User login
‘No Pulse’: An MD’s First Night Off in 2 Weeks Turns Grave
Emergencies happen anywhere, anytime, and sometimes, medical professionals find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a series by this news organization that tells these stories.
It was my first night off after 12 days. It was a Friday night, and I went to a bar in Naples to get a beer with some friends. As it turned out, it wasn’t a night off after all.
As soon as we got inside, we heard over the speaker that they needed medical personnel and to please go to the left side of the bar. I thought it would be syncope or something like that.
I went over there and saw a woman holding up a man. He was basically leaning all over her. The light was low, and the music was pounding. I started to assess him and tried to get him to answer me. No response. I checked for pulses — nothing.
The woman helped me lower him to the floor. I checked again for a pulse. Still nothing. I said, “Call 911,” and started compressions.
The difficult part was the place was completely dark. I knew where his body was on the floor. I could see his chest. But I couldn’t see his face at all.
It was also extremely loud with the music thumping. After a while, they finally shut it off.
Pretty soon, the security personnel from the bar brought me an automated external defibrillator, and it showed the man was having V-fib arrest. I shocked him. Still no pulse. I continued with cardiopulmonary resuscitation (CPR).
I hadn’t noticed, but lots of people were crowding around us. Somebody came up and said, “He’s my friend. He has a 9-year-old daughter. He can’t die. Let me help with the compressions.” I was like, “Go for it.”
The guy started kind of pushing on the man’s abdomen. He had no idea how to do compressions. I said, “Okay, let me take over again.”
Out of the crowd, nobody else volunteered to help. No one asked me, “Hey, what can I do?” Meanwhile, I found out later that someone was filming the whole thing on their phone.
But what the guy said about the man’s young daughter stayed in my brain. I thought, we need to keep going.
I did more compressions and shocked him again. Still no pulse. At that point, the police and emergency medical services showed up. They checked, nothing had changed, so they got him into the ambulance.
I asked one of the paramedics, “Where are you taking him? I can call ahead.”
But he said, “That’s HIPAA. We can’t tell you.” They also wouldn’t let me go with him in the ambulance.
“I have an active Florida license, and I work in the ICU [intensive care unit],” I said.
“No, we need to follow our protocol,” he replied.
I understood that, but I just wanted to help.
It was around 10:30 PM by then, and I was drenched in sweat. I had to go home. The first thing I did after taking a shower was open the computer and check my system. I needed to find out what happened to the guy.
I was looking for admissions, and I didn’t see him. I called the main hospital downtown and the one in North Naples. I couldn’t find him anywhere. I stayed up until almost 1:00 AM checking for his name. At that point I thought, okay, maybe he died.
The next night, Saturday, I was home and got a call from one of my colleagues. “Hey, were you in a bar yesterday? Did you do CPR on somebody?”
“How did you know?” I said.
He said the paramedics had described me — “a tall doctor with glasses who was a nice guy.” It was funny that he knew that was me.
He told me, “The guy’s alive. He’s sick and needs to be put on dialysis, but he’s alive.”
Apparently, the guy had gone to the emergency department at North Naples, and the doctors in the emergency room (ER) worked on him for over an hour. They did continuous CPR and shocked him for close to 40 minutes. They finally got his pulse back, and after that, he was transferred to the main hospital ICU. They didn’t admit him at the ER, which was why I couldn’t find his name.
On Sunday, I was checking my patients’ charts for the ICU that coming week. And there he was. I saw his name and the documentation by the ED that CPR was provided by a critical care doctor in the field. He was still alive. That gave me so much joy.
So, the man I had helped became my patient. When I saw him on Monday, he was intubated and needed dialysis. I finally saw his face and thought, Oh, so that’s what you look like. I hadn’t realized he was only 39 years old.
When he was awake, I explained to him I was the doctor that provided CPR at the bar. He was very grateful, but of course, he didn’t remember anything.
Eventually, I met his daughter, and she just said, “Thank you for allowing me to have my dad.”
The funny part is that he broke his leg. Well, that’s not funny, but no one had any idea how it happened. That was his only complaint. He was asking me, “Doctor, how did you break my leg?”
“Hey, I have no idea how you broke your leg,” I replied. “I was trying to save your life.”
He was in the hospital for almost a month but made a full recovery. The amazing part: After all the evaluations, he has no neurological deficits. He’s back to a normal life now.
They never found a cause for the cardiac arrest. I mean, he had an ejection fraction of 10%. All my money was on something drug related, but that wasn’t the case. They’d done a cardiac cut, and there was no obstruction. They couldn’t find a reason.
We’ve become friends. He still works as a DJ at the bar. He changed his name to “DJ the Survivor” or something like that.
Sometimes, he’ll text me: “Doctor, what are you doing? You want to come down to the bar?”
I’m like, “No. I don’t.”
It’s been more than a year, but I remember every detail. When you go into medicine, you dream that one day you’ll be able to say, “I saved somebody.”
He texted me a year later and told me he’s celebrating two birthdays now. He said, “I’m turning 1 year old today!”
I think about the value of life. How we can take it for granted. We think, I’m young, nothing is going to happen to me. But this guy was 39. He went to work and died that night.
I was able to help bring him back. That makes me thankful for every day.
Jose Valle Giler, MD, is a pulmonary, critical care, and sleep medicine physician at NCH Healthcare System in Naples, Florida.
A version of this article appeared on Medscape.com .
Emergencies happen anywhere, anytime, and sometimes, medical professionals find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a series by this news organization that tells these stories.
It was my first night off after 12 days. It was a Friday night, and I went to a bar in Naples to get a beer with some friends. As it turned out, it wasn’t a night off after all.
As soon as we got inside, we heard over the speaker that they needed medical personnel and to please go to the left side of the bar. I thought it would be syncope or something like that.
I went over there and saw a woman holding up a man. He was basically leaning all over her. The light was low, and the music was pounding. I started to assess him and tried to get him to answer me. No response. I checked for pulses — nothing.
The woman helped me lower him to the floor. I checked again for a pulse. Still nothing. I said, “Call 911,” and started compressions.
The difficult part was the place was completely dark. I knew where his body was on the floor. I could see his chest. But I couldn’t see his face at all.
It was also extremely loud with the music thumping. After a while, they finally shut it off.
Pretty soon, the security personnel from the bar brought me an automated external defibrillator, and it showed the man was having V-fib arrest. I shocked him. Still no pulse. I continued with cardiopulmonary resuscitation (CPR).
I hadn’t noticed, but lots of people were crowding around us. Somebody came up and said, “He’s my friend. He has a 9-year-old daughter. He can’t die. Let me help with the compressions.” I was like, “Go for it.”
The guy started kind of pushing on the man’s abdomen. He had no idea how to do compressions. I said, “Okay, let me take over again.”
Out of the crowd, nobody else volunteered to help. No one asked me, “Hey, what can I do?” Meanwhile, I found out later that someone was filming the whole thing on their phone.
But what the guy said about the man’s young daughter stayed in my brain. I thought, we need to keep going.
I did more compressions and shocked him again. Still no pulse. At that point, the police and emergency medical services showed up. They checked, nothing had changed, so they got him into the ambulance.
I asked one of the paramedics, “Where are you taking him? I can call ahead.”
But he said, “That’s HIPAA. We can’t tell you.” They also wouldn’t let me go with him in the ambulance.
“I have an active Florida license, and I work in the ICU [intensive care unit],” I said.
“No, we need to follow our protocol,” he replied.
I understood that, but I just wanted to help.
It was around 10:30 PM by then, and I was drenched in sweat. I had to go home. The first thing I did after taking a shower was open the computer and check my system. I needed to find out what happened to the guy.
I was looking for admissions, and I didn’t see him. I called the main hospital downtown and the one in North Naples. I couldn’t find him anywhere. I stayed up until almost 1:00 AM checking for his name. At that point I thought, okay, maybe he died.
The next night, Saturday, I was home and got a call from one of my colleagues. “Hey, were you in a bar yesterday? Did you do CPR on somebody?”
“How did you know?” I said.
He said the paramedics had described me — “a tall doctor with glasses who was a nice guy.” It was funny that he knew that was me.
He told me, “The guy’s alive. He’s sick and needs to be put on dialysis, but he’s alive.”
Apparently, the guy had gone to the emergency department at North Naples, and the doctors in the emergency room (ER) worked on him for over an hour. They did continuous CPR and shocked him for close to 40 minutes. They finally got his pulse back, and after that, he was transferred to the main hospital ICU. They didn’t admit him at the ER, which was why I couldn’t find his name.
On Sunday, I was checking my patients’ charts for the ICU that coming week. And there he was. I saw his name and the documentation by the ED that CPR was provided by a critical care doctor in the field. He was still alive. That gave me so much joy.
So, the man I had helped became my patient. When I saw him on Monday, he was intubated and needed dialysis. I finally saw his face and thought, Oh, so that’s what you look like. I hadn’t realized he was only 39 years old.
When he was awake, I explained to him I was the doctor that provided CPR at the bar. He was very grateful, but of course, he didn’t remember anything.
Eventually, I met his daughter, and she just said, “Thank you for allowing me to have my dad.”
The funny part is that he broke his leg. Well, that’s not funny, but no one had any idea how it happened. That was his only complaint. He was asking me, “Doctor, how did you break my leg?”
“Hey, I have no idea how you broke your leg,” I replied. “I was trying to save your life.”
He was in the hospital for almost a month but made a full recovery. The amazing part: After all the evaluations, he has no neurological deficits. He’s back to a normal life now.
They never found a cause for the cardiac arrest. I mean, he had an ejection fraction of 10%. All my money was on something drug related, but that wasn’t the case. They’d done a cardiac cut, and there was no obstruction. They couldn’t find a reason.
We’ve become friends. He still works as a DJ at the bar. He changed his name to “DJ the Survivor” or something like that.
Sometimes, he’ll text me: “Doctor, what are you doing? You want to come down to the bar?”
I’m like, “No. I don’t.”
It’s been more than a year, but I remember every detail. When you go into medicine, you dream that one day you’ll be able to say, “I saved somebody.”
He texted me a year later and told me he’s celebrating two birthdays now. He said, “I’m turning 1 year old today!”
I think about the value of life. How we can take it for granted. We think, I’m young, nothing is going to happen to me. But this guy was 39. He went to work and died that night.
I was able to help bring him back. That makes me thankful for every day.
Jose Valle Giler, MD, is a pulmonary, critical care, and sleep medicine physician at NCH Healthcare System in Naples, Florida.
A version of this article appeared on Medscape.com .
Emergencies happen anywhere, anytime, and sometimes, medical professionals find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a series by this news organization that tells these stories.
It was my first night off after 12 days. It was a Friday night, and I went to a bar in Naples to get a beer with some friends. As it turned out, it wasn’t a night off after all.
As soon as we got inside, we heard over the speaker that they needed medical personnel and to please go to the left side of the bar. I thought it would be syncope or something like that.
I went over there and saw a woman holding up a man. He was basically leaning all over her. The light was low, and the music was pounding. I started to assess him and tried to get him to answer me. No response. I checked for pulses — nothing.
The woman helped me lower him to the floor. I checked again for a pulse. Still nothing. I said, “Call 911,” and started compressions.
The difficult part was the place was completely dark. I knew where his body was on the floor. I could see his chest. But I couldn’t see his face at all.
It was also extremely loud with the music thumping. After a while, they finally shut it off.
Pretty soon, the security personnel from the bar brought me an automated external defibrillator, and it showed the man was having V-fib arrest. I shocked him. Still no pulse. I continued with cardiopulmonary resuscitation (CPR).
I hadn’t noticed, but lots of people were crowding around us. Somebody came up and said, “He’s my friend. He has a 9-year-old daughter. He can’t die. Let me help with the compressions.” I was like, “Go for it.”
The guy started kind of pushing on the man’s abdomen. He had no idea how to do compressions. I said, “Okay, let me take over again.”
Out of the crowd, nobody else volunteered to help. No one asked me, “Hey, what can I do?” Meanwhile, I found out later that someone was filming the whole thing on their phone.
But what the guy said about the man’s young daughter stayed in my brain. I thought, we need to keep going.
I did more compressions and shocked him again. Still no pulse. At that point, the police and emergency medical services showed up. They checked, nothing had changed, so they got him into the ambulance.
I asked one of the paramedics, “Where are you taking him? I can call ahead.”
But he said, “That’s HIPAA. We can’t tell you.” They also wouldn’t let me go with him in the ambulance.
“I have an active Florida license, and I work in the ICU [intensive care unit],” I said.
“No, we need to follow our protocol,” he replied.
I understood that, but I just wanted to help.
It was around 10:30 PM by then, and I was drenched in sweat. I had to go home. The first thing I did after taking a shower was open the computer and check my system. I needed to find out what happened to the guy.
I was looking for admissions, and I didn’t see him. I called the main hospital downtown and the one in North Naples. I couldn’t find him anywhere. I stayed up until almost 1:00 AM checking for his name. At that point I thought, okay, maybe he died.
The next night, Saturday, I was home and got a call from one of my colleagues. “Hey, were you in a bar yesterday? Did you do CPR on somebody?”
“How did you know?” I said.
He said the paramedics had described me — “a tall doctor with glasses who was a nice guy.” It was funny that he knew that was me.
He told me, “The guy’s alive. He’s sick and needs to be put on dialysis, but he’s alive.”
Apparently, the guy had gone to the emergency department at North Naples, and the doctors in the emergency room (ER) worked on him for over an hour. They did continuous CPR and shocked him for close to 40 minutes. They finally got his pulse back, and after that, he was transferred to the main hospital ICU. They didn’t admit him at the ER, which was why I couldn’t find his name.
On Sunday, I was checking my patients’ charts for the ICU that coming week. And there he was. I saw his name and the documentation by the ED that CPR was provided by a critical care doctor in the field. He was still alive. That gave me so much joy.
So, the man I had helped became my patient. When I saw him on Monday, he was intubated and needed dialysis. I finally saw his face and thought, Oh, so that’s what you look like. I hadn’t realized he was only 39 years old.
When he was awake, I explained to him I was the doctor that provided CPR at the bar. He was very grateful, but of course, he didn’t remember anything.
Eventually, I met his daughter, and she just said, “Thank you for allowing me to have my dad.”
The funny part is that he broke his leg. Well, that’s not funny, but no one had any idea how it happened. That was his only complaint. He was asking me, “Doctor, how did you break my leg?”
“Hey, I have no idea how you broke your leg,” I replied. “I was trying to save your life.”
He was in the hospital for almost a month but made a full recovery. The amazing part: After all the evaluations, he has no neurological deficits. He’s back to a normal life now.
They never found a cause for the cardiac arrest. I mean, he had an ejection fraction of 10%. All my money was on something drug related, but that wasn’t the case. They’d done a cardiac cut, and there was no obstruction. They couldn’t find a reason.
We’ve become friends. He still works as a DJ at the bar. He changed his name to “DJ the Survivor” or something like that.
Sometimes, he’ll text me: “Doctor, what are you doing? You want to come down to the bar?”
I’m like, “No. I don’t.”
It’s been more than a year, but I remember every detail. When you go into medicine, you dream that one day you’ll be able to say, “I saved somebody.”
He texted me a year later and told me he’s celebrating two birthdays now. He said, “I’m turning 1 year old today!”
I think about the value of life. How we can take it for granted. We think, I’m young, nothing is going to happen to me. But this guy was 39. He went to work and died that night.
I was able to help bring him back. That makes me thankful for every day.
Jose Valle Giler, MD, is a pulmonary, critical care, and sleep medicine physician at NCH Healthcare System in Naples, Florida.
A version of this article appeared on Medscape.com .
Moral Injury in Health Care: A Unified Definition and its Relationship to Burnout
Moral injury was identified by health care professionals (HCPs) as a driver of occupational distress prior to the COVID-19 pandemic, but the crisis expanded the appeal and investigation of the term.1 HCPs now consider moral injury an essential component of the framework to describe their distress, because using the term burnout alone fails to capture their full experience and has proven resistant to interventions.2 Moral injury goes beyond the transdiagnostic symptoms of exhaustion and cynicism and beyond operational, demand-resource mismatches that characterize burnout. It describes the frustration, anger, and helplessness associated with relational ruptures and the existential threats to a clinician’s professional identity as business interests erode their ability to put their patients’ needs ahead of corporate and health care system obligations.3
Proper characterization of moral injury in health care—separate from the military environments where it originated—is stymied by an ill-defined relationship between 2 definitions of the term and by an unclear relationship between moral injury and the long-standing body of scholarship in burnout. To clarify the concept, inform research agendas, and open avenues for more effective solutions to the crisis of HCP distress, we propose a unified conceptualization of moral injury and its association with burnout in health care.
CONTEXTUAL DISTINCTIONS
It is important to properly distinguish between the original use of moral injury in the military and its expanded use in civilian circumstances. Health care and the military are both professions whereupon donning the “uniform” of a physician—or soldier, sailor, airman, or marine—members must comport with strict expectations of behavior, including the refusal to engage in illegal actions or those contrary to professional ethics. Individuals in both professions acquire a highly specialized body of knowledge and enter an implied contract to provide critical services to society, specifically healing and protection, respectively. Members of both professions are trained to make complex judgments with integrity under conditions of technical and ethical uncertainty, upon which they take highly skilled action. Medical and military professionals must be free to act on their ethical principles, without confounding demands.4 However, the context of each profession’s commitment to society carries different moral implications.
The risk of moral injury is inherent in military service. The military promises protection with an implicit acknowledgment of the need to use lethal force to uphold the agreement. In contrast, HCPs promise healing and care. The military promises to protect our society, with an implicit acknowledgment of the need to use lethal force to uphold the agreement. Some military actions may inflict harm without the hope of benefitting an individual, and are therefore potentially morally injurious. The health care contract with society, promising healing and care, is devoid of inherent moral injury due to harm without potential individual benefit. Therefore, the presence of moral injury in health care settings are warning signs of a dysfunctional environment.
One complex example of the dysfunctional environments is illustrative. The military and health care are among the few industries where supply creates demand. For example, the more bad state actors there are, the more demand for the military. As we have seen since the 1950s, the more technology and therapeutics we create in health care, coupled with a larger share paid for by third parties, the greater the demand for and use of them.5 In a fee for service environment, corporate greed feeds on this reality. In most other environments, more technological and therapeutic options inevitably pit clinicians against multiple other factions: payers, who do not want to underwrite them; patients, who sometimes demand them without justification or later rail against spiraling health care costs; and administrators, especially in capitated systems, who watch their bottom lines erode. The moral injury risk in this instance demands a collective conversation among stakeholders regarding the structural determinants of health—how we choose to distribute limited resources. The intermediary of moral injury is a useful measure of the harm that results from ignoring or avoiding such challenges.
HARMONIZING DEFINITIONS
Moral injury is inherently nuanced. The 2 dominant definitions arise from work with combat veterans and create additional and perhaps unnecessary complexity. Unifying these 2 definitions eliminates inadvertent confusion, preventing the risk of unbridled interdisciplinary investigation which leads to a lack of precision in the meaning of moral injury and other related concepts, such as burnout.6
The first definition was developed by Jonathan Shay in 1994 and outlines 3 necessarycomponents, viewing the violator as a powerholder: (1) betrayal of what is right, (2) by someone who holds legitimate authority, (3) in a high stakes situation.7 Litz and colleagues describe moral injury another way: “Perpetrating, failing to prevent, bearing witness to, or learning about acts that transgress deeply held moral beliefs and expectations.”8 The violator is posited to be either the self or others.
Rather than representing “self” or “other” imposed moral injury, we propose the 2 definitions are related as exposure (ie, the perceived betrayal) and response (ie, the resulting transgression). An individual who experiences a betrayal by a legitimate authority has an opportunity to choose their response. They may acquiesce and transgress their moral beliefs (eg, their oath to provide ethical health care), or they could refuse, by speaking out, or in some way resisting the authority’s betrayal. The case of Ray Brovont is a useful illustration of reconciling the definitions (Box).9
Myriad factors—known as potentially morally injurious events—drive moral injury, such as resource-constrained decision making, witnessing the behaviors of colleagues that violate deeply held moral beliefs, questionable billing practices, and more. Each begins with a betrayal. Spotlighting the betrayal, refusing to perpetuate it, or taking actions toward change, may reduce the risk of experiencing moral injury.9 Conversely, acquiescing and transgressing one’s oath, the profession’s covenant with society, increases the risk of experiencing moral injury.8
Many HCPs believe they are not always free to resist betrayal, fearing retaliation, job loss, blacklisting, or worse. They feel constrained by debt accrued while receiving their education, being their household’s primary earner, community ties, practicing a niche specialty that requires working for a tertiary referral center, or perhaps believing the situation will be the same elsewhere. To not stand up or speak out is to choose complicity with corporate greed that uses HCPs to undermine their professional duties, which significantly increases the risk of experiencing moral injury.
MORAL INJURY AND BURNOUT
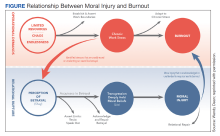
In addition to reconciling the definitions of moral injury, the relationship between moral injury and burnout are still being elucidated. We suggest that moral injury and burnout represent independent and potentially interrelated pathways to distress (Figure). Exposure to chronic, inconsonant, and transactional demands, which things like shorter work hours, better self-care, or improved health system operations might mitigate, manifests as burnout. In contrast, moral injury arises when a superior’s actions or a system’s policies and practices—such as justifiable but unnecessary testing, or referral restrictions to prevent revenue leakage—undermine one’s professional obligations to prioritize the patient’s best interest.
If concerns from HCPs about transactional demands are persistently dismissed, such inaction may be perceived as a betrayal, raising the risk of moral injury. Additionally, the resignation or helplessness of moral injury perceived as inescapable may present with emotional exhaustion, ineffectiveness, and depersonalization, all hallmarks of burnout. Both conditions can mediate and moderate the relationship between triggers for workplace distress and resulting psychological, physical, and existential harm.
CONCLUSIONS
Moral injury is increasingly recognized as a source of distress among HCPs, resulting from structural constraints on their ability to deliver optimal care and their own unwillingness to stand up for their patients, their oaths, and their professions.1 Unlike the military, where moral injury is inherent in the contract with society, moral injury in health care (and the relational rupture it connotes) is a signal of systemic dysfunction, fractured trust, and the need for relational repair.
Health care is at a crossroads, experiencing a workforce retention crisis while simultaneously predicting a significant increase in care needs by Baby Boomers over the next 3 decades.
Health care does not have the luxury of experimenting another 30 years with interventions that have limited impact. We must design a new generation of approaches, shaped by lessons learned from the pandemic while acknowledging that prepandemic standards were already failing the workforce. A unified definition of moral injury must be integrated to frame clinician distress alongside burnout, recentering ethical decision making, rather than profit, at the heart of health care. Harmonizing the definitions of moral injury and clarifying the relationship of moral injury with burnout reduces the need for further reinterpretations, allowing for more robust, easily comparable studies focused on identifying risk factors, as well as rapidly implementing effective mitigation strategies.
1. Griffin BJ, Weber MC, Hinkson KD, et al. Toward a dimensional contextual model of moral injury: a scoping review on healthcare workers. Curr Treat Options Psych. 2023;10:199-216. doi:10.1007/s40501-023-00296-4
2. National Academies of Sciences, Engineering, and Medicine; National Academy of Medicine; Committee on Systems Approaches to Improve Patient Care by Supporting Clinician Well-Being. Taking Action Against Clinician Burnout: A Systems Approach to Professional Well-Being. The National Academies Press; 2019. doi:10.17226/25521
3. Dean W, Talbot S, Dean A. Reframing clinician distress: moral injury not burnout. Fed Pract. 2019;36(9):400-402.
4. Gardner HE, Schulman LS. The professions in America today: crucial but fragile. Daedalus. 2005;134(3):13-18. doi:10.1162/0011526054622132
5. Fuchs VR. Major trends in the U.S. health economy since 1950. N Engl J Med. 2012;366(11):973-977. doi:10.1056/NEJMp1200478
6. Molendijk T. Warnings against romanticising moral injury. Br J Psychiatry. 2022;220(1):1-3. doi:10.1192/bjp.2021.114
7. Shay J. Moral injury. Psychoanalytic Psychol. 2014;31(2):182-191. doi:10.1037/a0036090
8. Litz BT, Stein N, Delaney E, et al. Moral injury and moral repair in war veterans: a preliminary model and intervention strategy. Clin Psychol Rev. 2009;29(8):695-706. doi:10.1016/j.cpr.2009.07.003
9. Brovont v KS-I Med. Servs., P.A., 622 SW3d 671 (Mo Ct App 2020).
Moral injury was identified by health care professionals (HCPs) as a driver of occupational distress prior to the COVID-19 pandemic, but the crisis expanded the appeal and investigation of the term.1 HCPs now consider moral injury an essential component of the framework to describe their distress, because using the term burnout alone fails to capture their full experience and has proven resistant to interventions.2 Moral injury goes beyond the transdiagnostic symptoms of exhaustion and cynicism and beyond operational, demand-resource mismatches that characterize burnout. It describes the frustration, anger, and helplessness associated with relational ruptures and the existential threats to a clinician’s professional identity as business interests erode their ability to put their patients’ needs ahead of corporate and health care system obligations.3
Proper characterization of moral injury in health care—separate from the military environments where it originated—is stymied by an ill-defined relationship between 2 definitions of the term and by an unclear relationship between moral injury and the long-standing body of scholarship in burnout. To clarify the concept, inform research agendas, and open avenues for more effective solutions to the crisis of HCP distress, we propose a unified conceptualization of moral injury and its association with burnout in health care.
CONTEXTUAL DISTINCTIONS
It is important to properly distinguish between the original use of moral injury in the military and its expanded use in civilian circumstances. Health care and the military are both professions whereupon donning the “uniform” of a physician—or soldier, sailor, airman, or marine—members must comport with strict expectations of behavior, including the refusal to engage in illegal actions or those contrary to professional ethics. Individuals in both professions acquire a highly specialized body of knowledge and enter an implied contract to provide critical services to society, specifically healing and protection, respectively. Members of both professions are trained to make complex judgments with integrity under conditions of technical and ethical uncertainty, upon which they take highly skilled action. Medical and military professionals must be free to act on their ethical principles, without confounding demands.4 However, the context of each profession’s commitment to society carries different moral implications.
The risk of moral injury is inherent in military service. The military promises protection with an implicit acknowledgment of the need to use lethal force to uphold the agreement. In contrast, HCPs promise healing and care. The military promises to protect our society, with an implicit acknowledgment of the need to use lethal force to uphold the agreement. Some military actions may inflict harm without the hope of benefitting an individual, and are therefore potentially morally injurious. The health care contract with society, promising healing and care, is devoid of inherent moral injury due to harm without potential individual benefit. Therefore, the presence of moral injury in health care settings are warning signs of a dysfunctional environment.
One complex example of the dysfunctional environments is illustrative. The military and health care are among the few industries where supply creates demand. For example, the more bad state actors there are, the more demand for the military. As we have seen since the 1950s, the more technology and therapeutics we create in health care, coupled with a larger share paid for by third parties, the greater the demand for and use of them.5 In a fee for service environment, corporate greed feeds on this reality. In most other environments, more technological and therapeutic options inevitably pit clinicians against multiple other factions: payers, who do not want to underwrite them; patients, who sometimes demand them without justification or later rail against spiraling health care costs; and administrators, especially in capitated systems, who watch their bottom lines erode. The moral injury risk in this instance demands a collective conversation among stakeholders regarding the structural determinants of health—how we choose to distribute limited resources. The intermediary of moral injury is a useful measure of the harm that results from ignoring or avoiding such challenges.
HARMONIZING DEFINITIONS
Moral injury is inherently nuanced. The 2 dominant definitions arise from work with combat veterans and create additional and perhaps unnecessary complexity. Unifying these 2 definitions eliminates inadvertent confusion, preventing the risk of unbridled interdisciplinary investigation which leads to a lack of precision in the meaning of moral injury and other related concepts, such as burnout.6
The first definition was developed by Jonathan Shay in 1994 and outlines 3 necessarycomponents, viewing the violator as a powerholder: (1) betrayal of what is right, (2) by someone who holds legitimate authority, (3) in a high stakes situation.7 Litz and colleagues describe moral injury another way: “Perpetrating, failing to prevent, bearing witness to, or learning about acts that transgress deeply held moral beliefs and expectations.”8 The violator is posited to be either the self or others.
Rather than representing “self” or “other” imposed moral injury, we propose the 2 definitions are related as exposure (ie, the perceived betrayal) and response (ie, the resulting transgression). An individual who experiences a betrayal by a legitimate authority has an opportunity to choose their response. They may acquiesce and transgress their moral beliefs (eg, their oath to provide ethical health care), or they could refuse, by speaking out, or in some way resisting the authority’s betrayal. The case of Ray Brovont is a useful illustration of reconciling the definitions (Box).9
Myriad factors—known as potentially morally injurious events—drive moral injury, such as resource-constrained decision making, witnessing the behaviors of colleagues that violate deeply held moral beliefs, questionable billing practices, and more. Each begins with a betrayal. Spotlighting the betrayal, refusing to perpetuate it, or taking actions toward change, may reduce the risk of experiencing moral injury.9 Conversely, acquiescing and transgressing one’s oath, the profession’s covenant with society, increases the risk of experiencing moral injury.8
Many HCPs believe they are not always free to resist betrayal, fearing retaliation, job loss, blacklisting, or worse. They feel constrained by debt accrued while receiving their education, being their household’s primary earner, community ties, practicing a niche specialty that requires working for a tertiary referral center, or perhaps believing the situation will be the same elsewhere. To not stand up or speak out is to choose complicity with corporate greed that uses HCPs to undermine their professional duties, which significantly increases the risk of experiencing moral injury.
MORAL INJURY AND BURNOUT
In addition to reconciling the definitions of moral injury, the relationship between moral injury and burnout are still being elucidated. We suggest that moral injury and burnout represent independent and potentially interrelated pathways to distress (Figure). Exposure to chronic, inconsonant, and transactional demands, which things like shorter work hours, better self-care, or improved health system operations might mitigate, manifests as burnout. In contrast, moral injury arises when a superior’s actions or a system’s policies and practices—such as justifiable but unnecessary testing, or referral restrictions to prevent revenue leakage—undermine one’s professional obligations to prioritize the patient’s best interest.
If concerns from HCPs about transactional demands are persistently dismissed, such inaction may be perceived as a betrayal, raising the risk of moral injury. Additionally, the resignation or helplessness of moral injury perceived as inescapable may present with emotional exhaustion, ineffectiveness, and depersonalization, all hallmarks of burnout. Both conditions can mediate and moderate the relationship between triggers for workplace distress and resulting psychological, physical, and existential harm.
CONCLUSIONS
Moral injury is increasingly recognized as a source of distress among HCPs, resulting from structural constraints on their ability to deliver optimal care and their own unwillingness to stand up for their patients, their oaths, and their professions.1 Unlike the military, where moral injury is inherent in the contract with society, moral injury in health care (and the relational rupture it connotes) is a signal of systemic dysfunction, fractured trust, and the need for relational repair.
Health care is at a crossroads, experiencing a workforce retention crisis while simultaneously predicting a significant increase in care needs by Baby Boomers over the next 3 decades.
Health care does not have the luxury of experimenting another 30 years with interventions that have limited impact. We must design a new generation of approaches, shaped by lessons learned from the pandemic while acknowledging that prepandemic standards were already failing the workforce. A unified definition of moral injury must be integrated to frame clinician distress alongside burnout, recentering ethical decision making, rather than profit, at the heart of health care. Harmonizing the definitions of moral injury and clarifying the relationship of moral injury with burnout reduces the need for further reinterpretations, allowing for more robust, easily comparable studies focused on identifying risk factors, as well as rapidly implementing effective mitigation strategies.
Moral injury was identified by health care professionals (HCPs) as a driver of occupational distress prior to the COVID-19 pandemic, but the crisis expanded the appeal and investigation of the term.1 HCPs now consider moral injury an essential component of the framework to describe their distress, because using the term burnout alone fails to capture their full experience and has proven resistant to interventions.2 Moral injury goes beyond the transdiagnostic symptoms of exhaustion and cynicism and beyond operational, demand-resource mismatches that characterize burnout. It describes the frustration, anger, and helplessness associated with relational ruptures and the existential threats to a clinician’s professional identity as business interests erode their ability to put their patients’ needs ahead of corporate and health care system obligations.3
Proper characterization of moral injury in health care—separate from the military environments where it originated—is stymied by an ill-defined relationship between 2 definitions of the term and by an unclear relationship between moral injury and the long-standing body of scholarship in burnout. To clarify the concept, inform research agendas, and open avenues for more effective solutions to the crisis of HCP distress, we propose a unified conceptualization of moral injury and its association with burnout in health care.
CONTEXTUAL DISTINCTIONS
It is important to properly distinguish between the original use of moral injury in the military and its expanded use in civilian circumstances. Health care and the military are both professions whereupon donning the “uniform” of a physician—or soldier, sailor, airman, or marine—members must comport with strict expectations of behavior, including the refusal to engage in illegal actions or those contrary to professional ethics. Individuals in both professions acquire a highly specialized body of knowledge and enter an implied contract to provide critical services to society, specifically healing and protection, respectively. Members of both professions are trained to make complex judgments with integrity under conditions of technical and ethical uncertainty, upon which they take highly skilled action. Medical and military professionals must be free to act on their ethical principles, without confounding demands.4 However, the context of each profession’s commitment to society carries different moral implications.
The risk of moral injury is inherent in military service. The military promises protection with an implicit acknowledgment of the need to use lethal force to uphold the agreement. In contrast, HCPs promise healing and care. The military promises to protect our society, with an implicit acknowledgment of the need to use lethal force to uphold the agreement. Some military actions may inflict harm without the hope of benefitting an individual, and are therefore potentially morally injurious. The health care contract with society, promising healing and care, is devoid of inherent moral injury due to harm without potential individual benefit. Therefore, the presence of moral injury in health care settings are warning signs of a dysfunctional environment.
One complex example of the dysfunctional environments is illustrative. The military and health care are among the few industries where supply creates demand. For example, the more bad state actors there are, the more demand for the military. As we have seen since the 1950s, the more technology and therapeutics we create in health care, coupled with a larger share paid for by third parties, the greater the demand for and use of them.5 In a fee for service environment, corporate greed feeds on this reality. In most other environments, more technological and therapeutic options inevitably pit clinicians against multiple other factions: payers, who do not want to underwrite them; patients, who sometimes demand them without justification or later rail against spiraling health care costs; and administrators, especially in capitated systems, who watch their bottom lines erode. The moral injury risk in this instance demands a collective conversation among stakeholders regarding the structural determinants of health—how we choose to distribute limited resources. The intermediary of moral injury is a useful measure of the harm that results from ignoring or avoiding such challenges.
HARMONIZING DEFINITIONS
Moral injury is inherently nuanced. The 2 dominant definitions arise from work with combat veterans and create additional and perhaps unnecessary complexity. Unifying these 2 definitions eliminates inadvertent confusion, preventing the risk of unbridled interdisciplinary investigation which leads to a lack of precision in the meaning of moral injury and other related concepts, such as burnout.6
The first definition was developed by Jonathan Shay in 1994 and outlines 3 necessarycomponents, viewing the violator as a powerholder: (1) betrayal of what is right, (2) by someone who holds legitimate authority, (3) in a high stakes situation.7 Litz and colleagues describe moral injury another way: “Perpetrating, failing to prevent, bearing witness to, or learning about acts that transgress deeply held moral beliefs and expectations.”8 The violator is posited to be either the self or others.
Rather than representing “self” or “other” imposed moral injury, we propose the 2 definitions are related as exposure (ie, the perceived betrayal) and response (ie, the resulting transgression). An individual who experiences a betrayal by a legitimate authority has an opportunity to choose their response. They may acquiesce and transgress their moral beliefs (eg, their oath to provide ethical health care), or they could refuse, by speaking out, or in some way resisting the authority’s betrayal. The case of Ray Brovont is a useful illustration of reconciling the definitions (Box).9
Myriad factors—known as potentially morally injurious events—drive moral injury, such as resource-constrained decision making, witnessing the behaviors of colleagues that violate deeply held moral beliefs, questionable billing practices, and more. Each begins with a betrayal. Spotlighting the betrayal, refusing to perpetuate it, or taking actions toward change, may reduce the risk of experiencing moral injury.9 Conversely, acquiescing and transgressing one’s oath, the profession’s covenant with society, increases the risk of experiencing moral injury.8
Many HCPs believe they are not always free to resist betrayal, fearing retaliation, job loss, blacklisting, or worse. They feel constrained by debt accrued while receiving their education, being their household’s primary earner, community ties, practicing a niche specialty that requires working for a tertiary referral center, or perhaps believing the situation will be the same elsewhere. To not stand up or speak out is to choose complicity with corporate greed that uses HCPs to undermine their professional duties, which significantly increases the risk of experiencing moral injury.
MORAL INJURY AND BURNOUT
In addition to reconciling the definitions of moral injury, the relationship between moral injury and burnout are still being elucidated. We suggest that moral injury and burnout represent independent and potentially interrelated pathways to distress (Figure). Exposure to chronic, inconsonant, and transactional demands, which things like shorter work hours, better self-care, or improved health system operations might mitigate, manifests as burnout. In contrast, moral injury arises when a superior’s actions or a system’s policies and practices—such as justifiable but unnecessary testing, or referral restrictions to prevent revenue leakage—undermine one’s professional obligations to prioritize the patient’s best interest.
If concerns from HCPs about transactional demands are persistently dismissed, such inaction may be perceived as a betrayal, raising the risk of moral injury. Additionally, the resignation or helplessness of moral injury perceived as inescapable may present with emotional exhaustion, ineffectiveness, and depersonalization, all hallmarks of burnout. Both conditions can mediate and moderate the relationship between triggers for workplace distress and resulting psychological, physical, and existential harm.
CONCLUSIONS
Moral injury is increasingly recognized as a source of distress among HCPs, resulting from structural constraints on their ability to deliver optimal care and their own unwillingness to stand up for their patients, their oaths, and their professions.1 Unlike the military, where moral injury is inherent in the contract with society, moral injury in health care (and the relational rupture it connotes) is a signal of systemic dysfunction, fractured trust, and the need for relational repair.
Health care is at a crossroads, experiencing a workforce retention crisis while simultaneously predicting a significant increase in care needs by Baby Boomers over the next 3 decades.
Health care does not have the luxury of experimenting another 30 years with interventions that have limited impact. We must design a new generation of approaches, shaped by lessons learned from the pandemic while acknowledging that prepandemic standards were already failing the workforce. A unified definition of moral injury must be integrated to frame clinician distress alongside burnout, recentering ethical decision making, rather than profit, at the heart of health care. Harmonizing the definitions of moral injury and clarifying the relationship of moral injury with burnout reduces the need for further reinterpretations, allowing for more robust, easily comparable studies focused on identifying risk factors, as well as rapidly implementing effective mitigation strategies.
1. Griffin BJ, Weber MC, Hinkson KD, et al. Toward a dimensional contextual model of moral injury: a scoping review on healthcare workers. Curr Treat Options Psych. 2023;10:199-216. doi:10.1007/s40501-023-00296-4
2. National Academies of Sciences, Engineering, and Medicine; National Academy of Medicine; Committee on Systems Approaches to Improve Patient Care by Supporting Clinician Well-Being. Taking Action Against Clinician Burnout: A Systems Approach to Professional Well-Being. The National Academies Press; 2019. doi:10.17226/25521
3. Dean W, Talbot S, Dean A. Reframing clinician distress: moral injury not burnout. Fed Pract. 2019;36(9):400-402.
4. Gardner HE, Schulman LS. The professions in America today: crucial but fragile. Daedalus. 2005;134(3):13-18. doi:10.1162/0011526054622132
5. Fuchs VR. Major trends in the U.S. health economy since 1950. N Engl J Med. 2012;366(11):973-977. doi:10.1056/NEJMp1200478
6. Molendijk T. Warnings against romanticising moral injury. Br J Psychiatry. 2022;220(1):1-3. doi:10.1192/bjp.2021.114
7. Shay J. Moral injury. Psychoanalytic Psychol. 2014;31(2):182-191. doi:10.1037/a0036090
8. Litz BT, Stein N, Delaney E, et al. Moral injury and moral repair in war veterans: a preliminary model and intervention strategy. Clin Psychol Rev. 2009;29(8):695-706. doi:10.1016/j.cpr.2009.07.003
9. Brovont v KS-I Med. Servs., P.A., 622 SW3d 671 (Mo Ct App 2020).
1. Griffin BJ, Weber MC, Hinkson KD, et al. Toward a dimensional contextual model of moral injury: a scoping review on healthcare workers. Curr Treat Options Psych. 2023;10:199-216. doi:10.1007/s40501-023-00296-4
2. National Academies of Sciences, Engineering, and Medicine; National Academy of Medicine; Committee on Systems Approaches to Improve Patient Care by Supporting Clinician Well-Being. Taking Action Against Clinician Burnout: A Systems Approach to Professional Well-Being. The National Academies Press; 2019. doi:10.17226/25521
3. Dean W, Talbot S, Dean A. Reframing clinician distress: moral injury not burnout. Fed Pract. 2019;36(9):400-402.
4. Gardner HE, Schulman LS. The professions in America today: crucial but fragile. Daedalus. 2005;134(3):13-18. doi:10.1162/0011526054622132
5. Fuchs VR. Major trends in the U.S. health economy since 1950. N Engl J Med. 2012;366(11):973-977. doi:10.1056/NEJMp1200478
6. Molendijk T. Warnings against romanticising moral injury. Br J Psychiatry. 2022;220(1):1-3. doi:10.1192/bjp.2021.114
7. Shay J. Moral injury. Psychoanalytic Psychol. 2014;31(2):182-191. doi:10.1037/a0036090
8. Litz BT, Stein N, Delaney E, et al. Moral injury and moral repair in war veterans: a preliminary model and intervention strategy. Clin Psychol Rev. 2009;29(8):695-706. doi:10.1016/j.cpr.2009.07.003
9. Brovont v KS-I Med. Servs., P.A., 622 SW3d 671 (Mo Ct App 2020).
How to Cure Hedonic Eating?
Logan is a 62-year-old woman who has reached the pinnacle of professional success. She started a $50 million consumer products company and, after selling it, managed to develop another successful brand. She is healthy and happily married, with four adult children. And yet, despite all her achievements and stable family life, Logan was always bothered by her inability to lose weight.
Despite peddling in beauty, she felt perpetually overweight and, frankly, unattractive. She has no family history of obesity, drinks minimal alcohol, and follows an (allegedly) healthy diet. Logan had tried “everything” to lose weight — human growth hormone injections (not prescribed by me), Ozempic-like medications, Belviq, etc. — all to no avail.
Here’s the catch: After she finished with her busy days of meetings and spreadsheets, Logan sat down to read through countless emails and rewarded herself with all her favorite foods. Without realizing it, she often doubled her daily caloric intake in one sitting. She wasn’t hungry in these moments, rather just a little worn out and perhaps a little careless. She then proceeded to email her doctor (me) to report on this endless cycle of unwanted behavior.
In January 2024, a novel study from Turkey examined the relationship between hedonic eating, self-condemnation, and self-esteem. Surprising to no one, the study determined that higher hedonic hunger scores were associated with lower self-esteem and an increased propensity to self-stigmatize.
Oprah could have handily predicted this conclusion. Many years ago, she described food as a fake friend: Perhaps you’ve had a long and difficult day. While you’re busy eating your feelings, the heaping plate of pasta feels like your best buddy in the world. However, the moment the plate is empty, you realize that you feel worse than before. Not only do you have to unbutton your new jeans, but you also realize that you have just lost your ability to self-regulate.
While the positive association between hedonic eating and low self-esteem may seem self-evident, the solution is less obvious. Mindfulness is one possible approach to this issue. Mindfulness has been described as “paying attention in a particular way: on purpose, in the present moment, and nonjudgmentally” and has existed for thousands of years. Mindful eating, in particular, involves paying close attention to our food choices and how they affect our emotions, and typically includes some combination of:
- Slowing down eating/chewing thoroughly
- Eliminating distractions such as TV, computers, and phones — perhaps even eating in silence
- Eating only until physically satiated
- Distinguishing between true hunger and cravings
- Noticing the texture, flavors, and smell of food
- Paying attention to the effect of food on your mood
- Appreciating food
In our society, where processed food is so readily available and stress is so ubiquitous, eating can become a hedonic and fast-paced activity. Our brains don’t have time to process our bodies’ signals of fullness and, as a result, we often ingest many more calories than we need for a healthy lifestyle.
If mindless eating is part of the problem, mindful eating is part of the solution. Indeed, a meta-review of 10 scientific studies showed that mindful eating is as effective as conventional weight loss programs in regard to body mass index and waist circumference. On the basis of these studies — as well as some good old-fashioned common sense — intuitive eating is an important component of sustainable weight reduction.
Eventually, I convinced Logan to meet up with the psychologist in our group who specializes in emotional eating. Through weekly cognitive-behavioral therapy sessions, Logan was able to understand the impetus behind her self-defeating behavior and has finally been able to reverse some of her lifelong habits. Once she started practicing mindful eating, I was able to introduce Ozempic, and now Logan is happily shedding several pounds a week.
Dr. Messer has disclosed no relevant financial relationships.
Dr. Messer is clinical assistant professor, Mount Sinai School of Medicine and associate professor, Hofstra School of Medicine, both in New York City.
A version of this article first appeared on Medscape.com.
Logan is a 62-year-old woman who has reached the pinnacle of professional success. She started a $50 million consumer products company and, after selling it, managed to develop another successful brand. She is healthy and happily married, with four adult children. And yet, despite all her achievements and stable family life, Logan was always bothered by her inability to lose weight.
Despite peddling in beauty, she felt perpetually overweight and, frankly, unattractive. She has no family history of obesity, drinks minimal alcohol, and follows an (allegedly) healthy diet. Logan had tried “everything” to lose weight — human growth hormone injections (not prescribed by me), Ozempic-like medications, Belviq, etc. — all to no avail.
Here’s the catch: After she finished with her busy days of meetings and spreadsheets, Logan sat down to read through countless emails and rewarded herself with all her favorite foods. Without realizing it, she often doubled her daily caloric intake in one sitting. She wasn’t hungry in these moments, rather just a little worn out and perhaps a little careless. She then proceeded to email her doctor (me) to report on this endless cycle of unwanted behavior.
In January 2024, a novel study from Turkey examined the relationship between hedonic eating, self-condemnation, and self-esteem. Surprising to no one, the study determined that higher hedonic hunger scores were associated with lower self-esteem and an increased propensity to self-stigmatize.
Oprah could have handily predicted this conclusion. Many years ago, she described food as a fake friend: Perhaps you’ve had a long and difficult day. While you’re busy eating your feelings, the heaping plate of pasta feels like your best buddy in the world. However, the moment the plate is empty, you realize that you feel worse than before. Not only do you have to unbutton your new jeans, but you also realize that you have just lost your ability to self-regulate.
While the positive association between hedonic eating and low self-esteem may seem self-evident, the solution is less obvious. Mindfulness is one possible approach to this issue. Mindfulness has been described as “paying attention in a particular way: on purpose, in the present moment, and nonjudgmentally” and has existed for thousands of years. Mindful eating, in particular, involves paying close attention to our food choices and how they affect our emotions, and typically includes some combination of:
- Slowing down eating/chewing thoroughly
- Eliminating distractions such as TV, computers, and phones — perhaps even eating in silence
- Eating only until physically satiated
- Distinguishing between true hunger and cravings
- Noticing the texture, flavors, and smell of food
- Paying attention to the effect of food on your mood
- Appreciating food
In our society, where processed food is so readily available and stress is so ubiquitous, eating can become a hedonic and fast-paced activity. Our brains don’t have time to process our bodies’ signals of fullness and, as a result, we often ingest many more calories than we need for a healthy lifestyle.
If mindless eating is part of the problem, mindful eating is part of the solution. Indeed, a meta-review of 10 scientific studies showed that mindful eating is as effective as conventional weight loss programs in regard to body mass index and waist circumference. On the basis of these studies — as well as some good old-fashioned common sense — intuitive eating is an important component of sustainable weight reduction.
Eventually, I convinced Logan to meet up with the psychologist in our group who specializes in emotional eating. Through weekly cognitive-behavioral therapy sessions, Logan was able to understand the impetus behind her self-defeating behavior and has finally been able to reverse some of her lifelong habits. Once she started practicing mindful eating, I was able to introduce Ozempic, and now Logan is happily shedding several pounds a week.
Dr. Messer has disclosed no relevant financial relationships.
Dr. Messer is clinical assistant professor, Mount Sinai School of Medicine and associate professor, Hofstra School of Medicine, both in New York City.
A version of this article first appeared on Medscape.com.
Logan is a 62-year-old woman who has reached the pinnacle of professional success. She started a $50 million consumer products company and, after selling it, managed to develop another successful brand. She is healthy and happily married, with four adult children. And yet, despite all her achievements and stable family life, Logan was always bothered by her inability to lose weight.
Despite peddling in beauty, she felt perpetually overweight and, frankly, unattractive. She has no family history of obesity, drinks minimal alcohol, and follows an (allegedly) healthy diet. Logan had tried “everything” to lose weight — human growth hormone injections (not prescribed by me), Ozempic-like medications, Belviq, etc. — all to no avail.
Here’s the catch: After she finished with her busy days of meetings and spreadsheets, Logan sat down to read through countless emails and rewarded herself with all her favorite foods. Without realizing it, she often doubled her daily caloric intake in one sitting. She wasn’t hungry in these moments, rather just a little worn out and perhaps a little careless. She then proceeded to email her doctor (me) to report on this endless cycle of unwanted behavior.
In January 2024, a novel study from Turkey examined the relationship between hedonic eating, self-condemnation, and self-esteem. Surprising to no one, the study determined that higher hedonic hunger scores were associated with lower self-esteem and an increased propensity to self-stigmatize.
Oprah could have handily predicted this conclusion. Many years ago, she described food as a fake friend: Perhaps you’ve had a long and difficult day. While you’re busy eating your feelings, the heaping plate of pasta feels like your best buddy in the world. However, the moment the plate is empty, you realize that you feel worse than before. Not only do you have to unbutton your new jeans, but you also realize that you have just lost your ability to self-regulate.
While the positive association between hedonic eating and low self-esteem may seem self-evident, the solution is less obvious. Mindfulness is one possible approach to this issue. Mindfulness has been described as “paying attention in a particular way: on purpose, in the present moment, and nonjudgmentally” and has existed for thousands of years. Mindful eating, in particular, involves paying close attention to our food choices and how they affect our emotions, and typically includes some combination of:
- Slowing down eating/chewing thoroughly
- Eliminating distractions such as TV, computers, and phones — perhaps even eating in silence
- Eating only until physically satiated
- Distinguishing between true hunger and cravings
- Noticing the texture, flavors, and smell of food
- Paying attention to the effect of food on your mood
- Appreciating food
In our society, where processed food is so readily available and stress is so ubiquitous, eating can become a hedonic and fast-paced activity. Our brains don’t have time to process our bodies’ signals of fullness and, as a result, we often ingest many more calories than we need for a healthy lifestyle.
If mindless eating is part of the problem, mindful eating is part of the solution. Indeed, a meta-review of 10 scientific studies showed that mindful eating is as effective as conventional weight loss programs in regard to body mass index and waist circumference. On the basis of these studies — as well as some good old-fashioned common sense — intuitive eating is an important component of sustainable weight reduction.
Eventually, I convinced Logan to meet up with the psychologist in our group who specializes in emotional eating. Through weekly cognitive-behavioral therapy sessions, Logan was able to understand the impetus behind her self-defeating behavior and has finally been able to reverse some of her lifelong habits. Once she started practicing mindful eating, I was able to introduce Ozempic, and now Logan is happily shedding several pounds a week.
Dr. Messer has disclosed no relevant financial relationships.
Dr. Messer is clinical assistant professor, Mount Sinai School of Medicine and associate professor, Hofstra School of Medicine, both in New York City.
A version of this article first appeared on Medscape.com.
Why We Need to Know About Our Patients’ History of Trauma
This case is a little out of the ordinary, but we would love to find out how readers would handle it.
Diana is a 51-year-old woman with a history of depression, obesity, hypertension, type 2 diabetes, and coronary artery disease. She has come in for a routine visit for her chronic illnesses. She seems very distant and has a flat affect during the initial interview. When you ask about any recent stressful events, she begins crying and explains that her daughter was just deported, leaving behind a child and boyfriend.
Their country of origin suffers from chronic instability and violence. Diana’s father was murdered there, and Diana was the victim of sexual assault. “I escaped when I was 18, and I tried to never look back. Until now.” Diana is very worried about her daughter’s return to that country. “I don’t want her to have to endure what I have endured.”
You spend some time discussing the patient’s mental health burden and identify a counselor and online resources that might help. You wonder if Diana’s adverse childhood experiences (ACEs) might have contributed to some of her physical illnesses.
ACEs and Adult Health
One of the most pronounced and straightforward links is that between ACEs and depression. In the Southern Community Cohort Study of more than 38,200 US adults, the highest odds ratio between ACEs and chronic disease was for depression. Persons who reported more than three ACEs had about a twofold increase in the risk for depression compared with persons without ACEs. There was a monotonic increase in the risk for depression and other chronic illnesses as the burden of ACEs increased.
In another study from the United Kingdom, each additional ACE was associated with a significant 11% increase in the risk for incident diabetes during adulthood. Researchers found that both depression symptoms and cardiometabolic dysfunction mediated the effects of ACEs in promoting higher rates of diabetes.
Depression and diabetes are significant risk factors for coronary artery disease, so it is not surprising that ACEs are also associated with a higher risk for coronary events. A review by Godoy and colleagues described how ACEs promote neuroendocrine, autonomic, and inflammatory dysfunction, which in turn leads to higher rates of traditional cardiovascular risk factors such as diabetes and obesity. Ultimately, the presence of four or more ACEs is associated with more than a twofold higher risk for cardiovascular disease compared with no ACEs.
Many of the pathologic processes that promote cardiovascular disease also increase the risk for dementia. Could the reach of ACEs span decades to promote a higher risk for dementia among older adults? A study by Yuan and colleagues of 7222 Chinese adults suggests that the answer is yes. This study divided the cohort into persons with a history of no ACEs, household dysfunction during childhood, or mistreatment during childhood. Child mistreatment was associated with higher rates of diabetes, depression, and cardiovascular disease, as well as an odds ratio of 1.37 (95% CI, 1.12 to 1.68) for cognitive impairment.
The magnitude of the effects ACEs can have on well-being is reinforced by epidemiologic data surrounding ACEs. According to the US Centers for Disease Control and Prevention (CDC), 64% of US adults report at least one ACE and 17% experienced at least four ACEs. Risk factors for ACEs include being female, American Indian or Alaska Native, or unemployed.
How do we reduce the impact of ACEs? Prevention is key. The CDC estimates that nearly 2 million cases of adult heart disease and more than 20 million cases of adult depression could be avoided if ACEs were eliminated.
But what is the best means to pragmatically reduce ACEs in our current practice models? How do we discover a history of ACEs in patients, and what are the best practices in managing persons with a positive history? We will cover these critical subjects in a future article, but for now, please provide your own comments and pearls regarding the prevention and management of ACEs.
Dr. Vega, health sciences clinical professor, family medicine, University of California, Irvine, disclosed ties with GlaxoSmithKline and Johnson and Johnson. Ms. Hurtado, MD candidate, University of California, Irvine School of Medicine, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This case is a little out of the ordinary, but we would love to find out how readers would handle it.
Diana is a 51-year-old woman with a history of depression, obesity, hypertension, type 2 diabetes, and coronary artery disease. She has come in for a routine visit for her chronic illnesses. She seems very distant and has a flat affect during the initial interview. When you ask about any recent stressful events, she begins crying and explains that her daughter was just deported, leaving behind a child and boyfriend.
Their country of origin suffers from chronic instability and violence. Diana’s father was murdered there, and Diana was the victim of sexual assault. “I escaped when I was 18, and I tried to never look back. Until now.” Diana is very worried about her daughter’s return to that country. “I don’t want her to have to endure what I have endured.”
You spend some time discussing the patient’s mental health burden and identify a counselor and online resources that might help. You wonder if Diana’s adverse childhood experiences (ACEs) might have contributed to some of her physical illnesses.
ACEs and Adult Health
One of the most pronounced and straightforward links is that between ACEs and depression. In the Southern Community Cohort Study of more than 38,200 US adults, the highest odds ratio between ACEs and chronic disease was for depression. Persons who reported more than three ACEs had about a twofold increase in the risk for depression compared with persons without ACEs. There was a monotonic increase in the risk for depression and other chronic illnesses as the burden of ACEs increased.
In another study from the United Kingdom, each additional ACE was associated with a significant 11% increase in the risk for incident diabetes during adulthood. Researchers found that both depression symptoms and cardiometabolic dysfunction mediated the effects of ACEs in promoting higher rates of diabetes.
Depression and diabetes are significant risk factors for coronary artery disease, so it is not surprising that ACEs are also associated with a higher risk for coronary events. A review by Godoy and colleagues described how ACEs promote neuroendocrine, autonomic, and inflammatory dysfunction, which in turn leads to higher rates of traditional cardiovascular risk factors such as diabetes and obesity. Ultimately, the presence of four or more ACEs is associated with more than a twofold higher risk for cardiovascular disease compared with no ACEs.
Many of the pathologic processes that promote cardiovascular disease also increase the risk for dementia. Could the reach of ACEs span decades to promote a higher risk for dementia among older adults? A study by Yuan and colleagues of 7222 Chinese adults suggests that the answer is yes. This study divided the cohort into persons with a history of no ACEs, household dysfunction during childhood, or mistreatment during childhood. Child mistreatment was associated with higher rates of diabetes, depression, and cardiovascular disease, as well as an odds ratio of 1.37 (95% CI, 1.12 to 1.68) for cognitive impairment.
The magnitude of the effects ACEs can have on well-being is reinforced by epidemiologic data surrounding ACEs. According to the US Centers for Disease Control and Prevention (CDC), 64% of US adults report at least one ACE and 17% experienced at least four ACEs. Risk factors for ACEs include being female, American Indian or Alaska Native, or unemployed.
How do we reduce the impact of ACEs? Prevention is key. The CDC estimates that nearly 2 million cases of adult heart disease and more than 20 million cases of adult depression could be avoided if ACEs were eliminated.
But what is the best means to pragmatically reduce ACEs in our current practice models? How do we discover a history of ACEs in patients, and what are the best practices in managing persons with a positive history? We will cover these critical subjects in a future article, but for now, please provide your own comments and pearls regarding the prevention and management of ACEs.
Dr. Vega, health sciences clinical professor, family medicine, University of California, Irvine, disclosed ties with GlaxoSmithKline and Johnson and Johnson. Ms. Hurtado, MD candidate, University of California, Irvine School of Medicine, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This case is a little out of the ordinary, but we would love to find out how readers would handle it.
Diana is a 51-year-old woman with a history of depression, obesity, hypertension, type 2 diabetes, and coronary artery disease. She has come in for a routine visit for her chronic illnesses. She seems very distant and has a flat affect during the initial interview. When you ask about any recent stressful events, she begins crying and explains that her daughter was just deported, leaving behind a child and boyfriend.
Their country of origin suffers from chronic instability and violence. Diana’s father was murdered there, and Diana was the victim of sexual assault. “I escaped when I was 18, and I tried to never look back. Until now.” Diana is very worried about her daughter’s return to that country. “I don’t want her to have to endure what I have endured.”
You spend some time discussing the patient’s mental health burden and identify a counselor and online resources that might help. You wonder if Diana’s adverse childhood experiences (ACEs) might have contributed to some of her physical illnesses.
ACEs and Adult Health
One of the most pronounced and straightforward links is that between ACEs and depression. In the Southern Community Cohort Study of more than 38,200 US adults, the highest odds ratio between ACEs and chronic disease was for depression. Persons who reported more than three ACEs had about a twofold increase in the risk for depression compared with persons without ACEs. There was a monotonic increase in the risk for depression and other chronic illnesses as the burden of ACEs increased.
In another study from the United Kingdom, each additional ACE was associated with a significant 11% increase in the risk for incident diabetes during adulthood. Researchers found that both depression symptoms and cardiometabolic dysfunction mediated the effects of ACEs in promoting higher rates of diabetes.
Depression and diabetes are significant risk factors for coronary artery disease, so it is not surprising that ACEs are also associated with a higher risk for coronary events. A review by Godoy and colleagues described how ACEs promote neuroendocrine, autonomic, and inflammatory dysfunction, which in turn leads to higher rates of traditional cardiovascular risk factors such as diabetes and obesity. Ultimately, the presence of four or more ACEs is associated with more than a twofold higher risk for cardiovascular disease compared with no ACEs.
Many of the pathologic processes that promote cardiovascular disease also increase the risk for dementia. Could the reach of ACEs span decades to promote a higher risk for dementia among older adults? A study by Yuan and colleagues of 7222 Chinese adults suggests that the answer is yes. This study divided the cohort into persons with a history of no ACEs, household dysfunction during childhood, or mistreatment during childhood. Child mistreatment was associated with higher rates of diabetes, depression, and cardiovascular disease, as well as an odds ratio of 1.37 (95% CI, 1.12 to 1.68) for cognitive impairment.
The magnitude of the effects ACEs can have on well-being is reinforced by epidemiologic data surrounding ACEs. According to the US Centers for Disease Control and Prevention (CDC), 64% of US adults report at least one ACE and 17% experienced at least four ACEs. Risk factors for ACEs include being female, American Indian or Alaska Native, or unemployed.
How do we reduce the impact of ACEs? Prevention is key. The CDC estimates that nearly 2 million cases of adult heart disease and more than 20 million cases of adult depression could be avoided if ACEs were eliminated.
But what is the best means to pragmatically reduce ACEs in our current practice models? How do we discover a history of ACEs in patients, and what are the best practices in managing persons with a positive history? We will cover these critical subjects in a future article, but for now, please provide your own comments and pearls regarding the prevention and management of ACEs.
Dr. Vega, health sciences clinical professor, family medicine, University of California, Irvine, disclosed ties with GlaxoSmithKline and Johnson and Johnson. Ms. Hurtado, MD candidate, University of California, Irvine School of Medicine, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
A Banned Chemical That Is Still Causing Cancer
This transcript has been edited for clarity.
These types of stories usually end with a call for regulation — to ban said chemical or substance, or to regulate it — but in this case, that has already happened. This new carcinogen I’m telling you about is actually an old chemical. And it has not been manufactured or legally imported in the US since 2013.
So, why bother? Because in this case, the chemical — or, really, a group of chemicals called polybrominated diphenyl ethers (PBDEs) — are still around: in our soil, in our food, and in our blood.
PBDEs are a group of compounds that confer flame-retardant properties to plastics, and they were used extensively in the latter part of the 20th century in electronic enclosures, business equipment, and foam cushioning in upholstery.
But there was a problem. They don’t chemically bond to plastics; they are just sort of mixed in, which means they can leach out. They are hydrophobic, meaning they don’t get washed out of soil, and, when ingested or inhaled by humans, they dissolve in our fat stores, making it difficult for our normal excretory systems to excrete them.
PBDEs biomagnify. Small animals can take them up from contaminated soil or water, and those animals are eaten by larger animals, which accumulate higher concentrations of the chemicals. This bioaccumulation increases as you move up the food web until you get to an apex predator — like you and me.
This is true of lots of chemicals, of course. The concern arises when these chemicals are toxic. To date, the toxicity data for PBDEs were pretty limited. There were some animal studies where rats were exposed to extremely high doses and they developed liver lesions — but I am always very wary of extrapolating high-dose rat toxicity studies to humans. There was also some suggestion that the chemicals could be endocrine disruptors, affecting breast and thyroid tissue.
What about cancer? In 2016, the International Agency for Research on Cancer concluded there was “inadequate evidence in humans for the carcinogencity of” PBDEs.
In the same report, though, they suggested PBDEs are “probably carcinogenic to humans” based on mechanistic studies.
In other words, we can’t prove they’re cancerous — but come on, they probably are.
Finally, we have some evidence that really pushes us toward the carcinogenic conclusion, in the form of this study, appearing in JAMA Network Open. It’s a nice bit of epidemiology leveraging the population-based National Health and Nutrition Examination Survey (NHANES).
Researchers measured PBDE levels in blood samples from 1100 people enrolled in NHANES in 2003 and 2004 and linked them to death records collected over the next 20 years or so.
The first thing to note is that the researchers were able to measure PBDEs in the blood samples. They were in there. They were detectable. And they were variable. Dividing the 1100 participants into low, medium, and high PBDE tertiles, you can see a nearly 10-fold difference across the population.
Importantly, not many baseline variables correlated with PBDE levels. People in the highest group were a bit younger but had a fairly similar sex distribution, race, ethnicity, education, income, physical activity, smoking status, and body mass index.
This is not a randomized trial, of course — but at least based on these data, exposure levels do seem fairly random, which is what you would expect from an environmental toxin that percolates up through the food chain. They are often somewhat indiscriminate.
This similarity in baseline characteristics between people with low or high blood levels of PBDE also allows us to make some stronger inferences about the observed outcomes. Let’s take a look at them.
After adjustment for baseline factors, individuals in the highest PBDE group had a 43% higher rate of death from any cause over the follow-up period. This was not enough to achieve statistical significance, but it was close.
But the key finding is deaths due to cancer. After adjustment, cancer deaths occurred four times as frequently among those in the high PBDE group, and that is a statistically significant difference.
To be fair, cancer deaths were rare in this cohort. The vast majority of people did not die of anything during the follow-up period regardless of PBDE level. But the data are strongly suggestive of the carcinogenicity of these chemicals.
I should also point out that the researchers are linking the PBDE level at a single time point to all these future events. If PBDE levels remain relatively stable within an individual over time, that’s fine, but if they tend to vary with intake of different foods for example, this would not be captured and would actually lead to an underestimation of the cancer risk.
The researchers also didn’t have granular enough data to determine the type of cancer, but they do show that rates are similar between men and women, which might point away from the more sex-specific cancer etiologies. Clearly, some more work is needed.
Of course, I started this piece by telling you that these chemicals are already pretty much banned in the United States. What are we supposed to do about these findings? Studies have examined the primary ongoing sources of PBDE in our environment and it seems like most of our exposure will be coming from the food we eat due to that biomagnification thing: high-fat fish, meat and dairy products, and fish oil supplements. It may be worth some investigation into the relative adulteration of these products with this new old carcinogen.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
These types of stories usually end with a call for regulation — to ban said chemical or substance, or to regulate it — but in this case, that has already happened. This new carcinogen I’m telling you about is actually an old chemical. And it has not been manufactured or legally imported in the US since 2013.
So, why bother? Because in this case, the chemical — or, really, a group of chemicals called polybrominated diphenyl ethers (PBDEs) — are still around: in our soil, in our food, and in our blood.
PBDEs are a group of compounds that confer flame-retardant properties to plastics, and they were used extensively in the latter part of the 20th century in electronic enclosures, business equipment, and foam cushioning in upholstery.
But there was a problem. They don’t chemically bond to plastics; they are just sort of mixed in, which means they can leach out. They are hydrophobic, meaning they don’t get washed out of soil, and, when ingested or inhaled by humans, they dissolve in our fat stores, making it difficult for our normal excretory systems to excrete them.
PBDEs biomagnify. Small animals can take them up from contaminated soil or water, and those animals are eaten by larger animals, which accumulate higher concentrations of the chemicals. This bioaccumulation increases as you move up the food web until you get to an apex predator — like you and me.
This is true of lots of chemicals, of course. The concern arises when these chemicals are toxic. To date, the toxicity data for PBDEs were pretty limited. There were some animal studies where rats were exposed to extremely high doses and they developed liver lesions — but I am always very wary of extrapolating high-dose rat toxicity studies to humans. There was also some suggestion that the chemicals could be endocrine disruptors, affecting breast and thyroid tissue.
What about cancer? In 2016, the International Agency for Research on Cancer concluded there was “inadequate evidence in humans for the carcinogencity of” PBDEs.
In the same report, though, they suggested PBDEs are “probably carcinogenic to humans” based on mechanistic studies.
In other words, we can’t prove they’re cancerous — but come on, they probably are.
Finally, we have some evidence that really pushes us toward the carcinogenic conclusion, in the form of this study, appearing in JAMA Network Open. It’s a nice bit of epidemiology leveraging the population-based National Health and Nutrition Examination Survey (NHANES).
Researchers measured PBDE levels in blood samples from 1100 people enrolled in NHANES in 2003 and 2004 and linked them to death records collected over the next 20 years or so.
The first thing to note is that the researchers were able to measure PBDEs in the blood samples. They were in there. They were detectable. And they were variable. Dividing the 1100 participants into low, medium, and high PBDE tertiles, you can see a nearly 10-fold difference across the population.
Importantly, not many baseline variables correlated with PBDE levels. People in the highest group were a bit younger but had a fairly similar sex distribution, race, ethnicity, education, income, physical activity, smoking status, and body mass index.
This is not a randomized trial, of course — but at least based on these data, exposure levels do seem fairly random, which is what you would expect from an environmental toxin that percolates up through the food chain. They are often somewhat indiscriminate.
This similarity in baseline characteristics between people with low or high blood levels of PBDE also allows us to make some stronger inferences about the observed outcomes. Let’s take a look at them.
After adjustment for baseline factors, individuals in the highest PBDE group had a 43% higher rate of death from any cause over the follow-up period. This was not enough to achieve statistical significance, but it was close.
But the key finding is deaths due to cancer. After adjustment, cancer deaths occurred four times as frequently among those in the high PBDE group, and that is a statistically significant difference.
To be fair, cancer deaths were rare in this cohort. The vast majority of people did not die of anything during the follow-up period regardless of PBDE level. But the data are strongly suggestive of the carcinogenicity of these chemicals.
I should also point out that the researchers are linking the PBDE level at a single time point to all these future events. If PBDE levels remain relatively stable within an individual over time, that’s fine, but if they tend to vary with intake of different foods for example, this would not be captured and would actually lead to an underestimation of the cancer risk.
The researchers also didn’t have granular enough data to determine the type of cancer, but they do show that rates are similar between men and women, which might point away from the more sex-specific cancer etiologies. Clearly, some more work is needed.
Of course, I started this piece by telling you that these chemicals are already pretty much banned in the United States. What are we supposed to do about these findings? Studies have examined the primary ongoing sources of PBDE in our environment and it seems like most of our exposure will be coming from the food we eat due to that biomagnification thing: high-fat fish, meat and dairy products, and fish oil supplements. It may be worth some investigation into the relative adulteration of these products with this new old carcinogen.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
These types of stories usually end with a call for regulation — to ban said chemical or substance, or to regulate it — but in this case, that has already happened. This new carcinogen I’m telling you about is actually an old chemical. And it has not been manufactured or legally imported in the US since 2013.
So, why bother? Because in this case, the chemical — or, really, a group of chemicals called polybrominated diphenyl ethers (PBDEs) — are still around: in our soil, in our food, and in our blood.
PBDEs are a group of compounds that confer flame-retardant properties to plastics, and they were used extensively in the latter part of the 20th century in electronic enclosures, business equipment, and foam cushioning in upholstery.
But there was a problem. They don’t chemically bond to plastics; they are just sort of mixed in, which means they can leach out. They are hydrophobic, meaning they don’t get washed out of soil, and, when ingested or inhaled by humans, they dissolve in our fat stores, making it difficult for our normal excretory systems to excrete them.
PBDEs biomagnify. Small animals can take them up from contaminated soil or water, and those animals are eaten by larger animals, which accumulate higher concentrations of the chemicals. This bioaccumulation increases as you move up the food web until you get to an apex predator — like you and me.
This is true of lots of chemicals, of course. The concern arises when these chemicals are toxic. To date, the toxicity data for PBDEs were pretty limited. There were some animal studies where rats were exposed to extremely high doses and they developed liver lesions — but I am always very wary of extrapolating high-dose rat toxicity studies to humans. There was also some suggestion that the chemicals could be endocrine disruptors, affecting breast and thyroid tissue.
What about cancer? In 2016, the International Agency for Research on Cancer concluded there was “inadequate evidence in humans for the carcinogencity of” PBDEs.
In the same report, though, they suggested PBDEs are “probably carcinogenic to humans” based on mechanistic studies.
In other words, we can’t prove they’re cancerous — but come on, they probably are.
Finally, we have some evidence that really pushes us toward the carcinogenic conclusion, in the form of this study, appearing in JAMA Network Open. It’s a nice bit of epidemiology leveraging the population-based National Health and Nutrition Examination Survey (NHANES).
Researchers measured PBDE levels in blood samples from 1100 people enrolled in NHANES in 2003 and 2004 and linked them to death records collected over the next 20 years or so.
The first thing to note is that the researchers were able to measure PBDEs in the blood samples. They were in there. They were detectable. And they were variable. Dividing the 1100 participants into low, medium, and high PBDE tertiles, you can see a nearly 10-fold difference across the population.
Importantly, not many baseline variables correlated with PBDE levels. People in the highest group were a bit younger but had a fairly similar sex distribution, race, ethnicity, education, income, physical activity, smoking status, and body mass index.
This is not a randomized trial, of course — but at least based on these data, exposure levels do seem fairly random, which is what you would expect from an environmental toxin that percolates up through the food chain. They are often somewhat indiscriminate.
This similarity in baseline characteristics between people with low or high blood levels of PBDE also allows us to make some stronger inferences about the observed outcomes. Let’s take a look at them.
After adjustment for baseline factors, individuals in the highest PBDE group had a 43% higher rate of death from any cause over the follow-up period. This was not enough to achieve statistical significance, but it was close.
But the key finding is deaths due to cancer. After adjustment, cancer deaths occurred four times as frequently among those in the high PBDE group, and that is a statistically significant difference.
To be fair, cancer deaths were rare in this cohort. The vast majority of people did not die of anything during the follow-up period regardless of PBDE level. But the data are strongly suggestive of the carcinogenicity of these chemicals.
I should also point out that the researchers are linking the PBDE level at a single time point to all these future events. If PBDE levels remain relatively stable within an individual over time, that’s fine, but if they tend to vary with intake of different foods for example, this would not be captured and would actually lead to an underestimation of the cancer risk.
The researchers also didn’t have granular enough data to determine the type of cancer, but they do show that rates are similar between men and women, which might point away from the more sex-specific cancer etiologies. Clearly, some more work is needed.
Of course, I started this piece by telling you that these chemicals are already pretty much banned in the United States. What are we supposed to do about these findings? Studies have examined the primary ongoing sources of PBDE in our environment and it seems like most of our exposure will be coming from the food we eat due to that biomagnification thing: high-fat fish, meat and dairy products, and fish oil supplements. It may be worth some investigation into the relative adulteration of these products with this new old carcinogen.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
What We’ve Learned About Remote Learning
I would have preferred to start this Letter reporting to you that the pandemic is fading out of sight in our rear view mirror. However, I think it is more accurate to say the pandemic is sitting in that blind spot off our passenger side rear fender. Unless you’re like one of those cars with “blind spot detection” blinking a warning, you probably aren’t giving the pandemic much thought. However, three journalists at The New York Times have taken this lull in the pandemic’s newsworthiness to consider the consequences of school closure and remote learning.
From what you may have read and heard, and possibly experienced firsthand, you have a sense that keeping children out of school has been awash in negatives. These journalists looked at all the data they could find and their article is replete with graphs and references. I will just summarize some of what they discovered.
“While poverty and other factors played a role, remote learning was a key driver in academic declines ...” They found there was a direct relationship between the length of school closure and the severity of academic skill loss. The journalists noted that “some time in school was better than no time.” And sadly, “most students have not caught up.”
Poverty played a significant role, with students in economically challenged communities experiencing steeper losses in academics. The reporters quoted Stanford Professor Sean F. Reardon, EdD, who has said “A community’s poverty rate and length of school closures had a ‘roughly equal’ effect.” Poorer school districts tended to continue remote learning longer than those in more well off communities.
At the very beginning of the pandemic, when we were floating in a sea of unknowns, the decision to close schools and take advantage of the new technology that made remote learning possible sounded like the best and maybe only option. However, looking back, Dr. Sean O’Leary, who helped craft AAP guidelines, admits “we probably kept schools closed longer than we should have.”
Early signs that children were not as likely as adults to get sick, and that students posed little threat to others in the school environment, were not taken seriously enough. Too much time and energy was wasted in deep cleaning even after it was clear the virus was airborne. Opening windows that had been painted shut would have been a much better investment.
As it became more apparent that school closures were not having the deterrent effect we had hoped for, there were still communities that resisted. The Times’ reporters noted that teachers’ unions and Democratic cities tended to be more cautious about reopening. And clearly there was political flavor to how communities responded. Masking is probably one of the best examples where emotions and politics colored our responses.
Are there things we could have done differently? One can certainly understand why teachers might have been cautious about returning to in-school learning. With more than a quarter of teachers in this country being older than 50 (16% over 55) and nearly 80% of elementary and middle school teachers self-reporting that they are obese or overweight, educators represent a group that we know now is more vulnerable to complications from COVID. In retrospect, had we understood more about the virus and the downsides of remote learning, the government could have offered paid leave to teachers who felt vulnerable. Then, by expediting the transition of the younger, less vulnerable college students in their final years of training into the workforce earlier could have kept schools open until we were up to speed with vaccines and treatment. But the water has spilled over the dam. We can hope that we as a nation have learned that making frequent evaluations of our strategies and being flexible enough to make changes will help in future pandemics. Unfortunately, those RNA viruses are fast mutators and clever adapters. Strategies we thought were working the first time may not succeed with new variants.
We have now learned that, in general, remote learning was a bust. My grandkids knew it at the time. It’s not just the learning piece. It’s about the social contact with peers that can provide comfort and support when the adults around at home may be anxious and depressed. School is a place you can be physically active away from 24/7 television at home. Adapting to going to school can be difficult for some young children in the beginning because of separation anxiety, but for the vast majority of children doing the school thing is a habit that is quickly rewarded and reinforced daily.
Children learn in school because they are rubbing elbows with other kids who are learning. While some peers may be distracting, the data suggest the distractions of home are far more of a problem. Most children I know were eager to get back in school because that’s where their friends were. But, getting back in the habit of going to school can be difficult for some, especially those who have been less successful in the past. Not surprisingly, the longer the hiatus the more difficult the reentry becomes.
The big lesson we mustn’t forget is that being in school is far more valuable than we ever imagined. And, when we are considering our options in future pandemics and natural disasters, we should be giving much more weight to in-school learning than we have in the past.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I would have preferred to start this Letter reporting to you that the pandemic is fading out of sight in our rear view mirror. However, I think it is more accurate to say the pandemic is sitting in that blind spot off our passenger side rear fender. Unless you’re like one of those cars with “blind spot detection” blinking a warning, you probably aren’t giving the pandemic much thought. However, three journalists at The New York Times have taken this lull in the pandemic’s newsworthiness to consider the consequences of school closure and remote learning.
From what you may have read and heard, and possibly experienced firsthand, you have a sense that keeping children out of school has been awash in negatives. These journalists looked at all the data they could find and their article is replete with graphs and references. I will just summarize some of what they discovered.
“While poverty and other factors played a role, remote learning was a key driver in academic declines ...” They found there was a direct relationship between the length of school closure and the severity of academic skill loss. The journalists noted that “some time in school was better than no time.” And sadly, “most students have not caught up.”
Poverty played a significant role, with students in economically challenged communities experiencing steeper losses in academics. The reporters quoted Stanford Professor Sean F. Reardon, EdD, who has said “A community’s poverty rate and length of school closures had a ‘roughly equal’ effect.” Poorer school districts tended to continue remote learning longer than those in more well off communities.
At the very beginning of the pandemic, when we were floating in a sea of unknowns, the decision to close schools and take advantage of the new technology that made remote learning possible sounded like the best and maybe only option. However, looking back, Dr. Sean O’Leary, who helped craft AAP guidelines, admits “we probably kept schools closed longer than we should have.”
Early signs that children were not as likely as adults to get sick, and that students posed little threat to others in the school environment, were not taken seriously enough. Too much time and energy was wasted in deep cleaning even after it was clear the virus was airborne. Opening windows that had been painted shut would have been a much better investment.
As it became more apparent that school closures were not having the deterrent effect we had hoped for, there were still communities that resisted. The Times’ reporters noted that teachers’ unions and Democratic cities tended to be more cautious about reopening. And clearly there was political flavor to how communities responded. Masking is probably one of the best examples where emotions and politics colored our responses.
Are there things we could have done differently? One can certainly understand why teachers might have been cautious about returning to in-school learning. With more than a quarter of teachers in this country being older than 50 (16% over 55) and nearly 80% of elementary and middle school teachers self-reporting that they are obese or overweight, educators represent a group that we know now is more vulnerable to complications from COVID. In retrospect, had we understood more about the virus and the downsides of remote learning, the government could have offered paid leave to teachers who felt vulnerable. Then, by expediting the transition of the younger, less vulnerable college students in their final years of training into the workforce earlier could have kept schools open until we were up to speed with vaccines and treatment. But the water has spilled over the dam. We can hope that we as a nation have learned that making frequent evaluations of our strategies and being flexible enough to make changes will help in future pandemics. Unfortunately, those RNA viruses are fast mutators and clever adapters. Strategies we thought were working the first time may not succeed with new variants.
We have now learned that, in general, remote learning was a bust. My grandkids knew it at the time. It’s not just the learning piece. It’s about the social contact with peers that can provide comfort and support when the adults around at home may be anxious and depressed. School is a place you can be physically active away from 24/7 television at home. Adapting to going to school can be difficult for some young children in the beginning because of separation anxiety, but for the vast majority of children doing the school thing is a habit that is quickly rewarded and reinforced daily.
Children learn in school because they are rubbing elbows with other kids who are learning. While some peers may be distracting, the data suggest the distractions of home are far more of a problem. Most children I know were eager to get back in school because that’s where their friends were. But, getting back in the habit of going to school can be difficult for some, especially those who have been less successful in the past. Not surprisingly, the longer the hiatus the more difficult the reentry becomes.
The big lesson we mustn’t forget is that being in school is far more valuable than we ever imagined. And, when we are considering our options in future pandemics and natural disasters, we should be giving much more weight to in-school learning than we have in the past.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I would have preferred to start this Letter reporting to you that the pandemic is fading out of sight in our rear view mirror. However, I think it is more accurate to say the pandemic is sitting in that blind spot off our passenger side rear fender. Unless you’re like one of those cars with “blind spot detection” blinking a warning, you probably aren’t giving the pandemic much thought. However, three journalists at The New York Times have taken this lull in the pandemic’s newsworthiness to consider the consequences of school closure and remote learning.
From what you may have read and heard, and possibly experienced firsthand, you have a sense that keeping children out of school has been awash in negatives. These journalists looked at all the data they could find and their article is replete with graphs and references. I will just summarize some of what they discovered.
“While poverty and other factors played a role, remote learning was a key driver in academic declines ...” They found there was a direct relationship between the length of school closure and the severity of academic skill loss. The journalists noted that “some time in school was better than no time.” And sadly, “most students have not caught up.”
Poverty played a significant role, with students in economically challenged communities experiencing steeper losses in academics. The reporters quoted Stanford Professor Sean F. Reardon, EdD, who has said “A community’s poverty rate and length of school closures had a ‘roughly equal’ effect.” Poorer school districts tended to continue remote learning longer than those in more well off communities.
At the very beginning of the pandemic, when we were floating in a sea of unknowns, the decision to close schools and take advantage of the new technology that made remote learning possible sounded like the best and maybe only option. However, looking back, Dr. Sean O’Leary, who helped craft AAP guidelines, admits “we probably kept schools closed longer than we should have.”
Early signs that children were not as likely as adults to get sick, and that students posed little threat to others in the school environment, were not taken seriously enough. Too much time and energy was wasted in deep cleaning even after it was clear the virus was airborne. Opening windows that had been painted shut would have been a much better investment.
As it became more apparent that school closures were not having the deterrent effect we had hoped for, there were still communities that resisted. The Times’ reporters noted that teachers’ unions and Democratic cities tended to be more cautious about reopening. And clearly there was political flavor to how communities responded. Masking is probably one of the best examples where emotions and politics colored our responses.
Are there things we could have done differently? One can certainly understand why teachers might have been cautious about returning to in-school learning. With more than a quarter of teachers in this country being older than 50 (16% over 55) and nearly 80% of elementary and middle school teachers self-reporting that they are obese or overweight, educators represent a group that we know now is more vulnerable to complications from COVID. In retrospect, had we understood more about the virus and the downsides of remote learning, the government could have offered paid leave to teachers who felt vulnerable. Then, by expediting the transition of the younger, less vulnerable college students in their final years of training into the workforce earlier could have kept schools open until we were up to speed with vaccines and treatment. But the water has spilled over the dam. We can hope that we as a nation have learned that making frequent evaluations of our strategies and being flexible enough to make changes will help in future pandemics. Unfortunately, those RNA viruses are fast mutators and clever adapters. Strategies we thought were working the first time may not succeed with new variants.
We have now learned that, in general, remote learning was a bust. My grandkids knew it at the time. It’s not just the learning piece. It’s about the social contact with peers that can provide comfort and support when the adults around at home may be anxious and depressed. School is a place you can be physically active away from 24/7 television at home. Adapting to going to school can be difficult for some young children in the beginning because of separation anxiety, but for the vast majority of children doing the school thing is a habit that is quickly rewarded and reinforced daily.
Children learn in school because they are rubbing elbows with other kids who are learning. While some peers may be distracting, the data suggest the distractions of home are far more of a problem. Most children I know were eager to get back in school because that’s where their friends were. But, getting back in the habit of going to school can be difficult for some, especially those who have been less successful in the past. Not surprisingly, the longer the hiatus the more difficult the reentry becomes.
The big lesson we mustn’t forget is that being in school is far more valuable than we ever imagined. And, when we are considering our options in future pandemics and natural disasters, we should be giving much more weight to in-school learning than we have in the past.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
When Does a Disease Become Its Own Specialty?
Once upon a time, treating multiple sclerosis (MS) was easy — steroids.
Then, in the 1990s, came Betaseron, then Avonex, then Copaxone. Suddenly we had three options to choose from, though overall roughly similar in efficacy (yeah, I’m leaving Novantrone out; it’s a niche drug). Treatment required some decision making, though not a huge amount. I usually laid out the different schedules and side effect to patients and let them decide.
MS treatment was uncomplicated enough that I knew family doctors who treated MS patients on their own, and I can’t say I could have done any better. If you’ve got a clear MRI, then prescribe Betaseron and hope.
Then came Rebif, then Tysabri, and then pretty much an explosion of new drugs which hasn’t slowed down. Next up are the BTK agents. An embarrassment of riches, though for patients, their families, and neurologists, a very welcome one.
But as more drugs come out, with different mechanisms of action and monitoring requirements, the treatment of MS becomes more complicated, slowly moving from the realm of a general neurologist to an MS subspecialist.
At some point it raises the question of when does a disease become its own specialty? Perhaps this is a bit of hyperbole — I’m pretty sure I’ll be seeing MS patients for a long time to come — but it’s a valid point. Especially as further research may subdivide MS treatment by genetics and other breakdowns.
Alzheimer’s disease may follow a similar (albeit very welcome) trajectory. While nothing really game-changing has come out in the 20 years, the number of new drugs and different mechanisms of action in development is large. Granted, not all of them will work, but hopefully some will. At some point it may come down to treating patients with a cocktail of drugs with separate ways of managing the disease, with guidance based on genetic or clinical profiles.
And that’s a good thing, but it may, again, move the disease from the province of general neurologists to subspecialists. Maybe that would be a good, maybe not. Probably will depend on the patient, their families, and other factors.
Of course, I may be overthinking this. The number of drugs we have for MS is nothing compared with the available treatments we have for hypertension, yet it’s certainly well within the capabilities of most internists to treat without referring to a cardiologist or nephrologist.
Perhaps the new drugs won’t make a difference except in a handful of cases. As new drugs come out we also move on from the old ones, dropping them from our mental armamentarium except in rare cases. When was the last time you prescribed Betaseron?
These drugs are very welcome, and very needed. I will be happy if we can beat back some of the diseases neurologist see, regardless of whom the patients and up seeing.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Once upon a time, treating multiple sclerosis (MS) was easy — steroids.
Then, in the 1990s, came Betaseron, then Avonex, then Copaxone. Suddenly we had three options to choose from, though overall roughly similar in efficacy (yeah, I’m leaving Novantrone out; it’s a niche drug). Treatment required some decision making, though not a huge amount. I usually laid out the different schedules and side effect to patients and let them decide.
MS treatment was uncomplicated enough that I knew family doctors who treated MS patients on their own, and I can’t say I could have done any better. If you’ve got a clear MRI, then prescribe Betaseron and hope.
Then came Rebif, then Tysabri, and then pretty much an explosion of new drugs which hasn’t slowed down. Next up are the BTK agents. An embarrassment of riches, though for patients, their families, and neurologists, a very welcome one.
But as more drugs come out, with different mechanisms of action and monitoring requirements, the treatment of MS becomes more complicated, slowly moving from the realm of a general neurologist to an MS subspecialist.
At some point it raises the question of when does a disease become its own specialty? Perhaps this is a bit of hyperbole — I’m pretty sure I’ll be seeing MS patients for a long time to come — but it’s a valid point. Especially as further research may subdivide MS treatment by genetics and other breakdowns.
Alzheimer’s disease may follow a similar (albeit very welcome) trajectory. While nothing really game-changing has come out in the 20 years, the number of new drugs and different mechanisms of action in development is large. Granted, not all of them will work, but hopefully some will. At some point it may come down to treating patients with a cocktail of drugs with separate ways of managing the disease, with guidance based on genetic or clinical profiles.
And that’s a good thing, but it may, again, move the disease from the province of general neurologists to subspecialists. Maybe that would be a good, maybe not. Probably will depend on the patient, their families, and other factors.
Of course, I may be overthinking this. The number of drugs we have for MS is nothing compared with the available treatments we have for hypertension, yet it’s certainly well within the capabilities of most internists to treat without referring to a cardiologist or nephrologist.
Perhaps the new drugs won’t make a difference except in a handful of cases. As new drugs come out we also move on from the old ones, dropping them from our mental armamentarium except in rare cases. When was the last time you prescribed Betaseron?
These drugs are very welcome, and very needed. I will be happy if we can beat back some of the diseases neurologist see, regardless of whom the patients and up seeing.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Once upon a time, treating multiple sclerosis (MS) was easy — steroids.
Then, in the 1990s, came Betaseron, then Avonex, then Copaxone. Suddenly we had three options to choose from, though overall roughly similar in efficacy (yeah, I’m leaving Novantrone out; it’s a niche drug). Treatment required some decision making, though not a huge amount. I usually laid out the different schedules and side effect to patients and let them decide.
MS treatment was uncomplicated enough that I knew family doctors who treated MS patients on their own, and I can’t say I could have done any better. If you’ve got a clear MRI, then prescribe Betaseron and hope.
Then came Rebif, then Tysabri, and then pretty much an explosion of new drugs which hasn’t slowed down. Next up are the BTK agents. An embarrassment of riches, though for patients, their families, and neurologists, a very welcome one.
But as more drugs come out, with different mechanisms of action and monitoring requirements, the treatment of MS becomes more complicated, slowly moving from the realm of a general neurologist to an MS subspecialist.
At some point it raises the question of when does a disease become its own specialty? Perhaps this is a bit of hyperbole — I’m pretty sure I’ll be seeing MS patients for a long time to come — but it’s a valid point. Especially as further research may subdivide MS treatment by genetics and other breakdowns.
Alzheimer’s disease may follow a similar (albeit very welcome) trajectory. While nothing really game-changing has come out in the 20 years, the number of new drugs and different mechanisms of action in development is large. Granted, not all of them will work, but hopefully some will. At some point it may come down to treating patients with a cocktail of drugs with separate ways of managing the disease, with guidance based on genetic or clinical profiles.
And that’s a good thing, but it may, again, move the disease from the province of general neurologists to subspecialists. Maybe that would be a good, maybe not. Probably will depend on the patient, their families, and other factors.
Of course, I may be overthinking this. The number of drugs we have for MS is nothing compared with the available treatments we have for hypertension, yet it’s certainly well within the capabilities of most internists to treat without referring to a cardiologist or nephrologist.
Perhaps the new drugs won’t make a difference except in a handful of cases. As new drugs come out we also move on from the old ones, dropping them from our mental armamentarium except in rare cases. When was the last time you prescribed Betaseron?
These drugs are very welcome, and very needed. I will be happy if we can beat back some of the diseases neurologist see, regardless of whom the patients and up seeing.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Ultrasound and Its Role In Diagnosing and Managing Endometriosis
Introduction: Imaging for Endometriosis — A Necessary Prerequisite
While the gold standard in the diagnosis of endometriosis remains laparoscopy, it is now recognized that thorough evaluation via ultrasound offers an acceptable, less expensive, and less invasive alternative. It is especially useful for the diagnosis of deep infiltrative disease, which penetrates more than 5 mm into the peritoneum, ovarian endometrioma, and when anatomic distortion occurs, such as to the path of the ureter.
Besides establishing the diagnosis, ultrasound imaging has become, along with MRI, the most important aid for proper preoperative planning. Not only does imaging provide the surgeon and patient with knowledge regarding the extent of the upcoming procedure, but it also allows the minimally invasive gynecologic (MIG) surgeon to involve colleagues, such as colorectal surgeons or urologists. For example, deep infiltrative endometriosis penetrating into the bowel mucosa will require a discoid or segmental bowel resection.
While many endometriosis experts rely on MRI, many MIG surgeons are dependent on ultrasound. I would not consider taking a patient with signs and symptoms suggestive of endometriosis to surgery without 2D/3D transvaginal ultrasound. If the patient possesses a uterus, a saline-infused sonogram is performed to potentially diagnose adenomyosis.
It is a pleasure and honor to welcome Professor Caterina Exacoustos MD, PhD, associate professor of ob.gyn. at the University of Rome “Tor Vergata,” to this edition of the Master Class in Gynecologic Surgery to discuss “Ultrasound and Its Role in the Diagnosis of and Management of Endometriosis, Including DIE.”
Prof. Exacoustos’ main areas of interest are endometriosis and benign diseases including uterine pathology and infertility. Her extensive body of work comprises over 120 scientific publications and numerous book chapters both in English and in Italian.
Prof. Exacoustos continues to be one of the most well respected lecturers speaking about ultrasound throughout the world.
Dr. Miller is professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago. Dr. Miller has no conflicts of interest to report.
Ultrasound and Its Role In Diagnosing and Managing Endometriosis
Endometriosis affects approximately 10%-20% of premenopausal women worldwide. It is the leading cause of chronic pelvic pain, is often associated with infertility, and has a significant impact on quality of life. Although the natural history of endometriosis remains unknown, emerging evidence suggests that the pathophysiological steps of initiation and development of endometriosis must occur earlier in the lifespan. Most notably, the onset of endometriosis-associated pain symptoms is often reported during adolescence and young adulthood.1
While many patients with endometriosis are referred with dysmenorrhea at a young age, at age ≤ 25 years,2 symptoms are often highly underestimated and considered to be normal and transient.3,4 Clinical and pelvic exams are often negative in young women, and delays in endometriosis diagnosis are well known.
The presentation of primary dysmenorrhea with no anatomical cause embodies the paradigm that dysmenorrhea in adolescents is most often an insignificant disorder. This perspective is probably a root cause of delayed endometriosis diagnosis in young patients. However, another issue behind delayed diagnosis is the reluctance of the physician to perform a diagnostic laparoscopy — historically the gold standard for diagnosing endometriosis — for seemingly common symptoms such as dysmenorrhea in young patients.
Today we know that there are typical aspects of ultrasound imaging that identify endometriosis in the pelvis, and notably, the 2022 European Society for Human Reproduction and Embryology (ESHRE) endometriosis guideline5 recognizes imaging (ultrasound or MRI) as the standard for endometriosis diagnosis without requiring laparoscopic or histological confirmation.
An early and noninvasive method of diagnosis aids in timely diagnosis and provides for the timely initiation of medical management to improve quality of life and prevent progression of disease (Figure 1).
(A. Transvaginal ultrasound appearance of a small ovarian endometrioma in a 16-year-old girl. Note the unilocular cyst with ground glass echogenicity surrounded by multifollicular ovarian tissue. B. Ultrasound image of a retroverted uterus of an 18-year-old girl with focal adenomyosis of the posterior wall. Note the round cystic anechoic areas in the inner myometrium or junctional zone. The small intra-myometrial cyst is surrounded by a hyperechoic ring).
Indeed, the typical appearance of endometriotic pelvic lesions on transvaginal sonography, such as endometriomas and rectal deep infiltrating endometriosis (DIE) — as well as adenomyosis – can be medically treated without histologic confirmation .
When surgery is advisable, ultrasound findings also play a valuable role in presurgical staging, planning, and counseling for patients of all ages. Determining the extent and location of DIE preoperatively, for instance, facilitates the engagement of the appropriate surgical specialists so that multiple surgeries can be avoided. It also enables patients to be optimally informed before surgery of possible outcomes and complications.
Moreover, in the context of infertility, ultrasound can be a valuable tool for understanding uterine pathology and assessing for adenomyosis so that affected patients may be treated surgically or medically before turning to assisted reproductive technology.
Uniformity, Standardization in the Sonographic Assessment
In Europe, as in the United States, transvaginal sonography (TVS) is the first-line imaging tool for the diagnosis and management of endometriosis. In Europe, many ob.gyns. perform ultrasound themselves, as do treating surgeons. When diagnostic findings are negative but clinical suspicion is high, MRI is often utilized. Laparoscopy may then be considered in patients with negative imaging results.
Efforts to standardize terms, definitions, measurements, and sonographic features of different types of endometriosis have been made to make it easier for physicians to share data and communicate with each other. A lack of uniformity has contributed to variability in the reported diagnostic accuracy of TVS.
About 10 years ago, in one such effort, we assessed the accuracy of TVS for DIE by comparing TVS results with laparoscopic/histologic findings, and developed an ultrasound mapping system to accurately record the location, size and depth of lesions visualized by TVS. The accuracy of TVS ranged from 76% for the diagnosis of vaginal endometriosis to 97% for the diagnosis of bladder lesions and posterior cul-de-sac obliteration. Accuracy was 93% and 91% for detecting ureteral involvement (right and left); 87% for uterosacral ligament endometriotic lesions; and 87% for parametrial involvement.6
Shortly after, with a focus on DIE, expert sonographers and physician-sonographers from across Europe — as well as some experts from Australia, Japan, Brazil, Chile, and the United States (Y. Osuga from Brigham and Women’s Hospital and Harvard Medical School) — came together to agree on a uniform approach to the sonographic evaluation for suspected endometriosis and a standardization of terminology.
The consensus opinion from the International Deep Endometriosis Analysis (IDEA) group details four steps for examining women with suspected DIE: 1) Evaluation of the uterus and adnexa, 2) evaluation of transvaginal sonographic “soft markers” (ie. site-specific tenderness and ovarian mobility), 3) assessment of the status of the posterior cul-de-sac using real-time ultrasound-based “sliding sign,” and 4) assessment for DIE nodules in the anterior and posterior compartments.7
Our paper describing a mapping system and the IDEA paper describe how to detect deep endometriosis in the pelvis by utilizing an ultrasound view of normal anatomy and pelvic organ structure to provide landmarks for accurately defining the site of DIE lesions (Figure 2).
(A. Ultrasound appearance of a small DIE lesion of the retrocervical area [white arrows], which involved the torus uterinum and the right uterosacral ligament [USL]. The lesion appears as hypoechoic tissue with irregular margins caused by the fibrosis induced by the DIE. B. TVS appearance of small nodules of DIE of the left USL. Note the small retrocervical DIE lesion [white arrows], which appears hypoechoic due to the infiltration of the hyperechoic USL. C) Ultrasound appearance of a DIE nodule of the recto-sigmoid wall. Note the hypoechoic thickening of the muscular layers of the bowel wall attached to the corpus of the uterus and the adenomyosis of the posterior wall. The retrocervical area is free. D. TVS appearance of nodules of DIE of the lower rectal wall. Note the hypoechoic lesion [white arrows] of the rectum is attached to a retrocervical DIE fibrosis of the torus and USL [white dotted line]).
So-called rectovaginal endometriosis can be well assessed, for instance, since the involvement of the rectum, sigmoid colon, vaginal wall, rectovaginal septum, and posterior cul-de-sac uterosacral ligament can be seen by ultrasound as a single structure, making the location, size, and depth of any lesions discernible.
Again, this evaluation of the extent of disease is important for presurgical assessment so the surgeon can organize the right team and time of surgery and so the patient can be counseled on the advantages and possible complications of the treatment.
Notably, an accurate ultrasound description of pelvic endometriosis is helpful for accurate classification of disease. Endometriosis classification systems such as that of the American Association of Gynecologic Laparoscopists (AAGL)8 and the American Society of Reproductive Medicine (ASRM),9 as well as the #Enzian surgical description system,10 have been adapted to cover findings from ultrasound as well as MRI imaging.
A Systematic Evaluation
In keeping with the IDEA consensus opinion and based on our years of experience at the University of Rome, I advise that patients with typical pain symptoms of endometriosis or infertility undergo an accurate sonographic assessment of the pelvis with particular evaluation not only of the uterus and ovaries but of all pelvic retroperitoneal spaces.
The TVS examination should start with a slightly filled bladder, which permits a better evaluation of the bladder walls and the presence of endometriotic nodules. These nodules appear as hyperechoic linear or spherical lesions bulging toward the lumen and involving the serosa, muscularis, or (sub)mucosa of the bladder.
Then, an accurate evaluation of the uterus in 2D and 3D permits the diagnosis of adenomyosis. 3D sonographic evaluation of the myometrium and of the junctional zone are important; alteration and infiltration of the junctional zone and the presence of small adenomyotic cysts in the inner or outer myometrium are direct, specific signs of adenomyosis and should be ruled out in patients with dysmenorrhea, heavy menstrual bleeding, infertility, and pregnancy complications.
Endometriomas of the ovaries can be easily detected as having the typical appearance of a cyst with ground glass content. Adhesions of the ovaries and the uterus also should be evaluated with a dynamic ultrasound approach that utilizes the sliding sign and mobilization by palpation of the organs during the TVS scan.
Finally, the posterior and lateral retroperitoneal compartments should be carefully evaluated, with symptoms guiding the TVS examination whenever possible. Deep endometriotic nodules of the rectum appear as hypoechoic lesions or linear or nodular retroperitoneal thickening with irregular borders, penetrating into the intestinal wall and distorting its normal structure. In young patients, it seems very important to assess for small lesions below the peritoneum between the vagina and rectum, and in the parametria and around the ureter and nerves — lesions that, notably, would not be seen by diagnostic laparoscopy.
The Evaluation of Young Patients
In adolescent and young patients, endometriosis and adenomyosis are often present with small lesions and shallow tissue invasion, making a very careful and experienced approach to ultrasound essential for detection. Endometriomas are often of small diameter, and DIE is not always easily diagnosed because retroperitoneal lesions are similarly very small.
In a series of 270 adolescents (ages 12-20) who were referred to our outpatient gynecologic ultrasound unit over a 5-year period for various indications, at least one ultrasound feature of endometriosis was observed in 13.3%. In those with dysmenorrhea, the detection of endometriosis increased to 21%. Endometrioma was the most common type of endometriosis we found in the study, but DIE and adenomyosis were found in 4%-11%.
Although endometriotic lesions typically are small in young patients, they are often associated with severe pain symptoms, including chronic pelvic pain, dysmenorrhea, dyspareunia, dysuria, and dyschezia, all of which can have a serious effect on the quality of life of these young women. These symptoms keep them away from school during menstruation, away from sports, and cause painful intercourse and infertility. In young patients, an accurate TVS can provide a lot of information, and the ability to detect retroperitoneal endometriotic lesions and adenomyosis is probably better than with purely diagnostic laparoscopy, which would evaluate only superficial lesions.
TVS or, when needed, transrectal ultrasound, can enable adequate treatment and follow-up of the disease and its symptoms. There are no guidelines recommending adequate follow-up times to evaluate the effectiveness of medical therapy in patients with ultrasound signs of endometriosis. (Likewise, there are no indications for follow-up in patients with severe dysmenorrhea without ultrasound signs of endometriosis.) Certainly, our studies suggest careful evaluation over time of young patients with severe dysmenorrhea by serial ultrasound scans. With such follow-up, disease progress can be monitored and the medical or surgical treatment approach modified if needed.
The diagnosis of endometriosis at a young age has significant benefits not only in avoiding or reducing progression of the disease, but also in improving quality of life and aiding women in their desire for pregnancy.
Dr. Exacoustos is associate professor of ob.gyn. at the University of Rome “Tor Vergata.” She has no conflicts of interest to report.
References
1. Zondervan KT et al. N Engl J Med. 2020;382:1244-56.
2. Greene R et al. Fertil Steril. 2009;91:32-9.
3. Chapron C et al. J Pediatr Adolesc Gynecol. 2011;24:S7-12.
4. Randhawa AE et al. J Pediatr Adolesc Gynecol. 2021;34:643-8.
5. Becker CM et al. Hum Reprod Open. 2022(2):hoac009.
6. Exacoustos C et al. Fertil Steril. 2014;102:143-9. 7. Guerriero S et al. Ultrasound Obstet Gynecol. 2016;48(3):318-32.
8. Abrao MS et al. J Minim Invasive Gynecol. 2021;28:1941-50.9. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817-21. 10. Keckstein J et al. Acta Obstet Gynecol Scand. 2021;100:1165-75.
11. Martire FG et al. Fertil Steril. 2020;114(5):1049-57.
Introduction: Imaging for Endometriosis — A Necessary Prerequisite
While the gold standard in the diagnosis of endometriosis remains laparoscopy, it is now recognized that thorough evaluation via ultrasound offers an acceptable, less expensive, and less invasive alternative. It is especially useful for the diagnosis of deep infiltrative disease, which penetrates more than 5 mm into the peritoneum, ovarian endometrioma, and when anatomic distortion occurs, such as to the path of the ureter.
Besides establishing the diagnosis, ultrasound imaging has become, along with MRI, the most important aid for proper preoperative planning. Not only does imaging provide the surgeon and patient with knowledge regarding the extent of the upcoming procedure, but it also allows the minimally invasive gynecologic (MIG) surgeon to involve colleagues, such as colorectal surgeons or urologists. For example, deep infiltrative endometriosis penetrating into the bowel mucosa will require a discoid or segmental bowel resection.
While many endometriosis experts rely on MRI, many MIG surgeons are dependent on ultrasound. I would not consider taking a patient with signs and symptoms suggestive of endometriosis to surgery without 2D/3D transvaginal ultrasound. If the patient possesses a uterus, a saline-infused sonogram is performed to potentially diagnose adenomyosis.
It is a pleasure and honor to welcome Professor Caterina Exacoustos MD, PhD, associate professor of ob.gyn. at the University of Rome “Tor Vergata,” to this edition of the Master Class in Gynecologic Surgery to discuss “Ultrasound and Its Role in the Diagnosis of and Management of Endometriosis, Including DIE.”
Prof. Exacoustos’ main areas of interest are endometriosis and benign diseases including uterine pathology and infertility. Her extensive body of work comprises over 120 scientific publications and numerous book chapters both in English and in Italian.
Prof. Exacoustos continues to be one of the most well respected lecturers speaking about ultrasound throughout the world.
Dr. Miller is professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago. Dr. Miller has no conflicts of interest to report.
Ultrasound and Its Role In Diagnosing and Managing Endometriosis
Endometriosis affects approximately 10%-20% of premenopausal women worldwide. It is the leading cause of chronic pelvic pain, is often associated with infertility, and has a significant impact on quality of life. Although the natural history of endometriosis remains unknown, emerging evidence suggests that the pathophysiological steps of initiation and development of endometriosis must occur earlier in the lifespan. Most notably, the onset of endometriosis-associated pain symptoms is often reported during adolescence and young adulthood.1
While many patients with endometriosis are referred with dysmenorrhea at a young age, at age ≤ 25 years,2 symptoms are often highly underestimated and considered to be normal and transient.3,4 Clinical and pelvic exams are often negative in young women, and delays in endometriosis diagnosis are well known.
The presentation of primary dysmenorrhea with no anatomical cause embodies the paradigm that dysmenorrhea in adolescents is most often an insignificant disorder. This perspective is probably a root cause of delayed endometriosis diagnosis in young patients. However, another issue behind delayed diagnosis is the reluctance of the physician to perform a diagnostic laparoscopy — historically the gold standard for diagnosing endometriosis — for seemingly common symptoms such as dysmenorrhea in young patients.
Today we know that there are typical aspects of ultrasound imaging that identify endometriosis in the pelvis, and notably, the 2022 European Society for Human Reproduction and Embryology (ESHRE) endometriosis guideline5 recognizes imaging (ultrasound or MRI) as the standard for endometriosis diagnosis without requiring laparoscopic or histological confirmation.
An early and noninvasive method of diagnosis aids in timely diagnosis and provides for the timely initiation of medical management to improve quality of life and prevent progression of disease (Figure 1).

(A. Transvaginal ultrasound appearance of a small ovarian endometrioma in a 16-year-old girl. Note the unilocular cyst with ground glass echogenicity surrounded by multifollicular ovarian tissue. B. Ultrasound image of a retroverted uterus of an 18-year-old girl with focal adenomyosis of the posterior wall. Note the round cystic anechoic areas in the inner myometrium or junctional zone. The small intra-myometrial cyst is surrounded by a hyperechoic ring).
Indeed, the typical appearance of endometriotic pelvic lesions on transvaginal sonography, such as endometriomas and rectal deep infiltrating endometriosis (DIE) — as well as adenomyosis – can be medically treated without histologic confirmation .
When surgery is advisable, ultrasound findings also play a valuable role in presurgical staging, planning, and counseling for patients of all ages. Determining the extent and location of DIE preoperatively, for instance, facilitates the engagement of the appropriate surgical specialists so that multiple surgeries can be avoided. It also enables patients to be optimally informed before surgery of possible outcomes and complications.
Moreover, in the context of infertility, ultrasound can be a valuable tool for understanding uterine pathology and assessing for adenomyosis so that affected patients may be treated surgically or medically before turning to assisted reproductive technology.
Uniformity, Standardization in the Sonographic Assessment
In Europe, as in the United States, transvaginal sonography (TVS) is the first-line imaging tool for the diagnosis and management of endometriosis. In Europe, many ob.gyns. perform ultrasound themselves, as do treating surgeons. When diagnostic findings are negative but clinical suspicion is high, MRI is often utilized. Laparoscopy may then be considered in patients with negative imaging results.
Efforts to standardize terms, definitions, measurements, and sonographic features of different types of endometriosis have been made to make it easier for physicians to share data and communicate with each other. A lack of uniformity has contributed to variability in the reported diagnostic accuracy of TVS.
About 10 years ago, in one such effort, we assessed the accuracy of TVS for DIE by comparing TVS results with laparoscopic/histologic findings, and developed an ultrasound mapping system to accurately record the location, size and depth of lesions visualized by TVS. The accuracy of TVS ranged from 76% for the diagnosis of vaginal endometriosis to 97% for the diagnosis of bladder lesions and posterior cul-de-sac obliteration. Accuracy was 93% and 91% for detecting ureteral involvement (right and left); 87% for uterosacral ligament endometriotic lesions; and 87% for parametrial involvement.6
Shortly after, with a focus on DIE, expert sonographers and physician-sonographers from across Europe — as well as some experts from Australia, Japan, Brazil, Chile, and the United States (Y. Osuga from Brigham and Women’s Hospital and Harvard Medical School) — came together to agree on a uniform approach to the sonographic evaluation for suspected endometriosis and a standardization of terminology.
The consensus opinion from the International Deep Endometriosis Analysis (IDEA) group details four steps for examining women with suspected DIE: 1) Evaluation of the uterus and adnexa, 2) evaluation of transvaginal sonographic “soft markers” (ie. site-specific tenderness and ovarian mobility), 3) assessment of the status of the posterior cul-de-sac using real-time ultrasound-based “sliding sign,” and 4) assessment for DIE nodules in the anterior and posterior compartments.7
Our paper describing a mapping system and the IDEA paper describe how to detect deep endometriosis in the pelvis by utilizing an ultrasound view of normal anatomy and pelvic organ structure to provide landmarks for accurately defining the site of DIE lesions (Figure 2).

(A. Ultrasound appearance of a small DIE lesion of the retrocervical area [white arrows], which involved the torus uterinum and the right uterosacral ligament [USL]. The lesion appears as hypoechoic tissue with irregular margins caused by the fibrosis induced by the DIE. B. TVS appearance of small nodules of DIE of the left USL. Note the small retrocervical DIE lesion [white arrows], which appears hypoechoic due to the infiltration of the hyperechoic USL. C) Ultrasound appearance of a DIE nodule of the recto-sigmoid wall. Note the hypoechoic thickening of the muscular layers of the bowel wall attached to the corpus of the uterus and the adenomyosis of the posterior wall. The retrocervical area is free. D. TVS appearance of nodules of DIE of the lower rectal wall. Note the hypoechoic lesion [white arrows] of the rectum is attached to a retrocervical DIE fibrosis of the torus and USL [white dotted line]).
So-called rectovaginal endometriosis can be well assessed, for instance, since the involvement of the rectum, sigmoid colon, vaginal wall, rectovaginal septum, and posterior cul-de-sac uterosacral ligament can be seen by ultrasound as a single structure, making the location, size, and depth of any lesions discernible.
Again, this evaluation of the extent of disease is important for presurgical assessment so the surgeon can organize the right team and time of surgery and so the patient can be counseled on the advantages and possible complications of the treatment.
Notably, an accurate ultrasound description of pelvic endometriosis is helpful for accurate classification of disease. Endometriosis classification systems such as that of the American Association of Gynecologic Laparoscopists (AAGL)8 and the American Society of Reproductive Medicine (ASRM),9 as well as the #Enzian surgical description system,10 have been adapted to cover findings from ultrasound as well as MRI imaging.
A Systematic Evaluation
In keeping with the IDEA consensus opinion and based on our years of experience at the University of Rome, I advise that patients with typical pain symptoms of endometriosis or infertility undergo an accurate sonographic assessment of the pelvis with particular evaluation not only of the uterus and ovaries but of all pelvic retroperitoneal spaces.
The TVS examination should start with a slightly filled bladder, which permits a better evaluation of the bladder walls and the presence of endometriotic nodules. These nodules appear as hyperechoic linear or spherical lesions bulging toward the lumen and involving the serosa, muscularis, or (sub)mucosa of the bladder.
Then, an accurate evaluation of the uterus in 2D and 3D permits the diagnosis of adenomyosis. 3D sonographic evaluation of the myometrium and of the junctional zone are important; alteration and infiltration of the junctional zone and the presence of small adenomyotic cysts in the inner or outer myometrium are direct, specific signs of adenomyosis and should be ruled out in patients with dysmenorrhea, heavy menstrual bleeding, infertility, and pregnancy complications.
Endometriomas of the ovaries can be easily detected as having the typical appearance of a cyst with ground glass content. Adhesions of the ovaries and the uterus also should be evaluated with a dynamic ultrasound approach that utilizes the sliding sign and mobilization by palpation of the organs during the TVS scan.
Finally, the posterior and lateral retroperitoneal compartments should be carefully evaluated, with symptoms guiding the TVS examination whenever possible. Deep endometriotic nodules of the rectum appear as hypoechoic lesions or linear or nodular retroperitoneal thickening with irregular borders, penetrating into the intestinal wall and distorting its normal structure. In young patients, it seems very important to assess for small lesions below the peritoneum between the vagina and rectum, and in the parametria and around the ureter and nerves — lesions that, notably, would not be seen by diagnostic laparoscopy.
The Evaluation of Young Patients
In adolescent and young patients, endometriosis and adenomyosis are often present with small lesions and shallow tissue invasion, making a very careful and experienced approach to ultrasound essential for detection. Endometriomas are often of small diameter, and DIE is not always easily diagnosed because retroperitoneal lesions are similarly very small.
In a series of 270 adolescents (ages 12-20) who were referred to our outpatient gynecologic ultrasound unit over a 5-year period for various indications, at least one ultrasound feature of endometriosis was observed in 13.3%. In those with dysmenorrhea, the detection of endometriosis increased to 21%. Endometrioma was the most common type of endometriosis we found in the study, but DIE and adenomyosis were found in 4%-11%.
Although endometriotic lesions typically are small in young patients, they are often associated with severe pain symptoms, including chronic pelvic pain, dysmenorrhea, dyspareunia, dysuria, and dyschezia, all of which can have a serious effect on the quality of life of these young women. These symptoms keep them away from school during menstruation, away from sports, and cause painful intercourse and infertility. In young patients, an accurate TVS can provide a lot of information, and the ability to detect retroperitoneal endometriotic lesions and adenomyosis is probably better than with purely diagnostic laparoscopy, which would evaluate only superficial lesions.
TVS or, when needed, transrectal ultrasound, can enable adequate treatment and follow-up of the disease and its symptoms. There are no guidelines recommending adequate follow-up times to evaluate the effectiveness of medical therapy in patients with ultrasound signs of endometriosis. (Likewise, there are no indications for follow-up in patients with severe dysmenorrhea without ultrasound signs of endometriosis.) Certainly, our studies suggest careful evaluation over time of young patients with severe dysmenorrhea by serial ultrasound scans. With such follow-up, disease progress can be monitored and the medical or surgical treatment approach modified if needed.
The diagnosis of endometriosis at a young age has significant benefits not only in avoiding or reducing progression of the disease, but also in improving quality of life and aiding women in their desire for pregnancy.
Dr. Exacoustos is associate professor of ob.gyn. at the University of Rome “Tor Vergata.” She has no conflicts of interest to report.
References
1. Zondervan KT et al. N Engl J Med. 2020;382:1244-56.
2. Greene R et al. Fertil Steril. 2009;91:32-9.
3. Chapron C et al. J Pediatr Adolesc Gynecol. 2011;24:S7-12.
4. Randhawa AE et al. J Pediatr Adolesc Gynecol. 2021;34:643-8.
5. Becker CM et al. Hum Reprod Open. 2022(2):hoac009.
6. Exacoustos C et al. Fertil Steril. 2014;102:143-9. 7. Guerriero S et al. Ultrasound Obstet Gynecol. 2016;48(3):318-32.
8. Abrao MS et al. J Minim Invasive Gynecol. 2021;28:1941-50.9. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817-21. 10. Keckstein J et al. Acta Obstet Gynecol Scand. 2021;100:1165-75.
11. Martire FG et al. Fertil Steril. 2020;114(5):1049-57.
Introduction: Imaging for Endometriosis — A Necessary Prerequisite
While the gold standard in the diagnosis of endometriosis remains laparoscopy, it is now recognized that thorough evaluation via ultrasound offers an acceptable, less expensive, and less invasive alternative. It is especially useful for the diagnosis of deep infiltrative disease, which penetrates more than 5 mm into the peritoneum, ovarian endometrioma, and when anatomic distortion occurs, such as to the path of the ureter.
Besides establishing the diagnosis, ultrasound imaging has become, along with MRI, the most important aid for proper preoperative planning. Not only does imaging provide the surgeon and patient with knowledge regarding the extent of the upcoming procedure, but it also allows the minimally invasive gynecologic (MIG) surgeon to involve colleagues, such as colorectal surgeons or urologists. For example, deep infiltrative endometriosis penetrating into the bowel mucosa will require a discoid or segmental bowel resection.
While many endometriosis experts rely on MRI, many MIG surgeons are dependent on ultrasound. I would not consider taking a patient with signs and symptoms suggestive of endometriosis to surgery without 2D/3D transvaginal ultrasound. If the patient possesses a uterus, a saline-infused sonogram is performed to potentially diagnose adenomyosis.
It is a pleasure and honor to welcome Professor Caterina Exacoustos MD, PhD, associate professor of ob.gyn. at the University of Rome “Tor Vergata,” to this edition of the Master Class in Gynecologic Surgery to discuss “Ultrasound and Its Role in the Diagnosis of and Management of Endometriosis, Including DIE.”
Prof. Exacoustos’ main areas of interest are endometriosis and benign diseases including uterine pathology and infertility. Her extensive body of work comprises over 120 scientific publications and numerous book chapters both in English and in Italian.
Prof. Exacoustos continues to be one of the most well respected lecturers speaking about ultrasound throughout the world.
Dr. Miller is professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago. Dr. Miller has no conflicts of interest to report.
Ultrasound and Its Role In Diagnosing and Managing Endometriosis
Endometriosis affects approximately 10%-20% of premenopausal women worldwide. It is the leading cause of chronic pelvic pain, is often associated with infertility, and has a significant impact on quality of life. Although the natural history of endometriosis remains unknown, emerging evidence suggests that the pathophysiological steps of initiation and development of endometriosis must occur earlier in the lifespan. Most notably, the onset of endometriosis-associated pain symptoms is often reported during adolescence and young adulthood.1
While many patients with endometriosis are referred with dysmenorrhea at a young age, at age ≤ 25 years,2 symptoms are often highly underestimated and considered to be normal and transient.3,4 Clinical and pelvic exams are often negative in young women, and delays in endometriosis diagnosis are well known.
The presentation of primary dysmenorrhea with no anatomical cause embodies the paradigm that dysmenorrhea in adolescents is most often an insignificant disorder. This perspective is probably a root cause of delayed endometriosis diagnosis in young patients. However, another issue behind delayed diagnosis is the reluctance of the physician to perform a diagnostic laparoscopy — historically the gold standard for diagnosing endometriosis — for seemingly common symptoms such as dysmenorrhea in young patients.
Today we know that there are typical aspects of ultrasound imaging that identify endometriosis in the pelvis, and notably, the 2022 European Society for Human Reproduction and Embryology (ESHRE) endometriosis guideline5 recognizes imaging (ultrasound or MRI) as the standard for endometriosis diagnosis without requiring laparoscopic or histological confirmation.
An early and noninvasive method of diagnosis aids in timely diagnosis and provides for the timely initiation of medical management to improve quality of life and prevent progression of disease (Figure 1).

(A. Transvaginal ultrasound appearance of a small ovarian endometrioma in a 16-year-old girl. Note the unilocular cyst with ground glass echogenicity surrounded by multifollicular ovarian tissue. B. Ultrasound image of a retroverted uterus of an 18-year-old girl with focal adenomyosis of the posterior wall. Note the round cystic anechoic areas in the inner myometrium or junctional zone. The small intra-myometrial cyst is surrounded by a hyperechoic ring).
Indeed, the typical appearance of endometriotic pelvic lesions on transvaginal sonography, such as endometriomas and rectal deep infiltrating endometriosis (DIE) — as well as adenomyosis – can be medically treated without histologic confirmation .
When surgery is advisable, ultrasound findings also play a valuable role in presurgical staging, planning, and counseling for patients of all ages. Determining the extent and location of DIE preoperatively, for instance, facilitates the engagement of the appropriate surgical specialists so that multiple surgeries can be avoided. It also enables patients to be optimally informed before surgery of possible outcomes and complications.
Moreover, in the context of infertility, ultrasound can be a valuable tool for understanding uterine pathology and assessing for adenomyosis so that affected patients may be treated surgically or medically before turning to assisted reproductive technology.
Uniformity, Standardization in the Sonographic Assessment
In Europe, as in the United States, transvaginal sonography (TVS) is the first-line imaging tool for the diagnosis and management of endometriosis. In Europe, many ob.gyns. perform ultrasound themselves, as do treating surgeons. When diagnostic findings are negative but clinical suspicion is high, MRI is often utilized. Laparoscopy may then be considered in patients with negative imaging results.
Efforts to standardize terms, definitions, measurements, and sonographic features of different types of endometriosis have been made to make it easier for physicians to share data and communicate with each other. A lack of uniformity has contributed to variability in the reported diagnostic accuracy of TVS.
About 10 years ago, in one such effort, we assessed the accuracy of TVS for DIE by comparing TVS results with laparoscopic/histologic findings, and developed an ultrasound mapping system to accurately record the location, size and depth of lesions visualized by TVS. The accuracy of TVS ranged from 76% for the diagnosis of vaginal endometriosis to 97% for the diagnosis of bladder lesions and posterior cul-de-sac obliteration. Accuracy was 93% and 91% for detecting ureteral involvement (right and left); 87% for uterosacral ligament endometriotic lesions; and 87% for parametrial involvement.6
Shortly after, with a focus on DIE, expert sonographers and physician-sonographers from across Europe — as well as some experts from Australia, Japan, Brazil, Chile, and the United States (Y. Osuga from Brigham and Women’s Hospital and Harvard Medical School) — came together to agree on a uniform approach to the sonographic evaluation for suspected endometriosis and a standardization of terminology.
The consensus opinion from the International Deep Endometriosis Analysis (IDEA) group details four steps for examining women with suspected DIE: 1) Evaluation of the uterus and adnexa, 2) evaluation of transvaginal sonographic “soft markers” (ie. site-specific tenderness and ovarian mobility), 3) assessment of the status of the posterior cul-de-sac using real-time ultrasound-based “sliding sign,” and 4) assessment for DIE nodules in the anterior and posterior compartments.7
Our paper describing a mapping system and the IDEA paper describe how to detect deep endometriosis in the pelvis by utilizing an ultrasound view of normal anatomy and pelvic organ structure to provide landmarks for accurately defining the site of DIE lesions (Figure 2).

(A. Ultrasound appearance of a small DIE lesion of the retrocervical area [white arrows], which involved the torus uterinum and the right uterosacral ligament [USL]. The lesion appears as hypoechoic tissue with irregular margins caused by the fibrosis induced by the DIE. B. TVS appearance of small nodules of DIE of the left USL. Note the small retrocervical DIE lesion [white arrows], which appears hypoechoic due to the infiltration of the hyperechoic USL. C) Ultrasound appearance of a DIE nodule of the recto-sigmoid wall. Note the hypoechoic thickening of the muscular layers of the bowel wall attached to the corpus of the uterus and the adenomyosis of the posterior wall. The retrocervical area is free. D. TVS appearance of nodules of DIE of the lower rectal wall. Note the hypoechoic lesion [white arrows] of the rectum is attached to a retrocervical DIE fibrosis of the torus and USL [white dotted line]).
So-called rectovaginal endometriosis can be well assessed, for instance, since the involvement of the rectum, sigmoid colon, vaginal wall, rectovaginal septum, and posterior cul-de-sac uterosacral ligament can be seen by ultrasound as a single structure, making the location, size, and depth of any lesions discernible.
Again, this evaluation of the extent of disease is important for presurgical assessment so the surgeon can organize the right team and time of surgery and so the patient can be counseled on the advantages and possible complications of the treatment.
Notably, an accurate ultrasound description of pelvic endometriosis is helpful for accurate classification of disease. Endometriosis classification systems such as that of the American Association of Gynecologic Laparoscopists (AAGL)8 and the American Society of Reproductive Medicine (ASRM),9 as well as the #Enzian surgical description system,10 have been adapted to cover findings from ultrasound as well as MRI imaging.
A Systematic Evaluation
In keeping with the IDEA consensus opinion and based on our years of experience at the University of Rome, I advise that patients with typical pain symptoms of endometriosis or infertility undergo an accurate sonographic assessment of the pelvis with particular evaluation not only of the uterus and ovaries but of all pelvic retroperitoneal spaces.
The TVS examination should start with a slightly filled bladder, which permits a better evaluation of the bladder walls and the presence of endometriotic nodules. These nodules appear as hyperechoic linear or spherical lesions bulging toward the lumen and involving the serosa, muscularis, or (sub)mucosa of the bladder.
Then, an accurate evaluation of the uterus in 2D and 3D permits the diagnosis of adenomyosis. 3D sonographic evaluation of the myometrium and of the junctional zone are important; alteration and infiltration of the junctional zone and the presence of small adenomyotic cysts in the inner or outer myometrium are direct, specific signs of adenomyosis and should be ruled out in patients with dysmenorrhea, heavy menstrual bleeding, infertility, and pregnancy complications.
Endometriomas of the ovaries can be easily detected as having the typical appearance of a cyst with ground glass content. Adhesions of the ovaries and the uterus also should be evaluated with a dynamic ultrasound approach that utilizes the sliding sign and mobilization by palpation of the organs during the TVS scan.
Finally, the posterior and lateral retroperitoneal compartments should be carefully evaluated, with symptoms guiding the TVS examination whenever possible. Deep endometriotic nodules of the rectum appear as hypoechoic lesions or linear or nodular retroperitoneal thickening with irregular borders, penetrating into the intestinal wall and distorting its normal structure. In young patients, it seems very important to assess for small lesions below the peritoneum between the vagina and rectum, and in the parametria and around the ureter and nerves — lesions that, notably, would not be seen by diagnostic laparoscopy.
The Evaluation of Young Patients
In adolescent and young patients, endometriosis and adenomyosis are often present with small lesions and shallow tissue invasion, making a very careful and experienced approach to ultrasound essential for detection. Endometriomas are often of small diameter, and DIE is not always easily diagnosed because retroperitoneal lesions are similarly very small.
In a series of 270 adolescents (ages 12-20) who were referred to our outpatient gynecologic ultrasound unit over a 5-year period for various indications, at least one ultrasound feature of endometriosis was observed in 13.3%. In those with dysmenorrhea, the detection of endometriosis increased to 21%. Endometrioma was the most common type of endometriosis we found in the study, but DIE and adenomyosis were found in 4%-11%.
Although endometriotic lesions typically are small in young patients, they are often associated with severe pain symptoms, including chronic pelvic pain, dysmenorrhea, dyspareunia, dysuria, and dyschezia, all of which can have a serious effect on the quality of life of these young women. These symptoms keep them away from school during menstruation, away from sports, and cause painful intercourse and infertility. In young patients, an accurate TVS can provide a lot of information, and the ability to detect retroperitoneal endometriotic lesions and adenomyosis is probably better than with purely diagnostic laparoscopy, which would evaluate only superficial lesions.
TVS or, when needed, transrectal ultrasound, can enable adequate treatment and follow-up of the disease and its symptoms. There are no guidelines recommending adequate follow-up times to evaluate the effectiveness of medical therapy in patients with ultrasound signs of endometriosis. (Likewise, there are no indications for follow-up in patients with severe dysmenorrhea without ultrasound signs of endometriosis.) Certainly, our studies suggest careful evaluation over time of young patients with severe dysmenorrhea by serial ultrasound scans. With such follow-up, disease progress can be monitored and the medical or surgical treatment approach modified if needed.
The diagnosis of endometriosis at a young age has significant benefits not only in avoiding or reducing progression of the disease, but also in improving quality of life and aiding women in their desire for pregnancy.
Dr. Exacoustos is associate professor of ob.gyn. at the University of Rome “Tor Vergata.” She has no conflicts of interest to report.
References
1. Zondervan KT et al. N Engl J Med. 2020;382:1244-56.
2. Greene R et al. Fertil Steril. 2009;91:32-9.
3. Chapron C et al. J Pediatr Adolesc Gynecol. 2011;24:S7-12.
4. Randhawa AE et al. J Pediatr Adolesc Gynecol. 2021;34:643-8.
5. Becker CM et al. Hum Reprod Open. 2022(2):hoac009.
6. Exacoustos C et al. Fertil Steril. 2014;102:143-9. 7. Guerriero S et al. Ultrasound Obstet Gynecol. 2016;48(3):318-32.
8. Abrao MS et al. J Minim Invasive Gynecol. 2021;28:1941-50.9. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817-21. 10. Keckstein J et al. Acta Obstet Gynecol Scand. 2021;100:1165-75.
11. Martire FG et al. Fertil Steril. 2020;114(5):1049-57.
You Can’t Spell ‘Medicine’ Without D, E, and I
Please note that this is a commentary, an opinion piece: my opinion. The statements here do not necessarily represent those of this news organization or any of the myriad people or institutions that comprise this corner of the human universe.
Some days, speaking as a long-time physician and editor, I wish that there were no such things as race or ethnicity or even geographic origin for that matter. We can’t get away from sex, gender, disability, age, or culture. I’m not sure about religion. I wish people were just people.
But race is deeply embedded in the American experience — an almost invisible but inevitable presence in all of our thoughts and expressions about human activities.
In medical education (for eons it seems) the student has been taught to mention race in the first sentence of a given patient presentation, along with age and sex. In human epidemiologic research, race is almost always a studied variable. In clinical and basic medical research, looking at the impact of race on this, that, or the other is commonplace. “Mixed race not otherwise specified” is ubiquitous in the United States yet blithely ignored by most who tally these statistics. Race is rarely gene-specific. It is more of a social and cultural construct but with plainly visible overt phenotypic markers — an almost infinite mix of daily reality.
Our country, and much of Western civilization in 2024, is based on the principle that all men are created equal, although the originators of that notion were unaware of their own “equity-challenged” situation.
Many organizations, in and out of government, are now understanding, developing, and implementing programs (and thought/language patterns) to socialize diversity, equity, and inclusion (known as DEI) into their culture. It should not be surprising that many who prefer the status quo are not happy with the pressure from this movement and are using whatever methods are available to them to prevent full DEI. Such it always is.
The trusty Copilot from Bing provides these definitions:
- Diversity refers to the presence of variety within the organizational workforce. This includes aspects such as gender, culture, ethnicity, religion, disability, age, and opinion.
- Equity encompasses concepts of fairness and justice. It involves fair compensation, substantive equality, and addressing societal disparities. Equity also considers unique circumstances and adjusts treatment to achieve equal outcomes.
- Inclusion focuses on creating an organizational culture where all employees feel heard, fostering a sense of belonging and integration.
I am more than proud that my old domain of peer-reviewed, primary source, medical (and science) journals is taking a leading role in this noble, necessary, and long overdue movement for medicine.
As the central repository and transmitter of new medical information, including scientific studies, clinical medicine reports, ethics measures, and education, medical journals (including those deemed prestigious) have historically been among the worst offenders in perpetuating non-DEI objectives in their leadership, staffing, focus, instructions for authors, style manuals, and published materials.
This issue came to a head in March 2021 when a JAMA podcast about racism in American medicine was followed by this promotional tweet: “No physician is racist, so how can there be structural racism in health care?”
Reactions and actions were rapid, strong, and decisive. After an interregnum at JAMA, a new editor in chief, Kirsten Bibbins-Domingo, PhD, MD, MAS, was named. She and her large staff of editors and editorial board members from the multijournal JAMA Network joined a worldwide movement of (currently) 56 publishing organizations representing 15,000 journals called the Joint Commitment for Action on Inclusion and Diversity in Publishing.
A recent JAMA editorial with 29 authors describes the entire commitment initiative of publishers-editors. It reports JAMA Network data from 2023 and 2024 from surveys of 455 editors (a 91% response rate) about their own gender (five choices), ethnic origins or geographic ancestry (13 choices), and race (eight choices), demonstrating considerable progress toward DEI goals. The survey’s complex multinational classifications may not jibe with the categorizations used in some countries (too bad that “mixed” is not “mixed in” — a missed opportunity).
This encouraging movement will not fix it all. But when people of certain groups are represented at the table, that point of view is far more likely to make it into the lexicon, language, and omnipresent work products, potentially changing cultural norms. Even the measurement of movement related to disparity in healthcare is marred by frequent variations of data accuracy. More consistency in what to measure can help a lot, and the medical literature can be very influential.
A personal anecdote: When I was a professor at UC Davis in 1978, Allan Bakke, MD, was my student. Some of you will remember the saga of affirmative action on admissions, which was just revisited in the light of a recent decision by the US Supreme Court.
Back in 1978, the dean at UC Davis told me that he kept two file folders on the admission processes in different desk drawers. One categorized all applicants and enrollees by race, and the other did not. Depending on who came to visit and ask questions, he would choose one or the other file to share once he figured out what they were looking for (this is not a joke).
The strength of the current active political pushback against the entire DEI movement has deep roots and should not be underestimated. There will be a lot of to-ing and fro-ing.
French writer Victor Hugo is credited with stating, “There is nothing as powerful as an idea whose time has come.” A majority of Americans, physicians, and other healthcare professionals believe in basic fairness. The time for DEI in all aspects of medicine is now.
Dr. Lundberg, editor in chief of Cancer Commons, disclosed having no relevant financial relationships.
A version of this article appeared on Medscape.com.
Please note that this is a commentary, an opinion piece: my opinion. The statements here do not necessarily represent those of this news organization or any of the myriad people or institutions that comprise this corner of the human universe.
Some days, speaking as a long-time physician and editor, I wish that there were no such things as race or ethnicity or even geographic origin for that matter. We can’t get away from sex, gender, disability, age, or culture. I’m not sure about religion. I wish people were just people.
But race is deeply embedded in the American experience — an almost invisible but inevitable presence in all of our thoughts and expressions about human activities.
In medical education (for eons it seems) the student has been taught to mention race in the first sentence of a given patient presentation, along with age and sex. In human epidemiologic research, race is almost always a studied variable. In clinical and basic medical research, looking at the impact of race on this, that, or the other is commonplace. “Mixed race not otherwise specified” is ubiquitous in the United States yet blithely ignored by most who tally these statistics. Race is rarely gene-specific. It is more of a social and cultural construct but with plainly visible overt phenotypic markers — an almost infinite mix of daily reality.
Our country, and much of Western civilization in 2024, is based on the principle that all men are created equal, although the originators of that notion were unaware of their own “equity-challenged” situation.
Many organizations, in and out of government, are now understanding, developing, and implementing programs (and thought/language patterns) to socialize diversity, equity, and inclusion (known as DEI) into their culture. It should not be surprising that many who prefer the status quo are not happy with the pressure from this movement and are using whatever methods are available to them to prevent full DEI. Such it always is.
The trusty Copilot from Bing provides these definitions:
- Diversity refers to the presence of variety within the organizational workforce. This includes aspects such as gender, culture, ethnicity, religion, disability, age, and opinion.
- Equity encompasses concepts of fairness and justice. It involves fair compensation, substantive equality, and addressing societal disparities. Equity also considers unique circumstances and adjusts treatment to achieve equal outcomes.
- Inclusion focuses on creating an organizational culture where all employees feel heard, fostering a sense of belonging and integration.
I am more than proud that my old domain of peer-reviewed, primary source, medical (and science) journals is taking a leading role in this noble, necessary, and long overdue movement for medicine.
As the central repository and transmitter of new medical information, including scientific studies, clinical medicine reports, ethics measures, and education, medical journals (including those deemed prestigious) have historically been among the worst offenders in perpetuating non-DEI objectives in their leadership, staffing, focus, instructions for authors, style manuals, and published materials.
This issue came to a head in March 2021 when a JAMA podcast about racism in American medicine was followed by this promotional tweet: “No physician is racist, so how can there be structural racism in health care?”
Reactions and actions were rapid, strong, and decisive. After an interregnum at JAMA, a new editor in chief, Kirsten Bibbins-Domingo, PhD, MD, MAS, was named. She and her large staff of editors and editorial board members from the multijournal JAMA Network joined a worldwide movement of (currently) 56 publishing organizations representing 15,000 journals called the Joint Commitment for Action on Inclusion and Diversity in Publishing.
A recent JAMA editorial with 29 authors describes the entire commitment initiative of publishers-editors. It reports JAMA Network data from 2023 and 2024 from surveys of 455 editors (a 91% response rate) about their own gender (five choices), ethnic origins or geographic ancestry (13 choices), and race (eight choices), demonstrating considerable progress toward DEI goals. The survey’s complex multinational classifications may not jibe with the categorizations used in some countries (too bad that “mixed” is not “mixed in” — a missed opportunity).
This encouraging movement will not fix it all. But when people of certain groups are represented at the table, that point of view is far more likely to make it into the lexicon, language, and omnipresent work products, potentially changing cultural norms. Even the measurement of movement related to disparity in healthcare is marred by frequent variations of data accuracy. More consistency in what to measure can help a lot, and the medical literature can be very influential.
A personal anecdote: When I was a professor at UC Davis in 1978, Allan Bakke, MD, was my student. Some of you will remember the saga of affirmative action on admissions, which was just revisited in the light of a recent decision by the US Supreme Court.
Back in 1978, the dean at UC Davis told me that he kept two file folders on the admission processes in different desk drawers. One categorized all applicants and enrollees by race, and the other did not. Depending on who came to visit and ask questions, he would choose one or the other file to share once he figured out what they were looking for (this is not a joke).
The strength of the current active political pushback against the entire DEI movement has deep roots and should not be underestimated. There will be a lot of to-ing and fro-ing.
French writer Victor Hugo is credited with stating, “There is nothing as powerful as an idea whose time has come.” A majority of Americans, physicians, and other healthcare professionals believe in basic fairness. The time for DEI in all aspects of medicine is now.
Dr. Lundberg, editor in chief of Cancer Commons, disclosed having no relevant financial relationships.
A version of this article appeared on Medscape.com.
Please note that this is a commentary, an opinion piece: my opinion. The statements here do not necessarily represent those of this news organization or any of the myriad people or institutions that comprise this corner of the human universe.
Some days, speaking as a long-time physician and editor, I wish that there were no such things as race or ethnicity or even geographic origin for that matter. We can’t get away from sex, gender, disability, age, or culture. I’m not sure about religion. I wish people were just people.
But race is deeply embedded in the American experience — an almost invisible but inevitable presence in all of our thoughts and expressions about human activities.
In medical education (for eons it seems) the student has been taught to mention race in the first sentence of a given patient presentation, along with age and sex. In human epidemiologic research, race is almost always a studied variable. In clinical and basic medical research, looking at the impact of race on this, that, or the other is commonplace. “Mixed race not otherwise specified” is ubiquitous in the United States yet blithely ignored by most who tally these statistics. Race is rarely gene-specific. It is more of a social and cultural construct but with plainly visible overt phenotypic markers — an almost infinite mix of daily reality.
Our country, and much of Western civilization in 2024, is based on the principle that all men are created equal, although the originators of that notion were unaware of their own “equity-challenged” situation.
Many organizations, in and out of government, are now understanding, developing, and implementing programs (and thought/language patterns) to socialize diversity, equity, and inclusion (known as DEI) into their culture. It should not be surprising that many who prefer the status quo are not happy with the pressure from this movement and are using whatever methods are available to them to prevent full DEI. Such it always is.
The trusty Copilot from Bing provides these definitions:
- Diversity refers to the presence of variety within the organizational workforce. This includes aspects such as gender, culture, ethnicity, religion, disability, age, and opinion.
- Equity encompasses concepts of fairness and justice. It involves fair compensation, substantive equality, and addressing societal disparities. Equity also considers unique circumstances and adjusts treatment to achieve equal outcomes.
- Inclusion focuses on creating an organizational culture where all employees feel heard, fostering a sense of belonging and integration.
I am more than proud that my old domain of peer-reviewed, primary source, medical (and science) journals is taking a leading role in this noble, necessary, and long overdue movement for medicine.
As the central repository and transmitter of new medical information, including scientific studies, clinical medicine reports, ethics measures, and education, medical journals (including those deemed prestigious) have historically been among the worst offenders in perpetuating non-DEI objectives in their leadership, staffing, focus, instructions for authors, style manuals, and published materials.
This issue came to a head in March 2021 when a JAMA podcast about racism in American medicine was followed by this promotional tweet: “No physician is racist, so how can there be structural racism in health care?”
Reactions and actions were rapid, strong, and decisive. After an interregnum at JAMA, a new editor in chief, Kirsten Bibbins-Domingo, PhD, MD, MAS, was named. She and her large staff of editors and editorial board members from the multijournal JAMA Network joined a worldwide movement of (currently) 56 publishing organizations representing 15,000 journals called the Joint Commitment for Action on Inclusion and Diversity in Publishing.
A recent JAMA editorial with 29 authors describes the entire commitment initiative of publishers-editors. It reports JAMA Network data from 2023 and 2024 from surveys of 455 editors (a 91% response rate) about their own gender (five choices), ethnic origins or geographic ancestry (13 choices), and race (eight choices), demonstrating considerable progress toward DEI goals. The survey’s complex multinational classifications may not jibe with the categorizations used in some countries (too bad that “mixed” is not “mixed in” — a missed opportunity).
This encouraging movement will not fix it all. But when people of certain groups are represented at the table, that point of view is far more likely to make it into the lexicon, language, and omnipresent work products, potentially changing cultural norms. Even the measurement of movement related to disparity in healthcare is marred by frequent variations of data accuracy. More consistency in what to measure can help a lot, and the medical literature can be very influential.
A personal anecdote: When I was a professor at UC Davis in 1978, Allan Bakke, MD, was my student. Some of you will remember the saga of affirmative action on admissions, which was just revisited in the light of a recent decision by the US Supreme Court.
Back in 1978, the dean at UC Davis told me that he kept two file folders on the admission processes in different desk drawers. One categorized all applicants and enrollees by race, and the other did not. Depending on who came to visit and ask questions, he would choose one or the other file to share once he figured out what they were looking for (this is not a joke).
The strength of the current active political pushback against the entire DEI movement has deep roots and should not be underestimated. There will be a lot of to-ing and fro-ing.
French writer Victor Hugo is credited with stating, “There is nothing as powerful as an idea whose time has come.” A majority of Americans, physicians, and other healthcare professionals believe in basic fairness. The time for DEI in all aspects of medicine is now.
Dr. Lundberg, editor in chief of Cancer Commons, disclosed having no relevant financial relationships.
A version of this article appeared on Medscape.com.
Losing More Than Fat
Whether you have totally bought into the “obesity is a disease” paradigm or are still in denial, you must admit that the development of a suite of effective weight loss medications has created a tsunami of interest and economic activity in this country on a scale not seen since the Beanie Baby craze of the mid-1990s. But, obesity management is serious business. While most of those soft cuddly toys are gathering dust in shoeboxes across this country, weight loss medications are likely to be the vanguard of rapidly evolving revolution in healthcare management that will be with us for the foreseeable future.
Most thoughtful folks who purchased Beanie Babies in 1994 had no illusions and knew that in a few short years this bubble of soft cuddly toys was going to burst. However, do those of us on the front line of medical care know what the future holds for the patients who are being prescribed or are scavenging those too-good-to-be-true medications?
My guess is that in the long run we will need a combination of some serious tinkering by the pharmaceutical industry and a trek up some steep learning curves before we eventually arrive at a safe and effective chemical management for obese patients. I recently read an article by an obesity management specialist at Harvard Medical School who voiced her concerns that we are missing an opportunity to make this explosion of popularity in GLP-1 drugs into an important learning experience.
In an opinion piece in JAMA Internal Medicine, Dr. Fatima Cody Stanford and her coauthors argue that we, actually the US Food and Drug Administration (FDA), is over-focused on weight loss in determining the efficacy of anti-obesity medications. Dr. Stanford and colleagues point out that when a patient loses weight it isn’t just fat — it is complex process that may include muscle and bone mineralization as well. She has consulted for at least one obesity-drug manufacturer and says that these companies have the resources to produce data on body composition that could help clinicians create management plans that would address the patients’ overall health. However, the FDA has not demanded this broader and deeper assessment of general health when reviewing the drug trials.
I don’t think we can blame the patients for not asking whether they will healthier while taking these medications. They have already spent a lifetime, even if it is just a decade, of suffering as the “fat one.” A new outfit and a look in the mirror can’t help but make them feel better ... in the short term anyway. We as physicians must shoulder some of the blame for focusing on weight. Our spoken or unspoken message has been “Lose weight and you will be healthier.” We may make our message sound more professional by tossing around terms like “BMI,” but as Dr. Stanford points out, “we have known BMI is a flawed metric for a long time.”
There is the notion that obese people have had to build more muscle to help them carry around the extra weight, so that we should expect them to lose that extra muscle along with the fat. However, in older adults there is an entity called sarcopenic obesity, in which the patient doesn’t have that extra muscle to lose.
In a brief Internet research venture, I could find little on the subject of muscle loss and GLP-1s, other than “it can happen.” And, nothing on the effect in adolescents. And that is one of Dr. Stanford’s points. We just don’t know. She said that looking at body composition can be costly and not something that the clinician can do. However, as far as muscle mass is concerned, we need to be alert to the potential for loss. Simple assessments of strength can help us tailor our management to the specific patient’s need.
The bottom line is this ... now that we have effective medications for “weight loss,” we need to redefine the relationship between weight and health. “We” means us as clinicians. It means the folks at FDA. And, if we can improve our messaging, it will osmose to the rest of the population. Just because you’ve dropped two dress sizes doesn’t mean you’re healthy.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Whether you have totally bought into the “obesity is a disease” paradigm or are still in denial, you must admit that the development of a suite of effective weight loss medications has created a tsunami of interest and economic activity in this country on a scale not seen since the Beanie Baby craze of the mid-1990s. But, obesity management is serious business. While most of those soft cuddly toys are gathering dust in shoeboxes across this country, weight loss medications are likely to be the vanguard of rapidly evolving revolution in healthcare management that will be with us for the foreseeable future.
Most thoughtful folks who purchased Beanie Babies in 1994 had no illusions and knew that in a few short years this bubble of soft cuddly toys was going to burst. However, do those of us on the front line of medical care know what the future holds for the patients who are being prescribed or are scavenging those too-good-to-be-true medications?
My guess is that in the long run we will need a combination of some serious tinkering by the pharmaceutical industry and a trek up some steep learning curves before we eventually arrive at a safe and effective chemical management for obese patients. I recently read an article by an obesity management specialist at Harvard Medical School who voiced her concerns that we are missing an opportunity to make this explosion of popularity in GLP-1 drugs into an important learning experience.
In an opinion piece in JAMA Internal Medicine, Dr. Fatima Cody Stanford and her coauthors argue that we, actually the US Food and Drug Administration (FDA), is over-focused on weight loss in determining the efficacy of anti-obesity medications. Dr. Stanford and colleagues point out that when a patient loses weight it isn’t just fat — it is complex process that may include muscle and bone mineralization as well. She has consulted for at least one obesity-drug manufacturer and says that these companies have the resources to produce data on body composition that could help clinicians create management plans that would address the patients’ overall health. However, the FDA has not demanded this broader and deeper assessment of general health when reviewing the drug trials.
I don’t think we can blame the patients for not asking whether they will healthier while taking these medications. They have already spent a lifetime, even if it is just a decade, of suffering as the “fat one.” A new outfit and a look in the mirror can’t help but make them feel better ... in the short term anyway. We as physicians must shoulder some of the blame for focusing on weight. Our spoken or unspoken message has been “Lose weight and you will be healthier.” We may make our message sound more professional by tossing around terms like “BMI,” but as Dr. Stanford points out, “we have known BMI is a flawed metric for a long time.”
There is the notion that obese people have had to build more muscle to help them carry around the extra weight, so that we should expect them to lose that extra muscle along with the fat. However, in older adults there is an entity called sarcopenic obesity, in which the patient doesn’t have that extra muscle to lose.
In a brief Internet research venture, I could find little on the subject of muscle loss and GLP-1s, other than “it can happen.” And, nothing on the effect in adolescents. And that is one of Dr. Stanford’s points. We just don’t know. She said that looking at body composition can be costly and not something that the clinician can do. However, as far as muscle mass is concerned, we need to be alert to the potential for loss. Simple assessments of strength can help us tailor our management to the specific patient’s need.
The bottom line is this ... now that we have effective medications for “weight loss,” we need to redefine the relationship between weight and health. “We” means us as clinicians. It means the folks at FDA. And, if we can improve our messaging, it will osmose to the rest of the population. Just because you’ve dropped two dress sizes doesn’t mean you’re healthy.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Whether you have totally bought into the “obesity is a disease” paradigm or are still in denial, you must admit that the development of a suite of effective weight loss medications has created a tsunami of interest and economic activity in this country on a scale not seen since the Beanie Baby craze of the mid-1990s. But, obesity management is serious business. While most of those soft cuddly toys are gathering dust in shoeboxes across this country, weight loss medications are likely to be the vanguard of rapidly evolving revolution in healthcare management that will be with us for the foreseeable future.
Most thoughtful folks who purchased Beanie Babies in 1994 had no illusions and knew that in a few short years this bubble of soft cuddly toys was going to burst. However, do those of us on the front line of medical care know what the future holds for the patients who are being prescribed or are scavenging those too-good-to-be-true medications?
My guess is that in the long run we will need a combination of some serious tinkering by the pharmaceutical industry and a trek up some steep learning curves before we eventually arrive at a safe and effective chemical management for obese patients. I recently read an article by an obesity management specialist at Harvard Medical School who voiced her concerns that we are missing an opportunity to make this explosion of popularity in GLP-1 drugs into an important learning experience.
In an opinion piece in JAMA Internal Medicine, Dr. Fatima Cody Stanford and her coauthors argue that we, actually the US Food and Drug Administration (FDA), is over-focused on weight loss in determining the efficacy of anti-obesity medications. Dr. Stanford and colleagues point out that when a patient loses weight it isn’t just fat — it is complex process that may include muscle and bone mineralization as well. She has consulted for at least one obesity-drug manufacturer and says that these companies have the resources to produce data on body composition that could help clinicians create management plans that would address the patients’ overall health. However, the FDA has not demanded this broader and deeper assessment of general health when reviewing the drug trials.
I don’t think we can blame the patients for not asking whether they will healthier while taking these medications. They have already spent a lifetime, even if it is just a decade, of suffering as the “fat one.” A new outfit and a look in the mirror can’t help but make them feel better ... in the short term anyway. We as physicians must shoulder some of the blame for focusing on weight. Our spoken or unspoken message has been “Lose weight and you will be healthier.” We may make our message sound more professional by tossing around terms like “BMI,” but as Dr. Stanford points out, “we have known BMI is a flawed metric for a long time.”
There is the notion that obese people have had to build more muscle to help them carry around the extra weight, so that we should expect them to lose that extra muscle along with the fat. However, in older adults there is an entity called sarcopenic obesity, in which the patient doesn’t have that extra muscle to lose.
In a brief Internet research venture, I could find little on the subject of muscle loss and GLP-1s, other than “it can happen.” And, nothing on the effect in adolescents. And that is one of Dr. Stanford’s points. We just don’t know. She said that looking at body composition can be costly and not something that the clinician can do. However, as far as muscle mass is concerned, we need to be alert to the potential for loss. Simple assessments of strength can help us tailor our management to the specific patient’s need.
The bottom line is this ... now that we have effective medications for “weight loss,” we need to redefine the relationship between weight and health. “We” means us as clinicians. It means the folks at FDA. And, if we can improve our messaging, it will osmose to the rest of the population. Just because you’ve dropped two dress sizes doesn’t mean you’re healthy.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].