User login
Using Active Surveillance to Identify Monoclonal Antibody Candidates Among COVID-19–Positive Veterans in the Atlanta VA Health Care System
Early in the COVID-19 pandemic, monoclonal antibody (Mab) therapy was the only outpatient therapy for patients with COVID-19 experiencing mild-to-moderate symptoms. The Blocking Viral Attachment and Cell Entry with SARS-CoV-2 Neutralizing Antibodies (BLAZE-1) and the REGN-COV2 (Regeneron) clinical trials found participants treated with Mab had a shorter duration of symptoms and fewer hospitalizations compared with those receiving placebo.1,2 Mab therapy was most efficacious early in the disease course, and the initial US Food and Drug Administration (FDA) Emergency Use Authorization (EUA) of Mab therapies required use within 10 days of symptom onset.3
The impact of the COVID-19 pandemic has been felt disproportionately among marginalized racial and ethnic groups in the US. The COVID-19 Associated Hospitalization Surveillance Network found that non-Hispanic Black persons have significantly higher rates of hospitalization and death by COVID-19 compared with White persons.4-7 However, marginalized groups are underrepresented in the receipt of therapeutic agents for COVID-19. From March 2020 through August 2021, the mean monthly Mab use among Black patients (2.8%) was lower compared with White patients (4.0%), and Black patients received Mab 22.4% less often than White patients.7
The Mab clinical trials BLAZE-1 and REGN-COV2 study populations consisted of > 80% White participants.1,2 Receipt of COVID-19 outpatient treatments may not align with the disease burden in marginalized racial and ethnic groups, leading to health disparities. Although not exhaustive, reasons for these disparities include patient, health care practitioner, and systems-level issues: patient awareness, trust, and engagement with the health care system; health care practitioner awareness and advocacy to pursue COVID-19 treatment for the patient; and health care capacity to provide the medication and service.7
Here, we describe a novel, quality improvement initiative at the Atlanta Veterans Affairs Health Care System (AVAHCS) in Georgia that paired a proactive laboratory-based surveillance strategy to identify and engage veterans for Mab. By centralizing the surveillance and outreach process, we sought to reduce barriers to the Mab referral process and optimize access to life-saving medication.
Implementation
AVAHCS serves a diverse population of more than 129,000 (50.8% non-Hispanic Black veterans, 37.5% White veterans, and 11.7% of other races) at a main medical campus and 18 surrounding community-based outpatient clinics. From December 28, 2020, to August 31, 2021, veterans with a positive COVID-19 nasopharyngeal polymerase chain reaction (PCR) test at AVAHCS were screened daily. A central Mab team consisting of infectious disease (ID) clinical pharmacists and physicians reviewed daily lists of positive laboratory results and identified high-risk individuals for Mab eligibility, using the FDA EUA inclusion criteria. Eligible patients were called by a Mab team member to discuss Mab treatment, provide anticipatory guidance, obtain verbal consent, and schedule the infusion. Conventional referrals from non-Mab team members (eg, primary care physicians) were also accepted into the screening process and underwent the same procedures and risk prioritization strategy as those identified by the Mab team.
Clinic resources allowed for 1 to 2 patients per day to be given Mab, increasing to a maximum of 5 patients per day during the COVID-19 Delta variant surge. We followed our best clinical judgment in prioritizing patient selection, and we aligned our practice with the standards of our affiliated partner, Emory University. In circumstances where patients who were Mab-eligible outnumbered infusion availability, patients were prioritized using the Veterans Health Administration (VHA) COVID-19 (VACO) Index for 30-day COVID-19 mortality.8 As COVID-19 variants developed resistance to the recommended Mab infusions, bamlanivimab, bamlanivimab-etesevimab, or casirivimab-imdevimab, local protocols adapted to EUA revisions. The Mab team also adopted FDA eligibility criteria revisions as they were available.9,10
We describe the outcomes of our centralized screening process for Mab therapy, as measured by screening, uptake, and time to receipt of Mab from screening. We also describe the demographic and clinical characteristics of Mab recipients. Clinical outcomes include postinfusion adverse events (AEs) at day 1 and day 7, emergency department (ED) visits, inpatient hospitalization, and death.
Results
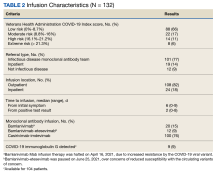
The Mab team screened 2028 veterans who were COVID-19 positive between December 28, 2020, and August 31, 2021, and identified 289 veterans (14%) who met the EUA criteria. One hundred thirty-two veterans (46%) completed Mab infusion, and of the remaining 145 veterans, 124 (86%) declined treatment, and 21 (14%) veterans did not complete Mab infusion largely due to not keeping the appointment. The Mab team active surveillance strategy identified 101 of 132 infusion candidates (77%); 82% had outpatient Mab infusion.
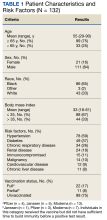
The mean age of veterans who received Mab was 55 years (range, 29-90), and 75% of veterans were aged ≥ 65 years; most were male (84%) and 86 (65%) identified as non-Hispanic Black individuals (Table 1).
Postinfusion AEs reported at day 1 and day 7 occurred for 38 veterans (29%) and 11 veterans (8%), respectively. Sixteen patients (12%) had postinfusion ED visit, and 12 patients (9%) required hospitalization. Eleven of the 12 hospitalized patients (92%) had worsening respiratory symptoms. No deaths occurred in the 132 patients who received Mab.
Discussion
This novel initiative to optimize access to outpatient COVID-19 treatment demonstrated how the Mab team proactively screened and reached out to eligible veterans with COVID-19 promptly. This approach removed layers in the traditional referral process that could be barriers to accessing care. More than three-quarters of patients who received Mab were identified through this strategy, and the uptake was high at 46%. Conventional passive referrals were suboptimal for identifying candidates, which was also the case at a neighboring institution.
In an Emory University study, referrals to the Mab clinic were made through a traditional, decentralized referral system and resulted in a lower uptake of Mab treatment (4.6%).11 One of the key advantages of the AVAHCS program was that we were able to provide individual education about COVID-19 and counsel on the benefits and risks of therapy. Having a structured, telehealth follow-up plan provided additional reassurance and support to the patient. These personalized patient connections likely helped increase acceptance of the Mab therapy.
Our surveillance and outreach strategy had high uptake among Black patients (65%), which exceeded the proportion of AVAHCS Black veterans (54%).12 In the Emory study, just 30% of the participants were Black patients.11 In a study of bamlanivimab use in Chicago, Black individuals represented just 11% of the study population. White patients were more likely to receive bamlanivimab compared with others races, and the likelihood of receiving bamlanivimab was significantly worse for Black patients (odds ratio, 0.28) compared with White patients.13 These studies highlight the disparity in COVID-19 outpatient treatment that does not reflect the racial and minority group representation of the community at large.
Limitations
The VHA medication allocation system at times created a significant mismatch in supply and demand, which significantly limited the AVAHCS Mab program. VHA facilities nationwide with Mab programs received discrete allocations through the US Department of Health and Human Services via VHA pharmacy benefits management services. Despite our large catchment, AVAHCS was allocated 6 or fewer doses of Mab per week during the evaluated period.
Without formal national guidance in the early period of Mab, the AVAHCS Mab team conferred with Emory University Mab clinicians as well as at other VHA facilities in the country to develop an optimal approach to resource allocation. The Mab team considered all EUA criteria to be as inclusive as possible. However, during times of high demand, our utilitarian approach tried to identify the highest-risk patients who would benefit the most from Mab. The VACO index was validated in early 2021, which facilitated decision making when demand was greater than supply. One limitation of the VACO index is its exclusion of several original Mab EUA criteria, including weight, hypertension, and nonmalignancy-related immunosuppression, into its algorithm.3,8
Conclusions
Through proactive screening and direct outreach to patients, the AVAHCS was able to achieve timely administration of Mab infusion that was well within the initial EUA time frame of 10 days and comparable with the time frame in the REGN-COV2 and BLAZE-1 trials. Improving access to resources by changing the referral structure helped engage veterans who may have otherwise missed the time frame for Mab therapy. The experience of the Mab infusion program at the AVAHCS provided valuable insight into how a health care system could effectively screen a large population and distribute the limited resource of Mab therapy in a timely and proportionate fashion among its represented demographic groups.
Acknowledgments
The authors acknowledge the Veterans Health Administration VISN 7 Clinical Resource Hub and Tele Primary Care group for their support.
1. Chen P, Nirula A, Heller B, et al; BLAZE-1 Investigators. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19. N Engl J Med. 2021;384(3):229-237. doi:10.1056/NEJMoa2029849
2. Weinreich DM, Sivapalasingam S, Norton T, et al; Trial Investigators. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. N Engl J Med. 2021;384(3):238-251. doi:10.1056/NEJMoa2035002
3. US Food and Drug Administration. Fact sheet for health care providers, emergency use authorization (EUA) of bamlanivimab and etesevimab. Accessed August 6, 2023. https://www.fda.gov/media/145802/download
4. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-net). Updated March 24, 2023. Accessed August 6, 2023. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covid-net/purpose-methods.html
5. Centers for Disease Control and Prevention, National Center for Health Statistics. Provisional COVID-19 deaths: distribution of deaths by race and Hispanic origin. Updated July 26, 2023. Accessed August 8, 2023. https://data.cdc.gov/NCHS/Provisional-COVID-19-Deaths-Distribution-of-Deaths/pj7m-y5uh
6. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among Black patients and White patients with COVID-19. N Engl J Med. 2020;382(26):2534-2543. doi:10.1056NEJMsa2011686
7. Wiltz JL, Feehan AK, Mollinari AM, et al. Racial and ethnic disparities in receipt of medications for treatment of COVID-19 - United States, March 2020-August 2021. MMWR Morb Mortal Wkly Rep. 2022;71(3):96-102. doi:10.15585/mmwr.mm7103e1
8. King JT Jr, Yoon JS, Rentsch CT, et al. Development and validation of a 30-day mortality index based on pre-existing medical administrative data from 13,323 COVID-19 patients: the Veterans Health Administration COVID-19 (VACO) Index. PLoS One. 2020;15(11):e0241825. doi:10.1371/journal.pone.0241825
9. US Food and Drug Administration, Office of Media Affairs. Coronavirus (COVID-19) update: FDA revokes emergency use authorization for monoclonal antibody bamlanivimab. Accessed August 8, 2023. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-monoclonal-antibody-bamlanivimab
10. National Institutes of Health. Information on COVID-19 treatment, prevention and research. Accessed August 8, 2023. https://www.covid19treatmentguidelines.nih.gov
11. Anderson B, Smith Z, Edupuganti S, Yan X, Masi CM, Wu HM. Effect of monoclonal antibody treatment on clinical outcomes in ambulatory patients with coronavirus disease 2019. Open Forum Infect Dis. 2021;8(7):ofab315. Published 2021 Jun 12. doi:10.1093/ofid/ofab315
12. United States Census Bureau. Quick facts: DeKalb County, Georgia. Updated July 1, 2022. Accessed August 8, 2023. www.census.gov/quickfacts/dekalbcountygeorgia
13. Kumar R, Wu EL, Stosor V, et al. Real-world experience of bamlanivimab for coronavirus disease 2019 (COVID-19): a case-control study. Clin Infect Dis. 2022;74(1):24-31. doi:10.1093/cid/ciab305
Early in the COVID-19 pandemic, monoclonal antibody (Mab) therapy was the only outpatient therapy for patients with COVID-19 experiencing mild-to-moderate symptoms. The Blocking Viral Attachment and Cell Entry with SARS-CoV-2 Neutralizing Antibodies (BLAZE-1) and the REGN-COV2 (Regeneron) clinical trials found participants treated with Mab had a shorter duration of symptoms and fewer hospitalizations compared with those receiving placebo.1,2 Mab therapy was most efficacious early in the disease course, and the initial US Food and Drug Administration (FDA) Emergency Use Authorization (EUA) of Mab therapies required use within 10 days of symptom onset.3
The impact of the COVID-19 pandemic has been felt disproportionately among marginalized racial and ethnic groups in the US. The COVID-19 Associated Hospitalization Surveillance Network found that non-Hispanic Black persons have significantly higher rates of hospitalization and death by COVID-19 compared with White persons.4-7 However, marginalized groups are underrepresented in the receipt of therapeutic agents for COVID-19. From March 2020 through August 2021, the mean monthly Mab use among Black patients (2.8%) was lower compared with White patients (4.0%), and Black patients received Mab 22.4% less often than White patients.7
The Mab clinical trials BLAZE-1 and REGN-COV2 study populations consisted of > 80% White participants.1,2 Receipt of COVID-19 outpatient treatments may not align with the disease burden in marginalized racial and ethnic groups, leading to health disparities. Although not exhaustive, reasons for these disparities include patient, health care practitioner, and systems-level issues: patient awareness, trust, and engagement with the health care system; health care practitioner awareness and advocacy to pursue COVID-19 treatment for the patient; and health care capacity to provide the medication and service.7
Here, we describe a novel, quality improvement initiative at the Atlanta Veterans Affairs Health Care System (AVAHCS) in Georgia that paired a proactive laboratory-based surveillance strategy to identify and engage veterans for Mab. By centralizing the surveillance and outreach process, we sought to reduce barriers to the Mab referral process and optimize access to life-saving medication.
Implementation
AVAHCS serves a diverse population of more than 129,000 (50.8% non-Hispanic Black veterans, 37.5% White veterans, and 11.7% of other races) at a main medical campus and 18 surrounding community-based outpatient clinics. From December 28, 2020, to August 31, 2021, veterans with a positive COVID-19 nasopharyngeal polymerase chain reaction (PCR) test at AVAHCS were screened daily. A central Mab team consisting of infectious disease (ID) clinical pharmacists and physicians reviewed daily lists of positive laboratory results and identified high-risk individuals for Mab eligibility, using the FDA EUA inclusion criteria. Eligible patients were called by a Mab team member to discuss Mab treatment, provide anticipatory guidance, obtain verbal consent, and schedule the infusion. Conventional referrals from non-Mab team members (eg, primary care physicians) were also accepted into the screening process and underwent the same procedures and risk prioritization strategy as those identified by the Mab team.
Clinic resources allowed for 1 to 2 patients per day to be given Mab, increasing to a maximum of 5 patients per day during the COVID-19 Delta variant surge. We followed our best clinical judgment in prioritizing patient selection, and we aligned our practice with the standards of our affiliated partner, Emory University. In circumstances where patients who were Mab-eligible outnumbered infusion availability, patients were prioritized using the Veterans Health Administration (VHA) COVID-19 (VACO) Index for 30-day COVID-19 mortality.8 As COVID-19 variants developed resistance to the recommended Mab infusions, bamlanivimab, bamlanivimab-etesevimab, or casirivimab-imdevimab, local protocols adapted to EUA revisions. The Mab team also adopted FDA eligibility criteria revisions as they were available.9,10
We describe the outcomes of our centralized screening process for Mab therapy, as measured by screening, uptake, and time to receipt of Mab from screening. We also describe the demographic and clinical characteristics of Mab recipients. Clinical outcomes include postinfusion adverse events (AEs) at day 1 and day 7, emergency department (ED) visits, inpatient hospitalization, and death.
Results
The Mab team screened 2028 veterans who were COVID-19 positive between December 28, 2020, and August 31, 2021, and identified 289 veterans (14%) who met the EUA criteria. One hundred thirty-two veterans (46%) completed Mab infusion, and of the remaining 145 veterans, 124 (86%) declined treatment, and 21 (14%) veterans did not complete Mab infusion largely due to not keeping the appointment. The Mab team active surveillance strategy identified 101 of 132 infusion candidates (77%); 82% had outpatient Mab infusion.
The mean age of veterans who received Mab was 55 years (range, 29-90), and 75% of veterans were aged ≥ 65 years; most were male (84%) and 86 (65%) identified as non-Hispanic Black individuals (Table 1).
Postinfusion AEs reported at day 1 and day 7 occurred for 38 veterans (29%) and 11 veterans (8%), respectively. Sixteen patients (12%) had postinfusion ED visit, and 12 patients (9%) required hospitalization. Eleven of the 12 hospitalized patients (92%) had worsening respiratory symptoms. No deaths occurred in the 132 patients who received Mab.
Discussion
This novel initiative to optimize access to outpatient COVID-19 treatment demonstrated how the Mab team proactively screened and reached out to eligible veterans with COVID-19 promptly. This approach removed layers in the traditional referral process that could be barriers to accessing care. More than three-quarters of patients who received Mab were identified through this strategy, and the uptake was high at 46%. Conventional passive referrals were suboptimal for identifying candidates, which was also the case at a neighboring institution.
In an Emory University study, referrals to the Mab clinic were made through a traditional, decentralized referral system and resulted in a lower uptake of Mab treatment (4.6%).11 One of the key advantages of the AVAHCS program was that we were able to provide individual education about COVID-19 and counsel on the benefits and risks of therapy. Having a structured, telehealth follow-up plan provided additional reassurance and support to the patient. These personalized patient connections likely helped increase acceptance of the Mab therapy.
Our surveillance and outreach strategy had high uptake among Black patients (65%), which exceeded the proportion of AVAHCS Black veterans (54%).12 In the Emory study, just 30% of the participants were Black patients.11 In a study of bamlanivimab use in Chicago, Black individuals represented just 11% of the study population. White patients were more likely to receive bamlanivimab compared with others races, and the likelihood of receiving bamlanivimab was significantly worse for Black patients (odds ratio, 0.28) compared with White patients.13 These studies highlight the disparity in COVID-19 outpatient treatment that does not reflect the racial and minority group representation of the community at large.
Limitations
The VHA medication allocation system at times created a significant mismatch in supply and demand, which significantly limited the AVAHCS Mab program. VHA facilities nationwide with Mab programs received discrete allocations through the US Department of Health and Human Services via VHA pharmacy benefits management services. Despite our large catchment, AVAHCS was allocated 6 or fewer doses of Mab per week during the evaluated period.
Without formal national guidance in the early period of Mab, the AVAHCS Mab team conferred with Emory University Mab clinicians as well as at other VHA facilities in the country to develop an optimal approach to resource allocation. The Mab team considered all EUA criteria to be as inclusive as possible. However, during times of high demand, our utilitarian approach tried to identify the highest-risk patients who would benefit the most from Mab. The VACO index was validated in early 2021, which facilitated decision making when demand was greater than supply. One limitation of the VACO index is its exclusion of several original Mab EUA criteria, including weight, hypertension, and nonmalignancy-related immunosuppression, into its algorithm.3,8
Conclusions
Through proactive screening and direct outreach to patients, the AVAHCS was able to achieve timely administration of Mab infusion that was well within the initial EUA time frame of 10 days and comparable with the time frame in the REGN-COV2 and BLAZE-1 trials. Improving access to resources by changing the referral structure helped engage veterans who may have otherwise missed the time frame for Mab therapy. The experience of the Mab infusion program at the AVAHCS provided valuable insight into how a health care system could effectively screen a large population and distribute the limited resource of Mab therapy in a timely and proportionate fashion among its represented demographic groups.
Acknowledgments
The authors acknowledge the Veterans Health Administration VISN 7 Clinical Resource Hub and Tele Primary Care group for their support.
Early in the COVID-19 pandemic, monoclonal antibody (Mab) therapy was the only outpatient therapy for patients with COVID-19 experiencing mild-to-moderate symptoms. The Blocking Viral Attachment and Cell Entry with SARS-CoV-2 Neutralizing Antibodies (BLAZE-1) and the REGN-COV2 (Regeneron) clinical trials found participants treated with Mab had a shorter duration of symptoms and fewer hospitalizations compared with those receiving placebo.1,2 Mab therapy was most efficacious early in the disease course, and the initial US Food and Drug Administration (FDA) Emergency Use Authorization (EUA) of Mab therapies required use within 10 days of symptom onset.3
The impact of the COVID-19 pandemic has been felt disproportionately among marginalized racial and ethnic groups in the US. The COVID-19 Associated Hospitalization Surveillance Network found that non-Hispanic Black persons have significantly higher rates of hospitalization and death by COVID-19 compared with White persons.4-7 However, marginalized groups are underrepresented in the receipt of therapeutic agents for COVID-19. From March 2020 through August 2021, the mean monthly Mab use among Black patients (2.8%) was lower compared with White patients (4.0%), and Black patients received Mab 22.4% less often than White patients.7
The Mab clinical trials BLAZE-1 and REGN-COV2 study populations consisted of > 80% White participants.1,2 Receipt of COVID-19 outpatient treatments may not align with the disease burden in marginalized racial and ethnic groups, leading to health disparities. Although not exhaustive, reasons for these disparities include patient, health care practitioner, and systems-level issues: patient awareness, trust, and engagement with the health care system; health care practitioner awareness and advocacy to pursue COVID-19 treatment for the patient; and health care capacity to provide the medication and service.7
Here, we describe a novel, quality improvement initiative at the Atlanta Veterans Affairs Health Care System (AVAHCS) in Georgia that paired a proactive laboratory-based surveillance strategy to identify and engage veterans for Mab. By centralizing the surveillance and outreach process, we sought to reduce barriers to the Mab referral process and optimize access to life-saving medication.
Implementation
AVAHCS serves a diverse population of more than 129,000 (50.8% non-Hispanic Black veterans, 37.5% White veterans, and 11.7% of other races) at a main medical campus and 18 surrounding community-based outpatient clinics. From December 28, 2020, to August 31, 2021, veterans with a positive COVID-19 nasopharyngeal polymerase chain reaction (PCR) test at AVAHCS were screened daily. A central Mab team consisting of infectious disease (ID) clinical pharmacists and physicians reviewed daily lists of positive laboratory results and identified high-risk individuals for Mab eligibility, using the FDA EUA inclusion criteria. Eligible patients were called by a Mab team member to discuss Mab treatment, provide anticipatory guidance, obtain verbal consent, and schedule the infusion. Conventional referrals from non-Mab team members (eg, primary care physicians) were also accepted into the screening process and underwent the same procedures and risk prioritization strategy as those identified by the Mab team.
Clinic resources allowed for 1 to 2 patients per day to be given Mab, increasing to a maximum of 5 patients per day during the COVID-19 Delta variant surge. We followed our best clinical judgment in prioritizing patient selection, and we aligned our practice with the standards of our affiliated partner, Emory University. In circumstances where patients who were Mab-eligible outnumbered infusion availability, patients were prioritized using the Veterans Health Administration (VHA) COVID-19 (VACO) Index for 30-day COVID-19 mortality.8 As COVID-19 variants developed resistance to the recommended Mab infusions, bamlanivimab, bamlanivimab-etesevimab, or casirivimab-imdevimab, local protocols adapted to EUA revisions. The Mab team also adopted FDA eligibility criteria revisions as they were available.9,10
We describe the outcomes of our centralized screening process for Mab therapy, as measured by screening, uptake, and time to receipt of Mab from screening. We also describe the demographic and clinical characteristics of Mab recipients. Clinical outcomes include postinfusion adverse events (AEs) at day 1 and day 7, emergency department (ED) visits, inpatient hospitalization, and death.
Results
The Mab team screened 2028 veterans who were COVID-19 positive between December 28, 2020, and August 31, 2021, and identified 289 veterans (14%) who met the EUA criteria. One hundred thirty-two veterans (46%) completed Mab infusion, and of the remaining 145 veterans, 124 (86%) declined treatment, and 21 (14%) veterans did not complete Mab infusion largely due to not keeping the appointment. The Mab team active surveillance strategy identified 101 of 132 infusion candidates (77%); 82% had outpatient Mab infusion.
The mean age of veterans who received Mab was 55 years (range, 29-90), and 75% of veterans were aged ≥ 65 years; most were male (84%) and 86 (65%) identified as non-Hispanic Black individuals (Table 1).
Postinfusion AEs reported at day 1 and day 7 occurred for 38 veterans (29%) and 11 veterans (8%), respectively. Sixteen patients (12%) had postinfusion ED visit, and 12 patients (9%) required hospitalization. Eleven of the 12 hospitalized patients (92%) had worsening respiratory symptoms. No deaths occurred in the 132 patients who received Mab.
Discussion
This novel initiative to optimize access to outpatient COVID-19 treatment demonstrated how the Mab team proactively screened and reached out to eligible veterans with COVID-19 promptly. This approach removed layers in the traditional referral process that could be barriers to accessing care. More than three-quarters of patients who received Mab were identified through this strategy, and the uptake was high at 46%. Conventional passive referrals were suboptimal for identifying candidates, which was also the case at a neighboring institution.
In an Emory University study, referrals to the Mab clinic were made through a traditional, decentralized referral system and resulted in a lower uptake of Mab treatment (4.6%).11 One of the key advantages of the AVAHCS program was that we were able to provide individual education about COVID-19 and counsel on the benefits and risks of therapy. Having a structured, telehealth follow-up plan provided additional reassurance and support to the patient. These personalized patient connections likely helped increase acceptance of the Mab therapy.
Our surveillance and outreach strategy had high uptake among Black patients (65%), which exceeded the proportion of AVAHCS Black veterans (54%).12 In the Emory study, just 30% of the participants were Black patients.11 In a study of bamlanivimab use in Chicago, Black individuals represented just 11% of the study population. White patients were more likely to receive bamlanivimab compared with others races, and the likelihood of receiving bamlanivimab was significantly worse for Black patients (odds ratio, 0.28) compared with White patients.13 These studies highlight the disparity in COVID-19 outpatient treatment that does not reflect the racial and minority group representation of the community at large.
Limitations
The VHA medication allocation system at times created a significant mismatch in supply and demand, which significantly limited the AVAHCS Mab program. VHA facilities nationwide with Mab programs received discrete allocations through the US Department of Health and Human Services via VHA pharmacy benefits management services. Despite our large catchment, AVAHCS was allocated 6 or fewer doses of Mab per week during the evaluated period.
Without formal national guidance in the early period of Mab, the AVAHCS Mab team conferred with Emory University Mab clinicians as well as at other VHA facilities in the country to develop an optimal approach to resource allocation. The Mab team considered all EUA criteria to be as inclusive as possible. However, during times of high demand, our utilitarian approach tried to identify the highest-risk patients who would benefit the most from Mab. The VACO index was validated in early 2021, which facilitated decision making when demand was greater than supply. One limitation of the VACO index is its exclusion of several original Mab EUA criteria, including weight, hypertension, and nonmalignancy-related immunosuppression, into its algorithm.3,8
Conclusions
Through proactive screening and direct outreach to patients, the AVAHCS was able to achieve timely administration of Mab infusion that was well within the initial EUA time frame of 10 days and comparable with the time frame in the REGN-COV2 and BLAZE-1 trials. Improving access to resources by changing the referral structure helped engage veterans who may have otherwise missed the time frame for Mab therapy. The experience of the Mab infusion program at the AVAHCS provided valuable insight into how a health care system could effectively screen a large population and distribute the limited resource of Mab therapy in a timely and proportionate fashion among its represented demographic groups.
Acknowledgments
The authors acknowledge the Veterans Health Administration VISN 7 Clinical Resource Hub and Tele Primary Care group for their support.
1. Chen P, Nirula A, Heller B, et al; BLAZE-1 Investigators. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19. N Engl J Med. 2021;384(3):229-237. doi:10.1056/NEJMoa2029849
2. Weinreich DM, Sivapalasingam S, Norton T, et al; Trial Investigators. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. N Engl J Med. 2021;384(3):238-251. doi:10.1056/NEJMoa2035002
3. US Food and Drug Administration. Fact sheet for health care providers, emergency use authorization (EUA) of bamlanivimab and etesevimab. Accessed August 6, 2023. https://www.fda.gov/media/145802/download
4. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-net). Updated March 24, 2023. Accessed August 6, 2023. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covid-net/purpose-methods.html
5. Centers for Disease Control and Prevention, National Center for Health Statistics. Provisional COVID-19 deaths: distribution of deaths by race and Hispanic origin. Updated July 26, 2023. Accessed August 8, 2023. https://data.cdc.gov/NCHS/Provisional-COVID-19-Deaths-Distribution-of-Deaths/pj7m-y5uh
6. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among Black patients and White patients with COVID-19. N Engl J Med. 2020;382(26):2534-2543. doi:10.1056NEJMsa2011686
7. Wiltz JL, Feehan AK, Mollinari AM, et al. Racial and ethnic disparities in receipt of medications for treatment of COVID-19 - United States, March 2020-August 2021. MMWR Morb Mortal Wkly Rep. 2022;71(3):96-102. doi:10.15585/mmwr.mm7103e1
8. King JT Jr, Yoon JS, Rentsch CT, et al. Development and validation of a 30-day mortality index based on pre-existing medical administrative data from 13,323 COVID-19 patients: the Veterans Health Administration COVID-19 (VACO) Index. PLoS One. 2020;15(11):e0241825. doi:10.1371/journal.pone.0241825
9. US Food and Drug Administration, Office of Media Affairs. Coronavirus (COVID-19) update: FDA revokes emergency use authorization for monoclonal antibody bamlanivimab. Accessed August 8, 2023. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-monoclonal-antibody-bamlanivimab
10. National Institutes of Health. Information on COVID-19 treatment, prevention and research. Accessed August 8, 2023. https://www.covid19treatmentguidelines.nih.gov
11. Anderson B, Smith Z, Edupuganti S, Yan X, Masi CM, Wu HM. Effect of monoclonal antibody treatment on clinical outcomes in ambulatory patients with coronavirus disease 2019. Open Forum Infect Dis. 2021;8(7):ofab315. Published 2021 Jun 12. doi:10.1093/ofid/ofab315
12. United States Census Bureau. Quick facts: DeKalb County, Georgia. Updated July 1, 2022. Accessed August 8, 2023. www.census.gov/quickfacts/dekalbcountygeorgia
13. Kumar R, Wu EL, Stosor V, et al. Real-world experience of bamlanivimab for coronavirus disease 2019 (COVID-19): a case-control study. Clin Infect Dis. 2022;74(1):24-31. doi:10.1093/cid/ciab305
1. Chen P, Nirula A, Heller B, et al; BLAZE-1 Investigators. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19. N Engl J Med. 2021;384(3):229-237. doi:10.1056/NEJMoa2029849
2. Weinreich DM, Sivapalasingam S, Norton T, et al; Trial Investigators. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. N Engl J Med. 2021;384(3):238-251. doi:10.1056/NEJMoa2035002
3. US Food and Drug Administration. Fact sheet for health care providers, emergency use authorization (EUA) of bamlanivimab and etesevimab. Accessed August 6, 2023. https://www.fda.gov/media/145802/download
4. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-net). Updated March 24, 2023. Accessed August 6, 2023. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covid-net/purpose-methods.html
5. Centers for Disease Control and Prevention, National Center for Health Statistics. Provisional COVID-19 deaths: distribution of deaths by race and Hispanic origin. Updated July 26, 2023. Accessed August 8, 2023. https://data.cdc.gov/NCHS/Provisional-COVID-19-Deaths-Distribution-of-Deaths/pj7m-y5uh
6. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among Black patients and White patients with COVID-19. N Engl J Med. 2020;382(26):2534-2543. doi:10.1056NEJMsa2011686
7. Wiltz JL, Feehan AK, Mollinari AM, et al. Racial and ethnic disparities in receipt of medications for treatment of COVID-19 - United States, March 2020-August 2021. MMWR Morb Mortal Wkly Rep. 2022;71(3):96-102. doi:10.15585/mmwr.mm7103e1
8. King JT Jr, Yoon JS, Rentsch CT, et al. Development and validation of a 30-day mortality index based on pre-existing medical administrative data from 13,323 COVID-19 patients: the Veterans Health Administration COVID-19 (VACO) Index. PLoS One. 2020;15(11):e0241825. doi:10.1371/journal.pone.0241825
9. US Food and Drug Administration, Office of Media Affairs. Coronavirus (COVID-19) update: FDA revokes emergency use authorization for monoclonal antibody bamlanivimab. Accessed August 8, 2023. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-monoclonal-antibody-bamlanivimab
10. National Institutes of Health. Information on COVID-19 treatment, prevention and research. Accessed August 8, 2023. https://www.covid19treatmentguidelines.nih.gov
11. Anderson B, Smith Z, Edupuganti S, Yan X, Masi CM, Wu HM. Effect of monoclonal antibody treatment on clinical outcomes in ambulatory patients with coronavirus disease 2019. Open Forum Infect Dis. 2021;8(7):ofab315. Published 2021 Jun 12. doi:10.1093/ofid/ofab315
12. United States Census Bureau. Quick facts: DeKalb County, Georgia. Updated July 1, 2022. Accessed August 8, 2023. www.census.gov/quickfacts/dekalbcountygeorgia
13. Kumar R, Wu EL, Stosor V, et al. Real-world experience of bamlanivimab for coronavirus disease 2019 (COVID-19): a case-control study. Clin Infect Dis. 2022;74(1):24-31. doi:10.1093/cid/ciab305
Olfactory Hallucinations Following COVID-19 Vaccination
The rapid development of multiple vaccines for COVID-19 significantly contributed to reducing the morbidity and mortality associated with COVID-19 infection.1 The vaccination campaign against COVID-19 started in December 2020 within the US Department of Veterans Affairs (VA) health care system with the Pfizer-BioNTech and Moderna COVID-19 mRNA vaccines followed by the Johnson & Johnson (J&J) vaccine in March 2021.2,3
Because of the importance of maintaining a safe vaccination campaign, surveillance reports documenting cases of malignant or benign adverse effects (AEs) are fundamental to generate awareness and accurate knowledge on these newly developed vaccines. Here we report the case of a veteran who developed olfactory hallucinations following the administration of the J&J COVID-19 vaccine.
Case Presentation
A 39-year-old veteran with a history of tension-type headaches presented to the neurology clinic with concern of a burning smell sensation in the absence of an identifiable source. He first noticed this symptom approximately 3 weeks after he received the J&J COVID-19 vaccine about 4 months prior. At the symptom’s first occurrence, he underwent a nasal swab antigen COVID-19 test, which was negative. Initially, symptoms would occur daily lasting about 1 hour. Thereafter, they started to decrease in duration, frequency, and intensity, and about 11 months postvaccination, milder episodes were occurring 1 to 2 times weekly. These episodes lasted nearly 2 years (21 months postvaccination). They happened randomly during the day and were not associated with any other symptoms. Specifically, there were no headaches, loss of consciousness, abnormal movements, nausea, vomiting, photophobia or phonophobia, or alteration of consciousness, such as confusion or drowsiness during or after the events. Additionally, there were no clear triggers the veteran could identify. The veteran did not sustain any head injuries or exposure to toxic odors before the onset of symptoms.
At the time of his presentation to the clinic, both his general and neurological examinations were unremarkable.
Discussion
It has been previously observed that infection with COVID-19 can lead to the loss of taste and smell, but only less commonly olfactory hallucination.4 The pathophysiology of olfactory hallucinations following COVID-19 infection is unknown, but several mechanisms have been proposed. These include obstruction of the olfactory cleft; infection of the sustentacular supporting cells, which express angiotensin‐converting enzyme 2 (ACE‐2); injury to olfactory sensory cells via neuropilin‐1 receptors (NRP1); and injury to the olfactory bulb.5
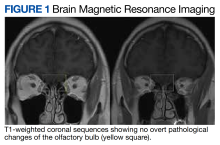
The case we present represents the only report of phantosmia following a J&J COVID-19 vaccination. Phantosmia, featured by a burning or smoke odor, has been reported prior in a case of a 57-year-old woman following the administration of the Pfizer-BioNTech mRNA vaccine.6 Similar to our case, symptoms were not associated with a concurrent COVID-19 infection ruled out via a COVID-19 polymerase chain reaction test. For the Pfizer-BioNTech phantosmia case, a 3 Tesla (T) brain MRI showed left greater than right olfactory bulb and tract gadolinium enhancement on T1-weighted postcontrast images. On axial T2-weighted fluid-attenuated inversion recovery images, hyperintensity along the left olfactory bulb and bilateral olfactory tracts was noted and interpreted as edema. On sagittal thin sections of T2-weighted images, the olfactory nerve filia were thickened and clumped.6 On the contrary, in the case we present, a brain MRI obtained with a 1.5 T magnet showed no abnormalities. It is possible that a high-resolution scan targeting the olfactory bulb could have disclosed pathological changes. At the time when the veteran presented to the neurology clinic, symptoms were already improving, and repeat MRI was deferred as it would not have changed the clinical management.
Konstantinidis and colleagues reported hyposmia in 2 patients following Pfizer-BioNTech COVID-19 vaccination.5 Both patients, 42- and 39-year-old women, experienced hyposmia following their second dose of the vaccine with symptom onset 3 and 5 days after vaccination, respectively. The first patient reported improvement of symptoms after 1 week, while the second patient participated in olfactory training and experienced only partial recovery after 1 month. Multiple studies have reported cranial nerve involvement secondary to other COVID-19 vaccines, including olfactory dysfunction, optic neuritis, acute abducens nerve palsy, Bell palsy, tinnitus, and cochleopathy.7
There are no previous reports of phantosmia following the J&J COVID-19 vaccine. In our case, reported symptoms were mild, although they persisted for nearly 2 years following vaccination.
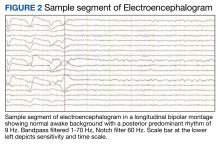
In the evaluation of this veteran, although the timing between symptom onset and vaccination was indicative of a possible link between the 2, other etiologies of phantosmia were ruled out. Isolated olfactory hallucination is most associated with temporal lobe epilepsy, which is the most common form of epilepsy to present in adulthood. However, given the absence of other symptoms suggestive of epilepsy and the duration of the episodes (approximately 1 hour), the clinical suspicion was low. This was reinforced by the EEG that showed no abnormalities in the temporal region. Notwithstanding these considerations, one must keep in mind that no episodes of phantosmia occurred during the EEG recording, the correlates of which are the gold standard to rule out a diagnosis of epilepsy.
A normal brain MRI argued against possible structural abnormalities leading to these symptoms. Thus, the origin of these symptoms remains unknown.
Conclusions
The emergency approval and use of vaccines against COVID-19 was a major victory for public health in 2021. However, given the rapid rollout of these vaccines, the medical community is responsible for reporting adverse effects as they are observed. The authors believe that the clinical events featuring the J&J COVID-19 vaccine in this veteran should not discourage the use of the COVID-19 vaccine. However, sharing the clinical outcome of this veteran is relevant to inform the community regarding this rare and benign possible adverse effect of the J&J COVID-19 vaccine.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Tennessee Valley Veteran Healthcare System (Nashville). The authors thank Dr. Martin Gallagher (Tennessee Valley Veteran Healthcare System) for providing clinical expertise with electroencephalogram interpretation.
1. Xu S, Huang R, Sy LS, et al. COVID-19 vaccination and non-COVID-19 mortality risk - seven integrated health care organizations, United States, December 14, 2020-July 31, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(43):1520-1524. Published 2021 Oct 29. doi:10.15585/mmwr.mm7043e2
2. Der-Martirosian C, Steers WN, Northcraft H, Chu K, Dobalian A. Vaccinating veterans for COVID-19 at the U.S. Department of Veterans Affairs. Am J Prev Med. 2022;62(6):e317-e324. doi:10.1016/j.amepre.2021.12.016
3. Bagnato F, Wallin M. COVID-19 vaccine in veterans with multiple sclerosis: protect the vulnerable. Fed Pract. 2021;38(suppl 1):S28-S32. doi:10.12788/fp.0113
4. Işlek A, Balcı MK. Phantosmia with COVID-19 related olfactory dysfunction: report of nine cases. Indian J Otolaryngol Head Neck Surg. 2022;74(suppl 2):2891-2893. doi:10.1007/s12070-021-02505-z
5. Konstantinidis I, Tsakiropoulou E, Hähner A, de With K, Poulas K, Hummel T. Olfactory dysfunction after coronavirus disease 2019 (COVID-19) vaccination. Int Forum Allergy Rhinol. 2021;11(9):1399-1401. doi:10.1002/alr.22809
6. Keir G, Maria NI, Kirsch CFE. Unique imaging findings of neurologic phantosmia following Pfizer-BioNtech COVID-19 vaccination: a case report. Top Magn Reson Imaging. 2021;30(3):133-137. doi:10.1097/RMR.0000000000000287
7. Garg RK, Paliwal VK. Spectrum of neurological complications following COVID-19 vaccination. Neurol Sci. 2022;43(1):3-40. doi:10.1007/s10072-021-05662-9
The rapid development of multiple vaccines for COVID-19 significantly contributed to reducing the morbidity and mortality associated with COVID-19 infection.1 The vaccination campaign against COVID-19 started in December 2020 within the US Department of Veterans Affairs (VA) health care system with the Pfizer-BioNTech and Moderna COVID-19 mRNA vaccines followed by the Johnson & Johnson (J&J) vaccine in March 2021.2,3
Because of the importance of maintaining a safe vaccination campaign, surveillance reports documenting cases of malignant or benign adverse effects (AEs) are fundamental to generate awareness and accurate knowledge on these newly developed vaccines. Here we report the case of a veteran who developed olfactory hallucinations following the administration of the J&J COVID-19 vaccine.
Case Presentation
A 39-year-old veteran with a history of tension-type headaches presented to the neurology clinic with concern of a burning smell sensation in the absence of an identifiable source. He first noticed this symptom approximately 3 weeks after he received the J&J COVID-19 vaccine about 4 months prior. At the symptom’s first occurrence, he underwent a nasal swab antigen COVID-19 test, which was negative. Initially, symptoms would occur daily lasting about 1 hour. Thereafter, they started to decrease in duration, frequency, and intensity, and about 11 months postvaccination, milder episodes were occurring 1 to 2 times weekly. These episodes lasted nearly 2 years (21 months postvaccination). They happened randomly during the day and were not associated with any other symptoms. Specifically, there were no headaches, loss of consciousness, abnormal movements, nausea, vomiting, photophobia or phonophobia, or alteration of consciousness, such as confusion or drowsiness during or after the events. Additionally, there were no clear triggers the veteran could identify. The veteran did not sustain any head injuries or exposure to toxic odors before the onset of symptoms.
At the time of his presentation to the clinic, both his general and neurological examinations were unremarkable.
Discussion
It has been previously observed that infection with COVID-19 can lead to the loss of taste and smell, but only less commonly olfactory hallucination.4 The pathophysiology of olfactory hallucinations following COVID-19 infection is unknown, but several mechanisms have been proposed. These include obstruction of the olfactory cleft; infection of the sustentacular supporting cells, which express angiotensin‐converting enzyme 2 (ACE‐2); injury to olfactory sensory cells via neuropilin‐1 receptors (NRP1); and injury to the olfactory bulb.5
The case we present represents the only report of phantosmia following a J&J COVID-19 vaccination. Phantosmia, featured by a burning or smoke odor, has been reported prior in a case of a 57-year-old woman following the administration of the Pfizer-BioNTech mRNA vaccine.6 Similar to our case, symptoms were not associated with a concurrent COVID-19 infection ruled out via a COVID-19 polymerase chain reaction test. For the Pfizer-BioNTech phantosmia case, a 3 Tesla (T) brain MRI showed left greater than right olfactory bulb and tract gadolinium enhancement on T1-weighted postcontrast images. On axial T2-weighted fluid-attenuated inversion recovery images, hyperintensity along the left olfactory bulb and bilateral olfactory tracts was noted and interpreted as edema. On sagittal thin sections of T2-weighted images, the olfactory nerve filia were thickened and clumped.6 On the contrary, in the case we present, a brain MRI obtained with a 1.5 T magnet showed no abnormalities. It is possible that a high-resolution scan targeting the olfactory bulb could have disclosed pathological changes. At the time when the veteran presented to the neurology clinic, symptoms were already improving, and repeat MRI was deferred as it would not have changed the clinical management.
Konstantinidis and colleagues reported hyposmia in 2 patients following Pfizer-BioNTech COVID-19 vaccination.5 Both patients, 42- and 39-year-old women, experienced hyposmia following their second dose of the vaccine with symptom onset 3 and 5 days after vaccination, respectively. The first patient reported improvement of symptoms after 1 week, while the second patient participated in olfactory training and experienced only partial recovery after 1 month. Multiple studies have reported cranial nerve involvement secondary to other COVID-19 vaccines, including olfactory dysfunction, optic neuritis, acute abducens nerve palsy, Bell palsy, tinnitus, and cochleopathy.7
There are no previous reports of phantosmia following the J&J COVID-19 vaccine. In our case, reported symptoms were mild, although they persisted for nearly 2 years following vaccination.
In the evaluation of this veteran, although the timing between symptom onset and vaccination was indicative of a possible link between the 2, other etiologies of phantosmia were ruled out. Isolated olfactory hallucination is most associated with temporal lobe epilepsy, which is the most common form of epilepsy to present in adulthood. However, given the absence of other symptoms suggestive of epilepsy and the duration of the episodes (approximately 1 hour), the clinical suspicion was low. This was reinforced by the EEG that showed no abnormalities in the temporal region. Notwithstanding these considerations, one must keep in mind that no episodes of phantosmia occurred during the EEG recording, the correlates of which are the gold standard to rule out a diagnosis of epilepsy.
A normal brain MRI argued against possible structural abnormalities leading to these symptoms. Thus, the origin of these symptoms remains unknown.
Conclusions
The emergency approval and use of vaccines against COVID-19 was a major victory for public health in 2021. However, given the rapid rollout of these vaccines, the medical community is responsible for reporting adverse effects as they are observed. The authors believe that the clinical events featuring the J&J COVID-19 vaccine in this veteran should not discourage the use of the COVID-19 vaccine. However, sharing the clinical outcome of this veteran is relevant to inform the community regarding this rare and benign possible adverse effect of the J&J COVID-19 vaccine.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Tennessee Valley Veteran Healthcare System (Nashville). The authors thank Dr. Martin Gallagher (Tennessee Valley Veteran Healthcare System) for providing clinical expertise with electroencephalogram interpretation.
The rapid development of multiple vaccines for COVID-19 significantly contributed to reducing the morbidity and mortality associated with COVID-19 infection.1 The vaccination campaign against COVID-19 started in December 2020 within the US Department of Veterans Affairs (VA) health care system with the Pfizer-BioNTech and Moderna COVID-19 mRNA vaccines followed by the Johnson & Johnson (J&J) vaccine in March 2021.2,3
Because of the importance of maintaining a safe vaccination campaign, surveillance reports documenting cases of malignant or benign adverse effects (AEs) are fundamental to generate awareness and accurate knowledge on these newly developed vaccines. Here we report the case of a veteran who developed olfactory hallucinations following the administration of the J&J COVID-19 vaccine.
Case Presentation
A 39-year-old veteran with a history of tension-type headaches presented to the neurology clinic with concern of a burning smell sensation in the absence of an identifiable source. He first noticed this symptom approximately 3 weeks after he received the J&J COVID-19 vaccine about 4 months prior. At the symptom’s first occurrence, he underwent a nasal swab antigen COVID-19 test, which was negative. Initially, symptoms would occur daily lasting about 1 hour. Thereafter, they started to decrease in duration, frequency, and intensity, and about 11 months postvaccination, milder episodes were occurring 1 to 2 times weekly. These episodes lasted nearly 2 years (21 months postvaccination). They happened randomly during the day and were not associated with any other symptoms. Specifically, there were no headaches, loss of consciousness, abnormal movements, nausea, vomiting, photophobia or phonophobia, or alteration of consciousness, such as confusion or drowsiness during or after the events. Additionally, there were no clear triggers the veteran could identify. The veteran did not sustain any head injuries or exposure to toxic odors before the onset of symptoms.
At the time of his presentation to the clinic, both his general and neurological examinations were unremarkable.
Discussion
It has been previously observed that infection with COVID-19 can lead to the loss of taste and smell, but only less commonly olfactory hallucination.4 The pathophysiology of olfactory hallucinations following COVID-19 infection is unknown, but several mechanisms have been proposed. These include obstruction of the olfactory cleft; infection of the sustentacular supporting cells, which express angiotensin‐converting enzyme 2 (ACE‐2); injury to olfactory sensory cells via neuropilin‐1 receptors (NRP1); and injury to the olfactory bulb.5
The case we present represents the only report of phantosmia following a J&J COVID-19 vaccination. Phantosmia, featured by a burning or smoke odor, has been reported prior in a case of a 57-year-old woman following the administration of the Pfizer-BioNTech mRNA vaccine.6 Similar to our case, symptoms were not associated with a concurrent COVID-19 infection ruled out via a COVID-19 polymerase chain reaction test. For the Pfizer-BioNTech phantosmia case, a 3 Tesla (T) brain MRI showed left greater than right olfactory bulb and tract gadolinium enhancement on T1-weighted postcontrast images. On axial T2-weighted fluid-attenuated inversion recovery images, hyperintensity along the left olfactory bulb and bilateral olfactory tracts was noted and interpreted as edema. On sagittal thin sections of T2-weighted images, the olfactory nerve filia were thickened and clumped.6 On the contrary, in the case we present, a brain MRI obtained with a 1.5 T magnet showed no abnormalities. It is possible that a high-resolution scan targeting the olfactory bulb could have disclosed pathological changes. At the time when the veteran presented to the neurology clinic, symptoms were already improving, and repeat MRI was deferred as it would not have changed the clinical management.
Konstantinidis and colleagues reported hyposmia in 2 patients following Pfizer-BioNTech COVID-19 vaccination.5 Both patients, 42- and 39-year-old women, experienced hyposmia following their second dose of the vaccine with symptom onset 3 and 5 days after vaccination, respectively. The first patient reported improvement of symptoms after 1 week, while the second patient participated in olfactory training and experienced only partial recovery after 1 month. Multiple studies have reported cranial nerve involvement secondary to other COVID-19 vaccines, including olfactory dysfunction, optic neuritis, acute abducens nerve palsy, Bell palsy, tinnitus, and cochleopathy.7
There are no previous reports of phantosmia following the J&J COVID-19 vaccine. In our case, reported symptoms were mild, although they persisted for nearly 2 years following vaccination.
In the evaluation of this veteran, although the timing between symptom onset and vaccination was indicative of a possible link between the 2, other etiologies of phantosmia were ruled out. Isolated olfactory hallucination is most associated with temporal lobe epilepsy, which is the most common form of epilepsy to present in adulthood. However, given the absence of other symptoms suggestive of epilepsy and the duration of the episodes (approximately 1 hour), the clinical suspicion was low. This was reinforced by the EEG that showed no abnormalities in the temporal region. Notwithstanding these considerations, one must keep in mind that no episodes of phantosmia occurred during the EEG recording, the correlates of which are the gold standard to rule out a diagnosis of epilepsy.
A normal brain MRI argued against possible structural abnormalities leading to these symptoms. Thus, the origin of these symptoms remains unknown.
Conclusions
The emergency approval and use of vaccines against COVID-19 was a major victory for public health in 2021. However, given the rapid rollout of these vaccines, the medical community is responsible for reporting adverse effects as they are observed. The authors believe that the clinical events featuring the J&J COVID-19 vaccine in this veteran should not discourage the use of the COVID-19 vaccine. However, sharing the clinical outcome of this veteran is relevant to inform the community regarding this rare and benign possible adverse effect of the J&J COVID-19 vaccine.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Tennessee Valley Veteran Healthcare System (Nashville). The authors thank Dr. Martin Gallagher (Tennessee Valley Veteran Healthcare System) for providing clinical expertise with electroencephalogram interpretation.
1. Xu S, Huang R, Sy LS, et al. COVID-19 vaccination and non-COVID-19 mortality risk - seven integrated health care organizations, United States, December 14, 2020-July 31, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(43):1520-1524. Published 2021 Oct 29. doi:10.15585/mmwr.mm7043e2
2. Der-Martirosian C, Steers WN, Northcraft H, Chu K, Dobalian A. Vaccinating veterans for COVID-19 at the U.S. Department of Veterans Affairs. Am J Prev Med. 2022;62(6):e317-e324. doi:10.1016/j.amepre.2021.12.016
3. Bagnato F, Wallin M. COVID-19 vaccine in veterans with multiple sclerosis: protect the vulnerable. Fed Pract. 2021;38(suppl 1):S28-S32. doi:10.12788/fp.0113
4. Işlek A, Balcı MK. Phantosmia with COVID-19 related olfactory dysfunction: report of nine cases. Indian J Otolaryngol Head Neck Surg. 2022;74(suppl 2):2891-2893. doi:10.1007/s12070-021-02505-z
5. Konstantinidis I, Tsakiropoulou E, Hähner A, de With K, Poulas K, Hummel T. Olfactory dysfunction after coronavirus disease 2019 (COVID-19) vaccination. Int Forum Allergy Rhinol. 2021;11(9):1399-1401. doi:10.1002/alr.22809
6. Keir G, Maria NI, Kirsch CFE. Unique imaging findings of neurologic phantosmia following Pfizer-BioNtech COVID-19 vaccination: a case report. Top Magn Reson Imaging. 2021;30(3):133-137. doi:10.1097/RMR.0000000000000287
7. Garg RK, Paliwal VK. Spectrum of neurological complications following COVID-19 vaccination. Neurol Sci. 2022;43(1):3-40. doi:10.1007/s10072-021-05662-9
1. Xu S, Huang R, Sy LS, et al. COVID-19 vaccination and non-COVID-19 mortality risk - seven integrated health care organizations, United States, December 14, 2020-July 31, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(43):1520-1524. Published 2021 Oct 29. doi:10.15585/mmwr.mm7043e2
2. Der-Martirosian C, Steers WN, Northcraft H, Chu K, Dobalian A. Vaccinating veterans for COVID-19 at the U.S. Department of Veterans Affairs. Am J Prev Med. 2022;62(6):e317-e324. doi:10.1016/j.amepre.2021.12.016
3. Bagnato F, Wallin M. COVID-19 vaccine in veterans with multiple sclerosis: protect the vulnerable. Fed Pract. 2021;38(suppl 1):S28-S32. doi:10.12788/fp.0113
4. Işlek A, Balcı MK. Phantosmia with COVID-19 related olfactory dysfunction: report of nine cases. Indian J Otolaryngol Head Neck Surg. 2022;74(suppl 2):2891-2893. doi:10.1007/s12070-021-02505-z
5. Konstantinidis I, Tsakiropoulou E, Hähner A, de With K, Poulas K, Hummel T. Olfactory dysfunction after coronavirus disease 2019 (COVID-19) vaccination. Int Forum Allergy Rhinol. 2021;11(9):1399-1401. doi:10.1002/alr.22809
6. Keir G, Maria NI, Kirsch CFE. Unique imaging findings of neurologic phantosmia following Pfizer-BioNtech COVID-19 vaccination: a case report. Top Magn Reson Imaging. 2021;30(3):133-137. doi:10.1097/RMR.0000000000000287
7. Garg RK, Paliwal VK. Spectrum of neurological complications following COVID-19 vaccination. Neurol Sci. 2022;43(1):3-40. doi:10.1007/s10072-021-05662-9
Children and COVID: Does latest rise in new cases point toward stabilization?
New COVID-19 cases rose for the second time in 3 weeks, as the effort to vaccinate the youngest children continued to slow after just 3 full weeks.
Nationally, over 75,000 children under age 5 years received their first dose of COVID-19 vaccine during the week of July 7-13. That number is down from the previous week – 118,000 from June 30 to July 6 – which, in turn, was lower than the 206,000 doses administered through the first 10 days after approval, based on data from the Centers for Disease Control and Prevention. That all adds up to just under 400,000 vaccinated children, or 2% of the eligible population under age 5, as of July 13.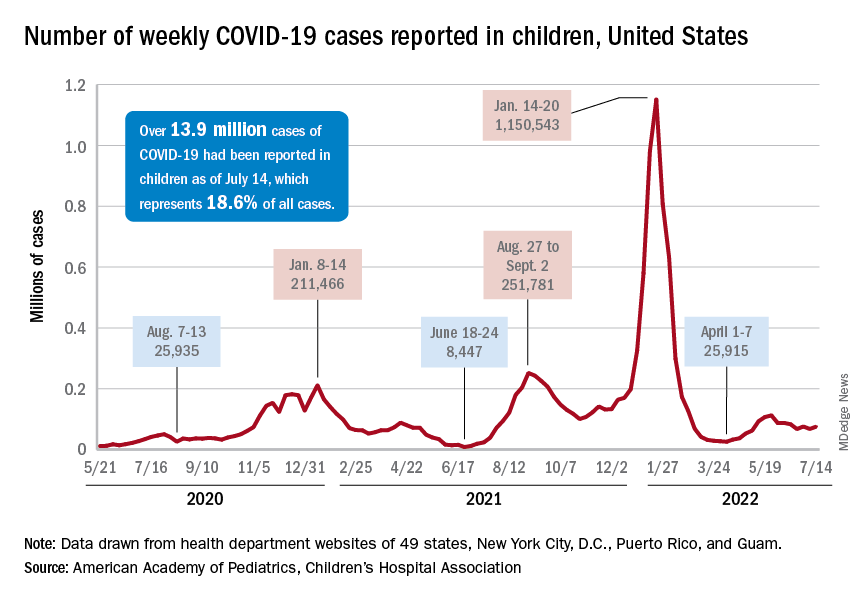
State-level data, meanwhile, show considerable variation, the American Academy of Pediatrics noted in its weekly analysis of the CDC vaccine data. Vermont has already vaccinated 10.0% of children under age 5 years, and Massachusetts is at 9.5%, while Mississippi (0.3%), Alabama (0.5%), and Louisiana (0.8%) are still below 1%, the AAP said.
New cases show signs of steadying
The national count was up by 11.1% for the week of July 8-14, rising to 75,000 new cases, compared with 68,000 the previous week, but the recent trend seems to be leaning toward steadiness. The overall number has been between 67,000 and 76,000 over the past 4 weeks, alternating between rising and falling in that time span, according to data gathered by the AAP and the Children’s Hospital Association from state and territorial health departments.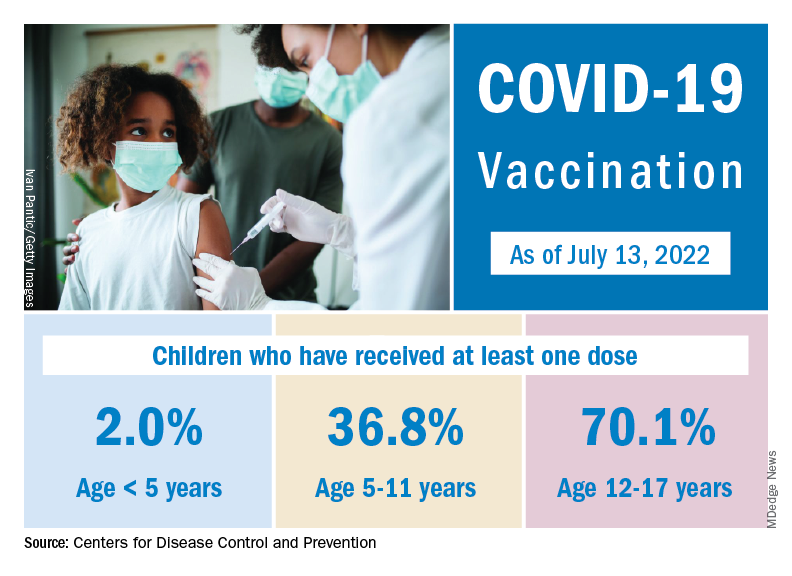
the two groups said, also noting that several states have stopped updating their online dashboards over the past year, making the current total artificially low in comparison.
Taken with that grain of salt, the cumulative number of child cases since the start of the pandemic is just over 13.9 million, which represents 18.6% of all cases in the United States. That proportion has been declining in recent weeks and was as high as 19.0% as late as mid-May. “While COVID-19 cases are likely increasingly underreported for all age groups, this decline indicates that children are disproportionately undercounted in reported COVID-19 cases,” the AAP and CHA said.
New COVID-19 cases rose for the second time in 3 weeks, as the effort to vaccinate the youngest children continued to slow after just 3 full weeks.
Nationally, over 75,000 children under age 5 years received their first dose of COVID-19 vaccine during the week of July 7-13. That number is down from the previous week – 118,000 from June 30 to July 6 – which, in turn, was lower than the 206,000 doses administered through the first 10 days after approval, based on data from the Centers for Disease Control and Prevention. That all adds up to just under 400,000 vaccinated children, or 2% of the eligible population under age 5, as of July 13.
State-level data, meanwhile, show considerable variation, the American Academy of Pediatrics noted in its weekly analysis of the CDC vaccine data. Vermont has already vaccinated 10.0% of children under age 5 years, and Massachusetts is at 9.5%, while Mississippi (0.3%), Alabama (0.5%), and Louisiana (0.8%) are still below 1%, the AAP said.
New cases show signs of steadying
The national count was up by 11.1% for the week of July 8-14, rising to 75,000 new cases, compared with 68,000 the previous week, but the recent trend seems to be leaning toward steadiness. The overall number has been between 67,000 and 76,000 over the past 4 weeks, alternating between rising and falling in that time span, according to data gathered by the AAP and the Children’s Hospital Association from state and territorial health departments.
the two groups said, also noting that several states have stopped updating their online dashboards over the past year, making the current total artificially low in comparison.
Taken with that grain of salt, the cumulative number of child cases since the start of the pandemic is just over 13.9 million, which represents 18.6% of all cases in the United States. That proportion has been declining in recent weeks and was as high as 19.0% as late as mid-May. “While COVID-19 cases are likely increasingly underreported for all age groups, this decline indicates that children are disproportionately undercounted in reported COVID-19 cases,” the AAP and CHA said.
New COVID-19 cases rose for the second time in 3 weeks, as the effort to vaccinate the youngest children continued to slow after just 3 full weeks.
Nationally, over 75,000 children under age 5 years received their first dose of COVID-19 vaccine during the week of July 7-13. That number is down from the previous week – 118,000 from June 30 to July 6 – which, in turn, was lower than the 206,000 doses administered through the first 10 days after approval, based on data from the Centers for Disease Control and Prevention. That all adds up to just under 400,000 vaccinated children, or 2% of the eligible population under age 5, as of July 13.
State-level data, meanwhile, show considerable variation, the American Academy of Pediatrics noted in its weekly analysis of the CDC vaccine data. Vermont has already vaccinated 10.0% of children under age 5 years, and Massachusetts is at 9.5%, while Mississippi (0.3%), Alabama (0.5%), and Louisiana (0.8%) are still below 1%, the AAP said.
New cases show signs of steadying
The national count was up by 11.1% for the week of July 8-14, rising to 75,000 new cases, compared with 68,000 the previous week, but the recent trend seems to be leaning toward steadiness. The overall number has been between 67,000 and 76,000 over the past 4 weeks, alternating between rising and falling in that time span, according to data gathered by the AAP and the Children’s Hospital Association from state and territorial health departments.
the two groups said, also noting that several states have stopped updating their online dashboards over the past year, making the current total artificially low in comparison.
Taken with that grain of salt, the cumulative number of child cases since the start of the pandemic is just over 13.9 million, which represents 18.6% of all cases in the United States. That proportion has been declining in recent weeks and was as high as 19.0% as late as mid-May. “While COVID-19 cases are likely increasingly underreported for all age groups, this decline indicates that children are disproportionately undercounted in reported COVID-19 cases,” the AAP and CHA said.
Meet the JCOM Author with Dr. Barkoudah: IVIG in Treating Nonventilated COVID-19 Patients With Moderate-to-Severe Hypoxia
Why mRNA COVID vaccines are preferred (and why patients should be reassured)
On December 16, 2021, the Advisory Committee on Immunization Practices (ACIP) voted to preferentially recommend messenger RNA (mRNA) vaccines over the Johnson & Johnson/Janssen (J&J) COVID-19 (Ad.26.COV2.S) adenovirus vector vaccine for prevention of COVID-19.1 The mRNA vaccines include Pfizer-BioNTech COVID-19 (BNT162b2) and Moderna COVID-19 (mRNA-1273).
The reason for this preferential recommendation is a rare but serious adverse reaction—thrombosis with thrombocytopenia (TTS) —that has been associated with the J&J vaccine. As of December 8, 2021, more than 16.9 million doses of the J&J COVID-19 vaccine have been given in the United States. The CDC has identified 57 confirmed reports of people who received this vaccine and later developed TTS.2 The known incidence of TTS is thus 1 per ~ 300,000 doses, although the rate may actually be higher.2 All cases have been documented as having occurred after administration of the J&J primary single-dose vaccine; none have been documented (so far) after the booster—although the number of booster doses of the J&J COVID-19 vaccine has been small.
Women between the ages of 30 and 50 years have the highest risk for TTS, with rates of 1 per 94,000 in those ages 30-39 and 1 per 111,000 for those ages 40-49.2,3 All those with TTS have been hospitalized, and 9 have died.2,3 While this adverse reaction is rare, the seriousness of it led the ACIP to state a preference for the mRNA vaccines.
The significance of the recommendation:
- Unless a person has a contraindication to an mRNA vaccine, they should receive 1 of these 2 vaccines for their primary series and boosters.
- The only “Mix and Match” that should occur with boosters is to follow a J&J/Janssen COVID-19 vaccine with an mRNA booster. At this time, booster doses following a 2-dose mRNA primary series should be with an mRNA vaccine.
- The recommendation is for adults ages 18 and older; however, the J&J/Janssen COVID-19 vaccine is not yet approved for younger age-groups.
- The J&J/Janssen COVID-19 vaccine remains an option for those who cannot receive an mRNA vaccine, but it should be administered only after full informed consent.
The J&J/Janssen COVID-19 vaccine initially looked promising a year ago because of its single-dose primary series and its much less stringent storage requirements. However, things have not quite panned out for the vaccine. Its effectiveness after a single dose has proven to be significantly inferior to the 2-dose mRNA vaccines, and it has now been associated with a very serious, albeit rare, adverse reaction.
The major take-home point for physicians to pass on to their patients is that the nation’s system for monitoring vaccine safety works. It can pick up serious adverse reactions that occur at a rate as low as 1/300,000. This should be reassuring.
1. CDC. CDC Endorses ACIP’s Updated COVID-19 Vaccine Recommendations [press release]. December 16, 2021. Accessed December 22, 2021. www.cdc.gov/media/releases/2021/s1216-covid-19-vaccines.html
2. CDC. Selected Adverse Events Reported after COVID-19 Vaccination. December 20, 2021. Accessed December 22, 2021. www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html
3. See I. Updates on thrombosis with thrombocytopenia syndrome (TTS). Presented to the Advisory Committee on Immunization Practices. December 16, 2021. Accessed December 22, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-12-16/02-COVID-See-508.pdf
On December 16, 2021, the Advisory Committee on Immunization Practices (ACIP) voted to preferentially recommend messenger RNA (mRNA) vaccines over the Johnson & Johnson/Janssen (J&J) COVID-19 (Ad.26.COV2.S) adenovirus vector vaccine for prevention of COVID-19.1 The mRNA vaccines include Pfizer-BioNTech COVID-19 (BNT162b2) and Moderna COVID-19 (mRNA-1273).
The reason for this preferential recommendation is a rare but serious adverse reaction—thrombosis with thrombocytopenia (TTS) —that has been associated with the J&J vaccine. As of December 8, 2021, more than 16.9 million doses of the J&J COVID-19 vaccine have been given in the United States. The CDC has identified 57 confirmed reports of people who received this vaccine and later developed TTS.2 The known incidence of TTS is thus 1 per ~ 300,000 doses, although the rate may actually be higher.2 All cases have been documented as having occurred after administration of the J&J primary single-dose vaccine; none have been documented (so far) after the booster—although the number of booster doses of the J&J COVID-19 vaccine has been small.
Women between the ages of 30 and 50 years have the highest risk for TTS, with rates of 1 per 94,000 in those ages 30-39 and 1 per 111,000 for those ages 40-49.2,3 All those with TTS have been hospitalized, and 9 have died.2,3 While this adverse reaction is rare, the seriousness of it led the ACIP to state a preference for the mRNA vaccines.
The significance of the recommendation:
- Unless a person has a contraindication to an mRNA vaccine, they should receive 1 of these 2 vaccines for their primary series and boosters.
- The only “Mix and Match” that should occur with boosters is to follow a J&J/Janssen COVID-19 vaccine with an mRNA booster. At this time, booster doses following a 2-dose mRNA primary series should be with an mRNA vaccine.
- The recommendation is for adults ages 18 and older; however, the J&J/Janssen COVID-19 vaccine is not yet approved for younger age-groups.
- The J&J/Janssen COVID-19 vaccine remains an option for those who cannot receive an mRNA vaccine, but it should be administered only after full informed consent.
The J&J/Janssen COVID-19 vaccine initially looked promising a year ago because of its single-dose primary series and its much less stringent storage requirements. However, things have not quite panned out for the vaccine. Its effectiveness after a single dose has proven to be significantly inferior to the 2-dose mRNA vaccines, and it has now been associated with a very serious, albeit rare, adverse reaction.
The major take-home point for physicians to pass on to their patients is that the nation’s system for monitoring vaccine safety works. It can pick up serious adverse reactions that occur at a rate as low as 1/300,000. This should be reassuring.
On December 16, 2021, the Advisory Committee on Immunization Practices (ACIP) voted to preferentially recommend messenger RNA (mRNA) vaccines over the Johnson & Johnson/Janssen (J&J) COVID-19 (Ad.26.COV2.S) adenovirus vector vaccine for prevention of COVID-19.1 The mRNA vaccines include Pfizer-BioNTech COVID-19 (BNT162b2) and Moderna COVID-19 (mRNA-1273).
The reason for this preferential recommendation is a rare but serious adverse reaction—thrombosis with thrombocytopenia (TTS) —that has been associated with the J&J vaccine. As of December 8, 2021, more than 16.9 million doses of the J&J COVID-19 vaccine have been given in the United States. The CDC has identified 57 confirmed reports of people who received this vaccine and later developed TTS.2 The known incidence of TTS is thus 1 per ~ 300,000 doses, although the rate may actually be higher.2 All cases have been documented as having occurred after administration of the J&J primary single-dose vaccine; none have been documented (so far) after the booster—although the number of booster doses of the J&J COVID-19 vaccine has been small.
Women between the ages of 30 and 50 years have the highest risk for TTS, with rates of 1 per 94,000 in those ages 30-39 and 1 per 111,000 for those ages 40-49.2,3 All those with TTS have been hospitalized, and 9 have died.2,3 While this adverse reaction is rare, the seriousness of it led the ACIP to state a preference for the mRNA vaccines.
The significance of the recommendation:
- Unless a person has a contraindication to an mRNA vaccine, they should receive 1 of these 2 vaccines for their primary series and boosters.
- The only “Mix and Match” that should occur with boosters is to follow a J&J/Janssen COVID-19 vaccine with an mRNA booster. At this time, booster doses following a 2-dose mRNA primary series should be with an mRNA vaccine.
- The recommendation is for adults ages 18 and older; however, the J&J/Janssen COVID-19 vaccine is not yet approved for younger age-groups.
- The J&J/Janssen COVID-19 vaccine remains an option for those who cannot receive an mRNA vaccine, but it should be administered only after full informed consent.
The J&J/Janssen COVID-19 vaccine initially looked promising a year ago because of its single-dose primary series and its much less stringent storage requirements. However, things have not quite panned out for the vaccine. Its effectiveness after a single dose has proven to be significantly inferior to the 2-dose mRNA vaccines, and it has now been associated with a very serious, albeit rare, adverse reaction.
The major take-home point for physicians to pass on to their patients is that the nation’s system for monitoring vaccine safety works. It can pick up serious adverse reactions that occur at a rate as low as 1/300,000. This should be reassuring.
1. CDC. CDC Endorses ACIP’s Updated COVID-19 Vaccine Recommendations [press release]. December 16, 2021. Accessed December 22, 2021. www.cdc.gov/media/releases/2021/s1216-covid-19-vaccines.html
2. CDC. Selected Adverse Events Reported after COVID-19 Vaccination. December 20, 2021. Accessed December 22, 2021. www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html
3. See I. Updates on thrombosis with thrombocytopenia syndrome (TTS). Presented to the Advisory Committee on Immunization Practices. December 16, 2021. Accessed December 22, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-12-16/02-COVID-See-508.pdf
1. CDC. CDC Endorses ACIP’s Updated COVID-19 Vaccine Recommendations [press release]. December 16, 2021. Accessed December 22, 2021. www.cdc.gov/media/releases/2021/s1216-covid-19-vaccines.html
2. CDC. Selected Adverse Events Reported after COVID-19 Vaccination. December 20, 2021. Accessed December 22, 2021. www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html
3. See I. Updates on thrombosis with thrombocytopenia syndrome (TTS). Presented to the Advisory Committee on Immunization Practices. December 16, 2021. Accessed December 22, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-12-16/02-COVID-See-508.pdf
Virtual Visitation: Exploring the Impact on Patients and Families During COVID-19 and Beyond
From Northwell Health, Lake Success, NY.
Objective: Northwell Health, New York’s largest health care organization, rapidly adopted technology solutions to support patient and family communication during the COVID-19 pandemic.
Methods: This case series outlines the pragmatic, interdisciplinary approach Northwell underwent to rapidly implement patient virtual visitation processes during the peak of the initial crisis.
Results: Implementation of large-scale virtual visitation required leadership, technology, and dedicated, empathetic frontline professionals. Patient and family feedback uncovered varied feelings and perspectives, from confusion to gratitude.
Conclusion: Subsequent efforts to obtain direct patient and family perspectives and insights helped Northwell identify areas of strength and ongoing performance improvement.
Keywords: virtual visitation; COVID-19; technology; communication; patient experience.
The power of human connection has become increasingly apparent throughout the COVID-19 pandemic and subsequent recovery phases. Due to the need for social distancing, people worldwide have turned to virtual means of communication, staying in touch with family, friends, and colleagues via digital technology platforms. On March 18, 2020, the New York State Department of Health (NYSDOH) issued a health advisory, suspending all hospital visitation.1 As a result, hospitals rapidly transformed existing in-person visitation practices to meet large-scale virtual programming needs.
Family members often take on various roles—such as advocate, emotional support person, and postdischarge caregiver—for an ill or injured loved one.2 The Institute for Patient- and Family-Centered Care, a nonprofit organization founded in 1992, has been leading a cultural transformation where families are valued as care partners, as opposed to “visitors.”3 Although widely adopted and well-received in specialized units, such as neonatal intensive care units,4 virtual visitation had not been widely implemented across adult care settings. The NYSDOH guidance therefore required organizational leadership, innovation, flexibility, and systems ingenuity to meet the evolving needs of patients, families, and health care professionals. An overarching goal was ensuring patients and families were afforded opportunities to stay connected throughout hospitalization.
Reflecting the impact of COVID-19 surges, hospital environments became increasingly depersonalized, with health care providers wearing extensive personal protective equipment (PPE) and taking remarkable measures to socially distance and minimize exposure. Patients’ room doors were kept primarily closed, while codes and alerts blared in the halls overhead. The lack of families and visitors became increasingly obvious, aiding feelings of isolation and confinement. With fear of nosocomial transmission, impactful modalities (such as sitting at the bedside) and empathetic, therapeutic touch were no longer taking place.
With those scenarios—common to so many health care systems during the pandemic—as a backdrop, comes our experience. Northwell Health is the largest health care system in New York State, geographically spread throughout New York City’s 5 boroughs, Westchester County, and Long Island. With 23 hospitals, approximately 820 medical practices, and over 72 000 employees, Northwell has cared for more than 100 000 COVID-positive patients to date. This case series outlines a pragmatic approach to implementing virtual visitation during the initial peak and obtaining patient and family perspectives to help inform performance improvement and future programming.
Methods
Implementing virtual visitation
Through swift and focused multidisciplinary collaboration, numerous Northwell teams came together to implement large-scale virtual visitation across the organization during the first wave of the COVID crisis. The initial priority involved securing devices that could support patient-family communication. Prior to COVID, each facility had only a handful of tablets that were used primarily during leadership rounding, so once visitation was restricted, we needed a large quantity of devices within a matter of days. Through diligent work from System Procurement and internal Foundation, Northwell was able to acquire nearly 900 devices, including iPads, PadInMotion tablets, and Samsung tablets.
Typically, the benefits of using wireless tablets within a health care setting include long battery life, powerful data processing, advanced operating systems, large screens, and easy end-user navigation.4 During COVID-19 and its associated isolation precautions, tablets offered a lifeline for effective and socially distant communication. With new devices in hand, the system Office of the Chief Information Officer (OCIO) and site-based Information Technology (IT) teams were engaged. They worked tirelessly to streamline connectivity, download necessary apps, test devices on approved WiFi networks, and troubleshoot issues. Once set up, devices were strategically deployed across all Northwell hospitals and post-acute rehabilitation facilities.
Frontline teams quickly realized that a model similar to mobile proning teams, who focus solely on turning and positioning COVID patients to promote optimal respiratory ventilation,5 was needed to support virtual visitation. During the initial COVID wave, elective surgeries were not permissible, as per the NYSDOH. As a result, large numbers of clinical and nonclinical ambulatory surgery employees were redeployed throughout the organization, with many assigned and dedicated to facilitating newly created virtual visitation processes. These employees were primarily responsible for creating unit-based schedules, coordinating logistics, navigating devices on behalf of patients, being present during video calls, and sanitizing the devices between uses. Finally, if necessary, virtual interpretation services were used to overcome language barriers between staff and patients.
What began as an ad hoc function quickly became a valued and meaningful role. Utilizing triage mentality, virtual visitation was first offered during unit-based rounding protocols to those patients with the highest acuity and need to connect with family. We had no formal script; instead, unit-based leaders and frontline team members had open dialogues with patients and families to gauge their interest in virtual visitation. That included patients with an active end-of-life care plan, critically ill patients within intensive care units, and those soon to be intubated or recently extubated. Utilization also occurred within specialty areas such as labor and delivery, pediatrics, inpatient psychiatry, medical units, and long-term rehab facilities. Frontline teams appreciated the supplementary support so they could prioritize ongoing physical assessments and medical interventions. Donned in PPE, virtual visitation team members often served as physical extensions of the patient’s loved ones—holding their hand, offering prayers, and, at times, bearing witness to a last breath. In reflecting on that time, this role required absolute professionalism, empathy, and compassion.
In summer 2020, although demand for virtual visitation was still at an all-time high when ambulatory surgery was reinstated, redeployed staff returned to their responsibilities. To fill this void without interruption to patients and their families, site leaders quickly pivoted and refined processes and protocols utilizing Patient & Customer Experience and Hospitality department team members. Throughout spring 2021, the NYSDOH offered guidance to open in-person visitation, and the institution’s Clinical Advisory Group has been taking a pragmatic approach to doing that in a measured and safe manner across care settings.
Listening to the ‘voice’ of patients and families
Our institution’s mission is grounded in providing “quality service and patient-centered care.” Honoring those tenets, during the initial COVID wave, the system “Voice of the Customer End User Device Workgroup” was created with system and site-based interdisciplinary representation. Despite challenging and unprecedented times, conscious attention and effort was undertaken to assess the use and impact of virtual devices. One of the major work streams was to capture and examine patient and family thoughts, feedback, and the overall experience as it relates to virtual visitation.
The system Office of Patient & Customer Experience (OPCE), led by Sven Gierlinger, SVP Chief Experience Officer, reached out to our colleagues at Press Ganey to add a custom question to patient experience surveys. Beginning on December 1, 2020, discharged inpatients were asked to rate the “Degree to which you were able to stay connected with your family/caregiver during your stay.” Potential answers include the Likert scale responses of Always, Usually, Sometimes, and Never, with “Always” representing the Top Box score. The OPCE team believes these quantitative insights are important to track and trend, particularly since in-person and virtual visitation remain in constant flux.
In an effort to obtain additional, focused, qualitative feedback, OPCE partnered with our institution’s Digital Patient Experience (dPX) colleagues. The approach consisted of voluntary, semistructured, interview-type conversations with patients and family members who engaged in virtual visitation multiple times while the patient was hospitalized. OPCE contacted site-based Patient Experience leads, also known as Culture Leaders, at 3 hospitals, asking them to identify potential participants. This convenience sample excluded instances where the patient passed away during and/or immediately following hospitalization.
The OPCE team phoned potential interview candidates to make a personalized connection, explain the purpose of the interviews, and schedule them, if interested. For consistency, the same Digital Customer Experience Researcher on the dPX team facilitated all sessions, which were 30-minute, semiscripted interviews conducted virtually via Microsoft Teams. The tone was intentionally conversational so that patients and family members would feel comfortable delving into themes that were most impactful during their experience. After some initial ice breakers, such as “What were some of your feelings about being a patient/having a loved one in the hospital during the early days of the COVID-19 pandemic?” we moved on to some more pragmatic, implementation questions and rating scales. These included questions such as “How did you first learn about the option for virtual visitation? Was it something you inquired about or did someone offer it to you? How was it explained to you?” Patients were also asked, on a scale of 1 (easy) to 5 (difficult), to rate their experience with the technology aspect when connecting with their loved ones. They also provided verbal consent to be recorded and were given a $15 gift card upon completion of the interview.
Transcriptions were generated by uploading the interview recordings to a platform called UserTesting. In addition to these transcriptions, this platform also allowed for a keyword mapping tool that organized high-level themes and adjectives into groupings along a sentiment axis from negative to neutral to positive. Transcripts were then read carefully and annotated by the Digital Customer Experience Researcher, which allowed for strengthening of some of the automated themes as well as the emergence of new, more nuanced themes. These themes were organized into those that we could address with design and/or procedure updates (actionable insights), those that came up most frequently overall (frequency), and those that came up across our 3 interview sessions (commonality).
This feedback, along with the responses to the new Press Ganey question, was presented to the system Voice of the Customer End User Device Workgroup. The results led to robust discussion and brainstorming regarding how to improve the process to be more patient-centered. Findings were also shared with our hospital-based Culture Leaders. As many of their local strategic plans focused on patient-family communication, this information was helpful to them in considering plans for expansion and/or sustaining virtual visitation efforts. The process map in the Figure outlines key milestones within this feedback loop.
Outcomes
During the height of the initial COVID-19 crisis, virtual visitation was a new and ever-evolving process. Amidst the chaos, mechanisms to capture the quantity and quality of virtual visits were not in place. Based on informal observation, a majority of patients utilized personal devices to connect with loved ones, and staff even offered their own cellular devices to facilitate timely patient-family communication. The technology primarily used included FaceTime, Zoom, and EZCall, as there was much public awareness and comfort with those platforms.
In the first quarter of 2021, our institution overall performed at a Top Box score of 60.2 for our ability to assist patients with staying connected to their family/caregiver during their inpatient visit. With more than 6700 returned surveys during that time period, our hospitals earned Top Box scores ranging between 48.0 and 75.3. At this time, obtaining a national benchmark ranking is not possible, because the question regarding connectedness is unique to Northwell inpatient settings. As other health care organizations adopt this customized question, further peer-to-peer measurements can be established.
Regarding virtual interviews, 25 patients were initially contacted to determine their interest in participating. Of that sample, 17 patients were engaged over the phone, representing a reach rate of 68%. Overall, 10 interviews were scheduled; 7 patients did not show up, resulting in 3 completed interviews. During follow-up, “no-show” participants either gave no response or stated they had a conflict at their originally scheduled time but were not interested in rescheduling due to personal circumstances. Through such conversations, ongoing health complications were found to be a reoccurring barrier to participation.
Each of the participating patients had experienced being placed on a ventilator. They described their hospitalization as a time of “confusion and despair” in the first days after extubation. After we reviewed interview recordings, a reoccurring theme across all interviews was the feeling of gratitude. Patients expressed deep and heartfelt appreciation for being given the opportunity to connect as a family. One patient described virtual visitation sessions as her “only tether to reality when nothing else made sense.”
Interestingly enough, none of the participants knew that virtual visitation was an option and/or thought to inquire about it before a hospital staff member offered to set up a session. Patients recounted how they were weak and physically unable to connect to the sessions without significant assistance. They reported examples of not having the physical strength to hold up the tablet or needing a staff member to facilitate the conversation because the patient could not speak loudly enough and/or they were having difficulty hearing over background medical equipment noises. Participants also described times when a nurse or social worker would stand and hold the tablet for 20 to 30 minutes at a time, further describing mixed feelings of gratitude, guilt for “taking up their time,” and a desire for more privacy to have those precious conversations.
Discussion
Our institution encountered various barriers when establishing, implementing, and sustaining virtual visitation. The acquisition and bulk purchasing of devices, so that each hospital unit and department had adequate par levels during a high-demand time frame, was an initial challenge. Ensuring appropriate safeguards, software programming, and access to WiFi required ingenuity from IT teams. Leaders sought to advocate for the importance of prioritizing virtual visitation alongside clinical interventions. For team members, education was needed to build awareness, learn how to navigate technology, and troubleshoot, in real-time, issues such as poor connectivity. However, despite these organizational struggles, the hospital’s frontline professionals fully recognized and understood the humanistic value of connecting ill patients with their loved ones. Harnessing their teamwork, empathy, and innovative spirits, they forged through such difficulties to create meaningful interactions.
Although virtual visitation occurred prior to the COVID-19 pandemic, particularly in subspecialty areas such as neonatal intensive care units,6 it was not commonplace in most adult inpatient care settings. However, now that virtual means to communication are widely accepted and preferred, our hospital anticipates these offerings will become a broad patient expectation and, therefore, part of standard hospital care and operations. Health care leaders and interdisciplinary teams must therefore prioritize virtual visitation protocols, efforts, and future programming. It is no longer an exception to the rule, but rather a critical approach when ensuring quality communication between patients, families, and care teams.
We strive to continually improve by including user feedback as part of an interactive design process. For a broader, more permanent installation of virtual visitation, health care organizations must proactively promote this capability as a valued option. Considering health literacy and comfort with technology, functionality, and logistics must be carefully explained to patients and their families. This may require additional staff training so that they are knowledgeable, comfortable with, and able to troubleshoot questions/concerns in real time. There needs to be an adequate number of mobile devices available at a unit or departmental level to meet short-term and long-term demands. Additionally, now that we have emerged from our initial crisis-based mentality, it is time to consider alternatives to alleviate the need for staff assistance, such as mounts to hold devices and enabling voice controls.
Conclusion
As an organization grounded in the spirit of innovation, Northwell has been able to quickly pivot, adopting virtual visitation to address emerging and complex communication needs. Taking a best practice established during a crisis period and engraining it into sustainable organizational culture and operations requires visionary leadership, strong teamwork, and an unbridled commitment to patient and family centeredness. Despite unprecedented challenges, our commitment to listening to the “voice” of patients and families never wavered. Using their insights and feedback as critical components to the decision-making process, there is much work ahead within the realm of virtual visitation.
Acknowledgements: The authors would like to acknowledge the Northwell Health providers, frontline health care professionals, and team members who worked tirelessly to care for its community during initial COVID-19 waves and every day thereafter. Heartfelt gratitude to Northwell’s senior leaders for the visionary leadership; the OCIO and hospital-based IT teams for their swift collaboration; and dedicated Culture Leaders, Patient Experience team members, and redeployed staff for their unbridled passion for caring for patients and families. Special thanks to Agnes Barden, DNP, RN, CPXP, Joseph Narvaez, MBA, and Natalie Bashkin, MBA, from the system Office of Patient & Customer Experience, and Carolyne Burgess, MPH, from the Digital Patient Experience teams, for their participation, leadership, and syngeristic partnerships.
Corresponding Author: Nicole Giammarinaro, MSN, RN, CPXP, Director, Patient & Customer Experience, Northwell Health, 2000 Marcus Ave, Lake Success, NY 11042; [email protected].
Financial disclosures: Sven Gierlinger serves on the Speakers Bureau for Northwell Health and as an Executive Board Member for The Beryl Institute.
1. New York State Department of Health. Health advisory: COVID-19 guidance for hospital operators regarding visitation. March 18, 2020. https://coronavirus.health.ny.gov/system/files/documents/2020/03/covid19-hospital-visitation-guidance-3.18.20.pdf
2. Zhang Y. Family functioning in the context of an adult family member with illness: a concept analysis. J Clin Nurs. 2018;27(15-16):3205-3224. doi:10.1111/jocn.14500
3. Institute for Patient- & Family-Centered Care. Better Together: Partnering with Families. https://www.ipfcc.org/bestpractices/better-together-ny.html
4. Marceglia S, Bonacina S, Zaccaria V, et al. How might the iPad change healthcare? J R Soc Med. 2012;105(6):233-241. doi:10.1258/jrsm.2012.110296
5. Short B, Parekh M, Ryan P, et al. Rapid implementation of a mobile prone team during the COVID-19 pandemic. J Crit Care. 2020;60:230-234. doi:10.1016/j.jcrc.2020.08.020
6. Yeo C, Ho SK, Khong K, Lau Y. Virtual visitation in the neonatal intensive care: experience with the use of internet and telemedicine in a tertiary neonatal unit. Perm J. 2011;15(3):32-36.
From Northwell Health, Lake Success, NY.
Objective: Northwell Health, New York’s largest health care organization, rapidly adopted technology solutions to support patient and family communication during the COVID-19 pandemic.
Methods: This case series outlines the pragmatic, interdisciplinary approach Northwell underwent to rapidly implement patient virtual visitation processes during the peak of the initial crisis.
Results: Implementation of large-scale virtual visitation required leadership, technology, and dedicated, empathetic frontline professionals. Patient and family feedback uncovered varied feelings and perspectives, from confusion to gratitude.
Conclusion: Subsequent efforts to obtain direct patient and family perspectives and insights helped Northwell identify areas of strength and ongoing performance improvement.
Keywords: virtual visitation; COVID-19; technology; communication; patient experience.
The power of human connection has become increasingly apparent throughout the COVID-19 pandemic and subsequent recovery phases. Due to the need for social distancing, people worldwide have turned to virtual means of communication, staying in touch with family, friends, and colleagues via digital technology platforms. On March 18, 2020, the New York State Department of Health (NYSDOH) issued a health advisory, suspending all hospital visitation.1 As a result, hospitals rapidly transformed existing in-person visitation practices to meet large-scale virtual programming needs.
Family members often take on various roles—such as advocate, emotional support person, and postdischarge caregiver—for an ill or injured loved one.2 The Institute for Patient- and Family-Centered Care, a nonprofit organization founded in 1992, has been leading a cultural transformation where families are valued as care partners, as opposed to “visitors.”3 Although widely adopted and well-received in specialized units, such as neonatal intensive care units,4 virtual visitation had not been widely implemented across adult care settings. The NYSDOH guidance therefore required organizational leadership, innovation, flexibility, and systems ingenuity to meet the evolving needs of patients, families, and health care professionals. An overarching goal was ensuring patients and families were afforded opportunities to stay connected throughout hospitalization.
Reflecting the impact of COVID-19 surges, hospital environments became increasingly depersonalized, with health care providers wearing extensive personal protective equipment (PPE) and taking remarkable measures to socially distance and minimize exposure. Patients’ room doors were kept primarily closed, while codes and alerts blared in the halls overhead. The lack of families and visitors became increasingly obvious, aiding feelings of isolation and confinement. With fear of nosocomial transmission, impactful modalities (such as sitting at the bedside) and empathetic, therapeutic touch were no longer taking place.
With those scenarios—common to so many health care systems during the pandemic—as a backdrop, comes our experience. Northwell Health is the largest health care system in New York State, geographically spread throughout New York City’s 5 boroughs, Westchester County, and Long Island. With 23 hospitals, approximately 820 medical practices, and over 72 000 employees, Northwell has cared for more than 100 000 COVID-positive patients to date. This case series outlines a pragmatic approach to implementing virtual visitation during the initial peak and obtaining patient and family perspectives to help inform performance improvement and future programming.
Methods
Implementing virtual visitation
Through swift and focused multidisciplinary collaboration, numerous Northwell teams came together to implement large-scale virtual visitation across the organization during the first wave of the COVID crisis. The initial priority involved securing devices that could support patient-family communication. Prior to COVID, each facility had only a handful of tablets that were used primarily during leadership rounding, so once visitation was restricted, we needed a large quantity of devices within a matter of days. Through diligent work from System Procurement and internal Foundation, Northwell was able to acquire nearly 900 devices, including iPads, PadInMotion tablets, and Samsung tablets.
Typically, the benefits of using wireless tablets within a health care setting include long battery life, powerful data processing, advanced operating systems, large screens, and easy end-user navigation.4 During COVID-19 and its associated isolation precautions, tablets offered a lifeline for effective and socially distant communication. With new devices in hand, the system Office of the Chief Information Officer (OCIO) and site-based Information Technology (IT) teams were engaged. They worked tirelessly to streamline connectivity, download necessary apps, test devices on approved WiFi networks, and troubleshoot issues. Once set up, devices were strategically deployed across all Northwell hospitals and post-acute rehabilitation facilities.
Frontline teams quickly realized that a model similar to mobile proning teams, who focus solely on turning and positioning COVID patients to promote optimal respiratory ventilation,5 was needed to support virtual visitation. During the initial COVID wave, elective surgeries were not permissible, as per the NYSDOH. As a result, large numbers of clinical and nonclinical ambulatory surgery employees were redeployed throughout the organization, with many assigned and dedicated to facilitating newly created virtual visitation processes. These employees were primarily responsible for creating unit-based schedules, coordinating logistics, navigating devices on behalf of patients, being present during video calls, and sanitizing the devices between uses. Finally, if necessary, virtual interpretation services were used to overcome language barriers between staff and patients.
What began as an ad hoc function quickly became a valued and meaningful role. Utilizing triage mentality, virtual visitation was first offered during unit-based rounding protocols to those patients with the highest acuity and need to connect with family. We had no formal script; instead, unit-based leaders and frontline team members had open dialogues with patients and families to gauge their interest in virtual visitation. That included patients with an active end-of-life care plan, critically ill patients within intensive care units, and those soon to be intubated or recently extubated. Utilization also occurred within specialty areas such as labor and delivery, pediatrics, inpatient psychiatry, medical units, and long-term rehab facilities. Frontline teams appreciated the supplementary support so they could prioritize ongoing physical assessments and medical interventions. Donned in PPE, virtual visitation team members often served as physical extensions of the patient’s loved ones—holding their hand, offering prayers, and, at times, bearing witness to a last breath. In reflecting on that time, this role required absolute professionalism, empathy, and compassion.
In summer 2020, although demand for virtual visitation was still at an all-time high when ambulatory surgery was reinstated, redeployed staff returned to their responsibilities. To fill this void without interruption to patients and their families, site leaders quickly pivoted and refined processes and protocols utilizing Patient & Customer Experience and Hospitality department team members. Throughout spring 2021, the NYSDOH offered guidance to open in-person visitation, and the institution’s Clinical Advisory Group has been taking a pragmatic approach to doing that in a measured and safe manner across care settings.
Listening to the ‘voice’ of patients and families
Our institution’s mission is grounded in providing “quality service and patient-centered care.” Honoring those tenets, during the initial COVID wave, the system “Voice of the Customer End User Device Workgroup” was created with system and site-based interdisciplinary representation. Despite challenging and unprecedented times, conscious attention and effort was undertaken to assess the use and impact of virtual devices. One of the major work streams was to capture and examine patient and family thoughts, feedback, and the overall experience as it relates to virtual visitation.
The system Office of Patient & Customer Experience (OPCE), led by Sven Gierlinger, SVP Chief Experience Officer, reached out to our colleagues at Press Ganey to add a custom question to patient experience surveys. Beginning on December 1, 2020, discharged inpatients were asked to rate the “Degree to which you were able to stay connected with your family/caregiver during your stay.” Potential answers include the Likert scale responses of Always, Usually, Sometimes, and Never, with “Always” representing the Top Box score. The OPCE team believes these quantitative insights are important to track and trend, particularly since in-person and virtual visitation remain in constant flux.
In an effort to obtain additional, focused, qualitative feedback, OPCE partnered with our institution’s Digital Patient Experience (dPX) colleagues. The approach consisted of voluntary, semistructured, interview-type conversations with patients and family members who engaged in virtual visitation multiple times while the patient was hospitalized. OPCE contacted site-based Patient Experience leads, also known as Culture Leaders, at 3 hospitals, asking them to identify potential participants. This convenience sample excluded instances where the patient passed away during and/or immediately following hospitalization.
The OPCE team phoned potential interview candidates to make a personalized connection, explain the purpose of the interviews, and schedule them, if interested. For consistency, the same Digital Customer Experience Researcher on the dPX team facilitated all sessions, which were 30-minute, semiscripted interviews conducted virtually via Microsoft Teams. The tone was intentionally conversational so that patients and family members would feel comfortable delving into themes that were most impactful during their experience. After some initial ice breakers, such as “What were some of your feelings about being a patient/having a loved one in the hospital during the early days of the COVID-19 pandemic?” we moved on to some more pragmatic, implementation questions and rating scales. These included questions such as “How did you first learn about the option for virtual visitation? Was it something you inquired about or did someone offer it to you? How was it explained to you?” Patients were also asked, on a scale of 1 (easy) to 5 (difficult), to rate their experience with the technology aspect when connecting with their loved ones. They also provided verbal consent to be recorded and were given a $15 gift card upon completion of the interview.
Transcriptions were generated by uploading the interview recordings to a platform called UserTesting. In addition to these transcriptions, this platform also allowed for a keyword mapping tool that organized high-level themes and adjectives into groupings along a sentiment axis from negative to neutral to positive. Transcripts were then read carefully and annotated by the Digital Customer Experience Researcher, which allowed for strengthening of some of the automated themes as well as the emergence of new, more nuanced themes. These themes were organized into those that we could address with design and/or procedure updates (actionable insights), those that came up most frequently overall (frequency), and those that came up across our 3 interview sessions (commonality).
This feedback, along with the responses to the new Press Ganey question, was presented to the system Voice of the Customer End User Device Workgroup. The results led to robust discussion and brainstorming regarding how to improve the process to be more patient-centered. Findings were also shared with our hospital-based Culture Leaders. As many of their local strategic plans focused on patient-family communication, this information was helpful to them in considering plans for expansion and/or sustaining virtual visitation efforts. The process map in the Figure outlines key milestones within this feedback loop.

Outcomes
During the height of the initial COVID-19 crisis, virtual visitation was a new and ever-evolving process. Amidst the chaos, mechanisms to capture the quantity and quality of virtual visits were not in place. Based on informal observation, a majority of patients utilized personal devices to connect with loved ones, and staff even offered their own cellular devices to facilitate timely patient-family communication. The technology primarily used included FaceTime, Zoom, and EZCall, as there was much public awareness and comfort with those platforms.
In the first quarter of 2021, our institution overall performed at a Top Box score of 60.2 for our ability to assist patients with staying connected to their family/caregiver during their inpatient visit. With more than 6700 returned surveys during that time period, our hospitals earned Top Box scores ranging between 48.0 and 75.3. At this time, obtaining a national benchmark ranking is not possible, because the question regarding connectedness is unique to Northwell inpatient settings. As other health care organizations adopt this customized question, further peer-to-peer measurements can be established.
Regarding virtual interviews, 25 patients were initially contacted to determine their interest in participating. Of that sample, 17 patients were engaged over the phone, representing a reach rate of 68%. Overall, 10 interviews were scheduled; 7 patients did not show up, resulting in 3 completed interviews. During follow-up, “no-show” participants either gave no response or stated they had a conflict at their originally scheduled time but were not interested in rescheduling due to personal circumstances. Through such conversations, ongoing health complications were found to be a reoccurring barrier to participation.
Each of the participating patients had experienced being placed on a ventilator. They described their hospitalization as a time of “confusion and despair” in the first days after extubation. After we reviewed interview recordings, a reoccurring theme across all interviews was the feeling of gratitude. Patients expressed deep and heartfelt appreciation for being given the opportunity to connect as a family. One patient described virtual visitation sessions as her “only tether to reality when nothing else made sense.”
Interestingly enough, none of the participants knew that virtual visitation was an option and/or thought to inquire about it before a hospital staff member offered to set up a session. Patients recounted how they were weak and physically unable to connect to the sessions without significant assistance. They reported examples of not having the physical strength to hold up the tablet or needing a staff member to facilitate the conversation because the patient could not speak loudly enough and/or they were having difficulty hearing over background medical equipment noises. Participants also described times when a nurse or social worker would stand and hold the tablet for 20 to 30 minutes at a time, further describing mixed feelings of gratitude, guilt for “taking up their time,” and a desire for more privacy to have those precious conversations.
Discussion
Our institution encountered various barriers when establishing, implementing, and sustaining virtual visitation. The acquisition and bulk purchasing of devices, so that each hospital unit and department had adequate par levels during a high-demand time frame, was an initial challenge. Ensuring appropriate safeguards, software programming, and access to WiFi required ingenuity from IT teams. Leaders sought to advocate for the importance of prioritizing virtual visitation alongside clinical interventions. For team members, education was needed to build awareness, learn how to navigate technology, and troubleshoot, in real-time, issues such as poor connectivity. However, despite these organizational struggles, the hospital’s frontline professionals fully recognized and understood the humanistic value of connecting ill patients with their loved ones. Harnessing their teamwork, empathy, and innovative spirits, they forged through such difficulties to create meaningful interactions.
Although virtual visitation occurred prior to the COVID-19 pandemic, particularly in subspecialty areas such as neonatal intensive care units,6 it was not commonplace in most adult inpatient care settings. However, now that virtual means to communication are widely accepted and preferred, our hospital anticipates these offerings will become a broad patient expectation and, therefore, part of standard hospital care and operations. Health care leaders and interdisciplinary teams must therefore prioritize virtual visitation protocols, efforts, and future programming. It is no longer an exception to the rule, but rather a critical approach when ensuring quality communication between patients, families, and care teams.
We strive to continually improve by including user feedback as part of an interactive design process. For a broader, more permanent installation of virtual visitation, health care organizations must proactively promote this capability as a valued option. Considering health literacy and comfort with technology, functionality, and logistics must be carefully explained to patients and their families. This may require additional staff training so that they are knowledgeable, comfortable with, and able to troubleshoot questions/concerns in real time. There needs to be an adequate number of mobile devices available at a unit or departmental level to meet short-term and long-term demands. Additionally, now that we have emerged from our initial crisis-based mentality, it is time to consider alternatives to alleviate the need for staff assistance, such as mounts to hold devices and enabling voice controls.
Conclusion
As an organization grounded in the spirit of innovation, Northwell has been able to quickly pivot, adopting virtual visitation to address emerging and complex communication needs. Taking a best practice established during a crisis period and engraining it into sustainable organizational culture and operations requires visionary leadership, strong teamwork, and an unbridled commitment to patient and family centeredness. Despite unprecedented challenges, our commitment to listening to the “voice” of patients and families never wavered. Using their insights and feedback as critical components to the decision-making process, there is much work ahead within the realm of virtual visitation.
Acknowledgements: The authors would like to acknowledge the Northwell Health providers, frontline health care professionals, and team members who worked tirelessly to care for its community during initial COVID-19 waves and every day thereafter. Heartfelt gratitude to Northwell’s senior leaders for the visionary leadership; the OCIO and hospital-based IT teams for their swift collaboration; and dedicated Culture Leaders, Patient Experience team members, and redeployed staff for their unbridled passion for caring for patients and families. Special thanks to Agnes Barden, DNP, RN, CPXP, Joseph Narvaez, MBA, and Natalie Bashkin, MBA, from the system Office of Patient & Customer Experience, and Carolyne Burgess, MPH, from the Digital Patient Experience teams, for their participation, leadership, and syngeristic partnerships.
Corresponding Author: Nicole Giammarinaro, MSN, RN, CPXP, Director, Patient & Customer Experience, Northwell Health, 2000 Marcus Ave, Lake Success, NY 11042; [email protected].
Financial disclosures: Sven Gierlinger serves on the Speakers Bureau for Northwell Health and as an Executive Board Member for The Beryl Institute.
From Northwell Health, Lake Success, NY.
Objective: Northwell Health, New York’s largest health care organization, rapidly adopted technology solutions to support patient and family communication during the COVID-19 pandemic.
Methods: This case series outlines the pragmatic, interdisciplinary approach Northwell underwent to rapidly implement patient virtual visitation processes during the peak of the initial crisis.
Results: Implementation of large-scale virtual visitation required leadership, technology, and dedicated, empathetic frontline professionals. Patient and family feedback uncovered varied feelings and perspectives, from confusion to gratitude.
Conclusion: Subsequent efforts to obtain direct patient and family perspectives and insights helped Northwell identify areas of strength and ongoing performance improvement.
Keywords: virtual visitation; COVID-19; technology; communication; patient experience.
The power of human connection has become increasingly apparent throughout the COVID-19 pandemic and subsequent recovery phases. Due to the need for social distancing, people worldwide have turned to virtual means of communication, staying in touch with family, friends, and colleagues via digital technology platforms. On March 18, 2020, the New York State Department of Health (NYSDOH) issued a health advisory, suspending all hospital visitation.1 As a result, hospitals rapidly transformed existing in-person visitation practices to meet large-scale virtual programming needs.
Family members often take on various roles—such as advocate, emotional support person, and postdischarge caregiver—for an ill or injured loved one.2 The Institute for Patient- and Family-Centered Care, a nonprofit organization founded in 1992, has been leading a cultural transformation where families are valued as care partners, as opposed to “visitors.”3 Although widely adopted and well-received in specialized units, such as neonatal intensive care units,4 virtual visitation had not been widely implemented across adult care settings. The NYSDOH guidance therefore required organizational leadership, innovation, flexibility, and systems ingenuity to meet the evolving needs of patients, families, and health care professionals. An overarching goal was ensuring patients and families were afforded opportunities to stay connected throughout hospitalization.
Reflecting the impact of COVID-19 surges, hospital environments became increasingly depersonalized, with health care providers wearing extensive personal protective equipment (PPE) and taking remarkable measures to socially distance and minimize exposure. Patients’ room doors were kept primarily closed, while codes and alerts blared in the halls overhead. The lack of families and visitors became increasingly obvious, aiding feelings of isolation and confinement. With fear of nosocomial transmission, impactful modalities (such as sitting at the bedside) and empathetic, therapeutic touch were no longer taking place.
With those scenarios—common to so many health care systems during the pandemic—as a backdrop, comes our experience. Northwell Health is the largest health care system in New York State, geographically spread throughout New York City’s 5 boroughs, Westchester County, and Long Island. With 23 hospitals, approximately 820 medical practices, and over 72 000 employees, Northwell has cared for more than 100 000 COVID-positive patients to date. This case series outlines a pragmatic approach to implementing virtual visitation during the initial peak and obtaining patient and family perspectives to help inform performance improvement and future programming.
Methods
Implementing virtual visitation
Through swift and focused multidisciplinary collaboration, numerous Northwell teams came together to implement large-scale virtual visitation across the organization during the first wave of the COVID crisis. The initial priority involved securing devices that could support patient-family communication. Prior to COVID, each facility had only a handful of tablets that were used primarily during leadership rounding, so once visitation was restricted, we needed a large quantity of devices within a matter of days. Through diligent work from System Procurement and internal Foundation, Northwell was able to acquire nearly 900 devices, including iPads, PadInMotion tablets, and Samsung tablets.
Typically, the benefits of using wireless tablets within a health care setting include long battery life, powerful data processing, advanced operating systems, large screens, and easy end-user navigation.4 During COVID-19 and its associated isolation precautions, tablets offered a lifeline for effective and socially distant communication. With new devices in hand, the system Office of the Chief Information Officer (OCIO) and site-based Information Technology (IT) teams were engaged. They worked tirelessly to streamline connectivity, download necessary apps, test devices on approved WiFi networks, and troubleshoot issues. Once set up, devices were strategically deployed across all Northwell hospitals and post-acute rehabilitation facilities.
Frontline teams quickly realized that a model similar to mobile proning teams, who focus solely on turning and positioning COVID patients to promote optimal respiratory ventilation,5 was needed to support virtual visitation. During the initial COVID wave, elective surgeries were not permissible, as per the NYSDOH. As a result, large numbers of clinical and nonclinical ambulatory surgery employees were redeployed throughout the organization, with many assigned and dedicated to facilitating newly created virtual visitation processes. These employees were primarily responsible for creating unit-based schedules, coordinating logistics, navigating devices on behalf of patients, being present during video calls, and sanitizing the devices between uses. Finally, if necessary, virtual interpretation services were used to overcome language barriers between staff and patients.
What began as an ad hoc function quickly became a valued and meaningful role. Utilizing triage mentality, virtual visitation was first offered during unit-based rounding protocols to those patients with the highest acuity and need to connect with family. We had no formal script; instead, unit-based leaders and frontline team members had open dialogues with patients and families to gauge their interest in virtual visitation. That included patients with an active end-of-life care plan, critically ill patients within intensive care units, and those soon to be intubated or recently extubated. Utilization also occurred within specialty areas such as labor and delivery, pediatrics, inpatient psychiatry, medical units, and long-term rehab facilities. Frontline teams appreciated the supplementary support so they could prioritize ongoing physical assessments and medical interventions. Donned in PPE, virtual visitation team members often served as physical extensions of the patient’s loved ones—holding their hand, offering prayers, and, at times, bearing witness to a last breath. In reflecting on that time, this role required absolute professionalism, empathy, and compassion.
In summer 2020, although demand for virtual visitation was still at an all-time high when ambulatory surgery was reinstated, redeployed staff returned to their responsibilities. To fill this void without interruption to patients and their families, site leaders quickly pivoted and refined processes and protocols utilizing Patient & Customer Experience and Hospitality department team members. Throughout spring 2021, the NYSDOH offered guidance to open in-person visitation, and the institution’s Clinical Advisory Group has been taking a pragmatic approach to doing that in a measured and safe manner across care settings.
Listening to the ‘voice’ of patients and families
Our institution’s mission is grounded in providing “quality service and patient-centered care.” Honoring those tenets, during the initial COVID wave, the system “Voice of the Customer End User Device Workgroup” was created with system and site-based interdisciplinary representation. Despite challenging and unprecedented times, conscious attention and effort was undertaken to assess the use and impact of virtual devices. One of the major work streams was to capture and examine patient and family thoughts, feedback, and the overall experience as it relates to virtual visitation.
The system Office of Patient & Customer Experience (OPCE), led by Sven Gierlinger, SVP Chief Experience Officer, reached out to our colleagues at Press Ganey to add a custom question to patient experience surveys. Beginning on December 1, 2020, discharged inpatients were asked to rate the “Degree to which you were able to stay connected with your family/caregiver during your stay.” Potential answers include the Likert scale responses of Always, Usually, Sometimes, and Never, with “Always” representing the Top Box score. The OPCE team believes these quantitative insights are important to track and trend, particularly since in-person and virtual visitation remain in constant flux.
In an effort to obtain additional, focused, qualitative feedback, OPCE partnered with our institution’s Digital Patient Experience (dPX) colleagues. The approach consisted of voluntary, semistructured, interview-type conversations with patients and family members who engaged in virtual visitation multiple times while the patient was hospitalized. OPCE contacted site-based Patient Experience leads, also known as Culture Leaders, at 3 hospitals, asking them to identify potential participants. This convenience sample excluded instances where the patient passed away during and/or immediately following hospitalization.
The OPCE team phoned potential interview candidates to make a personalized connection, explain the purpose of the interviews, and schedule them, if interested. For consistency, the same Digital Customer Experience Researcher on the dPX team facilitated all sessions, which were 30-minute, semiscripted interviews conducted virtually via Microsoft Teams. The tone was intentionally conversational so that patients and family members would feel comfortable delving into themes that were most impactful during their experience. After some initial ice breakers, such as “What were some of your feelings about being a patient/having a loved one in the hospital during the early days of the COVID-19 pandemic?” we moved on to some more pragmatic, implementation questions and rating scales. These included questions such as “How did you first learn about the option for virtual visitation? Was it something you inquired about or did someone offer it to you? How was it explained to you?” Patients were also asked, on a scale of 1 (easy) to 5 (difficult), to rate their experience with the technology aspect when connecting with their loved ones. They also provided verbal consent to be recorded and were given a $15 gift card upon completion of the interview.
Transcriptions were generated by uploading the interview recordings to a platform called UserTesting. In addition to these transcriptions, this platform also allowed for a keyword mapping tool that organized high-level themes and adjectives into groupings along a sentiment axis from negative to neutral to positive. Transcripts were then read carefully and annotated by the Digital Customer Experience Researcher, which allowed for strengthening of some of the automated themes as well as the emergence of new, more nuanced themes. These themes were organized into those that we could address with design and/or procedure updates (actionable insights), those that came up most frequently overall (frequency), and those that came up across our 3 interview sessions (commonality).
This feedback, along with the responses to the new Press Ganey question, was presented to the system Voice of the Customer End User Device Workgroup. The results led to robust discussion and brainstorming regarding how to improve the process to be more patient-centered. Findings were also shared with our hospital-based Culture Leaders. As many of their local strategic plans focused on patient-family communication, this information was helpful to them in considering plans for expansion and/or sustaining virtual visitation efforts. The process map in the Figure outlines key milestones within this feedback loop.

Outcomes
During the height of the initial COVID-19 crisis, virtual visitation was a new and ever-evolving process. Amidst the chaos, mechanisms to capture the quantity and quality of virtual visits were not in place. Based on informal observation, a majority of patients utilized personal devices to connect with loved ones, and staff even offered their own cellular devices to facilitate timely patient-family communication. The technology primarily used included FaceTime, Zoom, and EZCall, as there was much public awareness and comfort with those platforms.
In the first quarter of 2021, our institution overall performed at a Top Box score of 60.2 for our ability to assist patients with staying connected to their family/caregiver during their inpatient visit. With more than 6700 returned surveys during that time period, our hospitals earned Top Box scores ranging between 48.0 and 75.3. At this time, obtaining a national benchmark ranking is not possible, because the question regarding connectedness is unique to Northwell inpatient settings. As other health care organizations adopt this customized question, further peer-to-peer measurements can be established.
Regarding virtual interviews, 25 patients were initially contacted to determine their interest in participating. Of that sample, 17 patients were engaged over the phone, representing a reach rate of 68%. Overall, 10 interviews were scheduled; 7 patients did not show up, resulting in 3 completed interviews. During follow-up, “no-show” participants either gave no response or stated they had a conflict at their originally scheduled time but were not interested in rescheduling due to personal circumstances. Through such conversations, ongoing health complications were found to be a reoccurring barrier to participation.
Each of the participating patients had experienced being placed on a ventilator. They described their hospitalization as a time of “confusion and despair” in the first days after extubation. After we reviewed interview recordings, a reoccurring theme across all interviews was the feeling of gratitude. Patients expressed deep and heartfelt appreciation for being given the opportunity to connect as a family. One patient described virtual visitation sessions as her “only tether to reality when nothing else made sense.”
Interestingly enough, none of the participants knew that virtual visitation was an option and/or thought to inquire about it before a hospital staff member offered to set up a session. Patients recounted how they were weak and physically unable to connect to the sessions without significant assistance. They reported examples of not having the physical strength to hold up the tablet or needing a staff member to facilitate the conversation because the patient could not speak loudly enough and/or they were having difficulty hearing over background medical equipment noises. Participants also described times when a nurse or social worker would stand and hold the tablet for 20 to 30 minutes at a time, further describing mixed feelings of gratitude, guilt for “taking up their time,” and a desire for more privacy to have those precious conversations.
Discussion
Our institution encountered various barriers when establishing, implementing, and sustaining virtual visitation. The acquisition and bulk purchasing of devices, so that each hospital unit and department had adequate par levels during a high-demand time frame, was an initial challenge. Ensuring appropriate safeguards, software programming, and access to WiFi required ingenuity from IT teams. Leaders sought to advocate for the importance of prioritizing virtual visitation alongside clinical interventions. For team members, education was needed to build awareness, learn how to navigate technology, and troubleshoot, in real-time, issues such as poor connectivity. However, despite these organizational struggles, the hospital’s frontline professionals fully recognized and understood the humanistic value of connecting ill patients with their loved ones. Harnessing their teamwork, empathy, and innovative spirits, they forged through such difficulties to create meaningful interactions.
Although virtual visitation occurred prior to the COVID-19 pandemic, particularly in subspecialty areas such as neonatal intensive care units,6 it was not commonplace in most adult inpatient care settings. However, now that virtual means to communication are widely accepted and preferred, our hospital anticipates these offerings will become a broad patient expectation and, therefore, part of standard hospital care and operations. Health care leaders and interdisciplinary teams must therefore prioritize virtual visitation protocols, efforts, and future programming. It is no longer an exception to the rule, but rather a critical approach when ensuring quality communication between patients, families, and care teams.
We strive to continually improve by including user feedback as part of an interactive design process. For a broader, more permanent installation of virtual visitation, health care organizations must proactively promote this capability as a valued option. Considering health literacy and comfort with technology, functionality, and logistics must be carefully explained to patients and their families. This may require additional staff training so that they are knowledgeable, comfortable with, and able to troubleshoot questions/concerns in real time. There needs to be an adequate number of mobile devices available at a unit or departmental level to meet short-term and long-term demands. Additionally, now that we have emerged from our initial crisis-based mentality, it is time to consider alternatives to alleviate the need for staff assistance, such as mounts to hold devices and enabling voice controls.
Conclusion
As an organization grounded in the spirit of innovation, Northwell has been able to quickly pivot, adopting virtual visitation to address emerging and complex communication needs. Taking a best practice established during a crisis period and engraining it into sustainable organizational culture and operations requires visionary leadership, strong teamwork, and an unbridled commitment to patient and family centeredness. Despite unprecedented challenges, our commitment to listening to the “voice” of patients and families never wavered. Using their insights and feedback as critical components to the decision-making process, there is much work ahead within the realm of virtual visitation.
Acknowledgements: The authors would like to acknowledge the Northwell Health providers, frontline health care professionals, and team members who worked tirelessly to care for its community during initial COVID-19 waves and every day thereafter. Heartfelt gratitude to Northwell’s senior leaders for the visionary leadership; the OCIO and hospital-based IT teams for their swift collaboration; and dedicated Culture Leaders, Patient Experience team members, and redeployed staff for their unbridled passion for caring for patients and families. Special thanks to Agnes Barden, DNP, RN, CPXP, Joseph Narvaez, MBA, and Natalie Bashkin, MBA, from the system Office of Patient & Customer Experience, and Carolyne Burgess, MPH, from the Digital Patient Experience teams, for their participation, leadership, and syngeristic partnerships.
Corresponding Author: Nicole Giammarinaro, MSN, RN, CPXP, Director, Patient & Customer Experience, Northwell Health, 2000 Marcus Ave, Lake Success, NY 11042; [email protected].
Financial disclosures: Sven Gierlinger serves on the Speakers Bureau for Northwell Health and as an Executive Board Member for The Beryl Institute.
1. New York State Department of Health. Health advisory: COVID-19 guidance for hospital operators regarding visitation. March 18, 2020. https://coronavirus.health.ny.gov/system/files/documents/2020/03/covid19-hospital-visitation-guidance-3.18.20.pdf
2. Zhang Y. Family functioning in the context of an adult family member with illness: a concept analysis. J Clin Nurs. 2018;27(15-16):3205-3224. doi:10.1111/jocn.14500
3. Institute for Patient- & Family-Centered Care. Better Together: Partnering with Families. https://www.ipfcc.org/bestpractices/better-together-ny.html
4. Marceglia S, Bonacina S, Zaccaria V, et al. How might the iPad change healthcare? J R Soc Med. 2012;105(6):233-241. doi:10.1258/jrsm.2012.110296
5. Short B, Parekh M, Ryan P, et al. Rapid implementation of a mobile prone team during the COVID-19 pandemic. J Crit Care. 2020;60:230-234. doi:10.1016/j.jcrc.2020.08.020
6. Yeo C, Ho SK, Khong K, Lau Y. Virtual visitation in the neonatal intensive care: experience with the use of internet and telemedicine in a tertiary neonatal unit. Perm J. 2011;15(3):32-36.
1. New York State Department of Health. Health advisory: COVID-19 guidance for hospital operators regarding visitation. March 18, 2020. https://coronavirus.health.ny.gov/system/files/documents/2020/03/covid19-hospital-visitation-guidance-3.18.20.pdf
2. Zhang Y. Family functioning in the context of an adult family member with illness: a concept analysis. J Clin Nurs. 2018;27(15-16):3205-3224. doi:10.1111/jocn.14500
3. Institute for Patient- & Family-Centered Care. Better Together: Partnering with Families. https://www.ipfcc.org/bestpractices/better-together-ny.html
4. Marceglia S, Bonacina S, Zaccaria V, et al. How might the iPad change healthcare? J R Soc Med. 2012;105(6):233-241. doi:10.1258/jrsm.2012.110296
5. Short B, Parekh M, Ryan P, et al. Rapid implementation of a mobile prone team during the COVID-19 pandemic. J Crit Care. 2020;60:230-234. doi:10.1016/j.jcrc.2020.08.020
6. Yeo C, Ho SK, Khong K, Lau Y. Virtual visitation in the neonatal intensive care: experience with the use of internet and telemedicine in a tertiary neonatal unit. Perm J. 2011;15(3):32-36.
Feasibility of a Saliva-Based COVID-19 Screening Program in Abu Dhabi Primary Schools
From Health Center, New York University Abu Dhabi, Abu Dhabi, United Arab Emirates (Dr. Virji and Aisha Al Hamiz), Public Health, Abu Dhabi Public Health Center, Abu Dhabi, United Arab Emirates (Drs. Al Hajeri, Al Shehhi, Al Memari, and Ahlam Al Maskari), College of Medicine and Health Sciences, Khalifa University, Abu Dhabi, United Arab Emirates, Department of Medicine, Sheikh Shakhbout Medical City, Abu Dhabi, United Arab Emirates (Dr. Alhajri), Public Health Research Center, New York University Abu Dhabi, Abu Dhabi, United Arab Emirates, Oxford University Hospitals NHS Foundation Trust, Oxford, England, and the MRC Epidemiology Unit, University of Cambridge, Cambridge, England (Dr. Ali).
Objective: The pandemic has forced closures of primary schools, resulting in loss of learning time on a global scale. In addition to face coverings, social distancing, and hand hygiene, an efficient testing method is important to mitigate the spread of COVID-19 in schools. We evaluated the feasibility of a saliva-based SARS-CoV-2 polymerase chain reaction testing program among 18 primary schools in the Emirate of Abu Dhabi, United Arab Emirates. Qualitative results show that children 4 to 5 years old had difficulty producing an adequate saliva specimen compared to those 6 to 12 years old.
Methods: A short training video on saliva collection beforehand helps demystify the process for students and parents alike. Informed consent was challenging yet should be done beforehand by school health nurses or other medical professionals to reassure parents and maximize participation.
Results: Telephone interviews with school administrators resulted in an 83% response rate. Overall, 93% of school administrators had a positive experience with saliva testing and felt the program improved the safety of their schools. The ongoing use of saliva testing for SARS-CoV-2 was supported by 73% of respondents.
Conclusion: On-campus saliva testing is a feasible option for primary schools to screen for COVID-19 in their student population to help keep their campuses safe and open for learning.
Keywords: COVID-19; saliva testing; mitigation; primary school.
The COVID-19 pandemic is a leading cause of morbidity and mortality worldwide and continues to exhaust health care resources on a large scale.1 Efficient testing is critical to identify cases early and to help mitigate the deleterious effects of the pandemic.2 Saliva polymerase chain reaction (PCR) nucleic acid amplification testing (NAAT) is more comfortable than nasopharyngeal (NP) NAAT and has been validated as a test for SARS-CoV-2.1 Although children are less susceptible to severe disease, primary schools are considered a vector for transmission and community spread.3 Efficient and scalable methods of routine testing are needed globally to help keep schools open. Saliva testing has proven a useful resource for this population.4,5
Abu Dhabi is the largest Emirate in the United Arab Emirates (UAE), with an estimated population of 2.5 million.6 The first case of COVID-19 was discovered in the UAE on January 29, 2020.7 The UAE has been recognized worldwide for its robust pandemic response. Along with the coordinated and swift application of public health measures, the country has one of the highest COVID-19 testing rates per capita and one of the highest vaccination rates worldwide.8,9 The Abu Dhabi Public Health Center (ADPHC) works alongside the Ministry of Education (MOE) to establish testing, quarantine, and general safety guidelines for primary schools. In December 2020, the ADPHC partnered with a local, accredited diagnostic laboratory to test the feasibility of a saliva-based screening program for COVID-19 directly on school campuses for 18 primary schools in the Emirate.
Saliva-based PCR testing for COVID-19 was approved for use in schools in the UAE on January 24, 2021.10 As part of a greater mitigation strategy to reduce both school-based transmission and, hence, community spread, the ADPHC focused its on-site testing program on children aged 4 to 12 years. The program required collaboration among medical professionals, school administrators and teachers, students, and parents. Our study evaluates the feasibility of implementing a saliva-based COVID-19 screening program directly on primary school campuses involving children as young as 4 years of age.
Methods
The ADPHC, in collaboration with G42 Biogenix Labs, conducted a saliva SARS-CoV-2 NAAT testing program in 18 primary schools in the Emirate. Schools were selected based on outbreak prevalence at the time and focused on “hot spot” areas. The school on-site saliva testing program included children aged 4 to 12 years old in a “bubble” attendance model during the school day. This model involved children being assigned to groups or “pods.” This allowed us to limit a potential outbreak to a single pod, as opposed to risk exposing the entire school, should a single student test positive. The well-established SalivaDirect protocol developed at Yale University was used for testing and included an RNA extraction-free, RT-qPCR method for SARS-CoV-2 detection.11
We conducted a qualitative study involving telephone interviews of school administrators to evaluate their experience with the ADPHC testing program at their schools. In addition, we interviewed the G42 Biogenix Lab providers to understand the logistics that supported on-campus collection of saliva specimens for this age group. We also gathered the attitudes of school children before and after testing. This study was reviewed and approved by the Abu Dhabi Health Research and Technology Committee and the Institutional Review Board (IRB), New York University Abu Dhabi (NYUAD).
Sample and recruitment
The original sample collection of saliva specimens was performed by the ADPHC in collaboration with G42 Biogenix Lab providers on school campuses between December 6 and December 10, 2020. During this time, schools operated in a hybrid teaching model, where learning took place both online and in person. Infection control measures were deployed based on ADPHC standards and guidelines. Nurses utilized appropriate patient protective equipment, frequent hand hygiene, and social distancing during the collection process. Inclusion criteria included asymptomatic students aged 4 to 12 years attending in-person classes on campus. Students with respiratory symptoms who were asked to stay home or those not attending in-person classes were excluded.
Data collection
Data with regard to school children’s attitudes before and after testing were compiled through an online survey sent randomly to participants postintervention. Data from school administrators were collected through video and telephone interviews between April 14 and April 29, 2021. We first interviewed G42 Biogenix Lab providers to obtain previously acquired qualitative and quantitative data, which were collected during the intervention itself. After obtaining this information, we designed a questionnaire and proceeded with a structured interview process for school officials.
We interviewed school principals and administrators to collect their overall experiences with the saliva testing program. Before starting each interview, we established the interviewees preferred language, either English or Arabic. We then introduced the meeting attendees and provided study details, aims, and objectives, and described collaborating entities. We obtained verbal informed consent from a script approved by the NYUAD IRB and then proceeded with the interview, which included 4 questions. The first 3 questions were answered on a 5-point Likert scale model that consisted of 5 answer options: 5 being completely agree, 4 agree, 3 somewhat agree, 2 somewhat disagree, and 1 completely disagree. The fourth question invited open-ended feedback and comments on the following statements:
- I believe the COVID-19 saliva testing program improved the safety for my school campus.
- Our community had an overall positive experience with the COVID saliva testing.
- We would like to continue a saliva-based COVID testing program on our school campus.
- Please provide any additional comments you feel important about the program.
During the interview, we transcribed the answers as the interviewee was answering. We then translated those in Arabic into English and collected the data in 1 Excel spreadsheet. School interviewees and school names were de-identified in the collection and storage process.
Results
A total of 2011 saliva samples were collected from 18 different primary school campuses. Samples were sent the same day to G42 Biogenix Labs in Abu Dhabi for COVID PCR testing. A team consisting of 5 doctors providing general oversight, along with 2 to 6 nurses per site, were able to manage the collection process for all 18 school campuses. Samples were collected between 8
Sample stations were set up in either the school auditorium or gymnasium to ensure appropriate crowd control and ventilation. Teachers and other school staff, including public safety, were able to manage lines and the shuttling of students back and forth from classes to testing stations, which allowed medical staff to focus on sample collection.
Informed consent was obtained by prior electronic communication to parents from school staff, asking them to agree to allow their child to participate in the testing program. Informed consent was identified as a challenge: Getting parents to understand that saliva testing was more comfortable than NP testing, and that the results were only being used to help keep the school safe, took time. School staff are used to obtaining consent from parents for field trips, but this was clearly more challenging for them.
The saliva collection process per child took more time than expected. Children fasted for 45 minutes before saliva collection. We used an active drool technique, which required children to pool saliva in their mouth then express it into a collection tube. Adults can generally do this on command, but we found it took 10 to 12 minutes per child. Saliva production was cued by asking the children to think about food, and by showing them pictures and TV commercials depicting food. Children 4 to 5 years old had more difficulty with the process despite active cueing, while those 6 to 12 years old had an easier time with the process. We collected data on a cohort of 80 children regarding their attitudes pre (Figure 1) and post collection (Figure 2). Children felt happier, less nervous, and less scared after collection than before collection. This trend reassured us that future collections would be easier for students.
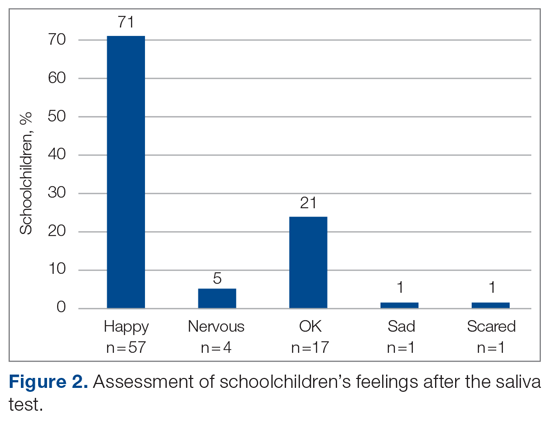
A total of 15 of 18 school principals completed the telephone interview, yielding a response rate of 83%. Overall, 93% of the school principals agreed or completely agreed that the COVID-19 saliva testing program improved school safety; 93% agreed or completely agreed that they had an overall positive experience with the program; and 73% supported the ongoing use of saliva testing in their schools (Table 1). Administrators’ open-ended comments on their experience were positive overall (Table 2).
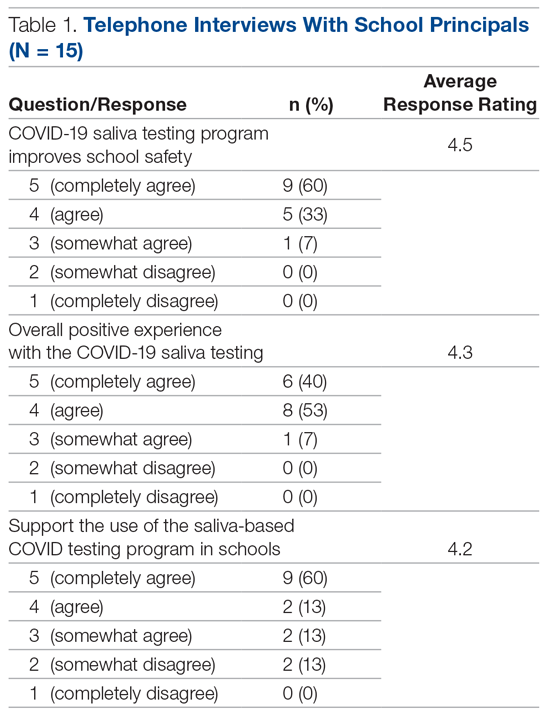
Discussion
By March 2020, many kindergarten to grade 12 public and private schools suspended in-person classes due to the pandemic and turned to online learning platforms. The negative impact of school closures on academic achievement is projected to be significant.7,12,13 Ensuring schools can stay open and run operations safely will require routine SARS-CoV-2 testing. Our study investigated the feasibility of routine saliva testing on children aged 4 to 12 years on their school campuses. The ADPHC school on-site saliva testing program involved bringing lab providers onto 18 primary school campuses and required cooperation among parents, students, school administrators, and health care professionals.

Children younger than 6 years had difficulty producing an adequate saliva specimen, whereas those 6 to 12 years did so with relative ease when cued by thoughts or pictures of food while waiting in line for collection. Schools considering on-site testing programs should consider the age range of 6 to 12 years as a viable age range for saliva screening. Children should fast for a minimum of 45 minutes prior to saliva collection and should be cued by thoughts of food, food pictures, or food commercials. Setting up a sampling station close to the cafeteria where students can smell meal preparation may also help.14,15 Sampling before breakfast or lunch, when children are potentially at their hungriest, should also be considered.
The greatest challenge was obtaining informed consent from parents who were not yet familiar with the reliability of saliva testing as a tool for SARS-CoV-2 screening or with the saliva collection process as a whole. Informed consent was initially done electronically, lacking direct human interaction to answer parents’ questions. Parents who refused had a follow-up call from the school nurse to further explain the logistics and rationale for saliva screening. Having medical professionals directly answer parents’ questions was helpful. Parents were reassured that the process was painless, confidential, and only to be used for school safety purposes. Despite school administrators being experienced in obtaining consent from parents for field trips, obtaining informed consent for a medical testing procedure is more complicated, and parents aren’t accustomed to providing such consent in a school environment. Schools considering on-site testing should ensure that their school nurse or other health care providers are on the front line obtaining informed consent and allaying parents’ fears.
School staff were able to effectively provide crowd control for testing, and children felt at ease being in a familiar environment. Teachers and public safety officers are well-equipped at managing the shuttling of students to class, to lunch, to physical education, and, finally, to dismissal. They were equally equipped at handling the logistics of students to and from testing, including minimizing crowds and helping students feel at ease during the process. This effective collaboration allowed the lab personnel to focus on sample collection and storage, while school staff managed all other aspects of the children’s safety and care.
Conclusion
Overall, school administrators had a positive experience with the testing program, felt the program improved the safety of their schools, and supported the ongoing use of saliva testing for SARS-CoV-2 on their school campuses. Children aged 6 years and older were able to provide adequate saliva samples, and children felt happier and less nervous after the process, indicating repeatability. Our findings highlight the feasibility of an integrated on-site saliva testing model for primary school campuses. Further research is needed to determine the scalability of such a model and whether the added compliance and safety of on-site testing compensates for the potential loss of learning time that testing during school hours would require.
Corresponding author: Ayaz Virji, MD, New York University Abu Dhabi, PO Box 129188, Abu Dhabi, United Arab Emirates; [email protected].
Financial disclosures: None.
1. Kuehn BM. Despite improvements, COVID-19’s health care disruptions persist. JAMA. 2021;325(23):2335. doi:10.1001/jama.2021.9134
2. National Institute on Aging. Why COVID-19 testing is the key to getting back to normal. September 4, 2020. Accessed September 8, 2021. https://www.nia.nih.gov/news/why-covid-19-testing-key-getting-back-normal
3. Centers for Disease Control and Prevention. Science brief: Transmission of SARS-CoV-2 in K-12 schools. Updated July 9, 2021. Accessed September 8, 2021. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/transmission_k_12_schools.html
4. Butler-Laporte G, Lawandi A, Schiller I, et al. Comparison of saliva and nasopharyngeal swab nucleic acid amplification testing for detection of SARS-CoV-2: a systematic review and meta-analysis. JAMA Intern Med. 2021;181(3):353-360. doi:10.1001/jamainternmed.2020.8876
5. Al Suwaidi H, Senok A, Varghese R, et al. Saliva for molecular detection of SARS-CoV-2 in school-age children. Clin Microbiol Infect. 2021;27(9):1330-1335. doi:10.1016/j.cmi.2021.02.009
6. Abu Dhabi. Accessed September 8, 2021. https://u.ae/en/about-the-uae/the-seven-emirates/abu-dhabi
7. Alsuwaidi AR, Al Hosani FI, Al Memari S, et al. Seroprevalence of COVID-19 infection in the Emirate of Abu Dhabi, United Arab Emirates: a population-based cross-sectional study. Int J Epidemiol. 2021;50(4):1077-1090. doi:10.1093/ije/dyab077
8. Al Hosany F, Ganesan S, Al Memari S, et al. Response to COVID-19 pandemic in the UAE: a public health perspective. J Glob Health. 2021;11:03050. doi:10.7189/jogh.11.03050
9. Bremmer I. The best global responses to the COVID-19 pandemic, 1 year later. Time Magazine. Updated February 23, 2021. Accessed September 8, 2021. https://time.com/5851633/best-global-responses-covid-19/
10. Department of Health, Abu Dhabi. Laboratory diagnostic test for COVID-19: update regarding saliva-based testing using RT-PCR test. 2021.
11. Vogels C, Brackney DE, Kalinich CC, et al. SalivaDirect: RNA extraction-free SARS-CoV-2 diagnostics. Protocols.io. Accessed September 8, 2021. https://www.protocols.io/view/salivadirect-rna-extraction-free-sars-cov-2-diagno-bh6jj9cn?version_warning=no
12. Education Endowment Foundation. Impact of school closures on the attainment gap: rapid evidence assessment. June 2020. Accessed September 8, 2021. https://www.researchgate.net/publication/342501263_EEF_2020_-_Impact_of_School_Closures_on_the_Attainment_Gap
13. United Nations. Policy brief: Education during COVID-19 and beyond. Accessed September 8, 2021. https://www.un.org/development/desa/dspd/wp-content/uploads/sites/22/2020/08/sg_policy_brief_covid-19_and_education_august_2020.pdf
14. Schiffman SS, Miletic ID. Effect of taste and smell on secretion rate of salivary IgA in elderly and young persons. J Nutr Health Aging. 1999;3(3):158-164.
15. Lee VM, Linden RW. The effect of odours on stimulated parotid salivary flow in humans. Physiol Behav. 1992;52(6):1121-1125. doi:10.1016/0031-9384(92)90470-m
From Health Center, New York University Abu Dhabi, Abu Dhabi, United Arab Emirates (Dr. Virji and Aisha Al Hamiz), Public Health, Abu Dhabi Public Health Center, Abu Dhabi, United Arab Emirates (Drs. Al Hajeri, Al Shehhi, Al Memari, and Ahlam Al Maskari), College of Medicine and Health Sciences, Khalifa University, Abu Dhabi, United Arab Emirates, Department of Medicine, Sheikh Shakhbout Medical City, Abu Dhabi, United Arab Emirates (Dr. Alhajri), Public Health Research Center, New York University Abu Dhabi, Abu Dhabi, United Arab Emirates, Oxford University Hospitals NHS Foundation Trust, Oxford, England, and the MRC Epidemiology Unit, University of Cambridge, Cambridge, England (Dr. Ali).
Objective: The pandemic has forced closures of primary schools, resulting in loss of learning time on a global scale. In addition to face coverings, social distancing, and hand hygiene, an efficient testing method is important to mitigate the spread of COVID-19 in schools. We evaluated the feasibility of a saliva-based SARS-CoV-2 polymerase chain reaction testing program among 18 primary schools in the Emirate of Abu Dhabi, United Arab Emirates. Qualitative results show that children 4 to 5 years old had difficulty producing an adequate saliva specimen compared to those 6 to 12 years old.
Methods: A short training video on saliva collection beforehand helps demystify the process for students and parents alike. Informed consent was challenging yet should be done beforehand by school health nurses or other medical professionals to reassure parents and maximize participation.
Results: Telephone interviews with school administrators resulted in an 83% response rate. Overall, 93% of school administrators had a positive experience with saliva testing and felt the program improved the safety of their schools. The ongoing use of saliva testing for SARS-CoV-2 was supported by 73% of respondents.
Conclusion: On-campus saliva testing is a feasible option for primary schools to screen for COVID-19 in their student population to help keep their campuses safe and open for learning.
Keywords: COVID-19; saliva testing; mitigation; primary school.
The COVID-19 pandemic is a leading cause of morbidity and mortality worldwide and continues to exhaust health care resources on a large scale.1 Efficient testing is critical to identify cases early and to help mitigate the deleterious effects of the pandemic.2 Saliva polymerase chain reaction (PCR) nucleic acid amplification testing (NAAT) is more comfortable than nasopharyngeal (NP) NAAT and has been validated as a test for SARS-CoV-2.1 Although children are less susceptible to severe disease, primary schools are considered a vector for transmission and community spread.3 Efficient and scalable methods of routine testing are needed globally to help keep schools open. Saliva testing has proven a useful resource for this population.4,5
Abu Dhabi is the largest Emirate in the United Arab Emirates (UAE), with an estimated population of 2.5 million.6 The first case of COVID-19 was discovered in the UAE on January 29, 2020.7 The UAE has been recognized worldwide for its robust pandemic response. Along with the coordinated and swift application of public health measures, the country has one of the highest COVID-19 testing rates per capita and one of the highest vaccination rates worldwide.8,9 The Abu Dhabi Public Health Center (ADPHC) works alongside the Ministry of Education (MOE) to establish testing, quarantine, and general safety guidelines for primary schools. In December 2020, the ADPHC partnered with a local, accredited diagnostic laboratory to test the feasibility of a saliva-based screening program for COVID-19 directly on school campuses for 18 primary schools in the Emirate.
Saliva-based PCR testing for COVID-19 was approved for use in schools in the UAE on January 24, 2021.10 As part of a greater mitigation strategy to reduce both school-based transmission and, hence, community spread, the ADPHC focused its on-site testing program on children aged 4 to 12 years. The program required collaboration among medical professionals, school administrators and teachers, students, and parents. Our study evaluates the feasibility of implementing a saliva-based COVID-19 screening program directly on primary school campuses involving children as young as 4 years of age.
Methods
The ADPHC, in collaboration with G42 Biogenix Labs, conducted a saliva SARS-CoV-2 NAAT testing program in 18 primary schools in the Emirate. Schools were selected based on outbreak prevalence at the time and focused on “hot spot” areas. The school on-site saliva testing program included children aged 4 to 12 years old in a “bubble” attendance model during the school day. This model involved children being assigned to groups or “pods.” This allowed us to limit a potential outbreak to a single pod, as opposed to risk exposing the entire school, should a single student test positive. The well-established SalivaDirect protocol developed at Yale University was used for testing and included an RNA extraction-free, RT-qPCR method for SARS-CoV-2 detection.11
We conducted a qualitative study involving telephone interviews of school administrators to evaluate their experience with the ADPHC testing program at their schools. In addition, we interviewed the G42 Biogenix Lab providers to understand the logistics that supported on-campus collection of saliva specimens for this age group. We also gathered the attitudes of school children before and after testing. This study was reviewed and approved by the Abu Dhabi Health Research and Technology Committee and the Institutional Review Board (IRB), New York University Abu Dhabi (NYUAD).
Sample and recruitment
The original sample collection of saliva specimens was performed by the ADPHC in collaboration with G42 Biogenix Lab providers on school campuses between December 6 and December 10, 2020. During this time, schools operated in a hybrid teaching model, where learning took place both online and in person. Infection control measures were deployed based on ADPHC standards and guidelines. Nurses utilized appropriate patient protective equipment, frequent hand hygiene, and social distancing during the collection process. Inclusion criteria included asymptomatic students aged 4 to 12 years attending in-person classes on campus. Students with respiratory symptoms who were asked to stay home or those not attending in-person classes were excluded.
Data collection
Data with regard to school children’s attitudes before and after testing were compiled through an online survey sent randomly to participants postintervention. Data from school administrators were collected through video and telephone interviews between April 14 and April 29, 2021. We first interviewed G42 Biogenix Lab providers to obtain previously acquired qualitative and quantitative data, which were collected during the intervention itself. After obtaining this information, we designed a questionnaire and proceeded with a structured interview process for school officials.
We interviewed school principals and administrators to collect their overall experiences with the saliva testing program. Before starting each interview, we established the interviewees preferred language, either English or Arabic. We then introduced the meeting attendees and provided study details, aims, and objectives, and described collaborating entities. We obtained verbal informed consent from a script approved by the NYUAD IRB and then proceeded with the interview, which included 4 questions. The first 3 questions were answered on a 5-point Likert scale model that consisted of 5 answer options: 5 being completely agree, 4 agree, 3 somewhat agree, 2 somewhat disagree, and 1 completely disagree. The fourth question invited open-ended feedback and comments on the following statements:
- I believe the COVID-19 saliva testing program improved the safety for my school campus.
- Our community had an overall positive experience with the COVID saliva testing.
- We would like to continue a saliva-based COVID testing program on our school campus.
- Please provide any additional comments you feel important about the program.
During the interview, we transcribed the answers as the interviewee was answering. We then translated those in Arabic into English and collected the data in 1 Excel spreadsheet. School interviewees and school names were de-identified in the collection and storage process.
Results
A total of 2011 saliva samples were collected from 18 different primary school campuses. Samples were sent the same day to G42 Biogenix Labs in Abu Dhabi for COVID PCR testing. A team consisting of 5 doctors providing general oversight, along with 2 to 6 nurses per site, were able to manage the collection process for all 18 school campuses. Samples were collected between 8
Sample stations were set up in either the school auditorium or gymnasium to ensure appropriate crowd control and ventilation. Teachers and other school staff, including public safety, were able to manage lines and the shuttling of students back and forth from classes to testing stations, which allowed medical staff to focus on sample collection.
Informed consent was obtained by prior electronic communication to parents from school staff, asking them to agree to allow their child to participate in the testing program. Informed consent was identified as a challenge: Getting parents to understand that saliva testing was more comfortable than NP testing, and that the results were only being used to help keep the school safe, took time. School staff are used to obtaining consent from parents for field trips, but this was clearly more challenging for them.

The saliva collection process per child took more time than expected. Children fasted for 45 minutes before saliva collection. We used an active drool technique, which required children to pool saliva in their mouth then express it into a collection tube. Adults can generally do this on command, but we found it took 10 to 12 minutes per child. Saliva production was cued by asking the children to think about food, and by showing them pictures and TV commercials depicting food. Children 4 to 5 years old had more difficulty with the process despite active cueing, while those 6 to 12 years old had an easier time with the process. We collected data on a cohort of 80 children regarding their attitudes pre (Figure 1) and post collection (Figure 2). Children felt happier, less nervous, and less scared after collection than before collection. This trend reassured us that future collections would be easier for students.

A total of 15 of 18 school principals completed the telephone interview, yielding a response rate of 83%. Overall, 93% of the school principals agreed or completely agreed that the COVID-19 saliva testing program improved school safety; 93% agreed or completely agreed that they had an overall positive experience with the program; and 73% supported the ongoing use of saliva testing in their schools (Table 1). Administrators’ open-ended comments on their experience were positive overall (Table 2).

Discussion
By March 2020, many kindergarten to grade 12 public and private schools suspended in-person classes due to the pandemic and turned to online learning platforms. The negative impact of school closures on academic achievement is projected to be significant.7,12,13 Ensuring schools can stay open and run operations safely will require routine SARS-CoV-2 testing. Our study investigated the feasibility of routine saliva testing on children aged 4 to 12 years on their school campuses. The ADPHC school on-site saliva testing program involved bringing lab providers onto 18 primary school campuses and required cooperation among parents, students, school administrators, and health care professionals.

Children younger than 6 years had difficulty producing an adequate saliva specimen, whereas those 6 to 12 years did so with relative ease when cued by thoughts or pictures of food while waiting in line for collection. Schools considering on-site testing programs should consider the age range of 6 to 12 years as a viable age range for saliva screening. Children should fast for a minimum of 45 minutes prior to saliva collection and should be cued by thoughts of food, food pictures, or food commercials. Setting up a sampling station close to the cafeteria where students can smell meal preparation may also help.14,15 Sampling before breakfast or lunch, when children are potentially at their hungriest, should also be considered.
The greatest challenge was obtaining informed consent from parents who were not yet familiar with the reliability of saliva testing as a tool for SARS-CoV-2 screening or with the saliva collection process as a whole. Informed consent was initially done electronically, lacking direct human interaction to answer parents’ questions. Parents who refused had a follow-up call from the school nurse to further explain the logistics and rationale for saliva screening. Having medical professionals directly answer parents’ questions was helpful. Parents were reassured that the process was painless, confidential, and only to be used for school safety purposes. Despite school administrators being experienced in obtaining consent from parents for field trips, obtaining informed consent for a medical testing procedure is more complicated, and parents aren’t accustomed to providing such consent in a school environment. Schools considering on-site testing should ensure that their school nurse or other health care providers are on the front line obtaining informed consent and allaying parents’ fears.
School staff were able to effectively provide crowd control for testing, and children felt at ease being in a familiar environment. Teachers and public safety officers are well-equipped at managing the shuttling of students to class, to lunch, to physical education, and, finally, to dismissal. They were equally equipped at handling the logistics of students to and from testing, including minimizing crowds and helping students feel at ease during the process. This effective collaboration allowed the lab personnel to focus on sample collection and storage, while school staff managed all other aspects of the children’s safety and care.
Conclusion
Overall, school administrators had a positive experience with the testing program, felt the program improved the safety of their schools, and supported the ongoing use of saliva testing for SARS-CoV-2 on their school campuses. Children aged 6 years and older were able to provide adequate saliva samples, and children felt happier and less nervous after the process, indicating repeatability. Our findings highlight the feasibility of an integrated on-site saliva testing model for primary school campuses. Further research is needed to determine the scalability of such a model and whether the added compliance and safety of on-site testing compensates for the potential loss of learning time that testing during school hours would require.
Corresponding author: Ayaz Virji, MD, New York University Abu Dhabi, PO Box 129188, Abu Dhabi, United Arab Emirates; [email protected].
Financial disclosures: None.
From Health Center, New York University Abu Dhabi, Abu Dhabi, United Arab Emirates (Dr. Virji and Aisha Al Hamiz), Public Health, Abu Dhabi Public Health Center, Abu Dhabi, United Arab Emirates (Drs. Al Hajeri, Al Shehhi, Al Memari, and Ahlam Al Maskari), College of Medicine and Health Sciences, Khalifa University, Abu Dhabi, United Arab Emirates, Department of Medicine, Sheikh Shakhbout Medical City, Abu Dhabi, United Arab Emirates (Dr. Alhajri), Public Health Research Center, New York University Abu Dhabi, Abu Dhabi, United Arab Emirates, Oxford University Hospitals NHS Foundation Trust, Oxford, England, and the MRC Epidemiology Unit, University of Cambridge, Cambridge, England (Dr. Ali).
Objective: The pandemic has forced closures of primary schools, resulting in loss of learning time on a global scale. In addition to face coverings, social distancing, and hand hygiene, an efficient testing method is important to mitigate the spread of COVID-19 in schools. We evaluated the feasibility of a saliva-based SARS-CoV-2 polymerase chain reaction testing program among 18 primary schools in the Emirate of Abu Dhabi, United Arab Emirates. Qualitative results show that children 4 to 5 years old had difficulty producing an adequate saliva specimen compared to those 6 to 12 years old.
Methods: A short training video on saliva collection beforehand helps demystify the process for students and parents alike. Informed consent was challenging yet should be done beforehand by school health nurses or other medical professionals to reassure parents and maximize participation.
Results: Telephone interviews with school administrators resulted in an 83% response rate. Overall, 93% of school administrators had a positive experience with saliva testing and felt the program improved the safety of their schools. The ongoing use of saliva testing for SARS-CoV-2 was supported by 73% of respondents.
Conclusion: On-campus saliva testing is a feasible option for primary schools to screen for COVID-19 in their student population to help keep their campuses safe and open for learning.
Keywords: COVID-19; saliva testing; mitigation; primary school.
The COVID-19 pandemic is a leading cause of morbidity and mortality worldwide and continues to exhaust health care resources on a large scale.1 Efficient testing is critical to identify cases early and to help mitigate the deleterious effects of the pandemic.2 Saliva polymerase chain reaction (PCR) nucleic acid amplification testing (NAAT) is more comfortable than nasopharyngeal (NP) NAAT and has been validated as a test for SARS-CoV-2.1 Although children are less susceptible to severe disease, primary schools are considered a vector for transmission and community spread.3 Efficient and scalable methods of routine testing are needed globally to help keep schools open. Saliva testing has proven a useful resource for this population.4,5
Abu Dhabi is the largest Emirate in the United Arab Emirates (UAE), with an estimated population of 2.5 million.6 The first case of COVID-19 was discovered in the UAE on January 29, 2020.7 The UAE has been recognized worldwide for its robust pandemic response. Along with the coordinated and swift application of public health measures, the country has one of the highest COVID-19 testing rates per capita and one of the highest vaccination rates worldwide.8,9 The Abu Dhabi Public Health Center (ADPHC) works alongside the Ministry of Education (MOE) to establish testing, quarantine, and general safety guidelines for primary schools. In December 2020, the ADPHC partnered with a local, accredited diagnostic laboratory to test the feasibility of a saliva-based screening program for COVID-19 directly on school campuses for 18 primary schools in the Emirate.
Saliva-based PCR testing for COVID-19 was approved for use in schools in the UAE on January 24, 2021.10 As part of a greater mitigation strategy to reduce both school-based transmission and, hence, community spread, the ADPHC focused its on-site testing program on children aged 4 to 12 years. The program required collaboration among medical professionals, school administrators and teachers, students, and parents. Our study evaluates the feasibility of implementing a saliva-based COVID-19 screening program directly on primary school campuses involving children as young as 4 years of age.
Methods
The ADPHC, in collaboration with G42 Biogenix Labs, conducted a saliva SARS-CoV-2 NAAT testing program in 18 primary schools in the Emirate. Schools were selected based on outbreak prevalence at the time and focused on “hot spot” areas. The school on-site saliva testing program included children aged 4 to 12 years old in a “bubble” attendance model during the school day. This model involved children being assigned to groups or “pods.” This allowed us to limit a potential outbreak to a single pod, as opposed to risk exposing the entire school, should a single student test positive. The well-established SalivaDirect protocol developed at Yale University was used for testing and included an RNA extraction-free, RT-qPCR method for SARS-CoV-2 detection.11
We conducted a qualitative study involving telephone interviews of school administrators to evaluate their experience with the ADPHC testing program at their schools. In addition, we interviewed the G42 Biogenix Lab providers to understand the logistics that supported on-campus collection of saliva specimens for this age group. We also gathered the attitudes of school children before and after testing. This study was reviewed and approved by the Abu Dhabi Health Research and Technology Committee and the Institutional Review Board (IRB), New York University Abu Dhabi (NYUAD).
Sample and recruitment
The original sample collection of saliva specimens was performed by the ADPHC in collaboration with G42 Biogenix Lab providers on school campuses between December 6 and December 10, 2020. During this time, schools operated in a hybrid teaching model, where learning took place both online and in person. Infection control measures were deployed based on ADPHC standards and guidelines. Nurses utilized appropriate patient protective equipment, frequent hand hygiene, and social distancing during the collection process. Inclusion criteria included asymptomatic students aged 4 to 12 years attending in-person classes on campus. Students with respiratory symptoms who were asked to stay home or those not attending in-person classes were excluded.
Data collection
Data with regard to school children’s attitudes before and after testing were compiled through an online survey sent randomly to participants postintervention. Data from school administrators were collected through video and telephone interviews between April 14 and April 29, 2021. We first interviewed G42 Biogenix Lab providers to obtain previously acquired qualitative and quantitative data, which were collected during the intervention itself. After obtaining this information, we designed a questionnaire and proceeded with a structured interview process for school officials.
We interviewed school principals and administrators to collect their overall experiences with the saliva testing program. Before starting each interview, we established the interviewees preferred language, either English or Arabic. We then introduced the meeting attendees and provided study details, aims, and objectives, and described collaborating entities. We obtained verbal informed consent from a script approved by the NYUAD IRB and then proceeded with the interview, which included 4 questions. The first 3 questions were answered on a 5-point Likert scale model that consisted of 5 answer options: 5 being completely agree, 4 agree, 3 somewhat agree, 2 somewhat disagree, and 1 completely disagree. The fourth question invited open-ended feedback and comments on the following statements:
- I believe the COVID-19 saliva testing program improved the safety for my school campus.
- Our community had an overall positive experience with the COVID saliva testing.
- We would like to continue a saliva-based COVID testing program on our school campus.
- Please provide any additional comments you feel important about the program.
During the interview, we transcribed the answers as the interviewee was answering. We then translated those in Arabic into English and collected the data in 1 Excel spreadsheet. School interviewees and school names were de-identified in the collection and storage process.
Results
A total of 2011 saliva samples were collected from 18 different primary school campuses. Samples were sent the same day to G42 Biogenix Labs in Abu Dhabi for COVID PCR testing. A team consisting of 5 doctors providing general oversight, along with 2 to 6 nurses per site, were able to manage the collection process for all 18 school campuses. Samples were collected between 8
Sample stations were set up in either the school auditorium or gymnasium to ensure appropriate crowd control and ventilation. Teachers and other school staff, including public safety, were able to manage lines and the shuttling of students back and forth from classes to testing stations, which allowed medical staff to focus on sample collection.
Informed consent was obtained by prior electronic communication to parents from school staff, asking them to agree to allow their child to participate in the testing program. Informed consent was identified as a challenge: Getting parents to understand that saliva testing was more comfortable than NP testing, and that the results were only being used to help keep the school safe, took time. School staff are used to obtaining consent from parents for field trips, but this was clearly more challenging for them.

The saliva collection process per child took more time than expected. Children fasted for 45 minutes before saliva collection. We used an active drool technique, which required children to pool saliva in their mouth then express it into a collection tube. Adults can generally do this on command, but we found it took 10 to 12 minutes per child. Saliva production was cued by asking the children to think about food, and by showing them pictures and TV commercials depicting food. Children 4 to 5 years old had more difficulty with the process despite active cueing, while those 6 to 12 years old had an easier time with the process. We collected data on a cohort of 80 children regarding their attitudes pre (Figure 1) and post collection (Figure 2). Children felt happier, less nervous, and less scared after collection than before collection. This trend reassured us that future collections would be easier for students.

A total of 15 of 18 school principals completed the telephone interview, yielding a response rate of 83%. Overall, 93% of the school principals agreed or completely agreed that the COVID-19 saliva testing program improved school safety; 93% agreed or completely agreed that they had an overall positive experience with the program; and 73% supported the ongoing use of saliva testing in their schools (Table 1). Administrators’ open-ended comments on their experience were positive overall (Table 2).

Discussion
By March 2020, many kindergarten to grade 12 public and private schools suspended in-person classes due to the pandemic and turned to online learning platforms. The negative impact of school closures on academic achievement is projected to be significant.7,12,13 Ensuring schools can stay open and run operations safely will require routine SARS-CoV-2 testing. Our study investigated the feasibility of routine saliva testing on children aged 4 to 12 years on their school campuses. The ADPHC school on-site saliva testing program involved bringing lab providers onto 18 primary school campuses and required cooperation among parents, students, school administrators, and health care professionals.

Children younger than 6 years had difficulty producing an adequate saliva specimen, whereas those 6 to 12 years did so with relative ease when cued by thoughts or pictures of food while waiting in line for collection. Schools considering on-site testing programs should consider the age range of 6 to 12 years as a viable age range for saliva screening. Children should fast for a minimum of 45 minutes prior to saliva collection and should be cued by thoughts of food, food pictures, or food commercials. Setting up a sampling station close to the cafeteria where students can smell meal preparation may also help.14,15 Sampling before breakfast or lunch, when children are potentially at their hungriest, should also be considered.
The greatest challenge was obtaining informed consent from parents who were not yet familiar with the reliability of saliva testing as a tool for SARS-CoV-2 screening or with the saliva collection process as a whole. Informed consent was initially done electronically, lacking direct human interaction to answer parents’ questions. Parents who refused had a follow-up call from the school nurse to further explain the logistics and rationale for saliva screening. Having medical professionals directly answer parents’ questions was helpful. Parents were reassured that the process was painless, confidential, and only to be used for school safety purposes. Despite school administrators being experienced in obtaining consent from parents for field trips, obtaining informed consent for a medical testing procedure is more complicated, and parents aren’t accustomed to providing such consent in a school environment. Schools considering on-site testing should ensure that their school nurse or other health care providers are on the front line obtaining informed consent and allaying parents’ fears.
School staff were able to effectively provide crowd control for testing, and children felt at ease being in a familiar environment. Teachers and public safety officers are well-equipped at managing the shuttling of students to class, to lunch, to physical education, and, finally, to dismissal. They were equally equipped at handling the logistics of students to and from testing, including minimizing crowds and helping students feel at ease during the process. This effective collaboration allowed the lab personnel to focus on sample collection and storage, while school staff managed all other aspects of the children’s safety and care.
Conclusion
Overall, school administrators had a positive experience with the testing program, felt the program improved the safety of their schools, and supported the ongoing use of saliva testing for SARS-CoV-2 on their school campuses. Children aged 6 years and older were able to provide adequate saliva samples, and children felt happier and less nervous after the process, indicating repeatability. Our findings highlight the feasibility of an integrated on-site saliva testing model for primary school campuses. Further research is needed to determine the scalability of such a model and whether the added compliance and safety of on-site testing compensates for the potential loss of learning time that testing during school hours would require.
Corresponding author: Ayaz Virji, MD, New York University Abu Dhabi, PO Box 129188, Abu Dhabi, United Arab Emirates; [email protected].
Financial disclosures: None.
1. Kuehn BM. Despite improvements, COVID-19’s health care disruptions persist. JAMA. 2021;325(23):2335. doi:10.1001/jama.2021.9134
2. National Institute on Aging. Why COVID-19 testing is the key to getting back to normal. September 4, 2020. Accessed September 8, 2021. https://www.nia.nih.gov/news/why-covid-19-testing-key-getting-back-normal
3. Centers for Disease Control and Prevention. Science brief: Transmission of SARS-CoV-2 in K-12 schools. Updated July 9, 2021. Accessed September 8, 2021. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/transmission_k_12_schools.html
4. Butler-Laporte G, Lawandi A, Schiller I, et al. Comparison of saliva and nasopharyngeal swab nucleic acid amplification testing for detection of SARS-CoV-2: a systematic review and meta-analysis. JAMA Intern Med. 2021;181(3):353-360. doi:10.1001/jamainternmed.2020.8876
5. Al Suwaidi H, Senok A, Varghese R, et al. Saliva for molecular detection of SARS-CoV-2 in school-age children. Clin Microbiol Infect. 2021;27(9):1330-1335. doi:10.1016/j.cmi.2021.02.009
6. Abu Dhabi. Accessed September 8, 2021. https://u.ae/en/about-the-uae/the-seven-emirates/abu-dhabi
7. Alsuwaidi AR, Al Hosani FI, Al Memari S, et al. Seroprevalence of COVID-19 infection in the Emirate of Abu Dhabi, United Arab Emirates: a population-based cross-sectional study. Int J Epidemiol. 2021;50(4):1077-1090. doi:10.1093/ije/dyab077
8. Al Hosany F, Ganesan S, Al Memari S, et al. Response to COVID-19 pandemic in the UAE: a public health perspective. J Glob Health. 2021;11:03050. doi:10.7189/jogh.11.03050
9. Bremmer I. The best global responses to the COVID-19 pandemic, 1 year later. Time Magazine. Updated February 23, 2021. Accessed September 8, 2021. https://time.com/5851633/best-global-responses-covid-19/
10. Department of Health, Abu Dhabi. Laboratory diagnostic test for COVID-19: update regarding saliva-based testing using RT-PCR test. 2021.
11. Vogels C, Brackney DE, Kalinich CC, et al. SalivaDirect: RNA extraction-free SARS-CoV-2 diagnostics. Protocols.io. Accessed September 8, 2021. https://www.protocols.io/view/salivadirect-rna-extraction-free-sars-cov-2-diagno-bh6jj9cn?version_warning=no
12. Education Endowment Foundation. Impact of school closures on the attainment gap: rapid evidence assessment. June 2020. Accessed September 8, 2021. https://www.researchgate.net/publication/342501263_EEF_2020_-_Impact_of_School_Closures_on_the_Attainment_Gap
13. United Nations. Policy brief: Education during COVID-19 and beyond. Accessed September 8, 2021. https://www.un.org/development/desa/dspd/wp-content/uploads/sites/22/2020/08/sg_policy_brief_covid-19_and_education_august_2020.pdf
14. Schiffman SS, Miletic ID. Effect of taste and smell on secretion rate of salivary IgA in elderly and young persons. J Nutr Health Aging. 1999;3(3):158-164.
15. Lee VM, Linden RW. The effect of odours on stimulated parotid salivary flow in humans. Physiol Behav. 1992;52(6):1121-1125. doi:10.1016/0031-9384(92)90470-m
1. Kuehn BM. Despite improvements, COVID-19’s health care disruptions persist. JAMA. 2021;325(23):2335. doi:10.1001/jama.2021.9134
2. National Institute on Aging. Why COVID-19 testing is the key to getting back to normal. September 4, 2020. Accessed September 8, 2021. https://www.nia.nih.gov/news/why-covid-19-testing-key-getting-back-normal
3. Centers for Disease Control and Prevention. Science brief: Transmission of SARS-CoV-2 in K-12 schools. Updated July 9, 2021. Accessed September 8, 2021. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/transmission_k_12_schools.html
4. Butler-Laporte G, Lawandi A, Schiller I, et al. Comparison of saliva and nasopharyngeal swab nucleic acid amplification testing for detection of SARS-CoV-2: a systematic review and meta-analysis. JAMA Intern Med. 2021;181(3):353-360. doi:10.1001/jamainternmed.2020.8876
5. Al Suwaidi H, Senok A, Varghese R, et al. Saliva for molecular detection of SARS-CoV-2 in school-age children. Clin Microbiol Infect. 2021;27(9):1330-1335. doi:10.1016/j.cmi.2021.02.009
6. Abu Dhabi. Accessed September 8, 2021. https://u.ae/en/about-the-uae/the-seven-emirates/abu-dhabi
7. Alsuwaidi AR, Al Hosani FI, Al Memari S, et al. Seroprevalence of COVID-19 infection in the Emirate of Abu Dhabi, United Arab Emirates: a population-based cross-sectional study. Int J Epidemiol. 2021;50(4):1077-1090. doi:10.1093/ije/dyab077
8. Al Hosany F, Ganesan S, Al Memari S, et al. Response to COVID-19 pandemic in the UAE: a public health perspective. J Glob Health. 2021;11:03050. doi:10.7189/jogh.11.03050
9. Bremmer I. The best global responses to the COVID-19 pandemic, 1 year later. Time Magazine. Updated February 23, 2021. Accessed September 8, 2021. https://time.com/5851633/best-global-responses-covid-19/
10. Department of Health, Abu Dhabi. Laboratory diagnostic test for COVID-19: update regarding saliva-based testing using RT-PCR test. 2021.
11. Vogels C, Brackney DE, Kalinich CC, et al. SalivaDirect: RNA extraction-free SARS-CoV-2 diagnostics. Protocols.io. Accessed September 8, 2021. https://www.protocols.io/view/salivadirect-rna-extraction-free-sars-cov-2-diagno-bh6jj9cn?version_warning=no
12. Education Endowment Foundation. Impact of school closures on the attainment gap: rapid evidence assessment. June 2020. Accessed September 8, 2021. https://www.researchgate.net/publication/342501263_EEF_2020_-_Impact_of_School_Closures_on_the_Attainment_Gap
13. United Nations. Policy brief: Education during COVID-19 and beyond. Accessed September 8, 2021. https://www.un.org/development/desa/dspd/wp-content/uploads/sites/22/2020/08/sg_policy_brief_covid-19_and_education_august_2020.pdf
14. Schiffman SS, Miletic ID. Effect of taste and smell on secretion rate of salivary IgA in elderly and young persons. J Nutr Health Aging. 1999;3(3):158-164.
15. Lee VM, Linden RW. The effect of odours on stimulated parotid salivary flow in humans. Physiol Behav. 1992;52(6):1121-1125. doi:10.1016/0031-9384(92)90470-m
What I Learned About Change From Practicing During the COVID-19 Surge
While sick at home with a 26-day symptomatic course of COVID-19 in March 2020, I watched the surge unfold in my state and the hospital where I work as an inpatient adult medicine physician. Although the preponderance of my professional life is dedicated to leading teams in implementing delivery system transformation, the hat I wore in that moment involved living through and keeping up with the changes around me. Once I recovered and returned to the arena as a COVID doctor, I adapted to and made changes during constant shifts in how we provided care.
Looking back on those months during the worst of the COVID-19 hospital surge in my region, I reflect on the factors that helped me, as a frontline and shift-work clinician, adapt to and make those changes. In reflecting on the elements that were meaningful to me during the crisis, I recognize a set of change-enabling factors that have broad relevance for those of us who work to improve outcomes for patients and populations.
Confidence engendered by liberating data
In the early days of the surge, there was much uncertainty, and unfortunately, some seriously imperfect messaging. Trust was broken or badly bruised for many frontline clinicians. I share this painful phase not to criticize, but rather reflect on what mattered to me during that crisis of confidence. It was data. Raw, unadjusted, best-available data. Produced and pushed out. Available, trended over time, telling the story of where we are, now. Counts of tests, beds, and ventilators. The consistent, transparent availability of relevant and straightforward data provided an active antidote to a sense of uncertainty during a crisis of confidence.
Personal practice change stimulated by relevance and urgency
For half a decade, I have been encouraging interdisciplinary inpatient teams to identify and actively engage the family and/or care partner as a member of the care team. Despite even the American Association of Retired Persons mobilizing an impressive regulatory approach in 32 states to require that family and/or care partners are included as such, the practice change efforts continued on a slow and steady path. Why? We just didn’t believe it was of urgent, relevant, mission-critical importance to our daily practice to do so. That all changed in March 2020.
Without needing to be told, educated, or incentivized, my first night as a COVID doctor found me calling every single patient’s family upon admission, regardless of what time it was. It was critical to review the diagnosis, transparently discuss the uncertainty regarding the upcoming hours and days, review the potential contingencies, and ask, right there and then, whether intubation is consistent with goals of care. It was that urgent and relevant. Without exception, families were grateful for the effort and candor.
The significance of this practice—undoubtedly adopted by every inpatient provider who has worked a COVID surge—is rooted in decades of academic deliberation on which is the “right” doctor to have these discussions. None of that mattered. Historical opinions changed due to what was urgent and relevant given the situation at hand and the job we had to do. Imagine, for example, what we could do and how we could change if we now consider it urgent and relevant to identify and mobilize enhanced services and supports to patients who experience inequities because we believe it to be mission-critical to the job we show up to do every day.
Change fostered by a creative problem-solving ecosystem
Embracing personal practice change was made easier and implicitly affirmed by the creative problem solving that occurred everywhere. Tents, drive-throughs, and even college field houses were now settings of care. Primary care physicians, cardiologists, and gastrointestinal (GI) and postanesthesia care nurses staffed the COVID floors. Rolling stands held iPads so staff could communicate with patients without entering the room. This creative ecosystem fostered individual practice change. No debates were needed to recognize that standard processes were inadequate. No single role or service of any discipline was singularly asked to change to meet the needs of the moment. Because of this ecosystem of creative, active change, there was a much greater flexibility among individuals, role types, departments, and disciplines to change. This is particularly poignant to me in light of the work I lead to improve care for patients who experience systemic inequities in our health care system. When we ask a single role type or discipline to change, it can be met with resistance; far more success is achieved when we engage an interdisciplinary and interdepartmental approach to change. When surrounded by others making change, it makes us more willing to change, too.
Change catalyzed teamwork
It is so often invoked that health care is a team sport. In practicality, while we may aspire to work as a team, health care delivery is still all too often comprised of a set of individual actors with individualized responsibilities trying to communicate the best they can with each other.
What I experienced during the surge at my hospital was the very best version of teamwork I have ever been a part of in health care: empathetic, mutually interdependent strangers coming together during daily changes in staffing, processes, and resources. I will never forget nights walking into the pediatric floor or day surgery recovery area—now repurposed as a COVID unit—to entirely new faces comprised of GI suite nurses, outpatient doctors, and moonlighting intensivists.
We were all new to each other, all new to working in this setting, and all new to whatever the newest changes of the day brought. I will never forget how we greeted each other and introduced ourselves. We asked each other where we were “from,” and held a genuine appreciation to each other for being there. Imagine how this impacted how we worked together. Looking back on those night shifts, I remember us as a truly interdependent team. I will endeavor to bring that sense of mutual regard and interdependency into my work to foster effective interdisciplinary and cross-continuum teamwork.
Takeaways
As a student and practitioner of delivery system transformation, I am often in conversations about imperfect data, incomplete evidence, and role-specific and organizational resistance to change. As an acute care provider during the COVID-19 hospital surge in my region, the experiences I had as a participant in the COVID-related delivery system change will stay with me as I lead value-based delivery system change. What worked in an infectious disease crisis holds great relevance to our pressing, urgent, relevant work to create a more person-centered, equitable, and value-based delivery system.
I am confident that if those of us seeking to improve outcomes use visible and accessible data to engender confidence, clearly link practice change to the relevant and urgent issue at hand, promote broadly visible creative problem solving to foster an ecosystem of change, and cultivate empathy and mutual interdependence to promote the teamwork we aspire to have, that we will foster meaningful progress in our efforts to improve care for patients and populations.
Corresponding author: Amy Boutwell, MD, MPP, President, Collaborative Healthcare Strategies, Lexington, MA; [email protected].
Financial disclosures: None.
While sick at home with a 26-day symptomatic course of COVID-19 in March 2020, I watched the surge unfold in my state and the hospital where I work as an inpatient adult medicine physician. Although the preponderance of my professional life is dedicated to leading teams in implementing delivery system transformation, the hat I wore in that moment involved living through and keeping up with the changes around me. Once I recovered and returned to the arena as a COVID doctor, I adapted to and made changes during constant shifts in how we provided care.
Looking back on those months during the worst of the COVID-19 hospital surge in my region, I reflect on the factors that helped me, as a frontline and shift-work clinician, adapt to and make those changes. In reflecting on the elements that were meaningful to me during the crisis, I recognize a set of change-enabling factors that have broad relevance for those of us who work to improve outcomes for patients and populations.
Confidence engendered by liberating data
In the early days of the surge, there was much uncertainty, and unfortunately, some seriously imperfect messaging. Trust was broken or badly bruised for many frontline clinicians. I share this painful phase not to criticize, but rather reflect on what mattered to me during that crisis of confidence. It was data. Raw, unadjusted, best-available data. Produced and pushed out. Available, trended over time, telling the story of where we are, now. Counts of tests, beds, and ventilators. The consistent, transparent availability of relevant and straightforward data provided an active antidote to a sense of uncertainty during a crisis of confidence.
Personal practice change stimulated by relevance and urgency
For half a decade, I have been encouraging interdisciplinary inpatient teams to identify and actively engage the family and/or care partner as a member of the care team. Despite even the American Association of Retired Persons mobilizing an impressive regulatory approach in 32 states to require that family and/or care partners are included as such, the practice change efforts continued on a slow and steady path. Why? We just didn’t believe it was of urgent, relevant, mission-critical importance to our daily practice to do so. That all changed in March 2020.
Without needing to be told, educated, or incentivized, my first night as a COVID doctor found me calling every single patient’s family upon admission, regardless of what time it was. It was critical to review the diagnosis, transparently discuss the uncertainty regarding the upcoming hours and days, review the potential contingencies, and ask, right there and then, whether intubation is consistent with goals of care. It was that urgent and relevant. Without exception, families were grateful for the effort and candor.
The significance of this practice—undoubtedly adopted by every inpatient provider who has worked a COVID surge—is rooted in decades of academic deliberation on which is the “right” doctor to have these discussions. None of that mattered. Historical opinions changed due to what was urgent and relevant given the situation at hand and the job we had to do. Imagine, for example, what we could do and how we could change if we now consider it urgent and relevant to identify and mobilize enhanced services and supports to patients who experience inequities because we believe it to be mission-critical to the job we show up to do every day.
Change fostered by a creative problem-solving ecosystem
Embracing personal practice change was made easier and implicitly affirmed by the creative problem solving that occurred everywhere. Tents, drive-throughs, and even college field houses were now settings of care. Primary care physicians, cardiologists, and gastrointestinal (GI) and postanesthesia care nurses staffed the COVID floors. Rolling stands held iPads so staff could communicate with patients without entering the room. This creative ecosystem fostered individual practice change. No debates were needed to recognize that standard processes were inadequate. No single role or service of any discipline was singularly asked to change to meet the needs of the moment. Because of this ecosystem of creative, active change, there was a much greater flexibility among individuals, role types, departments, and disciplines to change. This is particularly poignant to me in light of the work I lead to improve care for patients who experience systemic inequities in our health care system. When we ask a single role type or discipline to change, it can be met with resistance; far more success is achieved when we engage an interdisciplinary and interdepartmental approach to change. When surrounded by others making change, it makes us more willing to change, too.
Change catalyzed teamwork
It is so often invoked that health care is a team sport. In practicality, while we may aspire to work as a team, health care delivery is still all too often comprised of a set of individual actors with individualized responsibilities trying to communicate the best they can with each other.
What I experienced during the surge at my hospital was the very best version of teamwork I have ever been a part of in health care: empathetic, mutually interdependent strangers coming together during daily changes in staffing, processes, and resources. I will never forget nights walking into the pediatric floor or day surgery recovery area—now repurposed as a COVID unit—to entirely new faces comprised of GI suite nurses, outpatient doctors, and moonlighting intensivists.
We were all new to each other, all new to working in this setting, and all new to whatever the newest changes of the day brought. I will never forget how we greeted each other and introduced ourselves. We asked each other where we were “from,” and held a genuine appreciation to each other for being there. Imagine how this impacted how we worked together. Looking back on those night shifts, I remember us as a truly interdependent team. I will endeavor to bring that sense of mutual regard and interdependency into my work to foster effective interdisciplinary and cross-continuum teamwork.
Takeaways
As a student and practitioner of delivery system transformation, I am often in conversations about imperfect data, incomplete evidence, and role-specific and organizational resistance to change. As an acute care provider during the COVID-19 hospital surge in my region, the experiences I had as a participant in the COVID-related delivery system change will stay with me as I lead value-based delivery system change. What worked in an infectious disease crisis holds great relevance to our pressing, urgent, relevant work to create a more person-centered, equitable, and value-based delivery system.
I am confident that if those of us seeking to improve outcomes use visible and accessible data to engender confidence, clearly link practice change to the relevant and urgent issue at hand, promote broadly visible creative problem solving to foster an ecosystem of change, and cultivate empathy and mutual interdependence to promote the teamwork we aspire to have, that we will foster meaningful progress in our efforts to improve care for patients and populations.
Corresponding author: Amy Boutwell, MD, MPP, President, Collaborative Healthcare Strategies, Lexington, MA; [email protected].
Financial disclosures: None.
While sick at home with a 26-day symptomatic course of COVID-19 in March 2020, I watched the surge unfold in my state and the hospital where I work as an inpatient adult medicine physician. Although the preponderance of my professional life is dedicated to leading teams in implementing delivery system transformation, the hat I wore in that moment involved living through and keeping up with the changes around me. Once I recovered and returned to the arena as a COVID doctor, I adapted to and made changes during constant shifts in how we provided care.
Looking back on those months during the worst of the COVID-19 hospital surge in my region, I reflect on the factors that helped me, as a frontline and shift-work clinician, adapt to and make those changes. In reflecting on the elements that were meaningful to me during the crisis, I recognize a set of change-enabling factors that have broad relevance for those of us who work to improve outcomes for patients and populations.
Confidence engendered by liberating data
In the early days of the surge, there was much uncertainty, and unfortunately, some seriously imperfect messaging. Trust was broken or badly bruised for many frontline clinicians. I share this painful phase not to criticize, but rather reflect on what mattered to me during that crisis of confidence. It was data. Raw, unadjusted, best-available data. Produced and pushed out. Available, trended over time, telling the story of where we are, now. Counts of tests, beds, and ventilators. The consistent, transparent availability of relevant and straightforward data provided an active antidote to a sense of uncertainty during a crisis of confidence.
Personal practice change stimulated by relevance and urgency
For half a decade, I have been encouraging interdisciplinary inpatient teams to identify and actively engage the family and/or care partner as a member of the care team. Despite even the American Association of Retired Persons mobilizing an impressive regulatory approach in 32 states to require that family and/or care partners are included as such, the practice change efforts continued on a slow and steady path. Why? We just didn’t believe it was of urgent, relevant, mission-critical importance to our daily practice to do so. That all changed in March 2020.
Without needing to be told, educated, or incentivized, my first night as a COVID doctor found me calling every single patient’s family upon admission, regardless of what time it was. It was critical to review the diagnosis, transparently discuss the uncertainty regarding the upcoming hours and days, review the potential contingencies, and ask, right there and then, whether intubation is consistent with goals of care. It was that urgent and relevant. Without exception, families were grateful for the effort and candor.
The significance of this practice—undoubtedly adopted by every inpatient provider who has worked a COVID surge—is rooted in decades of academic deliberation on which is the “right” doctor to have these discussions. None of that mattered. Historical opinions changed due to what was urgent and relevant given the situation at hand and the job we had to do. Imagine, for example, what we could do and how we could change if we now consider it urgent and relevant to identify and mobilize enhanced services and supports to patients who experience inequities because we believe it to be mission-critical to the job we show up to do every day.
Change fostered by a creative problem-solving ecosystem
Embracing personal practice change was made easier and implicitly affirmed by the creative problem solving that occurred everywhere. Tents, drive-throughs, and even college field houses were now settings of care. Primary care physicians, cardiologists, and gastrointestinal (GI) and postanesthesia care nurses staffed the COVID floors. Rolling stands held iPads so staff could communicate with patients without entering the room. This creative ecosystem fostered individual practice change. No debates were needed to recognize that standard processes were inadequate. No single role or service of any discipline was singularly asked to change to meet the needs of the moment. Because of this ecosystem of creative, active change, there was a much greater flexibility among individuals, role types, departments, and disciplines to change. This is particularly poignant to me in light of the work I lead to improve care for patients who experience systemic inequities in our health care system. When we ask a single role type or discipline to change, it can be met with resistance; far more success is achieved when we engage an interdisciplinary and interdepartmental approach to change. When surrounded by others making change, it makes us more willing to change, too.
Change catalyzed teamwork
It is so often invoked that health care is a team sport. In practicality, while we may aspire to work as a team, health care delivery is still all too often comprised of a set of individual actors with individualized responsibilities trying to communicate the best they can with each other.
What I experienced during the surge at my hospital was the very best version of teamwork I have ever been a part of in health care: empathetic, mutually interdependent strangers coming together during daily changes in staffing, processes, and resources. I will never forget nights walking into the pediatric floor or day surgery recovery area—now repurposed as a COVID unit—to entirely new faces comprised of GI suite nurses, outpatient doctors, and moonlighting intensivists.
We were all new to each other, all new to working in this setting, and all new to whatever the newest changes of the day brought. I will never forget how we greeted each other and introduced ourselves. We asked each other where we were “from,” and held a genuine appreciation to each other for being there. Imagine how this impacted how we worked together. Looking back on those night shifts, I remember us as a truly interdependent team. I will endeavor to bring that sense of mutual regard and interdependency into my work to foster effective interdisciplinary and cross-continuum teamwork.
Takeaways
As a student and practitioner of delivery system transformation, I am often in conversations about imperfect data, incomplete evidence, and role-specific and organizational resistance to change. As an acute care provider during the COVID-19 hospital surge in my region, the experiences I had as a participant in the COVID-related delivery system change will stay with me as I lead value-based delivery system change. What worked in an infectious disease crisis holds great relevance to our pressing, urgent, relevant work to create a more person-centered, equitable, and value-based delivery system.
I am confident that if those of us seeking to improve outcomes use visible and accessible data to engender confidence, clearly link practice change to the relevant and urgent issue at hand, promote broadly visible creative problem solving to foster an ecosystem of change, and cultivate empathy and mutual interdependence to promote the teamwork we aspire to have, that we will foster meaningful progress in our efforts to improve care for patients and populations.
Corresponding author: Amy Boutwell, MD, MPP, President, Collaborative Healthcare Strategies, Lexington, MA; [email protected].
Financial disclosures: None.
COVID-19 Vaccine in Veterans with Multiple Sclerosis: Protect the Vulnerable
Older veterans with progressive MS and associated comorbidities are at higher risk of death should they be infected by COVID-19 and we urge health care providers to educate every veteran about the benefits of being vaccinated against COVID-19.
This article has been updated to reflect new US Food and Drug Administration and Centers for Disease Control and Prevention recommendations to pause administration of the Johnson and Johnson Jansen (JNJ-78436735) COVID-19 vaccine.1
Since the outbreak of the pandemic caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2),a plethora of studies have been performed to increase our knowledge of its associated illness COVID-19.2 There is no cure for COVID-19, which can be lethal. In the absence of a cure, preventive measures are of vital importance. In order to help prevent the spread of the virus, the Centers for Diseases Control and Prevention (CDC) advocates for: (1) the use of a face mask over the mouth and nose; (2) a minimum of 6-foot distance between individuals; and (3) avoidance of gatherings.As of March 2021, the US Food and Drug Administration (FDA) approved 3 vaccines for the prevention of COVID-19, under an emergency use authorization (EUA).3-5
COVID-19 and Multiple Sclerosis
Since the beginning of the pandemic, neurologists have faced a new challenge—determining whether persons with multiple sclerosis (pwMS) were more at risk than others of becoming ill from COVID-19 or were destined for a worse outcome. The National MS Society has advised a personalized approach in relation to particularly vulnerable persons when needed and has also initiated worldwide registries to collect information regarding incidence and outcome of COVID-19 in pwMS. Accordingly, through the MS Center of Excellence (MSCoE), the Veterans Health Administration (VHA) has established a national registry assembling data regarding COVID-19 in veterans with MS.
A recent descriptive literature review summarized the outcomes of 873 persons with both MS and COVID-19 and reported that about 36% of COVID-19 cases were treated with B-cell depleting therapies (ocrelizumab or rituximab).6 This proportion was relatively higher when compared with other disease modifying agents. Of those who became infected with SARS-CoV-2, death from COVID-19 occurred in about 4%, and an additional 3% required assisted invasive or noninvasive ventilation. Persons reported to have passed away from COVID-19 generally were older; had progressive MS; or had associated comorbidities such as obesity, hypertension, heart or lung conditions, or cancers. Of these, 50% were not on any disease modifying agent, 25% were on B-cell depleting therapies (ocrelizumab or rituximab), and the remaining 25% were on various medications for MS. It is important to highlight that no formal statistical analyses were performed in this review. On the contrary, in the recently published Italian report on 844 pwMS who had suspected or confirmed COVID-19, the authors used univariate and multivariate models to analyze their findings and noted that the use of ocrelizumab was significantly associated with a worse clinical outcome.7 These authors also identified age, sex, disability score, and recent (within 1 month) use of steroids as risk factors for a severe COVID-19 outcome. The incidence of death from COVID-19 in this cohort was 1.54%.
The recently published data from the North American Registry of the National MS Society based on 1,626 patients reported a 3.3% incidence of death from COVID-19.8 The following factors were identified as risks for worse outcome: male sex, nonambulatory status, age, Black race, and cardiovascular disease. The use of rituximab, ocrelizumab, and steroids (the latter medication over the preceding 2 months) increased the risks of hospitalization for COVID-19.
COVID-19 Vaccines
Of the 3 available vaccines, the Pfizer-BioNTech COVID-19 (BNT162b2) vaccine is approved for individuals aged ≥ 16 years, while the Moderna COVID-19 (mRNA-1273) and the Johnson and Johnson/Jannsen COVID-19 (JNJ-78436735) vaccines are approved for individuals aged ≥ 18 years, though the latter vaccine has been temporarily suspended.1,3-5 The EUAs were released following the disclosure of the results of 3 phase 3 clinical trials and several phase 1 and 2 clinical trials.9-16
The BNT162b2 vaccine from Pfizer-BioNTech encodes the SARS-CoV-2 full-length spike protein (S) in prefusion conformation locked by the mutation in 2 prolines.9 Differently from the BNT162b2 vaccine, the BNT162b1 vaccine encodes a secreted trimerized SARS-CoV-2 receptor–binding domain. The S-glycoprotein is required for viral entry, as implicated in host cell attachment, and is the target of the neutralizing antibodies. In a phase 1 clinical study on 195 volunteers treated with BNT162b1 (10 mg, 20 mg, 30 mg, or 100 mg doses) or BNT162b2 (10 mg, 20 mg, or 30 mg doses) vaccines or placebo 21 days apart, both the binding and neutralizing antibody response was found to be age and “somewhat” dose dependent.9
Higher neutralization titers were measured at day 28 and 35 (7 and 14 days after the second dose, respectively) and compared with titers of persons who recovered from a COVID-19 infection.9 Serum neutralization was measured using a fluorescence-based high-throughput neutralization assay, while binding activity was assessed using the receptor-binding domain (RBD)–binding or S1-binding IgG direct Luminex immunoassays.
The overall reactogenicity/immunogenicity profile of BNT162b2 administered twice (30 mg each time) led to its selection for the phase 3 clinical trial.9,10 In a large phase 3 clinical trial on 43,458 participants, the BNT162b2 vaccine given at 30 mg doses 21 days apart conferred 95% clinical efficacy in reducing the likelihood of being affected by symptomatic COVID-19.10 No safety concerns to stop the trial were identified, though related severe and life-threatening events were reported in 0.3% and 0.1% of the volunteers, respectively. We note that these incidence rates were the same for the treated and the placebo group.
The mRNA-1273 vaccine from Moderna also encodes the SARS-CoV-2 S-glycoprotein. In a dose escalation phase 1 trial of 45 participants aged between 18 and 55 years (25 mg, 100 mg or 250 mg, given at days 1 and 29) and 40 participants aged ≥ 57 years (25 mg and 100 mg, given at days 1 and 29), a dose-dependent effect was observed for both binding (receptor-binding domain and S-2p IgG on enzyme-linked immunosorbent assay [ELISA])and neutralizing antibodies (SARS-CoV-2 nanoluciferase high-throughput neutralization assay, focus reduction neutralization test mNeonGreen and SARS-CoV-2 plaque-reduction neutralization testing assay) development.11,12 The geometric mean of both binding and neutralizing antibodies declined over time but persisted high as late as 119 days after the first burst of 100 mg dose.13 The same dose of the vaccine also elicited a strong T helper-1 response with little T helper-2 response across all ages.11 The strength of the memory cellular response remains to be defined and is the subject of ongoing investigations. In a large phase 3 clinical trial with 30,420 participants, the Moderna COVID-19 mRNA-1273 vaccine, given 28 days apart at the dose of 100 mg, met 94.1% clinical efficacy in reducing the likelihood of being affected by symptomatic COVID-19.14
Less than 0.1% of volunteers in both groups withdrew from the trial due to adverse effects (AEs); 0.5% in the placebo group and 0.3% in the treated group had AEs after the first dose, which precluded receiving the second dose.14
The Johnson and Johnson/Jannsen JNJ-78436735 vaccine is based upon a recombinant, replication-incompetent adenovirus serotype 26 (Ad26) vector, which encodes the full-length, stabilized S-glycoprotein of SARS-CoV-2. The currently reported results of the phase 1 and 2 clinical study indicated that 805 volunteers (402 participants between ages 18 and 55 years and 403 individuals aged ≥ 65 years) were randomized to receive a single or double dose of either 5 x 1010 viral particles per 0.5 mL (low dose) or 1 x 1011 viral particles per 0.5 mL (high dose), each compared with a placebo group. Incidence of seroconversion to binding antibodies against the full-length stabilized S-glycoprotein, as measured by ELISA, showed ≥ 96% seroconversion by day 29 after the first dose. The incidence of seroconversion to neutralizing antibodies was ≥ 90% as early as early as 29 days after the first of either dose. In this study, neutralization activity was measured using the wild-type virus microneutralization assay based on the Victoria/1/2020/ SARS-CoV-2 strain.15 We note that the data related to this study have been partially reported and additional information will be available when each participant will have received the second dose.
In a large phase 3 clinical trial with 40,000 participants aged between 18 and 100 years, the Johnson and Johnson/Jannsen JNJ-78436735 vaccine, given as single dose of 5 x 1010 viral particles per 0.5 mL, met 65.5% clinical efficacy in the likelihood of being affected by symptomatic COVID-19 ≥ 28 days postimmunization.16 In this study, the vaccine efficacy was found to have a geographic distribution with highest efficacy in the US (74.4%), followed by Latin America (64.7%) where Brazil showed a predominance of the P2 COVID-19 lineage (64.7%), and Africa (52%) where the B.1.351 lineage was most frequent (94.5%). The vaccine also proved to be effective in reducing the likelihood of asymptomatic seroconversion, as measured by the level of a non-S protein, eg, 0.7% of positive cases in the vaccine group vs 2.8% in the placebo group. Immunological data indicated that the vaccine response was mainly driven by T-helper 1 lymphocytes. As of April 13, 2021 the FDA has recommend suspending the administration of the Johnson and Johnson/Janssen vaccine due to the occurrence of severe blood clots reported in a 6 subjects out of ~6.8 millions administered doses.1
It is noteworthy to highlight that all vaccines reduced the likelihood of hospitalizations and deaths due to COVID-19.
As of April 17, 2021, the CDC reports that more than 130 million (40%) Americans, nearly 1/3 of the population, have received at least 1 dose of any of the 3 available vaccines, including 4.6 million at the VHA.17 Using the Vaccine Adverse Event Reporting System and v-safe, the US is conducting what has been defined the most “intense and comprehensive safety monitoring in the US history.”18 Thus far, data affirm the overall safety of the available vaccines against COVID-19. Individuals should not receive the COVID-19 vaccines if they have had a severe allergic reaction to any ingredient in the vaccine or a severe allergic reaction to a prior dose of the vaccine. Additionally, individuals who have received convalescent plasma should wait 90 days before getting the COVID-19 vaccine.
Vaccination for Persons with MS
PwMS or those on immunosuppressive medications were excluded from the clinical trial led by Pfizer-BioNTech. There is no mention of MS as comorbidity in the study from Moderna, although this condition is not listed as an exclusion criterion either. The results of the phase 3 clinical trial for the Johnson and Johnson/Janssen vaccine are not fully public yet, thus this information is not known as well. As a result, the use of this vaccine in pwMS under immunomodulatory agents is based on previous knowledge of other vaccines. Evidence is growing for the safety of the BNT162b2 COVID-19 vaccination in pwMS.19 Data regarding COVID-19 efficacy and safety are still largely based on previous knowledge on other vaccines.20,21
Immunization of pwMS is considered safe and should proceed with confidence in those persons who have no other contraindication to receive a vaccine. A fundamental problem for pwMS treated with immunomodulatory or immunosuppressive medications is whether the vaccine will remain safe or be able to solicit an adequate immune response.20,21 As of the time of publication 2021, there is consensus that mRNA based or inactivated vaccines are also considered safe in pwMS undergoing immunomodulatory or immunosuppressive treatments.20-23 We advise a one-on-one conversation between each veteran with MS and their primary neurologist to understand the importance of the vaccination, the minimal risks associated with it and if any specific treatment modification should be made.
To provide guidance, the National MS Society released a position statement that is regularly updated.22 Given the risks associated with discontinuation of disease modifying agents, pwMS opting to receive a COVID-19 vaccine should continue taking their medications unless recommended otherwise by their primary neurologist. In addition, on the basis of available literature and the American Academy of Neurology recommendations on the use of vaccines in general, the following recommendations are proposed.20-23
Recommendation 1: injections, orals, and natalizumab. Given the risks associated with discontinuation of disease modifying agents, pwMS opting to receive a COVID-19 vaccine should continue taking their medications unless recommended otherwise by their primary neurologist. Neither delay in start nor adjustments in dosing or timing of administration are advised for pwMS taking currently available either generic or brand formulations of β interferons, glatiramer acetate, teriflunomide, dimethyl or monomethyl fumarate, or natalizumab.22
Recommendation 2: anti-CD20 monoclonal infusions. As an attenuated humoral response is predicted in pwMS treated with anti-CD20 monoclonal infusions, coordinating the timing of vaccination with treatment schedule may maximize efficacy of the vaccine. Whenever possible, it is advised to be vaccinated ≥ 12 weeks after the last infusion and to resume infusion 4 weeks after the last dose of the vaccine. PwMS starting anti-CD20 monoclonal infusions are advised to be fully vaccinated first and start these medications ≥ 2 to 4 weeks later.22
Recommendation 3: alemtuzumab infusion. Given its effect on CD52+ cells, it is advised to be vaccinated ≥ 24 weeks after the last infusion and to resume infusion 4 weeks after the last dose of the vaccine. PwMS starting alemtuzumab infusions are advised to get fully vaccinated first and start this medication 4 weeks or more after completing the vaccine.22
Recommendation 4: sphingosine 1 phosphate receptor modulators, oral cladribine, and ofatumumab. PwMS starting any of these medications are advised to be fully vaccinated first and start these medications 2 to 4 weeks after completing the vaccine. PwMS already on those medications are not advised to change the schedule of administration. When possible, though, one should resume the dose of cladribine or ofatumumab 2 to 4 weeks after the last dose of the vaccine. 20
Notably, all these recommendations hold true when there is enough disease stability to allow delaying treatment. We also add that it remains unclear if persons with an overall very low number of lymphocytes will be able to elicit a strong reaction to the vaccine. Blood collection and analysis of white blood cell count and lymphocyte subset estimates should be obtained in those persons with a markedly suppressed immune system. Whenever possible, to maximize outcome, timing the vaccination with treatment should be considered in those persons with a markedly reduced number of T-helper 1 cells.
Vaccination for Veterans
Currently the VHA is offering to veterans the Pfizer and Moderna COVID-19 vaccines with FDA EUAs. In accordance with FDA regulations, the VHA has paused administration of the Johnson and Johnson/Janssen vaccine. The VHA has launched its vaccination program in December 2020 by first providing the vaccine to health care personnel, nursing home patients, spinal cord injury patients, chemotherapy patients, dialysis and transplant patients, as well as homeless veterans. Most VA health care systems have passed this phase and are now able to provide vaccines to veterans with MS.
In December 2020, the MSCoE released a position statement regarding the importance and safety of the COVID-19 vaccine for veterans with MS.24 This statement will be updated on a regular basis as new information becomes available from major organizations like the National MS Society, FDA, CDC, and World Health Organization (WHO) or relevant literature.
Conclusions
Older veterans with progressive MS and associated comorbidities are at higher risk of death should they be infected by COVID-19. Fortunately, we live in a time where vaccines are recognized as a critical tool to prevent this infection and to significantly reduce its morbidity and mortality. Yet, hesitancy to vaccinate has been identified as one of the most important threats to public health by the WHO in 2019.25 Understandably such hesitancy is even more profound for the COVID-19 vaccine, which is being administered under an EUA. In light of this indecision, and given the current state of the pandemic, we urge health care providers to educate every veteran about the benefits of being vaccinated against COVID-19. Within the VHA, a solid campaign of vaccination has been put in place at an unprecedented speed.
Health care providers interacting with veterans with MS are encouraged to use the MSCoE website (www.va.gov/ms) for any questions or concerns, or to reach out to MSCoE staff. It is vitally important that our community of veterans receives appropriate education on the importance of this vaccination for their own safety, for that of their household and society.
1. Centers for Disease Control and Prevention. Recommendation to pause use of Johnson & Johnson’s Janssen COVID-19 vaccine. Updated April 16, 2021. Accessed April 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/JJUpdate.html
2. World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it. Accessed March 9, 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it
3. US Food and Drug Administration. Pfizer-BioNTech COVID-19 vaccine. Updated February 3, 2021. Accessed March 22, 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine
4. US Food and Drug Administration. Moderna COVID-19 vaccine. Updated February 3, 2021. Accessed March 22, 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccine
5. US Food and Drug Administration. FDA issues emergency use authorization for third COVID-19 vaccine [press release]. Published February 27, 2021. Accessed March 22, 2021. https://www.fda.gov/news-events/press-announcements/fda-issues-emergency-use-authorization-third-covid-19-vaccine
6. Möhn N, Konen FF, Pul R, et al. Experience in multiple sclerosis patients with COVID-19 and disease-modifying therapies: a review of 873 published cases. J Clin Med. 2020;9(12):4067. Published 2020 Dec 16. doi:10.3390/jcm9124067
7. Sormani MP, De Rossi N, Schiavetti I, et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol. 2021;89(4):780-789. doi:10.1002/ana.26028
8. Salter A, Fox RJ, Newsome SD, et al. Outcomes and risk factors associated with SARS-CoV-2 infection in a North American registry of patients with multiple sclerosis [published online ahead of print, 2021 Mar 19]. JAMA Neurol. 2021;10.1001/jamaneurol.2021.0688. doi:10.1001/jamaneurol.2021.0688
9. Walsh EE, Frenck RW Jr, Falsey AR, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439-2450. doi:10.1056/NEJMoa2027906
10. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi:10.1056/NEJMoa2034577
11. Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2 - preliminary Report. N Engl J Med. 2020;383(20):1920-1931. doi:10.1056/NEJMoa2022483
12. Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383(25):2427-2438. doi:10.1056/NEJMoa2028436
13. Widge AT, Rouphael NG, Jackson LA, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med. 2021;384(1):80-82. doi:10.1056/NEJMc2032195
14. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. doi:10.1056/NEJMoa2035389
15. Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a phase 1-2a Trial of Ad26.COV2.S Covid-19 Vaccine [published online ahead of print, 2021 Jan 13]. N Engl J Med. 2021;NEJMoa2034201. doi:10.1056/NEJMoa2034201
16. Oliver SE, Gargano JW, Scobie H, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Janssen COVID-19 vaccine - United States, February 2021. MMWR Morb Mortal Wkly Rep. 2021;70(9):329-332. Published 2021 Mar 5. doi:10.15585/mmwr.mm7009e4
17. US Centers for Disease Control and Prevention. COVID-19 vaccinations in the United States. Updated March 21, 2021. Accessed March 22, 2021. https://covid.cdc.gov/covid-data-tracker/#vaccinations
18. Gee J, Marquez P, Su J, et al. First month of COVID-19 vaccine safety monitoring - United States, December 14, 2020-January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(8):283-288. Published 2021 Feb 26. doi:10.15585/mmwr.mm7008e3
19. Achiron A, Dolev M, Menascu S, et al. COVID-19 vaccination in patients with multiple sclerosis: What we have learnt by February 2021 [published online ahead of print, 2021 Apr 15]. Mult Scler. 2021;13524585211003476. doi:10.1177/13524585211003476
20. Righi E, Gallo T, Azzini AM, et al. A review of vaccinations in adult patients with secondary immunodeficiency [published online ahead of print, 2021 Mar 9]. Infect Dis Ther. 2021;1-25. doi:10.1007/s40121-021-00404-y
21. Ciotti JR, Valtcheva MV, Cross AH. Effects of MS disease-modifying therapies on responses to vaccinations: A review. Mult Scler Relat Disord. 2020;45:102439. doi:10.1016/j.msard.2020.102439
22. National Multiple Sclerosis Society. COVID-19 vaccine guidance for people living with MS. Accessed March 22, 2021. https://www.nationalmssociety.org/coronavirus-covid-19-information/multiple-sclerosis-and-coronavirus/covid-19-vaccine-guidance
23. Farez MF, Correale J, Armstrong MJ, et al. Practice guideline update summary: vaccine-preventable infections and immunization in multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2019;93(13):584-594. doi:10.1212/WNL.0000000000008157
24. US Department of Veterans Affairs, Multiple Sclerosis Centers of Excellence. Coronavirus (COVID-19) and vaccine information. Updated February 25. 2021. Accessed March 9, 2021. https://www.va.gov/ms
25. World Health Organization. Ten threats to global health in 2019. Accessed March 18, 2021. https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019.
Older veterans with progressive MS and associated comorbidities are at higher risk of death should they be infected by COVID-19 and we urge health care providers to educate every veteran about the benefits of being vaccinated against COVID-19.
Older veterans with progressive MS and associated comorbidities are at higher risk of death should they be infected by COVID-19 and we urge health care providers to educate every veteran about the benefits of being vaccinated against COVID-19.
This article has been updated to reflect new US Food and Drug Administration and Centers for Disease Control and Prevention recommendations to pause administration of the Johnson and Johnson Jansen (JNJ-78436735) COVID-19 vaccine.1
Since the outbreak of the pandemic caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2),a plethora of studies have been performed to increase our knowledge of its associated illness COVID-19.2 There is no cure for COVID-19, which can be lethal. In the absence of a cure, preventive measures are of vital importance. In order to help prevent the spread of the virus, the Centers for Diseases Control and Prevention (CDC) advocates for: (1) the use of a face mask over the mouth and nose; (2) a minimum of 6-foot distance between individuals; and (3) avoidance of gatherings.As of March 2021, the US Food and Drug Administration (FDA) approved 3 vaccines for the prevention of COVID-19, under an emergency use authorization (EUA).3-5
COVID-19 and Multiple Sclerosis
Since the beginning of the pandemic, neurologists have faced a new challenge—determining whether persons with multiple sclerosis (pwMS) were more at risk than others of becoming ill from COVID-19 or were destined for a worse outcome. The National MS Society has advised a personalized approach in relation to particularly vulnerable persons when needed and has also initiated worldwide registries to collect information regarding incidence and outcome of COVID-19 in pwMS. Accordingly, through the MS Center of Excellence (MSCoE), the Veterans Health Administration (VHA) has established a national registry assembling data regarding COVID-19 in veterans with MS.
A recent descriptive literature review summarized the outcomes of 873 persons with both MS and COVID-19 and reported that about 36% of COVID-19 cases were treated with B-cell depleting therapies (ocrelizumab or rituximab).6 This proportion was relatively higher when compared with other disease modifying agents. Of those who became infected with SARS-CoV-2, death from COVID-19 occurred in about 4%, and an additional 3% required assisted invasive or noninvasive ventilation. Persons reported to have passed away from COVID-19 generally were older; had progressive MS; or had associated comorbidities such as obesity, hypertension, heart or lung conditions, or cancers. Of these, 50% were not on any disease modifying agent, 25% were on B-cell depleting therapies (ocrelizumab or rituximab), and the remaining 25% were on various medications for MS. It is important to highlight that no formal statistical analyses were performed in this review. On the contrary, in the recently published Italian report on 844 pwMS who had suspected or confirmed COVID-19, the authors used univariate and multivariate models to analyze their findings and noted that the use of ocrelizumab was significantly associated with a worse clinical outcome.7 These authors also identified age, sex, disability score, and recent (within 1 month) use of steroids as risk factors for a severe COVID-19 outcome. The incidence of death from COVID-19 in this cohort was 1.54%.
The recently published data from the North American Registry of the National MS Society based on 1,626 patients reported a 3.3% incidence of death from COVID-19.8 The following factors were identified as risks for worse outcome: male sex, nonambulatory status, age, Black race, and cardiovascular disease. The use of rituximab, ocrelizumab, and steroids (the latter medication over the preceding 2 months) increased the risks of hospitalization for COVID-19.
COVID-19 Vaccines
Of the 3 available vaccines, the Pfizer-BioNTech COVID-19 (BNT162b2) vaccine is approved for individuals aged ≥ 16 years, while the Moderna COVID-19 (mRNA-1273) and the Johnson and Johnson/Jannsen COVID-19 (JNJ-78436735) vaccines are approved for individuals aged ≥ 18 years, though the latter vaccine has been temporarily suspended.1,3-5 The EUAs were released following the disclosure of the results of 3 phase 3 clinical trials and several phase 1 and 2 clinical trials.9-16
The BNT162b2 vaccine from Pfizer-BioNTech encodes the SARS-CoV-2 full-length spike protein (S) in prefusion conformation locked by the mutation in 2 prolines.9 Differently from the BNT162b2 vaccine, the BNT162b1 vaccine encodes a secreted trimerized SARS-CoV-2 receptor–binding domain. The S-glycoprotein is required for viral entry, as implicated in host cell attachment, and is the target of the neutralizing antibodies. In a phase 1 clinical study on 195 volunteers treated with BNT162b1 (10 mg, 20 mg, 30 mg, or 100 mg doses) or BNT162b2 (10 mg, 20 mg, or 30 mg doses) vaccines or placebo 21 days apart, both the binding and neutralizing antibody response was found to be age and “somewhat” dose dependent.9
Higher neutralization titers were measured at day 28 and 35 (7 and 14 days after the second dose, respectively) and compared with titers of persons who recovered from a COVID-19 infection.9 Serum neutralization was measured using a fluorescence-based high-throughput neutralization assay, while binding activity was assessed using the receptor-binding domain (RBD)–binding or S1-binding IgG direct Luminex immunoassays.
The overall reactogenicity/immunogenicity profile of BNT162b2 administered twice (30 mg each time) led to its selection for the phase 3 clinical trial.9,10 In a large phase 3 clinical trial on 43,458 participants, the BNT162b2 vaccine given at 30 mg doses 21 days apart conferred 95% clinical efficacy in reducing the likelihood of being affected by symptomatic COVID-19.10 No safety concerns to stop the trial were identified, though related severe and life-threatening events were reported in 0.3% and 0.1% of the volunteers, respectively. We note that these incidence rates were the same for the treated and the placebo group.
The mRNA-1273 vaccine from Moderna also encodes the SARS-CoV-2 S-glycoprotein. In a dose escalation phase 1 trial of 45 participants aged between 18 and 55 years (25 mg, 100 mg or 250 mg, given at days 1 and 29) and 40 participants aged ≥ 57 years (25 mg and 100 mg, given at days 1 and 29), a dose-dependent effect was observed for both binding (receptor-binding domain and S-2p IgG on enzyme-linked immunosorbent assay [ELISA])and neutralizing antibodies (SARS-CoV-2 nanoluciferase high-throughput neutralization assay, focus reduction neutralization test mNeonGreen and SARS-CoV-2 plaque-reduction neutralization testing assay) development.11,12 The geometric mean of both binding and neutralizing antibodies declined over time but persisted high as late as 119 days after the first burst of 100 mg dose.13 The same dose of the vaccine also elicited a strong T helper-1 response with little T helper-2 response across all ages.11 The strength of the memory cellular response remains to be defined and is the subject of ongoing investigations. In a large phase 3 clinical trial with 30,420 participants, the Moderna COVID-19 mRNA-1273 vaccine, given 28 days apart at the dose of 100 mg, met 94.1% clinical efficacy in reducing the likelihood of being affected by symptomatic COVID-19.14
Less than 0.1% of volunteers in both groups withdrew from the trial due to adverse effects (AEs); 0.5% in the placebo group and 0.3% in the treated group had AEs after the first dose, which precluded receiving the second dose.14
The Johnson and Johnson/Jannsen JNJ-78436735 vaccine is based upon a recombinant, replication-incompetent adenovirus serotype 26 (Ad26) vector, which encodes the full-length, stabilized S-glycoprotein of SARS-CoV-2. The currently reported results of the phase 1 and 2 clinical study indicated that 805 volunteers (402 participants between ages 18 and 55 years and 403 individuals aged ≥ 65 years) were randomized to receive a single or double dose of either 5 x 1010 viral particles per 0.5 mL (low dose) or 1 x 1011 viral particles per 0.5 mL (high dose), each compared with a placebo group. Incidence of seroconversion to binding antibodies against the full-length stabilized S-glycoprotein, as measured by ELISA, showed ≥ 96% seroconversion by day 29 after the first dose. The incidence of seroconversion to neutralizing antibodies was ≥ 90% as early as early as 29 days after the first of either dose. In this study, neutralization activity was measured using the wild-type virus microneutralization assay based on the Victoria/1/2020/ SARS-CoV-2 strain.15 We note that the data related to this study have been partially reported and additional information will be available when each participant will have received the second dose.
In a large phase 3 clinical trial with 40,000 participants aged between 18 and 100 years, the Johnson and Johnson/Jannsen JNJ-78436735 vaccine, given as single dose of 5 x 1010 viral particles per 0.5 mL, met 65.5% clinical efficacy in the likelihood of being affected by symptomatic COVID-19 ≥ 28 days postimmunization.16 In this study, the vaccine efficacy was found to have a geographic distribution with highest efficacy in the US (74.4%), followed by Latin America (64.7%) where Brazil showed a predominance of the P2 COVID-19 lineage (64.7%), and Africa (52%) where the B.1.351 lineage was most frequent (94.5%). The vaccine also proved to be effective in reducing the likelihood of asymptomatic seroconversion, as measured by the level of a non-S protein, eg, 0.7% of positive cases in the vaccine group vs 2.8% in the placebo group. Immunological data indicated that the vaccine response was mainly driven by T-helper 1 lymphocytes. As of April 13, 2021 the FDA has recommend suspending the administration of the Johnson and Johnson/Janssen vaccine due to the occurrence of severe blood clots reported in a 6 subjects out of ~6.8 millions administered doses.1
It is noteworthy to highlight that all vaccines reduced the likelihood of hospitalizations and deaths due to COVID-19.
As of April 17, 2021, the CDC reports that more than 130 million (40%) Americans, nearly 1/3 of the population, have received at least 1 dose of any of the 3 available vaccines, including 4.6 million at the VHA.17 Using the Vaccine Adverse Event Reporting System and v-safe, the US is conducting what has been defined the most “intense and comprehensive safety monitoring in the US history.”18 Thus far, data affirm the overall safety of the available vaccines against COVID-19. Individuals should not receive the COVID-19 vaccines if they have had a severe allergic reaction to any ingredient in the vaccine or a severe allergic reaction to a prior dose of the vaccine. Additionally, individuals who have received convalescent plasma should wait 90 days before getting the COVID-19 vaccine.
Vaccination for Persons with MS
PwMS or those on immunosuppressive medications were excluded from the clinical trial led by Pfizer-BioNTech. There is no mention of MS as comorbidity in the study from Moderna, although this condition is not listed as an exclusion criterion either. The results of the phase 3 clinical trial for the Johnson and Johnson/Janssen vaccine are not fully public yet, thus this information is not known as well. As a result, the use of this vaccine in pwMS under immunomodulatory agents is based on previous knowledge of other vaccines. Evidence is growing for the safety of the BNT162b2 COVID-19 vaccination in pwMS.19 Data regarding COVID-19 efficacy and safety are still largely based on previous knowledge on other vaccines.20,21
Immunization of pwMS is considered safe and should proceed with confidence in those persons who have no other contraindication to receive a vaccine. A fundamental problem for pwMS treated with immunomodulatory or immunosuppressive medications is whether the vaccine will remain safe or be able to solicit an adequate immune response.20,21 As of the time of publication 2021, there is consensus that mRNA based or inactivated vaccines are also considered safe in pwMS undergoing immunomodulatory or immunosuppressive treatments.20-23 We advise a one-on-one conversation between each veteran with MS and their primary neurologist to understand the importance of the vaccination, the minimal risks associated with it and if any specific treatment modification should be made.
To provide guidance, the National MS Society released a position statement that is regularly updated.22 Given the risks associated with discontinuation of disease modifying agents, pwMS opting to receive a COVID-19 vaccine should continue taking their medications unless recommended otherwise by their primary neurologist. In addition, on the basis of available literature and the American Academy of Neurology recommendations on the use of vaccines in general, the following recommendations are proposed.20-23
Recommendation 1: injections, orals, and natalizumab. Given the risks associated with discontinuation of disease modifying agents, pwMS opting to receive a COVID-19 vaccine should continue taking their medications unless recommended otherwise by their primary neurologist. Neither delay in start nor adjustments in dosing or timing of administration are advised for pwMS taking currently available either generic or brand formulations of β interferons, glatiramer acetate, teriflunomide, dimethyl or monomethyl fumarate, or natalizumab.22
Recommendation 2: anti-CD20 monoclonal infusions. As an attenuated humoral response is predicted in pwMS treated with anti-CD20 monoclonal infusions, coordinating the timing of vaccination with treatment schedule may maximize efficacy of the vaccine. Whenever possible, it is advised to be vaccinated ≥ 12 weeks after the last infusion and to resume infusion 4 weeks after the last dose of the vaccine. PwMS starting anti-CD20 monoclonal infusions are advised to be fully vaccinated first and start these medications ≥ 2 to 4 weeks later.22
Recommendation 3: alemtuzumab infusion. Given its effect on CD52+ cells, it is advised to be vaccinated ≥ 24 weeks after the last infusion and to resume infusion 4 weeks after the last dose of the vaccine. PwMS starting alemtuzumab infusions are advised to get fully vaccinated first and start this medication 4 weeks or more after completing the vaccine.22
Recommendation 4: sphingosine 1 phosphate receptor modulators, oral cladribine, and ofatumumab. PwMS starting any of these medications are advised to be fully vaccinated first and start these medications 2 to 4 weeks after completing the vaccine. PwMS already on those medications are not advised to change the schedule of administration. When possible, though, one should resume the dose of cladribine or ofatumumab 2 to 4 weeks after the last dose of the vaccine. 20
Notably, all these recommendations hold true when there is enough disease stability to allow delaying treatment. We also add that it remains unclear if persons with an overall very low number of lymphocytes will be able to elicit a strong reaction to the vaccine. Blood collection and analysis of white blood cell count and lymphocyte subset estimates should be obtained in those persons with a markedly suppressed immune system. Whenever possible, to maximize outcome, timing the vaccination with treatment should be considered in those persons with a markedly reduced number of T-helper 1 cells.
Vaccination for Veterans
Currently the VHA is offering to veterans the Pfizer and Moderna COVID-19 vaccines with FDA EUAs. In accordance with FDA regulations, the VHA has paused administration of the Johnson and Johnson/Janssen vaccine. The VHA has launched its vaccination program in December 2020 by first providing the vaccine to health care personnel, nursing home patients, spinal cord injury patients, chemotherapy patients, dialysis and transplant patients, as well as homeless veterans. Most VA health care systems have passed this phase and are now able to provide vaccines to veterans with MS.
In December 2020, the MSCoE released a position statement regarding the importance and safety of the COVID-19 vaccine for veterans with MS.24 This statement will be updated on a regular basis as new information becomes available from major organizations like the National MS Society, FDA, CDC, and World Health Organization (WHO) or relevant literature.
Conclusions
Older veterans with progressive MS and associated comorbidities are at higher risk of death should they be infected by COVID-19. Fortunately, we live in a time where vaccines are recognized as a critical tool to prevent this infection and to significantly reduce its morbidity and mortality. Yet, hesitancy to vaccinate has been identified as one of the most important threats to public health by the WHO in 2019.25 Understandably such hesitancy is even more profound for the COVID-19 vaccine, which is being administered under an EUA. In light of this indecision, and given the current state of the pandemic, we urge health care providers to educate every veteran about the benefits of being vaccinated against COVID-19. Within the VHA, a solid campaign of vaccination has been put in place at an unprecedented speed.
Health care providers interacting with veterans with MS are encouraged to use the MSCoE website (www.va.gov/ms) for any questions or concerns, or to reach out to MSCoE staff. It is vitally important that our community of veterans receives appropriate education on the importance of this vaccination for their own safety, for that of their household and society.
This article has been updated to reflect new US Food and Drug Administration and Centers for Disease Control and Prevention recommendations to pause administration of the Johnson and Johnson Jansen (JNJ-78436735) COVID-19 vaccine.1
Since the outbreak of the pandemic caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2),a plethora of studies have been performed to increase our knowledge of its associated illness COVID-19.2 There is no cure for COVID-19, which can be lethal. In the absence of a cure, preventive measures are of vital importance. In order to help prevent the spread of the virus, the Centers for Diseases Control and Prevention (CDC) advocates for: (1) the use of a face mask over the mouth and nose; (2) a minimum of 6-foot distance between individuals; and (3) avoidance of gatherings.As of March 2021, the US Food and Drug Administration (FDA) approved 3 vaccines for the prevention of COVID-19, under an emergency use authorization (EUA).3-5
COVID-19 and Multiple Sclerosis
Since the beginning of the pandemic, neurologists have faced a new challenge—determining whether persons with multiple sclerosis (pwMS) were more at risk than others of becoming ill from COVID-19 or were destined for a worse outcome. The National MS Society has advised a personalized approach in relation to particularly vulnerable persons when needed and has also initiated worldwide registries to collect information regarding incidence and outcome of COVID-19 in pwMS. Accordingly, through the MS Center of Excellence (MSCoE), the Veterans Health Administration (VHA) has established a national registry assembling data regarding COVID-19 in veterans with MS.
A recent descriptive literature review summarized the outcomes of 873 persons with both MS and COVID-19 and reported that about 36% of COVID-19 cases were treated with B-cell depleting therapies (ocrelizumab or rituximab).6 This proportion was relatively higher when compared with other disease modifying agents. Of those who became infected with SARS-CoV-2, death from COVID-19 occurred in about 4%, and an additional 3% required assisted invasive or noninvasive ventilation. Persons reported to have passed away from COVID-19 generally were older; had progressive MS; or had associated comorbidities such as obesity, hypertension, heart or lung conditions, or cancers. Of these, 50% were not on any disease modifying agent, 25% were on B-cell depleting therapies (ocrelizumab or rituximab), and the remaining 25% were on various medications for MS. It is important to highlight that no formal statistical analyses were performed in this review. On the contrary, in the recently published Italian report on 844 pwMS who had suspected or confirmed COVID-19, the authors used univariate and multivariate models to analyze their findings and noted that the use of ocrelizumab was significantly associated with a worse clinical outcome.7 These authors also identified age, sex, disability score, and recent (within 1 month) use of steroids as risk factors for a severe COVID-19 outcome. The incidence of death from COVID-19 in this cohort was 1.54%.
The recently published data from the North American Registry of the National MS Society based on 1,626 patients reported a 3.3% incidence of death from COVID-19.8 The following factors were identified as risks for worse outcome: male sex, nonambulatory status, age, Black race, and cardiovascular disease. The use of rituximab, ocrelizumab, and steroids (the latter medication over the preceding 2 months) increased the risks of hospitalization for COVID-19.
COVID-19 Vaccines
Of the 3 available vaccines, the Pfizer-BioNTech COVID-19 (BNT162b2) vaccine is approved for individuals aged ≥ 16 years, while the Moderna COVID-19 (mRNA-1273) and the Johnson and Johnson/Jannsen COVID-19 (JNJ-78436735) vaccines are approved for individuals aged ≥ 18 years, though the latter vaccine has been temporarily suspended.1,3-5 The EUAs were released following the disclosure of the results of 3 phase 3 clinical trials and several phase 1 and 2 clinical trials.9-16
The BNT162b2 vaccine from Pfizer-BioNTech encodes the SARS-CoV-2 full-length spike protein (S) in prefusion conformation locked by the mutation in 2 prolines.9 Differently from the BNT162b2 vaccine, the BNT162b1 vaccine encodes a secreted trimerized SARS-CoV-2 receptor–binding domain. The S-glycoprotein is required for viral entry, as implicated in host cell attachment, and is the target of the neutralizing antibodies. In a phase 1 clinical study on 195 volunteers treated with BNT162b1 (10 mg, 20 mg, 30 mg, or 100 mg doses) or BNT162b2 (10 mg, 20 mg, or 30 mg doses) vaccines or placebo 21 days apart, both the binding and neutralizing antibody response was found to be age and “somewhat” dose dependent.9
Higher neutralization titers were measured at day 28 and 35 (7 and 14 days after the second dose, respectively) and compared with titers of persons who recovered from a COVID-19 infection.9 Serum neutralization was measured using a fluorescence-based high-throughput neutralization assay, while binding activity was assessed using the receptor-binding domain (RBD)–binding or S1-binding IgG direct Luminex immunoassays.
The overall reactogenicity/immunogenicity profile of BNT162b2 administered twice (30 mg each time) led to its selection for the phase 3 clinical trial.9,10 In a large phase 3 clinical trial on 43,458 participants, the BNT162b2 vaccine given at 30 mg doses 21 days apart conferred 95% clinical efficacy in reducing the likelihood of being affected by symptomatic COVID-19.10 No safety concerns to stop the trial were identified, though related severe and life-threatening events were reported in 0.3% and 0.1% of the volunteers, respectively. We note that these incidence rates were the same for the treated and the placebo group.
The mRNA-1273 vaccine from Moderna also encodes the SARS-CoV-2 S-glycoprotein. In a dose escalation phase 1 trial of 45 participants aged between 18 and 55 years (25 mg, 100 mg or 250 mg, given at days 1 and 29) and 40 participants aged ≥ 57 years (25 mg and 100 mg, given at days 1 and 29), a dose-dependent effect was observed for both binding (receptor-binding domain and S-2p IgG on enzyme-linked immunosorbent assay [ELISA])and neutralizing antibodies (SARS-CoV-2 nanoluciferase high-throughput neutralization assay, focus reduction neutralization test mNeonGreen and SARS-CoV-2 plaque-reduction neutralization testing assay) development.11,12 The geometric mean of both binding and neutralizing antibodies declined over time but persisted high as late as 119 days after the first burst of 100 mg dose.13 The same dose of the vaccine also elicited a strong T helper-1 response with little T helper-2 response across all ages.11 The strength of the memory cellular response remains to be defined and is the subject of ongoing investigations. In a large phase 3 clinical trial with 30,420 participants, the Moderna COVID-19 mRNA-1273 vaccine, given 28 days apart at the dose of 100 mg, met 94.1% clinical efficacy in reducing the likelihood of being affected by symptomatic COVID-19.14
Less than 0.1% of volunteers in both groups withdrew from the trial due to adverse effects (AEs); 0.5% in the placebo group and 0.3% in the treated group had AEs after the first dose, which precluded receiving the second dose.14
The Johnson and Johnson/Jannsen JNJ-78436735 vaccine is based upon a recombinant, replication-incompetent adenovirus serotype 26 (Ad26) vector, which encodes the full-length, stabilized S-glycoprotein of SARS-CoV-2. The currently reported results of the phase 1 and 2 clinical study indicated that 805 volunteers (402 participants between ages 18 and 55 years and 403 individuals aged ≥ 65 years) were randomized to receive a single or double dose of either 5 x 1010 viral particles per 0.5 mL (low dose) or 1 x 1011 viral particles per 0.5 mL (high dose), each compared with a placebo group. Incidence of seroconversion to binding antibodies against the full-length stabilized S-glycoprotein, as measured by ELISA, showed ≥ 96% seroconversion by day 29 after the first dose. The incidence of seroconversion to neutralizing antibodies was ≥ 90% as early as early as 29 days after the first of either dose. In this study, neutralization activity was measured using the wild-type virus microneutralization assay based on the Victoria/1/2020/ SARS-CoV-2 strain.15 We note that the data related to this study have been partially reported and additional information will be available when each participant will have received the second dose.
In a large phase 3 clinical trial with 40,000 participants aged between 18 and 100 years, the Johnson and Johnson/Jannsen JNJ-78436735 vaccine, given as single dose of 5 x 1010 viral particles per 0.5 mL, met 65.5% clinical efficacy in the likelihood of being affected by symptomatic COVID-19 ≥ 28 days postimmunization.16 In this study, the vaccine efficacy was found to have a geographic distribution with highest efficacy in the US (74.4%), followed by Latin America (64.7%) where Brazil showed a predominance of the P2 COVID-19 lineage (64.7%), and Africa (52%) where the B.1.351 lineage was most frequent (94.5%). The vaccine also proved to be effective in reducing the likelihood of asymptomatic seroconversion, as measured by the level of a non-S protein, eg, 0.7% of positive cases in the vaccine group vs 2.8% in the placebo group. Immunological data indicated that the vaccine response was mainly driven by T-helper 1 lymphocytes. As of April 13, 2021 the FDA has recommend suspending the administration of the Johnson and Johnson/Janssen vaccine due to the occurrence of severe blood clots reported in a 6 subjects out of ~6.8 millions administered doses.1
It is noteworthy to highlight that all vaccines reduced the likelihood of hospitalizations and deaths due to COVID-19.
As of April 17, 2021, the CDC reports that more than 130 million (40%) Americans, nearly 1/3 of the population, have received at least 1 dose of any of the 3 available vaccines, including 4.6 million at the VHA.17 Using the Vaccine Adverse Event Reporting System and v-safe, the US is conducting what has been defined the most “intense and comprehensive safety monitoring in the US history.”18 Thus far, data affirm the overall safety of the available vaccines against COVID-19. Individuals should not receive the COVID-19 vaccines if they have had a severe allergic reaction to any ingredient in the vaccine or a severe allergic reaction to a prior dose of the vaccine. Additionally, individuals who have received convalescent plasma should wait 90 days before getting the COVID-19 vaccine.
Vaccination for Persons with MS
PwMS or those on immunosuppressive medications were excluded from the clinical trial led by Pfizer-BioNTech. There is no mention of MS as comorbidity in the study from Moderna, although this condition is not listed as an exclusion criterion either. The results of the phase 3 clinical trial for the Johnson and Johnson/Janssen vaccine are not fully public yet, thus this information is not known as well. As a result, the use of this vaccine in pwMS under immunomodulatory agents is based on previous knowledge of other vaccines. Evidence is growing for the safety of the BNT162b2 COVID-19 vaccination in pwMS.19 Data regarding COVID-19 efficacy and safety are still largely based on previous knowledge on other vaccines.20,21
Immunization of pwMS is considered safe and should proceed with confidence in those persons who have no other contraindication to receive a vaccine. A fundamental problem for pwMS treated with immunomodulatory or immunosuppressive medications is whether the vaccine will remain safe or be able to solicit an adequate immune response.20,21 As of the time of publication 2021, there is consensus that mRNA based or inactivated vaccines are also considered safe in pwMS undergoing immunomodulatory or immunosuppressive treatments.20-23 We advise a one-on-one conversation between each veteran with MS and their primary neurologist to understand the importance of the vaccination, the minimal risks associated with it and if any specific treatment modification should be made.
To provide guidance, the National MS Society released a position statement that is regularly updated.22 Given the risks associated with discontinuation of disease modifying agents, pwMS opting to receive a COVID-19 vaccine should continue taking their medications unless recommended otherwise by their primary neurologist. In addition, on the basis of available literature and the American Academy of Neurology recommendations on the use of vaccines in general, the following recommendations are proposed.20-23
Recommendation 1: injections, orals, and natalizumab. Given the risks associated with discontinuation of disease modifying agents, pwMS opting to receive a COVID-19 vaccine should continue taking their medications unless recommended otherwise by their primary neurologist. Neither delay in start nor adjustments in dosing or timing of administration are advised for pwMS taking currently available either generic or brand formulations of β interferons, glatiramer acetate, teriflunomide, dimethyl or monomethyl fumarate, or natalizumab.22
Recommendation 2: anti-CD20 monoclonal infusions. As an attenuated humoral response is predicted in pwMS treated with anti-CD20 monoclonal infusions, coordinating the timing of vaccination with treatment schedule may maximize efficacy of the vaccine. Whenever possible, it is advised to be vaccinated ≥ 12 weeks after the last infusion and to resume infusion 4 weeks after the last dose of the vaccine. PwMS starting anti-CD20 monoclonal infusions are advised to be fully vaccinated first and start these medications ≥ 2 to 4 weeks later.22
Recommendation 3: alemtuzumab infusion. Given its effect on CD52+ cells, it is advised to be vaccinated ≥ 24 weeks after the last infusion and to resume infusion 4 weeks after the last dose of the vaccine. PwMS starting alemtuzumab infusions are advised to get fully vaccinated first and start this medication 4 weeks or more after completing the vaccine.22
Recommendation 4: sphingosine 1 phosphate receptor modulators, oral cladribine, and ofatumumab. PwMS starting any of these medications are advised to be fully vaccinated first and start these medications 2 to 4 weeks after completing the vaccine. PwMS already on those medications are not advised to change the schedule of administration. When possible, though, one should resume the dose of cladribine or ofatumumab 2 to 4 weeks after the last dose of the vaccine. 20
Notably, all these recommendations hold true when there is enough disease stability to allow delaying treatment. We also add that it remains unclear if persons with an overall very low number of lymphocytes will be able to elicit a strong reaction to the vaccine. Blood collection and analysis of white blood cell count and lymphocyte subset estimates should be obtained in those persons with a markedly suppressed immune system. Whenever possible, to maximize outcome, timing the vaccination with treatment should be considered in those persons with a markedly reduced number of T-helper 1 cells.
Vaccination for Veterans
Currently the VHA is offering to veterans the Pfizer and Moderna COVID-19 vaccines with FDA EUAs. In accordance with FDA regulations, the VHA has paused administration of the Johnson and Johnson/Janssen vaccine. The VHA has launched its vaccination program in December 2020 by first providing the vaccine to health care personnel, nursing home patients, spinal cord injury patients, chemotherapy patients, dialysis and transplant patients, as well as homeless veterans. Most VA health care systems have passed this phase and are now able to provide vaccines to veterans with MS.
In December 2020, the MSCoE released a position statement regarding the importance and safety of the COVID-19 vaccine for veterans with MS.24 This statement will be updated on a regular basis as new information becomes available from major organizations like the National MS Society, FDA, CDC, and World Health Organization (WHO) or relevant literature.
Conclusions
Older veterans with progressive MS and associated comorbidities are at higher risk of death should they be infected by COVID-19. Fortunately, we live in a time where vaccines are recognized as a critical tool to prevent this infection and to significantly reduce its morbidity and mortality. Yet, hesitancy to vaccinate has been identified as one of the most important threats to public health by the WHO in 2019.25 Understandably such hesitancy is even more profound for the COVID-19 vaccine, which is being administered under an EUA. In light of this indecision, and given the current state of the pandemic, we urge health care providers to educate every veteran about the benefits of being vaccinated against COVID-19. Within the VHA, a solid campaign of vaccination has been put in place at an unprecedented speed.
Health care providers interacting with veterans with MS are encouraged to use the MSCoE website (www.va.gov/ms) for any questions or concerns, or to reach out to MSCoE staff. It is vitally important that our community of veterans receives appropriate education on the importance of this vaccination for their own safety, for that of their household and society.
1. Centers for Disease Control and Prevention. Recommendation to pause use of Johnson & Johnson’s Janssen COVID-19 vaccine. Updated April 16, 2021. Accessed April 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/JJUpdate.html
2. World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it. Accessed March 9, 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it
3. US Food and Drug Administration. Pfizer-BioNTech COVID-19 vaccine. Updated February 3, 2021. Accessed March 22, 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine
4. US Food and Drug Administration. Moderna COVID-19 vaccine. Updated February 3, 2021. Accessed March 22, 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccine
5. US Food and Drug Administration. FDA issues emergency use authorization for third COVID-19 vaccine [press release]. Published February 27, 2021. Accessed March 22, 2021. https://www.fda.gov/news-events/press-announcements/fda-issues-emergency-use-authorization-third-covid-19-vaccine
6. Möhn N, Konen FF, Pul R, et al. Experience in multiple sclerosis patients with COVID-19 and disease-modifying therapies: a review of 873 published cases. J Clin Med. 2020;9(12):4067. Published 2020 Dec 16. doi:10.3390/jcm9124067
7. Sormani MP, De Rossi N, Schiavetti I, et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol. 2021;89(4):780-789. doi:10.1002/ana.26028
8. Salter A, Fox RJ, Newsome SD, et al. Outcomes and risk factors associated with SARS-CoV-2 infection in a North American registry of patients with multiple sclerosis [published online ahead of print, 2021 Mar 19]. JAMA Neurol. 2021;10.1001/jamaneurol.2021.0688. doi:10.1001/jamaneurol.2021.0688
9. Walsh EE, Frenck RW Jr, Falsey AR, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439-2450. doi:10.1056/NEJMoa2027906
10. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi:10.1056/NEJMoa2034577
11. Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2 - preliminary Report. N Engl J Med. 2020;383(20):1920-1931. doi:10.1056/NEJMoa2022483
12. Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383(25):2427-2438. doi:10.1056/NEJMoa2028436
13. Widge AT, Rouphael NG, Jackson LA, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med. 2021;384(1):80-82. doi:10.1056/NEJMc2032195
14. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. doi:10.1056/NEJMoa2035389
15. Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a phase 1-2a Trial of Ad26.COV2.S Covid-19 Vaccine [published online ahead of print, 2021 Jan 13]. N Engl J Med. 2021;NEJMoa2034201. doi:10.1056/NEJMoa2034201
16. Oliver SE, Gargano JW, Scobie H, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Janssen COVID-19 vaccine - United States, February 2021. MMWR Morb Mortal Wkly Rep. 2021;70(9):329-332. Published 2021 Mar 5. doi:10.15585/mmwr.mm7009e4
17. US Centers for Disease Control and Prevention. COVID-19 vaccinations in the United States. Updated March 21, 2021. Accessed March 22, 2021. https://covid.cdc.gov/covid-data-tracker/#vaccinations
18. Gee J, Marquez P, Su J, et al. First month of COVID-19 vaccine safety monitoring - United States, December 14, 2020-January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(8):283-288. Published 2021 Feb 26. doi:10.15585/mmwr.mm7008e3
19. Achiron A, Dolev M, Menascu S, et al. COVID-19 vaccination in patients with multiple sclerosis: What we have learnt by February 2021 [published online ahead of print, 2021 Apr 15]. Mult Scler. 2021;13524585211003476. doi:10.1177/13524585211003476
20. Righi E, Gallo T, Azzini AM, et al. A review of vaccinations in adult patients with secondary immunodeficiency [published online ahead of print, 2021 Mar 9]. Infect Dis Ther. 2021;1-25. doi:10.1007/s40121-021-00404-y
21. Ciotti JR, Valtcheva MV, Cross AH. Effects of MS disease-modifying therapies on responses to vaccinations: A review. Mult Scler Relat Disord. 2020;45:102439. doi:10.1016/j.msard.2020.102439
22. National Multiple Sclerosis Society. COVID-19 vaccine guidance for people living with MS. Accessed March 22, 2021. https://www.nationalmssociety.org/coronavirus-covid-19-information/multiple-sclerosis-and-coronavirus/covid-19-vaccine-guidance
23. Farez MF, Correale J, Armstrong MJ, et al. Practice guideline update summary: vaccine-preventable infections and immunization in multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2019;93(13):584-594. doi:10.1212/WNL.0000000000008157
24. US Department of Veterans Affairs, Multiple Sclerosis Centers of Excellence. Coronavirus (COVID-19) and vaccine information. Updated February 25. 2021. Accessed March 9, 2021. https://www.va.gov/ms
25. World Health Organization. Ten threats to global health in 2019. Accessed March 18, 2021. https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019.
1. Centers for Disease Control and Prevention. Recommendation to pause use of Johnson & Johnson’s Janssen COVID-19 vaccine. Updated April 16, 2021. Accessed April 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/JJUpdate.html
2. World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it. Accessed March 9, 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it
3. US Food and Drug Administration. Pfizer-BioNTech COVID-19 vaccine. Updated February 3, 2021. Accessed March 22, 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine
4. US Food and Drug Administration. Moderna COVID-19 vaccine. Updated February 3, 2021. Accessed March 22, 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccine
5. US Food and Drug Administration. FDA issues emergency use authorization for third COVID-19 vaccine [press release]. Published February 27, 2021. Accessed March 22, 2021. https://www.fda.gov/news-events/press-announcements/fda-issues-emergency-use-authorization-third-covid-19-vaccine
6. Möhn N, Konen FF, Pul R, et al. Experience in multiple sclerosis patients with COVID-19 and disease-modifying therapies: a review of 873 published cases. J Clin Med. 2020;9(12):4067. Published 2020 Dec 16. doi:10.3390/jcm9124067
7. Sormani MP, De Rossi N, Schiavetti I, et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol. 2021;89(4):780-789. doi:10.1002/ana.26028
8. Salter A, Fox RJ, Newsome SD, et al. Outcomes and risk factors associated with SARS-CoV-2 infection in a North American registry of patients with multiple sclerosis [published online ahead of print, 2021 Mar 19]. JAMA Neurol. 2021;10.1001/jamaneurol.2021.0688. doi:10.1001/jamaneurol.2021.0688
9. Walsh EE, Frenck RW Jr, Falsey AR, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439-2450. doi:10.1056/NEJMoa2027906
10. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi:10.1056/NEJMoa2034577
11. Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2 - preliminary Report. N Engl J Med. 2020;383(20):1920-1931. doi:10.1056/NEJMoa2022483
12. Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383(25):2427-2438. doi:10.1056/NEJMoa2028436
13. Widge AT, Rouphael NG, Jackson LA, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med. 2021;384(1):80-82. doi:10.1056/NEJMc2032195
14. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. doi:10.1056/NEJMoa2035389
15. Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a phase 1-2a Trial of Ad26.COV2.S Covid-19 Vaccine [published online ahead of print, 2021 Jan 13]. N Engl J Med. 2021;NEJMoa2034201. doi:10.1056/NEJMoa2034201
16. Oliver SE, Gargano JW, Scobie H, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Janssen COVID-19 vaccine - United States, February 2021. MMWR Morb Mortal Wkly Rep. 2021;70(9):329-332. Published 2021 Mar 5. doi:10.15585/mmwr.mm7009e4
17. US Centers for Disease Control and Prevention. COVID-19 vaccinations in the United States. Updated March 21, 2021. Accessed March 22, 2021. https://covid.cdc.gov/covid-data-tracker/#vaccinations
18. Gee J, Marquez P, Su J, et al. First month of COVID-19 vaccine safety monitoring - United States, December 14, 2020-January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(8):283-288. Published 2021 Feb 26. doi:10.15585/mmwr.mm7008e3
19. Achiron A, Dolev M, Menascu S, et al. COVID-19 vaccination in patients with multiple sclerosis: What we have learnt by February 2021 [published online ahead of print, 2021 Apr 15]. Mult Scler. 2021;13524585211003476. doi:10.1177/13524585211003476
20. Righi E, Gallo T, Azzini AM, et al. A review of vaccinations in adult patients with secondary immunodeficiency [published online ahead of print, 2021 Mar 9]. Infect Dis Ther. 2021;1-25. doi:10.1007/s40121-021-00404-y
21. Ciotti JR, Valtcheva MV, Cross AH. Effects of MS disease-modifying therapies on responses to vaccinations: A review. Mult Scler Relat Disord. 2020;45:102439. doi:10.1016/j.msard.2020.102439
22. National Multiple Sclerosis Society. COVID-19 vaccine guidance for people living with MS. Accessed March 22, 2021. https://www.nationalmssociety.org/coronavirus-covid-19-information/multiple-sclerosis-and-coronavirus/covid-19-vaccine-guidance
23. Farez MF, Correale J, Armstrong MJ, et al. Practice guideline update summary: vaccine-preventable infections and immunization in multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2019;93(13):584-594. doi:10.1212/WNL.0000000000008157
24. US Department of Veterans Affairs, Multiple Sclerosis Centers of Excellence. Coronavirus (COVID-19) and vaccine information. Updated February 25. 2021. Accessed March 9, 2021. https://www.va.gov/ms
25. World Health Organization. Ten threats to global health in 2019. Accessed March 18, 2021. https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019.
Assessing Risk for Amputation Patients During a Pandemic
Risk assessment becomes more complex during a pandemic—but even more necessary. Researchers from Virginia Commonwealth University and Hunter Holmes McGuire Veterans Afffairs Medical Center who studied a population of veterans who underwent leg amputation found that “preoperative testing may not be a feasible and well-applied standard, making risk assessment in the setting of a pandemic even more crucial for surgeons undertaking lower extremity amputations in this high-risk population.”
In their study, the researchers found that a majority of the patients had one or more risk factor from the list published by the European Centre for Disease Prevention and Control (ECDC). What’s more, based on their data, the researchers say veteran amputees are at a much higher risk for complications and negative outcomes if infected with COVID-19, compared with the general population.
Of 50,083 veterans who needed nontraumatic lower extremity amputations between 1999 and 2018, 82% of those with above-knee amputations and 89% of those with below-knee amputations had at least one ECDC risk factor comorbidity. Hypertension and diabetes were the two most prevalent conditions in all cohorts, regardless of race.
Between 40% and 50% of the patients studied were current or past smokers, “well beyond the prevalence of smoking” in the general US population,” the researchers say. One quarter of the veterans were Black. That also is a greater proportion than the proportion of Black patients in the national male veteran population; race is an “especially concerning” potential COVID-19 progression factor, the researchers say.
A year after the COVID-19 pandemic began, the researchers examined the association of Risk Analysis Index scores with postoperative outcomes in 47,197 patients who underwent lower extremity amputation: 27,098 below the knee and 20,099 above the knee amputations.
Frailty was associated with increased rates of major cardiac, pulmonary, and renal complications, as well as sepsis, intubation greater than 48 hours, reintubation, and increased length of stay. Higher frailty scores were associated with up to triple the likelihood of a postoperative complication and up to 32 times likelihood of death within 30 days.
In a previous study, the researchers concluded that standardized frailty indicators might be particularly relevant in a pandemic that has a heavy impact in elderly patients with comorbidities. The risk factors for COVID-19, they note, are similar to many of the factors assessed in surgical frailty scores. Surgical frailty and its assessment, they add, have become “essential considerations” in perioperative management for aging patients.
Risk assessment becomes more complex during a pandemic—but even more necessary. Researchers from Virginia Commonwealth University and Hunter Holmes McGuire Veterans Afffairs Medical Center who studied a population of veterans who underwent leg amputation found that “preoperative testing may not be a feasible and well-applied standard, making risk assessment in the setting of a pandemic even more crucial for surgeons undertaking lower extremity amputations in this high-risk population.”
In their study, the researchers found that a majority of the patients had one or more risk factor from the list published by the European Centre for Disease Prevention and Control (ECDC). What’s more, based on their data, the researchers say veteran amputees are at a much higher risk for complications and negative outcomes if infected with COVID-19, compared with the general population.
Of 50,083 veterans who needed nontraumatic lower extremity amputations between 1999 and 2018, 82% of those with above-knee amputations and 89% of those with below-knee amputations had at least one ECDC risk factor comorbidity. Hypertension and diabetes were the two most prevalent conditions in all cohorts, regardless of race.
Between 40% and 50% of the patients studied were current or past smokers, “well beyond the prevalence of smoking” in the general US population,” the researchers say. One quarter of the veterans were Black. That also is a greater proportion than the proportion of Black patients in the national male veteran population; race is an “especially concerning” potential COVID-19 progression factor, the researchers say.
A year after the COVID-19 pandemic began, the researchers examined the association of Risk Analysis Index scores with postoperative outcomes in 47,197 patients who underwent lower extremity amputation: 27,098 below the knee and 20,099 above the knee amputations.
Frailty was associated with increased rates of major cardiac, pulmonary, and renal complications, as well as sepsis, intubation greater than 48 hours, reintubation, and increased length of stay. Higher frailty scores were associated with up to triple the likelihood of a postoperative complication and up to 32 times likelihood of death within 30 days.
In a previous study, the researchers concluded that standardized frailty indicators might be particularly relevant in a pandemic that has a heavy impact in elderly patients with comorbidities. The risk factors for COVID-19, they note, are similar to many of the factors assessed in surgical frailty scores. Surgical frailty and its assessment, they add, have become “essential considerations” in perioperative management for aging patients.
Risk assessment becomes more complex during a pandemic—but even more necessary. Researchers from Virginia Commonwealth University and Hunter Holmes McGuire Veterans Afffairs Medical Center who studied a population of veterans who underwent leg amputation found that “preoperative testing may not be a feasible and well-applied standard, making risk assessment in the setting of a pandemic even more crucial for surgeons undertaking lower extremity amputations in this high-risk population.”
In their study, the researchers found that a majority of the patients had one or more risk factor from the list published by the European Centre for Disease Prevention and Control (ECDC). What’s more, based on their data, the researchers say veteran amputees are at a much higher risk for complications and negative outcomes if infected with COVID-19, compared with the general population.
Of 50,083 veterans who needed nontraumatic lower extremity amputations between 1999 and 2018, 82% of those with above-knee amputations and 89% of those with below-knee amputations had at least one ECDC risk factor comorbidity. Hypertension and diabetes were the two most prevalent conditions in all cohorts, regardless of race.
Between 40% and 50% of the patients studied were current or past smokers, “well beyond the prevalence of smoking” in the general US population,” the researchers say. One quarter of the veterans were Black. That also is a greater proportion than the proportion of Black patients in the national male veteran population; race is an “especially concerning” potential COVID-19 progression factor, the researchers say.
A year after the COVID-19 pandemic began, the researchers examined the association of Risk Analysis Index scores with postoperative outcomes in 47,197 patients who underwent lower extremity amputation: 27,098 below the knee and 20,099 above the knee amputations.
Frailty was associated with increased rates of major cardiac, pulmonary, and renal complications, as well as sepsis, intubation greater than 48 hours, reintubation, and increased length of stay. Higher frailty scores were associated with up to triple the likelihood of a postoperative complication and up to 32 times likelihood of death within 30 days.
In a previous study, the researchers concluded that standardized frailty indicators might be particularly relevant in a pandemic that has a heavy impact in elderly patients with comorbidities. The risk factors for COVID-19, they note, are similar to many of the factors assessed in surgical frailty scores. Surgical frailty and its assessment, they add, have become “essential considerations” in perioperative management for aging patients.