User login
Treat acne aggressively upfront, expert advises
First, determine the types of lesions they have. “Do they have comedones, papules/pustules, and nodules present?” she asked during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. Second, quantify the number of lesions that they have. Is it few? Several? Many? Third, determine the extent of their acne. “Is it limited to half the face, or is it generalized to the face, back, chest, and shoulders?” added Dr. Zaenglein, professor of dermatology and pediatrics at Penn State University, Hershey.
Fourth, identify postinflammatory changes such as erythema, hyperpigmentation, and scarring “because that’s going to influence your management,” she said. “Finally, you want to give a quick investigative global assessment of the acne severity where you quantify them as being clear, almost clear, mild, moderate, or severe. You want to do this with each patient at every visit so you can determine what their initial treatment’s going to be and what their management going forward is going to be.”
According to Dr. Zaenglein, the best acne treatments are based on the pathogenesis of the skin condition and trying to target as many pathogenic factors as possible. The four main pathogenic factors in acne include hyperkeratinization, increased sebum production, cutibacterium, and inflammation. “This is not a stepwise process; there’s an interplay between all of those factors,” she said. “All acne is inflammatory, but each of the treatments we have target specific factors. Retinoids target hyperkeratinization and inflammation, whereas the hormonal therapies will address decreased sebum production. Antimicrobial agents like benzoyl peroxide and antibiotics will work to decrease cutibacterium acnes. All of these are influenced by the exposome. This includes your genetics, external factors like pollution or changes in seasons that can affect your skin and the severity of your acne.” A state of hyperandrogenism, she added, “can definitely increase acne” and is seen in patients with polycystic ovary syndrome (PCOS).
For patients with mild acne, initial treatment should consist of a topical retinoid and, almost always, benzoyl peroxide, “unless it’s a pure comedonal form of acne,” Dr. Zaenglein said. She recommended using the combination of a topical retinoid and benzoyl peroxide, noting that while it used to be difficult to find benzoyl peroxide, “nowadays there are numerous manufacturers and different formulations of benzoyl peroxide. We also have over-the-counter adapalene now, which is great. So now we have a complete routine for patients with adapalene and benzoyl peroxide that you can combine together in a cost-effective way.”
If the initial regimen fails to improve the patient’s mild acne, a second-line treatment would be to change the retinoid and continue on the existing benzoyl peroxide formulation or to add dapsone gel if the patient is experiencing skin irritation. The four retinoids currently available include adapalene, tretinoin, tazarotene, and trifarotene. “These normalize keratinocyte differentiation, reduce keratinocyte proliferation, and decrease expression of inflammatory markers,” Dr. Zaenglein noted. “They also prevent scarring. Adapalene is considered to be the most tolerable, whereas tazarotene may have an edge on efficacy. There’s a lot of overlap; head-to-head studies may not always match them up exactly, but generally this is how it’s considered. Picking the right retinoid for your patient based on efficacy and tolerability is most important.”
The newest topical retinoid, trifarotene 50 mcg/g cream, is a fourth-generation retinoid which is retinoic acid receptor gamma selective. Pivotal trials were conducted in patients aged 9 years and older with moderate facial and truncal acne. With monotherapy there was a success rate of 36% at 12 weeks and 60% at 52 weeks based on the Investigator’s Global Assessment. Another newcomer, tazarotene 0.045% lotion, is a third-generation retinoid which is retinoic acid receptor alpha beta gamma selective. It’s approved for moderate to severe facial acne in patients 9 years and older.
To optimize tolerance to retinoids, Dr. Zaenglein asks patients about their typical skin care regimen. “I ask them what they’re washing their face with,” she said. “Are they using apricot scrubs or harsh cleansers? Make sure they’re applying it to the entire face and not spot-treating. You get less irritation when it’s applied to dry skin, so you can recommend that. Make sure that they use a bland unscented moisturizer in the morning and apply it over top of their retinoid. I always warn them that irritation usually peaks at about 2 weeks. If they can power through, the irritation will improve with continued use.”
To optimize adherence to retinoids, she asks patients how many nights per week that they apply it. If they are using it all seven nights, “they’re good at using it,” she said. “If they say three nights, then they need to work on getting it on more frequently.”
Topical dapsone gel (5% and 7.5%) is mainly used for patients with papular-pustular acne. “Its mechanism of action for acne is not known, but presumptively it’s anti-inflammatory,” Dr. Zaenglein said. “It doesn’t require G6PD [glucose-6-phosphate dehydrogenase] testing. It can cause some orange discoloration of your skin or fabrics if you use it with benzoyl peroxide, so you want to apply them at different times of the day. It’s well tolerated. I tend to use it in patients who have problems tolerating any topical retinoid or any benzoyl peroxide but have mild to moderate acne.”
For patients with moderate acne, consider combination therapy to target as many pathogenic factors as possible. “Use a topical retinoid plus benzoyl peroxide with or without a systemic antibiotic,” Dr. Zaenglein advised. “I may give them an oral antibiotic if their acne is not responsive to the routine. But you wouldn’t want to combine the systemic antibiotic with a topical antibiotic, like clindamycin with doxycycline, because you don’t need two antibiotics. Make sure that you treat aggressively up front. It can take up to 3 months to see improvement. I counsel my patients that we’ll rescue with the antibiotic and then we maintain, but we’re going to stop that antibiotic after 3 months.”
Systemic antibiotic options for acne include tetracyclines, doxycycline, minocycline, and sarecycline. “Tetracycline itself we don’t use too much because you have to take it on an empty stomach, and availability is sometimes an issue,” she said. “Primarily, we use doxycycline. You can take it with food, so that helps. The main side effects are gastrointestinal upset and photosensitivity. Alternately, you can use minocycline, which is also okay to take with food. It does have more potentially worrisome side effects, including pseudotumor cerebri, blue pigmentation, autoimmune hepatitis, and DRESS [drug reaction with eosinophilia and systemic symptoms].”
Sarecycline is the first narrow spectrum tetracycline for acne, with fewer vestibular and phototoxic side effects, compared with other tetracyclines. “It also has less effect on the GI flora,” Dr. Zaenglein said. “It’s a good alternative but it can be costly, so make sure to check the pricing for your patients.” She does not use other antibiotics such as TMP/SMX, penicillins, or cephalosporins for acne patients. “The reason is, the tetracyclines are not only antibacterial, but they’re anti-inflammatory,” she explained. “They also are lipophilic, so they will penetrate into the sebaceous unit where the heart of the acne is.”
For patients who don’t want to take an oral antibiotic, consider minocycline 4% foam, which was studied in moderate to severe acne in patients aged 9 years and older. The pooled results from the three studies showed a 47% mean improvement in inflammatory acne, compared with 37% among those in the vehicle arm. “You wouldn’t use this as monotherapy; you’d use this in combination with the topical retinoid and the benzoyl peroxide,” Dr. Zaenglein said.
Most primary care providers do not prescribe isotretinoin for patients with severe acne, but they can start patients on triple therapy with a topical retinoid, benzoyl peroxide, and a systemic antibiotic at its full dose. “The efficacy of triple therapy in patients you would typically deem as isotretinoin worthy is actually pretty good,” she said. “There have been several studies looking at this, and about 70%-80% of patients will respond to triple therapy, where they are no longer deemed isotretinoin candidates. They still may need to move on to isotretinoin, but they will be improved.”
Dr. Zaenglein disclosed that she is a consultant for Cassiopea, Novartis, and Pfizer. She has also received grants or research support from AbbVie, Incyte, and Pfizer.
First, determine the types of lesions they have. “Do they have comedones, papules/pustules, and nodules present?” she asked during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. Second, quantify the number of lesions that they have. Is it few? Several? Many? Third, determine the extent of their acne. “Is it limited to half the face, or is it generalized to the face, back, chest, and shoulders?” added Dr. Zaenglein, professor of dermatology and pediatrics at Penn State University, Hershey.
Fourth, identify postinflammatory changes such as erythema, hyperpigmentation, and scarring “because that’s going to influence your management,” she said. “Finally, you want to give a quick investigative global assessment of the acne severity where you quantify them as being clear, almost clear, mild, moderate, or severe. You want to do this with each patient at every visit so you can determine what their initial treatment’s going to be and what their management going forward is going to be.”
According to Dr. Zaenglein, the best acne treatments are based on the pathogenesis of the skin condition and trying to target as many pathogenic factors as possible. The four main pathogenic factors in acne include hyperkeratinization, increased sebum production, cutibacterium, and inflammation. “This is not a stepwise process; there’s an interplay between all of those factors,” she said. “All acne is inflammatory, but each of the treatments we have target specific factors. Retinoids target hyperkeratinization and inflammation, whereas the hormonal therapies will address decreased sebum production. Antimicrobial agents like benzoyl peroxide and antibiotics will work to decrease cutibacterium acnes. All of these are influenced by the exposome. This includes your genetics, external factors like pollution or changes in seasons that can affect your skin and the severity of your acne.” A state of hyperandrogenism, she added, “can definitely increase acne” and is seen in patients with polycystic ovary syndrome (PCOS).
For patients with mild acne, initial treatment should consist of a topical retinoid and, almost always, benzoyl peroxide, “unless it’s a pure comedonal form of acne,” Dr. Zaenglein said. She recommended using the combination of a topical retinoid and benzoyl peroxide, noting that while it used to be difficult to find benzoyl peroxide, “nowadays there are numerous manufacturers and different formulations of benzoyl peroxide. We also have over-the-counter adapalene now, which is great. So now we have a complete routine for patients with adapalene and benzoyl peroxide that you can combine together in a cost-effective way.”
If the initial regimen fails to improve the patient’s mild acne, a second-line treatment would be to change the retinoid and continue on the existing benzoyl peroxide formulation or to add dapsone gel if the patient is experiencing skin irritation. The four retinoids currently available include adapalene, tretinoin, tazarotene, and trifarotene. “These normalize keratinocyte differentiation, reduce keratinocyte proliferation, and decrease expression of inflammatory markers,” Dr. Zaenglein noted. “They also prevent scarring. Adapalene is considered to be the most tolerable, whereas tazarotene may have an edge on efficacy. There’s a lot of overlap; head-to-head studies may not always match them up exactly, but generally this is how it’s considered. Picking the right retinoid for your patient based on efficacy and tolerability is most important.”
The newest topical retinoid, trifarotene 50 mcg/g cream, is a fourth-generation retinoid which is retinoic acid receptor gamma selective. Pivotal trials were conducted in patients aged 9 years and older with moderate facial and truncal acne. With monotherapy there was a success rate of 36% at 12 weeks and 60% at 52 weeks based on the Investigator’s Global Assessment. Another newcomer, tazarotene 0.045% lotion, is a third-generation retinoid which is retinoic acid receptor alpha beta gamma selective. It’s approved for moderate to severe facial acne in patients 9 years and older.
To optimize tolerance to retinoids, Dr. Zaenglein asks patients about their typical skin care regimen. “I ask them what they’re washing their face with,” she said. “Are they using apricot scrubs or harsh cleansers? Make sure they’re applying it to the entire face and not spot-treating. You get less irritation when it’s applied to dry skin, so you can recommend that. Make sure that they use a bland unscented moisturizer in the morning and apply it over top of their retinoid. I always warn them that irritation usually peaks at about 2 weeks. If they can power through, the irritation will improve with continued use.”
To optimize adherence to retinoids, she asks patients how many nights per week that they apply it. If they are using it all seven nights, “they’re good at using it,” she said. “If they say three nights, then they need to work on getting it on more frequently.”
Topical dapsone gel (5% and 7.5%) is mainly used for patients with papular-pustular acne. “Its mechanism of action for acne is not known, but presumptively it’s anti-inflammatory,” Dr. Zaenglein said. “It doesn’t require G6PD [glucose-6-phosphate dehydrogenase] testing. It can cause some orange discoloration of your skin or fabrics if you use it with benzoyl peroxide, so you want to apply them at different times of the day. It’s well tolerated. I tend to use it in patients who have problems tolerating any topical retinoid or any benzoyl peroxide but have mild to moderate acne.”
For patients with moderate acne, consider combination therapy to target as many pathogenic factors as possible. “Use a topical retinoid plus benzoyl peroxide with or without a systemic antibiotic,” Dr. Zaenglein advised. “I may give them an oral antibiotic if their acne is not responsive to the routine. But you wouldn’t want to combine the systemic antibiotic with a topical antibiotic, like clindamycin with doxycycline, because you don’t need two antibiotics. Make sure that you treat aggressively up front. It can take up to 3 months to see improvement. I counsel my patients that we’ll rescue with the antibiotic and then we maintain, but we’re going to stop that antibiotic after 3 months.”
Systemic antibiotic options for acne include tetracyclines, doxycycline, minocycline, and sarecycline. “Tetracycline itself we don’t use too much because you have to take it on an empty stomach, and availability is sometimes an issue,” she said. “Primarily, we use doxycycline. You can take it with food, so that helps. The main side effects are gastrointestinal upset and photosensitivity. Alternately, you can use minocycline, which is also okay to take with food. It does have more potentially worrisome side effects, including pseudotumor cerebri, blue pigmentation, autoimmune hepatitis, and DRESS [drug reaction with eosinophilia and systemic symptoms].”
Sarecycline is the first narrow spectrum tetracycline for acne, with fewer vestibular and phototoxic side effects, compared with other tetracyclines. “It also has less effect on the GI flora,” Dr. Zaenglein said. “It’s a good alternative but it can be costly, so make sure to check the pricing for your patients.” She does not use other antibiotics such as TMP/SMX, penicillins, or cephalosporins for acne patients. “The reason is, the tetracyclines are not only antibacterial, but they’re anti-inflammatory,” she explained. “They also are lipophilic, so they will penetrate into the sebaceous unit where the heart of the acne is.”
For patients who don’t want to take an oral antibiotic, consider minocycline 4% foam, which was studied in moderate to severe acne in patients aged 9 years and older. The pooled results from the three studies showed a 47% mean improvement in inflammatory acne, compared with 37% among those in the vehicle arm. “You wouldn’t use this as monotherapy; you’d use this in combination with the topical retinoid and the benzoyl peroxide,” Dr. Zaenglein said.
Most primary care providers do not prescribe isotretinoin for patients with severe acne, but they can start patients on triple therapy with a topical retinoid, benzoyl peroxide, and a systemic antibiotic at its full dose. “The efficacy of triple therapy in patients you would typically deem as isotretinoin worthy is actually pretty good,” she said. “There have been several studies looking at this, and about 70%-80% of patients will respond to triple therapy, where they are no longer deemed isotretinoin candidates. They still may need to move on to isotretinoin, but they will be improved.”
Dr. Zaenglein disclosed that she is a consultant for Cassiopea, Novartis, and Pfizer. She has also received grants or research support from AbbVie, Incyte, and Pfizer.
First, determine the types of lesions they have. “Do they have comedones, papules/pustules, and nodules present?” she asked during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. Second, quantify the number of lesions that they have. Is it few? Several? Many? Third, determine the extent of their acne. “Is it limited to half the face, or is it generalized to the face, back, chest, and shoulders?” added Dr. Zaenglein, professor of dermatology and pediatrics at Penn State University, Hershey.
Fourth, identify postinflammatory changes such as erythema, hyperpigmentation, and scarring “because that’s going to influence your management,” she said. “Finally, you want to give a quick investigative global assessment of the acne severity where you quantify them as being clear, almost clear, mild, moderate, or severe. You want to do this with each patient at every visit so you can determine what their initial treatment’s going to be and what their management going forward is going to be.”
According to Dr. Zaenglein, the best acne treatments are based on the pathogenesis of the skin condition and trying to target as many pathogenic factors as possible. The four main pathogenic factors in acne include hyperkeratinization, increased sebum production, cutibacterium, and inflammation. “This is not a stepwise process; there’s an interplay between all of those factors,” she said. “All acne is inflammatory, but each of the treatments we have target specific factors. Retinoids target hyperkeratinization and inflammation, whereas the hormonal therapies will address decreased sebum production. Antimicrobial agents like benzoyl peroxide and antibiotics will work to decrease cutibacterium acnes. All of these are influenced by the exposome. This includes your genetics, external factors like pollution or changes in seasons that can affect your skin and the severity of your acne.” A state of hyperandrogenism, she added, “can definitely increase acne” and is seen in patients with polycystic ovary syndrome (PCOS).
For patients with mild acne, initial treatment should consist of a topical retinoid and, almost always, benzoyl peroxide, “unless it’s a pure comedonal form of acne,” Dr. Zaenglein said. She recommended using the combination of a topical retinoid and benzoyl peroxide, noting that while it used to be difficult to find benzoyl peroxide, “nowadays there are numerous manufacturers and different formulations of benzoyl peroxide. We also have over-the-counter adapalene now, which is great. So now we have a complete routine for patients with adapalene and benzoyl peroxide that you can combine together in a cost-effective way.”
If the initial regimen fails to improve the patient’s mild acne, a second-line treatment would be to change the retinoid and continue on the existing benzoyl peroxide formulation or to add dapsone gel if the patient is experiencing skin irritation. The four retinoids currently available include adapalene, tretinoin, tazarotene, and trifarotene. “These normalize keratinocyte differentiation, reduce keratinocyte proliferation, and decrease expression of inflammatory markers,” Dr. Zaenglein noted. “They also prevent scarring. Adapalene is considered to be the most tolerable, whereas tazarotene may have an edge on efficacy. There’s a lot of overlap; head-to-head studies may not always match them up exactly, but generally this is how it’s considered. Picking the right retinoid for your patient based on efficacy and tolerability is most important.”
The newest topical retinoid, trifarotene 50 mcg/g cream, is a fourth-generation retinoid which is retinoic acid receptor gamma selective. Pivotal trials were conducted in patients aged 9 years and older with moderate facial and truncal acne. With monotherapy there was a success rate of 36% at 12 weeks and 60% at 52 weeks based on the Investigator’s Global Assessment. Another newcomer, tazarotene 0.045% lotion, is a third-generation retinoid which is retinoic acid receptor alpha beta gamma selective. It’s approved for moderate to severe facial acne in patients 9 years and older.
To optimize tolerance to retinoids, Dr. Zaenglein asks patients about their typical skin care regimen. “I ask them what they’re washing their face with,” she said. “Are they using apricot scrubs or harsh cleansers? Make sure they’re applying it to the entire face and not spot-treating. You get less irritation when it’s applied to dry skin, so you can recommend that. Make sure that they use a bland unscented moisturizer in the morning and apply it over top of their retinoid. I always warn them that irritation usually peaks at about 2 weeks. If they can power through, the irritation will improve with continued use.”
To optimize adherence to retinoids, she asks patients how many nights per week that they apply it. If they are using it all seven nights, “they’re good at using it,” she said. “If they say three nights, then they need to work on getting it on more frequently.”
Topical dapsone gel (5% and 7.5%) is mainly used for patients with papular-pustular acne. “Its mechanism of action for acne is not known, but presumptively it’s anti-inflammatory,” Dr. Zaenglein said. “It doesn’t require G6PD [glucose-6-phosphate dehydrogenase] testing. It can cause some orange discoloration of your skin or fabrics if you use it with benzoyl peroxide, so you want to apply them at different times of the day. It’s well tolerated. I tend to use it in patients who have problems tolerating any topical retinoid or any benzoyl peroxide but have mild to moderate acne.”
For patients with moderate acne, consider combination therapy to target as many pathogenic factors as possible. “Use a topical retinoid plus benzoyl peroxide with or without a systemic antibiotic,” Dr. Zaenglein advised. “I may give them an oral antibiotic if their acne is not responsive to the routine. But you wouldn’t want to combine the systemic antibiotic with a topical antibiotic, like clindamycin with doxycycline, because you don’t need two antibiotics. Make sure that you treat aggressively up front. It can take up to 3 months to see improvement. I counsel my patients that we’ll rescue with the antibiotic and then we maintain, but we’re going to stop that antibiotic after 3 months.”
Systemic antibiotic options for acne include tetracyclines, doxycycline, minocycline, and sarecycline. “Tetracycline itself we don’t use too much because you have to take it on an empty stomach, and availability is sometimes an issue,” she said. “Primarily, we use doxycycline. You can take it with food, so that helps. The main side effects are gastrointestinal upset and photosensitivity. Alternately, you can use minocycline, which is also okay to take with food. It does have more potentially worrisome side effects, including pseudotumor cerebri, blue pigmentation, autoimmune hepatitis, and DRESS [drug reaction with eosinophilia and systemic symptoms].”
Sarecycline is the first narrow spectrum tetracycline for acne, with fewer vestibular and phototoxic side effects, compared with other tetracyclines. “It also has less effect on the GI flora,” Dr. Zaenglein said. “It’s a good alternative but it can be costly, so make sure to check the pricing for your patients.” She does not use other antibiotics such as TMP/SMX, penicillins, or cephalosporins for acne patients. “The reason is, the tetracyclines are not only antibacterial, but they’re anti-inflammatory,” she explained. “They also are lipophilic, so they will penetrate into the sebaceous unit where the heart of the acne is.”
For patients who don’t want to take an oral antibiotic, consider minocycline 4% foam, which was studied in moderate to severe acne in patients aged 9 years and older. The pooled results from the three studies showed a 47% mean improvement in inflammatory acne, compared with 37% among those in the vehicle arm. “You wouldn’t use this as monotherapy; you’d use this in combination with the topical retinoid and the benzoyl peroxide,” Dr. Zaenglein said.
Most primary care providers do not prescribe isotretinoin for patients with severe acne, but they can start patients on triple therapy with a topical retinoid, benzoyl peroxide, and a systemic antibiotic at its full dose. “The efficacy of triple therapy in patients you would typically deem as isotretinoin worthy is actually pretty good,” she said. “There have been several studies looking at this, and about 70%-80% of patients will respond to triple therapy, where they are no longer deemed isotretinoin candidates. They still may need to move on to isotretinoin, but they will be improved.”
Dr. Zaenglein disclosed that she is a consultant for Cassiopea, Novartis, and Pfizer. She has also received grants or research support from AbbVie, Incyte, and Pfizer.
FROM PEDIATRIC DERMATOLOGY 2020
Trifarotene sails through 52-week acne trial
James Q. Del Rosso, MD, reported at the virtual annual meeting of the American Academy of Dermatology.
The study is noteworthy because, even though roughly half of patients with facial acne also have truncal acne, there is actually very little clinical trial data on the treatment of truncal acne other than this new long-term study and the two earlier pivotal phase 3, 12-week trials which led to the October 2019 approval of trifarotene 50 mcg/g cream (Aklief) as the first novel retinoid for acne to reach the market in 20 years, observed Dr. Del Rosso, research director at JDR Research in Las Vegas and a member of the dermatology faculty at Touro University in Henderson, Nev.
The 52-week study, known as SATISFY, began with 454 patients with moderate facial and truncal acne who treated themselves with trifarotene once daily. Among the 348 patients who completed the full year, 67% achieved a score of 0 or 1 – clear or almost clear – with at least a 2-grade improvement from baseline by Investigator’s Global Assessment on their facial acne, and 65% met the same measure of success on the trunk. Moreover, 58% of patients met that standard at both acne sites.
The IGA success rate rose throughout the study period without ever reaching a plateau. However, it should be noted that 23% of participants dropped out of the study over the course of the year.
Mean tolerability scores reflecting redness, scaling, stinging or burning, and skin dryness remained well below the threshold for mild severity, peaking at weeks 2-4 of the study. The most common treatment-related adverse events were mild to moderate itching and irritation, each occurring in less than 5% of subjects.
Trifarotene is a first-in-class retinoid that specifically targets the retinoic acid receptor gamma, the most common cutaneous retinoic acid receptor.
Dr. Del Rosso reported serving as an investigator and consultant for Galderma, which sponsored the study and markets trifarotene cream.
James Q. Del Rosso, MD, reported at the virtual annual meeting of the American Academy of Dermatology.
The study is noteworthy because, even though roughly half of patients with facial acne also have truncal acne, there is actually very little clinical trial data on the treatment of truncal acne other than this new long-term study and the two earlier pivotal phase 3, 12-week trials which led to the October 2019 approval of trifarotene 50 mcg/g cream (Aklief) as the first novel retinoid for acne to reach the market in 20 years, observed Dr. Del Rosso, research director at JDR Research in Las Vegas and a member of the dermatology faculty at Touro University in Henderson, Nev.
The 52-week study, known as SATISFY, began with 454 patients with moderate facial and truncal acne who treated themselves with trifarotene once daily. Among the 348 patients who completed the full year, 67% achieved a score of 0 or 1 – clear or almost clear – with at least a 2-grade improvement from baseline by Investigator’s Global Assessment on their facial acne, and 65% met the same measure of success on the trunk. Moreover, 58% of patients met that standard at both acne sites.
The IGA success rate rose throughout the study period without ever reaching a plateau. However, it should be noted that 23% of participants dropped out of the study over the course of the year.
Mean tolerability scores reflecting redness, scaling, stinging or burning, and skin dryness remained well below the threshold for mild severity, peaking at weeks 2-4 of the study. The most common treatment-related adverse events were mild to moderate itching and irritation, each occurring in less than 5% of subjects.
Trifarotene is a first-in-class retinoid that specifically targets the retinoic acid receptor gamma, the most common cutaneous retinoic acid receptor.
Dr. Del Rosso reported serving as an investigator and consultant for Galderma, which sponsored the study and markets trifarotene cream.
James Q. Del Rosso, MD, reported at the virtual annual meeting of the American Academy of Dermatology.
The study is noteworthy because, even though roughly half of patients with facial acne also have truncal acne, there is actually very little clinical trial data on the treatment of truncal acne other than this new long-term study and the two earlier pivotal phase 3, 12-week trials which led to the October 2019 approval of trifarotene 50 mcg/g cream (Aklief) as the first novel retinoid for acne to reach the market in 20 years, observed Dr. Del Rosso, research director at JDR Research in Las Vegas and a member of the dermatology faculty at Touro University in Henderson, Nev.
The 52-week study, known as SATISFY, began with 454 patients with moderate facial and truncal acne who treated themselves with trifarotene once daily. Among the 348 patients who completed the full year, 67% achieved a score of 0 or 1 – clear or almost clear – with at least a 2-grade improvement from baseline by Investigator’s Global Assessment on their facial acne, and 65% met the same measure of success on the trunk. Moreover, 58% of patients met that standard at both acne sites.
The IGA success rate rose throughout the study period without ever reaching a plateau. However, it should be noted that 23% of participants dropped out of the study over the course of the year.
Mean tolerability scores reflecting redness, scaling, stinging or burning, and skin dryness remained well below the threshold for mild severity, peaking at weeks 2-4 of the study. The most common treatment-related adverse events were mild to moderate itching and irritation, each occurring in less than 5% of subjects.
Trifarotene is a first-in-class retinoid that specifically targets the retinoic acid receptor gamma, the most common cutaneous retinoic acid receptor.
Dr. Del Rosso reported serving as an investigator and consultant for Galderma, which sponsored the study and markets trifarotene cream.
FROM AAD 2020
Daily Recap 6/17
Here are the stories our MDedge editors across specialties think you need to know about today:
Comorbidities increase COVID-19 deaths by factor of 12
COVID-19 patients with an underlying condition are 6 times as likely to be hospitalized and 12 times as likely to die, compared with those who have no such condition, according to the CDC.
The most frequently reported underlying conditions were cardiovascular disease (32%), diabetes (30%), chronic lung disease (18%), and renal disease (7.6%), and there were no significant differences between males and females.
The pandemic “continues to affect all populations and result in severe outcomes including death,” noted the CDC, emphasizing “the continued need for community mitigation strategies, especially for vulnerable populations, to slow COVID-19 transmission.” Read more.
Preventive services coalition recommends routine anxiety screening for women
Women and girls aged 13 years and older with no current diagnosis of anxiety should be screened routinely for anxiety, according to a new recommendation from the Women’s Preventive Services Initiative.
The lifetime prevalence of anxiety disorders in women in the United States is 40%, approximately twice that of men, and anxiety can be a manifestation of underlying issues including posttraumatic stress, sexual harassment, and assault.
“The WPSI based its rationale for anxiety screening on several considerations,” the researchers noted. “Anxiety disorders are the most prevalent mental health disorders in women, and the problems created by untreated anxiety can impair function in all areas of a woman’s life.” Read more.
High-fat, high-sugar diet may promote adult acne
A diet higher in fat, sugar, and milk was associated with having acne in a cross-sectional study of approximately 24,000 adults in France.
Although acne patients may believe that eating certain foods exacerbates acne, data on the effects of nutrition on acne, including associations between acne and a high-glycemic diet, are limited and have produced conflicting results, noted investigators.
“The results of our study appear to support the hypothesis that the Western diet (rich in animal products and fatty and sugary foods) is associated with the presence of acne in adulthood,” the researchers concluded.
Population study supports migraine-dementia link
Preliminary results from a population-based cohort study support previous reports that migraine is a midlife risk factor for dementia later in life, but further determined that migraine with aura and frequent hospital contacts significantly increased dementia risk after age 60 years, according to results from a Danish registry presented at the virtual annual meeting of the American Headache Society.
The preliminary findings revealed that the median age at diagnosis was 49 years and about 70% of the migraine population were women. “There was a 50% higher dementia rate in individuals who had any migraine diagnosis,” Dr. Islamoska said.
“To the best of our knowledge, no previous national register–based studies have investigated the risk of dementia among individuals who suffer from migraine with aura,” Dr. Sabrina Islamoska said.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Comorbidities increase COVID-19 deaths by factor of 12
COVID-19 patients with an underlying condition are 6 times as likely to be hospitalized and 12 times as likely to die, compared with those who have no such condition, according to the CDC.
The most frequently reported underlying conditions were cardiovascular disease (32%), diabetes (30%), chronic lung disease (18%), and renal disease (7.6%), and there were no significant differences between males and females.
The pandemic “continues to affect all populations and result in severe outcomes including death,” noted the CDC, emphasizing “the continued need for community mitigation strategies, especially for vulnerable populations, to slow COVID-19 transmission.” Read more.
Preventive services coalition recommends routine anxiety screening for women
Women and girls aged 13 years and older with no current diagnosis of anxiety should be screened routinely for anxiety, according to a new recommendation from the Women’s Preventive Services Initiative.
The lifetime prevalence of anxiety disorders in women in the United States is 40%, approximately twice that of men, and anxiety can be a manifestation of underlying issues including posttraumatic stress, sexual harassment, and assault.
“The WPSI based its rationale for anxiety screening on several considerations,” the researchers noted. “Anxiety disorders are the most prevalent mental health disorders in women, and the problems created by untreated anxiety can impair function in all areas of a woman’s life.” Read more.
High-fat, high-sugar diet may promote adult acne
A diet higher in fat, sugar, and milk was associated with having acne in a cross-sectional study of approximately 24,000 adults in France.
Although acne patients may believe that eating certain foods exacerbates acne, data on the effects of nutrition on acne, including associations between acne and a high-glycemic diet, are limited and have produced conflicting results, noted investigators.
“The results of our study appear to support the hypothesis that the Western diet (rich in animal products and fatty and sugary foods) is associated with the presence of acne in adulthood,” the researchers concluded.
Population study supports migraine-dementia link
Preliminary results from a population-based cohort study support previous reports that migraine is a midlife risk factor for dementia later in life, but further determined that migraine with aura and frequent hospital contacts significantly increased dementia risk after age 60 years, according to results from a Danish registry presented at the virtual annual meeting of the American Headache Society.
The preliminary findings revealed that the median age at diagnosis was 49 years and about 70% of the migraine population were women. “There was a 50% higher dementia rate in individuals who had any migraine diagnosis,” Dr. Islamoska said.
“To the best of our knowledge, no previous national register–based studies have investigated the risk of dementia among individuals who suffer from migraine with aura,” Dr. Sabrina Islamoska said.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Comorbidities increase COVID-19 deaths by factor of 12
COVID-19 patients with an underlying condition are 6 times as likely to be hospitalized and 12 times as likely to die, compared with those who have no such condition, according to the CDC.
The most frequently reported underlying conditions were cardiovascular disease (32%), diabetes (30%), chronic lung disease (18%), and renal disease (7.6%), and there were no significant differences between males and females.
The pandemic “continues to affect all populations and result in severe outcomes including death,” noted the CDC, emphasizing “the continued need for community mitigation strategies, especially for vulnerable populations, to slow COVID-19 transmission.” Read more.
Preventive services coalition recommends routine anxiety screening for women
Women and girls aged 13 years and older with no current diagnosis of anxiety should be screened routinely for anxiety, according to a new recommendation from the Women’s Preventive Services Initiative.
The lifetime prevalence of anxiety disorders in women in the United States is 40%, approximately twice that of men, and anxiety can be a manifestation of underlying issues including posttraumatic stress, sexual harassment, and assault.
“The WPSI based its rationale for anxiety screening on several considerations,” the researchers noted. “Anxiety disorders are the most prevalent mental health disorders in women, and the problems created by untreated anxiety can impair function in all areas of a woman’s life.” Read more.
High-fat, high-sugar diet may promote adult acne
A diet higher in fat, sugar, and milk was associated with having acne in a cross-sectional study of approximately 24,000 adults in France.
Although acne patients may believe that eating certain foods exacerbates acne, data on the effects of nutrition on acne, including associations between acne and a high-glycemic diet, are limited and have produced conflicting results, noted investigators.
“The results of our study appear to support the hypothesis that the Western diet (rich in animal products and fatty and sugary foods) is associated with the presence of acne in adulthood,” the researchers concluded.
Population study supports migraine-dementia link
Preliminary results from a population-based cohort study support previous reports that migraine is a midlife risk factor for dementia later in life, but further determined that migraine with aura and frequent hospital contacts significantly increased dementia risk after age 60 years, according to results from a Danish registry presented at the virtual annual meeting of the American Headache Society.
The preliminary findings revealed that the median age at diagnosis was 49 years and about 70% of the migraine population were women. “There was a 50% higher dementia rate in individuals who had any migraine diagnosis,” Dr. Islamoska said.
“To the best of our knowledge, no previous national register–based studies have investigated the risk of dementia among individuals who suffer from migraine with aura,” Dr. Sabrina Islamoska said.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
High-fat, high-sugar diet may promote adult acne
A diet higher in fat, sugar, and milk was associated with having acne in a cross-sectional study of approximately 24,000 adults in France.
Acne in adults has been associated with social, emotional, and psychological consequences similar to those found with chronic diseases such as asthma, arthritis, epilepsy, and diabetes, wrote Laetitia Penso, MSc, of the University of Paris in Bobigny, France, and colleagues.
Although acne patients may believe that eating certain foods exacerbates acne, data on the effects of nutrition on acne, including associations between acne and a high-glycemic diet, are limited and have produced conflicting results, they noted.
In their study, published in JAMA Dermatology, the researchers identified 24,452 adults who participated in the NutriNet-Santé study, an ongoing, web-based study in France. Approximately 75% of the participants were women, the average age was 57 years, and 46% reported past or current acne.
Participants responded to an 11-item questionnaire between November 2008 and July 2019. Questions were related to the occurrence and diagnosis of acne, as well as medical history. Based on their acne status, participants were identified as falling into the categories of never acne, past acne, or current acne, and their dietary intake was assessed at baseline and every 6 months using three nonconsecutive 24-hour dietary records for 2 weekdays and 1 weekend day.
In an analysis, after adjustment for confounders, current acne was significantly associated with consumption of fatty and sugary foods (per portion, adjusted odds ratio, 1.54; P = .01), as well as with consumption of sugary drinks (per glass, aOR, 1.18; P = .04) and milk (per glass, aOR, 1.12; P = .04). In addition, carbohydrate intake and saturated fatty acid intake were significantly associated with current acne (aOR, 1.43; P = .02; and aOR, 3.90; P = .048, respectively).
Three dietary patterns accounted for 42% of the total variability, the researchers said. A healthy pattern of higher fruit, vegetable, and fish intake accounted for 18%, a fatty and sugary pattern of higher fat and sugar intake (including chocolate) accounted for 13%, and an animal product and cereal pattern of higher intake of meat, milk, and refined cereals accounted for 11%, they explained.
“The results of our study appear to support the hypothesis that the Western diet (rich in animal products and fatty and sugary foods) is associated with the presence of acne in adulthood,” the researchers concluded. Possible explanations for the findings include the effects of a high glycemic-load diet on circulating IGF-1 and insulin, which ultimately increases both oxidative stress and inflammation that promotes the development of acne, they noted.
The study findings were limited by several factors including the use of relatively homogenous younger and female patient population and the reliance on self-reported acne, as well as the observational design, which did not allow for identification of direct, causal associations between diet and acne, the researchers noted. Larger studies are needed to examine the relationship between diet and adult acne to inform prevention and treatment, they wrote.
“Much of the previous literature on the role of diet in acne has focused on the association of milk consumption and high glycemic-load diet with acne,” John S. Barbieri, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, wrote in an accompanying editorial.
Dr. Barbieri acknowledged the inability to make causal associations given the study design and noted that dietary interventions should be implemented with caution because of the potential for other effects such as reduced calcium or vitamin D.
“Nevertheless, given the potential overall health benefits of a healthy or low glycemic-load diet, and 2 small trials supporting its effectiveness in acne, a low glycemic-load diet is a reasonable recommendation for patients looking for dietary modifications that may improve their acne,” he said.
Dr. Barbieri said that he was encouraged to see that the study findings reflected previous research identifying an association between acne and high-glycemic load foods, as well as milk consumption, but he emphasized that more research is needed before general recommendations about diet and acne can be made.
“Trials are needed to evaluate whether dietary interventions can improve or prevent acne and how the effect size of such interventions compares with other standard treatment modalities,” he emphasized.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Barbieri disclosed support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health and from a Pfizer Fellowship grant to the Trustees of the University of Pennsylvania.
SOURCE: Penso L et al. JAMA Dermatol. 2020 June 10. doi: 10.1001/jamadermatol.2020.1602.
A diet higher in fat, sugar, and milk was associated with having acne in a cross-sectional study of approximately 24,000 adults in France.
Acne in adults has been associated with social, emotional, and psychological consequences similar to those found with chronic diseases such as asthma, arthritis, epilepsy, and diabetes, wrote Laetitia Penso, MSc, of the University of Paris in Bobigny, France, and colleagues.
Although acne patients may believe that eating certain foods exacerbates acne, data on the effects of nutrition on acne, including associations between acne and a high-glycemic diet, are limited and have produced conflicting results, they noted.
In their study, published in JAMA Dermatology, the researchers identified 24,452 adults who participated in the NutriNet-Santé study, an ongoing, web-based study in France. Approximately 75% of the participants were women, the average age was 57 years, and 46% reported past or current acne.
Participants responded to an 11-item questionnaire between November 2008 and July 2019. Questions were related to the occurrence and diagnosis of acne, as well as medical history. Based on their acne status, participants were identified as falling into the categories of never acne, past acne, or current acne, and their dietary intake was assessed at baseline and every 6 months using three nonconsecutive 24-hour dietary records for 2 weekdays and 1 weekend day.
In an analysis, after adjustment for confounders, current acne was significantly associated with consumption of fatty and sugary foods (per portion, adjusted odds ratio, 1.54; P = .01), as well as with consumption of sugary drinks (per glass, aOR, 1.18; P = .04) and milk (per glass, aOR, 1.12; P = .04). In addition, carbohydrate intake and saturated fatty acid intake were significantly associated with current acne (aOR, 1.43; P = .02; and aOR, 3.90; P = .048, respectively).
Three dietary patterns accounted for 42% of the total variability, the researchers said. A healthy pattern of higher fruit, vegetable, and fish intake accounted for 18%, a fatty and sugary pattern of higher fat and sugar intake (including chocolate) accounted for 13%, and an animal product and cereal pattern of higher intake of meat, milk, and refined cereals accounted for 11%, they explained.
“The results of our study appear to support the hypothesis that the Western diet (rich in animal products and fatty and sugary foods) is associated with the presence of acne in adulthood,” the researchers concluded. Possible explanations for the findings include the effects of a high glycemic-load diet on circulating IGF-1 and insulin, which ultimately increases both oxidative stress and inflammation that promotes the development of acne, they noted.
The study findings were limited by several factors including the use of relatively homogenous younger and female patient population and the reliance on self-reported acne, as well as the observational design, which did not allow for identification of direct, causal associations between diet and acne, the researchers noted. Larger studies are needed to examine the relationship between diet and adult acne to inform prevention and treatment, they wrote.
“Much of the previous literature on the role of diet in acne has focused on the association of milk consumption and high glycemic-load diet with acne,” John S. Barbieri, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, wrote in an accompanying editorial.
Dr. Barbieri acknowledged the inability to make causal associations given the study design and noted that dietary interventions should be implemented with caution because of the potential for other effects such as reduced calcium or vitamin D.
“Nevertheless, given the potential overall health benefits of a healthy or low glycemic-load diet, and 2 small trials supporting its effectiveness in acne, a low glycemic-load diet is a reasonable recommendation for patients looking for dietary modifications that may improve their acne,” he said.
Dr. Barbieri said that he was encouraged to see that the study findings reflected previous research identifying an association between acne and high-glycemic load foods, as well as milk consumption, but he emphasized that more research is needed before general recommendations about diet and acne can be made.
“Trials are needed to evaluate whether dietary interventions can improve or prevent acne and how the effect size of such interventions compares with other standard treatment modalities,” he emphasized.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Barbieri disclosed support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health and from a Pfizer Fellowship grant to the Trustees of the University of Pennsylvania.
SOURCE: Penso L et al. JAMA Dermatol. 2020 June 10. doi: 10.1001/jamadermatol.2020.1602.
A diet higher in fat, sugar, and milk was associated with having acne in a cross-sectional study of approximately 24,000 adults in France.
Acne in adults has been associated with social, emotional, and psychological consequences similar to those found with chronic diseases such as asthma, arthritis, epilepsy, and diabetes, wrote Laetitia Penso, MSc, of the University of Paris in Bobigny, France, and colleagues.
Although acne patients may believe that eating certain foods exacerbates acne, data on the effects of nutrition on acne, including associations between acne and a high-glycemic diet, are limited and have produced conflicting results, they noted.
In their study, published in JAMA Dermatology, the researchers identified 24,452 adults who participated in the NutriNet-Santé study, an ongoing, web-based study in France. Approximately 75% of the participants were women, the average age was 57 years, and 46% reported past or current acne.
Participants responded to an 11-item questionnaire between November 2008 and July 2019. Questions were related to the occurrence and diagnosis of acne, as well as medical history. Based on their acne status, participants were identified as falling into the categories of never acne, past acne, or current acne, and their dietary intake was assessed at baseline and every 6 months using three nonconsecutive 24-hour dietary records for 2 weekdays and 1 weekend day.
In an analysis, after adjustment for confounders, current acne was significantly associated with consumption of fatty and sugary foods (per portion, adjusted odds ratio, 1.54; P = .01), as well as with consumption of sugary drinks (per glass, aOR, 1.18; P = .04) and milk (per glass, aOR, 1.12; P = .04). In addition, carbohydrate intake and saturated fatty acid intake were significantly associated with current acne (aOR, 1.43; P = .02; and aOR, 3.90; P = .048, respectively).
Three dietary patterns accounted for 42% of the total variability, the researchers said. A healthy pattern of higher fruit, vegetable, and fish intake accounted for 18%, a fatty and sugary pattern of higher fat and sugar intake (including chocolate) accounted for 13%, and an animal product and cereal pattern of higher intake of meat, milk, and refined cereals accounted for 11%, they explained.
“The results of our study appear to support the hypothesis that the Western diet (rich in animal products and fatty and sugary foods) is associated with the presence of acne in adulthood,” the researchers concluded. Possible explanations for the findings include the effects of a high glycemic-load diet on circulating IGF-1 and insulin, which ultimately increases both oxidative stress and inflammation that promotes the development of acne, they noted.
The study findings were limited by several factors including the use of relatively homogenous younger and female patient population and the reliance on self-reported acne, as well as the observational design, which did not allow for identification of direct, causal associations between diet and acne, the researchers noted. Larger studies are needed to examine the relationship between diet and adult acne to inform prevention and treatment, they wrote.
“Much of the previous literature on the role of diet in acne has focused on the association of milk consumption and high glycemic-load diet with acne,” John S. Barbieri, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, wrote in an accompanying editorial.
Dr. Barbieri acknowledged the inability to make causal associations given the study design and noted that dietary interventions should be implemented with caution because of the potential for other effects such as reduced calcium or vitamin D.
“Nevertheless, given the potential overall health benefits of a healthy or low glycemic-load diet, and 2 small trials supporting its effectiveness in acne, a low glycemic-load diet is a reasonable recommendation for patients looking for dietary modifications that may improve their acne,” he said.
Dr. Barbieri said that he was encouraged to see that the study findings reflected previous research identifying an association between acne and high-glycemic load foods, as well as milk consumption, but he emphasized that more research is needed before general recommendations about diet and acne can be made.
“Trials are needed to evaluate whether dietary interventions can improve or prevent acne and how the effect size of such interventions compares with other standard treatment modalities,” he emphasized.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Barbieri disclosed support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health and from a Pfizer Fellowship grant to the Trustees of the University of Pennsylvania.
SOURCE: Penso L et al. JAMA Dermatol. 2020 June 10. doi: 10.1001/jamadermatol.2020.1602.
FROM JAMA DERMATOLOGY
Pediatric Dermatology: A Supplement to Pediatric News & Dermatology News
Content includes:
- Early onset of atopic dermatitis linked to poorer control, could signify more persistent disease
- Patients with actopic dermatitis should be routinely asked about conjunctivitis
- Hope on the horizon: New cantharidin formulation alleviates molluscum contagiosum in pivotal trials
- Patch testing in atopic dermatitis: When and how
- Topical calcineurin inhibitors are an effective treatment option for periorificial dermatitis
- Psychology consults for children’s skin issues can boost adherence, wellness
Content includes:
- Early onset of atopic dermatitis linked to poorer control, could signify more persistent disease
- Patients with actopic dermatitis should be routinely asked about conjunctivitis
- Hope on the horizon: New cantharidin formulation alleviates molluscum contagiosum in pivotal trials
- Patch testing in atopic dermatitis: When and how
- Topical calcineurin inhibitors are an effective treatment option for periorificial dermatitis
- Psychology consults for children’s skin issues can boost adherence, wellness
Content includes:
- Early onset of atopic dermatitis linked to poorer control, could signify more persistent disease
- Patients with actopic dermatitis should be routinely asked about conjunctivitis
- Hope on the horizon: New cantharidin formulation alleviates molluscum contagiosum in pivotal trials
- Patch testing in atopic dermatitis: When and how
- Topical calcineurin inhibitors are an effective treatment option for periorificial dermatitis
- Psychology consults for children’s skin issues can boost adherence, wellness
Hyperkalemia most common adverse event in women taking spironolactone
, according to new research.
Spironolactone, which is approved to treat heart failure, hypertension, edema, and primary hyperaldosteronism, has antagonistic effects on progesterone and androgen receptors and has been used as an off-label treatment for acne in women. “Numerous guidelines have recommended its off-label use for acne therapy to avoid antibiotic resistance and potential side effects,” wrote Yu Wang of Stony Brook (N.Y.) University and Shari R. Lipner MD, PhD, of Weill Cornell Medicine, New York. Their report is in the International Journal of Women’s Dermatology.
In a retrospective study, the investigators analyzed 7,920 adverse events with spironolactone reported by women of all ages between Jan. 1, 1969, and Dec. 30, 2018, to the Food and Drug Administration’s Adverse Event Reporting System database, for all indications. The most common adverse event was hyperkalemia, reported in 16.1%, followed by kidney injury (15.2%) and drug interactions (9%). Of the 1,272 cases of hyperkalemia reported, 25 occurred in women aged 45 years or younger; 59.3% occurred in women aged 65-85 years.
While spironolactone prescribing information was not available, the investigators compared yearly reports of adverse events with annual public interest in spironolactone using the Google Trends search term spironolactone and annual scholarly mentions of spironolactone in the Altmetric database. There was a strong correlation between the number of cases reported to the FDA and the Google Trends search (Spearman coefficient, 0.94; P less than .001) and to the Altmetric database (Spearman coefficient, 0.64; P less than .01).
Noting that hyperkalemia is “exceptionally uncommon” in women aged 45 years and younger, the investigators concluded that “in the absence of risk factors for hyperkalemia or reduced renal function, potassium laboratory monitoring is unnecessary in younger females taking spironolactone.” Because the incidence increases with age, “interval laboratory monitoring is recommended for females older than 45 years old,” they noted.
Limitations of the study, they noted, include the retrospective design and no available data before 1969. “In addition, since the [FDA Adverse Event Reporting System] data does not differentiate whether spironolactone was prescribed for heart failure, hypertension, edema, primary hyperaldosteronism, or for acne,” the study could not control for these or other confounding comorbidities or associated therapies.
“For future studies, it is important to analyze drug interactions more carefully to determine which other medications may potentiate the risk for hyperkalemia in patients taking spironolactone. It is also important to quantitate overall U.S. prescription data to better understand the relative frequency of these adverse effects reported to the FDA,” they wrote.
The investigators reported that they had no conflicts of interest; the study had no funding.
SOURCE: Wang Y, Lipner SR. Int J Womens Dermatol. 2020 May 18. doi: 10.1016/j.ijwd.2020.05.002.
, according to new research.
Spironolactone, which is approved to treat heart failure, hypertension, edema, and primary hyperaldosteronism, has antagonistic effects on progesterone and androgen receptors and has been used as an off-label treatment for acne in women. “Numerous guidelines have recommended its off-label use for acne therapy to avoid antibiotic resistance and potential side effects,” wrote Yu Wang of Stony Brook (N.Y.) University and Shari R. Lipner MD, PhD, of Weill Cornell Medicine, New York. Their report is in the International Journal of Women’s Dermatology.
In a retrospective study, the investigators analyzed 7,920 adverse events with spironolactone reported by women of all ages between Jan. 1, 1969, and Dec. 30, 2018, to the Food and Drug Administration’s Adverse Event Reporting System database, for all indications. The most common adverse event was hyperkalemia, reported in 16.1%, followed by kidney injury (15.2%) and drug interactions (9%). Of the 1,272 cases of hyperkalemia reported, 25 occurred in women aged 45 years or younger; 59.3% occurred in women aged 65-85 years.
While spironolactone prescribing information was not available, the investigators compared yearly reports of adverse events with annual public interest in spironolactone using the Google Trends search term spironolactone and annual scholarly mentions of spironolactone in the Altmetric database. There was a strong correlation between the number of cases reported to the FDA and the Google Trends search (Spearman coefficient, 0.94; P less than .001) and to the Altmetric database (Spearman coefficient, 0.64; P less than .01).
Noting that hyperkalemia is “exceptionally uncommon” in women aged 45 years and younger, the investigators concluded that “in the absence of risk factors for hyperkalemia or reduced renal function, potassium laboratory monitoring is unnecessary in younger females taking spironolactone.” Because the incidence increases with age, “interval laboratory monitoring is recommended for females older than 45 years old,” they noted.
Limitations of the study, they noted, include the retrospective design and no available data before 1969. “In addition, since the [FDA Adverse Event Reporting System] data does not differentiate whether spironolactone was prescribed for heart failure, hypertension, edema, primary hyperaldosteronism, or for acne,” the study could not control for these or other confounding comorbidities or associated therapies.
“For future studies, it is important to analyze drug interactions more carefully to determine which other medications may potentiate the risk for hyperkalemia in patients taking spironolactone. It is also important to quantitate overall U.S. prescription data to better understand the relative frequency of these adverse effects reported to the FDA,” they wrote.
The investigators reported that they had no conflicts of interest; the study had no funding.
SOURCE: Wang Y, Lipner SR. Int J Womens Dermatol. 2020 May 18. doi: 10.1016/j.ijwd.2020.05.002.
, according to new research.
Spironolactone, which is approved to treat heart failure, hypertension, edema, and primary hyperaldosteronism, has antagonistic effects on progesterone and androgen receptors and has been used as an off-label treatment for acne in women. “Numerous guidelines have recommended its off-label use for acne therapy to avoid antibiotic resistance and potential side effects,” wrote Yu Wang of Stony Brook (N.Y.) University and Shari R. Lipner MD, PhD, of Weill Cornell Medicine, New York. Their report is in the International Journal of Women’s Dermatology.
In a retrospective study, the investigators analyzed 7,920 adverse events with spironolactone reported by women of all ages between Jan. 1, 1969, and Dec. 30, 2018, to the Food and Drug Administration’s Adverse Event Reporting System database, for all indications. The most common adverse event was hyperkalemia, reported in 16.1%, followed by kidney injury (15.2%) and drug interactions (9%). Of the 1,272 cases of hyperkalemia reported, 25 occurred in women aged 45 years or younger; 59.3% occurred in women aged 65-85 years.
While spironolactone prescribing information was not available, the investigators compared yearly reports of adverse events with annual public interest in spironolactone using the Google Trends search term spironolactone and annual scholarly mentions of spironolactone in the Altmetric database. There was a strong correlation between the number of cases reported to the FDA and the Google Trends search (Spearman coefficient, 0.94; P less than .001) and to the Altmetric database (Spearman coefficient, 0.64; P less than .01).
Noting that hyperkalemia is “exceptionally uncommon” in women aged 45 years and younger, the investigators concluded that “in the absence of risk factors for hyperkalemia or reduced renal function, potassium laboratory monitoring is unnecessary in younger females taking spironolactone.” Because the incidence increases with age, “interval laboratory monitoring is recommended for females older than 45 years old,” they noted.
Limitations of the study, they noted, include the retrospective design and no available data before 1969. “In addition, since the [FDA Adverse Event Reporting System] data does not differentiate whether spironolactone was prescribed for heart failure, hypertension, edema, primary hyperaldosteronism, or for acne,” the study could not control for these or other confounding comorbidities or associated therapies.
“For future studies, it is important to analyze drug interactions more carefully to determine which other medications may potentiate the risk for hyperkalemia in patients taking spironolactone. It is also important to quantitate overall U.S. prescription data to better understand the relative frequency of these adverse effects reported to the FDA,” they wrote.
The investigators reported that they had no conflicts of interest; the study had no funding.
SOURCE: Wang Y, Lipner SR. Int J Womens Dermatol. 2020 May 18. doi: 10.1016/j.ijwd.2020.05.002.
FROM THE INTERNATIONAL JOURNAL OF WOMEN’S DERMATOLOGY
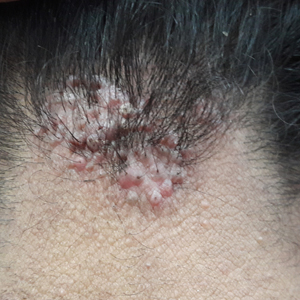
Acne Keloidalis Nuchae in the Armed Forces
Acne keloidalis nuchae (AKN) is a chronic inflammatory disorder most commonly involving the occipital scalp and posterior neck characterized by the development of keloidlike papules, pustules, and plaques. If left untreated, this condition may progress to scarring alopecia. It primarily affects males of African descent, but it also may occur in females and in other ethnic groups. Although the exact underlying pathogenesis is unclear, close haircuts and chronic mechanical irritation to the posterior neck and scalp are known inciting factors. For this reason, AKN disproportionately affects active-duty military servicemembers who are held to strict grooming standards. The US Military maintains these grooming standards to ensure uniformity, self-discipline, and serviceability in operational settings.1 Regulations dictate short tapered hair, particularly on the back of the neck, which can require weekly to biweekly haircuts to maintain.1-5
First-line treatment of AKN is prevention by avoiding short haircuts and other forms of mechanical irritation.1,6,7 However, there are considerable barriers to this strategy within the military due to uniform regulations as well as personal appearance and grooming standards. Early identification and treatment are of utmost importance in managing AKN in the military population to ensure reduction of morbidity, prevention of late-stage disease, and continued fitness for duty. This article reviews the clinical features, epidemiology, and treatments available for management of AKN, with a special focus on the active-duty military population.
Clinical Features and Epidemiology
Acne keloidalis nuchae is a chronic inflammatory disorder characterized by the development of keloidlike papules, pustules, and plaques on the posterior neck and occipital scalp.6 Also known as folliculitis keloidalis nuchae, AKN is seen primarily in men of African descent, though cases also have been reported in females and in a few other ethnic groups.6,7 In black males, the AKN prevalence worldwide ranges from 0.5% to 13.6%. The male to female ratio is 20 to 1.7 Although the exact cause is unknown, AKN appears to develop from chronic irritation and inflammation following localized skin injury and/or trauma. Chronic irritation from close-shaved haircuts, tight-fitting shirt collars, caps, and helmets have all been implicated as considerable risk factors.6-8
Symptoms generally develop hours to days following a close haircut and begin with the early formation of inflamed irritated papules and notable erythema.6,7 These papules may become secondarily infected and develop into pustules and/or abscesses, especially in cases in which the affected individual continues to have the hair shaved. Continued use of shared razors increases the risk for secondary infection and also raises the concern for transmission of blood-borne pathogens, as AKN lesions are quick to bleed with minor trauma.7
Over time, chronic inflammation and continued trauma of the AKN papules leads to widespread fibrosis and scar formation, as the papules coalesce into larger plaques and nodules. If left untreated, these later stages of disease can progress to chronic scarring alopecia.6
Prevention
In the general population, first-line therapy of AKN is preventative. The goal is to break the cycle of chronic inflammation, thereby preventing the development of additional lesions and subsequent scarring.7 Patients should be encouraged to avoid frequent haircuts, close shaves, hats, helmets, and tight shirt collars.6-8
A 2017 cross-sectional study by Adotama et al9 investigated recognition and management of AKN in predominantly black barbershops in an urban setting. Fifty barbers from barbershops in Oklahoma City, Oklahoma, were enrolled and interviewed for the study. Of these barbers, only 44% (22/50) were able to properly identify AKN from a photograph. Although the vast majority (94% [47/50]) were aware that razor use would aggravate the condition, only 46% (23/50) reported avoidance of cutting hair for clients with active AKN.9 This study, while limited by its small sample size, showed that many barbers may be unaware of AKN and therefore unknowingly contribute to the disease process by performing haircuts on actively inflamed scalps. For this reason, it is important to educate patients about their condition and strongly recommend lifestyle and hairstyle modifications in the management of their disease.
Acne keloidalis nuchae that is severe enough to interfere with the proper use and wear of military equipment (eg, Kevlar helmets) or maintenance of regulation grooming standards does not meet military admission standards.10,11 However, mild undiagnosed cases may be overlooked during entrance physical examinations, while many servicemembers develop AKN after entering the military.10 For these individuals, long-term avoidance of haircuts is not a realistic or obtainable therapeutic option.
Treatment
Topical Therapy
Early mild to moderate cases of AKN—papules less than 3 mm, no nodules present—may be treated with potent topical steroids. Studies have shown 2-week alternating cycles of high-potency topical steroids (2 weeks of twice-daily application followed by 2 weeks without application) for 8 to 12 weeks to be effective in reducing AKN lesions.8,12 Topical clindamycin also may be added and has demonstrated efficacy particularly when pustules are present.7,8
Intralesional Steroids
For moderate cases of AKN—papules more than 3 mm, plaques, and nodules—intralesional steroid injections may be considered. Triamcinolone may be used at a dose of 5 to 40 mg/mL administered at 4-week intervals.7 More concentrated doses will produce faster responses but also carry the known risk of side effects such as hypopigmentation in darker-skinned individuals and skin atrophy.
Systemic Therapy
Systemic therapy with oral antibiotics may be warranted as an adjunct to mild to moderate cases of AKN or in cases with clear evidence of secondary infection. Long-term tetracycline antibiotics, such as minocycline and doxycycline, may be used concurrently with topical and/or intralesional steroids.6,7 Their antibacterial and anti-inflammatory effects are useful in controlling secondary infections and reducing overall chronic inflammation.
When selecting an appropriate antibiotic for long-term use in active-duty military patients, it is important to consider their effects on duty status. Doxycycline is preferred for active-duty servicemembers because it is not duty limiting or medically disqualifying.10,13-15 However, minocycline, is restricted for use in aviators and aircrew members due to the risk for central nervous system side effects, which may include light-headedness, dizziness, and vertigo.
UV Light Therapy
UV radiation has known anti-inflammatory, immunosuppressive, and antifibrotic effects and commonly is used in the treatment of many dermatologic conditions.16 Within the last decade, targeted UVB (tUVB) radiation has shown promise as an effective alternative therapy for AKN. In 2014, Okoye et al16 conducted a prospective, randomized, split-scalp study in 11 patients with AKN. Each patient underwent treatment with a tUVB device (with peaks at 303 and 313 nm) to a randomly selected side of the scalp 3 times weekly for 16 weeks. Significant reductions in lesion count were seen on the treated side after 8 (P=.03) and 16 weeks (P=.04), with no change noted on the control side. Aside from objective lesion counts, patients completed questionnaires (n=6) regarding their treatment outcomes. Notably, 83.3% (5/6) reported marked improvement in their condition. Aside from mild transient burning and erythema of the treated area, no serious side effects were reported.16
Targeted UVB phototherapy has limited utility in an operational setting due to accessibility and operational tempo. Phototherapy units typically are available only at commands in close proximity to large medical treatment facilities. Further, the vast majority of servicemembers have duty hours that are not amenable to multiple treatment sessions per week for several months. For servicemembers in administrative roles or serving in garrison or shore billets, tUVB or narrowband UV phototherapy may be viable treatment options.
Laser Therapy
Various lasers have been used to treat AKN, including the CO2 laser, pulsed dye laser, 810-nm diode laser, and 1064-nm Nd:YAG laser.6 Kantor et al17 utilized a CO2 laser with a focused beam for surgical excision of a late-stage AKN case as early as 1986. In these patients, it was demonstrated that focused CO2 laser could be used to remove fibrotic lesions in an outpatient setting with only local anesthesia. Although only 8 patients were treated in this report, no relapses occurred.17
CO2 laser evaporation using the unfocused beam setting with 130 to 150 J/cm2 has been less successful, with relapses reported in multiple cases.6 Dragoni et al18 attempted treatment with a 595-nm pulsed dye laser with 6.5-J/cm2 fluence and 0.5-millisecond pulse but faced similar results, with lesions returning within 1 month.
There have been numerous reports of clinical improvement of AKN with the use of the 1064-nm Nd:YAG laser.6,19 Esmat et al19 treated 16 patients with a fluence of 35 to 45 J/cm2 and pulse duration of 10 to 30 milliseconds adjusted to skin type and hair thickness. An overall 82% reduction in lesion count was observed after 5 treatment sessions. Biopsies following the treatment course demonstrated a significant reduction in papule and plaque count (P=.001 and P=.011, respectively), and no clinical recurrences were noted at 12 months posttreatment.19 Similarly, Woo et al20 conducted a single-blinded, randomized, controlled trial to assess the efficacy of the Nd:YAG laser in combination with topical corticosteroid therapy vs topical corticosteroid monotherapy. Of the 20 patients treated, there was a statistically significant improvement in patients with papule-only AKN who received the laser and topical combination treatment (P=.031).20
Laser therapy may be an available treatment option for military servicemembers stationed within close proximity to military treatment facilities, with the Nd:YAG laser typically having the widest availability. Although laser therapy may be effective in early stages of disease, servicemembers would have to be amenable to limitation of future hair growth in the treated areas.
Surgical Excision
Surgical excision may be considered for large, extensive, disfiguring, and/or refractory lesions. Excision is a safe and effective method to remove tender, inflamed, keloidlike masses. Techniques for excision include electrosurgical excision with secondary intention healing, excision of a horizontal ellipse involving the posterior hairline with either primary closure or secondary intention healing, and use of a semilunar tissue expander prior to excision and closure.6 Regardless of the technique, it is important to ensure that affected tissue is excised at a depth that includes the base of the hair follicles to prevent recurrence.21
Final Thoughts
Acne keloidalis nuchae is a chronic inflammatory disease that causes considerable morbidity and can lead to chronic infection, alopecia, and disfigurement of the occipital scalp and posterior neck. Although easily preventable through the avoidance of mechanical trauma, irritation, and frequent short haircuts, the active-duty military population is restricted in their preventive measures due to current grooming and uniform standards. In this population, early identification and treatment are necessary to manage the disease to reduce patient morbidity and ensure continued operational and medical readiness. Topical and intralesional steroids may be used in mild to moderate cases. Topical and/or systemic antibiotics may be added to the treatment regimen in cases of secondary bacterial infection. For more severe refractory cases, laser therapy or complete surgical excision may be warranted.
- Weiss AN, Arballo OM, Miletta NR, et al. Military grooming standards and their impact on skin diseases of the head and neck. Cutis. 2018;102:328, 331-333.
- US Department of the Army. Wear and Appearance of Army Uniforms and Insignia: Army Regulation 670-1. Washington, DC: Department of the Army; 2017. https://history.army.mil/html/forcestruc/docs/AR670-1.pdf. Accessed April 14, 2020.
- U.S. Headquarters Marine Corps. Marine Corps Uniform Regulations: Marine Corps Order 1020.34H. Quantico, VA: United States Marine Corps, 2018. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137. Accessed April 14, 2020.
- Grooming standards. In: US Department of the Navy. United States Navy Uniform Regulations: NAVPERS 15665I. https://www.public.navy.mil/bupers-npc/support/uniforms/uniformregulations/chapter2/Pages/2201PersonalAppearance.aspx. Updated May 2019. Accessed April 14, 2020.
- Department of the Air Force. AFT 36-2903, Dress and Personal Appearance of Air Force Personnel. Washington, DC: Department of the Air Force, 2019. https://static.e-publishing.af.mil/production/1/af_a1/publication/afi36-2903/afi36-2903.pdf. Accessed April 14, 2020.
- Maranda EL, Simmons BJ, Nguyen AH, et al. Treatment of acne keloidalis nuchae: a systemic review of the literature. Dermatol Ther (Heidelb). 2016;6:362-378.
- Ogunbiyi A. Acne keloidalis nuchae: prevalence, impact, and management challenges. Clin Cosmet Investig Dermatol. 2016;9:483-489.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191.
- Adotama P, Tinker D, Mitchell K, et al. Barber knowledge and recommendations regarding pseudofolliculitis barbae and acne keloidalis nuchae in an urban setting. JAMA Dermatol. 2017;12:1325.
- Burke KR, Larrymore DC, Cho S. Treatment considerations for US military members with sin disease. Cutis. 2019;6:329-332.
- Medical standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; May 6, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf. Accessed April 27, 2020.
- Callender VD, Young CM, Haverstock CL, et al. An open label study of clobetasol propionate 0.05% and betamethasone valerate 0.12% foams in treatment of mild to moderate acne keloidalis. Cutis. 2005;75:317-321.
- US Department of the Army. Standards of medical fitness. https://www.qmo.amedd.army.mil/diabetes/AR40_5012011.pdf. Published December 14, 2007. Accessed April 27, 2020.
- US Department of the Air Force. Medical examinations and standards. https://static.e-publishing.af.mil/production/1/af_sg/publication/afi48-123/afi48-123.pdf. Published November 5, 2013. Accessed April 27, 2020.
- US Navy Aeromedical Reference and Waiver Guide. https://www.med.navy.mil/sites/nmotc/nami/arwg/Documents/WaiverGuide/Complete_Waiver_Guide.pdf. Published September 4, 2019. Accessed April 14, 2020.
- Okoye GA, Rainer BM, Leung SG, et al. Improving acne keloidalis nuchae with targeted ultraviolet B treatment: a prospective, randomized split-scalp study. Br J Dermatol. 2014;17:1156-1163.
- Kantor GR, Ratz JL, Wheeland RG. Treatment of acne keloidalis nuchae with carbon dioxide laser. J Am Acad Dermatol. 1986;14(2, pt 1):263-267.
- 18. Dragoni F, Bassi A, Cannarozzo G, et al. Successful treatment of acne keloidalis nuchae resistant to conventional therapy with 1064-nm Nd:YAG laser. G Ital Dermatol Venereol. 2013;148:231-232.
- Esmat SM, Hay RMA, Zeid OMA, et al. The efficacy of laser assisted hair removal in the treatment of acne keloidalis nuchae; a pilot study. Eur J Dermatol. 2012;22:645-650.
- Woo DK, Treyger G, Henderson M, et al. Prospective controlled trial for the treatment of acne keloidalis nuchae with a long-pulsed neodymium-doped yttrium-aluminum-garnet laser. J Cutan Med Surg. 2018;22:236-238.
- Beckett N, Lawson C, Cohen G. Electrosurgical excision of acne keloidalis nuchae with secondary intention healing. J Clin Aesthet Dermatol. 2011;4:36-39.
Acne keloidalis nuchae (AKN) is a chronic inflammatory disorder most commonly involving the occipital scalp and posterior neck characterized by the development of keloidlike papules, pustules, and plaques. If left untreated, this condition may progress to scarring alopecia. It primarily affects males of African descent, but it also may occur in females and in other ethnic groups. Although the exact underlying pathogenesis is unclear, close haircuts and chronic mechanical irritation to the posterior neck and scalp are known inciting factors. For this reason, AKN disproportionately affects active-duty military servicemembers who are held to strict grooming standards. The US Military maintains these grooming standards to ensure uniformity, self-discipline, and serviceability in operational settings.1 Regulations dictate short tapered hair, particularly on the back of the neck, which can require weekly to biweekly haircuts to maintain.1-5
First-line treatment of AKN is prevention by avoiding short haircuts and other forms of mechanical irritation.1,6,7 However, there are considerable barriers to this strategy within the military due to uniform regulations as well as personal appearance and grooming standards. Early identification and treatment are of utmost importance in managing AKN in the military population to ensure reduction of morbidity, prevention of late-stage disease, and continued fitness for duty. This article reviews the clinical features, epidemiology, and treatments available for management of AKN, with a special focus on the active-duty military population.
Clinical Features and Epidemiology
Acne keloidalis nuchae is a chronic inflammatory disorder characterized by the development of keloidlike papules, pustules, and plaques on the posterior neck and occipital scalp.6 Also known as folliculitis keloidalis nuchae, AKN is seen primarily in men of African descent, though cases also have been reported in females and in a few other ethnic groups.6,7 In black males, the AKN prevalence worldwide ranges from 0.5% to 13.6%. The male to female ratio is 20 to 1.7 Although the exact cause is unknown, AKN appears to develop from chronic irritation and inflammation following localized skin injury and/or trauma. Chronic irritation from close-shaved haircuts, tight-fitting shirt collars, caps, and helmets have all been implicated as considerable risk factors.6-8
Symptoms generally develop hours to days following a close haircut and begin with the early formation of inflamed irritated papules and notable erythema.6,7 These papules may become secondarily infected and develop into pustules and/or abscesses, especially in cases in which the affected individual continues to have the hair shaved. Continued use of shared razors increases the risk for secondary infection and also raises the concern for transmission of blood-borne pathogens, as AKN lesions are quick to bleed with minor trauma.7
Over time, chronic inflammation and continued trauma of the AKN papules leads to widespread fibrosis and scar formation, as the papules coalesce into larger plaques and nodules. If left untreated, these later stages of disease can progress to chronic scarring alopecia.6
Prevention
In the general population, first-line therapy of AKN is preventative. The goal is to break the cycle of chronic inflammation, thereby preventing the development of additional lesions and subsequent scarring.7 Patients should be encouraged to avoid frequent haircuts, close shaves, hats, helmets, and tight shirt collars.6-8
A 2017 cross-sectional study by Adotama et al9 investigated recognition and management of AKN in predominantly black barbershops in an urban setting. Fifty barbers from barbershops in Oklahoma City, Oklahoma, were enrolled and interviewed for the study. Of these barbers, only 44% (22/50) were able to properly identify AKN from a photograph. Although the vast majority (94% [47/50]) were aware that razor use would aggravate the condition, only 46% (23/50) reported avoidance of cutting hair for clients with active AKN.9 This study, while limited by its small sample size, showed that many barbers may be unaware of AKN and therefore unknowingly contribute to the disease process by performing haircuts on actively inflamed scalps. For this reason, it is important to educate patients about their condition and strongly recommend lifestyle and hairstyle modifications in the management of their disease.
Acne keloidalis nuchae that is severe enough to interfere with the proper use and wear of military equipment (eg, Kevlar helmets) or maintenance of regulation grooming standards does not meet military admission standards.10,11 However, mild undiagnosed cases may be overlooked during entrance physical examinations, while many servicemembers develop AKN after entering the military.10 For these individuals, long-term avoidance of haircuts is not a realistic or obtainable therapeutic option.
Treatment
Topical Therapy
Early mild to moderate cases of AKN—papules less than 3 mm, no nodules present—may be treated with potent topical steroids. Studies have shown 2-week alternating cycles of high-potency topical steroids (2 weeks of twice-daily application followed by 2 weeks without application) for 8 to 12 weeks to be effective in reducing AKN lesions.8,12 Topical clindamycin also may be added and has demonstrated efficacy particularly when pustules are present.7,8
Intralesional Steroids
For moderate cases of AKN—papules more than 3 mm, plaques, and nodules—intralesional steroid injections may be considered. Triamcinolone may be used at a dose of 5 to 40 mg/mL administered at 4-week intervals.7 More concentrated doses will produce faster responses but also carry the known risk of side effects such as hypopigmentation in darker-skinned individuals and skin atrophy.
Systemic Therapy
Systemic therapy with oral antibiotics may be warranted as an adjunct to mild to moderate cases of AKN or in cases with clear evidence of secondary infection. Long-term tetracycline antibiotics, such as minocycline and doxycycline, may be used concurrently with topical and/or intralesional steroids.6,7 Their antibacterial and anti-inflammatory effects are useful in controlling secondary infections and reducing overall chronic inflammation.
When selecting an appropriate antibiotic for long-term use in active-duty military patients, it is important to consider their effects on duty status. Doxycycline is preferred for active-duty servicemembers because it is not duty limiting or medically disqualifying.10,13-15 However, minocycline, is restricted for use in aviators and aircrew members due to the risk for central nervous system side effects, which may include light-headedness, dizziness, and vertigo.
UV Light Therapy
UV radiation has known anti-inflammatory, immunosuppressive, and antifibrotic effects and commonly is used in the treatment of many dermatologic conditions.16 Within the last decade, targeted UVB (tUVB) radiation has shown promise as an effective alternative therapy for AKN. In 2014, Okoye et al16 conducted a prospective, randomized, split-scalp study in 11 patients with AKN. Each patient underwent treatment with a tUVB device (with peaks at 303 and 313 nm) to a randomly selected side of the scalp 3 times weekly for 16 weeks. Significant reductions in lesion count were seen on the treated side after 8 (P=.03) and 16 weeks (P=.04), with no change noted on the control side. Aside from objective lesion counts, patients completed questionnaires (n=6) regarding their treatment outcomes. Notably, 83.3% (5/6) reported marked improvement in their condition. Aside from mild transient burning and erythema of the treated area, no serious side effects were reported.16
Targeted UVB phototherapy has limited utility in an operational setting due to accessibility and operational tempo. Phototherapy units typically are available only at commands in close proximity to large medical treatment facilities. Further, the vast majority of servicemembers have duty hours that are not amenable to multiple treatment sessions per week for several months. For servicemembers in administrative roles or serving in garrison or shore billets, tUVB or narrowband UV phototherapy may be viable treatment options.
Laser Therapy
Various lasers have been used to treat AKN, including the CO2 laser, pulsed dye laser, 810-nm diode laser, and 1064-nm Nd:YAG laser.6 Kantor et al17 utilized a CO2 laser with a focused beam for surgical excision of a late-stage AKN case as early as 1986. In these patients, it was demonstrated that focused CO2 laser could be used to remove fibrotic lesions in an outpatient setting with only local anesthesia. Although only 8 patients were treated in this report, no relapses occurred.17
CO2 laser evaporation using the unfocused beam setting with 130 to 150 J/cm2 has been less successful, with relapses reported in multiple cases.6 Dragoni et al18 attempted treatment with a 595-nm pulsed dye laser with 6.5-J/cm2 fluence and 0.5-millisecond pulse but faced similar results, with lesions returning within 1 month.
There have been numerous reports of clinical improvement of AKN with the use of the 1064-nm Nd:YAG laser.6,19 Esmat et al19 treated 16 patients with a fluence of 35 to 45 J/cm2 and pulse duration of 10 to 30 milliseconds adjusted to skin type and hair thickness. An overall 82% reduction in lesion count was observed after 5 treatment sessions. Biopsies following the treatment course demonstrated a significant reduction in papule and plaque count (P=.001 and P=.011, respectively), and no clinical recurrences were noted at 12 months posttreatment.19 Similarly, Woo et al20 conducted a single-blinded, randomized, controlled trial to assess the efficacy of the Nd:YAG laser in combination with topical corticosteroid therapy vs topical corticosteroid monotherapy. Of the 20 patients treated, there was a statistically significant improvement in patients with papule-only AKN who received the laser and topical combination treatment (P=.031).20
Laser therapy may be an available treatment option for military servicemembers stationed within close proximity to military treatment facilities, with the Nd:YAG laser typically having the widest availability. Although laser therapy may be effective in early stages of disease, servicemembers would have to be amenable to limitation of future hair growth in the treated areas.
Surgical Excision
Surgical excision may be considered for large, extensive, disfiguring, and/or refractory lesions. Excision is a safe and effective method to remove tender, inflamed, keloidlike masses. Techniques for excision include electrosurgical excision with secondary intention healing, excision of a horizontal ellipse involving the posterior hairline with either primary closure or secondary intention healing, and use of a semilunar tissue expander prior to excision and closure.6 Regardless of the technique, it is important to ensure that affected tissue is excised at a depth that includes the base of the hair follicles to prevent recurrence.21
Final Thoughts
Acne keloidalis nuchae is a chronic inflammatory disease that causes considerable morbidity and can lead to chronic infection, alopecia, and disfigurement of the occipital scalp and posterior neck. Although easily preventable through the avoidance of mechanical trauma, irritation, and frequent short haircuts, the active-duty military population is restricted in their preventive measures due to current grooming and uniform standards. In this population, early identification and treatment are necessary to manage the disease to reduce patient morbidity and ensure continued operational and medical readiness. Topical and intralesional steroids may be used in mild to moderate cases. Topical and/or systemic antibiotics may be added to the treatment regimen in cases of secondary bacterial infection. For more severe refractory cases, laser therapy or complete surgical excision may be warranted.
Acne keloidalis nuchae (AKN) is a chronic inflammatory disorder most commonly involving the occipital scalp and posterior neck characterized by the development of keloidlike papules, pustules, and plaques. If left untreated, this condition may progress to scarring alopecia. It primarily affects males of African descent, but it also may occur in females and in other ethnic groups. Although the exact underlying pathogenesis is unclear, close haircuts and chronic mechanical irritation to the posterior neck and scalp are known inciting factors. For this reason, AKN disproportionately affects active-duty military servicemembers who are held to strict grooming standards. The US Military maintains these grooming standards to ensure uniformity, self-discipline, and serviceability in operational settings.1 Regulations dictate short tapered hair, particularly on the back of the neck, which can require weekly to biweekly haircuts to maintain.1-5
First-line treatment of AKN is prevention by avoiding short haircuts and other forms of mechanical irritation.1,6,7 However, there are considerable barriers to this strategy within the military due to uniform regulations as well as personal appearance and grooming standards. Early identification and treatment are of utmost importance in managing AKN in the military population to ensure reduction of morbidity, prevention of late-stage disease, and continued fitness for duty. This article reviews the clinical features, epidemiology, and treatments available for management of AKN, with a special focus on the active-duty military population.
Clinical Features and Epidemiology
Acne keloidalis nuchae is a chronic inflammatory disorder characterized by the development of keloidlike papules, pustules, and plaques on the posterior neck and occipital scalp.6 Also known as folliculitis keloidalis nuchae, AKN is seen primarily in men of African descent, though cases also have been reported in females and in a few other ethnic groups.6,7 In black males, the AKN prevalence worldwide ranges from 0.5% to 13.6%. The male to female ratio is 20 to 1.7 Although the exact cause is unknown, AKN appears to develop from chronic irritation and inflammation following localized skin injury and/or trauma. Chronic irritation from close-shaved haircuts, tight-fitting shirt collars, caps, and helmets have all been implicated as considerable risk factors.6-8
Symptoms generally develop hours to days following a close haircut and begin with the early formation of inflamed irritated papules and notable erythema.6,7 These papules may become secondarily infected and develop into pustules and/or abscesses, especially in cases in which the affected individual continues to have the hair shaved. Continued use of shared razors increases the risk for secondary infection and also raises the concern for transmission of blood-borne pathogens, as AKN lesions are quick to bleed with minor trauma.7
Over time, chronic inflammation and continued trauma of the AKN papules leads to widespread fibrosis and scar formation, as the papules coalesce into larger plaques and nodules. If left untreated, these later stages of disease can progress to chronic scarring alopecia.6
Prevention
In the general population, first-line therapy of AKN is preventative. The goal is to break the cycle of chronic inflammation, thereby preventing the development of additional lesions and subsequent scarring.7 Patients should be encouraged to avoid frequent haircuts, close shaves, hats, helmets, and tight shirt collars.6-8
A 2017 cross-sectional study by Adotama et al9 investigated recognition and management of AKN in predominantly black barbershops in an urban setting. Fifty barbers from barbershops in Oklahoma City, Oklahoma, were enrolled and interviewed for the study. Of these barbers, only 44% (22/50) were able to properly identify AKN from a photograph. Although the vast majority (94% [47/50]) were aware that razor use would aggravate the condition, only 46% (23/50) reported avoidance of cutting hair for clients with active AKN.9 This study, while limited by its small sample size, showed that many barbers may be unaware of AKN and therefore unknowingly contribute to the disease process by performing haircuts on actively inflamed scalps. For this reason, it is important to educate patients about their condition and strongly recommend lifestyle and hairstyle modifications in the management of their disease.
Acne keloidalis nuchae that is severe enough to interfere with the proper use and wear of military equipment (eg, Kevlar helmets) or maintenance of regulation grooming standards does not meet military admission standards.10,11 However, mild undiagnosed cases may be overlooked during entrance physical examinations, while many servicemembers develop AKN after entering the military.10 For these individuals, long-term avoidance of haircuts is not a realistic or obtainable therapeutic option.
Treatment
Topical Therapy
Early mild to moderate cases of AKN—papules less than 3 mm, no nodules present—may be treated with potent topical steroids. Studies have shown 2-week alternating cycles of high-potency topical steroids (2 weeks of twice-daily application followed by 2 weeks without application) for 8 to 12 weeks to be effective in reducing AKN lesions.8,12 Topical clindamycin also may be added and has demonstrated efficacy particularly when pustules are present.7,8
Intralesional Steroids
For moderate cases of AKN—papules more than 3 mm, plaques, and nodules—intralesional steroid injections may be considered. Triamcinolone may be used at a dose of 5 to 40 mg/mL administered at 4-week intervals.7 More concentrated doses will produce faster responses but also carry the known risk of side effects such as hypopigmentation in darker-skinned individuals and skin atrophy.
Systemic Therapy
Systemic therapy with oral antibiotics may be warranted as an adjunct to mild to moderate cases of AKN or in cases with clear evidence of secondary infection. Long-term tetracycline antibiotics, such as minocycline and doxycycline, may be used concurrently with topical and/or intralesional steroids.6,7 Their antibacterial and anti-inflammatory effects are useful in controlling secondary infections and reducing overall chronic inflammation.
When selecting an appropriate antibiotic for long-term use in active-duty military patients, it is important to consider their effects on duty status. Doxycycline is preferred for active-duty servicemembers because it is not duty limiting or medically disqualifying.10,13-15 However, minocycline, is restricted for use in aviators and aircrew members due to the risk for central nervous system side effects, which may include light-headedness, dizziness, and vertigo.
UV Light Therapy
UV radiation has known anti-inflammatory, immunosuppressive, and antifibrotic effects and commonly is used in the treatment of many dermatologic conditions.16 Within the last decade, targeted UVB (tUVB) radiation has shown promise as an effective alternative therapy for AKN. In 2014, Okoye et al16 conducted a prospective, randomized, split-scalp study in 11 patients with AKN. Each patient underwent treatment with a tUVB device (with peaks at 303 and 313 nm) to a randomly selected side of the scalp 3 times weekly for 16 weeks. Significant reductions in lesion count were seen on the treated side after 8 (P=.03) and 16 weeks (P=.04), with no change noted on the control side. Aside from objective lesion counts, patients completed questionnaires (n=6) regarding their treatment outcomes. Notably, 83.3% (5/6) reported marked improvement in their condition. Aside from mild transient burning and erythema of the treated area, no serious side effects were reported.16
Targeted UVB phototherapy has limited utility in an operational setting due to accessibility and operational tempo. Phototherapy units typically are available only at commands in close proximity to large medical treatment facilities. Further, the vast majority of servicemembers have duty hours that are not amenable to multiple treatment sessions per week for several months. For servicemembers in administrative roles or serving in garrison or shore billets, tUVB or narrowband UV phototherapy may be viable treatment options.
Laser Therapy
Various lasers have been used to treat AKN, including the CO2 laser, pulsed dye laser, 810-nm diode laser, and 1064-nm Nd:YAG laser.6 Kantor et al17 utilized a CO2 laser with a focused beam for surgical excision of a late-stage AKN case as early as 1986. In these patients, it was demonstrated that focused CO2 laser could be used to remove fibrotic lesions in an outpatient setting with only local anesthesia. Although only 8 patients were treated in this report, no relapses occurred.17
CO2 laser evaporation using the unfocused beam setting with 130 to 150 J/cm2 has been less successful, with relapses reported in multiple cases.6 Dragoni et al18 attempted treatment with a 595-nm pulsed dye laser with 6.5-J/cm2 fluence and 0.5-millisecond pulse but faced similar results, with lesions returning within 1 month.
There have been numerous reports of clinical improvement of AKN with the use of the 1064-nm Nd:YAG laser.6,19 Esmat et al19 treated 16 patients with a fluence of 35 to 45 J/cm2 and pulse duration of 10 to 30 milliseconds adjusted to skin type and hair thickness. An overall 82% reduction in lesion count was observed after 5 treatment sessions. Biopsies following the treatment course demonstrated a significant reduction in papule and plaque count (P=.001 and P=.011, respectively), and no clinical recurrences were noted at 12 months posttreatment.19 Similarly, Woo et al20 conducted a single-blinded, randomized, controlled trial to assess the efficacy of the Nd:YAG laser in combination with topical corticosteroid therapy vs topical corticosteroid monotherapy. Of the 20 patients treated, there was a statistically significant improvement in patients with papule-only AKN who received the laser and topical combination treatment (P=.031).20
Laser therapy may be an available treatment option for military servicemembers stationed within close proximity to military treatment facilities, with the Nd:YAG laser typically having the widest availability. Although laser therapy may be effective in early stages of disease, servicemembers would have to be amenable to limitation of future hair growth in the treated areas.
Surgical Excision
Surgical excision may be considered for large, extensive, disfiguring, and/or refractory lesions. Excision is a safe and effective method to remove tender, inflamed, keloidlike masses. Techniques for excision include electrosurgical excision with secondary intention healing, excision of a horizontal ellipse involving the posterior hairline with either primary closure or secondary intention healing, and use of a semilunar tissue expander prior to excision and closure.6 Regardless of the technique, it is important to ensure that affected tissue is excised at a depth that includes the base of the hair follicles to prevent recurrence.21
Final Thoughts
Acne keloidalis nuchae is a chronic inflammatory disease that causes considerable morbidity and can lead to chronic infection, alopecia, and disfigurement of the occipital scalp and posterior neck. Although easily preventable through the avoidance of mechanical trauma, irritation, and frequent short haircuts, the active-duty military population is restricted in their preventive measures due to current grooming and uniform standards. In this population, early identification and treatment are necessary to manage the disease to reduce patient morbidity and ensure continued operational and medical readiness. Topical and intralesional steroids may be used in mild to moderate cases. Topical and/or systemic antibiotics may be added to the treatment regimen in cases of secondary bacterial infection. For more severe refractory cases, laser therapy or complete surgical excision may be warranted.
- Weiss AN, Arballo OM, Miletta NR, et al. Military grooming standards and their impact on skin diseases of the head and neck. Cutis. 2018;102:328, 331-333.
- US Department of the Army. Wear and Appearance of Army Uniforms and Insignia: Army Regulation 670-1. Washington, DC: Department of the Army; 2017. https://history.army.mil/html/forcestruc/docs/AR670-1.pdf. Accessed April 14, 2020.
- U.S. Headquarters Marine Corps. Marine Corps Uniform Regulations: Marine Corps Order 1020.34H. Quantico, VA: United States Marine Corps, 2018. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137. Accessed April 14, 2020.
- Grooming standards. In: US Department of the Navy. United States Navy Uniform Regulations: NAVPERS 15665I. https://www.public.navy.mil/bupers-npc/support/uniforms/uniformregulations/chapter2/Pages/2201PersonalAppearance.aspx. Updated May 2019. Accessed April 14, 2020.
- Department of the Air Force. AFT 36-2903, Dress and Personal Appearance of Air Force Personnel. Washington, DC: Department of the Air Force, 2019. https://static.e-publishing.af.mil/production/1/af_a1/publication/afi36-2903/afi36-2903.pdf. Accessed April 14, 2020.
- Maranda EL, Simmons BJ, Nguyen AH, et al. Treatment of acne keloidalis nuchae: a systemic review of the literature. Dermatol Ther (Heidelb). 2016;6:362-378.
- Ogunbiyi A. Acne keloidalis nuchae: prevalence, impact, and management challenges. Clin Cosmet Investig Dermatol. 2016;9:483-489.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191.
- Adotama P, Tinker D, Mitchell K, et al. Barber knowledge and recommendations regarding pseudofolliculitis barbae and acne keloidalis nuchae in an urban setting. JAMA Dermatol. 2017;12:1325.
- Burke KR, Larrymore DC, Cho S. Treatment considerations for US military members with sin disease. Cutis. 2019;6:329-332.
- Medical standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; May 6, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf. Accessed April 27, 2020.
- Callender VD, Young CM, Haverstock CL, et al. An open label study of clobetasol propionate 0.05% and betamethasone valerate 0.12% foams in treatment of mild to moderate acne keloidalis. Cutis. 2005;75:317-321.
- US Department of the Army. Standards of medical fitness. https://www.qmo.amedd.army.mil/diabetes/AR40_5012011.pdf. Published December 14, 2007. Accessed April 27, 2020.
- US Department of the Air Force. Medical examinations and standards. https://static.e-publishing.af.mil/production/1/af_sg/publication/afi48-123/afi48-123.pdf. Published November 5, 2013. Accessed April 27, 2020.
- US Navy Aeromedical Reference and Waiver Guide. https://www.med.navy.mil/sites/nmotc/nami/arwg/Documents/WaiverGuide/Complete_Waiver_Guide.pdf. Published September 4, 2019. Accessed April 14, 2020.
- Okoye GA, Rainer BM, Leung SG, et al. Improving acne keloidalis nuchae with targeted ultraviolet B treatment: a prospective, randomized split-scalp study. Br J Dermatol. 2014;17:1156-1163.
- Kantor GR, Ratz JL, Wheeland RG. Treatment of acne keloidalis nuchae with carbon dioxide laser. J Am Acad Dermatol. 1986;14(2, pt 1):263-267.
- 18. Dragoni F, Bassi A, Cannarozzo G, et al. Successful treatment of acne keloidalis nuchae resistant to conventional therapy with 1064-nm Nd:YAG laser. G Ital Dermatol Venereol. 2013;148:231-232.
- Esmat SM, Hay RMA, Zeid OMA, et al. The efficacy of laser assisted hair removal in the treatment of acne keloidalis nuchae; a pilot study. Eur J Dermatol. 2012;22:645-650.
- Woo DK, Treyger G, Henderson M, et al. Prospective controlled trial for the treatment of acne keloidalis nuchae with a long-pulsed neodymium-doped yttrium-aluminum-garnet laser. J Cutan Med Surg. 2018;22:236-238.
- Beckett N, Lawson C, Cohen G. Electrosurgical excision of acne keloidalis nuchae with secondary intention healing. J Clin Aesthet Dermatol. 2011;4:36-39.
- Weiss AN, Arballo OM, Miletta NR, et al. Military grooming standards and their impact on skin diseases of the head and neck. Cutis. 2018;102:328, 331-333.
- US Department of the Army. Wear and Appearance of Army Uniforms and Insignia: Army Regulation 670-1. Washington, DC: Department of the Army; 2017. https://history.army.mil/html/forcestruc/docs/AR670-1.pdf. Accessed April 14, 2020.
- U.S. Headquarters Marine Corps. Marine Corps Uniform Regulations: Marine Corps Order 1020.34H. Quantico, VA: United States Marine Corps, 2018. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137. Accessed April 14, 2020.
- Grooming standards. In: US Department of the Navy. United States Navy Uniform Regulations: NAVPERS 15665I. https://www.public.navy.mil/bupers-npc/support/uniforms/uniformregulations/chapter2/Pages/2201PersonalAppearance.aspx. Updated May 2019. Accessed April 14, 2020.
- Department of the Air Force. AFT 36-2903, Dress and Personal Appearance of Air Force Personnel. Washington, DC: Department of the Air Force, 2019. https://static.e-publishing.af.mil/production/1/af_a1/publication/afi36-2903/afi36-2903.pdf. Accessed April 14, 2020.
- Maranda EL, Simmons BJ, Nguyen AH, et al. Treatment of acne keloidalis nuchae: a systemic review of the literature. Dermatol Ther (Heidelb). 2016;6:362-378.
- Ogunbiyi A. Acne keloidalis nuchae: prevalence, impact, and management challenges. Clin Cosmet Investig Dermatol. 2016;9:483-489.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191.
- Adotama P, Tinker D, Mitchell K, et al. Barber knowledge and recommendations regarding pseudofolliculitis barbae and acne keloidalis nuchae in an urban setting. JAMA Dermatol. 2017;12:1325.
- Burke KR, Larrymore DC, Cho S. Treatment considerations for US military members with sin disease. Cutis. 2019;6:329-332.
- Medical standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; May 6, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf. Accessed April 27, 2020.
- Callender VD, Young CM, Haverstock CL, et al. An open label study of clobetasol propionate 0.05% and betamethasone valerate 0.12% foams in treatment of mild to moderate acne keloidalis. Cutis. 2005;75:317-321.
- US Department of the Army. Standards of medical fitness. https://www.qmo.amedd.army.mil/diabetes/AR40_5012011.pdf. Published December 14, 2007. Accessed April 27, 2020.
- US Department of the Air Force. Medical examinations and standards. https://static.e-publishing.af.mil/production/1/af_sg/publication/afi48-123/afi48-123.pdf. Published November 5, 2013. Accessed April 27, 2020.
- US Navy Aeromedical Reference and Waiver Guide. https://www.med.navy.mil/sites/nmotc/nami/arwg/Documents/WaiverGuide/Complete_Waiver_Guide.pdf. Published September 4, 2019. Accessed April 14, 2020.
- Okoye GA, Rainer BM, Leung SG, et al. Improving acne keloidalis nuchae with targeted ultraviolet B treatment: a prospective, randomized split-scalp study. Br J Dermatol. 2014;17:1156-1163.
- Kantor GR, Ratz JL, Wheeland RG. Treatment of acne keloidalis nuchae with carbon dioxide laser. J Am Acad Dermatol. 1986;14(2, pt 1):263-267.
- 18. Dragoni F, Bassi A, Cannarozzo G, et al. Successful treatment of acne keloidalis nuchae resistant to conventional therapy with 1064-nm Nd:YAG laser. G Ital Dermatol Venereol. 2013;148:231-232.
- Esmat SM, Hay RMA, Zeid OMA, et al. The efficacy of laser assisted hair removal in the treatment of acne keloidalis nuchae; a pilot study. Eur J Dermatol. 2012;22:645-650.
- Woo DK, Treyger G, Henderson M, et al. Prospective controlled trial for the treatment of acne keloidalis nuchae with a long-pulsed neodymium-doped yttrium-aluminum-garnet laser. J Cutan Med Surg. 2018;22:236-238.
- Beckett N, Lawson C, Cohen G. Electrosurgical excision of acne keloidalis nuchae with secondary intention healing. J Clin Aesthet Dermatol. 2011;4:36-39.
Practice Points
- Acne keloidalis nuchae (AKN) is a chronic inflammatory disorder of the occipital scalp and posterior neck characterized by keloidlike papules, pustules, and plaques that develop following mechanical irritation.
- Military members are required to maintain short haircuts and may be disproportionately affected by AKN.
- In the military population, early identification and treatment, which includes topical steroids, oral antibiotics, UV light therapy, lasers, and surgical excision, can prevent further scarring, permanent hair loss, and disfigurement from AKN.
Hydrogen peroxide reduces C. acnes cultures following shoulder surgery
Prior to shoulder surgery, application of 3% hydrogen peroxide is a simple and inexpensive strategy to reduce the risk of postoperative cultures of Cutibacterium acnes, according to findings from a prospective randomized trial. The results were reported in an abstract scheduled for release at the annual meeting of the American Academy of Orthopaedic Surgeons. The meeting was canceled because of COVID-19.
“This approach is simple, cheap, and does not rely on patient compliance,” explained Surena Namdari, MD, associate professor of orthopedic surgery at Thomas Jefferson University, Philadelphia.
C. acnes, formerly known as Propionibacterium acnes, is increasingly seen as an important target for prevention of postoperative shoulder infections because of published reports that it is the most commonly isolated bacterium from such infections, Dr. Namdari said in an interview.
In the prospective, randomized trial, male patients scheduled for shoulder arthroscopy were recruited if they did not have active acne, history of psoriatic or eczematous lesions, or recent antibiotic use. Most of the preoperative preparation of the surgical site was the same in the experimental and control arms. This included hair clipping, application of 2% chlorhexidine, and cleansing with saturated 7.5% povidone-iodine solution surgical scrub brushes.
The difference was that 3% hydrogen peroxide–soaked gauzes were applied to perioperative skin of those randomized to the experimental group but not to controls. All patients received routine preoperative oral antibiotics as well as perioperative applications of a formulation containing 2% chlorhexidine gluconate and 70% isopropyl alcohol.
Following surgery, 11 (18.6%) of the 59 patients in the experimental arm versus 23 (34.8%) of the 66 patients randomized to the control group had positive cultures for C. acnes (P = .047), according to the trial results, which have now been published (J Shoulder Elbow Surg. 2020;29:212-6).
There were no cases of skin reactions in either the experimental or control groups.
Topical skin cleansers that contain peroxide, such as benzoyl peroxide, have been shown to have a C. acnes decolonizing effect if applied repeatedly in the days prior to surgery, but Dr. Namdari suggested the problem with this approach is that it depends on patient compliance. A prophylaxis included in the preoperative routine eliminates this potential problem.
C. acnes is an anaerobic bacterium that is part of the resident flora of the skin around several joints, including the knee and the hip, but it is particularly common in the posterior shoulder. Colonization has been found substantially more common in men than in women, according to Dr. Namdari.
The specific threat posed by C. acnes to risk of postoperative infections “is still being defined,” and this trial was not large enough to associate the reduction in postoperative C. acnes cultures with a reduced risk of an adverse clinical outcome, but Dr. Namdari says that the data do show that the nearly 50% reduction in positive cultures was achieved efficiently and inexpensively with no apparent risk.
Several previous studies have also evaluated strategies for reducing C. acnes skin burden on the basis of expected protection against postoperative infection. In one, which associated a 3-day preoperative course of benzoyl peroxide with a reduction in the skin burden of C. acnes, the authors also concluded that this approach deserves consideration in routine skin preparation for shoulder arthroplasty (J Shoulder Elbow Surg. 2018;27:1539-44).
“We believe that a preoperative skin prep protocol that reduces C. acnes load on the skin would likely lead to reduced postoperative infections,” reported the senior author, Mohit N. Gilotra, MD, assistant professor, University of Maryland, Baltimore. Contacted about the rationale for reducing C. acnes skin burden without objective evidence of an impact on postoperative infection risk, Dr. Gilotra indicated these strategies make sense.
“It seems to be true for staph infections and is a reasonable assumption to make here,” he added. “Future work will help determine how much benzoyl peroxide, hydrogen peroxide, or other skin prep can reduce surgical site infection.”
Dr. Namdari reports financial relationships with multiple device and pharmaceutical companies but none relevant to this study.
SOURCE: Namdari S et al. AAOS 2020. Abstract P0808.
Prior to shoulder surgery, application of 3% hydrogen peroxide is a simple and inexpensive strategy to reduce the risk of postoperative cultures of Cutibacterium acnes, according to findings from a prospective randomized trial. The results were reported in an abstract scheduled for release at the annual meeting of the American Academy of Orthopaedic Surgeons. The meeting was canceled because of COVID-19.
“This approach is simple, cheap, and does not rely on patient compliance,” explained Surena Namdari, MD, associate professor of orthopedic surgery at Thomas Jefferson University, Philadelphia.
C. acnes, formerly known as Propionibacterium acnes, is increasingly seen as an important target for prevention of postoperative shoulder infections because of published reports that it is the most commonly isolated bacterium from such infections, Dr. Namdari said in an interview.
In the prospective, randomized trial, male patients scheduled for shoulder arthroscopy were recruited if they did not have active acne, history of psoriatic or eczematous lesions, or recent antibiotic use. Most of the preoperative preparation of the surgical site was the same in the experimental and control arms. This included hair clipping, application of 2% chlorhexidine, and cleansing with saturated 7.5% povidone-iodine solution surgical scrub brushes.
The difference was that 3% hydrogen peroxide–soaked gauzes were applied to perioperative skin of those randomized to the experimental group but not to controls. All patients received routine preoperative oral antibiotics as well as perioperative applications of a formulation containing 2% chlorhexidine gluconate and 70% isopropyl alcohol.
Following surgery, 11 (18.6%) of the 59 patients in the experimental arm versus 23 (34.8%) of the 66 patients randomized to the control group had positive cultures for C. acnes (P = .047), according to the trial results, which have now been published (J Shoulder Elbow Surg. 2020;29:212-6).
There were no cases of skin reactions in either the experimental or control groups.
Topical skin cleansers that contain peroxide, such as benzoyl peroxide, have been shown to have a C. acnes decolonizing effect if applied repeatedly in the days prior to surgery, but Dr. Namdari suggested the problem with this approach is that it depends on patient compliance. A prophylaxis included in the preoperative routine eliminates this potential problem.
C. acnes is an anaerobic bacterium that is part of the resident flora of the skin around several joints, including the knee and the hip, but it is particularly common in the posterior shoulder. Colonization has been found substantially more common in men than in women, according to Dr. Namdari.
The specific threat posed by C. acnes to risk of postoperative infections “is still being defined,” and this trial was not large enough to associate the reduction in postoperative C. acnes cultures with a reduced risk of an adverse clinical outcome, but Dr. Namdari says that the data do show that the nearly 50% reduction in positive cultures was achieved efficiently and inexpensively with no apparent risk.
Several previous studies have also evaluated strategies for reducing C. acnes skin burden on the basis of expected protection against postoperative infection. In one, which associated a 3-day preoperative course of benzoyl peroxide with a reduction in the skin burden of C. acnes, the authors also concluded that this approach deserves consideration in routine skin preparation for shoulder arthroplasty (J Shoulder Elbow Surg. 2018;27:1539-44).
“We believe that a preoperative skin prep protocol that reduces C. acnes load on the skin would likely lead to reduced postoperative infections,” reported the senior author, Mohit N. Gilotra, MD, assistant professor, University of Maryland, Baltimore. Contacted about the rationale for reducing C. acnes skin burden without objective evidence of an impact on postoperative infection risk, Dr. Gilotra indicated these strategies make sense.
“It seems to be true for staph infections and is a reasonable assumption to make here,” he added. “Future work will help determine how much benzoyl peroxide, hydrogen peroxide, or other skin prep can reduce surgical site infection.”
Dr. Namdari reports financial relationships with multiple device and pharmaceutical companies but none relevant to this study.
SOURCE: Namdari S et al. AAOS 2020. Abstract P0808.
Prior to shoulder surgery, application of 3% hydrogen peroxide is a simple and inexpensive strategy to reduce the risk of postoperative cultures of Cutibacterium acnes, according to findings from a prospective randomized trial. The results were reported in an abstract scheduled for release at the annual meeting of the American Academy of Orthopaedic Surgeons. The meeting was canceled because of COVID-19.
“This approach is simple, cheap, and does not rely on patient compliance,” explained Surena Namdari, MD, associate professor of orthopedic surgery at Thomas Jefferson University, Philadelphia.
C. acnes, formerly known as Propionibacterium acnes, is increasingly seen as an important target for prevention of postoperative shoulder infections because of published reports that it is the most commonly isolated bacterium from such infections, Dr. Namdari said in an interview.
In the prospective, randomized trial, male patients scheduled for shoulder arthroscopy were recruited if they did not have active acne, history of psoriatic or eczematous lesions, or recent antibiotic use. Most of the preoperative preparation of the surgical site was the same in the experimental and control arms. This included hair clipping, application of 2% chlorhexidine, and cleansing with saturated 7.5% povidone-iodine solution surgical scrub brushes.
The difference was that 3% hydrogen peroxide–soaked gauzes were applied to perioperative skin of those randomized to the experimental group but not to controls. All patients received routine preoperative oral antibiotics as well as perioperative applications of a formulation containing 2% chlorhexidine gluconate and 70% isopropyl alcohol.
Following surgery, 11 (18.6%) of the 59 patients in the experimental arm versus 23 (34.8%) of the 66 patients randomized to the control group had positive cultures for C. acnes (P = .047), according to the trial results, which have now been published (J Shoulder Elbow Surg. 2020;29:212-6).
There were no cases of skin reactions in either the experimental or control groups.
Topical skin cleansers that contain peroxide, such as benzoyl peroxide, have been shown to have a C. acnes decolonizing effect if applied repeatedly in the days prior to surgery, but Dr. Namdari suggested the problem with this approach is that it depends on patient compliance. A prophylaxis included in the preoperative routine eliminates this potential problem.
C. acnes is an anaerobic bacterium that is part of the resident flora of the skin around several joints, including the knee and the hip, but it is particularly common in the posterior shoulder. Colonization has been found substantially more common in men than in women, according to Dr. Namdari.
The specific threat posed by C. acnes to risk of postoperative infections “is still being defined,” and this trial was not large enough to associate the reduction in postoperative C. acnes cultures with a reduced risk of an adverse clinical outcome, but Dr. Namdari says that the data do show that the nearly 50% reduction in positive cultures was achieved efficiently and inexpensively with no apparent risk.
Several previous studies have also evaluated strategies for reducing C. acnes skin burden on the basis of expected protection against postoperative infection. In one, which associated a 3-day preoperative course of benzoyl peroxide with a reduction in the skin burden of C. acnes, the authors also concluded that this approach deserves consideration in routine skin preparation for shoulder arthroplasty (J Shoulder Elbow Surg. 2018;27:1539-44).
“We believe that a preoperative skin prep protocol that reduces C. acnes load on the skin would likely lead to reduced postoperative infections,” reported the senior author, Mohit N. Gilotra, MD, assistant professor, University of Maryland, Baltimore. Contacted about the rationale for reducing C. acnes skin burden without objective evidence of an impact on postoperative infection risk, Dr. Gilotra indicated these strategies make sense.
“It seems to be true for staph infections and is a reasonable assumption to make here,” he added. “Future work will help determine how much benzoyl peroxide, hydrogen peroxide, or other skin prep can reduce surgical site infection.”
Dr. Namdari reports financial relationships with multiple device and pharmaceutical companies but none relevant to this study.
SOURCE: Namdari S et al. AAOS 2020. Abstract P0808.
FROM AAOS 2020
Evidence on spironolactone safety, COVID-19 reassuring for acne patients
according to a report in the Journal of the American Academy of Dermatology.
The virus needs androgens to infect cells, and uses androgen-dependent transmembrane protease serine 2 to prime viral protein spikes to anchor onto ACE2 receptors. Without that step, the virus can’t enter cells. Androgens are the only known activator in humans, so androgen blockers like spironolactone probably short-circuit the process, said the report’s lead author Carlos Wambier, MD, PhD, of the department of dermatology at Brown University, Providence, R.I (J Am Acad Dermatol. 2020 Apr 10. doi: 10.1016/j.jaad.2020.04.032).
The lack of androgens could be a possible explanation as to why mortality is so rare among children with COVID-19, and why fatalities among men are higher than among women with COVID-19, he said in an interview.
There are a lot of androgen blocker candidates, but he said spironolactone – a mainstay of acne treatment – might well be the best for the pandemic because of its concomitant lung and heart benefits.
The message counters a post on Instagram in March from a New York City dermatologist in private practice, Ellen Marmur, MD, that raised a question about spironolactone. Concerned about the situation in New York, she reviewed the literature and found a 2005 study that reported that macrophages drawn from 10 heart failure patients had increased ACE2 activity and increased messenger RNA expression after the subjects had been on spironolactone 25 mg per day for a month.
In an interview, she said she has been sharing her concerns with patients on spironolactone and offering them an alternative, such as minocycline, until this issue is better elucidated. To date, she has had one young patient who declined to switch to another treatment, and about six patients who were comfortable switching to another treatment for 1-2 months. She said that she is “clearly cautious yet uncertain about the influence of chronic spironolactone for acne on COVID infection in acne patients,” and that eventually she would be interested in seeing retrospective data on outcomes of patients on spironolactone for hypertension versus acne during the pandemic.
Dr. Marmur’s post was spread on social media and was picked up by a few online news outlets.
In an interview, Adam Friedman, MD, professor and interim chair of dermatology at George Washington University, Washington, said he’s been addressing concerns about spironolactone in educational webinars because of it.
He tells his audience that “you can’t make any claims” for COVID-19 based on the 2005 study. It was a small clinical study in heart failure patients and only assessed ACE2 expression on macrophages, not respiratory, cardiac, or mesangial cells, which are the relevant locations for viral invasion and damage. In fact, there are studies showing that spironolactone reduced ACE2 in renal mesangial cells. Also of note, spironolactone has been used with no indication of virus risk since the 1950s, he pointed out. The American Academy of Dermatology has not said to stop spironolactone.
At least one study is underway to see if spironolactone is beneficial: 100 mg twice a day for 5 days is being pitted against placebo in Turkey among people hospitalized with acute respiratory distress. The study will evaluate the effect of spironolactone on oxygenation.
“There’s no evidence to show spironolactone can increase mortality levels,” Dr. Wambier said. He is using it more now in patients with acne – a sign of androgen hyperactivity – convinced that it will protect against COVID-19. He even started his sister on it to help with androgenic hair loss, and maybe the virus.
Observations in Spain – increased prevalence of androgenic alopecia among hospitalized patients – support the androgen link; 29 of 41 men (71%) hospitalized with bilateral pneumonia had male pattern baldness, which was severe in 16 (39%), according to a recent report (J Cosmet Dermatol. 2020 Apr 16. doi: 10.1111/jocd.13443). The expected prevalence in a similar age-matched population is 31%-53%.
“Based on the scientific rationale combined with this preliminary observation, we believe investigating the potential association between androgens and COVID‐19 disease severity warrants further merit,” concluded the authors, who included Dr. Wambier, and other dermatologists from the United States, as well as Spain, Australia, Croatia, and Switzerland. “If such an association is confirmed, antiandrogens could be evaluated as a potential treatment for COVID‐19 infection,” they wrote.
The numbers are holding up in a larger series from three Spanish hospitals, and also showing a greater prevalence of androgenic hair loss among hospitalized women, Dr. Wambier said in the interview.
Authors of the two studies include an employee of Applied Biology. No conflicts were declared in the Journal of Cosmetic Dermatology study; no disclosures were listed in the JAAD study. Dr. Friedman had no disclosures.
according to a report in the Journal of the American Academy of Dermatology.
The virus needs androgens to infect cells, and uses androgen-dependent transmembrane protease serine 2 to prime viral protein spikes to anchor onto ACE2 receptors. Without that step, the virus can’t enter cells. Androgens are the only known activator in humans, so androgen blockers like spironolactone probably short-circuit the process, said the report’s lead author Carlos Wambier, MD, PhD, of the department of dermatology at Brown University, Providence, R.I (J Am Acad Dermatol. 2020 Apr 10. doi: 10.1016/j.jaad.2020.04.032).
The lack of androgens could be a possible explanation as to why mortality is so rare among children with COVID-19, and why fatalities among men are higher than among women with COVID-19, he said in an interview.
There are a lot of androgen blocker candidates, but he said spironolactone – a mainstay of acne treatment – might well be the best for the pandemic because of its concomitant lung and heart benefits.
The message counters a post on Instagram in March from a New York City dermatologist in private practice, Ellen Marmur, MD, that raised a question about spironolactone. Concerned about the situation in New York, she reviewed the literature and found a 2005 study that reported that macrophages drawn from 10 heart failure patients had increased ACE2 activity and increased messenger RNA expression after the subjects had been on spironolactone 25 mg per day for a month.
In an interview, she said she has been sharing her concerns with patients on spironolactone and offering them an alternative, such as minocycline, until this issue is better elucidated. To date, she has had one young patient who declined to switch to another treatment, and about six patients who were comfortable switching to another treatment for 1-2 months. She said that she is “clearly cautious yet uncertain about the influence of chronic spironolactone for acne on COVID infection in acne patients,” and that eventually she would be interested in seeing retrospective data on outcomes of patients on spironolactone for hypertension versus acne during the pandemic.
Dr. Marmur’s post was spread on social media and was picked up by a few online news outlets.
In an interview, Adam Friedman, MD, professor and interim chair of dermatology at George Washington University, Washington, said he’s been addressing concerns about spironolactone in educational webinars because of it.
He tells his audience that “you can’t make any claims” for COVID-19 based on the 2005 study. It was a small clinical study in heart failure patients and only assessed ACE2 expression on macrophages, not respiratory, cardiac, or mesangial cells, which are the relevant locations for viral invasion and damage. In fact, there are studies showing that spironolactone reduced ACE2 in renal mesangial cells. Also of note, spironolactone has been used with no indication of virus risk since the 1950s, he pointed out. The American Academy of Dermatology has not said to stop spironolactone.
At least one study is underway to see if spironolactone is beneficial: 100 mg twice a day for 5 days is being pitted against placebo in Turkey among people hospitalized with acute respiratory distress. The study will evaluate the effect of spironolactone on oxygenation.
“There’s no evidence to show spironolactone can increase mortality levels,” Dr. Wambier said. He is using it more now in patients with acne – a sign of androgen hyperactivity – convinced that it will protect against COVID-19. He even started his sister on it to help with androgenic hair loss, and maybe the virus.
Observations in Spain – increased prevalence of androgenic alopecia among hospitalized patients – support the androgen link; 29 of 41 men (71%) hospitalized with bilateral pneumonia had male pattern baldness, which was severe in 16 (39%), according to a recent report (J Cosmet Dermatol. 2020 Apr 16. doi: 10.1111/jocd.13443). The expected prevalence in a similar age-matched population is 31%-53%.
“Based on the scientific rationale combined with this preliminary observation, we believe investigating the potential association between androgens and COVID‐19 disease severity warrants further merit,” concluded the authors, who included Dr. Wambier, and other dermatologists from the United States, as well as Spain, Australia, Croatia, and Switzerland. “If such an association is confirmed, antiandrogens could be evaluated as a potential treatment for COVID‐19 infection,” they wrote.
The numbers are holding up in a larger series from three Spanish hospitals, and also showing a greater prevalence of androgenic hair loss among hospitalized women, Dr. Wambier said in the interview.
Authors of the two studies include an employee of Applied Biology. No conflicts were declared in the Journal of Cosmetic Dermatology study; no disclosures were listed in the JAAD study. Dr. Friedman had no disclosures.
according to a report in the Journal of the American Academy of Dermatology.
The virus needs androgens to infect cells, and uses androgen-dependent transmembrane protease serine 2 to prime viral protein spikes to anchor onto ACE2 receptors. Without that step, the virus can’t enter cells. Androgens are the only known activator in humans, so androgen blockers like spironolactone probably short-circuit the process, said the report’s lead author Carlos Wambier, MD, PhD, of the department of dermatology at Brown University, Providence, R.I (J Am Acad Dermatol. 2020 Apr 10. doi: 10.1016/j.jaad.2020.04.032).
The lack of androgens could be a possible explanation as to why mortality is so rare among children with COVID-19, and why fatalities among men are higher than among women with COVID-19, he said in an interview.
There are a lot of androgen blocker candidates, but he said spironolactone – a mainstay of acne treatment – might well be the best for the pandemic because of its concomitant lung and heart benefits.
The message counters a post on Instagram in March from a New York City dermatologist in private practice, Ellen Marmur, MD, that raised a question about spironolactone. Concerned about the situation in New York, she reviewed the literature and found a 2005 study that reported that macrophages drawn from 10 heart failure patients had increased ACE2 activity and increased messenger RNA expression after the subjects had been on spironolactone 25 mg per day for a month.
In an interview, she said she has been sharing her concerns with patients on spironolactone and offering them an alternative, such as minocycline, until this issue is better elucidated. To date, she has had one young patient who declined to switch to another treatment, and about six patients who were comfortable switching to another treatment for 1-2 months. She said that she is “clearly cautious yet uncertain about the influence of chronic spironolactone for acne on COVID infection in acne patients,” and that eventually she would be interested in seeing retrospective data on outcomes of patients on spironolactone for hypertension versus acne during the pandemic.
Dr. Marmur’s post was spread on social media and was picked up by a few online news outlets.
In an interview, Adam Friedman, MD, professor and interim chair of dermatology at George Washington University, Washington, said he’s been addressing concerns about spironolactone in educational webinars because of it.
He tells his audience that “you can’t make any claims” for COVID-19 based on the 2005 study. It was a small clinical study in heart failure patients and only assessed ACE2 expression on macrophages, not respiratory, cardiac, or mesangial cells, which are the relevant locations for viral invasion and damage. In fact, there are studies showing that spironolactone reduced ACE2 in renal mesangial cells. Also of note, spironolactone has been used with no indication of virus risk since the 1950s, he pointed out. The American Academy of Dermatology has not said to stop spironolactone.
At least one study is underway to see if spironolactone is beneficial: 100 mg twice a day for 5 days is being pitted against placebo in Turkey among people hospitalized with acute respiratory distress. The study will evaluate the effect of spironolactone on oxygenation.
“There’s no evidence to show spironolactone can increase mortality levels,” Dr. Wambier said. He is using it more now in patients with acne – a sign of androgen hyperactivity – convinced that it will protect against COVID-19. He even started his sister on it to help with androgenic hair loss, and maybe the virus.
Observations in Spain – increased prevalence of androgenic alopecia among hospitalized patients – support the androgen link; 29 of 41 men (71%) hospitalized with bilateral pneumonia had male pattern baldness, which was severe in 16 (39%), according to a recent report (J Cosmet Dermatol. 2020 Apr 16. doi: 10.1111/jocd.13443). The expected prevalence in a similar age-matched population is 31%-53%.
“Based on the scientific rationale combined with this preliminary observation, we believe investigating the potential association between androgens and COVID‐19 disease severity warrants further merit,” concluded the authors, who included Dr. Wambier, and other dermatologists from the United States, as well as Spain, Australia, Croatia, and Switzerland. “If such an association is confirmed, antiandrogens could be evaluated as a potential treatment for COVID‐19 infection,” they wrote.
The numbers are holding up in a larger series from three Spanish hospitals, and also showing a greater prevalence of androgenic hair loss among hospitalized women, Dr. Wambier said in the interview.
Authors of the two studies include an employee of Applied Biology. No conflicts were declared in the Journal of Cosmetic Dermatology study; no disclosures were listed in the JAAD study. Dr. Friedman had no disclosures.
Novel acne drug now under review at the FDA
LAHAINA, HAWAII – by the Food and Drug Administration, is already generating considerable buzz in the patient-advocacy community even though the agency won’t issue its decision until August.
“I’ve actually had a lot of interest in this already from parents, especially regarding girls who have very hormonal acne but the parents are really not interested in starting them on a systemic hormonal therapy at their age,” Jessica Sprague, MD, said at the SDEF Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Clascoterone targets androgen receptors in the skin in order to reduce cutaneous 5-alpha dihydrotestosterone.
“It’s being developed for use in both males and females, which is great because at this point there’s no hormonal treatment for males,” noted Dr. Sprague, a pediatric dermatologist at Rady Children’s Hospital and the University of California, both in San Diego.
The manufacturer’s application for marketing approval of clascoterone cream 1% under FDA review includes evidence from two identical phase-3, double-blind, vehicle-controlled, 12-week, randomized trials. The two studies included a total of 1,440 patients aged 9 years through adulthood with moderate to severe facial acne vulgaris who were randomized to twice-daily application of clascoterone or its vehicle.
The primary outcome was the reduction in inflammatory lesions at week 12: a 46.2% decline from baseline with clascoterone 1% cream, which was a significantly greater improvement than the 32.7% reduction for vehicle. The secondary outcome – change in noninflammatory lesion counts at week 12 – was also positive for the topical androgen receptor inhibitor, which achieved a 29.8% reduction, compared with 18.9% for vehicle. Clascoterone exhibited a favorable safety and tolerability profile, with numerically fewer treatment-emergent adverse events than in the vehicle control group. A stronger formulation of the topical agent is in advanced clinical trials for the treatment of androgenetic alopecia in both males and females.
Dr. Sprague reported having no financial conflicts regarding her presentation.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – by the Food and Drug Administration, is already generating considerable buzz in the patient-advocacy community even though the agency won’t issue its decision until August.
“I’ve actually had a lot of interest in this already from parents, especially regarding girls who have very hormonal acne but the parents are really not interested in starting them on a systemic hormonal therapy at their age,” Jessica Sprague, MD, said at the SDEF Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Clascoterone targets androgen receptors in the skin in order to reduce cutaneous 5-alpha dihydrotestosterone.
“It’s being developed for use in both males and females, which is great because at this point there’s no hormonal treatment for males,” noted Dr. Sprague, a pediatric dermatologist at Rady Children’s Hospital and the University of California, both in San Diego.
The manufacturer’s application for marketing approval of clascoterone cream 1% under FDA review includes evidence from two identical phase-3, double-blind, vehicle-controlled, 12-week, randomized trials. The two studies included a total of 1,440 patients aged 9 years through adulthood with moderate to severe facial acne vulgaris who were randomized to twice-daily application of clascoterone or its vehicle.
The primary outcome was the reduction in inflammatory lesions at week 12: a 46.2% decline from baseline with clascoterone 1% cream, which was a significantly greater improvement than the 32.7% reduction for vehicle. The secondary outcome – change in noninflammatory lesion counts at week 12 – was also positive for the topical androgen receptor inhibitor, which achieved a 29.8% reduction, compared with 18.9% for vehicle. Clascoterone exhibited a favorable safety and tolerability profile, with numerically fewer treatment-emergent adverse events than in the vehicle control group. A stronger formulation of the topical agent is in advanced clinical trials for the treatment of androgenetic alopecia in both males and females.
Dr. Sprague reported having no financial conflicts regarding her presentation.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – by the Food and Drug Administration, is already generating considerable buzz in the patient-advocacy community even though the agency won’t issue its decision until August.
“I’ve actually had a lot of interest in this already from parents, especially regarding girls who have very hormonal acne but the parents are really not interested in starting them on a systemic hormonal therapy at their age,” Jessica Sprague, MD, said at the SDEF Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Clascoterone targets androgen receptors in the skin in order to reduce cutaneous 5-alpha dihydrotestosterone.
“It’s being developed for use in both males and females, which is great because at this point there’s no hormonal treatment for males,” noted Dr. Sprague, a pediatric dermatologist at Rady Children’s Hospital and the University of California, both in San Diego.
The manufacturer’s application for marketing approval of clascoterone cream 1% under FDA review includes evidence from two identical phase-3, double-blind, vehicle-controlled, 12-week, randomized trials. The two studies included a total of 1,440 patients aged 9 years through adulthood with moderate to severe facial acne vulgaris who were randomized to twice-daily application of clascoterone or its vehicle.
The primary outcome was the reduction in inflammatory lesions at week 12: a 46.2% decline from baseline with clascoterone 1% cream, which was a significantly greater improvement than the 32.7% reduction for vehicle. The secondary outcome – change in noninflammatory lesion counts at week 12 – was also positive for the topical androgen receptor inhibitor, which achieved a 29.8% reduction, compared with 18.9% for vehicle. Clascoterone exhibited a favorable safety and tolerability profile, with numerically fewer treatment-emergent adverse events than in the vehicle control group. A stronger formulation of the topical agent is in advanced clinical trials for the treatment of androgenetic alopecia in both males and females.
Dr. Sprague reported having no financial conflicts regarding her presentation.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM THE SDEF HAWAII DERMATOLOGY SEMINAR