User login
Comparing Outcomes and Toxicities With Standard and Reduced Dose Melphalan in Autologous Stem Cell Transplant Patients With Multiple Myeloma
BACKGROUND
Multiple myeloma, an incurable plasma cell malignancy, has an average age of diagnosis over 65 years. For transplant-eligible patients, high-dose melphalan 200 mg/m2 (MEL200), followed by autologous stem cell rescue (ASCR) is the standard in consolidation therapy. Most clinical trials evaluating MEL200 with ASCR excluded patients over 65 due to concerns for toxicity and treatment-related mortality, leading to use of reduced dose melphalan 140 mg/m2 (MEL140) in clinical practice for older patients. As this dose has limited studies surrounding its reduction, the purpose of this study was to compare outcomes and toxicities of MEL140 in patients over the age of 65 to MEL200 in patients 65 and under.
METHODS
This single-center institutional review board approved retrospective study was conducted at VA Tennessee Valley Healthcare System. All multiple myeloma patients greater than 18 years of age who received melphalan with ASCR from January 1, 2018, to December 31, 2021, were included. Patients were divided into two arms: age < 65 treated with MEL200 and age >65 treated with MEL140. The primary endpoint was oneyear progression-free survival (PFS). The secondary endpoints were one-year overall survival (OS), treatment related mortality, time to neutrophil engraftment, and toxicities including febrile neutropenia, diarrhea, mucositis, infection, and intensive care unit transfers.
RESULTS
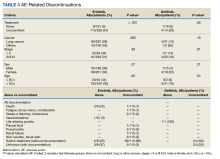
A total of 222 patients were included, 114 patients in the MEL200 arm and 108 patients in the MEL140 arm. The primary endpoint of one-year PFS had no significant difference, with 103 (90.4%) patients in the MEL200 group compared to 99 (91.7%) patients in the MEL140 group (p=0.732). Similarly, there was no statistically significant difference in the secondary endpoint of one-year OS with 112 (98.3%) patients in the MEL200 group compared to 106 (98.2%) in the MEL140 group (p=0.956). Toxicities were similar; however, grade 3 mucositis was higher in the MEL200 arm.
CONCLUSIONS
Our study found no difference in oneyear PFS or one-year OS when comparing MEL140 to MEL200 with minimal differences in regimen-related toxicities. Although not powered to detect statistical difference, results suggests that dose reduction with MEL140 in patients >65 years does not impact one-year PFS when compared to patients <65 receiving standard MEL200.
BACKGROUND
Multiple myeloma, an incurable plasma cell malignancy, has an average age of diagnosis over 65 years. For transplant-eligible patients, high-dose melphalan 200 mg/m2 (MEL200), followed by autologous stem cell rescue (ASCR) is the standard in consolidation therapy. Most clinical trials evaluating MEL200 with ASCR excluded patients over 65 due to concerns for toxicity and treatment-related mortality, leading to use of reduced dose melphalan 140 mg/m2 (MEL140) in clinical practice for older patients. As this dose has limited studies surrounding its reduction, the purpose of this study was to compare outcomes and toxicities of MEL140 in patients over the age of 65 to MEL200 in patients 65 and under.
METHODS
This single-center institutional review board approved retrospective study was conducted at VA Tennessee Valley Healthcare System. All multiple myeloma patients greater than 18 years of age who received melphalan with ASCR from January 1, 2018, to December 31, 2021, were included. Patients were divided into two arms: age < 65 treated with MEL200 and age >65 treated with MEL140. The primary endpoint was oneyear progression-free survival (PFS). The secondary endpoints were one-year overall survival (OS), treatment related mortality, time to neutrophil engraftment, and toxicities including febrile neutropenia, diarrhea, mucositis, infection, and intensive care unit transfers.
RESULTS
A total of 222 patients were included, 114 patients in the MEL200 arm and 108 patients in the MEL140 arm. The primary endpoint of one-year PFS had no significant difference, with 103 (90.4%) patients in the MEL200 group compared to 99 (91.7%) patients in the MEL140 group (p=0.732). Similarly, there was no statistically significant difference in the secondary endpoint of one-year OS with 112 (98.3%) patients in the MEL200 group compared to 106 (98.2%) in the MEL140 group (p=0.956). Toxicities were similar; however, grade 3 mucositis was higher in the MEL200 arm.
CONCLUSIONS
Our study found no difference in oneyear PFS or one-year OS when comparing MEL140 to MEL200 with minimal differences in regimen-related toxicities. Although not powered to detect statistical difference, results suggests that dose reduction with MEL140 in patients >65 years does not impact one-year PFS when compared to patients <65 receiving standard MEL200.
BACKGROUND
Multiple myeloma, an incurable plasma cell malignancy, has an average age of diagnosis over 65 years. For transplant-eligible patients, high-dose melphalan 200 mg/m2 (MEL200), followed by autologous stem cell rescue (ASCR) is the standard in consolidation therapy. Most clinical trials evaluating MEL200 with ASCR excluded patients over 65 due to concerns for toxicity and treatment-related mortality, leading to use of reduced dose melphalan 140 mg/m2 (MEL140) in clinical practice for older patients. As this dose has limited studies surrounding its reduction, the purpose of this study was to compare outcomes and toxicities of MEL140 in patients over the age of 65 to MEL200 in patients 65 and under.
METHODS
This single-center institutional review board approved retrospective study was conducted at VA Tennessee Valley Healthcare System. All multiple myeloma patients greater than 18 years of age who received melphalan with ASCR from January 1, 2018, to December 31, 2021, were included. Patients were divided into two arms: age < 65 treated with MEL200 and age >65 treated with MEL140. The primary endpoint was oneyear progression-free survival (PFS). The secondary endpoints were one-year overall survival (OS), treatment related mortality, time to neutrophil engraftment, and toxicities including febrile neutropenia, diarrhea, mucositis, infection, and intensive care unit transfers.
RESULTS
A total of 222 patients were included, 114 patients in the MEL200 arm and 108 patients in the MEL140 arm. The primary endpoint of one-year PFS had no significant difference, with 103 (90.4%) patients in the MEL200 group compared to 99 (91.7%) patients in the MEL140 group (p=0.732). Similarly, there was no statistically significant difference in the secondary endpoint of one-year OS with 112 (98.3%) patients in the MEL200 group compared to 106 (98.2%) in the MEL140 group (p=0.956). Toxicities were similar; however, grade 3 mucositis was higher in the MEL200 arm.
CONCLUSIONS
Our study found no difference in oneyear PFS or one-year OS when comparing MEL140 to MEL200 with minimal differences in regimen-related toxicities. Although not powered to detect statistical difference, results suggests that dose reduction with MEL140 in patients >65 years does not impact one-year PFS when compared to patients <65 receiving standard MEL200.
Quality Improvement Project of All Resected Lung Specimens for Pathologic Findings and Synoptic Surgical Reports for Accuracy in Staging: A Critical Review of 91 Specimens
BACKGROUND
In 2017, the Thoracic Tumor Board realized that there were patients whose lung resections had critical review of the slides and reports prior to presentation. Errors were found which resulted in a change of the pathology Tumor Nodal Metastases (pTNM) staging for the patient. The impacts were important for determining appropriate therapy. It was decided to systematically review all lung cancer resections for accuracy before determining definitive therapy recommendations.
METHODS
All lung resections for malignancy were examined prior and up to 2 days of completion for accuracy of tumor type, tumor size, tumor grade, lymph node metastases and pathologic stage (pTNM). Any errors found were given to the original pathologist for a change in the report before release or for a modified report to be issued.
RESULTS
From June 2017 to December 2020, there were 91 lung resections with 28 (30.77%) errors. Errors included: 16 incorrect pathologic staging, 5 missed tumors in lung and lymph nodes, 2 unexamined stapled surgical margins, 1 wrong site, 1 incorrect lymph node number and 2 missed tumor vascular invasion.
IMPLICATIONS
Quality improvement (QI) review of lung resections by a second pathologist is important and may clearly improve pathologic staging for lung cancer patients. It can be added to QI programs currently used in Surgical Pathology. It is important in directing appropriate postsurgical therapies.
BACKGROUND
In 2017, the Thoracic Tumor Board realized that there were patients whose lung resections had critical review of the slides and reports prior to presentation. Errors were found which resulted in a change of the pathology Tumor Nodal Metastases (pTNM) staging for the patient. The impacts were important for determining appropriate therapy. It was decided to systematically review all lung cancer resections for accuracy before determining definitive therapy recommendations.
METHODS
All lung resections for malignancy were examined prior and up to 2 days of completion for accuracy of tumor type, tumor size, tumor grade, lymph node metastases and pathologic stage (pTNM). Any errors found were given to the original pathologist for a change in the report before release or for a modified report to be issued.
RESULTS
From June 2017 to December 2020, there were 91 lung resections with 28 (30.77%) errors. Errors included: 16 incorrect pathologic staging, 5 missed tumors in lung and lymph nodes, 2 unexamined stapled surgical margins, 1 wrong site, 1 incorrect lymph node number and 2 missed tumor vascular invasion.
IMPLICATIONS
Quality improvement (QI) review of lung resections by a second pathologist is important and may clearly improve pathologic staging for lung cancer patients. It can be added to QI programs currently used in Surgical Pathology. It is important in directing appropriate postsurgical therapies.
BACKGROUND
In 2017, the Thoracic Tumor Board realized that there were patients whose lung resections had critical review of the slides and reports prior to presentation. Errors were found which resulted in a change of the pathology Tumor Nodal Metastases (pTNM) staging for the patient. The impacts were important for determining appropriate therapy. It was decided to systematically review all lung cancer resections for accuracy before determining definitive therapy recommendations.
METHODS
All lung resections for malignancy were examined prior and up to 2 days of completion for accuracy of tumor type, tumor size, tumor grade, lymph node metastases and pathologic stage (pTNM). Any errors found were given to the original pathologist for a change in the report before release or for a modified report to be issued.
RESULTS
From June 2017 to December 2020, there were 91 lung resections with 28 (30.77%) errors. Errors included: 16 incorrect pathologic staging, 5 missed tumors in lung and lymph nodes, 2 unexamined stapled surgical margins, 1 wrong site, 1 incorrect lymph node number and 2 missed tumor vascular invasion.
IMPLICATIONS
Quality improvement (QI) review of lung resections by a second pathologist is important and may clearly improve pathologic staging for lung cancer patients. It can be added to QI programs currently used in Surgical Pathology. It is important in directing appropriate postsurgical therapies.
A Multi-Disciplinary Approach to Increasing Germline Genetic Testing for Prostate Cancer
PURPOSE
This quality improvement project aims to enhance the rate of germline genetic testing for prostate cancer at the Stratton VA Medical Center, improving risk reduction strategies and therapeutic options for patients.
BACKGROUND
Prostate cancer is prevalent at the Stratton VA Medical Center, yet the rate of genetic evaluation for prostate cancer remains suboptimal. National guidelines recommend genetic counseling and testing in specific patient populations. To address this gap, an interdisciplinary working group conducted gap analysis and root cause analysis, identifying four significant barriers.
METHODS
The working group comprised medical oncologists, urologists, primary care physicians, genetics counselors, data experts, and a LEAN coach. Interventions included implementing a prostate cancer pathway to educate staff on genetic testing indications and integrating genetic testing screening into clinic visits. After the interventions were implemented in January 2022, patient charts were reviewed for all genetic referrals and new prostate cancer diagnoses from January to December 2022.
DATA ANALYSIS
Descriptive analysis was conducted on referral rates, evaluation visit completion rates, and genetic testing outcomes among prostate cancer patients.
RESULTS
During the study period, 59 prostate cancer patients were referred for genetic evaluation. Notably, this was a large increase from no genetic referrals for prostate cancer in the previous year. Among them, 43 completed the evaluation visit, and 34 underwent genetic testing. Noteworthy findings were observed in 5 patients, including 3 variants of unknown significance and 2 pathogenic germline variants: HOXB13 and BRCA2 mutations.
IMPLICATIONS
This project highlights the power of a collaborative, multidisciplinary approach to overcome barriers and enhance the quality of care for prostate cancer patients. The team’s use of gap analysis and root cause analysis successfully identified barriers and proposed solutions, leading to increased referrals and the identification of significant genetic findings. Continued efforts to improve access to germline genetic testing are crucial for enhanced patient care and improved outcomes.
PURPOSE
This quality improvement project aims to enhance the rate of germline genetic testing for prostate cancer at the Stratton VA Medical Center, improving risk reduction strategies and therapeutic options for patients.
BACKGROUND
Prostate cancer is prevalent at the Stratton VA Medical Center, yet the rate of genetic evaluation for prostate cancer remains suboptimal. National guidelines recommend genetic counseling and testing in specific patient populations. To address this gap, an interdisciplinary working group conducted gap analysis and root cause analysis, identifying four significant barriers.
METHODS
The working group comprised medical oncologists, urologists, primary care physicians, genetics counselors, data experts, and a LEAN coach. Interventions included implementing a prostate cancer pathway to educate staff on genetic testing indications and integrating genetic testing screening into clinic visits. After the interventions were implemented in January 2022, patient charts were reviewed for all genetic referrals and new prostate cancer diagnoses from January to December 2022.
DATA ANALYSIS
Descriptive analysis was conducted on referral rates, evaluation visit completion rates, and genetic testing outcomes among prostate cancer patients.
RESULTS
During the study period, 59 prostate cancer patients were referred for genetic evaluation. Notably, this was a large increase from no genetic referrals for prostate cancer in the previous year. Among them, 43 completed the evaluation visit, and 34 underwent genetic testing. Noteworthy findings were observed in 5 patients, including 3 variants of unknown significance and 2 pathogenic germline variants: HOXB13 and BRCA2 mutations.
IMPLICATIONS
This project highlights the power of a collaborative, multidisciplinary approach to overcome barriers and enhance the quality of care for prostate cancer patients. The team’s use of gap analysis and root cause analysis successfully identified barriers and proposed solutions, leading to increased referrals and the identification of significant genetic findings. Continued efforts to improve access to germline genetic testing are crucial for enhanced patient care and improved outcomes.
PURPOSE
This quality improvement project aims to enhance the rate of germline genetic testing for prostate cancer at the Stratton VA Medical Center, improving risk reduction strategies and therapeutic options for patients.
BACKGROUND
Prostate cancer is prevalent at the Stratton VA Medical Center, yet the rate of genetic evaluation for prostate cancer remains suboptimal. National guidelines recommend genetic counseling and testing in specific patient populations. To address this gap, an interdisciplinary working group conducted gap analysis and root cause analysis, identifying four significant barriers.
METHODS
The working group comprised medical oncologists, urologists, primary care physicians, genetics counselors, data experts, and a LEAN coach. Interventions included implementing a prostate cancer pathway to educate staff on genetic testing indications and integrating genetic testing screening into clinic visits. After the interventions were implemented in January 2022, patient charts were reviewed for all genetic referrals and new prostate cancer diagnoses from January to December 2022.
DATA ANALYSIS
Descriptive analysis was conducted on referral rates, evaluation visit completion rates, and genetic testing outcomes among prostate cancer patients.
RESULTS
During the study period, 59 prostate cancer patients were referred for genetic evaluation. Notably, this was a large increase from no genetic referrals for prostate cancer in the previous year. Among them, 43 completed the evaluation visit, and 34 underwent genetic testing. Noteworthy findings were observed in 5 patients, including 3 variants of unknown significance and 2 pathogenic germline variants: HOXB13 and BRCA2 mutations.
IMPLICATIONS
This project highlights the power of a collaborative, multidisciplinary approach to overcome barriers and enhance the quality of care for prostate cancer patients. The team’s use of gap analysis and root cause analysis successfully identified barriers and proposed solutions, leading to increased referrals and the identification of significant genetic findings. Continued efforts to improve access to germline genetic testing are crucial for enhanced patient care and improved outcomes.
Results From the First Annual Association of Professors of Dermatology Program Directors Survey
Educational organizations across several specialties, including internal medicine and obstetrics and gynecology, have formal surveys1; however, the field of dermatology has been without one. This study aimed to establish a formal survey for dermatology program directors (PDs) and clinician-educators. Because the Accreditation Council for Graduate Medical Education (ACGME) and American Board of Dermatology surveys do not capture all metrics relevant to dermatology residency educators, an annual survey for our specialty may be helpful to compare dermatology-specific data among programs. Responses could provide context and perspective to faculty and residents who respond to the ACGME annual survey, as our Association of Professors of Dermatology (APD) survey asks more in-depth questions, such as how often didactics occur and who leads them. Resident commute time and faculty demographics and training also are covered. Current ad hoc surveys disseminated through listserves of various medical associations contain overlapping questions and reflect relatively low response rates; dermatology PDs may benefit from a survey with a high response rate to which they can contribute future questions and topics that reflect recent trends and current needs in graduate medical education. As future surveys are administered, the results can be captured in a centralized database accessible by dermatology PDs.
Methods
A survey of PDs from 141 ACGME-accredited dermatology residency programs was conducted by the Residency Program Director Steering Committee of the APD from November 2022 to January 2023 using a prevalidated questionnaire. Personalized survey links were created and sent individually to each PD’s email listed in the ACGME accreditation data system. All survey responses were captured anonymously, with a number assigned to keep de-identified responses separate and organized. The survey consisted of 137 survey questions addressing topics that included program characteristics, PD demographics, the impact of the COVID-19 pandemic on clinical rotation and educational conferences, available resident resources, quality improvement, clinical and didactic instruction, research content, diversity and inclusion, wellness, professionalism, evaluation systems, and graduate outcomes.
Data were collected using Qualtrics survey tools. After removing duplicate and incomplete surveys, data were analyzed using Qualtrics reports and Microsoft Excel for data plotting, averages, and range calculations.
Results
One hundred forty-one personalized survey links were created and sent individually to each program’s filed email obtained from the APD listserv. Fifty-three responses were recorded after removing duplicate or incomplete surveys (38% [53/141] response rate). As of May 2023, there were 144 ACGME-accredited dermatology residency programs due to 3 newly accredited programs in 2022-2023 academic year, which were not included in our survey population.
Program Characteristics—Forty-four respondents (83%) were from a university-based program. Fifty respondents (94%) were from programs that were ACGME accredited prior to 2020, while 3 programs (6%) were American Osteopathic Association accredited prior to singular accreditation. Seventy-one percent (38/53) of respondents had 1 or more associate PDs.
PD Demographics—Eighty-seven percent (45/52) of PDs who responded to the survey graduated from a US allopathic medical school (MD), 10% (5/52) graduated from a US osteopathic medical school (DO), and 4% (2/52) graduated from an international medical school. Seventy-four percent (35/47) of respondents were White, 17% (8/47) were Asian, and 2% (1/47) were Black or African American; this data was not provided for 4 respondents. Forty-eight percent (23/48) of PDs identified as cisgender man, 48% (23/48) identified as cisgender woman, and 4% (2/48) preferred not to answer. Eighty-one percent (38/47) of PDs identified as heterosexual or straight, 15% (7/47) identified as gay or lesbian, and 4% (2/47) preferred not to answer.
Impact of COVID-19 Pandemic on Residency Training—Due to the COVID-19 pandemic, 88% (45/51) of respondents incorporated telemedicine into the resident clinical rotation schedule. Moving forward, 75% (38/51) of respondents indicated that their programs plan to continue to incorporate telemedicine into the rotation schedule. Based on 50 responses, the average of educational conferences that became virtual at the start of the COVID-19 pandemic was 87%; based on 46 responses, the percentage of educational conferences that will remain virtual moving forward is 46%, while 90% (46/51) of respondents indicated that their programs plan to use virtual conferences in some capacity moving forward. Seventy-three percent (37/51) of respondents indicated that they plan to use virtual interviews as part of residency recruitment moving forward.
Available Resources—Twenty-four percent (11/46) of respondents indicated that residents in their program do not get protected time or time off for CORE examinations. Seventy-five percent (33/44) of PDs said their program provides funding for residents to participate in board review courses. The chief residents at 63% (31/49) of programs receive additional compensation, and 69% (34/49) provide additional administrative time to chief residents. Seventy-one percent (24/34) of PDs reported their programs have scribes for attendings, and 12% (4/34) have scribes for residents. Support staff help residents with callbacks and in-basket messages according to 76% (35/46) of respondents. The majority (98% [45/46]) of PDs indicated that residents follow-up on results and messages from patients seen in resident clinics, and 43% (20/46) of programs have residents follow-up with patients seen in faculty clinics. Only 15% (7/46) of PDs responded they have schedules with residents dedicated to handle these tasks. According to respondents, 33% (17/52) have residents who are required to travel more than 25 miles to distant clinical sites. Of them, 35% (6/17) provide accommodations.
Quality Improvement—Seventy-one percent (35/49) of respondents indicated their department has a quality improvement/patient safety team or committee, and 94% (33/35) of these teams include residents. A lecture series on quality improvement and patient safety is offered at 67% (33/49) of the respondents’ programs, while morbidity and mortality conferences are offered in 73% (36/49).
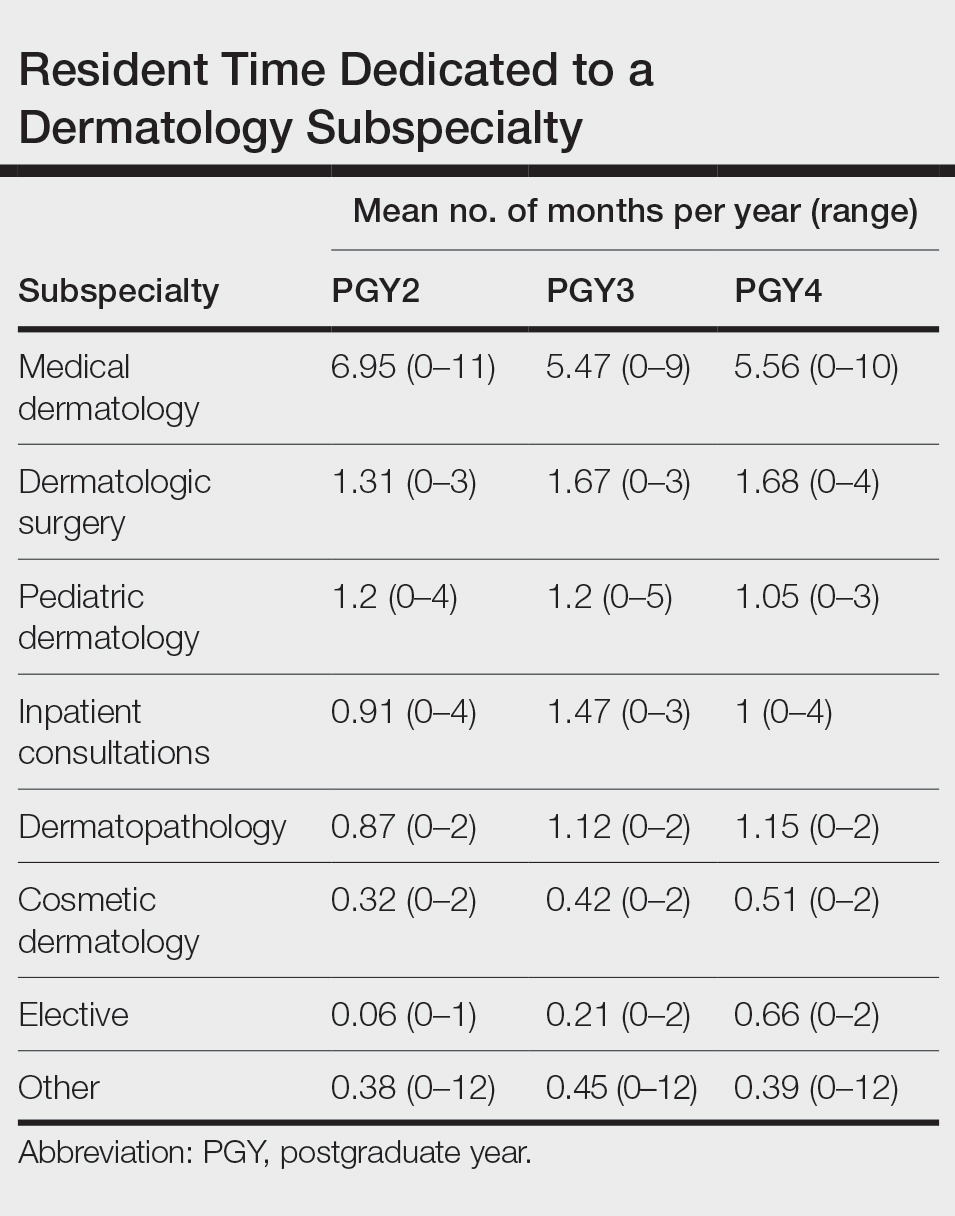
Clinical Instruction—Our survey asked PDs how many months each residency year spends on a certain rotational service. Based on 46 respondents, the average number of months dedicated to medical dermatology is 7, 5, and 6 months for postgraduate year (PGY) 2, PGY3, and PGY4, respectively. The average number of months spent in other subspecialties is provided in the Table. On average, PGY2 residents spend 8 half-days per week seeing patients in clinic, while PGY3 and PGY4 residents see patients for 7 half-days. The median and mean number of patients staffed by a single attending per hour in teaching clinics are 6 and 5.88, respectively. Respondents indicated that residents participate in the following specialty clinics: pediatric dermatology (96% [44/46]), laser/cosmetic (87% [40/44]), high-risk skin cancer (ie, immunosuppressed/transplant patient)(65% [30/44]), pigmented lesion/melanoma (52% [24/44]), connective tissue disease (52% [24/44]), teledermatology (50% [23/44]), free clinic for homeless and/or indigent populations (48% [22/44]), contact dermatitis (43% [20/44]), skin of color (43% [20/44]), oncodermatology (41% [19/44]), and bullous disease (33% [15/44]).
Additionally, in 87% (40/46) of programs, residents participate in a dedicated inpatient consultation service. Most respondents (98% [45/46]) responded that they utilize in-person consultations with a teledermatology supplement. Fifteen percent (7/46) utilize virtual teledermatology (live video-based consultations), and 57% (26/46) utilize asynchronous teledermatology (picture-based consultations). All respondents (n=46) indicated that 0% to 25% of patient encounters involving residents are teledermatology visits. Thirty-three percent (6/18) of programs have a global health special training track, 56% (10/18) have a Specialty Training and Advanced Research/Physician-Scientist Research Training track, 28% (5/18) have a diversity training track, and 50% (9/18) have a clinician educator training track.
Didactic Instruction—Five programs have a full day per week dedicated to didactics, while 36 programs have at least one half-day per week for didactics. On average, didactics in 57% (26/46) of programs are led by faculty alone, while 43% (20/46) are led at least in part by residents or fellows.
Research Content—Fifty percent (23/46) of programs have a specific research requirement for residents beyond general ACGME requirements, and 35% (16/46) require residents to participate in a longitudinal research project over the course of residency. There is a dedicated research coordinator for resident support at 63% (29/46) of programs. Dedicated biostatistics research support is available for resident projects at 42% (19/45) of programs. Additionally, at 42% (19/45) of programs, there is a dedicated faculty member for oversight of resident research.
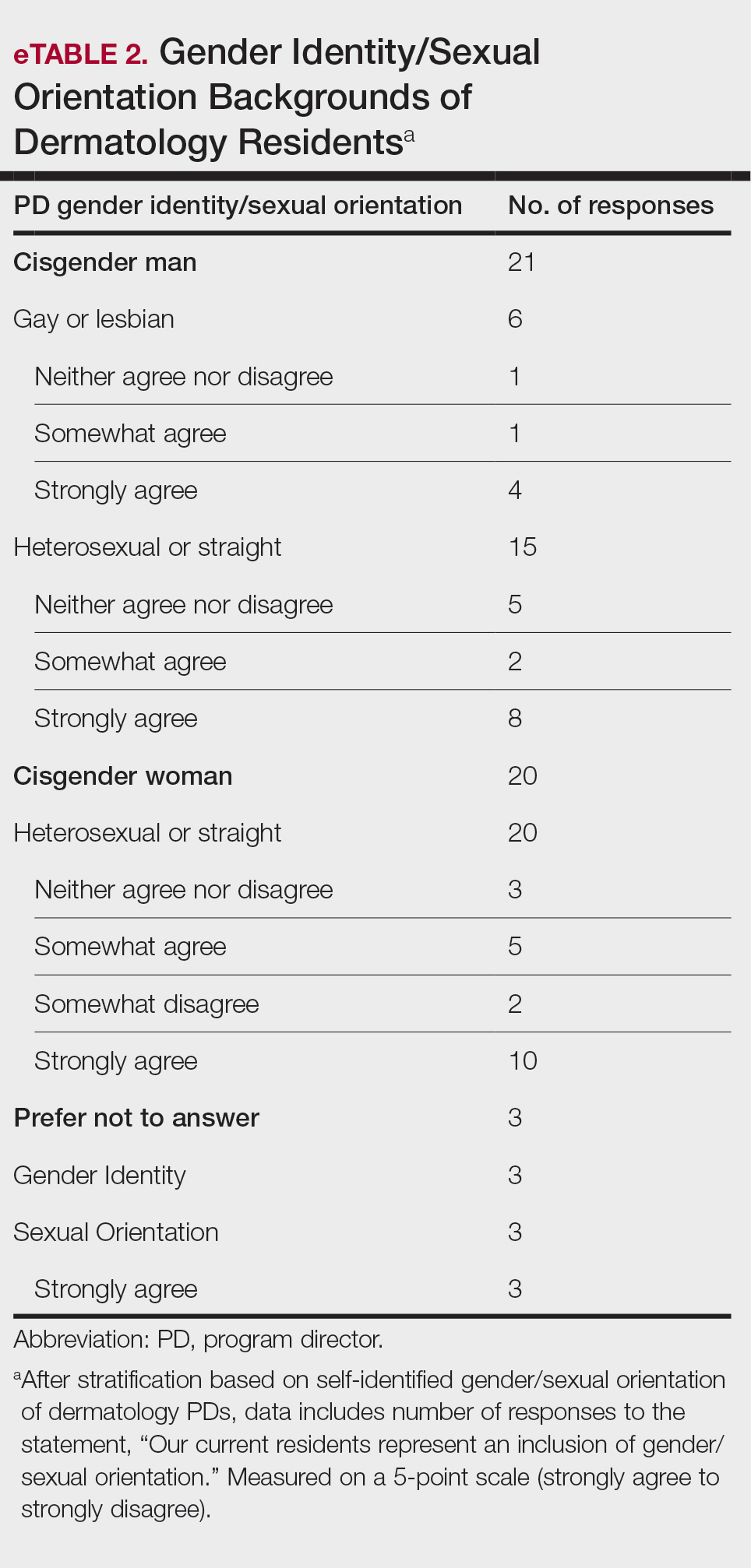
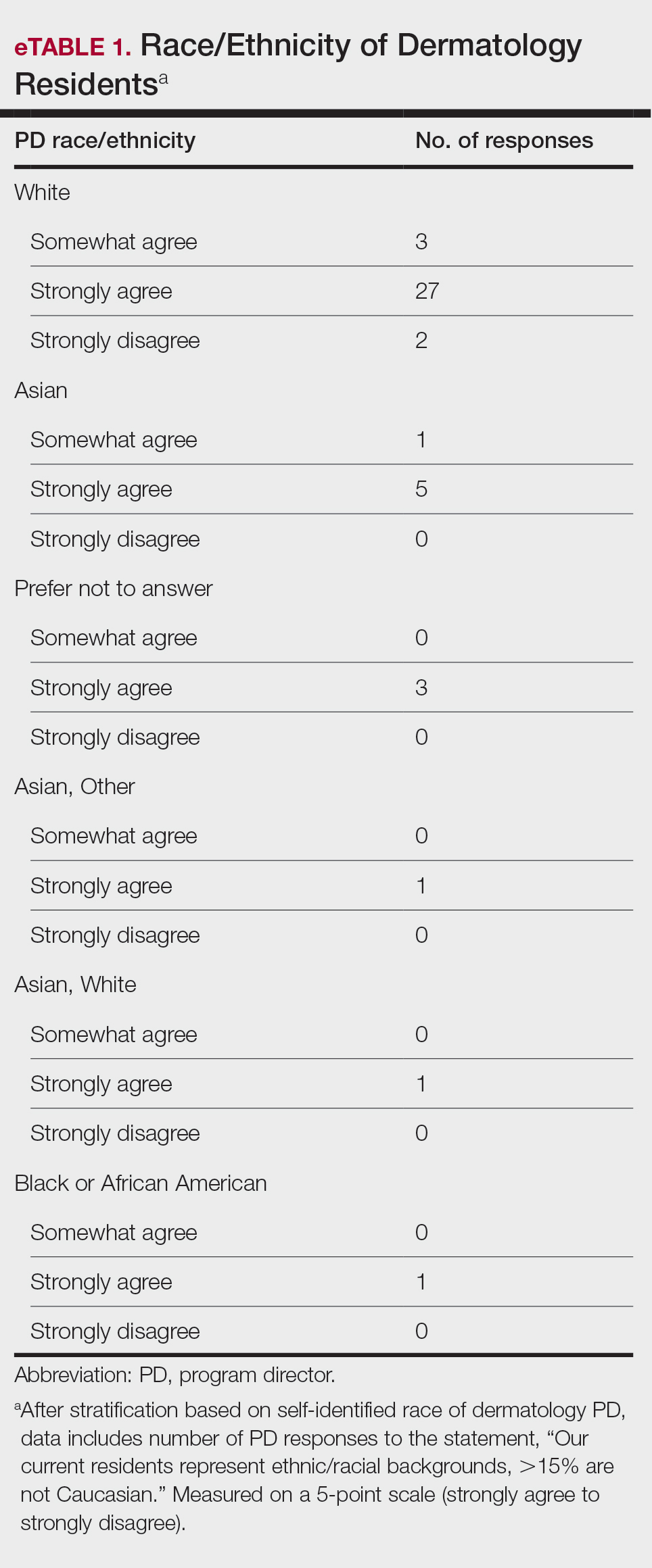
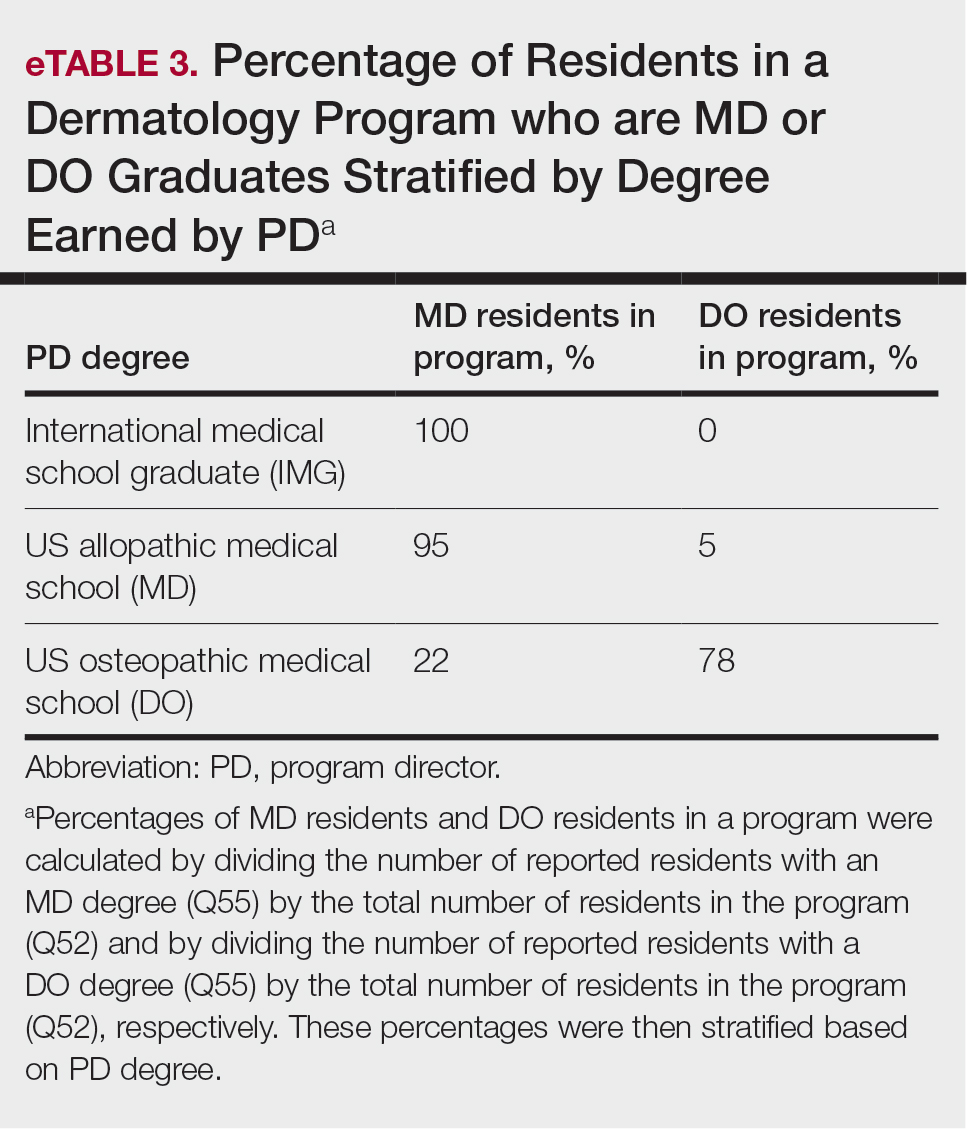
Diversity, Equity, and Inclusion—Seventy-three percent (29/40) of programs have special diversity, equity, and inclusion programs or meetings specific to residency, 60% (24/40) have residency initiatives, and 55% (22/40) have a residency diversity committee. Eighty-six percent (42/49) of respondents strongly agreed that their current residents represent diverse ethnic and racial backgrounds (ie, >15% are not White). eTable 1 shows PD responses to this statement, which were stratified based on self-identified race. eTable 2 shows PD responses to the statement, “Our current residents represent an inclusion of gender/sexual orientation,” which were stratified based on self-identified gender identity/sexual orientation. Lastly, eTable 3 highlights the percentage of residents with an MD and DO degree, stratified based on PD degree.
Wellness—Forty-eight percent (20/42) of respondents indicated they are under stress and do not always have as much energy as before becoming a PD but do not feel burned out. Thirty-one percent (13/42) indicated they have 1 or more symptoms of burnout, such as emotional exhaustion. Eighty-six percent (36/42) are satisfied with their jobs overall (43% agree and 43% strongly agree [18/42 each]).
Evaluation System—Seventy-five percent (33/44) of programs deliver evaluations of residents by faculty online, 86% (38/44) of programs have PDs discuss evaluations in-person, and 20% (9/44) of programs have faculty evaluators discuss evaluations in-person. Seventy-seven percent (34/44) of programs have formal faculty-resident mentor-mentee programs. Clinical competency committee chair positions are filled by PDs, assistant PDs, or core faculty members 47%, 38%, and 16% of the time, respectively.
Graduation Outcomes of PGY4 Residents—About 28% (55/199) of graduating residents applied to a fellowship position, with the majority (15% [29/55]) matching into Mohs micrographic surgery and dermatologic oncology (MSDO) fellowships. Approximately 5% (9/199) and 4% (7/199) of graduates matched into dermatopathology and pediatric dermatology, respectively. The remaining 5% (10/199) of graduating residents applied to a fellowship but did not match. The majority (45% [91/199]) of residency graduates entered private practice after graduation. Approximately 21% (42/199) of graduating residents chose an academic practice with 17% (33/199), 2% (4/199), and 2% (3/199) of those positions being full-time, part-time, and adjunct, respectively.
Comment
The first annual APD survey is a novel data source and provides opportunities for areas of discussion and investigation. Evaluating the similarities and differences among dermatology residency programs across the United States can strengthen individual programs through collaboration and provide areas of cohesion among programs.
Diversity of PDs—An important area of discussion is diversity and PD demographics. Although DO students make up 1 in 4 US graduating medical students, they are not interviewed or ranked as often as MD students.2 Diversity in PD race and ethnicity may be worthy of investigation in future studies, as match rates and recruitment of diverse medical school applicants may be impacted by these demographics.
Continued Use of Telemedicine in Training—Since 2020, the benefits of virtual residency recruitment have been debated among PDs across all medical specialties. Points in favor of virtual interviews include cost savings for programs and especially for applicants, as well as time efficiency, reduced burden of travel, and reduced carbon footprint. A problem posed by virtual interviews is that candidates are unable to fully learn institutional cultures and social environments of the programs.3 Likewise, telehealth was an important means of clinical teaching for residents during the height of the COVID-19 pandemic, with benefits that included cost-effectiveness and reduction of disparities in access to dermatologic care.4 Seventy-five percent (38/51) of PDs indicated that their program plans to include telemedicine in resident clinical rotation moving forward.
Resources Available—Our survey showed that resources available for residents, delivery of lectures and program time allocated to didactics, protected academic or study time for residents, and allocation of program time for CORE examinations are highly variable across programs. This could inspire future studies to be done to determine the differences in success of the resident on CORE examinations and in digesting material.
Postgraduate Career Plans and Fellowship Matches—Residents of programs that have a home MSDO fellowship are more likely to successfully match into a MSDO fellowship.5 Based on this survey, approximately 28% of graduating residents applied to a fellowship position, with 15%, 5%, and 3% matching into desired MSDO, dermatopathology, and pediatric dermatology fellowships, respectively. Additional studies are needed to determine advantages and disadvantages that lead to residents reaching their career goals.
Limitations—Limitations of this study include a small sample size that may not adequately represent all ACGME-accredited dermatology residency programs and selection bias toward respondents who are more likely to participate in survey-based research.
Conclusion
The APD plans to continue to administer this survey on an annual basis, with updates to the content and questions based on input from PDs. This survey will continue to provide valuable information to drive collaboration among residency programs and optimize the learning experience for residents. Our hope is that the response rate will increase in coming years, allowing us to draw more generalizable conclusions. Nonetheless, the survey data allow individual dermatology residency programs to compare their specific characteristics to other programs.
- Maciejko L, Cope A, Mara K, et al. A national survey of obstetrics and gynecology emergency training and deficits in office emergency preparation [A53]. Obstet Gynecol. 2022;139:16S. doi:10.1097/01.AOG.0000826548.05758.26
- Lavertue SM, Terry R. A comparison of surgical subspecialty match rates in 2022 in the United States. Cureus. 2023;15:E37178. doi:10.7759/cureus.37178
- Domingo A, Rdesinski RE, Stenson A, et al. Virtual residency interviews: applicant perceptions regarding virtual interview effectiveness, advantages, and barriers. J Grad Med Educ. 2022;14:224-228. doi:10.4300/JGME-D-21-00675.1
- Rustad AM, Lio PA. Pandemic pressure: teledermatology and health care disparities. J Patient Exp. 2021;8:2374373521996982. doi:10.1177/2374373521996982
- Rickstrew J, Rajpara A, Hocker TLH. Dermatology residency program influences chance of successful surgery fellowship match. Dermatol Surg. 2021;47:1040-1042. doi:10.1097/DSS.0000000000002859
Educational organizations across several specialties, including internal medicine and obstetrics and gynecology, have formal surveys1; however, the field of dermatology has been without one. This study aimed to establish a formal survey for dermatology program directors (PDs) and clinician-educators. Because the Accreditation Council for Graduate Medical Education (ACGME) and American Board of Dermatology surveys do not capture all metrics relevant to dermatology residency educators, an annual survey for our specialty may be helpful to compare dermatology-specific data among programs. Responses could provide context and perspective to faculty and residents who respond to the ACGME annual survey, as our Association of Professors of Dermatology (APD) survey asks more in-depth questions, such as how often didactics occur and who leads them. Resident commute time and faculty demographics and training also are covered. Current ad hoc surveys disseminated through listserves of various medical associations contain overlapping questions and reflect relatively low response rates; dermatology PDs may benefit from a survey with a high response rate to which they can contribute future questions and topics that reflect recent trends and current needs in graduate medical education. As future surveys are administered, the results can be captured in a centralized database accessible by dermatology PDs.
Methods
A survey of PDs from 141 ACGME-accredited dermatology residency programs was conducted by the Residency Program Director Steering Committee of the APD from November 2022 to January 2023 using a prevalidated questionnaire. Personalized survey links were created and sent individually to each PD’s email listed in the ACGME accreditation data system. All survey responses were captured anonymously, with a number assigned to keep de-identified responses separate and organized. The survey consisted of 137 survey questions addressing topics that included program characteristics, PD demographics, the impact of the COVID-19 pandemic on clinical rotation and educational conferences, available resident resources, quality improvement, clinical and didactic instruction, research content, diversity and inclusion, wellness, professionalism, evaluation systems, and graduate outcomes.
Data were collected using Qualtrics survey tools. After removing duplicate and incomplete surveys, data were analyzed using Qualtrics reports and Microsoft Excel for data plotting, averages, and range calculations.
Results
One hundred forty-one personalized survey links were created and sent individually to each program’s filed email obtained from the APD listserv. Fifty-three responses were recorded after removing duplicate or incomplete surveys (38% [53/141] response rate). As of May 2023, there were 144 ACGME-accredited dermatology residency programs due to 3 newly accredited programs in 2022-2023 academic year, which were not included in our survey population.
Program Characteristics—Forty-four respondents (83%) were from a university-based program. Fifty respondents (94%) were from programs that were ACGME accredited prior to 2020, while 3 programs (6%) were American Osteopathic Association accredited prior to singular accreditation. Seventy-one percent (38/53) of respondents had 1 or more associate PDs.
PD Demographics—Eighty-seven percent (45/52) of PDs who responded to the survey graduated from a US allopathic medical school (MD), 10% (5/52) graduated from a US osteopathic medical school (DO), and 4% (2/52) graduated from an international medical school. Seventy-four percent (35/47) of respondents were White, 17% (8/47) were Asian, and 2% (1/47) were Black or African American; this data was not provided for 4 respondents. Forty-eight percent (23/48) of PDs identified as cisgender man, 48% (23/48) identified as cisgender woman, and 4% (2/48) preferred not to answer. Eighty-one percent (38/47) of PDs identified as heterosexual or straight, 15% (7/47) identified as gay or lesbian, and 4% (2/47) preferred not to answer.
Impact of COVID-19 Pandemic on Residency Training—Due to the COVID-19 pandemic, 88% (45/51) of respondents incorporated telemedicine into the resident clinical rotation schedule. Moving forward, 75% (38/51) of respondents indicated that their programs plan to continue to incorporate telemedicine into the rotation schedule. Based on 50 responses, the average of educational conferences that became virtual at the start of the COVID-19 pandemic was 87%; based on 46 responses, the percentage of educational conferences that will remain virtual moving forward is 46%, while 90% (46/51) of respondents indicated that their programs plan to use virtual conferences in some capacity moving forward. Seventy-three percent (37/51) of respondents indicated that they plan to use virtual interviews as part of residency recruitment moving forward.
Available Resources—Twenty-four percent (11/46) of respondents indicated that residents in their program do not get protected time or time off for CORE examinations. Seventy-five percent (33/44) of PDs said their program provides funding for residents to participate in board review courses. The chief residents at 63% (31/49) of programs receive additional compensation, and 69% (34/49) provide additional administrative time to chief residents. Seventy-one percent (24/34) of PDs reported their programs have scribes for attendings, and 12% (4/34) have scribes for residents. Support staff help residents with callbacks and in-basket messages according to 76% (35/46) of respondents. The majority (98% [45/46]) of PDs indicated that residents follow-up on results and messages from patients seen in resident clinics, and 43% (20/46) of programs have residents follow-up with patients seen in faculty clinics. Only 15% (7/46) of PDs responded they have schedules with residents dedicated to handle these tasks. According to respondents, 33% (17/52) have residents who are required to travel more than 25 miles to distant clinical sites. Of them, 35% (6/17) provide accommodations.
Quality Improvement—Seventy-one percent (35/49) of respondents indicated their department has a quality improvement/patient safety team or committee, and 94% (33/35) of these teams include residents. A lecture series on quality improvement and patient safety is offered at 67% (33/49) of the respondents’ programs, while morbidity and mortality conferences are offered in 73% (36/49).
Clinical Instruction—Our survey asked PDs how many months each residency year spends on a certain rotational service. Based on 46 respondents, the average number of months dedicated to medical dermatology is 7, 5, and 6 months for postgraduate year (PGY) 2, PGY3, and PGY4, respectively. The average number of months spent in other subspecialties is provided in the Table. On average, PGY2 residents spend 8 half-days per week seeing patients in clinic, while PGY3 and PGY4 residents see patients for 7 half-days. The median and mean number of patients staffed by a single attending per hour in teaching clinics are 6 and 5.88, respectively. Respondents indicated that residents participate in the following specialty clinics: pediatric dermatology (96% [44/46]), laser/cosmetic (87% [40/44]), high-risk skin cancer (ie, immunosuppressed/transplant patient)(65% [30/44]), pigmented lesion/melanoma (52% [24/44]), connective tissue disease (52% [24/44]), teledermatology (50% [23/44]), free clinic for homeless and/or indigent populations (48% [22/44]), contact dermatitis (43% [20/44]), skin of color (43% [20/44]), oncodermatology (41% [19/44]), and bullous disease (33% [15/44]).

Additionally, in 87% (40/46) of programs, residents participate in a dedicated inpatient consultation service. Most respondents (98% [45/46]) responded that they utilize in-person consultations with a teledermatology supplement. Fifteen percent (7/46) utilize virtual teledermatology (live video-based consultations), and 57% (26/46) utilize asynchronous teledermatology (picture-based consultations). All respondents (n=46) indicated that 0% to 25% of patient encounters involving residents are teledermatology visits. Thirty-three percent (6/18) of programs have a global health special training track, 56% (10/18) have a Specialty Training and Advanced Research/Physician-Scientist Research Training track, 28% (5/18) have a diversity training track, and 50% (9/18) have a clinician educator training track.
Didactic Instruction—Five programs have a full day per week dedicated to didactics, while 36 programs have at least one half-day per week for didactics. On average, didactics in 57% (26/46) of programs are led by faculty alone, while 43% (20/46) are led at least in part by residents or fellows.

Research Content—Fifty percent (23/46) of programs have a specific research requirement for residents beyond general ACGME requirements, and 35% (16/46) require residents to participate in a longitudinal research project over the course of residency. There is a dedicated research coordinator for resident support at 63% (29/46) of programs. Dedicated biostatistics research support is available for resident projects at 42% (19/45) of programs. Additionally, at 42% (19/45) of programs, there is a dedicated faculty member for oversight of resident research.

Diversity, Equity, and Inclusion—Seventy-three percent (29/40) of programs have special diversity, equity, and inclusion programs or meetings specific to residency, 60% (24/40) have residency initiatives, and 55% (22/40) have a residency diversity committee. Eighty-six percent (42/49) of respondents strongly agreed that their current residents represent diverse ethnic and racial backgrounds (ie, >15% are not White). eTable 1 shows PD responses to this statement, which were stratified based on self-identified race. eTable 2 shows PD responses to the statement, “Our current residents represent an inclusion of gender/sexual orientation,” which were stratified based on self-identified gender identity/sexual orientation. Lastly, eTable 3 highlights the percentage of residents with an MD and DO degree, stratified based on PD degree.

Wellness—Forty-eight percent (20/42) of respondents indicated they are under stress and do not always have as much energy as before becoming a PD but do not feel burned out. Thirty-one percent (13/42) indicated they have 1 or more symptoms of burnout, such as emotional exhaustion. Eighty-six percent (36/42) are satisfied with their jobs overall (43% agree and 43% strongly agree [18/42 each]).
Evaluation System—Seventy-five percent (33/44) of programs deliver evaluations of residents by faculty online, 86% (38/44) of programs have PDs discuss evaluations in-person, and 20% (9/44) of programs have faculty evaluators discuss evaluations in-person. Seventy-seven percent (34/44) of programs have formal faculty-resident mentor-mentee programs. Clinical competency committee chair positions are filled by PDs, assistant PDs, or core faculty members 47%, 38%, and 16% of the time, respectively.
Graduation Outcomes of PGY4 Residents—About 28% (55/199) of graduating residents applied to a fellowship position, with the majority (15% [29/55]) matching into Mohs micrographic surgery and dermatologic oncology (MSDO) fellowships. Approximately 5% (9/199) and 4% (7/199) of graduates matched into dermatopathology and pediatric dermatology, respectively. The remaining 5% (10/199) of graduating residents applied to a fellowship but did not match. The majority (45% [91/199]) of residency graduates entered private practice after graduation. Approximately 21% (42/199) of graduating residents chose an academic practice with 17% (33/199), 2% (4/199), and 2% (3/199) of those positions being full-time, part-time, and adjunct, respectively.
Comment
The first annual APD survey is a novel data source and provides opportunities for areas of discussion and investigation. Evaluating the similarities and differences among dermatology residency programs across the United States can strengthen individual programs through collaboration and provide areas of cohesion among programs.
Diversity of PDs—An important area of discussion is diversity and PD demographics. Although DO students make up 1 in 4 US graduating medical students, they are not interviewed or ranked as often as MD students.2 Diversity in PD race and ethnicity may be worthy of investigation in future studies, as match rates and recruitment of diverse medical school applicants may be impacted by these demographics.
Continued Use of Telemedicine in Training—Since 2020, the benefits of virtual residency recruitment have been debated among PDs across all medical specialties. Points in favor of virtual interviews include cost savings for programs and especially for applicants, as well as time efficiency, reduced burden of travel, and reduced carbon footprint. A problem posed by virtual interviews is that candidates are unable to fully learn institutional cultures and social environments of the programs.3 Likewise, telehealth was an important means of clinical teaching for residents during the height of the COVID-19 pandemic, with benefits that included cost-effectiveness and reduction of disparities in access to dermatologic care.4 Seventy-five percent (38/51) of PDs indicated that their program plans to include telemedicine in resident clinical rotation moving forward.
Resources Available—Our survey showed that resources available for residents, delivery of lectures and program time allocated to didactics, protected academic or study time for residents, and allocation of program time for CORE examinations are highly variable across programs. This could inspire future studies to be done to determine the differences in success of the resident on CORE examinations and in digesting material.
Postgraduate Career Plans and Fellowship Matches—Residents of programs that have a home MSDO fellowship are more likely to successfully match into a MSDO fellowship.5 Based on this survey, approximately 28% of graduating residents applied to a fellowship position, with 15%, 5%, and 3% matching into desired MSDO, dermatopathology, and pediatric dermatology fellowships, respectively. Additional studies are needed to determine advantages and disadvantages that lead to residents reaching their career goals.
Limitations—Limitations of this study include a small sample size that may not adequately represent all ACGME-accredited dermatology residency programs and selection bias toward respondents who are more likely to participate in survey-based research.
Conclusion
The APD plans to continue to administer this survey on an annual basis, with updates to the content and questions based on input from PDs. This survey will continue to provide valuable information to drive collaboration among residency programs and optimize the learning experience for residents. Our hope is that the response rate will increase in coming years, allowing us to draw more generalizable conclusions. Nonetheless, the survey data allow individual dermatology residency programs to compare their specific characteristics to other programs.
Educational organizations across several specialties, including internal medicine and obstetrics and gynecology, have formal surveys1; however, the field of dermatology has been without one. This study aimed to establish a formal survey for dermatology program directors (PDs) and clinician-educators. Because the Accreditation Council for Graduate Medical Education (ACGME) and American Board of Dermatology surveys do not capture all metrics relevant to dermatology residency educators, an annual survey for our specialty may be helpful to compare dermatology-specific data among programs. Responses could provide context and perspective to faculty and residents who respond to the ACGME annual survey, as our Association of Professors of Dermatology (APD) survey asks more in-depth questions, such as how often didactics occur and who leads them. Resident commute time and faculty demographics and training also are covered. Current ad hoc surveys disseminated through listserves of various medical associations contain overlapping questions and reflect relatively low response rates; dermatology PDs may benefit from a survey with a high response rate to which they can contribute future questions and topics that reflect recent trends and current needs in graduate medical education. As future surveys are administered, the results can be captured in a centralized database accessible by dermatology PDs.
Methods
A survey of PDs from 141 ACGME-accredited dermatology residency programs was conducted by the Residency Program Director Steering Committee of the APD from November 2022 to January 2023 using a prevalidated questionnaire. Personalized survey links were created and sent individually to each PD’s email listed in the ACGME accreditation data system. All survey responses were captured anonymously, with a number assigned to keep de-identified responses separate and organized. The survey consisted of 137 survey questions addressing topics that included program characteristics, PD demographics, the impact of the COVID-19 pandemic on clinical rotation and educational conferences, available resident resources, quality improvement, clinical and didactic instruction, research content, diversity and inclusion, wellness, professionalism, evaluation systems, and graduate outcomes.
Data were collected using Qualtrics survey tools. After removing duplicate and incomplete surveys, data were analyzed using Qualtrics reports and Microsoft Excel for data plotting, averages, and range calculations.
Results
One hundred forty-one personalized survey links were created and sent individually to each program’s filed email obtained from the APD listserv. Fifty-three responses were recorded after removing duplicate or incomplete surveys (38% [53/141] response rate). As of May 2023, there were 144 ACGME-accredited dermatology residency programs due to 3 newly accredited programs in 2022-2023 academic year, which were not included in our survey population.
Program Characteristics—Forty-four respondents (83%) were from a university-based program. Fifty respondents (94%) were from programs that were ACGME accredited prior to 2020, while 3 programs (6%) were American Osteopathic Association accredited prior to singular accreditation. Seventy-one percent (38/53) of respondents had 1 or more associate PDs.
PD Demographics—Eighty-seven percent (45/52) of PDs who responded to the survey graduated from a US allopathic medical school (MD), 10% (5/52) graduated from a US osteopathic medical school (DO), and 4% (2/52) graduated from an international medical school. Seventy-four percent (35/47) of respondents were White, 17% (8/47) were Asian, and 2% (1/47) were Black or African American; this data was not provided for 4 respondents. Forty-eight percent (23/48) of PDs identified as cisgender man, 48% (23/48) identified as cisgender woman, and 4% (2/48) preferred not to answer. Eighty-one percent (38/47) of PDs identified as heterosexual or straight, 15% (7/47) identified as gay or lesbian, and 4% (2/47) preferred not to answer.
Impact of COVID-19 Pandemic on Residency Training—Due to the COVID-19 pandemic, 88% (45/51) of respondents incorporated telemedicine into the resident clinical rotation schedule. Moving forward, 75% (38/51) of respondents indicated that their programs plan to continue to incorporate telemedicine into the rotation schedule. Based on 50 responses, the average of educational conferences that became virtual at the start of the COVID-19 pandemic was 87%; based on 46 responses, the percentage of educational conferences that will remain virtual moving forward is 46%, while 90% (46/51) of respondents indicated that their programs plan to use virtual conferences in some capacity moving forward. Seventy-three percent (37/51) of respondents indicated that they plan to use virtual interviews as part of residency recruitment moving forward.
Available Resources—Twenty-four percent (11/46) of respondents indicated that residents in their program do not get protected time or time off for CORE examinations. Seventy-five percent (33/44) of PDs said their program provides funding for residents to participate in board review courses. The chief residents at 63% (31/49) of programs receive additional compensation, and 69% (34/49) provide additional administrative time to chief residents. Seventy-one percent (24/34) of PDs reported their programs have scribes for attendings, and 12% (4/34) have scribes for residents. Support staff help residents with callbacks and in-basket messages according to 76% (35/46) of respondents. The majority (98% [45/46]) of PDs indicated that residents follow-up on results and messages from patients seen in resident clinics, and 43% (20/46) of programs have residents follow-up with patients seen in faculty clinics. Only 15% (7/46) of PDs responded they have schedules with residents dedicated to handle these tasks. According to respondents, 33% (17/52) have residents who are required to travel more than 25 miles to distant clinical sites. Of them, 35% (6/17) provide accommodations.
Quality Improvement—Seventy-one percent (35/49) of respondents indicated their department has a quality improvement/patient safety team or committee, and 94% (33/35) of these teams include residents. A lecture series on quality improvement and patient safety is offered at 67% (33/49) of the respondents’ programs, while morbidity and mortality conferences are offered in 73% (36/49).
Clinical Instruction—Our survey asked PDs how many months each residency year spends on a certain rotational service. Based on 46 respondents, the average number of months dedicated to medical dermatology is 7, 5, and 6 months for postgraduate year (PGY) 2, PGY3, and PGY4, respectively. The average number of months spent in other subspecialties is provided in the Table. On average, PGY2 residents spend 8 half-days per week seeing patients in clinic, while PGY3 and PGY4 residents see patients for 7 half-days. The median and mean number of patients staffed by a single attending per hour in teaching clinics are 6 and 5.88, respectively. Respondents indicated that residents participate in the following specialty clinics: pediatric dermatology (96% [44/46]), laser/cosmetic (87% [40/44]), high-risk skin cancer (ie, immunosuppressed/transplant patient)(65% [30/44]), pigmented lesion/melanoma (52% [24/44]), connective tissue disease (52% [24/44]), teledermatology (50% [23/44]), free clinic for homeless and/or indigent populations (48% [22/44]), contact dermatitis (43% [20/44]), skin of color (43% [20/44]), oncodermatology (41% [19/44]), and bullous disease (33% [15/44]).

Additionally, in 87% (40/46) of programs, residents participate in a dedicated inpatient consultation service. Most respondents (98% [45/46]) responded that they utilize in-person consultations with a teledermatology supplement. Fifteen percent (7/46) utilize virtual teledermatology (live video-based consultations), and 57% (26/46) utilize asynchronous teledermatology (picture-based consultations). All respondents (n=46) indicated that 0% to 25% of patient encounters involving residents are teledermatology visits. Thirty-three percent (6/18) of programs have a global health special training track, 56% (10/18) have a Specialty Training and Advanced Research/Physician-Scientist Research Training track, 28% (5/18) have a diversity training track, and 50% (9/18) have a clinician educator training track.
Didactic Instruction—Five programs have a full day per week dedicated to didactics, while 36 programs have at least one half-day per week for didactics. On average, didactics in 57% (26/46) of programs are led by faculty alone, while 43% (20/46) are led at least in part by residents or fellows.

Research Content—Fifty percent (23/46) of programs have a specific research requirement for residents beyond general ACGME requirements, and 35% (16/46) require residents to participate in a longitudinal research project over the course of residency. There is a dedicated research coordinator for resident support at 63% (29/46) of programs. Dedicated biostatistics research support is available for resident projects at 42% (19/45) of programs. Additionally, at 42% (19/45) of programs, there is a dedicated faculty member for oversight of resident research.

Diversity, Equity, and Inclusion—Seventy-three percent (29/40) of programs have special diversity, equity, and inclusion programs or meetings specific to residency, 60% (24/40) have residency initiatives, and 55% (22/40) have a residency diversity committee. Eighty-six percent (42/49) of respondents strongly agreed that their current residents represent diverse ethnic and racial backgrounds (ie, >15% are not White). eTable 1 shows PD responses to this statement, which were stratified based on self-identified race. eTable 2 shows PD responses to the statement, “Our current residents represent an inclusion of gender/sexual orientation,” which were stratified based on self-identified gender identity/sexual orientation. Lastly, eTable 3 highlights the percentage of residents with an MD and DO degree, stratified based on PD degree.

Wellness—Forty-eight percent (20/42) of respondents indicated they are under stress and do not always have as much energy as before becoming a PD but do not feel burned out. Thirty-one percent (13/42) indicated they have 1 or more symptoms of burnout, such as emotional exhaustion. Eighty-six percent (36/42) are satisfied with their jobs overall (43% agree and 43% strongly agree [18/42 each]).
Evaluation System—Seventy-five percent (33/44) of programs deliver evaluations of residents by faculty online, 86% (38/44) of programs have PDs discuss evaluations in-person, and 20% (9/44) of programs have faculty evaluators discuss evaluations in-person. Seventy-seven percent (34/44) of programs have formal faculty-resident mentor-mentee programs. Clinical competency committee chair positions are filled by PDs, assistant PDs, or core faculty members 47%, 38%, and 16% of the time, respectively.
Graduation Outcomes of PGY4 Residents—About 28% (55/199) of graduating residents applied to a fellowship position, with the majority (15% [29/55]) matching into Mohs micrographic surgery and dermatologic oncology (MSDO) fellowships. Approximately 5% (9/199) and 4% (7/199) of graduates matched into dermatopathology and pediatric dermatology, respectively. The remaining 5% (10/199) of graduating residents applied to a fellowship but did not match. The majority (45% [91/199]) of residency graduates entered private practice after graduation. Approximately 21% (42/199) of graduating residents chose an academic practice with 17% (33/199), 2% (4/199), and 2% (3/199) of those positions being full-time, part-time, and adjunct, respectively.
Comment
The first annual APD survey is a novel data source and provides opportunities for areas of discussion and investigation. Evaluating the similarities and differences among dermatology residency programs across the United States can strengthen individual programs through collaboration and provide areas of cohesion among programs.
Diversity of PDs—An important area of discussion is diversity and PD demographics. Although DO students make up 1 in 4 US graduating medical students, they are not interviewed or ranked as often as MD students.2 Diversity in PD race and ethnicity may be worthy of investigation in future studies, as match rates and recruitment of diverse medical school applicants may be impacted by these demographics.
Continued Use of Telemedicine in Training—Since 2020, the benefits of virtual residency recruitment have been debated among PDs across all medical specialties. Points in favor of virtual interviews include cost savings for programs and especially for applicants, as well as time efficiency, reduced burden of travel, and reduced carbon footprint. A problem posed by virtual interviews is that candidates are unable to fully learn institutional cultures and social environments of the programs.3 Likewise, telehealth was an important means of clinical teaching for residents during the height of the COVID-19 pandemic, with benefits that included cost-effectiveness and reduction of disparities in access to dermatologic care.4 Seventy-five percent (38/51) of PDs indicated that their program plans to include telemedicine in resident clinical rotation moving forward.
Resources Available—Our survey showed that resources available for residents, delivery of lectures and program time allocated to didactics, protected academic or study time for residents, and allocation of program time for CORE examinations are highly variable across programs. This could inspire future studies to be done to determine the differences in success of the resident on CORE examinations and in digesting material.
Postgraduate Career Plans and Fellowship Matches—Residents of programs that have a home MSDO fellowship are more likely to successfully match into a MSDO fellowship.5 Based on this survey, approximately 28% of graduating residents applied to a fellowship position, with 15%, 5%, and 3% matching into desired MSDO, dermatopathology, and pediatric dermatology fellowships, respectively. Additional studies are needed to determine advantages and disadvantages that lead to residents reaching their career goals.
Limitations—Limitations of this study include a small sample size that may not adequately represent all ACGME-accredited dermatology residency programs and selection bias toward respondents who are more likely to participate in survey-based research.
Conclusion
The APD plans to continue to administer this survey on an annual basis, with updates to the content and questions based on input from PDs. This survey will continue to provide valuable information to drive collaboration among residency programs and optimize the learning experience for residents. Our hope is that the response rate will increase in coming years, allowing us to draw more generalizable conclusions. Nonetheless, the survey data allow individual dermatology residency programs to compare their specific characteristics to other programs.
- Maciejko L, Cope A, Mara K, et al. A national survey of obstetrics and gynecology emergency training and deficits in office emergency preparation [A53]. Obstet Gynecol. 2022;139:16S. doi:10.1097/01.AOG.0000826548.05758.26
- Lavertue SM, Terry R. A comparison of surgical subspecialty match rates in 2022 in the United States. Cureus. 2023;15:E37178. doi:10.7759/cureus.37178
- Domingo A, Rdesinski RE, Stenson A, et al. Virtual residency interviews: applicant perceptions regarding virtual interview effectiveness, advantages, and barriers. J Grad Med Educ. 2022;14:224-228. doi:10.4300/JGME-D-21-00675.1
- Rustad AM, Lio PA. Pandemic pressure: teledermatology and health care disparities. J Patient Exp. 2021;8:2374373521996982. doi:10.1177/2374373521996982
- Rickstrew J, Rajpara A, Hocker TLH. Dermatology residency program influences chance of successful surgery fellowship match. Dermatol Surg. 2021;47:1040-1042. doi:10.1097/DSS.0000000000002859
- Maciejko L, Cope A, Mara K, et al. A national survey of obstetrics and gynecology emergency training and deficits in office emergency preparation [A53]. Obstet Gynecol. 2022;139:16S. doi:10.1097/01.AOG.0000826548.05758.26
- Lavertue SM, Terry R. A comparison of surgical subspecialty match rates in 2022 in the United States. Cureus. 2023;15:E37178. doi:10.7759/cureus.37178
- Domingo A, Rdesinski RE, Stenson A, et al. Virtual residency interviews: applicant perceptions regarding virtual interview effectiveness, advantages, and barriers. J Grad Med Educ. 2022;14:224-228. doi:10.4300/JGME-D-21-00675.1
- Rustad AM, Lio PA. Pandemic pressure: teledermatology and health care disparities. J Patient Exp. 2021;8:2374373521996982. doi:10.1177/2374373521996982
- Rickstrew J, Rajpara A, Hocker TLH. Dermatology residency program influences chance of successful surgery fellowship match. Dermatol Surg. 2021;47:1040-1042. doi:10.1097/DSS.0000000000002859
Practice Points
- The first annual Association of Professors of Dermatology program directors survey allows faculty to compare their programs to other dermatology residency programs across the United States.
- The results should inspire opportunities for growth, improvement, and collaboration among dermatology residency programs.
Effect of COVID-19 Vaccination on Disease Severity in Patients With Stable Plaque Psoriasis: A Cross-sectional Study
To the Editor:
COVID-19 infection has resulted in 6.9 million deaths worldwide. India has the third highest mortality from COVID-19 infection after the United States and Brazil.1 Vaccination plays a crucial role in containing COVID-19 infection and reducing its severity. At present, 11 vaccines have been approved by the World Health Organization. India started its vaccination program on January 16, 2021, with approval for use of Covaxin (Bharat Biotech) and Covishield (Oxford/AstraZeneca formulation)(Serum Institute of India). More than 2 billion doses have been administered since then.2,3
Patients with psoriasis are prone to develop a severe form of COVID-19 due to comorbidities and the intake of immunosuppressive drugs.4 These patients often are hesitant to receive the vaccine without an expert opinion. COVID-19 vaccines are considered to increase tumor necrosis factor α (TNF-α) and IFN-γ production by CD4+ T cells. Tumor necrosis factor α is a key proinflammatory cytokine implicated in the pathogenesis of psoriasis. COVID-19 messenger RNA vaccines induce elevation of IL-6 and helper T cells (TH17), which can induce a flare of psoriasis in a subset of patients.5The International Psoriasis Council recommends that patients with psoriasis receive one of the vaccines approved to prevent COVID-19 infection as soon as possible.6 Reports of new-onset psoriasis and flare of psoriasis after the use of COVID-19 vaccines, such as those manufactured by Pfizer-BioNTech, Moderna, and AstraZeneca, have been published from different parts of the world.7 India used locally developed whole virion inactivated BBV152 (Covaxin) and nonreplicating viral vaccine ChAdOx1 nCoV-19 (Covishield) in its vaccination program and exported them to other developing countries. There is a dearth of data on the safety of these vaccines in patients with psoriasis, which needs to be assessed. Later, Covaxin, ZyCoV-D (DNA plasmid vaccine; Cadila Healthcare), and CorbeVax (protein subunit vaccine; Biological E) were approved for usage in children.8 We conducted a cross-sectional study using the direct interview method.
Patients with psoriasis who attended the outpatient department of the Postgraduate Institute of Medical Education and Research (Chandigarh, India) from April 2022 to June 2022 were invited to participate in the study after written informed consent was received. Patients 18 years and older with chronic plaque psoriasis who had received a COVID-19 vaccine dose in the last 90 days were enrolled. Data on demographics, comorbidities, treatment received for psoriasis, vaccination concerns, history of COVID-19 infection, type of vaccine received with doses, adverse effects, and psoriasis flare after receiving the vaccine (considered up to 2 weeks from the date of vaccination) were collected. Ordinal logistic regression was used to identify factors associated with a psoriasis flare following vaccination. P<.05 was considered statistically significant.
A total of 202 patients with chronic plaque psoriasis who received either Covaxin or Covishield were enrolled during the study period. The mean age (SD) was 40.3 (13.1) years, and 149 (73.8%) patients were male. One hundred thirty-five (66.8%) patients completed 2 doses of the vaccine. eTable 1 provides the clinicodemographic details of the patients. Eighty-three (41.1%) patients had a fear of psoriasis flare after vaccination. Seventy-two (35.6%) patients received the vaccine after clearance from their treating physician/dermatologist. One hundred sixty-four (81.2%) patients received the Covishield vaccine, and 38 (18.8%) patients received Covaxin. Eighty-three (41.1%) patients reported flulike symptoms, such as fever, myalgia, or body pain, within the first week of vaccination. Sixty-one (30.2%) patients reported a psoriasis flare after vaccination in the form of new lesions or worsening of pre-existing lesions. Of these patients, 51 reported a flare after receiving the first dose of vaccine, 8 patients reported a flare after receiving the second dose of vaccine, and 2 patients reported a flare after receiving both doses of vaccine. The mean (SD) flare onset was 8.1 (3.4) days after the vaccination. Eighteen patients considered the flare to be severe. Seventeen (8.4%) patients reported a positive history of COVID-19 infection before vaccination. None of the patients reported breakthrough COVID-19 infection or pustular aggravation of psoriasis following the vaccination.
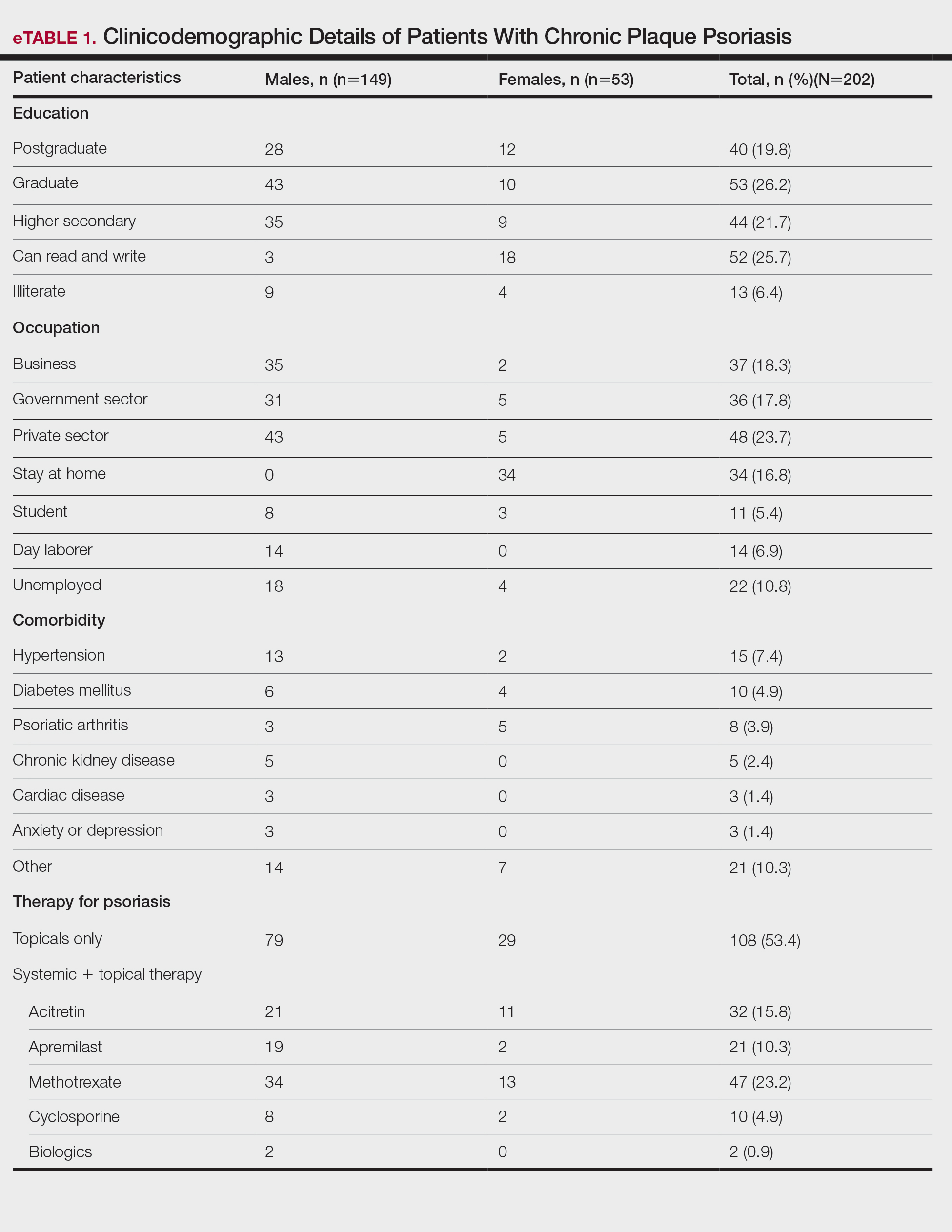
The self-reported psoriasis flare after receiving the COVID-19 vaccine was significantly higher in patients who experienced immediate adverse effects (P=.005), which included fever, myalgia, joint pain, and injection-site reaction. The reported postvaccination psoriasis flare was not significantly associated with patient sex, history of COVID-19 infection, type of vaccine received, comorbidities, or therapy for psoriasis (eTable 2).

Nearly 30% of our patients reported a postvaccination psoriasis flare, which was more common after the first vaccine dose. Sotiriou et al7 reported 14 cases of psoriasis flare in patients after receiving Pfizer-BioNTech, Moderna, and AstraZeneca COVID-19 vaccines. These patients experienced an exacerbation of disease soon after the second dose of vaccine (mean [SD], 10.36 [7.71] days), and 21% of the 713 enrolled patients wanted to forego the immunization due to concern of a postvaccination psoriasis flare.7 In another report, 14 (27%) patients developed a psoriasis flare after COVID-19 vaccination; the mean (SD) flare onset was 9.3 (4.3) days after vaccination.9
Data on the safety of the COVID-19 vaccine in patients using immunosuppressive drugs are limited. We did not find a significant association between the psoriasis flare and use of immunosuppressive drugs or type of vaccine received. Huang and Tsai9 observed similar results, with no association between psoriasis flare and use of immunosuppressive drugs or biologics, while Damiani et al10 demonstrated a protective role of biologics in preventing vaccine-induced psoriasis flare.
Similar to another study from India,11 the immediate adverse effects due to immunization with Covaxin and Covishield were mild in our study and resolved within a week. The incidence of psoriasis flare was significantly higher in patients who reported adverse effects (P=.005). Activation of immune response after vaccination leads to the release of proinflammatory and pyrogenic cytokines (ie, IL-1, IL-6, TNF-α), which may explain the higher incidence of psoriasis flare in patients experiencing adverse effects to vaccination.12
Our study showed approximately 30% of patients developed a psoriasis flare after COVID-19 vaccination, with no patients experiencing any vaccine-related serious adverse events, which suggests that Covaxin and Covishield are safe for patients with psoriasis in India. Limitations of our study include potential inaccuracy of the patient’s self-assessment of symptoms and disease flare, recall bias that may lead to errors in estimating patient-reported outcomes, the flare of psoriasis potentially being a part of disease fluctuation, and flare being enhanced by the psychological stress of vaccination.
Considering a high risk for severe COVID-19 infection in patients with psoriasis with comorbidities and those using immunosuppressive drugs, Covaxin and Covishield can be safely recommended in India. However, caution needs to be exercised when vaccinating patients with an unstable disease or severe psoriasis.
- COVID-19 coronavirus pandemic: weekly trends. Worldometer. Accessed August 21, 2023. https://www.worldometers.info/coronavirus/
- National COVID-19 vaccination programme meets its goals by overcoming R&D and logistical challenges, says economic survey 2022-23. Government of India Press Information Bureau website. Published January 31, 2023. Accessed August 24, 2023. https://pib.gov.in/PressReleasePage.aspx?PRID=1894907
- Ministry of Health and Family Welfare. CoWIN. Accessed August 21, 2023. https://www.cowin.gov.in/
- Griffiths CEM, Armstrong AW, Gudjonsson JE, et al. Psoriasis. Lancet. 2021;397:1301-1315.
- Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: anemerging target of JAK2 inhibitor fedratinib. J Microbiol Immunol Infect. 2020;53:368-370.
- International Psoriasis Council. Revised IPC statement on COVID-19. Published December 19, 2022. Accessed August 24, 2023. https://psoriasiscouncil.org/covid-19/revised-statement-covid-19/
- Sotiriou E, Tsentemeidou A, Bakirtzi K, et al. Psoriasis exacerbation after COVID-19 vaccination: a report of 14 cases from a single centre. J Eur Acad Dermatol Venereol. 2021;35:E857-E859.
- Kaul R. India clears 2 vaccines for kids under 12 years. Hindustan Times. Published April 27, 2022. Accessed August 24, 2023. https://www.hindustantimes.com/india-news/india-clears-2-vaccines-for-kids-under-12-years-101650998027336.html
- Huang YW, Tsai TF. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med (Lausanne). 2021;8:812010.
- Damiani G, Allocco F, Young Dermatologists Italian Network, et al. COVID-19 vaccination and patients with psoriasis under biologics: real-life evidence on safety and effectiveness from Italian vaccinated healthcare workers. Clin Exp Dermatol. 2021;460:1106-1108.
- Joshi RK, Muralidharan CG, Gulati DS, et al. Higher incidence of reported adverse events following immunisation (AEFI) after first dose of COVID-19 vaccine among previously infected health care workers. Med J Armed Forces India. 2021;77(suppl 2):S505-S507.
- Hervé C, Laupèze B, Del Giudice G, et al. The how’s and what’s of vaccine reactogenicity. NPJ Vaccines. 2019;4:39.
To the Editor:
COVID-19 infection has resulted in 6.9 million deaths worldwide. India has the third highest mortality from COVID-19 infection after the United States and Brazil.1 Vaccination plays a crucial role in containing COVID-19 infection and reducing its severity. At present, 11 vaccines have been approved by the World Health Organization. India started its vaccination program on January 16, 2021, with approval for use of Covaxin (Bharat Biotech) and Covishield (Oxford/AstraZeneca formulation)(Serum Institute of India). More than 2 billion doses have been administered since then.2,3
Patients with psoriasis are prone to develop a severe form of COVID-19 due to comorbidities and the intake of immunosuppressive drugs.4 These patients often are hesitant to receive the vaccine without an expert opinion. COVID-19 vaccines are considered to increase tumor necrosis factor α (TNF-α) and IFN-γ production by CD4+ T cells. Tumor necrosis factor α is a key proinflammatory cytokine implicated in the pathogenesis of psoriasis. COVID-19 messenger RNA vaccines induce elevation of IL-6 and helper T cells (TH17), which can induce a flare of psoriasis in a subset of patients.5The International Psoriasis Council recommends that patients with psoriasis receive one of the vaccines approved to prevent COVID-19 infection as soon as possible.6 Reports of new-onset psoriasis and flare of psoriasis after the use of COVID-19 vaccines, such as those manufactured by Pfizer-BioNTech, Moderna, and AstraZeneca, have been published from different parts of the world.7 India used locally developed whole virion inactivated BBV152 (Covaxin) and nonreplicating viral vaccine ChAdOx1 nCoV-19 (Covishield) in its vaccination program and exported them to other developing countries. There is a dearth of data on the safety of these vaccines in patients with psoriasis, which needs to be assessed. Later, Covaxin, ZyCoV-D (DNA plasmid vaccine; Cadila Healthcare), and CorbeVax (protein subunit vaccine; Biological E) were approved for usage in children.8 We conducted a cross-sectional study using the direct interview method.
Patients with psoriasis who attended the outpatient department of the Postgraduate Institute of Medical Education and Research (Chandigarh, India) from April 2022 to June 2022 were invited to participate in the study after written informed consent was received. Patients 18 years and older with chronic plaque psoriasis who had received a COVID-19 vaccine dose in the last 90 days were enrolled. Data on demographics, comorbidities, treatment received for psoriasis, vaccination concerns, history of COVID-19 infection, type of vaccine received with doses, adverse effects, and psoriasis flare after receiving the vaccine (considered up to 2 weeks from the date of vaccination) were collected. Ordinal logistic regression was used to identify factors associated with a psoriasis flare following vaccination. P<.05 was considered statistically significant.
A total of 202 patients with chronic plaque psoriasis who received either Covaxin or Covishield were enrolled during the study period. The mean age (SD) was 40.3 (13.1) years, and 149 (73.8%) patients were male. One hundred thirty-five (66.8%) patients completed 2 doses of the vaccine. eTable 1 provides the clinicodemographic details of the patients. Eighty-three (41.1%) patients had a fear of psoriasis flare after vaccination. Seventy-two (35.6%) patients received the vaccine after clearance from their treating physician/dermatologist. One hundred sixty-four (81.2%) patients received the Covishield vaccine, and 38 (18.8%) patients received Covaxin. Eighty-three (41.1%) patients reported flulike symptoms, such as fever, myalgia, or body pain, within the first week of vaccination. Sixty-one (30.2%) patients reported a psoriasis flare after vaccination in the form of new lesions or worsening of pre-existing lesions. Of these patients, 51 reported a flare after receiving the first dose of vaccine, 8 patients reported a flare after receiving the second dose of vaccine, and 2 patients reported a flare after receiving both doses of vaccine. The mean (SD) flare onset was 8.1 (3.4) days after the vaccination. Eighteen patients considered the flare to be severe. Seventeen (8.4%) patients reported a positive history of COVID-19 infection before vaccination. None of the patients reported breakthrough COVID-19 infection or pustular aggravation of psoriasis following the vaccination.

The self-reported psoriasis flare after receiving the COVID-19 vaccine was significantly higher in patients who experienced immediate adverse effects (P=.005), which included fever, myalgia, joint pain, and injection-site reaction. The reported postvaccination psoriasis flare was not significantly associated with patient sex, history of COVID-19 infection, type of vaccine received, comorbidities, or therapy for psoriasis (eTable 2).

Nearly 30% of our patients reported a postvaccination psoriasis flare, which was more common after the first vaccine dose. Sotiriou et al7 reported 14 cases of psoriasis flare in patients after receiving Pfizer-BioNTech, Moderna, and AstraZeneca COVID-19 vaccines. These patients experienced an exacerbation of disease soon after the second dose of vaccine (mean [SD], 10.36 [7.71] days), and 21% of the 713 enrolled patients wanted to forego the immunization due to concern of a postvaccination psoriasis flare.7 In another report, 14 (27%) patients developed a psoriasis flare after COVID-19 vaccination; the mean (SD) flare onset was 9.3 (4.3) days after vaccination.9
Data on the safety of the COVID-19 vaccine in patients using immunosuppressive drugs are limited. We did not find a significant association between the psoriasis flare and use of immunosuppressive drugs or type of vaccine received. Huang and Tsai9 observed similar results, with no association between psoriasis flare and use of immunosuppressive drugs or biologics, while Damiani et al10 demonstrated a protective role of biologics in preventing vaccine-induced psoriasis flare.
Similar to another study from India,11 the immediate adverse effects due to immunization with Covaxin and Covishield were mild in our study and resolved within a week. The incidence of psoriasis flare was significantly higher in patients who reported adverse effects (P=.005). Activation of immune response after vaccination leads to the release of proinflammatory and pyrogenic cytokines (ie, IL-1, IL-6, TNF-α), which may explain the higher incidence of psoriasis flare in patients experiencing adverse effects to vaccination.12
Our study showed approximately 30% of patients developed a psoriasis flare after COVID-19 vaccination, with no patients experiencing any vaccine-related serious adverse events, which suggests that Covaxin and Covishield are safe for patients with psoriasis in India. Limitations of our study include potential inaccuracy of the patient’s self-assessment of symptoms and disease flare, recall bias that may lead to errors in estimating patient-reported outcomes, the flare of psoriasis potentially being a part of disease fluctuation, and flare being enhanced by the psychological stress of vaccination.
Considering a high risk for severe COVID-19 infection in patients with psoriasis with comorbidities and those using immunosuppressive drugs, Covaxin and Covishield can be safely recommended in India. However, caution needs to be exercised when vaccinating patients with an unstable disease or severe psoriasis.
To the Editor:
COVID-19 infection has resulted in 6.9 million deaths worldwide. India has the third highest mortality from COVID-19 infection after the United States and Brazil.1 Vaccination plays a crucial role in containing COVID-19 infection and reducing its severity. At present, 11 vaccines have been approved by the World Health Organization. India started its vaccination program on January 16, 2021, with approval for use of Covaxin (Bharat Biotech) and Covishield (Oxford/AstraZeneca formulation)(Serum Institute of India). More than 2 billion doses have been administered since then.2,3
Patients with psoriasis are prone to develop a severe form of COVID-19 due to comorbidities and the intake of immunosuppressive drugs.4 These patients often are hesitant to receive the vaccine without an expert opinion. COVID-19 vaccines are considered to increase tumor necrosis factor α (TNF-α) and IFN-γ production by CD4+ T cells. Tumor necrosis factor α is a key proinflammatory cytokine implicated in the pathogenesis of psoriasis. COVID-19 messenger RNA vaccines induce elevation of IL-6 and helper T cells (TH17), which can induce a flare of psoriasis in a subset of patients.5The International Psoriasis Council recommends that patients with psoriasis receive one of the vaccines approved to prevent COVID-19 infection as soon as possible.6 Reports of new-onset psoriasis and flare of psoriasis after the use of COVID-19 vaccines, such as those manufactured by Pfizer-BioNTech, Moderna, and AstraZeneca, have been published from different parts of the world.7 India used locally developed whole virion inactivated BBV152 (Covaxin) and nonreplicating viral vaccine ChAdOx1 nCoV-19 (Covishield) in its vaccination program and exported them to other developing countries. There is a dearth of data on the safety of these vaccines in patients with psoriasis, which needs to be assessed. Later, Covaxin, ZyCoV-D (DNA plasmid vaccine; Cadila Healthcare), and CorbeVax (protein subunit vaccine; Biological E) were approved for usage in children.8 We conducted a cross-sectional study using the direct interview method.
Patients with psoriasis who attended the outpatient department of the Postgraduate Institute of Medical Education and Research (Chandigarh, India) from April 2022 to June 2022 were invited to participate in the study after written informed consent was received. Patients 18 years and older with chronic plaque psoriasis who had received a COVID-19 vaccine dose in the last 90 days were enrolled. Data on demographics, comorbidities, treatment received for psoriasis, vaccination concerns, history of COVID-19 infection, type of vaccine received with doses, adverse effects, and psoriasis flare after receiving the vaccine (considered up to 2 weeks from the date of vaccination) were collected. Ordinal logistic regression was used to identify factors associated with a psoriasis flare following vaccination. P<.05 was considered statistically significant.
A total of 202 patients with chronic plaque psoriasis who received either Covaxin or Covishield were enrolled during the study period. The mean age (SD) was 40.3 (13.1) years, and 149 (73.8%) patients were male. One hundred thirty-five (66.8%) patients completed 2 doses of the vaccine. eTable 1 provides the clinicodemographic details of the patients. Eighty-three (41.1%) patients had a fear of psoriasis flare after vaccination. Seventy-two (35.6%) patients received the vaccine after clearance from their treating physician/dermatologist. One hundred sixty-four (81.2%) patients received the Covishield vaccine, and 38 (18.8%) patients received Covaxin. Eighty-three (41.1%) patients reported flulike symptoms, such as fever, myalgia, or body pain, within the first week of vaccination. Sixty-one (30.2%) patients reported a psoriasis flare after vaccination in the form of new lesions or worsening of pre-existing lesions. Of these patients, 51 reported a flare after receiving the first dose of vaccine, 8 patients reported a flare after receiving the second dose of vaccine, and 2 patients reported a flare after receiving both doses of vaccine. The mean (SD) flare onset was 8.1 (3.4) days after the vaccination. Eighteen patients considered the flare to be severe. Seventeen (8.4%) patients reported a positive history of COVID-19 infection before vaccination. None of the patients reported breakthrough COVID-19 infection or pustular aggravation of psoriasis following the vaccination.

The self-reported psoriasis flare after receiving the COVID-19 vaccine was significantly higher in patients who experienced immediate adverse effects (P=.005), which included fever, myalgia, joint pain, and injection-site reaction. The reported postvaccination psoriasis flare was not significantly associated with patient sex, history of COVID-19 infection, type of vaccine received, comorbidities, or therapy for psoriasis (eTable 2).

Nearly 30% of our patients reported a postvaccination psoriasis flare, which was more common after the first vaccine dose. Sotiriou et al7 reported 14 cases of psoriasis flare in patients after receiving Pfizer-BioNTech, Moderna, and AstraZeneca COVID-19 vaccines. These patients experienced an exacerbation of disease soon after the second dose of vaccine (mean [SD], 10.36 [7.71] days), and 21% of the 713 enrolled patients wanted to forego the immunization due to concern of a postvaccination psoriasis flare.7 In another report, 14 (27%) patients developed a psoriasis flare after COVID-19 vaccination; the mean (SD) flare onset was 9.3 (4.3) days after vaccination.9
Data on the safety of the COVID-19 vaccine in patients using immunosuppressive drugs are limited. We did not find a significant association between the psoriasis flare and use of immunosuppressive drugs or type of vaccine received. Huang and Tsai9 observed similar results, with no association between psoriasis flare and use of immunosuppressive drugs or biologics, while Damiani et al10 demonstrated a protective role of biologics in preventing vaccine-induced psoriasis flare.
Similar to another study from India,11 the immediate adverse effects due to immunization with Covaxin and Covishield were mild in our study and resolved within a week. The incidence of psoriasis flare was significantly higher in patients who reported adverse effects (P=.005). Activation of immune response after vaccination leads to the release of proinflammatory and pyrogenic cytokines (ie, IL-1, IL-6, TNF-α), which may explain the higher incidence of psoriasis flare in patients experiencing adverse effects to vaccination.12
Our study showed approximately 30% of patients developed a psoriasis flare after COVID-19 vaccination, with no patients experiencing any vaccine-related serious adverse events, which suggests that Covaxin and Covishield are safe for patients with psoriasis in India. Limitations of our study include potential inaccuracy of the patient’s self-assessment of symptoms and disease flare, recall bias that may lead to errors in estimating patient-reported outcomes, the flare of psoriasis potentially being a part of disease fluctuation, and flare being enhanced by the psychological stress of vaccination.
Considering a high risk for severe COVID-19 infection in patients with psoriasis with comorbidities and those using immunosuppressive drugs, Covaxin and Covishield can be safely recommended in India. However, caution needs to be exercised when vaccinating patients with an unstable disease or severe psoriasis.
- COVID-19 coronavirus pandemic: weekly trends. Worldometer. Accessed August 21, 2023. https://www.worldometers.info/coronavirus/
- National COVID-19 vaccination programme meets its goals by overcoming R&D and logistical challenges, says economic survey 2022-23. Government of India Press Information Bureau website. Published January 31, 2023. Accessed August 24, 2023. https://pib.gov.in/PressReleasePage.aspx?PRID=1894907
- Ministry of Health and Family Welfare. CoWIN. Accessed August 21, 2023. https://www.cowin.gov.in/
- Griffiths CEM, Armstrong AW, Gudjonsson JE, et al. Psoriasis. Lancet. 2021;397:1301-1315.
- Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: anemerging target of JAK2 inhibitor fedratinib. J Microbiol Immunol Infect. 2020;53:368-370.
- International Psoriasis Council. Revised IPC statement on COVID-19. Published December 19, 2022. Accessed August 24, 2023. https://psoriasiscouncil.org/covid-19/revised-statement-covid-19/
- Sotiriou E, Tsentemeidou A, Bakirtzi K, et al. Psoriasis exacerbation after COVID-19 vaccination: a report of 14 cases from a single centre. J Eur Acad Dermatol Venereol. 2021;35:E857-E859.
- Kaul R. India clears 2 vaccines for kids under 12 years. Hindustan Times. Published April 27, 2022. Accessed August 24, 2023. https://www.hindustantimes.com/india-news/india-clears-2-vaccines-for-kids-under-12-years-101650998027336.html
- Huang YW, Tsai TF. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med (Lausanne). 2021;8:812010.
- Damiani G, Allocco F, Young Dermatologists Italian Network, et al. COVID-19 vaccination and patients with psoriasis under biologics: real-life evidence on safety and effectiveness from Italian vaccinated healthcare workers. Clin Exp Dermatol. 2021;460:1106-1108.
- Joshi RK, Muralidharan CG, Gulati DS, et al. Higher incidence of reported adverse events following immunisation (AEFI) after first dose of COVID-19 vaccine among previously infected health care workers. Med J Armed Forces India. 2021;77(suppl 2):S505-S507.
- Hervé C, Laupèze B, Del Giudice G, et al. The how’s and what’s of vaccine reactogenicity. NPJ Vaccines. 2019;4:39.
- COVID-19 coronavirus pandemic: weekly trends. Worldometer. Accessed August 21, 2023. https://www.worldometers.info/coronavirus/
- National COVID-19 vaccination programme meets its goals by overcoming R&D and logistical challenges, says economic survey 2022-23. Government of India Press Information Bureau website. Published January 31, 2023. Accessed August 24, 2023. https://pib.gov.in/PressReleasePage.aspx?PRID=1894907
- Ministry of Health and Family Welfare. CoWIN. Accessed August 21, 2023. https://www.cowin.gov.in/
- Griffiths CEM, Armstrong AW, Gudjonsson JE, et al. Psoriasis. Lancet. 2021;397:1301-1315.
- Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: anemerging target of JAK2 inhibitor fedratinib. J Microbiol Immunol Infect. 2020;53:368-370.
- International Psoriasis Council. Revised IPC statement on COVID-19. Published December 19, 2022. Accessed August 24, 2023. https://psoriasiscouncil.org/covid-19/revised-statement-covid-19/
- Sotiriou E, Tsentemeidou A, Bakirtzi K, et al. Psoriasis exacerbation after COVID-19 vaccination: a report of 14 cases from a single centre. J Eur Acad Dermatol Venereol. 2021;35:E857-E859.
- Kaul R. India clears 2 vaccines for kids under 12 years. Hindustan Times. Published April 27, 2022. Accessed August 24, 2023. https://www.hindustantimes.com/india-news/india-clears-2-vaccines-for-kids-under-12-years-101650998027336.html
- Huang YW, Tsai TF. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med (Lausanne). 2021;8:812010.
- Damiani G, Allocco F, Young Dermatologists Italian Network, et al. COVID-19 vaccination and patients with psoriasis under biologics: real-life evidence on safety and effectiveness from Italian vaccinated healthcare workers. Clin Exp Dermatol. 2021;460:1106-1108.
- Joshi RK, Muralidharan CG, Gulati DS, et al. Higher incidence of reported adverse events following immunisation (AEFI) after first dose of COVID-19 vaccine among previously infected health care workers. Med J Armed Forces India. 2021;77(suppl 2):S505-S507.
- Hervé C, Laupèze B, Del Giudice G, et al. The how’s and what’s of vaccine reactogenicity. NPJ Vaccines. 2019;4:39.
Practice Points
- Vaccines are known to induce a psoriasis flare.
- Given the high risk for severe COVID infection in individuals with psoriasis who have comorbidities, vaccination with Covaxin and Covishield can be safely recommended in India for this population.
Financial Insecurity Among US Adults With Psoriasis
To the Editor:
Approximately 3% of the US population, or 6.9 million adults, is affected by psoriasis.1 Psoriasis has a substantial impact on quality of life and is associated with increased health care expenses and medication costs. In 2013, it was reported that the estimated US annual cost—direct, indirect, intangible, and comorbidity costs—of psoriasis for adults was $112 billion.2 We investigated the prevalence and sociodemographic characteristics of adult psoriasis patients (aged ≥20 years) with financial insecurity utilizing the 2009–2014 National Health and Nutrition Examination Survey (NHANES) data.3
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 2009-2014 NHANES database to evaluate financial insecurity. Financial insecurity was evaluated by 2 outcome variables. The primary outcome variable was assessed by the question “Are you covered by health insurance or some other kind of health care plan (including health insurance obtained through employment or purchased directly as well as government programs like Medicare and Medicaid that provide medical care or help pay medical bills)?”3 Our secondary outcome variable was evaluated by a reported annual household income of less than $20,000. P values in Table 1 were calculated using Pearson χ2 tests. In Table 2, multivariate logistic regressions were performed using Stata/MP 17 (StataCorp LLC) to analyze associations between outcome variables and sociodemographic characteristics. Additionally, we controlled for age, race/ethnicity, sex, education, marital status, US citizenship status, and tobacco use. Subsequently, relationships with P<.05 were considered statistically significant.
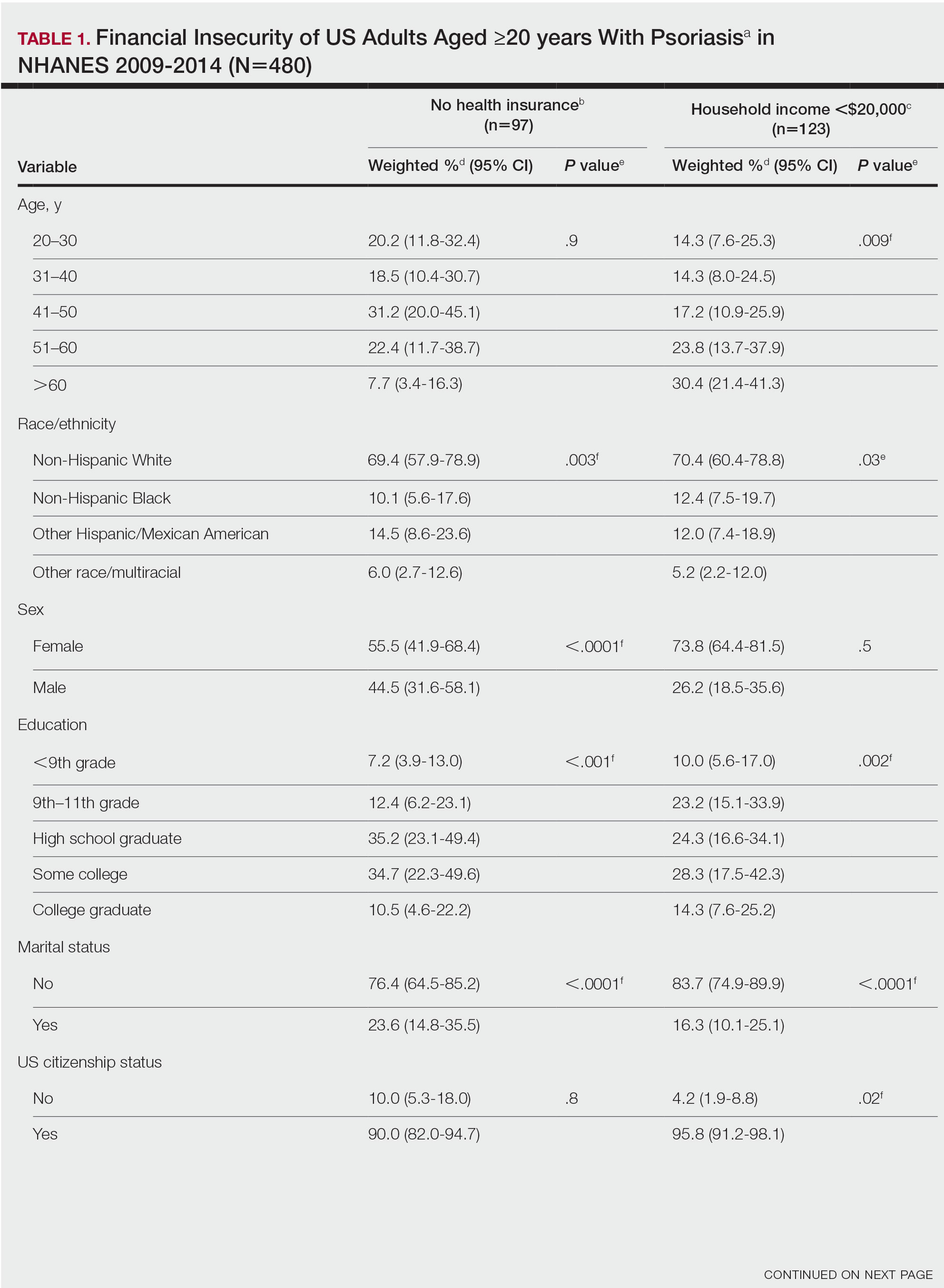

Our analysis comprised 480 individuals with psoriasis; 40 individuals were excluded from our analysis because they did not report annual household income and health insurance status (Table 1). Among the 480 individuals with psoriasis, approximately 16% (weighted) reported a lack of health insurance, and approximately 17% (weighted) reported an annual household income of less than $20,000. Among those who reported an annual household income of less than $20,000, approximately 38% (weighted) of them reported that they did not have health insurance.
Multivariate logistic regression analyses revealed that elderly individuals (aged >60 years), college graduates, married individuals, and US citizens had decreased odds of lacking health insurance (Table 2). Additionally, those with a history of tobacco use (adjusted odds ratio [AOR] 2.02; 95% CI, 1.00-4.05) were associated with lacking health insurance. Non-Hispanic Black individuals (AOR 2.26; 95% CI, 1.09-4.71) and US citizens (AOR 5.01; 95% CI, 1.28-19.63) had a significant association with an annual household income of less than $20,000 (P<.05). Lastly, males, those with education beyond ninth grade, and married individuals had a significantly decreased odds of having an annual household income of less than $20,000 (P<.05)(Table 2).
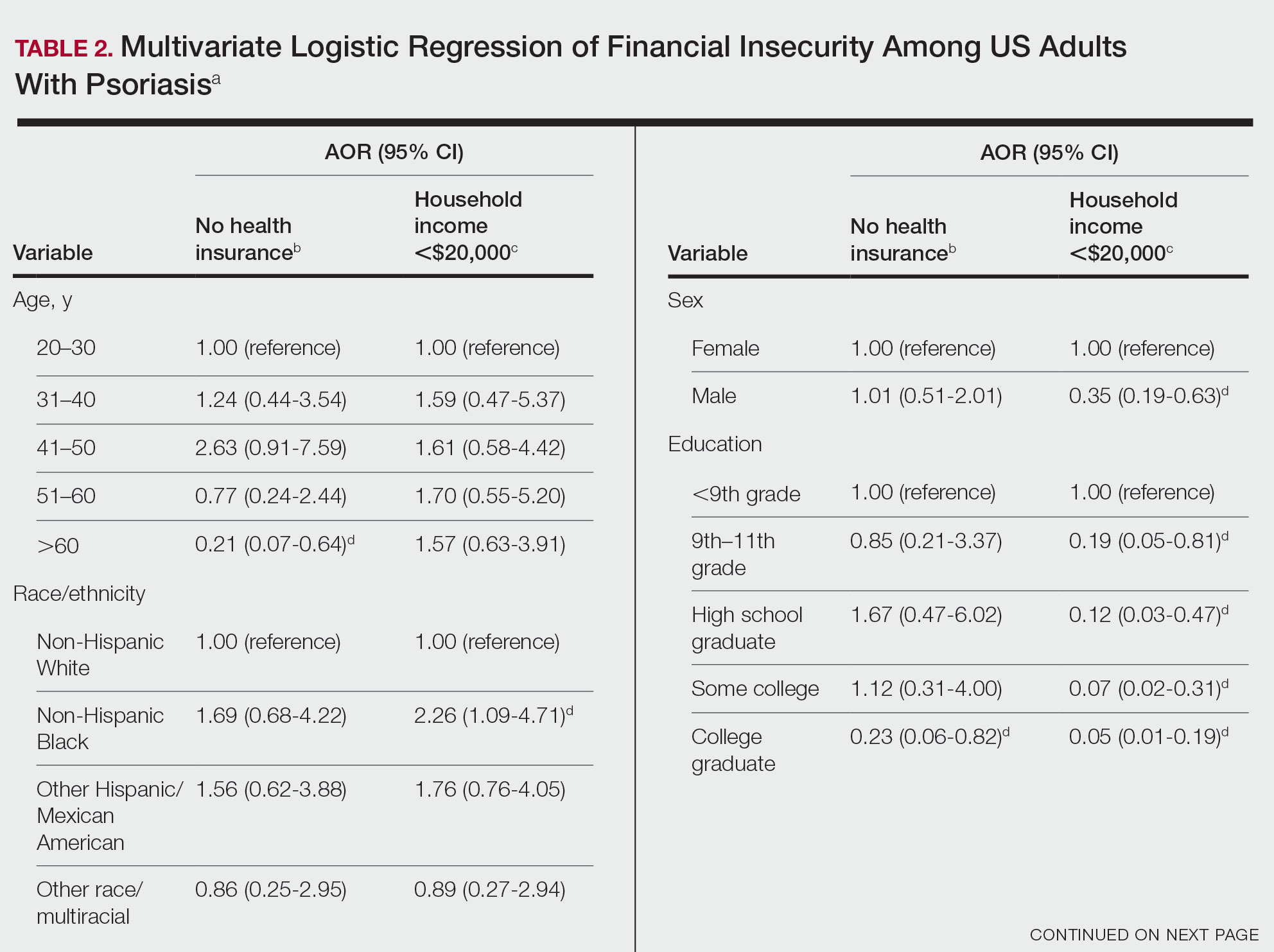
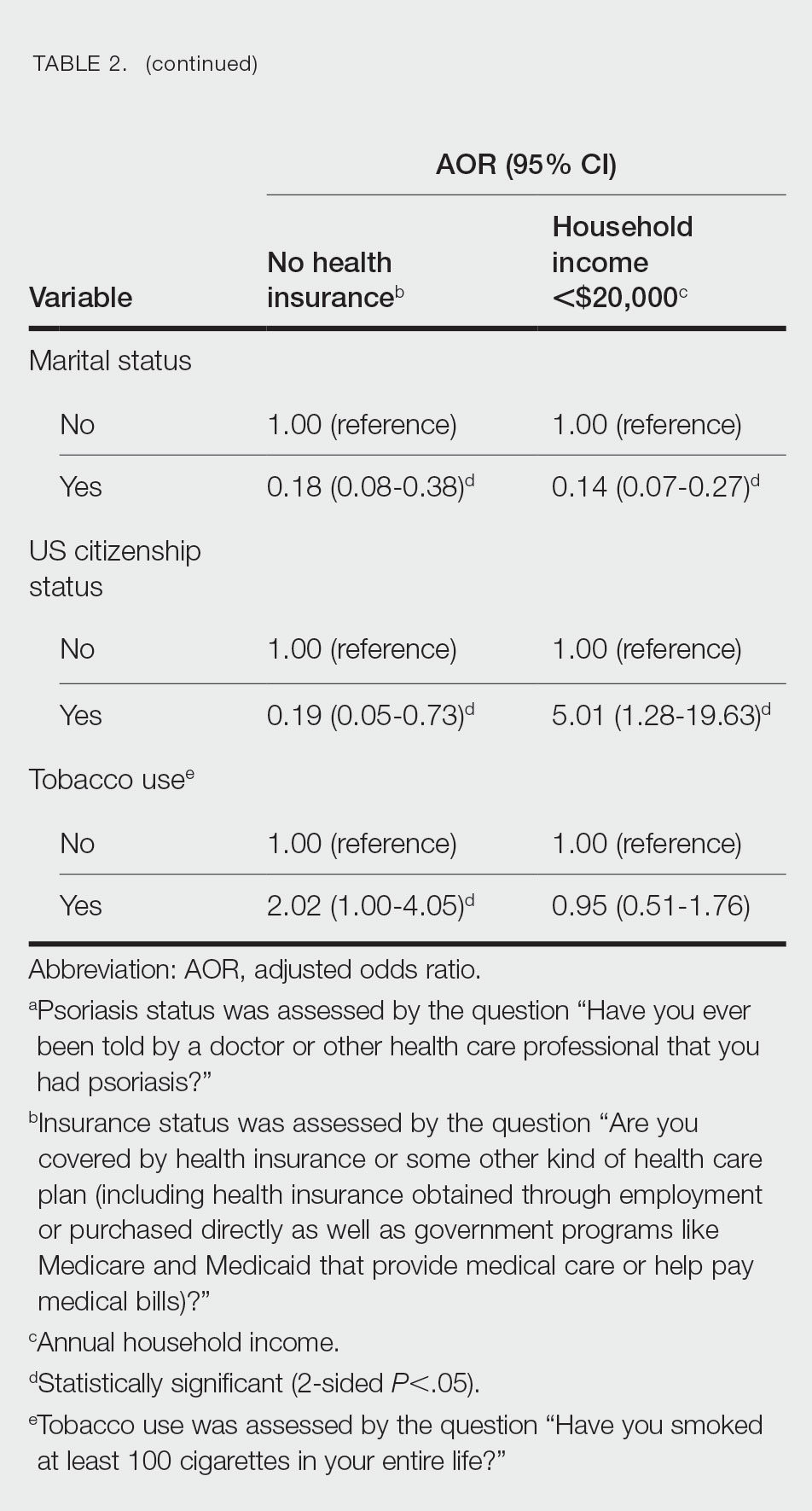
Our findings indicate that certain sociodemographic groups of psoriasis patients have an increased risk for being financially insecure. It is important to evaluate the cost of treatment, number of necessary visits to the office, and cost of transportation, as these factors can serve as a major economic burden to patients being managed for psoriasis.4 Additionally, the cost of biologics has been increasing over time.5 Taking all of this into account when caring for psoriasis patients is crucial, as understanding the financial status of patients can assist with determining appropriate individualized treatment regimens.
- Liu J, Thatiparthi A, Martin A, et al. Prevalence of psoriasis among adults in the US 2009-2010 and 2013-2014 National Health and Nutrition Examination Surveys. J Am Acad Dermatol. 2021;84:767-769. doi:10.1016/j.jaad.2020.10.035
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658. doi:10.1001/jamadermatol.2014.3593
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed June 22, 2023. https://wwwn.cdc.govnchs/nhanes/Default.aspx
- Maya-Rico AM, Londoño-García Á, Palacios-Barahona AU, et al. Out-of-pocket costs for patients with psoriasis in an outpatient dermatology referral service. An Bras Dermatol. 2021;96:295-300. doi:10.1016/j.abd.2020.09.004
- Cheng J, Feldman SR. The cost of biologics for psoriasis is increasing. Drugs Context. 2014;3:212266. doi:10.7573/dic.212266
To the Editor:
Approximately 3% of the US population, or 6.9 million adults, is affected by psoriasis.1 Psoriasis has a substantial impact on quality of life and is associated with increased health care expenses and medication costs. In 2013, it was reported that the estimated US annual cost—direct, indirect, intangible, and comorbidity costs—of psoriasis for adults was $112 billion.2 We investigated the prevalence and sociodemographic characteristics of adult psoriasis patients (aged ≥20 years) with financial insecurity utilizing the 2009–2014 National Health and Nutrition Examination Survey (NHANES) data.3
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 2009-2014 NHANES database to evaluate financial insecurity. Financial insecurity was evaluated by 2 outcome variables. The primary outcome variable was assessed by the question “Are you covered by health insurance or some other kind of health care plan (including health insurance obtained through employment or purchased directly as well as government programs like Medicare and Medicaid that provide medical care or help pay medical bills)?”3 Our secondary outcome variable was evaluated by a reported annual household income of less than $20,000. P values in Table 1 were calculated using Pearson χ2 tests. In Table 2, multivariate logistic regressions were performed using Stata/MP 17 (StataCorp LLC) to analyze associations between outcome variables and sociodemographic characteristics. Additionally, we controlled for age, race/ethnicity, sex, education, marital status, US citizenship status, and tobacco use. Subsequently, relationships with P<.05 were considered statistically significant.


Our analysis comprised 480 individuals with psoriasis; 40 individuals were excluded from our analysis because they did not report annual household income and health insurance status (Table 1). Among the 480 individuals with psoriasis, approximately 16% (weighted) reported a lack of health insurance, and approximately 17% (weighted) reported an annual household income of less than $20,000. Among those who reported an annual household income of less than $20,000, approximately 38% (weighted) of them reported that they did not have health insurance.
Multivariate logistic regression analyses revealed that elderly individuals (aged >60 years), college graduates, married individuals, and US citizens had decreased odds of lacking health insurance (Table 2). Additionally, those with a history of tobacco use (adjusted odds ratio [AOR] 2.02; 95% CI, 1.00-4.05) were associated with lacking health insurance. Non-Hispanic Black individuals (AOR 2.26; 95% CI, 1.09-4.71) and US citizens (AOR 5.01; 95% CI, 1.28-19.63) had a significant association with an annual household income of less than $20,000 (P<.05). Lastly, males, those with education beyond ninth grade, and married individuals had a significantly decreased odds of having an annual household income of less than $20,000 (P<.05)(Table 2).


Our findings indicate that certain sociodemographic groups of psoriasis patients have an increased risk for being financially insecure. It is important to evaluate the cost of treatment, number of necessary visits to the office, and cost of transportation, as these factors can serve as a major economic burden to patients being managed for psoriasis.4 Additionally, the cost of biologics has been increasing over time.5 Taking all of this into account when caring for psoriasis patients is crucial, as understanding the financial status of patients can assist with determining appropriate individualized treatment regimens.
To the Editor:
Approximately 3% of the US population, or 6.9 million adults, is affected by psoriasis.1 Psoriasis has a substantial impact on quality of life and is associated with increased health care expenses and medication costs. In 2013, it was reported that the estimated US annual cost—direct, indirect, intangible, and comorbidity costs—of psoriasis for adults was $112 billion.2 We investigated the prevalence and sociodemographic characteristics of adult psoriasis patients (aged ≥20 years) with financial insecurity utilizing the 2009–2014 National Health and Nutrition Examination Survey (NHANES) data.3
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 2009-2014 NHANES database to evaluate financial insecurity. Financial insecurity was evaluated by 2 outcome variables. The primary outcome variable was assessed by the question “Are you covered by health insurance or some other kind of health care plan (including health insurance obtained through employment or purchased directly as well as government programs like Medicare and Medicaid that provide medical care or help pay medical bills)?”3 Our secondary outcome variable was evaluated by a reported annual household income of less than $20,000. P values in Table 1 were calculated using Pearson χ2 tests. In Table 2, multivariate logistic regressions were performed using Stata/MP 17 (StataCorp LLC) to analyze associations between outcome variables and sociodemographic characteristics. Additionally, we controlled for age, race/ethnicity, sex, education, marital status, US citizenship status, and tobacco use. Subsequently, relationships with P<.05 were considered statistically significant.


Our analysis comprised 480 individuals with psoriasis; 40 individuals were excluded from our analysis because they did not report annual household income and health insurance status (Table 1). Among the 480 individuals with psoriasis, approximately 16% (weighted) reported a lack of health insurance, and approximately 17% (weighted) reported an annual household income of less than $20,000. Among those who reported an annual household income of less than $20,000, approximately 38% (weighted) of them reported that they did not have health insurance.
Multivariate logistic regression analyses revealed that elderly individuals (aged >60 years), college graduates, married individuals, and US citizens had decreased odds of lacking health insurance (Table 2). Additionally, those with a history of tobacco use (adjusted odds ratio [AOR] 2.02; 95% CI, 1.00-4.05) were associated with lacking health insurance. Non-Hispanic Black individuals (AOR 2.26; 95% CI, 1.09-4.71) and US citizens (AOR 5.01; 95% CI, 1.28-19.63) had a significant association with an annual household income of less than $20,000 (P<.05). Lastly, males, those with education beyond ninth grade, and married individuals had a significantly decreased odds of having an annual household income of less than $20,000 (P<.05)(Table 2).


Our findings indicate that certain sociodemographic groups of psoriasis patients have an increased risk for being financially insecure. It is important to evaluate the cost of treatment, number of necessary visits to the office, and cost of transportation, as these factors can serve as a major economic burden to patients being managed for psoriasis.4 Additionally, the cost of biologics has been increasing over time.5 Taking all of this into account when caring for psoriasis patients is crucial, as understanding the financial status of patients can assist with determining appropriate individualized treatment regimens.
- Liu J, Thatiparthi A, Martin A, et al. Prevalence of psoriasis among adults in the US 2009-2010 and 2013-2014 National Health and Nutrition Examination Surveys. J Am Acad Dermatol. 2021;84:767-769. doi:10.1016/j.jaad.2020.10.035
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658. doi:10.1001/jamadermatol.2014.3593
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed June 22, 2023. https://wwwn.cdc.govnchs/nhanes/Default.aspx
- Maya-Rico AM, Londoño-García Á, Palacios-Barahona AU, et al. Out-of-pocket costs for patients with psoriasis in an outpatient dermatology referral service. An Bras Dermatol. 2021;96:295-300. doi:10.1016/j.abd.2020.09.004
- Cheng J, Feldman SR. The cost of biologics for psoriasis is increasing. Drugs Context. 2014;3:212266. doi:10.7573/dic.212266
- Liu J, Thatiparthi A, Martin A, et al. Prevalence of psoriasis among adults in the US 2009-2010 and 2013-2014 National Health and Nutrition Examination Surveys. J Am Acad Dermatol. 2021;84:767-769. doi:10.1016/j.jaad.2020.10.035
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658. doi:10.1001/jamadermatol.2014.3593
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed June 22, 2023. https://wwwn.cdc.govnchs/nhanes/Default.aspx
- Maya-Rico AM, Londoño-García Á, Palacios-Barahona AU, et al. Out-of-pocket costs for patients with psoriasis in an outpatient dermatology referral service. An Bras Dermatol. 2021;96:295-300. doi:10.1016/j.abd.2020.09.004
- Cheng J, Feldman SR. The cost of biologics for psoriasis is increasing. Drugs Context. 2014;3:212266. doi:10.7573/dic.212266
Practice Points
- The economic burden on patients with psoriasis has been rising over time, as the disease impacts many aspects of patients’ lives.
- Various sociodemographic groups among patients with psoriasis are financially insecure. Knowing which groups are at higher risk for poor outcomes due to financial insecurity can assist with appropriate treatment regimens.
Evaluating Pharmacists’ Time Collecting Self-Monitoring Blood Glucose Data
The American Diabetes Association recommends that patients on intensive insulin regimens self-monitor blood glucose (SMBG) to assist in therapy optimization.1 To be useful, SMBG data must be captured by patients, shared with care teams, and used and interpreted by patients and practitioners.2,3 Communication of SMBG data from the patient to practitioner can be challenging. Although technology can help in this process, limitations exist, such as manual data entry into systems, patient and/or practitioner technological challenges (eg, accessing interface), and compatibility and integration between SMBG devices and electronic health record (EHR) systems.4
The Boise Veterans Affairs Medical Center (BVAMC) in Idaho serves more than 100,000 veterans. It includes a main site, community-based outpatient clinics, and a clinical resource hub that provides telehealth services to veterans residing in rural neighboring states. The BVAMC pharmacy department provides both inpatient and outpatient services. At the BVAMC, clinical pharmacist practitioners (CPPs) are independent practitioners who support their care teams in comprehensive medication management and have the ability to initiate, modify, and discontinue drug therapy for referred patients.5 A prominent role of CPPs in primary care teams is to manage patients with uncontrolled diabetes and intensive insulin regimens, in which SMBG data are vital to therapy optimization. As collecting SMBG data from patients is seen anecdotally as time intensive, we determined the mean time spent by CPPs collecting patient SMBG data and its potential implications.
Methods
Pharmacists at BVAMC were asked to estimate and record the following: SMBG data collection method, time spent collecting data, extra time spent documenting or formatting SMBG readings, total patient visit time, and visit type. Time was collected in minutes. Extra time spent documenting or formatting SMBG readings included any additional time formatting or entering data in the clinical note after talking to the patient; if this was done while multitasking and talking to the patient, it was not considered extra time. For total patient visit time, pharmacists were asked to estimate only time spent discussing diabetes care and collecting SMBG data. Visit types were categorized as in-person/face-to-face, telephone, and telehealth using clinical video telehealth (CVT)/VA Video Connect (VVC). Data were collected using a standardized spreadsheet. The spreadsheet was pilot tested by a CPP before distribution to all pharmacists.
CPPs were educated about the project in March 2021 and were asked to record data for a 1-week period between April 5, 2021, and April 30, 2021. One CPP also provided delayed data collected from May 17 to 21, 2021, and these data were included in our analysis.
Descriptive statistics were used to determine the mean time spent by CPPs collecting SMBG data. Unpaired t tests were used to compare time spent collecting SMBG data by different collection methods and patient visit types. A P value of ≤ .05 was considered statistically significant. Data were organized in Microsoft Excel, and statistics were completed with JMP Pro v15.
Results
Eight CPPs provided data from 120 patient encounters. For all pa
When compared by the SMBG collection method, the longest time spent collecting SMBG data was with patient report (3.7 minutes), and the longest time spent documenting/formatting time was with meter download/home telehealth (2 minutes). There was no statistically significant difference in the time to collect SMBG data between patient report and other methods (3.7 minutes vs 2.8 minutes; P = .07).
When compared by visit type, there was not a statistically significant difference between time spent collecting SMBG data (3.8 minutes vs 3.2 minutes; P = .39) (Table 2).
Discussion
We found that the mean amount of time spent collecting and documenting/formatting SMBG data was only 4.6 minutes; however, this still represented a substantial portion of visit time. For telephone and CVT/VVC appointments, this represented > 25% of total visit time. While CPPs make important contributions to interprofessional team management of patients with diabetes, their cost is not trivial.6-8 It is worth exploring the most effective and efficient ways to use CPPs. Our results indicate that streamlining SMBG data collection may be beneficial.
Pharmacy technicians, licensed practical nurses/clinical associates, registered nurses/nurse care managers, or other team members could help improve SMBG data collection. Using other team members is also an opportunity for comanagement, for team collaboration, and for more patients to be seen. For example, if a CPP currently has 12 patient encounters that last 20 minutes each, this results in about 240 minutes of direct patient care. If patient encounters were 16 minutes, CPPS could have 15 patient encounters in 240 minutes. Saved time could be used for other clinical tasks involved in disease management or clinical reminder reviews. While there are benefits to CPPs collecting SMBG data, such as further inquiry about patient-reported values, other team members could also be trained to ask appropriate follow-up questions for abnormal blood glucose readings. In addition, leveraging current team members and optimizing their roles could prevent the need to acquire additional full-time equivalent employees.
Another opportunity to increase efficiency in SMBG data collection is with SMBG devices and EHR integration.4,9 However, integration can be difficult with different types of SMBG devices and EHR platforms. Education for patients and practitioners could help to ensure accurate and reliable data uploads; patient internet availability; data protection, privacy, and sharing; workflow management; and clear patient-practitioner expectations.10 For example, if patient SMBG data are automatically uploaded to practitioners, patients’ expectations for practitioner review of data and follow-up need to be determined.
We found a subset of patient encounters (n = 23) where data collection and documenting/formatting represented more than half of the total visit time. In this subset, 13 SMBG reports were pulled from a log or meter, 8 were patient reported, and 3 were meter download or home telehealth.
Limitations
A potential reason for the lack of statistically significant differences in SMBG collection method or visit type in this study includes the small sample size. Participation in this work was voluntary, and all participating CPPs had ≥ 3 years of practice in their current setting, which includes a heavy workload of diabetes management. These pharmacists noted self-established procedures/systems for SMBG data collection, including the use of Excel spreadsheets with pregenerated formulas. For less experienced CPPs, SMBG data collection time may be even longer. Pharmacists also noted that they may limit time spent collecting SMBG data depending on the patient encounter and whether they have gathered sufficient data to guide clinical care. Other limitations of this work include data collection from a single institution and that the time documented represented estimates; there was no external monitor.
Conclusions
In this analysis, we found that CPPs spend about 3 minutes collecting SMBG data from patients, and about an additional 1 minute documenting and formatting data. While 4 to 5 minutes may not represent a substantial amount of time for one patient, it can be when multiplied by several patient encounters. The time spent collecting SMBG data did not significantly differ by collection method or visit type. Opportunities to increase efficiency in SMBG data collection, such as the use of nonpharmacist team members are worth exploring.
Acknowledgments
Thank you to the pharmacists at the Boise Veterans Affairs Medical Center for their time and support of this work: Danielle Ahlstrom, Paul Black, Robyn Cruz, Sarah Naidoo, Anthony Nelson, Laura Spoutz, Eileen Twomey, Donovan Victorine, and Michelle Wilkin.
1. American Diabetes Association. 7. Diabetes Technology: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44(suppl 1):S85-S99. doi:10.2337/dc21-S007
2. Austin MM. The two skill sets of self-monitoring of blood glucose education: the operational and the interpretive. Diabetes Spectr. 2013;26(2):83-90. doi:10.2337/diaspect.26.2.83
3. Gallichan M. Self monitoring of glucose by people with diabetes: evidence based practice. BMJ. 1997;314(7085):964-967. doi:10.1136/bmj.314.7085.964
4. Lewinski AA, Drake C, Shaw RJ, et al. Bridging the integration gap between patient-generated blood glucose data and electronic health records. J Am Med Inform Assoc. 2019;26(7):667-672. doi:10.1093/jamia/ocz039
5. McFarland MS, Groppi J, Jorgenson T, et al. Role of the US Veterans Health Administration clinical pharmacy specialist provider: shaping the future of comprehensive medication management. Can J Hosp Pharm. 2020;73(2):152-158. doi:10.4212/cjhp.v73i2.2982
6. Schmidt K, Caudill J. Hamilton T. Impact of clinical pharmacy specialists on glycemic control in veterans with type 2 diabetes. Am J Health Syst Pharm. 2019;76(suppl 1):S9-S14. doi:10.1093/ajhp/zxy015
7. Sullivan J, Jett BP, Cradick M, Zuber J. Effect of clinical pharmacist intervention on hemoglobin A1c reduction in veteran patients with type 2 diabetes in a rural setting. Ann Pharmacother. 2016;50(12):1023-1027. doi:10.1177/1060028016663564
8. Bloom CI, Ku M, Williams M. Clinical pharmacy specialists’ impact in patient aligned care teams for type 2 diabetes management. J Am Pharm Assoc (2003). 2019;59(5):717-721. doi:10.1016/j.japh.2019.05.002
9. Kumar RB, Goren ND, Stark DE, Wall DP, Longhurst CA. Automated integration of continuous glucose monitor data in the electronic health record using consumer technology. J Am Med Inform Assoc. 2016;23(3):532-537. doi:10.1093/jamia/ocv206
10. Reading MJ, Merrill JA. Converging and diverging needs between patients and providers who are collecting and using patient-generated health data: an integrative review. J Am Med Inform Assoc. 2018;25(6):759-771. doi:10.1093/jamia/ocy006
The American Diabetes Association recommends that patients on intensive insulin regimens self-monitor blood glucose (SMBG) to assist in therapy optimization.1 To be useful, SMBG data must be captured by patients, shared with care teams, and used and interpreted by patients and practitioners.2,3 Communication of SMBG data from the patient to practitioner can be challenging. Although technology can help in this process, limitations exist, such as manual data entry into systems, patient and/or practitioner technological challenges (eg, accessing interface), and compatibility and integration between SMBG devices and electronic health record (EHR) systems.4
The Boise Veterans Affairs Medical Center (BVAMC) in Idaho serves more than 100,000 veterans. It includes a main site, community-based outpatient clinics, and a clinical resource hub that provides telehealth services to veterans residing in rural neighboring states. The BVAMC pharmacy department provides both inpatient and outpatient services. At the BVAMC, clinical pharmacist practitioners (CPPs) are independent practitioners who support their care teams in comprehensive medication management and have the ability to initiate, modify, and discontinue drug therapy for referred patients.5 A prominent role of CPPs in primary care teams is to manage patients with uncontrolled diabetes and intensive insulin regimens, in which SMBG data are vital to therapy optimization. As collecting SMBG data from patients is seen anecdotally as time intensive, we determined the mean time spent by CPPs collecting patient SMBG data and its potential implications.
Methods
Pharmacists at BVAMC were asked to estimate and record the following: SMBG data collection method, time spent collecting data, extra time spent documenting or formatting SMBG readings, total patient visit time, and visit type. Time was collected in minutes. Extra time spent documenting or formatting SMBG readings included any additional time formatting or entering data in the clinical note after talking to the patient; if this was done while multitasking and talking to the patient, it was not considered extra time. For total patient visit time, pharmacists were asked to estimate only time spent discussing diabetes care and collecting SMBG data. Visit types were categorized as in-person/face-to-face, telephone, and telehealth using clinical video telehealth (CVT)/VA Video Connect (VVC). Data were collected using a standardized spreadsheet. The spreadsheet was pilot tested by a CPP before distribution to all pharmacists.
CPPs were educated about the project in March 2021 and were asked to record data for a 1-week period between April 5, 2021, and April 30, 2021. One CPP also provided delayed data collected from May 17 to 21, 2021, and these data were included in our analysis.
Descriptive statistics were used to determine the mean time spent by CPPs collecting SMBG data. Unpaired t tests were used to compare time spent collecting SMBG data by different collection methods and patient visit types. A P value of ≤ .05 was considered statistically significant. Data were organized in Microsoft Excel, and statistics were completed with JMP Pro v15.
Results
Eight CPPs provided data from 120 patient encounters. For all pa
When compared by the SMBG collection method, the longest time spent collecting SMBG data was with patient report (3.7 minutes), and the longest time spent documenting/formatting time was with meter download/home telehealth (2 minutes). There was no statistically significant difference in the time to collect SMBG data between patient report and other methods (3.7 minutes vs 2.8 minutes; P = .07).
When compared by visit type, there was not a statistically significant difference between time spent collecting SMBG data (3.8 minutes vs 3.2 minutes; P = .39) (Table 2).
Discussion
We found that the mean amount of time spent collecting and documenting/formatting SMBG data was only 4.6 minutes; however, this still represented a substantial portion of visit time. For telephone and CVT/VVC appointments, this represented > 25% of total visit time. While CPPs make important contributions to interprofessional team management of patients with diabetes, their cost is not trivial.6-8 It is worth exploring the most effective and efficient ways to use CPPs. Our results indicate that streamlining SMBG data collection may be beneficial.
Pharmacy technicians, licensed practical nurses/clinical associates, registered nurses/nurse care managers, or other team members could help improve SMBG data collection. Using other team members is also an opportunity for comanagement, for team collaboration, and for more patients to be seen. For example, if a CPP currently has 12 patient encounters that last 20 minutes each, this results in about 240 minutes of direct patient care. If patient encounters were 16 minutes, CPPS could have 15 patient encounters in 240 minutes. Saved time could be used for other clinical tasks involved in disease management or clinical reminder reviews. While there are benefits to CPPs collecting SMBG data, such as further inquiry about patient-reported values, other team members could also be trained to ask appropriate follow-up questions for abnormal blood glucose readings. In addition, leveraging current team members and optimizing their roles could prevent the need to acquire additional full-time equivalent employees.
Another opportunity to increase efficiency in SMBG data collection is with SMBG devices and EHR integration.4,9 However, integration can be difficult with different types of SMBG devices and EHR platforms. Education for patients and practitioners could help to ensure accurate and reliable data uploads; patient internet availability; data protection, privacy, and sharing; workflow management; and clear patient-practitioner expectations.10 For example, if patient SMBG data are automatically uploaded to practitioners, patients’ expectations for practitioner review of data and follow-up need to be determined.
We found a subset of patient encounters (n = 23) where data collection and documenting/formatting represented more than half of the total visit time. In this subset, 13 SMBG reports were pulled from a log or meter, 8 were patient reported, and 3 were meter download or home telehealth.
Limitations
A potential reason for the lack of statistically significant differences in SMBG collection method or visit type in this study includes the small sample size. Participation in this work was voluntary, and all participating CPPs had ≥ 3 years of practice in their current setting, which includes a heavy workload of diabetes management. These pharmacists noted self-established procedures/systems for SMBG data collection, including the use of Excel spreadsheets with pregenerated formulas. For less experienced CPPs, SMBG data collection time may be even longer. Pharmacists also noted that they may limit time spent collecting SMBG data depending on the patient encounter and whether they have gathered sufficient data to guide clinical care. Other limitations of this work include data collection from a single institution and that the time documented represented estimates; there was no external monitor.
Conclusions
In this analysis, we found that CPPs spend about 3 minutes collecting SMBG data from patients, and about an additional 1 minute documenting and formatting data. While 4 to 5 minutes may not represent a substantial amount of time for one patient, it can be when multiplied by several patient encounters. The time spent collecting SMBG data did not significantly differ by collection method or visit type. Opportunities to increase efficiency in SMBG data collection, such as the use of nonpharmacist team members are worth exploring.
Acknowledgments
Thank you to the pharmacists at the Boise Veterans Affairs Medical Center for their time and support of this work: Danielle Ahlstrom, Paul Black, Robyn Cruz, Sarah Naidoo, Anthony Nelson, Laura Spoutz, Eileen Twomey, Donovan Victorine, and Michelle Wilkin.
The American Diabetes Association recommends that patients on intensive insulin regimens self-monitor blood glucose (SMBG) to assist in therapy optimization.1 To be useful, SMBG data must be captured by patients, shared with care teams, and used and interpreted by patients and practitioners.2,3 Communication of SMBG data from the patient to practitioner can be challenging. Although technology can help in this process, limitations exist, such as manual data entry into systems, patient and/or practitioner technological challenges (eg, accessing interface), and compatibility and integration between SMBG devices and electronic health record (EHR) systems.4
The Boise Veterans Affairs Medical Center (BVAMC) in Idaho serves more than 100,000 veterans. It includes a main site, community-based outpatient clinics, and a clinical resource hub that provides telehealth services to veterans residing in rural neighboring states. The BVAMC pharmacy department provides both inpatient and outpatient services. At the BVAMC, clinical pharmacist practitioners (CPPs) are independent practitioners who support their care teams in comprehensive medication management and have the ability to initiate, modify, and discontinue drug therapy for referred patients.5 A prominent role of CPPs in primary care teams is to manage patients with uncontrolled diabetes and intensive insulin regimens, in which SMBG data are vital to therapy optimization. As collecting SMBG data from patients is seen anecdotally as time intensive, we determined the mean time spent by CPPs collecting patient SMBG data and its potential implications.
Methods
Pharmacists at BVAMC were asked to estimate and record the following: SMBG data collection method, time spent collecting data, extra time spent documenting or formatting SMBG readings, total patient visit time, and visit type. Time was collected in minutes. Extra time spent documenting or formatting SMBG readings included any additional time formatting or entering data in the clinical note after talking to the patient; if this was done while multitasking and talking to the patient, it was not considered extra time. For total patient visit time, pharmacists were asked to estimate only time spent discussing diabetes care and collecting SMBG data. Visit types were categorized as in-person/face-to-face, telephone, and telehealth using clinical video telehealth (CVT)/VA Video Connect (VVC). Data were collected using a standardized spreadsheet. The spreadsheet was pilot tested by a CPP before distribution to all pharmacists.
CPPs were educated about the project in March 2021 and were asked to record data for a 1-week period between April 5, 2021, and April 30, 2021. One CPP also provided delayed data collected from May 17 to 21, 2021, and these data were included in our analysis.
Descriptive statistics were used to determine the mean time spent by CPPs collecting SMBG data. Unpaired t tests were used to compare time spent collecting SMBG data by different collection methods and patient visit types. A P value of ≤ .05 was considered statistically significant. Data were organized in Microsoft Excel, and statistics were completed with JMP Pro v15.
Results
Eight CPPs provided data from 120 patient encounters. For all pa
When compared by the SMBG collection method, the longest time spent collecting SMBG data was with patient report (3.7 minutes), and the longest time spent documenting/formatting time was with meter download/home telehealth (2 minutes). There was no statistically significant difference in the time to collect SMBG data between patient report and other methods (3.7 minutes vs 2.8 minutes; P = .07).
When compared by visit type, there was not a statistically significant difference between time spent collecting SMBG data (3.8 minutes vs 3.2 minutes; P = .39) (Table 2).
Discussion
We found that the mean amount of time spent collecting and documenting/formatting SMBG data was only 4.6 minutes; however, this still represented a substantial portion of visit time. For telephone and CVT/VVC appointments, this represented > 25% of total visit time. While CPPs make important contributions to interprofessional team management of patients with diabetes, their cost is not trivial.6-8 It is worth exploring the most effective and efficient ways to use CPPs. Our results indicate that streamlining SMBG data collection may be beneficial.
Pharmacy technicians, licensed practical nurses/clinical associates, registered nurses/nurse care managers, or other team members could help improve SMBG data collection. Using other team members is also an opportunity for comanagement, for team collaboration, and for more patients to be seen. For example, if a CPP currently has 12 patient encounters that last 20 minutes each, this results in about 240 minutes of direct patient care. If patient encounters were 16 minutes, CPPS could have 15 patient encounters in 240 minutes. Saved time could be used for other clinical tasks involved in disease management or clinical reminder reviews. While there are benefits to CPPs collecting SMBG data, such as further inquiry about patient-reported values, other team members could also be trained to ask appropriate follow-up questions for abnormal blood glucose readings. In addition, leveraging current team members and optimizing their roles could prevent the need to acquire additional full-time equivalent employees.
Another opportunity to increase efficiency in SMBG data collection is with SMBG devices and EHR integration.4,9 However, integration can be difficult with different types of SMBG devices and EHR platforms. Education for patients and practitioners could help to ensure accurate and reliable data uploads; patient internet availability; data protection, privacy, and sharing; workflow management; and clear patient-practitioner expectations.10 For example, if patient SMBG data are automatically uploaded to practitioners, patients’ expectations for practitioner review of data and follow-up need to be determined.
We found a subset of patient encounters (n = 23) where data collection and documenting/formatting represented more than half of the total visit time. In this subset, 13 SMBG reports were pulled from a log or meter, 8 were patient reported, and 3 were meter download or home telehealth.
Limitations
A potential reason for the lack of statistically significant differences in SMBG collection method or visit type in this study includes the small sample size. Participation in this work was voluntary, and all participating CPPs had ≥ 3 years of practice in their current setting, which includes a heavy workload of diabetes management. These pharmacists noted self-established procedures/systems for SMBG data collection, including the use of Excel spreadsheets with pregenerated formulas. For less experienced CPPs, SMBG data collection time may be even longer. Pharmacists also noted that they may limit time spent collecting SMBG data depending on the patient encounter and whether they have gathered sufficient data to guide clinical care. Other limitations of this work include data collection from a single institution and that the time documented represented estimates; there was no external monitor.
Conclusions
In this analysis, we found that CPPs spend about 3 minutes collecting SMBG data from patients, and about an additional 1 minute documenting and formatting data. While 4 to 5 minutes may not represent a substantial amount of time for one patient, it can be when multiplied by several patient encounters. The time spent collecting SMBG data did not significantly differ by collection method or visit type. Opportunities to increase efficiency in SMBG data collection, such as the use of nonpharmacist team members are worth exploring.
Acknowledgments
Thank you to the pharmacists at the Boise Veterans Affairs Medical Center for their time and support of this work: Danielle Ahlstrom, Paul Black, Robyn Cruz, Sarah Naidoo, Anthony Nelson, Laura Spoutz, Eileen Twomey, Donovan Victorine, and Michelle Wilkin.
1. American Diabetes Association. 7. Diabetes Technology: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44(suppl 1):S85-S99. doi:10.2337/dc21-S007
2. Austin MM. The two skill sets of self-monitoring of blood glucose education: the operational and the interpretive. Diabetes Spectr. 2013;26(2):83-90. doi:10.2337/diaspect.26.2.83
3. Gallichan M. Self monitoring of glucose by people with diabetes: evidence based practice. BMJ. 1997;314(7085):964-967. doi:10.1136/bmj.314.7085.964
4. Lewinski AA, Drake C, Shaw RJ, et al. Bridging the integration gap between patient-generated blood glucose data and electronic health records. J Am Med Inform Assoc. 2019;26(7):667-672. doi:10.1093/jamia/ocz039
5. McFarland MS, Groppi J, Jorgenson T, et al. Role of the US Veterans Health Administration clinical pharmacy specialist provider: shaping the future of comprehensive medication management. Can J Hosp Pharm. 2020;73(2):152-158. doi:10.4212/cjhp.v73i2.2982
6. Schmidt K, Caudill J. Hamilton T. Impact of clinical pharmacy specialists on glycemic control in veterans with type 2 diabetes. Am J Health Syst Pharm. 2019;76(suppl 1):S9-S14. doi:10.1093/ajhp/zxy015
7. Sullivan J, Jett BP, Cradick M, Zuber J. Effect of clinical pharmacist intervention on hemoglobin A1c reduction in veteran patients with type 2 diabetes in a rural setting. Ann Pharmacother. 2016;50(12):1023-1027. doi:10.1177/1060028016663564
8. Bloom CI, Ku M, Williams M. Clinical pharmacy specialists’ impact in patient aligned care teams for type 2 diabetes management. J Am Pharm Assoc (2003). 2019;59(5):717-721. doi:10.1016/j.japh.2019.05.002
9. Kumar RB, Goren ND, Stark DE, Wall DP, Longhurst CA. Automated integration of continuous glucose monitor data in the electronic health record using consumer technology. J Am Med Inform Assoc. 2016;23(3):532-537. doi:10.1093/jamia/ocv206
10. Reading MJ, Merrill JA. Converging and diverging needs between patients and providers who are collecting and using patient-generated health data: an integrative review. J Am Med Inform Assoc. 2018;25(6):759-771. doi:10.1093/jamia/ocy006
1. American Diabetes Association. 7. Diabetes Technology: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44(suppl 1):S85-S99. doi:10.2337/dc21-S007
2. Austin MM. The two skill sets of self-monitoring of blood glucose education: the operational and the interpretive. Diabetes Spectr. 2013;26(2):83-90. doi:10.2337/diaspect.26.2.83
3. Gallichan M. Self monitoring of glucose by people with diabetes: evidence based practice. BMJ. 1997;314(7085):964-967. doi:10.1136/bmj.314.7085.964
4. Lewinski AA, Drake C, Shaw RJ, et al. Bridging the integration gap between patient-generated blood glucose data and electronic health records. J Am Med Inform Assoc. 2019;26(7):667-672. doi:10.1093/jamia/ocz039
5. McFarland MS, Groppi J, Jorgenson T, et al. Role of the US Veterans Health Administration clinical pharmacy specialist provider: shaping the future of comprehensive medication management. Can J Hosp Pharm. 2020;73(2):152-158. doi:10.4212/cjhp.v73i2.2982
6. Schmidt K, Caudill J. Hamilton T. Impact of clinical pharmacy specialists on glycemic control in veterans with type 2 diabetes. Am J Health Syst Pharm. 2019;76(suppl 1):S9-S14. doi:10.1093/ajhp/zxy015
7. Sullivan J, Jett BP, Cradick M, Zuber J. Effect of clinical pharmacist intervention on hemoglobin A1c reduction in veteran patients with type 2 diabetes in a rural setting. Ann Pharmacother. 2016;50(12):1023-1027. doi:10.1177/1060028016663564
8. Bloom CI, Ku M, Williams M. Clinical pharmacy specialists’ impact in patient aligned care teams for type 2 diabetes management. J Am Pharm Assoc (2003). 2019;59(5):717-721. doi:10.1016/j.japh.2019.05.002
9. Kumar RB, Goren ND, Stark DE, Wall DP, Longhurst CA. Automated integration of continuous glucose monitor data in the electronic health record using consumer technology. J Am Med Inform Assoc. 2016;23(3):532-537. doi:10.1093/jamia/ocv206
10. Reading MJ, Merrill JA. Converging and diverging needs between patients and providers who are collecting and using patient-generated health data: an integrative review. J Am Med Inform Assoc. 2018;25(6):759-771. doi:10.1093/jamia/ocy006
The Use of Magnets, Magnetic Fields, and Copper Devices in a Veteran Population
Complementary and alternative medicine (CAM) is a therapeutic approach to health care used in association with or in place of standard medical therapeutic approaches. When describing CAM, the terms complementary and alternative are often used interchangeably, but the terms refer to different concepts. A nonmainstream approach used together with conventional medicine is considered complementary, whereas an approach used in place of conventional medicine is considered alternative. Most people who use nonmainstream approaches also use conventional health care.1
Integrative medicine represents therapeutic interventions that bring conventional and complementary approaches together in a coordinated way. Integrative health also emphasizes multimodal interventions, which are ≥ 2 interventions such as conventional (eg, medication, physical rehabilitation, psychotherapy) and complementary health approaches (eg, acupuncture, yoga, and probiotics) in various combinations, with an emphasis on treating the whole person rather than 1 organ system. Integrative health aims for well-coordinated care among different practitioners and institutions.1
Functional medicine requires an individualized assessment and therapeutic plan for each patient, including optimizing the function of each organ system. It uses research to understand a patient’s unique needs and formulates a plan that often uses diet, exercise, and stress reduction methods. Functional medicine may use combinations of naturopathic, osteopathic, and chiropractic medicine, among other therapies. Functional medicine has been called a systems biology model, and patients and practitioners work together to achieve the highest expression of health by addressing the underlying causes of disease.2,3
According to a 2012 national survey, more than 30% of adults and about 12% of children use health care approaches that are not part of conventional medical care or that may have unconventional origins. A National Center for Health Statistics study found that the most common complementary medical interventions from 2002 to 2012 included natural products, deep breathing, yoga and other movement programs, and chiropractic, among others. Magnets, magnetic fields, and copper devices (MMFC), which are the focus of this study, were not among the top listed interventions.4 Recent data showed that individuals in the United States are high users of CAM, including many patients who have neoplastic disease.5,6
MMFCs are a part of CAM and are reported to be a billion-dollar industry worldwide, although it is not well studied.7,8 In our study, magnet refers to the use of a magnet in contact with the body, magnetic field refers to exposure to a magnetic field administered without direct contact with the body, and copper devices refer to devices that are in contact with the body, such as bracelets, necklaces, wraps, and joint braces. These devices are often constructed using copper mesh, or weaved copper wires. Advertising has helped to increase interest in the use of these devices for musculoskeletal pain and restricted joint movement therapies. However, it is less clear whether MMFCs are being used to provide therapy for other medical conditions, such as neoplastic disease.
It is unclear how widespread MMFC use is or how it is accessed. A 2016 study of veterans and CAM use did not specifically address MMFCs.9 A Japanese study of the use of CAM provided or prescribed by a physician found that just 12 of 1575 respondents (0.7%) described using magnetic therapy.10 A Korean internet study that assessed the use of CAM found that of 1668 respondents who received CAM therapy by practice or advice of a physician, 1.2% used magnet therapy.11,12 An online study of CAM use in patients with multiple sclerosis found that 9 of 1286 respondents (0.7%) had used magnetic field therapy in the previous 3 months.13
In this study, we aimed to assess MMFC use and perspectives in a veteran population at the Carl T. Hayden Veterans Affairs Medical Center (CTHVAMC) in Phoenix, Arizona.
METHODS
We created a brief questionnaire regarding MMFC use and perspectives and distributed it to veteran patients at the infusion center at the CTHVAMC. The study was approved by the CTHVAMC department of research, and the institutional review board determined that informed consent was not required. The questionnaire did not collect any specific personal identifying data but included the participant’s sex, age, and diagnosis. Although there are standardized questionnaires concerning the use of CAM, we designed a new survey for MMFCs. The participants in the study were consecutive patients referred to the CTHVAMC infusion center for IV or other nonoral therapies. Referrals came from endocrinology, gastroenterology, hematology/oncology, neurology, rheumatology, and other specialties (eg, allergy/immunology).
The questionnaire was 1 page (front and back) and was completed anonymously without involvement by the study investigators or infusion center staff. Dated and consecutively numbered questionnaires were given to patients receiving therapy regardless of their diagnosis. Ages were categorized into groups: 18 to 30 years; 31 to 50 years; 51 to 65 years; and ≥ 66 years. Diagnoses were categorized by specialty: endocrinology, gastroenterology, hematology/oncology, neurology, rheumatology, and other. We noted in a previous similar study that the exact diagnosis was often left blank, but the specialty was more often completed.9 Since some patients required multiple visits to the infusion center, respondents were asked whether they had previously answered the questionnaire; there were no duplications.
The population we studied was under stress while receiving therapy for underlying illnesses. To improve the response rate and accuracy of the responses, we limited the number of survey questions. Since many of the respondents in the infusion center for therapy received medications that could alter their ability to respond, all questionnaires were administered prior to therapeutic intervention. In addition to the background data, respondents were asked: Do you apply magnets to your body, use magnetic field therapy, or copper devices? If you use any of these therapies, is it for pain, your diagnosis, or other? Would you consider participating in a clinical trial using magnets applied to the body or magnetic therapy?
RESULTS
We collected 210 surveys. Four surveys were missing data and were excluded. The majority of respondents (n = 133, 64%) were in the hematology/oncology diagnostic group and 121 (59%) were aged ≥ 66 years (Table 1).
Respondents were asked whether they were using MMFC therapies. The results from all age groups showed an 18% overall use and in the diagnosis groups an overall use of 23%. Eighteen respondents (35%) aged 51 to 65 years reported using MMFC, followed by 6 respondents (21%) aged 31 to 50 years. Patients with an endocrinology diagnosis had the highest rate of MMFC use (6 of 11 patients; 55%) but more patients (33 of 133 [25%]) with a hematology/oncology diagnosis used MMFCs.
Copper was the most widely used MMFC therapy among individuals who used a single MMFC therapy. Twenty respondents reported copper use, 6 used magnets, and no respondents used magnetic field therapy (Table 2).
Although we were interested in understanding veterans’ use of these therapies, we were also interested in whether the respondent group would see MMFC as a potential therapy. The highest level of interest in participation in magnet clinical trials was reported by patients aged 31 to 50 years (64%) age group, followed by those aged 51 to 65 (62%). All of the respondents in hematology/oncology, rheumatology, neurology, endocrinology, and gastroenterology groups indicated that they would consider participating in clinical studies using magnets.
DISCUSSION
We surveyed a population of veterans at the CTHVAMC infusion center who were receiving antineoplastic chemotherapy, biologic therapy, immunomodulatory therapy, transfusion, and other therapies to evaluate their use of MMFC. We chose this group to sample because of how accessible this group was and the belief that there would be an adequate survey response. We hypothesized that by asking about a specific group of CAM therapies and not, as in many surveys, multiple CAM therapies, there would be an improved response rate. We expected that very few respondents would indicate MMFC use because in a similar study conducted in 2003 to 2004 at CTHVAMC, none of the 380 survey respondents (all with a hematology/oncology diagnosis) indicated magnet or magnetic field use (JR Salvatore, unpublished data). Although copper devices were available at that time, they were not included in that study. The current survey added copper devices and showed a greater use of MMFC, including copper devices. We identified veterans who used either 1 MMFC or multiple therapies. In both groups, copper devices were the most common. This may be due to the ubiquity and availability of copper devices. These devices are highly visible and promoted by professional athletes and other well-known personalities.
Our findings showed 2 unexpected results. First, there was greater than expected use of magnets and copper devices. Second, an even less expected result that there was considerable interest in participating in clinical research that used magnets or magnetic fields.
Respondents indicated a high interest in participating in clinical trials using magnets or magnetic fields regardless of their history of MMFC use. We did not ask about a trial using copper devices because there is less scientific/medical research to justify studying those devices as opposed to data that support the use of magnets or magnetic fields. The data presented in this study suggest interest in participating in clinical trials using magnets or magnetic field therapy. One clinical trial combined static magnets as an adjuvant to antineoplastic chemotherapy.14 We believe this is the first publication to specifically quantify both MMFC use in a veteran (or any) population, and to identify the desire to participate in clinical studies that would utilize magnets or magnetic fields, whether or not they currently use magnets or magnetic fields. Based on current knowledge, it is not clear whether use of MMFC by patients represents a risk or a benefit to the population studied, and seeking that information is part of the continuation of our work. We also believe that the data in this study will help practitioners to consider asking patients specifically whether they are using these therapies, and if so why and with what result. We are extending our work to a more generalized patient population.
The use of copper devices relates to beliefs (dating to the mid-1800s) that there was a relationship between copper deficiency and rheumatologic disorders. Copper devices are used as therapies because of the belief that small amounts of copper are absorbed through the skin, decreasing inflammation, particularly around joint spaces.15 Recent data suggest a mechanism for copper-induced cell death.16 Although this recent research suggests a mechanism for how copper might induce cell death, it is unclear how this would be applied to establishing a mechanism for the health effects of wearing copper devices. Since copper devices are thought to decrease inflammation, they may have a theoretical function by decreasing the number of inflammatory cells in an affected space.
CAM magnetics are typically of lower strength. The field generated by magnets is measured and reported in Tesla. Magnetic resonance imaging typically generates from 1.5 to 3 Tesla. A refrigerator magnet is about 1 milliTesla.17 In a study conducted at the CTHVAMC, the strength of the magnets used was measured at distances from the magnet. For example, at 2 cm from the magnet, the measured strength was 18 milliTesla.14 Many MMFC devices approved by the US Food and Drug Administration are pulsed electromagnetic fields (PEMF) devices for healing of nonunion fractures (approved in 1979); cervical and lumbar fusion therapies (approved in 2004); and therapy for anxiety and depression (approved in 2006).18
Limitations
Patients with endocrinology diagnoses were the most likely to use MMFCs but were a very small percentage of the infusion center population, which could skew the data. The surveyed individuals may not have been representative of the overall patient population. Similarly, the patient population at CTHVAMC, which is primarily male and aged ≥ 66 years, may not be representative of other veteran and nonveteran patient populations.
Conclusions
MMFC devices are being used regularly by patients as a form of CAM therapy, but few studies researching the use of CAM therapy have generated data that are as specific as this study is about the use of these MMFC devices. Although there is considerable general public awareness of MMFC therapies and devices, we believe that there is a need to quantify the use of these devices. We further believe that our study is one of the first to look specifically at the use of MMFCs in a veteran population. We have found a considerable use of MMFCs in the veteran population studied, and we also showed that whether or not veterans are using these devices, they are willing to be part of research that uses the devices. Further studies would look at a more general veteran population, look more in depth at the way and for what purpose these devices are being used, and consider the development of clinical research studies that use MMFCs.
1. National Institute of Health. National Center for Complementary and Integrative Health. Updated April 2021. Accessed June 26, 2023. https://www.nccih.nih.gov/health/complementary-alternative-or-integrative-health-whats-in-a-name
2. Hanaway P. Form follows function: a functional medicine overview. Perm J. 2016;20(4):16-109. doi:10.7812/TPP/16-109
3. Bland JS. Functional medicine past, present, and future. Integr Med (Encinitas). 2022;21(2):22-26.
4. Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002-2012. Natl Health Stat Report. 2015;(79):1-16.
5. Horneber M, Bueschel G, Dennert G, Less D, Ritter E, Zwahlen M. How many cancer patients use complementary and alternative medicine: a systematic review and metaanalysis. Integr Cancer Ther. 2012;11(3):187-203. doi:10.1177/1534735411423920
6. Buckner CA, Lafrenie RM, Dénommée JA, Caswell JM, Want DA. Complementary and alternative medicine use in patients before and after a cancer diagnosis. Curr Oncol. 2018;25(4):e275-e281. doi:10.3747/co.25.3884
7. Weintraub MI. Magnetic bio-stimulation in painful diabetic peripheral neuropathy: a novel intervention–a randomized, double-placebo crossover study. Am J Pain Manage. 1999; 9(1):8-17.
8. Colbert AP, Wahbeh H, Harling N, et al. Static magnetic field therapy: a critical review of treatment parameters. Evid Based Complement Alternat Med. 2009;6(2):133-139. doi:10.1093/ecam/nem131
9. Held RF, Santos S, Marki M, Helmer D. Veteran perceptions, interest, and use of complementary and alternative medicine. Fed Pract. 2016;33(9):41-47.
10. Motoo Y, Yukawa K, Arai I, Hisamura K, Tsutani K. Use of complementary and alternative medicine in Japan: a cross-sectional internet survey using the Japanese version of the International Complementary and Alternative Medicine Questionnaire. JMAJ. 2019;2(1):35-46. doi:10.31662/jmaj.2018-0044
11. Quandt SA, Verhoef MJ, Arcury TA, et al. Development of an international questionnaire to measure use of complementary and alternative medicine (I-CAM-Q). J Altern Complement Med. 2009;15(4):331-339. doi:10.1089/acm.2008.0521
12. Lee JA, Sasaki Y, Arai I, et al. An assessment of the use of complementary and alternative medicine by Korean people using an adapted version of the standardized international questionnaire (I-CAM-QK): a cross-sectional study of an internet survey. BMC Complement Altern Med. 2018;18(1):238. Published 2018 Aug 13. doi:10.1186/s12906-018-2294-6
13. Campbell E, Coulter E, Mattison P, McFadyen A, Miller L, Paul L. Access, delivery and perceived efficacy of physiotherapy and use of complementary and alternative therapies by people with progressive multiple sclerosis in the United Kingdom: An online survey. Mult Scler Relat Disord. 2017;12:64-69. doi:10.1016/j.msard.2017.01.002
14. Salvatore JR, Harrington J, Kummet T. Phase I clinical study of a static magnetic field combined with anti-neoplastic chemotherapy in the treatment of human malignancy: initial safety and toxicity data. Bioelectromagnetics. 2003;24(7):524-527. doi:10.1002/bem.10149
15. Richmond SJ, Gunadasa S, Bland M, Macpherson H. Copper bracelets and magnetic wrist straps for rheumatoid arthritis--analgesic and anti-inflammatory effects: a randomised double-blind placebo controlled crossover trial. PLoS One. 2013;8(9):e71529. Published 2013 Sep 16. doi:10.1371/journal.pone.0071529
16. Tsvetkov P, Coy S, Petrova B, et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science. 2022;375(6586):1254-1261. doi:10.1126/science.abf0529
17. Simon NJ. Biological Effects of Static Magnetic Fields: A Review. International Cryogenic Materials Commission; 1992:179.
18. Waldorff EI, Zhang N, Ryaby JT. Pulsed electromagnetic field applications: a corporate perspective. J Orthop Translat. 2017;9:60-68. Published 2017 Mar 31. doi:10.1016/j.jot.2017.02.006
Complementary and alternative medicine (CAM) is a therapeutic approach to health care used in association with or in place of standard medical therapeutic approaches. When describing CAM, the terms complementary and alternative are often used interchangeably, but the terms refer to different concepts. A nonmainstream approach used together with conventional medicine is considered complementary, whereas an approach used in place of conventional medicine is considered alternative. Most people who use nonmainstream approaches also use conventional health care.1
Integrative medicine represents therapeutic interventions that bring conventional and complementary approaches together in a coordinated way. Integrative health also emphasizes multimodal interventions, which are ≥ 2 interventions such as conventional (eg, medication, physical rehabilitation, psychotherapy) and complementary health approaches (eg, acupuncture, yoga, and probiotics) in various combinations, with an emphasis on treating the whole person rather than 1 organ system. Integrative health aims for well-coordinated care among different practitioners and institutions.1
Functional medicine requires an individualized assessment and therapeutic plan for each patient, including optimizing the function of each organ system. It uses research to understand a patient’s unique needs and formulates a plan that often uses diet, exercise, and stress reduction methods. Functional medicine may use combinations of naturopathic, osteopathic, and chiropractic medicine, among other therapies. Functional medicine has been called a systems biology model, and patients and practitioners work together to achieve the highest expression of health by addressing the underlying causes of disease.2,3
According to a 2012 national survey, more than 30% of adults and about 12% of children use health care approaches that are not part of conventional medical care or that may have unconventional origins. A National Center for Health Statistics study found that the most common complementary medical interventions from 2002 to 2012 included natural products, deep breathing, yoga and other movement programs, and chiropractic, among others. Magnets, magnetic fields, and copper devices (MMFC), which are the focus of this study, were not among the top listed interventions.4 Recent data showed that individuals in the United States are high users of CAM, including many patients who have neoplastic disease.5,6
MMFCs are a part of CAM and are reported to be a billion-dollar industry worldwide, although it is not well studied.7,8 In our study, magnet refers to the use of a magnet in contact with the body, magnetic field refers to exposure to a magnetic field administered without direct contact with the body, and copper devices refer to devices that are in contact with the body, such as bracelets, necklaces, wraps, and joint braces. These devices are often constructed using copper mesh, or weaved copper wires. Advertising has helped to increase interest in the use of these devices for musculoskeletal pain and restricted joint movement therapies. However, it is less clear whether MMFCs are being used to provide therapy for other medical conditions, such as neoplastic disease.
It is unclear how widespread MMFC use is or how it is accessed. A 2016 study of veterans and CAM use did not specifically address MMFCs.9 A Japanese study of the use of CAM provided or prescribed by a physician found that just 12 of 1575 respondents (0.7%) described using magnetic therapy.10 A Korean internet study that assessed the use of CAM found that of 1668 respondents who received CAM therapy by practice or advice of a physician, 1.2% used magnet therapy.11,12 An online study of CAM use in patients with multiple sclerosis found that 9 of 1286 respondents (0.7%) had used magnetic field therapy in the previous 3 months.13
In this study, we aimed to assess MMFC use and perspectives in a veteran population at the Carl T. Hayden Veterans Affairs Medical Center (CTHVAMC) in Phoenix, Arizona.
METHODS
We created a brief questionnaire regarding MMFC use and perspectives and distributed it to veteran patients at the infusion center at the CTHVAMC. The study was approved by the CTHVAMC department of research, and the institutional review board determined that informed consent was not required. The questionnaire did not collect any specific personal identifying data but included the participant’s sex, age, and diagnosis. Although there are standardized questionnaires concerning the use of CAM, we designed a new survey for MMFCs. The participants in the study were consecutive patients referred to the CTHVAMC infusion center for IV or other nonoral therapies. Referrals came from endocrinology, gastroenterology, hematology/oncology, neurology, rheumatology, and other specialties (eg, allergy/immunology).
The questionnaire was 1 page (front and back) and was completed anonymously without involvement by the study investigators or infusion center staff. Dated and consecutively numbered questionnaires were given to patients receiving therapy regardless of their diagnosis. Ages were categorized into groups: 18 to 30 years; 31 to 50 years; 51 to 65 years; and ≥ 66 years. Diagnoses were categorized by specialty: endocrinology, gastroenterology, hematology/oncology, neurology, rheumatology, and other. We noted in a previous similar study that the exact diagnosis was often left blank, but the specialty was more often completed.9 Since some patients required multiple visits to the infusion center, respondents were asked whether they had previously answered the questionnaire; there were no duplications.
The population we studied was under stress while receiving therapy for underlying illnesses. To improve the response rate and accuracy of the responses, we limited the number of survey questions. Since many of the respondents in the infusion center for therapy received medications that could alter their ability to respond, all questionnaires were administered prior to therapeutic intervention. In addition to the background data, respondents were asked: Do you apply magnets to your body, use magnetic field therapy, or copper devices? If you use any of these therapies, is it for pain, your diagnosis, or other? Would you consider participating in a clinical trial using magnets applied to the body or magnetic therapy?
RESULTS
We collected 210 surveys. Four surveys were missing data and were excluded. The majority of respondents (n = 133, 64%) were in the hematology/oncology diagnostic group and 121 (59%) were aged ≥ 66 years (Table 1).
Respondents were asked whether they were using MMFC therapies. The results from all age groups showed an 18% overall use and in the diagnosis groups an overall use of 23%. Eighteen respondents (35%) aged 51 to 65 years reported using MMFC, followed by 6 respondents (21%) aged 31 to 50 years. Patients with an endocrinology diagnosis had the highest rate of MMFC use (6 of 11 patients; 55%) but more patients (33 of 133 [25%]) with a hematology/oncology diagnosis used MMFCs.
Copper was the most widely used MMFC therapy among individuals who used a single MMFC therapy. Twenty respondents reported copper use, 6 used magnets, and no respondents used magnetic field therapy (Table 2).
Although we were interested in understanding veterans’ use of these therapies, we were also interested in whether the respondent group would see MMFC as a potential therapy. The highest level of interest in participation in magnet clinical trials was reported by patients aged 31 to 50 years (64%) age group, followed by those aged 51 to 65 (62%). All of the respondents in hematology/oncology, rheumatology, neurology, endocrinology, and gastroenterology groups indicated that they would consider participating in clinical studies using magnets.
DISCUSSION
We surveyed a population of veterans at the CTHVAMC infusion center who were receiving antineoplastic chemotherapy, biologic therapy, immunomodulatory therapy, transfusion, and other therapies to evaluate their use of MMFC. We chose this group to sample because of how accessible this group was and the belief that there would be an adequate survey response. We hypothesized that by asking about a specific group of CAM therapies and not, as in many surveys, multiple CAM therapies, there would be an improved response rate. We expected that very few respondents would indicate MMFC use because in a similar study conducted in 2003 to 2004 at CTHVAMC, none of the 380 survey respondents (all with a hematology/oncology diagnosis) indicated magnet or magnetic field use (JR Salvatore, unpublished data). Although copper devices were available at that time, they were not included in that study. The current survey added copper devices and showed a greater use of MMFC, including copper devices. We identified veterans who used either 1 MMFC or multiple therapies. In both groups, copper devices were the most common. This may be due to the ubiquity and availability of copper devices. These devices are highly visible and promoted by professional athletes and other well-known personalities.
Our findings showed 2 unexpected results. First, there was greater than expected use of magnets and copper devices. Second, an even less expected result that there was considerable interest in participating in clinical research that used magnets or magnetic fields.
Respondents indicated a high interest in participating in clinical trials using magnets or magnetic fields regardless of their history of MMFC use. We did not ask about a trial using copper devices because there is less scientific/medical research to justify studying those devices as opposed to data that support the use of magnets or magnetic fields. The data presented in this study suggest interest in participating in clinical trials using magnets or magnetic field therapy. One clinical trial combined static magnets as an adjuvant to antineoplastic chemotherapy.14 We believe this is the first publication to specifically quantify both MMFC use in a veteran (or any) population, and to identify the desire to participate in clinical studies that would utilize magnets or magnetic fields, whether or not they currently use magnets or magnetic fields. Based on current knowledge, it is not clear whether use of MMFC by patients represents a risk or a benefit to the population studied, and seeking that information is part of the continuation of our work. We also believe that the data in this study will help practitioners to consider asking patients specifically whether they are using these therapies, and if so why and with what result. We are extending our work to a more generalized patient population.
The use of copper devices relates to beliefs (dating to the mid-1800s) that there was a relationship between copper deficiency and rheumatologic disorders. Copper devices are used as therapies because of the belief that small amounts of copper are absorbed through the skin, decreasing inflammation, particularly around joint spaces.15 Recent data suggest a mechanism for copper-induced cell death.16 Although this recent research suggests a mechanism for how copper might induce cell death, it is unclear how this would be applied to establishing a mechanism for the health effects of wearing copper devices. Since copper devices are thought to decrease inflammation, they may have a theoretical function by decreasing the number of inflammatory cells in an affected space.
CAM magnetics are typically of lower strength. The field generated by magnets is measured and reported in Tesla. Magnetic resonance imaging typically generates from 1.5 to 3 Tesla. A refrigerator magnet is about 1 milliTesla.17 In a study conducted at the CTHVAMC, the strength of the magnets used was measured at distances from the magnet. For example, at 2 cm from the magnet, the measured strength was 18 milliTesla.14 Many MMFC devices approved by the US Food and Drug Administration are pulsed electromagnetic fields (PEMF) devices for healing of nonunion fractures (approved in 1979); cervical and lumbar fusion therapies (approved in 2004); and therapy for anxiety and depression (approved in 2006).18
Limitations
Patients with endocrinology diagnoses were the most likely to use MMFCs but were a very small percentage of the infusion center population, which could skew the data. The surveyed individuals may not have been representative of the overall patient population. Similarly, the patient population at CTHVAMC, which is primarily male and aged ≥ 66 years, may not be representative of other veteran and nonveteran patient populations.
Conclusions
MMFC devices are being used regularly by patients as a form of CAM therapy, but few studies researching the use of CAM therapy have generated data that are as specific as this study is about the use of these MMFC devices. Although there is considerable general public awareness of MMFC therapies and devices, we believe that there is a need to quantify the use of these devices. We further believe that our study is one of the first to look specifically at the use of MMFCs in a veteran population. We have found a considerable use of MMFCs in the veteran population studied, and we also showed that whether or not veterans are using these devices, they are willing to be part of research that uses the devices. Further studies would look at a more general veteran population, look more in depth at the way and for what purpose these devices are being used, and consider the development of clinical research studies that use MMFCs.
Complementary and alternative medicine (CAM) is a therapeutic approach to health care used in association with or in place of standard medical therapeutic approaches. When describing CAM, the terms complementary and alternative are often used interchangeably, but the terms refer to different concepts. A nonmainstream approach used together with conventional medicine is considered complementary, whereas an approach used in place of conventional medicine is considered alternative. Most people who use nonmainstream approaches also use conventional health care.1
Integrative medicine represents therapeutic interventions that bring conventional and complementary approaches together in a coordinated way. Integrative health also emphasizes multimodal interventions, which are ≥ 2 interventions such as conventional (eg, medication, physical rehabilitation, psychotherapy) and complementary health approaches (eg, acupuncture, yoga, and probiotics) in various combinations, with an emphasis on treating the whole person rather than 1 organ system. Integrative health aims for well-coordinated care among different practitioners and institutions.1
Functional medicine requires an individualized assessment and therapeutic plan for each patient, including optimizing the function of each organ system. It uses research to understand a patient’s unique needs and formulates a plan that often uses diet, exercise, and stress reduction methods. Functional medicine may use combinations of naturopathic, osteopathic, and chiropractic medicine, among other therapies. Functional medicine has been called a systems biology model, and patients and practitioners work together to achieve the highest expression of health by addressing the underlying causes of disease.2,3
According to a 2012 national survey, more than 30% of adults and about 12% of children use health care approaches that are not part of conventional medical care or that may have unconventional origins. A National Center for Health Statistics study found that the most common complementary medical interventions from 2002 to 2012 included natural products, deep breathing, yoga and other movement programs, and chiropractic, among others. Magnets, magnetic fields, and copper devices (MMFC), which are the focus of this study, were not among the top listed interventions.4 Recent data showed that individuals in the United States are high users of CAM, including many patients who have neoplastic disease.5,6
MMFCs are a part of CAM and are reported to be a billion-dollar industry worldwide, although it is not well studied.7,8 In our study, magnet refers to the use of a magnet in contact with the body, magnetic field refers to exposure to a magnetic field administered without direct contact with the body, and copper devices refer to devices that are in contact with the body, such as bracelets, necklaces, wraps, and joint braces. These devices are often constructed using copper mesh, or weaved copper wires. Advertising has helped to increase interest in the use of these devices for musculoskeletal pain and restricted joint movement therapies. However, it is less clear whether MMFCs are being used to provide therapy for other medical conditions, such as neoplastic disease.
It is unclear how widespread MMFC use is or how it is accessed. A 2016 study of veterans and CAM use did not specifically address MMFCs.9 A Japanese study of the use of CAM provided or prescribed by a physician found that just 12 of 1575 respondents (0.7%) described using magnetic therapy.10 A Korean internet study that assessed the use of CAM found that of 1668 respondents who received CAM therapy by practice or advice of a physician, 1.2% used magnet therapy.11,12 An online study of CAM use in patients with multiple sclerosis found that 9 of 1286 respondents (0.7%) had used magnetic field therapy in the previous 3 months.13
In this study, we aimed to assess MMFC use and perspectives in a veteran population at the Carl T. Hayden Veterans Affairs Medical Center (CTHVAMC) in Phoenix, Arizona.
METHODS
We created a brief questionnaire regarding MMFC use and perspectives and distributed it to veteran patients at the infusion center at the CTHVAMC. The study was approved by the CTHVAMC department of research, and the institutional review board determined that informed consent was not required. The questionnaire did not collect any specific personal identifying data but included the participant’s sex, age, and diagnosis. Although there are standardized questionnaires concerning the use of CAM, we designed a new survey for MMFCs. The participants in the study were consecutive patients referred to the CTHVAMC infusion center for IV or other nonoral therapies. Referrals came from endocrinology, gastroenterology, hematology/oncology, neurology, rheumatology, and other specialties (eg, allergy/immunology).
The questionnaire was 1 page (front and back) and was completed anonymously without involvement by the study investigators or infusion center staff. Dated and consecutively numbered questionnaires were given to patients receiving therapy regardless of their diagnosis. Ages were categorized into groups: 18 to 30 years; 31 to 50 years; 51 to 65 years; and ≥ 66 years. Diagnoses were categorized by specialty: endocrinology, gastroenterology, hematology/oncology, neurology, rheumatology, and other. We noted in a previous similar study that the exact diagnosis was often left blank, but the specialty was more often completed.9 Since some patients required multiple visits to the infusion center, respondents were asked whether they had previously answered the questionnaire; there were no duplications.
The population we studied was under stress while receiving therapy for underlying illnesses. To improve the response rate and accuracy of the responses, we limited the number of survey questions. Since many of the respondents in the infusion center for therapy received medications that could alter their ability to respond, all questionnaires were administered prior to therapeutic intervention. In addition to the background data, respondents were asked: Do you apply magnets to your body, use magnetic field therapy, or copper devices? If you use any of these therapies, is it for pain, your diagnosis, or other? Would you consider participating in a clinical trial using magnets applied to the body or magnetic therapy?
RESULTS
We collected 210 surveys. Four surveys were missing data and were excluded. The majority of respondents (n = 133, 64%) were in the hematology/oncology diagnostic group and 121 (59%) were aged ≥ 66 years (Table 1).
Respondents were asked whether they were using MMFC therapies. The results from all age groups showed an 18% overall use and in the diagnosis groups an overall use of 23%. Eighteen respondents (35%) aged 51 to 65 years reported using MMFC, followed by 6 respondents (21%) aged 31 to 50 years. Patients with an endocrinology diagnosis had the highest rate of MMFC use (6 of 11 patients; 55%) but more patients (33 of 133 [25%]) with a hematology/oncology diagnosis used MMFCs.
Copper was the most widely used MMFC therapy among individuals who used a single MMFC therapy. Twenty respondents reported copper use, 6 used magnets, and no respondents used magnetic field therapy (Table 2).
Although we were interested in understanding veterans’ use of these therapies, we were also interested in whether the respondent group would see MMFC as a potential therapy. The highest level of interest in participation in magnet clinical trials was reported by patients aged 31 to 50 years (64%) age group, followed by those aged 51 to 65 (62%). All of the respondents in hematology/oncology, rheumatology, neurology, endocrinology, and gastroenterology groups indicated that they would consider participating in clinical studies using magnets.
DISCUSSION
We surveyed a population of veterans at the CTHVAMC infusion center who were receiving antineoplastic chemotherapy, biologic therapy, immunomodulatory therapy, transfusion, and other therapies to evaluate their use of MMFC. We chose this group to sample because of how accessible this group was and the belief that there would be an adequate survey response. We hypothesized that by asking about a specific group of CAM therapies and not, as in many surveys, multiple CAM therapies, there would be an improved response rate. We expected that very few respondents would indicate MMFC use because in a similar study conducted in 2003 to 2004 at CTHVAMC, none of the 380 survey respondents (all with a hematology/oncology diagnosis) indicated magnet or magnetic field use (JR Salvatore, unpublished data). Although copper devices were available at that time, they were not included in that study. The current survey added copper devices and showed a greater use of MMFC, including copper devices. We identified veterans who used either 1 MMFC or multiple therapies. In both groups, copper devices were the most common. This may be due to the ubiquity and availability of copper devices. These devices are highly visible and promoted by professional athletes and other well-known personalities.
Our findings showed 2 unexpected results. First, there was greater than expected use of magnets and copper devices. Second, an even less expected result that there was considerable interest in participating in clinical research that used magnets or magnetic fields.
Respondents indicated a high interest in participating in clinical trials using magnets or magnetic fields regardless of their history of MMFC use. We did not ask about a trial using copper devices because there is less scientific/medical research to justify studying those devices as opposed to data that support the use of magnets or magnetic fields. The data presented in this study suggest interest in participating in clinical trials using magnets or magnetic field therapy. One clinical trial combined static magnets as an adjuvant to antineoplastic chemotherapy.14 We believe this is the first publication to specifically quantify both MMFC use in a veteran (or any) population, and to identify the desire to participate in clinical studies that would utilize magnets or magnetic fields, whether or not they currently use magnets or magnetic fields. Based on current knowledge, it is not clear whether use of MMFC by patients represents a risk or a benefit to the population studied, and seeking that information is part of the continuation of our work. We also believe that the data in this study will help practitioners to consider asking patients specifically whether they are using these therapies, and if so why and with what result. We are extending our work to a more generalized patient population.
The use of copper devices relates to beliefs (dating to the mid-1800s) that there was a relationship between copper deficiency and rheumatologic disorders. Copper devices are used as therapies because of the belief that small amounts of copper are absorbed through the skin, decreasing inflammation, particularly around joint spaces.15 Recent data suggest a mechanism for copper-induced cell death.16 Although this recent research suggests a mechanism for how copper might induce cell death, it is unclear how this would be applied to establishing a mechanism for the health effects of wearing copper devices. Since copper devices are thought to decrease inflammation, they may have a theoretical function by decreasing the number of inflammatory cells in an affected space.
CAM magnetics are typically of lower strength. The field generated by magnets is measured and reported in Tesla. Magnetic resonance imaging typically generates from 1.5 to 3 Tesla. A refrigerator magnet is about 1 milliTesla.17 In a study conducted at the CTHVAMC, the strength of the magnets used was measured at distances from the magnet. For example, at 2 cm from the magnet, the measured strength was 18 milliTesla.14 Many MMFC devices approved by the US Food and Drug Administration are pulsed electromagnetic fields (PEMF) devices for healing of nonunion fractures (approved in 1979); cervical and lumbar fusion therapies (approved in 2004); and therapy for anxiety and depression (approved in 2006).18
Limitations
Patients with endocrinology diagnoses were the most likely to use MMFCs but were a very small percentage of the infusion center population, which could skew the data. The surveyed individuals may not have been representative of the overall patient population. Similarly, the patient population at CTHVAMC, which is primarily male and aged ≥ 66 years, may not be representative of other veteran and nonveteran patient populations.
Conclusions
MMFC devices are being used regularly by patients as a form of CAM therapy, but few studies researching the use of CAM therapy have generated data that are as specific as this study is about the use of these MMFC devices. Although there is considerable general public awareness of MMFC therapies and devices, we believe that there is a need to quantify the use of these devices. We further believe that our study is one of the first to look specifically at the use of MMFCs in a veteran population. We have found a considerable use of MMFCs in the veteran population studied, and we also showed that whether or not veterans are using these devices, they are willing to be part of research that uses the devices. Further studies would look at a more general veteran population, look more in depth at the way and for what purpose these devices are being used, and consider the development of clinical research studies that use MMFCs.
1. National Institute of Health. National Center for Complementary and Integrative Health. Updated April 2021. Accessed June 26, 2023. https://www.nccih.nih.gov/health/complementary-alternative-or-integrative-health-whats-in-a-name
2. Hanaway P. Form follows function: a functional medicine overview. Perm J. 2016;20(4):16-109. doi:10.7812/TPP/16-109
3. Bland JS. Functional medicine past, present, and future. Integr Med (Encinitas). 2022;21(2):22-26.
4. Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002-2012. Natl Health Stat Report. 2015;(79):1-16.
5. Horneber M, Bueschel G, Dennert G, Less D, Ritter E, Zwahlen M. How many cancer patients use complementary and alternative medicine: a systematic review and metaanalysis. Integr Cancer Ther. 2012;11(3):187-203. doi:10.1177/1534735411423920
6. Buckner CA, Lafrenie RM, Dénommée JA, Caswell JM, Want DA. Complementary and alternative medicine use in patients before and after a cancer diagnosis. Curr Oncol. 2018;25(4):e275-e281. doi:10.3747/co.25.3884
7. Weintraub MI. Magnetic bio-stimulation in painful diabetic peripheral neuropathy: a novel intervention–a randomized, double-placebo crossover study. Am J Pain Manage. 1999; 9(1):8-17.
8. Colbert AP, Wahbeh H, Harling N, et al. Static magnetic field therapy: a critical review of treatment parameters. Evid Based Complement Alternat Med. 2009;6(2):133-139. doi:10.1093/ecam/nem131
9. Held RF, Santos S, Marki M, Helmer D. Veteran perceptions, interest, and use of complementary and alternative medicine. Fed Pract. 2016;33(9):41-47.
10. Motoo Y, Yukawa K, Arai I, Hisamura K, Tsutani K. Use of complementary and alternative medicine in Japan: a cross-sectional internet survey using the Japanese version of the International Complementary and Alternative Medicine Questionnaire. JMAJ. 2019;2(1):35-46. doi:10.31662/jmaj.2018-0044
11. Quandt SA, Verhoef MJ, Arcury TA, et al. Development of an international questionnaire to measure use of complementary and alternative medicine (I-CAM-Q). J Altern Complement Med. 2009;15(4):331-339. doi:10.1089/acm.2008.0521
12. Lee JA, Sasaki Y, Arai I, et al. An assessment of the use of complementary and alternative medicine by Korean people using an adapted version of the standardized international questionnaire (I-CAM-QK): a cross-sectional study of an internet survey. BMC Complement Altern Med. 2018;18(1):238. Published 2018 Aug 13. doi:10.1186/s12906-018-2294-6
13. Campbell E, Coulter E, Mattison P, McFadyen A, Miller L, Paul L. Access, delivery and perceived efficacy of physiotherapy and use of complementary and alternative therapies by people with progressive multiple sclerosis in the United Kingdom: An online survey. Mult Scler Relat Disord. 2017;12:64-69. doi:10.1016/j.msard.2017.01.002
14. Salvatore JR, Harrington J, Kummet T. Phase I clinical study of a static magnetic field combined with anti-neoplastic chemotherapy in the treatment of human malignancy: initial safety and toxicity data. Bioelectromagnetics. 2003;24(7):524-527. doi:10.1002/bem.10149
15. Richmond SJ, Gunadasa S, Bland M, Macpherson H. Copper bracelets and magnetic wrist straps for rheumatoid arthritis--analgesic and anti-inflammatory effects: a randomised double-blind placebo controlled crossover trial. PLoS One. 2013;8(9):e71529. Published 2013 Sep 16. doi:10.1371/journal.pone.0071529
16. Tsvetkov P, Coy S, Petrova B, et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science. 2022;375(6586):1254-1261. doi:10.1126/science.abf0529
17. Simon NJ. Biological Effects of Static Magnetic Fields: A Review. International Cryogenic Materials Commission; 1992:179.
18. Waldorff EI, Zhang N, Ryaby JT. Pulsed electromagnetic field applications: a corporate perspective. J Orthop Translat. 2017;9:60-68. Published 2017 Mar 31. doi:10.1016/j.jot.2017.02.006
1. National Institute of Health. National Center for Complementary and Integrative Health. Updated April 2021. Accessed June 26, 2023. https://www.nccih.nih.gov/health/complementary-alternative-or-integrative-health-whats-in-a-name
2. Hanaway P. Form follows function: a functional medicine overview. Perm J. 2016;20(4):16-109. doi:10.7812/TPP/16-109
3. Bland JS. Functional medicine past, present, and future. Integr Med (Encinitas). 2022;21(2):22-26.
4. Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002-2012. Natl Health Stat Report. 2015;(79):1-16.
5. Horneber M, Bueschel G, Dennert G, Less D, Ritter E, Zwahlen M. How many cancer patients use complementary and alternative medicine: a systematic review and metaanalysis. Integr Cancer Ther. 2012;11(3):187-203. doi:10.1177/1534735411423920
6. Buckner CA, Lafrenie RM, Dénommée JA, Caswell JM, Want DA. Complementary and alternative medicine use in patients before and after a cancer diagnosis. Curr Oncol. 2018;25(4):e275-e281. doi:10.3747/co.25.3884
7. Weintraub MI. Magnetic bio-stimulation in painful diabetic peripheral neuropathy: a novel intervention–a randomized, double-placebo crossover study. Am J Pain Manage. 1999; 9(1):8-17.
8. Colbert AP, Wahbeh H, Harling N, et al. Static magnetic field therapy: a critical review of treatment parameters. Evid Based Complement Alternat Med. 2009;6(2):133-139. doi:10.1093/ecam/nem131
9. Held RF, Santos S, Marki M, Helmer D. Veteran perceptions, interest, and use of complementary and alternative medicine. Fed Pract. 2016;33(9):41-47.
10. Motoo Y, Yukawa K, Arai I, Hisamura K, Tsutani K. Use of complementary and alternative medicine in Japan: a cross-sectional internet survey using the Japanese version of the International Complementary and Alternative Medicine Questionnaire. JMAJ. 2019;2(1):35-46. doi:10.31662/jmaj.2018-0044
11. Quandt SA, Verhoef MJ, Arcury TA, et al. Development of an international questionnaire to measure use of complementary and alternative medicine (I-CAM-Q). J Altern Complement Med. 2009;15(4):331-339. doi:10.1089/acm.2008.0521
12. Lee JA, Sasaki Y, Arai I, et al. An assessment of the use of complementary and alternative medicine by Korean people using an adapted version of the standardized international questionnaire (I-CAM-QK): a cross-sectional study of an internet survey. BMC Complement Altern Med. 2018;18(1):238. Published 2018 Aug 13. doi:10.1186/s12906-018-2294-6
13. Campbell E, Coulter E, Mattison P, McFadyen A, Miller L, Paul L. Access, delivery and perceived efficacy of physiotherapy and use of complementary and alternative therapies by people with progressive multiple sclerosis in the United Kingdom: An online survey. Mult Scler Relat Disord. 2017;12:64-69. doi:10.1016/j.msard.2017.01.002
14. Salvatore JR, Harrington J, Kummet T. Phase I clinical study of a static magnetic field combined with anti-neoplastic chemotherapy in the treatment of human malignancy: initial safety and toxicity data. Bioelectromagnetics. 2003;24(7):524-527. doi:10.1002/bem.10149
15. Richmond SJ, Gunadasa S, Bland M, Macpherson H. Copper bracelets and magnetic wrist straps for rheumatoid arthritis--analgesic and anti-inflammatory effects: a randomised double-blind placebo controlled crossover trial. PLoS One. 2013;8(9):e71529. Published 2013 Sep 16. doi:10.1371/journal.pone.0071529
16. Tsvetkov P, Coy S, Petrova B, et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science. 2022;375(6586):1254-1261. doi:10.1126/science.abf0529
17. Simon NJ. Biological Effects of Static Magnetic Fields: A Review. International Cryogenic Materials Commission; 1992:179.
18. Waldorff EI, Zhang N, Ryaby JT. Pulsed electromagnetic field applications: a corporate perspective. J Orthop Translat. 2017;9:60-68. Published 2017 Mar 31. doi:10.1016/j.jot.2017.02.006
Race and Age-Related PSA Testing Disparities in Spinal Cord Injured Men: Analysis of National Veterans Health Administration Data
Prostate cancer will be diagnosed in 12.5% of men during their lifetime. It is the most commonly diagnosed solid organ cancer in men.1 However, prostate cancer screening for prostate-specific antigen (PSA) remains controversial due to concerns about overdiagnosis, as the overall risk of dying of prostate cancer is only 2.4%.1
To address the risk and benefits of PSA testing, in 2012 the US Preventive Services Task Force (USPSTF) recommended against routine PSA testing.2 Updated 2018 recommendations continued this recommendation in men aged > 70 years but acknowledged a small potential benefit in men aged 55 to 69 years and suggested individualized shared decision making between patient and clinician.3 In addition, American Urological Association (AUA) guidelines for the early detection of prostate cancer recommend against PSA screening in men aged < 40 years or those aged > 70 years, shared decision making for individuals aged 55 to 70 years or in high-risk men aged 40 to 55 years (ie, family history of prostate cancer or African American race).4 PSA screening is not recommended for men with a life expectancy shorter than 10 to 15 years aged > 70 years.4
The Veterans Health Administration (VHA) is the largest integrated health care system in the US.5 In addition, the US Department of Veterans Affairs (VA) Spinal Cord Injury and Disorders System of Care operates 25 centers throughout the US.6 Life expectancy following spinal cord injury (SCI) increased significantly through the 1980s but has since plateaued, with life expectancy being impacted by age at injury, completeness of injury, and neurologic level.7,8 As part of a program of uniform care, all persons with SCI followed at the Spinal Cord Injury and Disorders System of Care centers are offered comprehensive annual evaluations, including screening laboratory tests, such as PSA level.9
Patients with SCI present a unique challenge when interpreting PSA levels, given potentially confounding factors, including neurogenic bladder management, high rates of bacteriuria, urinary tract infections (UTIs), testosterone deficiency, and pelvic innervation that differs from the noninjured population.10,11 Unfortunately, the literature on prostate cancer prevalence and average PSA levels in patients with SCI is limited by the small scope of studies and inconsistent data.10-16 Therefore, the purpose of the current investigation was to quantify and analyze the rates of annual PSA testing for all men with SCI in the VHA.
Methods
Approval was granted by the Richmond VA Medical Center (VAMC) Institutional Review Board in Virginia, and by the VA Informatics and Computing Infrastructure (VINCI) data access request tracker system for extraction of data from the VA Corporate Data Warehouse. Microsoft Structured Query Language was used for data programming and query design. Statistical analysis was conducted using Stata version 15.1 with assistance from professional biostatisticians.
Only male veterans with a nervous system disorder affecting the spinal cord or with myelopathy were included, based on International Classification of Diseases (ICD) version 9 and 10 codes, corresponding to traumatic and nontraumatic myelopathy. Veterans diagnosed with myelopathy based on ICD codes corresponding to progressive or degenerative myelopathies, such as multiple sclerosis or amyotrophic lateral sclerosis, were excluded.
For each veteran, extracted data included the unique identification number, date of birth, ICD code, date ICD code first appeared, race, gender, death status (yes/no), date of death (when applicable), date of each PSA test, PSA test values, and the VAMC where each test was performed. Only tests for total PSA were included. The date that the ICD code first appeared served as an approximation for the date of SCI. The time frame for the study included all PSA tests in the VINCI database for 2000 through 2017. However, only post-SCI PSA tests were included in the analysis. Duplicate tests (same date/time) were eliminated.
Race is considered a risk factor for prostate cancer only for African American patients, likely due to racial health disparities.17 Given this, we chose to categorize race as either African American or other, with a third category for missing/inconsistent reporting. Age at time of the PSA test was categorized into 4 groups (≤ 39, 40-54, 55-69, and ≥ 70 years) based on AUA guidelines.4 The annual PSA testing rate was calculated for each veteran with SCI as the number of PSA tests per year. A mean annual PSA test rate was then calculated as the weighted (by exposure time) mean value for all annual PSA testing rates from 2000 through 2017 for each age group and race. Annual exposure was calculated for each veteran and defined as the number of days a veteran was eligible to have a PSA test. This started with the date of SCI diagnosis and ended with either the date of death or the date of last PSA. If a veteran moved from one age group to another in 1 year, the first part of this year’s exposure was included in the calculation of the annual PSA testing rate for the younger group and the second part was included for the calculation of the older group. For deceased veterans, the death date was excluded from the exposure period, and their exposure period ended on the day before death.
Statistical Analysis
To compare PSA testing rates between African American race and other races, Poisson regression was used with exposure treated as an offset (exposures were summed across years for each veteran). An indicator (dummy) variable for African American race vs other races was coded, and statistical significance was set at P < .05. To check sensitivity for the Poisson assumption that the mean was equal to the variance, negative binomial regression was used. To assess for geographic PSA testing rate variability, the data were further analyzed based on the locations where PSA tests were performed. This subanalysis was limited to veterans who had all PSA tests in a single station. For each station, the average PSA testing rate was calculated for each veteran, and the mean for all annual PSA testing rates was used to determine station-specific PSA testing rates.
Results
A total of 45,274 veterans were initially identified of which 367 females were excluded (Figure 1).
The PSA testing rate rose for veterans in the age groups ≤ 39, 40 to 54, and 55 to 69 years (Figure 2A).
Of the cohort of 37,243 veterans, 28,396 (76.2%) had their post-SCI tests done at a single facility, 6770 (18.1%) at 2 locations, and 2077 (5.5%) at > 2 locations. Single-station group data were included in a subanalysis to determine the mean (SD) PSA testing rates, which for the 123 locations was 0.98 (0.36) tests per veteran per year (range, 0.2-3.0 tests per veteran per year).
To assess the impact of the 2012 USPSTF recommendations on PSA testing rates in veterans with SCI, mean PSA testing rates were calculated for 5 years before the recommendations (2007-2011) and compared with the average PSA testing rate for 5 years following the updated recommendations (2013-2017). The USPSTF updated its recommendation again in 2018 and acknowledged the potential benefit for PSA screening in certain patient populations.2,3 Surprisingly, and despite recommendations, the results show a significant increase in PSA testing rates in all age groups for all races (P < .001) (Figure 4).
Discussion
The goal of this study was to establish testing rates and analyze PSA testing trends across races and age groups in veterans with SCI. This is the largest cohort of patients with SCI analyzed in the literature. The key findings of this study were that despite clear AUA guidelines recommending against PSA testing in patients aged ≤ 39 years and ≥ 70 years, there are high rates of testing in veterans with SCI in these age groups (0.46 tests per year in those aged ≤ 39 years and 0.91 tests per year in those aged ≥ 70 years). In terms of race, as expected based on increased risk,
Prostate Cancer Incidence
Although the exact mechanism behind alterations in prostate function in the SCI population have yet to be fully elucidated, research suggests that the prostate behaves differently after SCI. Animal models of prostate gland denervation show decreased prostate volume and suggest that SCI may lead to a reduction in prostatic secretory function associated with autonomic dysfunction. Shim and colleagues hypothesized that impaired autonomic prostate innervation alters the prostatic volume and PSA in patients with SCI.10
Additional studies looking at actual PSA levels in men with SCI reveal conflicting data.10-15,20 Toricelli and colleagues retrospectively studied 140 men with SCI, of whom 34 had PSA levels available and found that mean PSA was not significantly different for patients with SCI compared with controls, but patients using clean intermittent catheterization had 2-fold higher PSA levels.21 In contrast, Konety and colleagues found that mean PSA was not significantly different from uninjured controls in their cohort of 79 patients with SCI, though they did find a correlation between indwelling catheter use and a higher PSA.22
Studies have shown an overall decreased risk of prostate cancer in patients with SCI, though the mechanism remains unclear. A large cohort study from Taiwan showed a lower risk of prostate cancer for 54,401 patients with SCI with an adjusted hazard ratio of 0.73.23 Patel and colleagues found the overall rate of prostate cancer in the population of veterans with SCI was lower than the general uninjured VA population, though this study was limited by scope with only 350 patients with SCI.24 A more recent systematic review and meta-analysis of 9 studies evaluating the prevalence of prostate cancer in men with SCI found a reduction of up to 65% in the risk of prostate cancer in men with SCI, and PSA was found to be a poor screening tool for prostate cancer due to large study heterogeneity.16
PSA Screening
This study identified widespread overscreening using the PSA test in veterans with SCI, which is likely attributable to many factors. Per VHA Directive 1176, all eligible veterans are offered yearly interdisciplinary comprehensive evaluations, including laboratory testing, and as such veterans with SCI have high rates of annual visit attendance due to the complexity of their care.9 PSA testing is included in the standard battery of laboratory tests ordered for all patients with SCI during their annual examinations. Additionally, many SCI specialists use the PSA level in patients with SCI for identifying cystitis or prostatitis in patients with colonization who may not experience typical symptoms. Everaert and colleagues demonstrated the clinical utility for localizing UTIs to the upper or lower tract, with elevated PSA indicating prostatitis. They found that serum PSA has a sensitivity of 68% and a specificity of 100% in the differential diagnosis of prostatitis and pyelonephritis.25 As such, the high PSA screening rates may be reflective of diagnostic use for infection rather than for cancer screening.
Likely as a response to the USPSTF recommendations, there has been a national slow decline in overall PSA screening rates since 2012.26-28 A study from Vetterlein and colleagues examining changes in the PSA screening trends related to USPSTF recommendations found an 8.5% decline in overall PSA screening from 2012 to 2014.29 However, the increase in PSA testing across all ages and races in the VA population with SCI over the same period is not entirely understood and suggests the need for further research and education in this area.
Limitations
This study is limited by the use of data identified by ICD codes rather than by review of individual health records. This required the use of decision algorithms for data points, such as the date of SCI. In addition, analysis was not able to capture shared decision making that may have contributed to PSA screening outside the recommended age ranges based on additional risk factors, such as family history of lethal malignancy. Furthermore, a detailed attempt to define specific age-adjusted PSA levels was beyond the scope of this study but will be addressed in later publications. In addition, we did not exclude individuals with a diagnosis of prostate adenocarcinoma, prostatitis, or recurrent UTIs because the onset, duration, and severity of disease could not be definitively ascertained. Finally, veterans with SCI are unique and may not be reflective of individuals with SCI who do not receive care within the VA. However, despite these limitations, this is, to our knowledge, the largest and most comprehensive study evaluating PSA testing rates in individuals with SCI.
Conclusions
Currently, PSA screening is recommended following shared decision making for patients at average risk aged 55 to 70 years. Patients with SCI experience many conditions that may affect PSA values, but data regarding normal PSA ranges and rates of prostate cancer in this population remain sparse. The study demonstrated high rates of overtesting in veterans with SCI, higher than expected testing rates in African American veterans, a paradoxical increase in PSA testing rates after the 2012 publication of the USPSTF PSA guidelines, and wide variability in testing rates depending on VA location.
African American men were tested at higher rates across all age groups, including in patients aged > 70 years. To balance the benefits of detecting clinically significant prostate cancer vs the risks of invasive testing in high-risk populations with SCI, more work is needed to determine the clinical impact of screening practices. Future work is currently ongoing to define age-based PSA values in patients with SCI.
Acknowledgments
This research was supported in part through funding from the Center for Rehabilitation Science and Engineering, Virginia Commonwealth University Health System.
1. American Cancer Society. Key statistics for prostate cancer. Updated January 12, 2023. Accessed June 2, 2023. https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html
2. Moyer VA; U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(2):120-134. doi:10.7326/0003-4819-157-2-201207170-00459
3. US Preventive Services Task Force, Grossman DC, Curry SJ, et al. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319(18):1901-1913. doi:10.1001/jama.2018.3710
4. Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA Guideline. J Urol. 2013;190(2):419-426. doi:10.1016/j.juro.2013.04.119
5. US Department of Veterans Affairs, Veterans Health Administration. Updated August 15, 2022. Accessed June 2, 2023. https://www.va.gov/health/aboutVHA.asp
6. US Department of Veterans Affairs. Spinal cord injuries and disorders system of care. Updated January 31, 2022. Accessed June 2, 2023. https://www.sci.va.gov/VAs_SCID_System_of_Care.asp
7. DeVivo MJ, Chen Y, Wen H. Cause of death trends among persons with spinal cord injury in the United States: 1960-2017. Arch Phys Med Rehabil. 2022;103(4):634-641. doi:10.1016/j.apmr.2021.09.019
8. Cao Y, DiPiro N, Krause JS. Health factors and spinal cord injury: a prospective study of risk of cause-specific mortality. Spinal Cord. 2019;57(7):594-602. doi:10.1038/s41393-019-0264-6
9. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1176(2): Spinal Cord Injuries and Disorders System of Care. Published September 30, 2019. Accessed June 2, 2023. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8523
10. Shim HB, Jung TY, Lee JK, Ku JH. Prostate activity and prostate cancer in spinal cord injury. Prostate Cancer Prostatic Dis. 2006;9(2):115-120. doi:10.1038/sj.pcan.4500865
11. Lynne CM, Aballa TC, Wang TJ, Rittenhouse HG, Ferrell SM, Brackett NL. Serum and semen prostate specific antigen concentrations are different in young spinal cord injured men compared to normal controls. J Urol. 1999;162(1):89-91. doi:10.1097/00005392-199907000-00022
12. Bartoletti R, Gavazzi A, Cai T, et al. Prostate growth and prevalence of prostate diseases in early onset spinal cord injuries. Eur Urol. 2009;56(1):142-148. doi:10.1016/j.eururo.2008.01.088
13. Pannek J, Berges RR, Cubick G, Meindl R, Senge T. Prostate size and PSA serum levels in male patients with spinal cord injury. Urology. 2003;62(5):845-848. doi:10.1016/s0090-4295(03)00654-x
14. Pramudji CK, Mutchnik SE, DeConcini D, Boone TB. Prostate cancer screening with prostate specific antigen in spinal cord injured men. J Urol. 2002;167(3):1303-1305.
15. Alexandrino AP, Rodrigues MA, Matsuo T. Evaluation of serum and seminal levels of prostate specific antigen in men with spinal cord injury. J Urol. 2004;171(6 Pt 1):2230-2232. doi:10.1097/01.ju.0000125241.77517.10
16. Barbonetti A, D’Andrea S, Martorella A, Felzani G, Francavilla S, Francavilla F. Risk of prostate cancer in men with spinal cord injury: a systematic review and meta-analysis. Asian J Androl. 2018;20(6):555-560. doi:10.4103/aja.aja_31_18
17. Vince RA Jr, Jiang R, Bank M, et al. Evaluation of social determinants of health and prostate cancer outcomes among black and white patients: a systematic review and meta-analysis. JAMA Netw Open. 2023;6(1):e2250416. Published 2023 Jan 3. doi:10.1001/jamanetworkopen.2022.50416
18. Smith ZL, Eggener SE, Murphy AB. African-American prostate cancer disparities. Curr Urol Rep. 2017;18(10):81. Published 2017 Aug 14. doi:10.1007/s11934-017-0724-5
19. Jeong SH, Werneburg GT, Abouassaly R, Wood H. Acquired and congenital spinal cord injury is associated with lower likelihood of prostate specific antigen screening. Urology. 2022;164:178-183. doi:10.1016/j.urology.2022.01.044
20. Benaim EA, Montoya JD, Saboorian MH, Litwiller S, Roehrborn CG. Characterization of prostate size, PSA and endocrine profiles in patients with spinal cord injuries. Prostate Cancer Prostatic Dis. 1998;1(5):250-255. doi:10.1038/sj.pcan.4500246
21. Torricelli FC, Lucon M, Vicentini F, Gomes CM, Srougi M, Bruschini H. PSA levels in men with spinal cord injury and under intermittent catheterization. Neurourol Urodyn. 2011;30(8):1522-1524. doi:10.1002/nau.21119
22. Konety BR, Nguyen TT, Brenes G, et al. Evaluation of the effect of spinal cord injury on serum PSA levels. Urology. 2000;56(1):82-86. doi:10.1016/s0090-4295(00)00548-3
23. Lee WY, Sun LM, Lin CL, et al. Risk of prostate and bladder cancers in patients with spinal cord injury: a population-based cohort study. Urol Oncol. 2014;32(1):51.e1-51.e517. doi:10.1016/j.urolonc.2013.07.019
24. Patel N, Ngo K, Hastings J, Ketchum N, Sepahpanah F. Prevalence of prostate cancer in patients with chronic spinal cord injury. PM R. 2011;3(7):633-636. doi:10.1016/j.pmrj.2011.04.024
25. Everaert K, Oostra C, Delanghe J, Vande Walle J, Van Laere M, Oosterlinck W. Diagnosis and localization of a complicated urinary tract infection in neurogenic bladder disease by tubular proteinuria and serum prostate specific antigen. Spinal Cord. 1998;36(1):33-38. doi:10.1038/sj.sc.3100520
26. Drazer MW, Huo D, Eggener SE. National prostate cancer screening rates after the 2012 US Preventive Services Task Force recommendation discouraging prostate-specific antigen-based screening. J Clin Oncol. 2015;33(22):2416-2423. doi:10.1200/JCO.2015.61.6532
27. Sammon JD, Abdollah F, Choueiri TK, et al. Prostate-specific antigen screening after 2012 US Preventive Services Task Force recommendations. JAMA. 2015;314(19):2077-2079. doi:10.1001/jama.2015.7273
28. Jemal A, Fedewa SA, Ma J, et al. Prostate cancer incidence and PSA testing patterns in relation to USPSTF screening recommendations. JAMA. 2015;314(19):2054-2061. doi:10.1001/jama.2015.14905
29. Vetterlein MW, Dalela D, Sammon JD, et al. State-by-state variation in prostate-specific antigen screening trends following the 2011 United States Preventive Services Task Force panel update. Urology. 2018;112:56-65. doi:10.1016/j.urology.2017.08.055
Prostate cancer will be diagnosed in 12.5% of men during their lifetime. It is the most commonly diagnosed solid organ cancer in men.1 However, prostate cancer screening for prostate-specific antigen (PSA) remains controversial due to concerns about overdiagnosis, as the overall risk of dying of prostate cancer is only 2.4%.1
To address the risk and benefits of PSA testing, in 2012 the US Preventive Services Task Force (USPSTF) recommended against routine PSA testing.2 Updated 2018 recommendations continued this recommendation in men aged > 70 years but acknowledged a small potential benefit in men aged 55 to 69 years and suggested individualized shared decision making between patient and clinician.3 In addition, American Urological Association (AUA) guidelines for the early detection of prostate cancer recommend against PSA screening in men aged < 40 years or those aged > 70 years, shared decision making for individuals aged 55 to 70 years or in high-risk men aged 40 to 55 years (ie, family history of prostate cancer or African American race).4 PSA screening is not recommended for men with a life expectancy shorter than 10 to 15 years aged > 70 years.4
The Veterans Health Administration (VHA) is the largest integrated health care system in the US.5 In addition, the US Department of Veterans Affairs (VA) Spinal Cord Injury and Disorders System of Care operates 25 centers throughout the US.6 Life expectancy following spinal cord injury (SCI) increased significantly through the 1980s but has since plateaued, with life expectancy being impacted by age at injury, completeness of injury, and neurologic level.7,8 As part of a program of uniform care, all persons with SCI followed at the Spinal Cord Injury and Disorders System of Care centers are offered comprehensive annual evaluations, including screening laboratory tests, such as PSA level.9
Patients with SCI present a unique challenge when interpreting PSA levels, given potentially confounding factors, including neurogenic bladder management, high rates of bacteriuria, urinary tract infections (UTIs), testosterone deficiency, and pelvic innervation that differs from the noninjured population.10,11 Unfortunately, the literature on prostate cancer prevalence and average PSA levels in patients with SCI is limited by the small scope of studies and inconsistent data.10-16 Therefore, the purpose of the current investigation was to quantify and analyze the rates of annual PSA testing for all men with SCI in the VHA.
Methods
Approval was granted by the Richmond VA Medical Center (VAMC) Institutional Review Board in Virginia, and by the VA Informatics and Computing Infrastructure (VINCI) data access request tracker system for extraction of data from the VA Corporate Data Warehouse. Microsoft Structured Query Language was used for data programming and query design. Statistical analysis was conducted using Stata version 15.1 with assistance from professional biostatisticians.
Only male veterans with a nervous system disorder affecting the spinal cord or with myelopathy were included, based on International Classification of Diseases (ICD) version 9 and 10 codes, corresponding to traumatic and nontraumatic myelopathy. Veterans diagnosed with myelopathy based on ICD codes corresponding to progressive or degenerative myelopathies, such as multiple sclerosis or amyotrophic lateral sclerosis, were excluded.
For each veteran, extracted data included the unique identification number, date of birth, ICD code, date ICD code first appeared, race, gender, death status (yes/no), date of death (when applicable), date of each PSA test, PSA test values, and the VAMC where each test was performed. Only tests for total PSA were included. The date that the ICD code first appeared served as an approximation for the date of SCI. The time frame for the study included all PSA tests in the VINCI database for 2000 through 2017. However, only post-SCI PSA tests were included in the analysis. Duplicate tests (same date/time) were eliminated.
Race is considered a risk factor for prostate cancer only for African American patients, likely due to racial health disparities.17 Given this, we chose to categorize race as either African American or other, with a third category for missing/inconsistent reporting. Age at time of the PSA test was categorized into 4 groups (≤ 39, 40-54, 55-69, and ≥ 70 years) based on AUA guidelines.4 The annual PSA testing rate was calculated for each veteran with SCI as the number of PSA tests per year. A mean annual PSA test rate was then calculated as the weighted (by exposure time) mean value for all annual PSA testing rates from 2000 through 2017 for each age group and race. Annual exposure was calculated for each veteran and defined as the number of days a veteran was eligible to have a PSA test. This started with the date of SCI diagnosis and ended with either the date of death or the date of last PSA. If a veteran moved from one age group to another in 1 year, the first part of this year’s exposure was included in the calculation of the annual PSA testing rate for the younger group and the second part was included for the calculation of the older group. For deceased veterans, the death date was excluded from the exposure period, and their exposure period ended on the day before death.
Statistical Analysis
To compare PSA testing rates between African American race and other races, Poisson regression was used with exposure treated as an offset (exposures were summed across years for each veteran). An indicator (dummy) variable for African American race vs other races was coded, and statistical significance was set at P < .05. To check sensitivity for the Poisson assumption that the mean was equal to the variance, negative binomial regression was used. To assess for geographic PSA testing rate variability, the data were further analyzed based on the locations where PSA tests were performed. This subanalysis was limited to veterans who had all PSA tests in a single station. For each station, the average PSA testing rate was calculated for each veteran, and the mean for all annual PSA testing rates was used to determine station-specific PSA testing rates.
Results
A total of 45,274 veterans were initially identified of which 367 females were excluded (Figure 1).
The PSA testing rate rose for veterans in the age groups ≤ 39, 40 to 54, and 55 to 69 years (Figure 2A).
Of the cohort of 37,243 veterans, 28,396 (76.2%) had their post-SCI tests done at a single facility, 6770 (18.1%) at 2 locations, and 2077 (5.5%) at > 2 locations. Single-station group data were included in a subanalysis to determine the mean (SD) PSA testing rates, which for the 123 locations was 0.98 (0.36) tests per veteran per year (range, 0.2-3.0 tests per veteran per year).
To assess the impact of the 2012 USPSTF recommendations on PSA testing rates in veterans with SCI, mean PSA testing rates were calculated for 5 years before the recommendations (2007-2011) and compared with the average PSA testing rate for 5 years following the updated recommendations (2013-2017). The USPSTF updated its recommendation again in 2018 and acknowledged the potential benefit for PSA screening in certain patient populations.2,3 Surprisingly, and despite recommendations, the results show a significant increase in PSA testing rates in all age groups for all races (P < .001) (Figure 4).
Discussion
The goal of this study was to establish testing rates and analyze PSA testing trends across races and age groups in veterans with SCI. This is the largest cohort of patients with SCI analyzed in the literature. The key findings of this study were that despite clear AUA guidelines recommending against PSA testing in patients aged ≤ 39 years and ≥ 70 years, there are high rates of testing in veterans with SCI in these age groups (0.46 tests per year in those aged ≤ 39 years and 0.91 tests per year in those aged ≥ 70 years). In terms of race, as expected based on increased risk,
Prostate Cancer Incidence
Although the exact mechanism behind alterations in prostate function in the SCI population have yet to be fully elucidated, research suggests that the prostate behaves differently after SCI. Animal models of prostate gland denervation show decreased prostate volume and suggest that SCI may lead to a reduction in prostatic secretory function associated with autonomic dysfunction. Shim and colleagues hypothesized that impaired autonomic prostate innervation alters the prostatic volume and PSA in patients with SCI.10
Additional studies looking at actual PSA levels in men with SCI reveal conflicting data.10-15,20 Toricelli and colleagues retrospectively studied 140 men with SCI, of whom 34 had PSA levels available and found that mean PSA was not significantly different for patients with SCI compared with controls, but patients using clean intermittent catheterization had 2-fold higher PSA levels.21 In contrast, Konety and colleagues found that mean PSA was not significantly different from uninjured controls in their cohort of 79 patients with SCI, though they did find a correlation between indwelling catheter use and a higher PSA.22
Studies have shown an overall decreased risk of prostate cancer in patients with SCI, though the mechanism remains unclear. A large cohort study from Taiwan showed a lower risk of prostate cancer for 54,401 patients with SCI with an adjusted hazard ratio of 0.73.23 Patel and colleagues found the overall rate of prostate cancer in the population of veterans with SCI was lower than the general uninjured VA population, though this study was limited by scope with only 350 patients with SCI.24 A more recent systematic review and meta-analysis of 9 studies evaluating the prevalence of prostate cancer in men with SCI found a reduction of up to 65% in the risk of prostate cancer in men with SCI, and PSA was found to be a poor screening tool for prostate cancer due to large study heterogeneity.16
PSA Screening
This study identified widespread overscreening using the PSA test in veterans with SCI, which is likely attributable to many factors. Per VHA Directive 1176, all eligible veterans are offered yearly interdisciplinary comprehensive evaluations, including laboratory testing, and as such veterans with SCI have high rates of annual visit attendance due to the complexity of their care.9 PSA testing is included in the standard battery of laboratory tests ordered for all patients with SCI during their annual examinations. Additionally, many SCI specialists use the PSA level in patients with SCI for identifying cystitis or prostatitis in patients with colonization who may not experience typical symptoms. Everaert and colleagues demonstrated the clinical utility for localizing UTIs to the upper or lower tract, with elevated PSA indicating prostatitis. They found that serum PSA has a sensitivity of 68% and a specificity of 100% in the differential diagnosis of prostatitis and pyelonephritis.25 As such, the high PSA screening rates may be reflective of diagnostic use for infection rather than for cancer screening.
Likely as a response to the USPSTF recommendations, there has been a national slow decline in overall PSA screening rates since 2012.26-28 A study from Vetterlein and colleagues examining changes in the PSA screening trends related to USPSTF recommendations found an 8.5% decline in overall PSA screening from 2012 to 2014.29 However, the increase in PSA testing across all ages and races in the VA population with SCI over the same period is not entirely understood and suggests the need for further research and education in this area.
Limitations
This study is limited by the use of data identified by ICD codes rather than by review of individual health records. This required the use of decision algorithms for data points, such as the date of SCI. In addition, analysis was not able to capture shared decision making that may have contributed to PSA screening outside the recommended age ranges based on additional risk factors, such as family history of lethal malignancy. Furthermore, a detailed attempt to define specific age-adjusted PSA levels was beyond the scope of this study but will be addressed in later publications. In addition, we did not exclude individuals with a diagnosis of prostate adenocarcinoma, prostatitis, or recurrent UTIs because the onset, duration, and severity of disease could not be definitively ascertained. Finally, veterans with SCI are unique and may not be reflective of individuals with SCI who do not receive care within the VA. However, despite these limitations, this is, to our knowledge, the largest and most comprehensive study evaluating PSA testing rates in individuals with SCI.
Conclusions
Currently, PSA screening is recommended following shared decision making for patients at average risk aged 55 to 70 years. Patients with SCI experience many conditions that may affect PSA values, but data regarding normal PSA ranges and rates of prostate cancer in this population remain sparse. The study demonstrated high rates of overtesting in veterans with SCI, higher than expected testing rates in African American veterans, a paradoxical increase in PSA testing rates after the 2012 publication of the USPSTF PSA guidelines, and wide variability in testing rates depending on VA location.
African American men were tested at higher rates across all age groups, including in patients aged > 70 years. To balance the benefits of detecting clinically significant prostate cancer vs the risks of invasive testing in high-risk populations with SCI, more work is needed to determine the clinical impact of screening practices. Future work is currently ongoing to define age-based PSA values in patients with SCI.
Acknowledgments
This research was supported in part through funding from the Center for Rehabilitation Science and Engineering, Virginia Commonwealth University Health System.
Prostate cancer will be diagnosed in 12.5% of men during their lifetime. It is the most commonly diagnosed solid organ cancer in men.1 However, prostate cancer screening for prostate-specific antigen (PSA) remains controversial due to concerns about overdiagnosis, as the overall risk of dying of prostate cancer is only 2.4%.1
To address the risk and benefits of PSA testing, in 2012 the US Preventive Services Task Force (USPSTF) recommended against routine PSA testing.2 Updated 2018 recommendations continued this recommendation in men aged > 70 years but acknowledged a small potential benefit in men aged 55 to 69 years and suggested individualized shared decision making between patient and clinician.3 In addition, American Urological Association (AUA) guidelines for the early detection of prostate cancer recommend against PSA screening in men aged < 40 years or those aged > 70 years, shared decision making for individuals aged 55 to 70 years or in high-risk men aged 40 to 55 years (ie, family history of prostate cancer or African American race).4 PSA screening is not recommended for men with a life expectancy shorter than 10 to 15 years aged > 70 years.4
The Veterans Health Administration (VHA) is the largest integrated health care system in the US.5 In addition, the US Department of Veterans Affairs (VA) Spinal Cord Injury and Disorders System of Care operates 25 centers throughout the US.6 Life expectancy following spinal cord injury (SCI) increased significantly through the 1980s but has since plateaued, with life expectancy being impacted by age at injury, completeness of injury, and neurologic level.7,8 As part of a program of uniform care, all persons with SCI followed at the Spinal Cord Injury and Disorders System of Care centers are offered comprehensive annual evaluations, including screening laboratory tests, such as PSA level.9
Patients with SCI present a unique challenge when interpreting PSA levels, given potentially confounding factors, including neurogenic bladder management, high rates of bacteriuria, urinary tract infections (UTIs), testosterone deficiency, and pelvic innervation that differs from the noninjured population.10,11 Unfortunately, the literature on prostate cancer prevalence and average PSA levels in patients with SCI is limited by the small scope of studies and inconsistent data.10-16 Therefore, the purpose of the current investigation was to quantify and analyze the rates of annual PSA testing for all men with SCI in the VHA.
Methods
Approval was granted by the Richmond VA Medical Center (VAMC) Institutional Review Board in Virginia, and by the VA Informatics and Computing Infrastructure (VINCI) data access request tracker system for extraction of data from the VA Corporate Data Warehouse. Microsoft Structured Query Language was used for data programming and query design. Statistical analysis was conducted using Stata version 15.1 with assistance from professional biostatisticians.
Only male veterans with a nervous system disorder affecting the spinal cord or with myelopathy were included, based on International Classification of Diseases (ICD) version 9 and 10 codes, corresponding to traumatic and nontraumatic myelopathy. Veterans diagnosed with myelopathy based on ICD codes corresponding to progressive or degenerative myelopathies, such as multiple sclerosis or amyotrophic lateral sclerosis, were excluded.
For each veteran, extracted data included the unique identification number, date of birth, ICD code, date ICD code first appeared, race, gender, death status (yes/no), date of death (when applicable), date of each PSA test, PSA test values, and the VAMC where each test was performed. Only tests for total PSA were included. The date that the ICD code first appeared served as an approximation for the date of SCI. The time frame for the study included all PSA tests in the VINCI database for 2000 through 2017. However, only post-SCI PSA tests were included in the analysis. Duplicate tests (same date/time) were eliminated.
Race is considered a risk factor for prostate cancer only for African American patients, likely due to racial health disparities.17 Given this, we chose to categorize race as either African American or other, with a third category for missing/inconsistent reporting. Age at time of the PSA test was categorized into 4 groups (≤ 39, 40-54, 55-69, and ≥ 70 years) based on AUA guidelines.4 The annual PSA testing rate was calculated for each veteran with SCI as the number of PSA tests per year. A mean annual PSA test rate was then calculated as the weighted (by exposure time) mean value for all annual PSA testing rates from 2000 through 2017 for each age group and race. Annual exposure was calculated for each veteran and defined as the number of days a veteran was eligible to have a PSA test. This started with the date of SCI diagnosis and ended with either the date of death or the date of last PSA. If a veteran moved from one age group to another in 1 year, the first part of this year’s exposure was included in the calculation of the annual PSA testing rate for the younger group and the second part was included for the calculation of the older group. For deceased veterans, the death date was excluded from the exposure period, and their exposure period ended on the day before death.
Statistical Analysis
To compare PSA testing rates between African American race and other races, Poisson regression was used with exposure treated as an offset (exposures were summed across years for each veteran). An indicator (dummy) variable for African American race vs other races was coded, and statistical significance was set at P < .05. To check sensitivity for the Poisson assumption that the mean was equal to the variance, negative binomial regression was used. To assess for geographic PSA testing rate variability, the data were further analyzed based on the locations where PSA tests were performed. This subanalysis was limited to veterans who had all PSA tests in a single station. For each station, the average PSA testing rate was calculated for each veteran, and the mean for all annual PSA testing rates was used to determine station-specific PSA testing rates.
Results
A total of 45,274 veterans were initially identified of which 367 females were excluded (Figure 1).
The PSA testing rate rose for veterans in the age groups ≤ 39, 40 to 54, and 55 to 69 years (Figure 2A).
Of the cohort of 37,243 veterans, 28,396 (76.2%) had their post-SCI tests done at a single facility, 6770 (18.1%) at 2 locations, and 2077 (5.5%) at > 2 locations. Single-station group data were included in a subanalysis to determine the mean (SD) PSA testing rates, which for the 123 locations was 0.98 (0.36) tests per veteran per year (range, 0.2-3.0 tests per veteran per year).
To assess the impact of the 2012 USPSTF recommendations on PSA testing rates in veterans with SCI, mean PSA testing rates were calculated for 5 years before the recommendations (2007-2011) and compared with the average PSA testing rate for 5 years following the updated recommendations (2013-2017). The USPSTF updated its recommendation again in 2018 and acknowledged the potential benefit for PSA screening in certain patient populations.2,3 Surprisingly, and despite recommendations, the results show a significant increase in PSA testing rates in all age groups for all races (P < .001) (Figure 4).
Discussion
The goal of this study was to establish testing rates and analyze PSA testing trends across races and age groups in veterans with SCI. This is the largest cohort of patients with SCI analyzed in the literature. The key findings of this study were that despite clear AUA guidelines recommending against PSA testing in patients aged ≤ 39 years and ≥ 70 years, there are high rates of testing in veterans with SCI in these age groups (0.46 tests per year in those aged ≤ 39 years and 0.91 tests per year in those aged ≥ 70 years). In terms of race, as expected based on increased risk,
Prostate Cancer Incidence
Although the exact mechanism behind alterations in prostate function in the SCI population have yet to be fully elucidated, research suggests that the prostate behaves differently after SCI. Animal models of prostate gland denervation show decreased prostate volume and suggest that SCI may lead to a reduction in prostatic secretory function associated with autonomic dysfunction. Shim and colleagues hypothesized that impaired autonomic prostate innervation alters the prostatic volume and PSA in patients with SCI.10
Additional studies looking at actual PSA levels in men with SCI reveal conflicting data.10-15,20 Toricelli and colleagues retrospectively studied 140 men with SCI, of whom 34 had PSA levels available and found that mean PSA was not significantly different for patients with SCI compared with controls, but patients using clean intermittent catheterization had 2-fold higher PSA levels.21 In contrast, Konety and colleagues found that mean PSA was not significantly different from uninjured controls in their cohort of 79 patients with SCI, though they did find a correlation between indwelling catheter use and a higher PSA.22
Studies have shown an overall decreased risk of prostate cancer in patients with SCI, though the mechanism remains unclear. A large cohort study from Taiwan showed a lower risk of prostate cancer for 54,401 patients with SCI with an adjusted hazard ratio of 0.73.23 Patel and colleagues found the overall rate of prostate cancer in the population of veterans with SCI was lower than the general uninjured VA population, though this study was limited by scope with only 350 patients with SCI.24 A more recent systematic review and meta-analysis of 9 studies evaluating the prevalence of prostate cancer in men with SCI found a reduction of up to 65% in the risk of prostate cancer in men with SCI, and PSA was found to be a poor screening tool for prostate cancer due to large study heterogeneity.16
PSA Screening
This study identified widespread overscreening using the PSA test in veterans with SCI, which is likely attributable to many factors. Per VHA Directive 1176, all eligible veterans are offered yearly interdisciplinary comprehensive evaluations, including laboratory testing, and as such veterans with SCI have high rates of annual visit attendance due to the complexity of their care.9 PSA testing is included in the standard battery of laboratory tests ordered for all patients with SCI during their annual examinations. Additionally, many SCI specialists use the PSA level in patients with SCI for identifying cystitis or prostatitis in patients with colonization who may not experience typical symptoms. Everaert and colleagues demonstrated the clinical utility for localizing UTIs to the upper or lower tract, with elevated PSA indicating prostatitis. They found that serum PSA has a sensitivity of 68% and a specificity of 100% in the differential diagnosis of prostatitis and pyelonephritis.25 As such, the high PSA screening rates may be reflective of diagnostic use for infection rather than for cancer screening.
Likely as a response to the USPSTF recommendations, there has been a national slow decline in overall PSA screening rates since 2012.26-28 A study from Vetterlein and colleagues examining changes in the PSA screening trends related to USPSTF recommendations found an 8.5% decline in overall PSA screening from 2012 to 2014.29 However, the increase in PSA testing across all ages and races in the VA population with SCI over the same period is not entirely understood and suggests the need for further research and education in this area.
Limitations
This study is limited by the use of data identified by ICD codes rather than by review of individual health records. This required the use of decision algorithms for data points, such as the date of SCI. In addition, analysis was not able to capture shared decision making that may have contributed to PSA screening outside the recommended age ranges based on additional risk factors, such as family history of lethal malignancy. Furthermore, a detailed attempt to define specific age-adjusted PSA levels was beyond the scope of this study but will be addressed in later publications. In addition, we did not exclude individuals with a diagnosis of prostate adenocarcinoma, prostatitis, or recurrent UTIs because the onset, duration, and severity of disease could not be definitively ascertained. Finally, veterans with SCI are unique and may not be reflective of individuals with SCI who do not receive care within the VA. However, despite these limitations, this is, to our knowledge, the largest and most comprehensive study evaluating PSA testing rates in individuals with SCI.
Conclusions
Currently, PSA screening is recommended following shared decision making for patients at average risk aged 55 to 70 years. Patients with SCI experience many conditions that may affect PSA values, but data regarding normal PSA ranges and rates of prostate cancer in this population remain sparse. The study demonstrated high rates of overtesting in veterans with SCI, higher than expected testing rates in African American veterans, a paradoxical increase in PSA testing rates after the 2012 publication of the USPSTF PSA guidelines, and wide variability in testing rates depending on VA location.
African American men were tested at higher rates across all age groups, including in patients aged > 70 years. To balance the benefits of detecting clinically significant prostate cancer vs the risks of invasive testing in high-risk populations with SCI, more work is needed to determine the clinical impact of screening practices. Future work is currently ongoing to define age-based PSA values in patients with SCI.
Acknowledgments
This research was supported in part through funding from the Center for Rehabilitation Science and Engineering, Virginia Commonwealth University Health System.
1. American Cancer Society. Key statistics for prostate cancer. Updated January 12, 2023. Accessed June 2, 2023. https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html
2. Moyer VA; U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(2):120-134. doi:10.7326/0003-4819-157-2-201207170-00459
3. US Preventive Services Task Force, Grossman DC, Curry SJ, et al. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319(18):1901-1913. doi:10.1001/jama.2018.3710
4. Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA Guideline. J Urol. 2013;190(2):419-426. doi:10.1016/j.juro.2013.04.119
5. US Department of Veterans Affairs, Veterans Health Administration. Updated August 15, 2022. Accessed June 2, 2023. https://www.va.gov/health/aboutVHA.asp
6. US Department of Veterans Affairs. Spinal cord injuries and disorders system of care. Updated January 31, 2022. Accessed June 2, 2023. https://www.sci.va.gov/VAs_SCID_System_of_Care.asp
7. DeVivo MJ, Chen Y, Wen H. Cause of death trends among persons with spinal cord injury in the United States: 1960-2017. Arch Phys Med Rehabil. 2022;103(4):634-641. doi:10.1016/j.apmr.2021.09.019
8. Cao Y, DiPiro N, Krause JS. Health factors and spinal cord injury: a prospective study of risk of cause-specific mortality. Spinal Cord. 2019;57(7):594-602. doi:10.1038/s41393-019-0264-6
9. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1176(2): Spinal Cord Injuries and Disorders System of Care. Published September 30, 2019. Accessed June 2, 2023. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8523
10. Shim HB, Jung TY, Lee JK, Ku JH. Prostate activity and prostate cancer in spinal cord injury. Prostate Cancer Prostatic Dis. 2006;9(2):115-120. doi:10.1038/sj.pcan.4500865
11. Lynne CM, Aballa TC, Wang TJ, Rittenhouse HG, Ferrell SM, Brackett NL. Serum and semen prostate specific antigen concentrations are different in young spinal cord injured men compared to normal controls. J Urol. 1999;162(1):89-91. doi:10.1097/00005392-199907000-00022
12. Bartoletti R, Gavazzi A, Cai T, et al. Prostate growth and prevalence of prostate diseases in early onset spinal cord injuries. Eur Urol. 2009;56(1):142-148. doi:10.1016/j.eururo.2008.01.088
13. Pannek J, Berges RR, Cubick G, Meindl R, Senge T. Prostate size and PSA serum levels in male patients with spinal cord injury. Urology. 2003;62(5):845-848. doi:10.1016/s0090-4295(03)00654-x
14. Pramudji CK, Mutchnik SE, DeConcini D, Boone TB. Prostate cancer screening with prostate specific antigen in spinal cord injured men. J Urol. 2002;167(3):1303-1305.
15. Alexandrino AP, Rodrigues MA, Matsuo T. Evaluation of serum and seminal levels of prostate specific antigen in men with spinal cord injury. J Urol. 2004;171(6 Pt 1):2230-2232. doi:10.1097/01.ju.0000125241.77517.10
16. Barbonetti A, D’Andrea S, Martorella A, Felzani G, Francavilla S, Francavilla F. Risk of prostate cancer in men with spinal cord injury: a systematic review and meta-analysis. Asian J Androl. 2018;20(6):555-560. doi:10.4103/aja.aja_31_18
17. Vince RA Jr, Jiang R, Bank M, et al. Evaluation of social determinants of health and prostate cancer outcomes among black and white patients: a systematic review and meta-analysis. JAMA Netw Open. 2023;6(1):e2250416. Published 2023 Jan 3. doi:10.1001/jamanetworkopen.2022.50416
18. Smith ZL, Eggener SE, Murphy AB. African-American prostate cancer disparities. Curr Urol Rep. 2017;18(10):81. Published 2017 Aug 14. doi:10.1007/s11934-017-0724-5
19. Jeong SH, Werneburg GT, Abouassaly R, Wood H. Acquired and congenital spinal cord injury is associated with lower likelihood of prostate specific antigen screening. Urology. 2022;164:178-183. doi:10.1016/j.urology.2022.01.044
20. Benaim EA, Montoya JD, Saboorian MH, Litwiller S, Roehrborn CG. Characterization of prostate size, PSA and endocrine profiles in patients with spinal cord injuries. Prostate Cancer Prostatic Dis. 1998;1(5):250-255. doi:10.1038/sj.pcan.4500246
21. Torricelli FC, Lucon M, Vicentini F, Gomes CM, Srougi M, Bruschini H. PSA levels in men with spinal cord injury and under intermittent catheterization. Neurourol Urodyn. 2011;30(8):1522-1524. doi:10.1002/nau.21119
22. Konety BR, Nguyen TT, Brenes G, et al. Evaluation of the effect of spinal cord injury on serum PSA levels. Urology. 2000;56(1):82-86. doi:10.1016/s0090-4295(00)00548-3
23. Lee WY, Sun LM, Lin CL, et al. Risk of prostate and bladder cancers in patients with spinal cord injury: a population-based cohort study. Urol Oncol. 2014;32(1):51.e1-51.e517. doi:10.1016/j.urolonc.2013.07.019
24. Patel N, Ngo K, Hastings J, Ketchum N, Sepahpanah F. Prevalence of prostate cancer in patients with chronic spinal cord injury. PM R. 2011;3(7):633-636. doi:10.1016/j.pmrj.2011.04.024
25. Everaert K, Oostra C, Delanghe J, Vande Walle J, Van Laere M, Oosterlinck W. Diagnosis and localization of a complicated urinary tract infection in neurogenic bladder disease by tubular proteinuria and serum prostate specific antigen. Spinal Cord. 1998;36(1):33-38. doi:10.1038/sj.sc.3100520
26. Drazer MW, Huo D, Eggener SE. National prostate cancer screening rates after the 2012 US Preventive Services Task Force recommendation discouraging prostate-specific antigen-based screening. J Clin Oncol. 2015;33(22):2416-2423. doi:10.1200/JCO.2015.61.6532
27. Sammon JD, Abdollah F, Choueiri TK, et al. Prostate-specific antigen screening after 2012 US Preventive Services Task Force recommendations. JAMA. 2015;314(19):2077-2079. doi:10.1001/jama.2015.7273
28. Jemal A, Fedewa SA, Ma J, et al. Prostate cancer incidence and PSA testing patterns in relation to USPSTF screening recommendations. JAMA. 2015;314(19):2054-2061. doi:10.1001/jama.2015.14905
29. Vetterlein MW, Dalela D, Sammon JD, et al. State-by-state variation in prostate-specific antigen screening trends following the 2011 United States Preventive Services Task Force panel update. Urology. 2018;112:56-65. doi:10.1016/j.urology.2017.08.055
1. American Cancer Society. Key statistics for prostate cancer. Updated January 12, 2023. Accessed June 2, 2023. https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html
2. Moyer VA; U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(2):120-134. doi:10.7326/0003-4819-157-2-201207170-00459
3. US Preventive Services Task Force, Grossman DC, Curry SJ, et al. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319(18):1901-1913. doi:10.1001/jama.2018.3710
4. Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA Guideline. J Urol. 2013;190(2):419-426. doi:10.1016/j.juro.2013.04.119
5. US Department of Veterans Affairs, Veterans Health Administration. Updated August 15, 2022. Accessed June 2, 2023. https://www.va.gov/health/aboutVHA.asp
6. US Department of Veterans Affairs. Spinal cord injuries and disorders system of care. Updated January 31, 2022. Accessed June 2, 2023. https://www.sci.va.gov/VAs_SCID_System_of_Care.asp
7. DeVivo MJ, Chen Y, Wen H. Cause of death trends among persons with spinal cord injury in the United States: 1960-2017. Arch Phys Med Rehabil. 2022;103(4):634-641. doi:10.1016/j.apmr.2021.09.019
8. Cao Y, DiPiro N, Krause JS. Health factors and spinal cord injury: a prospective study of risk of cause-specific mortality. Spinal Cord. 2019;57(7):594-602. doi:10.1038/s41393-019-0264-6
9. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1176(2): Spinal Cord Injuries and Disorders System of Care. Published September 30, 2019. Accessed June 2, 2023. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8523
10. Shim HB, Jung TY, Lee JK, Ku JH. Prostate activity and prostate cancer in spinal cord injury. Prostate Cancer Prostatic Dis. 2006;9(2):115-120. doi:10.1038/sj.pcan.4500865
11. Lynne CM, Aballa TC, Wang TJ, Rittenhouse HG, Ferrell SM, Brackett NL. Serum and semen prostate specific antigen concentrations are different in young spinal cord injured men compared to normal controls. J Urol. 1999;162(1):89-91. doi:10.1097/00005392-199907000-00022
12. Bartoletti R, Gavazzi A, Cai T, et al. Prostate growth and prevalence of prostate diseases in early onset spinal cord injuries. Eur Urol. 2009;56(1):142-148. doi:10.1016/j.eururo.2008.01.088
13. Pannek J, Berges RR, Cubick G, Meindl R, Senge T. Prostate size and PSA serum levels in male patients with spinal cord injury. Urology. 2003;62(5):845-848. doi:10.1016/s0090-4295(03)00654-x
14. Pramudji CK, Mutchnik SE, DeConcini D, Boone TB. Prostate cancer screening with prostate specific antigen in spinal cord injured men. J Urol. 2002;167(3):1303-1305.
15. Alexandrino AP, Rodrigues MA, Matsuo T. Evaluation of serum and seminal levels of prostate specific antigen in men with spinal cord injury. J Urol. 2004;171(6 Pt 1):2230-2232. doi:10.1097/01.ju.0000125241.77517.10
16. Barbonetti A, D’Andrea S, Martorella A, Felzani G, Francavilla S, Francavilla F. Risk of prostate cancer in men with spinal cord injury: a systematic review and meta-analysis. Asian J Androl. 2018;20(6):555-560. doi:10.4103/aja.aja_31_18
17. Vince RA Jr, Jiang R, Bank M, et al. Evaluation of social determinants of health and prostate cancer outcomes among black and white patients: a systematic review and meta-analysis. JAMA Netw Open. 2023;6(1):e2250416. Published 2023 Jan 3. doi:10.1001/jamanetworkopen.2022.50416
18. Smith ZL, Eggener SE, Murphy AB. African-American prostate cancer disparities. Curr Urol Rep. 2017;18(10):81. Published 2017 Aug 14. doi:10.1007/s11934-017-0724-5
19. Jeong SH, Werneburg GT, Abouassaly R, Wood H. Acquired and congenital spinal cord injury is associated with lower likelihood of prostate specific antigen screening. Urology. 2022;164:178-183. doi:10.1016/j.urology.2022.01.044
20. Benaim EA, Montoya JD, Saboorian MH, Litwiller S, Roehrborn CG. Characterization of prostate size, PSA and endocrine profiles in patients with spinal cord injuries. Prostate Cancer Prostatic Dis. 1998;1(5):250-255. doi:10.1038/sj.pcan.4500246
21. Torricelli FC, Lucon M, Vicentini F, Gomes CM, Srougi M, Bruschini H. PSA levels in men with spinal cord injury and under intermittent catheterization. Neurourol Urodyn. 2011;30(8):1522-1524. doi:10.1002/nau.21119
22. Konety BR, Nguyen TT, Brenes G, et al. Evaluation of the effect of spinal cord injury on serum PSA levels. Urology. 2000;56(1):82-86. doi:10.1016/s0090-4295(00)00548-3
23. Lee WY, Sun LM, Lin CL, et al. Risk of prostate and bladder cancers in patients with spinal cord injury: a population-based cohort study. Urol Oncol. 2014;32(1):51.e1-51.e517. doi:10.1016/j.urolonc.2013.07.019
24. Patel N, Ngo K, Hastings J, Ketchum N, Sepahpanah F. Prevalence of prostate cancer in patients with chronic spinal cord injury. PM R. 2011;3(7):633-636. doi:10.1016/j.pmrj.2011.04.024
25. Everaert K, Oostra C, Delanghe J, Vande Walle J, Van Laere M, Oosterlinck W. Diagnosis and localization of a complicated urinary tract infection in neurogenic bladder disease by tubular proteinuria and serum prostate specific antigen. Spinal Cord. 1998;36(1):33-38. doi:10.1038/sj.sc.3100520
26. Drazer MW, Huo D, Eggener SE. National prostate cancer screening rates after the 2012 US Preventive Services Task Force recommendation discouraging prostate-specific antigen-based screening. J Clin Oncol. 2015;33(22):2416-2423. doi:10.1200/JCO.2015.61.6532
27. Sammon JD, Abdollah F, Choueiri TK, et al. Prostate-specific antigen screening after 2012 US Preventive Services Task Force recommendations. JAMA. 2015;314(19):2077-2079. doi:10.1001/jama.2015.7273
28. Jemal A, Fedewa SA, Ma J, et al. Prostate cancer incidence and PSA testing patterns in relation to USPSTF screening recommendations. JAMA. 2015;314(19):2054-2061. doi:10.1001/jama.2015.14905
29. Vetterlein MW, Dalela D, Sammon JD, et al. State-by-state variation in prostate-specific antigen screening trends following the 2011 United States Preventive Services Task Force panel update. Urology. 2018;112:56-65. doi:10.1016/j.urology.2017.08.055
Retrospective Evaluation of Drug-Drug Interactions With Erlotinib and Gefitinib Use in the Military Health System
Most cancer treatment regimens include the administration of several chemotherapeutic agents. Drug-drug interactions (DDIs) can increase the risk of fatal adverse events and reduce therapeutic efficacy.1,2 Erlotinib, gefitinib, afatinib, osimertinib, and icotinib are epidermal growth factor receptor–tyrosine kinase inhibitors (EGFR-TKIs) that have proven efficacy for treating advanced non–small cell lung cancer (NSCLC). Erlotinib strongly inhibits cytochrome P450 (CYP) isoenzymes CYP 1A1, moderately inhibits CYP 3A4 and 2C8, and induces CYP 1A1 and 1A2.2 Gefitinib weakly inhibits CYP 2C19 and 2D6.2 CYP 3A4 inducers and inhibitors affect metabolism of both erlotinib and gefitinib.3,4
Erlotinib and gefitinib are first-generation EGFR-TKIs and have been approved for NSCLC treatment by the US Food and Drug Administration (FDA). These agents have been used since the early 2000s and increase the possibility of long-term response and survival.2,5,6 EGFR-TKIs have a range of potential DDIs, including interactions with CYP-dependent metabolism, uridine diphosphate-glucuronosyltransferase, and transporter proteins.2 Few retrospective studies have focused on the therapeutic efficacy of erlotinib, gefitinib, or the combination of these agents.7-14
DDIs from cancer and noncancer therapies could lead to treatment discontinuation and affect patient outcomes. The goals for this study were to perform a broad-scale retrospective analysis focused on investigating prescribed drugs used with erlotinib and gefitinib and determine patient outcomes as obtained through several Military Health System (MHS) databases. Our investigation focused on (1) the functions of these drugs; (2) identifying adverse effects (AEs) that patients experienced; (3) evaluating differences when these drugs are used alone vs concomitantly, and between the completed vs discontinued treatment groups; (4) identifying all drugs used during erlotinib or gefitinib treatment; and (5) evaluating DDIs with antidepressants.
This retrospective study was performed at the Department of Research Programs at Walter Reed National Military Medical Center (WRNMMC) in Bethesda, Maryland. The WRNMMC Institutional Review Board approved the study protocol and ensured compliance with the Health Insurance Portability and Accountability Act as an exempt protocol. The Joint Pathology Center of the US Department of Defense (DoD) Cancer Registry and MHS data experts from the Comprehensive Ambulatory/Professional Encounter Record (CAPER) and the Pharmacy Data Transaction Service (PDTS) provided data for the analysis.
Methods
The DoD Cancer Registry Program was established in 1986 by the Assistant Secretary of Defense for Health Affairs. The registry currently contains data from 1998 to 2023. CAPER and PDTS are part of the MHS Data Repository/Management Analysis and Reporting Tool database. Each observation in the CAPER record represents an ambulatory encounter at a military treatment facility (MTF). CAPER records are available from 2003 to 2023.
Each observation in the PDTS record represents an outpatient prescription filled for an MHS beneficiary at MTFs through the TRICARE mail-order program or a retail pharmacy in the United States. Missing from this record are prescriptions filled at civilian pharmacies outside the United States and inpatient pharmacy prescriptions. The MHS Data Repository PDTS record is available from 2002 to 2023. The Composite Health Care System—the legacy system—is being replaced by GENESIS at MTFs.
Data Extraction Design
The study design involved a cross-sectional analysis. We requested data extraction for erlotinib and gefitinib from 1998 to 2021. Data from the DoD Cancer Registry were used to identify patients who received cancer treatment. Once patients were identified, the CAPER database was searched for diagnoses to identify other health conditions, while the PDTS database was used to populate a list of prescription medications filled during chemotherapy treatment.
Data collected from the Joint Pathology Center included cancer treatment (alone or concomitant), cancer information (cancer types and stages), demographics (sex, age at diagnosis), and physicians’ comments on AEs. Collected data from the MHS include diagnosis and filled prescription history from initiation to completion of the therapy period (or a buffer of 6 months after the initial period). We used all collected data in this analysis. The only exclusion criterion was a provided physician’s note commenting that the patient did not use erlotinib or gefitinib.
Data Extraction Analysis
The Surveillance, Epidemiology, and End Results Program Coding and Staging Manual 2016 and the International Classification of Diseases for Oncology (ICD-O) were used to decode disease and cancer types.15,16 Data sorting and analysis were performed using Microsoft Excel. The percentage for the total was calculated by using the total number of patients or data available within the gefitinib and erlotinib groups divided by total number of patients or data variables. The subgroup percentage was calculated by using the number of patients or data available within the subgroup divided by the total number of patients in that subgroup.
In alone vs concomitant and completed vs discontinued treatment groups, a 2-tailed, 2-sample z test was used to calculate P to determine statistical significance (P < .05) using a statistics website.17 Concomitant was defined as erlotinib or gefitinib taken with other medication(s) before, after, or at the same time as cancer therapy. For the retrospective data analysis, physicians’ notes with “.”, “,”, “/”, “;”, (period, comma, forward slash, semicolon) or space between medication names were interpreted as concurrent, while “+”, “-/+” (plus, minus/plus), or and between drug names were interpreted as combined. Completed treatment was defined as erlotinib or gefitinib as the last medication the patient took without recorded AEs; switching or experiencing AEs was defined as discontinued treatment.
Results
Erlotinib
The Joint Pathology Center provided 387 entries for 382 patients aged 21 to 93 years (mean, 65 years) who were treated systemically with erlotinib from January 1, 2001, to December 31, 2020. Five patients had duplicate entries because they had different cancer sites. There were 287 patients (74%) with lung cancer, 61 (16%) with pancreatic cancer, and 39 (10%) with other cancers. For lung cancer, there were 118 patients (30%) for the upper lobe, 78 (20%) for the lower lobe, and 60 (16%) not otherwise specified (NOS). Other lung cancer sites had fewer patients: 21 (5%) middle lobe lung, 6 (2%) overlapping lung lesion(s), and 4 (1%) main bronchus of the lung. For pancreatic cancer, there were 27 patients (7%) for the head of the pancreas, 10 (3%) pancreas NOS, 9 (2%) body of the pancreas, 9 (2%) tail of the pancreas, 4 (1%) overlapping lesions of the pancreas, 1 (< 1%) pancreatic duct, and 1 (< 1%) other specified parts of the pancreas
There were 342 patients (88%) who were aged > 50 years; 186 male patients (48%) and 201 female patients (52%). There were 293 patients (76%) who had a cancer diagnosis of stage III or IV disease and 94 (24%) who had a cancer diagnosis of stage ≤ II (combination of data for stage 0, 1, and 2, not applicable, and unknown). For their systemic treatment, 161 patients (42%) were treated with erlotinib alone and 226 (58%) received erlotinib concomitantly with additional chemotherapy.
Patients were more likely to discontinue erlotinib for chemotherapy if they received concomitant treatment. Among the patients receiving erlotinib monotherapy, 5% stopped the treatment, whereas 51% of patients treated concomitantly discontinued (P < .001).
Among the 123 patients who discontinued their treatment, 101 switched treatment with no AEs notes, 22 died or experienced fatigue with blurry vision, constipation, nonspecific gastrointestinal effects, grade-4 diarrhea (as defined by the Common Terminology Criteria for Adverse Events), or developed a pleural fluid, pneumonitis, renal failure, skin swelling and facial rash, and unknown AEs of discontinuation. Patients who discontinued treatment because of unknown AEs had physicians’ notes that detailed emergency department visits, peripheral vascular disease, progressive disease, and treatment cessation, but did not specify the exact symptom(s) that led to discontinuation. The causes of death are unknown because they were not detailed in the available notes or databases. The overall results in this retrospective review cannot establish causality between taking erlotinib or gefitinib and death.
Gefitinib
In September 2021, the Joint Pathology Center provided 33 entries for 33 patients who were systemically treated with gefitinib from January 1, 2002, to December 31, 2017. The patient ages ranged from 49 to 89 years with a mean age of 66 years. There were 31 (94%) and 2 (6%) patients with lung and other cancers, respectively. The upper lobe, lower lobe, and lung NOS had the most patients: 14 (42%), 8 (24%), and 6 (18%), respectively.
There were 31 patients (94%) who were aged > 50 years; 15 were male (45%) and 18 were female (55%). There were 26 patients (79%) who had a cancer diagnosis of stage III or IV disease. Nineteen patients (58%) were treated with gefitinib alone, and 14 (42%) were treated with gefitinib concomitantly with additional chemotherapy. Thirty-one patients (94%) were treated for lung cancer (Table 2). Thirty-three patients are a small sample size to determine whether patients were likely to stop gefitinib if used concomitantly with other drugs. Among the patients treated with gefitinib monotherapy, 5% (n = 1) stopped treatment, whereas 29% (n = 4) of patients treated concomitantly discontinued treatment (P = .06). All comparisons for gefitinib yielded insignificant P values. Physicians’ notes indicated that the reasons for gefitinib discontinuation were life-altering pruritis and unknown (progressive disease outcome) (Table 3).
Management Analysis and Reporting Tool Database
MHS data analysts provided data on diagnoses for 348 patients among 415 submitted, with 232 and 112 patients completing and discontinuing erlotinib or gefitinib treatment, respectively. Each patient had 1 to 104 (completed treatment group) and 1 to 157 (discontinued treatment group) unique health conditions documented. The MHS reported 1319 unique-diagnosis conditions for the completed group and 1266 for the discontinued group. Patients with additional health issues stopped chemotherapy use more often than those without; P < .001 for the completed group (232 patients, 1319 diagnoses) vs the discontinued group (112 patients, 1266 diagnoses). The mean (SD) number of diagnoses was 19 (17) for the completed and 30 (22) for the discontinued treatment groups (Figure).
MHS data was provided for patients who filled erlotinib (n = 240) or gefitinib (n = 18). Among the 258 patients, there were 179 and 79 patients in the completed and discontinued treatment groups, respectively. Each patient filled 1 to 75 (for the completed treatment group) and 3 to 103 (for the discontinued treatment group) prescription drugs. There were 805 unique-filled prescriptions for the completed and 670 for the discontinued group. Patients in the discontinued group filled more prescriptions than those who completed treatment; P < .001 for the completed group (179 patients,805 drugs) vs the discontinued group (79 patients, 670 drugs).
The mean (SD) number of filled prescription drugs was 19 (11) for the completed group and 29 (18) for the discontinued treatment group. The 5 most filled prescriptions with erlotinib from 258 patients with PDTS data were ondansetron (151 prescriptions, 10 recorded AEs), dexamethasone (119 prescriptions, 9 recorded AEs), prochlorperazine (105 prescriptions, 15 recorded AEs), oxycodone (99 prescriptions, 1 AE), and docusate (96 prescriptions, 7 recorded AEs).
Discussion
The difference between erlotinib and gefitinib data can be attributed to the FDA approval date and gefitinib’s association with a higher frequency of hepatotoxicity.18-20 The FDA designated gefitinib as an orphan drug for EGFR mutation–positive NSCLC treatment. Gefitinib first received accelerated approval in 2003 for the treatment of locally advanced or metastatic NSCLC. Gefitinib then was voluntarily withdrawn from the market following confirmatory clinical trials that did not verify clinical benefit.
The current approval is for a different patient population—previously untreated, metastatic EGFR exon 19 or 21 L858R mutation—than the 2003 approval.4,6 There was no record of gefitinib use after 2017 in our study.
Erlotinib is a reversible EGFR-TKI that is approved by the FDA as first-line (maintenance) or second-line treatment (after progression following at least 1 earlier chemotherapy regimen) for patients with metastatic NSCLC who harbor EGFR exon 19 deletions or exon 21 L858R substitution mutations, as detected by an FDA-approved test.3 Since 2005, the FDA also approved erlotinib for first-line treatment of patients with locally advanced, unresectable, or metastatic pancreatic cancer in combination with gemcitabine.3 Without FDA indication, erlotinib is used for colorectal, head and neck, ovarian carcinoma, pancreatic carcinoma, and breast cancer.21
Erlotinib and gefitinib are not considered first-line treatments in EGFR exon 19 or 21–mutated NSCLC because osimertinib was approved in 2018. Targeted therapies for EGFR mutation continue to advance at a fast pace, with amivantamab and mobocertinib now FDA approved for EGFR exon 20 insertion–mutated NSCLC.
Erlotinib Use
Thirty-nine patients (10%) in this study were prescribed erlotinib for off-label indications. Erlotinib was used alone or in combination with bevacizumab, capecitabine, cisplatin, denosumab, docetaxel, gemcitabine, and the MEK-inhibitor selumetinib. Erlotinib combined with cisplatin, denosumab, docetaxel, and gemcitabine had no recorded AEs, with 10 data entries for gemcitabine and 1 for other drugs. Three patients received bevacizumab and erlotinib, and 1 patient (diagnosed with kidney NOS) showed rash or facial swelling/erythema and diffuse body itching then stable disease after 2 cycles.
One patient (diagnosed with cancer located at the pancreas head) was bridged with capecitabine and erlotinib when going on a vacation, then received FOLFIRINOX (a combination chemotherapy regimen containing folinic acid [leucovorin], fluorouracil, irinotecan, and oxaliplatin), which led to significant fatigue, blurry vision, and constipation. One patient was treated for lung NOS with the MEK-inhibitor selumetinib plus erlotinib and developed pneumonitis following treatment.
Because oncologists followed guidelines and protocols in systemic treatment, DDIs of erlotinib concurrently (before or after) and in combination with cancer drugs were unlikely. Further investigation is needed for several 1:1:1 DDIs with noncancer drugs. A retrospective overview is not a randomized clinical study; therefore, analysis is limited. Data from the MHS were obtained solely from notes from physicians who treated the patients; therefore, exact information explaining whether a patient completed treatment or had to withdraw could not be extrapolated (ie, blood/plasma samples were not obtained to confirm).
Discontinued Treatment
The reasons for treatment discontinuation with erlotinib or gefitinib varied among patients, with no consistent AE or cause. Most data were for switching treatments after discontinuing treatment with erlotinib (101 of 123 patients) and gefitinib (2 of 5 patients). This is not surprising given the widely recognized pillars of therapy for NSCLC: chemotherapy, target therapy, and immunotherapy.22 From the MHS records, the reasons patients switched treatment of erlotinib or gefitinib were not listed or listed as due to negative EGFR testing, lack of responsiveness, or enrollment in a different treatment.
Physicians’ notes on AEs were not detailed in most cases. Notes for gastrointestinal effects, life-altering pruritis, intolerance, peripheral vascular disease, pneumonitis, and progressive disease described the change in status or appearance of a new medical condition but did not indicate whether erlotinib or gefitinib caused the changes or worsened a pre-existing condition.
The causes of AEs were not described in the available notes or the databases. This retrospective data analysis only focused on identifying drugs involved with erlotinib and gefitinib treatment; further mapping of DDIs among patients experiencing AEs needs to be performed, then in vitro data testing before researchers can reach a conclusion.
DDIs With Antidepressants
We used the PDTS database to evaluate patients who experienced AEs, excluding patients who switched treatment. Thirteen patients filled a prescription for erlotinib and reported taking 220 cancer and noncancer prescription drugs. One patient (pruritis) was taking gefitinib along with 16 noncancer prescription drugs.
Selective serotonin reuptake inhibitors and other antidepressants have been implicated in CYP 2D6 inhibition and DDIs.48,49 Losartan is a widely used antihypertensive drug with a favorable DDI profile
Our data showed that 16 antidepressants (amitriptyline, bupropion, citalopram, desvenlafaxine, duloxetine, escitalopram, imipramine, fluoxetine, fluvoxamine, mirtazapine, nortriptyline, paroxetine, phenelzine, sertraline, trazodone, and venlafaxine) were recorded with concomitant erlotinib or gefitinib from initiation to completion of therapy or a buffer of 6 months from the first diagnosis date. Based on the date dispensed and days’ supply, only escitalopram could be used in combination with gefitinib treatment. The one patient who filled a prescription for gefitinib and escitalopram completed treatment without recorded AEs. PDTS database confirmed that patients experienced AEs with 5 antidepressants (amitriptyline, mirtazapine, paroxetine, trazodone, and venlafaxine) with concomitant erlotinib use.
Based on the date dispensed and days’ supply, only trazodone could be used in combination with erlotinib. PDTS database showed that cancer drugs (erlotinib and megestrol) and 39 noncancer drugs (including acetaminophen, azithromycin, dexamethasone, hydrocortisone, and polyethylene glycol) were filled by 1 patient whose physician noted skin rash. Another limitation of using databases to reflect clinical practice is that although megestrol is listed as a cancer drug by code in the PDTS database, it is not used for nonendometrial or gynecologic cancers. However, because of the PDTS database classification, megestrol is classified as a cancer drug in this retrospective review.
This retrospective review found no significant DDIs for erlotinib or gefitinib, with 1 antidepressant taken by 1 patient for each respective treatment. The degree of inhibition and induction for escitalopram and trazodone are categorized as weak, minimal, or none; therefore, while 1:1 DDIs might be little or no effect, 1:1:1 combination DDIs could have a different outcome. This retrospective data collection cannot be linked to the in vitro hepatocyte DDIs from erlotinib and gefitinib in previous studies.51,52
Conclusions
This retrospective study describes erlotinib and gefitinib use in the MHS and their potential for DDIs. Because of military service requirements, people who are qualified to serve must be healthy or have either controlled or nonactive medical diagnoses and be physically fit. Consequently, our patient population had fewer common medical illnesses, such as diabetes and obesity, compared with the general population. Most noncancer drugs mentioned in this study are not known CYP metabolizers; therefore, recorded AEs alone cannot conclusively determine whether there is a DDI among erlotinib or gefitinib and noncancer drugs. Antidepressants generally are safe but have boxed warnings in the US for increased risk of suicidal ideation in young people.53,54 This retrospective study did not find statistically significant DDIs for erlotinib or gefitinib with antidepressants. Based on this retrospective data analysis, future in vitro testing is needed to assess DDIs for erlotinib or gefitinib and cancer or noncancer drugs identified in this study.
Acknowledgments
The Department of Research Program funds at Walter Reed National Military Medical Center supported this protocol. We sincerely appreciate the contribution of data extraction from the Joint Pathology Center teams (Francisco J. Rentas, John D. McGeeney, Kimberly M. Greenfield, Beatriz A. Hallo, and Johnny P. Beason) and the MHS database personnel (Maj Ryan Costantino, Lee Ann Zarzabal, Brandon Jenkins, and Alex Rittel). We gratefully thank you for the protocol support from the Department of Research programs: CDR Wesley R. Campbell, CDR Ling Ye, Yaling Zhou, Elizabeth Schafer, Robert Roogow, Micah Stretch, Diane Beaner, Adrienne Woodard, David L. Evers, and Paula Amann.
1. van Leeuwen RW, van Gelder T, Mathijssen RH, Jansman FG. Drug-drug interactions with tyrosine-kinase inhibitors: a clinical perspective. Lancet Oncol. 2014;15(8):e315-e326. doi:10.1016/S1470-2045(13)70579-5
2. Xu ZY, Li JL. Comparative review of drug-drug interactions with epidermal growth factor receptor tyrosine kinase inhibitors for the treatment of non-small-cell lung cancer. Onco Targets Ther. 2019;12:5467-5484. doi:10.2147/OTT.S194870
3. Tarceva (erlotinib). Prescribing Information. Genetech, Astellas Pharma; 2016. Accessed June 28, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021743s025lbl.pdf
4. Iressa (gefitinib). Prescribing Information. AstraZeneca; 2018. Accessed June 28, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/206995s003lbl.pdf
5. Cohen MH, Williams GA, Sridhara R, Chen G, Pazdur R. FDA drug approval summary: gefitinib (ZD1839) (Iressa) tablets. Oncologist. 2003;8(4):303-306. doi:10.1634/theoncologist.8-4-303
6. Cohen MH, Williams GA, Sridhara R, Chen G, et al. United States Food and Drug Administration Drug Approval summary: gefitinib (ZD1839; Iressa) tablets. Clin Cancer Res. 2004;10(4):1212-8. doi:10.1158/1078-0432.ccr-03-0564
7. Fiala O, Pesek M, Finek J, et al. Erlotinib in the treatment of advanced squamous cell NSCLC. Neoplasma. 2013;60(6):676-682. doi:10.4149/neo_2013_086
8. Platania M, Agustoni F, Formisano B, et al. Clinical retrospective analysis of erlotinib in the treatment of elderly patients with advanced non-small cell lung cancer. Target Oncol. 2011;6(3):181-186. doi:10.1007/s11523-011-0185-6
9. Tseng JS, Yang TY, Chen KC, Hsu KH, Chen HY, Chang GC. Retrospective study of erlotinib in patients with advanced squamous lung cancer. Lung Cancer. 2012;77(1):128-133. doi:10.1016/j.lungcan.2012.02.012
10. Sim EH, Yang IA, Wood-Baker R, Bowman RV, Fong KM. Gefitinib for advanced non-small cell lung cancer. Cochrane Database Syst Rev. 2018;1(1):CD006847. doi:10.1002/14651858.CD006847.pub2
11. Shrestha S, Joshi P. Gefitinib monotherapy in advanced non-small-cell lung cancer: a retrospective analysis. JNMA J Nepal Med Assoc. 2012;52(186):66-71.
12. Nakamura H, Azuma M, Namisato S, et al. A retrospective study of gefitinib effective cases in non-small cell lung cancer patients with poor performance status. J. Clin. Oncol. 2004 22:14_suppl, 8177-8177. doi:10.1200/jco.2004.22.90140.8177
13. Pui C, Gregory C, Lunqing Z, Long LJ, Tou CH, Hong CT. Retrospective analysis of gefitinib and erlotinib in EGFR-mutated non-small-cell lung cancer patients. J Lung Health Dis. 2017;1(1):16-24. doi:10.29245/2689-999X/2017/1.1105
14. Yoshida T, Yamada K, Azuma K, et al. Comparison of adverse events and efficacy between gefitinib and erlotinib in patients with non-small-cell lung cancer: a retrospective analysis. Med Oncol. 2013;30(1):349. doi:10.1007/s12032-012-0349-y
15. Adamo M, Dickie L, Ruhl J. SEER program coding and staging manual 2016. National Cancer Institute; 2016. Accessed June 28, 2023. https://seer.cancer.gov/archive/manuals/2016/SPCSM_2016_maindoc.pdf
16. World Health Organization. International classification of diseases for oncology (ICD-O) 3rd ed, 1st revision. World Health Organization; 2013. Accessed June 28, 2023. https://apps.who.int/iris/handle/10665/96612
17. Z Score Calculator for 2 population proportions. Social science statistics. Accessed April 25, 2023. https://www.socscistatistics.com/tests/ztest/default2.aspx
18. Takeda M, Okamoto I, Nakagawa K. Pooled safety analysis of EGFR-TKI treatment for EGFR mutation-positive non-small cell lung cancer. Lung Cancer. 2015;88(1):74-79. doi:10.1016/j.lungcan.2015.01.026
19. Burotto M, Manasanch EE, Wilkerson J, Fojo T. Gefitinib and erlotinib in metastatic non-small cell lung cancer: a meta-analysis of toxicity and efficacy of randomized clinical trials. Oncologist. 2015;20(4):400-410. doi:10.1634/theoncologist.2014-0154
20. Yang Z, Hackshaw A, Feng Q, et al. Comparison of gefitinib, erlotinib and afatinib in non-small cell lung cancer: a meta-analysis. Int J Cancer. 2017;140(12):2805-2819. doi:10.1002/ijc.30691
21. Mack JT. Erlotinib. xPharm: The comprehensive pharmacology reference, 2007. Accessed June 28, 2023. https://www.sciencedirect.com/topics/chemistry/erlotinib
22. Melosky B. Rapidly changing treatment algorithms for metastatic nonsquamous non-small-cell lung cancer. Curr Oncol. 2018;25(suppl 1):S68-S76. doi:10.3747/co.25.3839
23. Xeloda (capecitabine). Prescribing Information. Hoffmann-La Roche, Genetech; 2015. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/020896s037lbl.pdf
24. Paraplatin (carboplatin). Prescribing Information. Bristol-Myers Squibb; 2010. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020452s005lbl.pdf
25. Gemzar (gemcitabine). Prescribing Information. Eli Lilly and Company; 1996. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020509s064lbl.pdf
26. Megace (megestrol). Prescribing Information. Par Pharmaceutical, Bristol-Myers Squibb; 2013. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021778s016lbl.pdf
27. Taxol (paclitaxel). Prescribing Information. BASF Aktiengesellschaft, Bristol-Myers Squibb; 2011. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020262s049lbl.pdf
28. Abraxane (paclitaxel). Prescribing Information. Celgene; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/021660s047lbl.pdf
29. Alima (pemetrexed). Prescribing Information. Sindan Pharma, Actavis Pharma; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208419s000lbl.pdf
30. Tagrisso (Osimertinib). Prescribing Information. AstraZeneca; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208065s021lbl.pdf
31. Elavil (amitriptyline). Prescribing Information. Sandoz; 2014. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/085966s095,085969s084,085968s096,085971s075,085967s076,085970s072lbl.pdf
32. Lexapro (escitalopram). Prescribing Information. H. Lundbeck, Allergan; 2017. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021323s047lbl.pdf

33. Remeron (mirtazapine). Prescribing Information. Merck; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020415s029,%20021208s019lbl.pdf
34. Paxil (paroxetine). Prescribing Information. Apotex; 2021. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/020031s077lbl.pdf
35. Desyrel (trazodone). Prescribing Information. Pragma Pharmaceuticals; 2017. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018207s032lbl.pdf
36. Effexor (venlafaxine). Prescribing Information. Norwich Pharmaceuticals, Almatica Pharma; 2022. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215429s000lbl.pdf
37. Sofran (ondansetron). Prescribing Information. GlaxoSmithKline; 2010. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020007s040,020403s018lbl.pdf
38. Hemady (dexamethasone). Prescribing Information. Dexcel Pharma; 2019. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211379s000lbl.pdf
39. Levaquin (levofloxacin). Prescribing Information. Janssen Pharmaceuticals; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020634s073lbl.pdf
40. Percocet (Oxycodone and Acetaminophen). Prescribing Information. Endo Pharmaceuticals; 2006. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/040330s015,040341s013,040434s003lbl.pdf
41. Docusate Sodium usage information. Spirit Pharmaceuticals; 2010. Accessed June 29, 2023. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=84ee7230-0bf6-4107-b5fa-d6fa265139d0
42. Golytely (polyethylene glycol 3350). Prescribing Information. Sebela Pharmaceuticals; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/019011s031lbl.pdf
43. Zithomax (azithromycin). Prescribing Information. Pliva, Pfizer; 2013. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/050710s039,050711s036,050784s023lbl.pdf
44. Acetaminophen. Prescribing Information. Fresenius Kabi; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/204767s003lbl.pdf
45. Compazine (prochlorperazine). Prescribing Information. GlaxoSmithKline; 2004. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/010571s096lbl.pdf
46. Rayos (prednisone). Prescribing Information. Horizon Pharma; 2012. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202020s000lbl.pdf
47. Cortef (hydrocortisone). Prescribing Information. Pfizer; 2019. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/008697s036lbl.pdf
48. Brown CH. Overview of drug–drug interactions with SSRIs. US Pharm. 2008;33(1):HS-3-HS-19. Accessed June 28, 2023. https://www.uspharmacist.com/article/overview-of-drugdrug-interactions-with-ssris
49. Jin X, Potter B, Luong TL, et al. Pre-clinical evaluation of CYP 2D6 dependent drug-drug interactions between primaquine and SSRI/SNRI antidepressants. Malar J. 2016;15(1):280. doi:10.1186/s12936-016-1329-z
50. Sica DA, Gehr TW, Ghosh S. Clinical pharmacokinetics of losartan. Clin Pharmacokinet. 2005;44(8):797-814. doi:10.2165/00003088-200544080-00003
51. Luong TT, Powers CN, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interactions (DDIs) of gefitinib with/without losartan and selective serotonin reuptake inhibitors (SSRIs): citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and venlafaxine. Curr Res Pharmacol Drug Discov. 2022;3:100112. doi:10.1016/j.crphar.2022.100112
52. Luong TT, McAnulty MJ, Evers DL, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interaction (DDI) of gefitinib or erlotinib with Cytochrome P450 (CYP) inhibiting drugs, fluoxetine and/or losartan. Curr Res Toxicol. 2021;2:217-224. doi:10.1016/j.crtox.2021.05.006
53. Lu CY, Zhang F, Lakoma MD, et al. Changes in antidepressant use by young people and suicidal behavior after FDA warnings and media coverage: quasi-experimental study. BMJ. 2014;348:g3596. Published 2014 Jun 18. doi:10.1136/bmj.g359654. Friedman RA. Antidepressants’ black-box warning--10 years later. N Engl J Med. 2014;371(18):1666-1668. doi:10.1056/NEJMp1408480
Most cancer treatment regimens include the administration of several chemotherapeutic agents. Drug-drug interactions (DDIs) can increase the risk of fatal adverse events and reduce therapeutic efficacy.1,2 Erlotinib, gefitinib, afatinib, osimertinib, and icotinib are epidermal growth factor receptor–tyrosine kinase inhibitors (EGFR-TKIs) that have proven efficacy for treating advanced non–small cell lung cancer (NSCLC). Erlotinib strongly inhibits cytochrome P450 (CYP) isoenzymes CYP 1A1, moderately inhibits CYP 3A4 and 2C8, and induces CYP 1A1 and 1A2.2 Gefitinib weakly inhibits CYP 2C19 and 2D6.2 CYP 3A4 inducers and inhibitors affect metabolism of both erlotinib and gefitinib.3,4
Erlotinib and gefitinib are first-generation EGFR-TKIs and have been approved for NSCLC treatment by the US Food and Drug Administration (FDA). These agents have been used since the early 2000s and increase the possibility of long-term response and survival.2,5,6 EGFR-TKIs have a range of potential DDIs, including interactions with CYP-dependent metabolism, uridine diphosphate-glucuronosyltransferase, and transporter proteins.2 Few retrospective studies have focused on the therapeutic efficacy of erlotinib, gefitinib, or the combination of these agents.7-14
DDIs from cancer and noncancer therapies could lead to treatment discontinuation and affect patient outcomes. The goals for this study were to perform a broad-scale retrospective analysis focused on investigating prescribed drugs used with erlotinib and gefitinib and determine patient outcomes as obtained through several Military Health System (MHS) databases. Our investigation focused on (1) the functions of these drugs; (2) identifying adverse effects (AEs) that patients experienced; (3) evaluating differences when these drugs are used alone vs concomitantly, and between the completed vs discontinued treatment groups; (4) identifying all drugs used during erlotinib or gefitinib treatment; and (5) evaluating DDIs with antidepressants.
This retrospective study was performed at the Department of Research Programs at Walter Reed National Military Medical Center (WRNMMC) in Bethesda, Maryland. The WRNMMC Institutional Review Board approved the study protocol and ensured compliance with the Health Insurance Portability and Accountability Act as an exempt protocol. The Joint Pathology Center of the US Department of Defense (DoD) Cancer Registry and MHS data experts from the Comprehensive Ambulatory/Professional Encounter Record (CAPER) and the Pharmacy Data Transaction Service (PDTS) provided data for the analysis.
Methods
The DoD Cancer Registry Program was established in 1986 by the Assistant Secretary of Defense for Health Affairs. The registry currently contains data from 1998 to 2023. CAPER and PDTS are part of the MHS Data Repository/Management Analysis and Reporting Tool database. Each observation in the CAPER record represents an ambulatory encounter at a military treatment facility (MTF). CAPER records are available from 2003 to 2023.
Each observation in the PDTS record represents an outpatient prescription filled for an MHS beneficiary at MTFs through the TRICARE mail-order program or a retail pharmacy in the United States. Missing from this record are prescriptions filled at civilian pharmacies outside the United States and inpatient pharmacy prescriptions. The MHS Data Repository PDTS record is available from 2002 to 2023. The Composite Health Care System—the legacy system—is being replaced by GENESIS at MTFs.
Data Extraction Design
The study design involved a cross-sectional analysis. We requested data extraction for erlotinib and gefitinib from 1998 to 2021. Data from the DoD Cancer Registry were used to identify patients who received cancer treatment. Once patients were identified, the CAPER database was searched for diagnoses to identify other health conditions, while the PDTS database was used to populate a list of prescription medications filled during chemotherapy treatment.
Data collected from the Joint Pathology Center included cancer treatment (alone or concomitant), cancer information (cancer types and stages), demographics (sex, age at diagnosis), and physicians’ comments on AEs. Collected data from the MHS include diagnosis and filled prescription history from initiation to completion of the therapy period (or a buffer of 6 months after the initial period). We used all collected data in this analysis. The only exclusion criterion was a provided physician’s note commenting that the patient did not use erlotinib or gefitinib.
Data Extraction Analysis
The Surveillance, Epidemiology, and End Results Program Coding and Staging Manual 2016 and the International Classification of Diseases for Oncology (ICD-O) were used to decode disease and cancer types.15,16 Data sorting and analysis were performed using Microsoft Excel. The percentage for the total was calculated by using the total number of patients or data available within the gefitinib and erlotinib groups divided by total number of patients or data variables. The subgroup percentage was calculated by using the number of patients or data available within the subgroup divided by the total number of patients in that subgroup.
In alone vs concomitant and completed vs discontinued treatment groups, a 2-tailed, 2-sample z test was used to calculate P to determine statistical significance (P < .05) using a statistics website.17 Concomitant was defined as erlotinib or gefitinib taken with other medication(s) before, after, or at the same time as cancer therapy. For the retrospective data analysis, physicians’ notes with “.”, “,”, “/”, “;”, (period, comma, forward slash, semicolon) or space between medication names were interpreted as concurrent, while “+”, “-/+” (plus, minus/plus), or and between drug names were interpreted as combined. Completed treatment was defined as erlotinib or gefitinib as the last medication the patient took without recorded AEs; switching or experiencing AEs was defined as discontinued treatment.
Results
Erlotinib
The Joint Pathology Center provided 387 entries for 382 patients aged 21 to 93 years (mean, 65 years) who were treated systemically with erlotinib from January 1, 2001, to December 31, 2020. Five patients had duplicate entries because they had different cancer sites. There were 287 patients (74%) with lung cancer, 61 (16%) with pancreatic cancer, and 39 (10%) with other cancers. For lung cancer, there were 118 patients (30%) for the upper lobe, 78 (20%) for the lower lobe, and 60 (16%) not otherwise specified (NOS). Other lung cancer sites had fewer patients: 21 (5%) middle lobe lung, 6 (2%) overlapping lung lesion(s), and 4 (1%) main bronchus of the lung. For pancreatic cancer, there were 27 patients (7%) for the head of the pancreas, 10 (3%) pancreas NOS, 9 (2%) body of the pancreas, 9 (2%) tail of the pancreas, 4 (1%) overlapping lesions of the pancreas, 1 (< 1%) pancreatic duct, and 1 (< 1%) other specified parts of the pancreas
There were 342 patients (88%) who were aged > 50 years; 186 male patients (48%) and 201 female patients (52%). There were 293 patients (76%) who had a cancer diagnosis of stage III or IV disease and 94 (24%) who had a cancer diagnosis of stage ≤ II (combination of data for stage 0, 1, and 2, not applicable, and unknown). For their systemic treatment, 161 patients (42%) were treated with erlotinib alone and 226 (58%) received erlotinib concomitantly with additional chemotherapy.
Patients were more likely to discontinue erlotinib for chemotherapy if they received concomitant treatment. Among the patients receiving erlotinib monotherapy, 5% stopped the treatment, whereas 51% of patients treated concomitantly discontinued (P < .001).
Among the 123 patients who discontinued their treatment, 101 switched treatment with no AEs notes, 22 died or experienced fatigue with blurry vision, constipation, nonspecific gastrointestinal effects, grade-4 diarrhea (as defined by the Common Terminology Criteria for Adverse Events), or developed a pleural fluid, pneumonitis, renal failure, skin swelling and facial rash, and unknown AEs of discontinuation. Patients who discontinued treatment because of unknown AEs had physicians’ notes that detailed emergency department visits, peripheral vascular disease, progressive disease, and treatment cessation, but did not specify the exact symptom(s) that led to discontinuation. The causes of death are unknown because they were not detailed in the available notes or databases. The overall results in this retrospective review cannot establish causality between taking erlotinib or gefitinib and death.
Gefitinib
In September 2021, the Joint Pathology Center provided 33 entries for 33 patients who were systemically treated with gefitinib from January 1, 2002, to December 31, 2017. The patient ages ranged from 49 to 89 years with a mean age of 66 years. There were 31 (94%) and 2 (6%) patients with lung and other cancers, respectively. The upper lobe, lower lobe, and lung NOS had the most patients: 14 (42%), 8 (24%), and 6 (18%), respectively.
There were 31 patients (94%) who were aged > 50 years; 15 were male (45%) and 18 were female (55%). There were 26 patients (79%) who had a cancer diagnosis of stage III or IV disease. Nineteen patients (58%) were treated with gefitinib alone, and 14 (42%) were treated with gefitinib concomitantly with additional chemotherapy. Thirty-one patients (94%) were treated for lung cancer (Table 2). Thirty-three patients are a small sample size to determine whether patients were likely to stop gefitinib if used concomitantly with other drugs. Among the patients treated with gefitinib monotherapy, 5% (n = 1) stopped treatment, whereas 29% (n = 4) of patients treated concomitantly discontinued treatment (P = .06). All comparisons for gefitinib yielded insignificant P values. Physicians’ notes indicated that the reasons for gefitinib discontinuation were life-altering pruritis and unknown (progressive disease outcome) (Table 3).
Management Analysis and Reporting Tool Database
MHS data analysts provided data on diagnoses for 348 patients among 415 submitted, with 232 and 112 patients completing and discontinuing erlotinib or gefitinib treatment, respectively. Each patient had 1 to 104 (completed treatment group) and 1 to 157 (discontinued treatment group) unique health conditions documented. The MHS reported 1319 unique-diagnosis conditions for the completed group and 1266 for the discontinued group. Patients with additional health issues stopped chemotherapy use more often than those without; P < .001 for the completed group (232 patients, 1319 diagnoses) vs the discontinued group (112 patients, 1266 diagnoses). The mean (SD) number of diagnoses was 19 (17) for the completed and 30 (22) for the discontinued treatment groups (Figure).
MHS data was provided for patients who filled erlotinib (n = 240) or gefitinib (n = 18). Among the 258 patients, there were 179 and 79 patients in the completed and discontinued treatment groups, respectively. Each patient filled 1 to 75 (for the completed treatment group) and 3 to 103 (for the discontinued treatment group) prescription drugs. There were 805 unique-filled prescriptions for the completed and 670 for the discontinued group. Patients in the discontinued group filled more prescriptions than those who completed treatment; P < .001 for the completed group (179 patients,805 drugs) vs the discontinued group (79 patients, 670 drugs).
The mean (SD) number of filled prescription drugs was 19 (11) for the completed group and 29 (18) for the discontinued treatment group. The 5 most filled prescriptions with erlotinib from 258 patients with PDTS data were ondansetron (151 prescriptions, 10 recorded AEs), dexamethasone (119 prescriptions, 9 recorded AEs), prochlorperazine (105 prescriptions, 15 recorded AEs), oxycodone (99 prescriptions, 1 AE), and docusate (96 prescriptions, 7 recorded AEs).
Discussion
The difference between erlotinib and gefitinib data can be attributed to the FDA approval date and gefitinib’s association with a higher frequency of hepatotoxicity.18-20 The FDA designated gefitinib as an orphan drug for EGFR mutation–positive NSCLC treatment. Gefitinib first received accelerated approval in 2003 for the treatment of locally advanced or metastatic NSCLC. Gefitinib then was voluntarily withdrawn from the market following confirmatory clinical trials that did not verify clinical benefit.
The current approval is for a different patient population—previously untreated, metastatic EGFR exon 19 or 21 L858R mutation—than the 2003 approval.4,6 There was no record of gefitinib use after 2017 in our study.
Erlotinib is a reversible EGFR-TKI that is approved by the FDA as first-line (maintenance) or second-line treatment (after progression following at least 1 earlier chemotherapy regimen) for patients with metastatic NSCLC who harbor EGFR exon 19 deletions or exon 21 L858R substitution mutations, as detected by an FDA-approved test.3 Since 2005, the FDA also approved erlotinib for first-line treatment of patients with locally advanced, unresectable, or metastatic pancreatic cancer in combination with gemcitabine.3 Without FDA indication, erlotinib is used for colorectal, head and neck, ovarian carcinoma, pancreatic carcinoma, and breast cancer.21
Erlotinib and gefitinib are not considered first-line treatments in EGFR exon 19 or 21–mutated NSCLC because osimertinib was approved in 2018. Targeted therapies for EGFR mutation continue to advance at a fast pace, with amivantamab and mobocertinib now FDA approved for EGFR exon 20 insertion–mutated NSCLC.
Erlotinib Use
Thirty-nine patients (10%) in this study were prescribed erlotinib for off-label indications. Erlotinib was used alone or in combination with bevacizumab, capecitabine, cisplatin, denosumab, docetaxel, gemcitabine, and the MEK-inhibitor selumetinib. Erlotinib combined with cisplatin, denosumab, docetaxel, and gemcitabine had no recorded AEs, with 10 data entries for gemcitabine and 1 for other drugs. Three patients received bevacizumab and erlotinib, and 1 patient (diagnosed with kidney NOS) showed rash or facial swelling/erythema and diffuse body itching then stable disease after 2 cycles.
One patient (diagnosed with cancer located at the pancreas head) was bridged with capecitabine and erlotinib when going on a vacation, then received FOLFIRINOX (a combination chemotherapy regimen containing folinic acid [leucovorin], fluorouracil, irinotecan, and oxaliplatin), which led to significant fatigue, blurry vision, and constipation. One patient was treated for lung NOS with the MEK-inhibitor selumetinib plus erlotinib and developed pneumonitis following treatment.
Because oncologists followed guidelines and protocols in systemic treatment, DDIs of erlotinib concurrently (before or after) and in combination with cancer drugs were unlikely. Further investigation is needed for several 1:1:1 DDIs with noncancer drugs. A retrospective overview is not a randomized clinical study; therefore, analysis is limited. Data from the MHS were obtained solely from notes from physicians who treated the patients; therefore, exact information explaining whether a patient completed treatment or had to withdraw could not be extrapolated (ie, blood/plasma samples were not obtained to confirm).
Discontinued Treatment
The reasons for treatment discontinuation with erlotinib or gefitinib varied among patients, with no consistent AE or cause. Most data were for switching treatments after discontinuing treatment with erlotinib (101 of 123 patients) and gefitinib (2 of 5 patients). This is not surprising given the widely recognized pillars of therapy for NSCLC: chemotherapy, target therapy, and immunotherapy.22 From the MHS records, the reasons patients switched treatment of erlotinib or gefitinib were not listed or listed as due to negative EGFR testing, lack of responsiveness, or enrollment in a different treatment.
Physicians’ notes on AEs were not detailed in most cases. Notes for gastrointestinal effects, life-altering pruritis, intolerance, peripheral vascular disease, pneumonitis, and progressive disease described the change in status or appearance of a new medical condition but did not indicate whether erlotinib or gefitinib caused the changes or worsened a pre-existing condition.
The causes of AEs were not described in the available notes or the databases. This retrospective data analysis only focused on identifying drugs involved with erlotinib and gefitinib treatment; further mapping of DDIs among patients experiencing AEs needs to be performed, then in vitro data testing before researchers can reach a conclusion.
DDIs With Antidepressants
We used the PDTS database to evaluate patients who experienced AEs, excluding patients who switched treatment. Thirteen patients filled a prescription for erlotinib and reported taking 220 cancer and noncancer prescription drugs. One patient (pruritis) was taking gefitinib along with 16 noncancer prescription drugs.
Selective serotonin reuptake inhibitors and other antidepressants have been implicated in CYP 2D6 inhibition and DDIs.48,49 Losartan is a widely used antihypertensive drug with a favorable DDI profile
Our data showed that 16 antidepressants (amitriptyline, bupropion, citalopram, desvenlafaxine, duloxetine, escitalopram, imipramine, fluoxetine, fluvoxamine, mirtazapine, nortriptyline, paroxetine, phenelzine, sertraline, trazodone, and venlafaxine) were recorded with concomitant erlotinib or gefitinib from initiation to completion of therapy or a buffer of 6 months from the first diagnosis date. Based on the date dispensed and days’ supply, only escitalopram could be used in combination with gefitinib treatment. The one patient who filled a prescription for gefitinib and escitalopram completed treatment without recorded AEs. PDTS database confirmed that patients experienced AEs with 5 antidepressants (amitriptyline, mirtazapine, paroxetine, trazodone, and venlafaxine) with concomitant erlotinib use.
Based on the date dispensed and days’ supply, only trazodone could be used in combination with erlotinib. PDTS database showed that cancer drugs (erlotinib and megestrol) and 39 noncancer drugs (including acetaminophen, azithromycin, dexamethasone, hydrocortisone, and polyethylene glycol) were filled by 1 patient whose physician noted skin rash. Another limitation of using databases to reflect clinical practice is that although megestrol is listed as a cancer drug by code in the PDTS database, it is not used for nonendometrial or gynecologic cancers. However, because of the PDTS database classification, megestrol is classified as a cancer drug in this retrospective review.
This retrospective review found no significant DDIs for erlotinib or gefitinib, with 1 antidepressant taken by 1 patient for each respective treatment. The degree of inhibition and induction for escitalopram and trazodone are categorized as weak, minimal, or none; therefore, while 1:1 DDIs might be little or no effect, 1:1:1 combination DDIs could have a different outcome. This retrospective data collection cannot be linked to the in vitro hepatocyte DDIs from erlotinib and gefitinib in previous studies.51,52
Conclusions
This retrospective study describes erlotinib and gefitinib use in the MHS and their potential for DDIs. Because of military service requirements, people who are qualified to serve must be healthy or have either controlled or nonactive medical diagnoses and be physically fit. Consequently, our patient population had fewer common medical illnesses, such as diabetes and obesity, compared with the general population. Most noncancer drugs mentioned in this study are not known CYP metabolizers; therefore, recorded AEs alone cannot conclusively determine whether there is a DDI among erlotinib or gefitinib and noncancer drugs. Antidepressants generally are safe but have boxed warnings in the US for increased risk of suicidal ideation in young people.53,54 This retrospective study did not find statistically significant DDIs for erlotinib or gefitinib with antidepressants. Based on this retrospective data analysis, future in vitro testing is needed to assess DDIs for erlotinib or gefitinib and cancer or noncancer drugs identified in this study.
Acknowledgments
The Department of Research Program funds at Walter Reed National Military Medical Center supported this protocol. We sincerely appreciate the contribution of data extraction from the Joint Pathology Center teams (Francisco J. Rentas, John D. McGeeney, Kimberly M. Greenfield, Beatriz A. Hallo, and Johnny P. Beason) and the MHS database personnel (Maj Ryan Costantino, Lee Ann Zarzabal, Brandon Jenkins, and Alex Rittel). We gratefully thank you for the protocol support from the Department of Research programs: CDR Wesley R. Campbell, CDR Ling Ye, Yaling Zhou, Elizabeth Schafer, Robert Roogow, Micah Stretch, Diane Beaner, Adrienne Woodard, David L. Evers, and Paula Amann.
Most cancer treatment regimens include the administration of several chemotherapeutic agents. Drug-drug interactions (DDIs) can increase the risk of fatal adverse events and reduce therapeutic efficacy.1,2 Erlotinib, gefitinib, afatinib, osimertinib, and icotinib are epidermal growth factor receptor–tyrosine kinase inhibitors (EGFR-TKIs) that have proven efficacy for treating advanced non–small cell lung cancer (NSCLC). Erlotinib strongly inhibits cytochrome P450 (CYP) isoenzymes CYP 1A1, moderately inhibits CYP 3A4 and 2C8, and induces CYP 1A1 and 1A2.2 Gefitinib weakly inhibits CYP 2C19 and 2D6.2 CYP 3A4 inducers and inhibitors affect metabolism of both erlotinib and gefitinib.3,4
Erlotinib and gefitinib are first-generation EGFR-TKIs and have been approved for NSCLC treatment by the US Food and Drug Administration (FDA). These agents have been used since the early 2000s and increase the possibility of long-term response and survival.2,5,6 EGFR-TKIs have a range of potential DDIs, including interactions with CYP-dependent metabolism, uridine diphosphate-glucuronosyltransferase, and transporter proteins.2 Few retrospective studies have focused on the therapeutic efficacy of erlotinib, gefitinib, or the combination of these agents.7-14
DDIs from cancer and noncancer therapies could lead to treatment discontinuation and affect patient outcomes. The goals for this study were to perform a broad-scale retrospective analysis focused on investigating prescribed drugs used with erlotinib and gefitinib and determine patient outcomes as obtained through several Military Health System (MHS) databases. Our investigation focused on (1) the functions of these drugs; (2) identifying adverse effects (AEs) that patients experienced; (3) evaluating differences when these drugs are used alone vs concomitantly, and between the completed vs discontinued treatment groups; (4) identifying all drugs used during erlotinib or gefitinib treatment; and (5) evaluating DDIs with antidepressants.
This retrospective study was performed at the Department of Research Programs at Walter Reed National Military Medical Center (WRNMMC) in Bethesda, Maryland. The WRNMMC Institutional Review Board approved the study protocol and ensured compliance with the Health Insurance Portability and Accountability Act as an exempt protocol. The Joint Pathology Center of the US Department of Defense (DoD) Cancer Registry and MHS data experts from the Comprehensive Ambulatory/Professional Encounter Record (CAPER) and the Pharmacy Data Transaction Service (PDTS) provided data for the analysis.
Methods
The DoD Cancer Registry Program was established in 1986 by the Assistant Secretary of Defense for Health Affairs. The registry currently contains data from 1998 to 2023. CAPER and PDTS are part of the MHS Data Repository/Management Analysis and Reporting Tool database. Each observation in the CAPER record represents an ambulatory encounter at a military treatment facility (MTF). CAPER records are available from 2003 to 2023.
Each observation in the PDTS record represents an outpatient prescription filled for an MHS beneficiary at MTFs through the TRICARE mail-order program or a retail pharmacy in the United States. Missing from this record are prescriptions filled at civilian pharmacies outside the United States and inpatient pharmacy prescriptions. The MHS Data Repository PDTS record is available from 2002 to 2023. The Composite Health Care System—the legacy system—is being replaced by GENESIS at MTFs.
Data Extraction Design
The study design involved a cross-sectional analysis. We requested data extraction for erlotinib and gefitinib from 1998 to 2021. Data from the DoD Cancer Registry were used to identify patients who received cancer treatment. Once patients were identified, the CAPER database was searched for diagnoses to identify other health conditions, while the PDTS database was used to populate a list of prescription medications filled during chemotherapy treatment.
Data collected from the Joint Pathology Center included cancer treatment (alone or concomitant), cancer information (cancer types and stages), demographics (sex, age at diagnosis), and physicians’ comments on AEs. Collected data from the MHS include diagnosis and filled prescription history from initiation to completion of the therapy period (or a buffer of 6 months after the initial period). We used all collected data in this analysis. The only exclusion criterion was a provided physician’s note commenting that the patient did not use erlotinib or gefitinib.
Data Extraction Analysis
The Surveillance, Epidemiology, and End Results Program Coding and Staging Manual 2016 and the International Classification of Diseases for Oncology (ICD-O) were used to decode disease and cancer types.15,16 Data sorting and analysis were performed using Microsoft Excel. The percentage for the total was calculated by using the total number of patients or data available within the gefitinib and erlotinib groups divided by total number of patients or data variables. The subgroup percentage was calculated by using the number of patients or data available within the subgroup divided by the total number of patients in that subgroup.
In alone vs concomitant and completed vs discontinued treatment groups, a 2-tailed, 2-sample z test was used to calculate P to determine statistical significance (P < .05) using a statistics website.17 Concomitant was defined as erlotinib or gefitinib taken with other medication(s) before, after, or at the same time as cancer therapy. For the retrospective data analysis, physicians’ notes with “.”, “,”, “/”, “;”, (period, comma, forward slash, semicolon) or space between medication names were interpreted as concurrent, while “+”, “-/+” (plus, minus/plus), or and between drug names were interpreted as combined. Completed treatment was defined as erlotinib or gefitinib as the last medication the patient took without recorded AEs; switching or experiencing AEs was defined as discontinued treatment.
Results
Erlotinib
The Joint Pathology Center provided 387 entries for 382 patients aged 21 to 93 years (mean, 65 years) who were treated systemically with erlotinib from January 1, 2001, to December 31, 2020. Five patients had duplicate entries because they had different cancer sites. There were 287 patients (74%) with lung cancer, 61 (16%) with pancreatic cancer, and 39 (10%) with other cancers. For lung cancer, there were 118 patients (30%) for the upper lobe, 78 (20%) for the lower lobe, and 60 (16%) not otherwise specified (NOS). Other lung cancer sites had fewer patients: 21 (5%) middle lobe lung, 6 (2%) overlapping lung lesion(s), and 4 (1%) main bronchus of the lung. For pancreatic cancer, there were 27 patients (7%) for the head of the pancreas, 10 (3%) pancreas NOS, 9 (2%) body of the pancreas, 9 (2%) tail of the pancreas, 4 (1%) overlapping lesions of the pancreas, 1 (< 1%) pancreatic duct, and 1 (< 1%) other specified parts of the pancreas
There were 342 patients (88%) who were aged > 50 years; 186 male patients (48%) and 201 female patients (52%). There were 293 patients (76%) who had a cancer diagnosis of stage III or IV disease and 94 (24%) who had a cancer diagnosis of stage ≤ II (combination of data for stage 0, 1, and 2, not applicable, and unknown). For their systemic treatment, 161 patients (42%) were treated with erlotinib alone and 226 (58%) received erlotinib concomitantly with additional chemotherapy.
Patients were more likely to discontinue erlotinib for chemotherapy if they received concomitant treatment. Among the patients receiving erlotinib monotherapy, 5% stopped the treatment, whereas 51% of patients treated concomitantly discontinued (P < .001).
Among the 123 patients who discontinued their treatment, 101 switched treatment with no AEs notes, 22 died or experienced fatigue with blurry vision, constipation, nonspecific gastrointestinal effects, grade-4 diarrhea (as defined by the Common Terminology Criteria for Adverse Events), or developed a pleural fluid, pneumonitis, renal failure, skin swelling and facial rash, and unknown AEs of discontinuation. Patients who discontinued treatment because of unknown AEs had physicians’ notes that detailed emergency department visits, peripheral vascular disease, progressive disease, and treatment cessation, but did not specify the exact symptom(s) that led to discontinuation. The causes of death are unknown because they were not detailed in the available notes or databases. The overall results in this retrospective review cannot establish causality between taking erlotinib or gefitinib and death.
Gefitinib
In September 2021, the Joint Pathology Center provided 33 entries for 33 patients who were systemically treated with gefitinib from January 1, 2002, to December 31, 2017. The patient ages ranged from 49 to 89 years with a mean age of 66 years. There were 31 (94%) and 2 (6%) patients with lung and other cancers, respectively. The upper lobe, lower lobe, and lung NOS had the most patients: 14 (42%), 8 (24%), and 6 (18%), respectively.
There were 31 patients (94%) who were aged > 50 years; 15 were male (45%) and 18 were female (55%). There were 26 patients (79%) who had a cancer diagnosis of stage III or IV disease. Nineteen patients (58%) were treated with gefitinib alone, and 14 (42%) were treated with gefitinib concomitantly with additional chemotherapy. Thirty-one patients (94%) were treated for lung cancer (Table 2). Thirty-three patients are a small sample size to determine whether patients were likely to stop gefitinib if used concomitantly with other drugs. Among the patients treated with gefitinib monotherapy, 5% (n = 1) stopped treatment, whereas 29% (n = 4) of patients treated concomitantly discontinued treatment (P = .06). All comparisons for gefitinib yielded insignificant P values. Physicians’ notes indicated that the reasons for gefitinib discontinuation were life-altering pruritis and unknown (progressive disease outcome) (Table 3).
Management Analysis and Reporting Tool Database
MHS data analysts provided data on diagnoses for 348 patients among 415 submitted, with 232 and 112 patients completing and discontinuing erlotinib or gefitinib treatment, respectively. Each patient had 1 to 104 (completed treatment group) and 1 to 157 (discontinued treatment group) unique health conditions documented. The MHS reported 1319 unique-diagnosis conditions for the completed group and 1266 for the discontinued group. Patients with additional health issues stopped chemotherapy use more often than those without; P < .001 for the completed group (232 patients, 1319 diagnoses) vs the discontinued group (112 patients, 1266 diagnoses). The mean (SD) number of diagnoses was 19 (17) for the completed and 30 (22) for the discontinued treatment groups (Figure).
MHS data was provided for patients who filled erlotinib (n = 240) or gefitinib (n = 18). Among the 258 patients, there were 179 and 79 patients in the completed and discontinued treatment groups, respectively. Each patient filled 1 to 75 (for the completed treatment group) and 3 to 103 (for the discontinued treatment group) prescription drugs. There were 805 unique-filled prescriptions for the completed and 670 for the discontinued group. Patients in the discontinued group filled more prescriptions than those who completed treatment; P < .001 for the completed group (179 patients,805 drugs) vs the discontinued group (79 patients, 670 drugs).
The mean (SD) number of filled prescription drugs was 19 (11) for the completed group and 29 (18) for the discontinued treatment group. The 5 most filled prescriptions with erlotinib from 258 patients with PDTS data were ondansetron (151 prescriptions, 10 recorded AEs), dexamethasone (119 prescriptions, 9 recorded AEs), prochlorperazine (105 prescriptions, 15 recorded AEs), oxycodone (99 prescriptions, 1 AE), and docusate (96 prescriptions, 7 recorded AEs).
Discussion
The difference between erlotinib and gefitinib data can be attributed to the FDA approval date and gefitinib’s association with a higher frequency of hepatotoxicity.18-20 The FDA designated gefitinib as an orphan drug for EGFR mutation–positive NSCLC treatment. Gefitinib first received accelerated approval in 2003 for the treatment of locally advanced or metastatic NSCLC. Gefitinib then was voluntarily withdrawn from the market following confirmatory clinical trials that did not verify clinical benefit.
The current approval is for a different patient population—previously untreated, metastatic EGFR exon 19 or 21 L858R mutation—than the 2003 approval.4,6 There was no record of gefitinib use after 2017 in our study.
Erlotinib is a reversible EGFR-TKI that is approved by the FDA as first-line (maintenance) or second-line treatment (after progression following at least 1 earlier chemotherapy regimen) for patients with metastatic NSCLC who harbor EGFR exon 19 deletions or exon 21 L858R substitution mutations, as detected by an FDA-approved test.3 Since 2005, the FDA also approved erlotinib for first-line treatment of patients with locally advanced, unresectable, or metastatic pancreatic cancer in combination with gemcitabine.3 Without FDA indication, erlotinib is used for colorectal, head and neck, ovarian carcinoma, pancreatic carcinoma, and breast cancer.21
Erlotinib and gefitinib are not considered first-line treatments in EGFR exon 19 or 21–mutated NSCLC because osimertinib was approved in 2018. Targeted therapies for EGFR mutation continue to advance at a fast pace, with amivantamab and mobocertinib now FDA approved for EGFR exon 20 insertion–mutated NSCLC.
Erlotinib Use
Thirty-nine patients (10%) in this study were prescribed erlotinib for off-label indications. Erlotinib was used alone or in combination with bevacizumab, capecitabine, cisplatin, denosumab, docetaxel, gemcitabine, and the MEK-inhibitor selumetinib. Erlotinib combined with cisplatin, denosumab, docetaxel, and gemcitabine had no recorded AEs, with 10 data entries for gemcitabine and 1 for other drugs. Three patients received bevacizumab and erlotinib, and 1 patient (diagnosed with kidney NOS) showed rash or facial swelling/erythema and diffuse body itching then stable disease after 2 cycles.
One patient (diagnosed with cancer located at the pancreas head) was bridged with capecitabine and erlotinib when going on a vacation, then received FOLFIRINOX (a combination chemotherapy regimen containing folinic acid [leucovorin], fluorouracil, irinotecan, and oxaliplatin), which led to significant fatigue, blurry vision, and constipation. One patient was treated for lung NOS with the MEK-inhibitor selumetinib plus erlotinib and developed pneumonitis following treatment.
Because oncologists followed guidelines and protocols in systemic treatment, DDIs of erlotinib concurrently (before or after) and in combination with cancer drugs were unlikely. Further investigation is needed for several 1:1:1 DDIs with noncancer drugs. A retrospective overview is not a randomized clinical study; therefore, analysis is limited. Data from the MHS were obtained solely from notes from physicians who treated the patients; therefore, exact information explaining whether a patient completed treatment or had to withdraw could not be extrapolated (ie, blood/plasma samples were not obtained to confirm).
Discontinued Treatment
The reasons for treatment discontinuation with erlotinib or gefitinib varied among patients, with no consistent AE or cause. Most data were for switching treatments after discontinuing treatment with erlotinib (101 of 123 patients) and gefitinib (2 of 5 patients). This is not surprising given the widely recognized pillars of therapy for NSCLC: chemotherapy, target therapy, and immunotherapy.22 From the MHS records, the reasons patients switched treatment of erlotinib or gefitinib were not listed or listed as due to negative EGFR testing, lack of responsiveness, or enrollment in a different treatment.
Physicians’ notes on AEs were not detailed in most cases. Notes for gastrointestinal effects, life-altering pruritis, intolerance, peripheral vascular disease, pneumonitis, and progressive disease described the change in status or appearance of a new medical condition but did not indicate whether erlotinib or gefitinib caused the changes or worsened a pre-existing condition.
The causes of AEs were not described in the available notes or the databases. This retrospective data analysis only focused on identifying drugs involved with erlotinib and gefitinib treatment; further mapping of DDIs among patients experiencing AEs needs to be performed, then in vitro data testing before researchers can reach a conclusion.
DDIs With Antidepressants
We used the PDTS database to evaluate patients who experienced AEs, excluding patients who switched treatment. Thirteen patients filled a prescription for erlotinib and reported taking 220 cancer and noncancer prescription drugs. One patient (pruritis) was taking gefitinib along with 16 noncancer prescription drugs.
Selective serotonin reuptake inhibitors and other antidepressants have been implicated in CYP 2D6 inhibition and DDIs.48,49 Losartan is a widely used antihypertensive drug with a favorable DDI profile
Our data showed that 16 antidepressants (amitriptyline, bupropion, citalopram, desvenlafaxine, duloxetine, escitalopram, imipramine, fluoxetine, fluvoxamine, mirtazapine, nortriptyline, paroxetine, phenelzine, sertraline, trazodone, and venlafaxine) were recorded with concomitant erlotinib or gefitinib from initiation to completion of therapy or a buffer of 6 months from the first diagnosis date. Based on the date dispensed and days’ supply, only escitalopram could be used in combination with gefitinib treatment. The one patient who filled a prescription for gefitinib and escitalopram completed treatment without recorded AEs. PDTS database confirmed that patients experienced AEs with 5 antidepressants (amitriptyline, mirtazapine, paroxetine, trazodone, and venlafaxine) with concomitant erlotinib use.
Based on the date dispensed and days’ supply, only trazodone could be used in combination with erlotinib. PDTS database showed that cancer drugs (erlotinib and megestrol) and 39 noncancer drugs (including acetaminophen, azithromycin, dexamethasone, hydrocortisone, and polyethylene glycol) were filled by 1 patient whose physician noted skin rash. Another limitation of using databases to reflect clinical practice is that although megestrol is listed as a cancer drug by code in the PDTS database, it is not used for nonendometrial or gynecologic cancers. However, because of the PDTS database classification, megestrol is classified as a cancer drug in this retrospective review.
This retrospective review found no significant DDIs for erlotinib or gefitinib, with 1 antidepressant taken by 1 patient for each respective treatment. The degree of inhibition and induction for escitalopram and trazodone are categorized as weak, minimal, or none; therefore, while 1:1 DDIs might be little or no effect, 1:1:1 combination DDIs could have a different outcome. This retrospective data collection cannot be linked to the in vitro hepatocyte DDIs from erlotinib and gefitinib in previous studies.51,52
Conclusions
This retrospective study describes erlotinib and gefitinib use in the MHS and their potential for DDIs. Because of military service requirements, people who are qualified to serve must be healthy or have either controlled or nonactive medical diagnoses and be physically fit. Consequently, our patient population had fewer common medical illnesses, such as diabetes and obesity, compared with the general population. Most noncancer drugs mentioned in this study are not known CYP metabolizers; therefore, recorded AEs alone cannot conclusively determine whether there is a DDI among erlotinib or gefitinib and noncancer drugs. Antidepressants generally are safe but have boxed warnings in the US for increased risk of suicidal ideation in young people.53,54 This retrospective study did not find statistically significant DDIs for erlotinib or gefitinib with antidepressants. Based on this retrospective data analysis, future in vitro testing is needed to assess DDIs for erlotinib or gefitinib and cancer or noncancer drugs identified in this study.
Acknowledgments
The Department of Research Program funds at Walter Reed National Military Medical Center supported this protocol. We sincerely appreciate the contribution of data extraction from the Joint Pathology Center teams (Francisco J. Rentas, John D. McGeeney, Kimberly M. Greenfield, Beatriz A. Hallo, and Johnny P. Beason) and the MHS database personnel (Maj Ryan Costantino, Lee Ann Zarzabal, Brandon Jenkins, and Alex Rittel). We gratefully thank you for the protocol support from the Department of Research programs: CDR Wesley R. Campbell, CDR Ling Ye, Yaling Zhou, Elizabeth Schafer, Robert Roogow, Micah Stretch, Diane Beaner, Adrienne Woodard, David L. Evers, and Paula Amann.
1. van Leeuwen RW, van Gelder T, Mathijssen RH, Jansman FG. Drug-drug interactions with tyrosine-kinase inhibitors: a clinical perspective. Lancet Oncol. 2014;15(8):e315-e326. doi:10.1016/S1470-2045(13)70579-5
2. Xu ZY, Li JL. Comparative review of drug-drug interactions with epidermal growth factor receptor tyrosine kinase inhibitors for the treatment of non-small-cell lung cancer. Onco Targets Ther. 2019;12:5467-5484. doi:10.2147/OTT.S194870
3. Tarceva (erlotinib). Prescribing Information. Genetech, Astellas Pharma; 2016. Accessed June 28, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021743s025lbl.pdf
4. Iressa (gefitinib). Prescribing Information. AstraZeneca; 2018. Accessed June 28, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/206995s003lbl.pdf
5. Cohen MH, Williams GA, Sridhara R, Chen G, Pazdur R. FDA drug approval summary: gefitinib (ZD1839) (Iressa) tablets. Oncologist. 2003;8(4):303-306. doi:10.1634/theoncologist.8-4-303
6. Cohen MH, Williams GA, Sridhara R, Chen G, et al. United States Food and Drug Administration Drug Approval summary: gefitinib (ZD1839; Iressa) tablets. Clin Cancer Res. 2004;10(4):1212-8. doi:10.1158/1078-0432.ccr-03-0564
7. Fiala O, Pesek M, Finek J, et al. Erlotinib in the treatment of advanced squamous cell NSCLC. Neoplasma. 2013;60(6):676-682. doi:10.4149/neo_2013_086
8. Platania M, Agustoni F, Formisano B, et al. Clinical retrospective analysis of erlotinib in the treatment of elderly patients with advanced non-small cell lung cancer. Target Oncol. 2011;6(3):181-186. doi:10.1007/s11523-011-0185-6
9. Tseng JS, Yang TY, Chen KC, Hsu KH, Chen HY, Chang GC. Retrospective study of erlotinib in patients with advanced squamous lung cancer. Lung Cancer. 2012;77(1):128-133. doi:10.1016/j.lungcan.2012.02.012
10. Sim EH, Yang IA, Wood-Baker R, Bowman RV, Fong KM. Gefitinib for advanced non-small cell lung cancer. Cochrane Database Syst Rev. 2018;1(1):CD006847. doi:10.1002/14651858.CD006847.pub2
11. Shrestha S, Joshi P. Gefitinib monotherapy in advanced non-small-cell lung cancer: a retrospective analysis. JNMA J Nepal Med Assoc. 2012;52(186):66-71.
12. Nakamura H, Azuma M, Namisato S, et al. A retrospective study of gefitinib effective cases in non-small cell lung cancer patients with poor performance status. J. Clin. Oncol. 2004 22:14_suppl, 8177-8177. doi:10.1200/jco.2004.22.90140.8177
13. Pui C, Gregory C, Lunqing Z, Long LJ, Tou CH, Hong CT. Retrospective analysis of gefitinib and erlotinib in EGFR-mutated non-small-cell lung cancer patients. J Lung Health Dis. 2017;1(1):16-24. doi:10.29245/2689-999X/2017/1.1105
14. Yoshida T, Yamada K, Azuma K, et al. Comparison of adverse events and efficacy between gefitinib and erlotinib in patients with non-small-cell lung cancer: a retrospective analysis. Med Oncol. 2013;30(1):349. doi:10.1007/s12032-012-0349-y
15. Adamo M, Dickie L, Ruhl J. SEER program coding and staging manual 2016. National Cancer Institute; 2016. Accessed June 28, 2023. https://seer.cancer.gov/archive/manuals/2016/SPCSM_2016_maindoc.pdf
16. World Health Organization. International classification of diseases for oncology (ICD-O) 3rd ed, 1st revision. World Health Organization; 2013. Accessed June 28, 2023. https://apps.who.int/iris/handle/10665/96612
17. Z Score Calculator for 2 population proportions. Social science statistics. Accessed April 25, 2023. https://www.socscistatistics.com/tests/ztest/default2.aspx
18. Takeda M, Okamoto I, Nakagawa K. Pooled safety analysis of EGFR-TKI treatment for EGFR mutation-positive non-small cell lung cancer. Lung Cancer. 2015;88(1):74-79. doi:10.1016/j.lungcan.2015.01.026
19. Burotto M, Manasanch EE, Wilkerson J, Fojo T. Gefitinib and erlotinib in metastatic non-small cell lung cancer: a meta-analysis of toxicity and efficacy of randomized clinical trials. Oncologist. 2015;20(4):400-410. doi:10.1634/theoncologist.2014-0154
20. Yang Z, Hackshaw A, Feng Q, et al. Comparison of gefitinib, erlotinib and afatinib in non-small cell lung cancer: a meta-analysis. Int J Cancer. 2017;140(12):2805-2819. doi:10.1002/ijc.30691
21. Mack JT. Erlotinib. xPharm: The comprehensive pharmacology reference, 2007. Accessed June 28, 2023. https://www.sciencedirect.com/topics/chemistry/erlotinib
22. Melosky B. Rapidly changing treatment algorithms for metastatic nonsquamous non-small-cell lung cancer. Curr Oncol. 2018;25(suppl 1):S68-S76. doi:10.3747/co.25.3839
23. Xeloda (capecitabine). Prescribing Information. Hoffmann-La Roche, Genetech; 2015. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/020896s037lbl.pdf
24. Paraplatin (carboplatin). Prescribing Information. Bristol-Myers Squibb; 2010. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020452s005lbl.pdf
25. Gemzar (gemcitabine). Prescribing Information. Eli Lilly and Company; 1996. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020509s064lbl.pdf
26. Megace (megestrol). Prescribing Information. Par Pharmaceutical, Bristol-Myers Squibb; 2013. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021778s016lbl.pdf
27. Taxol (paclitaxel). Prescribing Information. BASF Aktiengesellschaft, Bristol-Myers Squibb; 2011. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020262s049lbl.pdf
28. Abraxane (paclitaxel). Prescribing Information. Celgene; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/021660s047lbl.pdf
29. Alima (pemetrexed). Prescribing Information. Sindan Pharma, Actavis Pharma; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208419s000lbl.pdf
30. Tagrisso (Osimertinib). Prescribing Information. AstraZeneca; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208065s021lbl.pdf
31. Elavil (amitriptyline). Prescribing Information. Sandoz; 2014. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/085966s095,085969s084,085968s096,085971s075,085967s076,085970s072lbl.pdf
32. Lexapro (escitalopram). Prescribing Information. H. Lundbeck, Allergan; 2017. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021323s047lbl.pdf

33. Remeron (mirtazapine). Prescribing Information. Merck; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020415s029,%20021208s019lbl.pdf
34. Paxil (paroxetine). Prescribing Information. Apotex; 2021. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/020031s077lbl.pdf
35. Desyrel (trazodone). Prescribing Information. Pragma Pharmaceuticals; 2017. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018207s032lbl.pdf
36. Effexor (venlafaxine). Prescribing Information. Norwich Pharmaceuticals, Almatica Pharma; 2022. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215429s000lbl.pdf
37. Sofran (ondansetron). Prescribing Information. GlaxoSmithKline; 2010. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020007s040,020403s018lbl.pdf
38. Hemady (dexamethasone). Prescribing Information. Dexcel Pharma; 2019. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211379s000lbl.pdf
39. Levaquin (levofloxacin). Prescribing Information. Janssen Pharmaceuticals; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020634s073lbl.pdf
40. Percocet (Oxycodone and Acetaminophen). Prescribing Information. Endo Pharmaceuticals; 2006. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/040330s015,040341s013,040434s003lbl.pdf
41. Docusate Sodium usage information. Spirit Pharmaceuticals; 2010. Accessed June 29, 2023. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=84ee7230-0bf6-4107-b5fa-d6fa265139d0
42. Golytely (polyethylene glycol 3350). Prescribing Information. Sebela Pharmaceuticals; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/019011s031lbl.pdf
43. Zithomax (azithromycin). Prescribing Information. Pliva, Pfizer; 2013. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/050710s039,050711s036,050784s023lbl.pdf
44. Acetaminophen. Prescribing Information. Fresenius Kabi; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/204767s003lbl.pdf
45. Compazine (prochlorperazine). Prescribing Information. GlaxoSmithKline; 2004. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/010571s096lbl.pdf
46. Rayos (prednisone). Prescribing Information. Horizon Pharma; 2012. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202020s000lbl.pdf
47. Cortef (hydrocortisone). Prescribing Information. Pfizer; 2019. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/008697s036lbl.pdf
48. Brown CH. Overview of drug–drug interactions with SSRIs. US Pharm. 2008;33(1):HS-3-HS-19. Accessed June 28, 2023. https://www.uspharmacist.com/article/overview-of-drugdrug-interactions-with-ssris
49. Jin X, Potter B, Luong TL, et al. Pre-clinical evaluation of CYP 2D6 dependent drug-drug interactions between primaquine and SSRI/SNRI antidepressants. Malar J. 2016;15(1):280. doi:10.1186/s12936-016-1329-z
50. Sica DA, Gehr TW, Ghosh S. Clinical pharmacokinetics of losartan. Clin Pharmacokinet. 2005;44(8):797-814. doi:10.2165/00003088-200544080-00003
51. Luong TT, Powers CN, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interactions (DDIs) of gefitinib with/without losartan and selective serotonin reuptake inhibitors (SSRIs): citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and venlafaxine. Curr Res Pharmacol Drug Discov. 2022;3:100112. doi:10.1016/j.crphar.2022.100112
52. Luong TT, McAnulty MJ, Evers DL, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interaction (DDI) of gefitinib or erlotinib with Cytochrome P450 (CYP) inhibiting drugs, fluoxetine and/or losartan. Curr Res Toxicol. 2021;2:217-224. doi:10.1016/j.crtox.2021.05.006
53. Lu CY, Zhang F, Lakoma MD, et al. Changes in antidepressant use by young people and suicidal behavior after FDA warnings and media coverage: quasi-experimental study. BMJ. 2014;348:g3596. Published 2014 Jun 18. doi:10.1136/bmj.g359654. Friedman RA. Antidepressants’ black-box warning--10 years later. N Engl J Med. 2014;371(18):1666-1668. doi:10.1056/NEJMp1408480
1. van Leeuwen RW, van Gelder T, Mathijssen RH, Jansman FG. Drug-drug interactions with tyrosine-kinase inhibitors: a clinical perspective. Lancet Oncol. 2014;15(8):e315-e326. doi:10.1016/S1470-2045(13)70579-5
2. Xu ZY, Li JL. Comparative review of drug-drug interactions with epidermal growth factor receptor tyrosine kinase inhibitors for the treatment of non-small-cell lung cancer. Onco Targets Ther. 2019;12:5467-5484. doi:10.2147/OTT.S194870
3. Tarceva (erlotinib). Prescribing Information. Genetech, Astellas Pharma; 2016. Accessed June 28, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021743s025lbl.pdf
4. Iressa (gefitinib). Prescribing Information. AstraZeneca; 2018. Accessed June 28, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/206995s003lbl.pdf
5. Cohen MH, Williams GA, Sridhara R, Chen G, Pazdur R. FDA drug approval summary: gefitinib (ZD1839) (Iressa) tablets. Oncologist. 2003;8(4):303-306. doi:10.1634/theoncologist.8-4-303
6. Cohen MH, Williams GA, Sridhara R, Chen G, et al. United States Food and Drug Administration Drug Approval summary: gefitinib (ZD1839; Iressa) tablets. Clin Cancer Res. 2004;10(4):1212-8. doi:10.1158/1078-0432.ccr-03-0564
7. Fiala O, Pesek M, Finek J, et al. Erlotinib in the treatment of advanced squamous cell NSCLC. Neoplasma. 2013;60(6):676-682. doi:10.4149/neo_2013_086
8. Platania M, Agustoni F, Formisano B, et al. Clinical retrospective analysis of erlotinib in the treatment of elderly patients with advanced non-small cell lung cancer. Target Oncol. 2011;6(3):181-186. doi:10.1007/s11523-011-0185-6
9. Tseng JS, Yang TY, Chen KC, Hsu KH, Chen HY, Chang GC. Retrospective study of erlotinib in patients with advanced squamous lung cancer. Lung Cancer. 2012;77(1):128-133. doi:10.1016/j.lungcan.2012.02.012
10. Sim EH, Yang IA, Wood-Baker R, Bowman RV, Fong KM. Gefitinib for advanced non-small cell lung cancer. Cochrane Database Syst Rev. 2018;1(1):CD006847. doi:10.1002/14651858.CD006847.pub2
11. Shrestha S, Joshi P. Gefitinib monotherapy in advanced non-small-cell lung cancer: a retrospective analysis. JNMA J Nepal Med Assoc. 2012;52(186):66-71.
12. Nakamura H, Azuma M, Namisato S, et al. A retrospective study of gefitinib effective cases in non-small cell lung cancer patients with poor performance status. J. Clin. Oncol. 2004 22:14_suppl, 8177-8177. doi:10.1200/jco.2004.22.90140.8177
13. Pui C, Gregory C, Lunqing Z, Long LJ, Tou CH, Hong CT. Retrospective analysis of gefitinib and erlotinib in EGFR-mutated non-small-cell lung cancer patients. J Lung Health Dis. 2017;1(1):16-24. doi:10.29245/2689-999X/2017/1.1105
14. Yoshida T, Yamada K, Azuma K, et al. Comparison of adverse events and efficacy between gefitinib and erlotinib in patients with non-small-cell lung cancer: a retrospective analysis. Med Oncol. 2013;30(1):349. doi:10.1007/s12032-012-0349-y
15. Adamo M, Dickie L, Ruhl J. SEER program coding and staging manual 2016. National Cancer Institute; 2016. Accessed June 28, 2023. https://seer.cancer.gov/archive/manuals/2016/SPCSM_2016_maindoc.pdf
16. World Health Organization. International classification of diseases for oncology (ICD-O) 3rd ed, 1st revision. World Health Organization; 2013. Accessed June 28, 2023. https://apps.who.int/iris/handle/10665/96612
17. Z Score Calculator for 2 population proportions. Social science statistics. Accessed April 25, 2023. https://www.socscistatistics.com/tests/ztest/default2.aspx
18. Takeda M, Okamoto I, Nakagawa K. Pooled safety analysis of EGFR-TKI treatment for EGFR mutation-positive non-small cell lung cancer. Lung Cancer. 2015;88(1):74-79. doi:10.1016/j.lungcan.2015.01.026
19. Burotto M, Manasanch EE, Wilkerson J, Fojo T. Gefitinib and erlotinib in metastatic non-small cell lung cancer: a meta-analysis of toxicity and efficacy of randomized clinical trials. Oncologist. 2015;20(4):400-410. doi:10.1634/theoncologist.2014-0154
20. Yang Z, Hackshaw A, Feng Q, et al. Comparison of gefitinib, erlotinib and afatinib in non-small cell lung cancer: a meta-analysis. Int J Cancer. 2017;140(12):2805-2819. doi:10.1002/ijc.30691
21. Mack JT. Erlotinib. xPharm: The comprehensive pharmacology reference, 2007. Accessed June 28, 2023. https://www.sciencedirect.com/topics/chemistry/erlotinib
22. Melosky B. Rapidly changing treatment algorithms for metastatic nonsquamous non-small-cell lung cancer. Curr Oncol. 2018;25(suppl 1):S68-S76. doi:10.3747/co.25.3839
23. Xeloda (capecitabine). Prescribing Information. Hoffmann-La Roche, Genetech; 2015. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/020896s037lbl.pdf
24. Paraplatin (carboplatin). Prescribing Information. Bristol-Myers Squibb; 2010. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020452s005lbl.pdf
25. Gemzar (gemcitabine). Prescribing Information. Eli Lilly and Company; 1996. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020509s064lbl.pdf
26. Megace (megestrol). Prescribing Information. Par Pharmaceutical, Bristol-Myers Squibb; 2013. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021778s016lbl.pdf
27. Taxol (paclitaxel). Prescribing Information. BASF Aktiengesellschaft, Bristol-Myers Squibb; 2011. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020262s049lbl.pdf
28. Abraxane (paclitaxel). Prescribing Information. Celgene; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/021660s047lbl.pdf
29. Alima (pemetrexed). Prescribing Information. Sindan Pharma, Actavis Pharma; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208419s000lbl.pdf
30. Tagrisso (Osimertinib). Prescribing Information. AstraZeneca; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208065s021lbl.pdf
31. Elavil (amitriptyline). Prescribing Information. Sandoz; 2014. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/085966s095,085969s084,085968s096,085971s075,085967s076,085970s072lbl.pdf
32. Lexapro (escitalopram). Prescribing Information. H. Lundbeck, Allergan; 2017. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021323s047lbl.pdf

33. Remeron (mirtazapine). Prescribing Information. Merck; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020415s029,%20021208s019lbl.pdf
34. Paxil (paroxetine). Prescribing Information. Apotex; 2021. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/020031s077lbl.pdf
35. Desyrel (trazodone). Prescribing Information. Pragma Pharmaceuticals; 2017. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018207s032lbl.pdf
36. Effexor (venlafaxine). Prescribing Information. Norwich Pharmaceuticals, Almatica Pharma; 2022. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215429s000lbl.pdf
37. Sofran (ondansetron). Prescribing Information. GlaxoSmithKline; 2010. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020007s040,020403s018lbl.pdf
38. Hemady (dexamethasone). Prescribing Information. Dexcel Pharma; 2019. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211379s000lbl.pdf
39. Levaquin (levofloxacin). Prescribing Information. Janssen Pharmaceuticals; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020634s073lbl.pdf
40. Percocet (Oxycodone and Acetaminophen). Prescribing Information. Endo Pharmaceuticals; 2006. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/040330s015,040341s013,040434s003lbl.pdf
41. Docusate Sodium usage information. Spirit Pharmaceuticals; 2010. Accessed June 29, 2023. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=84ee7230-0bf6-4107-b5fa-d6fa265139d0
42. Golytely (polyethylene glycol 3350). Prescribing Information. Sebela Pharmaceuticals; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/019011s031lbl.pdf
43. Zithomax (azithromycin). Prescribing Information. Pliva, Pfizer; 2013. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/050710s039,050711s036,050784s023lbl.pdf
44. Acetaminophen. Prescribing Information. Fresenius Kabi; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/204767s003lbl.pdf
45. Compazine (prochlorperazine). Prescribing Information. GlaxoSmithKline; 2004. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/010571s096lbl.pdf
46. Rayos (prednisone). Prescribing Information. Horizon Pharma; 2012. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202020s000lbl.pdf
47. Cortef (hydrocortisone). Prescribing Information. Pfizer; 2019. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/008697s036lbl.pdf
48. Brown CH. Overview of drug–drug interactions with SSRIs. US Pharm. 2008;33(1):HS-3-HS-19. Accessed June 28, 2023. https://www.uspharmacist.com/article/overview-of-drugdrug-interactions-with-ssris
49. Jin X, Potter B, Luong TL, et al. Pre-clinical evaluation of CYP 2D6 dependent drug-drug interactions between primaquine and SSRI/SNRI antidepressants. Malar J. 2016;15(1):280. doi:10.1186/s12936-016-1329-z
50. Sica DA, Gehr TW, Ghosh S. Clinical pharmacokinetics of losartan. Clin Pharmacokinet. 2005;44(8):797-814. doi:10.2165/00003088-200544080-00003
51. Luong TT, Powers CN, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interactions (DDIs) of gefitinib with/without losartan and selective serotonin reuptake inhibitors (SSRIs): citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and venlafaxine. Curr Res Pharmacol Drug Discov. 2022;3:100112. doi:10.1016/j.crphar.2022.100112
52. Luong TT, McAnulty MJ, Evers DL, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interaction (DDI) of gefitinib or erlotinib with Cytochrome P450 (CYP) inhibiting drugs, fluoxetine and/or losartan. Curr Res Toxicol. 2021;2:217-224. doi:10.1016/j.crtox.2021.05.006
53. Lu CY, Zhang F, Lakoma MD, et al. Changes in antidepressant use by young people and suicidal behavior after FDA warnings and media coverage: quasi-experimental study. BMJ. 2014;348:g3596. Published 2014 Jun 18. doi:10.1136/bmj.g359654. Friedman RA. Antidepressants’ black-box warning--10 years later. N Engl J Med. 2014;371(18):1666-1668. doi:10.1056/NEJMp1408480