User login
Pharmacist-Led Deprescribing of Aspirin for Primary Prevention of Cardiovascular Disease Among Geriatric Veterans
Pharmacist-Led Deprescribing of Aspirin for Primary Prevention of Cardiovascular Disease Among Geriatric Veterans
Low-dose aspirin commonly is used for the prevention of cardiovascular disease (CVD) but is associated with an increased risk of major bleeding.1 The use of aspirin for primary prevention is largely extrapolated from clinical trials showing benefit in the secondary prevention of myocardial infarction and ischemic stroke. However, results from the Aspirin in Reducing Events in the Elderly (ASPREE) trial challenged this practice.2 The ASPREE trial, conducted in the United States and Australia from 2010 to 2014, sought to determine whether daily 100 mg aspirin, was superior to placebo in promoting disability-free survival among older adults. Participants were aged ≥ 70 years (≥ 65 years for Hispanic and Black US participants), living in the community, and were free from preexisting CVD, cerebrovascular disease, or any chronic condition likely to limit survival to < 5 years. The study found no significant difference in the primary endpoints of death, dementia, or persistent physical disability, but there was a significantly higher risk of major hemorrhage in the aspirin group (3.8% vs 2.8%; hazard ratio, 1.38; 95% CI, 1.18-1.62; P < .001).
Several medical societies have updated their guideline recommendations for aspirin for primary prevention of CVD. The 2022 United States Public Service Task Force (USPSTF) provides a grade C recommendation (at least moderate certainty that the net benefit is small) to consider low-dose aspirin for the primary prevention of CVD on an individual patient basis for adults aged 40 to 59 years who have a ≥ 10% 10-year CVD risk. For adults aged ≥ 60 years, the USPSTF recommendation is grade D (moderate or high certainty that the practice has no net benefit or that harms outweigh the benefits) for low-dose aspirin use.1,3 The American College of Cardiology and American Heart Association (ACC/AHA) recommend considering low-dose aspirin for primary prevention of atherosclerotic cardiovascular disease (ASCVD) among select adults aged 40 to 70 years at higher CVD risk but not at increased risk of bleeding.4 The American Diabetes Association (ADA) recommends low-dose aspirin for primary prevention of CVD in patients with diabetes and additional risk factors such as family history of premature ASCVD, hypertension, dyslipidemia, smoking, or chronic kidney disease, and who are not at higher risk of bleeding.5 The ADA standards also caution against the use of aspirin as primary prevention in patients aged > 70 years. Low-dose aspirin use is not recommended for the primary prevention of CVD in older adults or adults of any age who are at increased risk of bleeding.
Recent literature using the US Department of Veterans Affairs (VA) Corporate Data Warehouse database confirms 86,555 of 1.8 million veterans aged > 70 years (5%) were taking low-dose aspirin for primary prevention of ASCVD despite guideline recommendations.6 Higher risk of gastrointestinal and other major bleeding from low-dose aspirin has been reported in the literature.1 Major bleeds represent a significant burden to the health care system with an estimated mean $13,093 cost for gastrointestinal bleed hospitalization.7
Considering the large scale aspirin use without appropriate indication within the veteran population, the risk of adverse effects, and the significant cost to patients and the health care system, it is imperative to determine the best approach to efficiently deprescribe aspirin for primary prevention among geriatric patients. Deprescribing refers to the systematic and supervised process of dose reduction or drug discontinuation with the goal of improving health and/or reducing the risk of adverse effects.8 During patient visits, primary care practitioners (PCPs) have opportunities to discontinue aspirin, but these encounters are time-limited and deprescribing might be secondary to more acute primary care needs. The shortage of PCPs is expected to worsen in coming years, which could further reduce their availability to assess inappropriate aspirin use.9
VA clinical pharmacist practitioners (CPPs) serve as medication experts and work autonomously under a broad scope of practice as part of the patient aligned care team.10-12 CPPs can free up time for PCPs and facilitate deprescribing efforts, especially for older adults. One retrospective cohort study conducted at a VA medical center found that CPPs deprescribed more potentially inappropriate medications among individuals aged ≥ 80 years compared with usual care with PCPs (26.8% vs 16.1%; P < .001).12,13 An aspirin deprescribing protocol conducted in 2022 resulted in nearly half of veterans aged ≥ 70 years contacted by phone agreeing to stop aspirin. Although this study supports the role pharmacists can play in reducing aspirin use in accordance with guidelines, the authors acknowledge that their interventions had a mean time of 12 minutes per patient and would require workflow changes.14 The purpose of this study is to evaluate the efficiency of aspirin deprescribing through 2 approaches: direct deprescribing by pharmacists using populationlevel review compared with clinicians following a pharmacist-led education.
Methods
This was a single-center quality improvement cohort study at the Durham VA Health Care System (DVAHCS) in North Carolina. Patients included were aged ≥ 70 years without known ASCVD who received care at any of 3 DVAHCS community-based outpatient clinics and prescribed aspirin. Patient data was obtained using the VIONE (Deprescribing Dashboard called Vital, Important, Optional, Not indicated, and Every medication has a specific indication or diagnosis) dashboard.15 VIONE was developed to identify potentially inappropriate medications (PIMs) that are eligible to deprescribe based on Beers Criteria, Screening Tool of Older Personsf Prescriptions criteria, and common clinical scenarios when clinicians determine the risk outweighs the benefit to continue a specific medication. 16,17 VIONE is used to reduce polypharmacy and improve patient safety, comfort, and medication adherence. Aspirin for patients aged ≥ 70 years without a history of ASCVD is a PIM identified by VIONE. Patients aged ≥ 70 years were chosen as an inclusion criteria in this study to match the ASPREE trial inclusion criteria and age inclusion criteria in the VIONE dashboard for aspirin deprescribing.2 Patient lists were generated for these potentially inappropriate aspirin prescriptions for 3 months before clinician staff education presentations, the day of the presentations, and 3 months after.
The primary endpoint was the number of veterans with aspirin deprescribed directly by 2 pharmacists over 12 weeks, divided by total patient care time spent, compared with the change in number of veterans with aspirin deprescribed by any DVAHCS physician, nurse practitioner, physician assistant, or CPP over 12 weeks, divided by the total pharmacist time spent on PCP education. Secondary endpoints were the number of aspirin orders discontinued by pharmacists and CPPs, the number of aspirin orders discontinued 12 weeks before pharmacist-led education compared with the number of aspirin orders discontinued 12 weeks after CPP-led education, average and median pharmacist time spent per patient encounter, and time of direct patient encounters vs time spent on PCP education.
Pharmacists reviewed each patient who met the inclusion criteria from the list generated by VIONE on December 1, 2022, for aspirin appropriateness according to the ACC/AHA and USPSTF guidelines, with the goal to discontinue aspirin for primary prevention of ASCVD and no other indications.1,4 Pharmacists documented their visits using VIONE methodology in the Computerized Patient Record System (CPRS) using a polypharmacy review note. CPPs contacted patients who were taking aspirin for primary prevention by unscheduled telephone call to assess for aspirin adherence, undocumented history of ASCVD, cardiovascular risk factors, and history of bleeding. Aspirin was discontinued if patients met guideline criteria recommendations and agreed to discontinuation. Risk-benefit discussions were completed when patients without known ASCVD were considered high risk because the ACC/AHA guidelines mention there is insufficient evidence of safety and efficacy of aspirin for primary prevention for patients with other known ASCVD risk factors (eg, strong family history of premature myocardial infarction, inability to achieve lipid, blood pressure, or glucose targets, or significant elevation in coronary artery calcium score).
High risk was defined as family history of premature ASCVD (in a male first-degree relative aged < 55 years or a female first-degree relative aged < 65 years), most recent blood pressure or 2 blood pressure results in the last 12 months > 160/100 mm Hg, recent hemoglobin A1c > 9%, and/or low-density lipoprotein > 190 mg/dL or not prescribed an indicated statin.3 Aspirin was continued or discontinued according to patient preference after the personalized risk-benefit discussion.
For patients with a clinical indication for aspirin use other than ASCVD (eg, atrial fibrillation not on anticoagulation, venous thromboembolism prophylaxis, carotid artery disease), CPPs documented their assessment and when appropriate deferred to the PCP for consideration of stopping aspirin. For patients with undocumented ASCVD, CPPs added their ASCVD history to their problem list and aspirin was continued. PCPs were notified by alert when aspirin was discontinued and when patients could not be reached by telephone.
presented a review of recent guideline updates and supporting literature at 2 online staff meetings. The education sessions lasted about 10 minutes and were presented to PCPs across 3 community-based outpatient clinics. An estimated 40 minutes were spent creating the PowerPoint education materials, seeking feedback, making edits, and answering questions or emails from PCPs after the presentation. During the presentation, pharmacists encouraged PCPs to discontinue aspirin (active VA prescriptions and reported over-the-counter use) for primary prevention of ASCVD in patients aged ≥ 70 years during their upcoming appointments and consider risk factors recommended by the ACC/AHA guidelines when applicable. PCPs were notified that CPPs planned to start a population review for discontinuing active VA aspirin prescriptions on December 1, 2022. The primary endpoint and secondary endpoints were analyzed using descriptive statistics. All data were analyzed using Microsoft Excel.
Results
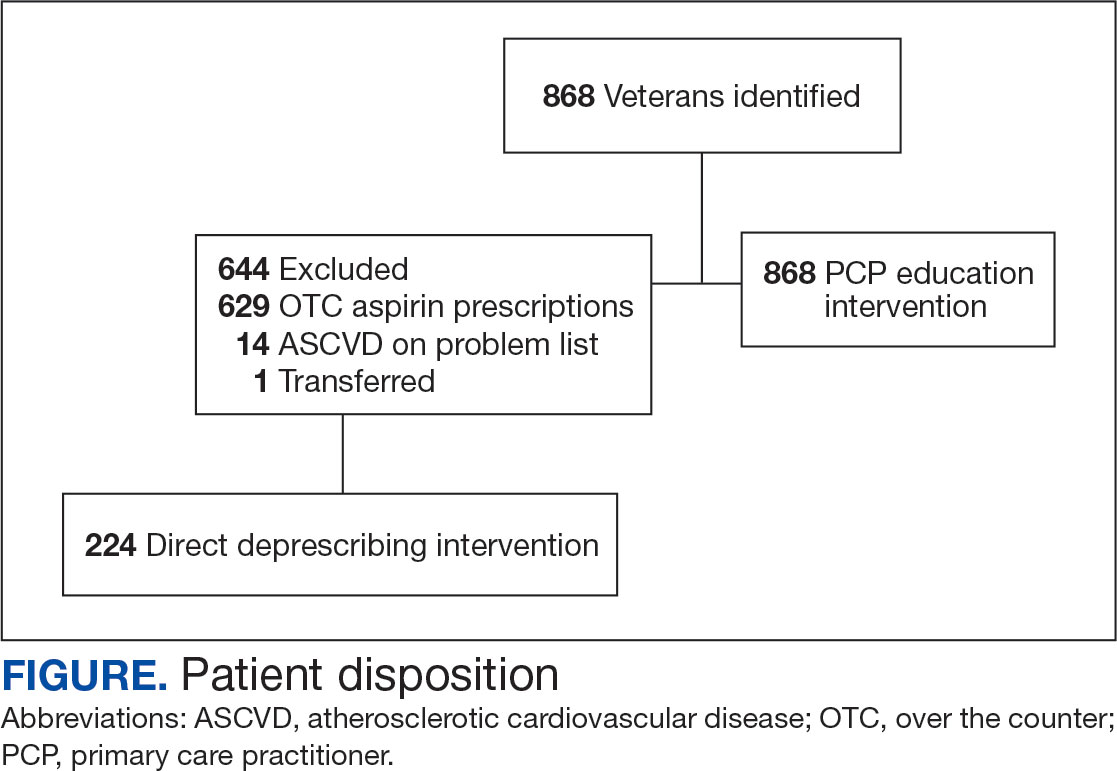
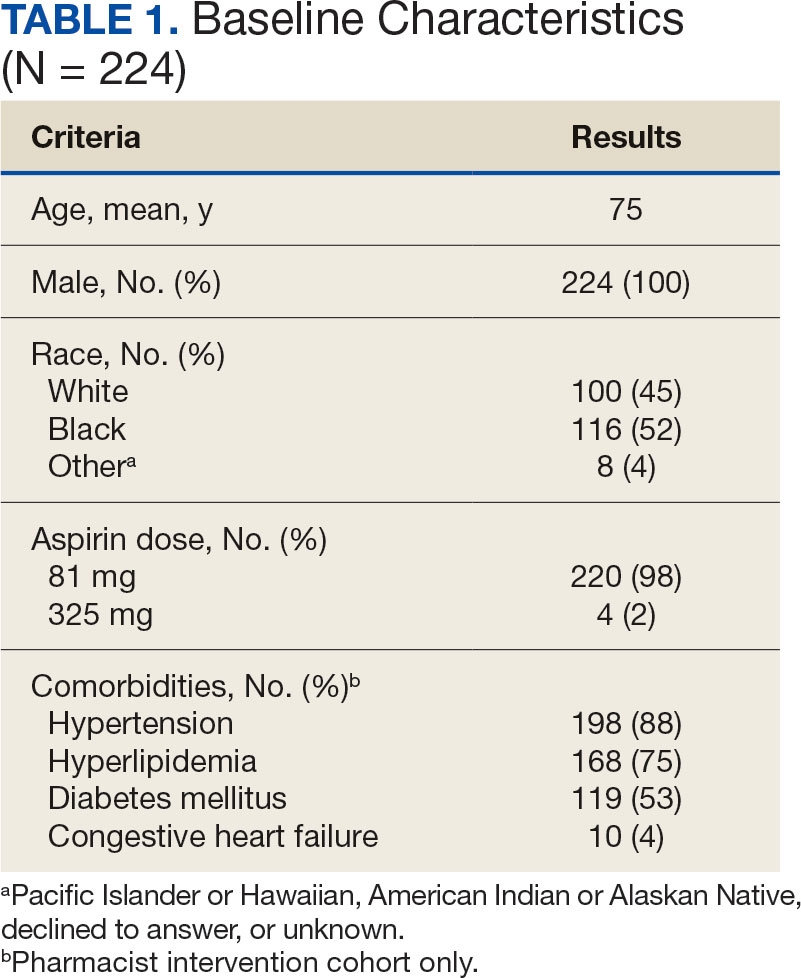
A total of 868 patients aged ≥ 70 years with active prescriptions for aspirin were identified on December 1, 2022. After applying inclusion and exclusion criteria for the pharmacist population review, 224 patients were included for cohort final analysis (Figure). All 868 patients were eligible for the CPP intervention. Primary reasons for exclusion from the CPP population included over-thecounter aspirin and a history of ASCVD in the patient’s problem list. All patients were male, with a mean (SD) age of 75 (4.4) years (Table 1). Most patients were prescribed aspirin, 81 mg daily (n = 220; 98%).
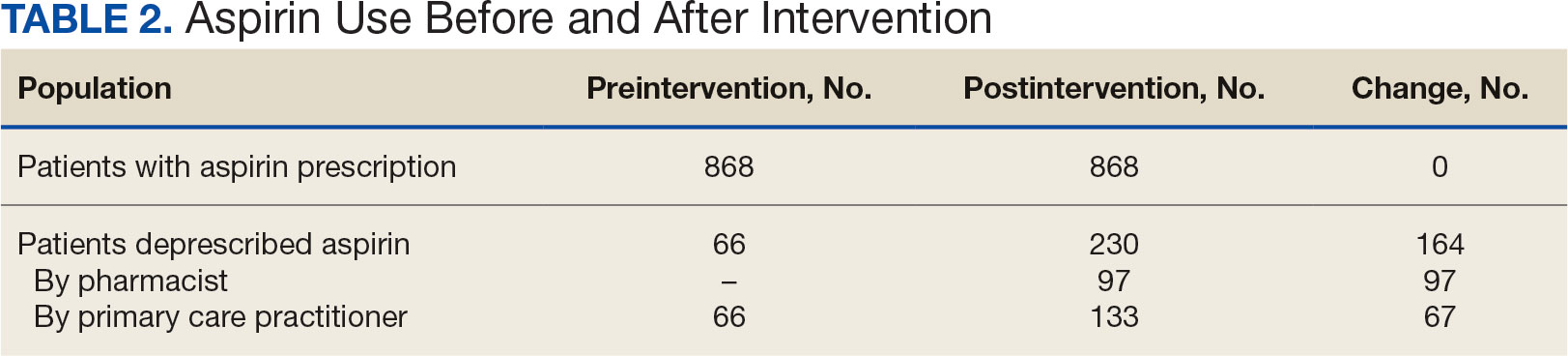
The direct CPP deprescribing intervention resulted in 2 aspirin prescriptions discontinued per hour of pharmacist time and 67 aspirin prescriptions discontinued per hour of pharmacist time via the PCP education intervention. CPPs discontinued 66 aspirin orders in the 12 weeks before the PCP education sessions. A total of 230 aspirin prescriptions were discontinued in the 12 weeks following the PCP education sessions, with 97 discontinued directly by CPPs and 133 discontinued by PCPs. The PCP education session yielded an additional 67 discontinued aspirin orders compared with the 12 weeks before the education sessions (Table 2).
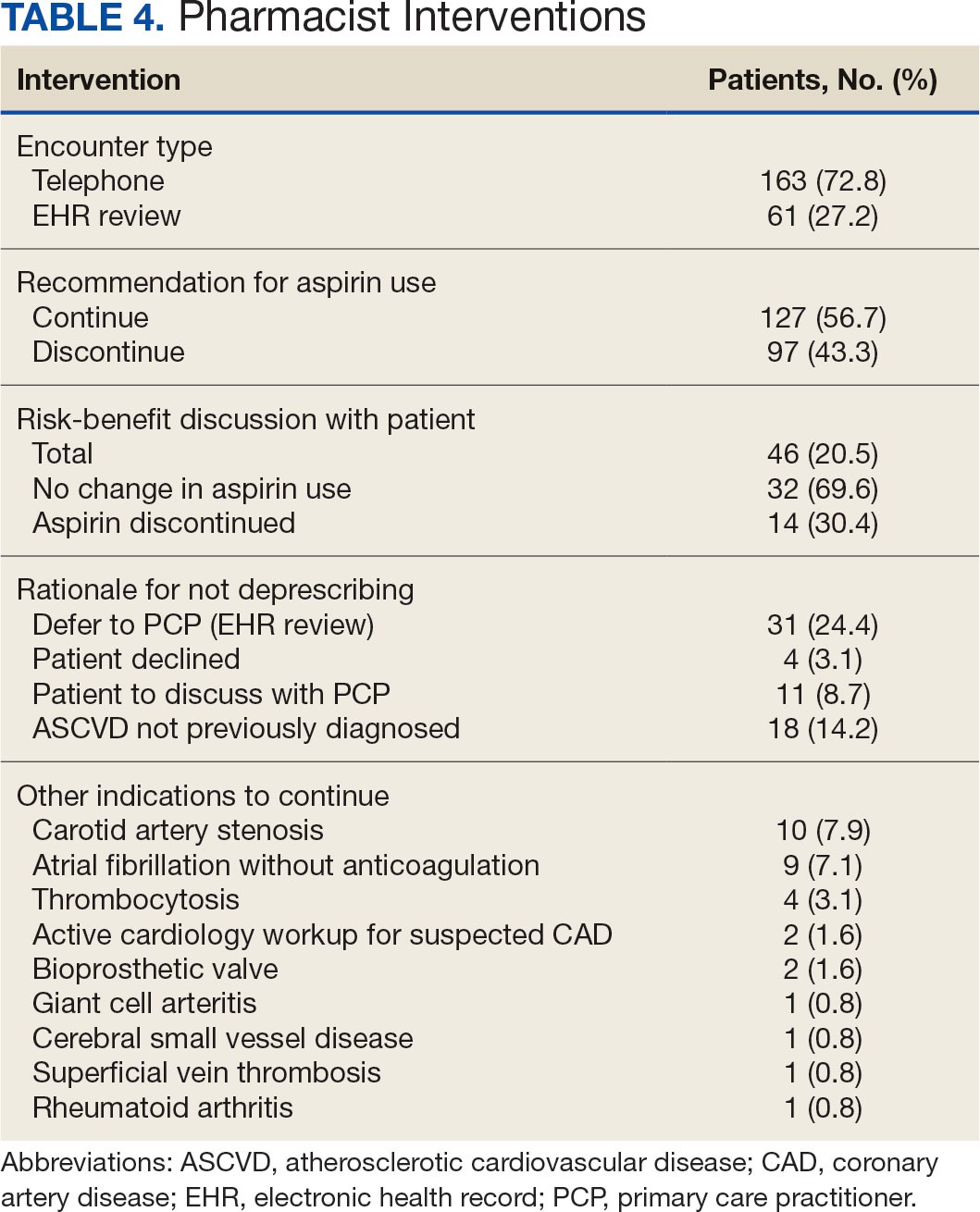
The CPP direct deprescribing intervention took about 48.3 hours, accounting for health record review and time interacting with patients. The PCP education intervention took about 60 minutes, which included time for preparing and delivering education materials (Table 3). CPP deprescribing encounter types, interventions, and related subcategories, and other identified indications to continue aspirin are listed in Table 4.

Discussion
Compared with direct deprescribing by pharmacists, the PCP education intervention was more efficient based on number of aspirin orders discontinued by pharmacist time. PCPs discontinued twice as many aspirin prescriptions in the 12 weeks after pharmacist-led education compared with the 12 weeks before.
Patients were primarily contacted by telephone (73%) for deprescribing. Among the 163 patients reached by phone and encouraged to discontinue aspirin, 97 patients (60%) accepted the recommendation, which was similar to the acceptance rates found in the literature (48% to 55%).14,18 Although many veterans continued taking aspirin (78%), most had indications for its continued use, such as a history of ASCVD, atrial fibrillation without anticoagulation, and carotid artery stenosis, and complex comorbidities that required further discussion with their PCP. Less common uses for aspirin were identified through CPRS review or patient reports included cerebral small vessel disease without history of ASCVD, subclavian artery stenosis, thrombocytosis, bioprosthetic valve replacement, giant cell arteritis, rheumatoid arthritis, and prevention of second eye involvement of ischemic optic neuropathy.
to describe the benefit of clinical pharmacy services for deprescribing aspirin for primary prevention of ASCVD through PCP education. Previously published literature has assessed alternative ways to identify or discontinue PIMs—including aspirin—among geriatric patients. One study evaluated the use of marking inappropriate aspirin prescriptions in the electronic health database, leading to a significant reduction in incidence of inappropriate aspirin prescribing; however, it did not assess changes in discontinuation rates of existing aspirin prescriptions.19 The previous VA pharmacist aspirin deprescribing protocol demonstrated pharmacists’ aptitude at discontinuing aspirin for primary prevention but only used direct patient contact and did not compare efficiency with other methods, including PCP education.14
This quality improvement project contributes new data to the existing literature to support the use of clinical pharmacists to discontinue aspirin for primary prevention and suggests a strong role for pharmacists as educators on clinical guidelines, in addition to their roles directly deprescribing PIMs in clinical practice. This study is further strengthened by its use of VIONE, which previously has demonstrated effectiveness in deprescribing a variety of PIMs in primary care settings.20
Despite using VIONE for generating a list of patients eligible for deprescription, our CPRS review found that this list was frequently inaccurate. For example, a small portion of patients were on the VIONE generated list indicating they had no ASCVD history, but had transient ischemic attack listed in their problem lists. Patient problem lists often were missing documented ASCVD history that was revealed by patient interview or CPRS review. It is possible that patients interviewed might have omitted relevant ASCVD history because of low health literacy, conditions affecting memory, or use of health care services outside the VA system.
There were several instances of aspirin used for other non-ASCVD indications, such as primary stroke prevention in atrial fibrillation. The ACC/AHA atrial fibrillation guidelines previously provided a Class IIb recommendation (benefit is greater than risk but additional studies are needed) for considering no antithrombic therapy or treatment with oral anticoagulant or aspirin for nonvalvular atrial fibrillation with CHA2DS2-VASc (Congestive heart failure, Hypertension, Age [> 65 y, 1 point; > 75 y, 2 points], Diabetes, previous Stroke/transient ischemic attack [2 points]) score of 1.21 The ACC/ AHA guidelines were updated in 2023 to recommend against antiplatelet therapy as an alternative to anticoagulation for reducing cardioembolic stroke risk among patients with atrial fibrillation with no indication for antiplatelet therapy because of risk of harm.22 If a patient has no risk factors for stroke, aspirin is not recommended to prevent thromboembolic events because of a lack of benefit. Interventions from this quality improvement study were completed before the 2023 atrial fibrillation guideline was published and therefore in this study aspirin was not discontinued when used for atrial fibrillation. Aspirin use for atrial fibrillation might benefit from similar discontinuation efforts analyzed within this study. Beyond atrial fibrillation, major guidelines do not comment on the use of aspirin for any other indications in the absence of clinical ASCVD.
Limitations
This study is limited by the lack of clinical consensus for complex patients and demonstrates the importance of individualized patient assessment when considering discontinuing aspirin. Because of the project’s relatively short intervention period, aspirin deprescribing rates could decrease over time and repeated education efforts might be necessary to see lasting impact. Health care professionals from services outside of primary care also might have discontinued aspirin during the study period unrelated to the education and these discontinued aspirin prescriptions could contribute to the higher rate observed among PCPs. This study included a specific population cohort of male, US veterans and might not reflect other populations where these interventions could be implemented.
The measurement of time spent by pharmacists and PCPs is an additional limitation. Although it is expected that PCPs attempt to discontinue aspirin during their existing patient care appointments, the time spent during visits was not measured or documented. Direct deprescribing by pharmacist CPRS review required a significant amount of time and could be a barrier to successful intervention by CPPs in patient aligned care teams.
To reduce the time pharmacists spent completing CPRS reviews, an aspirin deprescribing clinical reminder tool could be used to assess use and appropriate indication quickly during any primary care visit led by a PCP or CPP. In addition, it is recommended that pharmacists regularly educate health care professionals on guideline recommendations for aspirin use among geriatric patients. Future studies of the incidence of major cardiovascular events after aspirin deprescribing among geriatric patients and a longitudinal cost/benefit analysis could support these initiatives.
Conclusions
In this study, pharmacists successfully deprescribed inappropriate medications, such as aspirin. However, pharmacist-led PCP education is more efficient compared with direct deprescribing using a population-level review. PCP education requires less time and could allow ambulatory care pharmacists to spend more time on other direct patient care interventions to improve quality and access to care in primary care clinics. This study’s results further support the role of pharmacists in deprescribing PIMs for older adults and the use of a deprescribing tool, such as VIONE, in a primary care setting.
- US Preventive Services Task Force; Davidson KW, Barry MJ, et al. Aspirin use to prevent cardiovascular disease: US Preventive Services Task Force recommendation statement. JAMA. 2022;327(16):1577-1584. doi:10.1001/jama.2022.4983
- McNeil JJ, Nelson MR, Woods RL, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379(16):1519-1528. doi:10.1056/NEJMoa1803955
- Barry MJ, Wolff TA, Pbert L, et al. Putting evidence into practice: an update on the US Preventive Services Task Force methods for developing recommendations for preventive services. Ann Fam Med. 2023;21(2):165-171. doi:10.1370/afm.2946
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/ AHA Guideline on the Primary Prevention of Cardiovascular Disease: A report of the American College of Cardiology/ American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596-e646. doi:10.1161/CIR.0000000000000678
- American Diabetes Association Professional Practice Committee. 10. Cardiovascular disease and risk management: Standards of care in diabetes-2024. Diabetes Care. 2024;47(Suppl 1):S179-S218. doi:10.2337/dc24-S010
- Ong SY, Chui P, Bhargava A, Justice A, Hauser RG. Estimating aspirin overuse for primary prevention of atherosclerotic cardiovascular disease (from a nationwide healthcare system). Am J Cardiol. 2020;137:25-30. doi:10.1016/j.amjcard.2020.09.042
- Weiss AJ, Jiang HJ. Overview of clinical conditions with frequent and costly hospital readmissions by payer, 2018. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality (US); July 20, 2021.
- Krishnaswami A, Steinman MA, Goyal P, et al. Deprescribing in older adults with cardiovascular disease. J Am Coll Cardiol. 2019;73(20):2584-2595. doi:10.1016/j.jacc.2019.03.467
- Association of American Medical Colleges. The complexities of physician supply and demand: projections from 2019 to 2034. Accessed March 17, 2024. https://www.aamc.org/media/54681/download
- US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1108.07(1): General pharmacy service requirements. November 28, 2022. Accessed March 17, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=10045
- US Department of Veterans Affairs, Veterans Health Administration. VHA Handbook 1108.11(3): Clinical pharmacy services. July 1, 2015. Accessed March 17, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3120
- US Department of Veterans Affairs. Clinical pharmacist practitioner (CPP) to improve access to and quality of care August 2021. August 2021. Accessed May 19, 2023. https://www.pbm.va.gov/PBM/CPPO/Documents/ExternalFactSheet_OptimizingtheCPPToImproveAccess_508.pdf
- Ammerman CA, Simpkins BA, Warman N, Downs TN. Potentially inappropriate medications in older adults: Deprescribing with a clinical pharmacist. J Am Geriatr Soc. 2019;67(1):115-118. doi:10.1111/jgs.15623
- Rothbauer K, Siodlak M, Dreischmeier E, Ranola TS, Welch L. Evaluation of a pharmacist-driven ambulatory aspirin deprescribing protocol. Fed Pract. 2022;39(suppl 5):S37- S41a. doi:10.12788/fp.0294
- US Department of Veterans Affairs. VIONE changes the way VA handles prescriptions. January 25, 2020. Accessed May 21, 2023. https://news.va.gov/70709/vione-changes-way-va-handles-prescriptions/
- 2023 American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052- 2081. doi:10.1111/jgs.18372
- O’Mahony D, Cherubini A, Guiteras AR, et al. STOPP/ START criteria for potentially inappropriate prescribing in older people: version 3. Eur Geriatr Med. 2023;14(4):625- 632. doi:10.1007/s41999-023-00777-y
- Draeger C, Lodhi F, Geissinger N, Larson T, Griesbach S. Interdisciplinary deprescribing of aspirin through prescriber education and provision of patient-specific recommendations. WMJ. 2022;121(3):220-225
- de Lusignan S, Hinton W, Seidu S, et al. Dashboards to reduce inappropriate prescribing of metformin and aspirin: A quality assurance programme in a primary care sentinel network. Prim Care Diabetes. 2021;15(6):1075-1079. doi:10.1016/j.pcd.2021.06.003
- Nelson MW, Downs TN, Puglisi GM, Simpkins BA, Collier AS. Use of a deprescribing tool in an interdisciplinary primary-care patient-aligned care team. Sr Care Pharm. 2022;37(1):34-43. doi:10.4140/TCP.n.2022.34
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):e199-e267. doi:10.1161/CIR.0000000000000041
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/ AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines Circulation. 2024;149(1):e1- e156. doi:10.1161/CIR.0000000000001193
Low-dose aspirin commonly is used for the prevention of cardiovascular disease (CVD) but is associated with an increased risk of major bleeding.1 The use of aspirin for primary prevention is largely extrapolated from clinical trials showing benefit in the secondary prevention of myocardial infarction and ischemic stroke. However, results from the Aspirin in Reducing Events in the Elderly (ASPREE) trial challenged this practice.2 The ASPREE trial, conducted in the United States and Australia from 2010 to 2014, sought to determine whether daily 100 mg aspirin, was superior to placebo in promoting disability-free survival among older adults. Participants were aged ≥ 70 years (≥ 65 years for Hispanic and Black US participants), living in the community, and were free from preexisting CVD, cerebrovascular disease, or any chronic condition likely to limit survival to < 5 years. The study found no significant difference in the primary endpoints of death, dementia, or persistent physical disability, but there was a significantly higher risk of major hemorrhage in the aspirin group (3.8% vs 2.8%; hazard ratio, 1.38; 95% CI, 1.18-1.62; P < .001).
Several medical societies have updated their guideline recommendations for aspirin for primary prevention of CVD. The 2022 United States Public Service Task Force (USPSTF) provides a grade C recommendation (at least moderate certainty that the net benefit is small) to consider low-dose aspirin for the primary prevention of CVD on an individual patient basis for adults aged 40 to 59 years who have a ≥ 10% 10-year CVD risk. For adults aged ≥ 60 years, the USPSTF recommendation is grade D (moderate or high certainty that the practice has no net benefit or that harms outweigh the benefits) for low-dose aspirin use.1,3 The American College of Cardiology and American Heart Association (ACC/AHA) recommend considering low-dose aspirin for primary prevention of atherosclerotic cardiovascular disease (ASCVD) among select adults aged 40 to 70 years at higher CVD risk but not at increased risk of bleeding.4 The American Diabetes Association (ADA) recommends low-dose aspirin for primary prevention of CVD in patients with diabetes and additional risk factors such as family history of premature ASCVD, hypertension, dyslipidemia, smoking, or chronic kidney disease, and who are not at higher risk of bleeding.5 The ADA standards also caution against the use of aspirin as primary prevention in patients aged > 70 years. Low-dose aspirin use is not recommended for the primary prevention of CVD in older adults or adults of any age who are at increased risk of bleeding.
Recent literature using the US Department of Veterans Affairs (VA) Corporate Data Warehouse database confirms 86,555 of 1.8 million veterans aged > 70 years (5%) were taking low-dose aspirin for primary prevention of ASCVD despite guideline recommendations.6 Higher risk of gastrointestinal and other major bleeding from low-dose aspirin has been reported in the literature.1 Major bleeds represent a significant burden to the health care system with an estimated mean $13,093 cost for gastrointestinal bleed hospitalization.7
Considering the large scale aspirin use without appropriate indication within the veteran population, the risk of adverse effects, and the significant cost to patients and the health care system, it is imperative to determine the best approach to efficiently deprescribe aspirin for primary prevention among geriatric patients. Deprescribing refers to the systematic and supervised process of dose reduction or drug discontinuation with the goal of improving health and/or reducing the risk of adverse effects.8 During patient visits, primary care practitioners (PCPs) have opportunities to discontinue aspirin, but these encounters are time-limited and deprescribing might be secondary to more acute primary care needs. The shortage of PCPs is expected to worsen in coming years, which could further reduce their availability to assess inappropriate aspirin use.9
VA clinical pharmacist practitioners (CPPs) serve as medication experts and work autonomously under a broad scope of practice as part of the patient aligned care team.10-12 CPPs can free up time for PCPs and facilitate deprescribing efforts, especially for older adults. One retrospective cohort study conducted at a VA medical center found that CPPs deprescribed more potentially inappropriate medications among individuals aged ≥ 80 years compared with usual care with PCPs (26.8% vs 16.1%; P < .001).12,13 An aspirin deprescribing protocol conducted in 2022 resulted in nearly half of veterans aged ≥ 70 years contacted by phone agreeing to stop aspirin. Although this study supports the role pharmacists can play in reducing aspirin use in accordance with guidelines, the authors acknowledge that their interventions had a mean time of 12 minutes per patient and would require workflow changes.14 The purpose of this study is to evaluate the efficiency of aspirin deprescribing through 2 approaches: direct deprescribing by pharmacists using populationlevel review compared with clinicians following a pharmacist-led education.
Methods
This was a single-center quality improvement cohort study at the Durham VA Health Care System (DVAHCS) in North Carolina. Patients included were aged ≥ 70 years without known ASCVD who received care at any of 3 DVAHCS community-based outpatient clinics and prescribed aspirin. Patient data was obtained using the VIONE (Deprescribing Dashboard called Vital, Important, Optional, Not indicated, and Every medication has a specific indication or diagnosis) dashboard.15 VIONE was developed to identify potentially inappropriate medications (PIMs) that are eligible to deprescribe based on Beers Criteria, Screening Tool of Older Personsf Prescriptions criteria, and common clinical scenarios when clinicians determine the risk outweighs the benefit to continue a specific medication. 16,17 VIONE is used to reduce polypharmacy and improve patient safety, comfort, and medication adherence. Aspirin for patients aged ≥ 70 years without a history of ASCVD is a PIM identified by VIONE. Patients aged ≥ 70 years were chosen as an inclusion criteria in this study to match the ASPREE trial inclusion criteria and age inclusion criteria in the VIONE dashboard for aspirin deprescribing.2 Patient lists were generated for these potentially inappropriate aspirin prescriptions for 3 months before clinician staff education presentations, the day of the presentations, and 3 months after.
The primary endpoint was the number of veterans with aspirin deprescribed directly by 2 pharmacists over 12 weeks, divided by total patient care time spent, compared with the change in number of veterans with aspirin deprescribed by any DVAHCS physician, nurse practitioner, physician assistant, or CPP over 12 weeks, divided by the total pharmacist time spent on PCP education. Secondary endpoints were the number of aspirin orders discontinued by pharmacists and CPPs, the number of aspirin orders discontinued 12 weeks before pharmacist-led education compared with the number of aspirin orders discontinued 12 weeks after CPP-led education, average and median pharmacist time spent per patient encounter, and time of direct patient encounters vs time spent on PCP education.
Pharmacists reviewed each patient who met the inclusion criteria from the list generated by VIONE on December 1, 2022, for aspirin appropriateness according to the ACC/AHA and USPSTF guidelines, with the goal to discontinue aspirin for primary prevention of ASCVD and no other indications.1,4 Pharmacists documented their visits using VIONE methodology in the Computerized Patient Record System (CPRS) using a polypharmacy review note. CPPs contacted patients who were taking aspirin for primary prevention by unscheduled telephone call to assess for aspirin adherence, undocumented history of ASCVD, cardiovascular risk factors, and history of bleeding. Aspirin was discontinued if patients met guideline criteria recommendations and agreed to discontinuation. Risk-benefit discussions were completed when patients without known ASCVD were considered high risk because the ACC/AHA guidelines mention there is insufficient evidence of safety and efficacy of aspirin for primary prevention for patients with other known ASCVD risk factors (eg, strong family history of premature myocardial infarction, inability to achieve lipid, blood pressure, or glucose targets, or significant elevation in coronary artery calcium score).
High risk was defined as family history of premature ASCVD (in a male first-degree relative aged < 55 years or a female first-degree relative aged < 65 years), most recent blood pressure or 2 blood pressure results in the last 12 months > 160/100 mm Hg, recent hemoglobin A1c > 9%, and/or low-density lipoprotein > 190 mg/dL or not prescribed an indicated statin.3 Aspirin was continued or discontinued according to patient preference after the personalized risk-benefit discussion.
For patients with a clinical indication for aspirin use other than ASCVD (eg, atrial fibrillation not on anticoagulation, venous thromboembolism prophylaxis, carotid artery disease), CPPs documented their assessment and when appropriate deferred to the PCP for consideration of stopping aspirin. For patients with undocumented ASCVD, CPPs added their ASCVD history to their problem list and aspirin was continued. PCPs were notified by alert when aspirin was discontinued and when patients could not be reached by telephone.
presented a review of recent guideline updates and supporting literature at 2 online staff meetings. The education sessions lasted about 10 minutes and were presented to PCPs across 3 community-based outpatient clinics. An estimated 40 minutes were spent creating the PowerPoint education materials, seeking feedback, making edits, and answering questions or emails from PCPs after the presentation. During the presentation, pharmacists encouraged PCPs to discontinue aspirin (active VA prescriptions and reported over-the-counter use) for primary prevention of ASCVD in patients aged ≥ 70 years during their upcoming appointments and consider risk factors recommended by the ACC/AHA guidelines when applicable. PCPs were notified that CPPs planned to start a population review for discontinuing active VA aspirin prescriptions on December 1, 2022. The primary endpoint and secondary endpoints were analyzed using descriptive statistics. All data were analyzed using Microsoft Excel.

Results
A total of 868 patients aged ≥ 70 years with active prescriptions for aspirin were identified on December 1, 2022. After applying inclusion and exclusion criteria for the pharmacist population review, 224 patients were included for cohort final analysis (Figure). All 868 patients were eligible for the CPP intervention. Primary reasons for exclusion from the CPP population included over-thecounter aspirin and a history of ASCVD in the patient’s problem list. All patients were male, with a mean (SD) age of 75 (4.4) years (Table 1). Most patients were prescribed aspirin, 81 mg daily (n = 220; 98%).

The direct CPP deprescribing intervention resulted in 2 aspirin prescriptions discontinued per hour of pharmacist time and 67 aspirin prescriptions discontinued per hour of pharmacist time via the PCP education intervention. CPPs discontinued 66 aspirin orders in the 12 weeks before the PCP education sessions. A total of 230 aspirin prescriptions were discontinued in the 12 weeks following the PCP education sessions, with 97 discontinued directly by CPPs and 133 discontinued by PCPs. The PCP education session yielded an additional 67 discontinued aspirin orders compared with the 12 weeks before the education sessions (Table 2).

The CPP direct deprescribing intervention took about 48.3 hours, accounting for health record review and time interacting with patients. The PCP education intervention took about 60 minutes, which included time for preparing and delivering education materials (Table 3). CPP deprescribing encounter types, interventions, and related subcategories, and other identified indications to continue aspirin are listed in Table 4.


Discussion
Compared with direct deprescribing by pharmacists, the PCP education intervention was more efficient based on number of aspirin orders discontinued by pharmacist time. PCPs discontinued twice as many aspirin prescriptions in the 12 weeks after pharmacist-led education compared with the 12 weeks before.
Patients were primarily contacted by telephone (73%) for deprescribing. Among the 163 patients reached by phone and encouraged to discontinue aspirin, 97 patients (60%) accepted the recommendation, which was similar to the acceptance rates found in the literature (48% to 55%).14,18 Although many veterans continued taking aspirin (78%), most had indications for its continued use, such as a history of ASCVD, atrial fibrillation without anticoagulation, and carotid artery stenosis, and complex comorbidities that required further discussion with their PCP. Less common uses for aspirin were identified through CPRS review or patient reports included cerebral small vessel disease without history of ASCVD, subclavian artery stenosis, thrombocytosis, bioprosthetic valve replacement, giant cell arteritis, rheumatoid arthritis, and prevention of second eye involvement of ischemic optic neuropathy.
to describe the benefit of clinical pharmacy services for deprescribing aspirin for primary prevention of ASCVD through PCP education. Previously published literature has assessed alternative ways to identify or discontinue PIMs—including aspirin—among geriatric patients. One study evaluated the use of marking inappropriate aspirin prescriptions in the electronic health database, leading to a significant reduction in incidence of inappropriate aspirin prescribing; however, it did not assess changes in discontinuation rates of existing aspirin prescriptions.19 The previous VA pharmacist aspirin deprescribing protocol demonstrated pharmacists’ aptitude at discontinuing aspirin for primary prevention but only used direct patient contact and did not compare efficiency with other methods, including PCP education.14
This quality improvement project contributes new data to the existing literature to support the use of clinical pharmacists to discontinue aspirin for primary prevention and suggests a strong role for pharmacists as educators on clinical guidelines, in addition to their roles directly deprescribing PIMs in clinical practice. This study is further strengthened by its use of VIONE, which previously has demonstrated effectiveness in deprescribing a variety of PIMs in primary care settings.20
Despite using VIONE for generating a list of patients eligible for deprescription, our CPRS review found that this list was frequently inaccurate. For example, a small portion of patients were on the VIONE generated list indicating they had no ASCVD history, but had transient ischemic attack listed in their problem lists. Patient problem lists often were missing documented ASCVD history that was revealed by patient interview or CPRS review. It is possible that patients interviewed might have omitted relevant ASCVD history because of low health literacy, conditions affecting memory, or use of health care services outside the VA system.
There were several instances of aspirin used for other non-ASCVD indications, such as primary stroke prevention in atrial fibrillation. The ACC/AHA atrial fibrillation guidelines previously provided a Class IIb recommendation (benefit is greater than risk but additional studies are needed) for considering no antithrombic therapy or treatment with oral anticoagulant or aspirin for nonvalvular atrial fibrillation with CHA2DS2-VASc (Congestive heart failure, Hypertension, Age [> 65 y, 1 point; > 75 y, 2 points], Diabetes, previous Stroke/transient ischemic attack [2 points]) score of 1.21 The ACC/ AHA guidelines were updated in 2023 to recommend against antiplatelet therapy as an alternative to anticoagulation for reducing cardioembolic stroke risk among patients with atrial fibrillation with no indication for antiplatelet therapy because of risk of harm.22 If a patient has no risk factors for stroke, aspirin is not recommended to prevent thromboembolic events because of a lack of benefit. Interventions from this quality improvement study were completed before the 2023 atrial fibrillation guideline was published and therefore in this study aspirin was not discontinued when used for atrial fibrillation. Aspirin use for atrial fibrillation might benefit from similar discontinuation efforts analyzed within this study. Beyond atrial fibrillation, major guidelines do not comment on the use of aspirin for any other indications in the absence of clinical ASCVD.
Limitations
This study is limited by the lack of clinical consensus for complex patients and demonstrates the importance of individualized patient assessment when considering discontinuing aspirin. Because of the project’s relatively short intervention period, aspirin deprescribing rates could decrease over time and repeated education efforts might be necessary to see lasting impact. Health care professionals from services outside of primary care also might have discontinued aspirin during the study period unrelated to the education and these discontinued aspirin prescriptions could contribute to the higher rate observed among PCPs. This study included a specific population cohort of male, US veterans and might not reflect other populations where these interventions could be implemented.
The measurement of time spent by pharmacists and PCPs is an additional limitation. Although it is expected that PCPs attempt to discontinue aspirin during their existing patient care appointments, the time spent during visits was not measured or documented. Direct deprescribing by pharmacist CPRS review required a significant amount of time and could be a barrier to successful intervention by CPPs in patient aligned care teams.
To reduce the time pharmacists spent completing CPRS reviews, an aspirin deprescribing clinical reminder tool could be used to assess use and appropriate indication quickly during any primary care visit led by a PCP or CPP. In addition, it is recommended that pharmacists regularly educate health care professionals on guideline recommendations for aspirin use among geriatric patients. Future studies of the incidence of major cardiovascular events after aspirin deprescribing among geriatric patients and a longitudinal cost/benefit analysis could support these initiatives.
Conclusions
In this study, pharmacists successfully deprescribed inappropriate medications, such as aspirin. However, pharmacist-led PCP education is more efficient compared with direct deprescribing using a population-level review. PCP education requires less time and could allow ambulatory care pharmacists to spend more time on other direct patient care interventions to improve quality and access to care in primary care clinics. This study’s results further support the role of pharmacists in deprescribing PIMs for older adults and the use of a deprescribing tool, such as VIONE, in a primary care setting.
Low-dose aspirin commonly is used for the prevention of cardiovascular disease (CVD) but is associated with an increased risk of major bleeding.1 The use of aspirin for primary prevention is largely extrapolated from clinical trials showing benefit in the secondary prevention of myocardial infarction and ischemic stroke. However, results from the Aspirin in Reducing Events in the Elderly (ASPREE) trial challenged this practice.2 The ASPREE trial, conducted in the United States and Australia from 2010 to 2014, sought to determine whether daily 100 mg aspirin, was superior to placebo in promoting disability-free survival among older adults. Participants were aged ≥ 70 years (≥ 65 years for Hispanic and Black US participants), living in the community, and were free from preexisting CVD, cerebrovascular disease, or any chronic condition likely to limit survival to < 5 years. The study found no significant difference in the primary endpoints of death, dementia, or persistent physical disability, but there was a significantly higher risk of major hemorrhage in the aspirin group (3.8% vs 2.8%; hazard ratio, 1.38; 95% CI, 1.18-1.62; P < .001).
Several medical societies have updated their guideline recommendations for aspirin for primary prevention of CVD. The 2022 United States Public Service Task Force (USPSTF) provides a grade C recommendation (at least moderate certainty that the net benefit is small) to consider low-dose aspirin for the primary prevention of CVD on an individual patient basis for adults aged 40 to 59 years who have a ≥ 10% 10-year CVD risk. For adults aged ≥ 60 years, the USPSTF recommendation is grade D (moderate or high certainty that the practice has no net benefit or that harms outweigh the benefits) for low-dose aspirin use.1,3 The American College of Cardiology and American Heart Association (ACC/AHA) recommend considering low-dose aspirin for primary prevention of atherosclerotic cardiovascular disease (ASCVD) among select adults aged 40 to 70 years at higher CVD risk but not at increased risk of bleeding.4 The American Diabetes Association (ADA) recommends low-dose aspirin for primary prevention of CVD in patients with diabetes and additional risk factors such as family history of premature ASCVD, hypertension, dyslipidemia, smoking, or chronic kidney disease, and who are not at higher risk of bleeding.5 The ADA standards also caution against the use of aspirin as primary prevention in patients aged > 70 years. Low-dose aspirin use is not recommended for the primary prevention of CVD in older adults or adults of any age who are at increased risk of bleeding.
Recent literature using the US Department of Veterans Affairs (VA) Corporate Data Warehouse database confirms 86,555 of 1.8 million veterans aged > 70 years (5%) were taking low-dose aspirin for primary prevention of ASCVD despite guideline recommendations.6 Higher risk of gastrointestinal and other major bleeding from low-dose aspirin has been reported in the literature.1 Major bleeds represent a significant burden to the health care system with an estimated mean $13,093 cost for gastrointestinal bleed hospitalization.7
Considering the large scale aspirin use without appropriate indication within the veteran population, the risk of adverse effects, and the significant cost to patients and the health care system, it is imperative to determine the best approach to efficiently deprescribe aspirin for primary prevention among geriatric patients. Deprescribing refers to the systematic and supervised process of dose reduction or drug discontinuation with the goal of improving health and/or reducing the risk of adverse effects.8 During patient visits, primary care practitioners (PCPs) have opportunities to discontinue aspirin, but these encounters are time-limited and deprescribing might be secondary to more acute primary care needs. The shortage of PCPs is expected to worsen in coming years, which could further reduce their availability to assess inappropriate aspirin use.9
VA clinical pharmacist practitioners (CPPs) serve as medication experts and work autonomously under a broad scope of practice as part of the patient aligned care team.10-12 CPPs can free up time for PCPs and facilitate deprescribing efforts, especially for older adults. One retrospective cohort study conducted at a VA medical center found that CPPs deprescribed more potentially inappropriate medications among individuals aged ≥ 80 years compared with usual care with PCPs (26.8% vs 16.1%; P < .001).12,13 An aspirin deprescribing protocol conducted in 2022 resulted in nearly half of veterans aged ≥ 70 years contacted by phone agreeing to stop aspirin. Although this study supports the role pharmacists can play in reducing aspirin use in accordance with guidelines, the authors acknowledge that their interventions had a mean time of 12 minutes per patient and would require workflow changes.14 The purpose of this study is to evaluate the efficiency of aspirin deprescribing through 2 approaches: direct deprescribing by pharmacists using populationlevel review compared with clinicians following a pharmacist-led education.
Methods
This was a single-center quality improvement cohort study at the Durham VA Health Care System (DVAHCS) in North Carolina. Patients included were aged ≥ 70 years without known ASCVD who received care at any of 3 DVAHCS community-based outpatient clinics and prescribed aspirin. Patient data was obtained using the VIONE (Deprescribing Dashboard called Vital, Important, Optional, Not indicated, and Every medication has a specific indication or diagnosis) dashboard.15 VIONE was developed to identify potentially inappropriate medications (PIMs) that are eligible to deprescribe based on Beers Criteria, Screening Tool of Older Personsf Prescriptions criteria, and common clinical scenarios when clinicians determine the risk outweighs the benefit to continue a specific medication. 16,17 VIONE is used to reduce polypharmacy and improve patient safety, comfort, and medication adherence. Aspirin for patients aged ≥ 70 years without a history of ASCVD is a PIM identified by VIONE. Patients aged ≥ 70 years were chosen as an inclusion criteria in this study to match the ASPREE trial inclusion criteria and age inclusion criteria in the VIONE dashboard for aspirin deprescribing.2 Patient lists were generated for these potentially inappropriate aspirin prescriptions for 3 months before clinician staff education presentations, the day of the presentations, and 3 months after.
The primary endpoint was the number of veterans with aspirin deprescribed directly by 2 pharmacists over 12 weeks, divided by total patient care time spent, compared with the change in number of veterans with aspirin deprescribed by any DVAHCS physician, nurse practitioner, physician assistant, or CPP over 12 weeks, divided by the total pharmacist time spent on PCP education. Secondary endpoints were the number of aspirin orders discontinued by pharmacists and CPPs, the number of aspirin orders discontinued 12 weeks before pharmacist-led education compared with the number of aspirin orders discontinued 12 weeks after CPP-led education, average and median pharmacist time spent per patient encounter, and time of direct patient encounters vs time spent on PCP education.
Pharmacists reviewed each patient who met the inclusion criteria from the list generated by VIONE on December 1, 2022, for aspirin appropriateness according to the ACC/AHA and USPSTF guidelines, with the goal to discontinue aspirin for primary prevention of ASCVD and no other indications.1,4 Pharmacists documented their visits using VIONE methodology in the Computerized Patient Record System (CPRS) using a polypharmacy review note. CPPs contacted patients who were taking aspirin for primary prevention by unscheduled telephone call to assess for aspirin adherence, undocumented history of ASCVD, cardiovascular risk factors, and history of bleeding. Aspirin was discontinued if patients met guideline criteria recommendations and agreed to discontinuation. Risk-benefit discussions were completed when patients without known ASCVD were considered high risk because the ACC/AHA guidelines mention there is insufficient evidence of safety and efficacy of aspirin for primary prevention for patients with other known ASCVD risk factors (eg, strong family history of premature myocardial infarction, inability to achieve lipid, blood pressure, or glucose targets, or significant elevation in coronary artery calcium score).
High risk was defined as family history of premature ASCVD (in a male first-degree relative aged < 55 years or a female first-degree relative aged < 65 years), most recent blood pressure or 2 blood pressure results in the last 12 months > 160/100 mm Hg, recent hemoglobin A1c > 9%, and/or low-density lipoprotein > 190 mg/dL or not prescribed an indicated statin.3 Aspirin was continued or discontinued according to patient preference after the personalized risk-benefit discussion.
For patients with a clinical indication for aspirin use other than ASCVD (eg, atrial fibrillation not on anticoagulation, venous thromboembolism prophylaxis, carotid artery disease), CPPs documented their assessment and when appropriate deferred to the PCP for consideration of stopping aspirin. For patients with undocumented ASCVD, CPPs added their ASCVD history to their problem list and aspirin was continued. PCPs were notified by alert when aspirin was discontinued and when patients could not be reached by telephone.
presented a review of recent guideline updates and supporting literature at 2 online staff meetings. The education sessions lasted about 10 minutes and were presented to PCPs across 3 community-based outpatient clinics. An estimated 40 minutes were spent creating the PowerPoint education materials, seeking feedback, making edits, and answering questions or emails from PCPs after the presentation. During the presentation, pharmacists encouraged PCPs to discontinue aspirin (active VA prescriptions and reported over-the-counter use) for primary prevention of ASCVD in patients aged ≥ 70 years during their upcoming appointments and consider risk factors recommended by the ACC/AHA guidelines when applicable. PCPs were notified that CPPs planned to start a population review for discontinuing active VA aspirin prescriptions on December 1, 2022. The primary endpoint and secondary endpoints were analyzed using descriptive statistics. All data were analyzed using Microsoft Excel.

Results
A total of 868 patients aged ≥ 70 years with active prescriptions for aspirin were identified on December 1, 2022. After applying inclusion and exclusion criteria for the pharmacist population review, 224 patients were included for cohort final analysis (Figure). All 868 patients were eligible for the CPP intervention. Primary reasons for exclusion from the CPP population included over-thecounter aspirin and a history of ASCVD in the patient’s problem list. All patients were male, with a mean (SD) age of 75 (4.4) years (Table 1). Most patients were prescribed aspirin, 81 mg daily (n = 220; 98%).

The direct CPP deprescribing intervention resulted in 2 aspirin prescriptions discontinued per hour of pharmacist time and 67 aspirin prescriptions discontinued per hour of pharmacist time via the PCP education intervention. CPPs discontinued 66 aspirin orders in the 12 weeks before the PCP education sessions. A total of 230 aspirin prescriptions were discontinued in the 12 weeks following the PCP education sessions, with 97 discontinued directly by CPPs and 133 discontinued by PCPs. The PCP education session yielded an additional 67 discontinued aspirin orders compared with the 12 weeks before the education sessions (Table 2).

The CPP direct deprescribing intervention took about 48.3 hours, accounting for health record review and time interacting with patients. The PCP education intervention took about 60 minutes, which included time for preparing and delivering education materials (Table 3). CPP deprescribing encounter types, interventions, and related subcategories, and other identified indications to continue aspirin are listed in Table 4.


Discussion
Compared with direct deprescribing by pharmacists, the PCP education intervention was more efficient based on number of aspirin orders discontinued by pharmacist time. PCPs discontinued twice as many aspirin prescriptions in the 12 weeks after pharmacist-led education compared with the 12 weeks before.
Patients were primarily contacted by telephone (73%) for deprescribing. Among the 163 patients reached by phone and encouraged to discontinue aspirin, 97 patients (60%) accepted the recommendation, which was similar to the acceptance rates found in the literature (48% to 55%).14,18 Although many veterans continued taking aspirin (78%), most had indications for its continued use, such as a history of ASCVD, atrial fibrillation without anticoagulation, and carotid artery stenosis, and complex comorbidities that required further discussion with their PCP. Less common uses for aspirin were identified through CPRS review or patient reports included cerebral small vessel disease without history of ASCVD, subclavian artery stenosis, thrombocytosis, bioprosthetic valve replacement, giant cell arteritis, rheumatoid arthritis, and prevention of second eye involvement of ischemic optic neuropathy.
to describe the benefit of clinical pharmacy services for deprescribing aspirin for primary prevention of ASCVD through PCP education. Previously published literature has assessed alternative ways to identify or discontinue PIMs—including aspirin—among geriatric patients. One study evaluated the use of marking inappropriate aspirin prescriptions in the electronic health database, leading to a significant reduction in incidence of inappropriate aspirin prescribing; however, it did not assess changes in discontinuation rates of existing aspirin prescriptions.19 The previous VA pharmacist aspirin deprescribing protocol demonstrated pharmacists’ aptitude at discontinuing aspirin for primary prevention but only used direct patient contact and did not compare efficiency with other methods, including PCP education.14
This quality improvement project contributes new data to the existing literature to support the use of clinical pharmacists to discontinue aspirin for primary prevention and suggests a strong role for pharmacists as educators on clinical guidelines, in addition to their roles directly deprescribing PIMs in clinical practice. This study is further strengthened by its use of VIONE, which previously has demonstrated effectiveness in deprescribing a variety of PIMs in primary care settings.20
Despite using VIONE for generating a list of patients eligible for deprescription, our CPRS review found that this list was frequently inaccurate. For example, a small portion of patients were on the VIONE generated list indicating they had no ASCVD history, but had transient ischemic attack listed in their problem lists. Patient problem lists often were missing documented ASCVD history that was revealed by patient interview or CPRS review. It is possible that patients interviewed might have omitted relevant ASCVD history because of low health literacy, conditions affecting memory, or use of health care services outside the VA system.
There were several instances of aspirin used for other non-ASCVD indications, such as primary stroke prevention in atrial fibrillation. The ACC/AHA atrial fibrillation guidelines previously provided a Class IIb recommendation (benefit is greater than risk but additional studies are needed) for considering no antithrombic therapy or treatment with oral anticoagulant or aspirin for nonvalvular atrial fibrillation with CHA2DS2-VASc (Congestive heart failure, Hypertension, Age [> 65 y, 1 point; > 75 y, 2 points], Diabetes, previous Stroke/transient ischemic attack [2 points]) score of 1.21 The ACC/ AHA guidelines were updated in 2023 to recommend against antiplatelet therapy as an alternative to anticoagulation for reducing cardioembolic stroke risk among patients with atrial fibrillation with no indication for antiplatelet therapy because of risk of harm.22 If a patient has no risk factors for stroke, aspirin is not recommended to prevent thromboembolic events because of a lack of benefit. Interventions from this quality improvement study were completed before the 2023 atrial fibrillation guideline was published and therefore in this study aspirin was not discontinued when used for atrial fibrillation. Aspirin use for atrial fibrillation might benefit from similar discontinuation efforts analyzed within this study. Beyond atrial fibrillation, major guidelines do not comment on the use of aspirin for any other indications in the absence of clinical ASCVD.
Limitations
This study is limited by the lack of clinical consensus for complex patients and demonstrates the importance of individualized patient assessment when considering discontinuing aspirin. Because of the project’s relatively short intervention period, aspirin deprescribing rates could decrease over time and repeated education efforts might be necessary to see lasting impact. Health care professionals from services outside of primary care also might have discontinued aspirin during the study period unrelated to the education and these discontinued aspirin prescriptions could contribute to the higher rate observed among PCPs. This study included a specific population cohort of male, US veterans and might not reflect other populations where these interventions could be implemented.
The measurement of time spent by pharmacists and PCPs is an additional limitation. Although it is expected that PCPs attempt to discontinue aspirin during their existing patient care appointments, the time spent during visits was not measured or documented. Direct deprescribing by pharmacist CPRS review required a significant amount of time and could be a barrier to successful intervention by CPPs in patient aligned care teams.
To reduce the time pharmacists spent completing CPRS reviews, an aspirin deprescribing clinical reminder tool could be used to assess use and appropriate indication quickly during any primary care visit led by a PCP or CPP. In addition, it is recommended that pharmacists regularly educate health care professionals on guideline recommendations for aspirin use among geriatric patients. Future studies of the incidence of major cardiovascular events after aspirin deprescribing among geriatric patients and a longitudinal cost/benefit analysis could support these initiatives.
Conclusions
In this study, pharmacists successfully deprescribed inappropriate medications, such as aspirin. However, pharmacist-led PCP education is more efficient compared with direct deprescribing using a population-level review. PCP education requires less time and could allow ambulatory care pharmacists to spend more time on other direct patient care interventions to improve quality and access to care in primary care clinics. This study’s results further support the role of pharmacists in deprescribing PIMs for older adults and the use of a deprescribing tool, such as VIONE, in a primary care setting.
- US Preventive Services Task Force; Davidson KW, Barry MJ, et al. Aspirin use to prevent cardiovascular disease: US Preventive Services Task Force recommendation statement. JAMA. 2022;327(16):1577-1584. doi:10.1001/jama.2022.4983
- McNeil JJ, Nelson MR, Woods RL, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379(16):1519-1528. doi:10.1056/NEJMoa1803955
- Barry MJ, Wolff TA, Pbert L, et al. Putting evidence into practice: an update on the US Preventive Services Task Force methods for developing recommendations for preventive services. Ann Fam Med. 2023;21(2):165-171. doi:10.1370/afm.2946
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/ AHA Guideline on the Primary Prevention of Cardiovascular Disease: A report of the American College of Cardiology/ American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596-e646. doi:10.1161/CIR.0000000000000678
- American Diabetes Association Professional Practice Committee. 10. Cardiovascular disease and risk management: Standards of care in diabetes-2024. Diabetes Care. 2024;47(Suppl 1):S179-S218. doi:10.2337/dc24-S010
- Ong SY, Chui P, Bhargava A, Justice A, Hauser RG. Estimating aspirin overuse for primary prevention of atherosclerotic cardiovascular disease (from a nationwide healthcare system). Am J Cardiol. 2020;137:25-30. doi:10.1016/j.amjcard.2020.09.042
- Weiss AJ, Jiang HJ. Overview of clinical conditions with frequent and costly hospital readmissions by payer, 2018. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality (US); July 20, 2021.
- Krishnaswami A, Steinman MA, Goyal P, et al. Deprescribing in older adults with cardiovascular disease. J Am Coll Cardiol. 2019;73(20):2584-2595. doi:10.1016/j.jacc.2019.03.467
- Association of American Medical Colleges. The complexities of physician supply and demand: projections from 2019 to 2034. Accessed March 17, 2024. https://www.aamc.org/media/54681/download
- US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1108.07(1): General pharmacy service requirements. November 28, 2022. Accessed March 17, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=10045
- US Department of Veterans Affairs, Veterans Health Administration. VHA Handbook 1108.11(3): Clinical pharmacy services. July 1, 2015. Accessed March 17, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3120
- US Department of Veterans Affairs. Clinical pharmacist practitioner (CPP) to improve access to and quality of care August 2021. August 2021. Accessed May 19, 2023. https://www.pbm.va.gov/PBM/CPPO/Documents/ExternalFactSheet_OptimizingtheCPPToImproveAccess_508.pdf
- Ammerman CA, Simpkins BA, Warman N, Downs TN. Potentially inappropriate medications in older adults: Deprescribing with a clinical pharmacist. J Am Geriatr Soc. 2019;67(1):115-118. doi:10.1111/jgs.15623
- Rothbauer K, Siodlak M, Dreischmeier E, Ranola TS, Welch L. Evaluation of a pharmacist-driven ambulatory aspirin deprescribing protocol. Fed Pract. 2022;39(suppl 5):S37- S41a. doi:10.12788/fp.0294
- US Department of Veterans Affairs. VIONE changes the way VA handles prescriptions. January 25, 2020. Accessed May 21, 2023. https://news.va.gov/70709/vione-changes-way-va-handles-prescriptions/
- 2023 American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052- 2081. doi:10.1111/jgs.18372
- O’Mahony D, Cherubini A, Guiteras AR, et al. STOPP/ START criteria for potentially inappropriate prescribing in older people: version 3. Eur Geriatr Med. 2023;14(4):625- 632. doi:10.1007/s41999-023-00777-y
- Draeger C, Lodhi F, Geissinger N, Larson T, Griesbach S. Interdisciplinary deprescribing of aspirin through prescriber education and provision of patient-specific recommendations. WMJ. 2022;121(3):220-225
- de Lusignan S, Hinton W, Seidu S, et al. Dashboards to reduce inappropriate prescribing of metformin and aspirin: A quality assurance programme in a primary care sentinel network. Prim Care Diabetes. 2021;15(6):1075-1079. doi:10.1016/j.pcd.2021.06.003
- Nelson MW, Downs TN, Puglisi GM, Simpkins BA, Collier AS. Use of a deprescribing tool in an interdisciplinary primary-care patient-aligned care team. Sr Care Pharm. 2022;37(1):34-43. doi:10.4140/TCP.n.2022.34
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):e199-e267. doi:10.1161/CIR.0000000000000041
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/ AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines Circulation. 2024;149(1):e1- e156. doi:10.1161/CIR.0000000000001193
- US Preventive Services Task Force; Davidson KW, Barry MJ, et al. Aspirin use to prevent cardiovascular disease: US Preventive Services Task Force recommendation statement. JAMA. 2022;327(16):1577-1584. doi:10.1001/jama.2022.4983
- McNeil JJ, Nelson MR, Woods RL, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379(16):1519-1528. doi:10.1056/NEJMoa1803955
- Barry MJ, Wolff TA, Pbert L, et al. Putting evidence into practice: an update on the US Preventive Services Task Force methods for developing recommendations for preventive services. Ann Fam Med. 2023;21(2):165-171. doi:10.1370/afm.2946
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/ AHA Guideline on the Primary Prevention of Cardiovascular Disease: A report of the American College of Cardiology/ American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596-e646. doi:10.1161/CIR.0000000000000678
- American Diabetes Association Professional Practice Committee. 10. Cardiovascular disease and risk management: Standards of care in diabetes-2024. Diabetes Care. 2024;47(Suppl 1):S179-S218. doi:10.2337/dc24-S010
- Ong SY, Chui P, Bhargava A, Justice A, Hauser RG. Estimating aspirin overuse for primary prevention of atherosclerotic cardiovascular disease (from a nationwide healthcare system). Am J Cardiol. 2020;137:25-30. doi:10.1016/j.amjcard.2020.09.042
- Weiss AJ, Jiang HJ. Overview of clinical conditions with frequent and costly hospital readmissions by payer, 2018. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality (US); July 20, 2021.
- Krishnaswami A, Steinman MA, Goyal P, et al. Deprescribing in older adults with cardiovascular disease. J Am Coll Cardiol. 2019;73(20):2584-2595. doi:10.1016/j.jacc.2019.03.467
- Association of American Medical Colleges. The complexities of physician supply and demand: projections from 2019 to 2034. Accessed March 17, 2024. https://www.aamc.org/media/54681/download
- US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1108.07(1): General pharmacy service requirements. November 28, 2022. Accessed March 17, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=10045
- US Department of Veterans Affairs, Veterans Health Administration. VHA Handbook 1108.11(3): Clinical pharmacy services. July 1, 2015. Accessed March 17, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3120
- US Department of Veterans Affairs. Clinical pharmacist practitioner (CPP) to improve access to and quality of care August 2021. August 2021. Accessed May 19, 2023. https://www.pbm.va.gov/PBM/CPPO/Documents/ExternalFactSheet_OptimizingtheCPPToImproveAccess_508.pdf
- Ammerman CA, Simpkins BA, Warman N, Downs TN. Potentially inappropriate medications in older adults: Deprescribing with a clinical pharmacist. J Am Geriatr Soc. 2019;67(1):115-118. doi:10.1111/jgs.15623
- Rothbauer K, Siodlak M, Dreischmeier E, Ranola TS, Welch L. Evaluation of a pharmacist-driven ambulatory aspirin deprescribing protocol. Fed Pract. 2022;39(suppl 5):S37- S41a. doi:10.12788/fp.0294
- US Department of Veterans Affairs. VIONE changes the way VA handles prescriptions. January 25, 2020. Accessed May 21, 2023. https://news.va.gov/70709/vione-changes-way-va-handles-prescriptions/
- 2023 American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052- 2081. doi:10.1111/jgs.18372
- O’Mahony D, Cherubini A, Guiteras AR, et al. STOPP/ START criteria for potentially inappropriate prescribing in older people: version 3. Eur Geriatr Med. 2023;14(4):625- 632. doi:10.1007/s41999-023-00777-y
- Draeger C, Lodhi F, Geissinger N, Larson T, Griesbach S. Interdisciplinary deprescribing of aspirin through prescriber education and provision of patient-specific recommendations. WMJ. 2022;121(3):220-225
- de Lusignan S, Hinton W, Seidu S, et al. Dashboards to reduce inappropriate prescribing of metformin and aspirin: A quality assurance programme in a primary care sentinel network. Prim Care Diabetes. 2021;15(6):1075-1079. doi:10.1016/j.pcd.2021.06.003
- Nelson MW, Downs TN, Puglisi GM, Simpkins BA, Collier AS. Use of a deprescribing tool in an interdisciplinary primary-care patient-aligned care team. Sr Care Pharm. 2022;37(1):34-43. doi:10.4140/TCP.n.2022.34
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):e199-e267. doi:10.1161/CIR.0000000000000041
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/ AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines Circulation. 2024;149(1):e1- e156. doi:10.1161/CIR.0000000000001193
Pharmacist-Led Deprescribing of Aspirin for Primary Prevention of Cardiovascular Disease Among Geriatric Veterans
Pharmacist-Led Deprescribing of Aspirin for Primary Prevention of Cardiovascular Disease Among Geriatric Veterans
Weighted Blankets May Help Reduce Preoperative Anxiety During Mohs Micrographic Surgery
Weighted Blankets May Help Reduce Preoperative Anxiety During Mohs Micrographic Surgery
To the Editor:
Patients with nonmelanoma skin cancers exhibit high quality-of-life satisfaction after treatment with Mohs micrographic surgery (MMS) or excision.1,2 However, perioperative anxiety in patients undergoing MMS is common, especially during the immediate preoperative period.3 Anxiety activates the sympathetic nervous system, resulting in physiologic changes such as tachycardia and hypertension.4,5 These sequelae may not only increase patient distress but also increase intraoperative bleeding, complication rates, and recovery times.4,5 Thus, the preoperative period represents a critical window for interventions aimed at reducing anxiety. Anxiety peaks during the perioperative period for a myriad of reasons, including anticipation of pain or potential complications. Enhancing patient comfort and well-being during the procedure may help reduce negative emotional sequelae, alleviate fear during procedures, and increase patient satisfaction.3
Weighted blankets (WBs) frequently are utilized in occupational and physical therapy as a deep pressure stimulation tool to alleviate anxiety by mimicking the experience of being massaged or swaddled.6 Deep pressure tools increase parasympathetic tone, help reduce anxiety, and provide a calming effect.7,8 Nonhospitalized individuals were more relaxed during mental health evaluations when using a WB, and deep pressure tools have frequently been used to calm individuals with autism spectrum disorders or attention-deficit/hyperactivity disorders.6 Furthermore, WBs have successfully been used to reduce anxiety in mental health care settings, as well as during chemotherapy infusions.6,9 The literature is sparse regarding the use of WB in the perioperative setting. Potential benefit has been demonstrated in the setting of dental cleanings and wisdom teeth extractions.7,8 In the current study, we investigated whether use of a WB could reduce preoperative anxiety in the setting of MMS.
Institutional review board approval was obtained from the University of Virginia (Charlottesville, Virginia), and adult patients undergoing MMS to the head or neck were recruited to participate in a single-blind randomized controlled trial in the spring of 2023. Patients undergoing MMS on other areas of the body were excluded because the placement of the WB could interfere with the procedure. Other exclusion criteria included pregnancy, dementia, or current treatment with an anxiolytic medication.
Twenty-seven patients were included in the study, and informed consent was obtained. Patients were randomized to use a WB or standard hospital towel (control). The medical-grade WBs weighed 8.5 pounds, while the cotton hospital towels weighed less than 1 pound. The WBs were cleaned in between patients with standard germicidal disposable wipes.
Patient data were collected from electronic medical records including age, sex, weight, history of prior MMS, and current use of antihypertensives and/or beta-blockers. Data also were collected on the presence of anxiety disorders, major depression, fibromyalgia, tobacco and alcohol use, hyperthyroidism, hyperhidrosis, cardiac arrhythmias (including atrial fibrillation), chronic obstructive pulmonary disease, asthma, coronary artery disease, diabetes mellitus, peripheral neuropathy, and menopausal symptoms.
During the procedure, anxiety was monitored using the State-Trait Anxiety Inventory (STAI) Form Y-1, the visual analogue scale for anxiety (VAS-A), and vital signs including heart rate, blood pressure, and respiratory rate. Vital signs were evaluated by nursing staff with the patient sitting up and the WB or hospital towel removed. Using these assessments, anxiety was measured at 3 different timepoints: upon arrival to the clinic (timepoint A), after the patient rested in a reclined beach-chair position with the WB or hospital towel placed over them for 10 minutes before administration of local anesthetic and starting the procedure (timepoint B), and after the first MMS stage was taken (timepoint C).
A power analysis was not completed due to a lack of previous studies on the use of WBs during MMS. Group means were analyzed using two-tailed t-tests and one-way analysis of variance. A P value of .05 indicated statistical significance.
Fourteen patients were randomized to the WB group and 13 were randomized to the control group. Patient demographics are outlined in the eTable. In the WB group, mean STAI scores progressively decreased at each timepoint (A: 15.3, B: 13.6, C: 12.7) and mean VAS-A scores followed a similar trend (A: 24.2, B: 19.3, C: 10.5). In the control group, the mean STAI scores remained stable at timepoints A and B (17.7) and then decreased at timepoint C (14.8). The mean VAS-A scores in the control group followed a similar pattern, remaining stable at timepoints A (22.9) and B (22.8) and then decreasing at timepoint C (14.4). These changes were not statistically significant.
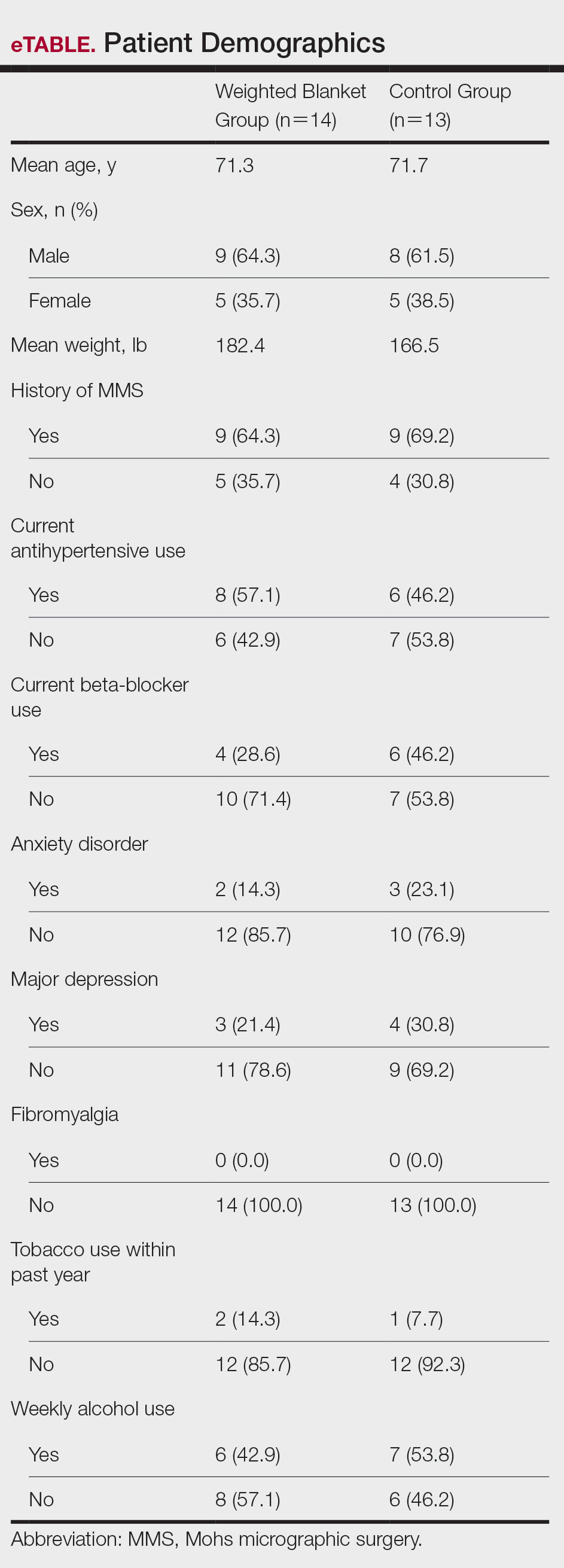
Mean vital signs for both the WB and control groups were relatively stable across all timepoints, although they tended to decrease by timepoint C. In the WB group, mean heart rates were 69, 69, and 67 beats per minute at timepoints A, B, and C, respectively. Mean systolic blood pressures were 137 mm Hg, 138 mm Hg, and 136 mm Hg and mean diastolic pressures were 71 mm Hg, 68 mm Hg, and 66 mm Hg at timepoints A, B, and C, respectively. Mean respiratory rates were 20, 19, and 18 breaths per minute at timepoints A, B, and C, respectively. In the control group, mean heart rates were 70, 69, and 68 beats per minute across timepoints A, B, and C, respectively. Mean systolic blood pressures were 137 mm Hg, 138 mm Hg, and 133 mm Hg and mean diastolic pressures were 71 mm Hg, 74 mm Hg, and 68 mm Hg at timepoints A, B, and C, respectively. Mean respiratory rates were 19, 18, and 18 breaths per minute at timepoints A, B, and C, respectively. These changes were not statistically significant.
Our pilot study examined the effects of using a WB to alleviate preoperative anxiety during MMS. Our results suggest that WBs may modestly improve subjective anxiety immediately prior to undergoing MMS. Mean STAI and VAS-A scores decreased from timepoint A to timepoint B in the WB group vs the control group in which these scores remained stable. Although our study was not powered to determine statistical differences and significance was not reached, our results suggest a favorable trend in decreased anxiety scores. Our analysis was limited by a small sample size; therefore, additional larger-scale studies will be needed to confirm this trend.
Our results are broadly consistent with earlier studies that found improvement in physiologic proxies of anxiety with the use of WBs during chemotherapy infusions, dental procedures, and acute inpatient mental health hospitalizations.7-10 During periods of high anxiety, use of WBs shifts the autonomic nervous system from a sympathetic to a parasympathetic state, as demonstrated by increased high-frequency heart rate variability, a marker of parasympathetic activity.6,11 While the exact mechanism of how WBs and other deep pressure stimulation tools affect high-frequency heart rate variability is unclear, one study showed that patients undergoing dental extractions were better equipped when using deep pressure stimulation tools to utilize calming techniques and regulate stress.12 The use of WBs and other deep pressure stimulation tools may extend beyond the perioperative setting and also may be an effective tool for clinicians in other settings (eg, clinic visits, physical examinations).
In our study, all participants demonstrated the greatest reduction in anxiety at timepoint C after the first MMS stage, likely related to patients relaxing more after knowing what to expect from the surgery; this also may have been reflected somewhat in the slight downward trend noted in vital signs across both study groups. One concern regarding WB use in surgical settings is whether the added pressure could trigger unfavorable circulatory effects, such as elevated blood pressure. In our study, with the exception of diastolic blood pressure, vital signs appeared unaffected by the type of blanket used and remained relatively stable from timepoint A to timepoint B and decreased at timepoint C. Diastolic blood pressure in the WB group decreased from timepoint A to timepoint B, then decreased further from timepoint B to timepoint C. This mirrored the decreasing STAI score trend, compared to the control group who increased from timepoint A to timepoint B and reached a nadir at timepoint C. Consistent with prior WB studies, there were no adverse effects from WBs, including adverse impacts on vital signs.6,9
The original recruitment goal was not met due to staffing issues related to the COVID-19 pandemic, and subgroup analyses were deferred as a result of sample size limitations. It is possible that the WB intervention may have a larger impact on subpopulations more prone to perioperative anxiety (eg, patients undergoing MMS for the first time). However, the results of our pilot study suggest a beneficial effect from the use of WBs. While these preliminary data are promising, additional studies in the perioperative setting are needed to more accurately determine the clinical utility of WBs during MMS and other procedures.
- Eberle FC, Schippert W, Trilling B, et al. Cosmetic results of histographically controlled excision of non-melanoma skin cancer in the head and neck region. J Dtsch Dermatol Ges. 2005;3:109-112. doi:10.1111/j.1610-0378.2005.04738.x
- Chren MM, Sahay AP, Bertenthal DS, et al. Quality-of-life outcomes of treatments for cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2007;127:1351-1357. doi:10.1038/sj.jid.5700740
- Kossintseva I, Zloty D. Determinants and timeline of perioperative anxiety in Mohs surgery. Dermatol Surg. 2017;43:1029-1035. doi:10.1097 /DSS.0000000000001152
- Pritchard MJ. Identifying and assessing anxiety in pre-operative patients. Nurs Stand. 2009;23:35-40. doi:10.7748/ns2009.08.23.51.35.c7222.
- Mavros MN, Athanasiou S, Gkegkes ID, et al. Do psychological variables affect early surgical recovery? PLoS One. 2011;6:E20306. doi:10.1371/journal.pone.0020306
- Mullen B, Champagne T, Krishnamurty S, et al. Exploring the safety and therapeutic effects of deep pressure stimulation using a weighted blanket. Occup Ther Ment Health. 2008;24:65-89. doi:10.1300/ J004v24n01_05
- Chen HY, Yang H, Chi HJ, et al. Physiological effects of deep touch pressure on anxiety alleviation: the weighted blanket approach. J Med Biol Eng. 2013;33:463-470. doi:10.5405/jmbe.1043
- Chen HY, Yang H, Meng LF, et al. Effect of deep pressure input on parasympathetic system in patients with wisdom tooth surgery. J Formos Med Assoc. 2016;115:853-859. doi:10.1016 /j.jfma.2016.07.008
- Vinson J, Powers J, Mosesso K. Weighted blankets: anxiety reduction in adult patients receiving chemotherapy. Clin J Oncol Nurs. 2020; 24:360-368. doi:10.1188/20.CJON.360-368
- Champagne T, Mullen B, Dickson D, et al. Evaluating the safety and effectiveness of the weighted blanket with adults during an inpatient mental health hospitalization. Occup Ther Ment Health. 2015;31:211-233. doi:10.1080/0164212X.2015.1066220
- Lane RD, McRae K, Reiman EM, et al. Neural correlates of heart rate variability during emotion. Neuroimage. 2009;44:213-222. doi: 10.1016/j.neuroimage.2008.07.056
- Moyer CA, Rounds J, Hannum JW. A meta-analysis of massage therapy research. Psychol Bull. 2004;130:3-18. doi: 10.1037 /0033-2909.130.1.3
To the Editor:
Patients with nonmelanoma skin cancers exhibit high quality-of-life satisfaction after treatment with Mohs micrographic surgery (MMS) or excision.1,2 However, perioperative anxiety in patients undergoing MMS is common, especially during the immediate preoperative period.3 Anxiety activates the sympathetic nervous system, resulting in physiologic changes such as tachycardia and hypertension.4,5 These sequelae may not only increase patient distress but also increase intraoperative bleeding, complication rates, and recovery times.4,5 Thus, the preoperative period represents a critical window for interventions aimed at reducing anxiety. Anxiety peaks during the perioperative period for a myriad of reasons, including anticipation of pain or potential complications. Enhancing patient comfort and well-being during the procedure may help reduce negative emotional sequelae, alleviate fear during procedures, and increase patient satisfaction.3
Weighted blankets (WBs) frequently are utilized in occupational and physical therapy as a deep pressure stimulation tool to alleviate anxiety by mimicking the experience of being massaged or swaddled.6 Deep pressure tools increase parasympathetic tone, help reduce anxiety, and provide a calming effect.7,8 Nonhospitalized individuals were more relaxed during mental health evaluations when using a WB, and deep pressure tools have frequently been used to calm individuals with autism spectrum disorders or attention-deficit/hyperactivity disorders.6 Furthermore, WBs have successfully been used to reduce anxiety in mental health care settings, as well as during chemotherapy infusions.6,9 The literature is sparse regarding the use of WB in the perioperative setting. Potential benefit has been demonstrated in the setting of dental cleanings and wisdom teeth extractions.7,8 In the current study, we investigated whether use of a WB could reduce preoperative anxiety in the setting of MMS.
Institutional review board approval was obtained from the University of Virginia (Charlottesville, Virginia), and adult patients undergoing MMS to the head or neck were recruited to participate in a single-blind randomized controlled trial in the spring of 2023. Patients undergoing MMS on other areas of the body were excluded because the placement of the WB could interfere with the procedure. Other exclusion criteria included pregnancy, dementia, or current treatment with an anxiolytic medication.
Twenty-seven patients were included in the study, and informed consent was obtained. Patients were randomized to use a WB or standard hospital towel (control). The medical-grade WBs weighed 8.5 pounds, while the cotton hospital towels weighed less than 1 pound. The WBs were cleaned in between patients with standard germicidal disposable wipes.
Patient data were collected from electronic medical records including age, sex, weight, history of prior MMS, and current use of antihypertensives and/or beta-blockers. Data also were collected on the presence of anxiety disorders, major depression, fibromyalgia, tobacco and alcohol use, hyperthyroidism, hyperhidrosis, cardiac arrhythmias (including atrial fibrillation), chronic obstructive pulmonary disease, asthma, coronary artery disease, diabetes mellitus, peripheral neuropathy, and menopausal symptoms.
During the procedure, anxiety was monitored using the State-Trait Anxiety Inventory (STAI) Form Y-1, the visual analogue scale for anxiety (VAS-A), and vital signs including heart rate, blood pressure, and respiratory rate. Vital signs were evaluated by nursing staff with the patient sitting up and the WB or hospital towel removed. Using these assessments, anxiety was measured at 3 different timepoints: upon arrival to the clinic (timepoint A), after the patient rested in a reclined beach-chair position with the WB or hospital towel placed over them for 10 minutes before administration of local anesthetic and starting the procedure (timepoint B), and after the first MMS stage was taken (timepoint C).
A power analysis was not completed due to a lack of previous studies on the use of WBs during MMS. Group means were analyzed using two-tailed t-tests and one-way analysis of variance. A P value of .05 indicated statistical significance.
Fourteen patients were randomized to the WB group and 13 were randomized to the control group. Patient demographics are outlined in the eTable. In the WB group, mean STAI scores progressively decreased at each timepoint (A: 15.3, B: 13.6, C: 12.7) and mean VAS-A scores followed a similar trend (A: 24.2, B: 19.3, C: 10.5). In the control group, the mean STAI scores remained stable at timepoints A and B (17.7) and then decreased at timepoint C (14.8). The mean VAS-A scores in the control group followed a similar pattern, remaining stable at timepoints A (22.9) and B (22.8) and then decreasing at timepoint C (14.4). These changes were not statistically significant.

Mean vital signs for both the WB and control groups were relatively stable across all timepoints, although they tended to decrease by timepoint C. In the WB group, mean heart rates were 69, 69, and 67 beats per minute at timepoints A, B, and C, respectively. Mean systolic blood pressures were 137 mm Hg, 138 mm Hg, and 136 mm Hg and mean diastolic pressures were 71 mm Hg, 68 mm Hg, and 66 mm Hg at timepoints A, B, and C, respectively. Mean respiratory rates were 20, 19, and 18 breaths per minute at timepoints A, B, and C, respectively. In the control group, mean heart rates were 70, 69, and 68 beats per minute across timepoints A, B, and C, respectively. Mean systolic blood pressures were 137 mm Hg, 138 mm Hg, and 133 mm Hg and mean diastolic pressures were 71 mm Hg, 74 mm Hg, and 68 mm Hg at timepoints A, B, and C, respectively. Mean respiratory rates were 19, 18, and 18 breaths per minute at timepoints A, B, and C, respectively. These changes were not statistically significant.
Our pilot study examined the effects of using a WB to alleviate preoperative anxiety during MMS. Our results suggest that WBs may modestly improve subjective anxiety immediately prior to undergoing MMS. Mean STAI and VAS-A scores decreased from timepoint A to timepoint B in the WB group vs the control group in which these scores remained stable. Although our study was not powered to determine statistical differences and significance was not reached, our results suggest a favorable trend in decreased anxiety scores. Our analysis was limited by a small sample size; therefore, additional larger-scale studies will be needed to confirm this trend.
Our results are broadly consistent with earlier studies that found improvement in physiologic proxies of anxiety with the use of WBs during chemotherapy infusions, dental procedures, and acute inpatient mental health hospitalizations.7-10 During periods of high anxiety, use of WBs shifts the autonomic nervous system from a sympathetic to a parasympathetic state, as demonstrated by increased high-frequency heart rate variability, a marker of parasympathetic activity.6,11 While the exact mechanism of how WBs and other deep pressure stimulation tools affect high-frequency heart rate variability is unclear, one study showed that patients undergoing dental extractions were better equipped when using deep pressure stimulation tools to utilize calming techniques and regulate stress.12 The use of WBs and other deep pressure stimulation tools may extend beyond the perioperative setting and also may be an effective tool for clinicians in other settings (eg, clinic visits, physical examinations).
In our study, all participants demonstrated the greatest reduction in anxiety at timepoint C after the first MMS stage, likely related to patients relaxing more after knowing what to expect from the surgery; this also may have been reflected somewhat in the slight downward trend noted in vital signs across both study groups. One concern regarding WB use in surgical settings is whether the added pressure could trigger unfavorable circulatory effects, such as elevated blood pressure. In our study, with the exception of diastolic blood pressure, vital signs appeared unaffected by the type of blanket used and remained relatively stable from timepoint A to timepoint B and decreased at timepoint C. Diastolic blood pressure in the WB group decreased from timepoint A to timepoint B, then decreased further from timepoint B to timepoint C. This mirrored the decreasing STAI score trend, compared to the control group who increased from timepoint A to timepoint B and reached a nadir at timepoint C. Consistent with prior WB studies, there were no adverse effects from WBs, including adverse impacts on vital signs.6,9
The original recruitment goal was not met due to staffing issues related to the COVID-19 pandemic, and subgroup analyses were deferred as a result of sample size limitations. It is possible that the WB intervention may have a larger impact on subpopulations more prone to perioperative anxiety (eg, patients undergoing MMS for the first time). However, the results of our pilot study suggest a beneficial effect from the use of WBs. While these preliminary data are promising, additional studies in the perioperative setting are needed to more accurately determine the clinical utility of WBs during MMS and other procedures.
To the Editor:
Patients with nonmelanoma skin cancers exhibit high quality-of-life satisfaction after treatment with Mohs micrographic surgery (MMS) or excision.1,2 However, perioperative anxiety in patients undergoing MMS is common, especially during the immediate preoperative period.3 Anxiety activates the sympathetic nervous system, resulting in physiologic changes such as tachycardia and hypertension.4,5 These sequelae may not only increase patient distress but also increase intraoperative bleeding, complication rates, and recovery times.4,5 Thus, the preoperative period represents a critical window for interventions aimed at reducing anxiety. Anxiety peaks during the perioperative period for a myriad of reasons, including anticipation of pain or potential complications. Enhancing patient comfort and well-being during the procedure may help reduce negative emotional sequelae, alleviate fear during procedures, and increase patient satisfaction.3
Weighted blankets (WBs) frequently are utilized in occupational and physical therapy as a deep pressure stimulation tool to alleviate anxiety by mimicking the experience of being massaged or swaddled.6 Deep pressure tools increase parasympathetic tone, help reduce anxiety, and provide a calming effect.7,8 Nonhospitalized individuals were more relaxed during mental health evaluations when using a WB, and deep pressure tools have frequently been used to calm individuals with autism spectrum disorders or attention-deficit/hyperactivity disorders.6 Furthermore, WBs have successfully been used to reduce anxiety in mental health care settings, as well as during chemotherapy infusions.6,9 The literature is sparse regarding the use of WB in the perioperative setting. Potential benefit has been demonstrated in the setting of dental cleanings and wisdom teeth extractions.7,8 In the current study, we investigated whether use of a WB could reduce preoperative anxiety in the setting of MMS.
Institutional review board approval was obtained from the University of Virginia (Charlottesville, Virginia), and adult patients undergoing MMS to the head or neck were recruited to participate in a single-blind randomized controlled trial in the spring of 2023. Patients undergoing MMS on other areas of the body were excluded because the placement of the WB could interfere with the procedure. Other exclusion criteria included pregnancy, dementia, or current treatment with an anxiolytic medication.
Twenty-seven patients were included in the study, and informed consent was obtained. Patients were randomized to use a WB or standard hospital towel (control). The medical-grade WBs weighed 8.5 pounds, while the cotton hospital towels weighed less than 1 pound. The WBs were cleaned in between patients with standard germicidal disposable wipes.
Patient data were collected from electronic medical records including age, sex, weight, history of prior MMS, and current use of antihypertensives and/or beta-blockers. Data also were collected on the presence of anxiety disorders, major depression, fibromyalgia, tobacco and alcohol use, hyperthyroidism, hyperhidrosis, cardiac arrhythmias (including atrial fibrillation), chronic obstructive pulmonary disease, asthma, coronary artery disease, diabetes mellitus, peripheral neuropathy, and menopausal symptoms.
During the procedure, anxiety was monitored using the State-Trait Anxiety Inventory (STAI) Form Y-1, the visual analogue scale for anxiety (VAS-A), and vital signs including heart rate, blood pressure, and respiratory rate. Vital signs were evaluated by nursing staff with the patient sitting up and the WB or hospital towel removed. Using these assessments, anxiety was measured at 3 different timepoints: upon arrival to the clinic (timepoint A), after the patient rested in a reclined beach-chair position with the WB or hospital towel placed over them for 10 minutes before administration of local anesthetic and starting the procedure (timepoint B), and after the first MMS stage was taken (timepoint C).
A power analysis was not completed due to a lack of previous studies on the use of WBs during MMS. Group means were analyzed using two-tailed t-tests and one-way analysis of variance. A P value of .05 indicated statistical significance.
Fourteen patients were randomized to the WB group and 13 were randomized to the control group. Patient demographics are outlined in the eTable. In the WB group, mean STAI scores progressively decreased at each timepoint (A: 15.3, B: 13.6, C: 12.7) and mean VAS-A scores followed a similar trend (A: 24.2, B: 19.3, C: 10.5). In the control group, the mean STAI scores remained stable at timepoints A and B (17.7) and then decreased at timepoint C (14.8). The mean VAS-A scores in the control group followed a similar pattern, remaining stable at timepoints A (22.9) and B (22.8) and then decreasing at timepoint C (14.4). These changes were not statistically significant.

Mean vital signs for both the WB and control groups were relatively stable across all timepoints, although they tended to decrease by timepoint C. In the WB group, mean heart rates were 69, 69, and 67 beats per minute at timepoints A, B, and C, respectively. Mean systolic blood pressures were 137 mm Hg, 138 mm Hg, and 136 mm Hg and mean diastolic pressures were 71 mm Hg, 68 mm Hg, and 66 mm Hg at timepoints A, B, and C, respectively. Mean respiratory rates were 20, 19, and 18 breaths per minute at timepoints A, B, and C, respectively. In the control group, mean heart rates were 70, 69, and 68 beats per minute across timepoints A, B, and C, respectively. Mean systolic blood pressures were 137 mm Hg, 138 mm Hg, and 133 mm Hg and mean diastolic pressures were 71 mm Hg, 74 mm Hg, and 68 mm Hg at timepoints A, B, and C, respectively. Mean respiratory rates were 19, 18, and 18 breaths per minute at timepoints A, B, and C, respectively. These changes were not statistically significant.
Our pilot study examined the effects of using a WB to alleviate preoperative anxiety during MMS. Our results suggest that WBs may modestly improve subjective anxiety immediately prior to undergoing MMS. Mean STAI and VAS-A scores decreased from timepoint A to timepoint B in the WB group vs the control group in which these scores remained stable. Although our study was not powered to determine statistical differences and significance was not reached, our results suggest a favorable trend in decreased anxiety scores. Our analysis was limited by a small sample size; therefore, additional larger-scale studies will be needed to confirm this trend.
Our results are broadly consistent with earlier studies that found improvement in physiologic proxies of anxiety with the use of WBs during chemotherapy infusions, dental procedures, and acute inpatient mental health hospitalizations.7-10 During periods of high anxiety, use of WBs shifts the autonomic nervous system from a sympathetic to a parasympathetic state, as demonstrated by increased high-frequency heart rate variability, a marker of parasympathetic activity.6,11 While the exact mechanism of how WBs and other deep pressure stimulation tools affect high-frequency heart rate variability is unclear, one study showed that patients undergoing dental extractions were better equipped when using deep pressure stimulation tools to utilize calming techniques and regulate stress.12 The use of WBs and other deep pressure stimulation tools may extend beyond the perioperative setting and also may be an effective tool for clinicians in other settings (eg, clinic visits, physical examinations).
In our study, all participants demonstrated the greatest reduction in anxiety at timepoint C after the first MMS stage, likely related to patients relaxing more after knowing what to expect from the surgery; this also may have been reflected somewhat in the slight downward trend noted in vital signs across both study groups. One concern regarding WB use in surgical settings is whether the added pressure could trigger unfavorable circulatory effects, such as elevated blood pressure. In our study, with the exception of diastolic blood pressure, vital signs appeared unaffected by the type of blanket used and remained relatively stable from timepoint A to timepoint B and decreased at timepoint C. Diastolic blood pressure in the WB group decreased from timepoint A to timepoint B, then decreased further from timepoint B to timepoint C. This mirrored the decreasing STAI score trend, compared to the control group who increased from timepoint A to timepoint B and reached a nadir at timepoint C. Consistent with prior WB studies, there were no adverse effects from WBs, including adverse impacts on vital signs.6,9
The original recruitment goal was not met due to staffing issues related to the COVID-19 pandemic, and subgroup analyses were deferred as a result of sample size limitations. It is possible that the WB intervention may have a larger impact on subpopulations more prone to perioperative anxiety (eg, patients undergoing MMS for the first time). However, the results of our pilot study suggest a beneficial effect from the use of WBs. While these preliminary data are promising, additional studies in the perioperative setting are needed to more accurately determine the clinical utility of WBs during MMS and other procedures.
- Eberle FC, Schippert W, Trilling B, et al. Cosmetic results of histographically controlled excision of non-melanoma skin cancer in the head and neck region. J Dtsch Dermatol Ges. 2005;3:109-112. doi:10.1111/j.1610-0378.2005.04738.x
- Chren MM, Sahay AP, Bertenthal DS, et al. Quality-of-life outcomes of treatments for cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2007;127:1351-1357. doi:10.1038/sj.jid.5700740
- Kossintseva I, Zloty D. Determinants and timeline of perioperative anxiety in Mohs surgery. Dermatol Surg. 2017;43:1029-1035. doi:10.1097 /DSS.0000000000001152
- Pritchard MJ. Identifying and assessing anxiety in pre-operative patients. Nurs Stand. 2009;23:35-40. doi:10.7748/ns2009.08.23.51.35.c7222.
- Mavros MN, Athanasiou S, Gkegkes ID, et al. Do psychological variables affect early surgical recovery? PLoS One. 2011;6:E20306. doi:10.1371/journal.pone.0020306
- Mullen B, Champagne T, Krishnamurty S, et al. Exploring the safety and therapeutic effects of deep pressure stimulation using a weighted blanket. Occup Ther Ment Health. 2008;24:65-89. doi:10.1300/ J004v24n01_05
- Chen HY, Yang H, Chi HJ, et al. Physiological effects of deep touch pressure on anxiety alleviation: the weighted blanket approach. J Med Biol Eng. 2013;33:463-470. doi:10.5405/jmbe.1043
- Chen HY, Yang H, Meng LF, et al. Effect of deep pressure input on parasympathetic system in patients with wisdom tooth surgery. J Formos Med Assoc. 2016;115:853-859. doi:10.1016 /j.jfma.2016.07.008
- Vinson J, Powers J, Mosesso K. Weighted blankets: anxiety reduction in adult patients receiving chemotherapy. Clin J Oncol Nurs. 2020; 24:360-368. doi:10.1188/20.CJON.360-368
- Champagne T, Mullen B, Dickson D, et al. Evaluating the safety and effectiveness of the weighted blanket with adults during an inpatient mental health hospitalization. Occup Ther Ment Health. 2015;31:211-233. doi:10.1080/0164212X.2015.1066220
- Lane RD, McRae K, Reiman EM, et al. Neural correlates of heart rate variability during emotion. Neuroimage. 2009;44:213-222. doi: 10.1016/j.neuroimage.2008.07.056
- Moyer CA, Rounds J, Hannum JW. A meta-analysis of massage therapy research. Psychol Bull. 2004;130:3-18. doi: 10.1037 /0033-2909.130.1.3
- Eberle FC, Schippert W, Trilling B, et al. Cosmetic results of histographically controlled excision of non-melanoma skin cancer in the head and neck region. J Dtsch Dermatol Ges. 2005;3:109-112. doi:10.1111/j.1610-0378.2005.04738.x
- Chren MM, Sahay AP, Bertenthal DS, et al. Quality-of-life outcomes of treatments for cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2007;127:1351-1357. doi:10.1038/sj.jid.5700740
- Kossintseva I, Zloty D. Determinants and timeline of perioperative anxiety in Mohs surgery. Dermatol Surg. 2017;43:1029-1035. doi:10.1097 /DSS.0000000000001152
- Pritchard MJ. Identifying and assessing anxiety in pre-operative patients. Nurs Stand. 2009;23:35-40. doi:10.7748/ns2009.08.23.51.35.c7222.
- Mavros MN, Athanasiou S, Gkegkes ID, et al. Do psychological variables affect early surgical recovery? PLoS One. 2011;6:E20306. doi:10.1371/journal.pone.0020306
- Mullen B, Champagne T, Krishnamurty S, et al. Exploring the safety and therapeutic effects of deep pressure stimulation using a weighted blanket. Occup Ther Ment Health. 2008;24:65-89. doi:10.1300/ J004v24n01_05
- Chen HY, Yang H, Chi HJ, et al. Physiological effects of deep touch pressure on anxiety alleviation: the weighted blanket approach. J Med Biol Eng. 2013;33:463-470. doi:10.5405/jmbe.1043
- Chen HY, Yang H, Meng LF, et al. Effect of deep pressure input on parasympathetic system in patients with wisdom tooth surgery. J Formos Med Assoc. 2016;115:853-859. doi:10.1016 /j.jfma.2016.07.008
- Vinson J, Powers J, Mosesso K. Weighted blankets: anxiety reduction in adult patients receiving chemotherapy. Clin J Oncol Nurs. 2020; 24:360-368. doi:10.1188/20.CJON.360-368
- Champagne T, Mullen B, Dickson D, et al. Evaluating the safety and effectiveness of the weighted blanket with adults during an inpatient mental health hospitalization. Occup Ther Ment Health. 2015;31:211-233. doi:10.1080/0164212X.2015.1066220
- Lane RD, McRae K, Reiman EM, et al. Neural correlates of heart rate variability during emotion. Neuroimage. 2009;44:213-222. doi: 10.1016/j.neuroimage.2008.07.056
- Moyer CA, Rounds J, Hannum JW. A meta-analysis of massage therapy research. Psychol Bull. 2004;130:3-18. doi: 10.1037 /0033-2909.130.1.3
Weighted Blankets May Help Reduce Preoperative Anxiety During Mohs Micrographic Surgery
Weighted Blankets May Help Reduce Preoperative Anxiety During Mohs Micrographic Surgery
PRACTICE POINTS
- Preoperative anxiety in patients during Mohs micrographic surgery (MMS) may increase intraoperative bleeding, complication rates, and recovery times.
- Using weighted blankets may reduce anxiety in patients undergoing MMS of the head and neck.
Impact of NSAID Use on Bleeding Rates for Patients Taking Rivaroxaban or Apixaban
Impact of NSAID Use on Bleeding Rates for Patients Taking Rivaroxaban or Apixaban
Clinical practice has shifted from vitamin K antagonists to direct oral anticoagulants (DOACs) for atrial fibrillation treatment due to their more favorable risk-benefit profile and less lifestyle modification required.1,2 However, the advantage of a lower bleeding risk with DOACs could be compromised by potentially problematic pharmacokinetic interactions like those conferred by antiplatelets or nonsteroidal anti-inflammatory drugs (NSAIDs).3,4 Treating a patient needing anticoagulation with a DOAC who has comorbidities may introduce unavoidable drug-drug interactions. This particularly happens with over-the-counter and prescription NSAIDs used for the management of pain and inflammatory conditions.5
NSAIDs primarily affect 2 cyclooxygenase (COX) enzyme isomers, COX-1 and COX-2.6 COX-1 helps maintain gastrointestinal (GI) mucosa integrity and platelet aggregation processes, whereas COX-2 is engaged in pain signaling and inflammation mediation. COX-1 inhibition is associated with more bleeding-related adverse events (AEs), especially in the GI tract. COX-2 inhibition is thought to provide analgesia and anti-inflammatory properties without elevating bleeding risk. This premise is responsible for the preferential use of celecoxib, a COX-2 selective NSAID, which should confer a lower bleeding risk compared to nonselective NSAIDs such as ibuprofen and naproxen.7 NSAIDs have been documented as independent risk factors for bleeding. NSAID users are about 3 times as likely to develop GI AEs compared to nonNSAID users.8
Many clinicians aim to further mitigate NSAID-associated bleeding risk by coprescribing a proton pump inhibitor (PPI). PPIs provide gastroprotection against NSAID-induced mucosal injury and sequential complication of GI bleeding. In a multicenter randomized control trial, patients who received concomitant PPI therapy while undergoing chronic NSAID therapy—including nonselective and COX-2 selective NSAIDs—had a significantly lower risk of GI ulcer development (placebo, 17.0%; 20 mg esomeprazole, 5.2%; 40 mg esomeprazole, 4.6%).9 Current clinical guidelines for preventing NSAIDassociated bleeding complications recommend using a COX-2 selective NSAID in combination with PPI therapy for patients at high risk for GI-related bleeding, including the concomitant use of anticoagulants.10
There is evidence suggesting an increased bleeding risk with NSAIDs when used in combination with vitamin K antagonists such as warfarin.11,12 A systematic review of warfarin and concomitant NSAID use found an increased risk of overall bleeding with NSAID use in combination with warfarin (odds ratio 1.58; 95% CI, 1.18-2.12), compared to warfarin alone.12
Posthoc analyses of randomized clinical trials have also demonstrated an increased bleeding risk with oral anticoagulation and concomitant NSAID use.13,14 In the RE-LY trial, NSAID users on warfarin or dabigatran had a statistically significant increased risk of major bleeding compared to non-NSAID users (hazard ratio [HR] 1.68; 95% CI, 1.40- 2.02; P < .001).13 In the ARISTOTLE trial, patients on warfarin or apixaban who were incident NSAID users were found to have an increased risk of major bleeding (HR 1.61; 95% CI, 1.11-2.33) and clinically relevant nonmajor bleeding (HR 1.70; 95% CI, 1.16- 2.48).14 These trials found a statistically significant increased bleeding risk associated with NSAID use, though the populations evaluated included patients taking warfarin and patients taking DOACs. These trials did not evaluate the bleeding risk of concomitant NSAID use among DOACs alone.
Evidence on NSAID-associated bleeding risk with DOACs is lacking in settings where the patient population, prescribing practices, and monitoring levels are variable. Within the Veterans Health Administration, clinical pharmacist practitioners (CPPs) in anticoagulation clinics oversee DOAC therapy management. CPPs monitor safety and efficacy of DOAC therapies through a population health management tool, the DOAC Dashboard.15 The DOAC Dashboard creates alerts for patients who may require an intervention based on certain clinical parameters, such as drug-drug interactions.16 Whenever a patient on a DOAC is prescribed an NSAID, an alert is generated on the DOAC Dashboard to flag the CPPs for the potential need for an intervention. If NSAID therapy remains clinically indicated, CPPs may recommend risk reduction strategies such as a COX-2 selective NSAID or coprescribing a PPI.10
The DOAC Dashboard provides an ideal setting for investigating the effects of NSAID use, NSAID selectivity, and PPI coprescribing on DOAC bleeding rates. With an increasing population of patients receiving anticoagulation therapy with a DOAC, more guidance regarding the bleeding risk of concomitant NSAID use with DOACs is needed. Studies evaluating the bleeding risk with concomitant NSAID use in patients on a DOAC alone are limited. This is the first study to date to compare bleeding risk with concomitant NSAID use between DOACs. This study provides information on bleeding risk with NSAID use among commonly prescribed DOACs, rivaroxaban and apixaban, and the potential impacts of current risk reduction strategies.
METHODS
This single-center retrospective cohort review was performed using the electronic health records (EHRs) of patients enrolled in the US Department of Veterans Affairs (VA) Mountain Home Healthcare System who received rivaroxaban or apixaban from December 2020 to December 2022. This study received approval from the East Tennessee State University/VA Institutional Review Board committee.
Patients were identified through the DOAC Dashboard, aged 21 to 100 years, and received rivaroxaban or apixaban at a therapeutic dose: rivaroxaban 10 to 20 mg daily or apixaban 2.5 to 5 mg twice daily. Patients were excluded if they were prescribed dual antiplatelet therapy, received rivaroxaban at dosing indicated for peripheral vascular disease, were undergoing dialysis, had evidence of moderate to severe hepatic impairment or any hepatic disease with coagulopathy, were undergoing chemotherapy or radiation, or had hematological conditions with predisposed bleeding risk. These patients were excluded to mitigate the potential confounding impact from nontherapeutic DOAC dosing strategies and conditions associated with an increased bleeding risk.
Eligible patients were stratified based on NSAID use. NSAID users were defined as patients prescribed an oral NSAID, including both acute and chronic courses, at any point during the study time frame while actively on a DOAC. Bleeding events were reviewed to evaluate rates between rivaroxaban and apixaban among NSAID and nonNSAID users. Identified NSAID users were further assessed for NSAID selectivity and PPI coprescribing as a subgroup analysis for the secondary assessment.
Data Collection
Baseline data were collected, including age, body mass index, anticoagulation indication, DOAC agent, DOAC dose, and DOAC total daily dose. Baseline serum creatinine levels, liver function tests, hemoglobin levels, and platelet counts were collected from the most recent data available immediately prior to the bleeding event, if applicable.
The DOAC Dashboard was reviewed for active and dismissed drug interaction alerts to identify patients taking rivaroxaban or apixaban who were prescribed an NSAID. Patients were categorized in the NSAID group if an interacting drug alert with an NSAID was reported during the study time frame. Data available through the interacting drug alerts on NSAID use were limited to the interacting drug name and date of the reported flag. Manual EHR review was required to confirm dates of NSAID therapy initiation and NSAID discontinuation, if applicable.
Data regarding concomitant antiplatelet use were obtained through review of the active and dismissed drug interaction alerts on the DOAC Dashboard. Concomitant antiplatelet use was defined as the prescribing of a single antiplatelet agent at any point while receiving DOAC therapy. Data on concomitant antiplatelets were collected regardless of NSAID status.
Data on coprescribed PPI therapy were obtained through manual EHR review of identified NSAID users. Coprescribed PPI therapy was defined as the prescribing of a PPI at any point during NSAID therapy. Data regarding PPI use among non-NSAID users were not collected because the secondary endpoint was designed to assess PPI use only among patients coprescribed a DOAC and NSAID.
Outcomes
Bleeding events were identified through an outcomes report generated by the DOAC Dashboard based on International Classification of Diseases, Tenth Revision diagnosis codes associated with a bleeding event. The outcomes report captures diagnoses from the outpatient and inpatient care settings. Reported bleeding events were limited to patients who received a DOAC at any point in the 6 months prior to the event and excluded patients with recent DOAC initiation within 7 days of the event, as these patients are not captured on the DOAC Dashboard.
All reported bleeding events were manually reviewed in the EHR and categorized as a major or clinically relevant nonmajor bleed, according to International Society of Thrombosis and Haemostasis criteria. Validated bleeding events were then crossreferenced with the interacting drug alerts report to identify events with potentially overlapping NSAID therapy at the time of the event. Overlapping NSAID therapy was defined as the prescribing of an NSAID at any point in the 6 months prior to the event. All events with potential overlapping NSAID therapies were manually reviewed for confirmation of NSAID status at the time of the event.
The primary endpoint was a composite of any bleeding event per International Society of Thrombosis and Haemostasis criteria. The secondary endpoint evaluated the potential impact of NSAID selectivity or PPI coprescribing on the bleeding rate among the NSAID user groups.
Statistical Analysis
Analyses were performed consistent with the methods used in the ARISTOTLE and RE-LY trials. It was determined that a sample size of 504 patients, with ≥ 168 patients in each group, would provide 80% power using a 2-sided a of 0.05. HRs with 95% CIs and respective P values were calculated using a SPSS-adapted online calculator.
RESULTS
The DOAC Dashboard identified 681 patients on rivaroxaban and 3225 patients on apixaban; 72 patients on rivaroxaban (10.6%) and 300 patients on apixaban (9.3%) were NSAID users. The mean age of NSAID users was 66.9 years in the rivaroxaban group and 72.4 years in the apixaban group. The mean age of non-NSAID users was 71.5 years in the rivaroxaban group and 75.6 years in the apixaban group. No appreciable differences were observed among subgroups in body mass index, renal function, hepatic function, hemoglobin, or platelet counts, and no statistically significant differences were identified (Table 1). Antiplatelet agents identified included aspirin, clopidogrel, prasugrel, and ticagrelor. Fifteen patients (20.3%) in the rivaroxaban group and 87 patients (28.7%) in the apixaban group had concomitant antiplatelet and NSAID use. Forty-five patients on rivaroxaban (60.8%) and 170 (55.9%) on apixaban were prescribed concomitant PPI and NSAID at baseline. Among non-NSAID users, there was concomitant antiplatelet use for 265 patients (43.6%) in the rivaroxaban group and 1401 patients (47.9%) in the apixaban group. Concomitant PPI use was identified among 63 patients (60.0%) taking selective NSAIDs and 182 (57.2%) taking nonselective NSAIDs.
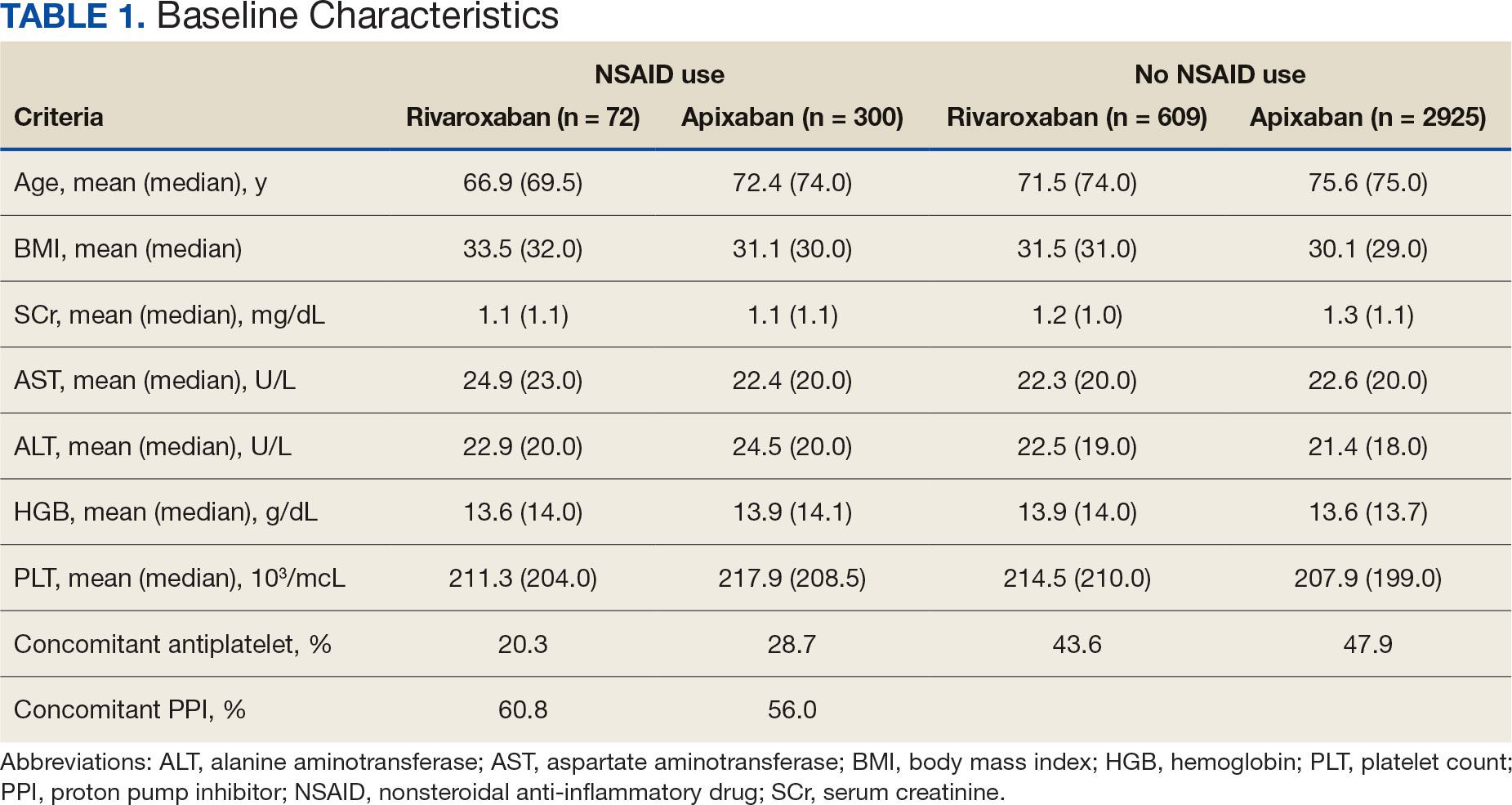
A total of 423 courses of NSAIDs were identified: 85 NSAID courses in the rivaroxaban group and 338 NSAID courses in the apixaban group. Most NSAID courses involved a nonselective NSAID in the rivaroxaban and apixaban NSAID user groups: 75.2% (n = 318) aggregately compared to 71.8% (n = 61) and 76.0% (n = 257) in the rivaroxaban and apixaban groups, respectively. The most frequent NSAID courses identified were meloxicam (26.7%; n = 113), celecoxib (24.8%; n = 105), ibuprofen (19.1%; n = 81), and naproxen (13.5%; n = 57). Data regarding NSAID therapy initiation and discontinuation dates were not readily available. As a result, the duration of NSAID courses was not captured.
There was no statistically significant difference in bleeding rates between rivaroxaban and apixaban among NSAID users (HR 1.04; 95% CI, 0.98-1.12) or non-NSAID users (HR 1.15; 95% CI, 0.80-1.66) (Table 2). Apixaban non-NSAID users had a higher rate of major bleeds (HR 0.32; 95% CI, 0.17-0.61) while rivaroxaban non-NSAID users had a higher rate of clinically relevant nonmajor bleeds (HR 1.63; 95% CI, 1.10-2.54).
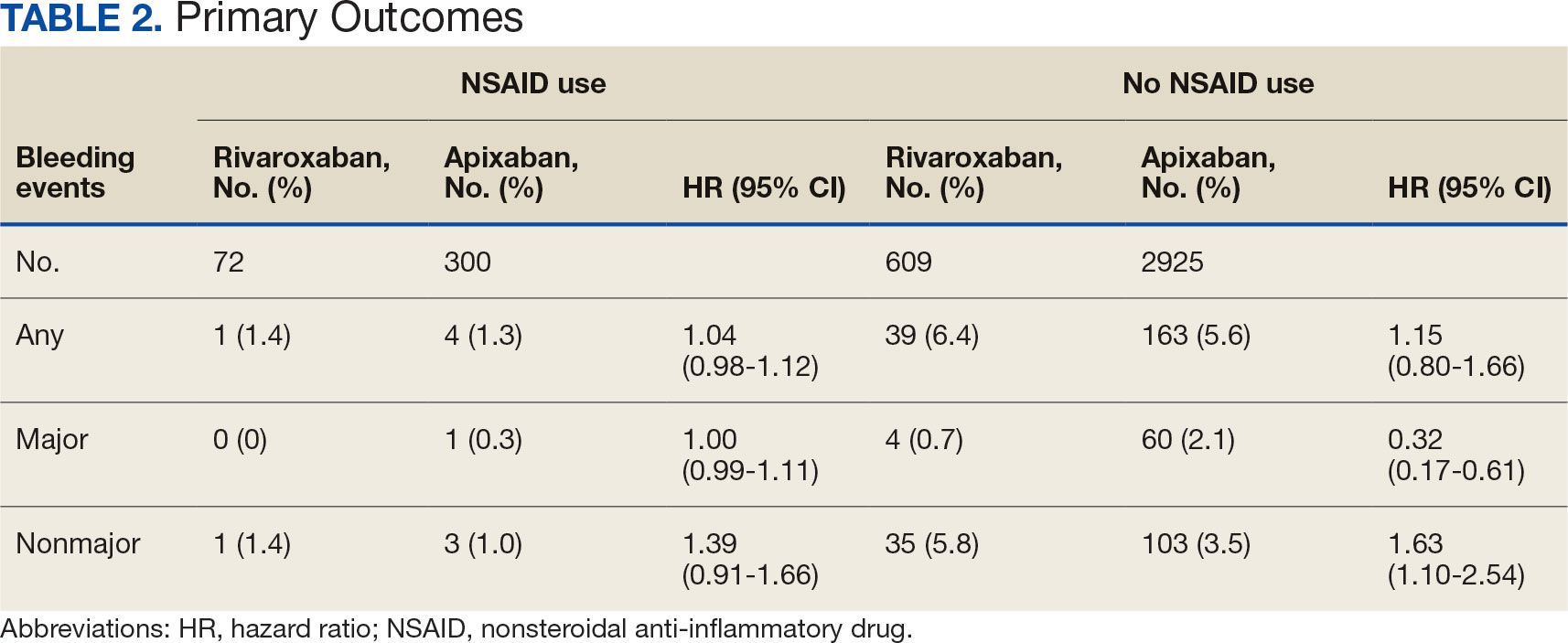
The sample size for the secondary endpoint consisted of bleeding events that were confirmed to have had an overlapping NSAID prescribed at the time of the event. For this secondary assessment, there was 1 rivaroxaban NSAID user bleeding event and 4 apixaban NSAID user bleeding events. For the rivaroxaban NSAID user bleeding event, the NSAID was nonselective and a PPI was not coprescribed. For the apixaban NSAID user bleeding events, 2 NSAIDs were nonselective and 2 were selective. All patients with apixaban and NSAID bleeding events had a coprescribed PPI. There was no clinically significant difference in the bleeding rates observed for NSAID selectivity or PPI coprescribing among the NSAID user subgroups.
DISCUSSION
This study found that there was no statistically significant difference for bleeding rates of major and nonmajor bleeding events between rivaroxaban and apixaban among NSAID users and non-NSAID users. This study did not identify a clinically significant impact on bleeding rates from NSAID selectivity or PPI coprescribing among the NSAID users.
There were notable but not statistically significant differences in baseline characteristics observed between the NSAID and non-NSAID user groups. On average, the rivaroxaban and apixaban NSAID users were younger compared with those not taking NSAIDs. NSAIDs, specifically nonselective NSAIDs, are recognized as potentially inappropriate medications for older adults given that this population is at an increased risk for GI ulcer development and/or GI bleeding.17 The non-NSAID user group likely consisted of older patients compared to the NSAID user group as clinicians may avoid prescribing NSAIDs to older adults regardless of concomitant DOAC therapy.
In addition to having an older patient population, non-NSAID users were more frequently prescribed a concomitant antiplatelet when compared with NSAID users. This prescribing pattern may be due to clinicians avoiding the use of NSAIDs in patients receiving DOAC therapy in combination with antiplatelet therapy, as these patients have been found to have an increased bleeding rate compared to DOAC therapy alone.18
Non-NSAID users had an overall higher bleeding rate for both major and nonmajor bleeding events. Based on this observation, it could be hypothesized that antiplatelet agents have a higher risk of bleeding in comparison to NSAIDs. In a subanalysis of the EXPAND study evaluating risk factors of major bleeding in patients receiving rivaroxaban, concomitant use of antiplatelet agents demonstrated a statistically significant increased risk of bleeding (HR 1.6; 95% CI, 1.2-2.3; P = .003) while concomitant use of NSAIDs did not (HR 0.8; 95% CI, 0.3-2.2; P = .67).19
In assessing PPI status at baseline, a majority of both rivaroxaban and apixaban NSAID users were coprescribed a PPI. This trend aligns with current clinical guideline recommendations for the prescribing of PPI therapy for GI protection in high-risk patients, such as those on DOAC therapy and concomitant NSAID therapy.10 Given the high proportion of NSAID users coprescribed a PPI at baseline, it may be possible that the true incidence of NSAID-associated bleeding events was higher than what this study found. This observation may reflect the impact from timely implementation of risk mitigation strategies by CPPs in the anticoagulation clinic. However, this study was not constructed to assess the efficacy of PPI use in this manner.
It is important to note the patients included in this study were followed by a pharmacist in an anticoagulation clinic using the DOAC Dashboard.15 This population management tool allows CPPs to make proactive interventions when a patient taking a DOAC receives an NSAID prescription, such as recommending the coprescribing of a PPI or use of a selective NSAID.10,16 These standards of care may have contributed to an overall reduced bleeding rate among the NSAID user group and may not be reflective of private practice.
The planned analysis of this study was modeled after the posthoc analysis of the RE-LY and ARISTOTLE trials. Both trials demonstrated an increased risk of bleeding with oral anticoagulation, including DOAC and warfarin, in combination with NSAID use. However, both trials found that NSAID use in patients treated with a DOAC was not independently associated with increased bleeding events compared with warfarin.13,14 The results of this study are comparable to the RE-LY and ARISTOTLE findings that NSAID use among patients treated with rivaroxaban or apixaban did not demonstrate a statistically significant increased bleeding risk.
Studies of NSAID use in combination with DOAC therapy have been limited to patient populations consisting of both DOAC and warfarin. Evidence from these trials outlines the increased bleeding risk associated with NSAID use in combination with oral anticoagulation; however, these patient populations include those on a DOAC and warfarin.13,14,19,20 Given the limited evidence on NSAID use among DOACs alone, it is assumed NSAID use in combination with DOACs has a similar risk of bleeding as warfarin use. This may cause clinicians to automatically exclude NSAID therapy as a treatment option for patients on a DOAC who are otherwise clinically appropriate candidates, such as those with underlying inflammatory conditions. Avoiding NSAID therapy in this patient population may lead to suboptimal pain management and increase the risk of patient harm from methods such as inappropriate opioid therapy prescribing.
DOAC therapy should not be a universal limitation to the use of NSAIDs. Although the risk of bleeding with NSAID therapy is always present, deliberate NSAID prescribing in addition to the timely implementation of risk mitigation strategies may provide an avenue for safe NSAID prescribing in patients receiving a DOAC. A population health-based approach to DOAC management, such as the DOAC Dashboard, appears to be effective at preventing patient harm when NSAIDs are prescribed in conjunction with DOACs.
Limitations
The DOAC Dashboard has been shown to be effective and efficient at monitoring DOAC therapy from a population-based approach.16 Reports generated through the DOAC Dashboard provide convenient access to patient data which allows for timely interventions; however, there are limits to its use for data collection. All the data elements necessary to properly assess bleeding risk with validated tools, such as HAS-BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, drugs/ alcohol concomitantly), are not available on DOAC Dashboard reports. Due to this constraint, bleeding risk assessments were not conducted at baseline and this study was unable to include risk modeling. Additionally, data elements like initiation and discontinuation dates and duration of therapies were not readily available. As a result, this study was unable to incorporate time as a data point.
This was a retrospective study that relied on manual review of chart documentation to verify bleeding events, but data obtained through the DOAC Dashboard were transferred directly from the EHR.15 Bleeding events available for evaluation were restricted to those that occurred at a VA facility. Additionally, the sample size within the rivaroxaban NSAID user group did not reach the predefined sample size required to reach power and may have been too small to detect a difference if one did exist. The secondary assessment had a low sample size of NSAID user bleeding events, making it difficult to fully assess its impact on NSAID selectivity and PPI coprescribing on bleeding rates. All courses of NSAIDs were equally valued regardless of the dose or therapy duration; however, this is consistent with how NSAID use was defined in the RE-LY and ARISTOTLE trials.
CONCLUSIONS
This retrospective cohort review found no statistically significant difference in the composite bleeding rates between rivaroxaban and apixaban among NSAID users and non-NSAID users. Moreover, there was no clinically significant impact observed for bleeding rates in regard to NSAID selectivity and PPI coprescribing among NSAID users. However, coprescribing of PPI therapy to patients on a DOAC who are clinically indicated for an NSAID may reduce the risk of bleeding. Population health management tools, such as the DOAC Dashboard, may also allow clinicians to safely prescribe NSAIDs to patients on a DOAC. Further large-scale observational studies are needed to quantify the real-world risk of bleeding with concomitant NSAID use among DOACs alone and to evaluate the impact from NSAID selectivity or PPI coprescribing.
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-962. doi:10.1016/S0140-6736(13)62343-0
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e44S-e88S. doi:10.1378/chest.11-2292
- Eikelboom J, Merli G. Bleeding with direct oral anticoagulants vs warfarin: clinical experience. Am J Med. 2016;129(11S):S33-S40. doi:10.1016/j.amjmed.2016.06.003
- Vranckx P, Valgimigli M, Heidbuchel H. The significance of drug-drug and drug-food interactions of oral anticoagulation. Arrhythm Electrophysiol Rev. 2018;7(1):55-61. doi:10.15420/aer.2017.50.1
- Davis JS, Lee HY, Kim J, et al. Use of non-steroidal antiinflammatory drugs in US adults: changes over time and by demographic. Open Heart. 2017;4(1):e000550. doi:10.1136/openhrt-2016-000550
- Schafer AI. Effects of nonsteroidal antiinflammatory drugs on platelet function and systemic hemostasis. J Clin Pharmacol. 1995;35(3):209-219. doi:10.1002/j.1552-4604.1995.tb04050.x
- Al-Saeed A. Gastrointestinal and cardiovascular risk of nonsteroidal anti-inflammatory drugs. Oman Med J. 2011;26(6):385-391. doi:10.5001/omj.2011.101
- Gabriel SE, Jaakkimainen L, Bombardier C. Risk for serious gastrointestinal complications related to use of nonsteroidal anti-inflammatory drugs. Ann Intern Med. 1991;115(10):787-796. doi:10.7326/0003-4819-115-10-787
- Scheiman JM, Yeomans ND, Talley NJ, et al. Prevention of ulcers by esomeprazole in at-risk patients using non-selective NSAIDs and COX-2 inhibitors. Am J Gastroenterol. 2006;101(4):701-710. doi:10.1111/j.1572-0241.2006.00499.x
- Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152(4):706-715. doi:10.1053/j.gastro.2017.01.031
- Lamberts M, Lip GYH, Hansen ML, et al. Relation of nonsteroidal anti-inflammatory drugs to serious bleeding and thromboembolism risk in patients with atrial fibrillation receiving antithrombotic therapy: a nationwide cohort study. Ann Intern Med. 2014;161(10):690-698. doi:10.7326/M13-1581
- Villa Zapata L, Hansten PD, Panic J, et al. Risk of bleeding with exposure to warfarin and nonsteroidal anti-inflammatory drugs: a systematic review and metaanalysis. Thromb Haemost. 2020;120(7):1066-1074. doi:10.1055/s-0040-1710592
- Kent AP, Brueckmann M, Fraessdorf M, et al. Concomitant oral anticoagulant and nonsteroidal anti-inflammatory drug therapy in patients with atrial fibrillation. J Am Coll Cardiol. 2018;72(3):255-267. doi:10.1016/j.jacc.2018.04.063
- Dalgaard F, Mulder H, Wojdyla DM, et al. Patients with atrial fibrillation taking nonsteroidal antiinflammatory drugs and oral anticoagulants in the ARISTOTLE Trial. Circulation. 2020;141(1):10-20. doi:10.1161/CIRCULATIONAHA.119.041296
- Allen AL, Lucas J, Parra D, et al. Shifting the paradigm: a population health approach to the management of direct oral anticoagulants. J Am Heart Asssoc. 2021;10(24):e022758. doi:10.1161/JAHA.121.022758
- . Valencia D, Spoutz P, Stoppi J, et al. Impact of a direct oral anticoagulant population management tool on anticoagulation therapy monitoring in clinical practice. Ann Pharmacother. 2019;53(8):806-811. doi:10.1177/1060028019835843
- By the 2023 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2023 Updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052-2081. doi:10.1111/jgs.18372
- Kumar S, Danik SB, Altman RK, et al. Non-vitamin K antagonist oral anticoagulants and antiplatelet therapy for stroke prevention in patients with atrial fibrillation. Cardiol Rev. 2016;24(5):218-223. doi:10.1097/CRD.0000000000000088
- Sakuma I, Uchiyama S, Atarashi H, et al. Clinical risk factors of stroke and major bleeding in patients with nonvalvular atrial fibrillation under rivaroxaban: the EXPAND study sub-analysis. Heart Vessels. 2019;34(11):1839-1851. doi:10.1007/s00380-019-01425-x
- Davidson BL, Verheijen S, Lensing AWA, et al. Bleeding risk of patients with acute venous thromboembolism taking nonsteroidal anti-inflammatory drugs or aspirin. JAMA Intern Med. 2014;174(6):947-953. doi:10.1001/jamainternmed.2014.946
Clinical practice has shifted from vitamin K antagonists to direct oral anticoagulants (DOACs) for atrial fibrillation treatment due to their more favorable risk-benefit profile and less lifestyle modification required.1,2 However, the advantage of a lower bleeding risk with DOACs could be compromised by potentially problematic pharmacokinetic interactions like those conferred by antiplatelets or nonsteroidal anti-inflammatory drugs (NSAIDs).3,4 Treating a patient needing anticoagulation with a DOAC who has comorbidities may introduce unavoidable drug-drug interactions. This particularly happens with over-the-counter and prescription NSAIDs used for the management of pain and inflammatory conditions.5
NSAIDs primarily affect 2 cyclooxygenase (COX) enzyme isomers, COX-1 and COX-2.6 COX-1 helps maintain gastrointestinal (GI) mucosa integrity and platelet aggregation processes, whereas COX-2 is engaged in pain signaling and inflammation mediation. COX-1 inhibition is associated with more bleeding-related adverse events (AEs), especially in the GI tract. COX-2 inhibition is thought to provide analgesia and anti-inflammatory properties without elevating bleeding risk. This premise is responsible for the preferential use of celecoxib, a COX-2 selective NSAID, which should confer a lower bleeding risk compared to nonselective NSAIDs such as ibuprofen and naproxen.7 NSAIDs have been documented as independent risk factors for bleeding. NSAID users are about 3 times as likely to develop GI AEs compared to nonNSAID users.8
Many clinicians aim to further mitigate NSAID-associated bleeding risk by coprescribing a proton pump inhibitor (PPI). PPIs provide gastroprotection against NSAID-induced mucosal injury and sequential complication of GI bleeding. In a multicenter randomized control trial, patients who received concomitant PPI therapy while undergoing chronic NSAID therapy—including nonselective and COX-2 selective NSAIDs—had a significantly lower risk of GI ulcer development (placebo, 17.0%; 20 mg esomeprazole, 5.2%; 40 mg esomeprazole, 4.6%).9 Current clinical guidelines for preventing NSAIDassociated bleeding complications recommend using a COX-2 selective NSAID in combination with PPI therapy for patients at high risk for GI-related bleeding, including the concomitant use of anticoagulants.10
There is evidence suggesting an increased bleeding risk with NSAIDs when used in combination with vitamin K antagonists such as warfarin.11,12 A systematic review of warfarin and concomitant NSAID use found an increased risk of overall bleeding with NSAID use in combination with warfarin (odds ratio 1.58; 95% CI, 1.18-2.12), compared to warfarin alone.12
Posthoc analyses of randomized clinical trials have also demonstrated an increased bleeding risk with oral anticoagulation and concomitant NSAID use.13,14 In the RE-LY trial, NSAID users on warfarin or dabigatran had a statistically significant increased risk of major bleeding compared to non-NSAID users (hazard ratio [HR] 1.68; 95% CI, 1.40- 2.02; P < .001).13 In the ARISTOTLE trial, patients on warfarin or apixaban who were incident NSAID users were found to have an increased risk of major bleeding (HR 1.61; 95% CI, 1.11-2.33) and clinically relevant nonmajor bleeding (HR 1.70; 95% CI, 1.16- 2.48).14 These trials found a statistically significant increased bleeding risk associated with NSAID use, though the populations evaluated included patients taking warfarin and patients taking DOACs. These trials did not evaluate the bleeding risk of concomitant NSAID use among DOACs alone.
Evidence on NSAID-associated bleeding risk with DOACs is lacking in settings where the patient population, prescribing practices, and monitoring levels are variable. Within the Veterans Health Administration, clinical pharmacist practitioners (CPPs) in anticoagulation clinics oversee DOAC therapy management. CPPs monitor safety and efficacy of DOAC therapies through a population health management tool, the DOAC Dashboard.15 The DOAC Dashboard creates alerts for patients who may require an intervention based on certain clinical parameters, such as drug-drug interactions.16 Whenever a patient on a DOAC is prescribed an NSAID, an alert is generated on the DOAC Dashboard to flag the CPPs for the potential need for an intervention. If NSAID therapy remains clinically indicated, CPPs may recommend risk reduction strategies such as a COX-2 selective NSAID or coprescribing a PPI.10
The DOAC Dashboard provides an ideal setting for investigating the effects of NSAID use, NSAID selectivity, and PPI coprescribing on DOAC bleeding rates. With an increasing population of patients receiving anticoagulation therapy with a DOAC, more guidance regarding the bleeding risk of concomitant NSAID use with DOACs is needed. Studies evaluating the bleeding risk with concomitant NSAID use in patients on a DOAC alone are limited. This is the first study to date to compare bleeding risk with concomitant NSAID use between DOACs. This study provides information on bleeding risk with NSAID use among commonly prescribed DOACs, rivaroxaban and apixaban, and the potential impacts of current risk reduction strategies.
METHODS
This single-center retrospective cohort review was performed using the electronic health records (EHRs) of patients enrolled in the US Department of Veterans Affairs (VA) Mountain Home Healthcare System who received rivaroxaban or apixaban from December 2020 to December 2022. This study received approval from the East Tennessee State University/VA Institutional Review Board committee.
Patients were identified through the DOAC Dashboard, aged 21 to 100 years, and received rivaroxaban or apixaban at a therapeutic dose: rivaroxaban 10 to 20 mg daily or apixaban 2.5 to 5 mg twice daily. Patients were excluded if they were prescribed dual antiplatelet therapy, received rivaroxaban at dosing indicated for peripheral vascular disease, were undergoing dialysis, had evidence of moderate to severe hepatic impairment or any hepatic disease with coagulopathy, were undergoing chemotherapy or radiation, or had hematological conditions with predisposed bleeding risk. These patients were excluded to mitigate the potential confounding impact from nontherapeutic DOAC dosing strategies and conditions associated with an increased bleeding risk.
Eligible patients were stratified based on NSAID use. NSAID users were defined as patients prescribed an oral NSAID, including both acute and chronic courses, at any point during the study time frame while actively on a DOAC. Bleeding events were reviewed to evaluate rates between rivaroxaban and apixaban among NSAID and nonNSAID users. Identified NSAID users were further assessed for NSAID selectivity and PPI coprescribing as a subgroup analysis for the secondary assessment.
Data Collection
Baseline data were collected, including age, body mass index, anticoagulation indication, DOAC agent, DOAC dose, and DOAC total daily dose. Baseline serum creatinine levels, liver function tests, hemoglobin levels, and platelet counts were collected from the most recent data available immediately prior to the bleeding event, if applicable.
The DOAC Dashboard was reviewed for active and dismissed drug interaction alerts to identify patients taking rivaroxaban or apixaban who were prescribed an NSAID. Patients were categorized in the NSAID group if an interacting drug alert with an NSAID was reported during the study time frame. Data available through the interacting drug alerts on NSAID use were limited to the interacting drug name and date of the reported flag. Manual EHR review was required to confirm dates of NSAID therapy initiation and NSAID discontinuation, if applicable.
Data regarding concomitant antiplatelet use were obtained through review of the active and dismissed drug interaction alerts on the DOAC Dashboard. Concomitant antiplatelet use was defined as the prescribing of a single antiplatelet agent at any point while receiving DOAC therapy. Data on concomitant antiplatelets were collected regardless of NSAID status.
Data on coprescribed PPI therapy were obtained through manual EHR review of identified NSAID users. Coprescribed PPI therapy was defined as the prescribing of a PPI at any point during NSAID therapy. Data regarding PPI use among non-NSAID users were not collected because the secondary endpoint was designed to assess PPI use only among patients coprescribed a DOAC and NSAID.
Outcomes
Bleeding events were identified through an outcomes report generated by the DOAC Dashboard based on International Classification of Diseases, Tenth Revision diagnosis codes associated with a bleeding event. The outcomes report captures diagnoses from the outpatient and inpatient care settings. Reported bleeding events were limited to patients who received a DOAC at any point in the 6 months prior to the event and excluded patients with recent DOAC initiation within 7 days of the event, as these patients are not captured on the DOAC Dashboard.
All reported bleeding events were manually reviewed in the EHR and categorized as a major or clinically relevant nonmajor bleed, according to International Society of Thrombosis and Haemostasis criteria. Validated bleeding events were then crossreferenced with the interacting drug alerts report to identify events with potentially overlapping NSAID therapy at the time of the event. Overlapping NSAID therapy was defined as the prescribing of an NSAID at any point in the 6 months prior to the event. All events with potential overlapping NSAID therapies were manually reviewed for confirmation of NSAID status at the time of the event.
The primary endpoint was a composite of any bleeding event per International Society of Thrombosis and Haemostasis criteria. The secondary endpoint evaluated the potential impact of NSAID selectivity or PPI coprescribing on the bleeding rate among the NSAID user groups.
Statistical Analysis
Analyses were performed consistent with the methods used in the ARISTOTLE and RE-LY trials. It was determined that a sample size of 504 patients, with ≥ 168 patients in each group, would provide 80% power using a 2-sided a of 0.05. HRs with 95% CIs and respective P values were calculated using a SPSS-adapted online calculator.
RESULTS
The DOAC Dashboard identified 681 patients on rivaroxaban and 3225 patients on apixaban; 72 patients on rivaroxaban (10.6%) and 300 patients on apixaban (9.3%) were NSAID users. The mean age of NSAID users was 66.9 years in the rivaroxaban group and 72.4 years in the apixaban group. The mean age of non-NSAID users was 71.5 years in the rivaroxaban group and 75.6 years in the apixaban group. No appreciable differences were observed among subgroups in body mass index, renal function, hepatic function, hemoglobin, or platelet counts, and no statistically significant differences were identified (Table 1). Antiplatelet agents identified included aspirin, clopidogrel, prasugrel, and ticagrelor. Fifteen patients (20.3%) in the rivaroxaban group and 87 patients (28.7%) in the apixaban group had concomitant antiplatelet and NSAID use. Forty-five patients on rivaroxaban (60.8%) and 170 (55.9%) on apixaban were prescribed concomitant PPI and NSAID at baseline. Among non-NSAID users, there was concomitant antiplatelet use for 265 patients (43.6%) in the rivaroxaban group and 1401 patients (47.9%) in the apixaban group. Concomitant PPI use was identified among 63 patients (60.0%) taking selective NSAIDs and 182 (57.2%) taking nonselective NSAIDs.

A total of 423 courses of NSAIDs were identified: 85 NSAID courses in the rivaroxaban group and 338 NSAID courses in the apixaban group. Most NSAID courses involved a nonselective NSAID in the rivaroxaban and apixaban NSAID user groups: 75.2% (n = 318) aggregately compared to 71.8% (n = 61) and 76.0% (n = 257) in the rivaroxaban and apixaban groups, respectively. The most frequent NSAID courses identified were meloxicam (26.7%; n = 113), celecoxib (24.8%; n = 105), ibuprofen (19.1%; n = 81), and naproxen (13.5%; n = 57). Data regarding NSAID therapy initiation and discontinuation dates were not readily available. As a result, the duration of NSAID courses was not captured.
There was no statistically significant difference in bleeding rates between rivaroxaban and apixaban among NSAID users (HR 1.04; 95% CI, 0.98-1.12) or non-NSAID users (HR 1.15; 95% CI, 0.80-1.66) (Table 2). Apixaban non-NSAID users had a higher rate of major bleeds (HR 0.32; 95% CI, 0.17-0.61) while rivaroxaban non-NSAID users had a higher rate of clinically relevant nonmajor bleeds (HR 1.63; 95% CI, 1.10-2.54).

The sample size for the secondary endpoint consisted of bleeding events that were confirmed to have had an overlapping NSAID prescribed at the time of the event. For this secondary assessment, there was 1 rivaroxaban NSAID user bleeding event and 4 apixaban NSAID user bleeding events. For the rivaroxaban NSAID user bleeding event, the NSAID was nonselective and a PPI was not coprescribed. For the apixaban NSAID user bleeding events, 2 NSAIDs were nonselective and 2 were selective. All patients with apixaban and NSAID bleeding events had a coprescribed PPI. There was no clinically significant difference in the bleeding rates observed for NSAID selectivity or PPI coprescribing among the NSAID user subgroups.
DISCUSSION
This study found that there was no statistically significant difference for bleeding rates of major and nonmajor bleeding events between rivaroxaban and apixaban among NSAID users and non-NSAID users. This study did not identify a clinically significant impact on bleeding rates from NSAID selectivity or PPI coprescribing among the NSAID users.
There were notable but not statistically significant differences in baseline characteristics observed between the NSAID and non-NSAID user groups. On average, the rivaroxaban and apixaban NSAID users were younger compared with those not taking NSAIDs. NSAIDs, specifically nonselective NSAIDs, are recognized as potentially inappropriate medications for older adults given that this population is at an increased risk for GI ulcer development and/or GI bleeding.17 The non-NSAID user group likely consisted of older patients compared to the NSAID user group as clinicians may avoid prescribing NSAIDs to older adults regardless of concomitant DOAC therapy.
In addition to having an older patient population, non-NSAID users were more frequently prescribed a concomitant antiplatelet when compared with NSAID users. This prescribing pattern may be due to clinicians avoiding the use of NSAIDs in patients receiving DOAC therapy in combination with antiplatelet therapy, as these patients have been found to have an increased bleeding rate compared to DOAC therapy alone.18
Non-NSAID users had an overall higher bleeding rate for both major and nonmajor bleeding events. Based on this observation, it could be hypothesized that antiplatelet agents have a higher risk of bleeding in comparison to NSAIDs. In a subanalysis of the EXPAND study evaluating risk factors of major bleeding in patients receiving rivaroxaban, concomitant use of antiplatelet agents demonstrated a statistically significant increased risk of bleeding (HR 1.6; 95% CI, 1.2-2.3; P = .003) while concomitant use of NSAIDs did not (HR 0.8; 95% CI, 0.3-2.2; P = .67).19
In assessing PPI status at baseline, a majority of both rivaroxaban and apixaban NSAID users were coprescribed a PPI. This trend aligns with current clinical guideline recommendations for the prescribing of PPI therapy for GI protection in high-risk patients, such as those on DOAC therapy and concomitant NSAID therapy.10 Given the high proportion of NSAID users coprescribed a PPI at baseline, it may be possible that the true incidence of NSAID-associated bleeding events was higher than what this study found. This observation may reflect the impact from timely implementation of risk mitigation strategies by CPPs in the anticoagulation clinic. However, this study was not constructed to assess the efficacy of PPI use in this manner.
It is important to note the patients included in this study were followed by a pharmacist in an anticoagulation clinic using the DOAC Dashboard.15 This population management tool allows CPPs to make proactive interventions when a patient taking a DOAC receives an NSAID prescription, such as recommending the coprescribing of a PPI or use of a selective NSAID.10,16 These standards of care may have contributed to an overall reduced bleeding rate among the NSAID user group and may not be reflective of private practice.
The planned analysis of this study was modeled after the posthoc analysis of the RE-LY and ARISTOTLE trials. Both trials demonstrated an increased risk of bleeding with oral anticoagulation, including DOAC and warfarin, in combination with NSAID use. However, both trials found that NSAID use in patients treated with a DOAC was not independently associated with increased bleeding events compared with warfarin.13,14 The results of this study are comparable to the RE-LY and ARISTOTLE findings that NSAID use among patients treated with rivaroxaban or apixaban did not demonstrate a statistically significant increased bleeding risk.
Studies of NSAID use in combination with DOAC therapy have been limited to patient populations consisting of both DOAC and warfarin. Evidence from these trials outlines the increased bleeding risk associated with NSAID use in combination with oral anticoagulation; however, these patient populations include those on a DOAC and warfarin.13,14,19,20 Given the limited evidence on NSAID use among DOACs alone, it is assumed NSAID use in combination with DOACs has a similar risk of bleeding as warfarin use. This may cause clinicians to automatically exclude NSAID therapy as a treatment option for patients on a DOAC who are otherwise clinically appropriate candidates, such as those with underlying inflammatory conditions. Avoiding NSAID therapy in this patient population may lead to suboptimal pain management and increase the risk of patient harm from methods such as inappropriate opioid therapy prescribing.
DOAC therapy should not be a universal limitation to the use of NSAIDs. Although the risk of bleeding with NSAID therapy is always present, deliberate NSAID prescribing in addition to the timely implementation of risk mitigation strategies may provide an avenue for safe NSAID prescribing in patients receiving a DOAC. A population health-based approach to DOAC management, such as the DOAC Dashboard, appears to be effective at preventing patient harm when NSAIDs are prescribed in conjunction with DOACs.
Limitations
The DOAC Dashboard has been shown to be effective and efficient at monitoring DOAC therapy from a population-based approach.16 Reports generated through the DOAC Dashboard provide convenient access to patient data which allows for timely interventions; however, there are limits to its use for data collection. All the data elements necessary to properly assess bleeding risk with validated tools, such as HAS-BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, drugs/ alcohol concomitantly), are not available on DOAC Dashboard reports. Due to this constraint, bleeding risk assessments were not conducted at baseline and this study was unable to include risk modeling. Additionally, data elements like initiation and discontinuation dates and duration of therapies were not readily available. As a result, this study was unable to incorporate time as a data point.
This was a retrospective study that relied on manual review of chart documentation to verify bleeding events, but data obtained through the DOAC Dashboard were transferred directly from the EHR.15 Bleeding events available for evaluation were restricted to those that occurred at a VA facility. Additionally, the sample size within the rivaroxaban NSAID user group did not reach the predefined sample size required to reach power and may have been too small to detect a difference if one did exist. The secondary assessment had a low sample size of NSAID user bleeding events, making it difficult to fully assess its impact on NSAID selectivity and PPI coprescribing on bleeding rates. All courses of NSAIDs were equally valued regardless of the dose or therapy duration; however, this is consistent with how NSAID use was defined in the RE-LY and ARISTOTLE trials.
CONCLUSIONS
This retrospective cohort review found no statistically significant difference in the composite bleeding rates between rivaroxaban and apixaban among NSAID users and non-NSAID users. Moreover, there was no clinically significant impact observed for bleeding rates in regard to NSAID selectivity and PPI coprescribing among NSAID users. However, coprescribing of PPI therapy to patients on a DOAC who are clinically indicated for an NSAID may reduce the risk of bleeding. Population health management tools, such as the DOAC Dashboard, may also allow clinicians to safely prescribe NSAIDs to patients on a DOAC. Further large-scale observational studies are needed to quantify the real-world risk of bleeding with concomitant NSAID use among DOACs alone and to evaluate the impact from NSAID selectivity or PPI coprescribing.
Clinical practice has shifted from vitamin K antagonists to direct oral anticoagulants (DOACs) for atrial fibrillation treatment due to their more favorable risk-benefit profile and less lifestyle modification required.1,2 However, the advantage of a lower bleeding risk with DOACs could be compromised by potentially problematic pharmacokinetic interactions like those conferred by antiplatelets or nonsteroidal anti-inflammatory drugs (NSAIDs).3,4 Treating a patient needing anticoagulation with a DOAC who has comorbidities may introduce unavoidable drug-drug interactions. This particularly happens with over-the-counter and prescription NSAIDs used for the management of pain and inflammatory conditions.5
NSAIDs primarily affect 2 cyclooxygenase (COX) enzyme isomers, COX-1 and COX-2.6 COX-1 helps maintain gastrointestinal (GI) mucosa integrity and platelet aggregation processes, whereas COX-2 is engaged in pain signaling and inflammation mediation. COX-1 inhibition is associated with more bleeding-related adverse events (AEs), especially in the GI tract. COX-2 inhibition is thought to provide analgesia and anti-inflammatory properties without elevating bleeding risk. This premise is responsible for the preferential use of celecoxib, a COX-2 selective NSAID, which should confer a lower bleeding risk compared to nonselective NSAIDs such as ibuprofen and naproxen.7 NSAIDs have been documented as independent risk factors for bleeding. NSAID users are about 3 times as likely to develop GI AEs compared to nonNSAID users.8
Many clinicians aim to further mitigate NSAID-associated bleeding risk by coprescribing a proton pump inhibitor (PPI). PPIs provide gastroprotection against NSAID-induced mucosal injury and sequential complication of GI bleeding. In a multicenter randomized control trial, patients who received concomitant PPI therapy while undergoing chronic NSAID therapy—including nonselective and COX-2 selective NSAIDs—had a significantly lower risk of GI ulcer development (placebo, 17.0%; 20 mg esomeprazole, 5.2%; 40 mg esomeprazole, 4.6%).9 Current clinical guidelines for preventing NSAIDassociated bleeding complications recommend using a COX-2 selective NSAID in combination with PPI therapy for patients at high risk for GI-related bleeding, including the concomitant use of anticoagulants.10
There is evidence suggesting an increased bleeding risk with NSAIDs when used in combination with vitamin K antagonists such as warfarin.11,12 A systematic review of warfarin and concomitant NSAID use found an increased risk of overall bleeding with NSAID use in combination with warfarin (odds ratio 1.58; 95% CI, 1.18-2.12), compared to warfarin alone.12
Posthoc analyses of randomized clinical trials have also demonstrated an increased bleeding risk with oral anticoagulation and concomitant NSAID use.13,14 In the RE-LY trial, NSAID users on warfarin or dabigatran had a statistically significant increased risk of major bleeding compared to non-NSAID users (hazard ratio [HR] 1.68; 95% CI, 1.40- 2.02; P < .001).13 In the ARISTOTLE trial, patients on warfarin or apixaban who were incident NSAID users were found to have an increased risk of major bleeding (HR 1.61; 95% CI, 1.11-2.33) and clinically relevant nonmajor bleeding (HR 1.70; 95% CI, 1.16- 2.48).14 These trials found a statistically significant increased bleeding risk associated with NSAID use, though the populations evaluated included patients taking warfarin and patients taking DOACs. These trials did not evaluate the bleeding risk of concomitant NSAID use among DOACs alone.
Evidence on NSAID-associated bleeding risk with DOACs is lacking in settings where the patient population, prescribing practices, and monitoring levels are variable. Within the Veterans Health Administration, clinical pharmacist practitioners (CPPs) in anticoagulation clinics oversee DOAC therapy management. CPPs monitor safety and efficacy of DOAC therapies through a population health management tool, the DOAC Dashboard.15 The DOAC Dashboard creates alerts for patients who may require an intervention based on certain clinical parameters, such as drug-drug interactions.16 Whenever a patient on a DOAC is prescribed an NSAID, an alert is generated on the DOAC Dashboard to flag the CPPs for the potential need for an intervention. If NSAID therapy remains clinically indicated, CPPs may recommend risk reduction strategies such as a COX-2 selective NSAID or coprescribing a PPI.10
The DOAC Dashboard provides an ideal setting for investigating the effects of NSAID use, NSAID selectivity, and PPI coprescribing on DOAC bleeding rates. With an increasing population of patients receiving anticoagulation therapy with a DOAC, more guidance regarding the bleeding risk of concomitant NSAID use with DOACs is needed. Studies evaluating the bleeding risk with concomitant NSAID use in patients on a DOAC alone are limited. This is the first study to date to compare bleeding risk with concomitant NSAID use between DOACs. This study provides information on bleeding risk with NSAID use among commonly prescribed DOACs, rivaroxaban and apixaban, and the potential impacts of current risk reduction strategies.
METHODS
This single-center retrospective cohort review was performed using the electronic health records (EHRs) of patients enrolled in the US Department of Veterans Affairs (VA) Mountain Home Healthcare System who received rivaroxaban or apixaban from December 2020 to December 2022. This study received approval from the East Tennessee State University/VA Institutional Review Board committee.
Patients were identified through the DOAC Dashboard, aged 21 to 100 years, and received rivaroxaban or apixaban at a therapeutic dose: rivaroxaban 10 to 20 mg daily or apixaban 2.5 to 5 mg twice daily. Patients were excluded if they were prescribed dual antiplatelet therapy, received rivaroxaban at dosing indicated for peripheral vascular disease, were undergoing dialysis, had evidence of moderate to severe hepatic impairment or any hepatic disease with coagulopathy, were undergoing chemotherapy or radiation, or had hematological conditions with predisposed bleeding risk. These patients were excluded to mitigate the potential confounding impact from nontherapeutic DOAC dosing strategies and conditions associated with an increased bleeding risk.
Eligible patients were stratified based on NSAID use. NSAID users were defined as patients prescribed an oral NSAID, including both acute and chronic courses, at any point during the study time frame while actively on a DOAC. Bleeding events were reviewed to evaluate rates between rivaroxaban and apixaban among NSAID and nonNSAID users. Identified NSAID users were further assessed for NSAID selectivity and PPI coprescribing as a subgroup analysis for the secondary assessment.
Data Collection
Baseline data were collected, including age, body mass index, anticoagulation indication, DOAC agent, DOAC dose, and DOAC total daily dose. Baseline serum creatinine levels, liver function tests, hemoglobin levels, and platelet counts were collected from the most recent data available immediately prior to the bleeding event, if applicable.
The DOAC Dashboard was reviewed for active and dismissed drug interaction alerts to identify patients taking rivaroxaban or apixaban who were prescribed an NSAID. Patients were categorized in the NSAID group if an interacting drug alert with an NSAID was reported during the study time frame. Data available through the interacting drug alerts on NSAID use were limited to the interacting drug name and date of the reported flag. Manual EHR review was required to confirm dates of NSAID therapy initiation and NSAID discontinuation, if applicable.
Data regarding concomitant antiplatelet use were obtained through review of the active and dismissed drug interaction alerts on the DOAC Dashboard. Concomitant antiplatelet use was defined as the prescribing of a single antiplatelet agent at any point while receiving DOAC therapy. Data on concomitant antiplatelets were collected regardless of NSAID status.
Data on coprescribed PPI therapy were obtained through manual EHR review of identified NSAID users. Coprescribed PPI therapy was defined as the prescribing of a PPI at any point during NSAID therapy. Data regarding PPI use among non-NSAID users were not collected because the secondary endpoint was designed to assess PPI use only among patients coprescribed a DOAC and NSAID.
Outcomes
Bleeding events were identified through an outcomes report generated by the DOAC Dashboard based on International Classification of Diseases, Tenth Revision diagnosis codes associated with a bleeding event. The outcomes report captures diagnoses from the outpatient and inpatient care settings. Reported bleeding events were limited to patients who received a DOAC at any point in the 6 months prior to the event and excluded patients with recent DOAC initiation within 7 days of the event, as these patients are not captured on the DOAC Dashboard.
All reported bleeding events were manually reviewed in the EHR and categorized as a major or clinically relevant nonmajor bleed, according to International Society of Thrombosis and Haemostasis criteria. Validated bleeding events were then crossreferenced with the interacting drug alerts report to identify events with potentially overlapping NSAID therapy at the time of the event. Overlapping NSAID therapy was defined as the prescribing of an NSAID at any point in the 6 months prior to the event. All events with potential overlapping NSAID therapies were manually reviewed for confirmation of NSAID status at the time of the event.
The primary endpoint was a composite of any bleeding event per International Society of Thrombosis and Haemostasis criteria. The secondary endpoint evaluated the potential impact of NSAID selectivity or PPI coprescribing on the bleeding rate among the NSAID user groups.
Statistical Analysis
Analyses were performed consistent with the methods used in the ARISTOTLE and RE-LY trials. It was determined that a sample size of 504 patients, with ≥ 168 patients in each group, would provide 80% power using a 2-sided a of 0.05. HRs with 95% CIs and respective P values were calculated using a SPSS-adapted online calculator.
RESULTS
The DOAC Dashboard identified 681 patients on rivaroxaban and 3225 patients on apixaban; 72 patients on rivaroxaban (10.6%) and 300 patients on apixaban (9.3%) were NSAID users. The mean age of NSAID users was 66.9 years in the rivaroxaban group and 72.4 years in the apixaban group. The mean age of non-NSAID users was 71.5 years in the rivaroxaban group and 75.6 years in the apixaban group. No appreciable differences were observed among subgroups in body mass index, renal function, hepatic function, hemoglobin, or platelet counts, and no statistically significant differences were identified (Table 1). Antiplatelet agents identified included aspirin, clopidogrel, prasugrel, and ticagrelor. Fifteen patients (20.3%) in the rivaroxaban group and 87 patients (28.7%) in the apixaban group had concomitant antiplatelet and NSAID use. Forty-five patients on rivaroxaban (60.8%) and 170 (55.9%) on apixaban were prescribed concomitant PPI and NSAID at baseline. Among non-NSAID users, there was concomitant antiplatelet use for 265 patients (43.6%) in the rivaroxaban group and 1401 patients (47.9%) in the apixaban group. Concomitant PPI use was identified among 63 patients (60.0%) taking selective NSAIDs and 182 (57.2%) taking nonselective NSAIDs.

A total of 423 courses of NSAIDs were identified: 85 NSAID courses in the rivaroxaban group and 338 NSAID courses in the apixaban group. Most NSAID courses involved a nonselective NSAID in the rivaroxaban and apixaban NSAID user groups: 75.2% (n = 318) aggregately compared to 71.8% (n = 61) and 76.0% (n = 257) in the rivaroxaban and apixaban groups, respectively. The most frequent NSAID courses identified were meloxicam (26.7%; n = 113), celecoxib (24.8%; n = 105), ibuprofen (19.1%; n = 81), and naproxen (13.5%; n = 57). Data regarding NSAID therapy initiation and discontinuation dates were not readily available. As a result, the duration of NSAID courses was not captured.
There was no statistically significant difference in bleeding rates between rivaroxaban and apixaban among NSAID users (HR 1.04; 95% CI, 0.98-1.12) or non-NSAID users (HR 1.15; 95% CI, 0.80-1.66) (Table 2). Apixaban non-NSAID users had a higher rate of major bleeds (HR 0.32; 95% CI, 0.17-0.61) while rivaroxaban non-NSAID users had a higher rate of clinically relevant nonmajor bleeds (HR 1.63; 95% CI, 1.10-2.54).

The sample size for the secondary endpoint consisted of bleeding events that were confirmed to have had an overlapping NSAID prescribed at the time of the event. For this secondary assessment, there was 1 rivaroxaban NSAID user bleeding event and 4 apixaban NSAID user bleeding events. For the rivaroxaban NSAID user bleeding event, the NSAID was nonselective and a PPI was not coprescribed. For the apixaban NSAID user bleeding events, 2 NSAIDs were nonselective and 2 were selective. All patients with apixaban and NSAID bleeding events had a coprescribed PPI. There was no clinically significant difference in the bleeding rates observed for NSAID selectivity or PPI coprescribing among the NSAID user subgroups.
DISCUSSION
This study found that there was no statistically significant difference for bleeding rates of major and nonmajor bleeding events between rivaroxaban and apixaban among NSAID users and non-NSAID users. This study did not identify a clinically significant impact on bleeding rates from NSAID selectivity or PPI coprescribing among the NSAID users.
There were notable but not statistically significant differences in baseline characteristics observed between the NSAID and non-NSAID user groups. On average, the rivaroxaban and apixaban NSAID users were younger compared with those not taking NSAIDs. NSAIDs, specifically nonselective NSAIDs, are recognized as potentially inappropriate medications for older adults given that this population is at an increased risk for GI ulcer development and/or GI bleeding.17 The non-NSAID user group likely consisted of older patients compared to the NSAID user group as clinicians may avoid prescribing NSAIDs to older adults regardless of concomitant DOAC therapy.
In addition to having an older patient population, non-NSAID users were more frequently prescribed a concomitant antiplatelet when compared with NSAID users. This prescribing pattern may be due to clinicians avoiding the use of NSAIDs in patients receiving DOAC therapy in combination with antiplatelet therapy, as these patients have been found to have an increased bleeding rate compared to DOAC therapy alone.18
Non-NSAID users had an overall higher bleeding rate for both major and nonmajor bleeding events. Based on this observation, it could be hypothesized that antiplatelet agents have a higher risk of bleeding in comparison to NSAIDs. In a subanalysis of the EXPAND study evaluating risk factors of major bleeding in patients receiving rivaroxaban, concomitant use of antiplatelet agents demonstrated a statistically significant increased risk of bleeding (HR 1.6; 95% CI, 1.2-2.3; P = .003) while concomitant use of NSAIDs did not (HR 0.8; 95% CI, 0.3-2.2; P = .67).19
In assessing PPI status at baseline, a majority of both rivaroxaban and apixaban NSAID users were coprescribed a PPI. This trend aligns with current clinical guideline recommendations for the prescribing of PPI therapy for GI protection in high-risk patients, such as those on DOAC therapy and concomitant NSAID therapy.10 Given the high proportion of NSAID users coprescribed a PPI at baseline, it may be possible that the true incidence of NSAID-associated bleeding events was higher than what this study found. This observation may reflect the impact from timely implementation of risk mitigation strategies by CPPs in the anticoagulation clinic. However, this study was not constructed to assess the efficacy of PPI use in this manner.
It is important to note the patients included in this study were followed by a pharmacist in an anticoagulation clinic using the DOAC Dashboard.15 This population management tool allows CPPs to make proactive interventions when a patient taking a DOAC receives an NSAID prescription, such as recommending the coprescribing of a PPI or use of a selective NSAID.10,16 These standards of care may have contributed to an overall reduced bleeding rate among the NSAID user group and may not be reflective of private practice.
The planned analysis of this study was modeled after the posthoc analysis of the RE-LY and ARISTOTLE trials. Both trials demonstrated an increased risk of bleeding with oral anticoagulation, including DOAC and warfarin, in combination with NSAID use. However, both trials found that NSAID use in patients treated with a DOAC was not independently associated with increased bleeding events compared with warfarin.13,14 The results of this study are comparable to the RE-LY and ARISTOTLE findings that NSAID use among patients treated with rivaroxaban or apixaban did not demonstrate a statistically significant increased bleeding risk.
Studies of NSAID use in combination with DOAC therapy have been limited to patient populations consisting of both DOAC and warfarin. Evidence from these trials outlines the increased bleeding risk associated with NSAID use in combination with oral anticoagulation; however, these patient populations include those on a DOAC and warfarin.13,14,19,20 Given the limited evidence on NSAID use among DOACs alone, it is assumed NSAID use in combination with DOACs has a similar risk of bleeding as warfarin use. This may cause clinicians to automatically exclude NSAID therapy as a treatment option for patients on a DOAC who are otherwise clinically appropriate candidates, such as those with underlying inflammatory conditions. Avoiding NSAID therapy in this patient population may lead to suboptimal pain management and increase the risk of patient harm from methods such as inappropriate opioid therapy prescribing.
DOAC therapy should not be a universal limitation to the use of NSAIDs. Although the risk of bleeding with NSAID therapy is always present, deliberate NSAID prescribing in addition to the timely implementation of risk mitigation strategies may provide an avenue for safe NSAID prescribing in patients receiving a DOAC. A population health-based approach to DOAC management, such as the DOAC Dashboard, appears to be effective at preventing patient harm when NSAIDs are prescribed in conjunction with DOACs.
Limitations
The DOAC Dashboard has been shown to be effective and efficient at monitoring DOAC therapy from a population-based approach.16 Reports generated through the DOAC Dashboard provide convenient access to patient data which allows for timely interventions; however, there are limits to its use for data collection. All the data elements necessary to properly assess bleeding risk with validated tools, such as HAS-BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, drugs/ alcohol concomitantly), are not available on DOAC Dashboard reports. Due to this constraint, bleeding risk assessments were not conducted at baseline and this study was unable to include risk modeling. Additionally, data elements like initiation and discontinuation dates and duration of therapies were not readily available. As a result, this study was unable to incorporate time as a data point.
This was a retrospective study that relied on manual review of chart documentation to verify bleeding events, but data obtained through the DOAC Dashboard were transferred directly from the EHR.15 Bleeding events available for evaluation were restricted to those that occurred at a VA facility. Additionally, the sample size within the rivaroxaban NSAID user group did not reach the predefined sample size required to reach power and may have been too small to detect a difference if one did exist. The secondary assessment had a low sample size of NSAID user bleeding events, making it difficult to fully assess its impact on NSAID selectivity and PPI coprescribing on bleeding rates. All courses of NSAIDs were equally valued regardless of the dose or therapy duration; however, this is consistent with how NSAID use was defined in the RE-LY and ARISTOTLE trials.
CONCLUSIONS
This retrospective cohort review found no statistically significant difference in the composite bleeding rates between rivaroxaban and apixaban among NSAID users and non-NSAID users. Moreover, there was no clinically significant impact observed for bleeding rates in regard to NSAID selectivity and PPI coprescribing among NSAID users. However, coprescribing of PPI therapy to patients on a DOAC who are clinically indicated for an NSAID may reduce the risk of bleeding. Population health management tools, such as the DOAC Dashboard, may also allow clinicians to safely prescribe NSAIDs to patients on a DOAC. Further large-scale observational studies are needed to quantify the real-world risk of bleeding with concomitant NSAID use among DOACs alone and to evaluate the impact from NSAID selectivity or PPI coprescribing.
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-962. doi:10.1016/S0140-6736(13)62343-0
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e44S-e88S. doi:10.1378/chest.11-2292
- Eikelboom J, Merli G. Bleeding with direct oral anticoagulants vs warfarin: clinical experience. Am J Med. 2016;129(11S):S33-S40. doi:10.1016/j.amjmed.2016.06.003
- Vranckx P, Valgimigli M, Heidbuchel H. The significance of drug-drug and drug-food interactions of oral anticoagulation. Arrhythm Electrophysiol Rev. 2018;7(1):55-61. doi:10.15420/aer.2017.50.1
- Davis JS, Lee HY, Kim J, et al. Use of non-steroidal antiinflammatory drugs in US adults: changes over time and by demographic. Open Heart. 2017;4(1):e000550. doi:10.1136/openhrt-2016-000550
- Schafer AI. Effects of nonsteroidal antiinflammatory drugs on platelet function and systemic hemostasis. J Clin Pharmacol. 1995;35(3):209-219. doi:10.1002/j.1552-4604.1995.tb04050.x
- Al-Saeed A. Gastrointestinal and cardiovascular risk of nonsteroidal anti-inflammatory drugs. Oman Med J. 2011;26(6):385-391. doi:10.5001/omj.2011.101
- Gabriel SE, Jaakkimainen L, Bombardier C. Risk for serious gastrointestinal complications related to use of nonsteroidal anti-inflammatory drugs. Ann Intern Med. 1991;115(10):787-796. doi:10.7326/0003-4819-115-10-787
- Scheiman JM, Yeomans ND, Talley NJ, et al. Prevention of ulcers by esomeprazole in at-risk patients using non-selective NSAIDs and COX-2 inhibitors. Am J Gastroenterol. 2006;101(4):701-710. doi:10.1111/j.1572-0241.2006.00499.x
- Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152(4):706-715. doi:10.1053/j.gastro.2017.01.031
- Lamberts M, Lip GYH, Hansen ML, et al. Relation of nonsteroidal anti-inflammatory drugs to serious bleeding and thromboembolism risk in patients with atrial fibrillation receiving antithrombotic therapy: a nationwide cohort study. Ann Intern Med. 2014;161(10):690-698. doi:10.7326/M13-1581
- Villa Zapata L, Hansten PD, Panic J, et al. Risk of bleeding with exposure to warfarin and nonsteroidal anti-inflammatory drugs: a systematic review and metaanalysis. Thromb Haemost. 2020;120(7):1066-1074. doi:10.1055/s-0040-1710592
- Kent AP, Brueckmann M, Fraessdorf M, et al. Concomitant oral anticoagulant and nonsteroidal anti-inflammatory drug therapy in patients with atrial fibrillation. J Am Coll Cardiol. 2018;72(3):255-267. doi:10.1016/j.jacc.2018.04.063
- Dalgaard F, Mulder H, Wojdyla DM, et al. Patients with atrial fibrillation taking nonsteroidal antiinflammatory drugs and oral anticoagulants in the ARISTOTLE Trial. Circulation. 2020;141(1):10-20. doi:10.1161/CIRCULATIONAHA.119.041296
- Allen AL, Lucas J, Parra D, et al. Shifting the paradigm: a population health approach to the management of direct oral anticoagulants. J Am Heart Asssoc. 2021;10(24):e022758. doi:10.1161/JAHA.121.022758
- . Valencia D, Spoutz P, Stoppi J, et al. Impact of a direct oral anticoagulant population management tool on anticoagulation therapy monitoring in clinical practice. Ann Pharmacother. 2019;53(8):806-811. doi:10.1177/1060028019835843
- By the 2023 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2023 Updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052-2081. doi:10.1111/jgs.18372
- Kumar S, Danik SB, Altman RK, et al. Non-vitamin K antagonist oral anticoagulants and antiplatelet therapy for stroke prevention in patients with atrial fibrillation. Cardiol Rev. 2016;24(5):218-223. doi:10.1097/CRD.0000000000000088
- Sakuma I, Uchiyama S, Atarashi H, et al. Clinical risk factors of stroke and major bleeding in patients with nonvalvular atrial fibrillation under rivaroxaban: the EXPAND study sub-analysis. Heart Vessels. 2019;34(11):1839-1851. doi:10.1007/s00380-019-01425-x
- Davidson BL, Verheijen S, Lensing AWA, et al. Bleeding risk of patients with acute venous thromboembolism taking nonsteroidal anti-inflammatory drugs or aspirin. JAMA Intern Med. 2014;174(6):947-953. doi:10.1001/jamainternmed.2014.946
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-962. doi:10.1016/S0140-6736(13)62343-0
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e44S-e88S. doi:10.1378/chest.11-2292
- Eikelboom J, Merli G. Bleeding with direct oral anticoagulants vs warfarin: clinical experience. Am J Med. 2016;129(11S):S33-S40. doi:10.1016/j.amjmed.2016.06.003
- Vranckx P, Valgimigli M, Heidbuchel H. The significance of drug-drug and drug-food interactions of oral anticoagulation. Arrhythm Electrophysiol Rev. 2018;7(1):55-61. doi:10.15420/aer.2017.50.1
- Davis JS, Lee HY, Kim J, et al. Use of non-steroidal antiinflammatory drugs in US adults: changes over time and by demographic. Open Heart. 2017;4(1):e000550. doi:10.1136/openhrt-2016-000550
- Schafer AI. Effects of nonsteroidal antiinflammatory drugs on platelet function and systemic hemostasis. J Clin Pharmacol. 1995;35(3):209-219. doi:10.1002/j.1552-4604.1995.tb04050.x
- Al-Saeed A. Gastrointestinal and cardiovascular risk of nonsteroidal anti-inflammatory drugs. Oman Med J. 2011;26(6):385-391. doi:10.5001/omj.2011.101
- Gabriel SE, Jaakkimainen L, Bombardier C. Risk for serious gastrointestinal complications related to use of nonsteroidal anti-inflammatory drugs. Ann Intern Med. 1991;115(10):787-796. doi:10.7326/0003-4819-115-10-787
- Scheiman JM, Yeomans ND, Talley NJ, et al. Prevention of ulcers by esomeprazole in at-risk patients using non-selective NSAIDs and COX-2 inhibitors. Am J Gastroenterol. 2006;101(4):701-710. doi:10.1111/j.1572-0241.2006.00499.x
- Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152(4):706-715. doi:10.1053/j.gastro.2017.01.031
- Lamberts M, Lip GYH, Hansen ML, et al. Relation of nonsteroidal anti-inflammatory drugs to serious bleeding and thromboembolism risk in patients with atrial fibrillation receiving antithrombotic therapy: a nationwide cohort study. Ann Intern Med. 2014;161(10):690-698. doi:10.7326/M13-1581
- Villa Zapata L, Hansten PD, Panic J, et al. Risk of bleeding with exposure to warfarin and nonsteroidal anti-inflammatory drugs: a systematic review and metaanalysis. Thromb Haemost. 2020;120(7):1066-1074. doi:10.1055/s-0040-1710592
- Kent AP, Brueckmann M, Fraessdorf M, et al. Concomitant oral anticoagulant and nonsteroidal anti-inflammatory drug therapy in patients with atrial fibrillation. J Am Coll Cardiol. 2018;72(3):255-267. doi:10.1016/j.jacc.2018.04.063
- Dalgaard F, Mulder H, Wojdyla DM, et al. Patients with atrial fibrillation taking nonsteroidal antiinflammatory drugs and oral anticoagulants in the ARISTOTLE Trial. Circulation. 2020;141(1):10-20. doi:10.1161/CIRCULATIONAHA.119.041296
- Allen AL, Lucas J, Parra D, et al. Shifting the paradigm: a population health approach to the management of direct oral anticoagulants. J Am Heart Asssoc. 2021;10(24):e022758. doi:10.1161/JAHA.121.022758
- . Valencia D, Spoutz P, Stoppi J, et al. Impact of a direct oral anticoagulant population management tool on anticoagulation therapy monitoring in clinical practice. Ann Pharmacother. 2019;53(8):806-811. doi:10.1177/1060028019835843
- By the 2023 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2023 Updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052-2081. doi:10.1111/jgs.18372
- Kumar S, Danik SB, Altman RK, et al. Non-vitamin K antagonist oral anticoagulants and antiplatelet therapy for stroke prevention in patients with atrial fibrillation. Cardiol Rev. 2016;24(5):218-223. doi:10.1097/CRD.0000000000000088
- Sakuma I, Uchiyama S, Atarashi H, et al. Clinical risk factors of stroke and major bleeding in patients with nonvalvular atrial fibrillation under rivaroxaban: the EXPAND study sub-analysis. Heart Vessels. 2019;34(11):1839-1851. doi:10.1007/s00380-019-01425-x
- Davidson BL, Verheijen S, Lensing AWA, et al. Bleeding risk of patients with acute venous thromboembolism taking nonsteroidal anti-inflammatory drugs or aspirin. JAMA Intern Med. 2014;174(6):947-953. doi:10.1001/jamainternmed.2014.946
Impact of NSAID Use on Bleeding Rates for Patients Taking Rivaroxaban or Apixaban
Impact of NSAID Use on Bleeding Rates for Patients Taking Rivaroxaban or Apixaban
Patient and Support Person Satisfaction Following a Whole Health-Informed Interdisciplinary Pain Team Meeting
Patient and Support Person Satisfaction Following a Whole Health-Informed Interdisciplinary Pain Team Meeting
Chronic pain is one of the most prevalent public health concerns in the United States, affecting > 51 million adults with about $500 billion in health care costs.1 Military veterans are among the most vulnerable subpopulations, with 65% of veterans reporting chronic pain in the last 3 months.2 Chronic pain is complex, affecting the biopsychosocial-spiritual levels of human health, and requires multimodal and comprehensive treatment approaches.3 Hence, chronic pain treatment can be best delivered via interdisciplinary teams (IDTs) that use a patient-centered approach.4,5
The Veterans Healthcare Administration (VHA) is a leader in developing and delivering interdisciplinary pain care.6,7 VHA Directive 2009-053 requires every US Department of Veterans Affairs (VA) medical center to offer an IDT for chronic pain. However, VHA and non-VHA IDT programs vary significantly.8-11 A recent systematic review found a median of 5 disciplines included on IDTs (range, 2-8), and program content often included exercise and education; only 11% of included IDTs met simultaneously with patients.11 The heterogeneity of IDT programs has made determining best practices challenging.8,11 The Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials has denoted several core measures and measurement domains that were critical for determining the success of pain management interventions, including patient satisfaction.12,13 Nevertheless, the association of IDTs with high patient satisfaction and improvement in pain measures has been documented.5,11
The VHA has worked to implement the Whole Health System into health care, which considers well-being across physical, behavioral, spiritual, and socioeconomic domains. As such, the Whole Health System involves an interpersonal, team-based approach, “anchored in trusting longitudinal relationships to promote resilience, prevent disease, and restore health.”14 It aligns with the patient’s mission, aspiration, and purpose. Surgeon General VADM Vivek H. Murthy, MD, MBA, recently endorsed this approach.15,16 Other health care systems adopting whole health tend to have higher patient satisfaction, increased access to care, and improved patient-reported outcomes.15 Within the VHA, the Whole Health System has shifted the conversation between clinicians and patients from “What is the matter with you?” to “What is important to you?” while emphasizing a proactive and personalized approach to health care.17 Rather than emphasizing passive modalities such as medications and clinician-led services (eg, interventional pain service), the Whole Health System highlights self-care.3,17 Initial research findings within the VHA have been promising.18-21 Whole health peer coaching calls appear to be an effective approach for veterans diagnosed with PTSD, and the use of whole health services is associated with a decrease in opioid use.19,22 However, there are negligible data on patient experiences after meeting with a whole health-focused pain IDT, and studies to date have focused on urban populations.23 One approach to IDT that has shown promise for other health issues involves a patient meeting simultaneously with all members of the IDT.24-27 With the integration of the Whole Health System and the VHA priorities to provide veterans with the “soonest and best care,” more data are needed on the experiences of patients and support persons with various approaches to IDT pain care.28 This study aimed to evaluate patient and support person experiences with a whole health-focused pain IDT that met simultaneously with the patient and support person during an initial evaluation. This study was approved by the institutional review board at the Salem VA Health Care System (SVAHCS) in Virginia.
Methods
The PREVAIL IDT Track is a clinical program offered at SVAHCS with a whole health-focused approach that involves patients and their support persons meeting simultaneously with a pain IDT. PREVAIL IDT Track is designed to help veterans more effectively self-manage chronic pain (Table 1).6,29 Health care practitioners (HCPs) at SVAHCS recommended that veterans with pain persisting for > 3 months participate in PREVAIL IDT Track. After meeting with an advanced practice clinician for an intake, veterans elected to participate in the PREVAIL IDT Track program and completed the initial 6 weeks of pain education. Veterans were then invited to be evaluated by the pain IDT. A team including HCPs from interventional pain, psychology, pharmacy, nutrition, and physical therapy services met with the veteran for 60 minutes. Veterans were also invited to bring a support person to the IDT initial evaluation.
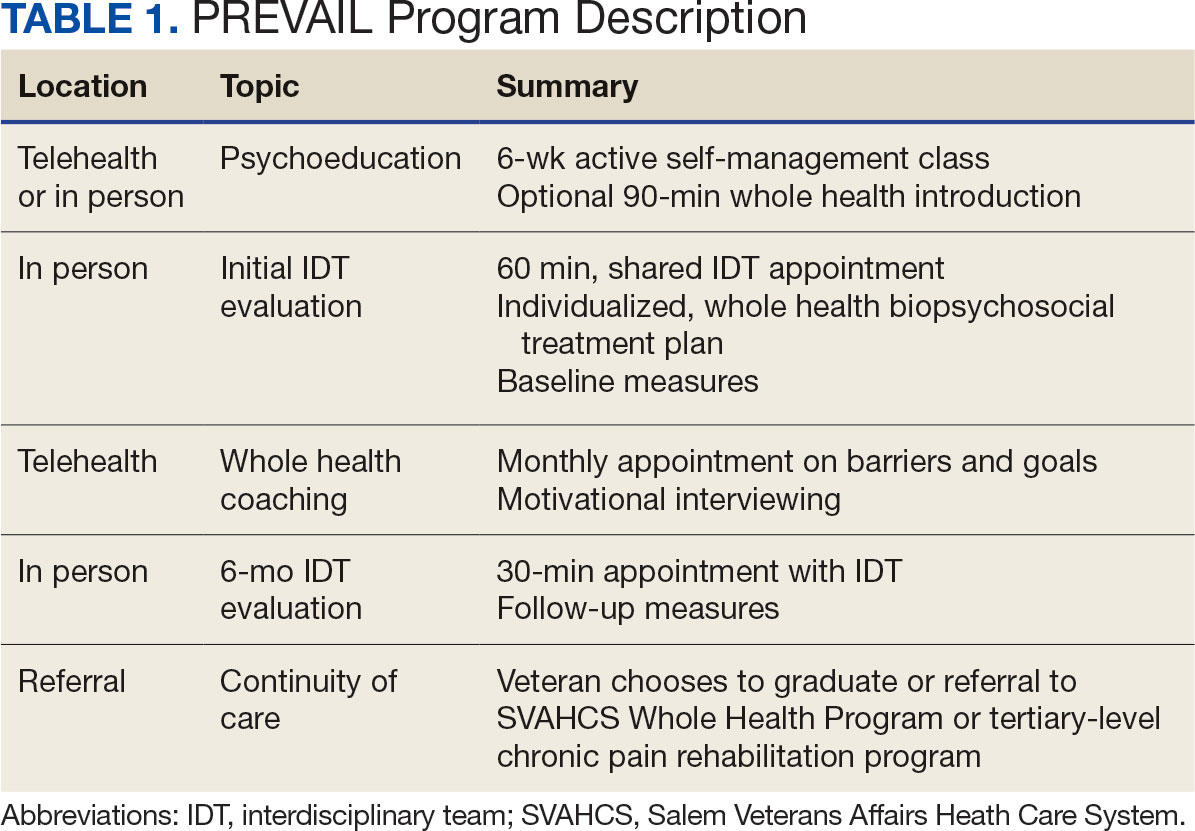
During the IDT initial evaluation, HCPs inquired about the patient’s mission, aspiration, and purpose (“If you were in less pain, what would you be doing more of?”) and about whole health self-care and wellness factors that may contribute to their chronic pain using the Personal Health Inventory.30,31 Veterans were then invited to select 3 whole health self-care areas to focus on during the 6-month program.3 The IDT HCPs worked with the veteran to establish the treatment plan for the first month in the areas of self-care selected by the patient and made recommendations for additional treatments. If the veteran brought a support person to the IDT initial evaluation, their feedback was elicited throughout and at the end to ensure the final treatment plan and first month’s goals were realistic. At the end of the appointment, the veteran and their support person were asked to complete a program-specific satisfaction survey. The HCPs on the team and the veteran executed the treatment plan developed during the appointment, except for medication prescribing. Recommendations for medication changes are included in clinical notes. Veterans then received 5 monthly coaching calls from a nurse navigator with training in whole health and a 6-month follow-up appointment with the IDT HCPs to discuss a plan for continuity of care.
Participant demographic information was not collected, and participants were not compensated for completing the survey. Veterans in PREVAIL IDT Track are predominantly residents of central Appalachia, White, male, unemployed, have ≥ 1 mental and physical health comorbidity, and have a history of mental health treatment.32 Veterans participating in PREVAIL IDT had a mean age of 57 years, and about 1 in 3 have opioid prescriptions.32
A program-specific 17-question satisfaction survey was developed, which included questions related to satisfaction with previous SVAHCS pain care and staff interactions. To assess the overall impression of the IDT initial evaluation, 3 yes/no questions and a 0 to 10-point scale were used. The 5 remaining open-ended questions allowed participants to give feedback about the IDT initial evaluation.
Data Analysis
A convergent mixed-methods approach was used to evaluate participant satisfaction with the initial IDT evaluation. The study team collected and analyzed quantitative and qualitative survey data and triangulated the findings.33 For quantitative responses, frequencies and means were calculated using Python. For qualitative responses, thematic data analysis was conducted by systematic coding, using inductive methods and allowing themes to emerge. Study team members performed a line-by-line analysis of responses using NVivo to identify important codes and reach a consensus. This study adhered to the Consolidated Criteria for Reporting Qualitative Research and followed the National Institute for Health Care Excellence checklist.34,35
Results
Quantitative Responses
In 2022, 168 veterans completed the initial IDT evaluation, and 144 (85.2%) completed the satisfaction survey and were included in this study. Thirty-two support persons who attended the initial IDT evaluation and completed the survey also were included. Of the 12 quantitative questions, 4 had a 100% completion rate, while 8 had ≤ 3% missing responses. When describing care prior to participating in PREVAIL, participants indicated a mean (SD) response of 4.6 (1.4) with the health care they received at SVAHCS and 4.3 (1.4) with SVAHCS pain management services, both on 6-point scales. All but 2 participants (98.9%) reported always being treated with courtesy and respect by PREVAIL HCPs during the initial IDT evaluation, with a mean (SD) score of 4.0 (0.2) on a 4-point scale. Most respondents (96.6%) reported that PREVAIL HCPs always listened carefully during the initial IDT evaluation, with a mean (SD) 4.0 (0.3) on a 4-point scale. Similarly, 92.6% reported that PREVAIL HCPs explained things clearly during the initial IDT evaluation, with a mean (SD) 3.9 (0.3) on a 4-point scale.
All respondents agreed that PREVAIL HCPs considered veteran preferences and those of their support persons in deciding their health care needs during the initial IDT evaluation, with a mean (SD) 3.7 (0.5) on a 4-point scale. Most respondents left the appointment with a good understanding of their responsibilities for chronic pain management with 99.4% (n = 169) strongly agreeing or agreeing (mean [SD] 3.6 [0.5]). A total of 135 respondents (79.4%) reported they left appointments with written information on their treatment plan. All 170 respondents reported that they would recommend PREVAIL to a friend, and 169 respondents (98.8%) felt that the initial PREVAIL IDT evaluation was a valuable use of time. Eighty-seven respondents (50.9%) rated the initial IDT evaluation as the “best clinical experience possible” with a mean (SD) score of 9.2 (1.1) on a 10-point scale (Table 2).
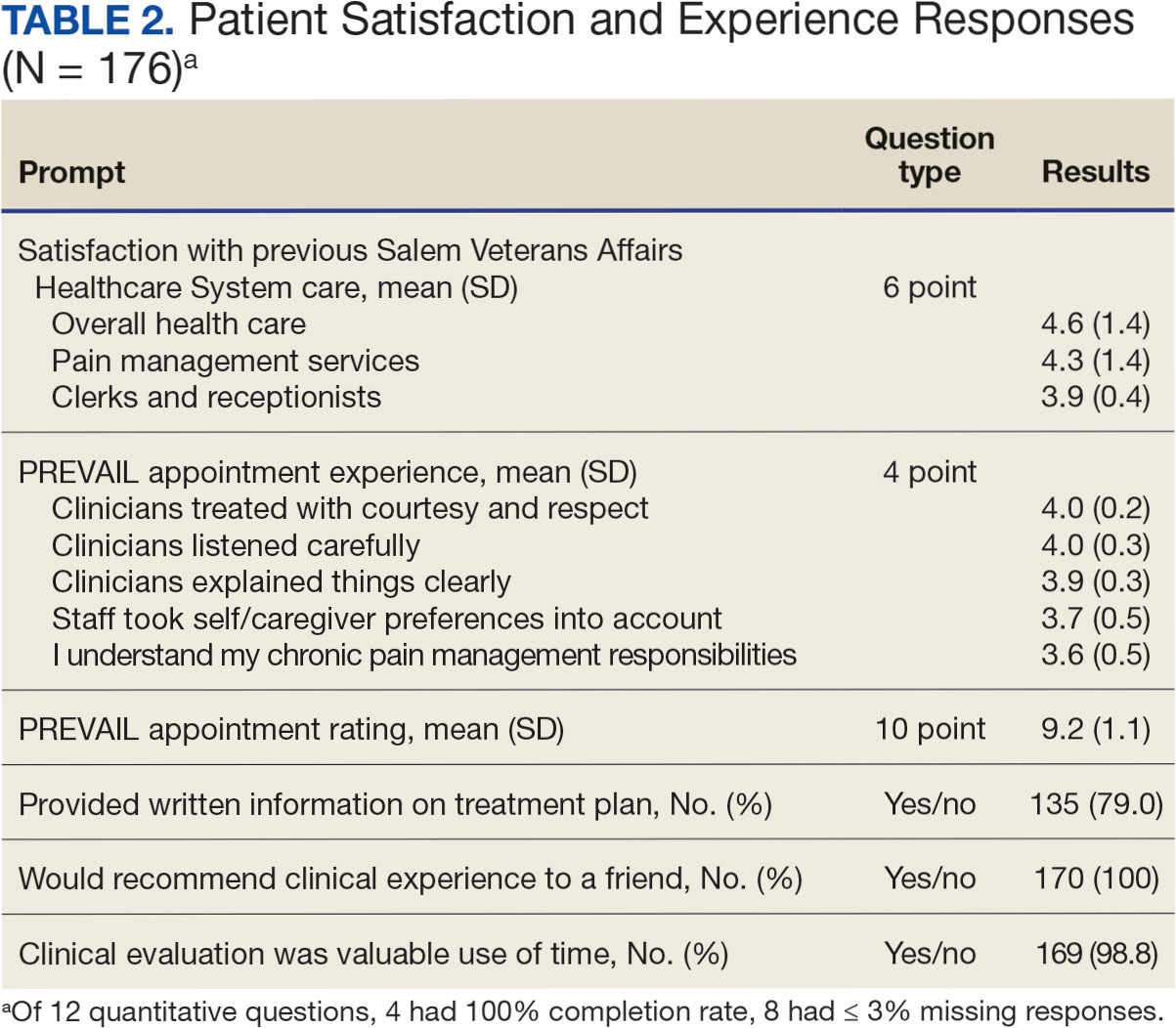
Qualitative Responses
Respondents provided complementary feedback on the program, with many participants stating that they enjoyed every aspect (eAppendix, available at doi:10.12788/fp.0503). In terms of positive aspects of the program, several themes emerged: participants appreciated meeting as an IDT, feeling cared for and listened to, learning more about their pain and ways to manage it, and specific services offered. Thirty-three of 144 respondents wanted longer appointment times. Twenty-two respondents suggested logistics improvements (eg, meeting in a larger room, having a written plan at the end, sending paperwork ahead of time, and later appointment times).
Discussion
Veterans and support persons were satisfied with the initial IDT evaluation for the PREVAIL whole health-focused pain clinical program for veterans predominantly residing in central Appalachia. These satisfaction findings are noteworthy since 20% of this same sample reported dissatisfaction with prior pain services, which could affect engagement and outcomes in pain care. In addition to high satisfaction levels, the PREVAIL IDT model may benefit veterans with limited resources. Rather than needing to attend several individual appointments, the PREVAIL IDT Track provides a 1-stop shop approach that decreases patient burden and barriers to care (eg, travel, transportation, and time) as well as health care system burden. For instance, schedulers need only to make 1 appointment for the veteran rather than several. This approach was highly acceptable to veterans served at SVAHCS and may increase the reach and impact of VHA IDT pain care.
The PREVAIL model may foster rapport with HCPs and encourage an active role in self-managing pain.36,37 Participants noted that their preferences were considered and that they had a good understanding of their responsibilities for managing their chronic pain. This patient-centered approach, emphasizing an active role for the patient, is a hallmark of the VHA Whole Health System and aligns with the overarching PREVAIL IDT Track goal to enhance self-management skills, thus improving functioning through decreased pain interference.14,38-41
Participants in PREVAIL provided substantial open-ended feedback that has contributed to the program’s improvement and may provide information into preferred components of pain IDT programs, particularly for rural veterans. When asked about their favorite component of the initial IDT evaluation, the most emergent theme was meeting simultaneously with HCPs on the IDT. This finding is significant, given that only 11% of IDTs involve direct patient interaction.
Furthermore, unlike most IDTs, PREVAIL IDT includes a dietitian.11,42 IDT programs may benefit from dietitian involvement given the importance of the anti-inflammatory diet on chronic pain.43-46 Participants recommended improvements, (eg, changes to the location and timing, adding a written treatment plan at the end of the appointment, and completing paperwork prior to the appointment) many of which have been addressed. The program now uses validated measures to track progress and comprehensive assessments of pain in response to calls for measurement-based care.13,47 These process improvement suggestions may be instructive for other VA medical centers with rural populations.
Limitations
This study used a program-specific satisfaction survey with open-ended questions to allow for rich responses; however, the survey has not been validated. It also sought to minimize bias by asking participants to give completed surveys to staff members who were not HCPs on the IDT. However, participants’ responses may still have been influenced by this process. Response rate and demographics for support persons were impossible to determine. The results analyzed the responses of veterans and support persons together, which may have skewed the data. Future studies of pain IDT programs should consider analyzing responses from veterans and their support persons separately and identifying factors (eg, demographics or clinical characteristics) that influence the patients’ experiences while participating.
Conclusions
The initial PREVAIL IDT evaluation at SVAHCS is associated with high-levels of satisfaction. These veterans living in rural Appalachia, similar to the 4.4 million rural US veterans, are more likely to encounter barriers to care (eg, drive time, or transportation concerns) and be prescribed opioids.48 These veterans are also at high risk of chronic physical and mental health comorbidities, drug misuse, overdose, and suicide.49,50 Providing veterans in rural communities the opportunity to attend a single appointment with a pain IDT instead of requiring several individual appointments could improve the reach of evidence-based pain care.
This model of meeting simultaneously with all HCPs on the pain IDT may connect all veterans to the most available and best care, something prioritized by the VHA.28 The initial PREVAIL IDT evaluation also utilizes the Personal Health Inventory and the VHA Whole Health System Circle of Health to design patient-centered treatment plans. Integration of the Whole Health System is currently a high priority within VHA. The PREVAIL IDT Track model warrants additional efficacy research.
- Rikard SM, Strahan AE, Schmit KM, Guy GP Jr. Chronic pain among adults - United States, 2019-2021. MMWR Morb Mortal Wkly Rep. 2023;72(15):379-385. doi:10.15585/mmwr.mm7215a1
- Nahin RL. Severe Pain in Veterans: The effect of age and sex, and comparisons with the general population. J Pain. 2017;18(3):247-254. doi:10.1016/j.jpain.2016.10.021
- Courtney RE, Schadegg MJ, Bolton R, Smith S, Harden SM. Using a whole health approach to build biopsychosocial-spiritual personal health plans for veterans with chronic pain. Pain Manag Nurs. 2024;25(1):69-74. doi:10.1016/j.pmn.2023.09.010
- Mackey SC, Pearl RG. Pain management: optimizing patient care through comprehensive, interdisciplinary models and continuous innovations. Anesthesiol Clin. 2023;41(2):xv-xvii. doi:10.1016/j.anclin.2023.03.011
- Gatchel RJ, McGeary DD, McGeary CA, Lippe B. Interdisciplinary chronic pain management: past, present, and future. Am Psychol. 2014;69(2):119-130. doi:10.1037/a0035514
- Courtney RE, Schadegg MJ. Chronic, noncancer pain care in the veterans administration: current trends and future directions. Anesthesiol Clin. 2023;41(2):519-529. doi:10.1016/j.anclin.2023.02.004
- Gallagher RM. Advancing the pain agenda in the veteran population. Anesthesiol Clin. 2016;34(2):357-378. doi:10.1016/j.anclin.2016.01.003
- Kamper SJ, Apeldoorn AT, Chiarotto A, et al. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain: cochrane systematic review and meta-analysis. BMJ. 2015;350:h444. doi:10.1136/bmj.h444
- Waterschoot FPC, Dijkstra PU, Hollak N, De Vries HJ, Geertzen JHB, Reneman MF. Dose or content? Effectiveness of pain rehabilitation programs for patients with chronic low back pain: a systematic review. Pain. 2014;155(1):179-189. doi:10.1016/j.pain.2013.10.006
- Scascighini L, Toma V, Dober-Spielmann S, Sprott H. Multidisciplinary treatment for chronic pain: a systematic review of interventions and outcomes. Rheumatology (Oxford). 2008;47(5):670-678. doi:10.1093/rheumatology/ken021
- Elbers S, Wittink H, Konings S, et al. Longitudinal outcome evaluations of interdisciplinary multimodal pain Treatment programmes for patients with chronic primary musculoskeletal pain: a systematic review and meta-analysis. Eur J Pain. 2022;26(2):310-335. doi:10.1002/ejp.1875
- Turk DC, Dworkin RH, Allen RR, et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain. 2003;106(3):337-345. doi:10.1016/j.pain.2003.08.001
- Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1-2):9-19. doi:10.1016/j.pain.2004.09.012
- Kligler B, Hyde J, Gantt C, Bokhour B. The whole health transformation at the Veterans Health Administration: moving from “what’s the matter with you?” to “what matters to you?”. Med Care. 2022;60(5):387-391. doi:10.1097/mlr.0000000000001706
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Care Services; Committee on Transforming Health Care to Create Whole Health: Strategies to Assess, Scale, and Spread the Whole Person Approach to Health, Meisnere M, SouthPaul J, Krist AH, eds. Achieving Whole Health: A New Approach for Veterans and the Nation. National Academies Press (US); February 15, 2023.
- The time Is now for a whole-person health approach to public health. Public Health Rep. 2023;138(4):561-564. doi:10.1177/00333549231154583
- Krejci LP, Carter K, Gaudet T. Whole health: the vision and implementation of personalized, proactive, patient-driven health care for veterans. Med Care. 2014;52(12 Suppl 5):S5-S8. doi:10.1097/mlr.0000000000000226
- Bokhour BG, Hyde J, Kligler B, et al. From patient outcomes to system change: evaluating the impact of VHA’s implementation of the whole health system of care. Health Serv Res. 2022;57 Suppl 1(Suppl 1):53-65. doi:10.1111/1475-6773.13938
- Zeliadt SB, Douglas JH, Gelman H, et al. Effectiveness of a whole health model of care emphasizing complementary and integrative health on reducing opioid use among patients with chronic pain. BMC Health Serv Res. 2022;22(1):1053. doi:10.1186/s12913-022-08388-2
- Reed DE 2nd, Bokhour BG, Gaj L, et al. Whole health use and interest across veterans with cooccurring chronic pain and PTSD: an examination of the 18 VA medical center flagship sites. Glob Adv Health Med. 2022;11:21649561211065374. doi:10.1177/21649561211065374
- Etingen B, Smith BM, Zeliadt SB, et al. VHA whole health services and complementary and integrative health therapies: a gateway to evidence-based mental health treatment. J Gen Intern Med. 2023;38(14):3144-3151. doi:10.1007/s11606-023-08296-z
- Johnson EM, Possemato K, Khan S, Chinman M, Maisto SA. Engagement, experience, and satisfaction with peerdelivered whole health coaching for veterans with PTSD: a mixed methods process evaluation. Psychol Serv. 2021;19(2):305-316. doi:10.1037/ser0000529
- Purcell N, Zamora K, Gibson C, et al. Patient experiences with integrated pain care: a qualitative evaluation of one VA’s biopsychosocial approach to chronic pain treatment and opioid safety. Glob Adv Health Med. 2019;8:2164956119838845. doi:10.1177/2164956119838845
- Will KK, Johnson ML, Lamb G. Team-based care and patient satisfaction in the hospital setting: a systematic review. J Patient Cent Res Rev. 2019;6(2):158-171. doi:10.17294/2330-0698.1695
- van Dongen JJJ, Habets IGJ, Beurskens A, van Bokhoven MA. Successful participation of patients in interprofessional team meetings: a qualitative study. Health Expect. 2017;20(4):724-733. doi:10.1111/hex.12511
- Oliver DP, Albright DL, Kruse RL, Wittenberg-Lyles E, Washington K, Demiris G. Caregiver evaluation of the ACTIVE intervention: “it was like we were sitting at the table with everyone.” Am J Hosp Palliat Care. 2014;31(4):444-453. doi:10.1177/1049909113490823
- Ansmann L, Heuser C, Diekmann A, et al. Patient participation in multidisciplinary tumor conferences: how is it implemented? What is the patients’ role? What are patients’ experiences? Cancer Med. 2021;10(19):6714-6724. doi:10.1002/cam4.4213
- US Department of Veterans Affairs, Veterans Health Administration. Updated March 20, 2023. Accessed June 11, 2024. https://www.va.gov/health/priorities/index.asp
- Darnall BD, Edwards KA, Courtney RE, Ziadni MS, Simons LE, Harrison LE. Innovative treatment formats, technologies, and clinician trainings that improve access to behavioral pain treatment for youth and adults. Front Pain Res (Lausanne). 2023;4:1223172. doi:10.3389/fpain.2023.1223172
- Kligler B. Whole health in the Veterans Health Administration. Glob Adv Health Med. 2022;11:2164957X221077214.
- Howe RJ, Poulin LM, Federman DG. The personal health inventory: current use, perceived barriers, and benefits. Fed Pract. 2017;34(5):23-26. doi:10.1177/2164957X221077214
- Hicks N, Harden S, Oursler KA, Courtney RE. Determining the representativeness of participants in a whole health interdisciplinary chronic pain program (PREVAIL) in a VA medical center: who did we reach? Presented at: PAINWeek 2022; September 6-9, 2022; Las Vegas, Nevada. Accessed September 10, 2024. https://www.tandfonline.com/doi/full/10.1080/00325481.2022.2116839
- Creswell JW, Creswell JD. Research Design: Qualitative, Quantitative, and Mixed Methods Approaches. SAGE Publications; 2018.
- Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual in Health Care. 2007;19(6):349-357. doi:10.1093/intqhc/mzm042
- National Institute for Health and Care Excellence. Methods for the development of NICE public health guidance, 3rd edition. Published September 26, 2012. Accessed June 11, 2024. https://www.nice.org.uk/process/pmg4/chapter/introduction
- Alexander JA, Hearld LR, Mittler JN, Harvey J. Patient-physician role relationships and patient activation among individuals with chronic illness. Health Serv Res. 2012;47(3 PART 1):1201-1223. doi:10.1111/j.1475-6773.2011.01354.x
- Fu Y, Yu G, McNichol E, Marczewski K, Closs SJ. The association between patient-professional partnerships and self-management of chronic back pain: a mixed methods study. Eur J Pain. 2018;22(7):1229-1244. doi:10.1002/ejp.1210
- Nicholas MK, Asghari A, Blyth FM, et al. Self-management intervention for chronic pain in older adults: a randomised controlled trial. Pain. 2013;154(6):824-835. doi:10.1016/j.pain.2013.02.009
- Nøst TH, Steinsbekk A, Bratås O, Grønning K. Twelvemonth effect of chronic pain self-management intervention delivered in an easily accessible primary healthcare service - a randomised controlled trial. BMC Health Serv Res. 2018;18(1):1012. doi:10.1186/s12913-018-3843-x
- Blyth FM, March LM, Nicholas MK, Cousins MJ. Selfmanagement of chronic pain: a population-based study. Pain. 2005;113(3):285-292. doi:10.1016/j.pain.2004.12.004
- Damush TM, Kroenke K, Bair MJ, et al. Pain self-management training increases self-efficacy, self-management behaviours and pain and depression outcomes. Eur J Pain. 2016;20(7):1070-1078. doi:10.1002/ejp.830
- Murphy JL, Palyo SA, Schmidt ZS, et al. The resurrection of interdisciplinary pain rehabilitation: outcomes across a veterans affairs collaborative. Pain Med. 2021;22(2):430- 443. doi:10.1093/pm/pnaa417
- Brain K, Burrows TL, Bruggink L, et al. Diet and chronic non-cancer pain: the state of the art and future directions. J Clin Med. 2021;10(21):5203. doi:10.3390/jcm10215203
- Field R, Pourkazemi F, Turton J, Rooney K. Dietary interventions are beneficial for patients with chronic pain: a systematic review with meta-analysis. Pain Med). 2021;22(3):694-714. doi:10.1093/pm/pnaa378
- Bjørklund G, Aaseth J, Do§a MD, et al. Does diet play a role in reducing nociception related to inflammation and chronic pain? Nutrition. 2019;66:153-165. doi:10.1016/j.nut.2019.04.007
- Kaushik AS, Strath LJ, Sorge RE. Dietary interventions for treatment of chronic pain: oxidative stress and inflammation. Pain Ther. 2020;9(2):487-498. doi:10.1007/s40122-020-00200-5
- Boswell JF, Hepner KA, Lysell K, et al. The need for a measurement-based care professional practice guideline. Psychotherapy (Chic). 2023;60(1):1-16. doi:10.1037/pst0000439
- Lund BC, Ohl ME, Hadlandsmyth K, Mosher HJ. Regional and rural-urban variation in opioid prescribing in the Veterans Health Administration. Mil Med. 2019;184(11-12):894- 900. doi:10.1093/milmed/usz104
- US Department of Veterans Affairs, Office of Rural Health. Rural veterans. Updated May 14, 2024. Accessed June 11, 2024. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp
- McCarthy JF, Blow FC, Ignacio R V., Ilgen MA, Austin KL, Valenstein M. Suicide among patients in the Veterans Affairs health system: rural-urban differences in rates, risks, and methods. Am J Public Health. 2012;102 Suppl 1(suppl 1):S111-S117. doi:10.2105/AJPH.2011.300463
Chronic pain is one of the most prevalent public health concerns in the United States, affecting > 51 million adults with about $500 billion in health care costs.1 Military veterans are among the most vulnerable subpopulations, with 65% of veterans reporting chronic pain in the last 3 months.2 Chronic pain is complex, affecting the biopsychosocial-spiritual levels of human health, and requires multimodal and comprehensive treatment approaches.3 Hence, chronic pain treatment can be best delivered via interdisciplinary teams (IDTs) that use a patient-centered approach.4,5
The Veterans Healthcare Administration (VHA) is a leader in developing and delivering interdisciplinary pain care.6,7 VHA Directive 2009-053 requires every US Department of Veterans Affairs (VA) medical center to offer an IDT for chronic pain. However, VHA and non-VHA IDT programs vary significantly.8-11 A recent systematic review found a median of 5 disciplines included on IDTs (range, 2-8), and program content often included exercise and education; only 11% of included IDTs met simultaneously with patients.11 The heterogeneity of IDT programs has made determining best practices challenging.8,11 The Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials has denoted several core measures and measurement domains that were critical for determining the success of pain management interventions, including patient satisfaction.12,13 Nevertheless, the association of IDTs with high patient satisfaction and improvement in pain measures has been documented.5,11
The VHA has worked to implement the Whole Health System into health care, which considers well-being across physical, behavioral, spiritual, and socioeconomic domains. As such, the Whole Health System involves an interpersonal, team-based approach, “anchored in trusting longitudinal relationships to promote resilience, prevent disease, and restore health.”14 It aligns with the patient’s mission, aspiration, and purpose. Surgeon General VADM Vivek H. Murthy, MD, MBA, recently endorsed this approach.15,16 Other health care systems adopting whole health tend to have higher patient satisfaction, increased access to care, and improved patient-reported outcomes.15 Within the VHA, the Whole Health System has shifted the conversation between clinicians and patients from “What is the matter with you?” to “What is important to you?” while emphasizing a proactive and personalized approach to health care.17 Rather than emphasizing passive modalities such as medications and clinician-led services (eg, interventional pain service), the Whole Health System highlights self-care.3,17 Initial research findings within the VHA have been promising.18-21 Whole health peer coaching calls appear to be an effective approach for veterans diagnosed with PTSD, and the use of whole health services is associated with a decrease in opioid use.19,22 However, there are negligible data on patient experiences after meeting with a whole health-focused pain IDT, and studies to date have focused on urban populations.23 One approach to IDT that has shown promise for other health issues involves a patient meeting simultaneously with all members of the IDT.24-27 With the integration of the Whole Health System and the VHA priorities to provide veterans with the “soonest and best care,” more data are needed on the experiences of patients and support persons with various approaches to IDT pain care.28 This study aimed to evaluate patient and support person experiences with a whole health-focused pain IDT that met simultaneously with the patient and support person during an initial evaluation. This study was approved by the institutional review board at the Salem VA Health Care System (SVAHCS) in Virginia.
Methods
The PREVAIL IDT Track is a clinical program offered at SVAHCS with a whole health-focused approach that involves patients and their support persons meeting simultaneously with a pain IDT. PREVAIL IDT Track is designed to help veterans more effectively self-manage chronic pain (Table 1).6,29 Health care practitioners (HCPs) at SVAHCS recommended that veterans with pain persisting for > 3 months participate in PREVAIL IDT Track. After meeting with an advanced practice clinician for an intake, veterans elected to participate in the PREVAIL IDT Track program and completed the initial 6 weeks of pain education. Veterans were then invited to be evaluated by the pain IDT. A team including HCPs from interventional pain, psychology, pharmacy, nutrition, and physical therapy services met with the veteran for 60 minutes. Veterans were also invited to bring a support person to the IDT initial evaluation.

During the IDT initial evaluation, HCPs inquired about the patient’s mission, aspiration, and purpose (“If you were in less pain, what would you be doing more of?”) and about whole health self-care and wellness factors that may contribute to their chronic pain using the Personal Health Inventory.30,31 Veterans were then invited to select 3 whole health self-care areas to focus on during the 6-month program.3 The IDT HCPs worked with the veteran to establish the treatment plan for the first month in the areas of self-care selected by the patient and made recommendations for additional treatments. If the veteran brought a support person to the IDT initial evaluation, their feedback was elicited throughout and at the end to ensure the final treatment plan and first month’s goals were realistic. At the end of the appointment, the veteran and their support person were asked to complete a program-specific satisfaction survey. The HCPs on the team and the veteran executed the treatment plan developed during the appointment, except for medication prescribing. Recommendations for medication changes are included in clinical notes. Veterans then received 5 monthly coaching calls from a nurse navigator with training in whole health and a 6-month follow-up appointment with the IDT HCPs to discuss a plan for continuity of care.
Participant demographic information was not collected, and participants were not compensated for completing the survey. Veterans in PREVAIL IDT Track are predominantly residents of central Appalachia, White, male, unemployed, have ≥ 1 mental and physical health comorbidity, and have a history of mental health treatment.32 Veterans participating in PREVAIL IDT had a mean age of 57 years, and about 1 in 3 have opioid prescriptions.32
A program-specific 17-question satisfaction survey was developed, which included questions related to satisfaction with previous SVAHCS pain care and staff interactions. To assess the overall impression of the IDT initial evaluation, 3 yes/no questions and a 0 to 10-point scale were used. The 5 remaining open-ended questions allowed participants to give feedback about the IDT initial evaluation.
Data Analysis
A convergent mixed-methods approach was used to evaluate participant satisfaction with the initial IDT evaluation. The study team collected and analyzed quantitative and qualitative survey data and triangulated the findings.33 For quantitative responses, frequencies and means were calculated using Python. For qualitative responses, thematic data analysis was conducted by systematic coding, using inductive methods and allowing themes to emerge. Study team members performed a line-by-line analysis of responses using NVivo to identify important codes and reach a consensus. This study adhered to the Consolidated Criteria for Reporting Qualitative Research and followed the National Institute for Health Care Excellence checklist.34,35
Results
Quantitative Responses
In 2022, 168 veterans completed the initial IDT evaluation, and 144 (85.2%) completed the satisfaction survey and were included in this study. Thirty-two support persons who attended the initial IDT evaluation and completed the survey also were included. Of the 12 quantitative questions, 4 had a 100% completion rate, while 8 had ≤ 3% missing responses. When describing care prior to participating in PREVAIL, participants indicated a mean (SD) response of 4.6 (1.4) with the health care they received at SVAHCS and 4.3 (1.4) with SVAHCS pain management services, both on 6-point scales. All but 2 participants (98.9%) reported always being treated with courtesy and respect by PREVAIL HCPs during the initial IDT evaluation, with a mean (SD) score of 4.0 (0.2) on a 4-point scale. Most respondents (96.6%) reported that PREVAIL HCPs always listened carefully during the initial IDT evaluation, with a mean (SD) 4.0 (0.3) on a 4-point scale. Similarly, 92.6% reported that PREVAIL HCPs explained things clearly during the initial IDT evaluation, with a mean (SD) 3.9 (0.3) on a 4-point scale.
All respondents agreed that PREVAIL HCPs considered veteran preferences and those of their support persons in deciding their health care needs during the initial IDT evaluation, with a mean (SD) 3.7 (0.5) on a 4-point scale. Most respondents left the appointment with a good understanding of their responsibilities for chronic pain management with 99.4% (n = 169) strongly agreeing or agreeing (mean [SD] 3.6 [0.5]). A total of 135 respondents (79.4%) reported they left appointments with written information on their treatment plan. All 170 respondents reported that they would recommend PREVAIL to a friend, and 169 respondents (98.8%) felt that the initial PREVAIL IDT evaluation was a valuable use of time. Eighty-seven respondents (50.9%) rated the initial IDT evaluation as the “best clinical experience possible” with a mean (SD) score of 9.2 (1.1) on a 10-point scale (Table 2).

Qualitative Responses
Respondents provided complementary feedback on the program, with many participants stating that they enjoyed every aspect (eAppendix, available at doi:10.12788/fp.0503). In terms of positive aspects of the program, several themes emerged: participants appreciated meeting as an IDT, feeling cared for and listened to, learning more about their pain and ways to manage it, and specific services offered. Thirty-three of 144 respondents wanted longer appointment times. Twenty-two respondents suggested logistics improvements (eg, meeting in a larger room, having a written plan at the end, sending paperwork ahead of time, and later appointment times).
Discussion
Veterans and support persons were satisfied with the initial IDT evaluation for the PREVAIL whole health-focused pain clinical program for veterans predominantly residing in central Appalachia. These satisfaction findings are noteworthy since 20% of this same sample reported dissatisfaction with prior pain services, which could affect engagement and outcomes in pain care. In addition to high satisfaction levels, the PREVAIL IDT model may benefit veterans with limited resources. Rather than needing to attend several individual appointments, the PREVAIL IDT Track provides a 1-stop shop approach that decreases patient burden and barriers to care (eg, travel, transportation, and time) as well as health care system burden. For instance, schedulers need only to make 1 appointment for the veteran rather than several. This approach was highly acceptable to veterans served at SVAHCS and may increase the reach and impact of VHA IDT pain care.
The PREVAIL model may foster rapport with HCPs and encourage an active role in self-managing pain.36,37 Participants noted that their preferences were considered and that they had a good understanding of their responsibilities for managing their chronic pain. This patient-centered approach, emphasizing an active role for the patient, is a hallmark of the VHA Whole Health System and aligns with the overarching PREVAIL IDT Track goal to enhance self-management skills, thus improving functioning through decreased pain interference.14,38-41
Participants in PREVAIL provided substantial open-ended feedback that has contributed to the program’s improvement and may provide information into preferred components of pain IDT programs, particularly for rural veterans. When asked about their favorite component of the initial IDT evaluation, the most emergent theme was meeting simultaneously with HCPs on the IDT. This finding is significant, given that only 11% of IDTs involve direct patient interaction.
Furthermore, unlike most IDTs, PREVAIL IDT includes a dietitian.11,42 IDT programs may benefit from dietitian involvement given the importance of the anti-inflammatory diet on chronic pain.43-46 Participants recommended improvements, (eg, changes to the location and timing, adding a written treatment plan at the end of the appointment, and completing paperwork prior to the appointment) many of which have been addressed. The program now uses validated measures to track progress and comprehensive assessments of pain in response to calls for measurement-based care.13,47 These process improvement suggestions may be instructive for other VA medical centers with rural populations.
Limitations
This study used a program-specific satisfaction survey with open-ended questions to allow for rich responses; however, the survey has not been validated. It also sought to minimize bias by asking participants to give completed surveys to staff members who were not HCPs on the IDT. However, participants’ responses may still have been influenced by this process. Response rate and demographics for support persons were impossible to determine. The results analyzed the responses of veterans and support persons together, which may have skewed the data. Future studies of pain IDT programs should consider analyzing responses from veterans and their support persons separately and identifying factors (eg, demographics or clinical characteristics) that influence the patients’ experiences while participating.
Conclusions
The initial PREVAIL IDT evaluation at SVAHCS is associated with high-levels of satisfaction. These veterans living in rural Appalachia, similar to the 4.4 million rural US veterans, are more likely to encounter barriers to care (eg, drive time, or transportation concerns) and be prescribed opioids.48 These veterans are also at high risk of chronic physical and mental health comorbidities, drug misuse, overdose, and suicide.49,50 Providing veterans in rural communities the opportunity to attend a single appointment with a pain IDT instead of requiring several individual appointments could improve the reach of evidence-based pain care.
This model of meeting simultaneously with all HCPs on the pain IDT may connect all veterans to the most available and best care, something prioritized by the VHA.28 The initial PREVAIL IDT evaluation also utilizes the Personal Health Inventory and the VHA Whole Health System Circle of Health to design patient-centered treatment plans. Integration of the Whole Health System is currently a high priority within VHA. The PREVAIL IDT Track model warrants additional efficacy research.
Chronic pain is one of the most prevalent public health concerns in the United States, affecting > 51 million adults with about $500 billion in health care costs.1 Military veterans are among the most vulnerable subpopulations, with 65% of veterans reporting chronic pain in the last 3 months.2 Chronic pain is complex, affecting the biopsychosocial-spiritual levels of human health, and requires multimodal and comprehensive treatment approaches.3 Hence, chronic pain treatment can be best delivered via interdisciplinary teams (IDTs) that use a patient-centered approach.4,5
The Veterans Healthcare Administration (VHA) is a leader in developing and delivering interdisciplinary pain care.6,7 VHA Directive 2009-053 requires every US Department of Veterans Affairs (VA) medical center to offer an IDT for chronic pain. However, VHA and non-VHA IDT programs vary significantly.8-11 A recent systematic review found a median of 5 disciplines included on IDTs (range, 2-8), and program content often included exercise and education; only 11% of included IDTs met simultaneously with patients.11 The heterogeneity of IDT programs has made determining best practices challenging.8,11 The Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials has denoted several core measures and measurement domains that were critical for determining the success of pain management interventions, including patient satisfaction.12,13 Nevertheless, the association of IDTs with high patient satisfaction and improvement in pain measures has been documented.5,11
The VHA has worked to implement the Whole Health System into health care, which considers well-being across physical, behavioral, spiritual, and socioeconomic domains. As such, the Whole Health System involves an interpersonal, team-based approach, “anchored in trusting longitudinal relationships to promote resilience, prevent disease, and restore health.”14 It aligns with the patient’s mission, aspiration, and purpose. Surgeon General VADM Vivek H. Murthy, MD, MBA, recently endorsed this approach.15,16 Other health care systems adopting whole health tend to have higher patient satisfaction, increased access to care, and improved patient-reported outcomes.15 Within the VHA, the Whole Health System has shifted the conversation between clinicians and patients from “What is the matter with you?” to “What is important to you?” while emphasizing a proactive and personalized approach to health care.17 Rather than emphasizing passive modalities such as medications and clinician-led services (eg, interventional pain service), the Whole Health System highlights self-care.3,17 Initial research findings within the VHA have been promising.18-21 Whole health peer coaching calls appear to be an effective approach for veterans diagnosed with PTSD, and the use of whole health services is associated with a decrease in opioid use.19,22 However, there are negligible data on patient experiences after meeting with a whole health-focused pain IDT, and studies to date have focused on urban populations.23 One approach to IDT that has shown promise for other health issues involves a patient meeting simultaneously with all members of the IDT.24-27 With the integration of the Whole Health System and the VHA priorities to provide veterans with the “soonest and best care,” more data are needed on the experiences of patients and support persons with various approaches to IDT pain care.28 This study aimed to evaluate patient and support person experiences with a whole health-focused pain IDT that met simultaneously with the patient and support person during an initial evaluation. This study was approved by the institutional review board at the Salem VA Health Care System (SVAHCS) in Virginia.
Methods
The PREVAIL IDT Track is a clinical program offered at SVAHCS with a whole health-focused approach that involves patients and their support persons meeting simultaneously with a pain IDT. PREVAIL IDT Track is designed to help veterans more effectively self-manage chronic pain (Table 1).6,29 Health care practitioners (HCPs) at SVAHCS recommended that veterans with pain persisting for > 3 months participate in PREVAIL IDT Track. After meeting with an advanced practice clinician for an intake, veterans elected to participate in the PREVAIL IDT Track program and completed the initial 6 weeks of pain education. Veterans were then invited to be evaluated by the pain IDT. A team including HCPs from interventional pain, psychology, pharmacy, nutrition, and physical therapy services met with the veteran for 60 minutes. Veterans were also invited to bring a support person to the IDT initial evaluation.

During the IDT initial evaluation, HCPs inquired about the patient’s mission, aspiration, and purpose (“If you were in less pain, what would you be doing more of?”) and about whole health self-care and wellness factors that may contribute to their chronic pain using the Personal Health Inventory.30,31 Veterans were then invited to select 3 whole health self-care areas to focus on during the 6-month program.3 The IDT HCPs worked with the veteran to establish the treatment plan for the first month in the areas of self-care selected by the patient and made recommendations for additional treatments. If the veteran brought a support person to the IDT initial evaluation, their feedback was elicited throughout and at the end to ensure the final treatment plan and first month’s goals were realistic. At the end of the appointment, the veteran and their support person were asked to complete a program-specific satisfaction survey. The HCPs on the team and the veteran executed the treatment plan developed during the appointment, except for medication prescribing. Recommendations for medication changes are included in clinical notes. Veterans then received 5 monthly coaching calls from a nurse navigator with training in whole health and a 6-month follow-up appointment with the IDT HCPs to discuss a plan for continuity of care.
Participant demographic information was not collected, and participants were not compensated for completing the survey. Veterans in PREVAIL IDT Track are predominantly residents of central Appalachia, White, male, unemployed, have ≥ 1 mental and physical health comorbidity, and have a history of mental health treatment.32 Veterans participating in PREVAIL IDT had a mean age of 57 years, and about 1 in 3 have opioid prescriptions.32
A program-specific 17-question satisfaction survey was developed, which included questions related to satisfaction with previous SVAHCS pain care and staff interactions. To assess the overall impression of the IDT initial evaluation, 3 yes/no questions and a 0 to 10-point scale were used. The 5 remaining open-ended questions allowed participants to give feedback about the IDT initial evaluation.
Data Analysis
A convergent mixed-methods approach was used to evaluate participant satisfaction with the initial IDT evaluation. The study team collected and analyzed quantitative and qualitative survey data and triangulated the findings.33 For quantitative responses, frequencies and means were calculated using Python. For qualitative responses, thematic data analysis was conducted by systematic coding, using inductive methods and allowing themes to emerge. Study team members performed a line-by-line analysis of responses using NVivo to identify important codes and reach a consensus. This study adhered to the Consolidated Criteria for Reporting Qualitative Research and followed the National Institute for Health Care Excellence checklist.34,35
Results
Quantitative Responses
In 2022, 168 veterans completed the initial IDT evaluation, and 144 (85.2%) completed the satisfaction survey and were included in this study. Thirty-two support persons who attended the initial IDT evaluation and completed the survey also were included. Of the 12 quantitative questions, 4 had a 100% completion rate, while 8 had ≤ 3% missing responses. When describing care prior to participating in PREVAIL, participants indicated a mean (SD) response of 4.6 (1.4) with the health care they received at SVAHCS and 4.3 (1.4) with SVAHCS pain management services, both on 6-point scales. All but 2 participants (98.9%) reported always being treated with courtesy and respect by PREVAIL HCPs during the initial IDT evaluation, with a mean (SD) score of 4.0 (0.2) on a 4-point scale. Most respondents (96.6%) reported that PREVAIL HCPs always listened carefully during the initial IDT evaluation, with a mean (SD) 4.0 (0.3) on a 4-point scale. Similarly, 92.6% reported that PREVAIL HCPs explained things clearly during the initial IDT evaluation, with a mean (SD) 3.9 (0.3) on a 4-point scale.
All respondents agreed that PREVAIL HCPs considered veteran preferences and those of their support persons in deciding their health care needs during the initial IDT evaluation, with a mean (SD) 3.7 (0.5) on a 4-point scale. Most respondents left the appointment with a good understanding of their responsibilities for chronic pain management with 99.4% (n = 169) strongly agreeing or agreeing (mean [SD] 3.6 [0.5]). A total of 135 respondents (79.4%) reported they left appointments with written information on their treatment plan. All 170 respondents reported that they would recommend PREVAIL to a friend, and 169 respondents (98.8%) felt that the initial PREVAIL IDT evaluation was a valuable use of time. Eighty-seven respondents (50.9%) rated the initial IDT evaluation as the “best clinical experience possible” with a mean (SD) score of 9.2 (1.1) on a 10-point scale (Table 2).

Qualitative Responses
Respondents provided complementary feedback on the program, with many participants stating that they enjoyed every aspect (eAppendix, available at doi:10.12788/fp.0503). In terms of positive aspects of the program, several themes emerged: participants appreciated meeting as an IDT, feeling cared for and listened to, learning more about their pain and ways to manage it, and specific services offered. Thirty-three of 144 respondents wanted longer appointment times. Twenty-two respondents suggested logistics improvements (eg, meeting in a larger room, having a written plan at the end, sending paperwork ahead of time, and later appointment times).
Discussion
Veterans and support persons were satisfied with the initial IDT evaluation for the PREVAIL whole health-focused pain clinical program for veterans predominantly residing in central Appalachia. These satisfaction findings are noteworthy since 20% of this same sample reported dissatisfaction with prior pain services, which could affect engagement and outcomes in pain care. In addition to high satisfaction levels, the PREVAIL IDT model may benefit veterans with limited resources. Rather than needing to attend several individual appointments, the PREVAIL IDT Track provides a 1-stop shop approach that decreases patient burden and barriers to care (eg, travel, transportation, and time) as well as health care system burden. For instance, schedulers need only to make 1 appointment for the veteran rather than several. This approach was highly acceptable to veterans served at SVAHCS and may increase the reach and impact of VHA IDT pain care.
The PREVAIL model may foster rapport with HCPs and encourage an active role in self-managing pain.36,37 Participants noted that their preferences were considered and that they had a good understanding of their responsibilities for managing their chronic pain. This patient-centered approach, emphasizing an active role for the patient, is a hallmark of the VHA Whole Health System and aligns with the overarching PREVAIL IDT Track goal to enhance self-management skills, thus improving functioning through decreased pain interference.14,38-41
Participants in PREVAIL provided substantial open-ended feedback that has contributed to the program’s improvement and may provide information into preferred components of pain IDT programs, particularly for rural veterans. When asked about their favorite component of the initial IDT evaluation, the most emergent theme was meeting simultaneously with HCPs on the IDT. This finding is significant, given that only 11% of IDTs involve direct patient interaction.
Furthermore, unlike most IDTs, PREVAIL IDT includes a dietitian.11,42 IDT programs may benefit from dietitian involvement given the importance of the anti-inflammatory diet on chronic pain.43-46 Participants recommended improvements, (eg, changes to the location and timing, adding a written treatment plan at the end of the appointment, and completing paperwork prior to the appointment) many of which have been addressed. The program now uses validated measures to track progress and comprehensive assessments of pain in response to calls for measurement-based care.13,47 These process improvement suggestions may be instructive for other VA medical centers with rural populations.
Limitations
This study used a program-specific satisfaction survey with open-ended questions to allow for rich responses; however, the survey has not been validated. It also sought to minimize bias by asking participants to give completed surveys to staff members who were not HCPs on the IDT. However, participants’ responses may still have been influenced by this process. Response rate and demographics for support persons were impossible to determine. The results analyzed the responses of veterans and support persons together, which may have skewed the data. Future studies of pain IDT programs should consider analyzing responses from veterans and their support persons separately and identifying factors (eg, demographics or clinical characteristics) that influence the patients’ experiences while participating.
Conclusions
The initial PREVAIL IDT evaluation at SVAHCS is associated with high-levels of satisfaction. These veterans living in rural Appalachia, similar to the 4.4 million rural US veterans, are more likely to encounter barriers to care (eg, drive time, or transportation concerns) and be prescribed opioids.48 These veterans are also at high risk of chronic physical and mental health comorbidities, drug misuse, overdose, and suicide.49,50 Providing veterans in rural communities the opportunity to attend a single appointment with a pain IDT instead of requiring several individual appointments could improve the reach of evidence-based pain care.
This model of meeting simultaneously with all HCPs on the pain IDT may connect all veterans to the most available and best care, something prioritized by the VHA.28 The initial PREVAIL IDT evaluation also utilizes the Personal Health Inventory and the VHA Whole Health System Circle of Health to design patient-centered treatment plans. Integration of the Whole Health System is currently a high priority within VHA. The PREVAIL IDT Track model warrants additional efficacy research.
- Rikard SM, Strahan AE, Schmit KM, Guy GP Jr. Chronic pain among adults - United States, 2019-2021. MMWR Morb Mortal Wkly Rep. 2023;72(15):379-385. doi:10.15585/mmwr.mm7215a1
- Nahin RL. Severe Pain in Veterans: The effect of age and sex, and comparisons with the general population. J Pain. 2017;18(3):247-254. doi:10.1016/j.jpain.2016.10.021
- Courtney RE, Schadegg MJ, Bolton R, Smith S, Harden SM. Using a whole health approach to build biopsychosocial-spiritual personal health plans for veterans with chronic pain. Pain Manag Nurs. 2024;25(1):69-74. doi:10.1016/j.pmn.2023.09.010
- Mackey SC, Pearl RG. Pain management: optimizing patient care through comprehensive, interdisciplinary models and continuous innovations. Anesthesiol Clin. 2023;41(2):xv-xvii. doi:10.1016/j.anclin.2023.03.011
- Gatchel RJ, McGeary DD, McGeary CA, Lippe B. Interdisciplinary chronic pain management: past, present, and future. Am Psychol. 2014;69(2):119-130. doi:10.1037/a0035514
- Courtney RE, Schadegg MJ. Chronic, noncancer pain care in the veterans administration: current trends and future directions. Anesthesiol Clin. 2023;41(2):519-529. doi:10.1016/j.anclin.2023.02.004
- Gallagher RM. Advancing the pain agenda in the veteran population. Anesthesiol Clin. 2016;34(2):357-378. doi:10.1016/j.anclin.2016.01.003
- Kamper SJ, Apeldoorn AT, Chiarotto A, et al. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain: cochrane systematic review and meta-analysis. BMJ. 2015;350:h444. doi:10.1136/bmj.h444
- Waterschoot FPC, Dijkstra PU, Hollak N, De Vries HJ, Geertzen JHB, Reneman MF. Dose or content? Effectiveness of pain rehabilitation programs for patients with chronic low back pain: a systematic review. Pain. 2014;155(1):179-189. doi:10.1016/j.pain.2013.10.006
- Scascighini L, Toma V, Dober-Spielmann S, Sprott H. Multidisciplinary treatment for chronic pain: a systematic review of interventions and outcomes. Rheumatology (Oxford). 2008;47(5):670-678. doi:10.1093/rheumatology/ken021
- Elbers S, Wittink H, Konings S, et al. Longitudinal outcome evaluations of interdisciplinary multimodal pain Treatment programmes for patients with chronic primary musculoskeletal pain: a systematic review and meta-analysis. Eur J Pain. 2022;26(2):310-335. doi:10.1002/ejp.1875
- Turk DC, Dworkin RH, Allen RR, et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain. 2003;106(3):337-345. doi:10.1016/j.pain.2003.08.001
- Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1-2):9-19. doi:10.1016/j.pain.2004.09.012
- Kligler B, Hyde J, Gantt C, Bokhour B. The whole health transformation at the Veterans Health Administration: moving from “what’s the matter with you?” to “what matters to you?”. Med Care. 2022;60(5):387-391. doi:10.1097/mlr.0000000000001706
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Care Services; Committee on Transforming Health Care to Create Whole Health: Strategies to Assess, Scale, and Spread the Whole Person Approach to Health, Meisnere M, SouthPaul J, Krist AH, eds. Achieving Whole Health: A New Approach for Veterans and the Nation. National Academies Press (US); February 15, 2023.
- The time Is now for a whole-person health approach to public health. Public Health Rep. 2023;138(4):561-564. doi:10.1177/00333549231154583
- Krejci LP, Carter K, Gaudet T. Whole health: the vision and implementation of personalized, proactive, patient-driven health care for veterans. Med Care. 2014;52(12 Suppl 5):S5-S8. doi:10.1097/mlr.0000000000000226
- Bokhour BG, Hyde J, Kligler B, et al. From patient outcomes to system change: evaluating the impact of VHA’s implementation of the whole health system of care. Health Serv Res. 2022;57 Suppl 1(Suppl 1):53-65. doi:10.1111/1475-6773.13938
- Zeliadt SB, Douglas JH, Gelman H, et al. Effectiveness of a whole health model of care emphasizing complementary and integrative health on reducing opioid use among patients with chronic pain. BMC Health Serv Res. 2022;22(1):1053. doi:10.1186/s12913-022-08388-2
- Reed DE 2nd, Bokhour BG, Gaj L, et al. Whole health use and interest across veterans with cooccurring chronic pain and PTSD: an examination of the 18 VA medical center flagship sites. Glob Adv Health Med. 2022;11:21649561211065374. doi:10.1177/21649561211065374
- Etingen B, Smith BM, Zeliadt SB, et al. VHA whole health services and complementary and integrative health therapies: a gateway to evidence-based mental health treatment. J Gen Intern Med. 2023;38(14):3144-3151. doi:10.1007/s11606-023-08296-z
- Johnson EM, Possemato K, Khan S, Chinman M, Maisto SA. Engagement, experience, and satisfaction with peerdelivered whole health coaching for veterans with PTSD: a mixed methods process evaluation. Psychol Serv. 2021;19(2):305-316. doi:10.1037/ser0000529
- Purcell N, Zamora K, Gibson C, et al. Patient experiences with integrated pain care: a qualitative evaluation of one VA’s biopsychosocial approach to chronic pain treatment and opioid safety. Glob Adv Health Med. 2019;8:2164956119838845. doi:10.1177/2164956119838845
- Will KK, Johnson ML, Lamb G. Team-based care and patient satisfaction in the hospital setting: a systematic review. J Patient Cent Res Rev. 2019;6(2):158-171. doi:10.17294/2330-0698.1695
- van Dongen JJJ, Habets IGJ, Beurskens A, van Bokhoven MA. Successful participation of patients in interprofessional team meetings: a qualitative study. Health Expect. 2017;20(4):724-733. doi:10.1111/hex.12511
- Oliver DP, Albright DL, Kruse RL, Wittenberg-Lyles E, Washington K, Demiris G. Caregiver evaluation of the ACTIVE intervention: “it was like we were sitting at the table with everyone.” Am J Hosp Palliat Care. 2014;31(4):444-453. doi:10.1177/1049909113490823
- Ansmann L, Heuser C, Diekmann A, et al. Patient participation in multidisciplinary tumor conferences: how is it implemented? What is the patients’ role? What are patients’ experiences? Cancer Med. 2021;10(19):6714-6724. doi:10.1002/cam4.4213
- US Department of Veterans Affairs, Veterans Health Administration. Updated March 20, 2023. Accessed June 11, 2024. https://www.va.gov/health/priorities/index.asp
- Darnall BD, Edwards KA, Courtney RE, Ziadni MS, Simons LE, Harrison LE. Innovative treatment formats, technologies, and clinician trainings that improve access to behavioral pain treatment for youth and adults. Front Pain Res (Lausanne). 2023;4:1223172. doi:10.3389/fpain.2023.1223172
- Kligler B. Whole health in the Veterans Health Administration. Glob Adv Health Med. 2022;11:2164957X221077214.
- Howe RJ, Poulin LM, Federman DG. The personal health inventory: current use, perceived barriers, and benefits. Fed Pract. 2017;34(5):23-26. doi:10.1177/2164957X221077214
- Hicks N, Harden S, Oursler KA, Courtney RE. Determining the representativeness of participants in a whole health interdisciplinary chronic pain program (PREVAIL) in a VA medical center: who did we reach? Presented at: PAINWeek 2022; September 6-9, 2022; Las Vegas, Nevada. Accessed September 10, 2024. https://www.tandfonline.com/doi/full/10.1080/00325481.2022.2116839
- Creswell JW, Creswell JD. Research Design: Qualitative, Quantitative, and Mixed Methods Approaches. SAGE Publications; 2018.
- Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual in Health Care. 2007;19(6):349-357. doi:10.1093/intqhc/mzm042
- National Institute for Health and Care Excellence. Methods for the development of NICE public health guidance, 3rd edition. Published September 26, 2012. Accessed June 11, 2024. https://www.nice.org.uk/process/pmg4/chapter/introduction
- Alexander JA, Hearld LR, Mittler JN, Harvey J. Patient-physician role relationships and patient activation among individuals with chronic illness. Health Serv Res. 2012;47(3 PART 1):1201-1223. doi:10.1111/j.1475-6773.2011.01354.x
- Fu Y, Yu G, McNichol E, Marczewski K, Closs SJ. The association between patient-professional partnerships and self-management of chronic back pain: a mixed methods study. Eur J Pain. 2018;22(7):1229-1244. doi:10.1002/ejp.1210
- Nicholas MK, Asghari A, Blyth FM, et al. Self-management intervention for chronic pain in older adults: a randomised controlled trial. Pain. 2013;154(6):824-835. doi:10.1016/j.pain.2013.02.009
- Nøst TH, Steinsbekk A, Bratås O, Grønning K. Twelvemonth effect of chronic pain self-management intervention delivered in an easily accessible primary healthcare service - a randomised controlled trial. BMC Health Serv Res. 2018;18(1):1012. doi:10.1186/s12913-018-3843-x
- Blyth FM, March LM, Nicholas MK, Cousins MJ. Selfmanagement of chronic pain: a population-based study. Pain. 2005;113(3):285-292. doi:10.1016/j.pain.2004.12.004
- Damush TM, Kroenke K, Bair MJ, et al. Pain self-management training increases self-efficacy, self-management behaviours and pain and depression outcomes. Eur J Pain. 2016;20(7):1070-1078. doi:10.1002/ejp.830
- Murphy JL, Palyo SA, Schmidt ZS, et al. The resurrection of interdisciplinary pain rehabilitation: outcomes across a veterans affairs collaborative. Pain Med. 2021;22(2):430- 443. doi:10.1093/pm/pnaa417
- Brain K, Burrows TL, Bruggink L, et al. Diet and chronic non-cancer pain: the state of the art and future directions. J Clin Med. 2021;10(21):5203. doi:10.3390/jcm10215203
- Field R, Pourkazemi F, Turton J, Rooney K. Dietary interventions are beneficial for patients with chronic pain: a systematic review with meta-analysis. Pain Med). 2021;22(3):694-714. doi:10.1093/pm/pnaa378
- Bjørklund G, Aaseth J, Do§a MD, et al. Does diet play a role in reducing nociception related to inflammation and chronic pain? Nutrition. 2019;66:153-165. doi:10.1016/j.nut.2019.04.007
- Kaushik AS, Strath LJ, Sorge RE. Dietary interventions for treatment of chronic pain: oxidative stress and inflammation. Pain Ther. 2020;9(2):487-498. doi:10.1007/s40122-020-00200-5
- Boswell JF, Hepner KA, Lysell K, et al. The need for a measurement-based care professional practice guideline. Psychotherapy (Chic). 2023;60(1):1-16. doi:10.1037/pst0000439
- Lund BC, Ohl ME, Hadlandsmyth K, Mosher HJ. Regional and rural-urban variation in opioid prescribing in the Veterans Health Administration. Mil Med. 2019;184(11-12):894- 900. doi:10.1093/milmed/usz104
- US Department of Veterans Affairs, Office of Rural Health. Rural veterans. Updated May 14, 2024. Accessed June 11, 2024. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp
- McCarthy JF, Blow FC, Ignacio R V., Ilgen MA, Austin KL, Valenstein M. Suicide among patients in the Veterans Affairs health system: rural-urban differences in rates, risks, and methods. Am J Public Health. 2012;102 Suppl 1(suppl 1):S111-S117. doi:10.2105/AJPH.2011.300463
- Rikard SM, Strahan AE, Schmit KM, Guy GP Jr. Chronic pain among adults - United States, 2019-2021. MMWR Morb Mortal Wkly Rep. 2023;72(15):379-385. doi:10.15585/mmwr.mm7215a1
- Nahin RL. Severe Pain in Veterans: The effect of age and sex, and comparisons with the general population. J Pain. 2017;18(3):247-254. doi:10.1016/j.jpain.2016.10.021
- Courtney RE, Schadegg MJ, Bolton R, Smith S, Harden SM. Using a whole health approach to build biopsychosocial-spiritual personal health plans for veterans with chronic pain. Pain Manag Nurs. 2024;25(1):69-74. doi:10.1016/j.pmn.2023.09.010
- Mackey SC, Pearl RG. Pain management: optimizing patient care through comprehensive, interdisciplinary models and continuous innovations. Anesthesiol Clin. 2023;41(2):xv-xvii. doi:10.1016/j.anclin.2023.03.011
- Gatchel RJ, McGeary DD, McGeary CA, Lippe B. Interdisciplinary chronic pain management: past, present, and future. Am Psychol. 2014;69(2):119-130. doi:10.1037/a0035514
- Courtney RE, Schadegg MJ. Chronic, noncancer pain care in the veterans administration: current trends and future directions. Anesthesiol Clin. 2023;41(2):519-529. doi:10.1016/j.anclin.2023.02.004
- Gallagher RM. Advancing the pain agenda in the veteran population. Anesthesiol Clin. 2016;34(2):357-378. doi:10.1016/j.anclin.2016.01.003
- Kamper SJ, Apeldoorn AT, Chiarotto A, et al. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain: cochrane systematic review and meta-analysis. BMJ. 2015;350:h444. doi:10.1136/bmj.h444
- Waterschoot FPC, Dijkstra PU, Hollak N, De Vries HJ, Geertzen JHB, Reneman MF. Dose or content? Effectiveness of pain rehabilitation programs for patients with chronic low back pain: a systematic review. Pain. 2014;155(1):179-189. doi:10.1016/j.pain.2013.10.006
- Scascighini L, Toma V, Dober-Spielmann S, Sprott H. Multidisciplinary treatment for chronic pain: a systematic review of interventions and outcomes. Rheumatology (Oxford). 2008;47(5):670-678. doi:10.1093/rheumatology/ken021
- Elbers S, Wittink H, Konings S, et al. Longitudinal outcome evaluations of interdisciplinary multimodal pain Treatment programmes for patients with chronic primary musculoskeletal pain: a systematic review and meta-analysis. Eur J Pain. 2022;26(2):310-335. doi:10.1002/ejp.1875
- Turk DC, Dworkin RH, Allen RR, et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain. 2003;106(3):337-345. doi:10.1016/j.pain.2003.08.001
- Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1-2):9-19. doi:10.1016/j.pain.2004.09.012
- Kligler B, Hyde J, Gantt C, Bokhour B. The whole health transformation at the Veterans Health Administration: moving from “what’s the matter with you?” to “what matters to you?”. Med Care. 2022;60(5):387-391. doi:10.1097/mlr.0000000000001706
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Care Services; Committee on Transforming Health Care to Create Whole Health: Strategies to Assess, Scale, and Spread the Whole Person Approach to Health, Meisnere M, SouthPaul J, Krist AH, eds. Achieving Whole Health: A New Approach for Veterans and the Nation. National Academies Press (US); February 15, 2023.
- The time Is now for a whole-person health approach to public health. Public Health Rep. 2023;138(4):561-564. doi:10.1177/00333549231154583
- Krejci LP, Carter K, Gaudet T. Whole health: the vision and implementation of personalized, proactive, patient-driven health care for veterans. Med Care. 2014;52(12 Suppl 5):S5-S8. doi:10.1097/mlr.0000000000000226
- Bokhour BG, Hyde J, Kligler B, et al. From patient outcomes to system change: evaluating the impact of VHA’s implementation of the whole health system of care. Health Serv Res. 2022;57 Suppl 1(Suppl 1):53-65. doi:10.1111/1475-6773.13938
- Zeliadt SB, Douglas JH, Gelman H, et al. Effectiveness of a whole health model of care emphasizing complementary and integrative health on reducing opioid use among patients with chronic pain. BMC Health Serv Res. 2022;22(1):1053. doi:10.1186/s12913-022-08388-2
- Reed DE 2nd, Bokhour BG, Gaj L, et al. Whole health use and interest across veterans with cooccurring chronic pain and PTSD: an examination of the 18 VA medical center flagship sites. Glob Adv Health Med. 2022;11:21649561211065374. doi:10.1177/21649561211065374
- Etingen B, Smith BM, Zeliadt SB, et al. VHA whole health services and complementary and integrative health therapies: a gateway to evidence-based mental health treatment. J Gen Intern Med. 2023;38(14):3144-3151. doi:10.1007/s11606-023-08296-z
- Johnson EM, Possemato K, Khan S, Chinman M, Maisto SA. Engagement, experience, and satisfaction with peerdelivered whole health coaching for veterans with PTSD: a mixed methods process evaluation. Psychol Serv. 2021;19(2):305-316. doi:10.1037/ser0000529
- Purcell N, Zamora K, Gibson C, et al. Patient experiences with integrated pain care: a qualitative evaluation of one VA’s biopsychosocial approach to chronic pain treatment and opioid safety. Glob Adv Health Med. 2019;8:2164956119838845. doi:10.1177/2164956119838845
- Will KK, Johnson ML, Lamb G. Team-based care and patient satisfaction in the hospital setting: a systematic review. J Patient Cent Res Rev. 2019;6(2):158-171. doi:10.17294/2330-0698.1695
- van Dongen JJJ, Habets IGJ, Beurskens A, van Bokhoven MA. Successful participation of patients in interprofessional team meetings: a qualitative study. Health Expect. 2017;20(4):724-733. doi:10.1111/hex.12511
- Oliver DP, Albright DL, Kruse RL, Wittenberg-Lyles E, Washington K, Demiris G. Caregiver evaluation of the ACTIVE intervention: “it was like we were sitting at the table with everyone.” Am J Hosp Palliat Care. 2014;31(4):444-453. doi:10.1177/1049909113490823
- Ansmann L, Heuser C, Diekmann A, et al. Patient participation in multidisciplinary tumor conferences: how is it implemented? What is the patients’ role? What are patients’ experiences? Cancer Med. 2021;10(19):6714-6724. doi:10.1002/cam4.4213
- US Department of Veterans Affairs, Veterans Health Administration. Updated March 20, 2023. Accessed June 11, 2024. https://www.va.gov/health/priorities/index.asp
- Darnall BD, Edwards KA, Courtney RE, Ziadni MS, Simons LE, Harrison LE. Innovative treatment formats, technologies, and clinician trainings that improve access to behavioral pain treatment for youth and adults. Front Pain Res (Lausanne). 2023;4:1223172. doi:10.3389/fpain.2023.1223172
- Kligler B. Whole health in the Veterans Health Administration. Glob Adv Health Med. 2022;11:2164957X221077214.
- Howe RJ, Poulin LM, Federman DG. The personal health inventory: current use, perceived barriers, and benefits. Fed Pract. 2017;34(5):23-26. doi:10.1177/2164957X221077214
- Hicks N, Harden S, Oursler KA, Courtney RE. Determining the representativeness of participants in a whole health interdisciplinary chronic pain program (PREVAIL) in a VA medical center: who did we reach? Presented at: PAINWeek 2022; September 6-9, 2022; Las Vegas, Nevada. Accessed September 10, 2024. https://www.tandfonline.com/doi/full/10.1080/00325481.2022.2116839
- Creswell JW, Creswell JD. Research Design: Qualitative, Quantitative, and Mixed Methods Approaches. SAGE Publications; 2018.
- Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual in Health Care. 2007;19(6):349-357. doi:10.1093/intqhc/mzm042
- National Institute for Health and Care Excellence. Methods for the development of NICE public health guidance, 3rd edition. Published September 26, 2012. Accessed June 11, 2024. https://www.nice.org.uk/process/pmg4/chapter/introduction
- Alexander JA, Hearld LR, Mittler JN, Harvey J. Patient-physician role relationships and patient activation among individuals with chronic illness. Health Serv Res. 2012;47(3 PART 1):1201-1223. doi:10.1111/j.1475-6773.2011.01354.x
- Fu Y, Yu G, McNichol E, Marczewski K, Closs SJ. The association between patient-professional partnerships and self-management of chronic back pain: a mixed methods study. Eur J Pain. 2018;22(7):1229-1244. doi:10.1002/ejp.1210
- Nicholas MK, Asghari A, Blyth FM, et al. Self-management intervention for chronic pain in older adults: a randomised controlled trial. Pain. 2013;154(6):824-835. doi:10.1016/j.pain.2013.02.009
- Nøst TH, Steinsbekk A, Bratås O, Grønning K. Twelvemonth effect of chronic pain self-management intervention delivered in an easily accessible primary healthcare service - a randomised controlled trial. BMC Health Serv Res. 2018;18(1):1012. doi:10.1186/s12913-018-3843-x
- Blyth FM, March LM, Nicholas MK, Cousins MJ. Selfmanagement of chronic pain: a population-based study. Pain. 2005;113(3):285-292. doi:10.1016/j.pain.2004.12.004
- Damush TM, Kroenke K, Bair MJ, et al. Pain self-management training increases self-efficacy, self-management behaviours and pain and depression outcomes. Eur J Pain. 2016;20(7):1070-1078. doi:10.1002/ejp.830
- Murphy JL, Palyo SA, Schmidt ZS, et al. The resurrection of interdisciplinary pain rehabilitation: outcomes across a veterans affairs collaborative. Pain Med. 2021;22(2):430- 443. doi:10.1093/pm/pnaa417
- Brain K, Burrows TL, Bruggink L, et al. Diet and chronic non-cancer pain: the state of the art and future directions. J Clin Med. 2021;10(21):5203. doi:10.3390/jcm10215203
- Field R, Pourkazemi F, Turton J, Rooney K. Dietary interventions are beneficial for patients with chronic pain: a systematic review with meta-analysis. Pain Med). 2021;22(3):694-714. doi:10.1093/pm/pnaa378
- Bjørklund G, Aaseth J, Do§a MD, et al. Does diet play a role in reducing nociception related to inflammation and chronic pain? Nutrition. 2019;66:153-165. doi:10.1016/j.nut.2019.04.007
- Kaushik AS, Strath LJ, Sorge RE. Dietary interventions for treatment of chronic pain: oxidative stress and inflammation. Pain Ther. 2020;9(2):487-498. doi:10.1007/s40122-020-00200-5
- Boswell JF, Hepner KA, Lysell K, et al. The need for a measurement-based care professional practice guideline. Psychotherapy (Chic). 2023;60(1):1-16. doi:10.1037/pst0000439
- Lund BC, Ohl ME, Hadlandsmyth K, Mosher HJ. Regional and rural-urban variation in opioid prescribing in the Veterans Health Administration. Mil Med. 2019;184(11-12):894- 900. doi:10.1093/milmed/usz104
- US Department of Veterans Affairs, Office of Rural Health. Rural veterans. Updated May 14, 2024. Accessed June 11, 2024. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp
- McCarthy JF, Blow FC, Ignacio R V., Ilgen MA, Austin KL, Valenstein M. Suicide among patients in the Veterans Affairs health system: rural-urban differences in rates, risks, and methods. Am J Public Health. 2012;102 Suppl 1(suppl 1):S111-S117. doi:10.2105/AJPH.2011.300463
Patient and Support Person Satisfaction Following a Whole Health-Informed Interdisciplinary Pain Team Meeting
Patient and Support Person Satisfaction Following a Whole Health-Informed Interdisciplinary Pain Team Meeting
Clinical, Laboratory, and Trichoscopic Features of Pediatric Androgenetic Alopecia
Clinical, Laboratory, and Trichoscopic Features of Pediatric Androgenetic Alopecia
Androgenetic alopecia (AGA) is the most common type of hair loss after adolescence, with a high prevalence of 21.3% among males and 6.0% among females in China.1 In men, AGA manifests as diffuse hair loss in the frontal and temporal areas of the scalp; in women, it is characterized by thinning of the hair on the top of the head with a wide part and less recession of the frontal line. Although the specific pathogenesis of AGA still is unclear, it is believed to be related mainly to genetics and androgen levels.1 Androgenetic alopecia is not considered a life-threatening medical condition, but it can have a major impact on patients’ self-esteem and quality of life.
The prevalence of pediatric AGA has been steadily rising over the past few decades and is thought to be correlated to a hyperinsulinemic diet and elevated circulating androgens at younger ages, resulting in early onset in genetically susceptible children and adolescents.2,3 Additionally, studies have shown that early-onset AGA is associated with metabolic syndrome,4-6 which includes conditions such as obesity, insulin resistance, hyperglycemia, and dyslipidemia.7,8 Furthermore, polycystic ovary syndrome (PCOS) is commonly observed in adolescent girls with early-onset AGA. The condition is associated with hormonal imbalances, particularly elevated androgens, which can contribute to the early onset of AGA. In girls, these hormonal changes may accelerate hair thinning and hair loss, making AGA a potential early indicator of underlying PCOS.9,10
Available research on early-onset AGA in pediatric patients is limited, with most studies having a relatively small sample size and generalized findings. Data on pediatric AGA in China is scarce; therefore, the objective of this retrospective study was to analyze the clinical, laboratory, and trichoscopic features of AGA in 133 pediatric patients with AGA who visited the hair disease clinic of the Department of Dermatology at the First Affiliated Hospital of Nanjing Medical University (Nanjing, China), from January 2010 to December 2023.
Methods
Study Population—Pediatric patients with early-onset AGA who were registered for outpatient consultations at the hair disease clinic of the Department of Dermatology at The First Affiliated Hospital of Nanjing Medical University from January 2010 to December 2023 were included. Patients aged 18 years and younger with a definitive diagnosis of AGA were selected for data collection and analysis. Any uncertain information was confirmed through telephone follow-up with patients.
Collection of Demographic Information and Laboratory Tests—Patient demographics and medical history including age, sex, age at disease onset, and duration of AGA were collected from the electronic medical record. Height and weight also were collected to calculate patients’ body mass index (BMI). Detailed laboratory test results were recorded, including assessments of sex hormone—binding globulin (SHBG), vitamin D, testosterone, and ferritin.
Analysis of Comorbidities—Due to the influence of genetic factors on body composition, there are differences in how obesity is defined across racial populations. The World Health Organization international standard defines the term overweight as a BMI greater than 25 and obese as a BMI greater than 30; however, the World Health Organization recommends a lower definition standard for these classifications in the Chinese population. China established specific BMI standards for classification of patients as overweight (24.0.27.9 kg/m2) and obese (≥28 kg/m2).11 During outpatient consultations, a comprehensive medical history was obtained from each patient, including the presence of PCOS, acne, seborrheic dermatitis, hirsutism, and sleeping disorders. During routine outpatient assessments, experienced dermatologists (including W.F.) determined the presence of symptoms and confirmed the diagnosis.
Hair Loss Classification and Trichoscopy—Hair loss patterns for male patients were assessed using the basic and specific classification system, while the Ludwig scale was utilized for female patients.12,13 Trichoscopy was utilized with high-resolution imaging systems and advanced software for image analysis, enabling precise assessment of hair in different scalp regions. Parameters such as hair density, hair diameter, percentage of terminal hairs, and percentage of vellus hair were recorded to monitor changes in hair growth for the patients.
Statistical Analysis—Categorical data were analyzed using the x2 test. A P value less than .05 was considered statistically significant. All statistical analyses were conducted using SPSS software version 26 (IBM).
Results
Patient Characteristics and Hair Loss Patterns—A sample of 133 pediatric patients (60 males, 73 females) who were diagnosed with AGA at the hair disease clinic of the Department of Dermatology at the First Affiliated Hospital of Nanjing Medical University from January 2010 to December 2023 were selected. The mean age of the patients was 15.5 years (range, 10–18 years). The mean age was slightly lower in females compared with males (15.05 vs 16.19 years, respectively). Additionally, females showed earlier onset of the disease, with a mean age at onset of 13.41 years compared to 14.44 years in males. The time between onset of AGA symptoms and first seeking medical care ranged from 4 months to 3 years, with a mean disease duration of 1.72 years. There was no significant difference in the duration of disease between males and females (1.76 and 1.70 years, respectively). Patient characteristics by age group are summarized in eTable 1.
The pediatric patients in our study exhibited hair loss patterns similar to those typically observed in adults. Male patients typically showed diffuse thinning on the crown and varying degrees of temporal thinning, while female patients demonstrated diffuse thinning on the crown with a preserved frontal hairline; however, 5 (8.3%) male patients presented with Christmas tree– like pattern of hair loss with a preserved hairline and a thinning crown (Figures 1 and 2).
Diffuse thinning of the hair on the crown demonstrated a Christmas tree-like pattern with a preserved frontal hairline.
BMI and Comorbidities—Among our study sample, 27.1% (36/133) of patients were identified as overweight or obese. It came to our attention that the prevalence of patients who were overweight and obese was notably higher in patients aged older than 14 but younger than 18 years compared with those aged 14 years or younger (24.1% vs 3.0% [32/133 vs 4/133]). A more detailed analysis of patients who were overweight and obese is outlined in eTable 2.
Seborrheic dermatitis was identified as the most prevalent comorbidity associated with pediatric AGA (51.9% [69/133]), followed by acne (42.8% [57/133]), hirsutism (33.1% [44/133]), and sleep disturbances/insomnia (28.6% [38/133]). The prevalence of these comorbidities varied by age group, with a higher incidence observed among patients aged older than 14 years as compared to those aged 14 years or younger.
Family History of AGA—Our study results indicated that most (78.2% [104/133]) patients had a family history of AGA. Among males and females, 81.7% and 75.3% (49/60 and 55/73) had a positive family history, respectively. Further analysis showed that 43.3% (26/60) of males and 21.9% (16/73) of females reported AGA in their father, while 16.7% (10/60) of males and 35.6% insert (26/73) of females reported AGA in their mother. Both parents were affected in 21.7% (13/60) of male patients and 17.8% (13/73) of female patients (eTable 3).
Related Laboratory Tests of Pediatric Patients With AGA—The results of laboratory testing for vitamin D deficiency, low SHBG, high testosterone, and low ferritin levels in the study sample are outlined in eTable 4. Among the study participants, 15.9% (10/63) of females exhibited increased levels of both free and total testosterone. Low SHBG was observed in 47.1% (56/119) of patients, with a slightly higher proportion in males (48.2% [27/56] than females (46.0% [29/63]). Vitamin D deficiency was prevalent in 60.5% (72/119) of the study population, with a higher incidence rate in females (71.4%[45/63]) compared to males (48.2%[27/56]). Moreover, 21.8% (26/119) of pediatric patients had low ferritin levels, with a higher incidence rate in females (33.3%[21/63]) compared to males (8.9%[5/56]).
Female Patients With PCOS—In our study, 6 (8.2%) female patients with AGA had been diagnosed with PCOS prior to their referral to the First Affiliated Hospital of Nanjing Medical University. Information regarding their age at treatment, hair loss grade, comorbidities, and laboratory test results is provided in eTable 5.
Degree of Hair Loss at First Visit—In male pediatric patients with AGA, the majority were classified as M type according to the basic and specific classification. Specifically, the main hairloss level in males was concentrated in M1 and M2 (80.0% [48/60]), while specific type F was mainly distributed in F1 and F2 (81.7% [49/60]), and specific type V was mainly distributed in V1 and V2 (80.0% [48/60]). On the other hand, female patients were mainly (87.7% [64/73]) classified as type I or II in the Ludwig scale.
Clinical Features of Trichoscopy Examinations at First Visit—We present the trichoscopic findings of our study regarding hair characteristics, including hair density, hair diameter, terminal hair ratio, and vellus hair ratio, among male and female pediatric participants stratified into 2 age groups: 14 years or younger, and older than 14 but younger than 18 years. In males, those aged 14 years or younger had a lower average hair density than those older than 14 years but thicker hair diameter. Conversely, males aged 14 years and older were more likely to seek treatment of hair loss than those aged 14 years or younger. Among females, those older than 14 years had higher hair density, hair diameter, and terminal hair ratio than those younger than 14 years. Hair trichoscopy characteristics among pediatric patients with AGA in our study population were similar to those of adults with AGA (Figure 3).
Efficacy and Adverse Effects of Topical Minoxidil—There were 56 (42.1%) patients who had used topical minoxidil for more than 6 months: 33 (58.9%) males and 23 (41.1%) females. In terms of efficacy, 51 (91.1%) patients responded positively, demonstrating improved scalp coverage, increased hair density, or greater hair diameter. There were 2 (3.6%) cases of minor adverse reactions: 1 case of scalp itching with increased dandruff that improved with local symptomatic treatment, and 1 case of hirsutism, which improved after discontinuing the drug. Among the 28 (50.0%) pediatric patients who used topical minoxidil for more than 12 months, there were no reported adverse reactions. Overall, topical minoxidil was effective and well tolerated in pediatric patients, with mild adverse reactions.
Comment
In our study, the youngest AGA patient was 10 years old, which is slightly older than a 6-year-old patient reported in the literature.14 Females showed a higher incidence of AGA compared to males, which is consistent with some previous studies14,15 but contradicts the findings of Gonzalez et al16 and Kim et al.17 We speculate that the differences in AGA incidence could be attributed to the diverse genetic background and racial disparities between the populations included in the study by Gonzalez et al16—primarily White patients from Europe and the United States—and our study, which included individuals from East Asia. Furthermore, variations in lifestyle and environment in Europe and the United States vs Asia (eg, dietary habits, stress, environmental pollution) may contribute to the differing sexspecific incidence rates. Additionally, our study showed that female patients tended to experience AGA at a younger age than male patients, as indicated by younger age of disease onset and at the initial visit. These findings are consistent with other studies reporting a slightly younger age of disease onset in female patients.14,16,17 The importance lies in raising awareness among both patients and physicians about early-onset AGA, facilitating earlier detection, diagnosis, and treatment. Furthermore, our study revealed a higher prevalence of a positive family history of AGA in our study population (78.2%) compared to other studies.14 Paternal family history was more commonly observed than maternal history (81.7% and 75.3%, respectively); moreover, 19.5% of patients reported a positive family history of AGA in both parents. Therefore, it is essential to raise awareness among pediatric patients with a positive family history of AGA, as they may experience hair loss at a younger age.
Patients with AGA commonly present with concurrent skin conditions, most notably acne, seborrheic dermatitis, and hirsutism. Therefore, it is important to monitor these associated diseases and adopt appropriate treatments. Moreover, it is worth mentioning that a considerable number of pediatric patients reported experiencing sleep difficulties. It is well known that sleep disturbances can lead to hormonal abnormalities, which are also a risk factor for AGA.18-20 Therefore, further research is needed to investigate whether treating sleep disturbances can delay onset or progression of pediatric AGA. A previous retrospective study reported a PCOS prevalence of 47.4% (9/19) in adolescent females with AGA,16 but our study observed a much lower incidence of 4.5%. This discrepancy may be due to the fact that diagnostic imaging was not required for all female patients suspected of having PCOS in our study, which may have resulted in the exclusion of some undiagnosed PCOS cases from the data analysis.
In our study, a considerable proportion of patients exhibited moderate hair loss at their first visit, and there were differences in hair density and diameter among different age groups, with female patients having finer hair than male patients. Therefore, it is necessary to raise awareness of and perform early diagnosis and treatment of AGA in pediatric patients presenting with hair loss. Upon evaluation of laboratory results, we observed a notable proportion of pediatric patients with AGA who had low levels of vitamin D, SHBG, and ferritin. Notably, female patients were more susceptible to low vitamin D levels compared with males. Screening for these indicators, particularly in female patients, could aid in the diagnosis and treatment of pediatric AGA. Surprisingly, testosterone levels did not show a significant increase in male patients with AGA. Furthermore, only a small percentage of female patients exhibited elevated testosterone levels, indicating that androgens may not play a dominant role in the pathogenesis of male pediatric AGA and that other factors and mechanisms may be involved. Although AGA has been extensively studied in adults, there is limited knowledge about its occurrence and characteristics in children and adolescents. Our study represents one of the few investigations into AGA in this population and is among the largest to explore the clinical features, laboratory testing and results, trichoscopic characteristics, and comorbidities in Chinese pediatric patients with AGA. Our findings offer valuable insights into early clinical characteristics of pediatric AGA in this specific demographic population to inform future research directions and clinical practice guidelines.
Given that we conducted a retrospective study with a relatively small sample size from a single clinic site, the generalizability of our research findings may be limited. In addition, the patients included in our study did not have frequent routine testing for metabolic and hormonal indicators to analyze further correlations between hormonal changes with severity of pediatric AGA. Future research with prospective multicenter designs and larger sample sizes are needed to increase representativeness and generalizability, and comprehensive testing is needed to validate and extend our findings. Furthermore, the psychological impact among pediatric patients with AGA warrants further investigation on early intervention to reduce psychological stress.
Besides enhancing the understanding of AGA in children and adolescents among dermatologists and pediatricians, there is a need for individualized, step-by-step, and comprehensive treatment. Initial assessment generally includes addressing hormonal disorders such as seborrheic dermatitis, folliculitis, PCOS, and acne. Some adult treatments may be effective in pediatric cases. In one study of 15 pediatric patients using minoxidil 5% daily (6 females, 4 males), 4 (66.7%) females had stable alopecia (follow-up, >6 months); 4 (44.4%) males using minoxidil 5% daily and 1 mg finasteride and 5 (55.6%) taking 1 mg of finasteride alone showed hair density gains.16 In another study,21 373 adolescents with AGA (286 boys, 87 girls; age range, 10–17 years) were treated with topical minoxidil solution over an 18-month period, with 95.0% responding positively: 54.0% showed improved scalp coverage, and 41.0% experienced slower hair thinning. Topical minoxidil generally is well tolerated in pediatric patients with no significant impact on blood pressure, pulse rate, or other vital signs.21 The primary adverse reactions to topical minoxidil observed in clinical practice are mild scalp irritation and increased facial hair, which usually resolve upon discontinuation.22 In China, topical minoxidil (available in 2% or 5% concentrations) commonly is used in children and adolescents, with adjustments made based on treatment response and adverse effects. Despite its proven efficacy and tolerability, it is essential that adverse effects be promptly communicated to health care providers for appropriate dosage adjustments, and that concurrent conditions, such as vitamin D and iron deficiencies, be adequately managed. Encouraging patients to adhere to prescribed medications and undergo long-term follow-up typically results in favorable outcomes.
- Jiang W, Yan Q, Tu P, et al. Chinese expert consensus on diagnosis and management of androgenic alopecia in both males and females. Int J Dermatol Venereol. 2019;3:195-202.
- Griggs J, Burroway B, Tosti A. Pediatric androgenetic alopecia: a review. J Am Acad Dermatol. 2021;85:1267-1273.
- Alfredo R, Andrea D, Flavia P. The diagnosis of androgenetic alopecia in children: considerations of pathophysiological plausibility. Australas J Dermatol. 2019;60:279-283.
- Sarkar P, Chakraborti K, Mondal S. Association of metabolic syndrome with early-onset androgenetic alopecia: a case-control study.
Iran J Dermatol. 2022;25:106-110. - Qiu Y, Zhou X, Fu S, et al. Systematic review and meta-analysis of the association between metabolic syndrome and androgenetic alopecia. Acta Derm Venereol. 2022;102:adv000645.
- Memon FH, Rahimoon AG. Androgenetic alopecia as a marker of metabolic syndrome. J Pharm Res Int. 2021;33:146-153.
- Rodríguez-Gutiérrez R, Salcido-Montenegro A, González-González JG. Early clinical expressions of insulin resistance: the real enemy to look for. Diabetes Ther. 2018;9:435-438.
- Wang YX, Chen XW, Wang SB, et al. Association between androgenic alopecia and coronary artery disease: a cross-sectional study of Han Chinese male population. Int J Gen Med. 2021;14:4809-4818.
- Tu YA, Lin SJ, Chen PL, et al. HSD3B1 gene polymorphism and female pattern hair loss in women with polycystic ovary syndrome. J Formos Med Assoc. 2019;118:1225-1231.
- Sanke S, Chander R, Jain A, et al. A comparison of the hormonal profile of early androgenetic alopecia in men with the phenotypic equivalent of polycystic ovarian syndrome in women. JAMA Dermatol. 2016;152:986-991.
- National Health Commission of the People’s Republic of China. (2021). Chinese Guidelines for the Prevention and Control of Overweight and Obesity in Adults.
- Lee WS, Ro BI, Hong SP. A new classification of pattern hair loss that is universal for men and women: basic and specific (BASP) classification. J Am Acad Dermatol. 2007;57:37-46.
- Ludwig, E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br J Dermatol. 1977;97:247-254.
- Tosti A, Iorizzo M, Piraccini BM. Androgenetic alopecia in children: report of 20 cases. Br J Dermatol. 2005;152:556-559.
- Özcan D. Pediatric androgenetic alopecia: a retrospective review of clinical characteristics, hormonal assays and metabolic syndrome risk factors in 23 patients. An Bras Dermatol. 2022;97:166-172.
- Gonzalez ME, Cantatore-Francis J, Orlow SJ. Androgenetic alopecia in the paediatric population: a retrospective review of 57 patients. Br J Dermatol. 2010;163:378-385.
- Kim BJ, Kim JY, Eun HC. Androgenetic alopecia in adolescents: a report of 43 cases. J Dermatol. 2006;33:696-699.
- B Liamsombut S, Pomsoong C, Kositkuljorn C. Sleep quality in men with androgenetic alopecia. Sleep Breath. 2023;27:371-378.
- Baik I, Lee S, Thomas RJ. Obstructive sleep apnea, low transferrin saturation levels, and male-pattern baldness. Int J Dermatol. 2019;58:67-74.
- Yi Y, Qiu J, Jia J. Severity of androgenetic alopecia associated with poor sleeping habits and carnivorous eating and junk food consumption—a web-based investigation of male pattern hair loss in China. Dermatol Ther. 2020;33:E13273.
- Price VH. Androgenetic alopecia in adolescents. Cutis. 2003;71:115-121.
- Gomes TF, Soares RO. Pediatric androgenetic alopecia: an updated review. J Dtsch Dermatol Ges. 2023;21:19-25.
Androgenetic alopecia (AGA) is the most common type of hair loss after adolescence, with a high prevalence of 21.3% among males and 6.0% among females in China.1 In men, AGA manifests as diffuse hair loss in the frontal and temporal areas of the scalp; in women, it is characterized by thinning of the hair on the top of the head with a wide part and less recession of the frontal line. Although the specific pathogenesis of AGA still is unclear, it is believed to be related mainly to genetics and androgen levels.1 Androgenetic alopecia is not considered a life-threatening medical condition, but it can have a major impact on patients’ self-esteem and quality of life.
The prevalence of pediatric AGA has been steadily rising over the past few decades and is thought to be correlated to a hyperinsulinemic diet and elevated circulating androgens at younger ages, resulting in early onset in genetically susceptible children and adolescents.2,3 Additionally, studies have shown that early-onset AGA is associated with metabolic syndrome,4-6 which includes conditions such as obesity, insulin resistance, hyperglycemia, and dyslipidemia.7,8 Furthermore, polycystic ovary syndrome (PCOS) is commonly observed in adolescent girls with early-onset AGA. The condition is associated with hormonal imbalances, particularly elevated androgens, which can contribute to the early onset of AGA. In girls, these hormonal changes may accelerate hair thinning and hair loss, making AGA a potential early indicator of underlying PCOS.9,10
Available research on early-onset AGA in pediatric patients is limited, with most studies having a relatively small sample size and generalized findings. Data on pediatric AGA in China is scarce; therefore, the objective of this retrospective study was to analyze the clinical, laboratory, and trichoscopic features of AGA in 133 pediatric patients with AGA who visited the hair disease clinic of the Department of Dermatology at the First Affiliated Hospital of Nanjing Medical University (Nanjing, China), from January 2010 to December 2023.
Methods
Study Population—Pediatric patients with early-onset AGA who were registered for outpatient consultations at the hair disease clinic of the Department of Dermatology at The First Affiliated Hospital of Nanjing Medical University from January 2010 to December 2023 were included. Patients aged 18 years and younger with a definitive diagnosis of AGA were selected for data collection and analysis. Any uncertain information was confirmed through telephone follow-up with patients.
Collection of Demographic Information and Laboratory Tests—Patient demographics and medical history including age, sex, age at disease onset, and duration of AGA were collected from the electronic medical record. Height and weight also were collected to calculate patients’ body mass index (BMI). Detailed laboratory test results were recorded, including assessments of sex hormone—binding globulin (SHBG), vitamin D, testosterone, and ferritin.
Analysis of Comorbidities—Due to the influence of genetic factors on body composition, there are differences in how obesity is defined across racial populations. The World Health Organization international standard defines the term overweight as a BMI greater than 25 and obese as a BMI greater than 30; however, the World Health Organization recommends a lower definition standard for these classifications in the Chinese population. China established specific BMI standards for classification of patients as overweight (24.0.27.9 kg/m2) and obese (≥28 kg/m2).11 During outpatient consultations, a comprehensive medical history was obtained from each patient, including the presence of PCOS, acne, seborrheic dermatitis, hirsutism, and sleeping disorders. During routine outpatient assessments, experienced dermatologists (including W.F.) determined the presence of symptoms and confirmed the diagnosis.
Hair Loss Classification and Trichoscopy—Hair loss patterns for male patients were assessed using the basic and specific classification system, while the Ludwig scale was utilized for female patients.12,13 Trichoscopy was utilized with high-resolution imaging systems and advanced software for image analysis, enabling precise assessment of hair in different scalp regions. Parameters such as hair density, hair diameter, percentage of terminal hairs, and percentage of vellus hair were recorded to monitor changes in hair growth for the patients.
Statistical Analysis—Categorical data were analyzed using the x2 test. A P value less than .05 was considered statistically significant. All statistical analyses were conducted using SPSS software version 26 (IBM).
Results
Patient Characteristics and Hair Loss Patterns—A sample of 133 pediatric patients (60 males, 73 females) who were diagnosed with AGA at the hair disease clinic of the Department of Dermatology at the First Affiliated Hospital of Nanjing Medical University from January 2010 to December 2023 were selected. The mean age of the patients was 15.5 years (range, 10–18 years). The mean age was slightly lower in females compared with males (15.05 vs 16.19 years, respectively). Additionally, females showed earlier onset of the disease, with a mean age at onset of 13.41 years compared to 14.44 years in males. The time between onset of AGA symptoms and first seeking medical care ranged from 4 months to 3 years, with a mean disease duration of 1.72 years. There was no significant difference in the duration of disease between males and females (1.76 and 1.70 years, respectively). Patient characteristics by age group are summarized in eTable 1.
The pediatric patients in our study exhibited hair loss patterns similar to those typically observed in adults. Male patients typically showed diffuse thinning on the crown and varying degrees of temporal thinning, while female patients demonstrated diffuse thinning on the crown with a preserved frontal hairline; however, 5 (8.3%) male patients presented with Christmas tree– like pattern of hair loss with a preserved hairline and a thinning crown (Figures 1 and 2).
Diffuse thinning of the hair on the crown demonstrated a Christmas tree-like pattern with a preserved frontal hairline.
BMI and Comorbidities—Among our study sample, 27.1% (36/133) of patients were identified as overweight or obese. It came to our attention that the prevalence of patients who were overweight and obese was notably higher in patients aged older than 14 but younger than 18 years compared with those aged 14 years or younger (24.1% vs 3.0% [32/133 vs 4/133]). A more detailed analysis of patients who were overweight and obese is outlined in eTable 2.
Seborrheic dermatitis was identified as the most prevalent comorbidity associated with pediatric AGA (51.9% [69/133]), followed by acne (42.8% [57/133]), hirsutism (33.1% [44/133]), and sleep disturbances/insomnia (28.6% [38/133]). The prevalence of these comorbidities varied by age group, with a higher incidence observed among patients aged older than 14 years as compared to those aged 14 years or younger.
Family History of AGA—Our study results indicated that most (78.2% [104/133]) patients had a family history of AGA. Among males and females, 81.7% and 75.3% (49/60 and 55/73) had a positive family history, respectively. Further analysis showed that 43.3% (26/60) of males and 21.9% (16/73) of females reported AGA in their father, while 16.7% (10/60) of males and 35.6% insert (26/73) of females reported AGA in their mother. Both parents were affected in 21.7% (13/60) of male patients and 17.8% (13/73) of female patients (eTable 3).
Related Laboratory Tests of Pediatric Patients With AGA—The results of laboratory testing for vitamin D deficiency, low SHBG, high testosterone, and low ferritin levels in the study sample are outlined in eTable 4. Among the study participants, 15.9% (10/63) of females exhibited increased levels of both free and total testosterone. Low SHBG was observed in 47.1% (56/119) of patients, with a slightly higher proportion in males (48.2% [27/56] than females (46.0% [29/63]). Vitamin D deficiency was prevalent in 60.5% (72/119) of the study population, with a higher incidence rate in females (71.4%[45/63]) compared to males (48.2%[27/56]). Moreover, 21.8% (26/119) of pediatric patients had low ferritin levels, with a higher incidence rate in females (33.3%[21/63]) compared to males (8.9%[5/56]).
Female Patients With PCOS—In our study, 6 (8.2%) female patients with AGA had been diagnosed with PCOS prior to their referral to the First Affiliated Hospital of Nanjing Medical University. Information regarding their age at treatment, hair loss grade, comorbidities, and laboratory test results is provided in eTable 5.
Degree of Hair Loss at First Visit—In male pediatric patients with AGA, the majority were classified as M type according to the basic and specific classification. Specifically, the main hairloss level in males was concentrated in M1 and M2 (80.0% [48/60]), while specific type F was mainly distributed in F1 and F2 (81.7% [49/60]), and specific type V was mainly distributed in V1 and V2 (80.0% [48/60]). On the other hand, female patients were mainly (87.7% [64/73]) classified as type I or II in the Ludwig scale.
Clinical Features of Trichoscopy Examinations at First Visit—We present the trichoscopic findings of our study regarding hair characteristics, including hair density, hair diameter, terminal hair ratio, and vellus hair ratio, among male and female pediatric participants stratified into 2 age groups: 14 years or younger, and older than 14 but younger than 18 years. In males, those aged 14 years or younger had a lower average hair density than those older than 14 years but thicker hair diameter. Conversely, males aged 14 years and older were more likely to seek treatment of hair loss than those aged 14 years or younger. Among females, those older than 14 years had higher hair density, hair diameter, and terminal hair ratio than those younger than 14 years. Hair trichoscopy characteristics among pediatric patients with AGA in our study population were similar to those of adults with AGA (Figure 3).
Efficacy and Adverse Effects of Topical Minoxidil—There were 56 (42.1%) patients who had used topical minoxidil for more than 6 months: 33 (58.9%) males and 23 (41.1%) females. In terms of efficacy, 51 (91.1%) patients responded positively, demonstrating improved scalp coverage, increased hair density, or greater hair diameter. There were 2 (3.6%) cases of minor adverse reactions: 1 case of scalp itching with increased dandruff that improved with local symptomatic treatment, and 1 case of hirsutism, which improved after discontinuing the drug. Among the 28 (50.0%) pediatric patients who used topical minoxidil for more than 12 months, there were no reported adverse reactions. Overall, topical minoxidil was effective and well tolerated in pediatric patients, with mild adverse reactions.
Comment
In our study, the youngest AGA patient was 10 years old, which is slightly older than a 6-year-old patient reported in the literature.14 Females showed a higher incidence of AGA compared to males, which is consistent with some previous studies14,15 but contradicts the findings of Gonzalez et al16 and Kim et al.17 We speculate that the differences in AGA incidence could be attributed to the diverse genetic background and racial disparities between the populations included in the study by Gonzalez et al16—primarily White patients from Europe and the United States—and our study, which included individuals from East Asia. Furthermore, variations in lifestyle and environment in Europe and the United States vs Asia (eg, dietary habits, stress, environmental pollution) may contribute to the differing sexspecific incidence rates. Additionally, our study showed that female patients tended to experience AGA at a younger age than male patients, as indicated by younger age of disease onset and at the initial visit. These findings are consistent with other studies reporting a slightly younger age of disease onset in female patients.14,16,17 The importance lies in raising awareness among both patients and physicians about early-onset AGA, facilitating earlier detection, diagnosis, and treatment. Furthermore, our study revealed a higher prevalence of a positive family history of AGA in our study population (78.2%) compared to other studies.14 Paternal family history was more commonly observed than maternal history (81.7% and 75.3%, respectively); moreover, 19.5% of patients reported a positive family history of AGA in both parents. Therefore, it is essential to raise awareness among pediatric patients with a positive family history of AGA, as they may experience hair loss at a younger age.
Patients with AGA commonly present with concurrent skin conditions, most notably acne, seborrheic dermatitis, and hirsutism. Therefore, it is important to monitor these associated diseases and adopt appropriate treatments. Moreover, it is worth mentioning that a considerable number of pediatric patients reported experiencing sleep difficulties. It is well known that sleep disturbances can lead to hormonal abnormalities, which are also a risk factor for AGA.18-20 Therefore, further research is needed to investigate whether treating sleep disturbances can delay onset or progression of pediatric AGA. A previous retrospective study reported a PCOS prevalence of 47.4% (9/19) in adolescent females with AGA,16 but our study observed a much lower incidence of 4.5%. This discrepancy may be due to the fact that diagnostic imaging was not required for all female patients suspected of having PCOS in our study, which may have resulted in the exclusion of some undiagnosed PCOS cases from the data analysis.
In our study, a considerable proportion of patients exhibited moderate hair loss at their first visit, and there were differences in hair density and diameter among different age groups, with female patients having finer hair than male patients. Therefore, it is necessary to raise awareness of and perform early diagnosis and treatment of AGA in pediatric patients presenting with hair loss. Upon evaluation of laboratory results, we observed a notable proportion of pediatric patients with AGA who had low levels of vitamin D, SHBG, and ferritin. Notably, female patients were more susceptible to low vitamin D levels compared with males. Screening for these indicators, particularly in female patients, could aid in the diagnosis and treatment of pediatric AGA. Surprisingly, testosterone levels did not show a significant increase in male patients with AGA. Furthermore, only a small percentage of female patients exhibited elevated testosterone levels, indicating that androgens may not play a dominant role in the pathogenesis of male pediatric AGA and that other factors and mechanisms may be involved. Although AGA has been extensively studied in adults, there is limited knowledge about its occurrence and characteristics in children and adolescents. Our study represents one of the few investigations into AGA in this population and is among the largest to explore the clinical features, laboratory testing and results, trichoscopic characteristics, and comorbidities in Chinese pediatric patients with AGA. Our findings offer valuable insights into early clinical characteristics of pediatric AGA in this specific demographic population to inform future research directions and clinical practice guidelines.
Given that we conducted a retrospective study with a relatively small sample size from a single clinic site, the generalizability of our research findings may be limited. In addition, the patients included in our study did not have frequent routine testing for metabolic and hormonal indicators to analyze further correlations between hormonal changes with severity of pediatric AGA. Future research with prospective multicenter designs and larger sample sizes are needed to increase representativeness and generalizability, and comprehensive testing is needed to validate and extend our findings. Furthermore, the psychological impact among pediatric patients with AGA warrants further investigation on early intervention to reduce psychological stress.
Besides enhancing the understanding of AGA in children and adolescents among dermatologists and pediatricians, there is a need for individualized, step-by-step, and comprehensive treatment. Initial assessment generally includes addressing hormonal disorders such as seborrheic dermatitis, folliculitis, PCOS, and acne. Some adult treatments may be effective in pediatric cases. In one study of 15 pediatric patients using minoxidil 5% daily (6 females, 4 males), 4 (66.7%) females had stable alopecia (follow-up, >6 months); 4 (44.4%) males using minoxidil 5% daily and 1 mg finasteride and 5 (55.6%) taking 1 mg of finasteride alone showed hair density gains.16 In another study,21 373 adolescents with AGA (286 boys, 87 girls; age range, 10–17 years) were treated with topical minoxidil solution over an 18-month period, with 95.0% responding positively: 54.0% showed improved scalp coverage, and 41.0% experienced slower hair thinning. Topical minoxidil generally is well tolerated in pediatric patients with no significant impact on blood pressure, pulse rate, or other vital signs.21 The primary adverse reactions to topical minoxidil observed in clinical practice are mild scalp irritation and increased facial hair, which usually resolve upon discontinuation.22 In China, topical minoxidil (available in 2% or 5% concentrations) commonly is used in children and adolescents, with adjustments made based on treatment response and adverse effects. Despite its proven efficacy and tolerability, it is essential that adverse effects be promptly communicated to health care providers for appropriate dosage adjustments, and that concurrent conditions, such as vitamin D and iron deficiencies, be adequately managed. Encouraging patients to adhere to prescribed medications and undergo long-term follow-up typically results in favorable outcomes.
Androgenetic alopecia (AGA) is the most common type of hair loss after adolescence, with a high prevalence of 21.3% among males and 6.0% among females in China.1 In men, AGA manifests as diffuse hair loss in the frontal and temporal areas of the scalp; in women, it is characterized by thinning of the hair on the top of the head with a wide part and less recession of the frontal line. Although the specific pathogenesis of AGA still is unclear, it is believed to be related mainly to genetics and androgen levels.1 Androgenetic alopecia is not considered a life-threatening medical condition, but it can have a major impact on patients’ self-esteem and quality of life.
The prevalence of pediatric AGA has been steadily rising over the past few decades and is thought to be correlated to a hyperinsulinemic diet and elevated circulating androgens at younger ages, resulting in early onset in genetically susceptible children and adolescents.2,3 Additionally, studies have shown that early-onset AGA is associated with metabolic syndrome,4-6 which includes conditions such as obesity, insulin resistance, hyperglycemia, and dyslipidemia.7,8 Furthermore, polycystic ovary syndrome (PCOS) is commonly observed in adolescent girls with early-onset AGA. The condition is associated with hormonal imbalances, particularly elevated androgens, which can contribute to the early onset of AGA. In girls, these hormonal changes may accelerate hair thinning and hair loss, making AGA a potential early indicator of underlying PCOS.9,10
Available research on early-onset AGA in pediatric patients is limited, with most studies having a relatively small sample size and generalized findings. Data on pediatric AGA in China is scarce; therefore, the objective of this retrospective study was to analyze the clinical, laboratory, and trichoscopic features of AGA in 133 pediatric patients with AGA who visited the hair disease clinic of the Department of Dermatology at the First Affiliated Hospital of Nanjing Medical University (Nanjing, China), from January 2010 to December 2023.
Methods
Study Population—Pediatric patients with early-onset AGA who were registered for outpatient consultations at the hair disease clinic of the Department of Dermatology at The First Affiliated Hospital of Nanjing Medical University from January 2010 to December 2023 were included. Patients aged 18 years and younger with a definitive diagnosis of AGA were selected for data collection and analysis. Any uncertain information was confirmed through telephone follow-up with patients.
Collection of Demographic Information and Laboratory Tests—Patient demographics and medical history including age, sex, age at disease onset, and duration of AGA were collected from the electronic medical record. Height and weight also were collected to calculate patients’ body mass index (BMI). Detailed laboratory test results were recorded, including assessments of sex hormone—binding globulin (SHBG), vitamin D, testosterone, and ferritin.
Analysis of Comorbidities—Due to the influence of genetic factors on body composition, there are differences in how obesity is defined across racial populations. The World Health Organization international standard defines the term overweight as a BMI greater than 25 and obese as a BMI greater than 30; however, the World Health Organization recommends a lower definition standard for these classifications in the Chinese population. China established specific BMI standards for classification of patients as overweight (24.0.27.9 kg/m2) and obese (≥28 kg/m2).11 During outpatient consultations, a comprehensive medical history was obtained from each patient, including the presence of PCOS, acne, seborrheic dermatitis, hirsutism, and sleeping disorders. During routine outpatient assessments, experienced dermatologists (including W.F.) determined the presence of symptoms and confirmed the diagnosis.
Hair Loss Classification and Trichoscopy—Hair loss patterns for male patients were assessed using the basic and specific classification system, while the Ludwig scale was utilized for female patients.12,13 Trichoscopy was utilized with high-resolution imaging systems and advanced software for image analysis, enabling precise assessment of hair in different scalp regions. Parameters such as hair density, hair diameter, percentage of terminal hairs, and percentage of vellus hair were recorded to monitor changes in hair growth for the patients.
Statistical Analysis—Categorical data were analyzed using the x2 test. A P value less than .05 was considered statistically significant. All statistical analyses were conducted using SPSS software version 26 (IBM).
Results
Patient Characteristics and Hair Loss Patterns—A sample of 133 pediatric patients (60 males, 73 females) who were diagnosed with AGA at the hair disease clinic of the Department of Dermatology at the First Affiliated Hospital of Nanjing Medical University from January 2010 to December 2023 were selected. The mean age of the patients was 15.5 years (range, 10–18 years). The mean age was slightly lower in females compared with males (15.05 vs 16.19 years, respectively). Additionally, females showed earlier onset of the disease, with a mean age at onset of 13.41 years compared to 14.44 years in males. The time between onset of AGA symptoms and first seeking medical care ranged from 4 months to 3 years, with a mean disease duration of 1.72 years. There was no significant difference in the duration of disease between males and females (1.76 and 1.70 years, respectively). Patient characteristics by age group are summarized in eTable 1.
The pediatric patients in our study exhibited hair loss patterns similar to those typically observed in adults. Male patients typically showed diffuse thinning on the crown and varying degrees of temporal thinning, while female patients demonstrated diffuse thinning on the crown with a preserved frontal hairline; however, 5 (8.3%) male patients presented with Christmas tree– like pattern of hair loss with a preserved hairline and a thinning crown (Figures 1 and 2).
Diffuse thinning of the hair on the crown demonstrated a Christmas tree-like pattern with a preserved frontal hairline.
BMI and Comorbidities—Among our study sample, 27.1% (36/133) of patients were identified as overweight or obese. It came to our attention that the prevalence of patients who were overweight and obese was notably higher in patients aged older than 14 but younger than 18 years compared with those aged 14 years or younger (24.1% vs 3.0% [32/133 vs 4/133]). A more detailed analysis of patients who were overweight and obese is outlined in eTable 2.
Seborrheic dermatitis was identified as the most prevalent comorbidity associated with pediatric AGA (51.9% [69/133]), followed by acne (42.8% [57/133]), hirsutism (33.1% [44/133]), and sleep disturbances/insomnia (28.6% [38/133]). The prevalence of these comorbidities varied by age group, with a higher incidence observed among patients aged older than 14 years as compared to those aged 14 years or younger.
Family History of AGA—Our study results indicated that most (78.2% [104/133]) patients had a family history of AGA. Among males and females, 81.7% and 75.3% (49/60 and 55/73) had a positive family history, respectively. Further analysis showed that 43.3% (26/60) of males and 21.9% (16/73) of females reported AGA in their father, while 16.7% (10/60) of males and 35.6% insert (26/73) of females reported AGA in their mother. Both parents were affected in 21.7% (13/60) of male patients and 17.8% (13/73) of female patients (eTable 3).
Related Laboratory Tests of Pediatric Patients With AGA—The results of laboratory testing for vitamin D deficiency, low SHBG, high testosterone, and low ferritin levels in the study sample are outlined in eTable 4. Among the study participants, 15.9% (10/63) of females exhibited increased levels of both free and total testosterone. Low SHBG was observed in 47.1% (56/119) of patients, with a slightly higher proportion in males (48.2% [27/56] than females (46.0% [29/63]). Vitamin D deficiency was prevalent in 60.5% (72/119) of the study population, with a higher incidence rate in females (71.4%[45/63]) compared to males (48.2%[27/56]). Moreover, 21.8% (26/119) of pediatric patients had low ferritin levels, with a higher incidence rate in females (33.3%[21/63]) compared to males (8.9%[5/56]).
Female Patients With PCOS—In our study, 6 (8.2%) female patients with AGA had been diagnosed with PCOS prior to their referral to the First Affiliated Hospital of Nanjing Medical University. Information regarding their age at treatment, hair loss grade, comorbidities, and laboratory test results is provided in eTable 5.
Degree of Hair Loss at First Visit—In male pediatric patients with AGA, the majority were classified as M type according to the basic and specific classification. Specifically, the main hairloss level in males was concentrated in M1 and M2 (80.0% [48/60]), while specific type F was mainly distributed in F1 and F2 (81.7% [49/60]), and specific type V was mainly distributed in V1 and V2 (80.0% [48/60]). On the other hand, female patients were mainly (87.7% [64/73]) classified as type I or II in the Ludwig scale.
Clinical Features of Trichoscopy Examinations at First Visit—We present the trichoscopic findings of our study regarding hair characteristics, including hair density, hair diameter, terminal hair ratio, and vellus hair ratio, among male and female pediatric participants stratified into 2 age groups: 14 years or younger, and older than 14 but younger than 18 years. In males, those aged 14 years or younger had a lower average hair density than those older than 14 years but thicker hair diameter. Conversely, males aged 14 years and older were more likely to seek treatment of hair loss than those aged 14 years or younger. Among females, those older than 14 years had higher hair density, hair diameter, and terminal hair ratio than those younger than 14 years. Hair trichoscopy characteristics among pediatric patients with AGA in our study population were similar to those of adults with AGA (Figure 3).
Efficacy and Adverse Effects of Topical Minoxidil—There were 56 (42.1%) patients who had used topical minoxidil for more than 6 months: 33 (58.9%) males and 23 (41.1%) females. In terms of efficacy, 51 (91.1%) patients responded positively, demonstrating improved scalp coverage, increased hair density, or greater hair diameter. There were 2 (3.6%) cases of minor adverse reactions: 1 case of scalp itching with increased dandruff that improved with local symptomatic treatment, and 1 case of hirsutism, which improved after discontinuing the drug. Among the 28 (50.0%) pediatric patients who used topical minoxidil for more than 12 months, there were no reported adverse reactions. Overall, topical minoxidil was effective and well tolerated in pediatric patients, with mild adverse reactions.
Comment
In our study, the youngest AGA patient was 10 years old, which is slightly older than a 6-year-old patient reported in the literature.14 Females showed a higher incidence of AGA compared to males, which is consistent with some previous studies14,15 but contradicts the findings of Gonzalez et al16 and Kim et al.17 We speculate that the differences in AGA incidence could be attributed to the diverse genetic background and racial disparities between the populations included in the study by Gonzalez et al16—primarily White patients from Europe and the United States—and our study, which included individuals from East Asia. Furthermore, variations in lifestyle and environment in Europe and the United States vs Asia (eg, dietary habits, stress, environmental pollution) may contribute to the differing sexspecific incidence rates. Additionally, our study showed that female patients tended to experience AGA at a younger age than male patients, as indicated by younger age of disease onset and at the initial visit. These findings are consistent with other studies reporting a slightly younger age of disease onset in female patients.14,16,17 The importance lies in raising awareness among both patients and physicians about early-onset AGA, facilitating earlier detection, diagnosis, and treatment. Furthermore, our study revealed a higher prevalence of a positive family history of AGA in our study population (78.2%) compared to other studies.14 Paternal family history was more commonly observed than maternal history (81.7% and 75.3%, respectively); moreover, 19.5% of patients reported a positive family history of AGA in both parents. Therefore, it is essential to raise awareness among pediatric patients with a positive family history of AGA, as they may experience hair loss at a younger age.
Patients with AGA commonly present with concurrent skin conditions, most notably acne, seborrheic dermatitis, and hirsutism. Therefore, it is important to monitor these associated diseases and adopt appropriate treatments. Moreover, it is worth mentioning that a considerable number of pediatric patients reported experiencing sleep difficulties. It is well known that sleep disturbances can lead to hormonal abnormalities, which are also a risk factor for AGA.18-20 Therefore, further research is needed to investigate whether treating sleep disturbances can delay onset or progression of pediatric AGA. A previous retrospective study reported a PCOS prevalence of 47.4% (9/19) in adolescent females with AGA,16 but our study observed a much lower incidence of 4.5%. This discrepancy may be due to the fact that diagnostic imaging was not required for all female patients suspected of having PCOS in our study, which may have resulted in the exclusion of some undiagnosed PCOS cases from the data analysis.
In our study, a considerable proportion of patients exhibited moderate hair loss at their first visit, and there were differences in hair density and diameter among different age groups, with female patients having finer hair than male patients. Therefore, it is necessary to raise awareness of and perform early diagnosis and treatment of AGA in pediatric patients presenting with hair loss. Upon evaluation of laboratory results, we observed a notable proportion of pediatric patients with AGA who had low levels of vitamin D, SHBG, and ferritin. Notably, female patients were more susceptible to low vitamin D levels compared with males. Screening for these indicators, particularly in female patients, could aid in the diagnosis and treatment of pediatric AGA. Surprisingly, testosterone levels did not show a significant increase in male patients with AGA. Furthermore, only a small percentage of female patients exhibited elevated testosterone levels, indicating that androgens may not play a dominant role in the pathogenesis of male pediatric AGA and that other factors and mechanisms may be involved. Although AGA has been extensively studied in adults, there is limited knowledge about its occurrence and characteristics in children and adolescents. Our study represents one of the few investigations into AGA in this population and is among the largest to explore the clinical features, laboratory testing and results, trichoscopic characteristics, and comorbidities in Chinese pediatric patients with AGA. Our findings offer valuable insights into early clinical characteristics of pediatric AGA in this specific demographic population to inform future research directions and clinical practice guidelines.
Given that we conducted a retrospective study with a relatively small sample size from a single clinic site, the generalizability of our research findings may be limited. In addition, the patients included in our study did not have frequent routine testing for metabolic and hormonal indicators to analyze further correlations between hormonal changes with severity of pediatric AGA. Future research with prospective multicenter designs and larger sample sizes are needed to increase representativeness and generalizability, and comprehensive testing is needed to validate and extend our findings. Furthermore, the psychological impact among pediatric patients with AGA warrants further investigation on early intervention to reduce psychological stress.
Besides enhancing the understanding of AGA in children and adolescents among dermatologists and pediatricians, there is a need for individualized, step-by-step, and comprehensive treatment. Initial assessment generally includes addressing hormonal disorders such as seborrheic dermatitis, folliculitis, PCOS, and acne. Some adult treatments may be effective in pediatric cases. In one study of 15 pediatric patients using minoxidil 5% daily (6 females, 4 males), 4 (66.7%) females had stable alopecia (follow-up, >6 months); 4 (44.4%) males using minoxidil 5% daily and 1 mg finasteride and 5 (55.6%) taking 1 mg of finasteride alone showed hair density gains.16 In another study,21 373 adolescents with AGA (286 boys, 87 girls; age range, 10–17 years) were treated with topical minoxidil solution over an 18-month period, with 95.0% responding positively: 54.0% showed improved scalp coverage, and 41.0% experienced slower hair thinning. Topical minoxidil generally is well tolerated in pediatric patients with no significant impact on blood pressure, pulse rate, or other vital signs.21 The primary adverse reactions to topical minoxidil observed in clinical practice are mild scalp irritation and increased facial hair, which usually resolve upon discontinuation.22 In China, topical minoxidil (available in 2% or 5% concentrations) commonly is used in children and adolescents, with adjustments made based on treatment response and adverse effects. Despite its proven efficacy and tolerability, it is essential that adverse effects be promptly communicated to health care providers for appropriate dosage adjustments, and that concurrent conditions, such as vitamin D and iron deficiencies, be adequately managed. Encouraging patients to adhere to prescribed medications and undergo long-term follow-up typically results in favorable outcomes.
- Jiang W, Yan Q, Tu P, et al. Chinese expert consensus on diagnosis and management of androgenic alopecia in both males and females. Int J Dermatol Venereol. 2019;3:195-202.
- Griggs J, Burroway B, Tosti A. Pediatric androgenetic alopecia: a review. J Am Acad Dermatol. 2021;85:1267-1273.
- Alfredo R, Andrea D, Flavia P. The diagnosis of androgenetic alopecia in children: considerations of pathophysiological plausibility. Australas J Dermatol. 2019;60:279-283.
- Sarkar P, Chakraborti K, Mondal S. Association of metabolic syndrome with early-onset androgenetic alopecia: a case-control study.
Iran J Dermatol. 2022;25:106-110. - Qiu Y, Zhou X, Fu S, et al. Systematic review and meta-analysis of the association between metabolic syndrome and androgenetic alopecia. Acta Derm Venereol. 2022;102:adv000645.
- Memon FH, Rahimoon AG. Androgenetic alopecia as a marker of metabolic syndrome. J Pharm Res Int. 2021;33:146-153.
- Rodríguez-Gutiérrez R, Salcido-Montenegro A, González-González JG. Early clinical expressions of insulin resistance: the real enemy to look for. Diabetes Ther. 2018;9:435-438.
- Wang YX, Chen XW, Wang SB, et al. Association between androgenic alopecia and coronary artery disease: a cross-sectional study of Han Chinese male population. Int J Gen Med. 2021;14:4809-4818.
- Tu YA, Lin SJ, Chen PL, et al. HSD3B1 gene polymorphism and female pattern hair loss in women with polycystic ovary syndrome. J Formos Med Assoc. 2019;118:1225-1231.
- Sanke S, Chander R, Jain A, et al. A comparison of the hormonal profile of early androgenetic alopecia in men with the phenotypic equivalent of polycystic ovarian syndrome in women. JAMA Dermatol. 2016;152:986-991.
- National Health Commission of the People’s Republic of China. (2021). Chinese Guidelines for the Prevention and Control of Overweight and Obesity in Adults.
- Lee WS, Ro BI, Hong SP. A new classification of pattern hair loss that is universal for men and women: basic and specific (BASP) classification. J Am Acad Dermatol. 2007;57:37-46.
- Ludwig, E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br J Dermatol. 1977;97:247-254.
- Tosti A, Iorizzo M, Piraccini BM. Androgenetic alopecia in children: report of 20 cases. Br J Dermatol. 2005;152:556-559.
- Özcan D. Pediatric androgenetic alopecia: a retrospective review of clinical characteristics, hormonal assays and metabolic syndrome risk factors in 23 patients. An Bras Dermatol. 2022;97:166-172.
- Gonzalez ME, Cantatore-Francis J, Orlow SJ. Androgenetic alopecia in the paediatric population: a retrospective review of 57 patients. Br J Dermatol. 2010;163:378-385.
- Kim BJ, Kim JY, Eun HC. Androgenetic alopecia in adolescents: a report of 43 cases. J Dermatol. 2006;33:696-699.
- B Liamsombut S, Pomsoong C, Kositkuljorn C. Sleep quality in men with androgenetic alopecia. Sleep Breath. 2023;27:371-378.
- Baik I, Lee S, Thomas RJ. Obstructive sleep apnea, low transferrin saturation levels, and male-pattern baldness. Int J Dermatol. 2019;58:67-74.
- Yi Y, Qiu J, Jia J. Severity of androgenetic alopecia associated with poor sleeping habits and carnivorous eating and junk food consumption—a web-based investigation of male pattern hair loss in China. Dermatol Ther. 2020;33:E13273.
- Price VH. Androgenetic alopecia in adolescents. Cutis. 2003;71:115-121.
- Gomes TF, Soares RO. Pediatric androgenetic alopecia: an updated review. J Dtsch Dermatol Ges. 2023;21:19-25.
- Jiang W, Yan Q, Tu P, et al. Chinese expert consensus on diagnosis and management of androgenic alopecia in both males and females. Int J Dermatol Venereol. 2019;3:195-202.
- Griggs J, Burroway B, Tosti A. Pediatric androgenetic alopecia: a review. J Am Acad Dermatol. 2021;85:1267-1273.
- Alfredo R, Andrea D, Flavia P. The diagnosis of androgenetic alopecia in children: considerations of pathophysiological plausibility. Australas J Dermatol. 2019;60:279-283.
- Sarkar P, Chakraborti K, Mondal S. Association of metabolic syndrome with early-onset androgenetic alopecia: a case-control study.
Iran J Dermatol. 2022;25:106-110. - Qiu Y, Zhou X, Fu S, et al. Systematic review and meta-analysis of the association between metabolic syndrome and androgenetic alopecia. Acta Derm Venereol. 2022;102:adv000645.
- Memon FH, Rahimoon AG. Androgenetic alopecia as a marker of metabolic syndrome. J Pharm Res Int. 2021;33:146-153.
- Rodríguez-Gutiérrez R, Salcido-Montenegro A, González-González JG. Early clinical expressions of insulin resistance: the real enemy to look for. Diabetes Ther. 2018;9:435-438.
- Wang YX, Chen XW, Wang SB, et al. Association between androgenic alopecia and coronary artery disease: a cross-sectional study of Han Chinese male population. Int J Gen Med. 2021;14:4809-4818.
- Tu YA, Lin SJ, Chen PL, et al. HSD3B1 gene polymorphism and female pattern hair loss in women with polycystic ovary syndrome. J Formos Med Assoc. 2019;118:1225-1231.
- Sanke S, Chander R, Jain A, et al. A comparison of the hormonal profile of early androgenetic alopecia in men with the phenotypic equivalent of polycystic ovarian syndrome in women. JAMA Dermatol. 2016;152:986-991.
- National Health Commission of the People’s Republic of China. (2021). Chinese Guidelines for the Prevention and Control of Overweight and Obesity in Adults.
- Lee WS, Ro BI, Hong SP. A new classification of pattern hair loss that is universal for men and women: basic and specific (BASP) classification. J Am Acad Dermatol. 2007;57:37-46.
- Ludwig, E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br J Dermatol. 1977;97:247-254.
- Tosti A, Iorizzo M, Piraccini BM. Androgenetic alopecia in children: report of 20 cases. Br J Dermatol. 2005;152:556-559.
- Özcan D. Pediatric androgenetic alopecia: a retrospective review of clinical characteristics, hormonal assays and metabolic syndrome risk factors in 23 patients. An Bras Dermatol. 2022;97:166-172.
- Gonzalez ME, Cantatore-Francis J, Orlow SJ. Androgenetic alopecia in the paediatric population: a retrospective review of 57 patients. Br J Dermatol. 2010;163:378-385.
- Kim BJ, Kim JY, Eun HC. Androgenetic alopecia in adolescents: a report of 43 cases. J Dermatol. 2006;33:696-699.
- B Liamsombut S, Pomsoong C, Kositkuljorn C. Sleep quality in men with androgenetic alopecia. Sleep Breath. 2023;27:371-378.
- Baik I, Lee S, Thomas RJ. Obstructive sleep apnea, low transferrin saturation levels, and male-pattern baldness. Int J Dermatol. 2019;58:67-74.
- Yi Y, Qiu J, Jia J. Severity of androgenetic alopecia associated with poor sleeping habits and carnivorous eating and junk food consumption—a web-based investigation of male pattern hair loss in China. Dermatol Ther. 2020;33:E13273.
- Price VH. Androgenetic alopecia in adolescents. Cutis. 2003;71:115-121.
- Gomes TF, Soares RO. Pediatric androgenetic alopecia: an updated review. J Dtsch Dermatol Ges. 2023;21:19-25.
Clinical, Laboratory, and Trichoscopic Features of Pediatric Androgenetic Alopecia
Clinical, Laboratory, and Trichoscopic Features of Pediatric Androgenetic Alopecia
PRACTICE POINTS
- Early identification of androgenetic alopecia (AGA) is key in pediatric patients, especially in those with a family history of AGA and comorbidities such as seborrheic dermatitis, acne, or sleep disturbances.
- It is important to evaluate pediatric patients with AGA for hormonal imbalances and deficiencies in vitamin D and iron to guide treatment.
- Use targeted therapies such as topical minoxidil to treat pediatric AGA while also monitoring for adverse effects. For optimal outcomes, encourage consistent medication use and regular follow-up.
Satisfaction With Department of Veterans Affairs Prosthetics and Support Services as Reported by Women and Men Veterans
Satisfaction With Department of Veterans Affairs Prosthetics and Support Services as Reported by Women and Men Veterans
Limb loss is a significant and growing concern in the United States. Nearly 2 million Americans are living with limb loss, and up to 185,000 people undergo amputations annually.1-4 Of these patients, about 35% are women.5 The Veterans Health Administration (VHA) provides about 10% of US amputations.6-8 Between 2015 and 2019, the number of prosthetic devices provided to female veterans increased from 3.3 million to 4.6 million.5,9,10
Previous research identified disparities in prosthetic care between men and women, both within and outside the VHA. These disparities include slower prosthesis prescription and receipt among women, in addition to differences in self-reported mobility, satisfaction, rates of prosthesis rejection, and challenges related to prosthesis appearance and fit.5,10,11 Recent studies suggest women tend to have worse outcomes following amputation, and are underrepresented in amputation research.12,13 However, these disparities are poorly described in a large, national sample. Because women represent a growing portion of patients with limb loss in the VHA, understanding their needs is critical.14
The Johnny Isakson and David P. Roe, MD Veterans Health Care and Benefits Improvement Act of 2020 was enacted, in part, to improve the care provided to women veterans.15 The law required the VHA to conduct a survey of ≥ 50,000 veterans to assess the satisfaction of women veterans with prostheses provided by the VHA. To comply with this legislation and understand how women veterans rate their prostheses and related care in the VHA, the US Department of Veterans Affairs (VA) Center for Collaborative Evaluation (VACE) conducted a large national survey of veterans with limb loss that oversampled women veterans. This article describes the survey results, including characteristics of female veterans with limb loss receiving care from the VHA, assesses their satisfaction with prostheses and prosthetic care, and highlights where their responses differ from those of male veterans.
Methods
We conducted a cross-sectional, mixedmode survey of eligible amputees in the VHA Support Service Capital Assets Amputee Data Cube. We identified a cohort of veterans with any major amputation (above the ankle or wrist) or partial hand or foot amputation who received VHA care between October 1, 2019, and September 30, 2020. The final cohort yielded 46,646 potentially eligible veterans. Thirty-three had invalid contact information, leaving 46,613 veterans who were asked to participate, including 1356 women.
Survey
We created a survey instrument de novo that included questions from validated instruments, including the Trinity Amputation Prosthesis and Experience Scales to assess prosthetic device satisfaction, the Prosthesis Evaluation Questionnaire to assess quality of life (QOL) satisfaction, and the Orthotics Prosthetics Users Survey to assess prosthesis-related care satisfaction. 16-18 Additional questions were incorporated from a survey of veterans with upper limb amputation to assess the importance of cosmetic considerations related to the prosthesis and comfort with prosthesis use in intimate relationships.19 Questions were also included to assess amputation type, year of amputation, if a prosthesis was currently used, reasons for ceasing use of a prosthesis, reasons for never using a prosthesis, the types of prostheses used, intensity of prosthesis use, satisfaction with time required to receive a prosthetic limb, and if the prosthesis reflected the veteran’s selfidentified gender. Veterans were asked to answer questions based on their most recent amputation.
We tested the survey using cognitive interviews with 6 veterans to refine the survey and better understand how veterans interpreted the questions. Pilot testers completed the survey and participated in individual interviews with experienced interviewers (CL and RRK) to describe how they selected their responses.20 This feedback was used to refine the survey. The online survey was programmed using Qualtrics Software and manually translated into Spanish.
Given the multimodal design, surveys were distributed by email, text message, and US Postal Service (USPS). Surveys were emailed to all veterans for whom a valid email address was available. If emails were undeliverable, veterans were contacted via text message or the USPS. Surveys were distributed by text message to all veterans without an email address but with a cellphone number. We were unable to consistently identify invalid numbers among all text message recipients. Invitations with a survey URL and QR code were sent via USPS to veterans who had no valid email address or cellphone number. Targeted efforts were made to increase the response rate for women. A random sample of 200 women who had not completed the survey 2 weeks prior to the closing date (15% of women in sample) was selected to receive personal phone calls. Another random sample of 400 women was selected to receive personalized outreach emails. The survey data were confidential, and responses could not be traced to identifying information.
Data Analyses
We conducted a descriptive analysis, including percentages and means for responses to variables focused on describing amputation characteristics, prosthesis characteristics, and QOL. All data, including missing values, were used to document the percentage of respondents for each question. Removing missing data from the denominator when calculating percentages could introduce bias to the analysis because we cannot be certain data are missing at random. Missing variables were removed to avoid underinflation of mean scores.
We compared responses across 2 groups: individuals who self-identified as men and individuals who self-identified as women. For each question, we assessed whether each of these groups differed significantly from the remaining sample. For example, we examined whether the percentage of men who answered affirmatively to a question was significantly higher or lower than that of individuals not identifying as male, and whether the percentage of women who answered affirmatively was significantly higher or lower than that of individuals not identifying as female. We utilized x2 tests to determine significant differences for percentage calculations and t tests to determine significant differences in means across gender.
Since conducting multiple comparisons within a dataset may result in inflating statistical significance (type 1 errors), we used a more conservative estimate of statistical significance (α = 0.01) and high significance (α = 0.001). This study was deemed quality improvement by the VHA Rehabilitation and Prosthetic Services (12RPS) and acknowledged by the VA Research Office at Eastern Colorado Health Care System and was not subject to institutional review board review.
Results
Surveys were distributed to 46,613 veterans and were completed by 4981 respondents for a 10.7% overall response rate. Survey respondents were generally similar to the eligible population invited to participate, but the proportion of women who completed the survey was higher than the proportion of women eligible to participate (2.0% of eligible population vs 16.7% of respondents), likely due to specific efforts to target women. Survey respondents were slightly younger than the general population (67.3 years vs 68.7 years), less likely to be male (97.1% vs 83.3%), showed similar representation of Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) veterans (4.4% vs 4.1%), and were less likely to have diabetes (58.0% vs 52.7% had diabetes) (Table 1).
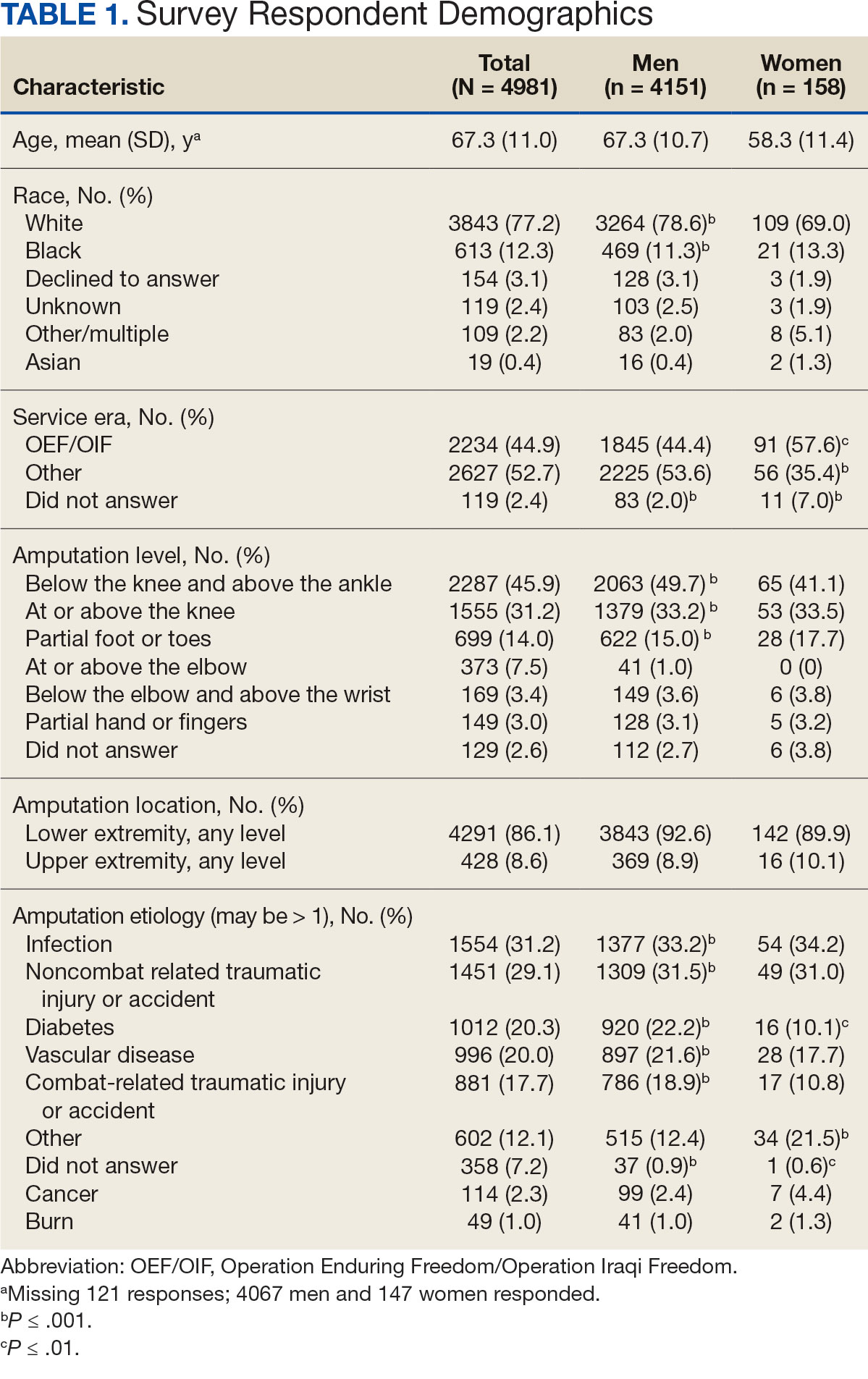
The mean age of male respondents was 67.3 years, while the mean age of female respondents was 58.3 years. The majority of respondents were male (83.3%) and White (77.2%). Female respondents were less likely to have diabetes (35.4% of women vs 53.5% of men) and less likely to report that their most recent amputation resulted from diabetes (10.1% of women vs 22.2% of men). Women respondents were more likely to report an amputation due to other causes, such as adverse results of surgery, neurologic disease, suicide attempt, blood clots, tumors, rheumatoid arthritis, and revisions of previous amputations. Most women respondents did not serve during the OEF or OIF eras. The most common amputation site for women respondents was lower limb, either below the knee and above the ankle or above the knee.
Most participants use an everyday prosthesis, but women were more likely to report using a sports-specific prosthesis (Table 2). Overall, most respondents report using a prosthesis (87.7%); however, women were more likely to report not using a prosthesis (19.4% of women vs 11.1% of men; P ≤ .01). Additionally, a lower proportion of women report using a prosthesis for < 12 hours per day (30.6% of women vs 46.4% of men; P ≤ .01) or using a prosthesis every day (54.8% of women vs 74.6% of men; P ≤ .001).
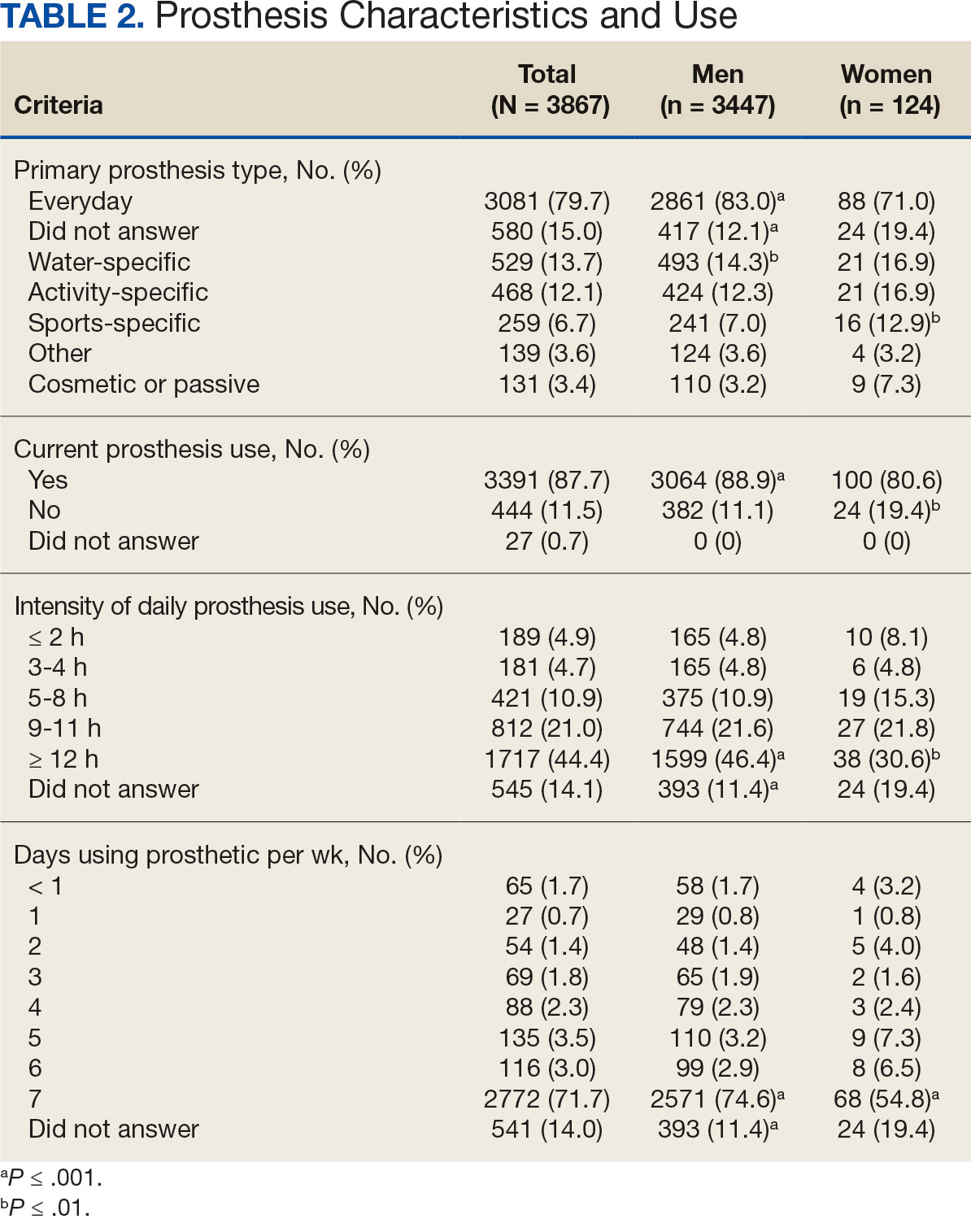
In the overall sample, the mean satisfaction score with a prosthesis was 2.7 on a 5-point scale, and women had slightly lower overall satisfaction scores (2.6 for women vs 2.7 for men; P ≤ .001) (Table 3). Women also had lower satisfaction scores related to appearance, usefulness, reliability, and comfort. Women were more likely to indicate that it was very important to be able to wear jewelry and accessories (20.2% of women vs 11.6% of men; P ≤ .01), while men were less likely to indicate that it was somewhat or very important that the prosthesis not restrict clothing or shoes (95.2% of women vs 82.9% of men; P ≤ .001). Men were more likely than women to report being comfortable or very comfortable using their prosthesis in intimate contact: 40.5% vs 29.0%, respectively (P ≤ .001).
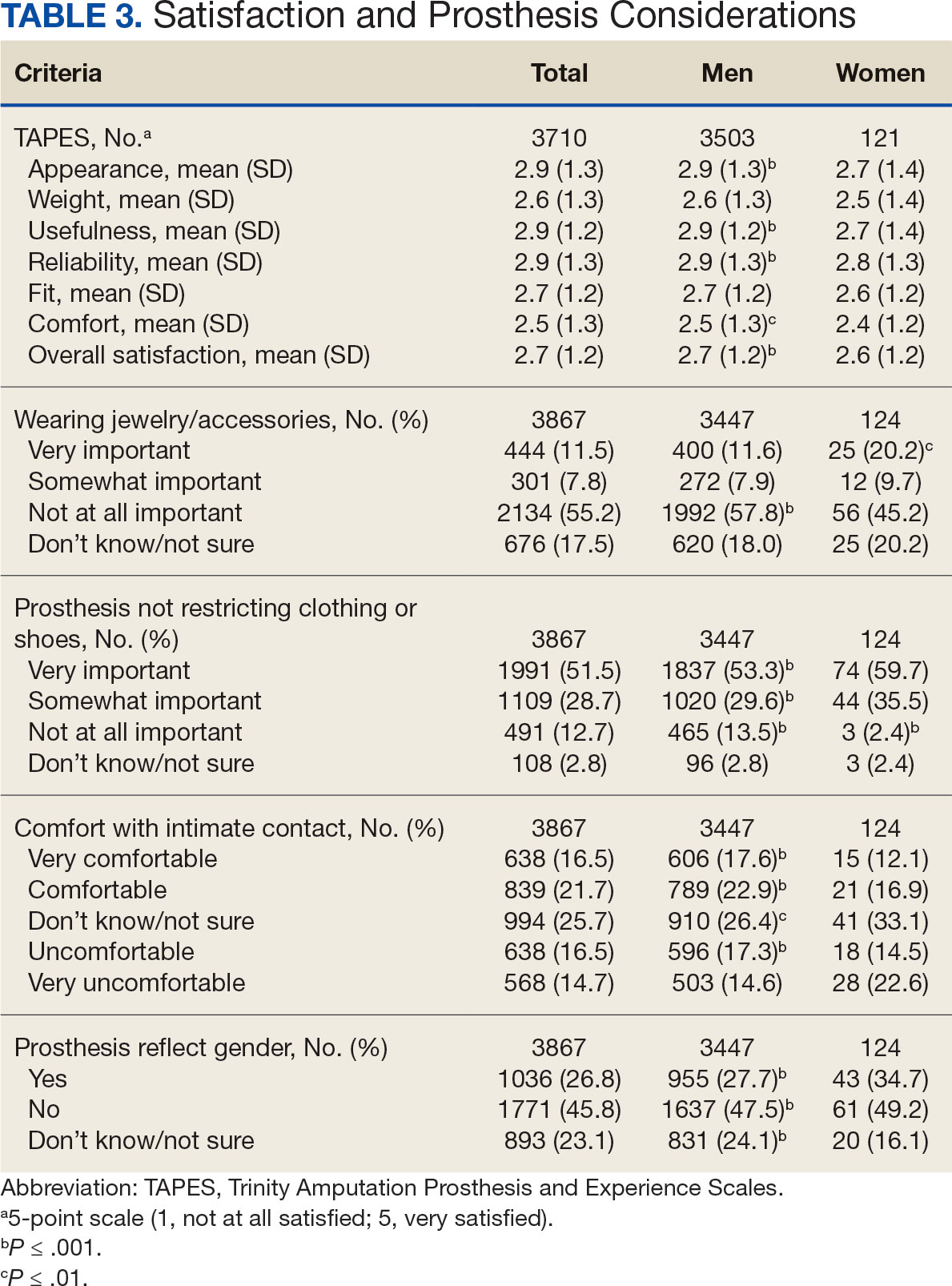
Overall, participants reported high satisfaction with appointment times, wait times, courteous treatment, opportunities to express concerns, and staff responsiveness. Men were slightly more likely than women to be satisfied with training (P ≤ 0.001) and problem discussion (P ≤ 0.01) (Table 4). There were no statistically significant differences in satisfaction or QOL ratings between women and men. The overall sample rated both QOL and satisfaction with QOL 6.7 on a 10-point scale.
Discussion
The goal of this study was to characterize the experience of veterans with limb loss receiving care in the VHA and assess their satisfaction with prostheses and prosthetic care. We received responses from nearly 5000 veterans, 158 of whom were women. Women veteran respondents were slightly younger and less likely to have an amputation due to diabetes. We did not observe significant differences in amputation level between men and women but women were less likely to use a prosthesis, reported lower intensity of prosthesis use, and were less satisfied with certain aspects of their prostheses. Women may also be less satisfied with prosthesis training and problem discussion. However, we found no differences in QOL ratings between men and women.
Findings indicating women were more likely to report not using a prosthesis and that a lower proportion of women report using a prosthesis for > 12 hours a day or every day are consistent with previous research. 21,22 Interestingly, women were more likely to report using a sports-specific prosthesis. This is notable because prior research suggests that individuals with amputations may avoid participating in sports and exercise, and a lack of access to sports-specific prostheses may inhibit physical activity.23,24 Women in this sample were slightly less satisfied with their prostheses overall and reported lower satisfaction scores regarding appearance, usefulness, reliability, and comfort, consistent with previous findings.25
A lower percentage of women in this sample reported being comfortable or very comfortable using their prosthesis during intimate contact. Previous research on prosthesis satisfaction suggests individuals who rate prosthesis satisfaction lower also report lower body image across genders. 26 While women in this sample did not rate their prosthesis satisfaction lower than men, they did report lower intensity of prosthesis use, suggesting potential issues with their prostheses this survey did not evaluate. Women indicated the importance of prostheses not restricting jewelry, accessories, clothing, or shoes. These results have significant clinical and social implications. A recent qualitative study emphasizes that women veterans feel prostheses are primarily designed for men and may not work well with their physiological needs.9 Research focused on limbs better suited to women’s bodies could result in better fitting sockets, lightweight limbs, or less bulky designs. Additional research has also explored the difficulties in accommodating a range of footwear for patients with lower limb amputation. One study found that varying footwear heights affect the function of adjustable prosthetic feet in ways that may not be optimal.27
Ratings of satisfaction with prosthesisrelated services between men and women in this sample are consistent with a recent study showing that women veterans do not have significant differences in satisfaction with prosthesis-related services.28 However, this study focused specifically on lower limb amputations, while the respondents of this study include those with both upper and lower limb amputations. Importantly, our findings that women are less likely to be satisfied with prosthesis training and problem discussions support recent qualitative findings in which women expressed a desire to work with prosthetists who listen to them, take their concerns seriously, and seek solutions that fit their needs. We did not observe a difference in QOL ratings between men and women in the sample despite lower satisfaction among women with some elements of prosthesis-related services. Previous research suggests many factors impact QOL after amputation, most notably time since amputation.16,29
Limitations
This survey was deployed in a short timeline that did not allow for careful sample selection or implementing strategies to increase response rate. Additionally, the study was conducted among veterans receiving care in the VHA, and findings may not be generalizable to limb loss in other settings. Finally, the discrepancy in number of respondents who identified as men vs women made it difficult to compare differences between the 2 groups.
Conclusions
This is the largest sample of survey respondents of veterans with limb loss to date. While the findings suggest veterans are generally satisfied with prosthetic-related services overall, they also highlight several areas for improvement with services or prostheses. Given that most veterans with limb loss are men, there is a significant discrepancy between the number of women and men respondents. Additional studies with more comparable numbers of men and women have found similar ratings of satisfaction with prostheses and services.28 Further research specifically focused on improving the experiences of women should focus on better characterizing their experiences and identifying how they differ from those of male veterans. For example, understanding how to engage female veterans with limb loss in prosthesis training and problem discussions may improve their experience with their care teams and improve their use of prostheses. Understanding experiences and needs that are specific to women could lead to the development of processes, resources, or devices that are tailored to the unique requirements of women with limb loss.
- Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89(3):422-429. doi:10.1016/j.apmr.2007.11.005
- Dillingham TR, Pezzin LE, MacKenzie EJ. Limb amputation and limb deficiency: epidemiology and recent trends in the united states. South Med J. 2002;95(8):875-883. doi:10.1097/00007611-200208000-00018
- Dillingham TR, Pezzin LE, Shore AD. Reamputation, mortality, and health care costs among persons with dysvascular lower-limb amputations. Arch Phys Med Rehabil. 2005;86(3):480-486. doi:10.1016/j.apmr.2004.06.072
- Centers for Disease Control and Prevention. Ambulatory and inpatient procedures in the United States. Accessed September 30, 2024. https://www.cdc.gov/nchs/pressroom/98facts/ambulat.htm
- Ljung J, Iacangelo A. Identifying and acknowledging a sex gap in lower-limb prosthetics. JPO. 2024;36(1):e18-e24. doi:10.1097/JPO.0000000000000470
- Feinglass J, Brown JL, LoSasso A, et al. Rates of lower-extremity amputation and arterial reconstruction in the united states, 1979 to 1996. Am J Public Health. 1999;89(8):1222- 1227. doi:10.2105/ajph.89.8.1222
- Mayfield JA, Reiber GE, Maynard C, Czerniecki JM, Caps MT, Sangeorzan BJ. Trends in lower limb amputation in the Veterans Health Administration, 1989-1998. J Rehabil Res Dev. 2000;37(1):23-30.
- Feinglass J, Pearce WH, Martin GJ, et al. Postoperative and late survival outcomes after major amputation: findings from the department of veterans affairs national surgical quality improvement program. Surgery. 2001;130(1):21-29. doi:10.1067/msy.2001.115359
- Lehavot K, Young JP, Thomas RM, et al. Voices of women veterans with lower limb prostheses: a qualitative study. J Gen Intern Med. 2022;37(3):799-805. doi:10.1007/s11606-022-07572-8
- US Government Accountability Office. COVID-19: Opportunities to improve federal response. GAO-21-60. Published November 12, 2020. Accessed September 30, 2024. https://www.gao.gov/products/gao-21-60
- Littman AJ, Peterson AC, Korpak A, et al. Differences in prosthetic prescription between men and women veterans after transtibial or transfemoral lowerextremity amputation: a longitudinal cohort study. Arch Phys Med Rehabil. 2023;104(8)1274-1281. doi:10.1016/j.amjsurg.2023.02.011
- Cimino SR, Vijayakumar A, MacKay C, Mayo AL, Hitzig SL, Guilcher SJT. Sex and gender differences in quality of life and related domains for individuals with adult acquired lower-limb amputation: a scoping review. Disabil Rehabil. 2022 Oct 23;44(22):6899-6925. doi:10.1080/09638288.2021.1974106
- DadeMatthews OO, Roper JA, Vazquez A, Shannon DM, Sefton JM. Prosthetic device and service satisfaction, quality of life, and functional performance in lower limb prosthesis clients. Prosthet Orthot Int. 2024;48(4):422-430. doi:10.1097/PXR.0000000000000285
- Hamilton AB, Schwarz EB, Thomas HN, Goldstein KM. Moving women veterans’ health research forward: a special supplement. J Gen Intern Med. 2022;37(Suppl3):665– 667. doi:10.1007/s11606-022-07606-1
- US Congress. Public Law 116-315: An Act to Improve the Lives of Veterans, S 5108 (2) (F). 116th Congress; 2021. Accessed September 30, 2024. https://www.congress.gov/116/plaws/publ315/PLAW-116publ315.pdf
- Gallagher P, MacLachlan M. The Trinity amputation and prosthesis experience scales and quality of life in people with lower-limb amputation. Arch Phys Med Rehabil. 2004;85(5):730-736. doi:10.1016/j.apmr.2003.07.009
- Legro MW, Reiber GD, Smith DG, del Aguila M, Larsen J, Boone D. Prosthesis evaluation questionnaire for persons with lower limb amputations: assessing prosthesis-related quality of life. Arch Phys Med Rehabil. 1998;79(8):931-938. doi:10.1016/s0003-9993(98)90090-9
- Legro MW, Reiber GD, Smith DG, del Aguila M, Larsen J, Boone D. Prosthesis evaluation questionnaire for persons with lower limb amputations: assessing prosthesis-related quality of life. Arch Phys Med Rehabil. 1998;79(8):931-938. doi:10.1016/s0003-9993(98)90090-9
- Heinemann AW, Bode RK, O’Reilly C. Development and measurement properties of the orthotics and prosthetics users’ survey (OPUS): a comprehensive set of clinical outcome instruments. Prosthet Orthot Int. 2003;27(3):191-206. doi:10.1080/03093640308726682
- Resnik LJ, Borgia ML, Clark MA. A national survey of prosthesis use in veterans with major upper limb amputation: comparisons by gender. PM R. 2020;12(11):1086-1098. doi:10.1002/pmrj.12351
- Collins D. Pretesting survey instruments: an overview of cognitive methods. Qual Life Res. 2003;12(3):229-238. doi:10.1023/a:1023254226592
- Østlie K, Lesjø IM, Franklin RJ, Garfelt B, Skjeldal OH, Magnus P. Prosthesis rejection in acquired major upper-limb amputees: a population-based survey. Disabil Rehabil Assist Technol. 2012;7(4):294-303. doi:10.3109/17483107.2011.635405
- Pezzin LE, Dillingham TR, MacKenzie EJ, Ephraim P, Rossbach P. Use and satisfaction with prosthetic limb devices and related services. Arch Phys Med Rehabil. 2004;85(5):723-729. doi:10.1016/j.apmr.2003.06.002
- Deans S, Burns D, McGarry A, Murray K, Mutrie N. Motivations and barriers to prosthesis users participation in physical activity, exercise and sport: a review of the literature. Prosthet Orthot Int. 2012;36(3):260-269. doi:10.1177/0309364612437905
- McDonald CL, Kahn A, Hafner BJ, Morgan SJ. Prevalence of secondary prosthesis use in lower limb prosthesis users. Disabil Rehabil. 2023;46(5):1016-1022. doi:10.1080/09638288.2023.2182919
- Baars EC, Schrier E, Dijkstra PU, Geertzen JHB. Prosthesis satisfaction in lower limb amputees: a systematic review of associated factors and questionnaires. Medicine (Baltimore). 2018;97(39):e12296. doi:10.1097/MD.0000000000012296
- Murray CD, Fox J. Body image and prosthesis satisfaction in the lower limb amputee. Disabil Rehabil. 2002;24(17):925–931. doi:10.1080/09638280210150014
- Major MJ, Quinlan J, Hansen AH, Esposito ER. Effects of women’s footwear on the mechanical function of heel-height accommodating prosthetic feet. PLoS One. 2022;17(1). doi:10.1371/journal.pone.0262910.
- Kuo PB, Lehavot K, Thomas RM, et al. Gender differences in prosthesis-related outcomes among veterans: results of a national survey of U.S. veterans. PM R. 2024;16(3):239- 249. doi:10.1002/pmrj.13028
- Asano M, Rushton P, Miller WC, Deathe BA. Predictors of quality of life among individuals who have a lower limb amputation. Prosthet Orthot Int. 2008;32(2):231-243. doi:10.1080/03093640802024955
Limb loss is a significant and growing concern in the United States. Nearly 2 million Americans are living with limb loss, and up to 185,000 people undergo amputations annually.1-4 Of these patients, about 35% are women.5 The Veterans Health Administration (VHA) provides about 10% of US amputations.6-8 Between 2015 and 2019, the number of prosthetic devices provided to female veterans increased from 3.3 million to 4.6 million.5,9,10
Previous research identified disparities in prosthetic care between men and women, both within and outside the VHA. These disparities include slower prosthesis prescription and receipt among women, in addition to differences in self-reported mobility, satisfaction, rates of prosthesis rejection, and challenges related to prosthesis appearance and fit.5,10,11 Recent studies suggest women tend to have worse outcomes following amputation, and are underrepresented in amputation research.12,13 However, these disparities are poorly described in a large, national sample. Because women represent a growing portion of patients with limb loss in the VHA, understanding their needs is critical.14
The Johnny Isakson and David P. Roe, MD Veterans Health Care and Benefits Improvement Act of 2020 was enacted, in part, to improve the care provided to women veterans.15 The law required the VHA to conduct a survey of ≥ 50,000 veterans to assess the satisfaction of women veterans with prostheses provided by the VHA. To comply with this legislation and understand how women veterans rate their prostheses and related care in the VHA, the US Department of Veterans Affairs (VA) Center for Collaborative Evaluation (VACE) conducted a large national survey of veterans with limb loss that oversampled women veterans. This article describes the survey results, including characteristics of female veterans with limb loss receiving care from the VHA, assesses their satisfaction with prostheses and prosthetic care, and highlights where their responses differ from those of male veterans.
Methods
We conducted a cross-sectional, mixedmode survey of eligible amputees in the VHA Support Service Capital Assets Amputee Data Cube. We identified a cohort of veterans with any major amputation (above the ankle or wrist) or partial hand or foot amputation who received VHA care between October 1, 2019, and September 30, 2020. The final cohort yielded 46,646 potentially eligible veterans. Thirty-three had invalid contact information, leaving 46,613 veterans who were asked to participate, including 1356 women.
Survey
We created a survey instrument de novo that included questions from validated instruments, including the Trinity Amputation Prosthesis and Experience Scales to assess prosthetic device satisfaction, the Prosthesis Evaluation Questionnaire to assess quality of life (QOL) satisfaction, and the Orthotics Prosthetics Users Survey to assess prosthesis-related care satisfaction. 16-18 Additional questions were incorporated from a survey of veterans with upper limb amputation to assess the importance of cosmetic considerations related to the prosthesis and comfort with prosthesis use in intimate relationships.19 Questions were also included to assess amputation type, year of amputation, if a prosthesis was currently used, reasons for ceasing use of a prosthesis, reasons for never using a prosthesis, the types of prostheses used, intensity of prosthesis use, satisfaction with time required to receive a prosthetic limb, and if the prosthesis reflected the veteran’s selfidentified gender. Veterans were asked to answer questions based on their most recent amputation.
We tested the survey using cognitive interviews with 6 veterans to refine the survey and better understand how veterans interpreted the questions. Pilot testers completed the survey and participated in individual interviews with experienced interviewers (CL and RRK) to describe how they selected their responses.20 This feedback was used to refine the survey. The online survey was programmed using Qualtrics Software and manually translated into Spanish.
Given the multimodal design, surveys were distributed by email, text message, and US Postal Service (USPS). Surveys were emailed to all veterans for whom a valid email address was available. If emails were undeliverable, veterans were contacted via text message or the USPS. Surveys were distributed by text message to all veterans without an email address but with a cellphone number. We were unable to consistently identify invalid numbers among all text message recipients. Invitations with a survey URL and QR code were sent via USPS to veterans who had no valid email address or cellphone number. Targeted efforts were made to increase the response rate for women. A random sample of 200 women who had not completed the survey 2 weeks prior to the closing date (15% of women in sample) was selected to receive personal phone calls. Another random sample of 400 women was selected to receive personalized outreach emails. The survey data were confidential, and responses could not be traced to identifying information.
Data Analyses
We conducted a descriptive analysis, including percentages and means for responses to variables focused on describing amputation characteristics, prosthesis characteristics, and QOL. All data, including missing values, were used to document the percentage of respondents for each question. Removing missing data from the denominator when calculating percentages could introduce bias to the analysis because we cannot be certain data are missing at random. Missing variables were removed to avoid underinflation of mean scores.
We compared responses across 2 groups: individuals who self-identified as men and individuals who self-identified as women. For each question, we assessed whether each of these groups differed significantly from the remaining sample. For example, we examined whether the percentage of men who answered affirmatively to a question was significantly higher or lower than that of individuals not identifying as male, and whether the percentage of women who answered affirmatively was significantly higher or lower than that of individuals not identifying as female. We utilized x2 tests to determine significant differences for percentage calculations and t tests to determine significant differences in means across gender.
Since conducting multiple comparisons within a dataset may result in inflating statistical significance (type 1 errors), we used a more conservative estimate of statistical significance (α = 0.01) and high significance (α = 0.001). This study was deemed quality improvement by the VHA Rehabilitation and Prosthetic Services (12RPS) and acknowledged by the VA Research Office at Eastern Colorado Health Care System and was not subject to institutional review board review.
Results
Surveys were distributed to 46,613 veterans and were completed by 4981 respondents for a 10.7% overall response rate. Survey respondents were generally similar to the eligible population invited to participate, but the proportion of women who completed the survey was higher than the proportion of women eligible to participate (2.0% of eligible population vs 16.7% of respondents), likely due to specific efforts to target women. Survey respondents were slightly younger than the general population (67.3 years vs 68.7 years), less likely to be male (97.1% vs 83.3%), showed similar representation of Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) veterans (4.4% vs 4.1%), and were less likely to have diabetes (58.0% vs 52.7% had diabetes) (Table 1).

The mean age of male respondents was 67.3 years, while the mean age of female respondents was 58.3 years. The majority of respondents were male (83.3%) and White (77.2%). Female respondents were less likely to have diabetes (35.4% of women vs 53.5% of men) and less likely to report that their most recent amputation resulted from diabetes (10.1% of women vs 22.2% of men). Women respondents were more likely to report an amputation due to other causes, such as adverse results of surgery, neurologic disease, suicide attempt, blood clots, tumors, rheumatoid arthritis, and revisions of previous amputations. Most women respondents did not serve during the OEF or OIF eras. The most common amputation site for women respondents was lower limb, either below the knee and above the ankle or above the knee.
Most participants use an everyday prosthesis, but women were more likely to report using a sports-specific prosthesis (Table 2). Overall, most respondents report using a prosthesis (87.7%); however, women were more likely to report not using a prosthesis (19.4% of women vs 11.1% of men; P ≤ .01). Additionally, a lower proportion of women report using a prosthesis for < 12 hours per day (30.6% of women vs 46.4% of men; P ≤ .01) or using a prosthesis every day (54.8% of women vs 74.6% of men; P ≤ .001).

In the overall sample, the mean satisfaction score with a prosthesis was 2.7 on a 5-point scale, and women had slightly lower overall satisfaction scores (2.6 for women vs 2.7 for men; P ≤ .001) (Table 3). Women also had lower satisfaction scores related to appearance, usefulness, reliability, and comfort. Women were more likely to indicate that it was very important to be able to wear jewelry and accessories (20.2% of women vs 11.6% of men; P ≤ .01), while men were less likely to indicate that it was somewhat or very important that the prosthesis not restrict clothing or shoes (95.2% of women vs 82.9% of men; P ≤ .001). Men were more likely than women to report being comfortable or very comfortable using their prosthesis in intimate contact: 40.5% vs 29.0%, respectively (P ≤ .001).

Overall, participants reported high satisfaction with appointment times, wait times, courteous treatment, opportunities to express concerns, and staff responsiveness. Men were slightly more likely than women to be satisfied with training (P ≤ 0.001) and problem discussion (P ≤ 0.01) (Table 4). There were no statistically significant differences in satisfaction or QOL ratings between women and men. The overall sample rated both QOL and satisfaction with QOL 6.7 on a 10-point scale.

Discussion
The goal of this study was to characterize the experience of veterans with limb loss receiving care in the VHA and assess their satisfaction with prostheses and prosthetic care. We received responses from nearly 5000 veterans, 158 of whom were women. Women veteran respondents were slightly younger and less likely to have an amputation due to diabetes. We did not observe significant differences in amputation level between men and women but women were less likely to use a prosthesis, reported lower intensity of prosthesis use, and were less satisfied with certain aspects of their prostheses. Women may also be less satisfied with prosthesis training and problem discussion. However, we found no differences in QOL ratings between men and women.
Findings indicating women were more likely to report not using a prosthesis and that a lower proportion of women report using a prosthesis for > 12 hours a day or every day are consistent with previous research. 21,22 Interestingly, women were more likely to report using a sports-specific prosthesis. This is notable because prior research suggests that individuals with amputations may avoid participating in sports and exercise, and a lack of access to sports-specific prostheses may inhibit physical activity.23,24 Women in this sample were slightly less satisfied with their prostheses overall and reported lower satisfaction scores regarding appearance, usefulness, reliability, and comfort, consistent with previous findings.25
A lower percentage of women in this sample reported being comfortable or very comfortable using their prosthesis during intimate contact. Previous research on prosthesis satisfaction suggests individuals who rate prosthesis satisfaction lower also report lower body image across genders. 26 While women in this sample did not rate their prosthesis satisfaction lower than men, they did report lower intensity of prosthesis use, suggesting potential issues with their prostheses this survey did not evaluate. Women indicated the importance of prostheses not restricting jewelry, accessories, clothing, or shoes. These results have significant clinical and social implications. A recent qualitative study emphasizes that women veterans feel prostheses are primarily designed for men and may not work well with their physiological needs.9 Research focused on limbs better suited to women’s bodies could result in better fitting sockets, lightweight limbs, or less bulky designs. Additional research has also explored the difficulties in accommodating a range of footwear for patients with lower limb amputation. One study found that varying footwear heights affect the function of adjustable prosthetic feet in ways that may not be optimal.27
Ratings of satisfaction with prosthesisrelated services between men and women in this sample are consistent with a recent study showing that women veterans do not have significant differences in satisfaction with prosthesis-related services.28 However, this study focused specifically on lower limb amputations, while the respondents of this study include those with both upper and lower limb amputations. Importantly, our findings that women are less likely to be satisfied with prosthesis training and problem discussions support recent qualitative findings in which women expressed a desire to work with prosthetists who listen to them, take their concerns seriously, and seek solutions that fit their needs. We did not observe a difference in QOL ratings between men and women in the sample despite lower satisfaction among women with some elements of prosthesis-related services. Previous research suggests many factors impact QOL after amputation, most notably time since amputation.16,29
Limitations
This survey was deployed in a short timeline that did not allow for careful sample selection or implementing strategies to increase response rate. Additionally, the study was conducted among veterans receiving care in the VHA, and findings may not be generalizable to limb loss in other settings. Finally, the discrepancy in number of respondents who identified as men vs women made it difficult to compare differences between the 2 groups.
Conclusions
This is the largest sample of survey respondents of veterans with limb loss to date. While the findings suggest veterans are generally satisfied with prosthetic-related services overall, they also highlight several areas for improvement with services or prostheses. Given that most veterans with limb loss are men, there is a significant discrepancy between the number of women and men respondents. Additional studies with more comparable numbers of men and women have found similar ratings of satisfaction with prostheses and services.28 Further research specifically focused on improving the experiences of women should focus on better characterizing their experiences and identifying how they differ from those of male veterans. For example, understanding how to engage female veterans with limb loss in prosthesis training and problem discussions may improve their experience with their care teams and improve their use of prostheses. Understanding experiences and needs that are specific to women could lead to the development of processes, resources, or devices that are tailored to the unique requirements of women with limb loss.
Limb loss is a significant and growing concern in the United States. Nearly 2 million Americans are living with limb loss, and up to 185,000 people undergo amputations annually.1-4 Of these patients, about 35% are women.5 The Veterans Health Administration (VHA) provides about 10% of US amputations.6-8 Between 2015 and 2019, the number of prosthetic devices provided to female veterans increased from 3.3 million to 4.6 million.5,9,10
Previous research identified disparities in prosthetic care between men and women, both within and outside the VHA. These disparities include slower prosthesis prescription and receipt among women, in addition to differences in self-reported mobility, satisfaction, rates of prosthesis rejection, and challenges related to prosthesis appearance and fit.5,10,11 Recent studies suggest women tend to have worse outcomes following amputation, and are underrepresented in amputation research.12,13 However, these disparities are poorly described in a large, national sample. Because women represent a growing portion of patients with limb loss in the VHA, understanding their needs is critical.14
The Johnny Isakson and David P. Roe, MD Veterans Health Care and Benefits Improvement Act of 2020 was enacted, in part, to improve the care provided to women veterans.15 The law required the VHA to conduct a survey of ≥ 50,000 veterans to assess the satisfaction of women veterans with prostheses provided by the VHA. To comply with this legislation and understand how women veterans rate their prostheses and related care in the VHA, the US Department of Veterans Affairs (VA) Center for Collaborative Evaluation (VACE) conducted a large national survey of veterans with limb loss that oversampled women veterans. This article describes the survey results, including characteristics of female veterans with limb loss receiving care from the VHA, assesses their satisfaction with prostheses and prosthetic care, and highlights where their responses differ from those of male veterans.
Methods
We conducted a cross-sectional, mixedmode survey of eligible amputees in the VHA Support Service Capital Assets Amputee Data Cube. We identified a cohort of veterans with any major amputation (above the ankle or wrist) or partial hand or foot amputation who received VHA care between October 1, 2019, and September 30, 2020. The final cohort yielded 46,646 potentially eligible veterans. Thirty-three had invalid contact information, leaving 46,613 veterans who were asked to participate, including 1356 women.
Survey
We created a survey instrument de novo that included questions from validated instruments, including the Trinity Amputation Prosthesis and Experience Scales to assess prosthetic device satisfaction, the Prosthesis Evaluation Questionnaire to assess quality of life (QOL) satisfaction, and the Orthotics Prosthetics Users Survey to assess prosthesis-related care satisfaction. 16-18 Additional questions were incorporated from a survey of veterans with upper limb amputation to assess the importance of cosmetic considerations related to the prosthesis and comfort with prosthesis use in intimate relationships.19 Questions were also included to assess amputation type, year of amputation, if a prosthesis was currently used, reasons for ceasing use of a prosthesis, reasons for never using a prosthesis, the types of prostheses used, intensity of prosthesis use, satisfaction with time required to receive a prosthetic limb, and if the prosthesis reflected the veteran’s selfidentified gender. Veterans were asked to answer questions based on their most recent amputation.
We tested the survey using cognitive interviews with 6 veterans to refine the survey and better understand how veterans interpreted the questions. Pilot testers completed the survey and participated in individual interviews with experienced interviewers (CL and RRK) to describe how they selected their responses.20 This feedback was used to refine the survey. The online survey was programmed using Qualtrics Software and manually translated into Spanish.
Given the multimodal design, surveys were distributed by email, text message, and US Postal Service (USPS). Surveys were emailed to all veterans for whom a valid email address was available. If emails were undeliverable, veterans were contacted via text message or the USPS. Surveys were distributed by text message to all veterans without an email address but with a cellphone number. We were unable to consistently identify invalid numbers among all text message recipients. Invitations with a survey URL and QR code were sent via USPS to veterans who had no valid email address or cellphone number. Targeted efforts were made to increase the response rate for women. A random sample of 200 women who had not completed the survey 2 weeks prior to the closing date (15% of women in sample) was selected to receive personal phone calls. Another random sample of 400 women was selected to receive personalized outreach emails. The survey data were confidential, and responses could not be traced to identifying information.
Data Analyses
We conducted a descriptive analysis, including percentages and means for responses to variables focused on describing amputation characteristics, prosthesis characteristics, and QOL. All data, including missing values, were used to document the percentage of respondents for each question. Removing missing data from the denominator when calculating percentages could introduce bias to the analysis because we cannot be certain data are missing at random. Missing variables were removed to avoid underinflation of mean scores.
We compared responses across 2 groups: individuals who self-identified as men and individuals who self-identified as women. For each question, we assessed whether each of these groups differed significantly from the remaining sample. For example, we examined whether the percentage of men who answered affirmatively to a question was significantly higher or lower than that of individuals not identifying as male, and whether the percentage of women who answered affirmatively was significantly higher or lower than that of individuals not identifying as female. We utilized x2 tests to determine significant differences for percentage calculations and t tests to determine significant differences in means across gender.
Since conducting multiple comparisons within a dataset may result in inflating statistical significance (type 1 errors), we used a more conservative estimate of statistical significance (α = 0.01) and high significance (α = 0.001). This study was deemed quality improvement by the VHA Rehabilitation and Prosthetic Services (12RPS) and acknowledged by the VA Research Office at Eastern Colorado Health Care System and was not subject to institutional review board review.
Results
Surveys were distributed to 46,613 veterans and were completed by 4981 respondents for a 10.7% overall response rate. Survey respondents were generally similar to the eligible population invited to participate, but the proportion of women who completed the survey was higher than the proportion of women eligible to participate (2.0% of eligible population vs 16.7% of respondents), likely due to specific efforts to target women. Survey respondents were slightly younger than the general population (67.3 years vs 68.7 years), less likely to be male (97.1% vs 83.3%), showed similar representation of Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) veterans (4.4% vs 4.1%), and were less likely to have diabetes (58.0% vs 52.7% had diabetes) (Table 1).

The mean age of male respondents was 67.3 years, while the mean age of female respondents was 58.3 years. The majority of respondents were male (83.3%) and White (77.2%). Female respondents were less likely to have diabetes (35.4% of women vs 53.5% of men) and less likely to report that their most recent amputation resulted from diabetes (10.1% of women vs 22.2% of men). Women respondents were more likely to report an amputation due to other causes, such as adverse results of surgery, neurologic disease, suicide attempt, blood clots, tumors, rheumatoid arthritis, and revisions of previous amputations. Most women respondents did not serve during the OEF or OIF eras. The most common amputation site for women respondents was lower limb, either below the knee and above the ankle or above the knee.
Most participants use an everyday prosthesis, but women were more likely to report using a sports-specific prosthesis (Table 2). Overall, most respondents report using a prosthesis (87.7%); however, women were more likely to report not using a prosthesis (19.4% of women vs 11.1% of men; P ≤ .01). Additionally, a lower proportion of women report using a prosthesis for < 12 hours per day (30.6% of women vs 46.4% of men; P ≤ .01) or using a prosthesis every day (54.8% of women vs 74.6% of men; P ≤ .001).

In the overall sample, the mean satisfaction score with a prosthesis was 2.7 on a 5-point scale, and women had slightly lower overall satisfaction scores (2.6 for women vs 2.7 for men; P ≤ .001) (Table 3). Women also had lower satisfaction scores related to appearance, usefulness, reliability, and comfort. Women were more likely to indicate that it was very important to be able to wear jewelry and accessories (20.2% of women vs 11.6% of men; P ≤ .01), while men were less likely to indicate that it was somewhat or very important that the prosthesis not restrict clothing or shoes (95.2% of women vs 82.9% of men; P ≤ .001). Men were more likely than women to report being comfortable or very comfortable using their prosthesis in intimate contact: 40.5% vs 29.0%, respectively (P ≤ .001).

Overall, participants reported high satisfaction with appointment times, wait times, courteous treatment, opportunities to express concerns, and staff responsiveness. Men were slightly more likely than women to be satisfied with training (P ≤ 0.001) and problem discussion (P ≤ 0.01) (Table 4). There were no statistically significant differences in satisfaction or QOL ratings between women and men. The overall sample rated both QOL and satisfaction with QOL 6.7 on a 10-point scale.

Discussion
The goal of this study was to characterize the experience of veterans with limb loss receiving care in the VHA and assess their satisfaction with prostheses and prosthetic care. We received responses from nearly 5000 veterans, 158 of whom were women. Women veteran respondents were slightly younger and less likely to have an amputation due to diabetes. We did not observe significant differences in amputation level between men and women but women were less likely to use a prosthesis, reported lower intensity of prosthesis use, and were less satisfied with certain aspects of their prostheses. Women may also be less satisfied with prosthesis training and problem discussion. However, we found no differences in QOL ratings between men and women.
Findings indicating women were more likely to report not using a prosthesis and that a lower proportion of women report using a prosthesis for > 12 hours a day or every day are consistent with previous research. 21,22 Interestingly, women were more likely to report using a sports-specific prosthesis. This is notable because prior research suggests that individuals with amputations may avoid participating in sports and exercise, and a lack of access to sports-specific prostheses may inhibit physical activity.23,24 Women in this sample were slightly less satisfied with their prostheses overall and reported lower satisfaction scores regarding appearance, usefulness, reliability, and comfort, consistent with previous findings.25
A lower percentage of women in this sample reported being comfortable or very comfortable using their prosthesis during intimate contact. Previous research on prosthesis satisfaction suggests individuals who rate prosthesis satisfaction lower also report lower body image across genders. 26 While women in this sample did not rate their prosthesis satisfaction lower than men, they did report lower intensity of prosthesis use, suggesting potential issues with their prostheses this survey did not evaluate. Women indicated the importance of prostheses not restricting jewelry, accessories, clothing, or shoes. These results have significant clinical and social implications. A recent qualitative study emphasizes that women veterans feel prostheses are primarily designed for men and may not work well with their physiological needs.9 Research focused on limbs better suited to women’s bodies could result in better fitting sockets, lightweight limbs, or less bulky designs. Additional research has also explored the difficulties in accommodating a range of footwear for patients with lower limb amputation. One study found that varying footwear heights affect the function of adjustable prosthetic feet in ways that may not be optimal.27
Ratings of satisfaction with prosthesisrelated services between men and women in this sample are consistent with a recent study showing that women veterans do not have significant differences in satisfaction with prosthesis-related services.28 However, this study focused specifically on lower limb amputations, while the respondents of this study include those with both upper and lower limb amputations. Importantly, our findings that women are less likely to be satisfied with prosthesis training and problem discussions support recent qualitative findings in which women expressed a desire to work with prosthetists who listen to them, take their concerns seriously, and seek solutions that fit their needs. We did not observe a difference in QOL ratings between men and women in the sample despite lower satisfaction among women with some elements of prosthesis-related services. Previous research suggests many factors impact QOL after amputation, most notably time since amputation.16,29
Limitations
This survey was deployed in a short timeline that did not allow for careful sample selection or implementing strategies to increase response rate. Additionally, the study was conducted among veterans receiving care in the VHA, and findings may not be generalizable to limb loss in other settings. Finally, the discrepancy in number of respondents who identified as men vs women made it difficult to compare differences between the 2 groups.
Conclusions
This is the largest sample of survey respondents of veterans with limb loss to date. While the findings suggest veterans are generally satisfied with prosthetic-related services overall, they also highlight several areas for improvement with services or prostheses. Given that most veterans with limb loss are men, there is a significant discrepancy between the number of women and men respondents. Additional studies with more comparable numbers of men and women have found similar ratings of satisfaction with prostheses and services.28 Further research specifically focused on improving the experiences of women should focus on better characterizing their experiences and identifying how they differ from those of male veterans. For example, understanding how to engage female veterans with limb loss in prosthesis training and problem discussions may improve their experience with their care teams and improve their use of prostheses. Understanding experiences and needs that are specific to women could lead to the development of processes, resources, or devices that are tailored to the unique requirements of women with limb loss.
- Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89(3):422-429. doi:10.1016/j.apmr.2007.11.005
- Dillingham TR, Pezzin LE, MacKenzie EJ. Limb amputation and limb deficiency: epidemiology and recent trends in the united states. South Med J. 2002;95(8):875-883. doi:10.1097/00007611-200208000-00018
- Dillingham TR, Pezzin LE, Shore AD. Reamputation, mortality, and health care costs among persons with dysvascular lower-limb amputations. Arch Phys Med Rehabil. 2005;86(3):480-486. doi:10.1016/j.apmr.2004.06.072
- Centers for Disease Control and Prevention. Ambulatory and inpatient procedures in the United States. Accessed September 30, 2024. https://www.cdc.gov/nchs/pressroom/98facts/ambulat.htm
- Ljung J, Iacangelo A. Identifying and acknowledging a sex gap in lower-limb prosthetics. JPO. 2024;36(1):e18-e24. doi:10.1097/JPO.0000000000000470
- Feinglass J, Brown JL, LoSasso A, et al. Rates of lower-extremity amputation and arterial reconstruction in the united states, 1979 to 1996. Am J Public Health. 1999;89(8):1222- 1227. doi:10.2105/ajph.89.8.1222
- Mayfield JA, Reiber GE, Maynard C, Czerniecki JM, Caps MT, Sangeorzan BJ. Trends in lower limb amputation in the Veterans Health Administration, 1989-1998. J Rehabil Res Dev. 2000;37(1):23-30.
- Feinglass J, Pearce WH, Martin GJ, et al. Postoperative and late survival outcomes after major amputation: findings from the department of veterans affairs national surgical quality improvement program. Surgery. 2001;130(1):21-29. doi:10.1067/msy.2001.115359
- Lehavot K, Young JP, Thomas RM, et al. Voices of women veterans with lower limb prostheses: a qualitative study. J Gen Intern Med. 2022;37(3):799-805. doi:10.1007/s11606-022-07572-8
- US Government Accountability Office. COVID-19: Opportunities to improve federal response. GAO-21-60. Published November 12, 2020. Accessed September 30, 2024. https://www.gao.gov/products/gao-21-60
- Littman AJ, Peterson AC, Korpak A, et al. Differences in prosthetic prescription between men and women veterans after transtibial or transfemoral lowerextremity amputation: a longitudinal cohort study. Arch Phys Med Rehabil. 2023;104(8)1274-1281. doi:10.1016/j.amjsurg.2023.02.011
- Cimino SR, Vijayakumar A, MacKay C, Mayo AL, Hitzig SL, Guilcher SJT. Sex and gender differences in quality of life and related domains for individuals with adult acquired lower-limb amputation: a scoping review. Disabil Rehabil. 2022 Oct 23;44(22):6899-6925. doi:10.1080/09638288.2021.1974106
- DadeMatthews OO, Roper JA, Vazquez A, Shannon DM, Sefton JM. Prosthetic device and service satisfaction, quality of life, and functional performance in lower limb prosthesis clients. Prosthet Orthot Int. 2024;48(4):422-430. doi:10.1097/PXR.0000000000000285
- Hamilton AB, Schwarz EB, Thomas HN, Goldstein KM. Moving women veterans’ health research forward: a special supplement. J Gen Intern Med. 2022;37(Suppl3):665– 667. doi:10.1007/s11606-022-07606-1
- US Congress. Public Law 116-315: An Act to Improve the Lives of Veterans, S 5108 (2) (F). 116th Congress; 2021. Accessed September 30, 2024. https://www.congress.gov/116/plaws/publ315/PLAW-116publ315.pdf
- Gallagher P, MacLachlan M. The Trinity amputation and prosthesis experience scales and quality of life in people with lower-limb amputation. Arch Phys Med Rehabil. 2004;85(5):730-736. doi:10.1016/j.apmr.2003.07.009
- Legro MW, Reiber GD, Smith DG, del Aguila M, Larsen J, Boone D. Prosthesis evaluation questionnaire for persons with lower limb amputations: assessing prosthesis-related quality of life. Arch Phys Med Rehabil. 1998;79(8):931-938. doi:10.1016/s0003-9993(98)90090-9
- Legro MW, Reiber GD, Smith DG, del Aguila M, Larsen J, Boone D. Prosthesis evaluation questionnaire for persons with lower limb amputations: assessing prosthesis-related quality of life. Arch Phys Med Rehabil. 1998;79(8):931-938. doi:10.1016/s0003-9993(98)90090-9
- Heinemann AW, Bode RK, O’Reilly C. Development and measurement properties of the orthotics and prosthetics users’ survey (OPUS): a comprehensive set of clinical outcome instruments. Prosthet Orthot Int. 2003;27(3):191-206. doi:10.1080/03093640308726682
- Resnik LJ, Borgia ML, Clark MA. A national survey of prosthesis use in veterans with major upper limb amputation: comparisons by gender. PM R. 2020;12(11):1086-1098. doi:10.1002/pmrj.12351
- Collins D. Pretesting survey instruments: an overview of cognitive methods. Qual Life Res. 2003;12(3):229-238. doi:10.1023/a:1023254226592
- Østlie K, Lesjø IM, Franklin RJ, Garfelt B, Skjeldal OH, Magnus P. Prosthesis rejection in acquired major upper-limb amputees: a population-based survey. Disabil Rehabil Assist Technol. 2012;7(4):294-303. doi:10.3109/17483107.2011.635405
- Pezzin LE, Dillingham TR, MacKenzie EJ, Ephraim P, Rossbach P. Use and satisfaction with prosthetic limb devices and related services. Arch Phys Med Rehabil. 2004;85(5):723-729. doi:10.1016/j.apmr.2003.06.002
- Deans S, Burns D, McGarry A, Murray K, Mutrie N. Motivations and barriers to prosthesis users participation in physical activity, exercise and sport: a review of the literature. Prosthet Orthot Int. 2012;36(3):260-269. doi:10.1177/0309364612437905
- McDonald CL, Kahn A, Hafner BJ, Morgan SJ. Prevalence of secondary prosthesis use in lower limb prosthesis users. Disabil Rehabil. 2023;46(5):1016-1022. doi:10.1080/09638288.2023.2182919
- Baars EC, Schrier E, Dijkstra PU, Geertzen JHB. Prosthesis satisfaction in lower limb amputees: a systematic review of associated factors and questionnaires. Medicine (Baltimore). 2018;97(39):e12296. doi:10.1097/MD.0000000000012296
- Murray CD, Fox J. Body image and prosthesis satisfaction in the lower limb amputee. Disabil Rehabil. 2002;24(17):925–931. doi:10.1080/09638280210150014
- Major MJ, Quinlan J, Hansen AH, Esposito ER. Effects of women’s footwear on the mechanical function of heel-height accommodating prosthetic feet. PLoS One. 2022;17(1). doi:10.1371/journal.pone.0262910.
- Kuo PB, Lehavot K, Thomas RM, et al. Gender differences in prosthesis-related outcomes among veterans: results of a national survey of U.S. veterans. PM R. 2024;16(3):239- 249. doi:10.1002/pmrj.13028
- Asano M, Rushton P, Miller WC, Deathe BA. Predictors of quality of life among individuals who have a lower limb amputation. Prosthet Orthot Int. 2008;32(2):231-243. doi:10.1080/03093640802024955
- Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89(3):422-429. doi:10.1016/j.apmr.2007.11.005
- Dillingham TR, Pezzin LE, MacKenzie EJ. Limb amputation and limb deficiency: epidemiology and recent trends in the united states. South Med J. 2002;95(8):875-883. doi:10.1097/00007611-200208000-00018
- Dillingham TR, Pezzin LE, Shore AD. Reamputation, mortality, and health care costs among persons with dysvascular lower-limb amputations. Arch Phys Med Rehabil. 2005;86(3):480-486. doi:10.1016/j.apmr.2004.06.072
- Centers for Disease Control and Prevention. Ambulatory and inpatient procedures in the United States. Accessed September 30, 2024. https://www.cdc.gov/nchs/pressroom/98facts/ambulat.htm
- Ljung J, Iacangelo A. Identifying and acknowledging a sex gap in lower-limb prosthetics. JPO. 2024;36(1):e18-e24. doi:10.1097/JPO.0000000000000470
- Feinglass J, Brown JL, LoSasso A, et al. Rates of lower-extremity amputation and arterial reconstruction in the united states, 1979 to 1996. Am J Public Health. 1999;89(8):1222- 1227. doi:10.2105/ajph.89.8.1222
- Mayfield JA, Reiber GE, Maynard C, Czerniecki JM, Caps MT, Sangeorzan BJ. Trends in lower limb amputation in the Veterans Health Administration, 1989-1998. J Rehabil Res Dev. 2000;37(1):23-30.
- Feinglass J, Pearce WH, Martin GJ, et al. Postoperative and late survival outcomes after major amputation: findings from the department of veterans affairs national surgical quality improvement program. Surgery. 2001;130(1):21-29. doi:10.1067/msy.2001.115359
- Lehavot K, Young JP, Thomas RM, et al. Voices of women veterans with lower limb prostheses: a qualitative study. J Gen Intern Med. 2022;37(3):799-805. doi:10.1007/s11606-022-07572-8
- US Government Accountability Office. COVID-19: Opportunities to improve federal response. GAO-21-60. Published November 12, 2020. Accessed September 30, 2024. https://www.gao.gov/products/gao-21-60
- Littman AJ, Peterson AC, Korpak A, et al. Differences in prosthetic prescription between men and women veterans after transtibial or transfemoral lowerextremity amputation: a longitudinal cohort study. Arch Phys Med Rehabil. 2023;104(8)1274-1281. doi:10.1016/j.amjsurg.2023.02.011
- Cimino SR, Vijayakumar A, MacKay C, Mayo AL, Hitzig SL, Guilcher SJT. Sex and gender differences in quality of life and related domains for individuals with adult acquired lower-limb amputation: a scoping review. Disabil Rehabil. 2022 Oct 23;44(22):6899-6925. doi:10.1080/09638288.2021.1974106
- DadeMatthews OO, Roper JA, Vazquez A, Shannon DM, Sefton JM. Prosthetic device and service satisfaction, quality of life, and functional performance in lower limb prosthesis clients. Prosthet Orthot Int. 2024;48(4):422-430. doi:10.1097/PXR.0000000000000285
- Hamilton AB, Schwarz EB, Thomas HN, Goldstein KM. Moving women veterans’ health research forward: a special supplement. J Gen Intern Med. 2022;37(Suppl3):665– 667. doi:10.1007/s11606-022-07606-1
- US Congress. Public Law 116-315: An Act to Improve the Lives of Veterans, S 5108 (2) (F). 116th Congress; 2021. Accessed September 30, 2024. https://www.congress.gov/116/plaws/publ315/PLAW-116publ315.pdf
- Gallagher P, MacLachlan M. The Trinity amputation and prosthesis experience scales and quality of life in people with lower-limb amputation. Arch Phys Med Rehabil. 2004;85(5):730-736. doi:10.1016/j.apmr.2003.07.009
- Legro MW, Reiber GD, Smith DG, del Aguila M, Larsen J, Boone D. Prosthesis evaluation questionnaire for persons with lower limb amputations: assessing prosthesis-related quality of life. Arch Phys Med Rehabil. 1998;79(8):931-938. doi:10.1016/s0003-9993(98)90090-9
- Legro MW, Reiber GD, Smith DG, del Aguila M, Larsen J, Boone D. Prosthesis evaluation questionnaire for persons with lower limb amputations: assessing prosthesis-related quality of life. Arch Phys Med Rehabil. 1998;79(8):931-938. doi:10.1016/s0003-9993(98)90090-9
- Heinemann AW, Bode RK, O’Reilly C. Development and measurement properties of the orthotics and prosthetics users’ survey (OPUS): a comprehensive set of clinical outcome instruments. Prosthet Orthot Int. 2003;27(3):191-206. doi:10.1080/03093640308726682
- Resnik LJ, Borgia ML, Clark MA. A national survey of prosthesis use in veterans with major upper limb amputation: comparisons by gender. PM R. 2020;12(11):1086-1098. doi:10.1002/pmrj.12351
- Collins D. Pretesting survey instruments: an overview of cognitive methods. Qual Life Res. 2003;12(3):229-238. doi:10.1023/a:1023254226592
- Østlie K, Lesjø IM, Franklin RJ, Garfelt B, Skjeldal OH, Magnus P. Prosthesis rejection in acquired major upper-limb amputees: a population-based survey. Disabil Rehabil Assist Technol. 2012;7(4):294-303. doi:10.3109/17483107.2011.635405
- Pezzin LE, Dillingham TR, MacKenzie EJ, Ephraim P, Rossbach P. Use and satisfaction with prosthetic limb devices and related services. Arch Phys Med Rehabil. 2004;85(5):723-729. doi:10.1016/j.apmr.2003.06.002
- Deans S, Burns D, McGarry A, Murray K, Mutrie N. Motivations and barriers to prosthesis users participation in physical activity, exercise and sport: a review of the literature. Prosthet Orthot Int. 2012;36(3):260-269. doi:10.1177/0309364612437905
- McDonald CL, Kahn A, Hafner BJ, Morgan SJ. Prevalence of secondary prosthesis use in lower limb prosthesis users. Disabil Rehabil. 2023;46(5):1016-1022. doi:10.1080/09638288.2023.2182919
- Baars EC, Schrier E, Dijkstra PU, Geertzen JHB. Prosthesis satisfaction in lower limb amputees: a systematic review of associated factors and questionnaires. Medicine (Baltimore). 2018;97(39):e12296. doi:10.1097/MD.0000000000012296
- Murray CD, Fox J. Body image and prosthesis satisfaction in the lower limb amputee. Disabil Rehabil. 2002;24(17):925–931. doi:10.1080/09638280210150014
- Major MJ, Quinlan J, Hansen AH, Esposito ER. Effects of women’s footwear on the mechanical function of heel-height accommodating prosthetic feet. PLoS One. 2022;17(1). doi:10.1371/journal.pone.0262910.
- Kuo PB, Lehavot K, Thomas RM, et al. Gender differences in prosthesis-related outcomes among veterans: results of a national survey of U.S. veterans. PM R. 2024;16(3):239- 249. doi:10.1002/pmrj.13028
- Asano M, Rushton P, Miller WC, Deathe BA. Predictors of quality of life among individuals who have a lower limb amputation. Prosthet Orthot Int. 2008;32(2):231-243. doi:10.1080/03093640802024955
Satisfaction With Department of Veterans Affairs Prosthetics and Support Services as Reported by Women and Men Veterans
Satisfaction With Department of Veterans Affairs Prosthetics and Support Services as Reported by Women and Men Veterans
Effect of Alirocumab Monotherapy vs Ezetimibe Plus Statin Therapy on LDL-C Lowering in Veterans With History of ASCVD
Atherosclerotic cardiovascular disease (ASCVD) is a significant cause of morbidity and mortality in the United States. ASCVD involves the buildup of cholesterol plaque in arteries and includes acute coronary syndrome, peripheral arterial disease, and events such as myocardial infarction and stroke.1 Cardiovascular disease (CVD) risk factors include high cholesterol levels, elevated blood pressure, insulin resistance, elevated blood glucose levels, smoking, poor dietary habits, and a sedentary lifestyle.2
According to the Centers for Disease Control and Prevention, about 86 million adults aged ≥ 20 years have total cholesterol levels > 200 mg/dL. More than half (54.5%) who could benefit are currently taking cholesterol-lowering medications.3 Controlling high cholesterol in American adults, especially veterans, is essential for reducing CVD morbidity and mortality.
The 2018 American College of Cardiology/American Heart Association (ACC/AHA) guideline recommends a low-density lipoprotein cholesterol (LDL-C) target goal of < 70 mg/dL for patients at high risk for ASCVD. Very high-risk ASCVD includes a history of multiple major ASCVD events or 1 major ASCVD event and multiple high-risk conditions (eg, age ≥ 65 years, smoking, or diabetes).4 Major ASCVD events include recent acute coronary syndrome (within the past 12 months), a history of myocardial infarction or ischemic stroke, and symptomatic peripheral artery disease.
The ACC/AHA guideline suggests that if the LDL-C level remains ≥ 70 mg/dL, adding ezetimibe (a dietary cholesterol absorption inhibitor) to maximally tolerated statin therapy is reasonable. If LDL-C levels remain ≥ 70 mg/dL, adding a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor, such as alirocumab, is reasonable.4 The US Departments of Veterans Affairs/US Department of Defense guidelines recommend using maximally tolerated statins and ezetimibe before PCSK9 inhibitors due to established long-term safety and reduction in CVD events.
Generic statins and ezetimibe are administered orally and widely available. In contrast, PCSK9 inhibitors have unknown long-term safety profiles, require subcutaneous injection once or twice monthly, and are significantly more expensive. They also require patient education on proper use while providing comparable or lesser relative risk reductions.2
These 3 classes of medication vary in their mechanisms of action to reduce LDL.5,6 Ezetimibe and several statin medications are included on the Veterans Affairs Sioux Falls Health Care System (VASFHCS) formulary and do not require review prior to prescribing. Alirocumab is available at VASFHCS but is restricted to patients with a history of ASCVD or a diagnosis of familial hypercholesterolemia, and who are receiving maximally tolerated statin and ezetimibe therapy but require further LDL-C lowering to reduce their ASCVD risk.
Studies have found ezetimibe monotherapy reduces LDL-C in patients with dyslipidemia by 18% after 12 weeks.7 One found that the percentage reduction in LDL-C was significantly greater (P < .001) with all doses of ezetimibe plus simvastatin (46% to 59%) compared with either atorvastatin 10 mg (37%) or simvastatin 20 mg (38%) monotherapy after 6 weeks.8
Although alirocumab can be added to other lipid therapies, most VASFHCS patients are prescribed alirocumab monotherapy. In the ODYSSEY CHOICE II study, patients were randomly assigned to receive either a placebo or alirocumab 150 mg every 4 weeks or alirocumab 75 mg every 2 weeks. The primary efficacy endpoint was LDL-C percentage change from baseline to week 24. In the alirocumab 150 mg every 4 weeks and 75 mg every 2 weeks groups, the least-squares mean LDL-C changes from baseline to week 24 were 51.7% and 53.5%, respectively, compared to a 4.7% increase in the placebo group (both groups P < .001 vs placebo). The authors also reported that alirocumab 150 mg every 4 weeks as monotherapy demonstrated a 47.4% reduction in LDL-C levels from baseline in a phase 1 study.9Although alirocumab monotherapy and ezetimibe plus statin therapy have been shown to effectively decrease LDL-C independently, a direct comparison of alirocumab monotherapy vs ezetimibe plus statin therapy has not been assessed, to our knowledge. Understanding the differences in effectiveness and safety between these 2 regimens will be valuable for clinicians when selecting a medication regimen for veterans with a history of ASCVD.
METHODS
This retrospective, single-center chart review used VASFHCS Computerized Patient Record System (CPRS) and Joint Longitudinal Viewer (JLV) records to compare patients with a history of ASCVD events who were treated with alirocumab monotherapy or ezetimibe plus statin. The 2 groups were randomized in a 1:3 ratio. The primary endpoint was achieving LDL-C < 70 mg/dL after 4 to 12 weeks, 13 to 24 weeks, and 25 to 52 weeks. Secondary endpoints included the mean percentage change from baseline in total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), LDL-C, and triglycerides (TG) over 52 weeks. The incidence of ASCVD events during this period was also assessed. If LDL-C < 70 mg/dL was achieved > 1 time during each time frame, only 1 incident was counted for analysis. Safety was assessed based on the incidence of any adverse event (AE) that led to treatment discontinuation.
Patients were identified by screening the prescription fill history between October 1, 2019, and December 31, 2022. The 52-week data collection period was counted from the first available fill date. Additionally, the prior authorization drug request file from January 1, 2017, to December 31, 2022, was used to obtain a list of patients prescribed alirocumab. Patients were included if they were veterans aged ≥ 18 years and had a history of an ASCVD event, had a alirocumab monotherapy or ezetimibe plus statin prescription between October 1, 2019, and December 31, 2022, or had an approved prior authorization drug request for alirocumab between January 1, 2017, and December 31, 2022. Patients missing a baseline or follow-up lipid panel and those with concurrent use of alirocumab and ezetimibe and/or statin were excluded.
Baseline characteristics collected for patients included age, sex, race, weight, body mass index, lipid parameters (LDL-C, TC, HDL-C, and TG), dosing of each type of statin before adding ezetimibe, and use of any other antihyperlipidemic medication. We also collected histories of hypertension, hyperlipidemia, diabetes, chronic kidney disease, congestive heart failure, and smoking or tobacco use status. The baseline lipid panel was the most recent lipid panel documented before starting alirocumab or ezetimibe plus statin therapy. Follow-up lipid panel values were gathered at 4 to 12 weeks, 13 to 24 weeks, and 25 to 52 weeks following initiation of either therapy.
High-, moderate-, and low-intensity dosing of statin therapy and alirocumab dosing (75 mg every 2 weeks, 150 mg every 2 weeks, or 300 mg every 4 weeks) were recorded at the specified intervals. However, no patients in this study received the latter dosing regimen. ASCVD events and safety endpoints were recorded based on a review of clinical notes over the 52 weeks following the first available start date.
Statistical Analysis
The primary endpoint of achieving the LDL-C < 70 mg/dL goal from baseline to 4 to 12 weeks, 13 to 24 weeks, and 25 to 52 weeks after initiation was compared between alirocumab monotherapy and ezetimibe plus statin therapy using the χ² test. Mean percentage change from baseline in TC, HDL-C, LDL-C, and TG were compared using the independent t test. P < .05 was considered statistically significant. Incidence of ASCVD events and the safety endpoint (incidence of AEs leading to treatment discontinuation) were also compared using the χ² test. Continuous baseline characteristics were reported mean (SD) and nominal baseline characteristics were reported as a percentage.
RESULTS
There were 80 participants in this study: 20 in the alirocumab monotherapy group and 60 in the ezetimibe plus statin therapy group. More than 100 patients did not meet the prespecified inclusion criteria and were excluded. Mean (SD) age was 75 (8) years in the alirocumab group and 74 (8) years in the ezetimibe plus statin group. There was no significant differences in mean (SD) weight or mean (SD) body mass index. All study participants identified as White and male except for 2 patients in the ezetimibe plus statin therapy group whose race was not documented. Differences in lipid parameters were observed between groups, with mean baseline LDL-C, HDL-C, and TC higher in the alirocumab monotherapy group than in the ezetimibe plus statin therapy group, with significant differences in LDL-C and TC (Table 1).
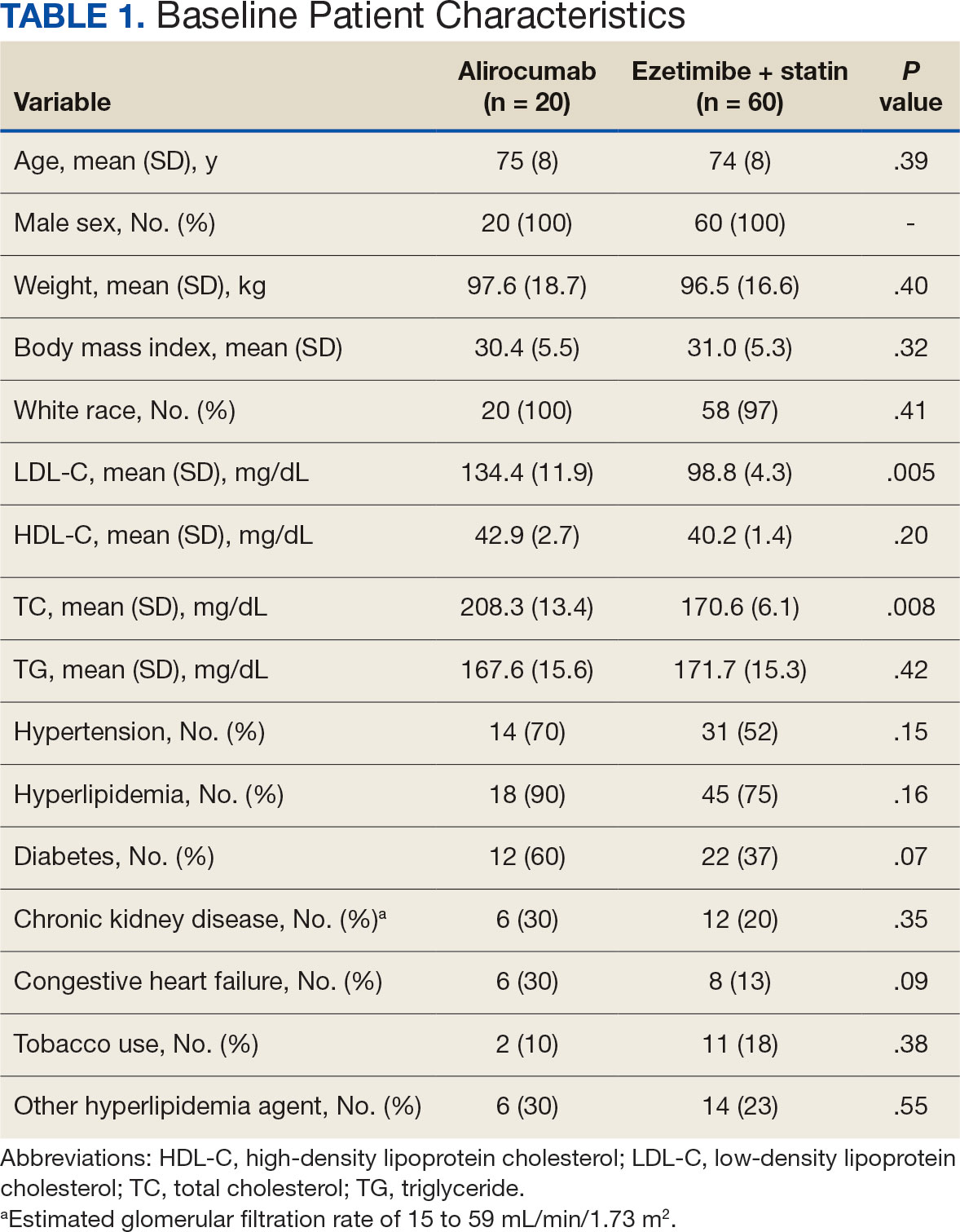
Fourteen patients (70%) in the alirocumab monotherapy group had hypertension, compared with 31 (52%) in the ezetimibe plus statin therapy group. In both groups, most patients had previously been diagnosed with hyperlipidemia. More patients (60%) in the alirocumab group had diabetes than in the ezetimibe plus statin therapy group (37%). The alirocumab monotherapy group also had a higher percentage of patients with diagnoses of congestive heart failure and used other antihyperlipidemic medications than in the ezetimibe plus statin therapy group. Five patients (25%) in the alirocumab monotherapy group and 12 patients (20%) in the ezetimibe plus statin therapy group took fish oil. In the ezetimibe plus statin therapy group, 2 patients (3%) took gemfibrozil, and 2 patients (3%) took fenofibrate. Six (30%) patients in the alirocumab monotherapy group and 12 (20%) patients in the ezetimibe plus statin therapy group had chronic kidney disease. Although the majority of patients in each group did not use tobacco products, there were more tobacco users in the ezetimibe plus statin therapy group.
In the alirocumab monotherapy group, 15 patients (75%) were prescribed 75 mg every 2 weeks and 5 patients (25%) were prescribed 150 mg every 2 weeks. In the ezetimibe plus statin therapy group, 59 patients (98%) were prescribed ezetimibe 10 mg/d (Table 2). Forty-three patients (72%) were prescribed a high-intensity statin 10 received moderate-intensity (17%) and 7 received low-intensity statin (12%). Most patients were prescribed rosuvastatin (45%), followed by atorvastatin (42%), pravastatin (10%), and simvastatin (3%).
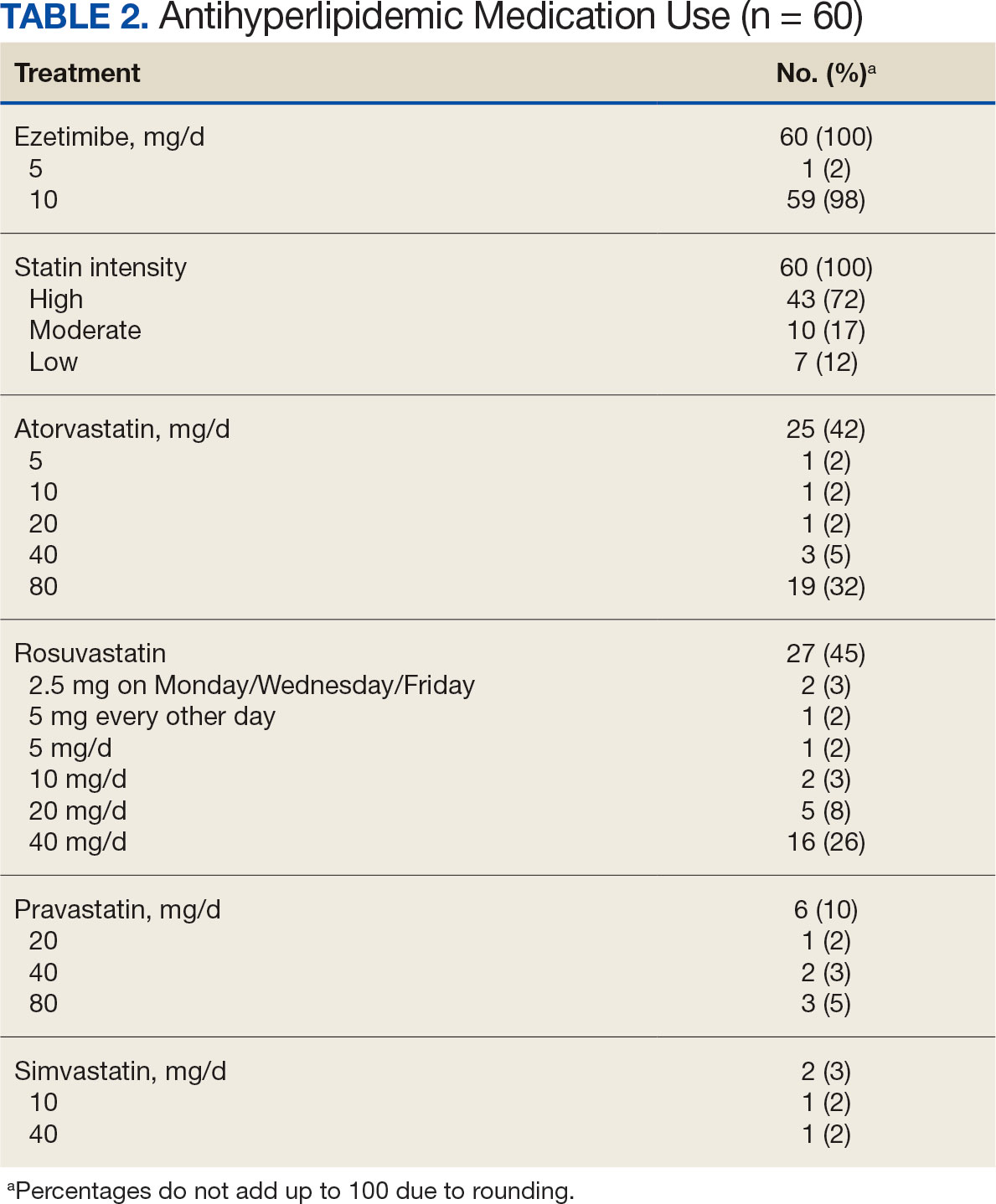
Primary Endpoint
During the 52-week study, more patients met the LDL-C goal of < 70 mg/dL in the alirocumab monotherapy group (70%) than in the ezetimibe plus statin therapy group (57%); however, the difference was not significant (P = .29). Of the patients prescribed alirocumab monotherapy who achieved LDL-C < 70 mg/dL, 15% achieved this goal in 4 to 12 weeks, 40% in 13 to 24 weeks, and 45% in 25 to 52 weeks. In the ezetimibe plus statin therapy group, 28% of patients achieved LDL-C < 70 mg/dL in 4 to 12 weeks, 31% in 13 to 24 weeks, and 41% in 25 to 52 weeks (Table 3).
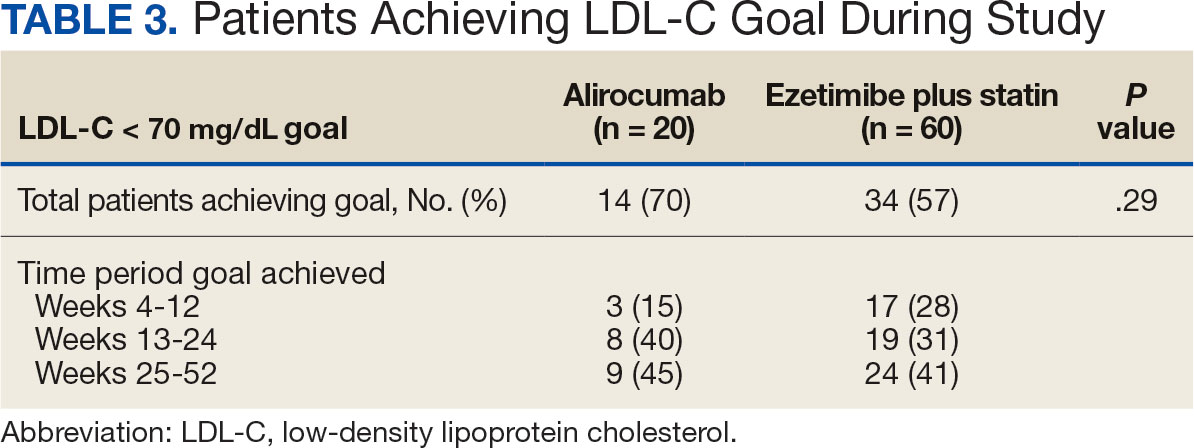
Secondary Endpoints
During weeks 4 to 52 of treatment, the mean percentage change decreased in LDL-C (37.7% vs 21.4%; P = .01), TC (24.7% vs 12.5%; P = .01), and TG (0.9% vs 7.0%; P = .28) in the alirocumab monotherapy group and the ezetimibe plus statin therapy group, respectively (Table 4). The mean percentage change increased in HDL-C by 3.6% in the alirocumab monotherapy group and 1.8% in the ezetimibe plus statin therapy group (P = .36). During the study, ASCVD events occurred in 1 patient (5%) in the alirocumab monotherapy group and 3 patients (5%) in the ezetimibe plus statin therapy group (P = .99). The patient in the alirocumab monotherapy group had unstable angina 1 month after taking alirocumab. One patient in the ezetimibe plus statin therapy group had coronary artery disease and 2 patients had coronary heart disease that required stents during the 52-week period. There was 1 patient in each group who reported an AE that led to treatment discontinuation (P = .41). One patient stopped alirocumab after a trial of 2 months due to intolerance, but no specific AE was reported in the CPRS. In the ezetimibe plus statin therapy group, 1 patient requested to discontinue ezetimibe after a trial of 3 months without a specific reason noted in the medical record.
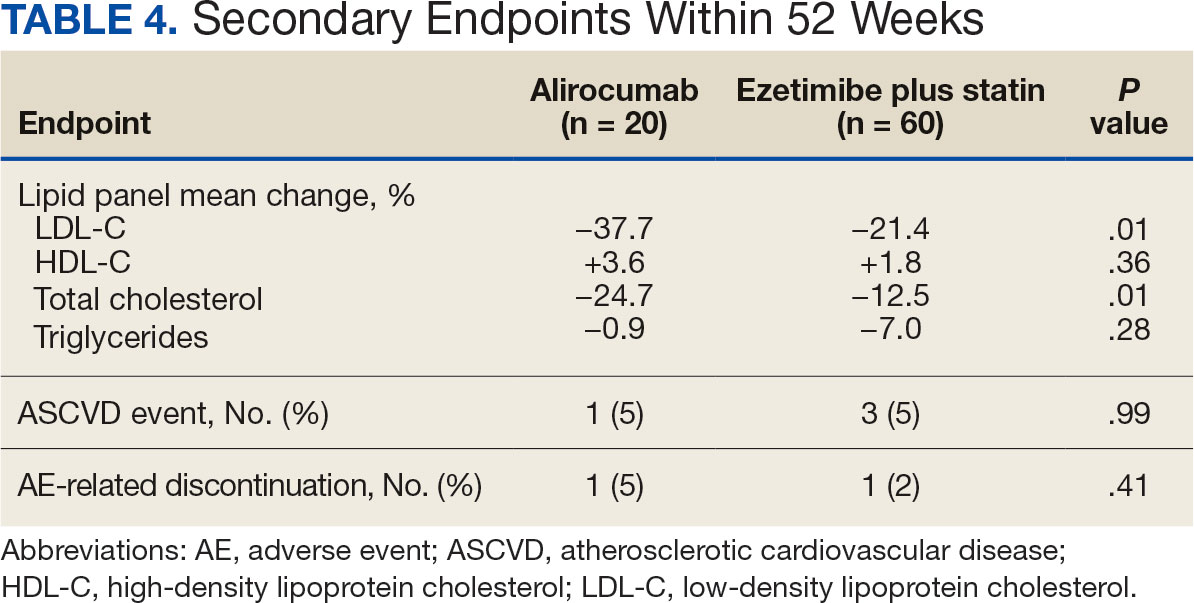
DISCUSSION
This study found no statistically significant difference in the incidence of reaching an LDL-C goal of < 70 mg/dL after alirocumab monotherapy initiation compared with ezetimibe plus statin therapy. This occurred despite baseline LDL-C being lower in the ezetimibe plus statin therapy group, which required a smaller reduction in LDL-C to reach the primary goal. Most patients on alirocumab monotherapy were prescribed a lower initial dose of 75 mg every 2 weeks. Of those patients, 30% did not achieve the LDL-C goal < 70 mg/dL. Thus, a higher dose may have led to more patients achieving the LDL-C goal.
Secondary endpoints, including mean percentage change in HDL-C and TG and incidence of ASCVD events during 52 weeks of treatment, were not statistically significant. The mean percentage increase in HDL-C was negligible in both groups, while the mean percentage reduction in TG favored the ezetimibe plus statin therapy group. In the ezetimibe plus statin therapy group, patients who also took fenofibrate experienced a significant reduction in TG while none of the patients in the alirocumab group were prescribed fenofibrate. Although the alirocumab monotherapy group had a statistically significant greater reduction in LDL-C and TC compared with those prescribed ezetimibe plus statin, the mean baseline LDL-C and TC were significantly greater in the alirocumab monotherapy group, which could contribute to higher reductions in LDL-C and TC after alirocumab monotherapy.Based on the available literature, we expected greater reductions in LDL-C in both study groups compared with statin therapy alone.8,9 However, it was unclear whether the LDL-C and TC reductions were clinically significant.
Limitations
The study design did not permit randomization prior to the treatments, restricting our ability to account for some confounding factors, such as diet, exercise, other antihyperlipidemic medication, and medication adherence, which may have affected LDL-C, HDL-C, TG, and TC levels. Differences in baseline characteristics—particularly major risk factors, such as hypertension, diabetes, and tobacco use—also could have confounding affect on lipid levels and ASCVD events. Additionally, patients prescribed alirocumab monotherapy may have switched from statin or ezetimibe therapy, and the washout period was not reviewed or recorded, which could have affected the lipid panel results.
The small sample size of this study also may have limited the ability to detect significant differences between groups. A direct comparison of alirocumab monotherapy vs ezetimibe plus statin therapy has not been performed, making it difficult to prospectively evaluate what sample size would be needed to power this study. A posthoc analysis was used to calculate power, which was found to be only 17%. Many patients were excluded due to a lack of laboratory results within the study period, contributing to the small sample size.
Another limitation was the reliance on documentation in CPRS and JLV. For example, having documentation of the specific AEs for the 2 patients who discontinued alirocumab or ezetimibe could have helped determine the severity of the AEs. Several patients were followed by non-VA clinicians, which could have contributed to limited documentation in the CPRS and JLV. It is difficult to draw any conclusions regarding ASCVD events and AEs that led to treatment discontinuation between alirocumab monotherapy and ezetimibe plus statin therapy based on the results of this retrospective study due to the limited number of events within the 52-week period.
CONCLUSIONS
This study found that there was no statistically significant difference in LDL-C reduction to < 70 mg/dL between alirocumab monotherapy and ezetimibe plus statin therapy in a small population of veterans with ASCVD, with a higher percentage of participants in both groups achieving that goal in 25 to 52 weeks. There also was no significant difference in percentage change in HDL-C or TG or in incidence of ASCVD events and AEs leading to treatment discontinuation. However, there was a statistically significant difference in percentage reduction for LDL-C and TC during 52 weeks of alirocumab monotherapy vs ezetimibe plus statin therapy.
Although there was no significant difference in LDL-C reduction to < 70 mg/dL, targeting this goal in patients with ASCVD is still clinically warranted. This study does not support a change in current VA criteria for use of alirocumab or a change in current guidelines for secondary prevention of ASCVD. Still, this study does indicate that the efficacy of alirocumab monotherapy is similar to that of ezetimibe plus statin therapy in patients with a history of ASCVD and may be useful in clinical settings when an alternative to ezetimibe plus statin therapy is needed. Alirocumab also may be more effective in lowering LDL-C and TC than ezetimibe plus statin therapy in veterans with ASCVD and could be added to statin therapy or ezetimibe when additional LDL-C or TC reduction is needed.
Lucchi T. Dyslipidemia and prevention of atherosclerotic cardiovascular disease in the elderly. Minerva Med. 2021;112:804-816. doi:10.23736/S0026-4806.21.07347-X
The Management of Dyslipidemia for Cardiovascular Risk Reduction Work Group. VA/DoD Clinical Practice Guideline for the Management of Dyslipidemia for Cardiovascular Risk Reduction. Version 4.0. June 2020. Accessed September 5, 2024. https://www.healthquality.va.gov/guidelines/CD/lipids/VADoDDyslipidemiaCPG5087212020.pdf
Centers for Disease Control and Prevention. High Cholesterol Facts. May 15, 2024. Accessed October 3, 2024. https://www.cdc.gov/cholesterol/data-research/facts-stats/index.html
Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082-e1143. doi:10.1161/CIR.0000000000000625
Vavlukis M, Vavlukis A. Statins alone or in combination with ezetimibe or PCSK9 inhibitors in atherosclerotic cardiovascular disease protection. IntechOpen. January 24, 2019. doi:10.5772/intechopen.82520
Alirocumab. Prescribing information. Regeneron Pharmaceuticals, Inc.; 2024. Accessed September 5, 2024. https://www.regeneron.com/downloads/praluent_pi.pdf
Pandor A, Ara RM, Tumur I, et al. Ezetimibe monotherapy for cholesterol lowering in 2,722 people: systematic review and meta-analysis of randomized controlled trials. J Intern Med. 2009;265(5):568-580. doi:10.1111/j.1365-2796.2008.02062.x
McKenney J, Ballantyne CM, Feldman TA, et al. LDL-C goal attainment with ezetimibe plus simvastatin coadministration vs atorvastatin or simvastatin monotherapy in patients at high risk of CHD. MedGenMed. 2005;7(3):3.
Stroes E, Guyton JR, Lepor N, et al. Efficacy and safety of alirocumab 150 mg every 4 weeks in patients with hypercholesterolemia not on statin therapy: the ODYSSEY CHOICE II study. J Am Heart Assoc. 2016;5(9):e003421. doi:10.1161/JAHA.116.003421
Atherosclerotic cardiovascular disease (ASCVD) is a significant cause of morbidity and mortality in the United States. ASCVD involves the buildup of cholesterol plaque in arteries and includes acute coronary syndrome, peripheral arterial disease, and events such as myocardial infarction and stroke.1 Cardiovascular disease (CVD) risk factors include high cholesterol levels, elevated blood pressure, insulin resistance, elevated blood glucose levels, smoking, poor dietary habits, and a sedentary lifestyle.2
According to the Centers for Disease Control and Prevention, about 86 million adults aged ≥ 20 years have total cholesterol levels > 200 mg/dL. More than half (54.5%) who could benefit are currently taking cholesterol-lowering medications.3 Controlling high cholesterol in American adults, especially veterans, is essential for reducing CVD morbidity and mortality.
The 2018 American College of Cardiology/American Heart Association (ACC/AHA) guideline recommends a low-density lipoprotein cholesterol (LDL-C) target goal of < 70 mg/dL for patients at high risk for ASCVD. Very high-risk ASCVD includes a history of multiple major ASCVD events or 1 major ASCVD event and multiple high-risk conditions (eg, age ≥ 65 years, smoking, or diabetes).4 Major ASCVD events include recent acute coronary syndrome (within the past 12 months), a history of myocardial infarction or ischemic stroke, and symptomatic peripheral artery disease.
The ACC/AHA guideline suggests that if the LDL-C level remains ≥ 70 mg/dL, adding ezetimibe (a dietary cholesterol absorption inhibitor) to maximally tolerated statin therapy is reasonable. If LDL-C levels remain ≥ 70 mg/dL, adding a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor, such as alirocumab, is reasonable.4 The US Departments of Veterans Affairs/US Department of Defense guidelines recommend using maximally tolerated statins and ezetimibe before PCSK9 inhibitors due to established long-term safety and reduction in CVD events.
Generic statins and ezetimibe are administered orally and widely available. In contrast, PCSK9 inhibitors have unknown long-term safety profiles, require subcutaneous injection once or twice monthly, and are significantly more expensive. They also require patient education on proper use while providing comparable or lesser relative risk reductions.2
These 3 classes of medication vary in their mechanisms of action to reduce LDL.5,6 Ezetimibe and several statin medications are included on the Veterans Affairs Sioux Falls Health Care System (VASFHCS) formulary and do not require review prior to prescribing. Alirocumab is available at VASFHCS but is restricted to patients with a history of ASCVD or a diagnosis of familial hypercholesterolemia, and who are receiving maximally tolerated statin and ezetimibe therapy but require further LDL-C lowering to reduce their ASCVD risk.
Studies have found ezetimibe monotherapy reduces LDL-C in patients with dyslipidemia by 18% after 12 weeks.7 One found that the percentage reduction in LDL-C was significantly greater (P < .001) with all doses of ezetimibe plus simvastatin (46% to 59%) compared with either atorvastatin 10 mg (37%) or simvastatin 20 mg (38%) monotherapy after 6 weeks.8
Although alirocumab can be added to other lipid therapies, most VASFHCS patients are prescribed alirocumab monotherapy. In the ODYSSEY CHOICE II study, patients were randomly assigned to receive either a placebo or alirocumab 150 mg every 4 weeks or alirocumab 75 mg every 2 weeks. The primary efficacy endpoint was LDL-C percentage change from baseline to week 24. In the alirocumab 150 mg every 4 weeks and 75 mg every 2 weeks groups, the least-squares mean LDL-C changes from baseline to week 24 were 51.7% and 53.5%, respectively, compared to a 4.7% increase in the placebo group (both groups P < .001 vs placebo). The authors also reported that alirocumab 150 mg every 4 weeks as monotherapy demonstrated a 47.4% reduction in LDL-C levels from baseline in a phase 1 study.9Although alirocumab monotherapy and ezetimibe plus statin therapy have been shown to effectively decrease LDL-C independently, a direct comparison of alirocumab monotherapy vs ezetimibe plus statin therapy has not been assessed, to our knowledge. Understanding the differences in effectiveness and safety between these 2 regimens will be valuable for clinicians when selecting a medication regimen for veterans with a history of ASCVD.
METHODS
This retrospective, single-center chart review used VASFHCS Computerized Patient Record System (CPRS) and Joint Longitudinal Viewer (JLV) records to compare patients with a history of ASCVD events who were treated with alirocumab monotherapy or ezetimibe plus statin. The 2 groups were randomized in a 1:3 ratio. The primary endpoint was achieving LDL-C < 70 mg/dL after 4 to 12 weeks, 13 to 24 weeks, and 25 to 52 weeks. Secondary endpoints included the mean percentage change from baseline in total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), LDL-C, and triglycerides (TG) over 52 weeks. The incidence of ASCVD events during this period was also assessed. If LDL-C < 70 mg/dL was achieved > 1 time during each time frame, only 1 incident was counted for analysis. Safety was assessed based on the incidence of any adverse event (AE) that led to treatment discontinuation.
Patients were identified by screening the prescription fill history between October 1, 2019, and December 31, 2022. The 52-week data collection period was counted from the first available fill date. Additionally, the prior authorization drug request file from January 1, 2017, to December 31, 2022, was used to obtain a list of patients prescribed alirocumab. Patients were included if they were veterans aged ≥ 18 years and had a history of an ASCVD event, had a alirocumab monotherapy or ezetimibe plus statin prescription between October 1, 2019, and December 31, 2022, or had an approved prior authorization drug request for alirocumab between January 1, 2017, and December 31, 2022. Patients missing a baseline or follow-up lipid panel and those with concurrent use of alirocumab and ezetimibe and/or statin were excluded.
Baseline characteristics collected for patients included age, sex, race, weight, body mass index, lipid parameters (LDL-C, TC, HDL-C, and TG), dosing of each type of statin before adding ezetimibe, and use of any other antihyperlipidemic medication. We also collected histories of hypertension, hyperlipidemia, diabetes, chronic kidney disease, congestive heart failure, and smoking or tobacco use status. The baseline lipid panel was the most recent lipid panel documented before starting alirocumab or ezetimibe plus statin therapy. Follow-up lipid panel values were gathered at 4 to 12 weeks, 13 to 24 weeks, and 25 to 52 weeks following initiation of either therapy.
High-, moderate-, and low-intensity dosing of statin therapy and alirocumab dosing (75 mg every 2 weeks, 150 mg every 2 weeks, or 300 mg every 4 weeks) were recorded at the specified intervals. However, no patients in this study received the latter dosing regimen. ASCVD events and safety endpoints were recorded based on a review of clinical notes over the 52 weeks following the first available start date.
Statistical Analysis
The primary endpoint of achieving the LDL-C < 70 mg/dL goal from baseline to 4 to 12 weeks, 13 to 24 weeks, and 25 to 52 weeks after initiation was compared between alirocumab monotherapy and ezetimibe plus statin therapy using the χ² test. Mean percentage change from baseline in TC, HDL-C, LDL-C, and TG were compared using the independent t test. P < .05 was considered statistically significant. Incidence of ASCVD events and the safety endpoint (incidence of AEs leading to treatment discontinuation) were also compared using the χ² test. Continuous baseline characteristics were reported mean (SD) and nominal baseline characteristics were reported as a percentage.
RESULTS
There were 80 participants in this study: 20 in the alirocumab monotherapy group and 60 in the ezetimibe plus statin therapy group. More than 100 patients did not meet the prespecified inclusion criteria and were excluded. Mean (SD) age was 75 (8) years in the alirocumab group and 74 (8) years in the ezetimibe plus statin group. There was no significant differences in mean (SD) weight or mean (SD) body mass index. All study participants identified as White and male except for 2 patients in the ezetimibe plus statin therapy group whose race was not documented. Differences in lipid parameters were observed between groups, with mean baseline LDL-C, HDL-C, and TC higher in the alirocumab monotherapy group than in the ezetimibe plus statin therapy group, with significant differences in LDL-C and TC (Table 1).

Fourteen patients (70%) in the alirocumab monotherapy group had hypertension, compared with 31 (52%) in the ezetimibe plus statin therapy group. In both groups, most patients had previously been diagnosed with hyperlipidemia. More patients (60%) in the alirocumab group had diabetes than in the ezetimibe plus statin therapy group (37%). The alirocumab monotherapy group also had a higher percentage of patients with diagnoses of congestive heart failure and used other antihyperlipidemic medications than in the ezetimibe plus statin therapy group. Five patients (25%) in the alirocumab monotherapy group and 12 patients (20%) in the ezetimibe plus statin therapy group took fish oil. In the ezetimibe plus statin therapy group, 2 patients (3%) took gemfibrozil, and 2 patients (3%) took fenofibrate. Six (30%) patients in the alirocumab monotherapy group and 12 (20%) patients in the ezetimibe plus statin therapy group had chronic kidney disease. Although the majority of patients in each group did not use tobacco products, there were more tobacco users in the ezetimibe plus statin therapy group.
In the alirocumab monotherapy group, 15 patients (75%) were prescribed 75 mg every 2 weeks and 5 patients (25%) were prescribed 150 mg every 2 weeks. In the ezetimibe plus statin therapy group, 59 patients (98%) were prescribed ezetimibe 10 mg/d (Table 2). Forty-three patients (72%) were prescribed a high-intensity statin 10 received moderate-intensity (17%) and 7 received low-intensity statin (12%). Most patients were prescribed rosuvastatin (45%), followed by atorvastatin (42%), pravastatin (10%), and simvastatin (3%).

Primary Endpoint
During the 52-week study, more patients met the LDL-C goal of < 70 mg/dL in the alirocumab monotherapy group (70%) than in the ezetimibe plus statin therapy group (57%); however, the difference was not significant (P = .29). Of the patients prescribed alirocumab monotherapy who achieved LDL-C < 70 mg/dL, 15% achieved this goal in 4 to 12 weeks, 40% in 13 to 24 weeks, and 45% in 25 to 52 weeks. In the ezetimibe plus statin therapy group, 28% of patients achieved LDL-C < 70 mg/dL in 4 to 12 weeks, 31% in 13 to 24 weeks, and 41% in 25 to 52 weeks (Table 3).

Secondary Endpoints
During weeks 4 to 52 of treatment, the mean percentage change decreased in LDL-C (37.7% vs 21.4%; P = .01), TC (24.7% vs 12.5%; P = .01), and TG (0.9% vs 7.0%; P = .28) in the alirocumab monotherapy group and the ezetimibe plus statin therapy group, respectively (Table 4). The mean percentage change increased in HDL-C by 3.6% in the alirocumab monotherapy group and 1.8% in the ezetimibe plus statin therapy group (P = .36). During the study, ASCVD events occurred in 1 patient (5%) in the alirocumab monotherapy group and 3 patients (5%) in the ezetimibe plus statin therapy group (P = .99). The patient in the alirocumab monotherapy group had unstable angina 1 month after taking alirocumab. One patient in the ezetimibe plus statin therapy group had coronary artery disease and 2 patients had coronary heart disease that required stents during the 52-week period. There was 1 patient in each group who reported an AE that led to treatment discontinuation (P = .41). One patient stopped alirocumab after a trial of 2 months due to intolerance, but no specific AE was reported in the CPRS. In the ezetimibe plus statin therapy group, 1 patient requested to discontinue ezetimibe after a trial of 3 months without a specific reason noted in the medical record.

DISCUSSION
This study found no statistically significant difference in the incidence of reaching an LDL-C goal of < 70 mg/dL after alirocumab monotherapy initiation compared with ezetimibe plus statin therapy. This occurred despite baseline LDL-C being lower in the ezetimibe plus statin therapy group, which required a smaller reduction in LDL-C to reach the primary goal. Most patients on alirocumab monotherapy were prescribed a lower initial dose of 75 mg every 2 weeks. Of those patients, 30% did not achieve the LDL-C goal < 70 mg/dL. Thus, a higher dose may have led to more patients achieving the LDL-C goal.
Secondary endpoints, including mean percentage change in HDL-C and TG and incidence of ASCVD events during 52 weeks of treatment, were not statistically significant. The mean percentage increase in HDL-C was negligible in both groups, while the mean percentage reduction in TG favored the ezetimibe plus statin therapy group. In the ezetimibe plus statin therapy group, patients who also took fenofibrate experienced a significant reduction in TG while none of the patients in the alirocumab group were prescribed fenofibrate. Although the alirocumab monotherapy group had a statistically significant greater reduction in LDL-C and TC compared with those prescribed ezetimibe plus statin, the mean baseline LDL-C and TC were significantly greater in the alirocumab monotherapy group, which could contribute to higher reductions in LDL-C and TC after alirocumab monotherapy.Based on the available literature, we expected greater reductions in LDL-C in both study groups compared with statin therapy alone.8,9 However, it was unclear whether the LDL-C and TC reductions were clinically significant.
Limitations
The study design did not permit randomization prior to the treatments, restricting our ability to account for some confounding factors, such as diet, exercise, other antihyperlipidemic medication, and medication adherence, which may have affected LDL-C, HDL-C, TG, and TC levels. Differences in baseline characteristics—particularly major risk factors, such as hypertension, diabetes, and tobacco use—also could have confounding affect on lipid levels and ASCVD events. Additionally, patients prescribed alirocumab monotherapy may have switched from statin or ezetimibe therapy, and the washout period was not reviewed or recorded, which could have affected the lipid panel results.
The small sample size of this study also may have limited the ability to detect significant differences between groups. A direct comparison of alirocumab monotherapy vs ezetimibe plus statin therapy has not been performed, making it difficult to prospectively evaluate what sample size would be needed to power this study. A posthoc analysis was used to calculate power, which was found to be only 17%. Many patients were excluded due to a lack of laboratory results within the study period, contributing to the small sample size.
Another limitation was the reliance on documentation in CPRS and JLV. For example, having documentation of the specific AEs for the 2 patients who discontinued alirocumab or ezetimibe could have helped determine the severity of the AEs. Several patients were followed by non-VA clinicians, which could have contributed to limited documentation in the CPRS and JLV. It is difficult to draw any conclusions regarding ASCVD events and AEs that led to treatment discontinuation between alirocumab monotherapy and ezetimibe plus statin therapy based on the results of this retrospective study due to the limited number of events within the 52-week period.
CONCLUSIONS
This study found that there was no statistically significant difference in LDL-C reduction to < 70 mg/dL between alirocumab monotherapy and ezetimibe plus statin therapy in a small population of veterans with ASCVD, with a higher percentage of participants in both groups achieving that goal in 25 to 52 weeks. There also was no significant difference in percentage change in HDL-C or TG or in incidence of ASCVD events and AEs leading to treatment discontinuation. However, there was a statistically significant difference in percentage reduction for LDL-C and TC during 52 weeks of alirocumab monotherapy vs ezetimibe plus statin therapy.
Although there was no significant difference in LDL-C reduction to < 70 mg/dL, targeting this goal in patients with ASCVD is still clinically warranted. This study does not support a change in current VA criteria for use of alirocumab or a change in current guidelines for secondary prevention of ASCVD. Still, this study does indicate that the efficacy of alirocumab monotherapy is similar to that of ezetimibe plus statin therapy in patients with a history of ASCVD and may be useful in clinical settings when an alternative to ezetimibe plus statin therapy is needed. Alirocumab also may be more effective in lowering LDL-C and TC than ezetimibe plus statin therapy in veterans with ASCVD and could be added to statin therapy or ezetimibe when additional LDL-C or TC reduction is needed.
Atherosclerotic cardiovascular disease (ASCVD) is a significant cause of morbidity and mortality in the United States. ASCVD involves the buildup of cholesterol plaque in arteries and includes acute coronary syndrome, peripheral arterial disease, and events such as myocardial infarction and stroke.1 Cardiovascular disease (CVD) risk factors include high cholesterol levels, elevated blood pressure, insulin resistance, elevated blood glucose levels, smoking, poor dietary habits, and a sedentary lifestyle.2
According to the Centers for Disease Control and Prevention, about 86 million adults aged ≥ 20 years have total cholesterol levels > 200 mg/dL. More than half (54.5%) who could benefit are currently taking cholesterol-lowering medications.3 Controlling high cholesterol in American adults, especially veterans, is essential for reducing CVD morbidity and mortality.
The 2018 American College of Cardiology/American Heart Association (ACC/AHA) guideline recommends a low-density lipoprotein cholesterol (LDL-C) target goal of < 70 mg/dL for patients at high risk for ASCVD. Very high-risk ASCVD includes a history of multiple major ASCVD events or 1 major ASCVD event and multiple high-risk conditions (eg, age ≥ 65 years, smoking, or diabetes).4 Major ASCVD events include recent acute coronary syndrome (within the past 12 months), a history of myocardial infarction or ischemic stroke, and symptomatic peripheral artery disease.
The ACC/AHA guideline suggests that if the LDL-C level remains ≥ 70 mg/dL, adding ezetimibe (a dietary cholesterol absorption inhibitor) to maximally tolerated statin therapy is reasonable. If LDL-C levels remain ≥ 70 mg/dL, adding a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor, such as alirocumab, is reasonable.4 The US Departments of Veterans Affairs/US Department of Defense guidelines recommend using maximally tolerated statins and ezetimibe before PCSK9 inhibitors due to established long-term safety and reduction in CVD events.
Generic statins and ezetimibe are administered orally and widely available. In contrast, PCSK9 inhibitors have unknown long-term safety profiles, require subcutaneous injection once or twice monthly, and are significantly more expensive. They also require patient education on proper use while providing comparable or lesser relative risk reductions.2
These 3 classes of medication vary in their mechanisms of action to reduce LDL.5,6 Ezetimibe and several statin medications are included on the Veterans Affairs Sioux Falls Health Care System (VASFHCS) formulary and do not require review prior to prescribing. Alirocumab is available at VASFHCS but is restricted to patients with a history of ASCVD or a diagnosis of familial hypercholesterolemia, and who are receiving maximally tolerated statin and ezetimibe therapy but require further LDL-C lowering to reduce their ASCVD risk.
Studies have found ezetimibe monotherapy reduces LDL-C in patients with dyslipidemia by 18% after 12 weeks.7 One found that the percentage reduction in LDL-C was significantly greater (P < .001) with all doses of ezetimibe plus simvastatin (46% to 59%) compared with either atorvastatin 10 mg (37%) or simvastatin 20 mg (38%) monotherapy after 6 weeks.8
Although alirocumab can be added to other lipid therapies, most VASFHCS patients are prescribed alirocumab monotherapy. In the ODYSSEY CHOICE II study, patients were randomly assigned to receive either a placebo or alirocumab 150 mg every 4 weeks or alirocumab 75 mg every 2 weeks. The primary efficacy endpoint was LDL-C percentage change from baseline to week 24. In the alirocumab 150 mg every 4 weeks and 75 mg every 2 weeks groups, the least-squares mean LDL-C changes from baseline to week 24 were 51.7% and 53.5%, respectively, compared to a 4.7% increase in the placebo group (both groups P < .001 vs placebo). The authors also reported that alirocumab 150 mg every 4 weeks as monotherapy demonstrated a 47.4% reduction in LDL-C levels from baseline in a phase 1 study.9Although alirocumab monotherapy and ezetimibe plus statin therapy have been shown to effectively decrease LDL-C independently, a direct comparison of alirocumab monotherapy vs ezetimibe plus statin therapy has not been assessed, to our knowledge. Understanding the differences in effectiveness and safety between these 2 regimens will be valuable for clinicians when selecting a medication regimen for veterans with a history of ASCVD.
METHODS
This retrospective, single-center chart review used VASFHCS Computerized Patient Record System (CPRS) and Joint Longitudinal Viewer (JLV) records to compare patients with a history of ASCVD events who were treated with alirocumab monotherapy or ezetimibe plus statin. The 2 groups were randomized in a 1:3 ratio. The primary endpoint was achieving LDL-C < 70 mg/dL after 4 to 12 weeks, 13 to 24 weeks, and 25 to 52 weeks. Secondary endpoints included the mean percentage change from baseline in total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), LDL-C, and triglycerides (TG) over 52 weeks. The incidence of ASCVD events during this period was also assessed. If LDL-C < 70 mg/dL was achieved > 1 time during each time frame, only 1 incident was counted for analysis. Safety was assessed based on the incidence of any adverse event (AE) that led to treatment discontinuation.
Patients were identified by screening the prescription fill history between October 1, 2019, and December 31, 2022. The 52-week data collection period was counted from the first available fill date. Additionally, the prior authorization drug request file from January 1, 2017, to December 31, 2022, was used to obtain a list of patients prescribed alirocumab. Patients were included if they were veterans aged ≥ 18 years and had a history of an ASCVD event, had a alirocumab monotherapy or ezetimibe plus statin prescription between October 1, 2019, and December 31, 2022, or had an approved prior authorization drug request for alirocumab between January 1, 2017, and December 31, 2022. Patients missing a baseline or follow-up lipid panel and those with concurrent use of alirocumab and ezetimibe and/or statin were excluded.
Baseline characteristics collected for patients included age, sex, race, weight, body mass index, lipid parameters (LDL-C, TC, HDL-C, and TG), dosing of each type of statin before adding ezetimibe, and use of any other antihyperlipidemic medication. We also collected histories of hypertension, hyperlipidemia, diabetes, chronic kidney disease, congestive heart failure, and smoking or tobacco use status. The baseline lipid panel was the most recent lipid panel documented before starting alirocumab or ezetimibe plus statin therapy. Follow-up lipid panel values were gathered at 4 to 12 weeks, 13 to 24 weeks, and 25 to 52 weeks following initiation of either therapy.
High-, moderate-, and low-intensity dosing of statin therapy and alirocumab dosing (75 mg every 2 weeks, 150 mg every 2 weeks, or 300 mg every 4 weeks) were recorded at the specified intervals. However, no patients in this study received the latter dosing regimen. ASCVD events and safety endpoints were recorded based on a review of clinical notes over the 52 weeks following the first available start date.
Statistical Analysis
The primary endpoint of achieving the LDL-C < 70 mg/dL goal from baseline to 4 to 12 weeks, 13 to 24 weeks, and 25 to 52 weeks after initiation was compared between alirocumab monotherapy and ezetimibe plus statin therapy using the χ² test. Mean percentage change from baseline in TC, HDL-C, LDL-C, and TG were compared using the independent t test. P < .05 was considered statistically significant. Incidence of ASCVD events and the safety endpoint (incidence of AEs leading to treatment discontinuation) were also compared using the χ² test. Continuous baseline characteristics were reported mean (SD) and nominal baseline characteristics were reported as a percentage.
RESULTS
There were 80 participants in this study: 20 in the alirocumab monotherapy group and 60 in the ezetimibe plus statin therapy group. More than 100 patients did not meet the prespecified inclusion criteria and were excluded. Mean (SD) age was 75 (8) years in the alirocumab group and 74 (8) years in the ezetimibe plus statin group. There was no significant differences in mean (SD) weight or mean (SD) body mass index. All study participants identified as White and male except for 2 patients in the ezetimibe plus statin therapy group whose race was not documented. Differences in lipid parameters were observed between groups, with mean baseline LDL-C, HDL-C, and TC higher in the alirocumab monotherapy group than in the ezetimibe plus statin therapy group, with significant differences in LDL-C and TC (Table 1).

Fourteen patients (70%) in the alirocumab monotherapy group had hypertension, compared with 31 (52%) in the ezetimibe plus statin therapy group. In both groups, most patients had previously been diagnosed with hyperlipidemia. More patients (60%) in the alirocumab group had diabetes than in the ezetimibe plus statin therapy group (37%). The alirocumab monotherapy group also had a higher percentage of patients with diagnoses of congestive heart failure and used other antihyperlipidemic medications than in the ezetimibe plus statin therapy group. Five patients (25%) in the alirocumab monotherapy group and 12 patients (20%) in the ezetimibe plus statin therapy group took fish oil. In the ezetimibe plus statin therapy group, 2 patients (3%) took gemfibrozil, and 2 patients (3%) took fenofibrate. Six (30%) patients in the alirocumab monotherapy group and 12 (20%) patients in the ezetimibe plus statin therapy group had chronic kidney disease. Although the majority of patients in each group did not use tobacco products, there were more tobacco users in the ezetimibe plus statin therapy group.
In the alirocumab monotherapy group, 15 patients (75%) were prescribed 75 mg every 2 weeks and 5 patients (25%) were prescribed 150 mg every 2 weeks. In the ezetimibe plus statin therapy group, 59 patients (98%) were prescribed ezetimibe 10 mg/d (Table 2). Forty-three patients (72%) were prescribed a high-intensity statin 10 received moderate-intensity (17%) and 7 received low-intensity statin (12%). Most patients were prescribed rosuvastatin (45%), followed by atorvastatin (42%), pravastatin (10%), and simvastatin (3%).

Primary Endpoint
During the 52-week study, more patients met the LDL-C goal of < 70 mg/dL in the alirocumab monotherapy group (70%) than in the ezetimibe plus statin therapy group (57%); however, the difference was not significant (P = .29). Of the patients prescribed alirocumab monotherapy who achieved LDL-C < 70 mg/dL, 15% achieved this goal in 4 to 12 weeks, 40% in 13 to 24 weeks, and 45% in 25 to 52 weeks. In the ezetimibe plus statin therapy group, 28% of patients achieved LDL-C < 70 mg/dL in 4 to 12 weeks, 31% in 13 to 24 weeks, and 41% in 25 to 52 weeks (Table 3).

Secondary Endpoints
During weeks 4 to 52 of treatment, the mean percentage change decreased in LDL-C (37.7% vs 21.4%; P = .01), TC (24.7% vs 12.5%; P = .01), and TG (0.9% vs 7.0%; P = .28) in the alirocumab monotherapy group and the ezetimibe plus statin therapy group, respectively (Table 4). The mean percentage change increased in HDL-C by 3.6% in the alirocumab monotherapy group and 1.8% in the ezetimibe plus statin therapy group (P = .36). During the study, ASCVD events occurred in 1 patient (5%) in the alirocumab monotherapy group and 3 patients (5%) in the ezetimibe plus statin therapy group (P = .99). The patient in the alirocumab monotherapy group had unstable angina 1 month after taking alirocumab. One patient in the ezetimibe plus statin therapy group had coronary artery disease and 2 patients had coronary heart disease that required stents during the 52-week period. There was 1 patient in each group who reported an AE that led to treatment discontinuation (P = .41). One patient stopped alirocumab after a trial of 2 months due to intolerance, but no specific AE was reported in the CPRS. In the ezetimibe plus statin therapy group, 1 patient requested to discontinue ezetimibe after a trial of 3 months without a specific reason noted in the medical record.

DISCUSSION
This study found no statistically significant difference in the incidence of reaching an LDL-C goal of < 70 mg/dL after alirocumab monotherapy initiation compared with ezetimibe plus statin therapy. This occurred despite baseline LDL-C being lower in the ezetimibe plus statin therapy group, which required a smaller reduction in LDL-C to reach the primary goal. Most patients on alirocumab monotherapy were prescribed a lower initial dose of 75 mg every 2 weeks. Of those patients, 30% did not achieve the LDL-C goal < 70 mg/dL. Thus, a higher dose may have led to more patients achieving the LDL-C goal.
Secondary endpoints, including mean percentage change in HDL-C and TG and incidence of ASCVD events during 52 weeks of treatment, were not statistically significant. The mean percentage increase in HDL-C was negligible in both groups, while the mean percentage reduction in TG favored the ezetimibe plus statin therapy group. In the ezetimibe plus statin therapy group, patients who also took fenofibrate experienced a significant reduction in TG while none of the patients in the alirocumab group were prescribed fenofibrate. Although the alirocumab monotherapy group had a statistically significant greater reduction in LDL-C and TC compared with those prescribed ezetimibe plus statin, the mean baseline LDL-C and TC were significantly greater in the alirocumab monotherapy group, which could contribute to higher reductions in LDL-C and TC after alirocumab monotherapy.Based on the available literature, we expected greater reductions in LDL-C in both study groups compared with statin therapy alone.8,9 However, it was unclear whether the LDL-C and TC reductions were clinically significant.
Limitations
The study design did not permit randomization prior to the treatments, restricting our ability to account for some confounding factors, such as diet, exercise, other antihyperlipidemic medication, and medication adherence, which may have affected LDL-C, HDL-C, TG, and TC levels. Differences in baseline characteristics—particularly major risk factors, such as hypertension, diabetes, and tobacco use—also could have confounding affect on lipid levels and ASCVD events. Additionally, patients prescribed alirocumab monotherapy may have switched from statin or ezetimibe therapy, and the washout period was not reviewed or recorded, which could have affected the lipid panel results.
The small sample size of this study also may have limited the ability to detect significant differences between groups. A direct comparison of alirocumab monotherapy vs ezetimibe plus statin therapy has not been performed, making it difficult to prospectively evaluate what sample size would be needed to power this study. A posthoc analysis was used to calculate power, which was found to be only 17%. Many patients were excluded due to a lack of laboratory results within the study period, contributing to the small sample size.
Another limitation was the reliance on documentation in CPRS and JLV. For example, having documentation of the specific AEs for the 2 patients who discontinued alirocumab or ezetimibe could have helped determine the severity of the AEs. Several patients were followed by non-VA clinicians, which could have contributed to limited documentation in the CPRS and JLV. It is difficult to draw any conclusions regarding ASCVD events and AEs that led to treatment discontinuation between alirocumab monotherapy and ezetimibe plus statin therapy based on the results of this retrospective study due to the limited number of events within the 52-week period.
CONCLUSIONS
This study found that there was no statistically significant difference in LDL-C reduction to < 70 mg/dL between alirocumab monotherapy and ezetimibe plus statin therapy in a small population of veterans with ASCVD, with a higher percentage of participants in both groups achieving that goal in 25 to 52 weeks. There also was no significant difference in percentage change in HDL-C or TG or in incidence of ASCVD events and AEs leading to treatment discontinuation. However, there was a statistically significant difference in percentage reduction for LDL-C and TC during 52 weeks of alirocumab monotherapy vs ezetimibe plus statin therapy.
Although there was no significant difference in LDL-C reduction to < 70 mg/dL, targeting this goal in patients with ASCVD is still clinically warranted. This study does not support a change in current VA criteria for use of alirocumab or a change in current guidelines for secondary prevention of ASCVD. Still, this study does indicate that the efficacy of alirocumab monotherapy is similar to that of ezetimibe plus statin therapy in patients with a history of ASCVD and may be useful in clinical settings when an alternative to ezetimibe plus statin therapy is needed. Alirocumab also may be more effective in lowering LDL-C and TC than ezetimibe plus statin therapy in veterans with ASCVD and could be added to statin therapy or ezetimibe when additional LDL-C or TC reduction is needed.
Lucchi T. Dyslipidemia and prevention of atherosclerotic cardiovascular disease in the elderly. Minerva Med. 2021;112:804-816. doi:10.23736/S0026-4806.21.07347-X
The Management of Dyslipidemia for Cardiovascular Risk Reduction Work Group. VA/DoD Clinical Practice Guideline for the Management of Dyslipidemia for Cardiovascular Risk Reduction. Version 4.0. June 2020. Accessed September 5, 2024. https://www.healthquality.va.gov/guidelines/CD/lipids/VADoDDyslipidemiaCPG5087212020.pdf
Centers for Disease Control and Prevention. High Cholesterol Facts. May 15, 2024. Accessed October 3, 2024. https://www.cdc.gov/cholesterol/data-research/facts-stats/index.html
Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082-e1143. doi:10.1161/CIR.0000000000000625
Vavlukis M, Vavlukis A. Statins alone or in combination with ezetimibe or PCSK9 inhibitors in atherosclerotic cardiovascular disease protection. IntechOpen. January 24, 2019. doi:10.5772/intechopen.82520
Alirocumab. Prescribing information. Regeneron Pharmaceuticals, Inc.; 2024. Accessed September 5, 2024. https://www.regeneron.com/downloads/praluent_pi.pdf
Pandor A, Ara RM, Tumur I, et al. Ezetimibe monotherapy for cholesterol lowering in 2,722 people: systematic review and meta-analysis of randomized controlled trials. J Intern Med. 2009;265(5):568-580. doi:10.1111/j.1365-2796.2008.02062.x
McKenney J, Ballantyne CM, Feldman TA, et al. LDL-C goal attainment with ezetimibe plus simvastatin coadministration vs atorvastatin or simvastatin monotherapy in patients at high risk of CHD. MedGenMed. 2005;7(3):3.
Stroes E, Guyton JR, Lepor N, et al. Efficacy and safety of alirocumab 150 mg every 4 weeks in patients with hypercholesterolemia not on statin therapy: the ODYSSEY CHOICE II study. J Am Heart Assoc. 2016;5(9):e003421. doi:10.1161/JAHA.116.003421
Lucchi T. Dyslipidemia and prevention of atherosclerotic cardiovascular disease in the elderly. Minerva Med. 2021;112:804-816. doi:10.23736/S0026-4806.21.07347-X
The Management of Dyslipidemia for Cardiovascular Risk Reduction Work Group. VA/DoD Clinical Practice Guideline for the Management of Dyslipidemia for Cardiovascular Risk Reduction. Version 4.0. June 2020. Accessed September 5, 2024. https://www.healthquality.va.gov/guidelines/CD/lipids/VADoDDyslipidemiaCPG5087212020.pdf
Centers for Disease Control and Prevention. High Cholesterol Facts. May 15, 2024. Accessed October 3, 2024. https://www.cdc.gov/cholesterol/data-research/facts-stats/index.html
Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082-e1143. doi:10.1161/CIR.0000000000000625
Vavlukis M, Vavlukis A. Statins alone or in combination with ezetimibe or PCSK9 inhibitors in atherosclerotic cardiovascular disease protection. IntechOpen. January 24, 2019. doi:10.5772/intechopen.82520
Alirocumab. Prescribing information. Regeneron Pharmaceuticals, Inc.; 2024. Accessed September 5, 2024. https://www.regeneron.com/downloads/praluent_pi.pdf
Pandor A, Ara RM, Tumur I, et al. Ezetimibe monotherapy for cholesterol lowering in 2,722 people: systematic review and meta-analysis of randomized controlled trials. J Intern Med. 2009;265(5):568-580. doi:10.1111/j.1365-2796.2008.02062.x
McKenney J, Ballantyne CM, Feldman TA, et al. LDL-C goal attainment with ezetimibe plus simvastatin coadministration vs atorvastatin or simvastatin monotherapy in patients at high risk of CHD. MedGenMed. 2005;7(3):3.
Stroes E, Guyton JR, Lepor N, et al. Efficacy and safety of alirocumab 150 mg every 4 weeks in patients with hypercholesterolemia not on statin therapy: the ODYSSEY CHOICE II study. J Am Heart Assoc. 2016;5(9):e003421. doi:10.1161/JAHA.116.003421
Impact and Recovery of VHA Epilepsy Care Services During the COVID-19 Pandemic
The COVID-19 pandemic affected diverse workplaces globally, leading to temporary and permanent changes across the health care landscape. Included among the impacted areas of care were epilepsy and electroencephalogram (EEG) clinicians and services. Surveys among epilepsy specialists and neurophysiologists conducted at the onset of the pandemic to evaluate working conditions include analyses from the American Epilepsy Society (AES), the National Association of Epilepsy Centers (NAEC), the International League Against Epilepsy, and an Italian national survey.1-4 These investigations revealed reductions in epilepsy monitoring unit (EMU) admissions (23% decline), epilepsy surgery (6% decline), inpatient EEG (22% of respondents reported decline), and patients having difficulty accessing epilepsy professionals (28% of respondents reported decline) or obtaining medications (20% of respondents reported decline).1-3
While such research provided evidence for changes to epilepsy care in 2020, there are limited data on subsequent adaptations during the pandemic. These studies did not incorporate data on the spread of COVID-19 or administrative workload numbers to analyze service delivery beyond self reports. This study aimed to address this gap in the literature by highlighting results from longitudinal national surveys conducted at the Epilepsy Centers of Excellence (ECoE), a specialty care service within the Veterans Health Administration (VHA), which annually serves > 9 million veterans.5 The ECoE represents epileptologists and neurophysiologists across the United States at the 17 primary facilities that were established at the time of this survey (2 ECoEs have been added since survey completion) in 4 geographical regions and for which other regional facilities refer patients for diagnostic services or specialty care.6
National surveys were conducted among the ECoE directors regarding adaptations made from May 2020 to June 2022 to provide a comprehensive account of limitations they experienced and how adjustments have been made to improve patient care. Survey responses were compared to administrative workload numbers and COVID-19 spread data from the Centers for Disease Control and Prevention (CDC) to provide a comprehensive analysis of performance during the pandemic.
METHODS
Data were collected as part of a quality improvement initiative by the VHA ECoE; institutional review board approval was not required. An 18-item survey covering 5 broad domains was sent to ECoE directors 4 separate times to accumulate data from 4 time periods: May to June 2020 (T1); December 2020 to February 2021 (T2); July to August 2021 (T3); and June to July 2022 (T4). These periods correspond to the following phases of the pandemic: T1, onset of pandemic; T2, vaccine availability; T3, Delta variant predominant; T4, Omicron variant predominant.
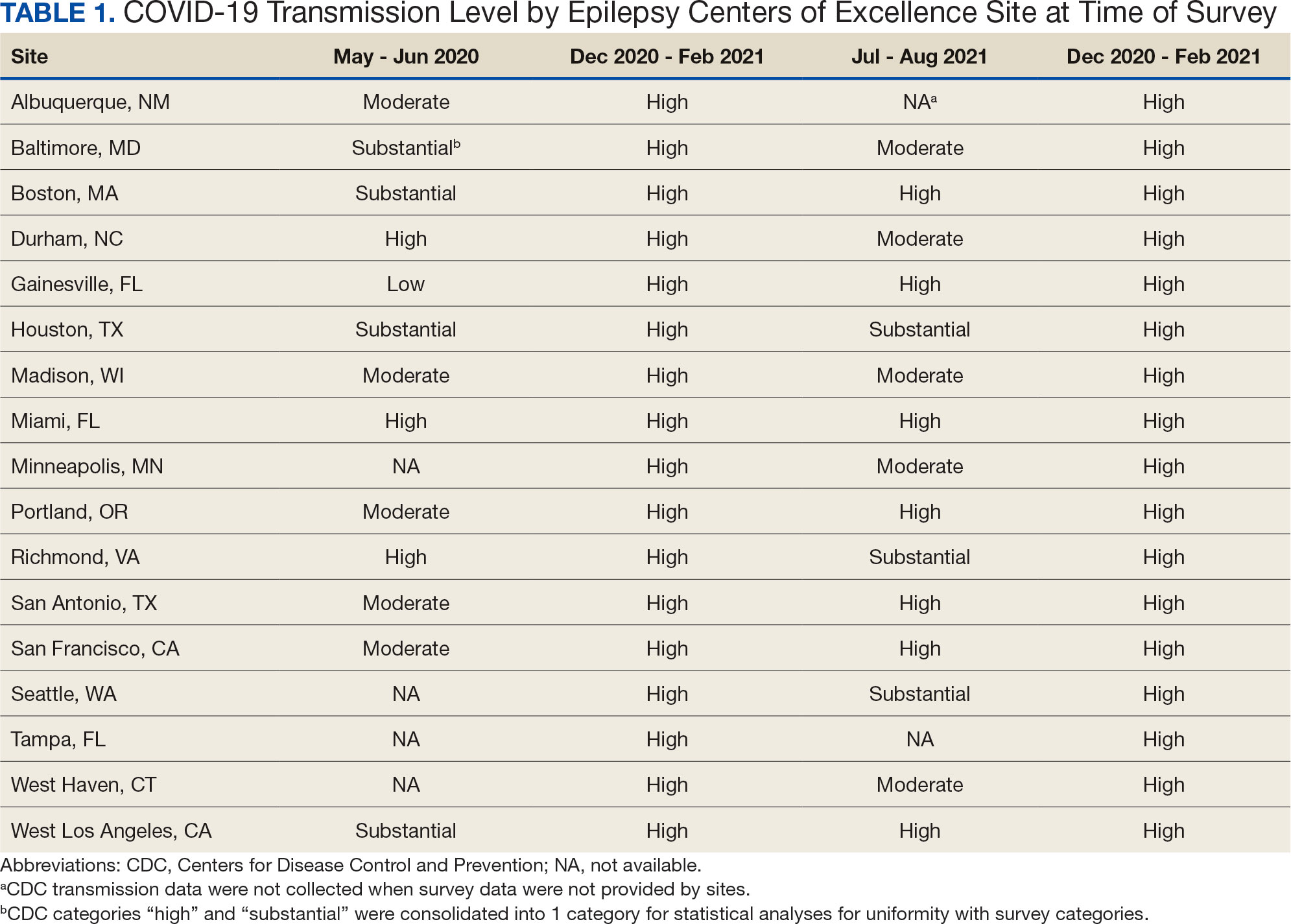
Data on the spread of COVID-19 were collected from the CDC archived dataset, US COVID-19 County Level of Community Transmission Historical Changes (Table 1).7 Administrative workload (patient counts) for EEG, EMU, and outpatient clinics were extracted from VHA administrative databases for the participating sites for the months prior to each survey: T1, April 2020; T2, November 2020; T3, June 2021; and T4, May 2022 (Table 2).
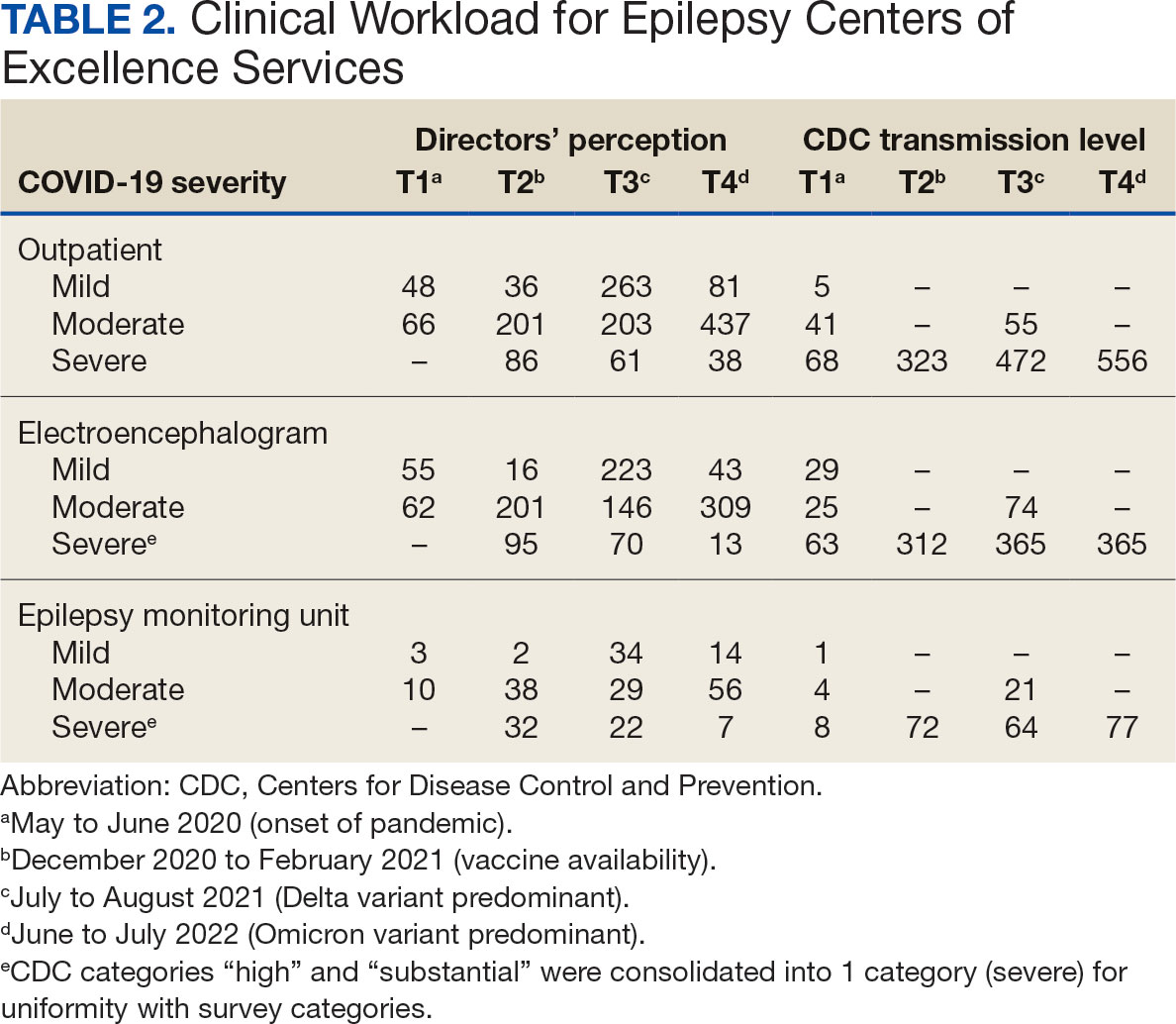
Survey Structure and Content
The survey was developed by the ECoE and was not validated prior to its use due to the time-sensitive nature of gathering information during the pandemic. The first survey (T1) was an emailed spreadsheet with open-ended questions to gauge availability of services (ie, outpatient clinic, EEG, EMU), assess whether safety precautions were being introduced, and understand whether national or local guidelines were thought to be helpful. Responses from this and subsequent surveys were standardized into yes/no and multiple choice formats. Subsequent surveys were administered online using a Research Electronic Data Capture tool.8,9
Availability of outpatient epilepsy services across the 4 time periods were categorized as unlimited (in-person with no restrictions), limited (in-person with restrictions), planned (not currently performed but scheduled for the near future), and unavailable (no in-person services offered) (eAppendices 1-6, available in article PDF).
Statistical Analyses
Analyses were performed to compare survey responses to workload and CDC data on COVID-19 community spread. The following associations were examined: (1) CDC COVID-19 spread vs respondents’ perception of spread; (2) respondents’ perception of spread vs availability of services; (3) CDC COVID-19 spread vs availability of services; (4) respondents’ perception of spread vs workload; and (5) CDC COVID-19 spread vs workload. Availability of services was dichotomized for analyses, with limited or fully available services classified as available. As services were mostly open at T3 regardless of the spread of the virus, and the CDC COVID-19 spread classification for all sites was severe or high at T2 and T4, corresponding associations were not tested at these time points. For associations 1 through 3, Fisher exact tests were used; for associations 4 and 5, Mann-Whitney U tests (where the COVID-19 spread fell into 2 categories) and Kruskal-Wallis tests (for 3 categories of COVID-19 spread) were performed. All tests were 2-tailed and performed at 0.05 error rate. Bonferroni corrections were applied to adjust P values for multiple hypotheses tests.
RESULTS
From the 17 sites invited, responses at each time point were obtained from 13 (T1),17 (T2), 15 (T3), and 16 (T4) centers. There was no significant association between self-reported COVID-19 spread and CDC classification of COVID spread. There were no associations between COVID-19 community spread (respondent reported or CDC severity level) and outpatient clinic availability (self-reported or workload captured). At T3, a positive association was found between the CDC spread level and workload (P = .008), but this was not significant after Bonferroni correction (P = .06).
EEG availability surpassed EMU availability at all time points, although EMU services made some recovery at T3 and T4. No associations were found between COVID-19 community spread (self-reported or CDC severity level) and outpatient EEG or EMU availability (self-reported or workload captured). At T3, there was a positive association between EEG workload and CDC COVID-19 severity level (P = .04), but this was not significant after Bonferroni correction (P = .30).
For outpatient EEG, staff and patient mask use were universally implemented by T2, while the use of full personal protective equipment (PPE) occurred at a subset of sites (T2, 6/17 [35%]; T3, 3/15 [20%]; T4: 4/16 [25%]). COVID-19 testing was rarely implemented prior to outpatient EEG (T1, 0 sites; T2, 1 site; T3, 1 site; T4, 0 sites). Within the EMU, safety precautions including COVID-19 testing, patient mask usage, staff mask usage, and aerosolization demonstrated a sustained majority usage across the 4 surveys.
National and Local Guidelines
The open-ended survey at T1 asked site directors, “Should there be national recommendations on how EEGs and related procedures should be done during the pandemic or should this be left to local conditions?” Responses were mixed, with 5 respondents desiring a national standard, 4 respondents favoring a local response, and 4 respondents believing a national standard should be in place but with modifications based on local outbreak levels and needs.
Surveys performed at T2 through T4 asked, “Which of the following do you feel was/will be helpful in adapting to COVID-19–related changes?” Overall, there was substantial agreement that guidelines were helpful. Most sites anticipated permanent changes in enhanced safety precautions and telehealth.
DISCUSSION
This longitudinal study across 4 time points describes how epilepsy services within the VHA and ECoE adapted to the COVID-19 pandemic. The first survey, conducted 2 months after COVID-19 was declared a pandemic, allowed a comparison with other concurrent US national surveys.1,2,10 The subsequent surveys describe longitudinal adaptations to balance patient and staff safety with service availability and is a unique feature of the current report. Results demonstrate flexibility and adaptability by the ECoEs surveyed, which surprisingly did not show significant associations between CDC COVID-19 spread data and administrative workload data.
Trends in Availability of Services
The most significant impact of COVID-19 restrictions was during T1. There were no significant relationships between service availability/workload and objective CDC COVID-19 spread levels or subjective self-reported COVID-19 spread. Respondents’ perceptions of local COVID-19 spread showed no association with CDC COVID-19 spread data. It appears that subjective perception of spread may be unreliable and factors other than actual or perceived COVID-19 spread were likely driving patterns for service availability.
In-person outpatient visits were most impacted at T1, similar to other civilian surveys, with only 1 site reporting in-person outpatient visits without limitations.1,2 These numbers significantly changed by T2, with all sites offering either limited or unlimited in-person visits. While the surveys did not evaluate factors leading to this rapid recovery, it may be related to the availability of COVID-19 vaccinations within the VHA during this time.11 The US Department of Veterans Affairs was the first federal agency to mandate employee vaccination.12 By the most recent time point (T4), all responding sites offered outpatient visits. Outpatient EEGs followed a similar trend, with T1 being the most restrictive and full, unrestricted outpatient EEGs available by T3.
Fiscal year (FY) trends from ECoE annual reports suggest that encounters slowly recovered over the course of the pandemic. In FY 2019 there were 13,143 outpatient encounters and 6394 EEGs, which dropped to 8097 outpatient encounters and 4432 EEGs in FY 2020 before rising to 8489 outpatient encounters and 5604 EEGs in FY 2021 and 9772 outpatient encounters and 5062 EEGs in FY 2022. Thus, while clinicians described availability of services, patients may have remained hesitant or were otherwise unable to fulfill in-person appointments. The increased availability of home EEG (145 encounter days in 2021 and 436 encounter days in 2022) may be filling this gap.
In contrast to outpatient clinics and EEG, EMU availability showed relatively slower reimplementation. In the last survey, about 30% of sites were still not offering EMU or had limited services. Early trends regarding reduced staffing and patient reluctance for elective admission cited in other surveys may have also affected EMU availability within the VHA.2,13 Consistent with trends in availability, ECoE annual report data suggest EMU patient participation was about one-half of prepandemic rates: 3069 encounters in FY 2019 dropped to 1614 encounters in 2020. By 2021, rates were about two-thirds of prepandemic rates with 2058 encounters in 2021 and 2101 encounters in 2022.
Early survey results (T1) from this study echo trends from other surveys. In the AES survey (April to June 2020), about a quarter of respondents (22%) reported doing fewer EEG studies than usual. The Italian national survey (April 2020) revealed reduced presurgical evaluations (81%), ambulatory EEG (78%), standard EEG (5%) and long-term EEG (32%).4 In the NAEC survey (end of 2020)—which roughly corresponded to T2—outpatient EEGs were still < 75% of pre-COVID levels in one-half of the centers.
National and Local Guidelines
Both national and local guidelines were perceived as useful by most respondents, with national guidelines being more beneficial. This aligns with the NAEC survey, where there was a perceived need for detailed recommendations for PPE and COVID-19 testing of patients, visitors, and staff. Based on national and local guidelines, ECoE implemented safety procedures, as reflected in responses. Staff masking procedures appeared to be the most widely adopted for all services, while the use of full PPE waned as the pandemic progressed. COVID-19 testing was rarely used for routine outpatient visits but common in EMU admissions. This is similar to a survey conducted by the American Academy of Neurology which found full PPE implementation intermittently in outpatient settings and more frequently in inpatient settings.14
Telehealth Attitudes
While most sites anticipated permanent implementation of safety precautions and telehealth, the latter was consistently reported as more likely to be sustained. The VHA had a large and well-developed system of telehealth services that considerably predated the pandemic.15,16 Through this established infrastructure, remote services were quickly increased across the VHA.17-19 This telehealth structure was supplemented by the ability of VHA clinicians to practice across state lines, following a 2018 federal rule.20 The AES survey noted the VHA ECoE's longstanding experience with telehealth as a model for telemedicine use in providing direct patient care, remote EEG analysis, and clinician-to-clinician consultation.1
Trends in the number of telehealth patients seen, observed through patterns in ECoE annual reports are consistent with positive views toward this method of service provision. Specifically, these annual reports capture trends in Video Telehealth Clinic (local station), Video Telehealth Clinic (different station), Home Video Telehealth, Telephone Clinic, and eConsults. Though video telehealth at in-person stations had a precipitous drop in 2020 that continued to wane in subsequent years (898 encounters in 2019; 455 encounters in 2020; 90 encounters in 2021; 88 encounters in 2022), use of home video telehealth rose over time (143 encounters in 2019; 1003 encounters in 2020; 3206 encounters in 2021; 3315 encounters in 2022). Use of telephone services rose drastically in 2020 but has since become a less frequently used service method (2636 in 2019; 5923 in 2020; 5319 in 2021; 3704 in 2022).
Limitations
While the survey encouraged a high response rate, this limited its scope and interpretability. While the availability of services was evaluated, the underlying reasons were not queried. Follow-up questions about barriers to reopening may have allowed for a better understanding of why some services, such as EMU, continued to operate suboptimally later in the pandemic. Similarly, asking about unique strategies or barriers for telehealth would have allowed for a better understanding of its current and future use. We hypothesize that staffing changes during the pandemic may have influenced the availability of services, but changes to staffing were not assessed via the survey and were not readily available via other sources (eg, ECoE annual reports) at the time of publication. An additional limitation is the lack of comparable surveys in the literature for time points T2 to T4, as most analogous surveys were performed early in 2020.
Conclusions
This longitudinal study performed at 4 time points during the COVID-19 pandemic is the first to offer a comprehensive picture of changes to epilepsy and EEG services over time, given that other similar surveys lacked follow-up. Results reveal a significant limitation of services at VHA ECoE shortly after the onset of the pandemic, with return to near-complete operational status 2 years later. While safety precautions and telehealth are predicted to continue, telehealth is perceived as a more permanent change in services.
Albert DVF, Das RR, Acharya JN, et al. The impact of COVID-19 on epilepsy care: a survey of the American Epilepsy Society membership. Epilepsy Curr. 2020;20(5):316-324. doi:10.1177/1535759720956994
Ahrens SM, Ostendorf AP, Lado FA, et al. Impact of the COVID-19 pandemic on epilepsy center practice in the United States. Neurology. 2022;98(19):e1893-e1901. doi:10.1212/WNL.0000000000200285
Cross JH, Kwon CS, Asadi-Pooya AA, et al. Epilepsy care during the COVID-19 pandemic. Epilepsia. 2021;62(10):2322-2332. doi:10.1111/epi.17045
Assenza G, Lanzone J, Ricci L, et al. Electroencephalography at the time of Covid-19 pandemic in Italy. Neurol Sci. 2020;41(8):1999-2004. doi:10.1007/s10072-020-04546-8
US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics. Veteran population. Updated September 7, 2022. Accessed October 25, 2024. https://www.va.gov/vetdata/veteran_population.asp
US Department of Veterans Affairs, Veterans Health Administration. Epilepsy Centers of Excellence (ECoE). Annual report fiscal year 2019. Accessed October 25, 2024. https://www.epilepsy.va.gov/docs/FY19AnnualReport-VHAEpilepsyCentersofExcellence.pdf
Centers for Disease Control and Prevention. United States COVID-19 county level of community transmission historical changes – ARCHIVED. Updated February 20, 2024. Accessed October 25, 2024. https://data.cdc.gov/Public-Health-Surveillance/United-States-COVID-19-County-Level-of-Community-T/nra9-vzzn
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi:10.1016/j.jbi.2008.08.010
Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi:10.1016/j.jbi.2019.103208
World Health Organization. Rolling updates on coronavirus disease (COVID-19). Updated July 31, 2020. Accessed October 25, 2024. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen
US Department of Veterans Affairs. VA announces initial plans for COVID-19 vaccine distribution. News release. December 10, 2020. Accessed October 25, 2024. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5580
Steinhauer J. V.A. Issues Vaccine Mandate for Health Care Workers, a First for a Federal Agency. The New York Times. August 16, 2021. Accessed October 25, 2024. https://www.nytimes.com/2021/07/26/us/politics/veterans-affairs-coronavirus-covid-19.html
Zafar SF, Khozein RJ, LaRoche SM, Westover MB, Gilmore EJ. Impact of the COVID-19 pandemic on continuous EEG utilization. J Clin Neurophysiol. 2022;39(7):567-574. doi:10.1097/WNP.0000000000000802
Qureshi AI, Rheaume C, Huang W, et al. COVID-19 exposure during neurology practice. Neurologist. 2021;26(6):225-230. doi:10.1097/NRL.0000000000000346
Darkins A, Cruise C, Armstrong M, Peters J, Finn M. Enhancing access of combat-wounded veterans to specialist rehabilitation services: the VA Polytrauma Telehealth Network. Arch Phys Med Rehabil. 2008;89(1):182-187. doi:10.1016/j.apmr.2007.07.027
Darkins A, Ryan P, Kobb R, et al. Care coordination/home telehealth: the systematic implementation of health informatics, home telehealth, and disease management to support the care of veteran patients with chronic conditions. Telemed J E Health. 2008;14(10):1118-1126. doi:10.1089/tmj.2008.0021
Gentry MT, Puspitasari AJ, McKean AJ, et al. Clinician satisfaction with rapid adoption and implementation of telehealth services during the COVID-19 pandemic. Telemed J E Health. 2021;27(12):1385-1392. doi:10.1089/tmj.2020.0575
Connolly SL, Stolzmann KL, Heyworth L, et al. Patient and provider predictors of telemental health use prior to and during the COVID-19 pandemic within the Department of Veterans Affairs. Am Psychol. 2022;77(2):249-261. doi:10.1037/amp0000895
Shelton CJ, Kim A, Hassan AM, Bhat A, Barnello J, Castro CA. System-wide implementation of telehealth to support military veterans and their families in response to COVID-19: a paradigm shift. J Mil Veteran Fam Health. 2020;6(S2):50-57. doi:10.3138/jmvfh-CO19-0003
VA expands telehealth by allowing health care providers to treat patients across state lines. News release. US Dept of Veterans Affairs. May 11, 2018. Accessed October 25, 2024. https://news.va.gov/press-room/va-expands-telehealth-by-allowing-health-care-providers-to-treat-patients-across-state-lines/
The COVID-19 pandemic affected diverse workplaces globally, leading to temporary and permanent changes across the health care landscape. Included among the impacted areas of care were epilepsy and electroencephalogram (EEG) clinicians and services. Surveys among epilepsy specialists and neurophysiologists conducted at the onset of the pandemic to evaluate working conditions include analyses from the American Epilepsy Society (AES), the National Association of Epilepsy Centers (NAEC), the International League Against Epilepsy, and an Italian national survey.1-4 These investigations revealed reductions in epilepsy monitoring unit (EMU) admissions (23% decline), epilepsy surgery (6% decline), inpatient EEG (22% of respondents reported decline), and patients having difficulty accessing epilepsy professionals (28% of respondents reported decline) or obtaining medications (20% of respondents reported decline).1-3
While such research provided evidence for changes to epilepsy care in 2020, there are limited data on subsequent adaptations during the pandemic. These studies did not incorporate data on the spread of COVID-19 or administrative workload numbers to analyze service delivery beyond self reports. This study aimed to address this gap in the literature by highlighting results from longitudinal national surveys conducted at the Epilepsy Centers of Excellence (ECoE), a specialty care service within the Veterans Health Administration (VHA), which annually serves > 9 million veterans.5 The ECoE represents epileptologists and neurophysiologists across the United States at the 17 primary facilities that were established at the time of this survey (2 ECoEs have been added since survey completion) in 4 geographical regions and for which other regional facilities refer patients for diagnostic services or specialty care.6
National surveys were conducted among the ECoE directors regarding adaptations made from May 2020 to June 2022 to provide a comprehensive account of limitations they experienced and how adjustments have been made to improve patient care. Survey responses were compared to administrative workload numbers and COVID-19 spread data from the Centers for Disease Control and Prevention (CDC) to provide a comprehensive analysis of performance during the pandemic.
METHODS
Data were collected as part of a quality improvement initiative by the VHA ECoE; institutional review board approval was not required. An 18-item survey covering 5 broad domains was sent to ECoE directors 4 separate times to accumulate data from 4 time periods: May to June 2020 (T1); December 2020 to February 2021 (T2); July to August 2021 (T3); and June to July 2022 (T4). These periods correspond to the following phases of the pandemic: T1, onset of pandemic; T2, vaccine availability; T3, Delta variant predominant; T4, Omicron variant predominant.

Data on the spread of COVID-19 were collected from the CDC archived dataset, US COVID-19 County Level of Community Transmission Historical Changes (Table 1).7 Administrative workload (patient counts) for EEG, EMU, and outpatient clinics were extracted from VHA administrative databases for the participating sites for the months prior to each survey: T1, April 2020; T2, November 2020; T3, June 2021; and T4, May 2022 (Table 2).

Survey Structure and Content
The survey was developed by the ECoE and was not validated prior to its use due to the time-sensitive nature of gathering information during the pandemic. The first survey (T1) was an emailed spreadsheet with open-ended questions to gauge availability of services (ie, outpatient clinic, EEG, EMU), assess whether safety precautions were being introduced, and understand whether national or local guidelines were thought to be helpful. Responses from this and subsequent surveys were standardized into yes/no and multiple choice formats. Subsequent surveys were administered online using a Research Electronic Data Capture tool.8,9
Availability of outpatient epilepsy services across the 4 time periods were categorized as unlimited (in-person with no restrictions), limited (in-person with restrictions), planned (not currently performed but scheduled for the near future), and unavailable (no in-person services offered) (eAppendices 1-6, available in article PDF).
Statistical Analyses
Analyses were performed to compare survey responses to workload and CDC data on COVID-19 community spread. The following associations were examined: (1) CDC COVID-19 spread vs respondents’ perception of spread; (2) respondents’ perception of spread vs availability of services; (3) CDC COVID-19 spread vs availability of services; (4) respondents’ perception of spread vs workload; and (5) CDC COVID-19 spread vs workload. Availability of services was dichotomized for analyses, with limited or fully available services classified as available. As services were mostly open at T3 regardless of the spread of the virus, and the CDC COVID-19 spread classification for all sites was severe or high at T2 and T4, corresponding associations were not tested at these time points. For associations 1 through 3, Fisher exact tests were used; for associations 4 and 5, Mann-Whitney U tests (where the COVID-19 spread fell into 2 categories) and Kruskal-Wallis tests (for 3 categories of COVID-19 spread) were performed. All tests were 2-tailed and performed at 0.05 error rate. Bonferroni corrections were applied to adjust P values for multiple hypotheses tests.
RESULTS
From the 17 sites invited, responses at each time point were obtained from 13 (T1),17 (T2), 15 (T3), and 16 (T4) centers. There was no significant association between self-reported COVID-19 spread and CDC classification of COVID spread. There were no associations between COVID-19 community spread (respondent reported or CDC severity level) and outpatient clinic availability (self-reported or workload captured). At T3, a positive association was found between the CDC spread level and workload (P = .008), but this was not significant after Bonferroni correction (P = .06).
EEG availability surpassed EMU availability at all time points, although EMU services made some recovery at T3 and T4. No associations were found between COVID-19 community spread (self-reported or CDC severity level) and outpatient EEG or EMU availability (self-reported or workload captured). At T3, there was a positive association between EEG workload and CDC COVID-19 severity level (P = .04), but this was not significant after Bonferroni correction (P = .30).
For outpatient EEG, staff and patient mask use were universally implemented by T2, while the use of full personal protective equipment (PPE) occurred at a subset of sites (T2, 6/17 [35%]; T3, 3/15 [20%]; T4: 4/16 [25%]). COVID-19 testing was rarely implemented prior to outpatient EEG (T1, 0 sites; T2, 1 site; T3, 1 site; T4, 0 sites). Within the EMU, safety precautions including COVID-19 testing, patient mask usage, staff mask usage, and aerosolization demonstrated a sustained majority usage across the 4 surveys.
National and Local Guidelines
The open-ended survey at T1 asked site directors, “Should there be national recommendations on how EEGs and related procedures should be done during the pandemic or should this be left to local conditions?” Responses were mixed, with 5 respondents desiring a national standard, 4 respondents favoring a local response, and 4 respondents believing a national standard should be in place but with modifications based on local outbreak levels and needs.
Surveys performed at T2 through T4 asked, “Which of the following do you feel was/will be helpful in adapting to COVID-19–related changes?” Overall, there was substantial agreement that guidelines were helpful. Most sites anticipated permanent changes in enhanced safety precautions and telehealth.
DISCUSSION
This longitudinal study across 4 time points describes how epilepsy services within the VHA and ECoE adapted to the COVID-19 pandemic. The first survey, conducted 2 months after COVID-19 was declared a pandemic, allowed a comparison with other concurrent US national surveys.1,2,10 The subsequent surveys describe longitudinal adaptations to balance patient and staff safety with service availability and is a unique feature of the current report. Results demonstrate flexibility and adaptability by the ECoEs surveyed, which surprisingly did not show significant associations between CDC COVID-19 spread data and administrative workload data.
Trends in Availability of Services
The most significant impact of COVID-19 restrictions was during T1. There were no significant relationships between service availability/workload and objective CDC COVID-19 spread levels or subjective self-reported COVID-19 spread. Respondents’ perceptions of local COVID-19 spread showed no association with CDC COVID-19 spread data. It appears that subjective perception of spread may be unreliable and factors other than actual or perceived COVID-19 spread were likely driving patterns for service availability.
In-person outpatient visits were most impacted at T1, similar to other civilian surveys, with only 1 site reporting in-person outpatient visits without limitations.1,2 These numbers significantly changed by T2, with all sites offering either limited or unlimited in-person visits. While the surveys did not evaluate factors leading to this rapid recovery, it may be related to the availability of COVID-19 vaccinations within the VHA during this time.11 The US Department of Veterans Affairs was the first federal agency to mandate employee vaccination.12 By the most recent time point (T4), all responding sites offered outpatient visits. Outpatient EEGs followed a similar trend, with T1 being the most restrictive and full, unrestricted outpatient EEGs available by T3.
Fiscal year (FY) trends from ECoE annual reports suggest that encounters slowly recovered over the course of the pandemic. In FY 2019 there were 13,143 outpatient encounters and 6394 EEGs, which dropped to 8097 outpatient encounters and 4432 EEGs in FY 2020 before rising to 8489 outpatient encounters and 5604 EEGs in FY 2021 and 9772 outpatient encounters and 5062 EEGs in FY 2022. Thus, while clinicians described availability of services, patients may have remained hesitant or were otherwise unable to fulfill in-person appointments. The increased availability of home EEG (145 encounter days in 2021 and 436 encounter days in 2022) may be filling this gap.
In contrast to outpatient clinics and EEG, EMU availability showed relatively slower reimplementation. In the last survey, about 30% of sites were still not offering EMU or had limited services. Early trends regarding reduced staffing and patient reluctance for elective admission cited in other surveys may have also affected EMU availability within the VHA.2,13 Consistent with trends in availability, ECoE annual report data suggest EMU patient participation was about one-half of prepandemic rates: 3069 encounters in FY 2019 dropped to 1614 encounters in 2020. By 2021, rates were about two-thirds of prepandemic rates with 2058 encounters in 2021 and 2101 encounters in 2022.
Early survey results (T1) from this study echo trends from other surveys. In the AES survey (April to June 2020), about a quarter of respondents (22%) reported doing fewer EEG studies than usual. The Italian national survey (April 2020) revealed reduced presurgical evaluations (81%), ambulatory EEG (78%), standard EEG (5%) and long-term EEG (32%).4 In the NAEC survey (end of 2020)—which roughly corresponded to T2—outpatient EEGs were still < 75% of pre-COVID levels in one-half of the centers.
National and Local Guidelines
Both national and local guidelines were perceived as useful by most respondents, with national guidelines being more beneficial. This aligns with the NAEC survey, where there was a perceived need for detailed recommendations for PPE and COVID-19 testing of patients, visitors, and staff. Based on national and local guidelines, ECoE implemented safety procedures, as reflected in responses. Staff masking procedures appeared to be the most widely adopted for all services, while the use of full PPE waned as the pandemic progressed. COVID-19 testing was rarely used for routine outpatient visits but common in EMU admissions. This is similar to a survey conducted by the American Academy of Neurology which found full PPE implementation intermittently in outpatient settings and more frequently in inpatient settings.14
Telehealth Attitudes
While most sites anticipated permanent implementation of safety precautions and telehealth, the latter was consistently reported as more likely to be sustained. The VHA had a large and well-developed system of telehealth services that considerably predated the pandemic.15,16 Through this established infrastructure, remote services were quickly increased across the VHA.17-19 This telehealth structure was supplemented by the ability of VHA clinicians to practice across state lines, following a 2018 federal rule.20 The AES survey noted the VHA ECoE's longstanding experience with telehealth as a model for telemedicine use in providing direct patient care, remote EEG analysis, and clinician-to-clinician consultation.1
Trends in the number of telehealth patients seen, observed through patterns in ECoE annual reports are consistent with positive views toward this method of service provision. Specifically, these annual reports capture trends in Video Telehealth Clinic (local station), Video Telehealth Clinic (different station), Home Video Telehealth, Telephone Clinic, and eConsults. Though video telehealth at in-person stations had a precipitous drop in 2020 that continued to wane in subsequent years (898 encounters in 2019; 455 encounters in 2020; 90 encounters in 2021; 88 encounters in 2022), use of home video telehealth rose over time (143 encounters in 2019; 1003 encounters in 2020; 3206 encounters in 2021; 3315 encounters in 2022). Use of telephone services rose drastically in 2020 but has since become a less frequently used service method (2636 in 2019; 5923 in 2020; 5319 in 2021; 3704 in 2022).
Limitations
While the survey encouraged a high response rate, this limited its scope and interpretability. While the availability of services was evaluated, the underlying reasons were not queried. Follow-up questions about barriers to reopening may have allowed for a better understanding of why some services, such as EMU, continued to operate suboptimally later in the pandemic. Similarly, asking about unique strategies or barriers for telehealth would have allowed for a better understanding of its current and future use. We hypothesize that staffing changes during the pandemic may have influenced the availability of services, but changes to staffing were not assessed via the survey and were not readily available via other sources (eg, ECoE annual reports) at the time of publication. An additional limitation is the lack of comparable surveys in the literature for time points T2 to T4, as most analogous surveys were performed early in 2020.
Conclusions
This longitudinal study performed at 4 time points during the COVID-19 pandemic is the first to offer a comprehensive picture of changes to epilepsy and EEG services over time, given that other similar surveys lacked follow-up. Results reveal a significant limitation of services at VHA ECoE shortly after the onset of the pandemic, with return to near-complete operational status 2 years later. While safety precautions and telehealth are predicted to continue, telehealth is perceived as a more permanent change in services.
The COVID-19 pandemic affected diverse workplaces globally, leading to temporary and permanent changes across the health care landscape. Included among the impacted areas of care were epilepsy and electroencephalogram (EEG) clinicians and services. Surveys among epilepsy specialists and neurophysiologists conducted at the onset of the pandemic to evaluate working conditions include analyses from the American Epilepsy Society (AES), the National Association of Epilepsy Centers (NAEC), the International League Against Epilepsy, and an Italian national survey.1-4 These investigations revealed reductions in epilepsy monitoring unit (EMU) admissions (23% decline), epilepsy surgery (6% decline), inpatient EEG (22% of respondents reported decline), and patients having difficulty accessing epilepsy professionals (28% of respondents reported decline) or obtaining medications (20% of respondents reported decline).1-3
While such research provided evidence for changes to epilepsy care in 2020, there are limited data on subsequent adaptations during the pandemic. These studies did not incorporate data on the spread of COVID-19 or administrative workload numbers to analyze service delivery beyond self reports. This study aimed to address this gap in the literature by highlighting results from longitudinal national surveys conducted at the Epilepsy Centers of Excellence (ECoE), a specialty care service within the Veterans Health Administration (VHA), which annually serves > 9 million veterans.5 The ECoE represents epileptologists and neurophysiologists across the United States at the 17 primary facilities that were established at the time of this survey (2 ECoEs have been added since survey completion) in 4 geographical regions and for which other regional facilities refer patients for diagnostic services or specialty care.6
National surveys were conducted among the ECoE directors regarding adaptations made from May 2020 to June 2022 to provide a comprehensive account of limitations they experienced and how adjustments have been made to improve patient care. Survey responses were compared to administrative workload numbers and COVID-19 spread data from the Centers for Disease Control and Prevention (CDC) to provide a comprehensive analysis of performance during the pandemic.
METHODS
Data were collected as part of a quality improvement initiative by the VHA ECoE; institutional review board approval was not required. An 18-item survey covering 5 broad domains was sent to ECoE directors 4 separate times to accumulate data from 4 time periods: May to June 2020 (T1); December 2020 to February 2021 (T2); July to August 2021 (T3); and June to July 2022 (T4). These periods correspond to the following phases of the pandemic: T1, onset of pandemic; T2, vaccine availability; T3, Delta variant predominant; T4, Omicron variant predominant.

Data on the spread of COVID-19 were collected from the CDC archived dataset, US COVID-19 County Level of Community Transmission Historical Changes (Table 1).7 Administrative workload (patient counts) for EEG, EMU, and outpatient clinics were extracted from VHA administrative databases for the participating sites for the months prior to each survey: T1, April 2020; T2, November 2020; T3, June 2021; and T4, May 2022 (Table 2).

Survey Structure and Content
The survey was developed by the ECoE and was not validated prior to its use due to the time-sensitive nature of gathering information during the pandemic. The first survey (T1) was an emailed spreadsheet with open-ended questions to gauge availability of services (ie, outpatient clinic, EEG, EMU), assess whether safety precautions were being introduced, and understand whether national or local guidelines were thought to be helpful. Responses from this and subsequent surveys were standardized into yes/no and multiple choice formats. Subsequent surveys were administered online using a Research Electronic Data Capture tool.8,9
Availability of outpatient epilepsy services across the 4 time periods were categorized as unlimited (in-person with no restrictions), limited (in-person with restrictions), planned (not currently performed but scheduled for the near future), and unavailable (no in-person services offered) (eAppendices 1-6, available in article PDF).
Statistical Analyses
Analyses were performed to compare survey responses to workload and CDC data on COVID-19 community spread. The following associations were examined: (1) CDC COVID-19 spread vs respondents’ perception of spread; (2) respondents’ perception of spread vs availability of services; (3) CDC COVID-19 spread vs availability of services; (4) respondents’ perception of spread vs workload; and (5) CDC COVID-19 spread vs workload. Availability of services was dichotomized for analyses, with limited or fully available services classified as available. As services were mostly open at T3 regardless of the spread of the virus, and the CDC COVID-19 spread classification for all sites was severe or high at T2 and T4, corresponding associations were not tested at these time points. For associations 1 through 3, Fisher exact tests were used; for associations 4 and 5, Mann-Whitney U tests (where the COVID-19 spread fell into 2 categories) and Kruskal-Wallis tests (for 3 categories of COVID-19 spread) were performed. All tests were 2-tailed and performed at 0.05 error rate. Bonferroni corrections were applied to adjust P values for multiple hypotheses tests.
RESULTS
From the 17 sites invited, responses at each time point were obtained from 13 (T1),17 (T2), 15 (T3), and 16 (T4) centers. There was no significant association between self-reported COVID-19 spread and CDC classification of COVID spread. There were no associations between COVID-19 community spread (respondent reported or CDC severity level) and outpatient clinic availability (self-reported or workload captured). At T3, a positive association was found between the CDC spread level and workload (P = .008), but this was not significant after Bonferroni correction (P = .06).
EEG availability surpassed EMU availability at all time points, although EMU services made some recovery at T3 and T4. No associations were found between COVID-19 community spread (self-reported or CDC severity level) and outpatient EEG or EMU availability (self-reported or workload captured). At T3, there was a positive association between EEG workload and CDC COVID-19 severity level (P = .04), but this was not significant after Bonferroni correction (P = .30).
For outpatient EEG, staff and patient mask use were universally implemented by T2, while the use of full personal protective equipment (PPE) occurred at a subset of sites (T2, 6/17 [35%]; T3, 3/15 [20%]; T4: 4/16 [25%]). COVID-19 testing was rarely implemented prior to outpatient EEG (T1, 0 sites; T2, 1 site; T3, 1 site; T4, 0 sites). Within the EMU, safety precautions including COVID-19 testing, patient mask usage, staff mask usage, and aerosolization demonstrated a sustained majority usage across the 4 surveys.
National and Local Guidelines
The open-ended survey at T1 asked site directors, “Should there be national recommendations on how EEGs and related procedures should be done during the pandemic or should this be left to local conditions?” Responses were mixed, with 5 respondents desiring a national standard, 4 respondents favoring a local response, and 4 respondents believing a national standard should be in place but with modifications based on local outbreak levels and needs.
Surveys performed at T2 through T4 asked, “Which of the following do you feel was/will be helpful in adapting to COVID-19–related changes?” Overall, there was substantial agreement that guidelines were helpful. Most sites anticipated permanent changes in enhanced safety precautions and telehealth.
DISCUSSION
This longitudinal study across 4 time points describes how epilepsy services within the VHA and ECoE adapted to the COVID-19 pandemic. The first survey, conducted 2 months after COVID-19 was declared a pandemic, allowed a comparison with other concurrent US national surveys.1,2,10 The subsequent surveys describe longitudinal adaptations to balance patient and staff safety with service availability and is a unique feature of the current report. Results demonstrate flexibility and adaptability by the ECoEs surveyed, which surprisingly did not show significant associations between CDC COVID-19 spread data and administrative workload data.
Trends in Availability of Services
The most significant impact of COVID-19 restrictions was during T1. There were no significant relationships between service availability/workload and objective CDC COVID-19 spread levels or subjective self-reported COVID-19 spread. Respondents’ perceptions of local COVID-19 spread showed no association with CDC COVID-19 spread data. It appears that subjective perception of spread may be unreliable and factors other than actual or perceived COVID-19 spread were likely driving patterns for service availability.
In-person outpatient visits were most impacted at T1, similar to other civilian surveys, with only 1 site reporting in-person outpatient visits without limitations.1,2 These numbers significantly changed by T2, with all sites offering either limited or unlimited in-person visits. While the surveys did not evaluate factors leading to this rapid recovery, it may be related to the availability of COVID-19 vaccinations within the VHA during this time.11 The US Department of Veterans Affairs was the first federal agency to mandate employee vaccination.12 By the most recent time point (T4), all responding sites offered outpatient visits. Outpatient EEGs followed a similar trend, with T1 being the most restrictive and full, unrestricted outpatient EEGs available by T3.
Fiscal year (FY) trends from ECoE annual reports suggest that encounters slowly recovered over the course of the pandemic. In FY 2019 there were 13,143 outpatient encounters and 6394 EEGs, which dropped to 8097 outpatient encounters and 4432 EEGs in FY 2020 before rising to 8489 outpatient encounters and 5604 EEGs in FY 2021 and 9772 outpatient encounters and 5062 EEGs in FY 2022. Thus, while clinicians described availability of services, patients may have remained hesitant or were otherwise unable to fulfill in-person appointments. The increased availability of home EEG (145 encounter days in 2021 and 436 encounter days in 2022) may be filling this gap.
In contrast to outpatient clinics and EEG, EMU availability showed relatively slower reimplementation. In the last survey, about 30% of sites were still not offering EMU or had limited services. Early trends regarding reduced staffing and patient reluctance for elective admission cited in other surveys may have also affected EMU availability within the VHA.2,13 Consistent with trends in availability, ECoE annual report data suggest EMU patient participation was about one-half of prepandemic rates: 3069 encounters in FY 2019 dropped to 1614 encounters in 2020. By 2021, rates were about two-thirds of prepandemic rates with 2058 encounters in 2021 and 2101 encounters in 2022.
Early survey results (T1) from this study echo trends from other surveys. In the AES survey (April to June 2020), about a quarter of respondents (22%) reported doing fewer EEG studies than usual. The Italian national survey (April 2020) revealed reduced presurgical evaluations (81%), ambulatory EEG (78%), standard EEG (5%) and long-term EEG (32%).4 In the NAEC survey (end of 2020)—which roughly corresponded to T2—outpatient EEGs were still < 75% of pre-COVID levels in one-half of the centers.
National and Local Guidelines
Both national and local guidelines were perceived as useful by most respondents, with national guidelines being more beneficial. This aligns with the NAEC survey, where there was a perceived need for detailed recommendations for PPE and COVID-19 testing of patients, visitors, and staff. Based on national and local guidelines, ECoE implemented safety procedures, as reflected in responses. Staff masking procedures appeared to be the most widely adopted for all services, while the use of full PPE waned as the pandemic progressed. COVID-19 testing was rarely used for routine outpatient visits but common in EMU admissions. This is similar to a survey conducted by the American Academy of Neurology which found full PPE implementation intermittently in outpatient settings and more frequently in inpatient settings.14
Telehealth Attitudes
While most sites anticipated permanent implementation of safety precautions and telehealth, the latter was consistently reported as more likely to be sustained. The VHA had a large and well-developed system of telehealth services that considerably predated the pandemic.15,16 Through this established infrastructure, remote services were quickly increased across the VHA.17-19 This telehealth structure was supplemented by the ability of VHA clinicians to practice across state lines, following a 2018 federal rule.20 The AES survey noted the VHA ECoE's longstanding experience with telehealth as a model for telemedicine use in providing direct patient care, remote EEG analysis, and clinician-to-clinician consultation.1
Trends in the number of telehealth patients seen, observed through patterns in ECoE annual reports are consistent with positive views toward this method of service provision. Specifically, these annual reports capture trends in Video Telehealth Clinic (local station), Video Telehealth Clinic (different station), Home Video Telehealth, Telephone Clinic, and eConsults. Though video telehealth at in-person stations had a precipitous drop in 2020 that continued to wane in subsequent years (898 encounters in 2019; 455 encounters in 2020; 90 encounters in 2021; 88 encounters in 2022), use of home video telehealth rose over time (143 encounters in 2019; 1003 encounters in 2020; 3206 encounters in 2021; 3315 encounters in 2022). Use of telephone services rose drastically in 2020 but has since become a less frequently used service method (2636 in 2019; 5923 in 2020; 5319 in 2021; 3704 in 2022).
Limitations
While the survey encouraged a high response rate, this limited its scope and interpretability. While the availability of services was evaluated, the underlying reasons were not queried. Follow-up questions about barriers to reopening may have allowed for a better understanding of why some services, such as EMU, continued to operate suboptimally later in the pandemic. Similarly, asking about unique strategies or barriers for telehealth would have allowed for a better understanding of its current and future use. We hypothesize that staffing changes during the pandemic may have influenced the availability of services, but changes to staffing were not assessed via the survey and were not readily available via other sources (eg, ECoE annual reports) at the time of publication. An additional limitation is the lack of comparable surveys in the literature for time points T2 to T4, as most analogous surveys were performed early in 2020.
Conclusions
This longitudinal study performed at 4 time points during the COVID-19 pandemic is the first to offer a comprehensive picture of changes to epilepsy and EEG services over time, given that other similar surveys lacked follow-up. Results reveal a significant limitation of services at VHA ECoE shortly after the onset of the pandemic, with return to near-complete operational status 2 years later. While safety precautions and telehealth are predicted to continue, telehealth is perceived as a more permanent change in services.
Albert DVF, Das RR, Acharya JN, et al. The impact of COVID-19 on epilepsy care: a survey of the American Epilepsy Society membership. Epilepsy Curr. 2020;20(5):316-324. doi:10.1177/1535759720956994
Ahrens SM, Ostendorf AP, Lado FA, et al. Impact of the COVID-19 pandemic on epilepsy center practice in the United States. Neurology. 2022;98(19):e1893-e1901. doi:10.1212/WNL.0000000000200285
Cross JH, Kwon CS, Asadi-Pooya AA, et al. Epilepsy care during the COVID-19 pandemic. Epilepsia. 2021;62(10):2322-2332. doi:10.1111/epi.17045
Assenza G, Lanzone J, Ricci L, et al. Electroencephalography at the time of Covid-19 pandemic in Italy. Neurol Sci. 2020;41(8):1999-2004. doi:10.1007/s10072-020-04546-8
US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics. Veteran population. Updated September 7, 2022. Accessed October 25, 2024. https://www.va.gov/vetdata/veteran_population.asp
US Department of Veterans Affairs, Veterans Health Administration. Epilepsy Centers of Excellence (ECoE). Annual report fiscal year 2019. Accessed October 25, 2024. https://www.epilepsy.va.gov/docs/FY19AnnualReport-VHAEpilepsyCentersofExcellence.pdf
Centers for Disease Control and Prevention. United States COVID-19 county level of community transmission historical changes – ARCHIVED. Updated February 20, 2024. Accessed October 25, 2024. https://data.cdc.gov/Public-Health-Surveillance/United-States-COVID-19-County-Level-of-Community-T/nra9-vzzn
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi:10.1016/j.jbi.2008.08.010
Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi:10.1016/j.jbi.2019.103208
World Health Organization. Rolling updates on coronavirus disease (COVID-19). Updated July 31, 2020. Accessed October 25, 2024. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen
US Department of Veterans Affairs. VA announces initial plans for COVID-19 vaccine distribution. News release. December 10, 2020. Accessed October 25, 2024. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5580
Steinhauer J. V.A. Issues Vaccine Mandate for Health Care Workers, a First for a Federal Agency. The New York Times. August 16, 2021. Accessed October 25, 2024. https://www.nytimes.com/2021/07/26/us/politics/veterans-affairs-coronavirus-covid-19.html
Zafar SF, Khozein RJ, LaRoche SM, Westover MB, Gilmore EJ. Impact of the COVID-19 pandemic on continuous EEG utilization. J Clin Neurophysiol. 2022;39(7):567-574. doi:10.1097/WNP.0000000000000802
Qureshi AI, Rheaume C, Huang W, et al. COVID-19 exposure during neurology practice. Neurologist. 2021;26(6):225-230. doi:10.1097/NRL.0000000000000346
Darkins A, Cruise C, Armstrong M, Peters J, Finn M. Enhancing access of combat-wounded veterans to specialist rehabilitation services: the VA Polytrauma Telehealth Network. Arch Phys Med Rehabil. 2008;89(1):182-187. doi:10.1016/j.apmr.2007.07.027
Darkins A, Ryan P, Kobb R, et al. Care coordination/home telehealth: the systematic implementation of health informatics, home telehealth, and disease management to support the care of veteran patients with chronic conditions. Telemed J E Health. 2008;14(10):1118-1126. doi:10.1089/tmj.2008.0021
Gentry MT, Puspitasari AJ, McKean AJ, et al. Clinician satisfaction with rapid adoption and implementation of telehealth services during the COVID-19 pandemic. Telemed J E Health. 2021;27(12):1385-1392. doi:10.1089/tmj.2020.0575
Connolly SL, Stolzmann KL, Heyworth L, et al. Patient and provider predictors of telemental health use prior to and during the COVID-19 pandemic within the Department of Veterans Affairs. Am Psychol. 2022;77(2):249-261. doi:10.1037/amp0000895
Shelton CJ, Kim A, Hassan AM, Bhat A, Barnello J, Castro CA. System-wide implementation of telehealth to support military veterans and their families in response to COVID-19: a paradigm shift. J Mil Veteran Fam Health. 2020;6(S2):50-57. doi:10.3138/jmvfh-CO19-0003
VA expands telehealth by allowing health care providers to treat patients across state lines. News release. US Dept of Veterans Affairs. May 11, 2018. Accessed October 25, 2024. https://news.va.gov/press-room/va-expands-telehealth-by-allowing-health-care-providers-to-treat-patients-across-state-lines/
Albert DVF, Das RR, Acharya JN, et al. The impact of COVID-19 on epilepsy care: a survey of the American Epilepsy Society membership. Epilepsy Curr. 2020;20(5):316-324. doi:10.1177/1535759720956994
Ahrens SM, Ostendorf AP, Lado FA, et al. Impact of the COVID-19 pandemic on epilepsy center practice in the United States. Neurology. 2022;98(19):e1893-e1901. doi:10.1212/WNL.0000000000200285
Cross JH, Kwon CS, Asadi-Pooya AA, et al. Epilepsy care during the COVID-19 pandemic. Epilepsia. 2021;62(10):2322-2332. doi:10.1111/epi.17045
Assenza G, Lanzone J, Ricci L, et al. Electroencephalography at the time of Covid-19 pandemic in Italy. Neurol Sci. 2020;41(8):1999-2004. doi:10.1007/s10072-020-04546-8
US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics. Veteran population. Updated September 7, 2022. Accessed October 25, 2024. https://www.va.gov/vetdata/veteran_population.asp
US Department of Veterans Affairs, Veterans Health Administration. Epilepsy Centers of Excellence (ECoE). Annual report fiscal year 2019. Accessed October 25, 2024. https://www.epilepsy.va.gov/docs/FY19AnnualReport-VHAEpilepsyCentersofExcellence.pdf
Centers for Disease Control and Prevention. United States COVID-19 county level of community transmission historical changes – ARCHIVED. Updated February 20, 2024. Accessed October 25, 2024. https://data.cdc.gov/Public-Health-Surveillance/United-States-COVID-19-County-Level-of-Community-T/nra9-vzzn
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi:10.1016/j.jbi.2008.08.010
Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi:10.1016/j.jbi.2019.103208
World Health Organization. Rolling updates on coronavirus disease (COVID-19). Updated July 31, 2020. Accessed October 25, 2024. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen
US Department of Veterans Affairs. VA announces initial plans for COVID-19 vaccine distribution. News release. December 10, 2020. Accessed October 25, 2024. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5580
Steinhauer J. V.A. Issues Vaccine Mandate for Health Care Workers, a First for a Federal Agency. The New York Times. August 16, 2021. Accessed October 25, 2024. https://www.nytimes.com/2021/07/26/us/politics/veterans-affairs-coronavirus-covid-19.html
Zafar SF, Khozein RJ, LaRoche SM, Westover MB, Gilmore EJ. Impact of the COVID-19 pandemic on continuous EEG utilization. J Clin Neurophysiol. 2022;39(7):567-574. doi:10.1097/WNP.0000000000000802
Qureshi AI, Rheaume C, Huang W, et al. COVID-19 exposure during neurology practice. Neurologist. 2021;26(6):225-230. doi:10.1097/NRL.0000000000000346
Darkins A, Cruise C, Armstrong M, Peters J, Finn M. Enhancing access of combat-wounded veterans to specialist rehabilitation services: the VA Polytrauma Telehealth Network. Arch Phys Med Rehabil. 2008;89(1):182-187. doi:10.1016/j.apmr.2007.07.027
Darkins A, Ryan P, Kobb R, et al. Care coordination/home telehealth: the systematic implementation of health informatics, home telehealth, and disease management to support the care of veteran patients with chronic conditions. Telemed J E Health. 2008;14(10):1118-1126. doi:10.1089/tmj.2008.0021
Gentry MT, Puspitasari AJ, McKean AJ, et al. Clinician satisfaction with rapid adoption and implementation of telehealth services during the COVID-19 pandemic. Telemed J E Health. 2021;27(12):1385-1392. doi:10.1089/tmj.2020.0575
Connolly SL, Stolzmann KL, Heyworth L, et al. Patient and provider predictors of telemental health use prior to and during the COVID-19 pandemic within the Department of Veterans Affairs. Am Psychol. 2022;77(2):249-261. doi:10.1037/amp0000895
Shelton CJ, Kim A, Hassan AM, Bhat A, Barnello J, Castro CA. System-wide implementation of telehealth to support military veterans and their families in response to COVID-19: a paradigm shift. J Mil Veteran Fam Health. 2020;6(S2):50-57. doi:10.3138/jmvfh-CO19-0003
VA expands telehealth by allowing health care providers to treat patients across state lines. News release. US Dept of Veterans Affairs. May 11, 2018. Accessed October 25, 2024. https://news.va.gov/press-room/va-expands-telehealth-by-allowing-health-care-providers-to-treat-patients-across-state-lines/
Comparing Patient Care Models at a Local Free Clinic vs an Insurance- Based University Medical Center
Comparing Patient Care Models at a Local Free Clinic vs an Insurance- Based University Medical Center
Approximately 25% of Americans have at least one skin condition, and 20% are estimated to develop skin cancer during their lifetime.1,2 However, 40% of the US population lives in areas underserved by dermatologists. 3 The severity and mortality of skin cancers such as melanoma and mycosis fungoides have been positively associated with minoritized race, lack of health insurance, and unstable housing status.4-6 Patients who receive health care at free clinics often are of a racial or ethnic minoritized social group, are uninsured, and/or lack stable housing; this underserved group also includes recent immigrants to the United States who have limited English proficiency (LEP).7 Only 25% of free clinics offer specialty care services such as dermatology.7,8
Of the 42 free clinics and Federally Qualified Health Centers in Pittsburgh, Pennsylvania, the Birmingham Free Clinic (BFC) is one of the few that offers specialty care services including dermatology.9 Founded in 1994, the BFC serves as a safety net for Pittsburgh’s medically underserved population, offering primary and acute care, medication access, and social services. From January 2020 to May 2022, the BFC offered 27 dermatology clinics that provided approximately 100 people with comprehensive care including full-body skin examinations, dermatologic diagnoses and treatments, minor procedures, and dermatopathology services.
In this study, we compared the BFC dermatology patient care model with that of the dermatology department at the University of Pittsburgh Medical Center (UPMC), an insurance-based tertiary referral health care system in western Pennsylvania. By analyzing the demographics, dermatologic diagnoses, and management strategies of both the BFC and UPMC, we gained an understanding of how these patient care models differ and how they can be improved to care for diverse patient populations.
Methods
A retrospective chart review of dermatology patients seen in person at the BFC and UPMC during the period from January 2020 to May 2022 was performed. The UPMC group included patients seen by 3 general dermatologists (including A.J.J.) at matched time points. Data were collected from patients’ first in-person visit during the study period. Variables of interest included patient age, sex, race, ethnicity, primary language, zip code, health insurance status, distance to clinic (estimated using Google Maps to calculate the shortest driving distance from the patient’s zip code to the clinic), history of skin cancer, dermatologic diagnoses, and management strategies. These variables were not collected for patients who cancelled or noshowed their first in-person appointments. All patient charts and notes corresponding to the date and visit of interest were accessed through the electronic medical record (EMR). Patient data were de-identified and stored in a password-protected spreadsheet. Comparisons between the BFC and UPMC patient populations were performed using X2 tests of independence, Fisher exact tests, and Mann-Whitney U tests via SPSS software (IBM). Statistical significance was set at P<.05.
Results
Patient Characteristics—Our analysis included 76 initial appointments at the BFC and 322 at UPMC (Table 1). The mean age for patients at the BFC and UPMC was 39.6 years and 47.8 years, respectively (P=.001). Males accounted for 39 (51.3%) and 112 (34.8%) of BFC and UPMC patients, respectively (P=.008); 2 (0.6%) patients from UPMC were transgender. Of the BFC and UPMC patients, 44.7% (34/76) and 0.9% (3/322) were Hispanic, respectively (P<.001). With regard to race, 52.6% (40/76) of BFC patients were White, 19.7% (15/76) were Black, 6.6% (5/76) were Asian/Pacific Islander (Chinese, 1.3% [1/76]; other Asian, 5.3% [4/76]), and 21.1% (16/76) were American Indian/other/unspecified (American Indian, 1.3% [1/76]; other, 13.2% [10/76]; unspecified, 6.6% [5/76]). At UPMC, 61.2% (197/322) of patients were White, 28.0% (90/322) were Black, 5.3% (17/322) were Asian/Pacific Islander (Chinese, 1.2% [4/322]; Indian [Asian], 1.9% [6/322]; Japanese, 0.3% [1/322]; other Asian, 1.6% [5/322]; other Asian/American Indian, 0.3% [1/322]), and 5.6% (18/322) were American Indian/other/ unspecified (American Indian, 0.3% [1/322]; other, 0.3% [1/322]; unspecified, 5.0% [16/322]). Overall, the BFC patient population was more ethnically and racially diverse than that of UPMC (P<.001).
Forty-six percent (35/76) of BFC patients and 4.3% (14/322) of UPMC patients had LEP (P<.001). Primary languages among BFC patients were 53.9% (41/76) English, 40.8% (31/76) Spanish, and 5.2% (4/76) other/ unspecified (Chinese, 1.3% [1/76]; Indonesian, 2.6% [2/76]; unspecified, 1.3% [1/76]). Primary languages among UPMC patients were 95.7% (308/322) English and 4.3% (14/322) other/unspecified (Chinese, 0.6% [2/322]; Nepali, 0.6% [2/322]; Pali, 0.3% [1/322]; Russian, 0.3% [1/322]; unspecified, 2.5% [8/322]). There were notable differences in insurance status at the BFC vs UPMC (P<.001), with more UPMC patients having private insurance (52.8% [170/322] vs 11.8% [9/76]) and more BFC patients being uninsured (52.8% [51/76] vs 1.9% [6/322]). There was no significant difference in distance to clinic between the 2 groups (P=.183). More UPMC patients had a history of skin cancer (P=.003). More patients at the BFC were no-shows for their appointments (P<.001), and UPMC patients more frequently canceled their appointments (P<.001).
Dermatologic Diagnoses—The most commonly diagnosed dermatologic conditions at the BFC were dermatitis (23.7% [18/76]), neoplasm of uncertain behavior (15.8% [12/76]), alopecia (11.8% [9/76]), and acne (10.5% [8/76]) (Table 2). The most commonly diagnosed conditions at UPMC were nevi (26.4% [85/322]), dermatitis (22.7% [73/322]), seborrheic keratosis (21.7% [70/322]), and skin cancer screening (21.4% [70/322]). Neoplasm of uncertain behavior was more common in BFC vs UPMC patients (P=.040), while UPMC patients were more frequently diagnosed with nevi (P<.001), seborrheic keratosis (P<.001), and skin cancer screening (P<.001). There was no significant difference between the incidence of skin cancer diagnoses in the BFC (1.3% [1/76]) and UPMC (0.6% [2/76]) patient populations (P=.471). Among the biopsied neoplasms, there was also no significant difference in malignant (BFC, 50.0% [5/10]; UPMC, 32.0% [8/25]) and benign (BFC, 50.0% [5/10]; UPMC, 36.0% [9/25]) neoplasms diagnosed at each clinic (P=.444).
Management Strategies—Systemic antibiotics were more frequently prescribed (P<.001) and laboratory testing/ imaging were more frequently ordered (P=.005) at the BFC vs UPMC (Table 3). Patients at the BFC also more frequently required emergency insurance (P=.036). Patients at UPMC were more frequently recommended sunscreen (P=.003) and received education about skin cancer signs by review of the ABCDEs of melanoma (P<.001), sun-protective behaviors (P=.001), and skin examination frequency (P<.001). Notes in the EMR for UPMC patients more frequently specified patient followup instructions (P<.001).
Comment
As of 2020, the city of Pittsburgh had an estimated population of nearly 303,000 based on US Census data.10 Its population is predominantly White (62.7%) followed by Black/African American (22.8%) and Asian (6.5%); 5.9% identify as 2 or more races. Approximately 3.8% identify as Hispanic or Latino. More than 11% of the Pittsburgh population aged 5 years and older speaks a language other than English as their primary language, including Spanish (2.3%), other Indo-European languages (3.9%), and Asian and Pacific Island languages (3.5%).11 More than 5% of the Pittsburgh population does not have health insurance.12
The BFC is located in Pittsburgh’s South Side area, while one of UPMC’s primary dermatology clinics is located in the Oakland district; however, most patients who seek care at these clinics live outside these areas. Our study results indicated that the BFC and UPMC serve distinct groups of people within the Pittsburgh population. The BFC patient population was younger with a higher percentage of patients who were male, Hispanic, racially diverse, and with LEP compared with the UPMC patient population. In this clinical setting, the BFC health care team engages with people from diverse backgrounds and requires greater interpreter and medical support services.
The BFC largely is supported by volunteers, UPMC, grants, and philanthropy. Dermatology clinics are staffed by paid and volunteer team members. Paid team members include 1 nurse and 1 access lead who operates the front desk and registration. Volunteer team members include 1 board-certified dermatologist from UPMC (A.J.J.), or an affiliate clinic and 1 or 2 of each of the following: UPMC dermatology residents, medical or undergraduate students from the University of Pittsburgh, AmeriCorps national service members, and student or community medical interpreters. The onsite pharmacy is run by volunteer faculty, resident, and student pharmacists from the University of Pittsburgh. Dermatology clinics are half-day clinics that occur monthly. Volunteers for each clinic are recruited approximately 1 month in advance.
Dermatology patients at the BFC are referred from the BFC general medicine clinic and nearby Federally Qualified Health Center s for simple to complex medical and surgical dermatologic skin conditions. Each BFC dermatology clinic schedules an average of 7 patients per clinic and places other patients on a wait-list unless more urgent triage is needed. Patients are notified when they are scheduled via phone or text message, and they receive a reminder call or text 1 or 2 days prior to their appointment that also asks them to confirm attendance. Patients with LEP are called with an interpreter and also may receive text reminders that can be translated using Google Translate. Patients are instructed to notify the BFC if they need to cancel or reschedule their appointment. At the end of each visit, patients are given an after-visit summary that lists follow-up instructions, medications prescribed during the visit, and upcoming appointments. The BFC offers bus tickets to help patients get to their appointments. In rare cases, the BFC may pay for a car service to drive patients to and from the clinic.
Dermatology clinics at UPMC use scheduling and self-scheduling systems through which patients can make appointments at a location of their choice with any available board-certified dermatologist or physician assistant. Patients receive a reminder phone call 3 days prior to their appointment instructing them to call the office if they are unable to keep their appointment. Patients signed up for the online portal also receive a reminder message and an option to confirm or cancel their appointment. Patients with cell phone numbers in the UPMC system receive a text message approximately 2 days prior to their appointment that allows them to preregister and pay their copayment in advance. They receive another text 20 minutes prior to their appointment with an option for contactless check-in. At the conclusion of their visit, patients can schedule a follow-up appointment and receive a printed copy of their after-visit summary that provides information about follow-up instructions, prescribed medications, and upcoming visits. They may alternatively access this summary via the online patient portal. Patients are not provided transportation to UPMC clinics, but they are offered parking validation.
Among the most common dermatologic diagnoses for each group, BFC patients presented for treatment of more acute dermatologic conditions, while UPMC patients presented for more benign and preventive-care conditions. This difference may be attributable to the BFC’s referral and triage system, wherein patients with more urgent problems are given scheduling priority. This patient care model contrasts with UPMC’s scheduling process in which no known formal triage system is utilized. Interestingly, there was no difference in skin cancer incidence despite a higher percentage of preventive skin cancer screenings at UPMC.
Patients at the BFC more often required emergency insurance for surgical interventions, which is consistent with the higher percentage of uninsured individuals in this population. Patients at UPMC more frequently were recommended sunscreen and were educated about skin cancer, sun protection, and skin examination, in part due to this group’s more extensive history of skin cancer and frequent presentation for skin cancer screenings. At the same time, educational materials for skin care at both the BFC and UPMC are populated into the EMR in English, whereas materials in other languages are less readily available.
Our retrospective study had several limitations. Demographic information that relied on clinic-dependent intake questionnaires may be limited due to variable intake processes and patients opting out of self-reporting. By comparing patient populations between 2 clinics, confounding variables such as location and hours of operation may impact the patient demographics recorded at the BFC vs UPMC. Resources and staff availability may affect the management strategies and follow-up care offered by each clinic. Our study period also was unique in that COVID-19 may have affected resources, staffing, scheduling, and logistics at both clinics.
Based on the aforementioned differences between the BFC and UPMC patient characteristics, care models should be strategically designed to support the needs of diverse populations. The BFC patient care model appropriately focuses on communication skills with patients with LEP by using interpreter services. Providing more skin care education and follow-up instructions in patients’ primary languages will help them develop a better understanding of their skin conditions. Another key asset of the BFC patient care model is its provision of social services such as transportation and insurance assistance.
To improve the UPMC patient care model, providing patients with bus tickets and car services may potentially reduce appointment cancellations. Using interpreter services to call and text appointment reminders, as well as interpreter resources to facilitate patient visits and patient instructions, also can mitigate language barriers for patients with LEP. Implementing a triage system into the UPMC scheduling system may help patients with more urgent skin conditions to be seen in a timely manner.
Other investigators have analyzed costs of care and proven the value of dermatologic services at free clinics to guide allocation of supplies and resources, demonstrating an area for future investigation at the BFC.13 A cost analysis of care provided at the BFC compared to UPMC could inform us about the value of the BFC’s services.
Conclusion
The dermatology clinics at the BFC and UPMC have distinct demographics, diagnoses, and management strategies to provide an inclusive patient care model. The services provided by both clinics are necessary to ensure that people in Pittsburgh have access to dermatologic care regardless of social barriers (eg, lack of health insurance, LEP). To achieve greater accessibility and health equity, dermatologic care at the BFC and UPMC can be improved by strengthening communication with people with LEP, providing skin care education, and offering social and scheduling services.
- Lim HW, Collins SAB, Resneck JS, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:958-972.e2. doi:10.1016/j.jaad.2016.12.043
- American Academy of Dermatology. Skin cancer. Accessed October 7, 2024. https://www.aad.org/media/stats-skin-cancer
- Suneja T, Smith ED, Chen GJ, et al. Waiting times to see a dermatologist are perceived as too long by dermatologists: implications for the dermatology workforce. Arch Dermatol. 2001;137:1303-1307. doi:10.1001/archderm.137.10.1303
- Grossberg AL, Carranza D, Lamp K, et al. Dermatologic care in the homeless and underserved populations: observations from the Venice Family Clinic. Cutis. 2012;89:25-32.
- Amini A, Rusthoven CG, Waxweiler TV, et al. Association of health insurance with outcomes in adults ages 18 to 64 years with melanoma in the United States. J Am Acad Dermatol. 2016;74:309-316. doi:10.1016/j.jaad.2015.09.054
- Su C, Nguyen KA, Bai HX, et al. Racial disparity in mycosis fungoides: an analysis of 4495 cases from the US National Cancer Database. J Am Acad Dermatol. 2017;77:497-502.e2. doi:10.1016/j.jaad .2017.04.1137
- Darnell JS. Free clinics in the United States: a nationwide survey. Arch Intern Med. 2010;170:946-953. doi:10.1001/archinternmed .2010.107
- Madray V, Ginjupalli S, Hashmi O, et al. Access to dermatology services at free medical clinics: a nationwide cross-sectional survey. J Am Acad Dermatol. 2019;81:245-246. doi:10.1016/j.jaad.2018.12.011
- Pennsylvania free and income-based clinics. Accessed October 7, 2024. https://www.freeclinics.com/sta/pennsylvania
- United States Census Bureau. Decennial census. P1: race. Accessed October 7, 2024. https://data.census.gov/table/DECENNIALPL2020.P1?g=160XX00US4261000
- United States Census Bureau. American community survey. S1601: language spoken at home. Accessed October 7, 2024. https://data.census.gov/table/ACSST5Y2020S1601?g=160XX00US4261000
- United States Census Bureau. S2701: selected characteristics of health insurance coverage in the United States. Accessed October 7, 2024. https://data.census.gov/table/ACSST5Y2020.S2701?g=160XX00US4261000
- Lin CP, Loy S, Boothe WD, et al. Value of Dermatology Nights at a student-run free clinic. Proc (Bayl Univ Med Cent). 2020;34:260-261. doi:10.1080/08998280.2020.1834771
Approximately 25% of Americans have at least one skin condition, and 20% are estimated to develop skin cancer during their lifetime.1,2 However, 40% of the US population lives in areas underserved by dermatologists. 3 The severity and mortality of skin cancers such as melanoma and mycosis fungoides have been positively associated with minoritized race, lack of health insurance, and unstable housing status.4-6 Patients who receive health care at free clinics often are of a racial or ethnic minoritized social group, are uninsured, and/or lack stable housing; this underserved group also includes recent immigrants to the United States who have limited English proficiency (LEP).7 Only 25% of free clinics offer specialty care services such as dermatology.7,8
Of the 42 free clinics and Federally Qualified Health Centers in Pittsburgh, Pennsylvania, the Birmingham Free Clinic (BFC) is one of the few that offers specialty care services including dermatology.9 Founded in 1994, the BFC serves as a safety net for Pittsburgh’s medically underserved population, offering primary and acute care, medication access, and social services. From January 2020 to May 2022, the BFC offered 27 dermatology clinics that provided approximately 100 people with comprehensive care including full-body skin examinations, dermatologic diagnoses and treatments, minor procedures, and dermatopathology services.
In this study, we compared the BFC dermatology patient care model with that of the dermatology department at the University of Pittsburgh Medical Center (UPMC), an insurance-based tertiary referral health care system in western Pennsylvania. By analyzing the demographics, dermatologic diagnoses, and management strategies of both the BFC and UPMC, we gained an understanding of how these patient care models differ and how they can be improved to care for diverse patient populations.
Methods
A retrospective chart review of dermatology patients seen in person at the BFC and UPMC during the period from January 2020 to May 2022 was performed. The UPMC group included patients seen by 3 general dermatologists (including A.J.J.) at matched time points. Data were collected from patients’ first in-person visit during the study period. Variables of interest included patient age, sex, race, ethnicity, primary language, zip code, health insurance status, distance to clinic (estimated using Google Maps to calculate the shortest driving distance from the patient’s zip code to the clinic), history of skin cancer, dermatologic diagnoses, and management strategies. These variables were not collected for patients who cancelled or noshowed their first in-person appointments. All patient charts and notes corresponding to the date and visit of interest were accessed through the electronic medical record (EMR). Patient data were de-identified and stored in a password-protected spreadsheet. Comparisons between the BFC and UPMC patient populations were performed using X2 tests of independence, Fisher exact tests, and Mann-Whitney U tests via SPSS software (IBM). Statistical significance was set at P<.05.
Results
Patient Characteristics—Our analysis included 76 initial appointments at the BFC and 322 at UPMC (Table 1). The mean age for patients at the BFC and UPMC was 39.6 years and 47.8 years, respectively (P=.001). Males accounted for 39 (51.3%) and 112 (34.8%) of BFC and UPMC patients, respectively (P=.008); 2 (0.6%) patients from UPMC were transgender. Of the BFC and UPMC patients, 44.7% (34/76) and 0.9% (3/322) were Hispanic, respectively (P<.001). With regard to race, 52.6% (40/76) of BFC patients were White, 19.7% (15/76) were Black, 6.6% (5/76) were Asian/Pacific Islander (Chinese, 1.3% [1/76]; other Asian, 5.3% [4/76]), and 21.1% (16/76) were American Indian/other/unspecified (American Indian, 1.3% [1/76]; other, 13.2% [10/76]; unspecified, 6.6% [5/76]). At UPMC, 61.2% (197/322) of patients were White, 28.0% (90/322) were Black, 5.3% (17/322) were Asian/Pacific Islander (Chinese, 1.2% [4/322]; Indian [Asian], 1.9% [6/322]; Japanese, 0.3% [1/322]; other Asian, 1.6% [5/322]; other Asian/American Indian, 0.3% [1/322]), and 5.6% (18/322) were American Indian/other/ unspecified (American Indian, 0.3% [1/322]; other, 0.3% [1/322]; unspecified, 5.0% [16/322]). Overall, the BFC patient population was more ethnically and racially diverse than that of UPMC (P<.001).
Forty-six percent (35/76) of BFC patients and 4.3% (14/322) of UPMC patients had LEP (P<.001). Primary languages among BFC patients were 53.9% (41/76) English, 40.8% (31/76) Spanish, and 5.2% (4/76) other/ unspecified (Chinese, 1.3% [1/76]; Indonesian, 2.6% [2/76]; unspecified, 1.3% [1/76]). Primary languages among UPMC patients were 95.7% (308/322) English and 4.3% (14/322) other/unspecified (Chinese, 0.6% [2/322]; Nepali, 0.6% [2/322]; Pali, 0.3% [1/322]; Russian, 0.3% [1/322]; unspecified, 2.5% [8/322]). There were notable differences in insurance status at the BFC vs UPMC (P<.001), with more UPMC patients having private insurance (52.8% [170/322] vs 11.8% [9/76]) and more BFC patients being uninsured (52.8% [51/76] vs 1.9% [6/322]). There was no significant difference in distance to clinic between the 2 groups (P=.183). More UPMC patients had a history of skin cancer (P=.003). More patients at the BFC were no-shows for their appointments (P<.001), and UPMC patients more frequently canceled their appointments (P<.001).
Dermatologic Diagnoses—The most commonly diagnosed dermatologic conditions at the BFC were dermatitis (23.7% [18/76]), neoplasm of uncertain behavior (15.8% [12/76]), alopecia (11.8% [9/76]), and acne (10.5% [8/76]) (Table 2). The most commonly diagnosed conditions at UPMC were nevi (26.4% [85/322]), dermatitis (22.7% [73/322]), seborrheic keratosis (21.7% [70/322]), and skin cancer screening (21.4% [70/322]). Neoplasm of uncertain behavior was more common in BFC vs UPMC patients (P=.040), while UPMC patients were more frequently diagnosed with nevi (P<.001), seborrheic keratosis (P<.001), and skin cancer screening (P<.001). There was no significant difference between the incidence of skin cancer diagnoses in the BFC (1.3% [1/76]) and UPMC (0.6% [2/76]) patient populations (P=.471). Among the biopsied neoplasms, there was also no significant difference in malignant (BFC, 50.0% [5/10]; UPMC, 32.0% [8/25]) and benign (BFC, 50.0% [5/10]; UPMC, 36.0% [9/25]) neoplasms diagnosed at each clinic (P=.444).
Management Strategies—Systemic antibiotics were more frequently prescribed (P<.001) and laboratory testing/ imaging were more frequently ordered (P=.005) at the BFC vs UPMC (Table 3). Patients at the BFC also more frequently required emergency insurance (P=.036). Patients at UPMC were more frequently recommended sunscreen (P=.003) and received education about skin cancer signs by review of the ABCDEs of melanoma (P<.001), sun-protective behaviors (P=.001), and skin examination frequency (P<.001). Notes in the EMR for UPMC patients more frequently specified patient followup instructions (P<.001).
Comment
As of 2020, the city of Pittsburgh had an estimated population of nearly 303,000 based on US Census data.10 Its population is predominantly White (62.7%) followed by Black/African American (22.8%) and Asian (6.5%); 5.9% identify as 2 or more races. Approximately 3.8% identify as Hispanic or Latino. More than 11% of the Pittsburgh population aged 5 years and older speaks a language other than English as their primary language, including Spanish (2.3%), other Indo-European languages (3.9%), and Asian and Pacific Island languages (3.5%).11 More than 5% of the Pittsburgh population does not have health insurance.12
The BFC is located in Pittsburgh’s South Side area, while one of UPMC’s primary dermatology clinics is located in the Oakland district; however, most patients who seek care at these clinics live outside these areas. Our study results indicated that the BFC and UPMC serve distinct groups of people within the Pittsburgh population. The BFC patient population was younger with a higher percentage of patients who were male, Hispanic, racially diverse, and with LEP compared with the UPMC patient population. In this clinical setting, the BFC health care team engages with people from diverse backgrounds and requires greater interpreter and medical support services.
The BFC largely is supported by volunteers, UPMC, grants, and philanthropy. Dermatology clinics are staffed by paid and volunteer team members. Paid team members include 1 nurse and 1 access lead who operates the front desk and registration. Volunteer team members include 1 board-certified dermatologist from UPMC (A.J.J.), or an affiliate clinic and 1 or 2 of each of the following: UPMC dermatology residents, medical or undergraduate students from the University of Pittsburgh, AmeriCorps national service members, and student or community medical interpreters. The onsite pharmacy is run by volunteer faculty, resident, and student pharmacists from the University of Pittsburgh. Dermatology clinics are half-day clinics that occur monthly. Volunteers for each clinic are recruited approximately 1 month in advance.
Dermatology patients at the BFC are referred from the BFC general medicine clinic and nearby Federally Qualified Health Center s for simple to complex medical and surgical dermatologic skin conditions. Each BFC dermatology clinic schedules an average of 7 patients per clinic and places other patients on a wait-list unless more urgent triage is needed. Patients are notified when they are scheduled via phone or text message, and they receive a reminder call or text 1 or 2 days prior to their appointment that also asks them to confirm attendance. Patients with LEP are called with an interpreter and also may receive text reminders that can be translated using Google Translate. Patients are instructed to notify the BFC if they need to cancel or reschedule their appointment. At the end of each visit, patients are given an after-visit summary that lists follow-up instructions, medications prescribed during the visit, and upcoming appointments. The BFC offers bus tickets to help patients get to their appointments. In rare cases, the BFC may pay for a car service to drive patients to and from the clinic.
Dermatology clinics at UPMC use scheduling and self-scheduling systems through which patients can make appointments at a location of their choice with any available board-certified dermatologist or physician assistant. Patients receive a reminder phone call 3 days prior to their appointment instructing them to call the office if they are unable to keep their appointment. Patients signed up for the online portal also receive a reminder message and an option to confirm or cancel their appointment. Patients with cell phone numbers in the UPMC system receive a text message approximately 2 days prior to their appointment that allows them to preregister and pay their copayment in advance. They receive another text 20 minutes prior to their appointment with an option for contactless check-in. At the conclusion of their visit, patients can schedule a follow-up appointment and receive a printed copy of their after-visit summary that provides information about follow-up instructions, prescribed medications, and upcoming visits. They may alternatively access this summary via the online patient portal. Patients are not provided transportation to UPMC clinics, but they are offered parking validation.
Among the most common dermatologic diagnoses for each group, BFC patients presented for treatment of more acute dermatologic conditions, while UPMC patients presented for more benign and preventive-care conditions. This difference may be attributable to the BFC’s referral and triage system, wherein patients with more urgent problems are given scheduling priority. This patient care model contrasts with UPMC’s scheduling process in which no known formal triage system is utilized. Interestingly, there was no difference in skin cancer incidence despite a higher percentage of preventive skin cancer screenings at UPMC.
Patients at the BFC more often required emergency insurance for surgical interventions, which is consistent with the higher percentage of uninsured individuals in this population. Patients at UPMC more frequently were recommended sunscreen and were educated about skin cancer, sun protection, and skin examination, in part due to this group’s more extensive history of skin cancer and frequent presentation for skin cancer screenings. At the same time, educational materials for skin care at both the BFC and UPMC are populated into the EMR in English, whereas materials in other languages are less readily available.
Our retrospective study had several limitations. Demographic information that relied on clinic-dependent intake questionnaires may be limited due to variable intake processes and patients opting out of self-reporting. By comparing patient populations between 2 clinics, confounding variables such as location and hours of operation may impact the patient demographics recorded at the BFC vs UPMC. Resources and staff availability may affect the management strategies and follow-up care offered by each clinic. Our study period also was unique in that COVID-19 may have affected resources, staffing, scheduling, and logistics at both clinics.
Based on the aforementioned differences between the BFC and UPMC patient characteristics, care models should be strategically designed to support the needs of diverse populations. The BFC patient care model appropriately focuses on communication skills with patients with LEP by using interpreter services. Providing more skin care education and follow-up instructions in patients’ primary languages will help them develop a better understanding of their skin conditions. Another key asset of the BFC patient care model is its provision of social services such as transportation and insurance assistance.
To improve the UPMC patient care model, providing patients with bus tickets and car services may potentially reduce appointment cancellations. Using interpreter services to call and text appointment reminders, as well as interpreter resources to facilitate patient visits and patient instructions, also can mitigate language barriers for patients with LEP. Implementing a triage system into the UPMC scheduling system may help patients with more urgent skin conditions to be seen in a timely manner.
Other investigators have analyzed costs of care and proven the value of dermatologic services at free clinics to guide allocation of supplies and resources, demonstrating an area for future investigation at the BFC.13 A cost analysis of care provided at the BFC compared to UPMC could inform us about the value of the BFC’s services.
Conclusion
The dermatology clinics at the BFC and UPMC have distinct demographics, diagnoses, and management strategies to provide an inclusive patient care model. The services provided by both clinics are necessary to ensure that people in Pittsburgh have access to dermatologic care regardless of social barriers (eg, lack of health insurance, LEP). To achieve greater accessibility and health equity, dermatologic care at the BFC and UPMC can be improved by strengthening communication with people with LEP, providing skin care education, and offering social and scheduling services.
Approximately 25% of Americans have at least one skin condition, and 20% are estimated to develop skin cancer during their lifetime.1,2 However, 40% of the US population lives in areas underserved by dermatologists. 3 The severity and mortality of skin cancers such as melanoma and mycosis fungoides have been positively associated with minoritized race, lack of health insurance, and unstable housing status.4-6 Patients who receive health care at free clinics often are of a racial or ethnic minoritized social group, are uninsured, and/or lack stable housing; this underserved group also includes recent immigrants to the United States who have limited English proficiency (LEP).7 Only 25% of free clinics offer specialty care services such as dermatology.7,8
Of the 42 free clinics and Federally Qualified Health Centers in Pittsburgh, Pennsylvania, the Birmingham Free Clinic (BFC) is one of the few that offers specialty care services including dermatology.9 Founded in 1994, the BFC serves as a safety net for Pittsburgh’s medically underserved population, offering primary and acute care, medication access, and social services. From January 2020 to May 2022, the BFC offered 27 dermatology clinics that provided approximately 100 people with comprehensive care including full-body skin examinations, dermatologic diagnoses and treatments, minor procedures, and dermatopathology services.
In this study, we compared the BFC dermatology patient care model with that of the dermatology department at the University of Pittsburgh Medical Center (UPMC), an insurance-based tertiary referral health care system in western Pennsylvania. By analyzing the demographics, dermatologic diagnoses, and management strategies of both the BFC and UPMC, we gained an understanding of how these patient care models differ and how they can be improved to care for diverse patient populations.
Methods
A retrospective chart review of dermatology patients seen in person at the BFC and UPMC during the period from January 2020 to May 2022 was performed. The UPMC group included patients seen by 3 general dermatologists (including A.J.J.) at matched time points. Data were collected from patients’ first in-person visit during the study period. Variables of interest included patient age, sex, race, ethnicity, primary language, zip code, health insurance status, distance to clinic (estimated using Google Maps to calculate the shortest driving distance from the patient’s zip code to the clinic), history of skin cancer, dermatologic diagnoses, and management strategies. These variables were not collected for patients who cancelled or noshowed their first in-person appointments. All patient charts and notes corresponding to the date and visit of interest were accessed through the electronic medical record (EMR). Patient data were de-identified and stored in a password-protected spreadsheet. Comparisons between the BFC and UPMC patient populations were performed using X2 tests of independence, Fisher exact tests, and Mann-Whitney U tests via SPSS software (IBM). Statistical significance was set at P<.05.
Results
Patient Characteristics—Our analysis included 76 initial appointments at the BFC and 322 at UPMC (Table 1). The mean age for patients at the BFC and UPMC was 39.6 years and 47.8 years, respectively (P=.001). Males accounted for 39 (51.3%) and 112 (34.8%) of BFC and UPMC patients, respectively (P=.008); 2 (0.6%) patients from UPMC were transgender. Of the BFC and UPMC patients, 44.7% (34/76) and 0.9% (3/322) were Hispanic, respectively (P<.001). With regard to race, 52.6% (40/76) of BFC patients were White, 19.7% (15/76) were Black, 6.6% (5/76) were Asian/Pacific Islander (Chinese, 1.3% [1/76]; other Asian, 5.3% [4/76]), and 21.1% (16/76) were American Indian/other/unspecified (American Indian, 1.3% [1/76]; other, 13.2% [10/76]; unspecified, 6.6% [5/76]). At UPMC, 61.2% (197/322) of patients were White, 28.0% (90/322) were Black, 5.3% (17/322) were Asian/Pacific Islander (Chinese, 1.2% [4/322]; Indian [Asian], 1.9% [6/322]; Japanese, 0.3% [1/322]; other Asian, 1.6% [5/322]; other Asian/American Indian, 0.3% [1/322]), and 5.6% (18/322) were American Indian/other/ unspecified (American Indian, 0.3% [1/322]; other, 0.3% [1/322]; unspecified, 5.0% [16/322]). Overall, the BFC patient population was more ethnically and racially diverse than that of UPMC (P<.001).
Forty-six percent (35/76) of BFC patients and 4.3% (14/322) of UPMC patients had LEP (P<.001). Primary languages among BFC patients were 53.9% (41/76) English, 40.8% (31/76) Spanish, and 5.2% (4/76) other/ unspecified (Chinese, 1.3% [1/76]; Indonesian, 2.6% [2/76]; unspecified, 1.3% [1/76]). Primary languages among UPMC patients were 95.7% (308/322) English and 4.3% (14/322) other/unspecified (Chinese, 0.6% [2/322]; Nepali, 0.6% [2/322]; Pali, 0.3% [1/322]; Russian, 0.3% [1/322]; unspecified, 2.5% [8/322]). There were notable differences in insurance status at the BFC vs UPMC (P<.001), with more UPMC patients having private insurance (52.8% [170/322] vs 11.8% [9/76]) and more BFC patients being uninsured (52.8% [51/76] vs 1.9% [6/322]). There was no significant difference in distance to clinic between the 2 groups (P=.183). More UPMC patients had a history of skin cancer (P=.003). More patients at the BFC were no-shows for their appointments (P<.001), and UPMC patients more frequently canceled their appointments (P<.001).
Dermatologic Diagnoses—The most commonly diagnosed dermatologic conditions at the BFC were dermatitis (23.7% [18/76]), neoplasm of uncertain behavior (15.8% [12/76]), alopecia (11.8% [9/76]), and acne (10.5% [8/76]) (Table 2). The most commonly diagnosed conditions at UPMC were nevi (26.4% [85/322]), dermatitis (22.7% [73/322]), seborrheic keratosis (21.7% [70/322]), and skin cancer screening (21.4% [70/322]). Neoplasm of uncertain behavior was more common in BFC vs UPMC patients (P=.040), while UPMC patients were more frequently diagnosed with nevi (P<.001), seborrheic keratosis (P<.001), and skin cancer screening (P<.001). There was no significant difference between the incidence of skin cancer diagnoses in the BFC (1.3% [1/76]) and UPMC (0.6% [2/76]) patient populations (P=.471). Among the biopsied neoplasms, there was also no significant difference in malignant (BFC, 50.0% [5/10]; UPMC, 32.0% [8/25]) and benign (BFC, 50.0% [5/10]; UPMC, 36.0% [9/25]) neoplasms diagnosed at each clinic (P=.444).
Management Strategies—Systemic antibiotics were more frequently prescribed (P<.001) and laboratory testing/ imaging were more frequently ordered (P=.005) at the BFC vs UPMC (Table 3). Patients at the BFC also more frequently required emergency insurance (P=.036). Patients at UPMC were more frequently recommended sunscreen (P=.003) and received education about skin cancer signs by review of the ABCDEs of melanoma (P<.001), sun-protective behaviors (P=.001), and skin examination frequency (P<.001). Notes in the EMR for UPMC patients more frequently specified patient followup instructions (P<.001).
Comment
As of 2020, the city of Pittsburgh had an estimated population of nearly 303,000 based on US Census data.10 Its population is predominantly White (62.7%) followed by Black/African American (22.8%) and Asian (6.5%); 5.9% identify as 2 or more races. Approximately 3.8% identify as Hispanic or Latino. More than 11% of the Pittsburgh population aged 5 years and older speaks a language other than English as their primary language, including Spanish (2.3%), other Indo-European languages (3.9%), and Asian and Pacific Island languages (3.5%).11 More than 5% of the Pittsburgh population does not have health insurance.12
The BFC is located in Pittsburgh’s South Side area, while one of UPMC’s primary dermatology clinics is located in the Oakland district; however, most patients who seek care at these clinics live outside these areas. Our study results indicated that the BFC and UPMC serve distinct groups of people within the Pittsburgh population. The BFC patient population was younger with a higher percentage of patients who were male, Hispanic, racially diverse, and with LEP compared with the UPMC patient population. In this clinical setting, the BFC health care team engages with people from diverse backgrounds and requires greater interpreter and medical support services.
The BFC largely is supported by volunteers, UPMC, grants, and philanthropy. Dermatology clinics are staffed by paid and volunteer team members. Paid team members include 1 nurse and 1 access lead who operates the front desk and registration. Volunteer team members include 1 board-certified dermatologist from UPMC (A.J.J.), or an affiliate clinic and 1 or 2 of each of the following: UPMC dermatology residents, medical or undergraduate students from the University of Pittsburgh, AmeriCorps national service members, and student or community medical interpreters. The onsite pharmacy is run by volunteer faculty, resident, and student pharmacists from the University of Pittsburgh. Dermatology clinics are half-day clinics that occur monthly. Volunteers for each clinic are recruited approximately 1 month in advance.
Dermatology patients at the BFC are referred from the BFC general medicine clinic and nearby Federally Qualified Health Center s for simple to complex medical and surgical dermatologic skin conditions. Each BFC dermatology clinic schedules an average of 7 patients per clinic and places other patients on a wait-list unless more urgent triage is needed. Patients are notified when they are scheduled via phone or text message, and they receive a reminder call or text 1 or 2 days prior to their appointment that also asks them to confirm attendance. Patients with LEP are called with an interpreter and also may receive text reminders that can be translated using Google Translate. Patients are instructed to notify the BFC if they need to cancel or reschedule their appointment. At the end of each visit, patients are given an after-visit summary that lists follow-up instructions, medications prescribed during the visit, and upcoming appointments. The BFC offers bus tickets to help patients get to their appointments. In rare cases, the BFC may pay for a car service to drive patients to and from the clinic.
Dermatology clinics at UPMC use scheduling and self-scheduling systems through which patients can make appointments at a location of their choice with any available board-certified dermatologist or physician assistant. Patients receive a reminder phone call 3 days prior to their appointment instructing them to call the office if they are unable to keep their appointment. Patients signed up for the online portal also receive a reminder message and an option to confirm or cancel their appointment. Patients with cell phone numbers in the UPMC system receive a text message approximately 2 days prior to their appointment that allows them to preregister and pay their copayment in advance. They receive another text 20 minutes prior to their appointment with an option for contactless check-in. At the conclusion of their visit, patients can schedule a follow-up appointment and receive a printed copy of their after-visit summary that provides information about follow-up instructions, prescribed medications, and upcoming visits. They may alternatively access this summary via the online patient portal. Patients are not provided transportation to UPMC clinics, but they are offered parking validation.
Among the most common dermatologic diagnoses for each group, BFC patients presented for treatment of more acute dermatologic conditions, while UPMC patients presented for more benign and preventive-care conditions. This difference may be attributable to the BFC’s referral and triage system, wherein patients with more urgent problems are given scheduling priority. This patient care model contrasts with UPMC’s scheduling process in which no known formal triage system is utilized. Interestingly, there was no difference in skin cancer incidence despite a higher percentage of preventive skin cancer screenings at UPMC.
Patients at the BFC more often required emergency insurance for surgical interventions, which is consistent with the higher percentage of uninsured individuals in this population. Patients at UPMC more frequently were recommended sunscreen and were educated about skin cancer, sun protection, and skin examination, in part due to this group’s more extensive history of skin cancer and frequent presentation for skin cancer screenings. At the same time, educational materials for skin care at both the BFC and UPMC are populated into the EMR in English, whereas materials in other languages are less readily available.
Our retrospective study had several limitations. Demographic information that relied on clinic-dependent intake questionnaires may be limited due to variable intake processes and patients opting out of self-reporting. By comparing patient populations between 2 clinics, confounding variables such as location and hours of operation may impact the patient demographics recorded at the BFC vs UPMC. Resources and staff availability may affect the management strategies and follow-up care offered by each clinic. Our study period also was unique in that COVID-19 may have affected resources, staffing, scheduling, and logistics at both clinics.
Based on the aforementioned differences between the BFC and UPMC patient characteristics, care models should be strategically designed to support the needs of diverse populations. The BFC patient care model appropriately focuses on communication skills with patients with LEP by using interpreter services. Providing more skin care education and follow-up instructions in patients’ primary languages will help them develop a better understanding of their skin conditions. Another key asset of the BFC patient care model is its provision of social services such as transportation and insurance assistance.
To improve the UPMC patient care model, providing patients with bus tickets and car services may potentially reduce appointment cancellations. Using interpreter services to call and text appointment reminders, as well as interpreter resources to facilitate patient visits and patient instructions, also can mitigate language barriers for patients with LEP. Implementing a triage system into the UPMC scheduling system may help patients with more urgent skin conditions to be seen in a timely manner.
Other investigators have analyzed costs of care and proven the value of dermatologic services at free clinics to guide allocation of supplies and resources, demonstrating an area for future investigation at the BFC.13 A cost analysis of care provided at the BFC compared to UPMC could inform us about the value of the BFC’s services.
Conclusion
The dermatology clinics at the BFC and UPMC have distinct demographics, diagnoses, and management strategies to provide an inclusive patient care model. The services provided by both clinics are necessary to ensure that people in Pittsburgh have access to dermatologic care regardless of social barriers (eg, lack of health insurance, LEP). To achieve greater accessibility and health equity, dermatologic care at the BFC and UPMC can be improved by strengthening communication with people with LEP, providing skin care education, and offering social and scheduling services.
- Lim HW, Collins SAB, Resneck JS, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:958-972.e2. doi:10.1016/j.jaad.2016.12.043
- American Academy of Dermatology. Skin cancer. Accessed October 7, 2024. https://www.aad.org/media/stats-skin-cancer
- Suneja T, Smith ED, Chen GJ, et al. Waiting times to see a dermatologist are perceived as too long by dermatologists: implications for the dermatology workforce. Arch Dermatol. 2001;137:1303-1307. doi:10.1001/archderm.137.10.1303
- Grossberg AL, Carranza D, Lamp K, et al. Dermatologic care in the homeless and underserved populations: observations from the Venice Family Clinic. Cutis. 2012;89:25-32.
- Amini A, Rusthoven CG, Waxweiler TV, et al. Association of health insurance with outcomes in adults ages 18 to 64 years with melanoma in the United States. J Am Acad Dermatol. 2016;74:309-316. doi:10.1016/j.jaad.2015.09.054
- Su C, Nguyen KA, Bai HX, et al. Racial disparity in mycosis fungoides: an analysis of 4495 cases from the US National Cancer Database. J Am Acad Dermatol. 2017;77:497-502.e2. doi:10.1016/j.jaad .2017.04.1137
- Darnell JS. Free clinics in the United States: a nationwide survey. Arch Intern Med. 2010;170:946-953. doi:10.1001/archinternmed .2010.107
- Madray V, Ginjupalli S, Hashmi O, et al. Access to dermatology services at free medical clinics: a nationwide cross-sectional survey. J Am Acad Dermatol. 2019;81:245-246. doi:10.1016/j.jaad.2018.12.011
- Pennsylvania free and income-based clinics. Accessed October 7, 2024. https://www.freeclinics.com/sta/pennsylvania
- United States Census Bureau. Decennial census. P1: race. Accessed October 7, 2024. https://data.census.gov/table/DECENNIALPL2020.P1?g=160XX00US4261000
- United States Census Bureau. American community survey. S1601: language spoken at home. Accessed October 7, 2024. https://data.census.gov/table/ACSST5Y2020S1601?g=160XX00US4261000
- United States Census Bureau. S2701: selected characteristics of health insurance coverage in the United States. Accessed October 7, 2024. https://data.census.gov/table/ACSST5Y2020.S2701?g=160XX00US4261000
- Lin CP, Loy S, Boothe WD, et al. Value of Dermatology Nights at a student-run free clinic. Proc (Bayl Univ Med Cent). 2020;34:260-261. doi:10.1080/08998280.2020.1834771
- Lim HW, Collins SAB, Resneck JS, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:958-972.e2. doi:10.1016/j.jaad.2016.12.043
- American Academy of Dermatology. Skin cancer. Accessed October 7, 2024. https://www.aad.org/media/stats-skin-cancer
- Suneja T, Smith ED, Chen GJ, et al. Waiting times to see a dermatologist are perceived as too long by dermatologists: implications for the dermatology workforce. Arch Dermatol. 2001;137:1303-1307. doi:10.1001/archderm.137.10.1303
- Grossberg AL, Carranza D, Lamp K, et al. Dermatologic care in the homeless and underserved populations: observations from the Venice Family Clinic. Cutis. 2012;89:25-32.
- Amini A, Rusthoven CG, Waxweiler TV, et al. Association of health insurance with outcomes in adults ages 18 to 64 years with melanoma in the United States. J Am Acad Dermatol. 2016;74:309-316. doi:10.1016/j.jaad.2015.09.054
- Su C, Nguyen KA, Bai HX, et al. Racial disparity in mycosis fungoides: an analysis of 4495 cases from the US National Cancer Database. J Am Acad Dermatol. 2017;77:497-502.e2. doi:10.1016/j.jaad .2017.04.1137
- Darnell JS. Free clinics in the United States: a nationwide survey. Arch Intern Med. 2010;170:946-953. doi:10.1001/archinternmed .2010.107
- Madray V, Ginjupalli S, Hashmi O, et al. Access to dermatology services at free medical clinics: a nationwide cross-sectional survey. J Am Acad Dermatol. 2019;81:245-246. doi:10.1016/j.jaad.2018.12.011
- Pennsylvania free and income-based clinics. Accessed October 7, 2024. https://www.freeclinics.com/sta/pennsylvania
- United States Census Bureau. Decennial census. P1: race. Accessed October 7, 2024. https://data.census.gov/table/DECENNIALPL2020.P1?g=160XX00US4261000
- United States Census Bureau. American community survey. S1601: language spoken at home. Accessed October 7, 2024. https://data.census.gov/table/ACSST5Y2020S1601?g=160XX00US4261000
- United States Census Bureau. S2701: selected characteristics of health insurance coverage in the United States. Accessed October 7, 2024. https://data.census.gov/table/ACSST5Y2020.S2701?g=160XX00US4261000
- Lin CP, Loy S, Boothe WD, et al. Value of Dermatology Nights at a student-run free clinic. Proc (Bayl Univ Med Cent). 2020;34:260-261. doi:10.1080/08998280.2020.1834771
Comparing Patient Care Models at a Local Free Clinic vs an Insurance- Based University Medical Center
Comparing Patient Care Models at a Local Free Clinic vs an Insurance- Based University Medical Center
PRACTICE POINTS
- Both free clinics and insurance-based health care systems serve dermatology patients with diverse characteristics, necessitating inclusive health care models.
- Dermatologic care can be improved at both free and insurance-based clinics by strengthening communication with individuals with limited English proficiency, providing skin care education, and offering social and scheduling services such as transportation, insurance assistance, and triage.
Multidisciplinary Amputation Prevention at the DeBakey VA Hospital: Our First Decade
Individuals with diabetes are at risk for developing foot ulcers or full-thickness defects in the epithelium of the foot. These defects can lead to bacterial invasion and foot infection, potentially resulting in leg amputation (Figure 1). Effective treatment to prevent leg amputation, known as limb salvage, requires management across multiple medical specialties including podiatry, vascular surgery, and infectious diseases. The multidisciplinary team approach to limb salvage was introduced in Boston in 1928 and has been the prevailing approach to this cross-specialty medical problem for at least a decade.1,2
The Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) has established an inpatient limb salvage program—a group of dedicated clinicians working collaboratively to provide evidence-guided management of patients hospitalized with foot ulcers, foot gangrene or any superimposed infection with the goal of avoiding leg amputations. We have seen a significant and durable reduction in the incidence of leg amputations among veterans at MEDVAMC.
This article describes the evolution and outcomes of the MEDVAMC limb salvage program over more than a decade. It includes changes to team structure and workflow, as well as past and present successes and challenges. The eAppendix provides a narrative summary with examples of how our clinical practice and research efforts have informed one another and how these findings are applied to clinical management. This process is part of the larger efforts of the Veterans Health Administration (VHA) to create a learning health system in which “internal data and experience are systematically integrated with external evidence, and that knowledge is put into practice.”3
Methods
Data from the VHA Support Service Center were used to obtain monthly major (leg) and minor (toe and partial foot) amputation records at MEDVAMC from October 2000 through May 2023. Yearly totals for the number of persons with diabetes and foot ulcers at MEDVAMC were also obtained from the support service center. Annual patient population sizes and number of persons with foot ulcers were converted to monthly estimates using cubic spline interpolation. Rates were calculated as 12-month rolling averages. Trend lines were created with locally weighted running line smoothing that used a span α of 0.1.
We characterized the patient population using data from cohorts of veterans treated for foot ulcers and foot infections at MEDVAMC. To compare the contemporary veteran population with nonveteran inpatients treated for foot ulcers and foot infections at other hospitals, we created a 2:1 nonveteran to veteran cohort matched by sex and zip code, using publicly available hospital admission data from the Texas Department of Health and State Health Services. Veterans used for this cohort comparison are consistent with the 100 consecutive patients who underwent angiography for limb salvage in 2022.
This research was approved by the Baylor College of Medicine Institutional Review Board (protocol H-34858) and the MEDVAMC Research Committee (IRBNet protocol 15A12. HB). All analyses used deidentified data in the R programming language version 4.2.2 using RStudio version 2022.06.0 Build 421.
Program Description
MEDVAMC is a 350-bed teaching hospital located in central Houston. Its hospital system includes 11 outpatient clinics, ranging from 28 to 126 miles (eAppendix, Supplemental Figure A) from MEDVAMC. MEDVAMC provides vascular, orthopedic, and podiatric surgery services, as well as many other highly specialized services such as liver and heart transplants. The hospital’s risk-adjusted rates of operative morbidity and mortality (observed-to-expected ratios) are significantly lower than expected.
Despite this, the incidence rate of leg amputations at MEDVAMC in early 2011 was nearly 3-times higher than the VHA average. The inpatient management of veterans with infected foot ulcers was fragmented, with the general, orthopedic, and vascular surgery teams separately providing siloed care. Delays in treatment were common. There was much service- and practitioner-level practice heterogeneity. No diagnostic or treatment protocols were used, and standard treatment components were sporadically provided.
Patient Population
Compared to the matched non-VHA patient cohort (Supplemental Table 1), veterans treated at MEDVAMC for limb salvage are older. Nearly half (46%) identify as Black, which is associated with a 2-fold higher riskadjusted rate of leg amputations.4 MEDVAMC patients also have significantly higher rates of diabetes, chronic kidney disease, and systolic heart failure. About 22% travel > 40 miles for treatment at MEDVAMC, double that of the matched cohort (10.7%). Additionally, 35% currently smoke and 37% have moderate to severe peripheral artery disease (PAD).5
Program Design
In late 2011, the MEDVAMC vascular surgery team led limb salvage efforts by implementing a single team model, which involved assuming the primary role of managing foot ulcers for all veterans, both infected and uninfected (eAppendix, Supplemental Figure B). Consultations were directed to a dedicated limb salvage pager. The vascular team provided interdisciplinary limb salvage management across the spectrum of disease, including the surgical treatment of infection, assessment for PAD, open surgical operations and endovascular interventions to treat PAD, and foot reconstruction (debridement, minor or partial foot amputations, and skin grafting). This care was complemented by frequent consultation with the infectious disease, vascular medicine, podiatry, and geriatric wound care teams. This approach streamlined the delivery of consistent multidisciplinary care.
This collaborative effort aimed to develop ideal multidisciplinary care plans through research spanning the spectrum of the diabetic foot infection disease process (eAppendix, Supplemental Table 1). Some of the most impactful practices were: (1) a proclivity towards surgical treatment of foot infections, especially osteomyelitis5; (2) improved identification of PAD6,7; (3) early surgical closure of foot wounds following revascularization8,9; and (4) palliative wound care as an alternative to leg amputation in veterans who are not candidates for revascularization and limb salvage.10 Initally, the vascular surgery team held monthly multidisciplinary limb salvage meetings to coordinate patient management, identify ways to streamline care and avoid waste, discuss research findings, and review the 12-month rolling average of the MEDVAMC leg amputation incidence rate.
During the study period, the MEDVAMC vascular surgery team consisted of 2 to 5 board certified vascular or general surgeons, 2 or 3 nurse practitioners, and 3 vascular ultrasound technologists. Associated specialists included 2 podiatrists, 3 geriatricians with wound care certification, as well as additional infectious diseases, vascular medicine, orthopedics, and general surgery specialists.
Program Assessment
We noted a significant and sustained decrease in the MEDVAMC leg amputation rate after implementing multidisciplinary meetings and a single- team model from early 2012 through 2017 (Figure 2). The amputation incidence rate decreased steadily over the period from a maximum of 160 per 100,000 per year in February 2012 to a nadir of 66 per 100,000 per year in April 2017, an overall 60% decrease. Increases were noted in early 2018 after ceasing the single- team model, and in the summer of 2022, following periods of bed shortages after the onset of the COVID-19 pandemic. Tracking this metric allowed clinicians to make course corrections.
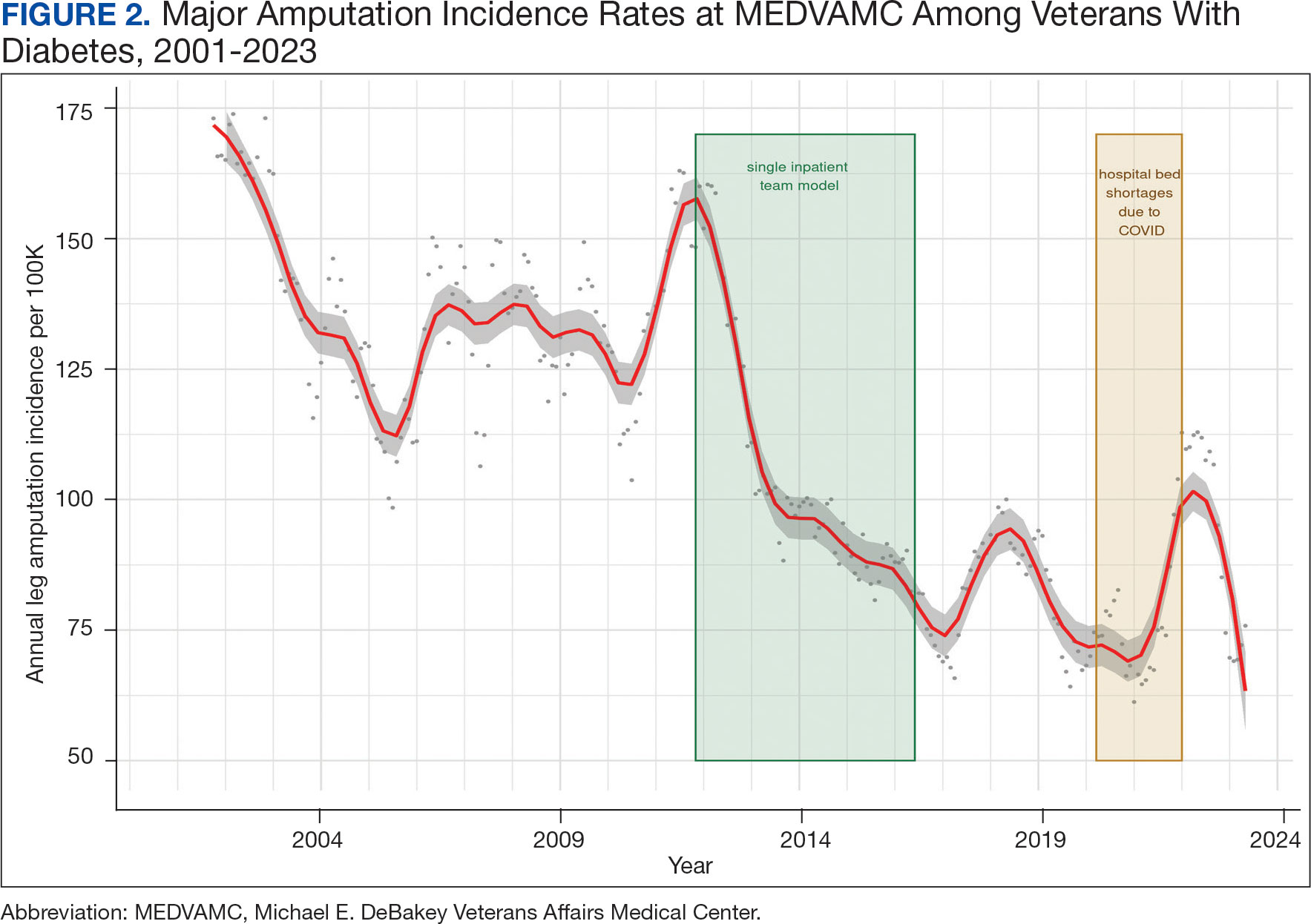
The decreased leg amputation rate at MEDVAMC does not seem to be mirroring national or regional trends. During this 10-year period, the VHA annualized amputation rate decreased minimally, from 58 to 54 per 100,000 (eAppendix Supplemental Figure C). Leg amputation incidence at non-VHA hospitals in Texas slightly increased over the same period.11
Value was also reflected in other metrics. MEDVAMC improved safety through a bundled strategy that reduced the risk-adjusted rate of surgical wound infections by 95%.12 MEDVAMC prioritized limb salvage when selecting patients for angiography and nearly eliminated using stent-grafts, cryopreserved allogeneic saphenous vein grafts, and expensive surgical and endovascular implants, which were identified as more expensive and less effective than other options (Figure 3).13-15 The MEDVAMC team achieved a > 90% patient trust rating on the Veterans Signals survey in fiscal years 2021 and 2022.
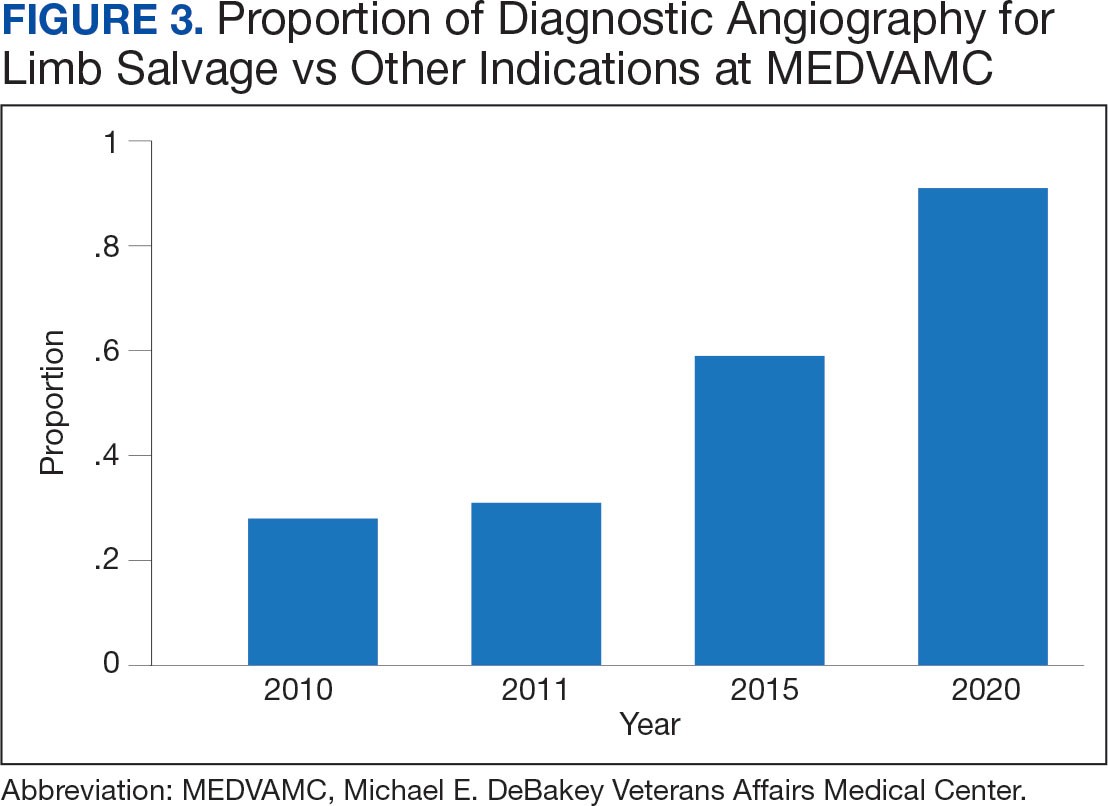
Challenges
A significant increase in the patient-physician ratio occurred 5 years into the program. In 2016, 2 vascular surgeons left MEDVAMC and a planned renovation of 1 of the 2 vascular surgery-assigned hybrid working facilities began even as the number of MEDVAMC patients with diabetes grew 120% (from 89,400 to 107,746 between 2010 and 2016), and the incidence rate of foot ulcers grew 300% (from 392 in 2010 to 1183 in 2016 per 100,000). The net result was a higher clinical workload among the remaining vascular surgeons with less operating room availability.
To stabilize surgeon retention, MEDVAMC reverted from the single team model back to inpatient care being distributed among general surgery, orthopedic surgery, and vascular surgery. After noting an increase in the leg amputation incidence rate, we adjusted the focus from multidisciplinary to interdisciplinary care (ie, majority of limb salvage clinical care can be provided by practitioners of any involved specialties). We worked to establish a local, written, interdisciplinary consensus on evaluating and managing veterans with nonhealing foot ulcers to mitigate the loss of a consolidated inpatient approach. Despite frequent staff turnover, ≥ 1 physician or surgeon from the core specialties of vascular surgery, podiatry, and infectious diseases remained throughout the study period.
The COVID-19 pandemic caused a shortage of hospital beds. This was followed by more bed shortages due to decreased nursing staff. Our health care system also had a period of restricted outpatient encounters early in the pandemic. During this time, we noted a delayed presentation of veterans with advanced infections and another increase in leg amputation incidence rate.
Like many health systems, MEDVAMC pivoted to telephone- and video-based outpatient encounters. Our team also used publicly available Texas hospitalization data to identify zip codes with particularly high leg amputation incidence rates, and > 3500 educational mailings to veterans categorized as moderate and high risk for leg amputation in these zip codes. These mailings provided information on recognizing foot ulcers and infections, emphasized timely evaluation, and named the MEDVAMC vascular surgery team as a point-of-contact. More recently, we have seen a further decrease in the MEDVAMC incidences of leg amputation to its lowest rate in > 20 years.
Discussion
A learning organization that directs its research based on clinical observations and informs its clinical care with research findings can produce palpable improvements in outcomes. Understanding the disease process and trying to better understand management across the entire range of this disease process has allowed our team to make consistent and systematic changes in care (Table). Consolidating inpatient care in a single team model seems to have been effective in reducing amputation rates among veterans with diabetes. The role the MEDVAMC vascular surgery team served for limb salvage patients may have been particularly beneficial because of the large impact untreated or unidentified PAD can have and because of the high prevalence of PAD among the limb salvage population seen at MEDVAMC. To be sustainable, though, a single-team model needs resources. A multiteam model can also be effective if the degree of multidisciplinary involvement for any given veteran is appropriate to the individual's clinical needs, teams are engaged and willing to contribute in a defined role within their specialty, and lines of communication remain open.
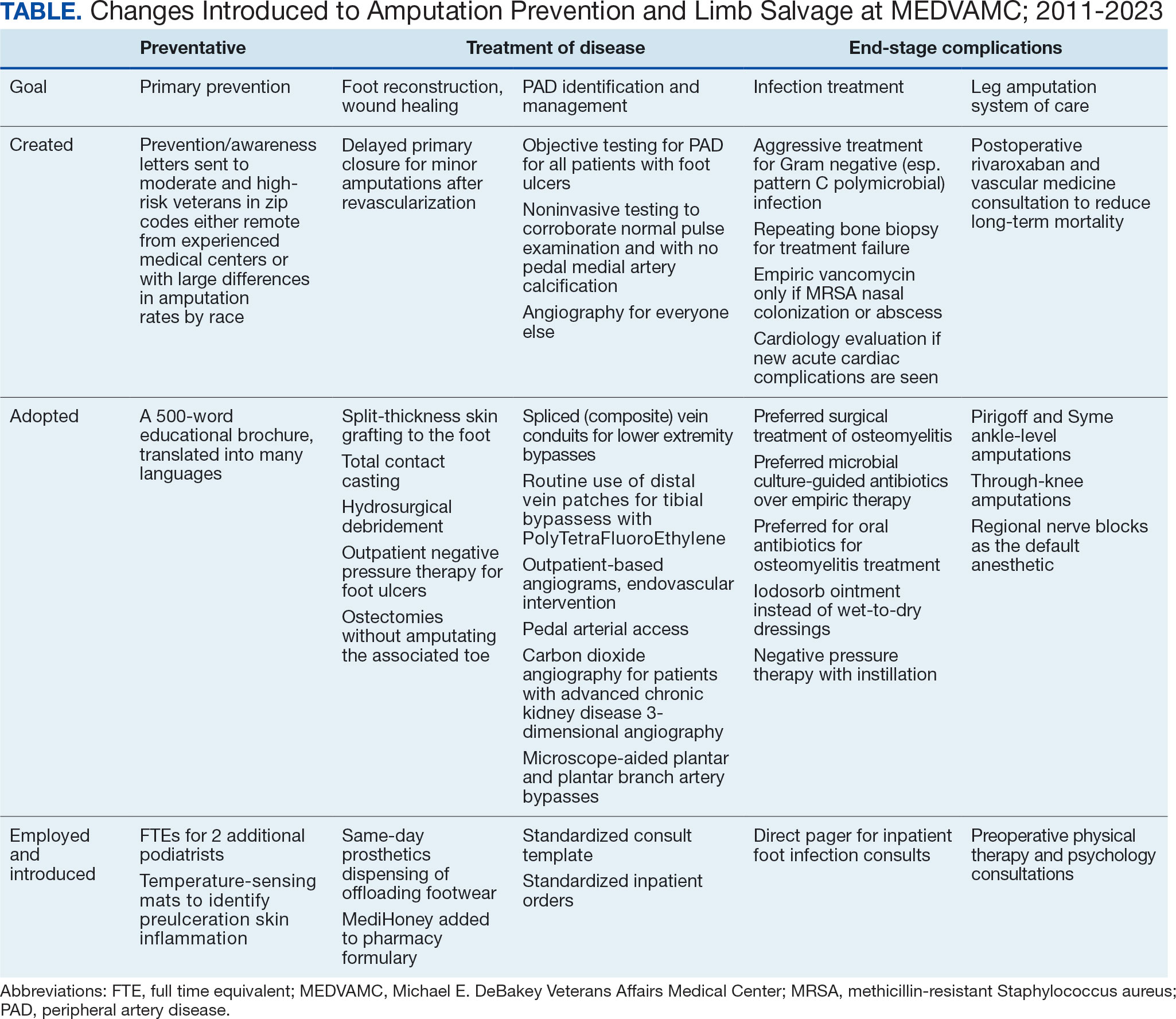
The primary challenge at MEDVAMC has been, and will continue to be, the retention of physicians and surgeons. MEDVAMC has excellent leadership and a collegial working environment, but better access to operating rooms for elective and time-sensitive operations, additional clinical staff support, and higher salary at non-VA positions have been the basis for many of physicians— especially surgeons—leaving MEDVAMC. Despite high staff turnover and a constant flow of resident and fellow trainees, MEDVAMC has been able to keep the clinical approach relatively consistent due to the use of written protocols and continuity of care as ≥ 1 physician or surgeon from each of the 4 main teams remained engaged with limb salvage throughout the entire period.
Going forward, we will work to ensure that all requirements of the 2022 Prevention of Amputation in Veterans Everywhere directive are incorporated into care.8 We plan to standardize MEDVAMC management algorithms further, both to streamline care and reduce the opportunity for disparities in treatment. More prophylactic podiatric procedures, surgical forms of offloading, and a shared multidisciplinary clinic space may also further help patients.
Conclusions
The introduction of multidisciplinary limb salvage at MEDVAMC has led to significant and sustained reductions in leg amputation incidence. These reductions do not seem dependent upon a specific team structure for inpatient care. To improve patient outcomes, efforts should focus on making improvements across the entire disease spectrum. For limb salvage, this includes primary prevention of foot ulcers, the treatment of foot infections, identification and management of PAD, surgical reconstruction/optimal wound healing, and care for patients who undergo leg amputation.
- Sanders LJ, Robbins JM, Edmonds ME. History of the team approach to amputation prevention: pioneers and milestones. J Am Podiatr Med Assoc. 2010;100(5):317- 334. doi:10.7547/1000317
- Sumpio BE, Armstrong DG, Lavery LA, Andros G. The role of interdisciplinary team approach in the management of the diabetic foot: a joint statement from the society for vascular surgery and the American podiatric medical association. J Am Podiatr Med Assoc. 2010;100(4):309-311. doi:10.7547/1000309
- About learning health systems. Agency for Healthcare Research and Quality. Published March 2019. Updated May 2019. Accessed October 9, 2024. https://www.ahrq.gov/learning-health-systems/about.html
- Barshes NR, Minc SD. Healthcare disparities in vascular surgery: a critical review. J Vasc Surg. 2021;74(2S):6S-14S.
- Barshes NR, Mindru C, Ashong C, Rodriguez-Barradas M, Trautner BW. Treatment failure and leg amputation among patients with foot osteomyelitis. Int J Low Extrem Wounds. 2016;15(4):303-312. doi:10.1177/1534734616661058
- Barshes NR, Flores E, Belkin M, Kougias P, Armstrong DG, Mills JL Sr. The accuracy and cost-effectiveness of strategies used to identify peripheral artery disease among patients with diabetic foot ulcers. J Vasc Surg. 2016;64(6):1682-1690.e3. doi:10.1016/j.jvs.2016.04.056 e1. doi:10.1016/j.jvs.2021.03.055
- Choi JC, Miranda J, Greenleaf E, et al. Lower-extremity pressure, staging, and grading thresholds to identify chronic limb-threatening ischemia. Vasc Med. 2023;28(1):45-53. doi:10.1177/1358863X221147945
- Barshes NR, Chambers JD, Cohen J, Belkin M; Model To Optimize Healthcare Value in Ischemic Extremities 1 (MOVIE) Study Collaborators. Cost-effectiveness in the contemporary management of critical limb ischemia with tissue loss. J Vasc Surg. 2012;56(4):1015-24.e1. doi:10.1016/j.jvs.2012.02.069
- Barshes NR, Bechara CF, Pisimisis G, Kougias P. Preliminary experiences with early primary closure of foot wounds after lower extremity revascularization. Ann Vasc Surg. 2014;28(1):48-52. doi:10.1016/j.avsg.2013.06.012
- Barshes NR, Gold B, Garcia A, Bechara CF, Pisimisis G, Kougias P. Minor amputation and palliative wound care as a strategy to avoid major amputation in patients with foot infections and severe peripheral arterial disease. Int J Low Extrem Wounds. 2014;13(3):211-219. doi:10.1177/1534734614543663
- Garcia M, Hernandez B, Ellington TG, et al. A lack of decline in major nontraumatic amputations in Texas: contemporary trends, risk factor associations, and impact of revascularization. Diabetes Care. 2019;42(6):1061-1066. doi:10.2337/dc19-0078
- Zamani N, Sharath SE, Vo E, Awad SS, Kougias P, Barshes NR. A multi-component strategy to decrease wound complications after open infra-inguinal re-vascularization. Surg Infect (Larchmt). 2018;19(1):87-94. doi:10.1089/sur.2017.193
- Barshes NR, Ozaki CK, Kougias P, Belkin M. A costeffectiveness analysis of infrainguinal bypass in the absence of great saphenous vein conduit. J Vasc Surg. 2013;57(6):1466-1470. doi:10.1016/j.jvs.2012.11.115
- Zamani N, Sharath S, Browder R, et al. PC158 longterm outcomes after endovascular stent placement for symptomatic, long-segment superficial femoral artery lesions. J Vasc Surg. 2017;65(6):182S-183S. doi:10.1016/j.jvs.2017.03.344
- Zamani N, Sharath SE, Browder RC, et al. Outcomes after endovascular stent placement for long-segment superficial femoral artery lesions. Ann Vasc Surg. 2021;71:298-307. doi:10.1016/j.avsg.2020.08.124
Individuals with diabetes are at risk for developing foot ulcers or full-thickness defects in the epithelium of the foot. These defects can lead to bacterial invasion and foot infection, potentially resulting in leg amputation (Figure 1). Effective treatment to prevent leg amputation, known as limb salvage, requires management across multiple medical specialties including podiatry, vascular surgery, and infectious diseases. The multidisciplinary team approach to limb salvage was introduced in Boston in 1928 and has been the prevailing approach to this cross-specialty medical problem for at least a decade.1,2

The Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) has established an inpatient limb salvage program—a group of dedicated clinicians working collaboratively to provide evidence-guided management of patients hospitalized with foot ulcers, foot gangrene or any superimposed infection with the goal of avoiding leg amputations. We have seen a significant and durable reduction in the incidence of leg amputations among veterans at MEDVAMC.
This article describes the evolution and outcomes of the MEDVAMC limb salvage program over more than a decade. It includes changes to team structure and workflow, as well as past and present successes and challenges. The eAppendix provides a narrative summary with examples of how our clinical practice and research efforts have informed one another and how these findings are applied to clinical management. This process is part of the larger efforts of the Veterans Health Administration (VHA) to create a learning health system in which “internal data and experience are systematically integrated with external evidence, and that knowledge is put into practice.”3
Methods
Data from the VHA Support Service Center were used to obtain monthly major (leg) and minor (toe and partial foot) amputation records at MEDVAMC from October 2000 through May 2023. Yearly totals for the number of persons with diabetes and foot ulcers at MEDVAMC were also obtained from the support service center. Annual patient population sizes and number of persons with foot ulcers were converted to monthly estimates using cubic spline interpolation. Rates were calculated as 12-month rolling averages. Trend lines were created with locally weighted running line smoothing that used a span α of 0.1.
We characterized the patient population using data from cohorts of veterans treated for foot ulcers and foot infections at MEDVAMC. To compare the contemporary veteran population with nonveteran inpatients treated for foot ulcers and foot infections at other hospitals, we created a 2:1 nonveteran to veteran cohort matched by sex and zip code, using publicly available hospital admission data from the Texas Department of Health and State Health Services. Veterans used for this cohort comparison are consistent with the 100 consecutive patients who underwent angiography for limb salvage in 2022.
This research was approved by the Baylor College of Medicine Institutional Review Board (protocol H-34858) and the MEDVAMC Research Committee (IRBNet protocol 15A12. HB). All analyses used deidentified data in the R programming language version 4.2.2 using RStudio version 2022.06.0 Build 421.
Program Description
MEDVAMC is a 350-bed teaching hospital located in central Houston. Its hospital system includes 11 outpatient clinics, ranging from 28 to 126 miles (eAppendix, Supplemental Figure A) from MEDVAMC. MEDVAMC provides vascular, orthopedic, and podiatric surgery services, as well as many other highly specialized services such as liver and heart transplants. The hospital’s risk-adjusted rates of operative morbidity and mortality (observed-to-expected ratios) are significantly lower than expected.
Despite this, the incidence rate of leg amputations at MEDVAMC in early 2011 was nearly 3-times higher than the VHA average. The inpatient management of veterans with infected foot ulcers was fragmented, with the general, orthopedic, and vascular surgery teams separately providing siloed care. Delays in treatment were common. There was much service- and practitioner-level practice heterogeneity. No diagnostic or treatment protocols were used, and standard treatment components were sporadically provided.
Patient Population
Compared to the matched non-VHA patient cohort (Supplemental Table 1), veterans treated at MEDVAMC for limb salvage are older. Nearly half (46%) identify as Black, which is associated with a 2-fold higher riskadjusted rate of leg amputations.4 MEDVAMC patients also have significantly higher rates of diabetes, chronic kidney disease, and systolic heart failure. About 22% travel > 40 miles for treatment at MEDVAMC, double that of the matched cohort (10.7%). Additionally, 35% currently smoke and 37% have moderate to severe peripheral artery disease (PAD).5
Program Design
In late 2011, the MEDVAMC vascular surgery team led limb salvage efforts by implementing a single team model, which involved assuming the primary role of managing foot ulcers for all veterans, both infected and uninfected (eAppendix, Supplemental Figure B). Consultations were directed to a dedicated limb salvage pager. The vascular team provided interdisciplinary limb salvage management across the spectrum of disease, including the surgical treatment of infection, assessment for PAD, open surgical operations and endovascular interventions to treat PAD, and foot reconstruction (debridement, minor or partial foot amputations, and skin grafting). This care was complemented by frequent consultation with the infectious disease, vascular medicine, podiatry, and geriatric wound care teams. This approach streamlined the delivery of consistent multidisciplinary care.
This collaborative effort aimed to develop ideal multidisciplinary care plans through research spanning the spectrum of the diabetic foot infection disease process (eAppendix, Supplemental Table 1). Some of the most impactful practices were: (1) a proclivity towards surgical treatment of foot infections, especially osteomyelitis5; (2) improved identification of PAD6,7; (3) early surgical closure of foot wounds following revascularization8,9; and (4) palliative wound care as an alternative to leg amputation in veterans who are not candidates for revascularization and limb salvage.10 Initally, the vascular surgery team held monthly multidisciplinary limb salvage meetings to coordinate patient management, identify ways to streamline care and avoid waste, discuss research findings, and review the 12-month rolling average of the MEDVAMC leg amputation incidence rate.
During the study period, the MEDVAMC vascular surgery team consisted of 2 to 5 board certified vascular or general surgeons, 2 or 3 nurse practitioners, and 3 vascular ultrasound technologists. Associated specialists included 2 podiatrists, 3 geriatricians with wound care certification, as well as additional infectious diseases, vascular medicine, orthopedics, and general surgery specialists.
Program Assessment
We noted a significant and sustained decrease in the MEDVAMC leg amputation rate after implementing multidisciplinary meetings and a single- team model from early 2012 through 2017 (Figure 2). The amputation incidence rate decreased steadily over the period from a maximum of 160 per 100,000 per year in February 2012 to a nadir of 66 per 100,000 per year in April 2017, an overall 60% decrease. Increases were noted in early 2018 after ceasing the single- team model, and in the summer of 2022, following periods of bed shortages after the onset of the COVID-19 pandemic. Tracking this metric allowed clinicians to make course corrections.

The decreased leg amputation rate at MEDVAMC does not seem to be mirroring national or regional trends. During this 10-year period, the VHA annualized amputation rate decreased minimally, from 58 to 54 per 100,000 (eAppendix Supplemental Figure C). Leg amputation incidence at non-VHA hospitals in Texas slightly increased over the same period.11
Value was also reflected in other metrics. MEDVAMC improved safety through a bundled strategy that reduced the risk-adjusted rate of surgical wound infections by 95%.12 MEDVAMC prioritized limb salvage when selecting patients for angiography and nearly eliminated using stent-grafts, cryopreserved allogeneic saphenous vein grafts, and expensive surgical and endovascular implants, which were identified as more expensive and less effective than other options (Figure 3).13-15 The MEDVAMC team achieved a > 90% patient trust rating on the Veterans Signals survey in fiscal years 2021 and 2022.

Challenges
A significant increase in the patient-physician ratio occurred 5 years into the program. In 2016, 2 vascular surgeons left MEDVAMC and a planned renovation of 1 of the 2 vascular surgery-assigned hybrid working facilities began even as the number of MEDVAMC patients with diabetes grew 120% (from 89,400 to 107,746 between 2010 and 2016), and the incidence rate of foot ulcers grew 300% (from 392 in 2010 to 1183 in 2016 per 100,000). The net result was a higher clinical workload among the remaining vascular surgeons with less operating room availability.
To stabilize surgeon retention, MEDVAMC reverted from the single team model back to inpatient care being distributed among general surgery, orthopedic surgery, and vascular surgery. After noting an increase in the leg amputation incidence rate, we adjusted the focus from multidisciplinary to interdisciplinary care (ie, majority of limb salvage clinical care can be provided by practitioners of any involved specialties). We worked to establish a local, written, interdisciplinary consensus on evaluating and managing veterans with nonhealing foot ulcers to mitigate the loss of a consolidated inpatient approach. Despite frequent staff turnover, ≥ 1 physician or surgeon from the core specialties of vascular surgery, podiatry, and infectious diseases remained throughout the study period.
The COVID-19 pandemic caused a shortage of hospital beds. This was followed by more bed shortages due to decreased nursing staff. Our health care system also had a period of restricted outpatient encounters early in the pandemic. During this time, we noted a delayed presentation of veterans with advanced infections and another increase in leg amputation incidence rate.
Like many health systems, MEDVAMC pivoted to telephone- and video-based outpatient encounters. Our team also used publicly available Texas hospitalization data to identify zip codes with particularly high leg amputation incidence rates, and > 3500 educational mailings to veterans categorized as moderate and high risk for leg amputation in these zip codes. These mailings provided information on recognizing foot ulcers and infections, emphasized timely evaluation, and named the MEDVAMC vascular surgery team as a point-of-contact. More recently, we have seen a further decrease in the MEDVAMC incidences of leg amputation to its lowest rate in > 20 years.
Discussion
A learning organization that directs its research based on clinical observations and informs its clinical care with research findings can produce palpable improvements in outcomes. Understanding the disease process and trying to better understand management across the entire range of this disease process has allowed our team to make consistent and systematic changes in care (Table). Consolidating inpatient care in a single team model seems to have been effective in reducing amputation rates among veterans with diabetes. The role the MEDVAMC vascular surgery team served for limb salvage patients may have been particularly beneficial because of the large impact untreated or unidentified PAD can have and because of the high prevalence of PAD among the limb salvage population seen at MEDVAMC. To be sustainable, though, a single-team model needs resources. A multiteam model can also be effective if the degree of multidisciplinary involvement for any given veteran is appropriate to the individual's clinical needs, teams are engaged and willing to contribute in a defined role within their specialty, and lines of communication remain open.

The primary challenge at MEDVAMC has been, and will continue to be, the retention of physicians and surgeons. MEDVAMC has excellent leadership and a collegial working environment, but better access to operating rooms for elective and time-sensitive operations, additional clinical staff support, and higher salary at non-VA positions have been the basis for many of physicians— especially surgeons—leaving MEDVAMC. Despite high staff turnover and a constant flow of resident and fellow trainees, MEDVAMC has been able to keep the clinical approach relatively consistent due to the use of written protocols and continuity of care as ≥ 1 physician or surgeon from each of the 4 main teams remained engaged with limb salvage throughout the entire period.
Going forward, we will work to ensure that all requirements of the 2022 Prevention of Amputation in Veterans Everywhere directive are incorporated into care.8 We plan to standardize MEDVAMC management algorithms further, both to streamline care and reduce the opportunity for disparities in treatment. More prophylactic podiatric procedures, surgical forms of offloading, and a shared multidisciplinary clinic space may also further help patients.
Conclusions
The introduction of multidisciplinary limb salvage at MEDVAMC has led to significant and sustained reductions in leg amputation incidence. These reductions do not seem dependent upon a specific team structure for inpatient care. To improve patient outcomes, efforts should focus on making improvements across the entire disease spectrum. For limb salvage, this includes primary prevention of foot ulcers, the treatment of foot infections, identification and management of PAD, surgical reconstruction/optimal wound healing, and care for patients who undergo leg amputation.
Individuals with diabetes are at risk for developing foot ulcers or full-thickness defects in the epithelium of the foot. These defects can lead to bacterial invasion and foot infection, potentially resulting in leg amputation (Figure 1). Effective treatment to prevent leg amputation, known as limb salvage, requires management across multiple medical specialties including podiatry, vascular surgery, and infectious diseases. The multidisciplinary team approach to limb salvage was introduced in Boston in 1928 and has been the prevailing approach to this cross-specialty medical problem for at least a decade.1,2

The Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) has established an inpatient limb salvage program—a group of dedicated clinicians working collaboratively to provide evidence-guided management of patients hospitalized with foot ulcers, foot gangrene or any superimposed infection with the goal of avoiding leg amputations. We have seen a significant and durable reduction in the incidence of leg amputations among veterans at MEDVAMC.
This article describes the evolution and outcomes of the MEDVAMC limb salvage program over more than a decade. It includes changes to team structure and workflow, as well as past and present successes and challenges. The eAppendix provides a narrative summary with examples of how our clinical practice and research efforts have informed one another and how these findings are applied to clinical management. This process is part of the larger efforts of the Veterans Health Administration (VHA) to create a learning health system in which “internal data and experience are systematically integrated with external evidence, and that knowledge is put into practice.”3
Methods
Data from the VHA Support Service Center were used to obtain monthly major (leg) and minor (toe and partial foot) amputation records at MEDVAMC from October 2000 through May 2023. Yearly totals for the number of persons with diabetes and foot ulcers at MEDVAMC were also obtained from the support service center. Annual patient population sizes and number of persons with foot ulcers were converted to monthly estimates using cubic spline interpolation. Rates were calculated as 12-month rolling averages. Trend lines were created with locally weighted running line smoothing that used a span α of 0.1.
We characterized the patient population using data from cohorts of veterans treated for foot ulcers and foot infections at MEDVAMC. To compare the contemporary veteran population with nonveteran inpatients treated for foot ulcers and foot infections at other hospitals, we created a 2:1 nonveteran to veteran cohort matched by sex and zip code, using publicly available hospital admission data from the Texas Department of Health and State Health Services. Veterans used for this cohort comparison are consistent with the 100 consecutive patients who underwent angiography for limb salvage in 2022.
This research was approved by the Baylor College of Medicine Institutional Review Board (protocol H-34858) and the MEDVAMC Research Committee (IRBNet protocol 15A12. HB). All analyses used deidentified data in the R programming language version 4.2.2 using RStudio version 2022.06.0 Build 421.
Program Description
MEDVAMC is a 350-bed teaching hospital located in central Houston. Its hospital system includes 11 outpatient clinics, ranging from 28 to 126 miles (eAppendix, Supplemental Figure A) from MEDVAMC. MEDVAMC provides vascular, orthopedic, and podiatric surgery services, as well as many other highly specialized services such as liver and heart transplants. The hospital’s risk-adjusted rates of operative morbidity and mortality (observed-to-expected ratios) are significantly lower than expected.
Despite this, the incidence rate of leg amputations at MEDVAMC in early 2011 was nearly 3-times higher than the VHA average. The inpatient management of veterans with infected foot ulcers was fragmented, with the general, orthopedic, and vascular surgery teams separately providing siloed care. Delays in treatment were common. There was much service- and practitioner-level practice heterogeneity. No diagnostic or treatment protocols were used, and standard treatment components were sporadically provided.
Patient Population
Compared to the matched non-VHA patient cohort (Supplemental Table 1), veterans treated at MEDVAMC for limb salvage are older. Nearly half (46%) identify as Black, which is associated with a 2-fold higher riskadjusted rate of leg amputations.4 MEDVAMC patients also have significantly higher rates of diabetes, chronic kidney disease, and systolic heart failure. About 22% travel > 40 miles for treatment at MEDVAMC, double that of the matched cohort (10.7%). Additionally, 35% currently smoke and 37% have moderate to severe peripheral artery disease (PAD).5
Program Design
In late 2011, the MEDVAMC vascular surgery team led limb salvage efforts by implementing a single team model, which involved assuming the primary role of managing foot ulcers for all veterans, both infected and uninfected (eAppendix, Supplemental Figure B). Consultations were directed to a dedicated limb salvage pager. The vascular team provided interdisciplinary limb salvage management across the spectrum of disease, including the surgical treatment of infection, assessment for PAD, open surgical operations and endovascular interventions to treat PAD, and foot reconstruction (debridement, minor or partial foot amputations, and skin grafting). This care was complemented by frequent consultation with the infectious disease, vascular medicine, podiatry, and geriatric wound care teams. This approach streamlined the delivery of consistent multidisciplinary care.
This collaborative effort aimed to develop ideal multidisciplinary care plans through research spanning the spectrum of the diabetic foot infection disease process (eAppendix, Supplemental Table 1). Some of the most impactful practices were: (1) a proclivity towards surgical treatment of foot infections, especially osteomyelitis5; (2) improved identification of PAD6,7; (3) early surgical closure of foot wounds following revascularization8,9; and (4) palliative wound care as an alternative to leg amputation in veterans who are not candidates for revascularization and limb salvage.10 Initally, the vascular surgery team held monthly multidisciplinary limb salvage meetings to coordinate patient management, identify ways to streamline care and avoid waste, discuss research findings, and review the 12-month rolling average of the MEDVAMC leg amputation incidence rate.
During the study period, the MEDVAMC vascular surgery team consisted of 2 to 5 board certified vascular or general surgeons, 2 or 3 nurse practitioners, and 3 vascular ultrasound technologists. Associated specialists included 2 podiatrists, 3 geriatricians with wound care certification, as well as additional infectious diseases, vascular medicine, orthopedics, and general surgery specialists.
Program Assessment
We noted a significant and sustained decrease in the MEDVAMC leg amputation rate after implementing multidisciplinary meetings and a single- team model from early 2012 through 2017 (Figure 2). The amputation incidence rate decreased steadily over the period from a maximum of 160 per 100,000 per year in February 2012 to a nadir of 66 per 100,000 per year in April 2017, an overall 60% decrease. Increases were noted in early 2018 after ceasing the single- team model, and in the summer of 2022, following periods of bed shortages after the onset of the COVID-19 pandemic. Tracking this metric allowed clinicians to make course corrections.

The decreased leg amputation rate at MEDVAMC does not seem to be mirroring national or regional trends. During this 10-year period, the VHA annualized amputation rate decreased minimally, from 58 to 54 per 100,000 (eAppendix Supplemental Figure C). Leg amputation incidence at non-VHA hospitals in Texas slightly increased over the same period.11
Value was also reflected in other metrics. MEDVAMC improved safety through a bundled strategy that reduced the risk-adjusted rate of surgical wound infections by 95%.12 MEDVAMC prioritized limb salvage when selecting patients for angiography and nearly eliminated using stent-grafts, cryopreserved allogeneic saphenous vein grafts, and expensive surgical and endovascular implants, which were identified as more expensive and less effective than other options (Figure 3).13-15 The MEDVAMC team achieved a > 90% patient trust rating on the Veterans Signals survey in fiscal years 2021 and 2022.

Challenges
A significant increase in the patient-physician ratio occurred 5 years into the program. In 2016, 2 vascular surgeons left MEDVAMC and a planned renovation of 1 of the 2 vascular surgery-assigned hybrid working facilities began even as the number of MEDVAMC patients with diabetes grew 120% (from 89,400 to 107,746 between 2010 and 2016), and the incidence rate of foot ulcers grew 300% (from 392 in 2010 to 1183 in 2016 per 100,000). The net result was a higher clinical workload among the remaining vascular surgeons with less operating room availability.
To stabilize surgeon retention, MEDVAMC reverted from the single team model back to inpatient care being distributed among general surgery, orthopedic surgery, and vascular surgery. After noting an increase in the leg amputation incidence rate, we adjusted the focus from multidisciplinary to interdisciplinary care (ie, majority of limb salvage clinical care can be provided by practitioners of any involved specialties). We worked to establish a local, written, interdisciplinary consensus on evaluating and managing veterans with nonhealing foot ulcers to mitigate the loss of a consolidated inpatient approach. Despite frequent staff turnover, ≥ 1 physician or surgeon from the core specialties of vascular surgery, podiatry, and infectious diseases remained throughout the study period.
The COVID-19 pandemic caused a shortage of hospital beds. This was followed by more bed shortages due to decreased nursing staff. Our health care system also had a period of restricted outpatient encounters early in the pandemic. During this time, we noted a delayed presentation of veterans with advanced infections and another increase in leg amputation incidence rate.
Like many health systems, MEDVAMC pivoted to telephone- and video-based outpatient encounters. Our team also used publicly available Texas hospitalization data to identify zip codes with particularly high leg amputation incidence rates, and > 3500 educational mailings to veterans categorized as moderate and high risk for leg amputation in these zip codes. These mailings provided information on recognizing foot ulcers and infections, emphasized timely evaluation, and named the MEDVAMC vascular surgery team as a point-of-contact. More recently, we have seen a further decrease in the MEDVAMC incidences of leg amputation to its lowest rate in > 20 years.
Discussion
A learning organization that directs its research based on clinical observations and informs its clinical care with research findings can produce palpable improvements in outcomes. Understanding the disease process and trying to better understand management across the entire range of this disease process has allowed our team to make consistent and systematic changes in care (Table). Consolidating inpatient care in a single team model seems to have been effective in reducing amputation rates among veterans with diabetes. The role the MEDVAMC vascular surgery team served for limb salvage patients may have been particularly beneficial because of the large impact untreated or unidentified PAD can have and because of the high prevalence of PAD among the limb salvage population seen at MEDVAMC. To be sustainable, though, a single-team model needs resources. A multiteam model can also be effective if the degree of multidisciplinary involvement for any given veteran is appropriate to the individual's clinical needs, teams are engaged and willing to contribute in a defined role within their specialty, and lines of communication remain open.

The primary challenge at MEDVAMC has been, and will continue to be, the retention of physicians and surgeons. MEDVAMC has excellent leadership and a collegial working environment, but better access to operating rooms for elective and time-sensitive operations, additional clinical staff support, and higher salary at non-VA positions have been the basis for many of physicians— especially surgeons—leaving MEDVAMC. Despite high staff turnover and a constant flow of resident and fellow trainees, MEDVAMC has been able to keep the clinical approach relatively consistent due to the use of written protocols and continuity of care as ≥ 1 physician or surgeon from each of the 4 main teams remained engaged with limb salvage throughout the entire period.
Going forward, we will work to ensure that all requirements of the 2022 Prevention of Amputation in Veterans Everywhere directive are incorporated into care.8 We plan to standardize MEDVAMC management algorithms further, both to streamline care and reduce the opportunity for disparities in treatment. More prophylactic podiatric procedures, surgical forms of offloading, and a shared multidisciplinary clinic space may also further help patients.
Conclusions
The introduction of multidisciplinary limb salvage at MEDVAMC has led to significant and sustained reductions in leg amputation incidence. These reductions do not seem dependent upon a specific team structure for inpatient care. To improve patient outcomes, efforts should focus on making improvements across the entire disease spectrum. For limb salvage, this includes primary prevention of foot ulcers, the treatment of foot infections, identification and management of PAD, surgical reconstruction/optimal wound healing, and care for patients who undergo leg amputation.
- Sanders LJ, Robbins JM, Edmonds ME. History of the team approach to amputation prevention: pioneers and milestones. J Am Podiatr Med Assoc. 2010;100(5):317- 334. doi:10.7547/1000317
- Sumpio BE, Armstrong DG, Lavery LA, Andros G. The role of interdisciplinary team approach in the management of the diabetic foot: a joint statement from the society for vascular surgery and the American podiatric medical association. J Am Podiatr Med Assoc. 2010;100(4):309-311. doi:10.7547/1000309
- About learning health systems. Agency for Healthcare Research and Quality. Published March 2019. Updated May 2019. Accessed October 9, 2024. https://www.ahrq.gov/learning-health-systems/about.html
- Barshes NR, Minc SD. Healthcare disparities in vascular surgery: a critical review. J Vasc Surg. 2021;74(2S):6S-14S.
- Barshes NR, Mindru C, Ashong C, Rodriguez-Barradas M, Trautner BW. Treatment failure and leg amputation among patients with foot osteomyelitis. Int J Low Extrem Wounds. 2016;15(4):303-312. doi:10.1177/1534734616661058
- Barshes NR, Flores E, Belkin M, Kougias P, Armstrong DG, Mills JL Sr. The accuracy and cost-effectiveness of strategies used to identify peripheral artery disease among patients with diabetic foot ulcers. J Vasc Surg. 2016;64(6):1682-1690.e3. doi:10.1016/j.jvs.2016.04.056 e1. doi:10.1016/j.jvs.2021.03.055
- Choi JC, Miranda J, Greenleaf E, et al. Lower-extremity pressure, staging, and grading thresholds to identify chronic limb-threatening ischemia. Vasc Med. 2023;28(1):45-53. doi:10.1177/1358863X221147945
- Barshes NR, Chambers JD, Cohen J, Belkin M; Model To Optimize Healthcare Value in Ischemic Extremities 1 (MOVIE) Study Collaborators. Cost-effectiveness in the contemporary management of critical limb ischemia with tissue loss. J Vasc Surg. 2012;56(4):1015-24.e1. doi:10.1016/j.jvs.2012.02.069
- Barshes NR, Bechara CF, Pisimisis G, Kougias P. Preliminary experiences with early primary closure of foot wounds after lower extremity revascularization. Ann Vasc Surg. 2014;28(1):48-52. doi:10.1016/j.avsg.2013.06.012
- Barshes NR, Gold B, Garcia A, Bechara CF, Pisimisis G, Kougias P. Minor amputation and palliative wound care as a strategy to avoid major amputation in patients with foot infections and severe peripheral arterial disease. Int J Low Extrem Wounds. 2014;13(3):211-219. doi:10.1177/1534734614543663
- Garcia M, Hernandez B, Ellington TG, et al. A lack of decline in major nontraumatic amputations in Texas: contemporary trends, risk factor associations, and impact of revascularization. Diabetes Care. 2019;42(6):1061-1066. doi:10.2337/dc19-0078
- Zamani N, Sharath SE, Vo E, Awad SS, Kougias P, Barshes NR. A multi-component strategy to decrease wound complications after open infra-inguinal re-vascularization. Surg Infect (Larchmt). 2018;19(1):87-94. doi:10.1089/sur.2017.193
- Barshes NR, Ozaki CK, Kougias P, Belkin M. A costeffectiveness analysis of infrainguinal bypass in the absence of great saphenous vein conduit. J Vasc Surg. 2013;57(6):1466-1470. doi:10.1016/j.jvs.2012.11.115
- Zamani N, Sharath S, Browder R, et al. PC158 longterm outcomes after endovascular stent placement for symptomatic, long-segment superficial femoral artery lesions. J Vasc Surg. 2017;65(6):182S-183S. doi:10.1016/j.jvs.2017.03.344
- Zamani N, Sharath SE, Browder RC, et al. Outcomes after endovascular stent placement for long-segment superficial femoral artery lesions. Ann Vasc Surg. 2021;71:298-307. doi:10.1016/j.avsg.2020.08.124
- Sanders LJ, Robbins JM, Edmonds ME. History of the team approach to amputation prevention: pioneers and milestones. J Am Podiatr Med Assoc. 2010;100(5):317- 334. doi:10.7547/1000317
- Sumpio BE, Armstrong DG, Lavery LA, Andros G. The role of interdisciplinary team approach in the management of the diabetic foot: a joint statement from the society for vascular surgery and the American podiatric medical association. J Am Podiatr Med Assoc. 2010;100(4):309-311. doi:10.7547/1000309
- About learning health systems. Agency for Healthcare Research and Quality. Published March 2019. Updated May 2019. Accessed October 9, 2024. https://www.ahrq.gov/learning-health-systems/about.html
- Barshes NR, Minc SD. Healthcare disparities in vascular surgery: a critical review. J Vasc Surg. 2021;74(2S):6S-14S.
- Barshes NR, Mindru C, Ashong C, Rodriguez-Barradas M, Trautner BW. Treatment failure and leg amputation among patients with foot osteomyelitis. Int J Low Extrem Wounds. 2016;15(4):303-312. doi:10.1177/1534734616661058
- Barshes NR, Flores E, Belkin M, Kougias P, Armstrong DG, Mills JL Sr. The accuracy and cost-effectiveness of strategies used to identify peripheral artery disease among patients with diabetic foot ulcers. J Vasc Surg. 2016;64(6):1682-1690.e3. doi:10.1016/j.jvs.2016.04.056 e1. doi:10.1016/j.jvs.2021.03.055
- Choi JC, Miranda J, Greenleaf E, et al. Lower-extremity pressure, staging, and grading thresholds to identify chronic limb-threatening ischemia. Vasc Med. 2023;28(1):45-53. doi:10.1177/1358863X221147945
- Barshes NR, Chambers JD, Cohen J, Belkin M; Model To Optimize Healthcare Value in Ischemic Extremities 1 (MOVIE) Study Collaborators. Cost-effectiveness in the contemporary management of critical limb ischemia with tissue loss. J Vasc Surg. 2012;56(4):1015-24.e1. doi:10.1016/j.jvs.2012.02.069
- Barshes NR, Bechara CF, Pisimisis G, Kougias P. Preliminary experiences with early primary closure of foot wounds after lower extremity revascularization. Ann Vasc Surg. 2014;28(1):48-52. doi:10.1016/j.avsg.2013.06.012
- Barshes NR, Gold B, Garcia A, Bechara CF, Pisimisis G, Kougias P. Minor amputation and palliative wound care as a strategy to avoid major amputation in patients with foot infections and severe peripheral arterial disease. Int J Low Extrem Wounds. 2014;13(3):211-219. doi:10.1177/1534734614543663
- Garcia M, Hernandez B, Ellington TG, et al. A lack of decline in major nontraumatic amputations in Texas: contemporary trends, risk factor associations, and impact of revascularization. Diabetes Care. 2019;42(6):1061-1066. doi:10.2337/dc19-0078
- Zamani N, Sharath SE, Vo E, Awad SS, Kougias P, Barshes NR. A multi-component strategy to decrease wound complications after open infra-inguinal re-vascularization. Surg Infect (Larchmt). 2018;19(1):87-94. doi:10.1089/sur.2017.193
- Barshes NR, Ozaki CK, Kougias P, Belkin M. A costeffectiveness analysis of infrainguinal bypass in the absence of great saphenous vein conduit. J Vasc Surg. 2013;57(6):1466-1470. doi:10.1016/j.jvs.2012.11.115
- Zamani N, Sharath S, Browder R, et al. PC158 longterm outcomes after endovascular stent placement for symptomatic, long-segment superficial femoral artery lesions. J Vasc Surg. 2017;65(6):182S-183S. doi:10.1016/j.jvs.2017.03.344
- Zamani N, Sharath SE, Browder RC, et al. Outcomes after endovascular stent placement for long-segment superficial femoral artery lesions. Ann Vasc Surg. 2021;71:298-307. doi:10.1016/j.avsg.2020.08.124