User login
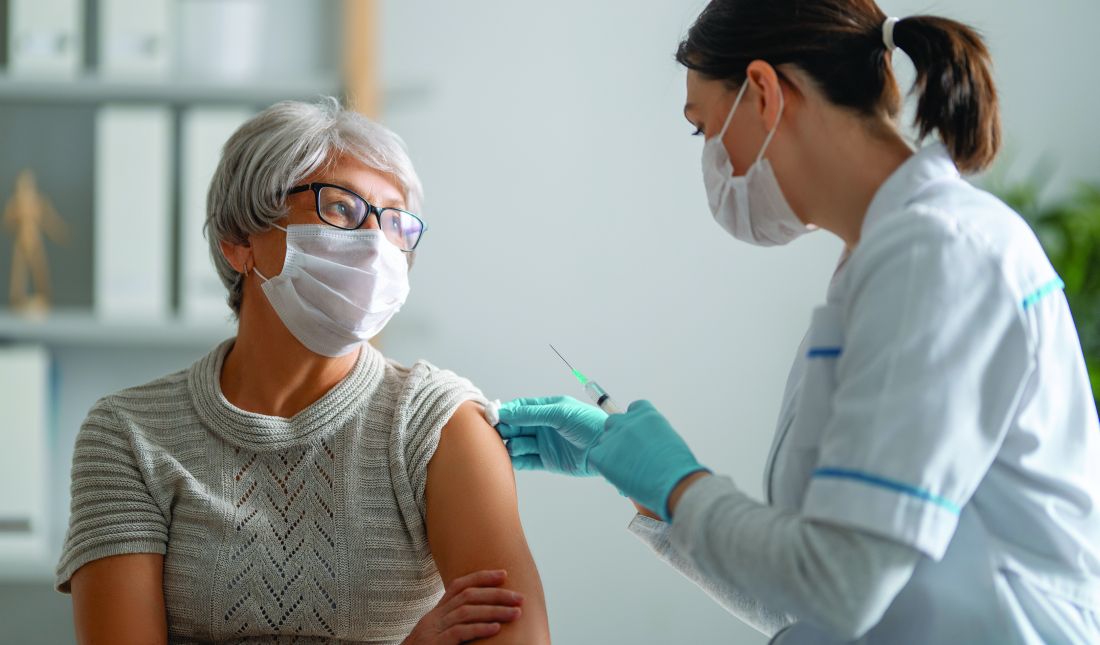
USPSTF expands criteria for lung cancer screening
“This is great news because it means that nearly twice as many people are eligible to be screened, which we hope will allow clinicians to save more lives and help people remain healthy longer,” commented John Wong, MD, chief science officer, vice chair for clinical affairs, and chief of the Division of Clinical Decision Making at USPSTF.
The updated final recommendations were published online on March 9 in JAMA.
The USPSTF recommends annual screening with low-dose CT for adults aged 50-80 years who have a 20 pack-year smoking history and currently smoke or have quit within the past 15 years.
This updates guidance issued in 2013, which recommended annual screening for lung cancer for adults aged 55-80 years who had a 30 pack-year smoking history and who were either current smokers or had quit within the past 15 years.
The move will nearly double the number of people are now eligible for screening, up to 14.5 million individuals – an increase of 81% (6.4 million adults) from the 2013 recommendations.
The expanded criteria may help increase screening among Black individuals and women. Data show that both groups tend to smoke fewer cigarettes than White men and that Black persons are at higher risk for lung cancer than White persons. In addition, research has shown that about one-third of Black patients with lung cancer were diagnosed before the age of 55 years, which means they would not have been recommended for screening under the previous guidelines.
Uptake has been limited
To date, uptake of lung cancer screening has been very limited, from 6% to 18% of individuals who meet the eligibility criteria.
The new recommendations will open up screening to many more people, but challenges to implementation remain.
“The science is clear that lung cancer screening has the potential to save lives,” Dr. Wong told this news organization. “We recognize that there are existing barriers to screening everyone who is eligible, but clinicians and patients both deserve to know that screening can detect lung cancer early, when treatment has the best chance of being beneficial.”
He added that the hope is that these recommendations will encourage clinicians to examine the barriers to effective lung cancer screening in their communities and to do what they can to improve implementation. “We also hope to encourage patients to have conversations with their clinicians about whether they are eligible for screening and to discuss smoking cessation treatments if they are still smoking,” Dr. Wong added.
In an accompanying editorial, Louise M. Henderson, PhD, M. Patricia Rivera, MD, FCCP, and Ethan Basch, MD, all from the University of North Carolina at Chapel Hill, address some of the current challenges in implementation.
They note that reimbursement for lung cancer screening by Medicare requires submission of data to a Centers for Medicare & Medicaid Services–approved registry, and this can present problems for facilities serving less affluent communities or that have limited resources.
Medicaid coverage is also uneven. As of September 2020, lung cancer screening was covered by 38 Medicaid programs, but not by 9. For three programs, data on coverage were not available.
“With the new recommendations lowering the screening-eligible age to 50 years, many eligible individuals who are uninsured or who are receiving Medicaid and living in states that do not cover screening will have financial barriers to undergo screening,” they write.
In addition, many individuals in at-risk populations lack adequate geographic access to comprehensive lung cancer screening programs.
Expanding eligibility criteria is important, the editorialists point out, but barriers to screening, which include lack of insurance coverage and limited physical access to high-quality screening programs, highlight the complex problems with implementation that need to be addressed.
“A concerted effort to increase the reach of lung cancer screening is needed,” they write. “The 2021 USPSTF recommendation statement represents a leap forward in evidence and offers promise to prevent more cancer deaths and address screening disparities. But the greatest work lies ahead to ensure this promise is actualized.”
Advocacy needed
When approached for comment, Jianjun Zhang, MD, PhD, from the department of thoracic/head and neck medical oncology, University of Texas MD Anderson Cancer Center, Houston, said he supports the new guidelines, and they will lower mortality. “The data are pretty strong overall,” he said in an interview.
Although the uptake of screening is currently very low, he pointed out that, even if uptake remains the same, more lives will be saved because eligibility has been expanded. “More people will be getting screened, so it’s a start,” he said.
Aside from factors such as insurance and access, another problem involves primary care. “Time is very limited in primary care,” he said. “You have about 15 minutes, and it can be really hard to fit everything into a visit. Screening may get left out or may only get a brief mention.”
Advocacy is needed, Dr. Zhang pointed out. “Breast cancer has strong voices and advocacy, and people are more aware of mammography,” he said. “The information is disseminated out into the community. We need the same for lung cancer.”
Dr. Zhang emphasized that, even with the expanded criteria, many individuals will still be missed. “There are other risk factors besides smoking,” he said. “About 10% of lung cancers occur in never-smokers.”
Other risk factors include a family history of lung cancer, exposure to certain materials and chemicals, working in the mining industry, and genetics.
“We will move on to more personalized screening at some point,” he said. “But right now, we can’t make it too complicated for patients and doctors. We need to concentrate on increasing screening rates within these current criteria.”
The updated guidelines have been given a B recommendation, meaning the USPSTF recommends that clinicians provide the service to eligible patients, there is at least fair evidence that this service improves important health outcomes, and benefits outweigh harms.
The USPSTF is an independent, voluntary body. The U.S. Congress mandates that the Agency for Healthcare Research and Quality support the operations of the USPSTF. All members of the USPSTF receive travel reimbursement and an honorarium for participating in USPSTF meetings. The original article lists relevant financial relationships of task force members. Dr. Zhang has received grants from Johnson & Johnson and Merck, and adversary/consulting/honoraria fees from AstraZeneca, Bristol-Myers Squibb, GenePlus, Innovent, OrigMed, and Roche.
A version of this article first appeared on Medscape.com.
“This is great news because it means that nearly twice as many people are eligible to be screened, which we hope will allow clinicians to save more lives and help people remain healthy longer,” commented John Wong, MD, chief science officer, vice chair for clinical affairs, and chief of the Division of Clinical Decision Making at USPSTF.
The updated final recommendations were published online on March 9 in JAMA.
The USPSTF recommends annual screening with low-dose CT for adults aged 50-80 years who have a 20 pack-year smoking history and currently smoke or have quit within the past 15 years.
This updates guidance issued in 2013, which recommended annual screening for lung cancer for adults aged 55-80 years who had a 30 pack-year smoking history and who were either current smokers or had quit within the past 15 years.
The move will nearly double the number of people are now eligible for screening, up to 14.5 million individuals – an increase of 81% (6.4 million adults) from the 2013 recommendations.
The expanded criteria may help increase screening among Black individuals and women. Data show that both groups tend to smoke fewer cigarettes than White men and that Black persons are at higher risk for lung cancer than White persons. In addition, research has shown that about one-third of Black patients with lung cancer were diagnosed before the age of 55 years, which means they would not have been recommended for screening under the previous guidelines.
Uptake has been limited
To date, uptake of lung cancer screening has been very limited, from 6% to 18% of individuals who meet the eligibility criteria.
The new recommendations will open up screening to many more people, but challenges to implementation remain.
“The science is clear that lung cancer screening has the potential to save lives,” Dr. Wong told this news organization. “We recognize that there are existing barriers to screening everyone who is eligible, but clinicians and patients both deserve to know that screening can detect lung cancer early, when treatment has the best chance of being beneficial.”
He added that the hope is that these recommendations will encourage clinicians to examine the barriers to effective lung cancer screening in their communities and to do what they can to improve implementation. “We also hope to encourage patients to have conversations with their clinicians about whether they are eligible for screening and to discuss smoking cessation treatments if they are still smoking,” Dr. Wong added.
In an accompanying editorial, Louise M. Henderson, PhD, M. Patricia Rivera, MD, FCCP, and Ethan Basch, MD, all from the University of North Carolina at Chapel Hill, address some of the current challenges in implementation.
They note that reimbursement for lung cancer screening by Medicare requires submission of data to a Centers for Medicare & Medicaid Services–approved registry, and this can present problems for facilities serving less affluent communities or that have limited resources.
Medicaid coverage is also uneven. As of September 2020, lung cancer screening was covered by 38 Medicaid programs, but not by 9. For three programs, data on coverage were not available.
“With the new recommendations lowering the screening-eligible age to 50 years, many eligible individuals who are uninsured or who are receiving Medicaid and living in states that do not cover screening will have financial barriers to undergo screening,” they write.
In addition, many individuals in at-risk populations lack adequate geographic access to comprehensive lung cancer screening programs.
Expanding eligibility criteria is important, the editorialists point out, but barriers to screening, which include lack of insurance coverage and limited physical access to high-quality screening programs, highlight the complex problems with implementation that need to be addressed.
“A concerted effort to increase the reach of lung cancer screening is needed,” they write. “The 2021 USPSTF recommendation statement represents a leap forward in evidence and offers promise to prevent more cancer deaths and address screening disparities. But the greatest work lies ahead to ensure this promise is actualized.”
Advocacy needed
When approached for comment, Jianjun Zhang, MD, PhD, from the department of thoracic/head and neck medical oncology, University of Texas MD Anderson Cancer Center, Houston, said he supports the new guidelines, and they will lower mortality. “The data are pretty strong overall,” he said in an interview.
Although the uptake of screening is currently very low, he pointed out that, even if uptake remains the same, more lives will be saved because eligibility has been expanded. “More people will be getting screened, so it’s a start,” he said.
Aside from factors such as insurance and access, another problem involves primary care. “Time is very limited in primary care,” he said. “You have about 15 minutes, and it can be really hard to fit everything into a visit. Screening may get left out or may only get a brief mention.”
Advocacy is needed, Dr. Zhang pointed out. “Breast cancer has strong voices and advocacy, and people are more aware of mammography,” he said. “The information is disseminated out into the community. We need the same for lung cancer.”
Dr. Zhang emphasized that, even with the expanded criteria, many individuals will still be missed. “There are other risk factors besides smoking,” he said. “About 10% of lung cancers occur in never-smokers.”
Other risk factors include a family history of lung cancer, exposure to certain materials and chemicals, working in the mining industry, and genetics.
“We will move on to more personalized screening at some point,” he said. “But right now, we can’t make it too complicated for patients and doctors. We need to concentrate on increasing screening rates within these current criteria.”
The updated guidelines have been given a B recommendation, meaning the USPSTF recommends that clinicians provide the service to eligible patients, there is at least fair evidence that this service improves important health outcomes, and benefits outweigh harms.
The USPSTF is an independent, voluntary body. The U.S. Congress mandates that the Agency for Healthcare Research and Quality support the operations of the USPSTF. All members of the USPSTF receive travel reimbursement and an honorarium for participating in USPSTF meetings. The original article lists relevant financial relationships of task force members. Dr. Zhang has received grants from Johnson & Johnson and Merck, and adversary/consulting/honoraria fees from AstraZeneca, Bristol-Myers Squibb, GenePlus, Innovent, OrigMed, and Roche.
A version of this article first appeared on Medscape.com.
“This is great news because it means that nearly twice as many people are eligible to be screened, which we hope will allow clinicians to save more lives and help people remain healthy longer,” commented John Wong, MD, chief science officer, vice chair for clinical affairs, and chief of the Division of Clinical Decision Making at USPSTF.
The updated final recommendations were published online on March 9 in JAMA.
The USPSTF recommends annual screening with low-dose CT for adults aged 50-80 years who have a 20 pack-year smoking history and currently smoke or have quit within the past 15 years.
This updates guidance issued in 2013, which recommended annual screening for lung cancer for adults aged 55-80 years who had a 30 pack-year smoking history and who were either current smokers or had quit within the past 15 years.
The move will nearly double the number of people are now eligible for screening, up to 14.5 million individuals – an increase of 81% (6.4 million adults) from the 2013 recommendations.
The expanded criteria may help increase screening among Black individuals and women. Data show that both groups tend to smoke fewer cigarettes than White men and that Black persons are at higher risk for lung cancer than White persons. In addition, research has shown that about one-third of Black patients with lung cancer were diagnosed before the age of 55 years, which means they would not have been recommended for screening under the previous guidelines.
Uptake has been limited
To date, uptake of lung cancer screening has been very limited, from 6% to 18% of individuals who meet the eligibility criteria.
The new recommendations will open up screening to many more people, but challenges to implementation remain.
“The science is clear that lung cancer screening has the potential to save lives,” Dr. Wong told this news organization. “We recognize that there are existing barriers to screening everyone who is eligible, but clinicians and patients both deserve to know that screening can detect lung cancer early, when treatment has the best chance of being beneficial.”
He added that the hope is that these recommendations will encourage clinicians to examine the barriers to effective lung cancer screening in their communities and to do what they can to improve implementation. “We also hope to encourage patients to have conversations with their clinicians about whether they are eligible for screening and to discuss smoking cessation treatments if they are still smoking,” Dr. Wong added.
In an accompanying editorial, Louise M. Henderson, PhD, M. Patricia Rivera, MD, FCCP, and Ethan Basch, MD, all from the University of North Carolina at Chapel Hill, address some of the current challenges in implementation.
They note that reimbursement for lung cancer screening by Medicare requires submission of data to a Centers for Medicare & Medicaid Services–approved registry, and this can present problems for facilities serving less affluent communities or that have limited resources.
Medicaid coverage is also uneven. As of September 2020, lung cancer screening was covered by 38 Medicaid programs, but not by 9. For three programs, data on coverage were not available.
“With the new recommendations lowering the screening-eligible age to 50 years, many eligible individuals who are uninsured or who are receiving Medicaid and living in states that do not cover screening will have financial barriers to undergo screening,” they write.
In addition, many individuals in at-risk populations lack adequate geographic access to comprehensive lung cancer screening programs.
Expanding eligibility criteria is important, the editorialists point out, but barriers to screening, which include lack of insurance coverage and limited physical access to high-quality screening programs, highlight the complex problems with implementation that need to be addressed.
“A concerted effort to increase the reach of lung cancer screening is needed,” they write. “The 2021 USPSTF recommendation statement represents a leap forward in evidence and offers promise to prevent more cancer deaths and address screening disparities. But the greatest work lies ahead to ensure this promise is actualized.”
Advocacy needed
When approached for comment, Jianjun Zhang, MD, PhD, from the department of thoracic/head and neck medical oncology, University of Texas MD Anderson Cancer Center, Houston, said he supports the new guidelines, and they will lower mortality. “The data are pretty strong overall,” he said in an interview.
Although the uptake of screening is currently very low, he pointed out that, even if uptake remains the same, more lives will be saved because eligibility has been expanded. “More people will be getting screened, so it’s a start,” he said.
Aside from factors such as insurance and access, another problem involves primary care. “Time is very limited in primary care,” he said. “You have about 15 minutes, and it can be really hard to fit everything into a visit. Screening may get left out or may only get a brief mention.”
Advocacy is needed, Dr. Zhang pointed out. “Breast cancer has strong voices and advocacy, and people are more aware of mammography,” he said. “The information is disseminated out into the community. We need the same for lung cancer.”
Dr. Zhang emphasized that, even with the expanded criteria, many individuals will still be missed. “There are other risk factors besides smoking,” he said. “About 10% of lung cancers occur in never-smokers.”
Other risk factors include a family history of lung cancer, exposure to certain materials and chemicals, working in the mining industry, and genetics.
“We will move on to more personalized screening at some point,” he said. “But right now, we can’t make it too complicated for patients and doctors. We need to concentrate on increasing screening rates within these current criteria.”
The updated guidelines have been given a B recommendation, meaning the USPSTF recommends that clinicians provide the service to eligible patients, there is at least fair evidence that this service improves important health outcomes, and benefits outweigh harms.
The USPSTF is an independent, voluntary body. The U.S. Congress mandates that the Agency for Healthcare Research and Quality support the operations of the USPSTF. All members of the USPSTF receive travel reimbursement and an honorarium for participating in USPSTF meetings. The original article lists relevant financial relationships of task force members. Dr. Zhang has received grants from Johnson & Johnson and Merck, and adversary/consulting/honoraria fees from AstraZeneca, Bristol-Myers Squibb, GenePlus, Innovent, OrigMed, and Roche.
A version of this article first appeared on Medscape.com.
ACOG advises on care for transgender patients
Transgender patients have unique needs regarding obstetric and gynecologic care as well as preventive care, and ob.gyns. can help by providing support, education, and understanding, according to new guidance from the American College of Obstetricians and Gynecologists.
“The American College of Obstetricians and Gynecologists opposes discrimination on the basis of gender identity, urges public and private health insurance plans to cover necessary services for individuals with gender dysphoria, and advocates for inclusive, thoughtful, and affirming care for transgender individuals,” according to the committee opinion, published in the March issue of Obstetrics & Gynecology. The opinion was developed jointly by ACOG’s Committee on Gynecologic Practice and Committee on Health Care for Underserved Women, led by Beth Cronin, MD, of Brown University, Providence, R.I., and Colleen K, Stockdale, MD, of the University of Iowa, Iowa City.
“Lack of awareness, knowledge, and sensitivity, as well as bias from health care professionals leads to inadequate access to, underuse of, and inequities within the health care system for transgender patients,” the authors wrote.
The committee opinion provides guidance for ob.gyns. on topics including inclusivity, routine screening, fertility and reproductive issues, hormone therapy, medication use, and surgery.
“One of the most incredible things about being an ob.gyn. is that this field is a hybrid of primary care and surgical practice,” said K. Ashley Brandt, DO, in an interview. “Many patients seek out care from ob.gyns. for routine screening such as a Pap test, for initiation of hormone therapy, or for postoperative management,” said Dr. Brandt, an ob.gyn. and a plastic surgeon at Reading Hospital/Tower Health System in West Reading, Pa. “Many of my colleagues are starting to see an increase in transgender and gender-nonconforming individuals and do not know where to access resources or information on basic care needs. I think ACOG issuing this guidance is a great first step in providing an overview for the ob.gyn., who otherwise haven’t had formal training in transgender medicine,” she emphasized.
Dr. Brandt said she was not surprised by any of the recommendations. “These recommendations, while evolving and updating as new data emerge, have been in place by WPATH (the World Professional Association for Transgender Health) and the Endocrine Society for quite some time,” she noted. “However, this updated committee opinion is a summary of recommendations that are relevant to the clinical practice of an ob.gyn.”
“Since the publication of Care for Transgender Adolescents (2017) and Healthcare for Transgender Individuals (2011), there has been an exponential increase in data that have helped to improve and guide best practices for this patient population including better defining risks, needs, therapy, and follow-up,” said Nancy Sokkary, MD, a specialist in pediatric and adolescent gynecology in Macon, Ga., in an interview. “This document also served as an opportunity for ACOG to educate ob.gyns. about health inequities and emphasize need for gender-affirming and inclusive care,” she said.
“These recommendations are consistent with literature that has been published over the last several years,” she added. “It is certainly important for ob.gyns. to have a document unequivocally supporting hysterectomies and bilateral salpingo-oophorectomy as medically necessary for transgender patients that desire these procedures for their transition.”
Inclusive environment
Approximately 1.4 million adults and 150,000 youth aged 13-17 years in the United States identify as transgender, but these individuals are often marginalized socially and economically, which can lead to worse health outcomes, according to the committee. “Creating a safe and affirming health care environment for all patients, including transgender individuals, is essential,” the authors said. Steps to create a supportive office setting include educating staff to avoid assumptions about sex and gender, and ask appropriately about choice of pronouns and orientation. Use patient forms that reflect a full range of options and places for patients to write in a response. Also, use electronic medical records to track information on use of names other than legal names. “Ob.gyns. play an important role in caring for gender-nonconforming people,” said Dr. Sokkary. “Ob.gyn. providers may have varying levels of participation in gender-affirming hormone or surgery provision, but they can universally conduct routine health maintenance, contraceptive and fertility counseling, and obstetric care in a respectful and inclusive environment,” she said.
Track transition issues
The opinion notes that many gender-transition medications can be prescribed not only by ob.gyns., but by a range of health care professionals with training and education. When it comes to medication and surgery, neither medication nor surgery is required for legally changing one’s name or gender, but patient desires vary from those seeking only letters of support for such legal changes to those who want to pursue hormone therapy or procedures such as chest surgery, hysterectomy, or phalloplasty.
Transgender patients seeking care from ob.gyns. include transmasculine and transfeminine individuals who are seeking various degrees of masculinizing or feminizing therapies.
Masculinizing therapies may result in development of facial hair, deepening voice, and changes in muscle mass, but patients undergoing masculinizing therapies should be reminded of the potential for continued ovulation, according to the opinion. “The only absolute contraindications to masculinizing hormone therapy are current pregnancy, unstable coronary artery disease, and polycythemia (hematocrit greater than 55%),” the authors wrote.
Feminizing therapies have no absolute contraindications, but “risks include venous thromboembolism (VTE), hypertriglyceridemia, development of gallstones, and elevated liver enzymes,” they noted.
Talk about sex and fertility
Clinicians treating transgender patients should discuss fertility and parenting early in the process of any gender transition, ideally before the patient undergoes hormone therapy or surgery, according to the opinion. Fertility preservation options for transgender patients are the same as for cisgender patients who wish to preserve fertility for various reasons, and include “sperm banking, oocyte preservation, embryo preservation, and in some cases, ovarian or testicular tissue cryopreservation,” the authors noted.
However, patients who do not desire pregnancy but may have the potential to become pregnant or impregnate others should be counseled on contraceptive options and reminded that gender-affirming hormone therapy alone does not provide effective contraception, they emphasized. In addition, “all patients should be counseled on barrier use for prevention of sexually transmitted diseases,” they said.
Consistent routine screening and preventive care
The committee opinion also states that transgender patients should undergo routine screening for any anatomical structures that are present, such as breast cancer screening for transmasculine individuals with breast tissue, and cervical cancer screening for those with a cervix. Transfeminine individuals should undergo prostate cancer screening in accordance with the recommendations for cisgender men, the authors said.
“As for all patients, transgender individuals should be counseled about the importance of routine preventive health care,” according to the opinion. “All individuals should be routinely screened for intimate partner violence, depression, substance use, cancer, and other health care needs and should be screened for sexually transmitted infections and counseled about appropriate immunizations based on age and risk factors, including HPV vaccination,” the authors said.
“We continue to see patient discrimination and discomfort with the medical system as a barrier to preventive care among gender-nonconforming individuals,” said Dr. Sokkary. “[Ensuring] that your clinic is a safe, inclusive place is a good start. Also, having providers such as ob.gyns. and family medicine physicians provide gender-affirming care in addition to routine screening and testing is helpful,” she said.
One of the ongoing challenges of counseling transgender patients across a range of age groups, from youth through menopause, is a lack of data on the long-term effects of hormone therapy or surgical intervention, Dr. Brandt noted. “Since there is a paucity of this information, many of the screening recommendations fall in line with that of cisgender patients; however, this is not always the case as screening is determined by hormonal usage, risk factors, and surgical state. It is important for clinicians to be aware of evolutions in screening that will continue to occur as more evidence becomes available,” she emphasized.
In addition, “This document did not include specific guidance for transgender and gender-diverse adolescents, and there are many factors and recommendations that are unique to this population,” Dr. Sokkary said.
Barriers and overcoming them
The main barrier to care with transgender and gender-nonconfirming patients is access to care and finding providers who are competent in gender-affirming health, Dr. Brandt noted. “Another significant barrier involves caring for transgender male patients in a traditionally ‘women’s health’ specialty,” she said. “While the office of an ob.gyn. can be very affirming for transgender women, it has the potential to exacerbate discomfort in transgender male patients,” she noted. “Having gender-affirming posters and pamphlets in the waiting area are ways to make patients feel more at ease. Another of the ways to overcome this barrier is education of the staff and health care providers,” added Dr. Brandt. “Fortunately, this is starting to occur at medical school and residency levels. For ob.gyns. already in practice, articles such as this committee opinion can serve as a resource for providers seeking to understand health care needs of this community,” she said.
“Cost and insurance coverage continue to be barriers, but this has improved immensely: There are now several local and national resources that can help with this depending on the issue,” said Dr. Sokkary. “Additionally, we still lack robust data that define cancer risk among transgender individuals, and until we have more evidence-based recommendations providers should follow screening outlined in this document,” she said.
Use the ACOG opinion as a starting point
“This committee opinion is a great introduction and summary for ob.gyns. seeking to understand basic care needs for gender-nonconforming individuals,” said Dr. Brandt. “However, I strongly encourage ob.gyns. who wish to truly incorporate gender-affirming care as part of their routine clinical practice to participate in continuing education, read the WPATH standards of care among many of the resources provided in the committee opinion, and attend conferences that are specific to transgender health and medicine,” she said.
The opinion received no outside funding. The authors were vetted by ACOG and had no relevant financial conflicts to disclose. Dr. Brandt had no financial conflicts to disclose. Dr. Sokkary had no financial conflicts to disclose.
Transgender patients have unique needs regarding obstetric and gynecologic care as well as preventive care, and ob.gyns. can help by providing support, education, and understanding, according to new guidance from the American College of Obstetricians and Gynecologists.
“The American College of Obstetricians and Gynecologists opposes discrimination on the basis of gender identity, urges public and private health insurance plans to cover necessary services for individuals with gender dysphoria, and advocates for inclusive, thoughtful, and affirming care for transgender individuals,” according to the committee opinion, published in the March issue of Obstetrics & Gynecology. The opinion was developed jointly by ACOG’s Committee on Gynecologic Practice and Committee on Health Care for Underserved Women, led by Beth Cronin, MD, of Brown University, Providence, R.I., and Colleen K, Stockdale, MD, of the University of Iowa, Iowa City.
“Lack of awareness, knowledge, and sensitivity, as well as bias from health care professionals leads to inadequate access to, underuse of, and inequities within the health care system for transgender patients,” the authors wrote.
The committee opinion provides guidance for ob.gyns. on topics including inclusivity, routine screening, fertility and reproductive issues, hormone therapy, medication use, and surgery.
“One of the most incredible things about being an ob.gyn. is that this field is a hybrid of primary care and surgical practice,” said K. Ashley Brandt, DO, in an interview. “Many patients seek out care from ob.gyns. for routine screening such as a Pap test, for initiation of hormone therapy, or for postoperative management,” said Dr. Brandt, an ob.gyn. and a plastic surgeon at Reading Hospital/Tower Health System in West Reading, Pa. “Many of my colleagues are starting to see an increase in transgender and gender-nonconforming individuals and do not know where to access resources or information on basic care needs. I think ACOG issuing this guidance is a great first step in providing an overview for the ob.gyn., who otherwise haven’t had formal training in transgender medicine,” she emphasized.
Dr. Brandt said she was not surprised by any of the recommendations. “These recommendations, while evolving and updating as new data emerge, have been in place by WPATH (the World Professional Association for Transgender Health) and the Endocrine Society for quite some time,” she noted. “However, this updated committee opinion is a summary of recommendations that are relevant to the clinical practice of an ob.gyn.”
“Since the publication of Care for Transgender Adolescents (2017) and Healthcare for Transgender Individuals (2011), there has been an exponential increase in data that have helped to improve and guide best practices for this patient population including better defining risks, needs, therapy, and follow-up,” said Nancy Sokkary, MD, a specialist in pediatric and adolescent gynecology in Macon, Ga., in an interview. “This document also served as an opportunity for ACOG to educate ob.gyns. about health inequities and emphasize need for gender-affirming and inclusive care,” she said.
“These recommendations are consistent with literature that has been published over the last several years,” she added. “It is certainly important for ob.gyns. to have a document unequivocally supporting hysterectomies and bilateral salpingo-oophorectomy as medically necessary for transgender patients that desire these procedures for their transition.”
Inclusive environment
Approximately 1.4 million adults and 150,000 youth aged 13-17 years in the United States identify as transgender, but these individuals are often marginalized socially and economically, which can lead to worse health outcomes, according to the committee. “Creating a safe and affirming health care environment for all patients, including transgender individuals, is essential,” the authors said. Steps to create a supportive office setting include educating staff to avoid assumptions about sex and gender, and ask appropriately about choice of pronouns and orientation. Use patient forms that reflect a full range of options and places for patients to write in a response. Also, use electronic medical records to track information on use of names other than legal names. “Ob.gyns. play an important role in caring for gender-nonconforming people,” said Dr. Sokkary. “Ob.gyn. providers may have varying levels of participation in gender-affirming hormone or surgery provision, but they can universally conduct routine health maintenance, contraceptive and fertility counseling, and obstetric care in a respectful and inclusive environment,” she said.
Track transition issues
The opinion notes that many gender-transition medications can be prescribed not only by ob.gyns., but by a range of health care professionals with training and education. When it comes to medication and surgery, neither medication nor surgery is required for legally changing one’s name or gender, but patient desires vary from those seeking only letters of support for such legal changes to those who want to pursue hormone therapy or procedures such as chest surgery, hysterectomy, or phalloplasty.
Transgender patients seeking care from ob.gyns. include transmasculine and transfeminine individuals who are seeking various degrees of masculinizing or feminizing therapies.
Masculinizing therapies may result in development of facial hair, deepening voice, and changes in muscle mass, but patients undergoing masculinizing therapies should be reminded of the potential for continued ovulation, according to the opinion. “The only absolute contraindications to masculinizing hormone therapy are current pregnancy, unstable coronary artery disease, and polycythemia (hematocrit greater than 55%),” the authors wrote.
Feminizing therapies have no absolute contraindications, but “risks include venous thromboembolism (VTE), hypertriglyceridemia, development of gallstones, and elevated liver enzymes,” they noted.
Talk about sex and fertility
Clinicians treating transgender patients should discuss fertility and parenting early in the process of any gender transition, ideally before the patient undergoes hormone therapy or surgery, according to the opinion. Fertility preservation options for transgender patients are the same as for cisgender patients who wish to preserve fertility for various reasons, and include “sperm banking, oocyte preservation, embryo preservation, and in some cases, ovarian or testicular tissue cryopreservation,” the authors noted.
However, patients who do not desire pregnancy but may have the potential to become pregnant or impregnate others should be counseled on contraceptive options and reminded that gender-affirming hormone therapy alone does not provide effective contraception, they emphasized. In addition, “all patients should be counseled on barrier use for prevention of sexually transmitted diseases,” they said.
Consistent routine screening and preventive care
The committee opinion also states that transgender patients should undergo routine screening for any anatomical structures that are present, such as breast cancer screening for transmasculine individuals with breast tissue, and cervical cancer screening for those with a cervix. Transfeminine individuals should undergo prostate cancer screening in accordance with the recommendations for cisgender men, the authors said.
“As for all patients, transgender individuals should be counseled about the importance of routine preventive health care,” according to the opinion. “All individuals should be routinely screened for intimate partner violence, depression, substance use, cancer, and other health care needs and should be screened for sexually transmitted infections and counseled about appropriate immunizations based on age and risk factors, including HPV vaccination,” the authors said.
“We continue to see patient discrimination and discomfort with the medical system as a barrier to preventive care among gender-nonconforming individuals,” said Dr. Sokkary. “[Ensuring] that your clinic is a safe, inclusive place is a good start. Also, having providers such as ob.gyns. and family medicine physicians provide gender-affirming care in addition to routine screening and testing is helpful,” she said.
One of the ongoing challenges of counseling transgender patients across a range of age groups, from youth through menopause, is a lack of data on the long-term effects of hormone therapy or surgical intervention, Dr. Brandt noted. “Since there is a paucity of this information, many of the screening recommendations fall in line with that of cisgender patients; however, this is not always the case as screening is determined by hormonal usage, risk factors, and surgical state. It is important for clinicians to be aware of evolutions in screening that will continue to occur as more evidence becomes available,” she emphasized.
In addition, “This document did not include specific guidance for transgender and gender-diverse adolescents, and there are many factors and recommendations that are unique to this population,” Dr. Sokkary said.
Barriers and overcoming them
The main barrier to care with transgender and gender-nonconfirming patients is access to care and finding providers who are competent in gender-affirming health, Dr. Brandt noted. “Another significant barrier involves caring for transgender male patients in a traditionally ‘women’s health’ specialty,” she said. “While the office of an ob.gyn. can be very affirming for transgender women, it has the potential to exacerbate discomfort in transgender male patients,” she noted. “Having gender-affirming posters and pamphlets in the waiting area are ways to make patients feel more at ease. Another of the ways to overcome this barrier is education of the staff and health care providers,” added Dr. Brandt. “Fortunately, this is starting to occur at medical school and residency levels. For ob.gyns. already in practice, articles such as this committee opinion can serve as a resource for providers seeking to understand health care needs of this community,” she said.
“Cost and insurance coverage continue to be barriers, but this has improved immensely: There are now several local and national resources that can help with this depending on the issue,” said Dr. Sokkary. “Additionally, we still lack robust data that define cancer risk among transgender individuals, and until we have more evidence-based recommendations providers should follow screening outlined in this document,” she said.
Use the ACOG opinion as a starting point
“This committee opinion is a great introduction and summary for ob.gyns. seeking to understand basic care needs for gender-nonconforming individuals,” said Dr. Brandt. “However, I strongly encourage ob.gyns. who wish to truly incorporate gender-affirming care as part of their routine clinical practice to participate in continuing education, read the WPATH standards of care among many of the resources provided in the committee opinion, and attend conferences that are specific to transgender health and medicine,” she said.
The opinion received no outside funding. The authors were vetted by ACOG and had no relevant financial conflicts to disclose. Dr. Brandt had no financial conflicts to disclose. Dr. Sokkary had no financial conflicts to disclose.
Transgender patients have unique needs regarding obstetric and gynecologic care as well as preventive care, and ob.gyns. can help by providing support, education, and understanding, according to new guidance from the American College of Obstetricians and Gynecologists.
“The American College of Obstetricians and Gynecologists opposes discrimination on the basis of gender identity, urges public and private health insurance plans to cover necessary services for individuals with gender dysphoria, and advocates for inclusive, thoughtful, and affirming care for transgender individuals,” according to the committee opinion, published in the March issue of Obstetrics & Gynecology. The opinion was developed jointly by ACOG’s Committee on Gynecologic Practice and Committee on Health Care for Underserved Women, led by Beth Cronin, MD, of Brown University, Providence, R.I., and Colleen K, Stockdale, MD, of the University of Iowa, Iowa City.
“Lack of awareness, knowledge, and sensitivity, as well as bias from health care professionals leads to inadequate access to, underuse of, and inequities within the health care system for transgender patients,” the authors wrote.
The committee opinion provides guidance for ob.gyns. on topics including inclusivity, routine screening, fertility and reproductive issues, hormone therapy, medication use, and surgery.
“One of the most incredible things about being an ob.gyn. is that this field is a hybrid of primary care and surgical practice,” said K. Ashley Brandt, DO, in an interview. “Many patients seek out care from ob.gyns. for routine screening such as a Pap test, for initiation of hormone therapy, or for postoperative management,” said Dr. Brandt, an ob.gyn. and a plastic surgeon at Reading Hospital/Tower Health System in West Reading, Pa. “Many of my colleagues are starting to see an increase in transgender and gender-nonconforming individuals and do not know where to access resources or information on basic care needs. I think ACOG issuing this guidance is a great first step in providing an overview for the ob.gyn., who otherwise haven’t had formal training in transgender medicine,” she emphasized.
Dr. Brandt said she was not surprised by any of the recommendations. “These recommendations, while evolving and updating as new data emerge, have been in place by WPATH (the World Professional Association for Transgender Health) and the Endocrine Society for quite some time,” she noted. “However, this updated committee opinion is a summary of recommendations that are relevant to the clinical practice of an ob.gyn.”
“Since the publication of Care for Transgender Adolescents (2017) and Healthcare for Transgender Individuals (2011), there has been an exponential increase in data that have helped to improve and guide best practices for this patient population including better defining risks, needs, therapy, and follow-up,” said Nancy Sokkary, MD, a specialist in pediatric and adolescent gynecology in Macon, Ga., in an interview. “This document also served as an opportunity for ACOG to educate ob.gyns. about health inequities and emphasize need for gender-affirming and inclusive care,” she said.
“These recommendations are consistent with literature that has been published over the last several years,” she added. “It is certainly important for ob.gyns. to have a document unequivocally supporting hysterectomies and bilateral salpingo-oophorectomy as medically necessary for transgender patients that desire these procedures for their transition.”
Inclusive environment
Approximately 1.4 million adults and 150,000 youth aged 13-17 years in the United States identify as transgender, but these individuals are often marginalized socially and economically, which can lead to worse health outcomes, according to the committee. “Creating a safe and affirming health care environment for all patients, including transgender individuals, is essential,” the authors said. Steps to create a supportive office setting include educating staff to avoid assumptions about sex and gender, and ask appropriately about choice of pronouns and orientation. Use patient forms that reflect a full range of options and places for patients to write in a response. Also, use electronic medical records to track information on use of names other than legal names. “Ob.gyns. play an important role in caring for gender-nonconforming people,” said Dr. Sokkary. “Ob.gyn. providers may have varying levels of participation in gender-affirming hormone or surgery provision, but they can universally conduct routine health maintenance, contraceptive and fertility counseling, and obstetric care in a respectful and inclusive environment,” she said.
Track transition issues
The opinion notes that many gender-transition medications can be prescribed not only by ob.gyns., but by a range of health care professionals with training and education. When it comes to medication and surgery, neither medication nor surgery is required for legally changing one’s name or gender, but patient desires vary from those seeking only letters of support for such legal changes to those who want to pursue hormone therapy or procedures such as chest surgery, hysterectomy, or phalloplasty.
Transgender patients seeking care from ob.gyns. include transmasculine and transfeminine individuals who are seeking various degrees of masculinizing or feminizing therapies.
Masculinizing therapies may result in development of facial hair, deepening voice, and changes in muscle mass, but patients undergoing masculinizing therapies should be reminded of the potential for continued ovulation, according to the opinion. “The only absolute contraindications to masculinizing hormone therapy are current pregnancy, unstable coronary artery disease, and polycythemia (hematocrit greater than 55%),” the authors wrote.
Feminizing therapies have no absolute contraindications, but “risks include venous thromboembolism (VTE), hypertriglyceridemia, development of gallstones, and elevated liver enzymes,” they noted.
Talk about sex and fertility
Clinicians treating transgender patients should discuss fertility and parenting early in the process of any gender transition, ideally before the patient undergoes hormone therapy or surgery, according to the opinion. Fertility preservation options for transgender patients are the same as for cisgender patients who wish to preserve fertility for various reasons, and include “sperm banking, oocyte preservation, embryo preservation, and in some cases, ovarian or testicular tissue cryopreservation,” the authors noted.
However, patients who do not desire pregnancy but may have the potential to become pregnant or impregnate others should be counseled on contraceptive options and reminded that gender-affirming hormone therapy alone does not provide effective contraception, they emphasized. In addition, “all patients should be counseled on barrier use for prevention of sexually transmitted diseases,” they said.
Consistent routine screening and preventive care
The committee opinion also states that transgender patients should undergo routine screening for any anatomical structures that are present, such as breast cancer screening for transmasculine individuals with breast tissue, and cervical cancer screening for those with a cervix. Transfeminine individuals should undergo prostate cancer screening in accordance with the recommendations for cisgender men, the authors said.
“As for all patients, transgender individuals should be counseled about the importance of routine preventive health care,” according to the opinion. “All individuals should be routinely screened for intimate partner violence, depression, substance use, cancer, and other health care needs and should be screened for sexually transmitted infections and counseled about appropriate immunizations based on age and risk factors, including HPV vaccination,” the authors said.
“We continue to see patient discrimination and discomfort with the medical system as a barrier to preventive care among gender-nonconforming individuals,” said Dr. Sokkary. “[Ensuring] that your clinic is a safe, inclusive place is a good start. Also, having providers such as ob.gyns. and family medicine physicians provide gender-affirming care in addition to routine screening and testing is helpful,” she said.
One of the ongoing challenges of counseling transgender patients across a range of age groups, from youth through menopause, is a lack of data on the long-term effects of hormone therapy or surgical intervention, Dr. Brandt noted. “Since there is a paucity of this information, many of the screening recommendations fall in line with that of cisgender patients; however, this is not always the case as screening is determined by hormonal usage, risk factors, and surgical state. It is important for clinicians to be aware of evolutions in screening that will continue to occur as more evidence becomes available,” she emphasized.
In addition, “This document did not include specific guidance for transgender and gender-diverse adolescents, and there are many factors and recommendations that are unique to this population,” Dr. Sokkary said.
Barriers and overcoming them
The main barrier to care with transgender and gender-nonconfirming patients is access to care and finding providers who are competent in gender-affirming health, Dr. Brandt noted. “Another significant barrier involves caring for transgender male patients in a traditionally ‘women’s health’ specialty,” she said. “While the office of an ob.gyn. can be very affirming for transgender women, it has the potential to exacerbate discomfort in transgender male patients,” she noted. “Having gender-affirming posters and pamphlets in the waiting area are ways to make patients feel more at ease. Another of the ways to overcome this barrier is education of the staff and health care providers,” added Dr. Brandt. “Fortunately, this is starting to occur at medical school and residency levels. For ob.gyns. already in practice, articles such as this committee opinion can serve as a resource for providers seeking to understand health care needs of this community,” she said.
“Cost and insurance coverage continue to be barriers, but this has improved immensely: There are now several local and national resources that can help with this depending on the issue,” said Dr. Sokkary. “Additionally, we still lack robust data that define cancer risk among transgender individuals, and until we have more evidence-based recommendations providers should follow screening outlined in this document,” she said.
Use the ACOG opinion as a starting point
“This committee opinion is a great introduction and summary for ob.gyns. seeking to understand basic care needs for gender-nonconforming individuals,” said Dr. Brandt. “However, I strongly encourage ob.gyns. who wish to truly incorporate gender-affirming care as part of their routine clinical practice to participate in continuing education, read the WPATH standards of care among many of the resources provided in the committee opinion, and attend conferences that are specific to transgender health and medicine,” she said.
The opinion received no outside funding. The authors were vetted by ACOG and had no relevant financial conflicts to disclose. Dr. Brandt had no financial conflicts to disclose. Dr. Sokkary had no financial conflicts to disclose.
COVID-19 vaccination recommended for rheumatology patients
People with rheumatic diseases should get vaccinated against SARS-CoV-2 as soon as possible, the American College of Rheumatology (ACR) recommends.
“It may be that people with rheumatic diseases are at increased risk of developing COVID or serious COVID-related complications,” Jonathan Hausmann, MD, assistant professor of medicine at Harvard Medical School, Boston, said in an ACR podcast. “So the need to prevent COVID-19 is incredibly important in this group of patients.”
The guidelines recommend a delay in vaccination only in rare circumstances, such as for patients with very severe illness or who have recently been administered rituximab, Jeffrey R. Curtis, MD, MPH, lead author of the guidelines, said in the podcast.
“Our members have been inundated with questions and concerns from their patients on whether they should receive the vaccine,” ACR President David Karp, MD, PhD, said in a press release.
So the ACR convened a panel of nine rheumatologists, two infectious disease specialists, and two public health experts. Over the course of 8 weeks, the task force reviewed the literature and agreed on recommendations. The organization posted a summary of the guidelines on its website after its board of directors approved it Feb. 8. The paper is pending journal peer review.
Some risks are real
The task force confined its research to the COVID-19 vaccines being offered by Pfizer and Moderna because they are currently the only ones approved by the Food and Drug Administration. It found no reason to distinguish between the two vaccines in its recommendations.
Because little research has directly addressed the question concerning COVID-19 vaccination for patients with rheumatic diseases, the task force extrapolated from data on other vaccinations in people with rheumatic disease and on the COVID-19 vaccinations in other populations.
It analyzed reports that other types of vaccination, such as for influenza, triggered flares of rheumatic conditions. “It is really individual case reports or small cohorts where there may be a somewhat higher incidence of flare, but it’s usually not very large in its magnitude nor duration,” said Dr. Curtis of the University of Alabama at Birmingham.
The task force also considered the possibility that vaccinations could lead to a new autoimmune disorder, such as Guillain-Barré syndrome or Bell palsy. The risk is real, the task force decided, but not significant enough to influence their recommendations.
Likewise, in immunocompromised people, vaccinations with live virus, such as those for shingles, might trigger the infection the vaccination is meant to prevent. But this can’t happen with the Pfizer and Moderna COVID-19 vaccines because they contain messenger RNA instead of live viruses, Dr. Curtis said.
Although it might be optimal to administer the vaccines when rheumatic diseases are quiescent, the urgency of getting vaccinated overrides that consideration, Dr. Curtis said. “By and large, there was a general consensus to not want to delay vaccination until somebody was stable and doing great, because you don’t know how long that’s going to be,” he said.
How well does it work?
One unanswered question is whether the COVID-19 vaccines work as well for patients with rheumatic diseases. The task force was reassured by data showing efficacy across a range of subgroups, including some with immunosenescence, Dr. Curtis said. “But until we have data in rheumatology patients, we’re just not going to know,” he said.
The guidelines specify that some drug regimens be modified when patients are vaccinated.
For patients taking rituximab, vaccination should be delayed, but only for those who are able to maintain safe social distancing to reduce the risk for COVID-19 exposure, Dr. Curtis said. “If somebody has just gotten rituximab recently, it might be more ideal to complete the vaccine series about 2-4 weeks before the next rituximab dose,” he said. “So if you are giving that therapy, say, at 6-month intervals, if you could vaccinate them at around month 5 from the most recent rituximab cycle, that might be more ideal.”
The guidance calls for withholding JAK inhibitors for a week after each vaccine dose is administered.
It calls for holding SQ abatacept 1 week prior and 1 week after the first COVID-19 vaccine dose, with no interruption after the second dose.
For abatacept IV, clinicians should “time vaccine administration so that the first vaccination will occur 4 weeks after abatacept infusion (i.e., the entire dosing interval), and postpone the subsequent abatacept infusion by 1 week (i.e., a 5-week gap in total).” It recommends no medication adjustment for the second vaccine dose.
For cyclophosphamide, the guidance recommends timing administration to occur about a week after each vaccine dose, when feasible.
None of this advice should supersede clinical judgment, Dr. Curtis said.
A version of this article first appeared on Medscape.com.
People with rheumatic diseases should get vaccinated against SARS-CoV-2 as soon as possible, the American College of Rheumatology (ACR) recommends.
“It may be that people with rheumatic diseases are at increased risk of developing COVID or serious COVID-related complications,” Jonathan Hausmann, MD, assistant professor of medicine at Harvard Medical School, Boston, said in an ACR podcast. “So the need to prevent COVID-19 is incredibly important in this group of patients.”
The guidelines recommend a delay in vaccination only in rare circumstances, such as for patients with very severe illness or who have recently been administered rituximab, Jeffrey R. Curtis, MD, MPH, lead author of the guidelines, said in the podcast.
“Our members have been inundated with questions and concerns from their patients on whether they should receive the vaccine,” ACR President David Karp, MD, PhD, said in a press release.
So the ACR convened a panel of nine rheumatologists, two infectious disease specialists, and two public health experts. Over the course of 8 weeks, the task force reviewed the literature and agreed on recommendations. The organization posted a summary of the guidelines on its website after its board of directors approved it Feb. 8. The paper is pending journal peer review.
Some risks are real
The task force confined its research to the COVID-19 vaccines being offered by Pfizer and Moderna because they are currently the only ones approved by the Food and Drug Administration. It found no reason to distinguish between the two vaccines in its recommendations.
Because little research has directly addressed the question concerning COVID-19 vaccination for patients with rheumatic diseases, the task force extrapolated from data on other vaccinations in people with rheumatic disease and on the COVID-19 vaccinations in other populations.
It analyzed reports that other types of vaccination, such as for influenza, triggered flares of rheumatic conditions. “It is really individual case reports or small cohorts where there may be a somewhat higher incidence of flare, but it’s usually not very large in its magnitude nor duration,” said Dr. Curtis of the University of Alabama at Birmingham.
The task force also considered the possibility that vaccinations could lead to a new autoimmune disorder, such as Guillain-Barré syndrome or Bell palsy. The risk is real, the task force decided, but not significant enough to influence their recommendations.
Likewise, in immunocompromised people, vaccinations with live virus, such as those for shingles, might trigger the infection the vaccination is meant to prevent. But this can’t happen with the Pfizer and Moderna COVID-19 vaccines because they contain messenger RNA instead of live viruses, Dr. Curtis said.
Although it might be optimal to administer the vaccines when rheumatic diseases are quiescent, the urgency of getting vaccinated overrides that consideration, Dr. Curtis said. “By and large, there was a general consensus to not want to delay vaccination until somebody was stable and doing great, because you don’t know how long that’s going to be,” he said.
How well does it work?
One unanswered question is whether the COVID-19 vaccines work as well for patients with rheumatic diseases. The task force was reassured by data showing efficacy across a range of subgroups, including some with immunosenescence, Dr. Curtis said. “But until we have data in rheumatology patients, we’re just not going to know,” he said.
The guidelines specify that some drug regimens be modified when patients are vaccinated.
For patients taking rituximab, vaccination should be delayed, but only for those who are able to maintain safe social distancing to reduce the risk for COVID-19 exposure, Dr. Curtis said. “If somebody has just gotten rituximab recently, it might be more ideal to complete the vaccine series about 2-4 weeks before the next rituximab dose,” he said. “So if you are giving that therapy, say, at 6-month intervals, if you could vaccinate them at around month 5 from the most recent rituximab cycle, that might be more ideal.”
The guidance calls for withholding JAK inhibitors for a week after each vaccine dose is administered.
It calls for holding SQ abatacept 1 week prior and 1 week after the first COVID-19 vaccine dose, with no interruption after the second dose.
For abatacept IV, clinicians should “time vaccine administration so that the first vaccination will occur 4 weeks after abatacept infusion (i.e., the entire dosing interval), and postpone the subsequent abatacept infusion by 1 week (i.e., a 5-week gap in total).” It recommends no medication adjustment for the second vaccine dose.
For cyclophosphamide, the guidance recommends timing administration to occur about a week after each vaccine dose, when feasible.
None of this advice should supersede clinical judgment, Dr. Curtis said.
A version of this article first appeared on Medscape.com.
People with rheumatic diseases should get vaccinated against SARS-CoV-2 as soon as possible, the American College of Rheumatology (ACR) recommends.
“It may be that people with rheumatic diseases are at increased risk of developing COVID or serious COVID-related complications,” Jonathan Hausmann, MD, assistant professor of medicine at Harvard Medical School, Boston, said in an ACR podcast. “So the need to prevent COVID-19 is incredibly important in this group of patients.”
The guidelines recommend a delay in vaccination only in rare circumstances, such as for patients with very severe illness or who have recently been administered rituximab, Jeffrey R. Curtis, MD, MPH, lead author of the guidelines, said in the podcast.
“Our members have been inundated with questions and concerns from their patients on whether they should receive the vaccine,” ACR President David Karp, MD, PhD, said in a press release.
So the ACR convened a panel of nine rheumatologists, two infectious disease specialists, and two public health experts. Over the course of 8 weeks, the task force reviewed the literature and agreed on recommendations. The organization posted a summary of the guidelines on its website after its board of directors approved it Feb. 8. The paper is pending journal peer review.
Some risks are real
The task force confined its research to the COVID-19 vaccines being offered by Pfizer and Moderna because they are currently the only ones approved by the Food and Drug Administration. It found no reason to distinguish between the two vaccines in its recommendations.
Because little research has directly addressed the question concerning COVID-19 vaccination for patients with rheumatic diseases, the task force extrapolated from data on other vaccinations in people with rheumatic disease and on the COVID-19 vaccinations in other populations.
It analyzed reports that other types of vaccination, such as for influenza, triggered flares of rheumatic conditions. “It is really individual case reports or small cohorts where there may be a somewhat higher incidence of flare, but it’s usually not very large in its magnitude nor duration,” said Dr. Curtis of the University of Alabama at Birmingham.
The task force also considered the possibility that vaccinations could lead to a new autoimmune disorder, such as Guillain-Barré syndrome or Bell palsy. The risk is real, the task force decided, but not significant enough to influence their recommendations.
Likewise, in immunocompromised people, vaccinations with live virus, such as those for shingles, might trigger the infection the vaccination is meant to prevent. But this can’t happen with the Pfizer and Moderna COVID-19 vaccines because they contain messenger RNA instead of live viruses, Dr. Curtis said.
Although it might be optimal to administer the vaccines when rheumatic diseases are quiescent, the urgency of getting vaccinated overrides that consideration, Dr. Curtis said. “By and large, there was a general consensus to not want to delay vaccination until somebody was stable and doing great, because you don’t know how long that’s going to be,” he said.
How well does it work?
One unanswered question is whether the COVID-19 vaccines work as well for patients with rheumatic diseases. The task force was reassured by data showing efficacy across a range of subgroups, including some with immunosenescence, Dr. Curtis said. “But until we have data in rheumatology patients, we’re just not going to know,” he said.
The guidelines specify that some drug regimens be modified when patients are vaccinated.
For patients taking rituximab, vaccination should be delayed, but only for those who are able to maintain safe social distancing to reduce the risk for COVID-19 exposure, Dr. Curtis said. “If somebody has just gotten rituximab recently, it might be more ideal to complete the vaccine series about 2-4 weeks before the next rituximab dose,” he said. “So if you are giving that therapy, say, at 6-month intervals, if you could vaccinate them at around month 5 from the most recent rituximab cycle, that might be more ideal.”
The guidance calls for withholding JAK inhibitors for a week after each vaccine dose is administered.
It calls for holding SQ abatacept 1 week prior and 1 week after the first COVID-19 vaccine dose, with no interruption after the second dose.
For abatacept IV, clinicians should “time vaccine administration so that the first vaccination will occur 4 weeks after abatacept infusion (i.e., the entire dosing interval), and postpone the subsequent abatacept infusion by 1 week (i.e., a 5-week gap in total).” It recommends no medication adjustment for the second vaccine dose.
For cyclophosphamide, the guidance recommends timing administration to occur about a week after each vaccine dose, when feasible.
None of this advice should supersede clinical judgment, Dr. Curtis said.
A version of this article first appeared on Medscape.com.
7 key changes: The 2021 child and adolescent immunization schedules
Each February, the Centers for Disease Control and Prevention, along with multiple professional organizations, releases an updated Recommended Child and Adolescent Immunization Schedule.
Recent years have seen fewer changes in the vaccine schedule, mostly with adjustments based on products coming on or off the market, and sometimes with slight changes in recommendations. This year is no different, with mostly minor changes in store. As most practitioners know, having quick access to the tables that accompany the recommendations is always handy. Table 1 contains the typical, recommended immunization schedule. Table 2 contains the catch-up provisions, and Table 3 provides guidance on vaccines for special circumstances and for children with specific medical conditions.
2021 childhood and adolescent immunization schedule
One update is a recommendation that patients with egg allergies who had symptoms more extensive than hives should receive the influenza vaccine in a medical setting where severe allergic reactions or anaphylaxis can be recognized and treated, with the exclusion of two specific preparations, Flublok and Flucelvax.
In regard to the live attenuated influenza vaccine (LAIV), there are several points of reinforcement. First, the nomenclature has generally been changed to “LAIV4” throughout the document because only quadrivalent preparations are available. There are specific recommendations that patients should not receive LAIV4 if they recently took antiviral medication for influenza, with “lockout” periods lasting from 2 days to 17 days, depending on the antiviral preparation used. In addition, there is an emphasis on not using LAIV4 for children younger than 2 years.
Two updates to the meningococcal group B vaccine are worth reviewing. The first is that children aged 10 years or older with complement deficiency, complement inhibitor use, or asplenia should receive a meningitis B booster dose beginning 1 year after completion of the primary series, with boosters thereafter every 2 or 3 years as long as that patient remains at greater risk. Another recommendation for patients 10 years or older is that, even if they have received a primary series of meningitis B vaccines, they should receive a booster dose in the setting of an outbreak if it has been 1 year or more since completion of their primary series.
Recommendations have generally been relaxed for tetanus prophylaxis in older children, indicating that individuals requiring tetanus prophylaxis or their 10-year tetanus booster after receipt of at least one Tdap vaccine can receive either tetanus-diphtheria toxoid or Tdap.
COVID-19 vaccines
Although childhood vaccination against COVID-19 is still currently limited to adolescents involved in clinical trials, pediatricians surely are getting peppered with questions from parents about whether they should be vaccinated and what to make of the recent reports about allergic reactions. Fortunately, there are several resources for pediatricians. First, two reports point out that true anaphylactic reactions to COVID-19 vaccines appear quite rare. The reported data on Pfizer-developed mRNA vaccine demonstrated an anaphylaxis rate of approximately 2 cases per 1 million doses administered. Among the 21 recipients who experienced anaphylaxis (out of over 11 million total doses administered), fully one third had a history of anaphylaxis episodes. The report also reviews vaccine reactions that were reported but were not classified as anaphylaxis, pointing out that when reporting vaccine reactions, we should be very careful in the nomenclature we use.
Reporting on the Moderna mRNA vaccine showed anaphylaxis rates of about 2.5 per 1 million doses, with 50% of the recipients who experienced true anaphylaxis having a history of anaphylaxis. Most of those who experienced anaphylaxis (90% in the Moderna group and 86% in the Pfizer group) exhibited symptoms of anaphylaxis within 30 minutes of receiving the vaccine. The take-home point, and the current CDC recommendation, is that many individuals, even those with a history of anaphylaxis, can still receive COVID-19 vaccines. The rates of observed anaphylaxis after COVID vaccination are far below population rates of a history of allergy or severe allergic reactions. When coupled with an estimated mortality rate of 0.5%-1% for SARS-CoV-2 disease, that CDC recommends that we encourage people, even those with severe allergies, to get vaccinated.
One clear caveat is that individuals with a history of severe anaphylaxis, and even those concerned about allergies, should be observed for a longer period after vaccination (at least 30 minutes) than the 15 minutes recommended for the general population. In addition, individuals with a specific anaphylactic reaction or severe allergic reaction to any injectable vaccine should confer with an immunologist before considering vaccination.
Another useful resource is a column published by the American Medical Association that walks through some talking points for providers when discussing whether a patient should receive COVID-19 vaccination. Advice is offered on answering patient questions about which preparation to get, what side effects to watch for, and how to report an adverse reaction. Providers are reminded to urge patients to complete whichever series they begin (get that second dose!), and that they currently should not have to pay for a vaccine. FAQ resource pages are available for patients and health care providers.
More vaccine news: HPV and influenza
Meanwhile, published vaccine reports provide evidence from the field to demonstrate the benefits of vaccination. A study published in the New England Journal of Medicine reported on the effectiveness of human papillomavirus (HPV) vaccine in a Swedish cohort. The report evaluated females aged between 10 and 30 years beginning in 2006 and followed them through 2017, comparing rates of invasive cervical cancer among the group who received one or more HPV vaccine doses with the group who receive none. Even without adjustment, the raw rate of invasive cervical cancer in the vaccinated group was half of that in the unvaccinated group. After full adjustment, some populations experienced incident rate ratios that were greater than 80% reduced. The largest reduction, and therefore the biggest benefit, was among those who received the HPV vaccine before age 17.
A report from the United States looking at the 2018-2019 influenza season demonstrated a vaccine effectiveness rate against hospitalization of 41% and 51% against any ED visit related to influenza. The authors note that there was considerable drift in the influenza A type that appeared late in the influenza season, reducing the overall effectiveness, but that the vaccine was still largely effective.
William T. Basco Jr, MD, MS, is a professor of pediatrics at the Medical University of South Carolina, Charleston, and director of the division of general pediatrics. He is an active health services researcher and has published more than 60 manuscripts in the peer-reviewed literature.
A version of this article first appeared on Medscape.com.
Each February, the Centers for Disease Control and Prevention, along with multiple professional organizations, releases an updated Recommended Child and Adolescent Immunization Schedule.
Recent years have seen fewer changes in the vaccine schedule, mostly with adjustments based on products coming on or off the market, and sometimes with slight changes in recommendations. This year is no different, with mostly minor changes in store. As most practitioners know, having quick access to the tables that accompany the recommendations is always handy. Table 1 contains the typical, recommended immunization schedule. Table 2 contains the catch-up provisions, and Table 3 provides guidance on vaccines for special circumstances and for children with specific medical conditions.
2021 childhood and adolescent immunization schedule
One update is a recommendation that patients with egg allergies who had symptoms more extensive than hives should receive the influenza vaccine in a medical setting where severe allergic reactions or anaphylaxis can be recognized and treated, with the exclusion of two specific preparations, Flublok and Flucelvax.
In regard to the live attenuated influenza vaccine (LAIV), there are several points of reinforcement. First, the nomenclature has generally been changed to “LAIV4” throughout the document because only quadrivalent preparations are available. There are specific recommendations that patients should not receive LAIV4 if they recently took antiviral medication for influenza, with “lockout” periods lasting from 2 days to 17 days, depending on the antiviral preparation used. In addition, there is an emphasis on not using LAIV4 for children younger than 2 years.
Two updates to the meningococcal group B vaccine are worth reviewing. The first is that children aged 10 years or older with complement deficiency, complement inhibitor use, or asplenia should receive a meningitis B booster dose beginning 1 year after completion of the primary series, with boosters thereafter every 2 or 3 years as long as that patient remains at greater risk. Another recommendation for patients 10 years or older is that, even if they have received a primary series of meningitis B vaccines, they should receive a booster dose in the setting of an outbreak if it has been 1 year or more since completion of their primary series.
Recommendations have generally been relaxed for tetanus prophylaxis in older children, indicating that individuals requiring tetanus prophylaxis or their 10-year tetanus booster after receipt of at least one Tdap vaccine can receive either tetanus-diphtheria toxoid or Tdap.
COVID-19 vaccines
Although childhood vaccination against COVID-19 is still currently limited to adolescents involved in clinical trials, pediatricians surely are getting peppered with questions from parents about whether they should be vaccinated and what to make of the recent reports about allergic reactions. Fortunately, there are several resources for pediatricians. First, two reports point out that true anaphylactic reactions to COVID-19 vaccines appear quite rare. The reported data on Pfizer-developed mRNA vaccine demonstrated an anaphylaxis rate of approximately 2 cases per 1 million doses administered. Among the 21 recipients who experienced anaphylaxis (out of over 11 million total doses administered), fully one third had a history of anaphylaxis episodes. The report also reviews vaccine reactions that were reported but were not classified as anaphylaxis, pointing out that when reporting vaccine reactions, we should be very careful in the nomenclature we use.
Reporting on the Moderna mRNA vaccine showed anaphylaxis rates of about 2.5 per 1 million doses, with 50% of the recipients who experienced true anaphylaxis having a history of anaphylaxis. Most of those who experienced anaphylaxis (90% in the Moderna group and 86% in the Pfizer group) exhibited symptoms of anaphylaxis within 30 minutes of receiving the vaccine. The take-home point, and the current CDC recommendation, is that many individuals, even those with a history of anaphylaxis, can still receive COVID-19 vaccines. The rates of observed anaphylaxis after COVID vaccination are far below population rates of a history of allergy or severe allergic reactions. When coupled with an estimated mortality rate of 0.5%-1% for SARS-CoV-2 disease, that CDC recommends that we encourage people, even those with severe allergies, to get vaccinated.
One clear caveat is that individuals with a history of severe anaphylaxis, and even those concerned about allergies, should be observed for a longer period after vaccination (at least 30 minutes) than the 15 minutes recommended for the general population. In addition, individuals with a specific anaphylactic reaction or severe allergic reaction to any injectable vaccine should confer with an immunologist before considering vaccination.
Another useful resource is a column published by the American Medical Association that walks through some talking points for providers when discussing whether a patient should receive COVID-19 vaccination. Advice is offered on answering patient questions about which preparation to get, what side effects to watch for, and how to report an adverse reaction. Providers are reminded to urge patients to complete whichever series they begin (get that second dose!), and that they currently should not have to pay for a vaccine. FAQ resource pages are available for patients and health care providers.
More vaccine news: HPV and influenza
Meanwhile, published vaccine reports provide evidence from the field to demonstrate the benefits of vaccination. A study published in the New England Journal of Medicine reported on the effectiveness of human papillomavirus (HPV) vaccine in a Swedish cohort. The report evaluated females aged between 10 and 30 years beginning in 2006 and followed them through 2017, comparing rates of invasive cervical cancer among the group who received one or more HPV vaccine doses with the group who receive none. Even without adjustment, the raw rate of invasive cervical cancer in the vaccinated group was half of that in the unvaccinated group. After full adjustment, some populations experienced incident rate ratios that were greater than 80% reduced. The largest reduction, and therefore the biggest benefit, was among those who received the HPV vaccine before age 17.
A report from the United States looking at the 2018-2019 influenza season demonstrated a vaccine effectiveness rate against hospitalization of 41% and 51% against any ED visit related to influenza. The authors note that there was considerable drift in the influenza A type that appeared late in the influenza season, reducing the overall effectiveness, but that the vaccine was still largely effective.
William T. Basco Jr, MD, MS, is a professor of pediatrics at the Medical University of South Carolina, Charleston, and director of the division of general pediatrics. He is an active health services researcher and has published more than 60 manuscripts in the peer-reviewed literature.
A version of this article first appeared on Medscape.com.
Each February, the Centers for Disease Control and Prevention, along with multiple professional organizations, releases an updated Recommended Child and Adolescent Immunization Schedule.
Recent years have seen fewer changes in the vaccine schedule, mostly with adjustments based on products coming on or off the market, and sometimes with slight changes in recommendations. This year is no different, with mostly minor changes in store. As most practitioners know, having quick access to the tables that accompany the recommendations is always handy. Table 1 contains the typical, recommended immunization schedule. Table 2 contains the catch-up provisions, and Table 3 provides guidance on vaccines for special circumstances and for children with specific medical conditions.
2021 childhood and adolescent immunization schedule
One update is a recommendation that patients with egg allergies who had symptoms more extensive than hives should receive the influenza vaccine in a medical setting where severe allergic reactions or anaphylaxis can be recognized and treated, with the exclusion of two specific preparations, Flublok and Flucelvax.
In regard to the live attenuated influenza vaccine (LAIV), there are several points of reinforcement. First, the nomenclature has generally been changed to “LAIV4” throughout the document because only quadrivalent preparations are available. There are specific recommendations that patients should not receive LAIV4 if they recently took antiviral medication for influenza, with “lockout” periods lasting from 2 days to 17 days, depending on the antiviral preparation used. In addition, there is an emphasis on not using LAIV4 for children younger than 2 years.
Two updates to the meningococcal group B vaccine are worth reviewing. The first is that children aged 10 years or older with complement deficiency, complement inhibitor use, or asplenia should receive a meningitis B booster dose beginning 1 year after completion of the primary series, with boosters thereafter every 2 or 3 years as long as that patient remains at greater risk. Another recommendation for patients 10 years or older is that, even if they have received a primary series of meningitis B vaccines, they should receive a booster dose in the setting of an outbreak if it has been 1 year or more since completion of their primary series.
Recommendations have generally been relaxed for tetanus prophylaxis in older children, indicating that individuals requiring tetanus prophylaxis or their 10-year tetanus booster after receipt of at least one Tdap vaccine can receive either tetanus-diphtheria toxoid or Tdap.
COVID-19 vaccines
Although childhood vaccination against COVID-19 is still currently limited to adolescents involved in clinical trials, pediatricians surely are getting peppered with questions from parents about whether they should be vaccinated and what to make of the recent reports about allergic reactions. Fortunately, there are several resources for pediatricians. First, two reports point out that true anaphylactic reactions to COVID-19 vaccines appear quite rare. The reported data on Pfizer-developed mRNA vaccine demonstrated an anaphylaxis rate of approximately 2 cases per 1 million doses administered. Among the 21 recipients who experienced anaphylaxis (out of over 11 million total doses administered), fully one third had a history of anaphylaxis episodes. The report also reviews vaccine reactions that were reported but were not classified as anaphylaxis, pointing out that when reporting vaccine reactions, we should be very careful in the nomenclature we use.
Reporting on the Moderna mRNA vaccine showed anaphylaxis rates of about 2.5 per 1 million doses, with 50% of the recipients who experienced true anaphylaxis having a history of anaphylaxis. Most of those who experienced anaphylaxis (90% in the Moderna group and 86% in the Pfizer group) exhibited symptoms of anaphylaxis within 30 minutes of receiving the vaccine. The take-home point, and the current CDC recommendation, is that many individuals, even those with a history of anaphylaxis, can still receive COVID-19 vaccines. The rates of observed anaphylaxis after COVID vaccination are far below population rates of a history of allergy or severe allergic reactions. When coupled with an estimated mortality rate of 0.5%-1% for SARS-CoV-2 disease, that CDC recommends that we encourage people, even those with severe allergies, to get vaccinated.
One clear caveat is that individuals with a history of severe anaphylaxis, and even those concerned about allergies, should be observed for a longer period after vaccination (at least 30 minutes) than the 15 minutes recommended for the general population. In addition, individuals with a specific anaphylactic reaction or severe allergic reaction to any injectable vaccine should confer with an immunologist before considering vaccination.
Another useful resource is a column published by the American Medical Association that walks through some talking points for providers when discussing whether a patient should receive COVID-19 vaccination. Advice is offered on answering patient questions about which preparation to get, what side effects to watch for, and how to report an adverse reaction. Providers are reminded to urge patients to complete whichever series they begin (get that second dose!), and that they currently should not have to pay for a vaccine. FAQ resource pages are available for patients and health care providers.
More vaccine news: HPV and influenza
Meanwhile, published vaccine reports provide evidence from the field to demonstrate the benefits of vaccination. A study published in the New England Journal of Medicine reported on the effectiveness of human papillomavirus (HPV) vaccine in a Swedish cohort. The report evaluated females aged between 10 and 30 years beginning in 2006 and followed them through 2017, comparing rates of invasive cervical cancer among the group who received one or more HPV vaccine doses with the group who receive none. Even without adjustment, the raw rate of invasive cervical cancer in the vaccinated group was half of that in the unvaccinated group. After full adjustment, some populations experienced incident rate ratios that were greater than 80% reduced. The largest reduction, and therefore the biggest benefit, was among those who received the HPV vaccine before age 17.
A report from the United States looking at the 2018-2019 influenza season demonstrated a vaccine effectiveness rate against hospitalization of 41% and 51% against any ED visit related to influenza. The authors note that there was considerable drift in the influenza A type that appeared late in the influenza season, reducing the overall effectiveness, but that the vaccine was still largely effective.
William T. Basco Jr, MD, MS, is a professor of pediatrics at the Medical University of South Carolina, Charleston, and director of the division of general pediatrics. He is an active health services researcher and has published more than 60 manuscripts in the peer-reviewed literature.
A version of this article first appeared on Medscape.com.
USPSTF plan for revising breast screening guidance questioned
The U.S. Preventive Services Task Force is planning to update its breast cancer screening guidelines, which were last issued in 2016. For transparency, it has released the draft research plan it will use for formulating the update, and this draft plan is open for comment until Feb. 17.
However, an expert in breast screening has taken issue with the whole plan.
Daniel Kopans, MD, professor of radiology at Harvard Medical School and founder of the Breast Imaging Division at Massachusetts General Hospital, Boston, argues that previous USPSTF guidelines on breast cancer screening “have been based on flawed analyses of scientific data” and the research plan, as outlined, perpetuates this.
He has also objected, yet again, to the USPSTF panel not having any experts in breast screening on the panel.
Writing in a commentary on Aunt Minnie, a radiology website, he warns about the dangers of not listening to experts: “The COVID-19 pandemic has demonstrated the tragic consequences that result from ignoring science, evidence, and the analysis and advice of experts while being guided by inexpert advice.”
Controversy over previous guidelines
The current USPSTF guidelines on breast cancer screening, which were issued in 2016, were largely unchanged from the previous guidelines that had been issued in 2009. They recommended mammography screening every 2 years for women 50-74 years of age but said that women aged 40-49 should make individual decisions about screening in partnership with their doctors.
The guidance on younger women was met with severe criticism from many experts, as previously reported by this news organization, and the every-2-year interval has also been questioned.
The American College of Radiology and Society of Breast Imaging both recommend annual mammograms starting at age 40.
In the update the USPSTF is now planning, it has an opportunity to “revisit the group’s flawed decision in 2009” about not recommending screening for women in their 40s, argues Dr. Kopans.
But to do that, a number of factors need to be addressed to present a fair and impartial review of the science and evidence in favor of breast screening, he continues, while worrying the draft plan, as currently outlined, will not do so.
One big problem, he argues, is that USPSTF, in its draft plan, has not included statistical models from the U.S. National Cancer Institute and Cancer Intervention and Surveillance Modeling Network to project the potential outcomes of various screening protocols. These NCI/CISNET models all predict that the most lives are saved by annual screening starting at age 40, he points out.
Without these models, the USPSTF will be “guessing in their predictions,” he argues.
Second, even though a reduction in advanced-stage disease is a potentially useful “surrogate endpoint,” Dr. Kopans points out that it is still crucial to remember that women diagnosed at all stages of breast cancer die of the disease. “It has been shown that reducing the size of cancers within stages is also a major benefit from screening that reduces deaths,” he says.
Third, he contends in his commentary that there is a “false claim that the background incidence of breast cancer has not increased over time.” Dr. Kopans says this has been the primary source of misinformation that has been used to promote “the false concepts of massive overdiagnosis” as well as a “false claim that there has not been a reduction in advanced cancers.”
To emphasize his point, Dr. Kopans explains that data clearly demonstrate that the baseline incidence of breast cancer has steadily risen by 1%-1.3% per year, going back at least 80 years. This increase predates screening, which didn’t really begin until the mid-1980s.
“If the correct increasing baseline is used, not only is there no apparent ‘overdiagnosis’ of invasive cancers, but it appears that there has been a major reduction in the incidence of invasive cancers,” he writes. “By using the correct baseline incidence and extrapolation, it is also clear that there has been a major reduction in the rate of advanced cancers.”
To date, there have not been any randomized controlled trials comparing screening intervals (for example, annual vs. every second or third year). But based on the CISNET models, Dr. Kopans emphasized that annual screening is estimated to provide the greatest reduction in deaths. “All women ages 40-74 should be encouraged to be screened every year,” he says.
A version of this article first appeared on Medscape.com.
The U.S. Preventive Services Task Force is planning to update its breast cancer screening guidelines, which were last issued in 2016. For transparency, it has released the draft research plan it will use for formulating the update, and this draft plan is open for comment until Feb. 17.
However, an expert in breast screening has taken issue with the whole plan.
Daniel Kopans, MD, professor of radiology at Harvard Medical School and founder of the Breast Imaging Division at Massachusetts General Hospital, Boston, argues that previous USPSTF guidelines on breast cancer screening “have been based on flawed analyses of scientific data” and the research plan, as outlined, perpetuates this.
He has also objected, yet again, to the USPSTF panel not having any experts in breast screening on the panel.
Writing in a commentary on Aunt Minnie, a radiology website, he warns about the dangers of not listening to experts: “The COVID-19 pandemic has demonstrated the tragic consequences that result from ignoring science, evidence, and the analysis and advice of experts while being guided by inexpert advice.”
Controversy over previous guidelines
The current USPSTF guidelines on breast cancer screening, which were issued in 2016, were largely unchanged from the previous guidelines that had been issued in 2009. They recommended mammography screening every 2 years for women 50-74 years of age but said that women aged 40-49 should make individual decisions about screening in partnership with their doctors.
The guidance on younger women was met with severe criticism from many experts, as previously reported by this news organization, and the every-2-year interval has also been questioned.
The American College of Radiology and Society of Breast Imaging both recommend annual mammograms starting at age 40.
In the update the USPSTF is now planning, it has an opportunity to “revisit the group’s flawed decision in 2009” about not recommending screening for women in their 40s, argues Dr. Kopans.
But to do that, a number of factors need to be addressed to present a fair and impartial review of the science and evidence in favor of breast screening, he continues, while worrying the draft plan, as currently outlined, will not do so.
One big problem, he argues, is that USPSTF, in its draft plan, has not included statistical models from the U.S. National Cancer Institute and Cancer Intervention and Surveillance Modeling Network to project the potential outcomes of various screening protocols. These NCI/CISNET models all predict that the most lives are saved by annual screening starting at age 40, he points out.
Without these models, the USPSTF will be “guessing in their predictions,” he argues.
Second, even though a reduction in advanced-stage disease is a potentially useful “surrogate endpoint,” Dr. Kopans points out that it is still crucial to remember that women diagnosed at all stages of breast cancer die of the disease. “It has been shown that reducing the size of cancers within stages is also a major benefit from screening that reduces deaths,” he says.
Third, he contends in his commentary that there is a “false claim that the background incidence of breast cancer has not increased over time.” Dr. Kopans says this has been the primary source of misinformation that has been used to promote “the false concepts of massive overdiagnosis” as well as a “false claim that there has not been a reduction in advanced cancers.”
To emphasize his point, Dr. Kopans explains that data clearly demonstrate that the baseline incidence of breast cancer has steadily risen by 1%-1.3% per year, going back at least 80 years. This increase predates screening, which didn’t really begin until the mid-1980s.
“If the correct increasing baseline is used, not only is there no apparent ‘overdiagnosis’ of invasive cancers, but it appears that there has been a major reduction in the incidence of invasive cancers,” he writes. “By using the correct baseline incidence and extrapolation, it is also clear that there has been a major reduction in the rate of advanced cancers.”
To date, there have not been any randomized controlled trials comparing screening intervals (for example, annual vs. every second or third year). But based on the CISNET models, Dr. Kopans emphasized that annual screening is estimated to provide the greatest reduction in deaths. “All women ages 40-74 should be encouraged to be screened every year,” he says.
A version of this article first appeared on Medscape.com.
The U.S. Preventive Services Task Force is planning to update its breast cancer screening guidelines, which were last issued in 2016. For transparency, it has released the draft research plan it will use for formulating the update, and this draft plan is open for comment until Feb. 17.
However, an expert in breast screening has taken issue with the whole plan.
Daniel Kopans, MD, professor of radiology at Harvard Medical School and founder of the Breast Imaging Division at Massachusetts General Hospital, Boston, argues that previous USPSTF guidelines on breast cancer screening “have been based on flawed analyses of scientific data” and the research plan, as outlined, perpetuates this.
He has also objected, yet again, to the USPSTF panel not having any experts in breast screening on the panel.
Writing in a commentary on Aunt Minnie, a radiology website, he warns about the dangers of not listening to experts: “The COVID-19 pandemic has demonstrated the tragic consequences that result from ignoring science, evidence, and the analysis and advice of experts while being guided by inexpert advice.”
Controversy over previous guidelines
The current USPSTF guidelines on breast cancer screening, which were issued in 2016, were largely unchanged from the previous guidelines that had been issued in 2009. They recommended mammography screening every 2 years for women 50-74 years of age but said that women aged 40-49 should make individual decisions about screening in partnership with their doctors.
The guidance on younger women was met with severe criticism from many experts, as previously reported by this news organization, and the every-2-year interval has also been questioned.
The American College of Radiology and Society of Breast Imaging both recommend annual mammograms starting at age 40.
In the update the USPSTF is now planning, it has an opportunity to “revisit the group’s flawed decision in 2009” about not recommending screening for women in their 40s, argues Dr. Kopans.
But to do that, a number of factors need to be addressed to present a fair and impartial review of the science and evidence in favor of breast screening, he continues, while worrying the draft plan, as currently outlined, will not do so.
One big problem, he argues, is that USPSTF, in its draft plan, has not included statistical models from the U.S. National Cancer Institute and Cancer Intervention and Surveillance Modeling Network to project the potential outcomes of various screening protocols. These NCI/CISNET models all predict that the most lives are saved by annual screening starting at age 40, he points out.
Without these models, the USPSTF will be “guessing in their predictions,” he argues.
Second, even though a reduction in advanced-stage disease is a potentially useful “surrogate endpoint,” Dr. Kopans points out that it is still crucial to remember that women diagnosed at all stages of breast cancer die of the disease. “It has been shown that reducing the size of cancers within stages is also a major benefit from screening that reduces deaths,” he says.
Third, he contends in his commentary that there is a “false claim that the background incidence of breast cancer has not increased over time.” Dr. Kopans says this has been the primary source of misinformation that has been used to promote “the false concepts of massive overdiagnosis” as well as a “false claim that there has not been a reduction in advanced cancers.”
To emphasize his point, Dr. Kopans explains that data clearly demonstrate that the baseline incidence of breast cancer has steadily risen by 1%-1.3% per year, going back at least 80 years. This increase predates screening, which didn’t really begin until the mid-1980s.
“If the correct increasing baseline is used, not only is there no apparent ‘overdiagnosis’ of invasive cancers, but it appears that there has been a major reduction in the incidence of invasive cancers,” he writes. “By using the correct baseline incidence and extrapolation, it is also clear that there has been a major reduction in the rate of advanced cancers.”
To date, there have not been any randomized controlled trials comparing screening intervals (for example, annual vs. every second or third year). But based on the CISNET models, Dr. Kopans emphasized that annual screening is estimated to provide the greatest reduction in deaths. “All women ages 40-74 should be encouraged to be screened every year,” he says.
A version of this article first appeared on Medscape.com.
BY ROXANNE NELSON, RN, BSN
AGA Clinical Practice Update: Diagnosis and management of immune checkpoint inhibitor enterocolitis and hepatitis
Endoscopy with biopsies is best for diagnosing immune-mediated enterocolitis in patients receiving immune checkpoint inhibitors (ICIs), but another option is to first test the stool for lactoferrin or calprotectin to identify patients with mild diarrhea who could benefit from endoscopy, according to a clinical practice update from the American Gastroenterological Association.
Writing in Gastroenterology, Michael Dougan, MD, PhD, of Harvard Medical School, Boston, and colleagues noted that stool lactoferrin had been found in one study to be 90% sensitive for detecting histologic inflammation, while another study found that mucosal inflammation is absent in 20%-30% of patients with suspected ICI enterocolitis. Nonetheless, clinicians should consider diagnostic endoscopy before starting high-dose corticosteroids for ICI enterocolitis, especially because “colonic ulceration identified by endoscopy is the only established factor that predicts how ICI enterocolitis will respond to treatment,” Dr. Dougan and colleagues wrote. If performed, endoscopy must be prompt because ICI colitis can progress within days, especially if patients are receiving ipilimumab.
ICIs can induce autoimmune inflammation in almost any organ system because they target pathways that play “key roles in regulating autoimmunity,” the experts wrote. The gastrointestinal tract is one of the most common sites of toxicity: One study from 2006 and another from 2019 suggested that colitis, with or without enteritis, affects up to 40% of patients depending on the pathway targeted by the treatment. Oncologists manage most gastrointestinal ICI toxicities, but gastroenterologists and hepatologists often help with diagnosis, risk assessment, and managing complex, atypical, or treatment-refractory cases; to help guide this process, the experts reviewed the literature and made 15 relevant recommendations.
The authors noted that the differential diagnosis is broad, but suggested that Clostridioides difficile testing and stool culture (or stool pathogen testing, where available) should be performed in all patients to rule out infectious causes prior to any immunosuppressive treatments, such as corticosteroids. Abdominal imaging is not recommended if a patient only has diarrhea but can help rule out complications if fever, bleeding, or abdominal pain are also present. Laboratory blood tests are rarely informative.
High-dose glucocorticoids are usually effective, often being started at 0.5-2.0 mg/kg prednisone or equivalent daily and tapered over 4-6 weeks after clinical improvement, but these doses and schedules have not been rigorously examined. For glucocorticoid-refractory ICI enterocolitis, infliximab and vedolizumab “are reasonable options” for second line immunosuppression and should be individualized based on the underlying cancer and other risk factors; patients usually respond to these immunomodulators in less than a week, “an important contrast with IBD,” the experts wrote. Most cases of ICI enterocolitis do not recur unless the ICI is restarted, but “many patients require the full loading dose for infliximab or vedolizumab, and maintenance therapy may still be required for certain cases.”
ICI-induced hepatitis is less common, affecting less than 5% of patients in clinical trials according to the authors, but incidence rises if patients are on ICI combinations or an ICI plus chemotherapy. Before starting any ICI, patients’ total bilirubin, alkaline phosphatase, AST, and ALT levels should be checked, as should testing for hepatitis B. Liver chemistries should be repeated before each ICI cycle, and rising chemistries should trigger an assessment for other causes of liver injury.
Patients with Common Terminology Criteria for Adverse Events (CTCAE) grade 1 hepatitis – defined as AST or ALT 1-3 times the upper limit of normal or total bilirubin 1-1.5 times upper limit of normal – should receive liver function tests once or twice weekly. For CTCAE grade 2 hepatitis, (AST/ALT more than 3-5 times upper limit of normal or total bilirubin more than 1.5-3 times upper limit of normal), ICI should be held until resolution to grade 1, and corticosteroids (prednisone or its equivalent dosed at 0.5-1.0 mg/kg daily) should be considered if there are clinical symptoms of liver toxicity. For grade 3 hepatitis (AST/ALT greater than 5-20 times upper limit of normal or total bilirubin more than 3-10 times upper limit of normal), ICI therapy should be halted, “and urgent consultation with a gastroenterologist/hepatologist is appropriate.” In this context, methylprednisone (1-2 mg/kg) is suggested, and azathioprine or mycophenolate mofetil can be considered if clinical hepatitis does not improve in 3-5 days. For CTCAE grade 4 hepatitis, hospitalization is recommended, and patients should permanently stop the ICI and receive 2 mg/kg per day of methylprednisolone or its equivalent.
The authors received no funding support. Dr. Dougan reported consulting or advisory relationships with Neoleukin Therapeutics, Genentech, Tillotts Pharma, and Partner Therapeutics and grant support from Novartis and Genentech. Two coauthors also reported ties to several pharmaceutical companies.
Endoscopy with biopsies is best for diagnosing immune-mediated enterocolitis in patients receiving immune checkpoint inhibitors (ICIs), but another option is to first test the stool for lactoferrin or calprotectin to identify patients with mild diarrhea who could benefit from endoscopy, according to a clinical practice update from the American Gastroenterological Association.
Writing in Gastroenterology, Michael Dougan, MD, PhD, of Harvard Medical School, Boston, and colleagues noted that stool lactoferrin had been found in one study to be 90% sensitive for detecting histologic inflammation, while another study found that mucosal inflammation is absent in 20%-30% of patients with suspected ICI enterocolitis. Nonetheless, clinicians should consider diagnostic endoscopy before starting high-dose corticosteroids for ICI enterocolitis, especially because “colonic ulceration identified by endoscopy is the only established factor that predicts how ICI enterocolitis will respond to treatment,” Dr. Dougan and colleagues wrote. If performed, endoscopy must be prompt because ICI colitis can progress within days, especially if patients are receiving ipilimumab.
ICIs can induce autoimmune inflammation in almost any organ system because they target pathways that play “key roles in regulating autoimmunity,” the experts wrote. The gastrointestinal tract is one of the most common sites of toxicity: One study from 2006 and another from 2019 suggested that colitis, with or without enteritis, affects up to 40% of patients depending on the pathway targeted by the treatment. Oncologists manage most gastrointestinal ICI toxicities, but gastroenterologists and hepatologists often help with diagnosis, risk assessment, and managing complex, atypical, or treatment-refractory cases; to help guide this process, the experts reviewed the literature and made 15 relevant recommendations.
The authors noted that the differential diagnosis is broad, but suggested that Clostridioides difficile testing and stool culture (or stool pathogen testing, where available) should be performed in all patients to rule out infectious causes prior to any immunosuppressive treatments, such as corticosteroids. Abdominal imaging is not recommended if a patient only has diarrhea but can help rule out complications if fever, bleeding, or abdominal pain are also present. Laboratory blood tests are rarely informative.
High-dose glucocorticoids are usually effective, often being started at 0.5-2.0 mg/kg prednisone or equivalent daily and tapered over 4-6 weeks after clinical improvement, but these doses and schedules have not been rigorously examined. For glucocorticoid-refractory ICI enterocolitis, infliximab and vedolizumab “are reasonable options” for second line immunosuppression and should be individualized based on the underlying cancer and other risk factors; patients usually respond to these immunomodulators in less than a week, “an important contrast with IBD,” the experts wrote. Most cases of ICI enterocolitis do not recur unless the ICI is restarted, but “many patients require the full loading dose for infliximab or vedolizumab, and maintenance therapy may still be required for certain cases.”
ICI-induced hepatitis is less common, affecting less than 5% of patients in clinical trials according to the authors, but incidence rises if patients are on ICI combinations or an ICI plus chemotherapy. Before starting any ICI, patients’ total bilirubin, alkaline phosphatase, AST, and ALT levels should be checked, as should testing for hepatitis B. Liver chemistries should be repeated before each ICI cycle, and rising chemistries should trigger an assessment for other causes of liver injury.
Patients with Common Terminology Criteria for Adverse Events (CTCAE) grade 1 hepatitis – defined as AST or ALT 1-3 times the upper limit of normal or total bilirubin 1-1.5 times upper limit of normal – should receive liver function tests once or twice weekly. For CTCAE grade 2 hepatitis, (AST/ALT more than 3-5 times upper limit of normal or total bilirubin more than 1.5-3 times upper limit of normal), ICI should be held until resolution to grade 1, and corticosteroids (prednisone or its equivalent dosed at 0.5-1.0 mg/kg daily) should be considered if there are clinical symptoms of liver toxicity. For grade 3 hepatitis (AST/ALT greater than 5-20 times upper limit of normal or total bilirubin more than 3-10 times upper limit of normal), ICI therapy should be halted, “and urgent consultation with a gastroenterologist/hepatologist is appropriate.” In this context, methylprednisone (1-2 mg/kg) is suggested, and azathioprine or mycophenolate mofetil can be considered if clinical hepatitis does not improve in 3-5 days. For CTCAE grade 4 hepatitis, hospitalization is recommended, and patients should permanently stop the ICI and receive 2 mg/kg per day of methylprednisolone or its equivalent.
The authors received no funding support. Dr. Dougan reported consulting or advisory relationships with Neoleukin Therapeutics, Genentech, Tillotts Pharma, and Partner Therapeutics and grant support from Novartis and Genentech. Two coauthors also reported ties to several pharmaceutical companies.
Endoscopy with biopsies is best for diagnosing immune-mediated enterocolitis in patients receiving immune checkpoint inhibitors (ICIs), but another option is to first test the stool for lactoferrin or calprotectin to identify patients with mild diarrhea who could benefit from endoscopy, according to a clinical practice update from the American Gastroenterological Association.
Writing in Gastroenterology, Michael Dougan, MD, PhD, of Harvard Medical School, Boston, and colleagues noted that stool lactoferrin had been found in one study to be 90% sensitive for detecting histologic inflammation, while another study found that mucosal inflammation is absent in 20%-30% of patients with suspected ICI enterocolitis. Nonetheless, clinicians should consider diagnostic endoscopy before starting high-dose corticosteroids for ICI enterocolitis, especially because “colonic ulceration identified by endoscopy is the only established factor that predicts how ICI enterocolitis will respond to treatment,” Dr. Dougan and colleagues wrote. If performed, endoscopy must be prompt because ICI colitis can progress within days, especially if patients are receiving ipilimumab.
ICIs can induce autoimmune inflammation in almost any organ system because they target pathways that play “key roles in regulating autoimmunity,” the experts wrote. The gastrointestinal tract is one of the most common sites of toxicity: One study from 2006 and another from 2019 suggested that colitis, with or without enteritis, affects up to 40% of patients depending on the pathway targeted by the treatment. Oncologists manage most gastrointestinal ICI toxicities, but gastroenterologists and hepatologists often help with diagnosis, risk assessment, and managing complex, atypical, or treatment-refractory cases; to help guide this process, the experts reviewed the literature and made 15 relevant recommendations.
The authors noted that the differential diagnosis is broad, but suggested that Clostridioides difficile testing and stool culture (or stool pathogen testing, where available) should be performed in all patients to rule out infectious causes prior to any immunosuppressive treatments, such as corticosteroids. Abdominal imaging is not recommended if a patient only has diarrhea but can help rule out complications if fever, bleeding, or abdominal pain are also present. Laboratory blood tests are rarely informative.
High-dose glucocorticoids are usually effective, often being started at 0.5-2.0 mg/kg prednisone or equivalent daily and tapered over 4-6 weeks after clinical improvement, but these doses and schedules have not been rigorously examined. For glucocorticoid-refractory ICI enterocolitis, infliximab and vedolizumab “are reasonable options” for second line immunosuppression and should be individualized based on the underlying cancer and other risk factors; patients usually respond to these immunomodulators in less than a week, “an important contrast with IBD,” the experts wrote. Most cases of ICI enterocolitis do not recur unless the ICI is restarted, but “many patients require the full loading dose for infliximab or vedolizumab, and maintenance therapy may still be required for certain cases.”
ICI-induced hepatitis is less common, affecting less than 5% of patients in clinical trials according to the authors, but incidence rises if patients are on ICI combinations or an ICI plus chemotherapy. Before starting any ICI, patients’ total bilirubin, alkaline phosphatase, AST, and ALT levels should be checked, as should testing for hepatitis B. Liver chemistries should be repeated before each ICI cycle, and rising chemistries should trigger an assessment for other causes of liver injury.
Patients with Common Terminology Criteria for Adverse Events (CTCAE) grade 1 hepatitis – defined as AST or ALT 1-3 times the upper limit of normal or total bilirubin 1-1.5 times upper limit of normal – should receive liver function tests once or twice weekly. For CTCAE grade 2 hepatitis, (AST/ALT more than 3-5 times upper limit of normal or total bilirubin more than 1.5-3 times upper limit of normal), ICI should be held until resolution to grade 1, and corticosteroids (prednisone or its equivalent dosed at 0.5-1.0 mg/kg daily) should be considered if there are clinical symptoms of liver toxicity. For grade 3 hepatitis (AST/ALT greater than 5-20 times upper limit of normal or total bilirubin more than 3-10 times upper limit of normal), ICI therapy should be halted, “and urgent consultation with a gastroenterologist/hepatologist is appropriate.” In this context, methylprednisone (1-2 mg/kg) is suggested, and azathioprine or mycophenolate mofetil can be considered if clinical hepatitis does not improve in 3-5 days. For CTCAE grade 4 hepatitis, hospitalization is recommended, and patients should permanently stop the ICI and receive 2 mg/kg per day of methylprednisolone or its equivalent.
The authors received no funding support. Dr. Dougan reported consulting or advisory relationships with Neoleukin Therapeutics, Genentech, Tillotts Pharma, and Partner Therapeutics and grant support from Novartis and Genentech. Two coauthors also reported ties to several pharmaceutical companies.
FROM GASTROENTEROLOGY
COVID-19 vaccination in cancer patients: NCCN outlines priorities
Vaccination timing considerations vary based on factors such as cancer and treatment type, and reasons for delaying vaccination in the general public also apply to cancer patients (recent COVID-19 exposure, for example).
In general, however, patients with cancer should be assigned to Centers for Disease Control and Prevention priority group 1 b/c and immunized when vaccination is available to them, the guidelines state. Exceptions to this recommendation include:
- Patients undergoing hematopoietic stem cell transplant or receiving engineered cellular therapy such as chimeric antigen receptor T-cell therapy. Vaccination should be delayed for at least 3 months in these patients to maximize vaccine efficacy. Caregivers of these patients, however, should be immunized when possible.
- Patients with hematologic malignancies who are receiving intensive cytotoxic chemotherapy, such as cytarabine- or anthracycline-based regimens for acute myeloid leukemia. Vaccination in these patients should be delayed until absolute neutrophil count recovery.
- Patients undergoing major surgery. Vaccination should occur at least a few days before or after surgery.
- Patients who have experienced a severe or immediate adverse reaction to any of the ingredients in the mRNA COVID-19 vaccines.
Conversely, vaccination should occur when available in patients with hematologic malignancies and marrow failure who are expected to have limited or no recovery, patients with hematologic malignancies who are on long-term maintenance therapy, and patients with solid tumors who are receiving cytotoxic chemotherapy, targeted therapy, checkpoint inhibitors and other immunotherapy, or radiotherapy.
Caregivers, household contacts, and other close contacts who are 16 years of age and older should be vaccinated whenever they are eligible.
Unique concerns in patients with cancer
The NCCN recommendations were developed to address the unique issues and concerns with respect to patients with cancer, who have an increased risk of severe illness from SARS-CoV-2 infection. But the guidelines come with a caveat: “[t]here are limited safety and efficacy data in these patients,” the NCCN emphasized in a press statement.
“Right now, there is urgent need and limited data,” Steven Pergam, MD, co-leader of the NCCN COVID-19 Vaccination Committee, said in the statement.
“Our number one goal is helping to get the vaccine to as many people as we can,” Dr. Pergam said. “That means following existing national and regional directions for prioritizing people who are more likely to face death or severe illness from COVID-19.”
Dr. Pergam, associate professor at Fred Hutchinson Cancer Research Center in Seattle, further explained that “people receiving active cancer treatment are at greater risk for worse outcomes from COVID-19, particularly if they are older and have additional comorbidities, like immunosuppression.”
NCCN’s recommendations couldn’t have come at a better time for patients with cancer, according to Nora Disis, MD, a professor at the University of Washington in Seattle.
“The NCCN’s recommendations to prioritize COVID vaccinations for cancer patients on active treatment is an important step forward in protecting our patients from the infection,” Dr. Disis said in an interview.
“Cancer patients may be at higher risk for the complications seen with infection. In addition, cancer is a disease of older people, and a good number of our patients have the comorbidities that would predict a poorer outcome if they should become sick,” Dr. Disis added. “With the correct treatment, many patients with cancer will be long-term survivors. It is important that they be protected from infection with COVID to realize their best outcome.”
Additional vaccine considerations
The NCCN recommendations also address several other issues of importance for cancer patients, including:
- Deprioritizing other vaccines. COVID-19 vaccines should take precedence over other vaccines because data on dual vaccination are lacking. The NCCN recommends waiting 14 days after COVID-19 vaccination to deliver other vaccines.
- Vaccinating clinical trial participants. Trial leads should be consulted to prevent protocol violations or exclusions.
- Decision-making in the setting of limited vaccine availability. The NCCN noted that decisions on allocation must be made in accordance with state and local vaccine guidance but suggests prioritizing appropriate patients on active treatment, those planning to start treatment, and those who have just completed treatment. Additional risk factors for these patients, as well as other factors associated with risk for adverse COVID-19 outcomes, should also be considered. These include advanced age, comorbidities, and adverse social and demographic factors such as poverty and limited health care access.
- The need for ongoing prevention measures. Vaccines have been shown to decrease the incidence of COVID-19 and related complications, but it remains unclear whether vaccines prevent infection and subsequent transmission. This means everyone should continue following prevention recommendations, such as wearing masks and avoiding crowds.
The NCCN stressed that these recommendations are “intended to be a living document that is constantly evolving – it will be updated rapidly whenever new data comes out, as well as any potential new vaccines that may get approved in the future.” The NCCN also noted that the advisory committee will meet regularly to refine the recommendations as needed.
Dr. Pergam disclosed relationships with Chimerix Inc., Merck & Co., Global Life Technologies Inc., and Sanofi-Aventis. Dr. Disis disclosed grants from Pfizer, Bavarian Nordisk, Janssen, and Precigen. She is the founder of EpiThany and editor-in-chief of JAMA Oncology.
Vaccination timing considerations vary based on factors such as cancer and treatment type, and reasons for delaying vaccination in the general public also apply to cancer patients (recent COVID-19 exposure, for example).
In general, however, patients with cancer should be assigned to Centers for Disease Control and Prevention priority group 1 b/c and immunized when vaccination is available to them, the guidelines state. Exceptions to this recommendation include:
- Patients undergoing hematopoietic stem cell transplant or receiving engineered cellular therapy such as chimeric antigen receptor T-cell therapy. Vaccination should be delayed for at least 3 months in these patients to maximize vaccine efficacy. Caregivers of these patients, however, should be immunized when possible.
- Patients with hematologic malignancies who are receiving intensive cytotoxic chemotherapy, such as cytarabine- or anthracycline-based regimens for acute myeloid leukemia. Vaccination in these patients should be delayed until absolute neutrophil count recovery.
- Patients undergoing major surgery. Vaccination should occur at least a few days before or after surgery.
- Patients who have experienced a severe or immediate adverse reaction to any of the ingredients in the mRNA COVID-19 vaccines.
Conversely, vaccination should occur when available in patients with hematologic malignancies and marrow failure who are expected to have limited or no recovery, patients with hematologic malignancies who are on long-term maintenance therapy, and patients with solid tumors who are receiving cytotoxic chemotherapy, targeted therapy, checkpoint inhibitors and other immunotherapy, or radiotherapy.
Caregivers, household contacts, and other close contacts who are 16 years of age and older should be vaccinated whenever they are eligible.
Unique concerns in patients with cancer
The NCCN recommendations were developed to address the unique issues and concerns with respect to patients with cancer, who have an increased risk of severe illness from SARS-CoV-2 infection. But the guidelines come with a caveat: “[t]here are limited safety and efficacy data in these patients,” the NCCN emphasized in a press statement.
“Right now, there is urgent need and limited data,” Steven Pergam, MD, co-leader of the NCCN COVID-19 Vaccination Committee, said in the statement.
“Our number one goal is helping to get the vaccine to as many people as we can,” Dr. Pergam said. “That means following existing national and regional directions for prioritizing people who are more likely to face death or severe illness from COVID-19.”
Dr. Pergam, associate professor at Fred Hutchinson Cancer Research Center in Seattle, further explained that “people receiving active cancer treatment are at greater risk for worse outcomes from COVID-19, particularly if they are older and have additional comorbidities, like immunosuppression.”
NCCN’s recommendations couldn’t have come at a better time for patients with cancer, according to Nora Disis, MD, a professor at the University of Washington in Seattle.
“The NCCN’s recommendations to prioritize COVID vaccinations for cancer patients on active treatment is an important step forward in protecting our patients from the infection,” Dr. Disis said in an interview.
“Cancer patients may be at higher risk for the complications seen with infection. In addition, cancer is a disease of older people, and a good number of our patients have the comorbidities that would predict a poorer outcome if they should become sick,” Dr. Disis added. “With the correct treatment, many patients with cancer will be long-term survivors. It is important that they be protected from infection with COVID to realize their best outcome.”
Additional vaccine considerations
The NCCN recommendations also address several other issues of importance for cancer patients, including:
- Deprioritizing other vaccines. COVID-19 vaccines should take precedence over other vaccines because data on dual vaccination are lacking. The NCCN recommends waiting 14 days after COVID-19 vaccination to deliver other vaccines.
- Vaccinating clinical trial participants. Trial leads should be consulted to prevent protocol violations or exclusions.
- Decision-making in the setting of limited vaccine availability. The NCCN noted that decisions on allocation must be made in accordance with state and local vaccine guidance but suggests prioritizing appropriate patients on active treatment, those planning to start treatment, and those who have just completed treatment. Additional risk factors for these patients, as well as other factors associated with risk for adverse COVID-19 outcomes, should also be considered. These include advanced age, comorbidities, and adverse social and demographic factors such as poverty and limited health care access.
- The need for ongoing prevention measures. Vaccines have been shown to decrease the incidence of COVID-19 and related complications, but it remains unclear whether vaccines prevent infection and subsequent transmission. This means everyone should continue following prevention recommendations, such as wearing masks and avoiding crowds.
The NCCN stressed that these recommendations are “intended to be a living document that is constantly evolving – it will be updated rapidly whenever new data comes out, as well as any potential new vaccines that may get approved in the future.” The NCCN also noted that the advisory committee will meet regularly to refine the recommendations as needed.
Dr. Pergam disclosed relationships with Chimerix Inc., Merck & Co., Global Life Technologies Inc., and Sanofi-Aventis. Dr. Disis disclosed grants from Pfizer, Bavarian Nordisk, Janssen, and Precigen. She is the founder of EpiThany and editor-in-chief of JAMA Oncology.
Vaccination timing considerations vary based on factors such as cancer and treatment type, and reasons for delaying vaccination in the general public also apply to cancer patients (recent COVID-19 exposure, for example).
In general, however, patients with cancer should be assigned to Centers for Disease Control and Prevention priority group 1 b/c and immunized when vaccination is available to them, the guidelines state. Exceptions to this recommendation include:
- Patients undergoing hematopoietic stem cell transplant or receiving engineered cellular therapy such as chimeric antigen receptor T-cell therapy. Vaccination should be delayed for at least 3 months in these patients to maximize vaccine efficacy. Caregivers of these patients, however, should be immunized when possible.
- Patients with hematologic malignancies who are receiving intensive cytotoxic chemotherapy, such as cytarabine- or anthracycline-based regimens for acute myeloid leukemia. Vaccination in these patients should be delayed until absolute neutrophil count recovery.
- Patients undergoing major surgery. Vaccination should occur at least a few days before or after surgery.
- Patients who have experienced a severe or immediate adverse reaction to any of the ingredients in the mRNA COVID-19 vaccines.
Conversely, vaccination should occur when available in patients with hematologic malignancies and marrow failure who are expected to have limited or no recovery, patients with hematologic malignancies who are on long-term maintenance therapy, and patients with solid tumors who are receiving cytotoxic chemotherapy, targeted therapy, checkpoint inhibitors and other immunotherapy, or radiotherapy.
Caregivers, household contacts, and other close contacts who are 16 years of age and older should be vaccinated whenever they are eligible.
Unique concerns in patients with cancer
The NCCN recommendations were developed to address the unique issues and concerns with respect to patients with cancer, who have an increased risk of severe illness from SARS-CoV-2 infection. But the guidelines come with a caveat: “[t]here are limited safety and efficacy data in these patients,” the NCCN emphasized in a press statement.
“Right now, there is urgent need and limited data,” Steven Pergam, MD, co-leader of the NCCN COVID-19 Vaccination Committee, said in the statement.
“Our number one goal is helping to get the vaccine to as many people as we can,” Dr. Pergam said. “That means following existing national and regional directions for prioritizing people who are more likely to face death or severe illness from COVID-19.”
Dr. Pergam, associate professor at Fred Hutchinson Cancer Research Center in Seattle, further explained that “people receiving active cancer treatment are at greater risk for worse outcomes from COVID-19, particularly if they are older and have additional comorbidities, like immunosuppression.”
NCCN’s recommendations couldn’t have come at a better time for patients with cancer, according to Nora Disis, MD, a professor at the University of Washington in Seattle.
“The NCCN’s recommendations to prioritize COVID vaccinations for cancer patients on active treatment is an important step forward in protecting our patients from the infection,” Dr. Disis said in an interview.
“Cancer patients may be at higher risk for the complications seen with infection. In addition, cancer is a disease of older people, and a good number of our patients have the comorbidities that would predict a poorer outcome if they should become sick,” Dr. Disis added. “With the correct treatment, many patients with cancer will be long-term survivors. It is important that they be protected from infection with COVID to realize their best outcome.”
Additional vaccine considerations
The NCCN recommendations also address several other issues of importance for cancer patients, including:
- Deprioritizing other vaccines. COVID-19 vaccines should take precedence over other vaccines because data on dual vaccination are lacking. The NCCN recommends waiting 14 days after COVID-19 vaccination to deliver other vaccines.
- Vaccinating clinical trial participants. Trial leads should be consulted to prevent protocol violations or exclusions.
- Decision-making in the setting of limited vaccine availability. The NCCN noted that decisions on allocation must be made in accordance with state and local vaccine guidance but suggests prioritizing appropriate patients on active treatment, those planning to start treatment, and those who have just completed treatment. Additional risk factors for these patients, as well as other factors associated with risk for adverse COVID-19 outcomes, should also be considered. These include advanced age, comorbidities, and adverse social and demographic factors such as poverty and limited health care access.
- The need for ongoing prevention measures. Vaccines have been shown to decrease the incidence of COVID-19 and related complications, but it remains unclear whether vaccines prevent infection and subsequent transmission. This means everyone should continue following prevention recommendations, such as wearing masks and avoiding crowds.
The NCCN stressed that these recommendations are “intended to be a living document that is constantly evolving – it will be updated rapidly whenever new data comes out, as well as any potential new vaccines that may get approved in the future.” The NCCN also noted that the advisory committee will meet regularly to refine the recommendations as needed.
Dr. Pergam disclosed relationships with Chimerix Inc., Merck & Co., Global Life Technologies Inc., and Sanofi-Aventis. Dr. Disis disclosed grants from Pfizer, Bavarian Nordisk, Janssen, and Precigen. She is the founder of EpiThany and editor-in-chief of JAMA Oncology.
First mammography guidelines for older breast cancer survivors
For women who have a life expectancy of 5-10 years, the guidelines recommend that consideration be given to discontinuing mammography.
Overall, the guidelines encourage shared decision-making that is individualized for each woman after weighing the benefits and harms associated with surveillance mammography and patient preferences.
The panel also recommended that patients with clinical findings and symptoms receive ongoing clinical breast examinations and diagnostic mammography and that patients be reassured that these practices will continue.
Guidelines on breast cancer screening for healthy women already “acknowledge the limitations of mammograms and the need to consider one’s health status and preferences when making decisions on how and when to stop routine mammograms,” said the article’s first author, Rachel A. Freedman, MD, MPH, of the Dana-Farber Cancer Institute, Boston.
However, “we don’t have this kind of consensus for women with a history of breast cancer,” she continued. “Current follow-up care guidelines simply state that women with a history of breast cancer with intact breasts should have annual mammography without any guidance.
“In practice, the use of mammograms is highly variable, with less than 50% of breast cancer survivors who have limited life expectancy having annual mammograms, according to survey data we have from prior work,” Dr. Freedman said in an interview.
The guidelines were published online Jan. 28 in JAMA Oncology.
Clinicians discuss how to have these discussions
As part of the process of developing these expert consensus guidelines, the researchers held several clinical focus groups that involved primary care physicians from Brigham and Women’s Hospital and oncology clinicians (including breast surgeons and medical oncologists) from the Dana-Farber Cancer Institute.
All clinicians felt that having expert guidelines and talking points to guide discussions would be helpful, the researchers report.
“However, some oncology clinicians felt that 75 years is often ‘too young’ to stop surveillance mammography and that 80 years may be a more comfortable age to stop routine testing,” they write. “Most clinicians felt that estimations of life expectancy, more than age, should inform the timing of this discussion.”
In contrast to primary and geriatric care clinicians, oncology clinicians reported discomfort with such discussions. They appreciated having the information but “felt it was easier to communicate findings indirectly, without specifically revealing life expectancy to patients. One oncology clinician, however, felt it would be ‘sneaky’ to calculate life expectancy without communicating this to patients, supporting more open discussions,” the authors report.
“All clinicians acknowledged that framing the conversation around patients’ low risk for in-breast cancer events and how mammography will not benefit them was more appealing than discussing life expectancy,” the researchers continue. Their literature review found that the risk of these individuals developing second breast cancers was similar to that of a healthy woman developing a first breast cancer, leading one clinician to comment: “If their risk is really equivalent to the general population – that is very powerful.”
“Some clinicians reported that they ‘focus on the risks’ or frame such discussions by asking: ‘If you were to find something on [a] mammogram, would you do anything about it?’ If a patient answered no, clinicians felt this was a signal to stop mammography,” they noted.
Literature review finds very low risk
Dr. Freedman and colleagues conducted a literature review of the risk for ipsilateral and contralateral breast cancer events among survivors and of the harms and benefits associated with mammography. Following the literature review, a multidisciplinary expert panel, which included patients and patient advocates, was convened to develop consensus guidelines.
The literature review confirmed that there was a low risk for in-breast cancer events in this population and that the risk was particularly low among patients who undergo treatment with endocrine therapy. Among those who did not receive systemic therapy for ERBB2-positive or triple-negative cancers, the rates of ipsilateral recurrence were estimated to be higher.
On the basis of the literature review, the estimated 10-year risk for in-breast cancer events ranged from 1% to 15% for ipsilateral breast cancers and from 1% to 5% for contralateral cancers. Among women in the same age group who did not have a history of breast cancer, the 5-year risk of developing the disease (average risk) was 2.2%.
The authors note that these findings mirror their estimates for new breast cancers among survivors who had low-risk disease. The findings are also similar to those cited in a large-scale mammography study, in which breast cancer survivors aged 70-80 years had a 1.1% annual risk for in-breast cancers. The risk was 0.7%-0.9% for similarly aged patients who did not have a history of breast cancer.
The benefits associated with mammography for older women are not well defined, but the literature suggests that mammography offers little to modest clinical benefit for patients in this age group. The limited benefits are likely because of the more than 10-year time lag that is needed to detect the small improvements in breast cancer mortality; slow-growing tumors generally do not affect the life expectancy of older women, they point out.
“Through our expert consensus process and after iterative feedback from clinicians, we created guidelines to support patients and clinicians in making individualized decisions on how and when to stop mammography,” said Dr. Freedman. “These guidelines are based on the risk of a breast cancer returning in the breast, one’s underlying health, and one’s preferences.”
The guidelines are also intended to provide information to patients on the benefits and harms of mammography in this setting, in addition to “how much we anticipate a mammogram may or may not continue to help a woman over time,” she said.
A companion guide for patients on these guidelines will be published in the coming months.
Dr. Freedman has received institutional clinical trial funding from Eisai and Puma Biotechnology outside the submitted work.
A version of this article first appeared on Medscape.com.
For women who have a life expectancy of 5-10 years, the guidelines recommend that consideration be given to discontinuing mammography.
Overall, the guidelines encourage shared decision-making that is individualized for each woman after weighing the benefits and harms associated with surveillance mammography and patient preferences.
The panel also recommended that patients with clinical findings and symptoms receive ongoing clinical breast examinations and diagnostic mammography and that patients be reassured that these practices will continue.
Guidelines on breast cancer screening for healthy women already “acknowledge the limitations of mammograms and the need to consider one’s health status and preferences when making decisions on how and when to stop routine mammograms,” said the article’s first author, Rachel A. Freedman, MD, MPH, of the Dana-Farber Cancer Institute, Boston.
However, “we don’t have this kind of consensus for women with a history of breast cancer,” she continued. “Current follow-up care guidelines simply state that women with a history of breast cancer with intact breasts should have annual mammography without any guidance.
“In practice, the use of mammograms is highly variable, with less than 50% of breast cancer survivors who have limited life expectancy having annual mammograms, according to survey data we have from prior work,” Dr. Freedman said in an interview.
The guidelines were published online Jan. 28 in JAMA Oncology.
Clinicians discuss how to have these discussions
As part of the process of developing these expert consensus guidelines, the researchers held several clinical focus groups that involved primary care physicians from Brigham and Women’s Hospital and oncology clinicians (including breast surgeons and medical oncologists) from the Dana-Farber Cancer Institute.
All clinicians felt that having expert guidelines and talking points to guide discussions would be helpful, the researchers report.
“However, some oncology clinicians felt that 75 years is often ‘too young’ to stop surveillance mammography and that 80 years may be a more comfortable age to stop routine testing,” they write. “Most clinicians felt that estimations of life expectancy, more than age, should inform the timing of this discussion.”
In contrast to primary and geriatric care clinicians, oncology clinicians reported discomfort with such discussions. They appreciated having the information but “felt it was easier to communicate findings indirectly, without specifically revealing life expectancy to patients. One oncology clinician, however, felt it would be ‘sneaky’ to calculate life expectancy without communicating this to patients, supporting more open discussions,” the authors report.
“All clinicians acknowledged that framing the conversation around patients’ low risk for in-breast cancer events and how mammography will not benefit them was more appealing than discussing life expectancy,” the researchers continue. Their literature review found that the risk of these individuals developing second breast cancers was similar to that of a healthy woman developing a first breast cancer, leading one clinician to comment: “If their risk is really equivalent to the general population – that is very powerful.”
“Some clinicians reported that they ‘focus on the risks’ or frame such discussions by asking: ‘If you were to find something on [a] mammogram, would you do anything about it?’ If a patient answered no, clinicians felt this was a signal to stop mammography,” they noted.
Literature review finds very low risk
Dr. Freedman and colleagues conducted a literature review of the risk for ipsilateral and contralateral breast cancer events among survivors and of the harms and benefits associated with mammography. Following the literature review, a multidisciplinary expert panel, which included patients and patient advocates, was convened to develop consensus guidelines.
The literature review confirmed that there was a low risk for in-breast cancer events in this population and that the risk was particularly low among patients who undergo treatment with endocrine therapy. Among those who did not receive systemic therapy for ERBB2-positive or triple-negative cancers, the rates of ipsilateral recurrence were estimated to be higher.
On the basis of the literature review, the estimated 10-year risk for in-breast cancer events ranged from 1% to 15% for ipsilateral breast cancers and from 1% to 5% for contralateral cancers. Among women in the same age group who did not have a history of breast cancer, the 5-year risk of developing the disease (average risk) was 2.2%.
The authors note that these findings mirror their estimates for new breast cancers among survivors who had low-risk disease. The findings are also similar to those cited in a large-scale mammography study, in which breast cancer survivors aged 70-80 years had a 1.1% annual risk for in-breast cancers. The risk was 0.7%-0.9% for similarly aged patients who did not have a history of breast cancer.
The benefits associated with mammography for older women are not well defined, but the literature suggests that mammography offers little to modest clinical benefit for patients in this age group. The limited benefits are likely because of the more than 10-year time lag that is needed to detect the small improvements in breast cancer mortality; slow-growing tumors generally do not affect the life expectancy of older women, they point out.
“Through our expert consensus process and after iterative feedback from clinicians, we created guidelines to support patients and clinicians in making individualized decisions on how and when to stop mammography,” said Dr. Freedman. “These guidelines are based on the risk of a breast cancer returning in the breast, one’s underlying health, and one’s preferences.”
The guidelines are also intended to provide information to patients on the benefits and harms of mammography in this setting, in addition to “how much we anticipate a mammogram may or may not continue to help a woman over time,” she said.
A companion guide for patients on these guidelines will be published in the coming months.
Dr. Freedman has received institutional clinical trial funding from Eisai and Puma Biotechnology outside the submitted work.
A version of this article first appeared on Medscape.com.
For women who have a life expectancy of 5-10 years, the guidelines recommend that consideration be given to discontinuing mammography.
Overall, the guidelines encourage shared decision-making that is individualized for each woman after weighing the benefits and harms associated with surveillance mammography and patient preferences.
The panel also recommended that patients with clinical findings and symptoms receive ongoing clinical breast examinations and diagnostic mammography and that patients be reassured that these practices will continue.
Guidelines on breast cancer screening for healthy women already “acknowledge the limitations of mammograms and the need to consider one’s health status and preferences when making decisions on how and when to stop routine mammograms,” said the article’s first author, Rachel A. Freedman, MD, MPH, of the Dana-Farber Cancer Institute, Boston.
However, “we don’t have this kind of consensus for women with a history of breast cancer,” she continued. “Current follow-up care guidelines simply state that women with a history of breast cancer with intact breasts should have annual mammography without any guidance.
“In practice, the use of mammograms is highly variable, with less than 50% of breast cancer survivors who have limited life expectancy having annual mammograms, according to survey data we have from prior work,” Dr. Freedman said in an interview.
The guidelines were published online Jan. 28 in JAMA Oncology.
Clinicians discuss how to have these discussions
As part of the process of developing these expert consensus guidelines, the researchers held several clinical focus groups that involved primary care physicians from Brigham and Women’s Hospital and oncology clinicians (including breast surgeons and medical oncologists) from the Dana-Farber Cancer Institute.
All clinicians felt that having expert guidelines and talking points to guide discussions would be helpful, the researchers report.
“However, some oncology clinicians felt that 75 years is often ‘too young’ to stop surveillance mammography and that 80 years may be a more comfortable age to stop routine testing,” they write. “Most clinicians felt that estimations of life expectancy, more than age, should inform the timing of this discussion.”
In contrast to primary and geriatric care clinicians, oncology clinicians reported discomfort with such discussions. They appreciated having the information but “felt it was easier to communicate findings indirectly, without specifically revealing life expectancy to patients. One oncology clinician, however, felt it would be ‘sneaky’ to calculate life expectancy without communicating this to patients, supporting more open discussions,” the authors report.
“All clinicians acknowledged that framing the conversation around patients’ low risk for in-breast cancer events and how mammography will not benefit them was more appealing than discussing life expectancy,” the researchers continue. Their literature review found that the risk of these individuals developing second breast cancers was similar to that of a healthy woman developing a first breast cancer, leading one clinician to comment: “If their risk is really equivalent to the general population – that is very powerful.”
“Some clinicians reported that they ‘focus on the risks’ or frame such discussions by asking: ‘If you were to find something on [a] mammogram, would you do anything about it?’ If a patient answered no, clinicians felt this was a signal to stop mammography,” they noted.
Literature review finds very low risk
Dr. Freedman and colleagues conducted a literature review of the risk for ipsilateral and contralateral breast cancer events among survivors and of the harms and benefits associated with mammography. Following the literature review, a multidisciplinary expert panel, which included patients and patient advocates, was convened to develop consensus guidelines.
The literature review confirmed that there was a low risk for in-breast cancer events in this population and that the risk was particularly low among patients who undergo treatment with endocrine therapy. Among those who did not receive systemic therapy for ERBB2-positive or triple-negative cancers, the rates of ipsilateral recurrence were estimated to be higher.
On the basis of the literature review, the estimated 10-year risk for in-breast cancer events ranged from 1% to 15% for ipsilateral breast cancers and from 1% to 5% for contralateral cancers. Among women in the same age group who did not have a history of breast cancer, the 5-year risk of developing the disease (average risk) was 2.2%.
The authors note that these findings mirror their estimates for new breast cancers among survivors who had low-risk disease. The findings are also similar to those cited in a large-scale mammography study, in which breast cancer survivors aged 70-80 years had a 1.1% annual risk for in-breast cancers. The risk was 0.7%-0.9% for similarly aged patients who did not have a history of breast cancer.
The benefits associated with mammography for older women are not well defined, but the literature suggests that mammography offers little to modest clinical benefit for patients in this age group. The limited benefits are likely because of the more than 10-year time lag that is needed to detect the small improvements in breast cancer mortality; slow-growing tumors generally do not affect the life expectancy of older women, they point out.
“Through our expert consensus process and after iterative feedback from clinicians, we created guidelines to support patients and clinicians in making individualized decisions on how and when to stop mammography,” said Dr. Freedman. “These guidelines are based on the risk of a breast cancer returning in the breast, one’s underlying health, and one’s preferences.”
The guidelines are also intended to provide information to patients on the benefits and harms of mammography in this setting, in addition to “how much we anticipate a mammogram may or may not continue to help a woman over time,” she said.
A companion guide for patients on these guidelines will be published in the coming months.
Dr. Freedman has received institutional clinical trial funding from Eisai and Puma Biotechnology outside the submitted work.
A version of this article first appeared on Medscape.com.
Von Willebrand disease guidelines address women’s bleeding concerns
New guidelines issued jointly by four major international hematology groups focus on the management of patients with von Willebrand disease (VWD), the most common bleeding disorder in the world.
The evidence-based guidelines, published in Blood Advances, were developed in collaboration by the American Society of Hematology (ASH), the International Society on Thrombosis and Haemostasis, the National Hemophilia Foundation, and the World Federation of Hemophilia. They outline key recommendations spanning the care of patients with a broad range of therapeutic needs.
“We addressed some of the questions that were most important to the community, but certainly there are a lot of areas that we couldn’t cover” said coauthor Veronica H. Flood, MD, of the Medical College of Wisconsin in Milwaukee.
The guidelines process began with a survey sent to the von Willebrand disease community, including patients, caregivers, nurses, physicians, and scientists. The respondents were asked to prioritize issues that they felt should be addressed in the guidelines.
“Interestingly, some of the issues were the same between patients and caregivers and physicians, and some were different, but there were obviously some areas that we just couldn’t cover,” she said in an interview.
One of the areas of greatest concern for respondents was bleeding in women, and many of the recommendations include specific considerations for management of gynecologic and obstetric patients, Dr. Flood said.
“We also tried to make the questions applicable to as many patients with von Willebrand disease as possible,” she added.
Some of the questions, such as recommendation 1, regarding prophylaxis, are geared toward management of patients with severe disease, while others, such as recommendations for treatment of menstrual bleeding, are more suited for patients with milder VWD.
All of the recommendations in the guidelines are “conditional” (suggested), due to very low certainty in the evidence of effects, the authors noted.
Prophylaxis
The guidelines suggest long-term prophylaxis for patients with a history of severe and frequent bleeds, with periodic assessment of the need for prophylaxis.
Desmopressin
For those patients who may benefit from the use of desmopressin, primarily those with type 1 VWD, and who have a baseline von Willebrand factor (VWF) level below 0.30 IU/mL, the panel issued a conditional recommendation for a desmopressin trial with treatment based on the patient’s results compared with not performing a trial and treating with tranexamic acid or factor concentrate. The guidelines also advise against treating with desmopressin in the absence of a trial. In a section of “good practice statements,” the guidelines indicate that using desmopressin in patients with type 2B VWD is generally contraindicated, because of the risk of thrombocytopenia as a result of increased platelet binding. In addition, desmopressin is generally contraindicated in patients with active cardiovascular disease, patients with seizure disorders, patients less than 2 years old, and patients with type 1C VWD in the setting of surgery.
Antithrombotic therapy
The guideline panelists conditionally recommend antithrombotic therapy with either antiplatelet agents or anticoagulants, with an emphasis on reassessing bleeding risk throughout the course of treatment.
An accompanying good practice statement calls for individualized assessments of risks and benefits of specific antithrombotic therapies by a multidisciplinary team including hematologists, cardiovascular specialists, and the patient.
Major surgery
This section includes a recommendation for targeting both factor VIII and VWF activity levels to a minimum of 50 IU/mL for at least 3 days after surgery, and a suggestion against using factor VIII target levels alone.
Minor surgery/invasive procedures
The panelists suggest increasing VWF activity levels to a minimum of 0.50 IU/mL with desmopressin or factor concentrate with the addition of tranexamic acid over raising VWF levels to at least 0.50 IU/mL with desmopressin or factor concentrate alone.
In addition, the panelists suggest “giving tranexamic acid alone over increasing VWF activity levels to a minimum threshold of 0.50 IU/mL with any intervention in patients with type 1 VWD with baseline VWF activity levels of 0.30 IU/mL and a mild bleeding phenotype undergoing minor mucosal procedures.”
Heavy menstrual bleeding
In women with heavy menstrual bleeding who do not plan to conceive, the panel suggests either combined hormonal therapy or levonorgestrel-releasing intrauterine system, or tranexamic acid over desmopressin.
In women who wish to conceive, the panel suggests using tranexamic acid over desmopressin.
Neuraxial anesthesia during labor
For women in labor for whom neuraxial anesthesia is considered, the guidelines suggest targeting a VWF activity level from 0.50 to 1.50 IU/mL over targeting a level above 1.50 IU/mL.
Postpartum management
“The guideline panel suggests the use of tranexamic acid over not using it in women with type 1 VWD or low VWF levels (and this may also apply to types 2 and 3 VWD) during the postpartum period,” the guidelines say.
An accompanying good practice statement says that tranexamic acid can be provided orally or intravenously. The oral dose is 25 mg/kg three times daily for 10-14 days, or longer if blood loss remains heavy.
Dr. Flood said that the guidelines were developed under the assumption that they would apply to care of patients in regions with a high or moderately high degree of clinical resources.
“We recognize that this eliminates a great deal of the globe, and our hope is that ASH and the other sponsoring organizations are going to let us revise this and do a version for lower-resourced settings,” she said.
New guidelines issued jointly by four major international hematology groups focus on the management of patients with von Willebrand disease (VWD), the most common bleeding disorder in the world.
The evidence-based guidelines, published in Blood Advances, were developed in collaboration by the American Society of Hematology (ASH), the International Society on Thrombosis and Haemostasis, the National Hemophilia Foundation, and the World Federation of Hemophilia. They outline key recommendations spanning the care of patients with a broad range of therapeutic needs.
“We addressed some of the questions that were most important to the community, but certainly there are a lot of areas that we couldn’t cover” said coauthor Veronica H. Flood, MD, of the Medical College of Wisconsin in Milwaukee.
The guidelines process began with a survey sent to the von Willebrand disease community, including patients, caregivers, nurses, physicians, and scientists. The respondents were asked to prioritize issues that they felt should be addressed in the guidelines.
“Interestingly, some of the issues were the same between patients and caregivers and physicians, and some were different, but there were obviously some areas that we just couldn’t cover,” she said in an interview.
One of the areas of greatest concern for respondents was bleeding in women, and many of the recommendations include specific considerations for management of gynecologic and obstetric patients, Dr. Flood said.
“We also tried to make the questions applicable to as many patients with von Willebrand disease as possible,” she added.
Some of the questions, such as recommendation 1, regarding prophylaxis, are geared toward management of patients with severe disease, while others, such as recommendations for treatment of menstrual bleeding, are more suited for patients with milder VWD.
All of the recommendations in the guidelines are “conditional” (suggested), due to very low certainty in the evidence of effects, the authors noted.
Prophylaxis
The guidelines suggest long-term prophylaxis for patients with a history of severe and frequent bleeds, with periodic assessment of the need for prophylaxis.
Desmopressin
For those patients who may benefit from the use of desmopressin, primarily those with type 1 VWD, and who have a baseline von Willebrand factor (VWF) level below 0.30 IU/mL, the panel issued a conditional recommendation for a desmopressin trial with treatment based on the patient’s results compared with not performing a trial and treating with tranexamic acid or factor concentrate. The guidelines also advise against treating with desmopressin in the absence of a trial. In a section of “good practice statements,” the guidelines indicate that using desmopressin in patients with type 2B VWD is generally contraindicated, because of the risk of thrombocytopenia as a result of increased platelet binding. In addition, desmopressin is generally contraindicated in patients with active cardiovascular disease, patients with seizure disorders, patients less than 2 years old, and patients with type 1C VWD in the setting of surgery.
Antithrombotic therapy
The guideline panelists conditionally recommend antithrombotic therapy with either antiplatelet agents or anticoagulants, with an emphasis on reassessing bleeding risk throughout the course of treatment.
An accompanying good practice statement calls for individualized assessments of risks and benefits of specific antithrombotic therapies by a multidisciplinary team including hematologists, cardiovascular specialists, and the patient.
Major surgery
This section includes a recommendation for targeting both factor VIII and VWF activity levels to a minimum of 50 IU/mL for at least 3 days after surgery, and a suggestion against using factor VIII target levels alone.
Minor surgery/invasive procedures
The panelists suggest increasing VWF activity levels to a minimum of 0.50 IU/mL with desmopressin or factor concentrate with the addition of tranexamic acid over raising VWF levels to at least 0.50 IU/mL with desmopressin or factor concentrate alone.
In addition, the panelists suggest “giving tranexamic acid alone over increasing VWF activity levels to a minimum threshold of 0.50 IU/mL with any intervention in patients with type 1 VWD with baseline VWF activity levels of 0.30 IU/mL and a mild bleeding phenotype undergoing minor mucosal procedures.”
Heavy menstrual bleeding
In women with heavy menstrual bleeding who do not plan to conceive, the panel suggests either combined hormonal therapy or levonorgestrel-releasing intrauterine system, or tranexamic acid over desmopressin.
In women who wish to conceive, the panel suggests using tranexamic acid over desmopressin.
Neuraxial anesthesia during labor
For women in labor for whom neuraxial anesthesia is considered, the guidelines suggest targeting a VWF activity level from 0.50 to 1.50 IU/mL over targeting a level above 1.50 IU/mL.
Postpartum management
“The guideline panel suggests the use of tranexamic acid over not using it in women with type 1 VWD or low VWF levels (and this may also apply to types 2 and 3 VWD) during the postpartum period,” the guidelines say.
An accompanying good practice statement says that tranexamic acid can be provided orally or intravenously. The oral dose is 25 mg/kg three times daily for 10-14 days, or longer if blood loss remains heavy.
Dr. Flood said that the guidelines were developed under the assumption that they would apply to care of patients in regions with a high or moderately high degree of clinical resources.
“We recognize that this eliminates a great deal of the globe, and our hope is that ASH and the other sponsoring organizations are going to let us revise this and do a version for lower-resourced settings,” she said.
New guidelines issued jointly by four major international hematology groups focus on the management of patients with von Willebrand disease (VWD), the most common bleeding disorder in the world.
The evidence-based guidelines, published in Blood Advances, were developed in collaboration by the American Society of Hematology (ASH), the International Society on Thrombosis and Haemostasis, the National Hemophilia Foundation, and the World Federation of Hemophilia. They outline key recommendations spanning the care of patients with a broad range of therapeutic needs.
“We addressed some of the questions that were most important to the community, but certainly there are a lot of areas that we couldn’t cover” said coauthor Veronica H. Flood, MD, of the Medical College of Wisconsin in Milwaukee.
The guidelines process began with a survey sent to the von Willebrand disease community, including patients, caregivers, nurses, physicians, and scientists. The respondents were asked to prioritize issues that they felt should be addressed in the guidelines.
“Interestingly, some of the issues were the same between patients and caregivers and physicians, and some were different, but there were obviously some areas that we just couldn’t cover,” she said in an interview.
One of the areas of greatest concern for respondents was bleeding in women, and many of the recommendations include specific considerations for management of gynecologic and obstetric patients, Dr. Flood said.
“We also tried to make the questions applicable to as many patients with von Willebrand disease as possible,” she added.
Some of the questions, such as recommendation 1, regarding prophylaxis, are geared toward management of patients with severe disease, while others, such as recommendations for treatment of menstrual bleeding, are more suited for patients with milder VWD.
All of the recommendations in the guidelines are “conditional” (suggested), due to very low certainty in the evidence of effects, the authors noted.
Prophylaxis
The guidelines suggest long-term prophylaxis for patients with a history of severe and frequent bleeds, with periodic assessment of the need for prophylaxis.
Desmopressin
For those patients who may benefit from the use of desmopressin, primarily those with type 1 VWD, and who have a baseline von Willebrand factor (VWF) level below 0.30 IU/mL, the panel issued a conditional recommendation for a desmopressin trial with treatment based on the patient’s results compared with not performing a trial and treating with tranexamic acid or factor concentrate. The guidelines also advise against treating with desmopressin in the absence of a trial. In a section of “good practice statements,” the guidelines indicate that using desmopressin in patients with type 2B VWD is generally contraindicated, because of the risk of thrombocytopenia as a result of increased platelet binding. In addition, desmopressin is generally contraindicated in patients with active cardiovascular disease, patients with seizure disorders, patients less than 2 years old, and patients with type 1C VWD in the setting of surgery.
Antithrombotic therapy
The guideline panelists conditionally recommend antithrombotic therapy with either antiplatelet agents or anticoagulants, with an emphasis on reassessing bleeding risk throughout the course of treatment.
An accompanying good practice statement calls for individualized assessments of risks and benefits of specific antithrombotic therapies by a multidisciplinary team including hematologists, cardiovascular specialists, and the patient.
Major surgery
This section includes a recommendation for targeting both factor VIII and VWF activity levels to a minimum of 50 IU/mL for at least 3 days after surgery, and a suggestion against using factor VIII target levels alone.
Minor surgery/invasive procedures
The panelists suggest increasing VWF activity levels to a minimum of 0.50 IU/mL with desmopressin or factor concentrate with the addition of tranexamic acid over raising VWF levels to at least 0.50 IU/mL with desmopressin or factor concentrate alone.
In addition, the panelists suggest “giving tranexamic acid alone over increasing VWF activity levels to a minimum threshold of 0.50 IU/mL with any intervention in patients with type 1 VWD with baseline VWF activity levels of 0.30 IU/mL and a mild bleeding phenotype undergoing minor mucosal procedures.”
Heavy menstrual bleeding
In women with heavy menstrual bleeding who do not plan to conceive, the panel suggests either combined hormonal therapy or levonorgestrel-releasing intrauterine system, or tranexamic acid over desmopressin.
In women who wish to conceive, the panel suggests using tranexamic acid over desmopressin.
Neuraxial anesthesia during labor
For women in labor for whom neuraxial anesthesia is considered, the guidelines suggest targeting a VWF activity level from 0.50 to 1.50 IU/mL over targeting a level above 1.50 IU/mL.
Postpartum management
“The guideline panel suggests the use of tranexamic acid over not using it in women with type 1 VWD or low VWF levels (and this may also apply to types 2 and 3 VWD) during the postpartum period,” the guidelines say.
An accompanying good practice statement says that tranexamic acid can be provided orally or intravenously. The oral dose is 25 mg/kg three times daily for 10-14 days, or longer if blood loss remains heavy.
Dr. Flood said that the guidelines were developed under the assumption that they would apply to care of patients in regions with a high or moderately high degree of clinical resources.
“We recognize that this eliminates a great deal of the globe, and our hope is that ASH and the other sponsoring organizations are going to let us revise this and do a version for lower-resourced settings,” she said.
FROM BLOOD ADVANCES
Consensus bundle has potential to affect postpartum morbidity, mortality
Health care professionals miss an important opportunity if they do not use a holistic patient-centered approach in providing support to their postpartum patients, advised Alison M. Stuebe, MD, MSc, of the division of maternal-fetal medicine at the University of North Carolina at Chapel Hill, and colleagues.
Too often, the emphasis for health care professionals is on less common, highly morbid complications, while their patients’ needs are focused more on daily functioning.
More than half of maternal deaths occur not during delivery, but in the days, weeks, and months following birth. Many serious complications and management of less-emergent conditions such as flu-like symptoms go unnoticed or result in treatment delays. Routinely asking women who present for care whether they have been pregnant during the past year could go a long way in helping clinicians connect the dots for conditions they might not otherwise consider, suggested Dr. Stuebe and colleagues.
In response to what is becoming a growing burden of postpartum maternal morbidity and mortality in the United States, Dr. Stuebe and associates, in coordination with the Alliance of Innovation in Maternal Health, prepared a comprehensive consensus statement on postpartum care basics. The work came out of a day-long planning meeting of an interdisciplinary work group funded by AIMH to develop the maternal safety bundle, which established 28 goals across four phases – readiness, recognition and prevention, response, and reporting. Each phase is intended to occur as part of a coordinated effort between women experiencing pregnancy and their health care providers, with involvement from multiple clinical settings and health systems.
America needs to change the way it treats its new mothers
In a separate interview, Dr. Stuebe shared additional insights into the development of the consensus statement: “This article is part of a broader movement to change the way America treats new mothers. For too long, we’ve lavished attention on women in the weeks before birth and then left them alone to manage recovery, plunging hormones, sleepless nights, and new baby care. The goal of this article is to set out the ways that we can better support women in this critical transition period.
“We need to start during pregnancy, by working with women to plan for the fourth trimester and identify a care team that will support her and her baby. After birth, we need to adapt those plans and make sure she has the emotional and material support she needs to navigate the coming months. Many of these decisions are deeply personal, and we need to center the values and preferences of the woman and her family.”
Dr. Stuebe also spotlighted a new University of North Carolina–based program designed to support shared decision-making. Their Trimester Project team partnered with mothers to design NewMomHealth.com, the first national postpartum resource designed by and for new mothers, which is in the process of launching a culturally adapted Spanish-language site, saludmadre.com, in partnership with Urban Strategies.
Readiness prepares mothers, HCPs, and clinical settings for the fourth trimester
In the first phase, Readiness, there are three key parties involved: the woman experiencing pregnancy, the health care providers (HCPs) supporting her, and the clinical settings involved in her care. Each player has specific goals to achieve in this phase, women are responsible for four goals, health care providers have two goals, and clinical settings have five.
Prenatally, every woman should work with health care providers to develop a comprehensive, individualized postpartum care plan that designates a postpartum clinical “home” for addressing any care needed between birth and the “comprehensive postpartum visit.” The plan encourages the patient and her health care team give attention to social support, birth recovery, infant care and feeding plans, thoughts concerning future pregnancies, and specifically use of contraception, as well as any chronic health concerns and overall emotional well-being.
The plan should consist of a postpartum care team that includes friends and family and ensures that medical, material, and social support are available in the weeks following birth for both mother and baby.
The authors emphasized the importance for clinicians to develop a keen awareness of “reproductive justice,” which respects a woman’s right “to maintain personal bodily autonomy, have children, not have children, and parent the children we have in safe and sustainable communities.” They encouraged adopting a sensitivity to concerns deeply rooted in the history of family planning, which is perceived to have been “fraught by coercive tactics to incentivize sterilization and contraceptive implants among marginalized groups.” With this in mind, they urged clinicians to respectfully ask about the patient’s intentions before making any suggestions concerning future births and family planning preferences.
HCP readiness ensures that each woman “has a documented postpartum care plan and care team identified in the prenatal period.” HCPs are also responsible for developing and maintaining “a working knowledge of evidence-based evaluation and management of common issues facing the mother-infant dyad.”
Clinical setting readiness ensures that woman-centered postpartum care and education have been developed and optimized using adult learning principles whenever possible. Diversity of family structures, cultural traditions, and parenting practices are fully embraced. Dr. Stuebe and colleagues emphasized that traditional top-down teaching methods are ineffective and should be avoided.
Clinical setting readiness also ensures that systems are developed to pair families with community-based resources that provide medical follow-up as well as social and material support. Clinical protocols and reimbursement options should be available that give women easy access to preferred contraception. Systems should be in place that facilitate prompt, pertinent communication between in- and outpatient providers. Lastly, and perhaps most importantly, clinical setting readiness demands the development of protocols to screen and treat key postpartum concerns, such as depression, substance use disorders, family violence, and even incontinence in coordination with locally identified specialists.
Recognition and prevention promotes accountability and establishes key guidelines
Recognition and prevention require coordinated participation between women experiencing pregnancy and the clinical environments supporting their care. In this phase, women are responsible for three goals; clinical environments have four goals.
Women are to be identified and respected as the expert most knowledgeable of their own needs, cautioned the authors. They should also be empowered to trust their instincts, seeking care as early and as often as needed in the weeks after they give birth. Postpartum care needs to be viewed as an ongoing process, not just a singular encounter.
Women also need to take ownership for their postpartum care plan, reviewing and revising it as needed in coordination with their health care providers before maternity discharge is complete. Every plan should include a comprehensive list of warning signs and recommended action plans when faced with life-threatening complications, a list of resources for lactation support, and a “first-call” phone number that identifies the postpartum medical providers available to address breastfeeding issues and for postpartum care visits, including prescheduled dates and times.
Lastly, women are to take responsibility for attending their postpartum visit. Although new guidance suggests that the comprehensive visit take place no later than 12 weeks post partum, it also recognizes multiple visits may be required to address individual needs. All women should have contact with a maternal care provider within 3 weeks post partum, the American College of Obstetricians and Gynecologists recommends.
Responsibility for recognition and prevention in the clinical setting begins with determining guidelines for early postpartum visits, considering chronic conditions such as hypertension, diabetes, or substance abuse disorder and risk for postpartum conditions such as wound complications or postpartum depression, the authors wrote. Ongoing care between inpatient and outpatient settings as well as between maternal and infant care providers is coordinated to ensure health and well-being of both patients. Screening and treatment of common comorbidities such as mental health issues, smoking, and substance use as well as issues related to unstable housing and food insecurities are also addressed in the clinical environment. Lastly, clinicians are tasked with ensuring that every patient has selected a primary care provider for ongoing care.
Response ensures key parties and resources are connected at every step
In the response phase, clinical settings and health care providers are the key participants and they have two goals each.
Every clinical setting is tasked with implementing treatment protocols and providing needed care or facilitating referral in a timely fashion. The importance of a “warm hand-off” and “face-to-face introduction to the specialist to whom the patient is being referred” is encouraged. They are also responsible for keeping an updated inventory of community resources on hand for such unmet needs as 24-hour hotlines, food banks, diaper banks, lactation support groups, and home-visiting programs.
Every health care provider is tasked with developing strategies designed to reach women who do not attend their comprehensive postpartum visit. They are also responsible for assessing the severity of identified needs, and arranging immediate transportation to appropriate facilities in life-threatening circumstances. In nonacute cases, the woman and her care providers work with her to make appropriate decisions.
Reporting gives health systems the opportunity to assess and improve
Sole responsibility in the reporting phase falls to every health system. In this phase, they have a total of six goals.
Health systems are ideally organized to convene strategy-sharing sessions with inpatient as well as outpatient professionals to evaluate successes and opportunities for improvement. They are equally qualified to identify and monitor such quality measures as postpartum emergency department utilization and readmission rates. They are tasked with working toward quality metrics that compare postpartum outcomes and prenatal intentions, including patient receipt of preferred contraception or completion of planned breastfeeding duration.
Health systems are expected to conduct quality improvement measures designed to reduce postpartum morbidity when preventable. They are also the logical choice for maintaining a clearinghouse for resources, in collaboration with the community, that is designed to meet the postpartum needs of women.
Lastly, they play an important role in ensuring that reimbursement policies are structured such that they do not disincentivize postpartum visits.
The consensus bundle encourages change in the way America treats its new mothers
Angela Bianco, MD, maternal and fetal medicine specialist at Mount Sinai Hospital, New York, also interviewed separately, noted: “I think the fact that ACOG has created a postpartum bundle for providers to use for guidance is long overdue. The current prenatal care paradigm focuses on numerous/intensive prenatal visits but only a single postpartum visit. Many women report feeling ill-equipped to navigate the challenges that arise post delivery, including self-care, newborn care, and breastfeeding. In addition, most women experience the impact of dramatic hormonal fluctuations resulting in mood alterations. Add profound sleep deprivation to this and the result is often some degree of postpartum blues and/or depression. The importance of anticipatory guidance for our patients cannot be underestimated. Helping to facilitate potential social support structures be in place prior to the birth is optimal. Providers should reinforce the importance of access to a variety of tools, including digital apps, community support groups, and 24/7 web access services. Moving forward, it should be considered unconscionable to send a new mother home without ensuring the appropriate resources are in place. Postpartum care should be tailored to a woman’s needs and may require multiple visits and/or referrals.”
Dr. Stuebe and colleagues, as well as Dr. Bianco, disclosed receiving honoraria for various projects. Funding for the study was supported by the Alliance for Innovation on Maternal and Child Health, which is funded by a grant from the Health Resources and Services Administration.
Health care professionals miss an important opportunity if they do not use a holistic patient-centered approach in providing support to their postpartum patients, advised Alison M. Stuebe, MD, MSc, of the division of maternal-fetal medicine at the University of North Carolina at Chapel Hill, and colleagues.
Too often, the emphasis for health care professionals is on less common, highly morbid complications, while their patients’ needs are focused more on daily functioning.
More than half of maternal deaths occur not during delivery, but in the days, weeks, and months following birth. Many serious complications and management of less-emergent conditions such as flu-like symptoms go unnoticed or result in treatment delays. Routinely asking women who present for care whether they have been pregnant during the past year could go a long way in helping clinicians connect the dots for conditions they might not otherwise consider, suggested Dr. Stuebe and colleagues.
In response to what is becoming a growing burden of postpartum maternal morbidity and mortality in the United States, Dr. Stuebe and associates, in coordination with the Alliance of Innovation in Maternal Health, prepared a comprehensive consensus statement on postpartum care basics. The work came out of a day-long planning meeting of an interdisciplinary work group funded by AIMH to develop the maternal safety bundle, which established 28 goals across four phases – readiness, recognition and prevention, response, and reporting. Each phase is intended to occur as part of a coordinated effort between women experiencing pregnancy and their health care providers, with involvement from multiple clinical settings and health systems.
America needs to change the way it treats its new mothers
In a separate interview, Dr. Stuebe shared additional insights into the development of the consensus statement: “This article is part of a broader movement to change the way America treats new mothers. For too long, we’ve lavished attention on women in the weeks before birth and then left them alone to manage recovery, plunging hormones, sleepless nights, and new baby care. The goal of this article is to set out the ways that we can better support women in this critical transition period.
“We need to start during pregnancy, by working with women to plan for the fourth trimester and identify a care team that will support her and her baby. After birth, we need to adapt those plans and make sure she has the emotional and material support she needs to navigate the coming months. Many of these decisions are deeply personal, and we need to center the values and preferences of the woman and her family.”
Dr. Stuebe also spotlighted a new University of North Carolina–based program designed to support shared decision-making. Their Trimester Project team partnered with mothers to design NewMomHealth.com, the first national postpartum resource designed by and for new mothers, which is in the process of launching a culturally adapted Spanish-language site, saludmadre.com, in partnership with Urban Strategies.
Readiness prepares mothers, HCPs, and clinical settings for the fourth trimester
In the first phase, Readiness, there are three key parties involved: the woman experiencing pregnancy, the health care providers (HCPs) supporting her, and the clinical settings involved in her care. Each player has specific goals to achieve in this phase, women are responsible for four goals, health care providers have two goals, and clinical settings have five.
Prenatally, every woman should work with health care providers to develop a comprehensive, individualized postpartum care plan that designates a postpartum clinical “home” for addressing any care needed between birth and the “comprehensive postpartum visit.” The plan encourages the patient and her health care team give attention to social support, birth recovery, infant care and feeding plans, thoughts concerning future pregnancies, and specifically use of contraception, as well as any chronic health concerns and overall emotional well-being.
The plan should consist of a postpartum care team that includes friends and family and ensures that medical, material, and social support are available in the weeks following birth for both mother and baby.
The authors emphasized the importance for clinicians to develop a keen awareness of “reproductive justice,” which respects a woman’s right “to maintain personal bodily autonomy, have children, not have children, and parent the children we have in safe and sustainable communities.” They encouraged adopting a sensitivity to concerns deeply rooted in the history of family planning, which is perceived to have been “fraught by coercive tactics to incentivize sterilization and contraceptive implants among marginalized groups.” With this in mind, they urged clinicians to respectfully ask about the patient’s intentions before making any suggestions concerning future births and family planning preferences.
HCP readiness ensures that each woman “has a documented postpartum care plan and care team identified in the prenatal period.” HCPs are also responsible for developing and maintaining “a working knowledge of evidence-based evaluation and management of common issues facing the mother-infant dyad.”
Clinical setting readiness ensures that woman-centered postpartum care and education have been developed and optimized using adult learning principles whenever possible. Diversity of family structures, cultural traditions, and parenting practices are fully embraced. Dr. Stuebe and colleagues emphasized that traditional top-down teaching methods are ineffective and should be avoided.
Clinical setting readiness also ensures that systems are developed to pair families with community-based resources that provide medical follow-up as well as social and material support. Clinical protocols and reimbursement options should be available that give women easy access to preferred contraception. Systems should be in place that facilitate prompt, pertinent communication between in- and outpatient providers. Lastly, and perhaps most importantly, clinical setting readiness demands the development of protocols to screen and treat key postpartum concerns, such as depression, substance use disorders, family violence, and even incontinence in coordination with locally identified specialists.
Recognition and prevention promotes accountability and establishes key guidelines
Recognition and prevention require coordinated participation between women experiencing pregnancy and the clinical environments supporting their care. In this phase, women are responsible for three goals; clinical environments have four goals.
Women are to be identified and respected as the expert most knowledgeable of their own needs, cautioned the authors. They should also be empowered to trust their instincts, seeking care as early and as often as needed in the weeks after they give birth. Postpartum care needs to be viewed as an ongoing process, not just a singular encounter.
Women also need to take ownership for their postpartum care plan, reviewing and revising it as needed in coordination with their health care providers before maternity discharge is complete. Every plan should include a comprehensive list of warning signs and recommended action plans when faced with life-threatening complications, a list of resources for lactation support, and a “first-call” phone number that identifies the postpartum medical providers available to address breastfeeding issues and for postpartum care visits, including prescheduled dates and times.
Lastly, women are to take responsibility for attending their postpartum visit. Although new guidance suggests that the comprehensive visit take place no later than 12 weeks post partum, it also recognizes multiple visits may be required to address individual needs. All women should have contact with a maternal care provider within 3 weeks post partum, the American College of Obstetricians and Gynecologists recommends.
Responsibility for recognition and prevention in the clinical setting begins with determining guidelines for early postpartum visits, considering chronic conditions such as hypertension, diabetes, or substance abuse disorder and risk for postpartum conditions such as wound complications or postpartum depression, the authors wrote. Ongoing care between inpatient and outpatient settings as well as between maternal and infant care providers is coordinated to ensure health and well-being of both patients. Screening and treatment of common comorbidities such as mental health issues, smoking, and substance use as well as issues related to unstable housing and food insecurities are also addressed in the clinical environment. Lastly, clinicians are tasked with ensuring that every patient has selected a primary care provider for ongoing care.
Response ensures key parties and resources are connected at every step
In the response phase, clinical settings and health care providers are the key participants and they have two goals each.
Every clinical setting is tasked with implementing treatment protocols and providing needed care or facilitating referral in a timely fashion. The importance of a “warm hand-off” and “face-to-face introduction to the specialist to whom the patient is being referred” is encouraged. They are also responsible for keeping an updated inventory of community resources on hand for such unmet needs as 24-hour hotlines, food banks, diaper banks, lactation support groups, and home-visiting programs.
Every health care provider is tasked with developing strategies designed to reach women who do not attend their comprehensive postpartum visit. They are also responsible for assessing the severity of identified needs, and arranging immediate transportation to appropriate facilities in life-threatening circumstances. In nonacute cases, the woman and her care providers work with her to make appropriate decisions.
Reporting gives health systems the opportunity to assess and improve
Sole responsibility in the reporting phase falls to every health system. In this phase, they have a total of six goals.
Health systems are ideally organized to convene strategy-sharing sessions with inpatient as well as outpatient professionals to evaluate successes and opportunities for improvement. They are equally qualified to identify and monitor such quality measures as postpartum emergency department utilization and readmission rates. They are tasked with working toward quality metrics that compare postpartum outcomes and prenatal intentions, including patient receipt of preferred contraception or completion of planned breastfeeding duration.
Health systems are expected to conduct quality improvement measures designed to reduce postpartum morbidity when preventable. They are also the logical choice for maintaining a clearinghouse for resources, in collaboration with the community, that is designed to meet the postpartum needs of women.
Lastly, they play an important role in ensuring that reimbursement policies are structured such that they do not disincentivize postpartum visits.
The consensus bundle encourages change in the way America treats its new mothers
Angela Bianco, MD, maternal and fetal medicine specialist at Mount Sinai Hospital, New York, also interviewed separately, noted: “I think the fact that ACOG has created a postpartum bundle for providers to use for guidance is long overdue. The current prenatal care paradigm focuses on numerous/intensive prenatal visits but only a single postpartum visit. Many women report feeling ill-equipped to navigate the challenges that arise post delivery, including self-care, newborn care, and breastfeeding. In addition, most women experience the impact of dramatic hormonal fluctuations resulting in mood alterations. Add profound sleep deprivation to this and the result is often some degree of postpartum blues and/or depression. The importance of anticipatory guidance for our patients cannot be underestimated. Helping to facilitate potential social support structures be in place prior to the birth is optimal. Providers should reinforce the importance of access to a variety of tools, including digital apps, community support groups, and 24/7 web access services. Moving forward, it should be considered unconscionable to send a new mother home without ensuring the appropriate resources are in place. Postpartum care should be tailored to a woman’s needs and may require multiple visits and/or referrals.”
Dr. Stuebe and colleagues, as well as Dr. Bianco, disclosed receiving honoraria for various projects. Funding for the study was supported by the Alliance for Innovation on Maternal and Child Health, which is funded by a grant from the Health Resources and Services Administration.
Health care professionals miss an important opportunity if they do not use a holistic patient-centered approach in providing support to their postpartum patients, advised Alison M. Stuebe, MD, MSc, of the division of maternal-fetal medicine at the University of North Carolina at Chapel Hill, and colleagues.
Too often, the emphasis for health care professionals is on less common, highly morbid complications, while their patients’ needs are focused more on daily functioning.
More than half of maternal deaths occur not during delivery, but in the days, weeks, and months following birth. Many serious complications and management of less-emergent conditions such as flu-like symptoms go unnoticed or result in treatment delays. Routinely asking women who present for care whether they have been pregnant during the past year could go a long way in helping clinicians connect the dots for conditions they might not otherwise consider, suggested Dr. Stuebe and colleagues.
In response to what is becoming a growing burden of postpartum maternal morbidity and mortality in the United States, Dr. Stuebe and associates, in coordination with the Alliance of Innovation in Maternal Health, prepared a comprehensive consensus statement on postpartum care basics. The work came out of a day-long planning meeting of an interdisciplinary work group funded by AIMH to develop the maternal safety bundle, which established 28 goals across four phases – readiness, recognition and prevention, response, and reporting. Each phase is intended to occur as part of a coordinated effort between women experiencing pregnancy and their health care providers, with involvement from multiple clinical settings and health systems.
America needs to change the way it treats its new mothers
In a separate interview, Dr. Stuebe shared additional insights into the development of the consensus statement: “This article is part of a broader movement to change the way America treats new mothers. For too long, we’ve lavished attention on women in the weeks before birth and then left them alone to manage recovery, plunging hormones, sleepless nights, and new baby care. The goal of this article is to set out the ways that we can better support women in this critical transition period.
“We need to start during pregnancy, by working with women to plan for the fourth trimester and identify a care team that will support her and her baby. After birth, we need to adapt those plans and make sure she has the emotional and material support she needs to navigate the coming months. Many of these decisions are deeply personal, and we need to center the values and preferences of the woman and her family.”
Dr. Stuebe also spotlighted a new University of North Carolina–based program designed to support shared decision-making. Their Trimester Project team partnered with mothers to design NewMomHealth.com, the first national postpartum resource designed by and for new mothers, which is in the process of launching a culturally adapted Spanish-language site, saludmadre.com, in partnership with Urban Strategies.
Readiness prepares mothers, HCPs, and clinical settings for the fourth trimester
In the first phase, Readiness, there are three key parties involved: the woman experiencing pregnancy, the health care providers (HCPs) supporting her, and the clinical settings involved in her care. Each player has specific goals to achieve in this phase, women are responsible for four goals, health care providers have two goals, and clinical settings have five.
Prenatally, every woman should work with health care providers to develop a comprehensive, individualized postpartum care plan that designates a postpartum clinical “home” for addressing any care needed between birth and the “comprehensive postpartum visit.” The plan encourages the patient and her health care team give attention to social support, birth recovery, infant care and feeding plans, thoughts concerning future pregnancies, and specifically use of contraception, as well as any chronic health concerns and overall emotional well-being.
The plan should consist of a postpartum care team that includes friends and family and ensures that medical, material, and social support are available in the weeks following birth for both mother and baby.
The authors emphasized the importance for clinicians to develop a keen awareness of “reproductive justice,” which respects a woman’s right “to maintain personal bodily autonomy, have children, not have children, and parent the children we have in safe and sustainable communities.” They encouraged adopting a sensitivity to concerns deeply rooted in the history of family planning, which is perceived to have been “fraught by coercive tactics to incentivize sterilization and contraceptive implants among marginalized groups.” With this in mind, they urged clinicians to respectfully ask about the patient’s intentions before making any suggestions concerning future births and family planning preferences.
HCP readiness ensures that each woman “has a documented postpartum care plan and care team identified in the prenatal period.” HCPs are also responsible for developing and maintaining “a working knowledge of evidence-based evaluation and management of common issues facing the mother-infant dyad.”
Clinical setting readiness ensures that woman-centered postpartum care and education have been developed and optimized using adult learning principles whenever possible. Diversity of family structures, cultural traditions, and parenting practices are fully embraced. Dr. Stuebe and colleagues emphasized that traditional top-down teaching methods are ineffective and should be avoided.
Clinical setting readiness also ensures that systems are developed to pair families with community-based resources that provide medical follow-up as well as social and material support. Clinical protocols and reimbursement options should be available that give women easy access to preferred contraception. Systems should be in place that facilitate prompt, pertinent communication between in- and outpatient providers. Lastly, and perhaps most importantly, clinical setting readiness demands the development of protocols to screen and treat key postpartum concerns, such as depression, substance use disorders, family violence, and even incontinence in coordination with locally identified specialists.
Recognition and prevention promotes accountability and establishes key guidelines
Recognition and prevention require coordinated participation between women experiencing pregnancy and the clinical environments supporting their care. In this phase, women are responsible for three goals; clinical environments have four goals.
Women are to be identified and respected as the expert most knowledgeable of their own needs, cautioned the authors. They should also be empowered to trust their instincts, seeking care as early and as often as needed in the weeks after they give birth. Postpartum care needs to be viewed as an ongoing process, not just a singular encounter.
Women also need to take ownership for their postpartum care plan, reviewing and revising it as needed in coordination with their health care providers before maternity discharge is complete. Every plan should include a comprehensive list of warning signs and recommended action plans when faced with life-threatening complications, a list of resources for lactation support, and a “first-call” phone number that identifies the postpartum medical providers available to address breastfeeding issues and for postpartum care visits, including prescheduled dates and times.
Lastly, women are to take responsibility for attending their postpartum visit. Although new guidance suggests that the comprehensive visit take place no later than 12 weeks post partum, it also recognizes multiple visits may be required to address individual needs. All women should have contact with a maternal care provider within 3 weeks post partum, the American College of Obstetricians and Gynecologists recommends.
Responsibility for recognition and prevention in the clinical setting begins with determining guidelines for early postpartum visits, considering chronic conditions such as hypertension, diabetes, or substance abuse disorder and risk for postpartum conditions such as wound complications or postpartum depression, the authors wrote. Ongoing care between inpatient and outpatient settings as well as between maternal and infant care providers is coordinated to ensure health and well-being of both patients. Screening and treatment of common comorbidities such as mental health issues, smoking, and substance use as well as issues related to unstable housing and food insecurities are also addressed in the clinical environment. Lastly, clinicians are tasked with ensuring that every patient has selected a primary care provider for ongoing care.
Response ensures key parties and resources are connected at every step
In the response phase, clinical settings and health care providers are the key participants and they have two goals each.
Every clinical setting is tasked with implementing treatment protocols and providing needed care or facilitating referral in a timely fashion. The importance of a “warm hand-off” and “face-to-face introduction to the specialist to whom the patient is being referred” is encouraged. They are also responsible for keeping an updated inventory of community resources on hand for such unmet needs as 24-hour hotlines, food banks, diaper banks, lactation support groups, and home-visiting programs.
Every health care provider is tasked with developing strategies designed to reach women who do not attend their comprehensive postpartum visit. They are also responsible for assessing the severity of identified needs, and arranging immediate transportation to appropriate facilities in life-threatening circumstances. In nonacute cases, the woman and her care providers work with her to make appropriate decisions.
Reporting gives health systems the opportunity to assess and improve
Sole responsibility in the reporting phase falls to every health system. In this phase, they have a total of six goals.
Health systems are ideally organized to convene strategy-sharing sessions with inpatient as well as outpatient professionals to evaluate successes and opportunities for improvement. They are equally qualified to identify and monitor such quality measures as postpartum emergency department utilization and readmission rates. They are tasked with working toward quality metrics that compare postpartum outcomes and prenatal intentions, including patient receipt of preferred contraception or completion of planned breastfeeding duration.
Health systems are expected to conduct quality improvement measures designed to reduce postpartum morbidity when preventable. They are also the logical choice for maintaining a clearinghouse for resources, in collaboration with the community, that is designed to meet the postpartum needs of women.
Lastly, they play an important role in ensuring that reimbursement policies are structured such that they do not disincentivize postpartum visits.
The consensus bundle encourages change in the way America treats its new mothers
Angela Bianco, MD, maternal and fetal medicine specialist at Mount Sinai Hospital, New York, also interviewed separately, noted: “I think the fact that ACOG has created a postpartum bundle for providers to use for guidance is long overdue. The current prenatal care paradigm focuses on numerous/intensive prenatal visits but only a single postpartum visit. Many women report feeling ill-equipped to navigate the challenges that arise post delivery, including self-care, newborn care, and breastfeeding. In addition, most women experience the impact of dramatic hormonal fluctuations resulting in mood alterations. Add profound sleep deprivation to this and the result is often some degree of postpartum blues and/or depression. The importance of anticipatory guidance for our patients cannot be underestimated. Helping to facilitate potential social support structures be in place prior to the birth is optimal. Providers should reinforce the importance of access to a variety of tools, including digital apps, community support groups, and 24/7 web access services. Moving forward, it should be considered unconscionable to send a new mother home without ensuring the appropriate resources are in place. Postpartum care should be tailored to a woman’s needs and may require multiple visits and/or referrals.”
Dr. Stuebe and colleagues, as well as Dr. Bianco, disclosed receiving honoraria for various projects. Funding for the study was supported by the Alliance for Innovation on Maternal and Child Health, which is funded by a grant from the Health Resources and Services Administration.
FROM OBSTETRICS AND GYNECOLOGY