User login
Dermatologists take to TikTok to share their own ‘hacks’
A young woman is having her lip swabbed with an unknown substance, smiling, on the TikTok video. Seconds later, another young woman, wearing gloves, pushes a hyaluron pen against the first woman’s lips, who, in the next cut, is smiling, happy. “My first syringe down and already 1,000x more confident,” the caption reads.
That video is one of thousands showing hyaluron pen use on TikTok. The pens are sold online and are unapproved – which led to a Food and Drug Administration warning in October 2021 that use could cause bleeding, infection, blood vessel occlusion that could result in blindness or stroke, allergic reactions, and other injuries.
The warning has not stopped many TikTokkers, who also use the medium to promote all sorts of skin and aesthetic products and procedures, a large number unproven, unapproved, or ill advised. which, more often than not, comes from “skinfluencers,” aestheticians, and other laypeople, not board-certified dermatologists.
The suggested “hacks” can be harmless or ineffective, but they also can be misleading, fraudulent, or even dangerous.
Skinfluencers take the lead
TikTok has a reported 1 billion monthly users. Two-thirds are aged 10-29 years, according to data reported in February 2021 in the Journal of the American Academy of Dermatology by David X. Zheng, BA, and colleagues at Case Western Reserve University, Cleveland, and the department of dermatology, Johns Hopkins University, Baltimore.
Visitors consume information in video bits that run from 15 seconds to up to 3 minutes and can follow their favorite TikTokkers, browse for people or hashtags with a search function, or click on content recommended by the platform, which uses algorithms based on the user’s viewing habits to determine what might be of interest.
Some of the biggest “skinfluencers” have millions of followers: Hyram Yarbro, (@skincarebyhyram) for instance, has 6.6 million followers and his own line of skin care products at Sephora. Mr. Yarbro is seen as a no-nonsense debunker of skin care myths, as is British influencer James Welsh, who has 124,000 followers.
“The reason why people trust your average influencer person who’s not a doctor is because they’re relatable,” said Muneeb Shah, MD, a dermatology resident at Atlantic Dermatology in Wilmington, N.C. – known to his 11.4 million TikTok followers as @dermdoctor.
To Sandra Lee, MD, the popularity of nonprofessionals is easy to explain. “You have to think about the fact that a lot of people can’t see dermatologists – they don’t have the money, they don’t have the time to travel there, they don’t have health insurance, or they’re scared of doctors, so they’re willing to try to find an answer, and one of the easiest ways, one of the more entertaining ways to get information, is on social media.”
Dr. Lee is in private practice in Upland, Calif., but is better known as “Dr. Pimple Popper,” through her television show of the same name and her social media accounts, including on TikTok, where she has 14.4 million followers after having started in 2020.
“We’re all looking for that no-down-time, no-expense, no-lines, no-wrinkles, stay-young-forever magic bullet,” said Dr. Lee.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, agreed that people are looking for a quick fix. They don’t want to wait 12 weeks for an acne medication or 16 weeks for a biologic to work. “They want something simple, easy, do-it-yourself,” and “natural,” he said.
Laypeople are still the dominant producers – and have the most views – of dermatology content.
Morgan Nguyen, BA, at Northwestern University, Chicago, and colleagues looked at hashtags for the top 10 dermatologic diagnoses and procedures and analyzed the content of the first 40 TikTok videos in each category. About half the videos were produced by an individual, and 39% by a health care provider, according to the study, published in the International Journal of Women’s Dermatology. About 40% of the videos were educational, focusing on skin care, procedures, and disease treatment.
Viewership was highest for videos by laypeople, followed by those produced by business or industry accounts. Those produced by health care providers received only 18% of the views.
The most popular videos were about dermatologic diagnoses, with 2.5 billion views, followed by dermatologic procedures, with 708 million views.
Ms. Nguyen noted in the study that the most liked and most viewed posts were related to #skincare but that board-certified dermatologists produced only 2.5% of the #skincare videos.
Dermatologists take to TikTok
Some dermatologists have started their own TikTok accounts, seeking both to counteract misinformation and provide education.
Dr. Shah has become one of the top influencers on the platform. In a year-end wrap, TikTok put Dr. Shah at No. 7 on its top creators list for 2021.

The dermatology resident said that TikTok is a good tool for reaching patients who might not otherwise interact with dermatologists. He recounted the story of an individual who came into his office with the idea that they had hidradenitis suppurativa.
The person had self-diagnosed after seeing one of Dr. Shah’s TikTok videos on the condition. It was a pleasant surprise, said Dr. Shah. People with hidradenitis suppurativa often avoid treatment, and it’s underdiagnosed and improperly treated, despite an American Academy of Dermatology awareness campaign.
“Dermatologists on social media are almost like the communications department for dermatology,” Dr. Shah commented.
A key to making TikTok work to advance dermatologists’ goals is knowing what makes it unique.
Dr. Lee said she prefers it to Instagram, because TikTok’s algorithms and its younger-skewing audience help her reach a more specific audience.
The algorithm “creates a positive feedback loop in which popular content creators or viral trends are prioritized on the users’ homepages, in turn providing the creators of these videos with an even larger audience,” Mr. Zheng, of University Hospitals Cleveland Medical Center, and coauthors noted in their letter in the Journal of the American Academy of Dermatology.
TikTok also celebrates the everyday – someone doesn’t have to be a celebrity to make something go viral, said Dr. Lee. She believes that TikTok users are more accepting of average people with real problems – which helps when someone is TikTokking about a skin condition.
Doris Day, MD, who goes by @drdorisday on TikTok, agreed with Dr. Lee. “There are so many creative ways you can convey information with it that’s different than what you have on Instagram,” said Dr. Day, who is in private practice in New York. And, she added, “it does really lend itself to getting points out super-fast.”
Dermatologists on TikTok also said they like the “duets” and the “stitch” features, which allow users to add on to an existing video, essentially chiming in or responding to what might have already been posted, in a side-by-side format.
Dr. Shah said he often duets videos that have questionable content. “It allows me to directly respond to people. A lot of times, if something is going really viral and it’s not accurate, you’ll have a response from me or one of the other doctors” within hours or days.
Dr. Shah’s duets are labeled with “DermDoctor Reacts” or “DermDoctor Explains.” In one duet, with more than 2.8 million views, the upper half of the video is someone squeezing a blackhead, while Dr. Shah, in the bottom half, in green scrubs, opines over some hip-hop music: “This is just a blackhead. But once it gets to this point, they do need to be extracted because topical treatments won’t help.”
Dr. Lee – whose TikTok and other accounts capitalize on teens’ obsession with popping pimples – has a duet in which she advised that although popping will leave scars, there are more ideal times to pop, if they must. The duet has at least 21 million views.
Sometimes a TikTok video effectively takes on a trend without being a duet. Nurse practitioner Uy Dam (@uy.np) has a video that demonstrates the dangers of hyaluron pens. He uses both a pen and a needle to inject fluid into a block of jello. The pen delivers a scattershot load of differing depths, while the needle is exact. It’s visual and easy to understand and has at least 1.3 million views.
Still, TikTok, like other forms of social media, is full of misinformation and false accounts, including people who claim to be doctors. “It’s hard for the regular person, myself included, sometimes to be able to root through that and find out whether something is real or not,” said Dr. Lee.
Dr. Friedman said he’s concerned about the lack of accountability. A doctor could lose his or her license for promoting unproven cures, especially if they are harmful. But for influencers, “there’s no accountability for posting information that can actually hurt people.”
TikTok trends gone bad
And some people are being hurt by emulating what they see on TikTok.
Dr. Friedman had a patient with extreme irritant contact dermatitis, “almost like chemical burns to her underarms,” he said. He determined that she saw a video “hack” that recommended using baking soda to stop hyperhidrosis. The patient used so much that it burned her skin.
In 2020, do-it-yourself freckles – with henna or sewing needles impregnated with ink – went viral. Tilly Whitfeld, a 21-year-old reality TV star on Australia’s Big Brother show, told the New York Times that she tried it at home after seeing a TikTok video. She ordered brown tattoo ink online and later found out that it was contaminated with lead, according to the Times. Ms. Whitfeld developed an infection and temporary vision loss and has permanent scarring.
She has since put out a cautionary TikTok video that’s been viewed some 300,000 times.
TikTokkers have also flocked to the idea of using sunscreen to “contour” the face. Selected areas are left without sunscreen to burn or tan. In a duet, a plastic surgeon shakes his head as a young woman explains that “it works.”
Scalp-popping – in which the hair is yanked so hard that it pulls the galea off the skull – has been mostly shut down by TikTok. A search of “scalp popping” brings up the message: “Learn how to recognize harmful challenges and hoaxes.” At-home mole and skin tag removal, pimple-popping, and supposed acne cures such as drinking chlorophyll are all avidly documented and shared on TikTok.
Dr. Shah had a back-and-forth video dialog with someone who had stubbed a toe and then drilled a hole into the nail to drain the hematoma. In a reaction video, Dr. Shah said it was likely to turn into an infection. When it did, the man revealed the infection in a video where he tagged Dr. Shah and later posted a video at the podiatrist’s office having his nail removed, again tagging Dr. Shah.
“I think that pretty much no procedure for skin is good to do at home,” said Dr. Shah, who repeatedly admonishes against mole removal by a nonphysician. He tells followers that “it’s extremely dangerous – not only is it going to cause scarring, but you are potentially discarding a cancerous lesion.”
Unfortunately, most will not follow the advice, said Dr. Shah. That’s especially true of pimple-popping. Aiming for the least harm, he suggests in some TikTok videos that poppers keep the area clean, wear gloves, and consult a physician to get an antibiotic prescription. “You might as well at least guide them in the right direction,” he added.
Dr. Lee believes that lack of access to physicians, insurance, or money may play into how TikTok trends evolve. “Probably those people who injected their lips with this air gun thing, maybe they didn’t have the money necessarily to get filler,” she said.
Also, she noted, while TikTok may try to police its content, creators are incentivized to be outrageous. “The more inflammatory your post is, the more engagement you get.”
Dr. Shah thinks TikTok is self-correcting. “If you’re not being ethical or contradicting yourself, putting out information that’s not accurate, people are going to catch on very quickly,” he said. “The only value, the only currency you have on social media is the trust that you build with people that follow you.”
What it takes to be a TikTokker
For dermatologists, conveying their credentials and experience is one way to build that currency. Dr. Lee advised fellow doctors on TikTok to “showcase your training and how many years it took to become a dermatologist.”
Plunging into TikTok is not for everyone, though. It’s time consuming, said Dr. Lee, who now devotes most of her nonclinical time to TikTok. She creates her own content, leaving others to manage her Instagram account.
Many of those in the medical field who have dived into TikTok are residents, like Dr. Shah. “They are attuned to it and understand it more,” said Dr. Lee. “It’s harder for a lot of us who are older, who really weren’t involved that much in social media at all. It’s very hard to jump in.” There’s a learning curve, and it takes hours to create a single video. “You have to enjoy it and it has to be a part of your life,” she said.
Dr. Shah started experimenting with TikTok at the beginning of the pandemic in 2020 and has never turned back. Fast-talking, curious, and with an infectious sense of fun, he shares tidbits about his personal life – putting his wife in some of his videos – and always seems upbeat.
He said that, as his following grew, users began to see him as an authority figure and started “tagging” him more often, seeking his opinion on other videos. Although still a resident, he believes he has specialized knowledge to share. “Even if you’re not the world’s leading expert in a particular topic, you’re still adding value for the person who doesn’t know much.”
Dr. Shah also occasionally does promotional TikToks, identified as sponsored content. He said he only works with companies that he believes have legitimate products. “You do have to monetize at some point,” he said, noting that many dermatologists, himself included, are trading clinic time for TikTok. “There’s no universe where they can do this for free.”
Product endorsements are likely more rewarding for influencers and other users like Dr. Shah than the remuneration from TikTok, the company. The platform pays user accounts $20 per 1 million views, Dr. Shah said. “Financially, it’s not a big winner for a practicing dermatologist, but the educational outreach is worthwhile.”
To be successful also means understanding what drives viewership.
Using “trending” sounds has “been shown to increase the likelihood of a video amassing millions of views” and may increase engagement with dermatologists’ TikTok videos, wrote Bina Kassamali, BA, and colleagues at the Brigham and Women’s Hospital in Boston and the Ponce Health Science University School of Medicine in Ponce, Puerto Rico, in a letter published in the Journal of the American Academy of Dermatology in July 2021.
Certain content is more likely to engage viewers. In their analysis of top trending dermatologic hashtags, acne-related content was viewed 6.7 billion times, followed by alopecia, with 1.1 billion views. Psoriasis content had 84 million views, putting it eighth on the list of topics.
Dermatologists are still cracking TikTok. They are accumulating more followers on TikTok than on Instagram but have greater engagement on Instagram reels, wrote Mindy D. Szeto, MS, and colleagues at the University of Colorado at Denver, Aurora, and Rocky Vista University in Parker, Colo., in the Journal of the American Academy of Dermatology in April 2021.
Dr. Lee and Dr. Shah had the highest engagement rate on TikTok, according to Ms. Szeto. The engagement rate is calculated as (likes + comments per post)/(total followers) x 100.
“TikTok may currently be the leading avenue for audience education by dermatologist influencers,” they wrote, urging dermatologists to use the platform to answer the call as more of the public “continues to turn to social media for medical advice.”
Dr. Day said she will keep trying to build her TikTok audience. She has just 239 followers, compared with her 44,500 on Instagram. “The more I do TikTok, the more I do any of these mediums, the better I get at it,” she said. “We just have to put a little time and effort into it and try to get more followers and just keep sharing the information.”
Dr. Friedman sees it as a positive that some dermatologists have taken to TikTok to dispel myths and put “good information out there in small bites.” But to be more effective, they need more followers.
“The truth is that 14-year-old is probably going to listen more to a Hyram than a dermatologist,” he said. “Maybe we need to work with these other individuals who know how to take these messages and convert them to a language that can be digested by a 14-year-old, by a 12-year-old, by a 23-year-old. We need to come to the table together and not fight.”
A version of this article first appeared on Medscape.com.
A young woman is having her lip swabbed with an unknown substance, smiling, on the TikTok video. Seconds later, another young woman, wearing gloves, pushes a hyaluron pen against the first woman’s lips, who, in the next cut, is smiling, happy. “My first syringe down and already 1,000x more confident,” the caption reads.
That video is one of thousands showing hyaluron pen use on TikTok. The pens are sold online and are unapproved – which led to a Food and Drug Administration warning in October 2021 that use could cause bleeding, infection, blood vessel occlusion that could result in blindness or stroke, allergic reactions, and other injuries.
The warning has not stopped many TikTokkers, who also use the medium to promote all sorts of skin and aesthetic products and procedures, a large number unproven, unapproved, or ill advised. which, more often than not, comes from “skinfluencers,” aestheticians, and other laypeople, not board-certified dermatologists.
The suggested “hacks” can be harmless or ineffective, but they also can be misleading, fraudulent, or even dangerous.
Skinfluencers take the lead
TikTok has a reported 1 billion monthly users. Two-thirds are aged 10-29 years, according to data reported in February 2021 in the Journal of the American Academy of Dermatology by David X. Zheng, BA, and colleagues at Case Western Reserve University, Cleveland, and the department of dermatology, Johns Hopkins University, Baltimore.
Visitors consume information in video bits that run from 15 seconds to up to 3 minutes and can follow their favorite TikTokkers, browse for people or hashtags with a search function, or click on content recommended by the platform, which uses algorithms based on the user’s viewing habits to determine what might be of interest.
Some of the biggest “skinfluencers” have millions of followers: Hyram Yarbro, (@skincarebyhyram) for instance, has 6.6 million followers and his own line of skin care products at Sephora. Mr. Yarbro is seen as a no-nonsense debunker of skin care myths, as is British influencer James Welsh, who has 124,000 followers.
“The reason why people trust your average influencer person who’s not a doctor is because they’re relatable,” said Muneeb Shah, MD, a dermatology resident at Atlantic Dermatology in Wilmington, N.C. – known to his 11.4 million TikTok followers as @dermdoctor.
To Sandra Lee, MD, the popularity of nonprofessionals is easy to explain. “You have to think about the fact that a lot of people can’t see dermatologists – they don’t have the money, they don’t have the time to travel there, they don’t have health insurance, or they’re scared of doctors, so they’re willing to try to find an answer, and one of the easiest ways, one of the more entertaining ways to get information, is on social media.”
Dr. Lee is in private practice in Upland, Calif., but is better known as “Dr. Pimple Popper,” through her television show of the same name and her social media accounts, including on TikTok, where she has 14.4 million followers after having started in 2020.
“We’re all looking for that no-down-time, no-expense, no-lines, no-wrinkles, stay-young-forever magic bullet,” said Dr. Lee.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, agreed that people are looking for a quick fix. They don’t want to wait 12 weeks for an acne medication or 16 weeks for a biologic to work. “They want something simple, easy, do-it-yourself,” and “natural,” he said.
Laypeople are still the dominant producers – and have the most views – of dermatology content.
Morgan Nguyen, BA, at Northwestern University, Chicago, and colleagues looked at hashtags for the top 10 dermatologic diagnoses and procedures and analyzed the content of the first 40 TikTok videos in each category. About half the videos were produced by an individual, and 39% by a health care provider, according to the study, published in the International Journal of Women’s Dermatology. About 40% of the videos were educational, focusing on skin care, procedures, and disease treatment.
Viewership was highest for videos by laypeople, followed by those produced by business or industry accounts. Those produced by health care providers received only 18% of the views.
The most popular videos were about dermatologic diagnoses, with 2.5 billion views, followed by dermatologic procedures, with 708 million views.
Ms. Nguyen noted in the study that the most liked and most viewed posts were related to #skincare but that board-certified dermatologists produced only 2.5% of the #skincare videos.
Dermatologists take to TikTok
Some dermatologists have started their own TikTok accounts, seeking both to counteract misinformation and provide education.
Dr. Shah has become one of the top influencers on the platform. In a year-end wrap, TikTok put Dr. Shah at No. 7 on its top creators list for 2021.

The dermatology resident said that TikTok is a good tool for reaching patients who might not otherwise interact with dermatologists. He recounted the story of an individual who came into his office with the idea that they had hidradenitis suppurativa.
The person had self-diagnosed after seeing one of Dr. Shah’s TikTok videos on the condition. It was a pleasant surprise, said Dr. Shah. People with hidradenitis suppurativa often avoid treatment, and it’s underdiagnosed and improperly treated, despite an American Academy of Dermatology awareness campaign.
“Dermatologists on social media are almost like the communications department for dermatology,” Dr. Shah commented.
A key to making TikTok work to advance dermatologists’ goals is knowing what makes it unique.
Dr. Lee said she prefers it to Instagram, because TikTok’s algorithms and its younger-skewing audience help her reach a more specific audience.
The algorithm “creates a positive feedback loop in which popular content creators or viral trends are prioritized on the users’ homepages, in turn providing the creators of these videos with an even larger audience,” Mr. Zheng, of University Hospitals Cleveland Medical Center, and coauthors noted in their letter in the Journal of the American Academy of Dermatology.
TikTok also celebrates the everyday – someone doesn’t have to be a celebrity to make something go viral, said Dr. Lee. She believes that TikTok users are more accepting of average people with real problems – which helps when someone is TikTokking about a skin condition.
Doris Day, MD, who goes by @drdorisday on TikTok, agreed with Dr. Lee. “There are so many creative ways you can convey information with it that’s different than what you have on Instagram,” said Dr. Day, who is in private practice in New York. And, she added, “it does really lend itself to getting points out super-fast.”
Dermatologists on TikTok also said they like the “duets” and the “stitch” features, which allow users to add on to an existing video, essentially chiming in or responding to what might have already been posted, in a side-by-side format.
Dr. Shah said he often duets videos that have questionable content. “It allows me to directly respond to people. A lot of times, if something is going really viral and it’s not accurate, you’ll have a response from me or one of the other doctors” within hours or days.
Dr. Shah’s duets are labeled with “DermDoctor Reacts” or “DermDoctor Explains.” In one duet, with more than 2.8 million views, the upper half of the video is someone squeezing a blackhead, while Dr. Shah, in the bottom half, in green scrubs, opines over some hip-hop music: “This is just a blackhead. But once it gets to this point, they do need to be extracted because topical treatments won’t help.”
Dr. Lee – whose TikTok and other accounts capitalize on teens’ obsession with popping pimples – has a duet in which she advised that although popping will leave scars, there are more ideal times to pop, if they must. The duet has at least 21 million views.
Sometimes a TikTok video effectively takes on a trend without being a duet. Nurse practitioner Uy Dam (@uy.np) has a video that demonstrates the dangers of hyaluron pens. He uses both a pen and a needle to inject fluid into a block of jello. The pen delivers a scattershot load of differing depths, while the needle is exact. It’s visual and easy to understand and has at least 1.3 million views.
Still, TikTok, like other forms of social media, is full of misinformation and false accounts, including people who claim to be doctors. “It’s hard for the regular person, myself included, sometimes to be able to root through that and find out whether something is real or not,” said Dr. Lee.
Dr. Friedman said he’s concerned about the lack of accountability. A doctor could lose his or her license for promoting unproven cures, especially if they are harmful. But for influencers, “there’s no accountability for posting information that can actually hurt people.”
TikTok trends gone bad
And some people are being hurt by emulating what they see on TikTok.
Dr. Friedman had a patient with extreme irritant contact dermatitis, “almost like chemical burns to her underarms,” he said. He determined that she saw a video “hack” that recommended using baking soda to stop hyperhidrosis. The patient used so much that it burned her skin.
In 2020, do-it-yourself freckles – with henna or sewing needles impregnated with ink – went viral. Tilly Whitfeld, a 21-year-old reality TV star on Australia’s Big Brother show, told the New York Times that she tried it at home after seeing a TikTok video. She ordered brown tattoo ink online and later found out that it was contaminated with lead, according to the Times. Ms. Whitfeld developed an infection and temporary vision loss and has permanent scarring.
She has since put out a cautionary TikTok video that’s been viewed some 300,000 times.
TikTokkers have also flocked to the idea of using sunscreen to “contour” the face. Selected areas are left without sunscreen to burn or tan. In a duet, a plastic surgeon shakes his head as a young woman explains that “it works.”
Scalp-popping – in which the hair is yanked so hard that it pulls the galea off the skull – has been mostly shut down by TikTok. A search of “scalp popping” brings up the message: “Learn how to recognize harmful challenges and hoaxes.” At-home mole and skin tag removal, pimple-popping, and supposed acne cures such as drinking chlorophyll are all avidly documented and shared on TikTok.
Dr. Shah had a back-and-forth video dialog with someone who had stubbed a toe and then drilled a hole into the nail to drain the hematoma. In a reaction video, Dr. Shah said it was likely to turn into an infection. When it did, the man revealed the infection in a video where he tagged Dr. Shah and later posted a video at the podiatrist’s office having his nail removed, again tagging Dr. Shah.
“I think that pretty much no procedure for skin is good to do at home,” said Dr. Shah, who repeatedly admonishes against mole removal by a nonphysician. He tells followers that “it’s extremely dangerous – not only is it going to cause scarring, but you are potentially discarding a cancerous lesion.”
Unfortunately, most will not follow the advice, said Dr. Shah. That’s especially true of pimple-popping. Aiming for the least harm, he suggests in some TikTok videos that poppers keep the area clean, wear gloves, and consult a physician to get an antibiotic prescription. “You might as well at least guide them in the right direction,” he added.
Dr. Lee believes that lack of access to physicians, insurance, or money may play into how TikTok trends evolve. “Probably those people who injected their lips with this air gun thing, maybe they didn’t have the money necessarily to get filler,” she said.
Also, she noted, while TikTok may try to police its content, creators are incentivized to be outrageous. “The more inflammatory your post is, the more engagement you get.”
Dr. Shah thinks TikTok is self-correcting. “If you’re not being ethical or contradicting yourself, putting out information that’s not accurate, people are going to catch on very quickly,” he said. “The only value, the only currency you have on social media is the trust that you build with people that follow you.”
What it takes to be a TikTokker
For dermatologists, conveying their credentials and experience is one way to build that currency. Dr. Lee advised fellow doctors on TikTok to “showcase your training and how many years it took to become a dermatologist.”
Plunging into TikTok is not for everyone, though. It’s time consuming, said Dr. Lee, who now devotes most of her nonclinical time to TikTok. She creates her own content, leaving others to manage her Instagram account.
Many of those in the medical field who have dived into TikTok are residents, like Dr. Shah. “They are attuned to it and understand it more,” said Dr. Lee. “It’s harder for a lot of us who are older, who really weren’t involved that much in social media at all. It’s very hard to jump in.” There’s a learning curve, and it takes hours to create a single video. “You have to enjoy it and it has to be a part of your life,” she said.
Dr. Shah started experimenting with TikTok at the beginning of the pandemic in 2020 and has never turned back. Fast-talking, curious, and with an infectious sense of fun, he shares tidbits about his personal life – putting his wife in some of his videos – and always seems upbeat.
He said that, as his following grew, users began to see him as an authority figure and started “tagging” him more often, seeking his opinion on other videos. Although still a resident, he believes he has specialized knowledge to share. “Even if you’re not the world’s leading expert in a particular topic, you’re still adding value for the person who doesn’t know much.”
Dr. Shah also occasionally does promotional TikToks, identified as sponsored content. He said he only works with companies that he believes have legitimate products. “You do have to monetize at some point,” he said, noting that many dermatologists, himself included, are trading clinic time for TikTok. “There’s no universe where they can do this for free.”
Product endorsements are likely more rewarding for influencers and other users like Dr. Shah than the remuneration from TikTok, the company. The platform pays user accounts $20 per 1 million views, Dr. Shah said. “Financially, it’s not a big winner for a practicing dermatologist, but the educational outreach is worthwhile.”
To be successful also means understanding what drives viewership.
Using “trending” sounds has “been shown to increase the likelihood of a video amassing millions of views” and may increase engagement with dermatologists’ TikTok videos, wrote Bina Kassamali, BA, and colleagues at the Brigham and Women’s Hospital in Boston and the Ponce Health Science University School of Medicine in Ponce, Puerto Rico, in a letter published in the Journal of the American Academy of Dermatology in July 2021.
Certain content is more likely to engage viewers. In their analysis of top trending dermatologic hashtags, acne-related content was viewed 6.7 billion times, followed by alopecia, with 1.1 billion views. Psoriasis content had 84 million views, putting it eighth on the list of topics.
Dermatologists are still cracking TikTok. They are accumulating more followers on TikTok than on Instagram but have greater engagement on Instagram reels, wrote Mindy D. Szeto, MS, and colleagues at the University of Colorado at Denver, Aurora, and Rocky Vista University in Parker, Colo., in the Journal of the American Academy of Dermatology in April 2021.
Dr. Lee and Dr. Shah had the highest engagement rate on TikTok, according to Ms. Szeto. The engagement rate is calculated as (likes + comments per post)/(total followers) x 100.
“TikTok may currently be the leading avenue for audience education by dermatologist influencers,” they wrote, urging dermatologists to use the platform to answer the call as more of the public “continues to turn to social media for medical advice.”
Dr. Day said she will keep trying to build her TikTok audience. She has just 239 followers, compared with her 44,500 on Instagram. “The more I do TikTok, the more I do any of these mediums, the better I get at it,” she said. “We just have to put a little time and effort into it and try to get more followers and just keep sharing the information.”
Dr. Friedman sees it as a positive that some dermatologists have taken to TikTok to dispel myths and put “good information out there in small bites.” But to be more effective, they need more followers.
“The truth is that 14-year-old is probably going to listen more to a Hyram than a dermatologist,” he said. “Maybe we need to work with these other individuals who know how to take these messages and convert them to a language that can be digested by a 14-year-old, by a 12-year-old, by a 23-year-old. We need to come to the table together and not fight.”
A version of this article first appeared on Medscape.com.
A young woman is having her lip swabbed with an unknown substance, smiling, on the TikTok video. Seconds later, another young woman, wearing gloves, pushes a hyaluron pen against the first woman’s lips, who, in the next cut, is smiling, happy. “My first syringe down and already 1,000x more confident,” the caption reads.
That video is one of thousands showing hyaluron pen use on TikTok. The pens are sold online and are unapproved – which led to a Food and Drug Administration warning in October 2021 that use could cause bleeding, infection, blood vessel occlusion that could result in blindness or stroke, allergic reactions, and other injuries.
The warning has not stopped many TikTokkers, who also use the medium to promote all sorts of skin and aesthetic products and procedures, a large number unproven, unapproved, or ill advised. which, more often than not, comes from “skinfluencers,” aestheticians, and other laypeople, not board-certified dermatologists.
The suggested “hacks” can be harmless or ineffective, but they also can be misleading, fraudulent, or even dangerous.
Skinfluencers take the lead
TikTok has a reported 1 billion monthly users. Two-thirds are aged 10-29 years, according to data reported in February 2021 in the Journal of the American Academy of Dermatology by David X. Zheng, BA, and colleagues at Case Western Reserve University, Cleveland, and the department of dermatology, Johns Hopkins University, Baltimore.
Visitors consume information in video bits that run from 15 seconds to up to 3 minutes and can follow their favorite TikTokkers, browse for people or hashtags with a search function, or click on content recommended by the platform, which uses algorithms based on the user’s viewing habits to determine what might be of interest.
Some of the biggest “skinfluencers” have millions of followers: Hyram Yarbro, (@skincarebyhyram) for instance, has 6.6 million followers and his own line of skin care products at Sephora. Mr. Yarbro is seen as a no-nonsense debunker of skin care myths, as is British influencer James Welsh, who has 124,000 followers.
“The reason why people trust your average influencer person who’s not a doctor is because they’re relatable,” said Muneeb Shah, MD, a dermatology resident at Atlantic Dermatology in Wilmington, N.C. – known to his 11.4 million TikTok followers as @dermdoctor.
To Sandra Lee, MD, the popularity of nonprofessionals is easy to explain. “You have to think about the fact that a lot of people can’t see dermatologists – they don’t have the money, they don’t have the time to travel there, they don’t have health insurance, or they’re scared of doctors, so they’re willing to try to find an answer, and one of the easiest ways, one of the more entertaining ways to get information, is on social media.”
Dr. Lee is in private practice in Upland, Calif., but is better known as “Dr. Pimple Popper,” through her television show of the same name and her social media accounts, including on TikTok, where she has 14.4 million followers after having started in 2020.
“We’re all looking for that no-down-time, no-expense, no-lines, no-wrinkles, stay-young-forever magic bullet,” said Dr. Lee.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, agreed that people are looking for a quick fix. They don’t want to wait 12 weeks for an acne medication or 16 weeks for a biologic to work. “They want something simple, easy, do-it-yourself,” and “natural,” he said.
Laypeople are still the dominant producers – and have the most views – of dermatology content.
Morgan Nguyen, BA, at Northwestern University, Chicago, and colleagues looked at hashtags for the top 10 dermatologic diagnoses and procedures and analyzed the content of the first 40 TikTok videos in each category. About half the videos were produced by an individual, and 39% by a health care provider, according to the study, published in the International Journal of Women’s Dermatology. About 40% of the videos were educational, focusing on skin care, procedures, and disease treatment.
Viewership was highest for videos by laypeople, followed by those produced by business or industry accounts. Those produced by health care providers received only 18% of the views.
The most popular videos were about dermatologic diagnoses, with 2.5 billion views, followed by dermatologic procedures, with 708 million views.
Ms. Nguyen noted in the study that the most liked and most viewed posts were related to #skincare but that board-certified dermatologists produced only 2.5% of the #skincare videos.
Dermatologists take to TikTok
Some dermatologists have started their own TikTok accounts, seeking both to counteract misinformation and provide education.
Dr. Shah has become one of the top influencers on the platform. In a year-end wrap, TikTok put Dr. Shah at No. 7 on its top creators list for 2021.

The dermatology resident said that TikTok is a good tool for reaching patients who might not otherwise interact with dermatologists. He recounted the story of an individual who came into his office with the idea that they had hidradenitis suppurativa.
The person had self-diagnosed after seeing one of Dr. Shah’s TikTok videos on the condition. It was a pleasant surprise, said Dr. Shah. People with hidradenitis suppurativa often avoid treatment, and it’s underdiagnosed and improperly treated, despite an American Academy of Dermatology awareness campaign.
“Dermatologists on social media are almost like the communications department for dermatology,” Dr. Shah commented.
A key to making TikTok work to advance dermatologists’ goals is knowing what makes it unique.
Dr. Lee said she prefers it to Instagram, because TikTok’s algorithms and its younger-skewing audience help her reach a more specific audience.
The algorithm “creates a positive feedback loop in which popular content creators or viral trends are prioritized on the users’ homepages, in turn providing the creators of these videos with an even larger audience,” Mr. Zheng, of University Hospitals Cleveland Medical Center, and coauthors noted in their letter in the Journal of the American Academy of Dermatology.
TikTok also celebrates the everyday – someone doesn’t have to be a celebrity to make something go viral, said Dr. Lee. She believes that TikTok users are more accepting of average people with real problems – which helps when someone is TikTokking about a skin condition.
Doris Day, MD, who goes by @drdorisday on TikTok, agreed with Dr. Lee. “There are so many creative ways you can convey information with it that’s different than what you have on Instagram,” said Dr. Day, who is in private practice in New York. And, she added, “it does really lend itself to getting points out super-fast.”
Dermatologists on TikTok also said they like the “duets” and the “stitch” features, which allow users to add on to an existing video, essentially chiming in or responding to what might have already been posted, in a side-by-side format.
Dr. Shah said he often duets videos that have questionable content. “It allows me to directly respond to people. A lot of times, if something is going really viral and it’s not accurate, you’ll have a response from me or one of the other doctors” within hours or days.
Dr. Shah’s duets are labeled with “DermDoctor Reacts” or “DermDoctor Explains.” In one duet, with more than 2.8 million views, the upper half of the video is someone squeezing a blackhead, while Dr. Shah, in the bottom half, in green scrubs, opines over some hip-hop music: “This is just a blackhead. But once it gets to this point, they do need to be extracted because topical treatments won’t help.”
Dr. Lee – whose TikTok and other accounts capitalize on teens’ obsession with popping pimples – has a duet in which she advised that although popping will leave scars, there are more ideal times to pop, if they must. The duet has at least 21 million views.
Sometimes a TikTok video effectively takes on a trend without being a duet. Nurse practitioner Uy Dam (@uy.np) has a video that demonstrates the dangers of hyaluron pens. He uses both a pen and a needle to inject fluid into a block of jello. The pen delivers a scattershot load of differing depths, while the needle is exact. It’s visual and easy to understand and has at least 1.3 million views.
Still, TikTok, like other forms of social media, is full of misinformation and false accounts, including people who claim to be doctors. “It’s hard for the regular person, myself included, sometimes to be able to root through that and find out whether something is real or not,” said Dr. Lee.
Dr. Friedman said he’s concerned about the lack of accountability. A doctor could lose his or her license for promoting unproven cures, especially if they are harmful. But for influencers, “there’s no accountability for posting information that can actually hurt people.”
TikTok trends gone bad
And some people are being hurt by emulating what they see on TikTok.
Dr. Friedman had a patient with extreme irritant contact dermatitis, “almost like chemical burns to her underarms,” he said. He determined that she saw a video “hack” that recommended using baking soda to stop hyperhidrosis. The patient used so much that it burned her skin.
In 2020, do-it-yourself freckles – with henna or sewing needles impregnated with ink – went viral. Tilly Whitfeld, a 21-year-old reality TV star on Australia’s Big Brother show, told the New York Times that she tried it at home after seeing a TikTok video. She ordered brown tattoo ink online and later found out that it was contaminated with lead, according to the Times. Ms. Whitfeld developed an infection and temporary vision loss and has permanent scarring.
She has since put out a cautionary TikTok video that’s been viewed some 300,000 times.
TikTokkers have also flocked to the idea of using sunscreen to “contour” the face. Selected areas are left without sunscreen to burn or tan. In a duet, a plastic surgeon shakes his head as a young woman explains that “it works.”
Scalp-popping – in which the hair is yanked so hard that it pulls the galea off the skull – has been mostly shut down by TikTok. A search of “scalp popping” brings up the message: “Learn how to recognize harmful challenges and hoaxes.” At-home mole and skin tag removal, pimple-popping, and supposed acne cures such as drinking chlorophyll are all avidly documented and shared on TikTok.
Dr. Shah had a back-and-forth video dialog with someone who had stubbed a toe and then drilled a hole into the nail to drain the hematoma. In a reaction video, Dr. Shah said it was likely to turn into an infection. When it did, the man revealed the infection in a video where he tagged Dr. Shah and later posted a video at the podiatrist’s office having his nail removed, again tagging Dr. Shah.
“I think that pretty much no procedure for skin is good to do at home,” said Dr. Shah, who repeatedly admonishes against mole removal by a nonphysician. He tells followers that “it’s extremely dangerous – not only is it going to cause scarring, but you are potentially discarding a cancerous lesion.”
Unfortunately, most will not follow the advice, said Dr. Shah. That’s especially true of pimple-popping. Aiming for the least harm, he suggests in some TikTok videos that poppers keep the area clean, wear gloves, and consult a physician to get an antibiotic prescription. “You might as well at least guide them in the right direction,” he added.
Dr. Lee believes that lack of access to physicians, insurance, or money may play into how TikTok trends evolve. “Probably those people who injected their lips with this air gun thing, maybe they didn’t have the money necessarily to get filler,” she said.
Also, she noted, while TikTok may try to police its content, creators are incentivized to be outrageous. “The more inflammatory your post is, the more engagement you get.”
Dr. Shah thinks TikTok is self-correcting. “If you’re not being ethical or contradicting yourself, putting out information that’s not accurate, people are going to catch on very quickly,” he said. “The only value, the only currency you have on social media is the trust that you build with people that follow you.”
What it takes to be a TikTokker
For dermatologists, conveying their credentials and experience is one way to build that currency. Dr. Lee advised fellow doctors on TikTok to “showcase your training and how many years it took to become a dermatologist.”
Plunging into TikTok is not for everyone, though. It’s time consuming, said Dr. Lee, who now devotes most of her nonclinical time to TikTok. She creates her own content, leaving others to manage her Instagram account.
Many of those in the medical field who have dived into TikTok are residents, like Dr. Shah. “They are attuned to it and understand it more,” said Dr. Lee. “It’s harder for a lot of us who are older, who really weren’t involved that much in social media at all. It’s very hard to jump in.” There’s a learning curve, and it takes hours to create a single video. “You have to enjoy it and it has to be a part of your life,” she said.
Dr. Shah started experimenting with TikTok at the beginning of the pandemic in 2020 and has never turned back. Fast-talking, curious, and with an infectious sense of fun, he shares tidbits about his personal life – putting his wife in some of his videos – and always seems upbeat.
He said that, as his following grew, users began to see him as an authority figure and started “tagging” him more often, seeking his opinion on other videos. Although still a resident, he believes he has specialized knowledge to share. “Even if you’re not the world’s leading expert in a particular topic, you’re still adding value for the person who doesn’t know much.”
Dr. Shah also occasionally does promotional TikToks, identified as sponsored content. He said he only works with companies that he believes have legitimate products. “You do have to monetize at some point,” he said, noting that many dermatologists, himself included, are trading clinic time for TikTok. “There’s no universe where they can do this for free.”
Product endorsements are likely more rewarding for influencers and other users like Dr. Shah than the remuneration from TikTok, the company. The platform pays user accounts $20 per 1 million views, Dr. Shah said. “Financially, it’s not a big winner for a practicing dermatologist, but the educational outreach is worthwhile.”
To be successful also means understanding what drives viewership.
Using “trending” sounds has “been shown to increase the likelihood of a video amassing millions of views” and may increase engagement with dermatologists’ TikTok videos, wrote Bina Kassamali, BA, and colleagues at the Brigham and Women’s Hospital in Boston and the Ponce Health Science University School of Medicine in Ponce, Puerto Rico, in a letter published in the Journal of the American Academy of Dermatology in July 2021.
Certain content is more likely to engage viewers. In their analysis of top trending dermatologic hashtags, acne-related content was viewed 6.7 billion times, followed by alopecia, with 1.1 billion views. Psoriasis content had 84 million views, putting it eighth on the list of topics.
Dermatologists are still cracking TikTok. They are accumulating more followers on TikTok than on Instagram but have greater engagement on Instagram reels, wrote Mindy D. Szeto, MS, and colleagues at the University of Colorado at Denver, Aurora, and Rocky Vista University in Parker, Colo., in the Journal of the American Academy of Dermatology in April 2021.
Dr. Lee and Dr. Shah had the highest engagement rate on TikTok, according to Ms. Szeto. The engagement rate is calculated as (likes + comments per post)/(total followers) x 100.
“TikTok may currently be the leading avenue for audience education by dermatologist influencers,” they wrote, urging dermatologists to use the platform to answer the call as more of the public “continues to turn to social media for medical advice.”
Dr. Day said she will keep trying to build her TikTok audience. She has just 239 followers, compared with her 44,500 on Instagram. “The more I do TikTok, the more I do any of these mediums, the better I get at it,” she said. “We just have to put a little time and effort into it and try to get more followers and just keep sharing the information.”
Dr. Friedman sees it as a positive that some dermatologists have taken to TikTok to dispel myths and put “good information out there in small bites.” But to be more effective, they need more followers.
“The truth is that 14-year-old is probably going to listen more to a Hyram than a dermatologist,” he said. “Maybe we need to work with these other individuals who know how to take these messages and convert them to a language that can be digested by a 14-year-old, by a 12-year-old, by a 23-year-old. We need to come to the table together and not fight.”
A version of this article first appeared on Medscape.com.
iPLEDGE rollout described as a failure, chaotic, and a disaster
The that launched on Dec. 13, and what can be done to fix it.
By most accounts, the rollout was disastrous, chaotic, and a failure. Dermatologists on Twitter and elsewhere are angry and frustrated, with some calling for a temporary halt to the program until the bugs can be ironed out.
On Twitter Dec. 15, the Academy posted: “Due to the unacceptable situation with #iPLEDGE, the @US_FDA has convened an emergency meeting with AADA representatives tomorrow, December 16.”
The switch to a new platform was met with frustration from physicians, pharmacists, and patients alike. The new website crashed repeatedly, with physicians and patients complaining they got locked out or bounced off the platform when they attempted to follow instructions to enter information. Calls to obtain support from a live person often required hours on hold, several said.
The new approach to the isotretinoin risk-mitigation program itself isn’t under fire. It was welcomed by dermatologists and others who had long requested the change. Instead of three risk categories (females of reproductive potential, females not of reproductive potential, and males), there are now two (those who can get pregnant and those who cannot). Advocates for the change said it will make the experience more inclusive for transgender patients. The previous categories, some contended, were a barrier to access to care.
Because isotretinoin (Absorica, Amnesteem, Claravis, others), an oral retinoid used to treat severe forms of acne, is teratogenic, with a high risk of birth defects, and has also been associated with other health issues, those who take the medication who are able to get pregnant must take contraceptive precautions. The risk evaluation and mitigation program (REMS), mandated by the FDA, stipulates that physicians, patients, and pharmacists prescribing, using, or dispensing the drug must all be registered with requirements that include the use of two forms of an effective contraceptive and regular pregnancy tests by those capable of becoming pregnant.
A day of frustration
Before navigating the new website, a new log-on name was needed, said Ilona J. Frieden, MD, chair of the AADA’s iPLEDGE Workgroup and professor of dermatology at the University of California, San Francisco. “They made you create a month-day-year date of personal significance.” When she tried to log on, she got locked out, she said in an interview.
The transition from the old website to the new, which Dr. Frieden said is now administered by a different vendor, was done quickly. The previous website shut down Dec. 10, and the new one launched Dec. 13, the first day for the new approach.
“A slower rollout would have helped,” Dr. Frieden said. While she and other dermatologists said they offered input previously on how to make the transition go more smoothly, no one seemed to want that help. “We did have a listening session with the FDA,” Dr. Frieden said. That was before the scheduled meeting of Dec. 16.
Neil S. Goldberg, MD, a dermatologist in Westchester County, New York, also was frustrated with the rollout. “The week before the transition, one of my staff had to call iPLEDGE. They had a 177-minute wait to get to a human.
“They want us to register patients online now instead of signing forms in the office, but the links to view, download, or print don’t work,” Dr. Goldberg said in an interview.
This was after receiving information from the iPLEDGE REMS program, which stated, “The iPLEDGE REMS website will be updated to a modernized platform. All program materials and educational tools will be now available to you at the click of a button.’’
Dr. Goldberg also received calls from three patients who reported that they couldn’t complete the quiz that is required of patients capable of reproducing to demonstrate their comprehension about risk. Without the completed quiz, required monthly, the prescription can’t be refilled.
“It’s chaotic,” said Howa Yeung, MD, assistant professor of dermatology at Emory University, Atlanta. “The change is sudden, it’s a major change in the workflow. The process of reverification [required] is not that hard, but a lot of people have trouble even logging into the platform.”
What would help? To have a human on the phone to help navigate the system, Dr. Yeung said.
The glitches are delaying prescriptions for established patients and new ones as well, Dr. Yeung said. Existing patients who can get pregnant have 7 days after their negative pregnancy test to get their prescription filled. “And over the weekend the website was down,” he said, so that was a 2-day delay.
“The information we have and were told to use doesn’t match what is in their database,” said Mitesh Patel, PharmD, owner of Sunshine Pharmacy in White Plains, N.Y., who said pharmacists are experiencing issues with the new platform similar to those of doctors.
Twitter users had a lot to say, as well. Jack Resneck Jr., MD, professor of dermatology at the University of California, San Francisco, tweeted: “#Accutane has basically been pulled from market by utter incompetence of @SyneosHealth hired by @US_FDA to administer risk mgmt program.”
Dr. Resneck, president-elect of the American Medical Association, noted the crashed website, help line with 6-hour hold times, and patients unable to get the drug.
Adewole Adamson, MD, a dermatologist at the University of Texas, Austin, tweeted, “Dermatologists around the US are BIG mad about the current accutane debacle brought on by @SyneosHealth and @US_FDA. What a disaster for patient care!”
Several called for the FDA to immediately halt the program and let physicians manage the risk until the platform could be improved.
Are fixes in sight?
On Tuesday, Dec. 14, AADA President Kenneth J. Tomecki, MD, issued a statement expressing disappointment about the transition.
“In advance of this transition, the AADA engaged the FDA and the iPLEDGE administrator, Syneos Health, about the numerous workflow concerns raised by dermatologists and how the impending changes would threaten patient access to necessary medication. Those concerns have become a reality across the country and we’re working to ensure patients can maintain safe and appropriate access to the treatment they need.”
The AADA, the statement continues, supports efforts to streamline the program while keeping patient safety and incorporating input from physicians.
“We are very aware of the problems with the implementation of the iPLEDGE program,” FDA spokesperson Charlie Kohler said in an email. “We are continuing to work closely with the isotretinoin manufacturers to ensure that they implement a smoothly functioning iPLEDGE REMS program and that patient care is not interrupted.”
“Syneos Health appreciates the concern about iPLEDGE,” said Gary Gatyas, a spokesperson for Syneos Health. “While Syneos Health does not maintain the iPLEDGE system or contact center, we are doing what we can to help the responsible parties with a resolution.” Meanwhile, he recommended that people contact the call center.
He did not respond immediately to questions about who is responsible for maintaining the system and call center.
Dr. Goldberg, Dr. Frieden, and Dr. Yeung have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The that launched on Dec. 13, and what can be done to fix it.
By most accounts, the rollout was disastrous, chaotic, and a failure. Dermatologists on Twitter and elsewhere are angry and frustrated, with some calling for a temporary halt to the program until the bugs can be ironed out.
On Twitter Dec. 15, the Academy posted: “Due to the unacceptable situation with #iPLEDGE, the @US_FDA has convened an emergency meeting with AADA representatives tomorrow, December 16.”
The switch to a new platform was met with frustration from physicians, pharmacists, and patients alike. The new website crashed repeatedly, with physicians and patients complaining they got locked out or bounced off the platform when they attempted to follow instructions to enter information. Calls to obtain support from a live person often required hours on hold, several said.
The new approach to the isotretinoin risk-mitigation program itself isn’t under fire. It was welcomed by dermatologists and others who had long requested the change. Instead of three risk categories (females of reproductive potential, females not of reproductive potential, and males), there are now two (those who can get pregnant and those who cannot). Advocates for the change said it will make the experience more inclusive for transgender patients. The previous categories, some contended, were a barrier to access to care.
Because isotretinoin (Absorica, Amnesteem, Claravis, others), an oral retinoid used to treat severe forms of acne, is teratogenic, with a high risk of birth defects, and has also been associated with other health issues, those who take the medication who are able to get pregnant must take contraceptive precautions. The risk evaluation and mitigation program (REMS), mandated by the FDA, stipulates that physicians, patients, and pharmacists prescribing, using, or dispensing the drug must all be registered with requirements that include the use of two forms of an effective contraceptive and regular pregnancy tests by those capable of becoming pregnant.
A day of frustration
Before navigating the new website, a new log-on name was needed, said Ilona J. Frieden, MD, chair of the AADA’s iPLEDGE Workgroup and professor of dermatology at the University of California, San Francisco. “They made you create a month-day-year date of personal significance.” When she tried to log on, she got locked out, she said in an interview.
The transition from the old website to the new, which Dr. Frieden said is now administered by a different vendor, was done quickly. The previous website shut down Dec. 10, and the new one launched Dec. 13, the first day for the new approach.
“A slower rollout would have helped,” Dr. Frieden said. While she and other dermatologists said they offered input previously on how to make the transition go more smoothly, no one seemed to want that help. “We did have a listening session with the FDA,” Dr. Frieden said. That was before the scheduled meeting of Dec. 16.
Neil S. Goldberg, MD, a dermatologist in Westchester County, New York, also was frustrated with the rollout. “The week before the transition, one of my staff had to call iPLEDGE. They had a 177-minute wait to get to a human.
“They want us to register patients online now instead of signing forms in the office, but the links to view, download, or print don’t work,” Dr. Goldberg said in an interview.
This was after receiving information from the iPLEDGE REMS program, which stated, “The iPLEDGE REMS website will be updated to a modernized platform. All program materials and educational tools will be now available to you at the click of a button.’’
Dr. Goldberg also received calls from three patients who reported that they couldn’t complete the quiz that is required of patients capable of reproducing to demonstrate their comprehension about risk. Without the completed quiz, required monthly, the prescription can’t be refilled.
“It’s chaotic,” said Howa Yeung, MD, assistant professor of dermatology at Emory University, Atlanta. “The change is sudden, it’s a major change in the workflow. The process of reverification [required] is not that hard, but a lot of people have trouble even logging into the platform.”
What would help? To have a human on the phone to help navigate the system, Dr. Yeung said.
The glitches are delaying prescriptions for established patients and new ones as well, Dr. Yeung said. Existing patients who can get pregnant have 7 days after their negative pregnancy test to get their prescription filled. “And over the weekend the website was down,” he said, so that was a 2-day delay.
“The information we have and were told to use doesn’t match what is in their database,” said Mitesh Patel, PharmD, owner of Sunshine Pharmacy in White Plains, N.Y., who said pharmacists are experiencing issues with the new platform similar to those of doctors.
Twitter users had a lot to say, as well. Jack Resneck Jr., MD, professor of dermatology at the University of California, San Francisco, tweeted: “#Accutane has basically been pulled from market by utter incompetence of @SyneosHealth hired by @US_FDA to administer risk mgmt program.”
Dr. Resneck, president-elect of the American Medical Association, noted the crashed website, help line with 6-hour hold times, and patients unable to get the drug.
Adewole Adamson, MD, a dermatologist at the University of Texas, Austin, tweeted, “Dermatologists around the US are BIG mad about the current accutane debacle brought on by @SyneosHealth and @US_FDA. What a disaster for patient care!”
Several called for the FDA to immediately halt the program and let physicians manage the risk until the platform could be improved.
Are fixes in sight?
On Tuesday, Dec. 14, AADA President Kenneth J. Tomecki, MD, issued a statement expressing disappointment about the transition.
“In advance of this transition, the AADA engaged the FDA and the iPLEDGE administrator, Syneos Health, about the numerous workflow concerns raised by dermatologists and how the impending changes would threaten patient access to necessary medication. Those concerns have become a reality across the country and we’re working to ensure patients can maintain safe and appropriate access to the treatment they need.”
The AADA, the statement continues, supports efforts to streamline the program while keeping patient safety and incorporating input from physicians.
“We are very aware of the problems with the implementation of the iPLEDGE program,” FDA spokesperson Charlie Kohler said in an email. “We are continuing to work closely with the isotretinoin manufacturers to ensure that they implement a smoothly functioning iPLEDGE REMS program and that patient care is not interrupted.”
“Syneos Health appreciates the concern about iPLEDGE,” said Gary Gatyas, a spokesperson for Syneos Health. “While Syneos Health does not maintain the iPLEDGE system or contact center, we are doing what we can to help the responsible parties with a resolution.” Meanwhile, he recommended that people contact the call center.
He did not respond immediately to questions about who is responsible for maintaining the system and call center.
Dr. Goldberg, Dr. Frieden, and Dr. Yeung have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The that launched on Dec. 13, and what can be done to fix it.
By most accounts, the rollout was disastrous, chaotic, and a failure. Dermatologists on Twitter and elsewhere are angry and frustrated, with some calling for a temporary halt to the program until the bugs can be ironed out.
On Twitter Dec. 15, the Academy posted: “Due to the unacceptable situation with #iPLEDGE, the @US_FDA has convened an emergency meeting with AADA representatives tomorrow, December 16.”
The switch to a new platform was met with frustration from physicians, pharmacists, and patients alike. The new website crashed repeatedly, with physicians and patients complaining they got locked out or bounced off the platform when they attempted to follow instructions to enter information. Calls to obtain support from a live person often required hours on hold, several said.
The new approach to the isotretinoin risk-mitigation program itself isn’t under fire. It was welcomed by dermatologists and others who had long requested the change. Instead of three risk categories (females of reproductive potential, females not of reproductive potential, and males), there are now two (those who can get pregnant and those who cannot). Advocates for the change said it will make the experience more inclusive for transgender patients. The previous categories, some contended, were a barrier to access to care.
Because isotretinoin (Absorica, Amnesteem, Claravis, others), an oral retinoid used to treat severe forms of acne, is teratogenic, with a high risk of birth defects, and has also been associated with other health issues, those who take the medication who are able to get pregnant must take contraceptive precautions. The risk evaluation and mitigation program (REMS), mandated by the FDA, stipulates that physicians, patients, and pharmacists prescribing, using, or dispensing the drug must all be registered with requirements that include the use of two forms of an effective contraceptive and regular pregnancy tests by those capable of becoming pregnant.
A day of frustration
Before navigating the new website, a new log-on name was needed, said Ilona J. Frieden, MD, chair of the AADA’s iPLEDGE Workgroup and professor of dermatology at the University of California, San Francisco. “They made you create a month-day-year date of personal significance.” When she tried to log on, she got locked out, she said in an interview.
The transition from the old website to the new, which Dr. Frieden said is now administered by a different vendor, was done quickly. The previous website shut down Dec. 10, and the new one launched Dec. 13, the first day for the new approach.
“A slower rollout would have helped,” Dr. Frieden said. While she and other dermatologists said they offered input previously on how to make the transition go more smoothly, no one seemed to want that help. “We did have a listening session with the FDA,” Dr. Frieden said. That was before the scheduled meeting of Dec. 16.
Neil S. Goldberg, MD, a dermatologist in Westchester County, New York, also was frustrated with the rollout. “The week before the transition, one of my staff had to call iPLEDGE. They had a 177-minute wait to get to a human.
“They want us to register patients online now instead of signing forms in the office, but the links to view, download, or print don’t work,” Dr. Goldberg said in an interview.
This was after receiving information from the iPLEDGE REMS program, which stated, “The iPLEDGE REMS website will be updated to a modernized platform. All program materials and educational tools will be now available to you at the click of a button.’’
Dr. Goldberg also received calls from three patients who reported that they couldn’t complete the quiz that is required of patients capable of reproducing to demonstrate their comprehension about risk. Without the completed quiz, required monthly, the prescription can’t be refilled.
“It’s chaotic,” said Howa Yeung, MD, assistant professor of dermatology at Emory University, Atlanta. “The change is sudden, it’s a major change in the workflow. The process of reverification [required] is not that hard, but a lot of people have trouble even logging into the platform.”
What would help? To have a human on the phone to help navigate the system, Dr. Yeung said.
The glitches are delaying prescriptions for established patients and new ones as well, Dr. Yeung said. Existing patients who can get pregnant have 7 days after their negative pregnancy test to get their prescription filled. “And over the weekend the website was down,” he said, so that was a 2-day delay.
“The information we have and were told to use doesn’t match what is in their database,” said Mitesh Patel, PharmD, owner of Sunshine Pharmacy in White Plains, N.Y., who said pharmacists are experiencing issues with the new platform similar to those of doctors.
Twitter users had a lot to say, as well. Jack Resneck Jr., MD, professor of dermatology at the University of California, San Francisco, tweeted: “#Accutane has basically been pulled from market by utter incompetence of @SyneosHealth hired by @US_FDA to administer risk mgmt program.”
Dr. Resneck, president-elect of the American Medical Association, noted the crashed website, help line with 6-hour hold times, and patients unable to get the drug.
Adewole Adamson, MD, a dermatologist at the University of Texas, Austin, tweeted, “Dermatologists around the US are BIG mad about the current accutane debacle brought on by @SyneosHealth and @US_FDA. What a disaster for patient care!”
Several called for the FDA to immediately halt the program and let physicians manage the risk until the platform could be improved.
Are fixes in sight?
On Tuesday, Dec. 14, AADA President Kenneth J. Tomecki, MD, issued a statement expressing disappointment about the transition.
“In advance of this transition, the AADA engaged the FDA and the iPLEDGE administrator, Syneos Health, about the numerous workflow concerns raised by dermatologists and how the impending changes would threaten patient access to necessary medication. Those concerns have become a reality across the country and we’re working to ensure patients can maintain safe and appropriate access to the treatment they need.”
The AADA, the statement continues, supports efforts to streamline the program while keeping patient safety and incorporating input from physicians.
“We are very aware of the problems with the implementation of the iPLEDGE program,” FDA spokesperson Charlie Kohler said in an email. “We are continuing to work closely with the isotretinoin manufacturers to ensure that they implement a smoothly functioning iPLEDGE REMS program and that patient care is not interrupted.”
“Syneos Health appreciates the concern about iPLEDGE,” said Gary Gatyas, a spokesperson for Syneos Health. “While Syneos Health does not maintain the iPLEDGE system or contact center, we are doing what we can to help the responsible parties with a resolution.” Meanwhile, he recommended that people contact the call center.
He did not respond immediately to questions about who is responsible for maintaining the system and call center.
Dr. Goldberg, Dr. Frieden, and Dr. Yeung have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
International panel backs energy-based devices as first-line treatment of acne scars
International consensus .
Peter R. Shumaker, MD, a dermatologist and dermatologic surgeon at the VA San Diego Healthcare System and one of the authors of the paper, noted that a panel of 24 international experts in dermatology and plastic surgery assembled to develop the recommendations for integrating EBDs into the management of acne scarring.
“The advent of fractional laser technology in the mid-2000s ushered in an exciting new period of exploration and advances in scar treatment with EBDs,” Dr. Shumaker said in an interview. “Despite intense interest and a wealth of available literature, international treatment guidelines and patient access to these potentially life-changing treatments are currently lagging behind our capabilities.”
One of the key recommendations of the paper is that EBDs should have an expanded role in the treatment of acne scars, according to Dr. Shumaker, associate clinical professor of dermatology at the University of California, San Diego. “Panel members were unanimous in their view that EBDs, particularly ablative and nonablative fractional lasers, vascular lasers, and fractional radiofrequency devices, have an important role in the management of acne scars and should be considered a first-line treatment for a variety of scar types,” he said.
The process leading to the recommendations included developing clinical questions, based on input from the panelists and a literature review, and using a two-step modified Delphi method, “an iterative process used to achieve consensus for a defined clinical problem where there is little or conflicting published evidence and where expert opinion is decisive,” the authors wrote. This involved email questionnaires highlighting different topics, including the role of EBDs in mitigating and treating acne scars in patients with active acne, the use of different EBDs for treating different types of acne scars, and considerations in treating skin of color.
The panel noted considerations in the treatment of acne scars in skin of color. “Regardless of the platform, patients with darker skin types may require treatment modifications including: a reduction in fluence/pulse energy; decreased microcolumn density; greater intervals between treatments; longer pulse durations; epidermal cooling with fastidious technique to ensure appropriate cooling, additional cooling in between passes to decrease bulk heating; and pretreatment and posttreatment topical regimens (e.g., retinoids, bleaching creams, etc.) and strict sun precautions,” wrote the authors.
Panelists agreed that there is an absence of large, well-controlled, multicenter comparative trials of various laser and energy treatments for acne scars. “Such trials would be helpful in establishing the relative utility and persistence of benefit of various laser treatments and also in comparing their effectiveness versus that of nonenergy treatments,” the authors noted.
Asked to comment on the paper, Andrei Metelitsa, MD, a dermatologist in Calgary, Alta., and clinical associate professor at the University of Calgary, said the consensus recommendations on EBDs in acne scarring are “providing an international expert perspective, potentially changing a long-perceived paradigm of treatments.”
Dr. Metelitsa pointed out that the authors are taking a solid position with respect to reducing the delay to initiation of laser treatment following isotretinoin therapy. “The authors take a strong stance against the old dogma of postponing laser resurfacing for at least 6 months post isotretinoin,” he said. “According to the authors, there is sufficient evidence to support the idea of safely starting laser therapies, including fractional ablative and nonablative, within 1 month post isotretinoin, much sooner than previously suggested.”
He added that the authors point to the fact most experts utilize vascular lasers, such as pulsed-dye, to treat active acne in combination with medical therapy, thus reducing duration and severity of inflammation and potentially reducing further scar formation. “According to this published consensus, such laser therapies can even be used while the patient is actively treated with isotretinoin,” he said.
Dr. Metelitsa noted that the consensus recommendations outline how the choice of device should be guided by the clinical subtype of acne scars.
Dr. Shumaker, Dr. Metelitsa, and the authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
International consensus .
Peter R. Shumaker, MD, a dermatologist and dermatologic surgeon at the VA San Diego Healthcare System and one of the authors of the paper, noted that a panel of 24 international experts in dermatology and plastic surgery assembled to develop the recommendations for integrating EBDs into the management of acne scarring.
“The advent of fractional laser technology in the mid-2000s ushered in an exciting new period of exploration and advances in scar treatment with EBDs,” Dr. Shumaker said in an interview. “Despite intense interest and a wealth of available literature, international treatment guidelines and patient access to these potentially life-changing treatments are currently lagging behind our capabilities.”
One of the key recommendations of the paper is that EBDs should have an expanded role in the treatment of acne scars, according to Dr. Shumaker, associate clinical professor of dermatology at the University of California, San Diego. “Panel members were unanimous in their view that EBDs, particularly ablative and nonablative fractional lasers, vascular lasers, and fractional radiofrequency devices, have an important role in the management of acne scars and should be considered a first-line treatment for a variety of scar types,” he said.
The process leading to the recommendations included developing clinical questions, based on input from the panelists and a literature review, and using a two-step modified Delphi method, “an iterative process used to achieve consensus for a defined clinical problem where there is little or conflicting published evidence and where expert opinion is decisive,” the authors wrote. This involved email questionnaires highlighting different topics, including the role of EBDs in mitigating and treating acne scars in patients with active acne, the use of different EBDs for treating different types of acne scars, and considerations in treating skin of color.
The panel noted considerations in the treatment of acne scars in skin of color. “Regardless of the platform, patients with darker skin types may require treatment modifications including: a reduction in fluence/pulse energy; decreased microcolumn density; greater intervals between treatments; longer pulse durations; epidermal cooling with fastidious technique to ensure appropriate cooling, additional cooling in between passes to decrease bulk heating; and pretreatment and posttreatment topical regimens (e.g., retinoids, bleaching creams, etc.) and strict sun precautions,” wrote the authors.
Panelists agreed that there is an absence of large, well-controlled, multicenter comparative trials of various laser and energy treatments for acne scars. “Such trials would be helpful in establishing the relative utility and persistence of benefit of various laser treatments and also in comparing their effectiveness versus that of nonenergy treatments,” the authors noted.
Asked to comment on the paper, Andrei Metelitsa, MD, a dermatologist in Calgary, Alta., and clinical associate professor at the University of Calgary, said the consensus recommendations on EBDs in acne scarring are “providing an international expert perspective, potentially changing a long-perceived paradigm of treatments.”
Dr. Metelitsa pointed out that the authors are taking a solid position with respect to reducing the delay to initiation of laser treatment following isotretinoin therapy. “The authors take a strong stance against the old dogma of postponing laser resurfacing for at least 6 months post isotretinoin,” he said. “According to the authors, there is sufficient evidence to support the idea of safely starting laser therapies, including fractional ablative and nonablative, within 1 month post isotretinoin, much sooner than previously suggested.”
He added that the authors point to the fact most experts utilize vascular lasers, such as pulsed-dye, to treat active acne in combination with medical therapy, thus reducing duration and severity of inflammation and potentially reducing further scar formation. “According to this published consensus, such laser therapies can even be used while the patient is actively treated with isotretinoin,” he said.
Dr. Metelitsa noted that the consensus recommendations outline how the choice of device should be guided by the clinical subtype of acne scars.
Dr. Shumaker, Dr. Metelitsa, and the authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
International consensus .
Peter R. Shumaker, MD, a dermatologist and dermatologic surgeon at the VA San Diego Healthcare System and one of the authors of the paper, noted that a panel of 24 international experts in dermatology and plastic surgery assembled to develop the recommendations for integrating EBDs into the management of acne scarring.
“The advent of fractional laser technology in the mid-2000s ushered in an exciting new period of exploration and advances in scar treatment with EBDs,” Dr. Shumaker said in an interview. “Despite intense interest and a wealth of available literature, international treatment guidelines and patient access to these potentially life-changing treatments are currently lagging behind our capabilities.”
One of the key recommendations of the paper is that EBDs should have an expanded role in the treatment of acne scars, according to Dr. Shumaker, associate clinical professor of dermatology at the University of California, San Diego. “Panel members were unanimous in their view that EBDs, particularly ablative and nonablative fractional lasers, vascular lasers, and fractional radiofrequency devices, have an important role in the management of acne scars and should be considered a first-line treatment for a variety of scar types,” he said.
The process leading to the recommendations included developing clinical questions, based on input from the panelists and a literature review, and using a two-step modified Delphi method, “an iterative process used to achieve consensus for a defined clinical problem where there is little or conflicting published evidence and where expert opinion is decisive,” the authors wrote. This involved email questionnaires highlighting different topics, including the role of EBDs in mitigating and treating acne scars in patients with active acne, the use of different EBDs for treating different types of acne scars, and considerations in treating skin of color.
The panel noted considerations in the treatment of acne scars in skin of color. “Regardless of the platform, patients with darker skin types may require treatment modifications including: a reduction in fluence/pulse energy; decreased microcolumn density; greater intervals between treatments; longer pulse durations; epidermal cooling with fastidious technique to ensure appropriate cooling, additional cooling in between passes to decrease bulk heating; and pretreatment and posttreatment topical regimens (e.g., retinoids, bleaching creams, etc.) and strict sun precautions,” wrote the authors.
Panelists agreed that there is an absence of large, well-controlled, multicenter comparative trials of various laser and energy treatments for acne scars. “Such trials would be helpful in establishing the relative utility and persistence of benefit of various laser treatments and also in comparing their effectiveness versus that of nonenergy treatments,” the authors noted.
Asked to comment on the paper, Andrei Metelitsa, MD, a dermatologist in Calgary, Alta., and clinical associate professor at the University of Calgary, said the consensus recommendations on EBDs in acne scarring are “providing an international expert perspective, potentially changing a long-perceived paradigm of treatments.”
Dr. Metelitsa pointed out that the authors are taking a solid position with respect to reducing the delay to initiation of laser treatment following isotretinoin therapy. “The authors take a strong stance against the old dogma of postponing laser resurfacing for at least 6 months post isotretinoin,” he said. “According to the authors, there is sufficient evidence to support the idea of safely starting laser therapies, including fractional ablative and nonablative, within 1 month post isotretinoin, much sooner than previously suggested.”
He added that the authors point to the fact most experts utilize vascular lasers, such as pulsed-dye, to treat active acne in combination with medical therapy, thus reducing duration and severity of inflammation and potentially reducing further scar formation. “According to this published consensus, such laser therapies can even be used while the patient is actively treated with isotretinoin,” he said.
Dr. Metelitsa noted that the consensus recommendations outline how the choice of device should be guided by the clinical subtype of acne scars.
Dr. Shumaker, Dr. Metelitsa, and the authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Acute Severe Urticaria From Minocycline
To the Editor:
Minocycline is a commonly prescribed semisynthetic tetracycline derivative used for long-term treatment of acne vulgaris.1 Given the continued popularity of minocycline and other tetracyclines in treating acne, more adverse side effects are being reported. We report a patient who experienced acute severe urticaria with angioedema from minocycline.
A 35-year-old woman with a history of acne vulgaris presented to the emergency department with urticaria and associated angioedema. Fifteen days after starting minocycline, she awoke with diffuse hives sparing only the abdomen that resolved with diphenhydramine. Later that day, she developed generalized pruritus, hives, and lip swelling. She received intravenous methylprednisolone, diphenhydramine, and famotidine in the emergency department. She returned to the emergency department the next day due to facial and lip swelling, diffuse urticaria that was most pronounced on the arms, and throat irritation. Intramuscular epinephrine was administered first followed by methylprednisolone, famotidine, and cetirizine. She was discharged and advised to start daily prednisone 50 mg and cetirizine 20 mg every evening.
She returned to the emergency department the following morning due to worsening generalized urticaria and angioedema of the lips. She denied any associated respiratory, joint, or gastrointestinal tract symptoms. She had several urticarial plaques on the scalp, face, and body (Figure), only sparing the abdomen. Her hives were erythematous, raised, pruritic, and blanching. There was no residual purpura, ecchymosis, or hyperpigmentation associated with the urticaria, and each lesion was present for less than 24 hours. There was no swelling on examination. Additionally, she was afebrile. The C4 level was 18 mg/dL (reference range, 15–45 mg/dL). She did not develop eosinophilia (absolute eosinophil count, 0/µL [reference range, 50–500/µL]), lymphocytosis (absolute lymphocyte count, 1300/µL [reference range, 1000–4800/µL]), or abnormal liver or renal function. She was hospitalized for 3 days with severe urticaria and required 7 days of prednisone 40 to 50 mg, fexofenadine 360 mg, and cetirizine 20 mg. A viral infection was considered as a possible etiology; however, she had no supporting signs or symptoms of an upper respiratory illness or other viral illness.
The patient’s minocycline use was considered the most likely etiology, as an oral contraceptive was the only other medication. She was labelled allergic to minocycline and discharged with intramuscular epinephrine. She was evaluated in the outpatient allergy immunology clinic 9 days later, and all her symptoms had resolved. Due to the severity of our patient’s reaction and the possibility of further severe reactions, an oral challenge was not carried out. Our patient was not interested in pursuing any further minocycline or other tetracycline-based therapy for her acne. She also was not interested in pursuing any minocycline skin-prick testing or oral challenge. One limitation to this case is our patient declining a confirmatory drug challenge; however, given the severity of the symptoms, the physicians involved agreed the patient's safety outweighed the benefits of confirmatory testing.
A PubMed search of articles indexed for MEDLINE and a Google Scholar search using the terms minocycline, drug hypersensitivity, urticaria, anaphylaxis, minocycline allergy, and angioedema yielded only 16 articles and correspondences. Reported adverse effects of minocycline included drug-induced lupus erythematosus, vasculitis, nausea, photosensitivity, and DRESS-like (drug reaction with eosinophilia and systemic symptoms syndrome) conditions. Three case reports of anaphylaxis/anaphylactoid reactions have been published,2-4 but cases of urticaria attributable to minocycline appear to be exceedingly rare.2,3 Reports of serum sickness in patients aged 15 to 62 years were rare. Women were noted to experience a higher frequency of adverse effects compared to men.5 Symptoms typically presented 3 to 28 days after initiation of minocycline. Data currently suggest that the pathogenesis of hypersensitivity reactions to minocycline remains unknown6; however, one hypothesis is that minocycline or its metabolites act as a superantigen, resulting in lymphocyte overactivation and massive cytokine release.7
Minocycline generally is well tolerated by patients. Physicians should be aware that minocycline is a possible causative agent of allergic drug reactions. Our patient’s presentation of severe acute urticaria with angioedema of the face and lips is a rarity.
- Levenson T, Masood D, Patterson R. Minocycline-induced serum sickness. Allergy Asthma Proc. 1996;17:79-81.
- Okano M, Imai S. Anaphylactoid symptoms due to oral minocycline. Acta Derm Venereol. 1996;76:164.
- Jang JW, Bae Y-J, Kim YG, et al. A case of anaphylaxis to oral minocycline. J Korean Med Sci. 2010;25:1233.
- Nakamura R, Tanaka A, Kinoshita H, et al. Minocycline-induced anaphylaxis mediated by antigen-specific immunoglobulin E [published online November 9, 2021]. J Dermatol. doi:10.1111/1346-8138.16228
- MacNeil M, Haase DA, Tremaine R, et al. Fever, lymphadenopathy, eosinophilia, lymphocytosis, hepatitis, and dermatitis: a severe adverse reaction to minocycline. J Am Acad Dermatol. 1997;36:347-350.
- DePaz S, Perez A, Gomez M, et al. Severe hypersensitivity reaction to minocycline. J Invest Allergol Clin Immunol. 1999;9:403-404.
- Somech R, Arav-Boger R, Assia A, et al. Complications of minocycline therapy for acne vulgaris: case reports and review of the literature. Pediatr Dermatol. 1999;16:469-472.
To the Editor:
Minocycline is a commonly prescribed semisynthetic tetracycline derivative used for long-term treatment of acne vulgaris.1 Given the continued popularity of minocycline and other tetracyclines in treating acne, more adverse side effects are being reported. We report a patient who experienced acute severe urticaria with angioedema from minocycline.
A 35-year-old woman with a history of acne vulgaris presented to the emergency department with urticaria and associated angioedema. Fifteen days after starting minocycline, she awoke with diffuse hives sparing only the abdomen that resolved with diphenhydramine. Later that day, she developed generalized pruritus, hives, and lip swelling. She received intravenous methylprednisolone, diphenhydramine, and famotidine in the emergency department. She returned to the emergency department the next day due to facial and lip swelling, diffuse urticaria that was most pronounced on the arms, and throat irritation. Intramuscular epinephrine was administered first followed by methylprednisolone, famotidine, and cetirizine. She was discharged and advised to start daily prednisone 50 mg and cetirizine 20 mg every evening.
She returned to the emergency department the following morning due to worsening generalized urticaria and angioedema of the lips. She denied any associated respiratory, joint, or gastrointestinal tract symptoms. She had several urticarial plaques on the scalp, face, and body (Figure), only sparing the abdomen. Her hives were erythematous, raised, pruritic, and blanching. There was no residual purpura, ecchymosis, or hyperpigmentation associated with the urticaria, and each lesion was present for less than 24 hours. There was no swelling on examination. Additionally, she was afebrile. The C4 level was 18 mg/dL (reference range, 15–45 mg/dL). She did not develop eosinophilia (absolute eosinophil count, 0/µL [reference range, 50–500/µL]), lymphocytosis (absolute lymphocyte count, 1300/µL [reference range, 1000–4800/µL]), or abnormal liver or renal function. She was hospitalized for 3 days with severe urticaria and required 7 days of prednisone 40 to 50 mg, fexofenadine 360 mg, and cetirizine 20 mg. A viral infection was considered as a possible etiology; however, she had no supporting signs or symptoms of an upper respiratory illness or other viral illness.

The patient’s minocycline use was considered the most likely etiology, as an oral contraceptive was the only other medication. She was labelled allergic to minocycline and discharged with intramuscular epinephrine. She was evaluated in the outpatient allergy immunology clinic 9 days later, and all her symptoms had resolved. Due to the severity of our patient’s reaction and the possibility of further severe reactions, an oral challenge was not carried out. Our patient was not interested in pursuing any further minocycline or other tetracycline-based therapy for her acne. She also was not interested in pursuing any minocycline skin-prick testing or oral challenge. One limitation to this case is our patient declining a confirmatory drug challenge; however, given the severity of the symptoms, the physicians involved agreed the patient's safety outweighed the benefits of confirmatory testing.
A PubMed search of articles indexed for MEDLINE and a Google Scholar search using the terms minocycline, drug hypersensitivity, urticaria, anaphylaxis, minocycline allergy, and angioedema yielded only 16 articles and correspondences. Reported adverse effects of minocycline included drug-induced lupus erythematosus, vasculitis, nausea, photosensitivity, and DRESS-like (drug reaction with eosinophilia and systemic symptoms syndrome) conditions. Three case reports of anaphylaxis/anaphylactoid reactions have been published,2-4 but cases of urticaria attributable to minocycline appear to be exceedingly rare.2,3 Reports of serum sickness in patients aged 15 to 62 years were rare. Women were noted to experience a higher frequency of adverse effects compared to men.5 Symptoms typically presented 3 to 28 days after initiation of minocycline. Data currently suggest that the pathogenesis of hypersensitivity reactions to minocycline remains unknown6; however, one hypothesis is that minocycline or its metabolites act as a superantigen, resulting in lymphocyte overactivation and massive cytokine release.7
Minocycline generally is well tolerated by patients. Physicians should be aware that minocycline is a possible causative agent of allergic drug reactions. Our patient’s presentation of severe acute urticaria with angioedema of the face and lips is a rarity.
To the Editor:
Minocycline is a commonly prescribed semisynthetic tetracycline derivative used for long-term treatment of acne vulgaris.1 Given the continued popularity of minocycline and other tetracyclines in treating acne, more adverse side effects are being reported. We report a patient who experienced acute severe urticaria with angioedema from minocycline.
A 35-year-old woman with a history of acne vulgaris presented to the emergency department with urticaria and associated angioedema. Fifteen days after starting minocycline, she awoke with diffuse hives sparing only the abdomen that resolved with diphenhydramine. Later that day, she developed generalized pruritus, hives, and lip swelling. She received intravenous methylprednisolone, diphenhydramine, and famotidine in the emergency department. She returned to the emergency department the next day due to facial and lip swelling, diffuse urticaria that was most pronounced on the arms, and throat irritation. Intramuscular epinephrine was administered first followed by methylprednisolone, famotidine, and cetirizine. She was discharged and advised to start daily prednisone 50 mg and cetirizine 20 mg every evening.
She returned to the emergency department the following morning due to worsening generalized urticaria and angioedema of the lips. She denied any associated respiratory, joint, or gastrointestinal tract symptoms. She had several urticarial plaques on the scalp, face, and body (Figure), only sparing the abdomen. Her hives were erythematous, raised, pruritic, and blanching. There was no residual purpura, ecchymosis, or hyperpigmentation associated with the urticaria, and each lesion was present for less than 24 hours. There was no swelling on examination. Additionally, she was afebrile. The C4 level was 18 mg/dL (reference range, 15–45 mg/dL). She did not develop eosinophilia (absolute eosinophil count, 0/µL [reference range, 50–500/µL]), lymphocytosis (absolute lymphocyte count, 1300/µL [reference range, 1000–4800/µL]), or abnormal liver or renal function. She was hospitalized for 3 days with severe urticaria and required 7 days of prednisone 40 to 50 mg, fexofenadine 360 mg, and cetirizine 20 mg. A viral infection was considered as a possible etiology; however, she had no supporting signs or symptoms of an upper respiratory illness or other viral illness.

The patient’s minocycline use was considered the most likely etiology, as an oral contraceptive was the only other medication. She was labelled allergic to minocycline and discharged with intramuscular epinephrine. She was evaluated in the outpatient allergy immunology clinic 9 days later, and all her symptoms had resolved. Due to the severity of our patient’s reaction and the possibility of further severe reactions, an oral challenge was not carried out. Our patient was not interested in pursuing any further minocycline or other tetracycline-based therapy for her acne. She also was not interested in pursuing any minocycline skin-prick testing or oral challenge. One limitation to this case is our patient declining a confirmatory drug challenge; however, given the severity of the symptoms, the physicians involved agreed the patient's safety outweighed the benefits of confirmatory testing.
A PubMed search of articles indexed for MEDLINE and a Google Scholar search using the terms minocycline, drug hypersensitivity, urticaria, anaphylaxis, minocycline allergy, and angioedema yielded only 16 articles and correspondences. Reported adverse effects of minocycline included drug-induced lupus erythematosus, vasculitis, nausea, photosensitivity, and DRESS-like (drug reaction with eosinophilia and systemic symptoms syndrome) conditions. Three case reports of anaphylaxis/anaphylactoid reactions have been published,2-4 but cases of urticaria attributable to minocycline appear to be exceedingly rare.2,3 Reports of serum sickness in patients aged 15 to 62 years were rare. Women were noted to experience a higher frequency of adverse effects compared to men.5 Symptoms typically presented 3 to 28 days after initiation of minocycline. Data currently suggest that the pathogenesis of hypersensitivity reactions to minocycline remains unknown6; however, one hypothesis is that minocycline or its metabolites act as a superantigen, resulting in lymphocyte overactivation and massive cytokine release.7
Minocycline generally is well tolerated by patients. Physicians should be aware that minocycline is a possible causative agent of allergic drug reactions. Our patient’s presentation of severe acute urticaria with angioedema of the face and lips is a rarity.
- Levenson T, Masood D, Patterson R. Minocycline-induced serum sickness. Allergy Asthma Proc. 1996;17:79-81.
- Okano M, Imai S. Anaphylactoid symptoms due to oral minocycline. Acta Derm Venereol. 1996;76:164.
- Jang JW, Bae Y-J, Kim YG, et al. A case of anaphylaxis to oral minocycline. J Korean Med Sci. 2010;25:1233.
- Nakamura R, Tanaka A, Kinoshita H, et al. Minocycline-induced anaphylaxis mediated by antigen-specific immunoglobulin E [published online November 9, 2021]. J Dermatol. doi:10.1111/1346-8138.16228
- MacNeil M, Haase DA, Tremaine R, et al. Fever, lymphadenopathy, eosinophilia, lymphocytosis, hepatitis, and dermatitis: a severe adverse reaction to minocycline. J Am Acad Dermatol. 1997;36:347-350.
- DePaz S, Perez A, Gomez M, et al. Severe hypersensitivity reaction to minocycline. J Invest Allergol Clin Immunol. 1999;9:403-404.
- Somech R, Arav-Boger R, Assia A, et al. Complications of minocycline therapy for acne vulgaris: case reports and review of the literature. Pediatr Dermatol. 1999;16:469-472.
- Levenson T, Masood D, Patterson R. Minocycline-induced serum sickness. Allergy Asthma Proc. 1996;17:79-81.
- Okano M, Imai S. Anaphylactoid symptoms due to oral minocycline. Acta Derm Venereol. 1996;76:164.
- Jang JW, Bae Y-J, Kim YG, et al. A case of anaphylaxis to oral minocycline. J Korean Med Sci. 2010;25:1233.
- Nakamura R, Tanaka A, Kinoshita H, et al. Minocycline-induced anaphylaxis mediated by antigen-specific immunoglobulin E [published online November 9, 2021]. J Dermatol. doi:10.1111/1346-8138.16228
- MacNeil M, Haase DA, Tremaine R, et al. Fever, lymphadenopathy, eosinophilia, lymphocytosis, hepatitis, and dermatitis: a severe adverse reaction to minocycline. J Am Acad Dermatol. 1997;36:347-350.
- DePaz S, Perez A, Gomez M, et al. Severe hypersensitivity reaction to minocycline. J Invest Allergol Clin Immunol. 1999;9:403-404.
- Somech R, Arav-Boger R, Assia A, et al. Complications of minocycline therapy for acne vulgaris: case reports and review of the literature. Pediatr Dermatol. 1999;16:469-472.
Practice Points
- Minocycline is a commonly prescribed long-term treatment for acne vulgaris.
- Minocycline-induced acute urticaria and anaphylaxis are rare adverse events.
Topical options for acne patients continue to expand
yet they are likely underused in today’s clinical practice.
A study of prescribing practices from 2012 to 2014 indicated that dermatologists prescribed retinoids for just 58.8% of acne cases, while nondermatologists prescribed them for only 32.4% of cases. “If the guidelines are telling us that we should use topical retinoids for almost all of our acne patients, why are we using them for half of the patients?” Emmy Graber, MD, MBA, asked during MedscapeLive’s annual Las Vegas Dermatology Seminar. “We have a lot of options today for topical retinoids,” she added, noting that, in the past few years, trifarotene cream 0.005% and new formulations of tazarotene lotion (0.045%) and tretinoin lotion (0.05%) have become available.
According to Dr. Graber, president of The Dermatology Institute of Boston, tazarotene has been considered the most efficacious topical retinoid but is generally the least well tolerated, while adapalene has often been considered to be one of the better-tolerated topical retinoids. “This is a broad generalization,” she said. “One should also take into account the concentration and formulation of the retinoid. Cutaneous adverse events increase in severity as the concentration increases regardless of the vehicle.” There are no studies comparing trifarotene with other topical retinoids, she added.
In two phase 2, double-blind, vehicle-controlled studies (PERFECT 1 and PERFECT 2), researchers randomized more than 2,400 patients with moderate facial or truncal acne to receive trifarotene cream or a vehicle for 12 weeks. The mean percent change from baseline in facial inflammatory lesions in the trifarotene-treated group was –54.4% and –66.2% in PERFECT 1, and PERFECT 2, respectively, while the mean percent change from baseline in facial noninflammatory lesions was –49.7% and –57.7%, respectively.
In addition, the mean percent change from baseline in truncal inflammatory lesions in the trifarotene-treated groups was –57.4% and –65.4%, respectively, while the mean percent change from baseline in truncal noninflammatory lesions was –49.1% and –55.2%, respectively.
The choice of vehicle may affect absorption of topical retinoids, and some formulations may increase skin hydration and decrease transepidermal water loss, “which is a good thing,” Dr. Graber said. “Also, vehicles aim to slow drug delivery over time while also making sure that the drug penetrates into the pilosebaceous unit.”
One recent advance is the honeycomb-like polymeric emulsion technology found in tretinoin 0.05% lotion and tazarotene 0.045% lotion. These formulations contain droplets of the tretinoin and tazarotene embedded in a honeycomb matrix with hydrating agents. “I think this is exciting and could enhance our patient compliance and tolerability,” she said. Another unique feature about these two products, especially the tretinoin product, is the very small particle size with this new formulation. “It’s small enough that it can penetrate down into the pilosebaceous unit,” which is different than with older formulations, in which the tretinoin “largely just sat on the surface of the skin and didn’t penetrate into the pilosebaceous unit.” In addition, she said, “there’s only 9% degradation of the tretinoin in UV light, compared to 72% degradation of standard tretinoin 0.025% gel, and with the new tretinoin formulation, there’s no degradation when used with benzoyl peroxide.”
Another new topical retinoid to consider is a fixed-dose combination of encapsulated benzoyl peroxide 3% and encapsulated tretinoin 0.1% cream (Twyneo), which was approved by the Food and Drug Administration in July 2021 for the treatment of acne in adults and children aged 9 years and older. “Typically, benzoyl peroxide and tretinoin cannot be mixed in the same tube to stability issues,” she said. “Here, each product is individually encapsulated in a silica shell so that they can be applied together.”
The approval was supported by positive results from two phase 3, randomized, double-blind, vehicle-controlled, multicenter studies (NCT03761784 and NCT03761810), in which Twyneo demonstrated efficacy and a favorable tolerability profile in patients aged 9 years and older with facial acne.
Another topical treatment option, dapsone, is now FDA approved for ages 9 and up, expanded from its initial indication for ages 12 and up. The new indication is based on a phase 4, multicenter, open-label study in which acne patients aged 9-11 years applied dapsone 7.5% gel once daily to the face and acne-affected areas on the upper chest, upper back, and shoulders for 12 weeks. After 12 weeks, facial acne was clear or almost clear in about 47% of patients. “Inflammatory, noninflammatory, and total lesions decreased from baseline, but there was a greater reduction in noninflammatory lesions, so if you have a very young patient with acne, now you can consider dapsone gel,” Dr. Graber said.
In August 2020, clascoterone cream became the first topical androgen receptor inhibitor approved for the treatment of acne in patients 12 years of age and older. It is a drug believed to address sebum and inflammation directly in the sebaceous gland and is structurally similar to dihydrotestosterone and spironolactone.
“This is a completely new drug category in acne,” she said. “Unlike all oral antiandrogen therapies, clascoterone cream can be used in both males and females with acne. It’s the first acne drug to have a new mechanism of action in almost 40 years, since isotretinoin was approved in 1982.”
In vitro, she continued, clascoterone competes with dihydrotestosterone for binding to the androgen receptor, inhibiting downstream signaling and leading to inhibited sebum production, reduced secretion of inflammatory cytokines, and inhibition of inflammatory pathways. Two phase 3 studies that led to its approval involved 1,440 patients with moderate to severe facial acne aged 9-58 years. The cream was applied twice a day for 12 weeks and treatment adherence was approximately 90%. The researchers found that clascoterone cream was significantly more effective than vehicle cream at achieving Investigator’s Global Assessment scores of 0 (clear) or 1 (almost clear), the definition of treatment success in the study, and reducing noninflammatory lesion and inflammatory lesion counts at week 12. “There were no safety issues noted during these studies, and clascoterone cream was well tolerated,” Dr. Graber said.
Dr. Graber disclosed that she is a consultant/adviser for Digital Diagnostics, Almirall, Hovione, Keratin Biosciences, La Roche Posay, Ortho Dermatologics, Sebacia, Sol-Gel, Verrica, and WebMD. She is also a research investigator for Hovione, Ortho Dermatologics, Sebacia, and she receives royalties from Wolters Kluwer Health.
MedscapeLive and this news organization are owned by the same parent company.
Commentary by Lawrence W. Eichenfield, MD
Acne vulgaris remains an issue of tremendous importance to preteens, teens, and young adults, with approximately 85% of individuals aged 12-24 being affected. Expanding options for topical treatments may help bring effective disease control. Dr. Graber pointed out that historically, pediatricians and other primary care practitioners utilize topical retinoids less often for acne care as compared with dermatologists or guidelines recommendations (either the AAP’s or AAD’s). There are now expanded options, including over-the-counter retinoids (adapalene 0.1% gel), generic and trade brand topical tretinoin products, prescription adapalene medications, older and recently approved tazarotene products, and a newer type of topical retinoid, trifarotene. Novel formulations and emulsion technology, as well as retinoid developed in combination products, give more options in patients down to 9 years of age. A novel topical anti-androgen, clascoterone, is in its own category, as the first topical “hormonal agent,” allowing hormonal therapy to be used for males as well as females (aged 12 years and up). A recent review in JAMA (2021 Nov 23;326[20]:2055-67) incorporates many of these newer medications into management suggestions, emphasizing that first-line therapies are topical retinoids, benzoyl peroxide, azelaic acid, or combinations of topicals, whereas in more severe disease, oral antibiotics such as doxycycline or minocycline, hormonal therapies such as combination oral conceptive agents or spironolactone, or isotretinoin are most effective.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. He disclosed that he has served as an investigator and/or consultant to AbbVie, Lilly, Pfizer, Regeneron, Sanofi-Genzyme, and Verrica.
A version of this article first appeared on Medscape.com.
This article was updated 6/18/22.
yet they are likely underused in today’s clinical practice.
A study of prescribing practices from 2012 to 2014 indicated that dermatologists prescribed retinoids for just 58.8% of acne cases, while nondermatologists prescribed them for only 32.4% of cases. “If the guidelines are telling us that we should use topical retinoids for almost all of our acne patients, why are we using them for half of the patients?” Emmy Graber, MD, MBA, asked during MedscapeLive’s annual Las Vegas Dermatology Seminar. “We have a lot of options today for topical retinoids,” she added, noting that, in the past few years, trifarotene cream 0.005% and new formulations of tazarotene lotion (0.045%) and tretinoin lotion (0.05%) have become available.
According to Dr. Graber, president of The Dermatology Institute of Boston, tazarotene has been considered the most efficacious topical retinoid but is generally the least well tolerated, while adapalene has often been considered to be one of the better-tolerated topical retinoids. “This is a broad generalization,” she said. “One should also take into account the concentration and formulation of the retinoid. Cutaneous adverse events increase in severity as the concentration increases regardless of the vehicle.” There are no studies comparing trifarotene with other topical retinoids, she added.
In two phase 2, double-blind, vehicle-controlled studies (PERFECT 1 and PERFECT 2), researchers randomized more than 2,400 patients with moderate facial or truncal acne to receive trifarotene cream or a vehicle for 12 weeks. The mean percent change from baseline in facial inflammatory lesions in the trifarotene-treated group was –54.4% and –66.2% in PERFECT 1, and PERFECT 2, respectively, while the mean percent change from baseline in facial noninflammatory lesions was –49.7% and –57.7%, respectively.
In addition, the mean percent change from baseline in truncal inflammatory lesions in the trifarotene-treated groups was –57.4% and –65.4%, respectively, while the mean percent change from baseline in truncal noninflammatory lesions was –49.1% and –55.2%, respectively.
The choice of vehicle may affect absorption of topical retinoids, and some formulations may increase skin hydration and decrease transepidermal water loss, “which is a good thing,” Dr. Graber said. “Also, vehicles aim to slow drug delivery over time while also making sure that the drug penetrates into the pilosebaceous unit.”
One recent advance is the honeycomb-like polymeric emulsion technology found in tretinoin 0.05% lotion and tazarotene 0.045% lotion. These formulations contain droplets of the tretinoin and tazarotene embedded in a honeycomb matrix with hydrating agents. “I think this is exciting and could enhance our patient compliance and tolerability,” she said. Another unique feature about these two products, especially the tretinoin product, is the very small particle size with this new formulation. “It’s small enough that it can penetrate down into the pilosebaceous unit,” which is different than with older formulations, in which the tretinoin “largely just sat on the surface of the skin and didn’t penetrate into the pilosebaceous unit.” In addition, she said, “there’s only 9% degradation of the tretinoin in UV light, compared to 72% degradation of standard tretinoin 0.025% gel, and with the new tretinoin formulation, there’s no degradation when used with benzoyl peroxide.”
Another new topical retinoid to consider is a fixed-dose combination of encapsulated benzoyl peroxide 3% and encapsulated tretinoin 0.1% cream (Twyneo), which was approved by the Food and Drug Administration in July 2021 for the treatment of acne in adults and children aged 9 years and older. “Typically, benzoyl peroxide and tretinoin cannot be mixed in the same tube to stability issues,” she said. “Here, each product is individually encapsulated in a silica shell so that they can be applied together.”
The approval was supported by positive results from two phase 3, randomized, double-blind, vehicle-controlled, multicenter studies (NCT03761784 and NCT03761810), in which Twyneo demonstrated efficacy and a favorable tolerability profile in patients aged 9 years and older with facial acne.
Another topical treatment option, dapsone, is now FDA approved for ages 9 and up, expanded from its initial indication for ages 12 and up. The new indication is based on a phase 4, multicenter, open-label study in which acne patients aged 9-11 years applied dapsone 7.5% gel once daily to the face and acne-affected areas on the upper chest, upper back, and shoulders for 12 weeks. After 12 weeks, facial acne was clear or almost clear in about 47% of patients. “Inflammatory, noninflammatory, and total lesions decreased from baseline, but there was a greater reduction in noninflammatory lesions, so if you have a very young patient with acne, now you can consider dapsone gel,” Dr. Graber said.
In August 2020, clascoterone cream became the first topical androgen receptor inhibitor approved for the treatment of acne in patients 12 years of age and older. It is a drug believed to address sebum and inflammation directly in the sebaceous gland and is structurally similar to dihydrotestosterone and spironolactone.
“This is a completely new drug category in acne,” she said. “Unlike all oral antiandrogen therapies, clascoterone cream can be used in both males and females with acne. It’s the first acne drug to have a new mechanism of action in almost 40 years, since isotretinoin was approved in 1982.”
In vitro, she continued, clascoterone competes with dihydrotestosterone for binding to the androgen receptor, inhibiting downstream signaling and leading to inhibited sebum production, reduced secretion of inflammatory cytokines, and inhibition of inflammatory pathways. Two phase 3 studies that led to its approval involved 1,440 patients with moderate to severe facial acne aged 9-58 years. The cream was applied twice a day for 12 weeks and treatment adherence was approximately 90%. The researchers found that clascoterone cream was significantly more effective than vehicle cream at achieving Investigator’s Global Assessment scores of 0 (clear) or 1 (almost clear), the definition of treatment success in the study, and reducing noninflammatory lesion and inflammatory lesion counts at week 12. “There were no safety issues noted during these studies, and clascoterone cream was well tolerated,” Dr. Graber said.
Dr. Graber disclosed that she is a consultant/adviser for Digital Diagnostics, Almirall, Hovione, Keratin Biosciences, La Roche Posay, Ortho Dermatologics, Sebacia, Sol-Gel, Verrica, and WebMD. She is also a research investigator for Hovione, Ortho Dermatologics, Sebacia, and she receives royalties from Wolters Kluwer Health.
MedscapeLive and this news organization are owned by the same parent company.
Commentary by Lawrence W. Eichenfield, MD
Acne vulgaris remains an issue of tremendous importance to preteens, teens, and young adults, with approximately 85% of individuals aged 12-24 being affected. Expanding options for topical treatments may help bring effective disease control. Dr. Graber pointed out that historically, pediatricians and other primary care practitioners utilize topical retinoids less often for acne care as compared with dermatologists or guidelines recommendations (either the AAP’s or AAD’s). There are now expanded options, including over-the-counter retinoids (adapalene 0.1% gel), generic and trade brand topical tretinoin products, prescription adapalene medications, older and recently approved tazarotene products, and a newer type of topical retinoid, trifarotene. Novel formulations and emulsion technology, as well as retinoid developed in combination products, give more options in patients down to 9 years of age. A novel topical anti-androgen, clascoterone, is in its own category, as the first topical “hormonal agent,” allowing hormonal therapy to be used for males as well as females (aged 12 years and up). A recent review in JAMA (2021 Nov 23;326[20]:2055-67) incorporates many of these newer medications into management suggestions, emphasizing that first-line therapies are topical retinoids, benzoyl peroxide, azelaic acid, or combinations of topicals, whereas in more severe disease, oral antibiotics such as doxycycline or minocycline, hormonal therapies such as combination oral conceptive agents or spironolactone, or isotretinoin are most effective.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. He disclosed that he has served as an investigator and/or consultant to AbbVie, Lilly, Pfizer, Regeneron, Sanofi-Genzyme, and Verrica.
A version of this article first appeared on Medscape.com.
This article was updated 6/18/22.
yet they are likely underused in today’s clinical practice.
A study of prescribing practices from 2012 to 2014 indicated that dermatologists prescribed retinoids for just 58.8% of acne cases, while nondermatologists prescribed them for only 32.4% of cases. “If the guidelines are telling us that we should use topical retinoids for almost all of our acne patients, why are we using them for half of the patients?” Emmy Graber, MD, MBA, asked during MedscapeLive’s annual Las Vegas Dermatology Seminar. “We have a lot of options today for topical retinoids,” she added, noting that, in the past few years, trifarotene cream 0.005% and new formulations of tazarotene lotion (0.045%) and tretinoin lotion (0.05%) have become available.
According to Dr. Graber, president of The Dermatology Institute of Boston, tazarotene has been considered the most efficacious topical retinoid but is generally the least well tolerated, while adapalene has often been considered to be one of the better-tolerated topical retinoids. “This is a broad generalization,” she said. “One should also take into account the concentration and formulation of the retinoid. Cutaneous adverse events increase in severity as the concentration increases regardless of the vehicle.” There are no studies comparing trifarotene with other topical retinoids, she added.
In two phase 2, double-blind, vehicle-controlled studies (PERFECT 1 and PERFECT 2), researchers randomized more than 2,400 patients with moderate facial or truncal acne to receive trifarotene cream or a vehicle for 12 weeks. The mean percent change from baseline in facial inflammatory lesions in the trifarotene-treated group was –54.4% and –66.2% in PERFECT 1, and PERFECT 2, respectively, while the mean percent change from baseline in facial noninflammatory lesions was –49.7% and –57.7%, respectively.
In addition, the mean percent change from baseline in truncal inflammatory lesions in the trifarotene-treated groups was –57.4% and –65.4%, respectively, while the mean percent change from baseline in truncal noninflammatory lesions was –49.1% and –55.2%, respectively.
The choice of vehicle may affect absorption of topical retinoids, and some formulations may increase skin hydration and decrease transepidermal water loss, “which is a good thing,” Dr. Graber said. “Also, vehicles aim to slow drug delivery over time while also making sure that the drug penetrates into the pilosebaceous unit.”
One recent advance is the honeycomb-like polymeric emulsion technology found in tretinoin 0.05% lotion and tazarotene 0.045% lotion. These formulations contain droplets of the tretinoin and tazarotene embedded in a honeycomb matrix with hydrating agents. “I think this is exciting and could enhance our patient compliance and tolerability,” she said. Another unique feature about these two products, especially the tretinoin product, is the very small particle size with this new formulation. “It’s small enough that it can penetrate down into the pilosebaceous unit,” which is different than with older formulations, in which the tretinoin “largely just sat on the surface of the skin and didn’t penetrate into the pilosebaceous unit.” In addition, she said, “there’s only 9% degradation of the tretinoin in UV light, compared to 72% degradation of standard tretinoin 0.025% gel, and with the new tretinoin formulation, there’s no degradation when used with benzoyl peroxide.”
Another new topical retinoid to consider is a fixed-dose combination of encapsulated benzoyl peroxide 3% and encapsulated tretinoin 0.1% cream (Twyneo), which was approved by the Food and Drug Administration in July 2021 for the treatment of acne in adults and children aged 9 years and older. “Typically, benzoyl peroxide and tretinoin cannot be mixed in the same tube to stability issues,” she said. “Here, each product is individually encapsulated in a silica shell so that they can be applied together.”
The approval was supported by positive results from two phase 3, randomized, double-blind, vehicle-controlled, multicenter studies (NCT03761784 and NCT03761810), in which Twyneo demonstrated efficacy and a favorable tolerability profile in patients aged 9 years and older with facial acne.
Another topical treatment option, dapsone, is now FDA approved for ages 9 and up, expanded from its initial indication for ages 12 and up. The new indication is based on a phase 4, multicenter, open-label study in which acne patients aged 9-11 years applied dapsone 7.5% gel once daily to the face and acne-affected areas on the upper chest, upper back, and shoulders for 12 weeks. After 12 weeks, facial acne was clear or almost clear in about 47% of patients. “Inflammatory, noninflammatory, and total lesions decreased from baseline, but there was a greater reduction in noninflammatory lesions, so if you have a very young patient with acne, now you can consider dapsone gel,” Dr. Graber said.
In August 2020, clascoterone cream became the first topical androgen receptor inhibitor approved for the treatment of acne in patients 12 years of age and older. It is a drug believed to address sebum and inflammation directly in the sebaceous gland and is structurally similar to dihydrotestosterone and spironolactone.
“This is a completely new drug category in acne,” she said. “Unlike all oral antiandrogen therapies, clascoterone cream can be used in both males and females with acne. It’s the first acne drug to have a new mechanism of action in almost 40 years, since isotretinoin was approved in 1982.”
In vitro, she continued, clascoterone competes with dihydrotestosterone for binding to the androgen receptor, inhibiting downstream signaling and leading to inhibited sebum production, reduced secretion of inflammatory cytokines, and inhibition of inflammatory pathways. Two phase 3 studies that led to its approval involved 1,440 patients with moderate to severe facial acne aged 9-58 years. The cream was applied twice a day for 12 weeks and treatment adherence was approximately 90%. The researchers found that clascoterone cream was significantly more effective than vehicle cream at achieving Investigator’s Global Assessment scores of 0 (clear) or 1 (almost clear), the definition of treatment success in the study, and reducing noninflammatory lesion and inflammatory lesion counts at week 12. “There were no safety issues noted during these studies, and clascoterone cream was well tolerated,” Dr. Graber said.
Dr. Graber disclosed that she is a consultant/adviser for Digital Diagnostics, Almirall, Hovione, Keratin Biosciences, La Roche Posay, Ortho Dermatologics, Sebacia, Sol-Gel, Verrica, and WebMD. She is also a research investigator for Hovione, Ortho Dermatologics, Sebacia, and she receives royalties from Wolters Kluwer Health.
MedscapeLive and this news organization are owned by the same parent company.
Commentary by Lawrence W. Eichenfield, MD
Acne vulgaris remains an issue of tremendous importance to preteens, teens, and young adults, with approximately 85% of individuals aged 12-24 being affected. Expanding options for topical treatments may help bring effective disease control. Dr. Graber pointed out that historically, pediatricians and other primary care practitioners utilize topical retinoids less often for acne care as compared with dermatologists or guidelines recommendations (either the AAP’s or AAD’s). There are now expanded options, including over-the-counter retinoids (adapalene 0.1% gel), generic and trade brand topical tretinoin products, prescription adapalene medications, older and recently approved tazarotene products, and a newer type of topical retinoid, trifarotene. Novel formulations and emulsion technology, as well as retinoid developed in combination products, give more options in patients down to 9 years of age. A novel topical anti-androgen, clascoterone, is in its own category, as the first topical “hormonal agent,” allowing hormonal therapy to be used for males as well as females (aged 12 years and up). A recent review in JAMA (2021 Nov 23;326[20]:2055-67) incorporates many of these newer medications into management suggestions, emphasizing that first-line therapies are topical retinoids, benzoyl peroxide, azelaic acid, or combinations of topicals, whereas in more severe disease, oral antibiotics such as doxycycline or minocycline, hormonal therapies such as combination oral conceptive agents or spironolactone, or isotretinoin are most effective.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. He disclosed that he has served as an investigator and/or consultant to AbbVie, Lilly, Pfizer, Regeneron, Sanofi-Genzyme, and Verrica.
A version of this article first appeared on Medscape.com.
This article was updated 6/18/22.
FROM THE MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Cannabinoids being studied for a variety of dermatologic conditions
.
“When you walk into places like CVS or Walgreens, you see lots of displays for CBD creams and oils,” Todd S. Anhalt, MD, said during the annual meeting of the Pacific Dermatologic Association. “The problem is, we don’t know what’s in them or who made them or how good they are. That’s going to be a problem for a while.”
According to Dr. Anhalt, clinical professor emeritus of dermatology at Stanford (Calif.) University, there are about 140 active cannabinoid compounds in cannabis, but the most important ones are THC and cannabidiol (CBD). There are three types of cannabinoids, based on where the cannabidiol is produced: endocannabinoids, which are produced in the human body; phytocannabinoids, which are derived from plants such as marijuana and hemp; and synthetic cannabinoids, which are derived in labs.
Dr. Anhalt described the endocannabinoid system as a conserved network of molecular signaling made of several components: signaling molecules (endocannabinoids), endocannabinoid receptors (CB-1 and CB-2), enzymes, and transporters. There is also overlap between cannabinoids and terpenes, which are responsible for flavor and aroma in plants and marijuana and can enhance the effects of CBD.
“For the most part, CB-1 receptors are in the central nervous system and CB-2 [receptors] are mostly in the periphery,” including the skin and digestive system, said Dr. Anhalt, who practices at the California Skin Institute in Los Altos, Calif. “This is interesting because one of the main conditions I recommend cannabidiol for is in patients with peripheral neuropathy, despite the fact they may be on all sorts of medications such as Neurontin and Lyrica or tricyclic antidepressants. Sometimes they don’t get much relief from those. I have had many patients tell me that they have had reduction of pain and increased functionality using the CBD creams.” CB-2 receptors, he noted, are located in keratinocytes, sensory receptors, sweat glands, fibroblasts, Langerhans cells, melanocytes, and sebaceous glands.
Recent research shows that the endocannabinoid system is involved in modulation of the CNS and in immune function, particularly skin homeostasis and barrier function. “We know that barrier function can be affected by the generation of oxidative species,” he said. “The stress that it causes can decrease barrier function and lead to cytokine release and itch. CBDs have been shown to enter cells, target and upregulate genes with decreased oxidation and inflammation, and protect membrane integrity in skin cells. Therefore, this might be helpful in atopic dermatitis.” Other potential uses in dermatology include wound healing, acne, hair growth modulation, skin and hair pigmentation, skin infections, psoriasis, and cutaneous malignancies, as well as neuropathic pain.
Evidence is strongest for neuropathic pain, he said, which is mediated by CB-1 receptors peripherally, followed by itch and atopic dermatitis. The authors of a 2017 systematic review concluded that “low-strength” evidence exists to suggest that cannabis alleviates neuropathic pain, with insufficient evidence for other types of pain.
Topical CBD comes in various forms: oils (usually hemp oil), creams, and lotions, Dr. Anhalt said. “I advise patients to apply it 2-4 times per day depending on how anxious or uncomfortable they are. It takes my patients 10 days to 2 weeks before they notice anything at all.”
For atopic dermatitis, it could be useful “not to use it instead of a moisturizer, but as a moisturizer,” Dr. Anhalt advised. “You can have a patient get big jars of CBD creams and lotions. They may have to try a few before they find one that they really like, but you can replace all of the other moisturizers that you’re using right now in patients who have a lot of itch.”
As for CBD’s effect on peripheral neuropathy, the medical literature is lacking, but some studies show low to moderate evidence of efficacy. For example, a Cochrane Review found that a 30% or greater pain reduction was achieved by 39% of patients who used cannabis-based treatments, vs. 33% of those on placebo.
“I would not suggest CBD as a first-line drug unless it’s very mild peripheral neuropathy, but for patients who are on gabapentin who are better but not better enough, this is an excellent adjunct,” Dr. Anhalt said. “It’s worth trying. It’s not too expensive and it’s really safe.”
The application of topical CBD to treat cutaneous malignancies has not yet shown evidence of significant efficacy, while using CBDs for acne holds promise. “The endogenous cannabinoid system is involved in the production of lipids,” he said. “Cannabinoids have an antilipogenic activity, so they decrease sebum production. CBD could help patients with mild acne who are reluctant to use other types of medications. For this and other potential dermatologic applications, lots more studies need to be done.”
Dr. Anhalt reported having no financial disclosures.
.
“When you walk into places like CVS or Walgreens, you see lots of displays for CBD creams and oils,” Todd S. Anhalt, MD, said during the annual meeting of the Pacific Dermatologic Association. “The problem is, we don’t know what’s in them or who made them or how good they are. That’s going to be a problem for a while.”
According to Dr. Anhalt, clinical professor emeritus of dermatology at Stanford (Calif.) University, there are about 140 active cannabinoid compounds in cannabis, but the most important ones are THC and cannabidiol (CBD). There are three types of cannabinoids, based on where the cannabidiol is produced: endocannabinoids, which are produced in the human body; phytocannabinoids, which are derived from plants such as marijuana and hemp; and synthetic cannabinoids, which are derived in labs.
Dr. Anhalt described the endocannabinoid system as a conserved network of molecular signaling made of several components: signaling molecules (endocannabinoids), endocannabinoid receptors (CB-1 and CB-2), enzymes, and transporters. There is also overlap between cannabinoids and terpenes, which are responsible for flavor and aroma in plants and marijuana and can enhance the effects of CBD.
“For the most part, CB-1 receptors are in the central nervous system and CB-2 [receptors] are mostly in the periphery,” including the skin and digestive system, said Dr. Anhalt, who practices at the California Skin Institute in Los Altos, Calif. “This is interesting because one of the main conditions I recommend cannabidiol for is in patients with peripheral neuropathy, despite the fact they may be on all sorts of medications such as Neurontin and Lyrica or tricyclic antidepressants. Sometimes they don’t get much relief from those. I have had many patients tell me that they have had reduction of pain and increased functionality using the CBD creams.” CB-2 receptors, he noted, are located in keratinocytes, sensory receptors, sweat glands, fibroblasts, Langerhans cells, melanocytes, and sebaceous glands.
Recent research shows that the endocannabinoid system is involved in modulation of the CNS and in immune function, particularly skin homeostasis and barrier function. “We know that barrier function can be affected by the generation of oxidative species,” he said. “The stress that it causes can decrease barrier function and lead to cytokine release and itch. CBDs have been shown to enter cells, target and upregulate genes with decreased oxidation and inflammation, and protect membrane integrity in skin cells. Therefore, this might be helpful in atopic dermatitis.” Other potential uses in dermatology include wound healing, acne, hair growth modulation, skin and hair pigmentation, skin infections, psoriasis, and cutaneous malignancies, as well as neuropathic pain.
Evidence is strongest for neuropathic pain, he said, which is mediated by CB-1 receptors peripherally, followed by itch and atopic dermatitis. The authors of a 2017 systematic review concluded that “low-strength” evidence exists to suggest that cannabis alleviates neuropathic pain, with insufficient evidence for other types of pain.
Topical CBD comes in various forms: oils (usually hemp oil), creams, and lotions, Dr. Anhalt said. “I advise patients to apply it 2-4 times per day depending on how anxious or uncomfortable they are. It takes my patients 10 days to 2 weeks before they notice anything at all.”
For atopic dermatitis, it could be useful “not to use it instead of a moisturizer, but as a moisturizer,” Dr. Anhalt advised. “You can have a patient get big jars of CBD creams and lotions. They may have to try a few before they find one that they really like, but you can replace all of the other moisturizers that you’re using right now in patients who have a lot of itch.”
As for CBD’s effect on peripheral neuropathy, the medical literature is lacking, but some studies show low to moderate evidence of efficacy. For example, a Cochrane Review found that a 30% or greater pain reduction was achieved by 39% of patients who used cannabis-based treatments, vs. 33% of those on placebo.
“I would not suggest CBD as a first-line drug unless it’s very mild peripheral neuropathy, but for patients who are on gabapentin who are better but not better enough, this is an excellent adjunct,” Dr. Anhalt said. “It’s worth trying. It’s not too expensive and it’s really safe.”
The application of topical CBD to treat cutaneous malignancies has not yet shown evidence of significant efficacy, while using CBDs for acne holds promise. “The endogenous cannabinoid system is involved in the production of lipids,” he said. “Cannabinoids have an antilipogenic activity, so they decrease sebum production. CBD could help patients with mild acne who are reluctant to use other types of medications. For this and other potential dermatologic applications, lots more studies need to be done.”
Dr. Anhalt reported having no financial disclosures.
.
“When you walk into places like CVS or Walgreens, you see lots of displays for CBD creams and oils,” Todd S. Anhalt, MD, said during the annual meeting of the Pacific Dermatologic Association. “The problem is, we don’t know what’s in them or who made them or how good they are. That’s going to be a problem for a while.”
According to Dr. Anhalt, clinical professor emeritus of dermatology at Stanford (Calif.) University, there are about 140 active cannabinoid compounds in cannabis, but the most important ones are THC and cannabidiol (CBD). There are three types of cannabinoids, based on where the cannabidiol is produced: endocannabinoids, which are produced in the human body; phytocannabinoids, which are derived from plants such as marijuana and hemp; and synthetic cannabinoids, which are derived in labs.
Dr. Anhalt described the endocannabinoid system as a conserved network of molecular signaling made of several components: signaling molecules (endocannabinoids), endocannabinoid receptors (CB-1 and CB-2), enzymes, and transporters. There is also overlap between cannabinoids and terpenes, which are responsible for flavor and aroma in plants and marijuana and can enhance the effects of CBD.
“For the most part, CB-1 receptors are in the central nervous system and CB-2 [receptors] are mostly in the periphery,” including the skin and digestive system, said Dr. Anhalt, who practices at the California Skin Institute in Los Altos, Calif. “This is interesting because one of the main conditions I recommend cannabidiol for is in patients with peripheral neuropathy, despite the fact they may be on all sorts of medications such as Neurontin and Lyrica or tricyclic antidepressants. Sometimes they don’t get much relief from those. I have had many patients tell me that they have had reduction of pain and increased functionality using the CBD creams.” CB-2 receptors, he noted, are located in keratinocytes, sensory receptors, sweat glands, fibroblasts, Langerhans cells, melanocytes, and sebaceous glands.
Recent research shows that the endocannabinoid system is involved in modulation of the CNS and in immune function, particularly skin homeostasis and barrier function. “We know that barrier function can be affected by the generation of oxidative species,” he said. “The stress that it causes can decrease barrier function and lead to cytokine release and itch. CBDs have been shown to enter cells, target and upregulate genes with decreased oxidation and inflammation, and protect membrane integrity in skin cells. Therefore, this might be helpful in atopic dermatitis.” Other potential uses in dermatology include wound healing, acne, hair growth modulation, skin and hair pigmentation, skin infections, psoriasis, and cutaneous malignancies, as well as neuropathic pain.
Evidence is strongest for neuropathic pain, he said, which is mediated by CB-1 receptors peripherally, followed by itch and atopic dermatitis. The authors of a 2017 systematic review concluded that “low-strength” evidence exists to suggest that cannabis alleviates neuropathic pain, with insufficient evidence for other types of pain.
Topical CBD comes in various forms: oils (usually hemp oil), creams, and lotions, Dr. Anhalt said. “I advise patients to apply it 2-4 times per day depending on how anxious or uncomfortable they are. It takes my patients 10 days to 2 weeks before they notice anything at all.”
For atopic dermatitis, it could be useful “not to use it instead of a moisturizer, but as a moisturizer,” Dr. Anhalt advised. “You can have a patient get big jars of CBD creams and lotions. They may have to try a few before they find one that they really like, but you can replace all of the other moisturizers that you’re using right now in patients who have a lot of itch.”
As for CBD’s effect on peripheral neuropathy, the medical literature is lacking, but some studies show low to moderate evidence of efficacy. For example, a Cochrane Review found that a 30% or greater pain reduction was achieved by 39% of patients who used cannabis-based treatments, vs. 33% of those on placebo.
“I would not suggest CBD as a first-line drug unless it’s very mild peripheral neuropathy, but for patients who are on gabapentin who are better but not better enough, this is an excellent adjunct,” Dr. Anhalt said. “It’s worth trying. It’s not too expensive and it’s really safe.”
The application of topical CBD to treat cutaneous malignancies has not yet shown evidence of significant efficacy, while using CBDs for acne holds promise. “The endogenous cannabinoid system is involved in the production of lipids,” he said. “Cannabinoids have an antilipogenic activity, so they decrease sebum production. CBD could help patients with mild acne who are reluctant to use other types of medications. For this and other potential dermatologic applications, lots more studies need to be done.”
Dr. Anhalt reported having no financial disclosures.
FROM PDA 2021
Contact Allergy to Topical Medicaments, Part 1: A Double-edged Sword
Topical medications frequently are prescribed in dermatology and provide the advantages of direct skin penetration and targeted application while typically sparing patients from systemic effects. Adverse cutaneous effects include allergic contact dermatitis (ACD), irritant contact dermatitis (ICD), photosensitivity, urticaria, hyperpigmentation or hypopigmentation, atrophy, periorificial dermatitis, and acneform eruptions. Allergic contact dermatitis can develop from the active drug or vehicle components.
Patients with medicament ACD often present with symptoms of pruritus and dermatitis at the site of topical application. They may express concern that the medication is no longer working or seems to be making things worse. Certain sites are more prone to developing medicament dermatitis, including the face, groin, and lower legs. Older adults may be more at risk. Other risk factors include pre-existing skin diseases such as stasis dermatitis, acne, psoriasis, atopic dermatitis, and genital dermatoses.1 A review of 14,911 patch-tested patients from a single referral clinic revealed that 17.4% had iatrogenic contact dermatitis, with the most common culprits being topical antibiotics, antiseptics, and steroids.2
In this 2-part series, we will focus on the active drug as a source of ACD. Part 1 explores ACD associated with acne and rosacea medications, antimicrobials, antihistamines, and topical pain preparations.
Acne and Rosacea Medications
Retinoids—Topical retinoids are first-line acne treatments that help normalize skin keratinization. Irritant contact dermatitis from retinoids is a well-known and common side effect. Although far less common than ICD, ACD from topical retinoid use has been reported.3,4 Reactions to tretinoin are most frequently reported in the literature compared to adapalene gel5 and tazarotene foam, which have lower potential for sensitization.6 Allergic contact dermatitis also has been reported from retinyl palmitate7,8 in cosmetic creams and from occupational exposure in settings of industrial vitamin A production.9 Both ICD and ACD from topical retinoids can present with pruritus, erythema, and scaling. Given this clinical overlap between ACD and ICD, patch testing is crucial in differentiating the underlying etiology of the dermatitis.
Benzoyl Peroxide—Benzoyl peroxide (BP) is another popular topical acne treatment that targets Cutibacterium acnes, a bacterium often implicated in the pathogenesis of acne vulgaris. Similar to retinoids, ICD is more common than ACD. Several cases of ACD to BP have been reported.10-14 Occasionally, honey-colored crusting associated with ACD to BP can mimic impetigo.10 Aside from use of BP as an acne treatment, other potential exposures to BP include bleached flour13 and orthopedic bone cement. Occupations at risk for potential BP exposure include dental technicians15 and those working in plastic manufacturing.
Brimonidine—Brimonidine tartrate is a selective α2-adrenergic agonist initially used to treat open-angle glaucoma and also is used as a topical treatment for rosacea. Allergic reactions to brimonidine eye drops may present with periorbital hyperpigmentation and pruritic bullous lesions.16 Case reports of topical brimonidine ACD have demonstrated mixed patch test results, with positive patch tests to Mirvaso (Galderma) as is but negative patch tests to pure brimonidine tartrate 0.33%.17,18 Ringuet and Houle19 reported the first known positive patch test reaction to pure topical brimonidine, testing with brimonidine tartrate 1% in petrolatum.20,21 Clinicians should be attuned to ACD to topical brimonidine in patients previously treated for glaucoma, as prior use of ophthalmic preparations may result in sensitization.18,20
Antimicrobials
Clindamycin—Clindamycin targets bacterial protein synthesis and is an effective adjunct in the treatment of acne. Despite its widespread and often long-term use, topical clindamycin is a weak sensitizer.22 To date, limited case reports on ACD to topical clindamycin exist.23-28 Rare clinical patterns of ACD to clindamycin include mimickers of irritant retinoid dermatitis, erythema multiforme, or pustular rosacea.25,26,29
Metronidazole—Metronidazole is a bactericidal agent that disrupts nucleic acid synthesis with additional anti-inflammatory properties used in the treatment of rosacea. Allergic contact dermatitis to topical metronidazole has been reported.30-34 In 2006, Beutner at al35 patch tested 215 patients using metronidazole gel 1%, which revealed no positive reactions to indicate contact sensitization. Similarly, Jappe et al36 found no positive reactions to metronidazole 2% in petrolatum in their prospective analysis of 78 rosacea patients, further highlighting the exceptionally low incidence of ACD. Cross-reaction with isothiazolinone, which shares structurally similar properties to metronidazole, has been speculated.31,34 One patient developed an acute reaction to metronidazole gel 0.75% within 24 hours of application, suggesting that isothiazolinone may act as a sensitizer, though this relationship has not been proven.31
Neomycin—Neomycin blocks bacterial protein synthesis and is available in both prescription and over-the-counter (OTC) formulations. It commonly is used to treat and prevent superficial wound infections as an OTC antibiotic and also has otic, ophthalmologic, gastroenterologic, urologic, and peritoneal formulations. It also can be used in the dental and veterinary fields and is present in some animal feeds and in trace amounts in some vaccines for humans. Neomycin is a common antibiotic contact allergen, and the most recently reported 2017-2018 North American Contact Dermatitis Group data cycle placed it at number 12 with 5.4% positivity.37 Co-reactions with bacitracin can occur, substantially limiting OTC topical antibiotic options for allergic patients. A safe alternative for patients with neomycin (and bacitracin and polymyxin) contact allergy is prescription mupirocin.
Bacitracin—Bacitracin interferes with peptidoglycan and cell-wall synthesis to treat superficial cutaneous infections. Similar to neomycin, it also can be found in OTC antibiotic ointments as well as in antibacterial bandages. There are several case reports of patients with both type IV delayed hypersensitivity (contact dermatitis) and type I anaphylactic reactions to bacitracin38-40; patch testers should be aware of this rare association. Bacitracin was positive in 5.5% of patch tested patients in the 2017-2018 North American Contact Dermatitis Group data cycle,37 and as with neomycin, bacitracin also is commonly patch tested in most screening patch test series.
Polymyxin—Polymyxin is a polypeptide topical antibiotic that is used to treat superficial wound infections and can be used in combination with neomycin and/or bacitracin. Historically, it is a less common antibiotic allergen; however, it is now frequently included in comprehensive patch test series, as the frequency of positive reactions seems to be increasing, probably due to polysensitization with neomycin and bacitracin.
Nystatin—Nystatin is an antifungal that binds to ergosterol and disrupts the cell wall. Cases exist of ACD to topical nystatin as well as systemic ACD from oral exposure, though both are quite rare. Authors have surmised that the overall low rates of ACD may be due to poor skin absorption of nystatin, which also can confound patch testing.41,42 For patients with suspected ACD to nystatin, repeat open application testing also can be performed to confirm allergy.
Imidazole Antifungals—Similar to nystatins, imidazole antifungals also work by disrupting the fungal cell wall. Imidazole antifungal preparations that have been reported to cause ACD include clotrimazole, miconazole, econazole, and isoconazole, and although cross-reactivity patterns have been described, they are not always reproducible with patch testing.43 In one reported case, tioconazole found in an antifungal nail lacquer triggered ACD involving not only the fingers and toes but also the trunk.44 Erythema multiforme–like reactions also have been described from topical use.45 Commercial patch test preparations of the most common imidazole allergens do exist. Nonimidazole antifungals remain a safe option for allergic patients.
Antihistamines
Antihistamines, or H1-receptor antagonists, are marketed to be applied topically for relief of pruritus associated with allergic cutaneous reactions. Ironically, they are known to be potent sensitizers themselves. There are 6 main chemical classes of antihistamines: phenothiazines, ethylenediamines, ethanolamines, alkylamines, piperazines, and piperidines. Goossens and Linsen46 patch tested 12,460 patients from 1978 to 1997 and found the most positive reactions to promethazine (phenothiazine)(n=12), followed by diphenhydramine (ethanolamine)(n=8) and clemizole (benzimidazole)(n=6). The authors also noted cross-reactions between diphenhydramine derivatives and between promethazine and chlorpromazine.46
Doxepin is a tricyclic antidepressant with antihistamine activity and is a well-documented sensitizer.47-52 Taylor et al47 evaluated 97 patients with chronic dermatoses, and patch testing revealed 17 (17.5%) positive reactions to doxepin cream, 13 (76.5%) of which were positive reactions to both the commercial cream and the active ingredient. Patch testing using doxepin dilution as low as 0.5% in petrolatum is sufficient to provoke a strong (++) allergic reaction.50,51 Early-onset ACD following the use of doxepin cream suggests the possibility of prior sensitization, perhaps with a structurally similar phenothiazine drug.51 A keen suspicion for ACD in patients using doxepin cream for longer than the recommended duration can help make the diagnosis.49,52
Topical Analgesics
Nonsteroidal Anti-inflammatory Drugs—Ketoprofen is one of the most frequent culprits of photoallergic contact dermatitis. Pruritic, papulovesicular, and bullous lesions typically develop acutely weeks after exposure. Prolonged photosensitivity is common and can last years after discontinuation of the nonsteroidal anti-inflammatory drug.53 Cases of cross-reactions and co-sensitization to structurally similar substances have been reported, including to benzophenone-related chemicals in sunscreen and aldehyde groups in fragrance mix.53,54
Diclofenac gel generally is well tolerated in the topical treatment of joint pain and inflammation. In the setting of ACD, patients typically present with dermatitis localized to the area of application.55 Immediate cessation and avoidance of topical diclofenac are crucial components of management. Although systemic contact dermatitis has been reported with oral diclofenac use,56 a recent report suggested that oral diclofenac may be well tolerated for some patients with topical ACD.57
Publications on bufexamac-induced ACD mainly consist of international reports, as this medication has been discontinued in the United States. Bufexamac is a highly sensitizing agent that can lead to severe polymorphic eruptions requiring treatment with prednisolone and even hospitalization.58 In one Australian case report, a mother developed an edematous, erythematous, papulovesicular eruption on the breast while breastfeeding her baby, who was being treated with bufexamac cream 5% for infantile eczema.59 Carprofen-induced photoallergic contact dermatitis is associated with occupational exposure in pharmaceutical workers.60,61 A few case reports on other nonsteroidal anti-inflammatory drugs, including etofenamate and aceclofenac, have been published.62,63
Compounded Medications—Compounded topical analgesics, which help to control pain via multiple combined effects, have gained increasing popularity in the management of chronic neuropathic pain disorders. Only a few recent retrospective studies assessing the efficacy and safety of these medications have mentioned suspected allergic cutaneous reactions.62,63 In 2015, Turrentine et al64 reported a case of ACD to cyclobenzaprine in a compound containing ketamine 10%, diclofenac 5%, baclofen 2%, bupivacaine 1%, cyclobenzaprine 2%, gabapentin 6%, ibuprofen 3%, and pentoxifylline 3% in a proprietary cream base. When patients present with suspected ACD to a compounded pain medication, obtaining individual components for patch testing is key to determining the allergic ingredient(s). We suspect that we will see a rise in reports of ACD as these topical compounds become readily adopted in clinical practices.
Patch Testing for Diagnosis
When patients present with symptoms concerning for ACD to medicaments, the astute clinician should promptly stop the suspected topical medication and consider patch testing. For common allergens such as neomycin, bacitracin, or ethylenediamine, commercial patch test preparations exist and should be used; however, for drugs that do not have a commercial patch test preparation, the patient’s product can be applied as is, keeping in mind that certain preparations (such as retinoids) can cause irritant patch test reactions, which may confound the reading. Alternatively, individual ingredients in the medication’s formulation can be requested from the manufacturer or a compounding pharmacy for targeted testing. Suggested concentrations for patch testing based on the literature and expert reference are listed in the Table. The authors (M.R., A.R.A.) frequently rely on an expert reference66 to determine ideal concentrations for patch testing. Referral to a specialized patch test clinic may be appropriate.
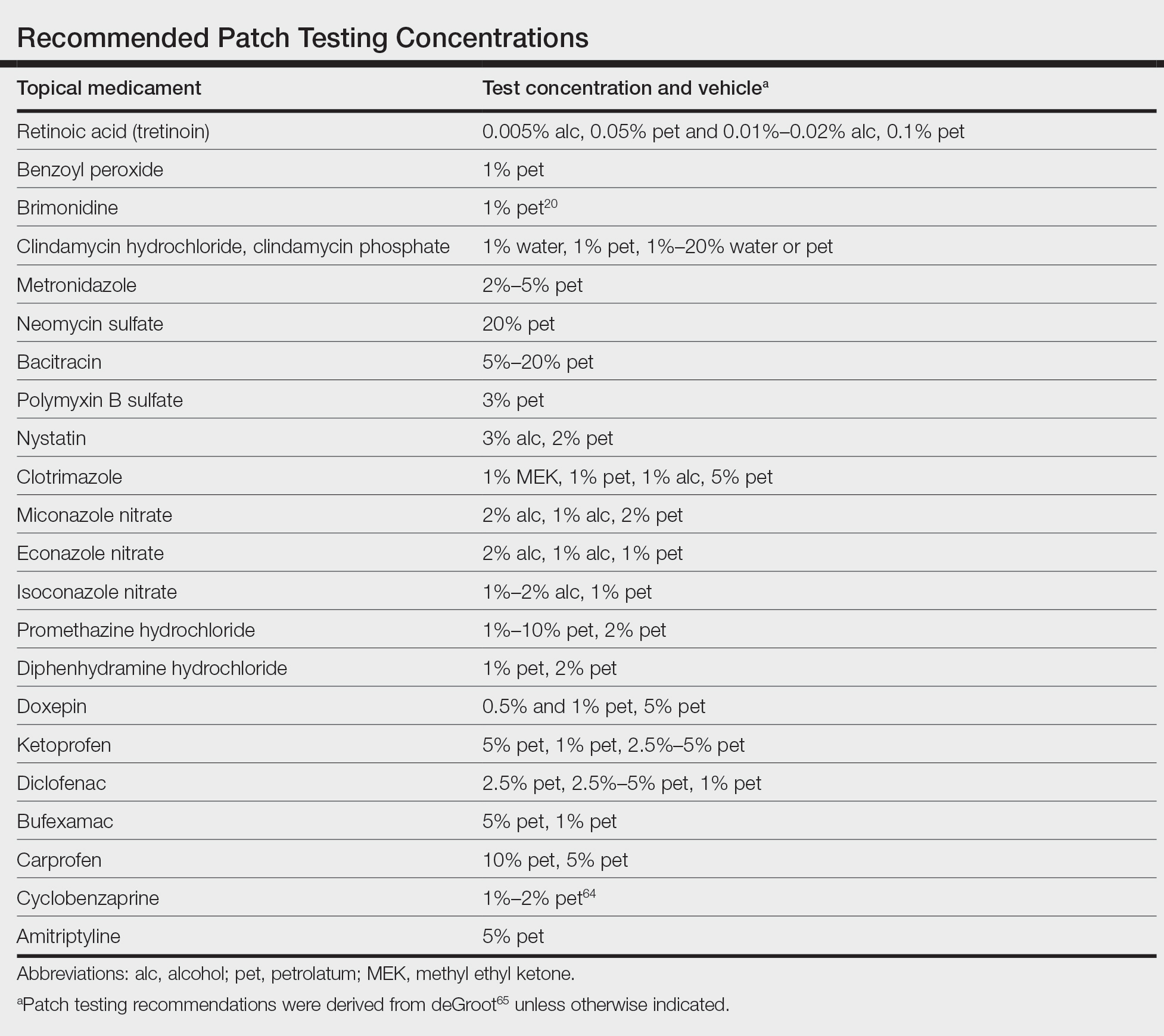
Final Interpretation
Although their intent is to heal, topical medicaments also can be a source of ACD. The astute clinician should consider ACD when topicals either no longer seem to help the patient or trigger new-onset dermatitis. Patch testing directly with the culprit medicament, or individual medication ingredients when needed, can lead to the diagnosis, though caution is advised. Stay tuned for part 2 of this series in which we will discuss ACD to topical steroids, immunomodulators, and anesthetic medications.
- Davis MD. Unusual patterns in contact dermatitis: medicaments. Dermatol Clin. 2009;27:289-297, vi. doi:10.1016/j.det.2009.05.003
- Gilissen L, Goossens A. Frequency and trends of contact allergy to and iatrogenic contact dermatitis caused by topical drugs over a 25-year period. Contact Dermatitis. 2016;75:290-302. doi:10.1111/cod.12621
- Balato N, Patruno C, Lembo G, et al. Allergic contact dermatitis from retinoic acid. Contact Dermatitis. 1995;32:51. doi:10.1111/j.1600-0536.1995.tb00846.x
- Berg JE, Bowman JP, Saenz AB. Cumulative irritation potential and contact sensitization potential of tazarotene foam 0.1% in 2 phase 1 patch studies. Cutis. 2012;90:206-211.
- Numata T, Jo R, Kobayashi Y, et al. Allergic contact dermatitis caused by adapalene. Contact Dermatitis. 2015;73:187-188. doi:10.1111/cod.12410
- Anderson A, Gebauer K. Periorbital allergic contact dermatitis resulting from topical retinoic acid use. Australas J Dermatol. 2014;55:152-153. doi:10.1111/ajd.12041
- Blondeel A. Contact allergy to vitamin A. Contact Dermatitis. 1984;11:191-192. doi:10.1111/j.1600-0536.1984.tb00976.x
- Manzano D, Aguirre A, Gardeazabal J, et al. Allergic contact dermatitis from tocopheryl acetate (vitamin E) and retinol palmitate (vitamin A) in a moisturizing cream. Contact Dermatitis. 1994;31:324. doi:10.1111/j.1600-0536.1994.tb02030.x
- Heidenheim M, Jemec GB. Occupational allergic contact dermatitis from vitamin A acetate. Contact Dermatitis. 1995;33:439. doi:10.1111/j.1600-0536.1995.tb02091.x
- Kim C, Craiglow BG, Watsky KL, et al. Allergic contact dermatitis to benzoyl peroxide resembling impetigo. Pediatr Dermatol. 2015;32:E161-E162. doi:10.1111/pde.12585
- Sandre M, Skotnicki-Grant S. A case of a paediatric patient with allergic contact dermatitis to benzoyl peroxide. J Cutan Med Surg. 2018;22:226-228. doi:10.1177/1203475417733462
- Corazza M, Amendolagine G, Musmeci D, et al. Sometimes even Dr Google is wrong: an unusual contact dermatitis caused by benzoyl peroxide. Contact Dermatitis. 2018;79:380-381. doi:10.1111/cod.13086
- Adelman M, Mohammad T, Kerr H. Allergic contact dermatitis due to benzoyl peroxide from an unlikely source. Dermatitis. 2019;30:230-231. doi:10.1097/DER.0000000000000470
- Gatica-Ortega ME, Pastor-Nieto MA. Allergic contact dermatitis to Glycyrrhiza inflata root extract in an anti-acne cosmetic product [published online April 28, 2021]. Contact Dermatitis. doi:10.1111/cod.13872
- Ockenfels HM, Uter W, Lessmann H, et al. Patch testing with benzoyl peroxide: reaction profile and interpretation of positive patch test reactions. Contact Dermatitis. 2009;61:209-216. doi:10.1111/j.1600-0536.2009.01603.x
- Sodhi PK, Verma L, Ratan J. Dermatological side effects of brimonidine: a report of three cases. J Dermatol. 2003;30:697-700. doi:10.1111/j.1346-8138.2003.tb00461.x
- Swanson LA, Warshaw EM. Allergic contact dermatitis to topical brimonidine tartrate gel 0.33% for treatment of rosacea. J Am Acad Dermatol. 2014;71:832-833. doi:10.1016/j.jaad.2014.05.073
- Bangsgaard N, Fischer LA, Zachariae C. Sensitization to and allergic contact dermatitis caused by Mirvaso(®)(brimonidine tartrate) for treatment of rosacea—2 cases. Contact Dermatitis. 2016;74:378-379. doi:10.1111/cod.12547
- Ringuet J, Houle MC. Case report: allergic contact dermatitis to topical brimonidine demonstrated with patch testing: insights on evaluation of brimonidine sensitization. J Cutan Med Surg. 2018;22:636-638. doi:10.1177/1203475418789020
- Cookson H, McFadden J, White J, et al. Allergic contact dermatitis caused by Mirvaso®, brimonidine tartrate gel 0.33%, a new topical treatment for rosaceal erythema. Contact Dermatitis. 2015;73:366-367. doi:10.1111/cod.12476
- Rajagopalan A, Rajagopalan B. Allergic contact dermatitis to topical brimonidine. Australas J Dermatol. 2015;56:235. doi:10.1111/ajd.12299
- Veraldi S, Brena M, Barbareschi M. Allergic contact dermatitis caused by topical antiacne drugs. Expert Rev Clin Pharmacol. 2015;8:377-381. doi:10.1586/17512433.2015.1046839
- Vejlstrup E, Menné T. Contact dermatitis from clindamycin. Contact Dermatitis. 1995;32:110. doi:10.1111/j.1600-0536.1995.tb00759.x
- García R, Galindo PA, Feo F, et al. Delayed allergic reactions to amoxycillin and clindamycin. Contact Dermatitis. 1996;35:116-117. doi:10.1111/j.1600-0536.1996.tb02312.x
- Muñoz D, Del Pozo MD, Audicana M, et al. Erythema-multiforme-like eruption from antibiotics of 3 different groups. Contact Dermatitis. 1996;34:227-228. doi:10.1111/j.1600-0536.1996.tb02187.x
- Romita P, Ettorre G, Corazza M, et al. Allergic contact dermatitis caused by clindamycin mimicking ‘retinoid flare.’ Contact Dermatitis. 2017;77:181-182. doi:10.1111/cod.12784
- Veraldi S, Guanziroli E, Ferrucci S, et al. Allergic contact dermatitis caused by clindamycin. Contact Dermatitis. 2019;80:68-69. doi:10.1111/cod.13133
- Voller LM, Kullberg SA, Warshaw EM. Axillary allergic contact dermatitis to topical clindamycin. Contact Dermatitis. 2020;82:313-314. doi:10.1111/cod.13465
- de Kort WJ, de Groot AC. Clindamycin allergy presenting as rosacea. Contact Dermatitis. 1989;20:72-73. doi:10.1111/j.1600-0536.1989.tb03108.x
- Vincenzi C, Lucente P, Ricci C, et al. Facial contact dermatitis due to metronidazole. Contact Dermatitis. 1997;36:116-117. doi:10.1111/j.1600-0536.1997.tb00434.x
- Wolf R, Orion E, Matz H. Co-existing sensitivity to metronidazole and isothiazolinone. Clin Exp Dermatol. 2003;28:506-507. doi:10.1046/j.1365-2230.2003.01364.x
- Madsen JT, Thormann J, Kerre S, et al. Allergic contact dermatitis to topical metronidazole—3 cases. Contact Dermatitis. 2007;56:364-366. doi:10.1111/j.1600-0536.2006.01064.x
- Fernández-Jorge B, Goday Buján J, Fernández-Torres R, et al. Concomitant allergic contact dermatitis from diphenhydramine and metronidazole. Contact Dermatitis. 2008;59:115-116. doi:10.1111/j.1600-0536.2008.01332.x
- Madsen JT, Lorentzen HF, Paulsen E. Contact sensitization to metronidazole from possible occupational exposure. Contact Dermatitis. 2009;60:117-118. doi:10.1111/j.1600-0536.2008.01490.x
- Beutner KR, Lemke S, Calvarese B. A look at the safety of metronidazole 1% gel: cumulative irritation, contact sensitization, phototoxicity, and photoallergy potential. Cutis. 2006;77(4 suppl):12-17.
- Jappe U, Schäfer T, Schnuch A, et al. Contact allergy in patients with rosacea: a clinic-based, prospective epidemiological study. J Eur Acad Dermatol Venereol. 2008;22:1208-1214. doi:10.1111/j.1468-3083.2008.02778.x
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group Patch Test Results: 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Comaish JS, Cunliffe WJ. Absorption of drugs from varicose ulcers: a cause of anaphylaxis. Br J Clin Pract. 1967;21:97-98.
- Roupe G, Strannegård O. Anaphylactic shock elicited by topical administration of bacitracin. Arch Dermatol. 1969;100:450-452.
- Farley M, Pak H, Carregal V, et al. Anaphylaxis to topically applied bacitracin. Am J Contact Dermat. 1995;6:28-31.
- Barranco R, Tornero P, de Barrio M, et al. Type IV hypersensitivity to oral nystatin. Contact Dermatitis. 2001;45:60. doi:10.1034/j.1600-0536.2001.045001060.x
- Cooper SM, Shaw S. Contact allergy to nystatin: an unusual allergen. Contact Dermatitis. 1999;41:120. doi:10.1111/j.1600-0536.1999.tb06254.x
- Dooms-Goossens A, Matura M, Drieghe J, et al. Contact allergy to imidazoles used as antimycotic agents. Contact Dermatitis. 1995;33:73-77. doi:10.1111/j.1600-0536.1995.tb00504.x
- Pérez-Mesonero R, Schneller-Pavelescu L, Ochando-Ibernón G, et al. Is tioconazole contact dermatitis still a concern? bringing allergic contact dermatitis caused by topical tioconazole back into the spotlight. Contact Dermatitis. 2019;80:168-169.
- Tang MM, Corti MA, Stirnimann R, et al. Severe cutaneous allergic reactions following topical antifungal therapy. Contact Dermatitis. 2013;68:56-57.
- Goossens A, Linsen G. Contact allergy to antihistamines is not common. Contact Dermatitis. 1998;39:38. doi:10.1111/j.1600-0536.1998.tb05817.x
- Taylor JS, Praditsuwan P, Handel D, et al. Allergic contact dermatitis from doxepin cream. one-year patch test clinic experience. Arch Dermatol. 1996;132:515-518.
- Bilbao I, Aguirre A, Vicente JM, et al. Allergic contact dermatitis due to 5% doxepin cream. Contact Dermatitis. 1996;35:254-255. doi:10.1111/j.1600-0536.1996.tb02374.x
- Shelley WB, Shelley ED, Talanin NY. Self-potentiating allergic contact dermatitis caused by doxepin hydrochloride cream. J Am Acad Dermatol. 1996;34:143-144. doi:10.1016/s0190-9622(96)90864-6
- Wakelin SH, Rycroft RJ. Allergic contact dermatitis from doxepin. Contact Dermatitis. 1999;40:214. doi:10.1111/j.1600-0536.1999.tb06037.x
- Horn HM, Tidman MJ, Aldridge RD. Allergic contact dermatitis due to doxepin cream in a patient with dystrophic epidermolysis bullosa. Contact Dermatitis. 2001;45:115. doi:10.1034/j.1600-0536.2001.045002115.x
- Bonnel RA, La Grenade L, Karwoski CB, et al. Allergic contact dermatitis from topical doxepin: Food and Drug Administration’s postmarketing surveillance experience. J Am Acad Dermatol. 2003;48:294-296. doi:10.1067/mjd.2003.46
- Devleeschouwer V, Roelandts R, Garmyn M, et al. Allergic and photoallergic contact dermatitis from ketoprofen: results of (photo) patch testing and follow-up of 42 patients. Contact Dermatitis. 2008;58:159-166. doi:10.1111/j.1600-0536.2007.01296.x
- Foti C, Bonamonte D, Conserva A, et al. Allergic and photoallergic contact dermatitis from ketoprofen: evaluation of cross-reactivities by a combination of photopatch testing and computerized conformational analysis. Curr Pharm Des. 2008;14:2833-2839. doi:10.2174/138161208786369696
- Gulin SJ, Chiriac A. Diclofenac-induced allergic contact dermatitis: a series of four patients. Drug Saf Case Rep. 2016;3:15. doi:10.1007/s40800-016-0039-3
- Lakshmi C, Srinivas CR. Systemic (allergic) contact dermatitis to diclofenac. Indian J Dermatol Venereol Leprol. 2011;77:536. doi:10.4103/0378-6323.82424
- Beutner C, Forkel S, Kreipe K, et al. Contact allergy to topical diclofenac with systemic tolerance [published online August 22, 2021]. Contact Dermatitis. doi:10.1111/cod.13961
- Pan Y, Nixon R. Allergic contact dermatitis to topical preparations of bufexamac. Australas J Dermatol. 2012;53:207-210. doi:10.1111/j.1440-0960.2012.00876.x
- Nakada T, Matsuzawa Y. Allergic contact dermatitis syndrome from bufexamac for nursing infant. Dermatitis. 2012;23:185-186. doi:10.1097/DER.0b013e318260d774
- Kerr AC, Muller F, Ferguson J, et al. Occupational carprofen photoallergic contact dermatitis. Br J Dermatol. 2008;159:1303-1308. doi:10.1111/j.1365-2133.2008.08847.x
- Kiely C, Murphy G. Photoallergic contact dermatitis caused by occupational exposure to the canine non-steroidal anti-inflammatory drug carprofen. Contact Dermatitis. 2010;63:364-365. doi:10.1111/j.1600-0536.2010.01820.x
- Somberg J, Molnar J. Retrospective evaluation on the analgesic activities of 2 compounded topical creams and voltaren gel in chronic noncancer pain. Am J Ther. 2015;22:342-349. doi:10.1097/MJT.0000000000000275
- Lee HG, Grossman SK, Valdes-Rodriguez R, et al. Topical ketamine-amitriptyline-lidocaine for chronic pruritus: a retrospective study assessing efficacy and tolerability. J Am Acad Dermatol. 2017;76:760-761. doi:10.1016/j.jaad.2016.10.030
- Turrentine JE, Marrazzo G, Cruz PD Jr. Novel use of patch testing in the first report of allergic contact dermatitis to cyclobenzaprine. Dermatitis. 2015;26:60-61. doi:10.1097/DER.0000000000000099
- de Groot A. Patch Testing. 3rd ed. acdegroot publishing; 2008.
- de Groot A. Patch Testing. 4th ed. acdegroot publishing; 2018.
Topical medications frequently are prescribed in dermatology and provide the advantages of direct skin penetration and targeted application while typically sparing patients from systemic effects. Adverse cutaneous effects include allergic contact dermatitis (ACD), irritant contact dermatitis (ICD), photosensitivity, urticaria, hyperpigmentation or hypopigmentation, atrophy, periorificial dermatitis, and acneform eruptions. Allergic contact dermatitis can develop from the active drug or vehicle components.
Patients with medicament ACD often present with symptoms of pruritus and dermatitis at the site of topical application. They may express concern that the medication is no longer working or seems to be making things worse. Certain sites are more prone to developing medicament dermatitis, including the face, groin, and lower legs. Older adults may be more at risk. Other risk factors include pre-existing skin diseases such as stasis dermatitis, acne, psoriasis, atopic dermatitis, and genital dermatoses.1 A review of 14,911 patch-tested patients from a single referral clinic revealed that 17.4% had iatrogenic contact dermatitis, with the most common culprits being topical antibiotics, antiseptics, and steroids.2
In this 2-part series, we will focus on the active drug as a source of ACD. Part 1 explores ACD associated with acne and rosacea medications, antimicrobials, antihistamines, and topical pain preparations.
Acne and Rosacea Medications
Retinoids—Topical retinoids are first-line acne treatments that help normalize skin keratinization. Irritant contact dermatitis from retinoids is a well-known and common side effect. Although far less common than ICD, ACD from topical retinoid use has been reported.3,4 Reactions to tretinoin are most frequently reported in the literature compared to adapalene gel5 and tazarotene foam, which have lower potential for sensitization.6 Allergic contact dermatitis also has been reported from retinyl palmitate7,8 in cosmetic creams and from occupational exposure in settings of industrial vitamin A production.9 Both ICD and ACD from topical retinoids can present with pruritus, erythema, and scaling. Given this clinical overlap between ACD and ICD, patch testing is crucial in differentiating the underlying etiology of the dermatitis.
Benzoyl Peroxide—Benzoyl peroxide (BP) is another popular topical acne treatment that targets Cutibacterium acnes, a bacterium often implicated in the pathogenesis of acne vulgaris. Similar to retinoids, ICD is more common than ACD. Several cases of ACD to BP have been reported.10-14 Occasionally, honey-colored crusting associated with ACD to BP can mimic impetigo.10 Aside from use of BP as an acne treatment, other potential exposures to BP include bleached flour13 and orthopedic bone cement. Occupations at risk for potential BP exposure include dental technicians15 and those working in plastic manufacturing.
Brimonidine—Brimonidine tartrate is a selective α2-adrenergic agonist initially used to treat open-angle glaucoma and also is used as a topical treatment for rosacea. Allergic reactions to brimonidine eye drops may present with periorbital hyperpigmentation and pruritic bullous lesions.16 Case reports of topical brimonidine ACD have demonstrated mixed patch test results, with positive patch tests to Mirvaso (Galderma) as is but negative patch tests to pure brimonidine tartrate 0.33%.17,18 Ringuet and Houle19 reported the first known positive patch test reaction to pure topical brimonidine, testing with brimonidine tartrate 1% in petrolatum.20,21 Clinicians should be attuned to ACD to topical brimonidine in patients previously treated for glaucoma, as prior use of ophthalmic preparations may result in sensitization.18,20
Antimicrobials
Clindamycin—Clindamycin targets bacterial protein synthesis and is an effective adjunct in the treatment of acne. Despite its widespread and often long-term use, topical clindamycin is a weak sensitizer.22 To date, limited case reports on ACD to topical clindamycin exist.23-28 Rare clinical patterns of ACD to clindamycin include mimickers of irritant retinoid dermatitis, erythema multiforme, or pustular rosacea.25,26,29
Metronidazole—Metronidazole is a bactericidal agent that disrupts nucleic acid synthesis with additional anti-inflammatory properties used in the treatment of rosacea. Allergic contact dermatitis to topical metronidazole has been reported.30-34 In 2006, Beutner at al35 patch tested 215 patients using metronidazole gel 1%, which revealed no positive reactions to indicate contact sensitization. Similarly, Jappe et al36 found no positive reactions to metronidazole 2% in petrolatum in their prospective analysis of 78 rosacea patients, further highlighting the exceptionally low incidence of ACD. Cross-reaction with isothiazolinone, which shares structurally similar properties to metronidazole, has been speculated.31,34 One patient developed an acute reaction to metronidazole gel 0.75% within 24 hours of application, suggesting that isothiazolinone may act as a sensitizer, though this relationship has not been proven.31
Neomycin—Neomycin blocks bacterial protein synthesis and is available in both prescription and over-the-counter (OTC) formulations. It commonly is used to treat and prevent superficial wound infections as an OTC antibiotic and also has otic, ophthalmologic, gastroenterologic, urologic, and peritoneal formulations. It also can be used in the dental and veterinary fields and is present in some animal feeds and in trace amounts in some vaccines for humans. Neomycin is a common antibiotic contact allergen, and the most recently reported 2017-2018 North American Contact Dermatitis Group data cycle placed it at number 12 with 5.4% positivity.37 Co-reactions with bacitracin can occur, substantially limiting OTC topical antibiotic options for allergic patients. A safe alternative for patients with neomycin (and bacitracin and polymyxin) contact allergy is prescription mupirocin.
Bacitracin—Bacitracin interferes with peptidoglycan and cell-wall synthesis to treat superficial cutaneous infections. Similar to neomycin, it also can be found in OTC antibiotic ointments as well as in antibacterial bandages. There are several case reports of patients with both type IV delayed hypersensitivity (contact dermatitis) and type I anaphylactic reactions to bacitracin38-40; patch testers should be aware of this rare association. Bacitracin was positive in 5.5% of patch tested patients in the 2017-2018 North American Contact Dermatitis Group data cycle,37 and as with neomycin, bacitracin also is commonly patch tested in most screening patch test series.
Polymyxin—Polymyxin is a polypeptide topical antibiotic that is used to treat superficial wound infections and can be used in combination with neomycin and/or bacitracin. Historically, it is a less common antibiotic allergen; however, it is now frequently included in comprehensive patch test series, as the frequency of positive reactions seems to be increasing, probably due to polysensitization with neomycin and bacitracin.
Nystatin—Nystatin is an antifungal that binds to ergosterol and disrupts the cell wall. Cases exist of ACD to topical nystatin as well as systemic ACD from oral exposure, though both are quite rare. Authors have surmised that the overall low rates of ACD may be due to poor skin absorption of nystatin, which also can confound patch testing.41,42 For patients with suspected ACD to nystatin, repeat open application testing also can be performed to confirm allergy.
Imidazole Antifungals—Similar to nystatins, imidazole antifungals also work by disrupting the fungal cell wall. Imidazole antifungal preparations that have been reported to cause ACD include clotrimazole, miconazole, econazole, and isoconazole, and although cross-reactivity patterns have been described, they are not always reproducible with patch testing.43 In one reported case, tioconazole found in an antifungal nail lacquer triggered ACD involving not only the fingers and toes but also the trunk.44 Erythema multiforme–like reactions also have been described from topical use.45 Commercial patch test preparations of the most common imidazole allergens do exist. Nonimidazole antifungals remain a safe option for allergic patients.
Antihistamines
Antihistamines, or H1-receptor antagonists, are marketed to be applied topically for relief of pruritus associated with allergic cutaneous reactions. Ironically, they are known to be potent sensitizers themselves. There are 6 main chemical classes of antihistamines: phenothiazines, ethylenediamines, ethanolamines, alkylamines, piperazines, and piperidines. Goossens and Linsen46 patch tested 12,460 patients from 1978 to 1997 and found the most positive reactions to promethazine (phenothiazine)(n=12), followed by diphenhydramine (ethanolamine)(n=8) and clemizole (benzimidazole)(n=6). The authors also noted cross-reactions between diphenhydramine derivatives and between promethazine and chlorpromazine.46
Doxepin is a tricyclic antidepressant with antihistamine activity and is a well-documented sensitizer.47-52 Taylor et al47 evaluated 97 patients with chronic dermatoses, and patch testing revealed 17 (17.5%) positive reactions to doxepin cream, 13 (76.5%) of which were positive reactions to both the commercial cream and the active ingredient. Patch testing using doxepin dilution as low as 0.5% in petrolatum is sufficient to provoke a strong (++) allergic reaction.50,51 Early-onset ACD following the use of doxepin cream suggests the possibility of prior sensitization, perhaps with a structurally similar phenothiazine drug.51 A keen suspicion for ACD in patients using doxepin cream for longer than the recommended duration can help make the diagnosis.49,52
Topical Analgesics
Nonsteroidal Anti-inflammatory Drugs—Ketoprofen is one of the most frequent culprits of photoallergic contact dermatitis. Pruritic, papulovesicular, and bullous lesions typically develop acutely weeks after exposure. Prolonged photosensitivity is common and can last years after discontinuation of the nonsteroidal anti-inflammatory drug.53 Cases of cross-reactions and co-sensitization to structurally similar substances have been reported, including to benzophenone-related chemicals in sunscreen and aldehyde groups in fragrance mix.53,54
Diclofenac gel generally is well tolerated in the topical treatment of joint pain and inflammation. In the setting of ACD, patients typically present with dermatitis localized to the area of application.55 Immediate cessation and avoidance of topical diclofenac are crucial components of management. Although systemic contact dermatitis has been reported with oral diclofenac use,56 a recent report suggested that oral diclofenac may be well tolerated for some patients with topical ACD.57
Publications on bufexamac-induced ACD mainly consist of international reports, as this medication has been discontinued in the United States. Bufexamac is a highly sensitizing agent that can lead to severe polymorphic eruptions requiring treatment with prednisolone and even hospitalization.58 In one Australian case report, a mother developed an edematous, erythematous, papulovesicular eruption on the breast while breastfeeding her baby, who was being treated with bufexamac cream 5% for infantile eczema.59 Carprofen-induced photoallergic contact dermatitis is associated with occupational exposure in pharmaceutical workers.60,61 A few case reports on other nonsteroidal anti-inflammatory drugs, including etofenamate and aceclofenac, have been published.62,63
Compounded Medications—Compounded topical analgesics, which help to control pain via multiple combined effects, have gained increasing popularity in the management of chronic neuropathic pain disorders. Only a few recent retrospective studies assessing the efficacy and safety of these medications have mentioned suspected allergic cutaneous reactions.62,63 In 2015, Turrentine et al64 reported a case of ACD to cyclobenzaprine in a compound containing ketamine 10%, diclofenac 5%, baclofen 2%, bupivacaine 1%, cyclobenzaprine 2%, gabapentin 6%, ibuprofen 3%, and pentoxifylline 3% in a proprietary cream base. When patients present with suspected ACD to a compounded pain medication, obtaining individual components for patch testing is key to determining the allergic ingredient(s). We suspect that we will see a rise in reports of ACD as these topical compounds become readily adopted in clinical practices.
Patch Testing for Diagnosis
When patients present with symptoms concerning for ACD to medicaments, the astute clinician should promptly stop the suspected topical medication and consider patch testing. For common allergens such as neomycin, bacitracin, or ethylenediamine, commercial patch test preparations exist and should be used; however, for drugs that do not have a commercial patch test preparation, the patient’s product can be applied as is, keeping in mind that certain preparations (such as retinoids) can cause irritant patch test reactions, which may confound the reading. Alternatively, individual ingredients in the medication’s formulation can be requested from the manufacturer or a compounding pharmacy for targeted testing. Suggested concentrations for patch testing based on the literature and expert reference are listed in the Table. The authors (M.R., A.R.A.) frequently rely on an expert reference66 to determine ideal concentrations for patch testing. Referral to a specialized patch test clinic may be appropriate.

Final Interpretation
Although their intent is to heal, topical medicaments also can be a source of ACD. The astute clinician should consider ACD when topicals either no longer seem to help the patient or trigger new-onset dermatitis. Patch testing directly with the culprit medicament, or individual medication ingredients when needed, can lead to the diagnosis, though caution is advised. Stay tuned for part 2 of this series in which we will discuss ACD to topical steroids, immunomodulators, and anesthetic medications.
Topical medications frequently are prescribed in dermatology and provide the advantages of direct skin penetration and targeted application while typically sparing patients from systemic effects. Adverse cutaneous effects include allergic contact dermatitis (ACD), irritant contact dermatitis (ICD), photosensitivity, urticaria, hyperpigmentation or hypopigmentation, atrophy, periorificial dermatitis, and acneform eruptions. Allergic contact dermatitis can develop from the active drug or vehicle components.
Patients with medicament ACD often present with symptoms of pruritus and dermatitis at the site of topical application. They may express concern that the medication is no longer working or seems to be making things worse. Certain sites are more prone to developing medicament dermatitis, including the face, groin, and lower legs. Older adults may be more at risk. Other risk factors include pre-existing skin diseases such as stasis dermatitis, acne, psoriasis, atopic dermatitis, and genital dermatoses.1 A review of 14,911 patch-tested patients from a single referral clinic revealed that 17.4% had iatrogenic contact dermatitis, with the most common culprits being topical antibiotics, antiseptics, and steroids.2
In this 2-part series, we will focus on the active drug as a source of ACD. Part 1 explores ACD associated with acne and rosacea medications, antimicrobials, antihistamines, and topical pain preparations.
Acne and Rosacea Medications
Retinoids—Topical retinoids are first-line acne treatments that help normalize skin keratinization. Irritant contact dermatitis from retinoids is a well-known and common side effect. Although far less common than ICD, ACD from topical retinoid use has been reported.3,4 Reactions to tretinoin are most frequently reported in the literature compared to adapalene gel5 and tazarotene foam, which have lower potential for sensitization.6 Allergic contact dermatitis also has been reported from retinyl palmitate7,8 in cosmetic creams and from occupational exposure in settings of industrial vitamin A production.9 Both ICD and ACD from topical retinoids can present with pruritus, erythema, and scaling. Given this clinical overlap between ACD and ICD, patch testing is crucial in differentiating the underlying etiology of the dermatitis.
Benzoyl Peroxide—Benzoyl peroxide (BP) is another popular topical acne treatment that targets Cutibacterium acnes, a bacterium often implicated in the pathogenesis of acne vulgaris. Similar to retinoids, ICD is more common than ACD. Several cases of ACD to BP have been reported.10-14 Occasionally, honey-colored crusting associated with ACD to BP can mimic impetigo.10 Aside from use of BP as an acne treatment, other potential exposures to BP include bleached flour13 and orthopedic bone cement. Occupations at risk for potential BP exposure include dental technicians15 and those working in plastic manufacturing.
Brimonidine—Brimonidine tartrate is a selective α2-adrenergic agonist initially used to treat open-angle glaucoma and also is used as a topical treatment for rosacea. Allergic reactions to brimonidine eye drops may present with periorbital hyperpigmentation and pruritic bullous lesions.16 Case reports of topical brimonidine ACD have demonstrated mixed patch test results, with positive patch tests to Mirvaso (Galderma) as is but negative patch tests to pure brimonidine tartrate 0.33%.17,18 Ringuet and Houle19 reported the first known positive patch test reaction to pure topical brimonidine, testing with brimonidine tartrate 1% in petrolatum.20,21 Clinicians should be attuned to ACD to topical brimonidine in patients previously treated for glaucoma, as prior use of ophthalmic preparations may result in sensitization.18,20
Antimicrobials
Clindamycin—Clindamycin targets bacterial protein synthesis and is an effective adjunct in the treatment of acne. Despite its widespread and often long-term use, topical clindamycin is a weak sensitizer.22 To date, limited case reports on ACD to topical clindamycin exist.23-28 Rare clinical patterns of ACD to clindamycin include mimickers of irritant retinoid dermatitis, erythema multiforme, or pustular rosacea.25,26,29
Metronidazole—Metronidazole is a bactericidal agent that disrupts nucleic acid synthesis with additional anti-inflammatory properties used in the treatment of rosacea. Allergic contact dermatitis to topical metronidazole has been reported.30-34 In 2006, Beutner at al35 patch tested 215 patients using metronidazole gel 1%, which revealed no positive reactions to indicate contact sensitization. Similarly, Jappe et al36 found no positive reactions to metronidazole 2% in petrolatum in their prospective analysis of 78 rosacea patients, further highlighting the exceptionally low incidence of ACD. Cross-reaction with isothiazolinone, which shares structurally similar properties to metronidazole, has been speculated.31,34 One patient developed an acute reaction to metronidazole gel 0.75% within 24 hours of application, suggesting that isothiazolinone may act as a sensitizer, though this relationship has not been proven.31
Neomycin—Neomycin blocks bacterial protein synthesis and is available in both prescription and over-the-counter (OTC) formulations. It commonly is used to treat and prevent superficial wound infections as an OTC antibiotic and also has otic, ophthalmologic, gastroenterologic, urologic, and peritoneal formulations. It also can be used in the dental and veterinary fields and is present in some animal feeds and in trace amounts in some vaccines for humans. Neomycin is a common antibiotic contact allergen, and the most recently reported 2017-2018 North American Contact Dermatitis Group data cycle placed it at number 12 with 5.4% positivity.37 Co-reactions with bacitracin can occur, substantially limiting OTC topical antibiotic options for allergic patients. A safe alternative for patients with neomycin (and bacitracin and polymyxin) contact allergy is prescription mupirocin.
Bacitracin—Bacitracin interferes with peptidoglycan and cell-wall synthesis to treat superficial cutaneous infections. Similar to neomycin, it also can be found in OTC antibiotic ointments as well as in antibacterial bandages. There are several case reports of patients with both type IV delayed hypersensitivity (contact dermatitis) and type I anaphylactic reactions to bacitracin38-40; patch testers should be aware of this rare association. Bacitracin was positive in 5.5% of patch tested patients in the 2017-2018 North American Contact Dermatitis Group data cycle,37 and as with neomycin, bacitracin also is commonly patch tested in most screening patch test series.
Polymyxin—Polymyxin is a polypeptide topical antibiotic that is used to treat superficial wound infections and can be used in combination with neomycin and/or bacitracin. Historically, it is a less common antibiotic allergen; however, it is now frequently included in comprehensive patch test series, as the frequency of positive reactions seems to be increasing, probably due to polysensitization with neomycin and bacitracin.
Nystatin—Nystatin is an antifungal that binds to ergosterol and disrupts the cell wall. Cases exist of ACD to topical nystatin as well as systemic ACD from oral exposure, though both are quite rare. Authors have surmised that the overall low rates of ACD may be due to poor skin absorption of nystatin, which also can confound patch testing.41,42 For patients with suspected ACD to nystatin, repeat open application testing also can be performed to confirm allergy.
Imidazole Antifungals—Similar to nystatins, imidazole antifungals also work by disrupting the fungal cell wall. Imidazole antifungal preparations that have been reported to cause ACD include clotrimazole, miconazole, econazole, and isoconazole, and although cross-reactivity patterns have been described, they are not always reproducible with patch testing.43 In one reported case, tioconazole found in an antifungal nail lacquer triggered ACD involving not only the fingers and toes but also the trunk.44 Erythema multiforme–like reactions also have been described from topical use.45 Commercial patch test preparations of the most common imidazole allergens do exist. Nonimidazole antifungals remain a safe option for allergic patients.
Antihistamines
Antihistamines, or H1-receptor antagonists, are marketed to be applied topically for relief of pruritus associated with allergic cutaneous reactions. Ironically, they are known to be potent sensitizers themselves. There are 6 main chemical classes of antihistamines: phenothiazines, ethylenediamines, ethanolamines, alkylamines, piperazines, and piperidines. Goossens and Linsen46 patch tested 12,460 patients from 1978 to 1997 and found the most positive reactions to promethazine (phenothiazine)(n=12), followed by diphenhydramine (ethanolamine)(n=8) and clemizole (benzimidazole)(n=6). The authors also noted cross-reactions between diphenhydramine derivatives and between promethazine and chlorpromazine.46
Doxepin is a tricyclic antidepressant with antihistamine activity and is a well-documented sensitizer.47-52 Taylor et al47 evaluated 97 patients with chronic dermatoses, and patch testing revealed 17 (17.5%) positive reactions to doxepin cream, 13 (76.5%) of which were positive reactions to both the commercial cream and the active ingredient. Patch testing using doxepin dilution as low as 0.5% in petrolatum is sufficient to provoke a strong (++) allergic reaction.50,51 Early-onset ACD following the use of doxepin cream suggests the possibility of prior sensitization, perhaps with a structurally similar phenothiazine drug.51 A keen suspicion for ACD in patients using doxepin cream for longer than the recommended duration can help make the diagnosis.49,52
Topical Analgesics
Nonsteroidal Anti-inflammatory Drugs—Ketoprofen is one of the most frequent culprits of photoallergic contact dermatitis. Pruritic, papulovesicular, and bullous lesions typically develop acutely weeks after exposure. Prolonged photosensitivity is common and can last years after discontinuation of the nonsteroidal anti-inflammatory drug.53 Cases of cross-reactions and co-sensitization to structurally similar substances have been reported, including to benzophenone-related chemicals in sunscreen and aldehyde groups in fragrance mix.53,54
Diclofenac gel generally is well tolerated in the topical treatment of joint pain and inflammation. In the setting of ACD, patients typically present with dermatitis localized to the area of application.55 Immediate cessation and avoidance of topical diclofenac are crucial components of management. Although systemic contact dermatitis has been reported with oral diclofenac use,56 a recent report suggested that oral diclofenac may be well tolerated for some patients with topical ACD.57
Publications on bufexamac-induced ACD mainly consist of international reports, as this medication has been discontinued in the United States. Bufexamac is a highly sensitizing agent that can lead to severe polymorphic eruptions requiring treatment with prednisolone and even hospitalization.58 In one Australian case report, a mother developed an edematous, erythematous, papulovesicular eruption on the breast while breastfeeding her baby, who was being treated with bufexamac cream 5% for infantile eczema.59 Carprofen-induced photoallergic contact dermatitis is associated with occupational exposure in pharmaceutical workers.60,61 A few case reports on other nonsteroidal anti-inflammatory drugs, including etofenamate and aceclofenac, have been published.62,63
Compounded Medications—Compounded topical analgesics, which help to control pain via multiple combined effects, have gained increasing popularity in the management of chronic neuropathic pain disorders. Only a few recent retrospective studies assessing the efficacy and safety of these medications have mentioned suspected allergic cutaneous reactions.62,63 In 2015, Turrentine et al64 reported a case of ACD to cyclobenzaprine in a compound containing ketamine 10%, diclofenac 5%, baclofen 2%, bupivacaine 1%, cyclobenzaprine 2%, gabapentin 6%, ibuprofen 3%, and pentoxifylline 3% in a proprietary cream base. When patients present with suspected ACD to a compounded pain medication, obtaining individual components for patch testing is key to determining the allergic ingredient(s). We suspect that we will see a rise in reports of ACD as these topical compounds become readily adopted in clinical practices.
Patch Testing for Diagnosis
When patients present with symptoms concerning for ACD to medicaments, the astute clinician should promptly stop the suspected topical medication and consider patch testing. For common allergens such as neomycin, bacitracin, or ethylenediamine, commercial patch test preparations exist and should be used; however, for drugs that do not have a commercial patch test preparation, the patient’s product can be applied as is, keeping in mind that certain preparations (such as retinoids) can cause irritant patch test reactions, which may confound the reading. Alternatively, individual ingredients in the medication’s formulation can be requested from the manufacturer or a compounding pharmacy for targeted testing. Suggested concentrations for patch testing based on the literature and expert reference are listed in the Table. The authors (M.R., A.R.A.) frequently rely on an expert reference66 to determine ideal concentrations for patch testing. Referral to a specialized patch test clinic may be appropriate.

Final Interpretation
Although their intent is to heal, topical medicaments also can be a source of ACD. The astute clinician should consider ACD when topicals either no longer seem to help the patient or trigger new-onset dermatitis. Patch testing directly with the culprit medicament, or individual medication ingredients when needed, can lead to the diagnosis, though caution is advised. Stay tuned for part 2 of this series in which we will discuss ACD to topical steroids, immunomodulators, and anesthetic medications.
- Davis MD. Unusual patterns in contact dermatitis: medicaments. Dermatol Clin. 2009;27:289-297, vi. doi:10.1016/j.det.2009.05.003
- Gilissen L, Goossens A. Frequency and trends of contact allergy to and iatrogenic contact dermatitis caused by topical drugs over a 25-year period. Contact Dermatitis. 2016;75:290-302. doi:10.1111/cod.12621
- Balato N, Patruno C, Lembo G, et al. Allergic contact dermatitis from retinoic acid. Contact Dermatitis. 1995;32:51. doi:10.1111/j.1600-0536.1995.tb00846.x
- Berg JE, Bowman JP, Saenz AB. Cumulative irritation potential and contact sensitization potential of tazarotene foam 0.1% in 2 phase 1 patch studies. Cutis. 2012;90:206-211.
- Numata T, Jo R, Kobayashi Y, et al. Allergic contact dermatitis caused by adapalene. Contact Dermatitis. 2015;73:187-188. doi:10.1111/cod.12410
- Anderson A, Gebauer K. Periorbital allergic contact dermatitis resulting from topical retinoic acid use. Australas J Dermatol. 2014;55:152-153. doi:10.1111/ajd.12041
- Blondeel A. Contact allergy to vitamin A. Contact Dermatitis. 1984;11:191-192. doi:10.1111/j.1600-0536.1984.tb00976.x
- Manzano D, Aguirre A, Gardeazabal J, et al. Allergic contact dermatitis from tocopheryl acetate (vitamin E) and retinol palmitate (vitamin A) in a moisturizing cream. Contact Dermatitis. 1994;31:324. doi:10.1111/j.1600-0536.1994.tb02030.x
- Heidenheim M, Jemec GB. Occupational allergic contact dermatitis from vitamin A acetate. Contact Dermatitis. 1995;33:439. doi:10.1111/j.1600-0536.1995.tb02091.x
- Kim C, Craiglow BG, Watsky KL, et al. Allergic contact dermatitis to benzoyl peroxide resembling impetigo. Pediatr Dermatol. 2015;32:E161-E162. doi:10.1111/pde.12585
- Sandre M, Skotnicki-Grant S. A case of a paediatric patient with allergic contact dermatitis to benzoyl peroxide. J Cutan Med Surg. 2018;22:226-228. doi:10.1177/1203475417733462
- Corazza M, Amendolagine G, Musmeci D, et al. Sometimes even Dr Google is wrong: an unusual contact dermatitis caused by benzoyl peroxide. Contact Dermatitis. 2018;79:380-381. doi:10.1111/cod.13086
- Adelman M, Mohammad T, Kerr H. Allergic contact dermatitis due to benzoyl peroxide from an unlikely source. Dermatitis. 2019;30:230-231. doi:10.1097/DER.0000000000000470
- Gatica-Ortega ME, Pastor-Nieto MA. Allergic contact dermatitis to Glycyrrhiza inflata root extract in an anti-acne cosmetic product [published online April 28, 2021]. Contact Dermatitis. doi:10.1111/cod.13872
- Ockenfels HM, Uter W, Lessmann H, et al. Patch testing with benzoyl peroxide: reaction profile and interpretation of positive patch test reactions. Contact Dermatitis. 2009;61:209-216. doi:10.1111/j.1600-0536.2009.01603.x
- Sodhi PK, Verma L, Ratan J. Dermatological side effects of brimonidine: a report of three cases. J Dermatol. 2003;30:697-700. doi:10.1111/j.1346-8138.2003.tb00461.x
- Swanson LA, Warshaw EM. Allergic contact dermatitis to topical brimonidine tartrate gel 0.33% for treatment of rosacea. J Am Acad Dermatol. 2014;71:832-833. doi:10.1016/j.jaad.2014.05.073
- Bangsgaard N, Fischer LA, Zachariae C. Sensitization to and allergic contact dermatitis caused by Mirvaso(®)(brimonidine tartrate) for treatment of rosacea—2 cases. Contact Dermatitis. 2016;74:378-379. doi:10.1111/cod.12547
- Ringuet J, Houle MC. Case report: allergic contact dermatitis to topical brimonidine demonstrated with patch testing: insights on evaluation of brimonidine sensitization. J Cutan Med Surg. 2018;22:636-638. doi:10.1177/1203475418789020
- Cookson H, McFadden J, White J, et al. Allergic contact dermatitis caused by Mirvaso®, brimonidine tartrate gel 0.33%, a new topical treatment for rosaceal erythema. Contact Dermatitis. 2015;73:366-367. doi:10.1111/cod.12476
- Rajagopalan A, Rajagopalan B. Allergic contact dermatitis to topical brimonidine. Australas J Dermatol. 2015;56:235. doi:10.1111/ajd.12299
- Veraldi S, Brena M, Barbareschi M. Allergic contact dermatitis caused by topical antiacne drugs. Expert Rev Clin Pharmacol. 2015;8:377-381. doi:10.1586/17512433.2015.1046839
- Vejlstrup E, Menné T. Contact dermatitis from clindamycin. Contact Dermatitis. 1995;32:110. doi:10.1111/j.1600-0536.1995.tb00759.x
- García R, Galindo PA, Feo F, et al. Delayed allergic reactions to amoxycillin and clindamycin. Contact Dermatitis. 1996;35:116-117. doi:10.1111/j.1600-0536.1996.tb02312.x
- Muñoz D, Del Pozo MD, Audicana M, et al. Erythema-multiforme-like eruption from antibiotics of 3 different groups. Contact Dermatitis. 1996;34:227-228. doi:10.1111/j.1600-0536.1996.tb02187.x
- Romita P, Ettorre G, Corazza M, et al. Allergic contact dermatitis caused by clindamycin mimicking ‘retinoid flare.’ Contact Dermatitis. 2017;77:181-182. doi:10.1111/cod.12784
- Veraldi S, Guanziroli E, Ferrucci S, et al. Allergic contact dermatitis caused by clindamycin. Contact Dermatitis. 2019;80:68-69. doi:10.1111/cod.13133
- Voller LM, Kullberg SA, Warshaw EM. Axillary allergic contact dermatitis to topical clindamycin. Contact Dermatitis. 2020;82:313-314. doi:10.1111/cod.13465
- de Kort WJ, de Groot AC. Clindamycin allergy presenting as rosacea. Contact Dermatitis. 1989;20:72-73. doi:10.1111/j.1600-0536.1989.tb03108.x
- Vincenzi C, Lucente P, Ricci C, et al. Facial contact dermatitis due to metronidazole. Contact Dermatitis. 1997;36:116-117. doi:10.1111/j.1600-0536.1997.tb00434.x
- Wolf R, Orion E, Matz H. Co-existing sensitivity to metronidazole and isothiazolinone. Clin Exp Dermatol. 2003;28:506-507. doi:10.1046/j.1365-2230.2003.01364.x
- Madsen JT, Thormann J, Kerre S, et al. Allergic contact dermatitis to topical metronidazole—3 cases. Contact Dermatitis. 2007;56:364-366. doi:10.1111/j.1600-0536.2006.01064.x
- Fernández-Jorge B, Goday Buján J, Fernández-Torres R, et al. Concomitant allergic contact dermatitis from diphenhydramine and metronidazole. Contact Dermatitis. 2008;59:115-116. doi:10.1111/j.1600-0536.2008.01332.x
- Madsen JT, Lorentzen HF, Paulsen E. Contact sensitization to metronidazole from possible occupational exposure. Contact Dermatitis. 2009;60:117-118. doi:10.1111/j.1600-0536.2008.01490.x
- Beutner KR, Lemke S, Calvarese B. A look at the safety of metronidazole 1% gel: cumulative irritation, contact sensitization, phototoxicity, and photoallergy potential. Cutis. 2006;77(4 suppl):12-17.
- Jappe U, Schäfer T, Schnuch A, et al. Contact allergy in patients with rosacea: a clinic-based, prospective epidemiological study. J Eur Acad Dermatol Venereol. 2008;22:1208-1214. doi:10.1111/j.1468-3083.2008.02778.x
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group Patch Test Results: 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Comaish JS, Cunliffe WJ. Absorption of drugs from varicose ulcers: a cause of anaphylaxis. Br J Clin Pract. 1967;21:97-98.
- Roupe G, Strannegård O. Anaphylactic shock elicited by topical administration of bacitracin. Arch Dermatol. 1969;100:450-452.
- Farley M, Pak H, Carregal V, et al. Anaphylaxis to topically applied bacitracin. Am J Contact Dermat. 1995;6:28-31.
- Barranco R, Tornero P, de Barrio M, et al. Type IV hypersensitivity to oral nystatin. Contact Dermatitis. 2001;45:60. doi:10.1034/j.1600-0536.2001.045001060.x
- Cooper SM, Shaw S. Contact allergy to nystatin: an unusual allergen. Contact Dermatitis. 1999;41:120. doi:10.1111/j.1600-0536.1999.tb06254.x
- Dooms-Goossens A, Matura M, Drieghe J, et al. Contact allergy to imidazoles used as antimycotic agents. Contact Dermatitis. 1995;33:73-77. doi:10.1111/j.1600-0536.1995.tb00504.x
- Pérez-Mesonero R, Schneller-Pavelescu L, Ochando-Ibernón G, et al. Is tioconazole contact dermatitis still a concern? bringing allergic contact dermatitis caused by topical tioconazole back into the spotlight. Contact Dermatitis. 2019;80:168-169.
- Tang MM, Corti MA, Stirnimann R, et al. Severe cutaneous allergic reactions following topical antifungal therapy. Contact Dermatitis. 2013;68:56-57.
- Goossens A, Linsen G. Contact allergy to antihistamines is not common. Contact Dermatitis. 1998;39:38. doi:10.1111/j.1600-0536.1998.tb05817.x
- Taylor JS, Praditsuwan P, Handel D, et al. Allergic contact dermatitis from doxepin cream. one-year patch test clinic experience. Arch Dermatol. 1996;132:515-518.
- Bilbao I, Aguirre A, Vicente JM, et al. Allergic contact dermatitis due to 5% doxepin cream. Contact Dermatitis. 1996;35:254-255. doi:10.1111/j.1600-0536.1996.tb02374.x
- Shelley WB, Shelley ED, Talanin NY. Self-potentiating allergic contact dermatitis caused by doxepin hydrochloride cream. J Am Acad Dermatol. 1996;34:143-144. doi:10.1016/s0190-9622(96)90864-6
- Wakelin SH, Rycroft RJ. Allergic contact dermatitis from doxepin. Contact Dermatitis. 1999;40:214. doi:10.1111/j.1600-0536.1999.tb06037.x
- Horn HM, Tidman MJ, Aldridge RD. Allergic contact dermatitis due to doxepin cream in a patient with dystrophic epidermolysis bullosa. Contact Dermatitis. 2001;45:115. doi:10.1034/j.1600-0536.2001.045002115.x
- Bonnel RA, La Grenade L, Karwoski CB, et al. Allergic contact dermatitis from topical doxepin: Food and Drug Administration’s postmarketing surveillance experience. J Am Acad Dermatol. 2003;48:294-296. doi:10.1067/mjd.2003.46
- Devleeschouwer V, Roelandts R, Garmyn M, et al. Allergic and photoallergic contact dermatitis from ketoprofen: results of (photo) patch testing and follow-up of 42 patients. Contact Dermatitis. 2008;58:159-166. doi:10.1111/j.1600-0536.2007.01296.x
- Foti C, Bonamonte D, Conserva A, et al. Allergic and photoallergic contact dermatitis from ketoprofen: evaluation of cross-reactivities by a combination of photopatch testing and computerized conformational analysis. Curr Pharm Des. 2008;14:2833-2839. doi:10.2174/138161208786369696
- Gulin SJ, Chiriac A. Diclofenac-induced allergic contact dermatitis: a series of four patients. Drug Saf Case Rep. 2016;3:15. doi:10.1007/s40800-016-0039-3
- Lakshmi C, Srinivas CR. Systemic (allergic) contact dermatitis to diclofenac. Indian J Dermatol Venereol Leprol. 2011;77:536. doi:10.4103/0378-6323.82424
- Beutner C, Forkel S, Kreipe K, et al. Contact allergy to topical diclofenac with systemic tolerance [published online August 22, 2021]. Contact Dermatitis. doi:10.1111/cod.13961
- Pan Y, Nixon R. Allergic contact dermatitis to topical preparations of bufexamac. Australas J Dermatol. 2012;53:207-210. doi:10.1111/j.1440-0960.2012.00876.x
- Nakada T, Matsuzawa Y. Allergic contact dermatitis syndrome from bufexamac for nursing infant. Dermatitis. 2012;23:185-186. doi:10.1097/DER.0b013e318260d774
- Kerr AC, Muller F, Ferguson J, et al. Occupational carprofen photoallergic contact dermatitis. Br J Dermatol. 2008;159:1303-1308. doi:10.1111/j.1365-2133.2008.08847.x
- Kiely C, Murphy G. Photoallergic contact dermatitis caused by occupational exposure to the canine non-steroidal anti-inflammatory drug carprofen. Contact Dermatitis. 2010;63:364-365. doi:10.1111/j.1600-0536.2010.01820.x
- Somberg J, Molnar J. Retrospective evaluation on the analgesic activities of 2 compounded topical creams and voltaren gel in chronic noncancer pain. Am J Ther. 2015;22:342-349. doi:10.1097/MJT.0000000000000275
- Lee HG, Grossman SK, Valdes-Rodriguez R, et al. Topical ketamine-amitriptyline-lidocaine for chronic pruritus: a retrospective study assessing efficacy and tolerability. J Am Acad Dermatol. 2017;76:760-761. doi:10.1016/j.jaad.2016.10.030
- Turrentine JE, Marrazzo G, Cruz PD Jr. Novel use of patch testing in the first report of allergic contact dermatitis to cyclobenzaprine. Dermatitis. 2015;26:60-61. doi:10.1097/DER.0000000000000099
- de Groot A. Patch Testing. 3rd ed. acdegroot publishing; 2008.
- de Groot A. Patch Testing. 4th ed. acdegroot publishing; 2018.
- Davis MD. Unusual patterns in contact dermatitis: medicaments. Dermatol Clin. 2009;27:289-297, vi. doi:10.1016/j.det.2009.05.003
- Gilissen L, Goossens A. Frequency and trends of contact allergy to and iatrogenic contact dermatitis caused by topical drugs over a 25-year period. Contact Dermatitis. 2016;75:290-302. doi:10.1111/cod.12621
- Balato N, Patruno C, Lembo G, et al. Allergic contact dermatitis from retinoic acid. Contact Dermatitis. 1995;32:51. doi:10.1111/j.1600-0536.1995.tb00846.x
- Berg JE, Bowman JP, Saenz AB. Cumulative irritation potential and contact sensitization potential of tazarotene foam 0.1% in 2 phase 1 patch studies. Cutis. 2012;90:206-211.
- Numata T, Jo R, Kobayashi Y, et al. Allergic contact dermatitis caused by adapalene. Contact Dermatitis. 2015;73:187-188. doi:10.1111/cod.12410
- Anderson A, Gebauer K. Periorbital allergic contact dermatitis resulting from topical retinoic acid use. Australas J Dermatol. 2014;55:152-153. doi:10.1111/ajd.12041
- Blondeel A. Contact allergy to vitamin A. Contact Dermatitis. 1984;11:191-192. doi:10.1111/j.1600-0536.1984.tb00976.x
- Manzano D, Aguirre A, Gardeazabal J, et al. Allergic contact dermatitis from tocopheryl acetate (vitamin E) and retinol palmitate (vitamin A) in a moisturizing cream. Contact Dermatitis. 1994;31:324. doi:10.1111/j.1600-0536.1994.tb02030.x
- Heidenheim M, Jemec GB. Occupational allergic contact dermatitis from vitamin A acetate. Contact Dermatitis. 1995;33:439. doi:10.1111/j.1600-0536.1995.tb02091.x
- Kim C, Craiglow BG, Watsky KL, et al. Allergic contact dermatitis to benzoyl peroxide resembling impetigo. Pediatr Dermatol. 2015;32:E161-E162. doi:10.1111/pde.12585
- Sandre M, Skotnicki-Grant S. A case of a paediatric patient with allergic contact dermatitis to benzoyl peroxide. J Cutan Med Surg. 2018;22:226-228. doi:10.1177/1203475417733462
- Corazza M, Amendolagine G, Musmeci D, et al. Sometimes even Dr Google is wrong: an unusual contact dermatitis caused by benzoyl peroxide. Contact Dermatitis. 2018;79:380-381. doi:10.1111/cod.13086
- Adelman M, Mohammad T, Kerr H. Allergic contact dermatitis due to benzoyl peroxide from an unlikely source. Dermatitis. 2019;30:230-231. doi:10.1097/DER.0000000000000470
- Gatica-Ortega ME, Pastor-Nieto MA. Allergic contact dermatitis to Glycyrrhiza inflata root extract in an anti-acne cosmetic product [published online April 28, 2021]. Contact Dermatitis. doi:10.1111/cod.13872
- Ockenfels HM, Uter W, Lessmann H, et al. Patch testing with benzoyl peroxide: reaction profile and interpretation of positive patch test reactions. Contact Dermatitis. 2009;61:209-216. doi:10.1111/j.1600-0536.2009.01603.x
- Sodhi PK, Verma L, Ratan J. Dermatological side effects of brimonidine: a report of three cases. J Dermatol. 2003;30:697-700. doi:10.1111/j.1346-8138.2003.tb00461.x
- Swanson LA, Warshaw EM. Allergic contact dermatitis to topical brimonidine tartrate gel 0.33% for treatment of rosacea. J Am Acad Dermatol. 2014;71:832-833. doi:10.1016/j.jaad.2014.05.073
- Bangsgaard N, Fischer LA, Zachariae C. Sensitization to and allergic contact dermatitis caused by Mirvaso(®)(brimonidine tartrate) for treatment of rosacea—2 cases. Contact Dermatitis. 2016;74:378-379. doi:10.1111/cod.12547
- Ringuet J, Houle MC. Case report: allergic contact dermatitis to topical brimonidine demonstrated with patch testing: insights on evaluation of brimonidine sensitization. J Cutan Med Surg. 2018;22:636-638. doi:10.1177/1203475418789020
- Cookson H, McFadden J, White J, et al. Allergic contact dermatitis caused by Mirvaso®, brimonidine tartrate gel 0.33%, a new topical treatment for rosaceal erythema. Contact Dermatitis. 2015;73:366-367. doi:10.1111/cod.12476
- Rajagopalan A, Rajagopalan B. Allergic contact dermatitis to topical brimonidine. Australas J Dermatol. 2015;56:235. doi:10.1111/ajd.12299
- Veraldi S, Brena M, Barbareschi M. Allergic contact dermatitis caused by topical antiacne drugs. Expert Rev Clin Pharmacol. 2015;8:377-381. doi:10.1586/17512433.2015.1046839
- Vejlstrup E, Menné T. Contact dermatitis from clindamycin. Contact Dermatitis. 1995;32:110. doi:10.1111/j.1600-0536.1995.tb00759.x
- García R, Galindo PA, Feo F, et al. Delayed allergic reactions to amoxycillin and clindamycin. Contact Dermatitis. 1996;35:116-117. doi:10.1111/j.1600-0536.1996.tb02312.x
- Muñoz D, Del Pozo MD, Audicana M, et al. Erythema-multiforme-like eruption from antibiotics of 3 different groups. Contact Dermatitis. 1996;34:227-228. doi:10.1111/j.1600-0536.1996.tb02187.x
- Romita P, Ettorre G, Corazza M, et al. Allergic contact dermatitis caused by clindamycin mimicking ‘retinoid flare.’ Contact Dermatitis. 2017;77:181-182. doi:10.1111/cod.12784
- Veraldi S, Guanziroli E, Ferrucci S, et al. Allergic contact dermatitis caused by clindamycin. Contact Dermatitis. 2019;80:68-69. doi:10.1111/cod.13133
- Voller LM, Kullberg SA, Warshaw EM. Axillary allergic contact dermatitis to topical clindamycin. Contact Dermatitis. 2020;82:313-314. doi:10.1111/cod.13465
- de Kort WJ, de Groot AC. Clindamycin allergy presenting as rosacea. Contact Dermatitis. 1989;20:72-73. doi:10.1111/j.1600-0536.1989.tb03108.x
- Vincenzi C, Lucente P, Ricci C, et al. Facial contact dermatitis due to metronidazole. Contact Dermatitis. 1997;36:116-117. doi:10.1111/j.1600-0536.1997.tb00434.x
- Wolf R, Orion E, Matz H. Co-existing sensitivity to metronidazole and isothiazolinone. Clin Exp Dermatol. 2003;28:506-507. doi:10.1046/j.1365-2230.2003.01364.x
- Madsen JT, Thormann J, Kerre S, et al. Allergic contact dermatitis to topical metronidazole—3 cases. Contact Dermatitis. 2007;56:364-366. doi:10.1111/j.1600-0536.2006.01064.x
- Fernández-Jorge B, Goday Buján J, Fernández-Torres R, et al. Concomitant allergic contact dermatitis from diphenhydramine and metronidazole. Contact Dermatitis. 2008;59:115-116. doi:10.1111/j.1600-0536.2008.01332.x
- Madsen JT, Lorentzen HF, Paulsen E. Contact sensitization to metronidazole from possible occupational exposure. Contact Dermatitis. 2009;60:117-118. doi:10.1111/j.1600-0536.2008.01490.x
- Beutner KR, Lemke S, Calvarese B. A look at the safety of metronidazole 1% gel: cumulative irritation, contact sensitization, phototoxicity, and photoallergy potential. Cutis. 2006;77(4 suppl):12-17.
- Jappe U, Schäfer T, Schnuch A, et al. Contact allergy in patients with rosacea: a clinic-based, prospective epidemiological study. J Eur Acad Dermatol Venereol. 2008;22:1208-1214. doi:10.1111/j.1468-3083.2008.02778.x
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group Patch Test Results: 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Comaish JS, Cunliffe WJ. Absorption of drugs from varicose ulcers: a cause of anaphylaxis. Br J Clin Pract. 1967;21:97-98.
- Roupe G, Strannegård O. Anaphylactic shock elicited by topical administration of bacitracin. Arch Dermatol. 1969;100:450-452.
- Farley M, Pak H, Carregal V, et al. Anaphylaxis to topically applied bacitracin. Am J Contact Dermat. 1995;6:28-31.
- Barranco R, Tornero P, de Barrio M, et al. Type IV hypersensitivity to oral nystatin. Contact Dermatitis. 2001;45:60. doi:10.1034/j.1600-0536.2001.045001060.x
- Cooper SM, Shaw S. Contact allergy to nystatin: an unusual allergen. Contact Dermatitis. 1999;41:120. doi:10.1111/j.1600-0536.1999.tb06254.x
- Dooms-Goossens A, Matura M, Drieghe J, et al. Contact allergy to imidazoles used as antimycotic agents. Contact Dermatitis. 1995;33:73-77. doi:10.1111/j.1600-0536.1995.tb00504.x
- Pérez-Mesonero R, Schneller-Pavelescu L, Ochando-Ibernón G, et al. Is tioconazole contact dermatitis still a concern? bringing allergic contact dermatitis caused by topical tioconazole back into the spotlight. Contact Dermatitis. 2019;80:168-169.
- Tang MM, Corti MA, Stirnimann R, et al. Severe cutaneous allergic reactions following topical antifungal therapy. Contact Dermatitis. 2013;68:56-57.
- Goossens A, Linsen G. Contact allergy to antihistamines is not common. Contact Dermatitis. 1998;39:38. doi:10.1111/j.1600-0536.1998.tb05817.x
- Taylor JS, Praditsuwan P, Handel D, et al. Allergic contact dermatitis from doxepin cream. one-year patch test clinic experience. Arch Dermatol. 1996;132:515-518.
- Bilbao I, Aguirre A, Vicente JM, et al. Allergic contact dermatitis due to 5% doxepin cream. Contact Dermatitis. 1996;35:254-255. doi:10.1111/j.1600-0536.1996.tb02374.x
- Shelley WB, Shelley ED, Talanin NY. Self-potentiating allergic contact dermatitis caused by doxepin hydrochloride cream. J Am Acad Dermatol. 1996;34:143-144. doi:10.1016/s0190-9622(96)90864-6
- Wakelin SH, Rycroft RJ. Allergic contact dermatitis from doxepin. Contact Dermatitis. 1999;40:214. doi:10.1111/j.1600-0536.1999.tb06037.x
- Horn HM, Tidman MJ, Aldridge RD. Allergic contact dermatitis due to doxepin cream in a patient with dystrophic epidermolysis bullosa. Contact Dermatitis. 2001;45:115. doi:10.1034/j.1600-0536.2001.045002115.x
- Bonnel RA, La Grenade L, Karwoski CB, et al. Allergic contact dermatitis from topical doxepin: Food and Drug Administration’s postmarketing surveillance experience. J Am Acad Dermatol. 2003;48:294-296. doi:10.1067/mjd.2003.46
- Devleeschouwer V, Roelandts R, Garmyn M, et al. Allergic and photoallergic contact dermatitis from ketoprofen: results of (photo) patch testing and follow-up of 42 patients. Contact Dermatitis. 2008;58:159-166. doi:10.1111/j.1600-0536.2007.01296.x
- Foti C, Bonamonte D, Conserva A, et al. Allergic and photoallergic contact dermatitis from ketoprofen: evaluation of cross-reactivities by a combination of photopatch testing and computerized conformational analysis. Curr Pharm Des. 2008;14:2833-2839. doi:10.2174/138161208786369696
- Gulin SJ, Chiriac A. Diclofenac-induced allergic contact dermatitis: a series of four patients. Drug Saf Case Rep. 2016;3:15. doi:10.1007/s40800-016-0039-3
- Lakshmi C, Srinivas CR. Systemic (allergic) contact dermatitis to diclofenac. Indian J Dermatol Venereol Leprol. 2011;77:536. doi:10.4103/0378-6323.82424
- Beutner C, Forkel S, Kreipe K, et al. Contact allergy to topical diclofenac with systemic tolerance [published online August 22, 2021]. Contact Dermatitis. doi:10.1111/cod.13961
- Pan Y, Nixon R. Allergic contact dermatitis to topical preparations of bufexamac. Australas J Dermatol. 2012;53:207-210. doi:10.1111/j.1440-0960.2012.00876.x
- Nakada T, Matsuzawa Y. Allergic contact dermatitis syndrome from bufexamac for nursing infant. Dermatitis. 2012;23:185-186. doi:10.1097/DER.0b013e318260d774
- Kerr AC, Muller F, Ferguson J, et al. Occupational carprofen photoallergic contact dermatitis. Br J Dermatol. 2008;159:1303-1308. doi:10.1111/j.1365-2133.2008.08847.x
- Kiely C, Murphy G. Photoallergic contact dermatitis caused by occupational exposure to the canine non-steroidal anti-inflammatory drug carprofen. Contact Dermatitis. 2010;63:364-365. doi:10.1111/j.1600-0536.2010.01820.x
- Somberg J, Molnar J. Retrospective evaluation on the analgesic activities of 2 compounded topical creams and voltaren gel in chronic noncancer pain. Am J Ther. 2015;22:342-349. doi:10.1097/MJT.0000000000000275
- Lee HG, Grossman SK, Valdes-Rodriguez R, et al. Topical ketamine-amitriptyline-lidocaine for chronic pruritus: a retrospective study assessing efficacy and tolerability. J Am Acad Dermatol. 2017;76:760-761. doi:10.1016/j.jaad.2016.10.030
- Turrentine JE, Marrazzo G, Cruz PD Jr. Novel use of patch testing in the first report of allergic contact dermatitis to cyclobenzaprine. Dermatitis. 2015;26:60-61. doi:10.1097/DER.0000000000000099
- de Groot A. Patch Testing. 3rd ed. acdegroot publishing; 2008.
- de Groot A. Patch Testing. 4th ed. acdegroot publishing; 2018.
Practice Points
- Allergic contact dermatitis should be suspected in patients with persistent or worsening dermatitis after use of topical medications.
- Prior sensitization is not always apparent, and cross-reactions may occur between structurally similar compounds.
- Although most medicaments can be patch tested as is, patch testing to the individual components may be necessary to identify the causative allergen.
Treatment with novel laser in acne studies targets sebaceous glands
at 12 months, a development that indicates the promise this has a treatment for acne in the future.
Currently, “there is no strong evidence that lasers are better than conventional treatments for acne,” Fernanda H. Sakamoto, MD, PhD, said during a virtual course on laser and aesthetic skin therapy. Some patients struggling with acne “search for so many different options and they end up spending a lot of money,” which, she said, includes an estimated $222 million for laser treatment alone in 2019.
Unlike other existing laser and light options for acne treatment, however, Accure is the first light-based platform to selectively target and injure sebaceous glands, the main source of sebum production and the key to a durable solution for acne. The laser, which uses a 1,726-nm wavelength, is being developed by researchers at the Wellman Center for Photomedicine, at Massachusetts General Hospital, Boston and was granted the European CE mark, which allows marketing of the product in Europe, in May of 2020.
In 2012, Dr. Sakamoto, a dermatologist at the center, and her Wellman colleagues were the first to describe the use of selective photothermolysis to target sebaceous glands. “We found that the peak absorption of lipids in sebaceous glands occurs between 1,700 and 1,720 nm,” she said. “Compared to water, the contrast is not high, so for us to develop a laser that is selective for acne, we needed to develop a strong cooling system and we had to create different methods to make it more selective.” She said that it took about 10 years to develop this laser.
The latest Accure prototype features a smart laser handpiece for real time thermal monitoring and precise delivery of laser emissions. “We have developed a mathematical model which permits us to predict safe and effective treatment patterns,” Dr. Sakamoto said at the meeting, which was named “Laser & Aesthetic Skin Therapy: What’s the Truth?” and was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “It has a unique cooling system that can control and protect the skin.”
The clinical trial for Food and Drug Administration clearance, which was delayed because of the COVID-19 pandemic, is still underway, she said, and the hope is that the laser will cleared by the FDA by next year. She and her Wellman colleagues have been working with four veteran dermatologists to conduct clinical trials of the device: Emil Tanghetti, MD, in California; Roy Geronemus, MD, in New York; Joel Cohen, MD, in Colorado; and Daniel Friedmann, MD, in Texas. As of Oct. 2, 2021, more than 50 patients were enrolled in four IRB-approved studies and an additional 30 are enrolled in a pilot facial acne trial, Dr. Sakamoto said. In the trials, patients are followed at 4, 8, 12, and 24 weeks post treatment.
Among patients enrolled in the pilot facial acne trial, researchers have observed a 100% responder rate for patients with more than five acne lesions at 4, 8, 12, and 24 weeks post treatment. The average lesion reduction at week 12 was 82% and the mean Visual Analog Scale score immediately after treatment was 2.10 out of 10. Each patient received more than 12,000 trigger pulls of energy from the device with no adverse events.
“This laser is absorbed in the near-infrared spectrum, so there is no melanin absorption,” Dr. Sakamoto explained. “It’s pretty much a color-blind laser, so we can treat darker skin types safely, with no side effects.” In other findings, researchers observed a 45% reduction in acne lesions after one treatment session, which “keeps improving over time,” she said. “At 12 weeks, we have clearance of over 80% of the lesions.”
At 12 months, they observed a 90% inflammatory lesion count reduction from baseline and a rapid response to treatment: a 73% reduction achieved after the first two treatment sessions. Histological studies revealed selective sebaceous gland destruction with no damage to the epidermis, surrounding dermis, or other follicular structures.
Dr. Sakamoto disclosed that she has received portions of patent royalties from Massachusetts General Hospital. Accure was cofounded by R. Rox Anderson, MD, the director of the Wellman Center.
at 12 months, a development that indicates the promise this has a treatment for acne in the future.
Currently, “there is no strong evidence that lasers are better than conventional treatments for acne,” Fernanda H. Sakamoto, MD, PhD, said during a virtual course on laser and aesthetic skin therapy. Some patients struggling with acne “search for so many different options and they end up spending a lot of money,” which, she said, includes an estimated $222 million for laser treatment alone in 2019.
Unlike other existing laser and light options for acne treatment, however, Accure is the first light-based platform to selectively target and injure sebaceous glands, the main source of sebum production and the key to a durable solution for acne. The laser, which uses a 1,726-nm wavelength, is being developed by researchers at the Wellman Center for Photomedicine, at Massachusetts General Hospital, Boston and was granted the European CE mark, which allows marketing of the product in Europe, in May of 2020.
In 2012, Dr. Sakamoto, a dermatologist at the center, and her Wellman colleagues were the first to describe the use of selective photothermolysis to target sebaceous glands. “We found that the peak absorption of lipids in sebaceous glands occurs between 1,700 and 1,720 nm,” she said. “Compared to water, the contrast is not high, so for us to develop a laser that is selective for acne, we needed to develop a strong cooling system and we had to create different methods to make it more selective.” She said that it took about 10 years to develop this laser.
The latest Accure prototype features a smart laser handpiece for real time thermal monitoring and precise delivery of laser emissions. “We have developed a mathematical model which permits us to predict safe and effective treatment patterns,” Dr. Sakamoto said at the meeting, which was named “Laser & Aesthetic Skin Therapy: What’s the Truth?” and was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “It has a unique cooling system that can control and protect the skin.”
The clinical trial for Food and Drug Administration clearance, which was delayed because of the COVID-19 pandemic, is still underway, she said, and the hope is that the laser will cleared by the FDA by next year. She and her Wellman colleagues have been working with four veteran dermatologists to conduct clinical trials of the device: Emil Tanghetti, MD, in California; Roy Geronemus, MD, in New York; Joel Cohen, MD, in Colorado; and Daniel Friedmann, MD, in Texas. As of Oct. 2, 2021, more than 50 patients were enrolled in four IRB-approved studies and an additional 30 are enrolled in a pilot facial acne trial, Dr. Sakamoto said. In the trials, patients are followed at 4, 8, 12, and 24 weeks post treatment.
Among patients enrolled in the pilot facial acne trial, researchers have observed a 100% responder rate for patients with more than five acne lesions at 4, 8, 12, and 24 weeks post treatment. The average lesion reduction at week 12 was 82% and the mean Visual Analog Scale score immediately after treatment was 2.10 out of 10. Each patient received more than 12,000 trigger pulls of energy from the device with no adverse events.
“This laser is absorbed in the near-infrared spectrum, so there is no melanin absorption,” Dr. Sakamoto explained. “It’s pretty much a color-blind laser, so we can treat darker skin types safely, with no side effects.” In other findings, researchers observed a 45% reduction in acne lesions after one treatment session, which “keeps improving over time,” she said. “At 12 weeks, we have clearance of over 80% of the lesions.”
At 12 months, they observed a 90% inflammatory lesion count reduction from baseline and a rapid response to treatment: a 73% reduction achieved after the first two treatment sessions. Histological studies revealed selective sebaceous gland destruction with no damage to the epidermis, surrounding dermis, or other follicular structures.
Dr. Sakamoto disclosed that she has received portions of patent royalties from Massachusetts General Hospital. Accure was cofounded by R. Rox Anderson, MD, the director of the Wellman Center.
at 12 months, a development that indicates the promise this has a treatment for acne in the future.
Currently, “there is no strong evidence that lasers are better than conventional treatments for acne,” Fernanda H. Sakamoto, MD, PhD, said during a virtual course on laser and aesthetic skin therapy. Some patients struggling with acne “search for so many different options and they end up spending a lot of money,” which, she said, includes an estimated $222 million for laser treatment alone in 2019.
Unlike other existing laser and light options for acne treatment, however, Accure is the first light-based platform to selectively target and injure sebaceous glands, the main source of sebum production and the key to a durable solution for acne. The laser, which uses a 1,726-nm wavelength, is being developed by researchers at the Wellman Center for Photomedicine, at Massachusetts General Hospital, Boston and was granted the European CE mark, which allows marketing of the product in Europe, in May of 2020.
In 2012, Dr. Sakamoto, a dermatologist at the center, and her Wellman colleagues were the first to describe the use of selective photothermolysis to target sebaceous glands. “We found that the peak absorption of lipids in sebaceous glands occurs between 1,700 and 1,720 nm,” she said. “Compared to water, the contrast is not high, so for us to develop a laser that is selective for acne, we needed to develop a strong cooling system and we had to create different methods to make it more selective.” She said that it took about 10 years to develop this laser.
The latest Accure prototype features a smart laser handpiece for real time thermal monitoring and precise delivery of laser emissions. “We have developed a mathematical model which permits us to predict safe and effective treatment patterns,” Dr. Sakamoto said at the meeting, which was named “Laser & Aesthetic Skin Therapy: What’s the Truth?” and was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “It has a unique cooling system that can control and protect the skin.”
The clinical trial for Food and Drug Administration clearance, which was delayed because of the COVID-19 pandemic, is still underway, she said, and the hope is that the laser will cleared by the FDA by next year. She and her Wellman colleagues have been working with four veteran dermatologists to conduct clinical trials of the device: Emil Tanghetti, MD, in California; Roy Geronemus, MD, in New York; Joel Cohen, MD, in Colorado; and Daniel Friedmann, MD, in Texas. As of Oct. 2, 2021, more than 50 patients were enrolled in four IRB-approved studies and an additional 30 are enrolled in a pilot facial acne trial, Dr. Sakamoto said. In the trials, patients are followed at 4, 8, 12, and 24 weeks post treatment.
Among patients enrolled in the pilot facial acne trial, researchers have observed a 100% responder rate for patients with more than five acne lesions at 4, 8, 12, and 24 weeks post treatment. The average lesion reduction at week 12 was 82% and the mean Visual Analog Scale score immediately after treatment was 2.10 out of 10. Each patient received more than 12,000 trigger pulls of energy from the device with no adverse events.
“This laser is absorbed in the near-infrared spectrum, so there is no melanin absorption,” Dr. Sakamoto explained. “It’s pretty much a color-blind laser, so we can treat darker skin types safely, with no side effects.” In other findings, researchers observed a 45% reduction in acne lesions after one treatment session, which “keeps improving over time,” she said. “At 12 weeks, we have clearance of over 80% of the lesions.”
At 12 months, they observed a 90% inflammatory lesion count reduction from baseline and a rapid response to treatment: a 73% reduction achieved after the first two treatment sessions. Histological studies revealed selective sebaceous gland destruction with no damage to the epidermis, surrounding dermis, or other follicular structures.
Dr. Sakamoto disclosed that she has received portions of patent royalties from Massachusetts General Hospital. Accure was cofounded by R. Rox Anderson, MD, the director of the Wellman Center.
FROM A LASER & AESTHETIC SKIN THERAPY COURSE
FDA OKs iPLEDGE change for gender-neutral language
The Food and Drug Administration has approved a modification to the isotretinoin risk-mitigation program to make it more inclusive for transgender patients.
Beginning on Dec. 13, 2021, Previously, there were three risk categories: females of reproductive potential, females not of reproductive potential, and males.
In recent years, dermatologists and others have advocated for the change, hoping to make the process more inclusive and less intrusive for their transgender patients.
Isotretinoin (Accutane, Absorica, Amnesteem, Claravis, others) has a high risk of severe birth defects, and has been linked with other health issues, making it crucial for those with the ability to become pregnant to take contraceptive precautions while on the medication. Under the iPLEDGE program, physicians, patients, and pharmacies prescribing, using, or dispensing the drug must all be registered, with requirements that include the use of two forms of an effective contraceptive and regular pregnancy testing for patients who can become pregnant.
The FDA had given notification in June 2018 that the REMS modification and labeling change would be required, replacing the gender-specific language with gender-neutral language, according to an FDA spokesperson. The change was based on feedback that the gender-specific language can be a barrier to access for some patients. The FDA approved the modification on Oct. 8.
Expert reactions
“This is an exciting and welcome change from the FDA on iPLEDGE that many dermatologists, myself included, have advocated for quite a few years,” Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, said in an interview.
In a report on the dermatologic care for lesbian, gay, bisexual, and transgender persons published in the Journal of the American Academy of Dermatology, Dr. Yeung and his colleagues noted that more than 10 million lesbian, gay, bisexual and transgender people live in the United States and that improving their health is a public health priority.
“For cisgender patients, nothing has changed – patients will continue to receive appropriate educational material related to isotretinoin based on their pregnancy potential,” Dr. Yeung said. “For transgender and gender diverse patients, this is a huge step forward.”
Under the previous system, doctors were asked to register patients using gender binary categories, “which were confusing when they did not reflect reality” for these patients, Dr. Yeung said. The new system, Dr. Yeung added, “will make my job easier. I no longer have to struggle between respecting the patient’s gender identity and providing medically necessary care for patients with severe acne.”
“The new terminology is not just respectful, it also is simpler and makes more sense,” agreed Joshua D. Safer, MD, executive director of the Center for Transgender Medicine and Surgery at Mount Sinai Health System and professor of medicine at the Icahn School of Medicine at Mount Sinai, New York. “As it stood, a transgender man with his uterus and ovaries in place might be missed in the pregnancy surveillance system because he could simply be labeled a man and not followed further. At the same time, both transgender women and cisgender women who were at no risk of pregnancy could be subject to more medical scrutiny that might have been consider intrusive.”
The change “validates the important point that pregnancy potential is not exclusively defined by sociocultural constructs of gender and allow dermatologists to focus purely on what matters when prescribing isotretinoin – whether an individual is able to become pregnant or not, regardless of their gender identity,” Klint Peebles, MD, a dermatologist at Kaiser Permanente in Washington, D.C., and suburban Maryland, who has also advocated for the change, said in an interview.
FDA elaborates
The modification includes important changes for doctors, pharmacists, and patients alike, according to the FDA.
Health care providers must assign and confirm their currently enrolled patient’s risk category when they first log in to the IPLEDGE REMS website on or after Dec. 13, the effective date. They should be sure any patient whose prescription RMA (iPLEDGE authorization) expires on Dec. 11-12 is told to obtain their prescription before midnight, Eastern time, Dec. 10.
Pharmacists will be affected, too, since the iPLEDGE REMS changed to a new platform vendor and the current “switch” pharmacy management system will be removed as a method to verify authorization to dispense isotretinoin. With these changes, as of Dec. 13, pharmacists can’t use the switch system to obtain a predispense authorization, or RMA (risk management authorization). They will need to obtain an RMA online by accessing the iPLEDGE REMS website or via telephone to the PLEDGE REMS center, 866-495-0654, before dispensing the prescription.
Patients, beginning Dec. 13, will have the option of presenting a unique QR code at the pharmacy on their smartphone rather than providing the iPLEDGE identification number. The code can be accessed by logging into their account on the iPLEDGE REMS website.
Patients with an isotretinoin prescription RMA that expires Dec. 11-12, must obtain the prescription before 11:59 p.m. Eastern time on Dec. 10. If the RMA expires before the prescription is picked up, the patient must begin the authorization process all over again.
Dr. Safer, Dr. Yeung, and Dr. Peebles have no relevant disclosures.
More information on the update and the isotretinoin REMS program is available on the FDA website.
The Food and Drug Administration has approved a modification to the isotretinoin risk-mitigation program to make it more inclusive for transgender patients.
Beginning on Dec. 13, 2021, Previously, there were three risk categories: females of reproductive potential, females not of reproductive potential, and males.
In recent years, dermatologists and others have advocated for the change, hoping to make the process more inclusive and less intrusive for their transgender patients.
Isotretinoin (Accutane, Absorica, Amnesteem, Claravis, others) has a high risk of severe birth defects, and has been linked with other health issues, making it crucial for those with the ability to become pregnant to take contraceptive precautions while on the medication. Under the iPLEDGE program, physicians, patients, and pharmacies prescribing, using, or dispensing the drug must all be registered, with requirements that include the use of two forms of an effective contraceptive and regular pregnancy testing for patients who can become pregnant.
The FDA had given notification in June 2018 that the REMS modification and labeling change would be required, replacing the gender-specific language with gender-neutral language, according to an FDA spokesperson. The change was based on feedback that the gender-specific language can be a barrier to access for some patients. The FDA approved the modification on Oct. 8.
Expert reactions
“This is an exciting and welcome change from the FDA on iPLEDGE that many dermatologists, myself included, have advocated for quite a few years,” Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, said in an interview.
In a report on the dermatologic care for lesbian, gay, bisexual, and transgender persons published in the Journal of the American Academy of Dermatology, Dr. Yeung and his colleagues noted that more than 10 million lesbian, gay, bisexual and transgender people live in the United States and that improving their health is a public health priority.
“For cisgender patients, nothing has changed – patients will continue to receive appropriate educational material related to isotretinoin based on their pregnancy potential,” Dr. Yeung said. “For transgender and gender diverse patients, this is a huge step forward.”
Under the previous system, doctors were asked to register patients using gender binary categories, “which were confusing when they did not reflect reality” for these patients, Dr. Yeung said. The new system, Dr. Yeung added, “will make my job easier. I no longer have to struggle between respecting the patient’s gender identity and providing medically necessary care for patients with severe acne.”
“The new terminology is not just respectful, it also is simpler and makes more sense,” agreed Joshua D. Safer, MD, executive director of the Center for Transgender Medicine and Surgery at Mount Sinai Health System and professor of medicine at the Icahn School of Medicine at Mount Sinai, New York. “As it stood, a transgender man with his uterus and ovaries in place might be missed in the pregnancy surveillance system because he could simply be labeled a man and not followed further. At the same time, both transgender women and cisgender women who were at no risk of pregnancy could be subject to more medical scrutiny that might have been consider intrusive.”
The change “validates the important point that pregnancy potential is not exclusively defined by sociocultural constructs of gender and allow dermatologists to focus purely on what matters when prescribing isotretinoin – whether an individual is able to become pregnant or not, regardless of their gender identity,” Klint Peebles, MD, a dermatologist at Kaiser Permanente in Washington, D.C., and suburban Maryland, who has also advocated for the change, said in an interview.
FDA elaborates
The modification includes important changes for doctors, pharmacists, and patients alike, according to the FDA.
Health care providers must assign and confirm their currently enrolled patient’s risk category when they first log in to the IPLEDGE REMS website on or after Dec. 13, the effective date. They should be sure any patient whose prescription RMA (iPLEDGE authorization) expires on Dec. 11-12 is told to obtain their prescription before midnight, Eastern time, Dec. 10.
Pharmacists will be affected, too, since the iPLEDGE REMS changed to a new platform vendor and the current “switch” pharmacy management system will be removed as a method to verify authorization to dispense isotretinoin. With these changes, as of Dec. 13, pharmacists can’t use the switch system to obtain a predispense authorization, or RMA (risk management authorization). They will need to obtain an RMA online by accessing the iPLEDGE REMS website or via telephone to the PLEDGE REMS center, 866-495-0654, before dispensing the prescription.
Patients, beginning Dec. 13, will have the option of presenting a unique QR code at the pharmacy on their smartphone rather than providing the iPLEDGE identification number. The code can be accessed by logging into their account on the iPLEDGE REMS website.
Patients with an isotretinoin prescription RMA that expires Dec. 11-12, must obtain the prescription before 11:59 p.m. Eastern time on Dec. 10. If the RMA expires before the prescription is picked up, the patient must begin the authorization process all over again.
Dr. Safer, Dr. Yeung, and Dr. Peebles have no relevant disclosures.
More information on the update and the isotretinoin REMS program is available on the FDA website.
The Food and Drug Administration has approved a modification to the isotretinoin risk-mitigation program to make it more inclusive for transgender patients.
Beginning on Dec. 13, 2021, Previously, there were three risk categories: females of reproductive potential, females not of reproductive potential, and males.
In recent years, dermatologists and others have advocated for the change, hoping to make the process more inclusive and less intrusive for their transgender patients.
Isotretinoin (Accutane, Absorica, Amnesteem, Claravis, others) has a high risk of severe birth defects, and has been linked with other health issues, making it crucial for those with the ability to become pregnant to take contraceptive precautions while on the medication. Under the iPLEDGE program, physicians, patients, and pharmacies prescribing, using, or dispensing the drug must all be registered, with requirements that include the use of two forms of an effective contraceptive and regular pregnancy testing for patients who can become pregnant.
The FDA had given notification in June 2018 that the REMS modification and labeling change would be required, replacing the gender-specific language with gender-neutral language, according to an FDA spokesperson. The change was based on feedback that the gender-specific language can be a barrier to access for some patients. The FDA approved the modification on Oct. 8.
Expert reactions
“This is an exciting and welcome change from the FDA on iPLEDGE that many dermatologists, myself included, have advocated for quite a few years,” Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, said in an interview.
In a report on the dermatologic care for lesbian, gay, bisexual, and transgender persons published in the Journal of the American Academy of Dermatology, Dr. Yeung and his colleagues noted that more than 10 million lesbian, gay, bisexual and transgender people live in the United States and that improving their health is a public health priority.
“For cisgender patients, nothing has changed – patients will continue to receive appropriate educational material related to isotretinoin based on their pregnancy potential,” Dr. Yeung said. “For transgender and gender diverse patients, this is a huge step forward.”
Under the previous system, doctors were asked to register patients using gender binary categories, “which were confusing when they did not reflect reality” for these patients, Dr. Yeung said. The new system, Dr. Yeung added, “will make my job easier. I no longer have to struggle between respecting the patient’s gender identity and providing medically necessary care for patients with severe acne.”
“The new terminology is not just respectful, it also is simpler and makes more sense,” agreed Joshua D. Safer, MD, executive director of the Center for Transgender Medicine and Surgery at Mount Sinai Health System and professor of medicine at the Icahn School of Medicine at Mount Sinai, New York. “As it stood, a transgender man with his uterus and ovaries in place might be missed in the pregnancy surveillance system because he could simply be labeled a man and not followed further. At the same time, both transgender women and cisgender women who were at no risk of pregnancy could be subject to more medical scrutiny that might have been consider intrusive.”
The change “validates the important point that pregnancy potential is not exclusively defined by sociocultural constructs of gender and allow dermatologists to focus purely on what matters when prescribing isotretinoin – whether an individual is able to become pregnant or not, regardless of their gender identity,” Klint Peebles, MD, a dermatologist at Kaiser Permanente in Washington, D.C., and suburban Maryland, who has also advocated for the change, said in an interview.
FDA elaborates
The modification includes important changes for doctors, pharmacists, and patients alike, according to the FDA.
Health care providers must assign and confirm their currently enrolled patient’s risk category when they first log in to the IPLEDGE REMS website on or after Dec. 13, the effective date. They should be sure any patient whose prescription RMA (iPLEDGE authorization) expires on Dec. 11-12 is told to obtain their prescription before midnight, Eastern time, Dec. 10.
Pharmacists will be affected, too, since the iPLEDGE REMS changed to a new platform vendor and the current “switch” pharmacy management system will be removed as a method to verify authorization to dispense isotretinoin. With these changes, as of Dec. 13, pharmacists can’t use the switch system to obtain a predispense authorization, or RMA (risk management authorization). They will need to obtain an RMA online by accessing the iPLEDGE REMS website or via telephone to the PLEDGE REMS center, 866-495-0654, before dispensing the prescription.
Patients, beginning Dec. 13, will have the option of presenting a unique QR code at the pharmacy on their smartphone rather than providing the iPLEDGE identification number. The code can be accessed by logging into their account on the iPLEDGE REMS website.
Patients with an isotretinoin prescription RMA that expires Dec. 11-12, must obtain the prescription before 11:59 p.m. Eastern time on Dec. 10. If the RMA expires before the prescription is picked up, the patient must begin the authorization process all over again.
Dr. Safer, Dr. Yeung, and Dr. Peebles have no relevant disclosures.
More information on the update and the isotretinoin REMS program is available on the FDA website.
Skin of Color in Preclinical Medical Education: A Cross-Institutional Comparison and A Call to Action
A ccording to the US Census Bureau, more than half of all Americans are projected to belong to a minority group, defined as any group other than non-Hispanic White alone, by 2044. 1 Consequently, the United States rapidly is becoming a country in which the majority of citizens will have skin of color. Individuals with skin of color are of diverse ethnic backgrounds and include people of African, Latin American, Native American, Pacific Islander, and Asian descent, as well as interethnic backgrounds. 2 Throughout the country, dermatologists along with primary care practitioners may be confronted with certain cutaneous conditions that have varying disease presentations or processes in patients with skin of color. It also is important to note that racial categories are socially rather than biologically constructed, and the term skin of color includes a wide variety of diverse skin types. Nevertheless, the current literature thoroughly supports unique pathophysiologic differences in skin of color as well as variations in disease manifestation compared to White patients. 3-5 For example, the increased lability of melanosomes in skin of color patients, which increases their risk for postinflammatory hyperpigmentation, has been well documented. 5-7 There are various dermatologic conditions that also occur with higher frequency and manifest uniquely in people with darker, more pigmented skin, 7-9 and dermatologists, along with primary care physicians, should feel prepared to recognize and address them.
Extensive evidence also indicates that there are unique aspects to consider while managing certain skin diseases in patients with skin of color.8,10,11 Consequently, as noted on the Skin of Color Society (SOCS) website, “[a]n increase in the body of dermatological literature concerning skin of color as well as the advancement of both basic science and clinical investigational research is necessary to meet the needs of the expanding skin of color population.”2 In the meantime, current knowledge regarding cutaneous conditions that diversely or disproportionately affect skin of color should be actively disseminated to physicians in training. Although patients with skin of color should always have access to comprehensive care and knowledgeable practitioners, the current changes in national and regional demographics further underscore the need for a more thorough understanding of skin of color with regard to disease pathogenesis, diagnosis, and treatment.
Several studies have found that medical students in the United States are minimally exposed to dermatology in general compared to other clinical specialties,12-14 which can easily lead to the underrecognition of disorders that may uniquely or disproportionately affect individuals with pigmented skin. Recent data showed that medical schools typically required fewer than 10 hours of dermatology instruction,12 and on average, dermatologic training made up less than 1% of a medical student’s undergraduate medical education.13,15,16 Consequently, less than 40% of primary care residents felt that their medical school curriculum adequately prepared them to manage common skin conditions.14 Although not all physicians should be expected to fully grasp the complexities of skin of color and its diagnostic and therapeutic implications, both practicing and training dermatologists have acknowledged a lack of exposure to skin of color. In one study, approximately 47% of dermatologists and dermatology residents reported that their medical training (medical school and/or residency) was inadequate in training them on skin conditions in Black patients. Furthermore, many who felt their training was lacking in skin of color identified the need for greater exposure to Black patients and training materials.15 The absence of comprehensive medical education regarding skin of color ultimately can be a disadvantage for both practitioners and patients, resulting in poorer outcomes. Furthermore, underrepresentation of skin of color may persist beyond undergraduate and graduate medical education. There also is evidence to suggest that noninclusion of skin of color pervades foundational dermatologic educational resources, including commonly used textbooks as well as continuing medical education disseminated at national conferences and meetings.17 Taken together, these findings highlight the need for more diverse and representative exposure to skin of color throughout medical training, which begins with a diverse inclusive undergraduate medical education in dermatology.
The objective of this study was to determine if the preclinical dermatology curriculum at 3 US medical schools provided adequate representation of skin of color patients in their didactic presentation slides.
Methods
Participants—Three US medical schools, a blend of private and public medical schools located across different geographic boundaries, agreed to participate in the study. All 3 institutions were current members of the American Medical Association (AMA) Accelerating Change in Medical Education consortium, whose primary goal is to create the medical school of the future and transform physician training.18 All 32 member institutions of the AMA consortium were contacted to request their participation in the study. As part of the consortium, these institutions have vowed to collectively work to develop and share the best models for educational advancement to improve care for patients, populations, and communities18 and would expectedly provide a more racially and ethnically inclusive curriculum than an institution not accountable to a group dedicated to identifying the best ways to deliver care for increasingly diverse communities.
Data Collection—Lectures were included if they were presented during dermatology preclinical courses in the 2015 to 2016 academic year. An uninvolved third party removed the names and identities of instructors to preserve anonymity. Two independent coders from different institutions extracted the data—lecture title, total number of clinical and histologic images, and number of skin of color images—from each of the anonymized lectures using a standardized coding form. We documented differences in skin of color noted in lectures and the disease context for the discussed differences, such as variations in clinical presentation, disease process, epidemiology/risk, and treatment between different skin phenotypes or ethnic groups. Photographs in which the coders were unable to differentiate whether the patient had skin of color were designated as indeterminate or unclear. Photographs appearing to represent Fitzpatrick skin types IV, V, and VI19 were categorically designated as skin of color, and those appearing to represent Fitzpatrick skin types I and II were described as not skin of color; however, images appearing to represent Fitzpatrick skin type III often were classified as not skin of color or indeterminate and occasionally skin of color. The Figure shows examples of images classified as skin of color, indeterminate, and not skin of color. Photographs often were classified as indeterminate due to poor lighting, close-up view photographs, or highlighted pathology obscuring the surrounding skin. We excluded duplicate photographs and histologic images from the analyses.
We also reviewed 19 conditions previously highlighted by the SOCS as areas of importance to skin of color patients.20 The coders tracked how many of these conditions were noted in each lecture. Duplicate discussion of these conditions was not included in the analyses. Any discrepancies between coders were resolved through additional slide review and discussion. The final coded data with the agreed upon changes were used for statistical analyses. Recent national demographic data from the US Census Bureau in 2019 describe approximately 39.9% of the population as belonging to racial/ethnic groups other than non-Hispanic/Latinx White.21 Consequently, the standard for adequate representation for skin of color photographs was set at 35% for the purpose of this study.
Results
Across all 3 institutions included in the study, the proportion of the total number of clinical photographs showing skin of color was 16% (290/1812). Eight percent of the total photographs (145/1812) were noted to be indeterminate (Table). For institution 1, 23.6% of photographs (155/658) showed skin of color, and 12.6% (83/658) were indeterminate. For institution 2, 13.1% (76/578) showed skin of color and 7.8% (45/578) were indeterminate. For institution 3, 10.2% (59/576) showed skin of color and 3% (17/576) were indeterminate.
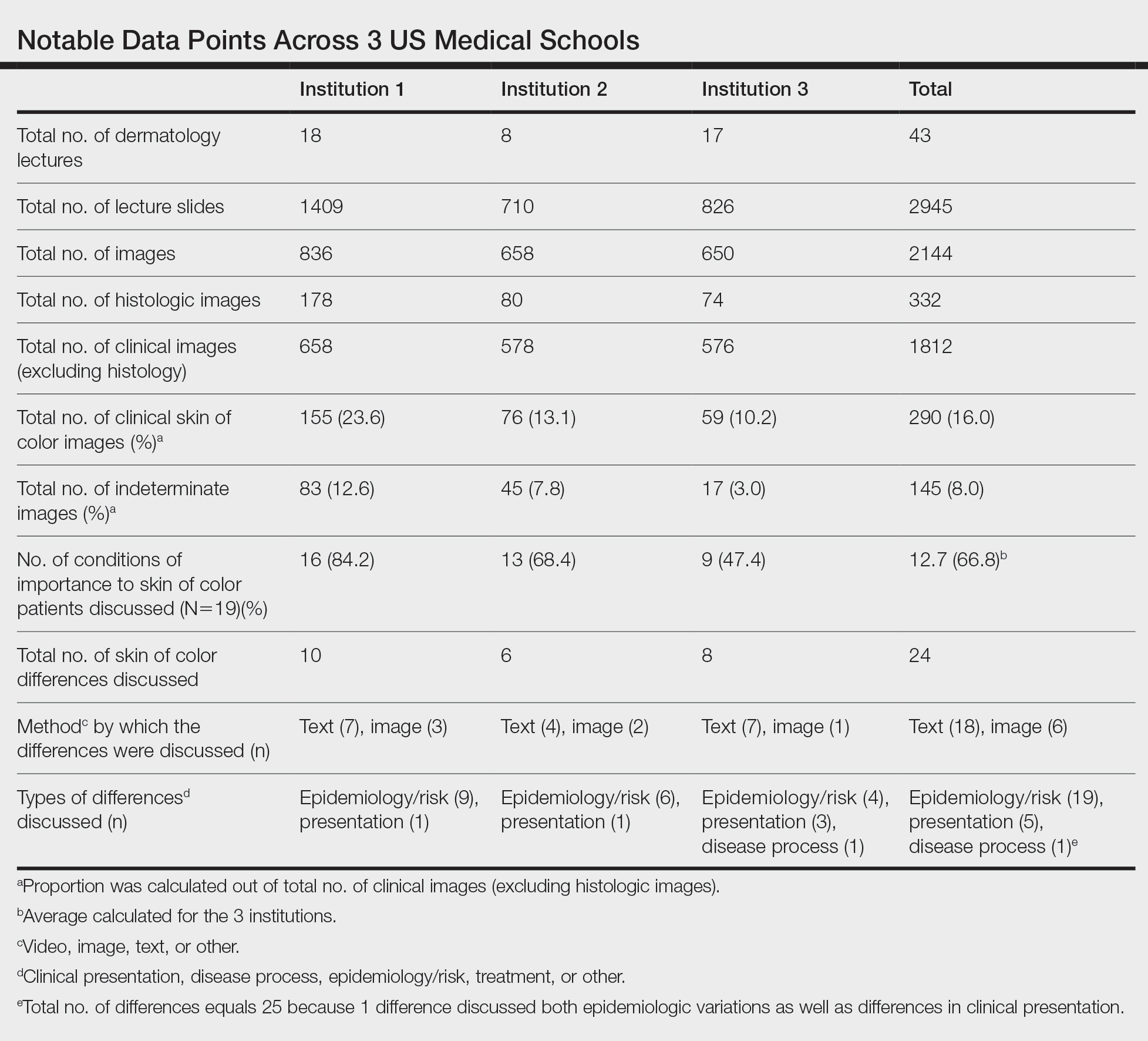
Institutions 1, 2, and 3 had 18, 8, and 17 total dermatology lectures, respectively. Of the 19 conditions designated as areas of importance to skin of color patients by the SOCS, 16 (84.2%) were discussed by institution 1, 11 (57.9%) by institution 2, and 9 (47.4%) by institution 3 (eTable 1). Institution 3 did not include photographs of skin of color patients in its acne, psoriasis, or cutaneous malignancy lectures. Institution 1 also did not include any skin of color patients in its malignancy lecture. Lectures that focused on pigmentary disorders, atopic dermatitis, infectious conditions, and benign cutaneous neoplasms were more likely to display photographs of skin of color patients; for example, lectures that discussed infectious conditions, such as superficial mycoses, herpes viruses, human papillomavirus, syphilis, and atypical mycobacterial infections, were consistently among those with higher proportions of photographs of skin of color patients.

Throughout the entire preclinical dermatology course at all 3 institutions, of 2945 lecture slides, only 24 (0.8%) unique differences were noted between skin color and non–skin of color patients, with 10 total differences noted by institution 1, 6 by institution 2, and 8 by institution 3 (Table). The majority of these differences (19/24) were related to epidemiologic differences in prevalence among varying racial/ethnic groups, with only 5 instances highlighting differences in clinical presentation. There was only a single instance that elaborated on the underlying pathophysiologic mechanisms of the discussed difference. Of all 24 unique differences discussed, 8 were related to skin cancer, 3 were related to dermatitis, and 2 were related to the difference in manifestation of erythema in patients with darker skin (eTable 2).
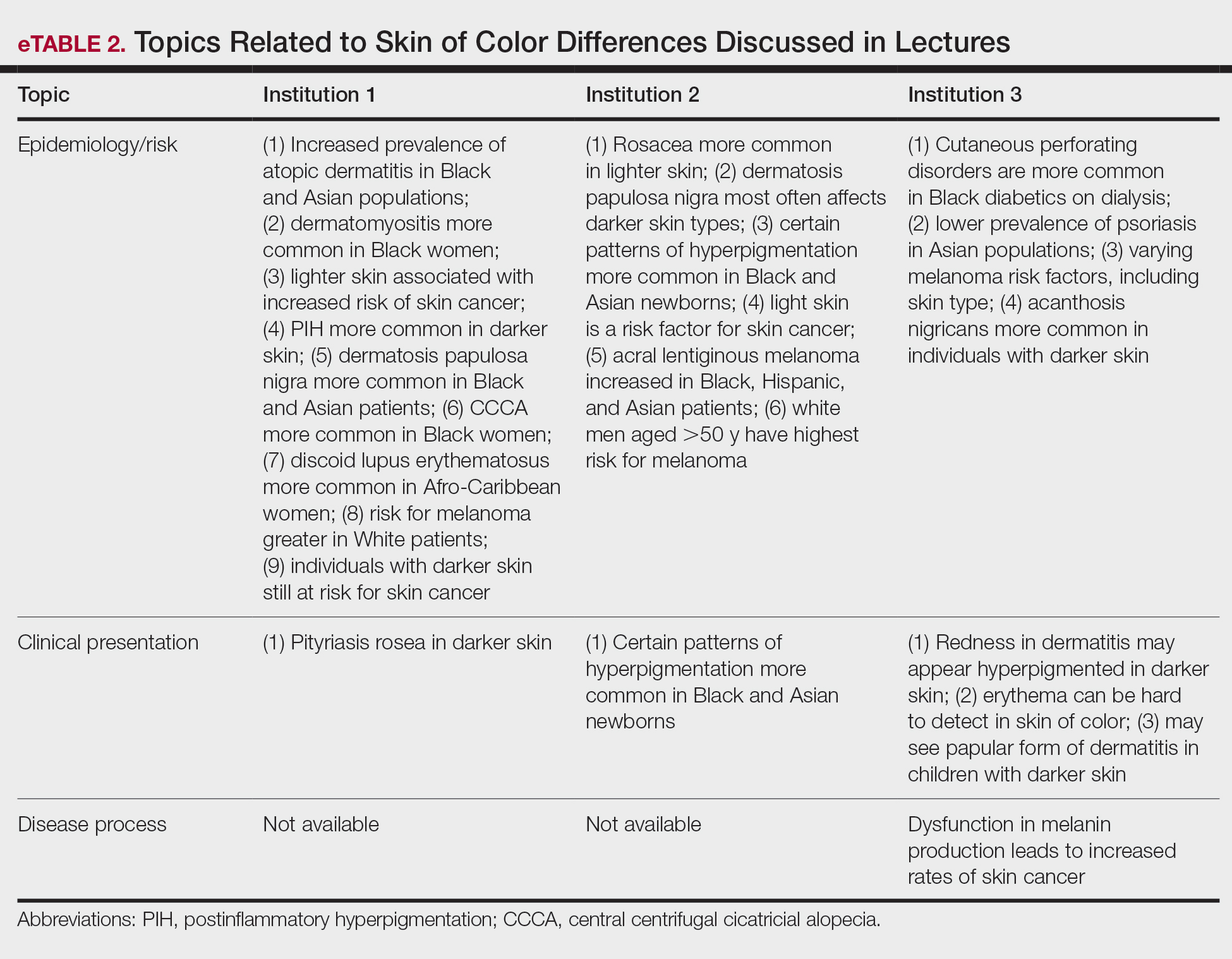
Comment
The results of this study demonstrated that skin of color is underrepresented in the preclinical dermatology curriculum at these 3 institutions. Although only 16% of all included clinical photographs were of skin of color, individuals with skin of color will soon represent more than half of the total US population within the next 2 decades.1 To increase representation of skin of color patients, teaching faculty should consciously and deliberately include more photographs of skin of color patients for a wider variety of common conditions, including atopic dermatitis and psoriasis, in addition to those that tend to disparately affect skin of color patients, such as pseudofolliculitis barbae or melasma. Furthermore, they also can incorporate more detailed discussions about important differences seen in skin of color patients.
More Skin of Color Photographs in Psoriasis Lectures—At institution 3, there were no skin of color patients included in the psoriasis lecture, even though there is considerable data in the literature indicating notable differences in the clinical presentation, quality-of-life impact, and treatment of psoriasis in skin of color patients.11,22 There are multiple nuances in psoriasis manifestation in patients with skin of color, including less-conspicuous erythema in darker skin, higher degrees of dyspigmentation, and greater body surface area involvement. For Black patients with scalp psoriasis, the impact of hair texture, styling practices, and washing frequency are additional considerations that may impact disease severity and selection of topical therapy.11 The lack of inclusion of any skin of color patients in the psoriasis lecture at one institution further underscores the pressing need to prioritize communities of color in medical education.
More Skin of Color Photographs in Cutaneous Malignancy Lectures—Similarly, while a lecturer at institution 2 noted that acral lentiginous melanoma accounts for a considerable proportion of melanoma among skin of color patients,23 there was no mention of how melanoma generally is substantially more deadly in this population, potentially due to decreased awareness and inconsistent screening.24 Furthermore, at institutions 1 and 3, there were no photographs or discussion of skin of color patients during the cutaneous malignancy lectures. Evidence shows that more emphasis is needed for melanoma screening and awareness in skin of color populations to improve survival outcomes,24 and this begins with educating not only future dermatologists but all future physicians as well. The failure to include photographs of skin of color patients in discussions or lectures regarding cutaneous malignancies may serve to further perpetuate the harmful misperception that individuals with skin of color are unaffected by skin cancer.25,26
Analysis of Skin of Color Photographs in Infectious Disease Lectures—In addition, lectures discussing infectious etiologies were among those with the highest proportion of skin of color photographs. This relatively disproportionate representation of skin of color compared to the other lectures may contribute to the development of harmful stereotypes or the stigmatization of skin of color patients. Although skin of color should continue to be represented in similar lectures, teaching faculty should remain mindful of the potential unintended impact from lectures including relatively disproportionate amounts of skin of color, particularly when other lectures may have sparse to absent representation of skin of color.
More Photographs Available for Education—Overall, our findings may help to inform changes to preclinical dermatology medical education at other institutions to create more inclusive and representative curricula for skin of color patients. The ability of instructors to provide visual representation of various dermatologic conditions may be limited by the photographs available in certain textbooks with few examples of patients with skin of color; however, concerns regarding the lack of skin of color representation in dermatology training is not a novel discussion.17 Although it is the responsibility of all dermatologists to advocate for the inclusion of skin of color, many dermatologists of color have been leading the way in this movement for decades, publishing several textbooks to document various skin conditions in those with darker skin types and discuss unique considerations for patients with skin of color.27-29 Images from these textbooks can be utilized by programs to increase representation of skin of color in dermatology training. There also are multiple expanding online dermatologic databases, such as VisualDx, with an increasing focus on skin of color patients, some of which allow users to filter images by degree of skin pigmentation.30 Moreover, instructors also can work to diversify their curricula by highlighting more of the SOCS conditions of importance to skin of color patients, which have since been renamed and highlighted on the Patient Dermatology Education section of the SOCS website.20 These conditions, while not completely comprehensive, provide a useful starting point for medical educators to reevaluate for potential areas of improvement and inclusion.
There are several potential strategies that can be used to better represent skin of color in dermatologic preclinical medical education, including increasing awareness, especially among dermatology teaching faculty, of existing disparities in the representation of skin of color in the preclinical curricula. Additionally, all dermatology teaching materials could be reviewed at the department level prior to being disseminated to medical students to assess for instances in which skin of color could be prioritized for discussion or varying disease presentations in skin of color could be demonstrated. Finally, teaching faculty may consider photographing more clinical images of their skin of color patients to further develop a catalog of diverse images that can be used to teach students.
Study Limitations—Our study was unable to account for verbal discussion of skin of color not otherwise denoted or captured in lecture slides. Additional limitations include the utilization of Fitzpatrick skin types to describe and differentiate varying skin tones, as the Fitzpatrick scale originally was developed as a method to describe an individual’s response to UV exposure.19 The inability to further delineate the representation of darker skin types, such as those that may be classified as Fitzpatrick skin types V or VI,19 compared to those with lighter skin of color also was a limiting factor. This study was unable to assess for discussion of other common conditions affecting skin of color patients that were not listed as one of the priority conditions by SOCS. Photographs that were designated as indeterminate were difficult to elucidate as skin of color; however, it is possible that instructors may have verbally described these images as skin of color during lectures. Nonetheless, it may be beneficial for learners if teaching faculty were to clearly label instances where skin of color patients are shown or when notable differences are present.
Conclusion
Future studies would benefit from the inclusion of audio data from lectures, syllabi, and small group teaching materials from preclinical courses to more accurately assess representation of skin of color in dermatology training. Additionally, future studies also may expand to include images from lectures of overlapping clinical specialties, particularly infectious disease and rheumatology, to provide a broader assessment of skin of color exposure. Furthermore, repeat assessment may be beneficial to assess the longitudinal effectiveness of curricular changes at the institutions included in this study, comparing older lectures to more recent, updated lectures. This study also may be replicated at other medical schools to allow for wider comparison of curricula.
Acknowledgment—The authors wish to thank the institutions that offered and agreed to participate in this study with the hopes of improving medical education.
- Colby SL, Ortman JM. Projections of the size and composition of the US population: 2014 to 2060. United States Census Bureau website. Published March 2015. Accessed September 14, 2021. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf
- Learn more about SOCS. Skin of Color Society website. Accessed September 14, 2021. http://skinofcolorsociety.org/about-socs/
- Taylor SC. Skin of color: biology, structure, function, and implications for dermatologic disease. J Am Acad Dermatol. 2002;46(suppl 2):S41-S62.
- Berardesca E, Maibach H. Ethnic skin: overview of structure and function. J Am Acad Dermatol. 2003;48(suppl 6):S139-S142.
- Callender VD, Surin-Lord SS, Davis EC, et al. Postinflammatory hyperpigmentation. Am J Clin Dermatol. 2011;12:87-99.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3:20-31.
- Grimes PE, Stockton T. Pigmentary disorders in blacks. Dermatol Clin. 1988;6:271-281.
- Halder RM, Nootheti PK. Ethnic skin disorders overview. J Am Acad Dermatol. 2003;48(suppl 6):S143-S148.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Callender VD. Acne in ethnic skin: special considerations for therapy. Dermatol Ther. 2004;17:184-195.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
- Ramsay DL, Mayer F. National survey of undergraduate dermatologic medical education. Arch Dermatol.1985;121:1529-1530.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii.
- Knable A, Hood AF, Pearson TG. Undergraduate medical education in dermatology: report from the AAD Interdisciplinary Education Committee, Subcommittee on Undergraduate Medical Education. J Am Acad Dermatol. 1997;36:467-470.
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690.
- Skochelak SE, Stack SJ. Creating the medical schools of the future. Acad Med. 2017;92:16-19.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871.
- Skin of Color Society. Patient dermatology education. Accessed September 22, 2021. https://skinofcolorsociety.org/patient-dermatology-education
- QuickFacts: United States. US Census Bureau website. Updated July 1, 2019. Accessed September 14, 2021. https://www.census.gov/quickfacts/fact/table/US#
- Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423.
- Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427-434.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Pipitone M, Robinson JK, Camara C, et al. Skin cancer awareness in suburban employees: a Hispanic perspective. J Am Acad Dermatol. 2002;47:118-123.
- Imahiyerobo-Ip J, Ip I, Jamal S, et al. Skin cancer awareness in communities of color. J Am Acad Dermatol. 2011;64:198-200.
- Taylor SSC, Serrano AMA, Kelly AP, et al, eds. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016.
- Dadzie OE, Petit A, Alexis AF, eds. Ethnic Dermatology: Principles and Practice. Wiley-Blackwell; 2013.
- Jackson-Richards D, Pandya AG, eds. Dermatology Atlas for Skin of Color. Springer; 2014.
- VisualDx. New VisualDx feature: skin of color sort. Published October 14, 2020. Accessed September 22, 2021. https://www.visualdx.com/blog/new-visualdx-feature-skin-of-color-sort/
A ccording to the US Census Bureau, more than half of all Americans are projected to belong to a minority group, defined as any group other than non-Hispanic White alone, by 2044. 1 Consequently, the United States rapidly is becoming a country in which the majority of citizens will have skin of color. Individuals with skin of color are of diverse ethnic backgrounds and include people of African, Latin American, Native American, Pacific Islander, and Asian descent, as well as interethnic backgrounds. 2 Throughout the country, dermatologists along with primary care practitioners may be confronted with certain cutaneous conditions that have varying disease presentations or processes in patients with skin of color. It also is important to note that racial categories are socially rather than biologically constructed, and the term skin of color includes a wide variety of diverse skin types. Nevertheless, the current literature thoroughly supports unique pathophysiologic differences in skin of color as well as variations in disease manifestation compared to White patients. 3-5 For example, the increased lability of melanosomes in skin of color patients, which increases their risk for postinflammatory hyperpigmentation, has been well documented. 5-7 There are various dermatologic conditions that also occur with higher frequency and manifest uniquely in people with darker, more pigmented skin, 7-9 and dermatologists, along with primary care physicians, should feel prepared to recognize and address them.
Extensive evidence also indicates that there are unique aspects to consider while managing certain skin diseases in patients with skin of color.8,10,11 Consequently, as noted on the Skin of Color Society (SOCS) website, “[a]n increase in the body of dermatological literature concerning skin of color as well as the advancement of both basic science and clinical investigational research is necessary to meet the needs of the expanding skin of color population.”2 In the meantime, current knowledge regarding cutaneous conditions that diversely or disproportionately affect skin of color should be actively disseminated to physicians in training. Although patients with skin of color should always have access to comprehensive care and knowledgeable practitioners, the current changes in national and regional demographics further underscore the need for a more thorough understanding of skin of color with regard to disease pathogenesis, diagnosis, and treatment.
Several studies have found that medical students in the United States are minimally exposed to dermatology in general compared to other clinical specialties,12-14 which can easily lead to the underrecognition of disorders that may uniquely or disproportionately affect individuals with pigmented skin. Recent data showed that medical schools typically required fewer than 10 hours of dermatology instruction,12 and on average, dermatologic training made up less than 1% of a medical student’s undergraduate medical education.13,15,16 Consequently, less than 40% of primary care residents felt that their medical school curriculum adequately prepared them to manage common skin conditions.14 Although not all physicians should be expected to fully grasp the complexities of skin of color and its diagnostic and therapeutic implications, both practicing and training dermatologists have acknowledged a lack of exposure to skin of color. In one study, approximately 47% of dermatologists and dermatology residents reported that their medical training (medical school and/or residency) was inadequate in training them on skin conditions in Black patients. Furthermore, many who felt their training was lacking in skin of color identified the need for greater exposure to Black patients and training materials.15 The absence of comprehensive medical education regarding skin of color ultimately can be a disadvantage for both practitioners and patients, resulting in poorer outcomes. Furthermore, underrepresentation of skin of color may persist beyond undergraduate and graduate medical education. There also is evidence to suggest that noninclusion of skin of color pervades foundational dermatologic educational resources, including commonly used textbooks as well as continuing medical education disseminated at national conferences and meetings.17 Taken together, these findings highlight the need for more diverse and representative exposure to skin of color throughout medical training, which begins with a diverse inclusive undergraduate medical education in dermatology.
The objective of this study was to determine if the preclinical dermatology curriculum at 3 US medical schools provided adequate representation of skin of color patients in their didactic presentation slides.
Methods
Participants—Three US medical schools, a blend of private and public medical schools located across different geographic boundaries, agreed to participate in the study. All 3 institutions were current members of the American Medical Association (AMA) Accelerating Change in Medical Education consortium, whose primary goal is to create the medical school of the future and transform physician training.18 All 32 member institutions of the AMA consortium were contacted to request their participation in the study. As part of the consortium, these institutions have vowed to collectively work to develop and share the best models for educational advancement to improve care for patients, populations, and communities18 and would expectedly provide a more racially and ethnically inclusive curriculum than an institution not accountable to a group dedicated to identifying the best ways to deliver care for increasingly diverse communities.
Data Collection—Lectures were included if they were presented during dermatology preclinical courses in the 2015 to 2016 academic year. An uninvolved third party removed the names and identities of instructors to preserve anonymity. Two independent coders from different institutions extracted the data—lecture title, total number of clinical and histologic images, and number of skin of color images—from each of the anonymized lectures using a standardized coding form. We documented differences in skin of color noted in lectures and the disease context for the discussed differences, such as variations in clinical presentation, disease process, epidemiology/risk, and treatment between different skin phenotypes or ethnic groups. Photographs in which the coders were unable to differentiate whether the patient had skin of color were designated as indeterminate or unclear. Photographs appearing to represent Fitzpatrick skin types IV, V, and VI19 were categorically designated as skin of color, and those appearing to represent Fitzpatrick skin types I and II were described as not skin of color; however, images appearing to represent Fitzpatrick skin type III often were classified as not skin of color or indeterminate and occasionally skin of color. The Figure shows examples of images classified as skin of color, indeterminate, and not skin of color. Photographs often were classified as indeterminate due to poor lighting, close-up view photographs, or highlighted pathology obscuring the surrounding skin. We excluded duplicate photographs and histologic images from the analyses.

We also reviewed 19 conditions previously highlighted by the SOCS as areas of importance to skin of color patients.20 The coders tracked how many of these conditions were noted in each lecture. Duplicate discussion of these conditions was not included in the analyses. Any discrepancies between coders were resolved through additional slide review and discussion. The final coded data with the agreed upon changes were used for statistical analyses. Recent national demographic data from the US Census Bureau in 2019 describe approximately 39.9% of the population as belonging to racial/ethnic groups other than non-Hispanic/Latinx White.21 Consequently, the standard for adequate representation for skin of color photographs was set at 35% for the purpose of this study.
Results
Across all 3 institutions included in the study, the proportion of the total number of clinical photographs showing skin of color was 16% (290/1812). Eight percent of the total photographs (145/1812) were noted to be indeterminate (Table). For institution 1, 23.6% of photographs (155/658) showed skin of color, and 12.6% (83/658) were indeterminate. For institution 2, 13.1% (76/578) showed skin of color and 7.8% (45/578) were indeterminate. For institution 3, 10.2% (59/576) showed skin of color and 3% (17/576) were indeterminate.

Institutions 1, 2, and 3 had 18, 8, and 17 total dermatology lectures, respectively. Of the 19 conditions designated as areas of importance to skin of color patients by the SOCS, 16 (84.2%) were discussed by institution 1, 11 (57.9%) by institution 2, and 9 (47.4%) by institution 3 (eTable 1). Institution 3 did not include photographs of skin of color patients in its acne, psoriasis, or cutaneous malignancy lectures. Institution 1 also did not include any skin of color patients in its malignancy lecture. Lectures that focused on pigmentary disorders, atopic dermatitis, infectious conditions, and benign cutaneous neoplasms were more likely to display photographs of skin of color patients; for example, lectures that discussed infectious conditions, such as superficial mycoses, herpes viruses, human papillomavirus, syphilis, and atypical mycobacterial infections, were consistently among those with higher proportions of photographs of skin of color patients.

Throughout the entire preclinical dermatology course at all 3 institutions, of 2945 lecture slides, only 24 (0.8%) unique differences were noted between skin color and non–skin of color patients, with 10 total differences noted by institution 1, 6 by institution 2, and 8 by institution 3 (Table). The majority of these differences (19/24) were related to epidemiologic differences in prevalence among varying racial/ethnic groups, with only 5 instances highlighting differences in clinical presentation. There was only a single instance that elaborated on the underlying pathophysiologic mechanisms of the discussed difference. Of all 24 unique differences discussed, 8 were related to skin cancer, 3 were related to dermatitis, and 2 were related to the difference in manifestation of erythema in patients with darker skin (eTable 2).

Comment
The results of this study demonstrated that skin of color is underrepresented in the preclinical dermatology curriculum at these 3 institutions. Although only 16% of all included clinical photographs were of skin of color, individuals with skin of color will soon represent more than half of the total US population within the next 2 decades.1 To increase representation of skin of color patients, teaching faculty should consciously and deliberately include more photographs of skin of color patients for a wider variety of common conditions, including atopic dermatitis and psoriasis, in addition to those that tend to disparately affect skin of color patients, such as pseudofolliculitis barbae or melasma. Furthermore, they also can incorporate more detailed discussions about important differences seen in skin of color patients.
More Skin of Color Photographs in Psoriasis Lectures—At institution 3, there were no skin of color patients included in the psoriasis lecture, even though there is considerable data in the literature indicating notable differences in the clinical presentation, quality-of-life impact, and treatment of psoriasis in skin of color patients.11,22 There are multiple nuances in psoriasis manifestation in patients with skin of color, including less-conspicuous erythema in darker skin, higher degrees of dyspigmentation, and greater body surface area involvement. For Black patients with scalp psoriasis, the impact of hair texture, styling practices, and washing frequency are additional considerations that may impact disease severity and selection of topical therapy.11 The lack of inclusion of any skin of color patients in the psoriasis lecture at one institution further underscores the pressing need to prioritize communities of color in medical education.
More Skin of Color Photographs in Cutaneous Malignancy Lectures—Similarly, while a lecturer at institution 2 noted that acral lentiginous melanoma accounts for a considerable proportion of melanoma among skin of color patients,23 there was no mention of how melanoma generally is substantially more deadly in this population, potentially due to decreased awareness and inconsistent screening.24 Furthermore, at institutions 1 and 3, there were no photographs or discussion of skin of color patients during the cutaneous malignancy lectures. Evidence shows that more emphasis is needed for melanoma screening and awareness in skin of color populations to improve survival outcomes,24 and this begins with educating not only future dermatologists but all future physicians as well. The failure to include photographs of skin of color patients in discussions or lectures regarding cutaneous malignancies may serve to further perpetuate the harmful misperception that individuals with skin of color are unaffected by skin cancer.25,26
Analysis of Skin of Color Photographs in Infectious Disease Lectures—In addition, lectures discussing infectious etiologies were among those with the highest proportion of skin of color photographs. This relatively disproportionate representation of skin of color compared to the other lectures may contribute to the development of harmful stereotypes or the stigmatization of skin of color patients. Although skin of color should continue to be represented in similar lectures, teaching faculty should remain mindful of the potential unintended impact from lectures including relatively disproportionate amounts of skin of color, particularly when other lectures may have sparse to absent representation of skin of color.
More Photographs Available for Education—Overall, our findings may help to inform changes to preclinical dermatology medical education at other institutions to create more inclusive and representative curricula for skin of color patients. The ability of instructors to provide visual representation of various dermatologic conditions may be limited by the photographs available in certain textbooks with few examples of patients with skin of color; however, concerns regarding the lack of skin of color representation in dermatology training is not a novel discussion.17 Although it is the responsibility of all dermatologists to advocate for the inclusion of skin of color, many dermatologists of color have been leading the way in this movement for decades, publishing several textbooks to document various skin conditions in those with darker skin types and discuss unique considerations for patients with skin of color.27-29 Images from these textbooks can be utilized by programs to increase representation of skin of color in dermatology training. There also are multiple expanding online dermatologic databases, such as VisualDx, with an increasing focus on skin of color patients, some of which allow users to filter images by degree of skin pigmentation.30 Moreover, instructors also can work to diversify their curricula by highlighting more of the SOCS conditions of importance to skin of color patients, which have since been renamed and highlighted on the Patient Dermatology Education section of the SOCS website.20 These conditions, while not completely comprehensive, provide a useful starting point for medical educators to reevaluate for potential areas of improvement and inclusion.
There are several potential strategies that can be used to better represent skin of color in dermatologic preclinical medical education, including increasing awareness, especially among dermatology teaching faculty, of existing disparities in the representation of skin of color in the preclinical curricula. Additionally, all dermatology teaching materials could be reviewed at the department level prior to being disseminated to medical students to assess for instances in which skin of color could be prioritized for discussion or varying disease presentations in skin of color could be demonstrated. Finally, teaching faculty may consider photographing more clinical images of their skin of color patients to further develop a catalog of diverse images that can be used to teach students.
Study Limitations—Our study was unable to account for verbal discussion of skin of color not otherwise denoted or captured in lecture slides. Additional limitations include the utilization of Fitzpatrick skin types to describe and differentiate varying skin tones, as the Fitzpatrick scale originally was developed as a method to describe an individual’s response to UV exposure.19 The inability to further delineate the representation of darker skin types, such as those that may be classified as Fitzpatrick skin types V or VI,19 compared to those with lighter skin of color also was a limiting factor. This study was unable to assess for discussion of other common conditions affecting skin of color patients that were not listed as one of the priority conditions by SOCS. Photographs that were designated as indeterminate were difficult to elucidate as skin of color; however, it is possible that instructors may have verbally described these images as skin of color during lectures. Nonetheless, it may be beneficial for learners if teaching faculty were to clearly label instances where skin of color patients are shown or when notable differences are present.
Conclusion
Future studies would benefit from the inclusion of audio data from lectures, syllabi, and small group teaching materials from preclinical courses to more accurately assess representation of skin of color in dermatology training. Additionally, future studies also may expand to include images from lectures of overlapping clinical specialties, particularly infectious disease and rheumatology, to provide a broader assessment of skin of color exposure. Furthermore, repeat assessment may be beneficial to assess the longitudinal effectiveness of curricular changes at the institutions included in this study, comparing older lectures to more recent, updated lectures. This study also may be replicated at other medical schools to allow for wider comparison of curricula.
Acknowledgment—The authors wish to thank the institutions that offered and agreed to participate in this study with the hopes of improving medical education.
A ccording to the US Census Bureau, more than half of all Americans are projected to belong to a minority group, defined as any group other than non-Hispanic White alone, by 2044. 1 Consequently, the United States rapidly is becoming a country in which the majority of citizens will have skin of color. Individuals with skin of color are of diverse ethnic backgrounds and include people of African, Latin American, Native American, Pacific Islander, and Asian descent, as well as interethnic backgrounds. 2 Throughout the country, dermatologists along with primary care practitioners may be confronted with certain cutaneous conditions that have varying disease presentations or processes in patients with skin of color. It also is important to note that racial categories are socially rather than biologically constructed, and the term skin of color includes a wide variety of diverse skin types. Nevertheless, the current literature thoroughly supports unique pathophysiologic differences in skin of color as well as variations in disease manifestation compared to White patients. 3-5 For example, the increased lability of melanosomes in skin of color patients, which increases their risk for postinflammatory hyperpigmentation, has been well documented. 5-7 There are various dermatologic conditions that also occur with higher frequency and manifest uniquely in people with darker, more pigmented skin, 7-9 and dermatologists, along with primary care physicians, should feel prepared to recognize and address them.
Extensive evidence also indicates that there are unique aspects to consider while managing certain skin diseases in patients with skin of color.8,10,11 Consequently, as noted on the Skin of Color Society (SOCS) website, “[a]n increase in the body of dermatological literature concerning skin of color as well as the advancement of both basic science and clinical investigational research is necessary to meet the needs of the expanding skin of color population.”2 In the meantime, current knowledge regarding cutaneous conditions that diversely or disproportionately affect skin of color should be actively disseminated to physicians in training. Although patients with skin of color should always have access to comprehensive care and knowledgeable practitioners, the current changes in national and regional demographics further underscore the need for a more thorough understanding of skin of color with regard to disease pathogenesis, diagnosis, and treatment.
Several studies have found that medical students in the United States are minimally exposed to dermatology in general compared to other clinical specialties,12-14 which can easily lead to the underrecognition of disorders that may uniquely or disproportionately affect individuals with pigmented skin. Recent data showed that medical schools typically required fewer than 10 hours of dermatology instruction,12 and on average, dermatologic training made up less than 1% of a medical student’s undergraduate medical education.13,15,16 Consequently, less than 40% of primary care residents felt that their medical school curriculum adequately prepared them to manage common skin conditions.14 Although not all physicians should be expected to fully grasp the complexities of skin of color and its diagnostic and therapeutic implications, both practicing and training dermatologists have acknowledged a lack of exposure to skin of color. In one study, approximately 47% of dermatologists and dermatology residents reported that their medical training (medical school and/or residency) was inadequate in training them on skin conditions in Black patients. Furthermore, many who felt their training was lacking in skin of color identified the need for greater exposure to Black patients and training materials.15 The absence of comprehensive medical education regarding skin of color ultimately can be a disadvantage for both practitioners and patients, resulting in poorer outcomes. Furthermore, underrepresentation of skin of color may persist beyond undergraduate and graduate medical education. There also is evidence to suggest that noninclusion of skin of color pervades foundational dermatologic educational resources, including commonly used textbooks as well as continuing medical education disseminated at national conferences and meetings.17 Taken together, these findings highlight the need for more diverse and representative exposure to skin of color throughout medical training, which begins with a diverse inclusive undergraduate medical education in dermatology.
The objective of this study was to determine if the preclinical dermatology curriculum at 3 US medical schools provided adequate representation of skin of color patients in their didactic presentation slides.
Methods
Participants—Three US medical schools, a blend of private and public medical schools located across different geographic boundaries, agreed to participate in the study. All 3 institutions were current members of the American Medical Association (AMA) Accelerating Change in Medical Education consortium, whose primary goal is to create the medical school of the future and transform physician training.18 All 32 member institutions of the AMA consortium were contacted to request their participation in the study. As part of the consortium, these institutions have vowed to collectively work to develop and share the best models for educational advancement to improve care for patients, populations, and communities18 and would expectedly provide a more racially and ethnically inclusive curriculum than an institution not accountable to a group dedicated to identifying the best ways to deliver care for increasingly diverse communities.
Data Collection—Lectures were included if they were presented during dermatology preclinical courses in the 2015 to 2016 academic year. An uninvolved third party removed the names and identities of instructors to preserve anonymity. Two independent coders from different institutions extracted the data—lecture title, total number of clinical and histologic images, and number of skin of color images—from each of the anonymized lectures using a standardized coding form. We documented differences in skin of color noted in lectures and the disease context for the discussed differences, such as variations in clinical presentation, disease process, epidemiology/risk, and treatment between different skin phenotypes or ethnic groups. Photographs in which the coders were unable to differentiate whether the patient had skin of color were designated as indeterminate or unclear. Photographs appearing to represent Fitzpatrick skin types IV, V, and VI19 were categorically designated as skin of color, and those appearing to represent Fitzpatrick skin types I and II were described as not skin of color; however, images appearing to represent Fitzpatrick skin type III often were classified as not skin of color or indeterminate and occasionally skin of color. The Figure shows examples of images classified as skin of color, indeterminate, and not skin of color. Photographs often were classified as indeterminate due to poor lighting, close-up view photographs, or highlighted pathology obscuring the surrounding skin. We excluded duplicate photographs and histologic images from the analyses.

We also reviewed 19 conditions previously highlighted by the SOCS as areas of importance to skin of color patients.20 The coders tracked how many of these conditions were noted in each lecture. Duplicate discussion of these conditions was not included in the analyses. Any discrepancies between coders were resolved through additional slide review and discussion. The final coded data with the agreed upon changes were used for statistical analyses. Recent national demographic data from the US Census Bureau in 2019 describe approximately 39.9% of the population as belonging to racial/ethnic groups other than non-Hispanic/Latinx White.21 Consequently, the standard for adequate representation for skin of color photographs was set at 35% for the purpose of this study.
Results
Across all 3 institutions included in the study, the proportion of the total number of clinical photographs showing skin of color was 16% (290/1812). Eight percent of the total photographs (145/1812) were noted to be indeterminate (Table). For institution 1, 23.6% of photographs (155/658) showed skin of color, and 12.6% (83/658) were indeterminate. For institution 2, 13.1% (76/578) showed skin of color and 7.8% (45/578) were indeterminate. For institution 3, 10.2% (59/576) showed skin of color and 3% (17/576) were indeterminate.

Institutions 1, 2, and 3 had 18, 8, and 17 total dermatology lectures, respectively. Of the 19 conditions designated as areas of importance to skin of color patients by the SOCS, 16 (84.2%) were discussed by institution 1, 11 (57.9%) by institution 2, and 9 (47.4%) by institution 3 (eTable 1). Institution 3 did not include photographs of skin of color patients in its acne, psoriasis, or cutaneous malignancy lectures. Institution 1 also did not include any skin of color patients in its malignancy lecture. Lectures that focused on pigmentary disorders, atopic dermatitis, infectious conditions, and benign cutaneous neoplasms were more likely to display photographs of skin of color patients; for example, lectures that discussed infectious conditions, such as superficial mycoses, herpes viruses, human papillomavirus, syphilis, and atypical mycobacterial infections, were consistently among those with higher proportions of photographs of skin of color patients.

Throughout the entire preclinical dermatology course at all 3 institutions, of 2945 lecture slides, only 24 (0.8%) unique differences were noted between skin color and non–skin of color patients, with 10 total differences noted by institution 1, 6 by institution 2, and 8 by institution 3 (Table). The majority of these differences (19/24) were related to epidemiologic differences in prevalence among varying racial/ethnic groups, with only 5 instances highlighting differences in clinical presentation. There was only a single instance that elaborated on the underlying pathophysiologic mechanisms of the discussed difference. Of all 24 unique differences discussed, 8 were related to skin cancer, 3 were related to dermatitis, and 2 were related to the difference in manifestation of erythema in patients with darker skin (eTable 2).

Comment
The results of this study demonstrated that skin of color is underrepresented in the preclinical dermatology curriculum at these 3 institutions. Although only 16% of all included clinical photographs were of skin of color, individuals with skin of color will soon represent more than half of the total US population within the next 2 decades.1 To increase representation of skin of color patients, teaching faculty should consciously and deliberately include more photographs of skin of color patients for a wider variety of common conditions, including atopic dermatitis and psoriasis, in addition to those that tend to disparately affect skin of color patients, such as pseudofolliculitis barbae or melasma. Furthermore, they also can incorporate more detailed discussions about important differences seen in skin of color patients.
More Skin of Color Photographs in Psoriasis Lectures—At institution 3, there were no skin of color patients included in the psoriasis lecture, even though there is considerable data in the literature indicating notable differences in the clinical presentation, quality-of-life impact, and treatment of psoriasis in skin of color patients.11,22 There are multiple nuances in psoriasis manifestation in patients with skin of color, including less-conspicuous erythema in darker skin, higher degrees of dyspigmentation, and greater body surface area involvement. For Black patients with scalp psoriasis, the impact of hair texture, styling practices, and washing frequency are additional considerations that may impact disease severity and selection of topical therapy.11 The lack of inclusion of any skin of color patients in the psoriasis lecture at one institution further underscores the pressing need to prioritize communities of color in medical education.
More Skin of Color Photographs in Cutaneous Malignancy Lectures—Similarly, while a lecturer at institution 2 noted that acral lentiginous melanoma accounts for a considerable proportion of melanoma among skin of color patients,23 there was no mention of how melanoma generally is substantially more deadly in this population, potentially due to decreased awareness and inconsistent screening.24 Furthermore, at institutions 1 and 3, there were no photographs or discussion of skin of color patients during the cutaneous malignancy lectures. Evidence shows that more emphasis is needed for melanoma screening and awareness in skin of color populations to improve survival outcomes,24 and this begins with educating not only future dermatologists but all future physicians as well. The failure to include photographs of skin of color patients in discussions or lectures regarding cutaneous malignancies may serve to further perpetuate the harmful misperception that individuals with skin of color are unaffected by skin cancer.25,26
Analysis of Skin of Color Photographs in Infectious Disease Lectures—In addition, lectures discussing infectious etiologies were among those with the highest proportion of skin of color photographs. This relatively disproportionate representation of skin of color compared to the other lectures may contribute to the development of harmful stereotypes or the stigmatization of skin of color patients. Although skin of color should continue to be represented in similar lectures, teaching faculty should remain mindful of the potential unintended impact from lectures including relatively disproportionate amounts of skin of color, particularly when other lectures may have sparse to absent representation of skin of color.
More Photographs Available for Education—Overall, our findings may help to inform changes to preclinical dermatology medical education at other institutions to create more inclusive and representative curricula for skin of color patients. The ability of instructors to provide visual representation of various dermatologic conditions may be limited by the photographs available in certain textbooks with few examples of patients with skin of color; however, concerns regarding the lack of skin of color representation in dermatology training is not a novel discussion.17 Although it is the responsibility of all dermatologists to advocate for the inclusion of skin of color, many dermatologists of color have been leading the way in this movement for decades, publishing several textbooks to document various skin conditions in those with darker skin types and discuss unique considerations for patients with skin of color.27-29 Images from these textbooks can be utilized by programs to increase representation of skin of color in dermatology training. There also are multiple expanding online dermatologic databases, such as VisualDx, with an increasing focus on skin of color patients, some of which allow users to filter images by degree of skin pigmentation.30 Moreover, instructors also can work to diversify their curricula by highlighting more of the SOCS conditions of importance to skin of color patients, which have since been renamed and highlighted on the Patient Dermatology Education section of the SOCS website.20 These conditions, while not completely comprehensive, provide a useful starting point for medical educators to reevaluate for potential areas of improvement and inclusion.
There are several potential strategies that can be used to better represent skin of color in dermatologic preclinical medical education, including increasing awareness, especially among dermatology teaching faculty, of existing disparities in the representation of skin of color in the preclinical curricula. Additionally, all dermatology teaching materials could be reviewed at the department level prior to being disseminated to medical students to assess for instances in which skin of color could be prioritized for discussion or varying disease presentations in skin of color could be demonstrated. Finally, teaching faculty may consider photographing more clinical images of their skin of color patients to further develop a catalog of diverse images that can be used to teach students.
Study Limitations—Our study was unable to account for verbal discussion of skin of color not otherwise denoted or captured in lecture slides. Additional limitations include the utilization of Fitzpatrick skin types to describe and differentiate varying skin tones, as the Fitzpatrick scale originally was developed as a method to describe an individual’s response to UV exposure.19 The inability to further delineate the representation of darker skin types, such as those that may be classified as Fitzpatrick skin types V or VI,19 compared to those with lighter skin of color also was a limiting factor. This study was unable to assess for discussion of other common conditions affecting skin of color patients that were not listed as one of the priority conditions by SOCS. Photographs that were designated as indeterminate were difficult to elucidate as skin of color; however, it is possible that instructors may have verbally described these images as skin of color during lectures. Nonetheless, it may be beneficial for learners if teaching faculty were to clearly label instances where skin of color patients are shown or when notable differences are present.
Conclusion
Future studies would benefit from the inclusion of audio data from lectures, syllabi, and small group teaching materials from preclinical courses to more accurately assess representation of skin of color in dermatology training. Additionally, future studies also may expand to include images from lectures of overlapping clinical specialties, particularly infectious disease and rheumatology, to provide a broader assessment of skin of color exposure. Furthermore, repeat assessment may be beneficial to assess the longitudinal effectiveness of curricular changes at the institutions included in this study, comparing older lectures to more recent, updated lectures. This study also may be replicated at other medical schools to allow for wider comparison of curricula.
Acknowledgment—The authors wish to thank the institutions that offered and agreed to participate in this study with the hopes of improving medical education.
- Colby SL, Ortman JM. Projections of the size and composition of the US population: 2014 to 2060. United States Census Bureau website. Published March 2015. Accessed September 14, 2021. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf
- Learn more about SOCS. Skin of Color Society website. Accessed September 14, 2021. http://skinofcolorsociety.org/about-socs/
- Taylor SC. Skin of color: biology, structure, function, and implications for dermatologic disease. J Am Acad Dermatol. 2002;46(suppl 2):S41-S62.
- Berardesca E, Maibach H. Ethnic skin: overview of structure and function. J Am Acad Dermatol. 2003;48(suppl 6):S139-S142.
- Callender VD, Surin-Lord SS, Davis EC, et al. Postinflammatory hyperpigmentation. Am J Clin Dermatol. 2011;12:87-99.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3:20-31.
- Grimes PE, Stockton T. Pigmentary disorders in blacks. Dermatol Clin. 1988;6:271-281.
- Halder RM, Nootheti PK. Ethnic skin disorders overview. J Am Acad Dermatol. 2003;48(suppl 6):S143-S148.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Callender VD. Acne in ethnic skin: special considerations for therapy. Dermatol Ther. 2004;17:184-195.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
- Ramsay DL, Mayer F. National survey of undergraduate dermatologic medical education. Arch Dermatol.1985;121:1529-1530.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii.
- Knable A, Hood AF, Pearson TG. Undergraduate medical education in dermatology: report from the AAD Interdisciplinary Education Committee, Subcommittee on Undergraduate Medical Education. J Am Acad Dermatol. 1997;36:467-470.
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690.
- Skochelak SE, Stack SJ. Creating the medical schools of the future. Acad Med. 2017;92:16-19.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871.
- Skin of Color Society. Patient dermatology education. Accessed September 22, 2021. https://skinofcolorsociety.org/patient-dermatology-education
- QuickFacts: United States. US Census Bureau website. Updated July 1, 2019. Accessed September 14, 2021. https://www.census.gov/quickfacts/fact/table/US#
- Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423.
- Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427-434.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Pipitone M, Robinson JK, Camara C, et al. Skin cancer awareness in suburban employees: a Hispanic perspective. J Am Acad Dermatol. 2002;47:118-123.
- Imahiyerobo-Ip J, Ip I, Jamal S, et al. Skin cancer awareness in communities of color. J Am Acad Dermatol. 2011;64:198-200.
- Taylor SSC, Serrano AMA, Kelly AP, et al, eds. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016.
- Dadzie OE, Petit A, Alexis AF, eds. Ethnic Dermatology: Principles and Practice. Wiley-Blackwell; 2013.
- Jackson-Richards D, Pandya AG, eds. Dermatology Atlas for Skin of Color. Springer; 2014.
- VisualDx. New VisualDx feature: skin of color sort. Published October 14, 2020. Accessed September 22, 2021. https://www.visualdx.com/blog/new-visualdx-feature-skin-of-color-sort/
- Colby SL, Ortman JM. Projections of the size and composition of the US population: 2014 to 2060. United States Census Bureau website. Published March 2015. Accessed September 14, 2021. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf
- Learn more about SOCS. Skin of Color Society website. Accessed September 14, 2021. http://skinofcolorsociety.org/about-socs/
- Taylor SC. Skin of color: biology, structure, function, and implications for dermatologic disease. J Am Acad Dermatol. 2002;46(suppl 2):S41-S62.
- Berardesca E, Maibach H. Ethnic skin: overview of structure and function. J Am Acad Dermatol. 2003;48(suppl 6):S139-S142.
- Callender VD, Surin-Lord SS, Davis EC, et al. Postinflammatory hyperpigmentation. Am J Clin Dermatol. 2011;12:87-99.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3:20-31.
- Grimes PE, Stockton T. Pigmentary disorders in blacks. Dermatol Clin. 1988;6:271-281.
- Halder RM, Nootheti PK. Ethnic skin disorders overview. J Am Acad Dermatol. 2003;48(suppl 6):S143-S148.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Callender VD. Acne in ethnic skin: special considerations for therapy. Dermatol Ther. 2004;17:184-195.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
- Ramsay DL, Mayer F. National survey of undergraduate dermatologic medical education. Arch Dermatol.1985;121:1529-1530.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii.
- Knable A, Hood AF, Pearson TG. Undergraduate medical education in dermatology: report from the AAD Interdisciplinary Education Committee, Subcommittee on Undergraduate Medical Education. J Am Acad Dermatol. 1997;36:467-470.
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690.
- Skochelak SE, Stack SJ. Creating the medical schools of the future. Acad Med. 2017;92:16-19.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871.
- Skin of Color Society. Patient dermatology education. Accessed September 22, 2021. https://skinofcolorsociety.org/patient-dermatology-education
- QuickFacts: United States. US Census Bureau website. Updated July 1, 2019. Accessed September 14, 2021. https://www.census.gov/quickfacts/fact/table/US#
- Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423.
- Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427-434.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Pipitone M, Robinson JK, Camara C, et al. Skin cancer awareness in suburban employees: a Hispanic perspective. J Am Acad Dermatol. 2002;47:118-123.
- Imahiyerobo-Ip J, Ip I, Jamal S, et al. Skin cancer awareness in communities of color. J Am Acad Dermatol. 2011;64:198-200.
- Taylor SSC, Serrano AMA, Kelly AP, et al, eds. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016.
- Dadzie OE, Petit A, Alexis AF, eds. Ethnic Dermatology: Principles and Practice. Wiley-Blackwell; 2013.
- Jackson-Richards D, Pandya AG, eds. Dermatology Atlas for Skin of Color. Springer; 2014.
- VisualDx. New VisualDx feature: skin of color sort. Published October 14, 2020. Accessed September 22, 2021. https://www.visualdx.com/blog/new-visualdx-feature-skin-of-color-sort/
Practice Points
- The United States rapidly is becoming a country in which the majority of citizens will have skin of color.
- Our study results strongly suggest that skin of color may be seriously underrepresented in medical education and can guide modifications to preclinical dermatology medical education to develop a more comprehensive and inclusive curriculum.
- Efforts should be made to increase images and discussion of skin of color in preclinical didactics.