User login
The ERAS Supplemental Application: Current Status and Recommendations for Dermatology Applicants and Programs
In the 2021-2022 residency application cycle, the Association of American Medical Colleges (AAMC) piloted a supplemental application to accompany the standard residency application submitted via the AAMC’s Electronic Residency Application Service (ERAS).1 Dermatology was 1 of 3 specialties to participate in the pilot alongside internal medicine and general surgery. The goal was to develop a tool that could align applicants with programs that best matched their career goals as well as program and geographic preferences. The Association of Professors of Dermatology Residency Program Directors Section was an early advocate for the supplemental application, and members of our leadership were involved in the design, implementation, and evaluation of the pilot supplemental application.
Participating in the supplemental application was optional for both applicants and programs. The supplemental application included a Past Experiences section, which allowed applicants to highlight their 5 most meaningful research, work, and/or volunteer experiences and to describe a challenging life event that might not otherwise be included with their application. The geographic preferences section permitted applicants to select up to 3 regions of interest as well as to indicate an urban vs rural preference. Lastly, a preference-signaling section allowed dermatology applicants to send signals to up to 3 programs of particular interest.
With the close of another application cycle, applicants and programs will begin preparing for the 2022-2023 recruitment season. In this column, we present dermatology-specific data regarding the supplemental application, highlight tentative changes for the upcoming application cycle, and offer tips for applicants and programs as we approach year 2 of the supplemental application.
Results of Supplemental Application Evaluation Surveys
During the 2021-2022 recruitment season, 93% (950/1019) of dermatology applicants submitted the supplemental application, and 87% (117/135) of dermatology residency programs participated in the pilot.2 Surveys conducted by the AAMC between October 2021 and January 2022 showed that a large majority of dermatology programs used supplemental application data during initial application review when deciding who to interview. Eighty-three percent (40/48) of program directors felt that preference signals in particular helped them identify applicants they would have otherwise overlooked. Fifty-seven percent (4288/7516) of applicants across all specialties that participated in the pilot felt that preference signals would help them be noticed by their preferred programs.2 Preference signals were not evenly distributed among dermatology programs. Programs received an average of 23 signals, with a range of 2 to 87 (AAMC, unpublished data, February 2022).
Additional questions remain to be answered: How does the number of signals received affect application review? How often do geographic and program signals convert to interview offers and matches? Regardless, enthusiasm among dermatology programs for the supplemental application remains. In a recent survey of Association of Professors of Dermatology program directors, all 43 respondents planned to participate in the supplemental application again in the upcoming year (Ilana Rosman, MD; unpublished data; February 2022). The pilot will be expanded to include at least 12 other specialties.1As many who reviewed residency applications in 2021-2022 will attest, there was difficulty accessing the supplemental application data because it was not integrated into the Program Directors’ Work Station, the ERAS platform for programs to access applications, which will be remedied for the 2022-2023 iteration. Other tentative changes include modifications to the past experiences sections and timeline of the application.2
Utilizing the Supplemental Application: Recommendations to Applicants
Format of the Application—Applicants should familiarize themselves with the format of the supplemental application in advance and give themselves sufficient time to complete the application. In general, 3 to 4 hours of focused work should be enough time. Applicants should proofread for grammar and spelling before submitting.
Past Experiences—The past experiences section is intended to provide a focused snapshot of an applicant’s most meaningful activities and unique path to residency. Applicants should answer honestly based on their interests. If a student’s focus has been on volunteerism, the bulk of their 5 experiences listed may be related to service. Similarly, a student who has focused on research may preferentially highlight those experiences. In place of the long list of research, volunteer, and work experiences in the traditional ERAS application, applicants can highlight those activities in which they have been most invested. Applicants are encouraged to reflect on all genres of activities at any stage of their careers, even those not medical in nature, including work experience, military service, college athletics, or sustained musical or artistic achievement. Applicants should explain why each experience is meaningful rather than simply describing the activity.
Applicants also have the option to share a notable challenge they have overcome. It is not expected that each applicant will complete this question; in general, applicants who have not faced notable personal or professional obstacles should avoid answering. Additionally, if these challenges have been discussed in other areas of the application—for example, in the personal statement or medical student performance evaluation—it is not necessary to restate them here, though applicants can choose to do so. Examples of topics a student might discuss include being a first-generation college or medical student, growing up in poverty, facing notable personal or family health challenges, or having limited educational opportunities. It is important to share how this experience impacted an applicant’s journey to dermatology residency.
Geographic Preferences—The geographic preferences section can be difficult for applicants to navigate, as it may involve balancing a desire to attend a residency program in a particular region vs a greater desire to simply match in dermatology. In the past, programs may have made assumptions about geographic preferences based on an applicant’s birthplace, hometown, or medical school. In the supplemental application, applicants have the opportunity to directly reveal their preferences. We encourage applicants to be candid. Selecting a geographic region will not necessarily exclude applicants from consideration at other programs. For some applicants, program qualities may be more important than geography, or there may be no regional preferences. Those applicants can choose “no geographic preference.” There is considerable variability in how programs use geographic preferences. For this reason, it is in the best interest of applicants to simply respond honestly.
Preference Signaling—Preference signaling allows applicants to signal up to 3 preferred programs. Dermatology program directors agree that applicants should not signal their home program or programs at which they did in-person away rotations, as those programs would already be aware of the applicant’s interest. Although a signal increases the chances that the application will be reviewed holistically, it does not guarantee an interview offer. Programs may differentially utilize signals depending on multiple factors, including the number of signals received. We encourage applicants to discuss preference signaling strategies with advisors and focus on signaling programs in which they have genuine interest.
Recommendations to Selection Committees and Program Directors
The intent of the supplemental application is to provide a more meaningful picture of applicants and their experiences and preferences, with the goal of optimizing applicant-program fit. Programs should explicitly define for themselves the applicant characteristics and experiences they prioritize as well as their program goals. The supplemental application offers the potential to streamline holistic application review based on these elements. The short essay answers in the past experiences section permit reviewers to quickly scan for important experiences that align with the program’s recruitment goals. Importantly, reviewers should not penalize applicants who have not completed the question regarding other impactful life experiences, as not all applicants will have relevant information to share.
Some programs may find the geographic preferences section more valuable than others. Multiple factors affect how much weight will be given to geographic preferences, including program location and other characteristics that affect the desirability of the program to applicants. The competitiveness of the field, relatively low match rate, and limited number of programs may lead to less emphasis on geographic preferences in dermatology compared to other specialties. The purpose of this section is not to exclude applicants but to give programs more information that may help with alignment.
Anecdotally, many dermatology program directors were most interested in the preference signaling section of the supplemental application. Programs should consider signals to be evidence of strong preliminary interest. Programs may utilize signals differently depending on many factors such as the overall competitiveness of the program, program location, and the total number of signals the program receives. We recommend that programs holistically review all applications accompanied by a signal. Programs that utilize a points system may choose to award a certain number of points for a signal to their program. A signal might have a higher value at a program that receives only a few signals; conversely, a program that receives a large number of signals might not place tremendous value on the signal but may use it as a tiebreaker between similarly qualified applicants. Preference signaling is solely a tool for application review; because applicants’ preferences may change after the interview process, signals should not be utilized during ranking.
Next Steps
For program directors who have excitedly awaited residency application reform, the supplemental ERAS application is an important first step. Ultimately, we hope the supplemental application supplants much of the current residency application, serving as an efficient high-yield tool for holistically evaluating applicants’ academic and service records, accomplishments, and training preferences. Arriving at a new application will undoubtedly take time and discussion among the various stakeholders. Please continue to complete surveys from the AAMC, as feedback is the best method for refining the tool to serve its intended purpose.
Optimization of the application content is only one component of the reforms needed to improve the application process. Even with a revamped application tool, holistic review is challenging when programs are inundated with an ever-increasing number of applications. As such, we encourage stakeholders to simultaneously consider other potential reforms, such as caps on the number of applications, to allow programs and applicants the best opportunity for a mutually successful match.
- Supplemental ERAS application. Association of American Medical Colleges website. Accessed May 9, 2022. https://students-residents.aamc.org/applying-residencies-eras/supplemental-eras-application-eras-2023-cycle
- Association of American Medical Colleges. Supplemental application data and reports. Accessed May 9, 2022. https://www.aamc.org/data-reports/students-residents/report/supplemental-eras-application-data-and-reports
In the 2021-2022 residency application cycle, the Association of American Medical Colleges (AAMC) piloted a supplemental application to accompany the standard residency application submitted via the AAMC’s Electronic Residency Application Service (ERAS).1 Dermatology was 1 of 3 specialties to participate in the pilot alongside internal medicine and general surgery. The goal was to develop a tool that could align applicants with programs that best matched their career goals as well as program and geographic preferences. The Association of Professors of Dermatology Residency Program Directors Section was an early advocate for the supplemental application, and members of our leadership were involved in the design, implementation, and evaluation of the pilot supplemental application.
Participating in the supplemental application was optional for both applicants and programs. The supplemental application included a Past Experiences section, which allowed applicants to highlight their 5 most meaningful research, work, and/or volunteer experiences and to describe a challenging life event that might not otherwise be included with their application. The geographic preferences section permitted applicants to select up to 3 regions of interest as well as to indicate an urban vs rural preference. Lastly, a preference-signaling section allowed dermatology applicants to send signals to up to 3 programs of particular interest.
With the close of another application cycle, applicants and programs will begin preparing for the 2022-2023 recruitment season. In this column, we present dermatology-specific data regarding the supplemental application, highlight tentative changes for the upcoming application cycle, and offer tips for applicants and programs as we approach year 2 of the supplemental application.
Results of Supplemental Application Evaluation Surveys
During the 2021-2022 recruitment season, 93% (950/1019) of dermatology applicants submitted the supplemental application, and 87% (117/135) of dermatology residency programs participated in the pilot.2 Surveys conducted by the AAMC between October 2021 and January 2022 showed that a large majority of dermatology programs used supplemental application data during initial application review when deciding who to interview. Eighty-three percent (40/48) of program directors felt that preference signals in particular helped them identify applicants they would have otherwise overlooked. Fifty-seven percent (4288/7516) of applicants across all specialties that participated in the pilot felt that preference signals would help them be noticed by their preferred programs.2 Preference signals were not evenly distributed among dermatology programs. Programs received an average of 23 signals, with a range of 2 to 87 (AAMC, unpublished data, February 2022).
Additional questions remain to be answered: How does the number of signals received affect application review? How often do geographic and program signals convert to interview offers and matches? Regardless, enthusiasm among dermatology programs for the supplemental application remains. In a recent survey of Association of Professors of Dermatology program directors, all 43 respondents planned to participate in the supplemental application again in the upcoming year (Ilana Rosman, MD; unpublished data; February 2022). The pilot will be expanded to include at least 12 other specialties.1As many who reviewed residency applications in 2021-2022 will attest, there was difficulty accessing the supplemental application data because it was not integrated into the Program Directors’ Work Station, the ERAS platform for programs to access applications, which will be remedied for the 2022-2023 iteration. Other tentative changes include modifications to the past experiences sections and timeline of the application.2
Utilizing the Supplemental Application: Recommendations to Applicants
Format of the Application—Applicants should familiarize themselves with the format of the supplemental application in advance and give themselves sufficient time to complete the application. In general, 3 to 4 hours of focused work should be enough time. Applicants should proofread for grammar and spelling before submitting.
Past Experiences—The past experiences section is intended to provide a focused snapshot of an applicant’s most meaningful activities and unique path to residency. Applicants should answer honestly based on their interests. If a student’s focus has been on volunteerism, the bulk of their 5 experiences listed may be related to service. Similarly, a student who has focused on research may preferentially highlight those experiences. In place of the long list of research, volunteer, and work experiences in the traditional ERAS application, applicants can highlight those activities in which they have been most invested. Applicants are encouraged to reflect on all genres of activities at any stage of their careers, even those not medical in nature, including work experience, military service, college athletics, or sustained musical or artistic achievement. Applicants should explain why each experience is meaningful rather than simply describing the activity.
Applicants also have the option to share a notable challenge they have overcome. It is not expected that each applicant will complete this question; in general, applicants who have not faced notable personal or professional obstacles should avoid answering. Additionally, if these challenges have been discussed in other areas of the application—for example, in the personal statement or medical student performance evaluation—it is not necessary to restate them here, though applicants can choose to do so. Examples of topics a student might discuss include being a first-generation college or medical student, growing up in poverty, facing notable personal or family health challenges, or having limited educational opportunities. It is important to share how this experience impacted an applicant’s journey to dermatology residency.
Geographic Preferences—The geographic preferences section can be difficult for applicants to navigate, as it may involve balancing a desire to attend a residency program in a particular region vs a greater desire to simply match in dermatology. In the past, programs may have made assumptions about geographic preferences based on an applicant’s birthplace, hometown, or medical school. In the supplemental application, applicants have the opportunity to directly reveal their preferences. We encourage applicants to be candid. Selecting a geographic region will not necessarily exclude applicants from consideration at other programs. For some applicants, program qualities may be more important than geography, or there may be no regional preferences. Those applicants can choose “no geographic preference.” There is considerable variability in how programs use geographic preferences. For this reason, it is in the best interest of applicants to simply respond honestly.
Preference Signaling—Preference signaling allows applicants to signal up to 3 preferred programs. Dermatology program directors agree that applicants should not signal their home program or programs at which they did in-person away rotations, as those programs would already be aware of the applicant’s interest. Although a signal increases the chances that the application will be reviewed holistically, it does not guarantee an interview offer. Programs may differentially utilize signals depending on multiple factors, including the number of signals received. We encourage applicants to discuss preference signaling strategies with advisors and focus on signaling programs in which they have genuine interest.
Recommendations to Selection Committees and Program Directors
The intent of the supplemental application is to provide a more meaningful picture of applicants and their experiences and preferences, with the goal of optimizing applicant-program fit. Programs should explicitly define for themselves the applicant characteristics and experiences they prioritize as well as their program goals. The supplemental application offers the potential to streamline holistic application review based on these elements. The short essay answers in the past experiences section permit reviewers to quickly scan for important experiences that align with the program’s recruitment goals. Importantly, reviewers should not penalize applicants who have not completed the question regarding other impactful life experiences, as not all applicants will have relevant information to share.
Some programs may find the geographic preferences section more valuable than others. Multiple factors affect how much weight will be given to geographic preferences, including program location and other characteristics that affect the desirability of the program to applicants. The competitiveness of the field, relatively low match rate, and limited number of programs may lead to less emphasis on geographic preferences in dermatology compared to other specialties. The purpose of this section is not to exclude applicants but to give programs more information that may help with alignment.
Anecdotally, many dermatology program directors were most interested in the preference signaling section of the supplemental application. Programs should consider signals to be evidence of strong preliminary interest. Programs may utilize signals differently depending on many factors such as the overall competitiveness of the program, program location, and the total number of signals the program receives. We recommend that programs holistically review all applications accompanied by a signal. Programs that utilize a points system may choose to award a certain number of points for a signal to their program. A signal might have a higher value at a program that receives only a few signals; conversely, a program that receives a large number of signals might not place tremendous value on the signal but may use it as a tiebreaker between similarly qualified applicants. Preference signaling is solely a tool for application review; because applicants’ preferences may change after the interview process, signals should not be utilized during ranking.
Next Steps
For program directors who have excitedly awaited residency application reform, the supplemental ERAS application is an important first step. Ultimately, we hope the supplemental application supplants much of the current residency application, serving as an efficient high-yield tool for holistically evaluating applicants’ academic and service records, accomplishments, and training preferences. Arriving at a new application will undoubtedly take time and discussion among the various stakeholders. Please continue to complete surveys from the AAMC, as feedback is the best method for refining the tool to serve its intended purpose.
Optimization of the application content is only one component of the reforms needed to improve the application process. Even with a revamped application tool, holistic review is challenging when programs are inundated with an ever-increasing number of applications. As such, we encourage stakeholders to simultaneously consider other potential reforms, such as caps on the number of applications, to allow programs and applicants the best opportunity for a mutually successful match.
In the 2021-2022 residency application cycle, the Association of American Medical Colleges (AAMC) piloted a supplemental application to accompany the standard residency application submitted via the AAMC’s Electronic Residency Application Service (ERAS).1 Dermatology was 1 of 3 specialties to participate in the pilot alongside internal medicine and general surgery. The goal was to develop a tool that could align applicants with programs that best matched their career goals as well as program and geographic preferences. The Association of Professors of Dermatology Residency Program Directors Section was an early advocate for the supplemental application, and members of our leadership were involved in the design, implementation, and evaluation of the pilot supplemental application.
Participating in the supplemental application was optional for both applicants and programs. The supplemental application included a Past Experiences section, which allowed applicants to highlight their 5 most meaningful research, work, and/or volunteer experiences and to describe a challenging life event that might not otherwise be included with their application. The geographic preferences section permitted applicants to select up to 3 regions of interest as well as to indicate an urban vs rural preference. Lastly, a preference-signaling section allowed dermatology applicants to send signals to up to 3 programs of particular interest.
With the close of another application cycle, applicants and programs will begin preparing for the 2022-2023 recruitment season. In this column, we present dermatology-specific data regarding the supplemental application, highlight tentative changes for the upcoming application cycle, and offer tips for applicants and programs as we approach year 2 of the supplemental application.
Results of Supplemental Application Evaluation Surveys
During the 2021-2022 recruitment season, 93% (950/1019) of dermatology applicants submitted the supplemental application, and 87% (117/135) of dermatology residency programs participated in the pilot.2 Surveys conducted by the AAMC between October 2021 and January 2022 showed that a large majority of dermatology programs used supplemental application data during initial application review when deciding who to interview. Eighty-three percent (40/48) of program directors felt that preference signals in particular helped them identify applicants they would have otherwise overlooked. Fifty-seven percent (4288/7516) of applicants across all specialties that participated in the pilot felt that preference signals would help them be noticed by their preferred programs.2 Preference signals were not evenly distributed among dermatology programs. Programs received an average of 23 signals, with a range of 2 to 87 (AAMC, unpublished data, February 2022).
Additional questions remain to be answered: How does the number of signals received affect application review? How often do geographic and program signals convert to interview offers and matches? Regardless, enthusiasm among dermatology programs for the supplemental application remains. In a recent survey of Association of Professors of Dermatology program directors, all 43 respondents planned to participate in the supplemental application again in the upcoming year (Ilana Rosman, MD; unpublished data; February 2022). The pilot will be expanded to include at least 12 other specialties.1As many who reviewed residency applications in 2021-2022 will attest, there was difficulty accessing the supplemental application data because it was not integrated into the Program Directors’ Work Station, the ERAS platform for programs to access applications, which will be remedied for the 2022-2023 iteration. Other tentative changes include modifications to the past experiences sections and timeline of the application.2
Utilizing the Supplemental Application: Recommendations to Applicants
Format of the Application—Applicants should familiarize themselves with the format of the supplemental application in advance and give themselves sufficient time to complete the application. In general, 3 to 4 hours of focused work should be enough time. Applicants should proofread for grammar and spelling before submitting.
Past Experiences—The past experiences section is intended to provide a focused snapshot of an applicant’s most meaningful activities and unique path to residency. Applicants should answer honestly based on their interests. If a student’s focus has been on volunteerism, the bulk of their 5 experiences listed may be related to service. Similarly, a student who has focused on research may preferentially highlight those experiences. In place of the long list of research, volunteer, and work experiences in the traditional ERAS application, applicants can highlight those activities in which they have been most invested. Applicants are encouraged to reflect on all genres of activities at any stage of their careers, even those not medical in nature, including work experience, military service, college athletics, or sustained musical or artistic achievement. Applicants should explain why each experience is meaningful rather than simply describing the activity.
Applicants also have the option to share a notable challenge they have overcome. It is not expected that each applicant will complete this question; in general, applicants who have not faced notable personal or professional obstacles should avoid answering. Additionally, if these challenges have been discussed in other areas of the application—for example, in the personal statement or medical student performance evaluation—it is not necessary to restate them here, though applicants can choose to do so. Examples of topics a student might discuss include being a first-generation college or medical student, growing up in poverty, facing notable personal or family health challenges, or having limited educational opportunities. It is important to share how this experience impacted an applicant’s journey to dermatology residency.
Geographic Preferences—The geographic preferences section can be difficult for applicants to navigate, as it may involve balancing a desire to attend a residency program in a particular region vs a greater desire to simply match in dermatology. In the past, programs may have made assumptions about geographic preferences based on an applicant’s birthplace, hometown, or medical school. In the supplemental application, applicants have the opportunity to directly reveal their preferences. We encourage applicants to be candid. Selecting a geographic region will not necessarily exclude applicants from consideration at other programs. For some applicants, program qualities may be more important than geography, or there may be no regional preferences. Those applicants can choose “no geographic preference.” There is considerable variability in how programs use geographic preferences. For this reason, it is in the best interest of applicants to simply respond honestly.
Preference Signaling—Preference signaling allows applicants to signal up to 3 preferred programs. Dermatology program directors agree that applicants should not signal their home program or programs at which they did in-person away rotations, as those programs would already be aware of the applicant’s interest. Although a signal increases the chances that the application will be reviewed holistically, it does not guarantee an interview offer. Programs may differentially utilize signals depending on multiple factors, including the number of signals received. We encourage applicants to discuss preference signaling strategies with advisors and focus on signaling programs in which they have genuine interest.
Recommendations to Selection Committees and Program Directors
The intent of the supplemental application is to provide a more meaningful picture of applicants and their experiences and preferences, with the goal of optimizing applicant-program fit. Programs should explicitly define for themselves the applicant characteristics and experiences they prioritize as well as their program goals. The supplemental application offers the potential to streamline holistic application review based on these elements. The short essay answers in the past experiences section permit reviewers to quickly scan for important experiences that align with the program’s recruitment goals. Importantly, reviewers should not penalize applicants who have not completed the question regarding other impactful life experiences, as not all applicants will have relevant information to share.
Some programs may find the geographic preferences section more valuable than others. Multiple factors affect how much weight will be given to geographic preferences, including program location and other characteristics that affect the desirability of the program to applicants. The competitiveness of the field, relatively low match rate, and limited number of programs may lead to less emphasis on geographic preferences in dermatology compared to other specialties. The purpose of this section is not to exclude applicants but to give programs more information that may help with alignment.
Anecdotally, many dermatology program directors were most interested in the preference signaling section of the supplemental application. Programs should consider signals to be evidence of strong preliminary interest. Programs may utilize signals differently depending on many factors such as the overall competitiveness of the program, program location, and the total number of signals the program receives. We recommend that programs holistically review all applications accompanied by a signal. Programs that utilize a points system may choose to award a certain number of points for a signal to their program. A signal might have a higher value at a program that receives only a few signals; conversely, a program that receives a large number of signals might not place tremendous value on the signal but may use it as a tiebreaker between similarly qualified applicants. Preference signaling is solely a tool for application review; because applicants’ preferences may change after the interview process, signals should not be utilized during ranking.
Next Steps
For program directors who have excitedly awaited residency application reform, the supplemental ERAS application is an important first step. Ultimately, we hope the supplemental application supplants much of the current residency application, serving as an efficient high-yield tool for holistically evaluating applicants’ academic and service records, accomplishments, and training preferences. Arriving at a new application will undoubtedly take time and discussion among the various stakeholders. Please continue to complete surveys from the AAMC, as feedback is the best method for refining the tool to serve its intended purpose.
Optimization of the application content is only one component of the reforms needed to improve the application process. Even with a revamped application tool, holistic review is challenging when programs are inundated with an ever-increasing number of applications. As such, we encourage stakeholders to simultaneously consider other potential reforms, such as caps on the number of applications, to allow programs and applicants the best opportunity for a mutually successful match.
- Supplemental ERAS application. Association of American Medical Colleges website. Accessed May 9, 2022. https://students-residents.aamc.org/applying-residencies-eras/supplemental-eras-application-eras-2023-cycle
- Association of American Medical Colleges. Supplemental application data and reports. Accessed May 9, 2022. https://www.aamc.org/data-reports/students-residents/report/supplemental-eras-application-data-and-reports
- Supplemental ERAS application. Association of American Medical Colleges website. Accessed May 9, 2022. https://students-residents.aamc.org/applying-residencies-eras/supplemental-eras-application-eras-2023-cycle
- Association of American Medical Colleges. Supplemental application data and reports. Accessed May 9, 2022. https://www.aamc.org/data-reports/students-residents/report/supplemental-eras-application-data-and-reports
Practice Points
- The Electronic Residency Application Service (ERAS) Supplemental Application was piloted in the 2021-2022 residency application cycle and was utilized by the vast majority of dermatology applicants and programs.
- Survey data suggested that both applicants and programs found the supplemental application useful, particularly the preference signaling portion.
- The supplemental application will return for the 2022-2023 application cycle and will be integrated into the MyERAS workstation platform for easier access by programs.
Caring for Muslim patients who fast during Ramadan
Ramadan is one of the obligatory pillars in Islam during which healthy Muslims are required to fast from dawn until sunset every day for 1 month. There are an estimated 3.45 million Muslims in the United States, and this population will continue to grow by 100,000 per year.1 With the increased growth of the Muslim population, it is important for clinicians to be aware of how patients of Muslim faith are affected during Ramadan. In this article, we explore the potential risks, as well as the benefits, the month of Ramadan brings to patients. We will also explain how being religiously aware is necessary to provide optimal care for these individuals.
For some patients, fasting may pose risks
Similar to other communities in the United States, individuals who are Muslim experience mood disorders, anxiety disorders, posttraumatic stress disorder, obsessive-compulsive disorder, schizophrenia, substance use disorders, and other psychiatric illnesses.2 During the month of Ramadan, Muslims are to abstain completely from eating and drinking from dawn until sunset. This includes medications as well as food and drink.
Due to these circumstances, patients will often change the timing, frequency, and dosing of their medications to allow them to fast. One study found 60% of Muslims made medication adjustments during Ramadan without seeking medical advice.3 It is possible that such alterations may be detrimental. During Ramadan, some Muslims wake up early in the morning to eat a pre-dawn meal, and often go back to sleep. This has been reported to cause a delay in sleep-wake times and to reduce rapid eye movement sleep.4 These circadian rhythm changes can be detrimental to patients with bipolar disorder. One study found higher rates of relapse to depression and mania in patients with bipolar disorder who were fasting during Ramadan.5 Circadian rhythm disturbances also may worsen depression.6 Another point of concern is patients with eating disorders. One small case series (N = 6) found that fasting during Ramadan exacerbated symptoms in patients with eating disorders.7
Another concern is that dehydration while fasting can lead to lithium toxicity. However, one study found lithium levels remained stable while fasting for 10 to 12 hours.5 Another showed that changing lithium dosing from twice a day to once a day allowed for easier administration without causing a subtherapeutic change in blood lithium levels.8
The practice also may have benefits for mental health
For many Muslims, Ramadan is the best time of the year, where they reconnect with their religion and experience the utmost spiritual growth. Studies have shown that the incidence of suicide is lowest during Ramadan compared to other months.9 A study of older men found that intermittent fasting and calorie restriction (not during Ramadan) resulted in decreases in tension, confusion, anger, and mood disturbance.10 Another study found that fasting during Ramadan had a positive impact on depression, anxiety, stress, and cognitive function.11
Clinical considerations
To provide the best care for Muslim patients during Ramadan, clinicians should take a holistic approach and take all factors into consideration. It is common for circadian rhythm disruptions to exacerbate mood disorders, so encourage patients to maintain healthy sleep hygiene to their best ability during this month. Another important consideration is medication timing and dosing.12 For patients prescribed a medication that typically is taken twice a day, determine if this dosing can be changed to once a day, or if both doses can be taken when it is permissible to eat (sunset to dawn). For medications that are absorbed with food, consider how these medications might be adjusted and maintained while a patient is fasting. Some medications may be sedating or activating, so the timing of administration may need to be adjusted to meet the patient’s needs. Lastly, keep in mind that certain medications can have withdrawal effects, and the likelihood of this occurring while a patient is fasting.
One vital point is that if a patient is at high risk of clinically decompensating due to fasting or medication adjustments or discontinuation, advise them to not fast. Muslims with physical or mental illnesses are excused from fasting. Bear in mind that because Ramadan is meant to be a month of heightened spirituality, many Muslims will prefer to fast.
1. Pew Research Center. Demographic portrait of Muslim Americans. Published July 26, 2017. Accessed January 15, 2019. https://www.pewforum.org/2017/07/26/demographic-portrait-of-muslim-americans
2. Basit A, Hamid M. Mental health issues of Muslim Americans. J IMA. 2010;42(3):106-110.
3. Aslam M, Assad A. Drug regimens and fasting during Ramadan: a survey in Kuwait. Public Health. 1986;100(1):49-53.
4. Qasrawi SO, Pandi-Perumal SR, BaHammam AS. The effect of intermittent fasting during Ramadan on sleep, sleepiness, cognitive function, and circadian rhythm. Sleep Breath. 2017;21(3):577-586.
5. Eddahby S, Kadri N, Moussaoui D. Fasting during Ramadan is associated with a higher recurrence rate in patients with bipolar disorder. World Psychiatry. 2014;13(1):97.
6. Germain A, Kupfer DJ. Circadian rhythm disturbances in depression. Hum Psychopharmacol. 2008;23(7):571-585.
7. Akgül S, Derman O, Kanbur NÖ. Fasting during Ramadan: a religious factor as a possible trigger or exacerbator for eating disorders in adolescents. Int J Eat Disord. 2014;47(8):905-910.
8. Kadri N, Mouchtaq N, Hakkou F, et al. Relapses in bipolar patients: changes in social rhythm? Int J Neuropsychopharmacol. 2000;3(1):45-49.
9. Taktak S, Kumral B, Unsal A, et al. Evidence for an association between suicide and religion: a 33-year retrospective autopsy analysis of suicide by hanging during the month of Ramadan in Istanbul. Aust J Forensic Sci. 2016;48(2):121-131.
10. Hussin NM, Shahar S, Teng NI, et al. Efficacy of fasting and calorie restriction (FCR) on mood and depression among ageing men. J Nutr Health Aging. 2013;17(8):674-680.
11. Amin A, Sai Sailesh K, Mishra S, et al. Effects of fasting during Ramadan month on depression, anxiety and stress and cognition. Int J Med Res Rev. 2016;4(5):771-774.
12. Furqan Z, Awaad R, Kurdyak P, et al. Considerations for clinicians treating Muslim patients with psychiatric disorders during Ramadan. Lancet Psychiatry. 2019;6(7):556-557.
Ramadan is one of the obligatory pillars in Islam during which healthy Muslims are required to fast from dawn until sunset every day for 1 month. There are an estimated 3.45 million Muslims in the United States, and this population will continue to grow by 100,000 per year.1 With the increased growth of the Muslim population, it is important for clinicians to be aware of how patients of Muslim faith are affected during Ramadan. In this article, we explore the potential risks, as well as the benefits, the month of Ramadan brings to patients. We will also explain how being religiously aware is necessary to provide optimal care for these individuals.
For some patients, fasting may pose risks
Similar to other communities in the United States, individuals who are Muslim experience mood disorders, anxiety disorders, posttraumatic stress disorder, obsessive-compulsive disorder, schizophrenia, substance use disorders, and other psychiatric illnesses.2 During the month of Ramadan, Muslims are to abstain completely from eating and drinking from dawn until sunset. This includes medications as well as food and drink.
Due to these circumstances, patients will often change the timing, frequency, and dosing of their medications to allow them to fast. One study found 60% of Muslims made medication adjustments during Ramadan without seeking medical advice.3 It is possible that such alterations may be detrimental. During Ramadan, some Muslims wake up early in the morning to eat a pre-dawn meal, and often go back to sleep. This has been reported to cause a delay in sleep-wake times and to reduce rapid eye movement sleep.4 These circadian rhythm changes can be detrimental to patients with bipolar disorder. One study found higher rates of relapse to depression and mania in patients with bipolar disorder who were fasting during Ramadan.5 Circadian rhythm disturbances also may worsen depression.6 Another point of concern is patients with eating disorders. One small case series (N = 6) found that fasting during Ramadan exacerbated symptoms in patients with eating disorders.7
Another concern is that dehydration while fasting can lead to lithium toxicity. However, one study found lithium levels remained stable while fasting for 10 to 12 hours.5 Another showed that changing lithium dosing from twice a day to once a day allowed for easier administration without causing a subtherapeutic change in blood lithium levels.8
The practice also may have benefits for mental health
For many Muslims, Ramadan is the best time of the year, where they reconnect with their religion and experience the utmost spiritual growth. Studies have shown that the incidence of suicide is lowest during Ramadan compared to other months.9 A study of older men found that intermittent fasting and calorie restriction (not during Ramadan) resulted in decreases in tension, confusion, anger, and mood disturbance.10 Another study found that fasting during Ramadan had a positive impact on depression, anxiety, stress, and cognitive function.11
Clinical considerations
To provide the best care for Muslim patients during Ramadan, clinicians should take a holistic approach and take all factors into consideration. It is common for circadian rhythm disruptions to exacerbate mood disorders, so encourage patients to maintain healthy sleep hygiene to their best ability during this month. Another important consideration is medication timing and dosing.12 For patients prescribed a medication that typically is taken twice a day, determine if this dosing can be changed to once a day, or if both doses can be taken when it is permissible to eat (sunset to dawn). For medications that are absorbed with food, consider how these medications might be adjusted and maintained while a patient is fasting. Some medications may be sedating or activating, so the timing of administration may need to be adjusted to meet the patient’s needs. Lastly, keep in mind that certain medications can have withdrawal effects, and the likelihood of this occurring while a patient is fasting.
One vital point is that if a patient is at high risk of clinically decompensating due to fasting or medication adjustments or discontinuation, advise them to not fast. Muslims with physical or mental illnesses are excused from fasting. Bear in mind that because Ramadan is meant to be a month of heightened spirituality, many Muslims will prefer to fast.
Ramadan is one of the obligatory pillars in Islam during which healthy Muslims are required to fast from dawn until sunset every day for 1 month. There are an estimated 3.45 million Muslims in the United States, and this population will continue to grow by 100,000 per year.1 With the increased growth of the Muslim population, it is important for clinicians to be aware of how patients of Muslim faith are affected during Ramadan. In this article, we explore the potential risks, as well as the benefits, the month of Ramadan brings to patients. We will also explain how being religiously aware is necessary to provide optimal care for these individuals.
For some patients, fasting may pose risks
Similar to other communities in the United States, individuals who are Muslim experience mood disorders, anxiety disorders, posttraumatic stress disorder, obsessive-compulsive disorder, schizophrenia, substance use disorders, and other psychiatric illnesses.2 During the month of Ramadan, Muslims are to abstain completely from eating and drinking from dawn until sunset. This includes medications as well as food and drink.
Due to these circumstances, patients will often change the timing, frequency, and dosing of their medications to allow them to fast. One study found 60% of Muslims made medication adjustments during Ramadan without seeking medical advice.3 It is possible that such alterations may be detrimental. During Ramadan, some Muslims wake up early in the morning to eat a pre-dawn meal, and often go back to sleep. This has been reported to cause a delay in sleep-wake times and to reduce rapid eye movement sleep.4 These circadian rhythm changes can be detrimental to patients with bipolar disorder. One study found higher rates of relapse to depression and mania in patients with bipolar disorder who were fasting during Ramadan.5 Circadian rhythm disturbances also may worsen depression.6 Another point of concern is patients with eating disorders. One small case series (N = 6) found that fasting during Ramadan exacerbated symptoms in patients with eating disorders.7
Another concern is that dehydration while fasting can lead to lithium toxicity. However, one study found lithium levels remained stable while fasting for 10 to 12 hours.5 Another showed that changing lithium dosing from twice a day to once a day allowed for easier administration without causing a subtherapeutic change in blood lithium levels.8
The practice also may have benefits for mental health
For many Muslims, Ramadan is the best time of the year, where they reconnect with their religion and experience the utmost spiritual growth. Studies have shown that the incidence of suicide is lowest during Ramadan compared to other months.9 A study of older men found that intermittent fasting and calorie restriction (not during Ramadan) resulted in decreases in tension, confusion, anger, and mood disturbance.10 Another study found that fasting during Ramadan had a positive impact on depression, anxiety, stress, and cognitive function.11
Clinical considerations
To provide the best care for Muslim patients during Ramadan, clinicians should take a holistic approach and take all factors into consideration. It is common for circadian rhythm disruptions to exacerbate mood disorders, so encourage patients to maintain healthy sleep hygiene to their best ability during this month. Another important consideration is medication timing and dosing.12 For patients prescribed a medication that typically is taken twice a day, determine if this dosing can be changed to once a day, or if both doses can be taken when it is permissible to eat (sunset to dawn). For medications that are absorbed with food, consider how these medications might be adjusted and maintained while a patient is fasting. Some medications may be sedating or activating, so the timing of administration may need to be adjusted to meet the patient’s needs. Lastly, keep in mind that certain medications can have withdrawal effects, and the likelihood of this occurring while a patient is fasting.
One vital point is that if a patient is at high risk of clinically decompensating due to fasting or medication adjustments or discontinuation, advise them to not fast. Muslims with physical or mental illnesses are excused from fasting. Bear in mind that because Ramadan is meant to be a month of heightened spirituality, many Muslims will prefer to fast.
1. Pew Research Center. Demographic portrait of Muslim Americans. Published July 26, 2017. Accessed January 15, 2019. https://www.pewforum.org/2017/07/26/demographic-portrait-of-muslim-americans
2. Basit A, Hamid M. Mental health issues of Muslim Americans. J IMA. 2010;42(3):106-110.
3. Aslam M, Assad A. Drug regimens and fasting during Ramadan: a survey in Kuwait. Public Health. 1986;100(1):49-53.
4. Qasrawi SO, Pandi-Perumal SR, BaHammam AS. The effect of intermittent fasting during Ramadan on sleep, sleepiness, cognitive function, and circadian rhythm. Sleep Breath. 2017;21(3):577-586.
5. Eddahby S, Kadri N, Moussaoui D. Fasting during Ramadan is associated with a higher recurrence rate in patients with bipolar disorder. World Psychiatry. 2014;13(1):97.
6. Germain A, Kupfer DJ. Circadian rhythm disturbances in depression. Hum Psychopharmacol. 2008;23(7):571-585.
7. Akgül S, Derman O, Kanbur NÖ. Fasting during Ramadan: a religious factor as a possible trigger or exacerbator for eating disorders in adolescents. Int J Eat Disord. 2014;47(8):905-910.
8. Kadri N, Mouchtaq N, Hakkou F, et al. Relapses in bipolar patients: changes in social rhythm? Int J Neuropsychopharmacol. 2000;3(1):45-49.
9. Taktak S, Kumral B, Unsal A, et al. Evidence for an association between suicide and religion: a 33-year retrospective autopsy analysis of suicide by hanging during the month of Ramadan in Istanbul. Aust J Forensic Sci. 2016;48(2):121-131.
10. Hussin NM, Shahar S, Teng NI, et al. Efficacy of fasting and calorie restriction (FCR) on mood and depression among ageing men. J Nutr Health Aging. 2013;17(8):674-680.
11. Amin A, Sai Sailesh K, Mishra S, et al. Effects of fasting during Ramadan month on depression, anxiety and stress and cognition. Int J Med Res Rev. 2016;4(5):771-774.
12. Furqan Z, Awaad R, Kurdyak P, et al. Considerations for clinicians treating Muslim patients with psychiatric disorders during Ramadan. Lancet Psychiatry. 2019;6(7):556-557.
1. Pew Research Center. Demographic portrait of Muslim Americans. Published July 26, 2017. Accessed January 15, 2019. https://www.pewforum.org/2017/07/26/demographic-portrait-of-muslim-americans
2. Basit A, Hamid M. Mental health issues of Muslim Americans. J IMA. 2010;42(3):106-110.
3. Aslam M, Assad A. Drug regimens and fasting during Ramadan: a survey in Kuwait. Public Health. 1986;100(1):49-53.
4. Qasrawi SO, Pandi-Perumal SR, BaHammam AS. The effect of intermittent fasting during Ramadan on sleep, sleepiness, cognitive function, and circadian rhythm. Sleep Breath. 2017;21(3):577-586.
5. Eddahby S, Kadri N, Moussaoui D. Fasting during Ramadan is associated with a higher recurrence rate in patients with bipolar disorder. World Psychiatry. 2014;13(1):97.
6. Germain A, Kupfer DJ. Circadian rhythm disturbances in depression. Hum Psychopharmacol. 2008;23(7):571-585.
7. Akgül S, Derman O, Kanbur NÖ. Fasting during Ramadan: a religious factor as a possible trigger or exacerbator for eating disorders in adolescents. Int J Eat Disord. 2014;47(8):905-910.
8. Kadri N, Mouchtaq N, Hakkou F, et al. Relapses in bipolar patients: changes in social rhythm? Int J Neuropsychopharmacol. 2000;3(1):45-49.
9. Taktak S, Kumral B, Unsal A, et al. Evidence for an association between suicide and religion: a 33-year retrospective autopsy analysis of suicide by hanging during the month of Ramadan in Istanbul. Aust J Forensic Sci. 2016;48(2):121-131.
10. Hussin NM, Shahar S, Teng NI, et al. Efficacy of fasting and calorie restriction (FCR) on mood and depression among ageing men. J Nutr Health Aging. 2013;17(8):674-680.
11. Amin A, Sai Sailesh K, Mishra S, et al. Effects of fasting during Ramadan month on depression, anxiety and stress and cognition. Int J Med Res Rev. 2016;4(5):771-774.
12. Furqan Z, Awaad R, Kurdyak P, et al. Considerations for clinicians treating Muslim patients with psychiatric disorders during Ramadan. Lancet Psychiatry. 2019;6(7):556-557.
Navigating Motherhood and Dermatology Residency
Motherhood and dermatology residency are both full-time jobs. The thought that a woman must either be superhuman to succeed at both or that success at one must come at the expense of the other is antiquated. With careful navigation and sufficient support, these two roles can complement and heighten one another. The most recent Accreditation Council for Graduate Medical Education (ACGME) report showed that nearly 60% of dermatology residents are women,1 with most women in training being of childbearing age. One study showed that female dermatologists were most likely to have children during residency (51% of those surveyed), despite residents reporting more barriers to childbearing at this career stage.2 Trainees thinking of starting a family have many considerations to navigate: timing of pregnancy, maternity leave scheduling, breastfeeding while working, and planning for childcare. For the first time in the history of the specialty, most active dermatologists in practice are women.3 Thus, the future of dermatology requires supportive policies and resources for the successful navigation of these issues by today’s trainees.
Timing of Pregnancy
Timing of pregnancy can be a source of stress to the female dermatology resident. Barriers to childbearing during residency include the perception that women who have children during residency training are less committed to their jobs; concerns of overburdening fellow residents; and fear that residency may need to be extended, thereby delaying the ability to sit for the board examination.2 However, the potential increased risk for infertility in delaying pregnancy adds to the stress of pregnancy planning. A 2016 survey of female physicians (N=327) showed that 24.1% of respondents who had attempted conception were diagnosed with infertility, with an average age at diagnosis of 33.7 years.4 This is higher than the national average, with the Centers for Disease Control and Prevention reporting that approximately 19% of women aged 15 to 49 years with no prior births experience infertility.5 In a 1992 survey of female physician residents (N=373) who gave birth during residency, 32% indicated that they would not recommend the experience to others; of the 68% who would recommend the experience, one-third encouraged timing delivery to occur in the last 2 months of residency due to benefits of continued insurance coverage, a decrease in clinic responsibilities, and the potential for extended maternity leave during hiatus between jobs.6 Although this may be a good strategy, studying and sitting for board examinations while caring for a newborn right after graduation may be overly difficult for some. The first year of residency was perceived as the most stressful time to be pregnant, with each subsequent year being less problematic.6 Planning pregnancy for delivery near the end of the second year and beginning of the third year of dermatology residency may be a reasonable choice.
Maternity Leave
The Family and Medical Leave Act entitles eligible employees of covered employers to take unpaid, job-protected leave, with 12 workweeks of leave in a 12-month period for the birth of a child and to care for the newborn child within 1 year of birth.7 The actual length of maternity leave taken by most surveyed female dermatologists (n=96) is shorter: 25% (24/96) took less than 4 weeks, 42.7% (41/96) took 4 to 8 weeks, 25% (24/96) took 9 to 12 weeks, and 7.3% (7/96) were able to take more than 12 weeks of maternity leave.2
The American Board of Dermatology implemented a new Resident Leave policy that went into effect July 1, 2021, stipulating that, within certain parameters, time spent away from training for family and parental leave would not exhaust vacation time or require an extension in training. Under this policy, absence from training exceeding 8 weeks (6 weeks leave, plus 2 weeks of vacation) in a given year should be approved only under exceptional circumstances and may necessitate additional training time to ensure that competency requirements are met.8 Although this policy is a step in the right direction, institutional policies still may vary. Dermatology residents planning to start a family during training should consider their plans for fellowship, as taking an extended maternity leave beyond 8 weeks may jeopardize a July fellowship start date.
Lactation and Residency
The American Academy of Pediatrics recommends exclusive breastfeeding for approximately 6 months, with continuation of breastfeeding for 1 year or longer as mutually desired by the mother and infant.9 Successful lactation and achieving breastfeeding goals can be difficult during medical training. A national cross-sectional survey of female residents (N=312) showed that the median total time of breastfeeding and pumping was 9 months, with 74% continuing after 6 months and 13% continuing past 12 months. Of those surveyed, 73% reported residency limited their ability to lactate, and 37% stopped prior to their desired goal.10 As of July 1, 2020, the ACGME requires that residency programs and sponsoring institutions provide clean and private facilities for lactation that have refrigeration capabilities, with proximity appropriate for safe patient care.11 There has been a call to dermatology program leadership to support breastfeeding residents by providing sufficient time and space to pump; a breastfeeding resident will need a 20- to 30-minute break to express milk approximately every 3 hours during the work day.12 One innovative initiative to meet the ACGME lactation requirement reported by the Kansas University Medical Center Graduate Medical Education program (Kansas City, Kansas) was the purchase of wearable breast pumps to loan to residents. The benefits of wearable breast pumps are that they are discreet and can allow mothers to express milk inconspicuously while working, can increase milk supply, require less set up and expression time than traditional pumps, and can allow the mother to manage time more efficiently.13 Breastfeeding plans and goals should be discussed with program leadership before return from leave to strategize and anticipate gaps in clinic scheduling to accommodate the lactating resident.
Planning for Childcare
Resident hours can be long and erratic, making choices for childcare difficult. In one survey of female residents, 61% of married or partnered respondents (n=447) were delaying childbearing, and 46% cited lack of access to childcare as a reason.14 Not all dermatology residents are fortunate enough to match to a program near family, but close family support can be an undeniable asset during childrearing and should be weighed heavily when ranking programs. Options for childcare include relying on a stay-at-home spouse or other family member, a live-in or live-out nanny, part-time babysitters, and daycare. It is crucial to have multiple layers and back-up options for childcare available at any given time when working as a resident. Even with a child enrolled in a full-time daycare and a live-in nanny, a daycare closure due to a COVID-19 exposure or sudden medical emergency in the nanny can still leave unpredicted holes in your childcare plan, leaving the resident to potentially miss work to fill the gap. A survey of residents at one institution showed that the most common backup childcare plan for situations in which either the child or the regular caregiver is ill is for the nontrainee parent or spouse to stay home (45%; n=101), with 25% of respondents staying home to care for a sick child themselves, which clearly has an impact on the hospital. The article proposed implementation of on-site or near-site childcare for residents with extended hours or a 24-hour emergency drop-in availability.15 One institution reported success with the development of a departmentally funded childcare supplementation stipend offered to residents to support daycare costs during the first 6 months of a baby’s life.16
Final Thoughts
Due to the competitiveness of the field, dermatology residents are by nature high performing and academically successful. For a high achiever, the idea of potentially disappointing faculty and colleagues by starting a family during residency can be guilt inducing. Concerns about one’s ability to adequately study the breadth of dermatology while simultaneously raising a child can be distressing; however, there are many ways in which motherhood can hone skills to become a better dermatology resident. Through motherhood one can enhance time management skills, increase efficiency, and improve rapport with pediatric patients and trust with their parents/guardians. A dermatology resident may be her own harshest critic, but it is time that the future generation of dermatologists become their own greatest advocates for establishing supportive policies and resources for the successful navigation of motherhood and dermatology residency.
- ACGME residents and fellows by sex and specialty, 2019. Association of American Medical Colleges website. Accessed April 21, 2022. https://www.aamc.org/data-reports/interactive-data/acgme-residents-and-fellows-sex-and-specialty-2019
- Mattessich S, Shea K, Whitaker-Worth D. Parenting and female dermatologists’ perceptions of work-life balance. Int J Womens Dermatol. 2017;3:127-130. doi:10.1016/j.ijwd.2017.04.001
- Active physicians by sex and specialty, 2019. Association of American Medical Colleges website. Accessed April 21, 2022. https://www.aamc.org/data-reports/workforce/interactive-data/active-physicians-sex-and-specialty-2019
- Stentz NC, Griffith KA, Perkins E, et al. Fertility and childbearing among American female physicians. J Womens Health. 2016;25:1059-1065. doi:10.1089/jwh.2015.5638
- Infertility. Centers for Disease Control and Prevention website. Updated March 1, 2022. Accessed April 21, 2022. https://www.cdc.gov/reproductivehealth/infertility/
- Phelan ST. Sources of stress and support for the pregnant resident. Acad Med. 1992;67:408-410. doi:10.1097/00001888-199206000-00014
- Family and Medical Leave Act. US Department of Labor website. Accessed April 21, 2022. https://www.dol.gov/agencies/whd/fmla
- American Board of Dermatology. Effective July 2021: new family leave policy. Accessed April 21, 2022. https://www.abderm.org/public/announcements/effective-july-2021-new-family-leave-policy.aspx
- Eidelman AI, Schanler RJ, Johnston M, et al. Breastfeeding and the use of human milk. Pediatrics. 2012;129:E827-E841. doi:10.1542/peds.2011-3552
- Peters GW, Kuczmarska-Haas A, Holliday EB, et al. Lactation challenges of resident physicians: results of a national survey. BMC Pregnancy Childbirth. 2020;20:762. doi:10.1186/s12884-020-03436-3
- Common program requirements (residency) sections I-V table of implementation dates. Accreditation Council for Graduate Medical Education website. Accessed April 21, 2022. https://www.acgme.org/globalassets/PFAssets/ProgramRequirements/CPRResidencyImplementationTable.pdf
- Gracey LE, Mathes EF, Shinkai K. Supporting breastfeeding mothers during dermatology residency—challenges and best practices. JAMA Dermatol. 2020;156:117-118. doi:10.1001/jamadermatol.2019.3759
- McMillin A, Behravesh B, Byrne P, et al. A GME wearable breast pump program: an innovative method to meet ACGME requirements and federal law. J Grad Med Educ. 2021;13:422-423. doi:10.4300/jgme-d-20-01275.1
- Stack SW, Jagsi R, Biermann JS, et al. Childbearing decisions in residency: a multicenter survey of female residents. Acad Med. 2020;95:1550-1557. doi:10.1097/acm.0000000000003549
- Snyder RA, Tarpley MJ, Phillips SE, et al. The case for on-site child care in residency training and afterward. J Grad Med Educ. 2013;5:365-367. doi:10.4300/jgme-d-12-00294.1
- Key LL. Child care supplementation: aid for residents and advantages for residency programs. J Pediatr. 2008;153:449-450. doi:10.1016/j.jpeds.2008.05.028
Motherhood and dermatology residency are both full-time jobs. The thought that a woman must either be superhuman to succeed at both or that success at one must come at the expense of the other is antiquated. With careful navigation and sufficient support, these two roles can complement and heighten one another. The most recent Accreditation Council for Graduate Medical Education (ACGME) report showed that nearly 60% of dermatology residents are women,1 with most women in training being of childbearing age. One study showed that female dermatologists were most likely to have children during residency (51% of those surveyed), despite residents reporting more barriers to childbearing at this career stage.2 Trainees thinking of starting a family have many considerations to navigate: timing of pregnancy, maternity leave scheduling, breastfeeding while working, and planning for childcare. For the first time in the history of the specialty, most active dermatologists in practice are women.3 Thus, the future of dermatology requires supportive policies and resources for the successful navigation of these issues by today’s trainees.
Timing of Pregnancy
Timing of pregnancy can be a source of stress to the female dermatology resident. Barriers to childbearing during residency include the perception that women who have children during residency training are less committed to their jobs; concerns of overburdening fellow residents; and fear that residency may need to be extended, thereby delaying the ability to sit for the board examination.2 However, the potential increased risk for infertility in delaying pregnancy adds to the stress of pregnancy planning. A 2016 survey of female physicians (N=327) showed that 24.1% of respondents who had attempted conception were diagnosed with infertility, with an average age at diagnosis of 33.7 years.4 This is higher than the national average, with the Centers for Disease Control and Prevention reporting that approximately 19% of women aged 15 to 49 years with no prior births experience infertility.5 In a 1992 survey of female physician residents (N=373) who gave birth during residency, 32% indicated that they would not recommend the experience to others; of the 68% who would recommend the experience, one-third encouraged timing delivery to occur in the last 2 months of residency due to benefits of continued insurance coverage, a decrease in clinic responsibilities, and the potential for extended maternity leave during hiatus between jobs.6 Although this may be a good strategy, studying and sitting for board examinations while caring for a newborn right after graduation may be overly difficult for some. The first year of residency was perceived as the most stressful time to be pregnant, with each subsequent year being less problematic.6 Planning pregnancy for delivery near the end of the second year and beginning of the third year of dermatology residency may be a reasonable choice.
Maternity Leave
The Family and Medical Leave Act entitles eligible employees of covered employers to take unpaid, job-protected leave, with 12 workweeks of leave in a 12-month period for the birth of a child and to care for the newborn child within 1 year of birth.7 The actual length of maternity leave taken by most surveyed female dermatologists (n=96) is shorter: 25% (24/96) took less than 4 weeks, 42.7% (41/96) took 4 to 8 weeks, 25% (24/96) took 9 to 12 weeks, and 7.3% (7/96) were able to take more than 12 weeks of maternity leave.2
The American Board of Dermatology implemented a new Resident Leave policy that went into effect July 1, 2021, stipulating that, within certain parameters, time spent away from training for family and parental leave would not exhaust vacation time or require an extension in training. Under this policy, absence from training exceeding 8 weeks (6 weeks leave, plus 2 weeks of vacation) in a given year should be approved only under exceptional circumstances and may necessitate additional training time to ensure that competency requirements are met.8 Although this policy is a step in the right direction, institutional policies still may vary. Dermatology residents planning to start a family during training should consider their plans for fellowship, as taking an extended maternity leave beyond 8 weeks may jeopardize a July fellowship start date.
Lactation and Residency
The American Academy of Pediatrics recommends exclusive breastfeeding for approximately 6 months, with continuation of breastfeeding for 1 year or longer as mutually desired by the mother and infant.9 Successful lactation and achieving breastfeeding goals can be difficult during medical training. A national cross-sectional survey of female residents (N=312) showed that the median total time of breastfeeding and pumping was 9 months, with 74% continuing after 6 months and 13% continuing past 12 months. Of those surveyed, 73% reported residency limited their ability to lactate, and 37% stopped prior to their desired goal.10 As of July 1, 2020, the ACGME requires that residency programs and sponsoring institutions provide clean and private facilities for lactation that have refrigeration capabilities, with proximity appropriate for safe patient care.11 There has been a call to dermatology program leadership to support breastfeeding residents by providing sufficient time and space to pump; a breastfeeding resident will need a 20- to 30-minute break to express milk approximately every 3 hours during the work day.12 One innovative initiative to meet the ACGME lactation requirement reported by the Kansas University Medical Center Graduate Medical Education program (Kansas City, Kansas) was the purchase of wearable breast pumps to loan to residents. The benefits of wearable breast pumps are that they are discreet and can allow mothers to express milk inconspicuously while working, can increase milk supply, require less set up and expression time than traditional pumps, and can allow the mother to manage time more efficiently.13 Breastfeeding plans and goals should be discussed with program leadership before return from leave to strategize and anticipate gaps in clinic scheduling to accommodate the lactating resident.
Planning for Childcare
Resident hours can be long and erratic, making choices for childcare difficult. In one survey of female residents, 61% of married or partnered respondents (n=447) were delaying childbearing, and 46% cited lack of access to childcare as a reason.14 Not all dermatology residents are fortunate enough to match to a program near family, but close family support can be an undeniable asset during childrearing and should be weighed heavily when ranking programs. Options for childcare include relying on a stay-at-home spouse or other family member, a live-in or live-out nanny, part-time babysitters, and daycare. It is crucial to have multiple layers and back-up options for childcare available at any given time when working as a resident. Even with a child enrolled in a full-time daycare and a live-in nanny, a daycare closure due to a COVID-19 exposure or sudden medical emergency in the nanny can still leave unpredicted holes in your childcare plan, leaving the resident to potentially miss work to fill the gap. A survey of residents at one institution showed that the most common backup childcare plan for situations in which either the child or the regular caregiver is ill is for the nontrainee parent or spouse to stay home (45%; n=101), with 25% of respondents staying home to care for a sick child themselves, which clearly has an impact on the hospital. The article proposed implementation of on-site or near-site childcare for residents with extended hours or a 24-hour emergency drop-in availability.15 One institution reported success with the development of a departmentally funded childcare supplementation stipend offered to residents to support daycare costs during the first 6 months of a baby’s life.16
Final Thoughts
Due to the competitiveness of the field, dermatology residents are by nature high performing and academically successful. For a high achiever, the idea of potentially disappointing faculty and colleagues by starting a family during residency can be guilt inducing. Concerns about one’s ability to adequately study the breadth of dermatology while simultaneously raising a child can be distressing; however, there are many ways in which motherhood can hone skills to become a better dermatology resident. Through motherhood one can enhance time management skills, increase efficiency, and improve rapport with pediatric patients and trust with their parents/guardians. A dermatology resident may be her own harshest critic, but it is time that the future generation of dermatologists become their own greatest advocates for establishing supportive policies and resources for the successful navigation of motherhood and dermatology residency.
Motherhood and dermatology residency are both full-time jobs. The thought that a woman must either be superhuman to succeed at both or that success at one must come at the expense of the other is antiquated. With careful navigation and sufficient support, these two roles can complement and heighten one another. The most recent Accreditation Council for Graduate Medical Education (ACGME) report showed that nearly 60% of dermatology residents are women,1 with most women in training being of childbearing age. One study showed that female dermatologists were most likely to have children during residency (51% of those surveyed), despite residents reporting more barriers to childbearing at this career stage.2 Trainees thinking of starting a family have many considerations to navigate: timing of pregnancy, maternity leave scheduling, breastfeeding while working, and planning for childcare. For the first time in the history of the specialty, most active dermatologists in practice are women.3 Thus, the future of dermatology requires supportive policies and resources for the successful navigation of these issues by today’s trainees.
Timing of Pregnancy
Timing of pregnancy can be a source of stress to the female dermatology resident. Barriers to childbearing during residency include the perception that women who have children during residency training are less committed to their jobs; concerns of overburdening fellow residents; and fear that residency may need to be extended, thereby delaying the ability to sit for the board examination.2 However, the potential increased risk for infertility in delaying pregnancy adds to the stress of pregnancy planning. A 2016 survey of female physicians (N=327) showed that 24.1% of respondents who had attempted conception were diagnosed with infertility, with an average age at diagnosis of 33.7 years.4 This is higher than the national average, with the Centers for Disease Control and Prevention reporting that approximately 19% of women aged 15 to 49 years with no prior births experience infertility.5 In a 1992 survey of female physician residents (N=373) who gave birth during residency, 32% indicated that they would not recommend the experience to others; of the 68% who would recommend the experience, one-third encouraged timing delivery to occur in the last 2 months of residency due to benefits of continued insurance coverage, a decrease in clinic responsibilities, and the potential for extended maternity leave during hiatus between jobs.6 Although this may be a good strategy, studying and sitting for board examinations while caring for a newborn right after graduation may be overly difficult for some. The first year of residency was perceived as the most stressful time to be pregnant, with each subsequent year being less problematic.6 Planning pregnancy for delivery near the end of the second year and beginning of the third year of dermatology residency may be a reasonable choice.
Maternity Leave
The Family and Medical Leave Act entitles eligible employees of covered employers to take unpaid, job-protected leave, with 12 workweeks of leave in a 12-month period for the birth of a child and to care for the newborn child within 1 year of birth.7 The actual length of maternity leave taken by most surveyed female dermatologists (n=96) is shorter: 25% (24/96) took less than 4 weeks, 42.7% (41/96) took 4 to 8 weeks, 25% (24/96) took 9 to 12 weeks, and 7.3% (7/96) were able to take more than 12 weeks of maternity leave.2
The American Board of Dermatology implemented a new Resident Leave policy that went into effect July 1, 2021, stipulating that, within certain parameters, time spent away from training for family and parental leave would not exhaust vacation time or require an extension in training. Under this policy, absence from training exceeding 8 weeks (6 weeks leave, plus 2 weeks of vacation) in a given year should be approved only under exceptional circumstances and may necessitate additional training time to ensure that competency requirements are met.8 Although this policy is a step in the right direction, institutional policies still may vary. Dermatology residents planning to start a family during training should consider their plans for fellowship, as taking an extended maternity leave beyond 8 weeks may jeopardize a July fellowship start date.
Lactation and Residency
The American Academy of Pediatrics recommends exclusive breastfeeding for approximately 6 months, with continuation of breastfeeding for 1 year or longer as mutually desired by the mother and infant.9 Successful lactation and achieving breastfeeding goals can be difficult during medical training. A national cross-sectional survey of female residents (N=312) showed that the median total time of breastfeeding and pumping was 9 months, with 74% continuing after 6 months and 13% continuing past 12 months. Of those surveyed, 73% reported residency limited their ability to lactate, and 37% stopped prior to their desired goal.10 As of July 1, 2020, the ACGME requires that residency programs and sponsoring institutions provide clean and private facilities for lactation that have refrigeration capabilities, with proximity appropriate for safe patient care.11 There has been a call to dermatology program leadership to support breastfeeding residents by providing sufficient time and space to pump; a breastfeeding resident will need a 20- to 30-minute break to express milk approximately every 3 hours during the work day.12 One innovative initiative to meet the ACGME lactation requirement reported by the Kansas University Medical Center Graduate Medical Education program (Kansas City, Kansas) was the purchase of wearable breast pumps to loan to residents. The benefits of wearable breast pumps are that they are discreet and can allow mothers to express milk inconspicuously while working, can increase milk supply, require less set up and expression time than traditional pumps, and can allow the mother to manage time more efficiently.13 Breastfeeding plans and goals should be discussed with program leadership before return from leave to strategize and anticipate gaps in clinic scheduling to accommodate the lactating resident.
Planning for Childcare
Resident hours can be long and erratic, making choices for childcare difficult. In one survey of female residents, 61% of married or partnered respondents (n=447) were delaying childbearing, and 46% cited lack of access to childcare as a reason.14 Not all dermatology residents are fortunate enough to match to a program near family, but close family support can be an undeniable asset during childrearing and should be weighed heavily when ranking programs. Options for childcare include relying on a stay-at-home spouse or other family member, a live-in or live-out nanny, part-time babysitters, and daycare. It is crucial to have multiple layers and back-up options for childcare available at any given time when working as a resident. Even with a child enrolled in a full-time daycare and a live-in nanny, a daycare closure due to a COVID-19 exposure or sudden medical emergency in the nanny can still leave unpredicted holes in your childcare plan, leaving the resident to potentially miss work to fill the gap. A survey of residents at one institution showed that the most common backup childcare plan for situations in which either the child or the regular caregiver is ill is for the nontrainee parent or spouse to stay home (45%; n=101), with 25% of respondents staying home to care for a sick child themselves, which clearly has an impact on the hospital. The article proposed implementation of on-site or near-site childcare for residents with extended hours or a 24-hour emergency drop-in availability.15 One institution reported success with the development of a departmentally funded childcare supplementation stipend offered to residents to support daycare costs during the first 6 months of a baby’s life.16
Final Thoughts
Due to the competitiveness of the field, dermatology residents are by nature high performing and academically successful. For a high achiever, the idea of potentially disappointing faculty and colleagues by starting a family during residency can be guilt inducing. Concerns about one’s ability to adequately study the breadth of dermatology while simultaneously raising a child can be distressing; however, there are many ways in which motherhood can hone skills to become a better dermatology resident. Through motherhood one can enhance time management skills, increase efficiency, and improve rapport with pediatric patients and trust with their parents/guardians. A dermatology resident may be her own harshest critic, but it is time that the future generation of dermatologists become their own greatest advocates for establishing supportive policies and resources for the successful navigation of motherhood and dermatology residency.
- ACGME residents and fellows by sex and specialty, 2019. Association of American Medical Colleges website. Accessed April 21, 2022. https://www.aamc.org/data-reports/interactive-data/acgme-residents-and-fellows-sex-and-specialty-2019
- Mattessich S, Shea K, Whitaker-Worth D. Parenting and female dermatologists’ perceptions of work-life balance. Int J Womens Dermatol. 2017;3:127-130. doi:10.1016/j.ijwd.2017.04.001
- Active physicians by sex and specialty, 2019. Association of American Medical Colleges website. Accessed April 21, 2022. https://www.aamc.org/data-reports/workforce/interactive-data/active-physicians-sex-and-specialty-2019
- Stentz NC, Griffith KA, Perkins E, et al. Fertility and childbearing among American female physicians. J Womens Health. 2016;25:1059-1065. doi:10.1089/jwh.2015.5638
- Infertility. Centers for Disease Control and Prevention website. Updated March 1, 2022. Accessed April 21, 2022. https://www.cdc.gov/reproductivehealth/infertility/
- Phelan ST. Sources of stress and support for the pregnant resident. Acad Med. 1992;67:408-410. doi:10.1097/00001888-199206000-00014
- Family and Medical Leave Act. US Department of Labor website. Accessed April 21, 2022. https://www.dol.gov/agencies/whd/fmla
- American Board of Dermatology. Effective July 2021: new family leave policy. Accessed April 21, 2022. https://www.abderm.org/public/announcements/effective-july-2021-new-family-leave-policy.aspx
- Eidelman AI, Schanler RJ, Johnston M, et al. Breastfeeding and the use of human milk. Pediatrics. 2012;129:E827-E841. doi:10.1542/peds.2011-3552
- Peters GW, Kuczmarska-Haas A, Holliday EB, et al. Lactation challenges of resident physicians: results of a national survey. BMC Pregnancy Childbirth. 2020;20:762. doi:10.1186/s12884-020-03436-3
- Common program requirements (residency) sections I-V table of implementation dates. Accreditation Council for Graduate Medical Education website. Accessed April 21, 2022. https://www.acgme.org/globalassets/PFAssets/ProgramRequirements/CPRResidencyImplementationTable.pdf
- Gracey LE, Mathes EF, Shinkai K. Supporting breastfeeding mothers during dermatology residency—challenges and best practices. JAMA Dermatol. 2020;156:117-118. doi:10.1001/jamadermatol.2019.3759
- McMillin A, Behravesh B, Byrne P, et al. A GME wearable breast pump program: an innovative method to meet ACGME requirements and federal law. J Grad Med Educ. 2021;13:422-423. doi:10.4300/jgme-d-20-01275.1
- Stack SW, Jagsi R, Biermann JS, et al. Childbearing decisions in residency: a multicenter survey of female residents. Acad Med. 2020;95:1550-1557. doi:10.1097/acm.0000000000003549
- Snyder RA, Tarpley MJ, Phillips SE, et al. The case for on-site child care in residency training and afterward. J Grad Med Educ. 2013;5:365-367. doi:10.4300/jgme-d-12-00294.1
- Key LL. Child care supplementation: aid for residents and advantages for residency programs. J Pediatr. 2008;153:449-450. doi:10.1016/j.jpeds.2008.05.028
- ACGME residents and fellows by sex and specialty, 2019. Association of American Medical Colleges website. Accessed April 21, 2022. https://www.aamc.org/data-reports/interactive-data/acgme-residents-and-fellows-sex-and-specialty-2019
- Mattessich S, Shea K, Whitaker-Worth D. Parenting and female dermatologists’ perceptions of work-life balance. Int J Womens Dermatol. 2017;3:127-130. doi:10.1016/j.ijwd.2017.04.001
- Active physicians by sex and specialty, 2019. Association of American Medical Colleges website. Accessed April 21, 2022. https://www.aamc.org/data-reports/workforce/interactive-data/active-physicians-sex-and-specialty-2019
- Stentz NC, Griffith KA, Perkins E, et al. Fertility and childbearing among American female physicians. J Womens Health. 2016;25:1059-1065. doi:10.1089/jwh.2015.5638
- Infertility. Centers for Disease Control and Prevention website. Updated March 1, 2022. Accessed April 21, 2022. https://www.cdc.gov/reproductivehealth/infertility/
- Phelan ST. Sources of stress and support for the pregnant resident. Acad Med. 1992;67:408-410. doi:10.1097/00001888-199206000-00014
- Family and Medical Leave Act. US Department of Labor website. Accessed April 21, 2022. https://www.dol.gov/agencies/whd/fmla
- American Board of Dermatology. Effective July 2021: new family leave policy. Accessed April 21, 2022. https://www.abderm.org/public/announcements/effective-july-2021-new-family-leave-policy.aspx
- Eidelman AI, Schanler RJ, Johnston M, et al. Breastfeeding and the use of human milk. Pediatrics. 2012;129:E827-E841. doi:10.1542/peds.2011-3552
- Peters GW, Kuczmarska-Haas A, Holliday EB, et al. Lactation challenges of resident physicians: results of a national survey. BMC Pregnancy Childbirth. 2020;20:762. doi:10.1186/s12884-020-03436-3
- Common program requirements (residency) sections I-V table of implementation dates. Accreditation Council for Graduate Medical Education website. Accessed April 21, 2022. https://www.acgme.org/globalassets/PFAssets/ProgramRequirements/CPRResidencyImplementationTable.pdf
- Gracey LE, Mathes EF, Shinkai K. Supporting breastfeeding mothers during dermatology residency—challenges and best practices. JAMA Dermatol. 2020;156:117-118. doi:10.1001/jamadermatol.2019.3759
- McMillin A, Behravesh B, Byrne P, et al. A GME wearable breast pump program: an innovative method to meet ACGME requirements and federal law. J Grad Med Educ. 2021;13:422-423. doi:10.4300/jgme-d-20-01275.1
- Stack SW, Jagsi R, Biermann JS, et al. Childbearing decisions in residency: a multicenter survey of female residents. Acad Med. 2020;95:1550-1557. doi:10.1097/acm.0000000000003549
- Snyder RA, Tarpley MJ, Phillips SE, et al. The case for on-site child care in residency training and afterward. J Grad Med Educ. 2013;5:365-367. doi:10.4300/jgme-d-12-00294.1
- Key LL. Child care supplementation: aid for residents and advantages for residency programs. J Pediatr. 2008;153:449-450. doi:10.1016/j.jpeds.2008.05.028
Resident Pearl
- Female dermatology residents seeking motherhood during training have many obstacles to navigate, including the timing of pregnancy, maternity leave scheduling, planning for breastfeeding while working, and arranging for childcare. With supportive policies and resources, motherhood and dermatology training can be rewarding complements to one another.
How Dermatology Residents Can Best Serve the Needs of the LGBT Community
The chances are good that at least one patient you saw today could have been provided a better environment to foster your patient-physician relationship. A 2020 Gallup poll revealed that an estimated 5.6% of US adults identified as lesbian, gay, bisexual, and transgender (LGBT).1 Based on the estimated US population of 331.7 million individuals on December 3, 2020, this means that approximately 18.6 million identified as LGBT and could potentially require health care services.2 These numbers highlight the increasing need within the medical community to provide quality and accessible care to the LGBT community, and dermatologists have a role to play. They treat conditions that are apparent to the patient and others around them, attracting those that may not be motivated to see different physicians. They can not only help with skin diseases that affect all patients but also can train other physicians to screen for some dermatologic diseases that may have a higher prevalence within the LGBT community. Dermatologists have a unique opportunity to help patients better reflect themselves through both surgical and nonsurgical modalities.
Demographics and Definitions
To discuss this topic effectively, it is important to define LGBT terms (Table).3 As a disclaimer, language is fluid. Despite a word or term currently being used and accepted, it quickly can become obsolete. A clinician can always do research, follow the lead of the patient, and respectfully ask questions if there is ever confusion surrounding terminology. Patients do not expect every physician they encounter to be an expert in this subject. What is most important is that patients are approached with an open mind and humility with the goal of providing optimal care.
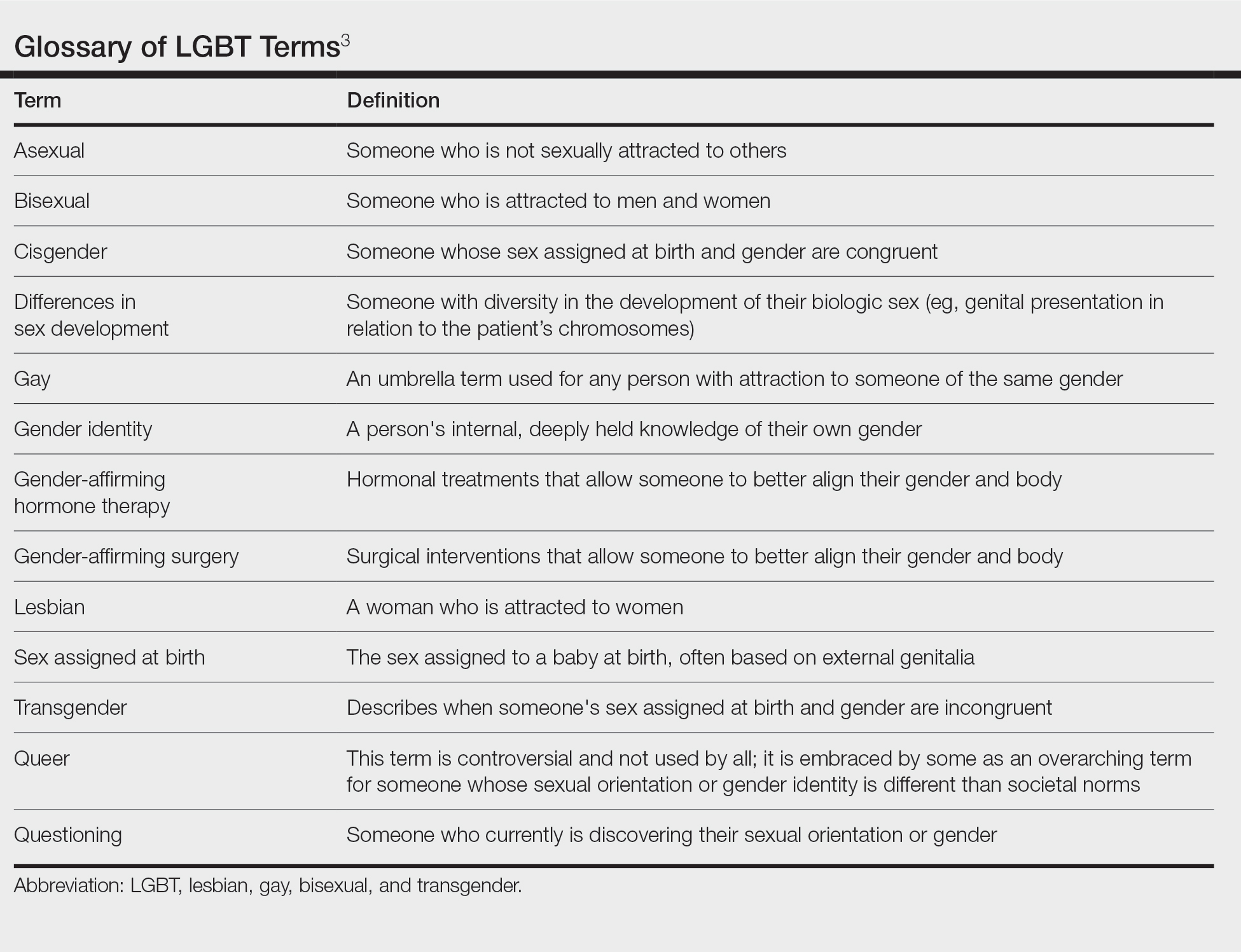
Although the federal government now uses the term sexual and gender minorities (SGM), the more specific terms lesbian, gay, bisexual, and transgender usually are preferred.3,4 Other letters are at times added to the acronym LGBT, including Q for questioning or queer, I for intersex, and A for asexual; all of these letters are under the larger SGM umbrella. Because LGBT is the most commonly used acronym in the daily vernacular, it will be the default for this article.
A term describing sexual orientation does not necessarily describe sexual practices. A woman who identifies as straight may have sex with both men and women, and a gay man may not have sex at all. To be more descriptive regarding sexual practices, one may use the terms men who have sex with men or women who have sex with women.3 Because of this nuance, it is important to elicit a sexual history when speaking to all patients in a forward nonjudgmental manner.
The term transgender is used to describe people whose gender identity differs from the sex they were assigned at birth. Two examples of transgender individuals would be transgender women who were assigned male at birth and transgender men who were assigned female at birth. The term transgender is used in opposition to the term cisgender, which is applied to a person whose gender and sex assigned at birth align.3 When a transgender patient presents to a physician, they may want to discuss methods of gender affirmation or transitioning. These terms encompass any action a person may take to align their body or gender expression with that of the gender they identify with. This could be in the form of gender-affirming hormone therapy (ie, estrogen or testosterone treatment) or gender-affirming surgery (ie, “top” and “bottom” surgeries, in which someone surgically treats their chest or genitals, respectively).3
Creating a Safe Space
The physician is responsible for providing a safe space for patients to disclose medically pertinent information. It is then the job of the dermatologist to be cognizant of health concerns that directly affect the LGBT population and to be prepared if one of these concerns should arise. A safe space consists of both the physical location in which the patient encounter will occur and the people that will be conducting and assisting in the patient encounter. Safe spaces provide a patient with reassurance that they will receive care in a judgement-free location. To create a safe space, both the physical and interpersonal aspects must be addressed to provide an environment that strengthens the patient-physician alliance.
Dermatology residents often spend more time with patients than their attending physicians, providing them the opportunity to foster robust relationships with those served. Although they may not be able to change the physical environment, residents can advocate for patients in their departments and show solidarity in subtle ways. One way to show support for the LGBT community is to publicly display a symbol of solidarity, which could be done by wearing a symbol of support on a white coat lapel. Although there are many designs and styles to choose from, one example is the American Medical Student Association pins that combine the caduceus (a common symbol for medicine) with a rainbow design.5 Whichever symbol is chosen, this small gesture allows patients to immediately know that their physician is an ally. Residents also can encourage their department to add a rainbow flag, a pink triangle, or another symbol somewhere prominent in the check-in area that conveys a message of support.6 Many institutions require residents to perform quality improvement projects. The resident can make a substantial difference in their patients’ experiences by revising their office’s intake forms as a quality improvement project, which can be done by including a section on assigned sex at birth separate from gender.7 When inquiring about gender, in addition to “male” and “female,” a space can be left for people that do not identify with the traditional binary. When asking about sexual orientation, inclusive language options can be provided with additional space for self-identification. Finally, residents can incorporate pronouns below their name in their email signature to normalize this disclosure of information.8 These small changes can have a substantial impact on the health care experience of SGM patients.
Medical Problems Encountered
The previously described changes can be implemented by residents to provide better care to SGM patients, a group usually considered to be more burdened by physical and psychological diseases.9 Furthermore, dermatologists can provide care for these patients in ways that other physicians cannot. There are special considerations for LGBT patients, as some dermatologic conditions may be more common in this patient population.
Prior studies have shown that men who have sex with men have a higher rate of HIV and other sexually transmitted infections, methicillin-resistant Staphylococcus aureus skin infections, and potentially nonmelanoma skin cancer.10-14 Transgender women also have been found to have higher rates of HIV, in addition to a higher incidence of anal human papillomavirus.15,16 Women who have sex with women have been shown to see physicians less frequently and to be less up to date on their pertinent cancer-related screenings.10,17 Although these associations should not dictate the patient encounter, awareness of them will lead to better patient care. Such awareness also can provide further motivation for dermatologists to discuss safe sexual practices, potential initiation of pre-exposure prophylactic antiretroviral therapy, sun-protective practices, and the importance of following up with a primary physician for examinations and age-specific cancer screening.
Transgender patients may present with unique dermatologic concerns. For transgender male patients, testosterone therapy can cause acne breakouts and androgenetic alopecia. Usually considered worse during the start of treatment, hormone-related acne can be managed with topical retinoids, topical and oral antibiotics, and isotretinoin (if severe).18,19 The iPLEDGE system necessary for prescribing isotretinoin to patients in the United States recently has changed its language to “patients who can get pregnant” and “patients who cannot get pregnant,” following urging by the medical community for inclusivity and progress.20,21 This change creates an inclusive space where registration is no longer centered around gender and instead focuses on the presence of anatomy. Although androgenetic alopecia is a side effect of hormone therapy, it may not be unwanted.18 Discussion about patient desires is important. If the alopecia is unwanted, the Endocrine Society recommends treating cisgender and transgender patients the same in terms of treatment modalities.22
Transgender female patients also can experience dermatologic manifestations of gender-affirming hormone therapy. Melasma may develop secondary to estrogen replacement and can be treated with topical bleaching creams, lasers, and phototherapy.23 Hair removal may be pursued for patients with refractory unwanted body hair, with laser hair removal being the most commonly pursued treatment. Patients also may desire cosmetic procedures, such as botulinum toxin or fillers, to augment their physical appearance.24 Providing these services to patients may allow them to better express themselves and live authentically.
Final Thoughts
There is no way to summarize the experience of everyone within a community. Each person has different thoughts, values, and goals. It also is impossible to encompass every topic that is important for SGM patients. The goal of this article is to empower clinicians to be comfortable discussing issues related to sexuality and gender while also offering resources to learn more, allowing optimal care to be provided to this population. Thus, this article is not comprehensive. There are articles to provide further resources and education, such as the continuing medical education series by Yeung et al10,25 in the Journal of the American Academy of Dermatology, as well as organizations within medicine, such as the GLMA: Health Professionals Advancing LGBTQ Equality (https://www.glma.org/), and in dermatology, such as GALDA, the Gay and Lesbian Dermatology Association (https://www.glderm.org/). By providing a safe space for our patients and learning about specific health-related risk factors, dermatologists can provide the best possible care to the LGBT community.
Acknowledgments—I thank Warren R. Heymann, MD (Camden, New Jersey), and Howa Yeung, MD, MSc (Atlanta, Georgia), for their guidance and mentorship in the creation of this article.
- Jones JM. LGBT identification rises to 5.6% in latest U.S. estimate. Gallup website. Published February 24, 2021. Accessed March 22, 2022. https://news.gallup.com/poll/329708/lgbt-identification-rises-latest-estimate.aspx
- U.S. and world population clock. US Census Bureau website. Accessed March 22, 2022. https://www.census.gov/popclock/
- National LGBTQIA+ Health Education Center. LGBTQIA+ glossary of terms for health care teams. Published February 2, 2022. Accessed April 11, 2022. https://www.lgbtqiahealtheducation.org/wp-content/uploads/2020/02/Glossary-2022.02.22-1.pdf
- National Institutes of Health Sexual and Gender Minority Research Coordinating Committee. NIH FY 2016-2020 strategic plan to advance research on the health and well-being of sexual and gender minorities. NIH website. Accessed March 23, 2022. https://www.edi.nih.gov/sites/default/files/EDI_Public_files/sgm-strategic-plan.pdf
- Caduceus pin—rainbow. American Medical Student Association website. Accessed March 23, 2022. https://www.amsa.org/member-center/store/Caduceus-Pin-Rainbow-p67375123
- 10 tips for caring for LGBTQIA+ patients. Nurse.org website. Accessed March 23, 2022. https://nurse.org/articles/culturally-competent-healthcare-for-LGBTQ-patients/
- Cartron AM, Raiciulescu S, Trinidad JC. Culturally competent care for LGBT patients in dermatology clinics. J Drugs Dermatol. 2020;19:786-787.
- Wareham J. Should you put pronouns in email signatures and social media bios? Forbes website. Published Dec 30, 2019. Accessed March 23, 2022. https://www.forbes.com/sites/jamiewareham/2020/12/30/should-you-put-pronouns-in-email-signatures-and-social-media-bios/?sh=5b74f1246320
- Hafeez H, Zeshan M, Tahir MA, et al. Healthcare disparities among lesbian, gay, bisexual, and transgender youth: a literature review. Cureus. 2017;9:E1184.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons. part II. epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602.
- Centers for Disease Control and Prevention. CDC fact sheet: HIV among gay and bisexual men. CDC website. Accessed April 14, 2022. https://www.cdc.gov/nchhstp/newsroom/docs/factsheets/cdc-msm-508.pdf
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2016. CDC website. Accessed April 14, 2022. https://www.cdc.gov/std/stats16/CDC_2016_STDS_Report-for508WebSep21_2017_1644.pdf
- Galindo GR, Casey AJ, Yeung A, et al. Community associated methicillin resistant Staphylococcus aureus among New York City men who have sex with men: qualitative research findings and implications for public health practice. J Community Health. 2012;37:458-467.
- Blashill AJ. Indoor tanning and skin cancer risk among diverse US youth: results from a national sample. JAMA Dermatol. 2017;153:344-345.
- Herbst JH, Jacobs ED, Finlayson TJ, et al. Estimating HIV prevalence and risk behaviors of transgender persons in the United States: a systematic review. AIDS Behav. 2008;12:1-17.
- Uaamnuichai S, Panyakhamlerd K, Suwan A, et al. Neovaginal and anal high-risk human papillomavirus DNA among Thai transgender women in gender health clinics. Sex Transm Dis. 2021;48:547-549.
- Valanis BG, Bowen DJ, Bassford T, et al. Sexual orientation and health: comparisons in the women’s health initiative sample. Arch Fam Med. 2000;9:843-853.
- Wierckx K, Van de Peer F, Verhaeghe E, et al. Short- and long-term clinical skin effects of testosterone treatment in trans men. J Sex Med. 2014;11:222-229.
- Turrion-Merino L, Urech-Garcia-de-la-Vega M, Miguel-Gomez L, et al. Severe acne in female-to-male transgender patients. JAMA Dermatol. 2015;151:1260-1261.
- Questions and answers on the iPLEDGE REMS. US Food and Drug Administration website. Published October 12, 2021. Accessed March 23, 2022. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/questions-and-answers-ipledge-rems#:~:text=The%20modification%20will%20become%20effective,verify%20authorization%20to%20dispense%20isotretinoin
- Gao JL, Thoreson N, Dommasch ED. Navigating iPLEDGE enrollment for transgender and gender diverse patients: a guide for providing culturally competent care. J Am Acad Dermatol. 2021;85:790-791.
- Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2017;102:3869-3903.
- Garcia-Rodriguez L, Spiegel JH. Melasma in a transgender woman. Am J Otolaryngol. 2018;39:788-790.
- Ginsberg BA, Calderon M, Seminara NM, et al. A potential role for the dermatologist in the physical transformation of transgender people: a survey of attitudes and practices within the transgender community.J Am Acad Dermatol. 2016;74:303-308.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian,gay, bisexual, and transgender persons. part I. terminology, demographics, health disparities, and approaches to care. J Am Acad Dermatol. 2019;80:581-589.
The chances are good that at least one patient you saw today could have been provided a better environment to foster your patient-physician relationship. A 2020 Gallup poll revealed that an estimated 5.6% of US adults identified as lesbian, gay, bisexual, and transgender (LGBT).1 Based on the estimated US population of 331.7 million individuals on December 3, 2020, this means that approximately 18.6 million identified as LGBT and could potentially require health care services.2 These numbers highlight the increasing need within the medical community to provide quality and accessible care to the LGBT community, and dermatologists have a role to play. They treat conditions that are apparent to the patient and others around them, attracting those that may not be motivated to see different physicians. They can not only help with skin diseases that affect all patients but also can train other physicians to screen for some dermatologic diseases that may have a higher prevalence within the LGBT community. Dermatologists have a unique opportunity to help patients better reflect themselves through both surgical and nonsurgical modalities.
Demographics and Definitions
To discuss this topic effectively, it is important to define LGBT terms (Table).3 As a disclaimer, language is fluid. Despite a word or term currently being used and accepted, it quickly can become obsolete. A clinician can always do research, follow the lead of the patient, and respectfully ask questions if there is ever confusion surrounding terminology. Patients do not expect every physician they encounter to be an expert in this subject. What is most important is that patients are approached with an open mind and humility with the goal of providing optimal care.

Although the federal government now uses the term sexual and gender minorities (SGM), the more specific terms lesbian, gay, bisexual, and transgender usually are preferred.3,4 Other letters are at times added to the acronym LGBT, including Q for questioning or queer, I for intersex, and A for asexual; all of these letters are under the larger SGM umbrella. Because LGBT is the most commonly used acronym in the daily vernacular, it will be the default for this article.
A term describing sexual orientation does not necessarily describe sexual practices. A woman who identifies as straight may have sex with both men and women, and a gay man may not have sex at all. To be more descriptive regarding sexual practices, one may use the terms men who have sex with men or women who have sex with women.3 Because of this nuance, it is important to elicit a sexual history when speaking to all patients in a forward nonjudgmental manner.
The term transgender is used to describe people whose gender identity differs from the sex they were assigned at birth. Two examples of transgender individuals would be transgender women who were assigned male at birth and transgender men who were assigned female at birth. The term transgender is used in opposition to the term cisgender, which is applied to a person whose gender and sex assigned at birth align.3 When a transgender patient presents to a physician, they may want to discuss methods of gender affirmation or transitioning. These terms encompass any action a person may take to align their body or gender expression with that of the gender they identify with. This could be in the form of gender-affirming hormone therapy (ie, estrogen or testosterone treatment) or gender-affirming surgery (ie, “top” and “bottom” surgeries, in which someone surgically treats their chest or genitals, respectively).3
Creating a Safe Space
The physician is responsible for providing a safe space for patients to disclose medically pertinent information. It is then the job of the dermatologist to be cognizant of health concerns that directly affect the LGBT population and to be prepared if one of these concerns should arise. A safe space consists of both the physical location in which the patient encounter will occur and the people that will be conducting and assisting in the patient encounter. Safe spaces provide a patient with reassurance that they will receive care in a judgement-free location. To create a safe space, both the physical and interpersonal aspects must be addressed to provide an environment that strengthens the patient-physician alliance.
Dermatology residents often spend more time with patients than their attending physicians, providing them the opportunity to foster robust relationships with those served. Although they may not be able to change the physical environment, residents can advocate for patients in their departments and show solidarity in subtle ways. One way to show support for the LGBT community is to publicly display a symbol of solidarity, which could be done by wearing a symbol of support on a white coat lapel. Although there are many designs and styles to choose from, one example is the American Medical Student Association pins that combine the caduceus (a common symbol for medicine) with a rainbow design.5 Whichever symbol is chosen, this small gesture allows patients to immediately know that their physician is an ally. Residents also can encourage their department to add a rainbow flag, a pink triangle, or another symbol somewhere prominent in the check-in area that conveys a message of support.6 Many institutions require residents to perform quality improvement projects. The resident can make a substantial difference in their patients’ experiences by revising their office’s intake forms as a quality improvement project, which can be done by including a section on assigned sex at birth separate from gender.7 When inquiring about gender, in addition to “male” and “female,” a space can be left for people that do not identify with the traditional binary. When asking about sexual orientation, inclusive language options can be provided with additional space for self-identification. Finally, residents can incorporate pronouns below their name in their email signature to normalize this disclosure of information.8 These small changes can have a substantial impact on the health care experience of SGM patients.
Medical Problems Encountered
The previously described changes can be implemented by residents to provide better care to SGM patients, a group usually considered to be more burdened by physical and psychological diseases.9 Furthermore, dermatologists can provide care for these patients in ways that other physicians cannot. There are special considerations for LGBT patients, as some dermatologic conditions may be more common in this patient population.
Prior studies have shown that men who have sex with men have a higher rate of HIV and other sexually transmitted infections, methicillin-resistant Staphylococcus aureus skin infections, and potentially nonmelanoma skin cancer.10-14 Transgender women also have been found to have higher rates of HIV, in addition to a higher incidence of anal human papillomavirus.15,16 Women who have sex with women have been shown to see physicians less frequently and to be less up to date on their pertinent cancer-related screenings.10,17 Although these associations should not dictate the patient encounter, awareness of them will lead to better patient care. Such awareness also can provide further motivation for dermatologists to discuss safe sexual practices, potential initiation of pre-exposure prophylactic antiretroviral therapy, sun-protective practices, and the importance of following up with a primary physician for examinations and age-specific cancer screening.
Transgender patients may present with unique dermatologic concerns. For transgender male patients, testosterone therapy can cause acne breakouts and androgenetic alopecia. Usually considered worse during the start of treatment, hormone-related acne can be managed with topical retinoids, topical and oral antibiotics, and isotretinoin (if severe).18,19 The iPLEDGE system necessary for prescribing isotretinoin to patients in the United States recently has changed its language to “patients who can get pregnant” and “patients who cannot get pregnant,” following urging by the medical community for inclusivity and progress.20,21 This change creates an inclusive space where registration is no longer centered around gender and instead focuses on the presence of anatomy. Although androgenetic alopecia is a side effect of hormone therapy, it may not be unwanted.18 Discussion about patient desires is important. If the alopecia is unwanted, the Endocrine Society recommends treating cisgender and transgender patients the same in terms of treatment modalities.22
Transgender female patients also can experience dermatologic manifestations of gender-affirming hormone therapy. Melasma may develop secondary to estrogen replacement and can be treated with topical bleaching creams, lasers, and phototherapy.23 Hair removal may be pursued for patients with refractory unwanted body hair, with laser hair removal being the most commonly pursued treatment. Patients also may desire cosmetic procedures, such as botulinum toxin or fillers, to augment their physical appearance.24 Providing these services to patients may allow them to better express themselves and live authentically.
Final Thoughts
There is no way to summarize the experience of everyone within a community. Each person has different thoughts, values, and goals. It also is impossible to encompass every topic that is important for SGM patients. The goal of this article is to empower clinicians to be comfortable discussing issues related to sexuality and gender while also offering resources to learn more, allowing optimal care to be provided to this population. Thus, this article is not comprehensive. There are articles to provide further resources and education, such as the continuing medical education series by Yeung et al10,25 in the Journal of the American Academy of Dermatology, as well as organizations within medicine, such as the GLMA: Health Professionals Advancing LGBTQ Equality (https://www.glma.org/), and in dermatology, such as GALDA, the Gay and Lesbian Dermatology Association (https://www.glderm.org/). By providing a safe space for our patients and learning about specific health-related risk factors, dermatologists can provide the best possible care to the LGBT community.
Acknowledgments—I thank Warren R. Heymann, MD (Camden, New Jersey), and Howa Yeung, MD, MSc (Atlanta, Georgia), for their guidance and mentorship in the creation of this article.
The chances are good that at least one patient you saw today could have been provided a better environment to foster your patient-physician relationship. A 2020 Gallup poll revealed that an estimated 5.6% of US adults identified as lesbian, gay, bisexual, and transgender (LGBT).1 Based on the estimated US population of 331.7 million individuals on December 3, 2020, this means that approximately 18.6 million identified as LGBT and could potentially require health care services.2 These numbers highlight the increasing need within the medical community to provide quality and accessible care to the LGBT community, and dermatologists have a role to play. They treat conditions that are apparent to the patient and others around them, attracting those that may not be motivated to see different physicians. They can not only help with skin diseases that affect all patients but also can train other physicians to screen for some dermatologic diseases that may have a higher prevalence within the LGBT community. Dermatologists have a unique opportunity to help patients better reflect themselves through both surgical and nonsurgical modalities.
Demographics and Definitions
To discuss this topic effectively, it is important to define LGBT terms (Table).3 As a disclaimer, language is fluid. Despite a word or term currently being used and accepted, it quickly can become obsolete. A clinician can always do research, follow the lead of the patient, and respectfully ask questions if there is ever confusion surrounding terminology. Patients do not expect every physician they encounter to be an expert in this subject. What is most important is that patients are approached with an open mind and humility with the goal of providing optimal care.

Although the federal government now uses the term sexual and gender minorities (SGM), the more specific terms lesbian, gay, bisexual, and transgender usually are preferred.3,4 Other letters are at times added to the acronym LGBT, including Q for questioning or queer, I for intersex, and A for asexual; all of these letters are under the larger SGM umbrella. Because LGBT is the most commonly used acronym in the daily vernacular, it will be the default for this article.
A term describing sexual orientation does not necessarily describe sexual practices. A woman who identifies as straight may have sex with both men and women, and a gay man may not have sex at all. To be more descriptive regarding sexual practices, one may use the terms men who have sex with men or women who have sex with women.3 Because of this nuance, it is important to elicit a sexual history when speaking to all patients in a forward nonjudgmental manner.
The term transgender is used to describe people whose gender identity differs from the sex they were assigned at birth. Two examples of transgender individuals would be transgender women who were assigned male at birth and transgender men who were assigned female at birth. The term transgender is used in opposition to the term cisgender, which is applied to a person whose gender and sex assigned at birth align.3 When a transgender patient presents to a physician, they may want to discuss methods of gender affirmation or transitioning. These terms encompass any action a person may take to align their body or gender expression with that of the gender they identify with. This could be in the form of gender-affirming hormone therapy (ie, estrogen or testosterone treatment) or gender-affirming surgery (ie, “top” and “bottom” surgeries, in which someone surgically treats their chest or genitals, respectively).3
Creating a Safe Space
The physician is responsible for providing a safe space for patients to disclose medically pertinent information. It is then the job of the dermatologist to be cognizant of health concerns that directly affect the LGBT population and to be prepared if one of these concerns should arise. A safe space consists of both the physical location in which the patient encounter will occur and the people that will be conducting and assisting in the patient encounter. Safe spaces provide a patient with reassurance that they will receive care in a judgement-free location. To create a safe space, both the physical and interpersonal aspects must be addressed to provide an environment that strengthens the patient-physician alliance.
Dermatology residents often spend more time with patients than their attending physicians, providing them the opportunity to foster robust relationships with those served. Although they may not be able to change the physical environment, residents can advocate for patients in their departments and show solidarity in subtle ways. One way to show support for the LGBT community is to publicly display a symbol of solidarity, which could be done by wearing a symbol of support on a white coat lapel. Although there are many designs and styles to choose from, one example is the American Medical Student Association pins that combine the caduceus (a common symbol for medicine) with a rainbow design.5 Whichever symbol is chosen, this small gesture allows patients to immediately know that their physician is an ally. Residents also can encourage their department to add a rainbow flag, a pink triangle, or another symbol somewhere prominent in the check-in area that conveys a message of support.6 Many institutions require residents to perform quality improvement projects. The resident can make a substantial difference in their patients’ experiences by revising their office’s intake forms as a quality improvement project, which can be done by including a section on assigned sex at birth separate from gender.7 When inquiring about gender, in addition to “male” and “female,” a space can be left for people that do not identify with the traditional binary. When asking about sexual orientation, inclusive language options can be provided with additional space for self-identification. Finally, residents can incorporate pronouns below their name in their email signature to normalize this disclosure of information.8 These small changes can have a substantial impact on the health care experience of SGM patients.
Medical Problems Encountered
The previously described changes can be implemented by residents to provide better care to SGM patients, a group usually considered to be more burdened by physical and psychological diseases.9 Furthermore, dermatologists can provide care for these patients in ways that other physicians cannot. There are special considerations for LGBT patients, as some dermatologic conditions may be more common in this patient population.
Prior studies have shown that men who have sex with men have a higher rate of HIV and other sexually transmitted infections, methicillin-resistant Staphylococcus aureus skin infections, and potentially nonmelanoma skin cancer.10-14 Transgender women also have been found to have higher rates of HIV, in addition to a higher incidence of anal human papillomavirus.15,16 Women who have sex with women have been shown to see physicians less frequently and to be less up to date on their pertinent cancer-related screenings.10,17 Although these associations should not dictate the patient encounter, awareness of them will lead to better patient care. Such awareness also can provide further motivation for dermatologists to discuss safe sexual practices, potential initiation of pre-exposure prophylactic antiretroviral therapy, sun-protective practices, and the importance of following up with a primary physician for examinations and age-specific cancer screening.
Transgender patients may present with unique dermatologic concerns. For transgender male patients, testosterone therapy can cause acne breakouts and androgenetic alopecia. Usually considered worse during the start of treatment, hormone-related acne can be managed with topical retinoids, topical and oral antibiotics, and isotretinoin (if severe).18,19 The iPLEDGE system necessary for prescribing isotretinoin to patients in the United States recently has changed its language to “patients who can get pregnant” and “patients who cannot get pregnant,” following urging by the medical community for inclusivity and progress.20,21 This change creates an inclusive space where registration is no longer centered around gender and instead focuses on the presence of anatomy. Although androgenetic alopecia is a side effect of hormone therapy, it may not be unwanted.18 Discussion about patient desires is important. If the alopecia is unwanted, the Endocrine Society recommends treating cisgender and transgender patients the same in terms of treatment modalities.22
Transgender female patients also can experience dermatologic manifestations of gender-affirming hormone therapy. Melasma may develop secondary to estrogen replacement and can be treated with topical bleaching creams, lasers, and phototherapy.23 Hair removal may be pursued for patients with refractory unwanted body hair, with laser hair removal being the most commonly pursued treatment. Patients also may desire cosmetic procedures, such as botulinum toxin or fillers, to augment their physical appearance.24 Providing these services to patients may allow them to better express themselves and live authentically.
Final Thoughts
There is no way to summarize the experience of everyone within a community. Each person has different thoughts, values, and goals. It also is impossible to encompass every topic that is important for SGM patients. The goal of this article is to empower clinicians to be comfortable discussing issues related to sexuality and gender while also offering resources to learn more, allowing optimal care to be provided to this population. Thus, this article is not comprehensive. There are articles to provide further resources and education, such as the continuing medical education series by Yeung et al10,25 in the Journal of the American Academy of Dermatology, as well as organizations within medicine, such as the GLMA: Health Professionals Advancing LGBTQ Equality (https://www.glma.org/), and in dermatology, such as GALDA, the Gay and Lesbian Dermatology Association (https://www.glderm.org/). By providing a safe space for our patients and learning about specific health-related risk factors, dermatologists can provide the best possible care to the LGBT community.
Acknowledgments—I thank Warren R. Heymann, MD (Camden, New Jersey), and Howa Yeung, MD, MSc (Atlanta, Georgia), for their guidance and mentorship in the creation of this article.
- Jones JM. LGBT identification rises to 5.6% in latest U.S. estimate. Gallup website. Published February 24, 2021. Accessed March 22, 2022. https://news.gallup.com/poll/329708/lgbt-identification-rises-latest-estimate.aspx
- U.S. and world population clock. US Census Bureau website. Accessed March 22, 2022. https://www.census.gov/popclock/
- National LGBTQIA+ Health Education Center. LGBTQIA+ glossary of terms for health care teams. Published February 2, 2022. Accessed April 11, 2022. https://www.lgbtqiahealtheducation.org/wp-content/uploads/2020/02/Glossary-2022.02.22-1.pdf
- National Institutes of Health Sexual and Gender Minority Research Coordinating Committee. NIH FY 2016-2020 strategic plan to advance research on the health and well-being of sexual and gender minorities. NIH website. Accessed March 23, 2022. https://www.edi.nih.gov/sites/default/files/EDI_Public_files/sgm-strategic-plan.pdf
- Caduceus pin—rainbow. American Medical Student Association website. Accessed March 23, 2022. https://www.amsa.org/member-center/store/Caduceus-Pin-Rainbow-p67375123
- 10 tips for caring for LGBTQIA+ patients. Nurse.org website. Accessed March 23, 2022. https://nurse.org/articles/culturally-competent-healthcare-for-LGBTQ-patients/
- Cartron AM, Raiciulescu S, Trinidad JC. Culturally competent care for LGBT patients in dermatology clinics. J Drugs Dermatol. 2020;19:786-787.
- Wareham J. Should you put pronouns in email signatures and social media bios? Forbes website. Published Dec 30, 2019. Accessed March 23, 2022. https://www.forbes.com/sites/jamiewareham/2020/12/30/should-you-put-pronouns-in-email-signatures-and-social-media-bios/?sh=5b74f1246320
- Hafeez H, Zeshan M, Tahir MA, et al. Healthcare disparities among lesbian, gay, bisexual, and transgender youth: a literature review. Cureus. 2017;9:E1184.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons. part II. epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602.
- Centers for Disease Control and Prevention. CDC fact sheet: HIV among gay and bisexual men. CDC website. Accessed April 14, 2022. https://www.cdc.gov/nchhstp/newsroom/docs/factsheets/cdc-msm-508.pdf
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2016. CDC website. Accessed April 14, 2022. https://www.cdc.gov/std/stats16/CDC_2016_STDS_Report-for508WebSep21_2017_1644.pdf
- Galindo GR, Casey AJ, Yeung A, et al. Community associated methicillin resistant Staphylococcus aureus among New York City men who have sex with men: qualitative research findings and implications for public health practice. J Community Health. 2012;37:458-467.
- Blashill AJ. Indoor tanning and skin cancer risk among diverse US youth: results from a national sample. JAMA Dermatol. 2017;153:344-345.
- Herbst JH, Jacobs ED, Finlayson TJ, et al. Estimating HIV prevalence and risk behaviors of transgender persons in the United States: a systematic review. AIDS Behav. 2008;12:1-17.
- Uaamnuichai S, Panyakhamlerd K, Suwan A, et al. Neovaginal and anal high-risk human papillomavirus DNA among Thai transgender women in gender health clinics. Sex Transm Dis. 2021;48:547-549.
- Valanis BG, Bowen DJ, Bassford T, et al. Sexual orientation and health: comparisons in the women’s health initiative sample. Arch Fam Med. 2000;9:843-853.
- Wierckx K, Van de Peer F, Verhaeghe E, et al. Short- and long-term clinical skin effects of testosterone treatment in trans men. J Sex Med. 2014;11:222-229.
- Turrion-Merino L, Urech-Garcia-de-la-Vega M, Miguel-Gomez L, et al. Severe acne in female-to-male transgender patients. JAMA Dermatol. 2015;151:1260-1261.
- Questions and answers on the iPLEDGE REMS. US Food and Drug Administration website. Published October 12, 2021. Accessed March 23, 2022. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/questions-and-answers-ipledge-rems#:~:text=The%20modification%20will%20become%20effective,verify%20authorization%20to%20dispense%20isotretinoin
- Gao JL, Thoreson N, Dommasch ED. Navigating iPLEDGE enrollment for transgender and gender diverse patients: a guide for providing culturally competent care. J Am Acad Dermatol. 2021;85:790-791.
- Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2017;102:3869-3903.
- Garcia-Rodriguez L, Spiegel JH. Melasma in a transgender woman. Am J Otolaryngol. 2018;39:788-790.
- Ginsberg BA, Calderon M, Seminara NM, et al. A potential role for the dermatologist in the physical transformation of transgender people: a survey of attitudes and practices within the transgender community.J Am Acad Dermatol. 2016;74:303-308.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian,gay, bisexual, and transgender persons. part I. terminology, demographics, health disparities, and approaches to care. J Am Acad Dermatol. 2019;80:581-589.
- Jones JM. LGBT identification rises to 5.6% in latest U.S. estimate. Gallup website. Published February 24, 2021. Accessed March 22, 2022. https://news.gallup.com/poll/329708/lgbt-identification-rises-latest-estimate.aspx
- U.S. and world population clock. US Census Bureau website. Accessed March 22, 2022. https://www.census.gov/popclock/
- National LGBTQIA+ Health Education Center. LGBTQIA+ glossary of terms for health care teams. Published February 2, 2022. Accessed April 11, 2022. https://www.lgbtqiahealtheducation.org/wp-content/uploads/2020/02/Glossary-2022.02.22-1.pdf
- National Institutes of Health Sexual and Gender Minority Research Coordinating Committee. NIH FY 2016-2020 strategic plan to advance research on the health and well-being of sexual and gender minorities. NIH website. Accessed March 23, 2022. https://www.edi.nih.gov/sites/default/files/EDI_Public_files/sgm-strategic-plan.pdf
- Caduceus pin—rainbow. American Medical Student Association website. Accessed March 23, 2022. https://www.amsa.org/member-center/store/Caduceus-Pin-Rainbow-p67375123
- 10 tips for caring for LGBTQIA+ patients. Nurse.org website. Accessed March 23, 2022. https://nurse.org/articles/culturally-competent-healthcare-for-LGBTQ-patients/
- Cartron AM, Raiciulescu S, Trinidad JC. Culturally competent care for LGBT patients in dermatology clinics. J Drugs Dermatol. 2020;19:786-787.
- Wareham J. Should you put pronouns in email signatures and social media bios? Forbes website. Published Dec 30, 2019. Accessed March 23, 2022. https://www.forbes.com/sites/jamiewareham/2020/12/30/should-you-put-pronouns-in-email-signatures-and-social-media-bios/?sh=5b74f1246320
- Hafeez H, Zeshan M, Tahir MA, et al. Healthcare disparities among lesbian, gay, bisexual, and transgender youth: a literature review. Cureus. 2017;9:E1184.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons. part II. epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602.
- Centers for Disease Control and Prevention. CDC fact sheet: HIV among gay and bisexual men. CDC website. Accessed April 14, 2022. https://www.cdc.gov/nchhstp/newsroom/docs/factsheets/cdc-msm-508.pdf
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2016. CDC website. Accessed April 14, 2022. https://www.cdc.gov/std/stats16/CDC_2016_STDS_Report-for508WebSep21_2017_1644.pdf
- Galindo GR, Casey AJ, Yeung A, et al. Community associated methicillin resistant Staphylococcus aureus among New York City men who have sex with men: qualitative research findings and implications for public health practice. J Community Health. 2012;37:458-467.
- Blashill AJ. Indoor tanning and skin cancer risk among diverse US youth: results from a national sample. JAMA Dermatol. 2017;153:344-345.
- Herbst JH, Jacobs ED, Finlayson TJ, et al. Estimating HIV prevalence and risk behaviors of transgender persons in the United States: a systematic review. AIDS Behav. 2008;12:1-17.
- Uaamnuichai S, Panyakhamlerd K, Suwan A, et al. Neovaginal and anal high-risk human papillomavirus DNA among Thai transgender women in gender health clinics. Sex Transm Dis. 2021;48:547-549.
- Valanis BG, Bowen DJ, Bassford T, et al. Sexual orientation and health: comparisons in the women’s health initiative sample. Arch Fam Med. 2000;9:843-853.
- Wierckx K, Van de Peer F, Verhaeghe E, et al. Short- and long-term clinical skin effects of testosterone treatment in trans men. J Sex Med. 2014;11:222-229.
- Turrion-Merino L, Urech-Garcia-de-la-Vega M, Miguel-Gomez L, et al. Severe acne in female-to-male transgender patients. JAMA Dermatol. 2015;151:1260-1261.
- Questions and answers on the iPLEDGE REMS. US Food and Drug Administration website. Published October 12, 2021. Accessed March 23, 2022. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/questions-and-answers-ipledge-rems#:~:text=The%20modification%20will%20become%20effective,verify%20authorization%20to%20dispense%20isotretinoin
- Gao JL, Thoreson N, Dommasch ED. Navigating iPLEDGE enrollment for transgender and gender diverse patients: a guide for providing culturally competent care. J Am Acad Dermatol. 2021;85:790-791.
- Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2017;102:3869-3903.
- Garcia-Rodriguez L, Spiegel JH. Melasma in a transgender woman. Am J Otolaryngol. 2018;39:788-790.
- Ginsberg BA, Calderon M, Seminara NM, et al. A potential role for the dermatologist in the physical transformation of transgender people: a survey of attitudes and practices within the transgender community.J Am Acad Dermatol. 2016;74:303-308.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian,gay, bisexual, and transgender persons. part I. terminology, demographics, health disparities, and approaches to care. J Am Acad Dermatol. 2019;80:581-589.
Resident Pearl
- Because of the longitudinal relationships dermatology residents make with their patients, they have a unique opportunity to provide a safe space and life-changing care to patients within the lesbian, gay, bisexual, and transgender community.
Borderline personality disorder: Remember empathy and compassion
Oh, great!” a senior resident sardonically remarked with a smirk as they read up on the next patient in the clinic. “A borderline patient. Get ready for a rough one ... Ugh.”
Before ever stepping foot into the patient’s room, this resident had prematurely established and demonstrated an unfortunate dynamic for any student or trainee within earshot. This is an all-too-familiar occurrence when caring for individuals with borderline personality disorder (BPD), or any other patients deemed to be “difficult.” The patient, however, likely walked into the room with a traumatic past that they continue to suffer from, in addition to any other issues for which they were seeking care.
Consider what these patients have experienced
A typical profile of these resilient patients with BPD: They were born emotionally sensitive. They grew up in homes with caretakers who knowingly or unknowingly invalidated their complaints about having their feelings hurt, about being abused emotionally, sexually, or otherwise, or about their worries concerning their interactions with peers at school. These caretakers may have been frightening and unpredictable, randomly showing affection or arbitrarily punishing for any perceived misstep, which led these patients to develop (for their own safety’s sake) a hypersensitivity to the affect of others. Their wariness and distrust of their social surroundings may have led to a skeptical view of kindness from others. Over time, without any guidance from prior demonstrations of healthy coping skills or interpersonal outlets from their caregivers, the emotional pressure builds. This pressure finally erupts in the form of impulsivity, self-harm, desperation, and defensiveness—in other words, survival. This is often followed by these patients’ first experience with receiving some degree of appropriate response to their complaints—their first experience with feeling seen and heard by their caretakers. They learn that their needs are met only when they cry out in desperation.1-3
These patients typically bring these maladaptive coping skills with them into adulthood, which often leads to a series of intense, unhealthy, and short-lived interpersonal and professional connections. They desire healthy, lasting connections with others, but through no fault of their own are unable to appropriately manage the normal stressors therein.1 Often, these patients do not know of their eventual BPD diagnosis, or even reject it due to its ever-negative valence. For other patients, receiving a personality disorder diagnosis is incredibly validating because they are no longer alone regarding this type of suffering, and a doctor—a caretaker—is finally making sense of this tumultuous world.
The countertransference of frustration, anxiety, doubt, and annoyance we may feel when caring for patients with BPD pales in comparison to living in their shoes and carrying the weight of what they have had to endure before presenting to our care. As these resilient patients wait in the exam room for the chance to be heard, let this be a reminder to greet them with the patience, understanding, empathy, and compassion that physicians are known to embody.
Suggestions for working with ‘difficult’ patients
The following tips may be helpful for building rapport with patients with BPD or other “difficult” patients:
- validate their complaints, and the difficulties they cause
- be genuine and honest when discussing their complaints
- acknowledge your own mistakes and misunderstandings in their care
- don’t be defensive—accept criticism with an open mind
- practice listening with intent, and reflective listening
- set ground rules and stick to them (eg, time limits, prescribing expectations, patient-physician relationship boundaries)
- educate and support the patient and their loved ones.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013:947.
2. Porter C, Palmier-Claus J, Branitsky A, et al. Childhood adversity and borderline personality disorder: a meta-analysis. Acta Psychiatr Scand. 2020;141(1):6-20.
3. Sansone RA, Sansone LA. Emotional hyper-reactivity in borderline personality disorder. Psychiatry (Edgmont). 2010;7(9):16-20.
Oh, great!” a senior resident sardonically remarked with a smirk as they read up on the next patient in the clinic. “A borderline patient. Get ready for a rough one ... Ugh.”
Before ever stepping foot into the patient’s room, this resident had prematurely established and demonstrated an unfortunate dynamic for any student or trainee within earshot. This is an all-too-familiar occurrence when caring for individuals with borderline personality disorder (BPD), or any other patients deemed to be “difficult.” The patient, however, likely walked into the room with a traumatic past that they continue to suffer from, in addition to any other issues for which they were seeking care.
Consider what these patients have experienced
A typical profile of these resilient patients with BPD: They were born emotionally sensitive. They grew up in homes with caretakers who knowingly or unknowingly invalidated their complaints about having their feelings hurt, about being abused emotionally, sexually, or otherwise, or about their worries concerning their interactions with peers at school. These caretakers may have been frightening and unpredictable, randomly showing affection or arbitrarily punishing for any perceived misstep, which led these patients to develop (for their own safety’s sake) a hypersensitivity to the affect of others. Their wariness and distrust of their social surroundings may have led to a skeptical view of kindness from others. Over time, without any guidance from prior demonstrations of healthy coping skills or interpersonal outlets from their caregivers, the emotional pressure builds. This pressure finally erupts in the form of impulsivity, self-harm, desperation, and defensiveness—in other words, survival. This is often followed by these patients’ first experience with receiving some degree of appropriate response to their complaints—their first experience with feeling seen and heard by their caretakers. They learn that their needs are met only when they cry out in desperation.1-3
These patients typically bring these maladaptive coping skills with them into adulthood, which often leads to a series of intense, unhealthy, and short-lived interpersonal and professional connections. They desire healthy, lasting connections with others, but through no fault of their own are unable to appropriately manage the normal stressors therein.1 Often, these patients do not know of their eventual BPD diagnosis, or even reject it due to its ever-negative valence. For other patients, receiving a personality disorder diagnosis is incredibly validating because they are no longer alone regarding this type of suffering, and a doctor—a caretaker—is finally making sense of this tumultuous world.
The countertransference of frustration, anxiety, doubt, and annoyance we may feel when caring for patients with BPD pales in comparison to living in their shoes and carrying the weight of what they have had to endure before presenting to our care. As these resilient patients wait in the exam room for the chance to be heard, let this be a reminder to greet them with the patience, understanding, empathy, and compassion that physicians are known to embody.
Suggestions for working with ‘difficult’ patients
The following tips may be helpful for building rapport with patients with BPD or other “difficult” patients:
- validate their complaints, and the difficulties they cause
- be genuine and honest when discussing their complaints
- acknowledge your own mistakes and misunderstandings in their care
- don’t be defensive—accept criticism with an open mind
- practice listening with intent, and reflective listening
- set ground rules and stick to them (eg, time limits, prescribing expectations, patient-physician relationship boundaries)
- educate and support the patient and their loved ones.
Oh, great!” a senior resident sardonically remarked with a smirk as they read up on the next patient in the clinic. “A borderline patient. Get ready for a rough one ... Ugh.”
Before ever stepping foot into the patient’s room, this resident had prematurely established and demonstrated an unfortunate dynamic for any student or trainee within earshot. This is an all-too-familiar occurrence when caring for individuals with borderline personality disorder (BPD), or any other patients deemed to be “difficult.” The patient, however, likely walked into the room with a traumatic past that they continue to suffer from, in addition to any other issues for which they were seeking care.
Consider what these patients have experienced
A typical profile of these resilient patients with BPD: They were born emotionally sensitive. They grew up in homes with caretakers who knowingly or unknowingly invalidated their complaints about having their feelings hurt, about being abused emotionally, sexually, or otherwise, or about their worries concerning their interactions with peers at school. These caretakers may have been frightening and unpredictable, randomly showing affection or arbitrarily punishing for any perceived misstep, which led these patients to develop (for their own safety’s sake) a hypersensitivity to the affect of others. Their wariness and distrust of their social surroundings may have led to a skeptical view of kindness from others. Over time, without any guidance from prior demonstrations of healthy coping skills or interpersonal outlets from their caregivers, the emotional pressure builds. This pressure finally erupts in the form of impulsivity, self-harm, desperation, and defensiveness—in other words, survival. This is often followed by these patients’ first experience with receiving some degree of appropriate response to their complaints—their first experience with feeling seen and heard by their caretakers. They learn that their needs are met only when they cry out in desperation.1-3
These patients typically bring these maladaptive coping skills with them into adulthood, which often leads to a series of intense, unhealthy, and short-lived interpersonal and professional connections. They desire healthy, lasting connections with others, but through no fault of their own are unable to appropriately manage the normal stressors therein.1 Often, these patients do not know of their eventual BPD diagnosis, or even reject it due to its ever-negative valence. For other patients, receiving a personality disorder diagnosis is incredibly validating because they are no longer alone regarding this type of suffering, and a doctor—a caretaker—is finally making sense of this tumultuous world.
The countertransference of frustration, anxiety, doubt, and annoyance we may feel when caring for patients with BPD pales in comparison to living in their shoes and carrying the weight of what they have had to endure before presenting to our care. As these resilient patients wait in the exam room for the chance to be heard, let this be a reminder to greet them with the patience, understanding, empathy, and compassion that physicians are known to embody.
Suggestions for working with ‘difficult’ patients
The following tips may be helpful for building rapport with patients with BPD or other “difficult” patients:
- validate their complaints, and the difficulties they cause
- be genuine and honest when discussing their complaints
- acknowledge your own mistakes and misunderstandings in their care
- don’t be defensive—accept criticism with an open mind
- practice listening with intent, and reflective listening
- set ground rules and stick to them (eg, time limits, prescribing expectations, patient-physician relationship boundaries)
- educate and support the patient and their loved ones.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013:947.
2. Porter C, Palmier-Claus J, Branitsky A, et al. Childhood adversity and borderline personality disorder: a meta-analysis. Acta Psychiatr Scand. 2020;141(1):6-20.
3. Sansone RA, Sansone LA. Emotional hyper-reactivity in borderline personality disorder. Psychiatry (Edgmont). 2010;7(9):16-20.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013:947.
2. Porter C, Palmier-Claus J, Branitsky A, et al. Childhood adversity and borderline personality disorder: a meta-analysis. Acta Psychiatr Scand. 2020;141(1):6-20.
3. Sansone RA, Sansone LA. Emotional hyper-reactivity in borderline personality disorder. Psychiatry (Edgmont). 2010;7(9):16-20.
Residency Roundup: Introducing a New Partnership Between Cutis and the APD-RPDS
We are excited to announce a new partnership between Cutis and the Association of Professors of Dermatology Residency Program Directors Section (APD-RPDS). The new APD-RPDS column Residency Roundup will contain quarterly communications and submissions that we hope will facilitate greater dissemination of information that is useful to the dermatology teaching community.
The APD is a group of academic dermatologists whose membership comprises chairs, chiefs, residency and fellowship program directors, and teaching faculty. Each fall, the group convenes in Chicago, Illinois, for a 2-day meeting centered around departmental and program leadership with a focus on education. The APD-RPDS was formed in 2020 and is led by a steering committee of 9 members, including our current Chair, Ilana S. Rosman, MD (Washington University School of Medicine, St. Louis, Missouri), and Vice Chair, Jo-Ann M. Latkowski, MD (New York University, New York). Committee members are elected from and by the APD membership and must serve in program leadership at their home programs. The APD-RPDS helps plan and coordinate breakout sessions and lectures at the annual APD meeting, which typically relate to program director duties, changing policies within the American Board of Dermatology or Accreditation Council for Graduate Medical Education, ideas for future growth, and changes in our specialty and in resident education. Members of the APD-RPDS have access to the APD listserv, a valuable resource for discussing issues affecting residency training. We also have work groups led by our members, which include diversity, equity, and inclusion; resource development; communications; and the annual survey. To join the APD, the RPDS, and/or any of our workgroups, please reach out to us or visit the APD website (https://www.dermatologyprofessors.org).
We look forward to welcoming and expediently reviewing members’ submissions to the new Residency Roundup column falling into 2 principal categories within the scope of dermatologic recruitment, didactic education, and clinical training. The first category will feature novel tools, programs, and platforms to improve dermatology training through collaboration. This could entail a description of a new platform designed for sharing resources among programs and specialties to enhance learning for trainees and faculty alike. For example, if a database is created that contains prerecorded lectures pertaining to alopecia, a potential article submission might introduce the database and provide information on what topics are covered and how to access these lectures for readers worldwide. Likewise, if a new technology emerges that allows for easier collaboration among programs, a possible submission would introduce the technology and discuss its potential benefits to trainees, faculty, and practicing dermatologists.
Secondly and more commonly, we anticipate the Residency Roundup column will feature articles that delve into the critical issues and challenges currently impacting recruitment, training, and administration in dermatology residency programs. Specific topics may include but are not limited to recruitment of underrepresented in medicine applicants to dermatology, technological advances to improve teaching methods within training programs, surveys delving into the dermatology match process, and educational gaps or future directions in the specialty. The column occasionally may be used to disseminate information from our section of the APD, including consensus statements or editorials related to changes implemented in the dermatology residency application process. A prospective editorial on this subject could explore varying viewpoints of implemented and proposed changes as well as the reasons behind the changes.
Our group is collaborative, and our aim is to improve education, equity, management of program director responsibilities, and the dermatology application process for programs and applicants alike. With your input, experience, and varied perspectives, we look forward to moving the field of dermatology to a better future by working together.
We are excited to announce a new partnership between Cutis and the Association of Professors of Dermatology Residency Program Directors Section (APD-RPDS). The new APD-RPDS column Residency Roundup will contain quarterly communications and submissions that we hope will facilitate greater dissemination of information that is useful to the dermatology teaching community.
The APD is a group of academic dermatologists whose membership comprises chairs, chiefs, residency and fellowship program directors, and teaching faculty. Each fall, the group convenes in Chicago, Illinois, for a 2-day meeting centered around departmental and program leadership with a focus on education. The APD-RPDS was formed in 2020 and is led by a steering committee of 9 members, including our current Chair, Ilana S. Rosman, MD (Washington University School of Medicine, St. Louis, Missouri), and Vice Chair, Jo-Ann M. Latkowski, MD (New York University, New York). Committee members are elected from and by the APD membership and must serve in program leadership at their home programs. The APD-RPDS helps plan and coordinate breakout sessions and lectures at the annual APD meeting, which typically relate to program director duties, changing policies within the American Board of Dermatology or Accreditation Council for Graduate Medical Education, ideas for future growth, and changes in our specialty and in resident education. Members of the APD-RPDS have access to the APD listserv, a valuable resource for discussing issues affecting residency training. We also have work groups led by our members, which include diversity, equity, and inclusion; resource development; communications; and the annual survey. To join the APD, the RPDS, and/or any of our workgroups, please reach out to us or visit the APD website (https://www.dermatologyprofessors.org).
We look forward to welcoming and expediently reviewing members’ submissions to the new Residency Roundup column falling into 2 principal categories within the scope of dermatologic recruitment, didactic education, and clinical training. The first category will feature novel tools, programs, and platforms to improve dermatology training through collaboration. This could entail a description of a new platform designed for sharing resources among programs and specialties to enhance learning for trainees and faculty alike. For example, if a database is created that contains prerecorded lectures pertaining to alopecia, a potential article submission might introduce the database and provide information on what topics are covered and how to access these lectures for readers worldwide. Likewise, if a new technology emerges that allows for easier collaboration among programs, a possible submission would introduce the technology and discuss its potential benefits to trainees, faculty, and practicing dermatologists.
Secondly and more commonly, we anticipate the Residency Roundup column will feature articles that delve into the critical issues and challenges currently impacting recruitment, training, and administration in dermatology residency programs. Specific topics may include but are not limited to recruitment of underrepresented in medicine applicants to dermatology, technological advances to improve teaching methods within training programs, surveys delving into the dermatology match process, and educational gaps or future directions in the specialty. The column occasionally may be used to disseminate information from our section of the APD, including consensus statements or editorials related to changes implemented in the dermatology residency application process. A prospective editorial on this subject could explore varying viewpoints of implemented and proposed changes as well as the reasons behind the changes.
Our group is collaborative, and our aim is to improve education, equity, management of program director responsibilities, and the dermatology application process for programs and applicants alike. With your input, experience, and varied perspectives, we look forward to moving the field of dermatology to a better future by working together.
We are excited to announce a new partnership between Cutis and the Association of Professors of Dermatology Residency Program Directors Section (APD-RPDS). The new APD-RPDS column Residency Roundup will contain quarterly communications and submissions that we hope will facilitate greater dissemination of information that is useful to the dermatology teaching community.
The APD is a group of academic dermatologists whose membership comprises chairs, chiefs, residency and fellowship program directors, and teaching faculty. Each fall, the group convenes in Chicago, Illinois, for a 2-day meeting centered around departmental and program leadership with a focus on education. The APD-RPDS was formed in 2020 and is led by a steering committee of 9 members, including our current Chair, Ilana S. Rosman, MD (Washington University School of Medicine, St. Louis, Missouri), and Vice Chair, Jo-Ann M. Latkowski, MD (New York University, New York). Committee members are elected from and by the APD membership and must serve in program leadership at their home programs. The APD-RPDS helps plan and coordinate breakout sessions and lectures at the annual APD meeting, which typically relate to program director duties, changing policies within the American Board of Dermatology or Accreditation Council for Graduate Medical Education, ideas for future growth, and changes in our specialty and in resident education. Members of the APD-RPDS have access to the APD listserv, a valuable resource for discussing issues affecting residency training. We also have work groups led by our members, which include diversity, equity, and inclusion; resource development; communications; and the annual survey. To join the APD, the RPDS, and/or any of our workgroups, please reach out to us or visit the APD website (https://www.dermatologyprofessors.org).
We look forward to welcoming and expediently reviewing members’ submissions to the new Residency Roundup column falling into 2 principal categories within the scope of dermatologic recruitment, didactic education, and clinical training. The first category will feature novel tools, programs, and platforms to improve dermatology training through collaboration. This could entail a description of a new platform designed for sharing resources among programs and specialties to enhance learning for trainees and faculty alike. For example, if a database is created that contains prerecorded lectures pertaining to alopecia, a potential article submission might introduce the database and provide information on what topics are covered and how to access these lectures for readers worldwide. Likewise, if a new technology emerges that allows for easier collaboration among programs, a possible submission would introduce the technology and discuss its potential benefits to trainees, faculty, and practicing dermatologists.
Secondly and more commonly, we anticipate the Residency Roundup column will feature articles that delve into the critical issues and challenges currently impacting recruitment, training, and administration in dermatology residency programs. Specific topics may include but are not limited to recruitment of underrepresented in medicine applicants to dermatology, technological advances to improve teaching methods within training programs, surveys delving into the dermatology match process, and educational gaps or future directions in the specialty. The column occasionally may be used to disseminate information from our section of the APD, including consensus statements or editorials related to changes implemented in the dermatology residency application process. A prospective editorial on this subject could explore varying viewpoints of implemented and proposed changes as well as the reasons behind the changes.
Our group is collaborative, and our aim is to improve education, equity, management of program director responsibilities, and the dermatology application process for programs and applicants alike. With your input, experience, and varied perspectives, we look forward to moving the field of dermatology to a better future by working together.
Managing a COVID-19–positive psychiatric patient on a medical unit
With the COVID-19 pandemic turning the world on its head, we have seen more first-episode psychotic breaks and quick deterioration in previously stable patients. Early in the pandemic, care was particularly complicated for psychiatric patients who had been infected with the virus. Many of these patients required immediate psychiatric hospitalization. At that time, many community hospital psychiatric inpatient units did not have the capacity, staffing, or infrastructure to safely admit such patients, so they needed to be managed on a medical unit. Here, I discuss the case of a COVID-19–positive woman with psychiatric illness who we managed while she was in quarantine on a medical unit.
Case report
Early in the COVID-19 pandemic, Ms. B, a 35-year-old teacher with a history of depression, was evaluated in the emergency department for bizarre behavior and paranoid delusions regarding her family. Initial laboratory and imaging testing was negative for any potential medical causes of her psychiatric symptoms. Psychiatric hospitalization was recommended, but before Ms. B could be transferred to the psychiatric unit, she tested positive for COVID-19. At that time, our community hospital did not have a designated wing on our psychiatric unit for patients infected with COVID-19. Thus, Ms. B was admitted to the medical floor, where she was quarantined in her room. She would need to remain asymptomatic and test negative for COVID-19 before she could be transferred to the psychiatric unit.
Upon arriving at the medical unit, Ms. B was hostile and uncooperative. She frequently attempted to leave her room and required restraints throughout the day. Our consultation-liaison (CL) team was consulted to assist in managing her. During the initial interview, we noticed that she had covered all 4 walls of her room with papers filled with handwritten notes. Ms. B had cut her gown to expose her stomach and legs. She had pressured speech, tangential thinking, and was religiously preoccupied. She denied any visual and auditory hallucinations, but her persecutory delusions involving her family persisted. We believed that her signs and symptoms were consistent with a manic episode from underlying, and likely undiagnosed, bipolar I disorder that was precipitated by her COVID-19 infection.
We first addressed Ms. B’s and the staff’s safety by transferring her to a larger room with a vestibule at the end of the hallway so she had more room to walk and minimal exposure to the stimuli of the medical unit. We initiated one-on-one observation to redirect her and prevent elopement. We incentivized her cooperation with staff by providing her with paper, pencils, reading material, and phone privileges. We started oral risperidone 2 mg twice daily and lorazepam 2 mg 3 times daily for short-term behavioral control and acute treatment of her symptoms, with the goal of deferring additional treatment decisions to the inpatient psychiatry team after she was transferred to the psychiatric unit. Ms. B’s agitation and impulsivity improved. She began participating with the medical team and was eventually transferred out of our medical unit to a psychiatric unit at a different facility.
COVID-19 and psychiatric illness: Clinical concerns
While infection from COVID-19 and widespread social distancing of the general population have been linked to depression and anxiety, manic and psychotic symptoms secondary to the COVID-19 pandemic have not been well described. The association between influenza infection and psychosis has been reported since the Spanish Flu pandemic,1 but there is limited data on the association between COVID-19 and psychosis. A review of 14 studies found that 0.9% to 4% of people exposed to a virus during an epidemic or pandemic develop psychosis or psychotic symptoms.1 Psychosis was associated with viral exposure, treatments used to manage the infection (steroid therapy), and psychosocial stress. This study also found that treatment with low doses of antipsychotic medication—notably aripiprazole—seemed to have been effective.1
Nonetheless, it is important to keep in mind a thorough differential diagnosis and rule out any potential organic etiologies in a COVID-19–positive patient who presents with psychiatric symptoms.2 For Ms. B, we began by ruling out drug-induced psychosis and electrolyte imbalance, and obtained brain imaging to rule out malignancy. We considered an interictal behavior syndrome of temporal lobe epilepsy, a neuropsychiatric disorder characterized by alterations in sexual behavior, religiosity, and extensive and compulsive writing and drawing.3 Neurology was consulted to evaluate the patient and possibly use EEG to detect interictal spikes, a tall task given the patient’s restlessness and paranoia. Ultimately, we determined the patient was most likely exhibiting symptoms of previously undetected bipolar disorder.
Managing patients with psychiatric illness on a medical floor during a pandemic such as COVID-19 requires the psychiatrist to truly serve as a consultant and liaison between the patient and the treatment team.4 Clinical management should address both infection control and psychiatric symptoms.5 We visited with Ms. B frequently, provided psychoeducation, engaged her in treatment, and updated her on the treatment plan.
As the medical world continues to adjust to treating patients during the pandemic, CL psychiatrists may be tasked with managing patients with acute psychiatric illness on the medical unit while they await transfer to a psychiatric unit. A creative, multifaceted, and team-based approach is key to ensure effective care and safety for all involved.
1. Brown E, Gray R, Lo Monaco S, et al. The potential impact of COVID-19 on psychosis: a rapid review of contemporary epidemic and pandemic research. Schizophr Res. 2020;222:79-87. doi:10.1016/j.schres.2020.05.005
2. Byrne P. Managing the acute psychotic episode. BMJ. 2007;334(7595):686-692. doi:10.1136/bmj.39148.668160.80
3. Waxman SG, Geschwind N. The interictal behavior syndrome of temporal lobe epilepsy. Arch Gen Psychiatry. 1975;32(12):1580-1586. doi:10.1001/archpsyc.1975.01760300118011
4. Stern TA, Freudenreich O, Smith FA, et al. Psychotic patients. In: Massachusetts General Hospital: Handbook of General Hospital Psychiatry. Mosby; 1997:109-121.
5. Deshpande S, Livingstone A. First-onset psychosis in older adults: social isolation influence during COVID pandemic—a UK case series. Progress in Neurology and Psychiatry. 2021;25(1):14-18. doi:10.1002/pnp.692
With the COVID-19 pandemic turning the world on its head, we have seen more first-episode psychotic breaks and quick deterioration in previously stable patients. Early in the pandemic, care was particularly complicated for psychiatric patients who had been infected with the virus. Many of these patients required immediate psychiatric hospitalization. At that time, many community hospital psychiatric inpatient units did not have the capacity, staffing, or infrastructure to safely admit such patients, so they needed to be managed on a medical unit. Here, I discuss the case of a COVID-19–positive woman with psychiatric illness who we managed while she was in quarantine on a medical unit.
Case report
Early in the COVID-19 pandemic, Ms. B, a 35-year-old teacher with a history of depression, was evaluated in the emergency department for bizarre behavior and paranoid delusions regarding her family. Initial laboratory and imaging testing was negative for any potential medical causes of her psychiatric symptoms. Psychiatric hospitalization was recommended, but before Ms. B could be transferred to the psychiatric unit, she tested positive for COVID-19. At that time, our community hospital did not have a designated wing on our psychiatric unit for patients infected with COVID-19. Thus, Ms. B was admitted to the medical floor, where she was quarantined in her room. She would need to remain asymptomatic and test negative for COVID-19 before she could be transferred to the psychiatric unit.
Upon arriving at the medical unit, Ms. B was hostile and uncooperative. She frequently attempted to leave her room and required restraints throughout the day. Our consultation-liaison (CL) team was consulted to assist in managing her. During the initial interview, we noticed that she had covered all 4 walls of her room with papers filled with handwritten notes. Ms. B had cut her gown to expose her stomach and legs. She had pressured speech, tangential thinking, and was religiously preoccupied. She denied any visual and auditory hallucinations, but her persecutory delusions involving her family persisted. We believed that her signs and symptoms were consistent with a manic episode from underlying, and likely undiagnosed, bipolar I disorder that was precipitated by her COVID-19 infection.
We first addressed Ms. B’s and the staff’s safety by transferring her to a larger room with a vestibule at the end of the hallway so she had more room to walk and minimal exposure to the stimuli of the medical unit. We initiated one-on-one observation to redirect her and prevent elopement. We incentivized her cooperation with staff by providing her with paper, pencils, reading material, and phone privileges. We started oral risperidone 2 mg twice daily and lorazepam 2 mg 3 times daily for short-term behavioral control and acute treatment of her symptoms, with the goal of deferring additional treatment decisions to the inpatient psychiatry team after she was transferred to the psychiatric unit. Ms. B’s agitation and impulsivity improved. She began participating with the medical team and was eventually transferred out of our medical unit to a psychiatric unit at a different facility.
COVID-19 and psychiatric illness: Clinical concerns
While infection from COVID-19 and widespread social distancing of the general population have been linked to depression and anxiety, manic and psychotic symptoms secondary to the COVID-19 pandemic have not been well described. The association between influenza infection and psychosis has been reported since the Spanish Flu pandemic,1 but there is limited data on the association between COVID-19 and psychosis. A review of 14 studies found that 0.9% to 4% of people exposed to a virus during an epidemic or pandemic develop psychosis or psychotic symptoms.1 Psychosis was associated with viral exposure, treatments used to manage the infection (steroid therapy), and psychosocial stress. This study also found that treatment with low doses of antipsychotic medication—notably aripiprazole—seemed to have been effective.1
Nonetheless, it is important to keep in mind a thorough differential diagnosis and rule out any potential organic etiologies in a COVID-19–positive patient who presents with psychiatric symptoms.2 For Ms. B, we began by ruling out drug-induced psychosis and electrolyte imbalance, and obtained brain imaging to rule out malignancy. We considered an interictal behavior syndrome of temporal lobe epilepsy, a neuropsychiatric disorder characterized by alterations in sexual behavior, religiosity, and extensive and compulsive writing and drawing.3 Neurology was consulted to evaluate the patient and possibly use EEG to detect interictal spikes, a tall task given the patient’s restlessness and paranoia. Ultimately, we determined the patient was most likely exhibiting symptoms of previously undetected bipolar disorder.
Managing patients with psychiatric illness on a medical floor during a pandemic such as COVID-19 requires the psychiatrist to truly serve as a consultant and liaison between the patient and the treatment team.4 Clinical management should address both infection control and psychiatric symptoms.5 We visited with Ms. B frequently, provided psychoeducation, engaged her in treatment, and updated her on the treatment plan.
As the medical world continues to adjust to treating patients during the pandemic, CL psychiatrists may be tasked with managing patients with acute psychiatric illness on the medical unit while they await transfer to a psychiatric unit. A creative, multifaceted, and team-based approach is key to ensure effective care and safety for all involved.
With the COVID-19 pandemic turning the world on its head, we have seen more first-episode psychotic breaks and quick deterioration in previously stable patients. Early in the pandemic, care was particularly complicated for psychiatric patients who had been infected with the virus. Many of these patients required immediate psychiatric hospitalization. At that time, many community hospital psychiatric inpatient units did not have the capacity, staffing, or infrastructure to safely admit such patients, so they needed to be managed on a medical unit. Here, I discuss the case of a COVID-19–positive woman with psychiatric illness who we managed while she was in quarantine on a medical unit.
Case report
Early in the COVID-19 pandemic, Ms. B, a 35-year-old teacher with a history of depression, was evaluated in the emergency department for bizarre behavior and paranoid delusions regarding her family. Initial laboratory and imaging testing was negative for any potential medical causes of her psychiatric symptoms. Psychiatric hospitalization was recommended, but before Ms. B could be transferred to the psychiatric unit, she tested positive for COVID-19. At that time, our community hospital did not have a designated wing on our psychiatric unit for patients infected with COVID-19. Thus, Ms. B was admitted to the medical floor, where she was quarantined in her room. She would need to remain asymptomatic and test negative for COVID-19 before she could be transferred to the psychiatric unit.
Upon arriving at the medical unit, Ms. B was hostile and uncooperative. She frequently attempted to leave her room and required restraints throughout the day. Our consultation-liaison (CL) team was consulted to assist in managing her. During the initial interview, we noticed that she had covered all 4 walls of her room with papers filled with handwritten notes. Ms. B had cut her gown to expose her stomach and legs. She had pressured speech, tangential thinking, and was religiously preoccupied. She denied any visual and auditory hallucinations, but her persecutory delusions involving her family persisted. We believed that her signs and symptoms were consistent with a manic episode from underlying, and likely undiagnosed, bipolar I disorder that was precipitated by her COVID-19 infection.
We first addressed Ms. B’s and the staff’s safety by transferring her to a larger room with a vestibule at the end of the hallway so she had more room to walk and minimal exposure to the stimuli of the medical unit. We initiated one-on-one observation to redirect her and prevent elopement. We incentivized her cooperation with staff by providing her with paper, pencils, reading material, and phone privileges. We started oral risperidone 2 mg twice daily and lorazepam 2 mg 3 times daily for short-term behavioral control and acute treatment of her symptoms, with the goal of deferring additional treatment decisions to the inpatient psychiatry team after she was transferred to the psychiatric unit. Ms. B’s agitation and impulsivity improved. She began participating with the medical team and was eventually transferred out of our medical unit to a psychiatric unit at a different facility.
COVID-19 and psychiatric illness: Clinical concerns
While infection from COVID-19 and widespread social distancing of the general population have been linked to depression and anxiety, manic and psychotic symptoms secondary to the COVID-19 pandemic have not been well described. The association between influenza infection and psychosis has been reported since the Spanish Flu pandemic,1 but there is limited data on the association between COVID-19 and psychosis. A review of 14 studies found that 0.9% to 4% of people exposed to a virus during an epidemic or pandemic develop psychosis or psychotic symptoms.1 Psychosis was associated with viral exposure, treatments used to manage the infection (steroid therapy), and psychosocial stress. This study also found that treatment with low doses of antipsychotic medication—notably aripiprazole—seemed to have been effective.1
Nonetheless, it is important to keep in mind a thorough differential diagnosis and rule out any potential organic etiologies in a COVID-19–positive patient who presents with psychiatric symptoms.2 For Ms. B, we began by ruling out drug-induced psychosis and electrolyte imbalance, and obtained brain imaging to rule out malignancy. We considered an interictal behavior syndrome of temporal lobe epilepsy, a neuropsychiatric disorder characterized by alterations in sexual behavior, religiosity, and extensive and compulsive writing and drawing.3 Neurology was consulted to evaluate the patient and possibly use EEG to detect interictal spikes, a tall task given the patient’s restlessness and paranoia. Ultimately, we determined the patient was most likely exhibiting symptoms of previously undetected bipolar disorder.
Managing patients with psychiatric illness on a medical floor during a pandemic such as COVID-19 requires the psychiatrist to truly serve as a consultant and liaison between the patient and the treatment team.4 Clinical management should address both infection control and psychiatric symptoms.5 We visited with Ms. B frequently, provided psychoeducation, engaged her in treatment, and updated her on the treatment plan.
As the medical world continues to adjust to treating patients during the pandemic, CL psychiatrists may be tasked with managing patients with acute psychiatric illness on the medical unit while they await transfer to a psychiatric unit. A creative, multifaceted, and team-based approach is key to ensure effective care and safety for all involved.
1. Brown E, Gray R, Lo Monaco S, et al. The potential impact of COVID-19 on psychosis: a rapid review of contemporary epidemic and pandemic research. Schizophr Res. 2020;222:79-87. doi:10.1016/j.schres.2020.05.005
2. Byrne P. Managing the acute psychotic episode. BMJ. 2007;334(7595):686-692. doi:10.1136/bmj.39148.668160.80
3. Waxman SG, Geschwind N. The interictal behavior syndrome of temporal lobe epilepsy. Arch Gen Psychiatry. 1975;32(12):1580-1586. doi:10.1001/archpsyc.1975.01760300118011
4. Stern TA, Freudenreich O, Smith FA, et al. Psychotic patients. In: Massachusetts General Hospital: Handbook of General Hospital Psychiatry. Mosby; 1997:109-121.
5. Deshpande S, Livingstone A. First-onset psychosis in older adults: social isolation influence during COVID pandemic—a UK case series. Progress in Neurology and Psychiatry. 2021;25(1):14-18. doi:10.1002/pnp.692
1. Brown E, Gray R, Lo Monaco S, et al. The potential impact of COVID-19 on psychosis: a rapid review of contemporary epidemic and pandemic research. Schizophr Res. 2020;222:79-87. doi:10.1016/j.schres.2020.05.005
2. Byrne P. Managing the acute psychotic episode. BMJ. 2007;334(7595):686-692. doi:10.1136/bmj.39148.668160.80
3. Waxman SG, Geschwind N. The interictal behavior syndrome of temporal lobe epilepsy. Arch Gen Psychiatry. 1975;32(12):1580-1586. doi:10.1001/archpsyc.1975.01760300118011
4. Stern TA, Freudenreich O, Smith FA, et al. Psychotic patients. In: Massachusetts General Hospital: Handbook of General Hospital Psychiatry. Mosby; 1997:109-121.
5. Deshpande S, Livingstone A. First-onset psychosis in older adults: social isolation influence during COVID pandemic—a UK case series. Progress in Neurology and Psychiatry. 2021;25(1):14-18. doi:10.1002/pnp.692
Removal of Isotretinoin Gender-Based Guidelines: Inclusivity Takes Precedence
Isotretinoin is one of the most highly regulated dermatologic medications on the market. The main reason for regulation through the US Food and Drug Administration (FDA)–managed iPLEDGE Risk Evaluation and Mitigation Strategy (REMS) is to minimize the drug’s teratogenic potential, as isotretinoin can cause profound birth defects. The program originally categorized patients into 1 of 3 categories: (1) females of reproductive potential, (2) females not of reproductive potential, and (3) males. Unless the patient commits to abstinence, the program required female patients of childbearing potential to be on 2 forms of birth control and undergo regular pregnancy testing before obtaining refills. Over the last few years, the American Academy of Dermatology Association (AADA) has been advocating for changes to the iPLEDGE system. Proposed changes have included decreasing attestation frequency for patients who cannot get pregnant, increasing contraception counseling and options, and changing enrollment guidelines to encompass all gender and sexual minorities. As of December 13, 2021, the iPLEDGE system changed enrollment categories to reflect the AADA’s wishes and rolled out gender-neutral categories for enrollment in iPLEDGE. This change will simplify and enhance patients’ experience when starting isotretinoin.
Developing Inclusive iPLEDGE Categories
In recent years, dermatologists and patients have viewed these strict gender-based categories as limiting and problematic, especially for their transgender patients and female patients of childbearing potential who exclusively engage in intercourse with cisgender females. The United States has more than 10 million LGBTQIA+ citizens and an estimated 1.4 million adults who identify as transgender individuals, rendering the previously established gender-binary iPLEDGE categories outdated.1,2
As a result, over the last few years, dermatologists, LGBTQIA+ allies, and patients have urged the FDA to create a gender-neutral registration process for iPLEDGE. With support from the AADA, the new modifications were approved for implementation and include 2 risk categories: (1) people who can get pregnant and (2) people who cannot get pregnant.3
As exciting as these changes are for the future of dermatologic practice, the actual transition to the new iPLEDGE system was described as a “failure, chaotic, and a disaster” due to additional changes made at the same time.4 The iPLEDGE system was switched to a new website administered by a different vendor and required providers to confirm each patient online by December 13, 2021. In addition, the new system required pharmacists to obtain risk management authorization via the iPLEDGE REMS website or by calling the iPLEDGE REMS center before dispensing isotretinoin. This overhaul did not work as planned, as the new website was constantly down and it was nearly impossible to reach a contact over the telephone. The complications resulted in major disruptions and delayed prescriptions for thousands of patients nationwide as well as a great disruption in workflow for physicians and pharmacists. The AADA subsequently met with the Isotretinoin Products Manufacturers Group to create workable solutions for these issues.
On January 14, 2022, the FDA posted updates regarding access to the iPLEDGE system. They have worked with the Isotretinoin Products Manufacturers Group to create workable solutions for patients and physicians while transferring the patients’ information to the new database. Their solution includes allowing physicians to send patients login links through their email to access their account instead of waiting for the call center. The majority of iPLEDGE users now have access to their accounts without issues, and the gender-neutral guidelines have been in place since the original change.
Impact of iPLEDGE Categories on Transgender Patients
These changes specifically will improve the experience of transgender men and cisgender women who are at no risk for pregnancy and could be subjected to monthly pregnancy testing when it is not medically necessary.
Consider the following patient scenario. A transgender man presents to your dermatology office seeking treatment of severe nodulocystic acne. He was placed on hormonal replacement therapy with exogenous testosterone—injections, oral pills, topical gel, topical patches, or subdermal pellets—to achieve secondary sex characteristics and promote gender congruence. The patient mentions he has been amenorrheic for several months now. He has tried many topical acne treatments as well as oral antibiotics without much benefit and is now interested in enrolling in iPLEDGE to obtain isotretinoin. With the prior iPLEDGE registration packets, how would this transgender man be classified? As a female with childbearing potential due to his retained ovaries and uterus? What if he did not endorse engaging in sexual intercourse that could result in pregnancy?
Transgender patients have unique and unmet needs that often are overlooked and prevent them from equitable, gender-affirming health care. For example, in a prospective study following 20 transgender men starting hormone replacement therapy, the percentage of patients with facial acne increased from 35% to 82% after 6 months of therapy.5 In addition, the increased psychosocial burden of acne may be especially difficult in these patients, as they already report higher rates of depression and suicidal ideation compared with their heterosexual cisgender peers.4 Further, the primary patient populations receiving isotretinoin typically are adolescents and young adults who are undergoing major physical, mental, and hormonal changes. Self-discovery and self-actualization develop over time, and our role as physicians is to advocate for all aspects of our patients’ health and eliminate barriers to optimal care.
Inclusive Language in iPLEDGE Categories
It is important to streamline access to care for all patients, and gender-affirming, culturally sensitive language is essential to building trust and understanding between patients and providers. Howa Yeung, MD, MSc, a dermatologist at Emory University (Atlanta, Georgia) who advocated for gender-neutral iPLEDGE registration, welcomes the change and stated it “will make my job easier. I no longer have to struggle between respecting the patient’s gender identity and providing medically necessary care for patients with severe acne.”3
Sanchez et al6 provided a list of structured questions providers can ask their patients to assess their risk regarding pregnancy: (1) Do you have a uterus and/or ovaries?, (2) Are you engaging in sexual intercourse with a person who has a penis?, and (3) If yes to these questions, what form(s) of birth control are you using? Providers should preface these questions with the following statement: “It is important that I ask these questions to assess your risk for becoming pregnant on this medication because isotretinoin can cause very serious birth defects.” It is important to review these questions and practice asking them so residents can operate from the same place of openness and understanding when caring for their patients.
Final Thoughts
The landscape of isotretinoin prescribing currently is changing on a day-to-day basis. As residents, it is important we stay up to date with the changes regarding our regularly dispensed medications. The main modification made to the iPLEDGE REMS system was switching the risk categories from 3 (females who can get pregnant, females who cannot get pregnant, males) to 2 (people who can get pregnant, people who cannot get pregnant). This change will make registration for iPLEDGE less complex and more inclusive for all patients. It is important for residents to stay at the forefront of these patient health issues and barriers to equal care, and this change represents a step in the right direction.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602. doi:10.1016/j.jaad.2018.02.045
- Flores AR, Herman JL, Gates GJ, et al. How many adults identify as transgender in the United States? UCLA Williams Institute website. Published June 2016. Accessed March 1, 2022. https://williamsinstitute.law.ucla.edu/publications/trans-adults-united-states/
- Doheny K. FDA OKs iPLEDGE change for gender-neutral language. Dermatology News. October 13, 2021. Accessed March 3, 2022. https://www.mdedge.com/dermatology/article/247352/acne/fda-oks-ipledge-change-gender-neutral-language/page/0/1
- Doheny K. iPLEDGE rollout described as a failure, chaotic, and a disaster. Medscape. December 16, 2021. Accessed March 1, 2022. https://www.medscape.com/viewarticle/964925?uac=423615MG
- Wierckx K, Van de Peer F, Verhaeghe E, et al. Short- and long-term clinical skin effects of testosterone treatment in trans men. J Sex Med. 2014;11:222-229.
- Sanchez DP, Brownstone N, Thibodeaux Q, et al. Prescribing isotretinoin for transgender patients: a call to action and recommendations. J Drugs Dermatol. 2021;20:106-108.
Isotretinoin is one of the most highly regulated dermatologic medications on the market. The main reason for regulation through the US Food and Drug Administration (FDA)–managed iPLEDGE Risk Evaluation and Mitigation Strategy (REMS) is to minimize the drug’s teratogenic potential, as isotretinoin can cause profound birth defects. The program originally categorized patients into 1 of 3 categories: (1) females of reproductive potential, (2) females not of reproductive potential, and (3) males. Unless the patient commits to abstinence, the program required female patients of childbearing potential to be on 2 forms of birth control and undergo regular pregnancy testing before obtaining refills. Over the last few years, the American Academy of Dermatology Association (AADA) has been advocating for changes to the iPLEDGE system. Proposed changes have included decreasing attestation frequency for patients who cannot get pregnant, increasing contraception counseling and options, and changing enrollment guidelines to encompass all gender and sexual minorities. As of December 13, 2021, the iPLEDGE system changed enrollment categories to reflect the AADA’s wishes and rolled out gender-neutral categories for enrollment in iPLEDGE. This change will simplify and enhance patients’ experience when starting isotretinoin.
Developing Inclusive iPLEDGE Categories
In recent years, dermatologists and patients have viewed these strict gender-based categories as limiting and problematic, especially for their transgender patients and female patients of childbearing potential who exclusively engage in intercourse with cisgender females. The United States has more than 10 million LGBTQIA+ citizens and an estimated 1.4 million adults who identify as transgender individuals, rendering the previously established gender-binary iPLEDGE categories outdated.1,2
As a result, over the last few years, dermatologists, LGBTQIA+ allies, and patients have urged the FDA to create a gender-neutral registration process for iPLEDGE. With support from the AADA, the new modifications were approved for implementation and include 2 risk categories: (1) people who can get pregnant and (2) people who cannot get pregnant.3
As exciting as these changes are for the future of dermatologic practice, the actual transition to the new iPLEDGE system was described as a “failure, chaotic, and a disaster” due to additional changes made at the same time.4 The iPLEDGE system was switched to a new website administered by a different vendor and required providers to confirm each patient online by December 13, 2021. In addition, the new system required pharmacists to obtain risk management authorization via the iPLEDGE REMS website or by calling the iPLEDGE REMS center before dispensing isotretinoin. This overhaul did not work as planned, as the new website was constantly down and it was nearly impossible to reach a contact over the telephone. The complications resulted in major disruptions and delayed prescriptions for thousands of patients nationwide as well as a great disruption in workflow for physicians and pharmacists. The AADA subsequently met with the Isotretinoin Products Manufacturers Group to create workable solutions for these issues.
On January 14, 2022, the FDA posted updates regarding access to the iPLEDGE system. They have worked with the Isotretinoin Products Manufacturers Group to create workable solutions for patients and physicians while transferring the patients’ information to the new database. Their solution includes allowing physicians to send patients login links through their email to access their account instead of waiting for the call center. The majority of iPLEDGE users now have access to their accounts without issues, and the gender-neutral guidelines have been in place since the original change.
Impact of iPLEDGE Categories on Transgender Patients
These changes specifically will improve the experience of transgender men and cisgender women who are at no risk for pregnancy and could be subjected to monthly pregnancy testing when it is not medically necessary.
Consider the following patient scenario. A transgender man presents to your dermatology office seeking treatment of severe nodulocystic acne. He was placed on hormonal replacement therapy with exogenous testosterone—injections, oral pills, topical gel, topical patches, or subdermal pellets—to achieve secondary sex characteristics and promote gender congruence. The patient mentions he has been amenorrheic for several months now. He has tried many topical acne treatments as well as oral antibiotics without much benefit and is now interested in enrolling in iPLEDGE to obtain isotretinoin. With the prior iPLEDGE registration packets, how would this transgender man be classified? As a female with childbearing potential due to his retained ovaries and uterus? What if he did not endorse engaging in sexual intercourse that could result in pregnancy?
Transgender patients have unique and unmet needs that often are overlooked and prevent them from equitable, gender-affirming health care. For example, in a prospective study following 20 transgender men starting hormone replacement therapy, the percentage of patients with facial acne increased from 35% to 82% after 6 months of therapy.5 In addition, the increased psychosocial burden of acne may be especially difficult in these patients, as they already report higher rates of depression and suicidal ideation compared with their heterosexual cisgender peers.4 Further, the primary patient populations receiving isotretinoin typically are adolescents and young adults who are undergoing major physical, mental, and hormonal changes. Self-discovery and self-actualization develop over time, and our role as physicians is to advocate for all aspects of our patients’ health and eliminate barriers to optimal care.
Inclusive Language in iPLEDGE Categories
It is important to streamline access to care for all patients, and gender-affirming, culturally sensitive language is essential to building trust and understanding between patients and providers. Howa Yeung, MD, MSc, a dermatologist at Emory University (Atlanta, Georgia) who advocated for gender-neutral iPLEDGE registration, welcomes the change and stated it “will make my job easier. I no longer have to struggle between respecting the patient’s gender identity and providing medically necessary care for patients with severe acne.”3
Sanchez et al6 provided a list of structured questions providers can ask their patients to assess their risk regarding pregnancy: (1) Do you have a uterus and/or ovaries?, (2) Are you engaging in sexual intercourse with a person who has a penis?, and (3) If yes to these questions, what form(s) of birth control are you using? Providers should preface these questions with the following statement: “It is important that I ask these questions to assess your risk for becoming pregnant on this medication because isotretinoin can cause very serious birth defects.” It is important to review these questions and practice asking them so residents can operate from the same place of openness and understanding when caring for their patients.
Final Thoughts
The landscape of isotretinoin prescribing currently is changing on a day-to-day basis. As residents, it is important we stay up to date with the changes regarding our regularly dispensed medications. The main modification made to the iPLEDGE REMS system was switching the risk categories from 3 (females who can get pregnant, females who cannot get pregnant, males) to 2 (people who can get pregnant, people who cannot get pregnant). This change will make registration for iPLEDGE less complex and more inclusive for all patients. It is important for residents to stay at the forefront of these patient health issues and barriers to equal care, and this change represents a step in the right direction.
Isotretinoin is one of the most highly regulated dermatologic medications on the market. The main reason for regulation through the US Food and Drug Administration (FDA)–managed iPLEDGE Risk Evaluation and Mitigation Strategy (REMS) is to minimize the drug’s teratogenic potential, as isotretinoin can cause profound birth defects. The program originally categorized patients into 1 of 3 categories: (1) females of reproductive potential, (2) females not of reproductive potential, and (3) males. Unless the patient commits to abstinence, the program required female patients of childbearing potential to be on 2 forms of birth control and undergo regular pregnancy testing before obtaining refills. Over the last few years, the American Academy of Dermatology Association (AADA) has been advocating for changes to the iPLEDGE system. Proposed changes have included decreasing attestation frequency for patients who cannot get pregnant, increasing contraception counseling and options, and changing enrollment guidelines to encompass all gender and sexual minorities. As of December 13, 2021, the iPLEDGE system changed enrollment categories to reflect the AADA’s wishes and rolled out gender-neutral categories for enrollment in iPLEDGE. This change will simplify and enhance patients’ experience when starting isotretinoin.
Developing Inclusive iPLEDGE Categories
In recent years, dermatologists and patients have viewed these strict gender-based categories as limiting and problematic, especially for their transgender patients and female patients of childbearing potential who exclusively engage in intercourse with cisgender females. The United States has more than 10 million LGBTQIA+ citizens and an estimated 1.4 million adults who identify as transgender individuals, rendering the previously established gender-binary iPLEDGE categories outdated.1,2
As a result, over the last few years, dermatologists, LGBTQIA+ allies, and patients have urged the FDA to create a gender-neutral registration process for iPLEDGE. With support from the AADA, the new modifications were approved for implementation and include 2 risk categories: (1) people who can get pregnant and (2) people who cannot get pregnant.3
As exciting as these changes are for the future of dermatologic practice, the actual transition to the new iPLEDGE system was described as a “failure, chaotic, and a disaster” due to additional changes made at the same time.4 The iPLEDGE system was switched to a new website administered by a different vendor and required providers to confirm each patient online by December 13, 2021. In addition, the new system required pharmacists to obtain risk management authorization via the iPLEDGE REMS website or by calling the iPLEDGE REMS center before dispensing isotretinoin. This overhaul did not work as planned, as the new website was constantly down and it was nearly impossible to reach a contact over the telephone. The complications resulted in major disruptions and delayed prescriptions for thousands of patients nationwide as well as a great disruption in workflow for physicians and pharmacists. The AADA subsequently met with the Isotretinoin Products Manufacturers Group to create workable solutions for these issues.
On January 14, 2022, the FDA posted updates regarding access to the iPLEDGE system. They have worked with the Isotretinoin Products Manufacturers Group to create workable solutions for patients and physicians while transferring the patients’ information to the new database. Their solution includes allowing physicians to send patients login links through their email to access their account instead of waiting for the call center. The majority of iPLEDGE users now have access to their accounts without issues, and the gender-neutral guidelines have been in place since the original change.
Impact of iPLEDGE Categories on Transgender Patients
These changes specifically will improve the experience of transgender men and cisgender women who are at no risk for pregnancy and could be subjected to monthly pregnancy testing when it is not medically necessary.
Consider the following patient scenario. A transgender man presents to your dermatology office seeking treatment of severe nodulocystic acne. He was placed on hormonal replacement therapy with exogenous testosterone—injections, oral pills, topical gel, topical patches, or subdermal pellets—to achieve secondary sex characteristics and promote gender congruence. The patient mentions he has been amenorrheic for several months now. He has tried many topical acne treatments as well as oral antibiotics without much benefit and is now interested in enrolling in iPLEDGE to obtain isotretinoin. With the prior iPLEDGE registration packets, how would this transgender man be classified? As a female with childbearing potential due to his retained ovaries and uterus? What if he did not endorse engaging in sexual intercourse that could result in pregnancy?
Transgender patients have unique and unmet needs that often are overlooked and prevent them from equitable, gender-affirming health care. For example, in a prospective study following 20 transgender men starting hormone replacement therapy, the percentage of patients with facial acne increased from 35% to 82% after 6 months of therapy.5 In addition, the increased psychosocial burden of acne may be especially difficult in these patients, as they already report higher rates of depression and suicidal ideation compared with their heterosexual cisgender peers.4 Further, the primary patient populations receiving isotretinoin typically are adolescents and young adults who are undergoing major physical, mental, and hormonal changes. Self-discovery and self-actualization develop over time, and our role as physicians is to advocate for all aspects of our patients’ health and eliminate barriers to optimal care.
Inclusive Language in iPLEDGE Categories
It is important to streamline access to care for all patients, and gender-affirming, culturally sensitive language is essential to building trust and understanding between patients and providers. Howa Yeung, MD, MSc, a dermatologist at Emory University (Atlanta, Georgia) who advocated for gender-neutral iPLEDGE registration, welcomes the change and stated it “will make my job easier. I no longer have to struggle between respecting the patient’s gender identity and providing medically necessary care for patients with severe acne.”3
Sanchez et al6 provided a list of structured questions providers can ask their patients to assess their risk regarding pregnancy: (1) Do you have a uterus and/or ovaries?, (2) Are you engaging in sexual intercourse with a person who has a penis?, and (3) If yes to these questions, what form(s) of birth control are you using? Providers should preface these questions with the following statement: “It is important that I ask these questions to assess your risk for becoming pregnant on this medication because isotretinoin can cause very serious birth defects.” It is important to review these questions and practice asking them so residents can operate from the same place of openness and understanding when caring for their patients.
Final Thoughts
The landscape of isotretinoin prescribing currently is changing on a day-to-day basis. As residents, it is important we stay up to date with the changes regarding our regularly dispensed medications. The main modification made to the iPLEDGE REMS system was switching the risk categories from 3 (females who can get pregnant, females who cannot get pregnant, males) to 2 (people who can get pregnant, people who cannot get pregnant). This change will make registration for iPLEDGE less complex and more inclusive for all patients. It is important for residents to stay at the forefront of these patient health issues and barriers to equal care, and this change represents a step in the right direction.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602. doi:10.1016/j.jaad.2018.02.045
- Flores AR, Herman JL, Gates GJ, et al. How many adults identify as transgender in the United States? UCLA Williams Institute website. Published June 2016. Accessed March 1, 2022. https://williamsinstitute.law.ucla.edu/publications/trans-adults-united-states/
- Doheny K. FDA OKs iPLEDGE change for gender-neutral language. Dermatology News. October 13, 2021. Accessed March 3, 2022. https://www.mdedge.com/dermatology/article/247352/acne/fda-oks-ipledge-change-gender-neutral-language/page/0/1
- Doheny K. iPLEDGE rollout described as a failure, chaotic, and a disaster. Medscape. December 16, 2021. Accessed March 1, 2022. https://www.medscape.com/viewarticle/964925?uac=423615MG
- Wierckx K, Van de Peer F, Verhaeghe E, et al. Short- and long-term clinical skin effects of testosterone treatment in trans men. J Sex Med. 2014;11:222-229.
- Sanchez DP, Brownstone N, Thibodeaux Q, et al. Prescribing isotretinoin for transgender patients: a call to action and recommendations. J Drugs Dermatol. 2021;20:106-108.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602. doi:10.1016/j.jaad.2018.02.045
- Flores AR, Herman JL, Gates GJ, et al. How many adults identify as transgender in the United States? UCLA Williams Institute website. Published June 2016. Accessed March 1, 2022. https://williamsinstitute.law.ucla.edu/publications/trans-adults-united-states/
- Doheny K. FDA OKs iPLEDGE change for gender-neutral language. Dermatology News. October 13, 2021. Accessed March 3, 2022. https://www.mdedge.com/dermatology/article/247352/acne/fda-oks-ipledge-change-gender-neutral-language/page/0/1
- Doheny K. iPLEDGE rollout described as a failure, chaotic, and a disaster. Medscape. December 16, 2021. Accessed March 1, 2022. https://www.medscape.com/viewarticle/964925?uac=423615MG
- Wierckx K, Van de Peer F, Verhaeghe E, et al. Short- and long-term clinical skin effects of testosterone treatment in trans men. J Sex Med. 2014;11:222-229.
- Sanchez DP, Brownstone N, Thibodeaux Q, et al. Prescribing isotretinoin for transgender patients: a call to action and recommendations. J Drugs Dermatol. 2021;20:106-108.
Resident Pearls
- Major changes in the iPLEDGE Risk Evaluation and Mitigation Strategy (REMS) system recently took place, including simplifying registration categories while making the process more inclusive for patients.
- It is important to practice culturally sensitive language when discussing subjects regarding gender identification and sexual practices. Sample questions have been provided to help familiarize practitioners with optimal ways to approach these patient encounters.
- There likely will be more changes with iPLEDGE REMS in the future as the American Academy of Dermatology Association continues to work on solutions regarding decreasing monthly qualifications for patients who cannot get pregnant and possible removal of patient attestation requirements.
Nuances in Training During the Age of Teledermatology
The COVID-19 pandemic largely altered the practice of medicine, including a rapid expansion of telemedicine following the March 2020 World Health Organization guidelines for social distancing, which recommended suspension of all nonurgent in-person visits.1 Expectedly, COVID-related urgent care visits initially comprised the bulk of the new telemedicine wave: NYU Langone Health (New York, New York), for example, saw a 683% increase in virtual visits between March and April 2020, most (55.3%) of which were for respiratory concerns. In-person visits, on the other hand, concurrently fell by more than 80%. Interestingly, nonurgent ambulatory care specialties also saw a considerable uptick in virtual encounters, from less than 50 visits in a typical day to an average of 7000 in a 10-day stretch.2
As a largely ambulatory specialty that relies on visual examination, dermatology was no exception to the swing toward telemedicine, or teledermatology (TD). Before the COVID-19 pandemic, 14.1% (82 of 582 respondents) of practicing US dermatologists reported having used teledermatology, compared to 96.9% (572/591) during the pandemic.3 Even at my home institution (Massachusetts General Hospital [Boston, Massachusetts] and its 12 affiliated dermatology clinics), the number of in-person visits in April 2020 (n=67) was less than 1% of that in April 2019 (n=7919), whereas there was a total of 1564 virtual visits in April 2020 compared to zero the year prior. Virtual provider-to-provider consults (e-consultations) also saw an increase of more than 20%, suggesting that dermatology’s avid adoption of TD also had improved the perceived accessibility of our specialty.4
The adoption and adaptation of TD are projected to continue to grow rapidly across the globe, as digitalization has enhanced access without increasing costs, shortened wait times, and even created opportunities for primary care providers based in rural or overseas locations to learn the diagnosis and treatment of skin disease.5 Residents and fellows should be privy to the nuances of training and practicing in this digital era, as our careers inevitably will involve some facet of TD.
The Art of Medicine
Touch, a sense that perhaps ranks second to sight in dermatology, is absent in TD. In either synchronous (live-interactive, face video visits) or asynchronous (store-and-forward, where digital photographs and clinical information sent by patients or referring physicians are assessed at a later time) TD, the skin cannot be rubbed for texture, pinched for thickness, or pushed for blanching. Instead, all we have is vision. Irwin Braverman, MD, Professor Emeritus of Dermatology at Yale University (New Haven, Connecticut), alongside Jacqueline Dolev, MD, dermatologist and Yale graduate, and Linda Friedlaender, curator at the Yale Center for British Art, founded an observational skills workshop in which trainees learn to observe and describe the paintings housed in the museum, noting all memorable details: the color of the sky, the actions of the animals, and the facial expressions of the people. A study of 90 participants over a 2-year period found that following the workshop, the ability to identify key diagnostic details from clinical photography improved by more than 10%.6 Other studies also utilizing fine art as a medical training tool to improve “visual literacy” saw similarly increased sophistication in the description of clinical imagery, which translated to better diagnostic acumen.7 Confined to video and photographs, TD necessitates trainees and practicing dermatologists to be excellent visual diagnosticians. Although surveyed dermatologists believe TD is presently appropriate for acne, benign lesions, or follow-up appointments,3 conditions for which patients have been examined via TD have included drug eruptions, premalignant or malignant neoplasms, infections, and papulosquamous or inflammatory dermatoses.8 At the very least, clinicians should be versed in identifying those conditions that require in-person evaluation, as patients cannot be held responsible to distinguish which situations can and cannot be addressed virtually.
Issues of Patient-Physician Confidentiality
Teledermatology is not without its shortcomings; critics have noted diagnostic challenges with poor quality photographs or videos, inability to perform total-body skin examinations, and socioeconomic limitations due to broadband availability and speed.5,9 Although most of these shortcomings are outside of our control, a key challenge within the purview of the provider is the protection of patient privacy.
Much of the salient concerns regarding patient-physician confidentiality involve asynchronous TD, where store-and-forward data sharing allows physicians to download patient photographs or information onto their personal email or smartphones.10 Although some hospital systems provide encryption software or hospital-sponsored devices to ensure security, physicians may opt to use their personal phones or laptops out of convenience or to save time.10,11 One study found that less than 30% of smartphone users choose to activate user authentication on their devices, even ones as simple as a passphrase.11 The digital exchange of information thus poses an immense risk for compromising protected health information (PHI), as personal devices can be easily lost, stolen, or hacked. Indeed, in 2015, more than 113 million individuals were affected by a breach of PHI, the majority over hacked network servers.12 With the growing diversity of mediums through which PHI is exchanged, such as videoconferencing and instant messaging, the potential medicolegal risks of information breach continue to climb. The US Department of Health & Human Services urges health care providers to uphold best practices for security, including encrypting data, updating all software including antivirus software, using multifactor authentication, and following local cybersecurity regulations or recommendations.13 For synchronous TD, suggested best practices include utilizing headphones during live appointments, avoiding public wireless networks, and ensuring the provider and patient both scan the room with their device’s camera before the start of the visit.14
On the Horizon of Teledermatology
What can we expect in the coming years? Increased utilization of telemedicine will translate into data that will help address questions surrounding safety, diagnostic accuracy, privacy, and accessibility. One aspect of TD in need of clarity is a guideline on payment and reimbursement, and whether TD can continue to be financially attractive to providers. Starting in 2020, the Centers for Medicare & Medicaid Services removed geographic restrictions for reimbursement of telemedicine visits, enabling even urban-residing patients to enjoy the convenience of TD. This followed a prior relaxation of restrictions, where even prerecorded patient information became eligible for Medicare reimbursement.9 However, as virtual visits tend to be shorter with fewer diagnostic services compared to in-person visits, the reimbursement structure of TD must be nuanced, which is the subject of ongoing study and modification in the wake of the COVID-19 pandemic.15
Another point to consider is the explosion of direct-to-consumer TD, which allows patients to receive virtual dermatologic care or prescription medication without a pre-established relationship with any physician. In 2017, there were 22 direct-to-consumer TD services available to US patients in 45 states, 16 (73%) of which provided dermatologic care for any concern while 6 (27%) were limited to acne or antiaging and were largely prescription oriented. Orchestrated mostly by the for-profit private sector, direct-to-consumer companies are poorly regulated and have raised concerns over questionable practices, such as the use of non–US board-certified physicians, exorbitant fees, and failure to disclose medication side effects.16 A study of 16 direct-to-consumer telemedicine sites found substantial discordance in the suggested management of the same patient, and many of the services relied heavily on patient-provided self-diagnoses, such as a case where psoriasis medication was dispensed for a psoriasis patient who submitted a photograph of his syphilitic rash.17 Despite these problems, consumers show a willingness to pay out of pocket to access these services for their shorter waiting times and convenience.18 Hence, we must learn to ask about direct-to-consumer service use when obtaining a thorough history and be open to counseling our patients on the proper use and potential risks of direct-to-consumer TD.
Final Thoughts
The telemedicine industry is expected to reach more than $130 billion by 2025, with more than 90% of surveyed health care executives planning for the adoption and incorporation of telemedicine into their business models.19 The COVID-19 pandemic was an impetus for an exponential adoption of TD, and it would behoove current residents to realize that the practice of dermatology will continue to be increasingly digitalized within the coming years. Whether through formal training or self-assessment, we must strive to grow as proficient virtual dermatologists while upholding professionalism, patient safety, and health information privacy.
- Yeboah CB, Harvey N, Krishnan R, et al. The impact of COVID-19 on teledermatology: a review. Dermatol Clin. 2021;39:599-608.
- Mann DM, Chen J, Chunara R, et al. COVID-19 transforms health care through telemedicine: evidence from the field. J Am Med Inform Assoc. 2020;27:1132-1135.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Su MY, Das S. Expansion of asynchronous teledermatology during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:E471-E472.
- Maddukuri S, Patel J, Lipoff JB. Teledermatology addressing disparities in health care access: a review [published online March 12, 2021]. Curr Dermatol Rep. doi:10.1007/s13671-021-00329-2
- Dolev JC, Friedlaender LK, Braverman IM. Use of fine art to enhance visual diagnostic skills. JAMA. 2001;286:1020-1021.
- Naghshineh S, Hafler JP, Miller AR, et al. Formal art observation training improves medical students’ visual diagnostic skills. J Gen Intern Med. 2008;23:991-997.
- Lee KJ, Finnane A, Soyer HP. Recent trends in teledermatology and teledermoscopy. Dermatol Pract Concept. 2018;8:214-223.
- Wang RH, Barbieri JS, Nguyen HP, et al. Clinical effectiveness and cost-effectiveness of teledermatology: where are we now, and what are the barriers to adoption? J Am Acad Dermatol. 2020;83:299-307.
- Stevenson P, Finnane AR, Soyer HP. Teledermatology and clinical photography: safeguarding patient privacy and mitigating medico-legal risk. Med J Aust. 2016;204:198-200e1.
- Smith KA, Zhou L, Watzlaf VJM. User authentication in smartphones for telehealth. Int J Telerehabil. 2017;9:3-12.
- Breaches of unsecured protected health information. Health IT website. Updated July 22, 2021. Accessed January 16, 2022. https://www.healthit.gov/data/quickstats/breaches-unsecured-protected-health-information
- Jalali MS, Landman A, Gordon WJ. Telemedicine, privacy, and information security in the age of COVID-19. J Am Med Inform Assoc. 2021;28:671-672.
- Telehealth for behavioral health care: protecting patients’ privacy. United States Department of Health and Human Services website. Updated July 2, 2021. Accessed January 16, 2022. https://telehealth.hhs.gov/providers/telehealth-for-behavioral-health/preparing-patients-for-telebehavioral-health/protecting-patients-privacy/
- Shachar C, Engel J, Elwyn G. Implications for telehealth in a postpandemic future: regulatory and privacy issues. JAMA. 2020;323:2375-2376.
- Fogel AL, Sarin KY. A survey of direct-to-consumer teledermatology services available to US patients: explosive growth, opportunities and controversy. J Telemed Telecare. 2017;23:19-25.
- Resneck JS Jr, Abrouk M, Steuer M, et al. Choice, transparency, coordination, and quality among direct-to-consumer telemedicine websites and apps treating skin disease. JAMA Dermatol. 2016;152:768-775.
- Snoswell CL, Whitty JA, Caffery LJ, et al. Consumer preference and willingness to pay for direct-to-consumer mobile teledermoscopy services in Australia [published online August 13, 2021]. Dermatology. doi:10.1159/000517257
- Elliott T, Yopes MC. Direct-to-consumer telemedicine. J Allergy Clin Immunol Pract. 2019;7:2546-2552.
The COVID-19 pandemic largely altered the practice of medicine, including a rapid expansion of telemedicine following the March 2020 World Health Organization guidelines for social distancing, which recommended suspension of all nonurgent in-person visits.1 Expectedly, COVID-related urgent care visits initially comprised the bulk of the new telemedicine wave: NYU Langone Health (New York, New York), for example, saw a 683% increase in virtual visits between March and April 2020, most (55.3%) of which were for respiratory concerns. In-person visits, on the other hand, concurrently fell by more than 80%. Interestingly, nonurgent ambulatory care specialties also saw a considerable uptick in virtual encounters, from less than 50 visits in a typical day to an average of 7000 in a 10-day stretch.2
As a largely ambulatory specialty that relies on visual examination, dermatology was no exception to the swing toward telemedicine, or teledermatology (TD). Before the COVID-19 pandemic, 14.1% (82 of 582 respondents) of practicing US dermatologists reported having used teledermatology, compared to 96.9% (572/591) during the pandemic.3 Even at my home institution (Massachusetts General Hospital [Boston, Massachusetts] and its 12 affiliated dermatology clinics), the number of in-person visits in April 2020 (n=67) was less than 1% of that in April 2019 (n=7919), whereas there was a total of 1564 virtual visits in April 2020 compared to zero the year prior. Virtual provider-to-provider consults (e-consultations) also saw an increase of more than 20%, suggesting that dermatology’s avid adoption of TD also had improved the perceived accessibility of our specialty.4
The adoption and adaptation of TD are projected to continue to grow rapidly across the globe, as digitalization has enhanced access without increasing costs, shortened wait times, and even created opportunities for primary care providers based in rural or overseas locations to learn the diagnosis and treatment of skin disease.5 Residents and fellows should be privy to the nuances of training and practicing in this digital era, as our careers inevitably will involve some facet of TD.
The Art of Medicine
Touch, a sense that perhaps ranks second to sight in dermatology, is absent in TD. In either synchronous (live-interactive, face video visits) or asynchronous (store-and-forward, where digital photographs and clinical information sent by patients or referring physicians are assessed at a later time) TD, the skin cannot be rubbed for texture, pinched for thickness, or pushed for blanching. Instead, all we have is vision. Irwin Braverman, MD, Professor Emeritus of Dermatology at Yale University (New Haven, Connecticut), alongside Jacqueline Dolev, MD, dermatologist and Yale graduate, and Linda Friedlaender, curator at the Yale Center for British Art, founded an observational skills workshop in which trainees learn to observe and describe the paintings housed in the museum, noting all memorable details: the color of the sky, the actions of the animals, and the facial expressions of the people. A study of 90 participants over a 2-year period found that following the workshop, the ability to identify key diagnostic details from clinical photography improved by more than 10%.6 Other studies also utilizing fine art as a medical training tool to improve “visual literacy” saw similarly increased sophistication in the description of clinical imagery, which translated to better diagnostic acumen.7 Confined to video and photographs, TD necessitates trainees and practicing dermatologists to be excellent visual diagnosticians. Although surveyed dermatologists believe TD is presently appropriate for acne, benign lesions, or follow-up appointments,3 conditions for which patients have been examined via TD have included drug eruptions, premalignant or malignant neoplasms, infections, and papulosquamous or inflammatory dermatoses.8 At the very least, clinicians should be versed in identifying those conditions that require in-person evaluation, as patients cannot be held responsible to distinguish which situations can and cannot be addressed virtually.
Issues of Patient-Physician Confidentiality
Teledermatology is not without its shortcomings; critics have noted diagnostic challenges with poor quality photographs or videos, inability to perform total-body skin examinations, and socioeconomic limitations due to broadband availability and speed.5,9 Although most of these shortcomings are outside of our control, a key challenge within the purview of the provider is the protection of patient privacy.
Much of the salient concerns regarding patient-physician confidentiality involve asynchronous TD, where store-and-forward data sharing allows physicians to download patient photographs or information onto their personal email or smartphones.10 Although some hospital systems provide encryption software or hospital-sponsored devices to ensure security, physicians may opt to use their personal phones or laptops out of convenience or to save time.10,11 One study found that less than 30% of smartphone users choose to activate user authentication on their devices, even ones as simple as a passphrase.11 The digital exchange of information thus poses an immense risk for compromising protected health information (PHI), as personal devices can be easily lost, stolen, or hacked. Indeed, in 2015, more than 113 million individuals were affected by a breach of PHI, the majority over hacked network servers.12 With the growing diversity of mediums through which PHI is exchanged, such as videoconferencing and instant messaging, the potential medicolegal risks of information breach continue to climb. The US Department of Health & Human Services urges health care providers to uphold best practices for security, including encrypting data, updating all software including antivirus software, using multifactor authentication, and following local cybersecurity regulations or recommendations.13 For synchronous TD, suggested best practices include utilizing headphones during live appointments, avoiding public wireless networks, and ensuring the provider and patient both scan the room with their device’s camera before the start of the visit.14
On the Horizon of Teledermatology
What can we expect in the coming years? Increased utilization of telemedicine will translate into data that will help address questions surrounding safety, diagnostic accuracy, privacy, and accessibility. One aspect of TD in need of clarity is a guideline on payment and reimbursement, and whether TD can continue to be financially attractive to providers. Starting in 2020, the Centers for Medicare & Medicaid Services removed geographic restrictions for reimbursement of telemedicine visits, enabling even urban-residing patients to enjoy the convenience of TD. This followed a prior relaxation of restrictions, where even prerecorded patient information became eligible for Medicare reimbursement.9 However, as virtual visits tend to be shorter with fewer diagnostic services compared to in-person visits, the reimbursement structure of TD must be nuanced, which is the subject of ongoing study and modification in the wake of the COVID-19 pandemic.15
Another point to consider is the explosion of direct-to-consumer TD, which allows patients to receive virtual dermatologic care or prescription medication without a pre-established relationship with any physician. In 2017, there were 22 direct-to-consumer TD services available to US patients in 45 states, 16 (73%) of which provided dermatologic care for any concern while 6 (27%) were limited to acne or antiaging and were largely prescription oriented. Orchestrated mostly by the for-profit private sector, direct-to-consumer companies are poorly regulated and have raised concerns over questionable practices, such as the use of non–US board-certified physicians, exorbitant fees, and failure to disclose medication side effects.16 A study of 16 direct-to-consumer telemedicine sites found substantial discordance in the suggested management of the same patient, and many of the services relied heavily on patient-provided self-diagnoses, such as a case where psoriasis medication was dispensed for a psoriasis patient who submitted a photograph of his syphilitic rash.17 Despite these problems, consumers show a willingness to pay out of pocket to access these services for their shorter waiting times and convenience.18 Hence, we must learn to ask about direct-to-consumer service use when obtaining a thorough history and be open to counseling our patients on the proper use and potential risks of direct-to-consumer TD.
Final Thoughts
The telemedicine industry is expected to reach more than $130 billion by 2025, with more than 90% of surveyed health care executives planning for the adoption and incorporation of telemedicine into their business models.19 The COVID-19 pandemic was an impetus for an exponential adoption of TD, and it would behoove current residents to realize that the practice of dermatology will continue to be increasingly digitalized within the coming years. Whether through formal training or self-assessment, we must strive to grow as proficient virtual dermatologists while upholding professionalism, patient safety, and health information privacy.
The COVID-19 pandemic largely altered the practice of medicine, including a rapid expansion of telemedicine following the March 2020 World Health Organization guidelines for social distancing, which recommended suspension of all nonurgent in-person visits.1 Expectedly, COVID-related urgent care visits initially comprised the bulk of the new telemedicine wave: NYU Langone Health (New York, New York), for example, saw a 683% increase in virtual visits between March and April 2020, most (55.3%) of which were for respiratory concerns. In-person visits, on the other hand, concurrently fell by more than 80%. Interestingly, nonurgent ambulatory care specialties also saw a considerable uptick in virtual encounters, from less than 50 visits in a typical day to an average of 7000 in a 10-day stretch.2
As a largely ambulatory specialty that relies on visual examination, dermatology was no exception to the swing toward telemedicine, or teledermatology (TD). Before the COVID-19 pandemic, 14.1% (82 of 582 respondents) of practicing US dermatologists reported having used teledermatology, compared to 96.9% (572/591) during the pandemic.3 Even at my home institution (Massachusetts General Hospital [Boston, Massachusetts] and its 12 affiliated dermatology clinics), the number of in-person visits in April 2020 (n=67) was less than 1% of that in April 2019 (n=7919), whereas there was a total of 1564 virtual visits in April 2020 compared to zero the year prior. Virtual provider-to-provider consults (e-consultations) also saw an increase of more than 20%, suggesting that dermatology’s avid adoption of TD also had improved the perceived accessibility of our specialty.4
The adoption and adaptation of TD are projected to continue to grow rapidly across the globe, as digitalization has enhanced access without increasing costs, shortened wait times, and even created opportunities for primary care providers based in rural or overseas locations to learn the diagnosis and treatment of skin disease.5 Residents and fellows should be privy to the nuances of training and practicing in this digital era, as our careers inevitably will involve some facet of TD.
The Art of Medicine
Touch, a sense that perhaps ranks second to sight in dermatology, is absent in TD. In either synchronous (live-interactive, face video visits) or asynchronous (store-and-forward, where digital photographs and clinical information sent by patients or referring physicians are assessed at a later time) TD, the skin cannot be rubbed for texture, pinched for thickness, or pushed for blanching. Instead, all we have is vision. Irwin Braverman, MD, Professor Emeritus of Dermatology at Yale University (New Haven, Connecticut), alongside Jacqueline Dolev, MD, dermatologist and Yale graduate, and Linda Friedlaender, curator at the Yale Center for British Art, founded an observational skills workshop in which trainees learn to observe and describe the paintings housed in the museum, noting all memorable details: the color of the sky, the actions of the animals, and the facial expressions of the people. A study of 90 participants over a 2-year period found that following the workshop, the ability to identify key diagnostic details from clinical photography improved by more than 10%.6 Other studies also utilizing fine art as a medical training tool to improve “visual literacy” saw similarly increased sophistication in the description of clinical imagery, which translated to better diagnostic acumen.7 Confined to video and photographs, TD necessitates trainees and practicing dermatologists to be excellent visual diagnosticians. Although surveyed dermatologists believe TD is presently appropriate for acne, benign lesions, or follow-up appointments,3 conditions for which patients have been examined via TD have included drug eruptions, premalignant or malignant neoplasms, infections, and papulosquamous or inflammatory dermatoses.8 At the very least, clinicians should be versed in identifying those conditions that require in-person evaluation, as patients cannot be held responsible to distinguish which situations can and cannot be addressed virtually.
Issues of Patient-Physician Confidentiality
Teledermatology is not without its shortcomings; critics have noted diagnostic challenges with poor quality photographs or videos, inability to perform total-body skin examinations, and socioeconomic limitations due to broadband availability and speed.5,9 Although most of these shortcomings are outside of our control, a key challenge within the purview of the provider is the protection of patient privacy.
Much of the salient concerns regarding patient-physician confidentiality involve asynchronous TD, where store-and-forward data sharing allows physicians to download patient photographs or information onto their personal email or smartphones.10 Although some hospital systems provide encryption software or hospital-sponsored devices to ensure security, physicians may opt to use their personal phones or laptops out of convenience or to save time.10,11 One study found that less than 30% of smartphone users choose to activate user authentication on their devices, even ones as simple as a passphrase.11 The digital exchange of information thus poses an immense risk for compromising protected health information (PHI), as personal devices can be easily lost, stolen, or hacked. Indeed, in 2015, more than 113 million individuals were affected by a breach of PHI, the majority over hacked network servers.12 With the growing diversity of mediums through which PHI is exchanged, such as videoconferencing and instant messaging, the potential medicolegal risks of information breach continue to climb. The US Department of Health & Human Services urges health care providers to uphold best practices for security, including encrypting data, updating all software including antivirus software, using multifactor authentication, and following local cybersecurity regulations or recommendations.13 For synchronous TD, suggested best practices include utilizing headphones during live appointments, avoiding public wireless networks, and ensuring the provider and patient both scan the room with their device’s camera before the start of the visit.14
On the Horizon of Teledermatology
What can we expect in the coming years? Increased utilization of telemedicine will translate into data that will help address questions surrounding safety, diagnostic accuracy, privacy, and accessibility. One aspect of TD in need of clarity is a guideline on payment and reimbursement, and whether TD can continue to be financially attractive to providers. Starting in 2020, the Centers for Medicare & Medicaid Services removed geographic restrictions for reimbursement of telemedicine visits, enabling even urban-residing patients to enjoy the convenience of TD. This followed a prior relaxation of restrictions, where even prerecorded patient information became eligible for Medicare reimbursement.9 However, as virtual visits tend to be shorter with fewer diagnostic services compared to in-person visits, the reimbursement structure of TD must be nuanced, which is the subject of ongoing study and modification in the wake of the COVID-19 pandemic.15
Another point to consider is the explosion of direct-to-consumer TD, which allows patients to receive virtual dermatologic care or prescription medication without a pre-established relationship with any physician. In 2017, there were 22 direct-to-consumer TD services available to US patients in 45 states, 16 (73%) of which provided dermatologic care for any concern while 6 (27%) were limited to acne or antiaging and were largely prescription oriented. Orchestrated mostly by the for-profit private sector, direct-to-consumer companies are poorly regulated and have raised concerns over questionable practices, such as the use of non–US board-certified physicians, exorbitant fees, and failure to disclose medication side effects.16 A study of 16 direct-to-consumer telemedicine sites found substantial discordance in the suggested management of the same patient, and many of the services relied heavily on patient-provided self-diagnoses, such as a case where psoriasis medication was dispensed for a psoriasis patient who submitted a photograph of his syphilitic rash.17 Despite these problems, consumers show a willingness to pay out of pocket to access these services for their shorter waiting times and convenience.18 Hence, we must learn to ask about direct-to-consumer service use when obtaining a thorough history and be open to counseling our patients on the proper use and potential risks of direct-to-consumer TD.
Final Thoughts
The telemedicine industry is expected to reach more than $130 billion by 2025, with more than 90% of surveyed health care executives planning for the adoption and incorporation of telemedicine into their business models.19 The COVID-19 pandemic was an impetus for an exponential adoption of TD, and it would behoove current residents to realize that the practice of dermatology will continue to be increasingly digitalized within the coming years. Whether through formal training or self-assessment, we must strive to grow as proficient virtual dermatologists while upholding professionalism, patient safety, and health information privacy.
- Yeboah CB, Harvey N, Krishnan R, et al. The impact of COVID-19 on teledermatology: a review. Dermatol Clin. 2021;39:599-608.
- Mann DM, Chen J, Chunara R, et al. COVID-19 transforms health care through telemedicine: evidence from the field. J Am Med Inform Assoc. 2020;27:1132-1135.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Su MY, Das S. Expansion of asynchronous teledermatology during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:E471-E472.
- Maddukuri S, Patel J, Lipoff JB. Teledermatology addressing disparities in health care access: a review [published online March 12, 2021]. Curr Dermatol Rep. doi:10.1007/s13671-021-00329-2
- Dolev JC, Friedlaender LK, Braverman IM. Use of fine art to enhance visual diagnostic skills. JAMA. 2001;286:1020-1021.
- Naghshineh S, Hafler JP, Miller AR, et al. Formal art observation training improves medical students’ visual diagnostic skills. J Gen Intern Med. 2008;23:991-997.
- Lee KJ, Finnane A, Soyer HP. Recent trends in teledermatology and teledermoscopy. Dermatol Pract Concept. 2018;8:214-223.
- Wang RH, Barbieri JS, Nguyen HP, et al. Clinical effectiveness and cost-effectiveness of teledermatology: where are we now, and what are the barriers to adoption? J Am Acad Dermatol. 2020;83:299-307.
- Stevenson P, Finnane AR, Soyer HP. Teledermatology and clinical photography: safeguarding patient privacy and mitigating medico-legal risk. Med J Aust. 2016;204:198-200e1.
- Smith KA, Zhou L, Watzlaf VJM. User authentication in smartphones for telehealth. Int J Telerehabil. 2017;9:3-12.
- Breaches of unsecured protected health information. Health IT website. Updated July 22, 2021. Accessed January 16, 2022. https://www.healthit.gov/data/quickstats/breaches-unsecured-protected-health-information
- Jalali MS, Landman A, Gordon WJ. Telemedicine, privacy, and information security in the age of COVID-19. J Am Med Inform Assoc. 2021;28:671-672.
- Telehealth for behavioral health care: protecting patients’ privacy. United States Department of Health and Human Services website. Updated July 2, 2021. Accessed January 16, 2022. https://telehealth.hhs.gov/providers/telehealth-for-behavioral-health/preparing-patients-for-telebehavioral-health/protecting-patients-privacy/
- Shachar C, Engel J, Elwyn G. Implications for telehealth in a postpandemic future: regulatory and privacy issues. JAMA. 2020;323:2375-2376.
- Fogel AL, Sarin KY. A survey of direct-to-consumer teledermatology services available to US patients: explosive growth, opportunities and controversy. J Telemed Telecare. 2017;23:19-25.
- Resneck JS Jr, Abrouk M, Steuer M, et al. Choice, transparency, coordination, and quality among direct-to-consumer telemedicine websites and apps treating skin disease. JAMA Dermatol. 2016;152:768-775.
- Snoswell CL, Whitty JA, Caffery LJ, et al. Consumer preference and willingness to pay for direct-to-consumer mobile teledermoscopy services in Australia [published online August 13, 2021]. Dermatology. doi:10.1159/000517257
- Elliott T, Yopes MC. Direct-to-consumer telemedicine. J Allergy Clin Immunol Pract. 2019;7:2546-2552.
- Yeboah CB, Harvey N, Krishnan R, et al. The impact of COVID-19 on teledermatology: a review. Dermatol Clin. 2021;39:599-608.
- Mann DM, Chen J, Chunara R, et al. COVID-19 transforms health care through telemedicine: evidence from the field. J Am Med Inform Assoc. 2020;27:1132-1135.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Su MY, Das S. Expansion of asynchronous teledermatology during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:E471-E472.
- Maddukuri S, Patel J, Lipoff JB. Teledermatology addressing disparities in health care access: a review [published online March 12, 2021]. Curr Dermatol Rep. doi:10.1007/s13671-021-00329-2
- Dolev JC, Friedlaender LK, Braverman IM. Use of fine art to enhance visual diagnostic skills. JAMA. 2001;286:1020-1021.
- Naghshineh S, Hafler JP, Miller AR, et al. Formal art observation training improves medical students’ visual diagnostic skills. J Gen Intern Med. 2008;23:991-997.
- Lee KJ, Finnane A, Soyer HP. Recent trends in teledermatology and teledermoscopy. Dermatol Pract Concept. 2018;8:214-223.
- Wang RH, Barbieri JS, Nguyen HP, et al. Clinical effectiveness and cost-effectiveness of teledermatology: where are we now, and what are the barriers to adoption? J Am Acad Dermatol. 2020;83:299-307.
- Stevenson P, Finnane AR, Soyer HP. Teledermatology and clinical photography: safeguarding patient privacy and mitigating medico-legal risk. Med J Aust. 2016;204:198-200e1.
- Smith KA, Zhou L, Watzlaf VJM. User authentication in smartphones for telehealth. Int J Telerehabil. 2017;9:3-12.
- Breaches of unsecured protected health information. Health IT website. Updated July 22, 2021. Accessed January 16, 2022. https://www.healthit.gov/data/quickstats/breaches-unsecured-protected-health-information
- Jalali MS, Landman A, Gordon WJ. Telemedicine, privacy, and information security in the age of COVID-19. J Am Med Inform Assoc. 2021;28:671-672.
- Telehealth for behavioral health care: protecting patients’ privacy. United States Department of Health and Human Services website. Updated July 2, 2021. Accessed January 16, 2022. https://telehealth.hhs.gov/providers/telehealth-for-behavioral-health/preparing-patients-for-telebehavioral-health/protecting-patients-privacy/
- Shachar C, Engel J, Elwyn G. Implications for telehealth in a postpandemic future: regulatory and privacy issues. JAMA. 2020;323:2375-2376.
- Fogel AL, Sarin KY. A survey of direct-to-consumer teledermatology services available to US patients: explosive growth, opportunities and controversy. J Telemed Telecare. 2017;23:19-25.
- Resneck JS Jr, Abrouk M, Steuer M, et al. Choice, transparency, coordination, and quality among direct-to-consumer telemedicine websites and apps treating skin disease. JAMA Dermatol. 2016;152:768-775.
- Snoswell CL, Whitty JA, Caffery LJ, et al. Consumer preference and willingness to pay for direct-to-consumer mobile teledermoscopy services in Australia [published online August 13, 2021]. Dermatology. doi:10.1159/000517257
- Elliott T, Yopes MC. Direct-to-consumer telemedicine. J Allergy Clin Immunol Pract. 2019;7:2546-2552.
Resident Pearl
- The COVID-19 pandemic has accelerated the adoption of teledermatology, enhancing patient access to dermatologic care while also facilitating multidisciplinary discourse and providing opportunities for education and training. However, these virtual interactions require a vigilance for patient privacy and security with an added emphasis on visual diagnostics to deliver high-quality care.
Teaching Evidence-Based Dermatology Using a Web-Based Journal Club: A Pilot Study and Survey
To the Editor:
With a steady increase in dermatology publications over recent decades, there is an expanding pool of evidence to address clinical questions.1 Residency training is the time when appraising the medical literature and practicing evidence-based medicine is most honed. Evidence-based medicine is an essential component of Practice-based Learning and Improvement, a required core competency of the Accreditation Council for Graduate Medical Education.2 Assimilation of new research evidence is traditionally taught through didactics and journal club discussions in residency.
However, at a time when the demand for information overwhelms safeguards that exist to evaluate its quality, it is more important than ever to be equipped with the proper tools to critically appraise novel literature. Beyond accepting a scientific article at face value, physicians must learn to ask targeted questions of the study design, results, and clinical relevance. These questions change based on the type of study, and organizations such as the Oxford Centre for Evidence-Based Medicine provide guidance through critical appraisal worksheets.3
To investigate the utility of using guided questions to evaluate the reliability, significance, and applicability of clinical evidence, we beta tested a novel web-based application in an academic dermatology setting to design and run a journal club for residents. Six dermatology residents participated in this institutional review board–approved study comprised of 3 phases: (1) independent article appraisal through the web-based application, (2) group discussion, and (3) anonymous postsurvey.
Using this platform, we uploaded a recent article into the interactive reader, which contained an integrated tool for appraisal based on specific questions. Because the article described the results of a randomized clinical trial, we used questions from the Centre for Evidence-Based Medicine’s Randomised Controlled Trials Critical Appraisal Worksheet, which has a series of questions to evaluate internal validity, results, and external validity and applicability.3
Residents used the platform to independently read the article, highlight areas of the text that corresponded to 8 critical appraisal questions, and answer yes or no to these questions. Based on residents’ answers, a final appraisal score (on a scale of 1% to 100%) was generated. Simultaneously, the attending dermatologist leading the journal club (C.W.) also completed the assignment to establish an expert score.
Scores from the residents’ independent appraisal ranged from 75% to 100% (mean, 85.4%). Upon discussing the article in a group setting, the residents established a consensus score of 75%. This consensus score matched the expert score, which suggested to us that both independently reviewing the article using guided questions and conducting a group debriefing were necessary to match the expert level of critical appraisal.
Of note, the residents’ average independent appraisal score was higher than both the consensus and expert scores, indicating that the residents evaluated the article less critically on their own. With more practice using this method, it is possible that the precision and accuracy of the residents’ critical appraisal of scientific articles will improve.
In the postsurvey, we asked residents about the critical appraisal of the medical literature. All residents agreed that evaluating the quality of evidence when reading a scientific article was somewhat important or very important to them; however, only 2 of 6 evaluated the quality of evidence all the time, and the other 4 did so half of the time or less than half of the time.
When critically appraising articles, 2 of 6 residents used specific rubrics half of the time; 4 of 6 less than half of the time. Most important, 5 of 6 residents agreed that the quality of evidence affected their management decisions more than half of the time or all of the time. Although it is clear that residents value evidence-based medicine and understand the importance of evaluating the quality of evidence, doing so currently might not be simple or practical.
An organized framework for appraising articles would streamline the process. Five of 6 residents agreed that the use of specific questions as a guide made it easier to appraise an article for the quality of its evidence. Four of 6 residents found that juxtaposing specific questions with the interactive reader was helpful; 5 of 6 agreed that they would use a web-based journal club platform if given the option.
Lastly, 5 of 6 residents agreed that if such a tool were available, a platform containing all major dermatology publications in an interactive reader format, along with relevant appraisal questions on the side, would be useful.
This pilot study augmented the typical journal club experience by emphasizing goal-directed reading and the importance of analyzing the quality of evidence. The combination of independent appraisal of an article using targeted questions and a group debrief led to better understanding of the evidence and its clinical applicability. The COVID-19 pandemic may be a better time than ever to explore innovative ways to teach evidence-based medicine in residency training.
- Mimouni D, Pavlovsky L, Akerman L, et al. Trends in dermatology publications over the past 15 years. Am J Clin Dermatol. 2010;11:55-58. doi:10.2165/11530190-000000000-00000.
- NEJM Knowledge+ Team. Exploring the ACGME Core Competencies: Practice-Based Learning and Improvement (part 2 of 7). Massachusetts Medical Society. NEJM Knowledge+ website. Published July 28, 2016. Accessed January 15, 2022. https://knowledgeplus.nejm.org/blog/practice-based-learning-and-improvement/
- University of Oxford. Critical appraisal tools. Centre for Evidence-Based Medicine website. Accessed January 2, 2022. www.cebm.ox.ac.uk/resources/ebm-tools/critical-appraisal-tools
To the Editor:
With a steady increase in dermatology publications over recent decades, there is an expanding pool of evidence to address clinical questions.1 Residency training is the time when appraising the medical literature and practicing evidence-based medicine is most honed. Evidence-based medicine is an essential component of Practice-based Learning and Improvement, a required core competency of the Accreditation Council for Graduate Medical Education.2 Assimilation of new research evidence is traditionally taught through didactics and journal club discussions in residency.
However, at a time when the demand for information overwhelms safeguards that exist to evaluate its quality, it is more important than ever to be equipped with the proper tools to critically appraise novel literature. Beyond accepting a scientific article at face value, physicians must learn to ask targeted questions of the study design, results, and clinical relevance. These questions change based on the type of study, and organizations such as the Oxford Centre for Evidence-Based Medicine provide guidance through critical appraisal worksheets.3
To investigate the utility of using guided questions to evaluate the reliability, significance, and applicability of clinical evidence, we beta tested a novel web-based application in an academic dermatology setting to design and run a journal club for residents. Six dermatology residents participated in this institutional review board–approved study comprised of 3 phases: (1) independent article appraisal through the web-based application, (2) group discussion, and (3) anonymous postsurvey.
Using this platform, we uploaded a recent article into the interactive reader, which contained an integrated tool for appraisal based on specific questions. Because the article described the results of a randomized clinical trial, we used questions from the Centre for Evidence-Based Medicine’s Randomised Controlled Trials Critical Appraisal Worksheet, which has a series of questions to evaluate internal validity, results, and external validity and applicability.3
Residents used the platform to independently read the article, highlight areas of the text that corresponded to 8 critical appraisal questions, and answer yes or no to these questions. Based on residents’ answers, a final appraisal score (on a scale of 1% to 100%) was generated. Simultaneously, the attending dermatologist leading the journal club (C.W.) also completed the assignment to establish an expert score.
Scores from the residents’ independent appraisal ranged from 75% to 100% (mean, 85.4%). Upon discussing the article in a group setting, the residents established a consensus score of 75%. This consensus score matched the expert score, which suggested to us that both independently reviewing the article using guided questions and conducting a group debriefing were necessary to match the expert level of critical appraisal.
Of note, the residents’ average independent appraisal score was higher than both the consensus and expert scores, indicating that the residents evaluated the article less critically on their own. With more practice using this method, it is possible that the precision and accuracy of the residents’ critical appraisal of scientific articles will improve.
In the postsurvey, we asked residents about the critical appraisal of the medical literature. All residents agreed that evaluating the quality of evidence when reading a scientific article was somewhat important or very important to them; however, only 2 of 6 evaluated the quality of evidence all the time, and the other 4 did so half of the time or less than half of the time.
When critically appraising articles, 2 of 6 residents used specific rubrics half of the time; 4 of 6 less than half of the time. Most important, 5 of 6 residents agreed that the quality of evidence affected their management decisions more than half of the time or all of the time. Although it is clear that residents value evidence-based medicine and understand the importance of evaluating the quality of evidence, doing so currently might not be simple or practical.
An organized framework for appraising articles would streamline the process. Five of 6 residents agreed that the use of specific questions as a guide made it easier to appraise an article for the quality of its evidence. Four of 6 residents found that juxtaposing specific questions with the interactive reader was helpful; 5 of 6 agreed that they would use a web-based journal club platform if given the option.
Lastly, 5 of 6 residents agreed that if such a tool were available, a platform containing all major dermatology publications in an interactive reader format, along with relevant appraisal questions on the side, would be useful.
This pilot study augmented the typical journal club experience by emphasizing goal-directed reading and the importance of analyzing the quality of evidence. The combination of independent appraisal of an article using targeted questions and a group debrief led to better understanding of the evidence and its clinical applicability. The COVID-19 pandemic may be a better time than ever to explore innovative ways to teach evidence-based medicine in residency training.
To the Editor:
With a steady increase in dermatology publications over recent decades, there is an expanding pool of evidence to address clinical questions.1 Residency training is the time when appraising the medical literature and practicing evidence-based medicine is most honed. Evidence-based medicine is an essential component of Practice-based Learning and Improvement, a required core competency of the Accreditation Council for Graduate Medical Education.2 Assimilation of new research evidence is traditionally taught through didactics and journal club discussions in residency.
However, at a time when the demand for information overwhelms safeguards that exist to evaluate its quality, it is more important than ever to be equipped with the proper tools to critically appraise novel literature. Beyond accepting a scientific article at face value, physicians must learn to ask targeted questions of the study design, results, and clinical relevance. These questions change based on the type of study, and organizations such as the Oxford Centre for Evidence-Based Medicine provide guidance through critical appraisal worksheets.3
To investigate the utility of using guided questions to evaluate the reliability, significance, and applicability of clinical evidence, we beta tested a novel web-based application in an academic dermatology setting to design and run a journal club for residents. Six dermatology residents participated in this institutional review board–approved study comprised of 3 phases: (1) independent article appraisal through the web-based application, (2) group discussion, and (3) anonymous postsurvey.
Using this platform, we uploaded a recent article into the interactive reader, which contained an integrated tool for appraisal based on specific questions. Because the article described the results of a randomized clinical trial, we used questions from the Centre for Evidence-Based Medicine’s Randomised Controlled Trials Critical Appraisal Worksheet, which has a series of questions to evaluate internal validity, results, and external validity and applicability.3
Residents used the platform to independently read the article, highlight areas of the text that corresponded to 8 critical appraisal questions, and answer yes or no to these questions. Based on residents’ answers, a final appraisal score (on a scale of 1% to 100%) was generated. Simultaneously, the attending dermatologist leading the journal club (C.W.) also completed the assignment to establish an expert score.
Scores from the residents’ independent appraisal ranged from 75% to 100% (mean, 85.4%). Upon discussing the article in a group setting, the residents established a consensus score of 75%. This consensus score matched the expert score, which suggested to us that both independently reviewing the article using guided questions and conducting a group debriefing were necessary to match the expert level of critical appraisal.
Of note, the residents’ average independent appraisal score was higher than both the consensus and expert scores, indicating that the residents evaluated the article less critically on their own. With more practice using this method, it is possible that the precision and accuracy of the residents’ critical appraisal of scientific articles will improve.
In the postsurvey, we asked residents about the critical appraisal of the medical literature. All residents agreed that evaluating the quality of evidence when reading a scientific article was somewhat important or very important to them; however, only 2 of 6 evaluated the quality of evidence all the time, and the other 4 did so half of the time or less than half of the time.
When critically appraising articles, 2 of 6 residents used specific rubrics half of the time; 4 of 6 less than half of the time. Most important, 5 of 6 residents agreed that the quality of evidence affected their management decisions more than half of the time or all of the time. Although it is clear that residents value evidence-based medicine and understand the importance of evaluating the quality of evidence, doing so currently might not be simple or practical.
An organized framework for appraising articles would streamline the process. Five of 6 residents agreed that the use of specific questions as a guide made it easier to appraise an article for the quality of its evidence. Four of 6 residents found that juxtaposing specific questions with the interactive reader was helpful; 5 of 6 agreed that they would use a web-based journal club platform if given the option.
Lastly, 5 of 6 residents agreed that if such a tool were available, a platform containing all major dermatology publications in an interactive reader format, along with relevant appraisal questions on the side, would be useful.
This pilot study augmented the typical journal club experience by emphasizing goal-directed reading and the importance of analyzing the quality of evidence. The combination of independent appraisal of an article using targeted questions and a group debrief led to better understanding of the evidence and its clinical applicability. The COVID-19 pandemic may be a better time than ever to explore innovative ways to teach evidence-based medicine in residency training.
- Mimouni D, Pavlovsky L, Akerman L, et al. Trends in dermatology publications over the past 15 years. Am J Clin Dermatol. 2010;11:55-58. doi:10.2165/11530190-000000000-00000.
- NEJM Knowledge+ Team. Exploring the ACGME Core Competencies: Practice-Based Learning and Improvement (part 2 of 7). Massachusetts Medical Society. NEJM Knowledge+ website. Published July 28, 2016. Accessed January 15, 2022. https://knowledgeplus.nejm.org/blog/practice-based-learning-and-improvement/
- University of Oxford. Critical appraisal tools. Centre for Evidence-Based Medicine website. Accessed January 2, 2022. www.cebm.ox.ac.uk/resources/ebm-tools/critical-appraisal-tools
- Mimouni D, Pavlovsky L, Akerman L, et al. Trends in dermatology publications over the past 15 years. Am J Clin Dermatol. 2010;11:55-58. doi:10.2165/11530190-000000000-00000.
- NEJM Knowledge+ Team. Exploring the ACGME Core Competencies: Practice-Based Learning and Improvement (part 2 of 7). Massachusetts Medical Society. NEJM Knowledge+ website. Published July 28, 2016. Accessed January 15, 2022. https://knowledgeplus.nejm.org/blog/practice-based-learning-and-improvement/
- University of Oxford. Critical appraisal tools. Centre for Evidence-Based Medicine website. Accessed January 2, 2022. www.cebm.ox.ac.uk/resources/ebm-tools/critical-appraisal-tools
Practice Points
- A novel web-based application was beta tested in an academic dermatology setting to design and run a journal club for residents.
- Goal-directed reading was emphasized by using guided questions to critically appraise literature based on reliability, significance, and applicability.
- The combination of independent appraisal of an article using targeted questions and a group debrief led to better understanding of the evidence and its clinical applicability.






