User login
Women struggle with benzodiazepine addiction post chemotherapy treatment
SAN ANTONIO – shows a new study.
While benzodiazepines and nonbenzodiazepine sedative-hypnotics are effective for these indications, misuse and increased health care utilization can ensue from their prolonged use, said Jacob C. Cogan, MD, a fellow in oncology/hematology at the Herbert Irving Comprehensive Cancer Center, Columbia University, New York. Dr. Cogan recently presented the results of the study at the San Antonio Breast Cancer Symposium.
The study included patients with breast cancer who received adjuvant chemotherapy between 2008 and 2017. Prescriptions for sedatives were divided into three periods: 365 days prior to chemotherapy to the start of chemotherapy (period one); start of chemotherapy to 90 days after the end of chemotherapy (period two); and 90-365 days after chemotherapy (period three). Patients who filled at least one benzodiazepine prescription in period two and patients who filled at least two benzodiazepine in period three were classified as new persistent benzodiazepine users. The same definitions were then used for nonbenzodiazepine sedative-hypnotics.
Among 17,532 benzodiazepine-naive patients (mean age, 57 years) and 21,863 nonbenzodiazepine sedative-hypnotic drug–naive patients (mean age, 56 years) who received adjuvant chemotherapy for breast cancer, lumpectomies were performed for a small majority (56.6% benzodiazepine naive, 55.1% nonbenzodiazepine sedative-hypnotics naive) versus mastectomy, and about half of patients received less than 4 months of chemotherapy (48.0% benzodiazepine naive, 48.6% nonbenzodiazepine sedative-hypnotics naive). Among benzodiazepine-naive patients, 4,447 (25.4%) filled at least one benzodiazepine prescription during chemotherapy, and 2,160 (9.9%) filled at least one nonbenzodiazepine sedative-hypnotic prescription during chemotherapy. The rate of new persistent benzodiazepine use after initial exposure during chemotherapy was 26.8% (n = 1,192). Similarly, 33.8% (n = 730) of nonbenzodiazepine sedative-hypnotics users became new persistent users. In addition, 115 patients became new persistent users of both types of sedative-hypnotics.
New persistent benzodiazepine use was associated with several characteristics: age 50-65 (odds ratio, 1.23; P = .01) and age greater than 65 (OR, 1.38, P = .005) relative to age less than 49; as well as Medicaid insurance, relative to commercial and Medicare insurance (OR, 1.68; P < .0001). Both new persistent benzodiazepine and nonbenzodiazepine sedative-hypnotics use was associated with chemotherapy duration of less than 4 months relative to 4 or more months of chemotherapy (OR, 1.17; P = .03 for benzodiazepines; OR, 1.58; P < .0001 for nonbenzodiazepine sedative-hypnotics).
It is not clear why shorter chemotherapy duration is associated with more new persistent use, Dr. Cogan said. “It may be that, paradoxically, a shorter duration of treatment could lead to more anxiety about recurrence. These patients may need closer monitoring of mental health symptoms and earlier referral for psychological services.”
Dr. Cogan said that providers should take steps to ensure that benzodiazepines and nonbenzodiazepine sedatives are used appropriately, which includes tapering dosages and, when appropriate, encouraging nonpharmacologic strategies.
There were no funding or other conflicts of interest associated with this study.
SAN ANTONIO – shows a new study.
While benzodiazepines and nonbenzodiazepine sedative-hypnotics are effective for these indications, misuse and increased health care utilization can ensue from their prolonged use, said Jacob C. Cogan, MD, a fellow in oncology/hematology at the Herbert Irving Comprehensive Cancer Center, Columbia University, New York. Dr. Cogan recently presented the results of the study at the San Antonio Breast Cancer Symposium.
The study included patients with breast cancer who received adjuvant chemotherapy between 2008 and 2017. Prescriptions for sedatives were divided into three periods: 365 days prior to chemotherapy to the start of chemotherapy (period one); start of chemotherapy to 90 days after the end of chemotherapy (period two); and 90-365 days after chemotherapy (period three). Patients who filled at least one benzodiazepine prescription in period two and patients who filled at least two benzodiazepine in period three were classified as new persistent benzodiazepine users. The same definitions were then used for nonbenzodiazepine sedative-hypnotics.
Among 17,532 benzodiazepine-naive patients (mean age, 57 years) and 21,863 nonbenzodiazepine sedative-hypnotic drug–naive patients (mean age, 56 years) who received adjuvant chemotherapy for breast cancer, lumpectomies were performed for a small majority (56.6% benzodiazepine naive, 55.1% nonbenzodiazepine sedative-hypnotics naive) versus mastectomy, and about half of patients received less than 4 months of chemotherapy (48.0% benzodiazepine naive, 48.6% nonbenzodiazepine sedative-hypnotics naive). Among benzodiazepine-naive patients, 4,447 (25.4%) filled at least one benzodiazepine prescription during chemotherapy, and 2,160 (9.9%) filled at least one nonbenzodiazepine sedative-hypnotic prescription during chemotherapy. The rate of new persistent benzodiazepine use after initial exposure during chemotherapy was 26.8% (n = 1,192). Similarly, 33.8% (n = 730) of nonbenzodiazepine sedative-hypnotics users became new persistent users. In addition, 115 patients became new persistent users of both types of sedative-hypnotics.
New persistent benzodiazepine use was associated with several characteristics: age 50-65 (odds ratio, 1.23; P = .01) and age greater than 65 (OR, 1.38, P = .005) relative to age less than 49; as well as Medicaid insurance, relative to commercial and Medicare insurance (OR, 1.68; P < .0001). Both new persistent benzodiazepine and nonbenzodiazepine sedative-hypnotics use was associated with chemotherapy duration of less than 4 months relative to 4 or more months of chemotherapy (OR, 1.17; P = .03 for benzodiazepines; OR, 1.58; P < .0001 for nonbenzodiazepine sedative-hypnotics).
It is not clear why shorter chemotherapy duration is associated with more new persistent use, Dr. Cogan said. “It may be that, paradoxically, a shorter duration of treatment could lead to more anxiety about recurrence. These patients may need closer monitoring of mental health symptoms and earlier referral for psychological services.”
Dr. Cogan said that providers should take steps to ensure that benzodiazepines and nonbenzodiazepine sedatives are used appropriately, which includes tapering dosages and, when appropriate, encouraging nonpharmacologic strategies.
There were no funding or other conflicts of interest associated with this study.
SAN ANTONIO – shows a new study.
While benzodiazepines and nonbenzodiazepine sedative-hypnotics are effective for these indications, misuse and increased health care utilization can ensue from their prolonged use, said Jacob C. Cogan, MD, a fellow in oncology/hematology at the Herbert Irving Comprehensive Cancer Center, Columbia University, New York. Dr. Cogan recently presented the results of the study at the San Antonio Breast Cancer Symposium.
The study included patients with breast cancer who received adjuvant chemotherapy between 2008 and 2017. Prescriptions for sedatives were divided into three periods: 365 days prior to chemotherapy to the start of chemotherapy (period one); start of chemotherapy to 90 days after the end of chemotherapy (period two); and 90-365 days after chemotherapy (period three). Patients who filled at least one benzodiazepine prescription in period two and patients who filled at least two benzodiazepine in period three were classified as new persistent benzodiazepine users. The same definitions were then used for nonbenzodiazepine sedative-hypnotics.
Among 17,532 benzodiazepine-naive patients (mean age, 57 years) and 21,863 nonbenzodiazepine sedative-hypnotic drug–naive patients (mean age, 56 years) who received adjuvant chemotherapy for breast cancer, lumpectomies were performed for a small majority (56.6% benzodiazepine naive, 55.1% nonbenzodiazepine sedative-hypnotics naive) versus mastectomy, and about half of patients received less than 4 months of chemotherapy (48.0% benzodiazepine naive, 48.6% nonbenzodiazepine sedative-hypnotics naive). Among benzodiazepine-naive patients, 4,447 (25.4%) filled at least one benzodiazepine prescription during chemotherapy, and 2,160 (9.9%) filled at least one nonbenzodiazepine sedative-hypnotic prescription during chemotherapy. The rate of new persistent benzodiazepine use after initial exposure during chemotherapy was 26.8% (n = 1,192). Similarly, 33.8% (n = 730) of nonbenzodiazepine sedative-hypnotics users became new persistent users. In addition, 115 patients became new persistent users of both types of sedative-hypnotics.
New persistent benzodiazepine use was associated with several characteristics: age 50-65 (odds ratio, 1.23; P = .01) and age greater than 65 (OR, 1.38, P = .005) relative to age less than 49; as well as Medicaid insurance, relative to commercial and Medicare insurance (OR, 1.68; P < .0001). Both new persistent benzodiazepine and nonbenzodiazepine sedative-hypnotics use was associated with chemotherapy duration of less than 4 months relative to 4 or more months of chemotherapy (OR, 1.17; P = .03 for benzodiazepines; OR, 1.58; P < .0001 for nonbenzodiazepine sedative-hypnotics).
It is not clear why shorter chemotherapy duration is associated with more new persistent use, Dr. Cogan said. “It may be that, paradoxically, a shorter duration of treatment could lead to more anxiety about recurrence. These patients may need closer monitoring of mental health symptoms and earlier referral for psychological services.”
Dr. Cogan said that providers should take steps to ensure that benzodiazepines and nonbenzodiazepine sedatives are used appropriately, which includes tapering dosages and, when appropriate, encouraging nonpharmacologic strategies.
There were no funding or other conflicts of interest associated with this study.
AT SABCS 2021
Multimodal Pain Management With Adductor Canal Block Decreases Opioid Consumption Following Total Knee Arthroplasty
Ease of access to opioids in the perioperative period is a risk factor for opioid misuse and has been identified as a strong risk factor for heroin use.1,2 Three-quarters of today’s heroin users were introduced to opioids through prescription medications.2 The United States accounts for about 80% of the global opioid supply consumption, and deaths from opioid overdose are increasing: 70,630 deaths in 2019 alone.3,4
The Centers for Disease Control and Prevention (CDC) has called for changes in opioid prescribing. The American Academy of Orthopaedic Surgeons (AAOS) also has published an information statement with strategies to decrease opioid misuse and abuse.5,6 Arthroplasty surgeons have recently focused on decreasing use of opioids in total knee arthroplasty (TKA), a procedure traditionally associated with high levels of opioid consumption and historical reliance on opioid monotherapy for postoperative analgesia.7,8 From a clinical perspective, prolonged postoperative opioid use contributes to poorer surgical outcomes due to increased risk of complications, including stiffness, infection, and revision TKA.9
Multimodal pain regimens are increasingly being used to control postoperative pain as data supports their efficacy.10,11 Previous studies have found that simultaneous modulation of multiple pain pathways decreases narcotics consumption and improves patient outcomes.12,13 Along with other adjuvant therapies, peripheral nerve blocks, such as adductor canal block (ACB) and femoral nerve block (FNB), have been used to decrease postoperative pain.14 Studies have shown that ACB has fewer complications and shorter functional recovery times compared with FNB.15,16 The distribution of the ACB excludes the femoral nerve, thus preserving greater quadriceps strength while providing equivalent levels of analgesia compared with FNB.15,17,18 The ACB has shown decreased near-fall events and improved balance scores in the immediate postoperative period.19
Our study analyzed opioid consumption patterns of TKA patients from a US Department of Veterans Affairs (VA) medical center before and after the institution of a multimodal analgesic protocol using ACB. The primary purpose of this study was to determine whether a protocol that included intraoperative spinal anesthesia with a postoperative multimodal analgesic regimen and ACB was associated with a decreased postoperative opioid requirement when compared with patients who received intraoperative general anesthesia and a traditional opioid regimen. Secondary outcomes included the effect of opioid consumption on range of motion on postoperative day (POD) 1 and number of opioid prescriptions written at the first postoperative clinic visit.
Methods
Approval for the study was obtained from the institutional review board at the Dayton Veterans Affairs Medical Center (DVAMC) in Ohio. A retrospective chart review was performed to collect data from all patients undergoing TKA at DVAMC from June 1, 2011, through December 31, 2015. Exclusion criteria included multiple surgeries in the study time frame, documented chronic pain, allergy to local anesthetics, daily preoperative use of opioids, and incomplete data in the health record.
All surgeries were performed by 2 staff arthroplasty surgeons at a single VAMC. All patients attended a preoperative visit where a history, physical, and anesthesia evaluation were performed, and watched an educational video detailing surgical indications and postoperative rehabilitation. All surgeries were performed with tourniquets and a periarticular injection was performed at the conclusion of each case. Surgeon 1 treatment of choice was 10 mL 0.5% bupivacaine, whereas surgeon 2 performed a posterior capsular injection of 30 mL 0.25% bupivacaine and a periarticular injection of 30 mg ketorolac in 10 mL 0.25% bupivacaine with epinephrine.
Prior to August 2014, general endotracheal anesthesia was used intraoperatively. A patient-controlled analgesia (PCA) pump of morphine or hydromorphone and additional oral oxycodone or hydrocodone was used for postoperative pain. PCA pumps were patient dependent. In the control group, 245 patients received the morphine PCA while 61 received the hydromorphone PCA. Morphine PCA dosing consisted of 1-mg doses every 10 minutes with potential baseline infusion rates of 0.5 to 1.0 mg/h and a 4-hour limit of 20 mg. Hydromorphone PCA dosing consisted of 0.2 to 0.4-mg doses with a potential continuous dose of 0.2 to 0.4 mg/h and a 4-hour limit of 4 mg.
In August 2014, a new analgesic protocol was adopted for TKA consisting of intraoperative spinal anesthesia (0.75% bupivacaine) with IV sedation (propofol), a postoperative multimodal analgesic regimen, an ACB performed in the postanesthesia care unit (PACU), and opioids as needed (protocol group). The ACB catheter was a 0.5% ropivo caine hydrochloride injection. It was attached to a local anesthetic fixed flow rate pump that administers 0.5% ropivacaine without epinephrine at 8 mL/h and was removed on POD 5 by the patient. The multimodal medication regimen included IV ketorolac 15 mg every 6 hours for 3 doses, gabapentin 300 mg every 8 hours, acetaminophen 975 mg every 8 hours, meloxicam 7.5 mg daily, tramadol 50 mg every 6 hours, oxycodone 5 mg 1 to 2 tabs every 4 hours as needed, and IV hydromorphone 0.5 mg every 4 hours as needed for breakthrough pain.
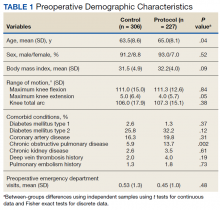
Preoperative demographic characteristics were collected (Table 1). Data on all IV and oral opioid requirements were collected for both groups, converted to morphine milligram equivalents (MME), and a total morphine equivalent dose (MED) was calculated.20,21
In April 2015, a separate protocol change occurred at the DVAMC with the goal of discharge on POD 1. To standardize outcomes before and after this change, data collection regarding opioid requirements was concluded at midnight on POD 1. If a patient was discharged before midnight on POD 1, opioid requirement through the time of discharge was collected. All surgeries were performed in the morning to early afternoon; however, specific surgical times were not collected. Patients were also evaluated by a physical therapist on POD 0, and maximal knee flexion and extension were measured on POD 1. Patients were discharged with prescriptions for oxycodone/acetaminophen and tramadol and were seen 3 weeks later for their first postoperative visit. Opioid refills at the first postoperative visit were recorded. All statistical analyses were performed in SAS 9.4 with significance set to α = 0.05. Between-groups differences in preoperative and perioperative characteristics as well as postoperative outcomes were analyzed using independent samples t tests for continuous variables and Fisher exact tests for dichotomous discrete variables. Where groups differed for a pre- or perioperative variable, linear mixed models analysis was used to determine whether IV, oral, and total MEDs were significantly affected by the interaction between the pre- or perioperative variable with analgesia group. For refills at the postoperative visit, the effects of pre- or perioperative differences were tested using χ2 tests. Effect sizes for outcome variables were estimated using Cohen d and probability of superiority (Δ) for continuous variables, and relative risk (RR) in the case of discrete variables.22
Results
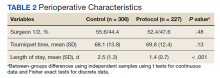
During the study period from June 1, 2011, through December 31, 2015, 533 eligible TKAs were performed, 306 in the control group and 227 in the protocol group. The groups had similar sex distribution; body mass index; knee range of motion; diagnoses of diabetes mellitus, coronary artery disease, and chronic kidney disease; and history of deep vein thrombosis (DVT) or pulmonary embolism (P ≥ .05). The protocol group was significantly older (P = .04) and had a significantly higher rate of chronic obstructive pulmonary disease (COPD) (P = .002). There were no significant differences between number of procedures performed by surgeon (P = .48) or total tourniquet time (P = .13) (Table 2). Mean (SD) length of stay was significantly greater in the control group compared with the protocol group (2.5 [1.3] vs 1.4 [0.7] days, P < .001).
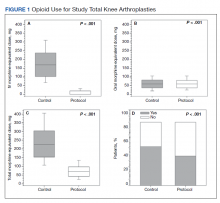
Figure 1 shows the distributions of each type of opioid used. Compared with the control group, the protocol group had a significantly lower mean (SD) IV opioid use: 178.2 (98.0) MED vs 12.0 (24.6) MED (P < .001; d = 2.19; Δ = 0.94) and mean (SD) total opioid use: 241.7 (120.1) MED vs 74.8 (42.7) MED (P < .001; d = 1.76; Δ = 0.89). Mean (SD) oral opioid use did not differ between groups (control, 63.6 [45.4] MED; protocol, 62.9 [31.4] MED; P = .85; d = 0.02; Δ = 0.51). A significantly lower percentage of patients in the protocol group received additional opioids at the 3-week follow-up when compared to the control group: 46.7% vs 61.3%, respectively (P < .001; RR, 0.76; 95% CI, 0.65-0.90).
There were no significant differences in postoperative mean (SD) maximum knee flexion (control, 67.2 [15.7]°; protocol, 67.8 [19.2]°; P = .72; d = 0.03; Δ = 0.51) or mean (SD) total flexion/extension arc (control, 66.2 [15.9]°; protocol, 67.9 [19.4]°; P = .32; d = 0.10; Δ = 0.53). Mean (SD) postoperative maximum knee extension was significantly higher in the protocol group compared with the control group (-0.1 [2.1]° vs 1.0 [3.7]°; P < .001; d = 0.35; Δ = 0.60). More patients in the protocol group (92.5%) were discharged to home compared with the control group (86.6%) (P = .02; RR, 1.07; 95% CI, 1.01-1.13).
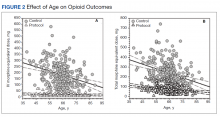
Because age and rates of COPD differed between groups, sensitivity analyses were conducted to determine whether these variables influenced postoperative opioid use. The relationship between age and group was significant for IV (P < .001) and total opioid use (P < .001). Younger patients received higher MED doses than older patients within the control group, while dosages were fairly consistent regardless of age in the protocol group (Figure 2). There was no significance in age interaction effect with regard to oral opioids (P = .83) nor opioid refills at 3-week follow-up (P = .24).
The sensitivity analysis for COPD found that a diagnosis of COPD did not significantly influence utilization of IV opioids (P = .10), or total opioids (P = .68). There was a significant interaction effect for oral opioids (Figure 3). Patients in the control group with COPD required significantly higher mean (SD) oral opioids than patients without COPD (91.5 [123.9] MED and 62.0 [36.0] MED, respectively; P = .03). In the control group, the χ2 test was significant regarding opioid prescription refills at the 3-week visit (P = .004) with 62.4% of patients with COPD requiring refills vs 44.4% without COPD (P = .004). There was no difference in refills in the protocol group (46.4% vs 48.4%).
Finally, 2-sided independent samples t test evaluated total MED use between the 2 surgeons. There was no difference in total MED per patient for the surgeons. In the control group, mean (SD) total MED for surgeon 1 was 232.9 (118.7) MED vs 252.8 (121.5) MED for surgeon 2 (P = .18). In the protocol group, the mean (SD) total MED was 72.5 (43.2) and 77.4 (42.1) for surgeon 1 and surgeon 2, respectively (P = .39).
Discussion
Coordinated efforts with major medical organizations are being made to decrease opioid prescriptions and exposure.5,6 To our knowledge, no study has quantified a decrease in opioid requirement in a VA population after implementation of a protocol that includes intraoperative spinal anesthesia and a postoperative multimodal analgesic regimen including ACB after TKA. The analgesic protocol described in this study aligns with recommendations from both the CDC and the AAOS to decrease opioid use and misuse by maximizing nonopioid medications and limiting the size and number of opioid prescriptions. However, public and medical opinion of opioids as well as prescribing practices have changed over time with a trend toward lower opioid use. The interventions, as part of the described protocol, are a result of these changes and attempt to minimize opioid use while maximizing postoperative analgesia.
Our data showed a significant decrease in total opioid use through POD 1, IV opioid use, and opioid prescriptions provided at the first postoperative visit. The protocol group used only 6.7% of the IV opioids and 30.9% of the total opioids that were used by the control group. The substantial difference in IV opioid requirement, 166.2 MED, is equivalent to 8 mg of IV hydromorphone or 55 mg of IV morphine. The difference in total opioid requirement was similar at 166.9 MED, equivalent to 111 mg of oral oxycodone.
Decreasing opioid use has the additional benefit of improving outcomes, as higher doses of opioids have been associated with increased length of stay, greater rates of DVT, and postoperative infection.23 These complications occurred in a stepwise manner, suggesting a dose-response gradient that makes the sizable decrease noted in our data of greater relevance.23 While the adverse effects (AEs) of opioids are well known, there are limited data on opioid dosing and its effect on perioperative outcomes.23
A significant decrease in the percentage of patients receiving an opioid prescription at the first postoperative visit suggests a decrease in the number of patients on prolonged opioids after TKA with implementation of modern analgesic modalities. The duration of postoperative opioid use has been found to be the strongest predictor of misuse, and each postoperative refill increases the probability of misuse by 44%.24 In addition, opioid use for > 3 months after TKA is associated with increased risk of periprosthetic infection, increased overall revision rate, and stiffness at 1 year postoperatively.9 While not entirely under the control of the surgeon, measures to decrease the number of postoperative opioid refills may lead to a decrease in opioid misuse.
In the control group, older patients tended to receive less opioids. This is likely due to physiologic changes in opioid metabolism associated with aging, including decreased renal and hepatic opioid metabolism and alterations in overall body composition that increase relative potency and duration of action of opioids in a geriatric population.25,26 No difference in opioid use by age was found for the protocol group.
Patients in the protocol group demonstrated significantly greater maximal knee extension on POD 1 compared with the control group. No difference in maximal flexion was found. This difference in extension may partially be explained by the use of an ACB. One benefit of ACB is greater quadriceps strength and fewer near-fall events when compared with FNB.15,19
Our results corroborate the findings of similar studies. A randomized controlled trial comparing a multimodal analgesic regimen with a periarticular injection without a postoperative ACB to a hydromorphone PCA revealed a significant decrease in opioid use in the multimodal analgesic group.27 Along with lower opioid requirements, the multimodal analgesic group had lower visual analog scale pain scores, fewer AEs, faster progression to physical therapy milestones, and higher satisfaction.27 Recent guidelines from the French Society of Anaesthesia and Intensive Care Medicine recommend against the use of gabapentin as a method of postoperative pain control. However, this specifically refers to the preoperative administration of gabapentin. This same set of guidelines later cites a high level of evidence suggesting patients undergoing arthroplasty benefit more from gabapentinoids.28 Multiple analgesic protocols that include gabapentin as a part of a multimodal approach have been shown to have positive results.13,29
In our study, patients receiving the multimodal analgesic regimen were significantly more likely to be discharged home rather than to postacute care facilities, which have been associated with increased rates of major complications, 30-day readmission, and 30-day reoperation.30,31 In addition, discharge to an inpatient rehabilitation or skilled nursing facility has not been found to result in higher functional outcomes, despite $3.2 billion spent yearly on rehabilitation services after primary TKA.32,33
A component of our described analgesic protocol included spinal anesthesia intraoperatively. The differences between groups regarding anesthesia type can be attributed to this protocol change. A significantly greater percentage of patients in the protocol group received spinal anesthesia, while more patients in the control group received general anesthesia. While patients who received spinal anesthesia may have enhanced analgesia in the immediate postoperative period, no differences in opioid outcomes were seen based on anesthesia type. Known benefits of intraoperative spinal anesthesia include decreased perioperative blood loss and a smaller decrease in hemoglobin postoperatively, as well as lower rates of in-hospital complications, including pulmonary embolism, pneumonia, cerebrovascular events, and acute renal failure.34
Limitations
A number of limitations of this study should be noted. One was a protocol change regarding length of stay, which occurred during the study period and resulted in a significantly shorter length of stay in the protocol group. As a result, opioid use data were analyzed only through midnight at the end of POD 1. Patients who were discharged on POD 1 did not have opioid use data available for the full duration of the first POD, which may exaggerate the decrease in opioid requirements, as opioids used after discharge but prior to midnight on POD 1 were not recorded. However, opioids taken at home are oral with a low MME compared with IV opioids received by hospitalized patients in the control group. In addition, if taken as prescribed, patients at home would only have enough time to take a few doses of opioids prior to the midnight cutoff. We do not believe this difference in time of opioid use meaningfully affected the data. An additional limitation includes the variability between periarticular injections between surgeons. While the percentage of patients that received injections from surgeon 1 vs surgeon 2 were similar, it cannot be ruled out as a potential confounding factor. Other limitations include a lack of pain scores to compare subjective pain ratings, the retrospective nature of the study, and a largely homogenous male VA population.
Conclusions
Ease of access to opioids is a risk factor for opioid abuse, which itself is a risk factor for subsequent heroin use.1,2 The CDC and AAOS have thus published recommendations regarding opioid prescribing practices to decrease opioid use and abuse.5,6 Our described protocol, which aligns with these recommendations, resulted in a significant decrease in IV opioid requirement, total opioid requirement, and lower rates of opioid prescriptions provided at the first postoperative visit. These promising findings demonstrate a lower percentage of patients on long-term opioids after TKA and a significantly decreased cumulative opioid exposure.
1. Lankenau SE, Teti M, Silva K, Jackson Bloom J, Harocopos A, Treese M. Initiation into prescription opioid misuse amongst young injection drug users. Int J Drug Policy. 2012;23(1):37-44. doi:10.1016/j.drugpo.2011.05.014
2. Jones CM. Heroin use and heroin use risk behaviors among nonmedical users of prescription opioid pain relievers - United States, 2002-2004 and 2008-2010. Drug Alcohol Depend. 2013;132(1-2):95-100. doi:10.1016/j.drugalcdep.2013.01.007
3. Manchikanti L, Singh A. Therapeutic opioids: a ten-year perspective on the complexities and complications of the escalating use, abuse, and nonmedical use of opioids. Pain Physician. 2008;11(suppl 2):S63-S88.
4. Seth P, Scholl L, Rudd RA, Bacon S. Overdose deaths involving opioids, cocaine, and psychostimulants - United States, 2015-2016. MMWR Morb Mortal Wkly Rep. 2018;67(12):349-358. Published 2018 Mar 30. doi:10.15585/mmwr.mm6712a1
5. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain-United States, 2016. JAMA. 2016;315(15):1624-1645. doi:10.1001/jama.2016.1464
6. American Academy of Orthopaedic Surgeons. Information statement: opioid use, misuse, and abuse in orthopaedic practice. Published October 2015. Accessed November 12, 2021. https://aaos.org/globalassets/about /bylaws-library/information-statements/1045-opioid-use -misuse-and-abuse-in-practice.pdf
7. Hernandez NM, Parry JA, Taunton MJ. Patients at risk: large opioid prescriptions after total knee arthroplasty. J Arthroplasty. 2017;32(8):2395-2398. doi:10.1016/j.arth.2017.02.060
8. Gerner P, Poeran J, Cozowicz C, Mörwald EE, Zubizarreta N, Mazumdar M, Memtsoudis SG, Multimodal pain management in total hip and knee arthroplasty: trends over the last 10 years. Abstract presented at: American Society of Anesthesiologists Annual Meeting; October 21, 2017; Boston, MA.
9. Cancienne JM, Patel KJ, Browne JA, Werner BC. Narcotic use and total knee arthroplasty. J Arthroplasty. 2018;33(1):113-118. doi:10.1016/j.arth.2017.08.006
10. Moucha CS, Weiser MC, Levin EJ. Current strategies in anesthesia and analgesia for total knee arthroplasty. J Am Acad Orthop Surg. 2016;24(2):60-73. doi:10.5435/JAAOS-D-14-00259
11. Wick EC, Grant MC, Wu CL. Postoperative multimodal analgesia pain management with nonopioid analgesics and techniques: a review. JAMA Surg. 2017;152(7):691-697.doi:10.1001/jamasurg.2017.0898
12. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthoplasty. 2014;29(2):329-334. doi:10.1016/j.arth.2013.06.005
13. Golladay GJ, Balch KR, Dalury DF, Satpathy J, Jiranek WA. Oral multimodal analgesia for total joint arthroplasty. J Arthroplasty. 2017;32(9S):S69-S73. doi:10.1016/j.arth.2017.05.002
14. Ardon AE, Clendenen SR, Porter SB, Robards CB, Greengrass RA. Opioid consumption in total knee arthroplasty patients: a retrospective comparison of adductor canal and femoral nerve continuous infusions in the presence of a sciatic nerve catheter. J Clin Anesth. 2016;31:19-26. doi:10.1016/j.jclinane.2015.12.014
15. Li D, Ma GG. Analgesic efficacy and quadriceps strength of adductor canal block versus femoral nerve block following total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2016;24(8):2614-2619. doi:10.1007/s00167-015-3874-3
16. Li D, Yang Z, Xie X, Zhao J, Kang P. Adductor canal block provides better performance after total knee arthroplasty compared with femoral nerve block: a systematic review and meta-analysis. Int Orthop. 2016;40(5):925-933. doi:10.1007/s00264-015-2998-x
17. Horner G, Dellon AL. Innervation of the human knee joint and implications for surgery. Clin Orthop Relat Res. 1994;(301):221-226.
18. Kim DH, Lin Y, Goytizolo EA, et al. Adductor canal block versus femoral nerve block for total knee arthroplasty: a prospective, randomized, controlled trial. Anesthesiology. 2014;120(3):540-550. doi:10.1097/ALN.0000000000000119
19. Thacher RR, Hickernell TR, Grosso MJ, et al. Decreased risk of knee buckling with adductor canal block versus femoral nerve block in total knee arthroplasty: a retrospective cohort study. Arthroplasty Today. 2017;3(4):281-285. Published 2017 Apr 15. doi:10.1016/j.artd.2017.02.008
20. Von Korff M, Saunders K, Thomas Ray G, et al. De facto long-term opioid therapy for noncancer pain [published correction appears in Clin J Pain. 2014 Sep;30(9):830. Korff, Michael Von [corrected to Von Korff, Michael]]. Clin J Pain. 2008;24(6):521-527. doi:10.1097/AJP.0b013e318169d03b
21. Kishner S. Opioid equivalents and conversions: overview. Published January 29, 2018. Accessed November 12, 2021. https://emedicine.medscape.com/article/2138678 -overview#a1
22. Ruscio J, Mullen T. Confidence intervals for the probability of superiority effect size measure and the area under a receiver operating characteristic curve. Multivariate Behav Res. 2012;47(2):201-223. doi:10.1080/00273171.2012.658329
23. Cozowicz C, Olson A, Poeran J, et al. Opioid prescription levels and postoperative outcomes in orthopedic orthopedic surgery. Pain. 2017;158(12):2422-2430. doi:10.1097/j.pain.0000000000001047
24. Brat GA, Agniel D, Beam A, et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: retrospective cohort study. BMJ. 2018;360:j5790. Published 2018 Jan 17. doi:10.1136/bmj.j5790
25. Tegeder I, Lötsch J, Geisslinger G. Pharmacokinetics of opioids in liver disease. Clin Pharmacokinet. 1999;37(1):17- 40. doi:10.2165/00003088-199937010-00002
26. Linnebur SA, O’Connell MB, Wessell AM, et al. Pharmacy practice, research, education, and advocacy for older adults. Pharmacotherapy. 2005;25(10):1396-1430. doi:10.1592/phco.2005.25.10.1396
27. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29(2):329- 334. doi:10.1016/j.arth.2013.06.005
28. Aubrun F, Nouette-Gaulain K, Fletcher D, et al. Revision of expert panel’s guidelines on postoperative pain management. Anaesth Crit Care Pain Med. 2019;38(4):405-411. doi:10.1016/j.accpm.2019.02.011
29. Han C, Li XD, Jiang HQ, Ma JX, Ma XL. The use of gabapentin in the management of postoperative pain after total knee arthroplasty: A PRISMA-compliant metaanalysis of randomized controlled trials [published correction appears in Medicine (Baltimore). 2016 Jul 18;95(28):e0916]. Medicine (Baltimore). 2016;95(23):e3883. doi:10.1097/MD.0000000000003883
30. McLawhorn AS, Fu MC, Schairer WW, Sculco PK, MacLean CH, Padgett DE. Continued inpatient care after primary total knee arthroplasty increases 30-day postdischarge complications: a propensity score-adjusted analysis. J Arthroplasty. 2017;32(9S):S113-S118. doi:10.1016/j.arth.2017.01.039
31. Pelt CE, Gililland JM, Erickson JA, Trimble DE, Anderson MB, Peters CL. Improving value in total joint arthroplasty: a comprehensive patient education and management program decreases discharge to post-acute care facilities and post-operative complications. J Arthroplasty. 2018;33(1):14-18. doi:10.1016/j.arth.2017.08.003
32. Padgett DE, Christ AB, Joseph AD, Lee YY, Haas SB, Lyman S. Discharge to inpatient rehab does not result in improved functional outcomes following primary total knee arthroplasty. J Arthroplasty. 2018;33(6):1663-1667. doi:10.1016/j.arth.2017.12.033
33. Lavernia CJ, D’Apuzzo MR, Hernandez VH, Lee DJ, Rossi MD. Postdischarge costs in arthroplasty surgery. J Arthroplasty. 2006;21(6 Suppl 2):144-150. doi:10.1016/j.arth.2006.05.003
Ease of access to opioids in the perioperative period is a risk factor for opioid misuse and has been identified as a strong risk factor for heroin use.1,2 Three-quarters of today’s heroin users were introduced to opioids through prescription medications.2 The United States accounts for about 80% of the global opioid supply consumption, and deaths from opioid overdose are increasing: 70,630 deaths in 2019 alone.3,4
The Centers for Disease Control and Prevention (CDC) has called for changes in opioid prescribing. The American Academy of Orthopaedic Surgeons (AAOS) also has published an information statement with strategies to decrease opioid misuse and abuse.5,6 Arthroplasty surgeons have recently focused on decreasing use of opioids in total knee arthroplasty (TKA), a procedure traditionally associated with high levels of opioid consumption and historical reliance on opioid monotherapy for postoperative analgesia.7,8 From a clinical perspective, prolonged postoperative opioid use contributes to poorer surgical outcomes due to increased risk of complications, including stiffness, infection, and revision TKA.9
Multimodal pain regimens are increasingly being used to control postoperative pain as data supports their efficacy.10,11 Previous studies have found that simultaneous modulation of multiple pain pathways decreases narcotics consumption and improves patient outcomes.12,13 Along with other adjuvant therapies, peripheral nerve blocks, such as adductor canal block (ACB) and femoral nerve block (FNB), have been used to decrease postoperative pain.14 Studies have shown that ACB has fewer complications and shorter functional recovery times compared with FNB.15,16 The distribution of the ACB excludes the femoral nerve, thus preserving greater quadriceps strength while providing equivalent levels of analgesia compared with FNB.15,17,18 The ACB has shown decreased near-fall events and improved balance scores in the immediate postoperative period.19
Our study analyzed opioid consumption patterns of TKA patients from a US Department of Veterans Affairs (VA) medical center before and after the institution of a multimodal analgesic protocol using ACB. The primary purpose of this study was to determine whether a protocol that included intraoperative spinal anesthesia with a postoperative multimodal analgesic regimen and ACB was associated with a decreased postoperative opioid requirement when compared with patients who received intraoperative general anesthesia and a traditional opioid regimen. Secondary outcomes included the effect of opioid consumption on range of motion on postoperative day (POD) 1 and number of opioid prescriptions written at the first postoperative clinic visit.
Methods
Approval for the study was obtained from the institutional review board at the Dayton Veterans Affairs Medical Center (DVAMC) in Ohio. A retrospective chart review was performed to collect data from all patients undergoing TKA at DVAMC from June 1, 2011, through December 31, 2015. Exclusion criteria included multiple surgeries in the study time frame, documented chronic pain, allergy to local anesthetics, daily preoperative use of opioids, and incomplete data in the health record.
All surgeries were performed by 2 staff arthroplasty surgeons at a single VAMC. All patients attended a preoperative visit where a history, physical, and anesthesia evaluation were performed, and watched an educational video detailing surgical indications and postoperative rehabilitation. All surgeries were performed with tourniquets and a periarticular injection was performed at the conclusion of each case. Surgeon 1 treatment of choice was 10 mL 0.5% bupivacaine, whereas surgeon 2 performed a posterior capsular injection of 30 mL 0.25% bupivacaine and a periarticular injection of 30 mg ketorolac in 10 mL 0.25% bupivacaine with epinephrine.
Prior to August 2014, general endotracheal anesthesia was used intraoperatively. A patient-controlled analgesia (PCA) pump of morphine or hydromorphone and additional oral oxycodone or hydrocodone was used for postoperative pain. PCA pumps were patient dependent. In the control group, 245 patients received the morphine PCA while 61 received the hydromorphone PCA. Morphine PCA dosing consisted of 1-mg doses every 10 minutes with potential baseline infusion rates of 0.5 to 1.0 mg/h and a 4-hour limit of 20 mg. Hydromorphone PCA dosing consisted of 0.2 to 0.4-mg doses with a potential continuous dose of 0.2 to 0.4 mg/h and a 4-hour limit of 4 mg.
In August 2014, a new analgesic protocol was adopted for TKA consisting of intraoperative spinal anesthesia (0.75% bupivacaine) with IV sedation (propofol), a postoperative multimodal analgesic regimen, an ACB performed in the postanesthesia care unit (PACU), and opioids as needed (protocol group). The ACB catheter was a 0.5% ropivo caine hydrochloride injection. It was attached to a local anesthetic fixed flow rate pump that administers 0.5% ropivacaine without epinephrine at 8 mL/h and was removed on POD 5 by the patient. The multimodal medication regimen included IV ketorolac 15 mg every 6 hours for 3 doses, gabapentin 300 mg every 8 hours, acetaminophen 975 mg every 8 hours, meloxicam 7.5 mg daily, tramadol 50 mg every 6 hours, oxycodone 5 mg 1 to 2 tabs every 4 hours as needed, and IV hydromorphone 0.5 mg every 4 hours as needed for breakthrough pain.
Preoperative demographic characteristics were collected (Table 1). Data on all IV and oral opioid requirements were collected for both groups, converted to morphine milligram equivalents (MME), and a total morphine equivalent dose (MED) was calculated.20,21
In April 2015, a separate protocol change occurred at the DVAMC with the goal of discharge on POD 1. To standardize outcomes before and after this change, data collection regarding opioid requirements was concluded at midnight on POD 1. If a patient was discharged before midnight on POD 1, opioid requirement through the time of discharge was collected. All surgeries were performed in the morning to early afternoon; however, specific surgical times were not collected. Patients were also evaluated by a physical therapist on POD 0, and maximal knee flexion and extension were measured on POD 1. Patients were discharged with prescriptions for oxycodone/acetaminophen and tramadol and were seen 3 weeks later for their first postoperative visit. Opioid refills at the first postoperative visit were recorded. All statistical analyses were performed in SAS 9.4 with significance set to α = 0.05. Between-groups differences in preoperative and perioperative characteristics as well as postoperative outcomes were analyzed using independent samples t tests for continuous variables and Fisher exact tests for dichotomous discrete variables. Where groups differed for a pre- or perioperative variable, linear mixed models analysis was used to determine whether IV, oral, and total MEDs were significantly affected by the interaction between the pre- or perioperative variable with analgesia group. For refills at the postoperative visit, the effects of pre- or perioperative differences were tested using χ2 tests. Effect sizes for outcome variables were estimated using Cohen d and probability of superiority (Δ) for continuous variables, and relative risk (RR) in the case of discrete variables.22
Results
During the study period from June 1, 2011, through December 31, 2015, 533 eligible TKAs were performed, 306 in the control group and 227 in the protocol group. The groups had similar sex distribution; body mass index; knee range of motion; diagnoses of diabetes mellitus, coronary artery disease, and chronic kidney disease; and history of deep vein thrombosis (DVT) or pulmonary embolism (P ≥ .05). The protocol group was significantly older (P = .04) and had a significantly higher rate of chronic obstructive pulmonary disease (COPD) (P = .002). There were no significant differences between number of procedures performed by surgeon (P = .48) or total tourniquet time (P = .13) (Table 2). Mean (SD) length of stay was significantly greater in the control group compared with the protocol group (2.5 [1.3] vs 1.4 [0.7] days, P < .001).
Figure 1 shows the distributions of each type of opioid used. Compared with the control group, the protocol group had a significantly lower mean (SD) IV opioid use: 178.2 (98.0) MED vs 12.0 (24.6) MED (P < .001; d = 2.19; Δ = 0.94) and mean (SD) total opioid use: 241.7 (120.1) MED vs 74.8 (42.7) MED (P < .001; d = 1.76; Δ = 0.89). Mean (SD) oral opioid use did not differ between groups (control, 63.6 [45.4] MED; protocol, 62.9 [31.4] MED; P = .85; d = 0.02; Δ = 0.51). A significantly lower percentage of patients in the protocol group received additional opioids at the 3-week follow-up when compared to the control group: 46.7% vs 61.3%, respectively (P < .001; RR, 0.76; 95% CI, 0.65-0.90).
There were no significant differences in postoperative mean (SD) maximum knee flexion (control, 67.2 [15.7]°; protocol, 67.8 [19.2]°; P = .72; d = 0.03; Δ = 0.51) or mean (SD) total flexion/extension arc (control, 66.2 [15.9]°; protocol, 67.9 [19.4]°; P = .32; d = 0.10; Δ = 0.53). Mean (SD) postoperative maximum knee extension was significantly higher in the protocol group compared with the control group (-0.1 [2.1]° vs 1.0 [3.7]°; P < .001; d = 0.35; Δ = 0.60). More patients in the protocol group (92.5%) were discharged to home compared with the control group (86.6%) (P = .02; RR, 1.07; 95% CI, 1.01-1.13).
Because age and rates of COPD differed between groups, sensitivity analyses were conducted to determine whether these variables influenced postoperative opioid use. The relationship between age and group was significant for IV (P < .001) and total opioid use (P < .001). Younger patients received higher MED doses than older patients within the control group, while dosages were fairly consistent regardless of age in the protocol group (Figure 2). There was no significance in age interaction effect with regard to oral opioids (P = .83) nor opioid refills at 3-week follow-up (P = .24).
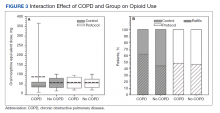
The sensitivity analysis for COPD found that a diagnosis of COPD did not significantly influence utilization of IV opioids (P = .10), or total opioids (P = .68). There was a significant interaction effect for oral opioids (Figure 3). Patients in the control group with COPD required significantly higher mean (SD) oral opioids than patients without COPD (91.5 [123.9] MED and 62.0 [36.0] MED, respectively; P = .03). In the control group, the χ2 test was significant regarding opioid prescription refills at the 3-week visit (P = .004) with 62.4% of patients with COPD requiring refills vs 44.4% without COPD (P = .004). There was no difference in refills in the protocol group (46.4% vs 48.4%).
Finally, 2-sided independent samples t test evaluated total MED use between the 2 surgeons. There was no difference in total MED per patient for the surgeons. In the control group, mean (SD) total MED for surgeon 1 was 232.9 (118.7) MED vs 252.8 (121.5) MED for surgeon 2 (P = .18). In the protocol group, the mean (SD) total MED was 72.5 (43.2) and 77.4 (42.1) for surgeon 1 and surgeon 2, respectively (P = .39).
Discussion
Coordinated efforts with major medical organizations are being made to decrease opioid prescriptions and exposure.5,6 To our knowledge, no study has quantified a decrease in opioid requirement in a VA population after implementation of a protocol that includes intraoperative spinal anesthesia and a postoperative multimodal analgesic regimen including ACB after TKA. The analgesic protocol described in this study aligns with recommendations from both the CDC and the AAOS to decrease opioid use and misuse by maximizing nonopioid medications and limiting the size and number of opioid prescriptions. However, public and medical opinion of opioids as well as prescribing practices have changed over time with a trend toward lower opioid use. The interventions, as part of the described protocol, are a result of these changes and attempt to minimize opioid use while maximizing postoperative analgesia.
Our data showed a significant decrease in total opioid use through POD 1, IV opioid use, and opioid prescriptions provided at the first postoperative visit. The protocol group used only 6.7% of the IV opioids and 30.9% of the total opioids that were used by the control group. The substantial difference in IV opioid requirement, 166.2 MED, is equivalent to 8 mg of IV hydromorphone or 55 mg of IV morphine. The difference in total opioid requirement was similar at 166.9 MED, equivalent to 111 mg of oral oxycodone.
Decreasing opioid use has the additional benefit of improving outcomes, as higher doses of opioids have been associated with increased length of stay, greater rates of DVT, and postoperative infection.23 These complications occurred in a stepwise manner, suggesting a dose-response gradient that makes the sizable decrease noted in our data of greater relevance.23 While the adverse effects (AEs) of opioids are well known, there are limited data on opioid dosing and its effect on perioperative outcomes.23
A significant decrease in the percentage of patients receiving an opioid prescription at the first postoperative visit suggests a decrease in the number of patients on prolonged opioids after TKA with implementation of modern analgesic modalities. The duration of postoperative opioid use has been found to be the strongest predictor of misuse, and each postoperative refill increases the probability of misuse by 44%.24 In addition, opioid use for > 3 months after TKA is associated with increased risk of periprosthetic infection, increased overall revision rate, and stiffness at 1 year postoperatively.9 While not entirely under the control of the surgeon, measures to decrease the number of postoperative opioid refills may lead to a decrease in opioid misuse.
In the control group, older patients tended to receive less opioids. This is likely due to physiologic changes in opioid metabolism associated with aging, including decreased renal and hepatic opioid metabolism and alterations in overall body composition that increase relative potency and duration of action of opioids in a geriatric population.25,26 No difference in opioid use by age was found for the protocol group.
Patients in the protocol group demonstrated significantly greater maximal knee extension on POD 1 compared with the control group. No difference in maximal flexion was found. This difference in extension may partially be explained by the use of an ACB. One benefit of ACB is greater quadriceps strength and fewer near-fall events when compared with FNB.15,19
Our results corroborate the findings of similar studies. A randomized controlled trial comparing a multimodal analgesic regimen with a periarticular injection without a postoperative ACB to a hydromorphone PCA revealed a significant decrease in opioid use in the multimodal analgesic group.27 Along with lower opioid requirements, the multimodal analgesic group had lower visual analog scale pain scores, fewer AEs, faster progression to physical therapy milestones, and higher satisfaction.27 Recent guidelines from the French Society of Anaesthesia and Intensive Care Medicine recommend against the use of gabapentin as a method of postoperative pain control. However, this specifically refers to the preoperative administration of gabapentin. This same set of guidelines later cites a high level of evidence suggesting patients undergoing arthroplasty benefit more from gabapentinoids.28 Multiple analgesic protocols that include gabapentin as a part of a multimodal approach have been shown to have positive results.13,29
In our study, patients receiving the multimodal analgesic regimen were significantly more likely to be discharged home rather than to postacute care facilities, which have been associated with increased rates of major complications, 30-day readmission, and 30-day reoperation.30,31 In addition, discharge to an inpatient rehabilitation or skilled nursing facility has not been found to result in higher functional outcomes, despite $3.2 billion spent yearly on rehabilitation services after primary TKA.32,33
A component of our described analgesic protocol included spinal anesthesia intraoperatively. The differences between groups regarding anesthesia type can be attributed to this protocol change. A significantly greater percentage of patients in the protocol group received spinal anesthesia, while more patients in the control group received general anesthesia. While patients who received spinal anesthesia may have enhanced analgesia in the immediate postoperative period, no differences in opioid outcomes were seen based on anesthesia type. Known benefits of intraoperative spinal anesthesia include decreased perioperative blood loss and a smaller decrease in hemoglobin postoperatively, as well as lower rates of in-hospital complications, including pulmonary embolism, pneumonia, cerebrovascular events, and acute renal failure.34
Limitations
A number of limitations of this study should be noted. One was a protocol change regarding length of stay, which occurred during the study period and resulted in a significantly shorter length of stay in the protocol group. As a result, opioid use data were analyzed only through midnight at the end of POD 1. Patients who were discharged on POD 1 did not have opioid use data available for the full duration of the first POD, which may exaggerate the decrease in opioid requirements, as opioids used after discharge but prior to midnight on POD 1 were not recorded. However, opioids taken at home are oral with a low MME compared with IV opioids received by hospitalized patients in the control group. In addition, if taken as prescribed, patients at home would only have enough time to take a few doses of opioids prior to the midnight cutoff. We do not believe this difference in time of opioid use meaningfully affected the data. An additional limitation includes the variability between periarticular injections between surgeons. While the percentage of patients that received injections from surgeon 1 vs surgeon 2 were similar, it cannot be ruled out as a potential confounding factor. Other limitations include a lack of pain scores to compare subjective pain ratings, the retrospective nature of the study, and a largely homogenous male VA population.
Conclusions
Ease of access to opioids is a risk factor for opioid abuse, which itself is a risk factor for subsequent heroin use.1,2 The CDC and AAOS have thus published recommendations regarding opioid prescribing practices to decrease opioid use and abuse.5,6 Our described protocol, which aligns with these recommendations, resulted in a significant decrease in IV opioid requirement, total opioid requirement, and lower rates of opioid prescriptions provided at the first postoperative visit. These promising findings demonstrate a lower percentage of patients on long-term opioids after TKA and a significantly decreased cumulative opioid exposure.
Ease of access to opioids in the perioperative period is a risk factor for opioid misuse and has been identified as a strong risk factor for heroin use.1,2 Three-quarters of today’s heroin users were introduced to opioids through prescription medications.2 The United States accounts for about 80% of the global opioid supply consumption, and deaths from opioid overdose are increasing: 70,630 deaths in 2019 alone.3,4
The Centers for Disease Control and Prevention (CDC) has called for changes in opioid prescribing. The American Academy of Orthopaedic Surgeons (AAOS) also has published an information statement with strategies to decrease opioid misuse and abuse.5,6 Arthroplasty surgeons have recently focused on decreasing use of opioids in total knee arthroplasty (TKA), a procedure traditionally associated with high levels of opioid consumption and historical reliance on opioid monotherapy for postoperative analgesia.7,8 From a clinical perspective, prolonged postoperative opioid use contributes to poorer surgical outcomes due to increased risk of complications, including stiffness, infection, and revision TKA.9
Multimodal pain regimens are increasingly being used to control postoperative pain as data supports their efficacy.10,11 Previous studies have found that simultaneous modulation of multiple pain pathways decreases narcotics consumption and improves patient outcomes.12,13 Along with other adjuvant therapies, peripheral nerve blocks, such as adductor canal block (ACB) and femoral nerve block (FNB), have been used to decrease postoperative pain.14 Studies have shown that ACB has fewer complications and shorter functional recovery times compared with FNB.15,16 The distribution of the ACB excludes the femoral nerve, thus preserving greater quadriceps strength while providing equivalent levels of analgesia compared with FNB.15,17,18 The ACB has shown decreased near-fall events and improved balance scores in the immediate postoperative period.19
Our study analyzed opioid consumption patterns of TKA patients from a US Department of Veterans Affairs (VA) medical center before and after the institution of a multimodal analgesic protocol using ACB. The primary purpose of this study was to determine whether a protocol that included intraoperative spinal anesthesia with a postoperative multimodal analgesic regimen and ACB was associated with a decreased postoperative opioid requirement when compared with patients who received intraoperative general anesthesia and a traditional opioid regimen. Secondary outcomes included the effect of opioid consumption on range of motion on postoperative day (POD) 1 and number of opioid prescriptions written at the first postoperative clinic visit.
Methods
Approval for the study was obtained from the institutional review board at the Dayton Veterans Affairs Medical Center (DVAMC) in Ohio. A retrospective chart review was performed to collect data from all patients undergoing TKA at DVAMC from June 1, 2011, through December 31, 2015. Exclusion criteria included multiple surgeries in the study time frame, documented chronic pain, allergy to local anesthetics, daily preoperative use of opioids, and incomplete data in the health record.
All surgeries were performed by 2 staff arthroplasty surgeons at a single VAMC. All patients attended a preoperative visit where a history, physical, and anesthesia evaluation were performed, and watched an educational video detailing surgical indications and postoperative rehabilitation. All surgeries were performed with tourniquets and a periarticular injection was performed at the conclusion of each case. Surgeon 1 treatment of choice was 10 mL 0.5% bupivacaine, whereas surgeon 2 performed a posterior capsular injection of 30 mL 0.25% bupivacaine and a periarticular injection of 30 mg ketorolac in 10 mL 0.25% bupivacaine with epinephrine.
Prior to August 2014, general endotracheal anesthesia was used intraoperatively. A patient-controlled analgesia (PCA) pump of morphine or hydromorphone and additional oral oxycodone or hydrocodone was used for postoperative pain. PCA pumps were patient dependent. In the control group, 245 patients received the morphine PCA while 61 received the hydromorphone PCA. Morphine PCA dosing consisted of 1-mg doses every 10 minutes with potential baseline infusion rates of 0.5 to 1.0 mg/h and a 4-hour limit of 20 mg. Hydromorphone PCA dosing consisted of 0.2 to 0.4-mg doses with a potential continuous dose of 0.2 to 0.4 mg/h and a 4-hour limit of 4 mg.
In August 2014, a new analgesic protocol was adopted for TKA consisting of intraoperative spinal anesthesia (0.75% bupivacaine) with IV sedation (propofol), a postoperative multimodal analgesic regimen, an ACB performed in the postanesthesia care unit (PACU), and opioids as needed (protocol group). The ACB catheter was a 0.5% ropivo caine hydrochloride injection. It was attached to a local anesthetic fixed flow rate pump that administers 0.5% ropivacaine without epinephrine at 8 mL/h and was removed on POD 5 by the patient. The multimodal medication regimen included IV ketorolac 15 mg every 6 hours for 3 doses, gabapentin 300 mg every 8 hours, acetaminophen 975 mg every 8 hours, meloxicam 7.5 mg daily, tramadol 50 mg every 6 hours, oxycodone 5 mg 1 to 2 tabs every 4 hours as needed, and IV hydromorphone 0.5 mg every 4 hours as needed for breakthrough pain.
Preoperative demographic characteristics were collected (Table 1). Data on all IV and oral opioid requirements were collected for both groups, converted to morphine milligram equivalents (MME), and a total morphine equivalent dose (MED) was calculated.20,21
In April 2015, a separate protocol change occurred at the DVAMC with the goal of discharge on POD 1. To standardize outcomes before and after this change, data collection regarding opioid requirements was concluded at midnight on POD 1. If a patient was discharged before midnight on POD 1, opioid requirement through the time of discharge was collected. All surgeries were performed in the morning to early afternoon; however, specific surgical times were not collected. Patients were also evaluated by a physical therapist on POD 0, and maximal knee flexion and extension were measured on POD 1. Patients were discharged with prescriptions for oxycodone/acetaminophen and tramadol and were seen 3 weeks later for their first postoperative visit. Opioid refills at the first postoperative visit were recorded. All statistical analyses were performed in SAS 9.4 with significance set to α = 0.05. Between-groups differences in preoperative and perioperative characteristics as well as postoperative outcomes were analyzed using independent samples t tests for continuous variables and Fisher exact tests for dichotomous discrete variables. Where groups differed for a pre- or perioperative variable, linear mixed models analysis was used to determine whether IV, oral, and total MEDs were significantly affected by the interaction between the pre- or perioperative variable with analgesia group. For refills at the postoperative visit, the effects of pre- or perioperative differences were tested using χ2 tests. Effect sizes for outcome variables were estimated using Cohen d and probability of superiority (Δ) for continuous variables, and relative risk (RR) in the case of discrete variables.22
Results
During the study period from June 1, 2011, through December 31, 2015, 533 eligible TKAs were performed, 306 in the control group and 227 in the protocol group. The groups had similar sex distribution; body mass index; knee range of motion; diagnoses of diabetes mellitus, coronary artery disease, and chronic kidney disease; and history of deep vein thrombosis (DVT) or pulmonary embolism (P ≥ .05). The protocol group was significantly older (P = .04) and had a significantly higher rate of chronic obstructive pulmonary disease (COPD) (P = .002). There were no significant differences between number of procedures performed by surgeon (P = .48) or total tourniquet time (P = .13) (Table 2). Mean (SD) length of stay was significantly greater in the control group compared with the protocol group (2.5 [1.3] vs 1.4 [0.7] days, P < .001).
Figure 1 shows the distributions of each type of opioid used. Compared with the control group, the protocol group had a significantly lower mean (SD) IV opioid use: 178.2 (98.0) MED vs 12.0 (24.6) MED (P < .001; d = 2.19; Δ = 0.94) and mean (SD) total opioid use: 241.7 (120.1) MED vs 74.8 (42.7) MED (P < .001; d = 1.76; Δ = 0.89). Mean (SD) oral opioid use did not differ between groups (control, 63.6 [45.4] MED; protocol, 62.9 [31.4] MED; P = .85; d = 0.02; Δ = 0.51). A significantly lower percentage of patients in the protocol group received additional opioids at the 3-week follow-up when compared to the control group: 46.7% vs 61.3%, respectively (P < .001; RR, 0.76; 95% CI, 0.65-0.90).
There were no significant differences in postoperative mean (SD) maximum knee flexion (control, 67.2 [15.7]°; protocol, 67.8 [19.2]°; P = .72; d = 0.03; Δ = 0.51) or mean (SD) total flexion/extension arc (control, 66.2 [15.9]°; protocol, 67.9 [19.4]°; P = .32; d = 0.10; Δ = 0.53). Mean (SD) postoperative maximum knee extension was significantly higher in the protocol group compared with the control group (-0.1 [2.1]° vs 1.0 [3.7]°; P < .001; d = 0.35; Δ = 0.60). More patients in the protocol group (92.5%) were discharged to home compared with the control group (86.6%) (P = .02; RR, 1.07; 95% CI, 1.01-1.13).
Because age and rates of COPD differed between groups, sensitivity analyses were conducted to determine whether these variables influenced postoperative opioid use. The relationship between age and group was significant for IV (P < .001) and total opioid use (P < .001). Younger patients received higher MED doses than older patients within the control group, while dosages were fairly consistent regardless of age in the protocol group (Figure 2). There was no significance in age interaction effect with regard to oral opioids (P = .83) nor opioid refills at 3-week follow-up (P = .24).
The sensitivity analysis for COPD found that a diagnosis of COPD did not significantly influence utilization of IV opioids (P = .10), or total opioids (P = .68). There was a significant interaction effect for oral opioids (Figure 3). Patients in the control group with COPD required significantly higher mean (SD) oral opioids than patients without COPD (91.5 [123.9] MED and 62.0 [36.0] MED, respectively; P = .03). In the control group, the χ2 test was significant regarding opioid prescription refills at the 3-week visit (P = .004) with 62.4% of patients with COPD requiring refills vs 44.4% without COPD (P = .004). There was no difference in refills in the protocol group (46.4% vs 48.4%).
Finally, 2-sided independent samples t test evaluated total MED use between the 2 surgeons. There was no difference in total MED per patient for the surgeons. In the control group, mean (SD) total MED for surgeon 1 was 232.9 (118.7) MED vs 252.8 (121.5) MED for surgeon 2 (P = .18). In the protocol group, the mean (SD) total MED was 72.5 (43.2) and 77.4 (42.1) for surgeon 1 and surgeon 2, respectively (P = .39).
Discussion
Coordinated efforts with major medical organizations are being made to decrease opioid prescriptions and exposure.5,6 To our knowledge, no study has quantified a decrease in opioid requirement in a VA population after implementation of a protocol that includes intraoperative spinal anesthesia and a postoperative multimodal analgesic regimen including ACB after TKA. The analgesic protocol described in this study aligns with recommendations from both the CDC and the AAOS to decrease opioid use and misuse by maximizing nonopioid medications and limiting the size and number of opioid prescriptions. However, public and medical opinion of opioids as well as prescribing practices have changed over time with a trend toward lower opioid use. The interventions, as part of the described protocol, are a result of these changes and attempt to minimize opioid use while maximizing postoperative analgesia.
Our data showed a significant decrease in total opioid use through POD 1, IV opioid use, and opioid prescriptions provided at the first postoperative visit. The protocol group used only 6.7% of the IV opioids and 30.9% of the total opioids that were used by the control group. The substantial difference in IV opioid requirement, 166.2 MED, is equivalent to 8 mg of IV hydromorphone or 55 mg of IV morphine. The difference in total opioid requirement was similar at 166.9 MED, equivalent to 111 mg of oral oxycodone.
Decreasing opioid use has the additional benefit of improving outcomes, as higher doses of opioids have been associated with increased length of stay, greater rates of DVT, and postoperative infection.23 These complications occurred in a stepwise manner, suggesting a dose-response gradient that makes the sizable decrease noted in our data of greater relevance.23 While the adverse effects (AEs) of opioids are well known, there are limited data on opioid dosing and its effect on perioperative outcomes.23
A significant decrease in the percentage of patients receiving an opioid prescription at the first postoperative visit suggests a decrease in the number of patients on prolonged opioids after TKA with implementation of modern analgesic modalities. The duration of postoperative opioid use has been found to be the strongest predictor of misuse, and each postoperative refill increases the probability of misuse by 44%.24 In addition, opioid use for > 3 months after TKA is associated with increased risk of periprosthetic infection, increased overall revision rate, and stiffness at 1 year postoperatively.9 While not entirely under the control of the surgeon, measures to decrease the number of postoperative opioid refills may lead to a decrease in opioid misuse.
In the control group, older patients tended to receive less opioids. This is likely due to physiologic changes in opioid metabolism associated with aging, including decreased renal and hepatic opioid metabolism and alterations in overall body composition that increase relative potency and duration of action of opioids in a geriatric population.25,26 No difference in opioid use by age was found for the protocol group.
Patients in the protocol group demonstrated significantly greater maximal knee extension on POD 1 compared with the control group. No difference in maximal flexion was found. This difference in extension may partially be explained by the use of an ACB. One benefit of ACB is greater quadriceps strength and fewer near-fall events when compared with FNB.15,19
Our results corroborate the findings of similar studies. A randomized controlled trial comparing a multimodal analgesic regimen with a periarticular injection without a postoperative ACB to a hydromorphone PCA revealed a significant decrease in opioid use in the multimodal analgesic group.27 Along with lower opioid requirements, the multimodal analgesic group had lower visual analog scale pain scores, fewer AEs, faster progression to physical therapy milestones, and higher satisfaction.27 Recent guidelines from the French Society of Anaesthesia and Intensive Care Medicine recommend against the use of gabapentin as a method of postoperative pain control. However, this specifically refers to the preoperative administration of gabapentin. This same set of guidelines later cites a high level of evidence suggesting patients undergoing arthroplasty benefit more from gabapentinoids.28 Multiple analgesic protocols that include gabapentin as a part of a multimodal approach have been shown to have positive results.13,29
In our study, patients receiving the multimodal analgesic regimen were significantly more likely to be discharged home rather than to postacute care facilities, which have been associated with increased rates of major complications, 30-day readmission, and 30-day reoperation.30,31 In addition, discharge to an inpatient rehabilitation or skilled nursing facility has not been found to result in higher functional outcomes, despite $3.2 billion spent yearly on rehabilitation services after primary TKA.32,33
A component of our described analgesic protocol included spinal anesthesia intraoperatively. The differences between groups regarding anesthesia type can be attributed to this protocol change. A significantly greater percentage of patients in the protocol group received spinal anesthesia, while more patients in the control group received general anesthesia. While patients who received spinal anesthesia may have enhanced analgesia in the immediate postoperative period, no differences in opioid outcomes were seen based on anesthesia type. Known benefits of intraoperative spinal anesthesia include decreased perioperative blood loss and a smaller decrease in hemoglobin postoperatively, as well as lower rates of in-hospital complications, including pulmonary embolism, pneumonia, cerebrovascular events, and acute renal failure.34
Limitations
A number of limitations of this study should be noted. One was a protocol change regarding length of stay, which occurred during the study period and resulted in a significantly shorter length of stay in the protocol group. As a result, opioid use data were analyzed only through midnight at the end of POD 1. Patients who were discharged on POD 1 did not have opioid use data available for the full duration of the first POD, which may exaggerate the decrease in opioid requirements, as opioids used after discharge but prior to midnight on POD 1 were not recorded. However, opioids taken at home are oral with a low MME compared with IV opioids received by hospitalized patients in the control group. In addition, if taken as prescribed, patients at home would only have enough time to take a few doses of opioids prior to the midnight cutoff. We do not believe this difference in time of opioid use meaningfully affected the data. An additional limitation includes the variability between periarticular injections between surgeons. While the percentage of patients that received injections from surgeon 1 vs surgeon 2 were similar, it cannot be ruled out as a potential confounding factor. Other limitations include a lack of pain scores to compare subjective pain ratings, the retrospective nature of the study, and a largely homogenous male VA population.
Conclusions
Ease of access to opioids is a risk factor for opioid abuse, which itself is a risk factor for subsequent heroin use.1,2 The CDC and AAOS have thus published recommendations regarding opioid prescribing practices to decrease opioid use and abuse.5,6 Our described protocol, which aligns with these recommendations, resulted in a significant decrease in IV opioid requirement, total opioid requirement, and lower rates of opioid prescriptions provided at the first postoperative visit. These promising findings demonstrate a lower percentage of patients on long-term opioids after TKA and a significantly decreased cumulative opioid exposure.
1. Lankenau SE, Teti M, Silva K, Jackson Bloom J, Harocopos A, Treese M. Initiation into prescription opioid misuse amongst young injection drug users. Int J Drug Policy. 2012;23(1):37-44. doi:10.1016/j.drugpo.2011.05.014
2. Jones CM. Heroin use and heroin use risk behaviors among nonmedical users of prescription opioid pain relievers - United States, 2002-2004 and 2008-2010. Drug Alcohol Depend. 2013;132(1-2):95-100. doi:10.1016/j.drugalcdep.2013.01.007
3. Manchikanti L, Singh A. Therapeutic opioids: a ten-year perspective on the complexities and complications of the escalating use, abuse, and nonmedical use of opioids. Pain Physician. 2008;11(suppl 2):S63-S88.
4. Seth P, Scholl L, Rudd RA, Bacon S. Overdose deaths involving opioids, cocaine, and psychostimulants - United States, 2015-2016. MMWR Morb Mortal Wkly Rep. 2018;67(12):349-358. Published 2018 Mar 30. doi:10.15585/mmwr.mm6712a1
5. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain-United States, 2016. JAMA. 2016;315(15):1624-1645. doi:10.1001/jama.2016.1464
6. American Academy of Orthopaedic Surgeons. Information statement: opioid use, misuse, and abuse in orthopaedic practice. Published October 2015. Accessed November 12, 2021. https://aaos.org/globalassets/about /bylaws-library/information-statements/1045-opioid-use -misuse-and-abuse-in-practice.pdf
7. Hernandez NM, Parry JA, Taunton MJ. Patients at risk: large opioid prescriptions after total knee arthroplasty. J Arthroplasty. 2017;32(8):2395-2398. doi:10.1016/j.arth.2017.02.060
8. Gerner P, Poeran J, Cozowicz C, Mörwald EE, Zubizarreta N, Mazumdar M, Memtsoudis SG, Multimodal pain management in total hip and knee arthroplasty: trends over the last 10 years. Abstract presented at: American Society of Anesthesiologists Annual Meeting; October 21, 2017; Boston, MA.
9. Cancienne JM, Patel KJ, Browne JA, Werner BC. Narcotic use and total knee arthroplasty. J Arthroplasty. 2018;33(1):113-118. doi:10.1016/j.arth.2017.08.006
10. Moucha CS, Weiser MC, Levin EJ. Current strategies in anesthesia and analgesia for total knee arthroplasty. J Am Acad Orthop Surg. 2016;24(2):60-73. doi:10.5435/JAAOS-D-14-00259
11. Wick EC, Grant MC, Wu CL. Postoperative multimodal analgesia pain management with nonopioid analgesics and techniques: a review. JAMA Surg. 2017;152(7):691-697.doi:10.1001/jamasurg.2017.0898
12. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthoplasty. 2014;29(2):329-334. doi:10.1016/j.arth.2013.06.005
13. Golladay GJ, Balch KR, Dalury DF, Satpathy J, Jiranek WA. Oral multimodal analgesia for total joint arthroplasty. J Arthroplasty. 2017;32(9S):S69-S73. doi:10.1016/j.arth.2017.05.002
14. Ardon AE, Clendenen SR, Porter SB, Robards CB, Greengrass RA. Opioid consumption in total knee arthroplasty patients: a retrospective comparison of adductor canal and femoral nerve continuous infusions in the presence of a sciatic nerve catheter. J Clin Anesth. 2016;31:19-26. doi:10.1016/j.jclinane.2015.12.014
15. Li D, Ma GG. Analgesic efficacy and quadriceps strength of adductor canal block versus femoral nerve block following total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2016;24(8):2614-2619. doi:10.1007/s00167-015-3874-3
16. Li D, Yang Z, Xie X, Zhao J, Kang P. Adductor canal block provides better performance after total knee arthroplasty compared with femoral nerve block: a systematic review and meta-analysis. Int Orthop. 2016;40(5):925-933. doi:10.1007/s00264-015-2998-x
17. Horner G, Dellon AL. Innervation of the human knee joint and implications for surgery. Clin Orthop Relat Res. 1994;(301):221-226.
18. Kim DH, Lin Y, Goytizolo EA, et al. Adductor canal block versus femoral nerve block for total knee arthroplasty: a prospective, randomized, controlled trial. Anesthesiology. 2014;120(3):540-550. doi:10.1097/ALN.0000000000000119
19. Thacher RR, Hickernell TR, Grosso MJ, et al. Decreased risk of knee buckling with adductor canal block versus femoral nerve block in total knee arthroplasty: a retrospective cohort study. Arthroplasty Today. 2017;3(4):281-285. Published 2017 Apr 15. doi:10.1016/j.artd.2017.02.008
20. Von Korff M, Saunders K, Thomas Ray G, et al. De facto long-term opioid therapy for noncancer pain [published correction appears in Clin J Pain. 2014 Sep;30(9):830. Korff, Michael Von [corrected to Von Korff, Michael]]. Clin J Pain. 2008;24(6):521-527. doi:10.1097/AJP.0b013e318169d03b
21. Kishner S. Opioid equivalents and conversions: overview. Published January 29, 2018. Accessed November 12, 2021. https://emedicine.medscape.com/article/2138678 -overview#a1
22. Ruscio J, Mullen T. Confidence intervals for the probability of superiority effect size measure and the area under a receiver operating characteristic curve. Multivariate Behav Res. 2012;47(2):201-223. doi:10.1080/00273171.2012.658329
23. Cozowicz C, Olson A, Poeran J, et al. Opioid prescription levels and postoperative outcomes in orthopedic orthopedic surgery. Pain. 2017;158(12):2422-2430. doi:10.1097/j.pain.0000000000001047
24. Brat GA, Agniel D, Beam A, et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: retrospective cohort study. BMJ. 2018;360:j5790. Published 2018 Jan 17. doi:10.1136/bmj.j5790
25. Tegeder I, Lötsch J, Geisslinger G. Pharmacokinetics of opioids in liver disease. Clin Pharmacokinet. 1999;37(1):17- 40. doi:10.2165/00003088-199937010-00002
26. Linnebur SA, O’Connell MB, Wessell AM, et al. Pharmacy practice, research, education, and advocacy for older adults. Pharmacotherapy. 2005;25(10):1396-1430. doi:10.1592/phco.2005.25.10.1396
27. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29(2):329- 334. doi:10.1016/j.arth.2013.06.005
28. Aubrun F, Nouette-Gaulain K, Fletcher D, et al. Revision of expert panel’s guidelines on postoperative pain management. Anaesth Crit Care Pain Med. 2019;38(4):405-411. doi:10.1016/j.accpm.2019.02.011
29. Han C, Li XD, Jiang HQ, Ma JX, Ma XL. The use of gabapentin in the management of postoperative pain after total knee arthroplasty: A PRISMA-compliant metaanalysis of randomized controlled trials [published correction appears in Medicine (Baltimore). 2016 Jul 18;95(28):e0916]. Medicine (Baltimore). 2016;95(23):e3883. doi:10.1097/MD.0000000000003883
30. McLawhorn AS, Fu MC, Schairer WW, Sculco PK, MacLean CH, Padgett DE. Continued inpatient care after primary total knee arthroplasty increases 30-day postdischarge complications: a propensity score-adjusted analysis. J Arthroplasty. 2017;32(9S):S113-S118. doi:10.1016/j.arth.2017.01.039
31. Pelt CE, Gililland JM, Erickson JA, Trimble DE, Anderson MB, Peters CL. Improving value in total joint arthroplasty: a comprehensive patient education and management program decreases discharge to post-acute care facilities and post-operative complications. J Arthroplasty. 2018;33(1):14-18. doi:10.1016/j.arth.2017.08.003
32. Padgett DE, Christ AB, Joseph AD, Lee YY, Haas SB, Lyman S. Discharge to inpatient rehab does not result in improved functional outcomes following primary total knee arthroplasty. J Arthroplasty. 2018;33(6):1663-1667. doi:10.1016/j.arth.2017.12.033
33. Lavernia CJ, D’Apuzzo MR, Hernandez VH, Lee DJ, Rossi MD. Postdischarge costs in arthroplasty surgery. J Arthroplasty. 2006;21(6 Suppl 2):144-150. doi:10.1016/j.arth.2006.05.003
1. Lankenau SE, Teti M, Silva K, Jackson Bloom J, Harocopos A, Treese M. Initiation into prescription opioid misuse amongst young injection drug users. Int J Drug Policy. 2012;23(1):37-44. doi:10.1016/j.drugpo.2011.05.014
2. Jones CM. Heroin use and heroin use risk behaviors among nonmedical users of prescription opioid pain relievers - United States, 2002-2004 and 2008-2010. Drug Alcohol Depend. 2013;132(1-2):95-100. doi:10.1016/j.drugalcdep.2013.01.007
3. Manchikanti L, Singh A. Therapeutic opioids: a ten-year perspective on the complexities and complications of the escalating use, abuse, and nonmedical use of opioids. Pain Physician. 2008;11(suppl 2):S63-S88.
4. Seth P, Scholl L, Rudd RA, Bacon S. Overdose deaths involving opioids, cocaine, and psychostimulants - United States, 2015-2016. MMWR Morb Mortal Wkly Rep. 2018;67(12):349-358. Published 2018 Mar 30. doi:10.15585/mmwr.mm6712a1
5. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain-United States, 2016. JAMA. 2016;315(15):1624-1645. doi:10.1001/jama.2016.1464
6. American Academy of Orthopaedic Surgeons. Information statement: opioid use, misuse, and abuse in orthopaedic practice. Published October 2015. Accessed November 12, 2021. https://aaos.org/globalassets/about /bylaws-library/information-statements/1045-opioid-use -misuse-and-abuse-in-practice.pdf
7. Hernandez NM, Parry JA, Taunton MJ. Patients at risk: large opioid prescriptions after total knee arthroplasty. J Arthroplasty. 2017;32(8):2395-2398. doi:10.1016/j.arth.2017.02.060
8. Gerner P, Poeran J, Cozowicz C, Mörwald EE, Zubizarreta N, Mazumdar M, Memtsoudis SG, Multimodal pain management in total hip and knee arthroplasty: trends over the last 10 years. Abstract presented at: American Society of Anesthesiologists Annual Meeting; October 21, 2017; Boston, MA.
9. Cancienne JM, Patel KJ, Browne JA, Werner BC. Narcotic use and total knee arthroplasty. J Arthroplasty. 2018;33(1):113-118. doi:10.1016/j.arth.2017.08.006
10. Moucha CS, Weiser MC, Levin EJ. Current strategies in anesthesia and analgesia for total knee arthroplasty. J Am Acad Orthop Surg. 2016;24(2):60-73. doi:10.5435/JAAOS-D-14-00259
11. Wick EC, Grant MC, Wu CL. Postoperative multimodal analgesia pain management with nonopioid analgesics and techniques: a review. JAMA Surg. 2017;152(7):691-697.doi:10.1001/jamasurg.2017.0898
12. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthoplasty. 2014;29(2):329-334. doi:10.1016/j.arth.2013.06.005
13. Golladay GJ, Balch KR, Dalury DF, Satpathy J, Jiranek WA. Oral multimodal analgesia for total joint arthroplasty. J Arthroplasty. 2017;32(9S):S69-S73. doi:10.1016/j.arth.2017.05.002
14. Ardon AE, Clendenen SR, Porter SB, Robards CB, Greengrass RA. Opioid consumption in total knee arthroplasty patients: a retrospective comparison of adductor canal and femoral nerve continuous infusions in the presence of a sciatic nerve catheter. J Clin Anesth. 2016;31:19-26. doi:10.1016/j.jclinane.2015.12.014
15. Li D, Ma GG. Analgesic efficacy and quadriceps strength of adductor canal block versus femoral nerve block following total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2016;24(8):2614-2619. doi:10.1007/s00167-015-3874-3
16. Li D, Yang Z, Xie X, Zhao J, Kang P. Adductor canal block provides better performance after total knee arthroplasty compared with femoral nerve block: a systematic review and meta-analysis. Int Orthop. 2016;40(5):925-933. doi:10.1007/s00264-015-2998-x
17. Horner G, Dellon AL. Innervation of the human knee joint and implications for surgery. Clin Orthop Relat Res. 1994;(301):221-226.
18. Kim DH, Lin Y, Goytizolo EA, et al. Adductor canal block versus femoral nerve block for total knee arthroplasty: a prospective, randomized, controlled trial. Anesthesiology. 2014;120(3):540-550. doi:10.1097/ALN.0000000000000119
19. Thacher RR, Hickernell TR, Grosso MJ, et al. Decreased risk of knee buckling with adductor canal block versus femoral nerve block in total knee arthroplasty: a retrospective cohort study. Arthroplasty Today. 2017;3(4):281-285. Published 2017 Apr 15. doi:10.1016/j.artd.2017.02.008
20. Von Korff M, Saunders K, Thomas Ray G, et al. De facto long-term opioid therapy for noncancer pain [published correction appears in Clin J Pain. 2014 Sep;30(9):830. Korff, Michael Von [corrected to Von Korff, Michael]]. Clin J Pain. 2008;24(6):521-527. doi:10.1097/AJP.0b013e318169d03b
21. Kishner S. Opioid equivalents and conversions: overview. Published January 29, 2018. Accessed November 12, 2021. https://emedicine.medscape.com/article/2138678 -overview#a1
22. Ruscio J, Mullen T. Confidence intervals for the probability of superiority effect size measure and the area under a receiver operating characteristic curve. Multivariate Behav Res. 2012;47(2):201-223. doi:10.1080/00273171.2012.658329
23. Cozowicz C, Olson A, Poeran J, et al. Opioid prescription levels and postoperative outcomes in orthopedic orthopedic surgery. Pain. 2017;158(12):2422-2430. doi:10.1097/j.pain.0000000000001047
24. Brat GA, Agniel D, Beam A, et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: retrospective cohort study. BMJ. 2018;360:j5790. Published 2018 Jan 17. doi:10.1136/bmj.j5790
25. Tegeder I, Lötsch J, Geisslinger G. Pharmacokinetics of opioids in liver disease. Clin Pharmacokinet. 1999;37(1):17- 40. doi:10.2165/00003088-199937010-00002
26. Linnebur SA, O’Connell MB, Wessell AM, et al. Pharmacy practice, research, education, and advocacy for older adults. Pharmacotherapy. 2005;25(10):1396-1430. doi:10.1592/phco.2005.25.10.1396
27. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29(2):329- 334. doi:10.1016/j.arth.2013.06.005
28. Aubrun F, Nouette-Gaulain K, Fletcher D, et al. Revision of expert panel’s guidelines on postoperative pain management. Anaesth Crit Care Pain Med. 2019;38(4):405-411. doi:10.1016/j.accpm.2019.02.011
29. Han C, Li XD, Jiang HQ, Ma JX, Ma XL. The use of gabapentin in the management of postoperative pain after total knee arthroplasty: A PRISMA-compliant metaanalysis of randomized controlled trials [published correction appears in Medicine (Baltimore). 2016 Jul 18;95(28):e0916]. Medicine (Baltimore). 2016;95(23):e3883. doi:10.1097/MD.0000000000003883
30. McLawhorn AS, Fu MC, Schairer WW, Sculco PK, MacLean CH, Padgett DE. Continued inpatient care after primary total knee arthroplasty increases 30-day postdischarge complications: a propensity score-adjusted analysis. J Arthroplasty. 2017;32(9S):S113-S118. doi:10.1016/j.arth.2017.01.039
31. Pelt CE, Gililland JM, Erickson JA, Trimble DE, Anderson MB, Peters CL. Improving value in total joint arthroplasty: a comprehensive patient education and management program decreases discharge to post-acute care facilities and post-operative complications. J Arthroplasty. 2018;33(1):14-18. doi:10.1016/j.arth.2017.08.003
32. Padgett DE, Christ AB, Joseph AD, Lee YY, Haas SB, Lyman S. Discharge to inpatient rehab does not result in improved functional outcomes following primary total knee arthroplasty. J Arthroplasty. 2018;33(6):1663-1667. doi:10.1016/j.arth.2017.12.033
33. Lavernia CJ, D’Apuzzo MR, Hernandez VH, Lee DJ, Rossi MD. Postdischarge costs in arthroplasty surgery. J Arthroplasty. 2006;21(6 Suppl 2):144-150. doi:10.1016/j.arth.2006.05.003
Using biomarkers to quantify problematic alcohol use
CASE A 34-year-old woman presents with fatigue. She appears defensive when asked about her alcohol use. She answers No to all questions on the CAGE (cut down, annoyed, guilty, eye-opener) screening tool, but acknowledges drinking excessively on rare occasions. Her physician has a high suspicion for alcohol use disorder (AUD) and recommends further testing. The patient agrees but denies having used alcohol over the past several days. Which of the following is most likely to help support the suspicion of a heavy drinking pattern?
- Routine lab tests (liver panel and complete blood count).
- Blood or urine alcohol level.
- Phosphatidylethanol (PEth) level in the blood.
- Ethyl glucuronide (EtG) in the urine.
- Carbohydrate-deficient transferrin (CDT) in the blood.
(See "Case answer.").
About 1 in 12 Americans have AUD,1 and 1 in 10 children live in a home with a parent who has a drinking problem.2 While the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) succinctly defines AUD with specific criteria,1 the term generally refers to an inability to control or stop drinking despite adverse social or health consequences. AUD is regarded as > 4 drinks per day for men and > 3 drinks per day for women.3 A “standard drink” would be a 12-oz bottle of beer, a 5-oz glass of wine, or 1.5 oz of distilled spirits. Effects of chronic alcohol use are vast and include malnutrition, alcohol withdrawal syndrome, alcoholic liver disease, pancreatitis/pancreatic cancer, cardiomyopathy, and stroke.4-6 Alcohol use by a pregnant woman can lead to fetal alcohol syndrome in her child.7
AUD may be more prevalent in the wake of COVID-19. Primary care practitioners tend to miss a large fraction of patients with AUD in their practice, especially younger patients and those without somatic comorbidities.8 Systematic screening for AUD can identify many of these people.8 Particularly as the COVID-19 pandemic continues to unfold and increases stress for everyone, risk of worsening drinking increases both in individuals with current AUD and for those in remission.9 Contrary to common belief, patients visiting primary care favor screening for at-risk drinking.10 Thus, awareness of the prevalence of AUD and patient acceptance of screening should encourage wider testing.
Screening tools. The 2014 guidelines published by the Centers for Disease Control and Prevention recommend using quick screening tools—ie, single question or AUDIT 1-3 (TABLE 111-18)—as an objective means of determining whether patients’ drinking creates a risk for themselves or others.11 Excessive drinking identified using alcohol questionnaires can help reduce medical complications and health care costs.19 The questionnaires we review do not provide a diagnosis but help identify individuals who might benefit from more thorough assessment.20 Following up, as needed, by testing for alcohol biomarkers can provide quantitative insight into problematic alcohol use.2
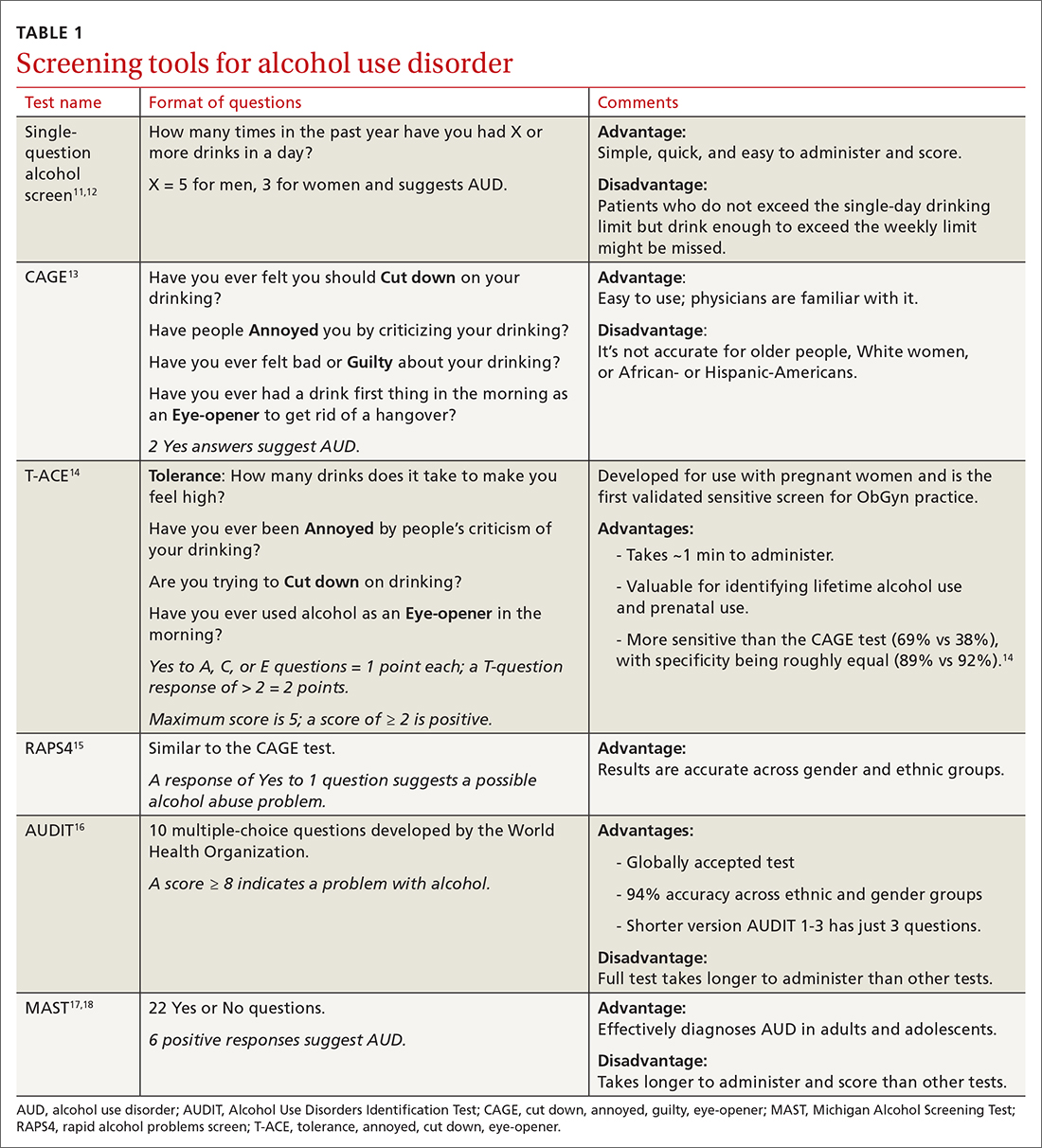
But before we discuss the utility of biomarkers, it’s important to quickly review how alcohol is eliminated from the body.
Alcohol elimination
The stomach and small intestine are the primary sites for alcohol absorption. Alcohol elimination from the body occurs through 3 pathways. The first involves oxidative metabolism, which eliminates most ethanol (95%) through the actions of alcohol dehydrogenase, cytochrome P4502E1, or catalase. A lesser amount of alcohol (2%-5%) is eliminated, unchanged, via the second pathway, which includes urine, sweat, and breath. Nonoxidative metabolism makes up the third pathway. Nonoxidative metabolism removes a very small amount (0.1%) of alcohol and involves the direct ethanol biomarkers PEth, EtG, ethyl sulfate (EtS), and fatty acid ethyl esters (FAEEs).21 Our emphasis in this article is on assays of direct metabolites of alcohol—particularly PEth.
Continue to: To understand the utility...
To understand the utility of these direct biomarkers, it is helpful to look at the indirect biomarkers first.
Indirect biomarkers have limited sensitivity and specificity
When alcohol is consumed in large enough quantities over time, indirect biomarkers of alcohol can become abnormal.22 The major indirect biomarkers are the liver enzymes aspartate and alanine aminotransferase (AST and ALT), gamma-glutamyl transferase (GGT), mean corpuscular volume (MCV) of red blood cells, and carbohydrate-deficient transferrin (CDT). Indirect biomarkers have limited sensitivity and specificity for AUD. (For specifics on sensitivity and specificity of indirect and direct biomarkers, see TABLE 2.23-31)
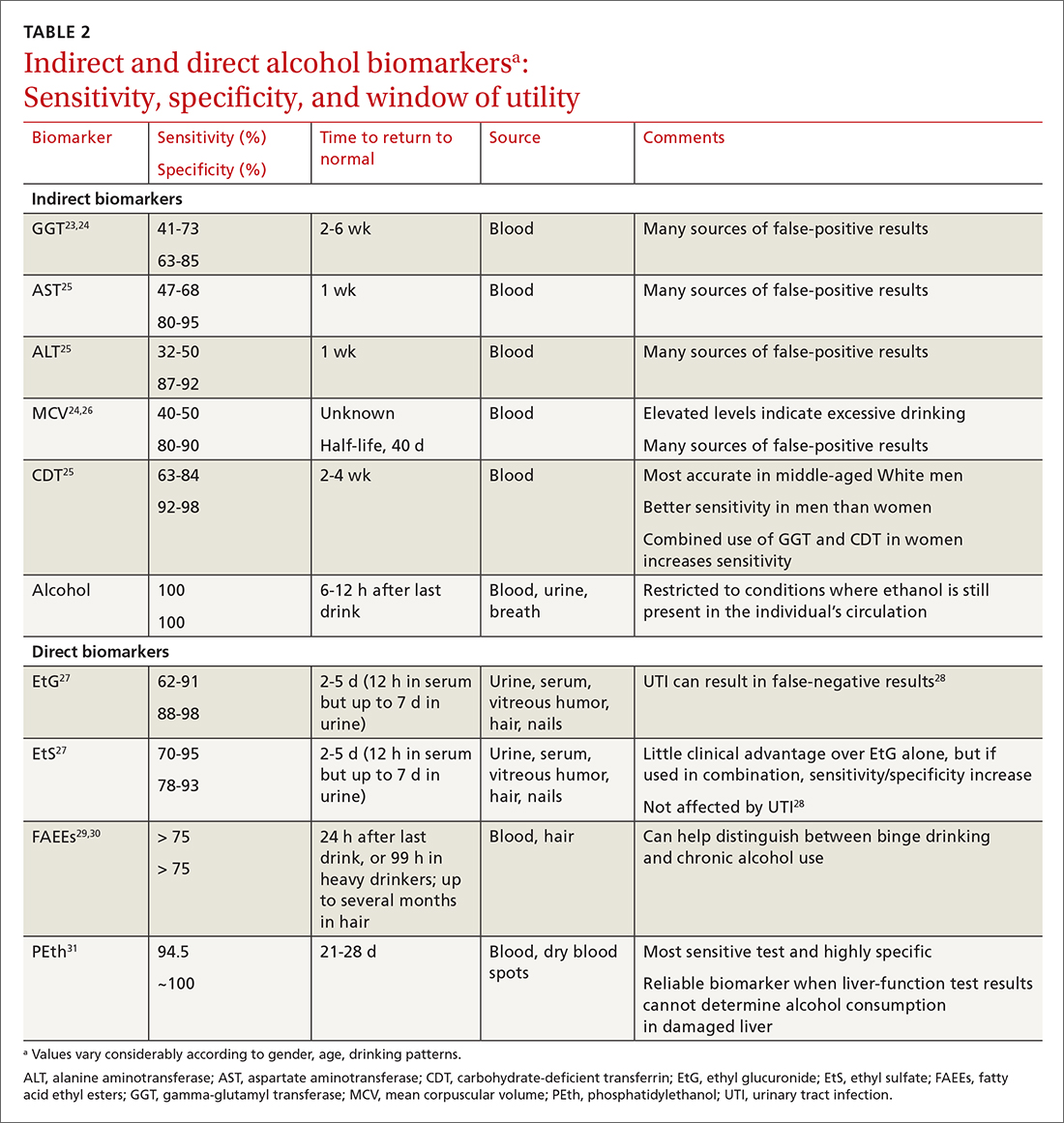
Liver enzymes. AST and ALT are also present in the heart, muscle, and kidneys. Elevated levels usually imply injury to hepatocytes, with ALT being more reflective of liver involvement than AST. Both AST and ALT are elevated in other common liver conditions including hepatitis C virus infection and fatty liver disease. In alcoholic liver disease (ALD), AST is elevated more than ALT; an AST-to-ALT ratio > 3 suggests ALD. An elevated GGT often indicates hepatic injury and is used to confirm that elevated alkaline phosphatase is of hepatic origin.3
MCV is the average volume of erythrocytes,33 and an elevated MCV is a potential indicator of excessive alcohol intake. Macrocytosis requires sustained alcohol use, and the test has low sensitivity. Other diseases such as vitamin B12 or folic acid deficiency, hypothyroidism, hematologic diseases (eg, cold agglutinin disease, multiple myeloma, amyloidosis), and certain medications can also increase MCV.34 Moreover, MCV responds slowly to alcohol use, abstinence, and relapse because red cells have a life span of 120 days.35
CDT. Transferrin is a glycoprotein produced in the liver. The level of transferrin with sialic acid chains increases with alcohol consumption as well as in the carbohydrate deficient glycoprotein syndrome, leading to so-called carbohydrate deficient transferrin.36 It is a sensitive marker for detecting alcohol relapse and monitoring sobriety. Moderate-to-heavy alcohol use, averaging ≥ 40 g of alcohol per day for 2 weeks,36 can decrease the amount of carbohydrate attached to transferrin. Two weeks after complete alcohol cessation, CDT levels will return to normal.37
Continue to: CDT is approved...
CDT is approved by the FDA as an assay for alcohol consumption.37 While CDT is felt to be one of the better indirect markers of AUD and can extend the window of detection, there are still issues with its sensitivity and specificity.38 This biomarker can be elevated with other liver diseases and can be affected by the patient’s age, body mass index, gender, and tobacco use.39,40 Testing for CDT has never achieved widespread clinical use and has been largely supplanted by the more accurate PEth test (described in a bit).
Direct biomarkers offer insight into recent alcohol use
Other than ethanol itself, direct biomarkers of alcohol use are minor ethanol metabolites created through biochemical reactions when ethanol is coupled to endogenous compounds. Hence, the presence of these metabolites is usually directly related to ethanol consumption.41 Direct alcohol biomarkers are EtG, EtS, FAEEs, and PEth (TABLE 223-31). They reflect alcohol consumption over a period of several days, making them useful when paired with questionnaire data, especially for identifying young adults who engage in binge drinking.42
Ethanol can be measured in blood, urine, and breath and is detectable a bit longer in urine than in blood. However, alcohol is detectable in the blood only for 6 to 12 hours after drinking. After alcohol consumption, concentrations peak in the blood within 2 hours. The window for detecting ethanol in the blood depends on the amount of alcohol consumed and the elimination rate of alcohol, which is about 12 mg/dL/h (or 0.012%)—approximately the same amount of alcohol contained in a standard drink (14 g).
Checking the blood alcohol level might be helpful in the office if a patient appears intoxicated but denies alcohol use. A blood alcohol level > 300 mg/dL, or > 150 mg/dL without gross evidence of intoxication, or > 100 mg/dL upon routine examination indicates AUD with a high degree of reliability.33,43 But the short half-life of ethanol in blood limits its use as a biomarker,33 and it is not a good indicator of chronic drinking.44
EtG and EtS. Less than 0.1% of ethanol is secreted as the metabolites EtG and EtS, which are generated, respectively, by the enzymes uridine diphosphate glucuronosyltransferase and sulfotransferase.45 They have value in the diagnosis of AUD because of the length of time in which they can be detected. Urinary EtG and EtS have been especially important biomarkers for monitoring relapse in outpatients treated for alcohol-related problems.46 Generally, EtG and EtS can be detected in urine for 13 to 20 hours after a single drink (0.1 g/kg), and for up to 4 to 5 days following ingestion of large amounts of alcohol.47
Continue to: EtG has been detectable...
EtG has been detectable in urine for ≥ 24 hours following only 1 or 2 drinks, and for up to 4 days following heavy consumption.48 Shortly after alcohol intake, even in small amounts, EtG is detectable. Analysis of EtG in urine is helpful in monitoring alcohol consumption during withdrawal treatment, for workplace testing, and to check for abstinence in legal matters. The EtG urine test is useful in detecting alcohol consumption in a person who claims to be abstinent but who drank 2 or 3 days before the evaluation. Although accurate, EtG’s window for detection is narrower than that of the PEth assay.
EtS is a good marker of acute short-term alcohol use, up to 12 hours in the blood (or longer in heavier drinkers) and up to 5 days in urine.49 Its sensitivity is highest in heavy drinkers. Post-sampling formation and degradation of EtS have not been known to occur in urine samples. Testing for this second metabolite of ethanol can slightly improve the sensitivity and specificity of the EtG test. A urine test for EtS has a wider detection window. But it has little practical advantage compared with EtG.50
For better clinical specificity, a combination of both EtG and EtS testing has been recommended. However, the EtS assay is more cumbersome and provides little advantage over EtG. EtG values do not correlate precisely with the amount or frequency of ethanol use, but the magnitude of the EtG finding roughly corresponds to the amount of alcohol recently consumed.
False-positive and false-negative results for EtG and EtS are uncommon in practice. However, false-positive results are possible with the EtG test in certain circumstances: presence of Escherichia coli in the specimen, use of ethanol-based hand sanitizers (> 20 times a day) or mouthwashes, and the consumption of substances like pralines, nonalcoholic beer, pharmaceutical products, and fruit juice. Similarly, false-negative results of EtG can occur from degradation if the samples are contaminated with other bacteria, transported without cooling, or stored improperly.51 In practice, this is uncommon, and the test is believed to be specific with few false-positive results. Commercially available EtG colorimetric test strips permit on-site analysis of urine samples.
FAEEs are a combination of different esters and products of alcohol metabolism through a nonoxidative pathway. They are formed by esterification of endogenous free fatty acids and ethanol in blood and several tissues.29 These are sensitive and specific markers of alcohol ingestion and can differentiate chronic alcohol consumption from binge drinking.29 It is elevated for up to 99 hours in heavy alcohol drinkers.30 It can be detected in hair for a longer period than in blood.52 Detection of FAEEs in meconium can help establish fetal alcohol exposure.53
Continue to: PEth
PEth. Use of the PEth assay has increased in recent years and its accuracy has had a transformative effect on the diagnosis of AUD.54 PEth is a phospholipid found in erythrocyte membranes, formed by an interaction between ethanol and phosphatidylcholine, catalyzed by phospholipase D.55,56 Major advantages of PEth include an unusually long half-life and specificity. Red cells lack enzymes to degrade PEth, therefore PEth accumulates in red cells and has a half-life of 4 to 10 days57,58 allowing for detection of significant ethanol consumption extending back 3 to 4 weeks.59 There is no evidence that PEth is formed in the absence of ethanol, making the test essentially 100% specific, particularly at higher cutoff values of ≥ 150 ng/mL.31,60
PEth levels are not affected by age, gender, or underlying liver or renal disease.61 PEth can differentiate between heavy alcohol use and social drinking and can therefore identify chronic excessive use.62 With chronic excessive alcohol consumption, PEth is detectable in blood up to 28 days after sobriety.63 A correlation exists between PEth concentrations in blood and the amount of consumed ethanol. PEth has increased specificity and sensitivity for the detection of latent ethanol use compared with other direct biomarkers.21 It can identify recent heavy drinking earlier than indirect biomarkers, as it does not rely on hepatic injury.
Using a cutoff level of 20 ng/mL, PEth assays have a sensitivity of 73% for any alcohol use in the past month; at 80 ng/mL, the sensitivity is 91% for > 4 drinks/d.61 PEth is considered semi-quantitative. The World Health Organization defines acceptable social alcohol use at a PEth value < 40 ng/dL for men and < 20 ng/dL for women. Chronic excessive use is defined by a level > 60 ng/dL.55 The cutoff levels tend to be arbitrary and vary with different guidelines.
Although false-positive PEth test results may be possible, most experts believe that dishonesty in self-reporting by test subjects is more likely. That said, the true specificity of PEth remains unknown; a lower value detected should not be regarded as absolute proof of relapse or chronic alcoholism.
Studies have shown a positive correlation between the AUDIT-C score and PEth values combined with self-reported alcohol consumption, indicating that PEth may be a useful marker in difficult-to-assess settings, or in confirming or invalidating self-reported alcohol consumption.61,64,65 The PEth test is now widely available and, in the authors’ experience, usually costs $100 to $200. Analysis typically costs $40 to $100,66 and costs could decrease as the test becomes more widely used. Turnaround time for PEth is 5 to 10 days. It is now the recommended assay by transplant hepatologists for detecting alcohol use.67

Continue to: CASE ANSWER
CASE
CORRESPONDENCE
Frederick Nunes, MD, Pennsylvania Hospital of University of Pennsylvania, 230 West Washington Square, 4th Floor, Philadelphia, PA 19104; [email protected]
1. APA. Diagnostic and Statistical Manual of Mental Disorders. 5th edition. American Psychiatric Publishing. 2013:490-497.
2. Fleming MF, Smith MJ, Oslakovic E, et al. Phosphatidylethanol detects moderate-to-heavy alcohol use in liver transplant recipients. Alcohol Clin Exp Res. 2017;41:857-862.
3. National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined. Accessed November 12, 2021. www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking
4. Herreros-Villanueva M, Hijona E, Bañales JM, et al. Alcohol consumption on pancreatic diseases. World J Gastroenterol. 2013;19:638-647.
5. Rocco A, Compare D, Angrisani D, et al. Alcoholic disease: liver and beyond. World J Gastroenterol. 2014;20:14652-14659.
6.
7. Sebastiani G, Borrás-Novell C, Casanova MA, et al. The effects of alcohol and drugs of abuse on maternal nutritional profile during pregnancy. Nutrients. 2018;10:1008.
8. Rehm J, Anderson P, Manthey J, et al. Alcohol use disorders in primary health care: what do we know and where do we go? Alcohol Alcohol. 2016;51:422-427. doi: 10.1093/alcalc/agv127
9. ASAM. Caring for patients during the COVID-19 pandemic. Accessed November 12, 2021. www.asam.org/docs/default-source/covid-19/acute-care_062620.pdf?sfvrsn=e66d54c2_10
10. Miller PM, Thomas SE, Mallin R. Patient attitudes towards self-report and biomarker alcohol screening by primary care physicians. Alcohol Alcohol. 2006;41:306-310. doi: 10.1093/alcalc/agl022
11. Zoorob R, Snell H, Kihlberg C, et al. Screening and brief intervention for risky alcohol use. Curr Probl Pediatr Adolesc Health Care. 2014;44:82-87.
12. Smith PC, Schmidt SM, Allensworth-Davies D, et al. Primary care validation of a single-question alcohol screening test. J Gen Intern Med. 2009;24:783-788.
13. Ewing JA. Detecting alcoholism. The CAGE questionnaire. JAMA. 1984;252:1905-1907.
14. Sokol RJ, Martier SS, Ager JW. The T-ACE questions: practical prenatal detection of risk-drinking. Am J Obstet Gynecol. 1989;160:863-868.
15. Cherpitel CJ. A brief screening instrument for problem drinking in the emergency room: the RAPS4. Rapid Alcohol Problems Screen. J Stud Alcohol. 2000;61:447-449.
16. WHO. AUDIT: The alcohol use identification test. Accessed November 14, 2021. http://apps.who.int/iris/bitstream/handle/10665/67205/WHO_MSD_MSB_01.6a.pdf?sequence=1
17. Westermeyer J, Yargic I, Thuras P. Michigan assessment-screening test for alcohol and drugs (MAST/AD): evaluation in a clinical sample. Am J Addict. 2004;13:151-162.
18. Powers JS, Spickard A. Michigan Alcoholism Screening Test to diagnose early alcoholism in a general practice. South Med J. 1984;77:852-856.
19. NIH. Treatment for alcohol problems: finding and getting help. Accessed November 12, 2021. www.niaaa.nih.gov/publications/brochures-and-fact-sheets/treatment-alcohol-problems-finding-and-getting-help
20. Kitchens JM. Does this patient have an alcohol problem? JAMA. 1994;272:1782-1787.
21. Kummer N, Lambert WE, Samyn N, et al. Alternative sampling strategies for the assessment of alcohol intake of living persons. Clin Biochem. 2016;49:1078-1091.
22. Ulwelling W, Smith K. The PEth blood test in the security environment: what it is; why it is important; and interpretative guidelines. J Forensic Sci. 2018;63:1634-1640.
23. Mundle G, Ackermann K, Munkes J, et al. Influence of age, alcohol consumption and abstinence on the sensitivity of carbohydrate‐deficient transferrin, gamma‐glutamyltransferase and mean corpuscular volume. Alcohol Alcohol. 1999;34:760-766.
24. Neumann T, Spies C. Use of biomarkers for alcohol use disorders in clinical practice. Addiction. 2003;98(suppl 2):81-91.
25. Torruellas C, French SW, Medici V. Diagnosis of alcoholic liver disease. World J Gastroenterol. 2014;20:11684-11699.
26. Helander A. Biological markers of alcohol use and abuse in theory and practice. In: Agarwal DP, Seitz HK, eds. Alcohol in Health and Disease. Marcel Dekker. 2001:177-205.
27. Stewart SH, Koch DG, Burgess DM, et al. Sensitivity and specificity of urinary ethyl glucuronide and ethyl sulfate in liver disease patients. Alcohol Clin Exp Res. 2013;37:150-155.
28. Helander A, Dahl H. Urinary tract infection: a risk factor for false-negative urinary ethyl glucuronide but not ethyl sulfate in the detection of recent alcohol consumption. Clin Chem. 2005;51:1728-1730.
29. Ghosh S, Jain R, Jhanjee S, et al. Alcohol biomarkers and their relevance in detection of alcohol consumption in clinical settings. Accessed November 12, 2021. https://www.clinmedjournals.org/articles/iasar/international-archives-of-substance-abuse-and-rehabilitation-iasar-1-002.php?jid=iasar
30. Borucki K, Dierkes J, Wartberg J, et al. In heavy drinkers, fatty acid ethyl esters remain elevated for up to 99 hours. Alcohol Clin Exp Res. 2007;31:423-427.
31. Hartmann S, Aradottir S, Graf M, et al. Phosphatidylethanol as a sensitive and specific biomarker: comparison with gamma-glutamyl transpeptidase, mean corpuscular volume and carbohydrate-deficient transferrin. Addict Biol. 2007;12:81-84.
32. Choe YM, Lee BC, Choi IG, et al. Combination of the CAGE and serum gamma-glutamyl transferase: an effective screening tool for alcohol use disorder and alcohol dependence. Neuropsychiatr Dis Treat. 2019 31;15:1507-1515.
33. Niemelä O. Biomarkers in alcoholism. Clin Chim Acta. 2007;377:39-49.
34. Kauffmann T, Evans DS. Macrocytosis. Accessed November 12, 2021. https://www.ncbi.nlm.nih.gov/books/NBK560908/
35. Maenhout TM, De Buyzere ML, Delanghe JR. Non-oxidative ethanol metabolites as a measure of alcohol intake. Clin Chim Acta. 2013;415:322-329.
36. Solomons HD. Carbohydrate deficient transferrin and alcoholism. Germs. 2012;2:75-78.
37. Allen JP, Wurst FM, Thon N, et al. Assessing the drinking status of liver transplant patients with alcoholic liver disease. Liver Transpl. 2013;19:369-376.
38. Bortolotti F, De Paoli G, Tagliaro F. Carbohydrate-deficient transferrin (CDT) as a marker of alcohol abuse: a critical review of the literature 2001-2005. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;841:96-109.
39. Hannuksela ML, Liisanantti MK, Nissinen AE, et al. Biochemical markers of alcoholism. Clin Chem Lab Med. 2007;45:953-961.
40. Arndt T. Carbohydrate-deficient transferrin as a marker of chronic alcohol abuse: a critical review of preanalysis, analysis, and interpretation. Clin Chem. 2001;47:13-27.
41. Cabarcos P, Hassan HM, Tabernero MJ, et al. Analysis of ethyl glucuronide in hair samples by liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS). J Appl Toxicol. 2013;33:638-643.
42. Piano MR, Mazzuco A, Kang M, et al. Binge drinking episodes in young adults: how should we measure them in a research setting? J Stud Alcohol Drugs. 2017;78:502-511.
43. Adinoff B, Bone GH, Linnoila M. Acute ethanol poisoning and the ethanol withdrawal syndrome. Med Toxicol Adverse Drug Exp. 1988;3:172-196.
44. Cabezas J, Lucey MR, Bataller R. Biomarkers for monitoring alcohol use. Clin Liver Dis (Hoboken). 2016;8:59-63.
45. Wurst FM, Alling C, Aradottir S, et al. Emerging biomarkers: new directions and clinical applications. Alcohol Clin Exp Res. 2005;29:465-473.
46. Helander A, Péter O, Zheng Y. Monitoring of the alcohol biomarkers PEth, CDT and EtG/EtS in an outpatient treatment setting. Alcohol Alcohol. 2012;47:552-557.
47. Helander A, Böttcher M, Fehr C, et al. Detection times for urinary ethyl glucuronide and ethyl sulfate in heavy drinkers during alcohol detoxification. Alcohol Alcohol. 2009;44:55-61.
48. Jatlow P, O’Malley SS. Clinical (nonforensic) application of ethyl glucuronide measurement: are we ready? Alcohol Clin Exp Res. 2010;34:968-975.
49. Jatlow PI, Agro A, Wu R, et al. Ethyl glucuronide and ethyl sulfate assays in clinical trials, interpretation, and limitations: results of a dose ranging alcohol challenge study and 2 clinical trials. Alcohol Clin Exp Res. 2014;38:2056-2065.
50. Gonzalo P, Radenne S, Gonzalo S. Biomarkers of chronic alcohol misuse. Curr Biomark Find. 2014;4:9-22.
51. Bornhorst JA, Mbughuni MM. Alcohol biomarkers: clinical issues and analytical methods. In: Critical Issues in Alcohol and Drugs of Abuse Testing. 2nd ed. Academic Press. 2019:25-42.
52. Soderberg BL, Salem RO, Best CA, et al. Fatty acid ethyl esters. Ethanol metabolites that reflect ethanol intake. Am J Clin Pathol. 2003;119(suppl):S94-S99.
53. Cheng CT, Ostrea EM Jr, Alviedo JN, et al. Fatty acid ethyl esters in meconium: a biomarker of fetal alcohol exposure and effect. Exp Biol Med (Maywood). 2021;246:380-386.
54. Andresen-Streichert H, Beres Y, Weinmann W, et al. Improved detection of alcohol consumption using the novel marker phosphatidylethanol in the transplant setting: results of a prospective study. Transpl Int. 2017;30:611-620.
55. Viel G, Boscolo-Berto R, Cecchetto G, et al. Phosphatidylethanol in blood as a marker of chronic alcohol use: a systematic review and meta-analysis. Int J Mol Sci. 2012;13:14788-14812.
56. Gnann H, Weinmann W, Thierauf A. Formation of phosphatidylethanol and its subsequent elimination during an extensive drinking experiment over 5 days. Alcohol Clin Exp Res. 2012;36:1507-1511.
57. Aradóttir S, Moller K, Alling C. Phosphatidylethanol formation and degradation in human and rat blood. Alcohol Alcohol. 2004;39:8-13.
58. Varga A, Alling C. Formation of phosphatidylethanol in vitro in red blood cells from healthy volunteers and chronic alcoholics. J Lab Clin Med. 2002;140:79-83.
59. Javors MA, Hill-Kapturczak N, Roache JD, et al. Characterization of the pharmacokinetics of phosphatidylethanol 16:0/18:1 and 16:0/18:2 in human whole blood after alcohol consumption in a clinical laboratory study. Alcohol Clin Exp Res. 2016;40:1228-1234.
60. Schröck A, Pfäffli M, König S, et al. Application of phosphatidylethanol (PEth) in whole blood in comparison to ethyl glucuronide in hair (hEtG) in driving aptitude assessment (DAA). Int J Legal Med. 2016;130:1527-1533.
61. Stewart SH, Koch DG, Willner IR, et al. Validation of blood phosphatidylethanol as an alcohol consumption biomarker in patients with chronic liver disease. Alcohol Clin Exp Res. 2014;38:1706-1711.
62. Nanau RM, Neuman MG. Biomolecules and biomarkers used in diagnosis of alcohol drinking and in monitoring therapeutic interventions. Biomolecules. 2015 29;5:1339-1385.
63. Hill-Kapturczak N, Dougherty DM, Roache JD, et al. Phosphatidylethanol homologs in blood as biomarkers for the time frame and amount of recent alcohol consumption. In: Preedy VR (ed) Neuroscience of Alcohol. Academic Press; 2019:567-576.
64. Jain J, Evans JL, Briceño A, et al. Comparison of phosphatidylethanol results to self-reported alcohol consumption among young injection drug users. Alcohol Alcohol. 2014;49:520-524.
65. Schröck A, Wurst FM, Thon N, et al. Assessing phosphatidylethanol (PEth) levels reflecting different drinking habits in comparison to the alcohol use disorders identification test - C (AUDIT-C). Drug Alcohol Depend. 2017;178:80-86.
66. McDonnell MG, Skalisky J, Leickly E, et al. Pilot investigation of a phosphatidylethanol-based contingency management intervention targeting alcohol use. Psychol Addict Behav. 2017;31:608-613.
67. Asrani SK, Trotter J, Lake J, et al. Meeting Report: The Dallas Consensus Conference on Liver Transplantation for Alcohol Associated Hepatitis. Liver Transpl. 2020;26:127-140.
68. WHO. International Guide for Monitoring Alcohol Consumption and Harm. 2000. Accessed November 12, 2021. http://apps.who.int/iris/bitstream/handle/10665/66529/WHO_MSD_MSB_00.4.pdf?sequence=1
CASE A 34-year-old woman presents with fatigue. She appears defensive when asked about her alcohol use. She answers No to all questions on the CAGE (cut down, annoyed, guilty, eye-opener) screening tool, but acknowledges drinking excessively on rare occasions. Her physician has a high suspicion for alcohol use disorder (AUD) and recommends further testing. The patient agrees but denies having used alcohol over the past several days. Which of the following is most likely to help support the suspicion of a heavy drinking pattern?
- Routine lab tests (liver panel and complete blood count).
- Blood or urine alcohol level.
- Phosphatidylethanol (PEth) level in the blood.
- Ethyl glucuronide (EtG) in the urine.
- Carbohydrate-deficient transferrin (CDT) in the blood.
(See "Case answer.").
About 1 in 12 Americans have AUD,1 and 1 in 10 children live in a home with a parent who has a drinking problem.2 While the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) succinctly defines AUD with specific criteria,1 the term generally refers to an inability to control or stop drinking despite adverse social or health consequences. AUD is regarded as > 4 drinks per day for men and > 3 drinks per day for women.3 A “standard drink” would be a 12-oz bottle of beer, a 5-oz glass of wine, or 1.5 oz of distilled spirits. Effects of chronic alcohol use are vast and include malnutrition, alcohol withdrawal syndrome, alcoholic liver disease, pancreatitis/pancreatic cancer, cardiomyopathy, and stroke.4-6 Alcohol use by a pregnant woman can lead to fetal alcohol syndrome in her child.7
AUD may be more prevalent in the wake of COVID-19. Primary care practitioners tend to miss a large fraction of patients with AUD in their practice, especially younger patients and those without somatic comorbidities.8 Systematic screening for AUD can identify many of these people.8 Particularly as the COVID-19 pandemic continues to unfold and increases stress for everyone, risk of worsening drinking increases both in individuals with current AUD and for those in remission.9 Contrary to common belief, patients visiting primary care favor screening for at-risk drinking.10 Thus, awareness of the prevalence of AUD and patient acceptance of screening should encourage wider testing.
Screening tools. The 2014 guidelines published by the Centers for Disease Control and Prevention recommend using quick screening tools—ie, single question or AUDIT 1-3 (TABLE 111-18)—as an objective means of determining whether patients’ drinking creates a risk for themselves or others.11 Excessive drinking identified using alcohol questionnaires can help reduce medical complications and health care costs.19 The questionnaires we review do not provide a diagnosis but help identify individuals who might benefit from more thorough assessment.20 Following up, as needed, by testing for alcohol biomarkers can provide quantitative insight into problematic alcohol use.2

But before we discuss the utility of biomarkers, it’s important to quickly review how alcohol is eliminated from the body.
Alcohol elimination
The stomach and small intestine are the primary sites for alcohol absorption. Alcohol elimination from the body occurs through 3 pathways. The first involves oxidative metabolism, which eliminates most ethanol (95%) through the actions of alcohol dehydrogenase, cytochrome P4502E1, or catalase. A lesser amount of alcohol (2%-5%) is eliminated, unchanged, via the second pathway, which includes urine, sweat, and breath. Nonoxidative metabolism makes up the third pathway. Nonoxidative metabolism removes a very small amount (0.1%) of alcohol and involves the direct ethanol biomarkers PEth, EtG, ethyl sulfate (EtS), and fatty acid ethyl esters (FAEEs).21 Our emphasis in this article is on assays of direct metabolites of alcohol—particularly PEth.
Continue to: To understand the utility...
To understand the utility of these direct biomarkers, it is helpful to look at the indirect biomarkers first.
Indirect biomarkers have limited sensitivity and specificity
When alcohol is consumed in large enough quantities over time, indirect biomarkers of alcohol can become abnormal.22 The major indirect biomarkers are the liver enzymes aspartate and alanine aminotransferase (AST and ALT), gamma-glutamyl transferase (GGT), mean corpuscular volume (MCV) of red blood cells, and carbohydrate-deficient transferrin (CDT). Indirect biomarkers have limited sensitivity and specificity for AUD. (For specifics on sensitivity and specificity of indirect and direct biomarkers, see TABLE 2.23-31)

Liver enzymes. AST and ALT are also present in the heart, muscle, and kidneys. Elevated levels usually imply injury to hepatocytes, with ALT being more reflective of liver involvement than AST. Both AST and ALT are elevated in other common liver conditions including hepatitis C virus infection and fatty liver disease. In alcoholic liver disease (ALD), AST is elevated more than ALT; an AST-to-ALT ratio > 3 suggests ALD. An elevated GGT often indicates hepatic injury and is used to confirm that elevated alkaline phosphatase is of hepatic origin.3
MCV is the average volume of erythrocytes,33 and an elevated MCV is a potential indicator of excessive alcohol intake. Macrocytosis requires sustained alcohol use, and the test has low sensitivity. Other diseases such as vitamin B12 or folic acid deficiency, hypothyroidism, hematologic diseases (eg, cold agglutinin disease, multiple myeloma, amyloidosis), and certain medications can also increase MCV.34 Moreover, MCV responds slowly to alcohol use, abstinence, and relapse because red cells have a life span of 120 days.35
CDT. Transferrin is a glycoprotein produced in the liver. The level of transferrin with sialic acid chains increases with alcohol consumption as well as in the carbohydrate deficient glycoprotein syndrome, leading to so-called carbohydrate deficient transferrin.36 It is a sensitive marker for detecting alcohol relapse and monitoring sobriety. Moderate-to-heavy alcohol use, averaging ≥ 40 g of alcohol per day for 2 weeks,36 can decrease the amount of carbohydrate attached to transferrin. Two weeks after complete alcohol cessation, CDT levels will return to normal.37
Continue to: CDT is approved...
CDT is approved by the FDA as an assay for alcohol consumption.37 While CDT is felt to be one of the better indirect markers of AUD and can extend the window of detection, there are still issues with its sensitivity and specificity.38 This biomarker can be elevated with other liver diseases and can be affected by the patient’s age, body mass index, gender, and tobacco use.39,40 Testing for CDT has never achieved widespread clinical use and has been largely supplanted by the more accurate PEth test (described in a bit).
Direct biomarkers offer insight into recent alcohol use
Other than ethanol itself, direct biomarkers of alcohol use are minor ethanol metabolites created through biochemical reactions when ethanol is coupled to endogenous compounds. Hence, the presence of these metabolites is usually directly related to ethanol consumption.41 Direct alcohol biomarkers are EtG, EtS, FAEEs, and PEth (TABLE 223-31). They reflect alcohol consumption over a period of several days, making them useful when paired with questionnaire data, especially for identifying young adults who engage in binge drinking.42
Ethanol can be measured in blood, urine, and breath and is detectable a bit longer in urine than in blood. However, alcohol is detectable in the blood only for 6 to 12 hours after drinking. After alcohol consumption, concentrations peak in the blood within 2 hours. The window for detecting ethanol in the blood depends on the amount of alcohol consumed and the elimination rate of alcohol, which is about 12 mg/dL/h (or 0.012%)—approximately the same amount of alcohol contained in a standard drink (14 g).
Checking the blood alcohol level might be helpful in the office if a patient appears intoxicated but denies alcohol use. A blood alcohol level > 300 mg/dL, or > 150 mg/dL without gross evidence of intoxication, or > 100 mg/dL upon routine examination indicates AUD with a high degree of reliability.33,43 But the short half-life of ethanol in blood limits its use as a biomarker,33 and it is not a good indicator of chronic drinking.44
EtG and EtS. Less than 0.1% of ethanol is secreted as the metabolites EtG and EtS, which are generated, respectively, by the enzymes uridine diphosphate glucuronosyltransferase and sulfotransferase.45 They have value in the diagnosis of AUD because of the length of time in which they can be detected. Urinary EtG and EtS have been especially important biomarkers for monitoring relapse in outpatients treated for alcohol-related problems.46 Generally, EtG and EtS can be detected in urine for 13 to 20 hours after a single drink (0.1 g/kg), and for up to 4 to 5 days following ingestion of large amounts of alcohol.47
Continue to: EtG has been detectable...
EtG has been detectable in urine for ≥ 24 hours following only 1 or 2 drinks, and for up to 4 days following heavy consumption.48 Shortly after alcohol intake, even in small amounts, EtG is detectable. Analysis of EtG in urine is helpful in monitoring alcohol consumption during withdrawal treatment, for workplace testing, and to check for abstinence in legal matters. The EtG urine test is useful in detecting alcohol consumption in a person who claims to be abstinent but who drank 2 or 3 days before the evaluation. Although accurate, EtG’s window for detection is narrower than that of the PEth assay.
EtS is a good marker of acute short-term alcohol use, up to 12 hours in the blood (or longer in heavier drinkers) and up to 5 days in urine.49 Its sensitivity is highest in heavy drinkers. Post-sampling formation and degradation of EtS have not been known to occur in urine samples. Testing for this second metabolite of ethanol can slightly improve the sensitivity and specificity of the EtG test. A urine test for EtS has a wider detection window. But it has little practical advantage compared with EtG.50
For better clinical specificity, a combination of both EtG and EtS testing has been recommended. However, the EtS assay is more cumbersome and provides little advantage over EtG. EtG values do not correlate precisely with the amount or frequency of ethanol use, but the magnitude of the EtG finding roughly corresponds to the amount of alcohol recently consumed.
False-positive and false-negative results for EtG and EtS are uncommon in practice. However, false-positive results are possible with the EtG test in certain circumstances: presence of Escherichia coli in the specimen, use of ethanol-based hand sanitizers (> 20 times a day) or mouthwashes, and the consumption of substances like pralines, nonalcoholic beer, pharmaceutical products, and fruit juice. Similarly, false-negative results of EtG can occur from degradation if the samples are contaminated with other bacteria, transported without cooling, or stored improperly.51 In practice, this is uncommon, and the test is believed to be specific with few false-positive results. Commercially available EtG colorimetric test strips permit on-site analysis of urine samples.
FAEEs are a combination of different esters and products of alcohol metabolism through a nonoxidative pathway. They are formed by esterification of endogenous free fatty acids and ethanol in blood and several tissues.29 These are sensitive and specific markers of alcohol ingestion and can differentiate chronic alcohol consumption from binge drinking.29 It is elevated for up to 99 hours in heavy alcohol drinkers.30 It can be detected in hair for a longer period than in blood.52 Detection of FAEEs in meconium can help establish fetal alcohol exposure.53
Continue to: PEth
PEth. Use of the PEth assay has increased in recent years and its accuracy has had a transformative effect on the diagnosis of AUD.54 PEth is a phospholipid found in erythrocyte membranes, formed by an interaction between ethanol and phosphatidylcholine, catalyzed by phospholipase D.55,56 Major advantages of PEth include an unusually long half-life and specificity. Red cells lack enzymes to degrade PEth, therefore PEth accumulates in red cells and has a half-life of 4 to 10 days57,58 allowing for detection of significant ethanol consumption extending back 3 to 4 weeks.59 There is no evidence that PEth is formed in the absence of ethanol, making the test essentially 100% specific, particularly at higher cutoff values of ≥ 150 ng/mL.31,60
PEth levels are not affected by age, gender, or underlying liver or renal disease.61 PEth can differentiate between heavy alcohol use and social drinking and can therefore identify chronic excessive use.62 With chronic excessive alcohol consumption, PEth is detectable in blood up to 28 days after sobriety.63 A correlation exists between PEth concentrations in blood and the amount of consumed ethanol. PEth has increased specificity and sensitivity for the detection of latent ethanol use compared with other direct biomarkers.21 It can identify recent heavy drinking earlier than indirect biomarkers, as it does not rely on hepatic injury.
Using a cutoff level of 20 ng/mL, PEth assays have a sensitivity of 73% for any alcohol use in the past month; at 80 ng/mL, the sensitivity is 91% for > 4 drinks/d.61 PEth is considered semi-quantitative. The World Health Organization defines acceptable social alcohol use at a PEth value < 40 ng/dL for men and < 20 ng/dL for women. Chronic excessive use is defined by a level > 60 ng/dL.55 The cutoff levels tend to be arbitrary and vary with different guidelines.
Although false-positive PEth test results may be possible, most experts believe that dishonesty in self-reporting by test subjects is more likely. That said, the true specificity of PEth remains unknown; a lower value detected should not be regarded as absolute proof of relapse or chronic alcoholism.
Studies have shown a positive correlation between the AUDIT-C score and PEth values combined with self-reported alcohol consumption, indicating that PEth may be a useful marker in difficult-to-assess settings, or in confirming or invalidating self-reported alcohol consumption.61,64,65 The PEth test is now widely available and, in the authors’ experience, usually costs $100 to $200. Analysis typically costs $40 to $100,66 and costs could decrease as the test becomes more widely used. Turnaround time for PEth is 5 to 10 days. It is now the recommended assay by transplant hepatologists for detecting alcohol use.67

Continue to: CASE ANSWER
CASE
CORRESPONDENCE
Frederick Nunes, MD, Pennsylvania Hospital of University of Pennsylvania, 230 West Washington Square, 4th Floor, Philadelphia, PA 19104; [email protected]
CASE A 34-year-old woman presents with fatigue. She appears defensive when asked about her alcohol use. She answers No to all questions on the CAGE (cut down, annoyed, guilty, eye-opener) screening tool, but acknowledges drinking excessively on rare occasions. Her physician has a high suspicion for alcohol use disorder (AUD) and recommends further testing. The patient agrees but denies having used alcohol over the past several days. Which of the following is most likely to help support the suspicion of a heavy drinking pattern?
- Routine lab tests (liver panel and complete blood count).
- Blood or urine alcohol level.
- Phosphatidylethanol (PEth) level in the blood.
- Ethyl glucuronide (EtG) in the urine.
- Carbohydrate-deficient transferrin (CDT) in the blood.
(See "Case answer.").
About 1 in 12 Americans have AUD,1 and 1 in 10 children live in a home with a parent who has a drinking problem.2 While the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) succinctly defines AUD with specific criteria,1 the term generally refers to an inability to control or stop drinking despite adverse social or health consequences. AUD is regarded as > 4 drinks per day for men and > 3 drinks per day for women.3 A “standard drink” would be a 12-oz bottle of beer, a 5-oz glass of wine, or 1.5 oz of distilled spirits. Effects of chronic alcohol use are vast and include malnutrition, alcohol withdrawal syndrome, alcoholic liver disease, pancreatitis/pancreatic cancer, cardiomyopathy, and stroke.4-6 Alcohol use by a pregnant woman can lead to fetal alcohol syndrome in her child.7
AUD may be more prevalent in the wake of COVID-19. Primary care practitioners tend to miss a large fraction of patients with AUD in their practice, especially younger patients and those without somatic comorbidities.8 Systematic screening for AUD can identify many of these people.8 Particularly as the COVID-19 pandemic continues to unfold and increases stress for everyone, risk of worsening drinking increases both in individuals with current AUD and for those in remission.9 Contrary to common belief, patients visiting primary care favor screening for at-risk drinking.10 Thus, awareness of the prevalence of AUD and patient acceptance of screening should encourage wider testing.
Screening tools. The 2014 guidelines published by the Centers for Disease Control and Prevention recommend using quick screening tools—ie, single question or AUDIT 1-3 (TABLE 111-18)—as an objective means of determining whether patients’ drinking creates a risk for themselves or others.11 Excessive drinking identified using alcohol questionnaires can help reduce medical complications and health care costs.19 The questionnaires we review do not provide a diagnosis but help identify individuals who might benefit from more thorough assessment.20 Following up, as needed, by testing for alcohol biomarkers can provide quantitative insight into problematic alcohol use.2

But before we discuss the utility of biomarkers, it’s important to quickly review how alcohol is eliminated from the body.
Alcohol elimination
The stomach and small intestine are the primary sites for alcohol absorption. Alcohol elimination from the body occurs through 3 pathways. The first involves oxidative metabolism, which eliminates most ethanol (95%) through the actions of alcohol dehydrogenase, cytochrome P4502E1, or catalase. A lesser amount of alcohol (2%-5%) is eliminated, unchanged, via the second pathway, which includes urine, sweat, and breath. Nonoxidative metabolism makes up the third pathway. Nonoxidative metabolism removes a very small amount (0.1%) of alcohol and involves the direct ethanol biomarkers PEth, EtG, ethyl sulfate (EtS), and fatty acid ethyl esters (FAEEs).21 Our emphasis in this article is on assays of direct metabolites of alcohol—particularly PEth.
Continue to: To understand the utility...
To understand the utility of these direct biomarkers, it is helpful to look at the indirect biomarkers first.
Indirect biomarkers have limited sensitivity and specificity
When alcohol is consumed in large enough quantities over time, indirect biomarkers of alcohol can become abnormal.22 The major indirect biomarkers are the liver enzymes aspartate and alanine aminotransferase (AST and ALT), gamma-glutamyl transferase (GGT), mean corpuscular volume (MCV) of red blood cells, and carbohydrate-deficient transferrin (CDT). Indirect biomarkers have limited sensitivity and specificity for AUD. (For specifics on sensitivity and specificity of indirect and direct biomarkers, see TABLE 2.23-31)

Liver enzymes. AST and ALT are also present in the heart, muscle, and kidneys. Elevated levels usually imply injury to hepatocytes, with ALT being more reflective of liver involvement than AST. Both AST and ALT are elevated in other common liver conditions including hepatitis C virus infection and fatty liver disease. In alcoholic liver disease (ALD), AST is elevated more than ALT; an AST-to-ALT ratio > 3 suggests ALD. An elevated GGT often indicates hepatic injury and is used to confirm that elevated alkaline phosphatase is of hepatic origin.3
MCV is the average volume of erythrocytes,33 and an elevated MCV is a potential indicator of excessive alcohol intake. Macrocytosis requires sustained alcohol use, and the test has low sensitivity. Other diseases such as vitamin B12 or folic acid deficiency, hypothyroidism, hematologic diseases (eg, cold agglutinin disease, multiple myeloma, amyloidosis), and certain medications can also increase MCV.34 Moreover, MCV responds slowly to alcohol use, abstinence, and relapse because red cells have a life span of 120 days.35
CDT. Transferrin is a glycoprotein produced in the liver. The level of transferrin with sialic acid chains increases with alcohol consumption as well as in the carbohydrate deficient glycoprotein syndrome, leading to so-called carbohydrate deficient transferrin.36 It is a sensitive marker for detecting alcohol relapse and monitoring sobriety. Moderate-to-heavy alcohol use, averaging ≥ 40 g of alcohol per day for 2 weeks,36 can decrease the amount of carbohydrate attached to transferrin. Two weeks after complete alcohol cessation, CDT levels will return to normal.37
Continue to: CDT is approved...
CDT is approved by the FDA as an assay for alcohol consumption.37 While CDT is felt to be one of the better indirect markers of AUD and can extend the window of detection, there are still issues with its sensitivity and specificity.38 This biomarker can be elevated with other liver diseases and can be affected by the patient’s age, body mass index, gender, and tobacco use.39,40 Testing for CDT has never achieved widespread clinical use and has been largely supplanted by the more accurate PEth test (described in a bit).
Direct biomarkers offer insight into recent alcohol use
Other than ethanol itself, direct biomarkers of alcohol use are minor ethanol metabolites created through biochemical reactions when ethanol is coupled to endogenous compounds. Hence, the presence of these metabolites is usually directly related to ethanol consumption.41 Direct alcohol biomarkers are EtG, EtS, FAEEs, and PEth (TABLE 223-31). They reflect alcohol consumption over a period of several days, making them useful when paired with questionnaire data, especially for identifying young adults who engage in binge drinking.42
Ethanol can be measured in blood, urine, and breath and is detectable a bit longer in urine than in blood. However, alcohol is detectable in the blood only for 6 to 12 hours after drinking. After alcohol consumption, concentrations peak in the blood within 2 hours. The window for detecting ethanol in the blood depends on the amount of alcohol consumed and the elimination rate of alcohol, which is about 12 mg/dL/h (or 0.012%)—approximately the same amount of alcohol contained in a standard drink (14 g).
Checking the blood alcohol level might be helpful in the office if a patient appears intoxicated but denies alcohol use. A blood alcohol level > 300 mg/dL, or > 150 mg/dL without gross evidence of intoxication, or > 100 mg/dL upon routine examination indicates AUD with a high degree of reliability.33,43 But the short half-life of ethanol in blood limits its use as a biomarker,33 and it is not a good indicator of chronic drinking.44
EtG and EtS. Less than 0.1% of ethanol is secreted as the metabolites EtG and EtS, which are generated, respectively, by the enzymes uridine diphosphate glucuronosyltransferase and sulfotransferase.45 They have value in the diagnosis of AUD because of the length of time in which they can be detected. Urinary EtG and EtS have been especially important biomarkers for monitoring relapse in outpatients treated for alcohol-related problems.46 Generally, EtG and EtS can be detected in urine for 13 to 20 hours after a single drink (0.1 g/kg), and for up to 4 to 5 days following ingestion of large amounts of alcohol.47
Continue to: EtG has been detectable...
EtG has been detectable in urine for ≥ 24 hours following only 1 or 2 drinks, and for up to 4 days following heavy consumption.48 Shortly after alcohol intake, even in small amounts, EtG is detectable. Analysis of EtG in urine is helpful in monitoring alcohol consumption during withdrawal treatment, for workplace testing, and to check for abstinence in legal matters. The EtG urine test is useful in detecting alcohol consumption in a person who claims to be abstinent but who drank 2 or 3 days before the evaluation. Although accurate, EtG’s window for detection is narrower than that of the PEth assay.
EtS is a good marker of acute short-term alcohol use, up to 12 hours in the blood (or longer in heavier drinkers) and up to 5 days in urine.49 Its sensitivity is highest in heavy drinkers. Post-sampling formation and degradation of EtS have not been known to occur in urine samples. Testing for this second metabolite of ethanol can slightly improve the sensitivity and specificity of the EtG test. A urine test for EtS has a wider detection window. But it has little practical advantage compared with EtG.50
For better clinical specificity, a combination of both EtG and EtS testing has been recommended. However, the EtS assay is more cumbersome and provides little advantage over EtG. EtG values do not correlate precisely with the amount or frequency of ethanol use, but the magnitude of the EtG finding roughly corresponds to the amount of alcohol recently consumed.
False-positive and false-negative results for EtG and EtS are uncommon in practice. However, false-positive results are possible with the EtG test in certain circumstances: presence of Escherichia coli in the specimen, use of ethanol-based hand sanitizers (> 20 times a day) or mouthwashes, and the consumption of substances like pralines, nonalcoholic beer, pharmaceutical products, and fruit juice. Similarly, false-negative results of EtG can occur from degradation if the samples are contaminated with other bacteria, transported without cooling, or stored improperly.51 In practice, this is uncommon, and the test is believed to be specific with few false-positive results. Commercially available EtG colorimetric test strips permit on-site analysis of urine samples.
FAEEs are a combination of different esters and products of alcohol metabolism through a nonoxidative pathway. They are formed by esterification of endogenous free fatty acids and ethanol in blood and several tissues.29 These are sensitive and specific markers of alcohol ingestion and can differentiate chronic alcohol consumption from binge drinking.29 It is elevated for up to 99 hours in heavy alcohol drinkers.30 It can be detected in hair for a longer period than in blood.52 Detection of FAEEs in meconium can help establish fetal alcohol exposure.53
Continue to: PEth
PEth. Use of the PEth assay has increased in recent years and its accuracy has had a transformative effect on the diagnosis of AUD.54 PEth is a phospholipid found in erythrocyte membranes, formed by an interaction between ethanol and phosphatidylcholine, catalyzed by phospholipase D.55,56 Major advantages of PEth include an unusually long half-life and specificity. Red cells lack enzymes to degrade PEth, therefore PEth accumulates in red cells and has a half-life of 4 to 10 days57,58 allowing for detection of significant ethanol consumption extending back 3 to 4 weeks.59 There is no evidence that PEth is formed in the absence of ethanol, making the test essentially 100% specific, particularly at higher cutoff values of ≥ 150 ng/mL.31,60
PEth levels are not affected by age, gender, or underlying liver or renal disease.61 PEth can differentiate between heavy alcohol use and social drinking and can therefore identify chronic excessive use.62 With chronic excessive alcohol consumption, PEth is detectable in blood up to 28 days after sobriety.63 A correlation exists between PEth concentrations in blood and the amount of consumed ethanol. PEth has increased specificity and sensitivity for the detection of latent ethanol use compared with other direct biomarkers.21 It can identify recent heavy drinking earlier than indirect biomarkers, as it does not rely on hepatic injury.
Using a cutoff level of 20 ng/mL, PEth assays have a sensitivity of 73% for any alcohol use in the past month; at 80 ng/mL, the sensitivity is 91% for > 4 drinks/d.61 PEth is considered semi-quantitative. The World Health Organization defines acceptable social alcohol use at a PEth value < 40 ng/dL for men and < 20 ng/dL for women. Chronic excessive use is defined by a level > 60 ng/dL.55 The cutoff levels tend to be arbitrary and vary with different guidelines.
Although false-positive PEth test results may be possible, most experts believe that dishonesty in self-reporting by test subjects is more likely. That said, the true specificity of PEth remains unknown; a lower value detected should not be regarded as absolute proof of relapse or chronic alcoholism.
Studies have shown a positive correlation between the AUDIT-C score and PEth values combined with self-reported alcohol consumption, indicating that PEth may be a useful marker in difficult-to-assess settings, or in confirming or invalidating self-reported alcohol consumption.61,64,65 The PEth test is now widely available and, in the authors’ experience, usually costs $100 to $200. Analysis typically costs $40 to $100,66 and costs could decrease as the test becomes more widely used. Turnaround time for PEth is 5 to 10 days. It is now the recommended assay by transplant hepatologists for detecting alcohol use.67

Continue to: CASE ANSWER
CASE
CORRESPONDENCE
Frederick Nunes, MD, Pennsylvania Hospital of University of Pennsylvania, 230 West Washington Square, 4th Floor, Philadelphia, PA 19104; [email protected]
1. APA. Diagnostic and Statistical Manual of Mental Disorders. 5th edition. American Psychiatric Publishing. 2013:490-497.
2. Fleming MF, Smith MJ, Oslakovic E, et al. Phosphatidylethanol detects moderate-to-heavy alcohol use in liver transplant recipients. Alcohol Clin Exp Res. 2017;41:857-862.
3. National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined. Accessed November 12, 2021. www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking
4. Herreros-Villanueva M, Hijona E, Bañales JM, et al. Alcohol consumption on pancreatic diseases. World J Gastroenterol. 2013;19:638-647.
5. Rocco A, Compare D, Angrisani D, et al. Alcoholic disease: liver and beyond. World J Gastroenterol. 2014;20:14652-14659.
6.
7. Sebastiani G, Borrás-Novell C, Casanova MA, et al. The effects of alcohol and drugs of abuse on maternal nutritional profile during pregnancy. Nutrients. 2018;10:1008.
8. Rehm J, Anderson P, Manthey J, et al. Alcohol use disorders in primary health care: what do we know and where do we go? Alcohol Alcohol. 2016;51:422-427. doi: 10.1093/alcalc/agv127
9. ASAM. Caring for patients during the COVID-19 pandemic. Accessed November 12, 2021. www.asam.org/docs/default-source/covid-19/acute-care_062620.pdf?sfvrsn=e66d54c2_10
10. Miller PM, Thomas SE, Mallin R. Patient attitudes towards self-report and biomarker alcohol screening by primary care physicians. Alcohol Alcohol. 2006;41:306-310. doi: 10.1093/alcalc/agl022
11. Zoorob R, Snell H, Kihlberg C, et al. Screening and brief intervention for risky alcohol use. Curr Probl Pediatr Adolesc Health Care. 2014;44:82-87.
12. Smith PC, Schmidt SM, Allensworth-Davies D, et al. Primary care validation of a single-question alcohol screening test. J Gen Intern Med. 2009;24:783-788.
13. Ewing JA. Detecting alcoholism. The CAGE questionnaire. JAMA. 1984;252:1905-1907.
14. Sokol RJ, Martier SS, Ager JW. The T-ACE questions: practical prenatal detection of risk-drinking. Am J Obstet Gynecol. 1989;160:863-868.
15. Cherpitel CJ. A brief screening instrument for problem drinking in the emergency room: the RAPS4. Rapid Alcohol Problems Screen. J Stud Alcohol. 2000;61:447-449.
16. WHO. AUDIT: The alcohol use identification test. Accessed November 14, 2021. http://apps.who.int/iris/bitstream/handle/10665/67205/WHO_MSD_MSB_01.6a.pdf?sequence=1
17. Westermeyer J, Yargic I, Thuras P. Michigan assessment-screening test for alcohol and drugs (MAST/AD): evaluation in a clinical sample. Am J Addict. 2004;13:151-162.
18. Powers JS, Spickard A. Michigan Alcoholism Screening Test to diagnose early alcoholism in a general practice. South Med J. 1984;77:852-856.
19. NIH. Treatment for alcohol problems: finding and getting help. Accessed November 12, 2021. www.niaaa.nih.gov/publications/brochures-and-fact-sheets/treatment-alcohol-problems-finding-and-getting-help
20. Kitchens JM. Does this patient have an alcohol problem? JAMA. 1994;272:1782-1787.
21. Kummer N, Lambert WE, Samyn N, et al. Alternative sampling strategies for the assessment of alcohol intake of living persons. Clin Biochem. 2016;49:1078-1091.
22. Ulwelling W, Smith K. The PEth blood test in the security environment: what it is; why it is important; and interpretative guidelines. J Forensic Sci. 2018;63:1634-1640.
23. Mundle G, Ackermann K, Munkes J, et al. Influence of age, alcohol consumption and abstinence on the sensitivity of carbohydrate‐deficient transferrin, gamma‐glutamyltransferase and mean corpuscular volume. Alcohol Alcohol. 1999;34:760-766.
24. Neumann T, Spies C. Use of biomarkers for alcohol use disorders in clinical practice. Addiction. 2003;98(suppl 2):81-91.
25. Torruellas C, French SW, Medici V. Diagnosis of alcoholic liver disease. World J Gastroenterol. 2014;20:11684-11699.
26. Helander A. Biological markers of alcohol use and abuse in theory and practice. In: Agarwal DP, Seitz HK, eds. Alcohol in Health and Disease. Marcel Dekker. 2001:177-205.
27. Stewart SH, Koch DG, Burgess DM, et al. Sensitivity and specificity of urinary ethyl glucuronide and ethyl sulfate in liver disease patients. Alcohol Clin Exp Res. 2013;37:150-155.
28. Helander A, Dahl H. Urinary tract infection: a risk factor for false-negative urinary ethyl glucuronide but not ethyl sulfate in the detection of recent alcohol consumption. Clin Chem. 2005;51:1728-1730.
29. Ghosh S, Jain R, Jhanjee S, et al. Alcohol biomarkers and their relevance in detection of alcohol consumption in clinical settings. Accessed November 12, 2021. https://www.clinmedjournals.org/articles/iasar/international-archives-of-substance-abuse-and-rehabilitation-iasar-1-002.php?jid=iasar
30. Borucki K, Dierkes J, Wartberg J, et al. In heavy drinkers, fatty acid ethyl esters remain elevated for up to 99 hours. Alcohol Clin Exp Res. 2007;31:423-427.
31. Hartmann S, Aradottir S, Graf M, et al. Phosphatidylethanol as a sensitive and specific biomarker: comparison with gamma-glutamyl transpeptidase, mean corpuscular volume and carbohydrate-deficient transferrin. Addict Biol. 2007;12:81-84.
32. Choe YM, Lee BC, Choi IG, et al. Combination of the CAGE and serum gamma-glutamyl transferase: an effective screening tool for alcohol use disorder and alcohol dependence. Neuropsychiatr Dis Treat. 2019 31;15:1507-1515.
33. Niemelä O. Biomarkers in alcoholism. Clin Chim Acta. 2007;377:39-49.
34. Kauffmann T, Evans DS. Macrocytosis. Accessed November 12, 2021. https://www.ncbi.nlm.nih.gov/books/NBK560908/
35. Maenhout TM, De Buyzere ML, Delanghe JR. Non-oxidative ethanol metabolites as a measure of alcohol intake. Clin Chim Acta. 2013;415:322-329.
36. Solomons HD. Carbohydrate deficient transferrin and alcoholism. Germs. 2012;2:75-78.
37. Allen JP, Wurst FM, Thon N, et al. Assessing the drinking status of liver transplant patients with alcoholic liver disease. Liver Transpl. 2013;19:369-376.
38. Bortolotti F, De Paoli G, Tagliaro F. Carbohydrate-deficient transferrin (CDT) as a marker of alcohol abuse: a critical review of the literature 2001-2005. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;841:96-109.
39. Hannuksela ML, Liisanantti MK, Nissinen AE, et al. Biochemical markers of alcoholism. Clin Chem Lab Med. 2007;45:953-961.
40. Arndt T. Carbohydrate-deficient transferrin as a marker of chronic alcohol abuse: a critical review of preanalysis, analysis, and interpretation. Clin Chem. 2001;47:13-27.
41. Cabarcos P, Hassan HM, Tabernero MJ, et al. Analysis of ethyl glucuronide in hair samples by liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS). J Appl Toxicol. 2013;33:638-643.
42. Piano MR, Mazzuco A, Kang M, et al. Binge drinking episodes in young adults: how should we measure them in a research setting? J Stud Alcohol Drugs. 2017;78:502-511.
43. Adinoff B, Bone GH, Linnoila M. Acute ethanol poisoning and the ethanol withdrawal syndrome. Med Toxicol Adverse Drug Exp. 1988;3:172-196.
44. Cabezas J, Lucey MR, Bataller R. Biomarkers for monitoring alcohol use. Clin Liver Dis (Hoboken). 2016;8:59-63.
45. Wurst FM, Alling C, Aradottir S, et al. Emerging biomarkers: new directions and clinical applications. Alcohol Clin Exp Res. 2005;29:465-473.
46. Helander A, Péter O, Zheng Y. Monitoring of the alcohol biomarkers PEth, CDT and EtG/EtS in an outpatient treatment setting. Alcohol Alcohol. 2012;47:552-557.
47. Helander A, Böttcher M, Fehr C, et al. Detection times for urinary ethyl glucuronide and ethyl sulfate in heavy drinkers during alcohol detoxification. Alcohol Alcohol. 2009;44:55-61.
48. Jatlow P, O’Malley SS. Clinical (nonforensic) application of ethyl glucuronide measurement: are we ready? Alcohol Clin Exp Res. 2010;34:968-975.
49. Jatlow PI, Agro A, Wu R, et al. Ethyl glucuronide and ethyl sulfate assays in clinical trials, interpretation, and limitations: results of a dose ranging alcohol challenge study and 2 clinical trials. Alcohol Clin Exp Res. 2014;38:2056-2065.
50. Gonzalo P, Radenne S, Gonzalo S. Biomarkers of chronic alcohol misuse. Curr Biomark Find. 2014;4:9-22.
51. Bornhorst JA, Mbughuni MM. Alcohol biomarkers: clinical issues and analytical methods. In: Critical Issues in Alcohol and Drugs of Abuse Testing. 2nd ed. Academic Press. 2019:25-42.
52. Soderberg BL, Salem RO, Best CA, et al. Fatty acid ethyl esters. Ethanol metabolites that reflect ethanol intake. Am J Clin Pathol. 2003;119(suppl):S94-S99.
53. Cheng CT, Ostrea EM Jr, Alviedo JN, et al. Fatty acid ethyl esters in meconium: a biomarker of fetal alcohol exposure and effect. Exp Biol Med (Maywood). 2021;246:380-386.
54. Andresen-Streichert H, Beres Y, Weinmann W, et al. Improved detection of alcohol consumption using the novel marker phosphatidylethanol in the transplant setting: results of a prospective study. Transpl Int. 2017;30:611-620.
55. Viel G, Boscolo-Berto R, Cecchetto G, et al. Phosphatidylethanol in blood as a marker of chronic alcohol use: a systematic review and meta-analysis. Int J Mol Sci. 2012;13:14788-14812.
56. Gnann H, Weinmann W, Thierauf A. Formation of phosphatidylethanol and its subsequent elimination during an extensive drinking experiment over 5 days. Alcohol Clin Exp Res. 2012;36:1507-1511.
57. Aradóttir S, Moller K, Alling C. Phosphatidylethanol formation and degradation in human and rat blood. Alcohol Alcohol. 2004;39:8-13.
58. Varga A, Alling C. Formation of phosphatidylethanol in vitro in red blood cells from healthy volunteers and chronic alcoholics. J Lab Clin Med. 2002;140:79-83.
59. Javors MA, Hill-Kapturczak N, Roache JD, et al. Characterization of the pharmacokinetics of phosphatidylethanol 16:0/18:1 and 16:0/18:2 in human whole blood after alcohol consumption in a clinical laboratory study. Alcohol Clin Exp Res. 2016;40:1228-1234.
60. Schröck A, Pfäffli M, König S, et al. Application of phosphatidylethanol (PEth) in whole blood in comparison to ethyl glucuronide in hair (hEtG) in driving aptitude assessment (DAA). Int J Legal Med. 2016;130:1527-1533.
61. Stewart SH, Koch DG, Willner IR, et al. Validation of blood phosphatidylethanol as an alcohol consumption biomarker in patients with chronic liver disease. Alcohol Clin Exp Res. 2014;38:1706-1711.
62. Nanau RM, Neuman MG. Biomolecules and biomarkers used in diagnosis of alcohol drinking and in monitoring therapeutic interventions. Biomolecules. 2015 29;5:1339-1385.
63. Hill-Kapturczak N, Dougherty DM, Roache JD, et al. Phosphatidylethanol homologs in blood as biomarkers for the time frame and amount of recent alcohol consumption. In: Preedy VR (ed) Neuroscience of Alcohol. Academic Press; 2019:567-576.
64. Jain J, Evans JL, Briceño A, et al. Comparison of phosphatidylethanol results to self-reported alcohol consumption among young injection drug users. Alcohol Alcohol. 2014;49:520-524.
65. Schröck A, Wurst FM, Thon N, et al. Assessing phosphatidylethanol (PEth) levels reflecting different drinking habits in comparison to the alcohol use disorders identification test - C (AUDIT-C). Drug Alcohol Depend. 2017;178:80-86.
66. McDonnell MG, Skalisky J, Leickly E, et al. Pilot investigation of a phosphatidylethanol-based contingency management intervention targeting alcohol use. Psychol Addict Behav. 2017;31:608-613.
67. Asrani SK, Trotter J, Lake J, et al. Meeting Report: The Dallas Consensus Conference on Liver Transplantation for Alcohol Associated Hepatitis. Liver Transpl. 2020;26:127-140.
68. WHO. International Guide for Monitoring Alcohol Consumption and Harm. 2000. Accessed November 12, 2021. http://apps.who.int/iris/bitstream/handle/10665/66529/WHO_MSD_MSB_00.4.pdf?sequence=1
1. APA. Diagnostic and Statistical Manual of Mental Disorders. 5th edition. American Psychiatric Publishing. 2013:490-497.
2. Fleming MF, Smith MJ, Oslakovic E, et al. Phosphatidylethanol detects moderate-to-heavy alcohol use in liver transplant recipients. Alcohol Clin Exp Res. 2017;41:857-862.
3. National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined. Accessed November 12, 2021. www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking
4. Herreros-Villanueva M, Hijona E, Bañales JM, et al. Alcohol consumption on pancreatic diseases. World J Gastroenterol. 2013;19:638-647.
5. Rocco A, Compare D, Angrisani D, et al. Alcoholic disease: liver and beyond. World J Gastroenterol. 2014;20:14652-14659.
6.
7. Sebastiani G, Borrás-Novell C, Casanova MA, et al. The effects of alcohol and drugs of abuse on maternal nutritional profile during pregnancy. Nutrients. 2018;10:1008.
8. Rehm J, Anderson P, Manthey J, et al. Alcohol use disorders in primary health care: what do we know and where do we go? Alcohol Alcohol. 2016;51:422-427. doi: 10.1093/alcalc/agv127
9. ASAM. Caring for patients during the COVID-19 pandemic. Accessed November 12, 2021. www.asam.org/docs/default-source/covid-19/acute-care_062620.pdf?sfvrsn=e66d54c2_10
10. Miller PM, Thomas SE, Mallin R. Patient attitudes towards self-report and biomarker alcohol screening by primary care physicians. Alcohol Alcohol. 2006;41:306-310. doi: 10.1093/alcalc/agl022
11. Zoorob R, Snell H, Kihlberg C, et al. Screening and brief intervention for risky alcohol use. Curr Probl Pediatr Adolesc Health Care. 2014;44:82-87.
12. Smith PC, Schmidt SM, Allensworth-Davies D, et al. Primary care validation of a single-question alcohol screening test. J Gen Intern Med. 2009;24:783-788.
13. Ewing JA. Detecting alcoholism. The CAGE questionnaire. JAMA. 1984;252:1905-1907.
14. Sokol RJ, Martier SS, Ager JW. The T-ACE questions: practical prenatal detection of risk-drinking. Am J Obstet Gynecol. 1989;160:863-868.
15. Cherpitel CJ. A brief screening instrument for problem drinking in the emergency room: the RAPS4. Rapid Alcohol Problems Screen. J Stud Alcohol. 2000;61:447-449.
16. WHO. AUDIT: The alcohol use identification test. Accessed November 14, 2021. http://apps.who.int/iris/bitstream/handle/10665/67205/WHO_MSD_MSB_01.6a.pdf?sequence=1
17. Westermeyer J, Yargic I, Thuras P. Michigan assessment-screening test for alcohol and drugs (MAST/AD): evaluation in a clinical sample. Am J Addict. 2004;13:151-162.
18. Powers JS, Spickard A. Michigan Alcoholism Screening Test to diagnose early alcoholism in a general practice. South Med J. 1984;77:852-856.
19. NIH. Treatment for alcohol problems: finding and getting help. Accessed November 12, 2021. www.niaaa.nih.gov/publications/brochures-and-fact-sheets/treatment-alcohol-problems-finding-and-getting-help
20. Kitchens JM. Does this patient have an alcohol problem? JAMA. 1994;272:1782-1787.
21. Kummer N, Lambert WE, Samyn N, et al. Alternative sampling strategies for the assessment of alcohol intake of living persons. Clin Biochem. 2016;49:1078-1091.
22. Ulwelling W, Smith K. The PEth blood test in the security environment: what it is; why it is important; and interpretative guidelines. J Forensic Sci. 2018;63:1634-1640.
23. Mundle G, Ackermann K, Munkes J, et al. Influence of age, alcohol consumption and abstinence on the sensitivity of carbohydrate‐deficient transferrin, gamma‐glutamyltransferase and mean corpuscular volume. Alcohol Alcohol. 1999;34:760-766.
24. Neumann T, Spies C. Use of biomarkers for alcohol use disorders in clinical practice. Addiction. 2003;98(suppl 2):81-91.
25. Torruellas C, French SW, Medici V. Diagnosis of alcoholic liver disease. World J Gastroenterol. 2014;20:11684-11699.
26. Helander A. Biological markers of alcohol use and abuse in theory and practice. In: Agarwal DP, Seitz HK, eds. Alcohol in Health and Disease. Marcel Dekker. 2001:177-205.
27. Stewart SH, Koch DG, Burgess DM, et al. Sensitivity and specificity of urinary ethyl glucuronide and ethyl sulfate in liver disease patients. Alcohol Clin Exp Res. 2013;37:150-155.
28. Helander A, Dahl H. Urinary tract infection: a risk factor for false-negative urinary ethyl glucuronide but not ethyl sulfate in the detection of recent alcohol consumption. Clin Chem. 2005;51:1728-1730.
29. Ghosh S, Jain R, Jhanjee S, et al. Alcohol biomarkers and their relevance in detection of alcohol consumption in clinical settings. Accessed November 12, 2021. https://www.clinmedjournals.org/articles/iasar/international-archives-of-substance-abuse-and-rehabilitation-iasar-1-002.php?jid=iasar
30. Borucki K, Dierkes J, Wartberg J, et al. In heavy drinkers, fatty acid ethyl esters remain elevated for up to 99 hours. Alcohol Clin Exp Res. 2007;31:423-427.
31. Hartmann S, Aradottir S, Graf M, et al. Phosphatidylethanol as a sensitive and specific biomarker: comparison with gamma-glutamyl transpeptidase, mean corpuscular volume and carbohydrate-deficient transferrin. Addict Biol. 2007;12:81-84.
32. Choe YM, Lee BC, Choi IG, et al. Combination of the CAGE and serum gamma-glutamyl transferase: an effective screening tool for alcohol use disorder and alcohol dependence. Neuropsychiatr Dis Treat. 2019 31;15:1507-1515.
33. Niemelä O. Biomarkers in alcoholism. Clin Chim Acta. 2007;377:39-49.
34. Kauffmann T, Evans DS. Macrocytosis. Accessed November 12, 2021. https://www.ncbi.nlm.nih.gov/books/NBK560908/
35. Maenhout TM, De Buyzere ML, Delanghe JR. Non-oxidative ethanol metabolites as a measure of alcohol intake. Clin Chim Acta. 2013;415:322-329.
36. Solomons HD. Carbohydrate deficient transferrin and alcoholism. Germs. 2012;2:75-78.
37. Allen JP, Wurst FM, Thon N, et al. Assessing the drinking status of liver transplant patients with alcoholic liver disease. Liver Transpl. 2013;19:369-376.
38. Bortolotti F, De Paoli G, Tagliaro F. Carbohydrate-deficient transferrin (CDT) as a marker of alcohol abuse: a critical review of the literature 2001-2005. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;841:96-109.
39. Hannuksela ML, Liisanantti MK, Nissinen AE, et al. Biochemical markers of alcoholism. Clin Chem Lab Med. 2007;45:953-961.
40. Arndt T. Carbohydrate-deficient transferrin as a marker of chronic alcohol abuse: a critical review of preanalysis, analysis, and interpretation. Clin Chem. 2001;47:13-27.
41. Cabarcos P, Hassan HM, Tabernero MJ, et al. Analysis of ethyl glucuronide in hair samples by liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS). J Appl Toxicol. 2013;33:638-643.
42. Piano MR, Mazzuco A, Kang M, et al. Binge drinking episodes in young adults: how should we measure them in a research setting? J Stud Alcohol Drugs. 2017;78:502-511.
43. Adinoff B, Bone GH, Linnoila M. Acute ethanol poisoning and the ethanol withdrawal syndrome. Med Toxicol Adverse Drug Exp. 1988;3:172-196.
44. Cabezas J, Lucey MR, Bataller R. Biomarkers for monitoring alcohol use. Clin Liver Dis (Hoboken). 2016;8:59-63.
45. Wurst FM, Alling C, Aradottir S, et al. Emerging biomarkers: new directions and clinical applications. Alcohol Clin Exp Res. 2005;29:465-473.
46. Helander A, Péter O, Zheng Y. Monitoring of the alcohol biomarkers PEth, CDT and EtG/EtS in an outpatient treatment setting. Alcohol Alcohol. 2012;47:552-557.
47. Helander A, Böttcher M, Fehr C, et al. Detection times for urinary ethyl glucuronide and ethyl sulfate in heavy drinkers during alcohol detoxification. Alcohol Alcohol. 2009;44:55-61.
48. Jatlow P, O’Malley SS. Clinical (nonforensic) application of ethyl glucuronide measurement: are we ready? Alcohol Clin Exp Res. 2010;34:968-975.
49. Jatlow PI, Agro A, Wu R, et al. Ethyl glucuronide and ethyl sulfate assays in clinical trials, interpretation, and limitations: results of a dose ranging alcohol challenge study and 2 clinical trials. Alcohol Clin Exp Res. 2014;38:2056-2065.
50. Gonzalo P, Radenne S, Gonzalo S. Biomarkers of chronic alcohol misuse. Curr Biomark Find. 2014;4:9-22.
51. Bornhorst JA, Mbughuni MM. Alcohol biomarkers: clinical issues and analytical methods. In: Critical Issues in Alcohol and Drugs of Abuse Testing. 2nd ed. Academic Press. 2019:25-42.
52. Soderberg BL, Salem RO, Best CA, et al. Fatty acid ethyl esters. Ethanol metabolites that reflect ethanol intake. Am J Clin Pathol. 2003;119(suppl):S94-S99.
53. Cheng CT, Ostrea EM Jr, Alviedo JN, et al. Fatty acid ethyl esters in meconium: a biomarker of fetal alcohol exposure and effect. Exp Biol Med (Maywood). 2021;246:380-386.
54. Andresen-Streichert H, Beres Y, Weinmann W, et al. Improved detection of alcohol consumption using the novel marker phosphatidylethanol in the transplant setting: results of a prospective study. Transpl Int. 2017;30:611-620.
55. Viel G, Boscolo-Berto R, Cecchetto G, et al. Phosphatidylethanol in blood as a marker of chronic alcohol use: a systematic review and meta-analysis. Int J Mol Sci. 2012;13:14788-14812.
56. Gnann H, Weinmann W, Thierauf A. Formation of phosphatidylethanol and its subsequent elimination during an extensive drinking experiment over 5 days. Alcohol Clin Exp Res. 2012;36:1507-1511.
57. Aradóttir S, Moller K, Alling C. Phosphatidylethanol formation and degradation in human and rat blood. Alcohol Alcohol. 2004;39:8-13.
58. Varga A, Alling C. Formation of phosphatidylethanol in vitro in red blood cells from healthy volunteers and chronic alcoholics. J Lab Clin Med. 2002;140:79-83.
59. Javors MA, Hill-Kapturczak N, Roache JD, et al. Characterization of the pharmacokinetics of phosphatidylethanol 16:0/18:1 and 16:0/18:2 in human whole blood after alcohol consumption in a clinical laboratory study. Alcohol Clin Exp Res. 2016;40:1228-1234.
60. Schröck A, Pfäffli M, König S, et al. Application of phosphatidylethanol (PEth) in whole blood in comparison to ethyl glucuronide in hair (hEtG) in driving aptitude assessment (DAA). Int J Legal Med. 2016;130:1527-1533.
61. Stewart SH, Koch DG, Willner IR, et al. Validation of blood phosphatidylethanol as an alcohol consumption biomarker in patients with chronic liver disease. Alcohol Clin Exp Res. 2014;38:1706-1711.
62. Nanau RM, Neuman MG. Biomolecules and biomarkers used in diagnosis of alcohol drinking and in monitoring therapeutic interventions. Biomolecules. 2015 29;5:1339-1385.
63. Hill-Kapturczak N, Dougherty DM, Roache JD, et al. Phosphatidylethanol homologs in blood as biomarkers for the time frame and amount of recent alcohol consumption. In: Preedy VR (ed) Neuroscience of Alcohol. Academic Press; 2019:567-576.
64. Jain J, Evans JL, Briceño A, et al. Comparison of phosphatidylethanol results to self-reported alcohol consumption among young injection drug users. Alcohol Alcohol. 2014;49:520-524.
65. Schröck A, Wurst FM, Thon N, et al. Assessing phosphatidylethanol (PEth) levels reflecting different drinking habits in comparison to the alcohol use disorders identification test - C (AUDIT-C). Drug Alcohol Depend. 2017;178:80-86.
66. McDonnell MG, Skalisky J, Leickly E, et al. Pilot investigation of a phosphatidylethanol-based contingency management intervention targeting alcohol use. Psychol Addict Behav. 2017;31:608-613.
67. Asrani SK, Trotter J, Lake J, et al. Meeting Report: The Dallas Consensus Conference on Liver Transplantation for Alcohol Associated Hepatitis. Liver Transpl. 2020;26:127-140.
68. WHO. International Guide for Monitoring Alcohol Consumption and Harm. 2000. Accessed November 12, 2021. http://apps.who.int/iris/bitstream/handle/10665/66529/WHO_MSD_MSB_00.4.pdf?sequence=1
PRACTICE RECOMMENDATIONS
› Use a quick screening instrument such as the single-question tool or the AUDIT 1-3 to objectively determine whether patients’ drinking is risky for themselves or for others. C
› Suspect alcoholic liver disease if the ratio of aspartate aminotransferase to alanine aminotransferase is > 3. C
› Consider using the PEth assay in high-risk patients to differentiate between heavy alcohol use and social drinking. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Boom in sports betting spurs new guidance on gambling disorder
Amid growing concerns about the impact of increased legalized online sports gambling, the American Psychiatric Association has issued an updated guide on gambling disorder.
The guide provides expert guidance based on current research and provides information on the etiology, psychopathology, neurobiology, and treatment of the disorder.
“For doctors who might think of gambling as either innocuous behavior or simply equivalent to, say, an alcohol problem, this guide not only shows the complexity and seriousness of gambling disorder but also evidence-based treatments that may help people actually get better,” the guide’s coeditor, Jon E. Grant, MD, MPH, JD, professor of psychiatry at the University of Chicago, said in an interview.
Online sports betting is booming. “It has really taken off” in recent years and is now a multibillion dollar industry worldwide, Dr. Grant added.
A recent CBS News report highlights a record volume of legally placed online sports bets during the first week of this year’s NFL season. All told, 26 states now have legalized sports betting.
said Dr. Grant. “They realized they could stay home, stay safe, and still gamble, so there was an uptick in that movement.”
However, the popularity of online gambling is also a sign of the times. “A whole generation of young adults have been raised on the Internet. A lot of companies realize this is not a market that would ever go to a land-based casino, so they essentially took their product to the young people,” said Dr. Grant.
Gambling meets technology
In addition to football, online gamblers can bet on other sports, including horse racing, or participate in “fantasy” sports where users assemble virtual teams of stand-ins for real professional players. There are also online casinos where users can play such things as blackjack and roulette.
The new guide devotes a chapter to online gambling and the complex interplay between gambling and technology. It highlights the growth of interactive platforms, the role of new player experiences and reward structures, and the integration into other online activities, such as social media.
Other chapters explore the interface between gambling and the legal system and differences in gender and between age groups.
There is also information on advances in treatments. Although there are no Food and Drug Administration–approved drugs for gambling disorder, new evidence supports the use of certain agents for this disorder, said Dr. Grant.
These include naltrexone, which has long been used for alcohol and drug addiction, and over-the-counter N-acetylcysteine (NAC), an amino acid that affects the reward system in the brain and has been used for cocaine and marijuana addiction.
Research also suggests that brief-format cognitive-behavioral therapy may be effective for gambling disorder, said Dr. Grant.
An estimated 1% of the population has such a disorder, which involves repeated, problem gambling with sufferers struggling to control their gambling behavior. Gambling disorder is associated with decreased self-esteem, comorbid substance abuse disorders, financial and legal difficulties, relationship and family stress, and suicidality.
Early intervention is key
Most gamblers don’t have a diagnosable disorder and can participate in the pastime without any long-term harm. However, some will show signs of problem gambling, Dr. Grant noted.
“We believe that’s where interventions may have an even bigger impact,” said Dr. Grant. “We want to get people early on in the illness.” He added that gambling “runs along a continuum” from simply dabbling to serious addiction.
Whereas previous versions of the DSM put gambling in an impulse control category, the latest version – DSM-5 – recognizes gambling as an addiction alongside substances.
“That shows greater awareness of the biological connection to substance addiction,” said Dr. Grant. “It’s important for clinicians who are screening substance use disorder folks to make sure they include gambling in that screening.”
The guide includes information on available screening and assessment instruments for diagnosing gambling disorder and for monitoring symptom changes.
Many clinicians may be unaware of the personal and social consequences of gambling disorder and its implications for public health. The new guide provides a detailed look at the effects of gambling on society and families, as well as on individual health and well-being.
A version of this article first appeared on Medscape.com.
Amid growing concerns about the impact of increased legalized online sports gambling, the American Psychiatric Association has issued an updated guide on gambling disorder.
The guide provides expert guidance based on current research and provides information on the etiology, psychopathology, neurobiology, and treatment of the disorder.
“For doctors who might think of gambling as either innocuous behavior or simply equivalent to, say, an alcohol problem, this guide not only shows the complexity and seriousness of gambling disorder but also evidence-based treatments that may help people actually get better,” the guide’s coeditor, Jon E. Grant, MD, MPH, JD, professor of psychiatry at the University of Chicago, said in an interview.
Online sports betting is booming. “It has really taken off” in recent years and is now a multibillion dollar industry worldwide, Dr. Grant added.
A recent CBS News report highlights a record volume of legally placed online sports bets during the first week of this year’s NFL season. All told, 26 states now have legalized sports betting.
said Dr. Grant. “They realized they could stay home, stay safe, and still gamble, so there was an uptick in that movement.”
However, the popularity of online gambling is also a sign of the times. “A whole generation of young adults have been raised on the Internet. A lot of companies realize this is not a market that would ever go to a land-based casino, so they essentially took their product to the young people,” said Dr. Grant.
Gambling meets technology
In addition to football, online gamblers can bet on other sports, including horse racing, or participate in “fantasy” sports where users assemble virtual teams of stand-ins for real professional players. There are also online casinos where users can play such things as blackjack and roulette.
The new guide devotes a chapter to online gambling and the complex interplay between gambling and technology. It highlights the growth of interactive platforms, the role of new player experiences and reward structures, and the integration into other online activities, such as social media.
Other chapters explore the interface between gambling and the legal system and differences in gender and between age groups.
There is also information on advances in treatments. Although there are no Food and Drug Administration–approved drugs for gambling disorder, new evidence supports the use of certain agents for this disorder, said Dr. Grant.
These include naltrexone, which has long been used for alcohol and drug addiction, and over-the-counter N-acetylcysteine (NAC), an amino acid that affects the reward system in the brain and has been used for cocaine and marijuana addiction.
Research also suggests that brief-format cognitive-behavioral therapy may be effective for gambling disorder, said Dr. Grant.
An estimated 1% of the population has such a disorder, which involves repeated, problem gambling with sufferers struggling to control their gambling behavior. Gambling disorder is associated with decreased self-esteem, comorbid substance abuse disorders, financial and legal difficulties, relationship and family stress, and suicidality.
Early intervention is key
Most gamblers don’t have a diagnosable disorder and can participate in the pastime without any long-term harm. However, some will show signs of problem gambling, Dr. Grant noted.
“We believe that’s where interventions may have an even bigger impact,” said Dr. Grant. “We want to get people early on in the illness.” He added that gambling “runs along a continuum” from simply dabbling to serious addiction.
Whereas previous versions of the DSM put gambling in an impulse control category, the latest version – DSM-5 – recognizes gambling as an addiction alongside substances.
“That shows greater awareness of the biological connection to substance addiction,” said Dr. Grant. “It’s important for clinicians who are screening substance use disorder folks to make sure they include gambling in that screening.”
The guide includes information on available screening and assessment instruments for diagnosing gambling disorder and for monitoring symptom changes.
Many clinicians may be unaware of the personal and social consequences of gambling disorder and its implications for public health. The new guide provides a detailed look at the effects of gambling on society and families, as well as on individual health and well-being.
A version of this article first appeared on Medscape.com.
Amid growing concerns about the impact of increased legalized online sports gambling, the American Psychiatric Association has issued an updated guide on gambling disorder.
The guide provides expert guidance based on current research and provides information on the etiology, psychopathology, neurobiology, and treatment of the disorder.
“For doctors who might think of gambling as either innocuous behavior or simply equivalent to, say, an alcohol problem, this guide not only shows the complexity and seriousness of gambling disorder but also evidence-based treatments that may help people actually get better,” the guide’s coeditor, Jon E. Grant, MD, MPH, JD, professor of psychiatry at the University of Chicago, said in an interview.
Online sports betting is booming. “It has really taken off” in recent years and is now a multibillion dollar industry worldwide, Dr. Grant added.
A recent CBS News report highlights a record volume of legally placed online sports bets during the first week of this year’s NFL season. All told, 26 states now have legalized sports betting.
said Dr. Grant. “They realized they could stay home, stay safe, and still gamble, so there was an uptick in that movement.”
However, the popularity of online gambling is also a sign of the times. “A whole generation of young adults have been raised on the Internet. A lot of companies realize this is not a market that would ever go to a land-based casino, so they essentially took their product to the young people,” said Dr. Grant.
Gambling meets technology
In addition to football, online gamblers can bet on other sports, including horse racing, or participate in “fantasy” sports where users assemble virtual teams of stand-ins for real professional players. There are also online casinos where users can play such things as blackjack and roulette.
The new guide devotes a chapter to online gambling and the complex interplay between gambling and technology. It highlights the growth of interactive platforms, the role of new player experiences and reward structures, and the integration into other online activities, such as social media.
Other chapters explore the interface between gambling and the legal system and differences in gender and between age groups.
There is also information on advances in treatments. Although there are no Food and Drug Administration–approved drugs for gambling disorder, new evidence supports the use of certain agents for this disorder, said Dr. Grant.
These include naltrexone, which has long been used for alcohol and drug addiction, and over-the-counter N-acetylcysteine (NAC), an amino acid that affects the reward system in the brain and has been used for cocaine and marijuana addiction.
Research also suggests that brief-format cognitive-behavioral therapy may be effective for gambling disorder, said Dr. Grant.
An estimated 1% of the population has such a disorder, which involves repeated, problem gambling with sufferers struggling to control their gambling behavior. Gambling disorder is associated with decreased self-esteem, comorbid substance abuse disorders, financial and legal difficulties, relationship and family stress, and suicidality.
Early intervention is key
Most gamblers don’t have a diagnosable disorder and can participate in the pastime without any long-term harm. However, some will show signs of problem gambling, Dr. Grant noted.
“We believe that’s where interventions may have an even bigger impact,” said Dr. Grant. “We want to get people early on in the illness.” He added that gambling “runs along a continuum” from simply dabbling to serious addiction.
Whereas previous versions of the DSM put gambling in an impulse control category, the latest version – DSM-5 – recognizes gambling as an addiction alongside substances.
“That shows greater awareness of the biological connection to substance addiction,” said Dr. Grant. “It’s important for clinicians who are screening substance use disorder folks to make sure they include gambling in that screening.”
The guide includes information on available screening and assessment instruments for diagnosing gambling disorder and for monitoring symptom changes.
Many clinicians may be unaware of the personal and social consequences of gambling disorder and its implications for public health. The new guide provides a detailed look at the effects of gambling on society and families, as well as on individual health and well-being.
A version of this article first appeared on Medscape.com.
Short-acting opioids needed for withdrawal in U.S. hospitals, say experts
The commentary by Robert A. Kleinman, MD, with the Centre for Addiction and Mental Health, and department of psychiatry, University of Toronto, and Sarah E. Wakeman, MD, with the division of general internal medicine at Massachusetts General Hospital, and Harvard Medical School, Boston, was published in Annals of Internal Medicine.
Currently, short-acting opioids are not recommended in the United States for opioid withdrawal symptoms (OWS) management in the hospital, the authors wrote. Instead, withdrawal symptoms are typically treated, followed by methadone or buprenorphine or nonopioid medications, but many patients don’t get enough relief. Undertreated withdrawal can result in patients leaving the hospital against medical advice, which is linked with higher risk of death.
Addiction specialist Elisabeth Poorman, MD, of the University of Illinois Chicago, said in an interview that she agrees it’s time to start shifting the thinking on using short-acting opioids for OWS in hospitals. Use varies greatly by hospital and by clinician, she said.
“It’s time to let evidence guide us and to be flexible,” Dr. Poorman said.
The commentary authors noted that with methadone, patients must wait several hours for maximal symptom reduction, and the full benefits of methadone treatment are not realized until days after initiation.
Rapid initiation of methadone may be feasible in hospitals and has been proposed as an option, but further study is necessary before widespread use, the authors wrote.
Short-acting opioids may address limitations of other opioids
Lofexidine, an alpha-2-adrenergic agonist, is the only drug approved by the Food and Drug Administration specifically for OWS.
“However,” the authors said, “more than half of patients with OWS treated with lofexidine in phase 3 efficacy trials dropped out by day five. Clonidine, another alpha-2-agonist used off label to treat OWS, has similar effects to those of lofexidine. “
Therefore, short-acting opioids may complement methadone and buprenorphine in treating OWS in the hospital by addressing their limitations, the authors wrote.
Dr. Kleinman and Dr. Wakeman also say short-acting opioids may help with starting buprenorphine for patients exposed to fentanyl, because short-acting opioids can relieve withdrawal symptoms while fentanyl is metabolized and excreted.
Supplementation with short-acting opioids within the hospital can relieve withdrawal symptoms and help keep patients comfortable while methadone is titrated to more effective doses for long-term treatment, they wrote.
With short-acting opioids, patients may become more engaged in their care with, for example, a tamper-proof, patient-controlled analgesia pump, which would allow them to have more autonomy in administration of opioids to relieve pain and withdrawal symptoms, the authors wrote.
Dr. Kleinman and Dr. Wakeman noted that many patients who inject drugs already consume short-acting illicit drugs in the hospital, typically in washrooms and smoking areas, so supervised use of short-acting opioids helps eliminate the risk for unwitnessed overdoses.
Barriers to short-acting opioid use
Despite use of short-acting opioids internationally, barriers in the United States include limited prospective, randomized, controlled research on their benefits. There is limited institutional support for such approaches, and concerns and stigma around providing opioids to patients with OUD.
“[M]any institutions have insufficient numbers of providers who are both confident and competent with standard buprenorphine and methadone initiation approaches, a prerequisite before adopting more complex regimens,” the authors wrote.
Short-acting, full-agonist opioids, as a complement to methadone or buprenorphine, is already recommended for inpatients with OUD who are experiencing acute pain.
But the authors argue it should be an option when pain is not present, but methadone or buprenorphine have not provided enough OWS relief.
When short-acting opioids are helpful, according to outside expert
Dr. Poorman agrees and says she has found short-acting opioids simple to use in the hospital and very helpful in two situations.
One is when patients are very clear that they don’t want any medication for opioid use disorder, but they do want to be treated for their acute medical issue.
“I thought that was a fantastic tool to have to demonstrate we’re listening to them and weren’t trying to impose something on them and left the door open to come back when they did want treatment, which many of them did,” Dr. Poorman said.
The second situation is when the patient is uncertain about options but very afraid of precipitated withdrawal from buprenorphine.
She said she then found it easy to switch from those medications to buprenorphine and methadone.
Dr. Poorman described a situation she encountered previously where the patient was injecting heroin several times a day for 30-40 years. He was very clear he wasn’t going to stop injecting heroin, but he needed medical attention. He was willing to get medical attention, but he told his doctor he didn’t want to be uncomfortable while in the hospital.
It was very hard for his doctor to accept relieving his symptoms of withdrawal as part of her job, because she felt as though she was condoning his drug use, Dr. Poorman explained.
But Dr. Poorman said it’s not realistic to think that someone who clearly does not want to stop using is going to stop using because a doctor made that person go through painful withdrawal “that they’ve structured their whole life around avoiding.”
Take-home message
“We need to understand that addiction is very complex. A lot of times people come to us distressed, and it’s a great time to engage them in care but engaging them in care doesn’t mean imposing discomfort or pain on them,” Dr. Poorman noted. Instead, it means “listening to them, helping them be comfortable in a really stressful situation and then letting them know we are always there for them wherever they are on their disease process or recovery journey so that they can come back to us.”
Dr. Wakeman previously served on clinical advisory board for Celero Systems and receives textbook royalties from Springer and author payment from UpToDate. Dr. Kleinman and Dr. Poorman declared no relevant financial relationships.
The commentary by Robert A. Kleinman, MD, with the Centre for Addiction and Mental Health, and department of psychiatry, University of Toronto, and Sarah E. Wakeman, MD, with the division of general internal medicine at Massachusetts General Hospital, and Harvard Medical School, Boston, was published in Annals of Internal Medicine.
Currently, short-acting opioids are not recommended in the United States for opioid withdrawal symptoms (OWS) management in the hospital, the authors wrote. Instead, withdrawal symptoms are typically treated, followed by methadone or buprenorphine or nonopioid medications, but many patients don’t get enough relief. Undertreated withdrawal can result in patients leaving the hospital against medical advice, which is linked with higher risk of death.
Addiction specialist Elisabeth Poorman, MD, of the University of Illinois Chicago, said in an interview that she agrees it’s time to start shifting the thinking on using short-acting opioids for OWS in hospitals. Use varies greatly by hospital and by clinician, she said.
“It’s time to let evidence guide us and to be flexible,” Dr. Poorman said.
The commentary authors noted that with methadone, patients must wait several hours for maximal symptom reduction, and the full benefits of methadone treatment are not realized until days after initiation.
Rapid initiation of methadone may be feasible in hospitals and has been proposed as an option, but further study is necessary before widespread use, the authors wrote.
Short-acting opioids may address limitations of other opioids
Lofexidine, an alpha-2-adrenergic agonist, is the only drug approved by the Food and Drug Administration specifically for OWS.
“However,” the authors said, “more than half of patients with OWS treated with lofexidine in phase 3 efficacy trials dropped out by day five. Clonidine, another alpha-2-agonist used off label to treat OWS, has similar effects to those of lofexidine. “
Therefore, short-acting opioids may complement methadone and buprenorphine in treating OWS in the hospital by addressing their limitations, the authors wrote.
Dr. Kleinman and Dr. Wakeman also say short-acting opioids may help with starting buprenorphine for patients exposed to fentanyl, because short-acting opioids can relieve withdrawal symptoms while fentanyl is metabolized and excreted.
Supplementation with short-acting opioids within the hospital can relieve withdrawal symptoms and help keep patients comfortable while methadone is titrated to more effective doses for long-term treatment, they wrote.
With short-acting opioids, patients may become more engaged in their care with, for example, a tamper-proof, patient-controlled analgesia pump, which would allow them to have more autonomy in administration of opioids to relieve pain and withdrawal symptoms, the authors wrote.
Dr. Kleinman and Dr. Wakeman noted that many patients who inject drugs already consume short-acting illicit drugs in the hospital, typically in washrooms and smoking areas, so supervised use of short-acting opioids helps eliminate the risk for unwitnessed overdoses.
Barriers to short-acting opioid use
Despite use of short-acting opioids internationally, barriers in the United States include limited prospective, randomized, controlled research on their benefits. There is limited institutional support for such approaches, and concerns and stigma around providing opioids to patients with OUD.
“[M]any institutions have insufficient numbers of providers who are both confident and competent with standard buprenorphine and methadone initiation approaches, a prerequisite before adopting more complex regimens,” the authors wrote.
Short-acting, full-agonist opioids, as a complement to methadone or buprenorphine, is already recommended for inpatients with OUD who are experiencing acute pain.
But the authors argue it should be an option when pain is not present, but methadone or buprenorphine have not provided enough OWS relief.
When short-acting opioids are helpful, according to outside expert
Dr. Poorman agrees and says she has found short-acting opioids simple to use in the hospital and very helpful in two situations.
One is when patients are very clear that they don’t want any medication for opioid use disorder, but they do want to be treated for their acute medical issue.
“I thought that was a fantastic tool to have to demonstrate we’re listening to them and weren’t trying to impose something on them and left the door open to come back when they did want treatment, which many of them did,” Dr. Poorman said.
The second situation is when the patient is uncertain about options but very afraid of precipitated withdrawal from buprenorphine.
She said she then found it easy to switch from those medications to buprenorphine and methadone.
Dr. Poorman described a situation she encountered previously where the patient was injecting heroin several times a day for 30-40 years. He was very clear he wasn’t going to stop injecting heroin, but he needed medical attention. He was willing to get medical attention, but he told his doctor he didn’t want to be uncomfortable while in the hospital.
It was very hard for his doctor to accept relieving his symptoms of withdrawal as part of her job, because she felt as though she was condoning his drug use, Dr. Poorman explained.
But Dr. Poorman said it’s not realistic to think that someone who clearly does not want to stop using is going to stop using because a doctor made that person go through painful withdrawal “that they’ve structured their whole life around avoiding.”
Take-home message
“We need to understand that addiction is very complex. A lot of times people come to us distressed, and it’s a great time to engage them in care but engaging them in care doesn’t mean imposing discomfort or pain on them,” Dr. Poorman noted. Instead, it means “listening to them, helping them be comfortable in a really stressful situation and then letting them know we are always there for them wherever they are on their disease process or recovery journey so that they can come back to us.”
Dr. Wakeman previously served on clinical advisory board for Celero Systems and receives textbook royalties from Springer and author payment from UpToDate. Dr. Kleinman and Dr. Poorman declared no relevant financial relationships.
The commentary by Robert A. Kleinman, MD, with the Centre for Addiction and Mental Health, and department of psychiatry, University of Toronto, and Sarah E. Wakeman, MD, with the division of general internal medicine at Massachusetts General Hospital, and Harvard Medical School, Boston, was published in Annals of Internal Medicine.
Currently, short-acting opioids are not recommended in the United States for opioid withdrawal symptoms (OWS) management in the hospital, the authors wrote. Instead, withdrawal symptoms are typically treated, followed by methadone or buprenorphine or nonopioid medications, but many patients don’t get enough relief. Undertreated withdrawal can result in patients leaving the hospital against medical advice, which is linked with higher risk of death.
Addiction specialist Elisabeth Poorman, MD, of the University of Illinois Chicago, said in an interview that she agrees it’s time to start shifting the thinking on using short-acting opioids for OWS in hospitals. Use varies greatly by hospital and by clinician, she said.
“It’s time to let evidence guide us and to be flexible,” Dr. Poorman said.
The commentary authors noted that with methadone, patients must wait several hours for maximal symptom reduction, and the full benefits of methadone treatment are not realized until days after initiation.
Rapid initiation of methadone may be feasible in hospitals and has been proposed as an option, but further study is necessary before widespread use, the authors wrote.
Short-acting opioids may address limitations of other opioids
Lofexidine, an alpha-2-adrenergic agonist, is the only drug approved by the Food and Drug Administration specifically for OWS.
“However,” the authors said, “more than half of patients with OWS treated with lofexidine in phase 3 efficacy trials dropped out by day five. Clonidine, another alpha-2-agonist used off label to treat OWS, has similar effects to those of lofexidine. “
Therefore, short-acting opioids may complement methadone and buprenorphine in treating OWS in the hospital by addressing their limitations, the authors wrote.
Dr. Kleinman and Dr. Wakeman also say short-acting opioids may help with starting buprenorphine for patients exposed to fentanyl, because short-acting opioids can relieve withdrawal symptoms while fentanyl is metabolized and excreted.
Supplementation with short-acting opioids within the hospital can relieve withdrawal symptoms and help keep patients comfortable while methadone is titrated to more effective doses for long-term treatment, they wrote.
With short-acting opioids, patients may become more engaged in their care with, for example, a tamper-proof, patient-controlled analgesia pump, which would allow them to have more autonomy in administration of opioids to relieve pain and withdrawal symptoms, the authors wrote.
Dr. Kleinman and Dr. Wakeman noted that many patients who inject drugs already consume short-acting illicit drugs in the hospital, typically in washrooms and smoking areas, so supervised use of short-acting opioids helps eliminate the risk for unwitnessed overdoses.
Barriers to short-acting opioid use
Despite use of short-acting opioids internationally, barriers in the United States include limited prospective, randomized, controlled research on their benefits. There is limited institutional support for such approaches, and concerns and stigma around providing opioids to patients with OUD.
“[M]any institutions have insufficient numbers of providers who are both confident and competent with standard buprenorphine and methadone initiation approaches, a prerequisite before adopting more complex regimens,” the authors wrote.
Short-acting, full-agonist opioids, as a complement to methadone or buprenorphine, is already recommended for inpatients with OUD who are experiencing acute pain.
But the authors argue it should be an option when pain is not present, but methadone or buprenorphine have not provided enough OWS relief.
When short-acting opioids are helpful, according to outside expert
Dr. Poorman agrees and says she has found short-acting opioids simple to use in the hospital and very helpful in two situations.
One is when patients are very clear that they don’t want any medication for opioid use disorder, but they do want to be treated for their acute medical issue.
“I thought that was a fantastic tool to have to demonstrate we’re listening to them and weren’t trying to impose something on them and left the door open to come back when they did want treatment, which many of them did,” Dr. Poorman said.
The second situation is when the patient is uncertain about options but very afraid of precipitated withdrawal from buprenorphine.
She said she then found it easy to switch from those medications to buprenorphine and methadone.
Dr. Poorman described a situation she encountered previously where the patient was injecting heroin several times a day for 30-40 years. He was very clear he wasn’t going to stop injecting heroin, but he needed medical attention. He was willing to get medical attention, but he told his doctor he didn’t want to be uncomfortable while in the hospital.
It was very hard for his doctor to accept relieving his symptoms of withdrawal as part of her job, because she felt as though she was condoning his drug use, Dr. Poorman explained.
But Dr. Poorman said it’s not realistic to think that someone who clearly does not want to stop using is going to stop using because a doctor made that person go through painful withdrawal “that they’ve structured their whole life around avoiding.”
Take-home message
“We need to understand that addiction is very complex. A lot of times people come to us distressed, and it’s a great time to engage them in care but engaging them in care doesn’t mean imposing discomfort or pain on them,” Dr. Poorman noted. Instead, it means “listening to them, helping them be comfortable in a really stressful situation and then letting them know we are always there for them wherever they are on their disease process or recovery journey so that they can come back to us.”
Dr. Wakeman previously served on clinical advisory board for Celero Systems and receives textbook royalties from Springer and author payment from UpToDate. Dr. Kleinman and Dr. Poorman declared no relevant financial relationships.
FROM ANNALS OF INTERNAL MEDICINE
Lithium’s antisuicidal effects questioned
Adding lithium to usual care does not decrease the risk of suicide-related events in those with major depressive disorder (MDD) or bipolar disorder (BD) who have survived a recent suicidal event, new research shows.
The results of a randomized, double-blind, placebo-controlled trial in veterans showed no apparent advantage of the drug in preventing self-injury, suicide attempts, or urgent hospitalization to prevent suicide.
“Lithium is an important therapy for bipolar disorders and depression subsets. Our study indicates that, in patients who are actively followed and treated in a system of care that the VA provides, simply adding lithium to their existing management, including medications, is unlikely to be effective for preventing a broad range of suicide-related events,” study investigator Ryan Ferguson, MPH, ScD, Boston Cooperative Studies Coordinating Center, VA Boston Healthcare System, told this news organization.
The study was published online JAMA Psychiatry.
Surprising findings
The results were somewhat surprising, Dr. Ferguson added. “Lithium showed little or no effect in our study, compared to observational data and results from previous trials. Many clinicians and practice guidelines had assumed that lithium was an effective agent in preventing suicide,” he said.
However, the authors of an accompanying editorial urge caution in concluding that lithium has no antisuicidal effects.
This “rigorously designed and conducted trial has much to teach but cannot be taken as evidence that lithium treatment is ineffective regarding suicidal risk,” write Ross Baldessarini, MD, and Leonardo Tondo, MD, department of psychiatry, Harvard Medical School, Boston.
Study participants were veterans with MDD or BD receiving care at one of 29 Veterans Administration medical centers who survived a recent suicide-related event. In addition to usual care, they were randomly assigned to receive oral extended-release lithium carbonate starting at 600 mg/day or matching placebo for 52 weeks.
The primary outcome was time to the first repeated suicide-related event, including suicide attempts, interrupted attempts, hospitalizations specifically to prevent suicide, and deaths from suicide.
The trial was stopped for futility after 519 veterans (mean age, 42.8 years; 84% male) were randomly assigned to receive lithium (n = 255) or placebo (n = 264). At 3 months, mean lithium concentrations were 0.54 mEq/L for patients with BD and 0.46 mEq/L for those with MDD.
There was no significant difference in the primary outcome (hazard ratio, 1.10; 95% confidence interval, 0.77-1.55; P = .61).
One death occurred in the lithium group and three in the placebo group. There were no unanticipated drug-related safety concerns.
Caveats, cautionary notes
The researchers note that the study did not reach its original recruitment goal. “One of the barriers to recruitment was the perception of many of the clinicians caring for potential participants that the effectiveness of lithium was already established; in fact, this perception was supported by the VA/U.S. Department of Defense Clinical Practice Guideline,” they point out.
They also note that most veterans in the study had depression rather than BD, which is the most common indication for lithium use. Most also had substance use disorders, posttraumatic stress disorder, or both, which could influence outcomes.
As a result of small numbers, it wasn’t possible to evaluate outcomes for patients with BD, test whether outcomes differed among patients with BD and MDD, or assess whether comorbidities attenuated the effects of lithium.
The study’s protocol increased participants’ contacts with the VA, which also may have affected outcomes, the researchers note.
In addition, high rates of attrition and low rates of substantial adherence to lithium meant only about half (48.1%) of the study population achieved target serum lithium concentrations.
Editorial writers Dr. Baldessarini and Dr. Tondo note that the low circulating concentrations of lithium and the fact that adherence to assigned treatment was considered adequate in only 17% of participants are key limitations of the study.
“In general, controlled treatment trials aimed at detecting suicide preventive effects are difficult to design, perform, and interpret,” they point out.
Evidence supporting an antisuicidal effect of lithium treatment includes nearly three dozen observational trials that have shown fewer suicides or attempts with lithium treatment, as well as “marked, temporary” increases in suicidal behavior soon after stopping lithium treatment.
Dr. Baldessarini and Dr. Tondo note the current findings “cannot be taken as evidence that lithium lacks antisuicidal effects. An ironic final note is that recruiting participants to such trials may be made difficult by an evidently prevalent belief that the question of antisuicidal effects of lithium is already settled, which it certainly is not,” they write.
Dr. Ferguson “agrees that more work needs to be done to understand the antisuicidal effect of lithium.
The study received financial and material support from a grant from the Cooperative Studies Program, Office of Research and Development, U.S. Department of Veterans Affairs. Dr. Ferguson has disclosed no relevant financial relationships. A complete list of author disclosures is available with the original article.
Dr. Baldessarini and Dr. Tondo have disclosed no relevant financial relationships. Their editorial was supported by grants from the Bruce J. Anderson Foundation, the McLean Private Donors Fund for Psychiatric Research, and the Aretaeus Foundation of Rome.
A version of this article first appeared on Medscape.com.
Adding lithium to usual care does not decrease the risk of suicide-related events in those with major depressive disorder (MDD) or bipolar disorder (BD) who have survived a recent suicidal event, new research shows.
The results of a randomized, double-blind, placebo-controlled trial in veterans showed no apparent advantage of the drug in preventing self-injury, suicide attempts, or urgent hospitalization to prevent suicide.
“Lithium is an important therapy for bipolar disorders and depression subsets. Our study indicates that, in patients who are actively followed and treated in a system of care that the VA provides, simply adding lithium to their existing management, including medications, is unlikely to be effective for preventing a broad range of suicide-related events,” study investigator Ryan Ferguson, MPH, ScD, Boston Cooperative Studies Coordinating Center, VA Boston Healthcare System, told this news organization.
The study was published online JAMA Psychiatry.
Surprising findings
The results were somewhat surprising, Dr. Ferguson added. “Lithium showed little or no effect in our study, compared to observational data and results from previous trials. Many clinicians and practice guidelines had assumed that lithium was an effective agent in preventing suicide,” he said.
However, the authors of an accompanying editorial urge caution in concluding that lithium has no antisuicidal effects.
This “rigorously designed and conducted trial has much to teach but cannot be taken as evidence that lithium treatment is ineffective regarding suicidal risk,” write Ross Baldessarini, MD, and Leonardo Tondo, MD, department of psychiatry, Harvard Medical School, Boston.
Study participants were veterans with MDD or BD receiving care at one of 29 Veterans Administration medical centers who survived a recent suicide-related event. In addition to usual care, they were randomly assigned to receive oral extended-release lithium carbonate starting at 600 mg/day or matching placebo for 52 weeks.
The primary outcome was time to the first repeated suicide-related event, including suicide attempts, interrupted attempts, hospitalizations specifically to prevent suicide, and deaths from suicide.
The trial was stopped for futility after 519 veterans (mean age, 42.8 years; 84% male) were randomly assigned to receive lithium (n = 255) or placebo (n = 264). At 3 months, mean lithium concentrations were 0.54 mEq/L for patients with BD and 0.46 mEq/L for those with MDD.
There was no significant difference in the primary outcome (hazard ratio, 1.10; 95% confidence interval, 0.77-1.55; P = .61).
One death occurred in the lithium group and three in the placebo group. There were no unanticipated drug-related safety concerns.
Caveats, cautionary notes
The researchers note that the study did not reach its original recruitment goal. “One of the barriers to recruitment was the perception of many of the clinicians caring for potential participants that the effectiveness of lithium was already established; in fact, this perception was supported by the VA/U.S. Department of Defense Clinical Practice Guideline,” they point out.
They also note that most veterans in the study had depression rather than BD, which is the most common indication for lithium use. Most also had substance use disorders, posttraumatic stress disorder, or both, which could influence outcomes.
As a result of small numbers, it wasn’t possible to evaluate outcomes for patients with BD, test whether outcomes differed among patients with BD and MDD, or assess whether comorbidities attenuated the effects of lithium.
The study’s protocol increased participants’ contacts with the VA, which also may have affected outcomes, the researchers note.
In addition, high rates of attrition and low rates of substantial adherence to lithium meant only about half (48.1%) of the study population achieved target serum lithium concentrations.
Editorial writers Dr. Baldessarini and Dr. Tondo note that the low circulating concentrations of lithium and the fact that adherence to assigned treatment was considered adequate in only 17% of participants are key limitations of the study.
“In general, controlled treatment trials aimed at detecting suicide preventive effects are difficult to design, perform, and interpret,” they point out.
Evidence supporting an antisuicidal effect of lithium treatment includes nearly three dozen observational trials that have shown fewer suicides or attempts with lithium treatment, as well as “marked, temporary” increases in suicidal behavior soon after stopping lithium treatment.
Dr. Baldessarini and Dr. Tondo note the current findings “cannot be taken as evidence that lithium lacks antisuicidal effects. An ironic final note is that recruiting participants to such trials may be made difficult by an evidently prevalent belief that the question of antisuicidal effects of lithium is already settled, which it certainly is not,” they write.
Dr. Ferguson “agrees that more work needs to be done to understand the antisuicidal effect of lithium.
The study received financial and material support from a grant from the Cooperative Studies Program, Office of Research and Development, U.S. Department of Veterans Affairs. Dr. Ferguson has disclosed no relevant financial relationships. A complete list of author disclosures is available with the original article.
Dr. Baldessarini and Dr. Tondo have disclosed no relevant financial relationships. Their editorial was supported by grants from the Bruce J. Anderson Foundation, the McLean Private Donors Fund for Psychiatric Research, and the Aretaeus Foundation of Rome.
A version of this article first appeared on Medscape.com.
Adding lithium to usual care does not decrease the risk of suicide-related events in those with major depressive disorder (MDD) or bipolar disorder (BD) who have survived a recent suicidal event, new research shows.
The results of a randomized, double-blind, placebo-controlled trial in veterans showed no apparent advantage of the drug in preventing self-injury, suicide attempts, or urgent hospitalization to prevent suicide.
“Lithium is an important therapy for bipolar disorders and depression subsets. Our study indicates that, in patients who are actively followed and treated in a system of care that the VA provides, simply adding lithium to their existing management, including medications, is unlikely to be effective for preventing a broad range of suicide-related events,” study investigator Ryan Ferguson, MPH, ScD, Boston Cooperative Studies Coordinating Center, VA Boston Healthcare System, told this news organization.
The study was published online JAMA Psychiatry.
Surprising findings
The results were somewhat surprising, Dr. Ferguson added. “Lithium showed little or no effect in our study, compared to observational data and results from previous trials. Many clinicians and practice guidelines had assumed that lithium was an effective agent in preventing suicide,” he said.
However, the authors of an accompanying editorial urge caution in concluding that lithium has no antisuicidal effects.
This “rigorously designed and conducted trial has much to teach but cannot be taken as evidence that lithium treatment is ineffective regarding suicidal risk,” write Ross Baldessarini, MD, and Leonardo Tondo, MD, department of psychiatry, Harvard Medical School, Boston.
Study participants were veterans with MDD or BD receiving care at one of 29 Veterans Administration medical centers who survived a recent suicide-related event. In addition to usual care, they were randomly assigned to receive oral extended-release lithium carbonate starting at 600 mg/day or matching placebo for 52 weeks.
The primary outcome was time to the first repeated suicide-related event, including suicide attempts, interrupted attempts, hospitalizations specifically to prevent suicide, and deaths from suicide.
The trial was stopped for futility after 519 veterans (mean age, 42.8 years; 84% male) were randomly assigned to receive lithium (n = 255) or placebo (n = 264). At 3 months, mean lithium concentrations were 0.54 mEq/L for patients with BD and 0.46 mEq/L for those with MDD.
There was no significant difference in the primary outcome (hazard ratio, 1.10; 95% confidence interval, 0.77-1.55; P = .61).
One death occurred in the lithium group and three in the placebo group. There were no unanticipated drug-related safety concerns.
Caveats, cautionary notes
The researchers note that the study did not reach its original recruitment goal. “One of the barriers to recruitment was the perception of many of the clinicians caring for potential participants that the effectiveness of lithium was already established; in fact, this perception was supported by the VA/U.S. Department of Defense Clinical Practice Guideline,” they point out.
They also note that most veterans in the study had depression rather than BD, which is the most common indication for lithium use. Most also had substance use disorders, posttraumatic stress disorder, or both, which could influence outcomes.
As a result of small numbers, it wasn’t possible to evaluate outcomes for patients with BD, test whether outcomes differed among patients with BD and MDD, or assess whether comorbidities attenuated the effects of lithium.
The study’s protocol increased participants’ contacts with the VA, which also may have affected outcomes, the researchers note.
In addition, high rates of attrition and low rates of substantial adherence to lithium meant only about half (48.1%) of the study population achieved target serum lithium concentrations.
Editorial writers Dr. Baldessarini and Dr. Tondo note that the low circulating concentrations of lithium and the fact that adherence to assigned treatment was considered adequate in only 17% of participants are key limitations of the study.
“In general, controlled treatment trials aimed at detecting suicide preventive effects are difficult to design, perform, and interpret,” they point out.
Evidence supporting an antisuicidal effect of lithium treatment includes nearly three dozen observational trials that have shown fewer suicides or attempts with lithium treatment, as well as “marked, temporary” increases in suicidal behavior soon after stopping lithium treatment.
Dr. Baldessarini and Dr. Tondo note the current findings “cannot be taken as evidence that lithium lacks antisuicidal effects. An ironic final note is that recruiting participants to such trials may be made difficult by an evidently prevalent belief that the question of antisuicidal effects of lithium is already settled, which it certainly is not,” they write.
Dr. Ferguson “agrees that more work needs to be done to understand the antisuicidal effect of lithium.
The study received financial and material support from a grant from the Cooperative Studies Program, Office of Research and Development, U.S. Department of Veterans Affairs. Dr. Ferguson has disclosed no relevant financial relationships. A complete list of author disclosures is available with the original article.
Dr. Baldessarini and Dr. Tondo have disclosed no relevant financial relationships. Their editorial was supported by grants from the Bruce J. Anderson Foundation, the McLean Private Donors Fund for Psychiatric Research, and the Aretaeus Foundation of Rome.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
Moms’ cannabis use in pregnancy tied to anxiety and hyperactivity in offspring
Mothers who use cannabis during pregnancy risk disrupting immune gene networks in the placenta and potentially increasing the risk of anxiety and hyperactivity in their children.
These findings emerged from a study led by Yasmin Hurd, PhD, a professor of psychiatry and director of the Addiction Institute at the Icahn School of Medicine at Mount Sinai, New York, and Yoko Nomura, PhD, a professor of behavioral neuroscience at Queen’s College, City University of New York, that was published online in Proceedings of the National Academy of Sciences.
The analysis assessed the effects of gestational maternal cannabis use on psychosocial and physiological measures in young children as well as its potentially immunomodulatory effect on the in utero environment as reflected in the placental transcriptome.
Participants were drawn from a larger cohort in a study launched in 2012; the investigators evaluated offspring aged 3-6 years for hair hormone levels, neurobehavioral traits on the Behavioral Assessment System for Children survey, and heart rate variability (HRV) at rest and during auditory startle.
The cohort consisted of 322 mother-child dyads and children with prenatal exposure to cannabis were compared with those having no exposure. The cohort consisted of 251 non–cannabis-using mothers and 71 cannabis-using mothers, with mean maternal ages in the two groups of 28.46 years and 25.91 years, respectively, The mothers gave birth at Mount Sinai and they and their children were assessed annually at affiliated medical centers in Mount Sinai’s catchment area.
For a subset of children with behavioral assessments, placental specimens collected at birth were processed for RNA sequencing.
Among the findings:
- Maternal cannabis use was associated with reduced maternal and paternal age, more single-mother pregnancies, state anxiety, trait anxiety, depression, cigarette smoking, and African American race.
- Hair hormone analysis revealed increased cortisol levels in the children of cannabis-using mothers, and was associated with greater anxiety, aggression, and hyperactivity.
- Affected children showed a reduction in the high-frequency component of HRV at baseline, reflecting reduced vagal tone.
- In the placenta, there was reduced expression of many genes involved in immune system function. These included genes for type I interferon, neutrophil, and cytokine-signaling pathways.
Several of these genes organized into coexpression networks that correlated with child anxiety and hyperactivity.
The principal active component of cannabis, tetrahydrocannabinol (THC), targets the endocannabinoid system in placental tissue and the developing brain, the authors noted. Exposure during pregnancy is associated with a range of adverse outcomes from fetal growth restriction to low birth weight and preterm birth.
“There are cannabinoid receptors on immune cells, and it is known that cannabinoids can alter immune function, which is important for maintaining maternal tolerance and protecting the fetus,” Dr. Hurd said. “It’s not surprising that something that affects the immune cells can have an impact on the developing fetus.”
“Overall, our findings reveal a relationship between [maternal cannabis use] and immune response gene networks in the placenta as a potential mediator of risk for anxiety-related problems in early childhood,” Dr. Hurd and colleagues wrote, adding that the results have significant implications for defining mental health issues in the children gestated by cannabis-smoking mothers.
Their results align with previous research indicating a greater risk for psychiatric illness in children with prenatal cannabis exposure from maternal use.
“While data are pretty limited in this realm, there are other studies that demonstrate a relationship between early child developmental and behavioral measures and cannabis use during pregnancy,” Camille Hoffman, MD, MSc, a high-risk obstetrics specialist and an associate professor at the University of Colorado at Denver, Aurora, said in an interview. “Our research group found children exposed to cannabis in utero at 10 weeks’ gestation and beyond were less interactive and more withdrawn than children who were not exposed.”
And THC remains in maternal breast milk even 6 weeks after usage stops.
The long-term effects of prenatal cannabis exposure remain to be determined and it is unknown whether the effects of gestational THC might attenuate as a child grows older. “We use early childhood measures in research as a proxy for the later development of diagnosed mental health conditions or behavioral problems,” Dr. Hoffman explained. “We know when we do this that not every child with an abnormal score early will go on to develop an actual condition. Fortunately, or unfortunately, other factors and exposures during childhood can change the trajectory for the better or worse.”
According to Dr. Hurd, child development is a dynamic process and epigenetic events in utero need not be deterministic. “The important thing is to identify children at risk early and to be able to go in and try to improve the environment they’re being raised in – not in terms of impoverishment but in terms of positive nurturing and giving the mother and family support.”
At the prenatal level, what’s the best advice for cannabis-using mothers-to-be? “If a woman doesn’t know she’s pregnant and has been using cannabis, taking extra choline for the remainder of the pregnancy can help buffer the potential negative impact of the cannabis exposure,” Dr. Hoffman said. The Food and Drug Administration and the American Medical Association recommend a dose of 550 mg daily. “The same is true for alcohol, which we know is also very bad for fetal brain development. This is not to say go ahead and use these substances and just take choline. The choline is more to try and salvage damage to the fetal brain that may have already occurred.”
This study was supported by the National Institute of Mental Health and the National Institute on Drug Abuse. The authors declared no competing interests. Dr. Hoffman disclosed no conflicts of interest with respect to her comments.
Mothers who use cannabis during pregnancy risk disrupting immune gene networks in the placenta and potentially increasing the risk of anxiety and hyperactivity in their children.
These findings emerged from a study led by Yasmin Hurd, PhD, a professor of psychiatry and director of the Addiction Institute at the Icahn School of Medicine at Mount Sinai, New York, and Yoko Nomura, PhD, a professor of behavioral neuroscience at Queen’s College, City University of New York, that was published online in Proceedings of the National Academy of Sciences.
The analysis assessed the effects of gestational maternal cannabis use on psychosocial and physiological measures in young children as well as its potentially immunomodulatory effect on the in utero environment as reflected in the placental transcriptome.
Participants were drawn from a larger cohort in a study launched in 2012; the investigators evaluated offspring aged 3-6 years for hair hormone levels, neurobehavioral traits on the Behavioral Assessment System for Children survey, and heart rate variability (HRV) at rest and during auditory startle.
The cohort consisted of 322 mother-child dyads and children with prenatal exposure to cannabis were compared with those having no exposure. The cohort consisted of 251 non–cannabis-using mothers and 71 cannabis-using mothers, with mean maternal ages in the two groups of 28.46 years and 25.91 years, respectively, The mothers gave birth at Mount Sinai and they and their children were assessed annually at affiliated medical centers in Mount Sinai’s catchment area.
For a subset of children with behavioral assessments, placental specimens collected at birth were processed for RNA sequencing.
Among the findings:
- Maternal cannabis use was associated with reduced maternal and paternal age, more single-mother pregnancies, state anxiety, trait anxiety, depression, cigarette smoking, and African American race.
- Hair hormone analysis revealed increased cortisol levels in the children of cannabis-using mothers, and was associated with greater anxiety, aggression, and hyperactivity.
- Affected children showed a reduction in the high-frequency component of HRV at baseline, reflecting reduced vagal tone.
- In the placenta, there was reduced expression of many genes involved in immune system function. These included genes for type I interferon, neutrophil, and cytokine-signaling pathways.
Several of these genes organized into coexpression networks that correlated with child anxiety and hyperactivity.
The principal active component of cannabis, tetrahydrocannabinol (THC), targets the endocannabinoid system in placental tissue and the developing brain, the authors noted. Exposure during pregnancy is associated with a range of adverse outcomes from fetal growth restriction to low birth weight and preterm birth.
“There are cannabinoid receptors on immune cells, and it is known that cannabinoids can alter immune function, which is important for maintaining maternal tolerance and protecting the fetus,” Dr. Hurd said. “It’s not surprising that something that affects the immune cells can have an impact on the developing fetus.”
“Overall, our findings reveal a relationship between [maternal cannabis use] and immune response gene networks in the placenta as a potential mediator of risk for anxiety-related problems in early childhood,” Dr. Hurd and colleagues wrote, adding that the results have significant implications for defining mental health issues in the children gestated by cannabis-smoking mothers.
Their results align with previous research indicating a greater risk for psychiatric illness in children with prenatal cannabis exposure from maternal use.
“While data are pretty limited in this realm, there are other studies that demonstrate a relationship between early child developmental and behavioral measures and cannabis use during pregnancy,” Camille Hoffman, MD, MSc, a high-risk obstetrics specialist and an associate professor at the University of Colorado at Denver, Aurora, said in an interview. “Our research group found children exposed to cannabis in utero at 10 weeks’ gestation and beyond were less interactive and more withdrawn than children who were not exposed.”
And THC remains in maternal breast milk even 6 weeks after usage stops.
The long-term effects of prenatal cannabis exposure remain to be determined and it is unknown whether the effects of gestational THC might attenuate as a child grows older. “We use early childhood measures in research as a proxy for the later development of diagnosed mental health conditions or behavioral problems,” Dr. Hoffman explained. “We know when we do this that not every child with an abnormal score early will go on to develop an actual condition. Fortunately, or unfortunately, other factors and exposures during childhood can change the trajectory for the better or worse.”
According to Dr. Hurd, child development is a dynamic process and epigenetic events in utero need not be deterministic. “The important thing is to identify children at risk early and to be able to go in and try to improve the environment they’re being raised in – not in terms of impoverishment but in terms of positive nurturing and giving the mother and family support.”
At the prenatal level, what’s the best advice for cannabis-using mothers-to-be? “If a woman doesn’t know she’s pregnant and has been using cannabis, taking extra choline for the remainder of the pregnancy can help buffer the potential negative impact of the cannabis exposure,” Dr. Hoffman said. The Food and Drug Administration and the American Medical Association recommend a dose of 550 mg daily. “The same is true for alcohol, which we know is also very bad for fetal brain development. This is not to say go ahead and use these substances and just take choline. The choline is more to try and salvage damage to the fetal brain that may have already occurred.”
This study was supported by the National Institute of Mental Health and the National Institute on Drug Abuse. The authors declared no competing interests. Dr. Hoffman disclosed no conflicts of interest with respect to her comments.
Mothers who use cannabis during pregnancy risk disrupting immune gene networks in the placenta and potentially increasing the risk of anxiety and hyperactivity in their children.
These findings emerged from a study led by Yasmin Hurd, PhD, a professor of psychiatry and director of the Addiction Institute at the Icahn School of Medicine at Mount Sinai, New York, and Yoko Nomura, PhD, a professor of behavioral neuroscience at Queen’s College, City University of New York, that was published online in Proceedings of the National Academy of Sciences.
The analysis assessed the effects of gestational maternal cannabis use on psychosocial and physiological measures in young children as well as its potentially immunomodulatory effect on the in utero environment as reflected in the placental transcriptome.
Participants were drawn from a larger cohort in a study launched in 2012; the investigators evaluated offspring aged 3-6 years for hair hormone levels, neurobehavioral traits on the Behavioral Assessment System for Children survey, and heart rate variability (HRV) at rest and during auditory startle.
The cohort consisted of 322 mother-child dyads and children with prenatal exposure to cannabis were compared with those having no exposure. The cohort consisted of 251 non–cannabis-using mothers and 71 cannabis-using mothers, with mean maternal ages in the two groups of 28.46 years and 25.91 years, respectively, The mothers gave birth at Mount Sinai and they and their children were assessed annually at affiliated medical centers in Mount Sinai’s catchment area.
For a subset of children with behavioral assessments, placental specimens collected at birth were processed for RNA sequencing.
Among the findings:
- Maternal cannabis use was associated with reduced maternal and paternal age, more single-mother pregnancies, state anxiety, trait anxiety, depression, cigarette smoking, and African American race.
- Hair hormone analysis revealed increased cortisol levels in the children of cannabis-using mothers, and was associated with greater anxiety, aggression, and hyperactivity.
- Affected children showed a reduction in the high-frequency component of HRV at baseline, reflecting reduced vagal tone.
- In the placenta, there was reduced expression of many genes involved in immune system function. These included genes for type I interferon, neutrophil, and cytokine-signaling pathways.
Several of these genes organized into coexpression networks that correlated with child anxiety and hyperactivity.
The principal active component of cannabis, tetrahydrocannabinol (THC), targets the endocannabinoid system in placental tissue and the developing brain, the authors noted. Exposure during pregnancy is associated with a range of adverse outcomes from fetal growth restriction to low birth weight and preterm birth.
“There are cannabinoid receptors on immune cells, and it is known that cannabinoids can alter immune function, which is important for maintaining maternal tolerance and protecting the fetus,” Dr. Hurd said. “It’s not surprising that something that affects the immune cells can have an impact on the developing fetus.”
“Overall, our findings reveal a relationship between [maternal cannabis use] and immune response gene networks in the placenta as a potential mediator of risk for anxiety-related problems in early childhood,” Dr. Hurd and colleagues wrote, adding that the results have significant implications for defining mental health issues in the children gestated by cannabis-smoking mothers.
Their results align with previous research indicating a greater risk for psychiatric illness in children with prenatal cannabis exposure from maternal use.
“While data are pretty limited in this realm, there are other studies that demonstrate a relationship between early child developmental and behavioral measures and cannabis use during pregnancy,” Camille Hoffman, MD, MSc, a high-risk obstetrics specialist and an associate professor at the University of Colorado at Denver, Aurora, said in an interview. “Our research group found children exposed to cannabis in utero at 10 weeks’ gestation and beyond were less interactive and more withdrawn than children who were not exposed.”
And THC remains in maternal breast milk even 6 weeks after usage stops.
The long-term effects of prenatal cannabis exposure remain to be determined and it is unknown whether the effects of gestational THC might attenuate as a child grows older. “We use early childhood measures in research as a proxy for the later development of diagnosed mental health conditions or behavioral problems,” Dr. Hoffman explained. “We know when we do this that not every child with an abnormal score early will go on to develop an actual condition. Fortunately, or unfortunately, other factors and exposures during childhood can change the trajectory for the better or worse.”
According to Dr. Hurd, child development is a dynamic process and epigenetic events in utero need not be deterministic. “The important thing is to identify children at risk early and to be able to go in and try to improve the environment they’re being raised in – not in terms of impoverishment but in terms of positive nurturing and giving the mother and family support.”
At the prenatal level, what’s the best advice for cannabis-using mothers-to-be? “If a woman doesn’t know she’s pregnant and has been using cannabis, taking extra choline for the remainder of the pregnancy can help buffer the potential negative impact of the cannabis exposure,” Dr. Hoffman said. The Food and Drug Administration and the American Medical Association recommend a dose of 550 mg daily. “The same is true for alcohol, which we know is also very bad for fetal brain development. This is not to say go ahead and use these substances and just take choline. The choline is more to try and salvage damage to the fetal brain that may have already occurred.”
This study was supported by the National Institute of Mental Health and the National Institute on Drug Abuse. The authors declared no competing interests. Dr. Hoffman disclosed no conflicts of interest with respect to her comments.
FROM PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES
U.S. overdose deaths hit an all-time high
a 28.5% increase from the previous year.
Deaths in some states rose even more precipitously. Vermont saw an almost 70% increase, and drug overdose deaths in West Virginia increased by 62%. Many states, including Alabama, California, Kansas, Kentucky, Louisiana, Tennessee, and Washington, had a 45%-50% rise in overdose deaths.
The data released by the CDC was provisional, as there is generally a lag between a reported overdose and confirmation of the death to the National Vital Statistics System. The agency uses statistical models that render the counts almost 100% accurate, the CDC says.
The vast majority (73,757) of overdose deaths involved opioids – with most of those (62,338) involving synthetic opioids such as fentanyl. Federal officials said that one American died every 5 minutes from an overdose, or 265 a day.
“We have to acknowledge what this is – it is a crisis,” Department of Health & Human Services Secretary Xavier Becerra told reporters on a call.
“As much as the numbers speak so vividly, they don’t tell the whole story. We see it in the faces of grieving families and all those overworked caregivers. You hear it every time you get that panicked 911 phone call, you read it in obituaries of sons and daughters who left us way too soon,” Mr. Becerra said.
Rahul Gupta, MD, director of the White House Office of National Drug Control Policy, said that “this is unacceptable, and it requires an unprecedented response.”
Dr. Gupta, who noted that he has a waiver to treat substance use disorder patients with buprenorphine, said he’s seen “first-hand the heartbreak of the overdose epidemic,” adding that, with 23 years in practice, “I’ve learned that an overdose is a cry for help and for far too many people that cry goes unanswered.”
Both Mr. Becerra and Dr. Gupta called on Congress to pass President Joe Biden’s fiscal 2022 budget request, noting that it calls for $41 billion – a $669 million increase from fiscal year 2021 – to go to agencies working on drug interdiction and substance use prevention, treatment, and recovery support.
Dr. Gupta also announced that the administration was releasing a model law that could be used by state legislatures to help standardize policies on making the overdose antidote naloxone more accessible. Currently, such policies are a patchwork across the nation.
In addition, the federal government is newly supporting harm reduction, Mr. Becerra said. This means federal money can be used by clinics and outreach programs to buy fentanyl test strips, which they can then distribute to drug users.
“It’s important for Americans to have the ability to make sure that they can test for fentanyl in the substance,” Dr. Gupta said.
Fake pills, fentanyl a huge issue
Federal officials said that both fentanyl and methamphetamine are contributing to rising numbers of fatalities.
“Drug cartels in Mexico are mass-producing fentanyl and methamphetamine largely sourced from chemicals in China and they are distributing these substances throughout the United States,” Anne Milgram, administrator of the Drug Enforcement Administration, said on the call.
Ms. Milgram said the agency had seized 12,000 pounds of fentanyl in 2021, enough to provide every American with a lethal dose. Fentanyl is also mixed in with cocaine, heroin, methamphetamine, and marijuana – often in counterfeit pills, Ms. Milgram said.
The DEA and other law enforcement agencies have seized more than 14 million such pills in 2021. “These types of pills are easily accessible today on social media and e-commerce platforms, Ms. Milgram said.
“Drug dealers are now in our homes,” she said. “Wherever there is a smart phone or a computer, a dealer is one click away,” Ms. Milgram said.
National Institute on Drug Abuse Director Nora D. Volkow, MD, said that dealers will continue to push both fentanyl and methamphetamine because they are among the most addictive substances. They also are more profitable because they don’t require cultivation and harvesting, she said on the call.
Dr. Volkow also noted that naloxone is not as effective in reversing fentanyl overdoses because fentanyl is more potent than heroin and other opioids, and “it gets into the brain extremely rapidly.”
Ongoing research is aimed at developing a faster delivery mechanism and a longer-lasting formulation to counter overdoses, Dr. Volkow said.
A version of this article first appeared on Medscape.com.
a 28.5% increase from the previous year.
Deaths in some states rose even more precipitously. Vermont saw an almost 70% increase, and drug overdose deaths in West Virginia increased by 62%. Many states, including Alabama, California, Kansas, Kentucky, Louisiana, Tennessee, and Washington, had a 45%-50% rise in overdose deaths.
The data released by the CDC was provisional, as there is generally a lag between a reported overdose and confirmation of the death to the National Vital Statistics System. The agency uses statistical models that render the counts almost 100% accurate, the CDC says.
The vast majority (73,757) of overdose deaths involved opioids – with most of those (62,338) involving synthetic opioids such as fentanyl. Federal officials said that one American died every 5 minutes from an overdose, or 265 a day.
“We have to acknowledge what this is – it is a crisis,” Department of Health & Human Services Secretary Xavier Becerra told reporters on a call.
“As much as the numbers speak so vividly, they don’t tell the whole story. We see it in the faces of grieving families and all those overworked caregivers. You hear it every time you get that panicked 911 phone call, you read it in obituaries of sons and daughters who left us way too soon,” Mr. Becerra said.
Rahul Gupta, MD, director of the White House Office of National Drug Control Policy, said that “this is unacceptable, and it requires an unprecedented response.”
Dr. Gupta, who noted that he has a waiver to treat substance use disorder patients with buprenorphine, said he’s seen “first-hand the heartbreak of the overdose epidemic,” adding that, with 23 years in practice, “I’ve learned that an overdose is a cry for help and for far too many people that cry goes unanswered.”
Both Mr. Becerra and Dr. Gupta called on Congress to pass President Joe Biden’s fiscal 2022 budget request, noting that it calls for $41 billion – a $669 million increase from fiscal year 2021 – to go to agencies working on drug interdiction and substance use prevention, treatment, and recovery support.
Dr. Gupta also announced that the administration was releasing a model law that could be used by state legislatures to help standardize policies on making the overdose antidote naloxone more accessible. Currently, such policies are a patchwork across the nation.
In addition, the federal government is newly supporting harm reduction, Mr. Becerra said. This means federal money can be used by clinics and outreach programs to buy fentanyl test strips, which they can then distribute to drug users.
“It’s important for Americans to have the ability to make sure that they can test for fentanyl in the substance,” Dr. Gupta said.
Fake pills, fentanyl a huge issue
Federal officials said that both fentanyl and methamphetamine are contributing to rising numbers of fatalities.
“Drug cartels in Mexico are mass-producing fentanyl and methamphetamine largely sourced from chemicals in China and they are distributing these substances throughout the United States,” Anne Milgram, administrator of the Drug Enforcement Administration, said on the call.
Ms. Milgram said the agency had seized 12,000 pounds of fentanyl in 2021, enough to provide every American with a lethal dose. Fentanyl is also mixed in with cocaine, heroin, methamphetamine, and marijuana – often in counterfeit pills, Ms. Milgram said.
The DEA and other law enforcement agencies have seized more than 14 million such pills in 2021. “These types of pills are easily accessible today on social media and e-commerce platforms, Ms. Milgram said.
“Drug dealers are now in our homes,” she said. “Wherever there is a smart phone or a computer, a dealer is one click away,” Ms. Milgram said.
National Institute on Drug Abuse Director Nora D. Volkow, MD, said that dealers will continue to push both fentanyl and methamphetamine because they are among the most addictive substances. They also are more profitable because they don’t require cultivation and harvesting, she said on the call.
Dr. Volkow also noted that naloxone is not as effective in reversing fentanyl overdoses because fentanyl is more potent than heroin and other opioids, and “it gets into the brain extremely rapidly.”
Ongoing research is aimed at developing a faster delivery mechanism and a longer-lasting formulation to counter overdoses, Dr. Volkow said.
A version of this article first appeared on Medscape.com.
a 28.5% increase from the previous year.
Deaths in some states rose even more precipitously. Vermont saw an almost 70% increase, and drug overdose deaths in West Virginia increased by 62%. Many states, including Alabama, California, Kansas, Kentucky, Louisiana, Tennessee, and Washington, had a 45%-50% rise in overdose deaths.
The data released by the CDC was provisional, as there is generally a lag between a reported overdose and confirmation of the death to the National Vital Statistics System. The agency uses statistical models that render the counts almost 100% accurate, the CDC says.
The vast majority (73,757) of overdose deaths involved opioids – with most of those (62,338) involving synthetic opioids such as fentanyl. Federal officials said that one American died every 5 minutes from an overdose, or 265 a day.
“We have to acknowledge what this is – it is a crisis,” Department of Health & Human Services Secretary Xavier Becerra told reporters on a call.
“As much as the numbers speak so vividly, they don’t tell the whole story. We see it in the faces of grieving families and all those overworked caregivers. You hear it every time you get that panicked 911 phone call, you read it in obituaries of sons and daughters who left us way too soon,” Mr. Becerra said.
Rahul Gupta, MD, director of the White House Office of National Drug Control Policy, said that “this is unacceptable, and it requires an unprecedented response.”
Dr. Gupta, who noted that he has a waiver to treat substance use disorder patients with buprenorphine, said he’s seen “first-hand the heartbreak of the overdose epidemic,” adding that, with 23 years in practice, “I’ve learned that an overdose is a cry for help and for far too many people that cry goes unanswered.”
Both Mr. Becerra and Dr. Gupta called on Congress to pass President Joe Biden’s fiscal 2022 budget request, noting that it calls for $41 billion – a $669 million increase from fiscal year 2021 – to go to agencies working on drug interdiction and substance use prevention, treatment, and recovery support.
Dr. Gupta also announced that the administration was releasing a model law that could be used by state legislatures to help standardize policies on making the overdose antidote naloxone more accessible. Currently, such policies are a patchwork across the nation.
In addition, the federal government is newly supporting harm reduction, Mr. Becerra said. This means federal money can be used by clinics and outreach programs to buy fentanyl test strips, which they can then distribute to drug users.
“It’s important for Americans to have the ability to make sure that they can test for fentanyl in the substance,” Dr. Gupta said.
Fake pills, fentanyl a huge issue
Federal officials said that both fentanyl and methamphetamine are contributing to rising numbers of fatalities.
“Drug cartels in Mexico are mass-producing fentanyl and methamphetamine largely sourced from chemicals in China and they are distributing these substances throughout the United States,” Anne Milgram, administrator of the Drug Enforcement Administration, said on the call.
Ms. Milgram said the agency had seized 12,000 pounds of fentanyl in 2021, enough to provide every American with a lethal dose. Fentanyl is also mixed in with cocaine, heroin, methamphetamine, and marijuana – often in counterfeit pills, Ms. Milgram said.
The DEA and other law enforcement agencies have seized more than 14 million such pills in 2021. “These types of pills are easily accessible today on social media and e-commerce platforms, Ms. Milgram said.
“Drug dealers are now in our homes,” she said. “Wherever there is a smart phone or a computer, a dealer is one click away,” Ms. Milgram said.
National Institute on Drug Abuse Director Nora D. Volkow, MD, said that dealers will continue to push both fentanyl and methamphetamine because they are among the most addictive substances. They also are more profitable because they don’t require cultivation and harvesting, she said on the call.
Dr. Volkow also noted that naloxone is not as effective in reversing fentanyl overdoses because fentanyl is more potent than heroin and other opioids, and “it gets into the brain extremely rapidly.”
Ongoing research is aimed at developing a faster delivery mechanism and a longer-lasting formulation to counter overdoses, Dr. Volkow said.
A version of this article first appeared on Medscape.com.
Britney Spears – Reflections on conservatorship
If you are a psychiatrist who has done a public lecture in the past year, you likely encountered the question, “What about Britney’s conservatorship?” Many psychiatrists are far removed from conservatorship evaluations, doing the different yet still important work of alleviating mental suffering without paddling in the controversial waters of involuntary treatment. Others judiciously hide behind the veil of the prudent Goldwater Rule in avoiding such discussions altogether. Regardless of whether psychiatry attempts to stay out of such affairs publicly, our field remains intimately involved in the process itself. This can lead to negative views of psychiatry among the public – that of a medical specialty with ulterior motives operating at the behest of the state.
Some psychiatrists simplistically advocate against any form of involuntary treatment.1 In many ways, this may appear noble. However, the reality of mental illness, with its potential harm to self and others, introduces the potential for dire consequences of such a position. If society is unwilling to accept behavior that may lead to harm, but psychiatry is unwilling to intervene, then other avenues of restricting such behavior will emerge. Those avenues traditionally have included conscription of law enforcement and the incarceration of patients with mental illness.
Yet, therein lies the conundrum of Ms. Spears and other celebrities on conservatorship. At face value, they do not appear to require conservatorship. We do not think it violates the Goldwater Rule to render this observation. In fact, it may reassure the public if the American Psychiatric Association, as well as individual psychiatrists, were more open about the goal, intent, and limitations of conservatorships.
The process of establishing conservatorships is not driven solely by mental health professionals. Rather, conservatorship laws permit society to enact, through psychiatrists, its desire to alleviate behaviors considered unacceptable in the context of mental illness.
In California, it has resulted in our famous or infamous “5150,” which asks psychiatrists to comment on the danger to self, danger to others, and grave disability of our patients. It can be helpful to frame these criteria regarding the relationship between society and our patients. The criteria of danger to self represents society’s wish to intervene in cases of patients with imminent intent of self-harm, operating under the presumption that a suicide can be prevented. Danger to others represents the societal angst, at times exaggerated,2 about people with mental illness perpetuating homicides, especially when off their medication. Grave disability represents public shame at the thought of persons so lost to mental illness they are unable to provide for themselves or even accept food, clothing, and shelter.
While an involuntary hold is necessary at times, working against our patients engenders revolting feelings. We often rationalize involuntary holds as illustrative of sincere compassion for our patients’ suffering and an attempt to lift them out of such tragic conditions. Our patients regularly do not feel our compassion when we are making an argument in a hearing for the restriction of their rights. They see our efforts as an attempt to lock them away “for their own good” because of society’s discomfort with homelessness. As such, we wonder whether our role becomes one of doctors for society, prescribing a treatment for the emotional distress of the community, and at times for ourselves, rather than that of the patient.
One may be perplexed as to how a celebrity could be considered gravely disabled. Celebrities generally have enough income to afford food, clothing, and shelter. One could justifiably ask why an individual with no history of violence would be considered a danger to others. Similarly, one may wonder how, in the absence of any reported attempts to engage in self-harm, with no visible marks of self-harm, someone is determined to be a danger to himself or herself. The bafflement on the part of one on the outside of these determinations can be sharply contrasted by the desperation felt by family members whose loved ones with mental illness appear to meet those criteria yet are consistently turned away by mental health programs and hospitals.
Not uncommonly, it is families advocating for involuntary hospitalization – while lamenting our strict criteria – that prevent doctors from intervening until some tragic fate befalls their loved ones. They criticize what they consider to be too-stringent mental health laws and are infuriated by seemingly obtuse insurance policies limiting care to patients. Most of our colleagues working with those who have severe mental illness share the frustration of these families over the scarcity of psychiatry beds. Therefore, it is particularly shocking when the most mediatized story about conservatorship is not about how hard it is to obtain. The story is about a singer who was seemingly safe, caring for herself, and yet still ended up on a conservatorship.
We wonder whether there is a question of magnitude. Are homeless patients more difficult to place on conservatorship because society sees a lesser stake? One could argue that Ms. Spears and other celebrities would have so much to lose in a single episode of mental illness. A week with mania or psychosis could cause irreparable damage to their persona, opportunity for employment, and their fortune. On the contrary, many of our patients on conservatorship have little to their names, and no one keeping up on their reputation. Triers of facts should ask themselves about the nature of their motivations. Envy, a desire to live vicariously through celebrities, or even less ethical motivations – such as a desire to control and exert authority over those individuals – can influence our decisions.
Throughout the past year, when asked about Ms. Spears, we have pointed out the obvious – she seemingly has a life incompatible with meeting criteria for a psychiatric conservatorship. We have outlined the role, history, and limitations of psychiatric conservatorship. We have shared how such cases are often approached, when required for our own patients or when asked by the court to do so. We have discussed the significant oversight of the system, including the public conservator’s office, which frequently refuses petitions outright. There are hearing officers, who, in the early stages of this process, weigh our case against that of the patients, aided by passionately driven patient advocates. There is the public defender’s office, which, at least in San Diego, vigorously defends the rights of those with mental illness. Most importantly, there are judges who adjudicate those cases with diligence and humility.
As the story has continued to be in the news, we have had numerous conversations about Ms. Spears’ conservatorship with colleagues sharing strong opinions on her case. Many of these colleagues do not have forensic practices and we inevitably find ourselves responding along the lines of, “It is easy to say this, but quite a different thing to prove it in court.” It is hard not to imagine testifying in such a high-profile conservatorship case; testifying, in front of jurors, about a celebrity who may have engaged in what some considered to be unusual behavior.
Conservatorship laws are not about the minutia or criteria of a specific mental health disorder. Patients do not meet criteria for conservatorship by having a certain number of delusional thoughts or a specific type of hallucination. Patients meet criteria for conservatorship because of state-enacted laws based on social factors – such as danger and self-care – the population wishes to treat, even if against the will of those treated. Under this light, one must recognize that a conservatorship trial is not just about mental illness but about how society wants to care for human beings. Psychiatric illness itself is not grounds for conservatorship. Oftentimes, severely ill patients win a hearing for grave disability by simply accepting a referral for housing, showing up to court clothed, and eating the meals provided at the hospital.
With understanding that these laws pertain specifically to behaviors resulting from mental illness that society finds unacceptable, the narrative of a celebrity conservatorship can be considered differently. The stories of celebrities being used and abused by deleterious beings and deleterious conditions have become a genre. Paul Prenter’s treatment of Freddie Mercury documented in the 2018 movie “Bohemian Rhapsody” and John Reid’s alleged betrayal of Elton John, who was suffering from a substance use disorder, documented in the 2019 movie “Rocketman,” are recent examples, among many.
Imagine yourself, as a juror, deciding on the fate of a celebrity. Would you require them to have lost all property, including the clothing on their backs, before intervening? Consider the next time you hear of a celebrity swindled from his or her fortune in a time of crisis and whether it would have been righteous to prevent it. We personally have, at times, argued for restraint in psychiatry’s desire to have more power. This concern extends not only to our ability to control people, but also our ability to force them into being subjected to psychotropic medications with well-known side effects.
At the same time, we remain cognizant of the magnified impact of adverse outcomes on public figures. John Hinckley Jr. did not attempt to murder a bystander; he attempted to kill the president of the United States when he shot at President Ronald Reagan in 1981. That incident led to considerable changes in our laws about insanity. More recently, society was particularly affected by Tom Hanks’ COVID-19 diagnosis. Mr. Hanks’ illness led to scientifically measurable changes in the public’s beliefs regarding the pandemic.3
On the other hand, and of equal importance to the desire to protect public figures from adverse events, is the risk that those same laws intended to protect will harm. From unsanitary asylums to disproportionate placements of minorities on psychiatric holds, we are concerned with unbridled control in the hands of those meant to cure and care. Sadly, there is also a cinematic genre of unprincipled and detrimental mental health treatment, from Brian Wilson’s treatment by his psychologist documented in “Love & Mercy,” to the upcoming “The Shrink Next Door,” featuring a psychiatrist swindling his patient.
With this additional understanding and analysis, we now ask our colleagues what it would take for them to intervene. Would a celebrity losing $100,000,000 because of mental illness constitute a form of grave disability despite remaining dressed? Would a celebrity engaging in significant drug use constitute a form of self-harm despite still recording albums? Would a celebrity failing to fulfill a social commitment to others, including children, constitute a form of harm to others? Those are not trivial questions to answer, and we are glad the Goldwater Rule reminds us of the limitations of speculating on people we do not know.
Nonetheless, the question of conservatorship is more complex than simply saying: “They make money; they have clothes on; this is absurd.” While this may be a catchy, compelling, and relevant argument, when confronted with a more complete narrative, triers of facts may feel compelled to intervene because, in the end, conservatorship laws are about what society is willing to accept rather than an enumeration of psychiatric symptoms.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Compton is a psychiatry resident at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research.
References
1. Badre N et al. “Coercion and the critical psychiatrist.” In Critical Psychiatry. Springer, Cham, 2019. doi: 10.1007/97-3-030-02732-2_7.
2. Barnes SS and Badre N. Psychiatr Serv. 2016 Jul 1;67(7)784-6.
3. Myrick JG and Willoughby JF. Health Commun. 2021 Jan 14;1-9.
If you are a psychiatrist who has done a public lecture in the past year, you likely encountered the question, “What about Britney’s conservatorship?” Many psychiatrists are far removed from conservatorship evaluations, doing the different yet still important work of alleviating mental suffering without paddling in the controversial waters of involuntary treatment. Others judiciously hide behind the veil of the prudent Goldwater Rule in avoiding such discussions altogether. Regardless of whether psychiatry attempts to stay out of such affairs publicly, our field remains intimately involved in the process itself. This can lead to negative views of psychiatry among the public – that of a medical specialty with ulterior motives operating at the behest of the state.
Some psychiatrists simplistically advocate against any form of involuntary treatment.1 In many ways, this may appear noble. However, the reality of mental illness, with its potential harm to self and others, introduces the potential for dire consequences of such a position. If society is unwilling to accept behavior that may lead to harm, but psychiatry is unwilling to intervene, then other avenues of restricting such behavior will emerge. Those avenues traditionally have included conscription of law enforcement and the incarceration of patients with mental illness.
Yet, therein lies the conundrum of Ms. Spears and other celebrities on conservatorship. At face value, they do not appear to require conservatorship. We do not think it violates the Goldwater Rule to render this observation. In fact, it may reassure the public if the American Psychiatric Association, as well as individual psychiatrists, were more open about the goal, intent, and limitations of conservatorships.
The process of establishing conservatorships is not driven solely by mental health professionals. Rather, conservatorship laws permit society to enact, through psychiatrists, its desire to alleviate behaviors considered unacceptable in the context of mental illness.
In California, it has resulted in our famous or infamous “5150,” which asks psychiatrists to comment on the danger to self, danger to others, and grave disability of our patients. It can be helpful to frame these criteria regarding the relationship between society and our patients. The criteria of danger to self represents society’s wish to intervene in cases of patients with imminent intent of self-harm, operating under the presumption that a suicide can be prevented. Danger to others represents the societal angst, at times exaggerated,2 about people with mental illness perpetuating homicides, especially when off their medication. Grave disability represents public shame at the thought of persons so lost to mental illness they are unable to provide for themselves or even accept food, clothing, and shelter.
While an involuntary hold is necessary at times, working against our patients engenders revolting feelings. We often rationalize involuntary holds as illustrative of sincere compassion for our patients’ suffering and an attempt to lift them out of such tragic conditions. Our patients regularly do not feel our compassion when we are making an argument in a hearing for the restriction of their rights. They see our efforts as an attempt to lock them away “for their own good” because of society’s discomfort with homelessness. As such, we wonder whether our role becomes one of doctors for society, prescribing a treatment for the emotional distress of the community, and at times for ourselves, rather than that of the patient.
One may be perplexed as to how a celebrity could be considered gravely disabled. Celebrities generally have enough income to afford food, clothing, and shelter. One could justifiably ask why an individual with no history of violence would be considered a danger to others. Similarly, one may wonder how, in the absence of any reported attempts to engage in self-harm, with no visible marks of self-harm, someone is determined to be a danger to himself or herself. The bafflement on the part of one on the outside of these determinations can be sharply contrasted by the desperation felt by family members whose loved ones with mental illness appear to meet those criteria yet are consistently turned away by mental health programs and hospitals.
Not uncommonly, it is families advocating for involuntary hospitalization – while lamenting our strict criteria – that prevent doctors from intervening until some tragic fate befalls their loved ones. They criticize what they consider to be too-stringent mental health laws and are infuriated by seemingly obtuse insurance policies limiting care to patients. Most of our colleagues working with those who have severe mental illness share the frustration of these families over the scarcity of psychiatry beds. Therefore, it is particularly shocking when the most mediatized story about conservatorship is not about how hard it is to obtain. The story is about a singer who was seemingly safe, caring for herself, and yet still ended up on a conservatorship.
We wonder whether there is a question of magnitude. Are homeless patients more difficult to place on conservatorship because society sees a lesser stake? One could argue that Ms. Spears and other celebrities would have so much to lose in a single episode of mental illness. A week with mania or psychosis could cause irreparable damage to their persona, opportunity for employment, and their fortune. On the contrary, many of our patients on conservatorship have little to their names, and no one keeping up on their reputation. Triers of facts should ask themselves about the nature of their motivations. Envy, a desire to live vicariously through celebrities, or even less ethical motivations – such as a desire to control and exert authority over those individuals – can influence our decisions.
Throughout the past year, when asked about Ms. Spears, we have pointed out the obvious – she seemingly has a life incompatible with meeting criteria for a psychiatric conservatorship. We have outlined the role, history, and limitations of psychiatric conservatorship. We have shared how such cases are often approached, when required for our own patients or when asked by the court to do so. We have discussed the significant oversight of the system, including the public conservator’s office, which frequently refuses petitions outright. There are hearing officers, who, in the early stages of this process, weigh our case against that of the patients, aided by passionately driven patient advocates. There is the public defender’s office, which, at least in San Diego, vigorously defends the rights of those with mental illness. Most importantly, there are judges who adjudicate those cases with diligence and humility.
As the story has continued to be in the news, we have had numerous conversations about Ms. Spears’ conservatorship with colleagues sharing strong opinions on her case. Many of these colleagues do not have forensic practices and we inevitably find ourselves responding along the lines of, “It is easy to say this, but quite a different thing to prove it in court.” It is hard not to imagine testifying in such a high-profile conservatorship case; testifying, in front of jurors, about a celebrity who may have engaged in what some considered to be unusual behavior.
Conservatorship laws are not about the minutia or criteria of a specific mental health disorder. Patients do not meet criteria for conservatorship by having a certain number of delusional thoughts or a specific type of hallucination. Patients meet criteria for conservatorship because of state-enacted laws based on social factors – such as danger and self-care – the population wishes to treat, even if against the will of those treated. Under this light, one must recognize that a conservatorship trial is not just about mental illness but about how society wants to care for human beings. Psychiatric illness itself is not grounds for conservatorship. Oftentimes, severely ill patients win a hearing for grave disability by simply accepting a referral for housing, showing up to court clothed, and eating the meals provided at the hospital.
With understanding that these laws pertain specifically to behaviors resulting from mental illness that society finds unacceptable, the narrative of a celebrity conservatorship can be considered differently. The stories of celebrities being used and abused by deleterious beings and deleterious conditions have become a genre. Paul Prenter’s treatment of Freddie Mercury documented in the 2018 movie “Bohemian Rhapsody” and John Reid’s alleged betrayal of Elton John, who was suffering from a substance use disorder, documented in the 2019 movie “Rocketman,” are recent examples, among many.
Imagine yourself, as a juror, deciding on the fate of a celebrity. Would you require them to have lost all property, including the clothing on their backs, before intervening? Consider the next time you hear of a celebrity swindled from his or her fortune in a time of crisis and whether it would have been righteous to prevent it. We personally have, at times, argued for restraint in psychiatry’s desire to have more power. This concern extends not only to our ability to control people, but also our ability to force them into being subjected to psychotropic medications with well-known side effects.
At the same time, we remain cognizant of the magnified impact of adverse outcomes on public figures. John Hinckley Jr. did not attempt to murder a bystander; he attempted to kill the president of the United States when he shot at President Ronald Reagan in 1981. That incident led to considerable changes in our laws about insanity. More recently, society was particularly affected by Tom Hanks’ COVID-19 diagnosis. Mr. Hanks’ illness led to scientifically measurable changes in the public’s beliefs regarding the pandemic.3
On the other hand, and of equal importance to the desire to protect public figures from adverse events, is the risk that those same laws intended to protect will harm. From unsanitary asylums to disproportionate placements of minorities on psychiatric holds, we are concerned with unbridled control in the hands of those meant to cure and care. Sadly, there is also a cinematic genre of unprincipled and detrimental mental health treatment, from Brian Wilson’s treatment by his psychologist documented in “Love & Mercy,” to the upcoming “The Shrink Next Door,” featuring a psychiatrist swindling his patient.
With this additional understanding and analysis, we now ask our colleagues what it would take for them to intervene. Would a celebrity losing $100,000,000 because of mental illness constitute a form of grave disability despite remaining dressed? Would a celebrity engaging in significant drug use constitute a form of self-harm despite still recording albums? Would a celebrity failing to fulfill a social commitment to others, including children, constitute a form of harm to others? Those are not trivial questions to answer, and we are glad the Goldwater Rule reminds us of the limitations of speculating on people we do not know.
Nonetheless, the question of conservatorship is more complex than simply saying: “They make money; they have clothes on; this is absurd.” While this may be a catchy, compelling, and relevant argument, when confronted with a more complete narrative, triers of facts may feel compelled to intervene because, in the end, conservatorship laws are about what society is willing to accept rather than an enumeration of psychiatric symptoms.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Compton is a psychiatry resident at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research.
References
1. Badre N et al. “Coercion and the critical psychiatrist.” In Critical Psychiatry. Springer, Cham, 2019. doi: 10.1007/97-3-030-02732-2_7.
2. Barnes SS and Badre N. Psychiatr Serv. 2016 Jul 1;67(7)784-6.
3. Myrick JG and Willoughby JF. Health Commun. 2021 Jan 14;1-9.
If you are a psychiatrist who has done a public lecture in the past year, you likely encountered the question, “What about Britney’s conservatorship?” Many psychiatrists are far removed from conservatorship evaluations, doing the different yet still important work of alleviating mental suffering without paddling in the controversial waters of involuntary treatment. Others judiciously hide behind the veil of the prudent Goldwater Rule in avoiding such discussions altogether. Regardless of whether psychiatry attempts to stay out of such affairs publicly, our field remains intimately involved in the process itself. This can lead to negative views of psychiatry among the public – that of a medical specialty with ulterior motives operating at the behest of the state.
Some psychiatrists simplistically advocate against any form of involuntary treatment.1 In many ways, this may appear noble. However, the reality of mental illness, with its potential harm to self and others, introduces the potential for dire consequences of such a position. If society is unwilling to accept behavior that may lead to harm, but psychiatry is unwilling to intervene, then other avenues of restricting such behavior will emerge. Those avenues traditionally have included conscription of law enforcement and the incarceration of patients with mental illness.
Yet, therein lies the conundrum of Ms. Spears and other celebrities on conservatorship. At face value, they do not appear to require conservatorship. We do not think it violates the Goldwater Rule to render this observation. In fact, it may reassure the public if the American Psychiatric Association, as well as individual psychiatrists, were more open about the goal, intent, and limitations of conservatorships.
The process of establishing conservatorships is not driven solely by mental health professionals. Rather, conservatorship laws permit society to enact, through psychiatrists, its desire to alleviate behaviors considered unacceptable in the context of mental illness.
In California, it has resulted in our famous or infamous “5150,” which asks psychiatrists to comment on the danger to self, danger to others, and grave disability of our patients. It can be helpful to frame these criteria regarding the relationship between society and our patients. The criteria of danger to self represents society’s wish to intervene in cases of patients with imminent intent of self-harm, operating under the presumption that a suicide can be prevented. Danger to others represents the societal angst, at times exaggerated,2 about people with mental illness perpetuating homicides, especially when off their medication. Grave disability represents public shame at the thought of persons so lost to mental illness they are unable to provide for themselves or even accept food, clothing, and shelter.
While an involuntary hold is necessary at times, working against our patients engenders revolting feelings. We often rationalize involuntary holds as illustrative of sincere compassion for our patients’ suffering and an attempt to lift them out of such tragic conditions. Our patients regularly do not feel our compassion when we are making an argument in a hearing for the restriction of their rights. They see our efforts as an attempt to lock them away “for their own good” because of society’s discomfort with homelessness. As such, we wonder whether our role becomes one of doctors for society, prescribing a treatment for the emotional distress of the community, and at times for ourselves, rather than that of the patient.
One may be perplexed as to how a celebrity could be considered gravely disabled. Celebrities generally have enough income to afford food, clothing, and shelter. One could justifiably ask why an individual with no history of violence would be considered a danger to others. Similarly, one may wonder how, in the absence of any reported attempts to engage in self-harm, with no visible marks of self-harm, someone is determined to be a danger to himself or herself. The bafflement on the part of one on the outside of these determinations can be sharply contrasted by the desperation felt by family members whose loved ones with mental illness appear to meet those criteria yet are consistently turned away by mental health programs and hospitals.
Not uncommonly, it is families advocating for involuntary hospitalization – while lamenting our strict criteria – that prevent doctors from intervening until some tragic fate befalls their loved ones. They criticize what they consider to be too-stringent mental health laws and are infuriated by seemingly obtuse insurance policies limiting care to patients. Most of our colleagues working with those who have severe mental illness share the frustration of these families over the scarcity of psychiatry beds. Therefore, it is particularly shocking when the most mediatized story about conservatorship is not about how hard it is to obtain. The story is about a singer who was seemingly safe, caring for herself, and yet still ended up on a conservatorship.
We wonder whether there is a question of magnitude. Are homeless patients more difficult to place on conservatorship because society sees a lesser stake? One could argue that Ms. Spears and other celebrities would have so much to lose in a single episode of mental illness. A week with mania or psychosis could cause irreparable damage to their persona, opportunity for employment, and their fortune. On the contrary, many of our patients on conservatorship have little to their names, and no one keeping up on their reputation. Triers of facts should ask themselves about the nature of their motivations. Envy, a desire to live vicariously through celebrities, or even less ethical motivations – such as a desire to control and exert authority over those individuals – can influence our decisions.
Throughout the past year, when asked about Ms. Spears, we have pointed out the obvious – she seemingly has a life incompatible with meeting criteria for a psychiatric conservatorship. We have outlined the role, history, and limitations of psychiatric conservatorship. We have shared how such cases are often approached, when required for our own patients or when asked by the court to do so. We have discussed the significant oversight of the system, including the public conservator’s office, which frequently refuses petitions outright. There are hearing officers, who, in the early stages of this process, weigh our case against that of the patients, aided by passionately driven patient advocates. There is the public defender’s office, which, at least in San Diego, vigorously defends the rights of those with mental illness. Most importantly, there are judges who adjudicate those cases with diligence and humility.
As the story has continued to be in the news, we have had numerous conversations about Ms. Spears’ conservatorship with colleagues sharing strong opinions on her case. Many of these colleagues do not have forensic practices and we inevitably find ourselves responding along the lines of, “It is easy to say this, but quite a different thing to prove it in court.” It is hard not to imagine testifying in such a high-profile conservatorship case; testifying, in front of jurors, about a celebrity who may have engaged in what some considered to be unusual behavior.
Conservatorship laws are not about the minutia or criteria of a specific mental health disorder. Patients do not meet criteria for conservatorship by having a certain number of delusional thoughts or a specific type of hallucination. Patients meet criteria for conservatorship because of state-enacted laws based on social factors – such as danger and self-care – the population wishes to treat, even if against the will of those treated. Under this light, one must recognize that a conservatorship trial is not just about mental illness but about how society wants to care for human beings. Psychiatric illness itself is not grounds for conservatorship. Oftentimes, severely ill patients win a hearing for grave disability by simply accepting a referral for housing, showing up to court clothed, and eating the meals provided at the hospital.
With understanding that these laws pertain specifically to behaviors resulting from mental illness that society finds unacceptable, the narrative of a celebrity conservatorship can be considered differently. The stories of celebrities being used and abused by deleterious beings and deleterious conditions have become a genre. Paul Prenter’s treatment of Freddie Mercury documented in the 2018 movie “Bohemian Rhapsody” and John Reid’s alleged betrayal of Elton John, who was suffering from a substance use disorder, documented in the 2019 movie “Rocketman,” are recent examples, among many.
Imagine yourself, as a juror, deciding on the fate of a celebrity. Would you require them to have lost all property, including the clothing on their backs, before intervening? Consider the next time you hear of a celebrity swindled from his or her fortune in a time of crisis and whether it would have been righteous to prevent it. We personally have, at times, argued for restraint in psychiatry’s desire to have more power. This concern extends not only to our ability to control people, but also our ability to force them into being subjected to psychotropic medications with well-known side effects.
At the same time, we remain cognizant of the magnified impact of adverse outcomes on public figures. John Hinckley Jr. did not attempt to murder a bystander; he attempted to kill the president of the United States when he shot at President Ronald Reagan in 1981. That incident led to considerable changes in our laws about insanity. More recently, society was particularly affected by Tom Hanks’ COVID-19 diagnosis. Mr. Hanks’ illness led to scientifically measurable changes in the public’s beliefs regarding the pandemic.3
On the other hand, and of equal importance to the desire to protect public figures from adverse events, is the risk that those same laws intended to protect will harm. From unsanitary asylums to disproportionate placements of minorities on psychiatric holds, we are concerned with unbridled control in the hands of those meant to cure and care. Sadly, there is also a cinematic genre of unprincipled and detrimental mental health treatment, from Brian Wilson’s treatment by his psychologist documented in “Love & Mercy,” to the upcoming “The Shrink Next Door,” featuring a psychiatrist swindling his patient.
With this additional understanding and analysis, we now ask our colleagues what it would take for them to intervene. Would a celebrity losing $100,000,000 because of mental illness constitute a form of grave disability despite remaining dressed? Would a celebrity engaging in significant drug use constitute a form of self-harm despite still recording albums? Would a celebrity failing to fulfill a social commitment to others, including children, constitute a form of harm to others? Those are not trivial questions to answer, and we are glad the Goldwater Rule reminds us of the limitations of speculating on people we do not know.
Nonetheless, the question of conservatorship is more complex than simply saying: “They make money; they have clothes on; this is absurd.” While this may be a catchy, compelling, and relevant argument, when confronted with a more complete narrative, triers of facts may feel compelled to intervene because, in the end, conservatorship laws are about what society is willing to accept rather than an enumeration of psychiatric symptoms.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Compton is a psychiatry resident at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research.
References
1. Badre N et al. “Coercion and the critical psychiatrist.” In Critical Psychiatry. Springer, Cham, 2019. doi: 10.1007/97-3-030-02732-2_7.
2. Barnes SS and Badre N. Psychiatr Serv. 2016 Jul 1;67(7)784-6.
3. Myrick JG and Willoughby JF. Health Commun. 2021 Jan 14;1-9.
Alcohol-related liver disease severity increased during COVID-19 pandemic
LAS VEGAS – Over the course of the COVID-19 pandemic, alcohol-related liver disease has increased in severity, a finding that is likely related to higher consumption of alcohol and reduced care. The difference was notable in higher Model for End-Stage Liver Disease–sodium (MELD-Na) scores, more signs of hepatic decompensation, and higher mortality rates.
“Alcohol consumption during the COVID-19 pandemic led to increased morbidity and mortality, specifically in patients that already had underlying liver disease. The importance of alcohol cessation, counseling, and close physician monitoring is emphasized, given continued or relapsed alcohol consumption can significantly affect quality of life, life expectancy, and liver transplantation candidacy,” research team member Lindsay A. Sobotka, DO, said in an interview. Dr. Sobotka is an assistant professor of gastroenterology, hepatology, and nutrition at the Ohio State University Wexner Medical Center, Columbus.
The research was presented by Ayushi Jain, MD, at the annual meeting of the American College of Gastroenterology. Dr. Jain is a resident at the Ohio State University Wexner Medical Center.
Dr. Jain noted that alcohol sales have gone up during the pandemic, with monthly sales up 14%-44% between February and September 2020, compared with the same months in previous years.
Decompensation rates rose
The researchers analyzed data from patients with alcoholic cirrhosis or alcoholic hepatitis who were seen at the Ohio State University Medical Center between March and August 2019, and between March and August 2020.
During the pandemic, the number of hospital admissions nearly doubled among alcoholic hepatitis patients (86 to 162), but declined slightly among patients with alcoholic cirrhosis (613 to 528), possibly because of efforts to manage decompensation and avoid hospitalizations during the pandemic, according to Dr. Jain. In total, 4 of 162 patients with alcoholic hepatitis and 14 of 528 patients with alcoholic cirrhosis had COVID-19 at the time of admission.
Higher mortality rates were seen during the pandemic, although this was only significant for alcoholic cirrhosis: 14.8% versus 7% for alcoholic hepatitis (P = .06) and 13.5% versus 7.4% for alcoholic cirrhosis (P = .001).
Among those with alcoholic hepatitis, there was no significant change in median Maddrey’s Discriminant Function during the pandemic (P = .51), but the researchers noted a significant decrease in steroid use, from 27 patients to 23 (P = .001). “This may be due to a statistically significant increase in GI bleeds and renal dysfunction that we noted during the pandemic,” said Dr. Jain.
Hepatic decompensation and critical care needs increased among patients admitted with alcoholic hepatitis, including hepatic encephalopathy (P = .037), gastrointestinal bleeding (P = .01), a need for increased oxygen (P = .024), vasopressor support (P = .005), and initiation of hemodialysis (P = .007). The median highest MELD-Na score during admission was also higher during the pandemic (24 vs. 23, P = .04).
Patients with alcoholic cirrhosis had greater decompensation as measured by ascites (P = .01), therapeutic paracentesis (P = .04), titration of diuretics (P = .005), acute kidney injury (P = .005), hepatorenal syndrome (P = .002), and spontaneous bacterial peritonitis (P = .04). They also had greater need for vasopressor support (9% to 14%; P = .006), were more likely to initiate hemodialysis (7% to 11%; P = .015), and had greater mortality (7% to 14%; P = .001).
In all, 212 patients reported increased alcohol intake, 161 reported little change over the past year, and 253 said they were abstinent. MELD-Na scores were highest in the increased group (27), compared with the unchanged group (24) and abstinent group (23) (P = .001).
More robust support needed
“This highlights that the increase in alcohol use seems to be associated with higher rates of more severe alcoholic hepatitis, and we are going to need to all be aware of and intervene in these individuals, and try to not only make health care more accessible, but help those with alcohol use disorder to reengage in some support systems [and] harm-reduction measures, to try to reduce the number of these episodes of admissions with severe alcoholic hepatitis,” said Paul Kwo, MD, who comoderated the session. Dr. Kwo is a professor of medicine at Stanford (Calif.) University.
Dr. Kwo suggested that the pandemic has presented dual challenges to patients with alcohol-related liver disease. One is that hospitals have filled up because of an influx of COVID-19 cases, which makes it hard for them to compete for limited resources. The other is that lockdowns and social interruptions may have interfered with the support systems that normally help them to keep sober and maintain health care. “The pandemic really disrupted everybody’s ecosystem substantially, and some of these individuals, as their ecosystems crumble, they don’t have other resources to engage in care, and then they present with far more advanced comorbidities than we might have seen prior to the pandemic,” said Dr. Kwo.
The findings underscore at least one lesson that can be drawn from the pandemic. “We now know that we have to develop more robust systems to provide support for all of these individuals,” said Dr. Kwo.
Comoderator Patricia D. Jones, MD, agreed, and expressed optimism. “We were forced develop more remote or virtual networks, so I think there are a lot of people that are taking advantage maybe of virtual [Alcoholics Anonymous], and that wasn’t something that they necessarily did [before the pandemic]. And so at least we’ve developed some parallel systems that hopefully people will benefit from,” said Dr. Jones, who is an assistant professor of medicine at the University of Miami.
She suggested that physicians should make inquiries about patients with alcohol-related liver disease and their social situations, and might consider trying to connect them to a social worker if called for. “I think that really speaking to the person about where they are would be beneficial,” said Dr. Jones.
Dr. Sobotka, Dr. Jain, Dr. Kwo, and Dr. Jones have no relevant financial disclosures.
LAS VEGAS – Over the course of the COVID-19 pandemic, alcohol-related liver disease has increased in severity, a finding that is likely related to higher consumption of alcohol and reduced care. The difference was notable in higher Model for End-Stage Liver Disease–sodium (MELD-Na) scores, more signs of hepatic decompensation, and higher mortality rates.
“Alcohol consumption during the COVID-19 pandemic led to increased morbidity and mortality, specifically in patients that already had underlying liver disease. The importance of alcohol cessation, counseling, and close physician monitoring is emphasized, given continued or relapsed alcohol consumption can significantly affect quality of life, life expectancy, and liver transplantation candidacy,” research team member Lindsay A. Sobotka, DO, said in an interview. Dr. Sobotka is an assistant professor of gastroenterology, hepatology, and nutrition at the Ohio State University Wexner Medical Center, Columbus.
The research was presented by Ayushi Jain, MD, at the annual meeting of the American College of Gastroenterology. Dr. Jain is a resident at the Ohio State University Wexner Medical Center.
Dr. Jain noted that alcohol sales have gone up during the pandemic, with monthly sales up 14%-44% between February and September 2020, compared with the same months in previous years.
Decompensation rates rose
The researchers analyzed data from patients with alcoholic cirrhosis or alcoholic hepatitis who were seen at the Ohio State University Medical Center between March and August 2019, and between March and August 2020.
During the pandemic, the number of hospital admissions nearly doubled among alcoholic hepatitis patients (86 to 162), but declined slightly among patients with alcoholic cirrhosis (613 to 528), possibly because of efforts to manage decompensation and avoid hospitalizations during the pandemic, according to Dr. Jain. In total, 4 of 162 patients with alcoholic hepatitis and 14 of 528 patients with alcoholic cirrhosis had COVID-19 at the time of admission.
Higher mortality rates were seen during the pandemic, although this was only significant for alcoholic cirrhosis: 14.8% versus 7% for alcoholic hepatitis (P = .06) and 13.5% versus 7.4% for alcoholic cirrhosis (P = .001).
Among those with alcoholic hepatitis, there was no significant change in median Maddrey’s Discriminant Function during the pandemic (P = .51), but the researchers noted a significant decrease in steroid use, from 27 patients to 23 (P = .001). “This may be due to a statistically significant increase in GI bleeds and renal dysfunction that we noted during the pandemic,” said Dr. Jain.
Hepatic decompensation and critical care needs increased among patients admitted with alcoholic hepatitis, including hepatic encephalopathy (P = .037), gastrointestinal bleeding (P = .01), a need for increased oxygen (P = .024), vasopressor support (P = .005), and initiation of hemodialysis (P = .007). The median highest MELD-Na score during admission was also higher during the pandemic (24 vs. 23, P = .04).
Patients with alcoholic cirrhosis had greater decompensation as measured by ascites (P = .01), therapeutic paracentesis (P = .04), titration of diuretics (P = .005), acute kidney injury (P = .005), hepatorenal syndrome (P = .002), and spontaneous bacterial peritonitis (P = .04). They also had greater need for vasopressor support (9% to 14%; P = .006), were more likely to initiate hemodialysis (7% to 11%; P = .015), and had greater mortality (7% to 14%; P = .001).
In all, 212 patients reported increased alcohol intake, 161 reported little change over the past year, and 253 said they were abstinent. MELD-Na scores were highest in the increased group (27), compared with the unchanged group (24) and abstinent group (23) (P = .001).
More robust support needed
“This highlights that the increase in alcohol use seems to be associated with higher rates of more severe alcoholic hepatitis, and we are going to need to all be aware of and intervene in these individuals, and try to not only make health care more accessible, but help those with alcohol use disorder to reengage in some support systems [and] harm-reduction measures, to try to reduce the number of these episodes of admissions with severe alcoholic hepatitis,” said Paul Kwo, MD, who comoderated the session. Dr. Kwo is a professor of medicine at Stanford (Calif.) University.
Dr. Kwo suggested that the pandemic has presented dual challenges to patients with alcohol-related liver disease. One is that hospitals have filled up because of an influx of COVID-19 cases, which makes it hard for them to compete for limited resources. The other is that lockdowns and social interruptions may have interfered with the support systems that normally help them to keep sober and maintain health care. “The pandemic really disrupted everybody’s ecosystem substantially, and some of these individuals, as their ecosystems crumble, they don’t have other resources to engage in care, and then they present with far more advanced comorbidities than we might have seen prior to the pandemic,” said Dr. Kwo.
The findings underscore at least one lesson that can be drawn from the pandemic. “We now know that we have to develop more robust systems to provide support for all of these individuals,” said Dr. Kwo.
Comoderator Patricia D. Jones, MD, agreed, and expressed optimism. “We were forced develop more remote or virtual networks, so I think there are a lot of people that are taking advantage maybe of virtual [Alcoholics Anonymous], and that wasn’t something that they necessarily did [before the pandemic]. And so at least we’ve developed some parallel systems that hopefully people will benefit from,” said Dr. Jones, who is an assistant professor of medicine at the University of Miami.
She suggested that physicians should make inquiries about patients with alcohol-related liver disease and their social situations, and might consider trying to connect them to a social worker if called for. “I think that really speaking to the person about where they are would be beneficial,” said Dr. Jones.
Dr. Sobotka, Dr. Jain, Dr. Kwo, and Dr. Jones have no relevant financial disclosures.
LAS VEGAS – Over the course of the COVID-19 pandemic, alcohol-related liver disease has increased in severity, a finding that is likely related to higher consumption of alcohol and reduced care. The difference was notable in higher Model for End-Stage Liver Disease–sodium (MELD-Na) scores, more signs of hepatic decompensation, and higher mortality rates.
“Alcohol consumption during the COVID-19 pandemic led to increased morbidity and mortality, specifically in patients that already had underlying liver disease. The importance of alcohol cessation, counseling, and close physician monitoring is emphasized, given continued or relapsed alcohol consumption can significantly affect quality of life, life expectancy, and liver transplantation candidacy,” research team member Lindsay A. Sobotka, DO, said in an interview. Dr. Sobotka is an assistant professor of gastroenterology, hepatology, and nutrition at the Ohio State University Wexner Medical Center, Columbus.
The research was presented by Ayushi Jain, MD, at the annual meeting of the American College of Gastroenterology. Dr. Jain is a resident at the Ohio State University Wexner Medical Center.
Dr. Jain noted that alcohol sales have gone up during the pandemic, with monthly sales up 14%-44% between February and September 2020, compared with the same months in previous years.
Decompensation rates rose
The researchers analyzed data from patients with alcoholic cirrhosis or alcoholic hepatitis who were seen at the Ohio State University Medical Center between March and August 2019, and between March and August 2020.
During the pandemic, the number of hospital admissions nearly doubled among alcoholic hepatitis patients (86 to 162), but declined slightly among patients with alcoholic cirrhosis (613 to 528), possibly because of efforts to manage decompensation and avoid hospitalizations during the pandemic, according to Dr. Jain. In total, 4 of 162 patients with alcoholic hepatitis and 14 of 528 patients with alcoholic cirrhosis had COVID-19 at the time of admission.
Higher mortality rates were seen during the pandemic, although this was only significant for alcoholic cirrhosis: 14.8% versus 7% for alcoholic hepatitis (P = .06) and 13.5% versus 7.4% for alcoholic cirrhosis (P = .001).
Among those with alcoholic hepatitis, there was no significant change in median Maddrey’s Discriminant Function during the pandemic (P = .51), but the researchers noted a significant decrease in steroid use, from 27 patients to 23 (P = .001). “This may be due to a statistically significant increase in GI bleeds and renal dysfunction that we noted during the pandemic,” said Dr. Jain.
Hepatic decompensation and critical care needs increased among patients admitted with alcoholic hepatitis, including hepatic encephalopathy (P = .037), gastrointestinal bleeding (P = .01), a need for increased oxygen (P = .024), vasopressor support (P = .005), and initiation of hemodialysis (P = .007). The median highest MELD-Na score during admission was also higher during the pandemic (24 vs. 23, P = .04).
Patients with alcoholic cirrhosis had greater decompensation as measured by ascites (P = .01), therapeutic paracentesis (P = .04), titration of diuretics (P = .005), acute kidney injury (P = .005), hepatorenal syndrome (P = .002), and spontaneous bacterial peritonitis (P = .04). They also had greater need for vasopressor support (9% to 14%; P = .006), were more likely to initiate hemodialysis (7% to 11%; P = .015), and had greater mortality (7% to 14%; P = .001).
In all, 212 patients reported increased alcohol intake, 161 reported little change over the past year, and 253 said they were abstinent. MELD-Na scores were highest in the increased group (27), compared with the unchanged group (24) and abstinent group (23) (P = .001).
More robust support needed
“This highlights that the increase in alcohol use seems to be associated with higher rates of more severe alcoholic hepatitis, and we are going to need to all be aware of and intervene in these individuals, and try to not only make health care more accessible, but help those with alcohol use disorder to reengage in some support systems [and] harm-reduction measures, to try to reduce the number of these episodes of admissions with severe alcoholic hepatitis,” said Paul Kwo, MD, who comoderated the session. Dr. Kwo is a professor of medicine at Stanford (Calif.) University.
Dr. Kwo suggested that the pandemic has presented dual challenges to patients with alcohol-related liver disease. One is that hospitals have filled up because of an influx of COVID-19 cases, which makes it hard for them to compete for limited resources. The other is that lockdowns and social interruptions may have interfered with the support systems that normally help them to keep sober and maintain health care. “The pandemic really disrupted everybody’s ecosystem substantially, and some of these individuals, as their ecosystems crumble, they don’t have other resources to engage in care, and then they present with far more advanced comorbidities than we might have seen prior to the pandemic,” said Dr. Kwo.
The findings underscore at least one lesson that can be drawn from the pandemic. “We now know that we have to develop more robust systems to provide support for all of these individuals,” said Dr. Kwo.
Comoderator Patricia D. Jones, MD, agreed, and expressed optimism. “We were forced develop more remote or virtual networks, so I think there are a lot of people that are taking advantage maybe of virtual [Alcoholics Anonymous], and that wasn’t something that they necessarily did [before the pandemic]. And so at least we’ve developed some parallel systems that hopefully people will benefit from,” said Dr. Jones, who is an assistant professor of medicine at the University of Miami.
She suggested that physicians should make inquiries about patients with alcohol-related liver disease and their social situations, and might consider trying to connect them to a social worker if called for. “I think that really speaking to the person about where they are would be beneficial,” said Dr. Jones.
Dr. Sobotka, Dr. Jain, Dr. Kwo, and Dr. Jones have no relevant financial disclosures.
AT ACG 2021