User login
Does taking isotretinoin worsen a patient’s baseline IBD symptoms?
A , results from a small retrospective study suggests.
“Early studies of isotretinoin for use in severe acne suggested the drug may serve as a trigger for new-onset inflammatory bowel disease (IBD),” researchers led by Christina G. Lopez, MD, of the Lewis Katz School of Medicine at Temple University, Philadelphia, wrote in an article published online , in the Journal of the American Academy of Dermatology. “While more recent studies have suggested no such causal relationship, little is known about the medication’s effect on patients with a preexisting IBD diagnosis.”
To investigate this topic further, the researchers identified 19 patients who were diagnosed with IBD and treated with isotretinoin between Jan. 1, 2006, and Jan. 1, 2020, at Mass General Brigham Hospitals, Boston. They determined severity of disease and degree of antecedent management of IBD by evaluating flaring two years prior to starting isotretinoin. The patients were considered to have a flare caused by isotretinoin if the IBD flare occurred during or up to 3 months following course completion.
The mean age of the 19 patients was 35 years, 26% were female, and 95% were White. Nearly half of the patients (42%) had ulcerative colitis, 37% had Crohn’s disease, and 21% had both. The researchers found that nine patients had flared two years before starting isotretinoin. Of these, five (56%) flared and four (44%) did not flare during treatment or within three months of completing the course of isotretinoin.
Of the 10 patients who did not flare two years before starting isotretinoin, seven (70%) did not flare during treatment and three (30%) flared during or within three months following completion of isotretinoin use. The researchers found no statistically significant association between isotretinoin use and flaring among patients with IBD (P = .76).
Dr. Lopez and her colleagues also assessed IBD maintenance therapy with respect to IBD flares in the study population. They observed no statistically significant association between the use of maintenance IBD therapy and the likelihood of having flares during isotretinoin treatment (P = .15).
“The results suggest limited association between isotretinoin and the worsening of a patient’s baseline IBD,” the authors concluded. They acknowledged certain limitations of the study, including its small sample size and retrospective design, and they called for larger and prospective studies to assess the relationship of IBD flaring in this population of patients.
Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, who was asked to comment on the results, characterized the trial as “an important study highlighting how we continue to understand the safe use of isotretinoin in the IBD cohort.”
Isotretinoin, she added, “continues to be a highly important treatment for acne and in patients such as these where oral antibiotics are relatively contraindicated due to risk of exacerbating their bowel disease.” Such data are reassuring, “albeit future studies with larger patient pools are desirable,” she added. “Future studies could also help to elucidate if diet, smoking, sleep, exercise, and medication adherence are potential confounding factors along with whether the cumulative isotretinoin dose has any effect on IBD flares in those who are susceptible.”
Neither the researchers nor Dr. Sodha had financial conflicts. The other authors were from Brigham and Women’s Hospital, Harvard University, Boston, and the University of Massachusetts, Worcester.
A , results from a small retrospective study suggests.
“Early studies of isotretinoin for use in severe acne suggested the drug may serve as a trigger for new-onset inflammatory bowel disease (IBD),” researchers led by Christina G. Lopez, MD, of the Lewis Katz School of Medicine at Temple University, Philadelphia, wrote in an article published online , in the Journal of the American Academy of Dermatology. “While more recent studies have suggested no such causal relationship, little is known about the medication’s effect on patients with a preexisting IBD diagnosis.”
To investigate this topic further, the researchers identified 19 patients who were diagnosed with IBD and treated with isotretinoin between Jan. 1, 2006, and Jan. 1, 2020, at Mass General Brigham Hospitals, Boston. They determined severity of disease and degree of antecedent management of IBD by evaluating flaring two years prior to starting isotretinoin. The patients were considered to have a flare caused by isotretinoin if the IBD flare occurred during or up to 3 months following course completion.
The mean age of the 19 patients was 35 years, 26% were female, and 95% were White. Nearly half of the patients (42%) had ulcerative colitis, 37% had Crohn’s disease, and 21% had both. The researchers found that nine patients had flared two years before starting isotretinoin. Of these, five (56%) flared and four (44%) did not flare during treatment or within three months of completing the course of isotretinoin.
Of the 10 patients who did not flare two years before starting isotretinoin, seven (70%) did not flare during treatment and three (30%) flared during or within three months following completion of isotretinoin use. The researchers found no statistically significant association between isotretinoin use and flaring among patients with IBD (P = .76).
Dr. Lopez and her colleagues also assessed IBD maintenance therapy with respect to IBD flares in the study population. They observed no statistically significant association between the use of maintenance IBD therapy and the likelihood of having flares during isotretinoin treatment (P = .15).
“The results suggest limited association between isotretinoin and the worsening of a patient’s baseline IBD,” the authors concluded. They acknowledged certain limitations of the study, including its small sample size and retrospective design, and they called for larger and prospective studies to assess the relationship of IBD flaring in this population of patients.
Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, who was asked to comment on the results, characterized the trial as “an important study highlighting how we continue to understand the safe use of isotretinoin in the IBD cohort.”
Isotretinoin, she added, “continues to be a highly important treatment for acne and in patients such as these where oral antibiotics are relatively contraindicated due to risk of exacerbating their bowel disease.” Such data are reassuring, “albeit future studies with larger patient pools are desirable,” she added. “Future studies could also help to elucidate if diet, smoking, sleep, exercise, and medication adherence are potential confounding factors along with whether the cumulative isotretinoin dose has any effect on IBD flares in those who are susceptible.”
Neither the researchers nor Dr. Sodha had financial conflicts. The other authors were from Brigham and Women’s Hospital, Harvard University, Boston, and the University of Massachusetts, Worcester.
A , results from a small retrospective study suggests.
“Early studies of isotretinoin for use in severe acne suggested the drug may serve as a trigger for new-onset inflammatory bowel disease (IBD),” researchers led by Christina G. Lopez, MD, of the Lewis Katz School of Medicine at Temple University, Philadelphia, wrote in an article published online , in the Journal of the American Academy of Dermatology. “While more recent studies have suggested no such causal relationship, little is known about the medication’s effect on patients with a preexisting IBD diagnosis.”
To investigate this topic further, the researchers identified 19 patients who were diagnosed with IBD and treated with isotretinoin between Jan. 1, 2006, and Jan. 1, 2020, at Mass General Brigham Hospitals, Boston. They determined severity of disease and degree of antecedent management of IBD by evaluating flaring two years prior to starting isotretinoin. The patients were considered to have a flare caused by isotretinoin if the IBD flare occurred during or up to 3 months following course completion.
The mean age of the 19 patients was 35 years, 26% were female, and 95% were White. Nearly half of the patients (42%) had ulcerative colitis, 37% had Crohn’s disease, and 21% had both. The researchers found that nine patients had flared two years before starting isotretinoin. Of these, five (56%) flared and four (44%) did not flare during treatment or within three months of completing the course of isotretinoin.
Of the 10 patients who did not flare two years before starting isotretinoin, seven (70%) did not flare during treatment and three (30%) flared during or within three months following completion of isotretinoin use. The researchers found no statistically significant association between isotretinoin use and flaring among patients with IBD (P = .76).
Dr. Lopez and her colleagues also assessed IBD maintenance therapy with respect to IBD flares in the study population. They observed no statistically significant association between the use of maintenance IBD therapy and the likelihood of having flares during isotretinoin treatment (P = .15).
“The results suggest limited association between isotretinoin and the worsening of a patient’s baseline IBD,” the authors concluded. They acknowledged certain limitations of the study, including its small sample size and retrospective design, and they called for larger and prospective studies to assess the relationship of IBD flaring in this population of patients.
Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, who was asked to comment on the results, characterized the trial as “an important study highlighting how we continue to understand the safe use of isotretinoin in the IBD cohort.”
Isotretinoin, she added, “continues to be a highly important treatment for acne and in patients such as these where oral antibiotics are relatively contraindicated due to risk of exacerbating their bowel disease.” Such data are reassuring, “albeit future studies with larger patient pools are desirable,” she added. “Future studies could also help to elucidate if diet, smoking, sleep, exercise, and medication adherence are potential confounding factors along with whether the cumulative isotretinoin dose has any effect on IBD flares in those who are susceptible.”
Neither the researchers nor Dr. Sodha had financial conflicts. The other authors were from Brigham and Women’s Hospital, Harvard University, Boston, and the University of Massachusetts, Worcester.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Study provides new analysis of isotretinoin and risk for adverse neuropsychiatric outcomes
The use of , in a large retrospective cohort study published in the British Journal of Dermatology.
Although severe neuropsychiatric effects associated with isotretinoin therapy in patients with acne have been reported, “the evidence base ... is mixed and inconclusive,” and many studies are small, Seena Fazel, MBChB, MD, of the department of psychiatry, Oxford University, England, and co-authors write in the study.
The study results suggest that isotretinoin is conferring protection against adverse neuropsychiatric outcomes, particularly when compared with using oral antibiotics to treat acne, Dr. Fazel, professor of forensic psychiatry at Oxford University and the study’s senior author, said in an interview.
In the study, the investigators reviewed electronic health records (2013-2019) from a primarily United States–based dataset (TriNetX) of patients with acne aged 12-27 who had been followed for up to 1 year after their prescriptions had been dispensed.
There were four arms: those prescribed isotretinoin (30,866), oral antibiotics (44,748), topical anti-acne treatments (108,367), and those who had not been prescribed any acne treatment (78,666). The primary outcomes were diagnoses of a neuropsychiatric disorder (psychotic, mood, anxiety, personality, behavioral, and sleep disorders; and non-fatal self-harm) within one year of being prescribed treatment.
After using propensity score matching to adjust for confounders at baseline, the investigators determined that the odds ratio for any incident neuropsychiatric outcomes among patients with acne treated with isotretinoin was 0.80 (95% confidence interval, 0.74-0.87), compared with patients on oral antibiotics; 0.94 (95% CI, 0.87-1.02), compared with patients on topical anti-acne medications; and 1.06 (95% CI, 0.97-1.16), compared with those without a prescription for anti-acne medicines.
Side effects of isotretinoin – such as headache, dry mouth, and fatigue – were higher among those on isotretinoin than in the other three groups.
The authors concluded that isotretinoin was not independently linked to excess adverse neuropsychiatric outcomes at a population level. “We observed a consistent association between increasing acne severity as indicated by anti-acne treatment options and incidence of adverse neuropsychiatric outcomes, but the findings showed that isotretinoin exposure did not add to the risk of neuropsychiatric adverse outcomes over and above what was associated with oral antibiotics,” they write.
Isotretinoin treatment “appeared to mitigate the excess neuropsychiatric risk associated with recalcitrant moderate-to-severe acne,” they add.
The dermatology community has been interested in the impact isotretinoin has on mental health, and “I think clinically, they see that people get better on isotretinoin and their mental health improves,” Dr. Fazel told this news organization.
Asked to comment on the study results, John Barbieri, MD, MBA, director of the Advanced Acne Therapeutics Clinic, Brigham and Women’s Hospital, Boston, commended the investigators for the design of the trial.
“One of the strengths of this study is that they use a technique called propensity-score matching, where you try to make the groups of patients similar with respect to their other characteristics to minimize the risks of confounding and bias in the study, which I think is a real strength,” he told this news organization. “The other thing that they do, which I think is a strength, is to think about the impact of acne severity on these outcomes, because we know acne itself is associated with depression and risk for suicide and other neuropsychiatric outcomes.”
Including a cohort of patients who had acne and received oral antibiotics for comparison “is a nice way to address the potential for confounding by severity and confounding by indication,” Dr. Barbieri said. “Those who get antibiotics usually have more severe acne. They may not have it as severely as those who get isotretinoin, but it is a nice approach to account for background levels of depression and neuropsychiatric outcomes in patients with acne. I think that is a real strength of the study. This is one of the best studies to have looked at this question.”
However, although the study found that isotretinoin decreased the excess psychiatric risk associated with refractory moderate-to-severe acne, it does not rule out the possibility that individuals may experience an adverse psychiatric outcome while on isotretinoin, Dr. Barbieri said.
“While I think on a population level, we absolutely can feel reassured by these data, I do think there are individual patients who have idiosyncratic, unpredictable reactions to isotretinoin where they have mood changes, whether it be irritability, depression, or other mood changes,” he cautioned. “Given the association of acne itself with mental health comorbidities, it is important to screen for comorbidities such as depression in all patients with acne.”
The study was funded by the Wellcome Trust, which provided Dr. Fazel and the first author with financial support for the study. One author is an employee of TriNetX; the other authors had no relevant disclosures. Dr. Barbieri reported no financial disclosures. He is cochair of the AAD’s Acne Guidelines Workgroup and associate editor at JAMA Dermatology.
A version of this article first appeared on Medscape.com.
The use of , in a large retrospective cohort study published in the British Journal of Dermatology.
Although severe neuropsychiatric effects associated with isotretinoin therapy in patients with acne have been reported, “the evidence base ... is mixed and inconclusive,” and many studies are small, Seena Fazel, MBChB, MD, of the department of psychiatry, Oxford University, England, and co-authors write in the study.
The study results suggest that isotretinoin is conferring protection against adverse neuropsychiatric outcomes, particularly when compared with using oral antibiotics to treat acne, Dr. Fazel, professor of forensic psychiatry at Oxford University and the study’s senior author, said in an interview.
In the study, the investigators reviewed electronic health records (2013-2019) from a primarily United States–based dataset (TriNetX) of patients with acne aged 12-27 who had been followed for up to 1 year after their prescriptions had been dispensed.
There were four arms: those prescribed isotretinoin (30,866), oral antibiotics (44,748), topical anti-acne treatments (108,367), and those who had not been prescribed any acne treatment (78,666). The primary outcomes were diagnoses of a neuropsychiatric disorder (psychotic, mood, anxiety, personality, behavioral, and sleep disorders; and non-fatal self-harm) within one year of being prescribed treatment.
After using propensity score matching to adjust for confounders at baseline, the investigators determined that the odds ratio for any incident neuropsychiatric outcomes among patients with acne treated with isotretinoin was 0.80 (95% confidence interval, 0.74-0.87), compared with patients on oral antibiotics; 0.94 (95% CI, 0.87-1.02), compared with patients on topical anti-acne medications; and 1.06 (95% CI, 0.97-1.16), compared with those without a prescription for anti-acne medicines.
Side effects of isotretinoin – such as headache, dry mouth, and fatigue – were higher among those on isotretinoin than in the other three groups.
The authors concluded that isotretinoin was not independently linked to excess adverse neuropsychiatric outcomes at a population level. “We observed a consistent association between increasing acne severity as indicated by anti-acne treatment options and incidence of adverse neuropsychiatric outcomes, but the findings showed that isotretinoin exposure did not add to the risk of neuropsychiatric adverse outcomes over and above what was associated with oral antibiotics,” they write.
Isotretinoin treatment “appeared to mitigate the excess neuropsychiatric risk associated with recalcitrant moderate-to-severe acne,” they add.
The dermatology community has been interested in the impact isotretinoin has on mental health, and “I think clinically, they see that people get better on isotretinoin and their mental health improves,” Dr. Fazel told this news organization.
Asked to comment on the study results, John Barbieri, MD, MBA, director of the Advanced Acne Therapeutics Clinic, Brigham and Women’s Hospital, Boston, commended the investigators for the design of the trial.
“One of the strengths of this study is that they use a technique called propensity-score matching, where you try to make the groups of patients similar with respect to their other characteristics to minimize the risks of confounding and bias in the study, which I think is a real strength,” he told this news organization. “The other thing that they do, which I think is a strength, is to think about the impact of acne severity on these outcomes, because we know acne itself is associated with depression and risk for suicide and other neuropsychiatric outcomes.”
Including a cohort of patients who had acne and received oral antibiotics for comparison “is a nice way to address the potential for confounding by severity and confounding by indication,” Dr. Barbieri said. “Those who get antibiotics usually have more severe acne. They may not have it as severely as those who get isotretinoin, but it is a nice approach to account for background levels of depression and neuropsychiatric outcomes in patients with acne. I think that is a real strength of the study. This is one of the best studies to have looked at this question.”
However, although the study found that isotretinoin decreased the excess psychiatric risk associated with refractory moderate-to-severe acne, it does not rule out the possibility that individuals may experience an adverse psychiatric outcome while on isotretinoin, Dr. Barbieri said.
“While I think on a population level, we absolutely can feel reassured by these data, I do think there are individual patients who have idiosyncratic, unpredictable reactions to isotretinoin where they have mood changes, whether it be irritability, depression, or other mood changes,” he cautioned. “Given the association of acne itself with mental health comorbidities, it is important to screen for comorbidities such as depression in all patients with acne.”
The study was funded by the Wellcome Trust, which provided Dr. Fazel and the first author with financial support for the study. One author is an employee of TriNetX; the other authors had no relevant disclosures. Dr. Barbieri reported no financial disclosures. He is cochair of the AAD’s Acne Guidelines Workgroup and associate editor at JAMA Dermatology.
A version of this article first appeared on Medscape.com.
The use of , in a large retrospective cohort study published in the British Journal of Dermatology.
Although severe neuropsychiatric effects associated with isotretinoin therapy in patients with acne have been reported, “the evidence base ... is mixed and inconclusive,” and many studies are small, Seena Fazel, MBChB, MD, of the department of psychiatry, Oxford University, England, and co-authors write in the study.
The study results suggest that isotretinoin is conferring protection against adverse neuropsychiatric outcomes, particularly when compared with using oral antibiotics to treat acne, Dr. Fazel, professor of forensic psychiatry at Oxford University and the study’s senior author, said in an interview.
In the study, the investigators reviewed electronic health records (2013-2019) from a primarily United States–based dataset (TriNetX) of patients with acne aged 12-27 who had been followed for up to 1 year after their prescriptions had been dispensed.
There were four arms: those prescribed isotretinoin (30,866), oral antibiotics (44,748), topical anti-acne treatments (108,367), and those who had not been prescribed any acne treatment (78,666). The primary outcomes were diagnoses of a neuropsychiatric disorder (psychotic, mood, anxiety, personality, behavioral, and sleep disorders; and non-fatal self-harm) within one year of being prescribed treatment.
After using propensity score matching to adjust for confounders at baseline, the investigators determined that the odds ratio for any incident neuropsychiatric outcomes among patients with acne treated with isotretinoin was 0.80 (95% confidence interval, 0.74-0.87), compared with patients on oral antibiotics; 0.94 (95% CI, 0.87-1.02), compared with patients on topical anti-acne medications; and 1.06 (95% CI, 0.97-1.16), compared with those without a prescription for anti-acne medicines.
Side effects of isotretinoin – such as headache, dry mouth, and fatigue – were higher among those on isotretinoin than in the other three groups.
The authors concluded that isotretinoin was not independently linked to excess adverse neuropsychiatric outcomes at a population level. “We observed a consistent association between increasing acne severity as indicated by anti-acne treatment options and incidence of adverse neuropsychiatric outcomes, but the findings showed that isotretinoin exposure did not add to the risk of neuropsychiatric adverse outcomes over and above what was associated with oral antibiotics,” they write.
Isotretinoin treatment “appeared to mitigate the excess neuropsychiatric risk associated with recalcitrant moderate-to-severe acne,” they add.
The dermatology community has been interested in the impact isotretinoin has on mental health, and “I think clinically, they see that people get better on isotretinoin and their mental health improves,” Dr. Fazel told this news organization.
Asked to comment on the study results, John Barbieri, MD, MBA, director of the Advanced Acne Therapeutics Clinic, Brigham and Women’s Hospital, Boston, commended the investigators for the design of the trial.
“One of the strengths of this study is that they use a technique called propensity-score matching, where you try to make the groups of patients similar with respect to their other characteristics to minimize the risks of confounding and bias in the study, which I think is a real strength,” he told this news organization. “The other thing that they do, which I think is a strength, is to think about the impact of acne severity on these outcomes, because we know acne itself is associated with depression and risk for suicide and other neuropsychiatric outcomes.”
Including a cohort of patients who had acne and received oral antibiotics for comparison “is a nice way to address the potential for confounding by severity and confounding by indication,” Dr. Barbieri said. “Those who get antibiotics usually have more severe acne. They may not have it as severely as those who get isotretinoin, but it is a nice approach to account for background levels of depression and neuropsychiatric outcomes in patients with acne. I think that is a real strength of the study. This is one of the best studies to have looked at this question.”
However, although the study found that isotretinoin decreased the excess psychiatric risk associated with refractory moderate-to-severe acne, it does not rule out the possibility that individuals may experience an adverse psychiatric outcome while on isotretinoin, Dr. Barbieri said.
“While I think on a population level, we absolutely can feel reassured by these data, I do think there are individual patients who have idiosyncratic, unpredictable reactions to isotretinoin where they have mood changes, whether it be irritability, depression, or other mood changes,” he cautioned. “Given the association of acne itself with mental health comorbidities, it is important to screen for comorbidities such as depression in all patients with acne.”
The study was funded by the Wellcome Trust, which provided Dr. Fazel and the first author with financial support for the study. One author is an employee of TriNetX; the other authors had no relevant disclosures. Dr. Barbieri reported no financial disclosures. He is cochair of the AAD’s Acne Guidelines Workgroup and associate editor at JAMA Dermatology.
A version of this article first appeared on Medscape.com.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
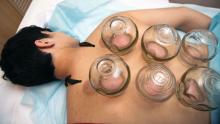
Cupping in dermatology
My inspiration to write about cupping this month stems from the perception that everyone seems to be talking about it, from a facialist who suggested it for me to a coworker who swears by cupping to treat her allergies. Cupping is by no means a novel procedure. Its use as a health therapy dates back thousands of years to ancient Egypt (1500 BCE), ancient Greece (described by Hippocrates), ancient Rome (described by the Greek physician Galen), China (during the Han dynasty, 206 BCE to 220 CE) and traditional Islamic culture.1 Over the past decade, the popularity of this ancient procedure has been increasing in the United States.1 Cupping has been applied as a remedy for various dermatologic and medical conditions, including herpes zoster, headaches, diminished appetite, maldigestion, abscess evacuation, narcolepsy, pain, fever, dysmenorrhea, and gout.1,2
Theories on the mechanism(s) of action
The practice of cupping is differentiated into dry and wet cupping.1,2 Traditionally, with dry cupping, a flame is applied to heat the air inside a thick glass cup (rather than the cup itself).1 The cup is placed on the skin surface, and negative pressure suctions the skin into the cup. Wet cupping differs mainly from dry cupping in that it involves blood-letting. Cups made of either silicone or glass of varying size and shapes are used. Modern adaptations to cupping include needle, herbal, and pulsatile cupping, as well as a “moving cupping” technique (vs. traditionally stationary cups).1
There are several theories, many of which are derived from the nondermatologic literature (that is, pain management), as to how cupping may deliver a clinical benefit. Some theories are based in scientific and medical principles, whereas other theories are more whimsical – specifically, that cupping draws out evil spirits.2 Studies of dry cupping have suggested that the procedure results in increased oxygenation of muscles via a local increase in oxygenated hemoglobin, which may help improve muscular activity and reduce pain.1 As theorized by Lowe in 2017, negative pressure exerted by dry cupping leads to stretching and dilation of capillaries, which increases blood flow.3 Wet cupping has been shown to increase heat shock protein 70 (HSP70) and beta-endorphin expression in rat models, which is thought to facilitate pain management.1 Removal of oxidants and reduction of reactive oxygen species in the blood is believed to be among the benefits of wet cupping.1
Cupping in general dermatology
While , as well as various inflammatory conditions.
Herpes zoster
In 2010, Cao et al. reported on their systematic review of wet cupping after completing searches of multiple databases (that is, PubMed, the Cochrane Library [Issue 3, 2008], China Network Knowledge Infrastructure, Chinese Scientific Journal Database, and Wan Fang Database). They identified eight randomized controlled trials involving 651 patients, with meta-analyses revealing that wet cupping performed better than medications in terms of the number of “cured” patients, number of patients with improved symptoms, and a lower incidence of postherpetic neuralgia. Wet cupping, in addition to medication, was also found to be superior to medication alone in multiple patients. The researchers concluded that wet cupping appears to effectively treat herpes zoster.4 However, the study failed to identify which medications were used to treat herpes zoster. In the United States, common medications for herpes zoster include acyclovir, valacyclovir, steroids, gabapentin, and other neuromodulators. Without knowing which medications were used, it is difficult to compare cupping to medication in terms of efficacy in treating herpes zoster.
Urticaria
Urticaria (hives) is an inflammatory skin condition that can be very uncomfortable for patients but often resolves without intervention within several months after onset. In 2001, Li and Ding reported on the treatment with cupping of 40 patients with urticaria. The cure rate among the treatment group was cited as 55%, compared with 30% in the control group, who were treated with a traditional Chinese remedy and an unidentified first-generation antihistamine.1,5 In 2020, Xiao et al. conducted a systematic review and meta-analysis of cupping therapy for patients with chronic urticaria. They identified 13 comparisons from 12 randomized controlled trials involving 842 subjects. The investigators found no significant differences between wet cupping and medication usage. They also found that cupping combined with antihistamine treatment was superior to antihistamines alone, and cupping therapy with acupuncture was more effective than acupuncture alone. The investigators did call for caution, citing the poor quality of the studies reviewed.6
It is important to note that it is difficult to attribute resolution of urticaria to the use of cupping given the self-resolution often associated with this condition. Antihistamines are the mainstay of therapy for urticaria, but in my personal experience, patients are not entirely satisfied with the level of symptom control with antihistamines alone and often search for alternative therapies to control the pesky hives and associated itch. In 2014, omalizumab (Xolair) was approved for treating chronic idiopathic urticaria, which has helped patients control symptoms of chronic idiopathic urticaria without needing to take antihistamines. There was no indication that the studies reviewed by Xiao et al. compared cupping against this new effective treatment. Therefore, these studies comparing cupping to medical management are outdated.
Acne, eczema, and psoriasis
Soliman’s 2018 review of cupping in dermatology included a few studies on these common cutaneous conditions. For instance, a 2013 single-blind prospective study by Xu et al. reported on the results of patients with moderate acne who received wet cupping (in the form of prickling bloodletting) twice weekly for 6 weeks.7 They reported that patients demonstrated improvement in the global acne grading system (GAGS) score by the end of the trial.1,7 Unfortunately, cupping was not compared with standard acne treatments (that is, benzoyl peroxide, topical and oral antibiotics, isotretinoin, topical retinoids, spironolactone).
In evaluating cupping for acute eczema, wet cupping was compared with oral loratadine and topical ointments in a 2007 study by Yao and Li. They divided 88 cases into treatment and control groups, with the former group (n = 46) receiving bloodletting puncturing and cupping and the control group (n = 42) receiving oral loratadine and topical Pairuisong (an herbal ointment used in Chinese medicine). The investigators observed no significant difference in total effective rates but a superior difference in the rates of responses that were considered “cured” and “markedly effective” in favor of the cupping treatment.1,8 However, a case report by Hon et al. has indicated that cupping therapy may be associated with more harm than benefit when used as an eczema treatment.1,9
In addition, it is important to note that the past 5 years have been gamechanging in the management of chronic eczema in terms of the array of novel and effective therapies (e.g., dupilumab and JAK inhibitors) and chronic moderate-to-severe eczema has become very treatable. Similarly, acute eczema is often successfully managed with topical steroids, calcineurin inhibitors, and emollients. As such, there is no compelling reason to consider an unproven treatment such as cupping.
In 2020, Xing et al. reviewed 16 randomized controlled trials assessing the use of “moving cupping” for plaque psoriasis, with 1,164 patients meeting inclusion criteria. Moving cupping was found to be significantly more effective than “no-moving” cupping therapy, and moving cupping, combined with medications, performed better than medications alone.10 None of the trials evaluated in this study included randomized controlled trials that compared patients using any of the more modern psoriasis medications, specifically biologics. And, again, the studies evaluated were not of the highest quality.
The data that support cupping, as summarized above, are based mostly on case reports, and strong double-blind prospective studies are lacking. Additionally, most of the studies cited gauged the efficacy of cupping using qualitative endpoints, rather than standardized quantitative endpoints and scales. Moreover, spontaneous remission of various dermatoses can occur, or they can improve over time, including acute eczema, psoriasis, and, especially, urticaria.
Adverse effects of cupping
Often alternative therapies are seen as “benign” and without adverse effects. However, complications can result from cupping. Trauma can be induced from the cupping itself by damaging superficial blood vessels and causing bruising.1,11 Blistering can also occur secondary to the suction effect, and the epidermal and dermal layers of the skin can be separated.1,11 Further, burns and discoloration have also been noted secondary to heat, trauma, and post inflammatory pigmentary changes.1,11 Another risk of cupping is the Koebner phenomenon, which occurs with psoriasis, with new lesions appearing in traumatized skin.12 Other adverse outcomes that have been reported with cupping include reactivation of herpes simplex virus secondary to skin trauma, iron deficiency anemia (secondary to blood loss), panniculitis, infections, and residual marks mistaken for signs of child abuse.1,11
Cupping in aesthetic dermatology
Facial cupping, a distinct practice from body cupping used to treat general dermatology conditions described previously, is also increasing in popularity. This practice is usually conducted in association with a facial or facial acupuncture by an aesthetician or other licensed professional. It can also be performed using at-home kits. The marketing claims for facial cupping cite improved tightening and contouring of facial skin, increased facial microcirculation and collagen synthesis, and enhanced lymphatic flow to aid with facial puffiness or swelling. One supposed mechanism for these benefits is that cupping increases blood flow. Interestingly, there was a 2020 animal study in which photoacoustic imaging of a mouse ear revealed increased temporary blood flow in the cupping microenvironment.13 Currently, however, there is no evidence in the English scientific literature that supports facial cupping. The benefits attributed to facial cupping for aesthetic purposes have emerged only in personal anecdotes. The temporary increase in blood flow may induce inflammation and swelling that adds volume to the face and temporarily diminishes wrinkles. However, this temporary plumpness may be associated with adverse effects, such as local trauma, irritation, bruising, postinflammatory pigmentary alteration, or even herpes reactivation. In my opinion, the possible adverse effects of cupping outweigh any potential benefit, especially given the insufficient evidence supporting the utility of cupping for cosmetic enhancement.
Summary
There is increasing interest among patients to incorporate complementary and alternative medicine – including the ancient tradition of cupping – in managing medical dermatologic conditions. However, current evidence supporting cupping as an effective therapeutic strategy is not strong, with most studies to date appearing to be of poor quality or not sufficiently convincing to displace standard therapies. Our medical strategies for managing chronic dermatologic conditions, particularly inflammatory disorders, continue to improve from both a safety and a proven efficacy standpoint. Therefore, I would not forgo medical management in favor of cupping. While cupping can be used as an adjunct therapy, I would caution patients about possible adverse side effects. In the aesthetic world, cupping is also gaining popularity, but this trend is also not supported by current evidence or studies, at least in the Western literature.
Dr. Goldman is a dermatologist in private practice in Miami and specializes in cosmetic and general dermatology. She practices at Baumann Cosmetic & Research Institute and is also opening a general dermatology practice. Write to her at [email protected] or message her on Instragram @DrChloeGoldman. Dr. Goldman receives compensation to create social media content for Replenix, a skin care company. She has no other disclosures.
References
1. Soliman Y et al. Acta Dermatovenerol Alp Pannonica Adriat. 2018 Jun;27(2):103-7.
2. França K and Lotti T. Advances in Integrative Dermatology. John Wiley & Sons, 2019.
3. Lowe DT. Complement Ther Clin Pract. 2017 Nov;29:162-8.
4.Cao H et al. Altern Ther Health Med. 2010 Nov-Dec;16(6):48-54.
5. Li L and Ding J. J Tradit Chin Med. 2001 Mar;21(1):37-8.
6. Xiao XJ et al. J Integr Med. 2020 Jul;18(4):303-12.
7. Xu J et al. J Tradit Chin Med. 2013 Dec;33(6):752-6.
8. Yao J et al. Zhongguo Zhen Jiu. 2007; Jun;27(6):424-6.
9. Hon KL et al. Case Rep Pediatr. 2013;2013:605829.
10. Xing M et al. Medicine (Baltimore). 2020 Oct 9;99(41):e22539.
11. Kim TH et al. Eur J Integr Med. 2014 Aug 1;6(4):434-40.
12. Vender R and Vender R. J Cutan Med Surg. 2015 May-Jun;19(3):320-2.
13. Zhou Y et al. Biomed Opt Express. 2020 Apr 6;11(5):2394-401.
This article was updated 4/25/22.
My inspiration to write about cupping this month stems from the perception that everyone seems to be talking about it, from a facialist who suggested it for me to a coworker who swears by cupping to treat her allergies. Cupping is by no means a novel procedure. Its use as a health therapy dates back thousands of years to ancient Egypt (1500 BCE), ancient Greece (described by Hippocrates), ancient Rome (described by the Greek physician Galen), China (during the Han dynasty, 206 BCE to 220 CE) and traditional Islamic culture.1 Over the past decade, the popularity of this ancient procedure has been increasing in the United States.1 Cupping has been applied as a remedy for various dermatologic and medical conditions, including herpes zoster, headaches, diminished appetite, maldigestion, abscess evacuation, narcolepsy, pain, fever, dysmenorrhea, and gout.1,2
Theories on the mechanism(s) of action
The practice of cupping is differentiated into dry and wet cupping.1,2 Traditionally, with dry cupping, a flame is applied to heat the air inside a thick glass cup (rather than the cup itself).1 The cup is placed on the skin surface, and negative pressure suctions the skin into the cup. Wet cupping differs mainly from dry cupping in that it involves blood-letting. Cups made of either silicone or glass of varying size and shapes are used. Modern adaptations to cupping include needle, herbal, and pulsatile cupping, as well as a “moving cupping” technique (vs. traditionally stationary cups).1
There are several theories, many of which are derived from the nondermatologic literature (that is, pain management), as to how cupping may deliver a clinical benefit. Some theories are based in scientific and medical principles, whereas other theories are more whimsical – specifically, that cupping draws out evil spirits.2 Studies of dry cupping have suggested that the procedure results in increased oxygenation of muscles via a local increase in oxygenated hemoglobin, which may help improve muscular activity and reduce pain.1 As theorized by Lowe in 2017, negative pressure exerted by dry cupping leads to stretching and dilation of capillaries, which increases blood flow.3 Wet cupping has been shown to increase heat shock protein 70 (HSP70) and beta-endorphin expression in rat models, which is thought to facilitate pain management.1 Removal of oxidants and reduction of reactive oxygen species in the blood is believed to be among the benefits of wet cupping.1
Cupping in general dermatology
While , as well as various inflammatory conditions.
Herpes zoster
In 2010, Cao et al. reported on their systematic review of wet cupping after completing searches of multiple databases (that is, PubMed, the Cochrane Library [Issue 3, 2008], China Network Knowledge Infrastructure, Chinese Scientific Journal Database, and Wan Fang Database). They identified eight randomized controlled trials involving 651 patients, with meta-analyses revealing that wet cupping performed better than medications in terms of the number of “cured” patients, number of patients with improved symptoms, and a lower incidence of postherpetic neuralgia. Wet cupping, in addition to medication, was also found to be superior to medication alone in multiple patients. The researchers concluded that wet cupping appears to effectively treat herpes zoster.4 However, the study failed to identify which medications were used to treat herpes zoster. In the United States, common medications for herpes zoster include acyclovir, valacyclovir, steroids, gabapentin, and other neuromodulators. Without knowing which medications were used, it is difficult to compare cupping to medication in terms of efficacy in treating herpes zoster.
Urticaria
Urticaria (hives) is an inflammatory skin condition that can be very uncomfortable for patients but often resolves without intervention within several months after onset. In 2001, Li and Ding reported on the treatment with cupping of 40 patients with urticaria. The cure rate among the treatment group was cited as 55%, compared with 30% in the control group, who were treated with a traditional Chinese remedy and an unidentified first-generation antihistamine.1,5 In 2020, Xiao et al. conducted a systematic review and meta-analysis of cupping therapy for patients with chronic urticaria. They identified 13 comparisons from 12 randomized controlled trials involving 842 subjects. The investigators found no significant differences between wet cupping and medication usage. They also found that cupping combined with antihistamine treatment was superior to antihistamines alone, and cupping therapy with acupuncture was more effective than acupuncture alone. The investigators did call for caution, citing the poor quality of the studies reviewed.6
It is important to note that it is difficult to attribute resolution of urticaria to the use of cupping given the self-resolution often associated with this condition. Antihistamines are the mainstay of therapy for urticaria, but in my personal experience, patients are not entirely satisfied with the level of symptom control with antihistamines alone and often search for alternative therapies to control the pesky hives and associated itch. In 2014, omalizumab (Xolair) was approved for treating chronic idiopathic urticaria, which has helped patients control symptoms of chronic idiopathic urticaria without needing to take antihistamines. There was no indication that the studies reviewed by Xiao et al. compared cupping against this new effective treatment. Therefore, these studies comparing cupping to medical management are outdated.
Acne, eczema, and psoriasis
Soliman’s 2018 review of cupping in dermatology included a few studies on these common cutaneous conditions. For instance, a 2013 single-blind prospective study by Xu et al. reported on the results of patients with moderate acne who received wet cupping (in the form of prickling bloodletting) twice weekly for 6 weeks.7 They reported that patients demonstrated improvement in the global acne grading system (GAGS) score by the end of the trial.1,7 Unfortunately, cupping was not compared with standard acne treatments (that is, benzoyl peroxide, topical and oral antibiotics, isotretinoin, topical retinoids, spironolactone).
In evaluating cupping for acute eczema, wet cupping was compared with oral loratadine and topical ointments in a 2007 study by Yao and Li. They divided 88 cases into treatment and control groups, with the former group (n = 46) receiving bloodletting puncturing and cupping and the control group (n = 42) receiving oral loratadine and topical Pairuisong (an herbal ointment used in Chinese medicine). The investigators observed no significant difference in total effective rates but a superior difference in the rates of responses that were considered “cured” and “markedly effective” in favor of the cupping treatment.1,8 However, a case report by Hon et al. has indicated that cupping therapy may be associated with more harm than benefit when used as an eczema treatment.1,9
In addition, it is important to note that the past 5 years have been gamechanging in the management of chronic eczema in terms of the array of novel and effective therapies (e.g., dupilumab and JAK inhibitors) and chronic moderate-to-severe eczema has become very treatable. Similarly, acute eczema is often successfully managed with topical steroids, calcineurin inhibitors, and emollients. As such, there is no compelling reason to consider an unproven treatment such as cupping.
In 2020, Xing et al. reviewed 16 randomized controlled trials assessing the use of “moving cupping” for plaque psoriasis, with 1,164 patients meeting inclusion criteria. Moving cupping was found to be significantly more effective than “no-moving” cupping therapy, and moving cupping, combined with medications, performed better than medications alone.10 None of the trials evaluated in this study included randomized controlled trials that compared patients using any of the more modern psoriasis medications, specifically biologics. And, again, the studies evaluated were not of the highest quality.
The data that support cupping, as summarized above, are based mostly on case reports, and strong double-blind prospective studies are lacking. Additionally, most of the studies cited gauged the efficacy of cupping using qualitative endpoints, rather than standardized quantitative endpoints and scales. Moreover, spontaneous remission of various dermatoses can occur, or they can improve over time, including acute eczema, psoriasis, and, especially, urticaria.
Adverse effects of cupping
Often alternative therapies are seen as “benign” and without adverse effects. However, complications can result from cupping. Trauma can be induced from the cupping itself by damaging superficial blood vessels and causing bruising.1,11 Blistering can also occur secondary to the suction effect, and the epidermal and dermal layers of the skin can be separated.1,11 Further, burns and discoloration have also been noted secondary to heat, trauma, and post inflammatory pigmentary changes.1,11 Another risk of cupping is the Koebner phenomenon, which occurs with psoriasis, with new lesions appearing in traumatized skin.12 Other adverse outcomes that have been reported with cupping include reactivation of herpes simplex virus secondary to skin trauma, iron deficiency anemia (secondary to blood loss), panniculitis, infections, and residual marks mistaken for signs of child abuse.1,11
Cupping in aesthetic dermatology
Facial cupping, a distinct practice from body cupping used to treat general dermatology conditions described previously, is also increasing in popularity. This practice is usually conducted in association with a facial or facial acupuncture by an aesthetician or other licensed professional. It can also be performed using at-home kits. The marketing claims for facial cupping cite improved tightening and contouring of facial skin, increased facial microcirculation and collagen synthesis, and enhanced lymphatic flow to aid with facial puffiness or swelling. One supposed mechanism for these benefits is that cupping increases blood flow. Interestingly, there was a 2020 animal study in which photoacoustic imaging of a mouse ear revealed increased temporary blood flow in the cupping microenvironment.13 Currently, however, there is no evidence in the English scientific literature that supports facial cupping. The benefits attributed to facial cupping for aesthetic purposes have emerged only in personal anecdotes. The temporary increase in blood flow may induce inflammation and swelling that adds volume to the face and temporarily diminishes wrinkles. However, this temporary plumpness may be associated with adverse effects, such as local trauma, irritation, bruising, postinflammatory pigmentary alteration, or even herpes reactivation. In my opinion, the possible adverse effects of cupping outweigh any potential benefit, especially given the insufficient evidence supporting the utility of cupping for cosmetic enhancement.
Summary
There is increasing interest among patients to incorporate complementary and alternative medicine – including the ancient tradition of cupping – in managing medical dermatologic conditions. However, current evidence supporting cupping as an effective therapeutic strategy is not strong, with most studies to date appearing to be of poor quality or not sufficiently convincing to displace standard therapies. Our medical strategies for managing chronic dermatologic conditions, particularly inflammatory disorders, continue to improve from both a safety and a proven efficacy standpoint. Therefore, I would not forgo medical management in favor of cupping. While cupping can be used as an adjunct therapy, I would caution patients about possible adverse side effects. In the aesthetic world, cupping is also gaining popularity, but this trend is also not supported by current evidence or studies, at least in the Western literature.
Dr. Goldman is a dermatologist in private practice in Miami and specializes in cosmetic and general dermatology. She practices at Baumann Cosmetic & Research Institute and is also opening a general dermatology practice. Write to her at [email protected] or message her on Instragram @DrChloeGoldman. Dr. Goldman receives compensation to create social media content for Replenix, a skin care company. She has no other disclosures.
References
1. Soliman Y et al. Acta Dermatovenerol Alp Pannonica Adriat. 2018 Jun;27(2):103-7.
2. França K and Lotti T. Advances in Integrative Dermatology. John Wiley & Sons, 2019.
3. Lowe DT. Complement Ther Clin Pract. 2017 Nov;29:162-8.
4.Cao H et al. Altern Ther Health Med. 2010 Nov-Dec;16(6):48-54.
5. Li L and Ding J. J Tradit Chin Med. 2001 Mar;21(1):37-8.
6. Xiao XJ et al. J Integr Med. 2020 Jul;18(4):303-12.
7. Xu J et al. J Tradit Chin Med. 2013 Dec;33(6):752-6.
8. Yao J et al. Zhongguo Zhen Jiu. 2007; Jun;27(6):424-6.
9. Hon KL et al. Case Rep Pediatr. 2013;2013:605829.
10. Xing M et al. Medicine (Baltimore). 2020 Oct 9;99(41):e22539.
11. Kim TH et al. Eur J Integr Med. 2014 Aug 1;6(4):434-40.
12. Vender R and Vender R. J Cutan Med Surg. 2015 May-Jun;19(3):320-2.
13. Zhou Y et al. Biomed Opt Express. 2020 Apr 6;11(5):2394-401.
This article was updated 4/25/22.
My inspiration to write about cupping this month stems from the perception that everyone seems to be talking about it, from a facialist who suggested it for me to a coworker who swears by cupping to treat her allergies. Cupping is by no means a novel procedure. Its use as a health therapy dates back thousands of years to ancient Egypt (1500 BCE), ancient Greece (described by Hippocrates), ancient Rome (described by the Greek physician Galen), China (during the Han dynasty, 206 BCE to 220 CE) and traditional Islamic culture.1 Over the past decade, the popularity of this ancient procedure has been increasing in the United States.1 Cupping has been applied as a remedy for various dermatologic and medical conditions, including herpes zoster, headaches, diminished appetite, maldigestion, abscess evacuation, narcolepsy, pain, fever, dysmenorrhea, and gout.1,2
Theories on the mechanism(s) of action
The practice of cupping is differentiated into dry and wet cupping.1,2 Traditionally, with dry cupping, a flame is applied to heat the air inside a thick glass cup (rather than the cup itself).1 The cup is placed on the skin surface, and negative pressure suctions the skin into the cup. Wet cupping differs mainly from dry cupping in that it involves blood-letting. Cups made of either silicone or glass of varying size and shapes are used. Modern adaptations to cupping include needle, herbal, and pulsatile cupping, as well as a “moving cupping” technique (vs. traditionally stationary cups).1
There are several theories, many of which are derived from the nondermatologic literature (that is, pain management), as to how cupping may deliver a clinical benefit. Some theories are based in scientific and medical principles, whereas other theories are more whimsical – specifically, that cupping draws out evil spirits.2 Studies of dry cupping have suggested that the procedure results in increased oxygenation of muscles via a local increase in oxygenated hemoglobin, which may help improve muscular activity and reduce pain.1 As theorized by Lowe in 2017, negative pressure exerted by dry cupping leads to stretching and dilation of capillaries, which increases blood flow.3 Wet cupping has been shown to increase heat shock protein 70 (HSP70) and beta-endorphin expression in rat models, which is thought to facilitate pain management.1 Removal of oxidants and reduction of reactive oxygen species in the blood is believed to be among the benefits of wet cupping.1
Cupping in general dermatology
While , as well as various inflammatory conditions.
Herpes zoster
In 2010, Cao et al. reported on their systematic review of wet cupping after completing searches of multiple databases (that is, PubMed, the Cochrane Library [Issue 3, 2008], China Network Knowledge Infrastructure, Chinese Scientific Journal Database, and Wan Fang Database). They identified eight randomized controlled trials involving 651 patients, with meta-analyses revealing that wet cupping performed better than medications in terms of the number of “cured” patients, number of patients with improved symptoms, and a lower incidence of postherpetic neuralgia. Wet cupping, in addition to medication, was also found to be superior to medication alone in multiple patients. The researchers concluded that wet cupping appears to effectively treat herpes zoster.4 However, the study failed to identify which medications were used to treat herpes zoster. In the United States, common medications for herpes zoster include acyclovir, valacyclovir, steroids, gabapentin, and other neuromodulators. Without knowing which medications were used, it is difficult to compare cupping to medication in terms of efficacy in treating herpes zoster.
Urticaria
Urticaria (hives) is an inflammatory skin condition that can be very uncomfortable for patients but often resolves without intervention within several months after onset. In 2001, Li and Ding reported on the treatment with cupping of 40 patients with urticaria. The cure rate among the treatment group was cited as 55%, compared with 30% in the control group, who were treated with a traditional Chinese remedy and an unidentified first-generation antihistamine.1,5 In 2020, Xiao et al. conducted a systematic review and meta-analysis of cupping therapy for patients with chronic urticaria. They identified 13 comparisons from 12 randomized controlled trials involving 842 subjects. The investigators found no significant differences between wet cupping and medication usage. They also found that cupping combined with antihistamine treatment was superior to antihistamines alone, and cupping therapy with acupuncture was more effective than acupuncture alone. The investigators did call for caution, citing the poor quality of the studies reviewed.6
It is important to note that it is difficult to attribute resolution of urticaria to the use of cupping given the self-resolution often associated with this condition. Antihistamines are the mainstay of therapy for urticaria, but in my personal experience, patients are not entirely satisfied with the level of symptom control with antihistamines alone and often search for alternative therapies to control the pesky hives and associated itch. In 2014, omalizumab (Xolair) was approved for treating chronic idiopathic urticaria, which has helped patients control symptoms of chronic idiopathic urticaria without needing to take antihistamines. There was no indication that the studies reviewed by Xiao et al. compared cupping against this new effective treatment. Therefore, these studies comparing cupping to medical management are outdated.
Acne, eczema, and psoriasis
Soliman’s 2018 review of cupping in dermatology included a few studies on these common cutaneous conditions. For instance, a 2013 single-blind prospective study by Xu et al. reported on the results of patients with moderate acne who received wet cupping (in the form of prickling bloodletting) twice weekly for 6 weeks.7 They reported that patients demonstrated improvement in the global acne grading system (GAGS) score by the end of the trial.1,7 Unfortunately, cupping was not compared with standard acne treatments (that is, benzoyl peroxide, topical and oral antibiotics, isotretinoin, topical retinoids, spironolactone).
In evaluating cupping for acute eczema, wet cupping was compared with oral loratadine and topical ointments in a 2007 study by Yao and Li. They divided 88 cases into treatment and control groups, with the former group (n = 46) receiving bloodletting puncturing and cupping and the control group (n = 42) receiving oral loratadine and topical Pairuisong (an herbal ointment used in Chinese medicine). The investigators observed no significant difference in total effective rates but a superior difference in the rates of responses that were considered “cured” and “markedly effective” in favor of the cupping treatment.1,8 However, a case report by Hon et al. has indicated that cupping therapy may be associated with more harm than benefit when used as an eczema treatment.1,9
In addition, it is important to note that the past 5 years have been gamechanging in the management of chronic eczema in terms of the array of novel and effective therapies (e.g., dupilumab and JAK inhibitors) and chronic moderate-to-severe eczema has become very treatable. Similarly, acute eczema is often successfully managed with topical steroids, calcineurin inhibitors, and emollients. As such, there is no compelling reason to consider an unproven treatment such as cupping.
In 2020, Xing et al. reviewed 16 randomized controlled trials assessing the use of “moving cupping” for plaque psoriasis, with 1,164 patients meeting inclusion criteria. Moving cupping was found to be significantly more effective than “no-moving” cupping therapy, and moving cupping, combined with medications, performed better than medications alone.10 None of the trials evaluated in this study included randomized controlled trials that compared patients using any of the more modern psoriasis medications, specifically biologics. And, again, the studies evaluated were not of the highest quality.
The data that support cupping, as summarized above, are based mostly on case reports, and strong double-blind prospective studies are lacking. Additionally, most of the studies cited gauged the efficacy of cupping using qualitative endpoints, rather than standardized quantitative endpoints and scales. Moreover, spontaneous remission of various dermatoses can occur, or they can improve over time, including acute eczema, psoriasis, and, especially, urticaria.
Adverse effects of cupping
Often alternative therapies are seen as “benign” and without adverse effects. However, complications can result from cupping. Trauma can be induced from the cupping itself by damaging superficial blood vessels and causing bruising.1,11 Blistering can also occur secondary to the suction effect, and the epidermal and dermal layers of the skin can be separated.1,11 Further, burns and discoloration have also been noted secondary to heat, trauma, and post inflammatory pigmentary changes.1,11 Another risk of cupping is the Koebner phenomenon, which occurs with psoriasis, with new lesions appearing in traumatized skin.12 Other adverse outcomes that have been reported with cupping include reactivation of herpes simplex virus secondary to skin trauma, iron deficiency anemia (secondary to blood loss), panniculitis, infections, and residual marks mistaken for signs of child abuse.1,11
Cupping in aesthetic dermatology
Facial cupping, a distinct practice from body cupping used to treat general dermatology conditions described previously, is also increasing in popularity. This practice is usually conducted in association with a facial or facial acupuncture by an aesthetician or other licensed professional. It can also be performed using at-home kits. The marketing claims for facial cupping cite improved tightening and contouring of facial skin, increased facial microcirculation and collagen synthesis, and enhanced lymphatic flow to aid with facial puffiness or swelling. One supposed mechanism for these benefits is that cupping increases blood flow. Interestingly, there was a 2020 animal study in which photoacoustic imaging of a mouse ear revealed increased temporary blood flow in the cupping microenvironment.13 Currently, however, there is no evidence in the English scientific literature that supports facial cupping. The benefits attributed to facial cupping for aesthetic purposes have emerged only in personal anecdotes. The temporary increase in blood flow may induce inflammation and swelling that adds volume to the face and temporarily diminishes wrinkles. However, this temporary plumpness may be associated with adverse effects, such as local trauma, irritation, bruising, postinflammatory pigmentary alteration, or even herpes reactivation. In my opinion, the possible adverse effects of cupping outweigh any potential benefit, especially given the insufficient evidence supporting the utility of cupping for cosmetic enhancement.
Summary
There is increasing interest among patients to incorporate complementary and alternative medicine – including the ancient tradition of cupping – in managing medical dermatologic conditions. However, current evidence supporting cupping as an effective therapeutic strategy is not strong, with most studies to date appearing to be of poor quality or not sufficiently convincing to displace standard therapies. Our medical strategies for managing chronic dermatologic conditions, particularly inflammatory disorders, continue to improve from both a safety and a proven efficacy standpoint. Therefore, I would not forgo medical management in favor of cupping. While cupping can be used as an adjunct therapy, I would caution patients about possible adverse side effects. In the aesthetic world, cupping is also gaining popularity, but this trend is also not supported by current evidence or studies, at least in the Western literature.
Dr. Goldman is a dermatologist in private practice in Miami and specializes in cosmetic and general dermatology. She practices at Baumann Cosmetic & Research Institute and is also opening a general dermatology practice. Write to her at [email protected] or message her on Instragram @DrChloeGoldman. Dr. Goldman receives compensation to create social media content for Replenix, a skin care company. She has no other disclosures.
References
1. Soliman Y et al. Acta Dermatovenerol Alp Pannonica Adriat. 2018 Jun;27(2):103-7.
2. França K and Lotti T. Advances in Integrative Dermatology. John Wiley & Sons, 2019.
3. Lowe DT. Complement Ther Clin Pract. 2017 Nov;29:162-8.
4.Cao H et al. Altern Ther Health Med. 2010 Nov-Dec;16(6):48-54.
5. Li L and Ding J. J Tradit Chin Med. 2001 Mar;21(1):37-8.
6. Xiao XJ et al. J Integr Med. 2020 Jul;18(4):303-12.
7. Xu J et al. J Tradit Chin Med. 2013 Dec;33(6):752-6.
8. Yao J et al. Zhongguo Zhen Jiu. 2007; Jun;27(6):424-6.
9. Hon KL et al. Case Rep Pediatr. 2013;2013:605829.
10. Xing M et al. Medicine (Baltimore). 2020 Oct 9;99(41):e22539.
11. Kim TH et al. Eur J Integr Med. 2014 Aug 1;6(4):434-40.
12. Vender R and Vender R. J Cutan Med Surg. 2015 May-Jun;19(3):320-2.
13. Zhou Y et al. Biomed Opt Express. 2020 Apr 6;11(5):2394-401.
This article was updated 4/25/22.
Removal of Isotretinoin Gender-Based Guidelines: Inclusivity Takes Precedence
Isotretinoin is one of the most highly regulated dermatologic medications on the market. The main reason for regulation through the US Food and Drug Administration (FDA)–managed iPLEDGE Risk Evaluation and Mitigation Strategy (REMS) is to minimize the drug’s teratogenic potential, as isotretinoin can cause profound birth defects. The program originally categorized patients into 1 of 3 categories: (1) females of reproductive potential, (2) females not of reproductive potential, and (3) males. Unless the patient commits to abstinence, the program required female patients of childbearing potential to be on 2 forms of birth control and undergo regular pregnancy testing before obtaining refills. Over the last few years, the American Academy of Dermatology Association (AADA) has been advocating for changes to the iPLEDGE system. Proposed changes have included decreasing attestation frequency for patients who cannot get pregnant, increasing contraception counseling and options, and changing enrollment guidelines to encompass all gender and sexual minorities. As of December 13, 2021, the iPLEDGE system changed enrollment categories to reflect the AADA’s wishes and rolled out gender-neutral categories for enrollment in iPLEDGE. This change will simplify and enhance patients’ experience when starting isotretinoin.
Developing Inclusive iPLEDGE Categories
In recent years, dermatologists and patients have viewed these strict gender-based categories as limiting and problematic, especially for their transgender patients and female patients of childbearing potential who exclusively engage in intercourse with cisgender females. The United States has more than 10 million LGBTQIA+ citizens and an estimated 1.4 million adults who identify as transgender individuals, rendering the previously established gender-binary iPLEDGE categories outdated.1,2
As a result, over the last few years, dermatologists, LGBTQIA+ allies, and patients have urged the FDA to create a gender-neutral registration process for iPLEDGE. With support from the AADA, the new modifications were approved for implementation and include 2 risk categories: (1) people who can get pregnant and (2) people who cannot get pregnant.3
As exciting as these changes are for the future of dermatologic practice, the actual transition to the new iPLEDGE system was described as a “failure, chaotic, and a disaster” due to additional changes made at the same time.4 The iPLEDGE system was switched to a new website administered by a different vendor and required providers to confirm each patient online by December 13, 2021. In addition, the new system required pharmacists to obtain risk management authorization via the iPLEDGE REMS website or by calling the iPLEDGE REMS center before dispensing isotretinoin. This overhaul did not work as planned, as the new website was constantly down and it was nearly impossible to reach a contact over the telephone. The complications resulted in major disruptions and delayed prescriptions for thousands of patients nationwide as well as a great disruption in workflow for physicians and pharmacists. The AADA subsequently met with the Isotretinoin Products Manufacturers Group to create workable solutions for these issues.
On January 14, 2022, the FDA posted updates regarding access to the iPLEDGE system. They have worked with the Isotretinoin Products Manufacturers Group to create workable solutions for patients and physicians while transferring the patients’ information to the new database. Their solution includes allowing physicians to send patients login links through their email to access their account instead of waiting for the call center. The majority of iPLEDGE users now have access to their accounts without issues, and the gender-neutral guidelines have been in place since the original change.
Impact of iPLEDGE Categories on Transgender Patients
These changes specifically will improve the experience of transgender men and cisgender women who are at no risk for pregnancy and could be subjected to monthly pregnancy testing when it is not medically necessary.
Consider the following patient scenario. A transgender man presents to your dermatology office seeking treatment of severe nodulocystic acne. He was placed on hormonal replacement therapy with exogenous testosterone—injections, oral pills, topical gel, topical patches, or subdermal pellets—to achieve secondary sex characteristics and promote gender congruence. The patient mentions he has been amenorrheic for several months now. He has tried many topical acne treatments as well as oral antibiotics without much benefit and is now interested in enrolling in iPLEDGE to obtain isotretinoin. With the prior iPLEDGE registration packets, how would this transgender man be classified? As a female with childbearing potential due to his retained ovaries and uterus? What if he did not endorse engaging in sexual intercourse that could result in pregnancy?
Transgender patients have unique and unmet needs that often are overlooked and prevent them from equitable, gender-affirming health care. For example, in a prospective study following 20 transgender men starting hormone replacement therapy, the percentage of patients with facial acne increased from 35% to 82% after 6 months of therapy.5 In addition, the increased psychosocial burden of acne may be especially difficult in these patients, as they already report higher rates of depression and suicidal ideation compared with their heterosexual cisgender peers.4 Further, the primary patient populations receiving isotretinoin typically are adolescents and young adults who are undergoing major physical, mental, and hormonal changes. Self-discovery and self-actualization develop over time, and our role as physicians is to advocate for all aspects of our patients’ health and eliminate barriers to optimal care.
Inclusive Language in iPLEDGE Categories
It is important to streamline access to care for all patients, and gender-affirming, culturally sensitive language is essential to building trust and understanding between patients and providers. Howa Yeung, MD, MSc, a dermatologist at Emory University (Atlanta, Georgia) who advocated for gender-neutral iPLEDGE registration, welcomes the change and stated it “will make my job easier. I no longer have to struggle between respecting the patient’s gender identity and providing medically necessary care for patients with severe acne.”3
Sanchez et al6 provided a list of structured questions providers can ask their patients to assess their risk regarding pregnancy: (1) Do you have a uterus and/or ovaries?, (2) Are you engaging in sexual intercourse with a person who has a penis?, and (3) If yes to these questions, what form(s) of birth control are you using? Providers should preface these questions with the following statement: “It is important that I ask these questions to assess your risk for becoming pregnant on this medication because isotretinoin can cause very serious birth defects.” It is important to review these questions and practice asking them so residents can operate from the same place of openness and understanding when caring for their patients.
Final Thoughts
The landscape of isotretinoin prescribing currently is changing on a day-to-day basis. As residents, it is important we stay up to date with the changes regarding our regularly dispensed medications. The main modification made to the iPLEDGE REMS system was switching the risk categories from 3 (females who can get pregnant, females who cannot get pregnant, males) to 2 (people who can get pregnant, people who cannot get pregnant). This change will make registration for iPLEDGE less complex and more inclusive for all patients. It is important for residents to stay at the forefront of these patient health issues and barriers to equal care, and this change represents a step in the right direction.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602. doi:10.1016/j.jaad.2018.02.045
- Flores AR, Herman JL, Gates GJ, et al. How many adults identify as transgender in the United States? UCLA Williams Institute website. Published June 2016. Accessed March 1, 2022. https://williamsinstitute.law.ucla.edu/publications/trans-adults-united-states/
- Doheny K. FDA OKs iPLEDGE change for gender-neutral language. Dermatology News. October 13, 2021. Accessed March 3, 2022. https://www.mdedge.com/dermatology/article/247352/acne/fda-oks-ipledge-change-gender-neutral-language/page/0/1
- Doheny K. iPLEDGE rollout described as a failure, chaotic, and a disaster. Medscape. December 16, 2021. Accessed March 1, 2022. https://www.medscape.com/viewarticle/964925?uac=423615MG
- Wierckx K, Van de Peer F, Verhaeghe E, et al. Short- and long-term clinical skin effects of testosterone treatment in trans men. J Sex Med. 2014;11:222-229.
- Sanchez DP, Brownstone N, Thibodeaux Q, et al. Prescribing isotretinoin for transgender patients: a call to action and recommendations. J Drugs Dermatol. 2021;20:106-108.
Isotretinoin is one of the most highly regulated dermatologic medications on the market. The main reason for regulation through the US Food and Drug Administration (FDA)–managed iPLEDGE Risk Evaluation and Mitigation Strategy (REMS) is to minimize the drug’s teratogenic potential, as isotretinoin can cause profound birth defects. The program originally categorized patients into 1 of 3 categories: (1) females of reproductive potential, (2) females not of reproductive potential, and (3) males. Unless the patient commits to abstinence, the program required female patients of childbearing potential to be on 2 forms of birth control and undergo regular pregnancy testing before obtaining refills. Over the last few years, the American Academy of Dermatology Association (AADA) has been advocating for changes to the iPLEDGE system. Proposed changes have included decreasing attestation frequency for patients who cannot get pregnant, increasing contraception counseling and options, and changing enrollment guidelines to encompass all gender and sexual minorities. As of December 13, 2021, the iPLEDGE system changed enrollment categories to reflect the AADA’s wishes and rolled out gender-neutral categories for enrollment in iPLEDGE. This change will simplify and enhance patients’ experience when starting isotretinoin.
Developing Inclusive iPLEDGE Categories
In recent years, dermatologists and patients have viewed these strict gender-based categories as limiting and problematic, especially for their transgender patients and female patients of childbearing potential who exclusively engage in intercourse with cisgender females. The United States has more than 10 million LGBTQIA+ citizens and an estimated 1.4 million adults who identify as transgender individuals, rendering the previously established gender-binary iPLEDGE categories outdated.1,2
As a result, over the last few years, dermatologists, LGBTQIA+ allies, and patients have urged the FDA to create a gender-neutral registration process for iPLEDGE. With support from the AADA, the new modifications were approved for implementation and include 2 risk categories: (1) people who can get pregnant and (2) people who cannot get pregnant.3
As exciting as these changes are for the future of dermatologic practice, the actual transition to the new iPLEDGE system was described as a “failure, chaotic, and a disaster” due to additional changes made at the same time.4 The iPLEDGE system was switched to a new website administered by a different vendor and required providers to confirm each patient online by December 13, 2021. In addition, the new system required pharmacists to obtain risk management authorization via the iPLEDGE REMS website or by calling the iPLEDGE REMS center before dispensing isotretinoin. This overhaul did not work as planned, as the new website was constantly down and it was nearly impossible to reach a contact over the telephone. The complications resulted in major disruptions and delayed prescriptions for thousands of patients nationwide as well as a great disruption in workflow for physicians and pharmacists. The AADA subsequently met with the Isotretinoin Products Manufacturers Group to create workable solutions for these issues.
On January 14, 2022, the FDA posted updates regarding access to the iPLEDGE system. They have worked with the Isotretinoin Products Manufacturers Group to create workable solutions for patients and physicians while transferring the patients’ information to the new database. Their solution includes allowing physicians to send patients login links through their email to access their account instead of waiting for the call center. The majority of iPLEDGE users now have access to their accounts without issues, and the gender-neutral guidelines have been in place since the original change.
Impact of iPLEDGE Categories on Transgender Patients
These changes specifically will improve the experience of transgender men and cisgender women who are at no risk for pregnancy and could be subjected to monthly pregnancy testing when it is not medically necessary.
Consider the following patient scenario. A transgender man presents to your dermatology office seeking treatment of severe nodulocystic acne. He was placed on hormonal replacement therapy with exogenous testosterone—injections, oral pills, topical gel, topical patches, or subdermal pellets—to achieve secondary sex characteristics and promote gender congruence. The patient mentions he has been amenorrheic for several months now. He has tried many topical acne treatments as well as oral antibiotics without much benefit and is now interested in enrolling in iPLEDGE to obtain isotretinoin. With the prior iPLEDGE registration packets, how would this transgender man be classified? As a female with childbearing potential due to his retained ovaries and uterus? What if he did not endorse engaging in sexual intercourse that could result in pregnancy?
Transgender patients have unique and unmet needs that often are overlooked and prevent them from equitable, gender-affirming health care. For example, in a prospective study following 20 transgender men starting hormone replacement therapy, the percentage of patients with facial acne increased from 35% to 82% after 6 months of therapy.5 In addition, the increased psychosocial burden of acne may be especially difficult in these patients, as they already report higher rates of depression and suicidal ideation compared with their heterosexual cisgender peers.4 Further, the primary patient populations receiving isotretinoin typically are adolescents and young adults who are undergoing major physical, mental, and hormonal changes. Self-discovery and self-actualization develop over time, and our role as physicians is to advocate for all aspects of our patients’ health and eliminate barriers to optimal care.
Inclusive Language in iPLEDGE Categories
It is important to streamline access to care for all patients, and gender-affirming, culturally sensitive language is essential to building trust and understanding between patients and providers. Howa Yeung, MD, MSc, a dermatologist at Emory University (Atlanta, Georgia) who advocated for gender-neutral iPLEDGE registration, welcomes the change and stated it “will make my job easier. I no longer have to struggle between respecting the patient’s gender identity and providing medically necessary care for patients with severe acne.”3
Sanchez et al6 provided a list of structured questions providers can ask their patients to assess their risk regarding pregnancy: (1) Do you have a uterus and/or ovaries?, (2) Are you engaging in sexual intercourse with a person who has a penis?, and (3) If yes to these questions, what form(s) of birth control are you using? Providers should preface these questions with the following statement: “It is important that I ask these questions to assess your risk for becoming pregnant on this medication because isotretinoin can cause very serious birth defects.” It is important to review these questions and practice asking them so residents can operate from the same place of openness and understanding when caring for their patients.
Final Thoughts
The landscape of isotretinoin prescribing currently is changing on a day-to-day basis. As residents, it is important we stay up to date with the changes regarding our regularly dispensed medications. The main modification made to the iPLEDGE REMS system was switching the risk categories from 3 (females who can get pregnant, females who cannot get pregnant, males) to 2 (people who can get pregnant, people who cannot get pregnant). This change will make registration for iPLEDGE less complex and more inclusive for all patients. It is important for residents to stay at the forefront of these patient health issues and barriers to equal care, and this change represents a step in the right direction.
Isotretinoin is one of the most highly regulated dermatologic medications on the market. The main reason for regulation through the US Food and Drug Administration (FDA)–managed iPLEDGE Risk Evaluation and Mitigation Strategy (REMS) is to minimize the drug’s teratogenic potential, as isotretinoin can cause profound birth defects. The program originally categorized patients into 1 of 3 categories: (1) females of reproductive potential, (2) females not of reproductive potential, and (3) males. Unless the patient commits to abstinence, the program required female patients of childbearing potential to be on 2 forms of birth control and undergo regular pregnancy testing before obtaining refills. Over the last few years, the American Academy of Dermatology Association (AADA) has been advocating for changes to the iPLEDGE system. Proposed changes have included decreasing attestation frequency for patients who cannot get pregnant, increasing contraception counseling and options, and changing enrollment guidelines to encompass all gender and sexual minorities. As of December 13, 2021, the iPLEDGE system changed enrollment categories to reflect the AADA’s wishes and rolled out gender-neutral categories for enrollment in iPLEDGE. This change will simplify and enhance patients’ experience when starting isotretinoin.
Developing Inclusive iPLEDGE Categories
In recent years, dermatologists and patients have viewed these strict gender-based categories as limiting and problematic, especially for their transgender patients and female patients of childbearing potential who exclusively engage in intercourse with cisgender females. The United States has more than 10 million LGBTQIA+ citizens and an estimated 1.4 million adults who identify as transgender individuals, rendering the previously established gender-binary iPLEDGE categories outdated.1,2
As a result, over the last few years, dermatologists, LGBTQIA+ allies, and patients have urged the FDA to create a gender-neutral registration process for iPLEDGE. With support from the AADA, the new modifications were approved for implementation and include 2 risk categories: (1) people who can get pregnant and (2) people who cannot get pregnant.3
As exciting as these changes are for the future of dermatologic practice, the actual transition to the new iPLEDGE system was described as a “failure, chaotic, and a disaster” due to additional changes made at the same time.4 The iPLEDGE system was switched to a new website administered by a different vendor and required providers to confirm each patient online by December 13, 2021. In addition, the new system required pharmacists to obtain risk management authorization via the iPLEDGE REMS website or by calling the iPLEDGE REMS center before dispensing isotretinoin. This overhaul did not work as planned, as the new website was constantly down and it was nearly impossible to reach a contact over the telephone. The complications resulted in major disruptions and delayed prescriptions for thousands of patients nationwide as well as a great disruption in workflow for physicians and pharmacists. The AADA subsequently met with the Isotretinoin Products Manufacturers Group to create workable solutions for these issues.
On January 14, 2022, the FDA posted updates regarding access to the iPLEDGE system. They have worked with the Isotretinoin Products Manufacturers Group to create workable solutions for patients and physicians while transferring the patients’ information to the new database. Their solution includes allowing physicians to send patients login links through their email to access their account instead of waiting for the call center. The majority of iPLEDGE users now have access to their accounts without issues, and the gender-neutral guidelines have been in place since the original change.
Impact of iPLEDGE Categories on Transgender Patients
These changes specifically will improve the experience of transgender men and cisgender women who are at no risk for pregnancy and could be subjected to monthly pregnancy testing when it is not medically necessary.
Consider the following patient scenario. A transgender man presents to your dermatology office seeking treatment of severe nodulocystic acne. He was placed on hormonal replacement therapy with exogenous testosterone—injections, oral pills, topical gel, topical patches, or subdermal pellets—to achieve secondary sex characteristics and promote gender congruence. The patient mentions he has been amenorrheic for several months now. He has tried many topical acne treatments as well as oral antibiotics without much benefit and is now interested in enrolling in iPLEDGE to obtain isotretinoin. With the prior iPLEDGE registration packets, how would this transgender man be classified? As a female with childbearing potential due to his retained ovaries and uterus? What if he did not endorse engaging in sexual intercourse that could result in pregnancy?
Transgender patients have unique and unmet needs that often are overlooked and prevent them from equitable, gender-affirming health care. For example, in a prospective study following 20 transgender men starting hormone replacement therapy, the percentage of patients with facial acne increased from 35% to 82% after 6 months of therapy.5 In addition, the increased psychosocial burden of acne may be especially difficult in these patients, as they already report higher rates of depression and suicidal ideation compared with their heterosexual cisgender peers.4 Further, the primary patient populations receiving isotretinoin typically are adolescents and young adults who are undergoing major physical, mental, and hormonal changes. Self-discovery and self-actualization develop over time, and our role as physicians is to advocate for all aspects of our patients’ health and eliminate barriers to optimal care.
Inclusive Language in iPLEDGE Categories
It is important to streamline access to care for all patients, and gender-affirming, culturally sensitive language is essential to building trust and understanding between patients and providers. Howa Yeung, MD, MSc, a dermatologist at Emory University (Atlanta, Georgia) who advocated for gender-neutral iPLEDGE registration, welcomes the change and stated it “will make my job easier. I no longer have to struggle between respecting the patient’s gender identity and providing medically necessary care for patients with severe acne.”3
Sanchez et al6 provided a list of structured questions providers can ask their patients to assess their risk regarding pregnancy: (1) Do you have a uterus and/or ovaries?, (2) Are you engaging in sexual intercourse with a person who has a penis?, and (3) If yes to these questions, what form(s) of birth control are you using? Providers should preface these questions with the following statement: “It is important that I ask these questions to assess your risk for becoming pregnant on this medication because isotretinoin can cause very serious birth defects.” It is important to review these questions and practice asking them so residents can operate from the same place of openness and understanding when caring for their patients.
Final Thoughts
The landscape of isotretinoin prescribing currently is changing on a day-to-day basis. As residents, it is important we stay up to date with the changes regarding our regularly dispensed medications. The main modification made to the iPLEDGE REMS system was switching the risk categories from 3 (females who can get pregnant, females who cannot get pregnant, males) to 2 (people who can get pregnant, people who cannot get pregnant). This change will make registration for iPLEDGE less complex and more inclusive for all patients. It is important for residents to stay at the forefront of these patient health issues and barriers to equal care, and this change represents a step in the right direction.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602. doi:10.1016/j.jaad.2018.02.045
- Flores AR, Herman JL, Gates GJ, et al. How many adults identify as transgender in the United States? UCLA Williams Institute website. Published June 2016. Accessed March 1, 2022. https://williamsinstitute.law.ucla.edu/publications/trans-adults-united-states/
- Doheny K. FDA OKs iPLEDGE change for gender-neutral language. Dermatology News. October 13, 2021. Accessed March 3, 2022. https://www.mdedge.com/dermatology/article/247352/acne/fda-oks-ipledge-change-gender-neutral-language/page/0/1
- Doheny K. iPLEDGE rollout described as a failure, chaotic, and a disaster. Medscape. December 16, 2021. Accessed March 1, 2022. https://www.medscape.com/viewarticle/964925?uac=423615MG
- Wierckx K, Van de Peer F, Verhaeghe E, et al. Short- and long-term clinical skin effects of testosterone treatment in trans men. J Sex Med. 2014;11:222-229.
- Sanchez DP, Brownstone N, Thibodeaux Q, et al. Prescribing isotretinoin for transgender patients: a call to action and recommendations. J Drugs Dermatol. 2021;20:106-108.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602. doi:10.1016/j.jaad.2018.02.045
- Flores AR, Herman JL, Gates GJ, et al. How many adults identify as transgender in the United States? UCLA Williams Institute website. Published June 2016. Accessed March 1, 2022. https://williamsinstitute.law.ucla.edu/publications/trans-adults-united-states/
- Doheny K. FDA OKs iPLEDGE change for gender-neutral language. Dermatology News. October 13, 2021. Accessed March 3, 2022. https://www.mdedge.com/dermatology/article/247352/acne/fda-oks-ipledge-change-gender-neutral-language/page/0/1
- Doheny K. iPLEDGE rollout described as a failure, chaotic, and a disaster. Medscape. December 16, 2021. Accessed March 1, 2022. https://www.medscape.com/viewarticle/964925?uac=423615MG
- Wierckx K, Van de Peer F, Verhaeghe E, et al. Short- and long-term clinical skin effects of testosterone treatment in trans men. J Sex Med. 2014;11:222-229.
- Sanchez DP, Brownstone N, Thibodeaux Q, et al. Prescribing isotretinoin for transgender patients: a call to action and recommendations. J Drugs Dermatol. 2021;20:106-108.
Resident Pearls
- Major changes in the iPLEDGE Risk Evaluation and Mitigation Strategy (REMS) system recently took place, including simplifying registration categories while making the process more inclusive for patients.
- It is important to practice culturally sensitive language when discussing subjects regarding gender identification and sexual practices. Sample questions have been provided to help familiarize practitioners with optimal ways to approach these patient encounters.
- There likely will be more changes with iPLEDGE REMS in the future as the American Academy of Dermatology Association continues to work on solutions regarding decreasing monthly qualifications for patients who cannot get pregnant and possible removal of patient attestation requirements.
Spironolactone not linked to increased cancer risk in systematic review and meta-analysis
covering seven observational studies and a total population of over 4.5 million people.
The data, published in JAMA Dermatology, are “reassuring,” the authors reported, considering that the spironolactone label carries a Food and Drug Administration warning regarding possible tumorigenicity, which is based on animal studies of doses up to 150-fold greater than doses used for humans. The drug’s antiandrogenic properties have driven its off-label use as a treatment for acne, hidradenitis, androgenetic alopecia, and hirsutism.
Spironolactone, a synthetic 17-lactone steroid, is approved for the treatment of heart failure, edema and ascites, hypertension, and primary hyperaldosteronism. Off label, it is also frequently used in gender-affirming care and is included in Endocrine Society guidelines as part of hormonal regimens for transgender women, the authors noted.
The seven eligible studies looked at the occurrence of cancer in men and women who had any exposure to the drug, regardless of the primary indication. Sample sizes ranged from 18,035 to 2.3 million, and the mean age across all studies was 62.6-72 years.
The researchers synthesized the studies, mostly of European individuals, using random effects meta-analysis and found no statistically significant association between spironolactone use and risk of breast cancer (risk ratio, 1.04; 95% confidence interval, 0.86-1.22). Three of the seven studies investigated breast cancer.
There was also no significant association between spironolactone use and risk of ovarian cancer (two studies), bladder cancer (three studies), kidney cancer (two studies), gastric cancer (two studies), or esophageal cancer (two studies).
For prostate cancer, investigated in four studies, use of the drug was associated with decreased risk (RR, 0.79, 95% CI, 0.68-0.90).
Kanthi Bommareddy, MD, of the University of Miami and coauthors concluded that all studies were at low risk of bias after appraising each one using a scale that looks at selection bias, confounding bias, and detection and outcome bias.
In dermatology, the results should “help us to take a collective sigh of relief,” said Julie C. Harper, MD, of the Dermatology and Skin Care Center of Birmingham, Ala., who was asked to comment on the study. The drug has been “safe and effective in our clinics and it is affordable and accessible to our patients,” she said, but with the FDA’s warning and the drug’s antiandrogen capacity, “there has been concern that we might be putting our patients at increased risk of breast cancer [in particular].”
The pooling of seven large studies together and the finding of no substantive increased risk of cancer “gives us evidence and comfort that spironolactone does not increase the risk of cancer in our dermatology patients,” said Dr. Harper, a past president of the American Acne & Rosacea Society.
“With every passing year,” she noted, “dermatologists are prescribing more and more spironolactone for acne, hidradenitis, androgenetic alopecia, and hirsutism.”
Four of the seven studies stratified analyses by sex, and in those without stratification by sex, women accounted for 17.2%-54.4% of the samples.
The studies had long follow-up periods of 5-20 years, but certainty of the evidence was low and since many of the studies included mostly older individuals, “they may not generalize to younger populations, such as those treated with spironolactone for acne,” the investigators wrote.
The authors also noted they were unable to look for dose-dependent associations between spironolactone and cancer risk, and that confidence intervals for rarer cancers like ovarian cancer were wide. “We cannot entirely exclude the potential for a meaningful increase in cancer risk,” and future studies are needed, in populations that include younger patients and those with acne or hirsutism.
Dr. Bommareddy reported no disclosures. Other coauthors reported grants from the National Cancer Institute outside the submitted work, and personal fees as a Cancer Prevention and Research Institute of Texas Scholar in Cancer Research. There was no funding reported for the study. Dr. Harper said she had no disclosures.
covering seven observational studies and a total population of over 4.5 million people.
The data, published in JAMA Dermatology, are “reassuring,” the authors reported, considering that the spironolactone label carries a Food and Drug Administration warning regarding possible tumorigenicity, which is based on animal studies of doses up to 150-fold greater than doses used for humans. The drug’s antiandrogenic properties have driven its off-label use as a treatment for acne, hidradenitis, androgenetic alopecia, and hirsutism.
Spironolactone, a synthetic 17-lactone steroid, is approved for the treatment of heart failure, edema and ascites, hypertension, and primary hyperaldosteronism. Off label, it is also frequently used in gender-affirming care and is included in Endocrine Society guidelines as part of hormonal regimens for transgender women, the authors noted.
The seven eligible studies looked at the occurrence of cancer in men and women who had any exposure to the drug, regardless of the primary indication. Sample sizes ranged from 18,035 to 2.3 million, and the mean age across all studies was 62.6-72 years.
The researchers synthesized the studies, mostly of European individuals, using random effects meta-analysis and found no statistically significant association between spironolactone use and risk of breast cancer (risk ratio, 1.04; 95% confidence interval, 0.86-1.22). Three of the seven studies investigated breast cancer.
There was also no significant association between spironolactone use and risk of ovarian cancer (two studies), bladder cancer (three studies), kidney cancer (two studies), gastric cancer (two studies), or esophageal cancer (two studies).
For prostate cancer, investigated in four studies, use of the drug was associated with decreased risk (RR, 0.79, 95% CI, 0.68-0.90).
Kanthi Bommareddy, MD, of the University of Miami and coauthors concluded that all studies were at low risk of bias after appraising each one using a scale that looks at selection bias, confounding bias, and detection and outcome bias.
In dermatology, the results should “help us to take a collective sigh of relief,” said Julie C. Harper, MD, of the Dermatology and Skin Care Center of Birmingham, Ala., who was asked to comment on the study. The drug has been “safe and effective in our clinics and it is affordable and accessible to our patients,” she said, but with the FDA’s warning and the drug’s antiandrogen capacity, “there has been concern that we might be putting our patients at increased risk of breast cancer [in particular].”
The pooling of seven large studies together and the finding of no substantive increased risk of cancer “gives us evidence and comfort that spironolactone does not increase the risk of cancer in our dermatology patients,” said Dr. Harper, a past president of the American Acne & Rosacea Society.
“With every passing year,” she noted, “dermatologists are prescribing more and more spironolactone for acne, hidradenitis, androgenetic alopecia, and hirsutism.”
Four of the seven studies stratified analyses by sex, and in those without stratification by sex, women accounted for 17.2%-54.4% of the samples.
The studies had long follow-up periods of 5-20 years, but certainty of the evidence was low and since many of the studies included mostly older individuals, “they may not generalize to younger populations, such as those treated with spironolactone for acne,” the investigators wrote.
The authors also noted they were unable to look for dose-dependent associations between spironolactone and cancer risk, and that confidence intervals for rarer cancers like ovarian cancer were wide. “We cannot entirely exclude the potential for a meaningful increase in cancer risk,” and future studies are needed, in populations that include younger patients and those with acne or hirsutism.
Dr. Bommareddy reported no disclosures. Other coauthors reported grants from the National Cancer Institute outside the submitted work, and personal fees as a Cancer Prevention and Research Institute of Texas Scholar in Cancer Research. There was no funding reported for the study. Dr. Harper said she had no disclosures.
covering seven observational studies and a total population of over 4.5 million people.
The data, published in JAMA Dermatology, are “reassuring,” the authors reported, considering that the spironolactone label carries a Food and Drug Administration warning regarding possible tumorigenicity, which is based on animal studies of doses up to 150-fold greater than doses used for humans. The drug’s antiandrogenic properties have driven its off-label use as a treatment for acne, hidradenitis, androgenetic alopecia, and hirsutism.
Spironolactone, a synthetic 17-lactone steroid, is approved for the treatment of heart failure, edema and ascites, hypertension, and primary hyperaldosteronism. Off label, it is also frequently used in gender-affirming care and is included in Endocrine Society guidelines as part of hormonal regimens for transgender women, the authors noted.
The seven eligible studies looked at the occurrence of cancer in men and women who had any exposure to the drug, regardless of the primary indication. Sample sizes ranged from 18,035 to 2.3 million, and the mean age across all studies was 62.6-72 years.
The researchers synthesized the studies, mostly of European individuals, using random effects meta-analysis and found no statistically significant association between spironolactone use and risk of breast cancer (risk ratio, 1.04; 95% confidence interval, 0.86-1.22). Three of the seven studies investigated breast cancer.
There was also no significant association between spironolactone use and risk of ovarian cancer (two studies), bladder cancer (three studies), kidney cancer (two studies), gastric cancer (two studies), or esophageal cancer (two studies).
For prostate cancer, investigated in four studies, use of the drug was associated with decreased risk (RR, 0.79, 95% CI, 0.68-0.90).
Kanthi Bommareddy, MD, of the University of Miami and coauthors concluded that all studies were at low risk of bias after appraising each one using a scale that looks at selection bias, confounding bias, and detection and outcome bias.
In dermatology, the results should “help us to take a collective sigh of relief,” said Julie C. Harper, MD, of the Dermatology and Skin Care Center of Birmingham, Ala., who was asked to comment on the study. The drug has been “safe and effective in our clinics and it is affordable and accessible to our patients,” she said, but with the FDA’s warning and the drug’s antiandrogen capacity, “there has been concern that we might be putting our patients at increased risk of breast cancer [in particular].”
The pooling of seven large studies together and the finding of no substantive increased risk of cancer “gives us evidence and comfort that spironolactone does not increase the risk of cancer in our dermatology patients,” said Dr. Harper, a past president of the American Acne & Rosacea Society.
“With every passing year,” she noted, “dermatologists are prescribing more and more spironolactone for acne, hidradenitis, androgenetic alopecia, and hirsutism.”
Four of the seven studies stratified analyses by sex, and in those without stratification by sex, women accounted for 17.2%-54.4% of the samples.
The studies had long follow-up periods of 5-20 years, but certainty of the evidence was low and since many of the studies included mostly older individuals, “they may not generalize to younger populations, such as those treated with spironolactone for acne,” the investigators wrote.
The authors also noted they were unable to look for dose-dependent associations between spironolactone and cancer risk, and that confidence intervals for rarer cancers like ovarian cancer were wide. “We cannot entirely exclude the potential for a meaningful increase in cancer risk,” and future studies are needed, in populations that include younger patients and those with acne or hirsutism.
Dr. Bommareddy reported no disclosures. Other coauthors reported grants from the National Cancer Institute outside the submitted work, and personal fees as a Cancer Prevention and Research Institute of Texas Scholar in Cancer Research. There was no funding reported for the study. Dr. Harper said she had no disclosures.
FROM JAMA DERMATOLOGY
Oral Isotretinoin for Acne in the US Military: How Accelerated Courses and Teledermatology Can Minimize the Duty-Limiting Impacts of Treatment
Acne vulgaris is an extremely common dermatologic disease affecting 40 to 50 million individuals in the United States each year, with a prevalence of 85% in adolescents and young adults aged 12 to 24 years. For some patients, the disease may persist well into adulthood, affecting 8% of adults aged 25 and 34 years.1 Acne negatively impacts patients’ quality of life and productivity, with an estimated direct and indirect cost of over $3 billion per year.2
Oral isotretinoin, a vitamin A derivative, is approved by the US Food and Drug Administration for the treatment of severe nodulocystic acne. Isotretinoin reduces the size and secretions of sebaceous glands, inhibits growth and resulting inflammation of Cutibacterium acnes, and normalizes the differentiation of follicular keratinocytes, resulting in permanent changes in the pathogenesis of acne that may lead to remission.3 The use of oral isotretinoin in the active-duty US Military population may cause service members to be nondeployable or limit their ability to function in special roles (eg, pilot, submariner).4 Treatment regimens that minimize the course duration of isotretinoin and reduce the risk for relapse that requires a retrial of isotretinoin may, in turn, increase a service member’s readiness, deployment availability, and ability to perform unique occupational roles.
Additionally, teledermatology has been increasingly utilized to maintain treatment continuity for patients on isotretinoin during the COVID-19 pandemic.5 Application of this technology in the military also may be used to facilitate timely isotretinoin treatment regimens in active-duty service members to minimize course duration and increase readiness.
In this article, we discuss an accelerated course of oral isotretinoin as a safe and effective option for military service members bound by duty restrictions and operational timelines and explore the role of teledermatology for the treatment of acne in military service members.
Isotretinoin for Acne
Isotretinoin typically is initiated at a dosage of 0.5 mg/kg daily, increasing to 1 mg/kg daily with a goal cumulative dose between 120 and 150 mg/kg. Relapse may occur after completing a treatment course and is associated with cumulative dosing less than 120 mg/kg.6 The average duration of acne treatment with oral isotretinoin is approximately 6 months.7 At therapeutic doses, nearly all patients experience side effects, most commonly dryness and desquamation of the skin and mucous membranes, as well as possible involvement of the lips, eyes, and nose. Notable extracutaneous side effects include headache, visual disturbances at night, idiopathic intracranial hypertension, and myalgia. Serum cholesterol, triglycerides, and transaminases may be increased in patients taking isotretinoin, which requires routine monitoring using serum lipid profiles and liver function studies. A potential association between isotretinoin and inflammatory bowel disease and changes in mood have been reported, but current data do not suggest an evidence-based link.6,8 Isotretinoin is a potent teratogen, and in the United States, all patients are required to enroll in iPLEDGE, a US Food and Drug Administration–approved pregnancy prevention program that monitors prescribing and dispensing of the medication. For patients who can become pregnant, iPLEDGE requires use of 2 forms of contraception as well as monthly pregnancy tests prior to dispensing the medication.
Acne in Military Service Members
Acne is exceedingly common in the active-duty military population. In 2018, more than 40% of soldiers, sailors, airmen, and marines were 25 years or younger, and 75% of all US service members were 35 years or younger, corresponding to acne peak incidences.1,9 Management of acne in this population requires unique treatment considerations due to distinctive occupational requirements of and hazards faced by military personnel. Use of personal protective equipment, including gas masks, safety restraints, parachute rigging, and flak jackets, may be limiting in individuals with moderate to severe acne.10 For example, severe nodulocystic acne on the chin and jawline can interfere with proper wear of the chin strap on a Kevlar helmet. The severity of acne often necessitates the use of oral isotretinoin therapy, which is considered disqualifying for many special military assignments, including submarine duty, nuclear field duty, and diving duty.11 In military aviation communities, oral isotretinoin requires grounding for the duration of therapy plus 3 months after cessation. Slit-lamp examination, triglycerides, and transaminase levels must be normal prior to returning to unrestricted duty.12 Furthermore, use of oral isotretinoin may limit overseas assignments or deployment eligibility.4
The high prevalence of acne and the operationally limiting consequences of isotretinoin therapy present a unique challenge for dermatologists treating military personnel. The average duration of isotretinoin treatment is approximately 6 months,7 which represents a considerable amount of time during an average 4-year enlistment contract. Therapeutic treatment strategies that (1) reduce the duration of oral isotretinoin therapy, (2) reduce the risk for relapse, and (3) increase medication compliance can reduce the operational impact of this acne treatment. Such treatment strategies are discussed below.
High-Dose Isotretinoin
An optimal isotretinoin dosing regimen would achieve swift resolution of acne lesions and reduce the overall relapse rate requiring retrial of isotretinoin, thereby minimizing the operational- and duty-limiting impacts of the medication. Cyrulnik et al13 studied treatment outcomes of high-dose isotretinoin for acne vulgaris using a mean dosage of 1.6 mg/kg daily with an average cumulative dosage of 290 mg/kg. They demonstrated 100% clearance of lesions over 6 months, with a 12.5% relapse rate at 3 years. Aside from an increased rate of elevated transaminases, incidence of adverse effects and laboratory abnormalities were not significantly increased compared to conventional dosing regimens.13 The goal cumulative dosing of 120 to 150 mg/kg can be achieved 1 to 2 months earlier using a dosage of 1.6 mg/kg daily vs a conventional dosage of 1 mg/kg daily.
It has been hypothesized that higher cumulative doses of oral isotretinoin reduce the risk for relapse of acne and retrial of oral isotretinoin.14 Blasiak et al15 studied relapse and retrial of oral isotretinoin in acne patients who received cumulative dosing higher or lower than 220 mg/kg. A clinically but not statistically significant reduced relapse rate was observed in the cohort that received cumulative dosing higher than 220 mg/kg. No statistically significant difference in rates of adverse advents was observed aside from an increase in retinoid dermatitis in the cohort that received cumulative dosing higher than 220 mg/kg. Higher but not statistically significant rates of adverse events were seen in the group that received dosing higher than 220 mg/kg.15 Cumulative doses of oral isotretinoin higher than the 120 to 150 mg/kg range may decrease the risk for acne relapse and the need for an additional course of oral isotretinoin, which would reduce a service member’s total time away from deployment and full duty.
Relapse requiring a retrial of oral isotretinoin not only increases the operational cost of acne treatment but also considerably increases the monetary cost to the health care system. In a cost-analysis model, cumulative doses of oral isotretinoin higher than 230 mg/kg have a decreased overall cost compared to traditional cumulative dosing of less than 150 mg/kg due to the cost of relapse.16
Limitations of high daily and cumulative dosing regimens of oral isotretinoin are chiefly the dose-dependent rate of adverse effects. Low-dose regimens are associated with a reduced risk of isotretinoin-related side effects.6,17 Acute acne flares may be seen following initial administration of oral isotretinoin and are aggravated by increases in dosage.18 Isotretinoin-induced acne fulminans is a rare but devastating complication observed with high initial doses of oral isotretinoin in patients with severe acne.19 The risks and benefits of high daily and cumulatively dosed isotretinoin must be carefully considered in patients with severe acne.
Teledermatology: A Force for Readiness
The COVID-19 pandemic drastically changed the dermatology practice landscape with recommendations to cancel all elective outpatient visits in favor of teledermatology encounters.20 This decreased access to care, which resulted in an increase in drug interruption for dermatology patients, including patients on oral isotretinoin.21 Teledermatology has been increasingly utilized to maintain continuity of care for the management of patients taking isotretinoin.5 Routine utilization of teledermatology evaluation in military practices could expedite care, decrease patient travel time, and allow for in-clinic visits to be utilized for higher-acuity concerns.22
The use of teledermatology for uncomplicated oral isotretinoin management has the potential to increase medication compliance and decrease the amount of travel time for active-duty service members; for example, consider a military dermatology practice based in San Diego, California, that accepts referrals from military bases 3 hours away by car. After an initial consultation for consideration and initiation of oral isotretinoin, teledermatology appointments can save the active-duty service member 3 hours of travel time for each follow-up visit per month. This ultimately increases operational productivity, reduces barriers to accessing care, and improves patient satisfaction.23
Although military personnel usually are located at duty stations for 2 to 4 years, training exercises and military vocational schools often temporarily take personnel away from their home station. These temporary-duty assignments have the potential to interrupt medical follow-up appointments and may cause delays in treatment for individuals who miss monthly isotretinoin visits. When deemed appropriate by the prescribing dermatologist, teledermatology allows for increased continuity of care for active-duty service members and maintenance of a therapeutic isotretinoin course despite temporary geographic displacement.
By facilitating regular follow-up appointments, teledermatology can minimize the amount of time an active-duty service member is on a course of oral isotretinoin, thereby reducing the operational and duty-limiting implications of the medication.
Final Thoughts
Acne is a common dermatologic concern within the active-duty military population. Oral isotretinoin is indicated for treatment-resistant moderate or severe acne; however, it limits the ability of service members to deploy and is disqualifying for special military assignments. High daily- and cumulative-dose isotretinoin treatment strategies can reduce the duration of therapy and may be associated with a decrease in acne relapse and the need for retrial. Teledermatology can increase access to care and facilitate the completion of oral isotretinoin courses in a timely manner. These treatment strategies may help mitigate the duty-limiting impact of oral isotretinoin therapy in military service members.
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39:S34-S37. doi:10.1016/s0190-9622(98)70442-6
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500. doi:10.1016/j.jaad.2006.05.048
- James WD. Clinical practice. acne. N Engl J Med. 2005;352:1463-1472. doi:10.1056/NEJMcp033487
- Burke KR, Larrymore DC, Cho SH. Treatment consideration for US military members with skin disease. Cutis. 2019;103:329-332.
- Rosamilia LL. Isotretinoin meets COVID-19: revisiting a fragmented paradigm. Cutis. 2021;108:8-12. doi:10.12788/cutis.0299
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33. doi:10.1016/j.jaad.2015.12.037
- Huang KE, Carstensen SE, Feldman SR. The duration of acne treatment. J Drugs Dermatol. 2014;13:655-656.
- Bettoli V, Guerra-Tapia A, Herane MI, et al. Challenges and solutions in oral isotretinoin in acne: reflections on 35 years of experience. Clin Cosmet Investig Dermatol. 2019;12:943-951. doi:10.2147/CCID.S234231
- US Department of Defense. 2018 demographics report: profile of the military community. Accessed January 18, 2022. https://download.militaryonesource.mil/12038/MOS/Reports/2018-demographics-report.pdf
- Brahe C, Peters K. Fighting acne for the fighting forces. Cutis. 2020;106:18-20, 22. doi:10.12788/cutis.0057
- US Department of the Navy. Change 167. manual of the medical department. Published February 15, 2019. Accessed January 18, 2022. https://www.med.navy.mil/Portals/62/Documents/BUMED/Directives/MANMED/Chapter%2015%20Medical%20Examinations%20(incorporates%20Changes%20126_135-138_140_145_150-152_154-156_160_164-167).pdf?ver=Rj7AoH54dNAX5uS3F1JUfw%3d%3d
- US Department of the Navy. US Navy aeromedical reference and waiver guide. Published August 11, 2021. Accessed January 18, 2022. https://www.med.navy.mil/Portals/62/Documents/NMFSC/NMOTC/NAMI/ARWG/Waiver%20Guide/ARWG%20COMPLETE_210811.pdf?ver=_pLPzFrtl8E2swFESnN4rA%3d%3d
- Cyrulnik AA, Viola KV, Gewirtzman AJ, et al. High-dose isotretinoin in acne vulgaris: improved treatment outcomes and quality of life. Int J Dermatol. 2012;51:1123-1130. doi:10.1111/j.1365-4632.2011.05409.x
- Coloe J, Du H, Morrell DS. Could higher doses of isotretinoin reduce the frequency of treatment failure in patients with acne? J Am Acad Dermatol. 2011;65:422-423. doi:10.1016/j.jaad.2010.06.025
- Blasiak RC, Stamey CR, Burkhart CN, et al. High-dose isotretinoin treatment and the rate of retrial, relapse, and adverse effects in patients with acne vulgaris. JAMA Dermatol. 2013;149:1392-1398. doi:10.1001/jamadermatol.2013.6746
- Zeitany AE, Bowers EV, Morrell DS. High-dose isotretinoin has lower impact on wallets: a cost analysis of dosing approaches. J Am Acad Dermatol. 2016;74:174-176. doi:10.1016/j.jaad.2015.08.012
- Amichai B, Shemer A, Grunwald MH. Low-dose isotretinoin in the treatment of acne vulgaris. J Am Acad Dermatol. 2006;54:644-666. doi:10.1016/j.jaad.2005.11.1061
- Borghi A, Mantovani L, Minghetti S, et al. Acute acne flare following isotretinoin administration: potential protective role of low starting dose. Dermatology. 2009;218:178-180. doi:10.1159/000182270
- Greywal T, Zaenglein AL, Baldwin HE, et al. Evidence-based recommendations for the management of acne fulminans and its variants. J Am Acad Dermatol. 2017;77:109-117. doi:10.1016/j.jaad.2016.11.028
- Kwatra SG, Sweren RJ, Grossberg AL. Dermatology practices as vectors for COVID-19 transmission: a call for immediate cessation of nonemergent dermatology visits. J Am Acad Dermatol. 2020;82:E179-E180. doi:10.1016/j.jaad.2020.03.037
- Alshiyab DM, Al-Qarqaz FA, Muhaidat JM. Impact of COVID-19 pandemic on the continuity of care for dermatologic patients on systemic therapy during the period of strict lockdown. Ann Med Surg (Lond). 2020;60:571-574. doi:10.1016/j.amsu.2020.11.056
- Hwang J, Kakimoto C. Teledermatology in the US military: a historic foundation for current and future applications. Cutis. 2018;101:335,337,345.
- Ruggiero A, Megna M, Annunziata MC, et al. Teledermatology for acne during COVID-19: high patients’ satisfaction in spite of the emergency. J Eur Acad Dermatol Venereol. 2020;34:E662-E663. doi:10.1111/jdv.16746
Acne vulgaris is an extremely common dermatologic disease affecting 40 to 50 million individuals in the United States each year, with a prevalence of 85% in adolescents and young adults aged 12 to 24 years. For some patients, the disease may persist well into adulthood, affecting 8% of adults aged 25 and 34 years.1 Acne negatively impacts patients’ quality of life and productivity, with an estimated direct and indirect cost of over $3 billion per year.2
Oral isotretinoin, a vitamin A derivative, is approved by the US Food and Drug Administration for the treatment of severe nodulocystic acne. Isotretinoin reduces the size and secretions of sebaceous glands, inhibits growth and resulting inflammation of Cutibacterium acnes, and normalizes the differentiation of follicular keratinocytes, resulting in permanent changes in the pathogenesis of acne that may lead to remission.3 The use of oral isotretinoin in the active-duty US Military population may cause service members to be nondeployable or limit their ability to function in special roles (eg, pilot, submariner).4 Treatment regimens that minimize the course duration of isotretinoin and reduce the risk for relapse that requires a retrial of isotretinoin may, in turn, increase a service member’s readiness, deployment availability, and ability to perform unique occupational roles.
Additionally, teledermatology has been increasingly utilized to maintain treatment continuity for patients on isotretinoin during the COVID-19 pandemic.5 Application of this technology in the military also may be used to facilitate timely isotretinoin treatment regimens in active-duty service members to minimize course duration and increase readiness.
In this article, we discuss an accelerated course of oral isotretinoin as a safe and effective option for military service members bound by duty restrictions and operational timelines and explore the role of teledermatology for the treatment of acne in military service members.
Isotretinoin for Acne
Isotretinoin typically is initiated at a dosage of 0.5 mg/kg daily, increasing to 1 mg/kg daily with a goal cumulative dose between 120 and 150 mg/kg. Relapse may occur after completing a treatment course and is associated with cumulative dosing less than 120 mg/kg.6 The average duration of acne treatment with oral isotretinoin is approximately 6 months.7 At therapeutic doses, nearly all patients experience side effects, most commonly dryness and desquamation of the skin and mucous membranes, as well as possible involvement of the lips, eyes, and nose. Notable extracutaneous side effects include headache, visual disturbances at night, idiopathic intracranial hypertension, and myalgia. Serum cholesterol, triglycerides, and transaminases may be increased in patients taking isotretinoin, which requires routine monitoring using serum lipid profiles and liver function studies. A potential association between isotretinoin and inflammatory bowel disease and changes in mood have been reported, but current data do not suggest an evidence-based link.6,8 Isotretinoin is a potent teratogen, and in the United States, all patients are required to enroll in iPLEDGE, a US Food and Drug Administration–approved pregnancy prevention program that monitors prescribing and dispensing of the medication. For patients who can become pregnant, iPLEDGE requires use of 2 forms of contraception as well as monthly pregnancy tests prior to dispensing the medication.
Acne in Military Service Members
Acne is exceedingly common in the active-duty military population. In 2018, more than 40% of soldiers, sailors, airmen, and marines were 25 years or younger, and 75% of all US service members were 35 years or younger, corresponding to acne peak incidences.1,9 Management of acne in this population requires unique treatment considerations due to distinctive occupational requirements of and hazards faced by military personnel. Use of personal protective equipment, including gas masks, safety restraints, parachute rigging, and flak jackets, may be limiting in individuals with moderate to severe acne.10 For example, severe nodulocystic acne on the chin and jawline can interfere with proper wear of the chin strap on a Kevlar helmet. The severity of acne often necessitates the use of oral isotretinoin therapy, which is considered disqualifying for many special military assignments, including submarine duty, nuclear field duty, and diving duty.11 In military aviation communities, oral isotretinoin requires grounding for the duration of therapy plus 3 months after cessation. Slit-lamp examination, triglycerides, and transaminase levels must be normal prior to returning to unrestricted duty.12 Furthermore, use of oral isotretinoin may limit overseas assignments or deployment eligibility.4
The high prevalence of acne and the operationally limiting consequences of isotretinoin therapy present a unique challenge for dermatologists treating military personnel. The average duration of isotretinoin treatment is approximately 6 months,7 which represents a considerable amount of time during an average 4-year enlistment contract. Therapeutic treatment strategies that (1) reduce the duration of oral isotretinoin therapy, (2) reduce the risk for relapse, and (3) increase medication compliance can reduce the operational impact of this acne treatment. Such treatment strategies are discussed below.
High-Dose Isotretinoin
An optimal isotretinoin dosing regimen would achieve swift resolution of acne lesions and reduce the overall relapse rate requiring retrial of isotretinoin, thereby minimizing the operational- and duty-limiting impacts of the medication. Cyrulnik et al13 studied treatment outcomes of high-dose isotretinoin for acne vulgaris using a mean dosage of 1.6 mg/kg daily with an average cumulative dosage of 290 mg/kg. They demonstrated 100% clearance of lesions over 6 months, with a 12.5% relapse rate at 3 years. Aside from an increased rate of elevated transaminases, incidence of adverse effects and laboratory abnormalities were not significantly increased compared to conventional dosing regimens.13 The goal cumulative dosing of 120 to 150 mg/kg can be achieved 1 to 2 months earlier using a dosage of 1.6 mg/kg daily vs a conventional dosage of 1 mg/kg daily.
It has been hypothesized that higher cumulative doses of oral isotretinoin reduce the risk for relapse of acne and retrial of oral isotretinoin.14 Blasiak et al15 studied relapse and retrial of oral isotretinoin in acne patients who received cumulative dosing higher or lower than 220 mg/kg. A clinically but not statistically significant reduced relapse rate was observed in the cohort that received cumulative dosing higher than 220 mg/kg. No statistically significant difference in rates of adverse advents was observed aside from an increase in retinoid dermatitis in the cohort that received cumulative dosing higher than 220 mg/kg. Higher but not statistically significant rates of adverse events were seen in the group that received dosing higher than 220 mg/kg.15 Cumulative doses of oral isotretinoin higher than the 120 to 150 mg/kg range may decrease the risk for acne relapse and the need for an additional course of oral isotretinoin, which would reduce a service member’s total time away from deployment and full duty.
Relapse requiring a retrial of oral isotretinoin not only increases the operational cost of acne treatment but also considerably increases the monetary cost to the health care system. In a cost-analysis model, cumulative doses of oral isotretinoin higher than 230 mg/kg have a decreased overall cost compared to traditional cumulative dosing of less than 150 mg/kg due to the cost of relapse.16
Limitations of high daily and cumulative dosing regimens of oral isotretinoin are chiefly the dose-dependent rate of adverse effects. Low-dose regimens are associated with a reduced risk of isotretinoin-related side effects.6,17 Acute acne flares may be seen following initial administration of oral isotretinoin and are aggravated by increases in dosage.18 Isotretinoin-induced acne fulminans is a rare but devastating complication observed with high initial doses of oral isotretinoin in patients with severe acne.19 The risks and benefits of high daily and cumulatively dosed isotretinoin must be carefully considered in patients with severe acne.
Teledermatology: A Force for Readiness
The COVID-19 pandemic drastically changed the dermatology practice landscape with recommendations to cancel all elective outpatient visits in favor of teledermatology encounters.20 This decreased access to care, which resulted in an increase in drug interruption for dermatology patients, including patients on oral isotretinoin.21 Teledermatology has been increasingly utilized to maintain continuity of care for the management of patients taking isotretinoin.5 Routine utilization of teledermatology evaluation in military practices could expedite care, decrease patient travel time, and allow for in-clinic visits to be utilized for higher-acuity concerns.22
The use of teledermatology for uncomplicated oral isotretinoin management has the potential to increase medication compliance and decrease the amount of travel time for active-duty service members; for example, consider a military dermatology practice based in San Diego, California, that accepts referrals from military bases 3 hours away by car. After an initial consultation for consideration and initiation of oral isotretinoin, teledermatology appointments can save the active-duty service member 3 hours of travel time for each follow-up visit per month. This ultimately increases operational productivity, reduces barriers to accessing care, and improves patient satisfaction.23
Although military personnel usually are located at duty stations for 2 to 4 years, training exercises and military vocational schools often temporarily take personnel away from their home station. These temporary-duty assignments have the potential to interrupt medical follow-up appointments and may cause delays in treatment for individuals who miss monthly isotretinoin visits. When deemed appropriate by the prescribing dermatologist, teledermatology allows for increased continuity of care for active-duty service members and maintenance of a therapeutic isotretinoin course despite temporary geographic displacement.
By facilitating regular follow-up appointments, teledermatology can minimize the amount of time an active-duty service member is on a course of oral isotretinoin, thereby reducing the operational and duty-limiting implications of the medication.
Final Thoughts
Acne is a common dermatologic concern within the active-duty military population. Oral isotretinoin is indicated for treatment-resistant moderate or severe acne; however, it limits the ability of service members to deploy and is disqualifying for special military assignments. High daily- and cumulative-dose isotretinoin treatment strategies can reduce the duration of therapy and may be associated with a decrease in acne relapse and the need for retrial. Teledermatology can increase access to care and facilitate the completion of oral isotretinoin courses in a timely manner. These treatment strategies may help mitigate the duty-limiting impact of oral isotretinoin therapy in military service members.
Acne vulgaris is an extremely common dermatologic disease affecting 40 to 50 million individuals in the United States each year, with a prevalence of 85% in adolescents and young adults aged 12 to 24 years. For some patients, the disease may persist well into adulthood, affecting 8% of adults aged 25 and 34 years.1 Acne negatively impacts patients’ quality of life and productivity, with an estimated direct and indirect cost of over $3 billion per year.2
Oral isotretinoin, a vitamin A derivative, is approved by the US Food and Drug Administration for the treatment of severe nodulocystic acne. Isotretinoin reduces the size and secretions of sebaceous glands, inhibits growth and resulting inflammation of Cutibacterium acnes, and normalizes the differentiation of follicular keratinocytes, resulting in permanent changes in the pathogenesis of acne that may lead to remission.3 The use of oral isotretinoin in the active-duty US Military population may cause service members to be nondeployable or limit their ability to function in special roles (eg, pilot, submariner).4 Treatment regimens that minimize the course duration of isotretinoin and reduce the risk for relapse that requires a retrial of isotretinoin may, in turn, increase a service member’s readiness, deployment availability, and ability to perform unique occupational roles.
Additionally, teledermatology has been increasingly utilized to maintain treatment continuity for patients on isotretinoin during the COVID-19 pandemic.5 Application of this technology in the military also may be used to facilitate timely isotretinoin treatment regimens in active-duty service members to minimize course duration and increase readiness.
In this article, we discuss an accelerated course of oral isotretinoin as a safe and effective option for military service members bound by duty restrictions and operational timelines and explore the role of teledermatology for the treatment of acne in military service members.
Isotretinoin for Acne
Isotretinoin typically is initiated at a dosage of 0.5 mg/kg daily, increasing to 1 mg/kg daily with a goal cumulative dose between 120 and 150 mg/kg. Relapse may occur after completing a treatment course and is associated with cumulative dosing less than 120 mg/kg.6 The average duration of acne treatment with oral isotretinoin is approximately 6 months.7 At therapeutic doses, nearly all patients experience side effects, most commonly dryness and desquamation of the skin and mucous membranes, as well as possible involvement of the lips, eyes, and nose. Notable extracutaneous side effects include headache, visual disturbances at night, idiopathic intracranial hypertension, and myalgia. Serum cholesterol, triglycerides, and transaminases may be increased in patients taking isotretinoin, which requires routine monitoring using serum lipid profiles and liver function studies. A potential association between isotretinoin and inflammatory bowel disease and changes in mood have been reported, but current data do not suggest an evidence-based link.6,8 Isotretinoin is a potent teratogen, and in the United States, all patients are required to enroll in iPLEDGE, a US Food and Drug Administration–approved pregnancy prevention program that monitors prescribing and dispensing of the medication. For patients who can become pregnant, iPLEDGE requires use of 2 forms of contraception as well as monthly pregnancy tests prior to dispensing the medication.
Acne in Military Service Members
Acne is exceedingly common in the active-duty military population. In 2018, more than 40% of soldiers, sailors, airmen, and marines were 25 years or younger, and 75% of all US service members were 35 years or younger, corresponding to acne peak incidences.1,9 Management of acne in this population requires unique treatment considerations due to distinctive occupational requirements of and hazards faced by military personnel. Use of personal protective equipment, including gas masks, safety restraints, parachute rigging, and flak jackets, may be limiting in individuals with moderate to severe acne.10 For example, severe nodulocystic acne on the chin and jawline can interfere with proper wear of the chin strap on a Kevlar helmet. The severity of acne often necessitates the use of oral isotretinoin therapy, which is considered disqualifying for many special military assignments, including submarine duty, nuclear field duty, and diving duty.11 In military aviation communities, oral isotretinoin requires grounding for the duration of therapy plus 3 months after cessation. Slit-lamp examination, triglycerides, and transaminase levels must be normal prior to returning to unrestricted duty.12 Furthermore, use of oral isotretinoin may limit overseas assignments or deployment eligibility.4
The high prevalence of acne and the operationally limiting consequences of isotretinoin therapy present a unique challenge for dermatologists treating military personnel. The average duration of isotretinoin treatment is approximately 6 months,7 which represents a considerable amount of time during an average 4-year enlistment contract. Therapeutic treatment strategies that (1) reduce the duration of oral isotretinoin therapy, (2) reduce the risk for relapse, and (3) increase medication compliance can reduce the operational impact of this acne treatment. Such treatment strategies are discussed below.
High-Dose Isotretinoin
An optimal isotretinoin dosing regimen would achieve swift resolution of acne lesions and reduce the overall relapse rate requiring retrial of isotretinoin, thereby minimizing the operational- and duty-limiting impacts of the medication. Cyrulnik et al13 studied treatment outcomes of high-dose isotretinoin for acne vulgaris using a mean dosage of 1.6 mg/kg daily with an average cumulative dosage of 290 mg/kg. They demonstrated 100% clearance of lesions over 6 months, with a 12.5% relapse rate at 3 years. Aside from an increased rate of elevated transaminases, incidence of adverse effects and laboratory abnormalities were not significantly increased compared to conventional dosing regimens.13 The goal cumulative dosing of 120 to 150 mg/kg can be achieved 1 to 2 months earlier using a dosage of 1.6 mg/kg daily vs a conventional dosage of 1 mg/kg daily.
It has been hypothesized that higher cumulative doses of oral isotretinoin reduce the risk for relapse of acne and retrial of oral isotretinoin.14 Blasiak et al15 studied relapse and retrial of oral isotretinoin in acne patients who received cumulative dosing higher or lower than 220 mg/kg. A clinically but not statistically significant reduced relapse rate was observed in the cohort that received cumulative dosing higher than 220 mg/kg. No statistically significant difference in rates of adverse advents was observed aside from an increase in retinoid dermatitis in the cohort that received cumulative dosing higher than 220 mg/kg. Higher but not statistically significant rates of adverse events were seen in the group that received dosing higher than 220 mg/kg.15 Cumulative doses of oral isotretinoin higher than the 120 to 150 mg/kg range may decrease the risk for acne relapse and the need for an additional course of oral isotretinoin, which would reduce a service member’s total time away from deployment and full duty.
Relapse requiring a retrial of oral isotretinoin not only increases the operational cost of acne treatment but also considerably increases the monetary cost to the health care system. In a cost-analysis model, cumulative doses of oral isotretinoin higher than 230 mg/kg have a decreased overall cost compared to traditional cumulative dosing of less than 150 mg/kg due to the cost of relapse.16
Limitations of high daily and cumulative dosing regimens of oral isotretinoin are chiefly the dose-dependent rate of adverse effects. Low-dose regimens are associated with a reduced risk of isotretinoin-related side effects.6,17 Acute acne flares may be seen following initial administration of oral isotretinoin and are aggravated by increases in dosage.18 Isotretinoin-induced acne fulminans is a rare but devastating complication observed with high initial doses of oral isotretinoin in patients with severe acne.19 The risks and benefits of high daily and cumulatively dosed isotretinoin must be carefully considered in patients with severe acne.
Teledermatology: A Force for Readiness
The COVID-19 pandemic drastically changed the dermatology practice landscape with recommendations to cancel all elective outpatient visits in favor of teledermatology encounters.20 This decreased access to care, which resulted in an increase in drug interruption for dermatology patients, including patients on oral isotretinoin.21 Teledermatology has been increasingly utilized to maintain continuity of care for the management of patients taking isotretinoin.5 Routine utilization of teledermatology evaluation in military practices could expedite care, decrease patient travel time, and allow for in-clinic visits to be utilized for higher-acuity concerns.22
The use of teledermatology for uncomplicated oral isotretinoin management has the potential to increase medication compliance and decrease the amount of travel time for active-duty service members; for example, consider a military dermatology practice based in San Diego, California, that accepts referrals from military bases 3 hours away by car. After an initial consultation for consideration and initiation of oral isotretinoin, teledermatology appointments can save the active-duty service member 3 hours of travel time for each follow-up visit per month. This ultimately increases operational productivity, reduces barriers to accessing care, and improves patient satisfaction.23
Although military personnel usually are located at duty stations for 2 to 4 years, training exercises and military vocational schools often temporarily take personnel away from their home station. These temporary-duty assignments have the potential to interrupt medical follow-up appointments and may cause delays in treatment for individuals who miss monthly isotretinoin visits. When deemed appropriate by the prescribing dermatologist, teledermatology allows for increased continuity of care for active-duty service members and maintenance of a therapeutic isotretinoin course despite temporary geographic displacement.
By facilitating regular follow-up appointments, teledermatology can minimize the amount of time an active-duty service member is on a course of oral isotretinoin, thereby reducing the operational and duty-limiting implications of the medication.
Final Thoughts
Acne is a common dermatologic concern within the active-duty military population. Oral isotretinoin is indicated for treatment-resistant moderate or severe acne; however, it limits the ability of service members to deploy and is disqualifying for special military assignments. High daily- and cumulative-dose isotretinoin treatment strategies can reduce the duration of therapy and may be associated with a decrease in acne relapse and the need for retrial. Teledermatology can increase access to care and facilitate the completion of oral isotretinoin courses in a timely manner. These treatment strategies may help mitigate the duty-limiting impact of oral isotretinoin therapy in military service members.
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39:S34-S37. doi:10.1016/s0190-9622(98)70442-6
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500. doi:10.1016/j.jaad.2006.05.048
- James WD. Clinical practice. acne. N Engl J Med. 2005;352:1463-1472. doi:10.1056/NEJMcp033487
- Burke KR, Larrymore DC, Cho SH. Treatment consideration for US military members with skin disease. Cutis. 2019;103:329-332.
- Rosamilia LL. Isotretinoin meets COVID-19: revisiting a fragmented paradigm. Cutis. 2021;108:8-12. doi:10.12788/cutis.0299
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33. doi:10.1016/j.jaad.2015.12.037
- Huang KE, Carstensen SE, Feldman SR. The duration of acne treatment. J Drugs Dermatol. 2014;13:655-656.
- Bettoli V, Guerra-Tapia A, Herane MI, et al. Challenges and solutions in oral isotretinoin in acne: reflections on 35 years of experience. Clin Cosmet Investig Dermatol. 2019;12:943-951. doi:10.2147/CCID.S234231
- US Department of Defense. 2018 demographics report: profile of the military community. Accessed January 18, 2022. https://download.militaryonesource.mil/12038/MOS/Reports/2018-demographics-report.pdf
- Brahe C, Peters K. Fighting acne for the fighting forces. Cutis. 2020;106:18-20, 22. doi:10.12788/cutis.0057
- US Department of the Navy. Change 167. manual of the medical department. Published February 15, 2019. Accessed January 18, 2022. https://www.med.navy.mil/Portals/62/Documents/BUMED/Directives/MANMED/Chapter%2015%20Medical%20Examinations%20(incorporates%20Changes%20126_135-138_140_145_150-152_154-156_160_164-167).pdf?ver=Rj7AoH54dNAX5uS3F1JUfw%3d%3d
- US Department of the Navy. US Navy aeromedical reference and waiver guide. Published August 11, 2021. Accessed January 18, 2022. https://www.med.navy.mil/Portals/62/Documents/NMFSC/NMOTC/NAMI/ARWG/Waiver%20Guide/ARWG%20COMPLETE_210811.pdf?ver=_pLPzFrtl8E2swFESnN4rA%3d%3d
- Cyrulnik AA, Viola KV, Gewirtzman AJ, et al. High-dose isotretinoin in acne vulgaris: improved treatment outcomes and quality of life. Int J Dermatol. 2012;51:1123-1130. doi:10.1111/j.1365-4632.2011.05409.x
- Coloe J, Du H, Morrell DS. Could higher doses of isotretinoin reduce the frequency of treatment failure in patients with acne? J Am Acad Dermatol. 2011;65:422-423. doi:10.1016/j.jaad.2010.06.025
- Blasiak RC, Stamey CR, Burkhart CN, et al. High-dose isotretinoin treatment and the rate of retrial, relapse, and adverse effects in patients with acne vulgaris. JAMA Dermatol. 2013;149:1392-1398. doi:10.1001/jamadermatol.2013.6746
- Zeitany AE, Bowers EV, Morrell DS. High-dose isotretinoin has lower impact on wallets: a cost analysis of dosing approaches. J Am Acad Dermatol. 2016;74:174-176. doi:10.1016/j.jaad.2015.08.012
- Amichai B, Shemer A, Grunwald MH. Low-dose isotretinoin in the treatment of acne vulgaris. J Am Acad Dermatol. 2006;54:644-666. doi:10.1016/j.jaad.2005.11.1061
- Borghi A, Mantovani L, Minghetti S, et al. Acute acne flare following isotretinoin administration: potential protective role of low starting dose. Dermatology. 2009;218:178-180. doi:10.1159/000182270
- Greywal T, Zaenglein AL, Baldwin HE, et al. Evidence-based recommendations for the management of acne fulminans and its variants. J Am Acad Dermatol. 2017;77:109-117. doi:10.1016/j.jaad.2016.11.028
- Kwatra SG, Sweren RJ, Grossberg AL. Dermatology practices as vectors for COVID-19 transmission: a call for immediate cessation of nonemergent dermatology visits. J Am Acad Dermatol. 2020;82:E179-E180. doi:10.1016/j.jaad.2020.03.037
- Alshiyab DM, Al-Qarqaz FA, Muhaidat JM. Impact of COVID-19 pandemic on the continuity of care for dermatologic patients on systemic therapy during the period of strict lockdown. Ann Med Surg (Lond). 2020;60:571-574. doi:10.1016/j.amsu.2020.11.056
- Hwang J, Kakimoto C. Teledermatology in the US military: a historic foundation for current and future applications. Cutis. 2018;101:335,337,345.
- Ruggiero A, Megna M, Annunziata MC, et al. Teledermatology for acne during COVID-19: high patients’ satisfaction in spite of the emergency. J Eur Acad Dermatol Venereol. 2020;34:E662-E663. doi:10.1111/jdv.16746
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39:S34-S37. doi:10.1016/s0190-9622(98)70442-6
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500. doi:10.1016/j.jaad.2006.05.048
- James WD. Clinical practice. acne. N Engl J Med. 2005;352:1463-1472. doi:10.1056/NEJMcp033487
- Burke KR, Larrymore DC, Cho SH. Treatment consideration for US military members with skin disease. Cutis. 2019;103:329-332.
- Rosamilia LL. Isotretinoin meets COVID-19: revisiting a fragmented paradigm. Cutis. 2021;108:8-12. doi:10.12788/cutis.0299
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33. doi:10.1016/j.jaad.2015.12.037
- Huang KE, Carstensen SE, Feldman SR. The duration of acne treatment. J Drugs Dermatol. 2014;13:655-656.
- Bettoli V, Guerra-Tapia A, Herane MI, et al. Challenges and solutions in oral isotretinoin in acne: reflections on 35 years of experience. Clin Cosmet Investig Dermatol. 2019;12:943-951. doi:10.2147/CCID.S234231
- US Department of Defense. 2018 demographics report: profile of the military community. Accessed January 18, 2022. https://download.militaryonesource.mil/12038/MOS/Reports/2018-demographics-report.pdf
- Brahe C, Peters K. Fighting acne for the fighting forces. Cutis. 2020;106:18-20, 22. doi:10.12788/cutis.0057
- US Department of the Navy. Change 167. manual of the medical department. Published February 15, 2019. Accessed January 18, 2022. https://www.med.navy.mil/Portals/62/Documents/BUMED/Directives/MANMED/Chapter%2015%20Medical%20Examinations%20(incorporates%20Changes%20126_135-138_140_145_150-152_154-156_160_164-167).pdf?ver=Rj7AoH54dNAX5uS3F1JUfw%3d%3d
- US Department of the Navy. US Navy aeromedical reference and waiver guide. Published August 11, 2021. Accessed January 18, 2022. https://www.med.navy.mil/Portals/62/Documents/NMFSC/NMOTC/NAMI/ARWG/Waiver%20Guide/ARWG%20COMPLETE_210811.pdf?ver=_pLPzFrtl8E2swFESnN4rA%3d%3d
- Cyrulnik AA, Viola KV, Gewirtzman AJ, et al. High-dose isotretinoin in acne vulgaris: improved treatment outcomes and quality of life. Int J Dermatol. 2012;51:1123-1130. doi:10.1111/j.1365-4632.2011.05409.x
- Coloe J, Du H, Morrell DS. Could higher doses of isotretinoin reduce the frequency of treatment failure in patients with acne? J Am Acad Dermatol. 2011;65:422-423. doi:10.1016/j.jaad.2010.06.025
- Blasiak RC, Stamey CR, Burkhart CN, et al. High-dose isotretinoin treatment and the rate of retrial, relapse, and adverse effects in patients with acne vulgaris. JAMA Dermatol. 2013;149:1392-1398. doi:10.1001/jamadermatol.2013.6746
- Zeitany AE, Bowers EV, Morrell DS. High-dose isotretinoin has lower impact on wallets: a cost analysis of dosing approaches. J Am Acad Dermatol. 2016;74:174-176. doi:10.1016/j.jaad.2015.08.012
- Amichai B, Shemer A, Grunwald MH. Low-dose isotretinoin in the treatment of acne vulgaris. J Am Acad Dermatol. 2006;54:644-666. doi:10.1016/j.jaad.2005.11.1061
- Borghi A, Mantovani L, Minghetti S, et al. Acute acne flare following isotretinoin administration: potential protective role of low starting dose. Dermatology. 2009;218:178-180. doi:10.1159/000182270
- Greywal T, Zaenglein AL, Baldwin HE, et al. Evidence-based recommendations for the management of acne fulminans and its variants. J Am Acad Dermatol. 2017;77:109-117. doi:10.1016/j.jaad.2016.11.028
- Kwatra SG, Sweren RJ, Grossberg AL. Dermatology practices as vectors for COVID-19 transmission: a call for immediate cessation of nonemergent dermatology visits. J Am Acad Dermatol. 2020;82:E179-E180. doi:10.1016/j.jaad.2020.03.037
- Alshiyab DM, Al-Qarqaz FA, Muhaidat JM. Impact of COVID-19 pandemic on the continuity of care for dermatologic patients on systemic therapy during the period of strict lockdown. Ann Med Surg (Lond). 2020;60:571-574. doi:10.1016/j.amsu.2020.11.056
- Hwang J, Kakimoto C. Teledermatology in the US military: a historic foundation for current and future applications. Cutis. 2018;101:335,337,345.
- Ruggiero A, Megna M, Annunziata MC, et al. Teledermatology for acne during COVID-19: high patients’ satisfaction in spite of the emergency. J Eur Acad Dermatol Venereol. 2020;34:E662-E663. doi:10.1111/jdv.16746
Practice Points
- Acne is a common skin disease with a high prevalence in the active-duty US Military population.
- Oral isotretinoin is a commonly utilized acne medication that can limit the ability for military service members to deploy and is considered disqualifying for some special duty assignments.
- High daily- and cumulative-dose oral isotretinoin therapy as well as teledermatology can minimize the duty-limiting impact of isotretinoin therapy for military service members.
Questions about optimal dosing of isotretinoin persist, expert says
Although the to this day, according to Diane M. Thiboutot, MD.
These include, what is the ideal daily dose of isotretinoin? What is the ideal cumulative dose of isotretinoin to minimize relapse of acne? What is the ideal duration of isotretinoin therapy? How do you define relapse?
“Initially, it was recommended as 1–mg/kg per day dosing,” Dr. Thiboutot, professor of dermatology at Penn State University, Hershey, said during the Orlando Dermatology Aesthetic and Clinical Conference. “As time went on and we became familiar with flares that some patients can have on that dose, it was recommended to start treatment at a dose of 0.5 mg/kg per day. Then there were trends for low dosing, intermittent dosing, and some of the more recent medical literature is talking about high dosing.”
Clinical studies
A multicenter study of 150 patients published in 1984 found that rates of relapse (retreatment needed) were 42% of patients of patients treated with 0.1 mg/kg daily, 20% in patients treated with 0.5 mg/kg daily, and 10% in patients treated with 1.0 mg/kg daily.
In a later study, researchers who followed 299 patients for 5 years post isotretinoin treatment found that there were more relapses in the 0.5-mg/kg group versus those treated with 1.0 mg/kg. Factors that contributed to the need for more treatment courses included lower-dose regimens (0.1 mg/kg and 0.5 mg/kg), having severe acne, being a female over the age of 25 at the onset of therapy, and having a prolonged history of acne.
More recently, investigators who conducted a single-center study of 1,453 patients treated with isotretinoin defined relapse as the need for a second course of isotretinoin. “They found that neither daily nor cumulative dosages influenced relapse of acne vulgaris in patients treated with varying doses of isotretinoin as long as treatment was continued for 2 or more months after the acne had completely resolved,” said Dr. Thiboutot, a past president of the American Acne and Rosacea Society.
“The current evidence underpinning the 120-150 mg/kg cumulative threshold–dosing regimen is equivocal and is based on two low-grade studies,” she noted. “Cumulative doses required for clearance appear lower for acne of mild to moderate severity and higher for more severe acne. Future investigations should use clinically relevant endpoints as end of treatment criteria and define treatment success in acne accurately.”
Other studies have looked at whether higher doses of isotretinoin could reduce treatment failures in patients with acne, Dr. Thiboutot said. In a retrospective chart review of 102 patients with acne who had been treated with isotretinoin for at least 4 consecutive months and followed for over a year, 45.1% required further treatment and 15.7% received a second course of isotretinoin. Cumulative dose (mg/kg), follow-up period, duration of treatment, and daily dose during the last month of treatment were not significantly different between those who relapsed and those who did not relapse.
However, “while the cumulative dose of isotretinoin did not significantly impact acne relapse, patients who received a higher cumulative dose were less likely to require a second course of treatment,” the authors wrote, adding that “female patients had a higher risk of needing retrial regardless of their cumulative dose.” They commented that “prescribing a higher dose per weight may result in less severe acne recurrences and the need for further isotretinoin therapy.”
Another study evaluated 116 patients who were treated to clearance with dosing at the discretion of the provider and defined relapse as subsequent treatment with an oral or topical agent. In the lower-dose treatment (less than 220 mg/kg; mean of 170 mg/kg) group, the relapse rate was 47.4%, compared with 26.9% in the higher-dose (greater than 220 mg/kg) group (P = .03). Cheilitis and xerosis during treatment was reported in nearly all patients in both treatment groups, but retinoid dermatitis was significantly more common among those in the higher-dose group (53.8% vs. 31.6%; P = .02). There were no significant differences in other adverse events between the two groups.
According to Dr. Thiboutot, variables to consider in selection of a daily dose include the presence of intense inflammation, cysts, nodules, potential difference in side-effect profiles with ethnicity, and an individual’s degree of side effects and their level of comfort. “What are some of the concerns with higher doses? Those of us who have treated a lot of acne patients have had the unfortunate occurrence where you start someone on isotretinoin and their acne explodes, as do their cysts and nodules. Once that happens it’s a bad situation and it takes you a while to get past it.”
Acne fulminans
Dr. Thiboutot was part of a panel of experts who assembled guidelines on the classification, management, and prevention of acne fulminans, published in 2017. “Acne fulminans can be induced by isotretinoin or it can occur on its own. If you have someone who has a lot of inflammation, a lot of cysts and nodules, it’s probably best to start them on a lower dose with or without prednisone,” she said.
Age at treatment is another variable that can affect the duration of response to isotretinoin. “If the patient is very young when they need isotretinoin, it’s highly likely that they will need it again,” Dr. Thiboutot said. “Also, adult females often need a repeat dose of isotretinoin. The cumulative dose is important, and the presence of truncal acne is a factor affecting duration of response. Truncal acne takes longer to clear, and it oftentimes needs a longer treatment course.”
Data from the iPledge database indicate that 37% of 10- to 11-year-olds needed a repeat course of isotretinoin, “and as you get older, the need for retreatment is less,” she said.
High-dose versus low-dose isotretinoin
A slow, low-dose approach to the use of isotretinoin in practice can minimize side effects and cystic flares, according to Dr. Thiboutot. “The cons are that you could have a longer treatment duration, you might need more patient visits, and it creates more prolonged drug exposure in patients who can become pregnant,” she said.
“The pros of a high-dose strategy include a shorter treatment duration and potential costs savings. The cons are that there is an increased risk of side effects and a risk of cystic flare. It seems that the general agreement is to treat until clearance and then treat for another 2 months. A clear definition of acne relapse is needed as well as prospective studies to optimize dosing.”
Dr. Thiboutot disclosed that she is a consultant Galderma and Novartis. She has also performed clinical trials for Galderma, Cassiopea, and Foamix.
Commentary by Robert Sidbury, MD, MPH
Isotretinoin has been around for a long time – 40-plus years, to be exact. Despite this cumulative experience, ideal dosing and duration remain uncertain. Many providers have evolved from a “one size fits all” approach (for example, 1 mg/kg of body weight per day for 4-6 months) to a more tailored and nuanced strategy. Dr. Thiboutot validates the need to individualize therapy and helps identify patients at higher risk for relapse. Younger patients are at greater risk for repeat courses of isotretinoin – Dr. Thiboutot cites a study showing 37% of 10- to 11-year-olds required a second course of isotretinoin. This could simply be a proxy for severity, which also augurs a more protracted course. Adult women and those with truncal acne also trend toward longer courses. Knowing these risk factors will help practitioners counsel proper expectations, tailor treatment courses, and thread the always challenging isotretinoin needle: Should one dose “low and slow” and court a longer risk window, or higher and faster, sometimes leading to a dryer disaster?
Dr. Sidbury is chief of dermatology at Seattle Children's Hospital and professor, department of pediatrics, University of Washington, Seattle. He is a site principal investigator for dupilumab trials, for which the hospital has a contract with Regeneron.
This article was updated 6/10/22.
Although the to this day, according to Diane M. Thiboutot, MD.
These include, what is the ideal daily dose of isotretinoin? What is the ideal cumulative dose of isotretinoin to minimize relapse of acne? What is the ideal duration of isotretinoin therapy? How do you define relapse?
“Initially, it was recommended as 1–mg/kg per day dosing,” Dr. Thiboutot, professor of dermatology at Penn State University, Hershey, said during the Orlando Dermatology Aesthetic and Clinical Conference. “As time went on and we became familiar with flares that some patients can have on that dose, it was recommended to start treatment at a dose of 0.5 mg/kg per day. Then there were trends for low dosing, intermittent dosing, and some of the more recent medical literature is talking about high dosing.”
Clinical studies
A multicenter study of 150 patients published in 1984 found that rates of relapse (retreatment needed) were 42% of patients of patients treated with 0.1 mg/kg daily, 20% in patients treated with 0.5 mg/kg daily, and 10% in patients treated with 1.0 mg/kg daily.
In a later study, researchers who followed 299 patients for 5 years post isotretinoin treatment found that there were more relapses in the 0.5-mg/kg group versus those treated with 1.0 mg/kg. Factors that contributed to the need for more treatment courses included lower-dose regimens (0.1 mg/kg and 0.5 mg/kg), having severe acne, being a female over the age of 25 at the onset of therapy, and having a prolonged history of acne.
More recently, investigators who conducted a single-center study of 1,453 patients treated with isotretinoin defined relapse as the need for a second course of isotretinoin. “They found that neither daily nor cumulative dosages influenced relapse of acne vulgaris in patients treated with varying doses of isotretinoin as long as treatment was continued for 2 or more months after the acne had completely resolved,” said Dr. Thiboutot, a past president of the American Acne and Rosacea Society.
“The current evidence underpinning the 120-150 mg/kg cumulative threshold–dosing regimen is equivocal and is based on two low-grade studies,” she noted. “Cumulative doses required for clearance appear lower for acne of mild to moderate severity and higher for more severe acne. Future investigations should use clinically relevant endpoints as end of treatment criteria and define treatment success in acne accurately.”
Other studies have looked at whether higher doses of isotretinoin could reduce treatment failures in patients with acne, Dr. Thiboutot said. In a retrospective chart review of 102 patients with acne who had been treated with isotretinoin for at least 4 consecutive months and followed for over a year, 45.1% required further treatment and 15.7% received a second course of isotretinoin. Cumulative dose (mg/kg), follow-up period, duration of treatment, and daily dose during the last month of treatment were not significantly different between those who relapsed and those who did not relapse.
However, “while the cumulative dose of isotretinoin did not significantly impact acne relapse, patients who received a higher cumulative dose were less likely to require a second course of treatment,” the authors wrote, adding that “female patients had a higher risk of needing retrial regardless of their cumulative dose.” They commented that “prescribing a higher dose per weight may result in less severe acne recurrences and the need for further isotretinoin therapy.”
Another study evaluated 116 patients who were treated to clearance with dosing at the discretion of the provider and defined relapse as subsequent treatment with an oral or topical agent. In the lower-dose treatment (less than 220 mg/kg; mean of 170 mg/kg) group, the relapse rate was 47.4%, compared with 26.9% in the higher-dose (greater than 220 mg/kg) group (P = .03). Cheilitis and xerosis during treatment was reported in nearly all patients in both treatment groups, but retinoid dermatitis was significantly more common among those in the higher-dose group (53.8% vs. 31.6%; P = .02). There were no significant differences in other adverse events between the two groups.
According to Dr. Thiboutot, variables to consider in selection of a daily dose include the presence of intense inflammation, cysts, nodules, potential difference in side-effect profiles with ethnicity, and an individual’s degree of side effects and their level of comfort. “What are some of the concerns with higher doses? Those of us who have treated a lot of acne patients have had the unfortunate occurrence where you start someone on isotretinoin and their acne explodes, as do their cysts and nodules. Once that happens it’s a bad situation and it takes you a while to get past it.”
Acne fulminans
Dr. Thiboutot was part of a panel of experts who assembled guidelines on the classification, management, and prevention of acne fulminans, published in 2017. “Acne fulminans can be induced by isotretinoin or it can occur on its own. If you have someone who has a lot of inflammation, a lot of cysts and nodules, it’s probably best to start them on a lower dose with or without prednisone,” she said.
Age at treatment is another variable that can affect the duration of response to isotretinoin. “If the patient is very young when they need isotretinoin, it’s highly likely that they will need it again,” Dr. Thiboutot said. “Also, adult females often need a repeat dose of isotretinoin. The cumulative dose is important, and the presence of truncal acne is a factor affecting duration of response. Truncal acne takes longer to clear, and it oftentimes needs a longer treatment course.”
Data from the iPledge database indicate that 37% of 10- to 11-year-olds needed a repeat course of isotretinoin, “and as you get older, the need for retreatment is less,” she said.
High-dose versus low-dose isotretinoin
A slow, low-dose approach to the use of isotretinoin in practice can minimize side effects and cystic flares, according to Dr. Thiboutot. “The cons are that you could have a longer treatment duration, you might need more patient visits, and it creates more prolonged drug exposure in patients who can become pregnant,” she said.
“The pros of a high-dose strategy include a shorter treatment duration and potential costs savings. The cons are that there is an increased risk of side effects and a risk of cystic flare. It seems that the general agreement is to treat until clearance and then treat for another 2 months. A clear definition of acne relapse is needed as well as prospective studies to optimize dosing.”
Dr. Thiboutot disclosed that she is a consultant Galderma and Novartis. She has also performed clinical trials for Galderma, Cassiopea, and Foamix.
Commentary by Robert Sidbury, MD, MPH
Isotretinoin has been around for a long time – 40-plus years, to be exact. Despite this cumulative experience, ideal dosing and duration remain uncertain. Many providers have evolved from a “one size fits all” approach (for example, 1 mg/kg of body weight per day for 4-6 months) to a more tailored and nuanced strategy. Dr. Thiboutot validates the need to individualize therapy and helps identify patients at higher risk for relapse. Younger patients are at greater risk for repeat courses of isotretinoin – Dr. Thiboutot cites a study showing 37% of 10- to 11-year-olds required a second course of isotretinoin. This could simply be a proxy for severity, which also augurs a more protracted course. Adult women and those with truncal acne also trend toward longer courses. Knowing these risk factors will help practitioners counsel proper expectations, tailor treatment courses, and thread the always challenging isotretinoin needle: Should one dose “low and slow” and court a longer risk window, or higher and faster, sometimes leading to a dryer disaster?
Dr. Sidbury is chief of dermatology at Seattle Children's Hospital and professor, department of pediatrics, University of Washington, Seattle. He is a site principal investigator for dupilumab trials, for which the hospital has a contract with Regeneron.
This article was updated 6/10/22.
Although the to this day, according to Diane M. Thiboutot, MD.
These include, what is the ideal daily dose of isotretinoin? What is the ideal cumulative dose of isotretinoin to minimize relapse of acne? What is the ideal duration of isotretinoin therapy? How do you define relapse?
“Initially, it was recommended as 1–mg/kg per day dosing,” Dr. Thiboutot, professor of dermatology at Penn State University, Hershey, said during the Orlando Dermatology Aesthetic and Clinical Conference. “As time went on and we became familiar with flares that some patients can have on that dose, it was recommended to start treatment at a dose of 0.5 mg/kg per day. Then there were trends for low dosing, intermittent dosing, and some of the more recent medical literature is talking about high dosing.”
Clinical studies
A multicenter study of 150 patients published in 1984 found that rates of relapse (retreatment needed) were 42% of patients of patients treated with 0.1 mg/kg daily, 20% in patients treated with 0.5 mg/kg daily, and 10% in patients treated with 1.0 mg/kg daily.
In a later study, researchers who followed 299 patients for 5 years post isotretinoin treatment found that there were more relapses in the 0.5-mg/kg group versus those treated with 1.0 mg/kg. Factors that contributed to the need for more treatment courses included lower-dose regimens (0.1 mg/kg and 0.5 mg/kg), having severe acne, being a female over the age of 25 at the onset of therapy, and having a prolonged history of acne.
More recently, investigators who conducted a single-center study of 1,453 patients treated with isotretinoin defined relapse as the need for a second course of isotretinoin. “They found that neither daily nor cumulative dosages influenced relapse of acne vulgaris in patients treated with varying doses of isotretinoin as long as treatment was continued for 2 or more months after the acne had completely resolved,” said Dr. Thiboutot, a past president of the American Acne and Rosacea Society.
“The current evidence underpinning the 120-150 mg/kg cumulative threshold–dosing regimen is equivocal and is based on two low-grade studies,” she noted. “Cumulative doses required for clearance appear lower for acne of mild to moderate severity and higher for more severe acne. Future investigations should use clinically relevant endpoints as end of treatment criteria and define treatment success in acne accurately.”
Other studies have looked at whether higher doses of isotretinoin could reduce treatment failures in patients with acne, Dr. Thiboutot said. In a retrospective chart review of 102 patients with acne who had been treated with isotretinoin for at least 4 consecutive months and followed for over a year, 45.1% required further treatment and 15.7% received a second course of isotretinoin. Cumulative dose (mg/kg), follow-up period, duration of treatment, and daily dose during the last month of treatment were not significantly different between those who relapsed and those who did not relapse.
However, “while the cumulative dose of isotretinoin did not significantly impact acne relapse, patients who received a higher cumulative dose were less likely to require a second course of treatment,” the authors wrote, adding that “female patients had a higher risk of needing retrial regardless of their cumulative dose.” They commented that “prescribing a higher dose per weight may result in less severe acne recurrences and the need for further isotretinoin therapy.”
Another study evaluated 116 patients who were treated to clearance with dosing at the discretion of the provider and defined relapse as subsequent treatment with an oral or topical agent. In the lower-dose treatment (less than 220 mg/kg; mean of 170 mg/kg) group, the relapse rate was 47.4%, compared with 26.9% in the higher-dose (greater than 220 mg/kg) group (P = .03). Cheilitis and xerosis during treatment was reported in nearly all patients in both treatment groups, but retinoid dermatitis was significantly more common among those in the higher-dose group (53.8% vs. 31.6%; P = .02). There were no significant differences in other adverse events between the two groups.
According to Dr. Thiboutot, variables to consider in selection of a daily dose include the presence of intense inflammation, cysts, nodules, potential difference in side-effect profiles with ethnicity, and an individual’s degree of side effects and their level of comfort. “What are some of the concerns with higher doses? Those of us who have treated a lot of acne patients have had the unfortunate occurrence where you start someone on isotretinoin and their acne explodes, as do their cysts and nodules. Once that happens it’s a bad situation and it takes you a while to get past it.”
Acne fulminans
Dr. Thiboutot was part of a panel of experts who assembled guidelines on the classification, management, and prevention of acne fulminans, published in 2017. “Acne fulminans can be induced by isotretinoin or it can occur on its own. If you have someone who has a lot of inflammation, a lot of cysts and nodules, it’s probably best to start them on a lower dose with or without prednisone,” she said.
Age at treatment is another variable that can affect the duration of response to isotretinoin. “If the patient is very young when they need isotretinoin, it’s highly likely that they will need it again,” Dr. Thiboutot said. “Also, adult females often need a repeat dose of isotretinoin. The cumulative dose is important, and the presence of truncal acne is a factor affecting duration of response. Truncal acne takes longer to clear, and it oftentimes needs a longer treatment course.”
Data from the iPledge database indicate that 37% of 10- to 11-year-olds needed a repeat course of isotretinoin, “and as you get older, the need for retreatment is less,” she said.
High-dose versus low-dose isotretinoin
A slow, low-dose approach to the use of isotretinoin in practice can minimize side effects and cystic flares, according to Dr. Thiboutot. “The cons are that you could have a longer treatment duration, you might need more patient visits, and it creates more prolonged drug exposure in patients who can become pregnant,” she said.
“The pros of a high-dose strategy include a shorter treatment duration and potential costs savings. The cons are that there is an increased risk of side effects and a risk of cystic flare. It seems that the general agreement is to treat until clearance and then treat for another 2 months. A clear definition of acne relapse is needed as well as prospective studies to optimize dosing.”
Dr. Thiboutot disclosed that she is a consultant Galderma and Novartis. She has also performed clinical trials for Galderma, Cassiopea, and Foamix.
Commentary by Robert Sidbury, MD, MPH
Isotretinoin has been around for a long time – 40-plus years, to be exact. Despite this cumulative experience, ideal dosing and duration remain uncertain. Many providers have evolved from a “one size fits all” approach (for example, 1 mg/kg of body weight per day for 4-6 months) to a more tailored and nuanced strategy. Dr. Thiboutot validates the need to individualize therapy and helps identify patients at higher risk for relapse. Younger patients are at greater risk for repeat courses of isotretinoin – Dr. Thiboutot cites a study showing 37% of 10- to 11-year-olds required a second course of isotretinoin. This could simply be a proxy for severity, which also augurs a more protracted course. Adult women and those with truncal acne also trend toward longer courses. Knowing these risk factors will help practitioners counsel proper expectations, tailor treatment courses, and thread the always challenging isotretinoin needle: Should one dose “low and slow” and court a longer risk window, or higher and faster, sometimes leading to a dryer disaster?
Dr. Sidbury is chief of dermatology at Seattle Children's Hospital and professor, department of pediatrics, University of Washington, Seattle. He is a site principal investigator for dupilumab trials, for which the hospital has a contract with Regeneron.
This article was updated 6/10/22.
FROM ODAC 2022
More than a month after launch, iPLEDGE glitches persist
which mandates the program to prevent fetal exposure to the teratogenic effects of isotretinoin, and by the American Academy of Dermatology Association, whose members have repeatedly asked the FDA for meetings to discuss solutions. The AADA is the legislative and advocacy arm of AAD.
When the new program launched Dec. 13, 2021, the website crashed repeatedly, with physicians and patients complaining they got locked out or bounced off the platform when they tried to follow instructions to enter information. Hold times to talk to a live person stretched to hours.
The latest improvement attempt, announced Jan. 14 by the FDA, is a tool created by the Isotretinoin Products Manufacturers Group, the manufacturers responsible for the FDA-mandated REMS program. It is meant to allow prescribers and designees to send log-in links directly to patients’ email accounts through the iPLEDGE REMS portal, bypassing the troublesome call center.
And it’s not the answer, dermatologists said.
“The new tool does not solve issues such as prescribers or pharmacies not being able to access the site, unacceptably long call center wait times, inefficiencies caused by frequent attestation requirements for those who cannot become pregnant, patients becoming ‘locked out’ because they missed a window period through no fault of their own, among others,” said John Barbieri, MD, MBA, director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital and instructor in dermatology at Harvard Medical School, both in Boston.
The day after the FDA update about the new tool, Klint Peebles, MD, a dermatologist at Kaiser Permanente in Washington, D.C., tweeted: “Lip service and empty words.” He noted that the situation has been “disastrous from the start” as the new platform launched.
Under the iPLEDGE program in place for the acne drug, physicians, patients, and pharmacies prescribing, using, or dispensing the drug must all be registered, with requirements that include the use of two forms of an effective contraceptive and regular pregnancy testing for patients who can become pregnant.
The aim of the new gender-neutral approach to the risk mitigation program is to make the experience more inclusive for transgender patients. The previous three risk categories (females of reproductive potential, females not of reproductive potential, and males) are now reduced to just two (those capable of getting pregnant and those not capable of getting pregnant).
The problem is the execution of the new platform. The transition from the old website to the new was done quickly. By most accounts, the Dec. 13 rollout was chaotic, a failure, and disastrous, triggering numerous expressions of frustration on Twitter and other social media, with some calling for the program to be halted until the bugs could be worked out.
“While the new gender-neutral categories are a welcome improvement to the system, the new categorization approach was not the underlying reason for the new platform and its failed rollout, which was instead due to a change in vendor,” Dr. Barbieri told this news organization.
AADA: More recent efforts to improve the system
“We have a letter to the FDA asking for a stakeholders meeting to include us, the IPMG, and pharmacists because there are ongoing problems, though there have been some improvement in terms of certain elements,” Ilona Frieden, MD, chair of the AADA’s iPLEDGE Workgroup and professor of dermatology at the University of California, San Francisco, said in an interview shortly after the FDA posted the update on the new tool. “That said, there are many patients who have not gotten isotretinoin during the 1 month since the roll-out of the new platform.”
What still needs to be fixed? “We have ongoing concerns about the lack of transparency of the IPMG, about call center wait times, actual number of prescriptions on the hands of patients compared to the previous month, and those patients who can get pregnant who – despite complying with all of the REMS requirements – are being locked out because of the lack of timely attestation to their negative pregnancy status due to the website, not the patients themselves,” Dr. Frieden told this news organization.
“We are continuing to advocate to have decreased attestation requirements for individuals who cannot become pregnant – because this will improve the efficiency of the system for those patients for whom the REMS program goals are truly intended – those who can become pregnant, since the primary aim of the REMS program is to minimize fetal exposure.”
An AADA spokesperson said that the IPMG has invited the AADA to a joint stakeholders meeting on Jan. 26, along with representatives from the FDA and pharmacy industry.
Spotty progress
“The iPLEDGE situation is as frustrating as ever,” said Neil S. Goldberg, MD, a dermatologist in Westchester County, New York, after the FDA’s Jan. 14 update was released. “It’s like they never tested the new website before deploying it.”
Among the issues he has experienced in his practice, he said, is an instance in which iPLEDGE swapped the first names of a mother and daughter, so it was impossible to fill the prescription. “It happened twice in the same day,” Dr. Goldberg said. The patient had to call iPLEDGE to fix this, but the call center wasn’t taking calls.
In today’s technology environment, he said, it’s hard to believe that “we have to put up with this.”
Some have seen success. ‘’The tool is working fine on our end,” said Mitesh Patel, PharmD, pharmacy manager at Sunshine Pharmacy in White Plains, N.Y. However, he added that some doctors and patients are still having issues. He encourages dermatologists still having issues with the system to reach out to independent pharmacies that have processed iPLEDGE prescriptions and ‘’lean on them to assist.”
This news organization contacted CVS and Walgreens about how the system is working at their locations, but has not yet received a response.
Dr. Goldberg, Dr. Frieden, Dr. Barbieri, and Dr. Peebles have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This story was updated on 1/24/22.
which mandates the program to prevent fetal exposure to the teratogenic effects of isotretinoin, and by the American Academy of Dermatology Association, whose members have repeatedly asked the FDA for meetings to discuss solutions. The AADA is the legislative and advocacy arm of AAD.
When the new program launched Dec. 13, 2021, the website crashed repeatedly, with physicians and patients complaining they got locked out or bounced off the platform when they tried to follow instructions to enter information. Hold times to talk to a live person stretched to hours.
The latest improvement attempt, announced Jan. 14 by the FDA, is a tool created by the Isotretinoin Products Manufacturers Group, the manufacturers responsible for the FDA-mandated REMS program. It is meant to allow prescribers and designees to send log-in links directly to patients’ email accounts through the iPLEDGE REMS portal, bypassing the troublesome call center.
And it’s not the answer, dermatologists said.
“The new tool does not solve issues such as prescribers or pharmacies not being able to access the site, unacceptably long call center wait times, inefficiencies caused by frequent attestation requirements for those who cannot become pregnant, patients becoming ‘locked out’ because they missed a window period through no fault of their own, among others,” said John Barbieri, MD, MBA, director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital and instructor in dermatology at Harvard Medical School, both in Boston.
The day after the FDA update about the new tool, Klint Peebles, MD, a dermatologist at Kaiser Permanente in Washington, D.C., tweeted: “Lip service and empty words.” He noted that the situation has been “disastrous from the start” as the new platform launched.
Under the iPLEDGE program in place for the acne drug, physicians, patients, and pharmacies prescribing, using, or dispensing the drug must all be registered, with requirements that include the use of two forms of an effective contraceptive and regular pregnancy testing for patients who can become pregnant.
The aim of the new gender-neutral approach to the risk mitigation program is to make the experience more inclusive for transgender patients. The previous three risk categories (females of reproductive potential, females not of reproductive potential, and males) are now reduced to just two (those capable of getting pregnant and those not capable of getting pregnant).
The problem is the execution of the new platform. The transition from the old website to the new was done quickly. By most accounts, the Dec. 13 rollout was chaotic, a failure, and disastrous, triggering numerous expressions of frustration on Twitter and other social media, with some calling for the program to be halted until the bugs could be worked out.
“While the new gender-neutral categories are a welcome improvement to the system, the new categorization approach was not the underlying reason for the new platform and its failed rollout, which was instead due to a change in vendor,” Dr. Barbieri told this news organization.
AADA: More recent efforts to improve the system
“We have a letter to the FDA asking for a stakeholders meeting to include us, the IPMG, and pharmacists because there are ongoing problems, though there have been some improvement in terms of certain elements,” Ilona Frieden, MD, chair of the AADA’s iPLEDGE Workgroup and professor of dermatology at the University of California, San Francisco, said in an interview shortly after the FDA posted the update on the new tool. “That said, there are many patients who have not gotten isotretinoin during the 1 month since the roll-out of the new platform.”
What still needs to be fixed? “We have ongoing concerns about the lack of transparency of the IPMG, about call center wait times, actual number of prescriptions on the hands of patients compared to the previous month, and those patients who can get pregnant who – despite complying with all of the REMS requirements – are being locked out because of the lack of timely attestation to their negative pregnancy status due to the website, not the patients themselves,” Dr. Frieden told this news organization.
“We are continuing to advocate to have decreased attestation requirements for individuals who cannot become pregnant – because this will improve the efficiency of the system for those patients for whom the REMS program goals are truly intended – those who can become pregnant, since the primary aim of the REMS program is to minimize fetal exposure.”
An AADA spokesperson said that the IPMG has invited the AADA to a joint stakeholders meeting on Jan. 26, along with representatives from the FDA and pharmacy industry.
Spotty progress
“The iPLEDGE situation is as frustrating as ever,” said Neil S. Goldberg, MD, a dermatologist in Westchester County, New York, after the FDA’s Jan. 14 update was released. “It’s like they never tested the new website before deploying it.”
Among the issues he has experienced in his practice, he said, is an instance in which iPLEDGE swapped the first names of a mother and daughter, so it was impossible to fill the prescription. “It happened twice in the same day,” Dr. Goldberg said. The patient had to call iPLEDGE to fix this, but the call center wasn’t taking calls.
In today’s technology environment, he said, it’s hard to believe that “we have to put up with this.”
Some have seen success. ‘’The tool is working fine on our end,” said Mitesh Patel, PharmD, pharmacy manager at Sunshine Pharmacy in White Plains, N.Y. However, he added that some doctors and patients are still having issues. He encourages dermatologists still having issues with the system to reach out to independent pharmacies that have processed iPLEDGE prescriptions and ‘’lean on them to assist.”
This news organization contacted CVS and Walgreens about how the system is working at their locations, but has not yet received a response.
Dr. Goldberg, Dr. Frieden, Dr. Barbieri, and Dr. Peebles have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This story was updated on 1/24/22.
which mandates the program to prevent fetal exposure to the teratogenic effects of isotretinoin, and by the American Academy of Dermatology Association, whose members have repeatedly asked the FDA for meetings to discuss solutions. The AADA is the legislative and advocacy arm of AAD.
When the new program launched Dec. 13, 2021, the website crashed repeatedly, with physicians and patients complaining they got locked out or bounced off the platform when they tried to follow instructions to enter information. Hold times to talk to a live person stretched to hours.
The latest improvement attempt, announced Jan. 14 by the FDA, is a tool created by the Isotretinoin Products Manufacturers Group, the manufacturers responsible for the FDA-mandated REMS program. It is meant to allow prescribers and designees to send log-in links directly to patients’ email accounts through the iPLEDGE REMS portal, bypassing the troublesome call center.
And it’s not the answer, dermatologists said.
“The new tool does not solve issues such as prescribers or pharmacies not being able to access the site, unacceptably long call center wait times, inefficiencies caused by frequent attestation requirements for those who cannot become pregnant, patients becoming ‘locked out’ because they missed a window period through no fault of their own, among others,” said John Barbieri, MD, MBA, director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital and instructor in dermatology at Harvard Medical School, both in Boston.
The day after the FDA update about the new tool, Klint Peebles, MD, a dermatologist at Kaiser Permanente in Washington, D.C., tweeted: “Lip service and empty words.” He noted that the situation has been “disastrous from the start” as the new platform launched.
Under the iPLEDGE program in place for the acne drug, physicians, patients, and pharmacies prescribing, using, or dispensing the drug must all be registered, with requirements that include the use of two forms of an effective contraceptive and regular pregnancy testing for patients who can become pregnant.
The aim of the new gender-neutral approach to the risk mitigation program is to make the experience more inclusive for transgender patients. The previous three risk categories (females of reproductive potential, females not of reproductive potential, and males) are now reduced to just two (those capable of getting pregnant and those not capable of getting pregnant).
The problem is the execution of the new platform. The transition from the old website to the new was done quickly. By most accounts, the Dec. 13 rollout was chaotic, a failure, and disastrous, triggering numerous expressions of frustration on Twitter and other social media, with some calling for the program to be halted until the bugs could be worked out.
“While the new gender-neutral categories are a welcome improvement to the system, the new categorization approach was not the underlying reason for the new platform and its failed rollout, which was instead due to a change in vendor,” Dr. Barbieri told this news organization.
AADA: More recent efforts to improve the system
“We have a letter to the FDA asking for a stakeholders meeting to include us, the IPMG, and pharmacists because there are ongoing problems, though there have been some improvement in terms of certain elements,” Ilona Frieden, MD, chair of the AADA’s iPLEDGE Workgroup and professor of dermatology at the University of California, San Francisco, said in an interview shortly after the FDA posted the update on the new tool. “That said, there are many patients who have not gotten isotretinoin during the 1 month since the roll-out of the new platform.”
What still needs to be fixed? “We have ongoing concerns about the lack of transparency of the IPMG, about call center wait times, actual number of prescriptions on the hands of patients compared to the previous month, and those patients who can get pregnant who – despite complying with all of the REMS requirements – are being locked out because of the lack of timely attestation to their negative pregnancy status due to the website, not the patients themselves,” Dr. Frieden told this news organization.
“We are continuing to advocate to have decreased attestation requirements for individuals who cannot become pregnant – because this will improve the efficiency of the system for those patients for whom the REMS program goals are truly intended – those who can become pregnant, since the primary aim of the REMS program is to minimize fetal exposure.”
An AADA spokesperson said that the IPMG has invited the AADA to a joint stakeholders meeting on Jan. 26, along with representatives from the FDA and pharmacy industry.
Spotty progress
“The iPLEDGE situation is as frustrating as ever,” said Neil S. Goldberg, MD, a dermatologist in Westchester County, New York, after the FDA’s Jan. 14 update was released. “It’s like they never tested the new website before deploying it.”
Among the issues he has experienced in his practice, he said, is an instance in which iPLEDGE swapped the first names of a mother and daughter, so it was impossible to fill the prescription. “It happened twice in the same day,” Dr. Goldberg said. The patient had to call iPLEDGE to fix this, but the call center wasn’t taking calls.
In today’s technology environment, he said, it’s hard to believe that “we have to put up with this.”
Some have seen success. ‘’The tool is working fine on our end,” said Mitesh Patel, PharmD, pharmacy manager at Sunshine Pharmacy in White Plains, N.Y. However, he added that some doctors and patients are still having issues. He encourages dermatologists still having issues with the system to reach out to independent pharmacies that have processed iPLEDGE prescriptions and ‘’lean on them to assist.”
This news organization contacted CVS and Walgreens about how the system is working at their locations, but has not yet received a response.
Dr. Goldberg, Dr. Frieden, Dr. Barbieri, and Dr. Peebles have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This story was updated on 1/24/22.
FDA updates status of iPLEDGE access problems
The, one month after a modified program was launched, the Food and Drug Administration announced on Jan. 14.
The IPMG has “created a new tool within the system to help resolve account access for some user groups without using the call center. This tool is intended to allow prescribers and designees to send login links directly to their patients’ desired email address through the Manage Patients page of the iPLEDGE REMS portal,” the FDA statement said.
“Prescribers can also send login links to their designees still having difficulty accessing their iPLEDGE account,” and users should check their emails for messages from iPLEDGE, including spam folders, the FDA advises. The iPLEDGE strategy is designed to prevent fetal exposure to isotretinoin, which is highly teratogenic.
Days after the new, gender-neutral approach to the isotretinoin risk mitigation program was launched on Dec. 13, the FDA convened an emergency meeting with representatives from the American Academy of Dermatology Association (AADA) to discuss the problematic rollout of the program, which was described as disastrous, chaotic, and a failure, with dermatologists on Twitter and elsewhere expressing anger and frustration over not being able to access the program or reach the call center.
A statement by the FDA on Dec. 23 followed, urging manufacturers to develop solutions for the website and to work with the AADA and pharmacy organizations to find solutions that would minimize treatment interruptions during the transition.
The modified REMS, launched on Dec. 13, is designed to make it more inclusive for transgender patients prescribed isotretinoin. Instead of three risk categories (females of reproductive potential, females not of reproductive potential, and males), patients who are prescribed isotretinoin for acne are assigned to one of two risk categories: those who can get pregnant and those who cannot get pregnant.
In the Jan. 14 statement, the FDA notes that the agency is continuing to work with the IPMG regarding the problems clinicians, pharmacists, and patients have had with accessing iPLEDGE over the last month.
“Although there has been progress, there is a significant amount of work still to be done,” the FDA acknowledged. “While we consider potential steps within the scope of FDA’s authorities, we will continue to meet with the IPMG for updates on the status of the problems with the iPLEDGE REMS and their progress towards having the system work as intended for all users.”
The, one month after a modified program was launched, the Food and Drug Administration announced on Jan. 14.
The IPMG has “created a new tool within the system to help resolve account access for some user groups without using the call center. This tool is intended to allow prescribers and designees to send login links directly to their patients’ desired email address through the Manage Patients page of the iPLEDGE REMS portal,” the FDA statement said.
“Prescribers can also send login links to their designees still having difficulty accessing their iPLEDGE account,” and users should check their emails for messages from iPLEDGE, including spam folders, the FDA advises. The iPLEDGE strategy is designed to prevent fetal exposure to isotretinoin, which is highly teratogenic.
Days after the new, gender-neutral approach to the isotretinoin risk mitigation program was launched on Dec. 13, the FDA convened an emergency meeting with representatives from the American Academy of Dermatology Association (AADA) to discuss the problematic rollout of the program, which was described as disastrous, chaotic, and a failure, with dermatologists on Twitter and elsewhere expressing anger and frustration over not being able to access the program or reach the call center.
A statement by the FDA on Dec. 23 followed, urging manufacturers to develop solutions for the website and to work with the AADA and pharmacy organizations to find solutions that would minimize treatment interruptions during the transition.
The modified REMS, launched on Dec. 13, is designed to make it more inclusive for transgender patients prescribed isotretinoin. Instead of three risk categories (females of reproductive potential, females not of reproductive potential, and males), patients who are prescribed isotretinoin for acne are assigned to one of two risk categories: those who can get pregnant and those who cannot get pregnant.
In the Jan. 14 statement, the FDA notes that the agency is continuing to work with the IPMG regarding the problems clinicians, pharmacists, and patients have had with accessing iPLEDGE over the last month.
“Although there has been progress, there is a significant amount of work still to be done,” the FDA acknowledged. “While we consider potential steps within the scope of FDA’s authorities, we will continue to meet with the IPMG for updates on the status of the problems with the iPLEDGE REMS and their progress towards having the system work as intended for all users.”
The, one month after a modified program was launched, the Food and Drug Administration announced on Jan. 14.
The IPMG has “created a new tool within the system to help resolve account access for some user groups without using the call center. This tool is intended to allow prescribers and designees to send login links directly to their patients’ desired email address through the Manage Patients page of the iPLEDGE REMS portal,” the FDA statement said.
“Prescribers can also send login links to their designees still having difficulty accessing their iPLEDGE account,” and users should check their emails for messages from iPLEDGE, including spam folders, the FDA advises. The iPLEDGE strategy is designed to prevent fetal exposure to isotretinoin, which is highly teratogenic.
Days after the new, gender-neutral approach to the isotretinoin risk mitigation program was launched on Dec. 13, the FDA convened an emergency meeting with representatives from the American Academy of Dermatology Association (AADA) to discuss the problematic rollout of the program, which was described as disastrous, chaotic, and a failure, with dermatologists on Twitter and elsewhere expressing anger and frustration over not being able to access the program or reach the call center.
A statement by the FDA on Dec. 23 followed, urging manufacturers to develop solutions for the website and to work with the AADA and pharmacy organizations to find solutions that would minimize treatment interruptions during the transition.
The modified REMS, launched on Dec. 13, is designed to make it more inclusive for transgender patients prescribed isotretinoin. Instead of three risk categories (females of reproductive potential, females not of reproductive potential, and males), patients who are prescribed isotretinoin for acne are assigned to one of two risk categories: those who can get pregnant and those who cannot get pregnant.
In the Jan. 14 statement, the FDA notes that the agency is continuing to work with the IPMG regarding the problems clinicians, pharmacists, and patients have had with accessing iPLEDGE over the last month.
“Although there has been progress, there is a significant amount of work still to be done,” the FDA acknowledged. “While we consider potential steps within the scope of FDA’s authorities, we will continue to meet with the IPMG for updates on the status of the problems with the iPLEDGE REMS and their progress towards having the system work as intended for all users.”
iPLEDGE rollout: As frustration mounts, FDA agrees to help solve issues
, according to dermatologists, pharmacists, and patients.
When the new website and call center launched Dec. 13, hours-long hold times and repeated crashing of the website were reported as the norm, not the exception, triggering the American Academy of Dermatology Association (AADA) to request – and get – an emergency meeting on Dec. 16 with the U.S. Food and Drug Administration, which mandates the risk evaluation and mitigation strategy (REMS) for isotretinoin due to the teratogenicity of the acne medication.
At that meeting, ‘’the FDA and HHS [U.S. Department of Health and Human Services] acknowledged the concerns of dermatologists and the need for stakeholders to work collaboratively to find a solution,” Ilona Frieden, MD, chair of the AADA’s iPLEDGE workgroup and professor of dermatology at the University of California, San Francisco, said in an email interview. At the meeting, the AADA representatives described the severe impact on patient access to treatment that is resulting from the issues. The AADA also ‘’reiterated our call for a temporary pause to the program while stakeholders work to resolve the urgent issues with the platform,” she said.
The new approach, which is intended to make the experience more inclusive for transgender patients, reduces the previous three risk categories (females of reproductive potential, females not of reproductive potential, and males) to just two (those capable of getting pregnant and those not capable). The program requires physicians, patients, and pharmacists who prescribe, use, or dispense the drug to be registered, with requirements that include the use of two forms of an effective contraceptive and regular pregnancy tests by patients capable of becoming pregnant.
With reduced or no access during the technology glitches, access to the medicine was delayed for some patients. And dermatologists, pharmacists, and their staffs reported grueling hold times trying to reach the call center when the website had issues.
While the FDA agreed to help find a solution, it noted that the solution ‘’was to be found with dermatologists and pharmacists who are on the ground living the program every day,” Dr. Frieden said. No timeline for solving the issues was provided, so on Dec. 21, the AADA asked the FDA for a constructive dialogue among stakeholders within the next 24 hours, Dr. Frieden told this news organization.
While Dr. Frieden sees progress, ‘’we are disappointed that this situation continues to drag on for more than a week later, with more patients losing access to their needed medication each day.” While some prescribers have been able to log onto the portal and enter the information required, confirming some patients, large gaps remain, she said. Patients and pharmacists still report difficulties logging on. When that happens and they try to reach the call center, there are often hours-long hold times, dropped calls, or a message saying to call back.
The iPLEDGE administrator is Syneos Health, but a spokesperson for Syneos, Gary Gatyas, said the company does not maintain the system or the contact center.
So who does manage the call center and website? “The AADA has asked stakeholders, including Syneos Health, for clarification on who manages the call center and website but has not received a response,” Dr. Frieden said. “In the meeting [Dec. 16], representatives from the FDA made clear that the iPLEDGE sponsors are ultimately responsible for this REMS program,” Dr. Frieden said.
According to the FDA, isotretinoin manufacturers are part of the iPLEDGE program. On the iPLEDGE website, 12 isotretinoin products are listed, made by eight different companies.
One dermatologist maneuvering the new website who registered successfully as a provider told this news organization that he received a follow-up survey from United BioSource about the new website. This news organization contacted that company to confirm it runs the website but has not yet received a response.
Meanwhile, dermatologists continue to help frustrated patients cope with the new website and registration details. Neil S. Goldberg, MD, a dermatologist in Westchester County, New York, heard from two mothers who helped their teen daughters complete the forms by attesting they would use abstinence as contraception but then couldn’t figure out how to answer another question. As a result, their answers were interpreted as the patients saying they were using abstinence but didn’t commit to not having sexual contact with a partner capable of impregnating them. So Dr. Goldberg got an automated message back from the iPLEDGE program that the answers were a mismatch.
And in the comments section following a previous story on the problematic rollout, one reader offered a suggestion for reducing hold times to the call center: choose the Spanish option.
Dr. Frieden and Dr. Goldberg have no relevant disclosures.
A version of this article first appeared on Medscape.com.
, according to dermatologists, pharmacists, and patients.
When the new website and call center launched Dec. 13, hours-long hold times and repeated crashing of the website were reported as the norm, not the exception, triggering the American Academy of Dermatology Association (AADA) to request – and get – an emergency meeting on Dec. 16 with the U.S. Food and Drug Administration, which mandates the risk evaluation and mitigation strategy (REMS) for isotretinoin due to the teratogenicity of the acne medication.
At that meeting, ‘’the FDA and HHS [U.S. Department of Health and Human Services] acknowledged the concerns of dermatologists and the need for stakeholders to work collaboratively to find a solution,” Ilona Frieden, MD, chair of the AADA’s iPLEDGE workgroup and professor of dermatology at the University of California, San Francisco, said in an email interview. At the meeting, the AADA representatives described the severe impact on patient access to treatment that is resulting from the issues. The AADA also ‘’reiterated our call for a temporary pause to the program while stakeholders work to resolve the urgent issues with the platform,” she said.
The new approach, which is intended to make the experience more inclusive for transgender patients, reduces the previous three risk categories (females of reproductive potential, females not of reproductive potential, and males) to just two (those capable of getting pregnant and those not capable). The program requires physicians, patients, and pharmacists who prescribe, use, or dispense the drug to be registered, with requirements that include the use of two forms of an effective contraceptive and regular pregnancy tests by patients capable of becoming pregnant.
With reduced or no access during the technology glitches, access to the medicine was delayed for some patients. And dermatologists, pharmacists, and their staffs reported grueling hold times trying to reach the call center when the website had issues.
While the FDA agreed to help find a solution, it noted that the solution ‘’was to be found with dermatologists and pharmacists who are on the ground living the program every day,” Dr. Frieden said. No timeline for solving the issues was provided, so on Dec. 21, the AADA asked the FDA for a constructive dialogue among stakeholders within the next 24 hours, Dr. Frieden told this news organization.
While Dr. Frieden sees progress, ‘’we are disappointed that this situation continues to drag on for more than a week later, with more patients losing access to their needed medication each day.” While some prescribers have been able to log onto the portal and enter the information required, confirming some patients, large gaps remain, she said. Patients and pharmacists still report difficulties logging on. When that happens and they try to reach the call center, there are often hours-long hold times, dropped calls, or a message saying to call back.
The iPLEDGE administrator is Syneos Health, but a spokesperson for Syneos, Gary Gatyas, said the company does not maintain the system or the contact center.
So who does manage the call center and website? “The AADA has asked stakeholders, including Syneos Health, for clarification on who manages the call center and website but has not received a response,” Dr. Frieden said. “In the meeting [Dec. 16], representatives from the FDA made clear that the iPLEDGE sponsors are ultimately responsible for this REMS program,” Dr. Frieden said.
According to the FDA, isotretinoin manufacturers are part of the iPLEDGE program. On the iPLEDGE website, 12 isotretinoin products are listed, made by eight different companies.
One dermatologist maneuvering the new website who registered successfully as a provider told this news organization that he received a follow-up survey from United BioSource about the new website. This news organization contacted that company to confirm it runs the website but has not yet received a response.
Meanwhile, dermatologists continue to help frustrated patients cope with the new website and registration details. Neil S. Goldberg, MD, a dermatologist in Westchester County, New York, heard from two mothers who helped their teen daughters complete the forms by attesting they would use abstinence as contraception but then couldn’t figure out how to answer another question. As a result, their answers were interpreted as the patients saying they were using abstinence but didn’t commit to not having sexual contact with a partner capable of impregnating them. So Dr. Goldberg got an automated message back from the iPLEDGE program that the answers were a mismatch.
And in the comments section following a previous story on the problematic rollout, one reader offered a suggestion for reducing hold times to the call center: choose the Spanish option.
Dr. Frieden and Dr. Goldberg have no relevant disclosures.
A version of this article first appeared on Medscape.com.
, according to dermatologists, pharmacists, and patients.
When the new website and call center launched Dec. 13, hours-long hold times and repeated crashing of the website were reported as the norm, not the exception, triggering the American Academy of Dermatology Association (AADA) to request – and get – an emergency meeting on Dec. 16 with the U.S. Food and Drug Administration, which mandates the risk evaluation and mitigation strategy (REMS) for isotretinoin due to the teratogenicity of the acne medication.
At that meeting, ‘’the FDA and HHS [U.S. Department of Health and Human Services] acknowledged the concerns of dermatologists and the need for stakeholders to work collaboratively to find a solution,” Ilona Frieden, MD, chair of the AADA’s iPLEDGE workgroup and professor of dermatology at the University of California, San Francisco, said in an email interview. At the meeting, the AADA representatives described the severe impact on patient access to treatment that is resulting from the issues. The AADA also ‘’reiterated our call for a temporary pause to the program while stakeholders work to resolve the urgent issues with the platform,” she said.
The new approach, which is intended to make the experience more inclusive for transgender patients, reduces the previous three risk categories (females of reproductive potential, females not of reproductive potential, and males) to just two (those capable of getting pregnant and those not capable). The program requires physicians, patients, and pharmacists who prescribe, use, or dispense the drug to be registered, with requirements that include the use of two forms of an effective contraceptive and regular pregnancy tests by patients capable of becoming pregnant.
With reduced or no access during the technology glitches, access to the medicine was delayed for some patients. And dermatologists, pharmacists, and their staffs reported grueling hold times trying to reach the call center when the website had issues.
While the FDA agreed to help find a solution, it noted that the solution ‘’was to be found with dermatologists and pharmacists who are on the ground living the program every day,” Dr. Frieden said. No timeline for solving the issues was provided, so on Dec. 21, the AADA asked the FDA for a constructive dialogue among stakeholders within the next 24 hours, Dr. Frieden told this news organization.
While Dr. Frieden sees progress, ‘’we are disappointed that this situation continues to drag on for more than a week later, with more patients losing access to their needed medication each day.” While some prescribers have been able to log onto the portal and enter the information required, confirming some patients, large gaps remain, she said. Patients and pharmacists still report difficulties logging on. When that happens and they try to reach the call center, there are often hours-long hold times, dropped calls, or a message saying to call back.
The iPLEDGE administrator is Syneos Health, but a spokesperson for Syneos, Gary Gatyas, said the company does not maintain the system or the contact center.
So who does manage the call center and website? “The AADA has asked stakeholders, including Syneos Health, for clarification on who manages the call center and website but has not received a response,” Dr. Frieden said. “In the meeting [Dec. 16], representatives from the FDA made clear that the iPLEDGE sponsors are ultimately responsible for this REMS program,” Dr. Frieden said.
According to the FDA, isotretinoin manufacturers are part of the iPLEDGE program. On the iPLEDGE website, 12 isotretinoin products are listed, made by eight different companies.
One dermatologist maneuvering the new website who registered successfully as a provider told this news organization that he received a follow-up survey from United BioSource about the new website. This news organization contacted that company to confirm it runs the website but has not yet received a response.
Meanwhile, dermatologists continue to help frustrated patients cope with the new website and registration details. Neil S. Goldberg, MD, a dermatologist in Westchester County, New York, heard from two mothers who helped their teen daughters complete the forms by attesting they would use abstinence as contraception but then couldn’t figure out how to answer another question. As a result, their answers were interpreted as the patients saying they were using abstinence but didn’t commit to not having sexual contact with a partner capable of impregnating them. So Dr. Goldberg got an automated message back from the iPLEDGE program that the answers were a mismatch.
And in the comments section following a previous story on the problematic rollout, one reader offered a suggestion for reducing hold times to the call center: choose the Spanish option.
Dr. Frieden and Dr. Goldberg have no relevant disclosures.
A version of this article first appeared on Medscape.com.