User login
Prevalence of psychiatric disorders higher in adult cerebral palsy patients
Adults with cerebral palsy, especially those with intellectual disabilities, are significantly more likely to be diagnosed with a psychiatric disorder, compared with the general population, a review of seven datasets shows.
The body of literature on psychiatric issues in children with cerebral palsy (CP) is increasing, but population-based studies of psychiatric issues in adults with CP have been limited in number and in scope. Most of those studies focus mainly on anxiety and depression, rather than on other issues such as psychosis or schizophrenia, Carly A. McMorris, PhD, of the University of Calgary (Alta.) and colleagues wrote.
In a retrospective, cross-sectional study published in Research in Developmental Disabilities, the researchers reviewed information from five health data sets, one registry, and census data for adults aged 18-64 years with a CP diagnosis living in Ontario, including those with and without diagnosed intellectual disabilities (ID) and a comparison group of individuals in the general population. The researchers examined the proportion of individuals with a psychiatric disorder in each of four groups: total CP, CP without ID, CP with ID, and the general population.
The study participants included 9,388 individuals with CP, 4,767 individuals with CP and ID, and a general population of 2,757,744 individuals. About half of the participants were male, and at least 85% lived in urban areas.
Overall, compared with the general population group, over a 2-year period (33.7 % vs. 24.7%). Also, the CP group was more than twice as likely to be diagnosed with a psychotic disorder, schizophrenia, personality disorder, or bipolar disorder, compared with the general population. Individuals with CP were significantly more likely to suffer from mood or affective disorders, and depression and anxiety disorders, compared with the general population, but less likely to suffer from substance use disorders.
When the data were assessed by ID status, disorders such as psychotic disorders, bipolar disorders, and schizophrenia were six times more common among individuals with CP and ID, compared with the general population (adjusted prevalence ratios, 6.26 and 6.46, respectively).
Individuals with CP and ID also had a notably higher prevalence of bipolar disorder (confidence interval, 2.06-2.89) and personality disorder, compared with the general population (aPR, 2.44 and 4.22, respectively), but this subgroup also was less likely than the general population to engage in substance use (aPR, 0.44).
The study findings were limited by several factors, including the absence of universal definitions for some of the conditions studied, potential misclassification of ID, the inclusion of data on specific psychiatric diagnoses but not elevated symptoms, and by the challenges of diagnosing psychiatric disorders in individuals with ID, the researchers noted.
However, “the present study contributes important information to the existing literature, highlighting that psychiatric issues are common in adults with CP, similar to what has been reported in children and youth,” they said. “Further research is needed to determine the validity and reliability of mental health assessment measures for this population, the efficacy of evidence-based psychotherapeutic approaches ... and the underlying causes or mechanisms of psychiatric issues in individuals with CP.”
The findings also highlight the need for health care clinicians to screen for psychiatric issues in CP patients, they said.
The study was supported in part by the Province of Ontario research grants and the Institute for Clinical Evaluative Sciences, funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. The researchers had no disclosures.
Adults with cerebral palsy, especially those with intellectual disabilities, are significantly more likely to be diagnosed with a psychiatric disorder, compared with the general population, a review of seven datasets shows.
The body of literature on psychiatric issues in children with cerebral palsy (CP) is increasing, but population-based studies of psychiatric issues in adults with CP have been limited in number and in scope. Most of those studies focus mainly on anxiety and depression, rather than on other issues such as psychosis or schizophrenia, Carly A. McMorris, PhD, of the University of Calgary (Alta.) and colleagues wrote.
In a retrospective, cross-sectional study published in Research in Developmental Disabilities, the researchers reviewed information from five health data sets, one registry, and census data for adults aged 18-64 years with a CP diagnosis living in Ontario, including those with and without diagnosed intellectual disabilities (ID) and a comparison group of individuals in the general population. The researchers examined the proportion of individuals with a psychiatric disorder in each of four groups: total CP, CP without ID, CP with ID, and the general population.
The study participants included 9,388 individuals with CP, 4,767 individuals with CP and ID, and a general population of 2,757,744 individuals. About half of the participants were male, and at least 85% lived in urban areas.
Overall, compared with the general population group, over a 2-year period (33.7 % vs. 24.7%). Also, the CP group was more than twice as likely to be diagnosed with a psychotic disorder, schizophrenia, personality disorder, or bipolar disorder, compared with the general population. Individuals with CP were significantly more likely to suffer from mood or affective disorders, and depression and anxiety disorders, compared with the general population, but less likely to suffer from substance use disorders.
When the data were assessed by ID status, disorders such as psychotic disorders, bipolar disorders, and schizophrenia were six times more common among individuals with CP and ID, compared with the general population (adjusted prevalence ratios, 6.26 and 6.46, respectively).
Individuals with CP and ID also had a notably higher prevalence of bipolar disorder (confidence interval, 2.06-2.89) and personality disorder, compared with the general population (aPR, 2.44 and 4.22, respectively), but this subgroup also was less likely than the general population to engage in substance use (aPR, 0.44).
The study findings were limited by several factors, including the absence of universal definitions for some of the conditions studied, potential misclassification of ID, the inclusion of data on specific psychiatric diagnoses but not elevated symptoms, and by the challenges of diagnosing psychiatric disorders in individuals with ID, the researchers noted.
However, “the present study contributes important information to the existing literature, highlighting that psychiatric issues are common in adults with CP, similar to what has been reported in children and youth,” they said. “Further research is needed to determine the validity and reliability of mental health assessment measures for this population, the efficacy of evidence-based psychotherapeutic approaches ... and the underlying causes or mechanisms of psychiatric issues in individuals with CP.”
The findings also highlight the need for health care clinicians to screen for psychiatric issues in CP patients, they said.
The study was supported in part by the Province of Ontario research grants and the Institute for Clinical Evaluative Sciences, funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. The researchers had no disclosures.
Adults with cerebral palsy, especially those with intellectual disabilities, are significantly more likely to be diagnosed with a psychiatric disorder, compared with the general population, a review of seven datasets shows.
The body of literature on psychiatric issues in children with cerebral palsy (CP) is increasing, but population-based studies of psychiatric issues in adults with CP have been limited in number and in scope. Most of those studies focus mainly on anxiety and depression, rather than on other issues such as psychosis or schizophrenia, Carly A. McMorris, PhD, of the University of Calgary (Alta.) and colleagues wrote.
In a retrospective, cross-sectional study published in Research in Developmental Disabilities, the researchers reviewed information from five health data sets, one registry, and census data for adults aged 18-64 years with a CP diagnosis living in Ontario, including those with and without diagnosed intellectual disabilities (ID) and a comparison group of individuals in the general population. The researchers examined the proportion of individuals with a psychiatric disorder in each of four groups: total CP, CP without ID, CP with ID, and the general population.
The study participants included 9,388 individuals with CP, 4,767 individuals with CP and ID, and a general population of 2,757,744 individuals. About half of the participants were male, and at least 85% lived in urban areas.
Overall, compared with the general population group, over a 2-year period (33.7 % vs. 24.7%). Also, the CP group was more than twice as likely to be diagnosed with a psychotic disorder, schizophrenia, personality disorder, or bipolar disorder, compared with the general population. Individuals with CP were significantly more likely to suffer from mood or affective disorders, and depression and anxiety disorders, compared with the general population, but less likely to suffer from substance use disorders.
When the data were assessed by ID status, disorders such as psychotic disorders, bipolar disorders, and schizophrenia were six times more common among individuals with CP and ID, compared with the general population (adjusted prevalence ratios, 6.26 and 6.46, respectively).
Individuals with CP and ID also had a notably higher prevalence of bipolar disorder (confidence interval, 2.06-2.89) and personality disorder, compared with the general population (aPR, 2.44 and 4.22, respectively), but this subgroup also was less likely than the general population to engage in substance use (aPR, 0.44).
The study findings were limited by several factors, including the absence of universal definitions for some of the conditions studied, potential misclassification of ID, the inclusion of data on specific psychiatric diagnoses but not elevated symptoms, and by the challenges of diagnosing psychiatric disorders in individuals with ID, the researchers noted.
However, “the present study contributes important information to the existing literature, highlighting that psychiatric issues are common in adults with CP, similar to what has been reported in children and youth,” they said. “Further research is needed to determine the validity and reliability of mental health assessment measures for this population, the efficacy of evidence-based psychotherapeutic approaches ... and the underlying causes or mechanisms of psychiatric issues in individuals with CP.”
The findings also highlight the need for health care clinicians to screen for psychiatric issues in CP patients, they said.
The study was supported in part by the Province of Ontario research grants and the Institute for Clinical Evaluative Sciences, funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. The researchers had no disclosures.
FROM RESEARCH IN DEVELOPMENTAL DISABILITIES
HHS to inject billions into mental health, substance use disorders
The U.S. Department of Health and Human Services will inject billions of dollars into programs designed to address mental health and substance use disorders, including $3 billion released to states as of May 18, said federal officials.
The American Rescue Plan, a COVID-relief package signed into law in March, contained the money, which will be divided equally between the Community Mental Health Services Block Grant Program and the Substance Abuse Prevention and Treatment Block Grant Program, said Tom Coderre, Acting Assistant Secretary for Mental Health and Substance Use, in a call with reporters.
The award amounts will vary by state.
The mental health program helps states and territories provide services for children with serious emotional issues and adults with serious mental illness.
The substance use program provides money to plan, implement, and evaluate prevention, intervention, treatment, and recovery services.
, which fueled an increase in anxiety, depression, and overdose, said Assistant Secretary for Health Rachel Levine, MD, on the call.
“We know multiple stressors during the pandemic – isolation, sickness, grief, job loss, food instability, and loss of routines – have devastated many Americans and presented the unprecedented behavioral health challenges across the nation,” said Dr. Levine.
The HHS also announced that it is re-establishing a Behavioral Health Coordinating Council (BHCC). Dr. Levine and Mr. Coderre will serve as cochairs of the Council, which will coordinate action-oriented approaches to addressing the HHS’s behavioral health efforts.
However, in 2014, the U.S. Government Accountability Office criticized the BHCC for only focusing on the HHS, and noted the lack of coordination across the federal government’s various efforts to address mental health.
‘A huge step forward’
The American Psychiatric Association welcomed the new money and the return of the council.
“In the wake of the pandemic an unprecedented, and as of yet untold, number of Americans are faced with mental health and substance use disorders, particularly in communities impacted by structural racism,” said APA President Vivian Pender, MD, in a statement. “With the creation of this Council and this investment in mental health, the administration is taking a huge step forward.”
APA CEO and Medical Director Saul Levin, MD, MPA, added: “This Council has great potential to ease the challenges we face as we begin to recover from the pandemic’s impact on our society, and [the] APA looks forward to assisting in their efforts.”
HHS Secretary Xavier Becerra noted in a statement that the COVID-19 pandemic “has made clear the need to invest resources in our nation’s mental health and address the inequities that still exist around behavioral health care.” He added, “This national problem calls for department-wide coordination to address the issue.”
Dr. Levine said the Council “will assure the right prioritization and guidelines are in place to provide pathways to prevention, intervention, treatment, and recovery services.”
A version of this article first appeared on Medscape.com.
The U.S. Department of Health and Human Services will inject billions of dollars into programs designed to address mental health and substance use disorders, including $3 billion released to states as of May 18, said federal officials.
The American Rescue Plan, a COVID-relief package signed into law in March, contained the money, which will be divided equally between the Community Mental Health Services Block Grant Program and the Substance Abuse Prevention and Treatment Block Grant Program, said Tom Coderre, Acting Assistant Secretary for Mental Health and Substance Use, in a call with reporters.
The award amounts will vary by state.
The mental health program helps states and territories provide services for children with serious emotional issues and adults with serious mental illness.
The substance use program provides money to plan, implement, and evaluate prevention, intervention, treatment, and recovery services.
, which fueled an increase in anxiety, depression, and overdose, said Assistant Secretary for Health Rachel Levine, MD, on the call.
“We know multiple stressors during the pandemic – isolation, sickness, grief, job loss, food instability, and loss of routines – have devastated many Americans and presented the unprecedented behavioral health challenges across the nation,” said Dr. Levine.
The HHS also announced that it is re-establishing a Behavioral Health Coordinating Council (BHCC). Dr. Levine and Mr. Coderre will serve as cochairs of the Council, which will coordinate action-oriented approaches to addressing the HHS’s behavioral health efforts.
However, in 2014, the U.S. Government Accountability Office criticized the BHCC for only focusing on the HHS, and noted the lack of coordination across the federal government’s various efforts to address mental health.
‘A huge step forward’
The American Psychiatric Association welcomed the new money and the return of the council.
“In the wake of the pandemic an unprecedented, and as of yet untold, number of Americans are faced with mental health and substance use disorders, particularly in communities impacted by structural racism,” said APA President Vivian Pender, MD, in a statement. “With the creation of this Council and this investment in mental health, the administration is taking a huge step forward.”
APA CEO and Medical Director Saul Levin, MD, MPA, added: “This Council has great potential to ease the challenges we face as we begin to recover from the pandemic’s impact on our society, and [the] APA looks forward to assisting in their efforts.”
HHS Secretary Xavier Becerra noted in a statement that the COVID-19 pandemic “has made clear the need to invest resources in our nation’s mental health and address the inequities that still exist around behavioral health care.” He added, “This national problem calls for department-wide coordination to address the issue.”
Dr. Levine said the Council “will assure the right prioritization and guidelines are in place to provide pathways to prevention, intervention, treatment, and recovery services.”
A version of this article first appeared on Medscape.com.
The U.S. Department of Health and Human Services will inject billions of dollars into programs designed to address mental health and substance use disorders, including $3 billion released to states as of May 18, said federal officials.
The American Rescue Plan, a COVID-relief package signed into law in March, contained the money, which will be divided equally between the Community Mental Health Services Block Grant Program and the Substance Abuse Prevention and Treatment Block Grant Program, said Tom Coderre, Acting Assistant Secretary for Mental Health and Substance Use, in a call with reporters.
The award amounts will vary by state.
The mental health program helps states and territories provide services for children with serious emotional issues and adults with serious mental illness.
The substance use program provides money to plan, implement, and evaluate prevention, intervention, treatment, and recovery services.
, which fueled an increase in anxiety, depression, and overdose, said Assistant Secretary for Health Rachel Levine, MD, on the call.
“We know multiple stressors during the pandemic – isolation, sickness, grief, job loss, food instability, and loss of routines – have devastated many Americans and presented the unprecedented behavioral health challenges across the nation,” said Dr. Levine.
The HHS also announced that it is re-establishing a Behavioral Health Coordinating Council (BHCC). Dr. Levine and Mr. Coderre will serve as cochairs of the Council, which will coordinate action-oriented approaches to addressing the HHS’s behavioral health efforts.
However, in 2014, the U.S. Government Accountability Office criticized the BHCC for only focusing on the HHS, and noted the lack of coordination across the federal government’s various efforts to address mental health.
‘A huge step forward’
The American Psychiatric Association welcomed the new money and the return of the council.
“In the wake of the pandemic an unprecedented, and as of yet untold, number of Americans are faced with mental health and substance use disorders, particularly in communities impacted by structural racism,” said APA President Vivian Pender, MD, in a statement. “With the creation of this Council and this investment in mental health, the administration is taking a huge step forward.”
APA CEO and Medical Director Saul Levin, MD, MPA, added: “This Council has great potential to ease the challenges we face as we begin to recover from the pandemic’s impact on our society, and [the] APA looks forward to assisting in their efforts.”
HHS Secretary Xavier Becerra noted in a statement that the COVID-19 pandemic “has made clear the need to invest resources in our nation’s mental health and address the inequities that still exist around behavioral health care.” He added, “This national problem calls for department-wide coordination to address the issue.”
Dr. Levine said the Council “will assure the right prioritization and guidelines are in place to provide pathways to prevention, intervention, treatment, and recovery services.”
A version of this article first appeared on Medscape.com.
Acts of kindness, empathy bolster mental health
Sigmund Freud said, “Out of your vulnerabilities will come greatest strength.” What exactly did Dr. Freud mean by this?
Many aspects of mental health treatment include cognitive restructuring, behavioral changes, emotion processing, and setting boundaries. These are all critical aspects of treatment, but what about kindness and compassion?
We often forget that kindness requires us to be vulnerable and take a risk at times. Being kind to others is not always easy, and it is not always an automatic reaction. Vulnerability often involves risk, but the outcomes often outweigh fear.
Dr. Freud was highlighting that being kind, open, and honest will often result in strong character and resilience. In turn, it will help others. Psychology and psychiatry have proved time and time again that empathy, compassion, and kindness have numerous benefits for mental and physical health for both the giver and the receiver.
From a biological perspective, we know that acts of kindness signal the brain to release serotonin and dopamine, known as “feel good transmitters,” and endorphins, which in turn lessen pain, depression, and anxiety. According to Waguih W. Ishak, MD, a psychiatrist affiliated with Cedars-Sinai in Los Angeles,1 in addition to boosting oxytocin and dopamine, being kind can increase serotonin, a neurotransmitter that helps regulate mood. Kindness and compassion have been shown to release oxytocin, known as the “love hormone,” which increases self-esteem, trust, connection, and optimism. Oxytocin also reduces blood pressure and has been dubbed the “cardioprotective” hormone. According to Kelli Harding, MD, MPH, a psychiatrist affiliated with Columbia University in New York,2 kindness can extend the lifespan. Research from Emory University in Atlanta has shown that, when an individual is kind to another, the brain’s reward centers light up – resulting in a “helper’s high.” Thus, kindness is self-reinforcing.3
Kindness leads to a greater sense of connection to others and a lessening in feelings of isolation. Small acts of kindness build up compassion in oneself. Research indicates that kindness doesn’t just positively affect the giver and receiver but can also benefit onlookers. An article in Psychology Today,4 suggests that those who witness acts of kindness are also more likely to “pay it forward,” resulting in a domino effect. Along these same lines, altruistic people, specifically those who engage in charitable donations, expressed higher levels of overall happiness according to a 2010 Harvard Business School survey.5
“You can’t pour from an empty cup” is a trendy quote making its way around social media. Before we can be kind and compassionate to others, we must first be kind and compassionate to ourselves. In today’s world, productivity and pressure-filled environments consume us daily. We often find ourselves skipping meals, forgetting to connect with loved ones, missing breaks, and even neglecting our sleep. It is virtually impossible to care for others when we are depleted ourselves. Sometimes not prioritizing ourselves can result in collateral damage. We may become short-tempered, irritable, moody, and overwhelmed. At this point kindness, compassion, and empathy toward others are likely to be absent. Once we replenish ourselves, by taking time off, indulging in a nice meal, exercising, we are more likely to respond as opposed to react, ask others about themselves, and engage in overall positive interactions throughout our day. Kindness is best fostered by being kind to ourselves to sustain our own well-being and by being kind to others in order to maintain the cycle. For clinicians who have been pushed to respond to various aspects of the COVID-19 pandemic, self-care has never been more important.
COVID-19 has been difficult for everyone, particularly the elderly and vulnerable populations. However, kindness has proved to be an overwhelming response as many businesses and individuals have taken to volunteering time and resources for those in need. Even big corporations have chipped in. For example, Lyft and Uber – in a partnership with the White House – are now offering free rides to vaccine sites, and several local businesses have donated personal protective equipment to hospitals and assisted living facilities.
Kindness and empathy are ever present in the field of mental health, medicine, and substance use treatment. The very act of caring for another involves kindness. In medicine, empathy has been defined as “an emotional experience between an observer and a subject in which the observer, based on visual and auditory cues, identifies and transiently experiences the subject’s emotional state.”6
As mental health professionals, we receive empathy training early on in our schooling – increasingly so over the last decade. Research has indicated that trusting relationships between clinicians and patients result in optimal care. Evidence-based communication styles are being widely implemented. This entails using nonjudgmental language, open-ended questions, and active listening skills, for example. In addition, the mental health professionals have our conscious and unconscious judgments. If empathy training is provided, we can learn to acknowledge our biases and mitigate them. Lastly, empathy training has been proven to assist with destigmatization, increase in treatment seeking, and overall better outcomes.
Substance use treatment, which often focuses on cognition and behavior changing, boundaries, and family dynamics, also requires support and kindness. Although it is not an empirically based “treatment,” Alcoholics Anonymous (AA) has used kindness for decades.
Step 12 of AA’s 12-step program, which was developed by two people with alcohol use disorder in 1935 in Akron, Ohio, is as follows: “Having had a spiritual awakening as the result of these steps, we tried to carry this message to alcoholics, and to practice these principles in all our affairs.”
Once AA members are on solid ground with their sobriety, they are urged to help others in their recovery. This process provides many benefits. When individuals are concerned about someone else, they are less focused on themselves. This helps the individuals in recovery to decrease their rumination and “get out of themselves.” It also allows for the AA member to be kind and helpful to an individual who is suffering, thereby expressing kindness, compassion, and empathy. This act of “paying it forward” produces a domino effect that has withstood the test of time as evidenced by the ever-growing fellowship of Alcoholics Anonymous.
Several small acts of kindness can help us as clinicians and our patients:
1. Practice self-care.
2. Take a half day off from your practice.
3. Give staff a half day off.
4. Call a family member or friend and ask them how they are doing. Then engage in active listening and refrain from giving advice.
5. Donate to a homeless shelter or volunteer your time at a charity.
6. Give a stranger a compliment.
7. Surprise someone with a small gift.
8. Send a loved one a letter instead of a text.
9. Pick up litter.
10. Acknowledge family and friends who gave you extra support during the pandemic.
11. Take baked goods to your office.
12. Help a neighbor with groceries.
13. Leave a generous tip.
14. Play soft music in your office.
In conclusion, kindness, empathy, and compassion are vital concepts that are not just fluffy theories. They have vast mental, physical, and social benefits for us and our patients.
References
1. Cedars-Sinai staff. The Science of Kindness. 2019 Feb 13. Cedars-Sinai blog.
2. Harding K. The Rabbit Effect: Live Longer, Happier, and Healthier with the Groundbreaking Science of Kindness. Atria Books, 2019.
3. Ritvo E. BeKindr. Momosa Publishing, 2017.
4. Svoboda E. “Pay it Forward.” Psychology Today. Last reviewed 2016 Jun 9.
5. Aknin LB et al. “Prosocial spending and well-being: Cross-Cultural Evidence for a Psychological Universal.” Harvard Business School. Working Paper 11-038. 2010.
6. Hirsch EM. AMA J Ethics. Virtual Mentor. 2007;9(6):423-7.
Dr. Haji is a licensed clinical psychologist specializing in psychodiagnostic assessment, forensic assessment, dual diagnosis, serious and persistent mental illness, depression, anxiety, personality disorders, and substance abuse treatment. She practices in Miami and has no conflicts of interest.
Sigmund Freud said, “Out of your vulnerabilities will come greatest strength.” What exactly did Dr. Freud mean by this?
Many aspects of mental health treatment include cognitive restructuring, behavioral changes, emotion processing, and setting boundaries. These are all critical aspects of treatment, but what about kindness and compassion?
We often forget that kindness requires us to be vulnerable and take a risk at times. Being kind to others is not always easy, and it is not always an automatic reaction. Vulnerability often involves risk, but the outcomes often outweigh fear.
Dr. Freud was highlighting that being kind, open, and honest will often result in strong character and resilience. In turn, it will help others. Psychology and psychiatry have proved time and time again that empathy, compassion, and kindness have numerous benefits for mental and physical health for both the giver and the receiver.
From a biological perspective, we know that acts of kindness signal the brain to release serotonin and dopamine, known as “feel good transmitters,” and endorphins, which in turn lessen pain, depression, and anxiety. According to Waguih W. Ishak, MD, a psychiatrist affiliated with Cedars-Sinai in Los Angeles,1 in addition to boosting oxytocin and dopamine, being kind can increase serotonin, a neurotransmitter that helps regulate mood. Kindness and compassion have been shown to release oxytocin, known as the “love hormone,” which increases self-esteem, trust, connection, and optimism. Oxytocin also reduces blood pressure and has been dubbed the “cardioprotective” hormone. According to Kelli Harding, MD, MPH, a psychiatrist affiliated with Columbia University in New York,2 kindness can extend the lifespan. Research from Emory University in Atlanta has shown that, when an individual is kind to another, the brain’s reward centers light up – resulting in a “helper’s high.” Thus, kindness is self-reinforcing.3
Kindness leads to a greater sense of connection to others and a lessening in feelings of isolation. Small acts of kindness build up compassion in oneself. Research indicates that kindness doesn’t just positively affect the giver and receiver but can also benefit onlookers. An article in Psychology Today,4 suggests that those who witness acts of kindness are also more likely to “pay it forward,” resulting in a domino effect. Along these same lines, altruistic people, specifically those who engage in charitable donations, expressed higher levels of overall happiness according to a 2010 Harvard Business School survey.5
“You can’t pour from an empty cup” is a trendy quote making its way around social media. Before we can be kind and compassionate to others, we must first be kind and compassionate to ourselves. In today’s world, productivity and pressure-filled environments consume us daily. We often find ourselves skipping meals, forgetting to connect with loved ones, missing breaks, and even neglecting our sleep. It is virtually impossible to care for others when we are depleted ourselves. Sometimes not prioritizing ourselves can result in collateral damage. We may become short-tempered, irritable, moody, and overwhelmed. At this point kindness, compassion, and empathy toward others are likely to be absent. Once we replenish ourselves, by taking time off, indulging in a nice meal, exercising, we are more likely to respond as opposed to react, ask others about themselves, and engage in overall positive interactions throughout our day. Kindness is best fostered by being kind to ourselves to sustain our own well-being and by being kind to others in order to maintain the cycle. For clinicians who have been pushed to respond to various aspects of the COVID-19 pandemic, self-care has never been more important.
COVID-19 has been difficult for everyone, particularly the elderly and vulnerable populations. However, kindness has proved to be an overwhelming response as many businesses and individuals have taken to volunteering time and resources for those in need. Even big corporations have chipped in. For example, Lyft and Uber – in a partnership with the White House – are now offering free rides to vaccine sites, and several local businesses have donated personal protective equipment to hospitals and assisted living facilities.
Kindness and empathy are ever present in the field of mental health, medicine, and substance use treatment. The very act of caring for another involves kindness. In medicine, empathy has been defined as “an emotional experience between an observer and a subject in which the observer, based on visual and auditory cues, identifies and transiently experiences the subject’s emotional state.”6
As mental health professionals, we receive empathy training early on in our schooling – increasingly so over the last decade. Research has indicated that trusting relationships between clinicians and patients result in optimal care. Evidence-based communication styles are being widely implemented. This entails using nonjudgmental language, open-ended questions, and active listening skills, for example. In addition, the mental health professionals have our conscious and unconscious judgments. If empathy training is provided, we can learn to acknowledge our biases and mitigate them. Lastly, empathy training has been proven to assist with destigmatization, increase in treatment seeking, and overall better outcomes.
Substance use treatment, which often focuses on cognition and behavior changing, boundaries, and family dynamics, also requires support and kindness. Although it is not an empirically based “treatment,” Alcoholics Anonymous (AA) has used kindness for decades.
Step 12 of AA’s 12-step program, which was developed by two people with alcohol use disorder in 1935 in Akron, Ohio, is as follows: “Having had a spiritual awakening as the result of these steps, we tried to carry this message to alcoholics, and to practice these principles in all our affairs.”
Once AA members are on solid ground with their sobriety, they are urged to help others in their recovery. This process provides many benefits. When individuals are concerned about someone else, they are less focused on themselves. This helps the individuals in recovery to decrease their rumination and “get out of themselves.” It also allows for the AA member to be kind and helpful to an individual who is suffering, thereby expressing kindness, compassion, and empathy. This act of “paying it forward” produces a domino effect that has withstood the test of time as evidenced by the ever-growing fellowship of Alcoholics Anonymous.
Several small acts of kindness can help us as clinicians and our patients:
1. Practice self-care.
2. Take a half day off from your practice.
3. Give staff a half day off.
4. Call a family member or friend and ask them how they are doing. Then engage in active listening and refrain from giving advice.
5. Donate to a homeless shelter or volunteer your time at a charity.
6. Give a stranger a compliment.
7. Surprise someone with a small gift.
8. Send a loved one a letter instead of a text.
9. Pick up litter.
10. Acknowledge family and friends who gave you extra support during the pandemic.
11. Take baked goods to your office.
12. Help a neighbor with groceries.
13. Leave a generous tip.
14. Play soft music in your office.
In conclusion, kindness, empathy, and compassion are vital concepts that are not just fluffy theories. They have vast mental, physical, and social benefits for us and our patients.
References
1. Cedars-Sinai staff. The Science of Kindness. 2019 Feb 13. Cedars-Sinai blog.
2. Harding K. The Rabbit Effect: Live Longer, Happier, and Healthier with the Groundbreaking Science of Kindness. Atria Books, 2019.
3. Ritvo E. BeKindr. Momosa Publishing, 2017.
4. Svoboda E. “Pay it Forward.” Psychology Today. Last reviewed 2016 Jun 9.
5. Aknin LB et al. “Prosocial spending and well-being: Cross-Cultural Evidence for a Psychological Universal.” Harvard Business School. Working Paper 11-038. 2010.
6. Hirsch EM. AMA J Ethics. Virtual Mentor. 2007;9(6):423-7.
Dr. Haji is a licensed clinical psychologist specializing in psychodiagnostic assessment, forensic assessment, dual diagnosis, serious and persistent mental illness, depression, anxiety, personality disorders, and substance abuse treatment. She practices in Miami and has no conflicts of interest.
Sigmund Freud said, “Out of your vulnerabilities will come greatest strength.” What exactly did Dr. Freud mean by this?
Many aspects of mental health treatment include cognitive restructuring, behavioral changes, emotion processing, and setting boundaries. These are all critical aspects of treatment, but what about kindness and compassion?
We often forget that kindness requires us to be vulnerable and take a risk at times. Being kind to others is not always easy, and it is not always an automatic reaction. Vulnerability often involves risk, but the outcomes often outweigh fear.
Dr. Freud was highlighting that being kind, open, and honest will often result in strong character and resilience. In turn, it will help others. Psychology and psychiatry have proved time and time again that empathy, compassion, and kindness have numerous benefits for mental and physical health for both the giver and the receiver.
From a biological perspective, we know that acts of kindness signal the brain to release serotonin and dopamine, known as “feel good transmitters,” and endorphins, which in turn lessen pain, depression, and anxiety. According to Waguih W. Ishak, MD, a psychiatrist affiliated with Cedars-Sinai in Los Angeles,1 in addition to boosting oxytocin and dopamine, being kind can increase serotonin, a neurotransmitter that helps regulate mood. Kindness and compassion have been shown to release oxytocin, known as the “love hormone,” which increases self-esteem, trust, connection, and optimism. Oxytocin also reduces blood pressure and has been dubbed the “cardioprotective” hormone. According to Kelli Harding, MD, MPH, a psychiatrist affiliated with Columbia University in New York,2 kindness can extend the lifespan. Research from Emory University in Atlanta has shown that, when an individual is kind to another, the brain’s reward centers light up – resulting in a “helper’s high.” Thus, kindness is self-reinforcing.3
Kindness leads to a greater sense of connection to others and a lessening in feelings of isolation. Small acts of kindness build up compassion in oneself. Research indicates that kindness doesn’t just positively affect the giver and receiver but can also benefit onlookers. An article in Psychology Today,4 suggests that those who witness acts of kindness are also more likely to “pay it forward,” resulting in a domino effect. Along these same lines, altruistic people, specifically those who engage in charitable donations, expressed higher levels of overall happiness according to a 2010 Harvard Business School survey.5
“You can’t pour from an empty cup” is a trendy quote making its way around social media. Before we can be kind and compassionate to others, we must first be kind and compassionate to ourselves. In today’s world, productivity and pressure-filled environments consume us daily. We often find ourselves skipping meals, forgetting to connect with loved ones, missing breaks, and even neglecting our sleep. It is virtually impossible to care for others when we are depleted ourselves. Sometimes not prioritizing ourselves can result in collateral damage. We may become short-tempered, irritable, moody, and overwhelmed. At this point kindness, compassion, and empathy toward others are likely to be absent. Once we replenish ourselves, by taking time off, indulging in a nice meal, exercising, we are more likely to respond as opposed to react, ask others about themselves, and engage in overall positive interactions throughout our day. Kindness is best fostered by being kind to ourselves to sustain our own well-being and by being kind to others in order to maintain the cycle. For clinicians who have been pushed to respond to various aspects of the COVID-19 pandemic, self-care has never been more important.
COVID-19 has been difficult for everyone, particularly the elderly and vulnerable populations. However, kindness has proved to be an overwhelming response as many businesses and individuals have taken to volunteering time and resources for those in need. Even big corporations have chipped in. For example, Lyft and Uber – in a partnership with the White House – are now offering free rides to vaccine sites, and several local businesses have donated personal protective equipment to hospitals and assisted living facilities.
Kindness and empathy are ever present in the field of mental health, medicine, and substance use treatment. The very act of caring for another involves kindness. In medicine, empathy has been defined as “an emotional experience between an observer and a subject in which the observer, based on visual and auditory cues, identifies and transiently experiences the subject’s emotional state.”6
As mental health professionals, we receive empathy training early on in our schooling – increasingly so over the last decade. Research has indicated that trusting relationships between clinicians and patients result in optimal care. Evidence-based communication styles are being widely implemented. This entails using nonjudgmental language, open-ended questions, and active listening skills, for example. In addition, the mental health professionals have our conscious and unconscious judgments. If empathy training is provided, we can learn to acknowledge our biases and mitigate them. Lastly, empathy training has been proven to assist with destigmatization, increase in treatment seeking, and overall better outcomes.
Substance use treatment, which often focuses on cognition and behavior changing, boundaries, and family dynamics, also requires support and kindness. Although it is not an empirically based “treatment,” Alcoholics Anonymous (AA) has used kindness for decades.
Step 12 of AA’s 12-step program, which was developed by two people with alcohol use disorder in 1935 in Akron, Ohio, is as follows: “Having had a spiritual awakening as the result of these steps, we tried to carry this message to alcoholics, and to practice these principles in all our affairs.”
Once AA members are on solid ground with their sobriety, they are urged to help others in their recovery. This process provides many benefits. When individuals are concerned about someone else, they are less focused on themselves. This helps the individuals in recovery to decrease their rumination and “get out of themselves.” It also allows for the AA member to be kind and helpful to an individual who is suffering, thereby expressing kindness, compassion, and empathy. This act of “paying it forward” produces a domino effect that has withstood the test of time as evidenced by the ever-growing fellowship of Alcoholics Anonymous.
Several small acts of kindness can help us as clinicians and our patients:
1. Practice self-care.
2. Take a half day off from your practice.
3. Give staff a half day off.
4. Call a family member or friend and ask them how they are doing. Then engage in active listening and refrain from giving advice.
5. Donate to a homeless shelter or volunteer your time at a charity.
6. Give a stranger a compliment.
7. Surprise someone with a small gift.
8. Send a loved one a letter instead of a text.
9. Pick up litter.
10. Acknowledge family and friends who gave you extra support during the pandemic.
11. Take baked goods to your office.
12. Help a neighbor with groceries.
13. Leave a generous tip.
14. Play soft music in your office.
In conclusion, kindness, empathy, and compassion are vital concepts that are not just fluffy theories. They have vast mental, physical, and social benefits for us and our patients.
References
1. Cedars-Sinai staff. The Science of Kindness. 2019 Feb 13. Cedars-Sinai blog.
2. Harding K. The Rabbit Effect: Live Longer, Happier, and Healthier with the Groundbreaking Science of Kindness. Atria Books, 2019.
3. Ritvo E. BeKindr. Momosa Publishing, 2017.
4. Svoboda E. “Pay it Forward.” Psychology Today. Last reviewed 2016 Jun 9.
5. Aknin LB et al. “Prosocial spending and well-being: Cross-Cultural Evidence for a Psychological Universal.” Harvard Business School. Working Paper 11-038. 2010.
6. Hirsch EM. AMA J Ethics. Virtual Mentor. 2007;9(6):423-7.
Dr. Haji is a licensed clinical psychologist specializing in psychodiagnostic assessment, forensic assessment, dual diagnosis, serious and persistent mental illness, depression, anxiety, personality disorders, and substance abuse treatment. She practices in Miami and has no conflicts of interest.
Depot buprenorphine a shot in the arm for opioid addiction?
Adults in treatment for opioid dependence report high satisfaction with buprenorphine injections, in new findings that researchers say could help improve treatment and management of patients with opioid dependence.
In the DEBUT trial, patients who received weekly or monthly depot buprenorphine had significantly higher overall treatment satisfaction, reduced treatment burden, and higher quality-of-life ratings than peers who received daily treatment with sublingual buprenorphine.
“The study’s focus on patient-reported outcomes (PROs) can help to better inform patients and clinicians when selecting treatment options than the clinical traditional outcomes of opioid dependence treatment studies,” lead investigator Fredrik Tiberg, PhD, president and CEO of Camurus, a pharmaceutical company in Lund, Sweden, said in an interview.
“The positive patient experiences with the depot buprenorphine injection reported in the DEBUT study indicate that long-acting treatments could contribute to advancing the quality of care and access to treatment for patients with opioid dependence/use disorder,” said Dr. Tiberg.
The study was published online May 10 in JAMA Network Open.
Novel study
The study was an open-label, parallel-group randomized controlled trial that included 119 patients from six outpatient clinics in Australia; 60 received weekly or monthly depot buprenorphine and 59 received sublingual buprenorphine for 24 weeks.
The primary outcome was global treatment satisfaction, as measured by the 14-question Treatment Satisfaction Questionnaire for Medication (TSQM) at the end of the study at week 24.
The study met its primary endpoint with a significantly higher TSQM global satisfaction score among adults who received depot injections, compared with those who received sublingual buprenorphine (mean score 82.5 vs. 74.3; difference, 8.2; 95% confidence interval, 1.7-14.6; P = .01).
Improvement was also observed for several secondary outcomes, including decreased treatment burden and higher quality of life.
The safety profile was consistent with the known safety profile of buprenorphine, aside from transient, mild-to-moderate injection site reactions.
“To our knowledge, this is the first randomized study that has used a range of PROs to compare outcomes between a long-acting injection and daily dosing of buprenorphine in the treatment of opioid dependence,” the investigators note.
“The study highlights the application of PROs as alternate endpoints to traditional markers of substance use in addiction treatment outcome studies,” they conclude.
Giving patients a voice
In an invited commentary, from most of the work in medication development, including for opioid use disorder.
The current study addresses this very issue in a “well designed and executed” fashion and the results “consistently demonstrated” the superiority of injectable buprenorphine across many outcomes.
The study highlights the importance of considering PRO measures in clinical trials, Dr. Volkow and Dr. Compton say.
“Even if efficacy is no different for various formulations, PROs may provide an important reason to select a new formulation. Patient preferences and apparently improved function may prove to be useful secondary outcomes in medication trials, and the measures used in this new study deserve consideration,” they write.
In addition, the greater treatment satisfaction by patients receiving extended-release buprenorphine suggests that these formulations “might help to improve long-term retention and, as such, be a valuable tool to help combat the current opioid epidemic and reduce its associated mortality,” they conclude.
This study was supported by Camurus AB. Dr. Tiberg is president and CEO of Camurus AB. A complete list of author disclosures is with the original article. Dr. Volkow and Dr. Compton have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Adults in treatment for opioid dependence report high satisfaction with buprenorphine injections, in new findings that researchers say could help improve treatment and management of patients with opioid dependence.
In the DEBUT trial, patients who received weekly or monthly depot buprenorphine had significantly higher overall treatment satisfaction, reduced treatment burden, and higher quality-of-life ratings than peers who received daily treatment with sublingual buprenorphine.
“The study’s focus on patient-reported outcomes (PROs) can help to better inform patients and clinicians when selecting treatment options than the clinical traditional outcomes of opioid dependence treatment studies,” lead investigator Fredrik Tiberg, PhD, president and CEO of Camurus, a pharmaceutical company in Lund, Sweden, said in an interview.
“The positive patient experiences with the depot buprenorphine injection reported in the DEBUT study indicate that long-acting treatments could contribute to advancing the quality of care and access to treatment for patients with opioid dependence/use disorder,” said Dr. Tiberg.
The study was published online May 10 in JAMA Network Open.
Novel study
The study was an open-label, parallel-group randomized controlled trial that included 119 patients from six outpatient clinics in Australia; 60 received weekly or monthly depot buprenorphine and 59 received sublingual buprenorphine for 24 weeks.
The primary outcome was global treatment satisfaction, as measured by the 14-question Treatment Satisfaction Questionnaire for Medication (TSQM) at the end of the study at week 24.
The study met its primary endpoint with a significantly higher TSQM global satisfaction score among adults who received depot injections, compared with those who received sublingual buprenorphine (mean score 82.5 vs. 74.3; difference, 8.2; 95% confidence interval, 1.7-14.6; P = .01).
Improvement was also observed for several secondary outcomes, including decreased treatment burden and higher quality of life.
The safety profile was consistent with the known safety profile of buprenorphine, aside from transient, mild-to-moderate injection site reactions.
“To our knowledge, this is the first randomized study that has used a range of PROs to compare outcomes between a long-acting injection and daily dosing of buprenorphine in the treatment of opioid dependence,” the investigators note.
“The study highlights the application of PROs as alternate endpoints to traditional markers of substance use in addiction treatment outcome studies,” they conclude.
Giving patients a voice
In an invited commentary, from most of the work in medication development, including for opioid use disorder.
The current study addresses this very issue in a “well designed and executed” fashion and the results “consistently demonstrated” the superiority of injectable buprenorphine across many outcomes.
The study highlights the importance of considering PRO measures in clinical trials, Dr. Volkow and Dr. Compton say.
“Even if efficacy is no different for various formulations, PROs may provide an important reason to select a new formulation. Patient preferences and apparently improved function may prove to be useful secondary outcomes in medication trials, and the measures used in this new study deserve consideration,” they write.
In addition, the greater treatment satisfaction by patients receiving extended-release buprenorphine suggests that these formulations “might help to improve long-term retention and, as such, be a valuable tool to help combat the current opioid epidemic and reduce its associated mortality,” they conclude.
This study was supported by Camurus AB. Dr. Tiberg is president and CEO of Camurus AB. A complete list of author disclosures is with the original article. Dr. Volkow and Dr. Compton have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Adults in treatment for opioid dependence report high satisfaction with buprenorphine injections, in new findings that researchers say could help improve treatment and management of patients with opioid dependence.
In the DEBUT trial, patients who received weekly or monthly depot buprenorphine had significantly higher overall treatment satisfaction, reduced treatment burden, and higher quality-of-life ratings than peers who received daily treatment with sublingual buprenorphine.
“The study’s focus on patient-reported outcomes (PROs) can help to better inform patients and clinicians when selecting treatment options than the clinical traditional outcomes of opioid dependence treatment studies,” lead investigator Fredrik Tiberg, PhD, president and CEO of Camurus, a pharmaceutical company in Lund, Sweden, said in an interview.
“The positive patient experiences with the depot buprenorphine injection reported in the DEBUT study indicate that long-acting treatments could contribute to advancing the quality of care and access to treatment for patients with opioid dependence/use disorder,” said Dr. Tiberg.
The study was published online May 10 in JAMA Network Open.
Novel study
The study was an open-label, parallel-group randomized controlled trial that included 119 patients from six outpatient clinics in Australia; 60 received weekly or monthly depot buprenorphine and 59 received sublingual buprenorphine for 24 weeks.
The primary outcome was global treatment satisfaction, as measured by the 14-question Treatment Satisfaction Questionnaire for Medication (TSQM) at the end of the study at week 24.
The study met its primary endpoint with a significantly higher TSQM global satisfaction score among adults who received depot injections, compared with those who received sublingual buprenorphine (mean score 82.5 vs. 74.3; difference, 8.2; 95% confidence interval, 1.7-14.6; P = .01).
Improvement was also observed for several secondary outcomes, including decreased treatment burden and higher quality of life.
The safety profile was consistent with the known safety profile of buprenorphine, aside from transient, mild-to-moderate injection site reactions.
“To our knowledge, this is the first randomized study that has used a range of PROs to compare outcomes between a long-acting injection and daily dosing of buprenorphine in the treatment of opioid dependence,” the investigators note.
“The study highlights the application of PROs as alternate endpoints to traditional markers of substance use in addiction treatment outcome studies,” they conclude.
Giving patients a voice
In an invited commentary, from most of the work in medication development, including for opioid use disorder.
The current study addresses this very issue in a “well designed and executed” fashion and the results “consistently demonstrated” the superiority of injectable buprenorphine across many outcomes.
The study highlights the importance of considering PRO measures in clinical trials, Dr. Volkow and Dr. Compton say.
“Even if efficacy is no different for various formulations, PROs may provide an important reason to select a new formulation. Patient preferences and apparently improved function may prove to be useful secondary outcomes in medication trials, and the measures used in this new study deserve consideration,” they write.
In addition, the greater treatment satisfaction by patients receiving extended-release buprenorphine suggests that these formulations “might help to improve long-term retention and, as such, be a valuable tool to help combat the current opioid epidemic and reduce its associated mortality,” they conclude.
This study was supported by Camurus AB. Dr. Tiberg is president and CEO of Camurus AB. A complete list of author disclosures is with the original article. Dr. Volkow and Dr. Compton have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Heavy cannabis use in pregnancy correlates with risks to infant
Cannabis use that interferes with a woman’s ability to function during pregnancy is a risk factor for severe health problems in the child, new research indicates.
Pregnant women with cannabis use disorder are more likely to have children with low birth weights and children who die within 1 year of birth, compared with matched controls, according to a study published online in Addiction.
The death rate among infants exposed to prenatal cannabis use disorder was 0.98%, compared with 0.75% among infants whose mothers did not have this diagnosis.
Cannabis use disorder during pregnancy “has increased dramatically in the past two decades,” but few studies have examined the health impacts on offspring, study author Yuyan Shi, PhD, said in an interview. “It is particularly concerning in states with cannabis legalization where cannabis is increasingly available.”
Dr. Shi, a researcher at the Herbert Wertheim School of Public Health and Human Longevity Science at the University of California, San Diego, and colleagues analyzed data from more than 4.8 million mothers who delivered a live singleton birth in California between 2001 and 2012 and their infants. They focused on 20,237 mothers who had a diagnosis of cannabis use disorder at delivery. The disorder is defined by continued use of the drug despite impairments in physical, psychological, and social functioning.
The researchers matched mothers with cannabis use disorder 1:2 to mothers who did not have this diagnosis. They aimed to balance factors such as maternal age, educational attainment, health insurance, physical and mental health conditions, prenatal care, and alcohol and opioid use disorder.
An increasingly common diagnosis
Over the study period, the rate of cannabis use disorder increased from 2.8 cases per 1,000 deliveries in 2001 to 6.9 cases per 1,000 deliveries in 2012.
Cannabis use disorder was associated with increased odds of preterm birth (odds ratio, 1.06), small for gestational age (OR, 1.13), low birth weight (OR, 1.13), and death within 1 year of birth (OR, 1.35), according to the researchers’ estimates. Cannabis use disorder was associated with lower odds of hospitalization within 1 year of birth, however (OR, 0.91).
“The most notable observation is that exposed infants were 35% more likely to die within 1 year of birth than unexposed infants,” Dr. Shi and colleagues wrote. More research is needed to understand the causes of death at different stages of infancy, they said.
The results “imply that cannabis use disorder screening as well as appropriate education, counseling, or referral to substance abuse treatment services should be encouraged among pregnant women,” Dr. Shi said.
The study does not establish that cannabis use disorder causes adverse effects, and it is not clear how the results might apply to mothers who use cannabis but do not meet diagnostic criteria for the disorder, the authors noted.
“Presumably the health consequences of mothers who use cannabis but do not meet the criteria ... are less severe than mothers with cannabis use disorder,” Dr. Shi said. “Unfortunately, no research has been conducted to test this hypothesis.”
Enough data to recommend abstaining
Many clinicians may not feel equipped to make a diagnosis of cannabis use disorder, said Jamie Lo, MD, assistant professor of obstetrics and gynecology at Oregon Health and Science University in Portland.
Although many clinicians ask patients about substance use in general, specifically screening for cannabis use is not necessarily routine practice. “I think people are starting to adopt that, but it probably will take a little bit of time,” Dr. Lo said.
Dr. Lo, who was not involved in the study, researches the effects of marijuana during pregnancy.
Confounding factors such as frequent co-use of tobacco have so far made it “difficult to suss out” whether observed effects are directly from cannabis use, other substances or exposures, or a combination, said Dr. Lo. The possibility that stigma may lead to inaccurate self-reporting poses another challenge. And the range of cannabis delivery devices further complicates matters.
“It is hard to compare smoking a bowl versus a joint versus using the oils or CBD or edibles,” Dr. Lo said. The data regarding cigarettes and alcohol are cleaner and more precise, in comparison.
Still, federal agencies and professional societies agree that “what we do know is enough to recommend that pregnant women abstain from using cannabis during pregnancy,” Dr. Lo said.
The National Institute on Drug Abuse, which funded the study, said the results add to the evidence that prenatal exposure to cannabis may be associated with poor birth outcomes and infant health.
“While we cannot establish that cannabis use caused negative outcomes in this study, these data reinforce the case for caution around using cannabis during pregnancy,” Nora D. Volkow, MD, the director of the agency, said in a news release.
“Careful analysis of data like these is one way we can responsibly study how cannabis use affects the developing child, all while a natural experiment is playing out across our country in places where cannabis is becoming widely available to pregnant consumers.”
The study authors and Dr. Lo had no disclosures.
Cannabis use that interferes with a woman’s ability to function during pregnancy is a risk factor for severe health problems in the child, new research indicates.
Pregnant women with cannabis use disorder are more likely to have children with low birth weights and children who die within 1 year of birth, compared with matched controls, according to a study published online in Addiction.
The death rate among infants exposed to prenatal cannabis use disorder was 0.98%, compared with 0.75% among infants whose mothers did not have this diagnosis.
Cannabis use disorder during pregnancy “has increased dramatically in the past two decades,” but few studies have examined the health impacts on offspring, study author Yuyan Shi, PhD, said in an interview. “It is particularly concerning in states with cannabis legalization where cannabis is increasingly available.”
Dr. Shi, a researcher at the Herbert Wertheim School of Public Health and Human Longevity Science at the University of California, San Diego, and colleagues analyzed data from more than 4.8 million mothers who delivered a live singleton birth in California between 2001 and 2012 and their infants. They focused on 20,237 mothers who had a diagnosis of cannabis use disorder at delivery. The disorder is defined by continued use of the drug despite impairments in physical, psychological, and social functioning.
The researchers matched mothers with cannabis use disorder 1:2 to mothers who did not have this diagnosis. They aimed to balance factors such as maternal age, educational attainment, health insurance, physical and mental health conditions, prenatal care, and alcohol and opioid use disorder.
An increasingly common diagnosis
Over the study period, the rate of cannabis use disorder increased from 2.8 cases per 1,000 deliveries in 2001 to 6.9 cases per 1,000 deliveries in 2012.
Cannabis use disorder was associated with increased odds of preterm birth (odds ratio, 1.06), small for gestational age (OR, 1.13), low birth weight (OR, 1.13), and death within 1 year of birth (OR, 1.35), according to the researchers’ estimates. Cannabis use disorder was associated with lower odds of hospitalization within 1 year of birth, however (OR, 0.91).
“The most notable observation is that exposed infants were 35% more likely to die within 1 year of birth than unexposed infants,” Dr. Shi and colleagues wrote. More research is needed to understand the causes of death at different stages of infancy, they said.
The results “imply that cannabis use disorder screening as well as appropriate education, counseling, or referral to substance abuse treatment services should be encouraged among pregnant women,” Dr. Shi said.
The study does not establish that cannabis use disorder causes adverse effects, and it is not clear how the results might apply to mothers who use cannabis but do not meet diagnostic criteria for the disorder, the authors noted.
“Presumably the health consequences of mothers who use cannabis but do not meet the criteria ... are less severe than mothers with cannabis use disorder,” Dr. Shi said. “Unfortunately, no research has been conducted to test this hypothesis.”
Enough data to recommend abstaining
Many clinicians may not feel equipped to make a diagnosis of cannabis use disorder, said Jamie Lo, MD, assistant professor of obstetrics and gynecology at Oregon Health and Science University in Portland.
Although many clinicians ask patients about substance use in general, specifically screening for cannabis use is not necessarily routine practice. “I think people are starting to adopt that, but it probably will take a little bit of time,” Dr. Lo said.
Dr. Lo, who was not involved in the study, researches the effects of marijuana during pregnancy.
Confounding factors such as frequent co-use of tobacco have so far made it “difficult to suss out” whether observed effects are directly from cannabis use, other substances or exposures, or a combination, said Dr. Lo. The possibility that stigma may lead to inaccurate self-reporting poses another challenge. And the range of cannabis delivery devices further complicates matters.
“It is hard to compare smoking a bowl versus a joint versus using the oils or CBD or edibles,” Dr. Lo said. The data regarding cigarettes and alcohol are cleaner and more precise, in comparison.
Still, federal agencies and professional societies agree that “what we do know is enough to recommend that pregnant women abstain from using cannabis during pregnancy,” Dr. Lo said.
The National Institute on Drug Abuse, which funded the study, said the results add to the evidence that prenatal exposure to cannabis may be associated with poor birth outcomes and infant health.
“While we cannot establish that cannabis use caused negative outcomes in this study, these data reinforce the case for caution around using cannabis during pregnancy,” Nora D. Volkow, MD, the director of the agency, said in a news release.
“Careful analysis of data like these is one way we can responsibly study how cannabis use affects the developing child, all while a natural experiment is playing out across our country in places where cannabis is becoming widely available to pregnant consumers.”
The study authors and Dr. Lo had no disclosures.
Cannabis use that interferes with a woman’s ability to function during pregnancy is a risk factor for severe health problems in the child, new research indicates.
Pregnant women with cannabis use disorder are more likely to have children with low birth weights and children who die within 1 year of birth, compared with matched controls, according to a study published online in Addiction.
The death rate among infants exposed to prenatal cannabis use disorder was 0.98%, compared with 0.75% among infants whose mothers did not have this diagnosis.
Cannabis use disorder during pregnancy “has increased dramatically in the past two decades,” but few studies have examined the health impacts on offspring, study author Yuyan Shi, PhD, said in an interview. “It is particularly concerning in states with cannabis legalization where cannabis is increasingly available.”
Dr. Shi, a researcher at the Herbert Wertheim School of Public Health and Human Longevity Science at the University of California, San Diego, and colleagues analyzed data from more than 4.8 million mothers who delivered a live singleton birth in California between 2001 and 2012 and their infants. They focused on 20,237 mothers who had a diagnosis of cannabis use disorder at delivery. The disorder is defined by continued use of the drug despite impairments in physical, psychological, and social functioning.
The researchers matched mothers with cannabis use disorder 1:2 to mothers who did not have this diagnosis. They aimed to balance factors such as maternal age, educational attainment, health insurance, physical and mental health conditions, prenatal care, and alcohol and opioid use disorder.
An increasingly common diagnosis
Over the study period, the rate of cannabis use disorder increased from 2.8 cases per 1,000 deliveries in 2001 to 6.9 cases per 1,000 deliveries in 2012.
Cannabis use disorder was associated with increased odds of preterm birth (odds ratio, 1.06), small for gestational age (OR, 1.13), low birth weight (OR, 1.13), and death within 1 year of birth (OR, 1.35), according to the researchers’ estimates. Cannabis use disorder was associated with lower odds of hospitalization within 1 year of birth, however (OR, 0.91).
“The most notable observation is that exposed infants were 35% more likely to die within 1 year of birth than unexposed infants,” Dr. Shi and colleagues wrote. More research is needed to understand the causes of death at different stages of infancy, they said.
The results “imply that cannabis use disorder screening as well as appropriate education, counseling, or referral to substance abuse treatment services should be encouraged among pregnant women,” Dr. Shi said.
The study does not establish that cannabis use disorder causes adverse effects, and it is not clear how the results might apply to mothers who use cannabis but do not meet diagnostic criteria for the disorder, the authors noted.
“Presumably the health consequences of mothers who use cannabis but do not meet the criteria ... are less severe than mothers with cannabis use disorder,” Dr. Shi said. “Unfortunately, no research has been conducted to test this hypothesis.”
Enough data to recommend abstaining
Many clinicians may not feel equipped to make a diagnosis of cannabis use disorder, said Jamie Lo, MD, assistant professor of obstetrics and gynecology at Oregon Health and Science University in Portland.
Although many clinicians ask patients about substance use in general, specifically screening for cannabis use is not necessarily routine practice. “I think people are starting to adopt that, but it probably will take a little bit of time,” Dr. Lo said.
Dr. Lo, who was not involved in the study, researches the effects of marijuana during pregnancy.
Confounding factors such as frequent co-use of tobacco have so far made it “difficult to suss out” whether observed effects are directly from cannabis use, other substances or exposures, or a combination, said Dr. Lo. The possibility that stigma may lead to inaccurate self-reporting poses another challenge. And the range of cannabis delivery devices further complicates matters.
“It is hard to compare smoking a bowl versus a joint versus using the oils or CBD or edibles,” Dr. Lo said. The data regarding cigarettes and alcohol are cleaner and more precise, in comparison.
Still, federal agencies and professional societies agree that “what we do know is enough to recommend that pregnant women abstain from using cannabis during pregnancy,” Dr. Lo said.
The National Institute on Drug Abuse, which funded the study, said the results add to the evidence that prenatal exposure to cannabis may be associated with poor birth outcomes and infant health.
“While we cannot establish that cannabis use caused negative outcomes in this study, these data reinforce the case for caution around using cannabis during pregnancy,” Nora D. Volkow, MD, the director of the agency, said in a news release.
“Careful analysis of data like these is one way we can responsibly study how cannabis use affects the developing child, all while a natural experiment is playing out across our country in places where cannabis is becoming widely available to pregnant consumers.”
The study authors and Dr. Lo had no disclosures.
Opioid addiction meds may curb growing problem of kratom dependence
Medications typically used to treat opioid use disorder (OUD) may* also be effective for the growing public health problem of kratom addiction, new research shows.
Results of a comprehensive literature review and an expert survey suggest buprenorphine, naltrexone, and methadone may be effective for patients seeking help for kratom addiction, and if further research confirms these findings, the indication for OUD medications could potentially be expanded to include moderate-to-severe kratom addiction, study investigator Saeed Ahmed, MD, medical director of West Ridge Center at Rutland Regional Medical Center, Rutland, Vermont, said in an interview.
Dr. Ahmed, who practices general psychiatry and addiction psychiatry, presented the findings at the virtual American Psychiatric Association 2021 Annual Meeting.
Emerging public health problem
Kratom can be ingested in pill or capsule form or as an extract. Its leaves can be chewed or dried and powdered to make a tea. It can also be incorporated into topical creams, balms, or tinctures.
Products containing the substance are “readily available and legal for sale in many states and cities in the U.S.,” said Dr. Ahmed, adding that it can be purchased online or at local smoke shops and is increasingly used by individuals to self-treat a variety of conditions including pain, anxiety, and mood conditions and as an opioid substitute.
As reported by this news organization, a 2018 analysis conducted by the U.S. Food and Drug Administration showed kratom is, in fact, an opioid, a finding that garnered significant push-back from the American Kratom Association.
Kratom addiction is an “emerging public health problem,” said Dr. Ahmed, adding that in recent years the number of calls to poison control centers across the country has increased 52-fold – from one per month to two per day. He believes misinformation through social media has helped fuel its use.
Kratom use, the investigators note, can lead to muscle pain, weight loss, insomnia, hallucinations and, in some cases (particularly when combined with synthetic opioids or benzodiazepines), it can lead to respiratory depression, seizures, coma, and death.
In addition,
To investigate, the researchers conducted a systematic literature search for cases pertaining to maintenance treatment for kratom dependence. They also tapped into case reports and scientific posters from reliable online sources and conference proceedings. In addition, they conducted a survey of members from the American Society of Addiction Medicine (ASAM).
The researchers found 14 reports of long-term management of kratom addiction, half of which did not involve an OUD. It’s important to exclude OUDs to avoid possible confounding.
In most cases, buprenorphine was used, but in a few cases naltrexone or methadone were prescribed. All cases had a favorable outcome. Dr. Ahmed noted that buprenorphine maintenance doses appear to be lower than those required to effectively treat OUD.
With a response rate of 11.5% (82 respondents) the ASAM survey results showed 82.6% of respondents (n = 57) had experience managing KUD, including 27.5% (n = 19) who had kratom addiction only. Of these, 89.5% (n = 17-19), used buprenorphine to manage KUD and of these, 6 combined it with talk therapy.
Dr. Ahmed cautioned that the included cases varied significantly in terms of relevant data, including kratom dose and route of administration, toxicology screening used to monitor abstinence, and duration of maintenance follow-up.
Despite these limitations, the review and survey underscore the importance of including moderate to severe kratom dependence as an indication for current OUD medications, the researchers note.
Including kratom addiction as an indication for these medications is important, especially for patients who are heavily addicted, to meet DSM-5 diagnostic criteria for moderate or severe SUD, they add.
In addition, the researchers recommend that clinicians consider referring patients with moderate to severe kratom dependence for counseling or enrollment in 12-step addiction treatment programs.
A separate diagnosis?
Dr. Ahmed said he would like to see kratom dependence included in the DSM-5 as a separate entity because it is a botanical with properties similar to, but different from, traditional opioids.
“This will not only help to better inform clinicians about a diagnostic criteria encompassing problematic use and facilitate screening, but it will also pave the way for treatments to be explored for this diagnosable condition,” he said. Dr. Ahmed pointed to a review published in the Wisconsin Medical Journal earlier this year that explored potential treatments for kratom dependence.
Commenting on the study for an interview, Petros Levounis, MD, professor and chair, department of psychiatry, and associate dean for professional development, Rutgers New Jersey Medical School, Newark, said the authors “have done a great job reviewing the literature and asking experts” about kratom addiction treatment.
“The punchline of their study is that kratom behaves very much like an opioid and is treated like an opioid.”
Dr. Levounis noted that kratom dependence is so new that experts don’t know much about it. However, he added, emerging evidence suggests that kratom “should be considered an opioid more than anything else,” but specified that he does not believe it warrants its own diagnosis.
He noted that individual opioids don’t have their own diagnostic category and that opioid use disorder is an umbrella term that covers all of these drugs.
Dr. Ahmed and Dr. Levounis have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
*Updated 5/18/2021
Medications typically used to treat opioid use disorder (OUD) may* also be effective for the growing public health problem of kratom addiction, new research shows.
Results of a comprehensive literature review and an expert survey suggest buprenorphine, naltrexone, and methadone may be effective for patients seeking help for kratom addiction, and if further research confirms these findings, the indication for OUD medications could potentially be expanded to include moderate-to-severe kratom addiction, study investigator Saeed Ahmed, MD, medical director of West Ridge Center at Rutland Regional Medical Center, Rutland, Vermont, said in an interview.
Dr. Ahmed, who practices general psychiatry and addiction psychiatry, presented the findings at the virtual American Psychiatric Association 2021 Annual Meeting.
Emerging public health problem
Kratom can be ingested in pill or capsule form or as an extract. Its leaves can be chewed or dried and powdered to make a tea. It can also be incorporated into topical creams, balms, or tinctures.
Products containing the substance are “readily available and legal for sale in many states and cities in the U.S.,” said Dr. Ahmed, adding that it can be purchased online or at local smoke shops and is increasingly used by individuals to self-treat a variety of conditions including pain, anxiety, and mood conditions and as an opioid substitute.
As reported by this news organization, a 2018 analysis conducted by the U.S. Food and Drug Administration showed kratom is, in fact, an opioid, a finding that garnered significant push-back from the American Kratom Association.
Kratom addiction is an “emerging public health problem,” said Dr. Ahmed, adding that in recent years the number of calls to poison control centers across the country has increased 52-fold – from one per month to two per day. He believes misinformation through social media has helped fuel its use.
Kratom use, the investigators note, can lead to muscle pain, weight loss, insomnia, hallucinations and, in some cases (particularly when combined with synthetic opioids or benzodiazepines), it can lead to respiratory depression, seizures, coma, and death.
In addition,
To investigate, the researchers conducted a systematic literature search for cases pertaining to maintenance treatment for kratom dependence. They also tapped into case reports and scientific posters from reliable online sources and conference proceedings. In addition, they conducted a survey of members from the American Society of Addiction Medicine (ASAM).
The researchers found 14 reports of long-term management of kratom addiction, half of which did not involve an OUD. It’s important to exclude OUDs to avoid possible confounding.
In most cases, buprenorphine was used, but in a few cases naltrexone or methadone were prescribed. All cases had a favorable outcome. Dr. Ahmed noted that buprenorphine maintenance doses appear to be lower than those required to effectively treat OUD.
With a response rate of 11.5% (82 respondents) the ASAM survey results showed 82.6% of respondents (n = 57) had experience managing KUD, including 27.5% (n = 19) who had kratom addiction only. Of these, 89.5% (n = 17-19), used buprenorphine to manage KUD and of these, 6 combined it with talk therapy.
Dr. Ahmed cautioned that the included cases varied significantly in terms of relevant data, including kratom dose and route of administration, toxicology screening used to monitor abstinence, and duration of maintenance follow-up.
Despite these limitations, the review and survey underscore the importance of including moderate to severe kratom dependence as an indication for current OUD medications, the researchers note.
Including kratom addiction as an indication for these medications is important, especially for patients who are heavily addicted, to meet DSM-5 diagnostic criteria for moderate or severe SUD, they add.
In addition, the researchers recommend that clinicians consider referring patients with moderate to severe kratom dependence for counseling or enrollment in 12-step addiction treatment programs.
A separate diagnosis?
Dr. Ahmed said he would like to see kratom dependence included in the DSM-5 as a separate entity because it is a botanical with properties similar to, but different from, traditional opioids.
“This will not only help to better inform clinicians about a diagnostic criteria encompassing problematic use and facilitate screening, but it will also pave the way for treatments to be explored for this diagnosable condition,” he said. Dr. Ahmed pointed to a review published in the Wisconsin Medical Journal earlier this year that explored potential treatments for kratom dependence.
Commenting on the study for an interview, Petros Levounis, MD, professor and chair, department of psychiatry, and associate dean for professional development, Rutgers New Jersey Medical School, Newark, said the authors “have done a great job reviewing the literature and asking experts” about kratom addiction treatment.
“The punchline of their study is that kratom behaves very much like an opioid and is treated like an opioid.”
Dr. Levounis noted that kratom dependence is so new that experts don’t know much about it. However, he added, emerging evidence suggests that kratom “should be considered an opioid more than anything else,” but specified that he does not believe it warrants its own diagnosis.
He noted that individual opioids don’t have their own diagnostic category and that opioid use disorder is an umbrella term that covers all of these drugs.
Dr. Ahmed and Dr. Levounis have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
*Updated 5/18/2021
Medications typically used to treat opioid use disorder (OUD) may* also be effective for the growing public health problem of kratom addiction, new research shows.
Results of a comprehensive literature review and an expert survey suggest buprenorphine, naltrexone, and methadone may be effective for patients seeking help for kratom addiction, and if further research confirms these findings, the indication for OUD medications could potentially be expanded to include moderate-to-severe kratom addiction, study investigator Saeed Ahmed, MD, medical director of West Ridge Center at Rutland Regional Medical Center, Rutland, Vermont, said in an interview.
Dr. Ahmed, who practices general psychiatry and addiction psychiatry, presented the findings at the virtual American Psychiatric Association 2021 Annual Meeting.
Emerging public health problem
Kratom can be ingested in pill or capsule form or as an extract. Its leaves can be chewed or dried and powdered to make a tea. It can also be incorporated into topical creams, balms, or tinctures.
Products containing the substance are “readily available and legal for sale in many states and cities in the U.S.,” said Dr. Ahmed, adding that it can be purchased online or at local smoke shops and is increasingly used by individuals to self-treat a variety of conditions including pain, anxiety, and mood conditions and as an opioid substitute.
As reported by this news organization, a 2018 analysis conducted by the U.S. Food and Drug Administration showed kratom is, in fact, an opioid, a finding that garnered significant push-back from the American Kratom Association.
Kratom addiction is an “emerging public health problem,” said Dr. Ahmed, adding that in recent years the number of calls to poison control centers across the country has increased 52-fold – from one per month to two per day. He believes misinformation through social media has helped fuel its use.
Kratom use, the investigators note, can lead to muscle pain, weight loss, insomnia, hallucinations and, in some cases (particularly when combined with synthetic opioids or benzodiazepines), it can lead to respiratory depression, seizures, coma, and death.
In addition,
To investigate, the researchers conducted a systematic literature search for cases pertaining to maintenance treatment for kratom dependence. They also tapped into case reports and scientific posters from reliable online sources and conference proceedings. In addition, they conducted a survey of members from the American Society of Addiction Medicine (ASAM).
The researchers found 14 reports of long-term management of kratom addiction, half of which did not involve an OUD. It’s important to exclude OUDs to avoid possible confounding.
In most cases, buprenorphine was used, but in a few cases naltrexone or methadone were prescribed. All cases had a favorable outcome. Dr. Ahmed noted that buprenorphine maintenance doses appear to be lower than those required to effectively treat OUD.
With a response rate of 11.5% (82 respondents) the ASAM survey results showed 82.6% of respondents (n = 57) had experience managing KUD, including 27.5% (n = 19) who had kratom addiction only. Of these, 89.5% (n = 17-19), used buprenorphine to manage KUD and of these, 6 combined it with talk therapy.
Dr. Ahmed cautioned that the included cases varied significantly in terms of relevant data, including kratom dose and route of administration, toxicology screening used to monitor abstinence, and duration of maintenance follow-up.
Despite these limitations, the review and survey underscore the importance of including moderate to severe kratom dependence as an indication for current OUD medications, the researchers note.
Including kratom addiction as an indication for these medications is important, especially for patients who are heavily addicted, to meet DSM-5 diagnostic criteria for moderate or severe SUD, they add.
In addition, the researchers recommend that clinicians consider referring patients with moderate to severe kratom dependence for counseling or enrollment in 12-step addiction treatment programs.
A separate diagnosis?
Dr. Ahmed said he would like to see kratom dependence included in the DSM-5 as a separate entity because it is a botanical with properties similar to, but different from, traditional opioids.
“This will not only help to better inform clinicians about a diagnostic criteria encompassing problematic use and facilitate screening, but it will also pave the way for treatments to be explored for this diagnosable condition,” he said. Dr. Ahmed pointed to a review published in the Wisconsin Medical Journal earlier this year that explored potential treatments for kratom dependence.
Commenting on the study for an interview, Petros Levounis, MD, professor and chair, department of psychiatry, and associate dean for professional development, Rutgers New Jersey Medical School, Newark, said the authors “have done a great job reviewing the literature and asking experts” about kratom addiction treatment.
“The punchline of their study is that kratom behaves very much like an opioid and is treated like an opioid.”
Dr. Levounis noted that kratom dependence is so new that experts don’t know much about it. However, he added, emerging evidence suggests that kratom “should be considered an opioid more than anything else,” but specified that he does not believe it warrants its own diagnosis.
He noted that individual opioids don’t have their own diagnostic category and that opioid use disorder is an umbrella term that covers all of these drugs.
Dr. Ahmed and Dr. Levounis have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
*Updated 5/18/2021
Blood biomarker a ‘promising’ predictor of psychosis relapse
Copeptin, a small peptide secreted with the hormone vasopressin, appears to be one of the first promising biomarkers for predicting psychosis relapse, results of an observational study suggest.
An analysis of plasma copeptin levels in patients with schizophrenia showed those with high plasma levels of the peptide were about three times more likely to experience psychotic relapse, compared with their counterparts with lower levels.
The results suggest, “copeptin could be a promising biomarker in predicting psychotic relapse in schizophrenia spectrum disorder,” said study investigator Jennifer Küster, MD, University Psychiatric Clinics Basel (Switzerland). Measuring copeptin levels upon hospital admission “could help to intensify” the care of at-risk patients, she added.
The findings were presented at the virtual Congress of the Schizophrenia International Research Society 2021.
Relapse prevention important
Two-thirds of patients with schizophrenia experience at least one relapse of a psychotic episode, which in turn increases the risk of the disorder having a chronic course, Dr. Küster noted.
In addition, a psychotic relapse is associated with deterioration of function and cognition and reduced treatment response, “so relapse prevention is important,” she said.
Previous research has explored various methods of predicting schizophrenia outcomes. These include measuring inflammatory markers, catecholamines, oxytocin, and cortisol in combination with imaging markers, “but so far no reliable biomarker has been found,” Dr. Küster said.
She noted that psychotic relapse is associated with increased psychological stress – and vasopressin, which is secreted by the pituitary gland, is a known marker of stress. It is involved in sodium homeostasis and higher brain function and is also elevated in acute psychosis.
However, vasopressin “is challenging to measure because assays are complicated and unreliable,” Dr. Küster said.
As a result, the researchers turned their attention to copeptin, a more stable, more reliable surrogate marker for vasopressin. Copeptin has been shown previously to be a predictor of outcomes in somatic diseases and is also increased during psychological distress.
To measure the utility of copeptin in predicting psychotic relapse,
Baseline characteristics were collected and fasting serum copeptin levels were measured. Disease severity was measured using a range of validated assessment scales.
Predictive factor
Among 69 patients available for analysis, 30 experienced psychotic relapse at 1-year follow-up. Relapse was defined as rehospitalization because of an acute psychotic episode.
There were no differences in baseline demographic characteristics between patients with, and without, psychotic relapse. There were also no differences in baseline psychopathology, including scores on the Positive and Negative Syndrome Scale, the Beck Depression Inventory, and the Global Assessment of Function.
Dr. Küster noted that there were no overall differences between patients with and without psychotic relapse in terms of their plasma copeptin or cortisol levels at baseline.
“The only difference we saw was in diagnosis,” she reported. Patients with psychotic relapse were significantly more likely to have comorbid drug abuse – 43% in patients who relapsed versus 15% of those who did not (P = .02).
However, when the investigators calculated the area under the receiver operating characteristics curve for copeptin levels, they found there was a significant difference in relapse rates in those with copeptin levels >6 pmol/L vs. those with lower levels (hazard ratio, 2.3; P = .039).
When the focus was on only patients with schizophrenia spectrum disorder, the results were even more pronounced. The HR for psychotic relapse in patients with higher vs. lower copeptin levels was 3.2 (P = .028).
“We also looked for other possible predicting factors,” Dr. Küster said. This included sex, age, duration of disease, reason for hospitalization, psychopathology, medication, comorbidities, and cortisol levels. “But none of these factors was associated with psychotic relapse,” she added.
The only factor positively associated with relapse was drug abuse, primarily via marijuana. However, the association with copeptin remained significant even after taking this factor into account.
In future studies, the researchers plan to examine whether copeptin levels could identify which patients at ultra-high risk will transition to first-episode psychosis, as well as to predict development of posttraumatic stress disorder, Dr. Küster said.
A proxy for ‘something simpler’?
Commenting on the findings for this news organization, Leah H. Rubin, PhD, associate professor of neurology, Johns Hopkins University, Baltimore, described the study as “interesting” – and noted that her own research has included measuring vasopressin in patients with untreated first-episode psychosis.
Dr. Rubin’s findings showed that levels of the hormone were associated with psychosis severity, and thus she is “not surprised that they found a marker” that may be promising in psychosis relapse prediction.
However, she took issue with the notion that vasopressin is an unreliable marker, pointing out that the work of her team demonstrates that it can be measured. Dr. Rubin added that she found it to be “pretty stable.”
In addition, because the current study had a small sample size, Dr. Rubin said she would be interested to see whether the findings can be replicated on a larger scale.
She also noted that more than two-thirds of the study population were men. “Vasopressin and oxytocin are sexually dimorphic neuropeptides,” she explained, “so I think it becomes important to ensure ... whether it’s the same for men and women.”
“Just from a psychosocial perspective, what’s going on in those folks’ lives?” Dr. Rubin asked. “Is it truly copeptin” or is it high stress levels that facilitate a relapse? Copeptin levels, she added, may be “a proxy for something simpler.”
The study authors and Dr. Rubin have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Copeptin, a small peptide secreted with the hormone vasopressin, appears to be one of the first promising biomarkers for predicting psychosis relapse, results of an observational study suggest.
An analysis of plasma copeptin levels in patients with schizophrenia showed those with high plasma levels of the peptide were about three times more likely to experience psychotic relapse, compared with their counterparts with lower levels.
The results suggest, “copeptin could be a promising biomarker in predicting psychotic relapse in schizophrenia spectrum disorder,” said study investigator Jennifer Küster, MD, University Psychiatric Clinics Basel (Switzerland). Measuring copeptin levels upon hospital admission “could help to intensify” the care of at-risk patients, she added.
The findings were presented at the virtual Congress of the Schizophrenia International Research Society 2021.
Relapse prevention important
Two-thirds of patients with schizophrenia experience at least one relapse of a psychotic episode, which in turn increases the risk of the disorder having a chronic course, Dr. Küster noted.
In addition, a psychotic relapse is associated with deterioration of function and cognition and reduced treatment response, “so relapse prevention is important,” she said.
Previous research has explored various methods of predicting schizophrenia outcomes. These include measuring inflammatory markers, catecholamines, oxytocin, and cortisol in combination with imaging markers, “but so far no reliable biomarker has been found,” Dr. Küster said.
She noted that psychotic relapse is associated with increased psychological stress – and vasopressin, which is secreted by the pituitary gland, is a known marker of stress. It is involved in sodium homeostasis and higher brain function and is also elevated in acute psychosis.
However, vasopressin “is challenging to measure because assays are complicated and unreliable,” Dr. Küster said.
As a result, the researchers turned their attention to copeptin, a more stable, more reliable surrogate marker for vasopressin. Copeptin has been shown previously to be a predictor of outcomes in somatic diseases and is also increased during psychological distress.
To measure the utility of copeptin in predicting psychotic relapse,
Baseline characteristics were collected and fasting serum copeptin levels were measured. Disease severity was measured using a range of validated assessment scales.
Predictive factor
Among 69 patients available for analysis, 30 experienced psychotic relapse at 1-year follow-up. Relapse was defined as rehospitalization because of an acute psychotic episode.
There were no differences in baseline demographic characteristics between patients with, and without, psychotic relapse. There were also no differences in baseline psychopathology, including scores on the Positive and Negative Syndrome Scale, the Beck Depression Inventory, and the Global Assessment of Function.
Dr. Küster noted that there were no overall differences between patients with and without psychotic relapse in terms of their plasma copeptin or cortisol levels at baseline.
“The only difference we saw was in diagnosis,” she reported. Patients with psychotic relapse were significantly more likely to have comorbid drug abuse – 43% in patients who relapsed versus 15% of those who did not (P = .02).
However, when the investigators calculated the area under the receiver operating characteristics curve for copeptin levels, they found there was a significant difference in relapse rates in those with copeptin levels >6 pmol/L vs. those with lower levels (hazard ratio, 2.3; P = .039).
When the focus was on only patients with schizophrenia spectrum disorder, the results were even more pronounced. The HR for psychotic relapse in patients with higher vs. lower copeptin levels was 3.2 (P = .028).
“We also looked for other possible predicting factors,” Dr. Küster said. This included sex, age, duration of disease, reason for hospitalization, psychopathology, medication, comorbidities, and cortisol levels. “But none of these factors was associated with psychotic relapse,” she added.
The only factor positively associated with relapse was drug abuse, primarily via marijuana. However, the association with copeptin remained significant even after taking this factor into account.
In future studies, the researchers plan to examine whether copeptin levels could identify which patients at ultra-high risk will transition to first-episode psychosis, as well as to predict development of posttraumatic stress disorder, Dr. Küster said.
A proxy for ‘something simpler’?
Commenting on the findings for this news organization, Leah H. Rubin, PhD, associate professor of neurology, Johns Hopkins University, Baltimore, described the study as “interesting” – and noted that her own research has included measuring vasopressin in patients with untreated first-episode psychosis.
Dr. Rubin’s findings showed that levels of the hormone were associated with psychosis severity, and thus she is “not surprised that they found a marker” that may be promising in psychosis relapse prediction.
However, she took issue with the notion that vasopressin is an unreliable marker, pointing out that the work of her team demonstrates that it can be measured. Dr. Rubin added that she found it to be “pretty stable.”
In addition, because the current study had a small sample size, Dr. Rubin said she would be interested to see whether the findings can be replicated on a larger scale.
She also noted that more than two-thirds of the study population were men. “Vasopressin and oxytocin are sexually dimorphic neuropeptides,” she explained, “so I think it becomes important to ensure ... whether it’s the same for men and women.”
“Just from a psychosocial perspective, what’s going on in those folks’ lives?” Dr. Rubin asked. “Is it truly copeptin” or is it high stress levels that facilitate a relapse? Copeptin levels, she added, may be “a proxy for something simpler.”
The study authors and Dr. Rubin have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Copeptin, a small peptide secreted with the hormone vasopressin, appears to be one of the first promising biomarkers for predicting psychosis relapse, results of an observational study suggest.
An analysis of plasma copeptin levels in patients with schizophrenia showed those with high plasma levels of the peptide were about three times more likely to experience psychotic relapse, compared with their counterparts with lower levels.
The results suggest, “copeptin could be a promising biomarker in predicting psychotic relapse in schizophrenia spectrum disorder,” said study investigator Jennifer Küster, MD, University Psychiatric Clinics Basel (Switzerland). Measuring copeptin levels upon hospital admission “could help to intensify” the care of at-risk patients, she added.
The findings were presented at the virtual Congress of the Schizophrenia International Research Society 2021.
Relapse prevention important
Two-thirds of patients with schizophrenia experience at least one relapse of a psychotic episode, which in turn increases the risk of the disorder having a chronic course, Dr. Küster noted.
In addition, a psychotic relapse is associated with deterioration of function and cognition and reduced treatment response, “so relapse prevention is important,” she said.
Previous research has explored various methods of predicting schizophrenia outcomes. These include measuring inflammatory markers, catecholamines, oxytocin, and cortisol in combination with imaging markers, “but so far no reliable biomarker has been found,” Dr. Küster said.
She noted that psychotic relapse is associated with increased psychological stress – and vasopressin, which is secreted by the pituitary gland, is a known marker of stress. It is involved in sodium homeostasis and higher brain function and is also elevated in acute psychosis.
However, vasopressin “is challenging to measure because assays are complicated and unreliable,” Dr. Küster said.
As a result, the researchers turned their attention to copeptin, a more stable, more reliable surrogate marker for vasopressin. Copeptin has been shown previously to be a predictor of outcomes in somatic diseases and is also increased during psychological distress.
To measure the utility of copeptin in predicting psychotic relapse,
Baseline characteristics were collected and fasting serum copeptin levels were measured. Disease severity was measured using a range of validated assessment scales.
Predictive factor
Among 69 patients available for analysis, 30 experienced psychotic relapse at 1-year follow-up. Relapse was defined as rehospitalization because of an acute psychotic episode.
There were no differences in baseline demographic characteristics between patients with, and without, psychotic relapse. There were also no differences in baseline psychopathology, including scores on the Positive and Negative Syndrome Scale, the Beck Depression Inventory, and the Global Assessment of Function.
Dr. Küster noted that there were no overall differences between patients with and without psychotic relapse in terms of their plasma copeptin or cortisol levels at baseline.
“The only difference we saw was in diagnosis,” she reported. Patients with psychotic relapse were significantly more likely to have comorbid drug abuse – 43% in patients who relapsed versus 15% of those who did not (P = .02).
However, when the investigators calculated the area under the receiver operating characteristics curve for copeptin levels, they found there was a significant difference in relapse rates in those with copeptin levels >6 pmol/L vs. those with lower levels (hazard ratio, 2.3; P = .039).
When the focus was on only patients with schizophrenia spectrum disorder, the results were even more pronounced. The HR for psychotic relapse in patients with higher vs. lower copeptin levels was 3.2 (P = .028).
“We also looked for other possible predicting factors,” Dr. Küster said. This included sex, age, duration of disease, reason for hospitalization, psychopathology, medication, comorbidities, and cortisol levels. “But none of these factors was associated with psychotic relapse,” she added.
The only factor positively associated with relapse was drug abuse, primarily via marijuana. However, the association with copeptin remained significant even after taking this factor into account.
In future studies, the researchers plan to examine whether copeptin levels could identify which patients at ultra-high risk will transition to first-episode psychosis, as well as to predict development of posttraumatic stress disorder, Dr. Küster said.
A proxy for ‘something simpler’?
Commenting on the findings for this news organization, Leah H. Rubin, PhD, associate professor of neurology, Johns Hopkins University, Baltimore, described the study as “interesting” – and noted that her own research has included measuring vasopressin in patients with untreated first-episode psychosis.
Dr. Rubin’s findings showed that levels of the hormone were associated with psychosis severity, and thus she is “not surprised that they found a marker” that may be promising in psychosis relapse prediction.
However, she took issue with the notion that vasopressin is an unreliable marker, pointing out that the work of her team demonstrates that it can be measured. Dr. Rubin added that she found it to be “pretty stable.”
In addition, because the current study had a small sample size, Dr. Rubin said she would be interested to see whether the findings can be replicated on a larger scale.
She also noted that more than two-thirds of the study population were men. “Vasopressin and oxytocin are sexually dimorphic neuropeptides,” she explained, “so I think it becomes important to ensure ... whether it’s the same for men and women.”
“Just from a psychosocial perspective, what’s going on in those folks’ lives?” Dr. Rubin asked. “Is it truly copeptin” or is it high stress levels that facilitate a relapse? Copeptin levels, she added, may be “a proxy for something simpler.”
The study authors and Dr. Rubin have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A review of the latest USPSTF recommendations
Since the last Practice Alert update on recommendations made by the US Preventive Services Task Force,1 the Task Force has completed work on 12 topics (TABLE 1).2-17 Five of these topics have been discussed in JFP audio recordings, and the links are provided in TABLE 1.
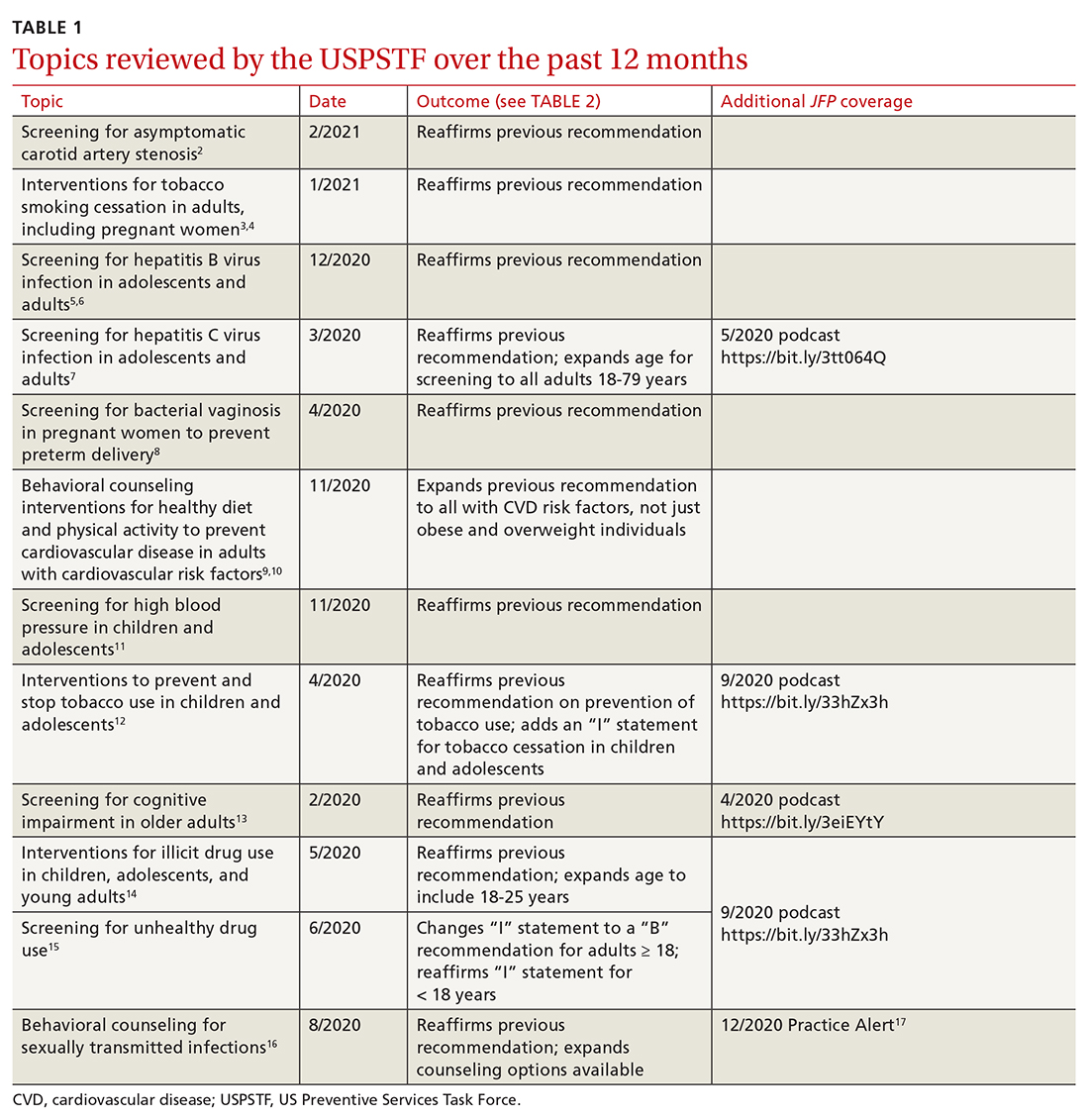
This latest Task Force endeavor resulted in 18 recommendations (TABLE 2), all of which reaffirm previous recommendations on these topics and expand the scope of 2. There were 2 “A” recommendations, 6 “B” recommendations, 2 “D” recommendations, and 8 “I” statements, indicating that there was insufficient evidence to assess effectiveness or harms. The willingness to make “I” statements when there is little or no evidence on the intervention being assessed distinguishes the USPSTF from other clinical guideline committees.

Screening for carotid artery stenosis
One of the “D” recommendations this past year reaffirms the prior recommendation against screening for carotid artery stenosis in asymptomatic adults—ie, those without a history of transient ischemic attack, stroke, or neurologic signs or symptoms that might be caused by carotid artery stenosis.2 The screening tests the Task Force researched included carotid duplex ultrasonography (DUS), magnetic resonance angiography, and computed tomography angiography. The Task Force did not look at the value of auscultation for carotid bruits because it has been proven to be inaccurate and they do not consider it to be a useful screening tool.
The Task Force based its “D” recommendation on a lack of evidence for any benefit in detecting asymptomatic carotid artery stenosis, and on evidence that screening can lead to harms through false-positive tests and potential complications from carotid endarterectomy and carotid artery angioplasty and stenting. In its clinical considerations, the Task Force emphasized the primary prevention of atherosclerotic disease by focusing on the following actions:
- screening for high blood pressure in adults
- encouraging tobacco smoking cessation in adults
- promoting a healthy diet and physical activity in adults with cardiovascular risk factors
- recommending aspirin use to prevent cardiovascular disease and colorectal cancer
- advising statin use for the primary prevention of cardiovascular disease in adults ages 45 to 75 years who have 1 or more risk factors (hyperlipidemia, diabetes, hypertension, smoking) and those with a 10-year risk of a cardiovascular event of 10% or greater.
This “D” recommendation differs from recommendations made by other professional organizations, some of which recommend testing with DUS for asymptomatic patients with a carotid bruit, and others that recommend DUS screening in patients with multiple risk factors for stroke and in those with known peripheral artery disease or other cardiovascular disease.18,19
Smoking cessation in adults
Smoking tobacco is the leading preventable cause of death in the United States, causing about 480,000 deaths annually.3 Smoking during pregnancy increases the risk of complications including miscarriage, congenital anomalies, stillbirth, fetal growth restriction, preterm birth, and placental abruption.
The Task Force published recommendations earlier this year advising all clinicians to ask all adult patients about tobacco use; and, for those who smoke, to provide (or refer them to) smoking cessation behavioral therapy. The Task Force also recommends prescribing pharmacotherapy approved by the Food and Drug Administration (FDA) for smoking cessation for nonpregnant adults. (There is a lack of information to assess the harms and benefits of smoking cessation pharmacotherapy during pregnancy.)
Continue to: FDA-approved medications...
FDA-approved medications for treating tobacco smoking dependence are nicotine replacement therapy (NRT), bupropion hydrochloride, and varenicline.3 NRT is available in transdermal patches, lozenges, gum, inhalers, and nasal sprays.
In addition, the Task Force indicates that there is insufficient evidence to assess the benefits and harms of e-cigarettes when used as a method of achieving smoking cessation: “Few randomized trials have evaluated the effectiveness of e-cigarettes to increase tobacco smoking cessation in nonpregnant adults, and no trials have evaluated e-cigarettes for tobacco smoking cessation in pregnant persons.”4
Hepatitis B infection screening
The Task Force reaffirmed a previous recommendation to screen for hepatitis B virus (HBV) infection only in adults who are at high risk,5 rather than universal screening that it recommends for hepatitis C virus infection (HCV).7 (See: https://bit.ly/3tt064Q). The Task Force has a separate recommendation to screen all pregnant women for hepatitis B at the first prenatal visit.6
Those at high risk for hepatitis B who should be screened include individuals born in countries or regions of the world with a hepatitis B surface antigen (HBsAg) prevalence ≥ 2% and individuals born in the United States who have not received HBV vaccine and whose parents were born in regions with an HBsAg prevalence ≥ 8%.5 (A table listing countries with HBsAg ≥ 8%—as well as those in lower prevalence categories—is included with the recommendation.5)
HBV screening should also be offered to other high-risk groups that have a prevalence of positive HBsAg ≥ 2%: those who have injected drugs in the past or are currently injecting drugs; men who have sex with men; individuals with HIV; and sex partners, needle-sharing contacts, and household contacts of people known to be HBsAg positive.5
Continue to: It is estimated that...
It is estimated that > 860,000 people in the United States have chronic HBV infection and that close to two-thirds of them are unaware of their infection.5 The screening test for HBV is highly accurate; sensitivity and specificity are both > 98%.5 While there is no direct evidence that screening, detecting, and treating asymptomatic HBV infection reduces morbidity and mortality, the Task Force felt that the evidence for improvement in multiple outcomes in those with HBV when treated with antiviral regimens was sufficient to support the recommendation.
Screening for bacterial vaginosis in pregnancy
While bacterial vaginosis (BV) is associated with a two-fold risk of preterm delivery, treating BV during pregnancy does not seem to reduce this risk, indicating that some other variable is involved.8 In addition, studies that looked at screening for, and treatment of, asymptomatic BV in pregnant women at high risk for preterm delivery (defined primarily as those with a previous preterm delivery) have shown inconsistent results. There is the potential for harm in treating BV in pregnancy, chiefly involving gastrointestinal upset caused by metronidazole or clindamycin.
Given that there are no benefits—and some harms—resulting from treatment, the Task Force recommends against screening for BV in non-high-risk pregnant women. A lack of sufficient information to assess any potential benefits to screening in high-risk pregnancies led the Task Force to an “I” statement on this question.8
Behavioral counseling on healthy diet, exercise for adults with CV risks
Cardiovascular disease (CVD) remains the number one cause of death in the United States. The major risk factors for CVD, which can be modified, are high blood pressure, hyperlipidemia, diabetes, smoking, obesity or overweight, and lack of physical activity.
The Task Force has previously recommended intensive behavioral interventions to improve nutrition and physical activity in those who are overweight/obese and in those with abnormal blood glucose levels,9 and has addressed smoking prevention and cessation.4 This new recommendation applies to those with other CVD risks such as high blood pressure and/or hyperlipidemia and those with an estimated 10-year CVD risk of ≥ 7.5%.10
Continue to: Behavioral interventions...
Behavioral interventions included in the Task Force analysis employed a median of 12 contacts and an estimated 6 hours of contact time over 6 to 18 months.10 Most interventions involved motivational interviewing and instruction on behavioral change methods. These interventions can be provided by primary care clinicians, as well as a wide range of other trained professionals. The Affordable Care Act dictates that all “A” and “B” recommendations must be provided by commercial health plans at no out-of-pocket expense for the patient.
Nutritional advice should include reductions in saturated fats, salt, and sugars and increases in fruits, vegetables, and whole grains. The Mediterranean diet and the Dietary Approaches to Stop Hypertension (DASH) diet are often recommended.10 Physical activity counseling should advocate for 90 to 180 minutes per week of moderate to vigorous activity.
This new recommendation, along with the previous ones pertaining to behavioral interventions for lifestyle changes, make it clear that intensive interventions are needed to achieve meaningful change. Simple advice from a clinician will have little to no effect.
Task Force reviews evidence on HTN, smoking cessation in young people
In 2020 the Task Force completed reviews of evidence relevant to screening for high blood pressure11 and
The 2 “I” statements are in disagreement with recommendations of other professional organizations. The American Academy of Pediatrics (AAP) and the American Heart Association recommend routine screening for high blood pressure starting at age 3 years. And the AAP recommends screening teenagers for tobacco use and offering tobacco dependence treatment, referral, or both (including pharmacotherapy) when indicated. E-cigarettes are not recommended as a treatment for tobacco dependence.20
Continue to: The difference between...
The difference between the methods used by the Task Force and other guideline-producing organizations becomes apparent when it comes to recommendations pertaining to children and adolescents, for whom long-term outcome-oriented studies on prevention issues are rare. The Task Force is unwilling to make recommendations when evidence does not exist. The AAP often makes recommendations based on expert opinion consensus in such situations. One notable part of each Task Force recommendation statement is a discussion of what other organizations recommend on the same topic so that these differences can be openly described.
Better Task Force funding could expand topic coverage
It is worth revisiting 2 issues that were pointed out in last year’s USPSTF summary in this column.1 First, the Task Force methods are robust and evidence based, and recommendations therefore are rarely changed once they are made at an “A”, “B”, or “D” level. Second, Task Force resources are finite, and thus, the group is currently unable to update previous recommendations with greater frequency or to consider many new topics. In the past 2 years, the Task Force has developed recommendations on only 2 completely new topics. Hopefully, its budget can be expanded so that new topics can be added in the future.
1. Campos-Outcalt D. USPSTF roundup. J Fam Pract. 2020;69:201-204.
2. USPSTF. Screening for asymptomatic carotid artery stenosis. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/carotid-artery-stenosis-screening
3. USPSTF. Interventions for tobacco smoking cessation in adults, including pregnant persons. Accessed April 30, 2021. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions
4. USPSTF. Interventions for tobacco smoking cessation in adults, including pregnant persons. JAMA. 2021;325:265-279.
5. USPSTF. Screening for Hepatitis B virus infection in adolescents and adults. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-b-virus-infection-screening
6. USPSTF. Hepatitis B virus infection in pregnant women: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-b-virus-infection-in-pregnant-women-screening
7. USPSTF. Hepatitis C virus infection in adolescents and adults: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-c-screening
8. USPSTF; Owens DK, Davidson KW, Krisk AH, et al. Screening for bacterial vaginosis in pregnant persons to prevent preterm delivery: US Preventive Services Task Force recommendation statement. JAMA. 2020;323:1286-1292.
9. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:587-593.
10. USPSTF. Behavioral counseling interventions to promote a healthy and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: US Preventive Services Task Force recommendation statement. JAMA. 2020;324:2069-2075.
11. USPSTF. High blood pressure in children and adolescents: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/blood-pressure-in-children-and-adolescents-hypertension-screening
12. USPSTF. Prevention and cessation of tobacco use in children and adolescents: primary care interventions. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions
13. USPSTF. Cognitive impairment in older adults: screening. Accessed March 26, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/cognitive-impairment-in-older-adults-screening
14. USPSTF. Illicit drug use in children, adolescents, and young adults: primary care-based interventions. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-primary-care-interventions-for-children-and-adolescents
15. USPSTF. Unhealthy drug use: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-screening
16. USPSTF. Sexually transmitted infections: behavioral counseling. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/sexually-transmitted-infections-behavioral-counseling.
17. Campos-Outcalt D. USPSTF update on sexually transmitted infections. J Fam Pract. 2020;69:514-517.
18. Brott TG, Halperin JL, Abbara S, et al; ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease. Catheter Cardiovasc Interv. 2013;81:E76-E123.
19. Ricotta JJ, Aburahma A, Ascher E, et al; Society for Vascular Surgery. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease. J Vasc Surg. 2011;54:e1-e31.
20. Farber HJ, Walley SC, Groner JA, et al; Section on Tobacco Control. Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics. 2015;136:1008-1017.
Since the last Practice Alert update on recommendations made by the US Preventive Services Task Force,1 the Task Force has completed work on 12 topics (TABLE 1).2-17 Five of these topics have been discussed in JFP audio recordings, and the links are provided in TABLE 1.

This latest Task Force endeavor resulted in 18 recommendations (TABLE 2), all of which reaffirm previous recommendations on these topics and expand the scope of 2. There were 2 “A” recommendations, 6 “B” recommendations, 2 “D” recommendations, and 8 “I” statements, indicating that there was insufficient evidence to assess effectiveness or harms. The willingness to make “I” statements when there is little or no evidence on the intervention being assessed distinguishes the USPSTF from other clinical guideline committees.

Screening for carotid artery stenosis
One of the “D” recommendations this past year reaffirms the prior recommendation against screening for carotid artery stenosis in asymptomatic adults—ie, those without a history of transient ischemic attack, stroke, or neurologic signs or symptoms that might be caused by carotid artery stenosis.2 The screening tests the Task Force researched included carotid duplex ultrasonography (DUS), magnetic resonance angiography, and computed tomography angiography. The Task Force did not look at the value of auscultation for carotid bruits because it has been proven to be inaccurate and they do not consider it to be a useful screening tool.
The Task Force based its “D” recommendation on a lack of evidence for any benefit in detecting asymptomatic carotid artery stenosis, and on evidence that screening can lead to harms through false-positive tests and potential complications from carotid endarterectomy and carotid artery angioplasty and stenting. In its clinical considerations, the Task Force emphasized the primary prevention of atherosclerotic disease by focusing on the following actions:
- screening for high blood pressure in adults
- encouraging tobacco smoking cessation in adults
- promoting a healthy diet and physical activity in adults with cardiovascular risk factors
- recommending aspirin use to prevent cardiovascular disease and colorectal cancer
- advising statin use for the primary prevention of cardiovascular disease in adults ages 45 to 75 years who have 1 or more risk factors (hyperlipidemia, diabetes, hypertension, smoking) and those with a 10-year risk of a cardiovascular event of 10% or greater.
This “D” recommendation differs from recommendations made by other professional organizations, some of which recommend testing with DUS for asymptomatic patients with a carotid bruit, and others that recommend DUS screening in patients with multiple risk factors for stroke and in those with known peripheral artery disease or other cardiovascular disease.18,19
Smoking cessation in adults
Smoking tobacco is the leading preventable cause of death in the United States, causing about 480,000 deaths annually.3 Smoking during pregnancy increases the risk of complications including miscarriage, congenital anomalies, stillbirth, fetal growth restriction, preterm birth, and placental abruption.
The Task Force published recommendations earlier this year advising all clinicians to ask all adult patients about tobacco use; and, for those who smoke, to provide (or refer them to) smoking cessation behavioral therapy. The Task Force also recommends prescribing pharmacotherapy approved by the Food and Drug Administration (FDA) for smoking cessation for nonpregnant adults. (There is a lack of information to assess the harms and benefits of smoking cessation pharmacotherapy during pregnancy.)
Continue to: FDA-approved medications...
FDA-approved medications for treating tobacco smoking dependence are nicotine replacement therapy (NRT), bupropion hydrochloride, and varenicline.3 NRT is available in transdermal patches, lozenges, gum, inhalers, and nasal sprays.
In addition, the Task Force indicates that there is insufficient evidence to assess the benefits and harms of e-cigarettes when used as a method of achieving smoking cessation: “Few randomized trials have evaluated the effectiveness of e-cigarettes to increase tobacco smoking cessation in nonpregnant adults, and no trials have evaluated e-cigarettes for tobacco smoking cessation in pregnant persons.”4
Hepatitis B infection screening
The Task Force reaffirmed a previous recommendation to screen for hepatitis B virus (HBV) infection only in adults who are at high risk,5 rather than universal screening that it recommends for hepatitis C virus infection (HCV).7 (See: https://bit.ly/3tt064Q). The Task Force has a separate recommendation to screen all pregnant women for hepatitis B at the first prenatal visit.6
Those at high risk for hepatitis B who should be screened include individuals born in countries or regions of the world with a hepatitis B surface antigen (HBsAg) prevalence ≥ 2% and individuals born in the United States who have not received HBV vaccine and whose parents were born in regions with an HBsAg prevalence ≥ 8%.5 (A table listing countries with HBsAg ≥ 8%—as well as those in lower prevalence categories—is included with the recommendation.5)
HBV screening should also be offered to other high-risk groups that have a prevalence of positive HBsAg ≥ 2%: those who have injected drugs in the past or are currently injecting drugs; men who have sex with men; individuals with HIV; and sex partners, needle-sharing contacts, and household contacts of people known to be HBsAg positive.5
Continue to: It is estimated that...
It is estimated that > 860,000 people in the United States have chronic HBV infection and that close to two-thirds of them are unaware of their infection.5 The screening test for HBV is highly accurate; sensitivity and specificity are both > 98%.5 While there is no direct evidence that screening, detecting, and treating asymptomatic HBV infection reduces morbidity and mortality, the Task Force felt that the evidence for improvement in multiple outcomes in those with HBV when treated with antiviral regimens was sufficient to support the recommendation.
Screening for bacterial vaginosis in pregnancy
While bacterial vaginosis (BV) is associated with a two-fold risk of preterm delivery, treating BV during pregnancy does not seem to reduce this risk, indicating that some other variable is involved.8 In addition, studies that looked at screening for, and treatment of, asymptomatic BV in pregnant women at high risk for preterm delivery (defined primarily as those with a previous preterm delivery) have shown inconsistent results. There is the potential for harm in treating BV in pregnancy, chiefly involving gastrointestinal upset caused by metronidazole or clindamycin.
Given that there are no benefits—and some harms—resulting from treatment, the Task Force recommends against screening for BV in non-high-risk pregnant women. A lack of sufficient information to assess any potential benefits to screening in high-risk pregnancies led the Task Force to an “I” statement on this question.8
Behavioral counseling on healthy diet, exercise for adults with CV risks
Cardiovascular disease (CVD) remains the number one cause of death in the United States. The major risk factors for CVD, which can be modified, are high blood pressure, hyperlipidemia, diabetes, smoking, obesity or overweight, and lack of physical activity.
The Task Force has previously recommended intensive behavioral interventions to improve nutrition and physical activity in those who are overweight/obese and in those with abnormal blood glucose levels,9 and has addressed smoking prevention and cessation.4 This new recommendation applies to those with other CVD risks such as high blood pressure and/or hyperlipidemia and those with an estimated 10-year CVD risk of ≥ 7.5%.10
Continue to: Behavioral interventions...
Behavioral interventions included in the Task Force analysis employed a median of 12 contacts and an estimated 6 hours of contact time over 6 to 18 months.10 Most interventions involved motivational interviewing and instruction on behavioral change methods. These interventions can be provided by primary care clinicians, as well as a wide range of other trained professionals. The Affordable Care Act dictates that all “A” and “B” recommendations must be provided by commercial health plans at no out-of-pocket expense for the patient.
Nutritional advice should include reductions in saturated fats, salt, and sugars and increases in fruits, vegetables, and whole grains. The Mediterranean diet and the Dietary Approaches to Stop Hypertension (DASH) diet are often recommended.10 Physical activity counseling should advocate for 90 to 180 minutes per week of moderate to vigorous activity.
This new recommendation, along with the previous ones pertaining to behavioral interventions for lifestyle changes, make it clear that intensive interventions are needed to achieve meaningful change. Simple advice from a clinician will have little to no effect.
Task Force reviews evidence on HTN, smoking cessation in young people
In 2020 the Task Force completed reviews of evidence relevant to screening for high blood pressure11 and
The 2 “I” statements are in disagreement with recommendations of other professional organizations. The American Academy of Pediatrics (AAP) and the American Heart Association recommend routine screening for high blood pressure starting at age 3 years. And the AAP recommends screening teenagers for tobacco use and offering tobacco dependence treatment, referral, or both (including pharmacotherapy) when indicated. E-cigarettes are not recommended as a treatment for tobacco dependence.20
Continue to: The difference between...
The difference between the methods used by the Task Force and other guideline-producing organizations becomes apparent when it comes to recommendations pertaining to children and adolescents, for whom long-term outcome-oriented studies on prevention issues are rare. The Task Force is unwilling to make recommendations when evidence does not exist. The AAP often makes recommendations based on expert opinion consensus in such situations. One notable part of each Task Force recommendation statement is a discussion of what other organizations recommend on the same topic so that these differences can be openly described.
Better Task Force funding could expand topic coverage
It is worth revisiting 2 issues that were pointed out in last year’s USPSTF summary in this column.1 First, the Task Force methods are robust and evidence based, and recommendations therefore are rarely changed once they are made at an “A”, “B”, or “D” level. Second, Task Force resources are finite, and thus, the group is currently unable to update previous recommendations with greater frequency or to consider many new topics. In the past 2 years, the Task Force has developed recommendations on only 2 completely new topics. Hopefully, its budget can be expanded so that new topics can be added in the future.
Since the last Practice Alert update on recommendations made by the US Preventive Services Task Force,1 the Task Force has completed work on 12 topics (TABLE 1).2-17 Five of these topics have been discussed in JFP audio recordings, and the links are provided in TABLE 1.

This latest Task Force endeavor resulted in 18 recommendations (TABLE 2), all of which reaffirm previous recommendations on these topics and expand the scope of 2. There were 2 “A” recommendations, 6 “B” recommendations, 2 “D” recommendations, and 8 “I” statements, indicating that there was insufficient evidence to assess effectiveness or harms. The willingness to make “I” statements when there is little or no evidence on the intervention being assessed distinguishes the USPSTF from other clinical guideline committees.

Screening for carotid artery stenosis
One of the “D” recommendations this past year reaffirms the prior recommendation against screening for carotid artery stenosis in asymptomatic adults—ie, those without a history of transient ischemic attack, stroke, or neurologic signs or symptoms that might be caused by carotid artery stenosis.2 The screening tests the Task Force researched included carotid duplex ultrasonography (DUS), magnetic resonance angiography, and computed tomography angiography. The Task Force did not look at the value of auscultation for carotid bruits because it has been proven to be inaccurate and they do not consider it to be a useful screening tool.
The Task Force based its “D” recommendation on a lack of evidence for any benefit in detecting asymptomatic carotid artery stenosis, and on evidence that screening can lead to harms through false-positive tests and potential complications from carotid endarterectomy and carotid artery angioplasty and stenting. In its clinical considerations, the Task Force emphasized the primary prevention of atherosclerotic disease by focusing on the following actions:
- screening for high blood pressure in adults
- encouraging tobacco smoking cessation in adults
- promoting a healthy diet and physical activity in adults with cardiovascular risk factors
- recommending aspirin use to prevent cardiovascular disease and colorectal cancer
- advising statin use for the primary prevention of cardiovascular disease in adults ages 45 to 75 years who have 1 or more risk factors (hyperlipidemia, diabetes, hypertension, smoking) and those with a 10-year risk of a cardiovascular event of 10% or greater.
This “D” recommendation differs from recommendations made by other professional organizations, some of which recommend testing with DUS for asymptomatic patients with a carotid bruit, and others that recommend DUS screening in patients with multiple risk factors for stroke and in those with known peripheral artery disease or other cardiovascular disease.18,19
Smoking cessation in adults
Smoking tobacco is the leading preventable cause of death in the United States, causing about 480,000 deaths annually.3 Smoking during pregnancy increases the risk of complications including miscarriage, congenital anomalies, stillbirth, fetal growth restriction, preterm birth, and placental abruption.
The Task Force published recommendations earlier this year advising all clinicians to ask all adult patients about tobacco use; and, for those who smoke, to provide (or refer them to) smoking cessation behavioral therapy. The Task Force also recommends prescribing pharmacotherapy approved by the Food and Drug Administration (FDA) for smoking cessation for nonpregnant adults. (There is a lack of information to assess the harms and benefits of smoking cessation pharmacotherapy during pregnancy.)
Continue to: FDA-approved medications...
FDA-approved medications for treating tobacco smoking dependence are nicotine replacement therapy (NRT), bupropion hydrochloride, and varenicline.3 NRT is available in transdermal patches, lozenges, gum, inhalers, and nasal sprays.
In addition, the Task Force indicates that there is insufficient evidence to assess the benefits and harms of e-cigarettes when used as a method of achieving smoking cessation: “Few randomized trials have evaluated the effectiveness of e-cigarettes to increase tobacco smoking cessation in nonpregnant adults, and no trials have evaluated e-cigarettes for tobacco smoking cessation in pregnant persons.”4
Hepatitis B infection screening
The Task Force reaffirmed a previous recommendation to screen for hepatitis B virus (HBV) infection only in adults who are at high risk,5 rather than universal screening that it recommends for hepatitis C virus infection (HCV).7 (See: https://bit.ly/3tt064Q). The Task Force has a separate recommendation to screen all pregnant women for hepatitis B at the first prenatal visit.6
Those at high risk for hepatitis B who should be screened include individuals born in countries or regions of the world with a hepatitis B surface antigen (HBsAg) prevalence ≥ 2% and individuals born in the United States who have not received HBV vaccine and whose parents were born in regions with an HBsAg prevalence ≥ 8%.5 (A table listing countries with HBsAg ≥ 8%—as well as those in lower prevalence categories—is included with the recommendation.5)
HBV screening should also be offered to other high-risk groups that have a prevalence of positive HBsAg ≥ 2%: those who have injected drugs in the past or are currently injecting drugs; men who have sex with men; individuals with HIV; and sex partners, needle-sharing contacts, and household contacts of people known to be HBsAg positive.5
Continue to: It is estimated that...
It is estimated that > 860,000 people in the United States have chronic HBV infection and that close to two-thirds of them are unaware of their infection.5 The screening test for HBV is highly accurate; sensitivity and specificity are both > 98%.5 While there is no direct evidence that screening, detecting, and treating asymptomatic HBV infection reduces morbidity and mortality, the Task Force felt that the evidence for improvement in multiple outcomes in those with HBV when treated with antiviral regimens was sufficient to support the recommendation.
Screening for bacterial vaginosis in pregnancy
While bacterial vaginosis (BV) is associated with a two-fold risk of preterm delivery, treating BV during pregnancy does not seem to reduce this risk, indicating that some other variable is involved.8 In addition, studies that looked at screening for, and treatment of, asymptomatic BV in pregnant women at high risk for preterm delivery (defined primarily as those with a previous preterm delivery) have shown inconsistent results. There is the potential for harm in treating BV in pregnancy, chiefly involving gastrointestinal upset caused by metronidazole or clindamycin.
Given that there are no benefits—and some harms—resulting from treatment, the Task Force recommends against screening for BV in non-high-risk pregnant women. A lack of sufficient information to assess any potential benefits to screening in high-risk pregnancies led the Task Force to an “I” statement on this question.8
Behavioral counseling on healthy diet, exercise for adults with CV risks
Cardiovascular disease (CVD) remains the number one cause of death in the United States. The major risk factors for CVD, which can be modified, are high blood pressure, hyperlipidemia, diabetes, smoking, obesity or overweight, and lack of physical activity.
The Task Force has previously recommended intensive behavioral interventions to improve nutrition and physical activity in those who are overweight/obese and in those with abnormal blood glucose levels,9 and has addressed smoking prevention and cessation.4 This new recommendation applies to those with other CVD risks such as high blood pressure and/or hyperlipidemia and those with an estimated 10-year CVD risk of ≥ 7.5%.10
Continue to: Behavioral interventions...
Behavioral interventions included in the Task Force analysis employed a median of 12 contacts and an estimated 6 hours of contact time over 6 to 18 months.10 Most interventions involved motivational interviewing and instruction on behavioral change methods. These interventions can be provided by primary care clinicians, as well as a wide range of other trained professionals. The Affordable Care Act dictates that all “A” and “B” recommendations must be provided by commercial health plans at no out-of-pocket expense for the patient.
Nutritional advice should include reductions in saturated fats, salt, and sugars and increases in fruits, vegetables, and whole grains. The Mediterranean diet and the Dietary Approaches to Stop Hypertension (DASH) diet are often recommended.10 Physical activity counseling should advocate for 90 to 180 minutes per week of moderate to vigorous activity.
This new recommendation, along with the previous ones pertaining to behavioral interventions for lifestyle changes, make it clear that intensive interventions are needed to achieve meaningful change. Simple advice from a clinician will have little to no effect.
Task Force reviews evidence on HTN, smoking cessation in young people
In 2020 the Task Force completed reviews of evidence relevant to screening for high blood pressure11 and
The 2 “I” statements are in disagreement with recommendations of other professional organizations. The American Academy of Pediatrics (AAP) and the American Heart Association recommend routine screening for high blood pressure starting at age 3 years. And the AAP recommends screening teenagers for tobacco use and offering tobacco dependence treatment, referral, or both (including pharmacotherapy) when indicated. E-cigarettes are not recommended as a treatment for tobacco dependence.20
Continue to: The difference between...
The difference between the methods used by the Task Force and other guideline-producing organizations becomes apparent when it comes to recommendations pertaining to children and adolescents, for whom long-term outcome-oriented studies on prevention issues are rare. The Task Force is unwilling to make recommendations when evidence does not exist. The AAP often makes recommendations based on expert opinion consensus in such situations. One notable part of each Task Force recommendation statement is a discussion of what other organizations recommend on the same topic so that these differences can be openly described.
Better Task Force funding could expand topic coverage
It is worth revisiting 2 issues that were pointed out in last year’s USPSTF summary in this column.1 First, the Task Force methods are robust and evidence based, and recommendations therefore are rarely changed once they are made at an “A”, “B”, or “D” level. Second, Task Force resources are finite, and thus, the group is currently unable to update previous recommendations with greater frequency or to consider many new topics. In the past 2 years, the Task Force has developed recommendations on only 2 completely new topics. Hopefully, its budget can be expanded so that new topics can be added in the future.
1. Campos-Outcalt D. USPSTF roundup. J Fam Pract. 2020;69:201-204.
2. USPSTF. Screening for asymptomatic carotid artery stenosis. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/carotid-artery-stenosis-screening
3. USPSTF. Interventions for tobacco smoking cessation in adults, including pregnant persons. Accessed April 30, 2021. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions
4. USPSTF. Interventions for tobacco smoking cessation in adults, including pregnant persons. JAMA. 2021;325:265-279.
5. USPSTF. Screening for Hepatitis B virus infection in adolescents and adults. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-b-virus-infection-screening
6. USPSTF. Hepatitis B virus infection in pregnant women: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-b-virus-infection-in-pregnant-women-screening
7. USPSTF. Hepatitis C virus infection in adolescents and adults: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-c-screening
8. USPSTF; Owens DK, Davidson KW, Krisk AH, et al. Screening for bacterial vaginosis in pregnant persons to prevent preterm delivery: US Preventive Services Task Force recommendation statement. JAMA. 2020;323:1286-1292.
9. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:587-593.
10. USPSTF. Behavioral counseling interventions to promote a healthy and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: US Preventive Services Task Force recommendation statement. JAMA. 2020;324:2069-2075.
11. USPSTF. High blood pressure in children and adolescents: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/blood-pressure-in-children-and-adolescents-hypertension-screening
12. USPSTF. Prevention and cessation of tobacco use in children and adolescents: primary care interventions. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions
13. USPSTF. Cognitive impairment in older adults: screening. Accessed March 26, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/cognitive-impairment-in-older-adults-screening
14. USPSTF. Illicit drug use in children, adolescents, and young adults: primary care-based interventions. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-primary-care-interventions-for-children-and-adolescents
15. USPSTF. Unhealthy drug use: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-screening
16. USPSTF. Sexually transmitted infections: behavioral counseling. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/sexually-transmitted-infections-behavioral-counseling.
17. Campos-Outcalt D. USPSTF update on sexually transmitted infections. J Fam Pract. 2020;69:514-517.
18. Brott TG, Halperin JL, Abbara S, et al; ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease. Catheter Cardiovasc Interv. 2013;81:E76-E123.
19. Ricotta JJ, Aburahma A, Ascher E, et al; Society for Vascular Surgery. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease. J Vasc Surg. 2011;54:e1-e31.
20. Farber HJ, Walley SC, Groner JA, et al; Section on Tobacco Control. Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics. 2015;136:1008-1017.
1. Campos-Outcalt D. USPSTF roundup. J Fam Pract. 2020;69:201-204.
2. USPSTF. Screening for asymptomatic carotid artery stenosis. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/carotid-artery-stenosis-screening
3. USPSTF. Interventions for tobacco smoking cessation in adults, including pregnant persons. Accessed April 30, 2021. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions
4. USPSTF. Interventions for tobacco smoking cessation in adults, including pregnant persons. JAMA. 2021;325:265-279.
5. USPSTF. Screening for Hepatitis B virus infection in adolescents and adults. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-b-virus-infection-screening
6. USPSTF. Hepatitis B virus infection in pregnant women: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-b-virus-infection-in-pregnant-women-screening
7. USPSTF. Hepatitis C virus infection in adolescents and adults: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-c-screening
8. USPSTF; Owens DK, Davidson KW, Krisk AH, et al. Screening for bacterial vaginosis in pregnant persons to prevent preterm delivery: US Preventive Services Task Force recommendation statement. JAMA. 2020;323:1286-1292.
9. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:587-593.
10. USPSTF. Behavioral counseling interventions to promote a healthy and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: US Preventive Services Task Force recommendation statement. JAMA. 2020;324:2069-2075.
11. USPSTF. High blood pressure in children and adolescents: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/blood-pressure-in-children-and-adolescents-hypertension-screening
12. USPSTF. Prevention and cessation of tobacco use in children and adolescents: primary care interventions. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions
13. USPSTF. Cognitive impairment in older adults: screening. Accessed March 26, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/cognitive-impairment-in-older-adults-screening
14. USPSTF. Illicit drug use in children, adolescents, and young adults: primary care-based interventions. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-primary-care-interventions-for-children-and-adolescents
15. USPSTF. Unhealthy drug use: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-screening
16. USPSTF. Sexually transmitted infections: behavioral counseling. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/sexually-transmitted-infections-behavioral-counseling.
17. Campos-Outcalt D. USPSTF update on sexually transmitted infections. J Fam Pract. 2020;69:514-517.
18. Brott TG, Halperin JL, Abbara S, et al; ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease. Catheter Cardiovasc Interv. 2013;81:E76-E123.
19. Ricotta JJ, Aburahma A, Ascher E, et al; Society for Vascular Surgery. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease. J Vasc Surg. 2011;54:e1-e31.
20. Farber HJ, Walley SC, Groner JA, et al; Section on Tobacco Control. Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics. 2015;136:1008-1017.
Outcomes Associated With Pharmacist- Led Consult Service for Opioid Tapering and Pharmacotherapy
In the late 1980s and early 1990s, an emphasis on better pain management led health care professionals (HCPs) to increase prescribing of opioids to better manage patient’s pain. In 1991, 76 million prescriptions were written for opioids in the United States, and by 2011, the number had nearly tripled to 219 million.1 Overdose rates increased as well, nearly tripling from 1999 to 2014.2 Of the 52,404 US deaths from drug overdoses in the in 2015, 63% involved an opioid.2
Opioid Safety Initiative
In response to the growing opioid epidemic, the US Department of Veterans Affairs (VA) created the Opioid Safety Initiative in 2014.3 This comprehensive, multifaceted initiative was designed to improve the care and safety of veterans managed with opioid therapy and promote rational opioid prescribing and monitoring. In 2016 the Centers for Disease Control and Prevention (CDC) issued guidelines for opioid prescriptions, and the following year the VA and the US Department of Defense (DoD) updated the VA/DoD Clinical Practice Guidelines for Opioid Therapy for Chronic Pain (VA/DoD guidelines).4,5 After the release of these guidelines, the use of opioid tapers expanded. However, due to public outcry of forced opioid tapering in 2019, the US Food and Drug Administration updated its opioid labeling requirements to provide clearer guidance on opioid tapers for tolerant patients.6,7
As a result, HCPs began to develop various strategies to balance the safety and efficacy of opioid use in patients with chronic pain. The West Palm Beach VA Medical Center (WPBVAMC) in Florida has a Pain Clinic that includes 2 pain management clinical pharmacy specialists (CPSs) with specialized training in pain management, who are uniquely qualified to assess and evaluate medication therapy in complex pain patient cases. These CPSs were involved in the face-to-face management of patients requiring specialized pain care and participated in a pain pharmacy electronic consult (eConsult) service to document pain management consultative recommendations for patients appropriate for management at the primary care level. This formalized process increased specialty pain care access for veterans whose pain was managed by primary care providers (PCPs).
The pain pharmacy eConsult service was initiated at the WPBVAMC in June 2013 to assist PCPs in the management of outpatients with chronic pain. The eConsult service includes evaluation of a patient’s electronic health records (EHRs) by CPSs. The eConsult service also provided PCPs with the option to engage a pharmacist who could provide recommendations for opioid dosing conversion, opioid tapering, pain pharmacotherapy, or drug screen interpretation, without the necessity for an additional patient visit.
Subsequent to the release of the 2016 CDC (and later the 2017 VA/DoD) guidelines recommending reducing morphine equivalent daily dose (MEDD) levels, the WPBVAMC had a large increase in pain eConsult requests for opioid tapering and opioid pharmacotherapy. A 3.4-fold increase in requests occurred in March, April, and May vs the following 9 months, and a nearly 4-fold increase in requests for opioid tapers during the same period. However, the impact of the completed eConsults was unclear. Therefore, the primary objective of this study was to assess the effect of CPS services for opioid tapering and opioid pharmacotherapy by quantifying the number of recommendations accepted/implemented by PCPs. The secondary objectives included evaluating harms associated with the recommendations (eg, increase in visits to the emergency department [ED], hospitalizations, suicide attempts, or PCP visits) and provider satisfaction.
Methods
A retrospective chart review was completed to assess data of patients from the WPBVAMC and its associated community-based outpatient clinics (CBOCs). The project was approved by the WPBVAMC Scientific Advisory Committee as part of the facility’s performance improvement efforts.
Included patients had a pain pharmacy eConsult placed between April 1, 2016 and March 31, 2017. EHRs were reviewed and only eConsults for opioid pharmacotherapy recommendation or opioid tapers were evaluated. eConsults were excluded if the request was discontinued, completed by a HCP other than the pain CPS, or placed for an opioid dose conversion, nonopioid pharmacotherapy, or drug screen interpretation.
Data for analyses were entered into Microsoft Excel 2016 and were securely saved and accessible to relevant researchers. Patient protected health information used during patient care remained confidential.
Demographic data were collected, including age, gender, race, pertinent medical comorbidities (eg, diabetes mellitus, sleep apnea), and mental health comorbidities. Pain scores were collected at baseline and 6-months postconsult. Pain medications used by patients were noted at baseline and 6 months postconsult, including concomitant opioid and benzodiazepine use, MEDD, and other pain medication. The duration of time needed by pain CPS to complete each eConsult and total time from eConsult entered to HCP implementation of the initial recommendation was collected. The number of actionable recommendations (eg, changes in drug therapy, urine drug screens [UDSs], and referrals to other services also were recorded and reviewed 6 months postconsult to determine the number and percentage of recommendations implemented by the HCP. The EHR was examined to determine adverse events (AEs) (eg, any documentation of suicide attempt, calls to the Veterans Crisis Line, or death 6 month postconsult). Collected data also included new eConsults, the reason for opioid tapering either by HCP or patient, and assessment of economic harms (count of the number of visits to ED, hospitalizations, or unscheduled PCP visits with uncontrolled pain as chief reason within 6 months postconsult). Last, PCPs were sent a survey to assess their satisfaction with the pain eConsult service.
Results
Of 517 eConsults received from April 1, 2016 to March 31, 2017, 285 (55.1%) met inclusion criteria (Figure). Using a random number generator, 100 eConsults were further reviewed for outcomes of interest.
In this cohort, the mean age was 61 years, 87% were male, and 80% were White individuals. Most patients (83%) had ≥ 1 mental health comorbidity, and 53% had ≥ 2, with depressive symptoms, tobacco use, and/or posttraumatic stress disorder the most common diagnoses (Table 1). Eighty-seven percent of eConsults were for opioid tapers and the remaining 13% were for opioid pharmacotherapy.
The median pain score at time of consult was 6 on a 10-point scale, with no change at 6 months postconsult. However, 41% of patients overall had a median 3.3-point drop in pain score, 17% had no change in pain score, and 42% had a median 2.6-point increase in pain score.
At time of consult, 24% of patients had an opioid and benzodiazepine prescribed concurrently. At the time of the initial request, the mean MEDD was 177.5 mg (median, 165; range, 0-577.5). At 6 months postconsult, the average MEDD was 71 mg (median, 90; range, 0-450) for a mean 44% MEDD decrease. Eighteen percent of patients had no change in MEDD, and 5% had an increase.
One concern was the number of patients whose pain management regimen consisted of either opioids as monotherapy or a combination of opioids and skeletal muscle relaxants (SMRs), which can increase the opioid overdose risk and are not indicated for long-term use (except for baclofen for spasticity). Thirty-five percent of patients were taking either opioid monotherapy or opioids and SMRs for chronic pain management at time of consult and 28% were taking opioid monotherapy or opioids and SMRs 6 months postconsult.
Electronic Consults
Table 2 describes the reasons eConsults were requested. The most common reason was to taper the dose to be in compliance with the CDC 2016 guideline recommendation of MEDD < 90 mg, which was later increased to 100 mg by the VA/DoD guideline.
On average, eConsults were completed within a mean of 11.5 days of the PCP request, including nights and weekends. The CPS spent a mean 66.8 minutes to complete each eConsult. Once the eConsult was completed, PCPs took a mean of 9 days to initiate the primary recommendation. This 9-day average does not include 11 eConsults with no accepted recommendations and 11 eConsults for which the PCP implemented the primary recommendation before the CPS completed the consult, most likely due to a phone call or direct contact with the CPS at the time the eConsult was ordered.
A mean 3.5 actionable recommendations were made by the CPS and a mean 1.6 recommendations were implemented within 6 months by the PCP. At least 1 recommendation was accepted/implemented for 89% of patients, with a mean 55% recommendations that were accepted/implemented. Eleven percent of the eConsult final recommendations were not accepted by PCPs and clear documentation of the reasons were not provided.
Adverse Outcomes
In the 6 months postconsult, 11 patients (7 men and 4 women) experienced 32 AEs (Table 3). Eight patients had 15 ED visits, with 3 of the visits resulting in hospitalizations, 8 patients had 9 unscheduled PCP visits, 1 patient reported suicidal ideation and 2 patients made a total of 4 calls to the Veterans Crisis Line. There were also 2 deaths; however, both were due to end-stage disease (cirrhosis and amyotrophic lateral sclerosis) and not believed to be related to eConsult recommendations.
Eight patients had a history of substance use disorders (SUDs) and 8 had a history of a mood disorder or psychosis. One patient had both SUD and a mood/psychosis-related mental health disorder, including a reported suicidal attempt/ideation at an ED visit and a subsequent hospitalization. A similar number of AEs occurred in patients with decreases in MEDD of 0 to 24% compared with those that received more aggressive tapers of 75 to 100% (Table 4).
Nine patients were reconsulted, with only 1 secondary to the PCP not implementing recommendations from the initial consult. No factors were found that correlated with likelihood of a patient being reconsulted.
Surveys on PCP satisfaction with the eConsult service were completed by 29 of the 55 PCPs. PCP feedback was generally positive with nearly 90% of PCPs planning to use the service in the future as well as recommending use to other providers.
PCPs also were given the option to indicate the most important factor for overall satisfaction with eConsult service (time, access, safety, expectations or confidence). Safety was provider’s top choice with time being a close second.
Discussion
Most (89%) PCPs accepted at least 1 recommendation from the completed eConsult, and MEDDs decreased by 60%, likely reducing the patient’s risk of overdose or other AEs from opioids. There also was a slight reduction in patient’s mean pain scores; however, 41% had a decrease and 42% had an increase in pain scores. There was no clear relationship when pain scores were compared with MEDDs, likely giving credence to the idea that pain scores are largely subjective and an unreliable surrogate marker for assessing effectiveness of analgesic regimens.
Eleven patients experienced AEs, including 1 patient for whom the recommendations were not implemented by the PCP. Eight of the 11 had multiple AEs. One interesting finding was that 7 of the 11 patients with an AE tested positive for unexpected substances on routine UDS or were arrested for driving while intoxicated (DWI). However, only 3 of the 7 had an active SUD diagnosis. With 25% of the AEs coming from patients with a history of SUD, it is important that any history of SUD be documented in the EHR. Maintaining this documentation can be especially difficult if patients switch VA medical centers or receive services outside the VA. Thorough and accurate history and chart review should ideally be completed before prescribing opioids.
Guidelines
While the PCPs were following VA/DoD and CDC recommendations for opioid tapering to < 100 or 90 mg MEDD, respectively, there is weak evidence in these guidelines to support specific MEDD cutoffs. The CDC guidelines even state, “a single dosage threshold for safe opioid use could not be identified.”5 One of the largest issues when using MEDD as a cutoff is the lack of agreement on its calculation. In 2014, Nuckols and colleagues al conducted a study to compare the existing guidelines on the use of opioids for chronic pain. While 13 guidelines were considered eligible, most recommendations were supported only by observational data or expert recommendations, and there was no consensus on what constitutes a “morphine equivalent.”8 Currently there is no universally accepted opioid-conversion method, resulting in a substantial problem when calculating a MEDD.9 A survey of 8 online opioid dose conversion tools found a -55% to +242% variation.10 As Fudin and colleagues concluded in response to the large variations found in these various analyses, the studies “unequivocally disqualify the validity of embracing MEDD to assess risk in any meaningful statistical way.”11 Pharmacogenetics, drug tolerance, drug-drug interactions, body surface area, and organ function are patient- specific factors that are not taken into consideration when relying solely on a MEDD calculation. Tapering to lowest functional dose rather than a specific number or cutoff may be a more effective way to treat patients, and providers should use the guidelines as recommendations and not a hardline mandate.
At 6 months, 6 patients were receiving no pain medications from the VA, and 24 of the patients were tapered from their opiate to discontinuation. It is unclear whether patients are no longer taking opioids or switched their care to non-VA providers to receive medications, including opioids, privately. This is difficult to verify, though a prescription drug monitoring program (PDMP) could be used to assess patient adherence. As many of the patients that were tapered due to identification of aberrant behaviors, lack of continuity of care across health care systems may result in future patient harm.
The results of this analysis highlight the importance of checking PDMP databases and routine UDSs when prescribing opioids—there can be serious safety concerns if patients are taking other prescribed or illicit medications. However, care must be taken; there were 2 instances of patients’ chronic opioid prescriptions discontinued by their VA provider after a review of the PDMP showed they had received non-VA opioids. In both cases, the quantity and doses received were small (counts of ≤ 12) and were received more than 6 months prior to the check of the PDMP. While this constitutes a breach of the Informed Consent for long-term opioid use, if there are no other concerning behaviors, it may be more prudent to review the informed consent with the patient and discuss why the behavior is a breach to ensure that patients and PCPs continue to work as a team to manage chronic pain.
Limitations
The study population was one limitation of this project. While data suggest that chronic pain affects women more than men, this study’s population was only 13% female. Thirty percent of the women in this study had an AE compared with only 8% of the men. Additional limitations included use of problem list for comorbidities, as lists may be inaccurate or outdated, and limiting the monitoring of AE to only 6 months. As some tapers were not initiated immediately and some taper schedules can last several months to years; therefor, outcomes may have been higher if patients were followed longer. Many of the patients with AEs had increased ED visits or unscheduled primary care visits as the tapers went on and their pain worsened, but the visits were outside the 6-month time frame for data collection. An additional weakness of this review included assessing a pain score, but not functional status, which may be a better predictor of the effectiveness of a patient’s pain management regimen. This assessment is needed in future studies for more reliable data. Finally, PCP survey results also should be viewed with caution. The current survey had only 29 respondents, and the 2014 survey had only 10 respondents and did not include CBOC providers.
Conclusion
A pain eConsult service managed by CPSs specializing in pain management can assist patients and PCPs with opioid therapy recommendations in a safe and timely manner, reducing risk of overdose secondary to high dose opioid therapy and with limited harm to patients.
1. National Institute on Drug Abuse. Increased drug availability is associated with increased use and overdose. Published June 9, 2020. Accessed February 19, 2021. https://www.drugabuse.gov/publications/research-reports/prescription-opioids-heroin/increased-drug-availability-associated-increased-use-overdose
2. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452. Published 2016 Dec 30.doi:10.15585/mmwr.mm655051e1
3. US Department of Veterans Affairs, Office of Inspector General. Healthcare inspection – VA patterns of dispensing take-home opioids and monitoring patients on opioid therapy. Report 14-00895-163. Published May 14, 2014. Accessed February 2, 2021. https://www.va.gov/oig/pubs/VAOIG-14-00895-163.pdf
4. US Department of Veterans Affairs, US Department of Defense, Opioid Therapy for Chronic Pain Work Group. VA/DoD clinical practice guidelines for opioid therapy for chronic pain. Version 3.0. Published December 2017. Accessed February 2, 2021. https://www.va.gov/HOMELESS/nchav/resources/docs/mental-health/substance-abuse/VA_DoD-CLINICAL-PRACTICE-GUIDELINE-FOR-OPIOID-THERAPY-FOR-CHRONIC-PAIN-508.pdf
5. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain - United States, 2016 [published correction appears in MMWR Recomm Rep. 2016;65(11):295]. MMWR Recomm Rep. 2016;65(1):1-49. Published 2016 Mar 18. doi:10.15585/mmwr.rr6501e1.
6. US Food and Drug Administration. (2019). FDA identifies harm reported from sudden discontinuation of opioid pain medicines and requires label changes to guide prescribers on gradual, individualized tapering. Updated April 17, 2019. Accessed February 2, 2021. https://www.fda.gov/drugs/fda-drug-safety-podcasts/fda-identifies-harm-reported-sudden-discontinuation-opioid-pain-medicines-and-requires-label-changes
7. Dowell D, Haegerich T, Chou R. No Shortcuts to Safer Opioid Prescribing. N Engl J Med. 2019;380(24):2285-2287. doi:10.1056/NEJMp1904190
8. Nuckols TK, Anderson L, Popescu I, et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160(1):38-47. doi:10.7326/0003-4819-160-1-201401070-00732
9. Rennick A, Atkinson T, Cimino NM, Strassels SA, McPherson ML, Fudin J. Variability in Opioid Equivalence Calculations. Pain Med. 2016;17(5):892-898. doi:10.1111/pme.12920
10. Shaw K, Fudin J. Evaluation and comparison of online equianalgesic opioid dose conversion calculators. Pract Pain Manag. 2013;13(7):61-66.
11. Fudin J, Pratt Cleary J, Schatman ME. The MEDD myth: the impact of pseudoscience on pain research and prescribing-guideline development. J Pain Res. 2016;9:153-156. Published 2016 Mar 23. doi:10.2147/JPR.S107794
In the late 1980s and early 1990s, an emphasis on better pain management led health care professionals (HCPs) to increase prescribing of opioids to better manage patient’s pain. In 1991, 76 million prescriptions were written for opioids in the United States, and by 2011, the number had nearly tripled to 219 million.1 Overdose rates increased as well, nearly tripling from 1999 to 2014.2 Of the 52,404 US deaths from drug overdoses in the in 2015, 63% involved an opioid.2
Opioid Safety Initiative
In response to the growing opioid epidemic, the US Department of Veterans Affairs (VA) created the Opioid Safety Initiative in 2014.3 This comprehensive, multifaceted initiative was designed to improve the care and safety of veterans managed with opioid therapy and promote rational opioid prescribing and monitoring. In 2016 the Centers for Disease Control and Prevention (CDC) issued guidelines for opioid prescriptions, and the following year the VA and the US Department of Defense (DoD) updated the VA/DoD Clinical Practice Guidelines for Opioid Therapy for Chronic Pain (VA/DoD guidelines).4,5 After the release of these guidelines, the use of opioid tapers expanded. However, due to public outcry of forced opioid tapering in 2019, the US Food and Drug Administration updated its opioid labeling requirements to provide clearer guidance on opioid tapers for tolerant patients.6,7
As a result, HCPs began to develop various strategies to balance the safety and efficacy of opioid use in patients with chronic pain. The West Palm Beach VA Medical Center (WPBVAMC) in Florida has a Pain Clinic that includes 2 pain management clinical pharmacy specialists (CPSs) with specialized training in pain management, who are uniquely qualified to assess and evaluate medication therapy in complex pain patient cases. These CPSs were involved in the face-to-face management of patients requiring specialized pain care and participated in a pain pharmacy electronic consult (eConsult) service to document pain management consultative recommendations for patients appropriate for management at the primary care level. This formalized process increased specialty pain care access for veterans whose pain was managed by primary care providers (PCPs).
The pain pharmacy eConsult service was initiated at the WPBVAMC in June 2013 to assist PCPs in the management of outpatients with chronic pain. The eConsult service includes evaluation of a patient’s electronic health records (EHRs) by CPSs. The eConsult service also provided PCPs with the option to engage a pharmacist who could provide recommendations for opioid dosing conversion, opioid tapering, pain pharmacotherapy, or drug screen interpretation, without the necessity for an additional patient visit.
Subsequent to the release of the 2016 CDC (and later the 2017 VA/DoD) guidelines recommending reducing morphine equivalent daily dose (MEDD) levels, the WPBVAMC had a large increase in pain eConsult requests for opioid tapering and opioid pharmacotherapy. A 3.4-fold increase in requests occurred in March, April, and May vs the following 9 months, and a nearly 4-fold increase in requests for opioid tapers during the same period. However, the impact of the completed eConsults was unclear. Therefore, the primary objective of this study was to assess the effect of CPS services for opioid tapering and opioid pharmacotherapy by quantifying the number of recommendations accepted/implemented by PCPs. The secondary objectives included evaluating harms associated with the recommendations (eg, increase in visits to the emergency department [ED], hospitalizations, suicide attempts, or PCP visits) and provider satisfaction.
Methods
A retrospective chart review was completed to assess data of patients from the WPBVAMC and its associated community-based outpatient clinics (CBOCs). The project was approved by the WPBVAMC Scientific Advisory Committee as part of the facility’s performance improvement efforts.
Included patients had a pain pharmacy eConsult placed between April 1, 2016 and March 31, 2017. EHRs were reviewed and only eConsults for opioid pharmacotherapy recommendation or opioid tapers were evaluated. eConsults were excluded if the request was discontinued, completed by a HCP other than the pain CPS, or placed for an opioid dose conversion, nonopioid pharmacotherapy, or drug screen interpretation.
Data for analyses were entered into Microsoft Excel 2016 and were securely saved and accessible to relevant researchers. Patient protected health information used during patient care remained confidential.
Demographic data were collected, including age, gender, race, pertinent medical comorbidities (eg, diabetes mellitus, sleep apnea), and mental health comorbidities. Pain scores were collected at baseline and 6-months postconsult. Pain medications used by patients were noted at baseline and 6 months postconsult, including concomitant opioid and benzodiazepine use, MEDD, and other pain medication. The duration of time needed by pain CPS to complete each eConsult and total time from eConsult entered to HCP implementation of the initial recommendation was collected. The number of actionable recommendations (eg, changes in drug therapy, urine drug screens [UDSs], and referrals to other services also were recorded and reviewed 6 months postconsult to determine the number and percentage of recommendations implemented by the HCP. The EHR was examined to determine adverse events (AEs) (eg, any documentation of suicide attempt, calls to the Veterans Crisis Line, or death 6 month postconsult). Collected data also included new eConsults, the reason for opioid tapering either by HCP or patient, and assessment of economic harms (count of the number of visits to ED, hospitalizations, or unscheduled PCP visits with uncontrolled pain as chief reason within 6 months postconsult). Last, PCPs were sent a survey to assess their satisfaction with the pain eConsult service.
Results
Of 517 eConsults received from April 1, 2016 to March 31, 2017, 285 (55.1%) met inclusion criteria (Figure). Using a random number generator, 100 eConsults were further reviewed for outcomes of interest.
In this cohort, the mean age was 61 years, 87% were male, and 80% were White individuals. Most patients (83%) had ≥ 1 mental health comorbidity, and 53% had ≥ 2, with depressive symptoms, tobacco use, and/or posttraumatic stress disorder the most common diagnoses (Table 1). Eighty-seven percent of eConsults were for opioid tapers and the remaining 13% were for opioid pharmacotherapy.
The median pain score at time of consult was 6 on a 10-point scale, with no change at 6 months postconsult. However, 41% of patients overall had a median 3.3-point drop in pain score, 17% had no change in pain score, and 42% had a median 2.6-point increase in pain score.
At time of consult, 24% of patients had an opioid and benzodiazepine prescribed concurrently. At the time of the initial request, the mean MEDD was 177.5 mg (median, 165; range, 0-577.5). At 6 months postconsult, the average MEDD was 71 mg (median, 90; range, 0-450) for a mean 44% MEDD decrease. Eighteen percent of patients had no change in MEDD, and 5% had an increase.
One concern was the number of patients whose pain management regimen consisted of either opioids as monotherapy or a combination of opioids and skeletal muscle relaxants (SMRs), which can increase the opioid overdose risk and are not indicated for long-term use (except for baclofen for spasticity). Thirty-five percent of patients were taking either opioid monotherapy or opioids and SMRs for chronic pain management at time of consult and 28% were taking opioid monotherapy or opioids and SMRs 6 months postconsult.
Electronic Consults
Table 2 describes the reasons eConsults were requested. The most common reason was to taper the dose to be in compliance with the CDC 2016 guideline recommendation of MEDD < 90 mg, which was later increased to 100 mg by the VA/DoD guideline.
On average, eConsults were completed within a mean of 11.5 days of the PCP request, including nights and weekends. The CPS spent a mean 66.8 minutes to complete each eConsult. Once the eConsult was completed, PCPs took a mean of 9 days to initiate the primary recommendation. This 9-day average does not include 11 eConsults with no accepted recommendations and 11 eConsults for which the PCP implemented the primary recommendation before the CPS completed the consult, most likely due to a phone call or direct contact with the CPS at the time the eConsult was ordered.
A mean 3.5 actionable recommendations were made by the CPS and a mean 1.6 recommendations were implemented within 6 months by the PCP. At least 1 recommendation was accepted/implemented for 89% of patients, with a mean 55% recommendations that were accepted/implemented. Eleven percent of the eConsult final recommendations were not accepted by PCPs and clear documentation of the reasons were not provided.
Adverse Outcomes
In the 6 months postconsult, 11 patients (7 men and 4 women) experienced 32 AEs (Table 3). Eight patients had 15 ED visits, with 3 of the visits resulting in hospitalizations, 8 patients had 9 unscheduled PCP visits, 1 patient reported suicidal ideation and 2 patients made a total of 4 calls to the Veterans Crisis Line. There were also 2 deaths; however, both were due to end-stage disease (cirrhosis and amyotrophic lateral sclerosis) and not believed to be related to eConsult recommendations.
Eight patients had a history of substance use disorders (SUDs) and 8 had a history of a mood disorder or psychosis. One patient had both SUD and a mood/psychosis-related mental health disorder, including a reported suicidal attempt/ideation at an ED visit and a subsequent hospitalization. A similar number of AEs occurred in patients with decreases in MEDD of 0 to 24% compared with those that received more aggressive tapers of 75 to 100% (Table 4).
Nine patients were reconsulted, with only 1 secondary to the PCP not implementing recommendations from the initial consult. No factors were found that correlated with likelihood of a patient being reconsulted.
Surveys on PCP satisfaction with the eConsult service were completed by 29 of the 55 PCPs. PCP feedback was generally positive with nearly 90% of PCPs planning to use the service in the future as well as recommending use to other providers.
PCPs also were given the option to indicate the most important factor for overall satisfaction with eConsult service (time, access, safety, expectations or confidence). Safety was provider’s top choice with time being a close second.
Discussion
Most (89%) PCPs accepted at least 1 recommendation from the completed eConsult, and MEDDs decreased by 60%, likely reducing the patient’s risk of overdose or other AEs from opioids. There also was a slight reduction in patient’s mean pain scores; however, 41% had a decrease and 42% had an increase in pain scores. There was no clear relationship when pain scores were compared with MEDDs, likely giving credence to the idea that pain scores are largely subjective and an unreliable surrogate marker for assessing effectiveness of analgesic regimens.
Eleven patients experienced AEs, including 1 patient for whom the recommendations were not implemented by the PCP. Eight of the 11 had multiple AEs. One interesting finding was that 7 of the 11 patients with an AE tested positive for unexpected substances on routine UDS or were arrested for driving while intoxicated (DWI). However, only 3 of the 7 had an active SUD diagnosis. With 25% of the AEs coming from patients with a history of SUD, it is important that any history of SUD be documented in the EHR. Maintaining this documentation can be especially difficult if patients switch VA medical centers or receive services outside the VA. Thorough and accurate history and chart review should ideally be completed before prescribing opioids.
Guidelines
While the PCPs were following VA/DoD and CDC recommendations for opioid tapering to < 100 or 90 mg MEDD, respectively, there is weak evidence in these guidelines to support specific MEDD cutoffs. The CDC guidelines even state, “a single dosage threshold for safe opioid use could not be identified.”5 One of the largest issues when using MEDD as a cutoff is the lack of agreement on its calculation. In 2014, Nuckols and colleagues al conducted a study to compare the existing guidelines on the use of opioids for chronic pain. While 13 guidelines were considered eligible, most recommendations were supported only by observational data or expert recommendations, and there was no consensus on what constitutes a “morphine equivalent.”8 Currently there is no universally accepted opioid-conversion method, resulting in a substantial problem when calculating a MEDD.9 A survey of 8 online opioid dose conversion tools found a -55% to +242% variation.10 As Fudin and colleagues concluded in response to the large variations found in these various analyses, the studies “unequivocally disqualify the validity of embracing MEDD to assess risk in any meaningful statistical way.”11 Pharmacogenetics, drug tolerance, drug-drug interactions, body surface area, and organ function are patient- specific factors that are not taken into consideration when relying solely on a MEDD calculation. Tapering to lowest functional dose rather than a specific number or cutoff may be a more effective way to treat patients, and providers should use the guidelines as recommendations and not a hardline mandate.
At 6 months, 6 patients were receiving no pain medications from the VA, and 24 of the patients were tapered from their opiate to discontinuation. It is unclear whether patients are no longer taking opioids or switched their care to non-VA providers to receive medications, including opioids, privately. This is difficult to verify, though a prescription drug monitoring program (PDMP) could be used to assess patient adherence. As many of the patients that were tapered due to identification of aberrant behaviors, lack of continuity of care across health care systems may result in future patient harm.
The results of this analysis highlight the importance of checking PDMP databases and routine UDSs when prescribing opioids—there can be serious safety concerns if patients are taking other prescribed or illicit medications. However, care must be taken; there were 2 instances of patients’ chronic opioid prescriptions discontinued by their VA provider after a review of the PDMP showed they had received non-VA opioids. In both cases, the quantity and doses received were small (counts of ≤ 12) and were received more than 6 months prior to the check of the PDMP. While this constitutes a breach of the Informed Consent for long-term opioid use, if there are no other concerning behaviors, it may be more prudent to review the informed consent with the patient and discuss why the behavior is a breach to ensure that patients and PCPs continue to work as a team to manage chronic pain.
Limitations
The study population was one limitation of this project. While data suggest that chronic pain affects women more than men, this study’s population was only 13% female. Thirty percent of the women in this study had an AE compared with only 8% of the men. Additional limitations included use of problem list for comorbidities, as lists may be inaccurate or outdated, and limiting the monitoring of AE to only 6 months. As some tapers were not initiated immediately and some taper schedules can last several months to years; therefor, outcomes may have been higher if patients were followed longer. Many of the patients with AEs had increased ED visits or unscheduled primary care visits as the tapers went on and their pain worsened, but the visits were outside the 6-month time frame for data collection. An additional weakness of this review included assessing a pain score, but not functional status, which may be a better predictor of the effectiveness of a patient’s pain management regimen. This assessment is needed in future studies for more reliable data. Finally, PCP survey results also should be viewed with caution. The current survey had only 29 respondents, and the 2014 survey had only 10 respondents and did not include CBOC providers.
Conclusion
A pain eConsult service managed by CPSs specializing in pain management can assist patients and PCPs with opioid therapy recommendations in a safe and timely manner, reducing risk of overdose secondary to high dose opioid therapy and with limited harm to patients.
In the late 1980s and early 1990s, an emphasis on better pain management led health care professionals (HCPs) to increase prescribing of opioids to better manage patient’s pain. In 1991, 76 million prescriptions were written for opioids in the United States, and by 2011, the number had nearly tripled to 219 million.1 Overdose rates increased as well, nearly tripling from 1999 to 2014.2 Of the 52,404 US deaths from drug overdoses in the in 2015, 63% involved an opioid.2
Opioid Safety Initiative
In response to the growing opioid epidemic, the US Department of Veterans Affairs (VA) created the Opioid Safety Initiative in 2014.3 This comprehensive, multifaceted initiative was designed to improve the care and safety of veterans managed with opioid therapy and promote rational opioid prescribing and monitoring. In 2016 the Centers for Disease Control and Prevention (CDC) issued guidelines for opioid prescriptions, and the following year the VA and the US Department of Defense (DoD) updated the VA/DoD Clinical Practice Guidelines for Opioid Therapy for Chronic Pain (VA/DoD guidelines).4,5 After the release of these guidelines, the use of opioid tapers expanded. However, due to public outcry of forced opioid tapering in 2019, the US Food and Drug Administration updated its opioid labeling requirements to provide clearer guidance on opioid tapers for tolerant patients.6,7
As a result, HCPs began to develop various strategies to balance the safety and efficacy of opioid use in patients with chronic pain. The West Palm Beach VA Medical Center (WPBVAMC) in Florida has a Pain Clinic that includes 2 pain management clinical pharmacy specialists (CPSs) with specialized training in pain management, who are uniquely qualified to assess and evaluate medication therapy in complex pain patient cases. These CPSs were involved in the face-to-face management of patients requiring specialized pain care and participated in a pain pharmacy electronic consult (eConsult) service to document pain management consultative recommendations for patients appropriate for management at the primary care level. This formalized process increased specialty pain care access for veterans whose pain was managed by primary care providers (PCPs).
The pain pharmacy eConsult service was initiated at the WPBVAMC in June 2013 to assist PCPs in the management of outpatients with chronic pain. The eConsult service includes evaluation of a patient’s electronic health records (EHRs) by CPSs. The eConsult service also provided PCPs with the option to engage a pharmacist who could provide recommendations for opioid dosing conversion, opioid tapering, pain pharmacotherapy, or drug screen interpretation, without the necessity for an additional patient visit.
Subsequent to the release of the 2016 CDC (and later the 2017 VA/DoD) guidelines recommending reducing morphine equivalent daily dose (MEDD) levels, the WPBVAMC had a large increase in pain eConsult requests for opioid tapering and opioid pharmacotherapy. A 3.4-fold increase in requests occurred in March, April, and May vs the following 9 months, and a nearly 4-fold increase in requests for opioid tapers during the same period. However, the impact of the completed eConsults was unclear. Therefore, the primary objective of this study was to assess the effect of CPS services for opioid tapering and opioid pharmacotherapy by quantifying the number of recommendations accepted/implemented by PCPs. The secondary objectives included evaluating harms associated with the recommendations (eg, increase in visits to the emergency department [ED], hospitalizations, suicide attempts, or PCP visits) and provider satisfaction.
Methods
A retrospective chart review was completed to assess data of patients from the WPBVAMC and its associated community-based outpatient clinics (CBOCs). The project was approved by the WPBVAMC Scientific Advisory Committee as part of the facility’s performance improvement efforts.
Included patients had a pain pharmacy eConsult placed between April 1, 2016 and March 31, 2017. EHRs were reviewed and only eConsults for opioid pharmacotherapy recommendation or opioid tapers were evaluated. eConsults were excluded if the request was discontinued, completed by a HCP other than the pain CPS, or placed for an opioid dose conversion, nonopioid pharmacotherapy, or drug screen interpretation.
Data for analyses were entered into Microsoft Excel 2016 and were securely saved and accessible to relevant researchers. Patient protected health information used during patient care remained confidential.
Demographic data were collected, including age, gender, race, pertinent medical comorbidities (eg, diabetes mellitus, sleep apnea), and mental health comorbidities. Pain scores were collected at baseline and 6-months postconsult. Pain medications used by patients were noted at baseline and 6 months postconsult, including concomitant opioid and benzodiazepine use, MEDD, and other pain medication. The duration of time needed by pain CPS to complete each eConsult and total time from eConsult entered to HCP implementation of the initial recommendation was collected. The number of actionable recommendations (eg, changes in drug therapy, urine drug screens [UDSs], and referrals to other services also were recorded and reviewed 6 months postconsult to determine the number and percentage of recommendations implemented by the HCP. The EHR was examined to determine adverse events (AEs) (eg, any documentation of suicide attempt, calls to the Veterans Crisis Line, or death 6 month postconsult). Collected data also included new eConsults, the reason for opioid tapering either by HCP or patient, and assessment of economic harms (count of the number of visits to ED, hospitalizations, or unscheduled PCP visits with uncontrolled pain as chief reason within 6 months postconsult). Last, PCPs were sent a survey to assess their satisfaction with the pain eConsult service.
Results
Of 517 eConsults received from April 1, 2016 to March 31, 2017, 285 (55.1%) met inclusion criteria (Figure). Using a random number generator, 100 eConsults were further reviewed for outcomes of interest.
In this cohort, the mean age was 61 years, 87% were male, and 80% were White individuals. Most patients (83%) had ≥ 1 mental health comorbidity, and 53% had ≥ 2, with depressive symptoms, tobacco use, and/or posttraumatic stress disorder the most common diagnoses (Table 1). Eighty-seven percent of eConsults were for opioid tapers and the remaining 13% were for opioid pharmacotherapy.
The median pain score at time of consult was 6 on a 10-point scale, with no change at 6 months postconsult. However, 41% of patients overall had a median 3.3-point drop in pain score, 17% had no change in pain score, and 42% had a median 2.6-point increase in pain score.
At time of consult, 24% of patients had an opioid and benzodiazepine prescribed concurrently. At the time of the initial request, the mean MEDD was 177.5 mg (median, 165; range, 0-577.5). At 6 months postconsult, the average MEDD was 71 mg (median, 90; range, 0-450) for a mean 44% MEDD decrease. Eighteen percent of patients had no change in MEDD, and 5% had an increase.
One concern was the number of patients whose pain management regimen consisted of either opioids as monotherapy or a combination of opioids and skeletal muscle relaxants (SMRs), which can increase the opioid overdose risk and are not indicated for long-term use (except for baclofen for spasticity). Thirty-five percent of patients were taking either opioid monotherapy or opioids and SMRs for chronic pain management at time of consult and 28% were taking opioid monotherapy or opioids and SMRs 6 months postconsult.
Electronic Consults
Table 2 describes the reasons eConsults were requested. The most common reason was to taper the dose to be in compliance with the CDC 2016 guideline recommendation of MEDD < 90 mg, which was later increased to 100 mg by the VA/DoD guideline.
On average, eConsults were completed within a mean of 11.5 days of the PCP request, including nights and weekends. The CPS spent a mean 66.8 minutes to complete each eConsult. Once the eConsult was completed, PCPs took a mean of 9 days to initiate the primary recommendation. This 9-day average does not include 11 eConsults with no accepted recommendations and 11 eConsults for which the PCP implemented the primary recommendation before the CPS completed the consult, most likely due to a phone call or direct contact with the CPS at the time the eConsult was ordered.
A mean 3.5 actionable recommendations were made by the CPS and a mean 1.6 recommendations were implemented within 6 months by the PCP. At least 1 recommendation was accepted/implemented for 89% of patients, with a mean 55% recommendations that were accepted/implemented. Eleven percent of the eConsult final recommendations were not accepted by PCPs and clear documentation of the reasons were not provided.
Adverse Outcomes
In the 6 months postconsult, 11 patients (7 men and 4 women) experienced 32 AEs (Table 3). Eight patients had 15 ED visits, with 3 of the visits resulting in hospitalizations, 8 patients had 9 unscheduled PCP visits, 1 patient reported suicidal ideation and 2 patients made a total of 4 calls to the Veterans Crisis Line. There were also 2 deaths; however, both were due to end-stage disease (cirrhosis and amyotrophic lateral sclerosis) and not believed to be related to eConsult recommendations.
Eight patients had a history of substance use disorders (SUDs) and 8 had a history of a mood disorder or psychosis. One patient had both SUD and a mood/psychosis-related mental health disorder, including a reported suicidal attempt/ideation at an ED visit and a subsequent hospitalization. A similar number of AEs occurred in patients with decreases in MEDD of 0 to 24% compared with those that received more aggressive tapers of 75 to 100% (Table 4).
Nine patients were reconsulted, with only 1 secondary to the PCP not implementing recommendations from the initial consult. No factors were found that correlated with likelihood of a patient being reconsulted.
Surveys on PCP satisfaction with the eConsult service were completed by 29 of the 55 PCPs. PCP feedback was generally positive with nearly 90% of PCPs planning to use the service in the future as well as recommending use to other providers.
PCPs also were given the option to indicate the most important factor for overall satisfaction with eConsult service (time, access, safety, expectations or confidence). Safety was provider’s top choice with time being a close second.
Discussion
Most (89%) PCPs accepted at least 1 recommendation from the completed eConsult, and MEDDs decreased by 60%, likely reducing the patient’s risk of overdose or other AEs from opioids. There also was a slight reduction in patient’s mean pain scores; however, 41% had a decrease and 42% had an increase in pain scores. There was no clear relationship when pain scores were compared with MEDDs, likely giving credence to the idea that pain scores are largely subjective and an unreliable surrogate marker for assessing effectiveness of analgesic regimens.
Eleven patients experienced AEs, including 1 patient for whom the recommendations were not implemented by the PCP. Eight of the 11 had multiple AEs. One interesting finding was that 7 of the 11 patients with an AE tested positive for unexpected substances on routine UDS or were arrested for driving while intoxicated (DWI). However, only 3 of the 7 had an active SUD diagnosis. With 25% of the AEs coming from patients with a history of SUD, it is important that any history of SUD be documented in the EHR. Maintaining this documentation can be especially difficult if patients switch VA medical centers or receive services outside the VA. Thorough and accurate history and chart review should ideally be completed before prescribing opioids.
Guidelines
While the PCPs were following VA/DoD and CDC recommendations for opioid tapering to < 100 or 90 mg MEDD, respectively, there is weak evidence in these guidelines to support specific MEDD cutoffs. The CDC guidelines even state, “a single dosage threshold for safe opioid use could not be identified.”5 One of the largest issues when using MEDD as a cutoff is the lack of agreement on its calculation. In 2014, Nuckols and colleagues al conducted a study to compare the existing guidelines on the use of opioids for chronic pain. While 13 guidelines were considered eligible, most recommendations were supported only by observational data or expert recommendations, and there was no consensus on what constitutes a “morphine equivalent.”8 Currently there is no universally accepted opioid-conversion method, resulting in a substantial problem when calculating a MEDD.9 A survey of 8 online opioid dose conversion tools found a -55% to +242% variation.10 As Fudin and colleagues concluded in response to the large variations found in these various analyses, the studies “unequivocally disqualify the validity of embracing MEDD to assess risk in any meaningful statistical way.”11 Pharmacogenetics, drug tolerance, drug-drug interactions, body surface area, and organ function are patient- specific factors that are not taken into consideration when relying solely on a MEDD calculation. Tapering to lowest functional dose rather than a specific number or cutoff may be a more effective way to treat patients, and providers should use the guidelines as recommendations and not a hardline mandate.
At 6 months, 6 patients were receiving no pain medications from the VA, and 24 of the patients were tapered from their opiate to discontinuation. It is unclear whether patients are no longer taking opioids or switched their care to non-VA providers to receive medications, including opioids, privately. This is difficult to verify, though a prescription drug monitoring program (PDMP) could be used to assess patient adherence. As many of the patients that were tapered due to identification of aberrant behaviors, lack of continuity of care across health care systems may result in future patient harm.
The results of this analysis highlight the importance of checking PDMP databases and routine UDSs when prescribing opioids—there can be serious safety concerns if patients are taking other prescribed or illicit medications. However, care must be taken; there were 2 instances of patients’ chronic opioid prescriptions discontinued by their VA provider after a review of the PDMP showed they had received non-VA opioids. In both cases, the quantity and doses received were small (counts of ≤ 12) and were received more than 6 months prior to the check of the PDMP. While this constitutes a breach of the Informed Consent for long-term opioid use, if there are no other concerning behaviors, it may be more prudent to review the informed consent with the patient and discuss why the behavior is a breach to ensure that patients and PCPs continue to work as a team to manage chronic pain.
Limitations
The study population was one limitation of this project. While data suggest that chronic pain affects women more than men, this study’s population was only 13% female. Thirty percent of the women in this study had an AE compared with only 8% of the men. Additional limitations included use of problem list for comorbidities, as lists may be inaccurate or outdated, and limiting the monitoring of AE to only 6 months. As some tapers were not initiated immediately and some taper schedules can last several months to years; therefor, outcomes may have been higher if patients were followed longer. Many of the patients with AEs had increased ED visits or unscheduled primary care visits as the tapers went on and their pain worsened, but the visits were outside the 6-month time frame for data collection. An additional weakness of this review included assessing a pain score, but not functional status, which may be a better predictor of the effectiveness of a patient’s pain management regimen. This assessment is needed in future studies for more reliable data. Finally, PCP survey results also should be viewed with caution. The current survey had only 29 respondents, and the 2014 survey had only 10 respondents and did not include CBOC providers.
Conclusion
A pain eConsult service managed by CPSs specializing in pain management can assist patients and PCPs with opioid therapy recommendations in a safe and timely manner, reducing risk of overdose secondary to high dose opioid therapy and with limited harm to patients.
1. National Institute on Drug Abuse. Increased drug availability is associated with increased use and overdose. Published June 9, 2020. Accessed February 19, 2021. https://www.drugabuse.gov/publications/research-reports/prescription-opioids-heroin/increased-drug-availability-associated-increased-use-overdose
2. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452. Published 2016 Dec 30.doi:10.15585/mmwr.mm655051e1
3. US Department of Veterans Affairs, Office of Inspector General. Healthcare inspection – VA patterns of dispensing take-home opioids and monitoring patients on opioid therapy. Report 14-00895-163. Published May 14, 2014. Accessed February 2, 2021. https://www.va.gov/oig/pubs/VAOIG-14-00895-163.pdf
4. US Department of Veterans Affairs, US Department of Defense, Opioid Therapy for Chronic Pain Work Group. VA/DoD clinical practice guidelines for opioid therapy for chronic pain. Version 3.0. Published December 2017. Accessed February 2, 2021. https://www.va.gov/HOMELESS/nchav/resources/docs/mental-health/substance-abuse/VA_DoD-CLINICAL-PRACTICE-GUIDELINE-FOR-OPIOID-THERAPY-FOR-CHRONIC-PAIN-508.pdf
5. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain - United States, 2016 [published correction appears in MMWR Recomm Rep. 2016;65(11):295]. MMWR Recomm Rep. 2016;65(1):1-49. Published 2016 Mar 18. doi:10.15585/mmwr.rr6501e1.
6. US Food and Drug Administration. (2019). FDA identifies harm reported from sudden discontinuation of opioid pain medicines and requires label changes to guide prescribers on gradual, individualized tapering. Updated April 17, 2019. Accessed February 2, 2021. https://www.fda.gov/drugs/fda-drug-safety-podcasts/fda-identifies-harm-reported-sudden-discontinuation-opioid-pain-medicines-and-requires-label-changes
7. Dowell D, Haegerich T, Chou R. No Shortcuts to Safer Opioid Prescribing. N Engl J Med. 2019;380(24):2285-2287. doi:10.1056/NEJMp1904190
8. Nuckols TK, Anderson L, Popescu I, et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160(1):38-47. doi:10.7326/0003-4819-160-1-201401070-00732
9. Rennick A, Atkinson T, Cimino NM, Strassels SA, McPherson ML, Fudin J. Variability in Opioid Equivalence Calculations. Pain Med. 2016;17(5):892-898. doi:10.1111/pme.12920
10. Shaw K, Fudin J. Evaluation and comparison of online equianalgesic opioid dose conversion calculators. Pract Pain Manag. 2013;13(7):61-66.
11. Fudin J, Pratt Cleary J, Schatman ME. The MEDD myth: the impact of pseudoscience on pain research and prescribing-guideline development. J Pain Res. 2016;9:153-156. Published 2016 Mar 23. doi:10.2147/JPR.S107794
1. National Institute on Drug Abuse. Increased drug availability is associated with increased use and overdose. Published June 9, 2020. Accessed February 19, 2021. https://www.drugabuse.gov/publications/research-reports/prescription-opioids-heroin/increased-drug-availability-associated-increased-use-overdose
2. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452. Published 2016 Dec 30.doi:10.15585/mmwr.mm655051e1
3. US Department of Veterans Affairs, Office of Inspector General. Healthcare inspection – VA patterns of dispensing take-home opioids and monitoring patients on opioid therapy. Report 14-00895-163. Published May 14, 2014. Accessed February 2, 2021. https://www.va.gov/oig/pubs/VAOIG-14-00895-163.pdf
4. US Department of Veterans Affairs, US Department of Defense, Opioid Therapy for Chronic Pain Work Group. VA/DoD clinical practice guidelines for opioid therapy for chronic pain. Version 3.0. Published December 2017. Accessed February 2, 2021. https://www.va.gov/HOMELESS/nchav/resources/docs/mental-health/substance-abuse/VA_DoD-CLINICAL-PRACTICE-GUIDELINE-FOR-OPIOID-THERAPY-FOR-CHRONIC-PAIN-508.pdf
5. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain - United States, 2016 [published correction appears in MMWR Recomm Rep. 2016;65(11):295]. MMWR Recomm Rep. 2016;65(1):1-49. Published 2016 Mar 18. doi:10.15585/mmwr.rr6501e1.
6. US Food and Drug Administration. (2019). FDA identifies harm reported from sudden discontinuation of opioid pain medicines and requires label changes to guide prescribers on gradual, individualized tapering. Updated April 17, 2019. Accessed February 2, 2021. https://www.fda.gov/drugs/fda-drug-safety-podcasts/fda-identifies-harm-reported-sudden-discontinuation-opioid-pain-medicines-and-requires-label-changes
7. Dowell D, Haegerich T, Chou R. No Shortcuts to Safer Opioid Prescribing. N Engl J Med. 2019;380(24):2285-2287. doi:10.1056/NEJMp1904190
8. Nuckols TK, Anderson L, Popescu I, et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160(1):38-47. doi:10.7326/0003-4819-160-1-201401070-00732
9. Rennick A, Atkinson T, Cimino NM, Strassels SA, McPherson ML, Fudin J. Variability in Opioid Equivalence Calculations. Pain Med. 2016;17(5):892-898. doi:10.1111/pme.12920
10. Shaw K, Fudin J. Evaluation and comparison of online equianalgesic opioid dose conversion calculators. Pract Pain Manag. 2013;13(7):61-66.
11. Fudin J, Pratt Cleary J, Schatman ME. The MEDD myth: the impact of pseudoscience on pain research and prescribing-guideline development. J Pain Res. 2016;9:153-156. Published 2016 Mar 23. doi:10.2147/JPR.S107794
Adulterants in street drugs could increase susceptibility to COVID
The composition of street drugs like heroin and cocaine are changing. According to a new analysis, almost all contain at least one toxic adulterant, and many contain a plethora. Most adulterants have pharmacologic activities and toxicities. Their presence has added impact in the context of the COVID-19 pandemic, since some may cause a drastic drop in white blood cells that could leave drug users more vulnerable to infection.
“It’s remarkable that we just forgot to notice, in the horrendous transition from prescription opioid epidemic to the illicit opioid and psychostimulant epidemics, that we would have to pay special attention to what the medications are in the drugs that the person was exposed to – and for how long,” said Mark S. Gold, MD, a coauthor of the review.
The analysis showed that adulterants include new psychoactive substances, industrial compounds, fungicides, veterinary medications, and various impurities. In addition, other various medications are being found in street drugs, such as antipsychotics, antidepressants, anxiolytics, antihistamines, anthelmintics, anesthetics, anti-inflammatory agents, antipyretics, analgesics, antispasmodics, antiarrhythmics, antimalarials, bronchodilators, decongestants, expectorants, muscle relaxers, natural/synthetic hallucinogens, and sedatives.
Illicit drugs are by nature manufactured without Food and Drug Administration oversight, and it is becoming increasingly common that substances like leftover medicines and other active drugs are added to illicit drug batches to add weight, said Dr. Gold, a professor at Washington University,St. Louis. The study appeared in Current Psychopharmacology.
Effects of adulterants ‘terrifying’
The findings of adulterants and their consequences are concerning, according to Jean Lud Cadet, MD, who was asked to comment on the findings. “The blood dysplasia, the pulmonary problems that some of those adulterants can cause – it’s actually terrifying, to put it bluntly,” said Dr. Cadet, who is a senior investigator and chief of the Molecular Neuropsychiatry Research Branch at the National Institute on Drug Abuse.
Before 2000, street drugs were generally diluted with comparatively benign substances such as caffeine, sugars, or lidocaine. Drugs like phenacetin, levamisole, acetaminophen, and diltiazem began to appear in heroin and cocaine in the late 1990s, and by 2010, more powerful adulterants like fentanyl, ketamine, and quetiapine became common.
In 2015, the U.S. Department of State partnered with the Colombo Plan, an international organization based in Sri Lanka, to use field spectroscopy to detect toxins directly in cocaine and heroin samples found in Argentina, Brazil, Ecuador, Peru, Sri Lanka, Thailand, Honduras, Guatemala, Mexico, Colombia, and South Africa. They found a range of adulterants such as aminopyrine, diltiazem, metamizole, levamisole, and phenacetin.
A similar project with 431 heroin and cocaine samples from Vermont and Kentucky found that 69% of samples had five or more controlled drugs, toxic adulterants, or impurities. About 15% had nine or more, and 95% of samples had at least one toxic adulterant.
In the midst of the COVID-19 pandemic, these adulterants take on even greater significance. Individuals with substance use disorders often have other health conditions that can make them more vulnerable to viral infections, and this could be exacerbated by the effects of adulterants on white blood cells or other systems. The pandemic has also had an indirect effect by causing a shortage of street drugs. During production shortages, traffickers might boost potency by adding more cutting agents and adulterants. As a result, COVID-19 and opioid addiction tend to reinforce each other.
“The clinical message would be that our [substance use] patients will contract infectious disease and need to be prioritized for [COVID-19] vaccination,” said Dr. Gold.
The findings came as a surprise to Dr. Cadet, and that illustrates a need to publicize the presence of adulterants in street drugs.
“If I wasn’t aware of many of these, then the general public is also not going to be aware of them,” Dr. Cadet said. “Scientists, including myself, and government agencies need to do a better job [of communicating this issue].”
The study references individuals with substance use disorder, but Dr. Cadet cautioned that anyone who uses street drugs, even once or twice, could be a victim of adulterants. “You don’t need to have met criteria for diagnosis in order to suffer the consequences.”
The study had no funding. Dr. Gold and Dr. Cadet have no relevant financial disclosures.
The composition of street drugs like heroin and cocaine are changing. According to a new analysis, almost all contain at least one toxic adulterant, and many contain a plethora. Most adulterants have pharmacologic activities and toxicities. Their presence has added impact in the context of the COVID-19 pandemic, since some may cause a drastic drop in white blood cells that could leave drug users more vulnerable to infection.
“It’s remarkable that we just forgot to notice, in the horrendous transition from prescription opioid epidemic to the illicit opioid and psychostimulant epidemics, that we would have to pay special attention to what the medications are in the drugs that the person was exposed to – and for how long,” said Mark S. Gold, MD, a coauthor of the review.
The analysis showed that adulterants include new psychoactive substances, industrial compounds, fungicides, veterinary medications, and various impurities. In addition, other various medications are being found in street drugs, such as antipsychotics, antidepressants, anxiolytics, antihistamines, anthelmintics, anesthetics, anti-inflammatory agents, antipyretics, analgesics, antispasmodics, antiarrhythmics, antimalarials, bronchodilators, decongestants, expectorants, muscle relaxers, natural/synthetic hallucinogens, and sedatives.
Illicit drugs are by nature manufactured without Food and Drug Administration oversight, and it is becoming increasingly common that substances like leftover medicines and other active drugs are added to illicit drug batches to add weight, said Dr. Gold, a professor at Washington University,St. Louis. The study appeared in Current Psychopharmacology.
Effects of adulterants ‘terrifying’
The findings of adulterants and their consequences are concerning, according to Jean Lud Cadet, MD, who was asked to comment on the findings. “The blood dysplasia, the pulmonary problems that some of those adulterants can cause – it’s actually terrifying, to put it bluntly,” said Dr. Cadet, who is a senior investigator and chief of the Molecular Neuropsychiatry Research Branch at the National Institute on Drug Abuse.
Before 2000, street drugs were generally diluted with comparatively benign substances such as caffeine, sugars, or lidocaine. Drugs like phenacetin, levamisole, acetaminophen, and diltiazem began to appear in heroin and cocaine in the late 1990s, and by 2010, more powerful adulterants like fentanyl, ketamine, and quetiapine became common.
In 2015, the U.S. Department of State partnered with the Colombo Plan, an international organization based in Sri Lanka, to use field spectroscopy to detect toxins directly in cocaine and heroin samples found in Argentina, Brazil, Ecuador, Peru, Sri Lanka, Thailand, Honduras, Guatemala, Mexico, Colombia, and South Africa. They found a range of adulterants such as aminopyrine, diltiazem, metamizole, levamisole, and phenacetin.
A similar project with 431 heroin and cocaine samples from Vermont and Kentucky found that 69% of samples had five or more controlled drugs, toxic adulterants, or impurities. About 15% had nine or more, and 95% of samples had at least one toxic adulterant.
In the midst of the COVID-19 pandemic, these adulterants take on even greater significance. Individuals with substance use disorders often have other health conditions that can make them more vulnerable to viral infections, and this could be exacerbated by the effects of adulterants on white blood cells or other systems. The pandemic has also had an indirect effect by causing a shortage of street drugs. During production shortages, traffickers might boost potency by adding more cutting agents and adulterants. As a result, COVID-19 and opioid addiction tend to reinforce each other.
“The clinical message would be that our [substance use] patients will contract infectious disease and need to be prioritized for [COVID-19] vaccination,” said Dr. Gold.
The findings came as a surprise to Dr. Cadet, and that illustrates a need to publicize the presence of adulterants in street drugs.
“If I wasn’t aware of many of these, then the general public is also not going to be aware of them,” Dr. Cadet said. “Scientists, including myself, and government agencies need to do a better job [of communicating this issue].”
The study references individuals with substance use disorder, but Dr. Cadet cautioned that anyone who uses street drugs, even once or twice, could be a victim of adulterants. “You don’t need to have met criteria for diagnosis in order to suffer the consequences.”
The study had no funding. Dr. Gold and Dr. Cadet have no relevant financial disclosures.
The composition of street drugs like heroin and cocaine are changing. According to a new analysis, almost all contain at least one toxic adulterant, and many contain a plethora. Most adulterants have pharmacologic activities and toxicities. Their presence has added impact in the context of the COVID-19 pandemic, since some may cause a drastic drop in white blood cells that could leave drug users more vulnerable to infection.
“It’s remarkable that we just forgot to notice, in the horrendous transition from prescription opioid epidemic to the illicit opioid and psychostimulant epidemics, that we would have to pay special attention to what the medications are in the drugs that the person was exposed to – and for how long,” said Mark S. Gold, MD, a coauthor of the review.
The analysis showed that adulterants include new psychoactive substances, industrial compounds, fungicides, veterinary medications, and various impurities. In addition, other various medications are being found in street drugs, such as antipsychotics, antidepressants, anxiolytics, antihistamines, anthelmintics, anesthetics, anti-inflammatory agents, antipyretics, analgesics, antispasmodics, antiarrhythmics, antimalarials, bronchodilators, decongestants, expectorants, muscle relaxers, natural/synthetic hallucinogens, and sedatives.
Illicit drugs are by nature manufactured without Food and Drug Administration oversight, and it is becoming increasingly common that substances like leftover medicines and other active drugs are added to illicit drug batches to add weight, said Dr. Gold, a professor at Washington University,St. Louis. The study appeared in Current Psychopharmacology.
Effects of adulterants ‘terrifying’
The findings of adulterants and their consequences are concerning, according to Jean Lud Cadet, MD, who was asked to comment on the findings. “The blood dysplasia, the pulmonary problems that some of those adulterants can cause – it’s actually terrifying, to put it bluntly,” said Dr. Cadet, who is a senior investigator and chief of the Molecular Neuropsychiatry Research Branch at the National Institute on Drug Abuse.
Before 2000, street drugs were generally diluted with comparatively benign substances such as caffeine, sugars, or lidocaine. Drugs like phenacetin, levamisole, acetaminophen, and diltiazem began to appear in heroin and cocaine in the late 1990s, and by 2010, more powerful adulterants like fentanyl, ketamine, and quetiapine became common.
In 2015, the U.S. Department of State partnered with the Colombo Plan, an international organization based in Sri Lanka, to use field spectroscopy to detect toxins directly in cocaine and heroin samples found in Argentina, Brazil, Ecuador, Peru, Sri Lanka, Thailand, Honduras, Guatemala, Mexico, Colombia, and South Africa. They found a range of adulterants such as aminopyrine, diltiazem, metamizole, levamisole, and phenacetin.
A similar project with 431 heroin and cocaine samples from Vermont and Kentucky found that 69% of samples had five or more controlled drugs, toxic adulterants, or impurities. About 15% had nine or more, and 95% of samples had at least one toxic adulterant.
In the midst of the COVID-19 pandemic, these adulterants take on even greater significance. Individuals with substance use disorders often have other health conditions that can make them more vulnerable to viral infections, and this could be exacerbated by the effects of adulterants on white blood cells or other systems. The pandemic has also had an indirect effect by causing a shortage of street drugs. During production shortages, traffickers might boost potency by adding more cutting agents and adulterants. As a result, COVID-19 and opioid addiction tend to reinforce each other.
“The clinical message would be that our [substance use] patients will contract infectious disease and need to be prioritized for [COVID-19] vaccination,” said Dr. Gold.
The findings came as a surprise to Dr. Cadet, and that illustrates a need to publicize the presence of adulterants in street drugs.
“If I wasn’t aware of many of these, then the general public is also not going to be aware of them,” Dr. Cadet said. “Scientists, including myself, and government agencies need to do a better job [of communicating this issue].”
The study references individuals with substance use disorder, but Dr. Cadet cautioned that anyone who uses street drugs, even once or twice, could be a victim of adulterants. “You don’t need to have met criteria for diagnosis in order to suffer the consequences.”
The study had no funding. Dr. Gold and Dr. Cadet have no relevant financial disclosures.
FROM CURRENT PSYCHOPHARMACOLOGY