User login
Veteran and Provider Perspectives on Telehealth for Vocational Rehabilitation Services
Vocational rehabilitation (VR) interventions are offered through Compensated Work Therapy (CWT) as part of clinical care in the Veterans Health Administration (VHA) to improve employment and quality of life outcomes for veterans with life-altering disabilities.1–5 CWT vocational services range from assessment, vocational counseling, and treatment plan development to job placement, coaching, and follow-along support.1 However, many veterans receive care in community-based clinics that are not staffed with a VR specialist (VRS) to provide these services.6–8 Telehealth may increase patient access to VR, especially for rural veterans and those with travel barriers, but it is not known whether veterans and VRS would find this to be a satisfactory service delivery method.8,9 This paper examines veteran and VRS provider perspectives on VR provided by telehealth (VRtele) as part of a VHA clinical demonstration project. To our knowledge, this is the first report of using real-time, clinic-based VRtele.
Methods
The Rural Veterans Supported Employment Telerehabilitation Initiative (RVSETI) was conducted as a field-initiated demonstration project at 2 US Department of Veterans Affairs (VA) medical centers (VAMCs) in Florida between 2014 and 2016: James A. Haley Veterans’ Hospital & Clinics (Tampa) and Malcom Randall VAMC (Gainesville). This retrospective evaluation of its first year did not require institutional review board approval as it was determined to be a quality improvement project by the local research service.
The patient population for the project was veterans with disabilities who were referred by clinical consults to the CWT service, a recovery-oriented vocational program. During the project years, veterans were offered the option of receiving VR services, such as supported employment, community-based employment services, or vocational assistance, through VRtele rather than traditional face-to-face meetings. The specific interventions delivered included patient orientation, interview assessment, treatment plan development, referral activities, vocational counseling, assessment of workplace for accommodation needs, vocational case management, and other employment supports. VR staff participating in the project included 2 VR supervisors, 1 supported employment mentor trainer, and 5 VRSs.
Each clinic was set up for VRtele, and codes were added to the electronic health record (EHR) to ensure proper documentation. Participating VRSs completed teleconferencing training, including a skills assessment using the equipment for real-time, high-quality video streaming over an encrypted network to provide services in a patient’s home or other remote locations. VRS staff provided veterans with instructions on using a VA-provided tablet or their own device and assisted them with establishing connectivity with the network. Video equipment included speakers, camera, and headphones connected to the desktop computer or laptop of the VRS. A patient’s first VRtele
Demographic data, primary diagnosis, VR usage data, and zip codes of participating veterans were extracted from the EHR. Veterans completed a 2-part satisfaction survey administered 90 days after enrollment and at discharge. Part 1 was composed of 15 items, most with a 5-point Likert scale (higher ratings indicated greater satisfaction), on various aspects of the VRtele experience, such as audio and video quality and wait times.10 Part 2 addressed VR services and the VRS and consisted of 8 Likert scale items with the option to add a comment for each and 2 open-ended items that asked the participant to list what they liked best and least about VRtele.
Semistructured, in-person 30- to 60-minute interviews were conducted with VRSs at the initiation of VRtele
After ≥ 2 months of VRtele use
Analyses
Descriptive statistics were used for EHR data and satisfaction surveys. For qualitative analysis, each transcript was read in full by 2 researchers to get an overview of the data, and a rapid analysis approach was used to identify central themes focused on how technology was used and the experiences of the participants.11,12 Relevant text for each topic was tabulated, and a summary table was created that highlighted overlapping ideas discussed by the interviewees as well as differences.
Results
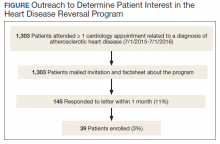
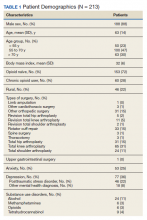
Of the 22 veterans who participated in the project, 11 completed satisfaction surveys and 4 participated in qualitative interviews. The rural and nonrural groups did not differ demographically or by diagnosis, which was predominantly mental health related. Only 1 veteran in each group owned a tablet; the majority of both groups required VA-issued devices: 80% (n = 8) rural and 91.7% (n = 11) nonrural. The number of VRtele sessions for the groups also was similar, 53 for rural and 60 for nonrural, as was the mean (SD) number of sessions per veteran: 5.3 (SD, 3.2) rural and 5.0 (SD, 2.5) urban. Overall, 63 miles per session were saved, mostly for rural veterans, and the number of mean (SD) miles saved per veteran was greater for rural than nonrural veterans: 379.2 (243.0) and 256.1 (275.9), respectively. One veteran who moved to a different state during the program continued VRtele at the new location. In a qualitative sampling of 5 VRtele sessions, all the VRSs used office desktop computers.
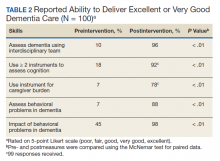
Level of satisfaction with aspects of VRtele related to the technology rated was consistently > 4 on the Likert scale. The lowest mean (SD) ratings were 4.2 (1.0) for audio quality and 4.4 (0.5) for video quality, and the highest rating was given for equipment operation explanation and privacy was respected, 4.9 (0.3) for both. All questions related to satisfaction with services were also rated high: The mean (SD) lowest ratings were 4.3 (1.0) given to both vocational needs 4.3 (1.0) and tasks effectively helped achieve goals 4.3 (0.7). The highest mean (SD) ratings were 4.6 (0.5) given to VR program service explained and 4.7 (0.5) for appointment timeliness.
Qualitative Results
At first, some VRSs thought the teleconferencing system might be difficult or awkward to use, but they found it easier to set up than expected and seamless to use. VRS staff reported being surprised at how well it worked despite some issues that occurred with loading the software. Once loaded, however, the connection worked well, one VRS noting that following step-by-step instructions solved the problem. Some VRSs indicated they did not invite all the veterans on their caseload to participate in VRtele due to concerns with the patient’s familiarity with technology, but one VRS stated, “I haven’t had anybody that failed to do a [session] that I couldn’t get them up and running within a few minutes.”
When working in the community, VRSs reported using laptops for VRtele but found that these devices were unreliable due to lack of internet access and were slow to start; several VRSs thought tablets would have been more helpful. Some veterans reported technical glitches, lack of comfort with technology, or a problem with sound due to a tablet’s protective case blocking the speakers. To solve the sound issue, a veteran used headphones. This veteran also explained that the log-on process required a new password every time, so he would keep a pen and paper ready to write it down. Because signing in and setting up takes a little time, this veteran and his VRS agreed to start connecting 5 minutes before their meeting time to allow for that set- up time.
Initially, some VRSs expressed concern that transitioning to VRtele would affect the quality of interactions with the veterans. However, VRSs also identified strengths of VRtele, including flexibility, saved time, and increased interaction. One VRS discussed a veteran’s adaptation by saying, “I think he feels even more involved in his plan [and] enjoys the increased interaction.” Veterans reported enjoying using tablets and identified the main strength of VRtele as being able to talk face-to-face with the VRS. Echoing the VRSs, veterans reported teleconferencing saved time by avoiding travel and enabled spontaneous meetings. One of the veterans summed up the benefits of using VRtele: “I’d rather just connect. It’s going to take us 40 to 50 minutes [to meet in person] when we can just connect right here and it takes 15 to 20. We don’t have to go through the driving.… So this right here, doing it ahead of time and having the appointment, it’s a lot easier.”
In their interviews, VRSs talked about enjoying VRtele. A VRS explained: “It makes it a lot easier. It makes me feel less guilty. This way [veterans] don’t have to use their gas money, use their time. I know [the veteran] had something else he needed to do today.” Thus, both veterans and VRSs were satisfied with their VRtele experiences.
Discussion
This first report on the perspective of providers and veterans using VRtele suggests that it is a viable option for service delivery and that is highly satisfactory for serving veterans with disabilities, many of whom live in rural areas or have travel barriers. These findings are consistent with data on telerehabilitation for veterans with cognitive, physical, and mental disabilities.13-22 Further, the data support the notion of using VRtele to facilitate long-term VR follow-up for persons with disabilities, as illustrated by successful continuation of vocational services after a veteran moved out of state.23
Similar to other reports, our experience highlighted 2 factors that affect successful VRtele: (1) Troubleshooting technology barriers for both VR providers and clients; and (2) supportive leadership to facilitate implementation
Changes to technology and increased usage of VA Video Connect may indicate that the barriers identified from the earlier process described here have been diminished or eliminated. More evaluation is needed to assess whether system upgrades have increased ease of use and access for veterans with disabilities.
Conclusions
Encouragingly, this clinical demonstration project showed that both providers and clients recognize the benefits of VRtele. Patient satisfaction and decreased travel costs were clear advantages to using VRtele for this small group of veterans who had barriers to care due to travel or disability barriers. As this program evaluation was limited by a small sample, absence of a comparison group, and lack of outcome data (eg, employment rates, hours, wages, retention), future research is needed on implementation and outcomes of VRtele
Acknowledgments
The authors thank Lynn Dirk, MAMC, for substantial editorial assistance. This material was based on work supported by Rural Veterans Supported Employment TeleRehabilitation Initiative (RVSETI), funded by the VA Office of Rural Health (Project # N08-FY14Q3-S2-P01222) and by support of the VA Health Services Research and Development Service. This work was presented in part at the 114th Annual Meeting of the American Anthropological Association at Denver, Colorado, November 21, 2015; a field-based Health Services Research and Development Service meeting, US Department of Veterans Affairs at Washington, DC, September 12, 2016; and the 2016 Annual Conference of the American Congress for Rehabilitation Medicine at Chicago, Illinois, October-November 2016.
1. Abraham KM, Yosef M, Resnick SG, Zivin K. Competitive employment outcomes among veterans in VHA therapeutic and supported employment services programs. Psychiatr Serv. 2017;68(9):938-946. doi:10.1176/appi.ps.201600412
2. Davis LL, Kyriakides TC, Suris AM, et al. Effect of evidence-based supported employment vs transitional work on achieving steady work among veterans with posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2018;75(4):316. doi:10.1001/jamapsychiatry.2017.4472
3. Ottomanelli L, Goetz LL, Suris A, et al. Effectiveness of supported employment for veterans with spinal cord injuries: results from a randomized multisite study. Arch Phys Med Rehabil. 2012;93(5):740-747. doi:10.1016/j.apmr.2012.01.002
4. Ottomanelli L, Goetz LL, Barnett SD, et al. Individual placement and support in spinal cord injury: a longitudinal observational study of employment outcomes. Arch Phys Med Rehabil. 2017;98(8):1567-1575. doi:10.1016/j.apmr.2016.12.010
5. Cotner BA, Ottomanelli L, O’Connor DR, Njoh EN, Barnett SD, Miech EJ. Quality of life outcomes for veterans with spinal cord injury receiving individual placement and support (IPS). Top Spinal Cord Inj Rehabil. 2018;24(4):325-335. doi:10.1310/sci17-00046
6. Metzel DS, Giordano A. Locations of employment services and people with disabilities: a geographical analysis of accessibility. J Disabil Policy Stud. 2007;18(2):88-97. doi:10.1177/10442073070180020501
7. Landon T, Connor A, McKnight-Lizotte M, Peña J. Rehabilitation counseling in rural settings: a phenomenological study on barriers and supports. J Rehabil. 2019;85(2):47-57.
8. Riemer-Reiss M. Vocational rehabilitation counseling at a distance: Challenges, strategies and ethics to consider. J Rehabil. 2000;66(1):11-17.
9. Schmeler MR, Schein RM, McCue M, Betz K. Telerehabilitation clinical and vocational applications for assistive technology: research, opportunities, and challenges. Int J Telerehabilitation. 2009;1(1):59-72.
10. Levy CE, Silverman E, Jia H, Geiss M, Omura D. Effects of physical therapy delivery via home video telerehabilitation on functional and health-related quality of life outcomes. J Rehabil Res Dev. 2015;52(3):361-370. doi:10.1682/JRRD.2014.10.0239
11. McMullen CK, Ash JS, Sittig DF, et al. Rapid assessment of clinical information systems in the healthcare setting: an efficient method for time-pressed evaluation. Methods Inf Med. 2011;50(4):299-307. doi:10.3414/ME10-01-0042
12. Averill JB. Matrix analysis as a complementary analytic strategy in qualitative inquiry. Qual Health Res. 2002;12(6):855-866.
13. Egede LE, Acierno R, Knapp RG, et al. Psychotherapy for depression in older veterans via telemedicine: a randomised, open-label, non-inferiority trial. Lancet Psychiatry. 2015;2(8):693-701. doi:10.1016/S2215-0366(15)00122-4
14. Fortney JC, Pyne JM, Edlund MJ, et al. A randomized trial of telemedicine-based collaborative care for depression. J Gen Intern Med. 2007;22(8):1086-1093. doi:10.1007/s11606-007-0201-9
15. Fortney JC, Pyne JM, Kimbrell TA, et al. Telemedicine-based collaborative care for posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2015;72(1):58. doi:10.1001/jamapsychiatry.2014.1575
16. Grubbs KM, Fortney JC, Dean T, Williams JS, Godleski L. A comparison of mental health diagnoses treated via interactive video and face to face in the Veterans Healthcare Administration. Telemed E-Health. 2015;21(7):564-566. doi:10.1089/tmj.2014.0152
17. Agostini M, Moja L, Banzi R, et al. Telerehabilitation and recovery of motor function: a systematic review and meta-analysis. J Telemed Telecare. 2015;21(4):202-213. doi:10.1177/1357633X15572201
18. Bergquist TF, Thompson K, Gehl C, Munoz Pineda J. Satisfaction ratings after receiving internet-based cognitive rehabilitation in persons with memory impairments after severe acquired brain injury. Telemed E-Health. 2010;16(4):417-423. doi:10.1089/tmj.2009.0118
19. Brennan DM, Georgeadis AC, Baron CR, Barker LM. The effect of videoconference-based telerehabilitation on story retelling performance by brain-injured subjects and its implications for remote speech-language therapy. Telemed J E Health. 2004;10(2):147-154. doi:10.1089/tmj.2004.10.147
20. Dallolio L, Menarini M, China S, et al. Functional and clinical outcomes of telemedicine in patients with spinal cord injury. Arch Phys Med Rehabil. 2008;89(12):2332-2341. doi:10.1016/j.apmr.2008.06.012
21. Houlihan BV, Jette A, Friedman RH, et al. A pilot study of a telehealth intervention for persons with spinal cord dysfunction. Spinal Cord. 2013;51(9):715-720.doi:10.1038/sc.2013.45
22. Smith MW, Hill ML, Hopkins KL, Kiratli BJ, Cronkite RC. A modeled analysis of telehealth methods for treating pressure ulcers after spinal cord injury. Int J Telemed Appl. 2012;2012:1-10. doi:10.1155/2012/729492
23. Balcazar FE, Keys CB, Davis M, Lardon C, Jones C. Strengths and challenges of intervention research in vocational rehabilitation: an illustration of agency-university collaboration. J Rehabil. 2005;71(2):40-48.
24. Martinez RN, Hogan TP, Balbale S, et al. Sociotechnical perspective on implementing clinical video telehealth for veterans with spinal cord injuries and disorders. Telemed J E Health. 2017;23(7):567-576. doi:10.1089/tmj.2016.0200
25. Martinez RN, Hogan TP, Lones K, et al. Evaluation and treatment of mild traumatic brain injury through the implementation of clinical video telehealth: provider perspectives from the Veterans Health Administration. PM R. 2017;9(3):231-240. doi:10.1016/j.pmrj.2016.07.002
26. Smith AC, Thomas E, Snoswell CL, et al. Telehealth for global emergencies: implications for coronavirus disease 2019 (COVID-19). J Telemed Telecare. 2020;26(5):309-313. doi:10.1177/1357633X20916567
27. Cowper-Ripley DC, Jia H, Wang X, et al. Trends in VA telerehabilitation patients and encounters over time and by rurality. Fed Pract. 2019; 36(3):122-128.
28. US Department of Veterans Affairs. Veterans VA Video Connect. Published May 22, 2020. Accessed May 29, 2020. https://mobile.va.gov/app/va-video-connect#AppDescription.
29. US Department of Veterans Affairs. VA telehealth at home. Accessed May 29, 2020. https://telehealth.va.gov/type/home
Vocational rehabilitation (VR) interventions are offered through Compensated Work Therapy (CWT) as part of clinical care in the Veterans Health Administration (VHA) to improve employment and quality of life outcomes for veterans with life-altering disabilities.1–5 CWT vocational services range from assessment, vocational counseling, and treatment plan development to job placement, coaching, and follow-along support.1 However, many veterans receive care in community-based clinics that are not staffed with a VR specialist (VRS) to provide these services.6–8 Telehealth may increase patient access to VR, especially for rural veterans and those with travel barriers, but it is not known whether veterans and VRS would find this to be a satisfactory service delivery method.8,9 This paper examines veteran and VRS provider perspectives on VR provided by telehealth (VRtele) as part of a VHA clinical demonstration project. To our knowledge, this is the first report of using real-time, clinic-based VRtele.
Methods
The Rural Veterans Supported Employment Telerehabilitation Initiative (RVSETI) was conducted as a field-initiated demonstration project at 2 US Department of Veterans Affairs (VA) medical centers (VAMCs) in Florida between 2014 and 2016: James A. Haley Veterans’ Hospital & Clinics (Tampa) and Malcom Randall VAMC (Gainesville). This retrospective evaluation of its first year did not require institutional review board approval as it was determined to be a quality improvement project by the local research service.
The patient population for the project was veterans with disabilities who were referred by clinical consults to the CWT service, a recovery-oriented vocational program. During the project years, veterans were offered the option of receiving VR services, such as supported employment, community-based employment services, or vocational assistance, through VRtele rather than traditional face-to-face meetings. The specific interventions delivered included patient orientation, interview assessment, treatment plan development, referral activities, vocational counseling, assessment of workplace for accommodation needs, vocational case management, and other employment supports. VR staff participating in the project included 2 VR supervisors, 1 supported employment mentor trainer, and 5 VRSs.
Each clinic was set up for VRtele, and codes were added to the electronic health record (EHR) to ensure proper documentation. Participating VRSs completed teleconferencing training, including a skills assessment using the equipment for real-time, high-quality video streaming over an encrypted network to provide services in a patient’s home or other remote locations. VRS staff provided veterans with instructions on using a VA-provided tablet or their own device and assisted them with establishing connectivity with the network. Video equipment included speakers, camera, and headphones connected to the desktop computer or laptop of the VRS. A patient’s first VRtele
Demographic data, primary diagnosis, VR usage data, and zip codes of participating veterans were extracted from the EHR. Veterans completed a 2-part satisfaction survey administered 90 days after enrollment and at discharge. Part 1 was composed of 15 items, most with a 5-point Likert scale (higher ratings indicated greater satisfaction), on various aspects of the VRtele experience, such as audio and video quality and wait times.10 Part 2 addressed VR services and the VRS and consisted of 8 Likert scale items with the option to add a comment for each and 2 open-ended items that asked the participant to list what they liked best and least about VRtele.
Semistructured, in-person 30- to 60-minute interviews were conducted with VRSs at the initiation of VRtele
After ≥ 2 months of VRtele use
Analyses
Descriptive statistics were used for EHR data and satisfaction surveys. For qualitative analysis, each transcript was read in full by 2 researchers to get an overview of the data, and a rapid analysis approach was used to identify central themes focused on how technology was used and the experiences of the participants.11,12 Relevant text for each topic was tabulated, and a summary table was created that highlighted overlapping ideas discussed by the interviewees as well as differences.
Results
Of the 22 veterans who participated in the project, 11 completed satisfaction surveys and 4 participated in qualitative interviews. The rural and nonrural groups did not differ demographically or by diagnosis, which was predominantly mental health related. Only 1 veteran in each group owned a tablet; the majority of both groups required VA-issued devices: 80% (n = 8) rural and 91.7% (n = 11) nonrural. The number of VRtele sessions for the groups also was similar, 53 for rural and 60 for nonrural, as was the mean (SD) number of sessions per veteran: 5.3 (SD, 3.2) rural and 5.0 (SD, 2.5) urban. Overall, 63 miles per session were saved, mostly for rural veterans, and the number of mean (SD) miles saved per veteran was greater for rural than nonrural veterans: 379.2 (243.0) and 256.1 (275.9), respectively. One veteran who moved to a different state during the program continued VRtele at the new location. In a qualitative sampling of 5 VRtele sessions, all the VRSs used office desktop computers.
Level of satisfaction with aspects of VRtele related to the technology rated was consistently > 4 on the Likert scale. The lowest mean (SD) ratings were 4.2 (1.0) for audio quality and 4.4 (0.5) for video quality, and the highest rating was given for equipment operation explanation and privacy was respected, 4.9 (0.3) for both. All questions related to satisfaction with services were also rated high: The mean (SD) lowest ratings were 4.3 (1.0) given to both vocational needs 4.3 (1.0) and tasks effectively helped achieve goals 4.3 (0.7). The highest mean (SD) ratings were 4.6 (0.5) given to VR program service explained and 4.7 (0.5) for appointment timeliness.
Qualitative Results
At first, some VRSs thought the teleconferencing system might be difficult or awkward to use, but they found it easier to set up than expected and seamless to use. VRS staff reported being surprised at how well it worked despite some issues that occurred with loading the software. Once loaded, however, the connection worked well, one VRS noting that following step-by-step instructions solved the problem. Some VRSs indicated they did not invite all the veterans on their caseload to participate in VRtele due to concerns with the patient’s familiarity with technology, but one VRS stated, “I haven’t had anybody that failed to do a [session] that I couldn’t get them up and running within a few minutes.”
When working in the community, VRSs reported using laptops for VRtele but found that these devices were unreliable due to lack of internet access and were slow to start; several VRSs thought tablets would have been more helpful. Some veterans reported technical glitches, lack of comfort with technology, or a problem with sound due to a tablet’s protective case blocking the speakers. To solve the sound issue, a veteran used headphones. This veteran also explained that the log-on process required a new password every time, so he would keep a pen and paper ready to write it down. Because signing in and setting up takes a little time, this veteran and his VRS agreed to start connecting 5 minutes before their meeting time to allow for that set- up time.
Initially, some VRSs expressed concern that transitioning to VRtele would affect the quality of interactions with the veterans. However, VRSs also identified strengths of VRtele, including flexibility, saved time, and increased interaction. One VRS discussed a veteran’s adaptation by saying, “I think he feels even more involved in his plan [and] enjoys the increased interaction.” Veterans reported enjoying using tablets and identified the main strength of VRtele as being able to talk face-to-face with the VRS. Echoing the VRSs, veterans reported teleconferencing saved time by avoiding travel and enabled spontaneous meetings. One of the veterans summed up the benefits of using VRtele: “I’d rather just connect. It’s going to take us 40 to 50 minutes [to meet in person] when we can just connect right here and it takes 15 to 20. We don’t have to go through the driving.… So this right here, doing it ahead of time and having the appointment, it’s a lot easier.”
In their interviews, VRSs talked about enjoying VRtele. A VRS explained: “It makes it a lot easier. It makes me feel less guilty. This way [veterans] don’t have to use their gas money, use their time. I know [the veteran] had something else he needed to do today.” Thus, both veterans and VRSs were satisfied with their VRtele experiences.
Discussion
This first report on the perspective of providers and veterans using VRtele suggests that it is a viable option for service delivery and that is highly satisfactory for serving veterans with disabilities, many of whom live in rural areas or have travel barriers. These findings are consistent with data on telerehabilitation for veterans with cognitive, physical, and mental disabilities.13-22 Further, the data support the notion of using VRtele to facilitate long-term VR follow-up for persons with disabilities, as illustrated by successful continuation of vocational services after a veteran moved out of state.23
Similar to other reports, our experience highlighted 2 factors that affect successful VRtele: (1) Troubleshooting technology barriers for both VR providers and clients; and (2) supportive leadership to facilitate implementation
Changes to technology and increased usage of VA Video Connect may indicate that the barriers identified from the earlier process described here have been diminished or eliminated. More evaluation is needed to assess whether system upgrades have increased ease of use and access for veterans with disabilities.
Conclusions
Encouragingly, this clinical demonstration project showed that both providers and clients recognize the benefits of VRtele. Patient satisfaction and decreased travel costs were clear advantages to using VRtele for this small group of veterans who had barriers to care due to travel or disability barriers. As this program evaluation was limited by a small sample, absence of a comparison group, and lack of outcome data (eg, employment rates, hours, wages, retention), future research is needed on implementation and outcomes of VRtele
Acknowledgments
The authors thank Lynn Dirk, MAMC, for substantial editorial assistance. This material was based on work supported by Rural Veterans Supported Employment TeleRehabilitation Initiative (RVSETI), funded by the VA Office of Rural Health (Project # N08-FY14Q3-S2-P01222) and by support of the VA Health Services Research and Development Service. This work was presented in part at the 114th Annual Meeting of the American Anthropological Association at Denver, Colorado, November 21, 2015; a field-based Health Services Research and Development Service meeting, US Department of Veterans Affairs at Washington, DC, September 12, 2016; and the 2016 Annual Conference of the American Congress for Rehabilitation Medicine at Chicago, Illinois, October-November 2016.
Vocational rehabilitation (VR) interventions are offered through Compensated Work Therapy (CWT) as part of clinical care in the Veterans Health Administration (VHA) to improve employment and quality of life outcomes for veterans with life-altering disabilities.1–5 CWT vocational services range from assessment, vocational counseling, and treatment plan development to job placement, coaching, and follow-along support.1 However, many veterans receive care in community-based clinics that are not staffed with a VR specialist (VRS) to provide these services.6–8 Telehealth may increase patient access to VR, especially for rural veterans and those with travel barriers, but it is not known whether veterans and VRS would find this to be a satisfactory service delivery method.8,9 This paper examines veteran and VRS provider perspectives on VR provided by telehealth (VRtele) as part of a VHA clinical demonstration project. To our knowledge, this is the first report of using real-time, clinic-based VRtele.
Methods
The Rural Veterans Supported Employment Telerehabilitation Initiative (RVSETI) was conducted as a field-initiated demonstration project at 2 US Department of Veterans Affairs (VA) medical centers (VAMCs) in Florida between 2014 and 2016: James A. Haley Veterans’ Hospital & Clinics (Tampa) and Malcom Randall VAMC (Gainesville). This retrospective evaluation of its first year did not require institutional review board approval as it was determined to be a quality improvement project by the local research service.
The patient population for the project was veterans with disabilities who were referred by clinical consults to the CWT service, a recovery-oriented vocational program. During the project years, veterans were offered the option of receiving VR services, such as supported employment, community-based employment services, or vocational assistance, through VRtele rather than traditional face-to-face meetings. The specific interventions delivered included patient orientation, interview assessment, treatment plan development, referral activities, vocational counseling, assessment of workplace for accommodation needs, vocational case management, and other employment supports. VR staff participating in the project included 2 VR supervisors, 1 supported employment mentor trainer, and 5 VRSs.
Each clinic was set up for VRtele, and codes were added to the electronic health record (EHR) to ensure proper documentation. Participating VRSs completed teleconferencing training, including a skills assessment using the equipment for real-time, high-quality video streaming over an encrypted network to provide services in a patient’s home or other remote locations. VRS staff provided veterans with instructions on using a VA-provided tablet or their own device and assisted them with establishing connectivity with the network. Video equipment included speakers, camera, and headphones connected to the desktop computer or laptop of the VRS. A patient’s first VRtele
Demographic data, primary diagnosis, VR usage data, and zip codes of participating veterans were extracted from the EHR. Veterans completed a 2-part satisfaction survey administered 90 days after enrollment and at discharge. Part 1 was composed of 15 items, most with a 5-point Likert scale (higher ratings indicated greater satisfaction), on various aspects of the VRtele experience, such as audio and video quality and wait times.10 Part 2 addressed VR services and the VRS and consisted of 8 Likert scale items with the option to add a comment for each and 2 open-ended items that asked the participant to list what they liked best and least about VRtele.
Semistructured, in-person 30- to 60-minute interviews were conducted with VRSs at the initiation of VRtele
After ≥ 2 months of VRtele use
Analyses
Descriptive statistics were used for EHR data and satisfaction surveys. For qualitative analysis, each transcript was read in full by 2 researchers to get an overview of the data, and a rapid analysis approach was used to identify central themes focused on how technology was used and the experiences of the participants.11,12 Relevant text for each topic was tabulated, and a summary table was created that highlighted overlapping ideas discussed by the interviewees as well as differences.
Results
Of the 22 veterans who participated in the project, 11 completed satisfaction surveys and 4 participated in qualitative interviews. The rural and nonrural groups did not differ demographically or by diagnosis, which was predominantly mental health related. Only 1 veteran in each group owned a tablet; the majority of both groups required VA-issued devices: 80% (n = 8) rural and 91.7% (n = 11) nonrural. The number of VRtele sessions for the groups also was similar, 53 for rural and 60 for nonrural, as was the mean (SD) number of sessions per veteran: 5.3 (SD, 3.2) rural and 5.0 (SD, 2.5) urban. Overall, 63 miles per session were saved, mostly for rural veterans, and the number of mean (SD) miles saved per veteran was greater for rural than nonrural veterans: 379.2 (243.0) and 256.1 (275.9), respectively. One veteran who moved to a different state during the program continued VRtele at the new location. In a qualitative sampling of 5 VRtele sessions, all the VRSs used office desktop computers.
Level of satisfaction with aspects of VRtele related to the technology rated was consistently > 4 on the Likert scale. The lowest mean (SD) ratings were 4.2 (1.0) for audio quality and 4.4 (0.5) for video quality, and the highest rating was given for equipment operation explanation and privacy was respected, 4.9 (0.3) for both. All questions related to satisfaction with services were also rated high: The mean (SD) lowest ratings were 4.3 (1.0) given to both vocational needs 4.3 (1.0) and tasks effectively helped achieve goals 4.3 (0.7). The highest mean (SD) ratings were 4.6 (0.5) given to VR program service explained and 4.7 (0.5) for appointment timeliness.
Qualitative Results
At first, some VRSs thought the teleconferencing system might be difficult or awkward to use, but they found it easier to set up than expected and seamless to use. VRS staff reported being surprised at how well it worked despite some issues that occurred with loading the software. Once loaded, however, the connection worked well, one VRS noting that following step-by-step instructions solved the problem. Some VRSs indicated they did not invite all the veterans on their caseload to participate in VRtele due to concerns with the patient’s familiarity with technology, but one VRS stated, “I haven’t had anybody that failed to do a [session] that I couldn’t get them up and running within a few minutes.”
When working in the community, VRSs reported using laptops for VRtele but found that these devices were unreliable due to lack of internet access and were slow to start; several VRSs thought tablets would have been more helpful. Some veterans reported technical glitches, lack of comfort with technology, or a problem with sound due to a tablet’s protective case blocking the speakers. To solve the sound issue, a veteran used headphones. This veteran also explained that the log-on process required a new password every time, so he would keep a pen and paper ready to write it down. Because signing in and setting up takes a little time, this veteran and his VRS agreed to start connecting 5 minutes before their meeting time to allow for that set- up time.
Initially, some VRSs expressed concern that transitioning to VRtele would affect the quality of interactions with the veterans. However, VRSs also identified strengths of VRtele, including flexibility, saved time, and increased interaction. One VRS discussed a veteran’s adaptation by saying, “I think he feels even more involved in his plan [and] enjoys the increased interaction.” Veterans reported enjoying using tablets and identified the main strength of VRtele as being able to talk face-to-face with the VRS. Echoing the VRSs, veterans reported teleconferencing saved time by avoiding travel and enabled spontaneous meetings. One of the veterans summed up the benefits of using VRtele: “I’d rather just connect. It’s going to take us 40 to 50 minutes [to meet in person] when we can just connect right here and it takes 15 to 20. We don’t have to go through the driving.… So this right here, doing it ahead of time and having the appointment, it’s a lot easier.”
In their interviews, VRSs talked about enjoying VRtele. A VRS explained: “It makes it a lot easier. It makes me feel less guilty. This way [veterans] don’t have to use their gas money, use their time. I know [the veteran] had something else he needed to do today.” Thus, both veterans and VRSs were satisfied with their VRtele experiences.
Discussion
This first report on the perspective of providers and veterans using VRtele suggests that it is a viable option for service delivery and that is highly satisfactory for serving veterans with disabilities, many of whom live in rural areas or have travel barriers. These findings are consistent with data on telerehabilitation for veterans with cognitive, physical, and mental disabilities.13-22 Further, the data support the notion of using VRtele to facilitate long-term VR follow-up for persons with disabilities, as illustrated by successful continuation of vocational services after a veteran moved out of state.23
Similar to other reports, our experience highlighted 2 factors that affect successful VRtele: (1) Troubleshooting technology barriers for both VR providers and clients; and (2) supportive leadership to facilitate implementation
Changes to technology and increased usage of VA Video Connect may indicate that the barriers identified from the earlier process described here have been diminished or eliminated. More evaluation is needed to assess whether system upgrades have increased ease of use and access for veterans with disabilities.
Conclusions
Encouragingly, this clinical demonstration project showed that both providers and clients recognize the benefits of VRtele. Patient satisfaction and decreased travel costs were clear advantages to using VRtele for this small group of veterans who had barriers to care due to travel or disability barriers. As this program evaluation was limited by a small sample, absence of a comparison group, and lack of outcome data (eg, employment rates, hours, wages, retention), future research is needed on implementation and outcomes of VRtele
Acknowledgments
The authors thank Lynn Dirk, MAMC, for substantial editorial assistance. This material was based on work supported by Rural Veterans Supported Employment TeleRehabilitation Initiative (RVSETI), funded by the VA Office of Rural Health (Project # N08-FY14Q3-S2-P01222) and by support of the VA Health Services Research and Development Service. This work was presented in part at the 114th Annual Meeting of the American Anthropological Association at Denver, Colorado, November 21, 2015; a field-based Health Services Research and Development Service meeting, US Department of Veterans Affairs at Washington, DC, September 12, 2016; and the 2016 Annual Conference of the American Congress for Rehabilitation Medicine at Chicago, Illinois, October-November 2016.
1. Abraham KM, Yosef M, Resnick SG, Zivin K. Competitive employment outcomes among veterans in VHA therapeutic and supported employment services programs. Psychiatr Serv. 2017;68(9):938-946. doi:10.1176/appi.ps.201600412
2. Davis LL, Kyriakides TC, Suris AM, et al. Effect of evidence-based supported employment vs transitional work on achieving steady work among veterans with posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2018;75(4):316. doi:10.1001/jamapsychiatry.2017.4472
3. Ottomanelli L, Goetz LL, Suris A, et al. Effectiveness of supported employment for veterans with spinal cord injuries: results from a randomized multisite study. Arch Phys Med Rehabil. 2012;93(5):740-747. doi:10.1016/j.apmr.2012.01.002
4. Ottomanelli L, Goetz LL, Barnett SD, et al. Individual placement and support in spinal cord injury: a longitudinal observational study of employment outcomes. Arch Phys Med Rehabil. 2017;98(8):1567-1575. doi:10.1016/j.apmr.2016.12.010
5. Cotner BA, Ottomanelli L, O’Connor DR, Njoh EN, Barnett SD, Miech EJ. Quality of life outcomes for veterans with spinal cord injury receiving individual placement and support (IPS). Top Spinal Cord Inj Rehabil. 2018;24(4):325-335. doi:10.1310/sci17-00046
6. Metzel DS, Giordano A. Locations of employment services and people with disabilities: a geographical analysis of accessibility. J Disabil Policy Stud. 2007;18(2):88-97. doi:10.1177/10442073070180020501
7. Landon T, Connor A, McKnight-Lizotte M, Peña J. Rehabilitation counseling in rural settings: a phenomenological study on barriers and supports. J Rehabil. 2019;85(2):47-57.
8. Riemer-Reiss M. Vocational rehabilitation counseling at a distance: Challenges, strategies and ethics to consider. J Rehabil. 2000;66(1):11-17.
9. Schmeler MR, Schein RM, McCue M, Betz K. Telerehabilitation clinical and vocational applications for assistive technology: research, opportunities, and challenges. Int J Telerehabilitation. 2009;1(1):59-72.
10. Levy CE, Silverman E, Jia H, Geiss M, Omura D. Effects of physical therapy delivery via home video telerehabilitation on functional and health-related quality of life outcomes. J Rehabil Res Dev. 2015;52(3):361-370. doi:10.1682/JRRD.2014.10.0239
11. McMullen CK, Ash JS, Sittig DF, et al. Rapid assessment of clinical information systems in the healthcare setting: an efficient method for time-pressed evaluation. Methods Inf Med. 2011;50(4):299-307. doi:10.3414/ME10-01-0042
12. Averill JB. Matrix analysis as a complementary analytic strategy in qualitative inquiry. Qual Health Res. 2002;12(6):855-866.
13. Egede LE, Acierno R, Knapp RG, et al. Psychotherapy for depression in older veterans via telemedicine: a randomised, open-label, non-inferiority trial. Lancet Psychiatry. 2015;2(8):693-701. doi:10.1016/S2215-0366(15)00122-4
14. Fortney JC, Pyne JM, Edlund MJ, et al. A randomized trial of telemedicine-based collaborative care for depression. J Gen Intern Med. 2007;22(8):1086-1093. doi:10.1007/s11606-007-0201-9
15. Fortney JC, Pyne JM, Kimbrell TA, et al. Telemedicine-based collaborative care for posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2015;72(1):58. doi:10.1001/jamapsychiatry.2014.1575
16. Grubbs KM, Fortney JC, Dean T, Williams JS, Godleski L. A comparison of mental health diagnoses treated via interactive video and face to face in the Veterans Healthcare Administration. Telemed E-Health. 2015;21(7):564-566. doi:10.1089/tmj.2014.0152
17. Agostini M, Moja L, Banzi R, et al. Telerehabilitation and recovery of motor function: a systematic review and meta-analysis. J Telemed Telecare. 2015;21(4):202-213. doi:10.1177/1357633X15572201
18. Bergquist TF, Thompson K, Gehl C, Munoz Pineda J. Satisfaction ratings after receiving internet-based cognitive rehabilitation in persons with memory impairments after severe acquired brain injury. Telemed E-Health. 2010;16(4):417-423. doi:10.1089/tmj.2009.0118
19. Brennan DM, Georgeadis AC, Baron CR, Barker LM. The effect of videoconference-based telerehabilitation on story retelling performance by brain-injured subjects and its implications for remote speech-language therapy. Telemed J E Health. 2004;10(2):147-154. doi:10.1089/tmj.2004.10.147
20. Dallolio L, Menarini M, China S, et al. Functional and clinical outcomes of telemedicine in patients with spinal cord injury. Arch Phys Med Rehabil. 2008;89(12):2332-2341. doi:10.1016/j.apmr.2008.06.012
21. Houlihan BV, Jette A, Friedman RH, et al. A pilot study of a telehealth intervention for persons with spinal cord dysfunction. Spinal Cord. 2013;51(9):715-720.doi:10.1038/sc.2013.45
22. Smith MW, Hill ML, Hopkins KL, Kiratli BJ, Cronkite RC. A modeled analysis of telehealth methods for treating pressure ulcers after spinal cord injury. Int J Telemed Appl. 2012;2012:1-10. doi:10.1155/2012/729492
23. Balcazar FE, Keys CB, Davis M, Lardon C, Jones C. Strengths and challenges of intervention research in vocational rehabilitation: an illustration of agency-university collaboration. J Rehabil. 2005;71(2):40-48.
24. Martinez RN, Hogan TP, Balbale S, et al. Sociotechnical perspective on implementing clinical video telehealth for veterans with spinal cord injuries and disorders. Telemed J E Health. 2017;23(7):567-576. doi:10.1089/tmj.2016.0200
25. Martinez RN, Hogan TP, Lones K, et al. Evaluation and treatment of mild traumatic brain injury through the implementation of clinical video telehealth: provider perspectives from the Veterans Health Administration. PM R. 2017;9(3):231-240. doi:10.1016/j.pmrj.2016.07.002
26. Smith AC, Thomas E, Snoswell CL, et al. Telehealth for global emergencies: implications for coronavirus disease 2019 (COVID-19). J Telemed Telecare. 2020;26(5):309-313. doi:10.1177/1357633X20916567
27. Cowper-Ripley DC, Jia H, Wang X, et al. Trends in VA telerehabilitation patients and encounters over time and by rurality. Fed Pract. 2019; 36(3):122-128.
28. US Department of Veterans Affairs. Veterans VA Video Connect. Published May 22, 2020. Accessed May 29, 2020. https://mobile.va.gov/app/va-video-connect#AppDescription.
29. US Department of Veterans Affairs. VA telehealth at home. Accessed May 29, 2020. https://telehealth.va.gov/type/home
1. Abraham KM, Yosef M, Resnick SG, Zivin K. Competitive employment outcomes among veterans in VHA therapeutic and supported employment services programs. Psychiatr Serv. 2017;68(9):938-946. doi:10.1176/appi.ps.201600412
2. Davis LL, Kyriakides TC, Suris AM, et al. Effect of evidence-based supported employment vs transitional work on achieving steady work among veterans with posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2018;75(4):316. doi:10.1001/jamapsychiatry.2017.4472
3. Ottomanelli L, Goetz LL, Suris A, et al. Effectiveness of supported employment for veterans with spinal cord injuries: results from a randomized multisite study. Arch Phys Med Rehabil. 2012;93(5):740-747. doi:10.1016/j.apmr.2012.01.002
4. Ottomanelli L, Goetz LL, Barnett SD, et al. Individual placement and support in spinal cord injury: a longitudinal observational study of employment outcomes. Arch Phys Med Rehabil. 2017;98(8):1567-1575. doi:10.1016/j.apmr.2016.12.010
5. Cotner BA, Ottomanelli L, O’Connor DR, Njoh EN, Barnett SD, Miech EJ. Quality of life outcomes for veterans with spinal cord injury receiving individual placement and support (IPS). Top Spinal Cord Inj Rehabil. 2018;24(4):325-335. doi:10.1310/sci17-00046
6. Metzel DS, Giordano A. Locations of employment services and people with disabilities: a geographical analysis of accessibility. J Disabil Policy Stud. 2007;18(2):88-97. doi:10.1177/10442073070180020501
7. Landon T, Connor A, McKnight-Lizotte M, Peña J. Rehabilitation counseling in rural settings: a phenomenological study on barriers and supports. J Rehabil. 2019;85(2):47-57.
8. Riemer-Reiss M. Vocational rehabilitation counseling at a distance: Challenges, strategies and ethics to consider. J Rehabil. 2000;66(1):11-17.
9. Schmeler MR, Schein RM, McCue M, Betz K. Telerehabilitation clinical and vocational applications for assistive technology: research, opportunities, and challenges. Int J Telerehabilitation. 2009;1(1):59-72.
10. Levy CE, Silverman E, Jia H, Geiss M, Omura D. Effects of physical therapy delivery via home video telerehabilitation on functional and health-related quality of life outcomes. J Rehabil Res Dev. 2015;52(3):361-370. doi:10.1682/JRRD.2014.10.0239
11. McMullen CK, Ash JS, Sittig DF, et al. Rapid assessment of clinical information systems in the healthcare setting: an efficient method for time-pressed evaluation. Methods Inf Med. 2011;50(4):299-307. doi:10.3414/ME10-01-0042
12. Averill JB. Matrix analysis as a complementary analytic strategy in qualitative inquiry. Qual Health Res. 2002;12(6):855-866.
13. Egede LE, Acierno R, Knapp RG, et al. Psychotherapy for depression in older veterans via telemedicine: a randomised, open-label, non-inferiority trial. Lancet Psychiatry. 2015;2(8):693-701. doi:10.1016/S2215-0366(15)00122-4
14. Fortney JC, Pyne JM, Edlund MJ, et al. A randomized trial of telemedicine-based collaborative care for depression. J Gen Intern Med. 2007;22(8):1086-1093. doi:10.1007/s11606-007-0201-9
15. Fortney JC, Pyne JM, Kimbrell TA, et al. Telemedicine-based collaborative care for posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2015;72(1):58. doi:10.1001/jamapsychiatry.2014.1575
16. Grubbs KM, Fortney JC, Dean T, Williams JS, Godleski L. A comparison of mental health diagnoses treated via interactive video and face to face in the Veterans Healthcare Administration. Telemed E-Health. 2015;21(7):564-566. doi:10.1089/tmj.2014.0152
17. Agostini M, Moja L, Banzi R, et al. Telerehabilitation and recovery of motor function: a systematic review and meta-analysis. J Telemed Telecare. 2015;21(4):202-213. doi:10.1177/1357633X15572201
18. Bergquist TF, Thompson K, Gehl C, Munoz Pineda J. Satisfaction ratings after receiving internet-based cognitive rehabilitation in persons with memory impairments after severe acquired brain injury. Telemed E-Health. 2010;16(4):417-423. doi:10.1089/tmj.2009.0118
19. Brennan DM, Georgeadis AC, Baron CR, Barker LM. The effect of videoconference-based telerehabilitation on story retelling performance by brain-injured subjects and its implications for remote speech-language therapy. Telemed J E Health. 2004;10(2):147-154. doi:10.1089/tmj.2004.10.147
20. Dallolio L, Menarini M, China S, et al. Functional and clinical outcomes of telemedicine in patients with spinal cord injury. Arch Phys Med Rehabil. 2008;89(12):2332-2341. doi:10.1016/j.apmr.2008.06.012
21. Houlihan BV, Jette A, Friedman RH, et al. A pilot study of a telehealth intervention for persons with spinal cord dysfunction. Spinal Cord. 2013;51(9):715-720.doi:10.1038/sc.2013.45
22. Smith MW, Hill ML, Hopkins KL, Kiratli BJ, Cronkite RC. A modeled analysis of telehealth methods for treating pressure ulcers after spinal cord injury. Int J Telemed Appl. 2012;2012:1-10. doi:10.1155/2012/729492
23. Balcazar FE, Keys CB, Davis M, Lardon C, Jones C. Strengths and challenges of intervention research in vocational rehabilitation: an illustration of agency-university collaboration. J Rehabil. 2005;71(2):40-48.
24. Martinez RN, Hogan TP, Balbale S, et al. Sociotechnical perspective on implementing clinical video telehealth for veterans with spinal cord injuries and disorders. Telemed J E Health. 2017;23(7):567-576. doi:10.1089/tmj.2016.0200
25. Martinez RN, Hogan TP, Lones K, et al. Evaluation and treatment of mild traumatic brain injury through the implementation of clinical video telehealth: provider perspectives from the Veterans Health Administration. PM R. 2017;9(3):231-240. doi:10.1016/j.pmrj.2016.07.002
26. Smith AC, Thomas E, Snoswell CL, et al. Telehealth for global emergencies: implications for coronavirus disease 2019 (COVID-19). J Telemed Telecare. 2020;26(5):309-313. doi:10.1177/1357633X20916567
27. Cowper-Ripley DC, Jia H, Wang X, et al. Trends in VA telerehabilitation patients and encounters over time and by rurality. Fed Pract. 2019; 36(3):122-128.
28. US Department of Veterans Affairs. Veterans VA Video Connect. Published May 22, 2020. Accessed May 29, 2020. https://mobile.va.gov/app/va-video-connect#AppDescription.
29. US Department of Veterans Affairs. VA telehealth at home. Accessed May 29, 2020. https://telehealth.va.gov/type/home
Creating a Sustainable and Reliable Emergency Preparedness Program to Promote Appropriate Health Care Resources Use
Over the past decade, natural disasters and health care emergencies have increased 74%, averaging 400 documented events per year.1 These unpredictable and sometimes devastating events negatively impact the physical and mental health of communities, taxing already stretched health care system resources and the economy.2,3 During many of these events, patients inappropriately use hospitals, emergency departments (EDs), and critical care resources for chronic disease and elective health care management, resulting in medication shortages, health care access concerns, and treatment delays.4
Most available emergency preparedness programs rely solely on volunteers and/or public health providers to address the resultant coverage gap; however, instability in state and federal funding can make it difficult to maintain and sustain focused preparedness and response efforts. Alaska’s vast geography, low population density (1.2 people per square mile), and access limitations (about 200 villages only reachable by air or boat) make it especially challenging to provide reliable and sustained emergency preparedness and response support. Therefore, all eligible health care providers (HCPs) in Alaska must be involved in preparedness and response efforts.
Despite being the most accessible HCPs, pharmacists and student pharmacists, have not been actively involved in statewide emergency preparedness planning and disaster management efforts in Alaska. In preparation for and during disasters, for example, pharmacists may administer vaccinations, conduct point of care testing, dispense emergency medications, provide emergency medication refills, help mitigate medication shortages, and provide reliable health information to other health care professionals, patients, and their families as they prepare for and manage care during the event.4
The goal of this paper is to share the experience at the University of Alaska Anchorage/Idaho State University College of Pharmacy (UAA/ISU) in the development and implementation of a sustainable emergency preparedness and response support network (EPRSN) model; leveraging an established university student leadership structure and Doctor of Pharmacy (PharmD) students to support sharing of information among community pharmacies, state emergency response teams, and community members.
2018 Alaska Earthquake
On November 30, 2018, southcentral Alaska experienced a magnitude 7.1 earthquake, affecting nearly 295,000 people (approximately 40% of Alaska’s population) damaging roads, buildings, homes, and health care facilities. Emergency response efforts were quickly overwhelmed and hospital EDs became overburdened with patients seeking not only emergent, but also chronic care along with requests for prescription refills.
During disasters, disruptions in medication access and adherence are common. Disruptions can lead to disease exacerbation or progression, hospitalization, and/or death; all of which further contribute to the health care system and economic health burden. For example, after Hurricane Katrina, 46% of patients on hypertension medications had less than perfect adherence due to a variety of reasons (eg, not bringing any or enough medications during evacuation, lack of access to refills).5 Nonadherence to prescription hypertension medication specifically can lead to stroke, heart attack, and more rapidly progressing kidney dysfunction. Patients with diabetes mellitus (DM) also experience negative consequences due to disruptions in medication adherence.6 Lack of access to medications and supplies for DM can likewise lead to significant health sequelae, including acute hyperglycemic events, which can be life-threatening; ongoing hyperglycemia can lead to higher rates of cardiovascular disease, kidney disease, nerve damage, and diabetic retinopathy.7 However, the long-term effects of a natural disaster on health in terms of morbidity and mortality often go unreported, and their impact on chronic health conditions may be underestimated and last for years after the event.
As future health care professionals, student pharmacists continually seek opportunities to engage with and support communities; including preparing for, responding to, mitigating against, and recovering from disasters that affect the health care system and access to needed drug therapies. After the earthquake, student pharmacists reached out to state and local emergency response programs detailed within The State of Alaska Emergency Operations Plan to find opportunities to volunteer.
Agencies contacted included the Office of Emergency Management (OEM) for the Municipality of Anchorage. OEM partners with local health, fire, and police departments, the Alaska Department of Health and Social Services and Emergency Management, the Federal Emergency Management Agency, Centers for Disease Control and Prevention, American Red Cross, and the Salvation Army. It is important to note, due to lack of funding, Alaska no longer has a Medical Reserve Corps, which significantly impacts community emergency response and resilience efforts. After the earthquake, the emergency program manager extended an invitation to student pharmacists to join the joint medical emergency conference call, where local HCPs discuss emergency protocols, identify gaps, and work together to identify solutions.
During this call there was a consensus among HCPs that many patients were inappropriately seeking to fill and refill prescription medications in the ED, and staff were ill-prepared to guide patients to the appropriate services, unaware of which pharmacies were impacted by the earthquake; therefore unable to direct patients to still-operational pharmacies in the area. Together faculty and students discussed how student pharmacists could be involved in filling these identified information gaps and enhance communication among HCPs and entities. It was determined that if student pharmacists established and maintained open lines of communication with community pharmacists, they could efficiently determine which pharmacies were open and operational after disasters and disseminate that information to EDs and health care facilities in order to better direct patients to appropriate health care services.
Observations
A question/answer format and time line approach was used to review the steps leading to EPRSN program development and establishment of project/model deliverables.
Identified gaps
Chronic disease management. According to interviews conducted by the National Center for Disaster Preparedness, people often inappropriately use EDs during disasters.8 EDs do not stock enough medications to refill prescriptions for patients outside of their emergent care needs and are typically ill-suited for patients’ chronic disease management. At the time of the earthquake in Alaska no specific place/organization had been established to collect, store, or disseminate information regarding available pharmacy resources in an emergency. Had such a system been in place to actively inform HCPs and community members which pharmacies were open and operational, it is likely that many negative consequences related to health care utilization could have been reduced or avoided, including the number of people inappropriately using EDs for chronic prescription medication refills. This would not only reduce the burden on the health care system but allow for patients with both emergency and chronic needs to be seen quickly and prevent unnecessary health care costs.
Pharmacists play a vital role in managing chronic diseases.9 Due to extensive education and training, they are considered medication experts, ideally suited to manage chronic medication therapy, help prevent or minimize disease exacerbation and/or progression, reduce preventable health care costs, improve patient quality of life, and reduce morbidity and mortality.9 Pharmacists are accessible and strategically located throughout communities and provide patients with continuity of care other HCPs may be unable to provide. For example, during the COVID-19 pandemic, pharmacies remained open when other primary care providers (PCPs) were not. In addition, during times of natural disasters pharmacies tend to remain open unless there are extenuating circumstances (eg, unsafe building infrastructure, unsafe drug supply).
Emergency Response. To determine the role pharmacists play in emergency preparedness efforts we looked initially to the peer-reviewed literature (search terms: emergency preparedness, natural disasters, pharmacy/pharmacies) then turned to materials and research produced by organizations outside of the traditional commercial and academic publishing channels; however, most emergency preparedness protocols and standard operating procedures (SOPs) did not pertain to pharmacies or acknowledge the contribution of pharmacists. Researchers urge both state and federal governments to foster relationships with and use community pharmacist’s expertise and expanded roles in order to improve the nation’s public health.10
Historically, pharmacists within the US Public Health Service (PHS) have responded alongside local HCPs to meet the needs of communities during public health emergencies. Pharmacists were pivotal in the 2009 response to H1N1 influenza and the 2015 Ebola response, both abroad and within the United States.6 Pharmacists screened and triaged patients, provided life-saving vaccinations, and supported community and health care system education initiatives. However, as the COVID-19 pandemic has demonstrated, responding to a public health crisis takes more than the 1,000 pharmacists serving in the PHS.11 The American Society of Health-System Pharmacists argues that all pharmacists should be involved in working with public health planners.12
Community and health-systems pharmacists are vital to current and future public health responses and represent a largely untapped resource. Pharmacists across the country, especially in rural and underserved communities, have the potential to significantly impact emergency preparedness and response efforts. The > 319,000 US pharmacists comprise a sizable portion of the population and can play vital roles during emergency situations or disasters.13 Often after catastrophic events, community pharmacists provide first-aid, emergency refills, medication counseling, point of care testing, triage patients and serve on emergency response teams.14 However, pharmacists alone cannot address all medication-related patient needs and student pharmacists likewise have a role in emergency preparedness and response efforts. By participating in these efforts and learning these roles as students, they are better prepared to engage in emergency efforts as pharmacists.
Student pharmacist support. There are more than 140 accredited pharmacy schools across the United States, employing > 6,500 pharmacy faculty, and teaching > 63,000 student pharmacists.15 The majority of schools provide free and volunteer-based health care services and collaborate with local, regional, and national entities such as state boards of pharmacy, professional pharmacy organizations, and the American Pharmacist Association (APhA). Through the APhA Academy of Student Pharmacists (ASP), in 2018 and 2019 Operation Heart Campaign, 4,239 patients were referred to a PCP for follow-up care, 117,251 patients received health and wellness services, and 2,772,179 patients were educated regarding cardiovascular disease, the most common noncommunicable disease in the United States.16,17 Also, in 2018 and 2019, APhA-ASPs Operation Diabetes Campaign referred 3,785 patients to their PCP, provided health and wellness services to 36,334 patients, and educated 1,114,281 patients regarding DM.18
Student pharmacists are positioned across the country with reach to rural and underserved communities and have student organizational structures in place to manage student volunteers and support health care service opportunities. These structures could readily be used to augment and provide emergency pharmacy services and the coordination of chronic care services during times of emergency or disaster. Student leaders are well situated to coordinate communication and cooperation across health care disciplines and to facilitate local community pharmacy resource information collection and distribution.
Emergency Preparation Program
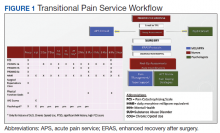
To address gaps in emergency preparedness and response, student pharmacists at UAA/ISU took the following steps to develop the EPRSN. Planning involved a multistep process. Step 1 identified important uncaptured data (eg, operational status, staffing, hours of operation, continuity and safety of drug supply chain, building/parking lot damage) required to direct patients to the appropriate medication-related care during an emergency. For step 2, student pharmacists obtained a list of the 138 pharmacies in Alaska from the state board of pharmacy. Pharmacies were contacted by student pharmacists using an established telephone script and updated contact information collected was stored on a secure, online drive accessible to UAA/ISU College of Pharmacy faculty and students using their UAA/ISU email address. In step 3, the APhA-ASP president elect and 3 leaders in each of the 16 APhA-ASP operation in charge of the EPRSN Alaska initiative, surveyed student leaders to determine student willingness to participate. Step 4 was to develop an organizational structure using established leadership structure to collect, capture, update, and share pharmacy data with state emergency response teams. Sustainability from year to year will be ensured through incorporation into the APhA-ASP student engagement framework (eg, annual training led by the president elect, contact information updated biyearly by student leaders, and oversight provided by College of Pharmacy faculty). Step 5 was to create SOPs, flowcharts, telephone scripts, talking points, and student training materials. And in the final preparatory step, plan documents and deliverables were provided to faculty administration and advisors within the College of Pharmacy for initial approval and presented to the student leadership for final approval.
EPRSN will be activated in the case of a natural disaster or state of emergency. Pharmacy students will contact all pharmacies within the designated area to collect up-to-date vital information (eg, operational status, staffing, hours of operation, safe drug supply, building/parking lot damage). Collected information will be disseminated to appropriate community members, HCPs, health care facilities, and emergency preparedness officials, under the direction of the Emergency Program Manager.
Discussion
In order to make informed and timely decisions during emergency situations, patients, HCPs, and health care systems must have appropriate situational awareness. The ability of decision makers to respond is directly dependent on timeliness and relevance of the information collected and shared and greatly contributes to this awareness. Accurate, effective, and consistent information collection has historically been one of the greatest challenges to situational awareness. This is particularly important in times of disaster when necessary emergency situation data may not exist, tools to collect data are inefficient and/or ineffective, and/or current data are inaccessible to relevant parties.19 This was the case in the Alaska earthquake of 2018 and more recently the COVID-19 pandemic of 2020 where information sharing deficits and structural barriers became even more evident.
Transfer of knowledge and information is especially critical during an emergency situation. Ineffective communication and information sharing results in transfer gaps. Gaps that result from inadequate transfers of care between HCPs are referred to as hand-off gaps. Training gaps result from inadequate preparation on the part of HCPs and civic leaders as well as in public health policies and procedures and in understanding of needs in emergent situations. Organization gaps occur when an individual changes positions or leaves a given institution and the acquired knowledge is not shared with others before departure or the replacement individual does not receive necessary training.
In both the Alaska earthquake and the COVID-19 pandemic, gaps in hand-offs, training, and organization were identified. Pharmacists were involved in the solution, providing care, addressing unmet health needs, and supporting the health care system. Many patients and HCPs remain unaware of the services pharmacists are capable and willing to provide, but at even a more basic level they are unsure of what services may be needed in emergency situations. Pharmacists are often used and considered vital HCPs after natural disasters or emergency situations, providing services that extend beyond their normal duties, yet remain within their SOP and expertise and address the medication management needs of their patients, ensuring safe, effective, and continuous access to needed pharmaceuticals.
It is vital that pharmacists and student pharmacists take an active role in emergency preparedness, that students get involved early in outreach and engagement initiatives for which they are ideally suited to coordinate in their communities, and that College of Pharmacy faculty support student pharmacist efforts to continue to highlight the professional roles of pharmacists, in routine health care as well as during times of crisis or disaster. It is important to note that an indirect but important cause of patient mortality related to an emergency event is the inability to access routine health care. If pharmacists and student pharmacists were more involved in emergency preparedness and response efforts, they could play an even greater role in providing much needed health care to patients during times when the health care system is overtaxed (facilitating medication refills and providing administrative and health care support).
Conclusions
Emergency and disaster preparedness are vital to promote the appropriate use of health care resources and prevent health-related complications. Student pharmacists represent a sustainable resource, uniquely positioned to identify community needs, support emergency efforts, coordinate with local pharmacies, and work with pharmacists and others to ensure patients receive the care they need. This work has the potential to improve utilization of health care resources and service delivery during natural disasters and emergencies, on a local, state, and regional level, with the overall goal of maintaining patient health and well-being.
1. Ritchie H, Roser M. Natural disasters. Updated November 2019. Accessed March 12, 2021. https://ourworldindata.org/natural-disasters
2. Freedy JR, Simpson WM Jr. Disaster-related physical and mental health: a role for the family physician. Am Fam Physician. 2007;75(6):841-846.
3. Martin U. Health after disaster: a perspective of psychological/health reactions to disaster. Cogent Psychol. 2015;2(1):1053741. doi:10.1080/23311908.2015.1053741
4. Joy K. Ripple effect: how hurricanes and other disasters affect hospital care. Published September 11, 2017. Accessed March 12, 2021. https://labblog.uofmhealth.org/industry-dx/ripple-effect-how-hurricanes-and-other-disasters-affect-hospital-care
5. Krousel-Wood MA, Islam T, Muntner P, et al. Medication adherence in older clinic patients with hypertension after Hurricane Katrina: implications for clinical practice and disaster management. Am J Med Sci. 2008;336(2):99-104. doi:10.1097/MAJ.0b013e318180f14f
6. Cefalu WT, Smith SR, Blonde L, Fonseca V. The Hurricane Katrina aftermath and its impact on diabetes care: observations from “ground zero”: lessons in disaster preparedness of people with diabetes. Diabetes Care. 2006;29(1):158-160. doi:10.2337/diacare.29.1.158
7. Fonseca VA, Smith H, Kuhadiya N, et al. Impact of a natural disaster on diabetes: exacerbation of disparities and long-term consequences. Diabetes Care. 2009;32(9):1632-1638. doi:10.2337/dc09-0670
8. Suneja A, Chandler TE, Schlegelmilch J, May M, Redlener IE; Columbia University Earth Institute. Chronic disease after natural disasters: public health, policy, and provider perspectives. Published November 12, 2018. Accessed March 12, 2021. doi:10.7916/D8ZP5Q23
9. Kehrer JP, Eberhart G, Wing M, Horon K. Pharmacy’s role in a modern health continuum. Can Pharm J (Ott). 2013;146(6):321-324. doi:10.1177/1715163513506370
10. Shearer MP, Geleta A, Adalja A, Gronvall GK; Johns Hopkins Bloomberg School of Public Health Center for Health Security. Serving the greater good: public health & community pharmacy partnerships. Published October 2017. Accessed March 12, 2021. https://www.centerforhealthsecurity.org/our-work/pubs_archive/pubs-pdfs/2017/public-health-and-community-pharmacy-partnerships-report.pdf
11. Flowers L, Wick J, Figg WD Sr, et al. U.S. Public Health Service Commissioned Corps pharmacists: making a difference in advancing the nation’s health. J Am Pharm Assoc (2003). 2009;49(3):446-452. doi:10.1331/JAPhA.2009.08036
12. American Society of Health-System Pharmacists. ASHP Statement on the Role of Health-System Pharmacists in Public Health. Am J Health Syst Pharm. 2008;65(5):462-467. doi:10.2146/ajhp070399
13. Deloitte. Data USA: pharmacists. Accessed June 2, 2020. https://datausa.io/profile/soc/pharmacists
14. Menighan TE. Pharmacists have major role in emergency response. Pharmacy Today. 2016;22(8):8. doi:10.1016/j.ptdy.2016.07.009
15. American Association of Colleges of Pharmacy. Academic pharmacy’s vital statistics. Updated July 2020. Accessed March 12, 2021. https://www.aacp.org/article/academic-pharmacys-vital-statistics
16. American Pharmacists Association. APhA-ASP Operation Heart. Accessed March 12, 2021. https://www.pharmacist.com/apha-asp-operation-heart
17. World Health Organization. Noncommunicable diseases. Updated June 1, 2018. Accessed March 12, 2021. https://www.who.int/en/news-room/fact-sheets/detail/noncommunicable-diseases
18. American Pharmacists Association. APhA-ASP Operation Diabetes. Accessed March 12, 2021. https://www.pharmacist.com/apha-asp-operation-diabetes
19. Reeve M, Wizemann T, Altevogt B. Enabling Rapid and Sustainable Public Health Research During Disasters: Summary of a Joint Workshop by the Institute of Medicine and the U.S. Department of Health and Human Services. National Academies Press; 2015.
Over the past decade, natural disasters and health care emergencies have increased 74%, averaging 400 documented events per year.1 These unpredictable and sometimes devastating events negatively impact the physical and mental health of communities, taxing already stretched health care system resources and the economy.2,3 During many of these events, patients inappropriately use hospitals, emergency departments (EDs), and critical care resources for chronic disease and elective health care management, resulting in medication shortages, health care access concerns, and treatment delays.4
Most available emergency preparedness programs rely solely on volunteers and/or public health providers to address the resultant coverage gap; however, instability in state and federal funding can make it difficult to maintain and sustain focused preparedness and response efforts. Alaska’s vast geography, low population density (1.2 people per square mile), and access limitations (about 200 villages only reachable by air or boat) make it especially challenging to provide reliable and sustained emergency preparedness and response support. Therefore, all eligible health care providers (HCPs) in Alaska must be involved in preparedness and response efforts.
Despite being the most accessible HCPs, pharmacists and student pharmacists, have not been actively involved in statewide emergency preparedness planning and disaster management efforts in Alaska. In preparation for and during disasters, for example, pharmacists may administer vaccinations, conduct point of care testing, dispense emergency medications, provide emergency medication refills, help mitigate medication shortages, and provide reliable health information to other health care professionals, patients, and their families as they prepare for and manage care during the event.4
The goal of this paper is to share the experience at the University of Alaska Anchorage/Idaho State University College of Pharmacy (UAA/ISU) in the development and implementation of a sustainable emergency preparedness and response support network (EPRSN) model; leveraging an established university student leadership structure and Doctor of Pharmacy (PharmD) students to support sharing of information among community pharmacies, state emergency response teams, and community members.
2018 Alaska Earthquake
On November 30, 2018, southcentral Alaska experienced a magnitude 7.1 earthquake, affecting nearly 295,000 people (approximately 40% of Alaska’s population) damaging roads, buildings, homes, and health care facilities. Emergency response efforts were quickly overwhelmed and hospital EDs became overburdened with patients seeking not only emergent, but also chronic care along with requests for prescription refills.
During disasters, disruptions in medication access and adherence are common. Disruptions can lead to disease exacerbation or progression, hospitalization, and/or death; all of which further contribute to the health care system and economic health burden. For example, after Hurricane Katrina, 46% of patients on hypertension medications had less than perfect adherence due to a variety of reasons (eg, not bringing any or enough medications during evacuation, lack of access to refills).5 Nonadherence to prescription hypertension medication specifically can lead to stroke, heart attack, and more rapidly progressing kidney dysfunction. Patients with diabetes mellitus (DM) also experience negative consequences due to disruptions in medication adherence.6 Lack of access to medications and supplies for DM can likewise lead to significant health sequelae, including acute hyperglycemic events, which can be life-threatening; ongoing hyperglycemia can lead to higher rates of cardiovascular disease, kidney disease, nerve damage, and diabetic retinopathy.7 However, the long-term effects of a natural disaster on health in terms of morbidity and mortality often go unreported, and their impact on chronic health conditions may be underestimated and last for years after the event.
As future health care professionals, student pharmacists continually seek opportunities to engage with and support communities; including preparing for, responding to, mitigating against, and recovering from disasters that affect the health care system and access to needed drug therapies. After the earthquake, student pharmacists reached out to state and local emergency response programs detailed within The State of Alaska Emergency Operations Plan to find opportunities to volunteer.
Agencies contacted included the Office of Emergency Management (OEM) for the Municipality of Anchorage. OEM partners with local health, fire, and police departments, the Alaska Department of Health and Social Services and Emergency Management, the Federal Emergency Management Agency, Centers for Disease Control and Prevention, American Red Cross, and the Salvation Army. It is important to note, due to lack of funding, Alaska no longer has a Medical Reserve Corps, which significantly impacts community emergency response and resilience efforts. After the earthquake, the emergency program manager extended an invitation to student pharmacists to join the joint medical emergency conference call, where local HCPs discuss emergency protocols, identify gaps, and work together to identify solutions.
During this call there was a consensus among HCPs that many patients were inappropriately seeking to fill and refill prescription medications in the ED, and staff were ill-prepared to guide patients to the appropriate services, unaware of which pharmacies were impacted by the earthquake; therefore unable to direct patients to still-operational pharmacies in the area. Together faculty and students discussed how student pharmacists could be involved in filling these identified information gaps and enhance communication among HCPs and entities. It was determined that if student pharmacists established and maintained open lines of communication with community pharmacists, they could efficiently determine which pharmacies were open and operational after disasters and disseminate that information to EDs and health care facilities in order to better direct patients to appropriate health care services.
Observations
A question/answer format and time line approach was used to review the steps leading to EPRSN program development and establishment of project/model deliverables.
Identified gaps
Chronic disease management. According to interviews conducted by the National Center for Disaster Preparedness, people often inappropriately use EDs during disasters.8 EDs do not stock enough medications to refill prescriptions for patients outside of their emergent care needs and are typically ill-suited for patients’ chronic disease management. At the time of the earthquake in Alaska no specific place/organization had been established to collect, store, or disseminate information regarding available pharmacy resources in an emergency. Had such a system been in place to actively inform HCPs and community members which pharmacies were open and operational, it is likely that many negative consequences related to health care utilization could have been reduced or avoided, including the number of people inappropriately using EDs for chronic prescription medication refills. This would not only reduce the burden on the health care system but allow for patients with both emergency and chronic needs to be seen quickly and prevent unnecessary health care costs.
Pharmacists play a vital role in managing chronic diseases.9 Due to extensive education and training, they are considered medication experts, ideally suited to manage chronic medication therapy, help prevent or minimize disease exacerbation and/or progression, reduce preventable health care costs, improve patient quality of life, and reduce morbidity and mortality.9 Pharmacists are accessible and strategically located throughout communities and provide patients with continuity of care other HCPs may be unable to provide. For example, during the COVID-19 pandemic, pharmacies remained open when other primary care providers (PCPs) were not. In addition, during times of natural disasters pharmacies tend to remain open unless there are extenuating circumstances (eg, unsafe building infrastructure, unsafe drug supply).
Emergency Response. To determine the role pharmacists play in emergency preparedness efforts we looked initially to the peer-reviewed literature (search terms: emergency preparedness, natural disasters, pharmacy/pharmacies) then turned to materials and research produced by organizations outside of the traditional commercial and academic publishing channels; however, most emergency preparedness protocols and standard operating procedures (SOPs) did not pertain to pharmacies or acknowledge the contribution of pharmacists. Researchers urge both state and federal governments to foster relationships with and use community pharmacist’s expertise and expanded roles in order to improve the nation’s public health.10
Historically, pharmacists within the US Public Health Service (PHS) have responded alongside local HCPs to meet the needs of communities during public health emergencies. Pharmacists were pivotal in the 2009 response to H1N1 influenza and the 2015 Ebola response, both abroad and within the United States.6 Pharmacists screened and triaged patients, provided life-saving vaccinations, and supported community and health care system education initiatives. However, as the COVID-19 pandemic has demonstrated, responding to a public health crisis takes more than the 1,000 pharmacists serving in the PHS.11 The American Society of Health-System Pharmacists argues that all pharmacists should be involved in working with public health planners.12
Community and health-systems pharmacists are vital to current and future public health responses and represent a largely untapped resource. Pharmacists across the country, especially in rural and underserved communities, have the potential to significantly impact emergency preparedness and response efforts. The > 319,000 US pharmacists comprise a sizable portion of the population and can play vital roles during emergency situations or disasters.13 Often after catastrophic events, community pharmacists provide first-aid, emergency refills, medication counseling, point of care testing, triage patients and serve on emergency response teams.14 However, pharmacists alone cannot address all medication-related patient needs and student pharmacists likewise have a role in emergency preparedness and response efforts. By participating in these efforts and learning these roles as students, they are better prepared to engage in emergency efforts as pharmacists.
Student pharmacist support. There are more than 140 accredited pharmacy schools across the United States, employing > 6,500 pharmacy faculty, and teaching > 63,000 student pharmacists.15 The majority of schools provide free and volunteer-based health care services and collaborate with local, regional, and national entities such as state boards of pharmacy, professional pharmacy organizations, and the American Pharmacist Association (APhA). Through the APhA Academy of Student Pharmacists (ASP), in 2018 and 2019 Operation Heart Campaign, 4,239 patients were referred to a PCP for follow-up care, 117,251 patients received health and wellness services, and 2,772,179 patients were educated regarding cardiovascular disease, the most common noncommunicable disease in the United States.16,17 Also, in 2018 and 2019, APhA-ASPs Operation Diabetes Campaign referred 3,785 patients to their PCP, provided health and wellness services to 36,334 patients, and educated 1,114,281 patients regarding DM.18
Student pharmacists are positioned across the country with reach to rural and underserved communities and have student organizational structures in place to manage student volunteers and support health care service opportunities. These structures could readily be used to augment and provide emergency pharmacy services and the coordination of chronic care services during times of emergency or disaster. Student leaders are well situated to coordinate communication and cooperation across health care disciplines and to facilitate local community pharmacy resource information collection and distribution.
Emergency Preparation Program
To address gaps in emergency preparedness and response, student pharmacists at UAA/ISU took the following steps to develop the EPRSN. Planning involved a multistep process. Step 1 identified important uncaptured data (eg, operational status, staffing, hours of operation, continuity and safety of drug supply chain, building/parking lot damage) required to direct patients to the appropriate medication-related care during an emergency. For step 2, student pharmacists obtained a list of the 138 pharmacies in Alaska from the state board of pharmacy. Pharmacies were contacted by student pharmacists using an established telephone script and updated contact information collected was stored on a secure, online drive accessible to UAA/ISU College of Pharmacy faculty and students using their UAA/ISU email address. In step 3, the APhA-ASP president elect and 3 leaders in each of the 16 APhA-ASP operation in charge of the EPRSN Alaska initiative, surveyed student leaders to determine student willingness to participate. Step 4 was to develop an organizational structure using established leadership structure to collect, capture, update, and share pharmacy data with state emergency response teams. Sustainability from year to year will be ensured through incorporation into the APhA-ASP student engagement framework (eg, annual training led by the president elect, contact information updated biyearly by student leaders, and oversight provided by College of Pharmacy faculty). Step 5 was to create SOPs, flowcharts, telephone scripts, talking points, and student training materials. And in the final preparatory step, plan documents and deliverables were provided to faculty administration and advisors within the College of Pharmacy for initial approval and presented to the student leadership for final approval.
EPRSN will be activated in the case of a natural disaster or state of emergency. Pharmacy students will contact all pharmacies within the designated area to collect up-to-date vital information (eg, operational status, staffing, hours of operation, safe drug supply, building/parking lot damage). Collected information will be disseminated to appropriate community members, HCPs, health care facilities, and emergency preparedness officials, under the direction of the Emergency Program Manager.
Discussion
In order to make informed and timely decisions during emergency situations, patients, HCPs, and health care systems must have appropriate situational awareness. The ability of decision makers to respond is directly dependent on timeliness and relevance of the information collected and shared and greatly contributes to this awareness. Accurate, effective, and consistent information collection has historically been one of the greatest challenges to situational awareness. This is particularly important in times of disaster when necessary emergency situation data may not exist, tools to collect data are inefficient and/or ineffective, and/or current data are inaccessible to relevant parties.19 This was the case in the Alaska earthquake of 2018 and more recently the COVID-19 pandemic of 2020 where information sharing deficits and structural barriers became even more evident.
Transfer of knowledge and information is especially critical during an emergency situation. Ineffective communication and information sharing results in transfer gaps. Gaps that result from inadequate transfers of care between HCPs are referred to as hand-off gaps. Training gaps result from inadequate preparation on the part of HCPs and civic leaders as well as in public health policies and procedures and in understanding of needs in emergent situations. Organization gaps occur when an individual changes positions or leaves a given institution and the acquired knowledge is not shared with others before departure or the replacement individual does not receive necessary training.
In both the Alaska earthquake and the COVID-19 pandemic, gaps in hand-offs, training, and organization were identified. Pharmacists were involved in the solution, providing care, addressing unmet health needs, and supporting the health care system. Many patients and HCPs remain unaware of the services pharmacists are capable and willing to provide, but at even a more basic level they are unsure of what services may be needed in emergency situations. Pharmacists are often used and considered vital HCPs after natural disasters or emergency situations, providing services that extend beyond their normal duties, yet remain within their SOP and expertise and address the medication management needs of their patients, ensuring safe, effective, and continuous access to needed pharmaceuticals.
It is vital that pharmacists and student pharmacists take an active role in emergency preparedness, that students get involved early in outreach and engagement initiatives for which they are ideally suited to coordinate in their communities, and that College of Pharmacy faculty support student pharmacist efforts to continue to highlight the professional roles of pharmacists, in routine health care as well as during times of crisis or disaster. It is important to note that an indirect but important cause of patient mortality related to an emergency event is the inability to access routine health care. If pharmacists and student pharmacists were more involved in emergency preparedness and response efforts, they could play an even greater role in providing much needed health care to patients during times when the health care system is overtaxed (facilitating medication refills and providing administrative and health care support).
Conclusions
Emergency and disaster preparedness are vital to promote the appropriate use of health care resources and prevent health-related complications. Student pharmacists represent a sustainable resource, uniquely positioned to identify community needs, support emergency efforts, coordinate with local pharmacies, and work with pharmacists and others to ensure patients receive the care they need. This work has the potential to improve utilization of health care resources and service delivery during natural disasters and emergencies, on a local, state, and regional level, with the overall goal of maintaining patient health and well-being.
Over the past decade, natural disasters and health care emergencies have increased 74%, averaging 400 documented events per year.1 These unpredictable and sometimes devastating events negatively impact the physical and mental health of communities, taxing already stretched health care system resources and the economy.2,3 During many of these events, patients inappropriately use hospitals, emergency departments (EDs), and critical care resources for chronic disease and elective health care management, resulting in medication shortages, health care access concerns, and treatment delays.4
Most available emergency preparedness programs rely solely on volunteers and/or public health providers to address the resultant coverage gap; however, instability in state and federal funding can make it difficult to maintain and sustain focused preparedness and response efforts. Alaska’s vast geography, low population density (1.2 people per square mile), and access limitations (about 200 villages only reachable by air or boat) make it especially challenging to provide reliable and sustained emergency preparedness and response support. Therefore, all eligible health care providers (HCPs) in Alaska must be involved in preparedness and response efforts.
Despite being the most accessible HCPs, pharmacists and student pharmacists, have not been actively involved in statewide emergency preparedness planning and disaster management efforts in Alaska. In preparation for and during disasters, for example, pharmacists may administer vaccinations, conduct point of care testing, dispense emergency medications, provide emergency medication refills, help mitigate medication shortages, and provide reliable health information to other health care professionals, patients, and their families as they prepare for and manage care during the event.4
The goal of this paper is to share the experience at the University of Alaska Anchorage/Idaho State University College of Pharmacy (UAA/ISU) in the development and implementation of a sustainable emergency preparedness and response support network (EPRSN) model; leveraging an established university student leadership structure and Doctor of Pharmacy (PharmD) students to support sharing of information among community pharmacies, state emergency response teams, and community members.
2018 Alaska Earthquake
On November 30, 2018, southcentral Alaska experienced a magnitude 7.1 earthquake, affecting nearly 295,000 people (approximately 40% of Alaska’s population) damaging roads, buildings, homes, and health care facilities. Emergency response efforts were quickly overwhelmed and hospital EDs became overburdened with patients seeking not only emergent, but also chronic care along with requests for prescription refills.
During disasters, disruptions in medication access and adherence are common. Disruptions can lead to disease exacerbation or progression, hospitalization, and/or death; all of which further contribute to the health care system and economic health burden. For example, after Hurricane Katrina, 46% of patients on hypertension medications had less than perfect adherence due to a variety of reasons (eg, not bringing any or enough medications during evacuation, lack of access to refills).5 Nonadherence to prescription hypertension medication specifically can lead to stroke, heart attack, and more rapidly progressing kidney dysfunction. Patients with diabetes mellitus (DM) also experience negative consequences due to disruptions in medication adherence.6 Lack of access to medications and supplies for DM can likewise lead to significant health sequelae, including acute hyperglycemic events, which can be life-threatening; ongoing hyperglycemia can lead to higher rates of cardiovascular disease, kidney disease, nerve damage, and diabetic retinopathy.7 However, the long-term effects of a natural disaster on health in terms of morbidity and mortality often go unreported, and their impact on chronic health conditions may be underestimated and last for years after the event.
As future health care professionals, student pharmacists continually seek opportunities to engage with and support communities; including preparing for, responding to, mitigating against, and recovering from disasters that affect the health care system and access to needed drug therapies. After the earthquake, student pharmacists reached out to state and local emergency response programs detailed within The State of Alaska Emergency Operations Plan to find opportunities to volunteer.
Agencies contacted included the Office of Emergency Management (OEM) for the Municipality of Anchorage. OEM partners with local health, fire, and police departments, the Alaska Department of Health and Social Services and Emergency Management, the Federal Emergency Management Agency, Centers for Disease Control and Prevention, American Red Cross, and the Salvation Army. It is important to note, due to lack of funding, Alaska no longer has a Medical Reserve Corps, which significantly impacts community emergency response and resilience efforts. After the earthquake, the emergency program manager extended an invitation to student pharmacists to join the joint medical emergency conference call, where local HCPs discuss emergency protocols, identify gaps, and work together to identify solutions.
During this call there was a consensus among HCPs that many patients were inappropriately seeking to fill and refill prescription medications in the ED, and staff were ill-prepared to guide patients to the appropriate services, unaware of which pharmacies were impacted by the earthquake; therefore unable to direct patients to still-operational pharmacies in the area. Together faculty and students discussed how student pharmacists could be involved in filling these identified information gaps and enhance communication among HCPs and entities. It was determined that if student pharmacists established and maintained open lines of communication with community pharmacists, they could efficiently determine which pharmacies were open and operational after disasters and disseminate that information to EDs and health care facilities in order to better direct patients to appropriate health care services.
Observations
A question/answer format and time line approach was used to review the steps leading to EPRSN program development and establishment of project/model deliverables.
Identified gaps
Chronic disease management. According to interviews conducted by the National Center for Disaster Preparedness, people often inappropriately use EDs during disasters.8 EDs do not stock enough medications to refill prescriptions for patients outside of their emergent care needs and are typically ill-suited for patients’ chronic disease management. At the time of the earthquake in Alaska no specific place/organization had been established to collect, store, or disseminate information regarding available pharmacy resources in an emergency. Had such a system been in place to actively inform HCPs and community members which pharmacies were open and operational, it is likely that many negative consequences related to health care utilization could have been reduced or avoided, including the number of people inappropriately using EDs for chronic prescription medication refills. This would not only reduce the burden on the health care system but allow for patients with both emergency and chronic needs to be seen quickly and prevent unnecessary health care costs.
Pharmacists play a vital role in managing chronic diseases.9 Due to extensive education and training, they are considered medication experts, ideally suited to manage chronic medication therapy, help prevent or minimize disease exacerbation and/or progression, reduce preventable health care costs, improve patient quality of life, and reduce morbidity and mortality.9 Pharmacists are accessible and strategically located throughout communities and provide patients with continuity of care other HCPs may be unable to provide. For example, during the COVID-19 pandemic, pharmacies remained open when other primary care providers (PCPs) were not. In addition, during times of natural disasters pharmacies tend to remain open unless there are extenuating circumstances (eg, unsafe building infrastructure, unsafe drug supply).
Emergency Response. To determine the role pharmacists play in emergency preparedness efforts we looked initially to the peer-reviewed literature (search terms: emergency preparedness, natural disasters, pharmacy/pharmacies) then turned to materials and research produced by organizations outside of the traditional commercial and academic publishing channels; however, most emergency preparedness protocols and standard operating procedures (SOPs) did not pertain to pharmacies or acknowledge the contribution of pharmacists. Researchers urge both state and federal governments to foster relationships with and use community pharmacist’s expertise and expanded roles in order to improve the nation’s public health.10
Historically, pharmacists within the US Public Health Service (PHS) have responded alongside local HCPs to meet the needs of communities during public health emergencies. Pharmacists were pivotal in the 2009 response to H1N1 influenza and the 2015 Ebola response, both abroad and within the United States.6 Pharmacists screened and triaged patients, provided life-saving vaccinations, and supported community and health care system education initiatives. However, as the COVID-19 pandemic has demonstrated, responding to a public health crisis takes more than the 1,000 pharmacists serving in the PHS.11 The American Society of Health-System Pharmacists argues that all pharmacists should be involved in working with public health planners.12
Community and health-systems pharmacists are vital to current and future public health responses and represent a largely untapped resource. Pharmacists across the country, especially in rural and underserved communities, have the potential to significantly impact emergency preparedness and response efforts. The > 319,000 US pharmacists comprise a sizable portion of the population and can play vital roles during emergency situations or disasters.13 Often after catastrophic events, community pharmacists provide first-aid, emergency refills, medication counseling, point of care testing, triage patients and serve on emergency response teams.14 However, pharmacists alone cannot address all medication-related patient needs and student pharmacists likewise have a role in emergency preparedness and response efforts. By participating in these efforts and learning these roles as students, they are better prepared to engage in emergency efforts as pharmacists.
Student pharmacist support. There are more than 140 accredited pharmacy schools across the United States, employing > 6,500 pharmacy faculty, and teaching > 63,000 student pharmacists.15 The majority of schools provide free and volunteer-based health care services and collaborate with local, regional, and national entities such as state boards of pharmacy, professional pharmacy organizations, and the American Pharmacist Association (APhA). Through the APhA Academy of Student Pharmacists (ASP), in 2018 and 2019 Operation Heart Campaign, 4,239 patients were referred to a PCP for follow-up care, 117,251 patients received health and wellness services, and 2,772,179 patients were educated regarding cardiovascular disease, the most common noncommunicable disease in the United States.16,17 Also, in 2018 and 2019, APhA-ASPs Operation Diabetes Campaign referred 3,785 patients to their PCP, provided health and wellness services to 36,334 patients, and educated 1,114,281 patients regarding DM.18
Student pharmacists are positioned across the country with reach to rural and underserved communities and have student organizational structures in place to manage student volunteers and support health care service opportunities. These structures could readily be used to augment and provide emergency pharmacy services and the coordination of chronic care services during times of emergency or disaster. Student leaders are well situated to coordinate communication and cooperation across health care disciplines and to facilitate local community pharmacy resource information collection and distribution.
Emergency Preparation Program
To address gaps in emergency preparedness and response, student pharmacists at UAA/ISU took the following steps to develop the EPRSN. Planning involved a multistep process. Step 1 identified important uncaptured data (eg, operational status, staffing, hours of operation, continuity and safety of drug supply chain, building/parking lot damage) required to direct patients to the appropriate medication-related care during an emergency. For step 2, student pharmacists obtained a list of the 138 pharmacies in Alaska from the state board of pharmacy. Pharmacies were contacted by student pharmacists using an established telephone script and updated contact information collected was stored on a secure, online drive accessible to UAA/ISU College of Pharmacy faculty and students using their UAA/ISU email address. In step 3, the APhA-ASP president elect and 3 leaders in each of the 16 APhA-ASP operation in charge of the EPRSN Alaska initiative, surveyed student leaders to determine student willingness to participate. Step 4 was to develop an organizational structure using established leadership structure to collect, capture, update, and share pharmacy data with state emergency response teams. Sustainability from year to year will be ensured through incorporation into the APhA-ASP student engagement framework (eg, annual training led by the president elect, contact information updated biyearly by student leaders, and oversight provided by College of Pharmacy faculty). Step 5 was to create SOPs, flowcharts, telephone scripts, talking points, and student training materials. And in the final preparatory step, plan documents and deliverables were provided to faculty administration and advisors within the College of Pharmacy for initial approval and presented to the student leadership for final approval.
EPRSN will be activated in the case of a natural disaster or state of emergency. Pharmacy students will contact all pharmacies within the designated area to collect up-to-date vital information (eg, operational status, staffing, hours of operation, safe drug supply, building/parking lot damage). Collected information will be disseminated to appropriate community members, HCPs, health care facilities, and emergency preparedness officials, under the direction of the Emergency Program Manager.
Discussion
In order to make informed and timely decisions during emergency situations, patients, HCPs, and health care systems must have appropriate situational awareness. The ability of decision makers to respond is directly dependent on timeliness and relevance of the information collected and shared and greatly contributes to this awareness. Accurate, effective, and consistent information collection has historically been one of the greatest challenges to situational awareness. This is particularly important in times of disaster when necessary emergency situation data may not exist, tools to collect data are inefficient and/or ineffective, and/or current data are inaccessible to relevant parties.19 This was the case in the Alaska earthquake of 2018 and more recently the COVID-19 pandemic of 2020 where information sharing deficits and structural barriers became even more evident.
Transfer of knowledge and information is especially critical during an emergency situation. Ineffective communication and information sharing results in transfer gaps. Gaps that result from inadequate transfers of care between HCPs are referred to as hand-off gaps. Training gaps result from inadequate preparation on the part of HCPs and civic leaders as well as in public health policies and procedures and in understanding of needs in emergent situations. Organization gaps occur when an individual changes positions or leaves a given institution and the acquired knowledge is not shared with others before departure or the replacement individual does not receive necessary training.
In both the Alaska earthquake and the COVID-19 pandemic, gaps in hand-offs, training, and organization were identified. Pharmacists were involved in the solution, providing care, addressing unmet health needs, and supporting the health care system. Many patients and HCPs remain unaware of the services pharmacists are capable and willing to provide, but at even a more basic level they are unsure of what services may be needed in emergency situations. Pharmacists are often used and considered vital HCPs after natural disasters or emergency situations, providing services that extend beyond their normal duties, yet remain within their SOP and expertise and address the medication management needs of their patients, ensuring safe, effective, and continuous access to needed pharmaceuticals.
It is vital that pharmacists and student pharmacists take an active role in emergency preparedness, that students get involved early in outreach and engagement initiatives for which they are ideally suited to coordinate in their communities, and that College of Pharmacy faculty support student pharmacist efforts to continue to highlight the professional roles of pharmacists, in routine health care as well as during times of crisis or disaster. It is important to note that an indirect but important cause of patient mortality related to an emergency event is the inability to access routine health care. If pharmacists and student pharmacists were more involved in emergency preparedness and response efforts, they could play an even greater role in providing much needed health care to patients during times when the health care system is overtaxed (facilitating medication refills and providing administrative and health care support).
Conclusions
Emergency and disaster preparedness are vital to promote the appropriate use of health care resources and prevent health-related complications. Student pharmacists represent a sustainable resource, uniquely positioned to identify community needs, support emergency efforts, coordinate with local pharmacies, and work with pharmacists and others to ensure patients receive the care they need. This work has the potential to improve utilization of health care resources and service delivery during natural disasters and emergencies, on a local, state, and regional level, with the overall goal of maintaining patient health and well-being.
1. Ritchie H, Roser M. Natural disasters. Updated November 2019. Accessed March 12, 2021. https://ourworldindata.org/natural-disasters
2. Freedy JR, Simpson WM Jr. Disaster-related physical and mental health: a role for the family physician. Am Fam Physician. 2007;75(6):841-846.
3. Martin U. Health after disaster: a perspective of psychological/health reactions to disaster. Cogent Psychol. 2015;2(1):1053741. doi:10.1080/23311908.2015.1053741
4. Joy K. Ripple effect: how hurricanes and other disasters affect hospital care. Published September 11, 2017. Accessed March 12, 2021. https://labblog.uofmhealth.org/industry-dx/ripple-effect-how-hurricanes-and-other-disasters-affect-hospital-care
5. Krousel-Wood MA, Islam T, Muntner P, et al. Medication adherence in older clinic patients with hypertension after Hurricane Katrina: implications for clinical practice and disaster management. Am J Med Sci. 2008;336(2):99-104. doi:10.1097/MAJ.0b013e318180f14f
6. Cefalu WT, Smith SR, Blonde L, Fonseca V. The Hurricane Katrina aftermath and its impact on diabetes care: observations from “ground zero”: lessons in disaster preparedness of people with diabetes. Diabetes Care. 2006;29(1):158-160. doi:10.2337/diacare.29.1.158
7. Fonseca VA, Smith H, Kuhadiya N, et al. Impact of a natural disaster on diabetes: exacerbation of disparities and long-term consequences. Diabetes Care. 2009;32(9):1632-1638. doi:10.2337/dc09-0670
8. Suneja A, Chandler TE, Schlegelmilch J, May M, Redlener IE; Columbia University Earth Institute. Chronic disease after natural disasters: public health, policy, and provider perspectives. Published November 12, 2018. Accessed March 12, 2021. doi:10.7916/D8ZP5Q23
9. Kehrer JP, Eberhart G, Wing M, Horon K. Pharmacy’s role in a modern health continuum. Can Pharm J (Ott). 2013;146(6):321-324. doi:10.1177/1715163513506370
10. Shearer MP, Geleta A, Adalja A, Gronvall GK; Johns Hopkins Bloomberg School of Public Health Center for Health Security. Serving the greater good: public health & community pharmacy partnerships. Published October 2017. Accessed March 12, 2021. https://www.centerforhealthsecurity.org/our-work/pubs_archive/pubs-pdfs/2017/public-health-and-community-pharmacy-partnerships-report.pdf
11. Flowers L, Wick J, Figg WD Sr, et al. U.S. Public Health Service Commissioned Corps pharmacists: making a difference in advancing the nation’s health. J Am Pharm Assoc (2003). 2009;49(3):446-452. doi:10.1331/JAPhA.2009.08036
12. American Society of Health-System Pharmacists. ASHP Statement on the Role of Health-System Pharmacists in Public Health. Am J Health Syst Pharm. 2008;65(5):462-467. doi:10.2146/ajhp070399
13. Deloitte. Data USA: pharmacists. Accessed June 2, 2020. https://datausa.io/profile/soc/pharmacists
14. Menighan TE. Pharmacists have major role in emergency response. Pharmacy Today. 2016;22(8):8. doi:10.1016/j.ptdy.2016.07.009
15. American Association of Colleges of Pharmacy. Academic pharmacy’s vital statistics. Updated July 2020. Accessed March 12, 2021. https://www.aacp.org/article/academic-pharmacys-vital-statistics
16. American Pharmacists Association. APhA-ASP Operation Heart. Accessed March 12, 2021. https://www.pharmacist.com/apha-asp-operation-heart
17. World Health Organization. Noncommunicable diseases. Updated June 1, 2018. Accessed March 12, 2021. https://www.who.int/en/news-room/fact-sheets/detail/noncommunicable-diseases
18. American Pharmacists Association. APhA-ASP Operation Diabetes. Accessed March 12, 2021. https://www.pharmacist.com/apha-asp-operation-diabetes
19. Reeve M, Wizemann T, Altevogt B. Enabling Rapid and Sustainable Public Health Research During Disasters: Summary of a Joint Workshop by the Institute of Medicine and the U.S. Department of Health and Human Services. National Academies Press; 2015.
1. Ritchie H, Roser M. Natural disasters. Updated November 2019. Accessed March 12, 2021. https://ourworldindata.org/natural-disasters
2. Freedy JR, Simpson WM Jr. Disaster-related physical and mental health: a role for the family physician. Am Fam Physician. 2007;75(6):841-846.
3. Martin U. Health after disaster: a perspective of psychological/health reactions to disaster. Cogent Psychol. 2015;2(1):1053741. doi:10.1080/23311908.2015.1053741
4. Joy K. Ripple effect: how hurricanes and other disasters affect hospital care. Published September 11, 2017. Accessed March 12, 2021. https://labblog.uofmhealth.org/industry-dx/ripple-effect-how-hurricanes-and-other-disasters-affect-hospital-care
5. Krousel-Wood MA, Islam T, Muntner P, et al. Medication adherence in older clinic patients with hypertension after Hurricane Katrina: implications for clinical practice and disaster management. Am J Med Sci. 2008;336(2):99-104. doi:10.1097/MAJ.0b013e318180f14f
6. Cefalu WT, Smith SR, Blonde L, Fonseca V. The Hurricane Katrina aftermath and its impact on diabetes care: observations from “ground zero”: lessons in disaster preparedness of people with diabetes. Diabetes Care. 2006;29(1):158-160. doi:10.2337/diacare.29.1.158
7. Fonseca VA, Smith H, Kuhadiya N, et al. Impact of a natural disaster on diabetes: exacerbation of disparities and long-term consequences. Diabetes Care. 2009;32(9):1632-1638. doi:10.2337/dc09-0670
8. Suneja A, Chandler TE, Schlegelmilch J, May M, Redlener IE; Columbia University Earth Institute. Chronic disease after natural disasters: public health, policy, and provider perspectives. Published November 12, 2018. Accessed March 12, 2021. doi:10.7916/D8ZP5Q23
9. Kehrer JP, Eberhart G, Wing M, Horon K. Pharmacy’s role in a modern health continuum. Can Pharm J (Ott). 2013;146(6):321-324. doi:10.1177/1715163513506370
10. Shearer MP, Geleta A, Adalja A, Gronvall GK; Johns Hopkins Bloomberg School of Public Health Center for Health Security. Serving the greater good: public health & community pharmacy partnerships. Published October 2017. Accessed March 12, 2021. https://www.centerforhealthsecurity.org/our-work/pubs_archive/pubs-pdfs/2017/public-health-and-community-pharmacy-partnerships-report.pdf
11. Flowers L, Wick J, Figg WD Sr, et al. U.S. Public Health Service Commissioned Corps pharmacists: making a difference in advancing the nation’s health. J Am Pharm Assoc (2003). 2009;49(3):446-452. doi:10.1331/JAPhA.2009.08036
12. American Society of Health-System Pharmacists. ASHP Statement on the Role of Health-System Pharmacists in Public Health. Am J Health Syst Pharm. 2008;65(5):462-467. doi:10.2146/ajhp070399
13. Deloitte. Data USA: pharmacists. Accessed June 2, 2020. https://datausa.io/profile/soc/pharmacists
14. Menighan TE. Pharmacists have major role in emergency response. Pharmacy Today. 2016;22(8):8. doi:10.1016/j.ptdy.2016.07.009
15. American Association of Colleges of Pharmacy. Academic pharmacy’s vital statistics. Updated July 2020. Accessed March 12, 2021. https://www.aacp.org/article/academic-pharmacys-vital-statistics
16. American Pharmacists Association. APhA-ASP Operation Heart. Accessed March 12, 2021. https://www.pharmacist.com/apha-asp-operation-heart
17. World Health Organization. Noncommunicable diseases. Updated June 1, 2018. Accessed March 12, 2021. https://www.who.int/en/news-room/fact-sheets/detail/noncommunicable-diseases
18. American Pharmacists Association. APhA-ASP Operation Diabetes. Accessed March 12, 2021. https://www.pharmacist.com/apha-asp-operation-diabetes
19. Reeve M, Wizemann T, Altevogt B. Enabling Rapid and Sustainable Public Health Research During Disasters: Summary of a Joint Workshop by the Institute of Medicine and the U.S. Department of Health and Human Services. National Academies Press; 2015.
The Design and Implementation of a Heart Disease Reversal Program in the Veterans Health Administration: Before and During the COVID-19 Pandemic
While cardiovascular mortality rates have declined, heart disease continues to be the leading cause of death in the US, and the number of people with cardiovascular disease (CVD) is rising.1 CVD is more prevalent among military veterans than it is among nonveterans aged ≥ 25 years, and veteran status is associated with higher risk of incident heart disease after controlling for socioeconomic status, other medical diseases, depression, and lifestyle.2-4 Combat exposure, posttraumatic stress disorder (PTSD), and Purple Heart commendation are associated with higher rates of CVD, including adverse cardiovascular events.5-7 Many patients seeking care in the Veterans Health Administration (VHA), including those who undergo cardiac catheterization, meet the criteria for multimorbidity (defined as having ≥ 2 chronic diseases8), which is common among veterans.9,10 Multimorbidity presents a challenge for lifestyle intervention, as different diets may be prescribed to treat different conditions, such as Dietary Approaches to Stop Hypertension, and low-glycemic diet for diabetes mellitus (DM). Veterans with CVD are often clinically complex and may require more multifaceted secondary prevention programs.
During the coronavirus 2019 (COVID-19) pandemic, effective secondary prevention intervention is needed more than ever. Older age, CVD, and common comorbidities, including hypertension, DM, and obesity, place patients at the highest risk for severe COVID-19 infection.11 COVID-19 social distancing encourages vulnerable populations to stay home, which can make engaging in any levels of physical activity more challenging. The International Food Council found that 85% of adults have made a change to their food consumption pattern, including eating more, during the COVID-19 pandemic.12 Thus, secondary CVD prevention programs for veterans need to provide treatment that addresses these specific challenges and can be delivered via telehealth for continuity of care after disruption of traditional services.
Clinical practice guidelines for the treatment of patients with recent cardiovascular adverse events (AEs) include a referral to cardiac rehabilitation (CR).13 CR emphasizes exercise as the main intervention, along with coaching to promote multiple risk reduction. The most comprehensive CR program is intensive CR (ICR), including the Ornish ICR program.14 ICR includes 4 components: vegetarian diet, exercise, stress management (yoga, meditation), and group support. Ornish ICR has been shown to be efficacious in randomized controlled trials (RCTs).15-17 Three effectiveness studies, with 5,372 participants, demonstrated the real-world effectiveness of Ornish ICR in US hospitals.14,18,19 The program also was adapted successfully for the active-duty military and veteran population.20,21 Yet Ornish ICR is time intensive, and there are no certified VHA ICR Ornish sites. Furthermore, there is no formal strategy for targeting people with atherosclerotic CVD who no longer meet the criteria for CR or ICR. While Ornish ICR is highly effective for patients who are eligible and have access, a more effective and streamlined approach is needed for targeting many patients.
Nutrition may be the most powerful Ornish ICR component. The initial RCT conducted by Ornish and colleagues included only stress management training and a whole-food, plant-based (WFPB) diet, including grains, legumes, vegetables, fruits, nuts, and seeds. The trial found 91% of participants experienced reduced angina after only 24 days.15 The only single-component intervention study resulting in partial reversal of atherosclerosis was a WFPB diet-only study, which documented regression of atherosclerotic plaques after 5 years, using coronary angiography in 73% of participants, with arrested progression in the other 27%.22 Participants reported no cardiovascular AEs after 12 years.23 Furthermore, a number of other recent studies have demonstrated the benefits of WFPB diet-only interventions for type 2 DM (T2DM), hypertension, and obesity.24-27 The Heart Disease Reversal Program (HDRP) was developed to create an interdisciplinary lifestyle intervention that emphasized nutrition for a broad population of veterans with atherosclerotic CVD, of varying levels of functional ability, to promote comprehensive CVD risk reduction and bring heart disease reversal intervention into routine clinical practice.
Program Description
The Mental Health, Cardiology, and Nutrition and Food services all approved the launch of HDRP. We contacted veterans by mail, and 11% expressed interest (Figure). Among patients who received the initial mailed letter (prior to our accepting staff referrals), only 5% of patients who enrolled in HDRP reported previously being told about or prescribed a WFPB diet by any health care provider (HCP). Currently, patients are primarily referred to HDRP by Cardiology, Primary Care, and Mental Health services.
Design
HDRP is an adaptation of interdisciplinary lifestyle interventions that have resulted in regression of atherosclerotic blockages confirmed with invasive coronary angiography.15-17,22,28 HDRP currently is offered in a Behavioral Medicine Clinic at the Sacramento US Department of Veterans Affairs (VA) Medical Center (VAMC) in California. Program staff include a clinical health psychologist who organizes, coordinates, and act as the lead facilitator of the program; registered dietitians; clinical pharmacists; and a consulting physician. Patients engage in the 4-month core HDRP program in small cohorts (ie, 6-10 patients), and spouses/partners are highly encouraged to attend all sessions.
Components
Telephone screening. Patients are screened for the inclusion and exclusion criteria (Table 1). Patients engaging in a traditional CR program are included in the screening. Patients are informed that the program consists of lifestyle intervention, including emphasis on following a WFPB diet.
Health assessment. Once approved, all patients are instructed to complete baseline laboratory tests and questionnaires. Along with an electronic health record (EHR) review, a psychosocial assessment is completed by a licensed clinical health psychologist who assesses CVD history, eating behavior, exercise/physical activity, sleep, mental health, substance use, and social history, with the aim of enhancing our ability to help the patient to benefit from HDRP.29 The patient data are used to develop a case conceptualization (ie, integrated understanding of the particular patient’s psychiatric and medical diagnoses, behavioral patterns, social supports, lifestyle habits, strengths and weaknesses, and their interrelationships with each other and the patient’s environment), resulting in an individualized plan. Patients are encouraged to ask questions about the program, and those who are still interested are invited to attend a seminar. A request for medical clearance to participate in the program is initiated through the EHR or by patients scheduling an appointment with their HCP. All patients are medically cleared by their HCP for participation. Safe exercise recommendations also are provided and guide patient goals.
CVD risk profile. Patients complete psychosocial questionnaires and fasting laboratory tests to produce a tailored CVD risk profile. Laboratory tests include fasting lipid, fasting glucose, hemoglobin A1c (HbA1c) C-reactive protein, vitamin B12, and vitamin D. The same tests (excluding HbA1c) are completed 1 month later (after completing 4 group sessions) and again posttreatment (including HbA1c). Self-reported questionnaires are completed at the same time points, which include the Rate Your Plate dietary composition questionnaire, CHAMPS physical activity questionnaire for older adults, Beck Depression Inventory-II, and the Perceived Stress Scale.
Seminar. A 2-hour seminar provides patients and families with an opportunity to meet HDRP program staff, learn the background and rationale for chronic disease reversal, obtain a summary of the program, and hear a patient testimonial. Patients are asked to make a commitment, and the informed consent process includes all patients signing a behavioral contract.
Assessment and feedback. A licensed clinical health psychologist provides feedback to patients on their comprehensive CVD risk profile, using motivational interviewing.30,31 Smokers are encouraged to quit, and those interested are referred to their HCP and/or facility smoking cessation program.
Group sessions. Twelve weekly group sessions cover nutrition education and cooking, physical activity and exercise, stress management training, and medication reconciliation and adjustment. The nutrition component is the centerpiece of HDRP and is delivered by registered dietitians (Table 2). Patients are instructed to use the 3-week period between the HDRP seminar and the first core group session to try new recipes and prepare their kitchens, pantries, and mind-set to adopt the HDRP diet with 100% adherence. The WFPB diet used is consistent with the current guidelines of Caldwell Esselstyn, MD, and Dean Ornish, MD.32-34
A psychologist delivers the physical activity component. Patients are encouraged to meet the American Heart Association/American College of Cardiology recommendations for aerobic exercise (at least 150 minutes of moderate intensity physical activity per week) through a walking program.35 Patients with medical contraindications (eg, severe pain, mobility restrictions) are encouraged to follow the exercise/activity recommendations they had been given by their primary care provider (PCP), physical therapist, or other HCP.
A psychologist provides evidence-based cognitive behavioral stress management (CBSM) training, adapted from models developed for patients with stable ischemic heart disease, HIV/AIDS, and cancer.36-38 CBSM is a psychotherapy grounded in stress/coping theory and cognitive behavioral theory of psychopathology that integrates cognitive restructuring, coping skills training, communication/assertiveness training, anger management, and mindfulness/acceptance-based approaches. Additional emphasis is placed on assisting patients’ adjustment to the lifestyle challenges for following a plant-based diet, dealing with food cravings and emotional eating, and connecting lifestyle change to patients’ deepest values and goals.
A clinical pharmacist conducts a medication reconciliation for each patient at baseline. The pharmacist consults with each patient’s PCP, cardiologist, and HDRP consulting physician, as needed, to ensure safe adjustments to medications. Pharmacists also provide education on medications at group sessions.
After completion of the 12-week core program, graduates are encouraged to attend the monthly graduates’ group indefinitely, and as often as they desire to promote maintenance of the disease reversal lifestyle. Patients are encouraged to complete our recommended fasting laboratory work every 3 to 6 months to facilitate maintenance of treatment gains.
Program Evaluation
Patients frequently reported that the group format was vital to their success. Patients requested a cooking class, yet we lacked a full teaching kitchen. Integrating plant-based meal samples at every session and cooking videos helped. Patients reported that 100% adherence to the WFPB diet led to significant changes in their food preferences, including a loss of interest in meat.39 Patients encouraged us to keep the “disease reversal” language and focus. One veteran stated: “Disease reversal, that is the reason I called you when I got your letter.” Showing before and after images of coronary angiograms and cardiac positron emission tomography scans depicting regression of atherosclerotic plaque and restored myocardial perfusion were described as highly motivating and generated willingness to commit to a more aggressive lifestyle change.31
Patients routinely stated that they lacked understanding of their laboratory test results, which HDRP remedied. Some patients reported their adult children followed a plant-based diet, and our program resulted in a new commonality and source of bonding that was highly valued. Some patients reported that HDRP was helpful for controlling their COVID-19 anxiety and feeling in control of their health. Satisfaction surveys were completed by participants at the end of the core program, which demonstrated very high satisfaction with and acceptability of HDRP (Table 3).
The program also has received positive feedback from HCPs when we alert them to improvements in outcome measures for their patients. These HCPs expressed satisfaction with having a program to refer patients to that can help with chronic illness in more depth.
COVID-19 Response
Face-to-face group appointments were converted to videoconferencing as a result of the COVID-19 pandemic. While HDRP always promoted the use of technology and mHealth tools, the pandemic led us to develop novel technology-based interventions.40 One cohort transitioned from in-person to videoconferencing sessions, and 2 cohorts recently started this format and are ongoing. We have successfully used videoconferencing with Cisco Webex, the VA-approved backup platform, as we encountered technical barriers when using VA Video Connect. Program materials were shared electronically, and participants sent blood pressure/sugar logs by secure messaging. Guidance for online grocery shopping with home delivery was provided, and research on the benefits of the HDRP lifestyle on immune function was incorporated.
The stress management component incorporated coping with COVID-19, including normalizing common emotional difficulties with sheltering-in-place and quarantine, acknowledging and processing fear and anxiety related to being at very high risk for severe COVID-19. We presented heart disease reversal as an urgent and feasible goal during the pandemic both reducing risk of premature death and major adverse cardiovascular events in the long-term and also reducing personal risk of severe COVID complications. The new VA COVID Coach app was also presented as a resource. Reputable sources of COVID-19 and public health information were shared. Walking continued to be the primary recommended form of exercise, while indoor home exercise options were promoted during the periods of very poor air quality due to the widespread California fires and smoke.
Considering the research suggesting benefits of our intervention for treating T2DM,promoting sustained weight loss, and promoting comprehensive cardiometabolic risk reduction, we have begun accepting referrals for patients with any type of atherosclerotic CVD (eg, peripheral artery disease, carotid artery disease), patients with T2DM (without CVD), and patients with only a history of ischemic stroke or transient ischemic attack.24-27 Vascular surgery has become a new referral source, primarily for patients with peripheral and carotid artery diseases. Finally, we are leveraging videoconferencing and accepting referrals across the VA Northern California Health Care System (VANCHCS)catchment (from the California-Oregon state border to the San Francisco Bay Area). This also helps address a long-standing problem with reaching the many rural veterans who live far from a VA clinic. We successfully implemented a consult/referral process within the EHR that is available to providers across VANCHCS.
Discussion
The efficacy and effectiveness of reversal programs are well established in intensive programs (eg, ICR), yet such programs have yet to be streamlined and disseminated broadly into routine clinical care. HDRP has endeavored to address this by emphasizing nutrition relative to other program components. We have learned that the words “disease reversal” are very often the reason patients initially reach out or accept referral to our program.
Consistent with past research on plant-based nutrition interventions, the group format was indispensable.41 Individual sessions with a clinical health psychologist enabled tailored feedback and education on how behavior changes could impact laboratory results and how certain psychosocial factors could support success. Participants reported that seeing significantly favorable laboratory results was highly motivating and confirmed the power of their lifestyle changes. Furthermore, a psychosocial health assessment with individual sessions promoted a tailored treatment plan with targeted clinical interventions, such as behavioral health education, motivational interviewing, and advanced methods, including cognitive behavioral therapy and techniques drawn from dialectical behavior therapy and acceptance and commitment therapy.
Veterans with multimorbidity face the difficult task of learning and maintaining a complex disease self-management program and implementing a lifestyle approach that is feasible, effective, promotes weight loss, and treats multiple conditions. HDRP is a model approach for this population, as demonstrated by a recent case report of a 65-year-old male veteran with atherosclerotic CVD, T2DM, hypertension, and myasthenia gravis who had 2 heart attacks within 2 months.42 His neurologic disease precluded significant physical activity. Although he achieved some initial weight loss through lifestyle changes, he continued to have daily angina despite optimal and aggressive cardiology management. After enrolling in HDRP and adopting the WFPB diet, the patient reported almost complete resolution of angina within 1 month, similar to that found in other studies.15
The literature suggests that concern over the acceptability of plant-based diets and patients’ ability to adhere to them long-term may be misplaced. A review paper on dietary interventions lasting > 1 year found that 51 to 61% of vegetarian and vegan study participants had maintained dietary adherence, while 20 to 55% of omnivorous diet intervention participants adhered to their study diets.43 Remarkably, there were no statistically significant differences in the acceptability of the vegan, vegetarian, or omnivorous diets in the studies reviewed.43 Recent dietary research also suggests that providing patients with higher goals (eg, adopting a vegan diet instead of only moderate dietary changes) results in greater weight loss and maintenance.26 HDRP provides training on consumption of whole plant foods, which may offer patients a unique advantage for maximizing results and higher adherence over time.
Limitations
Hands-on cooking instruction was not provided at our VAMC. The total time of the intervention was significantly less in HDRP (25 hours) than it was for the Ornish ICR program (72 hours), which may hinder long-term adherence. Without an exercise facility, we were not able to provide more detailed exercise instruction and supervised exercise.
Program Improvements Planned
There are a number of improvements that are planned for HDRP. First, the program anticipates requesting medical clearance at the telephone screening stage for self-referred patients. Second,
Conclusions
Although our patient population was self-selected for participation, early program evaluation demonstrates high acceptability. Very few patients had ever been told about a heart disease reversing lifestyle, and we found direct-to-patient clinical outreach an effective method for launching a disease reversal program (optimally timed with HCP presentations). Furthermore, the program is adaptable to current restrictions on in-person appointments due to the COVID-19 pandemic, and much more convenient for rural veterans who live far from any VA clinic. Being able to offer sustainable health care for individuals during unexpected public health crises is critically important. Additionally, treating veterans who are most vulnerable to pandemic illness due to existing medical conditions, such as CVD, should be a high priority. Last, HDRP also may represent a novel integrated treatment for COVID-19 anxiety and secondary CVD prevention, as lifestyle habits are optimized to improve chronic diseases that elevate risk for severe COVID-19 infection and mortality, as well as including coping strategies consistent with evidence-based psychotherapies for anxiety disorders.44
We believe that beyond the clinical benefits to patients, there is significant value and benefit added to the health care system by offering an intervention within the “disease reversal” paradigm. Efforts of the health care team to reverse a disease can be considered the highest aim of medicine and health care.45
Acknowledgments
This work was supported by the US Department of Veterans Affairs. We give special thanks to David M. Gellerman, MD, PhD, and David W. Schafer, PsyD, for providing Mental Health Service support for initiating the Heart Disease Reversal Program, and to Joseph Giorgio, PsyD (Program Manager, Integrated Care Program) for sustaining it. We thank Amogh Bhat, MD, Chief of Cardiology, for his continued support and partnership with the Cardiology Department. We express thanks to Stephanie Mohney, RDN (Chief, Nutrition and Food Service), Amy Klotz, RDN (Supervisory Dietician), Sian M. Carr-Lopez, PharmD (Associate Chief of Pharmacy, Primary Care), and Michelle Rand, PharmD, CACP (Anticoagulation Clinical Pharmacist-Supervisor) for their staff support of this interdisciplinary program. We thank the patients and their families for their participation in the program and commitment to the lifestyle changes. We also thank the following individuals for their contributions to this program: Lisa Wagaman, RDN, Karen Soong, PharmD, Sara S. Ali, PharmD, Suzan Hua, PharmD, and Stephen Cooperman.
1. Benjamin EJ, Virani SS, Callaway CW, et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association [published correction appears in Circulation. 2018 Mar 20;137(12 ): e493]. Circulation. 2018;137(12):e67-e492. doi:10.1161/CIR.0000000000000558
2. Hinojosa R. Cardiovascular disease among United States military veterans: evidence of a waning healthy soldier effect using the National Health Interview Survey. Chronic Illn. 2020;16(1):55-68. doi:10.1177/1742395318785237.
3. Hinojosa R. Sex, age, race/ethnicity, veteran status, and the likelihood of reporting cardiovascular conditions in the National Health Interview Survey. J Cardiovasc Nurs. 2019;34(3):215-221. doi:10.1097/JCN.0000000000000561 4. Assari S. Veterans and risk of heart disease in the United States: a cohort with 20 years of follow up. Int J Prev Med. 2014;5(6):703-709.
5. Thomas MM, Harpaz-Rotem I, Tsai J, Southwick SM, Pietrzak RH. Mental and physical health conditions in US combat veterans: results from the National Health and Resilience in Veterans Study. Prim Care Companion CNS Disord. 2017;19(3):10.4088/PCC.17m02118. Published 2017 Jun 22. doi:10.4088/PCC.17m02118
6. Bukhbinder AS, Wang AC, Qureshi SU, et al. Increased vascular pathology in older veterans with a purple heart commendation or chronic post-traumatic stress disorder. J Geriatr Psychiatry Neurol. 2020;33(4):195-206. doi:10.1177/0891988719868308
7. Edmondson D, von Känel R. Post-traumatic stress disorder and cardiovascular disease. Lancet Psychiatry. 2017;4(4):320-329. doi:10.1016/S2215-0366(16)30377-7
8. Forman DE, Maurer MS, Boyd C, et a;. Multimorbidity in older adults with cardiovascular disease. J Am Coll Cardiol. 2018;71(19):2149-2161. doi:10.1016/j.jacc.2018.03.022
9. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
10. Maddox TM, Plomondon ME, Petrich M, et al. A national clinical quality program for Veterans Affairs catheterization laboratories (from the Veterans Affairs clinical assessment, reporting, and tracking program). Am J Cardiol. 2014;114(11):1750-1757. doi:10.1016/j.amjcard.2014.08.045
11. Centers for Disease Control and Prevention. Coronavirus 2019 (COVID-19):people at increased risk and other people who need to take extra precautions. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/index.html. Updated September 11, 2020. Accessed November 12, 2020.
12. International Food Information Council. 2020 food and health survey. https://foodinsight.org/2020-food-and-health-survey. Updated June 9, 2020. Accessed November 12, 2020.
13. American Association of Cardiovascular and Pulmonary Rehabilitation. Guidelines for Cardiac Rehabilitation and Secondary Prevention Programs. 5th ed. Champaign, IL: Human Kinetics; 2013.
14. Silberman A, Banthia R, Estay IS, et al. The effectiveness and efficacy of an intensive cardiac rehabilitation program in 24 sites. Am J Health Promot. 2010;24(4):260-266. doi:10.4278/ajhp.24.4.arb
15. Ornish D, Scherwitz LW, Doody RS, et al. Effects of stress management training and dietary changes in treating ischemic heart disease. JAMA. 1983;249(1):54-59.
16. Ornish D, Brown SE, Scherwitz LW, et al. Can lifestyle changes reverse coronary heart disease? The Lifestyle Heart Trial. Lancet. 1990;336(8708):129-133. doi:10.1016/0140-6736(90)91656-u.
17. Ornish D, Scherwitz LW, Billings JH, et al. Intensive lifestyle changes for reversal of coronary heart disease [published correction appears in JAMA 1999 Apr 21;281(15):1380]. JAMA. 1998;280(23):2001-2007. doi:10.1001/jama.280.23.2001
18. Frattaroli J, Weidner G, Merritt-Worden TA, Frenda S, Ornish D. Angina pectoris and atherosclerotic risk factors in the multisite cardiac lifestyle intervention program. Am J Cardiol. 2008;101(7):911-918. doi:10.1016/j.amjcard.2007.11.039
19. Koertge J, Weidner G, Elliott-Eller M, et al. Improvement in medical risk factors and quality of life in women and men with coronary artery disease in the Multicenter Lifestyle Demonstration Project. Am J Cardiol. 2003;91(11):1316-1322. doi:10.1016/s0002-9149(03)00320-5
20. Marshall DA, Walizer EM, Vernalis MN. Achievement of heart health characteristics through participation in an intensive lifestyle change program (Coronary Artery Disease Reversal Study). J Cardiopulm Rehabil Prev. 2009;29(2):84-96. doi:10.1097/HCR.0b013e31819a00b2
21. Marshall D, Elaine W, Vernalis M. The effect of a one-year lifestyle intervention program on carotid intima media thickness. Mil Med. 2011;176(7):798-804. doi:10.7205/milmed-d-10-00447
22. Esselstyn CB Jr, Ellis SG, Medendorp SV, Crowe TD. A strategy to arrest and reverse coronary artery disease: a 5-year longitudinal study of a single physician’s practice. J Fam Pract. 1995;41(6):560-568.
23. Esselstyn CB Jr. Updating a 12-year experience with arrest and reversal therapy for coronary heart disease (an overdue requiem for palliative cardiology). Am J Cardiol. 1999;84(3):339-A8. doi:10.1016/s0002-9149(99)00290-8
24. Barnard ND, Cohen J, Jenkins DJ, et al. A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes Care. 2006;29(8):1777-1783. doi:10.2337/dc06-0606
25. McDougall J, Thomas LE, McDougall C, et al. Effects of 7 days on an ad libitum low-fat vegan diet: the McDougall Program cohort [published correction appears in Nutr J. 2017 Feb 10;16(1):12]. Nutr J. 2014;13:99. Published 2014 Oct 14. doi:10.1186/1475-2891-13-99
26. Turner-McGrievy GM, Davidson CR, Wingard EE, Wilcox S, Frongillo EA. Comparative effectiveness of plant-based diets for weight loss: a randomized controlled trial of five different diets. Nutrition. 2015;31(2):350-358. doi:10.1016/j.nut.2014.09.002
27. Wright N, Wilson L, Smith M, Duncan B, McHugh P. The BROAD study: a randomised controlled trial using a whole food plant-based diet in the community for obesity, ischaemic heart disease or diabetes. Nutr Diabetes. 2017;7(3):e256. Published 2017 Mar 20. doi:10.1038/nutd.2017.3

28. Schaefer S, Hussein H, Gershony GR, Rutledge JC, Kappagoda CT. Regression of severe atherosclerotic plaque in patients with mild elevation of LDL cholesterol. J Investig Med. 1997;45(9):536-541.
29. Kitazono R. Know thy patient: Enhancing lifestyle interventions with psychological assessment. Int J Dis Rev Prev. 2020;2(1):76-81.
30. Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3rd ed. New York, NY: Guilford Press; 2013.
31. Mascola AJ, Yiaslas TA, Meir RL, et al. Framing physical activity as a distinct and uniquely valuable behavior independent of weight management: A pilot randomized controlled trial for overweight and obese sedentary persons. Eat Weight Disord. 2009;14(2-3):e148-e152. doi:10.1007/BF03327814
32. Esselstyn AC, Esselstyn J. The Prevent and Reverse Heart Disease Cookbook: Over 125 Delicious, Life-Changing, Plant-Based Recipes. New York, NY: Avery; 2014.
33. Esselstyn CB Jr, Gendy G, Doyle J, Golubic M, Roizen MF. A way to reverse CAD? J Fam Pract. 2014;63(7):356-364.
34. Ornish D, Ornish A. Undo It! How Simple Lifestyle Changes Can Reverse Most Chronic Diseases. New York, NY: Ballantine Books; 2019.
35. Smith SC Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association [published correction appears in J Am Coll Cardiol. 2015 Apr 14;65(14):1495. Dosage error in article text.]. J Am Coll Cardiol. 2011;58(23):2432-2446. doi:10.1016/j.jacc.2011.10.824
36. Blumenthal JA, Babyak M, Wei J, et al. Usefulness of psychosocial treatment of mental stress-induced myocardial ischemia in men. Am J Cardiol. 2002;89(2):164-168. doi:10.1016/s0002-9149(01)02194-4
37. Antoni MH. Stress management effects on psychological, endocrinological, and immune functioning in men with HIV infection: empirical support for a psychoneuroimmunological model. Stress. 2003;6(3):173-188. doi:10.1080/1025389031000156727
38. Penedo FJ, Molton I, Dahn JR, et al. A randomized clinical trial of group-based cognitive-behavioral stress management in localized prostate cancer: development of stress management skills improves quality of life and benefit finding. Ann Behav Med. 2006;31(3):261-270. doi:10.1207/s15324796abm3103_8
39. Yiaslas TA. “Look doctor, I’m a carnivore.” Int J Dis Rev Prev. 2020;2(2):35-39.
40. Khaylis A, Yiaslas T, Bergstrom J, Gore-Felton C. A review of efficacious technology-based weight-loss interventions: five key components. Telemed J E Health. 2010;16(9):931-938. doi:10.1089/tmj.2010.0065
41. Barnard ND, Sherwitz L, Ornish D. Adherence and acceptability of a low-fat, vegetarian diet among patients with cardiac disease. J Cardiopulm Rehabil. 1992;12(6):423-431.
42. Yiaslas TA, Taylor J, Embree J, Schaefer S. Elimination of angina, comprehensive cardio-metabolic risk reduction, and 50-pound weight loss in a US Navy veteran with myasthenia gravis. Int J Dis Rev Prev. 2019;1(1):77-83.
43. Berkow SE, Barnard N, Eckart J, Katcher H. Four therapeutic diets: adherence and acceptability. Can J Diet Pract Res. 2010;71(4):199-204. doi:10.3148/71.4.2010.199
44. Carpenter JK, Andrews LA, Witcraft SM, Powers MB, Smits JAJ, Hofmann SG. Cognitive behavioral therapy for anxiety and related disorders: A meta-analysis of randomized placebo-controlled trials. Depress Anxiety. 2018;35(6):502-514. doi:10.1002/da.22728
45. Yiaslas TA. The pursuit of arete in medicine and health care. Int J Dis Rev Prev. 2019;1(2):53-56.
While cardiovascular mortality rates have declined, heart disease continues to be the leading cause of death in the US, and the number of people with cardiovascular disease (CVD) is rising.1 CVD is more prevalent among military veterans than it is among nonveterans aged ≥ 25 years, and veteran status is associated with higher risk of incident heart disease after controlling for socioeconomic status, other medical diseases, depression, and lifestyle.2-4 Combat exposure, posttraumatic stress disorder (PTSD), and Purple Heart commendation are associated with higher rates of CVD, including adverse cardiovascular events.5-7 Many patients seeking care in the Veterans Health Administration (VHA), including those who undergo cardiac catheterization, meet the criteria for multimorbidity (defined as having ≥ 2 chronic diseases8), which is common among veterans.9,10 Multimorbidity presents a challenge for lifestyle intervention, as different diets may be prescribed to treat different conditions, such as Dietary Approaches to Stop Hypertension, and low-glycemic diet for diabetes mellitus (DM). Veterans with CVD are often clinically complex and may require more multifaceted secondary prevention programs.
During the coronavirus 2019 (COVID-19) pandemic, effective secondary prevention intervention is needed more than ever. Older age, CVD, and common comorbidities, including hypertension, DM, and obesity, place patients at the highest risk for severe COVID-19 infection.11 COVID-19 social distancing encourages vulnerable populations to stay home, which can make engaging in any levels of physical activity more challenging. The International Food Council found that 85% of adults have made a change to their food consumption pattern, including eating more, during the COVID-19 pandemic.12 Thus, secondary CVD prevention programs for veterans need to provide treatment that addresses these specific challenges and can be delivered via telehealth for continuity of care after disruption of traditional services.
Clinical practice guidelines for the treatment of patients with recent cardiovascular adverse events (AEs) include a referral to cardiac rehabilitation (CR).13 CR emphasizes exercise as the main intervention, along with coaching to promote multiple risk reduction. The most comprehensive CR program is intensive CR (ICR), including the Ornish ICR program.14 ICR includes 4 components: vegetarian diet, exercise, stress management (yoga, meditation), and group support. Ornish ICR has been shown to be efficacious in randomized controlled trials (RCTs).15-17 Three effectiveness studies, with 5,372 participants, demonstrated the real-world effectiveness of Ornish ICR in US hospitals.14,18,19 The program also was adapted successfully for the active-duty military and veteran population.20,21 Yet Ornish ICR is time intensive, and there are no certified VHA ICR Ornish sites. Furthermore, there is no formal strategy for targeting people with atherosclerotic CVD who no longer meet the criteria for CR or ICR. While Ornish ICR is highly effective for patients who are eligible and have access, a more effective and streamlined approach is needed for targeting many patients.
Nutrition may be the most powerful Ornish ICR component. The initial RCT conducted by Ornish and colleagues included only stress management training and a whole-food, plant-based (WFPB) diet, including grains, legumes, vegetables, fruits, nuts, and seeds. The trial found 91% of participants experienced reduced angina after only 24 days.15 The only single-component intervention study resulting in partial reversal of atherosclerosis was a WFPB diet-only study, which documented regression of atherosclerotic plaques after 5 years, using coronary angiography in 73% of participants, with arrested progression in the other 27%.22 Participants reported no cardiovascular AEs after 12 years.23 Furthermore, a number of other recent studies have demonstrated the benefits of WFPB diet-only interventions for type 2 DM (T2DM), hypertension, and obesity.24-27 The Heart Disease Reversal Program (HDRP) was developed to create an interdisciplinary lifestyle intervention that emphasized nutrition for a broad population of veterans with atherosclerotic CVD, of varying levels of functional ability, to promote comprehensive CVD risk reduction and bring heart disease reversal intervention into routine clinical practice.
Program Description
The Mental Health, Cardiology, and Nutrition and Food services all approved the launch of HDRP. We contacted veterans by mail, and 11% expressed interest (Figure). Among patients who received the initial mailed letter (prior to our accepting staff referrals), only 5% of patients who enrolled in HDRP reported previously being told about or prescribed a WFPB diet by any health care provider (HCP). Currently, patients are primarily referred to HDRP by Cardiology, Primary Care, and Mental Health services.
Design
HDRP is an adaptation of interdisciplinary lifestyle interventions that have resulted in regression of atherosclerotic blockages confirmed with invasive coronary angiography.15-17,22,28 HDRP currently is offered in a Behavioral Medicine Clinic at the Sacramento US Department of Veterans Affairs (VA) Medical Center (VAMC) in California. Program staff include a clinical health psychologist who organizes, coordinates, and act as the lead facilitator of the program; registered dietitians; clinical pharmacists; and a consulting physician. Patients engage in the 4-month core HDRP program in small cohorts (ie, 6-10 patients), and spouses/partners are highly encouraged to attend all sessions.
Components
Telephone screening. Patients are screened for the inclusion and exclusion criteria (Table 1). Patients engaging in a traditional CR program are included in the screening. Patients are informed that the program consists of lifestyle intervention, including emphasis on following a WFPB diet.
Health assessment. Once approved, all patients are instructed to complete baseline laboratory tests and questionnaires. Along with an electronic health record (EHR) review, a psychosocial assessment is completed by a licensed clinical health psychologist who assesses CVD history, eating behavior, exercise/physical activity, sleep, mental health, substance use, and social history, with the aim of enhancing our ability to help the patient to benefit from HDRP.29 The patient data are used to develop a case conceptualization (ie, integrated understanding of the particular patient’s psychiatric and medical diagnoses, behavioral patterns, social supports, lifestyle habits, strengths and weaknesses, and their interrelationships with each other and the patient’s environment), resulting in an individualized plan. Patients are encouraged to ask questions about the program, and those who are still interested are invited to attend a seminar. A request for medical clearance to participate in the program is initiated through the EHR or by patients scheduling an appointment with their HCP. All patients are medically cleared by their HCP for participation. Safe exercise recommendations also are provided and guide patient goals.
CVD risk profile. Patients complete psychosocial questionnaires and fasting laboratory tests to produce a tailored CVD risk profile. Laboratory tests include fasting lipid, fasting glucose, hemoglobin A1c (HbA1c) C-reactive protein, vitamin B12, and vitamin D. The same tests (excluding HbA1c) are completed 1 month later (after completing 4 group sessions) and again posttreatment (including HbA1c). Self-reported questionnaires are completed at the same time points, which include the Rate Your Plate dietary composition questionnaire, CHAMPS physical activity questionnaire for older adults, Beck Depression Inventory-II, and the Perceived Stress Scale.
Seminar. A 2-hour seminar provides patients and families with an opportunity to meet HDRP program staff, learn the background and rationale for chronic disease reversal, obtain a summary of the program, and hear a patient testimonial. Patients are asked to make a commitment, and the informed consent process includes all patients signing a behavioral contract.
Assessment and feedback. A licensed clinical health psychologist provides feedback to patients on their comprehensive CVD risk profile, using motivational interviewing.30,31 Smokers are encouraged to quit, and those interested are referred to their HCP and/or facility smoking cessation program.
Group sessions. Twelve weekly group sessions cover nutrition education and cooking, physical activity and exercise, stress management training, and medication reconciliation and adjustment. The nutrition component is the centerpiece of HDRP and is delivered by registered dietitians (Table 2). Patients are instructed to use the 3-week period between the HDRP seminar and the first core group session to try new recipes and prepare their kitchens, pantries, and mind-set to adopt the HDRP diet with 100% adherence. The WFPB diet used is consistent with the current guidelines of Caldwell Esselstyn, MD, and Dean Ornish, MD.32-34
A psychologist delivers the physical activity component. Patients are encouraged to meet the American Heart Association/American College of Cardiology recommendations for aerobic exercise (at least 150 minutes of moderate intensity physical activity per week) through a walking program.35 Patients with medical contraindications (eg, severe pain, mobility restrictions) are encouraged to follow the exercise/activity recommendations they had been given by their primary care provider (PCP), physical therapist, or other HCP.
A psychologist provides evidence-based cognitive behavioral stress management (CBSM) training, adapted from models developed for patients with stable ischemic heart disease, HIV/AIDS, and cancer.36-38 CBSM is a psychotherapy grounded in stress/coping theory and cognitive behavioral theory of psychopathology that integrates cognitive restructuring, coping skills training, communication/assertiveness training, anger management, and mindfulness/acceptance-based approaches. Additional emphasis is placed on assisting patients’ adjustment to the lifestyle challenges for following a plant-based diet, dealing with food cravings and emotional eating, and connecting lifestyle change to patients’ deepest values and goals.
A clinical pharmacist conducts a medication reconciliation for each patient at baseline. The pharmacist consults with each patient’s PCP, cardiologist, and HDRP consulting physician, as needed, to ensure safe adjustments to medications. Pharmacists also provide education on medications at group sessions.
After completion of the 12-week core program, graduates are encouraged to attend the monthly graduates’ group indefinitely, and as often as they desire to promote maintenance of the disease reversal lifestyle. Patients are encouraged to complete our recommended fasting laboratory work every 3 to 6 months to facilitate maintenance of treatment gains.
Program Evaluation
Patients frequently reported that the group format was vital to their success. Patients requested a cooking class, yet we lacked a full teaching kitchen. Integrating plant-based meal samples at every session and cooking videos helped. Patients reported that 100% adherence to the WFPB diet led to significant changes in their food preferences, including a loss of interest in meat.39 Patients encouraged us to keep the “disease reversal” language and focus. One veteran stated: “Disease reversal, that is the reason I called you when I got your letter.” Showing before and after images of coronary angiograms and cardiac positron emission tomography scans depicting regression of atherosclerotic plaque and restored myocardial perfusion were described as highly motivating and generated willingness to commit to a more aggressive lifestyle change.31
Patients routinely stated that they lacked understanding of their laboratory test results, which HDRP remedied. Some patients reported their adult children followed a plant-based diet, and our program resulted in a new commonality and source of bonding that was highly valued. Some patients reported that HDRP was helpful for controlling their COVID-19 anxiety and feeling in control of their health. Satisfaction surveys were completed by participants at the end of the core program, which demonstrated very high satisfaction with and acceptability of HDRP (Table 3).
The program also has received positive feedback from HCPs when we alert them to improvements in outcome measures for their patients. These HCPs expressed satisfaction with having a program to refer patients to that can help with chronic illness in more depth.
COVID-19 Response
Face-to-face group appointments were converted to videoconferencing as a result of the COVID-19 pandemic. While HDRP always promoted the use of technology and mHealth tools, the pandemic led us to develop novel technology-based interventions.40 One cohort transitioned from in-person to videoconferencing sessions, and 2 cohorts recently started this format and are ongoing. We have successfully used videoconferencing with Cisco Webex, the VA-approved backup platform, as we encountered technical barriers when using VA Video Connect. Program materials were shared electronically, and participants sent blood pressure/sugar logs by secure messaging. Guidance for online grocery shopping with home delivery was provided, and research on the benefits of the HDRP lifestyle on immune function was incorporated.
The stress management component incorporated coping with COVID-19, including normalizing common emotional difficulties with sheltering-in-place and quarantine, acknowledging and processing fear and anxiety related to being at very high risk for severe COVID-19. We presented heart disease reversal as an urgent and feasible goal during the pandemic both reducing risk of premature death and major adverse cardiovascular events in the long-term and also reducing personal risk of severe COVID complications. The new VA COVID Coach app was also presented as a resource. Reputable sources of COVID-19 and public health information were shared. Walking continued to be the primary recommended form of exercise, while indoor home exercise options were promoted during the periods of very poor air quality due to the widespread California fires and smoke.
Considering the research suggesting benefits of our intervention for treating T2DM,promoting sustained weight loss, and promoting comprehensive cardiometabolic risk reduction, we have begun accepting referrals for patients with any type of atherosclerotic CVD (eg, peripheral artery disease, carotid artery disease), patients with T2DM (without CVD), and patients with only a history of ischemic stroke or transient ischemic attack.24-27 Vascular surgery has become a new referral source, primarily for patients with peripheral and carotid artery diseases. Finally, we are leveraging videoconferencing and accepting referrals across the VA Northern California Health Care System (VANCHCS)catchment (from the California-Oregon state border to the San Francisco Bay Area). This also helps address a long-standing problem with reaching the many rural veterans who live far from a VA clinic. We successfully implemented a consult/referral process within the EHR that is available to providers across VANCHCS.
Discussion
The efficacy and effectiveness of reversal programs are well established in intensive programs (eg, ICR), yet such programs have yet to be streamlined and disseminated broadly into routine clinical care. HDRP has endeavored to address this by emphasizing nutrition relative to other program components. We have learned that the words “disease reversal” are very often the reason patients initially reach out or accept referral to our program.
Consistent with past research on plant-based nutrition interventions, the group format was indispensable.41 Individual sessions with a clinical health psychologist enabled tailored feedback and education on how behavior changes could impact laboratory results and how certain psychosocial factors could support success. Participants reported that seeing significantly favorable laboratory results was highly motivating and confirmed the power of their lifestyle changes. Furthermore, a psychosocial health assessment with individual sessions promoted a tailored treatment plan with targeted clinical interventions, such as behavioral health education, motivational interviewing, and advanced methods, including cognitive behavioral therapy and techniques drawn from dialectical behavior therapy and acceptance and commitment therapy.
Veterans with multimorbidity face the difficult task of learning and maintaining a complex disease self-management program and implementing a lifestyle approach that is feasible, effective, promotes weight loss, and treats multiple conditions. HDRP is a model approach for this population, as demonstrated by a recent case report of a 65-year-old male veteran with atherosclerotic CVD, T2DM, hypertension, and myasthenia gravis who had 2 heart attacks within 2 months.42 His neurologic disease precluded significant physical activity. Although he achieved some initial weight loss through lifestyle changes, he continued to have daily angina despite optimal and aggressive cardiology management. After enrolling in HDRP and adopting the WFPB diet, the patient reported almost complete resolution of angina within 1 month, similar to that found in other studies.15
The literature suggests that concern over the acceptability of plant-based diets and patients’ ability to adhere to them long-term may be misplaced. A review paper on dietary interventions lasting > 1 year found that 51 to 61% of vegetarian and vegan study participants had maintained dietary adherence, while 20 to 55% of omnivorous diet intervention participants adhered to their study diets.43 Remarkably, there were no statistically significant differences in the acceptability of the vegan, vegetarian, or omnivorous diets in the studies reviewed.43 Recent dietary research also suggests that providing patients with higher goals (eg, adopting a vegan diet instead of only moderate dietary changes) results in greater weight loss and maintenance.26 HDRP provides training on consumption of whole plant foods, which may offer patients a unique advantage for maximizing results and higher adherence over time.
Limitations
Hands-on cooking instruction was not provided at our VAMC. The total time of the intervention was significantly less in HDRP (25 hours) than it was for the Ornish ICR program (72 hours), which may hinder long-term adherence. Without an exercise facility, we were not able to provide more detailed exercise instruction and supervised exercise.
Program Improvements Planned
There are a number of improvements that are planned for HDRP. First, the program anticipates requesting medical clearance at the telephone screening stage for self-referred patients. Second,
Conclusions
Although our patient population was self-selected for participation, early program evaluation demonstrates high acceptability. Very few patients had ever been told about a heart disease reversing lifestyle, and we found direct-to-patient clinical outreach an effective method for launching a disease reversal program (optimally timed with HCP presentations). Furthermore, the program is adaptable to current restrictions on in-person appointments due to the COVID-19 pandemic, and much more convenient for rural veterans who live far from any VA clinic. Being able to offer sustainable health care for individuals during unexpected public health crises is critically important. Additionally, treating veterans who are most vulnerable to pandemic illness due to existing medical conditions, such as CVD, should be a high priority. Last, HDRP also may represent a novel integrated treatment for COVID-19 anxiety and secondary CVD prevention, as lifestyle habits are optimized to improve chronic diseases that elevate risk for severe COVID-19 infection and mortality, as well as including coping strategies consistent with evidence-based psychotherapies for anxiety disorders.44
We believe that beyond the clinical benefits to patients, there is significant value and benefit added to the health care system by offering an intervention within the “disease reversal” paradigm. Efforts of the health care team to reverse a disease can be considered the highest aim of medicine and health care.45
Acknowledgments
This work was supported by the US Department of Veterans Affairs. We give special thanks to David M. Gellerman, MD, PhD, and David W. Schafer, PsyD, for providing Mental Health Service support for initiating the Heart Disease Reversal Program, and to Joseph Giorgio, PsyD (Program Manager, Integrated Care Program) for sustaining it. We thank Amogh Bhat, MD, Chief of Cardiology, for his continued support and partnership with the Cardiology Department. We express thanks to Stephanie Mohney, RDN (Chief, Nutrition and Food Service), Amy Klotz, RDN (Supervisory Dietician), Sian M. Carr-Lopez, PharmD (Associate Chief of Pharmacy, Primary Care), and Michelle Rand, PharmD, CACP (Anticoagulation Clinical Pharmacist-Supervisor) for their staff support of this interdisciplinary program. We thank the patients and their families for their participation in the program and commitment to the lifestyle changes. We also thank the following individuals for their contributions to this program: Lisa Wagaman, RDN, Karen Soong, PharmD, Sara S. Ali, PharmD, Suzan Hua, PharmD, and Stephen Cooperman.
While cardiovascular mortality rates have declined, heart disease continues to be the leading cause of death in the US, and the number of people with cardiovascular disease (CVD) is rising.1 CVD is more prevalent among military veterans than it is among nonveterans aged ≥ 25 years, and veteran status is associated with higher risk of incident heart disease after controlling for socioeconomic status, other medical diseases, depression, and lifestyle.2-4 Combat exposure, posttraumatic stress disorder (PTSD), and Purple Heart commendation are associated with higher rates of CVD, including adverse cardiovascular events.5-7 Many patients seeking care in the Veterans Health Administration (VHA), including those who undergo cardiac catheterization, meet the criteria for multimorbidity (defined as having ≥ 2 chronic diseases8), which is common among veterans.9,10 Multimorbidity presents a challenge for lifestyle intervention, as different diets may be prescribed to treat different conditions, such as Dietary Approaches to Stop Hypertension, and low-glycemic diet for diabetes mellitus (DM). Veterans with CVD are often clinically complex and may require more multifaceted secondary prevention programs.
During the coronavirus 2019 (COVID-19) pandemic, effective secondary prevention intervention is needed more than ever. Older age, CVD, and common comorbidities, including hypertension, DM, and obesity, place patients at the highest risk for severe COVID-19 infection.11 COVID-19 social distancing encourages vulnerable populations to stay home, which can make engaging in any levels of physical activity more challenging. The International Food Council found that 85% of adults have made a change to their food consumption pattern, including eating more, during the COVID-19 pandemic.12 Thus, secondary CVD prevention programs for veterans need to provide treatment that addresses these specific challenges and can be delivered via telehealth for continuity of care after disruption of traditional services.
Clinical practice guidelines for the treatment of patients with recent cardiovascular adverse events (AEs) include a referral to cardiac rehabilitation (CR).13 CR emphasizes exercise as the main intervention, along with coaching to promote multiple risk reduction. The most comprehensive CR program is intensive CR (ICR), including the Ornish ICR program.14 ICR includes 4 components: vegetarian diet, exercise, stress management (yoga, meditation), and group support. Ornish ICR has been shown to be efficacious in randomized controlled trials (RCTs).15-17 Three effectiveness studies, with 5,372 participants, demonstrated the real-world effectiveness of Ornish ICR in US hospitals.14,18,19 The program also was adapted successfully for the active-duty military and veteran population.20,21 Yet Ornish ICR is time intensive, and there are no certified VHA ICR Ornish sites. Furthermore, there is no formal strategy for targeting people with atherosclerotic CVD who no longer meet the criteria for CR or ICR. While Ornish ICR is highly effective for patients who are eligible and have access, a more effective and streamlined approach is needed for targeting many patients.
Nutrition may be the most powerful Ornish ICR component. The initial RCT conducted by Ornish and colleagues included only stress management training and a whole-food, plant-based (WFPB) diet, including grains, legumes, vegetables, fruits, nuts, and seeds. The trial found 91% of participants experienced reduced angina after only 24 days.15 The only single-component intervention study resulting in partial reversal of atherosclerosis was a WFPB diet-only study, which documented regression of atherosclerotic plaques after 5 years, using coronary angiography in 73% of participants, with arrested progression in the other 27%.22 Participants reported no cardiovascular AEs after 12 years.23 Furthermore, a number of other recent studies have demonstrated the benefits of WFPB diet-only interventions for type 2 DM (T2DM), hypertension, and obesity.24-27 The Heart Disease Reversal Program (HDRP) was developed to create an interdisciplinary lifestyle intervention that emphasized nutrition for a broad population of veterans with atherosclerotic CVD, of varying levels of functional ability, to promote comprehensive CVD risk reduction and bring heart disease reversal intervention into routine clinical practice.
Program Description
The Mental Health, Cardiology, and Nutrition and Food services all approved the launch of HDRP. We contacted veterans by mail, and 11% expressed interest (Figure). Among patients who received the initial mailed letter (prior to our accepting staff referrals), only 5% of patients who enrolled in HDRP reported previously being told about or prescribed a WFPB diet by any health care provider (HCP). Currently, patients are primarily referred to HDRP by Cardiology, Primary Care, and Mental Health services.
Design
HDRP is an adaptation of interdisciplinary lifestyle interventions that have resulted in regression of atherosclerotic blockages confirmed with invasive coronary angiography.15-17,22,28 HDRP currently is offered in a Behavioral Medicine Clinic at the Sacramento US Department of Veterans Affairs (VA) Medical Center (VAMC) in California. Program staff include a clinical health psychologist who organizes, coordinates, and act as the lead facilitator of the program; registered dietitians; clinical pharmacists; and a consulting physician. Patients engage in the 4-month core HDRP program in small cohorts (ie, 6-10 patients), and spouses/partners are highly encouraged to attend all sessions.
Components
Telephone screening. Patients are screened for the inclusion and exclusion criteria (Table 1). Patients engaging in a traditional CR program are included in the screening. Patients are informed that the program consists of lifestyle intervention, including emphasis on following a WFPB diet.
Health assessment. Once approved, all patients are instructed to complete baseline laboratory tests and questionnaires. Along with an electronic health record (EHR) review, a psychosocial assessment is completed by a licensed clinical health psychologist who assesses CVD history, eating behavior, exercise/physical activity, sleep, mental health, substance use, and social history, with the aim of enhancing our ability to help the patient to benefit from HDRP.29 The patient data are used to develop a case conceptualization (ie, integrated understanding of the particular patient’s psychiatric and medical diagnoses, behavioral patterns, social supports, lifestyle habits, strengths and weaknesses, and their interrelationships with each other and the patient’s environment), resulting in an individualized plan. Patients are encouraged to ask questions about the program, and those who are still interested are invited to attend a seminar. A request for medical clearance to participate in the program is initiated through the EHR or by patients scheduling an appointment with their HCP. All patients are medically cleared by their HCP for participation. Safe exercise recommendations also are provided and guide patient goals.
CVD risk profile. Patients complete psychosocial questionnaires and fasting laboratory tests to produce a tailored CVD risk profile. Laboratory tests include fasting lipid, fasting glucose, hemoglobin A1c (HbA1c) C-reactive protein, vitamin B12, and vitamin D. The same tests (excluding HbA1c) are completed 1 month later (after completing 4 group sessions) and again posttreatment (including HbA1c). Self-reported questionnaires are completed at the same time points, which include the Rate Your Plate dietary composition questionnaire, CHAMPS physical activity questionnaire for older adults, Beck Depression Inventory-II, and the Perceived Stress Scale.
Seminar. A 2-hour seminar provides patients and families with an opportunity to meet HDRP program staff, learn the background and rationale for chronic disease reversal, obtain a summary of the program, and hear a patient testimonial. Patients are asked to make a commitment, and the informed consent process includes all patients signing a behavioral contract.
Assessment and feedback. A licensed clinical health psychologist provides feedback to patients on their comprehensive CVD risk profile, using motivational interviewing.30,31 Smokers are encouraged to quit, and those interested are referred to their HCP and/or facility smoking cessation program.
Group sessions. Twelve weekly group sessions cover nutrition education and cooking, physical activity and exercise, stress management training, and medication reconciliation and adjustment. The nutrition component is the centerpiece of HDRP and is delivered by registered dietitians (Table 2). Patients are instructed to use the 3-week period between the HDRP seminar and the first core group session to try new recipes and prepare their kitchens, pantries, and mind-set to adopt the HDRP diet with 100% adherence. The WFPB diet used is consistent with the current guidelines of Caldwell Esselstyn, MD, and Dean Ornish, MD.32-34
A psychologist delivers the physical activity component. Patients are encouraged to meet the American Heart Association/American College of Cardiology recommendations for aerobic exercise (at least 150 minutes of moderate intensity physical activity per week) through a walking program.35 Patients with medical contraindications (eg, severe pain, mobility restrictions) are encouraged to follow the exercise/activity recommendations they had been given by their primary care provider (PCP), physical therapist, or other HCP.
A psychologist provides evidence-based cognitive behavioral stress management (CBSM) training, adapted from models developed for patients with stable ischemic heart disease, HIV/AIDS, and cancer.36-38 CBSM is a psychotherapy grounded in stress/coping theory and cognitive behavioral theory of psychopathology that integrates cognitive restructuring, coping skills training, communication/assertiveness training, anger management, and mindfulness/acceptance-based approaches. Additional emphasis is placed on assisting patients’ adjustment to the lifestyle challenges for following a plant-based diet, dealing with food cravings and emotional eating, and connecting lifestyle change to patients’ deepest values and goals.
A clinical pharmacist conducts a medication reconciliation for each patient at baseline. The pharmacist consults with each patient’s PCP, cardiologist, and HDRP consulting physician, as needed, to ensure safe adjustments to medications. Pharmacists also provide education on medications at group sessions.
After completion of the 12-week core program, graduates are encouraged to attend the monthly graduates’ group indefinitely, and as often as they desire to promote maintenance of the disease reversal lifestyle. Patients are encouraged to complete our recommended fasting laboratory work every 3 to 6 months to facilitate maintenance of treatment gains.
Program Evaluation
Patients frequently reported that the group format was vital to their success. Patients requested a cooking class, yet we lacked a full teaching kitchen. Integrating plant-based meal samples at every session and cooking videos helped. Patients reported that 100% adherence to the WFPB diet led to significant changes in their food preferences, including a loss of interest in meat.39 Patients encouraged us to keep the “disease reversal” language and focus. One veteran stated: “Disease reversal, that is the reason I called you when I got your letter.” Showing before and after images of coronary angiograms and cardiac positron emission tomography scans depicting regression of atherosclerotic plaque and restored myocardial perfusion were described as highly motivating and generated willingness to commit to a more aggressive lifestyle change.31
Patients routinely stated that they lacked understanding of their laboratory test results, which HDRP remedied. Some patients reported their adult children followed a plant-based diet, and our program resulted in a new commonality and source of bonding that was highly valued. Some patients reported that HDRP was helpful for controlling their COVID-19 anxiety and feeling in control of their health. Satisfaction surveys were completed by participants at the end of the core program, which demonstrated very high satisfaction with and acceptability of HDRP (Table 3).
The program also has received positive feedback from HCPs when we alert them to improvements in outcome measures for their patients. These HCPs expressed satisfaction with having a program to refer patients to that can help with chronic illness in more depth.
COVID-19 Response
Face-to-face group appointments were converted to videoconferencing as a result of the COVID-19 pandemic. While HDRP always promoted the use of technology and mHealth tools, the pandemic led us to develop novel technology-based interventions.40 One cohort transitioned from in-person to videoconferencing sessions, and 2 cohorts recently started this format and are ongoing. We have successfully used videoconferencing with Cisco Webex, the VA-approved backup platform, as we encountered technical barriers when using VA Video Connect. Program materials were shared electronically, and participants sent blood pressure/sugar logs by secure messaging. Guidance for online grocery shopping with home delivery was provided, and research on the benefits of the HDRP lifestyle on immune function was incorporated.
The stress management component incorporated coping with COVID-19, including normalizing common emotional difficulties with sheltering-in-place and quarantine, acknowledging and processing fear and anxiety related to being at very high risk for severe COVID-19. We presented heart disease reversal as an urgent and feasible goal during the pandemic both reducing risk of premature death and major adverse cardiovascular events in the long-term and also reducing personal risk of severe COVID complications. The new VA COVID Coach app was also presented as a resource. Reputable sources of COVID-19 and public health information were shared. Walking continued to be the primary recommended form of exercise, while indoor home exercise options were promoted during the periods of very poor air quality due to the widespread California fires and smoke.
Considering the research suggesting benefits of our intervention for treating T2DM,promoting sustained weight loss, and promoting comprehensive cardiometabolic risk reduction, we have begun accepting referrals for patients with any type of atherosclerotic CVD (eg, peripheral artery disease, carotid artery disease), patients with T2DM (without CVD), and patients with only a history of ischemic stroke or transient ischemic attack.24-27 Vascular surgery has become a new referral source, primarily for patients with peripheral and carotid artery diseases. Finally, we are leveraging videoconferencing and accepting referrals across the VA Northern California Health Care System (VANCHCS)catchment (from the California-Oregon state border to the San Francisco Bay Area). This also helps address a long-standing problem with reaching the many rural veterans who live far from a VA clinic. We successfully implemented a consult/referral process within the EHR that is available to providers across VANCHCS.
Discussion
The efficacy and effectiveness of reversal programs are well established in intensive programs (eg, ICR), yet such programs have yet to be streamlined and disseminated broadly into routine clinical care. HDRP has endeavored to address this by emphasizing nutrition relative to other program components. We have learned that the words “disease reversal” are very often the reason patients initially reach out or accept referral to our program.
Consistent with past research on plant-based nutrition interventions, the group format was indispensable.41 Individual sessions with a clinical health psychologist enabled tailored feedback and education on how behavior changes could impact laboratory results and how certain psychosocial factors could support success. Participants reported that seeing significantly favorable laboratory results was highly motivating and confirmed the power of their lifestyle changes. Furthermore, a psychosocial health assessment with individual sessions promoted a tailored treatment plan with targeted clinical interventions, such as behavioral health education, motivational interviewing, and advanced methods, including cognitive behavioral therapy and techniques drawn from dialectical behavior therapy and acceptance and commitment therapy.
Veterans with multimorbidity face the difficult task of learning and maintaining a complex disease self-management program and implementing a lifestyle approach that is feasible, effective, promotes weight loss, and treats multiple conditions. HDRP is a model approach for this population, as demonstrated by a recent case report of a 65-year-old male veteran with atherosclerotic CVD, T2DM, hypertension, and myasthenia gravis who had 2 heart attacks within 2 months.42 His neurologic disease precluded significant physical activity. Although he achieved some initial weight loss through lifestyle changes, he continued to have daily angina despite optimal and aggressive cardiology management. After enrolling in HDRP and adopting the WFPB diet, the patient reported almost complete resolution of angina within 1 month, similar to that found in other studies.15
The literature suggests that concern over the acceptability of plant-based diets and patients’ ability to adhere to them long-term may be misplaced. A review paper on dietary interventions lasting > 1 year found that 51 to 61% of vegetarian and vegan study participants had maintained dietary adherence, while 20 to 55% of omnivorous diet intervention participants adhered to their study diets.43 Remarkably, there were no statistically significant differences in the acceptability of the vegan, vegetarian, or omnivorous diets in the studies reviewed.43 Recent dietary research also suggests that providing patients with higher goals (eg, adopting a vegan diet instead of only moderate dietary changes) results in greater weight loss and maintenance.26 HDRP provides training on consumption of whole plant foods, which may offer patients a unique advantage for maximizing results and higher adherence over time.
Limitations
Hands-on cooking instruction was not provided at our VAMC. The total time of the intervention was significantly less in HDRP (25 hours) than it was for the Ornish ICR program (72 hours), which may hinder long-term adherence. Without an exercise facility, we were not able to provide more detailed exercise instruction and supervised exercise.
Program Improvements Planned
There are a number of improvements that are planned for HDRP. First, the program anticipates requesting medical clearance at the telephone screening stage for self-referred patients. Second,
Conclusions
Although our patient population was self-selected for participation, early program evaluation demonstrates high acceptability. Very few patients had ever been told about a heart disease reversing lifestyle, and we found direct-to-patient clinical outreach an effective method for launching a disease reversal program (optimally timed with HCP presentations). Furthermore, the program is adaptable to current restrictions on in-person appointments due to the COVID-19 pandemic, and much more convenient for rural veterans who live far from any VA clinic. Being able to offer sustainable health care for individuals during unexpected public health crises is critically important. Additionally, treating veterans who are most vulnerable to pandemic illness due to existing medical conditions, such as CVD, should be a high priority. Last, HDRP also may represent a novel integrated treatment for COVID-19 anxiety and secondary CVD prevention, as lifestyle habits are optimized to improve chronic diseases that elevate risk for severe COVID-19 infection and mortality, as well as including coping strategies consistent with evidence-based psychotherapies for anxiety disorders.44
We believe that beyond the clinical benefits to patients, there is significant value and benefit added to the health care system by offering an intervention within the “disease reversal” paradigm. Efforts of the health care team to reverse a disease can be considered the highest aim of medicine and health care.45
Acknowledgments
This work was supported by the US Department of Veterans Affairs. We give special thanks to David M. Gellerman, MD, PhD, and David W. Schafer, PsyD, for providing Mental Health Service support for initiating the Heart Disease Reversal Program, and to Joseph Giorgio, PsyD (Program Manager, Integrated Care Program) for sustaining it. We thank Amogh Bhat, MD, Chief of Cardiology, for his continued support and partnership with the Cardiology Department. We express thanks to Stephanie Mohney, RDN (Chief, Nutrition and Food Service), Amy Klotz, RDN (Supervisory Dietician), Sian M. Carr-Lopez, PharmD (Associate Chief of Pharmacy, Primary Care), and Michelle Rand, PharmD, CACP (Anticoagulation Clinical Pharmacist-Supervisor) for their staff support of this interdisciplinary program. We thank the patients and their families for their participation in the program and commitment to the lifestyle changes. We also thank the following individuals for their contributions to this program: Lisa Wagaman, RDN, Karen Soong, PharmD, Sara S. Ali, PharmD, Suzan Hua, PharmD, and Stephen Cooperman.
1. Benjamin EJ, Virani SS, Callaway CW, et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association [published correction appears in Circulation. 2018 Mar 20;137(12 ): e493]. Circulation. 2018;137(12):e67-e492. doi:10.1161/CIR.0000000000000558
2. Hinojosa R. Cardiovascular disease among United States military veterans: evidence of a waning healthy soldier effect using the National Health Interview Survey. Chronic Illn. 2020;16(1):55-68. doi:10.1177/1742395318785237.
3. Hinojosa R. Sex, age, race/ethnicity, veteran status, and the likelihood of reporting cardiovascular conditions in the National Health Interview Survey. J Cardiovasc Nurs. 2019;34(3):215-221. doi:10.1097/JCN.0000000000000561 4. Assari S. Veterans and risk of heart disease in the United States: a cohort with 20 years of follow up. Int J Prev Med. 2014;5(6):703-709.
5. Thomas MM, Harpaz-Rotem I, Tsai J, Southwick SM, Pietrzak RH. Mental and physical health conditions in US combat veterans: results from the National Health and Resilience in Veterans Study. Prim Care Companion CNS Disord. 2017;19(3):10.4088/PCC.17m02118. Published 2017 Jun 22. doi:10.4088/PCC.17m02118
6. Bukhbinder AS, Wang AC, Qureshi SU, et al. Increased vascular pathology in older veterans with a purple heart commendation or chronic post-traumatic stress disorder. J Geriatr Psychiatry Neurol. 2020;33(4):195-206. doi:10.1177/0891988719868308
7. Edmondson D, von Känel R. Post-traumatic stress disorder and cardiovascular disease. Lancet Psychiatry. 2017;4(4):320-329. doi:10.1016/S2215-0366(16)30377-7
8. Forman DE, Maurer MS, Boyd C, et a;. Multimorbidity in older adults with cardiovascular disease. J Am Coll Cardiol. 2018;71(19):2149-2161. doi:10.1016/j.jacc.2018.03.022
9. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
10. Maddox TM, Plomondon ME, Petrich M, et al. A national clinical quality program for Veterans Affairs catheterization laboratories (from the Veterans Affairs clinical assessment, reporting, and tracking program). Am J Cardiol. 2014;114(11):1750-1757. doi:10.1016/j.amjcard.2014.08.045
11. Centers for Disease Control and Prevention. Coronavirus 2019 (COVID-19):people at increased risk and other people who need to take extra precautions. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/index.html. Updated September 11, 2020. Accessed November 12, 2020.
12. International Food Information Council. 2020 food and health survey. https://foodinsight.org/2020-food-and-health-survey. Updated June 9, 2020. Accessed November 12, 2020.
13. American Association of Cardiovascular and Pulmonary Rehabilitation. Guidelines for Cardiac Rehabilitation and Secondary Prevention Programs. 5th ed. Champaign, IL: Human Kinetics; 2013.
14. Silberman A, Banthia R, Estay IS, et al. The effectiveness and efficacy of an intensive cardiac rehabilitation program in 24 sites. Am J Health Promot. 2010;24(4):260-266. doi:10.4278/ajhp.24.4.arb
15. Ornish D, Scherwitz LW, Doody RS, et al. Effects of stress management training and dietary changes in treating ischemic heart disease. JAMA. 1983;249(1):54-59.
16. Ornish D, Brown SE, Scherwitz LW, et al. Can lifestyle changes reverse coronary heart disease? The Lifestyle Heart Trial. Lancet. 1990;336(8708):129-133. doi:10.1016/0140-6736(90)91656-u.
17. Ornish D, Scherwitz LW, Billings JH, et al. Intensive lifestyle changes for reversal of coronary heart disease [published correction appears in JAMA 1999 Apr 21;281(15):1380]. JAMA. 1998;280(23):2001-2007. doi:10.1001/jama.280.23.2001
18. Frattaroli J, Weidner G, Merritt-Worden TA, Frenda S, Ornish D. Angina pectoris and atherosclerotic risk factors in the multisite cardiac lifestyle intervention program. Am J Cardiol. 2008;101(7):911-918. doi:10.1016/j.amjcard.2007.11.039
19. Koertge J, Weidner G, Elliott-Eller M, et al. Improvement in medical risk factors and quality of life in women and men with coronary artery disease in the Multicenter Lifestyle Demonstration Project. Am J Cardiol. 2003;91(11):1316-1322. doi:10.1016/s0002-9149(03)00320-5
20. Marshall DA, Walizer EM, Vernalis MN. Achievement of heart health characteristics through participation in an intensive lifestyle change program (Coronary Artery Disease Reversal Study). J Cardiopulm Rehabil Prev. 2009;29(2):84-96. doi:10.1097/HCR.0b013e31819a00b2
21. Marshall D, Elaine W, Vernalis M. The effect of a one-year lifestyle intervention program on carotid intima media thickness. Mil Med. 2011;176(7):798-804. doi:10.7205/milmed-d-10-00447
22. Esselstyn CB Jr, Ellis SG, Medendorp SV, Crowe TD. A strategy to arrest and reverse coronary artery disease: a 5-year longitudinal study of a single physician’s practice. J Fam Pract. 1995;41(6):560-568.
23. Esselstyn CB Jr. Updating a 12-year experience with arrest and reversal therapy for coronary heart disease (an overdue requiem for palliative cardiology). Am J Cardiol. 1999;84(3):339-A8. doi:10.1016/s0002-9149(99)00290-8
24. Barnard ND, Cohen J, Jenkins DJ, et al. A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes Care. 2006;29(8):1777-1783. doi:10.2337/dc06-0606
25. McDougall J, Thomas LE, McDougall C, et al. Effects of 7 days on an ad libitum low-fat vegan diet: the McDougall Program cohort [published correction appears in Nutr J. 2017 Feb 10;16(1):12]. Nutr J. 2014;13:99. Published 2014 Oct 14. doi:10.1186/1475-2891-13-99
26. Turner-McGrievy GM, Davidson CR, Wingard EE, Wilcox S, Frongillo EA. Comparative effectiveness of plant-based diets for weight loss: a randomized controlled trial of five different diets. Nutrition. 2015;31(2):350-358. doi:10.1016/j.nut.2014.09.002
27. Wright N, Wilson L, Smith M, Duncan B, McHugh P. The BROAD study: a randomised controlled trial using a whole food plant-based diet in the community for obesity, ischaemic heart disease or diabetes. Nutr Diabetes. 2017;7(3):e256. Published 2017 Mar 20. doi:10.1038/nutd.2017.3

28. Schaefer S, Hussein H, Gershony GR, Rutledge JC, Kappagoda CT. Regression of severe atherosclerotic plaque in patients with mild elevation of LDL cholesterol. J Investig Med. 1997;45(9):536-541.
29. Kitazono R. Know thy patient: Enhancing lifestyle interventions with psychological assessment. Int J Dis Rev Prev. 2020;2(1):76-81.
30. Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3rd ed. New York, NY: Guilford Press; 2013.
31. Mascola AJ, Yiaslas TA, Meir RL, et al. Framing physical activity as a distinct and uniquely valuable behavior independent of weight management: A pilot randomized controlled trial for overweight and obese sedentary persons. Eat Weight Disord. 2009;14(2-3):e148-e152. doi:10.1007/BF03327814
32. Esselstyn AC, Esselstyn J. The Prevent and Reverse Heart Disease Cookbook: Over 125 Delicious, Life-Changing, Plant-Based Recipes. New York, NY: Avery; 2014.
33. Esselstyn CB Jr, Gendy G, Doyle J, Golubic M, Roizen MF. A way to reverse CAD? J Fam Pract. 2014;63(7):356-364.
34. Ornish D, Ornish A. Undo It! How Simple Lifestyle Changes Can Reverse Most Chronic Diseases. New York, NY: Ballantine Books; 2019.
35. Smith SC Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association [published correction appears in J Am Coll Cardiol. 2015 Apr 14;65(14):1495. Dosage error in article text.]. J Am Coll Cardiol. 2011;58(23):2432-2446. doi:10.1016/j.jacc.2011.10.824
36. Blumenthal JA, Babyak M, Wei J, et al. Usefulness of psychosocial treatment of mental stress-induced myocardial ischemia in men. Am J Cardiol. 2002;89(2):164-168. doi:10.1016/s0002-9149(01)02194-4
37. Antoni MH. Stress management effects on psychological, endocrinological, and immune functioning in men with HIV infection: empirical support for a psychoneuroimmunological model. Stress. 2003;6(3):173-188. doi:10.1080/1025389031000156727
38. Penedo FJ, Molton I, Dahn JR, et al. A randomized clinical trial of group-based cognitive-behavioral stress management in localized prostate cancer: development of stress management skills improves quality of life and benefit finding. Ann Behav Med. 2006;31(3):261-270. doi:10.1207/s15324796abm3103_8
39. Yiaslas TA. “Look doctor, I’m a carnivore.” Int J Dis Rev Prev. 2020;2(2):35-39.
40. Khaylis A, Yiaslas T, Bergstrom J, Gore-Felton C. A review of efficacious technology-based weight-loss interventions: five key components. Telemed J E Health. 2010;16(9):931-938. doi:10.1089/tmj.2010.0065
41. Barnard ND, Sherwitz L, Ornish D. Adherence and acceptability of a low-fat, vegetarian diet among patients with cardiac disease. J Cardiopulm Rehabil. 1992;12(6):423-431.
42. Yiaslas TA, Taylor J, Embree J, Schaefer S. Elimination of angina, comprehensive cardio-metabolic risk reduction, and 50-pound weight loss in a US Navy veteran with myasthenia gravis. Int J Dis Rev Prev. 2019;1(1):77-83.
43. Berkow SE, Barnard N, Eckart J, Katcher H. Four therapeutic diets: adherence and acceptability. Can J Diet Pract Res. 2010;71(4):199-204. doi:10.3148/71.4.2010.199
44. Carpenter JK, Andrews LA, Witcraft SM, Powers MB, Smits JAJ, Hofmann SG. Cognitive behavioral therapy for anxiety and related disorders: A meta-analysis of randomized placebo-controlled trials. Depress Anxiety. 2018;35(6):502-514. doi:10.1002/da.22728
45. Yiaslas TA. The pursuit of arete in medicine and health care. Int J Dis Rev Prev. 2019;1(2):53-56.
1. Benjamin EJ, Virani SS, Callaway CW, et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association [published correction appears in Circulation. 2018 Mar 20;137(12 ): e493]. Circulation. 2018;137(12):e67-e492. doi:10.1161/CIR.0000000000000558
2. Hinojosa R. Cardiovascular disease among United States military veterans: evidence of a waning healthy soldier effect using the National Health Interview Survey. Chronic Illn. 2020;16(1):55-68. doi:10.1177/1742395318785237.
3. Hinojosa R. Sex, age, race/ethnicity, veteran status, and the likelihood of reporting cardiovascular conditions in the National Health Interview Survey. J Cardiovasc Nurs. 2019;34(3):215-221. doi:10.1097/JCN.0000000000000561 4. Assari S. Veterans and risk of heart disease in the United States: a cohort with 20 years of follow up. Int J Prev Med. 2014;5(6):703-709.
5. Thomas MM, Harpaz-Rotem I, Tsai J, Southwick SM, Pietrzak RH. Mental and physical health conditions in US combat veterans: results from the National Health and Resilience in Veterans Study. Prim Care Companion CNS Disord. 2017;19(3):10.4088/PCC.17m02118. Published 2017 Jun 22. doi:10.4088/PCC.17m02118
6. Bukhbinder AS, Wang AC, Qureshi SU, et al. Increased vascular pathology in older veterans with a purple heart commendation or chronic post-traumatic stress disorder. J Geriatr Psychiatry Neurol. 2020;33(4):195-206. doi:10.1177/0891988719868308
7. Edmondson D, von Känel R. Post-traumatic stress disorder and cardiovascular disease. Lancet Psychiatry. 2017;4(4):320-329. doi:10.1016/S2215-0366(16)30377-7
8. Forman DE, Maurer MS, Boyd C, et a;. Multimorbidity in older adults with cardiovascular disease. J Am Coll Cardiol. 2018;71(19):2149-2161. doi:10.1016/j.jacc.2018.03.022
9. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
10. Maddox TM, Plomondon ME, Petrich M, et al. A national clinical quality program for Veterans Affairs catheterization laboratories (from the Veterans Affairs clinical assessment, reporting, and tracking program). Am J Cardiol. 2014;114(11):1750-1757. doi:10.1016/j.amjcard.2014.08.045
11. Centers for Disease Control and Prevention. Coronavirus 2019 (COVID-19):people at increased risk and other people who need to take extra precautions. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/index.html. Updated September 11, 2020. Accessed November 12, 2020.
12. International Food Information Council. 2020 food and health survey. https://foodinsight.org/2020-food-and-health-survey. Updated June 9, 2020. Accessed November 12, 2020.
13. American Association of Cardiovascular and Pulmonary Rehabilitation. Guidelines for Cardiac Rehabilitation and Secondary Prevention Programs. 5th ed. Champaign, IL: Human Kinetics; 2013.
14. Silberman A, Banthia R, Estay IS, et al. The effectiveness and efficacy of an intensive cardiac rehabilitation program in 24 sites. Am J Health Promot. 2010;24(4):260-266. doi:10.4278/ajhp.24.4.arb
15. Ornish D, Scherwitz LW, Doody RS, et al. Effects of stress management training and dietary changes in treating ischemic heart disease. JAMA. 1983;249(1):54-59.
16. Ornish D, Brown SE, Scherwitz LW, et al. Can lifestyle changes reverse coronary heart disease? The Lifestyle Heart Trial. Lancet. 1990;336(8708):129-133. doi:10.1016/0140-6736(90)91656-u.
17. Ornish D, Scherwitz LW, Billings JH, et al. Intensive lifestyle changes for reversal of coronary heart disease [published correction appears in JAMA 1999 Apr 21;281(15):1380]. JAMA. 1998;280(23):2001-2007. doi:10.1001/jama.280.23.2001
18. Frattaroli J, Weidner G, Merritt-Worden TA, Frenda S, Ornish D. Angina pectoris and atherosclerotic risk factors in the multisite cardiac lifestyle intervention program. Am J Cardiol. 2008;101(7):911-918. doi:10.1016/j.amjcard.2007.11.039
19. Koertge J, Weidner G, Elliott-Eller M, et al. Improvement in medical risk factors and quality of life in women and men with coronary artery disease in the Multicenter Lifestyle Demonstration Project. Am J Cardiol. 2003;91(11):1316-1322. doi:10.1016/s0002-9149(03)00320-5
20. Marshall DA, Walizer EM, Vernalis MN. Achievement of heart health characteristics through participation in an intensive lifestyle change program (Coronary Artery Disease Reversal Study). J Cardiopulm Rehabil Prev. 2009;29(2):84-96. doi:10.1097/HCR.0b013e31819a00b2
21. Marshall D, Elaine W, Vernalis M. The effect of a one-year lifestyle intervention program on carotid intima media thickness. Mil Med. 2011;176(7):798-804. doi:10.7205/milmed-d-10-00447
22. Esselstyn CB Jr, Ellis SG, Medendorp SV, Crowe TD. A strategy to arrest and reverse coronary artery disease: a 5-year longitudinal study of a single physician’s practice. J Fam Pract. 1995;41(6):560-568.
23. Esselstyn CB Jr. Updating a 12-year experience with arrest and reversal therapy for coronary heart disease (an overdue requiem for palliative cardiology). Am J Cardiol. 1999;84(3):339-A8. doi:10.1016/s0002-9149(99)00290-8
24. Barnard ND, Cohen J, Jenkins DJ, et al. A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes Care. 2006;29(8):1777-1783. doi:10.2337/dc06-0606
25. McDougall J, Thomas LE, McDougall C, et al. Effects of 7 days on an ad libitum low-fat vegan diet: the McDougall Program cohort [published correction appears in Nutr J. 2017 Feb 10;16(1):12]. Nutr J. 2014;13:99. Published 2014 Oct 14. doi:10.1186/1475-2891-13-99
26. Turner-McGrievy GM, Davidson CR, Wingard EE, Wilcox S, Frongillo EA. Comparative effectiveness of plant-based diets for weight loss: a randomized controlled trial of five different diets. Nutrition. 2015;31(2):350-358. doi:10.1016/j.nut.2014.09.002
27. Wright N, Wilson L, Smith M, Duncan B, McHugh P. The BROAD study: a randomised controlled trial using a whole food plant-based diet in the community for obesity, ischaemic heart disease or diabetes. Nutr Diabetes. 2017;7(3):e256. Published 2017 Mar 20. doi:10.1038/nutd.2017.3

28. Schaefer S, Hussein H, Gershony GR, Rutledge JC, Kappagoda CT. Regression of severe atherosclerotic plaque in patients with mild elevation of LDL cholesterol. J Investig Med. 1997;45(9):536-541.
29. Kitazono R. Know thy patient: Enhancing lifestyle interventions with psychological assessment. Int J Dis Rev Prev. 2020;2(1):76-81.
30. Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3rd ed. New York, NY: Guilford Press; 2013.
31. Mascola AJ, Yiaslas TA, Meir RL, et al. Framing physical activity as a distinct and uniquely valuable behavior independent of weight management: A pilot randomized controlled trial for overweight and obese sedentary persons. Eat Weight Disord. 2009;14(2-3):e148-e152. doi:10.1007/BF03327814
32. Esselstyn AC, Esselstyn J. The Prevent and Reverse Heart Disease Cookbook: Over 125 Delicious, Life-Changing, Plant-Based Recipes. New York, NY: Avery; 2014.
33. Esselstyn CB Jr, Gendy G, Doyle J, Golubic M, Roizen MF. A way to reverse CAD? J Fam Pract. 2014;63(7):356-364.
34. Ornish D, Ornish A. Undo It! How Simple Lifestyle Changes Can Reverse Most Chronic Diseases. New York, NY: Ballantine Books; 2019.
35. Smith SC Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association [published correction appears in J Am Coll Cardiol. 2015 Apr 14;65(14):1495. Dosage error in article text.]. J Am Coll Cardiol. 2011;58(23):2432-2446. doi:10.1016/j.jacc.2011.10.824
36. Blumenthal JA, Babyak M, Wei J, et al. Usefulness of psychosocial treatment of mental stress-induced myocardial ischemia in men. Am J Cardiol. 2002;89(2):164-168. doi:10.1016/s0002-9149(01)02194-4
37. Antoni MH. Stress management effects on psychological, endocrinological, and immune functioning in men with HIV infection: empirical support for a psychoneuroimmunological model. Stress. 2003;6(3):173-188. doi:10.1080/1025389031000156727
38. Penedo FJ, Molton I, Dahn JR, et al. A randomized clinical trial of group-based cognitive-behavioral stress management in localized prostate cancer: development of stress management skills improves quality of life and benefit finding. Ann Behav Med. 2006;31(3):261-270. doi:10.1207/s15324796abm3103_8
39. Yiaslas TA. “Look doctor, I’m a carnivore.” Int J Dis Rev Prev. 2020;2(2):35-39.
40. Khaylis A, Yiaslas T, Bergstrom J, Gore-Felton C. A review of efficacious technology-based weight-loss interventions: five key components. Telemed J E Health. 2010;16(9):931-938. doi:10.1089/tmj.2010.0065
41. Barnard ND, Sherwitz L, Ornish D. Adherence and acceptability of a low-fat, vegetarian diet among patients with cardiac disease. J Cardiopulm Rehabil. 1992;12(6):423-431.
42. Yiaslas TA, Taylor J, Embree J, Schaefer S. Elimination of angina, comprehensive cardio-metabolic risk reduction, and 50-pound weight loss in a US Navy veteran with myasthenia gravis. Int J Dis Rev Prev. 2019;1(1):77-83.
43. Berkow SE, Barnard N, Eckart J, Katcher H. Four therapeutic diets: adherence and acceptability. Can J Diet Pract Res. 2010;71(4):199-204. doi:10.3148/71.4.2010.199
44. Carpenter JK, Andrews LA, Witcraft SM, Powers MB, Smits JAJ, Hofmann SG. Cognitive behavioral therapy for anxiety and related disorders: A meta-analysis of randomized placebo-controlled trials. Depress Anxiety. 2018;35(6):502-514. doi:10.1002/da.22728
45. Yiaslas TA. The pursuit of arete in medicine and health care. Int J Dis Rev Prev. 2019;1(2):53-56.
An Interdisciplinary Approach to Educating Medical Students About Dementia Assessment and Treatment Planning
The global burden of dementia is increasing at an alarming pace and is estimated to soon affect 81 million individuals worldwide.1 The World Health Organization and the Institute of Medicine have recommended greater dementia awareness and education.2,3 Despite this emphasis on dementia education, many general practitioners consider dementia care beyond their clinical domain and feel that specialists, such as geriatricians, geriatric psychiatrists, or neurologists should address dementia assessment and treatment. 4 Unfortunately, the geriatric health care workforce has been shrinking. The American Geriatrics Society estimates the need for 30,000 geriatricians by 2030, although there are only 7,300 board-certified geriatricians currently in the US.5 There is an urgent need for educating all medical trainees in dementia care regardless of their specialization interest. As the largest underwriter of graduate medical education in the US, the US Department of Veterans Affairs (VA) is well placed for rolling out focused dementia education. Training needs to be practical, brief, and responsive to knowledge gaps to reach the most trainees.
Despite growing emphasis on geriatric training, many medical students have limited experience with patients with dementia or their caregivers, lack exposure to interdisciplinary teams, have a poor attitude toward geriatric patients, and display specific knowledge gaps in dementia assessment and management. 6-9 Other knowledge gaps noted in medical students included assessing behavioral problems, function, safety, and caregiver burden. Medical students also had limited exposure to interdisciplinary team dementia assessment and management.
Our goal was to develop a multicomponent, experiential, brief curriculum using team-based learning to expose senior medical students to interdisciplinary assessment of dementia. The curriculum was developed with input from the interdisciplinary team to address dementia knowledge gaps while providing an opportunity to interact with caregivers. The curriculum targeted all medical students regardless of their interest in geriatrics. Particular emphasis was placed on systems-based learning and the importance of teamwork in managing complex conditions such as dementia. Students were taught that incorporating interdisciplinary input would be more effective during dementia care planning rather than developing specialized knowledge.
Methods
Our team developed a curriculum for fourthyear medical students who rotated through the VA Memory Disorders Clinic as a part of their geriatric medicine clerkship at the University of Arkansas for Medical Sciences in Little Rock. The Memory Disorders Clinic is a consultation practice at the Central Arkansas Veterans Healthcare System (CAVHS) where patients with memory problems are evaluated by a team consisting of a geriatric psychiatrist, a geriatrician, a social worker, and a neuropsychologist. Each specialist addresses specific areas of dementia assessment and management. The curriculum included didactics, clinical experience, and team-based learning.
Didactics
An hour-long didactic session lead by the team geriatrician provided a general overview of interdisciplinary assessment of dementia to groups of 2 to 3 students at a time. The geriatrician presented an overview of dementia types, comorbidities, medications that affect memory, details of the physical examination, and laboratory, cognitive, and behavioral assessments along with treatment plan development. Students also learned about the roles of the social worker, geriatrician, neuropsychologist, and geriatric psychiatrist in the clinic. Pictographs and pie charts highlighted the role of disciplines in assessing and managing aspects of dementia.
The social work evaluation included advance care planning, functional assessment, safety assessment (driving, guns, wandering behaviors, etc), home safety evaluation, support system, and financial evaluation. Each medical student received a binder with local resources to become familiar with the depth and breadth of agencies involved in dementia care. Each medical student learned how to administer the Zarit Burden Scale to assess caregiver burden.10 The details of the geriatrician assessment included reviewing medical comorbidities and medications contributing to dementia, a physical examination, including a focused neurologic examination, laboratory assessment, and judicious use of neuroimaging.
The neuropsychology assessment education included a battery of tests and assessments. The global screening instruments included the Modified Mini-Mental State examination (3MS), Montreal Cognitive Assessment (MoCA), and Saint Louis University Mental Status examination (SLUMS).11-13 Executive function is evaluated using the Trails Making Test A and Trails Making Test B, Controlled Oral Word Association Test, Semantic Fluency Test, and Repeatable Battery for the Assessment of Neuropsychological Status test. Cognitive tests were compared and age- , education-, and race-adjusted norms for rating scales were listed if available. Each student was expected to show proficiency in ≥ 2 cognitive screening instruments (3MS, MoCA, or SLUMS). The geriatric psychiatry assessment included clinical history, onset, and course of memory problems from patient and caregiver perspectives, the Neuropsychiatric Inventory for assessing behavioral problems, employing the clinical dementia rating scale, integrating the team data, summarizing assessment, and formulating a treatment plan.14
Clinical
Students had a single clinical exposure. Students followed 1 patient and his or her caregiver through the team assessment and observed each provider’s assessment to learn interview techniques to adapt to the patient’s sensory or cognitive impairment and become familiar with different tools and devices used in the dementia clinic, such as hearing amplifiers. Each specialist provided hands-on experience. This encounter helped the students connect with caregivers and appreciate their role in patient care.
Systems learning was an important component integrated throughout the clinical experience. Examples include using video teleconferences to communicate findings among team members and electronic health records to seamlessly obtain and integrate data. Students learned how to create worksheets to graph laboratory data such as B12, thyroid-stimulating hormone, and rapid plasma regain levels. Student gained experience in using applications to retrieve neuroimaging data, results of sleep studies, and other data. Many patients had not received the results of their sleep study, and students had the responsibility to share these reports, including the number of apneic episodes. Students used the VA Computerized Patient Record System for reviewing patient records. One particularly useful tool was Joint Legacy Viewer, a remote access tool used to retrieve data on veterans from anywhere within the US. Students were also trained on medication and consult order menus in the system.
Team-Based
Learning The objectives of the team-based learning section were to teach students basic concepts of integrating the interdisciplinary assessment and formulating a treatment plan, to provide an opportunity to present their case in a group format, to discuss the differential diagnosis, management and treatment plan with a geriatrician in the team-based learning format, and to answer questions from other students. The instructors developed a set of prepared take-home points (Table 1). The team-based learning sessions were structured so that all take-home points were covered.
Evaluations
Evaluations were performed before and immediately after the clinical experience. In preevaluation, students reported the frequency of their participation in an interdisciplinary team assessment of any condition and specifically for dementia. In pre- and postevaluation, students rated their perception of the role of interdisciplinary team members in assessing and managing dementia, their personal abilities to assess cognition, behavioral problems, caregiver burden, and their perception of the impact of behavioral problems on dementia care. A Likert scale (poor = 1; fair = 2; good = 3; very good = 4; and excellent = 5) was employed (eApendices 1 and 2 can be found at doi:10.12788/fp.0052). The only demographic information collected was the student’s gender. Semistructured interviews were conducted to assess students’ current knowledge, experience, and needs. These interviews lasted about 20 minutes and collected information regarding the students’ knowledge about cognitive and behavioral problems in general and those occurring in dementia, their experience with screening, and any problems they encountered.
Statistical Analysis
Student baseline characteristics were assessed. Pre- and postassessments were analyzed with the McNemar test for paired data, and associations with experience were evaluated using χ2 tests. Ratings were dichotomized as very good/excellent vs poor/fair/ good because our educational goal was “very good” to “excellent” experience in dementia care and to avoid expected small cell counts. Two-sided P < .05 indicated statistical significance. Data were analyzed using SAS Enterprise Guide v5.1.
Results
One hundred fourth-year medical students participated, including 54 women. Thirtysix percent reported they had not previously attended an interdisciplinary team assessment for dementia, while 18% stated that they had attended only 1 interdisciplinary team assessment for dementia.
Before the education, students rated their dementia ability as poor. Only 2% (1 of 54), of those with 0 to 1 assessment experience rated their ability for assessing dementia with an interdisciplinary team format as very good/excellent compared with 20% (9/46) of those previously attending ≥ 2 assessments (P = .03); other ratings of ability were not associated with prior experience.
There was a significant change in the students’ self-efficacy ratings pre- to postassessment (P < .05) (Table 2). Only 10% rated their ability to assess for dementia as very good/excellent in before the intervention compared with 96% in postassessment (P < .01). Students’ perception of the impact of behavioral problems on dementia care improved significantly (45% to 98%, P < .01). Similarly, student’s perception of their ability to assess behavioral problems, caregiver burden, and cognition improved significantly from 7 to 88%; 7 to 78%, and 18 to 92%, respectively (P < .01). Students perception of the role of social worker, neuropsychologist, geriatrician, and geriatric psychiatrist also improved significantly for most measures from 81 to 98% (P = .02), 87 to 98% (P = .05), 94 to 99% (P = .06), and 88 to 100% (P = .01), respectively.
The semistructured interviews revealed that awareness of behavioral problems associated with dementia varied for different behavioral problems. Although many students showed familiarity with depression, agitation, and psychosis, they were not comfortable assessing them in a patient with dementia. These students were less aware of other behavioral problems such as disinhibition, apathy, and movement disorders. Deficits were noted in the skill of administering commonly used global cognitive screens, such as the Mini-Mental State Examination (MMSE).15
In semistructured interviews, only 7% of senior medical students were comfortable assessing behavioral problems associated with dementia. Most were not aware of any validated rating scale to assess neuropsychiatric symptoms. Similarly, only 7% of students were comfortable assessing caregiver burden, and most were not aware of any validated rating scale to assess caregiver burden. Only 1 in 5 students were comfortable using 2 cognitive screens to assess cognitive deficits. Many students stated that they were not routinely expected to perform common cognitive screens, such as the MMSE during their medical training except students who had expressed an interest in psychiatry and were expected to be proficient in the MMSE. Most students were making common mistakes, such as converting the 3-command task to 3 individual single commands, helping too much with serial 7s, and giving too much positive feedback throughout the test.
Discussion
Significant knowledge gaps regarding dementia were found in our study, which is in keeping with other studies in the area. Dementia knowledge deficits among medical trainees have been identified in the United Kingdom, Australia, and the US.6-9
In our study, a brief multicomponent experiential curriculum improved senior medical students’ perception and self-efficacy in diagnosing dementia. This is in keeping with other studies, such as the PAIRS Program.7 Findings from another study indicated that education for geriatric- oriented physicians should focus on experiential learning components through observation and interaction with older adults.16
A background of direct experience with older adults is associated with more positive attitudes toward older adults and increased interest in geriatric medicine.16 In our study, the exposure was brief; therefore, the results could not be compared with other long-term exposure studies. However, even with this brief intervention most students reported being comfortable with assessing caregiver burden (78%), behavioral problems of dementia (88%), and using ≥ 2 cognitive screens (92%). Comfortable in dementia assessment increased after the intervention from 10% to 96%. This finding is encouraging because brief multicomponent dementia education can be devised easily. This finding needs to be taken with caution because we did not conduct a formal skills evaluation.
A unique component of our experience was to learn medical students’ perception about the impact of neuropsychiatric symptoms on the trajectory, outcomes, and management of dementia. These symptoms included delusions, hallucinations, agitation, depression, anxiety, euphoria, apathy, disinhibition, irritability, motor disturbance, nighttime behaviors, and appetite and eating. Less than half the students thought that neuropsychiatric symptoms had a significant impact on dementia before the experience. Through didactics, systematic assessment of neuropsychiatric symptoms and interaction with caregivers, > 98% of students learned that these symptoms have a significant impact on dementia management.
This experience also emphasized the role of several disciplines in dementia assessment and management. Students’ experience positively influenced appreciation of the role of the memory clinic team. Our hope is that students will seek input from social workers, neuropsychologists, and other team members when working with patients with dementia or their caregivers. The common reason why primary care physicians focus on an exclusive medical model is the time commitment for communicating with an interdisciplinary team. Students experienced the feasibility of the interdisciplinary team involvement and how technology could be used for synchronous and asynchronous communication among team members. Medical students also were introduced to complex billing codes used when ≥ 3 disciplines assess/manage a geriatric patient.
Limitations
This study is limited by the lack of long-term follow-up evaluations, no metrics for practice changes clinical outcomes, and implementation in a single medical school. The postexperience evaluation in this study was performed immediately after the intervention. Long-term follow-up would inform whether the changes noted are durable. Because of the brief nature of our intervention, we do not believe that it would change practice in clinical care. It will be informative to follow this cohort of students to study whether their clinical approach to dementia care changes. The intervention needs to be replicated in other medical schools and in more heterogeneous groups to generalize the results of the study.
Conclusions
Senior medical students are not routinely exposed to interdisciplinary team assessments. Dementia knowledge gaps were prevalent in this cohort of senior medical students. Providing interdisciplinary geriatric educational experience improved their perception of their ability to assess for dementia and their recognition of the roles of interdisciplinary team members. Plans are in place to continue and expand the program to other complex geriatric syndromes.
Acknowledgments
Poster presented at the 2019 annual meeting of the American Geriatrics Society. Oral presentation at the same meeting as part of the select Geriatric Education Methods and Materials Swap workshop.
1. Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366(9503):2112-2117. doi:10.1016/S0140-6736(05)67889-0
2. Janca A, Aarli JA, Prilipko L, Dua T, Saxena S, Saraceno B. WHO/WFN survey of neurological services: a worldwide perspective. J Neurol Sci. 2006;247(1):29-34. doi:10.1016/j.jns.2006.03.003
3. Wilkins KM, Blazek MC, Brooks WB, Lehmann SW, Popeo D, Wagenaar D. Six things all medical students need to know about geriatric psychiatry (and how to teach them). Acad Psychiatry. 2017;41(5):693-700. doi:10.1007/s40596-017-0691-7
4. Turner S, Iliffe S, Downs M, et al. General practitioners’ knowledge, confidence and attitudes in the diagnosis and management of dementia. Age Ageing. 2004;33(5):461- 467. doi:10.1093/ageing/afh140
5. Lester PE, Dharmarajan TS, Weinstein E. The looming geriatrician shortage: ramifications and solutions. J Aging Health. 2019:898264319879325. doi:10.1177/0898264319879325
6. Struck BD, Bernard MA, Teasdale TA; Oklahoma University Geriatric Education G. Effect of a mandatory geriatric medicine clerkship on third-year students. J Am Geriatr Soc. 2005;53(11):2007-2011. doi:10.1111/j.1532-5415.2005.00473.x
7. Jefferson AL, Cantwell NG, Byerly LK, Morhardt D. Medical student education program in Alzheimer’s disease: the PAIRS Program. BMC Med Educ. 2012;12:80. doi:10.1186/1472-6920-12-80
8. Nagle BJ, Usita PM, Edland SD. United States medical students’ knowledge of Alzheimer disease. J Educ Eval Health Prof. 2013;10:4. doi:10.3352/jeehp.2013.10.4
9. Scott TL, Kugelman M, Tulloch K. How medical professional students view older people with dementia: Implications for education and practice. PLoS One. 2019;14(11):e0225329. doi:10.1371/journal.pone.0225329.
10. Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20(6):649-655. doi:10.1093/geront/20.6.649
11. McDowell I, Kristjansson B, Hill GB, Hebert R. Community screening for dementia: the Mini Mental State Exam (MMSE) and Modified Mini-Mental State Exam (3MS) compared. J Clin Epidemiol. 1997;50(4):377-383. doi:10.1016/s0895-4356(97)00060-7
12. Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Ger iatr Soc. 2005;53(4):695-699. doi:10.1111/j.1532-5415.2005.53221.x
13. Tariq SH, Tumosa N, Chibnall JT, Perry MH, 3rd, Morley JE. Comparison of the Saint Louis University mental status examination and the Mini-Mental State Examination for detecting dementia and mild neurocognitive disorder--a pilot study. Am J Geriatr Psychiatry. 2006;14(11):900-910. doi:10.1097/01.JGP.0000221510.33817.86
14. Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308-2314. doi:10.1212/wnl.44.12.2308
15. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. doi:10.1016/0022-3956(75)90026-6
16. Fitzgerald JT, Wray LA, Halter JB, Williams BC, Supiano MA. Relating medical students’ knowledge, attitudes, and experience to an interest in geriatric medicine. Gerontologist. 2003;43(6):849-855. doi:10.1093/geront/43.6.849
The global burden of dementia is increasing at an alarming pace and is estimated to soon affect 81 million individuals worldwide.1 The World Health Organization and the Institute of Medicine have recommended greater dementia awareness and education.2,3 Despite this emphasis on dementia education, many general practitioners consider dementia care beyond their clinical domain and feel that specialists, such as geriatricians, geriatric psychiatrists, or neurologists should address dementia assessment and treatment. 4 Unfortunately, the geriatric health care workforce has been shrinking. The American Geriatrics Society estimates the need for 30,000 geriatricians by 2030, although there are only 7,300 board-certified geriatricians currently in the US.5 There is an urgent need for educating all medical trainees in dementia care regardless of their specialization interest. As the largest underwriter of graduate medical education in the US, the US Department of Veterans Affairs (VA) is well placed for rolling out focused dementia education. Training needs to be practical, brief, and responsive to knowledge gaps to reach the most trainees.
Despite growing emphasis on geriatric training, many medical students have limited experience with patients with dementia or their caregivers, lack exposure to interdisciplinary teams, have a poor attitude toward geriatric patients, and display specific knowledge gaps in dementia assessment and management. 6-9 Other knowledge gaps noted in medical students included assessing behavioral problems, function, safety, and caregiver burden. Medical students also had limited exposure to interdisciplinary team dementia assessment and management.
Our goal was to develop a multicomponent, experiential, brief curriculum using team-based learning to expose senior medical students to interdisciplinary assessment of dementia. The curriculum was developed with input from the interdisciplinary team to address dementia knowledge gaps while providing an opportunity to interact with caregivers. The curriculum targeted all medical students regardless of their interest in geriatrics. Particular emphasis was placed on systems-based learning and the importance of teamwork in managing complex conditions such as dementia. Students were taught that incorporating interdisciplinary input would be more effective during dementia care planning rather than developing specialized knowledge.
Methods
Our team developed a curriculum for fourthyear medical students who rotated through the VA Memory Disorders Clinic as a part of their geriatric medicine clerkship at the University of Arkansas for Medical Sciences in Little Rock. The Memory Disorders Clinic is a consultation practice at the Central Arkansas Veterans Healthcare System (CAVHS) where patients with memory problems are evaluated by a team consisting of a geriatric psychiatrist, a geriatrician, a social worker, and a neuropsychologist. Each specialist addresses specific areas of dementia assessment and management. The curriculum included didactics, clinical experience, and team-based learning.
Didactics
An hour-long didactic session lead by the team geriatrician provided a general overview of interdisciplinary assessment of dementia to groups of 2 to 3 students at a time. The geriatrician presented an overview of dementia types, comorbidities, medications that affect memory, details of the physical examination, and laboratory, cognitive, and behavioral assessments along with treatment plan development. Students also learned about the roles of the social worker, geriatrician, neuropsychologist, and geriatric psychiatrist in the clinic. Pictographs and pie charts highlighted the role of disciplines in assessing and managing aspects of dementia.
The social work evaluation included advance care planning, functional assessment, safety assessment (driving, guns, wandering behaviors, etc), home safety evaluation, support system, and financial evaluation. Each medical student received a binder with local resources to become familiar with the depth and breadth of agencies involved in dementia care. Each medical student learned how to administer the Zarit Burden Scale to assess caregiver burden.10 The details of the geriatrician assessment included reviewing medical comorbidities and medications contributing to dementia, a physical examination, including a focused neurologic examination, laboratory assessment, and judicious use of neuroimaging.
The neuropsychology assessment education included a battery of tests and assessments. The global screening instruments included the Modified Mini-Mental State examination (3MS), Montreal Cognitive Assessment (MoCA), and Saint Louis University Mental Status examination (SLUMS).11-13 Executive function is evaluated using the Trails Making Test A and Trails Making Test B, Controlled Oral Word Association Test, Semantic Fluency Test, and Repeatable Battery for the Assessment of Neuropsychological Status test. Cognitive tests were compared and age- , education-, and race-adjusted norms for rating scales were listed if available. Each student was expected to show proficiency in ≥ 2 cognitive screening instruments (3MS, MoCA, or SLUMS). The geriatric psychiatry assessment included clinical history, onset, and course of memory problems from patient and caregiver perspectives, the Neuropsychiatric Inventory for assessing behavioral problems, employing the clinical dementia rating scale, integrating the team data, summarizing assessment, and formulating a treatment plan.14
Clinical
Students had a single clinical exposure. Students followed 1 patient and his or her caregiver through the team assessment and observed each provider’s assessment to learn interview techniques to adapt to the patient’s sensory or cognitive impairment and become familiar with different tools and devices used in the dementia clinic, such as hearing amplifiers. Each specialist provided hands-on experience. This encounter helped the students connect with caregivers and appreciate their role in patient care.
Systems learning was an important component integrated throughout the clinical experience. Examples include using video teleconferences to communicate findings among team members and electronic health records to seamlessly obtain and integrate data. Students learned how to create worksheets to graph laboratory data such as B12, thyroid-stimulating hormone, and rapid plasma regain levels. Student gained experience in using applications to retrieve neuroimaging data, results of sleep studies, and other data. Many patients had not received the results of their sleep study, and students had the responsibility to share these reports, including the number of apneic episodes. Students used the VA Computerized Patient Record System for reviewing patient records. One particularly useful tool was Joint Legacy Viewer, a remote access tool used to retrieve data on veterans from anywhere within the US. Students were also trained on medication and consult order menus in the system.
Team-Based
Learning The objectives of the team-based learning section were to teach students basic concepts of integrating the interdisciplinary assessment and formulating a treatment plan, to provide an opportunity to present their case in a group format, to discuss the differential diagnosis, management and treatment plan with a geriatrician in the team-based learning format, and to answer questions from other students. The instructors developed a set of prepared take-home points (Table 1). The team-based learning sessions were structured so that all take-home points were covered.
Evaluations
Evaluations were performed before and immediately after the clinical experience. In preevaluation, students reported the frequency of their participation in an interdisciplinary team assessment of any condition and specifically for dementia. In pre- and postevaluation, students rated their perception of the role of interdisciplinary team members in assessing and managing dementia, their personal abilities to assess cognition, behavioral problems, caregiver burden, and their perception of the impact of behavioral problems on dementia care. A Likert scale (poor = 1; fair = 2; good = 3; very good = 4; and excellent = 5) was employed (eApendices 1 and 2 can be found at doi:10.12788/fp.0052). The only demographic information collected was the student’s gender. Semistructured interviews were conducted to assess students’ current knowledge, experience, and needs. These interviews lasted about 20 minutes and collected information regarding the students’ knowledge about cognitive and behavioral problems in general and those occurring in dementia, their experience with screening, and any problems they encountered.
Statistical Analysis
Student baseline characteristics were assessed. Pre- and postassessments were analyzed with the McNemar test for paired data, and associations with experience were evaluated using χ2 tests. Ratings were dichotomized as very good/excellent vs poor/fair/ good because our educational goal was “very good” to “excellent” experience in dementia care and to avoid expected small cell counts. Two-sided P < .05 indicated statistical significance. Data were analyzed using SAS Enterprise Guide v5.1.
Results
One hundred fourth-year medical students participated, including 54 women. Thirtysix percent reported they had not previously attended an interdisciplinary team assessment for dementia, while 18% stated that they had attended only 1 interdisciplinary team assessment for dementia.
Before the education, students rated their dementia ability as poor. Only 2% (1 of 54), of those with 0 to 1 assessment experience rated their ability for assessing dementia with an interdisciplinary team format as very good/excellent compared with 20% (9/46) of those previously attending ≥ 2 assessments (P = .03); other ratings of ability were not associated with prior experience.
There was a significant change in the students’ self-efficacy ratings pre- to postassessment (P < .05) (Table 2). Only 10% rated their ability to assess for dementia as very good/excellent in before the intervention compared with 96% in postassessment (P < .01). Students’ perception of the impact of behavioral problems on dementia care improved significantly (45% to 98%, P < .01). Similarly, student’s perception of their ability to assess behavioral problems, caregiver burden, and cognition improved significantly from 7 to 88%; 7 to 78%, and 18 to 92%, respectively (P < .01). Students perception of the role of social worker, neuropsychologist, geriatrician, and geriatric psychiatrist also improved significantly for most measures from 81 to 98% (P = .02), 87 to 98% (P = .05), 94 to 99% (P = .06), and 88 to 100% (P = .01), respectively.
The semistructured interviews revealed that awareness of behavioral problems associated with dementia varied for different behavioral problems. Although many students showed familiarity with depression, agitation, and psychosis, they were not comfortable assessing them in a patient with dementia. These students were less aware of other behavioral problems such as disinhibition, apathy, and movement disorders. Deficits were noted in the skill of administering commonly used global cognitive screens, such as the Mini-Mental State Examination (MMSE).15
In semistructured interviews, only 7% of senior medical students were comfortable assessing behavioral problems associated with dementia. Most were not aware of any validated rating scale to assess neuropsychiatric symptoms. Similarly, only 7% of students were comfortable assessing caregiver burden, and most were not aware of any validated rating scale to assess caregiver burden. Only 1 in 5 students were comfortable using 2 cognitive screens to assess cognitive deficits. Many students stated that they were not routinely expected to perform common cognitive screens, such as the MMSE during their medical training except students who had expressed an interest in psychiatry and were expected to be proficient in the MMSE. Most students were making common mistakes, such as converting the 3-command task to 3 individual single commands, helping too much with serial 7s, and giving too much positive feedback throughout the test.
Discussion
Significant knowledge gaps regarding dementia were found in our study, which is in keeping with other studies in the area. Dementia knowledge deficits among medical trainees have been identified in the United Kingdom, Australia, and the US.6-9
In our study, a brief multicomponent experiential curriculum improved senior medical students’ perception and self-efficacy in diagnosing dementia. This is in keeping with other studies, such as the PAIRS Program.7 Findings from another study indicated that education for geriatric- oriented physicians should focus on experiential learning components through observation and interaction with older adults.16
A background of direct experience with older adults is associated with more positive attitudes toward older adults and increased interest in geriatric medicine.16 In our study, the exposure was brief; therefore, the results could not be compared with other long-term exposure studies. However, even with this brief intervention most students reported being comfortable with assessing caregiver burden (78%), behavioral problems of dementia (88%), and using ≥ 2 cognitive screens (92%). Comfortable in dementia assessment increased after the intervention from 10% to 96%. This finding is encouraging because brief multicomponent dementia education can be devised easily. This finding needs to be taken with caution because we did not conduct a formal skills evaluation.
A unique component of our experience was to learn medical students’ perception about the impact of neuropsychiatric symptoms on the trajectory, outcomes, and management of dementia. These symptoms included delusions, hallucinations, agitation, depression, anxiety, euphoria, apathy, disinhibition, irritability, motor disturbance, nighttime behaviors, and appetite and eating. Less than half the students thought that neuropsychiatric symptoms had a significant impact on dementia before the experience. Through didactics, systematic assessment of neuropsychiatric symptoms and interaction with caregivers, > 98% of students learned that these symptoms have a significant impact on dementia management.
This experience also emphasized the role of several disciplines in dementia assessment and management. Students’ experience positively influenced appreciation of the role of the memory clinic team. Our hope is that students will seek input from social workers, neuropsychologists, and other team members when working with patients with dementia or their caregivers. The common reason why primary care physicians focus on an exclusive medical model is the time commitment for communicating with an interdisciplinary team. Students experienced the feasibility of the interdisciplinary team involvement and how technology could be used for synchronous and asynchronous communication among team members. Medical students also were introduced to complex billing codes used when ≥ 3 disciplines assess/manage a geriatric patient.
Limitations
This study is limited by the lack of long-term follow-up evaluations, no metrics for practice changes clinical outcomes, and implementation in a single medical school. The postexperience evaluation in this study was performed immediately after the intervention. Long-term follow-up would inform whether the changes noted are durable. Because of the brief nature of our intervention, we do not believe that it would change practice in clinical care. It will be informative to follow this cohort of students to study whether their clinical approach to dementia care changes. The intervention needs to be replicated in other medical schools and in more heterogeneous groups to generalize the results of the study.
Conclusions
Senior medical students are not routinely exposed to interdisciplinary team assessments. Dementia knowledge gaps were prevalent in this cohort of senior medical students. Providing interdisciplinary geriatric educational experience improved their perception of their ability to assess for dementia and their recognition of the roles of interdisciplinary team members. Plans are in place to continue and expand the program to other complex geriatric syndromes.
Acknowledgments
Poster presented at the 2019 annual meeting of the American Geriatrics Society. Oral presentation at the same meeting as part of the select Geriatric Education Methods and Materials Swap workshop.
The global burden of dementia is increasing at an alarming pace and is estimated to soon affect 81 million individuals worldwide.1 The World Health Organization and the Institute of Medicine have recommended greater dementia awareness and education.2,3 Despite this emphasis on dementia education, many general practitioners consider dementia care beyond their clinical domain and feel that specialists, such as geriatricians, geriatric psychiatrists, or neurologists should address dementia assessment and treatment. 4 Unfortunately, the geriatric health care workforce has been shrinking. The American Geriatrics Society estimates the need for 30,000 geriatricians by 2030, although there are only 7,300 board-certified geriatricians currently in the US.5 There is an urgent need for educating all medical trainees in dementia care regardless of their specialization interest. As the largest underwriter of graduate medical education in the US, the US Department of Veterans Affairs (VA) is well placed for rolling out focused dementia education. Training needs to be practical, brief, and responsive to knowledge gaps to reach the most trainees.
Despite growing emphasis on geriatric training, many medical students have limited experience with patients with dementia or their caregivers, lack exposure to interdisciplinary teams, have a poor attitude toward geriatric patients, and display specific knowledge gaps in dementia assessment and management. 6-9 Other knowledge gaps noted in medical students included assessing behavioral problems, function, safety, and caregiver burden. Medical students also had limited exposure to interdisciplinary team dementia assessment and management.
Our goal was to develop a multicomponent, experiential, brief curriculum using team-based learning to expose senior medical students to interdisciplinary assessment of dementia. The curriculum was developed with input from the interdisciplinary team to address dementia knowledge gaps while providing an opportunity to interact with caregivers. The curriculum targeted all medical students regardless of their interest in geriatrics. Particular emphasis was placed on systems-based learning and the importance of teamwork in managing complex conditions such as dementia. Students were taught that incorporating interdisciplinary input would be more effective during dementia care planning rather than developing specialized knowledge.
Methods
Our team developed a curriculum for fourthyear medical students who rotated through the VA Memory Disorders Clinic as a part of their geriatric medicine clerkship at the University of Arkansas for Medical Sciences in Little Rock. The Memory Disorders Clinic is a consultation practice at the Central Arkansas Veterans Healthcare System (CAVHS) where patients with memory problems are evaluated by a team consisting of a geriatric psychiatrist, a geriatrician, a social worker, and a neuropsychologist. Each specialist addresses specific areas of dementia assessment and management. The curriculum included didactics, clinical experience, and team-based learning.
Didactics
An hour-long didactic session lead by the team geriatrician provided a general overview of interdisciplinary assessment of dementia to groups of 2 to 3 students at a time. The geriatrician presented an overview of dementia types, comorbidities, medications that affect memory, details of the physical examination, and laboratory, cognitive, and behavioral assessments along with treatment plan development. Students also learned about the roles of the social worker, geriatrician, neuropsychologist, and geriatric psychiatrist in the clinic. Pictographs and pie charts highlighted the role of disciplines in assessing and managing aspects of dementia.
The social work evaluation included advance care planning, functional assessment, safety assessment (driving, guns, wandering behaviors, etc), home safety evaluation, support system, and financial evaluation. Each medical student received a binder with local resources to become familiar with the depth and breadth of agencies involved in dementia care. Each medical student learned how to administer the Zarit Burden Scale to assess caregiver burden.10 The details of the geriatrician assessment included reviewing medical comorbidities and medications contributing to dementia, a physical examination, including a focused neurologic examination, laboratory assessment, and judicious use of neuroimaging.
The neuropsychology assessment education included a battery of tests and assessments. The global screening instruments included the Modified Mini-Mental State examination (3MS), Montreal Cognitive Assessment (MoCA), and Saint Louis University Mental Status examination (SLUMS).11-13 Executive function is evaluated using the Trails Making Test A and Trails Making Test B, Controlled Oral Word Association Test, Semantic Fluency Test, and Repeatable Battery for the Assessment of Neuropsychological Status test. Cognitive tests were compared and age- , education-, and race-adjusted norms for rating scales were listed if available. Each student was expected to show proficiency in ≥ 2 cognitive screening instruments (3MS, MoCA, or SLUMS). The geriatric psychiatry assessment included clinical history, onset, and course of memory problems from patient and caregiver perspectives, the Neuropsychiatric Inventory for assessing behavioral problems, employing the clinical dementia rating scale, integrating the team data, summarizing assessment, and formulating a treatment plan.14
Clinical
Students had a single clinical exposure. Students followed 1 patient and his or her caregiver through the team assessment and observed each provider’s assessment to learn interview techniques to adapt to the patient’s sensory or cognitive impairment and become familiar with different tools and devices used in the dementia clinic, such as hearing amplifiers. Each specialist provided hands-on experience. This encounter helped the students connect with caregivers and appreciate their role in patient care.
Systems learning was an important component integrated throughout the clinical experience. Examples include using video teleconferences to communicate findings among team members and electronic health records to seamlessly obtain and integrate data. Students learned how to create worksheets to graph laboratory data such as B12, thyroid-stimulating hormone, and rapid plasma regain levels. Student gained experience in using applications to retrieve neuroimaging data, results of sleep studies, and other data. Many patients had not received the results of their sleep study, and students had the responsibility to share these reports, including the number of apneic episodes. Students used the VA Computerized Patient Record System for reviewing patient records. One particularly useful tool was Joint Legacy Viewer, a remote access tool used to retrieve data on veterans from anywhere within the US. Students were also trained on medication and consult order menus in the system.
Team-Based
Learning The objectives of the team-based learning section were to teach students basic concepts of integrating the interdisciplinary assessment and formulating a treatment plan, to provide an opportunity to present their case in a group format, to discuss the differential diagnosis, management and treatment plan with a geriatrician in the team-based learning format, and to answer questions from other students. The instructors developed a set of prepared take-home points (Table 1). The team-based learning sessions were structured so that all take-home points were covered.
Evaluations
Evaluations were performed before and immediately after the clinical experience. In preevaluation, students reported the frequency of their participation in an interdisciplinary team assessment of any condition and specifically for dementia. In pre- and postevaluation, students rated their perception of the role of interdisciplinary team members in assessing and managing dementia, their personal abilities to assess cognition, behavioral problems, caregiver burden, and their perception of the impact of behavioral problems on dementia care. A Likert scale (poor = 1; fair = 2; good = 3; very good = 4; and excellent = 5) was employed (eApendices 1 and 2 can be found at doi:10.12788/fp.0052). The only demographic information collected was the student’s gender. Semistructured interviews were conducted to assess students’ current knowledge, experience, and needs. These interviews lasted about 20 minutes and collected information regarding the students’ knowledge about cognitive and behavioral problems in general and those occurring in dementia, their experience with screening, and any problems they encountered.
Statistical Analysis
Student baseline characteristics were assessed. Pre- and postassessments were analyzed with the McNemar test for paired data, and associations with experience were evaluated using χ2 tests. Ratings were dichotomized as very good/excellent vs poor/fair/ good because our educational goal was “very good” to “excellent” experience in dementia care and to avoid expected small cell counts. Two-sided P < .05 indicated statistical significance. Data were analyzed using SAS Enterprise Guide v5.1.
Results
One hundred fourth-year medical students participated, including 54 women. Thirtysix percent reported they had not previously attended an interdisciplinary team assessment for dementia, while 18% stated that they had attended only 1 interdisciplinary team assessment for dementia.
Before the education, students rated their dementia ability as poor. Only 2% (1 of 54), of those with 0 to 1 assessment experience rated their ability for assessing dementia with an interdisciplinary team format as very good/excellent compared with 20% (9/46) of those previously attending ≥ 2 assessments (P = .03); other ratings of ability were not associated with prior experience.
There was a significant change in the students’ self-efficacy ratings pre- to postassessment (P < .05) (Table 2). Only 10% rated their ability to assess for dementia as very good/excellent in before the intervention compared with 96% in postassessment (P < .01). Students’ perception of the impact of behavioral problems on dementia care improved significantly (45% to 98%, P < .01). Similarly, student’s perception of their ability to assess behavioral problems, caregiver burden, and cognition improved significantly from 7 to 88%; 7 to 78%, and 18 to 92%, respectively (P < .01). Students perception of the role of social worker, neuropsychologist, geriatrician, and geriatric psychiatrist also improved significantly for most measures from 81 to 98% (P = .02), 87 to 98% (P = .05), 94 to 99% (P = .06), and 88 to 100% (P = .01), respectively.
The semistructured interviews revealed that awareness of behavioral problems associated with dementia varied for different behavioral problems. Although many students showed familiarity with depression, agitation, and psychosis, they were not comfortable assessing them in a patient with dementia. These students were less aware of other behavioral problems such as disinhibition, apathy, and movement disorders. Deficits were noted in the skill of administering commonly used global cognitive screens, such as the Mini-Mental State Examination (MMSE).15
In semistructured interviews, only 7% of senior medical students were comfortable assessing behavioral problems associated with dementia. Most were not aware of any validated rating scale to assess neuropsychiatric symptoms. Similarly, only 7% of students were comfortable assessing caregiver burden, and most were not aware of any validated rating scale to assess caregiver burden. Only 1 in 5 students were comfortable using 2 cognitive screens to assess cognitive deficits. Many students stated that they were not routinely expected to perform common cognitive screens, such as the MMSE during their medical training except students who had expressed an interest in psychiatry and were expected to be proficient in the MMSE. Most students were making common mistakes, such as converting the 3-command task to 3 individual single commands, helping too much with serial 7s, and giving too much positive feedback throughout the test.
Discussion
Significant knowledge gaps regarding dementia were found in our study, which is in keeping with other studies in the area. Dementia knowledge deficits among medical trainees have been identified in the United Kingdom, Australia, and the US.6-9
In our study, a brief multicomponent experiential curriculum improved senior medical students’ perception and self-efficacy in diagnosing dementia. This is in keeping with other studies, such as the PAIRS Program.7 Findings from another study indicated that education for geriatric- oriented physicians should focus on experiential learning components through observation and interaction with older adults.16
A background of direct experience with older adults is associated with more positive attitudes toward older adults and increased interest in geriatric medicine.16 In our study, the exposure was brief; therefore, the results could not be compared with other long-term exposure studies. However, even with this brief intervention most students reported being comfortable with assessing caregiver burden (78%), behavioral problems of dementia (88%), and using ≥ 2 cognitive screens (92%). Comfortable in dementia assessment increased after the intervention from 10% to 96%. This finding is encouraging because brief multicomponent dementia education can be devised easily. This finding needs to be taken with caution because we did not conduct a formal skills evaluation.
A unique component of our experience was to learn medical students’ perception about the impact of neuropsychiatric symptoms on the trajectory, outcomes, and management of dementia. These symptoms included delusions, hallucinations, agitation, depression, anxiety, euphoria, apathy, disinhibition, irritability, motor disturbance, nighttime behaviors, and appetite and eating. Less than half the students thought that neuropsychiatric symptoms had a significant impact on dementia before the experience. Through didactics, systematic assessment of neuropsychiatric symptoms and interaction with caregivers, > 98% of students learned that these symptoms have a significant impact on dementia management.
This experience also emphasized the role of several disciplines in dementia assessment and management. Students’ experience positively influenced appreciation of the role of the memory clinic team. Our hope is that students will seek input from social workers, neuropsychologists, and other team members when working with patients with dementia or their caregivers. The common reason why primary care physicians focus on an exclusive medical model is the time commitment for communicating with an interdisciplinary team. Students experienced the feasibility of the interdisciplinary team involvement and how technology could be used for synchronous and asynchronous communication among team members. Medical students also were introduced to complex billing codes used when ≥ 3 disciplines assess/manage a geriatric patient.
Limitations
This study is limited by the lack of long-term follow-up evaluations, no metrics for practice changes clinical outcomes, and implementation in a single medical school. The postexperience evaluation in this study was performed immediately after the intervention. Long-term follow-up would inform whether the changes noted are durable. Because of the brief nature of our intervention, we do not believe that it would change practice in clinical care. It will be informative to follow this cohort of students to study whether their clinical approach to dementia care changes. The intervention needs to be replicated in other medical schools and in more heterogeneous groups to generalize the results of the study.
Conclusions
Senior medical students are not routinely exposed to interdisciplinary team assessments. Dementia knowledge gaps were prevalent in this cohort of senior medical students. Providing interdisciplinary geriatric educational experience improved their perception of their ability to assess for dementia and their recognition of the roles of interdisciplinary team members. Plans are in place to continue and expand the program to other complex geriatric syndromes.
Acknowledgments
Poster presented at the 2019 annual meeting of the American Geriatrics Society. Oral presentation at the same meeting as part of the select Geriatric Education Methods and Materials Swap workshop.
1. Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366(9503):2112-2117. doi:10.1016/S0140-6736(05)67889-0
2. Janca A, Aarli JA, Prilipko L, Dua T, Saxena S, Saraceno B. WHO/WFN survey of neurological services: a worldwide perspective. J Neurol Sci. 2006;247(1):29-34. doi:10.1016/j.jns.2006.03.003
3. Wilkins KM, Blazek MC, Brooks WB, Lehmann SW, Popeo D, Wagenaar D. Six things all medical students need to know about geriatric psychiatry (and how to teach them). Acad Psychiatry. 2017;41(5):693-700. doi:10.1007/s40596-017-0691-7
4. Turner S, Iliffe S, Downs M, et al. General practitioners’ knowledge, confidence and attitudes in the diagnosis and management of dementia. Age Ageing. 2004;33(5):461- 467. doi:10.1093/ageing/afh140
5. Lester PE, Dharmarajan TS, Weinstein E. The looming geriatrician shortage: ramifications and solutions. J Aging Health. 2019:898264319879325. doi:10.1177/0898264319879325
6. Struck BD, Bernard MA, Teasdale TA; Oklahoma University Geriatric Education G. Effect of a mandatory geriatric medicine clerkship on third-year students. J Am Geriatr Soc. 2005;53(11):2007-2011. doi:10.1111/j.1532-5415.2005.00473.x
7. Jefferson AL, Cantwell NG, Byerly LK, Morhardt D. Medical student education program in Alzheimer’s disease: the PAIRS Program. BMC Med Educ. 2012;12:80. doi:10.1186/1472-6920-12-80
8. Nagle BJ, Usita PM, Edland SD. United States medical students’ knowledge of Alzheimer disease. J Educ Eval Health Prof. 2013;10:4. doi:10.3352/jeehp.2013.10.4
9. Scott TL, Kugelman M, Tulloch K. How medical professional students view older people with dementia: Implications for education and practice. PLoS One. 2019;14(11):e0225329. doi:10.1371/journal.pone.0225329.
10. Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20(6):649-655. doi:10.1093/geront/20.6.649
11. McDowell I, Kristjansson B, Hill GB, Hebert R. Community screening for dementia: the Mini Mental State Exam (MMSE) and Modified Mini-Mental State Exam (3MS) compared. J Clin Epidemiol. 1997;50(4):377-383. doi:10.1016/s0895-4356(97)00060-7
12. Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Ger iatr Soc. 2005;53(4):695-699. doi:10.1111/j.1532-5415.2005.53221.x
13. Tariq SH, Tumosa N, Chibnall JT, Perry MH, 3rd, Morley JE. Comparison of the Saint Louis University mental status examination and the Mini-Mental State Examination for detecting dementia and mild neurocognitive disorder--a pilot study. Am J Geriatr Psychiatry. 2006;14(11):900-910. doi:10.1097/01.JGP.0000221510.33817.86
14. Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308-2314. doi:10.1212/wnl.44.12.2308
15. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. doi:10.1016/0022-3956(75)90026-6
16. Fitzgerald JT, Wray LA, Halter JB, Williams BC, Supiano MA. Relating medical students’ knowledge, attitudes, and experience to an interest in geriatric medicine. Gerontologist. 2003;43(6):849-855. doi:10.1093/geront/43.6.849
1. Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366(9503):2112-2117. doi:10.1016/S0140-6736(05)67889-0
2. Janca A, Aarli JA, Prilipko L, Dua T, Saxena S, Saraceno B. WHO/WFN survey of neurological services: a worldwide perspective. J Neurol Sci. 2006;247(1):29-34. doi:10.1016/j.jns.2006.03.003
3. Wilkins KM, Blazek MC, Brooks WB, Lehmann SW, Popeo D, Wagenaar D. Six things all medical students need to know about geriatric psychiatry (and how to teach them). Acad Psychiatry. 2017;41(5):693-700. doi:10.1007/s40596-017-0691-7
4. Turner S, Iliffe S, Downs M, et al. General practitioners’ knowledge, confidence and attitudes in the diagnosis and management of dementia. Age Ageing. 2004;33(5):461- 467. doi:10.1093/ageing/afh140
5. Lester PE, Dharmarajan TS, Weinstein E. The looming geriatrician shortage: ramifications and solutions. J Aging Health. 2019:898264319879325. doi:10.1177/0898264319879325
6. Struck BD, Bernard MA, Teasdale TA; Oklahoma University Geriatric Education G. Effect of a mandatory geriatric medicine clerkship on third-year students. J Am Geriatr Soc. 2005;53(11):2007-2011. doi:10.1111/j.1532-5415.2005.00473.x
7. Jefferson AL, Cantwell NG, Byerly LK, Morhardt D. Medical student education program in Alzheimer’s disease: the PAIRS Program. BMC Med Educ. 2012;12:80. doi:10.1186/1472-6920-12-80
8. Nagle BJ, Usita PM, Edland SD. United States medical students’ knowledge of Alzheimer disease. J Educ Eval Health Prof. 2013;10:4. doi:10.3352/jeehp.2013.10.4
9. Scott TL, Kugelman M, Tulloch K. How medical professional students view older people with dementia: Implications for education and practice. PLoS One. 2019;14(11):e0225329. doi:10.1371/journal.pone.0225329.
10. Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20(6):649-655. doi:10.1093/geront/20.6.649
11. McDowell I, Kristjansson B, Hill GB, Hebert R. Community screening for dementia: the Mini Mental State Exam (MMSE) and Modified Mini-Mental State Exam (3MS) compared. J Clin Epidemiol. 1997;50(4):377-383. doi:10.1016/s0895-4356(97)00060-7
12. Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Ger iatr Soc. 2005;53(4):695-699. doi:10.1111/j.1532-5415.2005.53221.x
13. Tariq SH, Tumosa N, Chibnall JT, Perry MH, 3rd, Morley JE. Comparison of the Saint Louis University mental status examination and the Mini-Mental State Examination for detecting dementia and mild neurocognitive disorder--a pilot study. Am J Geriatr Psychiatry. 2006;14(11):900-910. doi:10.1097/01.JGP.0000221510.33817.86
14. Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308-2314. doi:10.1212/wnl.44.12.2308
15. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. doi:10.1016/0022-3956(75)90026-6
16. Fitzgerald JT, Wray LA, Halter JB, Williams BC, Supiano MA. Relating medical students’ knowledge, attitudes, and experience to an interest in geriatric medicine. Gerontologist. 2003;43(6):849-855. doi:10.1093/geront/43.6.849
Multidisciplinary Transitional Pain Service for the Veteran Population
Despite advancements in techniques, postsurgical pain continues to be a prominent part of the patient experience. Often this experience can lead to developing chronic postsurgical pain that interferes with quality of life after the expected time to recovery.1-3 As many as 14% of patients who undergo surgery without any history of opioid use develop chronic opioid use that persists after recovery from their operation.4-8 For patients with existing chronic opioid use or a history of substance use disorder (SUD), surgeons, primary care providers, or addiction providers often do not provide sufficient presurgical planning or postsurgical coordination of care. This lack of pain care coordination can increase the risk of inadequate pain control, opioid use escalation, or SUD relapse after surgery.
Convincing arguments have been made that a perioperative surgical home can improve significantly the quality of perioperative care.9-14 This report describes our experience implementing a perioperative surgical home at the US Department of Veterans Affairs (VA) Salt Lake City VA Medical Center (SLCVAMC), focusing on pain management extending from the preoperative period until 6 months or more after surgery. This type of Transitional Pain Service (TPS) has been described previously.15-17 Our service differs from those described previously by enrolling all patients before surgery rather than select postsurgical enrollment of only patients with a history of opioid use or SUD or patients who struggle with persistent postsurgical pain.
Methods
In January 2018, we developed and implemented a new TPS at the SLCVAMC. The transitional pain team consisted of an anesthesiologist with specialization in acute pain management, a nurse practitioner (NP) with experience in both acute and chronic pain management, 2 nurse care coordinators, and a psychologist (Figure 1). Before implementation, a needs assessment took place with these key stakeholders and others at SLCVAMC to identify the following specific goals of the TPS: (1) reduce pain through pharmacologic and nonpharmacologic interventions; (2) eliminate new chronic opioid use in previously nonopioid user (NOU) patients; (3) address chronic opioid use in previous chronic opioid users (COUs) by providing support for opioid taper and alternative analgesic therapies for their chronic pain conditions; and (4) improve continuity of care by close coordination with the surgical team, primary care providers (PCPs), and mental health or chronic pain providers as needed.
Once these TPS goals were defined, the Consolidated Framework for Implementation Research (CFIR) guided the implementation. CFIR is a theory-based implementation framework consisting of 5 domains: intervention characteristics, inner setting, outer setting, characteristics of individuals, and process. These domains were used to identify barriers and facilitators during the early implementation process and helped refine TPS as it was put into clinical practice.
Patient Selection
During the initial implementation of TPS, enrollment was limited to patients scheduled for elective primary or revision knee, hip, or shoulder replacement as well as rotator cuff repair surgery. But as the TPS workflow became established after iterative refinement, we expanded the program to enroll patients with established risk factors for OUD having other types of surgery (Table 1). The diagnosis of risk factors, such as history of SUD, chronic opioid use, or significant mental health disorders (ie, history of suicidal ideation or attempt, posttraumatic stress disorder, and inpatient psychiatric care) were confirmed through both in-person interviews and electronic health record (EHR) documentation. The overall goal was to identify all at-risk patients as soon as they were indicated for surgery, to allow time for evaluation, education, developing an individualized pain plan, and opioid taper prior to surgery if indicated.
Preoperative Procedures
Once identified, patients were contacted by a TPS team member and invited to attend a onetime 90-minute presurgical expectations class held at SLCVAMC. The education curriculum was developed by the whole team, and classes were taught primarily by the TPS psychologist. The class included education about expectations for postoperative pain, available analgesic therapies, opioid education, appropriate use of opioids, and the effect of psychological factors on pain. Pain coping strategies were introduced using a mindfulness-based intervention (MBI) and the Acceptance and Commitment Therapy (ACT) matrix. Classes were offered multiple times a week to help maximize convenience for patients and were separate from the anesthesia preoperative evaluation. Patients attended class only once. High-risk patients (patients with chronic opioid therapy, recent history of or current SUDs, significant comorbid mental health issues) were encouraged to attend this class one-on-one with the TPS psychologist rather than in the group setting, so individual attention to mental health and SUD issues could be addressed directly.
Baseline history, morphine equivalent daily dose (MEDD), and patient-reported outcomes using measures from the Patient-Reported Outcome Measurement System (PROMIS) for pain intensity (PROMIS 3a), pain interference (PROMIS 6b), and physical function (PROMIS 8b), and a pain-catastrophizing scale (PCS) score were obtained on all patients.18 PROMIS measures are validated questionnaires developed with the National Institutes of Health to standardize and quantify patient-reported outcomes in many domains.19 Patients with a history of SUD or COU met with the anesthesiologist and/or NP, and a personalized pain plan was developed that included preoperative opioid taper, buprenorphine use strategy, or opioid-free strategies.
Hospital Procedures
On the day of surgery, the TPS team met with the patient preoperatively and implemented an individualized pain plan that included multimodal analgesic techniques with nonsteroidal anti-inflammatory drugs, acetaminophen, gabapentinoids, and regional anesthesia, where appropriate (Table 2). Enhanced recovery after surgery protocols were developed in conjunction with the surgeons to include local infiltration analgesia by the surgeon, postoperative multimodal analgesic strategies, and intensive physical therapy starting the day of surgery for inpatient procedures.
After surgery, the TPS team followed up with patients daily and provided recommendations for analgesic therapies. Patients were offered daily sessions with the psychologist to reinforce and practice nonpharmacologic pain-coping strategies, such as meditation and relaxation. Prior to patient discharge, the TPS team provided recommendations for discharge medications and an opioid taper plan. For some patients taking buprenorphine before surgery who had stopped this therapy prior to or during their hospital stay, TPS providers transitioned them back to buprenorphine before discharge.
Postoperative Procedures
Patients were called by the nurse care coordinators at postdischarge days 2, 7, 10, 14, 21, 28, and then monthly for ≥ 6 months. For patients who had not stopped opioid use or returned to their preoperative baseline opioid dose, weekly calls were made until opioid taper goals were achieved. At each call, nurses collected PROMIS scores for the previous 24 hours, the most recent 24-hour MEDD, the date of last opioid use, and the number of remaining opioid tablets after opioid cessation. In addition, nurses provided active listening and supportive care and encouragement as well as care coordination for issues related to rehabilitation facilities, physical therapy, transportation, medication questions, and wound questions. Nurses notified the anesthesiologist or NP when patients were unable to taper opioid use or had poor pain control as indicated by their PROMIS scores, opioid use, or directly expressed by the patient.
The TPS team prescribed alternative analgesic therapies, opioid taper plans, and communicated with surgeons and primary care providers if limited continued opioid therapy was recommended. Individual sessions with the psychologist were available to patients after discharge with a focus on ACT-matrix therapy and consultation with long-term mental health and/or substance abuse providers as indicated. Frequent communication and care coordination were maintained with the surgical team, the PCP, and other providers on the mental health or chronic pain services. This care coordination often included postsurgical joint clinic appointments in which TPS providers and nurses would be present with the surgeon or the PCP.
For patients with inadequately treated chronic pain conditions or who required long-term opioid tapers, we developed a combined clinic with the TPS and Anesthesia Chronic Pain group. This clinic allows patients to be seen by both services in the same setting, allowing a warm handoff by TPS to the chronic pain team.
Heath and Decision Support Tools
An electronic dashboard registry of surgical episodes managed by TPS was developed to achieve clinical, administrative, and quality improvement goals. The dashboard registry consists of surgical episode data, opioid doses, patient-reported outcomes, and clinical decision-making processes. Custom-built note templates capture pertinent data through embedded data labels, called health factors. Data are captured as part of routine clinical care, recorded in Computerized Patient Record System as health factors. They are available in the VA Corporate Data Warehouse as structured data. Workflows are executed daily to keep the dashboard registry current, clean, and able to process new data. Information displays direct daily clinical workflow and support point-of-care clinical decision making (Figures 2, 3, and 4). Data are aggregated across patient-care encounters and allow nurse care coordinators to concisely review pertinent patient data prior to delivering care. These data include surgical history, comorbidities, timeline of opioid use, and PROMIS scores during their course of recovery. This system allows TPS to optimize care delivery by providing longitudinal data across the surgical episode, thereby reducing the time needed to review records. Secondary purposes of captured data include measuring clinic performance and quality improvement to improve care delivery.
Results
The TPS intervention was implemented January 1, 2018. Two-hundred thirteen patients were enrolled between January and December 2018, which included 60 (28%) patients with a history of chronic opioid use and 153 (72%) patients who were considered opioid naïve. A total of 99% of patients had ≥ 1 successful follow-up within 14 days after discharge, 96% had ≥ 1 follow-up between 14 and 30 days after surgery, and 72% had completed personal follow-up 90 days after discharge (Table 3). For patients who TPS was unable to contact in person or by phone, 90-day MEDD was obtained using prescription and Controlled Substance Database reviews. The protocol for this retrospective analysis was approved by the University of Utah Institutional Review Board and the VA Research Review Committee.
By 90 days after surgery, 26 (43.3%) COUs were off opioids completely, 17 (28.3%) had decreased their opioid dose from their preoperative baseline MEDD (120 [SD, 108] vs 55 [SD, 45]), 14 (23.3%) returned to their baseline dose, and 3 (5%) increased from their baseline dose. Of the 153 patients who were NOUs before surgery, only 1 (0.7%) was taking opioids after 90 days. TPS continued to work closely with the patient and their PCP and that patient was finally able to stop opioid use 262 days after discharge. Ten patients had an additional surgery within 90 days of the initial surgery. Of these, 6 were COU, of whom 3 stopped all opioids by 90 days from their original surgery, 2 had no change in MEDD at 90 days, and 1 had a lower MEDD at 90 days. Of the 4 NOU who had additional surgery, all were off opioids by 90 days from the original surgery.
Although difficult to quantify, a meaningful outcome of TPS has been to improve satisfaction substantially among health care providers caring for complex patients at risk for chronic opioid abuse. This group includes the many members of the surgical team, PCPs, and addiction specialists who appreciate the close care coordination and assistance in caring for patients with difficult issues, especially with opioid tapers or SUDs. We also have noticed changes in prescribing practices among surgeons and PCPs for their patients who are not part of TPS.
Discussion
With any new clinical service, there are obstacles and challenges. TPS requires a considerable investment in personnel, and currently no mechanism is in place for obtaining payment for many of the provided services. We were fortunate the VA Whole Health Initiative, the VA Office of Rural Health, and the VA Centers of Innovation provided support for the development, implementation, and pilot evaluation of TPS. After we presented our initial results to hospital leadership, we also received hospital support to expand TPS service to include a total of 4 nurse care coordinators and 2 psychologists. We are currently performing a cost analysis of the service but recognize that this model may be difficult to reproduce at other institutions without a change in reimbursement standards.
Developing a working relationship with the surgical and primary care services required a concerted effort from the TPS team and a number of months to become effective. As most veterans receive primary care, mental health care, and surgical care within the VA system, this model lends itself to close care coordination. Initially there was skepticism about TPS recommendations to reduce opioid use, especially from PCPs who had cared for complex patients over many years. But this uncertainty went away as we showed evidence of close patient follow-up and detailed communication. TPS soon became the designated service for both primary care and surgical providers who were otherwise uncomfortable with how to approach opioid tapers and nonopioid pain strategies. In fact, a substantial portion of our referrals now come directly from the PCP who is referring a high-risk patient for evaluation for surgery rather than from the surgeons, and joint visits with TPS and primary care have become commonplace.
Challenges abound when working with patients with substance abuse history, opioid use history, high anxiety, significant pain catastrophizing, and those who have had previous negative experiences with surgery. We have found that the most important facet of our service comes from the amount of time and effort team members, especially the nurses, spend helping patients. Much of the nurses' work focuses on nonpain-related issues, such as assisting patients with finding transportation, housing issues, questions about medications, help scheduling appointments, etc. Through this concerted effort, patients gain trust in TPS providers and are willing to listen to and experiment with our recommendations. Many patients who were initially extremely unreceptive to the presurgery education asked for our support weeks after surgery to help with postsurgery pain.
Another challenge we continue to experience comes from the success of the program.
Conclusions
The multidisciplinary TPS supports greater preoperative to postoperative longitudinal care for surgical patients. This endeavor has resulted in better patient preparation before surgery and improved care coordination after surgery, with specific improvements in appropriate use of opioid medications and smooth transitions of care for patients with ongoing and complex needs. Development of sophisticated note templates and customized health information technology allows for accurate follow-through and data gathering for quality improvement, facilitating data-driven improvements and proving value to the facility.
Given that TPS is a multidisciplinary program with multiple interventions, it is difficult to pinpoint which specific aspects of TPS are most effective in achieving success. For example, although we have little doubt that the work our psychologists do with our patients is beneficial and even essential for the success we have had with some of our most difficult patients, it is less clear whether it matters if they use mindfulness, ACT matrix, or cognitive behavioral therapy. We think that an important part of TPS is the frequent human interaction with a caring individual. Therefore, as TPS continues to grow, maintaining the ability to provide frequent personal interaction is a priority.
The role of opioids in acute pain deserves further scrutiny. In 2018, with TPS use of opioids after orthopedic surgery decreased by > 40% from the previous year. Despite this more restricted use of opioids, pain interference and physical function scores indicated that surgical patients do not seem to experience increased pain or reduced physical function. In addition, stopping opioid use for COUs did not seem to affect the quality of recovery, pain, or physical function. Future prospective controlled studies of TPS are needed to confirm these findings and identify which aspects of TPS are most effective in improving functional recovery of patients. Also, more evidence is needed to determine the appropriateness or need for opioids in acute postsurgical pain.
TPS has expanded to include all surgical specialties. Given the high burden and limited resources, we have chosen to focus on patients at higher risk for chronic postsurgical pain by type of surgery (eg, thoracotomy, open abdominal, limb amputation, major joint surgery) and/or history of substance abuse or chronic opioid use. To better direct scarce resources where it would be of most benefit, we are now enrolling only NOUs without other risk factors postoperatively if they request a refill of opioids or are otherwise struggling with pain control after surgery. Whether this approach affects the success we had in the first year in preventing new COUs after surgery remains to be seen.
It is unlikely that any single model of a perioperative surgical home will fit the needs of the many different types of medical systems that exist. The TPS model fits well in large hospital systems, like the VA, where patients receive most of their care within the same system. However, it seems to us that the optimal TPS program in any health system will provide education, support, and care coordination beginning preoperatively to prepare the patient for surgery and then to facilitate care coordination to transition patients back to their PCPs or on to specialized chronic care.
Acknowledgments
We would like to acknowledge the contributions of Candice Harmon, RN; David Merrill, RN; Amy Beckstead, RN, who have provided invaluable assistance with establishing the TPS program at the VA Salt Lake City and helping with the evaluation process.
Funding for the implementation and evaluation of the TPS was received from the VA Whole Health Initiative, the VA Center of Innovation, the VA Office of Rural Health, and National Institutes of Health Grant UL1TR002538.
1. Ilfeld BM, Madison SJ, Suresh PJ. Persistent postmastectomy pain and pain-related physical and emotional functioning with and without a continuous paravertebral nerve block: a prospective 1-year follow-up assessment of a randomized, triple-masked, placebo-controlled study. Ann Surg Oncol. 2015;22(6):2017-2025. doi:10.1245/s10434-014-4248-7
2. Richebé P, Capdevila X, Rivat C. Persistent postsurgical pain. Anesthesiology. 2018;129(3):590-607. doi:10.1097/aln.0000000000002238
3. Glare P, Aubrey KR, Myles PS. Transition from acute to chronic pain after surgery. Lancet. 2019;393(10180):1537-1546. doi:10.1016/s0140-6736(19)30352-6
4. Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surgery. 2017;152(6):e170504-e170504. doi:10.1001/jamasurg.2017.0504
5. Swenson CW, Kamdar NS, Seiler K, Morgan DM, Lin P, As-Sanie S. Definition development and prevalence of new persistent opioid use following hysterectomy. Am J Obstet Gynecol. 2018;219(5):486.e1-486.e7. doi:10.1016/j.ajog.2018.06.010
6. Bartels K, Fernandez-Bustamante A, McWilliams SK, Hopfer CJ, Mikulich-Gilbertson SK. Long-term opioid use after inpatient surgery - a retrospective cohort study. Drug Alcohol Depend. 2018;187:61-65. doi:10.1016/j.drugalcdep.2018.02.013
7. Bedard N, DeMik D, Dowdle S, Callaghan J. Trends and risk factors for prolonged opioid use after unicompartmental knee arthroplasty. Bone Joint J. 2018;100-B(1)(suppl A):62-67. doi:10.1302/0301-620x.100b1.bjj-2017-0547.r1
8. Politzer CS, Kildow BJ, Goltz DE, Green CL, Bolognesi MP, Seyler T. Trends in opioid utilization before and after total knee arthroplasty. J Arthroplasty. 2018;33(7S):S147-S153.e1. doi:10.1016/j.arth.2017.10.060
9. Mariano ER, Walters TL, Kim ET, Kain ZN. Why the perioperative surgical home makes sense for Veterans Affairs health care. Anesth Analg. 2015;120(5):1163-1166. doi:10.1213/ane.0000000000000712
10. Walters TL, Howard SK, Kou A, et al. Design and implementation of a perioperative surgical home at a Veterans Affairs hospital. Semin Cardiothorac Vasc Anesth. 2016;20(2):133-140. doi:10.1177/1089253215607066
11. Walters TL, Mariano ER, Clark DJ. Perioperative surgical home and the integral role of pain medicine. Pain Med. 2015;16(9):1666-1672. doi:10.1111/pme.12796
12. Vetter TR, Kain ZN. Role of the perioperative surgical home in optimizing the perioperative use of opioids. Anesth Analg. 2017;125(5):1653-1657. doi:10.1213/ane.0000000000002280
13. Shafer SL. Anesthesia & Analgesia’s 2015 collection on the perioperative surgical home. Anesth Analg. 2015;120(5):966-967. doi:10.1213/ane.0000000000000696
14. Wenzel JT, Schwenk ES, Baratta JL, Viscusi ER. Managing opioid-tolerant patients in the perioperative surgical home. Anesthesiol Clin. 2016;34(2):287-301. doi:10.1016/j.anclin.2016.01.005
15. Katz J, Weinrib A, Fashler SR, et al. The Toronto General Hospital Transitional Pain Service: development and implementation of a multidisciplinary program to prevent chronic postsurgical pain. J Pain Res. 2015;8:695-702. doi:10.2147/jpr.s91924
16. Tiippana E, Hamunen K, Heiskanen T, Nieminen T, Kalso E, Kontinen VK. New approach for treatment of prolonged postoperative pain: APS Out-Patient Clinic. Scand J Pain. 2016;12(1):19-24. doi:10.1016/j.sjpain.2016.02.008
17. Katz J, Weinrib AZ, Clarke H. Chronic postsurgical pain: from risk factor identification to multidisciplinary management at the Toronto General Hospital Transitional Pain Service. Can J Pain. 2019;3(2):49-58. doi:10.1080/24740527.2019.1574537
18. Sullivan MJ, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess. 1995;7(4):524-532. doi:10.1037/1040-3590.7.4.524
19. HealthMeasures. Intro to PROMIS. https://www.healthmeasures.net/explore-measurement-systems/promis. Accessed September 28, 2020.
Despite advancements in techniques, postsurgical pain continues to be a prominent part of the patient experience. Often this experience can lead to developing chronic postsurgical pain that interferes with quality of life after the expected time to recovery.1-3 As many as 14% of patients who undergo surgery without any history of opioid use develop chronic opioid use that persists after recovery from their operation.4-8 For patients with existing chronic opioid use or a history of substance use disorder (SUD), surgeons, primary care providers, or addiction providers often do not provide sufficient presurgical planning or postsurgical coordination of care. This lack of pain care coordination can increase the risk of inadequate pain control, opioid use escalation, or SUD relapse after surgery.
Convincing arguments have been made that a perioperative surgical home can improve significantly the quality of perioperative care.9-14 This report describes our experience implementing a perioperative surgical home at the US Department of Veterans Affairs (VA) Salt Lake City VA Medical Center (SLCVAMC), focusing on pain management extending from the preoperative period until 6 months or more after surgery. This type of Transitional Pain Service (TPS) has been described previously.15-17 Our service differs from those described previously by enrolling all patients before surgery rather than select postsurgical enrollment of only patients with a history of opioid use or SUD or patients who struggle with persistent postsurgical pain.
Methods
In January 2018, we developed and implemented a new TPS at the SLCVAMC. The transitional pain team consisted of an anesthesiologist with specialization in acute pain management, a nurse practitioner (NP) with experience in both acute and chronic pain management, 2 nurse care coordinators, and a psychologist (Figure 1). Before implementation, a needs assessment took place with these key stakeholders and others at SLCVAMC to identify the following specific goals of the TPS: (1) reduce pain through pharmacologic and nonpharmacologic interventions; (2) eliminate new chronic opioid use in previously nonopioid user (NOU) patients; (3) address chronic opioid use in previous chronic opioid users (COUs) by providing support for opioid taper and alternative analgesic therapies for their chronic pain conditions; and (4) improve continuity of care by close coordination with the surgical team, primary care providers (PCPs), and mental health or chronic pain providers as needed.
Once these TPS goals were defined, the Consolidated Framework for Implementation Research (CFIR) guided the implementation. CFIR is a theory-based implementation framework consisting of 5 domains: intervention characteristics, inner setting, outer setting, characteristics of individuals, and process. These domains were used to identify barriers and facilitators during the early implementation process and helped refine TPS as it was put into clinical practice.
Patient Selection
During the initial implementation of TPS, enrollment was limited to patients scheduled for elective primary or revision knee, hip, or shoulder replacement as well as rotator cuff repair surgery. But as the TPS workflow became established after iterative refinement, we expanded the program to enroll patients with established risk factors for OUD having other types of surgery (Table 1). The diagnosis of risk factors, such as history of SUD, chronic opioid use, or significant mental health disorders (ie, history of suicidal ideation or attempt, posttraumatic stress disorder, and inpatient psychiatric care) were confirmed through both in-person interviews and electronic health record (EHR) documentation. The overall goal was to identify all at-risk patients as soon as they were indicated for surgery, to allow time for evaluation, education, developing an individualized pain plan, and opioid taper prior to surgery if indicated.
Preoperative Procedures
Once identified, patients were contacted by a TPS team member and invited to attend a onetime 90-minute presurgical expectations class held at SLCVAMC. The education curriculum was developed by the whole team, and classes were taught primarily by the TPS psychologist. The class included education about expectations for postoperative pain, available analgesic therapies, opioid education, appropriate use of opioids, and the effect of psychological factors on pain. Pain coping strategies were introduced using a mindfulness-based intervention (MBI) and the Acceptance and Commitment Therapy (ACT) matrix. Classes were offered multiple times a week to help maximize convenience for patients and were separate from the anesthesia preoperative evaluation. Patients attended class only once. High-risk patients (patients with chronic opioid therapy, recent history of or current SUDs, significant comorbid mental health issues) were encouraged to attend this class one-on-one with the TPS psychologist rather than in the group setting, so individual attention to mental health and SUD issues could be addressed directly.
Baseline history, morphine equivalent daily dose (MEDD), and patient-reported outcomes using measures from the Patient-Reported Outcome Measurement System (PROMIS) for pain intensity (PROMIS 3a), pain interference (PROMIS 6b), and physical function (PROMIS 8b), and a pain-catastrophizing scale (PCS) score were obtained on all patients.18 PROMIS measures are validated questionnaires developed with the National Institutes of Health to standardize and quantify patient-reported outcomes in many domains.19 Patients with a history of SUD or COU met with the anesthesiologist and/or NP, and a personalized pain plan was developed that included preoperative opioid taper, buprenorphine use strategy, or opioid-free strategies.
Hospital Procedures
On the day of surgery, the TPS team met with the patient preoperatively and implemented an individualized pain plan that included multimodal analgesic techniques with nonsteroidal anti-inflammatory drugs, acetaminophen, gabapentinoids, and regional anesthesia, where appropriate (Table 2). Enhanced recovery after surgery protocols were developed in conjunction with the surgeons to include local infiltration analgesia by the surgeon, postoperative multimodal analgesic strategies, and intensive physical therapy starting the day of surgery for inpatient procedures.
After surgery, the TPS team followed up with patients daily and provided recommendations for analgesic therapies. Patients were offered daily sessions with the psychologist to reinforce and practice nonpharmacologic pain-coping strategies, such as meditation and relaxation. Prior to patient discharge, the TPS team provided recommendations for discharge medications and an opioid taper plan. For some patients taking buprenorphine before surgery who had stopped this therapy prior to or during their hospital stay, TPS providers transitioned them back to buprenorphine before discharge.
Postoperative Procedures
Patients were called by the nurse care coordinators at postdischarge days 2, 7, 10, 14, 21, 28, and then monthly for ≥ 6 months. For patients who had not stopped opioid use or returned to their preoperative baseline opioid dose, weekly calls were made until opioid taper goals were achieved. At each call, nurses collected PROMIS scores for the previous 24 hours, the most recent 24-hour MEDD, the date of last opioid use, and the number of remaining opioid tablets after opioid cessation. In addition, nurses provided active listening and supportive care and encouragement as well as care coordination for issues related to rehabilitation facilities, physical therapy, transportation, medication questions, and wound questions. Nurses notified the anesthesiologist or NP when patients were unable to taper opioid use or had poor pain control as indicated by their PROMIS scores, opioid use, or directly expressed by the patient.
The TPS team prescribed alternative analgesic therapies, opioid taper plans, and communicated with surgeons and primary care providers if limited continued opioid therapy was recommended. Individual sessions with the psychologist were available to patients after discharge with a focus on ACT-matrix therapy and consultation with long-term mental health and/or substance abuse providers as indicated. Frequent communication and care coordination were maintained with the surgical team, the PCP, and other providers on the mental health or chronic pain services. This care coordination often included postsurgical joint clinic appointments in which TPS providers and nurses would be present with the surgeon or the PCP.
For patients with inadequately treated chronic pain conditions or who required long-term opioid tapers, we developed a combined clinic with the TPS and Anesthesia Chronic Pain group. This clinic allows patients to be seen by both services in the same setting, allowing a warm handoff by TPS to the chronic pain team.
Heath and Decision Support Tools
An electronic dashboard registry of surgical episodes managed by TPS was developed to achieve clinical, administrative, and quality improvement goals. The dashboard registry consists of surgical episode data, opioid doses, patient-reported outcomes, and clinical decision-making processes. Custom-built note templates capture pertinent data through embedded data labels, called health factors. Data are captured as part of routine clinical care, recorded in Computerized Patient Record System as health factors. They are available in the VA Corporate Data Warehouse as structured data. Workflows are executed daily to keep the dashboard registry current, clean, and able to process new data. Information displays direct daily clinical workflow and support point-of-care clinical decision making (Figures 2, 3, and 4). Data are aggregated across patient-care encounters and allow nurse care coordinators to concisely review pertinent patient data prior to delivering care. These data include surgical history, comorbidities, timeline of opioid use, and PROMIS scores during their course of recovery. This system allows TPS to optimize care delivery by providing longitudinal data across the surgical episode, thereby reducing the time needed to review records. Secondary purposes of captured data include measuring clinic performance and quality improvement to improve care delivery.
Results
The TPS intervention was implemented January 1, 2018. Two-hundred thirteen patients were enrolled between January and December 2018, which included 60 (28%) patients with a history of chronic opioid use and 153 (72%) patients who were considered opioid naïve. A total of 99% of patients had ≥ 1 successful follow-up within 14 days after discharge, 96% had ≥ 1 follow-up between 14 and 30 days after surgery, and 72% had completed personal follow-up 90 days after discharge (Table 3). For patients who TPS was unable to contact in person or by phone, 90-day MEDD was obtained using prescription and Controlled Substance Database reviews. The protocol for this retrospective analysis was approved by the University of Utah Institutional Review Board and the VA Research Review Committee.
By 90 days after surgery, 26 (43.3%) COUs were off opioids completely, 17 (28.3%) had decreased their opioid dose from their preoperative baseline MEDD (120 [SD, 108] vs 55 [SD, 45]), 14 (23.3%) returned to their baseline dose, and 3 (5%) increased from their baseline dose. Of the 153 patients who were NOUs before surgery, only 1 (0.7%) was taking opioids after 90 days. TPS continued to work closely with the patient and their PCP and that patient was finally able to stop opioid use 262 days after discharge. Ten patients had an additional surgery within 90 days of the initial surgery. Of these, 6 were COU, of whom 3 stopped all opioids by 90 days from their original surgery, 2 had no change in MEDD at 90 days, and 1 had a lower MEDD at 90 days. Of the 4 NOU who had additional surgery, all were off opioids by 90 days from the original surgery.
Although difficult to quantify, a meaningful outcome of TPS has been to improve satisfaction substantially among health care providers caring for complex patients at risk for chronic opioid abuse. This group includes the many members of the surgical team, PCPs, and addiction specialists who appreciate the close care coordination and assistance in caring for patients with difficult issues, especially with opioid tapers or SUDs. We also have noticed changes in prescribing practices among surgeons and PCPs for their patients who are not part of TPS.
Discussion
With any new clinical service, there are obstacles and challenges. TPS requires a considerable investment in personnel, and currently no mechanism is in place for obtaining payment for many of the provided services. We were fortunate the VA Whole Health Initiative, the VA Office of Rural Health, and the VA Centers of Innovation provided support for the development, implementation, and pilot evaluation of TPS. After we presented our initial results to hospital leadership, we also received hospital support to expand TPS service to include a total of 4 nurse care coordinators and 2 psychologists. We are currently performing a cost analysis of the service but recognize that this model may be difficult to reproduce at other institutions without a change in reimbursement standards.
Developing a working relationship with the surgical and primary care services required a concerted effort from the TPS team and a number of months to become effective. As most veterans receive primary care, mental health care, and surgical care within the VA system, this model lends itself to close care coordination. Initially there was skepticism about TPS recommendations to reduce opioid use, especially from PCPs who had cared for complex patients over many years. But this uncertainty went away as we showed evidence of close patient follow-up and detailed communication. TPS soon became the designated service for both primary care and surgical providers who were otherwise uncomfortable with how to approach opioid tapers and nonopioid pain strategies. In fact, a substantial portion of our referrals now come directly from the PCP who is referring a high-risk patient for evaluation for surgery rather than from the surgeons, and joint visits with TPS and primary care have become commonplace.
Challenges abound when working with patients with substance abuse history, opioid use history, high anxiety, significant pain catastrophizing, and those who have had previous negative experiences with surgery. We have found that the most important facet of our service comes from the amount of time and effort team members, especially the nurses, spend helping patients. Much of the nurses' work focuses on nonpain-related issues, such as assisting patients with finding transportation, housing issues, questions about medications, help scheduling appointments, etc. Through this concerted effort, patients gain trust in TPS providers and are willing to listen to and experiment with our recommendations. Many patients who were initially extremely unreceptive to the presurgery education asked for our support weeks after surgery to help with postsurgery pain.
Another challenge we continue to experience comes from the success of the program.
Conclusions
The multidisciplinary TPS supports greater preoperative to postoperative longitudinal care for surgical patients. This endeavor has resulted in better patient preparation before surgery and improved care coordination after surgery, with specific improvements in appropriate use of opioid medications and smooth transitions of care for patients with ongoing and complex needs. Development of sophisticated note templates and customized health information technology allows for accurate follow-through and data gathering for quality improvement, facilitating data-driven improvements and proving value to the facility.
Given that TPS is a multidisciplinary program with multiple interventions, it is difficult to pinpoint which specific aspects of TPS are most effective in achieving success. For example, although we have little doubt that the work our psychologists do with our patients is beneficial and even essential for the success we have had with some of our most difficult patients, it is less clear whether it matters if they use mindfulness, ACT matrix, or cognitive behavioral therapy. We think that an important part of TPS is the frequent human interaction with a caring individual. Therefore, as TPS continues to grow, maintaining the ability to provide frequent personal interaction is a priority.
The role of opioids in acute pain deserves further scrutiny. In 2018, with TPS use of opioids after orthopedic surgery decreased by > 40% from the previous year. Despite this more restricted use of opioids, pain interference and physical function scores indicated that surgical patients do not seem to experience increased pain or reduced physical function. In addition, stopping opioid use for COUs did not seem to affect the quality of recovery, pain, or physical function. Future prospective controlled studies of TPS are needed to confirm these findings and identify which aspects of TPS are most effective in improving functional recovery of patients. Also, more evidence is needed to determine the appropriateness or need for opioids in acute postsurgical pain.
TPS has expanded to include all surgical specialties. Given the high burden and limited resources, we have chosen to focus on patients at higher risk for chronic postsurgical pain by type of surgery (eg, thoracotomy, open abdominal, limb amputation, major joint surgery) and/or history of substance abuse or chronic opioid use. To better direct scarce resources where it would be of most benefit, we are now enrolling only NOUs without other risk factors postoperatively if they request a refill of opioids or are otherwise struggling with pain control after surgery. Whether this approach affects the success we had in the first year in preventing new COUs after surgery remains to be seen.
It is unlikely that any single model of a perioperative surgical home will fit the needs of the many different types of medical systems that exist. The TPS model fits well in large hospital systems, like the VA, where patients receive most of their care within the same system. However, it seems to us that the optimal TPS program in any health system will provide education, support, and care coordination beginning preoperatively to prepare the patient for surgery and then to facilitate care coordination to transition patients back to their PCPs or on to specialized chronic care.
Acknowledgments
We would like to acknowledge the contributions of Candice Harmon, RN; David Merrill, RN; Amy Beckstead, RN, who have provided invaluable assistance with establishing the TPS program at the VA Salt Lake City and helping with the evaluation process.
Funding for the implementation and evaluation of the TPS was received from the VA Whole Health Initiative, the VA Center of Innovation, the VA Office of Rural Health, and National Institutes of Health Grant UL1TR002538.
Despite advancements in techniques, postsurgical pain continues to be a prominent part of the patient experience. Often this experience can lead to developing chronic postsurgical pain that interferes with quality of life after the expected time to recovery.1-3 As many as 14% of patients who undergo surgery without any history of opioid use develop chronic opioid use that persists after recovery from their operation.4-8 For patients with existing chronic opioid use or a history of substance use disorder (SUD), surgeons, primary care providers, or addiction providers often do not provide sufficient presurgical planning or postsurgical coordination of care. This lack of pain care coordination can increase the risk of inadequate pain control, opioid use escalation, or SUD relapse after surgery.
Convincing arguments have been made that a perioperative surgical home can improve significantly the quality of perioperative care.9-14 This report describes our experience implementing a perioperative surgical home at the US Department of Veterans Affairs (VA) Salt Lake City VA Medical Center (SLCVAMC), focusing on pain management extending from the preoperative period until 6 months or more after surgery. This type of Transitional Pain Service (TPS) has been described previously.15-17 Our service differs from those described previously by enrolling all patients before surgery rather than select postsurgical enrollment of only patients with a history of opioid use or SUD or patients who struggle with persistent postsurgical pain.
Methods
In January 2018, we developed and implemented a new TPS at the SLCVAMC. The transitional pain team consisted of an anesthesiologist with specialization in acute pain management, a nurse practitioner (NP) with experience in both acute and chronic pain management, 2 nurse care coordinators, and a psychologist (Figure 1). Before implementation, a needs assessment took place with these key stakeholders and others at SLCVAMC to identify the following specific goals of the TPS: (1) reduce pain through pharmacologic and nonpharmacologic interventions; (2) eliminate new chronic opioid use in previously nonopioid user (NOU) patients; (3) address chronic opioid use in previous chronic opioid users (COUs) by providing support for opioid taper and alternative analgesic therapies for their chronic pain conditions; and (4) improve continuity of care by close coordination with the surgical team, primary care providers (PCPs), and mental health or chronic pain providers as needed.
Once these TPS goals were defined, the Consolidated Framework for Implementation Research (CFIR) guided the implementation. CFIR is a theory-based implementation framework consisting of 5 domains: intervention characteristics, inner setting, outer setting, characteristics of individuals, and process. These domains were used to identify barriers and facilitators during the early implementation process and helped refine TPS as it was put into clinical practice.
Patient Selection
During the initial implementation of TPS, enrollment was limited to patients scheduled for elective primary or revision knee, hip, or shoulder replacement as well as rotator cuff repair surgery. But as the TPS workflow became established after iterative refinement, we expanded the program to enroll patients with established risk factors for OUD having other types of surgery (Table 1). The diagnosis of risk factors, such as history of SUD, chronic opioid use, or significant mental health disorders (ie, history of suicidal ideation or attempt, posttraumatic stress disorder, and inpatient psychiatric care) were confirmed through both in-person interviews and electronic health record (EHR) documentation. The overall goal was to identify all at-risk patients as soon as they were indicated for surgery, to allow time for evaluation, education, developing an individualized pain plan, and opioid taper prior to surgery if indicated.
Preoperative Procedures
Once identified, patients were contacted by a TPS team member and invited to attend a onetime 90-minute presurgical expectations class held at SLCVAMC. The education curriculum was developed by the whole team, and classes were taught primarily by the TPS psychologist. The class included education about expectations for postoperative pain, available analgesic therapies, opioid education, appropriate use of opioids, and the effect of psychological factors on pain. Pain coping strategies were introduced using a mindfulness-based intervention (MBI) and the Acceptance and Commitment Therapy (ACT) matrix. Classes were offered multiple times a week to help maximize convenience for patients and were separate from the anesthesia preoperative evaluation. Patients attended class only once. High-risk patients (patients with chronic opioid therapy, recent history of or current SUDs, significant comorbid mental health issues) were encouraged to attend this class one-on-one with the TPS psychologist rather than in the group setting, so individual attention to mental health and SUD issues could be addressed directly.
Baseline history, morphine equivalent daily dose (MEDD), and patient-reported outcomes using measures from the Patient-Reported Outcome Measurement System (PROMIS) for pain intensity (PROMIS 3a), pain interference (PROMIS 6b), and physical function (PROMIS 8b), and a pain-catastrophizing scale (PCS) score were obtained on all patients.18 PROMIS measures are validated questionnaires developed with the National Institutes of Health to standardize and quantify patient-reported outcomes in many domains.19 Patients with a history of SUD or COU met with the anesthesiologist and/or NP, and a personalized pain plan was developed that included preoperative opioid taper, buprenorphine use strategy, or opioid-free strategies.
Hospital Procedures
On the day of surgery, the TPS team met with the patient preoperatively and implemented an individualized pain plan that included multimodal analgesic techniques with nonsteroidal anti-inflammatory drugs, acetaminophen, gabapentinoids, and regional anesthesia, where appropriate (Table 2). Enhanced recovery after surgery protocols were developed in conjunction with the surgeons to include local infiltration analgesia by the surgeon, postoperative multimodal analgesic strategies, and intensive physical therapy starting the day of surgery for inpatient procedures.
After surgery, the TPS team followed up with patients daily and provided recommendations for analgesic therapies. Patients were offered daily sessions with the psychologist to reinforce and practice nonpharmacologic pain-coping strategies, such as meditation and relaxation. Prior to patient discharge, the TPS team provided recommendations for discharge medications and an opioid taper plan. For some patients taking buprenorphine before surgery who had stopped this therapy prior to or during their hospital stay, TPS providers transitioned them back to buprenorphine before discharge.
Postoperative Procedures
Patients were called by the nurse care coordinators at postdischarge days 2, 7, 10, 14, 21, 28, and then monthly for ≥ 6 months. For patients who had not stopped opioid use or returned to their preoperative baseline opioid dose, weekly calls were made until opioid taper goals were achieved. At each call, nurses collected PROMIS scores for the previous 24 hours, the most recent 24-hour MEDD, the date of last opioid use, and the number of remaining opioid tablets after opioid cessation. In addition, nurses provided active listening and supportive care and encouragement as well as care coordination for issues related to rehabilitation facilities, physical therapy, transportation, medication questions, and wound questions. Nurses notified the anesthesiologist or NP when patients were unable to taper opioid use or had poor pain control as indicated by their PROMIS scores, opioid use, or directly expressed by the patient.
The TPS team prescribed alternative analgesic therapies, opioid taper plans, and communicated with surgeons and primary care providers if limited continued opioid therapy was recommended. Individual sessions with the psychologist were available to patients after discharge with a focus on ACT-matrix therapy and consultation with long-term mental health and/or substance abuse providers as indicated. Frequent communication and care coordination were maintained with the surgical team, the PCP, and other providers on the mental health or chronic pain services. This care coordination often included postsurgical joint clinic appointments in which TPS providers and nurses would be present with the surgeon or the PCP.
For patients with inadequately treated chronic pain conditions or who required long-term opioid tapers, we developed a combined clinic with the TPS and Anesthesia Chronic Pain group. This clinic allows patients to be seen by both services in the same setting, allowing a warm handoff by TPS to the chronic pain team.
Heath and Decision Support Tools
An electronic dashboard registry of surgical episodes managed by TPS was developed to achieve clinical, administrative, and quality improvement goals. The dashboard registry consists of surgical episode data, opioid doses, patient-reported outcomes, and clinical decision-making processes. Custom-built note templates capture pertinent data through embedded data labels, called health factors. Data are captured as part of routine clinical care, recorded in Computerized Patient Record System as health factors. They are available in the VA Corporate Data Warehouse as structured data. Workflows are executed daily to keep the dashboard registry current, clean, and able to process new data. Information displays direct daily clinical workflow and support point-of-care clinical decision making (Figures 2, 3, and 4). Data are aggregated across patient-care encounters and allow nurse care coordinators to concisely review pertinent patient data prior to delivering care. These data include surgical history, comorbidities, timeline of opioid use, and PROMIS scores during their course of recovery. This system allows TPS to optimize care delivery by providing longitudinal data across the surgical episode, thereby reducing the time needed to review records. Secondary purposes of captured data include measuring clinic performance and quality improvement to improve care delivery.
Results
The TPS intervention was implemented January 1, 2018. Two-hundred thirteen patients were enrolled between January and December 2018, which included 60 (28%) patients with a history of chronic opioid use and 153 (72%) patients who were considered opioid naïve. A total of 99% of patients had ≥ 1 successful follow-up within 14 days after discharge, 96% had ≥ 1 follow-up between 14 and 30 days after surgery, and 72% had completed personal follow-up 90 days after discharge (Table 3). For patients who TPS was unable to contact in person or by phone, 90-day MEDD was obtained using prescription and Controlled Substance Database reviews. The protocol for this retrospective analysis was approved by the University of Utah Institutional Review Board and the VA Research Review Committee.
By 90 days after surgery, 26 (43.3%) COUs were off opioids completely, 17 (28.3%) had decreased their opioid dose from their preoperative baseline MEDD (120 [SD, 108] vs 55 [SD, 45]), 14 (23.3%) returned to their baseline dose, and 3 (5%) increased from their baseline dose. Of the 153 patients who were NOUs before surgery, only 1 (0.7%) was taking opioids after 90 days. TPS continued to work closely with the patient and their PCP and that patient was finally able to stop opioid use 262 days after discharge. Ten patients had an additional surgery within 90 days of the initial surgery. Of these, 6 were COU, of whom 3 stopped all opioids by 90 days from their original surgery, 2 had no change in MEDD at 90 days, and 1 had a lower MEDD at 90 days. Of the 4 NOU who had additional surgery, all were off opioids by 90 days from the original surgery.
Although difficult to quantify, a meaningful outcome of TPS has been to improve satisfaction substantially among health care providers caring for complex patients at risk for chronic opioid abuse. This group includes the many members of the surgical team, PCPs, and addiction specialists who appreciate the close care coordination and assistance in caring for patients with difficult issues, especially with opioid tapers or SUDs. We also have noticed changes in prescribing practices among surgeons and PCPs for their patients who are not part of TPS.
Discussion
With any new clinical service, there are obstacles and challenges. TPS requires a considerable investment in personnel, and currently no mechanism is in place for obtaining payment for many of the provided services. We were fortunate the VA Whole Health Initiative, the VA Office of Rural Health, and the VA Centers of Innovation provided support for the development, implementation, and pilot evaluation of TPS. After we presented our initial results to hospital leadership, we also received hospital support to expand TPS service to include a total of 4 nurse care coordinators and 2 psychologists. We are currently performing a cost analysis of the service but recognize that this model may be difficult to reproduce at other institutions without a change in reimbursement standards.
Developing a working relationship with the surgical and primary care services required a concerted effort from the TPS team and a number of months to become effective. As most veterans receive primary care, mental health care, and surgical care within the VA system, this model lends itself to close care coordination. Initially there was skepticism about TPS recommendations to reduce opioid use, especially from PCPs who had cared for complex patients over many years. But this uncertainty went away as we showed evidence of close patient follow-up and detailed communication. TPS soon became the designated service for both primary care and surgical providers who were otherwise uncomfortable with how to approach opioid tapers and nonopioid pain strategies. In fact, a substantial portion of our referrals now come directly from the PCP who is referring a high-risk patient for evaluation for surgery rather than from the surgeons, and joint visits with TPS and primary care have become commonplace.
Challenges abound when working with patients with substance abuse history, opioid use history, high anxiety, significant pain catastrophizing, and those who have had previous negative experiences with surgery. We have found that the most important facet of our service comes from the amount of time and effort team members, especially the nurses, spend helping patients. Much of the nurses' work focuses on nonpain-related issues, such as assisting patients with finding transportation, housing issues, questions about medications, help scheduling appointments, etc. Through this concerted effort, patients gain trust in TPS providers and are willing to listen to and experiment with our recommendations. Many patients who were initially extremely unreceptive to the presurgery education asked for our support weeks after surgery to help with postsurgery pain.
Another challenge we continue to experience comes from the success of the program.
Conclusions
The multidisciplinary TPS supports greater preoperative to postoperative longitudinal care for surgical patients. This endeavor has resulted in better patient preparation before surgery and improved care coordination after surgery, with specific improvements in appropriate use of opioid medications and smooth transitions of care for patients with ongoing and complex needs. Development of sophisticated note templates and customized health information technology allows for accurate follow-through and data gathering for quality improvement, facilitating data-driven improvements and proving value to the facility.
Given that TPS is a multidisciplinary program with multiple interventions, it is difficult to pinpoint which specific aspects of TPS are most effective in achieving success. For example, although we have little doubt that the work our psychologists do with our patients is beneficial and even essential for the success we have had with some of our most difficult patients, it is less clear whether it matters if they use mindfulness, ACT matrix, or cognitive behavioral therapy. We think that an important part of TPS is the frequent human interaction with a caring individual. Therefore, as TPS continues to grow, maintaining the ability to provide frequent personal interaction is a priority.
The role of opioids in acute pain deserves further scrutiny. In 2018, with TPS use of opioids after orthopedic surgery decreased by > 40% from the previous year. Despite this more restricted use of opioids, pain interference and physical function scores indicated that surgical patients do not seem to experience increased pain or reduced physical function. In addition, stopping opioid use for COUs did not seem to affect the quality of recovery, pain, or physical function. Future prospective controlled studies of TPS are needed to confirm these findings and identify which aspects of TPS are most effective in improving functional recovery of patients. Also, more evidence is needed to determine the appropriateness or need for opioids in acute postsurgical pain.
TPS has expanded to include all surgical specialties. Given the high burden and limited resources, we have chosen to focus on patients at higher risk for chronic postsurgical pain by type of surgery (eg, thoracotomy, open abdominal, limb amputation, major joint surgery) and/or history of substance abuse or chronic opioid use. To better direct scarce resources where it would be of most benefit, we are now enrolling only NOUs without other risk factors postoperatively if they request a refill of opioids or are otherwise struggling with pain control after surgery. Whether this approach affects the success we had in the first year in preventing new COUs after surgery remains to be seen.
It is unlikely that any single model of a perioperative surgical home will fit the needs of the many different types of medical systems that exist. The TPS model fits well in large hospital systems, like the VA, where patients receive most of their care within the same system. However, it seems to us that the optimal TPS program in any health system will provide education, support, and care coordination beginning preoperatively to prepare the patient for surgery and then to facilitate care coordination to transition patients back to their PCPs or on to specialized chronic care.
Acknowledgments
We would like to acknowledge the contributions of Candice Harmon, RN; David Merrill, RN; Amy Beckstead, RN, who have provided invaluable assistance with establishing the TPS program at the VA Salt Lake City and helping with the evaluation process.
Funding for the implementation and evaluation of the TPS was received from the VA Whole Health Initiative, the VA Center of Innovation, the VA Office of Rural Health, and National Institutes of Health Grant UL1TR002538.
1. Ilfeld BM, Madison SJ, Suresh PJ. Persistent postmastectomy pain and pain-related physical and emotional functioning with and without a continuous paravertebral nerve block: a prospective 1-year follow-up assessment of a randomized, triple-masked, placebo-controlled study. Ann Surg Oncol. 2015;22(6):2017-2025. doi:10.1245/s10434-014-4248-7
2. Richebé P, Capdevila X, Rivat C. Persistent postsurgical pain. Anesthesiology. 2018;129(3):590-607. doi:10.1097/aln.0000000000002238
3. Glare P, Aubrey KR, Myles PS. Transition from acute to chronic pain after surgery. Lancet. 2019;393(10180):1537-1546. doi:10.1016/s0140-6736(19)30352-6
4. Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surgery. 2017;152(6):e170504-e170504. doi:10.1001/jamasurg.2017.0504
5. Swenson CW, Kamdar NS, Seiler K, Morgan DM, Lin P, As-Sanie S. Definition development and prevalence of new persistent opioid use following hysterectomy. Am J Obstet Gynecol. 2018;219(5):486.e1-486.e7. doi:10.1016/j.ajog.2018.06.010
6. Bartels K, Fernandez-Bustamante A, McWilliams SK, Hopfer CJ, Mikulich-Gilbertson SK. Long-term opioid use after inpatient surgery - a retrospective cohort study. Drug Alcohol Depend. 2018;187:61-65. doi:10.1016/j.drugalcdep.2018.02.013
7. Bedard N, DeMik D, Dowdle S, Callaghan J. Trends and risk factors for prolonged opioid use after unicompartmental knee arthroplasty. Bone Joint J. 2018;100-B(1)(suppl A):62-67. doi:10.1302/0301-620x.100b1.bjj-2017-0547.r1
8. Politzer CS, Kildow BJ, Goltz DE, Green CL, Bolognesi MP, Seyler T. Trends in opioid utilization before and after total knee arthroplasty. J Arthroplasty. 2018;33(7S):S147-S153.e1. doi:10.1016/j.arth.2017.10.060
9. Mariano ER, Walters TL, Kim ET, Kain ZN. Why the perioperative surgical home makes sense for Veterans Affairs health care. Anesth Analg. 2015;120(5):1163-1166. doi:10.1213/ane.0000000000000712
10. Walters TL, Howard SK, Kou A, et al. Design and implementation of a perioperative surgical home at a Veterans Affairs hospital. Semin Cardiothorac Vasc Anesth. 2016;20(2):133-140. doi:10.1177/1089253215607066
11. Walters TL, Mariano ER, Clark DJ. Perioperative surgical home and the integral role of pain medicine. Pain Med. 2015;16(9):1666-1672. doi:10.1111/pme.12796
12. Vetter TR, Kain ZN. Role of the perioperative surgical home in optimizing the perioperative use of opioids. Anesth Analg. 2017;125(5):1653-1657. doi:10.1213/ane.0000000000002280
13. Shafer SL. Anesthesia & Analgesia’s 2015 collection on the perioperative surgical home. Anesth Analg. 2015;120(5):966-967. doi:10.1213/ane.0000000000000696
14. Wenzel JT, Schwenk ES, Baratta JL, Viscusi ER. Managing opioid-tolerant patients in the perioperative surgical home. Anesthesiol Clin. 2016;34(2):287-301. doi:10.1016/j.anclin.2016.01.005
15. Katz J, Weinrib A, Fashler SR, et al. The Toronto General Hospital Transitional Pain Service: development and implementation of a multidisciplinary program to prevent chronic postsurgical pain. J Pain Res. 2015;8:695-702. doi:10.2147/jpr.s91924
16. Tiippana E, Hamunen K, Heiskanen T, Nieminen T, Kalso E, Kontinen VK. New approach for treatment of prolonged postoperative pain: APS Out-Patient Clinic. Scand J Pain. 2016;12(1):19-24. doi:10.1016/j.sjpain.2016.02.008
17. Katz J, Weinrib AZ, Clarke H. Chronic postsurgical pain: from risk factor identification to multidisciplinary management at the Toronto General Hospital Transitional Pain Service. Can J Pain. 2019;3(2):49-58. doi:10.1080/24740527.2019.1574537
18. Sullivan MJ, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess. 1995;7(4):524-532. doi:10.1037/1040-3590.7.4.524
19. HealthMeasures. Intro to PROMIS. https://www.healthmeasures.net/explore-measurement-systems/promis. Accessed September 28, 2020.
1. Ilfeld BM, Madison SJ, Suresh PJ. Persistent postmastectomy pain and pain-related physical and emotional functioning with and without a continuous paravertebral nerve block: a prospective 1-year follow-up assessment of a randomized, triple-masked, placebo-controlled study. Ann Surg Oncol. 2015;22(6):2017-2025. doi:10.1245/s10434-014-4248-7
2. Richebé P, Capdevila X, Rivat C. Persistent postsurgical pain. Anesthesiology. 2018;129(3):590-607. doi:10.1097/aln.0000000000002238
3. Glare P, Aubrey KR, Myles PS. Transition from acute to chronic pain after surgery. Lancet. 2019;393(10180):1537-1546. doi:10.1016/s0140-6736(19)30352-6
4. Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surgery. 2017;152(6):e170504-e170504. doi:10.1001/jamasurg.2017.0504
5. Swenson CW, Kamdar NS, Seiler K, Morgan DM, Lin P, As-Sanie S. Definition development and prevalence of new persistent opioid use following hysterectomy. Am J Obstet Gynecol. 2018;219(5):486.e1-486.e7. doi:10.1016/j.ajog.2018.06.010
6. Bartels K, Fernandez-Bustamante A, McWilliams SK, Hopfer CJ, Mikulich-Gilbertson SK. Long-term opioid use after inpatient surgery - a retrospective cohort study. Drug Alcohol Depend. 2018;187:61-65. doi:10.1016/j.drugalcdep.2018.02.013
7. Bedard N, DeMik D, Dowdle S, Callaghan J. Trends and risk factors for prolonged opioid use after unicompartmental knee arthroplasty. Bone Joint J. 2018;100-B(1)(suppl A):62-67. doi:10.1302/0301-620x.100b1.bjj-2017-0547.r1
8. Politzer CS, Kildow BJ, Goltz DE, Green CL, Bolognesi MP, Seyler T. Trends in opioid utilization before and after total knee arthroplasty. J Arthroplasty. 2018;33(7S):S147-S153.e1. doi:10.1016/j.arth.2017.10.060
9. Mariano ER, Walters TL, Kim ET, Kain ZN. Why the perioperative surgical home makes sense for Veterans Affairs health care. Anesth Analg. 2015;120(5):1163-1166. doi:10.1213/ane.0000000000000712
10. Walters TL, Howard SK, Kou A, et al. Design and implementation of a perioperative surgical home at a Veterans Affairs hospital. Semin Cardiothorac Vasc Anesth. 2016;20(2):133-140. doi:10.1177/1089253215607066
11. Walters TL, Mariano ER, Clark DJ. Perioperative surgical home and the integral role of pain medicine. Pain Med. 2015;16(9):1666-1672. doi:10.1111/pme.12796
12. Vetter TR, Kain ZN. Role of the perioperative surgical home in optimizing the perioperative use of opioids. Anesth Analg. 2017;125(5):1653-1657. doi:10.1213/ane.0000000000002280
13. Shafer SL. Anesthesia & Analgesia’s 2015 collection on the perioperative surgical home. Anesth Analg. 2015;120(5):966-967. doi:10.1213/ane.0000000000000696
14. Wenzel JT, Schwenk ES, Baratta JL, Viscusi ER. Managing opioid-tolerant patients in the perioperative surgical home. Anesthesiol Clin. 2016;34(2):287-301. doi:10.1016/j.anclin.2016.01.005
15. Katz J, Weinrib A, Fashler SR, et al. The Toronto General Hospital Transitional Pain Service: development and implementation of a multidisciplinary program to prevent chronic postsurgical pain. J Pain Res. 2015;8:695-702. doi:10.2147/jpr.s91924
16. Tiippana E, Hamunen K, Heiskanen T, Nieminen T, Kalso E, Kontinen VK. New approach for treatment of prolonged postoperative pain: APS Out-Patient Clinic. Scand J Pain. 2016;12(1):19-24. doi:10.1016/j.sjpain.2016.02.008
17. Katz J, Weinrib AZ, Clarke H. Chronic postsurgical pain: from risk factor identification to multidisciplinary management at the Toronto General Hospital Transitional Pain Service. Can J Pain. 2019;3(2):49-58. doi:10.1080/24740527.2019.1574537
18. Sullivan MJ, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess. 1995;7(4):524-532. doi:10.1037/1040-3590.7.4.524
19. HealthMeasures. Intro to PROMIS. https://www.healthmeasures.net/explore-measurement-systems/promis. Accessed September 28, 2020.
VA Connecticut Friendly Phone Call Program (FPCP): A Collaborative, Team-based Approach to Alleviating Loneliness related to Social Isolation in Veterans with Cancer During the Covid-19 Pandemic
BACKGROUND: At VACHS, we identified oncology patients at risk for loneliness subsequent to COVID-19 social distancing recommendations. Cancer patients are older, more physically frail and immune compromised, making them high-risk for complications related to covid-19 infection. Given this risk, social isolation might extend significantly beyond the initial period of lockdown for oncology patients. To protect this vulnerable population, we shifted from face-to-face visits to telemedicine. A workgroup formed to develop an intervention to support Veterans at-risk for prolonged isolation. Social isolation is a well-established risk factor for poor health and mortality (Caccioppo & Hawkley, 2003). For individuals with cancer, social isolation has been linked to poorer survival (Reynolds & Kaplan, 1990; Hislop, Waxler, Coldman, Elwood, and Kan, 1987). Research in woman with ovarian cancer suggests that limited social support is associated with higher angiogenic cytokine levels (Costanzo et al., 2005). Thus, bolstering social support for individuals with cancer is important for both psychological wellbeing as well as possibly for cancer outcomes.
METHODS: A Friendly Phone Call Program (FPCP) was developed in collaboration by the Cancer Coordinator, Health Psychologist, Recreation Therapist and Social Worker to support Veterans who live alone, are elderly, or are physically frail throughout the COVID- 19 pandemic. Oncology providers were educated to identify socially isolated Veterans at-risk for distress during their phone appointments. The FPCP was notified of referrals by alert in CPRS. Charts were reviewed and triaged to the appropriate team member (i.e., psychology, recreation therapy, or social work). Follow-up phone calls were made utilizing a script to introduce FPCP and educate patients on available psychosocial services. In concert with FPCP, recreation therapy groups were offered by phone.
RESULTS: From 4/1/20 to 6/30/20, oncology providers identified 45 patients with psychosocial needs related to social isolation. 23 received outreach from Recreation Therapy, 9 by mental health, 8 by Social Work and 3 get weekly check-in calls from the Cancer Coordinator. 2 patients have since passed away.
CONCLUSIONS: VACHS developed a collaborative, multidisciplinary intervention that identifies patients at risk for psychosocial distress related to loneliness and provides ongoing, individualized, emotional support using existing staff and technology that is replicable in any VA setting.
BACKGROUND: At VACHS, we identified oncology patients at risk for loneliness subsequent to COVID-19 social distancing recommendations. Cancer patients are older, more physically frail and immune compromised, making them high-risk for complications related to covid-19 infection. Given this risk, social isolation might extend significantly beyond the initial period of lockdown for oncology patients. To protect this vulnerable population, we shifted from face-to-face visits to telemedicine. A workgroup formed to develop an intervention to support Veterans at-risk for prolonged isolation. Social isolation is a well-established risk factor for poor health and mortality (Caccioppo & Hawkley, 2003). For individuals with cancer, social isolation has been linked to poorer survival (Reynolds & Kaplan, 1990; Hislop, Waxler, Coldman, Elwood, and Kan, 1987). Research in woman with ovarian cancer suggests that limited social support is associated with higher angiogenic cytokine levels (Costanzo et al., 2005). Thus, bolstering social support for individuals with cancer is important for both psychological wellbeing as well as possibly for cancer outcomes.
METHODS: A Friendly Phone Call Program (FPCP) was developed in collaboration by the Cancer Coordinator, Health Psychologist, Recreation Therapist and Social Worker to support Veterans who live alone, are elderly, or are physically frail throughout the COVID- 19 pandemic. Oncology providers were educated to identify socially isolated Veterans at-risk for distress during their phone appointments. The FPCP was notified of referrals by alert in CPRS. Charts were reviewed and triaged to the appropriate team member (i.e., psychology, recreation therapy, or social work). Follow-up phone calls were made utilizing a script to introduce FPCP and educate patients on available psychosocial services. In concert with FPCP, recreation therapy groups were offered by phone.
RESULTS: From 4/1/20 to 6/30/20, oncology providers identified 45 patients with psychosocial needs related to social isolation. 23 received outreach from Recreation Therapy, 9 by mental health, 8 by Social Work and 3 get weekly check-in calls from the Cancer Coordinator. 2 patients have since passed away.
CONCLUSIONS: VACHS developed a collaborative, multidisciplinary intervention that identifies patients at risk for psychosocial distress related to loneliness and provides ongoing, individualized, emotional support using existing staff and technology that is replicable in any VA setting.
BACKGROUND: At VACHS, we identified oncology patients at risk for loneliness subsequent to COVID-19 social distancing recommendations. Cancer patients are older, more physically frail and immune compromised, making them high-risk for complications related to covid-19 infection. Given this risk, social isolation might extend significantly beyond the initial period of lockdown for oncology patients. To protect this vulnerable population, we shifted from face-to-face visits to telemedicine. A workgroup formed to develop an intervention to support Veterans at-risk for prolonged isolation. Social isolation is a well-established risk factor for poor health and mortality (Caccioppo & Hawkley, 2003). For individuals with cancer, social isolation has been linked to poorer survival (Reynolds & Kaplan, 1990; Hislop, Waxler, Coldman, Elwood, and Kan, 1987). Research in woman with ovarian cancer suggests that limited social support is associated with higher angiogenic cytokine levels (Costanzo et al., 2005). Thus, bolstering social support for individuals with cancer is important for both psychological wellbeing as well as possibly for cancer outcomes.
METHODS: A Friendly Phone Call Program (FPCP) was developed in collaboration by the Cancer Coordinator, Health Psychologist, Recreation Therapist and Social Worker to support Veterans who live alone, are elderly, or are physically frail throughout the COVID- 19 pandemic. Oncology providers were educated to identify socially isolated Veterans at-risk for distress during their phone appointments. The FPCP was notified of referrals by alert in CPRS. Charts were reviewed and triaged to the appropriate team member (i.e., psychology, recreation therapy, or social work). Follow-up phone calls were made utilizing a script to introduce FPCP and educate patients on available psychosocial services. In concert with FPCP, recreation therapy groups were offered by phone.
RESULTS: From 4/1/20 to 6/30/20, oncology providers identified 45 patients with psychosocial needs related to social isolation. 23 received outreach from Recreation Therapy, 9 by mental health, 8 by Social Work and 3 get weekly check-in calls from the Cancer Coordinator. 2 patients have since passed away.
CONCLUSIONS: VACHS developed a collaborative, multidisciplinary intervention that identifies patients at risk for psychosocial distress related to loneliness and provides ongoing, individualized, emotional support using existing staff and technology that is replicable in any VA setting.
Short Story Clubs to Decrease Burnout
Burnout is common in hematology/oncology practice where work pressure is high, patients are complex, and outcomes are variable. We hypothesized that a short story club could be helpful to improve community, humanism, and transcendence; and thereby to decrease burnout. Most of the potential participants indicated little time for preparation and we, therefore, chose short stories rather than books as reading material. The meetings began in April 2019 and continued until April 2020 when they were suspended for the COVID-19 epidemic. Participants included oncologists (6), oncology fellows (2), psychologist (1), social workers (2), research writer (1) and, student (1). Of these, 7 were females and 6 were males; 4 in senior and 9 in junior positions. Country of origin of participants was USA (6), India (3), Syria (2), Pakistan (1) and, Poland (1). Meetings were held every two months, each time with different stories, focus, themes, and points of view. Readings included classical stories, modern stories, and personal essays, from the eyes of other oncologists, country doctor, patients, nurses, or students. Stories included “The Doctor” by Chekhov, “The Country Doctor” by Kafka, “Three Questions” by Tolstoy, “Elephant Hills” and “Indian Camp” each by Hemingway, “Interpreter of Maladies” by Lahiri, “Get your Own Fatal Disease” by Yalom, “Caves of Lascaux” by Karmel, “The Plagiarist” by Seamon and three essays on “undying,” end-of-life and love. Themes included falling in love with a patient, empathy, loneliness, burnout, communication, helplessness, and end-of-life issues. Discussions lasted two hours and promoted a sense of belonging and community; sharing of feelings and concerns; and transcendence of everyday burdens. Attendance was more than 80% at each meeting and all participants indicated an interest in continuing the club for the foreseeable future. Short story clubs may be one way to overcome or prevent burnout in oncology. Further quantitative and qualitative studies are needed.
Burnout is common in hematology/oncology practice where work pressure is high, patients are complex, and outcomes are variable. We hypothesized that a short story club could be helpful to improve community, humanism, and transcendence; and thereby to decrease burnout. Most of the potential participants indicated little time for preparation and we, therefore, chose short stories rather than books as reading material. The meetings began in April 2019 and continued until April 2020 when they were suspended for the COVID-19 epidemic. Participants included oncologists (6), oncology fellows (2), psychologist (1), social workers (2), research writer (1) and, student (1). Of these, 7 were females and 6 were males; 4 in senior and 9 in junior positions. Country of origin of participants was USA (6), India (3), Syria (2), Pakistan (1) and, Poland (1). Meetings were held every two months, each time with different stories, focus, themes, and points of view. Readings included classical stories, modern stories, and personal essays, from the eyes of other oncologists, country doctor, patients, nurses, or students. Stories included “The Doctor” by Chekhov, “The Country Doctor” by Kafka, “Three Questions” by Tolstoy, “Elephant Hills” and “Indian Camp” each by Hemingway, “Interpreter of Maladies” by Lahiri, “Get your Own Fatal Disease” by Yalom, “Caves of Lascaux” by Karmel, “The Plagiarist” by Seamon and three essays on “undying,” end-of-life and love. Themes included falling in love with a patient, empathy, loneliness, burnout, communication, helplessness, and end-of-life issues. Discussions lasted two hours and promoted a sense of belonging and community; sharing of feelings and concerns; and transcendence of everyday burdens. Attendance was more than 80% at each meeting and all participants indicated an interest in continuing the club for the foreseeable future. Short story clubs may be one way to overcome or prevent burnout in oncology. Further quantitative and qualitative studies are needed.
Burnout is common in hematology/oncology practice where work pressure is high, patients are complex, and outcomes are variable. We hypothesized that a short story club could be helpful to improve community, humanism, and transcendence; and thereby to decrease burnout. Most of the potential participants indicated little time for preparation and we, therefore, chose short stories rather than books as reading material. The meetings began in April 2019 and continued until April 2020 when they were suspended for the COVID-19 epidemic. Participants included oncologists (6), oncology fellows (2), psychologist (1), social workers (2), research writer (1) and, student (1). Of these, 7 were females and 6 were males; 4 in senior and 9 in junior positions. Country of origin of participants was USA (6), India (3), Syria (2), Pakistan (1) and, Poland (1). Meetings were held every two months, each time with different stories, focus, themes, and points of view. Readings included classical stories, modern stories, and personal essays, from the eyes of other oncologists, country doctor, patients, nurses, or students. Stories included “The Doctor” by Chekhov, “The Country Doctor” by Kafka, “Three Questions” by Tolstoy, “Elephant Hills” and “Indian Camp” each by Hemingway, “Interpreter of Maladies” by Lahiri, “Get your Own Fatal Disease” by Yalom, “Caves of Lascaux” by Karmel, “The Plagiarist” by Seamon and three essays on “undying,” end-of-life and love. Themes included falling in love with a patient, empathy, loneliness, burnout, communication, helplessness, and end-of-life issues. Discussions lasted two hours and promoted a sense of belonging and community; sharing of feelings and concerns; and transcendence of everyday burdens. Attendance was more than 80% at each meeting and all participants indicated an interest in continuing the club for the foreseeable future. Short story clubs may be one way to overcome or prevent burnout in oncology. Further quantitative and qualitative studies are needed.
Central Texas Veterans Health Care System’s Experiences With Hematology Oncology Clinical Trials
BACKGROUND: Availability of clinical trials for veterans is limited and more clinical trials are needed. Central Texas Veterans Health Care System (CTVHCS) has been actively involved with hematologic oncologic clinical trials over the last 10 years. This poster describes the number and types of hematology/oncology clinical trials that are either active or completed, and the processes of opening clinical trials, identifying patients, and trial management.
METHODS: Locating clinical trials is key to veteran enrollment into active trials and is accomplished through networking at medical meetings and VA work groups. Developing a clinical trial program requires working closely with the research department/foundations and becoming comfortable with the IRB oversight process. Conduct of a clinical trial is a team effort, with individual members having delegated responsibilities of patient care, data collection, and adverse effect reporting to the sponsors and IRB. The CTVHCS Oncology Section has been active in recruiting and enrolling veterans in clinical trials for treatment of many hematologic malignancies and solid tumors.
RESULTS: At the time of this presentation, 49 veterans have been successfully enrolled in 1 of 9 hematology/ oncology clinical trials ranging from phase Ib to phase III from 2011-2020. Advantages to opening clinical trials include academic scholarship, authorship in publications, generating revenue and most importantly to provide state of the art treatment for our cancer patients. We have been able to effectively accrue/enroll patients into clinical trials through a collaborative effort between the research department and our oncology department by identifying open clinical trials that fit our unique patient population and having a team of providers aiding in the management and care of these enrolled veterans.
BACKGROUND: Availability of clinical trials for veterans is limited and more clinical trials are needed. Central Texas Veterans Health Care System (CTVHCS) has been actively involved with hematologic oncologic clinical trials over the last 10 years. This poster describes the number and types of hematology/oncology clinical trials that are either active or completed, and the processes of opening clinical trials, identifying patients, and trial management.
METHODS: Locating clinical trials is key to veteran enrollment into active trials and is accomplished through networking at medical meetings and VA work groups. Developing a clinical trial program requires working closely with the research department/foundations and becoming comfortable with the IRB oversight process. Conduct of a clinical trial is a team effort, with individual members having delegated responsibilities of patient care, data collection, and adverse effect reporting to the sponsors and IRB. The CTVHCS Oncology Section has been active in recruiting and enrolling veterans in clinical trials for treatment of many hematologic malignancies and solid tumors.
RESULTS: At the time of this presentation, 49 veterans have been successfully enrolled in 1 of 9 hematology/ oncology clinical trials ranging from phase Ib to phase III from 2011-2020. Advantages to opening clinical trials include academic scholarship, authorship in publications, generating revenue and most importantly to provide state of the art treatment for our cancer patients. We have been able to effectively accrue/enroll patients into clinical trials through a collaborative effort between the research department and our oncology department by identifying open clinical trials that fit our unique patient population and having a team of providers aiding in the management and care of these enrolled veterans.
BACKGROUND: Availability of clinical trials for veterans is limited and more clinical trials are needed. Central Texas Veterans Health Care System (CTVHCS) has been actively involved with hematologic oncologic clinical trials over the last 10 years. This poster describes the number and types of hematology/oncology clinical trials that are either active or completed, and the processes of opening clinical trials, identifying patients, and trial management.
METHODS: Locating clinical trials is key to veteran enrollment into active trials and is accomplished through networking at medical meetings and VA work groups. Developing a clinical trial program requires working closely with the research department/foundations and becoming comfortable with the IRB oversight process. Conduct of a clinical trial is a team effort, with individual members having delegated responsibilities of patient care, data collection, and adverse effect reporting to the sponsors and IRB. The CTVHCS Oncology Section has been active in recruiting and enrolling veterans in clinical trials for treatment of many hematologic malignancies and solid tumors.
RESULTS: At the time of this presentation, 49 veterans have been successfully enrolled in 1 of 9 hematology/ oncology clinical trials ranging from phase Ib to phase III from 2011-2020. Advantages to opening clinical trials include academic scholarship, authorship in publications, generating revenue and most importantly to provide state of the art treatment for our cancer patients. We have been able to effectively accrue/enroll patients into clinical trials through a collaborative effort between the research department and our oncology department by identifying open clinical trials that fit our unique patient population and having a team of providers aiding in the management and care of these enrolled veterans.
Implementation of a Protocol to Manage Patients at Risk for Hospitalization Due to an Ambulatory Care Sensitive Condition
Hospitalizations related to ambulatory care sensitive conditions (ACSCs) are potentially avoidable if timely and effective care is provided to the patient. The Agency of Healthcare Research and Quality has identified type 2 diabetes mellitus (T2DM), chronic obstructive pulmonary disease (COPD), hypertension, congestive heart failure (CHF), urinary tract infections (UTIs), asthma, dehydration, bacterial pneumonia, angina without an inhospital procedure, and perforated appendix as ACSCs.1,2 Identifying patients with ACSCs who are at risk for hospitalization is a potential measure to enhance primary care delivery and reduce preventable hospitalizations
The US Department of Veterans Affairs (VA) Clinical Pharmacy Practice Office implemented a guidance statement describing the role and impact of a clinical pharmacy specialist (CPS) in managing ACSCs.1 Within the Veterans Health Administration, the CPS may function under a scope of practice within their area of expertise with the ability to prescribe medications, place consults, and order laboratory tests and additional referrals as appropriate. As hospitalizations related to ACSCs are potentially preventable with effective primary care, the CPS can play an essential primary care role to implement interventions targeted at reducing these hospitalizations.
At the William S. Middleton Memorial Veterans Hospital, in Madison, Wisconsin, multiple transitions of care and postdischarge services have been established to capture those patients who are at a high risk of rehospitalization. Studies have been completed regarding implementation of intensive case management programs for high-risk patients.3 Currently though, no standardized process or protocol exists that can identify and optimize primary care for patients with ACSCs who have been hospitalized but are predicted to be at low risk for rehospitalization. Although these patients may not require intensive case management like that of those at high risk, improvements can be made to optimize clinical resources, education, and patient self-monitoring to mitigate risk for hospitalization or rehospitalization. Therefore, this project aimed to evaluate the implementation of offering further referrals and care for patients who have been hospitalized but are considered low risk for hospitalization from ACSCs.
Methods
This quality improvement project to offer further referrals and care to patients considered low risk for hospitalization was implemented to enhance ambulatory-care provided services. All patients identified as being a low risk for hospitalization via a VA dashboard from July through September 2018 were included. Patients were identified based on age, chronic diseases, gender, and other patient-specific factors predetermined by the VA dashboard algorithm. Patients receiving hospice or palliative care and those no longer receiving primary care through the facility were excluded.
A pharmacy resident conducted a baseline chart review using a standardized template in the computerized patient record system (CPRS) to identify additional referrals or interventions a patient may benefit from based on any identified ACSC. Potential referral options included a CPS or nurse care manager disease management, whole health/wellness, educational classes, home monitoring equipment, specialty clinics, nutrition, cardiac or pulmonary rehabilitation, social work, and mental health. A pharmacy resident or the patient aligned care team (PACT) CPS reviewed the identified referrals with PACT members at interdisciplinary team meetings and determined which referrals to offer the patient. The pharmacy resident or designated PACT member reached out to the patient via telephone or during a clinic visit to offer and enter the referrals. If the patient agreed to any referrals, a chart review was conducted 3 months later to determine the percentage of initially agreed-upon referrals that the patient completed. Additionally, the number of emergency department (ED) visits and hospitalizations related to an ACSC at 3 months was collected.
Feasibility was assessed to evaluate potential service implementation and was measured by the time in minutes to complete the baseline chart review, time in minutes to offer referrals to the patient, and proportion of referrals that were completed at 3 months.4 As this quality improvement project was undertaken for programmatic evaluation, the University of Wisconsin-Madison Health Sciences Institutional Review Board determined that this project did not meet the federal definition of research and therefore review was not required. Data were analyzed using descriptive statistics.
Results
A total of 78 veterans who had ≥ 1 ACSC-related hospitalization in the past year and who were categorized as low risk were identified, and 69 veterans were reviewed. Nine patients were not included based on hospice care and no longer receiving primary care through the facility. Eight patients were found to have optimized care with no further action warranted after review. Based on their assigned PACT, there was a range of 0 to 5 patients identified per team. Fifty-one patients were contacted, and 37 accepted ≥ 1 referral. Most of the patients were white and male (Table). The most common ACSCs were hypertension (68%), COPD (46%), and T2DM (30%); additional ACSCs included angina (18%), pneumonia (15%), UTIs (10%), CHF (6%), and asthma, dehydration, and perforated appendix (1.5% for each). Any ACSC listed as a diagnosis for a patient was included, regardless of whether it was related to a hospitalization. Most referrals were offered by pharmacists (pharmacy resident, 41%; CPS, 29%), followed by the nurse care manager (18%) and the primary care provider (12%). One patient passed away related to heart failure complications prior to being contacted to offer additional referrals. Of the 9 patients that were unable to be contacted, 4 did not respond to 3 phone call attempts and 5 had no documentation of referrals being offered after the initial chart review and recommendation was completed.
Most of the initially accepted referrals (n = 68) were for CPS disease management, whole health/wellness, and educational classes (Figure). Of the 28 initially accepted referrals for CPS disease management, most were for COPD (10) and hypertension (8), followed by neuropathic pain (3), vitamin D deficiency (3), hyperlipidemia (2), and T2DM (2). At 3 months, all referrals were completed except for 1 hypertension, 1 vitamin D deficiency, and 2 hyperlipidemia referrals. There were 6 COPD, 4 T2DM self-management, and 1 chronic pain class referrals made with 3 COPD and 1 T2DM referrals completed at 3 months. Two tobacco treatment and 2 palliative care referrals were specialty referrals accepted by patients with 1 palliative care referral completed at 3 months.
In terms of feasibility, the chart review took an average (SD) of 13 (4) minutes, and contacting the patient to offer referrals took an average of 8 (5) minutes. Most of the accepted referrals were completed by 3 months (42/68, 62%).
Comparing the 3 months prior to and the 3 months after offering referrals, there was a cumulative quantitative decrease in the number of ED visits (5 to 1) and hospitalizations (11 to 5). The 1 ED visit was for a patient who was unable to be contacted to offer additional referrals as were 4 of the hospitalizations. One of the hospitalizations was for a patient who was deemed to have optimized care with no additional referrals necessary.
Discussion
Evaluation of the review and referral process for patients at low risk for hospitalization from an ACSC was a proactive approach toward optimizing primary care for veterans, and the process increased patient access to education and primary care. There was a high initial patient acceptance rate of referrals and a high completion rate when offered by PACT members. Based on the number of identified patients, the time spent completing chart reviews and contacting patients to offer referrals for each PACT CPS and team was feasible to conduct.
As there were 69 eligible patients identified over a 3-month period for a single VA facility, including all community-based outpatient clinics serving an estimated 130,000 veterans, the additional time and workload for an individual PACT to reach out to these patients is minimal. Completing the review and outreach process for an average of 21 minutes per patient for at most 5 patients per primary care provider team is feasible to complete during the recommended 4 hours of weekly CPS population health management responsibilities.
Limitations
Several limitations were identified with the implementation of the project. A variety of PACT members completed initial outreach to veterans regarding additional referrals, which may have resulted in a lack of consistency in the approach and discussion of offering referrals to patients. Although there may be a difference in how the team members made referral offers to patients and therefore varying acceptance rates by patients, the process was thought to be more generalizable to the PACT approach for providing care in the VA. In addition, the time to contact patients to offer referrals was not always documented in the electronic health record, making the documented time an estimate. Given that patients identified were managed by a variety of PACT members, there were differences noted among PACTs in terms of acceptability of offering referrals to patients.
While there was a decrease noted in ED visits and hospitalizations when comparing 3 months before and afterward, additional data are needed to provide further insight into this relationship. As the patients identified were at low risk for hospitalization from an ACSC and had 1 or 2 hospitalizations within the year prior, additional time is warranted to compare 12-month ED visits and hospitalization rates postintervention. Finally, these findings may be limited in generalizability to other health care systems as the project was conducted among a specific, veteran patient population with PACT CPSs practicing independently within an established broad scope of practice.
Future Directions
Future directions include incorporating the review and referral process into the PACT CPS population health management responsibilities as a way to use all PACT members to enhance primary care delivered to veterans. To further elucidate the relationship between the referral process and hospitalization rates, a longer data collection period is needed.
Conclusions
Identifying patients at risk for hospitalization from an ACSC via a review and referral process by using the VA PACT structure and team members was feasible and led to increased patient access to primary care and additional services. The PACT CPS would benefit from using a similar approach for population health management for low risk for hospitalization patients or other identified chronic conditions.
Acknowledgments
Presented at the Wisconsin Pharmacy Residency Conference at the Pharmacy Society of Wisconsin Educational Conference April 10, 2019, in Madison, Wisconsin.
1. US Department of Veterans Affairs, Veterans Health Administration, Pharmacy Benefits Management Service, Clinical Pharmacy Practice Office. Clinical pharmacy specialist (CPS) role in management of ambulatory care sensitive conditions (ACSC). [Nonpublic source.]
2. US Department of Health and Human Services, Agency for Healthcare Research and Quality. Guide to prevention quality indicators: hospital admission for ambulatory care sensitive conditions. https://www.ahrq.gov/downloads/pub/ahrqqi/pqiguide.pdf. Revised April 17, 2002. Accessed July 16, 2020.
3. Yoon J, Chang E, Rubenstein L, et al. Impact of primary care intensive management on high-risk veterans’ costs and utilization. Ann Intern Med. 2018;168(12):846-854. doi:10.7326/M17-3039
4. Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38:65-76. doi:10.1007/s10488-010-0319-7
Hospitalizations related to ambulatory care sensitive conditions (ACSCs) are potentially avoidable if timely and effective care is provided to the patient. The Agency of Healthcare Research and Quality has identified type 2 diabetes mellitus (T2DM), chronic obstructive pulmonary disease (COPD), hypertension, congestive heart failure (CHF), urinary tract infections (UTIs), asthma, dehydration, bacterial pneumonia, angina without an inhospital procedure, and perforated appendix as ACSCs.1,2 Identifying patients with ACSCs who are at risk for hospitalization is a potential measure to enhance primary care delivery and reduce preventable hospitalizations
The US Department of Veterans Affairs (VA) Clinical Pharmacy Practice Office implemented a guidance statement describing the role and impact of a clinical pharmacy specialist (CPS) in managing ACSCs.1 Within the Veterans Health Administration, the CPS may function under a scope of practice within their area of expertise with the ability to prescribe medications, place consults, and order laboratory tests and additional referrals as appropriate. As hospitalizations related to ACSCs are potentially preventable with effective primary care, the CPS can play an essential primary care role to implement interventions targeted at reducing these hospitalizations.
At the William S. Middleton Memorial Veterans Hospital, in Madison, Wisconsin, multiple transitions of care and postdischarge services have been established to capture those patients who are at a high risk of rehospitalization. Studies have been completed regarding implementation of intensive case management programs for high-risk patients.3 Currently though, no standardized process or protocol exists that can identify and optimize primary care for patients with ACSCs who have been hospitalized but are predicted to be at low risk for rehospitalization. Although these patients may not require intensive case management like that of those at high risk, improvements can be made to optimize clinical resources, education, and patient self-monitoring to mitigate risk for hospitalization or rehospitalization. Therefore, this project aimed to evaluate the implementation of offering further referrals and care for patients who have been hospitalized but are considered low risk for hospitalization from ACSCs.
Methods
This quality improvement project to offer further referrals and care to patients considered low risk for hospitalization was implemented to enhance ambulatory-care provided services. All patients identified as being a low risk for hospitalization via a VA dashboard from July through September 2018 were included. Patients were identified based on age, chronic diseases, gender, and other patient-specific factors predetermined by the VA dashboard algorithm. Patients receiving hospice or palliative care and those no longer receiving primary care through the facility were excluded.
A pharmacy resident conducted a baseline chart review using a standardized template in the computerized patient record system (CPRS) to identify additional referrals or interventions a patient may benefit from based on any identified ACSC. Potential referral options included a CPS or nurse care manager disease management, whole health/wellness, educational classes, home monitoring equipment, specialty clinics, nutrition, cardiac or pulmonary rehabilitation, social work, and mental health. A pharmacy resident or the patient aligned care team (PACT) CPS reviewed the identified referrals with PACT members at interdisciplinary team meetings and determined which referrals to offer the patient. The pharmacy resident or designated PACT member reached out to the patient via telephone or during a clinic visit to offer and enter the referrals. If the patient agreed to any referrals, a chart review was conducted 3 months later to determine the percentage of initially agreed-upon referrals that the patient completed. Additionally, the number of emergency department (ED) visits and hospitalizations related to an ACSC at 3 months was collected.
Feasibility was assessed to evaluate potential service implementation and was measured by the time in minutes to complete the baseline chart review, time in minutes to offer referrals to the patient, and proportion of referrals that were completed at 3 months.4 As this quality improvement project was undertaken for programmatic evaluation, the University of Wisconsin-Madison Health Sciences Institutional Review Board determined that this project did not meet the federal definition of research and therefore review was not required. Data were analyzed using descriptive statistics.
Results
A total of 78 veterans who had ≥ 1 ACSC-related hospitalization in the past year and who were categorized as low risk were identified, and 69 veterans were reviewed. Nine patients were not included based on hospice care and no longer receiving primary care through the facility. Eight patients were found to have optimized care with no further action warranted after review. Based on their assigned PACT, there was a range of 0 to 5 patients identified per team. Fifty-one patients were contacted, and 37 accepted ≥ 1 referral. Most of the patients were white and male (Table). The most common ACSCs were hypertension (68%), COPD (46%), and T2DM (30%); additional ACSCs included angina (18%), pneumonia (15%), UTIs (10%), CHF (6%), and asthma, dehydration, and perforated appendix (1.5% for each). Any ACSC listed as a diagnosis for a patient was included, regardless of whether it was related to a hospitalization. Most referrals were offered by pharmacists (pharmacy resident, 41%; CPS, 29%), followed by the nurse care manager (18%) and the primary care provider (12%). One patient passed away related to heart failure complications prior to being contacted to offer additional referrals. Of the 9 patients that were unable to be contacted, 4 did not respond to 3 phone call attempts and 5 had no documentation of referrals being offered after the initial chart review and recommendation was completed.
Most of the initially accepted referrals (n = 68) were for CPS disease management, whole health/wellness, and educational classes (Figure). Of the 28 initially accepted referrals for CPS disease management, most were for COPD (10) and hypertension (8), followed by neuropathic pain (3), vitamin D deficiency (3), hyperlipidemia (2), and T2DM (2). At 3 months, all referrals were completed except for 1 hypertension, 1 vitamin D deficiency, and 2 hyperlipidemia referrals. There were 6 COPD, 4 T2DM self-management, and 1 chronic pain class referrals made with 3 COPD and 1 T2DM referrals completed at 3 months. Two tobacco treatment and 2 palliative care referrals were specialty referrals accepted by patients with 1 palliative care referral completed at 3 months.
In terms of feasibility, the chart review took an average (SD) of 13 (4) minutes, and contacting the patient to offer referrals took an average of 8 (5) minutes. Most of the accepted referrals were completed by 3 months (42/68, 62%).
Comparing the 3 months prior to and the 3 months after offering referrals, there was a cumulative quantitative decrease in the number of ED visits (5 to 1) and hospitalizations (11 to 5). The 1 ED visit was for a patient who was unable to be contacted to offer additional referrals as were 4 of the hospitalizations. One of the hospitalizations was for a patient who was deemed to have optimized care with no additional referrals necessary.
Discussion
Evaluation of the review and referral process for patients at low risk for hospitalization from an ACSC was a proactive approach toward optimizing primary care for veterans, and the process increased patient access to education and primary care. There was a high initial patient acceptance rate of referrals and a high completion rate when offered by PACT members. Based on the number of identified patients, the time spent completing chart reviews and contacting patients to offer referrals for each PACT CPS and team was feasible to conduct.
As there were 69 eligible patients identified over a 3-month period for a single VA facility, including all community-based outpatient clinics serving an estimated 130,000 veterans, the additional time and workload for an individual PACT to reach out to these patients is minimal. Completing the review and outreach process for an average of 21 minutes per patient for at most 5 patients per primary care provider team is feasible to complete during the recommended 4 hours of weekly CPS population health management responsibilities.
Limitations
Several limitations were identified with the implementation of the project. A variety of PACT members completed initial outreach to veterans regarding additional referrals, which may have resulted in a lack of consistency in the approach and discussion of offering referrals to patients. Although there may be a difference in how the team members made referral offers to patients and therefore varying acceptance rates by patients, the process was thought to be more generalizable to the PACT approach for providing care in the VA. In addition, the time to contact patients to offer referrals was not always documented in the electronic health record, making the documented time an estimate. Given that patients identified were managed by a variety of PACT members, there were differences noted among PACTs in terms of acceptability of offering referrals to patients.
While there was a decrease noted in ED visits and hospitalizations when comparing 3 months before and afterward, additional data are needed to provide further insight into this relationship. As the patients identified were at low risk for hospitalization from an ACSC and had 1 or 2 hospitalizations within the year prior, additional time is warranted to compare 12-month ED visits and hospitalization rates postintervention. Finally, these findings may be limited in generalizability to other health care systems as the project was conducted among a specific, veteran patient population with PACT CPSs practicing independently within an established broad scope of practice.
Future Directions
Future directions include incorporating the review and referral process into the PACT CPS population health management responsibilities as a way to use all PACT members to enhance primary care delivered to veterans. To further elucidate the relationship between the referral process and hospitalization rates, a longer data collection period is needed.
Conclusions
Identifying patients at risk for hospitalization from an ACSC via a review and referral process by using the VA PACT structure and team members was feasible and led to increased patient access to primary care and additional services. The PACT CPS would benefit from using a similar approach for population health management for low risk for hospitalization patients or other identified chronic conditions.
Acknowledgments
Presented at the Wisconsin Pharmacy Residency Conference at the Pharmacy Society of Wisconsin Educational Conference April 10, 2019, in Madison, Wisconsin.
Hospitalizations related to ambulatory care sensitive conditions (ACSCs) are potentially avoidable if timely and effective care is provided to the patient. The Agency of Healthcare Research and Quality has identified type 2 diabetes mellitus (T2DM), chronic obstructive pulmonary disease (COPD), hypertension, congestive heart failure (CHF), urinary tract infections (UTIs), asthma, dehydration, bacterial pneumonia, angina without an inhospital procedure, and perforated appendix as ACSCs.1,2 Identifying patients with ACSCs who are at risk for hospitalization is a potential measure to enhance primary care delivery and reduce preventable hospitalizations
The US Department of Veterans Affairs (VA) Clinical Pharmacy Practice Office implemented a guidance statement describing the role and impact of a clinical pharmacy specialist (CPS) in managing ACSCs.1 Within the Veterans Health Administration, the CPS may function under a scope of practice within their area of expertise with the ability to prescribe medications, place consults, and order laboratory tests and additional referrals as appropriate. As hospitalizations related to ACSCs are potentially preventable with effective primary care, the CPS can play an essential primary care role to implement interventions targeted at reducing these hospitalizations.
At the William S. Middleton Memorial Veterans Hospital, in Madison, Wisconsin, multiple transitions of care and postdischarge services have been established to capture those patients who are at a high risk of rehospitalization. Studies have been completed regarding implementation of intensive case management programs for high-risk patients.3 Currently though, no standardized process or protocol exists that can identify and optimize primary care for patients with ACSCs who have been hospitalized but are predicted to be at low risk for rehospitalization. Although these patients may not require intensive case management like that of those at high risk, improvements can be made to optimize clinical resources, education, and patient self-monitoring to mitigate risk for hospitalization or rehospitalization. Therefore, this project aimed to evaluate the implementation of offering further referrals and care for patients who have been hospitalized but are considered low risk for hospitalization from ACSCs.
Methods
This quality improvement project to offer further referrals and care to patients considered low risk for hospitalization was implemented to enhance ambulatory-care provided services. All patients identified as being a low risk for hospitalization via a VA dashboard from July through September 2018 were included. Patients were identified based on age, chronic diseases, gender, and other patient-specific factors predetermined by the VA dashboard algorithm. Patients receiving hospice or palliative care and those no longer receiving primary care through the facility were excluded.
A pharmacy resident conducted a baseline chart review using a standardized template in the computerized patient record system (CPRS) to identify additional referrals or interventions a patient may benefit from based on any identified ACSC. Potential referral options included a CPS or nurse care manager disease management, whole health/wellness, educational classes, home monitoring equipment, specialty clinics, nutrition, cardiac or pulmonary rehabilitation, social work, and mental health. A pharmacy resident or the patient aligned care team (PACT) CPS reviewed the identified referrals with PACT members at interdisciplinary team meetings and determined which referrals to offer the patient. The pharmacy resident or designated PACT member reached out to the patient via telephone or during a clinic visit to offer and enter the referrals. If the patient agreed to any referrals, a chart review was conducted 3 months later to determine the percentage of initially agreed-upon referrals that the patient completed. Additionally, the number of emergency department (ED) visits and hospitalizations related to an ACSC at 3 months was collected.
Feasibility was assessed to evaluate potential service implementation and was measured by the time in minutes to complete the baseline chart review, time in minutes to offer referrals to the patient, and proportion of referrals that were completed at 3 months.4 As this quality improvement project was undertaken for programmatic evaluation, the University of Wisconsin-Madison Health Sciences Institutional Review Board determined that this project did not meet the federal definition of research and therefore review was not required. Data were analyzed using descriptive statistics.
Results
A total of 78 veterans who had ≥ 1 ACSC-related hospitalization in the past year and who were categorized as low risk were identified, and 69 veterans were reviewed. Nine patients were not included based on hospice care and no longer receiving primary care through the facility. Eight patients were found to have optimized care with no further action warranted after review. Based on their assigned PACT, there was a range of 0 to 5 patients identified per team. Fifty-one patients were contacted, and 37 accepted ≥ 1 referral. Most of the patients were white and male (Table). The most common ACSCs were hypertension (68%), COPD (46%), and T2DM (30%); additional ACSCs included angina (18%), pneumonia (15%), UTIs (10%), CHF (6%), and asthma, dehydration, and perforated appendix (1.5% for each). Any ACSC listed as a diagnosis for a patient was included, regardless of whether it was related to a hospitalization. Most referrals were offered by pharmacists (pharmacy resident, 41%; CPS, 29%), followed by the nurse care manager (18%) and the primary care provider (12%). One patient passed away related to heart failure complications prior to being contacted to offer additional referrals. Of the 9 patients that were unable to be contacted, 4 did not respond to 3 phone call attempts and 5 had no documentation of referrals being offered after the initial chart review and recommendation was completed.
Most of the initially accepted referrals (n = 68) were for CPS disease management, whole health/wellness, and educational classes (Figure). Of the 28 initially accepted referrals for CPS disease management, most were for COPD (10) and hypertension (8), followed by neuropathic pain (3), vitamin D deficiency (3), hyperlipidemia (2), and T2DM (2). At 3 months, all referrals were completed except for 1 hypertension, 1 vitamin D deficiency, and 2 hyperlipidemia referrals. There were 6 COPD, 4 T2DM self-management, and 1 chronic pain class referrals made with 3 COPD and 1 T2DM referrals completed at 3 months. Two tobacco treatment and 2 palliative care referrals were specialty referrals accepted by patients with 1 palliative care referral completed at 3 months.
In terms of feasibility, the chart review took an average (SD) of 13 (4) minutes, and contacting the patient to offer referrals took an average of 8 (5) minutes. Most of the accepted referrals were completed by 3 months (42/68, 62%).
Comparing the 3 months prior to and the 3 months after offering referrals, there was a cumulative quantitative decrease in the number of ED visits (5 to 1) and hospitalizations (11 to 5). The 1 ED visit was for a patient who was unable to be contacted to offer additional referrals as were 4 of the hospitalizations. One of the hospitalizations was for a patient who was deemed to have optimized care with no additional referrals necessary.
Discussion
Evaluation of the review and referral process for patients at low risk for hospitalization from an ACSC was a proactive approach toward optimizing primary care for veterans, and the process increased patient access to education and primary care. There was a high initial patient acceptance rate of referrals and a high completion rate when offered by PACT members. Based on the number of identified patients, the time spent completing chart reviews and contacting patients to offer referrals for each PACT CPS and team was feasible to conduct.
As there were 69 eligible patients identified over a 3-month period for a single VA facility, including all community-based outpatient clinics serving an estimated 130,000 veterans, the additional time and workload for an individual PACT to reach out to these patients is minimal. Completing the review and outreach process for an average of 21 minutes per patient for at most 5 patients per primary care provider team is feasible to complete during the recommended 4 hours of weekly CPS population health management responsibilities.
Limitations
Several limitations were identified with the implementation of the project. A variety of PACT members completed initial outreach to veterans regarding additional referrals, which may have resulted in a lack of consistency in the approach and discussion of offering referrals to patients. Although there may be a difference in how the team members made referral offers to patients and therefore varying acceptance rates by patients, the process was thought to be more generalizable to the PACT approach for providing care in the VA. In addition, the time to contact patients to offer referrals was not always documented in the electronic health record, making the documented time an estimate. Given that patients identified were managed by a variety of PACT members, there were differences noted among PACTs in terms of acceptability of offering referrals to patients.
While there was a decrease noted in ED visits and hospitalizations when comparing 3 months before and afterward, additional data are needed to provide further insight into this relationship. As the patients identified were at low risk for hospitalization from an ACSC and had 1 or 2 hospitalizations within the year prior, additional time is warranted to compare 12-month ED visits and hospitalization rates postintervention. Finally, these findings may be limited in generalizability to other health care systems as the project was conducted among a specific, veteran patient population with PACT CPSs practicing independently within an established broad scope of practice.
Future Directions
Future directions include incorporating the review and referral process into the PACT CPS population health management responsibilities as a way to use all PACT members to enhance primary care delivered to veterans. To further elucidate the relationship between the referral process and hospitalization rates, a longer data collection period is needed.
Conclusions
Identifying patients at risk for hospitalization from an ACSC via a review and referral process by using the VA PACT structure and team members was feasible and led to increased patient access to primary care and additional services. The PACT CPS would benefit from using a similar approach for population health management for low risk for hospitalization patients or other identified chronic conditions.
Acknowledgments
Presented at the Wisconsin Pharmacy Residency Conference at the Pharmacy Society of Wisconsin Educational Conference April 10, 2019, in Madison, Wisconsin.
1. US Department of Veterans Affairs, Veterans Health Administration, Pharmacy Benefits Management Service, Clinical Pharmacy Practice Office. Clinical pharmacy specialist (CPS) role in management of ambulatory care sensitive conditions (ACSC). [Nonpublic source.]
2. US Department of Health and Human Services, Agency for Healthcare Research and Quality. Guide to prevention quality indicators: hospital admission for ambulatory care sensitive conditions. https://www.ahrq.gov/downloads/pub/ahrqqi/pqiguide.pdf. Revised April 17, 2002. Accessed July 16, 2020.
3. Yoon J, Chang E, Rubenstein L, et al. Impact of primary care intensive management on high-risk veterans’ costs and utilization. Ann Intern Med. 2018;168(12):846-854. doi:10.7326/M17-3039
4. Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38:65-76. doi:10.1007/s10488-010-0319-7
1. US Department of Veterans Affairs, Veterans Health Administration, Pharmacy Benefits Management Service, Clinical Pharmacy Practice Office. Clinical pharmacy specialist (CPS) role in management of ambulatory care sensitive conditions (ACSC). [Nonpublic source.]
2. US Department of Health and Human Services, Agency for Healthcare Research and Quality. Guide to prevention quality indicators: hospital admission for ambulatory care sensitive conditions. https://www.ahrq.gov/downloads/pub/ahrqqi/pqiguide.pdf. Revised April 17, 2002. Accessed July 16, 2020.
3. Yoon J, Chang E, Rubenstein L, et al. Impact of primary care intensive management on high-risk veterans’ costs and utilization. Ann Intern Med. 2018;168(12):846-854. doi:10.7326/M17-3039
4. Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38:65-76. doi:10.1007/s10488-010-0319-7
Effects of Computer-Based Documentation Procedures on Health Care Workload Assessment and Resource Allocation: An Example From VA Sleep Medicine Programs
Health care systems are faced with the challenge of meeting increasing patient care demands with finite resources.1 Advocating for additional capital—specifically, human resources—requires compelling data that accurately capture workload credit. When workload is not captured accurately, clinicians may be tasked with providing care to a high volume of patients without appropriate resource allocation. This understaffing can delay care delivery and increase the risk of diagnostic and treatment errors.2 Furthermore, workers in understaffed medical facilities are more likely to experience burnout, which leads to high workforce turnover.
Computer based documentation (CBD) is used often in medical practices to track patient care and clinical workload. However, improperly designed and implemented CBD systems can contribute to cumbersome documentation tasks and inaccurate or incomplete data capture.3 Conversely, CBD can be a useful tool to capture workload credit and can subsequently facilitate justification for medical staff allocation to meet patient care demands. This article uses our experience with US Department of Veterans Affairs (VA) national sleep medicine programs to illustrate the impact of CBD procedures on health care workload assessment and allocation. Specifically, we examine how appropriate workload capture facilitates growth and improves the efficiency of health care programs.
The VA is the largest integrated health care system in the US, serving 9 million veterans at 1,255 facilities, including 170 VA Medical Centers (VAMCs).4 As veterans’ demands for VA medical services have outpaced available resources, there have been several media reports of lapses in timely care delivery.5-7 These lapses have been due, in part, to insufficient workforce resource allocation within the Veterans Health Administration (VHA) facilities. A 2012 audit of physician staffing levels conducted by the VA Inspector General concluded that the VA did not have an effective staffing methodology to ensure appropriate staffing levels for specialty care services.8 The lack of staffing plans and productivity standards limits the ability of medical facility officials to make informed business decisions regarding the appropriate number of specialty physicians required to meet patient care needs.8 In 2017, the Government Accountability Office (GAO) issued a report to Congress that stated the “VA’s productivity metrics and efficiency models do not provide complete and accurate information, they may misrepresent the true level of productivity and efficiency across VAMCs and limit the VA’s ability to determine the extent to which its resources are being used effectively.”9 To understand how and why many VA medical facilities remain understaffed, and therefore struggle to provide health care to veterans in a timely fashion, a description of VA CBD procedures is provided.
Background
VA Directive 1082 on Patient Care Data requires the capture of all outpatient and inpatient billable encounter data.10 Accurate capture of workload informs budget allocation models and is necessary for health care provider (HCP) productivity metrics. These data points help identify staff shortages relative to the generated workload. The Veterans Equitable Resource Allocation (VERA) model is used to allocate general purpose funds to the Veterans Integrated Service Networks (VISNs) regional network of VHA facilities. The underlying data components of the VERA model rely on comprehensive data systems that track and analyze the many management information systems used in VHA. Historically, at least 90% of the funds allocated by the VERA model have been attributed directly to patient care. All workload that is appropriately documented is accounted for in the VERA patient classification process, which is the official data source for funding patient care in VHA.
VA medical facilities use Stop Codes (formerly known as Decision Support System Identifiers) to identify workload for all outpatient encounters and inpatient professional services. Each code is composed of a 6-character descriptor that includes a primary Stop Code and a credit (secondary) Stop Code. Primary Stop Codes—the first 3 numbers in the sequence—designate the main clinical group responsible for patient care, such as sleep medicine or neurology. Secondary Stop Codes—the last 3 numbers in the sequence—further define the primary workgroup, such as the type of services provided (eg, telehealth) or the type of HCP (eg, nurse practitioner). These codes help ensure that workload and generated revenue are allocated or credited to the proper specialty care service.11 An example of how changes or inaccuracies in Stop Code reporting can affect VHA clinical workload assessment and resource allocation is provided by the VHA sleep medicine program.
The prevalence of sleep disorders—particularly apnea and insomnia—among US military service members and veterans has increased dramatically over the past 2 decades and continues to rise.12-14 Consequently, demand for sleep care services at VHA facilities also has increased substantially (Figure 1). Unfortunately, this demand has outpaced the VHA’s staffing models, sometimes resulting in long wait times for appointments.15 In fact, sleep medicine remains one of the most backlogged services in the VHA, despite significant improvements in program efficiency achieved by incorporating telehealth modalities.16 Untreated sleep disorders are associated with increased risk of depression, anxiety, impaired neurocognitive functions, cardiovascular disease, motor vehicle accidents, and premature death.17-23
A major contributor to understaffing of VHA sleep medicine programs is the CBD system’s historical inability to accurately track sleep resources and demand for sleep care services. For many years, Stop Codes attributed sleep workload credit primarily to pulmonary medicine, neurology, and internal medicine workgroups. Within these workgroups, few individuals contributed to sleep care, but the entire workgroup received credit for these services, masking the workload of sleep care providers. Additional barriers to accurate sleep medicine workload capture within the VHA included (1) inability to centrally identify personnel, including physicians, as providers of sleep care; (2) limited and variable understanding among VA sleep physicians of the importance of proper encounter form completion (the mechanism by which the cost of a service is calculated); and (3) a lack of awareness that encounter closure is directly linked to productivity measures such as relative value units (RVUs) that support sleep medicine programs and the salaries of those who provide care.
Methods
The critical role of accurate CBD in health care administration is illustrated by the proper use of Stop Codes as a foundational step in tracking services provided to justify adequate resource allocation within VA. A complete redesign of tracking sleep service documentation was initiated in 2014 and resulted in national changes to sleep medicine Stop Codes. The Stop Code initiative was the first step of several to improve CBD for VA sleep services.
Primary Stop Code 349 designates sleep medicine encounters in VA facilities (Table). However, before changes were implemented in fiscal year (FY) 2015, Stop Codes for VHA sleep care did not differentiate between specific services provided, such as laboratory-based sleep testing, at-home sleep testing, education/training sessions, follow-up appointments, equipment consults, telephone or video consults, or administrative tasks. In early FY 2015, several changes were made to Stop Codes used for VHA sleep medicine services nationwide to capture the breadth of services that were being provided; services that had previously been performed but were not documented. A new standardized coding methodology was established for continuous positive airway pressure (CPAP) clinics (349/116 or 349/117); telephone consults for sleep care (324/349); and store and forward sleep telehealth encounters (349/694, 349/695, or 349/696).
In the VA, store-and-forward telehealth refers to asynchronous telemedicine involving the acquisition and storing of clinical information (eg, data, image, sound, or video) that another site or clinician reviews later for evaluation and interpretation. In sleep medicine, data uploaded from home sleep apnea test units or CPAP devices are examples of this asynchronous telehealth model. The goal of these changes in VA Stop Codes was to accurately assess the volume of sleep care delivered and the demand for sleep care (consult volumes); enable planning for resource allocation and utilization appropriately; provide veterans with consistent access to sleep services across the country; and facilitate reductions in wait times for sleep care appointments. Results of these changes were immediate and dramatic in terms of data capture and reporting.
Results
Figure 1 illustrates an increase in patient encounters in VA sleep clinics by 24,197 (19.6%) in the first quarter of Stop Code change implementation (FY 2015, quarter 2) compared with those of the previous quarter. VHA sleep clinic patient encounters increased in subsequent quarters of FY 2015 by 29,910 (20.2%) and 11,206 (6.3%) respectively. By the end of FY 2015, reported sleep clinic encounters increased by 190,803 compared with the those at the end of FY 2014, an increase of 42.7%.
Figures 2, 3, and 4 show the additional effects of sleep Stop Code changes that were implemented in FY 2015 for CPAP clinics, telephone encounters, and store-and-forward telehealth encounters, respectively. The large increases in reported sleep patient encounters between FY 2014 and FY 2016 reflect changes in CBD and are not entirely due to actual changes in clinical workloads. These results indicate that workloads in many VHA sleep medicine clinics were grossly underreported or misallocated to other specialty services prior to the changes implemented in FY 2015. This discrepancy in care delivery vs workload capture is a contributing factor to the understaffing that continues to challenge VHA sleep programs. However, the improved accuracy of workload reporting that resulted from Stop Code modifications has resulted in only a small proportional increase in VHA clinical resources allocated to provide adequate services and care for veterans with sleep disorders.
In response to the substantial and increasing demand for sleep services by veterans, the VA Office of Rural Health (ORH) funded an enterprise-wide initiative (EWI) to develop and implement a national TeleSleep Program.16 The goal of this program is to improve the health and well-being of rural veterans by increasing their access to sleep care and services.
Discussion
Inaccuracies in CBD procedures can adversely affect health care workload assessment and allocation, contributing to ongoing challenges faced by sleep medicine clinics and other VHA programs that have limited staff yet strive to provide timely and high-quality care to veterans. “Not only does inaccurate coding contribute to miscalculations in staffing and resource allocation, it can also contribute to inaccuracies in overall measures of VA healthcare efficiency,” the GAO reported to Congress.9 The GAO went on to recommend that the VA should ensure the accuracy of underlying staffing and workload data. VHA sleep medicine programs have made efforts to educate HCPs and administrators on the importance of accurate CBD as a tool for accurate data capture that is necessary to facilitate improvements in health care availability and delivery.
In 2018, the VA Sleep Program Office released an updated set of Stop Code changes, including expansion of telehealth codes and improved designation of laboratory and home sleep testing services. These changes are anticipated to result in accurate documentation of VA sleep clinic workload and services, especially as the VA TeleSleep EWI to reach rural veterans expands.16 In light of the improved accuracy of reporting of delivered sleep services due to changes in Stop Codes over the past 4 years, VHA sleep medicine providers continue to advocate for allocation of resources commensurate with their clinical workload. An appropriate administrative response to the significant clinical workload performed by disproportionately few providers should include the authorization of increased resources and personnel for sleep medicine as well as providing the tools needed to further streamline workflow efficiency (eg, artificial intelligence, machine learning, and population health management).
Conclusions
Despite the barriers faced by many large integrated health care systems, VHA sleep medicine leadership continues to implement changes in CBD protocols that improve the accuracy of clinical workload tracking and reporting. Ultimately, these changes will support proposals for increased resources necessary to improve the quality and availability of sleep care for veterans. This example from VA illustrates the importance of accurate workload capture and its role in informing administrators of health care systems as they strive to meet the needs of patients. Although some VA sleep medicine programs continue to face challenges imposed by systemwide limitations, the ORH TeleSleep Program is a major initiative that improves veterans’ access to care by disseminating and implementing effective telehealth technologies and strategies.16
Acknowledgments
This work was supported by a VA Office of Rural Health Enterprise-Wide Initiative.
1. World Health Organization. Workload indicators of staffing need (WISN). https://www.who.int/hrh/resources/WISN_Eng_UsersManual.pdf?ua=1. Published December 2015. Accessed June 24, 2020.
2. American Association for Respiratory Care. Position statement: best practices in respiratory care productivity and staffing. https://www.aarc.org/wp-content/uploads/2017/03/statement-of-best-practices_productivity-and-staffing.pdf. Revised July 2015. Accessed June 24, 2020.
3. Wu DTY, Smart N, Ciemins EL, Lanham HJ, Lindberg C, Zheng K. Using EHR audit trail logs to analyze clinical workflow: a case study from community-based ambulatory clinics. AMIA Annu Symp Proc. 2018;2017:1820-1827. Published 2018 Apr 16.
4. US Department of Veterans Affairs, Veterans Health Administration. https://www.va.gov/health.
5. Cohen T. VA crisis: solutions exist, but haven’t happened, panel hears. https://www.cnn.com/2014/06/12/politics/va-reforms/index.html. Published June 12, 2014. Accessed June 24, 2020.
6. Richardson B. IG probes uncover more problems at VA hospitals. https://thehill.com/policy/defense/258652-ig-probes-uncover-more-problems-at-va-hospitals. Published October 30, 2015. Accessed June 24, 2020.
7. Slack D. Inaccurate VA wait times prelude thousands of vets from getting outside care, probe finds. USA Today. March 3, 2017. https://www.usatoday.com/story/news/politics/2017/03/03/veterans-affairs-inspector-general-widespread-inaccuracies-wait-times/98693856. Accessed June 24, 2020.
8. US Department of Veterans Affairs, Office of the Inspector General. Veterans Health Administration: audit of physician staffing levels for specialty care services. https://www.va.gov/oig/pubs/VAOIG-11-01827-36.pdf. Published December 27, 2012. Accessed June 24, 2020.
9. Government Accountability Office. VA health care: improvements needed in data and monitoring of clinical productivity and efficiency. https://www.gao.gov/assets/690/684869.pdf. Published May 2017. Accessed June 24, 2020.
10. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1082. Patient care data capture. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3091. Published March 24, 2015. Accessed June 24, 2020.
11. US Department of Veterans Affairs, Veterans Health Administration. VHA Handbook 1006.02. VHA site classifications and definitions. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2970. Published December 30, 2013. Accessed June 24, 2020.
12. Alexander M, Ray MA, Hébert JR, et al. The National Veteran Sleep Disorder Study: Descriptive Epidemiology and Secular Trends, 2000-2010. Sleep. 2016;39(7):1399-1410. Published 2016 Jul 1. doi:10.5665/sleep.5972.
13. A Caldwell J, Knapik JJ, Lieberman HR. Trends and factors associated with insomnia and sleep apnea in all United States military service members from 2005 to 2014. J Sleep Res. 2017;26(5):665-670. doi:10.1111/jsr.12543
14. Klingaman EA, Brownlow JA, Boland EM, Mosti C, Gehrman PR. Prevalence, predictors and correlates of insomnia in US army soldiers. J Sleep Res. 2018;27(3):e12612. doi:10.1111/jsr.12612
15. Sharafkhaneh A, Richardson P, Hirshkowitz M. Sleep apnea in a high risk population: a study of Veterans Health Administration beneficiaries. Sleep Med. 2004;5(4):345-350. doi:10.1016/j.sleep.2004.01.019.
16. Sarmiento KF, Folmer RL, Stepnowsky CJ, et al. National Expansion of Sleep Telemedicine for Veterans: The TeleSleep Program. J Clin Sleep Med. 2019;15(9):1355-1364. doi:10.5664/jcsm.7934
17. Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation [published correction appears in Sleep. 2004 Jun 15;27(4):600]. Sleep. 2003;26(2):117-126. doi:10.1093/sleep/26.2.117
18. Johnson EO, Roth T, Breslau N. The association of insomnia with anxiety disorders and depression: exploration of the direction of risk. J Psychiatr Res. 2006;40(8):700-708. doi:10.1016/j.jpsychires.2006.07.008
19. Léger D, Bayon V, Ohayon MM, et al. Insomnia and accidents: cross-sectional study (EQUINOX) on sleep-related home, work and car accidents in 5293 subjects with insomnia from 10 countries. J Sleep Res. 2014;23(2):143-152. doi:10.1111/jsr.12104
20. Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7(8):1311-1322. doi:10.3978/j.issn.2072-1439.2015.06.11
21. Javaheri S, Redline S. Insomnia and Risk of Cardiovascular Disease. Chest. 2017;152(2):435-444. doi:10.1016/j.chest.2017.01.026
22. Linz D, McEvoy RD, Cowie MR, et al. Associations of obstructivesSleepaApnea with atrial fibrillation and continuous positive airway pressure treatment: a review. JAMA Cardiol. 2018;3(6):532-540. doi:10.1001/jamacardio.2018.0095
23. Ogilvie RP, Lakshminarayan K, Iber C, Patel SR, Lutsey PL. Joint effects of OSA and self-reported sleepiness on incident CHD and stroke. Sleep Med. 2018;44:32-37. doi:10.1016/j.sleep.2018.01.004
Health care systems are faced with the challenge of meeting increasing patient care demands with finite resources.1 Advocating for additional capital—specifically, human resources—requires compelling data that accurately capture workload credit. When workload is not captured accurately, clinicians may be tasked with providing care to a high volume of patients without appropriate resource allocation. This understaffing can delay care delivery and increase the risk of diagnostic and treatment errors.2 Furthermore, workers in understaffed medical facilities are more likely to experience burnout, which leads to high workforce turnover.
Computer based documentation (CBD) is used often in medical practices to track patient care and clinical workload. However, improperly designed and implemented CBD systems can contribute to cumbersome documentation tasks and inaccurate or incomplete data capture.3 Conversely, CBD can be a useful tool to capture workload credit and can subsequently facilitate justification for medical staff allocation to meet patient care demands. This article uses our experience with US Department of Veterans Affairs (VA) national sleep medicine programs to illustrate the impact of CBD procedures on health care workload assessment and allocation. Specifically, we examine how appropriate workload capture facilitates growth and improves the efficiency of health care programs.
The VA is the largest integrated health care system in the US, serving 9 million veterans at 1,255 facilities, including 170 VA Medical Centers (VAMCs).4 As veterans’ demands for VA medical services have outpaced available resources, there have been several media reports of lapses in timely care delivery.5-7 These lapses have been due, in part, to insufficient workforce resource allocation within the Veterans Health Administration (VHA) facilities. A 2012 audit of physician staffing levels conducted by the VA Inspector General concluded that the VA did not have an effective staffing methodology to ensure appropriate staffing levels for specialty care services.8 The lack of staffing plans and productivity standards limits the ability of medical facility officials to make informed business decisions regarding the appropriate number of specialty physicians required to meet patient care needs.8 In 2017, the Government Accountability Office (GAO) issued a report to Congress that stated the “VA’s productivity metrics and efficiency models do not provide complete and accurate information, they may misrepresent the true level of productivity and efficiency across VAMCs and limit the VA’s ability to determine the extent to which its resources are being used effectively.”9 To understand how and why many VA medical facilities remain understaffed, and therefore struggle to provide health care to veterans in a timely fashion, a description of VA CBD procedures is provided.
Background
VA Directive 1082 on Patient Care Data requires the capture of all outpatient and inpatient billable encounter data.10 Accurate capture of workload informs budget allocation models and is necessary for health care provider (HCP) productivity metrics. These data points help identify staff shortages relative to the generated workload. The Veterans Equitable Resource Allocation (VERA) model is used to allocate general purpose funds to the Veterans Integrated Service Networks (VISNs) regional network of VHA facilities. The underlying data components of the VERA model rely on comprehensive data systems that track and analyze the many management information systems used in VHA. Historically, at least 90% of the funds allocated by the VERA model have been attributed directly to patient care. All workload that is appropriately documented is accounted for in the VERA patient classification process, which is the official data source for funding patient care in VHA.
VA medical facilities use Stop Codes (formerly known as Decision Support System Identifiers) to identify workload for all outpatient encounters and inpatient professional services. Each code is composed of a 6-character descriptor that includes a primary Stop Code and a credit (secondary) Stop Code. Primary Stop Codes—the first 3 numbers in the sequence—designate the main clinical group responsible for patient care, such as sleep medicine or neurology. Secondary Stop Codes—the last 3 numbers in the sequence—further define the primary workgroup, such as the type of services provided (eg, telehealth) or the type of HCP (eg, nurse practitioner). These codes help ensure that workload and generated revenue are allocated or credited to the proper specialty care service.11 An example of how changes or inaccuracies in Stop Code reporting can affect VHA clinical workload assessment and resource allocation is provided by the VHA sleep medicine program.
The prevalence of sleep disorders—particularly apnea and insomnia—among US military service members and veterans has increased dramatically over the past 2 decades and continues to rise.12-14 Consequently, demand for sleep care services at VHA facilities also has increased substantially (Figure 1). Unfortunately, this demand has outpaced the VHA’s staffing models, sometimes resulting in long wait times for appointments.15 In fact, sleep medicine remains one of the most backlogged services in the VHA, despite significant improvements in program efficiency achieved by incorporating telehealth modalities.16 Untreated sleep disorders are associated with increased risk of depression, anxiety, impaired neurocognitive functions, cardiovascular disease, motor vehicle accidents, and premature death.17-23
A major contributor to understaffing of VHA sleep medicine programs is the CBD system’s historical inability to accurately track sleep resources and demand for sleep care services. For many years, Stop Codes attributed sleep workload credit primarily to pulmonary medicine, neurology, and internal medicine workgroups. Within these workgroups, few individuals contributed to sleep care, but the entire workgroup received credit for these services, masking the workload of sleep care providers. Additional barriers to accurate sleep medicine workload capture within the VHA included (1) inability to centrally identify personnel, including physicians, as providers of sleep care; (2) limited and variable understanding among VA sleep physicians of the importance of proper encounter form completion (the mechanism by which the cost of a service is calculated); and (3) a lack of awareness that encounter closure is directly linked to productivity measures such as relative value units (RVUs) that support sleep medicine programs and the salaries of those who provide care.
Methods
The critical role of accurate CBD in health care administration is illustrated by the proper use of Stop Codes as a foundational step in tracking services provided to justify adequate resource allocation within VA. A complete redesign of tracking sleep service documentation was initiated in 2014 and resulted in national changes to sleep medicine Stop Codes. The Stop Code initiative was the first step of several to improve CBD for VA sleep services.
Primary Stop Code 349 designates sleep medicine encounters in VA facilities (Table). However, before changes were implemented in fiscal year (FY) 2015, Stop Codes for VHA sleep care did not differentiate between specific services provided, such as laboratory-based sleep testing, at-home sleep testing, education/training sessions, follow-up appointments, equipment consults, telephone or video consults, or administrative tasks. In early FY 2015, several changes were made to Stop Codes used for VHA sleep medicine services nationwide to capture the breadth of services that were being provided; services that had previously been performed but were not documented. A new standardized coding methodology was established for continuous positive airway pressure (CPAP) clinics (349/116 or 349/117); telephone consults for sleep care (324/349); and store and forward sleep telehealth encounters (349/694, 349/695, or 349/696).
In the VA, store-and-forward telehealth refers to asynchronous telemedicine involving the acquisition and storing of clinical information (eg, data, image, sound, or video) that another site or clinician reviews later for evaluation and interpretation. In sleep medicine, data uploaded from home sleep apnea test units or CPAP devices are examples of this asynchronous telehealth model. The goal of these changes in VA Stop Codes was to accurately assess the volume of sleep care delivered and the demand for sleep care (consult volumes); enable planning for resource allocation and utilization appropriately; provide veterans with consistent access to sleep services across the country; and facilitate reductions in wait times for sleep care appointments. Results of these changes were immediate and dramatic in terms of data capture and reporting.
Results
Figure 1 illustrates an increase in patient encounters in VA sleep clinics by 24,197 (19.6%) in the first quarter of Stop Code change implementation (FY 2015, quarter 2) compared with those of the previous quarter. VHA sleep clinic patient encounters increased in subsequent quarters of FY 2015 by 29,910 (20.2%) and 11,206 (6.3%) respectively. By the end of FY 2015, reported sleep clinic encounters increased by 190,803 compared with the those at the end of FY 2014, an increase of 42.7%.
Figures 2, 3, and 4 show the additional effects of sleep Stop Code changes that were implemented in FY 2015 for CPAP clinics, telephone encounters, and store-and-forward telehealth encounters, respectively. The large increases in reported sleep patient encounters between FY 2014 and FY 2016 reflect changes in CBD and are not entirely due to actual changes in clinical workloads. These results indicate that workloads in many VHA sleep medicine clinics were grossly underreported or misallocated to other specialty services prior to the changes implemented in FY 2015. This discrepancy in care delivery vs workload capture is a contributing factor to the understaffing that continues to challenge VHA sleep programs. However, the improved accuracy of workload reporting that resulted from Stop Code modifications has resulted in only a small proportional increase in VHA clinical resources allocated to provide adequate services and care for veterans with sleep disorders.
In response to the substantial and increasing demand for sleep services by veterans, the VA Office of Rural Health (ORH) funded an enterprise-wide initiative (EWI) to develop and implement a national TeleSleep Program.16 The goal of this program is to improve the health and well-being of rural veterans by increasing their access to sleep care and services.
Discussion
Inaccuracies in CBD procedures can adversely affect health care workload assessment and allocation, contributing to ongoing challenges faced by sleep medicine clinics and other VHA programs that have limited staff yet strive to provide timely and high-quality care to veterans. “Not only does inaccurate coding contribute to miscalculations in staffing and resource allocation, it can also contribute to inaccuracies in overall measures of VA healthcare efficiency,” the GAO reported to Congress.9 The GAO went on to recommend that the VA should ensure the accuracy of underlying staffing and workload data. VHA sleep medicine programs have made efforts to educate HCPs and administrators on the importance of accurate CBD as a tool for accurate data capture that is necessary to facilitate improvements in health care availability and delivery.
In 2018, the VA Sleep Program Office released an updated set of Stop Code changes, including expansion of telehealth codes and improved designation of laboratory and home sleep testing services. These changes are anticipated to result in accurate documentation of VA sleep clinic workload and services, especially as the VA TeleSleep EWI to reach rural veterans expands.16 In light of the improved accuracy of reporting of delivered sleep services due to changes in Stop Codes over the past 4 years, VHA sleep medicine providers continue to advocate for allocation of resources commensurate with their clinical workload. An appropriate administrative response to the significant clinical workload performed by disproportionately few providers should include the authorization of increased resources and personnel for sleep medicine as well as providing the tools needed to further streamline workflow efficiency (eg, artificial intelligence, machine learning, and population health management).
Conclusions
Despite the barriers faced by many large integrated health care systems, VHA sleep medicine leadership continues to implement changes in CBD protocols that improve the accuracy of clinical workload tracking and reporting. Ultimately, these changes will support proposals for increased resources necessary to improve the quality and availability of sleep care for veterans. This example from VA illustrates the importance of accurate workload capture and its role in informing administrators of health care systems as they strive to meet the needs of patients. Although some VA sleep medicine programs continue to face challenges imposed by systemwide limitations, the ORH TeleSleep Program is a major initiative that improves veterans’ access to care by disseminating and implementing effective telehealth technologies and strategies.16
Acknowledgments
This work was supported by a VA Office of Rural Health Enterprise-Wide Initiative.
Health care systems are faced with the challenge of meeting increasing patient care demands with finite resources.1 Advocating for additional capital—specifically, human resources—requires compelling data that accurately capture workload credit. When workload is not captured accurately, clinicians may be tasked with providing care to a high volume of patients without appropriate resource allocation. This understaffing can delay care delivery and increase the risk of diagnostic and treatment errors.2 Furthermore, workers in understaffed medical facilities are more likely to experience burnout, which leads to high workforce turnover.
Computer based documentation (CBD) is used often in medical practices to track patient care and clinical workload. However, improperly designed and implemented CBD systems can contribute to cumbersome documentation tasks and inaccurate or incomplete data capture.3 Conversely, CBD can be a useful tool to capture workload credit and can subsequently facilitate justification for medical staff allocation to meet patient care demands. This article uses our experience with US Department of Veterans Affairs (VA) national sleep medicine programs to illustrate the impact of CBD procedures on health care workload assessment and allocation. Specifically, we examine how appropriate workload capture facilitates growth and improves the efficiency of health care programs.
The VA is the largest integrated health care system in the US, serving 9 million veterans at 1,255 facilities, including 170 VA Medical Centers (VAMCs).4 As veterans’ demands for VA medical services have outpaced available resources, there have been several media reports of lapses in timely care delivery.5-7 These lapses have been due, in part, to insufficient workforce resource allocation within the Veterans Health Administration (VHA) facilities. A 2012 audit of physician staffing levels conducted by the VA Inspector General concluded that the VA did not have an effective staffing methodology to ensure appropriate staffing levels for specialty care services.8 The lack of staffing plans and productivity standards limits the ability of medical facility officials to make informed business decisions regarding the appropriate number of specialty physicians required to meet patient care needs.8 In 2017, the Government Accountability Office (GAO) issued a report to Congress that stated the “VA’s productivity metrics and efficiency models do not provide complete and accurate information, they may misrepresent the true level of productivity and efficiency across VAMCs and limit the VA’s ability to determine the extent to which its resources are being used effectively.”9 To understand how and why many VA medical facilities remain understaffed, and therefore struggle to provide health care to veterans in a timely fashion, a description of VA CBD procedures is provided.
Background
VA Directive 1082 on Patient Care Data requires the capture of all outpatient and inpatient billable encounter data.10 Accurate capture of workload informs budget allocation models and is necessary for health care provider (HCP) productivity metrics. These data points help identify staff shortages relative to the generated workload. The Veterans Equitable Resource Allocation (VERA) model is used to allocate general purpose funds to the Veterans Integrated Service Networks (VISNs) regional network of VHA facilities. The underlying data components of the VERA model rely on comprehensive data systems that track and analyze the many management information systems used in VHA. Historically, at least 90% of the funds allocated by the VERA model have been attributed directly to patient care. All workload that is appropriately documented is accounted for in the VERA patient classification process, which is the official data source for funding patient care in VHA.
VA medical facilities use Stop Codes (formerly known as Decision Support System Identifiers) to identify workload for all outpatient encounters and inpatient professional services. Each code is composed of a 6-character descriptor that includes a primary Stop Code and a credit (secondary) Stop Code. Primary Stop Codes—the first 3 numbers in the sequence—designate the main clinical group responsible for patient care, such as sleep medicine or neurology. Secondary Stop Codes—the last 3 numbers in the sequence—further define the primary workgroup, such as the type of services provided (eg, telehealth) or the type of HCP (eg, nurse practitioner). These codes help ensure that workload and generated revenue are allocated or credited to the proper specialty care service.11 An example of how changes or inaccuracies in Stop Code reporting can affect VHA clinical workload assessment and resource allocation is provided by the VHA sleep medicine program.
The prevalence of sleep disorders—particularly apnea and insomnia—among US military service members and veterans has increased dramatically over the past 2 decades and continues to rise.12-14 Consequently, demand for sleep care services at VHA facilities also has increased substantially (Figure 1). Unfortunately, this demand has outpaced the VHA’s staffing models, sometimes resulting in long wait times for appointments.15 In fact, sleep medicine remains one of the most backlogged services in the VHA, despite significant improvements in program efficiency achieved by incorporating telehealth modalities.16 Untreated sleep disorders are associated with increased risk of depression, anxiety, impaired neurocognitive functions, cardiovascular disease, motor vehicle accidents, and premature death.17-23
A major contributor to understaffing of VHA sleep medicine programs is the CBD system’s historical inability to accurately track sleep resources and demand for sleep care services. For many years, Stop Codes attributed sleep workload credit primarily to pulmonary medicine, neurology, and internal medicine workgroups. Within these workgroups, few individuals contributed to sleep care, but the entire workgroup received credit for these services, masking the workload of sleep care providers. Additional barriers to accurate sleep medicine workload capture within the VHA included (1) inability to centrally identify personnel, including physicians, as providers of sleep care; (2) limited and variable understanding among VA sleep physicians of the importance of proper encounter form completion (the mechanism by which the cost of a service is calculated); and (3) a lack of awareness that encounter closure is directly linked to productivity measures such as relative value units (RVUs) that support sleep medicine programs and the salaries of those who provide care.
Methods
The critical role of accurate CBD in health care administration is illustrated by the proper use of Stop Codes as a foundational step in tracking services provided to justify adequate resource allocation within VA. A complete redesign of tracking sleep service documentation was initiated in 2014 and resulted in national changes to sleep medicine Stop Codes. The Stop Code initiative was the first step of several to improve CBD for VA sleep services.
Primary Stop Code 349 designates sleep medicine encounters in VA facilities (Table). However, before changes were implemented in fiscal year (FY) 2015, Stop Codes for VHA sleep care did not differentiate between specific services provided, such as laboratory-based sleep testing, at-home sleep testing, education/training sessions, follow-up appointments, equipment consults, telephone or video consults, or administrative tasks. In early FY 2015, several changes were made to Stop Codes used for VHA sleep medicine services nationwide to capture the breadth of services that were being provided; services that had previously been performed but were not documented. A new standardized coding methodology was established for continuous positive airway pressure (CPAP) clinics (349/116 or 349/117); telephone consults for sleep care (324/349); and store and forward sleep telehealth encounters (349/694, 349/695, or 349/696).
In the VA, store-and-forward telehealth refers to asynchronous telemedicine involving the acquisition and storing of clinical information (eg, data, image, sound, or video) that another site or clinician reviews later for evaluation and interpretation. In sleep medicine, data uploaded from home sleep apnea test units or CPAP devices are examples of this asynchronous telehealth model. The goal of these changes in VA Stop Codes was to accurately assess the volume of sleep care delivered and the demand for sleep care (consult volumes); enable planning for resource allocation and utilization appropriately; provide veterans with consistent access to sleep services across the country; and facilitate reductions in wait times for sleep care appointments. Results of these changes were immediate and dramatic in terms of data capture and reporting.
Results
Figure 1 illustrates an increase in patient encounters in VA sleep clinics by 24,197 (19.6%) in the first quarter of Stop Code change implementation (FY 2015, quarter 2) compared with those of the previous quarter. VHA sleep clinic patient encounters increased in subsequent quarters of FY 2015 by 29,910 (20.2%) and 11,206 (6.3%) respectively. By the end of FY 2015, reported sleep clinic encounters increased by 190,803 compared with the those at the end of FY 2014, an increase of 42.7%.
Figures 2, 3, and 4 show the additional effects of sleep Stop Code changes that were implemented in FY 2015 for CPAP clinics, telephone encounters, and store-and-forward telehealth encounters, respectively. The large increases in reported sleep patient encounters between FY 2014 and FY 2016 reflect changes in CBD and are not entirely due to actual changes in clinical workloads. These results indicate that workloads in many VHA sleep medicine clinics were grossly underreported or misallocated to other specialty services prior to the changes implemented in FY 2015. This discrepancy in care delivery vs workload capture is a contributing factor to the understaffing that continues to challenge VHA sleep programs. However, the improved accuracy of workload reporting that resulted from Stop Code modifications has resulted in only a small proportional increase in VHA clinical resources allocated to provide adequate services and care for veterans with sleep disorders.
In response to the substantial and increasing demand for sleep services by veterans, the VA Office of Rural Health (ORH) funded an enterprise-wide initiative (EWI) to develop and implement a national TeleSleep Program.16 The goal of this program is to improve the health and well-being of rural veterans by increasing their access to sleep care and services.
Discussion
Inaccuracies in CBD procedures can adversely affect health care workload assessment and allocation, contributing to ongoing challenges faced by sleep medicine clinics and other VHA programs that have limited staff yet strive to provide timely and high-quality care to veterans. “Not only does inaccurate coding contribute to miscalculations in staffing and resource allocation, it can also contribute to inaccuracies in overall measures of VA healthcare efficiency,” the GAO reported to Congress.9 The GAO went on to recommend that the VA should ensure the accuracy of underlying staffing and workload data. VHA sleep medicine programs have made efforts to educate HCPs and administrators on the importance of accurate CBD as a tool for accurate data capture that is necessary to facilitate improvements in health care availability and delivery.
In 2018, the VA Sleep Program Office released an updated set of Stop Code changes, including expansion of telehealth codes and improved designation of laboratory and home sleep testing services. These changes are anticipated to result in accurate documentation of VA sleep clinic workload and services, especially as the VA TeleSleep EWI to reach rural veterans expands.16 In light of the improved accuracy of reporting of delivered sleep services due to changes in Stop Codes over the past 4 years, VHA sleep medicine providers continue to advocate for allocation of resources commensurate with their clinical workload. An appropriate administrative response to the significant clinical workload performed by disproportionately few providers should include the authorization of increased resources and personnel for sleep medicine as well as providing the tools needed to further streamline workflow efficiency (eg, artificial intelligence, machine learning, and population health management).
Conclusions
Despite the barriers faced by many large integrated health care systems, VHA sleep medicine leadership continues to implement changes in CBD protocols that improve the accuracy of clinical workload tracking and reporting. Ultimately, these changes will support proposals for increased resources necessary to improve the quality and availability of sleep care for veterans. This example from VA illustrates the importance of accurate workload capture and its role in informing administrators of health care systems as they strive to meet the needs of patients. Although some VA sleep medicine programs continue to face challenges imposed by systemwide limitations, the ORH TeleSleep Program is a major initiative that improves veterans’ access to care by disseminating and implementing effective telehealth technologies and strategies.16
Acknowledgments
This work was supported by a VA Office of Rural Health Enterprise-Wide Initiative.
1. World Health Organization. Workload indicators of staffing need (WISN). https://www.who.int/hrh/resources/WISN_Eng_UsersManual.pdf?ua=1. Published December 2015. Accessed June 24, 2020.
2. American Association for Respiratory Care. Position statement: best practices in respiratory care productivity and staffing. https://www.aarc.org/wp-content/uploads/2017/03/statement-of-best-practices_productivity-and-staffing.pdf. Revised July 2015. Accessed June 24, 2020.
3. Wu DTY, Smart N, Ciemins EL, Lanham HJ, Lindberg C, Zheng K. Using EHR audit trail logs to analyze clinical workflow: a case study from community-based ambulatory clinics. AMIA Annu Symp Proc. 2018;2017:1820-1827. Published 2018 Apr 16.
4. US Department of Veterans Affairs, Veterans Health Administration. https://www.va.gov/health.
5. Cohen T. VA crisis: solutions exist, but haven’t happened, panel hears. https://www.cnn.com/2014/06/12/politics/va-reforms/index.html. Published June 12, 2014. Accessed June 24, 2020.
6. Richardson B. IG probes uncover more problems at VA hospitals. https://thehill.com/policy/defense/258652-ig-probes-uncover-more-problems-at-va-hospitals. Published October 30, 2015. Accessed June 24, 2020.
7. Slack D. Inaccurate VA wait times prelude thousands of vets from getting outside care, probe finds. USA Today. March 3, 2017. https://www.usatoday.com/story/news/politics/2017/03/03/veterans-affairs-inspector-general-widespread-inaccuracies-wait-times/98693856. Accessed June 24, 2020.
8. US Department of Veterans Affairs, Office of the Inspector General. Veterans Health Administration: audit of physician staffing levels for specialty care services. https://www.va.gov/oig/pubs/VAOIG-11-01827-36.pdf. Published December 27, 2012. Accessed June 24, 2020.
9. Government Accountability Office. VA health care: improvements needed in data and monitoring of clinical productivity and efficiency. https://www.gao.gov/assets/690/684869.pdf. Published May 2017. Accessed June 24, 2020.
10. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1082. Patient care data capture. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3091. Published March 24, 2015. Accessed June 24, 2020.
11. US Department of Veterans Affairs, Veterans Health Administration. VHA Handbook 1006.02. VHA site classifications and definitions. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2970. Published December 30, 2013. Accessed June 24, 2020.
12. Alexander M, Ray MA, Hébert JR, et al. The National Veteran Sleep Disorder Study: Descriptive Epidemiology and Secular Trends, 2000-2010. Sleep. 2016;39(7):1399-1410. Published 2016 Jul 1. doi:10.5665/sleep.5972.
13. A Caldwell J, Knapik JJ, Lieberman HR. Trends and factors associated with insomnia and sleep apnea in all United States military service members from 2005 to 2014. J Sleep Res. 2017;26(5):665-670. doi:10.1111/jsr.12543
14. Klingaman EA, Brownlow JA, Boland EM, Mosti C, Gehrman PR. Prevalence, predictors and correlates of insomnia in US army soldiers. J Sleep Res. 2018;27(3):e12612. doi:10.1111/jsr.12612
15. Sharafkhaneh A, Richardson P, Hirshkowitz M. Sleep apnea in a high risk population: a study of Veterans Health Administration beneficiaries. Sleep Med. 2004;5(4):345-350. doi:10.1016/j.sleep.2004.01.019.
16. Sarmiento KF, Folmer RL, Stepnowsky CJ, et al. National Expansion of Sleep Telemedicine for Veterans: The TeleSleep Program. J Clin Sleep Med. 2019;15(9):1355-1364. doi:10.5664/jcsm.7934
17. Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation [published correction appears in Sleep. 2004 Jun 15;27(4):600]. Sleep. 2003;26(2):117-126. doi:10.1093/sleep/26.2.117
18. Johnson EO, Roth T, Breslau N. The association of insomnia with anxiety disorders and depression: exploration of the direction of risk. J Psychiatr Res. 2006;40(8):700-708. doi:10.1016/j.jpsychires.2006.07.008
19. Léger D, Bayon V, Ohayon MM, et al. Insomnia and accidents: cross-sectional study (EQUINOX) on sleep-related home, work and car accidents in 5293 subjects with insomnia from 10 countries. J Sleep Res. 2014;23(2):143-152. doi:10.1111/jsr.12104
20. Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7(8):1311-1322. doi:10.3978/j.issn.2072-1439.2015.06.11
21. Javaheri S, Redline S. Insomnia and Risk of Cardiovascular Disease. Chest. 2017;152(2):435-444. doi:10.1016/j.chest.2017.01.026
22. Linz D, McEvoy RD, Cowie MR, et al. Associations of obstructivesSleepaApnea with atrial fibrillation and continuous positive airway pressure treatment: a review. JAMA Cardiol. 2018;3(6):532-540. doi:10.1001/jamacardio.2018.0095
23. Ogilvie RP, Lakshminarayan K, Iber C, Patel SR, Lutsey PL. Joint effects of OSA and self-reported sleepiness on incident CHD and stroke. Sleep Med. 2018;44:32-37. doi:10.1016/j.sleep.2018.01.004
1. World Health Organization. Workload indicators of staffing need (WISN). https://www.who.int/hrh/resources/WISN_Eng_UsersManual.pdf?ua=1. Published December 2015. Accessed June 24, 2020.
2. American Association for Respiratory Care. Position statement: best practices in respiratory care productivity and staffing. https://www.aarc.org/wp-content/uploads/2017/03/statement-of-best-practices_productivity-and-staffing.pdf. Revised July 2015. Accessed June 24, 2020.
3. Wu DTY, Smart N, Ciemins EL, Lanham HJ, Lindberg C, Zheng K. Using EHR audit trail logs to analyze clinical workflow: a case study from community-based ambulatory clinics. AMIA Annu Symp Proc. 2018;2017:1820-1827. Published 2018 Apr 16.
4. US Department of Veterans Affairs, Veterans Health Administration. https://www.va.gov/health.
5. Cohen T. VA crisis: solutions exist, but haven’t happened, panel hears. https://www.cnn.com/2014/06/12/politics/va-reforms/index.html. Published June 12, 2014. Accessed June 24, 2020.
6. Richardson B. IG probes uncover more problems at VA hospitals. https://thehill.com/policy/defense/258652-ig-probes-uncover-more-problems-at-va-hospitals. Published October 30, 2015. Accessed June 24, 2020.
7. Slack D. Inaccurate VA wait times prelude thousands of vets from getting outside care, probe finds. USA Today. March 3, 2017. https://www.usatoday.com/story/news/politics/2017/03/03/veterans-affairs-inspector-general-widespread-inaccuracies-wait-times/98693856. Accessed June 24, 2020.
8. US Department of Veterans Affairs, Office of the Inspector General. Veterans Health Administration: audit of physician staffing levels for specialty care services. https://www.va.gov/oig/pubs/VAOIG-11-01827-36.pdf. Published December 27, 2012. Accessed June 24, 2020.
9. Government Accountability Office. VA health care: improvements needed in data and monitoring of clinical productivity and efficiency. https://www.gao.gov/assets/690/684869.pdf. Published May 2017. Accessed June 24, 2020.
10. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1082. Patient care data capture. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3091. Published March 24, 2015. Accessed June 24, 2020.
11. US Department of Veterans Affairs, Veterans Health Administration. VHA Handbook 1006.02. VHA site classifications and definitions. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2970. Published December 30, 2013. Accessed June 24, 2020.
12. Alexander M, Ray MA, Hébert JR, et al. The National Veteran Sleep Disorder Study: Descriptive Epidemiology and Secular Trends, 2000-2010. Sleep. 2016;39(7):1399-1410. Published 2016 Jul 1. doi:10.5665/sleep.5972.
13. A Caldwell J, Knapik JJ, Lieberman HR. Trends and factors associated with insomnia and sleep apnea in all United States military service members from 2005 to 2014. J Sleep Res. 2017;26(5):665-670. doi:10.1111/jsr.12543
14. Klingaman EA, Brownlow JA, Boland EM, Mosti C, Gehrman PR. Prevalence, predictors and correlates of insomnia in US army soldiers. J Sleep Res. 2018;27(3):e12612. doi:10.1111/jsr.12612
15. Sharafkhaneh A, Richardson P, Hirshkowitz M. Sleep apnea in a high risk population: a study of Veterans Health Administration beneficiaries. Sleep Med. 2004;5(4):345-350. doi:10.1016/j.sleep.2004.01.019.
16. Sarmiento KF, Folmer RL, Stepnowsky CJ, et al. National Expansion of Sleep Telemedicine for Veterans: The TeleSleep Program. J Clin Sleep Med. 2019;15(9):1355-1364. doi:10.5664/jcsm.7934
17. Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation [published correction appears in Sleep. 2004 Jun 15;27(4):600]. Sleep. 2003;26(2):117-126. doi:10.1093/sleep/26.2.117
18. Johnson EO, Roth T, Breslau N. The association of insomnia with anxiety disorders and depression: exploration of the direction of risk. J Psychiatr Res. 2006;40(8):700-708. doi:10.1016/j.jpsychires.2006.07.008
19. Léger D, Bayon V, Ohayon MM, et al. Insomnia and accidents: cross-sectional study (EQUINOX) on sleep-related home, work and car accidents in 5293 subjects with insomnia from 10 countries. J Sleep Res. 2014;23(2):143-152. doi:10.1111/jsr.12104
20. Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7(8):1311-1322. doi:10.3978/j.issn.2072-1439.2015.06.11
21. Javaheri S, Redline S. Insomnia and Risk of Cardiovascular Disease. Chest. 2017;152(2):435-444. doi:10.1016/j.chest.2017.01.026
22. Linz D, McEvoy RD, Cowie MR, et al. Associations of obstructivesSleepaApnea with atrial fibrillation and continuous positive airway pressure treatment: a review. JAMA Cardiol. 2018;3(6):532-540. doi:10.1001/jamacardio.2018.0095
23. Ogilvie RP, Lakshminarayan K, Iber C, Patel SR, Lutsey PL. Joint effects of OSA and self-reported sleepiness on incident CHD and stroke. Sleep Med. 2018;44:32-37. doi:10.1016/j.sleep.2018.01.004