User login
Bridging the Digital Divide in Teledermatology Usage: A Retrospective Review of Patient Visits
Teledermatology is an effective patient care model for the delivery of high-quality dermatologic care.1 Teledermatology can occur using synchronous, asynchronous, and hybrid models of care. In asynchronous visits (AVs), patients or health professionals submit photographs and information for dermatologists to review and provide treatment recommendations. With synchronous visits (SVs), patients have a visit with a dermatology health professional in real time via live video conferencing software. Hybrid models incorporate asynchronous strategies for patient intake forms and skin photograph submissions as well as synchronous methods for live video consultation in a single visit.1 However, remarkable inequities in internet access limit telemedicine usage among medically marginalized patient populations, including racialized, elderly, and low socioeconomic status groups.2
Synchronous visits, a relatively newer teledermatology format, allow for communication with dermatology professionals from the convenience of a patient’s selected location. The live interaction of SVs allows dermatology professionals to answer questions, provide treatment recommendations, and build therapeutic relationships with patients. Concerns for dermatologist reimbursement, malpractice/liability, and technological challenges stalled large-scale uptake of teledermatology platforms.3 The COVID-19 pandemic led to a drastic increase in teledermatology usage of approximately 587.2%, largely due to public safety measures and Medicaid reimbursement parity between SV and in-office visits (IVs).3,4
With the implementation of SVs as a patient care model, we investigated the demographics of patients who utilized SVs, AVs, or IVs, and we propose strategies to promote equity in dermatologic care access.
Methods
This study was approved by the University of Pittsburgh institutional review board (STUDY20110043). We performed a retrospective electronic medical record review of deidentified data from the University of Pittsburgh Medical Center, a tertiary care center in Allegheny County, Pennsylvania, with an established asynchronous teledermatology program. Hybrid SVs were integrated into the University of Pittsburgh Medical Center patient care visit options in March 2020. Patients were instructed to upload photographs of their skin conditions prior to SV appointments. The study included visits occurring between July and December 2020. Visit types included SVs, AVs, and IVs.
We analyzed the initial dermatology visits of 17,130 patients aged 17.5 years and older. Recorded data included diagnosis, age, sex, race, ethnicity, and insurance type for each visit type. Patients without a reported race (990 patients) or ethnicity (1712 patients) were excluded from analysis of race/ethnicity data. Patient zip codes were compared with the zip codes of Allegheny County municipalities as reported by the Allegheny County Elections Division.
Statistical Analysis—Descriptive statistics were calculated; frequency with percentage was used to report categorical variables, and the mean (SD) was used for normally distributed continuous variables. Univariate analysis was performed using the χ2 test for categorical variables. One-way analysis of variance was used to compare age among visit types. Statistical significance was defined as P<.05. IBM SPSS Statistics for Windows, Version 24 (IBM Corp) was used for all statistical analyses.
Results
In our study population, 81.2% (13,916) of patients were residents of Allegheny County, where 51.6% of residents are female and 81.4% are older than 18 years according to data from 2020.5 The racial and ethnic demographics of Allegheny County were 13.4% African American/Black, 0.2% American Indian/Alaska Native, 4.2% Asian, 2.3% Hispanic/Latino, and 79.6% White. The percentage of residents who identified as Native Hawaiian/Pacific Islander was reported to be greater than 0% but less than 0.5%.5
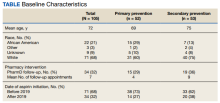
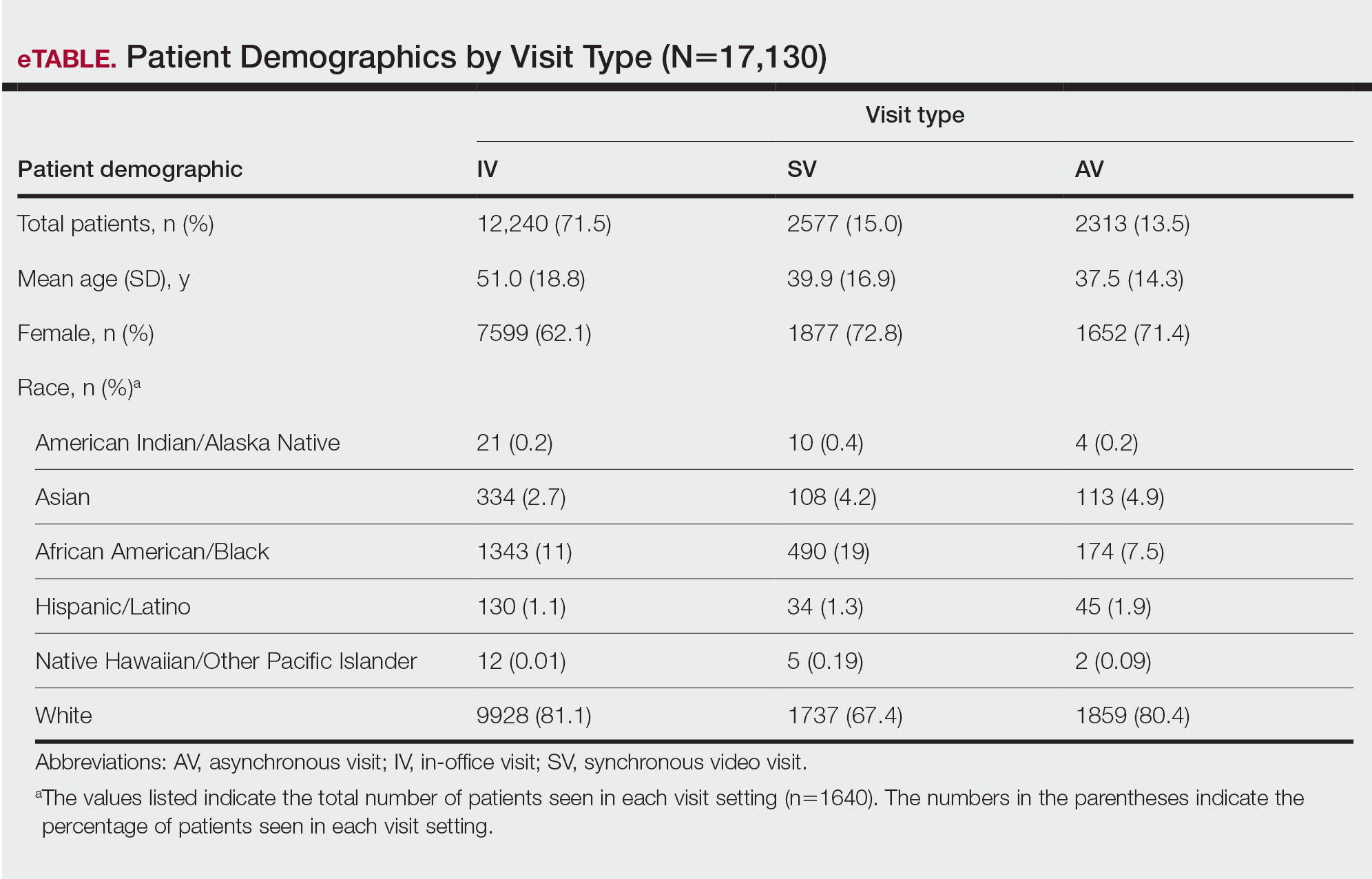
In our analysis, IVs were the most utilized visit type, accounting for 71.5% (12,240) of visits, followed by 15.0% (2577) for SVs and 13.5% (2313) for AVs. The mean age (SD) of IV patients was 51.0 (18.8) years compared with 39.9 (16.9) years for SV patients and 37.5 (14.3) years for AV patients (eTable). The majority of patients for all visits were female: 62.1% (7599) for IVs, 71.4% (1652) for AVs, and 72.8% (1877) for SVs. The largest racial or ethnic group for all visit types included White patients (83.8% [13,524] of all patients), followed by Black (12.4% [2007]), Hispanic/Latino (1.4% [209]), Asian (3.4% [555]), American Indian/Alaska Native (0.2% [35]), and Native Hawaiian/Other Pacific Islander patients (0.1% [19]).
Asian patients, who comprised 4.2% of Allegheny County residents,5 accounted for 2.7% (334) of IVs, 4.9% (113) of AVs, and 4.2% (108) of SVs. Black patients, who were reported as 13.4% of the Allegheny County population,5 were more likely to utilize SVs (19% [490])compared with AVs (7.5% [174]) and IVs (11% [1343]). Hispanic/Latino patients had a disproportionally lower utilization of dermatologic care in all settings, comprising 1.4% (209) of all patients in our study compared with 2.3% of Allegheny County residents.5 White patients, who comprised 79.6% of Allegheny County residents, accounted for 81.1% (9928) of IVs, 67.4% (1737) of SVs, and 80.4% (1859) of AVs. There was no significant difference in the percentage of American Indian/Alaska Native and Native Hawaiian/Other Pacific Islander patients among visit types.
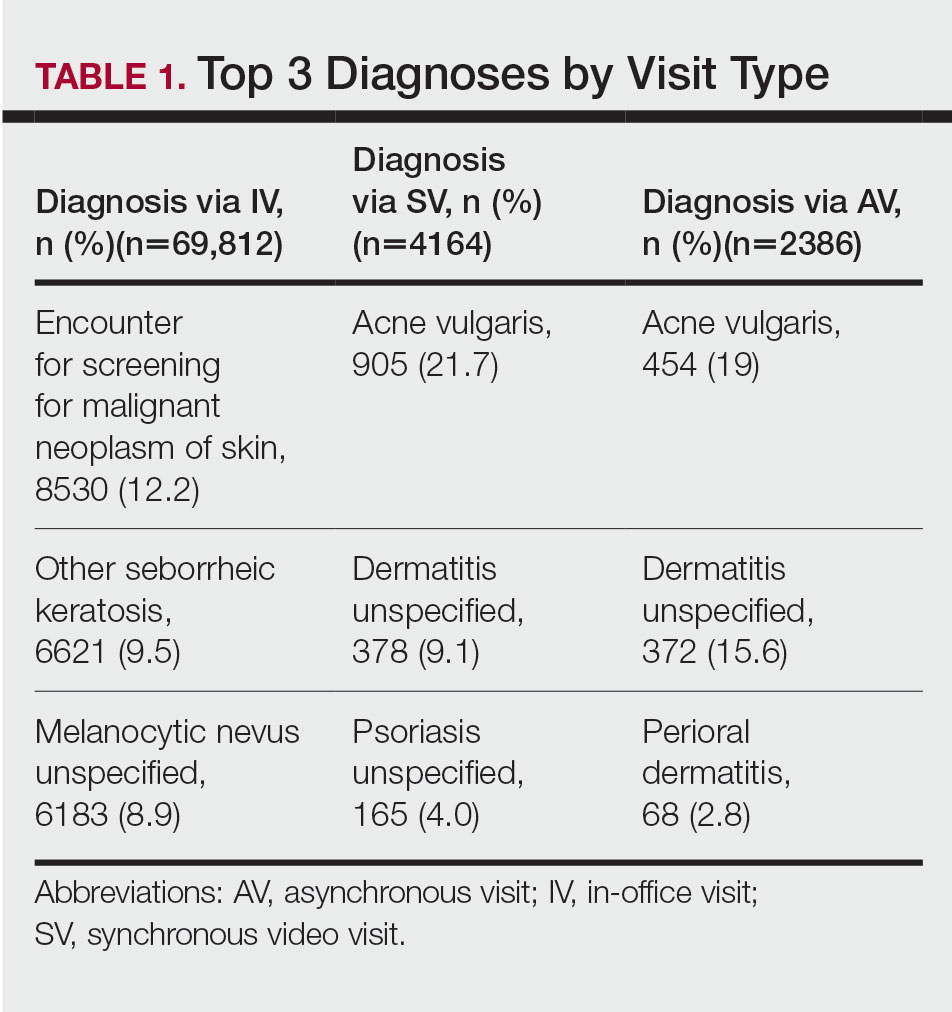
The 3 most common diagnoses for IVs were skin cancer screening, seborrheic keratosis, and melanocytic nevus (Table 1). Skin cancer screening was the most common diagnosis, accounting for 12.2% (8530) of 69,812 IVs. The 3 most common diagnoses for SVs were acne vulgaris, dermatitis, and psoriasis. The 3 most common diagnoses for AVs were acne vulgaris, dermatitis, and perioral dermatitis.
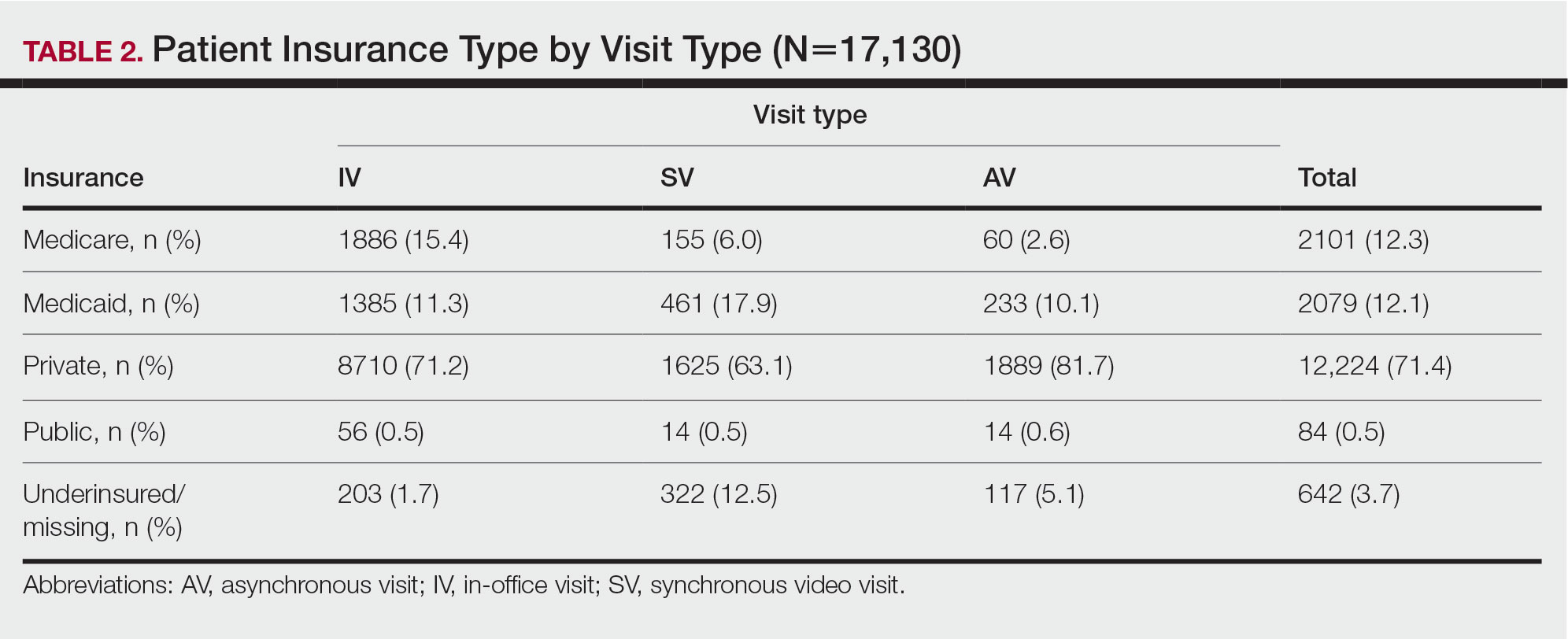
Private insurance was the most common insurance type among all patients (71.4% [12,224])(Table 2). A higher percentage of patients with Medicaid insurance (17.9% [461]) utilized SVs compared with AVs (10.1% [233]) and IVs (11.3% 1385]). Similarly, a higher percentage of patients with no insurance or no insurance listed were seen via SVs (12.5% [322]) compared with AVs (5.1% [117]) and IVs (1.7% [203]). Patients with Medicare insurance used IVs (15.4% [1886]) more than SVs (6.0% [155]) or AVs (2.6% [60]). There was no significant difference among visit type usage for patients with public insurance.
Comment
Teledermatology Benefits—In this retrospective review of medical records of patients who obtained dermatologic care after the implementation of SVs at our institution, we found a proportionally higher use of SVs among Black patients, patients with Medicaid, and patients who are underinsured. Benefits of teledermatology include decreases in patient transportation and associated costs, time away from work or home, and need for childcare.6 The SV format provides the additional advantage of direct live interaction and the development of a patient-physician or patient–physician assistant relationship. Although the prerequisite technology, internet, and broadband connectivity preclude use of teledermatology for many vulnerable patients,2 its convenience ultimately may reduce inequities in access.
Disparities in Dermatologic Care—Hispanic ethnicity and male sex are among described patient demographics associated with decreased rates of outpatient dermatologic care.7 We reported disparities in dermatologic care utilization across all visit types among Hispanic patients and males. Patients identifying as Hispanic/Latino composed only 1.4% (n=209) of our study population compared with 2.3% of Allegheny County residents.5 During our study period, most patients seen were female, accounting for 62.1% to 72.8% of visits, compared with 51.6% of Allegheny County residents.5 These disparities in dermatologic care use may have implications for increased skin-associated morbidity and provide impetus for dermatologists to increase engagement with these patient groups.
Characteristics of Patients Using Teledermatology—Patients using SVs and AVs were significantly younger (mean age [SD], 39.9 [16.9] years and 37.5 [14.3] years, respectively) compared with those using IVs (51.0 [18.8] years). This finding reflects known digital knowledge barriers among older patients.8,9 The synchronous communication format of SVs simulates the traditional visit style of IVs, which may be preferable for some patients. Continued patient education and advocacy for broadband access may increase teledermatology use among older patients and patients with limited technology resources.8
Teledermatology visits were used most frequently for acne and dermatitis, while IVs were used for skin cancer screenings and examination of concerning lesions. This usage pattern is consistent with a previously described consensus among dermatologists on the conditions most amenable to teledermatology evaluation.3
Medicaid reimbursement parity for SVs is in effect nationally until the end of the COVID-19 public health emergency declaration in the United States.10 As of February 2023, the public health emergency declaration has been renewed 12 times since January 2020, with the most recent renewal on January 11, 2023.11 As of January 2023, 21 states have enacted legislation providing permanent reimbursement parity for SV services. Six additional states have some payment parity in place, each with its own qualifying criteria, and 23 states have no payment parity.12 Only 25 Medicaid programs currently provide reimbursement for AV services.13
Study Limitations—Our study was limited by lack of data on patients who are multiracial and those who identify as nonbinary and transgender. Because of the low numbers of Hispanic patients associated with each race category and a high number of patients who did not report an ethnicity or race, race and ethnicity data were analyzed separately. For SVs, patients were instructed to upload photographs prior to their visit; however, the percentage of patients who uploaded photographs was not analyzed.
Conclusion
Expansion of teledermatology services, including SVs and AVs, patient outreach and education, advocacy for broadband access, and Medicaid payment parity, may improve dermatologic care access for medically marginalized groups. Teledermatology has the potential to serve as an effective health care option for patients who are racially minoritized, older, and underinsured. To further assess the effectiveness of teledermatology, we plan to analyze the number of SVs and AVs that were referred to IVs. Future studies also will investigate the impact of implementing patient education and patient-reported outcomes of teledermatology visits.
- Lee JJ, English JC. Teledermatology: a review and update. Am J Clin Dermatol. 2018;19:253-260.
- Bakhtiar M, Elbuluk N, Lipoff JB. The digital divide: how COVID-19’s telemedicine expansion could exacerbate disparities. J Am Acad Dermatol. 2020;83:E345-E346.
- Kennedy J, Arey S, Hopkins Z, et al. dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Centers for Disease Control and Prevention. Using telehealth to expand access to essential health services during the COVID-19 pandemic. Updated June 10, 2020. Accessed February 10, 2023. https://www.cdc.gov/coronavirus/2019-ncov/hcp/telehealth.html
- United States Census Bureau. QuickFacts: Allegheny County, Pennsylvania. Accessed August 12, 2021. https://www.census.gov/quickfacts/alleghenycountypennsylvania
- Moore HW. Teledermatology—access to specialized care via a different model. Dermatology Advisor. November 12, 2019. Accessed February 10, 2023. https://www.dermatologyadvisor.com/home/topics/practice-management/teledermatology-access-to-specialized-care-via-a-different-model/
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Nouri S, Khoong EC, Lyles CR, et al. Addressing equity in telemedicine for chronic disease management during the COVID-19 pandemic [published online May 4, 2020]. NEJM Catal Innov Care Deliv. doi:10.1056/CAT.20.0123
- Swenson K, Ghertner R. People in low-income households have less access to internet services—2019 update. Office of the Assistant Secretary for Planning and Evaluation; US Department of Health and Human Services. March 2021. Accessed February 10, 2023. https://aspe.hhs.gov/sites/default/files/private/pdf/263601/internet-access-among-low-income-2019.pdf
- Centers for Medicare and Medicaid Services. COVID-19 frequently asked questions (FAQs) on Medicare fee-for-service (FFS) billing. Updated August 16, 2022. Accessed February 10, 2023. https://www.cms.gov/files/document/03092020-covid-19-faqs-508.pdf
- US Department of Health and Human Services. Renewal of determination that a public health emergency exists. Updated February 9, 2023. Accessed February 20, 2023. https://aspr.hhs.gov/legal/PHE/Pages/COVID19-9Feb2023.aspx?
- Augenstein J, Smith JM. Executive summary: tracking telehealth changes state-by-state in response to COVID-19. Updated January 27, 2023. Accessed February 10, 2023. https://www.manatt.com/insights/newsletters/covid-19-update/executive-summary-tracking-telehealth-changes-stat
- Center for Connected Health Policy. Policy trend maps: store and forward Medicaid reimbursement. Accessed June 23, 2022. https://www.cchpca.org/policy-trends/
Teledermatology is an effective patient care model for the delivery of high-quality dermatologic care.1 Teledermatology can occur using synchronous, asynchronous, and hybrid models of care. In asynchronous visits (AVs), patients or health professionals submit photographs and information for dermatologists to review and provide treatment recommendations. With synchronous visits (SVs), patients have a visit with a dermatology health professional in real time via live video conferencing software. Hybrid models incorporate asynchronous strategies for patient intake forms and skin photograph submissions as well as synchronous methods for live video consultation in a single visit.1 However, remarkable inequities in internet access limit telemedicine usage among medically marginalized patient populations, including racialized, elderly, and low socioeconomic status groups.2
Synchronous visits, a relatively newer teledermatology format, allow for communication with dermatology professionals from the convenience of a patient’s selected location. The live interaction of SVs allows dermatology professionals to answer questions, provide treatment recommendations, and build therapeutic relationships with patients. Concerns for dermatologist reimbursement, malpractice/liability, and technological challenges stalled large-scale uptake of teledermatology platforms.3 The COVID-19 pandemic led to a drastic increase in teledermatology usage of approximately 587.2%, largely due to public safety measures and Medicaid reimbursement parity between SV and in-office visits (IVs).3,4
With the implementation of SVs as a patient care model, we investigated the demographics of patients who utilized SVs, AVs, or IVs, and we propose strategies to promote equity in dermatologic care access.
Methods
This study was approved by the University of Pittsburgh institutional review board (STUDY20110043). We performed a retrospective electronic medical record review of deidentified data from the University of Pittsburgh Medical Center, a tertiary care center in Allegheny County, Pennsylvania, with an established asynchronous teledermatology program. Hybrid SVs were integrated into the University of Pittsburgh Medical Center patient care visit options in March 2020. Patients were instructed to upload photographs of their skin conditions prior to SV appointments. The study included visits occurring between July and December 2020. Visit types included SVs, AVs, and IVs.
We analyzed the initial dermatology visits of 17,130 patients aged 17.5 years and older. Recorded data included diagnosis, age, sex, race, ethnicity, and insurance type for each visit type. Patients without a reported race (990 patients) or ethnicity (1712 patients) were excluded from analysis of race/ethnicity data. Patient zip codes were compared with the zip codes of Allegheny County municipalities as reported by the Allegheny County Elections Division.
Statistical Analysis—Descriptive statistics were calculated; frequency with percentage was used to report categorical variables, and the mean (SD) was used for normally distributed continuous variables. Univariate analysis was performed using the χ2 test for categorical variables. One-way analysis of variance was used to compare age among visit types. Statistical significance was defined as P<.05. IBM SPSS Statistics for Windows, Version 24 (IBM Corp) was used for all statistical analyses.
Results
In our study population, 81.2% (13,916) of patients were residents of Allegheny County, where 51.6% of residents are female and 81.4% are older than 18 years according to data from 2020.5 The racial and ethnic demographics of Allegheny County were 13.4% African American/Black, 0.2% American Indian/Alaska Native, 4.2% Asian, 2.3% Hispanic/Latino, and 79.6% White. The percentage of residents who identified as Native Hawaiian/Pacific Islander was reported to be greater than 0% but less than 0.5%.5
In our analysis, IVs were the most utilized visit type, accounting for 71.5% (12,240) of visits, followed by 15.0% (2577) for SVs and 13.5% (2313) for AVs. The mean age (SD) of IV patients was 51.0 (18.8) years compared with 39.9 (16.9) years for SV patients and 37.5 (14.3) years for AV patients (eTable). The majority of patients for all visits were female: 62.1% (7599) for IVs, 71.4% (1652) for AVs, and 72.8% (1877) for SVs. The largest racial or ethnic group for all visit types included White patients (83.8% [13,524] of all patients), followed by Black (12.4% [2007]), Hispanic/Latino (1.4% [209]), Asian (3.4% [555]), American Indian/Alaska Native (0.2% [35]), and Native Hawaiian/Other Pacific Islander patients (0.1% [19]).

Asian patients, who comprised 4.2% of Allegheny County residents,5 accounted for 2.7% (334) of IVs, 4.9% (113) of AVs, and 4.2% (108) of SVs. Black patients, who were reported as 13.4% of the Allegheny County population,5 were more likely to utilize SVs (19% [490])compared with AVs (7.5% [174]) and IVs (11% [1343]). Hispanic/Latino patients had a disproportionally lower utilization of dermatologic care in all settings, comprising 1.4% (209) of all patients in our study compared with 2.3% of Allegheny County residents.5 White patients, who comprised 79.6% of Allegheny County residents, accounted for 81.1% (9928) of IVs, 67.4% (1737) of SVs, and 80.4% (1859) of AVs. There was no significant difference in the percentage of American Indian/Alaska Native and Native Hawaiian/Other Pacific Islander patients among visit types.
The 3 most common diagnoses for IVs were skin cancer screening, seborrheic keratosis, and melanocytic nevus (Table 1). Skin cancer screening was the most common diagnosis, accounting for 12.2% (8530) of 69,812 IVs. The 3 most common diagnoses for SVs were acne vulgaris, dermatitis, and psoriasis. The 3 most common diagnoses for AVs were acne vulgaris, dermatitis, and perioral dermatitis.

Private insurance was the most common insurance type among all patients (71.4% [12,224])(Table 2). A higher percentage of patients with Medicaid insurance (17.9% [461]) utilized SVs compared with AVs (10.1% [233]) and IVs (11.3% 1385]). Similarly, a higher percentage of patients with no insurance or no insurance listed were seen via SVs (12.5% [322]) compared with AVs (5.1% [117]) and IVs (1.7% [203]). Patients with Medicare insurance used IVs (15.4% [1886]) more than SVs (6.0% [155]) or AVs (2.6% [60]). There was no significant difference among visit type usage for patients with public insurance.

Comment
Teledermatology Benefits—In this retrospective review of medical records of patients who obtained dermatologic care after the implementation of SVs at our institution, we found a proportionally higher use of SVs among Black patients, patients with Medicaid, and patients who are underinsured. Benefits of teledermatology include decreases in patient transportation and associated costs, time away from work or home, and need for childcare.6 The SV format provides the additional advantage of direct live interaction and the development of a patient-physician or patient–physician assistant relationship. Although the prerequisite technology, internet, and broadband connectivity preclude use of teledermatology for many vulnerable patients,2 its convenience ultimately may reduce inequities in access.
Disparities in Dermatologic Care—Hispanic ethnicity and male sex are among described patient demographics associated with decreased rates of outpatient dermatologic care.7 We reported disparities in dermatologic care utilization across all visit types among Hispanic patients and males. Patients identifying as Hispanic/Latino composed only 1.4% (n=209) of our study population compared with 2.3% of Allegheny County residents.5 During our study period, most patients seen were female, accounting for 62.1% to 72.8% of visits, compared with 51.6% of Allegheny County residents.5 These disparities in dermatologic care use may have implications for increased skin-associated morbidity and provide impetus for dermatologists to increase engagement with these patient groups.
Characteristics of Patients Using Teledermatology—Patients using SVs and AVs were significantly younger (mean age [SD], 39.9 [16.9] years and 37.5 [14.3] years, respectively) compared with those using IVs (51.0 [18.8] years). This finding reflects known digital knowledge barriers among older patients.8,9 The synchronous communication format of SVs simulates the traditional visit style of IVs, which may be preferable for some patients. Continued patient education and advocacy for broadband access may increase teledermatology use among older patients and patients with limited technology resources.8
Teledermatology visits were used most frequently for acne and dermatitis, while IVs were used for skin cancer screenings and examination of concerning lesions. This usage pattern is consistent with a previously described consensus among dermatologists on the conditions most amenable to teledermatology evaluation.3
Medicaid reimbursement parity for SVs is in effect nationally until the end of the COVID-19 public health emergency declaration in the United States.10 As of February 2023, the public health emergency declaration has been renewed 12 times since January 2020, with the most recent renewal on January 11, 2023.11 As of January 2023, 21 states have enacted legislation providing permanent reimbursement parity for SV services. Six additional states have some payment parity in place, each with its own qualifying criteria, and 23 states have no payment parity.12 Only 25 Medicaid programs currently provide reimbursement for AV services.13
Study Limitations—Our study was limited by lack of data on patients who are multiracial and those who identify as nonbinary and transgender. Because of the low numbers of Hispanic patients associated with each race category and a high number of patients who did not report an ethnicity or race, race and ethnicity data were analyzed separately. For SVs, patients were instructed to upload photographs prior to their visit; however, the percentage of patients who uploaded photographs was not analyzed.
Conclusion
Expansion of teledermatology services, including SVs and AVs, patient outreach and education, advocacy for broadband access, and Medicaid payment parity, may improve dermatologic care access for medically marginalized groups. Teledermatology has the potential to serve as an effective health care option for patients who are racially minoritized, older, and underinsured. To further assess the effectiveness of teledermatology, we plan to analyze the number of SVs and AVs that were referred to IVs. Future studies also will investigate the impact of implementing patient education and patient-reported outcomes of teledermatology visits.
Teledermatology is an effective patient care model for the delivery of high-quality dermatologic care.1 Teledermatology can occur using synchronous, asynchronous, and hybrid models of care. In asynchronous visits (AVs), patients or health professionals submit photographs and information for dermatologists to review and provide treatment recommendations. With synchronous visits (SVs), patients have a visit with a dermatology health professional in real time via live video conferencing software. Hybrid models incorporate asynchronous strategies for patient intake forms and skin photograph submissions as well as synchronous methods for live video consultation in a single visit.1 However, remarkable inequities in internet access limit telemedicine usage among medically marginalized patient populations, including racialized, elderly, and low socioeconomic status groups.2
Synchronous visits, a relatively newer teledermatology format, allow for communication with dermatology professionals from the convenience of a patient’s selected location. The live interaction of SVs allows dermatology professionals to answer questions, provide treatment recommendations, and build therapeutic relationships with patients. Concerns for dermatologist reimbursement, malpractice/liability, and technological challenges stalled large-scale uptake of teledermatology platforms.3 The COVID-19 pandemic led to a drastic increase in teledermatology usage of approximately 587.2%, largely due to public safety measures and Medicaid reimbursement parity between SV and in-office visits (IVs).3,4
With the implementation of SVs as a patient care model, we investigated the demographics of patients who utilized SVs, AVs, or IVs, and we propose strategies to promote equity in dermatologic care access.
Methods
This study was approved by the University of Pittsburgh institutional review board (STUDY20110043). We performed a retrospective electronic medical record review of deidentified data from the University of Pittsburgh Medical Center, a tertiary care center in Allegheny County, Pennsylvania, with an established asynchronous teledermatology program. Hybrid SVs were integrated into the University of Pittsburgh Medical Center patient care visit options in March 2020. Patients were instructed to upload photographs of their skin conditions prior to SV appointments. The study included visits occurring between July and December 2020. Visit types included SVs, AVs, and IVs.
We analyzed the initial dermatology visits of 17,130 patients aged 17.5 years and older. Recorded data included diagnosis, age, sex, race, ethnicity, and insurance type for each visit type. Patients without a reported race (990 patients) or ethnicity (1712 patients) were excluded from analysis of race/ethnicity data. Patient zip codes were compared with the zip codes of Allegheny County municipalities as reported by the Allegheny County Elections Division.
Statistical Analysis—Descriptive statistics were calculated; frequency with percentage was used to report categorical variables, and the mean (SD) was used for normally distributed continuous variables. Univariate analysis was performed using the χ2 test for categorical variables. One-way analysis of variance was used to compare age among visit types. Statistical significance was defined as P<.05. IBM SPSS Statistics for Windows, Version 24 (IBM Corp) was used for all statistical analyses.
Results
In our study population, 81.2% (13,916) of patients were residents of Allegheny County, where 51.6% of residents are female and 81.4% are older than 18 years according to data from 2020.5 The racial and ethnic demographics of Allegheny County were 13.4% African American/Black, 0.2% American Indian/Alaska Native, 4.2% Asian, 2.3% Hispanic/Latino, and 79.6% White. The percentage of residents who identified as Native Hawaiian/Pacific Islander was reported to be greater than 0% but less than 0.5%.5
In our analysis, IVs were the most utilized visit type, accounting for 71.5% (12,240) of visits, followed by 15.0% (2577) for SVs and 13.5% (2313) for AVs. The mean age (SD) of IV patients was 51.0 (18.8) years compared with 39.9 (16.9) years for SV patients and 37.5 (14.3) years for AV patients (eTable). The majority of patients for all visits were female: 62.1% (7599) for IVs, 71.4% (1652) for AVs, and 72.8% (1877) for SVs. The largest racial or ethnic group for all visit types included White patients (83.8% [13,524] of all patients), followed by Black (12.4% [2007]), Hispanic/Latino (1.4% [209]), Asian (3.4% [555]), American Indian/Alaska Native (0.2% [35]), and Native Hawaiian/Other Pacific Islander patients (0.1% [19]).

Asian patients, who comprised 4.2% of Allegheny County residents,5 accounted for 2.7% (334) of IVs, 4.9% (113) of AVs, and 4.2% (108) of SVs. Black patients, who were reported as 13.4% of the Allegheny County population,5 were more likely to utilize SVs (19% [490])compared with AVs (7.5% [174]) and IVs (11% [1343]). Hispanic/Latino patients had a disproportionally lower utilization of dermatologic care in all settings, comprising 1.4% (209) of all patients in our study compared with 2.3% of Allegheny County residents.5 White patients, who comprised 79.6% of Allegheny County residents, accounted for 81.1% (9928) of IVs, 67.4% (1737) of SVs, and 80.4% (1859) of AVs. There was no significant difference in the percentage of American Indian/Alaska Native and Native Hawaiian/Other Pacific Islander patients among visit types.
The 3 most common diagnoses for IVs were skin cancer screening, seborrheic keratosis, and melanocytic nevus (Table 1). Skin cancer screening was the most common diagnosis, accounting for 12.2% (8530) of 69,812 IVs. The 3 most common diagnoses for SVs were acne vulgaris, dermatitis, and psoriasis. The 3 most common diagnoses for AVs were acne vulgaris, dermatitis, and perioral dermatitis.

Private insurance was the most common insurance type among all patients (71.4% [12,224])(Table 2). A higher percentage of patients with Medicaid insurance (17.9% [461]) utilized SVs compared with AVs (10.1% [233]) and IVs (11.3% 1385]). Similarly, a higher percentage of patients with no insurance or no insurance listed were seen via SVs (12.5% [322]) compared with AVs (5.1% [117]) and IVs (1.7% [203]). Patients with Medicare insurance used IVs (15.4% [1886]) more than SVs (6.0% [155]) or AVs (2.6% [60]). There was no significant difference among visit type usage for patients with public insurance.

Comment
Teledermatology Benefits—In this retrospective review of medical records of patients who obtained dermatologic care after the implementation of SVs at our institution, we found a proportionally higher use of SVs among Black patients, patients with Medicaid, and patients who are underinsured. Benefits of teledermatology include decreases in patient transportation and associated costs, time away from work or home, and need for childcare.6 The SV format provides the additional advantage of direct live interaction and the development of a patient-physician or patient–physician assistant relationship. Although the prerequisite technology, internet, and broadband connectivity preclude use of teledermatology for many vulnerable patients,2 its convenience ultimately may reduce inequities in access.
Disparities in Dermatologic Care—Hispanic ethnicity and male sex are among described patient demographics associated with decreased rates of outpatient dermatologic care.7 We reported disparities in dermatologic care utilization across all visit types among Hispanic patients and males. Patients identifying as Hispanic/Latino composed only 1.4% (n=209) of our study population compared with 2.3% of Allegheny County residents.5 During our study period, most patients seen were female, accounting for 62.1% to 72.8% of visits, compared with 51.6% of Allegheny County residents.5 These disparities in dermatologic care use may have implications for increased skin-associated morbidity and provide impetus for dermatologists to increase engagement with these patient groups.
Characteristics of Patients Using Teledermatology—Patients using SVs and AVs were significantly younger (mean age [SD], 39.9 [16.9] years and 37.5 [14.3] years, respectively) compared with those using IVs (51.0 [18.8] years). This finding reflects known digital knowledge barriers among older patients.8,9 The synchronous communication format of SVs simulates the traditional visit style of IVs, which may be preferable for some patients. Continued patient education and advocacy for broadband access may increase teledermatology use among older patients and patients with limited technology resources.8
Teledermatology visits were used most frequently for acne and dermatitis, while IVs were used for skin cancer screenings and examination of concerning lesions. This usage pattern is consistent with a previously described consensus among dermatologists on the conditions most amenable to teledermatology evaluation.3
Medicaid reimbursement parity for SVs is in effect nationally until the end of the COVID-19 public health emergency declaration in the United States.10 As of February 2023, the public health emergency declaration has been renewed 12 times since January 2020, with the most recent renewal on January 11, 2023.11 As of January 2023, 21 states have enacted legislation providing permanent reimbursement parity for SV services. Six additional states have some payment parity in place, each with its own qualifying criteria, and 23 states have no payment parity.12 Only 25 Medicaid programs currently provide reimbursement for AV services.13
Study Limitations—Our study was limited by lack of data on patients who are multiracial and those who identify as nonbinary and transgender. Because of the low numbers of Hispanic patients associated with each race category and a high number of patients who did not report an ethnicity or race, race and ethnicity data were analyzed separately. For SVs, patients were instructed to upload photographs prior to their visit; however, the percentage of patients who uploaded photographs was not analyzed.
Conclusion
Expansion of teledermatology services, including SVs and AVs, patient outreach and education, advocacy for broadband access, and Medicaid payment parity, may improve dermatologic care access for medically marginalized groups. Teledermatology has the potential to serve as an effective health care option for patients who are racially minoritized, older, and underinsured. To further assess the effectiveness of teledermatology, we plan to analyze the number of SVs and AVs that were referred to IVs. Future studies also will investigate the impact of implementing patient education and patient-reported outcomes of teledermatology visits.
- Lee JJ, English JC. Teledermatology: a review and update. Am J Clin Dermatol. 2018;19:253-260.
- Bakhtiar M, Elbuluk N, Lipoff JB. The digital divide: how COVID-19’s telemedicine expansion could exacerbate disparities. J Am Acad Dermatol. 2020;83:E345-E346.
- Kennedy J, Arey S, Hopkins Z, et al. dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Centers for Disease Control and Prevention. Using telehealth to expand access to essential health services during the COVID-19 pandemic. Updated June 10, 2020. Accessed February 10, 2023. https://www.cdc.gov/coronavirus/2019-ncov/hcp/telehealth.html
- United States Census Bureau. QuickFacts: Allegheny County, Pennsylvania. Accessed August 12, 2021. https://www.census.gov/quickfacts/alleghenycountypennsylvania
- Moore HW. Teledermatology—access to specialized care via a different model. Dermatology Advisor. November 12, 2019. Accessed February 10, 2023. https://www.dermatologyadvisor.com/home/topics/practice-management/teledermatology-access-to-specialized-care-via-a-different-model/
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Nouri S, Khoong EC, Lyles CR, et al. Addressing equity in telemedicine for chronic disease management during the COVID-19 pandemic [published online May 4, 2020]. NEJM Catal Innov Care Deliv. doi:10.1056/CAT.20.0123
- Swenson K, Ghertner R. People in low-income households have less access to internet services—2019 update. Office of the Assistant Secretary for Planning and Evaluation; US Department of Health and Human Services. March 2021. Accessed February 10, 2023. https://aspe.hhs.gov/sites/default/files/private/pdf/263601/internet-access-among-low-income-2019.pdf
- Centers for Medicare and Medicaid Services. COVID-19 frequently asked questions (FAQs) on Medicare fee-for-service (FFS) billing. Updated August 16, 2022. Accessed February 10, 2023. https://www.cms.gov/files/document/03092020-covid-19-faqs-508.pdf
- US Department of Health and Human Services. Renewal of determination that a public health emergency exists. Updated February 9, 2023. Accessed February 20, 2023. https://aspr.hhs.gov/legal/PHE/Pages/COVID19-9Feb2023.aspx?
- Augenstein J, Smith JM. Executive summary: tracking telehealth changes state-by-state in response to COVID-19. Updated January 27, 2023. Accessed February 10, 2023. https://www.manatt.com/insights/newsletters/covid-19-update/executive-summary-tracking-telehealth-changes-stat
- Center for Connected Health Policy. Policy trend maps: store and forward Medicaid reimbursement. Accessed June 23, 2022. https://www.cchpca.org/policy-trends/
- Lee JJ, English JC. Teledermatology: a review and update. Am J Clin Dermatol. 2018;19:253-260.
- Bakhtiar M, Elbuluk N, Lipoff JB. The digital divide: how COVID-19’s telemedicine expansion could exacerbate disparities. J Am Acad Dermatol. 2020;83:E345-E346.
- Kennedy J, Arey S, Hopkins Z, et al. dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Centers for Disease Control and Prevention. Using telehealth to expand access to essential health services during the COVID-19 pandemic. Updated June 10, 2020. Accessed February 10, 2023. https://www.cdc.gov/coronavirus/2019-ncov/hcp/telehealth.html
- United States Census Bureau. QuickFacts: Allegheny County, Pennsylvania. Accessed August 12, 2021. https://www.census.gov/quickfacts/alleghenycountypennsylvania
- Moore HW. Teledermatology—access to specialized care via a different model. Dermatology Advisor. November 12, 2019. Accessed February 10, 2023. https://www.dermatologyadvisor.com/home/topics/practice-management/teledermatology-access-to-specialized-care-via-a-different-model/
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Nouri S, Khoong EC, Lyles CR, et al. Addressing equity in telemedicine for chronic disease management during the COVID-19 pandemic [published online May 4, 2020]. NEJM Catal Innov Care Deliv. doi:10.1056/CAT.20.0123
- Swenson K, Ghertner R. People in low-income households have less access to internet services—2019 update. Office of the Assistant Secretary for Planning and Evaluation; US Department of Health and Human Services. March 2021. Accessed February 10, 2023. https://aspe.hhs.gov/sites/default/files/private/pdf/263601/internet-access-among-low-income-2019.pdf
- Centers for Medicare and Medicaid Services. COVID-19 frequently asked questions (FAQs) on Medicare fee-for-service (FFS) billing. Updated August 16, 2022. Accessed February 10, 2023. https://www.cms.gov/files/document/03092020-covid-19-faqs-508.pdf
- US Department of Health and Human Services. Renewal of determination that a public health emergency exists. Updated February 9, 2023. Accessed February 20, 2023. https://aspr.hhs.gov/legal/PHE/Pages/COVID19-9Feb2023.aspx?
- Augenstein J, Smith JM. Executive summary: tracking telehealth changes state-by-state in response to COVID-19. Updated January 27, 2023. Accessed February 10, 2023. https://www.manatt.com/insights/newsletters/covid-19-update/executive-summary-tracking-telehealth-changes-stat
- Center for Connected Health Policy. Policy trend maps: store and forward Medicaid reimbursement. Accessed June 23, 2022. https://www.cchpca.org/policy-trends/
Practice Points
- There is increased use of synchronous video visits (SVs) among Black patients, patients with Medicaid, and patients who are underinsured.
- Synchronous video visits may increase dermatologic care utilization for medically marginalized groups.
- Efforts are needed to increase engagement with dermatologic care for Hispanic and male patients.
Muscle-Related Adverse Events Associated With PCSK9 Inhibitors in a Veteran Population
HMG-CoA reductase inhibitors (statins) have been shown to effectively reduce low-density lipoprotein cholesterol (LDL-C) as well as morbidity and mortality in patients who have either atherosclerotic cardiovascular disease (ASCVD) or risk factors for ASCVD.1-12 However, research shows that up to 20% of patients are unable to tolerate statin therapy due to muscle-related adverse events (AEs).13 This presents a substantial clinical challenge, as current management strategies for patients with statin-associated muscle symptoms, such as intermittent administration of statins and ezetimibe, seldom achieve the > 50% LDL-C reduction recommended by the 2018 American Heart Association/American College of Cardiology Clinical Practice Guidelines.14 Additionally, statin-intolerant patients who have antihyperlipidemic medication lowered or discontinued are at an increased risk of future cardiovascular events.15 Observational data also show that about 70% of adult patients (primarily with genetic lipid disorders such as heterozygous familial hypercholesterolemia) do not achieve an LDL-C level < 100 mg/dL despite treatment with maximum doses of statins with or without ezetimibe.16,17
PCSK9 inhibitors (PCSK9i) have robust efficacy data to support use in patients who do not meet their LDL-C goal despite maximally tolerated lipid therapy.14 However, long-term safety data for PCSK9i are not as robust as its efficacy data. Specifically, safety data relating to muscle-related AEs, which are the most widely recognized AE associated with statins, have only been reported in a few clinical trials with varying incidence rates, levels of significance, and relatively small study populations. Furthermore, the real-world prevalence of muscle-related PCSK9i AEs is unknown. Clinical guidance for management strategies for muscle-related AEs associated with PCSK9i is largely lacking. For this study, muscle-related AEs were defined as any new or unusual muscle soreness, weakness, cramping, aches, and stiffness that persists, is generally bilateral, and typically affects the large muscles. It is important to note, that muscle-related AEs associated with statins, ezetimibe, and PCSK9i can be attributed to the nocebo effect.
According to the prescribing information for alirocumab and evolocumab, myalgia, muscle spasms, and musculoskeletal pain each occurred in < 5% of the study populations.18,19 From these data, muscle-related PCSK9i AEs are thought to be relatively rare, based on the ODYSSEY-OUTCOME and FOURIER trials, which did not enroll statin-intolerant patients.20,21 However, currently available safety data from 3 small, randomized clinical trials specifically in statin-intolerant patients taking a PCSK9i suggest that muscle-related AEs occur at a rate of 12.2% to 32.5% and discontinuation rates varied from 0% to 15.9%.22-25 As the incidence rates of muscle-related AEs in the prescribing information and clinical trials varied widely, this study will provide quantitative data on the percentage of patients that developed muscle-related PCSK9i AEs in a veteran population to help shed light on a topic that is not well studied.
Methods
This was a single-center, retrospective chart review of patients prescribed a PCSK9i between December 1, 2017, and September 1, 2021, and were managed in a pharmacy-led patient aligned care team (PACT) clinic at the Wilkes-Barre US Department of Veterans Affairs (VA) Medical Center (WBVAMC) in Pennsylvania. This study was approved by the Coatesville VA Medical Center Institutional Review Board, which oversees research conducted at WBVAMC. Veterans aged ≥ 18 years were included in the study. Patients were excluded if they had a history of serious hypersensitivity reaction to a PCSK9i or rhabdomyolysis or did not meet the VA criteria for use.26
The primary outcome was the percentage of patients who developed a muscle-related AE while on a PCSK9i in a PACT clinic. Data were further analyzed based on patients who (1) tolerated a full PCSK9i dose; (2) tolerated alternative PCSK9i following initial intolerance; (3) required a PCSK9i dose reduction, or (4) discontinued PCSK9i. A secondary outcome was the percentage of statin- and/or ezetimibe-intolerant patients in these 4 groups. Another secondary outcome was the management strategies taken for patients who were on a reduced (monthly) dose of PCSK9i who did not reach their LDL-C goal. Management strategies that were assessed included restarting weekly statin, restarting weekly ezetimibe, increasing the dose of the same PCSK9i administered monthly, and switching to an alternative PCSK9i.
Data were collected using the VA Computerized Patient Record System (CPRS) and stored in a secure, locked spreadsheet. Baseline patient demographic characteristics collected included age (at PCSK9i start); sex; race; and PCSK9i name, dose, and frequency. We recorded when a patient switched PCSK9i, whether or not it was due to a muscle-related AE, and the name of the original PCSK9i. Also collected were lipid therapy intolerances prior to PCSK9i initiation (ie, intolerance to statin, ezetimibe, or both).
Patients were considered statin intolerant due to a muscle-related AE in accordance with the VA PCSK9i Criteria for Use, which requires trial of at least 3 statins, one of which was trialed at the lowest dosage approved by the US Food and Drug Administration (FDA) and resulted in intolerable skeletal muscle AEs that worsened during treatment and resolved when the statin was stopped. For our study purposes, patients taking alternative day dosing of statins due to muscle-related AEs (ie, 2- or 3-times weekly dosing) were not considered statin intolerant; however, patients taking once-weekly statin dosing were considered statin intolerant. Patients were considered ezetimibe intolerant due to a muscle-related AE if the intolerance was due to skeletal muscle concerns that worsened during treatment and resolved when ezetimibe was stopped. Patients were considered PCSK9i intolerant due to a muscle-related AE if the intolerance was due to skeletal muscle concerns that worsened during treatment and resolved when the PCSK9i was stopped. Patients with non–muscle-related intolerances to statins, ezetimibe, and PCSK9i were not considered statin, ezetimibe, and PCSK9i intolerant.
Alirocumab was initiated at 75 mg subcutaneous (SQ) once every 2 weeks or evolocumab 140 mg SQ once every 2 weeks in our study. The protocol allowed for a dose reduction of alirocumab 75 mg SQ once monthly if a patient experienced AEs, but this dose reduction strategy was not used for any patients on evolocumab in this study. Of note, alirocumab 75 mg SQ once monthly is not an FDA-approved dosing strategy. However, it is similar in concept to the alternative statin dosing (ie, alternate day dosing, once-weekly dosing) and may avoid the need to discontinue PCSK9i therapy altogether.
A review of the CPRS also documented whether a muscle-related AE occurred while the patient was on a PCSK9i (if yes, the specific AE was recorded), the result of PCSK9i therapy (tolerated full dose, required a dose reduction, switched medication, or discontinued), and management strategies taken for patients who did not meet their LDL-C goal while on a reduced (monthly) PCSK9i dose. Prior lipid therapy intolerances, PCSK9i-related AEs, results of PCSK9i therapy, and management strategies for patients who did not meet LDL-C goal while on a reduced PCSK9i dose were obtained by reviewing the PACT pharmacist’s clinic notes and assessment, along with clinic notes and medication history listed within the CPRS.
Statistical Analysis
Descriptive statistics were used for the demographic characteristics of study patients. The primary outcome was calculated as a binary measure (yes/no) of whether the patient developed a muscle-related AE while on a PCSK9i. The secondary outcome of statin, ezetimibe, or statin and ezetimibe intolerances in subgroups also was calculated as a binary measure.
Results
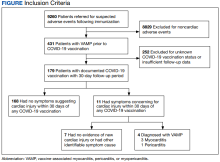
For the study, 156 charts were reviewed and 137 patients were included (Figure).
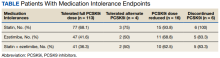
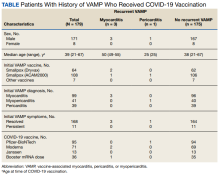
For the secondary results, 4 patients (2.9%) tolerated an alternate PCSK9i (evolocumab 140 mg SQ every 2 weeks) after initial intolerance to PCSK9i, 16 (11.7%) required a dose reduction, and 6 (4.4%) discontinued PCSK9i due to a muscle-related AE.
Statin intolerance was most common in all groups, followed by ezetimibe intolerance, and intolerance to statins + ezetimibe. Of the 113 patients who tolerated a full dose of PCSK9i, 77 (68.1%) had intolerance to statin, 47 (41.6%) to ezetimibe, and 41 (36.3%) to both statins and ezetimibe. Of the 6 patients who discontinued PCSK9i, all had intolerance to statins, 5 (83.3%) to ezetimibe, and 5 (83.3%) to statins and ezetimibe.
For patients who were on a reduced (monthly) dose of a PCSK9i who did not reach their LDL-C goal, we found that 16 patients (11.7%) required a PCSK9i dose reduction following muscle-related AEs. Of the patients who had their dose of PCSK9i reduced to monthly dosing, 5 (31%) met their LDL-C goal. For the 11 patients who did not meet their LDL-C goal, different management strategies were taken. Lifestyle modifications were made in 6 patients (54%), the monthly PCSK9i dose was increased to alirocumab 150 mg SQ monthly in 4 patients (36%), and 1 patient (9.1%) was switched to an alternative PCSK9i. There were no identified muscle-related AEs recorded in patients whose dose was increased to alirocumab 150 mg SQ monthly.
Discussion
This retrospective study found 17.5% of patients experienced muscle-related PCSK9i AEs. These occurred at a higher rate than reported in the prescribing information (< 5%) and were similar to the incidence rates reported in the GAUSS-2, GAUSS-3, and ODYSSEY-ALTERNATIVE clinical trials (12.0%-32.5%), which is what we hypothesized.18,19,22-25 It is important to note that the incidence rates of muscle-related AEs reported in the prescribing information for alirocumab and evolocumab were based on trials that did not include statin- and/or ezetimibe-intolerant patients; whereas many patients in our study and patients in the clinical trials were statin and/or ezetimibe intolerant.
Additionally, a new study by Donald and colleagues found an incidence rate of 32% to 36% for muscle-related PCSK9i AEs.27 Collectively, the data from clinical trials and our study indicate that patients with prior intolerances to statin and/or ezetimibe appear to have a higher likelihood of developing a muscle-related PCSK9i intolerance. In our study, 23 of 24 patients who developed a muscle-related PCSK9i AE had a prior history of statin and/or ezetimibe intolerances. This should alert clinicians prescribing PCSK9i in patients with a history of statin and/or ezetimibe intolerance to counsel their patients on the possibility of muscle-related PCSK9i AEs and management strategies. However, it is important to note that there was a substantial number of patients in our study who were statin and/or ezetimibe intolerant due to a prior muscle-related AE who tolerated the full dose of PCSK9i.
To our knowledge, this was the first trial to evaluate muscle-related PCSK9i AEs in a veteran population. Additionally, our study appears to be the first to use 2 PCSK9i dosing strategies that are not FDA approved: Dose reduction for patients who experienced a muscle-related AE on alirocumab 75 mg SQ every 2 weeks and dose escalation for patients who did not meet their LDL-C goal on alirocumab 75 mg SQ monthly following an initial intolerance to 2-week dosing. The dose-reduction strategy allowed patients who experienced a muscle-related AE to alirocumab 75 mg to reduce administration from every 2 weeks to monthly.
This strategy was only performed with alirocumab, the preferred PCSK9i at WBVAMC, but the same dose-reduction strategy can theoretically be used with evolocumab as well. Reduced monthly dosing of alirocumab allowed patients with a prior intolerance to remain on a lower dosage without discontinuation. This is important because as noted by Myers and colleagues, individuals without access to PCSK9i were found to have a significantly higher incidence ratio of cardiovascular events compared with those taking PCSK9i.15 Also of note, > 30% of patients on the reduced monthly dose of alirocumab still met their LDL-C goal. Therefore, using this dose-reduction strategy (instead of patients discontinuing therapy altogether due to a muscle-related intolerance) can lessen the risk of major adverse cardiovascular events (MACE) as well as mitigate muscle-related AEs that occurred while on 2-week PCSK9i dosing regimens. While we acknowledge that this reduced monthly dose of either alirocumab or evolocumab is not FDA approved, it is similar to alternative statin dosing that also is not FDA approved but may minimize the need to discontinue PCSK9i therapy. It would be beneficial if these dosing strategies were investigated by future research.
The dose-escalation strategy for patients who did not meet their LDL-C goal while on the reduced, monthly dose of alirocumab also was unique. Alirocumab was increased from 75 mg SQ once monthly to 150 mg SQ once monthly. Interestingly, we found that through the end of the chart review period, all patients tolerated the increase well, despite having an initial muscle-related AE to alirocumab 75 mg every 2 weeks, which is the same total monthly dosage. This approach is similar to that of once-weekly statin dosing or a drug holiday and may be explained by the long half-life of PCSK9i. Regardless of the mechanism, this finding suggests that an increased monthly dose of PCSK9i is a potential alternative for patients who cannot tolerate the FDA-approved dose. However, the ability for patients to achieve goal LDL-C on the monthly dosage requires future study.
In our study, only 6 patients (4.4%) discontinued PCSK9i therapy. This low discontinuation rate is largely attributable to our unique study design, which allowed for a dose reduction in patients who experienced muscle-related AEs. The earlier ODYSSEY-ALTERNATIVE trial evaluated the safety and efficacy of alirocumab compared with ezetimibe in confirmed statin-intolerant subjects after 24 weeks. This trial did not use a dose-reduction strategy and found 15.9% of patients discontinued alirocumab due to a muscle-related AE.24 This is notably higher than our discontinuation rate of 4.4%. If patients with a muscle-related AE discontinued PCKS9i instead of reducing the dose, they would likely return to their baseline LDL-C, which would increase the risk of MACE.
In general, myalgias due to antihyperlipidemic medications are not completely understood. One possible mechanism for statin-induced myalgias is the depletion of ubiquinone. However, this theory cannot explain muscle-related AEs associated with PCSK9i or ezetimibe, which have not been shown to deplete ubiquinone. We also found that the onset of muscle-related AEs associated with PCSK9i tends to appear later in therapy than what we know about statin therapy. Our study showed that the onset of a muscle-related PCSK9i AEs occurred a mean (SD) 8 (5.3) months after initiation (range, 1-19). Statin muscle-related AEs typically occur within the initial 4 to 8 weeks of treatment, although they can occur at any time.28
Limitations
The results of this study should be considered with the following limitations. First, this was a retrospective chart review performed over a prespecified period. Any muscle-related AEs or LDL-C lowering effects from PCSK9i that occurred outside the review period were not captured. Our study was small and only included 137 patients, though it was similar in size to the GAUSS-2, GAUSS-3, and ODYSSEY-ALTERNATIVE trials.22-24 Additionally, the study was primarily composed of White men and may not be representative of other populations. Some muscle-related PCSK9i AEs may be attributed to the nocebo. Last, our study did not capture patients on a PCSK9i who were not followed in the PACT clinic.
Conclusions
We found that muscle-related PCSK9i AEs occurred at a similar rate as those reported in previous clinical trials and exceeded the incidence rate reported in the prescribing information for alirocumab and evolocumab. It appears that patients who have a prior muscle-related intolerance to a statin and/or ezetimibe had a higher likelihood of developing a muscle-related PCSK9i AE. In our study, only 1 patient developed a muscle-related PCSK9i AE who did not have a prior history of muscle-related intolerance to either a statin or ezetimibe. However, in our study, a substantial percentage of patients with statin and/or ezetimibe intolerances tolerated the full PCSK9i dose well, proving that PCSK9i are still a reasonable alternative for patients with prior intolerances to statins and/or ezetimibe.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the US Department of Veterans Affairs Medical Center, Wilkes-Barre, Pennsylvania.
1. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344(8934):1383-1389.
2. Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335(14):1001-1009. doi:10.1056/NEJM199610033351401
3. Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339(19):1349-1357. doi:10.1056/NEJM199811053391902.
4. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7-22. doi:10.1016/S0140-6736(02)09327-3
5. Koren MJ, Hunninghake DB; ALLIANCE Investigators. Clinical outcomes in managed-care patients with coronary heart disease treated aggressively in lipid-lowering disease management clinics: the alliance study. J Am Coll Cardiol. 2004;44(9):1772-1779. doi:10.1016/j.jacc.2004.07.053
6. Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279(20):1615-1622. doi:10.1001/jama.279.20.1615
7. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). JAMA. 2002;288(23):2998-3007. doi:10.1001/jama.288.23.2998
8. Sever PS, Dahlöf B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361(9364):1149-1158. doi:10.1016/S0140-6736(03)12948-0
9. Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195-2207. doi:10.1056/NEJMoa0807646
10. Nakamura H, Arakawa K, Itakura H, et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet. 2006;368(9542):1155-1163. doi:10.1016/S0140-6736(06)69472-5
11. Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360(9346):1623-1630. doi:10.1016/s0140-6736(02)11600-x
12. Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333(20):1301-1307. doi:10.1056/NEJM199511163332001

13. Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36(17):1012-1022. doi:10.1093/eurheartj/ehv043
14. Grundy SM, Stone NJ, Bailey AL, et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24) e285-350. doi:10.1016/j.jacc.2018.11003
15. Myers KD, Farboodi N, Mwamburi M, et al. Effect of access to prescribed PCSK9 inhibitors on cardiovascular outcomes. Circ Cardiovasc Qual Outcomes. 2019;12(8):e005404. doi:10.1161/CIRCOUTCOMES.118.005404
16. Wong ND, Chuang J, Zhao Y, Rosenblit PD. Residual dyslipidemia according to low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B among statin-treated US adults: National Health and Nutrition Examination Survey 2009-2010. J Clin Lipidol. 2015;9(4):525-532. doi:10.1016/j.jacl.2015.05.003
17. Della Badia LA, Elshourbagy NA, Mousa SA. Targeting PCSK9 as a promising new mechanism for lowering low-density lipoprotein cholesterol. Pharmacol Ther. 2016;164:183-194. doi:10.1016/j.pharmthera.2016.04.011
18. Praluent (alirocumab) injection. Prescribing information. Regeneron Pharmaceuticals; 2021.
19. Repatha (evolocumab) injection. Prescribing information. Amgen; 2021.
20. Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097-2107. doi:10.1056/NEJMoa1801174
21. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713-1722. doi:10.1056/NEJMoa1615664
22. Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63(23):2541-2548. doi:10.1016/j.jacc.2014.03.019
23. Nissen SE, Stroes E, Dent-Acosta RE, et al. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: the GAUSS-3 randomized clinical trial. JAMA. 2016;315(15):1580-1590. doi:10.1001/jama.2016.3608
24. Moriarty PM, Thompson PD, Cannon CP, et al. Efficacy and safety of alirocumab vs ezetimibe in statin-intolerant patients, with a statin rechallenge arm: the ODYSSEY ALTERNATIVE randomized trial. J Clin Lipidol. 2015;9(6):758-769. doi:10.1016/j.jacl.2015.08.006
25. Mesi O, Lin C, Ahmed H, Cho LS. Statin intolerance and new lipid-lowering treatments. Cleve Clin J Med. 2021;88(7):381-387. Published 2021 Jul 1. doi:10.3949/ccjm.88a.20165
26. US Department of Veterans Affairs. Clinical Guidance - Criteria For Use. September 2016. Accessed January 23, 2023. https://www.pbm.va.gov/clinicalguidance/criteriaforuse.asp
27. Donald DR, Reynolds VW, Hall N, DeClercq J, Choi L. Exploring rates of PCSK9 inhibitor persistence and reasons for treatment non-persistence in an integrated specialty pharmacy model. J Clin Lipidol. 2022;16(3):315-324. doi:10.1016/j.jacl.2022.03.004
28. Warden BA, Guyton JR, Kovacs AC, et al. Assessment and management of statin-associated muscle symptoms: A clinical perspective from the National Lipid Association. J Clin Lipidol. Published online September 10, 2022. doi:10.1016/j.jacl.2022.09.001
HMG-CoA reductase inhibitors (statins) have been shown to effectively reduce low-density lipoprotein cholesterol (LDL-C) as well as morbidity and mortality in patients who have either atherosclerotic cardiovascular disease (ASCVD) or risk factors for ASCVD.1-12 However, research shows that up to 20% of patients are unable to tolerate statin therapy due to muscle-related adverse events (AEs).13 This presents a substantial clinical challenge, as current management strategies for patients with statin-associated muscle symptoms, such as intermittent administration of statins and ezetimibe, seldom achieve the > 50% LDL-C reduction recommended by the 2018 American Heart Association/American College of Cardiology Clinical Practice Guidelines.14 Additionally, statin-intolerant patients who have antihyperlipidemic medication lowered or discontinued are at an increased risk of future cardiovascular events.15 Observational data also show that about 70% of adult patients (primarily with genetic lipid disorders such as heterozygous familial hypercholesterolemia) do not achieve an LDL-C level < 100 mg/dL despite treatment with maximum doses of statins with or without ezetimibe.16,17
PCSK9 inhibitors (PCSK9i) have robust efficacy data to support use in patients who do not meet their LDL-C goal despite maximally tolerated lipid therapy.14 However, long-term safety data for PCSK9i are not as robust as its efficacy data. Specifically, safety data relating to muscle-related AEs, which are the most widely recognized AE associated with statins, have only been reported in a few clinical trials with varying incidence rates, levels of significance, and relatively small study populations. Furthermore, the real-world prevalence of muscle-related PCSK9i AEs is unknown. Clinical guidance for management strategies for muscle-related AEs associated with PCSK9i is largely lacking. For this study, muscle-related AEs were defined as any new or unusual muscle soreness, weakness, cramping, aches, and stiffness that persists, is generally bilateral, and typically affects the large muscles. It is important to note, that muscle-related AEs associated with statins, ezetimibe, and PCSK9i can be attributed to the nocebo effect.
According to the prescribing information for alirocumab and evolocumab, myalgia, muscle spasms, and musculoskeletal pain each occurred in < 5% of the study populations.18,19 From these data, muscle-related PCSK9i AEs are thought to be relatively rare, based on the ODYSSEY-OUTCOME and FOURIER trials, which did not enroll statin-intolerant patients.20,21 However, currently available safety data from 3 small, randomized clinical trials specifically in statin-intolerant patients taking a PCSK9i suggest that muscle-related AEs occur at a rate of 12.2% to 32.5% and discontinuation rates varied from 0% to 15.9%.22-25 As the incidence rates of muscle-related AEs in the prescribing information and clinical trials varied widely, this study will provide quantitative data on the percentage of patients that developed muscle-related PCSK9i AEs in a veteran population to help shed light on a topic that is not well studied.
Methods
This was a single-center, retrospective chart review of patients prescribed a PCSK9i between December 1, 2017, and September 1, 2021, and were managed in a pharmacy-led patient aligned care team (PACT) clinic at the Wilkes-Barre US Department of Veterans Affairs (VA) Medical Center (WBVAMC) in Pennsylvania. This study was approved by the Coatesville VA Medical Center Institutional Review Board, which oversees research conducted at WBVAMC. Veterans aged ≥ 18 years were included in the study. Patients were excluded if they had a history of serious hypersensitivity reaction to a PCSK9i or rhabdomyolysis or did not meet the VA criteria for use.26
The primary outcome was the percentage of patients who developed a muscle-related AE while on a PCSK9i in a PACT clinic. Data were further analyzed based on patients who (1) tolerated a full PCSK9i dose; (2) tolerated alternative PCSK9i following initial intolerance; (3) required a PCSK9i dose reduction, or (4) discontinued PCSK9i. A secondary outcome was the percentage of statin- and/or ezetimibe-intolerant patients in these 4 groups. Another secondary outcome was the management strategies taken for patients who were on a reduced (monthly) dose of PCSK9i who did not reach their LDL-C goal. Management strategies that were assessed included restarting weekly statin, restarting weekly ezetimibe, increasing the dose of the same PCSK9i administered monthly, and switching to an alternative PCSK9i.
Data were collected using the VA Computerized Patient Record System (CPRS) and stored in a secure, locked spreadsheet. Baseline patient demographic characteristics collected included age (at PCSK9i start); sex; race; and PCSK9i name, dose, and frequency. We recorded when a patient switched PCSK9i, whether or not it was due to a muscle-related AE, and the name of the original PCSK9i. Also collected were lipid therapy intolerances prior to PCSK9i initiation (ie, intolerance to statin, ezetimibe, or both).
Patients were considered statin intolerant due to a muscle-related AE in accordance with the VA PCSK9i Criteria for Use, which requires trial of at least 3 statins, one of which was trialed at the lowest dosage approved by the US Food and Drug Administration (FDA) and resulted in intolerable skeletal muscle AEs that worsened during treatment and resolved when the statin was stopped. For our study purposes, patients taking alternative day dosing of statins due to muscle-related AEs (ie, 2- or 3-times weekly dosing) were not considered statin intolerant; however, patients taking once-weekly statin dosing were considered statin intolerant. Patients were considered ezetimibe intolerant due to a muscle-related AE if the intolerance was due to skeletal muscle concerns that worsened during treatment and resolved when ezetimibe was stopped. Patients were considered PCSK9i intolerant due to a muscle-related AE if the intolerance was due to skeletal muscle concerns that worsened during treatment and resolved when the PCSK9i was stopped. Patients with non–muscle-related intolerances to statins, ezetimibe, and PCSK9i were not considered statin, ezetimibe, and PCSK9i intolerant.
Alirocumab was initiated at 75 mg subcutaneous (SQ) once every 2 weeks or evolocumab 140 mg SQ once every 2 weeks in our study. The protocol allowed for a dose reduction of alirocumab 75 mg SQ once monthly if a patient experienced AEs, but this dose reduction strategy was not used for any patients on evolocumab in this study. Of note, alirocumab 75 mg SQ once monthly is not an FDA-approved dosing strategy. However, it is similar in concept to the alternative statin dosing (ie, alternate day dosing, once-weekly dosing) and may avoid the need to discontinue PCSK9i therapy altogether.
A review of the CPRS also documented whether a muscle-related AE occurred while the patient was on a PCSK9i (if yes, the specific AE was recorded), the result of PCSK9i therapy (tolerated full dose, required a dose reduction, switched medication, or discontinued), and management strategies taken for patients who did not meet their LDL-C goal while on a reduced (monthly) PCSK9i dose. Prior lipid therapy intolerances, PCSK9i-related AEs, results of PCSK9i therapy, and management strategies for patients who did not meet LDL-C goal while on a reduced PCSK9i dose were obtained by reviewing the PACT pharmacist’s clinic notes and assessment, along with clinic notes and medication history listed within the CPRS.
Statistical Analysis
Descriptive statistics were used for the demographic characteristics of study patients. The primary outcome was calculated as a binary measure (yes/no) of whether the patient developed a muscle-related AE while on a PCSK9i. The secondary outcome of statin, ezetimibe, or statin and ezetimibe intolerances in subgroups also was calculated as a binary measure.
Results
For the study, 156 charts were reviewed and 137 patients were included (Figure).
For the secondary results, 4 patients (2.9%) tolerated an alternate PCSK9i (evolocumab 140 mg SQ every 2 weeks) after initial intolerance to PCSK9i, 16 (11.7%) required a dose reduction, and 6 (4.4%) discontinued PCSK9i due to a muscle-related AE.
Statin intolerance was most common in all groups, followed by ezetimibe intolerance, and intolerance to statins + ezetimibe. Of the 113 patients who tolerated a full dose of PCSK9i, 77 (68.1%) had intolerance to statin, 47 (41.6%) to ezetimibe, and 41 (36.3%) to both statins and ezetimibe. Of the 6 patients who discontinued PCSK9i, all had intolerance to statins, 5 (83.3%) to ezetimibe, and 5 (83.3%) to statins and ezetimibe.
For patients who were on a reduced (monthly) dose of a PCSK9i who did not reach their LDL-C goal, we found that 16 patients (11.7%) required a PCSK9i dose reduction following muscle-related AEs. Of the patients who had their dose of PCSK9i reduced to monthly dosing, 5 (31%) met their LDL-C goal. For the 11 patients who did not meet their LDL-C goal, different management strategies were taken. Lifestyle modifications were made in 6 patients (54%), the monthly PCSK9i dose was increased to alirocumab 150 mg SQ monthly in 4 patients (36%), and 1 patient (9.1%) was switched to an alternative PCSK9i. There were no identified muscle-related AEs recorded in patients whose dose was increased to alirocumab 150 mg SQ monthly.
Discussion
This retrospective study found 17.5% of patients experienced muscle-related PCSK9i AEs. These occurred at a higher rate than reported in the prescribing information (< 5%) and were similar to the incidence rates reported in the GAUSS-2, GAUSS-3, and ODYSSEY-ALTERNATIVE clinical trials (12.0%-32.5%), which is what we hypothesized.18,19,22-25 It is important to note that the incidence rates of muscle-related AEs reported in the prescribing information for alirocumab and evolocumab were based on trials that did not include statin- and/or ezetimibe-intolerant patients; whereas many patients in our study and patients in the clinical trials were statin and/or ezetimibe intolerant.
Additionally, a new study by Donald and colleagues found an incidence rate of 32% to 36% for muscle-related PCSK9i AEs.27 Collectively, the data from clinical trials and our study indicate that patients with prior intolerances to statin and/or ezetimibe appear to have a higher likelihood of developing a muscle-related PCSK9i intolerance. In our study, 23 of 24 patients who developed a muscle-related PCSK9i AE had a prior history of statin and/or ezetimibe intolerances. This should alert clinicians prescribing PCSK9i in patients with a history of statin and/or ezetimibe intolerance to counsel their patients on the possibility of muscle-related PCSK9i AEs and management strategies. However, it is important to note that there was a substantial number of patients in our study who were statin and/or ezetimibe intolerant due to a prior muscle-related AE who tolerated the full dose of PCSK9i.
To our knowledge, this was the first trial to evaluate muscle-related PCSK9i AEs in a veteran population. Additionally, our study appears to be the first to use 2 PCSK9i dosing strategies that are not FDA approved: Dose reduction for patients who experienced a muscle-related AE on alirocumab 75 mg SQ every 2 weeks and dose escalation for patients who did not meet their LDL-C goal on alirocumab 75 mg SQ monthly following an initial intolerance to 2-week dosing. The dose-reduction strategy allowed patients who experienced a muscle-related AE to alirocumab 75 mg to reduce administration from every 2 weeks to monthly.
This strategy was only performed with alirocumab, the preferred PCSK9i at WBVAMC, but the same dose-reduction strategy can theoretically be used with evolocumab as well. Reduced monthly dosing of alirocumab allowed patients with a prior intolerance to remain on a lower dosage without discontinuation. This is important because as noted by Myers and colleagues, individuals without access to PCSK9i were found to have a significantly higher incidence ratio of cardiovascular events compared with those taking PCSK9i.15 Also of note, > 30% of patients on the reduced monthly dose of alirocumab still met their LDL-C goal. Therefore, using this dose-reduction strategy (instead of patients discontinuing therapy altogether due to a muscle-related intolerance) can lessen the risk of major adverse cardiovascular events (MACE) as well as mitigate muscle-related AEs that occurred while on 2-week PCSK9i dosing regimens. While we acknowledge that this reduced monthly dose of either alirocumab or evolocumab is not FDA approved, it is similar to alternative statin dosing that also is not FDA approved but may minimize the need to discontinue PCSK9i therapy. It would be beneficial if these dosing strategies were investigated by future research.
The dose-escalation strategy for patients who did not meet their LDL-C goal while on the reduced, monthly dose of alirocumab also was unique. Alirocumab was increased from 75 mg SQ once monthly to 150 mg SQ once monthly. Interestingly, we found that through the end of the chart review period, all patients tolerated the increase well, despite having an initial muscle-related AE to alirocumab 75 mg every 2 weeks, which is the same total monthly dosage. This approach is similar to that of once-weekly statin dosing or a drug holiday and may be explained by the long half-life of PCSK9i. Regardless of the mechanism, this finding suggests that an increased monthly dose of PCSK9i is a potential alternative for patients who cannot tolerate the FDA-approved dose. However, the ability for patients to achieve goal LDL-C on the monthly dosage requires future study.
In our study, only 6 patients (4.4%) discontinued PCSK9i therapy. This low discontinuation rate is largely attributable to our unique study design, which allowed for a dose reduction in patients who experienced muscle-related AEs. The earlier ODYSSEY-ALTERNATIVE trial evaluated the safety and efficacy of alirocumab compared with ezetimibe in confirmed statin-intolerant subjects after 24 weeks. This trial did not use a dose-reduction strategy and found 15.9% of patients discontinued alirocumab due to a muscle-related AE.24 This is notably higher than our discontinuation rate of 4.4%. If patients with a muscle-related AE discontinued PCKS9i instead of reducing the dose, they would likely return to their baseline LDL-C, which would increase the risk of MACE.
In general, myalgias due to antihyperlipidemic medications are not completely understood. One possible mechanism for statin-induced myalgias is the depletion of ubiquinone. However, this theory cannot explain muscle-related AEs associated with PCSK9i or ezetimibe, which have not been shown to deplete ubiquinone. We also found that the onset of muscle-related AEs associated with PCSK9i tends to appear later in therapy than what we know about statin therapy. Our study showed that the onset of a muscle-related PCSK9i AEs occurred a mean (SD) 8 (5.3) months after initiation (range, 1-19). Statin muscle-related AEs typically occur within the initial 4 to 8 weeks of treatment, although they can occur at any time.28
Limitations
The results of this study should be considered with the following limitations. First, this was a retrospective chart review performed over a prespecified period. Any muscle-related AEs or LDL-C lowering effects from PCSK9i that occurred outside the review period were not captured. Our study was small and only included 137 patients, though it was similar in size to the GAUSS-2, GAUSS-3, and ODYSSEY-ALTERNATIVE trials.22-24 Additionally, the study was primarily composed of White men and may not be representative of other populations. Some muscle-related PCSK9i AEs may be attributed to the nocebo. Last, our study did not capture patients on a PCSK9i who were not followed in the PACT clinic.
Conclusions
We found that muscle-related PCSK9i AEs occurred at a similar rate as those reported in previous clinical trials and exceeded the incidence rate reported in the prescribing information for alirocumab and evolocumab. It appears that patients who have a prior muscle-related intolerance to a statin and/or ezetimibe had a higher likelihood of developing a muscle-related PCSK9i AE. In our study, only 1 patient developed a muscle-related PCSK9i AE who did not have a prior history of muscle-related intolerance to either a statin or ezetimibe. However, in our study, a substantial percentage of patients with statin and/or ezetimibe intolerances tolerated the full PCSK9i dose well, proving that PCSK9i are still a reasonable alternative for patients with prior intolerances to statins and/or ezetimibe.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the US Department of Veterans Affairs Medical Center, Wilkes-Barre, Pennsylvania.
HMG-CoA reductase inhibitors (statins) have been shown to effectively reduce low-density lipoprotein cholesterol (LDL-C) as well as morbidity and mortality in patients who have either atherosclerotic cardiovascular disease (ASCVD) or risk factors for ASCVD.1-12 However, research shows that up to 20% of patients are unable to tolerate statin therapy due to muscle-related adverse events (AEs).13 This presents a substantial clinical challenge, as current management strategies for patients with statin-associated muscle symptoms, such as intermittent administration of statins and ezetimibe, seldom achieve the > 50% LDL-C reduction recommended by the 2018 American Heart Association/American College of Cardiology Clinical Practice Guidelines.14 Additionally, statin-intolerant patients who have antihyperlipidemic medication lowered or discontinued are at an increased risk of future cardiovascular events.15 Observational data also show that about 70% of adult patients (primarily with genetic lipid disorders such as heterozygous familial hypercholesterolemia) do not achieve an LDL-C level < 100 mg/dL despite treatment with maximum doses of statins with or without ezetimibe.16,17
PCSK9 inhibitors (PCSK9i) have robust efficacy data to support use in patients who do not meet their LDL-C goal despite maximally tolerated lipid therapy.14 However, long-term safety data for PCSK9i are not as robust as its efficacy data. Specifically, safety data relating to muscle-related AEs, which are the most widely recognized AE associated with statins, have only been reported in a few clinical trials with varying incidence rates, levels of significance, and relatively small study populations. Furthermore, the real-world prevalence of muscle-related PCSK9i AEs is unknown. Clinical guidance for management strategies for muscle-related AEs associated with PCSK9i is largely lacking. For this study, muscle-related AEs were defined as any new or unusual muscle soreness, weakness, cramping, aches, and stiffness that persists, is generally bilateral, and typically affects the large muscles. It is important to note, that muscle-related AEs associated with statins, ezetimibe, and PCSK9i can be attributed to the nocebo effect.
According to the prescribing information for alirocumab and evolocumab, myalgia, muscle spasms, and musculoskeletal pain each occurred in < 5% of the study populations.18,19 From these data, muscle-related PCSK9i AEs are thought to be relatively rare, based on the ODYSSEY-OUTCOME and FOURIER trials, which did not enroll statin-intolerant patients.20,21 However, currently available safety data from 3 small, randomized clinical trials specifically in statin-intolerant patients taking a PCSK9i suggest that muscle-related AEs occur at a rate of 12.2% to 32.5% and discontinuation rates varied from 0% to 15.9%.22-25 As the incidence rates of muscle-related AEs in the prescribing information and clinical trials varied widely, this study will provide quantitative data on the percentage of patients that developed muscle-related PCSK9i AEs in a veteran population to help shed light on a topic that is not well studied.
Methods
This was a single-center, retrospective chart review of patients prescribed a PCSK9i between December 1, 2017, and September 1, 2021, and were managed in a pharmacy-led patient aligned care team (PACT) clinic at the Wilkes-Barre US Department of Veterans Affairs (VA) Medical Center (WBVAMC) in Pennsylvania. This study was approved by the Coatesville VA Medical Center Institutional Review Board, which oversees research conducted at WBVAMC. Veterans aged ≥ 18 years were included in the study. Patients were excluded if they had a history of serious hypersensitivity reaction to a PCSK9i or rhabdomyolysis or did not meet the VA criteria for use.26
The primary outcome was the percentage of patients who developed a muscle-related AE while on a PCSK9i in a PACT clinic. Data were further analyzed based on patients who (1) tolerated a full PCSK9i dose; (2) tolerated alternative PCSK9i following initial intolerance; (3) required a PCSK9i dose reduction, or (4) discontinued PCSK9i. A secondary outcome was the percentage of statin- and/or ezetimibe-intolerant patients in these 4 groups. Another secondary outcome was the management strategies taken for patients who were on a reduced (monthly) dose of PCSK9i who did not reach their LDL-C goal. Management strategies that were assessed included restarting weekly statin, restarting weekly ezetimibe, increasing the dose of the same PCSK9i administered monthly, and switching to an alternative PCSK9i.
Data were collected using the VA Computerized Patient Record System (CPRS) and stored in a secure, locked spreadsheet. Baseline patient demographic characteristics collected included age (at PCSK9i start); sex; race; and PCSK9i name, dose, and frequency. We recorded when a patient switched PCSK9i, whether or not it was due to a muscle-related AE, and the name of the original PCSK9i. Also collected were lipid therapy intolerances prior to PCSK9i initiation (ie, intolerance to statin, ezetimibe, or both).
Patients were considered statin intolerant due to a muscle-related AE in accordance with the VA PCSK9i Criteria for Use, which requires trial of at least 3 statins, one of which was trialed at the lowest dosage approved by the US Food and Drug Administration (FDA) and resulted in intolerable skeletal muscle AEs that worsened during treatment and resolved when the statin was stopped. For our study purposes, patients taking alternative day dosing of statins due to muscle-related AEs (ie, 2- or 3-times weekly dosing) were not considered statin intolerant; however, patients taking once-weekly statin dosing were considered statin intolerant. Patients were considered ezetimibe intolerant due to a muscle-related AE if the intolerance was due to skeletal muscle concerns that worsened during treatment and resolved when ezetimibe was stopped. Patients were considered PCSK9i intolerant due to a muscle-related AE if the intolerance was due to skeletal muscle concerns that worsened during treatment and resolved when the PCSK9i was stopped. Patients with non–muscle-related intolerances to statins, ezetimibe, and PCSK9i were not considered statin, ezetimibe, and PCSK9i intolerant.
Alirocumab was initiated at 75 mg subcutaneous (SQ) once every 2 weeks or evolocumab 140 mg SQ once every 2 weeks in our study. The protocol allowed for a dose reduction of alirocumab 75 mg SQ once monthly if a patient experienced AEs, but this dose reduction strategy was not used for any patients on evolocumab in this study. Of note, alirocumab 75 mg SQ once monthly is not an FDA-approved dosing strategy. However, it is similar in concept to the alternative statin dosing (ie, alternate day dosing, once-weekly dosing) and may avoid the need to discontinue PCSK9i therapy altogether.
A review of the CPRS also documented whether a muscle-related AE occurred while the patient was on a PCSK9i (if yes, the specific AE was recorded), the result of PCSK9i therapy (tolerated full dose, required a dose reduction, switched medication, or discontinued), and management strategies taken for patients who did not meet their LDL-C goal while on a reduced (monthly) PCSK9i dose. Prior lipid therapy intolerances, PCSK9i-related AEs, results of PCSK9i therapy, and management strategies for patients who did not meet LDL-C goal while on a reduced PCSK9i dose were obtained by reviewing the PACT pharmacist’s clinic notes and assessment, along with clinic notes and medication history listed within the CPRS.
Statistical Analysis
Descriptive statistics were used for the demographic characteristics of study patients. The primary outcome was calculated as a binary measure (yes/no) of whether the patient developed a muscle-related AE while on a PCSK9i. The secondary outcome of statin, ezetimibe, or statin and ezetimibe intolerances in subgroups also was calculated as a binary measure.
Results
For the study, 156 charts were reviewed and 137 patients were included (Figure).
For the secondary results, 4 patients (2.9%) tolerated an alternate PCSK9i (evolocumab 140 mg SQ every 2 weeks) after initial intolerance to PCSK9i, 16 (11.7%) required a dose reduction, and 6 (4.4%) discontinued PCSK9i due to a muscle-related AE.
Statin intolerance was most common in all groups, followed by ezetimibe intolerance, and intolerance to statins + ezetimibe. Of the 113 patients who tolerated a full dose of PCSK9i, 77 (68.1%) had intolerance to statin, 47 (41.6%) to ezetimibe, and 41 (36.3%) to both statins and ezetimibe. Of the 6 patients who discontinued PCSK9i, all had intolerance to statins, 5 (83.3%) to ezetimibe, and 5 (83.3%) to statins and ezetimibe.
For patients who were on a reduced (monthly) dose of a PCSK9i who did not reach their LDL-C goal, we found that 16 patients (11.7%) required a PCSK9i dose reduction following muscle-related AEs. Of the patients who had their dose of PCSK9i reduced to monthly dosing, 5 (31%) met their LDL-C goal. For the 11 patients who did not meet their LDL-C goal, different management strategies were taken. Lifestyle modifications were made in 6 patients (54%), the monthly PCSK9i dose was increased to alirocumab 150 mg SQ monthly in 4 patients (36%), and 1 patient (9.1%) was switched to an alternative PCSK9i. There were no identified muscle-related AEs recorded in patients whose dose was increased to alirocumab 150 mg SQ monthly.
Discussion
This retrospective study found 17.5% of patients experienced muscle-related PCSK9i AEs. These occurred at a higher rate than reported in the prescribing information (< 5%) and were similar to the incidence rates reported in the GAUSS-2, GAUSS-3, and ODYSSEY-ALTERNATIVE clinical trials (12.0%-32.5%), which is what we hypothesized.18,19,22-25 It is important to note that the incidence rates of muscle-related AEs reported in the prescribing information for alirocumab and evolocumab were based on trials that did not include statin- and/or ezetimibe-intolerant patients; whereas many patients in our study and patients in the clinical trials were statin and/or ezetimibe intolerant.
Additionally, a new study by Donald and colleagues found an incidence rate of 32% to 36% for muscle-related PCSK9i AEs.27 Collectively, the data from clinical trials and our study indicate that patients with prior intolerances to statin and/or ezetimibe appear to have a higher likelihood of developing a muscle-related PCSK9i intolerance. In our study, 23 of 24 patients who developed a muscle-related PCSK9i AE had a prior history of statin and/or ezetimibe intolerances. This should alert clinicians prescribing PCSK9i in patients with a history of statin and/or ezetimibe intolerance to counsel their patients on the possibility of muscle-related PCSK9i AEs and management strategies. However, it is important to note that there was a substantial number of patients in our study who were statin and/or ezetimibe intolerant due to a prior muscle-related AE who tolerated the full dose of PCSK9i.
To our knowledge, this was the first trial to evaluate muscle-related PCSK9i AEs in a veteran population. Additionally, our study appears to be the first to use 2 PCSK9i dosing strategies that are not FDA approved: Dose reduction for patients who experienced a muscle-related AE on alirocumab 75 mg SQ every 2 weeks and dose escalation for patients who did not meet their LDL-C goal on alirocumab 75 mg SQ monthly following an initial intolerance to 2-week dosing. The dose-reduction strategy allowed patients who experienced a muscle-related AE to alirocumab 75 mg to reduce administration from every 2 weeks to monthly.
This strategy was only performed with alirocumab, the preferred PCSK9i at WBVAMC, but the same dose-reduction strategy can theoretically be used with evolocumab as well. Reduced monthly dosing of alirocumab allowed patients with a prior intolerance to remain on a lower dosage without discontinuation. This is important because as noted by Myers and colleagues, individuals without access to PCSK9i were found to have a significantly higher incidence ratio of cardiovascular events compared with those taking PCSK9i.15 Also of note, > 30% of patients on the reduced monthly dose of alirocumab still met their LDL-C goal. Therefore, using this dose-reduction strategy (instead of patients discontinuing therapy altogether due to a muscle-related intolerance) can lessen the risk of major adverse cardiovascular events (MACE) as well as mitigate muscle-related AEs that occurred while on 2-week PCSK9i dosing regimens. While we acknowledge that this reduced monthly dose of either alirocumab or evolocumab is not FDA approved, it is similar to alternative statin dosing that also is not FDA approved but may minimize the need to discontinue PCSK9i therapy. It would be beneficial if these dosing strategies were investigated by future research.
The dose-escalation strategy for patients who did not meet their LDL-C goal while on the reduced, monthly dose of alirocumab also was unique. Alirocumab was increased from 75 mg SQ once monthly to 150 mg SQ once monthly. Interestingly, we found that through the end of the chart review period, all patients tolerated the increase well, despite having an initial muscle-related AE to alirocumab 75 mg every 2 weeks, which is the same total monthly dosage. This approach is similar to that of once-weekly statin dosing or a drug holiday and may be explained by the long half-life of PCSK9i. Regardless of the mechanism, this finding suggests that an increased monthly dose of PCSK9i is a potential alternative for patients who cannot tolerate the FDA-approved dose. However, the ability for patients to achieve goal LDL-C on the monthly dosage requires future study.
In our study, only 6 patients (4.4%) discontinued PCSK9i therapy. This low discontinuation rate is largely attributable to our unique study design, which allowed for a dose reduction in patients who experienced muscle-related AEs. The earlier ODYSSEY-ALTERNATIVE trial evaluated the safety and efficacy of alirocumab compared with ezetimibe in confirmed statin-intolerant subjects after 24 weeks. This trial did not use a dose-reduction strategy and found 15.9% of patients discontinued alirocumab due to a muscle-related AE.24 This is notably higher than our discontinuation rate of 4.4%. If patients with a muscle-related AE discontinued PCKS9i instead of reducing the dose, they would likely return to their baseline LDL-C, which would increase the risk of MACE.
In general, myalgias due to antihyperlipidemic medications are not completely understood. One possible mechanism for statin-induced myalgias is the depletion of ubiquinone. However, this theory cannot explain muscle-related AEs associated with PCSK9i or ezetimibe, which have not been shown to deplete ubiquinone. We also found that the onset of muscle-related AEs associated with PCSK9i tends to appear later in therapy than what we know about statin therapy. Our study showed that the onset of a muscle-related PCSK9i AEs occurred a mean (SD) 8 (5.3) months after initiation (range, 1-19). Statin muscle-related AEs typically occur within the initial 4 to 8 weeks of treatment, although they can occur at any time.28
Limitations
The results of this study should be considered with the following limitations. First, this was a retrospective chart review performed over a prespecified period. Any muscle-related AEs or LDL-C lowering effects from PCSK9i that occurred outside the review period were not captured. Our study was small and only included 137 patients, though it was similar in size to the GAUSS-2, GAUSS-3, and ODYSSEY-ALTERNATIVE trials.22-24 Additionally, the study was primarily composed of White men and may not be representative of other populations. Some muscle-related PCSK9i AEs may be attributed to the nocebo. Last, our study did not capture patients on a PCSK9i who were not followed in the PACT clinic.
Conclusions
We found that muscle-related PCSK9i AEs occurred at a similar rate as those reported in previous clinical trials and exceeded the incidence rate reported in the prescribing information for alirocumab and evolocumab. It appears that patients who have a prior muscle-related intolerance to a statin and/or ezetimibe had a higher likelihood of developing a muscle-related PCSK9i AE. In our study, only 1 patient developed a muscle-related PCSK9i AE who did not have a prior history of muscle-related intolerance to either a statin or ezetimibe. However, in our study, a substantial percentage of patients with statin and/or ezetimibe intolerances tolerated the full PCSK9i dose well, proving that PCSK9i are still a reasonable alternative for patients with prior intolerances to statins and/or ezetimibe.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the US Department of Veterans Affairs Medical Center, Wilkes-Barre, Pennsylvania.
1. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344(8934):1383-1389.
2. Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335(14):1001-1009. doi:10.1056/NEJM199610033351401
3. Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339(19):1349-1357. doi:10.1056/NEJM199811053391902.
4. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7-22. doi:10.1016/S0140-6736(02)09327-3
5. Koren MJ, Hunninghake DB; ALLIANCE Investigators. Clinical outcomes in managed-care patients with coronary heart disease treated aggressively in lipid-lowering disease management clinics: the alliance study. J Am Coll Cardiol. 2004;44(9):1772-1779. doi:10.1016/j.jacc.2004.07.053
6. Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279(20):1615-1622. doi:10.1001/jama.279.20.1615
7. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). JAMA. 2002;288(23):2998-3007. doi:10.1001/jama.288.23.2998
8. Sever PS, Dahlöf B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361(9364):1149-1158. doi:10.1016/S0140-6736(03)12948-0
9. Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195-2207. doi:10.1056/NEJMoa0807646
10. Nakamura H, Arakawa K, Itakura H, et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet. 2006;368(9542):1155-1163. doi:10.1016/S0140-6736(06)69472-5
11. Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360(9346):1623-1630. doi:10.1016/s0140-6736(02)11600-x
12. Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333(20):1301-1307. doi:10.1056/NEJM199511163332001

13. Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36(17):1012-1022. doi:10.1093/eurheartj/ehv043
14. Grundy SM, Stone NJ, Bailey AL, et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24) e285-350. doi:10.1016/j.jacc.2018.11003
15. Myers KD, Farboodi N, Mwamburi M, et al. Effect of access to prescribed PCSK9 inhibitors on cardiovascular outcomes. Circ Cardiovasc Qual Outcomes. 2019;12(8):e005404. doi:10.1161/CIRCOUTCOMES.118.005404
16. Wong ND, Chuang J, Zhao Y, Rosenblit PD. Residual dyslipidemia according to low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B among statin-treated US adults: National Health and Nutrition Examination Survey 2009-2010. J Clin Lipidol. 2015;9(4):525-532. doi:10.1016/j.jacl.2015.05.003
17. Della Badia LA, Elshourbagy NA, Mousa SA. Targeting PCSK9 as a promising new mechanism for lowering low-density lipoprotein cholesterol. Pharmacol Ther. 2016;164:183-194. doi:10.1016/j.pharmthera.2016.04.011
18. Praluent (alirocumab) injection. Prescribing information. Regeneron Pharmaceuticals; 2021.
19. Repatha (evolocumab) injection. Prescribing information. Amgen; 2021.
20. Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097-2107. doi:10.1056/NEJMoa1801174
21. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713-1722. doi:10.1056/NEJMoa1615664
22. Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63(23):2541-2548. doi:10.1016/j.jacc.2014.03.019
23. Nissen SE, Stroes E, Dent-Acosta RE, et al. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: the GAUSS-3 randomized clinical trial. JAMA. 2016;315(15):1580-1590. doi:10.1001/jama.2016.3608
24. Moriarty PM, Thompson PD, Cannon CP, et al. Efficacy and safety of alirocumab vs ezetimibe in statin-intolerant patients, with a statin rechallenge arm: the ODYSSEY ALTERNATIVE randomized trial. J Clin Lipidol. 2015;9(6):758-769. doi:10.1016/j.jacl.2015.08.006
25. Mesi O, Lin C, Ahmed H, Cho LS. Statin intolerance and new lipid-lowering treatments. Cleve Clin J Med. 2021;88(7):381-387. Published 2021 Jul 1. doi:10.3949/ccjm.88a.20165
26. US Department of Veterans Affairs. Clinical Guidance - Criteria For Use. September 2016. Accessed January 23, 2023. https://www.pbm.va.gov/clinicalguidance/criteriaforuse.asp
27. Donald DR, Reynolds VW, Hall N, DeClercq J, Choi L. Exploring rates of PCSK9 inhibitor persistence and reasons for treatment non-persistence in an integrated specialty pharmacy model. J Clin Lipidol. 2022;16(3):315-324. doi:10.1016/j.jacl.2022.03.004
28. Warden BA, Guyton JR, Kovacs AC, et al. Assessment and management of statin-associated muscle symptoms: A clinical perspective from the National Lipid Association. J Clin Lipidol. Published online September 10, 2022. doi:10.1016/j.jacl.2022.09.001
1. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344(8934):1383-1389.
2. Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335(14):1001-1009. doi:10.1056/NEJM199610033351401
3. Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339(19):1349-1357. doi:10.1056/NEJM199811053391902.
4. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7-22. doi:10.1016/S0140-6736(02)09327-3
5. Koren MJ, Hunninghake DB; ALLIANCE Investigators. Clinical outcomes in managed-care patients with coronary heart disease treated aggressively in lipid-lowering disease management clinics: the alliance study. J Am Coll Cardiol. 2004;44(9):1772-1779. doi:10.1016/j.jacc.2004.07.053
6. Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279(20):1615-1622. doi:10.1001/jama.279.20.1615
7. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). JAMA. 2002;288(23):2998-3007. doi:10.1001/jama.288.23.2998
8. Sever PS, Dahlöf B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361(9364):1149-1158. doi:10.1016/S0140-6736(03)12948-0
9. Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195-2207. doi:10.1056/NEJMoa0807646
10. Nakamura H, Arakawa K, Itakura H, et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet. 2006;368(9542):1155-1163. doi:10.1016/S0140-6736(06)69472-5
11. Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360(9346):1623-1630. doi:10.1016/s0140-6736(02)11600-x
12. Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333(20):1301-1307. doi:10.1056/NEJM199511163332001

13. Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36(17):1012-1022. doi:10.1093/eurheartj/ehv043
14. Grundy SM, Stone NJ, Bailey AL, et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24) e285-350. doi:10.1016/j.jacc.2018.11003
15. Myers KD, Farboodi N, Mwamburi M, et al. Effect of access to prescribed PCSK9 inhibitors on cardiovascular outcomes. Circ Cardiovasc Qual Outcomes. 2019;12(8):e005404. doi:10.1161/CIRCOUTCOMES.118.005404
16. Wong ND, Chuang J, Zhao Y, Rosenblit PD. Residual dyslipidemia according to low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B among statin-treated US adults: National Health and Nutrition Examination Survey 2009-2010. J Clin Lipidol. 2015;9(4):525-532. doi:10.1016/j.jacl.2015.05.003
17. Della Badia LA, Elshourbagy NA, Mousa SA. Targeting PCSK9 as a promising new mechanism for lowering low-density lipoprotein cholesterol. Pharmacol Ther. 2016;164:183-194. doi:10.1016/j.pharmthera.2016.04.011
18. Praluent (alirocumab) injection. Prescribing information. Regeneron Pharmaceuticals; 2021.
19. Repatha (evolocumab) injection. Prescribing information. Amgen; 2021.
20. Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097-2107. doi:10.1056/NEJMoa1801174
21. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713-1722. doi:10.1056/NEJMoa1615664
22. Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63(23):2541-2548. doi:10.1016/j.jacc.2014.03.019
23. Nissen SE, Stroes E, Dent-Acosta RE, et al. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: the GAUSS-3 randomized clinical trial. JAMA. 2016;315(15):1580-1590. doi:10.1001/jama.2016.3608
24. Moriarty PM, Thompson PD, Cannon CP, et al. Efficacy and safety of alirocumab vs ezetimibe in statin-intolerant patients, with a statin rechallenge arm: the ODYSSEY ALTERNATIVE randomized trial. J Clin Lipidol. 2015;9(6):758-769. doi:10.1016/j.jacl.2015.08.006
25. Mesi O, Lin C, Ahmed H, Cho LS. Statin intolerance and new lipid-lowering treatments. Cleve Clin J Med. 2021;88(7):381-387. Published 2021 Jul 1. doi:10.3949/ccjm.88a.20165
26. US Department of Veterans Affairs. Clinical Guidance - Criteria For Use. September 2016. Accessed January 23, 2023. https://www.pbm.va.gov/clinicalguidance/criteriaforuse.asp
27. Donald DR, Reynolds VW, Hall N, DeClercq J, Choi L. Exploring rates of PCSK9 inhibitor persistence and reasons for treatment non-persistence in an integrated specialty pharmacy model. J Clin Lipidol. 2022;16(3):315-324. doi:10.1016/j.jacl.2022.03.004
28. Warden BA, Guyton JR, Kovacs AC, et al. Assessment and management of statin-associated muscle symptoms: A clinical perspective from the National Lipid Association. J Clin Lipidol. Published online September 10, 2022. doi:10.1016/j.jacl.2022.09.001
Evaluation of the Appropriateness of Aspirin Therapy in a Veteran Population
Aspirin is an antiplatelet agent that binds irreversibly to COX-1 and COX-2 enzymes, which results in decreased prostaglandin and thromboxane A2 production and inhibition of platelet aggregation. Aspirin often is used for its antipyretic, analgesic, and antiplatelet properties. Its use in cardiovascular disease (CVD) has been studied extensively over the past few decades, and recent data are changing the framework for aspirin use in primary prevention of atherosclerotic cardiovascular disease (ASCVD). Primary prevention refers to efforts to prevent the incidence of cardiovascular events, whereas secondary prevention refers to efforts to prevent a cardiovascular event after one has occurred.1 This differentiation is important as it guides the course of treatment.
Three trials published in 2018 evaluated aspirin use in primary prevention of ASCVD. The ASCEND trial evaluated aspirin use for primary prevention of ASCVD in patients with diabetes mellitus (DM). This study concluded that although aspirin prevented serious vascular events in patients with DM, the benefit observed was largely counteracted by the bleeding hazard.2 The ARRIVE trial evaluated aspirin use for primary prevention in patients with a moderate CVD risk. The study concluded that aspirin use in patients at moderate risk of CVD could not be assessed due to the low incidence rate of CVD; however, the study concluded that aspirin did not reduce the incidence of cardiovascular events for patients at low CVD risk and that aspirin caused more mild gastrointestinal bleeds compared with placebo.3 The ASPREE trial evaluated aspirin use for primary prevention in patients aged > 70 years to determine whether its use prolonged a healthy lifespan. This trial concluded that patients who received daily aspirin were at a higher risk of major hemorrhage and that aspirin did not diminish CVD risk compared with placebo.4
These studies led to a paradigm shift in therapy to reevaluate aspirin use for primary prevention. Current indications for aspirin include secondary prevention of ASCVD (ie, myocardial infarction [MI], coronary artery bypass graft, transient ischemic attack [TIA], and stroke), venous thromboembolism prophylaxis in the setting of orthopedic surgery, or valvular disease with replacement and analgesia. It is important to note that certain clinical circumstances may warrant aspirin use for primary prevention of ASCVD on a patient-specific basis, and this decision should be made using a risk/benefit analysis with the patient.
In April 2022, the US Preventive Services Task Force (USPSTF) recommended against using low-dose aspirin for primary prevention of ASCVD in individuals aged ≥ 60 years. The USPSTF noted that for patients who have a ≥ 10%, 10-year CVD risk, the decision to initiate aspirin should be based on a risk/benefit discussion and may be beneficial in certain patient populations.5A 2019 National Heart, Lung, and Blood Institute survey found that 29 million Americans used aspirin for primary prevention of ASCVD, and 6.6 million of these Americans used aspirin for primary prevention without the recommendation of a health care professional (HCP). Almost half of these individuals were aged > 70 years and, therefore, at an increased risk for bleeding.6 With the recent studies and changes in guidelines highlighting a higher risk rather than benefit with the use of aspirin for primary prevention, the current use of aspirin for primary prevention in the United States needs to be readdressed.
HCPs should assess the appropriateness of aspirin use in their patients to ensure that the risks of aspirin do not outweigh the benefits. Pharmacists can play a vital role in the assessment of aspirin for primary prevention during patient visits and make recommendations to primary care practitioners to deprescribe aspirin when appropriate.
Methods
The objective of this study was to evaluate the appropriateness of aspirin therapy in patient aligned care team (PACT) clinics at the Captain James A. Lovell Federal Health Care Center (FHCC) in North Chicago, Illinois. The PACT clinics are a category of clinics that include all the primary care clinics at FHCC.
The primary outcome of this study was to determine the percentage of patients inappropriately on aspirin therapy. To assess the inappropriate use of aspirin, relevant history of ASCVD was collected. Patients were divided into 3 groups: those with a history of ASCVD, those with no risk factors or history of ASCVD, and those with risk factors and no history of ASCVD. Patients were then categorized for their indication for aspirin use, which included either primary or secondary prevention of ASCVD. Patients were categorized into the primary prevention group if they had no history of ASCVD, whereas patients with a history of ASCVD were placed into the secondary prevention group.
ASCVD was defined as patients with acute coronary syndrome (ACS), history of MI, stable or unstable angina, coronary or other arterial revascularization, stroke, TIA, or peripheral artery disease (PAD), including aortic aneurysm (all with an atherosclerotic origin). Possible ASCVD risk was defined as patients with DM with a major risk factor (family history of premature ASCVD, hypertension, dyslipidemia, smoking, chronic kidney disease [CKD]/albuminuria) or patients diagnosed with coronary artery disease without an event. The percentage of patients followed by a PACT pharmacist, the number of pharmacist follow-up visits during the study period, and the date of the first 81-mg aspirin pharmacy order that was filled at FHCC were also collected.
The secondary outcome of this study focused on patients who were using aspirin for primary prevention and assessed potential reasons that may warrant deprescribing aspirin therapy. One reason for deprescribing is that aspirin may not be indicated for some patients, including those with DM without cardiovascular complications, patients aged > 70 years, and/or patients with CKD (defined as estimated glomerular filtration rate < 60 mL/min). Another reason for deprescribing is contraindication, which included patients with coagulopathy, thrombocytopenia (defined as platelet count < 150,000 mL), a history of gastrointestinal bleeding, peptic ulcer disease or other major bleeds, and/or consistent use of medications that increase bleeding risk (such as nonsteroidal anti-inflammatory agents, steroids, or anticoagulants) for > 14 days.
The safety outcome of this study assessed bleeding events while on aspirin therapy. All patients were categorized depending on if they had a major, minor, or no bleeding event while on aspirin therapy. Hemorrhagic stroke, symptomatic intracranial bleeding, bleeds located in other critical sites or organs (intracranial, intraspinal, intraocular, retroperitoneal, intra‐articular or pericardial), bleeds causing hemodynamic instability requiring vasopressors, bleeds causing a > 2 g/dL hemoglobin drop since initiation of aspirin therapy, severe extracranial bleeding requiring transfusion or hospitalization, fatal bleeding, or bleeds requiring > 2 units of red blood cell transfusion were considered major bleeding events. Minor bleeding events were any events that did not meet the criteria for major bleeding, including bruising, bleeding gums, epistaxis, hemorrhoidal bleeds, and bleeding that did not require intervention or treatment.7
Patients were included if they were aged > 18 years, had an active prescription for 81-mg aspirin tablet on September 30, 2021, and were seen in FHCC PACT clinics or at affiliated community-based outpatient centers. Other doses of aspirin were excluded as the 81-mg dose is the standard dose for primary prevention of ASCVD in the United States. US Department of Defense patients, home-based primary care patients, and community living center patients were excluded in this study. Patients with an aspirin prescription from a non–US Department of Veterans Affairs (VA) facility and patients on aspirin for reasons other than cardiovascular protection (such as pain, fever, etc) also were excluded from this study.
Data were collected from the FHCC electronic health record. A list was generated to include all active prescriptions for aspirin filled at FHCC as of September 30, 2021. Data were reviewed before this date to capture primary and secondary outcomes. No information was gathered from the chart after that date. This project was approved by the Edward Hines, Jr. VA Hospital Institutional Review Board. The primary and secondary outcomes were reported using descriptive statistics.
Results
This study reviewed 140 patient records and 105 patients met inclusion criteria.
For the primary endpoint, 53 patients (50%) were on aspirin for secondary prevention and 52 (50%) were on aspirin for primary prevention. Of the 105 patients included in the study, 31 (30%) had a possible ASCVD risk and were taking aspirin for primary prevention, while 21 (20%) had no ASCVD and were taking aspirin for primary prevention. Of the 52 patients on aspirin for primary prevention, 31 patients (60%) had a possible risk for ASCVD. Of the 52 patients in the primary prevention group, 15 (29%) were followed by a pharmacist, and the average number of follow-up appointments was 4.
The secondary endpoint focused on patients taking aspirin for primary prevention and the factors that may warrant deprescribing aspirin. Of the 52 patients on aspirin for primary prevention, 25 patients were aged > 70 years, 15 patients were concurrently taking medications that may increase bleeding risk,
For the entire study group, 6 patients (6%) experienced a major bleeding event while on aspirin, 46 (44%) experienced a minor bleeding event while on aspirin, and 53 (50%) experienced no bleeding events while on aspirin. Of the 6 patients who experienced major bleeding events, 4 were on aspirin for secondary prevention, and 2 were on aspirin for primary prevention with ASCVD risk factors. The major bleeding events included 4 gastrointestinal bleeds, 1 intracranial hemorrhage, and 1 hemorrhagic stroke. Of the 46 who experienced minor bleeding events, 20 patients were on aspirin for primary prevention; 11 of those patients had possible ASCVD risk factors and 9 had no documented ASCVD. The minor bleeding events included hematuria, epistaxis, bleeding scabs, and dental bleeding.
Discussion
The majority of patients in this study were on aspirin appropriately. Indications deemed appropriate for aspirin therapy include secondary prevention and primary prevention with a possible ASCVD risk. About 20% of the total patient population in this study was taking aspirin for primary prevention with no ASCVD risk. For these patients, the risk of bleeding likely outweighs the benefits of aspirin therapy as patients are at low risk for ASCVD; therefore, aspirin therapy is likely inappropriate in this patient population. These patients may be unnecessarily at an increased risk for bleeding and may benefit from deprescribing aspirin. For the safety of patients, HCPs should be continuously assessing the appropriateness of aspirin for primary prevention and deprescribing when necessary.
About one-third of the patients using aspirin for primary prevention were followed by a pharmacist. Pharmacists can play a key role in deprescribing aspirin for primary prevention when aspirin use is deemed inappropriate. About 30% of the total patient population in this study was on aspirin for primary prevention with possible ASCVD risk. This patient population may benefit from aspirin therapy as they are at a higher risk for ASCVD. For these patients, a risk/benefit discussion is necessary to determine the appropriateness of aspirin for primary prevention. This risk/benefit discussion should be a continuous conversation between patients and HCPs as different factors such as age and changes in comorbid conditions and medications may increase bleeding risk.
The secondary endpoint focused on patients taking aspirin for primary prevention and the factors that may warrant deprescribing aspirin. The most common factors seen in this study included patients who were aged > 70 years, patients who were concurrently taking medications that may increase bleeding risk, and patients with CKD. All of these factors increase bleeding risk, making the risks potentially outweigh the benefits of aspirin for primary prevention. These factors should be the primary focus when assessing patients on aspirin for primary prevention to promote deprescribing aspirin if deemed appropriate as they were the most prevalent in this study.
The safety endpoints focused on bleeding events as a whole as well as the bleeding events seen in the primary prevention group. There were 2 major bleeding events and 20 minor bleeding events in the primary prevention group. The number of bleeding events both major and minor further shows the need for a continuous risk/benefit discussion between patients and HCPs on continued aspirin use for primary prevention. The bleeding risk with aspirin is prevalent. HCPs should continue to assess for factors that increase the bleeding risk that may warrant deprescribing aspirin to prevent future bleeding events in this patient population.
Strengths and Limitations
As there have been recent updates to guidelines on the use of aspirin for primary prevention, a strength of this study is that it evaluates a topic that is relevant in health care. Another strength of this study is that it focuses on specific patient factors that HCPs can assess when determining whether aspirin for primary prevention is appropriate in their patients. These specific patient factors can also be used as a guide to help HCPs deprescribe aspirin for primary prevention when appropriate.
One of the limitations of this study is that bleeding events that occurred outside of the FHCC were unable to be assessed unless the HCP specifically commented on the bleeding event in the chart. This could potentially underestimate the bleeding events seen in this study. Another limitation is that the bleeding risk for patients who were not on aspirin was not assessed. There was no comparison group to ascertain whether the bleeding risk was higher in the aspirin group compared with a no aspirin group. However, many of the major clinical trials saw an increased risk of bleeding in the aspirin group compared with placebo.
Conclusions
Aspirin therapy for secondary prevention remains an important part of treatment. Aspirin therapy for primary prevention may be appropriate for patients with a possible ASCVD risk. The therapy may be inappropriate in cases where patients have an increased bleeding risk and low or no ASCVD risk. It is important to continuously assess the need for aspirin therapy for patients in the setting of primary prevention. Common factors seen in this study to warrant deprescribing aspirin for primary prevention include patients aged > 70 years, concurrent use of medications that increase bleeding risk, and patients with CKD. By assessing ASCVD risk as well as bleeding risk and having a risk/benefit discussion between the HCP and patient, aspirin used for primary prevention can be appropriately deprescribed when the risks of bleeding outweigh the benefits.
Acknowledgments
The authors thank the Captain James A. Lovell Federal Health Care Center research committee (Hong-Yen Vi, PharmD, BCPS; Shaiza Khan, PharmD, BCPS; Yinka Alaka, BPharm, PharmD; Jennifer Kwon, PharmD, BCOP) and coinvestigator Aeman Choudhury, PharmD, BCPS, BCACP.
1. Warner TD, Nylander S, Whatling C. Anti-platelet therapy: cyclo-oxygenase inhibition and the use of aspirin with particular regard to dual anti-platelet therapy. Br J Clin Pharmacol. 2011;72(4):619-633. doi:10.1111/j.1365-2125.2011.03943.x
2. ASCEND Study Collaborative Group, Bowman L, Mafham M, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379(16):1529-1539. doi:10.1056/NEJMoa1804988
3. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392(10152):1036-1046. doi:10.1016/S0140-6736(18)31924-X
4. McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379(16):1509-1518. doi:10.1056/NEJMoa1805819
5. US Preventive Services Task Force, Davidson KW, Barry MJ, et al. Aspirin use to prevent cardiovascular disease: US Preventive Services Task Force Recommendation Statement. JAMA. 2022;327(16):1577-1584. doi:10.1001/jama.2022.4983
6. Murphy E, McEvoy JW. Does stopping aspirin differ fundamentally from not starting aspirin in the primary prevention of cardiovascular disease among older adults? Ann Intern Med. 2022;175(5):757-758. doi:10.7326/M22-0550
7. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694. doi:10.1111/j.1538-7836.2005.01204.
Aspirin is an antiplatelet agent that binds irreversibly to COX-1 and COX-2 enzymes, which results in decreased prostaglandin and thromboxane A2 production and inhibition of platelet aggregation. Aspirin often is used for its antipyretic, analgesic, and antiplatelet properties. Its use in cardiovascular disease (CVD) has been studied extensively over the past few decades, and recent data are changing the framework for aspirin use in primary prevention of atherosclerotic cardiovascular disease (ASCVD). Primary prevention refers to efforts to prevent the incidence of cardiovascular events, whereas secondary prevention refers to efforts to prevent a cardiovascular event after one has occurred.1 This differentiation is important as it guides the course of treatment.
Three trials published in 2018 evaluated aspirin use in primary prevention of ASCVD. The ASCEND trial evaluated aspirin use for primary prevention of ASCVD in patients with diabetes mellitus (DM). This study concluded that although aspirin prevented serious vascular events in patients with DM, the benefit observed was largely counteracted by the bleeding hazard.2 The ARRIVE trial evaluated aspirin use for primary prevention in patients with a moderate CVD risk. The study concluded that aspirin use in patients at moderate risk of CVD could not be assessed due to the low incidence rate of CVD; however, the study concluded that aspirin did not reduce the incidence of cardiovascular events for patients at low CVD risk and that aspirin caused more mild gastrointestinal bleeds compared with placebo.3 The ASPREE trial evaluated aspirin use for primary prevention in patients aged > 70 years to determine whether its use prolonged a healthy lifespan. This trial concluded that patients who received daily aspirin were at a higher risk of major hemorrhage and that aspirin did not diminish CVD risk compared with placebo.4
These studies led to a paradigm shift in therapy to reevaluate aspirin use for primary prevention. Current indications for aspirin include secondary prevention of ASCVD (ie, myocardial infarction [MI], coronary artery bypass graft, transient ischemic attack [TIA], and stroke), venous thromboembolism prophylaxis in the setting of orthopedic surgery, or valvular disease with replacement and analgesia. It is important to note that certain clinical circumstances may warrant aspirin use for primary prevention of ASCVD on a patient-specific basis, and this decision should be made using a risk/benefit analysis with the patient.
In April 2022, the US Preventive Services Task Force (USPSTF) recommended against using low-dose aspirin for primary prevention of ASCVD in individuals aged ≥ 60 years. The USPSTF noted that for patients who have a ≥ 10%, 10-year CVD risk, the decision to initiate aspirin should be based on a risk/benefit discussion and may be beneficial in certain patient populations.5A 2019 National Heart, Lung, and Blood Institute survey found that 29 million Americans used aspirin for primary prevention of ASCVD, and 6.6 million of these Americans used aspirin for primary prevention without the recommendation of a health care professional (HCP). Almost half of these individuals were aged > 70 years and, therefore, at an increased risk for bleeding.6 With the recent studies and changes in guidelines highlighting a higher risk rather than benefit with the use of aspirin for primary prevention, the current use of aspirin for primary prevention in the United States needs to be readdressed.
HCPs should assess the appropriateness of aspirin use in their patients to ensure that the risks of aspirin do not outweigh the benefits. Pharmacists can play a vital role in the assessment of aspirin for primary prevention during patient visits and make recommendations to primary care practitioners to deprescribe aspirin when appropriate.
Methods
The objective of this study was to evaluate the appropriateness of aspirin therapy in patient aligned care team (PACT) clinics at the Captain James A. Lovell Federal Health Care Center (FHCC) in North Chicago, Illinois. The PACT clinics are a category of clinics that include all the primary care clinics at FHCC.
The primary outcome of this study was to determine the percentage of patients inappropriately on aspirin therapy. To assess the inappropriate use of aspirin, relevant history of ASCVD was collected. Patients were divided into 3 groups: those with a history of ASCVD, those with no risk factors or history of ASCVD, and those with risk factors and no history of ASCVD. Patients were then categorized for their indication for aspirin use, which included either primary or secondary prevention of ASCVD. Patients were categorized into the primary prevention group if they had no history of ASCVD, whereas patients with a history of ASCVD were placed into the secondary prevention group.
ASCVD was defined as patients with acute coronary syndrome (ACS), history of MI, stable or unstable angina, coronary or other arterial revascularization, stroke, TIA, or peripheral artery disease (PAD), including aortic aneurysm (all with an atherosclerotic origin). Possible ASCVD risk was defined as patients with DM with a major risk factor (family history of premature ASCVD, hypertension, dyslipidemia, smoking, chronic kidney disease [CKD]/albuminuria) or patients diagnosed with coronary artery disease without an event. The percentage of patients followed by a PACT pharmacist, the number of pharmacist follow-up visits during the study period, and the date of the first 81-mg aspirin pharmacy order that was filled at FHCC were also collected.
The secondary outcome of this study focused on patients who were using aspirin for primary prevention and assessed potential reasons that may warrant deprescribing aspirin therapy. One reason for deprescribing is that aspirin may not be indicated for some patients, including those with DM without cardiovascular complications, patients aged > 70 years, and/or patients with CKD (defined as estimated glomerular filtration rate < 60 mL/min). Another reason for deprescribing is contraindication, which included patients with coagulopathy, thrombocytopenia (defined as platelet count < 150,000 mL), a history of gastrointestinal bleeding, peptic ulcer disease or other major bleeds, and/or consistent use of medications that increase bleeding risk (such as nonsteroidal anti-inflammatory agents, steroids, or anticoagulants) for > 14 days.
The safety outcome of this study assessed bleeding events while on aspirin therapy. All patients were categorized depending on if they had a major, minor, or no bleeding event while on aspirin therapy. Hemorrhagic stroke, symptomatic intracranial bleeding, bleeds located in other critical sites or organs (intracranial, intraspinal, intraocular, retroperitoneal, intra‐articular or pericardial), bleeds causing hemodynamic instability requiring vasopressors, bleeds causing a > 2 g/dL hemoglobin drop since initiation of aspirin therapy, severe extracranial bleeding requiring transfusion or hospitalization, fatal bleeding, or bleeds requiring > 2 units of red blood cell transfusion were considered major bleeding events. Minor bleeding events were any events that did not meet the criteria for major bleeding, including bruising, bleeding gums, epistaxis, hemorrhoidal bleeds, and bleeding that did not require intervention or treatment.7
Patients were included if they were aged > 18 years, had an active prescription for 81-mg aspirin tablet on September 30, 2021, and were seen in FHCC PACT clinics or at affiliated community-based outpatient centers. Other doses of aspirin were excluded as the 81-mg dose is the standard dose for primary prevention of ASCVD in the United States. US Department of Defense patients, home-based primary care patients, and community living center patients were excluded in this study. Patients with an aspirin prescription from a non–US Department of Veterans Affairs (VA) facility and patients on aspirin for reasons other than cardiovascular protection (such as pain, fever, etc) also were excluded from this study.
Data were collected from the FHCC electronic health record. A list was generated to include all active prescriptions for aspirin filled at FHCC as of September 30, 2021. Data were reviewed before this date to capture primary and secondary outcomes. No information was gathered from the chart after that date. This project was approved by the Edward Hines, Jr. VA Hospital Institutional Review Board. The primary and secondary outcomes were reported using descriptive statistics.
Results
This study reviewed 140 patient records and 105 patients met inclusion criteria.
For the primary endpoint, 53 patients (50%) were on aspirin for secondary prevention and 52 (50%) were on aspirin for primary prevention. Of the 105 patients included in the study, 31 (30%) had a possible ASCVD risk and were taking aspirin for primary prevention, while 21 (20%) had no ASCVD and were taking aspirin for primary prevention. Of the 52 patients on aspirin for primary prevention, 31 patients (60%) had a possible risk for ASCVD. Of the 52 patients in the primary prevention group, 15 (29%) were followed by a pharmacist, and the average number of follow-up appointments was 4.
The secondary endpoint focused on patients taking aspirin for primary prevention and the factors that may warrant deprescribing aspirin. Of the 52 patients on aspirin for primary prevention, 25 patients were aged > 70 years, 15 patients were concurrently taking medications that may increase bleeding risk,
For the entire study group, 6 patients (6%) experienced a major bleeding event while on aspirin, 46 (44%) experienced a minor bleeding event while on aspirin, and 53 (50%) experienced no bleeding events while on aspirin. Of the 6 patients who experienced major bleeding events, 4 were on aspirin for secondary prevention, and 2 were on aspirin for primary prevention with ASCVD risk factors. The major bleeding events included 4 gastrointestinal bleeds, 1 intracranial hemorrhage, and 1 hemorrhagic stroke. Of the 46 who experienced minor bleeding events, 20 patients were on aspirin for primary prevention; 11 of those patients had possible ASCVD risk factors and 9 had no documented ASCVD. The minor bleeding events included hematuria, epistaxis, bleeding scabs, and dental bleeding.
Discussion
The majority of patients in this study were on aspirin appropriately. Indications deemed appropriate for aspirin therapy include secondary prevention and primary prevention with a possible ASCVD risk. About 20% of the total patient population in this study was taking aspirin for primary prevention with no ASCVD risk. For these patients, the risk of bleeding likely outweighs the benefits of aspirin therapy as patients are at low risk for ASCVD; therefore, aspirin therapy is likely inappropriate in this patient population. These patients may be unnecessarily at an increased risk for bleeding and may benefit from deprescribing aspirin. For the safety of patients, HCPs should be continuously assessing the appropriateness of aspirin for primary prevention and deprescribing when necessary.
About one-third of the patients using aspirin for primary prevention were followed by a pharmacist. Pharmacists can play a key role in deprescribing aspirin for primary prevention when aspirin use is deemed inappropriate. About 30% of the total patient population in this study was on aspirin for primary prevention with possible ASCVD risk. This patient population may benefit from aspirin therapy as they are at a higher risk for ASCVD. For these patients, a risk/benefit discussion is necessary to determine the appropriateness of aspirin for primary prevention. This risk/benefit discussion should be a continuous conversation between patients and HCPs as different factors such as age and changes in comorbid conditions and medications may increase bleeding risk.
The secondary endpoint focused on patients taking aspirin for primary prevention and the factors that may warrant deprescribing aspirin. The most common factors seen in this study included patients who were aged > 70 years, patients who were concurrently taking medications that may increase bleeding risk, and patients with CKD. All of these factors increase bleeding risk, making the risks potentially outweigh the benefits of aspirin for primary prevention. These factors should be the primary focus when assessing patients on aspirin for primary prevention to promote deprescribing aspirin if deemed appropriate as they were the most prevalent in this study.
The safety endpoints focused on bleeding events as a whole as well as the bleeding events seen in the primary prevention group. There were 2 major bleeding events and 20 minor bleeding events in the primary prevention group. The number of bleeding events both major and minor further shows the need for a continuous risk/benefit discussion between patients and HCPs on continued aspirin use for primary prevention. The bleeding risk with aspirin is prevalent. HCPs should continue to assess for factors that increase the bleeding risk that may warrant deprescribing aspirin to prevent future bleeding events in this patient population.
Strengths and Limitations
As there have been recent updates to guidelines on the use of aspirin for primary prevention, a strength of this study is that it evaluates a topic that is relevant in health care. Another strength of this study is that it focuses on specific patient factors that HCPs can assess when determining whether aspirin for primary prevention is appropriate in their patients. These specific patient factors can also be used as a guide to help HCPs deprescribe aspirin for primary prevention when appropriate.
One of the limitations of this study is that bleeding events that occurred outside of the FHCC were unable to be assessed unless the HCP specifically commented on the bleeding event in the chart. This could potentially underestimate the bleeding events seen in this study. Another limitation is that the bleeding risk for patients who were not on aspirin was not assessed. There was no comparison group to ascertain whether the bleeding risk was higher in the aspirin group compared with a no aspirin group. However, many of the major clinical trials saw an increased risk of bleeding in the aspirin group compared with placebo.
Conclusions
Aspirin therapy for secondary prevention remains an important part of treatment. Aspirin therapy for primary prevention may be appropriate for patients with a possible ASCVD risk. The therapy may be inappropriate in cases where patients have an increased bleeding risk and low or no ASCVD risk. It is important to continuously assess the need for aspirin therapy for patients in the setting of primary prevention. Common factors seen in this study to warrant deprescribing aspirin for primary prevention include patients aged > 70 years, concurrent use of medications that increase bleeding risk, and patients with CKD. By assessing ASCVD risk as well as bleeding risk and having a risk/benefit discussion between the HCP and patient, aspirin used for primary prevention can be appropriately deprescribed when the risks of bleeding outweigh the benefits.
Acknowledgments
The authors thank the Captain James A. Lovell Federal Health Care Center research committee (Hong-Yen Vi, PharmD, BCPS; Shaiza Khan, PharmD, BCPS; Yinka Alaka, BPharm, PharmD; Jennifer Kwon, PharmD, BCOP) and coinvestigator Aeman Choudhury, PharmD, BCPS, BCACP.
Aspirin is an antiplatelet agent that binds irreversibly to COX-1 and COX-2 enzymes, which results in decreased prostaglandin and thromboxane A2 production and inhibition of platelet aggregation. Aspirin often is used for its antipyretic, analgesic, and antiplatelet properties. Its use in cardiovascular disease (CVD) has been studied extensively over the past few decades, and recent data are changing the framework for aspirin use in primary prevention of atherosclerotic cardiovascular disease (ASCVD). Primary prevention refers to efforts to prevent the incidence of cardiovascular events, whereas secondary prevention refers to efforts to prevent a cardiovascular event after one has occurred.1 This differentiation is important as it guides the course of treatment.
Three trials published in 2018 evaluated aspirin use in primary prevention of ASCVD. The ASCEND trial evaluated aspirin use for primary prevention of ASCVD in patients with diabetes mellitus (DM). This study concluded that although aspirin prevented serious vascular events in patients with DM, the benefit observed was largely counteracted by the bleeding hazard.2 The ARRIVE trial evaluated aspirin use for primary prevention in patients with a moderate CVD risk. The study concluded that aspirin use in patients at moderate risk of CVD could not be assessed due to the low incidence rate of CVD; however, the study concluded that aspirin did not reduce the incidence of cardiovascular events for patients at low CVD risk and that aspirin caused more mild gastrointestinal bleeds compared with placebo.3 The ASPREE trial evaluated aspirin use for primary prevention in patients aged > 70 years to determine whether its use prolonged a healthy lifespan. This trial concluded that patients who received daily aspirin were at a higher risk of major hemorrhage and that aspirin did not diminish CVD risk compared with placebo.4
These studies led to a paradigm shift in therapy to reevaluate aspirin use for primary prevention. Current indications for aspirin include secondary prevention of ASCVD (ie, myocardial infarction [MI], coronary artery bypass graft, transient ischemic attack [TIA], and stroke), venous thromboembolism prophylaxis in the setting of orthopedic surgery, or valvular disease with replacement and analgesia. It is important to note that certain clinical circumstances may warrant aspirin use for primary prevention of ASCVD on a patient-specific basis, and this decision should be made using a risk/benefit analysis with the patient.
In April 2022, the US Preventive Services Task Force (USPSTF) recommended against using low-dose aspirin for primary prevention of ASCVD in individuals aged ≥ 60 years. The USPSTF noted that for patients who have a ≥ 10%, 10-year CVD risk, the decision to initiate aspirin should be based on a risk/benefit discussion and may be beneficial in certain patient populations.5A 2019 National Heart, Lung, and Blood Institute survey found that 29 million Americans used aspirin for primary prevention of ASCVD, and 6.6 million of these Americans used aspirin for primary prevention without the recommendation of a health care professional (HCP). Almost half of these individuals were aged > 70 years and, therefore, at an increased risk for bleeding.6 With the recent studies and changes in guidelines highlighting a higher risk rather than benefit with the use of aspirin for primary prevention, the current use of aspirin for primary prevention in the United States needs to be readdressed.
HCPs should assess the appropriateness of aspirin use in their patients to ensure that the risks of aspirin do not outweigh the benefits. Pharmacists can play a vital role in the assessment of aspirin for primary prevention during patient visits and make recommendations to primary care practitioners to deprescribe aspirin when appropriate.
Methods
The objective of this study was to evaluate the appropriateness of aspirin therapy in patient aligned care team (PACT) clinics at the Captain James A. Lovell Federal Health Care Center (FHCC) in North Chicago, Illinois. The PACT clinics are a category of clinics that include all the primary care clinics at FHCC.
The primary outcome of this study was to determine the percentage of patients inappropriately on aspirin therapy. To assess the inappropriate use of aspirin, relevant history of ASCVD was collected. Patients were divided into 3 groups: those with a history of ASCVD, those with no risk factors or history of ASCVD, and those with risk factors and no history of ASCVD. Patients were then categorized for their indication for aspirin use, which included either primary or secondary prevention of ASCVD. Patients were categorized into the primary prevention group if they had no history of ASCVD, whereas patients with a history of ASCVD were placed into the secondary prevention group.
ASCVD was defined as patients with acute coronary syndrome (ACS), history of MI, stable or unstable angina, coronary or other arterial revascularization, stroke, TIA, or peripheral artery disease (PAD), including aortic aneurysm (all with an atherosclerotic origin). Possible ASCVD risk was defined as patients with DM with a major risk factor (family history of premature ASCVD, hypertension, dyslipidemia, smoking, chronic kidney disease [CKD]/albuminuria) or patients diagnosed with coronary artery disease without an event. The percentage of patients followed by a PACT pharmacist, the number of pharmacist follow-up visits during the study period, and the date of the first 81-mg aspirin pharmacy order that was filled at FHCC were also collected.
The secondary outcome of this study focused on patients who were using aspirin for primary prevention and assessed potential reasons that may warrant deprescribing aspirin therapy. One reason for deprescribing is that aspirin may not be indicated for some patients, including those with DM without cardiovascular complications, patients aged > 70 years, and/or patients with CKD (defined as estimated glomerular filtration rate < 60 mL/min). Another reason for deprescribing is contraindication, which included patients with coagulopathy, thrombocytopenia (defined as platelet count < 150,000 mL), a history of gastrointestinal bleeding, peptic ulcer disease or other major bleeds, and/or consistent use of medications that increase bleeding risk (such as nonsteroidal anti-inflammatory agents, steroids, or anticoagulants) for > 14 days.
The safety outcome of this study assessed bleeding events while on aspirin therapy. All patients were categorized depending on if they had a major, minor, or no bleeding event while on aspirin therapy. Hemorrhagic stroke, symptomatic intracranial bleeding, bleeds located in other critical sites or organs (intracranial, intraspinal, intraocular, retroperitoneal, intra‐articular or pericardial), bleeds causing hemodynamic instability requiring vasopressors, bleeds causing a > 2 g/dL hemoglobin drop since initiation of aspirin therapy, severe extracranial bleeding requiring transfusion or hospitalization, fatal bleeding, or bleeds requiring > 2 units of red blood cell transfusion were considered major bleeding events. Minor bleeding events were any events that did not meet the criteria for major bleeding, including bruising, bleeding gums, epistaxis, hemorrhoidal bleeds, and bleeding that did not require intervention or treatment.7
Patients were included if they were aged > 18 years, had an active prescription for 81-mg aspirin tablet on September 30, 2021, and were seen in FHCC PACT clinics or at affiliated community-based outpatient centers. Other doses of aspirin were excluded as the 81-mg dose is the standard dose for primary prevention of ASCVD in the United States. US Department of Defense patients, home-based primary care patients, and community living center patients were excluded in this study. Patients with an aspirin prescription from a non–US Department of Veterans Affairs (VA) facility and patients on aspirin for reasons other than cardiovascular protection (such as pain, fever, etc) also were excluded from this study.
Data were collected from the FHCC electronic health record. A list was generated to include all active prescriptions for aspirin filled at FHCC as of September 30, 2021. Data were reviewed before this date to capture primary and secondary outcomes. No information was gathered from the chart after that date. This project was approved by the Edward Hines, Jr. VA Hospital Institutional Review Board. The primary and secondary outcomes were reported using descriptive statistics.
Results
This study reviewed 140 patient records and 105 patients met inclusion criteria.
For the primary endpoint, 53 patients (50%) were on aspirin for secondary prevention and 52 (50%) were on aspirin for primary prevention. Of the 105 patients included in the study, 31 (30%) had a possible ASCVD risk and were taking aspirin for primary prevention, while 21 (20%) had no ASCVD and were taking aspirin for primary prevention. Of the 52 patients on aspirin for primary prevention, 31 patients (60%) had a possible risk for ASCVD. Of the 52 patients in the primary prevention group, 15 (29%) were followed by a pharmacist, and the average number of follow-up appointments was 4.
The secondary endpoint focused on patients taking aspirin for primary prevention and the factors that may warrant deprescribing aspirin. Of the 52 patients on aspirin for primary prevention, 25 patients were aged > 70 years, 15 patients were concurrently taking medications that may increase bleeding risk,
For the entire study group, 6 patients (6%) experienced a major bleeding event while on aspirin, 46 (44%) experienced a minor bleeding event while on aspirin, and 53 (50%) experienced no bleeding events while on aspirin. Of the 6 patients who experienced major bleeding events, 4 were on aspirin for secondary prevention, and 2 were on aspirin for primary prevention with ASCVD risk factors. The major bleeding events included 4 gastrointestinal bleeds, 1 intracranial hemorrhage, and 1 hemorrhagic stroke. Of the 46 who experienced minor bleeding events, 20 patients were on aspirin for primary prevention; 11 of those patients had possible ASCVD risk factors and 9 had no documented ASCVD. The minor bleeding events included hematuria, epistaxis, bleeding scabs, and dental bleeding.
Discussion
The majority of patients in this study were on aspirin appropriately. Indications deemed appropriate for aspirin therapy include secondary prevention and primary prevention with a possible ASCVD risk. About 20% of the total patient population in this study was taking aspirin for primary prevention with no ASCVD risk. For these patients, the risk of bleeding likely outweighs the benefits of aspirin therapy as patients are at low risk for ASCVD; therefore, aspirin therapy is likely inappropriate in this patient population. These patients may be unnecessarily at an increased risk for bleeding and may benefit from deprescribing aspirin. For the safety of patients, HCPs should be continuously assessing the appropriateness of aspirin for primary prevention and deprescribing when necessary.
About one-third of the patients using aspirin for primary prevention were followed by a pharmacist. Pharmacists can play a key role in deprescribing aspirin for primary prevention when aspirin use is deemed inappropriate. About 30% of the total patient population in this study was on aspirin for primary prevention with possible ASCVD risk. This patient population may benefit from aspirin therapy as they are at a higher risk for ASCVD. For these patients, a risk/benefit discussion is necessary to determine the appropriateness of aspirin for primary prevention. This risk/benefit discussion should be a continuous conversation between patients and HCPs as different factors such as age and changes in comorbid conditions and medications may increase bleeding risk.
The secondary endpoint focused on patients taking aspirin for primary prevention and the factors that may warrant deprescribing aspirin. The most common factors seen in this study included patients who were aged > 70 years, patients who were concurrently taking medications that may increase bleeding risk, and patients with CKD. All of these factors increase bleeding risk, making the risks potentially outweigh the benefits of aspirin for primary prevention. These factors should be the primary focus when assessing patients on aspirin for primary prevention to promote deprescribing aspirin if deemed appropriate as they were the most prevalent in this study.
The safety endpoints focused on bleeding events as a whole as well as the bleeding events seen in the primary prevention group. There were 2 major bleeding events and 20 minor bleeding events in the primary prevention group. The number of bleeding events both major and minor further shows the need for a continuous risk/benefit discussion between patients and HCPs on continued aspirin use for primary prevention. The bleeding risk with aspirin is prevalent. HCPs should continue to assess for factors that increase the bleeding risk that may warrant deprescribing aspirin to prevent future bleeding events in this patient population.
Strengths and Limitations
As there have been recent updates to guidelines on the use of aspirin for primary prevention, a strength of this study is that it evaluates a topic that is relevant in health care. Another strength of this study is that it focuses on specific patient factors that HCPs can assess when determining whether aspirin for primary prevention is appropriate in their patients. These specific patient factors can also be used as a guide to help HCPs deprescribe aspirin for primary prevention when appropriate.
One of the limitations of this study is that bleeding events that occurred outside of the FHCC were unable to be assessed unless the HCP specifically commented on the bleeding event in the chart. This could potentially underestimate the bleeding events seen in this study. Another limitation is that the bleeding risk for patients who were not on aspirin was not assessed. There was no comparison group to ascertain whether the bleeding risk was higher in the aspirin group compared with a no aspirin group. However, many of the major clinical trials saw an increased risk of bleeding in the aspirin group compared with placebo.
Conclusions
Aspirin therapy for secondary prevention remains an important part of treatment. Aspirin therapy for primary prevention may be appropriate for patients with a possible ASCVD risk. The therapy may be inappropriate in cases where patients have an increased bleeding risk and low or no ASCVD risk. It is important to continuously assess the need for aspirin therapy for patients in the setting of primary prevention. Common factors seen in this study to warrant deprescribing aspirin for primary prevention include patients aged > 70 years, concurrent use of medications that increase bleeding risk, and patients with CKD. By assessing ASCVD risk as well as bleeding risk and having a risk/benefit discussion between the HCP and patient, aspirin used for primary prevention can be appropriately deprescribed when the risks of bleeding outweigh the benefits.
Acknowledgments
The authors thank the Captain James A. Lovell Federal Health Care Center research committee (Hong-Yen Vi, PharmD, BCPS; Shaiza Khan, PharmD, BCPS; Yinka Alaka, BPharm, PharmD; Jennifer Kwon, PharmD, BCOP) and coinvestigator Aeman Choudhury, PharmD, BCPS, BCACP.
1. Warner TD, Nylander S, Whatling C. Anti-platelet therapy: cyclo-oxygenase inhibition and the use of aspirin with particular regard to dual anti-platelet therapy. Br J Clin Pharmacol. 2011;72(4):619-633. doi:10.1111/j.1365-2125.2011.03943.x
2. ASCEND Study Collaborative Group, Bowman L, Mafham M, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379(16):1529-1539. doi:10.1056/NEJMoa1804988
3. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392(10152):1036-1046. doi:10.1016/S0140-6736(18)31924-X
4. McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379(16):1509-1518. doi:10.1056/NEJMoa1805819
5. US Preventive Services Task Force, Davidson KW, Barry MJ, et al. Aspirin use to prevent cardiovascular disease: US Preventive Services Task Force Recommendation Statement. JAMA. 2022;327(16):1577-1584. doi:10.1001/jama.2022.4983
6. Murphy E, McEvoy JW. Does stopping aspirin differ fundamentally from not starting aspirin in the primary prevention of cardiovascular disease among older adults? Ann Intern Med. 2022;175(5):757-758. doi:10.7326/M22-0550
7. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694. doi:10.1111/j.1538-7836.2005.01204.
1. Warner TD, Nylander S, Whatling C. Anti-platelet therapy: cyclo-oxygenase inhibition and the use of aspirin with particular regard to dual anti-platelet therapy. Br J Clin Pharmacol. 2011;72(4):619-633. doi:10.1111/j.1365-2125.2011.03943.x
2. ASCEND Study Collaborative Group, Bowman L, Mafham M, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379(16):1529-1539. doi:10.1056/NEJMoa1804988
3. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392(10152):1036-1046. doi:10.1016/S0140-6736(18)31924-X
4. McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379(16):1509-1518. doi:10.1056/NEJMoa1805819
5. US Preventive Services Task Force, Davidson KW, Barry MJ, et al. Aspirin use to prevent cardiovascular disease: US Preventive Services Task Force Recommendation Statement. JAMA. 2022;327(16):1577-1584. doi:10.1001/jama.2022.4983
6. Murphy E, McEvoy JW. Does stopping aspirin differ fundamentally from not starting aspirin in the primary prevention of cardiovascular disease among older adults? Ann Intern Med. 2022;175(5):757-758. doi:10.7326/M22-0550
7. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694. doi:10.1111/j.1538-7836.2005.01204.
Dermatology Articles in Preprint Servers: A Cross-sectional Study
To the Editor:
Preprint servers allow researchers to post manuscripts before publication in peer-reviewed journals. As of January 2022, 41 public preprint servers accepted medicine/science submissions.1 We sought to analyze characteristics of dermatology manuscripts in preprint servers and assess preprint publication policies in top dermatology journals.
Thirty-five biology/health sciences preprint servers1 were searched (March 3 to March 24, 2021) with keywords dermatology, skin, and cutaneous. Preprint server, preprint post date, location, metrics, journal, impact factor (IF), and journal publication date were recorded. Preprint policies of the top 20 dermatology journals—determined by impact factor of the journal (https://www.scimagojr.com/)—were reviewed. Two-tailed t tests and χ2 tests were performed (P<.05).
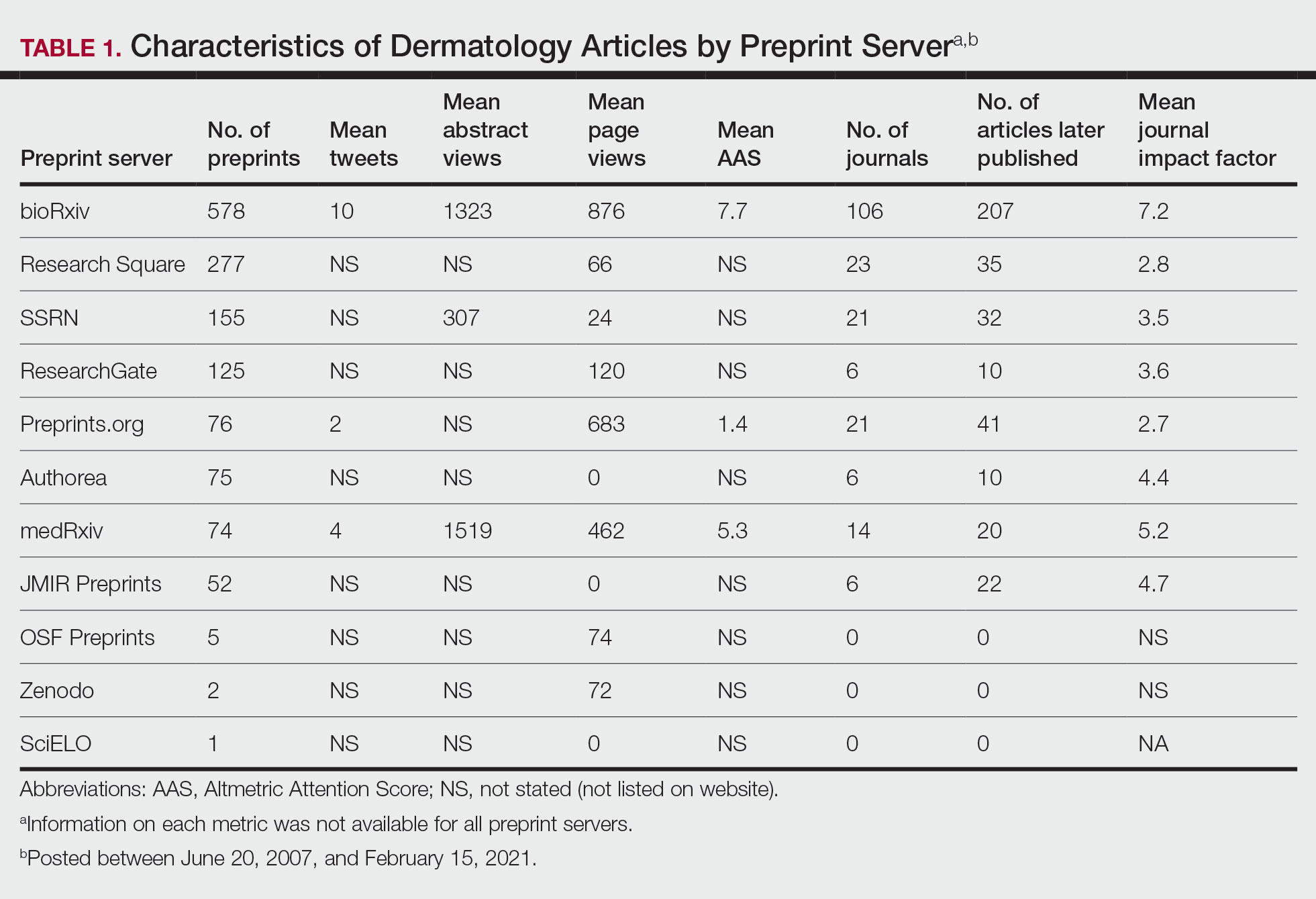
A total of 1420 articles were posted to 11 preprint servers between June 20, 2007, and February 15, 2021 (Table 1); 377 (27%) were published in peer-reviewed journals, with 350 (93%) of those published within 1 year of preprint post. Preprints were published in 203 journals with a mean IF of 6.2. Growth in preprint posts by year (2007-2020) was exponential (R2=0.78)(Figure). On average, preprints were viewed 424 times (Table 2), with published preprints viewed more often than unpublished preprints (596 vs 362 views)(P<.001). Only 23 of 786 (3%) preprints with comments enabled had feedback. Among the top 20 dermatology journals, 18 (90%) allowed preprints, 1 (5%) evaluated case by case, and 1 (5%) prohibited preprints.
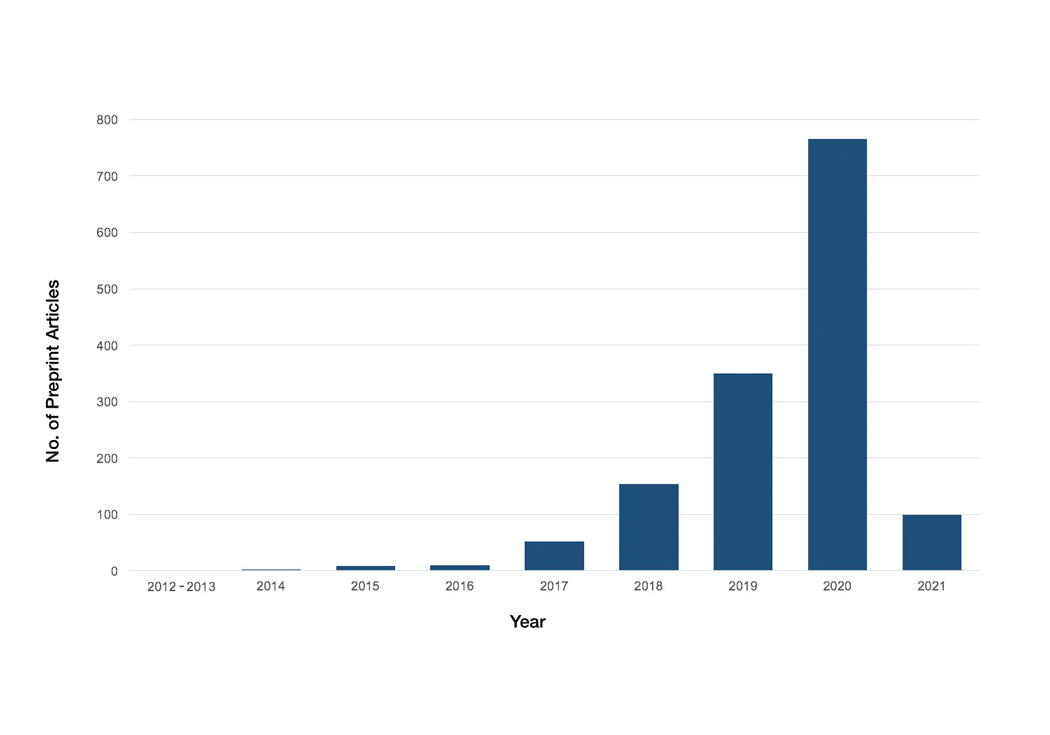
Our study showed exponential growth in dermatology preprints, a low proportion published in peer-reviewed journals with high IFs, and a substantial number of page views for both published and unpublished preprints. Very few preprints had feedback. We found that most of the top 20 dermatology journals accept preprints. An analysis of 61 dermatology articles in medRxiv found only 51% (31/61) of articles were subsequently published.2 The low rate of publication may be due to the quality of preprints that do not meet criteria to be published following peer review.
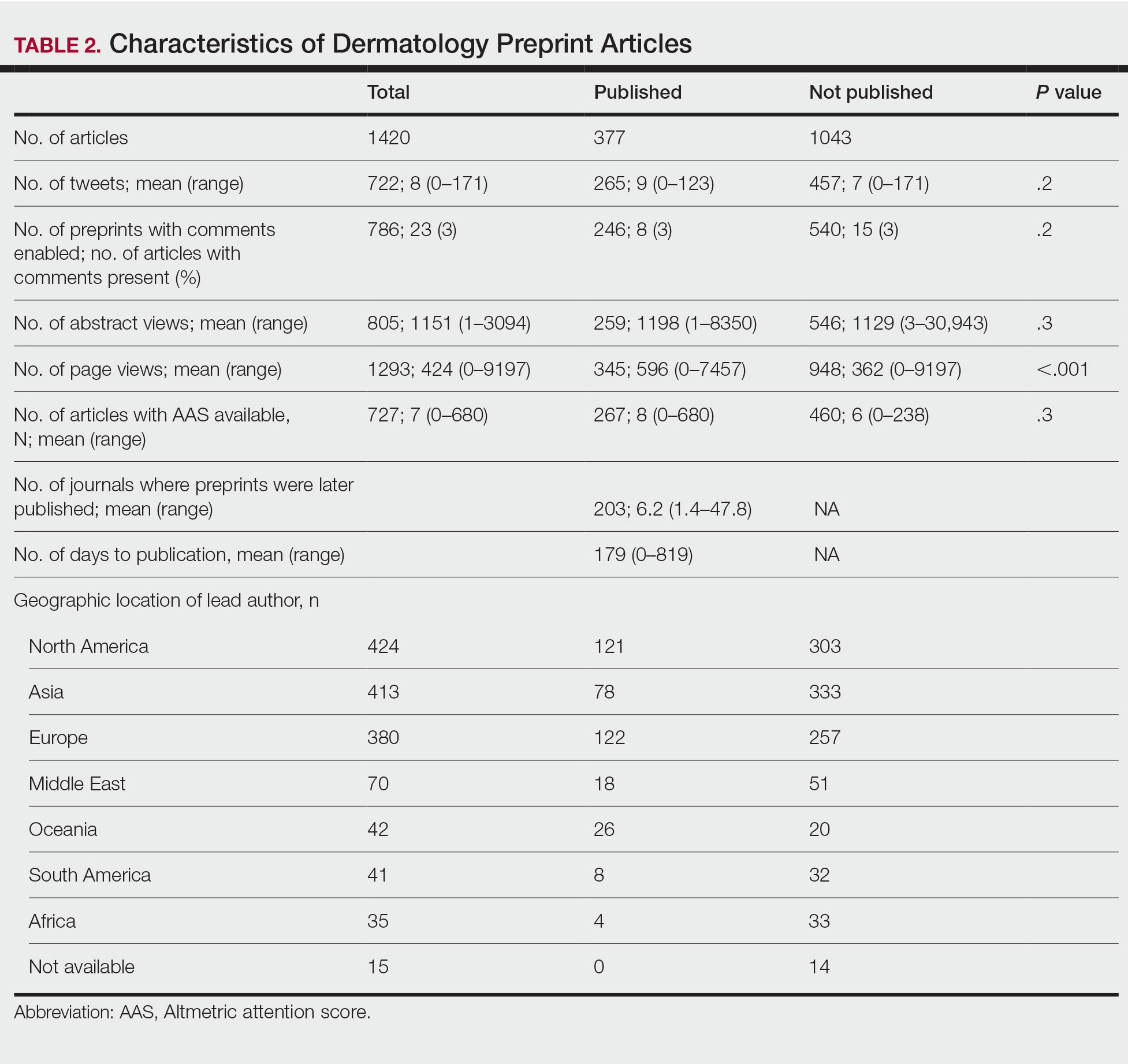
Preprint servers are fairly novel, with a majority launched within the last 5 years.1 The goal of preprints is to claim conception of an idea, solicit feedback prior to submission for peer review, and expedite research distribution.3 Because preprints are uploaded without peer review, manuscripts may lack quality and accuracy. An analysis of 57 of thelargest preprint servers found that few provided guidelines on authorship, image manipulation, or reporting of study limitations; however, most preprint servers do perform some screening.4 medRxiv requires full scientific research reports and absence of obscenity, plagiarism, and patient identifiers. In its first year, medRxiv rejected 34% of 176 submissios; reasons were not disclosed.5
The low rate of on-site comments suggests that preprint servers may not be effective for obtaining feedback to improve dermatology manuscripts prior to journal submission. Almost all of the top 20 dermatologyjournals accept preprints. Therefore, dermatologists may use these preprint servers to assert project ideas and disseminate research quickly and freely but may not receive constructive criticism.
Our study is subject to several limitations. Although our search was extensive, it is possible manuscripts were missed. Article metrics also were not available on all servers, and we could not account for accepted articles that were not yet indexed.
There has been a surge in posting of dermatology preprints in recent years. Preprints have not been peer reviewed, and data should be corroborated before incorporating new diagnostics or treatments into clinical practice. Utilization of preprint servers by dermatologists is increasing, but because the impact is still unknown, further studies on accuracy and reliability of preprints are warranted.
1. List of preprint servers: policies and practices across platforms. ASAPbio website. Accessed January 25, 2023. https://asapbio.org/preprint-servers
2. Jia JL, Hua VJ, Sarin KY. Journal attitudes and outcomes of preprints in dermatology. Br J Dermatol. 2021;185:230-232.
3. Chiarelli A, Johnson R, Richens E, et al. Accelerating scholarly communication: the transformative role of preprints. Copyright, Fair Use, Scholarly Communication, etc. 127. September 20, 2019. Accessed January 18, 2023. https://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1128&context=scholcom
4. Malicki M, Jeroncic A, Riet GT, et al. Preprint servers’ policies, submission requirements, and transparency in reporting and research integrity recommendations. JAMA. 2020;324:1901-1903.
5. Krumholz HM, Bloom T, Sever R, et al. Submissions and downloads of preprints in the first year of medRxiv. JAMA. 2020;324:1903-1905.
To the Editor:
Preprint servers allow researchers to post manuscripts before publication in peer-reviewed journals. As of January 2022, 41 public preprint servers accepted medicine/science submissions.1 We sought to analyze characteristics of dermatology manuscripts in preprint servers and assess preprint publication policies in top dermatology journals.
Thirty-five biology/health sciences preprint servers1 were searched (March 3 to March 24, 2021) with keywords dermatology, skin, and cutaneous. Preprint server, preprint post date, location, metrics, journal, impact factor (IF), and journal publication date were recorded. Preprint policies of the top 20 dermatology journals—determined by impact factor of the journal (https://www.scimagojr.com/)—were reviewed. Two-tailed t tests and χ2 tests were performed (P<.05).

A total of 1420 articles were posted to 11 preprint servers between June 20, 2007, and February 15, 2021 (Table 1); 377 (27%) were published in peer-reviewed journals, with 350 (93%) of those published within 1 year of preprint post. Preprints were published in 203 journals with a mean IF of 6.2. Growth in preprint posts by year (2007-2020) was exponential (R2=0.78)(Figure). On average, preprints were viewed 424 times (Table 2), with published preprints viewed more often than unpublished preprints (596 vs 362 views)(P<.001). Only 23 of 786 (3%) preprints with comments enabled had feedback. Among the top 20 dermatology journals, 18 (90%) allowed preprints, 1 (5%) evaluated case by case, and 1 (5%) prohibited preprints.

Our study showed exponential growth in dermatology preprints, a low proportion published in peer-reviewed journals with high IFs, and a substantial number of page views for both published and unpublished preprints. Very few preprints had feedback. We found that most of the top 20 dermatology journals accept preprints. An analysis of 61 dermatology articles in medRxiv found only 51% (31/61) of articles were subsequently published.2 The low rate of publication may be due to the quality of preprints that do not meet criteria to be published following peer review.

Preprint servers are fairly novel, with a majority launched within the last 5 years.1 The goal of preprints is to claim conception of an idea, solicit feedback prior to submission for peer review, and expedite research distribution.3 Because preprints are uploaded without peer review, manuscripts may lack quality and accuracy. An analysis of 57 of thelargest preprint servers found that few provided guidelines on authorship, image manipulation, or reporting of study limitations; however, most preprint servers do perform some screening.4 medRxiv requires full scientific research reports and absence of obscenity, plagiarism, and patient identifiers. In its first year, medRxiv rejected 34% of 176 submissios; reasons were not disclosed.5
The low rate of on-site comments suggests that preprint servers may not be effective for obtaining feedback to improve dermatology manuscripts prior to journal submission. Almost all of the top 20 dermatologyjournals accept preprints. Therefore, dermatologists may use these preprint servers to assert project ideas and disseminate research quickly and freely but may not receive constructive criticism.
Our study is subject to several limitations. Although our search was extensive, it is possible manuscripts were missed. Article metrics also were not available on all servers, and we could not account for accepted articles that were not yet indexed.
There has been a surge in posting of dermatology preprints in recent years. Preprints have not been peer reviewed, and data should be corroborated before incorporating new diagnostics or treatments into clinical practice. Utilization of preprint servers by dermatologists is increasing, but because the impact is still unknown, further studies on accuracy and reliability of preprints are warranted.
To the Editor:
Preprint servers allow researchers to post manuscripts before publication in peer-reviewed journals. As of January 2022, 41 public preprint servers accepted medicine/science submissions.1 We sought to analyze characteristics of dermatology manuscripts in preprint servers and assess preprint publication policies in top dermatology journals.
Thirty-five biology/health sciences preprint servers1 were searched (March 3 to March 24, 2021) with keywords dermatology, skin, and cutaneous. Preprint server, preprint post date, location, metrics, journal, impact factor (IF), and journal publication date were recorded. Preprint policies of the top 20 dermatology journals—determined by impact factor of the journal (https://www.scimagojr.com/)—were reviewed. Two-tailed t tests and χ2 tests were performed (P<.05).

A total of 1420 articles were posted to 11 preprint servers between June 20, 2007, and February 15, 2021 (Table 1); 377 (27%) were published in peer-reviewed journals, with 350 (93%) of those published within 1 year of preprint post. Preprints were published in 203 journals with a mean IF of 6.2. Growth in preprint posts by year (2007-2020) was exponential (R2=0.78)(Figure). On average, preprints were viewed 424 times (Table 2), with published preprints viewed more often than unpublished preprints (596 vs 362 views)(P<.001). Only 23 of 786 (3%) preprints with comments enabled had feedback. Among the top 20 dermatology journals, 18 (90%) allowed preprints, 1 (5%) evaluated case by case, and 1 (5%) prohibited preprints.

Our study showed exponential growth in dermatology preprints, a low proportion published in peer-reviewed journals with high IFs, and a substantial number of page views for both published and unpublished preprints. Very few preprints had feedback. We found that most of the top 20 dermatology journals accept preprints. An analysis of 61 dermatology articles in medRxiv found only 51% (31/61) of articles were subsequently published.2 The low rate of publication may be due to the quality of preprints that do not meet criteria to be published following peer review.

Preprint servers are fairly novel, with a majority launched within the last 5 years.1 The goal of preprints is to claim conception of an idea, solicit feedback prior to submission for peer review, and expedite research distribution.3 Because preprints are uploaded without peer review, manuscripts may lack quality and accuracy. An analysis of 57 of thelargest preprint servers found that few provided guidelines on authorship, image manipulation, or reporting of study limitations; however, most preprint servers do perform some screening.4 medRxiv requires full scientific research reports and absence of obscenity, plagiarism, and patient identifiers. In its first year, medRxiv rejected 34% of 176 submissios; reasons were not disclosed.5
The low rate of on-site comments suggests that preprint servers may not be effective for obtaining feedback to improve dermatology manuscripts prior to journal submission. Almost all of the top 20 dermatologyjournals accept preprints. Therefore, dermatologists may use these preprint servers to assert project ideas and disseminate research quickly and freely but may not receive constructive criticism.
Our study is subject to several limitations. Although our search was extensive, it is possible manuscripts were missed. Article metrics also were not available on all servers, and we could not account for accepted articles that were not yet indexed.
There has been a surge in posting of dermatology preprints in recent years. Preprints have not been peer reviewed, and data should be corroborated before incorporating new diagnostics or treatments into clinical practice. Utilization of preprint servers by dermatologists is increasing, but because the impact is still unknown, further studies on accuracy and reliability of preprints are warranted.
1. List of preprint servers: policies and practices across platforms. ASAPbio website. Accessed January 25, 2023. https://asapbio.org/preprint-servers
2. Jia JL, Hua VJ, Sarin KY. Journal attitudes and outcomes of preprints in dermatology. Br J Dermatol. 2021;185:230-232.
3. Chiarelli A, Johnson R, Richens E, et al. Accelerating scholarly communication: the transformative role of preprints. Copyright, Fair Use, Scholarly Communication, etc. 127. September 20, 2019. Accessed January 18, 2023. https://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1128&context=scholcom
4. Malicki M, Jeroncic A, Riet GT, et al. Preprint servers’ policies, submission requirements, and transparency in reporting and research integrity recommendations. JAMA. 2020;324:1901-1903.
5. Krumholz HM, Bloom T, Sever R, et al. Submissions and downloads of preprints in the first year of medRxiv. JAMA. 2020;324:1903-1905.
1. List of preprint servers: policies and practices across platforms. ASAPbio website. Accessed January 25, 2023. https://asapbio.org/preprint-servers
2. Jia JL, Hua VJ, Sarin KY. Journal attitudes and outcomes of preprints in dermatology. Br J Dermatol. 2021;185:230-232.
3. Chiarelli A, Johnson R, Richens E, et al. Accelerating scholarly communication: the transformative role of preprints. Copyright, Fair Use, Scholarly Communication, etc. 127. September 20, 2019. Accessed January 18, 2023. https://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1128&context=scholcom
4. Malicki M, Jeroncic A, Riet GT, et al. Preprint servers’ policies, submission requirements, and transparency in reporting and research integrity recommendations. JAMA. 2020;324:1901-1903.
5. Krumholz HM, Bloom T, Sever R, et al. Submissions and downloads of preprints in the first year of medRxiv. JAMA. 2020;324:1903-1905.
PRACTICE POINTS
- Preprint servers allow researchers to post manuscripts before publication in peer-reviewed journals.
- The low rate of on-site comments suggests that preprint servers may not be effective for obtaining feedback to improve dermatology manuscripts prior to journal submission; therefore, dermatologists may use these servers to disseminate research quickly and freely but may not receive constructive criticism.
- Preprints have not been peer reviewed, and data should be corroborated before incorporating new diagnostics or treatments into clinical practice.
Adverse Effects of the COVID-19 Vaccine in Patients With Psoriasis
To the Editor:
Because the SARS-CoV-2 virus is constantly changing, routine vaccination to prevent COVID-19 infection is recommended. The messenger RNA (mRNA) vaccines from Pfizer-BioNTech and Moderna as well as the Ad26.COV2.S (Johnson & Johnson) and NVX-CoV2373 (Novavax) vaccines are the most commonly used COVID-19 vaccines in the United States. Adverse effects following vaccination against SARS-CoV-2 are well documented; recent studies report a small incidence of adverse effects in the general population, with most being minor (eg, headache, fever, muscle pain).1,2 Interestingly, reports of exacerbation of psoriasis and new-onset psoriasis following COVID-19 vaccination suggest a potential association.3,4 However, the literature investigating the vaccine adverse effect profile in this demographic is scarce. We examined the incidence of adverse effects from SARS-CoV-2 vaccines in patients with psoriasis.
This retrospective cohort study used the COVID-19 Research Database (https://covid19researchdatabase.org/) to examine the adverse effects following the first and second doses of the mRNA vaccines in patients with and without psoriasis. The sample size for the Ad26.COV2.S vaccine was too small to analyze.
Claims were evaluated from August to October 2021 for 2 diagnoses of psoriasis prior to January 1, 2020, using the International Classification of Diseases, Tenth Revision (ICD-10) code L40.9 to increase the positive predictive value and ensure that the diagnosis preceded the COVID-19 pandemic. Patients younger than 18 years and those who did not receive 2 doses of a SARS-CoV-2 vaccine were excluded. Controls who did not have a diagnosis of psoriasis were matched for age, sex, and hypertension at a 4:1 ratio. Hypertension represented the most common comorbidity that could feasibly be controlled for in this study population. Other comorbidities recorded included obesity, type 2 diabetes mellitus, congestive heart failure, asthma, chronic obstructive pulmonary disease, chronic ischemic heart disease, rhinitis, and chronic kidney disease.
Common adverse effects as long as 30 days after vaccination were identified using ICD-10 codes. Adverse effects of interest were anaphylactic reaction, initial encounter of adverse effect of viral vaccines, fever, allergic urticaria, weakness, altered mental status, malaise, allergic reaction, chest pain, symptoms involving circulatory or respiratory systems, localized rash, axillary lymphadenopathy, infection, and myocarditis.5 Poisson regression was performed using Stata 17 analytical software.
We identified 4273 patients with psoriasis and 17,092 controls who received mRNA COVID-19 vaccines (Table). Adjusted odds ratios (aORs) for doses 1 and 2 were calculated for each vaccine (eTable). Adverse effects with sufficient data to generate an aOR included weakness, altered mental status, malaise, chest pain, and symptoms involving the circulatory or respiratory system. The aORs for allergic urticaria and initial encounter of adverse effect of viral vaccines were only calculated for the Moderna mRNA vaccine due to low sample size.
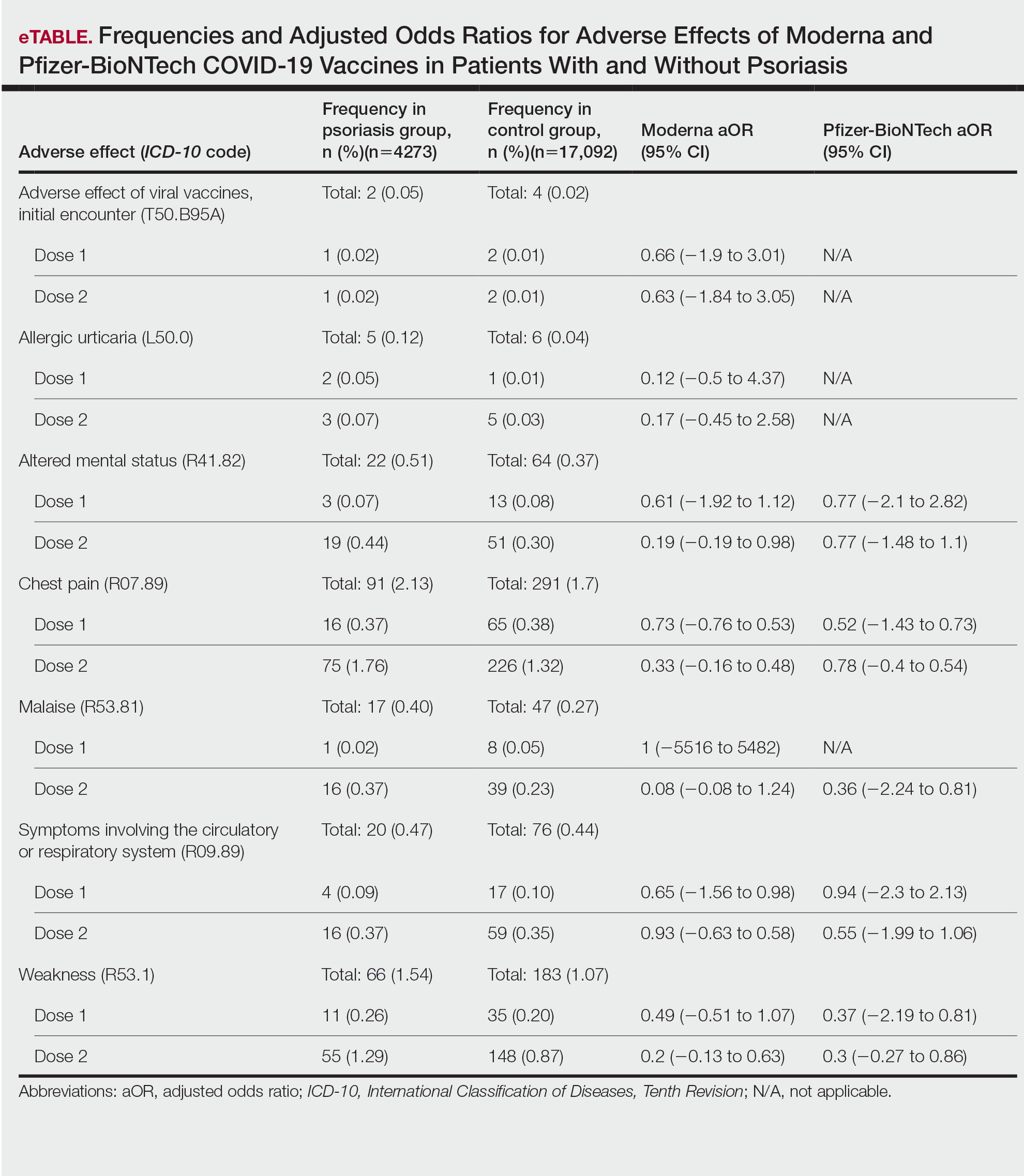
This study demonstrated that patients with psoriasis do not appear to have a significantly increased risk of adverse effects from mRNA SARS-CoV-2 vaccines. Although the ORs in this study were not significant, most recorded adverse effects demonstrated an aOR less than 1, suggesting that there might be a lower risk of certain adverse effects in psoriasis patients. This could be explained by the immunomodulatory effects of certain systemic psoriasis treatments that might influence the adverse effect presentation.

The study is limited by the lack of treatment data, small sample size, and the fact that it did not assess flares or worsening of psoriasis with the vaccines. Underreporting of adverse effects by patients and underdiagnosis of adverse effects secondary to SARS-CoV-2 vaccines due to its novel nature, incompletely understood consequences, and limited ICD-10 codes associated with adverse effects all contributed to the small sample size.
Our findings suggest that the risk for immediate adverse effects from the mRNA SARS-CoV-2 vaccines is not increased among psoriasis patients. However, the impact of immunomodulatory agents on vaccine efficacy and expected adverse effects should be investigated. As more individuals receive the COVID-19 vaccine, the adverse effect profile in patients with psoriasis is an important area of investigation.
- Singh A, Khillan R, Mishra Y, et al. The safety profile of COVID-19 vaccinations in the United States. Am J Infect Control. 2022;50:15-19. doi: 10.1016/j.ajic.2021.10.015
- Beatty AL, Peyser ND, Butcher XE, et al. Analysis of COVID-19 vaccine type and adverse effects following vaccination. JAMA Netw Open. 2021;4:e2140364. doi:10.1001/jamanetworkopen.2021.40364
- Bellinato F, Maurelli M, Gisondi P, et al. Cutaneous adverse reactions associated with SARS-CoV-2 vaccines. J Clin Med. 2021;10:5344. doi:10.3390/jcm10225344
- Elamin S, Hinds F, Tolland J. De novo generalized pustular psoriasis following Oxford-AstraZeneca COVID-19 vaccine. Clin Exp Dermatol. 2022;47:153-155. doi:10.1111/ced.14895
- Remer EE. Coding COVID-19 vaccination. ICD10monitor. Published March 2, 2021. Updated October 18, 2022. Accessed January 17, 2023. https://icd10monitor.medlearn.com/coding-covid-19-vaccination/
To the Editor:
Because the SARS-CoV-2 virus is constantly changing, routine vaccination to prevent COVID-19 infection is recommended. The messenger RNA (mRNA) vaccines from Pfizer-BioNTech and Moderna as well as the Ad26.COV2.S (Johnson & Johnson) and NVX-CoV2373 (Novavax) vaccines are the most commonly used COVID-19 vaccines in the United States. Adverse effects following vaccination against SARS-CoV-2 are well documented; recent studies report a small incidence of adverse effects in the general population, with most being minor (eg, headache, fever, muscle pain).1,2 Interestingly, reports of exacerbation of psoriasis and new-onset psoriasis following COVID-19 vaccination suggest a potential association.3,4 However, the literature investigating the vaccine adverse effect profile in this demographic is scarce. We examined the incidence of adverse effects from SARS-CoV-2 vaccines in patients with psoriasis.
This retrospective cohort study used the COVID-19 Research Database (https://covid19researchdatabase.org/) to examine the adverse effects following the first and second doses of the mRNA vaccines in patients with and without psoriasis. The sample size for the Ad26.COV2.S vaccine was too small to analyze.
Claims were evaluated from August to October 2021 for 2 diagnoses of psoriasis prior to January 1, 2020, using the International Classification of Diseases, Tenth Revision (ICD-10) code L40.9 to increase the positive predictive value and ensure that the diagnosis preceded the COVID-19 pandemic. Patients younger than 18 years and those who did not receive 2 doses of a SARS-CoV-2 vaccine were excluded. Controls who did not have a diagnosis of psoriasis were matched for age, sex, and hypertension at a 4:1 ratio. Hypertension represented the most common comorbidity that could feasibly be controlled for in this study population. Other comorbidities recorded included obesity, type 2 diabetes mellitus, congestive heart failure, asthma, chronic obstructive pulmonary disease, chronic ischemic heart disease, rhinitis, and chronic kidney disease.
Common adverse effects as long as 30 days after vaccination were identified using ICD-10 codes. Adverse effects of interest were anaphylactic reaction, initial encounter of adverse effect of viral vaccines, fever, allergic urticaria, weakness, altered mental status, malaise, allergic reaction, chest pain, symptoms involving circulatory or respiratory systems, localized rash, axillary lymphadenopathy, infection, and myocarditis.5 Poisson regression was performed using Stata 17 analytical software.
We identified 4273 patients with psoriasis and 17,092 controls who received mRNA COVID-19 vaccines (Table). Adjusted odds ratios (aORs) for doses 1 and 2 were calculated for each vaccine (eTable). Adverse effects with sufficient data to generate an aOR included weakness, altered mental status, malaise, chest pain, and symptoms involving the circulatory or respiratory system. The aORs for allergic urticaria and initial encounter of adverse effect of viral vaccines were only calculated for the Moderna mRNA vaccine due to low sample size.

This study demonstrated that patients with psoriasis do not appear to have a significantly increased risk of adverse effects from mRNA SARS-CoV-2 vaccines. Although the ORs in this study were not significant, most recorded adverse effects demonstrated an aOR less than 1, suggesting that there might be a lower risk of certain adverse effects in psoriasis patients. This could be explained by the immunomodulatory effects of certain systemic psoriasis treatments that might influence the adverse effect presentation.

The study is limited by the lack of treatment data, small sample size, and the fact that it did not assess flares or worsening of psoriasis with the vaccines. Underreporting of adverse effects by patients and underdiagnosis of adverse effects secondary to SARS-CoV-2 vaccines due to its novel nature, incompletely understood consequences, and limited ICD-10 codes associated with adverse effects all contributed to the small sample size.
Our findings suggest that the risk for immediate adverse effects from the mRNA SARS-CoV-2 vaccines is not increased among psoriasis patients. However, the impact of immunomodulatory agents on vaccine efficacy and expected adverse effects should be investigated. As more individuals receive the COVID-19 vaccine, the adverse effect profile in patients with psoriasis is an important area of investigation.
To the Editor:
Because the SARS-CoV-2 virus is constantly changing, routine vaccination to prevent COVID-19 infection is recommended. The messenger RNA (mRNA) vaccines from Pfizer-BioNTech and Moderna as well as the Ad26.COV2.S (Johnson & Johnson) and NVX-CoV2373 (Novavax) vaccines are the most commonly used COVID-19 vaccines in the United States. Adverse effects following vaccination against SARS-CoV-2 are well documented; recent studies report a small incidence of adverse effects in the general population, with most being minor (eg, headache, fever, muscle pain).1,2 Interestingly, reports of exacerbation of psoriasis and new-onset psoriasis following COVID-19 vaccination suggest a potential association.3,4 However, the literature investigating the vaccine adverse effect profile in this demographic is scarce. We examined the incidence of adverse effects from SARS-CoV-2 vaccines in patients with psoriasis.
This retrospective cohort study used the COVID-19 Research Database (https://covid19researchdatabase.org/) to examine the adverse effects following the first and second doses of the mRNA vaccines in patients with and without psoriasis. The sample size for the Ad26.COV2.S vaccine was too small to analyze.
Claims were evaluated from August to October 2021 for 2 diagnoses of psoriasis prior to January 1, 2020, using the International Classification of Diseases, Tenth Revision (ICD-10) code L40.9 to increase the positive predictive value and ensure that the diagnosis preceded the COVID-19 pandemic. Patients younger than 18 years and those who did not receive 2 doses of a SARS-CoV-2 vaccine were excluded. Controls who did not have a diagnosis of psoriasis were matched for age, sex, and hypertension at a 4:1 ratio. Hypertension represented the most common comorbidity that could feasibly be controlled for in this study population. Other comorbidities recorded included obesity, type 2 diabetes mellitus, congestive heart failure, asthma, chronic obstructive pulmonary disease, chronic ischemic heart disease, rhinitis, and chronic kidney disease.
Common adverse effects as long as 30 days after vaccination were identified using ICD-10 codes. Adverse effects of interest were anaphylactic reaction, initial encounter of adverse effect of viral vaccines, fever, allergic urticaria, weakness, altered mental status, malaise, allergic reaction, chest pain, symptoms involving circulatory or respiratory systems, localized rash, axillary lymphadenopathy, infection, and myocarditis.5 Poisson regression was performed using Stata 17 analytical software.
We identified 4273 patients with psoriasis and 17,092 controls who received mRNA COVID-19 vaccines (Table). Adjusted odds ratios (aORs) for doses 1 and 2 were calculated for each vaccine (eTable). Adverse effects with sufficient data to generate an aOR included weakness, altered mental status, malaise, chest pain, and symptoms involving the circulatory or respiratory system. The aORs for allergic urticaria and initial encounter of adverse effect of viral vaccines were only calculated for the Moderna mRNA vaccine due to low sample size.

This study demonstrated that patients with psoriasis do not appear to have a significantly increased risk of adverse effects from mRNA SARS-CoV-2 vaccines. Although the ORs in this study were not significant, most recorded adverse effects demonstrated an aOR less than 1, suggesting that there might be a lower risk of certain adverse effects in psoriasis patients. This could be explained by the immunomodulatory effects of certain systemic psoriasis treatments that might influence the adverse effect presentation.

The study is limited by the lack of treatment data, small sample size, and the fact that it did not assess flares or worsening of psoriasis with the vaccines. Underreporting of adverse effects by patients and underdiagnosis of adverse effects secondary to SARS-CoV-2 vaccines due to its novel nature, incompletely understood consequences, and limited ICD-10 codes associated with adverse effects all contributed to the small sample size.
Our findings suggest that the risk for immediate adverse effects from the mRNA SARS-CoV-2 vaccines is not increased among psoriasis patients. However, the impact of immunomodulatory agents on vaccine efficacy and expected adverse effects should be investigated. As more individuals receive the COVID-19 vaccine, the adverse effect profile in patients with psoriasis is an important area of investigation.
- Singh A, Khillan R, Mishra Y, et al. The safety profile of COVID-19 vaccinations in the United States. Am J Infect Control. 2022;50:15-19. doi: 10.1016/j.ajic.2021.10.015
- Beatty AL, Peyser ND, Butcher XE, et al. Analysis of COVID-19 vaccine type and adverse effects following vaccination. JAMA Netw Open. 2021;4:e2140364. doi:10.1001/jamanetworkopen.2021.40364
- Bellinato F, Maurelli M, Gisondi P, et al. Cutaneous adverse reactions associated with SARS-CoV-2 vaccines. J Clin Med. 2021;10:5344. doi:10.3390/jcm10225344
- Elamin S, Hinds F, Tolland J. De novo generalized pustular psoriasis following Oxford-AstraZeneca COVID-19 vaccine. Clin Exp Dermatol. 2022;47:153-155. doi:10.1111/ced.14895
- Remer EE. Coding COVID-19 vaccination. ICD10monitor. Published March 2, 2021. Updated October 18, 2022. Accessed January 17, 2023. https://icd10monitor.medlearn.com/coding-covid-19-vaccination/
- Singh A, Khillan R, Mishra Y, et al. The safety profile of COVID-19 vaccinations in the United States. Am J Infect Control. 2022;50:15-19. doi: 10.1016/j.ajic.2021.10.015
- Beatty AL, Peyser ND, Butcher XE, et al. Analysis of COVID-19 vaccine type and adverse effects following vaccination. JAMA Netw Open. 2021;4:e2140364. doi:10.1001/jamanetworkopen.2021.40364
- Bellinato F, Maurelli M, Gisondi P, et al. Cutaneous adverse reactions associated with SARS-CoV-2 vaccines. J Clin Med. 2021;10:5344. doi:10.3390/jcm10225344
- Elamin S, Hinds F, Tolland J. De novo generalized pustular psoriasis following Oxford-AstraZeneca COVID-19 vaccine. Clin Exp Dermatol. 2022;47:153-155. doi:10.1111/ced.14895
- Remer EE. Coding COVID-19 vaccination. ICD10monitor. Published March 2, 2021. Updated October 18, 2022. Accessed January 17, 2023. https://icd10monitor.medlearn.com/coding-covid-19-vaccination/
PRACTICE POINTS
- Patients who have psoriasis do not appear to have an increased incidence of adverse effects from messenger RNA COVID-19 vaccines.
- Clinicians can safely recommend COVID-19 vaccines to patients who have psoriasis.
Dissociating Fibroepithelioma of Pinkus From Internal Malignancy: A Single-Center Retrospective Study
Fibroepithelioma of Pinkus (FeP), or Pinkus tumor, is a rare tumor with a presentation similar to benign neoplasms such as acrochordons and seborrheic keratoses. Classically, FeP presents as a nontender, solitary, flesh-colored, firm, dome-shaped papule or plaque with a predilection for the lumbosacral region rather than sun-exposed areas. This tumor typically develops in fair-skinned older adults, more often in females.1
The association between cutaneous lesions and internal malignancies is well known to include dermatoses such as erythema repens in patients with lung cancer, or tripe palms and acanthosis nigricans in patients with gastrointestinal malignancy. Outside of paraneoplastic presentations, many syndromes have unique constellations of clinical findings that require the clinician to investigate for internal malignancy. Cancer-associated genodermatoses such as Birt-Hogg-Dubé, neurofibromatosis, and Cowden syndrome have key findings to alert the provider of potential internal malignancies.2 Given the rarity and relative novelty of FeP, few studies have been performed that evaluate for an association with internal malignancies.
There potentially is a common pathophysiologic mechanism between FeP and other benign and malignant tumors. Some have noted a possible common embryonic origin, such as Merkel cells, and even a common gene mutation involving tumor protein p53 or PTCH1 gene.3,4 Carcinoembryonic antigen is a glycoprotein often found in association with gastrointestinal tract tumors and also is elevated in some cases of FeP.5 A single-center retrospective study performed by Longo et al3 demonstrated an association between FeP and gastrointestinal malignancy by calculating a percentage of those with FeP who also had gastrointestinal tract tumors. Moreover, they noted that FeP preceded gastrointestinal tract tumors by up to 1 to 2 years. Using the results of this study, they suggested that a similar pathogenesis underlies the association between FeP and gastrointestinal malignancy, but a shared pathogenesis has not yet been elucidated.3
With a transition to preventive medicine and age-adjusted malignancy screening in the US medical community, the findings of FeP as a marker of gastrointestinal tract tumors could alter current recommendations of routine skin examinations and colorectal cancer screening. This study investigates the association between FeP and internal malignancy, especially gastrointestinal tract tumors.
Methods
Patient Selection—A single-center, retrospective, case-control study was designed to investigate an association between FeP and internal malignancy. The study protocol was approved by the institutional review board of the Naval Medical Center San Diego, California, in compliance with all applicable federal regulations governing the protection of human subjects. A medical record review was initiated using the Department of Defense (DoD) electronic health record to identify patients with a history of FeP. The query used a natural language search for patients who had received a histopathology report that included Fibroepithelioma of Pinkus, Pinkus, or Pinkus tumor within the diagnosis or comment section for pathology specimens processed at our institution (Naval Medical Center San Diego). A total of 45 patients evaluated at Naval Medical Center San Diego had biopsy specimens that met inclusion criteria. Only 42 electronic medical records were available to review between January 1, 2003, and March 1, 2020. Three patients were excluded from the study for absent or incomplete medical records.
Study Procedures—Data extracted by researchers were analyzed for statistical significance. All available data in current electronic health records prior to the FeP diagnosis until March 1, 2020, was reviewed for other documented malignancy or colonoscopy data. Data extracted included age, sex, date of diagnosis of FeP, location of FeP, social history, and medical and surgical history to identify prior malignancy. Colorectal cancer screening results were drawn from original reports, gastrointestinal clinic notes, biopsy results, and/or primary care provider documentation of colonoscopy results. If the exact date of internal tumor diagnosis could not be determined but the year was known, the value “July, year” was utilized as the diagnosis date.
Statistical Analysis—Data were reviewed for validity, and the Shapiro-Wilk test was used to test for normality. Graphical visualization assisted in reviewing the distribution of the data in relation to the internal tumors. The Fisher exact test was performed to test for associations, while continuous variables were assessed using the Student t test or the nonparametric Mann-Whitney U test. Analysis was conducted with StataCorp. 2017 Stata Statistical Software: Release 15 (StataCorp LLC). Significance was set at P<.05.
Results
Patient Demographics—Of the 42 patients with FeP included in this study, 28 (66.7%) were male and 14 (33.3%) were female. The overall mean age at FeP diagnosis was 56.83 years. The mean age (SD) at FeP diagnosis for males was 59.21 (19.00) years and 52.07 (21.61) for females (P=.2792)(Table 1). Other pertinent medical history, including alcohol and tobacco use, obesity, and diabetes mellitus, is included in Table 1.

Characterization of Tumors—The classification of the number of patients with any other nonskin neoplasm is presented in Table 2. Fifteen (35.7%) patients had 1 or more gastrointestinal tubular adenomas. Three patients were found to have colorectal adenocarcinoma. Karsenti et al6 published a large study of colonic adenoma detection rates in the World Journal of Gastroenterology stratified by age and found that the incidence of adenoma for those aged 55 to 59 years was 28.3% vs 35.7% in our study (P=.2978 [Fisher exact test]).
Given the number of gastrointestinal tract tumors detected, most of which were found during routine surveillance, and a prior study6 suggesting a relationship between FeP and gastrointestinal tract tumors, we analyzed the temporal relationship between the date of gastrointestinal tract tumor diagnosis and the date of FeP diagnosis to assess if gastrointestinal tract tumor or FeP might predict the onset of the other (Figure 1). By assigning a temporal category to each gastrointestinal tract tumor as occurring either before or after the FeP diagnosis by 0 to 3 years, 3 to 10 years, 10 to 15 years, and 15 or more years, the box plot in Figure 1 shows that gastrointestinal adenoma development had no significant temporal relationship to the presence of FeP, excluding any outliers (shown as dots). Additionally, in Figure 1, the same concept was applied to assess the relationship between the dates of all gastrointestinal tract tumors—benign, precancerous, or malignant—and the date of FeP diagnosis, which again showed that FeP and gastrointestinal tract tumors did not predict the onset of the other. Figure 2 showed the same for all nonskin tumor diagnoses and again demonstrated that FeP and all other nondermatologic tumors did not predict the onset of the other.
Comment
Malignancy Potential—The malignant potential of FeP—characterized as a trichoblastoma (an adnexal tumor) or a basal cell carcinoma (BCC) variant—has been documented.1 Haddock and Cohen1 noted that FeP can be considered as an intermediate variant between BCC and trichoblastomas. Furthermore, they questioned the relevance of differentiating FeP as benign or malignant.1 There are additional elements of FeP that currently are unknown, which can be partially attributed to its rarity. If we can clarify a more accurate pathogenic model of FeP, then common mutational pathways with other malignancies may be identified.
Screening for Malignancy in FeP Patients—Until recently, FeP has not been demonstrated to be associated with other cancers or to have increased metastatic potential.1 In a 1985 case series of 2 patients, FeP was found to be specifically overlying infiltrating ductal carcinoma of the breast. After a unilateral mastectomy, examination of the overlying skin of the breast showed a solitary, lightly pigmented nodule, which was identified as an FeP after histopathologic evaluation.7 There have been limited investigations of whether FeP is simply a solitary tumor or a harbinger for other malignancies, despite a study by Longo et al3 that attempted to establish this temporal relationship. They recommended that patients with FeP be clinically evaluated and screened for gastrointestinal tract tumors.3 Based on these recommendations, textbooks for dermatopathology now highlight the possible correlation of FeP and gastrointestinal malignancy,8 which may lead to earlier and unwarranted screening.
Comparison to the General Population—Although our analysis showed a portion of patients with FeP have gastrointestinal tract tumors, we do not detect a significant difference from the general population. The average age at the time of FeP diagnosis in our study was 56.83 years compared with the average age of 64.0 years by Longo et al,3 where they found an association with gastrointestinal adenocarcinoma and neuroendocrine tumors. As the rate of gastrointestinal adenoma and malignancy increases with age, the older population in the study by Longo et al3 may have developed colorectal cancer independent of FeP development. However, the rate of gastrointestinal or other malignancies in their study was substantially higher than that of the general population. The Longo et al3 study found that 22 of 49 patients developed nondermatologic malignancies within 2 years of FeP diagnosis. Additionally, no data were provided in the study regarding precancerous lesions.
In our study population, benign gastrointestinal tract tumors, specifically tubular adenomas, were noted in 35.7% of patients with FeP compared with 28.3% of the general population in the same age group reported by Karsenti et al.6 Although limited by our sample size, our study demonstrated that patients with FeP diagnosis showed no significant difference in age-stratified incidence of tubular adenoma compared with the general population (P=.2978). Figures 1 and 2 showed no obvious temporal relationship between the development of FeP and the diagnosis of gastrointestinal tumor—either precancerous or malignant lesions—suggesting that diagnosis of one does not indicate the presence of the other.
Relationship With Colonoscopy Results—By analyzing those patients with FeP who specifically had documented colonoscopy results, we did not find a correlation between FeP and gastrointestinal tubular adenoma or carcinoma at any time during the patients’ available records. Although some patients may have had undocumented colonoscopies performed outside the DoD medical system, most had evidence that these procedures were being performed by transcription into primary care provider notes, uploaded gastroenterologist clinical notes, or colonoscopy reports. It is unlikely a true colorectal or other malignancy would remain undocumented over years within the electronic medical record.
Study Limitations—Because of the nature of electronic medical records at multiple institutions, the quality and/or the quantity of medical documentation is not standardized across all patients. Not all pathology reports may include FeP as the primary diagnosis or description, as FeP may simply be reported as BCC. Despite thorough data extraction by physicians, we were limited to the data available within our electronic medical records. Colonoscopies and other specialty care often were performed by civilian providers. Documentation regarding where patients were referred for such procedures outside the DoD was not available unless reports were transmitted to the DoD or transcribed by primary care providers. Incomplete records may make it more difficult to identify and document the number and characteristics of patients’ tubular adenomas. Therefore, a complete review of civilian records was not possible, causing some patients’ medical records to be documented for a longer period of their lives than for others.
Conclusion
Given the discrepancies in our findings with the previous study,3 future investigations on FeP and associated tumors should focus on integrated health care systems with longitudinal data sets for all age-appropriate cancer screenings in a larger sample size. Another related study is needed to evaluate the pathophysiologic mechanisms of FeP development relative to known cancer lines.
- Haddock ES, Cohen PR. Fibroepithelioma of Pinkus revisited. Dermatol Ther (Heidelb). 2016;6:347-362.
- Ponti G, Pellacani G, Seidenari S, et al. Cancer-associated genodermatoses: skin neoplasms as clues to hereditary tumor syndromes. Crit Rev Oncol Hematol. 2013;85:239-256.
- Longo C, Pellacani G, Tomasi A, et al. Fibroepithelioma of Pinkus: solitary tumor or sign of a complex gastrointestinal syndrome. Mol Clin Oncol. 2016;4:797-800.
- Warner TF, Burgess H, Mohs FE. Extramammary Paget’s disease in fibroepithelioma of Pinkus. J Cutan Pathol. 1982;9:340-344.
- Stern JB, Haupt HM, Smith RR. Fibroepithelioma of Pinkus. eccrine duct spread of basal cell carcinoma. Am J Dermatopathol. 1994;16:585-587.
- Karsenti D, Tharsis G, Burtin P, et al. Adenoma and advanced neoplasia detection rates increase from 45 years of age. World J Gastroenterol. 2019;25:447-456.
- Bryant J. Fibroepithelioma of Pinkus overlying breast cancer. Arch Dermatol. 1985;121:310.
- Calonje E, Brenn T, Lazar A, et al. McKee’s Pathology of the Skin: With Clinical Correlations. 5th ed. Elsevier; 2020.
Fibroepithelioma of Pinkus (FeP), or Pinkus tumor, is a rare tumor with a presentation similar to benign neoplasms such as acrochordons and seborrheic keratoses. Classically, FeP presents as a nontender, solitary, flesh-colored, firm, dome-shaped papule or plaque with a predilection for the lumbosacral region rather than sun-exposed areas. This tumor typically develops in fair-skinned older adults, more often in females.1
The association between cutaneous lesions and internal malignancies is well known to include dermatoses such as erythema repens in patients with lung cancer, or tripe palms and acanthosis nigricans in patients with gastrointestinal malignancy. Outside of paraneoplastic presentations, many syndromes have unique constellations of clinical findings that require the clinician to investigate for internal malignancy. Cancer-associated genodermatoses such as Birt-Hogg-Dubé, neurofibromatosis, and Cowden syndrome have key findings to alert the provider of potential internal malignancies.2 Given the rarity and relative novelty of FeP, few studies have been performed that evaluate for an association with internal malignancies.
There potentially is a common pathophysiologic mechanism between FeP and other benign and malignant tumors. Some have noted a possible common embryonic origin, such as Merkel cells, and even a common gene mutation involving tumor protein p53 or PTCH1 gene.3,4 Carcinoembryonic antigen is a glycoprotein often found in association with gastrointestinal tract tumors and also is elevated in some cases of FeP.5 A single-center retrospective study performed by Longo et al3 demonstrated an association between FeP and gastrointestinal malignancy by calculating a percentage of those with FeP who also had gastrointestinal tract tumors. Moreover, they noted that FeP preceded gastrointestinal tract tumors by up to 1 to 2 years. Using the results of this study, they suggested that a similar pathogenesis underlies the association between FeP and gastrointestinal malignancy, but a shared pathogenesis has not yet been elucidated.3
With a transition to preventive medicine and age-adjusted malignancy screening in the US medical community, the findings of FeP as a marker of gastrointestinal tract tumors could alter current recommendations of routine skin examinations and colorectal cancer screening. This study investigates the association between FeP and internal malignancy, especially gastrointestinal tract tumors.
Methods
Patient Selection—A single-center, retrospective, case-control study was designed to investigate an association between FeP and internal malignancy. The study protocol was approved by the institutional review board of the Naval Medical Center San Diego, California, in compliance with all applicable federal regulations governing the protection of human subjects. A medical record review was initiated using the Department of Defense (DoD) electronic health record to identify patients with a history of FeP. The query used a natural language search for patients who had received a histopathology report that included Fibroepithelioma of Pinkus, Pinkus, or Pinkus tumor within the diagnosis or comment section for pathology specimens processed at our institution (Naval Medical Center San Diego). A total of 45 patients evaluated at Naval Medical Center San Diego had biopsy specimens that met inclusion criteria. Only 42 electronic medical records were available to review between January 1, 2003, and March 1, 2020. Three patients were excluded from the study for absent or incomplete medical records.
Study Procedures—Data extracted by researchers were analyzed for statistical significance. All available data in current electronic health records prior to the FeP diagnosis until March 1, 2020, was reviewed for other documented malignancy or colonoscopy data. Data extracted included age, sex, date of diagnosis of FeP, location of FeP, social history, and medical and surgical history to identify prior malignancy. Colorectal cancer screening results were drawn from original reports, gastrointestinal clinic notes, biopsy results, and/or primary care provider documentation of colonoscopy results. If the exact date of internal tumor diagnosis could not be determined but the year was known, the value “July, year” was utilized as the diagnosis date.
Statistical Analysis—Data were reviewed for validity, and the Shapiro-Wilk test was used to test for normality. Graphical visualization assisted in reviewing the distribution of the data in relation to the internal tumors. The Fisher exact test was performed to test for associations, while continuous variables were assessed using the Student t test or the nonparametric Mann-Whitney U test. Analysis was conducted with StataCorp. 2017 Stata Statistical Software: Release 15 (StataCorp LLC). Significance was set at P<.05.
Results
Patient Demographics—Of the 42 patients with FeP included in this study, 28 (66.7%) were male and 14 (33.3%) were female. The overall mean age at FeP diagnosis was 56.83 years. The mean age (SD) at FeP diagnosis for males was 59.21 (19.00) years and 52.07 (21.61) for females (P=.2792)(Table 1). Other pertinent medical history, including alcohol and tobacco use, obesity, and diabetes mellitus, is included in Table 1.

Characterization of Tumors—The classification of the number of patients with any other nonskin neoplasm is presented in Table 2. Fifteen (35.7%) patients had 1 or more gastrointestinal tubular adenomas. Three patients were found to have colorectal adenocarcinoma. Karsenti et al6 published a large study of colonic adenoma detection rates in the World Journal of Gastroenterology stratified by age and found that the incidence of adenoma for those aged 55 to 59 years was 28.3% vs 35.7% in our study (P=.2978 [Fisher exact test]).

Given the number of gastrointestinal tract tumors detected, most of which were found during routine surveillance, and a prior study6 suggesting a relationship between FeP and gastrointestinal tract tumors, we analyzed the temporal relationship between the date of gastrointestinal tract tumor diagnosis and the date of FeP diagnosis to assess if gastrointestinal tract tumor or FeP might predict the onset of the other (Figure 1). By assigning a temporal category to each gastrointestinal tract tumor as occurring either before or after the FeP diagnosis by 0 to 3 years, 3 to 10 years, 10 to 15 years, and 15 or more years, the box plot in Figure 1 shows that gastrointestinal adenoma development had no significant temporal relationship to the presence of FeP, excluding any outliers (shown as dots). Additionally, in Figure 1, the same concept was applied to assess the relationship between the dates of all gastrointestinal tract tumors—benign, precancerous, or malignant—and the date of FeP diagnosis, which again showed that FeP and gastrointestinal tract tumors did not predict the onset of the other. Figure 2 showed the same for all nonskin tumor diagnoses and again demonstrated that FeP and all other nondermatologic tumors did not predict the onset of the other.

Comment
Malignancy Potential—The malignant potential of FeP—characterized as a trichoblastoma (an adnexal tumor) or a basal cell carcinoma (BCC) variant—has been documented.1 Haddock and Cohen1 noted that FeP can be considered as an intermediate variant between BCC and trichoblastomas. Furthermore, they questioned the relevance of differentiating FeP as benign or malignant.1 There are additional elements of FeP that currently are unknown, which can be partially attributed to its rarity. If we can clarify a more accurate pathogenic model of FeP, then common mutational pathways with other malignancies may be identified.

Screening for Malignancy in FeP Patients—Until recently, FeP has not been demonstrated to be associated with other cancers or to have increased metastatic potential.1 In a 1985 case series of 2 patients, FeP was found to be specifically overlying infiltrating ductal carcinoma of the breast. After a unilateral mastectomy, examination of the overlying skin of the breast showed a solitary, lightly pigmented nodule, which was identified as an FeP after histopathologic evaluation.7 There have been limited investigations of whether FeP is simply a solitary tumor or a harbinger for other malignancies, despite a study by Longo et al3 that attempted to establish this temporal relationship. They recommended that patients with FeP be clinically evaluated and screened for gastrointestinal tract tumors.3 Based on these recommendations, textbooks for dermatopathology now highlight the possible correlation of FeP and gastrointestinal malignancy,8 which may lead to earlier and unwarranted screening.
Comparison to the General Population—Although our analysis showed a portion of patients with FeP have gastrointestinal tract tumors, we do not detect a significant difference from the general population. The average age at the time of FeP diagnosis in our study was 56.83 years compared with the average age of 64.0 years by Longo et al,3 where they found an association with gastrointestinal adenocarcinoma and neuroendocrine tumors. As the rate of gastrointestinal adenoma and malignancy increases with age, the older population in the study by Longo et al3 may have developed colorectal cancer independent of FeP development. However, the rate of gastrointestinal or other malignancies in their study was substantially higher than that of the general population. The Longo et al3 study found that 22 of 49 patients developed nondermatologic malignancies within 2 years of FeP diagnosis. Additionally, no data were provided in the study regarding precancerous lesions.
In our study population, benign gastrointestinal tract tumors, specifically tubular adenomas, were noted in 35.7% of patients with FeP compared with 28.3% of the general population in the same age group reported by Karsenti et al.6 Although limited by our sample size, our study demonstrated that patients with FeP diagnosis showed no significant difference in age-stratified incidence of tubular adenoma compared with the general population (P=.2978). Figures 1 and 2 showed no obvious temporal relationship between the development of FeP and the diagnosis of gastrointestinal tumor—either precancerous or malignant lesions—suggesting that diagnosis of one does not indicate the presence of the other.
Relationship With Colonoscopy Results—By analyzing those patients with FeP who specifically had documented colonoscopy results, we did not find a correlation between FeP and gastrointestinal tubular adenoma or carcinoma at any time during the patients’ available records. Although some patients may have had undocumented colonoscopies performed outside the DoD medical system, most had evidence that these procedures were being performed by transcription into primary care provider notes, uploaded gastroenterologist clinical notes, or colonoscopy reports. It is unlikely a true colorectal or other malignancy would remain undocumented over years within the electronic medical record.
Study Limitations—Because of the nature of electronic medical records at multiple institutions, the quality and/or the quantity of medical documentation is not standardized across all patients. Not all pathology reports may include FeP as the primary diagnosis or description, as FeP may simply be reported as BCC. Despite thorough data extraction by physicians, we were limited to the data available within our electronic medical records. Colonoscopies and other specialty care often were performed by civilian providers. Documentation regarding where patients were referred for such procedures outside the DoD was not available unless reports were transmitted to the DoD or transcribed by primary care providers. Incomplete records may make it more difficult to identify and document the number and characteristics of patients’ tubular adenomas. Therefore, a complete review of civilian records was not possible, causing some patients’ medical records to be documented for a longer period of their lives than for others.
Conclusion
Given the discrepancies in our findings with the previous study,3 future investigations on FeP and associated tumors should focus on integrated health care systems with longitudinal data sets for all age-appropriate cancer screenings in a larger sample size. Another related study is needed to evaluate the pathophysiologic mechanisms of FeP development relative to known cancer lines.
Fibroepithelioma of Pinkus (FeP), or Pinkus tumor, is a rare tumor with a presentation similar to benign neoplasms such as acrochordons and seborrheic keratoses. Classically, FeP presents as a nontender, solitary, flesh-colored, firm, dome-shaped papule or plaque with a predilection for the lumbosacral region rather than sun-exposed areas. This tumor typically develops in fair-skinned older adults, more often in females.1
The association between cutaneous lesions and internal malignancies is well known to include dermatoses such as erythema repens in patients with lung cancer, or tripe palms and acanthosis nigricans in patients with gastrointestinal malignancy. Outside of paraneoplastic presentations, many syndromes have unique constellations of clinical findings that require the clinician to investigate for internal malignancy. Cancer-associated genodermatoses such as Birt-Hogg-Dubé, neurofibromatosis, and Cowden syndrome have key findings to alert the provider of potential internal malignancies.2 Given the rarity and relative novelty of FeP, few studies have been performed that evaluate for an association with internal malignancies.
There potentially is a common pathophysiologic mechanism between FeP and other benign and malignant tumors. Some have noted a possible common embryonic origin, such as Merkel cells, and even a common gene mutation involving tumor protein p53 or PTCH1 gene.3,4 Carcinoembryonic antigen is a glycoprotein often found in association with gastrointestinal tract tumors and also is elevated in some cases of FeP.5 A single-center retrospective study performed by Longo et al3 demonstrated an association between FeP and gastrointestinal malignancy by calculating a percentage of those with FeP who also had gastrointestinal tract tumors. Moreover, they noted that FeP preceded gastrointestinal tract tumors by up to 1 to 2 years. Using the results of this study, they suggested that a similar pathogenesis underlies the association between FeP and gastrointestinal malignancy, but a shared pathogenesis has not yet been elucidated.3
With a transition to preventive medicine and age-adjusted malignancy screening in the US medical community, the findings of FeP as a marker of gastrointestinal tract tumors could alter current recommendations of routine skin examinations and colorectal cancer screening. This study investigates the association between FeP and internal malignancy, especially gastrointestinal tract tumors.
Methods
Patient Selection—A single-center, retrospective, case-control study was designed to investigate an association between FeP and internal malignancy. The study protocol was approved by the institutional review board of the Naval Medical Center San Diego, California, in compliance with all applicable federal regulations governing the protection of human subjects. A medical record review was initiated using the Department of Defense (DoD) electronic health record to identify patients with a history of FeP. The query used a natural language search for patients who had received a histopathology report that included Fibroepithelioma of Pinkus, Pinkus, or Pinkus tumor within the diagnosis or comment section for pathology specimens processed at our institution (Naval Medical Center San Diego). A total of 45 patients evaluated at Naval Medical Center San Diego had biopsy specimens that met inclusion criteria. Only 42 electronic medical records were available to review between January 1, 2003, and March 1, 2020. Three patients were excluded from the study for absent or incomplete medical records.
Study Procedures—Data extracted by researchers were analyzed for statistical significance. All available data in current electronic health records prior to the FeP diagnosis until March 1, 2020, was reviewed for other documented malignancy or colonoscopy data. Data extracted included age, sex, date of diagnosis of FeP, location of FeP, social history, and medical and surgical history to identify prior malignancy. Colorectal cancer screening results were drawn from original reports, gastrointestinal clinic notes, biopsy results, and/or primary care provider documentation of colonoscopy results. If the exact date of internal tumor diagnosis could not be determined but the year was known, the value “July, year” was utilized as the diagnosis date.
Statistical Analysis—Data were reviewed for validity, and the Shapiro-Wilk test was used to test for normality. Graphical visualization assisted in reviewing the distribution of the data in relation to the internal tumors. The Fisher exact test was performed to test for associations, while continuous variables were assessed using the Student t test or the nonparametric Mann-Whitney U test. Analysis was conducted with StataCorp. 2017 Stata Statistical Software: Release 15 (StataCorp LLC). Significance was set at P<.05.
Results
Patient Demographics—Of the 42 patients with FeP included in this study, 28 (66.7%) were male and 14 (33.3%) were female. The overall mean age at FeP diagnosis was 56.83 years. The mean age (SD) at FeP diagnosis for males was 59.21 (19.00) years and 52.07 (21.61) for females (P=.2792)(Table 1). Other pertinent medical history, including alcohol and tobacco use, obesity, and diabetes mellitus, is included in Table 1.

Characterization of Tumors—The classification of the number of patients with any other nonskin neoplasm is presented in Table 2. Fifteen (35.7%) patients had 1 or more gastrointestinal tubular adenomas. Three patients were found to have colorectal adenocarcinoma. Karsenti et al6 published a large study of colonic adenoma detection rates in the World Journal of Gastroenterology stratified by age and found that the incidence of adenoma for those aged 55 to 59 years was 28.3% vs 35.7% in our study (P=.2978 [Fisher exact test]).

Given the number of gastrointestinal tract tumors detected, most of which were found during routine surveillance, and a prior study6 suggesting a relationship between FeP and gastrointestinal tract tumors, we analyzed the temporal relationship between the date of gastrointestinal tract tumor diagnosis and the date of FeP diagnosis to assess if gastrointestinal tract tumor or FeP might predict the onset of the other (Figure 1). By assigning a temporal category to each gastrointestinal tract tumor as occurring either before or after the FeP diagnosis by 0 to 3 years, 3 to 10 years, 10 to 15 years, and 15 or more years, the box plot in Figure 1 shows that gastrointestinal adenoma development had no significant temporal relationship to the presence of FeP, excluding any outliers (shown as dots). Additionally, in Figure 1, the same concept was applied to assess the relationship between the dates of all gastrointestinal tract tumors—benign, precancerous, or malignant—and the date of FeP diagnosis, which again showed that FeP and gastrointestinal tract tumors did not predict the onset of the other. Figure 2 showed the same for all nonskin tumor diagnoses and again demonstrated that FeP and all other nondermatologic tumors did not predict the onset of the other.

Comment
Malignancy Potential—The malignant potential of FeP—characterized as a trichoblastoma (an adnexal tumor) or a basal cell carcinoma (BCC) variant—has been documented.1 Haddock and Cohen1 noted that FeP can be considered as an intermediate variant between BCC and trichoblastomas. Furthermore, they questioned the relevance of differentiating FeP as benign or malignant.1 There are additional elements of FeP that currently are unknown, which can be partially attributed to its rarity. If we can clarify a more accurate pathogenic model of FeP, then common mutational pathways with other malignancies may be identified.

Screening for Malignancy in FeP Patients—Until recently, FeP has not been demonstrated to be associated with other cancers or to have increased metastatic potential.1 In a 1985 case series of 2 patients, FeP was found to be specifically overlying infiltrating ductal carcinoma of the breast. After a unilateral mastectomy, examination of the overlying skin of the breast showed a solitary, lightly pigmented nodule, which was identified as an FeP after histopathologic evaluation.7 There have been limited investigations of whether FeP is simply a solitary tumor or a harbinger for other malignancies, despite a study by Longo et al3 that attempted to establish this temporal relationship. They recommended that patients with FeP be clinically evaluated and screened for gastrointestinal tract tumors.3 Based on these recommendations, textbooks for dermatopathology now highlight the possible correlation of FeP and gastrointestinal malignancy,8 which may lead to earlier and unwarranted screening.
Comparison to the General Population—Although our analysis showed a portion of patients with FeP have gastrointestinal tract tumors, we do not detect a significant difference from the general population. The average age at the time of FeP diagnosis in our study was 56.83 years compared with the average age of 64.0 years by Longo et al,3 where they found an association with gastrointestinal adenocarcinoma and neuroendocrine tumors. As the rate of gastrointestinal adenoma and malignancy increases with age, the older population in the study by Longo et al3 may have developed colorectal cancer independent of FeP development. However, the rate of gastrointestinal or other malignancies in their study was substantially higher than that of the general population. The Longo et al3 study found that 22 of 49 patients developed nondermatologic malignancies within 2 years of FeP diagnosis. Additionally, no data were provided in the study regarding precancerous lesions.
In our study population, benign gastrointestinal tract tumors, specifically tubular adenomas, were noted in 35.7% of patients with FeP compared with 28.3% of the general population in the same age group reported by Karsenti et al.6 Although limited by our sample size, our study demonstrated that patients with FeP diagnosis showed no significant difference in age-stratified incidence of tubular adenoma compared with the general population (P=.2978). Figures 1 and 2 showed no obvious temporal relationship between the development of FeP and the diagnosis of gastrointestinal tumor—either precancerous or malignant lesions—suggesting that diagnosis of one does not indicate the presence of the other.
Relationship With Colonoscopy Results—By analyzing those patients with FeP who specifically had documented colonoscopy results, we did not find a correlation between FeP and gastrointestinal tubular adenoma or carcinoma at any time during the patients’ available records. Although some patients may have had undocumented colonoscopies performed outside the DoD medical system, most had evidence that these procedures were being performed by transcription into primary care provider notes, uploaded gastroenterologist clinical notes, or colonoscopy reports. It is unlikely a true colorectal or other malignancy would remain undocumented over years within the electronic medical record.
Study Limitations—Because of the nature of electronic medical records at multiple institutions, the quality and/or the quantity of medical documentation is not standardized across all patients. Not all pathology reports may include FeP as the primary diagnosis or description, as FeP may simply be reported as BCC. Despite thorough data extraction by physicians, we were limited to the data available within our electronic medical records. Colonoscopies and other specialty care often were performed by civilian providers. Documentation regarding where patients were referred for such procedures outside the DoD was not available unless reports were transmitted to the DoD or transcribed by primary care providers. Incomplete records may make it more difficult to identify and document the number and characteristics of patients’ tubular adenomas. Therefore, a complete review of civilian records was not possible, causing some patients’ medical records to be documented for a longer period of their lives than for others.
Conclusion
Given the discrepancies in our findings with the previous study,3 future investigations on FeP and associated tumors should focus on integrated health care systems with longitudinal data sets for all age-appropriate cancer screenings in a larger sample size. Another related study is needed to evaluate the pathophysiologic mechanisms of FeP development relative to known cancer lines.
- Haddock ES, Cohen PR. Fibroepithelioma of Pinkus revisited. Dermatol Ther (Heidelb). 2016;6:347-362.
- Ponti G, Pellacani G, Seidenari S, et al. Cancer-associated genodermatoses: skin neoplasms as clues to hereditary tumor syndromes. Crit Rev Oncol Hematol. 2013;85:239-256.
- Longo C, Pellacani G, Tomasi A, et al. Fibroepithelioma of Pinkus: solitary tumor or sign of a complex gastrointestinal syndrome. Mol Clin Oncol. 2016;4:797-800.
- Warner TF, Burgess H, Mohs FE. Extramammary Paget’s disease in fibroepithelioma of Pinkus. J Cutan Pathol. 1982;9:340-344.
- Stern JB, Haupt HM, Smith RR. Fibroepithelioma of Pinkus. eccrine duct spread of basal cell carcinoma. Am J Dermatopathol. 1994;16:585-587.
- Karsenti D, Tharsis G, Burtin P, et al. Adenoma and advanced neoplasia detection rates increase from 45 years of age. World J Gastroenterol. 2019;25:447-456.
- Bryant J. Fibroepithelioma of Pinkus overlying breast cancer. Arch Dermatol. 1985;121:310.
- Calonje E, Brenn T, Lazar A, et al. McKee’s Pathology of the Skin: With Clinical Correlations. 5th ed. Elsevier; 2020.
- Haddock ES, Cohen PR. Fibroepithelioma of Pinkus revisited. Dermatol Ther (Heidelb). 2016;6:347-362.
- Ponti G, Pellacani G, Seidenari S, et al. Cancer-associated genodermatoses: skin neoplasms as clues to hereditary tumor syndromes. Crit Rev Oncol Hematol. 2013;85:239-256.
- Longo C, Pellacani G, Tomasi A, et al. Fibroepithelioma of Pinkus: solitary tumor or sign of a complex gastrointestinal syndrome. Mol Clin Oncol. 2016;4:797-800.
- Warner TF, Burgess H, Mohs FE. Extramammary Paget’s disease in fibroepithelioma of Pinkus. J Cutan Pathol. 1982;9:340-344.
- Stern JB, Haupt HM, Smith RR. Fibroepithelioma of Pinkus. eccrine duct spread of basal cell carcinoma. Am J Dermatopathol. 1994;16:585-587.
- Karsenti D, Tharsis G, Burtin P, et al. Adenoma and advanced neoplasia detection rates increase from 45 years of age. World J Gastroenterol. 2019;25:447-456.
- Bryant J. Fibroepithelioma of Pinkus overlying breast cancer. Arch Dermatol. 1985;121:310.
- Calonje E, Brenn T, Lazar A, et al. McKee’s Pathology of the Skin: With Clinical Correlations. 5th ed. Elsevier; 2020.
PRACTICE POINTS
- Dermatologic reactions may be the initial presentation of an internal malignancy.
- Fibroepithelioma of Pinkus is considered on the spectrum between adnexal neoplasms and a nonaggressive variant of basal cell carcinoma (BCC).
- Fibroepithelioma of Pinkus should be managed similar to nonaggressive variants of BCC such as nodular BCC.
- Fibroepithelioma of Pinkus is not associated with internal malignancy.
Cutaneous T-Cell Lymphoma Treatment: Case Series of Combination Therapy With Intralesional Injections of 5-Fluorouracil and Topical Imiquimod
Cutaneous T-cell lymphoma (CTCL) is a diverse group of skin-homing T-cell neoplasms with a wide array of clinical presentations, immunohistopathologic subtypes, and prognoses. The age-adjusted incidence of CTCL in the United States is 6.4 per million individuals.1 In the early stages of CTCL, the malignant lymphocytes are isolated to the skin, while more advanced disease involves metastatic spread to the lymphatic and peripheral blood compartments. Mycosis fungoides (MF) is the most common subtype of CTCL, comprising roughly 50% of all cases. The etiology of CTCL and MF remains poorly understood and no unifying driver mutation has been identified.2 However, recent sequencing efforts have revealed recurrent genomics alterations primarily in 3 pathways: constitutive T-cell activation, resistance to apoptosis/cell-cycle dysregulation, and DNA structural/gene expression dysregulation.3-8 These studies, among others, support the assertion that CTCL may be an epigenetic phenomenon.9-14
Most patients with MF will experience an indolent course of skin-limited disease with a favorable prognosis and a 5-year survival rate of 88%.15-17 A large study of patients with MF (N=525) followed for more than 40 years determined that approximately 20% of early-stage (IA-IIA) patients with MF progress to develop tumors, metastasis to the lymphatic tissue, and/or leukemic blood disease.18
Cutaneous T-cell lymphoma is a chronic disease, and most treatment responses are partial and short-lived. Allogenic hematopoietic transplantation is the only potentially curative option, and all other therapies are aimed at arresting progression and achieving remission.19 Skin-directed therapies include topical steroids, topical nitrogen mustard, phototherapy, and radiation. Systemic therapies such as oral retinoids, chemotherapy, and immunotherapy may be used alone or in combination with skin-directed therapies based on the overall disease stage and clinical presentation. Unfortunately, complete response (CR) to therapy is rare and fleeting, and most patients require multiple sequential treatments over their lifetimes.20
Across all stages of CTCL, there is a therapeutic push to combination and immune-based therapies to achieve more durable responses. The imidazoquinolines are a family of toll-like receptor (TLR) agonists including imiquimod (TLR7) and resiquimod (TLR7 and TLR8). Imiquimod (IMQ) is a topical immunomodulator, which increases the local cytotoxic helper T-cell profile (T
Skin-directed chemotherapy using 5-fluorouracil (5-FU) has shown activity against many cutaneous malignancies. 5-Fluorouracil is an antimetabolite drug that inhibits thymidylate synthase, resulting in interrupted DNA and RNA synthesis and leading to an apoptotic cell death (Figure 1). It has been administered via intravenous, oral (prodrug), intralesional (IL), and topical routes with well-documented success in treating cutaneous squamous cell carcinoma, keratoacanthoma, basal cell carcinoma, and precancerous actinic keratosis.35 As a topical, 5-FU has been shown to provide a good response in 6 patients with early MF.36 In late-stage MF, 5-FU has been used in combination with methotrexate as an infusion.37 We present a single-center case series of 9 patients with CTCL who received combination IL 5-FU and IMQ cream 5%.
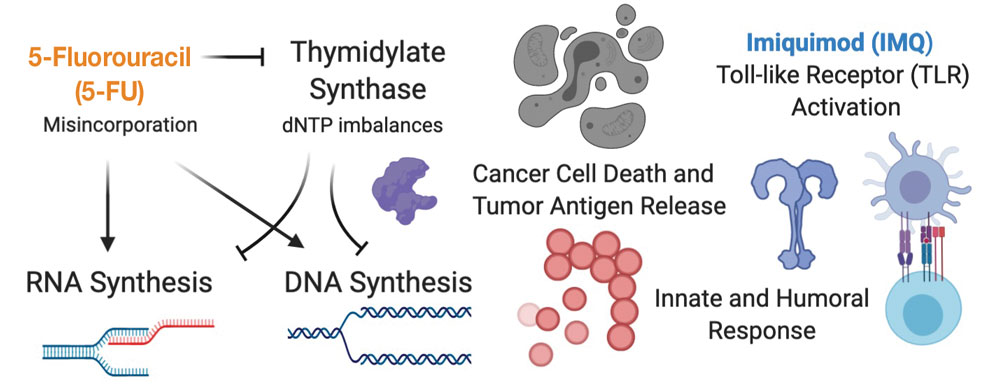
Methods
Patient Selection—Patients were selected from our multidisciplinary CTCL subspecialty clinic at the Inova Schar Cancer Institute (Fairfax, Virginia). Patients with single to few recalcitrant CTCL plaques or tumors that were symptomatic or otherwise bothersome were included. All patients had at least 2 prior skin-directed therapies that failed, and many had advanced-stage disease requiring systemic therapy. All patients provided verbal consent.
Study Materials and Evaluations—Patients received IL injections of 5-FU 50 mg/mL. The volume injected was approximately 0.2 cc per cubic centimeter of lesion tissue. Injections were repeated at 2- to 3-week intervals until the target lesions achieved an acute hemorrhagic phase characterized by erosion, flattening, and crust formation. The total number of serial injections administered ranged from 1 to 5. The patients concomitantly treated all lesions with IMQ cream 5% daily for a duration of 2 to 3 months.
Medical photography and physical examination were performed every 2 to 3 weeks until the hemorrhagic phase resolved and treated sites re-epithelialized. Index lesions were assessed using the Composite Assessment of Index Lesion Severity (CAILS) score by a single investigator for all patients.38 Scores were retrospectively assigned using the investigator’s detailed physical examination descriptions and extensive medical photography. Any hyperpigmentation was scored as residual disease, despite the fair interpretation of it as procedure-related postinflammatory dyspigmentation. Complete response was strictly defined as a CAILS score of 0. The patients were screened for possible systemic effects of IMQ, including the presence of fever, chills, fatigue, and myalgia. Patients were evaluated every 6 to 12 weeks as a standing follow-up.
Statistical Analysis—Reductions were calculated using local regression from baseline to the 4- to 7-week follow-up. Patients with multiple lesions had their CAILS score averaged at ea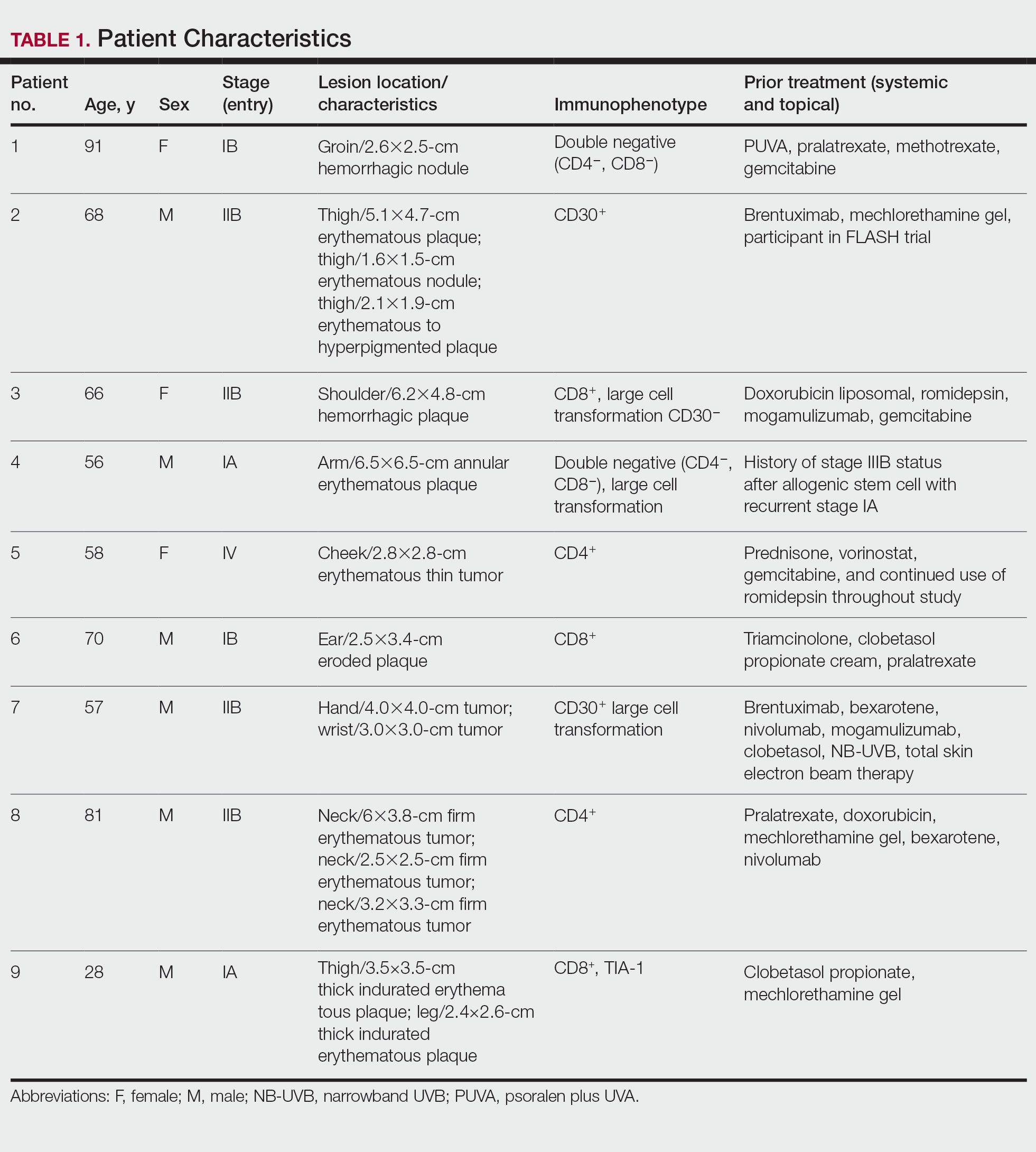
Results
Nine patients aged 28 to 91 years (median age, 66 years) with CTCL stages IA to IVA2, who had lesions located throughout their body, achieved CR; 3 patients were female (Table 1). The most common phenotype was CD8+ (n=3). All patients had at least 2 prior skin-directed therapies at treatment sites that failed, and 1 patient had 7 prior treatments that failed. Prior treatments included a variety of modalities, including all standard-of-care options and enrollment in clinical trials. One patient died from pneumonia following CR (Table 2). Seven patients had previously received systemic therapy for CTCL, and 1 patient was stable on romidepsin during our study. In patients who received more than 1 injection of 5-FU—1 injection: 3 patients; 2 injections: 3 patients; 3 injections: 1 patient; 4 injections: 1 patient; 5 injections: 1 patient—injections were spaced by 2 to 3 weeks. There was 1 patient who initially had an inadequate dosing of IL 5-FU and was restarted 14 months later; this was the patient with 5 total injections. This occurred in one of the first patients in the study, who presented with a facial lesion. The investigator used approximately 0.02 cc per cubic centimeter (dose reduction of nearly 90%), which was inadequate and did not achieve the requisite hemorrhagic phase.
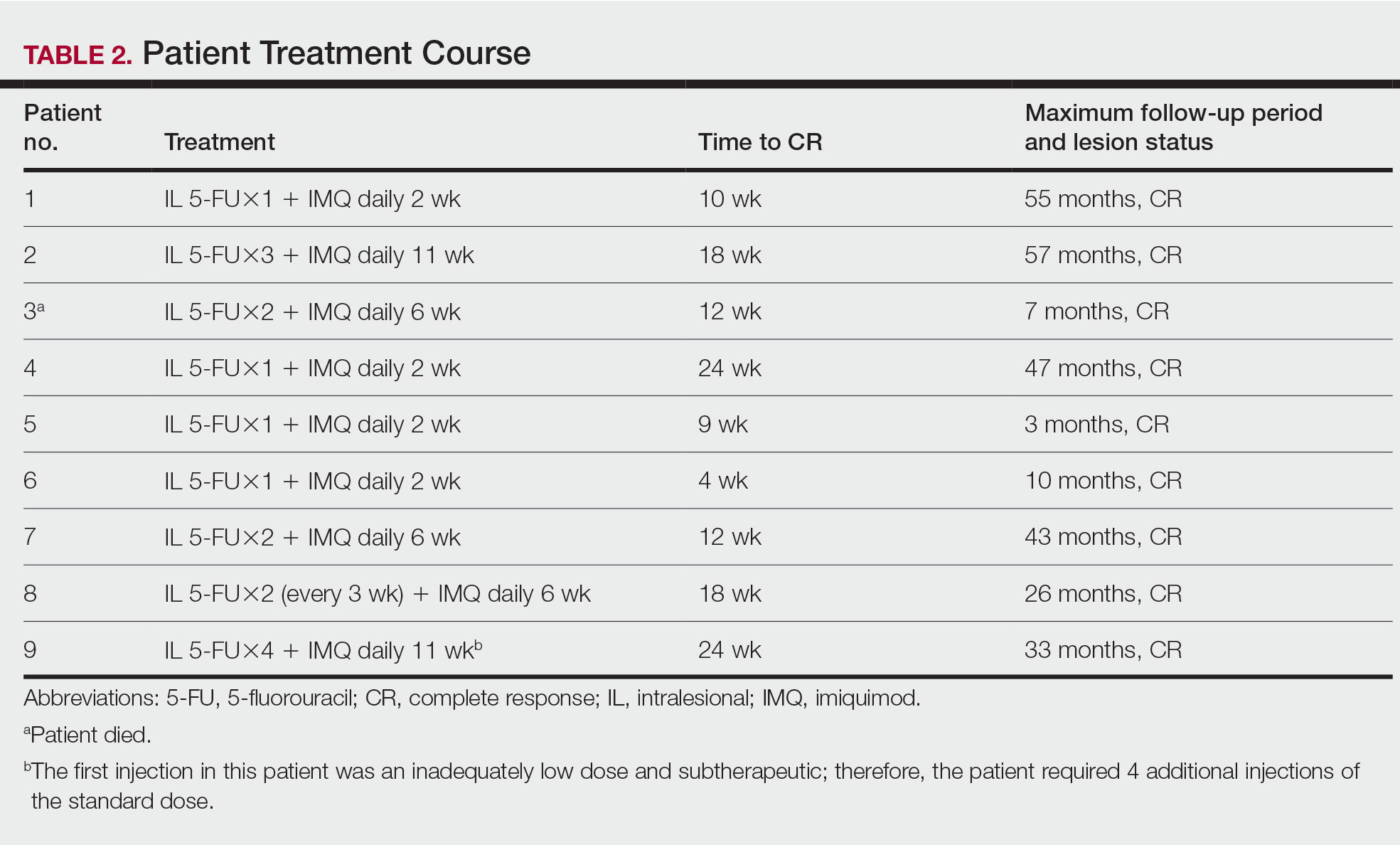
Treatment was well tolerated overall. In all cases, a hemorrhagic phase was achieved, characterized by erosion and crusting that was rated as mildly uncomfortable by 7 patients and moderately uncomfortable by 2 patients. In total, 15 lesions in all 9 patients achieved a CR within 24 weeks of the final injection. The longest treatment course required 12 weeks of therapy with IMQ and 5 IL injections of 5-FU. The fastest CR was achieved in patient 6 within 6 weeks following a single IL injection of 5-FU and 2 applications of IMQ. The average time to CR was 14.78 weeks (95% CI, 1.75-27.81)(Figure 2), and the time to CR ranged from 4 to 24 weeks. On average, patients achieved more than 50% reduction in CAILS score by 3.53 weeks (95% CI, 1.55-5.51) and nearly a 4-fold (74.7%) reduction at the time of initial follow-up (occurring at 4–7 weeks). By 7 weeks, patient 3 had the most modest improvement in CAILS score with a 2.75-fold reduction, while patient 5 had the largest decrease with a 5-fold reduction. Figure 3 shows representative clinical photographs of 2 patients before and after treatment, with all patients having similar results.
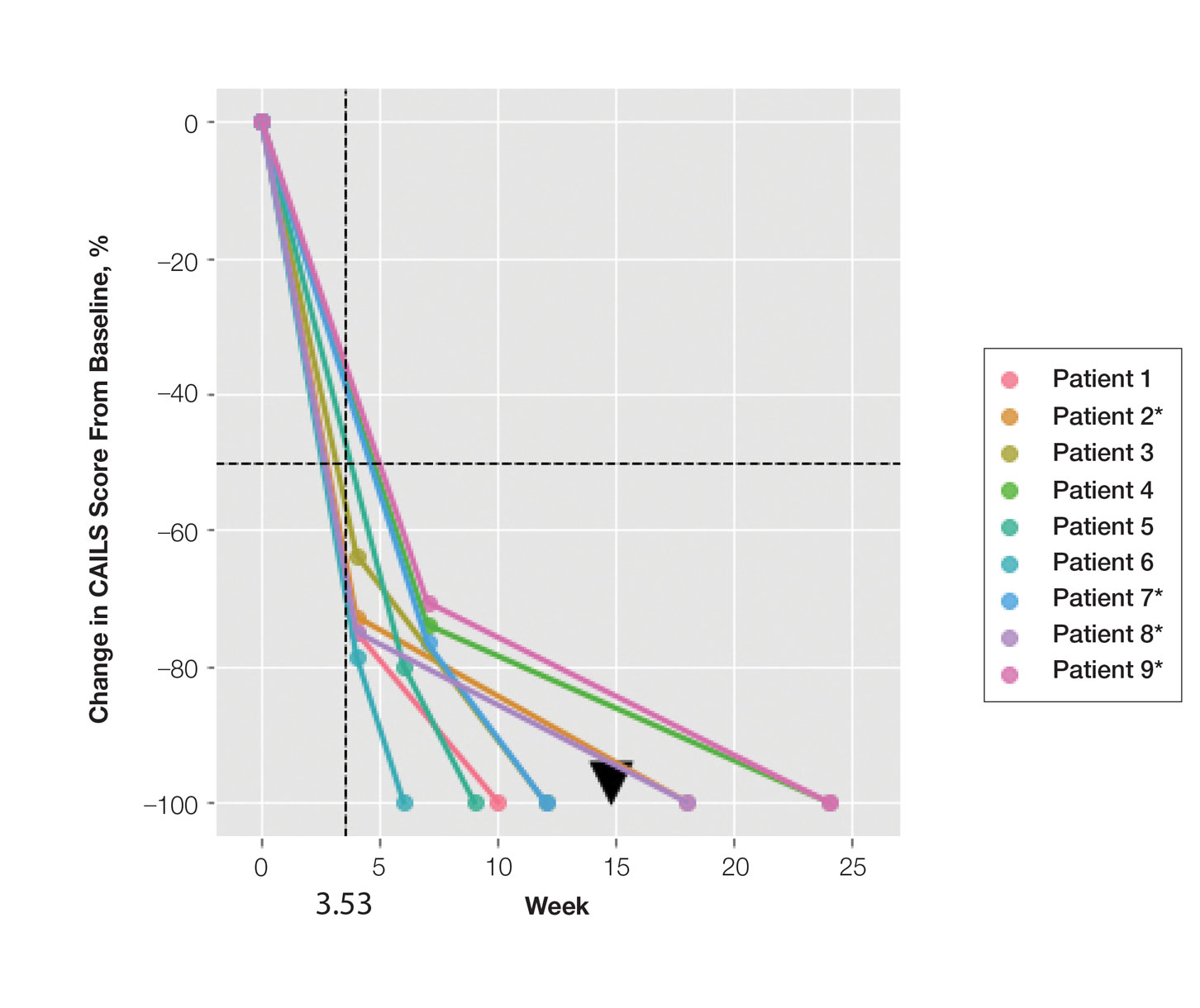
Comment
Cutaneous T-cell lymphoma is a chronic skin cancer with a pattern of limited response to therapy and frequent recurrence. Currently available skin-directed therapies function as temporizing measures rather than curative treatments. Immunotherapy offers the promise of lasting disease control even after cessation of treatment, as it may essentially awaken cutaneous immune surveillance to malignant lymphocytes.
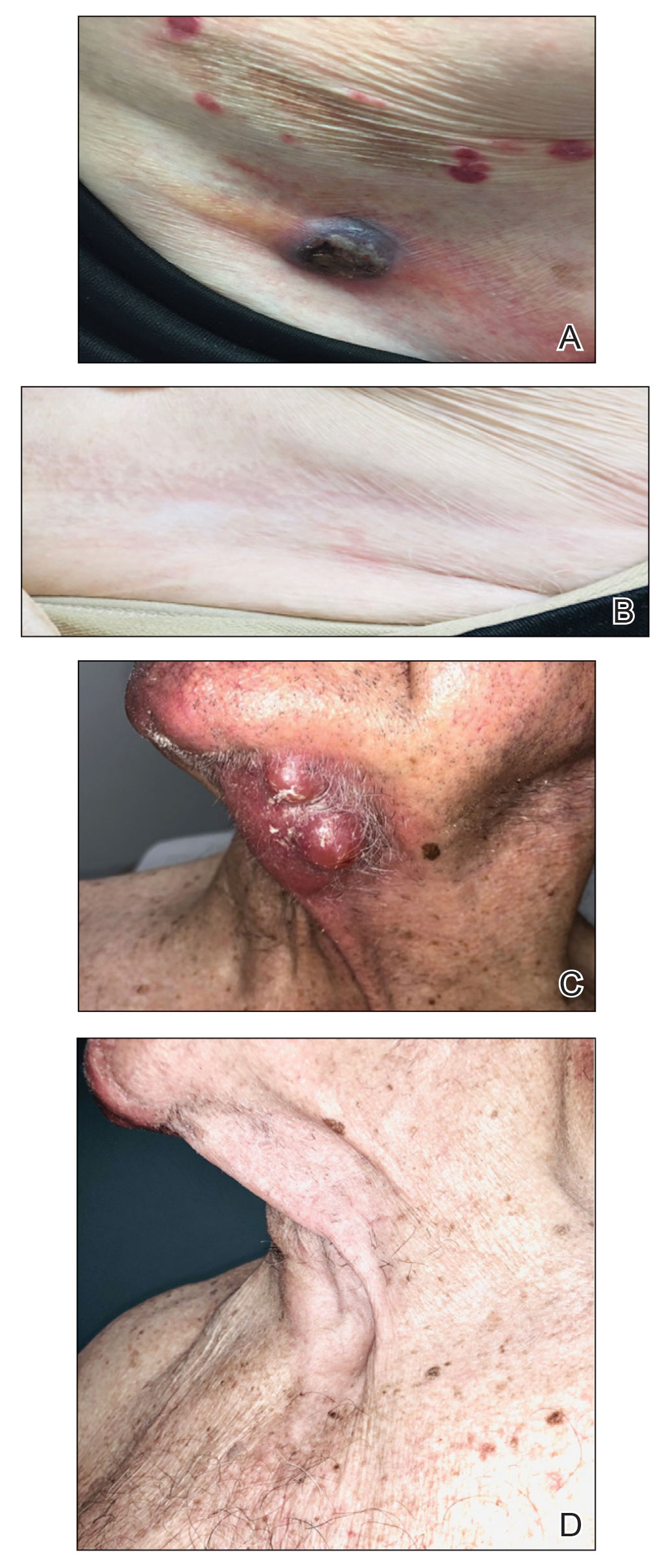
Several small observational studies have evaluated topical IMQ and TLR agonist therapy in CTCL. The construct of prior reports varies widely, including many different pretreatments, dosing schemes, and follow-up periods.24-33 Dosing intervals with IMQ ranged from daily to 3 times per week and treatment duration from 2 weeks to 1 year. Complete response rates from 50% to 100% were reported, and partial responses were observed in all but 1 patient, with recurrence-free follow-up ranging from 6 months to 8 years. Comparatively, combining IL 5-FU and IMQ appears to be at least as effective as IMQ alone or in other sequential treatments and combinations.24-33
Resiquimod, an experimental TLR7/8 agonist, has shown promising results in CTCL. Rook et al34 conducted a phase 1 trial of topical resiquimod in 12 early-stage patients with CTCL, all of whom responded to therapy. Two patients achieved CR, and 9 achieved a partial response, including 5 patients with the folliculotropic subtype. Interestingly, an abscopal effect was observed in 92% (11/12) of patients. Molecular evidence of reduction of the malignant clone was observed in 90% of patients via high-throughput sequencing of lesional tissue.34 These exciting findings suggest that topical immune therapy with TLR agonists may achieve robust, sustained, and possibly global disease control in CTCL.
Topical therapies are limited by depth of absorption, which can present a barrier to using these treatments for thicker plaques and tumors. Combining IL and topical routes was critical in our study design. Having good clinical experience using IL 5-FU in nonmelanoma skin cancers, we hypothesized that IL 5-FU would achieve a cytotoxic response through the full depth of thicker lesions and erode the surface of these lesions to facilitate penetration of topical IMQ. We additionally hypothesized that the combination of mechanisms of action would lead to an additive or synergistic response (Figure 1). By first inducing apoptotic cell death via 5-FU, we hoped to spill malignant lymphocyte neoantigens. Coupling that antigen exposure with an enhanced T
In our case series, all 15 lesions in 9 patients completely cleared, and no recurrences were observed at 26-month follow-up. No patients encountered any major adverse events, and the procedure was well tolerated by all.
Study Limitations—Limitations of this small study certainly exist. It is impossible to prove that our mechanistic theory is accurate given our strictly clinical assessment tools. We speculate that if our results had been achieved with IL 5-FU alone, future investigation with a prospective study using multiple treatment arms including a control would be warranted. Kannangara et al36 reported the use of topical 5-FU for MF and the drug’s utility in either topical or IL routes for CTCL, which deserves further study. It is less likely that results were achieved exclusively by IMQ because of the rapid tissue breakdown observed in the acute hemorrhagic phase. This phenomenon is best explained by the sudden apoptosis caused by DNA intercalation from 5-FU. The follow-up period is not uniform because this was a rolling enrollment study. Follow-up will be ongoing, and we aim to assess all patients up to at least the 5-year point. A final limitation of this study is the purely clinical end point. In the future, pretreatment and posttreatment biopsies would be useful in assessing proof of histologic response, and high-throughput sequencing may be used to look for molecular clearance via liquid biopsy. Lastly, careful observation for possible abscopal effect using the Severity-Weighted Assessment Tool score would be interesting and potentially contributory to our understanding of the impact of topical immune therapy on cutaneous tumor surveillance.
Conclusion
Combination IL 5-FU and topical IMQ is a well-tolerated, effective, and durable therapy for recalcitrant thick plaques and tumors of CTCL. This treatment is convenient and cost-effective. The procedure is performed in less than 5 minutes in an outpatient dermatology clinic. All patients received full insurance coverage for both drug and procedure fees under Medicare and commercial carriers.
- Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973-2002. Arch Dermatol. 2007;143:854-859.
- DeSimone JA, Sodha P, Ignatova D, et al. Recent advances in primary cutaneous T-cell lymphoma. Curr Opin Oncol. 2015;27:128-133.
- Choi J, Goh G, Walradt T, et al. Genomic landscape of cutaneous T cell lymphoma. Nat Genet. 2015;47:1011-1019.
- Ungewickell A, Bhaduri A, Rios E, et al. Genomic analysis of mycosis fungoides and Sézary syndrome identifies recurrent alterations in TNFR2. Nat Genet. 2015;47:1056-1060.
- McGirt LY, Jia P, Baerenwald DA, et al. Whole-genome sequencing reveals oncogenic mutations in mycosis fungoides. Blood. 2015;126:508-519.
- da Silva Almeida AC, Abate F, Khiabanian H, et al. The mutational landscape of cutaneous T cell lymphoma and Sézary syndrome. Nat Genet. 2015;47:1465-1470.
- Litvinov IV, Netchiporouk E, Cordeiro B, et al. The use oftranscriptional profiling to improve personalized diagnosis and management of cutaneous T-cell lymphoma (CTCL). Clin Cancer Res. 2015;21:2820-2829.
- Cyrenne BM, Lewis JM, Weed JG, et al. Synergy of BCL2 and histone deacetylase inhibition against leukemic cells from cutaneous T-cell lymphoma patients. Blood. 2017;130:2073-2083.
- Cancer Genome Atlas Research Network; Weinstein JN, Collisson EA, Mills GB, et al. The Cancer Genome Atlas Pan-Cancer analysis project. Nat Genet. 2013;45:1113-1120.
- Kiel MJ, Sahasrabuddhe AA, Rolland DCM, et al. Genomic analyses reveal recurrent mutations in epigenetic modifiers and the JAK-STAT pathway in Sézary syndrome. Nat Commun. 2015;6:8470.
- Wang L, Ni X, Covington KR, et al. Genomic profiling of Sézary syndrome identifies alterations of key T cell signaling and differentiation genes. Nat Genet. 2015;47:1426-1434.
- van Doorn R, Slieker RC, Boonk SE, et al. Epigenomic analysis of Sézary syndrome defines patterns of aberrant DNA methylation and identifies diagnostic markers. J Invest Dermatol. 2016;136:1876-1884.
- Qiu L, Liu F, Yi S, et al. Loss of 5-hydroxymethylcytosine is an epigenetic biomarker in cutaneous T-cell lymphoma. J Invest Dermatol. 2018;138:2388-2397.
- Kim SR, Lewis JM, Cyrenne BM, et al. BET inhibition in advanced cutaneous T cell lymphoma is synergistically potentiated by BCL2 inhibition or HDAC inhibition. Oncotarget. 2018;9:29193-29207.
- Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703-1714.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome, part I. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-16.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Kim YH, Liu HL, Mraz-Gernhard S, et al. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857-866.
- Lechowicz MJ, Lazarus HM, Carreras J, et al. Allogeneic hematopoietic cell transplantation for mycosis fungoides and Sezary syndrome. Bone Marrow Transplant. 2014;49:1360-1365.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome, part II: prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e1-17.
- Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196-200.
- Gibson SJ, Lindh JM, Riter TR, et al. Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cell Immunol. 2002;218:74-86.
- Schön MP, Schön M. TLR7 and TLR8 as targets in cancer therapy. Oncogene. 2008;27:190-199.
- Suchin KR, Junkins-Hopkins JM, Rook AH. Treatment of stage IA cutaneous T-cell lymphoma with topical application of the immune response modifier imiquimod. Arch Dermatol. 2002;138:1137-1139.
- Dummer R, Urosevic M, Kempf W, et al. Imiquimod induces complete clearance of a PUVA-resistant plaque in mycosis fungoides. Dermatology. 2003;207:116-118.
- Didona B, Benucci R, Amerio P, et al. Primary cutaneous CD30+ T-cell lymphoma responsive to topical imiquimod (Aldara). Br J Dermatol. 2004;150:1198-1201.
- Deeths MJ, Chapman JT, Dellavalle RP, et al. Treatment of patch and plaque stage mycosis fungoides with imiquimod 5% cream. J Am Acad Dermatol. 2005;52:275-280.
- Coors EA, Schuler G, Von Den Driesch P. Topical imiquimod as treatment for different kinds of cutaneous lymphoma. Eur J Dermatol. 2006;16:391-393.
- Chiam LYT, Chan YC. Solitary plaque mycosis fungoides on the penis responding to topical imiquimod therapy. Br J Dermatol. 2007;156:560-562.
- Soler-Machín J, Gilaberte-Calzada Y, Vera-Alvarez J, et al. Imiquimod in treatment of palpebral mycosis fungoides. Article in Spanish. Arch Soc Esp Oftalmol. 2006;81:221-223.
- Martínez-González MC, Verea-Hernando MM, Yebra-Pimentel MT, et al. Imiquimod in mycosis fungoides. Eur J Dermatol. 2008;18:148-152.
- Gordon MC, Sluzevich JC, Jambusaria-Pahlajani A. Clearance of folliculotropic and tumor mycosis fungoides with topical 5% imiquimod. JAAD Case Rep. 2015;1:348-350.
- Lewis DJ, Byekova YA, Emge DA, et al. Complete resolution of mycosis fungoides tumors with imiquimod 5% cream: a case series. J Dermatolog Treat. 2017;28:567-569.
- Rook AH, Gelfand JM, Wysocka M, et al. Topical resiquimod can induce disease regression and enhance T-cell effector functions in cutaneous T-cell lymphoma. Blood. 2015;126:1452-1461.
- Morse LG, Kendrick C, Hooper D, et al. Treatment of squamous cell carcinoma with intralesional 5-fluorouracil. Dermatol Surg. 2003;29:1150-1153.
- Kannangara AP, Levitan D, Fleischer AB Jr. Six patients with early-stage cutaneous T-cell lymphoma successfully treated with topical 5-fluorouracil. J Drugs Dermatol. 2010;9:1017-1018.
- Schappell DL, Alper JC, McDonald CJ. Treatment of advanced mycosis fungoides and Sézary syndrome with continuous infusions of methotrexate followed by fluorouracil and leucovorin rescue. Arch Dermatol. 1995;131:307-313.
- Olsen EA, Whittaker S, Kim YH, et al. Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29:2598-2607.
Cutaneous T-cell lymphoma (CTCL) is a diverse group of skin-homing T-cell neoplasms with a wide array of clinical presentations, immunohistopathologic subtypes, and prognoses. The age-adjusted incidence of CTCL in the United States is 6.4 per million individuals.1 In the early stages of CTCL, the malignant lymphocytes are isolated to the skin, while more advanced disease involves metastatic spread to the lymphatic and peripheral blood compartments. Mycosis fungoides (MF) is the most common subtype of CTCL, comprising roughly 50% of all cases. The etiology of CTCL and MF remains poorly understood and no unifying driver mutation has been identified.2 However, recent sequencing efforts have revealed recurrent genomics alterations primarily in 3 pathways: constitutive T-cell activation, resistance to apoptosis/cell-cycle dysregulation, and DNA structural/gene expression dysregulation.3-8 These studies, among others, support the assertion that CTCL may be an epigenetic phenomenon.9-14
Most patients with MF will experience an indolent course of skin-limited disease with a favorable prognosis and a 5-year survival rate of 88%.15-17 A large study of patients with MF (N=525) followed for more than 40 years determined that approximately 20% of early-stage (IA-IIA) patients with MF progress to develop tumors, metastasis to the lymphatic tissue, and/or leukemic blood disease.18
Cutaneous T-cell lymphoma is a chronic disease, and most treatment responses are partial and short-lived. Allogenic hematopoietic transplantation is the only potentially curative option, and all other therapies are aimed at arresting progression and achieving remission.19 Skin-directed therapies include topical steroids, topical nitrogen mustard, phototherapy, and radiation. Systemic therapies such as oral retinoids, chemotherapy, and immunotherapy may be used alone or in combination with skin-directed therapies based on the overall disease stage and clinical presentation. Unfortunately, complete response (CR) to therapy is rare and fleeting, and most patients require multiple sequential treatments over their lifetimes.20
Across all stages of CTCL, there is a therapeutic push to combination and immune-based therapies to achieve more durable responses. The imidazoquinolines are a family of toll-like receptor (TLR) agonists including imiquimod (TLR7) and resiquimod (TLR7 and TLR8). Imiquimod (IMQ) is a topical immunomodulator, which increases the local cytotoxic helper T-cell profile (T
Skin-directed chemotherapy using 5-fluorouracil (5-FU) has shown activity against many cutaneous malignancies. 5-Fluorouracil is an antimetabolite drug that inhibits thymidylate synthase, resulting in interrupted DNA and RNA synthesis and leading to an apoptotic cell death (Figure 1). It has been administered via intravenous, oral (prodrug), intralesional (IL), and topical routes with well-documented success in treating cutaneous squamous cell carcinoma, keratoacanthoma, basal cell carcinoma, and precancerous actinic keratosis.35 As a topical, 5-FU has been shown to provide a good response in 6 patients with early MF.36 In late-stage MF, 5-FU has been used in combination with methotrexate as an infusion.37 We present a single-center case series of 9 patients with CTCL who received combination IL 5-FU and IMQ cream 5%.

Methods
Patient Selection—Patients were selected from our multidisciplinary CTCL subspecialty clinic at the Inova Schar Cancer Institute (Fairfax, Virginia). Patients with single to few recalcitrant CTCL plaques or tumors that were symptomatic or otherwise bothersome were included. All patients had at least 2 prior skin-directed therapies that failed, and many had advanced-stage disease requiring systemic therapy. All patients provided verbal consent.
Study Materials and Evaluations—Patients received IL injections of 5-FU 50 mg/mL. The volume injected was approximately 0.2 cc per cubic centimeter of lesion tissue. Injections were repeated at 2- to 3-week intervals until the target lesions achieved an acute hemorrhagic phase characterized by erosion, flattening, and crust formation. The total number of serial injections administered ranged from 1 to 5. The patients concomitantly treated all lesions with IMQ cream 5% daily for a duration of 2 to 3 months.
Medical photography and physical examination were performed every 2 to 3 weeks until the hemorrhagic phase resolved and treated sites re-epithelialized. Index lesions were assessed using the Composite Assessment of Index Lesion Severity (CAILS) score by a single investigator for all patients.38 Scores were retrospectively assigned using the investigator’s detailed physical examination descriptions and extensive medical photography. Any hyperpigmentation was scored as residual disease, despite the fair interpretation of it as procedure-related postinflammatory dyspigmentation. Complete response was strictly defined as a CAILS score of 0. The patients were screened for possible systemic effects of IMQ, including the presence of fever, chills, fatigue, and myalgia. Patients were evaluated every 6 to 12 weeks as a standing follow-up.
Statistical Analysis—Reductions were calculated using local regression from baseline to the 4- to 7-week follow-up. Patients with multiple lesions had their CAILS score averaged at ea
Results
Nine patients aged 28 to 91 years (median age, 66 years) with CTCL stages IA to IVA2, who had lesions located throughout their body, achieved CR; 3 patients were female (Table 1). The most common phenotype was CD8+ (n=3). All patients had at least 2 prior skin-directed therapies at treatment sites that failed, and 1 patient had 7 prior treatments that failed. Prior treatments included a variety of modalities, including all standard-of-care options and enrollment in clinical trials. One patient died from pneumonia following CR (Table 2). Seven patients had previously received systemic therapy for CTCL, and 1 patient was stable on romidepsin during our study. In patients who received more than 1 injection of 5-FU—1 injection: 3 patients; 2 injections: 3 patients; 3 injections: 1 patient; 4 injections: 1 patient; 5 injections: 1 patient—injections were spaced by 2 to 3 weeks. There was 1 patient who initially had an inadequate dosing of IL 5-FU and was restarted 14 months later; this was the patient with 5 total injections. This occurred in one of the first patients in the study, who presented with a facial lesion. The investigator used approximately 0.02 cc per cubic centimeter (dose reduction of nearly 90%), which was inadequate and did not achieve the requisite hemorrhagic phase.

Treatment was well tolerated overall. In all cases, a hemorrhagic phase was achieved, characterized by erosion and crusting that was rated as mildly uncomfortable by 7 patients and moderately uncomfortable by 2 patients. In total, 15 lesions in all 9 patients achieved a CR within 24 weeks of the final injection. The longest treatment course required 12 weeks of therapy with IMQ and 5 IL injections of 5-FU. The fastest CR was achieved in patient 6 within 6 weeks following a single IL injection of 5-FU and 2 applications of IMQ. The average time to CR was 14.78 weeks (95% CI, 1.75-27.81)(Figure 2), and the time to CR ranged from 4 to 24 weeks. On average, patients achieved more than 50% reduction in CAILS score by 3.53 weeks (95% CI, 1.55-5.51) and nearly a 4-fold (74.7%) reduction at the time of initial follow-up (occurring at 4–7 weeks). By 7 weeks, patient 3 had the most modest improvement in CAILS score with a 2.75-fold reduction, while patient 5 had the largest decrease with a 5-fold reduction. Figure 3 shows representative clinical photographs of 2 patients before and after treatment, with all patients having similar results.

Comment
Cutaneous T-cell lymphoma is a chronic skin cancer with a pattern of limited response to therapy and frequent recurrence. Currently available skin-directed therapies function as temporizing measures rather than curative treatments. Immunotherapy offers the promise of lasting disease control even after cessation of treatment, as it may essentially awaken cutaneous immune surveillance to malignant lymphocytes.

Several small observational studies have evaluated topical IMQ and TLR agonist therapy in CTCL. The construct of prior reports varies widely, including many different pretreatments, dosing schemes, and follow-up periods.24-33 Dosing intervals with IMQ ranged from daily to 3 times per week and treatment duration from 2 weeks to 1 year. Complete response rates from 50% to 100% were reported, and partial responses were observed in all but 1 patient, with recurrence-free follow-up ranging from 6 months to 8 years. Comparatively, combining IL 5-FU and IMQ appears to be at least as effective as IMQ alone or in other sequential treatments and combinations.24-33
Resiquimod, an experimental TLR7/8 agonist, has shown promising results in CTCL. Rook et al34 conducted a phase 1 trial of topical resiquimod in 12 early-stage patients with CTCL, all of whom responded to therapy. Two patients achieved CR, and 9 achieved a partial response, including 5 patients with the folliculotropic subtype. Interestingly, an abscopal effect was observed in 92% (11/12) of patients. Molecular evidence of reduction of the malignant clone was observed in 90% of patients via high-throughput sequencing of lesional tissue.34 These exciting findings suggest that topical immune therapy with TLR agonists may achieve robust, sustained, and possibly global disease control in CTCL.
Topical therapies are limited by depth of absorption, which can present a barrier to using these treatments for thicker plaques and tumors. Combining IL and topical routes was critical in our study design. Having good clinical experience using IL 5-FU in nonmelanoma skin cancers, we hypothesized that IL 5-FU would achieve a cytotoxic response through the full depth of thicker lesions and erode the surface of these lesions to facilitate penetration of topical IMQ. We additionally hypothesized that the combination of mechanisms of action would lead to an additive or synergistic response (Figure 1). By first inducing apoptotic cell death via 5-FU, we hoped to spill malignant lymphocyte neoantigens. Coupling that antigen exposure with an enhanced T
In our case series, all 15 lesions in 9 patients completely cleared, and no recurrences were observed at 26-month follow-up. No patients encountered any major adverse events, and the procedure was well tolerated by all.
Study Limitations—Limitations of this small study certainly exist. It is impossible to prove that our mechanistic theory is accurate given our strictly clinical assessment tools. We speculate that if our results had been achieved with IL 5-FU alone, future investigation with a prospective study using multiple treatment arms including a control would be warranted. Kannangara et al36 reported the use of topical 5-FU for MF and the drug’s utility in either topical or IL routes for CTCL, which deserves further study. It is less likely that results were achieved exclusively by IMQ because of the rapid tissue breakdown observed in the acute hemorrhagic phase. This phenomenon is best explained by the sudden apoptosis caused by DNA intercalation from 5-FU. The follow-up period is not uniform because this was a rolling enrollment study. Follow-up will be ongoing, and we aim to assess all patients up to at least the 5-year point. A final limitation of this study is the purely clinical end point. In the future, pretreatment and posttreatment biopsies would be useful in assessing proof of histologic response, and high-throughput sequencing may be used to look for molecular clearance via liquid biopsy. Lastly, careful observation for possible abscopal effect using the Severity-Weighted Assessment Tool score would be interesting and potentially contributory to our understanding of the impact of topical immune therapy on cutaneous tumor surveillance.
Conclusion
Combination IL 5-FU and topical IMQ is a well-tolerated, effective, and durable therapy for recalcitrant thick plaques and tumors of CTCL. This treatment is convenient and cost-effective. The procedure is performed in less than 5 minutes in an outpatient dermatology clinic. All patients received full insurance coverage for both drug and procedure fees under Medicare and commercial carriers.
Cutaneous T-cell lymphoma (CTCL) is a diverse group of skin-homing T-cell neoplasms with a wide array of clinical presentations, immunohistopathologic subtypes, and prognoses. The age-adjusted incidence of CTCL in the United States is 6.4 per million individuals.1 In the early stages of CTCL, the malignant lymphocytes are isolated to the skin, while more advanced disease involves metastatic spread to the lymphatic and peripheral blood compartments. Mycosis fungoides (MF) is the most common subtype of CTCL, comprising roughly 50% of all cases. The etiology of CTCL and MF remains poorly understood and no unifying driver mutation has been identified.2 However, recent sequencing efforts have revealed recurrent genomics alterations primarily in 3 pathways: constitutive T-cell activation, resistance to apoptosis/cell-cycle dysregulation, and DNA structural/gene expression dysregulation.3-8 These studies, among others, support the assertion that CTCL may be an epigenetic phenomenon.9-14
Most patients with MF will experience an indolent course of skin-limited disease with a favorable prognosis and a 5-year survival rate of 88%.15-17 A large study of patients with MF (N=525) followed for more than 40 years determined that approximately 20% of early-stage (IA-IIA) patients with MF progress to develop tumors, metastasis to the lymphatic tissue, and/or leukemic blood disease.18
Cutaneous T-cell lymphoma is a chronic disease, and most treatment responses are partial and short-lived. Allogenic hematopoietic transplantation is the only potentially curative option, and all other therapies are aimed at arresting progression and achieving remission.19 Skin-directed therapies include topical steroids, topical nitrogen mustard, phototherapy, and radiation. Systemic therapies such as oral retinoids, chemotherapy, and immunotherapy may be used alone or in combination with skin-directed therapies based on the overall disease stage and clinical presentation. Unfortunately, complete response (CR) to therapy is rare and fleeting, and most patients require multiple sequential treatments over their lifetimes.20
Across all stages of CTCL, there is a therapeutic push to combination and immune-based therapies to achieve more durable responses. The imidazoquinolines are a family of toll-like receptor (TLR) agonists including imiquimod (TLR7) and resiquimod (TLR7 and TLR8). Imiquimod (IMQ) is a topical immunomodulator, which increases the local cytotoxic helper T-cell profile (T
Skin-directed chemotherapy using 5-fluorouracil (5-FU) has shown activity against many cutaneous malignancies. 5-Fluorouracil is an antimetabolite drug that inhibits thymidylate synthase, resulting in interrupted DNA and RNA synthesis and leading to an apoptotic cell death (Figure 1). It has been administered via intravenous, oral (prodrug), intralesional (IL), and topical routes with well-documented success in treating cutaneous squamous cell carcinoma, keratoacanthoma, basal cell carcinoma, and precancerous actinic keratosis.35 As a topical, 5-FU has been shown to provide a good response in 6 patients with early MF.36 In late-stage MF, 5-FU has been used in combination with methotrexate as an infusion.37 We present a single-center case series of 9 patients with CTCL who received combination IL 5-FU and IMQ cream 5%.

Methods
Patient Selection—Patients were selected from our multidisciplinary CTCL subspecialty clinic at the Inova Schar Cancer Institute (Fairfax, Virginia). Patients with single to few recalcitrant CTCL plaques or tumors that were symptomatic or otherwise bothersome were included. All patients had at least 2 prior skin-directed therapies that failed, and many had advanced-stage disease requiring systemic therapy. All patients provided verbal consent.
Study Materials and Evaluations—Patients received IL injections of 5-FU 50 mg/mL. The volume injected was approximately 0.2 cc per cubic centimeter of lesion tissue. Injections were repeated at 2- to 3-week intervals until the target lesions achieved an acute hemorrhagic phase characterized by erosion, flattening, and crust formation. The total number of serial injections administered ranged from 1 to 5. The patients concomitantly treated all lesions with IMQ cream 5% daily for a duration of 2 to 3 months.
Medical photography and physical examination were performed every 2 to 3 weeks until the hemorrhagic phase resolved and treated sites re-epithelialized. Index lesions were assessed using the Composite Assessment of Index Lesion Severity (CAILS) score by a single investigator for all patients.38 Scores were retrospectively assigned using the investigator’s detailed physical examination descriptions and extensive medical photography. Any hyperpigmentation was scored as residual disease, despite the fair interpretation of it as procedure-related postinflammatory dyspigmentation. Complete response was strictly defined as a CAILS score of 0. The patients were screened for possible systemic effects of IMQ, including the presence of fever, chills, fatigue, and myalgia. Patients were evaluated every 6 to 12 weeks as a standing follow-up.
Statistical Analysis—Reductions were calculated using local regression from baseline to the 4- to 7-week follow-up. Patients with multiple lesions had their CAILS score averaged at ea
Results
Nine patients aged 28 to 91 years (median age, 66 years) with CTCL stages IA to IVA2, who had lesions located throughout their body, achieved CR; 3 patients were female (Table 1). The most common phenotype was CD8+ (n=3). All patients had at least 2 prior skin-directed therapies at treatment sites that failed, and 1 patient had 7 prior treatments that failed. Prior treatments included a variety of modalities, including all standard-of-care options and enrollment in clinical trials. One patient died from pneumonia following CR (Table 2). Seven patients had previously received systemic therapy for CTCL, and 1 patient was stable on romidepsin during our study. In patients who received more than 1 injection of 5-FU—1 injection: 3 patients; 2 injections: 3 patients; 3 injections: 1 patient; 4 injections: 1 patient; 5 injections: 1 patient—injections were spaced by 2 to 3 weeks. There was 1 patient who initially had an inadequate dosing of IL 5-FU and was restarted 14 months later; this was the patient with 5 total injections. This occurred in one of the first patients in the study, who presented with a facial lesion. The investigator used approximately 0.02 cc per cubic centimeter (dose reduction of nearly 90%), which was inadequate and did not achieve the requisite hemorrhagic phase.

Treatment was well tolerated overall. In all cases, a hemorrhagic phase was achieved, characterized by erosion and crusting that was rated as mildly uncomfortable by 7 patients and moderately uncomfortable by 2 patients. In total, 15 lesions in all 9 patients achieved a CR within 24 weeks of the final injection. The longest treatment course required 12 weeks of therapy with IMQ and 5 IL injections of 5-FU. The fastest CR was achieved in patient 6 within 6 weeks following a single IL injection of 5-FU and 2 applications of IMQ. The average time to CR was 14.78 weeks (95% CI, 1.75-27.81)(Figure 2), and the time to CR ranged from 4 to 24 weeks. On average, patients achieved more than 50% reduction in CAILS score by 3.53 weeks (95% CI, 1.55-5.51) and nearly a 4-fold (74.7%) reduction at the time of initial follow-up (occurring at 4–7 weeks). By 7 weeks, patient 3 had the most modest improvement in CAILS score with a 2.75-fold reduction, while patient 5 had the largest decrease with a 5-fold reduction. Figure 3 shows representative clinical photographs of 2 patients before and after treatment, with all patients having similar results.

Comment
Cutaneous T-cell lymphoma is a chronic skin cancer with a pattern of limited response to therapy and frequent recurrence. Currently available skin-directed therapies function as temporizing measures rather than curative treatments. Immunotherapy offers the promise of lasting disease control even after cessation of treatment, as it may essentially awaken cutaneous immune surveillance to malignant lymphocytes.

Several small observational studies have evaluated topical IMQ and TLR agonist therapy in CTCL. The construct of prior reports varies widely, including many different pretreatments, dosing schemes, and follow-up periods.24-33 Dosing intervals with IMQ ranged from daily to 3 times per week and treatment duration from 2 weeks to 1 year. Complete response rates from 50% to 100% were reported, and partial responses were observed in all but 1 patient, with recurrence-free follow-up ranging from 6 months to 8 years. Comparatively, combining IL 5-FU and IMQ appears to be at least as effective as IMQ alone or in other sequential treatments and combinations.24-33
Resiquimod, an experimental TLR7/8 agonist, has shown promising results in CTCL. Rook et al34 conducted a phase 1 trial of topical resiquimod in 12 early-stage patients with CTCL, all of whom responded to therapy. Two patients achieved CR, and 9 achieved a partial response, including 5 patients with the folliculotropic subtype. Interestingly, an abscopal effect was observed in 92% (11/12) of patients. Molecular evidence of reduction of the malignant clone was observed in 90% of patients via high-throughput sequencing of lesional tissue.34 These exciting findings suggest that topical immune therapy with TLR agonists may achieve robust, sustained, and possibly global disease control in CTCL.
Topical therapies are limited by depth of absorption, which can present a barrier to using these treatments for thicker plaques and tumors. Combining IL and topical routes was critical in our study design. Having good clinical experience using IL 5-FU in nonmelanoma skin cancers, we hypothesized that IL 5-FU would achieve a cytotoxic response through the full depth of thicker lesions and erode the surface of these lesions to facilitate penetration of topical IMQ. We additionally hypothesized that the combination of mechanisms of action would lead to an additive or synergistic response (Figure 1). By first inducing apoptotic cell death via 5-FU, we hoped to spill malignant lymphocyte neoantigens. Coupling that antigen exposure with an enhanced T
In our case series, all 15 lesions in 9 patients completely cleared, and no recurrences were observed at 26-month follow-up. No patients encountered any major adverse events, and the procedure was well tolerated by all.
Study Limitations—Limitations of this small study certainly exist. It is impossible to prove that our mechanistic theory is accurate given our strictly clinical assessment tools. We speculate that if our results had been achieved with IL 5-FU alone, future investigation with a prospective study using multiple treatment arms including a control would be warranted. Kannangara et al36 reported the use of topical 5-FU for MF and the drug’s utility in either topical or IL routes for CTCL, which deserves further study. It is less likely that results were achieved exclusively by IMQ because of the rapid tissue breakdown observed in the acute hemorrhagic phase. This phenomenon is best explained by the sudden apoptosis caused by DNA intercalation from 5-FU. The follow-up period is not uniform because this was a rolling enrollment study. Follow-up will be ongoing, and we aim to assess all patients up to at least the 5-year point. A final limitation of this study is the purely clinical end point. In the future, pretreatment and posttreatment biopsies would be useful in assessing proof of histologic response, and high-throughput sequencing may be used to look for molecular clearance via liquid biopsy. Lastly, careful observation for possible abscopal effect using the Severity-Weighted Assessment Tool score would be interesting and potentially contributory to our understanding of the impact of topical immune therapy on cutaneous tumor surveillance.
Conclusion
Combination IL 5-FU and topical IMQ is a well-tolerated, effective, and durable therapy for recalcitrant thick plaques and tumors of CTCL. This treatment is convenient and cost-effective. The procedure is performed in less than 5 minutes in an outpatient dermatology clinic. All patients received full insurance coverage for both drug and procedure fees under Medicare and commercial carriers.
- Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973-2002. Arch Dermatol. 2007;143:854-859.
- DeSimone JA, Sodha P, Ignatova D, et al. Recent advances in primary cutaneous T-cell lymphoma. Curr Opin Oncol. 2015;27:128-133.
- Choi J, Goh G, Walradt T, et al. Genomic landscape of cutaneous T cell lymphoma. Nat Genet. 2015;47:1011-1019.
- Ungewickell A, Bhaduri A, Rios E, et al. Genomic analysis of mycosis fungoides and Sézary syndrome identifies recurrent alterations in TNFR2. Nat Genet. 2015;47:1056-1060.
- McGirt LY, Jia P, Baerenwald DA, et al. Whole-genome sequencing reveals oncogenic mutations in mycosis fungoides. Blood. 2015;126:508-519.
- da Silva Almeida AC, Abate F, Khiabanian H, et al. The mutational landscape of cutaneous T cell lymphoma and Sézary syndrome. Nat Genet. 2015;47:1465-1470.
- Litvinov IV, Netchiporouk E, Cordeiro B, et al. The use oftranscriptional profiling to improve personalized diagnosis and management of cutaneous T-cell lymphoma (CTCL). Clin Cancer Res. 2015;21:2820-2829.
- Cyrenne BM, Lewis JM, Weed JG, et al. Synergy of BCL2 and histone deacetylase inhibition against leukemic cells from cutaneous T-cell lymphoma patients. Blood. 2017;130:2073-2083.
- Cancer Genome Atlas Research Network; Weinstein JN, Collisson EA, Mills GB, et al. The Cancer Genome Atlas Pan-Cancer analysis project. Nat Genet. 2013;45:1113-1120.
- Kiel MJ, Sahasrabuddhe AA, Rolland DCM, et al. Genomic analyses reveal recurrent mutations in epigenetic modifiers and the JAK-STAT pathway in Sézary syndrome. Nat Commun. 2015;6:8470.
- Wang L, Ni X, Covington KR, et al. Genomic profiling of Sézary syndrome identifies alterations of key T cell signaling and differentiation genes. Nat Genet. 2015;47:1426-1434.
- van Doorn R, Slieker RC, Boonk SE, et al. Epigenomic analysis of Sézary syndrome defines patterns of aberrant DNA methylation and identifies diagnostic markers. J Invest Dermatol. 2016;136:1876-1884.
- Qiu L, Liu F, Yi S, et al. Loss of 5-hydroxymethylcytosine is an epigenetic biomarker in cutaneous T-cell lymphoma. J Invest Dermatol. 2018;138:2388-2397.
- Kim SR, Lewis JM, Cyrenne BM, et al. BET inhibition in advanced cutaneous T cell lymphoma is synergistically potentiated by BCL2 inhibition or HDAC inhibition. Oncotarget. 2018;9:29193-29207.
- Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703-1714.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome, part I. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-16.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Kim YH, Liu HL, Mraz-Gernhard S, et al. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857-866.
- Lechowicz MJ, Lazarus HM, Carreras J, et al. Allogeneic hematopoietic cell transplantation for mycosis fungoides and Sezary syndrome. Bone Marrow Transplant. 2014;49:1360-1365.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome, part II: prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e1-17.
- Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196-200.
- Gibson SJ, Lindh JM, Riter TR, et al. Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cell Immunol. 2002;218:74-86.
- Schön MP, Schön M. TLR7 and TLR8 as targets in cancer therapy. Oncogene. 2008;27:190-199.
- Suchin KR, Junkins-Hopkins JM, Rook AH. Treatment of stage IA cutaneous T-cell lymphoma with topical application of the immune response modifier imiquimod. Arch Dermatol. 2002;138:1137-1139.
- Dummer R, Urosevic M, Kempf W, et al. Imiquimod induces complete clearance of a PUVA-resistant plaque in mycosis fungoides. Dermatology. 2003;207:116-118.
- Didona B, Benucci R, Amerio P, et al. Primary cutaneous CD30+ T-cell lymphoma responsive to topical imiquimod (Aldara). Br J Dermatol. 2004;150:1198-1201.
- Deeths MJ, Chapman JT, Dellavalle RP, et al. Treatment of patch and plaque stage mycosis fungoides with imiquimod 5% cream. J Am Acad Dermatol. 2005;52:275-280.
- Coors EA, Schuler G, Von Den Driesch P. Topical imiquimod as treatment for different kinds of cutaneous lymphoma. Eur J Dermatol. 2006;16:391-393.
- Chiam LYT, Chan YC. Solitary plaque mycosis fungoides on the penis responding to topical imiquimod therapy. Br J Dermatol. 2007;156:560-562.
- Soler-Machín J, Gilaberte-Calzada Y, Vera-Alvarez J, et al. Imiquimod in treatment of palpebral mycosis fungoides. Article in Spanish. Arch Soc Esp Oftalmol. 2006;81:221-223.
- Martínez-González MC, Verea-Hernando MM, Yebra-Pimentel MT, et al. Imiquimod in mycosis fungoides. Eur J Dermatol. 2008;18:148-152.
- Gordon MC, Sluzevich JC, Jambusaria-Pahlajani A. Clearance of folliculotropic and tumor mycosis fungoides with topical 5% imiquimod. JAAD Case Rep. 2015;1:348-350.
- Lewis DJ, Byekova YA, Emge DA, et al. Complete resolution of mycosis fungoides tumors with imiquimod 5% cream: a case series. J Dermatolog Treat. 2017;28:567-569.
- Rook AH, Gelfand JM, Wysocka M, et al. Topical resiquimod can induce disease regression and enhance T-cell effector functions in cutaneous T-cell lymphoma. Blood. 2015;126:1452-1461.
- Morse LG, Kendrick C, Hooper D, et al. Treatment of squamous cell carcinoma with intralesional 5-fluorouracil. Dermatol Surg. 2003;29:1150-1153.
- Kannangara AP, Levitan D, Fleischer AB Jr. Six patients with early-stage cutaneous T-cell lymphoma successfully treated with topical 5-fluorouracil. J Drugs Dermatol. 2010;9:1017-1018.
- Schappell DL, Alper JC, McDonald CJ. Treatment of advanced mycosis fungoides and Sézary syndrome with continuous infusions of methotrexate followed by fluorouracil and leucovorin rescue. Arch Dermatol. 1995;131:307-313.
- Olsen EA, Whittaker S, Kim YH, et al. Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29:2598-2607.
- Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973-2002. Arch Dermatol. 2007;143:854-859.
- DeSimone JA, Sodha P, Ignatova D, et al. Recent advances in primary cutaneous T-cell lymphoma. Curr Opin Oncol. 2015;27:128-133.
- Choi J, Goh G, Walradt T, et al. Genomic landscape of cutaneous T cell lymphoma. Nat Genet. 2015;47:1011-1019.
- Ungewickell A, Bhaduri A, Rios E, et al. Genomic analysis of mycosis fungoides and Sézary syndrome identifies recurrent alterations in TNFR2. Nat Genet. 2015;47:1056-1060.
- McGirt LY, Jia P, Baerenwald DA, et al. Whole-genome sequencing reveals oncogenic mutations in mycosis fungoides. Blood. 2015;126:508-519.
- da Silva Almeida AC, Abate F, Khiabanian H, et al. The mutational landscape of cutaneous T cell lymphoma and Sézary syndrome. Nat Genet. 2015;47:1465-1470.
- Litvinov IV, Netchiporouk E, Cordeiro B, et al. The use oftranscriptional profiling to improve personalized diagnosis and management of cutaneous T-cell lymphoma (CTCL). Clin Cancer Res. 2015;21:2820-2829.
- Cyrenne BM, Lewis JM, Weed JG, et al. Synergy of BCL2 and histone deacetylase inhibition against leukemic cells from cutaneous T-cell lymphoma patients. Blood. 2017;130:2073-2083.
- Cancer Genome Atlas Research Network; Weinstein JN, Collisson EA, Mills GB, et al. The Cancer Genome Atlas Pan-Cancer analysis project. Nat Genet. 2013;45:1113-1120.
- Kiel MJ, Sahasrabuddhe AA, Rolland DCM, et al. Genomic analyses reveal recurrent mutations in epigenetic modifiers and the JAK-STAT pathway in Sézary syndrome. Nat Commun. 2015;6:8470.
- Wang L, Ni X, Covington KR, et al. Genomic profiling of Sézary syndrome identifies alterations of key T cell signaling and differentiation genes. Nat Genet. 2015;47:1426-1434.
- van Doorn R, Slieker RC, Boonk SE, et al. Epigenomic analysis of Sézary syndrome defines patterns of aberrant DNA methylation and identifies diagnostic markers. J Invest Dermatol. 2016;136:1876-1884.
- Qiu L, Liu F, Yi S, et al. Loss of 5-hydroxymethylcytosine is an epigenetic biomarker in cutaneous T-cell lymphoma. J Invest Dermatol. 2018;138:2388-2397.
- Kim SR, Lewis JM, Cyrenne BM, et al. BET inhibition in advanced cutaneous T cell lymphoma is synergistically potentiated by BCL2 inhibition or HDAC inhibition. Oncotarget. 2018;9:29193-29207.
- Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703-1714.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome, part I. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-16.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Kim YH, Liu HL, Mraz-Gernhard S, et al. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857-866.
- Lechowicz MJ, Lazarus HM, Carreras J, et al. Allogeneic hematopoietic cell transplantation for mycosis fungoides and Sezary syndrome. Bone Marrow Transplant. 2014;49:1360-1365.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome, part II: prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e1-17.
- Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196-200.
- Gibson SJ, Lindh JM, Riter TR, et al. Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cell Immunol. 2002;218:74-86.
- Schön MP, Schön M. TLR7 and TLR8 as targets in cancer therapy. Oncogene. 2008;27:190-199.
- Suchin KR, Junkins-Hopkins JM, Rook AH. Treatment of stage IA cutaneous T-cell lymphoma with topical application of the immune response modifier imiquimod. Arch Dermatol. 2002;138:1137-1139.
- Dummer R, Urosevic M, Kempf W, et al. Imiquimod induces complete clearance of a PUVA-resistant plaque in mycosis fungoides. Dermatology. 2003;207:116-118.
- Didona B, Benucci R, Amerio P, et al. Primary cutaneous CD30+ T-cell lymphoma responsive to topical imiquimod (Aldara). Br J Dermatol. 2004;150:1198-1201.
- Deeths MJ, Chapman JT, Dellavalle RP, et al. Treatment of patch and plaque stage mycosis fungoides with imiquimod 5% cream. J Am Acad Dermatol. 2005;52:275-280.
- Coors EA, Schuler G, Von Den Driesch P. Topical imiquimod as treatment for different kinds of cutaneous lymphoma. Eur J Dermatol. 2006;16:391-393.
- Chiam LYT, Chan YC. Solitary plaque mycosis fungoides on the penis responding to topical imiquimod therapy. Br J Dermatol. 2007;156:560-562.
- Soler-Machín J, Gilaberte-Calzada Y, Vera-Alvarez J, et al. Imiquimod in treatment of palpebral mycosis fungoides. Article in Spanish. Arch Soc Esp Oftalmol. 2006;81:221-223.
- Martínez-González MC, Verea-Hernando MM, Yebra-Pimentel MT, et al. Imiquimod in mycosis fungoides. Eur J Dermatol. 2008;18:148-152.
- Gordon MC, Sluzevich JC, Jambusaria-Pahlajani A. Clearance of folliculotropic and tumor mycosis fungoides with topical 5% imiquimod. JAAD Case Rep. 2015;1:348-350.
- Lewis DJ, Byekova YA, Emge DA, et al. Complete resolution of mycosis fungoides tumors with imiquimod 5% cream: a case series. J Dermatolog Treat. 2017;28:567-569.
- Rook AH, Gelfand JM, Wysocka M, et al. Topical resiquimod can induce disease regression and enhance T-cell effector functions in cutaneous T-cell lymphoma. Blood. 2015;126:1452-1461.
- Morse LG, Kendrick C, Hooper D, et al. Treatment of squamous cell carcinoma with intralesional 5-fluorouracil. Dermatol Surg. 2003;29:1150-1153.
- Kannangara AP, Levitan D, Fleischer AB Jr. Six patients with early-stage cutaneous T-cell lymphoma successfully treated with topical 5-fluorouracil. J Drugs Dermatol. 2010;9:1017-1018.
- Schappell DL, Alper JC, McDonald CJ. Treatment of advanced mycosis fungoides and Sézary syndrome with continuous infusions of methotrexate followed by fluorouracil and leucovorin rescue. Arch Dermatol. 1995;131:307-313.
- Olsen EA, Whittaker S, Kim YH, et al. Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29:2598-2607.
PRACTICE POINTS
- Cutaneous T-cell lymphoma (CTCL) is a chronic lymphoma affecting the skin with limited durable effective skin-directed therapies.
- Combination intralesional 5-fluorouracil and topical imiquimod is a well-tolerated, fast, convenient, and durable therapy for recalcitrant thick plaques and tumors of CTCL.
- This regimen may be utilized as monotherapy or as the skin-directed component of combination therapy based on disease stage.
Teaching Quality Improvement to Internal Medicine Residents to Address Patient Care Gaps in Ambulatory Quality Metrics
ABSTRACT
Objective: To teach internal medicine residents quality improvement (QI) principles in an effort to improve resident knowledge and comfort with QI, as well as address quality care gaps in resident clinic primary care patient panels.
Design: A QI curriculum was implemented for all residents rotating through a primary care block over a 6-month period. Residents completed Institute for Healthcare Improvement (IHI) modules, participated in a QI workshop, and received panel data reports, ultimately completing a plan-do-study-act (PDSA) cycle to improve colorectal cancer screening and hypertension control.
Setting and participants: This project was undertaken at Tufts Medical Center Primary Care, Boston, Massachusetts, the primary care teaching practice for all 75 internal medicine residents at Tufts Medical Center. All internal medicine residents were included, with 55 (73%) of the 75 residents completing the presurvey, and 39 (52%) completing the postsurvey.
Measurements: We administered a 10-question pre- and postsurvey looking at resident attitudes toward and comfort with QI and familiarity with their panel data as well as measured rates of colorectal cancer screening and hypertension control in resident panels.
Results: There was an increase in the numbers of residents who performed a PDSA cycle (P = .002), completed outreach based on their panel data (P = .02), and felt comfortable in both creating aim statements and designing and implementing PDSA cycles (P < .0001). The residents’ knowledge of their panel data significantly increased. There was no significant improvement in hypertension control, but there was an increase in colorectal cancer screening rates (P < .0001).
Conclusion: Providing panel data and performing targeted QI interventions can improve resident comfort with QI, translating to improvement in patient outcomes.
Keywords: quality improvement, resident education, medical education, care gaps, quality metrics.
As quality improvement (QI) has become an integral part of clinical practice, residency training programs have continued to evolve in how best to teach QI. The Accreditation Council for Graduate Medical Education (ACGME) Common Program requirements mandate that core competencies in residency programs include practice-based learning and improvement and systems-based practice.1 Residents should receive education in QI, receive data on quality metrics and benchmarks related to their patient population, and participate in QI activities. The Clinical Learning Environment Review (CLER) program was established to provide feedback to institutions on 6 focused areas, including patient safety and health care quality. In visits to institutions across the United States, the CLER committees found that many residents had limited knowledge of QI concepts and limited access to data on quality metrics and benchmarks.2
There are many barriers to implementing a QI curriculum in residency programs, and creating and maintaining successful strategies has proven challenging.3 Many QI curricula for internal medicine residents have been described in the literature, but the results of many of these studies focus on resident self-assessment of QI knowledge and numbers of projects rather than on patient outcomes.4-13 As there is some evidence suggesting that patients treated by residents have worse outcomes on ambulatory quality measures when compared with patients treated by staff physicians,14,15 it is important to also look at patient outcomes when evaluating a QI curriculum. Experts in education recommend the following to optimize learning: exposure to both didactic and experiential opportunities, connection to health system improvement efforts, and assessment of patient outcomes in addition to learner feedback.16,17 A study also found that providing panel data to residents could improve quality metrics.18
In this study, we sought to investigate the effects of a resident QI intervention during an ambulatory block on both residents’ self-assessments of QI knowledge and attitudes as well as on patient quality metrics.
Methods
Curriculum
We implemented this educational initiative at Tufts Medical Center Primary Care, Boston, Massachusetts, the primary care teaching practice for all 75 internal medicine residents at Tufts Medical Center. Co-located with the 415-bed academic medical center in downtown Boston, the practice serves more than 40,000 patients, approximately 7000 of whom are cared for by resident primary care physicians (PCPs). The internal medicine residents rotate through the primary care clinic as part of continuity clinic during ambulatory or elective blocks. In addition to continuity clinic, the residents have 2 dedicated 3-week primary care rotations during the course of an academic year. Primary care rotations consist of 5 clinic sessions a week as well as structured teaching sessions. Each resident inherits a panel of patients from an outgoing senior resident, with an average panel size of 96 patients per resident.
Prior to this study intervention, we did not do any formal QI teaching to our residents as part of their primary care curriculum, and previous panel management had focused more on chart reviews of patients whom residents perceived to be higher risk. Residents from all 3 years were included in the intervention. We taught a QI curriculum to our residents from January 2018 to June 2018 during the 3-week primary care rotation, which consisted of the following components:
- Institute for Healthcare Improvement (IHI) module QI 102 completed independently online.
- A 2-hour QI workshop led by 1 of 2 primary care faculty with backgrounds in QI, during which residents were taught basic principles of QI, including how to craft aim statements and design plan-do-study-act (PDSA) cycles, and participated in a hands-on QI activity designed to model rapid cycle improvement (the Paper Airplane Factory19).
- Distribution of individualized reports of residents’ patient panel data by email at the start of the primary care block that detailed patients’ overall rates of colorectal cancer screening and hypertension (HTN) control, along with the average resident panel rates and the average attending panel rates. The reports also included a list of all residents’ patients who were overdue for colorectal cancer screening or whose last blood pressure (BP) was uncontrolled (systolic BP ≥ 140 mm Hg or diastolic BP ≥ 90 mm Hg). These reports were originally designed by our practice’s QI team and run and exported in Microsoft Excel format monthly by our information technology (IT) administrator.
- Instruction on aim statements as a group, followed by the expectation that each resident create an individualized aim statement tailored to each resident’s patient panel rates, with the PDSA cycle to be implemented during the remainder of the primary care rotation, focusing on improvement of colorectal cancer screening and HTN control (see supplementary eFigure 1 online for the worksheet used for the workshop).
- Residents were held accountable for their interventions by various check-ins. At the end of the primary care block, residents were required to submit their completed worksheets showing the intervention they had undertaken and when it was performed. The 2 primary care attendings primarily responsible for QI education would review the resident’s work approximately 1 to 2 months after they submitted their worksheets describing their intervention. These attendings sent the residents personalized feedback based on whether the intervention had been completed or successful as evidenced by documentation in the chart, including direct patient outreach by phone, letter, or portal; outreach to the resident coordinator; scheduled follow-up appointment; or booking or completion of colorectal cancer screening. Along with this feedback, residents were also sent suggestions for next steps. Resident preceptors were copied on the email to facilitate reinforcement of the goals and plans. Finally, the resident preceptors also helped with accountability by going through the residents’ worksheets and patient panel metrics with the residents during biannual evaluations.
Evaluation
Residents were surveyed with a 10-item questionnaire pre and post intervention regarding their attitudes toward QI, understanding of QI principles, and familiarity with their patient panel data. Surveys were anonymous and distributed via the SurveyMonkey platform (see supplementary eFigure 2 online). Residents were asked if they had ever performed a PDSA cycle, performed patient outreach, or performed an intervention and whether they knew the rates of diabetes, HTN, and colorectal cancer screening in their patient panels. Questions rated on a 5-point Likert scale were used to assess comfort with panel management, developing an aim statement, designing and implementing a PDSA cycle, as well as interest in pursuing QI as a career. For the purposes of analysis, these questions were dichotomized into “somewhat comfortable” and “very comfortable” vs “neutral,” “somewhat uncomfortable,” and “very uncomfortable.” Similarly, we dichotomized the question about interest in QI as a career into “somewhat interested” and “very interested” vs “neutral,” “somewhat disinterested,” and “very disinterested.” As the surveys were anonymous, we were unable to pair the pre- and postintervention surveys and used a chi-square test to evaluate whether there was an association between survey assessments pre intervention vs post intervention and a positive or negative response to the question.
We also examined rates of HTN control and colorectal cancer screening in all 75 resident panels pre and post intervention. The paired t-test was used to determine whether the mean change from pre to post intervention was significant. SAS 9.4 (SAS Institute Inc.) was used for all analyses. Institutional Review Board exemption was obtained from the Tufts Medical Center IRB. There was no funding received for this study.
Results
Respondents
Of the 75 residents, 55 (73%) completed the survey prior to the intervention, and 39 (52%) completed the survey after the intervention.
Panel Knowledge and Intervention
Prior to the intervention, 45% of residents had performed a PDSA cycle, compared with 77% post intervention, which was a significant increase (P = .002) (Table 1). Sixty-two percent of residents had performed outreach or an intervention based on their patient panel reports prior to the intervention, compared with 85% of residents post intervention, which was also a significant increase (P = .02). The increase post intervention was not 100%, as there were residents who either missed the initial workshop or who did not follow through with their planned intervention. Common interventions included the residents giving their coordinators a list of patients to call to schedule appointments, utilizing fellow team members (eg, pharmacists, social workers) for targeted patient outreach, or calling patients themselves to reestablish a connection.
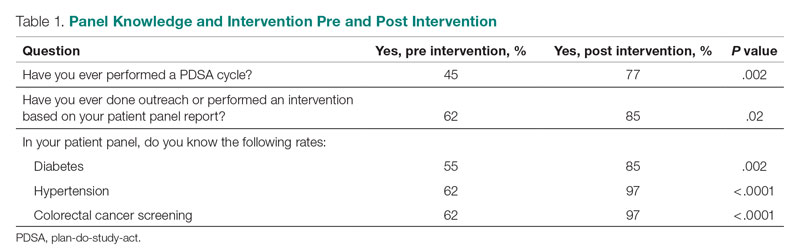
In terms of knowledge of their patient panels, prior to the intervention, 55%, 62%, and 62% of residents knew the rates of patients in their panel with diabetes, HTN, and colorectal cancer screening, respectively. After the intervention, the residents’ knowledge of these rates increased significantly, to 85% for diabetes (P = .002), 97% for HTN (P < .0001), and 97% for colorectal cancer screening (P < .0001).
Comfort With QI Approaches
Prior to the intervention, 82% of residents were comfortable managing their primary care panel, which did not change significantly post intervention (Table 2). The residents’ comfort with designing an aim statement did significantly increase, from 55% to 95% (P < .0001). The residents also had a significant increase in comfort with both designing and implementing a PDSA cycle. Prior to the intervention, 22% felt comfortable designing a PDSA cycle, which increased to 79% (P < .0001) post intervention, and 24% felt comfortable implementing a PDSA cycle, which increased to 77% (P < .0001) post intervention.
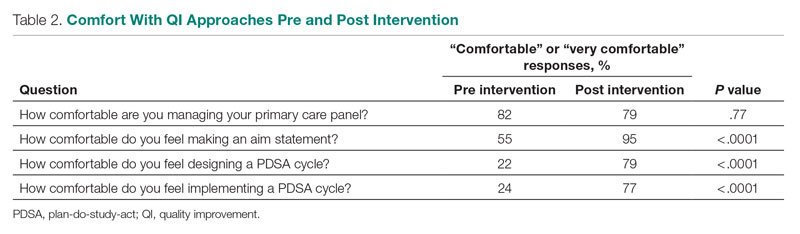
Patient Outcome Measures
The rate of HTN control in the residents' patient panels did not change significantly pre and post intervention (Table 3). The rate of resident patients who were up to date with colorectal cancer screening increased by 6.5% post intervention (P < .0001).
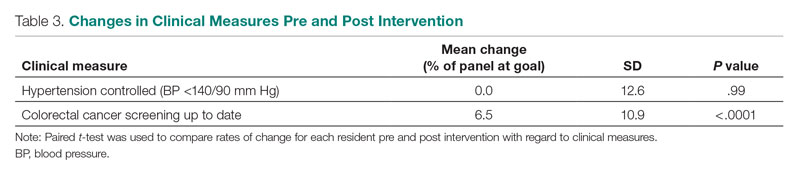
Interest in QI as a Career
As part of the survey, residents were asked how interested they were in making QI a part of their career. Fifty percent of residents indicated an interest in QI pre intervention, and 54% indicated an interest post intervention, which was not a significant difference (P = .72).
Discussion
In this study, we found that integration of a QI curriculum into a primary care rotation improved both residents’ knowledge of their patient panels and comfort with QI approaches, which translated to improvement in patient outcomes. Several previous studies have found improvements in resident self-assessment or knowledge after implementation of a QI curriculum.4-13 Liao et al implemented a longitudinal curriculum including both didactic and experiential components and found an improvement in both QI confidence and knowledge.3 Similarly, Duello et al8 found that a curriculum including both didactic lectures and QI projects improved subjective QI knowledge and comfort. Interestingly, Fok and Wong9 found that resident knowledge could be sustained post curriculum after completion of a QI project, suggesting that experiential learning may be helpful in maintaining knowledge.
Studies also have looked at providing performance data to residents. Hwang et al18 found that providing audit and feedback in the form of individual panel performance data to residents compared with practice targets led to statistically significant improvement in cancer screening rates and composite quality score, indicating that there is tremendous potential in providing residents with their data. While the ACGME mandates that residents should receive data on their quality metrics, on CLER visits, many residents interviewed noted limited access to data on their metrics and benchmarks.1,2
Though previous studies have individually looked at teaching QI concepts, providing panel data, or targeting select metrics, our study was unique in that it reviewed both self-reported resident outcomes data as well as actual patient outcomes. In addition to finding increased knowledge of patient panels and comfort with QI approaches, we found a significant increase in colorectal cancer screening rates post intervention. We thought this finding was particularly important given some data that residents' patients have been found to have worse outcomes on quality metrics compared with patients cared for by staff physicians.14,15 Given that having a resident physician as a PCP has been associated with failing to meet quality measures, it is especially important to focus targeted quality improvement initiatives in this patient population to reduce disparities in care.
We found that residents had improved knowledge on their patient panels as a result of this initiative. The residents were noted to have a higher knowledge of their HTN and colorectal cancer screening rates in comparison to their diabetes metrics. We suspect this is because residents are provided with multiple metrics related to diabetes, including process measures such as A1c testing, as well as outcome measures such as A1c control, so it may be harder for them to elucidate exactly how they are doing with their diabetes patients, whereas in HTN control and colorectal cancer screening, there is only 1 associated metric. Interestingly, even though HTN and colorectal cancer screening were the 2 measures focused on in the study, the residents had a significant improvement in knowledge of the rates of diabetes in their panel as well. This suggests that even just receiving data alone is valuable, hopefully translating to better outcomes with better baseline understanding of panels. We believe that our intervention was successful because it included both a didactic and an experiential component, as well as the use of individual panel performance data.
There were several limitations to our study. It was performed at a single institution, translating to a small sample size. Our data analysis was limited because we were unable to pair our pre- and postintervention survey responses because we used an anonymous survey. We also did not have full participation in postintervention surveys from all residents, which may have biased the study in favor of high performers. Another limitation was that our survey relied on self-reported outcomes for the questions about the residents knowing their patient panels.
This study required a 2-hour workshop every 3 weeks led by a faculty member trained in QI. Given the amount of time needed for the curriculum, this study may be difficult to replicate at other institutions, especially if faculty with an interest or training in QI are not available. Given our finding that residents had increased knowledge of their patient panels after receiving panel metrics, simply providing data with the goal of smaller, focused interventions may be easier to implement. At our institution, we discontinued the longer 2-hour QI workshops designed to teach QI approaches more broadly. We continue to provide individualized panel data to all residents during their primary care rotations and conduct half-hour, small group workshops with the interns that focus on drafting aim statements and planning interventions. All residents are required to submit worksheets to us at the end of their primary care blocks listing their current rates of each predetermined metric and laying out their aim statements and planned interventions. Residents also continue to receive feedback from our faculty with expertise in QI afterward on their plans and evidence of follow-through in the chart, with their preceptors included on the feedback emails. Even without the larger QI workshop, this approach has continued to be successful and appreciated. In fact, it does appear as though improvement in colorectal cancer screening has been sustained over several years. At the end of our study period, the resident patient colorectal cancer screening rate rose from 34% to 43%, and for the 2021-2022 academic year, the rate rose further, from 46% to 50%.
Given that the resident clinic patient population is at higher risk overall, targeted outreach and approaches to improve quality must be continued. Future areas of research include looking at which interventions, whether QI curriculum, provision of panel data, or required panel management interventions, translate to the greatest improvements in patient outcomes in this vulnerable population.
Conclusion
Our study showed that a dedicated QI curriculum for the residents and access to quality metric data improved both resident knowledge and comfort with QI approaches. Beyond resident-centered outcomes, there was also translation to improved patient outcomes, with a significant increase in colon cancer screening rates post intervention.
Corresponding author: Kinjalika Sathi, MD, 800 Washington St., Boston, MA 02111; [email protected]
Disclosures: None reported.
1. Accreditation Council for Graduate Medical Education. ACGME Common Program Requirements (Residency). Approved June 13, 2021. Updated July 1, 2022. Accessed December 29, 2022. https://www.acgme.org/globalassets/pfassets/programrequirements/cprresidency_2022v3.pdf
2. Koh NJ, Wagner R, Newton RC, et al; on behalf of the CLER Evaluation Committee and the CLER Program. CLER National Report of Findings 2021. Accreditation Council for Graduate Medical Education; 2021. Accessed December 29, 2022. https://www.acgme.org/globalassets/pdfs/cler/2021clernationalreportoffindings.pdf
3. Liao JM, Co JP, Kachalia A. Providing educational content and context for training the next generation of physicians in quality improvement. Acad Med. 2015;90(9):1241-1245. doi:10.1097/ACM.0000000000000799
4. Johnson KM, Fiordellisi W, Kuperman E, et al. X + Y = time for QI: meaningful engagement of residents in quality improvement during the ambulatory block. J Grad Med Educ. 2018;10(3):316-324. doi:10.4300/JGME-D-17-00761.1
5. Kesari K, Ali S, Smith S. Integrating residents with institutional quality improvement teams. Med Educ. 2017;51(11):1173. doi:10.1111/medu.13431
6. Ogrinc G, Cohen ES, van Aalst R, et al. Clinical and educational outcomes of an integrated inpatient quality improvement curriculum for internal medicine residents. J Grad Med Educ. 2016;8(4):563-568. doi:10.4300/JGME-D-15-00412.1
7. Malayala SV, Qazi KJ, Samdani AJ, et al. A multidisciplinary performance improvement rotation in an internal medicine training program. Int J Med Educ. 2016;7:212-213. doi:10.5116/ijme.5765.0bda
8. Duello K, Louh I, Greig H, et al. Residents’ knowledge of quality improvement: the impact of using a group project curriculum. Postgrad Med J. 2015;91(1078):431-435. doi:10.1136/postgradmedj-2014-132886
9. Fok MC, Wong RY. Impact of a competency based curriculum on quality improvement among internal medicine residents. BMC Med Educ. 2014;14:252. doi:10.1186/s12909-014-0252-7
10. Wilper AP, Smith CS, Weppner W. Instituting systems-based practice and practice-based learning and improvement: a curriculum of inquiry. Med Educ Online. 2013;18:21612. doi:10.3402/meo.v18i0.21612
11. Weigel C, Suen W, Gupte G. Using lean methodology to teach quality improvement to internal medicine residents at a safety net hospital. Am J Med Qual. 2013;28(5):392-399. doi:10.1177/1062860612474062
12. Tomolo AM, Lawrence RH, Watts B, et al. Pilot study evaluating a practice-based learning and improvement curriculum focusing on the development of system-level quality improvement skills. J Grad Med Educ. 2011;3(1):49-58. doi:10.4300/JGME-D-10-00104.1
13. Djuricich AM, Ciccarelli M, Swigonski NL. A continuous quality improvement curriculum for residents: addressing core competency, improving systems. Acad Med. 2004;79(10 Suppl):S65-S67. doi:10.1097/00001888-200410001-00020
14. Essien UR, He W, Ray A, et al. Disparities in quality of primary care by resident and staff physicians: is there a conflict between training and equity? J Gen Intern Med. 2019;34(7):1184-1191. doi:10.1007/s11606-019-04960-5
15. Amat M, Norian E, Graham KL. Unmasking a vulnerable patient care process: a qualitative study describing the current state of resident continuity clinic in a nationwide cohort of internal medicine residency programs. Am J Med. 2022;135(6):783-786. doi:10.1016/j.amjmed.2022.02.007
16. Wong BM, Etchells EE, Kuper A, et al. Teaching quality improvement and patient safety to trainees: a systematic review. Acad Med. 2010;85(9):1425-1439. doi:10.1097/ACM.0b013e3181e2d0c6
17. Armstrong G, Headrick L, Madigosky W, et al. Designing education to improve care. Jt Comm J Qual Patient Saf. 2012;38:5-14. doi:10.1016/s1553-7250(12)38002-1
18. Hwang AS, Harding AS, Chang Y, et al. An audit and feedback intervention to improve internal medicine residents’ performance on ambulatory quality measures: a randomized controlled trial. Popul Health Manag. 2019;22(6):529-535. doi:10.1089/pop.2018.0217
19. Institute for Healthcare Improvement. Open school. The paper airplane factory. Accessed December 29, 2022. https://www.ihi.org/education/IHIOpenSchool/resources/Pages/Activities/PaperAirplaneFactory.aspx
ABSTRACT
Objective: To teach internal medicine residents quality improvement (QI) principles in an effort to improve resident knowledge and comfort with QI, as well as address quality care gaps in resident clinic primary care patient panels.
Design: A QI curriculum was implemented for all residents rotating through a primary care block over a 6-month period. Residents completed Institute for Healthcare Improvement (IHI) modules, participated in a QI workshop, and received panel data reports, ultimately completing a plan-do-study-act (PDSA) cycle to improve colorectal cancer screening and hypertension control.
Setting and participants: This project was undertaken at Tufts Medical Center Primary Care, Boston, Massachusetts, the primary care teaching practice for all 75 internal medicine residents at Tufts Medical Center. All internal medicine residents were included, with 55 (73%) of the 75 residents completing the presurvey, and 39 (52%) completing the postsurvey.
Measurements: We administered a 10-question pre- and postsurvey looking at resident attitudes toward and comfort with QI and familiarity with their panel data as well as measured rates of colorectal cancer screening and hypertension control in resident panels.
Results: There was an increase in the numbers of residents who performed a PDSA cycle (P = .002), completed outreach based on their panel data (P = .02), and felt comfortable in both creating aim statements and designing and implementing PDSA cycles (P < .0001). The residents’ knowledge of their panel data significantly increased. There was no significant improvement in hypertension control, but there was an increase in colorectal cancer screening rates (P < .0001).
Conclusion: Providing panel data and performing targeted QI interventions can improve resident comfort with QI, translating to improvement in patient outcomes.
Keywords: quality improvement, resident education, medical education, care gaps, quality metrics.
As quality improvement (QI) has become an integral part of clinical practice, residency training programs have continued to evolve in how best to teach QI. The Accreditation Council for Graduate Medical Education (ACGME) Common Program requirements mandate that core competencies in residency programs include practice-based learning and improvement and systems-based practice.1 Residents should receive education in QI, receive data on quality metrics and benchmarks related to their patient population, and participate in QI activities. The Clinical Learning Environment Review (CLER) program was established to provide feedback to institutions on 6 focused areas, including patient safety and health care quality. In visits to institutions across the United States, the CLER committees found that many residents had limited knowledge of QI concepts and limited access to data on quality metrics and benchmarks.2
There are many barriers to implementing a QI curriculum in residency programs, and creating and maintaining successful strategies has proven challenging.3 Many QI curricula for internal medicine residents have been described in the literature, but the results of many of these studies focus on resident self-assessment of QI knowledge and numbers of projects rather than on patient outcomes.4-13 As there is some evidence suggesting that patients treated by residents have worse outcomes on ambulatory quality measures when compared with patients treated by staff physicians,14,15 it is important to also look at patient outcomes when evaluating a QI curriculum. Experts in education recommend the following to optimize learning: exposure to both didactic and experiential opportunities, connection to health system improvement efforts, and assessment of patient outcomes in addition to learner feedback.16,17 A study also found that providing panel data to residents could improve quality metrics.18
In this study, we sought to investigate the effects of a resident QI intervention during an ambulatory block on both residents’ self-assessments of QI knowledge and attitudes as well as on patient quality metrics.
Methods
Curriculum
We implemented this educational initiative at Tufts Medical Center Primary Care, Boston, Massachusetts, the primary care teaching practice for all 75 internal medicine residents at Tufts Medical Center. Co-located with the 415-bed academic medical center in downtown Boston, the practice serves more than 40,000 patients, approximately 7000 of whom are cared for by resident primary care physicians (PCPs). The internal medicine residents rotate through the primary care clinic as part of continuity clinic during ambulatory or elective blocks. In addition to continuity clinic, the residents have 2 dedicated 3-week primary care rotations during the course of an academic year. Primary care rotations consist of 5 clinic sessions a week as well as structured teaching sessions. Each resident inherits a panel of patients from an outgoing senior resident, with an average panel size of 96 patients per resident.
Prior to this study intervention, we did not do any formal QI teaching to our residents as part of their primary care curriculum, and previous panel management had focused more on chart reviews of patients whom residents perceived to be higher risk. Residents from all 3 years were included in the intervention. We taught a QI curriculum to our residents from January 2018 to June 2018 during the 3-week primary care rotation, which consisted of the following components:
- Institute for Healthcare Improvement (IHI) module QI 102 completed independently online.
- A 2-hour QI workshop led by 1 of 2 primary care faculty with backgrounds in QI, during which residents were taught basic principles of QI, including how to craft aim statements and design plan-do-study-act (PDSA) cycles, and participated in a hands-on QI activity designed to model rapid cycle improvement (the Paper Airplane Factory19).
- Distribution of individualized reports of residents’ patient panel data by email at the start of the primary care block that detailed patients’ overall rates of colorectal cancer screening and hypertension (HTN) control, along with the average resident panel rates and the average attending panel rates. The reports also included a list of all residents’ patients who were overdue for colorectal cancer screening or whose last blood pressure (BP) was uncontrolled (systolic BP ≥ 140 mm Hg or diastolic BP ≥ 90 mm Hg). These reports were originally designed by our practice’s QI team and run and exported in Microsoft Excel format monthly by our information technology (IT) administrator.
- Instruction on aim statements as a group, followed by the expectation that each resident create an individualized aim statement tailored to each resident’s patient panel rates, with the PDSA cycle to be implemented during the remainder of the primary care rotation, focusing on improvement of colorectal cancer screening and HTN control (see supplementary eFigure 1 online for the worksheet used for the workshop).
- Residents were held accountable for their interventions by various check-ins. At the end of the primary care block, residents were required to submit their completed worksheets showing the intervention they had undertaken and when it was performed. The 2 primary care attendings primarily responsible for QI education would review the resident’s work approximately 1 to 2 months after they submitted their worksheets describing their intervention. These attendings sent the residents personalized feedback based on whether the intervention had been completed or successful as evidenced by documentation in the chart, including direct patient outreach by phone, letter, or portal; outreach to the resident coordinator; scheduled follow-up appointment; or booking or completion of colorectal cancer screening. Along with this feedback, residents were also sent suggestions for next steps. Resident preceptors were copied on the email to facilitate reinforcement of the goals and plans. Finally, the resident preceptors also helped with accountability by going through the residents’ worksheets and patient panel metrics with the residents during biannual evaluations.

Evaluation
Residents were surveyed with a 10-item questionnaire pre and post intervention regarding their attitudes toward QI, understanding of QI principles, and familiarity with their patient panel data. Surveys were anonymous and distributed via the SurveyMonkey platform (see supplementary eFigure 2 online). Residents were asked if they had ever performed a PDSA cycle, performed patient outreach, or performed an intervention and whether they knew the rates of diabetes, HTN, and colorectal cancer screening in their patient panels. Questions rated on a 5-point Likert scale were used to assess comfort with panel management, developing an aim statement, designing and implementing a PDSA cycle, as well as interest in pursuing QI as a career. For the purposes of analysis, these questions were dichotomized into “somewhat comfortable” and “very comfortable” vs “neutral,” “somewhat uncomfortable,” and “very uncomfortable.” Similarly, we dichotomized the question about interest in QI as a career into “somewhat interested” and “very interested” vs “neutral,” “somewhat disinterested,” and “very disinterested.” As the surveys were anonymous, we were unable to pair the pre- and postintervention surveys and used a chi-square test to evaluate whether there was an association between survey assessments pre intervention vs post intervention and a positive or negative response to the question.

We also examined rates of HTN control and colorectal cancer screening in all 75 resident panels pre and post intervention. The paired t-test was used to determine whether the mean change from pre to post intervention was significant. SAS 9.4 (SAS Institute Inc.) was used for all analyses. Institutional Review Board exemption was obtained from the Tufts Medical Center IRB. There was no funding received for this study.
Results
Respondents
Of the 75 residents, 55 (73%) completed the survey prior to the intervention, and 39 (52%) completed the survey after the intervention.
Panel Knowledge and Intervention
Prior to the intervention, 45% of residents had performed a PDSA cycle, compared with 77% post intervention, which was a significant increase (P = .002) (Table 1). Sixty-two percent of residents had performed outreach or an intervention based on their patient panel reports prior to the intervention, compared with 85% of residents post intervention, which was also a significant increase (P = .02). The increase post intervention was not 100%, as there were residents who either missed the initial workshop or who did not follow through with their planned intervention. Common interventions included the residents giving their coordinators a list of patients to call to schedule appointments, utilizing fellow team members (eg, pharmacists, social workers) for targeted patient outreach, or calling patients themselves to reestablish a connection.

In terms of knowledge of their patient panels, prior to the intervention, 55%, 62%, and 62% of residents knew the rates of patients in their panel with diabetes, HTN, and colorectal cancer screening, respectively. After the intervention, the residents’ knowledge of these rates increased significantly, to 85% for diabetes (P = .002), 97% for HTN (P < .0001), and 97% for colorectal cancer screening (P < .0001).
Comfort With QI Approaches
Prior to the intervention, 82% of residents were comfortable managing their primary care panel, which did not change significantly post intervention (Table 2). The residents’ comfort with designing an aim statement did significantly increase, from 55% to 95% (P < .0001). The residents also had a significant increase in comfort with both designing and implementing a PDSA cycle. Prior to the intervention, 22% felt comfortable designing a PDSA cycle, which increased to 79% (P < .0001) post intervention, and 24% felt comfortable implementing a PDSA cycle, which increased to 77% (P < .0001) post intervention.

Patient Outcome Measures
The rate of HTN control in the residents' patient panels did not change significantly pre and post intervention (Table 3). The rate of resident patients who were up to date with colorectal cancer screening increased by 6.5% post intervention (P < .0001).

Interest in QI as a Career
As part of the survey, residents were asked how interested they were in making QI a part of their career. Fifty percent of residents indicated an interest in QI pre intervention, and 54% indicated an interest post intervention, which was not a significant difference (P = .72).
Discussion
In this study, we found that integration of a QI curriculum into a primary care rotation improved both residents’ knowledge of their patient panels and comfort with QI approaches, which translated to improvement in patient outcomes. Several previous studies have found improvements in resident self-assessment or knowledge after implementation of a QI curriculum.4-13 Liao et al implemented a longitudinal curriculum including both didactic and experiential components and found an improvement in both QI confidence and knowledge.3 Similarly, Duello et al8 found that a curriculum including both didactic lectures and QI projects improved subjective QI knowledge and comfort. Interestingly, Fok and Wong9 found that resident knowledge could be sustained post curriculum after completion of a QI project, suggesting that experiential learning may be helpful in maintaining knowledge.
Studies also have looked at providing performance data to residents. Hwang et al18 found that providing audit and feedback in the form of individual panel performance data to residents compared with practice targets led to statistically significant improvement in cancer screening rates and composite quality score, indicating that there is tremendous potential in providing residents with their data. While the ACGME mandates that residents should receive data on their quality metrics, on CLER visits, many residents interviewed noted limited access to data on their metrics and benchmarks.1,2
Though previous studies have individually looked at teaching QI concepts, providing panel data, or targeting select metrics, our study was unique in that it reviewed both self-reported resident outcomes data as well as actual patient outcomes. In addition to finding increased knowledge of patient panels and comfort with QI approaches, we found a significant increase in colorectal cancer screening rates post intervention. We thought this finding was particularly important given some data that residents' patients have been found to have worse outcomes on quality metrics compared with patients cared for by staff physicians.14,15 Given that having a resident physician as a PCP has been associated with failing to meet quality measures, it is especially important to focus targeted quality improvement initiatives in this patient population to reduce disparities in care.
We found that residents had improved knowledge on their patient panels as a result of this initiative. The residents were noted to have a higher knowledge of their HTN and colorectal cancer screening rates in comparison to their diabetes metrics. We suspect this is because residents are provided with multiple metrics related to diabetes, including process measures such as A1c testing, as well as outcome measures such as A1c control, so it may be harder for them to elucidate exactly how they are doing with their diabetes patients, whereas in HTN control and colorectal cancer screening, there is only 1 associated metric. Interestingly, even though HTN and colorectal cancer screening were the 2 measures focused on in the study, the residents had a significant improvement in knowledge of the rates of diabetes in their panel as well. This suggests that even just receiving data alone is valuable, hopefully translating to better outcomes with better baseline understanding of panels. We believe that our intervention was successful because it included both a didactic and an experiential component, as well as the use of individual panel performance data.
There were several limitations to our study. It was performed at a single institution, translating to a small sample size. Our data analysis was limited because we were unable to pair our pre- and postintervention survey responses because we used an anonymous survey. We also did not have full participation in postintervention surveys from all residents, which may have biased the study in favor of high performers. Another limitation was that our survey relied on self-reported outcomes for the questions about the residents knowing their patient panels.
This study required a 2-hour workshop every 3 weeks led by a faculty member trained in QI. Given the amount of time needed for the curriculum, this study may be difficult to replicate at other institutions, especially if faculty with an interest or training in QI are not available. Given our finding that residents had increased knowledge of their patient panels after receiving panel metrics, simply providing data with the goal of smaller, focused interventions may be easier to implement. At our institution, we discontinued the longer 2-hour QI workshops designed to teach QI approaches more broadly. We continue to provide individualized panel data to all residents during their primary care rotations and conduct half-hour, small group workshops with the interns that focus on drafting aim statements and planning interventions. All residents are required to submit worksheets to us at the end of their primary care blocks listing their current rates of each predetermined metric and laying out their aim statements and planned interventions. Residents also continue to receive feedback from our faculty with expertise in QI afterward on their plans and evidence of follow-through in the chart, with their preceptors included on the feedback emails. Even without the larger QI workshop, this approach has continued to be successful and appreciated. In fact, it does appear as though improvement in colorectal cancer screening has been sustained over several years. At the end of our study period, the resident patient colorectal cancer screening rate rose from 34% to 43%, and for the 2021-2022 academic year, the rate rose further, from 46% to 50%.
Given that the resident clinic patient population is at higher risk overall, targeted outreach and approaches to improve quality must be continued. Future areas of research include looking at which interventions, whether QI curriculum, provision of panel data, or required panel management interventions, translate to the greatest improvements in patient outcomes in this vulnerable population.
Conclusion
Our study showed that a dedicated QI curriculum for the residents and access to quality metric data improved both resident knowledge and comfort with QI approaches. Beyond resident-centered outcomes, there was also translation to improved patient outcomes, with a significant increase in colon cancer screening rates post intervention.
Corresponding author: Kinjalika Sathi, MD, 800 Washington St., Boston, MA 02111; [email protected]
Disclosures: None reported.
ABSTRACT
Objective: To teach internal medicine residents quality improvement (QI) principles in an effort to improve resident knowledge and comfort with QI, as well as address quality care gaps in resident clinic primary care patient panels.
Design: A QI curriculum was implemented for all residents rotating through a primary care block over a 6-month period. Residents completed Institute for Healthcare Improvement (IHI) modules, participated in a QI workshop, and received panel data reports, ultimately completing a plan-do-study-act (PDSA) cycle to improve colorectal cancer screening and hypertension control.
Setting and participants: This project was undertaken at Tufts Medical Center Primary Care, Boston, Massachusetts, the primary care teaching practice for all 75 internal medicine residents at Tufts Medical Center. All internal medicine residents were included, with 55 (73%) of the 75 residents completing the presurvey, and 39 (52%) completing the postsurvey.
Measurements: We administered a 10-question pre- and postsurvey looking at resident attitudes toward and comfort with QI and familiarity with their panel data as well as measured rates of colorectal cancer screening and hypertension control in resident panels.
Results: There was an increase in the numbers of residents who performed a PDSA cycle (P = .002), completed outreach based on their panel data (P = .02), and felt comfortable in both creating aim statements and designing and implementing PDSA cycles (P < .0001). The residents’ knowledge of their panel data significantly increased. There was no significant improvement in hypertension control, but there was an increase in colorectal cancer screening rates (P < .0001).
Conclusion: Providing panel data and performing targeted QI interventions can improve resident comfort with QI, translating to improvement in patient outcomes.
Keywords: quality improvement, resident education, medical education, care gaps, quality metrics.
As quality improvement (QI) has become an integral part of clinical practice, residency training programs have continued to evolve in how best to teach QI. The Accreditation Council for Graduate Medical Education (ACGME) Common Program requirements mandate that core competencies in residency programs include practice-based learning and improvement and systems-based practice.1 Residents should receive education in QI, receive data on quality metrics and benchmarks related to their patient population, and participate in QI activities. The Clinical Learning Environment Review (CLER) program was established to provide feedback to institutions on 6 focused areas, including patient safety and health care quality. In visits to institutions across the United States, the CLER committees found that many residents had limited knowledge of QI concepts and limited access to data on quality metrics and benchmarks.2
There are many barriers to implementing a QI curriculum in residency programs, and creating and maintaining successful strategies has proven challenging.3 Many QI curricula for internal medicine residents have been described in the literature, but the results of many of these studies focus on resident self-assessment of QI knowledge and numbers of projects rather than on patient outcomes.4-13 As there is some evidence suggesting that patients treated by residents have worse outcomes on ambulatory quality measures when compared with patients treated by staff physicians,14,15 it is important to also look at patient outcomes when evaluating a QI curriculum. Experts in education recommend the following to optimize learning: exposure to both didactic and experiential opportunities, connection to health system improvement efforts, and assessment of patient outcomes in addition to learner feedback.16,17 A study also found that providing panel data to residents could improve quality metrics.18
In this study, we sought to investigate the effects of a resident QI intervention during an ambulatory block on both residents’ self-assessments of QI knowledge and attitudes as well as on patient quality metrics.
Methods
Curriculum
We implemented this educational initiative at Tufts Medical Center Primary Care, Boston, Massachusetts, the primary care teaching practice for all 75 internal medicine residents at Tufts Medical Center. Co-located with the 415-bed academic medical center in downtown Boston, the practice serves more than 40,000 patients, approximately 7000 of whom are cared for by resident primary care physicians (PCPs). The internal medicine residents rotate through the primary care clinic as part of continuity clinic during ambulatory or elective blocks. In addition to continuity clinic, the residents have 2 dedicated 3-week primary care rotations during the course of an academic year. Primary care rotations consist of 5 clinic sessions a week as well as structured teaching sessions. Each resident inherits a panel of patients from an outgoing senior resident, with an average panel size of 96 patients per resident.
Prior to this study intervention, we did not do any formal QI teaching to our residents as part of their primary care curriculum, and previous panel management had focused more on chart reviews of patients whom residents perceived to be higher risk. Residents from all 3 years were included in the intervention. We taught a QI curriculum to our residents from January 2018 to June 2018 during the 3-week primary care rotation, which consisted of the following components:
- Institute for Healthcare Improvement (IHI) module QI 102 completed independently online.
- A 2-hour QI workshop led by 1 of 2 primary care faculty with backgrounds in QI, during which residents were taught basic principles of QI, including how to craft aim statements and design plan-do-study-act (PDSA) cycles, and participated in a hands-on QI activity designed to model rapid cycle improvement (the Paper Airplane Factory19).
- Distribution of individualized reports of residents’ patient panel data by email at the start of the primary care block that detailed patients’ overall rates of colorectal cancer screening and hypertension (HTN) control, along with the average resident panel rates and the average attending panel rates. The reports also included a list of all residents’ patients who were overdue for colorectal cancer screening or whose last blood pressure (BP) was uncontrolled (systolic BP ≥ 140 mm Hg or diastolic BP ≥ 90 mm Hg). These reports were originally designed by our practice’s QI team and run and exported in Microsoft Excel format monthly by our information technology (IT) administrator.
- Instruction on aim statements as a group, followed by the expectation that each resident create an individualized aim statement tailored to each resident’s patient panel rates, with the PDSA cycle to be implemented during the remainder of the primary care rotation, focusing on improvement of colorectal cancer screening and HTN control (see supplementary eFigure 1 online for the worksheet used for the workshop).
- Residents were held accountable for their interventions by various check-ins. At the end of the primary care block, residents were required to submit their completed worksheets showing the intervention they had undertaken and when it was performed. The 2 primary care attendings primarily responsible for QI education would review the resident’s work approximately 1 to 2 months after they submitted their worksheets describing their intervention. These attendings sent the residents personalized feedback based on whether the intervention had been completed or successful as evidenced by documentation in the chart, including direct patient outreach by phone, letter, or portal; outreach to the resident coordinator; scheduled follow-up appointment; or booking or completion of colorectal cancer screening. Along with this feedback, residents were also sent suggestions for next steps. Resident preceptors were copied on the email to facilitate reinforcement of the goals and plans. Finally, the resident preceptors also helped with accountability by going through the residents’ worksheets and patient panel metrics with the residents during biannual evaluations.

Evaluation
Residents were surveyed with a 10-item questionnaire pre and post intervention regarding their attitudes toward QI, understanding of QI principles, and familiarity with their patient panel data. Surveys were anonymous and distributed via the SurveyMonkey platform (see supplementary eFigure 2 online). Residents were asked if they had ever performed a PDSA cycle, performed patient outreach, or performed an intervention and whether they knew the rates of diabetes, HTN, and colorectal cancer screening in their patient panels. Questions rated on a 5-point Likert scale were used to assess comfort with panel management, developing an aim statement, designing and implementing a PDSA cycle, as well as interest in pursuing QI as a career. For the purposes of analysis, these questions were dichotomized into “somewhat comfortable” and “very comfortable” vs “neutral,” “somewhat uncomfortable,” and “very uncomfortable.” Similarly, we dichotomized the question about interest in QI as a career into “somewhat interested” and “very interested” vs “neutral,” “somewhat disinterested,” and “very disinterested.” As the surveys were anonymous, we were unable to pair the pre- and postintervention surveys and used a chi-square test to evaluate whether there was an association between survey assessments pre intervention vs post intervention and a positive or negative response to the question.

We also examined rates of HTN control and colorectal cancer screening in all 75 resident panels pre and post intervention. The paired t-test was used to determine whether the mean change from pre to post intervention was significant. SAS 9.4 (SAS Institute Inc.) was used for all analyses. Institutional Review Board exemption was obtained from the Tufts Medical Center IRB. There was no funding received for this study.
Results
Respondents
Of the 75 residents, 55 (73%) completed the survey prior to the intervention, and 39 (52%) completed the survey after the intervention.
Panel Knowledge and Intervention
Prior to the intervention, 45% of residents had performed a PDSA cycle, compared with 77% post intervention, which was a significant increase (P = .002) (Table 1). Sixty-two percent of residents had performed outreach or an intervention based on their patient panel reports prior to the intervention, compared with 85% of residents post intervention, which was also a significant increase (P = .02). The increase post intervention was not 100%, as there were residents who either missed the initial workshop or who did not follow through with their planned intervention. Common interventions included the residents giving their coordinators a list of patients to call to schedule appointments, utilizing fellow team members (eg, pharmacists, social workers) for targeted patient outreach, or calling patients themselves to reestablish a connection.

In terms of knowledge of their patient panels, prior to the intervention, 55%, 62%, and 62% of residents knew the rates of patients in their panel with diabetes, HTN, and colorectal cancer screening, respectively. After the intervention, the residents’ knowledge of these rates increased significantly, to 85% for diabetes (P = .002), 97% for HTN (P < .0001), and 97% for colorectal cancer screening (P < .0001).
Comfort With QI Approaches
Prior to the intervention, 82% of residents were comfortable managing their primary care panel, which did not change significantly post intervention (Table 2). The residents’ comfort with designing an aim statement did significantly increase, from 55% to 95% (P < .0001). The residents also had a significant increase in comfort with both designing and implementing a PDSA cycle. Prior to the intervention, 22% felt comfortable designing a PDSA cycle, which increased to 79% (P < .0001) post intervention, and 24% felt comfortable implementing a PDSA cycle, which increased to 77% (P < .0001) post intervention.

Patient Outcome Measures
The rate of HTN control in the residents' patient panels did not change significantly pre and post intervention (Table 3). The rate of resident patients who were up to date with colorectal cancer screening increased by 6.5% post intervention (P < .0001).

Interest in QI as a Career
As part of the survey, residents were asked how interested they were in making QI a part of their career. Fifty percent of residents indicated an interest in QI pre intervention, and 54% indicated an interest post intervention, which was not a significant difference (P = .72).
Discussion
In this study, we found that integration of a QI curriculum into a primary care rotation improved both residents’ knowledge of their patient panels and comfort with QI approaches, which translated to improvement in patient outcomes. Several previous studies have found improvements in resident self-assessment or knowledge after implementation of a QI curriculum.4-13 Liao et al implemented a longitudinal curriculum including both didactic and experiential components and found an improvement in both QI confidence and knowledge.3 Similarly, Duello et al8 found that a curriculum including both didactic lectures and QI projects improved subjective QI knowledge and comfort. Interestingly, Fok and Wong9 found that resident knowledge could be sustained post curriculum after completion of a QI project, suggesting that experiential learning may be helpful in maintaining knowledge.
Studies also have looked at providing performance data to residents. Hwang et al18 found that providing audit and feedback in the form of individual panel performance data to residents compared with practice targets led to statistically significant improvement in cancer screening rates and composite quality score, indicating that there is tremendous potential in providing residents with their data. While the ACGME mandates that residents should receive data on their quality metrics, on CLER visits, many residents interviewed noted limited access to data on their metrics and benchmarks.1,2
Though previous studies have individually looked at teaching QI concepts, providing panel data, or targeting select metrics, our study was unique in that it reviewed both self-reported resident outcomes data as well as actual patient outcomes. In addition to finding increased knowledge of patient panels and comfort with QI approaches, we found a significant increase in colorectal cancer screening rates post intervention. We thought this finding was particularly important given some data that residents' patients have been found to have worse outcomes on quality metrics compared with patients cared for by staff physicians.14,15 Given that having a resident physician as a PCP has been associated with failing to meet quality measures, it is especially important to focus targeted quality improvement initiatives in this patient population to reduce disparities in care.
We found that residents had improved knowledge on their patient panels as a result of this initiative. The residents were noted to have a higher knowledge of their HTN and colorectal cancer screening rates in comparison to their diabetes metrics. We suspect this is because residents are provided with multiple metrics related to diabetes, including process measures such as A1c testing, as well as outcome measures such as A1c control, so it may be harder for them to elucidate exactly how they are doing with their diabetes patients, whereas in HTN control and colorectal cancer screening, there is only 1 associated metric. Interestingly, even though HTN and colorectal cancer screening were the 2 measures focused on in the study, the residents had a significant improvement in knowledge of the rates of diabetes in their panel as well. This suggests that even just receiving data alone is valuable, hopefully translating to better outcomes with better baseline understanding of panels. We believe that our intervention was successful because it included both a didactic and an experiential component, as well as the use of individual panel performance data.
There were several limitations to our study. It was performed at a single institution, translating to a small sample size. Our data analysis was limited because we were unable to pair our pre- and postintervention survey responses because we used an anonymous survey. We also did not have full participation in postintervention surveys from all residents, which may have biased the study in favor of high performers. Another limitation was that our survey relied on self-reported outcomes for the questions about the residents knowing their patient panels.
This study required a 2-hour workshop every 3 weeks led by a faculty member trained in QI. Given the amount of time needed for the curriculum, this study may be difficult to replicate at other institutions, especially if faculty with an interest or training in QI are not available. Given our finding that residents had increased knowledge of their patient panels after receiving panel metrics, simply providing data with the goal of smaller, focused interventions may be easier to implement. At our institution, we discontinued the longer 2-hour QI workshops designed to teach QI approaches more broadly. We continue to provide individualized panel data to all residents during their primary care rotations and conduct half-hour, small group workshops with the interns that focus on drafting aim statements and planning interventions. All residents are required to submit worksheets to us at the end of their primary care blocks listing their current rates of each predetermined metric and laying out their aim statements and planned interventions. Residents also continue to receive feedback from our faculty with expertise in QI afterward on their plans and evidence of follow-through in the chart, with their preceptors included on the feedback emails. Even without the larger QI workshop, this approach has continued to be successful and appreciated. In fact, it does appear as though improvement in colorectal cancer screening has been sustained over several years. At the end of our study period, the resident patient colorectal cancer screening rate rose from 34% to 43%, and for the 2021-2022 academic year, the rate rose further, from 46% to 50%.
Given that the resident clinic patient population is at higher risk overall, targeted outreach and approaches to improve quality must be continued. Future areas of research include looking at which interventions, whether QI curriculum, provision of panel data, or required panel management interventions, translate to the greatest improvements in patient outcomes in this vulnerable population.
Conclusion
Our study showed that a dedicated QI curriculum for the residents and access to quality metric data improved both resident knowledge and comfort with QI approaches. Beyond resident-centered outcomes, there was also translation to improved patient outcomes, with a significant increase in colon cancer screening rates post intervention.
Corresponding author: Kinjalika Sathi, MD, 800 Washington St., Boston, MA 02111; [email protected]
Disclosures: None reported.
1. Accreditation Council for Graduate Medical Education. ACGME Common Program Requirements (Residency). Approved June 13, 2021. Updated July 1, 2022. Accessed December 29, 2022. https://www.acgme.org/globalassets/pfassets/programrequirements/cprresidency_2022v3.pdf
2. Koh NJ, Wagner R, Newton RC, et al; on behalf of the CLER Evaluation Committee and the CLER Program. CLER National Report of Findings 2021. Accreditation Council for Graduate Medical Education; 2021. Accessed December 29, 2022. https://www.acgme.org/globalassets/pdfs/cler/2021clernationalreportoffindings.pdf
3. Liao JM, Co JP, Kachalia A. Providing educational content and context for training the next generation of physicians in quality improvement. Acad Med. 2015;90(9):1241-1245. doi:10.1097/ACM.0000000000000799
4. Johnson KM, Fiordellisi W, Kuperman E, et al. X + Y = time for QI: meaningful engagement of residents in quality improvement during the ambulatory block. J Grad Med Educ. 2018;10(3):316-324. doi:10.4300/JGME-D-17-00761.1
5. Kesari K, Ali S, Smith S. Integrating residents with institutional quality improvement teams. Med Educ. 2017;51(11):1173. doi:10.1111/medu.13431
6. Ogrinc G, Cohen ES, van Aalst R, et al. Clinical and educational outcomes of an integrated inpatient quality improvement curriculum for internal medicine residents. J Grad Med Educ. 2016;8(4):563-568. doi:10.4300/JGME-D-15-00412.1
7. Malayala SV, Qazi KJ, Samdani AJ, et al. A multidisciplinary performance improvement rotation in an internal medicine training program. Int J Med Educ. 2016;7:212-213. doi:10.5116/ijme.5765.0bda
8. Duello K, Louh I, Greig H, et al. Residents’ knowledge of quality improvement: the impact of using a group project curriculum. Postgrad Med J. 2015;91(1078):431-435. doi:10.1136/postgradmedj-2014-132886
9. Fok MC, Wong RY. Impact of a competency based curriculum on quality improvement among internal medicine residents. BMC Med Educ. 2014;14:252. doi:10.1186/s12909-014-0252-7
10. Wilper AP, Smith CS, Weppner W. Instituting systems-based practice and practice-based learning and improvement: a curriculum of inquiry. Med Educ Online. 2013;18:21612. doi:10.3402/meo.v18i0.21612
11. Weigel C, Suen W, Gupte G. Using lean methodology to teach quality improvement to internal medicine residents at a safety net hospital. Am J Med Qual. 2013;28(5):392-399. doi:10.1177/1062860612474062
12. Tomolo AM, Lawrence RH, Watts B, et al. Pilot study evaluating a practice-based learning and improvement curriculum focusing on the development of system-level quality improvement skills. J Grad Med Educ. 2011;3(1):49-58. doi:10.4300/JGME-D-10-00104.1
13. Djuricich AM, Ciccarelli M, Swigonski NL. A continuous quality improvement curriculum for residents: addressing core competency, improving systems. Acad Med. 2004;79(10 Suppl):S65-S67. doi:10.1097/00001888-200410001-00020
14. Essien UR, He W, Ray A, et al. Disparities in quality of primary care by resident and staff physicians: is there a conflict between training and equity? J Gen Intern Med. 2019;34(7):1184-1191. doi:10.1007/s11606-019-04960-5
15. Amat M, Norian E, Graham KL. Unmasking a vulnerable patient care process: a qualitative study describing the current state of resident continuity clinic in a nationwide cohort of internal medicine residency programs. Am J Med. 2022;135(6):783-786. doi:10.1016/j.amjmed.2022.02.007
16. Wong BM, Etchells EE, Kuper A, et al. Teaching quality improvement and patient safety to trainees: a systematic review. Acad Med. 2010;85(9):1425-1439. doi:10.1097/ACM.0b013e3181e2d0c6
17. Armstrong G, Headrick L, Madigosky W, et al. Designing education to improve care. Jt Comm J Qual Patient Saf. 2012;38:5-14. doi:10.1016/s1553-7250(12)38002-1
18. Hwang AS, Harding AS, Chang Y, et al. An audit and feedback intervention to improve internal medicine residents’ performance on ambulatory quality measures: a randomized controlled trial. Popul Health Manag. 2019;22(6):529-535. doi:10.1089/pop.2018.0217
19. Institute for Healthcare Improvement. Open school. The paper airplane factory. Accessed December 29, 2022. https://www.ihi.org/education/IHIOpenSchool/resources/Pages/Activities/PaperAirplaneFactory.aspx
1. Accreditation Council for Graduate Medical Education. ACGME Common Program Requirements (Residency). Approved June 13, 2021. Updated July 1, 2022. Accessed December 29, 2022. https://www.acgme.org/globalassets/pfassets/programrequirements/cprresidency_2022v3.pdf
2. Koh NJ, Wagner R, Newton RC, et al; on behalf of the CLER Evaluation Committee and the CLER Program. CLER National Report of Findings 2021. Accreditation Council for Graduate Medical Education; 2021. Accessed December 29, 2022. https://www.acgme.org/globalassets/pdfs/cler/2021clernationalreportoffindings.pdf
3. Liao JM, Co JP, Kachalia A. Providing educational content and context for training the next generation of physicians in quality improvement. Acad Med. 2015;90(9):1241-1245. doi:10.1097/ACM.0000000000000799
4. Johnson KM, Fiordellisi W, Kuperman E, et al. X + Y = time for QI: meaningful engagement of residents in quality improvement during the ambulatory block. J Grad Med Educ. 2018;10(3):316-324. doi:10.4300/JGME-D-17-00761.1
5. Kesari K, Ali S, Smith S. Integrating residents with institutional quality improvement teams. Med Educ. 2017;51(11):1173. doi:10.1111/medu.13431
6. Ogrinc G, Cohen ES, van Aalst R, et al. Clinical and educational outcomes of an integrated inpatient quality improvement curriculum for internal medicine residents. J Grad Med Educ. 2016;8(4):563-568. doi:10.4300/JGME-D-15-00412.1
7. Malayala SV, Qazi KJ, Samdani AJ, et al. A multidisciplinary performance improvement rotation in an internal medicine training program. Int J Med Educ. 2016;7:212-213. doi:10.5116/ijme.5765.0bda
8. Duello K, Louh I, Greig H, et al. Residents’ knowledge of quality improvement: the impact of using a group project curriculum. Postgrad Med J. 2015;91(1078):431-435. doi:10.1136/postgradmedj-2014-132886
9. Fok MC, Wong RY. Impact of a competency based curriculum on quality improvement among internal medicine residents. BMC Med Educ. 2014;14:252. doi:10.1186/s12909-014-0252-7
10. Wilper AP, Smith CS, Weppner W. Instituting systems-based practice and practice-based learning and improvement: a curriculum of inquiry. Med Educ Online. 2013;18:21612. doi:10.3402/meo.v18i0.21612
11. Weigel C, Suen W, Gupte G. Using lean methodology to teach quality improvement to internal medicine residents at a safety net hospital. Am J Med Qual. 2013;28(5):392-399. doi:10.1177/1062860612474062
12. Tomolo AM, Lawrence RH, Watts B, et al. Pilot study evaluating a practice-based learning and improvement curriculum focusing on the development of system-level quality improvement skills. J Grad Med Educ. 2011;3(1):49-58. doi:10.4300/JGME-D-10-00104.1
13. Djuricich AM, Ciccarelli M, Swigonski NL. A continuous quality improvement curriculum for residents: addressing core competency, improving systems. Acad Med. 2004;79(10 Suppl):S65-S67. doi:10.1097/00001888-200410001-00020
14. Essien UR, He W, Ray A, et al. Disparities in quality of primary care by resident and staff physicians: is there a conflict between training and equity? J Gen Intern Med. 2019;34(7):1184-1191. doi:10.1007/s11606-019-04960-5
15. Amat M, Norian E, Graham KL. Unmasking a vulnerable patient care process: a qualitative study describing the current state of resident continuity clinic in a nationwide cohort of internal medicine residency programs. Am J Med. 2022;135(6):783-786. doi:10.1016/j.amjmed.2022.02.007
16. Wong BM, Etchells EE, Kuper A, et al. Teaching quality improvement and patient safety to trainees: a systematic review. Acad Med. 2010;85(9):1425-1439. doi:10.1097/ACM.0b013e3181e2d0c6
17. Armstrong G, Headrick L, Madigosky W, et al. Designing education to improve care. Jt Comm J Qual Patient Saf. 2012;38:5-14. doi:10.1016/s1553-7250(12)38002-1
18. Hwang AS, Harding AS, Chang Y, et al. An audit and feedback intervention to improve internal medicine residents’ performance on ambulatory quality measures: a randomized controlled trial. Popul Health Manag. 2019;22(6):529-535. doi:10.1089/pop.2018.0217
19. Institute for Healthcare Improvement. Open school. The paper airplane factory. Accessed December 29, 2022. https://www.ihi.org/education/IHIOpenSchool/resources/Pages/Activities/PaperAirplaneFactory.aspx
Characteristics of Matched vs Nonmatched Dermatology Applicants
Dermatology residency continues to be one of the most competitive specialties, with a match rate of 84.7% for US allopathic seniors in the 2019-2020 academic year.1 In the 2019-2020 cycle, dermatology applicants were tied with plastic surgery for the highest median US Medical Licensing Examination (USMLE) Step 1 score compared with other specialties, which suggests that the top medical students are applying, yet only approximately 5 of 6 students are matching.
Factors that have been cited with successful dermatology matching include USMLE Step 1 and Step 2 Clinical Knowledge (CK) scores,2 research accomplishments,3 letters of recommendation,4 medical school performance, personal statement, grades in required clerkships, and volunteer/extracurricular experiences, among others.5
The National Resident Matching Program (NRMP) publishes data each year regarding different academic factors—USMLE scores; number of abstracts, presentations, and papers; work, volunteer, and research experiences—and compares the mean between matched and nonmatched applicants.1 However, the USMLE does not report any demographic information of the applicants and the implication it has for matching. Additionally, the number of couples participating in the couples match continues to increase each year. In the 2019-2020 cycle, 1224 couples participated in the couples match.1 However, NRMP reports only limited data regarding the couples match, and it is not specialty specific.
We aimed to determine the characteristics of matched vs nonmatched dermatology applicants. Secondarily, we aimed to determine any differences among demographics regarding matching rates, academic performance, and research publications. We also aimed to characterize the strategy and outcomes of applicants that couples matched.
Materials and Methods
The Mayo Clinic institutional review board deemed this study exempt. All applicants who applied to Mayo Clinic dermatology residency in Scottsdale, Arizona, during the 2018-2019 cycle were emailed an initial survey (N=475) before Match Day that obtained demographic information, geographic information, gap-year information, USMLE Step 1 score, publications, medical school grades, number of away rotations, and number of interviews. A follow-up survey gathering match data and couples matching data was sent to the applicants who completed the first survey on Match Day. The survey was repeated for the 2019-2020 cycle. In the second survey, Step 2 CK data were obtained. The survey was sent to 629 applicants who applied to Mayo Clinic dermatology residencies in Arizona, Minnesota, and Florida to include a broader group of applicants. For publications, applicants were asked to count only published or accepted manuscripts, not abstracts, posters, conference presentations, or submitted manuscripts. Applicants who did not respond to the second survey (match data) were not included in that part of the analysis. One survey was excluded because of implausible answers (eg, scores outside of range for USMLE Step scores).
Statistical Analysis—For statistical analyses, the applicants from both applications cycles were combined. Descriptive statistics were reported in the form of mean, median, or counts (percentages), as applicable. Means were compared using 2-sided t tests. Group comparisons were examined using χ2 tests for categorical variables. Statistical analyses were performed using the BlueSky Statistics version 6.30. P<.05 was considered significant.
Results
In 2019, a total of 149 applicants completed the initial survey (31.4% response rate), and 112 completed the follow-up survey (75.2% response rate). In 2020, a total of 142 applicants completed the initial survey (22.6% response rate), and 124 completed the follow-up survey (87.3% response rate). Combining the 2 years, after removing 1 survey with implausible answers, there were 290 respondents from the initial survey and 235 from the follow-up survey. The median (SD) age for the total applicants over both years was 27 (3.0) years, and 180 applicants were female (61.9%).
USMLE Scores—The median USMLE Step 1 score was 250, and scores ranged from 196 to 271. The median USMLE Step 2 CK score was 257, and scores ranged from 213 to 281. Higher USMLE Step 1 and Step 2 CK scores and more interviews were associated with higher match rates (Table 1). In addition, students with a dermatology program at their medical school were more likely to match than those without a home dermatology program.
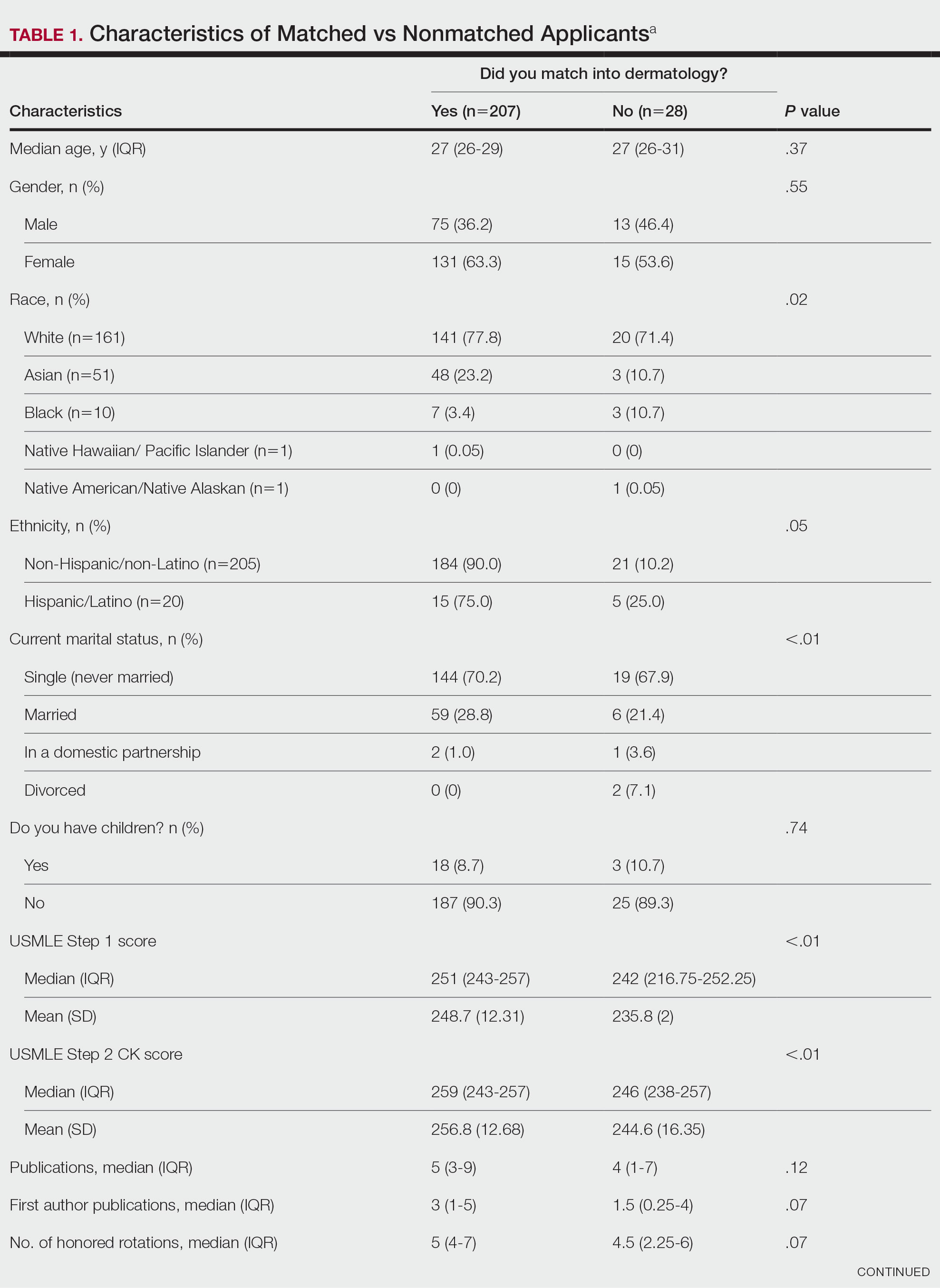
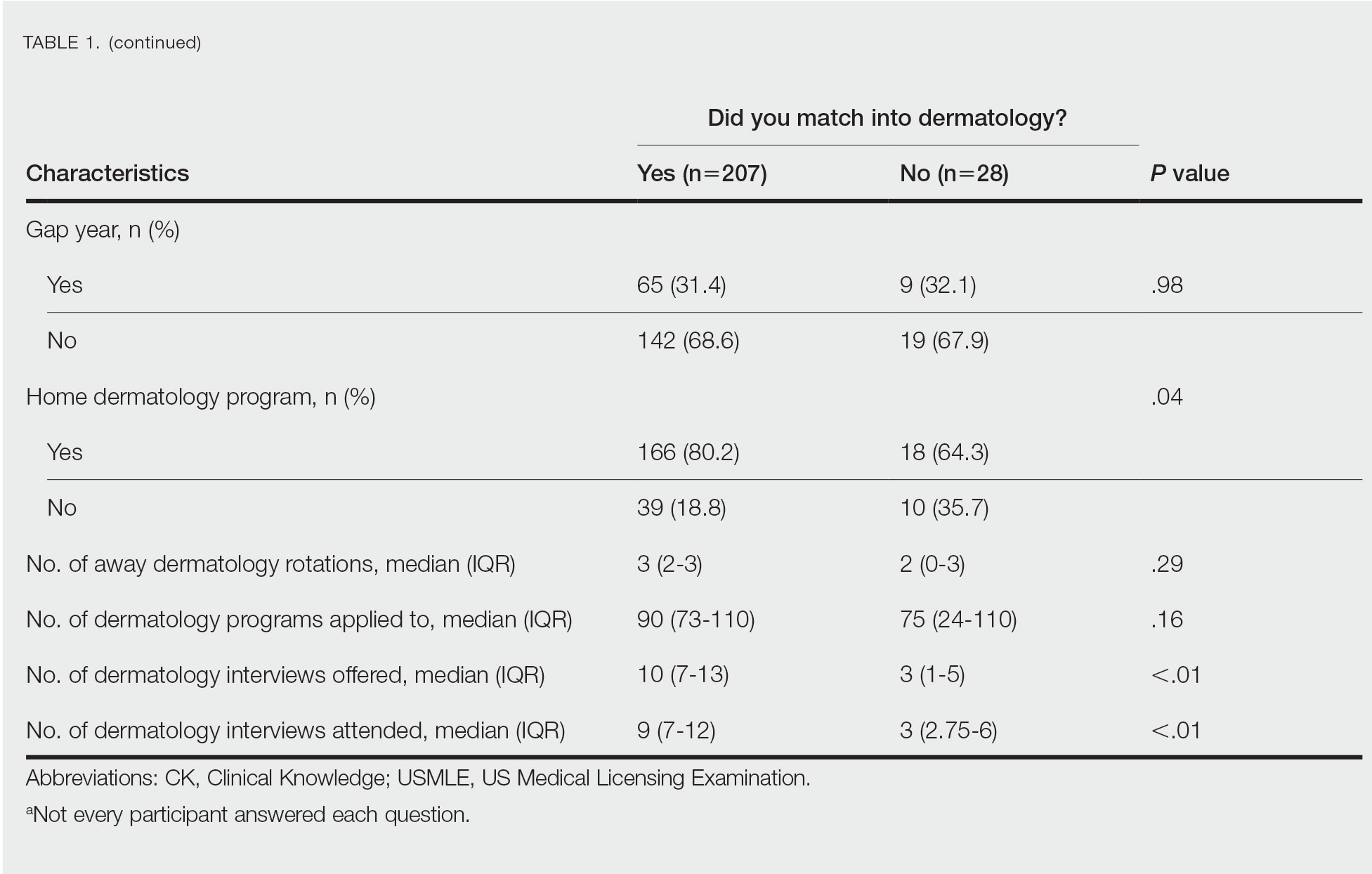
Gender Differences—There were 180 females and 110 males who completed the surveys. Males and females had similar match rates (85.2% vs 89.0%; P=.39)(Table 2).
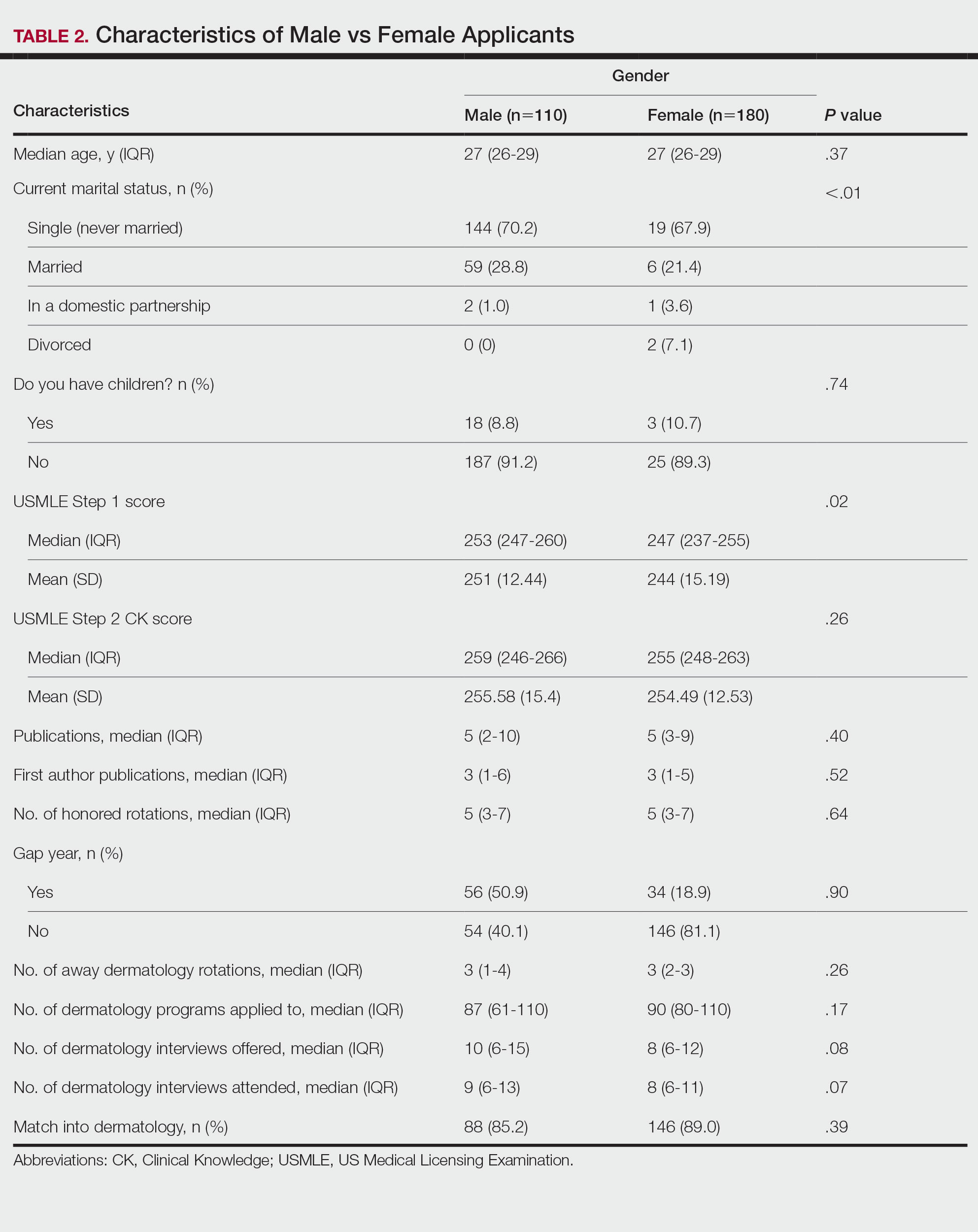
Family Life—In comparing marital status, applicants who were divorced had a higher median age (38.5 years) compared with applicants who were single, married, or in a domestic partnership (all 27 years; P<.01). Differences are outlined in Table 3.
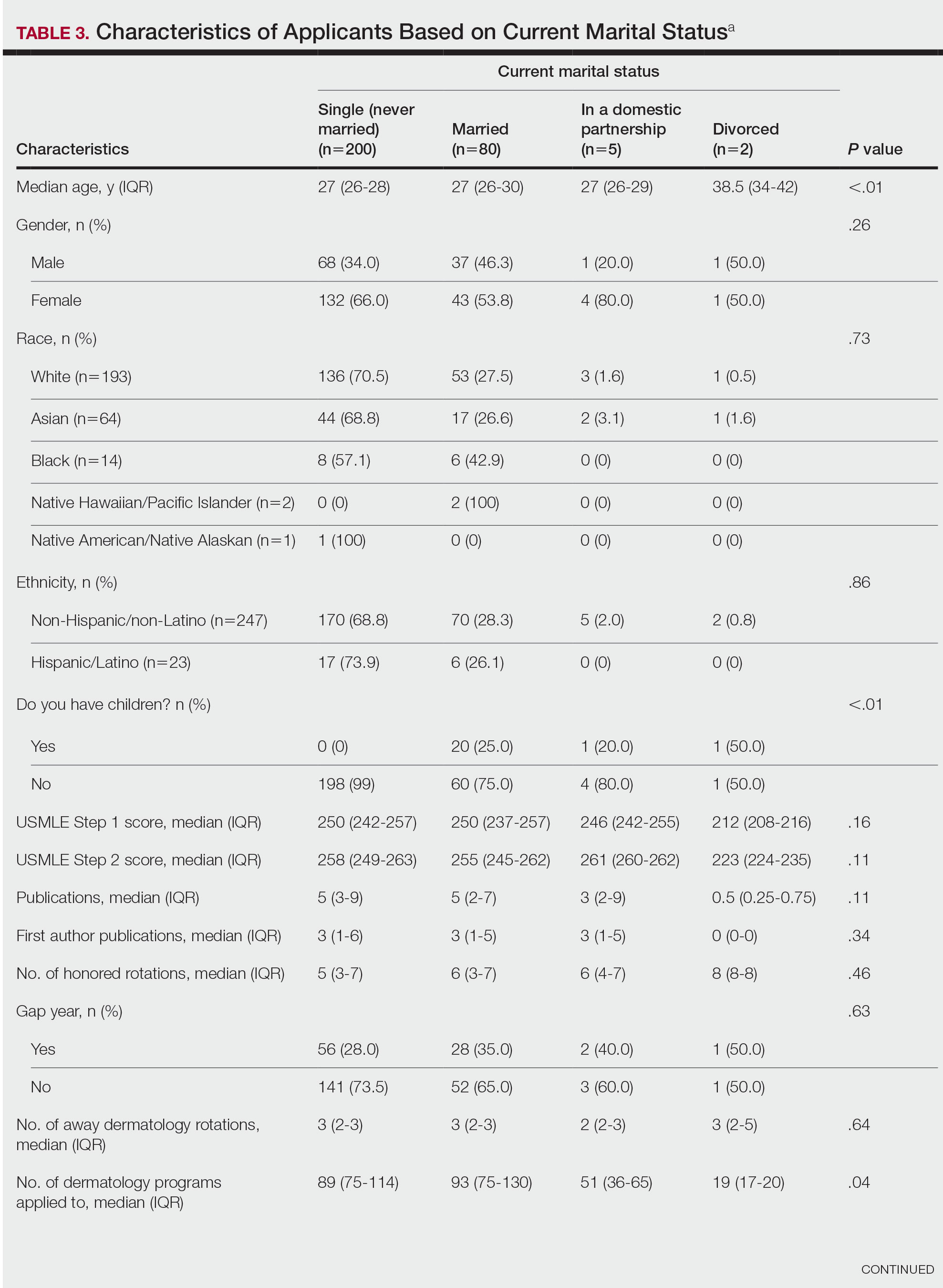
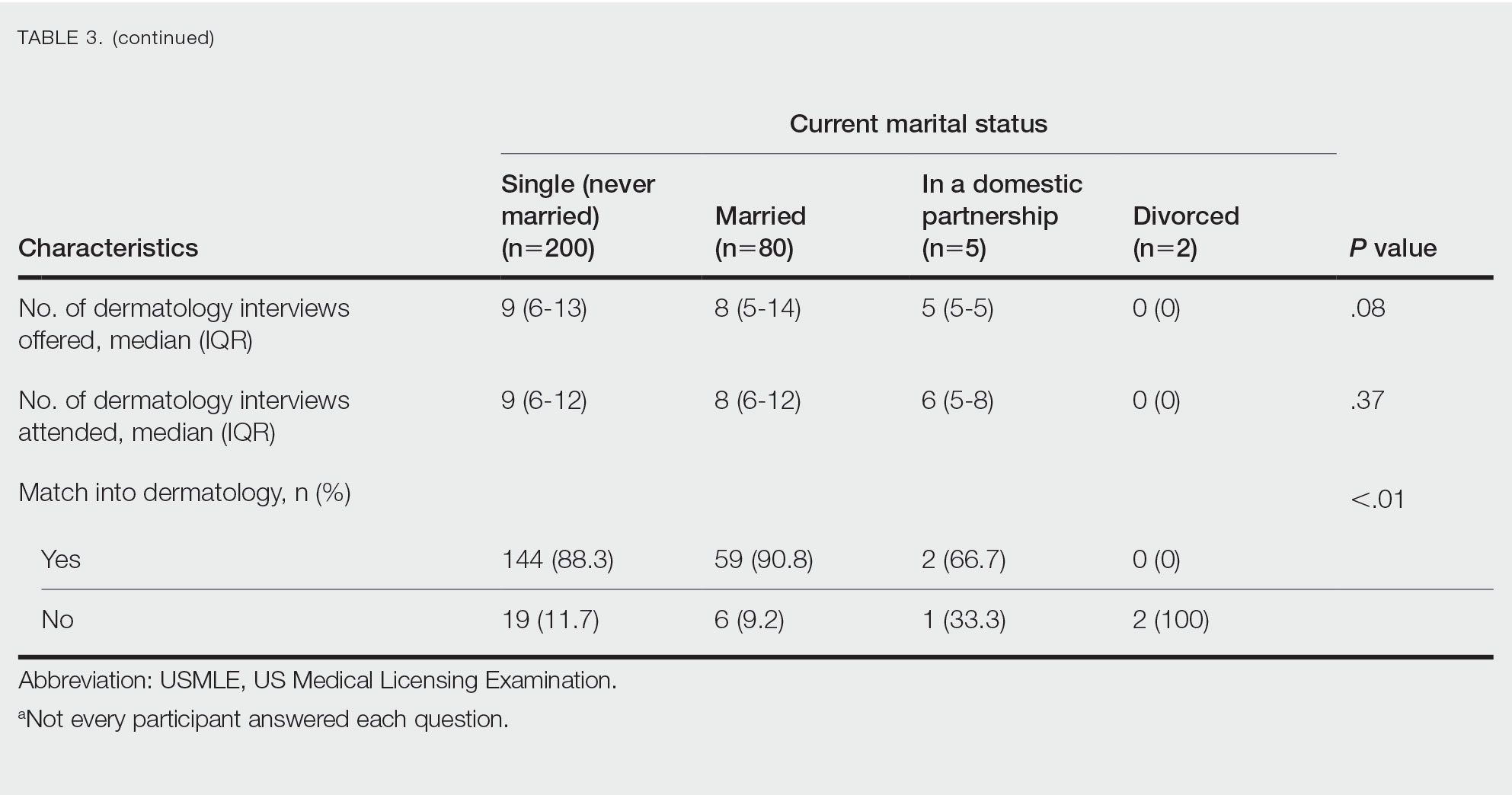
On average, applicants with children (n=27 [15 male, 12 female]; P=.13) were 3 years older than those without (30.5 vs 27; P<.01) and were more likely to be married (88.9% vs 21.5%; P<.01). Applicants with children had a mean USMLE Step 1 score of 241 compared to 251 for those without children (P=.02) and a mean USMLE Step 2 CK score of 246 compared to 258 for those without children (P<.01). Applicants with children had similar debt, number of publications, number of honored rotations, and match rates compared to applicants without children (Figure).
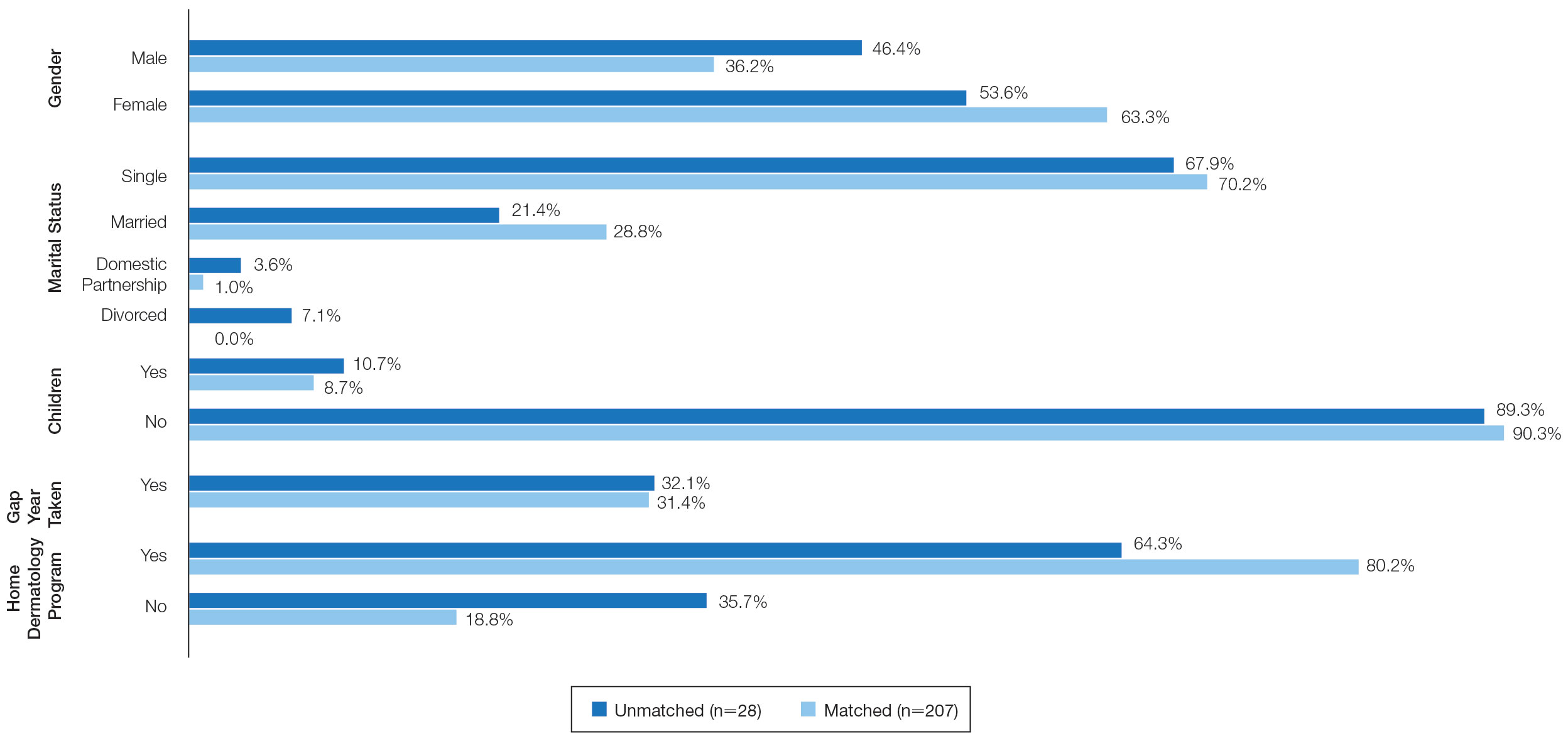
Couples Match—Seventeen individuals in our survey participated in the couples match (7.8%), and all 17 (100%) matched into dermatology. The mean age was 26.7 years, 12 applicants were female, 2 applicants were married, and 1 applicant had children. The mean number of interviews offered was 13.6, and the mean number of interviews attended was 11.3. This was higher than participants who were not couples matching (13.6 vs 9.8 [P=.02] and 11.3 vs 8.9 [P=.04], respectively). Applicants and their partners applied to programs and received interviews in a mean of 10 cities. Sixteen applicants reported that they contacted programs where their partner had interview offers. All participants’ rank lists included programs located in different cities than their partners’ ranked programs, and all but 1 participant ranked programs located in a different state than their partners’ ranked programs. Fifteen participants had options in their rank list for the applicant not to match, even if the partner would match. Similarly, 12 had the option for the applicant to match, even if the partner would not match. Fourteen (82.4%) matched at the same institution as their significant other. Three (17.6%) applicants matched to a program in a different state than the partner’s matched program. Two (11.8%) participants felt their relationship with their partner suffered because of the match, and 1 (5.9%) applicant was undetermined. One applicant described their relationship suffering from “unnecessary tension and anxiety” and noted “difficult conversations” about potentially matching into dermatology in a different location from their partner that could have been “devastating and not something [he or she] should have to choose.”
Comment
Factors for Matching in Dermatology—In our survey, we found the statistically significant factors of matching into dermatology included high USMLE Step 1 and Step 2 CK scores (P<.01), having a home dermatology program (P=.04), and attending a higher number of dermatology interviews (P<.01). These data are similar to NRMP results1; however, the higher likelihood of matching if the medical school has a home dermatology program has not been reported. This finding could be due to multiple factors such as students have less access to academic dermatologists for research projects, letters of recommendations, mentorship, and clinical rotations.
Gender and having children were factors that had no correlation with the match rate. There was a statistical difference of matching based on marital status (P<.01), but this is likely due to the low number of applicants in the divorced category. There were differences among demographics with USMLE Step 1 and Step 2 CK scores, which is a known factor in matching.1,2 Applicants with children had lower USMLE Step 1 and Step 2 CK scores compared to applicants without children. Females also had lower median USMLE Step 1 scores compared to males. This finding may serve as a reminder to programs when comparing USMLE Step examination scores that demographic factors may play a role. The race and ethnicity of applicants likely play a role. It has been reported that underrepresented minorities had lower match rates than White and Asian applicants in dermatology.6 There have been several published articles discussing the lack of diversity in dermatology, with a call to action.7-9
Factors for Couples Matching—The number of applicants participating in the couples match continues to increase yearly. The NMRP does publish data regarding “successful” couples matching but does not specify how many couples match together. There also is little published regarding advice for participation in the couples match. Although we had a limited number of couples that participated in the match, it is interesting to note they had similar strategies, including contacting programs at institutions that had offered interviews to their partners. This strategy may be effective, as dermatology programs offer interviews relatively late compared with other specialties.5 Additionally, this strategy may increase the number of interviews offered and received, as evidenced by the higher number of interviews offered compared with those who were not couples matching. Additionally, this survey highlights the sacrifice often needed by couples in the couples match as revealed by the inclusion of rank-list options in which the couples reside long distance or in which 1 partner does not match. This information may be helpful to applicants who are planning a strategy for the couples match in dermatology. Although this study does not encompass all dermatology applicants in the 2019-2020 cycle, we do believe it may be representative. The USMLE Step 1 scores in this study were similar to the published NRMP data.1,10 According to NRMP data from the 2019-2020 cycle, the mean USMLE Step 1 score was 248 for matched applicants and 239 for unmatched.1 The NRMP reported the mean USMLE Step 2 CK score for matched was 256 and 248 for unmatched, which also is similar to our data. The NRMP reported the mean number of programs ranked was 9.9 for matched and 4.5 for unmatched applicants.1 Again, our data were similar for number of dermatology interviews attended.
Limitations—There are limitations to this study. The main limitation is that the survey is from a single institution and had a limited number of respondents. Given the nature of the study, the accuracy of the data is dependent on the applicants’ honesty in self-reporting academic performance and other variables. There also may be a selection bias given the low response rate. The subanalyses—children and couples matching—were underpowered with the limited number of participants. Further studies that include multiple residency programs and multiple years could be helpful to provide more power and less risk of bias. We did not gather information such as the Medical Student Performance Evaluation letter, letters of recommendation, or personal statements, which do play an important role in the assessment of an applicant. However, because the applicants completed these surveys, and given these are largely blinded to applicants, we did not feel the applicants could accurately respond to those aspects of the application.
Conclusion
Our survey finds that factors associated with matching included a higher USMLE Step 1 score, having a home dermatology program, and a higher number of interviews offered and attended. Some demographics had varying USMLE Step 1 scores but similar match rates.
- National Resident Matching Program. Results and Data: 2020 Main Residency Match. National Resident Matching Program; May 2020. Accessed January 9, 2023. https://www.nrmp.org/wp-content/uploads/2021/12/MM_Results_and-Data_2020-1.pdf
- Gauer JL, Jackson JB. The association of USMLE Step 1 and Step 2 CK scores with residency match specialty and location. Med Educ Online. 2017;22:1358579.
- Wang JV, Keller M. Pressure to publish for residency applicants in dermatology. Dermatol Online J. 2016;22:13030/qt56x1t7ww.
- Wang RF, Zhang M, Kaffenberger JA. Does the dermatology standardized letter of recommendation alter applicants’ chances of matching into residency. J Am Acad Dermatol. 2017;77:e139-e140.
- National Resident Matching Program, Data Release and Research Committee: results of the 2018 NRMP Program Director Survey. Accessed December 19, 2022. https://www.nrmp.org/wp-content/uploads/2021/07/NRMP-2018-Program-Director-Survey-for-WWW.pdf
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role of race and ethnicity in the dermatology applicant match process. J Natl Med Assoc. 2022;113:666-670.
- Chen A, Shinkai K. Rethinking how we select dermatology applicants-turning the tide. JAMA Dermatol. 2017;153:259-260.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49.
- National Resident Matching Program. Charting outcomes in the match: U.S. allopathic seniors. Characteristics of U.S. allopathic seniors who matched to their preferred specialty in the 2018 main residency match. 2nd ed. Accessed December 19, 2022. https://www.nrmp.org/wp-content/uploads/2021/07/Charting-Outcomes-in-the-Match-2018_Seniors-1.pdf
Dermatology residency continues to be one of the most competitive specialties, with a match rate of 84.7% for US allopathic seniors in the 2019-2020 academic year.1 In the 2019-2020 cycle, dermatology applicants were tied with plastic surgery for the highest median US Medical Licensing Examination (USMLE) Step 1 score compared with other specialties, which suggests that the top medical students are applying, yet only approximately 5 of 6 students are matching.
Factors that have been cited with successful dermatology matching include USMLE Step 1 and Step 2 Clinical Knowledge (CK) scores,2 research accomplishments,3 letters of recommendation,4 medical school performance, personal statement, grades in required clerkships, and volunteer/extracurricular experiences, among others.5
The National Resident Matching Program (NRMP) publishes data each year regarding different academic factors—USMLE scores; number of abstracts, presentations, and papers; work, volunteer, and research experiences—and compares the mean between matched and nonmatched applicants.1 However, the USMLE does not report any demographic information of the applicants and the implication it has for matching. Additionally, the number of couples participating in the couples match continues to increase each year. In the 2019-2020 cycle, 1224 couples participated in the couples match.1 However, NRMP reports only limited data regarding the couples match, and it is not specialty specific.
We aimed to determine the characteristics of matched vs nonmatched dermatology applicants. Secondarily, we aimed to determine any differences among demographics regarding matching rates, academic performance, and research publications. We also aimed to characterize the strategy and outcomes of applicants that couples matched.
Materials and Methods
The Mayo Clinic institutional review board deemed this study exempt. All applicants who applied to Mayo Clinic dermatology residency in Scottsdale, Arizona, during the 2018-2019 cycle were emailed an initial survey (N=475) before Match Day that obtained demographic information, geographic information, gap-year information, USMLE Step 1 score, publications, medical school grades, number of away rotations, and number of interviews. A follow-up survey gathering match data and couples matching data was sent to the applicants who completed the first survey on Match Day. The survey was repeated for the 2019-2020 cycle. In the second survey, Step 2 CK data were obtained. The survey was sent to 629 applicants who applied to Mayo Clinic dermatology residencies in Arizona, Minnesota, and Florida to include a broader group of applicants. For publications, applicants were asked to count only published or accepted manuscripts, not abstracts, posters, conference presentations, or submitted manuscripts. Applicants who did not respond to the second survey (match data) were not included in that part of the analysis. One survey was excluded because of implausible answers (eg, scores outside of range for USMLE Step scores).
Statistical Analysis—For statistical analyses, the applicants from both applications cycles were combined. Descriptive statistics were reported in the form of mean, median, or counts (percentages), as applicable. Means were compared using 2-sided t tests. Group comparisons were examined using χ2 tests for categorical variables. Statistical analyses were performed using the BlueSky Statistics version 6.30. P<.05 was considered significant.
Results
In 2019, a total of 149 applicants completed the initial survey (31.4% response rate), and 112 completed the follow-up survey (75.2% response rate). In 2020, a total of 142 applicants completed the initial survey (22.6% response rate), and 124 completed the follow-up survey (87.3% response rate). Combining the 2 years, after removing 1 survey with implausible answers, there were 290 respondents from the initial survey and 235 from the follow-up survey. The median (SD) age for the total applicants over both years was 27 (3.0) years, and 180 applicants were female (61.9%).
USMLE Scores—The median USMLE Step 1 score was 250, and scores ranged from 196 to 271. The median USMLE Step 2 CK score was 257, and scores ranged from 213 to 281. Higher USMLE Step 1 and Step 2 CK scores and more interviews were associated with higher match rates (Table 1). In addition, students with a dermatology program at their medical school were more likely to match than those without a home dermatology program.


Gender Differences—There were 180 females and 110 males who completed the surveys. Males and females had similar match rates (85.2% vs 89.0%; P=.39)(Table 2).

Family Life—In comparing marital status, applicants who were divorced had a higher median age (38.5 years) compared with applicants who were single, married, or in a domestic partnership (all 27 years; P<.01). Differences are outlined in Table 3.


On average, applicants with children (n=27 [15 male, 12 female]; P=.13) were 3 years older than those without (30.5 vs 27; P<.01) and were more likely to be married (88.9% vs 21.5%; P<.01). Applicants with children had a mean USMLE Step 1 score of 241 compared to 251 for those without children (P=.02) and a mean USMLE Step 2 CK score of 246 compared to 258 for those without children (P<.01). Applicants with children had similar debt, number of publications, number of honored rotations, and match rates compared to applicants without children (Figure).

Couples Match—Seventeen individuals in our survey participated in the couples match (7.8%), and all 17 (100%) matched into dermatology. The mean age was 26.7 years, 12 applicants were female, 2 applicants were married, and 1 applicant had children. The mean number of interviews offered was 13.6, and the mean number of interviews attended was 11.3. This was higher than participants who were not couples matching (13.6 vs 9.8 [P=.02] and 11.3 vs 8.9 [P=.04], respectively). Applicants and their partners applied to programs and received interviews in a mean of 10 cities. Sixteen applicants reported that they contacted programs where their partner had interview offers. All participants’ rank lists included programs located in different cities than their partners’ ranked programs, and all but 1 participant ranked programs located in a different state than their partners’ ranked programs. Fifteen participants had options in their rank list for the applicant not to match, even if the partner would match. Similarly, 12 had the option for the applicant to match, even if the partner would not match. Fourteen (82.4%) matched at the same institution as their significant other. Three (17.6%) applicants matched to a program in a different state than the partner’s matched program. Two (11.8%) participants felt their relationship with their partner suffered because of the match, and 1 (5.9%) applicant was undetermined. One applicant described their relationship suffering from “unnecessary tension and anxiety” and noted “difficult conversations” about potentially matching into dermatology in a different location from their partner that could have been “devastating and not something [he or she] should have to choose.”
Comment
Factors for Matching in Dermatology—In our survey, we found the statistically significant factors of matching into dermatology included high USMLE Step 1 and Step 2 CK scores (P<.01), having a home dermatology program (P=.04), and attending a higher number of dermatology interviews (P<.01). These data are similar to NRMP results1; however, the higher likelihood of matching if the medical school has a home dermatology program has not been reported. This finding could be due to multiple factors such as students have less access to academic dermatologists for research projects, letters of recommendations, mentorship, and clinical rotations.
Gender and having children were factors that had no correlation with the match rate. There was a statistical difference of matching based on marital status (P<.01), but this is likely due to the low number of applicants in the divorced category. There were differences among demographics with USMLE Step 1 and Step 2 CK scores, which is a known factor in matching.1,2 Applicants with children had lower USMLE Step 1 and Step 2 CK scores compared to applicants without children. Females also had lower median USMLE Step 1 scores compared to males. This finding may serve as a reminder to programs when comparing USMLE Step examination scores that demographic factors may play a role. The race and ethnicity of applicants likely play a role. It has been reported that underrepresented minorities had lower match rates than White and Asian applicants in dermatology.6 There have been several published articles discussing the lack of diversity in dermatology, with a call to action.7-9
Factors for Couples Matching—The number of applicants participating in the couples match continues to increase yearly. The NMRP does publish data regarding “successful” couples matching but does not specify how many couples match together. There also is little published regarding advice for participation in the couples match. Although we had a limited number of couples that participated in the match, it is interesting to note they had similar strategies, including contacting programs at institutions that had offered interviews to their partners. This strategy may be effective, as dermatology programs offer interviews relatively late compared with other specialties.5 Additionally, this strategy may increase the number of interviews offered and received, as evidenced by the higher number of interviews offered compared with those who were not couples matching. Additionally, this survey highlights the sacrifice often needed by couples in the couples match as revealed by the inclusion of rank-list options in which the couples reside long distance or in which 1 partner does not match. This information may be helpful to applicants who are planning a strategy for the couples match in dermatology. Although this study does not encompass all dermatology applicants in the 2019-2020 cycle, we do believe it may be representative. The USMLE Step 1 scores in this study were similar to the published NRMP data.1,10 According to NRMP data from the 2019-2020 cycle, the mean USMLE Step 1 score was 248 for matched applicants and 239 for unmatched.1 The NRMP reported the mean USMLE Step 2 CK score for matched was 256 and 248 for unmatched, which also is similar to our data. The NRMP reported the mean number of programs ranked was 9.9 for matched and 4.5 for unmatched applicants.1 Again, our data were similar for number of dermatology interviews attended.
Limitations—There are limitations to this study. The main limitation is that the survey is from a single institution and had a limited number of respondents. Given the nature of the study, the accuracy of the data is dependent on the applicants’ honesty in self-reporting academic performance and other variables. There also may be a selection bias given the low response rate. The subanalyses—children and couples matching—were underpowered with the limited number of participants. Further studies that include multiple residency programs and multiple years could be helpful to provide more power and less risk of bias. We did not gather information such as the Medical Student Performance Evaluation letter, letters of recommendation, or personal statements, which do play an important role in the assessment of an applicant. However, because the applicants completed these surveys, and given these are largely blinded to applicants, we did not feel the applicants could accurately respond to those aspects of the application.
Conclusion
Our survey finds that factors associated with matching included a higher USMLE Step 1 score, having a home dermatology program, and a higher number of interviews offered and attended. Some demographics had varying USMLE Step 1 scores but similar match rates.
Dermatology residency continues to be one of the most competitive specialties, with a match rate of 84.7% for US allopathic seniors in the 2019-2020 academic year.1 In the 2019-2020 cycle, dermatology applicants were tied with plastic surgery for the highest median US Medical Licensing Examination (USMLE) Step 1 score compared with other specialties, which suggests that the top medical students are applying, yet only approximately 5 of 6 students are matching.
Factors that have been cited with successful dermatology matching include USMLE Step 1 and Step 2 Clinical Knowledge (CK) scores,2 research accomplishments,3 letters of recommendation,4 medical school performance, personal statement, grades in required clerkships, and volunteer/extracurricular experiences, among others.5
The National Resident Matching Program (NRMP) publishes data each year regarding different academic factors—USMLE scores; number of abstracts, presentations, and papers; work, volunteer, and research experiences—and compares the mean between matched and nonmatched applicants.1 However, the USMLE does not report any demographic information of the applicants and the implication it has for matching. Additionally, the number of couples participating in the couples match continues to increase each year. In the 2019-2020 cycle, 1224 couples participated in the couples match.1 However, NRMP reports only limited data regarding the couples match, and it is not specialty specific.
We aimed to determine the characteristics of matched vs nonmatched dermatology applicants. Secondarily, we aimed to determine any differences among demographics regarding matching rates, academic performance, and research publications. We also aimed to characterize the strategy and outcomes of applicants that couples matched.
Materials and Methods
The Mayo Clinic institutional review board deemed this study exempt. All applicants who applied to Mayo Clinic dermatology residency in Scottsdale, Arizona, during the 2018-2019 cycle were emailed an initial survey (N=475) before Match Day that obtained demographic information, geographic information, gap-year information, USMLE Step 1 score, publications, medical school grades, number of away rotations, and number of interviews. A follow-up survey gathering match data and couples matching data was sent to the applicants who completed the first survey on Match Day. The survey was repeated for the 2019-2020 cycle. In the second survey, Step 2 CK data were obtained. The survey was sent to 629 applicants who applied to Mayo Clinic dermatology residencies in Arizona, Minnesota, and Florida to include a broader group of applicants. For publications, applicants were asked to count only published or accepted manuscripts, not abstracts, posters, conference presentations, or submitted manuscripts. Applicants who did not respond to the second survey (match data) were not included in that part of the analysis. One survey was excluded because of implausible answers (eg, scores outside of range for USMLE Step scores).
Statistical Analysis—For statistical analyses, the applicants from both applications cycles were combined. Descriptive statistics were reported in the form of mean, median, or counts (percentages), as applicable. Means were compared using 2-sided t tests. Group comparisons were examined using χ2 tests for categorical variables. Statistical analyses were performed using the BlueSky Statistics version 6.30. P<.05 was considered significant.
Results
In 2019, a total of 149 applicants completed the initial survey (31.4% response rate), and 112 completed the follow-up survey (75.2% response rate). In 2020, a total of 142 applicants completed the initial survey (22.6% response rate), and 124 completed the follow-up survey (87.3% response rate). Combining the 2 years, after removing 1 survey with implausible answers, there were 290 respondents from the initial survey and 235 from the follow-up survey. The median (SD) age for the total applicants over both years was 27 (3.0) years, and 180 applicants were female (61.9%).
USMLE Scores—The median USMLE Step 1 score was 250, and scores ranged from 196 to 271. The median USMLE Step 2 CK score was 257, and scores ranged from 213 to 281. Higher USMLE Step 1 and Step 2 CK scores and more interviews were associated with higher match rates (Table 1). In addition, students with a dermatology program at their medical school were more likely to match than those without a home dermatology program.


Gender Differences—There were 180 females and 110 males who completed the surveys. Males and females had similar match rates (85.2% vs 89.0%; P=.39)(Table 2).

Family Life—In comparing marital status, applicants who were divorced had a higher median age (38.5 years) compared with applicants who were single, married, or in a domestic partnership (all 27 years; P<.01). Differences are outlined in Table 3.


On average, applicants with children (n=27 [15 male, 12 female]; P=.13) were 3 years older than those without (30.5 vs 27; P<.01) and were more likely to be married (88.9% vs 21.5%; P<.01). Applicants with children had a mean USMLE Step 1 score of 241 compared to 251 for those without children (P=.02) and a mean USMLE Step 2 CK score of 246 compared to 258 for those without children (P<.01). Applicants with children had similar debt, number of publications, number of honored rotations, and match rates compared to applicants without children (Figure).

Couples Match—Seventeen individuals in our survey participated in the couples match (7.8%), and all 17 (100%) matched into dermatology. The mean age was 26.7 years, 12 applicants were female, 2 applicants were married, and 1 applicant had children. The mean number of interviews offered was 13.6, and the mean number of interviews attended was 11.3. This was higher than participants who were not couples matching (13.6 vs 9.8 [P=.02] and 11.3 vs 8.9 [P=.04], respectively). Applicants and their partners applied to programs and received interviews in a mean of 10 cities. Sixteen applicants reported that they contacted programs where their partner had interview offers. All participants’ rank lists included programs located in different cities than their partners’ ranked programs, and all but 1 participant ranked programs located in a different state than their partners’ ranked programs. Fifteen participants had options in their rank list for the applicant not to match, even if the partner would match. Similarly, 12 had the option for the applicant to match, even if the partner would not match. Fourteen (82.4%) matched at the same institution as their significant other. Three (17.6%) applicants matched to a program in a different state than the partner’s matched program. Two (11.8%) participants felt their relationship with their partner suffered because of the match, and 1 (5.9%) applicant was undetermined. One applicant described their relationship suffering from “unnecessary tension and anxiety” and noted “difficult conversations” about potentially matching into dermatology in a different location from their partner that could have been “devastating and not something [he or she] should have to choose.”
Comment
Factors for Matching in Dermatology—In our survey, we found the statistically significant factors of matching into dermatology included high USMLE Step 1 and Step 2 CK scores (P<.01), having a home dermatology program (P=.04), and attending a higher number of dermatology interviews (P<.01). These data are similar to NRMP results1; however, the higher likelihood of matching if the medical school has a home dermatology program has not been reported. This finding could be due to multiple factors such as students have less access to academic dermatologists for research projects, letters of recommendations, mentorship, and clinical rotations.
Gender and having children were factors that had no correlation with the match rate. There was a statistical difference of matching based on marital status (P<.01), but this is likely due to the low number of applicants in the divorced category. There were differences among demographics with USMLE Step 1 and Step 2 CK scores, which is a known factor in matching.1,2 Applicants with children had lower USMLE Step 1 and Step 2 CK scores compared to applicants without children. Females also had lower median USMLE Step 1 scores compared to males. This finding may serve as a reminder to programs when comparing USMLE Step examination scores that demographic factors may play a role. The race and ethnicity of applicants likely play a role. It has been reported that underrepresented minorities had lower match rates than White and Asian applicants in dermatology.6 There have been several published articles discussing the lack of diversity in dermatology, with a call to action.7-9
Factors for Couples Matching—The number of applicants participating in the couples match continues to increase yearly. The NMRP does publish data regarding “successful” couples matching but does not specify how many couples match together. There also is little published regarding advice for participation in the couples match. Although we had a limited number of couples that participated in the match, it is interesting to note they had similar strategies, including contacting programs at institutions that had offered interviews to their partners. This strategy may be effective, as dermatology programs offer interviews relatively late compared with other specialties.5 Additionally, this strategy may increase the number of interviews offered and received, as evidenced by the higher number of interviews offered compared with those who were not couples matching. Additionally, this survey highlights the sacrifice often needed by couples in the couples match as revealed by the inclusion of rank-list options in which the couples reside long distance or in which 1 partner does not match. This information may be helpful to applicants who are planning a strategy for the couples match in dermatology. Although this study does not encompass all dermatology applicants in the 2019-2020 cycle, we do believe it may be representative. The USMLE Step 1 scores in this study were similar to the published NRMP data.1,10 According to NRMP data from the 2019-2020 cycle, the mean USMLE Step 1 score was 248 for matched applicants and 239 for unmatched.1 The NRMP reported the mean USMLE Step 2 CK score for matched was 256 and 248 for unmatched, which also is similar to our data. The NRMP reported the mean number of programs ranked was 9.9 for matched and 4.5 for unmatched applicants.1 Again, our data were similar for number of dermatology interviews attended.
Limitations—There are limitations to this study. The main limitation is that the survey is from a single institution and had a limited number of respondents. Given the nature of the study, the accuracy of the data is dependent on the applicants’ honesty in self-reporting academic performance and other variables. There also may be a selection bias given the low response rate. The subanalyses—children and couples matching—were underpowered with the limited number of participants. Further studies that include multiple residency programs and multiple years could be helpful to provide more power and less risk of bias. We did not gather information such as the Medical Student Performance Evaluation letter, letters of recommendation, or personal statements, which do play an important role in the assessment of an applicant. However, because the applicants completed these surveys, and given these are largely blinded to applicants, we did not feel the applicants could accurately respond to those aspects of the application.
Conclusion
Our survey finds that factors associated with matching included a higher USMLE Step 1 score, having a home dermatology program, and a higher number of interviews offered and attended. Some demographics had varying USMLE Step 1 scores but similar match rates.
- National Resident Matching Program. Results and Data: 2020 Main Residency Match. National Resident Matching Program; May 2020. Accessed January 9, 2023. https://www.nrmp.org/wp-content/uploads/2021/12/MM_Results_and-Data_2020-1.pdf
- Gauer JL, Jackson JB. The association of USMLE Step 1 and Step 2 CK scores with residency match specialty and location. Med Educ Online. 2017;22:1358579.
- Wang JV, Keller M. Pressure to publish for residency applicants in dermatology. Dermatol Online J. 2016;22:13030/qt56x1t7ww.
- Wang RF, Zhang M, Kaffenberger JA. Does the dermatology standardized letter of recommendation alter applicants’ chances of matching into residency. J Am Acad Dermatol. 2017;77:e139-e140.
- National Resident Matching Program, Data Release and Research Committee: results of the 2018 NRMP Program Director Survey. Accessed December 19, 2022. https://www.nrmp.org/wp-content/uploads/2021/07/NRMP-2018-Program-Director-Survey-for-WWW.pdf
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role of race and ethnicity in the dermatology applicant match process. J Natl Med Assoc. 2022;113:666-670.
- Chen A, Shinkai K. Rethinking how we select dermatology applicants-turning the tide. JAMA Dermatol. 2017;153:259-260.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49.
- National Resident Matching Program. Charting outcomes in the match: U.S. allopathic seniors. Characteristics of U.S. allopathic seniors who matched to their preferred specialty in the 2018 main residency match. 2nd ed. Accessed December 19, 2022. https://www.nrmp.org/wp-content/uploads/2021/07/Charting-Outcomes-in-the-Match-2018_Seniors-1.pdf
- National Resident Matching Program. Results and Data: 2020 Main Residency Match. National Resident Matching Program; May 2020. Accessed January 9, 2023. https://www.nrmp.org/wp-content/uploads/2021/12/MM_Results_and-Data_2020-1.pdf
- Gauer JL, Jackson JB. The association of USMLE Step 1 and Step 2 CK scores with residency match specialty and location. Med Educ Online. 2017;22:1358579.
- Wang JV, Keller M. Pressure to publish for residency applicants in dermatology. Dermatol Online J. 2016;22:13030/qt56x1t7ww.
- Wang RF, Zhang M, Kaffenberger JA. Does the dermatology standardized letter of recommendation alter applicants’ chances of matching into residency. J Am Acad Dermatol. 2017;77:e139-e140.
- National Resident Matching Program, Data Release and Research Committee: results of the 2018 NRMP Program Director Survey. Accessed December 19, 2022. https://www.nrmp.org/wp-content/uploads/2021/07/NRMP-2018-Program-Director-Survey-for-WWW.pdf
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role of race and ethnicity in the dermatology applicant match process. J Natl Med Assoc. 2022;113:666-670.
- Chen A, Shinkai K. Rethinking how we select dermatology applicants-turning the tide. JAMA Dermatol. 2017;153:259-260.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49.
- National Resident Matching Program. Charting outcomes in the match: U.S. allopathic seniors. Characteristics of U.S. allopathic seniors who matched to their preferred specialty in the 2018 main residency match. 2nd ed. Accessed December 19, 2022. https://www.nrmp.org/wp-content/uploads/2021/07/Charting-Outcomes-in-the-Match-2018_Seniors-1.pdf
PRACTICE POINTS
- Dermatology residency continues to be one of the most competitive specialties, with a match rate of 84.7% in 2019.
- A high US Medical Licensing Examination (USMLE) Step 1 score and having a home dermatology program and a greater number of interviews may lead to higher likeliness of matching in dermatology.
- Most applicants (82.4%) applied to programs their partner had interviews at, suggesting this may be a helpful strategy.
Cardiac Adverse Events Following COVID-19 Vaccination in Patients With Prior Vaccine-Associated Myocarditis
Vaccinations have substantially reduced morbidity and mortality from many infectious diseases. Despite the clear value of vaccinations in public health, efforts to better understand adverse events (AEs) following immunization are important to sustain public trust and vaccine confidence. Noninfectious inflammation of the heart may manifest as myocarditis or pericarditis, or occasionally, with shared signs and symptoms of each, as myopericarditis. This is a rare AE following some immunizations. Vaccine-associated myocarditis, pericarditis, or myopericarditis (VAMP) has been most clearly associated with smallpox vaccines and mRNA COVID-19 vaccines.1-6 Although extremely rare, VAMP also has been associated with other vaccines.7,8 Limited information exists to guide shared clinical decision making on COVID-19 vaccination in persons with a history of VAMP. It is unknown whether individuals with a history of VAMP are at higher risk for developing a recurrence or experiencing a more severe outcome following COVID-19 vaccination.
Methods
As part of the collaborative public health mission with the Centers for Disease Control and Prevention (CDC) for enhanced vaccine AE surveillance, the Defense Health Agency Immunization Healthcare Division (IHD) maintains a clinical database of service members and beneficiaries referred for suspected AEs following immunizations. A review of all AEs following immunization cases in this database from January 1, 2003, through February 28, 2022, identified individuals meeting the following criteria: (a) VAMP prior to receipt of COVID-19 vaccine; (b) receipt of COVID-19 vaccine in 2021; and (c) medical documentation in available electronic health records sufficient to describe health status at least 30 days following COVID-19 vaccination.9 If medical entries suggested cardiac symptoms following a COVID-19 vaccine, additional information was sought to verify VAMP based on current published criteria.10,11 Both the initial VAMP cases and the suspected COVID-19 VAMP cases were adjudicated by a team of vaccine experts and specialists in immunology, cardiology, and preventive medicine.
This retrospective review was approved and conducted in accordance with the Walter Reed National Military Medical Center Institutional Review Board protocol #20664. All individuals with recurrent VAMP consented to share their health records and clinical details.
Results
Among 9260 cases in the IHD database, 431 met the case definition for VAMP.
Among the 179 patients included in this analysis, 171 (96%) were male. Their median age was 39 years at the time of COVID-19 vaccination.
Within 1 month of receipt of any COVID-19 vaccine, 11 individuals had documented symptoms suggesting cardiac involvement, specifically, chest pain, palpitations, or dyspnea. After cardiac evaluation, 4 patients met the criteria for VAMP after COVID-19 vaccination.10,11 Seven patients either did not meet the criteria for VAMP or had alternative causes for their symptoms.
Two men aged 49 and 50 years with a history of vaccine-associated myocarditis following smallpox vaccination (Dryvax and ACAM2000) developed myocarditis 3 days after their second dose of the Moderna vaccine. One of these patients received a Pfizer-BioNTech booster 10 months later with no recurrence of symptoms. A 55-year-old man with a history of vaccine-associated myocarditis following Dryvax vaccination developed myocarditis 2 days after his Pfizer-BioNTech booster. None of the patients who developed post-COVID-19 VAMP reported residual symptoms from their initial VAMP episode, which occurred 12 to 18 years earlier. All were hospitalized briefly for observation and had complete symptom resolution within 6 weeks.
A 25-year-old man developed pericarditis 4 days after his second Pfizer-BioNTech vaccination. His previous ACAM2000 vaccine-associated myocarditis occurred 3 years earlier, with no residual symptoms. Of note, he had a mild COVID-19 infection 78 days before the onset of his pericarditis. After the onset of his COVID-19 vaccine-associated pericarditis, he continued to experience transient bouts of chest pressure and exertional dyspnea that resolved within 7 months of onset.
The median interval between COVID-19 vaccine doses in those who developed post-COVID-19 VAMP was within the recommended mRNA vaccine dosing intervals of 3 to 4 weeks and was consistent with the median mRNA vaccine dosing intervals among the entire cohort.
Due to the small cohort size and other limitations of this study, the suggested rate of cardiac injury in this review (4 cases in 179 persons, or 2.2%) is an imprecise estimate of risk in a small population (95% CI, 0.1%-4.4%). While this rate may seem higher than expected within the general population after COVID-19 vaccination, it is lower than the estimated lifetime risk of recurrent myocarditis from any cause.6,12
Discussion
To our knowledge, this is the first report describing cardiac outcomes after COVID-19 vaccination among a cohort of individuals with prior history of VAMP. Four cases of COVID-19 VAMP were identified among 179 patients with previous VAMP. All cases had experienced VAMP after the smallpox vaccine several years earlier, with complete resolution of symptoms. Three cases presented with recurrent VAMP after their second dose of an mRNA COVID-19 vaccine, and one after an mRNA booster dose. All fully recovered over the course of several months.
Myocarditis is a heterogeneous inflammatory injury with diverse, sometimes idiopathic, etiologies.13 In contrast to infection-related cardiac injury, prior reports of vaccine-associated myocarditis have suggested a hypersensitivity reaction characterized by patchy eosinophilic infiltrates, a benign clinical course, and good prognosis.2,3
There are several common features between VAMP after smallpox and COVID-19 vaccination. Cases occur predominantly in young men. The onset of symptoms after smallpox vaccine (mean, 10 days) and after mRNA COVID-19 vaccine (mean, 3 days) appears to correspond to the timing of peak postvaccination pro-inflammatory cytokine elevation.14 While all VAMP cases are serious events, the majority of patients appear to have a relatively benign clinical course with rapid and full recovery.13
Patients who have experienced an inflammatory cardiac injury may be at higher risk for recurrence, but quantifying risk of this rare phenomenon is challenging. Cases of VAMP after the COVID-19 vaccine have occasionally been reported in patients with previous cardiac injury unrelated to vaccination.15-17 The cases presented here represent the first report of recurrent VAMP following prior non-COVID-19 vaccinations.
Most patients with prior VAMP in this cohort did not experience cardiac-suggestive symptoms following COVID-19 vaccination. Among 11 patients who developed symptoms, 3 had confirmed myocarditis and 1 had confirmed pericarditis. The clinical course for these patients with recurrent VAMP was observed to be no different in severity or duration from those who experience new-onset VAMP.4 All other patients not meeting criteria for VAMP or having alternative explanations for their symptoms also had a benign clinical course. Nonetheless, of the study cohort of 179, recurrent VAMP was diagnosed in 4 of the 11 who developed cardiac-suggestive symptoms following COVID-19 vaccination. The importance of cardiac evaluation should be emphasized for any patient presenting with chest pain, dyspnea, or other cardiac-suggestive symptoms following vaccination.
Strengths and Limitations
The strength of this review of VAMP recurrence associated with COVID-19 vaccination derives from our large and unique longitudinal database of VAMP among current and prior service members. Additionally, the IHD’s ongoing enhanced vaccine AEs surveillance provides the opportunity to contact patients and review their electronic health records over an extended interval of time.
When interpreting this report’s implications, limitations inherent to any retrospective case review should be considered. The cohort of cases of prior VAMP included primarily healthy, fit, young service members; this population is not representative of the general population. The cohort included prior VAMP cases that generally occurred after smallpox vaccination. Experiences after smallpox vaccine may not apply to cardiac injury from other vaccines or etiologies. By the nature of this review, the population studied at the time of COVID-19 vaccination was somewhat older than those most likely to develop an initial bout of VAMP.2 This review was limited by information available in the electronic health records of a small number of patients. Subclinical cases of VAMP and cases without adequate clinical evaluation also could not be included.
Conclusions
Noninfectious inflammation of the heart (myocarditis, pericarditis, or myopericarditis) is a rare AE following certain vaccines, especially live replicating smallpox vaccine and mRNA COVID-19 vaccines. In this observational analysis, the majority of patients with previous VAMP successfully received a COVID-19 vaccine without recurrence. The 4 patients who were identified with recurrent VAMP following COVID-19 vaccination all recovered with supportive care. While the CDC endorses that individuals with a history of infectious myocarditis may receive COVID-19 vaccine after symptoms have resolved, there is currently insufficient safety data regarding COVID-19 vaccination of those with prior non-COVID-19 VAMP or following subsequent COVID-19 vaccination in those with prior VAMP related to COVID-19.10 For these individuals, COVID-19 vaccination is a precaution.10 Although insufficient to determine a precise level of risk, this report does provide data on which to base the CDC-recommended shared decision-making counseling of these patients. More research is needed to better define factors that increase risk for, or protection from, immune-mediated AEs following immunization, including VAMP. While benefits of vaccination have clearly outweighed risks during the COVID-19 pandemic, such research may optimize future vaccine recommendations.18
1. Decker MD, Garman PM, Hughes H, et al. Enhanced safety surveillance study of ACAM2000 smallpox vaccine among US military service members. Vaccine. 2021;39(39):5541-5547. doi:10.1016/j.vaccine.2021.08.041
2. Engler RJ, Nelson MR, Collins LC Jr, et al. A prospective study of the incidence of myocarditis/pericarditis and new onset cardiac symptoms following smallpox and influenza vaccination. PLoS One. 2015;10(3):e0118283. doi:10.1371/journal.pone.0118283
3. Faix DJ, Gordon DM, Perry LN, et al. Prospective safety surveillance study of ACAM2000 smallpox vaccine in deploying military personnel. Vaccine. 2020;38(46):7323-7330. doi:10.1016/j.vaccine.2020.09.037
4. Montgomery J, Ryan M, Engler R, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. 2021;6(10):1202-1206. doi:10.1001/jamacardio.2021.2833
5. Witberg G, Barda N, Hoss S, et al. Myocarditis after Covid-19 vaccination in a large health care organization. N Engl J Med. 2021;385(23):2132-2139. doi:10.1056/NEJMoa2110737
6. Oster ME, Shay DK, Su JR, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327(4):331-340. doi:10.1001/jama.2021.24110
7. Su JR, McNeil MM, Welsh KJ, et al. Myopericarditis after vaccination, Vaccine Adverse Event Reporting System (VAERS), 1990-2018. Vaccine. 2021;39(5):839-845. doi:10.1016/j.vaccine.2020.12.046
8. Mei R, Raschi E, Forcesi E, Diemberger I, De Ponti F, Poluzzi E. Myocarditis and pericarditis after immunization: gaining insights through the Vaccine Adverse Event Reporting System. Int J Cardiol. 2018;273:183-186. doi:10.1016/j.ijcard.2018.09.054
9. Centers for Disease Control and Prevention (CDC). Update: cardiac-related events during the civilian smallpox vaccination program—United States, 2003. MMWR Morb Mortal Wkly Rep. 2003;52(21):492-496.
10. Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70(27):977-982. doi:10.15585/mmwr.mm7027e2
11. Sexson Tejtel SK, Munoz FM, Al-Ammouri I, et al. Myocarditis and pericarditis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2022;40(10):1499-1511. doi:10.1016/j.vaccine.2021.11.074
12. Sagar S, Liu PP, Cooper LT Jr. Myocarditis. Lancet. 2012;379(9817):738-747. doi:10.1016/S0140-6736(11) 60648-X
13. Heymans S, Cooper LT. Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nat Rev Cardiol. 2022;19(2):75-77. doi:10.1038/s41569-021-00662-w
14. Cohen JI, Hohman P, Fulton R, et al. Kinetics of serum cytokines after primary or repeat vaccination with the smallpox vaccine. J Infect Dis. 2010;201(8):1183-1191. doi:10.1086/651453
15. Minocha PK, Better D, Singh RK, Hoque T. Recurrence of acute myocarditis temporally associated with receipt of the mRNA COVID-19 vaccine in an adolescent male. J Pediatr. 2021;238:321-323. doi:10.1016/j.jpeds.2021.06.035
16. Umei TC, Kishino Y, Watanabe K, et al. Recurrence of myopericarditis following mRNA COVID-19 vaccination in a male adolescent. CJC Open. 2022;4(3):350-352. doi:10.1016/j.cjco.2021.12.002
17. Pasha MA, Isaac S, Khan Z. Recurrent myocarditis following COVID-19 infection and the mRNA vaccine. Cureus. 2022;14(7):e26650. doi:10.7759/cureus.26650
18. Block JP, Boehmer TK, Forrest CB, et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination—PCORnet, United States, January 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(14):517-523. Published 2022 Apr 8. doi:10.15585/mmwr.mm7114e1
Vaccinations have substantially reduced morbidity and mortality from many infectious diseases. Despite the clear value of vaccinations in public health, efforts to better understand adverse events (AEs) following immunization are important to sustain public trust and vaccine confidence. Noninfectious inflammation of the heart may manifest as myocarditis or pericarditis, or occasionally, with shared signs and symptoms of each, as myopericarditis. This is a rare AE following some immunizations. Vaccine-associated myocarditis, pericarditis, or myopericarditis (VAMP) has been most clearly associated with smallpox vaccines and mRNA COVID-19 vaccines.1-6 Although extremely rare, VAMP also has been associated with other vaccines.7,8 Limited information exists to guide shared clinical decision making on COVID-19 vaccination in persons with a history of VAMP. It is unknown whether individuals with a history of VAMP are at higher risk for developing a recurrence or experiencing a more severe outcome following COVID-19 vaccination.
Methods
As part of the collaborative public health mission with the Centers for Disease Control and Prevention (CDC) for enhanced vaccine AE surveillance, the Defense Health Agency Immunization Healthcare Division (IHD) maintains a clinical database of service members and beneficiaries referred for suspected AEs following immunizations. A review of all AEs following immunization cases in this database from January 1, 2003, through February 28, 2022, identified individuals meeting the following criteria: (a) VAMP prior to receipt of COVID-19 vaccine; (b) receipt of COVID-19 vaccine in 2021; and (c) medical documentation in available electronic health records sufficient to describe health status at least 30 days following COVID-19 vaccination.9 If medical entries suggested cardiac symptoms following a COVID-19 vaccine, additional information was sought to verify VAMP based on current published criteria.10,11 Both the initial VAMP cases and the suspected COVID-19 VAMP cases were adjudicated by a team of vaccine experts and specialists in immunology, cardiology, and preventive medicine.
This retrospective review was approved and conducted in accordance with the Walter Reed National Military Medical Center Institutional Review Board protocol #20664. All individuals with recurrent VAMP consented to share their health records and clinical details.
Results
Among 9260 cases in the IHD database, 431 met the case definition for VAMP.
Among the 179 patients included in this analysis, 171 (96%) were male. Their median age was 39 years at the time of COVID-19 vaccination.
Within 1 month of receipt of any COVID-19 vaccine, 11 individuals had documented symptoms suggesting cardiac involvement, specifically, chest pain, palpitations, or dyspnea. After cardiac evaluation, 4 patients met the criteria for VAMP after COVID-19 vaccination.10,11 Seven patients either did not meet the criteria for VAMP or had alternative causes for their symptoms.
Two men aged 49 and 50 years with a history of vaccine-associated myocarditis following smallpox vaccination (Dryvax and ACAM2000) developed myocarditis 3 days after their second dose of the Moderna vaccine. One of these patients received a Pfizer-BioNTech booster 10 months later with no recurrence of symptoms. A 55-year-old man with a history of vaccine-associated myocarditis following Dryvax vaccination developed myocarditis 2 days after his Pfizer-BioNTech booster. None of the patients who developed post-COVID-19 VAMP reported residual symptoms from their initial VAMP episode, which occurred 12 to 18 years earlier. All were hospitalized briefly for observation and had complete symptom resolution within 6 weeks.
A 25-year-old man developed pericarditis 4 days after his second Pfizer-BioNTech vaccination. His previous ACAM2000 vaccine-associated myocarditis occurred 3 years earlier, with no residual symptoms. Of note, he had a mild COVID-19 infection 78 days before the onset of his pericarditis. After the onset of his COVID-19 vaccine-associated pericarditis, he continued to experience transient bouts of chest pressure and exertional dyspnea that resolved within 7 months of onset.
The median interval between COVID-19 vaccine doses in those who developed post-COVID-19 VAMP was within the recommended mRNA vaccine dosing intervals of 3 to 4 weeks and was consistent with the median mRNA vaccine dosing intervals among the entire cohort.
Due to the small cohort size and other limitations of this study, the suggested rate of cardiac injury in this review (4 cases in 179 persons, or 2.2%) is an imprecise estimate of risk in a small population (95% CI, 0.1%-4.4%). While this rate may seem higher than expected within the general population after COVID-19 vaccination, it is lower than the estimated lifetime risk of recurrent myocarditis from any cause.6,12
Discussion
To our knowledge, this is the first report describing cardiac outcomes after COVID-19 vaccination among a cohort of individuals with prior history of VAMP. Four cases of COVID-19 VAMP were identified among 179 patients with previous VAMP. All cases had experienced VAMP after the smallpox vaccine several years earlier, with complete resolution of symptoms. Three cases presented with recurrent VAMP after their second dose of an mRNA COVID-19 vaccine, and one after an mRNA booster dose. All fully recovered over the course of several months.
Myocarditis is a heterogeneous inflammatory injury with diverse, sometimes idiopathic, etiologies.13 In contrast to infection-related cardiac injury, prior reports of vaccine-associated myocarditis have suggested a hypersensitivity reaction characterized by patchy eosinophilic infiltrates, a benign clinical course, and good prognosis.2,3
There are several common features between VAMP after smallpox and COVID-19 vaccination. Cases occur predominantly in young men. The onset of symptoms after smallpox vaccine (mean, 10 days) and after mRNA COVID-19 vaccine (mean, 3 days) appears to correspond to the timing of peak postvaccination pro-inflammatory cytokine elevation.14 While all VAMP cases are serious events, the majority of patients appear to have a relatively benign clinical course with rapid and full recovery.13
Patients who have experienced an inflammatory cardiac injury may be at higher risk for recurrence, but quantifying risk of this rare phenomenon is challenging. Cases of VAMP after the COVID-19 vaccine have occasionally been reported in patients with previous cardiac injury unrelated to vaccination.15-17 The cases presented here represent the first report of recurrent VAMP following prior non-COVID-19 vaccinations.
Most patients with prior VAMP in this cohort did not experience cardiac-suggestive symptoms following COVID-19 vaccination. Among 11 patients who developed symptoms, 3 had confirmed myocarditis and 1 had confirmed pericarditis. The clinical course for these patients with recurrent VAMP was observed to be no different in severity or duration from those who experience new-onset VAMP.4 All other patients not meeting criteria for VAMP or having alternative explanations for their symptoms also had a benign clinical course. Nonetheless, of the study cohort of 179, recurrent VAMP was diagnosed in 4 of the 11 who developed cardiac-suggestive symptoms following COVID-19 vaccination. The importance of cardiac evaluation should be emphasized for any patient presenting with chest pain, dyspnea, or other cardiac-suggestive symptoms following vaccination.
Strengths and Limitations
The strength of this review of VAMP recurrence associated with COVID-19 vaccination derives from our large and unique longitudinal database of VAMP among current and prior service members. Additionally, the IHD’s ongoing enhanced vaccine AEs surveillance provides the opportunity to contact patients and review their electronic health records over an extended interval of time.
When interpreting this report’s implications, limitations inherent to any retrospective case review should be considered. The cohort of cases of prior VAMP included primarily healthy, fit, young service members; this population is not representative of the general population. The cohort included prior VAMP cases that generally occurred after smallpox vaccination. Experiences after smallpox vaccine may not apply to cardiac injury from other vaccines or etiologies. By the nature of this review, the population studied at the time of COVID-19 vaccination was somewhat older than those most likely to develop an initial bout of VAMP.2 This review was limited by information available in the electronic health records of a small number of patients. Subclinical cases of VAMP and cases without adequate clinical evaluation also could not be included.
Conclusions
Noninfectious inflammation of the heart (myocarditis, pericarditis, or myopericarditis) is a rare AE following certain vaccines, especially live replicating smallpox vaccine and mRNA COVID-19 vaccines. In this observational analysis, the majority of patients with previous VAMP successfully received a COVID-19 vaccine without recurrence. The 4 patients who were identified with recurrent VAMP following COVID-19 vaccination all recovered with supportive care. While the CDC endorses that individuals with a history of infectious myocarditis may receive COVID-19 vaccine after symptoms have resolved, there is currently insufficient safety data regarding COVID-19 vaccination of those with prior non-COVID-19 VAMP or following subsequent COVID-19 vaccination in those with prior VAMP related to COVID-19.10 For these individuals, COVID-19 vaccination is a precaution.10 Although insufficient to determine a precise level of risk, this report does provide data on which to base the CDC-recommended shared decision-making counseling of these patients. More research is needed to better define factors that increase risk for, or protection from, immune-mediated AEs following immunization, including VAMP. While benefits of vaccination have clearly outweighed risks during the COVID-19 pandemic, such research may optimize future vaccine recommendations.18
Vaccinations have substantially reduced morbidity and mortality from many infectious diseases. Despite the clear value of vaccinations in public health, efforts to better understand adverse events (AEs) following immunization are important to sustain public trust and vaccine confidence. Noninfectious inflammation of the heart may manifest as myocarditis or pericarditis, or occasionally, with shared signs and symptoms of each, as myopericarditis. This is a rare AE following some immunizations. Vaccine-associated myocarditis, pericarditis, or myopericarditis (VAMP) has been most clearly associated with smallpox vaccines and mRNA COVID-19 vaccines.1-6 Although extremely rare, VAMP also has been associated with other vaccines.7,8 Limited information exists to guide shared clinical decision making on COVID-19 vaccination in persons with a history of VAMP. It is unknown whether individuals with a history of VAMP are at higher risk for developing a recurrence or experiencing a more severe outcome following COVID-19 vaccination.
Methods
As part of the collaborative public health mission with the Centers for Disease Control and Prevention (CDC) for enhanced vaccine AE surveillance, the Defense Health Agency Immunization Healthcare Division (IHD) maintains a clinical database of service members and beneficiaries referred for suspected AEs following immunizations. A review of all AEs following immunization cases in this database from January 1, 2003, through February 28, 2022, identified individuals meeting the following criteria: (a) VAMP prior to receipt of COVID-19 vaccine; (b) receipt of COVID-19 vaccine in 2021; and (c) medical documentation in available electronic health records sufficient to describe health status at least 30 days following COVID-19 vaccination.9 If medical entries suggested cardiac symptoms following a COVID-19 vaccine, additional information was sought to verify VAMP based on current published criteria.10,11 Both the initial VAMP cases and the suspected COVID-19 VAMP cases were adjudicated by a team of vaccine experts and specialists in immunology, cardiology, and preventive medicine.
This retrospective review was approved and conducted in accordance with the Walter Reed National Military Medical Center Institutional Review Board protocol #20664. All individuals with recurrent VAMP consented to share their health records and clinical details.
Results
Among 9260 cases in the IHD database, 431 met the case definition for VAMP.
Among the 179 patients included in this analysis, 171 (96%) were male. Their median age was 39 years at the time of COVID-19 vaccination.
Within 1 month of receipt of any COVID-19 vaccine, 11 individuals had documented symptoms suggesting cardiac involvement, specifically, chest pain, palpitations, or dyspnea. After cardiac evaluation, 4 patients met the criteria for VAMP after COVID-19 vaccination.10,11 Seven patients either did not meet the criteria for VAMP or had alternative causes for their symptoms.
Two men aged 49 and 50 years with a history of vaccine-associated myocarditis following smallpox vaccination (Dryvax and ACAM2000) developed myocarditis 3 days after their second dose of the Moderna vaccine. One of these patients received a Pfizer-BioNTech booster 10 months later with no recurrence of symptoms. A 55-year-old man with a history of vaccine-associated myocarditis following Dryvax vaccination developed myocarditis 2 days after his Pfizer-BioNTech booster. None of the patients who developed post-COVID-19 VAMP reported residual symptoms from their initial VAMP episode, which occurred 12 to 18 years earlier. All were hospitalized briefly for observation and had complete symptom resolution within 6 weeks.
A 25-year-old man developed pericarditis 4 days after his second Pfizer-BioNTech vaccination. His previous ACAM2000 vaccine-associated myocarditis occurred 3 years earlier, with no residual symptoms. Of note, he had a mild COVID-19 infection 78 days before the onset of his pericarditis. After the onset of his COVID-19 vaccine-associated pericarditis, he continued to experience transient bouts of chest pressure and exertional dyspnea that resolved within 7 months of onset.
The median interval between COVID-19 vaccine doses in those who developed post-COVID-19 VAMP was within the recommended mRNA vaccine dosing intervals of 3 to 4 weeks and was consistent with the median mRNA vaccine dosing intervals among the entire cohort.
Due to the small cohort size and other limitations of this study, the suggested rate of cardiac injury in this review (4 cases in 179 persons, or 2.2%) is an imprecise estimate of risk in a small population (95% CI, 0.1%-4.4%). While this rate may seem higher than expected within the general population after COVID-19 vaccination, it is lower than the estimated lifetime risk of recurrent myocarditis from any cause.6,12
Discussion
To our knowledge, this is the first report describing cardiac outcomes after COVID-19 vaccination among a cohort of individuals with prior history of VAMP. Four cases of COVID-19 VAMP were identified among 179 patients with previous VAMP. All cases had experienced VAMP after the smallpox vaccine several years earlier, with complete resolution of symptoms. Three cases presented with recurrent VAMP after their second dose of an mRNA COVID-19 vaccine, and one after an mRNA booster dose. All fully recovered over the course of several months.
Myocarditis is a heterogeneous inflammatory injury with diverse, sometimes idiopathic, etiologies.13 In contrast to infection-related cardiac injury, prior reports of vaccine-associated myocarditis have suggested a hypersensitivity reaction characterized by patchy eosinophilic infiltrates, a benign clinical course, and good prognosis.2,3
There are several common features between VAMP after smallpox and COVID-19 vaccination. Cases occur predominantly in young men. The onset of symptoms after smallpox vaccine (mean, 10 days) and after mRNA COVID-19 vaccine (mean, 3 days) appears to correspond to the timing of peak postvaccination pro-inflammatory cytokine elevation.14 While all VAMP cases are serious events, the majority of patients appear to have a relatively benign clinical course with rapid and full recovery.13
Patients who have experienced an inflammatory cardiac injury may be at higher risk for recurrence, but quantifying risk of this rare phenomenon is challenging. Cases of VAMP after the COVID-19 vaccine have occasionally been reported in patients with previous cardiac injury unrelated to vaccination.15-17 The cases presented here represent the first report of recurrent VAMP following prior non-COVID-19 vaccinations.
Most patients with prior VAMP in this cohort did not experience cardiac-suggestive symptoms following COVID-19 vaccination. Among 11 patients who developed symptoms, 3 had confirmed myocarditis and 1 had confirmed pericarditis. The clinical course for these patients with recurrent VAMP was observed to be no different in severity or duration from those who experience new-onset VAMP.4 All other patients not meeting criteria for VAMP or having alternative explanations for their symptoms also had a benign clinical course. Nonetheless, of the study cohort of 179, recurrent VAMP was diagnosed in 4 of the 11 who developed cardiac-suggestive symptoms following COVID-19 vaccination. The importance of cardiac evaluation should be emphasized for any patient presenting with chest pain, dyspnea, or other cardiac-suggestive symptoms following vaccination.
Strengths and Limitations
The strength of this review of VAMP recurrence associated with COVID-19 vaccination derives from our large and unique longitudinal database of VAMP among current and prior service members. Additionally, the IHD’s ongoing enhanced vaccine AEs surveillance provides the opportunity to contact patients and review their electronic health records over an extended interval of time.
When interpreting this report’s implications, limitations inherent to any retrospective case review should be considered. The cohort of cases of prior VAMP included primarily healthy, fit, young service members; this population is not representative of the general population. The cohort included prior VAMP cases that generally occurred after smallpox vaccination. Experiences after smallpox vaccine may not apply to cardiac injury from other vaccines or etiologies. By the nature of this review, the population studied at the time of COVID-19 vaccination was somewhat older than those most likely to develop an initial bout of VAMP.2 This review was limited by information available in the electronic health records of a small number of patients. Subclinical cases of VAMP and cases without adequate clinical evaluation also could not be included.
Conclusions
Noninfectious inflammation of the heart (myocarditis, pericarditis, or myopericarditis) is a rare AE following certain vaccines, especially live replicating smallpox vaccine and mRNA COVID-19 vaccines. In this observational analysis, the majority of patients with previous VAMP successfully received a COVID-19 vaccine without recurrence. The 4 patients who were identified with recurrent VAMP following COVID-19 vaccination all recovered with supportive care. While the CDC endorses that individuals with a history of infectious myocarditis may receive COVID-19 vaccine after symptoms have resolved, there is currently insufficient safety data regarding COVID-19 vaccination of those with prior non-COVID-19 VAMP or following subsequent COVID-19 vaccination in those with prior VAMP related to COVID-19.10 For these individuals, COVID-19 vaccination is a precaution.10 Although insufficient to determine a precise level of risk, this report does provide data on which to base the CDC-recommended shared decision-making counseling of these patients. More research is needed to better define factors that increase risk for, or protection from, immune-mediated AEs following immunization, including VAMP. While benefits of vaccination have clearly outweighed risks during the COVID-19 pandemic, such research may optimize future vaccine recommendations.18
1. Decker MD, Garman PM, Hughes H, et al. Enhanced safety surveillance study of ACAM2000 smallpox vaccine among US military service members. Vaccine. 2021;39(39):5541-5547. doi:10.1016/j.vaccine.2021.08.041
2. Engler RJ, Nelson MR, Collins LC Jr, et al. A prospective study of the incidence of myocarditis/pericarditis and new onset cardiac symptoms following smallpox and influenza vaccination. PLoS One. 2015;10(3):e0118283. doi:10.1371/journal.pone.0118283
3. Faix DJ, Gordon DM, Perry LN, et al. Prospective safety surveillance study of ACAM2000 smallpox vaccine in deploying military personnel. Vaccine. 2020;38(46):7323-7330. doi:10.1016/j.vaccine.2020.09.037
4. Montgomery J, Ryan M, Engler R, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. 2021;6(10):1202-1206. doi:10.1001/jamacardio.2021.2833
5. Witberg G, Barda N, Hoss S, et al. Myocarditis after Covid-19 vaccination in a large health care organization. N Engl J Med. 2021;385(23):2132-2139. doi:10.1056/NEJMoa2110737
6. Oster ME, Shay DK, Su JR, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327(4):331-340. doi:10.1001/jama.2021.24110
7. Su JR, McNeil MM, Welsh KJ, et al. Myopericarditis after vaccination, Vaccine Adverse Event Reporting System (VAERS), 1990-2018. Vaccine. 2021;39(5):839-845. doi:10.1016/j.vaccine.2020.12.046
8. Mei R, Raschi E, Forcesi E, Diemberger I, De Ponti F, Poluzzi E. Myocarditis and pericarditis after immunization: gaining insights through the Vaccine Adverse Event Reporting System. Int J Cardiol. 2018;273:183-186. doi:10.1016/j.ijcard.2018.09.054
9. Centers for Disease Control and Prevention (CDC). Update: cardiac-related events during the civilian smallpox vaccination program—United States, 2003. MMWR Morb Mortal Wkly Rep. 2003;52(21):492-496.
10. Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70(27):977-982. doi:10.15585/mmwr.mm7027e2
11. Sexson Tejtel SK, Munoz FM, Al-Ammouri I, et al. Myocarditis and pericarditis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2022;40(10):1499-1511. doi:10.1016/j.vaccine.2021.11.074
12. Sagar S, Liu PP, Cooper LT Jr. Myocarditis. Lancet. 2012;379(9817):738-747. doi:10.1016/S0140-6736(11) 60648-X
13. Heymans S, Cooper LT. Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nat Rev Cardiol. 2022;19(2):75-77. doi:10.1038/s41569-021-00662-w
14. Cohen JI, Hohman P, Fulton R, et al. Kinetics of serum cytokines after primary or repeat vaccination with the smallpox vaccine. J Infect Dis. 2010;201(8):1183-1191. doi:10.1086/651453
15. Minocha PK, Better D, Singh RK, Hoque T. Recurrence of acute myocarditis temporally associated with receipt of the mRNA COVID-19 vaccine in an adolescent male. J Pediatr. 2021;238:321-323. doi:10.1016/j.jpeds.2021.06.035
16. Umei TC, Kishino Y, Watanabe K, et al. Recurrence of myopericarditis following mRNA COVID-19 vaccination in a male adolescent. CJC Open. 2022;4(3):350-352. doi:10.1016/j.cjco.2021.12.002
17. Pasha MA, Isaac S, Khan Z. Recurrent myocarditis following COVID-19 infection and the mRNA vaccine. Cureus. 2022;14(7):e26650. doi:10.7759/cureus.26650
18. Block JP, Boehmer TK, Forrest CB, et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination—PCORnet, United States, January 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(14):517-523. Published 2022 Apr 8. doi:10.15585/mmwr.mm7114e1
1. Decker MD, Garman PM, Hughes H, et al. Enhanced safety surveillance study of ACAM2000 smallpox vaccine among US military service members. Vaccine. 2021;39(39):5541-5547. doi:10.1016/j.vaccine.2021.08.041
2. Engler RJ, Nelson MR, Collins LC Jr, et al. A prospective study of the incidence of myocarditis/pericarditis and new onset cardiac symptoms following smallpox and influenza vaccination. PLoS One. 2015;10(3):e0118283. doi:10.1371/journal.pone.0118283
3. Faix DJ, Gordon DM, Perry LN, et al. Prospective safety surveillance study of ACAM2000 smallpox vaccine in deploying military personnel. Vaccine. 2020;38(46):7323-7330. doi:10.1016/j.vaccine.2020.09.037
4. Montgomery J, Ryan M, Engler R, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. 2021;6(10):1202-1206. doi:10.1001/jamacardio.2021.2833
5. Witberg G, Barda N, Hoss S, et al. Myocarditis after Covid-19 vaccination in a large health care organization. N Engl J Med. 2021;385(23):2132-2139. doi:10.1056/NEJMoa2110737
6. Oster ME, Shay DK, Su JR, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327(4):331-340. doi:10.1001/jama.2021.24110
7. Su JR, McNeil MM, Welsh KJ, et al. Myopericarditis after vaccination, Vaccine Adverse Event Reporting System (VAERS), 1990-2018. Vaccine. 2021;39(5):839-845. doi:10.1016/j.vaccine.2020.12.046
8. Mei R, Raschi E, Forcesi E, Diemberger I, De Ponti F, Poluzzi E. Myocarditis and pericarditis after immunization: gaining insights through the Vaccine Adverse Event Reporting System. Int J Cardiol. 2018;273:183-186. doi:10.1016/j.ijcard.2018.09.054
9. Centers for Disease Control and Prevention (CDC). Update: cardiac-related events during the civilian smallpox vaccination program—United States, 2003. MMWR Morb Mortal Wkly Rep. 2003;52(21):492-496.
10. Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70(27):977-982. doi:10.15585/mmwr.mm7027e2
11. Sexson Tejtel SK, Munoz FM, Al-Ammouri I, et al. Myocarditis and pericarditis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2022;40(10):1499-1511. doi:10.1016/j.vaccine.2021.11.074
12. Sagar S, Liu PP, Cooper LT Jr. Myocarditis. Lancet. 2012;379(9817):738-747. doi:10.1016/S0140-6736(11) 60648-X
13. Heymans S, Cooper LT. Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nat Rev Cardiol. 2022;19(2):75-77. doi:10.1038/s41569-021-00662-w
14. Cohen JI, Hohman P, Fulton R, et al. Kinetics of serum cytokines after primary or repeat vaccination with the smallpox vaccine. J Infect Dis. 2010;201(8):1183-1191. doi:10.1086/651453
15. Minocha PK, Better D, Singh RK, Hoque T. Recurrence of acute myocarditis temporally associated with receipt of the mRNA COVID-19 vaccine in an adolescent male. J Pediatr. 2021;238:321-323. doi:10.1016/j.jpeds.2021.06.035
16. Umei TC, Kishino Y, Watanabe K, et al. Recurrence of myopericarditis following mRNA COVID-19 vaccination in a male adolescent. CJC Open. 2022;4(3):350-352. doi:10.1016/j.cjco.2021.12.002
17. Pasha MA, Isaac S, Khan Z. Recurrent myocarditis following COVID-19 infection and the mRNA vaccine. Cureus. 2022;14(7):e26650. doi:10.7759/cureus.26650
18. Block JP, Boehmer TK, Forrest CB, et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination—PCORnet, United States, January 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(14):517-523. Published 2022 Apr 8. doi:10.15585/mmwr.mm7114e1