User login
FDA approves weekly contraceptive patch Twirla
in women whose body mass index is less than 30 kg/m2 and for whom a combined hormonal contraceptive is appropriate.
Applied weekly to the abdomen, buttock, or upper torso (excluding the breasts), Twirla delivers a 30-mcg daily dose of ethinyl estradiol and 120-mcg daily dose of levonorgestrel.
“Twirla is an important addition to available hormonal contraceptive methods, allowing prescribers to now offer appropriate U.S. women a weekly transdermal option that delivers estrogen levels in line with labeled doses of many commonly prescribed oral contraceptives, David Portman, MD, an obstetrician/gynecologist in Columbus, Ohio, and a primary investigator of the SECURE trial, said in a news release issued by the company.
Twirla was evaluated in “a diverse population providing important data to prescribers and to women seeking contraception. It is vital to expand the full range of contraceptive methods and inform the choices that fit an individual’s family planning needs and lifestyle,” Dr. Portman added.
As part of approval, the FDA will require Agile Therapeutics to conduct a long-term, prospective, observational postmarketing study to assess risks for venous thromboembolism and arterial thromboembolism in new users of Twirla, compared with new users of other combined hormonal contraceptives.
Twirla is contraindicated in women at high risk for arterial or venous thrombotic disease, including women with a BMI equal to or greater than 30 kg/m2; women who have headaches with focal neurologic symptoms or migraine with aura; and women older than 35 years who have any migraine headache.
Twirla also should be avoided in women who have liver tumors, acute viral hepatitis, decompensated cirrhosis, liver disease, or undiagnosed abnormal uterine bleeding. It also should be avoided during pregnancy; in women who currently have or who have history of breast cancer or other estrogen- or progestin-sensitive cancer; in women who are hypersensitivity to any components of Twirla; and in women who use hepatitis C drug combinations containing ombitasvir/paraparesis/ritonavir, with or without dasabuvir.
Because cigarette smoking increases the risk for serious cardiovascular events from combined hormonal contraceptive use, Twirla also is contraindicated in women older than 35 who smoke.
Twirla will contain a boxed warning that will include these risks about cigarette smoking and the serious cardiovascular events, and it will stipulate that Twirla is contraindicated in women with a BMI greater than 30 kg/m2.
This article first appeared on Medscape.com.
in women whose body mass index is less than 30 kg/m2 and for whom a combined hormonal contraceptive is appropriate.
Applied weekly to the abdomen, buttock, or upper torso (excluding the breasts), Twirla delivers a 30-mcg daily dose of ethinyl estradiol and 120-mcg daily dose of levonorgestrel.
“Twirla is an important addition to available hormonal contraceptive methods, allowing prescribers to now offer appropriate U.S. women a weekly transdermal option that delivers estrogen levels in line with labeled doses of many commonly prescribed oral contraceptives, David Portman, MD, an obstetrician/gynecologist in Columbus, Ohio, and a primary investigator of the SECURE trial, said in a news release issued by the company.
Twirla was evaluated in “a diverse population providing important data to prescribers and to women seeking contraception. It is vital to expand the full range of contraceptive methods and inform the choices that fit an individual’s family planning needs and lifestyle,” Dr. Portman added.
As part of approval, the FDA will require Agile Therapeutics to conduct a long-term, prospective, observational postmarketing study to assess risks for venous thromboembolism and arterial thromboembolism in new users of Twirla, compared with new users of other combined hormonal contraceptives.
Twirla is contraindicated in women at high risk for arterial or venous thrombotic disease, including women with a BMI equal to or greater than 30 kg/m2; women who have headaches with focal neurologic symptoms or migraine with aura; and women older than 35 years who have any migraine headache.
Twirla also should be avoided in women who have liver tumors, acute viral hepatitis, decompensated cirrhosis, liver disease, or undiagnosed abnormal uterine bleeding. It also should be avoided during pregnancy; in women who currently have or who have history of breast cancer or other estrogen- or progestin-sensitive cancer; in women who are hypersensitivity to any components of Twirla; and in women who use hepatitis C drug combinations containing ombitasvir/paraparesis/ritonavir, with or without dasabuvir.
Because cigarette smoking increases the risk for serious cardiovascular events from combined hormonal contraceptive use, Twirla also is contraindicated in women older than 35 who smoke.
Twirla will contain a boxed warning that will include these risks about cigarette smoking and the serious cardiovascular events, and it will stipulate that Twirla is contraindicated in women with a BMI greater than 30 kg/m2.
This article first appeared on Medscape.com.
in women whose body mass index is less than 30 kg/m2 and for whom a combined hormonal contraceptive is appropriate.
Applied weekly to the abdomen, buttock, or upper torso (excluding the breasts), Twirla delivers a 30-mcg daily dose of ethinyl estradiol and 120-mcg daily dose of levonorgestrel.
“Twirla is an important addition to available hormonal contraceptive methods, allowing prescribers to now offer appropriate U.S. women a weekly transdermal option that delivers estrogen levels in line with labeled doses of many commonly prescribed oral contraceptives, David Portman, MD, an obstetrician/gynecologist in Columbus, Ohio, and a primary investigator of the SECURE trial, said in a news release issued by the company.
Twirla was evaluated in “a diverse population providing important data to prescribers and to women seeking contraception. It is vital to expand the full range of contraceptive methods and inform the choices that fit an individual’s family planning needs and lifestyle,” Dr. Portman added.
As part of approval, the FDA will require Agile Therapeutics to conduct a long-term, prospective, observational postmarketing study to assess risks for venous thromboembolism and arterial thromboembolism in new users of Twirla, compared with new users of other combined hormonal contraceptives.
Twirla is contraindicated in women at high risk for arterial or venous thrombotic disease, including women with a BMI equal to or greater than 30 kg/m2; women who have headaches with focal neurologic symptoms or migraine with aura; and women older than 35 years who have any migraine headache.
Twirla also should be avoided in women who have liver tumors, acute viral hepatitis, decompensated cirrhosis, liver disease, or undiagnosed abnormal uterine bleeding. It also should be avoided during pregnancy; in women who currently have or who have history of breast cancer or other estrogen- or progestin-sensitive cancer; in women who are hypersensitivity to any components of Twirla; and in women who use hepatitis C drug combinations containing ombitasvir/paraparesis/ritonavir, with or without dasabuvir.
Because cigarette smoking increases the risk for serious cardiovascular events from combined hormonal contraceptive use, Twirla also is contraindicated in women older than 35 who smoke.
Twirla will contain a boxed warning that will include these risks about cigarette smoking and the serious cardiovascular events, and it will stipulate that Twirla is contraindicated in women with a BMI greater than 30 kg/m2.
This article first appeared on Medscape.com.
Flu increases activity but not its severity

The CDC’s latest report shows that 6.8% of outpatients visiting health care providers had influenza-like illness during the week ending Feb. 8. That’s up from the previous week’s 6.6%, but that rise of 0.2 percentage points is smaller than the 0.6-point rises that occurred each of the 2 weeks before, and that could mean that activity is slowing.
That slowing, however, is not noticeable from this week’s map, which puts 41 states (there were 35 last week) and Puerto Rico in the red at the highest level of activity on the CDC’s 1-10 scale and another three states in the “high” range with levels of 8 or 9, the CDC’s influenza division reported.
That leaves Nevada and Oregon at level 7; Alaska, Florida, and the District of Columbia at level 5; Idaho at level 3, and Delaware with insufficient data (it was at level 5 last week), the CDC said.
The 2019-2020 season’s high activity, fortunately, has not translated into high severity, as overall hospitalization and mortality rates continue to remain at fairly typical levels. Hospitalization rates are elevated among children and young adults, however, and pediatric deaths are now up to 92, the CDC said, which is high for this point in the season.

The CDC’s latest report shows that 6.8% of outpatients visiting health care providers had influenza-like illness during the week ending Feb. 8. That’s up from the previous week’s 6.6%, but that rise of 0.2 percentage points is smaller than the 0.6-point rises that occurred each of the 2 weeks before, and that could mean that activity is slowing.
That slowing, however, is not noticeable from this week’s map, which puts 41 states (there were 35 last week) and Puerto Rico in the red at the highest level of activity on the CDC’s 1-10 scale and another three states in the “high” range with levels of 8 or 9, the CDC’s influenza division reported.
That leaves Nevada and Oregon at level 7; Alaska, Florida, and the District of Columbia at level 5; Idaho at level 3, and Delaware with insufficient data (it was at level 5 last week), the CDC said.
The 2019-2020 season’s high activity, fortunately, has not translated into high severity, as overall hospitalization and mortality rates continue to remain at fairly typical levels. Hospitalization rates are elevated among children and young adults, however, and pediatric deaths are now up to 92, the CDC said, which is high for this point in the season.

The CDC’s latest report shows that 6.8% of outpatients visiting health care providers had influenza-like illness during the week ending Feb. 8. That’s up from the previous week’s 6.6%, but that rise of 0.2 percentage points is smaller than the 0.6-point rises that occurred each of the 2 weeks before, and that could mean that activity is slowing.
That slowing, however, is not noticeable from this week’s map, which puts 41 states (there were 35 last week) and Puerto Rico in the red at the highest level of activity on the CDC’s 1-10 scale and another three states in the “high” range with levels of 8 or 9, the CDC’s influenza division reported.
That leaves Nevada and Oregon at level 7; Alaska, Florida, and the District of Columbia at level 5; Idaho at level 3, and Delaware with insufficient data (it was at level 5 last week), the CDC said.
The 2019-2020 season’s high activity, fortunately, has not translated into high severity, as overall hospitalization and mortality rates continue to remain at fairly typical levels. Hospitalization rates are elevated among children and young adults, however, and pediatric deaths are now up to 92, the CDC said, which is high for this point in the season.
Lorcaserin withdrawn from U.S. market due to cancer risk
The Food and Drug Administration asked Eisai to voluntary withdraw the weight-loss drug lorcaserin (Belviq and Belviq XR) on Feb. 13 after a post-marketing trial with more than 12,000 subjects revealed an increased occurrence of cancer.
In a Drug Safety Communication, the agency said “health care professionals should stop prescribing and dispensing lorcaserin to patients. Contact patients currently taking lorcaserin, inform them of the increased occurrence of cancer seen in the clinical trial, and ask them to stop taking the medicine. Discuss alternative weight-loss medicines or strategies with your patients.”
Eisai is complying with the withdrawal request.
The decision is based on the agency’s review of the 5-year trial, which was designed to evaluate cardiac risk with the drug and ended in June 2018. In total, 7.7% of patients randomized to 10 mg lorcaserin twice daily were diagnosed with 520 primary cancers, compared with 7.1% of placebo subjects diagnosed with 470 cancers, over a median follow-up of 3 years and 3 months. There was one additional cancer observed for every 470 patients treated for 1 year.
“There was no apparent difference in the incidence of cancer over the initial months of treatment, but the imbalance increased with longer duration on lorcaserin,” FDA said. Pancreatic, colorectal, and lung cancers were among those diagnosed.
In short, “we believe that the risks of lorcaserin outweigh its benefits based on our completed review of” the data, the agency said. The FDA is not recommending special cancer screenings for patients who have taken lorcaserin.
The action follows an FDA alert in January about a possible elevated cancer risk based on its preliminary analysis of the study.
Patients were also advised Feb. 13 to stop taking the drug and talk to their providers about alternative weight-loss medications and weight-management programs.
They were also told to dispose of the pills at a drug take-back location if available, but if not, to mix them with an “unappealing substance” such as dirt, cat litter, or used coffee grounds; seal them in plastic bag; and put them in the trash.
The Food and Drug Administration asked Eisai to voluntary withdraw the weight-loss drug lorcaserin (Belviq and Belviq XR) on Feb. 13 after a post-marketing trial with more than 12,000 subjects revealed an increased occurrence of cancer.
In a Drug Safety Communication, the agency said “health care professionals should stop prescribing and dispensing lorcaserin to patients. Contact patients currently taking lorcaserin, inform them of the increased occurrence of cancer seen in the clinical trial, and ask them to stop taking the medicine. Discuss alternative weight-loss medicines or strategies with your patients.”
Eisai is complying with the withdrawal request.
The decision is based on the agency’s review of the 5-year trial, which was designed to evaluate cardiac risk with the drug and ended in June 2018. In total, 7.7% of patients randomized to 10 mg lorcaserin twice daily were diagnosed with 520 primary cancers, compared with 7.1% of placebo subjects diagnosed with 470 cancers, over a median follow-up of 3 years and 3 months. There was one additional cancer observed for every 470 patients treated for 1 year.
“There was no apparent difference in the incidence of cancer over the initial months of treatment, but the imbalance increased with longer duration on lorcaserin,” FDA said. Pancreatic, colorectal, and lung cancers were among those diagnosed.
In short, “we believe that the risks of lorcaserin outweigh its benefits based on our completed review of” the data, the agency said. The FDA is not recommending special cancer screenings for patients who have taken lorcaserin.
The action follows an FDA alert in January about a possible elevated cancer risk based on its preliminary analysis of the study.
Patients were also advised Feb. 13 to stop taking the drug and talk to their providers about alternative weight-loss medications and weight-management programs.
They were also told to dispose of the pills at a drug take-back location if available, but if not, to mix them with an “unappealing substance” such as dirt, cat litter, or used coffee grounds; seal them in plastic bag; and put them in the trash.
The Food and Drug Administration asked Eisai to voluntary withdraw the weight-loss drug lorcaserin (Belviq and Belviq XR) on Feb. 13 after a post-marketing trial with more than 12,000 subjects revealed an increased occurrence of cancer.
In a Drug Safety Communication, the agency said “health care professionals should stop prescribing and dispensing lorcaserin to patients. Contact patients currently taking lorcaserin, inform them of the increased occurrence of cancer seen in the clinical trial, and ask them to stop taking the medicine. Discuss alternative weight-loss medicines or strategies with your patients.”
Eisai is complying with the withdrawal request.
The decision is based on the agency’s review of the 5-year trial, which was designed to evaluate cardiac risk with the drug and ended in June 2018. In total, 7.7% of patients randomized to 10 mg lorcaserin twice daily were diagnosed with 520 primary cancers, compared with 7.1% of placebo subjects diagnosed with 470 cancers, over a median follow-up of 3 years and 3 months. There was one additional cancer observed for every 470 patients treated for 1 year.
“There was no apparent difference in the incidence of cancer over the initial months of treatment, but the imbalance increased with longer duration on lorcaserin,” FDA said. Pancreatic, colorectal, and lung cancers were among those diagnosed.
In short, “we believe that the risks of lorcaserin outweigh its benefits based on our completed review of” the data, the agency said. The FDA is not recommending special cancer screenings for patients who have taken lorcaserin.
The action follows an FDA alert in January about a possible elevated cancer risk based on its preliminary analysis of the study.
Patients were also advised Feb. 13 to stop taking the drug and talk to their providers about alternative weight-loss medications and weight-management programs.
They were also told to dispose of the pills at a drug take-back location if available, but if not, to mix them with an “unappealing substance” such as dirt, cat litter, or used coffee grounds; seal them in plastic bag; and put them in the trash.
Two new Novel Coronavirus cases confirmed among quarantined U.S. patients
The Centers for Disease Control and Prevention announced two new patients now have the 2019 Novel Coronavirus (2019-nCoV), bringing the case total in the United States to 15.

The 14th case was discovered in California among a group of people under federal quarantine after returning from the Hubei Province in China. That patient was on a U.S. State Department–chartered flight that arrived in the United States on Feb. 7.
The 15th case was discovered in Texas among a group of people who also are under federal quarantine. That patient arrived on a State Department–chartered flight that arrived on Feb. 7. It is the first person in Texas that has tested positive for 2019-nCoV.
CDC said in a statement announcing the Texas case that there “will likely be additional cases in the coming days and weeks, including among other people recently returned from Wuhan.” Officials noted that more than 600 people who have returned as part of State Department–chartered flights are currently under that 14-day quarantine.
The agency is preparing for more widespread cases of 2019-nCoV.
Nancy Messonnier, MD, director of the CDC National Center for Immunization and Respiratory Diseases, said that containment has been the early focus for the agency.
“The goal of the measures we have taken to date are to slow the introduction and impact of this disease in the United States, but at some point, we are likely to see community spread in the U.S.,” Dr. Messonnier said during a Feb. 12 teleconference with reporters. She added that the federal response will change over time as the virus spreads.
Dr. Messonnier noted that public health officials are planning for the increased demands that a wider outbreak of 2019-nCov would place on the health care delivery system, including ensuring an adequate supply of medical equipment.
The Centers for Disease Control and Prevention announced two new patients now have the 2019 Novel Coronavirus (2019-nCoV), bringing the case total in the United States to 15.

The 14th case was discovered in California among a group of people under federal quarantine after returning from the Hubei Province in China. That patient was on a U.S. State Department–chartered flight that arrived in the United States on Feb. 7.
The 15th case was discovered in Texas among a group of people who also are under federal quarantine. That patient arrived on a State Department–chartered flight that arrived on Feb. 7. It is the first person in Texas that has tested positive for 2019-nCoV.
CDC said in a statement announcing the Texas case that there “will likely be additional cases in the coming days and weeks, including among other people recently returned from Wuhan.” Officials noted that more than 600 people who have returned as part of State Department–chartered flights are currently under that 14-day quarantine.
The agency is preparing for more widespread cases of 2019-nCoV.
Nancy Messonnier, MD, director of the CDC National Center for Immunization and Respiratory Diseases, said that containment has been the early focus for the agency.
“The goal of the measures we have taken to date are to slow the introduction and impact of this disease in the United States, but at some point, we are likely to see community spread in the U.S.,” Dr. Messonnier said during a Feb. 12 teleconference with reporters. She added that the federal response will change over time as the virus spreads.
Dr. Messonnier noted that public health officials are planning for the increased demands that a wider outbreak of 2019-nCov would place on the health care delivery system, including ensuring an adequate supply of medical equipment.
The Centers for Disease Control and Prevention announced two new patients now have the 2019 Novel Coronavirus (2019-nCoV), bringing the case total in the United States to 15.

The 14th case was discovered in California among a group of people under federal quarantine after returning from the Hubei Province in China. That patient was on a U.S. State Department–chartered flight that arrived in the United States on Feb. 7.
The 15th case was discovered in Texas among a group of people who also are under federal quarantine. That patient arrived on a State Department–chartered flight that arrived on Feb. 7. It is the first person in Texas that has tested positive for 2019-nCoV.
CDC said in a statement announcing the Texas case that there “will likely be additional cases in the coming days and weeks, including among other people recently returned from Wuhan.” Officials noted that more than 600 people who have returned as part of State Department–chartered flights are currently under that 14-day quarantine.
The agency is preparing for more widespread cases of 2019-nCoV.
Nancy Messonnier, MD, director of the CDC National Center for Immunization and Respiratory Diseases, said that containment has been the early focus for the agency.
“The goal of the measures we have taken to date are to slow the introduction and impact of this disease in the United States, but at some point, we are likely to see community spread in the U.S.,” Dr. Messonnier said during a Feb. 12 teleconference with reporters. She added that the federal response will change over time as the virus spreads.
Dr. Messonnier noted that public health officials are planning for the increased demands that a wider outbreak of 2019-nCov would place on the health care delivery system, including ensuring an adequate supply of medical equipment.
Flu activity increases for third straight week
For the second time during the 2019-2020 flu season, activity measures have climbed into noteworthy territory.

The proportion of outpatient visits for influenza-like illness (ILI) reached its highest December level, 7.1%, since 2003 and then dropped for 2 weeks. Three weeks of increases since then, however, have the outpatient-visit rate at 6.7% for the week ending Feb. 1, 2020, the Centers for Disease Control and Prevention reported. The baseline rate for the United States is 2.4%.
That rate of 6.7% is already above the highest rates recorded in eight of the last nine flu seasons, and another increase could mean a second, separate trip above 7.0% in the 2019-2020 season – something that has not occurred since national tracking began in 1997, CDC data show.
Those same data also show that,
Another important measure on the rise, the proportion of respiratory specimens testing positive for influenza, reached a new high for the season, 29.8%, during the week of Feb. 1, the CDC’s influenza division said.
Tests at clinical laboratories also show that predominance is continuing to switch from type B (45.6%) to type A (54.4%), the influenza division noted. Overall predominance for the season, however, continues to favor type B, 59.3% to 40.7%.
The percentage of deaths caused by pneumonia and influenza, which passed the threshold for epidemic of 7.2% back in early January, has been trending downward for the last 3 weeks and was 7.1% as of Feb. 1, according to the influenza division.
ILI-related deaths among children continue to remain high, with a total count of 78 for the season after another 10 deaths were reported during the week ending Feb. 1, the CDC reported. Comparable numbers for the last three seasons are 44 (2018-2019), 97 (2017-2018), and 35 (2016-2017).
The CDC estimates put the total number of ILIs at around 22 million for the season so far, leading to 210,000 hospitalizations. The agency said that it expects to release estimates of vaccine effectiveness later this month.
For the second time during the 2019-2020 flu season, activity measures have climbed into noteworthy territory.

The proportion of outpatient visits for influenza-like illness (ILI) reached its highest December level, 7.1%, since 2003 and then dropped for 2 weeks. Three weeks of increases since then, however, have the outpatient-visit rate at 6.7% for the week ending Feb. 1, 2020, the Centers for Disease Control and Prevention reported. The baseline rate for the United States is 2.4%.
That rate of 6.7% is already above the highest rates recorded in eight of the last nine flu seasons, and another increase could mean a second, separate trip above 7.0% in the 2019-2020 season – something that has not occurred since national tracking began in 1997, CDC data show.
Those same data also show that,
Another important measure on the rise, the proportion of respiratory specimens testing positive for influenza, reached a new high for the season, 29.8%, during the week of Feb. 1, the CDC’s influenza division said.
Tests at clinical laboratories also show that predominance is continuing to switch from type B (45.6%) to type A (54.4%), the influenza division noted. Overall predominance for the season, however, continues to favor type B, 59.3% to 40.7%.
The percentage of deaths caused by pneumonia and influenza, which passed the threshold for epidemic of 7.2% back in early January, has been trending downward for the last 3 weeks and was 7.1% as of Feb. 1, according to the influenza division.
ILI-related deaths among children continue to remain high, with a total count of 78 for the season after another 10 deaths were reported during the week ending Feb. 1, the CDC reported. Comparable numbers for the last three seasons are 44 (2018-2019), 97 (2017-2018), and 35 (2016-2017).
The CDC estimates put the total number of ILIs at around 22 million for the season so far, leading to 210,000 hospitalizations. The agency said that it expects to release estimates of vaccine effectiveness later this month.
For the second time during the 2019-2020 flu season, activity measures have climbed into noteworthy territory.

The proportion of outpatient visits for influenza-like illness (ILI) reached its highest December level, 7.1%, since 2003 and then dropped for 2 weeks. Three weeks of increases since then, however, have the outpatient-visit rate at 6.7% for the week ending Feb. 1, 2020, the Centers for Disease Control and Prevention reported. The baseline rate for the United States is 2.4%.
That rate of 6.7% is already above the highest rates recorded in eight of the last nine flu seasons, and another increase could mean a second, separate trip above 7.0% in the 2019-2020 season – something that has not occurred since national tracking began in 1997, CDC data show.
Those same data also show that,
Another important measure on the rise, the proportion of respiratory specimens testing positive for influenza, reached a new high for the season, 29.8%, during the week of Feb. 1, the CDC’s influenza division said.
Tests at clinical laboratories also show that predominance is continuing to switch from type B (45.6%) to type A (54.4%), the influenza division noted. Overall predominance for the season, however, continues to favor type B, 59.3% to 40.7%.
The percentage of deaths caused by pneumonia and influenza, which passed the threshold for epidemic of 7.2% back in early January, has been trending downward for the last 3 weeks and was 7.1% as of Feb. 1, according to the influenza division.
ILI-related deaths among children continue to remain high, with a total count of 78 for the season after another 10 deaths were reported during the week ending Feb. 1, the CDC reported. Comparable numbers for the last three seasons are 44 (2018-2019), 97 (2017-2018), and 35 (2016-2017).
The CDC estimates put the total number of ILIs at around 22 million for the season so far, leading to 210,000 hospitalizations. The agency said that it expects to release estimates of vaccine effectiveness later this month.
CDC begins coronavirus diagnostic test kit distribution; new case confirmed in Wisconsin
The Centers for Disease Control and Prevention and the Wisconsin Department of Health Services confirmed a new case of the 2019 Novel Coronavirus (2019-nCoV) on Feb. 5, 2020, bringing the total number of cases in the United States to 12.*
Earlier in the day, Nancy Messonnier, MD, director of the CDC National Center for Immunization and Respiratory Diseases, told reporters that 206 individuals under investigation had tested negative for infection with the novel virus and that tests were pending on another 76 individuals.
The agency also announced during a press briefing call that diagnostic test kits will begin shipping on Feb. 5, less than 24 hours after receiving an emergency use authorization from the Food and Drug Administration. Full information is available in an article published in the Morbidity and Mortality Weekly Report.
The emergency use authorization will allow for broader use of the CDC’s 2019-nCoV Real Time RT-PCR Diagnostic Panel, which to date has been limited for use at CDC laboratories. Under the emergency use authorization, the diagnostic kit is authorized for patients who meed the CDC criteria for 2019-nCoV testing. The diagnostic test is a reverse transcriptase polymerase chain reaction test that provides presumptive detection of 2019-nCoV from respiratory secretions, such as nasal or oral swabs. A positive test indicates likely infection, although a negative test does not preclude infection and should not be the sole determination for patient management decisions.
“Today, the test kits will start shipping to over 100 U.S. public health labs,” she said. “Each of these labs is required to perform international verification for [Clinical Laboratory Improvement Amendments] compliance prior to reporting out. This process is expected to take a few days.”
Dr. Messonnier said that 200 test kits will be distributed to domestic labs and another 200 test kits will go to select international labs. Each kit can perform diagnostics on 700-800 patient samples.
“What that means is that, by the start of next week, we expect there to be much enhanced capacity for laboratory testing closer to our patients,” she said, adding that additional test kits are being produced and will be available for ordering in the future. Each laboratory that places an order will receive one test kit.
“Distribution of these tests will improve the global capacity to detect and respond to this new virus,” Dr. Messonnier said. “Availability of this test is a starting place for greater commercial availability of diagnostic testing for nCoV.”
The CDC also said that the next batch of passengers arriving from Wuhan, China, will be arriving in one of four locations: Travis Air Force Base, Fairfield, Calif.; Marine Corps Air Station Miramar, San Diego; Lackland Air Force Base, San Antonio; and Eppley Airfield, Omaha, Neb. Passengers will be quarantined for up to 14 days from the day the flight left Wuhan and medical care will be provided if needed.
“We do not believe these people pose a threat to the communities where they are being housed as we are taking measures to minimize any contact,” she said, adding that confirmed infections are expected among these and other returning travelers.
Dr. Messonnier warned that the quarantine measures “may not catch every single returning traveler returning with novel coronavirus, given the nature of this virus and how it is spreading. But if we can catch the majority of them, that will slow the entry of this virus into the United States.”
*This story was updated on 02/05/2020.
The Centers for Disease Control and Prevention and the Wisconsin Department of Health Services confirmed a new case of the 2019 Novel Coronavirus (2019-nCoV) on Feb. 5, 2020, bringing the total number of cases in the United States to 12.*
Earlier in the day, Nancy Messonnier, MD, director of the CDC National Center for Immunization and Respiratory Diseases, told reporters that 206 individuals under investigation had tested negative for infection with the novel virus and that tests were pending on another 76 individuals.
The agency also announced during a press briefing call that diagnostic test kits will begin shipping on Feb. 5, less than 24 hours after receiving an emergency use authorization from the Food and Drug Administration. Full information is available in an article published in the Morbidity and Mortality Weekly Report.
The emergency use authorization will allow for broader use of the CDC’s 2019-nCoV Real Time RT-PCR Diagnostic Panel, which to date has been limited for use at CDC laboratories. Under the emergency use authorization, the diagnostic kit is authorized for patients who meed the CDC criteria for 2019-nCoV testing. The diagnostic test is a reverse transcriptase polymerase chain reaction test that provides presumptive detection of 2019-nCoV from respiratory secretions, such as nasal or oral swabs. A positive test indicates likely infection, although a negative test does not preclude infection and should not be the sole determination for patient management decisions.
“Today, the test kits will start shipping to over 100 U.S. public health labs,” she said. “Each of these labs is required to perform international verification for [Clinical Laboratory Improvement Amendments] compliance prior to reporting out. This process is expected to take a few days.”
Dr. Messonnier said that 200 test kits will be distributed to domestic labs and another 200 test kits will go to select international labs. Each kit can perform diagnostics on 700-800 patient samples.
“What that means is that, by the start of next week, we expect there to be much enhanced capacity for laboratory testing closer to our patients,” she said, adding that additional test kits are being produced and will be available for ordering in the future. Each laboratory that places an order will receive one test kit.
“Distribution of these tests will improve the global capacity to detect and respond to this new virus,” Dr. Messonnier said. “Availability of this test is a starting place for greater commercial availability of diagnostic testing for nCoV.”
The CDC also said that the next batch of passengers arriving from Wuhan, China, will be arriving in one of four locations: Travis Air Force Base, Fairfield, Calif.; Marine Corps Air Station Miramar, San Diego; Lackland Air Force Base, San Antonio; and Eppley Airfield, Omaha, Neb. Passengers will be quarantined for up to 14 days from the day the flight left Wuhan and medical care will be provided if needed.
“We do not believe these people pose a threat to the communities where they are being housed as we are taking measures to minimize any contact,” she said, adding that confirmed infections are expected among these and other returning travelers.
Dr. Messonnier warned that the quarantine measures “may not catch every single returning traveler returning with novel coronavirus, given the nature of this virus and how it is spreading. But if we can catch the majority of them, that will slow the entry of this virus into the United States.”
*This story was updated on 02/05/2020.
The Centers for Disease Control and Prevention and the Wisconsin Department of Health Services confirmed a new case of the 2019 Novel Coronavirus (2019-nCoV) on Feb. 5, 2020, bringing the total number of cases in the United States to 12.*
Earlier in the day, Nancy Messonnier, MD, director of the CDC National Center for Immunization and Respiratory Diseases, told reporters that 206 individuals under investigation had tested negative for infection with the novel virus and that tests were pending on another 76 individuals.
The agency also announced during a press briefing call that diagnostic test kits will begin shipping on Feb. 5, less than 24 hours after receiving an emergency use authorization from the Food and Drug Administration. Full information is available in an article published in the Morbidity and Mortality Weekly Report.
The emergency use authorization will allow for broader use of the CDC’s 2019-nCoV Real Time RT-PCR Diagnostic Panel, which to date has been limited for use at CDC laboratories. Under the emergency use authorization, the diagnostic kit is authorized for patients who meed the CDC criteria for 2019-nCoV testing. The diagnostic test is a reverse transcriptase polymerase chain reaction test that provides presumptive detection of 2019-nCoV from respiratory secretions, such as nasal or oral swabs. A positive test indicates likely infection, although a negative test does not preclude infection and should not be the sole determination for patient management decisions.
“Today, the test kits will start shipping to over 100 U.S. public health labs,” she said. “Each of these labs is required to perform international verification for [Clinical Laboratory Improvement Amendments] compliance prior to reporting out. This process is expected to take a few days.”
Dr. Messonnier said that 200 test kits will be distributed to domestic labs and another 200 test kits will go to select international labs. Each kit can perform diagnostics on 700-800 patient samples.
“What that means is that, by the start of next week, we expect there to be much enhanced capacity for laboratory testing closer to our patients,” she said, adding that additional test kits are being produced and will be available for ordering in the future. Each laboratory that places an order will receive one test kit.
“Distribution of these tests will improve the global capacity to detect and respond to this new virus,” Dr. Messonnier said. “Availability of this test is a starting place for greater commercial availability of diagnostic testing for nCoV.”
The CDC also said that the next batch of passengers arriving from Wuhan, China, will be arriving in one of four locations: Travis Air Force Base, Fairfield, Calif.; Marine Corps Air Station Miramar, San Diego; Lackland Air Force Base, San Antonio; and Eppley Airfield, Omaha, Neb. Passengers will be quarantined for up to 14 days from the day the flight left Wuhan and medical care will be provided if needed.
“We do not believe these people pose a threat to the communities where they are being housed as we are taking measures to minimize any contact,” she said, adding that confirmed infections are expected among these and other returning travelers.
Dr. Messonnier warned that the quarantine measures “may not catch every single returning traveler returning with novel coronavirus, given the nature of this virus and how it is spreading. But if we can catch the majority of them, that will slow the entry of this virus into the United States.”
*This story was updated on 02/05/2020.
FDA issues public health warning recommending against cesium salt usage
The Food and Drug Administration has issued a public health alert warning consumers to avoid the use of dietary supplements that contain cesium chloride or any other cesium salt because of significant safety risks.
Cesium salts are sometimes advertised as an alternative treatment for cancer, the FDA said in the announcement, but these salts have never proved to be safe or effective at treating cancer or any other disease. Clinical case reports and nonclinical trials have shown that cesium salts are associated with a variety of adverse events, including cardiac arrhythmias, hypokalemia, seizures, syncope, and death.
The FDA warned health care providers that cesium salts presented a significant safety risk in compounding drugs in July 2018.
Health care providers should not recommend dietary supplements containing cesium salts to their patients, the FDA said, and if a patient experiences an adverse event while taking a supplement containing cesium salt, the event should be reported to the agency.
While there are few dietary supplements on the market that contain cesium salt, consumers should be aware of the risks and avoid these products. The FDA noted that “if claims sound too good to be true, they probably are.”
The Food and Drug Administration has issued a public health alert warning consumers to avoid the use of dietary supplements that contain cesium chloride or any other cesium salt because of significant safety risks.
Cesium salts are sometimes advertised as an alternative treatment for cancer, the FDA said in the announcement, but these salts have never proved to be safe or effective at treating cancer or any other disease. Clinical case reports and nonclinical trials have shown that cesium salts are associated with a variety of adverse events, including cardiac arrhythmias, hypokalemia, seizures, syncope, and death.
The FDA warned health care providers that cesium salts presented a significant safety risk in compounding drugs in July 2018.
Health care providers should not recommend dietary supplements containing cesium salts to their patients, the FDA said, and if a patient experiences an adverse event while taking a supplement containing cesium salt, the event should be reported to the agency.
While there are few dietary supplements on the market that contain cesium salt, consumers should be aware of the risks and avoid these products. The FDA noted that “if claims sound too good to be true, they probably are.”
The Food and Drug Administration has issued a public health alert warning consumers to avoid the use of dietary supplements that contain cesium chloride or any other cesium salt because of significant safety risks.
Cesium salts are sometimes advertised as an alternative treatment for cancer, the FDA said in the announcement, but these salts have never proved to be safe or effective at treating cancer or any other disease. Clinical case reports and nonclinical trials have shown that cesium salts are associated with a variety of adverse events, including cardiac arrhythmias, hypokalemia, seizures, syncope, and death.
The FDA warned health care providers that cesium salts presented a significant safety risk in compounding drugs in July 2018.
Health care providers should not recommend dietary supplements containing cesium salts to their patients, the FDA said, and if a patient experiences an adverse event while taking a supplement containing cesium salt, the event should be reported to the agency.
While there are few dietary supplements on the market that contain cesium salt, consumers should be aware of the risks and avoid these products. The FDA noted that “if claims sound too good to be true, they probably are.”
ACIP updates recommendations for adult vaccines
The Centers for Disease Control and Prevention has released an updated schedule for adult vaccines. The update includes changes regarding the administration of several vaccines, including those for influenza, human papillomavirus (HPV), hepatitis A and B, and meningitis B, as well as the pneumococcal 13-valent conjugate (PCV13) vaccine.
The schedule, revised annually by the Advisory Committee on Immunization Practices (ACIP) of the CDC, was simultaneously published online February 3, 2020, in the Annals of Internal Medicine and on the CDC website.
Perhaps the change most likely to raise questions is that concerning the PCV13 vaccine. “Owing to a decline in prevalence of the types covered by the PCV13 vaccine, this is no longer routinely recommended for all persons age 65 and older,” senior author Mark Freedman, DVM, MPH, of the immunization services division at the National Center for Immunization and Respiratory Disease, said in an interview.
For purposes of shared clinical decision, however, it should be discussed with previously unvaccinated seniors who do not have risk factors, such as an immunocompromising condition, a cerebrospinal fluid leak, or a cochlear implant.
“But the circumstances for use of the vaccine are not always clear even based on the detailed list of considerations provided, because it’s impossible to think of every conceivable combination of risk factors,” Mr. Freedman added.
Possible beneficiaries of this vaccine are vulnerable elderly people living in nursing homes and long-term care facilities and those living in or traveling to settings in which the rate of pediatric PCV13 uptake is low or zero.
All adults in this age group should continue to receive a single dose of the pneumococcal 23-valent polysaccharide vaccine.*
HPV
The advisory committee now recommends catch-up immunization for women and men through age 26 years (the previous cutoff for men was 21). And in another new recommendation, the ACIP advises considering vaccination for some patients aged 27-45 years who have not been adequately vaccinated.
“Most people ages 27-45 do not need vaccination, but some may benefit,” Mr. Freedman said. “For example, somebody who’s been in a prior long-term monogamous relationship and suddenly finds himself with a new sexual partner.”
“That makes very good sense for older people who haven’t been vaccinated and might continue to be exposed to HPV,” Daniel M. Musher, MD, a professor of medicine at Baylor College of Medicine and an infectious diseases physician at the Michael E. DeBakey Veterans Affairs Medical Center, both in Houston, said in an interview.
Here again, the ACIP advises taking a shared decision-making approach, with clinicians discussing the merits of vaccination in this and other scenarios with patients according to the talking points outlined in the HPV section.
Influenza, hepatitis A and B
For the 2019-2020 influenza season, routine influenza vaccination is recommended for all persons aged 6 months or older who have no contraindications. Where more than one appropriate option is available, the ACIP does not recommend any product over another.
Routine hepatitis A vaccination is recommended for all persons aged 1 year or older who have HIV infection regardless of their level of immune suppression.
For hepatitis B, a new addition to the list of vulnerable patients who may possibly benefit from vaccination is pregnant women at risk for infection or an adverse infection-related pregnancy outcome. Whereas older formulations are safe, the ACIP does not recommend the HepB-CpG (Heplisav-B) vaccine during pregnancy, owing to the fact that safety data are lacking.
Meningitis B
Individuals aged 10 years or older who have complement deficiency, who use a complement inhibitor, who have asplenia, or who are microbiologists should receive a meningitis B booster dose 1 year following completion of a primary series. After that, they should receive booster doses every 2-3 years for as long they are at elevated risk.
Vaccination should be discussed with individuals aged 16-23 years even if they are not at increased risk for meningococcal disease. Persons aged 10 years or older whom public health authorities deem to be at increased risk during an outbreak should have a one-time booster dose if at least 1 year has elapsed since completion of a meningitis B primary series.
Td/Tdap, varicella
The ACIP now recommends that either the Td or Tdap vaccine be given in cases in which currently just the Td vaccine is recommended; that is, for the 10-year booster shot as well as for tetanus prophylaxis in wound management and the catch-up immunization schedule, including that for pregnant women.
Vaccination against varicella should be considered for HIV-infected individuals who are without evidence of varicella immunity and whose CD4 counts are at least 200 cells/mL.
Dr. Musher, who was not involved in drafting the recommendations, takes issue generally with the addition of shared clinical decision making on vaccination. “Shared decision making is a problem for anyone practicing medicine. It places a terrible burden [on] the doctors to discuss these options with patients at great length. Most patients want the doctor to make the decision.”
In his view, this approach makes little sense in the case of the PCV13 vaccine because the strains it covers have disappeared from the population through the widespread vaccination of children. “But discussions are important for some vaccines, such as the herpes zoster vaccine, since patients can have a terrible reaction to the first dose and refuse to have the second,” he said.
Some of these new recommendations were released in 2019 after ACIP members met to vote on them in February, June, and October.
As in previous years, the schedule has been streamlined for easier reference. Physicians are reminded to closely read the details in the vaccine notes, as these specify who needs what vaccine, when, and at what dose.
The ACIP develops its recommendations after reviewing vaccine-related data, including the data regarding the epidemiology and burden of the vaccine-preventable disease, vaccine effectiveness and safety, the quality of evidence, implementability, and the economics of immunization policy.
The authors have received grants and expense payments from public and not-for-profit institutions. One coauthor has received fees from ACI Clinical for data and safety monitoring in an immunization trial. Dr. Musher has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Correction, 3/31/20: An earlier version of this article misstated the recommendation for administration of the pneumococcal 23-valent polysaccharide vaccine. All adults in this age group should continue to receive a single dose of this vaccine.
The Centers for Disease Control and Prevention has released an updated schedule for adult vaccines. The update includes changes regarding the administration of several vaccines, including those for influenza, human papillomavirus (HPV), hepatitis A and B, and meningitis B, as well as the pneumococcal 13-valent conjugate (PCV13) vaccine.
The schedule, revised annually by the Advisory Committee on Immunization Practices (ACIP) of the CDC, was simultaneously published online February 3, 2020, in the Annals of Internal Medicine and on the CDC website.
Perhaps the change most likely to raise questions is that concerning the PCV13 vaccine. “Owing to a decline in prevalence of the types covered by the PCV13 vaccine, this is no longer routinely recommended for all persons age 65 and older,” senior author Mark Freedman, DVM, MPH, of the immunization services division at the National Center for Immunization and Respiratory Disease, said in an interview.
For purposes of shared clinical decision, however, it should be discussed with previously unvaccinated seniors who do not have risk factors, such as an immunocompromising condition, a cerebrospinal fluid leak, or a cochlear implant.
“But the circumstances for use of the vaccine are not always clear even based on the detailed list of considerations provided, because it’s impossible to think of every conceivable combination of risk factors,” Mr. Freedman added.
Possible beneficiaries of this vaccine are vulnerable elderly people living in nursing homes and long-term care facilities and those living in or traveling to settings in which the rate of pediatric PCV13 uptake is low or zero.
All adults in this age group should continue to receive a single dose of the pneumococcal 23-valent polysaccharide vaccine.*
HPV
The advisory committee now recommends catch-up immunization for women and men through age 26 years (the previous cutoff for men was 21). And in another new recommendation, the ACIP advises considering vaccination for some patients aged 27-45 years who have not been adequately vaccinated.
“Most people ages 27-45 do not need vaccination, but some may benefit,” Mr. Freedman said. “For example, somebody who’s been in a prior long-term monogamous relationship and suddenly finds himself with a new sexual partner.”
“That makes very good sense for older people who haven’t been vaccinated and might continue to be exposed to HPV,” Daniel M. Musher, MD, a professor of medicine at Baylor College of Medicine and an infectious diseases physician at the Michael E. DeBakey Veterans Affairs Medical Center, both in Houston, said in an interview.
Here again, the ACIP advises taking a shared decision-making approach, with clinicians discussing the merits of vaccination in this and other scenarios with patients according to the talking points outlined in the HPV section.
Influenza, hepatitis A and B
For the 2019-2020 influenza season, routine influenza vaccination is recommended for all persons aged 6 months or older who have no contraindications. Where more than one appropriate option is available, the ACIP does not recommend any product over another.
Routine hepatitis A vaccination is recommended for all persons aged 1 year or older who have HIV infection regardless of their level of immune suppression.
For hepatitis B, a new addition to the list of vulnerable patients who may possibly benefit from vaccination is pregnant women at risk for infection or an adverse infection-related pregnancy outcome. Whereas older formulations are safe, the ACIP does not recommend the HepB-CpG (Heplisav-B) vaccine during pregnancy, owing to the fact that safety data are lacking.
Meningitis B
Individuals aged 10 years or older who have complement deficiency, who use a complement inhibitor, who have asplenia, or who are microbiologists should receive a meningitis B booster dose 1 year following completion of a primary series. After that, they should receive booster doses every 2-3 years for as long they are at elevated risk.
Vaccination should be discussed with individuals aged 16-23 years even if they are not at increased risk for meningococcal disease. Persons aged 10 years or older whom public health authorities deem to be at increased risk during an outbreak should have a one-time booster dose if at least 1 year has elapsed since completion of a meningitis B primary series.
Td/Tdap, varicella
The ACIP now recommends that either the Td or Tdap vaccine be given in cases in which currently just the Td vaccine is recommended; that is, for the 10-year booster shot as well as for tetanus prophylaxis in wound management and the catch-up immunization schedule, including that for pregnant women.
Vaccination against varicella should be considered for HIV-infected individuals who are without evidence of varicella immunity and whose CD4 counts are at least 200 cells/mL.
Dr. Musher, who was not involved in drafting the recommendations, takes issue generally with the addition of shared clinical decision making on vaccination. “Shared decision making is a problem for anyone practicing medicine. It places a terrible burden [on] the doctors to discuss these options with patients at great length. Most patients want the doctor to make the decision.”
In his view, this approach makes little sense in the case of the PCV13 vaccine because the strains it covers have disappeared from the population through the widespread vaccination of children. “But discussions are important for some vaccines, such as the herpes zoster vaccine, since patients can have a terrible reaction to the first dose and refuse to have the second,” he said.
Some of these new recommendations were released in 2019 after ACIP members met to vote on them in February, June, and October.
As in previous years, the schedule has been streamlined for easier reference. Physicians are reminded to closely read the details in the vaccine notes, as these specify who needs what vaccine, when, and at what dose.
The ACIP develops its recommendations after reviewing vaccine-related data, including the data regarding the epidemiology and burden of the vaccine-preventable disease, vaccine effectiveness and safety, the quality of evidence, implementability, and the economics of immunization policy.
The authors have received grants and expense payments from public and not-for-profit institutions. One coauthor has received fees from ACI Clinical for data and safety monitoring in an immunization trial. Dr. Musher has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Correction, 3/31/20: An earlier version of this article misstated the recommendation for administration of the pneumococcal 23-valent polysaccharide vaccine. All adults in this age group should continue to receive a single dose of this vaccine.
The Centers for Disease Control and Prevention has released an updated schedule for adult vaccines. The update includes changes regarding the administration of several vaccines, including those for influenza, human papillomavirus (HPV), hepatitis A and B, and meningitis B, as well as the pneumococcal 13-valent conjugate (PCV13) vaccine.
The schedule, revised annually by the Advisory Committee on Immunization Practices (ACIP) of the CDC, was simultaneously published online February 3, 2020, in the Annals of Internal Medicine and on the CDC website.
Perhaps the change most likely to raise questions is that concerning the PCV13 vaccine. “Owing to a decline in prevalence of the types covered by the PCV13 vaccine, this is no longer routinely recommended for all persons age 65 and older,” senior author Mark Freedman, DVM, MPH, of the immunization services division at the National Center for Immunization and Respiratory Disease, said in an interview.
For purposes of shared clinical decision, however, it should be discussed with previously unvaccinated seniors who do not have risk factors, such as an immunocompromising condition, a cerebrospinal fluid leak, or a cochlear implant.
“But the circumstances for use of the vaccine are not always clear even based on the detailed list of considerations provided, because it’s impossible to think of every conceivable combination of risk factors,” Mr. Freedman added.
Possible beneficiaries of this vaccine are vulnerable elderly people living in nursing homes and long-term care facilities and those living in or traveling to settings in which the rate of pediatric PCV13 uptake is low or zero.
All adults in this age group should continue to receive a single dose of the pneumococcal 23-valent polysaccharide vaccine.*
HPV
The advisory committee now recommends catch-up immunization for women and men through age 26 years (the previous cutoff for men was 21). And in another new recommendation, the ACIP advises considering vaccination for some patients aged 27-45 years who have not been adequately vaccinated.
“Most people ages 27-45 do not need vaccination, but some may benefit,” Mr. Freedman said. “For example, somebody who’s been in a prior long-term monogamous relationship and suddenly finds himself with a new sexual partner.”
“That makes very good sense for older people who haven’t been vaccinated and might continue to be exposed to HPV,” Daniel M. Musher, MD, a professor of medicine at Baylor College of Medicine and an infectious diseases physician at the Michael E. DeBakey Veterans Affairs Medical Center, both in Houston, said in an interview.
Here again, the ACIP advises taking a shared decision-making approach, with clinicians discussing the merits of vaccination in this and other scenarios with patients according to the talking points outlined in the HPV section.
Influenza, hepatitis A and B
For the 2019-2020 influenza season, routine influenza vaccination is recommended for all persons aged 6 months or older who have no contraindications. Where more than one appropriate option is available, the ACIP does not recommend any product over another.
Routine hepatitis A vaccination is recommended for all persons aged 1 year or older who have HIV infection regardless of their level of immune suppression.
For hepatitis B, a new addition to the list of vulnerable patients who may possibly benefit from vaccination is pregnant women at risk for infection or an adverse infection-related pregnancy outcome. Whereas older formulations are safe, the ACIP does not recommend the HepB-CpG (Heplisav-B) vaccine during pregnancy, owing to the fact that safety data are lacking.
Meningitis B
Individuals aged 10 years or older who have complement deficiency, who use a complement inhibitor, who have asplenia, or who are microbiologists should receive a meningitis B booster dose 1 year following completion of a primary series. After that, they should receive booster doses every 2-3 years for as long they are at elevated risk.
Vaccination should be discussed with individuals aged 16-23 years even if they are not at increased risk for meningococcal disease. Persons aged 10 years or older whom public health authorities deem to be at increased risk during an outbreak should have a one-time booster dose if at least 1 year has elapsed since completion of a meningitis B primary series.
Td/Tdap, varicella
The ACIP now recommends that either the Td or Tdap vaccine be given in cases in which currently just the Td vaccine is recommended; that is, for the 10-year booster shot as well as for tetanus prophylaxis in wound management and the catch-up immunization schedule, including that for pregnant women.
Vaccination against varicella should be considered for HIV-infected individuals who are without evidence of varicella immunity and whose CD4 counts are at least 200 cells/mL.
Dr. Musher, who was not involved in drafting the recommendations, takes issue generally with the addition of shared clinical decision making on vaccination. “Shared decision making is a problem for anyone practicing medicine. It places a terrible burden [on] the doctors to discuss these options with patients at great length. Most patients want the doctor to make the decision.”
In his view, this approach makes little sense in the case of the PCV13 vaccine because the strains it covers have disappeared from the population through the widespread vaccination of children. “But discussions are important for some vaccines, such as the herpes zoster vaccine, since patients can have a terrible reaction to the first dose and refuse to have the second,” he said.
Some of these new recommendations were released in 2019 after ACIP members met to vote on them in February, June, and October.
As in previous years, the schedule has been streamlined for easier reference. Physicians are reminded to closely read the details in the vaccine notes, as these specify who needs what vaccine, when, and at what dose.
The ACIP develops its recommendations after reviewing vaccine-related data, including the data regarding the epidemiology and burden of the vaccine-preventable disease, vaccine effectiveness and safety, the quality of evidence, implementability, and the economics of immunization policy.
The authors have received grants and expense payments from public and not-for-profit institutions. One coauthor has received fees from ACI Clinical for data and safety monitoring in an immunization trial. Dr. Musher has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Correction, 3/31/20: An earlier version of this article misstated the recommendation for administration of the pneumococcal 23-valent polysaccharide vaccine. All adults in this age group should continue to receive a single dose of this vaccine.
Novel coronavirus cases now at 11; entry ban and quarantine measures begin
, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during a Centers for Disease Control and Prevention press briefing.
Four of the new cases are in California, and one in Massachusetts. Although four of the new cases have recent travel history to Wuhan, China, the epicenter of the 2019-nCoV outbreak, the fifth is a close household contact of one of the other California patients, said Dr. Messonnier. This last case is the second instance of person-to-person spread of 2019-nCoV in the United States.
“We expect to find additional cases of the novel coronavirus in the United States,” she said. “We expect to see more cases of person-to-person spread among close contacts. And we continue to expect this will happen given the explosive nature of this outbreak in China.”
As of the morning of Feb. 3, 167 persons under investigation, or PUIs, for possible 2019-nCoV have tested negative for the virus, and an additional 82 PUIs have testing pending – this latter figure includes some tests that are still in transit to the CDC, said Dr. Messonnier.
During the briefing, Dr. Messonnier emphasized both the aggressive nature of the U.S. public health response and the rationale for quick and assertive action. “The goal of our public health response is to protect and contain,” she said. “Strong measures now may blunt the impact of this virus on the United States.”
She cited the intensity of transmission in Hubei Province, the expansion of transmission to other provinces in China, the expansion of cases outside of China, and sporadic ongoing deaths from 2019-nCoV as drivers of the aggressive U.S. public health response.
A presidential proclamation is currently in place that bars U.S. entry to foreign nationals who have visited mainland China within the past 14 days; the ban does not apply to travelers from Hong Kong and Macao. Immediate family members of U.S. citizens and individuals who have U.S. permanent resident status are exempted from the entry ban and will be allowed entry into the United States.
However, explained Dr. Messonnier, those who have traveled to China recently and are permitted entry will be subject to screening. All passengers with such recent travel will be directed to one of 11 U.S. airports set up to perform additional screening.
As of Feb 3, the list of airports includes:
- San Francisco International Airport in California.
- Los Angeles International Airport in California.
- Hartsfield-Jackson Atlanta International Airport in Georgia.
- Daniel K. Inouye International Airport in Hawaii.
- O’Hare International Airport in Illinois.
- Detroit Metropolitan Airport in Michigan.
- Newark Liberty International Airport in New Jersey.
- John F. Kennedy International Airport in New York.
- Dallas/Fort Worth International Airport in Texas.
- Washington Dulles International Airport in Virginia.
- Seattle-Tacoma International Airport in Washington.
Travelers who have been to Hubei Province in the previous 14 days will have an additional health assessment at which they will be screened for fever, cough, or difficulty breathing. Any American citizens or exempt individuals who are symptomatic would then be transferred for further medical evaluation. Asymptomatic travelers in this category will be subject to a mandatory 14-day quarantine near their point of entry, rather than continuing on to their final destinations.
Dr. Messonnier emphasized that the mandatory 14-day quarantine is specifically for Americans or exempt individuals returning from Hubei Province, adding that the CDC is presently working with individual states to determine the exact venues for quarantine.
American citizens and exempt individuals returning from other parts of mainland China will be routed to one of the 11 airports and will also receive additional health screening. Symptomatic individuals in this travel category would be referred for further evaluation before being able to complete their itinerary.
Asymptomatic American citizens and exempt individuals who are returning from mainland China – but not Hubei Province – will be allowed to travel on to their final destinations, but will be asked to stay home as much as possible and to monitor their health during the 14 days after their return.
The U.S. Department of State is bringing back more Americans from Wuhan province this week, and these individuals will also be kept under federal quarantine for 14 days.
“There are likely to be confirmed infections among returning travelers,” said Dr. Messonnier. “It is important to note that this strategy is not meant to catch every single traveler returning from China with novel coronavirus; given the nature of this virus and how it’s spreading, that would be impossible, but working together we can catch the majority of them.
“The goal here is to slow the entry of this virus into the United States,” she said, adding that the nation’s health care and public health systems stand on high alert to detect the virus in community settings. In response to questioning from the press, Dr. Messonnier defended the stringent quarantine measures, noting that they are in line with those taken by some other nations, and with the aggressive action being taken by the Chinese government itself. “These actions are science based and aimed at protecting the health of all Americans,” she said.
The real-time reverse transcription polymerase chain reaction (rRT-PCR) assay that the CDC has developed detects 2019-nCoV in both respiratory and serum specimens. Dr. Messonnier reported that the CDC is today filing an emergency use authorization (EUA) application to the U.S. Food and Drug Administration to expedite access to the assay for public health laboratories across the country. “This will greatly enhance our capacity to test for this virus,” she said, noting that EUA approval may come as soon as the end of this week.
Although the CDC is poised to send an expert team to China, it’s still awaiting favorable results from the international negotiations currently underway. “This is a horrible situation in China,” said Dr. Messonnier. “Our presence on the ground in China would be a help to China. ... Science should trump everything else; that’s what we’re hoping – that the scientific expertise of the global community can be brought to bear on the incredibly complicated, difficult situation that our colleagues in China are dealing with.”
, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during a Centers for Disease Control and Prevention press briefing.
Four of the new cases are in California, and one in Massachusetts. Although four of the new cases have recent travel history to Wuhan, China, the epicenter of the 2019-nCoV outbreak, the fifth is a close household contact of one of the other California patients, said Dr. Messonnier. This last case is the second instance of person-to-person spread of 2019-nCoV in the United States.
“We expect to find additional cases of the novel coronavirus in the United States,” she said. “We expect to see more cases of person-to-person spread among close contacts. And we continue to expect this will happen given the explosive nature of this outbreak in China.”
As of the morning of Feb. 3, 167 persons under investigation, or PUIs, for possible 2019-nCoV have tested negative for the virus, and an additional 82 PUIs have testing pending – this latter figure includes some tests that are still in transit to the CDC, said Dr. Messonnier.
During the briefing, Dr. Messonnier emphasized both the aggressive nature of the U.S. public health response and the rationale for quick and assertive action. “The goal of our public health response is to protect and contain,” she said. “Strong measures now may blunt the impact of this virus on the United States.”
She cited the intensity of transmission in Hubei Province, the expansion of transmission to other provinces in China, the expansion of cases outside of China, and sporadic ongoing deaths from 2019-nCoV as drivers of the aggressive U.S. public health response.
A presidential proclamation is currently in place that bars U.S. entry to foreign nationals who have visited mainland China within the past 14 days; the ban does not apply to travelers from Hong Kong and Macao. Immediate family members of U.S. citizens and individuals who have U.S. permanent resident status are exempted from the entry ban and will be allowed entry into the United States.
However, explained Dr. Messonnier, those who have traveled to China recently and are permitted entry will be subject to screening. All passengers with such recent travel will be directed to one of 11 U.S. airports set up to perform additional screening.
As of Feb 3, the list of airports includes:
- San Francisco International Airport in California.
- Los Angeles International Airport in California.
- Hartsfield-Jackson Atlanta International Airport in Georgia.
- Daniel K. Inouye International Airport in Hawaii.
- O’Hare International Airport in Illinois.
- Detroit Metropolitan Airport in Michigan.
- Newark Liberty International Airport in New Jersey.
- John F. Kennedy International Airport in New York.
- Dallas/Fort Worth International Airport in Texas.
- Washington Dulles International Airport in Virginia.
- Seattle-Tacoma International Airport in Washington.
Travelers who have been to Hubei Province in the previous 14 days will have an additional health assessment at which they will be screened for fever, cough, or difficulty breathing. Any American citizens or exempt individuals who are symptomatic would then be transferred for further medical evaluation. Asymptomatic travelers in this category will be subject to a mandatory 14-day quarantine near their point of entry, rather than continuing on to their final destinations.
Dr. Messonnier emphasized that the mandatory 14-day quarantine is specifically for Americans or exempt individuals returning from Hubei Province, adding that the CDC is presently working with individual states to determine the exact venues for quarantine.
American citizens and exempt individuals returning from other parts of mainland China will be routed to one of the 11 airports and will also receive additional health screening. Symptomatic individuals in this travel category would be referred for further evaluation before being able to complete their itinerary.
Asymptomatic American citizens and exempt individuals who are returning from mainland China – but not Hubei Province – will be allowed to travel on to their final destinations, but will be asked to stay home as much as possible and to monitor their health during the 14 days after their return.
The U.S. Department of State is bringing back more Americans from Wuhan province this week, and these individuals will also be kept under federal quarantine for 14 days.
“There are likely to be confirmed infections among returning travelers,” said Dr. Messonnier. “It is important to note that this strategy is not meant to catch every single traveler returning from China with novel coronavirus; given the nature of this virus and how it’s spreading, that would be impossible, but working together we can catch the majority of them.
“The goal here is to slow the entry of this virus into the United States,” she said, adding that the nation’s health care and public health systems stand on high alert to detect the virus in community settings. In response to questioning from the press, Dr. Messonnier defended the stringent quarantine measures, noting that they are in line with those taken by some other nations, and with the aggressive action being taken by the Chinese government itself. “These actions are science based and aimed at protecting the health of all Americans,” she said.
The real-time reverse transcription polymerase chain reaction (rRT-PCR) assay that the CDC has developed detects 2019-nCoV in both respiratory and serum specimens. Dr. Messonnier reported that the CDC is today filing an emergency use authorization (EUA) application to the U.S. Food and Drug Administration to expedite access to the assay for public health laboratories across the country. “This will greatly enhance our capacity to test for this virus,” she said, noting that EUA approval may come as soon as the end of this week.
Although the CDC is poised to send an expert team to China, it’s still awaiting favorable results from the international negotiations currently underway. “This is a horrible situation in China,” said Dr. Messonnier. “Our presence on the ground in China would be a help to China. ... Science should trump everything else; that’s what we’re hoping – that the scientific expertise of the global community can be brought to bear on the incredibly complicated, difficult situation that our colleagues in China are dealing with.”
, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during a Centers for Disease Control and Prevention press briefing.
Four of the new cases are in California, and one in Massachusetts. Although four of the new cases have recent travel history to Wuhan, China, the epicenter of the 2019-nCoV outbreak, the fifth is a close household contact of one of the other California patients, said Dr. Messonnier. This last case is the second instance of person-to-person spread of 2019-nCoV in the United States.
“We expect to find additional cases of the novel coronavirus in the United States,” she said. “We expect to see more cases of person-to-person spread among close contacts. And we continue to expect this will happen given the explosive nature of this outbreak in China.”
As of the morning of Feb. 3, 167 persons under investigation, or PUIs, for possible 2019-nCoV have tested negative for the virus, and an additional 82 PUIs have testing pending – this latter figure includes some tests that are still in transit to the CDC, said Dr. Messonnier.
During the briefing, Dr. Messonnier emphasized both the aggressive nature of the U.S. public health response and the rationale for quick and assertive action. “The goal of our public health response is to protect and contain,” she said. “Strong measures now may blunt the impact of this virus on the United States.”
She cited the intensity of transmission in Hubei Province, the expansion of transmission to other provinces in China, the expansion of cases outside of China, and sporadic ongoing deaths from 2019-nCoV as drivers of the aggressive U.S. public health response.
A presidential proclamation is currently in place that bars U.S. entry to foreign nationals who have visited mainland China within the past 14 days; the ban does not apply to travelers from Hong Kong and Macao. Immediate family members of U.S. citizens and individuals who have U.S. permanent resident status are exempted from the entry ban and will be allowed entry into the United States.
However, explained Dr. Messonnier, those who have traveled to China recently and are permitted entry will be subject to screening. All passengers with such recent travel will be directed to one of 11 U.S. airports set up to perform additional screening.
As of Feb 3, the list of airports includes:
- San Francisco International Airport in California.
- Los Angeles International Airport in California.
- Hartsfield-Jackson Atlanta International Airport in Georgia.
- Daniel K. Inouye International Airport in Hawaii.
- O’Hare International Airport in Illinois.
- Detroit Metropolitan Airport in Michigan.
- Newark Liberty International Airport in New Jersey.
- John F. Kennedy International Airport in New York.
- Dallas/Fort Worth International Airport in Texas.
- Washington Dulles International Airport in Virginia.
- Seattle-Tacoma International Airport in Washington.
Travelers who have been to Hubei Province in the previous 14 days will have an additional health assessment at which they will be screened for fever, cough, or difficulty breathing. Any American citizens or exempt individuals who are symptomatic would then be transferred for further medical evaluation. Asymptomatic travelers in this category will be subject to a mandatory 14-day quarantine near their point of entry, rather than continuing on to their final destinations.
Dr. Messonnier emphasized that the mandatory 14-day quarantine is specifically for Americans or exempt individuals returning from Hubei Province, adding that the CDC is presently working with individual states to determine the exact venues for quarantine.
American citizens and exempt individuals returning from other parts of mainland China will be routed to one of the 11 airports and will also receive additional health screening. Symptomatic individuals in this travel category would be referred for further evaluation before being able to complete their itinerary.
Asymptomatic American citizens and exempt individuals who are returning from mainland China – but not Hubei Province – will be allowed to travel on to their final destinations, but will be asked to stay home as much as possible and to monitor their health during the 14 days after their return.
The U.S. Department of State is bringing back more Americans from Wuhan province this week, and these individuals will also be kept under federal quarantine for 14 days.
“There are likely to be confirmed infections among returning travelers,” said Dr. Messonnier. “It is important to note that this strategy is not meant to catch every single traveler returning from China with novel coronavirus; given the nature of this virus and how it’s spreading, that would be impossible, but working together we can catch the majority of them.
“The goal here is to slow the entry of this virus into the United States,” she said, adding that the nation’s health care and public health systems stand on high alert to detect the virus in community settings. In response to questioning from the press, Dr. Messonnier defended the stringent quarantine measures, noting that they are in line with those taken by some other nations, and with the aggressive action being taken by the Chinese government itself. “These actions are science based and aimed at protecting the health of all Americans,” she said.
The real-time reverse transcription polymerase chain reaction (rRT-PCR) assay that the CDC has developed detects 2019-nCoV in both respiratory and serum specimens. Dr. Messonnier reported that the CDC is today filing an emergency use authorization (EUA) application to the U.S. Food and Drug Administration to expedite access to the assay for public health laboratories across the country. “This will greatly enhance our capacity to test for this virus,” she said, noting that EUA approval may come as soon as the end of this week.
Although the CDC is poised to send an expert team to China, it’s still awaiting favorable results from the international negotiations currently underway. “This is a horrible situation in China,” said Dr. Messonnier. “Our presence on the ground in China would be a help to China. ... Science should trump everything else; that’s what we’re hoping – that the scientific expertise of the global community can be brought to bear on the incredibly complicated, difficult situation that our colleagues in China are dealing with.”
FROM A CDC PRESS BRIEFING
Don’t forget about the flu: 2019-2010 season is not over
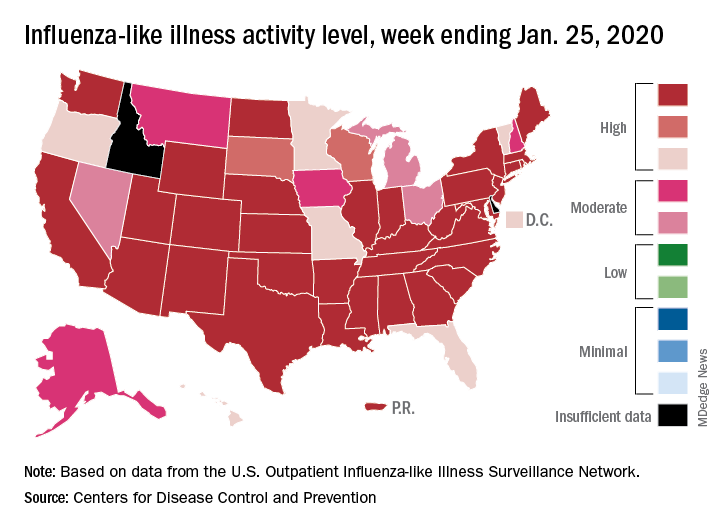
Nationally, an estimated 5.7% of all outpatients visiting health care providers had influenza-like illness (ILI) for the week ending Jan. 25, which was up from 5.1% the previous week but still lower than the current seasonal high of 7.1% recorded during the week of Dec. 22-28, the CDC’s influenza division reported.
Another key indicator of influenza activity, the percentage of respiratory specimens testing positive, also remains high as it rose from 25.7% the week before to 27.7% for the week ending Jan. 25, the influenza division said. That is the highest rate of the 2019-2020 season so far, surpassing the 26.8% reached during Dec. 22-28.
Another new seasonal high involves the number of states, 33 plus Puerto Rico, at the highest level of ILI activity on the CDC’s 1-10 scale for the latest reporting week, topping the 32 jurisdictions from the last full week of December. Another eight states and the District of Columbia were in the “high” range with activity levels of 8 and 9, and no state with available data was lower than level 6, the CDC data show.
Going along with the recent 2-week increase in activity is a large increase in the number of ILI-related pediatric deaths, which rose from 39 on Jan. 11 to the current count of 68, the CDC said. At the same point last year, there had been 36 pediatric deaths.
Other indicators of ILI severity, however, “are not high at this point in the season,” the influenza division noted. “Overall, hospitalization rates remain similar to what has been seen at this time during recent seasons, but rates among children and young adults are higher at this time than in recent seasons.” Overall pneumonia and influenza mortality is also low, the CDC added.

Nationally, an estimated 5.7% of all outpatients visiting health care providers had influenza-like illness (ILI) for the week ending Jan. 25, which was up from 5.1% the previous week but still lower than the current seasonal high of 7.1% recorded during the week of Dec. 22-28, the CDC’s influenza division reported.
Another key indicator of influenza activity, the percentage of respiratory specimens testing positive, also remains high as it rose from 25.7% the week before to 27.7% for the week ending Jan. 25, the influenza division said. That is the highest rate of the 2019-2020 season so far, surpassing the 26.8% reached during Dec. 22-28.
Another new seasonal high involves the number of states, 33 plus Puerto Rico, at the highest level of ILI activity on the CDC’s 1-10 scale for the latest reporting week, topping the 32 jurisdictions from the last full week of December. Another eight states and the District of Columbia were in the “high” range with activity levels of 8 and 9, and no state with available data was lower than level 6, the CDC data show.
Going along with the recent 2-week increase in activity is a large increase in the number of ILI-related pediatric deaths, which rose from 39 on Jan. 11 to the current count of 68, the CDC said. At the same point last year, there had been 36 pediatric deaths.
Other indicators of ILI severity, however, “are not high at this point in the season,” the influenza division noted. “Overall, hospitalization rates remain similar to what has been seen at this time during recent seasons, but rates among children and young adults are higher at this time than in recent seasons.” Overall pneumonia and influenza mortality is also low, the CDC added.

Nationally, an estimated 5.7% of all outpatients visiting health care providers had influenza-like illness (ILI) for the week ending Jan. 25, which was up from 5.1% the previous week but still lower than the current seasonal high of 7.1% recorded during the week of Dec. 22-28, the CDC’s influenza division reported.
Another key indicator of influenza activity, the percentage of respiratory specimens testing positive, also remains high as it rose from 25.7% the week before to 27.7% for the week ending Jan. 25, the influenza division said. That is the highest rate of the 2019-2020 season so far, surpassing the 26.8% reached during Dec. 22-28.
Another new seasonal high involves the number of states, 33 plus Puerto Rico, at the highest level of ILI activity on the CDC’s 1-10 scale for the latest reporting week, topping the 32 jurisdictions from the last full week of December. Another eight states and the District of Columbia were in the “high” range with activity levels of 8 and 9, and no state with available data was lower than level 6, the CDC data show.
Going along with the recent 2-week increase in activity is a large increase in the number of ILI-related pediatric deaths, which rose from 39 on Jan. 11 to the current count of 68, the CDC said. At the same point last year, there had been 36 pediatric deaths.
Other indicators of ILI severity, however, “are not high at this point in the season,” the influenza division noted. “Overall, hospitalization rates remain similar to what has been seen at this time during recent seasons, but rates among children and young adults are higher at this time than in recent seasons.” Overall pneumonia and influenza mortality is also low, the CDC added.