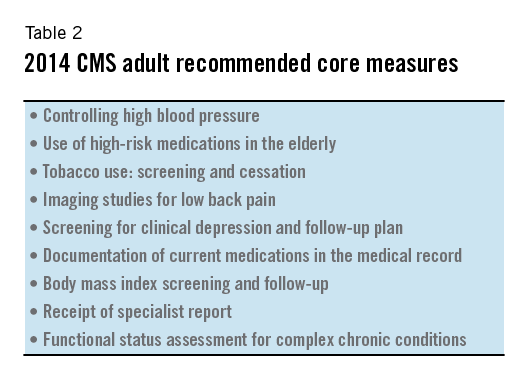
CMS encourages reporting on nine recommended core sets of Clinical Quality Measures, as long as those measures are relevant to a practitioner’s patient population. The recommended core measures focus on aspects of medical care that are felt to have the most significant effect on morbidity and mortality of Medicare and Medicaid beneficiaries.
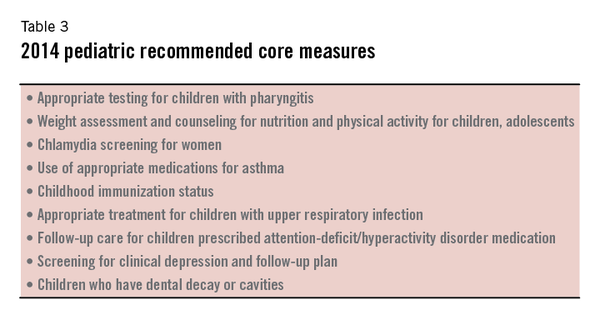
They also focus on aspects of medical care that are consistent with national public health priorities or that particularly increase healthcare costs. The nine measures recommended by CMS for adult and pediatric populations are listed in Tables 2 and 3.
Between Core Objectives, Menu Objectives, and CQMs, the requirements for Stage 2 meaningful use have gotten more complicated and perhaps more confusing to track and implement than before. We recommend that every practice has an identified individual who will become a resource to help others both understand and implement Stage 2 meaningful use. We anticipate a range of opinion about the challenges of Stage 2 and are interested in your thoughts. Please email us, and we will try to publish some of the comments in upcoming columns.
References:
1. An Introduction to EHR Incentive Programs 2014 Clincial Quality Measure (CQM) Electronic Reporting Guide for Eligible Professionals.
http://www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms/Downloads/CQM2014_GuideEP.pdf.
2. Eligible Professionals Guide to Stage 2 of the EHR Incentive Programs http://www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms/Downloads/Stage2_Guide_EPs_9_23_13.pdf.
3. For a comprehensive list of the CQMs, see the 2014 CQMs for eligible professionals PDF (available at http://www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms/Downloads/EP_MeasuresTable_Posting_CQMs.pdf).
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia.
In last month’s column, we began our discussion of Stage 2 of meaningful use. As a reminder, we noted that clinicians must meet or exceed the thresholds for the 17 core objectives and three of six menu objectives, as well as report on defined Clinical Quality Measures. We reviewed in detail the rationale for the program, as well as details of the core and menu measures.
For Stage 2 of meaningful use, the menu items and quality measures are aimed at enhancing actionable decision support to improve the quality of medical care and enable population management for patients who come into our office (and even for those who don’t). Stage 2 is also meant to facilitate physician-patient communication.

As a point of reminder and clarification, on August 29th the U.S. Department of Health and Human Services published a final rule allowing certain eligible providers the flexibility to continue using the Stage 1 criteria for the 2014 attestation year, even if they were due to start Stage 2. Unfortunately, this only applies to those who have been unable to obtain the 2014-certified software in time because of vendor delays. The reprieve does not extend to those who can’t meet Stage 2 due to measure difficulty or procrastination in purchasing software or adopting new work flow. As always, we recommend speaking with a meaningful use expert or consultant before attempting to take advantage of this flexibility. Either way, you’ll need to proceed with the 2014 Clinical Quality Measures, as these new definitions are now required by both the Stage 1 and Stage 2 goals. In this month’s EHR Report, we will highlight the most noteworthy 2014 Clinical Quality Measures.
Clinical Quality Measures are meant to measure and track the quality of health care services that are provided by the practitioner. Clinical Quality Measures are constructed to measure these aspects of care:
• Health outcomes
• Cinical processes
• Patient safety
• Efficient use of health care resources
• Care coordination
• Patient engagements
• Population and public health
• Adherence to clinical guidelines
Beginning in 2014, practitioners must select and report on 9 out of a list of 64 approved Clinical Quality Measures for the EHR Incentive Programs.
Clinical Quality Measures may be reported electronically through the EHR if this function is available through your EHR software. It can also be done through CMS’s Physician Quality Reporting System Portal. In order for a practice to report through the portal, the practice needs to sign up through CMS, which can be done through the CMS website. In addition, reporting can be done through a number of group reporting options if a practice is part of a large group of practices or an ACO, or via attestation as before. While the details go of how to report go beyond what we can cover in this column, your IT support person or consultant should be well acquainted with the process.

The Clinical Quality Measures are divided into six different domains of care, and providers must report on Clinical Quality Measures from at least three different domains (Table 1).
CMS encourages reporting on nine recommended core sets of Clinical Quality Measures, as long as those measures are relevant to a practitioner’s patient population. The recommended core measures focus on aspects of medical care that are felt to have the most significant effect on morbidity and mortality of Medicare and Medicaid beneficiaries.
They also focus on aspects of medical care that are consistent with national public health priorities or that particularly increase healthcare costs. The nine measures recommended by CMS for adult and pediatric populations are listed in Tables 2 and 3.

Between Core Objectives, Menu Objectives, and CQMs, the requirements for Stage 2 meaningful use have gotten more complicated and perhaps more confusing to track and implement than before. We recommend that every practice has an identified individual who will become a resource to help others both understand and implement Stage 2 meaningful use. We anticipate a range of opinion about the challenges of Stage 2 and are interested in your thoughts. Please email us, and we will try to publish some of the comments in upcoming columns.
References:
1. An Introduction to EHR Incentive Programs 2014 Clincial Quality Measure (CQM) Electronic Reporting Guide for Eligible Professionals.
http://www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms/Downloads/CQM2014_GuideEP.pdf.
2. Eligible Professionals Guide to Stage 2 of the EHR Incentive Programs http://www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms/Downloads/Stage2_Guide_EPs_9_23_13.pdf.
3. For a comprehensive list of the CQMs, see the 2014 CQMs for eligible professionals PDF (available at http://www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms/Downloads/EP_MeasuresTable_Posting_CQMs.pdf).
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia.
In last month’s column, we began our discussion of Stage 2 of meaningful use. As a reminder, we noted that clinicians must meet or exceed the thresholds for the 17 core objectives and three of six menu objectives, as well as report on defined Clinical Quality Measures. We reviewed in detail the rationale for the program, as well as details of the core and menu measures.
For Stage 2 of meaningful use, the menu items and quality measures are aimed at enhancing actionable decision support to improve the quality of medical care and enable population management for patients who come into our office (and even for those who don’t). Stage 2 is also meant to facilitate physician-patient communication.

As a point of reminder and clarification, on August 29th the U.S. Department of Health and Human Services published a final rule allowing certain eligible providers the flexibility to continue using the Stage 1 criteria for the 2014 attestation year, even if they were due to start Stage 2. Unfortunately, this only applies to those who have been unable to obtain the 2014-certified software in time because of vendor delays. The reprieve does not extend to those who can’t meet Stage 2 due to measure difficulty or procrastination in purchasing software or adopting new work flow. As always, we recommend speaking with a meaningful use expert or consultant before attempting to take advantage of this flexibility. Either way, you’ll need to proceed with the 2014 Clinical Quality Measures, as these new definitions are now required by both the Stage 1 and Stage 2 goals. In this month’s EHR Report, we will highlight the most noteworthy 2014 Clinical Quality Measures.
Clinical Quality Measures are meant to measure and track the quality of health care services that are provided by the practitioner. Clinical Quality Measures are constructed to measure these aspects of care:
• Health outcomes
• Cinical processes
• Patient safety
• Efficient use of health care resources
• Care coordination
• Patient engagements
• Population and public health
• Adherence to clinical guidelines
Beginning in 2014, practitioners must select and report on 9 out of a list of 64 approved Clinical Quality Measures for the EHR Incentive Programs.
Clinical Quality Measures may be reported electronically through the EHR if this function is available through your EHR software. It can also be done through CMS’s Physician Quality Reporting System Portal. In order for a practice to report through the portal, the practice needs to sign up through CMS, which can be done through the CMS website. In addition, reporting can be done through a number of group reporting options if a practice is part of a large group of practices or an ACO, or via attestation as before. While the details go of how to report go beyond what we can cover in this column, your IT support person or consultant should be well acquainted with the process.

The Clinical Quality Measures are divided into six different domains of care, and providers must report on Clinical Quality Measures from at least three different domains (Table 1).
CMS encourages reporting on nine recommended core sets of Clinical Quality Measures, as long as those measures are relevant to a practitioner’s patient population. The recommended core measures focus on aspects of medical care that are felt to have the most significant effect on morbidity and mortality of Medicare and Medicaid beneficiaries.
They also focus on aspects of medical care that are consistent with national public health priorities or that particularly increase healthcare costs. The nine measures recommended by CMS for adult and pediatric populations are listed in Tables 2 and 3.

Between Core Objectives, Menu Objectives, and CQMs, the requirements for Stage 2 meaningful use have gotten more complicated and perhaps more confusing to track and implement than before. We recommend that every practice has an identified individual who will become a resource to help others both understand and implement Stage 2 meaningful use. We anticipate a range of opinion about the challenges of Stage 2 and are interested in your thoughts. Please email us, and we will try to publish some of the comments in upcoming columns.
References:
1. An Introduction to EHR Incentive Programs 2014 Clincial Quality Measure (CQM) Electronic Reporting Guide for Eligible Professionals.
http://www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms/Downloads/CQM2014_GuideEP.pdf.
2. Eligible Professionals Guide to Stage 2 of the EHR Incentive Programs http://www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms/Downloads/Stage2_Guide_EPs_9_23_13.pdf.
3. For a comprehensive list of the CQMs, see the 2014 CQMs for eligible professionals PDF (available at http://www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms/Downloads/EP_MeasuresTable_Posting_CQMs.pdf).
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia.
Point/Counterpoint: Is TEVAR required for all Type B aortic dissections?
Yes, TEVAR is clearly indicated.
Aortic dissection is a devastating condition afflicting an estimated two to eight per 100,000 people annually and comprises a large portion of the clinical entity known as the acute aortic syndromes. Patients presenting with an uncomplicated type B acute aortic dissection (TBAD) generally have low in-hospital mortality rates (2.4%-9%) when managed appropriately with anti-impulse therapy. However, survival continues to decrease with follow-up, with survival ranging between 80% and more than 95% at 1 year, progressing to approximately 75% at 3-4 years, and 48%-65% at 10 years. In late follow-up, the development of a new dissection with complications is estimated to occur in 20%-50% of patients. Complicated aortic dissections affect between 22% and 47%, and when present, mortality reaches more than 50% within the first week. TEVAR in these patients has been shown to be clearly indicated in a variety of studies with marked improvements in early mortality and late survival. Thus, one can see that aortic dissection is a disease that needs to be managed lifelong, and is associated with a high risk of mortality for the next 10 years after the initial presentation.1,2,3
The long-term effects of a patent false lumen have been well documented. Several studies following patients with chronic TBAD have documented progressive enlargement in aortic diameter with a patent false lumen. The mean increase in maximum aortic diameter ranges from 3.8 to 7.1 mm annually with any flow in the false lumen (FL) versus 1-2 mm per year with a thrombosed FL. Patients with a patent FL had 7.5 times increased risk of a dissection-related death or need for surgery as compared to patients with thrombosis of the FL. Dissection-related death or need for surgery occurred at a significantly earlier follow-up period in the patients with a patent FL.1,2,3
The aortic diameter may also influence the patency of the FL at presentation. In a review of 110 patients presenting with acute uncomplicated TBAD, 44% were identified to have a patent FL on initial imaging. Thirty-one percent of these patients had a maximum aortic diameter of 45 mm or more versus 14% of patients with a thrombosed FL (P = .053). Incidentally, patients with FL patency were on average 4 years younger than their thrombosed counterparts (62 vs. 66 years, P = .009).
Moreover, it appears that the long-term risks associated with a patent FL are further augmented by aortic dilatation at presentation. When combining both risk factors (FL patency and aortic diameter of 40 mm or more), only 22% of patients are dissection-related event–free at 5-year follow-up.Onitsuka et al.4 substantiated this finding on multivariate analysis. Interestingly, 10 of the 76 patients included in that study met both conditions, and seven of those patients (70%) experienced a dissection-related death or surgical conversion. Certainly patients meeting both criteria merit close follow-up for the development of aortic enlargement or symptoms of impending rupture.
The natural history of TBAD lends itself to at least some thrombus formation within the FL and is a common finding as the dissection becomes chronic. But in fact, partial thrombosis of the FL is associated with higher mortality in patients discharged from the hospital with stable TBAD at 1- and 3-year follow-up (15.4% and 31.6%, respectively). Matched patients with a patent FL had a 5.4% and 13.7% rate of mortality at 1 and 3 years, and patients with complete FL thrombosis were found to have mortality rates of 0% and 22.6% at the same follow-up.
Aortic remodeling after TEVAR
Placement of a thoracic endograft under these acute circumstances can often significantly alter the preoperative morphology of the true and false lumen. Schoder and colleagues5 followed changes in the TL and FL diameter in 20 patients after TEVAR for acute complicated dissection. Ninety percent of patients were found to have complete FL thrombosis of the thoracic aorta at 1 year, with a mean decrease in FL diameter of 11.6 mm. Two patients with a patent FL showed a mean increase in the maximal aortic diameter of 4.5 mm. In a similar study, Conrad et al.6 documented aortic remodeling of 21 patients in the year following TEVAR, 88% of whom had thrombosis of the FL. Most often the mobile septum is easily displaced by the radial force of the stent graft, with minimal limitation of expansion to the design diameter. Thus, endograft selection should be directed by the diameter of the normal unaffected aorta with minimal oversizing commonly limited to 5%-10%. Balloon profiling is not typically necessary.
The INSTEAD trial7 evaluated the management of uncomplicated type B aortic dissection and compared optimum medical therapy (OMT) to OMT with TEVAR. A total of 140 subjects were enrolled at seven European sites with 68 patients enrolled in OMT and 72 in OMT with TEVAR. In patients treated with TEVAR there was 90.6% complete FL thrombosis with a maximum true lumen diameter of 32.6 mm as compared to 22% and 18.7 mm in those treated with medical therapy alone. Furthermore, there was a 12.4% absolute risk reduction in aortic specific mortality and a 19.1% absolute risk reduction in disease progression in patients treated with TEVAR.
It is clear that patients that present with complicated type B aortic dissections mandate intervention with TEVAR and potentially other interventions to alleviate the complications at presentation. INSTEAD demonstrates that elective TEVAR results in favorable aortic remodeling and long-term survival, reinterventions were low, and it prevents late expansion and malperfusion. TEVAR was also associated with improved 5-year aortic-specific survival. TEVAR appears to be beneficial in those patients who present initially with a false lumen diameter of greater than 22 mm and an aortic diameter of greater than 40 mm with a patent false lumen.
References
1. Circ. Cardiovasc. Interv. 2013;4:407-16.
2. J. Vasc. Surg. 2012;55:641-51.
3. J. Vasc. Surg. 2011;54:985-92
4. Ann. Thorac. Surg. 2004;78:1268-73.
5. Ann. Thorac. Surg. 2007;83:1059-66.
6. J. Vasc. Surg. 2009;50:510-17.
7. Circulation 2009;120:2519-28.
Dr. Arko is with the Aortic Institute, Sanger Heart & Vascular Institute, Charlotte, N.C. He reported no relevant conflicts.
No, evidence supports careful choice of patients.
While the role of TEVAR has been proven to treat complications of acute type B dissections,1 its value as a prophylactic treatment in uncomplicated cases remains controversial. Optimal medical treatment (OMT) with strict blood pressure (SBP less than 120 mm Hg) and heart rate control is associated with a low morbidity and mortality, despite the risk of progressive aortic dilation. On the other hand TEVAR can result in early death and significant neurologic complications; other devastating complications of TEVAR include retrograde aortic dissection and access vessel rupture with a high associated mortality.
A meta-analysis of the published literature reported a high technical success of TEVAR for uncomplicated type B dissection and a relatively high conversion rate (20%) for patient treated with OMT, however the results did not identify an advantage for TEVAR with respect to 30-day and 2-year mortality.2
An expert panel review of the world literature also did not find significant data to support use of TEVAR for uncomplicated type B dissection.3 In the only randomized prospective trial to examine the role of TEVAR for uncomplicated type B dissection, the INSTEAD trial randomized 140 patients to OMT vs. OMT and TEVAR.4 The study results also did not support the use of TEVAR for the treatment of uncomplicated type B dissection, there was no survival advantage at 2 years, while TEVAR was associated with a 11.1% overall mortality and 4.3% neurologic complication rate, compared with 4.4% and 1.4% in the OMT group. The initial study did however report improved aortic remodeling at 2 years with TEVAR. The results of INSTEAD have been challenged because critical analysis of the INSTEAD trial has determined that the results were underpowered and that there was a 21% crossover in the OMT group and four patients received TEVAR that should have been excluded.5
Subsequent long-term analysis of the INSTEAD XL data do demonstrate a significant survival benefit and freedom from aortic adverse events in the TEVAR group after the initial 2-year analysis.6 At the 5-year follow up only 27 patients remained without a TEVAR. Fortunately there were no adverse events in the patients that crossed over to TEVAR from the OMT group demonstrating the safety of delayed TEVAR in this group. The high rate of aortic associated adverse events may favor early TEVAR. The INSTEAD XL study did identify a large primary tear (more than 10 mm) and an initial aortic diameter of 40 mm as risk factors to crossover suggesting a more aggressive approach in this subset of patients.
So while the INSTEAD XL trial now supports the use of TEVAR for uncomplicated type B dissections this was a relatively small trial that was underpowered in its initial analysis. Expert review of the world literature still supports medical management in the initial phase of treatment. Obviously in cases of failure of medical management TEVAR provides an effective treatment to restore the true lumen and visceral perfusion with possible sustained remodeling of the false lumen.
Given the not insignificant morbidity associated with TEVAR placement, routine treatment of all acute, uncomplicated type B dissections cannot be supported with the current evidence. However, a strategy of selective treatment based on size of the entry tear, extent of dissection, false lumen diameter and extent of thrombosis, effectiveness of antihypertension medications, ability to comply with medical therapy, and surveillance may be implemented. Furthermore treatment at centers of excellence with extensive TEVAR experience based on established protocols favor improved patient outcomes.
References
1. N. Engl. J. Med. 199;340:1546-52
2. Vasc. Endovascular. Surg. 2013 Oct 12;47(7):497-501. Epub 2013 Jul 12.
3. J. Am. Coll. Cardiol. 2013;61(16):1661-78.
4. Circulation 2009;120:2519-28.
5. Circulation 2009;120:2513-14.
6. Circ. Cardiovasc. Interv. 2013;6:407-16.
Dr. Shames is professor of surgery and radiology and program director of vascular surgery at the University of South Florida, Tampa. He reported no relevant conflicts.
Yes, TEVAR is clearly indicated.
Aortic dissection is a devastating condition afflicting an estimated two to eight per 100,000 people annually and comprises a large portion of the clinical entity known as the acute aortic syndromes. Patients presenting with an uncomplicated type B acute aortic dissection (TBAD) generally have low in-hospital mortality rates (2.4%-9%) when managed appropriately with anti-impulse therapy. However, survival continues to decrease with follow-up, with survival ranging between 80% and more than 95% at 1 year, progressing to approximately 75% at 3-4 years, and 48%-65% at 10 years. In late follow-up, the development of a new dissection with complications is estimated to occur in 20%-50% of patients. Complicated aortic dissections affect between 22% and 47%, and when present, mortality reaches more than 50% within the first week. TEVAR in these patients has been shown to be clearly indicated in a variety of studies with marked improvements in early mortality and late survival. Thus, one can see that aortic dissection is a disease that needs to be managed lifelong, and is associated with a high risk of mortality for the next 10 years after the initial presentation.1,2,3
The long-term effects of a patent false lumen have been well documented. Several studies following patients with chronic TBAD have documented progressive enlargement in aortic diameter with a patent false lumen. The mean increase in maximum aortic diameter ranges from 3.8 to 7.1 mm annually with any flow in the false lumen (FL) versus 1-2 mm per year with a thrombosed FL. Patients with a patent FL had 7.5 times increased risk of a dissection-related death or need for surgery as compared to patients with thrombosis of the FL. Dissection-related death or need for surgery occurred at a significantly earlier follow-up period in the patients with a patent FL.1,2,3
The aortic diameter may also influence the patency of the FL at presentation. In a review of 110 patients presenting with acute uncomplicated TBAD, 44% were identified to have a patent FL on initial imaging. Thirty-one percent of these patients had a maximum aortic diameter of 45 mm or more versus 14% of patients with a thrombosed FL (P = .053). Incidentally, patients with FL patency were on average 4 years younger than their thrombosed counterparts (62 vs. 66 years, P = .009).
Moreover, it appears that the long-term risks associated with a patent FL are further augmented by aortic dilatation at presentation. When combining both risk factors (FL patency and aortic diameter of 40 mm or more), only 22% of patients are dissection-related event–free at 5-year follow-up.Onitsuka et al.4 substantiated this finding on multivariate analysis. Interestingly, 10 of the 76 patients included in that study met both conditions, and seven of those patients (70%) experienced a dissection-related death or surgical conversion. Certainly patients meeting both criteria merit close follow-up for the development of aortic enlargement or symptoms of impending rupture.
The natural history of TBAD lends itself to at least some thrombus formation within the FL and is a common finding as the dissection becomes chronic. But in fact, partial thrombosis of the FL is associated with higher mortality in patients discharged from the hospital with stable TBAD at 1- and 3-year follow-up (15.4% and 31.6%, respectively). Matched patients with a patent FL had a 5.4% and 13.7% rate of mortality at 1 and 3 years, and patients with complete FL thrombosis were found to have mortality rates of 0% and 22.6% at the same follow-up.
Aortic remodeling after TEVAR
Placement of a thoracic endograft under these acute circumstances can often significantly alter the preoperative morphology of the true and false lumen. Schoder and colleagues5 followed changes in the TL and FL diameter in 20 patients after TEVAR for acute complicated dissection. Ninety percent of patients were found to have complete FL thrombosis of the thoracic aorta at 1 year, with a mean decrease in FL diameter of 11.6 mm. Two patients with a patent FL showed a mean increase in the maximal aortic diameter of 4.5 mm. In a similar study, Conrad et al.6 documented aortic remodeling of 21 patients in the year following TEVAR, 88% of whom had thrombosis of the FL. Most often the mobile septum is easily displaced by the radial force of the stent graft, with minimal limitation of expansion to the design diameter. Thus, endograft selection should be directed by the diameter of the normal unaffected aorta with minimal oversizing commonly limited to 5%-10%. Balloon profiling is not typically necessary.
The INSTEAD trial7 evaluated the management of uncomplicated type B aortic dissection and compared optimum medical therapy (OMT) to OMT with TEVAR. A total of 140 subjects were enrolled at seven European sites with 68 patients enrolled in OMT and 72 in OMT with TEVAR. In patients treated with TEVAR there was 90.6% complete FL thrombosis with a maximum true lumen diameter of 32.6 mm as compared to 22% and 18.7 mm in those treated with medical therapy alone. Furthermore, there was a 12.4% absolute risk reduction in aortic specific mortality and a 19.1% absolute risk reduction in disease progression in patients treated with TEVAR.
It is clear that patients that present with complicated type B aortic dissections mandate intervention with TEVAR and potentially other interventions to alleviate the complications at presentation. INSTEAD demonstrates that elective TEVAR results in favorable aortic remodeling and long-term survival, reinterventions were low, and it prevents late expansion and malperfusion. TEVAR was also associated with improved 5-year aortic-specific survival. TEVAR appears to be beneficial in those patients who present initially with a false lumen diameter of greater than 22 mm and an aortic diameter of greater than 40 mm with a patent false lumen.
References
1. Circ. Cardiovasc. Interv. 2013;4:407-16.
2. J. Vasc. Surg. 2012;55:641-51.
3. J. Vasc. Surg. 2011;54:985-92
4. Ann. Thorac. Surg. 2004;78:1268-73.
5. Ann. Thorac. Surg. 2007;83:1059-66.
6. J. Vasc. Surg. 2009;50:510-17.
7. Circulation 2009;120:2519-28.
Dr. Arko is with the Aortic Institute, Sanger Heart & Vascular Institute, Charlotte, N.C. He reported no relevant conflicts.
No, evidence supports careful choice of patients.
While the role of TEVAR has been proven to treat complications of acute type B dissections,1 its value as a prophylactic treatment in uncomplicated cases remains controversial. Optimal medical treatment (OMT) with strict blood pressure (SBP less than 120 mm Hg) and heart rate control is associated with a low morbidity and mortality, despite the risk of progressive aortic dilation. On the other hand TEVAR can result in early death and significant neurologic complications; other devastating complications of TEVAR include retrograde aortic dissection and access vessel rupture with a high associated mortality.
A meta-analysis of the published literature reported a high technical success of TEVAR for uncomplicated type B dissection and a relatively high conversion rate (20%) for patient treated with OMT, however the results did not identify an advantage for TEVAR with respect to 30-day and 2-year mortality.2
An expert panel review of the world literature also did not find significant data to support use of TEVAR for uncomplicated type B dissection.3 In the only randomized prospective trial to examine the role of TEVAR for uncomplicated type B dissection, the INSTEAD trial randomized 140 patients to OMT vs. OMT and TEVAR.4 The study results also did not support the use of TEVAR for the treatment of uncomplicated type B dissection, there was no survival advantage at 2 years, while TEVAR was associated with a 11.1% overall mortality and 4.3% neurologic complication rate, compared with 4.4% and 1.4% in the OMT group. The initial study did however report improved aortic remodeling at 2 years with TEVAR. The results of INSTEAD have been challenged because critical analysis of the INSTEAD trial has determined that the results were underpowered and that there was a 21% crossover in the OMT group and four patients received TEVAR that should have been excluded.5
Subsequent long-term analysis of the INSTEAD XL data do demonstrate a significant survival benefit and freedom from aortic adverse events in the TEVAR group after the initial 2-year analysis.6 At the 5-year follow up only 27 patients remained without a TEVAR. Fortunately there were no adverse events in the patients that crossed over to TEVAR from the OMT group demonstrating the safety of delayed TEVAR in this group. The high rate of aortic associated adverse events may favor early TEVAR. The INSTEAD XL study did identify a large primary tear (more than 10 mm) and an initial aortic diameter of 40 mm as risk factors to crossover suggesting a more aggressive approach in this subset of patients.
So while the INSTEAD XL trial now supports the use of TEVAR for uncomplicated type B dissections this was a relatively small trial that was underpowered in its initial analysis. Expert review of the world literature still supports medical management in the initial phase of treatment. Obviously in cases of failure of medical management TEVAR provides an effective treatment to restore the true lumen and visceral perfusion with possible sustained remodeling of the false lumen.
Given the not insignificant morbidity associated with TEVAR placement, routine treatment of all acute, uncomplicated type B dissections cannot be supported with the current evidence. However, a strategy of selective treatment based on size of the entry tear, extent of dissection, false lumen diameter and extent of thrombosis, effectiveness of antihypertension medications, ability to comply with medical therapy, and surveillance may be implemented. Furthermore treatment at centers of excellence with extensive TEVAR experience based on established protocols favor improved patient outcomes.
References
1. N. Engl. J. Med. 199;340:1546-52
2. Vasc. Endovascular. Surg. 2013 Oct 12;47(7):497-501. Epub 2013 Jul 12.
3. J. Am. Coll. Cardiol. 2013;61(16):1661-78.
4. Circulation 2009;120:2519-28.
5. Circulation 2009;120:2513-14.
6. Circ. Cardiovasc. Interv. 2013;6:407-16.
Dr. Shames is professor of surgery and radiology and program director of vascular surgery at the University of South Florida, Tampa. He reported no relevant conflicts.
Yes, TEVAR is clearly indicated.
Aortic dissection is a devastating condition afflicting an estimated two to eight per 100,000 people annually and comprises a large portion of the clinical entity known as the acute aortic syndromes. Patients presenting with an uncomplicated type B acute aortic dissection (TBAD) generally have low in-hospital mortality rates (2.4%-9%) when managed appropriately with anti-impulse therapy. However, survival continues to decrease with follow-up, with survival ranging between 80% and more than 95% at 1 year, progressing to approximately 75% at 3-4 years, and 48%-65% at 10 years. In late follow-up, the development of a new dissection with complications is estimated to occur in 20%-50% of patients. Complicated aortic dissections affect between 22% and 47%, and when present, mortality reaches more than 50% within the first week. TEVAR in these patients has been shown to be clearly indicated in a variety of studies with marked improvements in early mortality and late survival. Thus, one can see that aortic dissection is a disease that needs to be managed lifelong, and is associated with a high risk of mortality for the next 10 years after the initial presentation.1,2,3
The long-term effects of a patent false lumen have been well documented. Several studies following patients with chronic TBAD have documented progressive enlargement in aortic diameter with a patent false lumen. The mean increase in maximum aortic diameter ranges from 3.8 to 7.1 mm annually with any flow in the false lumen (FL) versus 1-2 mm per year with a thrombosed FL. Patients with a patent FL had 7.5 times increased risk of a dissection-related death or need for surgery as compared to patients with thrombosis of the FL. Dissection-related death or need for surgery occurred at a significantly earlier follow-up period in the patients with a patent FL.1,2,3
The aortic diameter may also influence the patency of the FL at presentation. In a review of 110 patients presenting with acute uncomplicated TBAD, 44% were identified to have a patent FL on initial imaging. Thirty-one percent of these patients had a maximum aortic diameter of 45 mm or more versus 14% of patients with a thrombosed FL (P = .053). Incidentally, patients with FL patency were on average 4 years younger than their thrombosed counterparts (62 vs. 66 years, P = .009).
Moreover, it appears that the long-term risks associated with a patent FL are further augmented by aortic dilatation at presentation. When combining both risk factors (FL patency and aortic diameter of 40 mm or more), only 22% of patients are dissection-related event–free at 5-year follow-up.Onitsuka et al.4 substantiated this finding on multivariate analysis. Interestingly, 10 of the 76 patients included in that study met both conditions, and seven of those patients (70%) experienced a dissection-related death or surgical conversion. Certainly patients meeting both criteria merit close follow-up for the development of aortic enlargement or symptoms of impending rupture.
The natural history of TBAD lends itself to at least some thrombus formation within the FL and is a common finding as the dissection becomes chronic. But in fact, partial thrombosis of the FL is associated with higher mortality in patients discharged from the hospital with stable TBAD at 1- and 3-year follow-up (15.4% and 31.6%, respectively). Matched patients with a patent FL had a 5.4% and 13.7% rate of mortality at 1 and 3 years, and patients with complete FL thrombosis were found to have mortality rates of 0% and 22.6% at the same follow-up.
Aortic remodeling after TEVAR
Placement of a thoracic endograft under these acute circumstances can often significantly alter the preoperative morphology of the true and false lumen. Schoder and colleagues5 followed changes in the TL and FL diameter in 20 patients after TEVAR for acute complicated dissection. Ninety percent of patients were found to have complete FL thrombosis of the thoracic aorta at 1 year, with a mean decrease in FL diameter of 11.6 mm. Two patients with a patent FL showed a mean increase in the maximal aortic diameter of 4.5 mm. In a similar study, Conrad et al.6 documented aortic remodeling of 21 patients in the year following TEVAR, 88% of whom had thrombosis of the FL. Most often the mobile septum is easily displaced by the radial force of the stent graft, with minimal limitation of expansion to the design diameter. Thus, endograft selection should be directed by the diameter of the normal unaffected aorta with minimal oversizing commonly limited to 5%-10%. Balloon profiling is not typically necessary.
The INSTEAD trial7 evaluated the management of uncomplicated type B aortic dissection and compared optimum medical therapy (OMT) to OMT with TEVAR. A total of 140 subjects were enrolled at seven European sites with 68 patients enrolled in OMT and 72 in OMT with TEVAR. In patients treated with TEVAR there was 90.6% complete FL thrombosis with a maximum true lumen diameter of 32.6 mm as compared to 22% and 18.7 mm in those treated with medical therapy alone. Furthermore, there was a 12.4% absolute risk reduction in aortic specific mortality and a 19.1% absolute risk reduction in disease progression in patients treated with TEVAR.
It is clear that patients that present with complicated type B aortic dissections mandate intervention with TEVAR and potentially other interventions to alleviate the complications at presentation. INSTEAD demonstrates that elective TEVAR results in favorable aortic remodeling and long-term survival, reinterventions were low, and it prevents late expansion and malperfusion. TEVAR was also associated with improved 5-year aortic-specific survival. TEVAR appears to be beneficial in those patients who present initially with a false lumen diameter of greater than 22 mm and an aortic diameter of greater than 40 mm with a patent false lumen.
References
1. Circ. Cardiovasc. Interv. 2013;4:407-16.
2. J. Vasc. Surg. 2012;55:641-51.
3. J. Vasc. Surg. 2011;54:985-92
4. Ann. Thorac. Surg. 2004;78:1268-73.
5. Ann. Thorac. Surg. 2007;83:1059-66.
6. J. Vasc. Surg. 2009;50:510-17.
7. Circulation 2009;120:2519-28.
Dr. Arko is with the Aortic Institute, Sanger Heart & Vascular Institute, Charlotte, N.C. He reported no relevant conflicts.
No, evidence supports careful choice of patients.
While the role of TEVAR has been proven to treat complications of acute type B dissections,1 its value as a prophylactic treatment in uncomplicated cases remains controversial. Optimal medical treatment (OMT) with strict blood pressure (SBP less than 120 mm Hg) and heart rate control is associated with a low morbidity and mortality, despite the risk of progressive aortic dilation. On the other hand TEVAR can result in early death and significant neurologic complications; other devastating complications of TEVAR include retrograde aortic dissection and access vessel rupture with a high associated mortality.
A meta-analysis of the published literature reported a high technical success of TEVAR for uncomplicated type B dissection and a relatively high conversion rate (20%) for patient treated with OMT, however the results did not identify an advantage for TEVAR with respect to 30-day and 2-year mortality.2
An expert panel review of the world literature also did not find significant data to support use of TEVAR for uncomplicated type B dissection.3 In the only randomized prospective trial to examine the role of TEVAR for uncomplicated type B dissection, the INSTEAD trial randomized 140 patients to OMT vs. OMT and TEVAR.4 The study results also did not support the use of TEVAR for the treatment of uncomplicated type B dissection, there was no survival advantage at 2 years, while TEVAR was associated with a 11.1% overall mortality and 4.3% neurologic complication rate, compared with 4.4% and 1.4% in the OMT group. The initial study did however report improved aortic remodeling at 2 years with TEVAR. The results of INSTEAD have been challenged because critical analysis of the INSTEAD trial has determined that the results were underpowered and that there was a 21% crossover in the OMT group and four patients received TEVAR that should have been excluded.5
Subsequent long-term analysis of the INSTEAD XL data do demonstrate a significant survival benefit and freedom from aortic adverse events in the TEVAR group after the initial 2-year analysis.6 At the 5-year follow up only 27 patients remained without a TEVAR. Fortunately there were no adverse events in the patients that crossed over to TEVAR from the OMT group demonstrating the safety of delayed TEVAR in this group. The high rate of aortic associated adverse events may favor early TEVAR. The INSTEAD XL study did identify a large primary tear (more than 10 mm) and an initial aortic diameter of 40 mm as risk factors to crossover suggesting a more aggressive approach in this subset of patients.
So while the INSTEAD XL trial now supports the use of TEVAR for uncomplicated type B dissections this was a relatively small trial that was underpowered in its initial analysis. Expert review of the world literature still supports medical management in the initial phase of treatment. Obviously in cases of failure of medical management TEVAR provides an effective treatment to restore the true lumen and visceral perfusion with possible sustained remodeling of the false lumen.
Given the not insignificant morbidity associated with TEVAR placement, routine treatment of all acute, uncomplicated type B dissections cannot be supported with the current evidence. However, a strategy of selective treatment based on size of the entry tear, extent of dissection, false lumen diameter and extent of thrombosis, effectiveness of antihypertension medications, ability to comply with medical therapy, and surveillance may be implemented. Furthermore treatment at centers of excellence with extensive TEVAR experience based on established protocols favor improved patient outcomes.
References
1. N. Engl. J. Med. 199;340:1546-52
2. Vasc. Endovascular. Surg. 2013 Oct 12;47(7):497-501. Epub 2013 Jul 12.
3. J. Am. Coll. Cardiol. 2013;61(16):1661-78.
4. Circulation 2009;120:2519-28.
5. Circulation 2009;120:2513-14.
6. Circ. Cardiovasc. Interv. 2013;6:407-16.
Dr. Shames is professor of surgery and radiology and program director of vascular surgery at the University of South Florida, Tampa. He reported no relevant conflicts.
COSMECEUTICAL CRITIQUE: Master formulators: The ‘Julia Childs’ of skin care
In the multibillion-dollar skin care industry, there are many well-recognized brands. However, we sometimes forget that behind these products were formulators who took their scientific ideas and turned them into recipes for cosmetically elegant active formulations.
I have spent the last 15 years researching the activity of cosmeceutical ingredients for my new textbook, “Cosmeceuticals and Cosmetic Ingredients” (McGraw Hill, 2014). Each ingredient has its own quirks, and they all do not “play well in the sandbox” together. Formulation knowledge (cosmetic chemistry) is required to take these ingredients and combine them in a way that enhances rather than hinders their activity, just as a chef combines ingredients and cooking techniques to enhance the flavor and presentation of food. When I discuss cosmeceutical products, I always stress the importance of the ingredients and understanding ingredient interactions, because they determine the end product – how effective it is and how elegant it feels. If a product works well but smells bad and feels unpleasant, consumers will not use it.
Whom are we trusting when it comes to this science? The formulators, also known as cosmetic chemists, who put their blood, sweat, and tears into years of work to develop products that yield efficacious results. They are often behind the scenes, and their contributions are not always recognized. I refer to them as the “Julia Childs” of skin care, because they remind me of how Julia Child combined her knowledge of ingredients and aesthetic sensibilities to change the world of cooking.
I’d like to shine the spotlight on several top skin care formulators that I have met. Their relentless desire to perfect skin care recipes has helped the industry boom and has improved skin health.
Richard Parker
Location: Melbourne
Richard Parker is the CEO/founder of the Australia-based company Rationale. When he was unable to find skin care products that worked with his skin type, he decided to study cosmetic chemistry and create his own skin care line. Today, Rationale can be found in dermatologists’ and plastic surgeons’ offices across Australia. Parker’s passion for cosmetic science is evident. Australia has a high incidence of melanoma, and sunscreens undergo greater scrutiny there compared with other countries. One of the things that Parker is most proud of is his creation of SPF products that are “as elegant as they are effective.” This is a difficult combination to achieve, because sunscreens tend to be too white or too greasy; formulating them properly requires a “master chef.”
In addition to formulating effective and elegant sun protection, he has developed Essential Six: a combination of six products that work in synergy, delivering the perfect combination of active ingredients at the correct concentration to be recognized and utilized by skin cells.
In order to succeed in the formulations industry, you must possess a desire to make it better; and Parker does just that. It’s his wish for the industry to have an increased awareness of a holistic approach to skin care that includes immune protection, antioxidants, sunscreens, gentle cleansing, alpha-hydroxy acids, and vitamin A.
If being at the forefront of this evolution isn’t enough, Parker is devoted to continue his mission for years to come, all the while helping younger chemists/formulators embrace the culture.
“For the past 25 years, I have had the privilege to work with Australia’s leading dermatologists to create the best possible products and procedures,” he said. “At this stage of my career, it is so gratifying to see the younger generation of skin specialists embrace medical skin care as a part of best clinical practice.”
Chuck Friedman
Location: Wendell, N.C.
Chuck Friedman is a man who prides himself on the use of natural products – not a small achievement for a man who has been in the industry for almost half a century. His work as a formulation chemist has spanned globally recognized companies such as Lanvin-Charles of the Ritz, Almay, Estée Lauder, Burt’s Bees, and Polysciences.
Friedman prides himself on his natural products. His product list includes hypoallergenic and natural versions of cleansers; toners; exfoliators; moisturizers and masks; shampoos; conditioners; dandruff treatments and hair sprays; antiperspirants and deodorants; lip balms; salves and cuticle treatments; shaving creams and aftershaves; over-the-counter analgesics; acne treatments and sunscreens; toothpastes; and liquid soap.
Friedman has said that he is most proud of his Burt’s Bees Orange Essence Cleansing Cream, which won Health Magazine’s Healthiest Cleanser of the Year in 1999. The product is an anhydrous, 100% natural, self-preserving translucent gel-emulsion of vegetable oil and vegetable glycerin stabilized by a proprietary protein.
During his tenure in the industry, Friedman has faced many hurdles in creating his natural formulations – achieving esthetics, efficacy, and physical stability at temperature extremes while maintaining microbiological integrity and using more green, renewable ingredients while formulating with fewer petrochemicals. His breakthrough natural formulations developed at Burt’s Bees are emulated and marketed widely today.
Sergio Nacht
Location: Las Vegas
Sergio Nacht is a biochemist, researcher, and product developer with 48 years of formulation experience. Currently, he is chief scientific officer/cofounder at resolutionMD and Riley-Nacht.
“A better understanding of the structure and function of the skin has resulted in the development of better functional products that deliver clinically demonstrable benefits and not only ‘hope in a jar,’ ” he has said.
Nacht has coauthored more than 50 scientific papers, and he holds 17 international and U.S. patents.
Possibly his most significant accomplishment followed the discovery of what he believes is one of the biggest challenges in skin care formulation. Microsponge Technology is the first – and still the only – U.S. Food and Drug Administration–approved controlled-release technology for topical products that maximizes efficacy while minimizing side effects and optimizing cosmetic attributes by allowing slow release of ingredients. The microsponge is used to provide various therapeutic solutions for antiaging, acne treatment, skin firming, skin lightening, and mattifying – most notably as the lead technology behind Retin-A Micro.
Byeong-Deog Park
Location: Seoul, South Korea
Byeong-Deog Park holds a Ph.D. in industrial chemicals from Seoul National University, among his other achievements. Dr. Park’s company, Neopharm, is located in Seoul. He is a true scientist who has been awarded many patents in the areas of ceramides for the treatment of dry skin and atopic dermatitis; PPAR (peroxisome proliferator-activated receptor)-alpha in the treatment of inflammatory disorders; and an antimicrobial peptide, Defensamide, which has been shown to prevent colonization of Staphylococcus aureus. His research led to the development of a proprietary MLE (multilamellar emulsion) technology in which lipids and ceramides form the identical Maltese cross structure that is seen in the natural lipid barrier of the skin, allowing effective skin barrier repair.
With MLE technology, the ceramides, fatty acids, and cholesterol required for an intact skin barrier are replaced in the proper ratio and three-dimensional structure needed to emulate the skin’s natural structure. This reforms the skin’s barrier and prevents water evaporation from the skin’s surface. Dr. Park has said that he is most proud of his patented MLE technology, found in the brands Atopalm and Zerafite. He also combined MLE technology and Defensamide in an atopic dermatitis treatment known as Zeroid.
Dr. Park never ceases to impress me with his scientific knowledge and dedication to the scientific method. In a field where many products are considered “hope in a jar,” his cosmetically elegant products stand out as “verified science in a jar.”
Gordon Dow
Location: Petaluma, Calif.
Gordon Dow started Dow Pharmaceutical Sciences in his garage. Today, Dow Pharmaceutical Sciences (recently acquired by Valeant Pharmaceuticals International) is a leading company in the formulation and manufacturing of dermatological products.
Over the past 25 years, Dow has commanded the company’s evolution by carefully balancing science and business. He previously served as vice president of research and development for Ingram Pharmaceuticals, where he developed seven commercially successful products, including four dermatologicals. He also served as the executive secretary of the research advisory panel for the State of California. A few of Dow’s best-known products include MetroGel, Ziana, and Acanya.
The passion for science and skin care of these individuals has shaped the dermatologic landscape for the best. They would probably agree with Julia Child, who once said, “Find something you’re passionate about and keep tremendously interested in it.”
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She founded the cosmetic dermatology center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (McGraw-Hill, April 2002), and a book for consumers, “The Skin Type Solution” (Bantam, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever.
In the multibillion-dollar skin care industry, there are many well-recognized brands. However, we sometimes forget that behind these products were formulators who took their scientific ideas and turned them into recipes for cosmetically elegant active formulations.
I have spent the last 15 years researching the activity of cosmeceutical ingredients for my new textbook, “Cosmeceuticals and Cosmetic Ingredients” (McGraw Hill, 2014). Each ingredient has its own quirks, and they all do not “play well in the sandbox” together. Formulation knowledge (cosmetic chemistry) is required to take these ingredients and combine them in a way that enhances rather than hinders their activity, just as a chef combines ingredients and cooking techniques to enhance the flavor and presentation of food. When I discuss cosmeceutical products, I always stress the importance of the ingredients and understanding ingredient interactions, because they determine the end product – how effective it is and how elegant it feels. If a product works well but smells bad and feels unpleasant, consumers will not use it.
Whom are we trusting when it comes to this science? The formulators, also known as cosmetic chemists, who put their blood, sweat, and tears into years of work to develop products that yield efficacious results. They are often behind the scenes, and their contributions are not always recognized. I refer to them as the “Julia Childs” of skin care, because they remind me of how Julia Child combined her knowledge of ingredients and aesthetic sensibilities to change the world of cooking.
I’d like to shine the spotlight on several top skin care formulators that I have met. Their relentless desire to perfect skin care recipes has helped the industry boom and has improved skin health.
Richard Parker
Location: Melbourne
Richard Parker is the CEO/founder of the Australia-based company Rationale. When he was unable to find skin care products that worked with his skin type, he decided to study cosmetic chemistry and create his own skin care line. Today, Rationale can be found in dermatologists’ and plastic surgeons’ offices across Australia. Parker’s passion for cosmetic science is evident. Australia has a high incidence of melanoma, and sunscreens undergo greater scrutiny there compared with other countries. One of the things that Parker is most proud of is his creation of SPF products that are “as elegant as they are effective.” This is a difficult combination to achieve, because sunscreens tend to be too white or too greasy; formulating them properly requires a “master chef.”
In addition to formulating effective and elegant sun protection, he has developed Essential Six: a combination of six products that work in synergy, delivering the perfect combination of active ingredients at the correct concentration to be recognized and utilized by skin cells.
In order to succeed in the formulations industry, you must possess a desire to make it better; and Parker does just that. It’s his wish for the industry to have an increased awareness of a holistic approach to skin care that includes immune protection, antioxidants, sunscreens, gentle cleansing, alpha-hydroxy acids, and vitamin A.
If being at the forefront of this evolution isn’t enough, Parker is devoted to continue his mission for years to come, all the while helping younger chemists/formulators embrace the culture.
“For the past 25 years, I have had the privilege to work with Australia’s leading dermatologists to create the best possible products and procedures,” he said. “At this stage of my career, it is so gratifying to see the younger generation of skin specialists embrace medical skin care as a part of best clinical practice.”
Chuck Friedman
Location: Wendell, N.C.
Chuck Friedman is a man who prides himself on the use of natural products – not a small achievement for a man who has been in the industry for almost half a century. His work as a formulation chemist has spanned globally recognized companies such as Lanvin-Charles of the Ritz, Almay, Estée Lauder, Burt’s Bees, and Polysciences.
Friedman prides himself on his natural products. His product list includes hypoallergenic and natural versions of cleansers; toners; exfoliators; moisturizers and masks; shampoos; conditioners; dandruff treatments and hair sprays; antiperspirants and deodorants; lip balms; salves and cuticle treatments; shaving creams and aftershaves; over-the-counter analgesics; acne treatments and sunscreens; toothpastes; and liquid soap.
Friedman has said that he is most proud of his Burt’s Bees Orange Essence Cleansing Cream, which won Health Magazine’s Healthiest Cleanser of the Year in 1999. The product is an anhydrous, 100% natural, self-preserving translucent gel-emulsion of vegetable oil and vegetable glycerin stabilized by a proprietary protein.
During his tenure in the industry, Friedman has faced many hurdles in creating his natural formulations – achieving esthetics, efficacy, and physical stability at temperature extremes while maintaining microbiological integrity and using more green, renewable ingredients while formulating with fewer petrochemicals. His breakthrough natural formulations developed at Burt’s Bees are emulated and marketed widely today.
Sergio Nacht
Location: Las Vegas
Sergio Nacht is a biochemist, researcher, and product developer with 48 years of formulation experience. Currently, he is chief scientific officer/cofounder at resolutionMD and Riley-Nacht.
“A better understanding of the structure and function of the skin has resulted in the development of better functional products that deliver clinically demonstrable benefits and not only ‘hope in a jar,’ ” he has said.
Nacht has coauthored more than 50 scientific papers, and he holds 17 international and U.S. patents.
Possibly his most significant accomplishment followed the discovery of what he believes is one of the biggest challenges in skin care formulation. Microsponge Technology is the first – and still the only – U.S. Food and Drug Administration–approved controlled-release technology for topical products that maximizes efficacy while minimizing side effects and optimizing cosmetic attributes by allowing slow release of ingredients. The microsponge is used to provide various therapeutic solutions for antiaging, acne treatment, skin firming, skin lightening, and mattifying – most notably as the lead technology behind Retin-A Micro.
Byeong-Deog Park
Location: Seoul, South Korea
Byeong-Deog Park holds a Ph.D. in industrial chemicals from Seoul National University, among his other achievements. Dr. Park’s company, Neopharm, is located in Seoul. He is a true scientist who has been awarded many patents in the areas of ceramides for the treatment of dry skin and atopic dermatitis; PPAR (peroxisome proliferator-activated receptor)-alpha in the treatment of inflammatory disorders; and an antimicrobial peptide, Defensamide, which has been shown to prevent colonization of Staphylococcus aureus. His research led to the development of a proprietary MLE (multilamellar emulsion) technology in which lipids and ceramides form the identical Maltese cross structure that is seen in the natural lipid barrier of the skin, allowing effective skin barrier repair.
With MLE technology, the ceramides, fatty acids, and cholesterol required for an intact skin barrier are replaced in the proper ratio and three-dimensional structure needed to emulate the skin’s natural structure. This reforms the skin’s barrier and prevents water evaporation from the skin’s surface. Dr. Park has said that he is most proud of his patented MLE technology, found in the brands Atopalm and Zerafite. He also combined MLE technology and Defensamide in an atopic dermatitis treatment known as Zeroid.
Dr. Park never ceases to impress me with his scientific knowledge and dedication to the scientific method. In a field where many products are considered “hope in a jar,” his cosmetically elegant products stand out as “verified science in a jar.”
Gordon Dow
Location: Petaluma, Calif.
Gordon Dow started Dow Pharmaceutical Sciences in his garage. Today, Dow Pharmaceutical Sciences (recently acquired by Valeant Pharmaceuticals International) is a leading company in the formulation and manufacturing of dermatological products.
Over the past 25 years, Dow has commanded the company’s evolution by carefully balancing science and business. He previously served as vice president of research and development for Ingram Pharmaceuticals, where he developed seven commercially successful products, including four dermatologicals. He also served as the executive secretary of the research advisory panel for the State of California. A few of Dow’s best-known products include MetroGel, Ziana, and Acanya.
The passion for science and skin care of these individuals has shaped the dermatologic landscape for the best. They would probably agree with Julia Child, who once said, “Find something you’re passionate about and keep tremendously interested in it.”
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She founded the cosmetic dermatology center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (McGraw-Hill, April 2002), and a book for consumers, “The Skin Type Solution” (Bantam, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever.
In the multibillion-dollar skin care industry, there are many well-recognized brands. However, we sometimes forget that behind these products were formulators who took their scientific ideas and turned them into recipes for cosmetically elegant active formulations.
I have spent the last 15 years researching the activity of cosmeceutical ingredients for my new textbook, “Cosmeceuticals and Cosmetic Ingredients” (McGraw Hill, 2014). Each ingredient has its own quirks, and they all do not “play well in the sandbox” together. Formulation knowledge (cosmetic chemistry) is required to take these ingredients and combine them in a way that enhances rather than hinders their activity, just as a chef combines ingredients and cooking techniques to enhance the flavor and presentation of food. When I discuss cosmeceutical products, I always stress the importance of the ingredients and understanding ingredient interactions, because they determine the end product – how effective it is and how elegant it feels. If a product works well but smells bad and feels unpleasant, consumers will not use it.
Whom are we trusting when it comes to this science? The formulators, also known as cosmetic chemists, who put their blood, sweat, and tears into years of work to develop products that yield efficacious results. They are often behind the scenes, and their contributions are not always recognized. I refer to them as the “Julia Childs” of skin care, because they remind me of how Julia Child combined her knowledge of ingredients and aesthetic sensibilities to change the world of cooking.
I’d like to shine the spotlight on several top skin care formulators that I have met. Their relentless desire to perfect skin care recipes has helped the industry boom and has improved skin health.
Richard Parker
Location: Melbourne
Richard Parker is the CEO/founder of the Australia-based company Rationale. When he was unable to find skin care products that worked with his skin type, he decided to study cosmetic chemistry and create his own skin care line. Today, Rationale can be found in dermatologists’ and plastic surgeons’ offices across Australia. Parker’s passion for cosmetic science is evident. Australia has a high incidence of melanoma, and sunscreens undergo greater scrutiny there compared with other countries. One of the things that Parker is most proud of is his creation of SPF products that are “as elegant as they are effective.” This is a difficult combination to achieve, because sunscreens tend to be too white or too greasy; formulating them properly requires a “master chef.”
In addition to formulating effective and elegant sun protection, he has developed Essential Six: a combination of six products that work in synergy, delivering the perfect combination of active ingredients at the correct concentration to be recognized and utilized by skin cells.
In order to succeed in the formulations industry, you must possess a desire to make it better; and Parker does just that. It’s his wish for the industry to have an increased awareness of a holistic approach to skin care that includes immune protection, antioxidants, sunscreens, gentle cleansing, alpha-hydroxy acids, and vitamin A.
If being at the forefront of this evolution isn’t enough, Parker is devoted to continue his mission for years to come, all the while helping younger chemists/formulators embrace the culture.
“For the past 25 years, I have had the privilege to work with Australia’s leading dermatologists to create the best possible products and procedures,” he said. “At this stage of my career, it is so gratifying to see the younger generation of skin specialists embrace medical skin care as a part of best clinical practice.”
Chuck Friedman
Location: Wendell, N.C.
Chuck Friedman is a man who prides himself on the use of natural products – not a small achievement for a man who has been in the industry for almost half a century. His work as a formulation chemist has spanned globally recognized companies such as Lanvin-Charles of the Ritz, Almay, Estée Lauder, Burt’s Bees, and Polysciences.
Friedman prides himself on his natural products. His product list includes hypoallergenic and natural versions of cleansers; toners; exfoliators; moisturizers and masks; shampoos; conditioners; dandruff treatments and hair sprays; antiperspirants and deodorants; lip balms; salves and cuticle treatments; shaving creams and aftershaves; over-the-counter analgesics; acne treatments and sunscreens; toothpastes; and liquid soap.
Friedman has said that he is most proud of his Burt’s Bees Orange Essence Cleansing Cream, which won Health Magazine’s Healthiest Cleanser of the Year in 1999. The product is an anhydrous, 100% natural, self-preserving translucent gel-emulsion of vegetable oil and vegetable glycerin stabilized by a proprietary protein.
During his tenure in the industry, Friedman has faced many hurdles in creating his natural formulations – achieving esthetics, efficacy, and physical stability at temperature extremes while maintaining microbiological integrity and using more green, renewable ingredients while formulating with fewer petrochemicals. His breakthrough natural formulations developed at Burt’s Bees are emulated and marketed widely today.
Sergio Nacht
Location: Las Vegas
Sergio Nacht is a biochemist, researcher, and product developer with 48 years of formulation experience. Currently, he is chief scientific officer/cofounder at resolutionMD and Riley-Nacht.
“A better understanding of the structure and function of the skin has resulted in the development of better functional products that deliver clinically demonstrable benefits and not only ‘hope in a jar,’ ” he has said.
Nacht has coauthored more than 50 scientific papers, and he holds 17 international and U.S. patents.
Possibly his most significant accomplishment followed the discovery of what he believes is one of the biggest challenges in skin care formulation. Microsponge Technology is the first – and still the only – U.S. Food and Drug Administration–approved controlled-release technology for topical products that maximizes efficacy while minimizing side effects and optimizing cosmetic attributes by allowing slow release of ingredients. The microsponge is used to provide various therapeutic solutions for antiaging, acne treatment, skin firming, skin lightening, and mattifying – most notably as the lead technology behind Retin-A Micro.
Byeong-Deog Park
Location: Seoul, South Korea
Byeong-Deog Park holds a Ph.D. in industrial chemicals from Seoul National University, among his other achievements. Dr. Park’s company, Neopharm, is located in Seoul. He is a true scientist who has been awarded many patents in the areas of ceramides for the treatment of dry skin and atopic dermatitis; PPAR (peroxisome proliferator-activated receptor)-alpha in the treatment of inflammatory disorders; and an antimicrobial peptide, Defensamide, which has been shown to prevent colonization of Staphylococcus aureus. His research led to the development of a proprietary MLE (multilamellar emulsion) technology in which lipids and ceramides form the identical Maltese cross structure that is seen in the natural lipid barrier of the skin, allowing effective skin barrier repair.
With MLE technology, the ceramides, fatty acids, and cholesterol required for an intact skin barrier are replaced in the proper ratio and three-dimensional structure needed to emulate the skin’s natural structure. This reforms the skin’s barrier and prevents water evaporation from the skin’s surface. Dr. Park has said that he is most proud of his patented MLE technology, found in the brands Atopalm and Zerafite. He also combined MLE technology and Defensamide in an atopic dermatitis treatment known as Zeroid.
Dr. Park never ceases to impress me with his scientific knowledge and dedication to the scientific method. In a field where many products are considered “hope in a jar,” his cosmetically elegant products stand out as “verified science in a jar.”
Gordon Dow
Location: Petaluma, Calif.
Gordon Dow started Dow Pharmaceutical Sciences in his garage. Today, Dow Pharmaceutical Sciences (recently acquired by Valeant Pharmaceuticals International) is a leading company in the formulation and manufacturing of dermatological products.
Over the past 25 years, Dow has commanded the company’s evolution by carefully balancing science and business. He previously served as vice president of research and development for Ingram Pharmaceuticals, where he developed seven commercially successful products, including four dermatologicals. He also served as the executive secretary of the research advisory panel for the State of California. A few of Dow’s best-known products include MetroGel, Ziana, and Acanya.
The passion for science and skin care of these individuals has shaped the dermatologic landscape for the best. They would probably agree with Julia Child, who once said, “Find something you’re passionate about and keep tremendously interested in it.”
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She founded the cosmetic dermatology center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (McGraw-Hill, April 2002), and a book for consumers, “The Skin Type Solution” (Bantam, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever.
Helping history come alive online in Vascular Specialist
Medical history is more than just the praise of great heroes and heroines and the nearly mythical stories of serendipitous discovery and invention. It is the means by which we learn to understand and appreciate the qualities and conditions that lead to innovation and it illuminates the present by honoring the past.
Since its inception in 2005, Vascular Specialist has highlighted some of the most important persons and events in the history of vascular surgery and medicine in our column, Vascular Surgery Chronicles.
Most of these articles are out of print and have not been available online. But that has changed.
As part of our new and improved wesbite, we are now placing these articles on the Vascular Specialist web site, where they can be visited here, and we are renewing our committment to making the Vascular Surgery Chronicles column a more frequent feature of the print edition and also our special online-only newsletters.
Currently available articles on the web site highlight such notable figures as:
Look for these and other articles and stay tuned for more windows on the vascular past online and in the pages of Vascular Specialist.
Medical history is more than just the praise of great heroes and heroines and the nearly mythical stories of serendipitous discovery and invention. It is the means by which we learn to understand and appreciate the qualities and conditions that lead to innovation and it illuminates the present by honoring the past.
Since its inception in 2005, Vascular Specialist has highlighted some of the most important persons and events in the history of vascular surgery and medicine in our column, Vascular Surgery Chronicles.
Most of these articles are out of print and have not been available online. But that has changed.
As part of our new and improved wesbite, we are now placing these articles on the Vascular Specialist web site, where they can be visited here, and we are renewing our committment to making the Vascular Surgery Chronicles column a more frequent feature of the print edition and also our special online-only newsletters.
Currently available articles on the web site highlight such notable figures as:
Look for these and other articles and stay tuned for more windows on the vascular past online and in the pages of Vascular Specialist.
Medical history is more than just the praise of great heroes and heroines and the nearly mythical stories of serendipitous discovery and invention. It is the means by which we learn to understand and appreciate the qualities and conditions that lead to innovation and it illuminates the present by honoring the past.
Since its inception in 2005, Vascular Specialist has highlighted some of the most important persons and events in the history of vascular surgery and medicine in our column, Vascular Surgery Chronicles.
Most of these articles are out of print and have not been available online. But that has changed.
As part of our new and improved wesbite, we are now placing these articles on the Vascular Specialist web site, where they can be visited here, and we are renewing our committment to making the Vascular Surgery Chronicles column a more frequent feature of the print edition and also our special online-only newsletters.
Currently available articles on the web site highlight such notable figures as:
Look for these and other articles and stay tuned for more windows on the vascular past online and in the pages of Vascular Specialist.
Digital Dermatology: Online service recovery
We can learn a lot from a car rentals. Like medicine, they are a service industry. And all service industries have the same problem: Service is delivered in real time, and the quality of that service depends on variables that may or may not be in the company’s control.
Even so, one bad experience can result in termination of a life-long customer, or in our case, patient. Worse, the patient can now go online and write a scathing review, criticizing everything from your bedside manner to the artwork in your waiting room.
What can you do when a visit goes wrong? Employ service-recovery techniques. Service recovery is the act of trying to resuscitate an encounter once things have gone badly. It happens to physicians and to restaurants and to car rentals.
While on vacation with my wife in Salt Lake City, we rented a car from a company (let’s call them “Discount Cars”). We don’t usually book with them; however, we got double airline points for choosing them, so we bit.
At the airport rental terminal, we waited for 15 minutes before being helped. When we reached the counter, we were told that our reserved car was not ready yet. (I immediately thought of the Seinfeld episode when Jerry says: “So you can take a reservation, but you can’t keep a reservation?!”) We were advised that our car was being washed and would be ready in 15 minutes “tops.” Thirty minutes later, my miffed wife pushed through the line to the counter. “It’s still being washed,” she was told. So she asked for another car and was offered a full-size pickup truck. My wife, who drives a teeny Honda Fit at home, said no thanks. Another 30 minutes passed and my incensed wife returned to the counter. “It’s been over an hour! This is unacceptable!” A different representative replied it was our fault for declining the pickup truck. There would be more cars soon, so they promised.
We were too far to walk to any airport bars, and the situation was rapidly deteriorating. I decided to take action. I fired up Twitter and let her rip:
“Closing in on 1 hr for a car promised in 15 min. Which we reserved ahead. This isn’t the first time, @DiscountCars #operations #fail.”
Within minutes, they replied by Twitter:
Them: @Dermdoc We are so sorry for the wait! What location are you at?
My wife, along with five other equally incensed wives, continued to wait for a response (and a car) from the live representatives at the counter.
Nearly 1 hour and 20 minutes later, we got a car. It was much larger than we wanted, but we were done waiting. After signing the papers, we got inside – it reeked of smoke. Oh, this is no bueno, I thought. We requested a different car. Twenty more minutes passed before our smoke-free vehicle arrived. The gas tank was 7/8’s full. And the carpets were littered with twigs and leaves.
Now I’m thinking, this is so bad, I should write an article about it. From the front seat of our faulty but moving vehicle, I fired again: “Dear @DiscountCars we waited 1+ hrs. Not the car we wanted. Then tank not full. Yet, not a single apology from anyone. Really?”
Them: @Dermdoc, we are sorry.
Them: @Dermdoc Please e-mail us the details and your RA# to [email protected] so we can look into this for you!
Me: @Discount Thank you! Will do.
I sent a list of grievances to the e-mail as they requested. Within an hour they offered us a $50 credit on a future rental.
What’s remarkable about this story is that not a single live person was able to assuage us, but their digital team managed to apologize and save us as customers. There might have been legitimate reasons for their service failure, but it didn’t matter. What mattered was that they responded to me personally, apologized, and made amends. This is an important lesson for us physicians. Patients will expect that your digital channels are legitimate ways to express their level of satisfaction with your practice. The stakes are higher for us in health care in particular because of the risks of violating patients’ privacy. However, as you can see from the rental car example, it can effectively be done without revealing any information about the customer or the experience. The goal is to recover the service publicly and take all of the information offline and manage it in a secure, private fashion.
The formula is simple: Believe the customer. Listen. Apologize for not satisfying the customer. Even if you’ve done nothing wrong, you have in some way failed to satisfy the customer’s needs. Ask for more information in a secure, private manner, never on a public platform. Do what you can reasonably do to remedy the problem and remediate the situation.
Dr. Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego, and volunteer clinical assistant professor at the University of California, San Diego. Dr. Benabio is @dermdoc on Twitter.
We can learn a lot from a car rentals. Like medicine, they are a service industry. And all service industries have the same problem: Service is delivered in real time, and the quality of that service depends on variables that may or may not be in the company’s control.
Even so, one bad experience can result in termination of a life-long customer, or in our case, patient. Worse, the patient can now go online and write a scathing review, criticizing everything from your bedside manner to the artwork in your waiting room.
What can you do when a visit goes wrong? Employ service-recovery techniques. Service recovery is the act of trying to resuscitate an encounter once things have gone badly. It happens to physicians and to restaurants and to car rentals.
While on vacation with my wife in Salt Lake City, we rented a car from a company (let’s call them “Discount Cars”). We don’t usually book with them; however, we got double airline points for choosing them, so we bit.
At the airport rental terminal, we waited for 15 minutes before being helped. When we reached the counter, we were told that our reserved car was not ready yet. (I immediately thought of the Seinfeld episode when Jerry says: “So you can take a reservation, but you can’t keep a reservation?!”) We were advised that our car was being washed and would be ready in 15 minutes “tops.” Thirty minutes later, my miffed wife pushed through the line to the counter. “It’s still being washed,” she was told. So she asked for another car and was offered a full-size pickup truck. My wife, who drives a teeny Honda Fit at home, said no thanks. Another 30 minutes passed and my incensed wife returned to the counter. “It’s been over an hour! This is unacceptable!” A different representative replied it was our fault for declining the pickup truck. There would be more cars soon, so they promised.
We were too far to walk to any airport bars, and the situation was rapidly deteriorating. I decided to take action. I fired up Twitter and let her rip:
“Closing in on 1 hr for a car promised in 15 min. Which we reserved ahead. This isn’t the first time, @DiscountCars #operations #fail.”
Within minutes, they replied by Twitter:
Them: @Dermdoc We are so sorry for the wait! What location are you at?
My wife, along with five other equally incensed wives, continued to wait for a response (and a car) from the live representatives at the counter.
Nearly 1 hour and 20 minutes later, we got a car. It was much larger than we wanted, but we were done waiting. After signing the papers, we got inside – it reeked of smoke. Oh, this is no bueno, I thought. We requested a different car. Twenty more minutes passed before our smoke-free vehicle arrived. The gas tank was 7/8’s full. And the carpets were littered with twigs and leaves.
Now I’m thinking, this is so bad, I should write an article about it. From the front seat of our faulty but moving vehicle, I fired again: “Dear @DiscountCars we waited 1+ hrs. Not the car we wanted. Then tank not full. Yet, not a single apology from anyone. Really?”
Them: @Dermdoc, we are sorry.
Them: @Dermdoc Please e-mail us the details and your RA# to [email protected] so we can look into this for you!
Me: @Discount Thank you! Will do.
I sent a list of grievances to the e-mail as they requested. Within an hour they offered us a $50 credit on a future rental.
What’s remarkable about this story is that not a single live person was able to assuage us, but their digital team managed to apologize and save us as customers. There might have been legitimate reasons for their service failure, but it didn’t matter. What mattered was that they responded to me personally, apologized, and made amends. This is an important lesson for us physicians. Patients will expect that your digital channels are legitimate ways to express their level of satisfaction with your practice. The stakes are higher for us in health care in particular because of the risks of violating patients’ privacy. However, as you can see from the rental car example, it can effectively be done without revealing any information about the customer or the experience. The goal is to recover the service publicly and take all of the information offline and manage it in a secure, private fashion.
The formula is simple: Believe the customer. Listen. Apologize for not satisfying the customer. Even if you’ve done nothing wrong, you have in some way failed to satisfy the customer’s needs. Ask for more information in a secure, private manner, never on a public platform. Do what you can reasonably do to remedy the problem and remediate the situation.
Dr. Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego, and volunteer clinical assistant professor at the University of California, San Diego. Dr. Benabio is @dermdoc on Twitter.
We can learn a lot from a car rentals. Like medicine, they are a service industry. And all service industries have the same problem: Service is delivered in real time, and the quality of that service depends on variables that may or may not be in the company’s control.
Even so, one bad experience can result in termination of a life-long customer, or in our case, patient. Worse, the patient can now go online and write a scathing review, criticizing everything from your bedside manner to the artwork in your waiting room.
What can you do when a visit goes wrong? Employ service-recovery techniques. Service recovery is the act of trying to resuscitate an encounter once things have gone badly. It happens to physicians and to restaurants and to car rentals.
While on vacation with my wife in Salt Lake City, we rented a car from a company (let’s call them “Discount Cars”). We don’t usually book with them; however, we got double airline points for choosing them, so we bit.
At the airport rental terminal, we waited for 15 minutes before being helped. When we reached the counter, we were told that our reserved car was not ready yet. (I immediately thought of the Seinfeld episode when Jerry says: “So you can take a reservation, but you can’t keep a reservation?!”) We were advised that our car was being washed and would be ready in 15 minutes “tops.” Thirty minutes later, my miffed wife pushed through the line to the counter. “It’s still being washed,” she was told. So she asked for another car and was offered a full-size pickup truck. My wife, who drives a teeny Honda Fit at home, said no thanks. Another 30 minutes passed and my incensed wife returned to the counter. “It’s been over an hour! This is unacceptable!” A different representative replied it was our fault for declining the pickup truck. There would be more cars soon, so they promised.
We were too far to walk to any airport bars, and the situation was rapidly deteriorating. I decided to take action. I fired up Twitter and let her rip:
“Closing in on 1 hr for a car promised in 15 min. Which we reserved ahead. This isn’t the first time, @DiscountCars #operations #fail.”
Within minutes, they replied by Twitter:
Them: @Dermdoc We are so sorry for the wait! What location are you at?
My wife, along with five other equally incensed wives, continued to wait for a response (and a car) from the live representatives at the counter.
Nearly 1 hour and 20 minutes later, we got a car. It was much larger than we wanted, but we were done waiting. After signing the papers, we got inside – it reeked of smoke. Oh, this is no bueno, I thought. We requested a different car. Twenty more minutes passed before our smoke-free vehicle arrived. The gas tank was 7/8’s full. And the carpets were littered with twigs and leaves.
Now I’m thinking, this is so bad, I should write an article about it. From the front seat of our faulty but moving vehicle, I fired again: “Dear @DiscountCars we waited 1+ hrs. Not the car we wanted. Then tank not full. Yet, not a single apology from anyone. Really?”
Them: @Dermdoc, we are sorry.
Them: @Dermdoc Please e-mail us the details and your RA# to [email protected] so we can look into this for you!
Me: @Discount Thank you! Will do.
I sent a list of grievances to the e-mail as they requested. Within an hour they offered us a $50 credit on a future rental.
What’s remarkable about this story is that not a single live person was able to assuage us, but their digital team managed to apologize and save us as customers. There might have been legitimate reasons for their service failure, but it didn’t matter. What mattered was that they responded to me personally, apologized, and made amends. This is an important lesson for us physicians. Patients will expect that your digital channels are legitimate ways to express their level of satisfaction with your practice. The stakes are higher for us in health care in particular because of the risks of violating patients’ privacy. However, as you can see from the rental car example, it can effectively be done without revealing any information about the customer or the experience. The goal is to recover the service publicly and take all of the information offline and manage it in a secure, private fashion.
The formula is simple: Believe the customer. Listen. Apologize for not satisfying the customer. Even if you’ve done nothing wrong, you have in some way failed to satisfy the customer’s needs. Ask for more information in a secure, private manner, never on a public platform. Do what you can reasonably do to remedy the problem and remediate the situation.
Dr. Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego, and volunteer clinical assistant professor at the University of California, San Diego. Dr. Benabio is @dermdoc on Twitter.
Why screen if there are no services?
Do you remember the discussion of the ethical dilemma of Huntington’s disease you probably participated in during medical school? The question was whether you would want to know that you were at risk for a chronic debilitating condition that would develop at some later age if there was nothing that could be done about it. In that discussion, you also may have heard about individuals who, after hearing about their risk status, become depressed or suicidal, depending on the story line.
Some pediatricians seem to have taken this example too far in arguing that there is no point in screening for issues of development, autism, maternal depression, or child mental health because “there are no services” available to treat them.
Despair is understandable. Physicians’ lack of knowledge about resources in the community is often a sore point among local agencies, parents, and even pediatricians themselves. In spite of United Way, state 3-1-1 programs ,and the occasionally available social worker, the resources with which we are familiar sometimes come from hard-working parents telling us about a program they found on their own. It also seems that, just when we hit upon a valuable resource, it runs out of funding, changes eligibility requirements, or loses key staff. Worse yet, we may rely on resources we know about because of our own children’s problems, activities, or friends. While the Internet is an increasingly valuable method of finding resources, there is no filter of the evidence-basis of the care provided, and the process of searching, vetting, and informing your patients is extremely time consuming, and often the patient is not eligible or has a long waiting period after all that.
There are important reasons not to succumb to throwing up one’s hands about service availability. And more important reasons to still screen even if you do not know where to refer.
Screening using validated tools is recommended by the American Academy of Pediatrics because parent concern and even clinical observation are not adequately sensitive to detect significant problems of development and mental health, even when done by experienced physicians who know families well. The process of screening sends an important message to the parents – that you care about the child’s progress and are using proven methods to ensure that it is going well and consider it part of complete medical care.
And families often already think that their child may have a problem, even when they don’t bring it up. Perhaps deep down they are afraid that somehow raising the question of autism will make it true. They may be in denial, are feeling guilty, or are under pressure from their spouse, relatives, or friends not to worry, that “he will grow out” of it, that better discipline will fix the problem, etc. They may even care so much about your positive regard that they do not want to seem overly anxious, obsessive, or be regarded as a failure for having a “defective” child. They, like you, also may be in despair about finding effective help.
But there can be serious consequences to not screening, even when you are not sure what you will do with the results. The family may push the child with delays or mental health problems beyond his abilities, and even become negative and punitive in trying to make him succeed, in the process promoting unnecessary behavior problems, discouragement, and even defiance in the child. Failure to detect also means failure to list the child on a registry for follow-up to determine progress or refer when resources become available. Some problems of development or mental health that are detected by screening may have medical causes that you can treat, even though counseling or therapy interventions are not available. Examples include hearing or vision deficits causing delays or anemia, sleep apnea, or hypothyroidism or maternal depression or attention-deficit/hyperactivity disorder (ADHD). For issues with a genetic basis, siblings may be born with same problem during the period of delay in making a diagnosis, a prime example being Fragile X. In untold cases, the family loses trust in you and in the medical system for not acknowledging a problem.
In many cases, your acknowledgment, explanation, sympathy, and advice can help enormously. Families can cope better, garner support from family or friends, deal with the child’s behavior better, and find steps to take to help their child in their own ways, even without formal services, once told that their child has a specific problem.
On a system level, it is important to realize that how services are established and maintained is far less rational than might be imagined. State programs, schools, hospitals, and insurers all have legal requirements to provide services within a certain time frame once referred. Even if the services are not there to help a your child or family right now, the referral itself adds to the data used to determine if services are adequate and to plan for additional service types or capacity. The Autism Waiver is one such example where waits are years long, but getting on the list is crucial to the future of the program.
Until you screen and give parents information – especially middle-class parents – we will never have the resources. As it was for lead paint, until we identified prevalence of elevated lead levels and the harm associated, we got no action on lead paint removal policies. Another example where complaints about access made a difference, is the relatively new Paul Wellstone and Pete Domenici Mental Health Parity and Addiction Equity Act of 2008 that requires health insurers and health plans to guarantee that financial requirements on benefits for mental health, such as copays, deductibles, and limitations on treatment benefits, are not more restrictive than those that are for medical benefits. This does not guarantee that services will be available or of high quality, but is a step toward accessibility.
You may be one of the many pediatricians who consider advocacy a basic component of your professional responsibilities. If you cannot advocate for services that you see your patients in need of, you can pass your concerns onto a group that does. Many American Academy of Pediatrics state chapters have so-called Pediatric Councils that receive ideas about system problems and put group pressure on leaders in the state to address them.
As in the historic painting of the physician leaning over the ill child whom he could not cure, after detection through screening our thoughtful evaluation, explanations, shared concern, and our patients’ advocacy have great value even when specific services are not yet available.
Dr. Howard is an assistant professor of pediatrics at the Johns Hopkins University, Baltimore, and creator of CHADIS (www.chadis.com). She has no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to Frontline Medical News. E-mail her at [email protected].
Do you remember the discussion of the ethical dilemma of Huntington’s disease you probably participated in during medical school? The question was whether you would want to know that you were at risk for a chronic debilitating condition that would develop at some later age if there was nothing that could be done about it. In that discussion, you also may have heard about individuals who, after hearing about their risk status, become depressed or suicidal, depending on the story line.
Some pediatricians seem to have taken this example too far in arguing that there is no point in screening for issues of development, autism, maternal depression, or child mental health because “there are no services” available to treat them.
Despair is understandable. Physicians’ lack of knowledge about resources in the community is often a sore point among local agencies, parents, and even pediatricians themselves. In spite of United Way, state 3-1-1 programs ,and the occasionally available social worker, the resources with which we are familiar sometimes come from hard-working parents telling us about a program they found on their own. It also seems that, just when we hit upon a valuable resource, it runs out of funding, changes eligibility requirements, or loses key staff. Worse yet, we may rely on resources we know about because of our own children’s problems, activities, or friends. While the Internet is an increasingly valuable method of finding resources, there is no filter of the evidence-basis of the care provided, and the process of searching, vetting, and informing your patients is extremely time consuming, and often the patient is not eligible or has a long waiting period after all that.
There are important reasons not to succumb to throwing up one’s hands about service availability. And more important reasons to still screen even if you do not know where to refer.
Screening using validated tools is recommended by the American Academy of Pediatrics because parent concern and even clinical observation are not adequately sensitive to detect significant problems of development and mental health, even when done by experienced physicians who know families well. The process of screening sends an important message to the parents – that you care about the child’s progress and are using proven methods to ensure that it is going well and consider it part of complete medical care.
And families often already think that their child may have a problem, even when they don’t bring it up. Perhaps deep down they are afraid that somehow raising the question of autism will make it true. They may be in denial, are feeling guilty, or are under pressure from their spouse, relatives, or friends not to worry, that “he will grow out” of it, that better discipline will fix the problem, etc. They may even care so much about your positive regard that they do not want to seem overly anxious, obsessive, or be regarded as a failure for having a “defective” child. They, like you, also may be in despair about finding effective help.
But there can be serious consequences to not screening, even when you are not sure what you will do with the results. The family may push the child with delays or mental health problems beyond his abilities, and even become negative and punitive in trying to make him succeed, in the process promoting unnecessary behavior problems, discouragement, and even defiance in the child. Failure to detect also means failure to list the child on a registry for follow-up to determine progress or refer when resources become available. Some problems of development or mental health that are detected by screening may have medical causes that you can treat, even though counseling or therapy interventions are not available. Examples include hearing or vision deficits causing delays or anemia, sleep apnea, or hypothyroidism or maternal depression or attention-deficit/hyperactivity disorder (ADHD). For issues with a genetic basis, siblings may be born with same problem during the period of delay in making a diagnosis, a prime example being Fragile X. In untold cases, the family loses trust in you and in the medical system for not acknowledging a problem.
In many cases, your acknowledgment, explanation, sympathy, and advice can help enormously. Families can cope better, garner support from family or friends, deal with the child’s behavior better, and find steps to take to help their child in their own ways, even without formal services, once told that their child has a specific problem.
On a system level, it is important to realize that how services are established and maintained is far less rational than might be imagined. State programs, schools, hospitals, and insurers all have legal requirements to provide services within a certain time frame once referred. Even if the services are not there to help a your child or family right now, the referral itself adds to the data used to determine if services are adequate and to plan for additional service types or capacity. The Autism Waiver is one such example where waits are years long, but getting on the list is crucial to the future of the program.
Until you screen and give parents information – especially middle-class parents – we will never have the resources. As it was for lead paint, until we identified prevalence of elevated lead levels and the harm associated, we got no action on lead paint removal policies. Another example where complaints about access made a difference, is the relatively new Paul Wellstone and Pete Domenici Mental Health Parity and Addiction Equity Act of 2008 that requires health insurers and health plans to guarantee that financial requirements on benefits for mental health, such as copays, deductibles, and limitations on treatment benefits, are not more restrictive than those that are for medical benefits. This does not guarantee that services will be available or of high quality, but is a step toward accessibility.
You may be one of the many pediatricians who consider advocacy a basic component of your professional responsibilities. If you cannot advocate for services that you see your patients in need of, you can pass your concerns onto a group that does. Many American Academy of Pediatrics state chapters have so-called Pediatric Councils that receive ideas about system problems and put group pressure on leaders in the state to address them.
As in the historic painting of the physician leaning over the ill child whom he could not cure, after detection through screening our thoughtful evaluation, explanations, shared concern, and our patients’ advocacy have great value even when specific services are not yet available.
Dr. Howard is an assistant professor of pediatrics at the Johns Hopkins University, Baltimore, and creator of CHADIS (www.chadis.com). She has no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to Frontline Medical News. E-mail her at [email protected].
Do you remember the discussion of the ethical dilemma of Huntington’s disease you probably participated in during medical school? The question was whether you would want to know that you were at risk for a chronic debilitating condition that would develop at some later age if there was nothing that could be done about it. In that discussion, you also may have heard about individuals who, after hearing about their risk status, become depressed or suicidal, depending on the story line.
Some pediatricians seem to have taken this example too far in arguing that there is no point in screening for issues of development, autism, maternal depression, or child mental health because “there are no services” available to treat them.
Despair is understandable. Physicians’ lack of knowledge about resources in the community is often a sore point among local agencies, parents, and even pediatricians themselves. In spite of United Way, state 3-1-1 programs ,and the occasionally available social worker, the resources with which we are familiar sometimes come from hard-working parents telling us about a program they found on their own. It also seems that, just when we hit upon a valuable resource, it runs out of funding, changes eligibility requirements, or loses key staff. Worse yet, we may rely on resources we know about because of our own children’s problems, activities, or friends. While the Internet is an increasingly valuable method of finding resources, there is no filter of the evidence-basis of the care provided, and the process of searching, vetting, and informing your patients is extremely time consuming, and often the patient is not eligible or has a long waiting period after all that.
There are important reasons not to succumb to throwing up one’s hands about service availability. And more important reasons to still screen even if you do not know where to refer.
Screening using validated tools is recommended by the American Academy of Pediatrics because parent concern and even clinical observation are not adequately sensitive to detect significant problems of development and mental health, even when done by experienced physicians who know families well. The process of screening sends an important message to the parents – that you care about the child’s progress and are using proven methods to ensure that it is going well and consider it part of complete medical care.
And families often already think that their child may have a problem, even when they don’t bring it up. Perhaps deep down they are afraid that somehow raising the question of autism will make it true. They may be in denial, are feeling guilty, or are under pressure from their spouse, relatives, or friends not to worry, that “he will grow out” of it, that better discipline will fix the problem, etc. They may even care so much about your positive regard that they do not want to seem overly anxious, obsessive, or be regarded as a failure for having a “defective” child. They, like you, also may be in despair about finding effective help.
But there can be serious consequences to not screening, even when you are not sure what you will do with the results. The family may push the child with delays or mental health problems beyond his abilities, and even become negative and punitive in trying to make him succeed, in the process promoting unnecessary behavior problems, discouragement, and even defiance in the child. Failure to detect also means failure to list the child on a registry for follow-up to determine progress or refer when resources become available. Some problems of development or mental health that are detected by screening may have medical causes that you can treat, even though counseling or therapy interventions are not available. Examples include hearing or vision deficits causing delays or anemia, sleep apnea, or hypothyroidism or maternal depression or attention-deficit/hyperactivity disorder (ADHD). For issues with a genetic basis, siblings may be born with same problem during the period of delay in making a diagnosis, a prime example being Fragile X. In untold cases, the family loses trust in you and in the medical system for not acknowledging a problem.
In many cases, your acknowledgment, explanation, sympathy, and advice can help enormously. Families can cope better, garner support from family or friends, deal with the child’s behavior better, and find steps to take to help their child in their own ways, even without formal services, once told that their child has a specific problem.
On a system level, it is important to realize that how services are established and maintained is far less rational than might be imagined. State programs, schools, hospitals, and insurers all have legal requirements to provide services within a certain time frame once referred. Even if the services are not there to help a your child or family right now, the referral itself adds to the data used to determine if services are adequate and to plan for additional service types or capacity. The Autism Waiver is one such example where waits are years long, but getting on the list is crucial to the future of the program.
Until you screen and give parents information – especially middle-class parents – we will never have the resources. As it was for lead paint, until we identified prevalence of elevated lead levels and the harm associated, we got no action on lead paint removal policies. Another example where complaints about access made a difference, is the relatively new Paul Wellstone and Pete Domenici Mental Health Parity and Addiction Equity Act of 2008 that requires health insurers and health plans to guarantee that financial requirements on benefits for mental health, such as copays, deductibles, and limitations on treatment benefits, are not more restrictive than those that are for medical benefits. This does not guarantee that services will be available or of high quality, but is a step toward accessibility.
You may be one of the many pediatricians who consider advocacy a basic component of your professional responsibilities. If you cannot advocate for services that you see your patients in need of, you can pass your concerns onto a group that does. Many American Academy of Pediatrics state chapters have so-called Pediatric Councils that receive ideas about system problems and put group pressure on leaders in the state to address them.
As in the historic painting of the physician leaning over the ill child whom he could not cure, after detection through screening our thoughtful evaluation, explanations, shared concern, and our patients’ advocacy have great value even when specific services are not yet available.
Dr. Howard is an assistant professor of pediatrics at the Johns Hopkins University, Baltimore, and creator of CHADIS (www.chadis.com). She has no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to Frontline Medical News. E-mail her at [email protected].
VIDEO: Mayo Clinic app shortened hospitalizations
The Mayo Clinic handed iPads with an app called “myCare” to patients scheduled for surgery and showed that its use significantly reduced postsurgical length of stay, the total cost of care, and the need for home health care or skilled nursing care at discharge.
A study of 150 patients was so successful that the software, developed initially as an external software program for testing, is now being rebuilt into the institution’s systems so that it has a home in clinicians’ workflow, Dr. David J. Cook said at the Health 2.0 fall conference.
Dr. Cook, chair of cardiovascular anesthesiology at the Mayo Clinic, Rochester, Minn., describes the app in detail in this video interview. The app provides patients a customized plan of care including what they can expect daily, just-in-time education, self-assessment tools, and more. Results are transmitted wirelessly to a dashboard, where clinicians can track a patient’s progress, facilitating earlier intervention when needed.
Other investigators at the Mayo Clinic developed a separate online and smartphone-based app to help with rehabilitation of patients hospitalized after a heart attack and stent placement. The app functioned as a self-monitoring system that allowed patients to enter vital signs and to access educational content about steps they could take to reduce their risk of another heart attack.
During a 90-day study, 20% of 25 patients using the app were rehospitalized or admitted to an emergency department, compared with 60% of 19 patients in a control group who received conventional cardiac rehabilitation care without the app. The investigators reported the data at the American College of Cardiology earlier this year.
“We hope a tool like this will help us extend the reach of cardiac rehabilitation to all heart patients, but in particular, it could help patients in rural and underserved populations who might not be able to attend cardiac rehabilitation sessions,” Dr. R. Jay Widmer said in a statement released by the Mayo Clinic.
These and dozens of other apps and technological tools are being developed and tested is a systematic fashion through the Mayo Clinic’s Center for Innovation. In the video, Dr. Cook also describes the Center’s activities and previews another innovative tool in development.
Dr. Cook reported having no financial disclosures.
On Twitter @sherryboschert
The Mayo Clinic handed iPads with an app called “myCare” to patients scheduled for surgery and showed that its use significantly reduced postsurgical length of stay, the total cost of care, and the need for home health care or skilled nursing care at discharge.
A study of 150 patients was so successful that the software, developed initially as an external software program for testing, is now being rebuilt into the institution’s systems so that it has a home in clinicians’ workflow, Dr. David J. Cook said at the Health 2.0 fall conference.
Dr. Cook, chair of cardiovascular anesthesiology at the Mayo Clinic, Rochester, Minn., describes the app in detail in this video interview. The app provides patients a customized plan of care including what they can expect daily, just-in-time education, self-assessment tools, and more. Results are transmitted wirelessly to a dashboard, where clinicians can track a patient’s progress, facilitating earlier intervention when needed.
Other investigators at the Mayo Clinic developed a separate online and smartphone-based app to help with rehabilitation of patients hospitalized after a heart attack and stent placement. The app functioned as a self-monitoring system that allowed patients to enter vital signs and to access educational content about steps they could take to reduce their risk of another heart attack.
During a 90-day study, 20% of 25 patients using the app were rehospitalized or admitted to an emergency department, compared with 60% of 19 patients in a control group who received conventional cardiac rehabilitation care without the app. The investigators reported the data at the American College of Cardiology earlier this year.
“We hope a tool like this will help us extend the reach of cardiac rehabilitation to all heart patients, but in particular, it could help patients in rural and underserved populations who might not be able to attend cardiac rehabilitation sessions,” Dr. R. Jay Widmer said in a statement released by the Mayo Clinic.
These and dozens of other apps and technological tools are being developed and tested is a systematic fashion through the Mayo Clinic’s Center for Innovation. In the video, Dr. Cook also describes the Center’s activities and previews another innovative tool in development.
Dr. Cook reported having no financial disclosures.
On Twitter @sherryboschert
The Mayo Clinic handed iPads with an app called “myCare” to patients scheduled for surgery and showed that its use significantly reduced postsurgical length of stay, the total cost of care, and the need for home health care or skilled nursing care at discharge.
A study of 150 patients was so successful that the software, developed initially as an external software program for testing, is now being rebuilt into the institution’s systems so that it has a home in clinicians’ workflow, Dr. David J. Cook said at the Health 2.0 fall conference.
Dr. Cook, chair of cardiovascular anesthesiology at the Mayo Clinic, Rochester, Minn., describes the app in detail in this video interview. The app provides patients a customized plan of care including what they can expect daily, just-in-time education, self-assessment tools, and more. Results are transmitted wirelessly to a dashboard, where clinicians can track a patient’s progress, facilitating earlier intervention when needed.
Other investigators at the Mayo Clinic developed a separate online and smartphone-based app to help with rehabilitation of patients hospitalized after a heart attack and stent placement. The app functioned as a self-monitoring system that allowed patients to enter vital signs and to access educational content about steps they could take to reduce their risk of another heart attack.
During a 90-day study, 20% of 25 patients using the app were rehospitalized or admitted to an emergency department, compared with 60% of 19 patients in a control group who received conventional cardiac rehabilitation care without the app. The investigators reported the data at the American College of Cardiology earlier this year.
“We hope a tool like this will help us extend the reach of cardiac rehabilitation to all heart patients, but in particular, it could help patients in rural and underserved populations who might not be able to attend cardiac rehabilitation sessions,” Dr. R. Jay Widmer said in a statement released by the Mayo Clinic.
These and dozens of other apps and technological tools are being developed and tested is a systematic fashion through the Mayo Clinic’s Center for Innovation. In the video, Dr. Cook also describes the Center’s activities and previews another innovative tool in development.
Dr. Cook reported having no financial disclosures.
On Twitter @sherryboschert
AT HEALTH 2.0
Practical Parenting: Lice
As summer has come to a close and children are settling into their new classrooms and routines, there is one thing certain about back to school season – pediculosis capitis, more affectionately known as lice. My own children hadn’t been back in school 3 days when they came home with the dreaded note in their backpacks alerting parents that there had been cases of head lice identified in the school. While there are some variations in susceptibility (males and black children tend to be affected less frequently than are females and children of other races), head lice infestations are incredibly common in children, and are one of the most frequently seen communicable diseases in elementary school settings.
Many parents will appropriately treat their children with home remedies and/or over-the-counter medications, so most of these children aren’t seen in our offices. However, families frequently have questions about the best or most effective methods of treatment, or need help with cases that are difficult to resolve. So, what is the best method of treatment? As with many conditions, the answer is that it depends on parent preference and the resistance patterns in the community.
The first step in the process is twofold – make sure that treatment is needed, and reassure the parent if it is. The best way to diagnose head lice is visualization of a live, active louse. This is best done using a small, fine-toothed comb to systematically examine all areas of the scalp and hair at least twice over. Visual inspection without systematically combing through the hair misses a large number of cases. Relatedly, the presence of nits (or eggs – small whitish, oval capsules that firmly adhere to the base of the hair shaft) does not definitively indicate infection. A sizable percentage of children with nits do not go on to develop active lice infections, and nits may be present several months after effective treatment. That said, a high concentration of nits over a relatively small area (1/4 inch) makes the incidence of active infection more likely. Lastly, the presence of itching alone does not indicate active infection (as evidenced by the large number of you who are likely itching your heads just reading this article). If treatment is in fact needed, parents should be reassured that this is a very common condition, unrelated to cleanliness of the home or school, and something that almost everyone faces at sometime during their school years.
The most common first line treatments are the topical pediculicides, including the pyrethroids (available over the counter), malathion, benzyl alcohol, spinosad, and topical ivermectin. These medications are generally safe and well tolerated (alternately, lindane is only recommended as a second line treatment, and not recommended in children, because of its possible toxic side effects). The Centers for Disease Control and Prevention website has a nice summary of the available medications, their indications, and common side effects to help guide your decision making. The pyrethroids are generally well tolerated and are available over the counter and thus, despite increasing resistance, are commonly used first line medications. Malathion is also frequently used and recommended, especially as it likely has greater efficacy than the pyrethroids, but the fact that it requires a prescription and has a very strong odor can make it a less tolerable choice for families. Oral medications are available for use as a second line therapy or in recalcitrant cases.
For families who prefer not to use topical pediculocides, or for very young children, wet combing is a possible alternative. This technique involves wetting the air with a lubricant such as hair conditioner or olive oil and systematically combing the hair with a fine tooth comb until no lice are found. This should be repeated every 2-4 days and continue for 2 weeks after the last live louse is found. This technique also can be a useful adjunct to topical pediculocide treatment. The downside to this technique, as anyone who has ever done it can attest to, is that it is time consuming and difficult to get small children to sit still through it. Using topical agents such as petroleum jelly or Cetaphil cleanser to attempt to suffocate the lice is a common, but not well studied or validated approach. It is certainly harmless and possibly effective, but parents should be aware that there is not strong evidence to support this technique.
Additionally, at the time of diagnosis and treatment for all families, any bedding or towels the infected child has had contact with in the past 48-72 hours should be washed in very hot water and anything (such as stuffed animals or pillows) that can’t be washed should be put in an air-tight bag for 2 weeks. Vacuuming of carpets in the home also may be helpful. Close household contacts should be carefully monitored for infection, and any contacts who share a bed should be considered for prophylactic therapy. Children should be allowed to return to school as soon as they are treated. “No-nit” policies are not recommended or helpful, and if your school or school district has them it is an opportunity for education and advocacy to minimize the number of days children unnecessarily miss school.
This tiny parasite can be an enormous pest to parents, schools and physicians alike. With a calm, reassuring common sense approach, pediatricians can help kids get back to school itch free.
Dr. Beers is an assistant professor of pediatrics at Children’s National Medical Center and the George Washington University Medical Center, Washington. She is chair of the American Academy of Pediatrics Committee on Residency Scholarships and president of the District of Columbia chapter of the American Academy of Pediatrics.
As summer has come to a close and children are settling into their new classrooms and routines, there is one thing certain about back to school season – pediculosis capitis, more affectionately known as lice. My own children hadn’t been back in school 3 days when they came home with the dreaded note in their backpacks alerting parents that there had been cases of head lice identified in the school. While there are some variations in susceptibility (males and black children tend to be affected less frequently than are females and children of other races), head lice infestations are incredibly common in children, and are one of the most frequently seen communicable diseases in elementary school settings.
Many parents will appropriately treat their children with home remedies and/or over-the-counter medications, so most of these children aren’t seen in our offices. However, families frequently have questions about the best or most effective methods of treatment, or need help with cases that are difficult to resolve. So, what is the best method of treatment? As with many conditions, the answer is that it depends on parent preference and the resistance patterns in the community.
The first step in the process is twofold – make sure that treatment is needed, and reassure the parent if it is. The best way to diagnose head lice is visualization of a live, active louse. This is best done using a small, fine-toothed comb to systematically examine all areas of the scalp and hair at least twice over. Visual inspection without systematically combing through the hair misses a large number of cases. Relatedly, the presence of nits (or eggs – small whitish, oval capsules that firmly adhere to the base of the hair shaft) does not definitively indicate infection. A sizable percentage of children with nits do not go on to develop active lice infections, and nits may be present several months after effective treatment. That said, a high concentration of nits over a relatively small area (1/4 inch) makes the incidence of active infection more likely. Lastly, the presence of itching alone does not indicate active infection (as evidenced by the large number of you who are likely itching your heads just reading this article). If treatment is in fact needed, parents should be reassured that this is a very common condition, unrelated to cleanliness of the home or school, and something that almost everyone faces at sometime during their school years.
The most common first line treatments are the topical pediculicides, including the pyrethroids (available over the counter), malathion, benzyl alcohol, spinosad, and topical ivermectin. These medications are generally safe and well tolerated (alternately, lindane is only recommended as a second line treatment, and not recommended in children, because of its possible toxic side effects). The Centers for Disease Control and Prevention website has a nice summary of the available medications, their indications, and common side effects to help guide your decision making. The pyrethroids are generally well tolerated and are available over the counter and thus, despite increasing resistance, are commonly used first line medications. Malathion is also frequently used and recommended, especially as it likely has greater efficacy than the pyrethroids, but the fact that it requires a prescription and has a very strong odor can make it a less tolerable choice for families. Oral medications are available for use as a second line therapy or in recalcitrant cases.
For families who prefer not to use topical pediculocides, or for very young children, wet combing is a possible alternative. This technique involves wetting the air with a lubricant such as hair conditioner or olive oil and systematically combing the hair with a fine tooth comb until no lice are found. This should be repeated every 2-4 days and continue for 2 weeks after the last live louse is found. This technique also can be a useful adjunct to topical pediculocide treatment. The downside to this technique, as anyone who has ever done it can attest to, is that it is time consuming and difficult to get small children to sit still through it. Using topical agents such as petroleum jelly or Cetaphil cleanser to attempt to suffocate the lice is a common, but not well studied or validated approach. It is certainly harmless and possibly effective, but parents should be aware that there is not strong evidence to support this technique.
Additionally, at the time of diagnosis and treatment for all families, any bedding or towels the infected child has had contact with in the past 48-72 hours should be washed in very hot water and anything (such as stuffed animals or pillows) that can’t be washed should be put in an air-tight bag for 2 weeks. Vacuuming of carpets in the home also may be helpful. Close household contacts should be carefully monitored for infection, and any contacts who share a bed should be considered for prophylactic therapy. Children should be allowed to return to school as soon as they are treated. “No-nit” policies are not recommended or helpful, and if your school or school district has them it is an opportunity for education and advocacy to minimize the number of days children unnecessarily miss school.
This tiny parasite can be an enormous pest to parents, schools and physicians alike. With a calm, reassuring common sense approach, pediatricians can help kids get back to school itch free.
Dr. Beers is an assistant professor of pediatrics at Children’s National Medical Center and the George Washington University Medical Center, Washington. She is chair of the American Academy of Pediatrics Committee on Residency Scholarships and president of the District of Columbia chapter of the American Academy of Pediatrics.
As summer has come to a close and children are settling into their new classrooms and routines, there is one thing certain about back to school season – pediculosis capitis, more affectionately known as lice. My own children hadn’t been back in school 3 days when they came home with the dreaded note in their backpacks alerting parents that there had been cases of head lice identified in the school. While there are some variations in susceptibility (males and black children tend to be affected less frequently than are females and children of other races), head lice infestations are incredibly common in children, and are one of the most frequently seen communicable diseases in elementary school settings.
Many parents will appropriately treat their children with home remedies and/or over-the-counter medications, so most of these children aren’t seen in our offices. However, families frequently have questions about the best or most effective methods of treatment, or need help with cases that are difficult to resolve. So, what is the best method of treatment? As with many conditions, the answer is that it depends on parent preference and the resistance patterns in the community.
The first step in the process is twofold – make sure that treatment is needed, and reassure the parent if it is. The best way to diagnose head lice is visualization of a live, active louse. This is best done using a small, fine-toothed comb to systematically examine all areas of the scalp and hair at least twice over. Visual inspection without systematically combing through the hair misses a large number of cases. Relatedly, the presence of nits (or eggs – small whitish, oval capsules that firmly adhere to the base of the hair shaft) does not definitively indicate infection. A sizable percentage of children with nits do not go on to develop active lice infections, and nits may be present several months after effective treatment. That said, a high concentration of nits over a relatively small area (1/4 inch) makes the incidence of active infection more likely. Lastly, the presence of itching alone does not indicate active infection (as evidenced by the large number of you who are likely itching your heads just reading this article). If treatment is in fact needed, parents should be reassured that this is a very common condition, unrelated to cleanliness of the home or school, and something that almost everyone faces at sometime during their school years.
The most common first line treatments are the topical pediculicides, including the pyrethroids (available over the counter), malathion, benzyl alcohol, spinosad, and topical ivermectin. These medications are generally safe and well tolerated (alternately, lindane is only recommended as a second line treatment, and not recommended in children, because of its possible toxic side effects). The Centers for Disease Control and Prevention website has a nice summary of the available medications, their indications, and common side effects to help guide your decision making. The pyrethroids are generally well tolerated and are available over the counter and thus, despite increasing resistance, are commonly used first line medications. Malathion is also frequently used and recommended, especially as it likely has greater efficacy than the pyrethroids, but the fact that it requires a prescription and has a very strong odor can make it a less tolerable choice for families. Oral medications are available for use as a second line therapy or in recalcitrant cases.
For families who prefer not to use topical pediculocides, or for very young children, wet combing is a possible alternative. This technique involves wetting the air with a lubricant such as hair conditioner or olive oil and systematically combing the hair with a fine tooth comb until no lice are found. This should be repeated every 2-4 days and continue for 2 weeks after the last live louse is found. This technique also can be a useful adjunct to topical pediculocide treatment. The downside to this technique, as anyone who has ever done it can attest to, is that it is time consuming and difficult to get small children to sit still through it. Using topical agents such as petroleum jelly or Cetaphil cleanser to attempt to suffocate the lice is a common, but not well studied or validated approach. It is certainly harmless and possibly effective, but parents should be aware that there is not strong evidence to support this technique.
Additionally, at the time of diagnosis and treatment for all families, any bedding or towels the infected child has had contact with in the past 48-72 hours should be washed in very hot water and anything (such as stuffed animals or pillows) that can’t be washed should be put in an air-tight bag for 2 weeks. Vacuuming of carpets in the home also may be helpful. Close household contacts should be carefully monitored for infection, and any contacts who share a bed should be considered for prophylactic therapy. Children should be allowed to return to school as soon as they are treated. “No-nit” policies are not recommended or helpful, and if your school or school district has them it is an opportunity for education and advocacy to minimize the number of days children unnecessarily miss school.
This tiny parasite can be an enormous pest to parents, schools and physicians alike. With a calm, reassuring common sense approach, pediatricians can help kids get back to school itch free.
Dr. Beers is an assistant professor of pediatrics at Children’s National Medical Center and the George Washington University Medical Center, Washington. She is chair of the American Academy of Pediatrics Committee on Residency Scholarships and president of the District of Columbia chapter of the American Academy of Pediatrics.














