User login
Antidepressants and Dementia Risk: Reassuring Data
TOPLINE:
, new research suggests.
METHODOLOGY:
- Investigators studied 5511 individuals (58% women; mean age, 71 years) from the Rotterdam study, an ongoing prospective population-based cohort study.
- Participants were free from dementia at baseline, and incident dementia was monitored from baseline until 2018 with repeated cognitive assessments using the Mini-Mental Status Examination (MMSE) and the Geriatric Mental Schedule, as well as MRIs.
- Information on participants’ antidepressant use was extracted from pharmacy records from 1992 until baseline (2002-2008).
- During a mean follow-up of 10 years, 12% of participants developed dementia.
TAKEAWAY:
- Overall, 17% of participants had used antidepressants during the roughly 10-year period prior to baseline, and 4.1% were still using antidepressants at baseline.
- Medication use at baseline was more common in women than in men (21% vs 18%), and use increased with age: From 2.1% in participants aged between 45 and 50 years to 4.5% in those older than 80 years.
- After adjustment for confounders, there was no association between antidepressant use and dementia risk (hazard ratio [HR], 1.14; 95% CI, 0.92-1.41), accelerated cognitive decline, or atrophy of white and gray matter.
- However, tricyclic antidepressant use was associated with increased dementia risk (HR, 1.36; 95% CI, 1.01-1.83) compared with the use of selective serotonin reuptake inhibitors (HR, 1.12; 95% CI, 0.81-1.54).
IN PRACTICE:
“Although prescription of antidepressant medication in older individuals, in particular those with some cognitive impairment, may have acute symptomatic anticholinergic effects that warrant consideration in clinical practice, our results show that long-term antidepressant use does not have lasting effects on cognition or brain health in older adults without indication of cognitive impairment,” the authors wrote.
SOURCE:
Frank J. Wolters, MD, of the Department of Epidemiology and the Department of Radiology and Nuclear Medicine and Alzheimer Center, Erasmus University Medical Center, Rotterdam, the Netherlands, was the senior author on this study that was published online in Alzheimer’s and Dementia.
LIMITATIONS:
Limitations included the concern that although exclusion of participants with MMSE < 26 at baseline prevented reversed causation (ie, antidepressant use in response to depression during the prodromal phase of dementia), it may have introduced selection bias by disregarding the effects of antidepressant use prior to baseline and excluding participants with lower education.
DISCLOSURES:
This study was conducted as part of the Netherlands Consortium of Dementia Cohorts, which receives funding in the context of Deltaplan Dementie from ZonMW Memorabel and Alzheimer Nederland. Further funding was also obtained from the Stichting Erasmus Trustfonds. This study was further supported by a 2020 NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation. The authors reported no conflicts of interest or relevant financial relationships.
A version of this article appeared on Medscape.com.
TOPLINE:
, new research suggests.
METHODOLOGY:
- Investigators studied 5511 individuals (58% women; mean age, 71 years) from the Rotterdam study, an ongoing prospective population-based cohort study.
- Participants were free from dementia at baseline, and incident dementia was monitored from baseline until 2018 with repeated cognitive assessments using the Mini-Mental Status Examination (MMSE) and the Geriatric Mental Schedule, as well as MRIs.
- Information on participants’ antidepressant use was extracted from pharmacy records from 1992 until baseline (2002-2008).
- During a mean follow-up of 10 years, 12% of participants developed dementia.
TAKEAWAY:
- Overall, 17% of participants had used antidepressants during the roughly 10-year period prior to baseline, and 4.1% were still using antidepressants at baseline.
- Medication use at baseline was more common in women than in men (21% vs 18%), and use increased with age: From 2.1% in participants aged between 45 and 50 years to 4.5% in those older than 80 years.
- After adjustment for confounders, there was no association between antidepressant use and dementia risk (hazard ratio [HR], 1.14; 95% CI, 0.92-1.41), accelerated cognitive decline, or atrophy of white and gray matter.
- However, tricyclic antidepressant use was associated with increased dementia risk (HR, 1.36; 95% CI, 1.01-1.83) compared with the use of selective serotonin reuptake inhibitors (HR, 1.12; 95% CI, 0.81-1.54).
IN PRACTICE:
“Although prescription of antidepressant medication in older individuals, in particular those with some cognitive impairment, may have acute symptomatic anticholinergic effects that warrant consideration in clinical practice, our results show that long-term antidepressant use does not have lasting effects on cognition or brain health in older adults without indication of cognitive impairment,” the authors wrote.
SOURCE:
Frank J. Wolters, MD, of the Department of Epidemiology and the Department of Radiology and Nuclear Medicine and Alzheimer Center, Erasmus University Medical Center, Rotterdam, the Netherlands, was the senior author on this study that was published online in Alzheimer’s and Dementia.
LIMITATIONS:
Limitations included the concern that although exclusion of participants with MMSE < 26 at baseline prevented reversed causation (ie, antidepressant use in response to depression during the prodromal phase of dementia), it may have introduced selection bias by disregarding the effects of antidepressant use prior to baseline and excluding participants with lower education.
DISCLOSURES:
This study was conducted as part of the Netherlands Consortium of Dementia Cohorts, which receives funding in the context of Deltaplan Dementie from ZonMW Memorabel and Alzheimer Nederland. Further funding was also obtained from the Stichting Erasmus Trustfonds. This study was further supported by a 2020 NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation. The authors reported no conflicts of interest or relevant financial relationships.
A version of this article appeared on Medscape.com.
TOPLINE:
, new research suggests.
METHODOLOGY:
- Investigators studied 5511 individuals (58% women; mean age, 71 years) from the Rotterdam study, an ongoing prospective population-based cohort study.
- Participants were free from dementia at baseline, and incident dementia was monitored from baseline until 2018 with repeated cognitive assessments using the Mini-Mental Status Examination (MMSE) and the Geriatric Mental Schedule, as well as MRIs.
- Information on participants’ antidepressant use was extracted from pharmacy records from 1992 until baseline (2002-2008).
- During a mean follow-up of 10 years, 12% of participants developed dementia.
TAKEAWAY:
- Overall, 17% of participants had used antidepressants during the roughly 10-year period prior to baseline, and 4.1% were still using antidepressants at baseline.
- Medication use at baseline was more common in women than in men (21% vs 18%), and use increased with age: From 2.1% in participants aged between 45 and 50 years to 4.5% in those older than 80 years.
- After adjustment for confounders, there was no association between antidepressant use and dementia risk (hazard ratio [HR], 1.14; 95% CI, 0.92-1.41), accelerated cognitive decline, or atrophy of white and gray matter.
- However, tricyclic antidepressant use was associated with increased dementia risk (HR, 1.36; 95% CI, 1.01-1.83) compared with the use of selective serotonin reuptake inhibitors (HR, 1.12; 95% CI, 0.81-1.54).
IN PRACTICE:
“Although prescription of antidepressant medication in older individuals, in particular those with some cognitive impairment, may have acute symptomatic anticholinergic effects that warrant consideration in clinical practice, our results show that long-term antidepressant use does not have lasting effects on cognition or brain health in older adults without indication of cognitive impairment,” the authors wrote.
SOURCE:
Frank J. Wolters, MD, of the Department of Epidemiology and the Department of Radiology and Nuclear Medicine and Alzheimer Center, Erasmus University Medical Center, Rotterdam, the Netherlands, was the senior author on this study that was published online in Alzheimer’s and Dementia.
LIMITATIONS:
Limitations included the concern that although exclusion of participants with MMSE < 26 at baseline prevented reversed causation (ie, antidepressant use in response to depression during the prodromal phase of dementia), it may have introduced selection bias by disregarding the effects of antidepressant use prior to baseline and excluding participants with lower education.
DISCLOSURES:
This study was conducted as part of the Netherlands Consortium of Dementia Cohorts, which receives funding in the context of Deltaplan Dementie from ZonMW Memorabel and Alzheimer Nederland. Further funding was also obtained from the Stichting Erasmus Trustfonds. This study was further supported by a 2020 NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation. The authors reported no conflicts of interest or relevant financial relationships.
A version of this article appeared on Medscape.com.
Mandatory DMV Reporting Tied to Dementia Underdiagnosis
, new research suggests.
Investigators found that primary care physicians (PCPs) in states with clinician reporting mandates had a 59% higher probability of underdiagnosing dementia compared with their counterparts in states that require patients to self-report or that have no reporting mandates.
“Our findings in this cross-sectional study raise concerns about potential adverse effects of mandatory clinician reporting for dementia diagnosis and underscore the need for careful consideration of the effect of such policies,” wrote the investigators, led by Soeren Mattke, MD, DSc, director of the USC Brain Health Observatory and research professor of economics at the University of Southern California, Los Angeles.
The study was published online in JAMA Network Open.
Lack of Guidance
As the US population ages, the number of older drivers is increasing, with 55.8 million drivers 65 years old or older. Approximately 7 million people in this age group have dementia — an estimate that is expected to increase to nearly 12 million by 2040.
The aging population raises a “critical policy question” about how to ensure road safety. Although the American Medical Association’s Code of Ethics outlines a physician’s obligation to identify drivers with medical impairments that impede safe driving, guidance restricting cognitively impaired drivers from driving is lacking.
In addition, evidence as to whether cognitive impairment indeed poses a threat to driving safety is mixed and has led to a lack of uniform policies with respect to reporting dementia.
Four states explicitly require clinicians to report dementia diagnoses to the DMV, which will then determine the patient’s fitness to drive, whereas 14 states require people with dementia to self-report. The remaining states have no explicit reporting requirements.
The issue of mandatory reporting is controversial, the researchers noted. On the one hand, physicians could protect patients and others by reporting potentially unsafe drivers.
On the other hand, evidence of an association with lower accident risks in patients with dementia is sparse and mandatory reporting may adversely affect physician-patient relationships. Empirical evidence for unintended consequences of reporting laws is lacking.
To examine the potential link between dementia underdiagnosis and mandatory reporting policies, the investigators analyzed the 100% data from the Medicare fee-for-service program and Medicare Advantage plans from 2017 to 2019, which included 223,036 PCPs with a panel of 25 or more Medicare patients.
The researchers examined dementia diagnosis rates in the patient panel of PCPs, rather than neurologists or gerontologists, regardless of who documented the diagnosis. Dr. Mattke said that it is possible that the diagnosis was established after referral to a specialist.
Each physician’s expected number of dementia cases was estimated using a predictive model based on patient characteristics. The researchers then compared the estimate with observed dementia diagnoses, thereby identifying clinicians who underdiagnosed dementia after sampling errors were accounted for.
‘Heavy-Handed Interference’
The researchers adjusted for several covariates potentially associated with a clinician’s probability of underdiagnosing dementia. These included sex, office location, practice specialty, racial/ethnic composition of the patient panel, and percentage of patients dually eligible for Medicare and Medicaid. The table shows PCP characteristics.
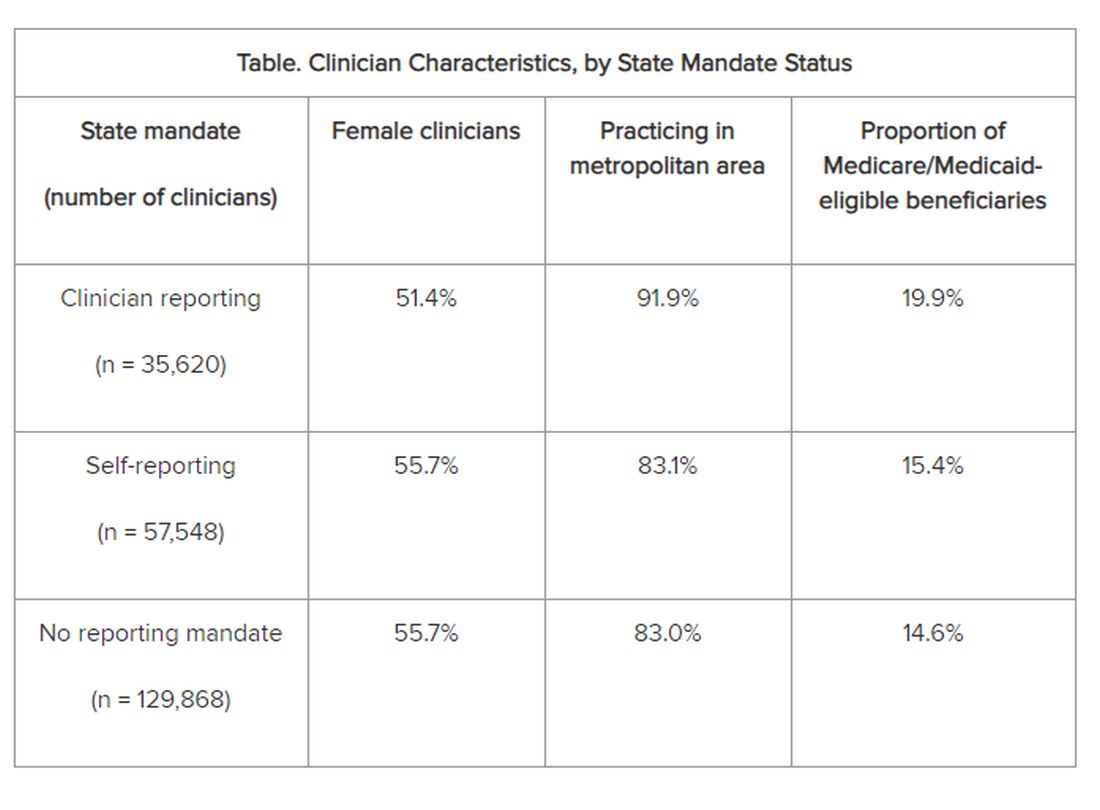
Adjusted results showed that PCPs practicing in states with clinician reporting mandates had a 12.4% (95% confidence interval [CI], 10.5%-14.2%) probability of underdiagnosing dementia versus 7.8% (95% CI, 6.9%-8.7%) in states with self-reporting and 7.7% (95% CI, 6.9%-8.4%) in states with no mandates, translating into a 4–percentage point difference (P < .001).
“Our study is the first to provide empirical evidence for the potential adverse effects of reporting policies,” the researchers noted. “Although we found that some clinicians underdiagnosed dementia regardless of state mandates, the key finding of this study reveals that primary care clinicians who practice in states with clinician reporting mandates were 59% more likely to do so…compared with those states with no reporting requirements…or driver self-reporting requirements.”
The investigators suggested that one potential explanation for underdiagnosis is patient resistance to cognitive testing. If patients were aware that the clinician was obligated by law to report their dementia diagnosis to the DMV, “they might be more inclined to conceal their symptoms or refuse further assessments, in addition to the general stigma and resistance to a formal assessment after a positive dementia screening result.”
“The findings suggest that policymakers might want to rethink those physician reporting mandates, since we also could not find conclusive evidence that they improve road safety,” Dr. Mattke said. “Maybe patients and their physicians can arrive at a sensible approach to determine driving fitness without such heavy-handed interference.”
However, he cautioned that the findings are not definitive and further study is needed before firm recommendations either for or against mandatory reporting.
In addition, the researchers noted several study limitations. One is that dementia underdiagnosis may also be associated with factors not captured in their model, including physician-patient relationships, health literacy, or language barriers.
However, Dr. Mattke noted, “ my sense is that those unobservable factors are not systematically related to state reporting policies and having omitted them would therefore not bias our results.”
Experts Weigh In
Commenting on the research, Morgan Daven, MA, the Alzheimer’s Association vice president of health systems, said that dementia is widely and significantly underdiagnosed, and not only in the states with dementia reporting mandates. Many factors may contribute to underdiagnosis, and although the study shows an association between reporting mandates and underdiagnosis, it does not demonstrate causation.
That said, Mr. Daven added, “fear and stigma related to dementia may inhibit the clinician, the patient, and their family from pursuing detection and diagnosis for dementia. As a society, we need to address dementia fear and stigma for all parties.”
He noted that useful tools include healthcare policies, workforce training, public awareness and education, and public policies to mitigate fear and stigma and their negative effects on diagnosis, care, support, and communication.
A potential study limitation is that it relied only on diagnoses by PCPs. Mr. Daven noted that the diagnosis of Alzheimer’ disease — the most common cause of dementia — is confirmation of amyloid buildup via a biomarker test, using PET or cerebrospinal fluid analysis.
“Both of these tests are extremely limited in their use and accessibility in a primary care setting. Inclusion of diagnoses by dementia specialists would provide a more complete picture,” he said.
Mr. Daven added that the Alzheimer’s Association encourages families to proactively discuss driving and other disease-related safety concerns as soon as possible. The Alzheimer’s Association Dementia and Driving webpage offers tips and strategies to discuss driving concerns with a family member.
In an accompanying editorial, Donald Redelmeier, MD, MS(HSR), and Vidhi Bhatt, BSc, both of the Department of Medicine, University of Toronto, differentiate the mandate for physicians to warn patients with dementia about traffic safety from the mandate for reporting child maltreatment, gunshot victims, or communicable diseases. They noted that mandated warnings “are not easy, can engender patient dissatisfaction, and need to be handled with tact.”
Yet, they pointed out, “breaking bad news is what practicing medicine entails.” They emphasized that, regardless of government mandates, “counseling patients for more road safety is an essential skill for clinicians in diverse states who hope to help their patients avoid becoming more traffic statistics.”
Research reported in this publication was supported by Genentech, a member of the Roche Group, and a grant from the National Institute on Aging of the National Institutes of Health. Dr. Mattke reported receiving grants from Genentech for a research contract with USC during the conduct of the study; personal fees from Eisai, Biogen, C2N, Novo Nordisk, Novartis, and Roche Genentech; and serving on the Senscio Systems board of directors, ALZpath scientific advisory board, AiCure scientific advisory board, and Boston Millennia Partners scientific advisory board outside the submitted work. The other authors’ disclosures are listed on the original paper. The editorial was supported by the Canada Research Chair in Medical Decision Sciences, the Canadian Institutes of Health Research, Kimel-Schatzky Traumatic Brain Injury Research Fund, and the Graduate Diploma Program in Health Research at the University of Toronto. The editorial authors report no other relevant financial relationships.
A version of this article appeared on Medscape.com.
, new research suggests.
Investigators found that primary care physicians (PCPs) in states with clinician reporting mandates had a 59% higher probability of underdiagnosing dementia compared with their counterparts in states that require patients to self-report or that have no reporting mandates.
“Our findings in this cross-sectional study raise concerns about potential adverse effects of mandatory clinician reporting for dementia diagnosis and underscore the need for careful consideration of the effect of such policies,” wrote the investigators, led by Soeren Mattke, MD, DSc, director of the USC Brain Health Observatory and research professor of economics at the University of Southern California, Los Angeles.
The study was published online in JAMA Network Open.
Lack of Guidance
As the US population ages, the number of older drivers is increasing, with 55.8 million drivers 65 years old or older. Approximately 7 million people in this age group have dementia — an estimate that is expected to increase to nearly 12 million by 2040.
The aging population raises a “critical policy question” about how to ensure road safety. Although the American Medical Association’s Code of Ethics outlines a physician’s obligation to identify drivers with medical impairments that impede safe driving, guidance restricting cognitively impaired drivers from driving is lacking.
In addition, evidence as to whether cognitive impairment indeed poses a threat to driving safety is mixed and has led to a lack of uniform policies with respect to reporting dementia.
Four states explicitly require clinicians to report dementia diagnoses to the DMV, which will then determine the patient’s fitness to drive, whereas 14 states require people with dementia to self-report. The remaining states have no explicit reporting requirements.
The issue of mandatory reporting is controversial, the researchers noted. On the one hand, physicians could protect patients and others by reporting potentially unsafe drivers.
On the other hand, evidence of an association with lower accident risks in patients with dementia is sparse and mandatory reporting may adversely affect physician-patient relationships. Empirical evidence for unintended consequences of reporting laws is lacking.
To examine the potential link between dementia underdiagnosis and mandatory reporting policies, the investigators analyzed the 100% data from the Medicare fee-for-service program and Medicare Advantage plans from 2017 to 2019, which included 223,036 PCPs with a panel of 25 or more Medicare patients.
The researchers examined dementia diagnosis rates in the patient panel of PCPs, rather than neurologists or gerontologists, regardless of who documented the diagnosis. Dr. Mattke said that it is possible that the diagnosis was established after referral to a specialist.
Each physician’s expected number of dementia cases was estimated using a predictive model based on patient characteristics. The researchers then compared the estimate with observed dementia diagnoses, thereby identifying clinicians who underdiagnosed dementia after sampling errors were accounted for.
‘Heavy-Handed Interference’
The researchers adjusted for several covariates potentially associated with a clinician’s probability of underdiagnosing dementia. These included sex, office location, practice specialty, racial/ethnic composition of the patient panel, and percentage of patients dually eligible for Medicare and Medicaid. The table shows PCP characteristics.

Adjusted results showed that PCPs practicing in states with clinician reporting mandates had a 12.4% (95% confidence interval [CI], 10.5%-14.2%) probability of underdiagnosing dementia versus 7.8% (95% CI, 6.9%-8.7%) in states with self-reporting and 7.7% (95% CI, 6.9%-8.4%) in states with no mandates, translating into a 4–percentage point difference (P < .001).
“Our study is the first to provide empirical evidence for the potential adverse effects of reporting policies,” the researchers noted. “Although we found that some clinicians underdiagnosed dementia regardless of state mandates, the key finding of this study reveals that primary care clinicians who practice in states with clinician reporting mandates were 59% more likely to do so…compared with those states with no reporting requirements…or driver self-reporting requirements.”
The investigators suggested that one potential explanation for underdiagnosis is patient resistance to cognitive testing. If patients were aware that the clinician was obligated by law to report their dementia diagnosis to the DMV, “they might be more inclined to conceal their symptoms or refuse further assessments, in addition to the general stigma and resistance to a formal assessment after a positive dementia screening result.”
“The findings suggest that policymakers might want to rethink those physician reporting mandates, since we also could not find conclusive evidence that they improve road safety,” Dr. Mattke said. “Maybe patients and their physicians can arrive at a sensible approach to determine driving fitness without such heavy-handed interference.”
However, he cautioned that the findings are not definitive and further study is needed before firm recommendations either for or against mandatory reporting.
In addition, the researchers noted several study limitations. One is that dementia underdiagnosis may also be associated with factors not captured in their model, including physician-patient relationships, health literacy, or language barriers.
However, Dr. Mattke noted, “ my sense is that those unobservable factors are not systematically related to state reporting policies and having omitted them would therefore not bias our results.”
Experts Weigh In
Commenting on the research, Morgan Daven, MA, the Alzheimer’s Association vice president of health systems, said that dementia is widely and significantly underdiagnosed, and not only in the states with dementia reporting mandates. Many factors may contribute to underdiagnosis, and although the study shows an association between reporting mandates and underdiagnosis, it does not demonstrate causation.
That said, Mr. Daven added, “fear and stigma related to dementia may inhibit the clinician, the patient, and their family from pursuing detection and diagnosis for dementia. As a society, we need to address dementia fear and stigma for all parties.”
He noted that useful tools include healthcare policies, workforce training, public awareness and education, and public policies to mitigate fear and stigma and their negative effects on diagnosis, care, support, and communication.
A potential study limitation is that it relied only on diagnoses by PCPs. Mr. Daven noted that the diagnosis of Alzheimer’ disease — the most common cause of dementia — is confirmation of amyloid buildup via a biomarker test, using PET or cerebrospinal fluid analysis.
“Both of these tests are extremely limited in their use and accessibility in a primary care setting. Inclusion of diagnoses by dementia specialists would provide a more complete picture,” he said.
Mr. Daven added that the Alzheimer’s Association encourages families to proactively discuss driving and other disease-related safety concerns as soon as possible. The Alzheimer’s Association Dementia and Driving webpage offers tips and strategies to discuss driving concerns with a family member.
In an accompanying editorial, Donald Redelmeier, MD, MS(HSR), and Vidhi Bhatt, BSc, both of the Department of Medicine, University of Toronto, differentiate the mandate for physicians to warn patients with dementia about traffic safety from the mandate for reporting child maltreatment, gunshot victims, or communicable diseases. They noted that mandated warnings “are not easy, can engender patient dissatisfaction, and need to be handled with tact.”
Yet, they pointed out, “breaking bad news is what practicing medicine entails.” They emphasized that, regardless of government mandates, “counseling patients for more road safety is an essential skill for clinicians in diverse states who hope to help their patients avoid becoming more traffic statistics.”
Research reported in this publication was supported by Genentech, a member of the Roche Group, and a grant from the National Institute on Aging of the National Institutes of Health. Dr. Mattke reported receiving grants from Genentech for a research contract with USC during the conduct of the study; personal fees from Eisai, Biogen, C2N, Novo Nordisk, Novartis, and Roche Genentech; and serving on the Senscio Systems board of directors, ALZpath scientific advisory board, AiCure scientific advisory board, and Boston Millennia Partners scientific advisory board outside the submitted work. The other authors’ disclosures are listed on the original paper. The editorial was supported by the Canada Research Chair in Medical Decision Sciences, the Canadian Institutes of Health Research, Kimel-Schatzky Traumatic Brain Injury Research Fund, and the Graduate Diploma Program in Health Research at the University of Toronto. The editorial authors report no other relevant financial relationships.
A version of this article appeared on Medscape.com.
, new research suggests.
Investigators found that primary care physicians (PCPs) in states with clinician reporting mandates had a 59% higher probability of underdiagnosing dementia compared with their counterparts in states that require patients to self-report or that have no reporting mandates.
“Our findings in this cross-sectional study raise concerns about potential adverse effects of mandatory clinician reporting for dementia diagnosis and underscore the need for careful consideration of the effect of such policies,” wrote the investigators, led by Soeren Mattke, MD, DSc, director of the USC Brain Health Observatory and research professor of economics at the University of Southern California, Los Angeles.
The study was published online in JAMA Network Open.
Lack of Guidance
As the US population ages, the number of older drivers is increasing, with 55.8 million drivers 65 years old or older. Approximately 7 million people in this age group have dementia — an estimate that is expected to increase to nearly 12 million by 2040.
The aging population raises a “critical policy question” about how to ensure road safety. Although the American Medical Association’s Code of Ethics outlines a physician’s obligation to identify drivers with medical impairments that impede safe driving, guidance restricting cognitively impaired drivers from driving is lacking.
In addition, evidence as to whether cognitive impairment indeed poses a threat to driving safety is mixed and has led to a lack of uniform policies with respect to reporting dementia.
Four states explicitly require clinicians to report dementia diagnoses to the DMV, which will then determine the patient’s fitness to drive, whereas 14 states require people with dementia to self-report. The remaining states have no explicit reporting requirements.
The issue of mandatory reporting is controversial, the researchers noted. On the one hand, physicians could protect patients and others by reporting potentially unsafe drivers.
On the other hand, evidence of an association with lower accident risks in patients with dementia is sparse and mandatory reporting may adversely affect physician-patient relationships. Empirical evidence for unintended consequences of reporting laws is lacking.
To examine the potential link between dementia underdiagnosis and mandatory reporting policies, the investigators analyzed the 100% data from the Medicare fee-for-service program and Medicare Advantage plans from 2017 to 2019, which included 223,036 PCPs with a panel of 25 or more Medicare patients.
The researchers examined dementia diagnosis rates in the patient panel of PCPs, rather than neurologists or gerontologists, regardless of who documented the diagnosis. Dr. Mattke said that it is possible that the diagnosis was established after referral to a specialist.
Each physician’s expected number of dementia cases was estimated using a predictive model based on patient characteristics. The researchers then compared the estimate with observed dementia diagnoses, thereby identifying clinicians who underdiagnosed dementia after sampling errors were accounted for.
‘Heavy-Handed Interference’
The researchers adjusted for several covariates potentially associated with a clinician’s probability of underdiagnosing dementia. These included sex, office location, practice specialty, racial/ethnic composition of the patient panel, and percentage of patients dually eligible for Medicare and Medicaid. The table shows PCP characteristics.

Adjusted results showed that PCPs practicing in states with clinician reporting mandates had a 12.4% (95% confidence interval [CI], 10.5%-14.2%) probability of underdiagnosing dementia versus 7.8% (95% CI, 6.9%-8.7%) in states with self-reporting and 7.7% (95% CI, 6.9%-8.4%) in states with no mandates, translating into a 4–percentage point difference (P < .001).
“Our study is the first to provide empirical evidence for the potential adverse effects of reporting policies,” the researchers noted. “Although we found that some clinicians underdiagnosed dementia regardless of state mandates, the key finding of this study reveals that primary care clinicians who practice in states with clinician reporting mandates were 59% more likely to do so…compared with those states with no reporting requirements…or driver self-reporting requirements.”
The investigators suggested that one potential explanation for underdiagnosis is patient resistance to cognitive testing. If patients were aware that the clinician was obligated by law to report their dementia diagnosis to the DMV, “they might be more inclined to conceal their symptoms or refuse further assessments, in addition to the general stigma and resistance to a formal assessment after a positive dementia screening result.”
“The findings suggest that policymakers might want to rethink those physician reporting mandates, since we also could not find conclusive evidence that they improve road safety,” Dr. Mattke said. “Maybe patients and their physicians can arrive at a sensible approach to determine driving fitness without such heavy-handed interference.”
However, he cautioned that the findings are not definitive and further study is needed before firm recommendations either for or against mandatory reporting.
In addition, the researchers noted several study limitations. One is that dementia underdiagnosis may also be associated with factors not captured in their model, including physician-patient relationships, health literacy, or language barriers.
However, Dr. Mattke noted, “ my sense is that those unobservable factors are not systematically related to state reporting policies and having omitted them would therefore not bias our results.”
Experts Weigh In
Commenting on the research, Morgan Daven, MA, the Alzheimer’s Association vice president of health systems, said that dementia is widely and significantly underdiagnosed, and not only in the states with dementia reporting mandates. Many factors may contribute to underdiagnosis, and although the study shows an association between reporting mandates and underdiagnosis, it does not demonstrate causation.
That said, Mr. Daven added, “fear and stigma related to dementia may inhibit the clinician, the patient, and their family from pursuing detection and diagnosis for dementia. As a society, we need to address dementia fear and stigma for all parties.”
He noted that useful tools include healthcare policies, workforce training, public awareness and education, and public policies to mitigate fear and stigma and their negative effects on diagnosis, care, support, and communication.
A potential study limitation is that it relied only on diagnoses by PCPs. Mr. Daven noted that the diagnosis of Alzheimer’ disease — the most common cause of dementia — is confirmation of amyloid buildup via a biomarker test, using PET or cerebrospinal fluid analysis.
“Both of these tests are extremely limited in their use and accessibility in a primary care setting. Inclusion of diagnoses by dementia specialists would provide a more complete picture,” he said.
Mr. Daven added that the Alzheimer’s Association encourages families to proactively discuss driving and other disease-related safety concerns as soon as possible. The Alzheimer’s Association Dementia and Driving webpage offers tips and strategies to discuss driving concerns with a family member.
In an accompanying editorial, Donald Redelmeier, MD, MS(HSR), and Vidhi Bhatt, BSc, both of the Department of Medicine, University of Toronto, differentiate the mandate for physicians to warn patients with dementia about traffic safety from the mandate for reporting child maltreatment, gunshot victims, or communicable diseases. They noted that mandated warnings “are not easy, can engender patient dissatisfaction, and need to be handled with tact.”
Yet, they pointed out, “breaking bad news is what practicing medicine entails.” They emphasized that, regardless of government mandates, “counseling patients for more road safety is an essential skill for clinicians in diverse states who hope to help their patients avoid becoming more traffic statistics.”
Research reported in this publication was supported by Genentech, a member of the Roche Group, and a grant from the National Institute on Aging of the National Institutes of Health. Dr. Mattke reported receiving grants from Genentech for a research contract with USC during the conduct of the study; personal fees from Eisai, Biogen, C2N, Novo Nordisk, Novartis, and Roche Genentech; and serving on the Senscio Systems board of directors, ALZpath scientific advisory board, AiCure scientific advisory board, and Boston Millennia Partners scientific advisory board outside the submitted work. The other authors’ disclosures are listed on the original paper. The editorial was supported by the Canada Research Chair in Medical Decision Sciences, the Canadian Institutes of Health Research, Kimel-Schatzky Traumatic Brain Injury Research Fund, and the Graduate Diploma Program in Health Research at the University of Toronto. The editorial authors report no other relevant financial relationships.
A version of this article appeared on Medscape.com.
From JAMA Network Open
Does ‘Brain Training’ Really Improve Cognition and Forestall Cognitive Decline?
The concept that cognitive health can be preserved or improved is often expressed as “use it or lose it.” Numerous modifiable risk factors are associated with “losing” cognitive abilities with age, and a cognitively active lifestyle may have a protective effect.
But what is a “cognitively active lifestyle” — do crosswords and Sudoku count?
One popular approach is “brain training.” While not a scientific term with an established definition, it “typically refers to tasks or drills that are designed to strengthen specific aspects of one’s cognitive function,” explained Yuko Hara, PhD, director of Aging and Alzheimer’s Prevention at the Alzheimer’s Drug Discovery Foundation.
Manuel Montero-Odasso, MD, PhD, director of the Gait and Brain Lab, Parkwood Institute, London, Ontario, Canada, elaborated: “Cognitive training involves performing a definitive task or set of tasks where you increase attentional demands to improve focus and concentration and memory. You try to execute the new things that you’ve learned and to remember them.”
In a commentary published by this news organization in 2022, neuroscientist Michael Merzenich, PhD, professor emeritus at University of California San Francisco, said that growing a person’s cognitive reserve and actively managing brain health can play an important role in preventing or delaying Alzheimer’s disease. Important components of this include brain training and physical exercise.
Brain Training: Mechanism of Action
Dr. Montero-Odasso, team leader at the Canadian Consortium on Neurodegeneration in Aging and team co-leader at the Ontario Neurodegenerative Research Initiative, explained that cognitive training creates new synapses in the brain, thus stimulating neuroplasticity.
“When we try to activate networks mainly in the frontal lobe, the prefrontal cortex, a key mechanism underlying this process is enhancement of the synaptic plasticity at excitatory synapses, which connect neurons into networks; in other words, we generate new synapses, and that’s how we enhance brain health and cognitive abilities.”
The more neural connections, the greater the processing speed of the brain, he continued. “Cognitive training creates an anatomical change in the brain.”
Executive functions, which include attention, inhibition, planning, and multitasking, are regulated predominantly by the prefrontal cortex. Damage in this region of the brain is also implicated in dementia. Alterations in the connectivity of this area are associated with cognitive impairment, independent of other structural pathological aberrations (eg, gray matter atrophy). These patterns may precede structural pathological changes associated with cognitive impairment and dementia.
Neuroplasticity changes have been corroborated through neuroimaging, which has demonstrated that after cognitive training, there is more activation in the prefrontal cortex that correlates with new synapses, Dr. Montero-Odasso said.
Henry Mahncke, PhD, CEO of the brain training company Posit Science/BrainHQ, explained that early research was conducted on rodents and monkeys, with Dr. Merzenich as one of the leading pioneers in developing the concept of brain plasticity. Dr. Merzenich cofounded Posit Science and is currently its chief scientific officer.
Dr. Mahncke recounted that as a graduate student, he had worked with Dr. Merzenich researching brain plasticity. When Dr. Merzenich founded Posit Science, he asked Dr. Mahncke to join the company to help develop approaches to enhance brain plasticity — building the brain-training exercises and running the clinical trials.
“It’s now well understood that the brain can rewire itself at any age and in almost any condition,” Dr. Mahncke said. “In kids and in younger and older adults, whether with healthy or unhealthy brains, the fundamental way the brain works is by continually rewiring and rebuilding itself, based on what we ask it to do.”
Dr. Mahncke said.
Unsubstantiated Claims and Controversy
Brain training is not without controversy, Dr. Hara pointed out. “Some manufacturers of brain games have been criticized and even fined for making unsubstantiated claims,” she said.
A 2016 review found that brain-training interventions do improve performance on specific trained tasks, but there is less evidence that they improve performance on closely related tasks and little evidence that training improves everyday cognitive performance. A 2017 review reached similar conclusions, calling evidence regarding prevention or delay of cognitive decline or dementia through brain games “insufficient,” although cognitive training could “improve cognition in the domain trained.”
“The general consensus is that for most brain-training programs, people may get better at specific tasks through practice, but these improvements don’t necessarily translate into improvement in other tasks that require other cognitive domains or prevention of dementia or age-related cognitive decline,” Dr. Hara said.
She noted that most brain-training programs “have not been rigorously tested in clinical trials” — although some, such as those featured in the ACTIVE trial, did show evidence of effectiveness.
Dr. Mahncke agreed. “Asking whether brain training works is like asking whether small molecules improve health,” he said noting that some brain-training programs are nonsense and not evidence based. He believes that his company’s product, BrainHQ, and some others are “backed by robust evidence in their ability to stave off, slow, or even reverse cognitive changes.”
BrainHQ is a web-based brain game suite that can be used independently as an app or in group settings (classes and webinars) and is covered by some Medicare Advantage insurance plans. It encompasses “dozens of individual brain-training exercises, linked by a common thread. Each one is intensively designed to make the brain faster and more accurate,” said Dr. Mahncke.
He explained that human brains “get noisy as people get older, like a radio which is wearing out, so there’s static in the background. This makes the music hard to hear, and in the case of the human brain, it makes it difficult to pay attention.” The exercises are “designed to tamp down the ‘noise,’ speed up the brain, and make information processing more accurate.”
Dr. Mahncke called this a “bottom-up” approach, in contrast to many previous cognitive-training approaches that come from the brain injury rehabilitation field. They teach “top-down” skills and strategies designed to compensate for deficits in specific domains, such as reading, concentration, or fine motor skills.
By contrast, the approach of BrainHQ is “to improve the overall processing system of the brain with speed, attention, working memory, and executive function, which will in turn impact all skills and activities.”
Supporting Evidence
Dr. Mahncke cited several supporting studies. For example, the IMPACT study randomized 487 adults (aged ≥ 65 years) to receive either a brain plasticity–based computerized cognitive training program (BrainHQ) or a novelty- and intensity-matched general cognitive stimulation treatment program (intervention and control group, respectively) for an 8-week period.
Those who underwent brain training showed significantly greater improvement in the repeatable Battery for the Assessment of Neuropsychological Status (RBANS Auditory Memory/Attention) compared with those in the control group (3.9 vs 1.8, respectively; P =.02). The intervention group also showed significant improvements on multiple secondary measures of attention and memory. The magnitude of the effect sizes suggests that the results are clinically significant, according to the authors.
The ACTIVE study tested the effects of different cognitive training programs on cognitive function and time to dementia. The researchers randomized 2802 healthy older adults (mean age, 74 years) to a control group with no cognitive training or one of three brain-training groups comprising:
1. In-person training on verbal memory skills
2. In-person training on reasoning and problem-solving
3. Computer-based speed-of-processing training on visual attention
Participants in the training groups completed 10 sessions, each lasting 60-75 minutes, over a 5- to 6-week period. A random subsample of each training group was selected to receive “booster” sessions, with four-session booster training delivered at 11 and 35 months. All study participants completed follow-up tests of cognition and function after 1, 2, 3, 5, and 10 years.
At the end of 10 years, those assigned to the speed-of-processing training, now part of BrainHQ, had a 29% lower risk for dementia than those in the control group who received no training. No reduction was found in the memory or reasoning training groups. Participants who completed the “booster” sessions had an even greater reduction: Each additional booster session was associated with a 10% lower risk for dementia.
Dr. Montero-Odasso was involved in the SYNERGIC study that randomized 175 participants with mild cognitive impairment (MCI; average age, 73 years) to one of five study arms:
1. Multidomain intervention with exercise, cognitive training, and vitamin D
2. Exercise, cognitive training, and placebo
3. Exercise, sham cognitive training, and vitamin D
4. Exercise, sham cognitive training, and placebo
5. Control group with balance-toning exercise, sham cognitive training, and placebo
“Sham” cognitive training consisted of alternating between two tasks (touristic search and video watching) performed on a tablet, with the same time exposure as the intervention training.
The researchers found that after 6 months of interventions, all active arms with aerobic-resistance exercise showed improvement in the ADAS-Cog-13, an established outcome to evaluate dementia treatments, when compared with the control group — regardless of the addition of cognitive training or vitamin D.
Compared with exercise alone (arms 3 and 4), those who did exercise plus cognitive training (arms 1 and 2) showed greater improvements in their ADAS-Cog-13l score, with a mean difference of −1.45 points (P = .02). The greatest improvement was seen in those who underwent the multidomain intervention in arm 1.
The authors noted that the mean 2.64-point improvement seen in the ADAS-Cog-13 for the multidomain intervention is actually larger than changes seen in previous pharmaceutical trials among individuals with MCI or mild dementia and “approaches” the three points considered clinically meaningful.
“We found that older adults with MCI who received aerobic-resistance exercise with sequential computerized cognitive training significantly improved cognition,” Dr. Montero-Odasso said. “The cognitive training we used was called Neuropeak, a multidomain lifestyle training delivered through a web-based platform developed by our co-leader Louis Bherer at Université de Montréal.”
He explained that the purpose “is to challenge your brain to the point where you need to make an effort to remember things, pay attention, and later to execute tasks. The evidence from clinical trials, including ours, shows this type of brain challenge is effective in slowing and even reversing cognitive decline.”
A follow-up study, SYNERGIC 2.0, is ongoing.
Puzzles, Board Games, and New Challenges
Formal brain-training programs aren’t the only way to improve brain plasticity, Dr. Hara said. Observational studies suggested an association between improved cognitive performance and/or lower dementia risk and engaging in number and word puzzles, such as crosswords, cards, or board games.
Some studies suggested that older adults who use technology might also protect their cognitive reserve. Dr. Hara cited a US longitudinal study of more than 18,000 older adults suggesting that regular Internet users had roughly half the risk for dementia compared to nonregular Internet users. Estimates of daily Internet use suggested a U-shaped relationship with dementia with 0.1-2.0 hours daily (excluding time spent watching television or movies online) associated with the lowest risk. Similar associations between Internet use and a lower risk for cognitive decline have been reported in the United Kingdom and Europe.
“Engaging in mentally stimulating activities can increase ‘cognitive reserve’ — meaning, capacity of the brain to resist the effects of age-related changes or disease-related pathology, such that one can maintain cognitive function for longer,” Dr. Hara said. “Cognitively stimulating activities, regardless of the type, may help delay the onset of cognitive decline.”
She listed several examples of activities that are stimulating to the brain, including learning a new game or puzzle, a new language, or a new dance, and learning how to play a musical instrument.
Dr. Montero-Odasso emphasized that the “newness” is key to increasing and preserving cognitive reserve. “Just surfing the Internet, playing word or board games, or doing crossword puzzles won’t be enough if you’ve been doing these things all your life,” he said. “It won’t hurt, of course, but it won’t necessarily increase your cognitive abilities.
“For example, a person who regularly engages in public speaking may not improve cognition by taking a public-speaking course, but someone who has never spoken before an audience might show cognitive improvements as a result of learning a new skill,” he said. “Or someone who knows several languages already might gain from learning a brand-new language.”
He cited research supporting the benefits of dancing, which he called “an ideal activity because it’s physical, so it provides the exercise that’s been associated with improved cognition. But it also requires learning new steps and moves, which builds the synapses in the brain. And the socialization of dance classes adds another component that can improve cognition.”
Dr. Mahncke hopes that beyond engaging in day-to-day new activities, seniors will participate in computerized brain training. “There’s no reason that evidence-based training can’t be offered in senior and community centers, as yoga and swimming are,” he said. “It doesn’t have to be simply something people do on their own virtually.”
Zoom classes and Medicare reimbursements are “good steps in the right direction, but it’s time to expand this potentially life-transformative intervention so that it reaches the ever-expanding population of seniors in the United States and beyond.”
Dr. Hara reported having no disclosures. Dr. Montero-Odasso reported having no commercial or financial interest related to this topic. He serves as the president of the Canadian Geriatrics Société and is team leader in the Canadian Consortium of Neurodegeneration in Aging. Dr. Mahncke is CEO of the brain training company Posit Science/BrainHQ.
A version of this article appeared on Medscape.com.
The concept that cognitive health can be preserved or improved is often expressed as “use it or lose it.” Numerous modifiable risk factors are associated with “losing” cognitive abilities with age, and a cognitively active lifestyle may have a protective effect.
But what is a “cognitively active lifestyle” — do crosswords and Sudoku count?
One popular approach is “brain training.” While not a scientific term with an established definition, it “typically refers to tasks or drills that are designed to strengthen specific aspects of one’s cognitive function,” explained Yuko Hara, PhD, director of Aging and Alzheimer’s Prevention at the Alzheimer’s Drug Discovery Foundation.
Manuel Montero-Odasso, MD, PhD, director of the Gait and Brain Lab, Parkwood Institute, London, Ontario, Canada, elaborated: “Cognitive training involves performing a definitive task or set of tasks where you increase attentional demands to improve focus and concentration and memory. You try to execute the new things that you’ve learned and to remember them.”
In a commentary published by this news organization in 2022, neuroscientist Michael Merzenich, PhD, professor emeritus at University of California San Francisco, said that growing a person’s cognitive reserve and actively managing brain health can play an important role in preventing or delaying Alzheimer’s disease. Important components of this include brain training and physical exercise.
Brain Training: Mechanism of Action
Dr. Montero-Odasso, team leader at the Canadian Consortium on Neurodegeneration in Aging and team co-leader at the Ontario Neurodegenerative Research Initiative, explained that cognitive training creates new synapses in the brain, thus stimulating neuroplasticity.
“When we try to activate networks mainly in the frontal lobe, the prefrontal cortex, a key mechanism underlying this process is enhancement of the synaptic plasticity at excitatory synapses, which connect neurons into networks; in other words, we generate new synapses, and that’s how we enhance brain health and cognitive abilities.”
The more neural connections, the greater the processing speed of the brain, he continued. “Cognitive training creates an anatomical change in the brain.”
Executive functions, which include attention, inhibition, planning, and multitasking, are regulated predominantly by the prefrontal cortex. Damage in this region of the brain is also implicated in dementia. Alterations in the connectivity of this area are associated with cognitive impairment, independent of other structural pathological aberrations (eg, gray matter atrophy). These patterns may precede structural pathological changes associated with cognitive impairment and dementia.
Neuroplasticity changes have been corroborated through neuroimaging, which has demonstrated that after cognitive training, there is more activation in the prefrontal cortex that correlates with new synapses, Dr. Montero-Odasso said.
Henry Mahncke, PhD, CEO of the brain training company Posit Science/BrainHQ, explained that early research was conducted on rodents and monkeys, with Dr. Merzenich as one of the leading pioneers in developing the concept of brain plasticity. Dr. Merzenich cofounded Posit Science and is currently its chief scientific officer.
Dr. Mahncke recounted that as a graduate student, he had worked with Dr. Merzenich researching brain plasticity. When Dr. Merzenich founded Posit Science, he asked Dr. Mahncke to join the company to help develop approaches to enhance brain plasticity — building the brain-training exercises and running the clinical trials.
“It’s now well understood that the brain can rewire itself at any age and in almost any condition,” Dr. Mahncke said. “In kids and in younger and older adults, whether with healthy or unhealthy brains, the fundamental way the brain works is by continually rewiring and rebuilding itself, based on what we ask it to do.”
Dr. Mahncke said.
Unsubstantiated Claims and Controversy
Brain training is not without controversy, Dr. Hara pointed out. “Some manufacturers of brain games have been criticized and even fined for making unsubstantiated claims,” she said.
A 2016 review found that brain-training interventions do improve performance on specific trained tasks, but there is less evidence that they improve performance on closely related tasks and little evidence that training improves everyday cognitive performance. A 2017 review reached similar conclusions, calling evidence regarding prevention or delay of cognitive decline or dementia through brain games “insufficient,” although cognitive training could “improve cognition in the domain trained.”
“The general consensus is that for most brain-training programs, people may get better at specific tasks through practice, but these improvements don’t necessarily translate into improvement in other tasks that require other cognitive domains or prevention of dementia or age-related cognitive decline,” Dr. Hara said.
She noted that most brain-training programs “have not been rigorously tested in clinical trials” — although some, such as those featured in the ACTIVE trial, did show evidence of effectiveness.
Dr. Mahncke agreed. “Asking whether brain training works is like asking whether small molecules improve health,” he said noting that some brain-training programs are nonsense and not evidence based. He believes that his company’s product, BrainHQ, and some others are “backed by robust evidence in their ability to stave off, slow, or even reverse cognitive changes.”
BrainHQ is a web-based brain game suite that can be used independently as an app or in group settings (classes and webinars) and is covered by some Medicare Advantage insurance plans. It encompasses “dozens of individual brain-training exercises, linked by a common thread. Each one is intensively designed to make the brain faster and more accurate,” said Dr. Mahncke.
He explained that human brains “get noisy as people get older, like a radio which is wearing out, so there’s static in the background. This makes the music hard to hear, and in the case of the human brain, it makes it difficult to pay attention.” The exercises are “designed to tamp down the ‘noise,’ speed up the brain, and make information processing more accurate.”
Dr. Mahncke called this a “bottom-up” approach, in contrast to many previous cognitive-training approaches that come from the brain injury rehabilitation field. They teach “top-down” skills and strategies designed to compensate for deficits in specific domains, such as reading, concentration, or fine motor skills.
By contrast, the approach of BrainHQ is “to improve the overall processing system of the brain with speed, attention, working memory, and executive function, which will in turn impact all skills and activities.”
Supporting Evidence
Dr. Mahncke cited several supporting studies. For example, the IMPACT study randomized 487 adults (aged ≥ 65 years) to receive either a brain plasticity–based computerized cognitive training program (BrainHQ) or a novelty- and intensity-matched general cognitive stimulation treatment program (intervention and control group, respectively) for an 8-week period.
Those who underwent brain training showed significantly greater improvement in the repeatable Battery for the Assessment of Neuropsychological Status (RBANS Auditory Memory/Attention) compared with those in the control group (3.9 vs 1.8, respectively; P =.02). The intervention group also showed significant improvements on multiple secondary measures of attention and memory. The magnitude of the effect sizes suggests that the results are clinically significant, according to the authors.
The ACTIVE study tested the effects of different cognitive training programs on cognitive function and time to dementia. The researchers randomized 2802 healthy older adults (mean age, 74 years) to a control group with no cognitive training or one of three brain-training groups comprising:
1. In-person training on verbal memory skills
2. In-person training on reasoning and problem-solving
3. Computer-based speed-of-processing training on visual attention
Participants in the training groups completed 10 sessions, each lasting 60-75 minutes, over a 5- to 6-week period. A random subsample of each training group was selected to receive “booster” sessions, with four-session booster training delivered at 11 and 35 months. All study participants completed follow-up tests of cognition and function after 1, 2, 3, 5, and 10 years.
At the end of 10 years, those assigned to the speed-of-processing training, now part of BrainHQ, had a 29% lower risk for dementia than those in the control group who received no training. No reduction was found in the memory or reasoning training groups. Participants who completed the “booster” sessions had an even greater reduction: Each additional booster session was associated with a 10% lower risk for dementia.
Dr. Montero-Odasso was involved in the SYNERGIC study that randomized 175 participants with mild cognitive impairment (MCI; average age, 73 years) to one of five study arms:
1. Multidomain intervention with exercise, cognitive training, and vitamin D
2. Exercise, cognitive training, and placebo
3. Exercise, sham cognitive training, and vitamin D
4. Exercise, sham cognitive training, and placebo
5. Control group with balance-toning exercise, sham cognitive training, and placebo
“Sham” cognitive training consisted of alternating between two tasks (touristic search and video watching) performed on a tablet, with the same time exposure as the intervention training.
The researchers found that after 6 months of interventions, all active arms with aerobic-resistance exercise showed improvement in the ADAS-Cog-13, an established outcome to evaluate dementia treatments, when compared with the control group — regardless of the addition of cognitive training or vitamin D.
Compared with exercise alone (arms 3 and 4), those who did exercise plus cognitive training (arms 1 and 2) showed greater improvements in their ADAS-Cog-13l score, with a mean difference of −1.45 points (P = .02). The greatest improvement was seen in those who underwent the multidomain intervention in arm 1.
The authors noted that the mean 2.64-point improvement seen in the ADAS-Cog-13 for the multidomain intervention is actually larger than changes seen in previous pharmaceutical trials among individuals with MCI or mild dementia and “approaches” the three points considered clinically meaningful.
“We found that older adults with MCI who received aerobic-resistance exercise with sequential computerized cognitive training significantly improved cognition,” Dr. Montero-Odasso said. “The cognitive training we used was called Neuropeak, a multidomain lifestyle training delivered through a web-based platform developed by our co-leader Louis Bherer at Université de Montréal.”
He explained that the purpose “is to challenge your brain to the point where you need to make an effort to remember things, pay attention, and later to execute tasks. The evidence from clinical trials, including ours, shows this type of brain challenge is effective in slowing and even reversing cognitive decline.”
A follow-up study, SYNERGIC 2.0, is ongoing.
Puzzles, Board Games, and New Challenges
Formal brain-training programs aren’t the only way to improve brain plasticity, Dr. Hara said. Observational studies suggested an association between improved cognitive performance and/or lower dementia risk and engaging in number and word puzzles, such as crosswords, cards, or board games.
Some studies suggested that older adults who use technology might also protect their cognitive reserve. Dr. Hara cited a US longitudinal study of more than 18,000 older adults suggesting that regular Internet users had roughly half the risk for dementia compared to nonregular Internet users. Estimates of daily Internet use suggested a U-shaped relationship with dementia with 0.1-2.0 hours daily (excluding time spent watching television or movies online) associated with the lowest risk. Similar associations between Internet use and a lower risk for cognitive decline have been reported in the United Kingdom and Europe.
“Engaging in mentally stimulating activities can increase ‘cognitive reserve’ — meaning, capacity of the brain to resist the effects of age-related changes or disease-related pathology, such that one can maintain cognitive function for longer,” Dr. Hara said. “Cognitively stimulating activities, regardless of the type, may help delay the onset of cognitive decline.”
She listed several examples of activities that are stimulating to the brain, including learning a new game or puzzle, a new language, or a new dance, and learning how to play a musical instrument.
Dr. Montero-Odasso emphasized that the “newness” is key to increasing and preserving cognitive reserve. “Just surfing the Internet, playing word or board games, or doing crossword puzzles won’t be enough if you’ve been doing these things all your life,” he said. “It won’t hurt, of course, but it won’t necessarily increase your cognitive abilities.
“For example, a person who regularly engages in public speaking may not improve cognition by taking a public-speaking course, but someone who has never spoken before an audience might show cognitive improvements as a result of learning a new skill,” he said. “Or someone who knows several languages already might gain from learning a brand-new language.”
He cited research supporting the benefits of dancing, which he called “an ideal activity because it’s physical, so it provides the exercise that’s been associated with improved cognition. But it also requires learning new steps and moves, which builds the synapses in the brain. And the socialization of dance classes adds another component that can improve cognition.”
Dr. Mahncke hopes that beyond engaging in day-to-day new activities, seniors will participate in computerized brain training. “There’s no reason that evidence-based training can’t be offered in senior and community centers, as yoga and swimming are,” he said. “It doesn’t have to be simply something people do on their own virtually.”
Zoom classes and Medicare reimbursements are “good steps in the right direction, but it’s time to expand this potentially life-transformative intervention so that it reaches the ever-expanding population of seniors in the United States and beyond.”
Dr. Hara reported having no disclosures. Dr. Montero-Odasso reported having no commercial or financial interest related to this topic. He serves as the president of the Canadian Geriatrics Société and is team leader in the Canadian Consortium of Neurodegeneration in Aging. Dr. Mahncke is CEO of the brain training company Posit Science/BrainHQ.
A version of this article appeared on Medscape.com.
The concept that cognitive health can be preserved or improved is often expressed as “use it or lose it.” Numerous modifiable risk factors are associated with “losing” cognitive abilities with age, and a cognitively active lifestyle may have a protective effect.
But what is a “cognitively active lifestyle” — do crosswords and Sudoku count?
One popular approach is “brain training.” While not a scientific term with an established definition, it “typically refers to tasks or drills that are designed to strengthen specific aspects of one’s cognitive function,” explained Yuko Hara, PhD, director of Aging and Alzheimer’s Prevention at the Alzheimer’s Drug Discovery Foundation.
Manuel Montero-Odasso, MD, PhD, director of the Gait and Brain Lab, Parkwood Institute, London, Ontario, Canada, elaborated: “Cognitive training involves performing a definitive task or set of tasks where you increase attentional demands to improve focus and concentration and memory. You try to execute the new things that you’ve learned and to remember them.”
In a commentary published by this news organization in 2022, neuroscientist Michael Merzenich, PhD, professor emeritus at University of California San Francisco, said that growing a person’s cognitive reserve and actively managing brain health can play an important role in preventing or delaying Alzheimer’s disease. Important components of this include brain training and physical exercise.
Brain Training: Mechanism of Action
Dr. Montero-Odasso, team leader at the Canadian Consortium on Neurodegeneration in Aging and team co-leader at the Ontario Neurodegenerative Research Initiative, explained that cognitive training creates new synapses in the brain, thus stimulating neuroplasticity.
“When we try to activate networks mainly in the frontal lobe, the prefrontal cortex, a key mechanism underlying this process is enhancement of the synaptic plasticity at excitatory synapses, which connect neurons into networks; in other words, we generate new synapses, and that’s how we enhance brain health and cognitive abilities.”
The more neural connections, the greater the processing speed of the brain, he continued. “Cognitive training creates an anatomical change in the brain.”
Executive functions, which include attention, inhibition, planning, and multitasking, are regulated predominantly by the prefrontal cortex. Damage in this region of the brain is also implicated in dementia. Alterations in the connectivity of this area are associated with cognitive impairment, independent of other structural pathological aberrations (eg, gray matter atrophy). These patterns may precede structural pathological changes associated with cognitive impairment and dementia.
Neuroplasticity changes have been corroborated through neuroimaging, which has demonstrated that after cognitive training, there is more activation in the prefrontal cortex that correlates with new synapses, Dr. Montero-Odasso said.
Henry Mahncke, PhD, CEO of the brain training company Posit Science/BrainHQ, explained that early research was conducted on rodents and monkeys, with Dr. Merzenich as one of the leading pioneers in developing the concept of brain plasticity. Dr. Merzenich cofounded Posit Science and is currently its chief scientific officer.
Dr. Mahncke recounted that as a graduate student, he had worked with Dr. Merzenich researching brain plasticity. When Dr. Merzenich founded Posit Science, he asked Dr. Mahncke to join the company to help develop approaches to enhance brain plasticity — building the brain-training exercises and running the clinical trials.
“It’s now well understood that the brain can rewire itself at any age and in almost any condition,” Dr. Mahncke said. “In kids and in younger and older adults, whether with healthy or unhealthy brains, the fundamental way the brain works is by continually rewiring and rebuilding itself, based on what we ask it to do.”
Dr. Mahncke said.
Unsubstantiated Claims and Controversy
Brain training is not without controversy, Dr. Hara pointed out. “Some manufacturers of brain games have been criticized and even fined for making unsubstantiated claims,” she said.
A 2016 review found that brain-training interventions do improve performance on specific trained tasks, but there is less evidence that they improve performance on closely related tasks and little evidence that training improves everyday cognitive performance. A 2017 review reached similar conclusions, calling evidence regarding prevention or delay of cognitive decline or dementia through brain games “insufficient,” although cognitive training could “improve cognition in the domain trained.”
“The general consensus is that for most brain-training programs, people may get better at specific tasks through practice, but these improvements don’t necessarily translate into improvement in other tasks that require other cognitive domains or prevention of dementia or age-related cognitive decline,” Dr. Hara said.
She noted that most brain-training programs “have not been rigorously tested in clinical trials” — although some, such as those featured in the ACTIVE trial, did show evidence of effectiveness.
Dr. Mahncke agreed. “Asking whether brain training works is like asking whether small molecules improve health,” he said noting that some brain-training programs are nonsense and not evidence based. He believes that his company’s product, BrainHQ, and some others are “backed by robust evidence in their ability to stave off, slow, or even reverse cognitive changes.”
BrainHQ is a web-based brain game suite that can be used independently as an app or in group settings (classes and webinars) and is covered by some Medicare Advantage insurance plans. It encompasses “dozens of individual brain-training exercises, linked by a common thread. Each one is intensively designed to make the brain faster and more accurate,” said Dr. Mahncke.
He explained that human brains “get noisy as people get older, like a radio which is wearing out, so there’s static in the background. This makes the music hard to hear, and in the case of the human brain, it makes it difficult to pay attention.” The exercises are “designed to tamp down the ‘noise,’ speed up the brain, and make information processing more accurate.”
Dr. Mahncke called this a “bottom-up” approach, in contrast to many previous cognitive-training approaches that come from the brain injury rehabilitation field. They teach “top-down” skills and strategies designed to compensate for deficits in specific domains, such as reading, concentration, or fine motor skills.
By contrast, the approach of BrainHQ is “to improve the overall processing system of the brain with speed, attention, working memory, and executive function, which will in turn impact all skills and activities.”
Supporting Evidence
Dr. Mahncke cited several supporting studies. For example, the IMPACT study randomized 487 adults (aged ≥ 65 years) to receive either a brain plasticity–based computerized cognitive training program (BrainHQ) or a novelty- and intensity-matched general cognitive stimulation treatment program (intervention and control group, respectively) for an 8-week period.
Those who underwent brain training showed significantly greater improvement in the repeatable Battery for the Assessment of Neuropsychological Status (RBANS Auditory Memory/Attention) compared with those in the control group (3.9 vs 1.8, respectively; P =.02). The intervention group also showed significant improvements on multiple secondary measures of attention and memory. The magnitude of the effect sizes suggests that the results are clinically significant, according to the authors.
The ACTIVE study tested the effects of different cognitive training programs on cognitive function and time to dementia. The researchers randomized 2802 healthy older adults (mean age, 74 years) to a control group with no cognitive training or one of three brain-training groups comprising:
1. In-person training on verbal memory skills
2. In-person training on reasoning and problem-solving
3. Computer-based speed-of-processing training on visual attention
Participants in the training groups completed 10 sessions, each lasting 60-75 minutes, over a 5- to 6-week period. A random subsample of each training group was selected to receive “booster” sessions, with four-session booster training delivered at 11 and 35 months. All study participants completed follow-up tests of cognition and function after 1, 2, 3, 5, and 10 years.
At the end of 10 years, those assigned to the speed-of-processing training, now part of BrainHQ, had a 29% lower risk for dementia than those in the control group who received no training. No reduction was found in the memory or reasoning training groups. Participants who completed the “booster” sessions had an even greater reduction: Each additional booster session was associated with a 10% lower risk for dementia.
Dr. Montero-Odasso was involved in the SYNERGIC study that randomized 175 participants with mild cognitive impairment (MCI; average age, 73 years) to one of five study arms:
1. Multidomain intervention with exercise, cognitive training, and vitamin D
2. Exercise, cognitive training, and placebo
3. Exercise, sham cognitive training, and vitamin D
4. Exercise, sham cognitive training, and placebo
5. Control group with balance-toning exercise, sham cognitive training, and placebo
“Sham” cognitive training consisted of alternating between two tasks (touristic search and video watching) performed on a tablet, with the same time exposure as the intervention training.
The researchers found that after 6 months of interventions, all active arms with aerobic-resistance exercise showed improvement in the ADAS-Cog-13, an established outcome to evaluate dementia treatments, when compared with the control group — regardless of the addition of cognitive training or vitamin D.
Compared with exercise alone (arms 3 and 4), those who did exercise plus cognitive training (arms 1 and 2) showed greater improvements in their ADAS-Cog-13l score, with a mean difference of −1.45 points (P = .02). The greatest improvement was seen in those who underwent the multidomain intervention in arm 1.
The authors noted that the mean 2.64-point improvement seen in the ADAS-Cog-13 for the multidomain intervention is actually larger than changes seen in previous pharmaceutical trials among individuals with MCI or mild dementia and “approaches” the three points considered clinically meaningful.
“We found that older adults with MCI who received aerobic-resistance exercise with sequential computerized cognitive training significantly improved cognition,” Dr. Montero-Odasso said. “The cognitive training we used was called Neuropeak, a multidomain lifestyle training delivered through a web-based platform developed by our co-leader Louis Bherer at Université de Montréal.”
He explained that the purpose “is to challenge your brain to the point where you need to make an effort to remember things, pay attention, and later to execute tasks. The evidence from clinical trials, including ours, shows this type of brain challenge is effective in slowing and even reversing cognitive decline.”
A follow-up study, SYNERGIC 2.0, is ongoing.
Puzzles, Board Games, and New Challenges
Formal brain-training programs aren’t the only way to improve brain plasticity, Dr. Hara said. Observational studies suggested an association between improved cognitive performance and/or lower dementia risk and engaging in number and word puzzles, such as crosswords, cards, or board games.
Some studies suggested that older adults who use technology might also protect their cognitive reserve. Dr. Hara cited a US longitudinal study of more than 18,000 older adults suggesting that regular Internet users had roughly half the risk for dementia compared to nonregular Internet users. Estimates of daily Internet use suggested a U-shaped relationship with dementia with 0.1-2.0 hours daily (excluding time spent watching television or movies online) associated with the lowest risk. Similar associations between Internet use and a lower risk for cognitive decline have been reported in the United Kingdom and Europe.
“Engaging in mentally stimulating activities can increase ‘cognitive reserve’ — meaning, capacity of the brain to resist the effects of age-related changes or disease-related pathology, such that one can maintain cognitive function for longer,” Dr. Hara said. “Cognitively stimulating activities, regardless of the type, may help delay the onset of cognitive decline.”
She listed several examples of activities that are stimulating to the brain, including learning a new game or puzzle, a new language, or a new dance, and learning how to play a musical instrument.
Dr. Montero-Odasso emphasized that the “newness” is key to increasing and preserving cognitive reserve. “Just surfing the Internet, playing word or board games, or doing crossword puzzles won’t be enough if you’ve been doing these things all your life,” he said. “It won’t hurt, of course, but it won’t necessarily increase your cognitive abilities.
“For example, a person who regularly engages in public speaking may not improve cognition by taking a public-speaking course, but someone who has never spoken before an audience might show cognitive improvements as a result of learning a new skill,” he said. “Or someone who knows several languages already might gain from learning a brand-new language.”
He cited research supporting the benefits of dancing, which he called “an ideal activity because it’s physical, so it provides the exercise that’s been associated with improved cognition. But it also requires learning new steps and moves, which builds the synapses in the brain. And the socialization of dance classes adds another component that can improve cognition.”
Dr. Mahncke hopes that beyond engaging in day-to-day new activities, seniors will participate in computerized brain training. “There’s no reason that evidence-based training can’t be offered in senior and community centers, as yoga and swimming are,” he said. “It doesn’t have to be simply something people do on their own virtually.”
Zoom classes and Medicare reimbursements are “good steps in the right direction, but it’s time to expand this potentially life-transformative intervention so that it reaches the ever-expanding population of seniors in the United States and beyond.”
Dr. Hara reported having no disclosures. Dr. Montero-Odasso reported having no commercial or financial interest related to this topic. He serves as the president of the Canadian Geriatrics Société and is team leader in the Canadian Consortium of Neurodegeneration in Aging. Dr. Mahncke is CEO of the brain training company Posit Science/BrainHQ.
A version of this article appeared on Medscape.com.
Novel Agent Curbs Alzheimer’s-Related Agitation
DENVER —
More than half of participants in the open-label extension period of the randomized clinical trial responded to the medication, which was associated with a 3.6-fold lower risk for relapse compared with placebo.
“The positive efficacy and favorable safety results with AXS-05 support its potential to fulfill a high unmet need for the treatment of Alzheimer’s disease agitation,” said Anton P. Porsteinsson, MD, director of the Alzheimer’s Disease Care, Research and Education Program, University of Rochester, New York.
The findings were presented at the 2024 annual meeting of the American Academy of Neurology.
Common and Disruptive
Agitation is reported in up to 70% of patients with Alzheimer’s disease and is characterized by emotional distress, aggressive behaviors, disruptive irritability, and disinhibition. Alzheimer’s disease-related agitation has been associated with increased caregiver burden, decreased functioning, accelerated cognitive decline, earlier nursing home placement, and increased mortality.
A previous phase 2/3 study of AXS-05 showed that the investigative agent led to rapid and significantly improvement in Alzheimer’s disease agitation, as measured by the Cohen-Mansfield Agitation Inventory (CMAI) total score, compared with placebo.
ACCORD was a phase 3, randomized, double-blind, placebo-controlled withdrawal trial evaluating the efficacy and safety of AXS-05 in patients with Alzheimer’s disease agitation.
In the open-label period, 178 adults with probable Alzheimer’s disease and clinically significant agitation received AXS-05 (titrated to 45 mg dextromethorphan/105 mg bupropion twice daily) for up to 9 weeks.
A total of 108 (61%) patients had a sustained response, with 30% or more improvement from baseline in the CMAI total score and improvement on the Patient Global Impression of Change that were both maintained for 4 or more consecutive weeks. These patients entered the double-blind phase and were randomly allocated to receive twice-daily AXS-05 or placebo for up to 26 weeks.
In the double-blind period, AXS-05 “substantially and statistically” increased the time to relapse of agitation symptoms compared with placebo (hazard ratio [HR], 0.275; P = .014).
“The risk of relapse was 3.6-fold lower with AXS-05 compared with placebo,” Dr. Porsteinsson reported.
AXS-05 was also associated with a significantly lower relapse rate compared with placebo (7.5% vs 25.9%; P = .018).
Rates of discontinuation in the double-blind period owing to adverse events (AEs) were low (0% for AXS-05 and 1.9% for placebo). Three serious AEs were reported: one in the AXS-05 group (fecaloma), which was not related to study medication, and two in the placebo group (cardiac arrest, femur fracture).
Falls were reported in four participants in the AXS-05 group, none of which were related to study medication or associated with serious AEs, and in two participants in the placebo group, one of which was associated with femur fracture.
One death was reported in the placebo group. There was no evidence of cognitive decline with AXS-05, and treatment was not associated with sedation.
Promising Agent
Commenting on this research, Glen R. Finney, MD, director of the Geisinger Memory and Cognition Clinic in Wilkes-Barre, Pennsylvania, said the data “look promising as a safe way to help address acute agitation and reduce agitation reoccurrence.
“Agitation is a common, distressing, and sometimes safety issue for people fighting Alzheimer’s disease, and there’s very little evidence for efficacy and significant side effect issues for current medical management of agitation in Alzheimer’s disease,” said Dr. Finney, who was not part of the study.
He noted that first-line strategies for addressing agitation involve behavioral and environmental interventions.
“See if there’s a reason for the agitation and address that. Look for triggers for agitation and avoid those. Find places, things, and interactions that help people with Alzheimer’s disease avoid agitation: familiar locations, music, simple engaging activities. Reassurance, redirection, and distraction can help de-escalate agitation. Provide a safe environment that reduces safety risks,” Dr. Finney explained.
The next step, when medically appropriate, is trying acetylcholinesterase inhibitors such as donepezil, rivastigmine, and galantamine, and then adding memantine, a weak N-methyl-D-aspartate receptor antagonist.
“These medications can help reduce the risk of agitation,” Dr. Finney said.
“Beyond that, the evidence becomes weaker for any specific treatments, and that is where treatments with emerging evidence of efficacy and safety like dextromethorphan-bupropion become important,” Dr. Finney added.
Last May, the US Food and Drug Administration (FDA) approved the antipsychotic brexpiprazole (Rexulti) for Alzheimer’s disease-related agitation, making it the first FDA-approved drug for this indication.
The drug includes a boxed warning for medications in this class that older patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk for death.
“There’s certainly a need to have multiple options for treating agitation in individuals with Alzheimer’s disease,” said Rebecca Edelmayer, PhD, senior director of scientific engagement for the Alzheimer’s Association.
Dr. Edelmayer, who was not part of the study, noted that in the ACCORD study, AXS-05 “significantly delayed the relapse or prevented the relapse with Alzheimer’s disease agitation compared with the placebo group and it was generally well tolerated, but it will be important to make sure that there’s more thorough review of the data overall to be sure that it’s both safe and effective.”
The study was funded by Axsome Therapeutics, the manufacturer of AXS-05. Dr. Porsteinsson has disclosed no relevant conflicts of interest. Dr. Finney and Dr. Edelmayer have no relevant disclosures.
A version of this article appeared on Medscape.com.
DENVER —
More than half of participants in the open-label extension period of the randomized clinical trial responded to the medication, which was associated with a 3.6-fold lower risk for relapse compared with placebo.
“The positive efficacy and favorable safety results with AXS-05 support its potential to fulfill a high unmet need for the treatment of Alzheimer’s disease agitation,” said Anton P. Porsteinsson, MD, director of the Alzheimer’s Disease Care, Research and Education Program, University of Rochester, New York.
The findings were presented at the 2024 annual meeting of the American Academy of Neurology.
Common and Disruptive
Agitation is reported in up to 70% of patients with Alzheimer’s disease and is characterized by emotional distress, aggressive behaviors, disruptive irritability, and disinhibition. Alzheimer’s disease-related agitation has been associated with increased caregiver burden, decreased functioning, accelerated cognitive decline, earlier nursing home placement, and increased mortality.
A previous phase 2/3 study of AXS-05 showed that the investigative agent led to rapid and significantly improvement in Alzheimer’s disease agitation, as measured by the Cohen-Mansfield Agitation Inventory (CMAI) total score, compared with placebo.
ACCORD was a phase 3, randomized, double-blind, placebo-controlled withdrawal trial evaluating the efficacy and safety of AXS-05 in patients with Alzheimer’s disease agitation.
In the open-label period, 178 adults with probable Alzheimer’s disease and clinically significant agitation received AXS-05 (titrated to 45 mg dextromethorphan/105 mg bupropion twice daily) for up to 9 weeks.
A total of 108 (61%) patients had a sustained response, with 30% or more improvement from baseline in the CMAI total score and improvement on the Patient Global Impression of Change that were both maintained for 4 or more consecutive weeks. These patients entered the double-blind phase and were randomly allocated to receive twice-daily AXS-05 or placebo for up to 26 weeks.
In the double-blind period, AXS-05 “substantially and statistically” increased the time to relapse of agitation symptoms compared with placebo (hazard ratio [HR], 0.275; P = .014).
“The risk of relapse was 3.6-fold lower with AXS-05 compared with placebo,” Dr. Porsteinsson reported.
AXS-05 was also associated with a significantly lower relapse rate compared with placebo (7.5% vs 25.9%; P = .018).
Rates of discontinuation in the double-blind period owing to adverse events (AEs) were low (0% for AXS-05 and 1.9% for placebo). Three serious AEs were reported: one in the AXS-05 group (fecaloma), which was not related to study medication, and two in the placebo group (cardiac arrest, femur fracture).
Falls were reported in four participants in the AXS-05 group, none of which were related to study medication or associated with serious AEs, and in two participants in the placebo group, one of which was associated with femur fracture.
One death was reported in the placebo group. There was no evidence of cognitive decline with AXS-05, and treatment was not associated with sedation.
Promising Agent
Commenting on this research, Glen R. Finney, MD, director of the Geisinger Memory and Cognition Clinic in Wilkes-Barre, Pennsylvania, said the data “look promising as a safe way to help address acute agitation and reduce agitation reoccurrence.
“Agitation is a common, distressing, and sometimes safety issue for people fighting Alzheimer’s disease, and there’s very little evidence for efficacy and significant side effect issues for current medical management of agitation in Alzheimer’s disease,” said Dr. Finney, who was not part of the study.
He noted that first-line strategies for addressing agitation involve behavioral and environmental interventions.
“See if there’s a reason for the agitation and address that. Look for triggers for agitation and avoid those. Find places, things, and interactions that help people with Alzheimer’s disease avoid agitation: familiar locations, music, simple engaging activities. Reassurance, redirection, and distraction can help de-escalate agitation. Provide a safe environment that reduces safety risks,” Dr. Finney explained.
The next step, when medically appropriate, is trying acetylcholinesterase inhibitors such as donepezil, rivastigmine, and galantamine, and then adding memantine, a weak N-methyl-D-aspartate receptor antagonist.
“These medications can help reduce the risk of agitation,” Dr. Finney said.
“Beyond that, the evidence becomes weaker for any specific treatments, and that is where treatments with emerging evidence of efficacy and safety like dextromethorphan-bupropion become important,” Dr. Finney added.
Last May, the US Food and Drug Administration (FDA) approved the antipsychotic brexpiprazole (Rexulti) for Alzheimer’s disease-related agitation, making it the first FDA-approved drug for this indication.
The drug includes a boxed warning for medications in this class that older patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk for death.
“There’s certainly a need to have multiple options for treating agitation in individuals with Alzheimer’s disease,” said Rebecca Edelmayer, PhD, senior director of scientific engagement for the Alzheimer’s Association.
Dr. Edelmayer, who was not part of the study, noted that in the ACCORD study, AXS-05 “significantly delayed the relapse or prevented the relapse with Alzheimer’s disease agitation compared with the placebo group and it was generally well tolerated, but it will be important to make sure that there’s more thorough review of the data overall to be sure that it’s both safe and effective.”
The study was funded by Axsome Therapeutics, the manufacturer of AXS-05. Dr. Porsteinsson has disclosed no relevant conflicts of interest. Dr. Finney and Dr. Edelmayer have no relevant disclosures.
A version of this article appeared on Medscape.com.
DENVER —
More than half of participants in the open-label extension period of the randomized clinical trial responded to the medication, which was associated with a 3.6-fold lower risk for relapse compared with placebo.
“The positive efficacy and favorable safety results with AXS-05 support its potential to fulfill a high unmet need for the treatment of Alzheimer’s disease agitation,” said Anton P. Porsteinsson, MD, director of the Alzheimer’s Disease Care, Research and Education Program, University of Rochester, New York.
The findings were presented at the 2024 annual meeting of the American Academy of Neurology.
Common and Disruptive
Agitation is reported in up to 70% of patients with Alzheimer’s disease and is characterized by emotional distress, aggressive behaviors, disruptive irritability, and disinhibition. Alzheimer’s disease-related agitation has been associated with increased caregiver burden, decreased functioning, accelerated cognitive decline, earlier nursing home placement, and increased mortality.
A previous phase 2/3 study of AXS-05 showed that the investigative agent led to rapid and significantly improvement in Alzheimer’s disease agitation, as measured by the Cohen-Mansfield Agitation Inventory (CMAI) total score, compared with placebo.
ACCORD was a phase 3, randomized, double-blind, placebo-controlled withdrawal trial evaluating the efficacy and safety of AXS-05 in patients with Alzheimer’s disease agitation.
In the open-label period, 178 adults with probable Alzheimer’s disease and clinically significant agitation received AXS-05 (titrated to 45 mg dextromethorphan/105 mg bupropion twice daily) for up to 9 weeks.
A total of 108 (61%) patients had a sustained response, with 30% or more improvement from baseline in the CMAI total score and improvement on the Patient Global Impression of Change that were both maintained for 4 or more consecutive weeks. These patients entered the double-blind phase and were randomly allocated to receive twice-daily AXS-05 or placebo for up to 26 weeks.
In the double-blind period, AXS-05 “substantially and statistically” increased the time to relapse of agitation symptoms compared with placebo (hazard ratio [HR], 0.275; P = .014).
“The risk of relapse was 3.6-fold lower with AXS-05 compared with placebo,” Dr. Porsteinsson reported.
AXS-05 was also associated with a significantly lower relapse rate compared with placebo (7.5% vs 25.9%; P = .018).
Rates of discontinuation in the double-blind period owing to adverse events (AEs) were low (0% for AXS-05 and 1.9% for placebo). Three serious AEs were reported: one in the AXS-05 group (fecaloma), which was not related to study medication, and two in the placebo group (cardiac arrest, femur fracture).
Falls were reported in four participants in the AXS-05 group, none of which were related to study medication or associated with serious AEs, and in two participants in the placebo group, one of which was associated with femur fracture.
One death was reported in the placebo group. There was no evidence of cognitive decline with AXS-05, and treatment was not associated with sedation.
Promising Agent
Commenting on this research, Glen R. Finney, MD, director of the Geisinger Memory and Cognition Clinic in Wilkes-Barre, Pennsylvania, said the data “look promising as a safe way to help address acute agitation and reduce agitation reoccurrence.
“Agitation is a common, distressing, and sometimes safety issue for people fighting Alzheimer’s disease, and there’s very little evidence for efficacy and significant side effect issues for current medical management of agitation in Alzheimer’s disease,” said Dr. Finney, who was not part of the study.
He noted that first-line strategies for addressing agitation involve behavioral and environmental interventions.
“See if there’s a reason for the agitation and address that. Look for triggers for agitation and avoid those. Find places, things, and interactions that help people with Alzheimer’s disease avoid agitation: familiar locations, music, simple engaging activities. Reassurance, redirection, and distraction can help de-escalate agitation. Provide a safe environment that reduces safety risks,” Dr. Finney explained.
The next step, when medically appropriate, is trying acetylcholinesterase inhibitors such as donepezil, rivastigmine, and galantamine, and then adding memantine, a weak N-methyl-D-aspartate receptor antagonist.
“These medications can help reduce the risk of agitation,” Dr. Finney said.
“Beyond that, the evidence becomes weaker for any specific treatments, and that is where treatments with emerging evidence of efficacy and safety like dextromethorphan-bupropion become important,” Dr. Finney added.
Last May, the US Food and Drug Administration (FDA) approved the antipsychotic brexpiprazole (Rexulti) for Alzheimer’s disease-related agitation, making it the first FDA-approved drug for this indication.
The drug includes a boxed warning for medications in this class that older patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk for death.
“There’s certainly a need to have multiple options for treating agitation in individuals with Alzheimer’s disease,” said Rebecca Edelmayer, PhD, senior director of scientific engagement for the Alzheimer’s Association.
Dr. Edelmayer, who was not part of the study, noted that in the ACCORD study, AXS-05 “significantly delayed the relapse or prevented the relapse with Alzheimer’s disease agitation compared with the placebo group and it was generally well tolerated, but it will be important to make sure that there’s more thorough review of the data overall to be sure that it’s both safe and effective.”
The study was funded by Axsome Therapeutics, the manufacturer of AXS-05. Dr. Porsteinsson has disclosed no relevant conflicts of interest. Dr. Finney and Dr. Edelmayer have no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM AAN 2024
Antipsychotics for Dementia Pose Wide-Ranging Health Risks
Antipsychotic use in older adults with dementia is associated with a significant increased risk for stroke, myocardial infarction, heart failure, pneumonia, fracture, acute kidney injury, and a range of other health problems compared with nonuse, new research showed.
The adverse events are far broader and pose more severe health risks than previously reported, investigators noted, and suggested greater caution is needed when prescribing antipsychotics to treat psychological symptoms of dementia.
The matched cohort study used patient registry data on nearly 174,000 people with dementia and compared those who were prescribed an antipsychotic on or after their dementia diagnosis with those who had not received a prescription for the drugs.
Any antipsychotic use was associated with double the risk for pneumonia, a 1.7-fold increased risk for acute kidney injury, and 1.6-fold higher odds of venous thromboembolism compared to nonuse.
Investigators found an increased risk for all outcomes studied, except for ventricular arrythmia, and risk was highest for most within the first week of treatment.
“Any potential benefits of antipsychotic treatment therefore need to be weighed against the risk of serious harm across multiple outcomes. Although there may be times when an antipsychotic prescription is the least bad option, clinicians should actively consider the risks, considering patients’ pre-existing comorbidities and living support,” lead investigator Pearl Mok, research fellow at the Centre for Pharmacoepidemiology and Drug Safety, The University of Manchester, Manchester, England, and colleagues wrote.
The findings were published online in The BMJ.
High Risk
Depression, aggression, anxiety, psychosis, and other behavioral and psychological symptoms are common in people with dementia. Despite earlier reports of increased risk for stroke and mortality with antipsychotic use, the drugs are frequently prescribed to treat these symptoms.
While some preliminary studies identified other adverse outcomes from antipsychotic use, results are limited and inconsistent.
Investigators used primary and secondary care data from the Clinical Practice Research Datalink in England. A total of 173,910 adults (63% women) had a dementia diagnosis between January 1998 and May 2018.
Of the total cohort, 35,339 patients were prescribed an antipsychotic on, or after, a dementia diagnosis. Each was matched with up to 15 patients with dementia with no history of antipsychotic use following diagnosis.
Almost 80% of antipsychotic prescriptions were for risperidone, quetiapine, haloperidol, and olanzapine.
Any antipsychotic use was associated with significantly higher risks for pneumonia (hazard ratio [HR], 2.03; 95% CI, 1.96-2.10), acute kidney injury (HR, 1.57; 95% CI, 1.48-1.66), stroke (HR, 1.54; 95% CI, 1.46-1.63), venous thromboembolism (HR, 1.52; 95% CI, 1.38-1.67), fracture (HR, 1.36; 95% CI, 1.30-1.44), myocardial infarction (HR, 1.22; 95% CI, 1.12-1.34), and heart failure (HR, 1.16; 95% CI, 1.09-1.24).
The risk for all conditions was highest within the first 3 months of treatment, with a cumulative incidence of pneumonia among antipsychotic users of 4.48% vs 1.49% among nonusers. At 1 year, this increased to 10.41% for users vs 5.63% for nonusers.
“Given the higher risks of adverse events in the early days after drug initiation, clinical examinations should be taken before, and clinical reviews conducted shortly after, the start of treatment,” the authors wrote. “Our study reaffirms that these drugs should only be prescribed for the shortest period possible.”
‘Serious Harms’
In an accompanying editorial, Raya Elfadel Kheirbek, MD, and Cristina LaFont, Department of Medicine, University of Maryland School of Medicine, Baltimore, Maryland, said the findings “highlight the need for careful justification of antipsychotic use in dementia care, including a comprehensive assessment of the benefits weighed against a broader range of serious harms than previously acknowledged.”
“Using antipsychotics for the management of dementia-related behaviors requires nuanced decision-making after careful assessment, informed by a personalized approach,” they continued. “Dr. Mok and colleagues call for a critical re-evaluation of antipsychotic use in this clinical setting.”
While the findings add to and expand what was already known, “we need to be clear that they don’t show antipsychotics cause all the adverse outcomes reported,” Masud Husain, DPhil, professor of neurology, University of Oxford, England, said in a statement.
While investigators attempted to use matched controls with dementia who had not received antipsychotics, “the people who were prescribed the drugs may simply have been more vulnerable to some of the conditions that occurred more frequently in them, such as pneumonia and cardiovascular disorders,” said Dr. Husain, who was not part of the research.
Although the study was not designed to explore reverse causality, the findings are important for clinicians who prescribe antipsychotics for patients with dementia, Robert Howard, professor of old age psychiatry, at the University of College London, London, England said in a statement.
“Initiation of these drugs in people with dementia should only ever be under specialist supervision, with involvement of patients and family members in informed discussion and review,” said Dr. Howard, who was not involved in the study.
The study was funded by the National Institute for Health and Care Research. Dr. Mok reported no relevant conflicts. Other authors’ disclosures are included in the original article. Dr. Hussain, Dr. Howard, Dr. Kheirbek, and Dr. LeFon reported no relevant conflicts.
A version of this article appeared on Medscape.com.
Antipsychotic use in older adults with dementia is associated with a significant increased risk for stroke, myocardial infarction, heart failure, pneumonia, fracture, acute kidney injury, and a range of other health problems compared with nonuse, new research showed.
The adverse events are far broader and pose more severe health risks than previously reported, investigators noted, and suggested greater caution is needed when prescribing antipsychotics to treat psychological symptoms of dementia.
The matched cohort study used patient registry data on nearly 174,000 people with dementia and compared those who were prescribed an antipsychotic on or after their dementia diagnosis with those who had not received a prescription for the drugs.
Any antipsychotic use was associated with double the risk for pneumonia, a 1.7-fold increased risk for acute kidney injury, and 1.6-fold higher odds of venous thromboembolism compared to nonuse.
Investigators found an increased risk for all outcomes studied, except for ventricular arrythmia, and risk was highest for most within the first week of treatment.
“Any potential benefits of antipsychotic treatment therefore need to be weighed against the risk of serious harm across multiple outcomes. Although there may be times when an antipsychotic prescription is the least bad option, clinicians should actively consider the risks, considering patients’ pre-existing comorbidities and living support,” lead investigator Pearl Mok, research fellow at the Centre for Pharmacoepidemiology and Drug Safety, The University of Manchester, Manchester, England, and colleagues wrote.
The findings were published online in The BMJ.
High Risk
Depression, aggression, anxiety, psychosis, and other behavioral and psychological symptoms are common in people with dementia. Despite earlier reports of increased risk for stroke and mortality with antipsychotic use, the drugs are frequently prescribed to treat these symptoms.
While some preliminary studies identified other adverse outcomes from antipsychotic use, results are limited and inconsistent.
Investigators used primary and secondary care data from the Clinical Practice Research Datalink in England. A total of 173,910 adults (63% women) had a dementia diagnosis between January 1998 and May 2018.
Of the total cohort, 35,339 patients were prescribed an antipsychotic on, or after, a dementia diagnosis. Each was matched with up to 15 patients with dementia with no history of antipsychotic use following diagnosis.
Almost 80% of antipsychotic prescriptions were for risperidone, quetiapine, haloperidol, and olanzapine.
Any antipsychotic use was associated with significantly higher risks for pneumonia (hazard ratio [HR], 2.03; 95% CI, 1.96-2.10), acute kidney injury (HR, 1.57; 95% CI, 1.48-1.66), stroke (HR, 1.54; 95% CI, 1.46-1.63), venous thromboembolism (HR, 1.52; 95% CI, 1.38-1.67), fracture (HR, 1.36; 95% CI, 1.30-1.44), myocardial infarction (HR, 1.22; 95% CI, 1.12-1.34), and heart failure (HR, 1.16; 95% CI, 1.09-1.24).
The risk for all conditions was highest within the first 3 months of treatment, with a cumulative incidence of pneumonia among antipsychotic users of 4.48% vs 1.49% among nonusers. At 1 year, this increased to 10.41% for users vs 5.63% for nonusers.
“Given the higher risks of adverse events in the early days after drug initiation, clinical examinations should be taken before, and clinical reviews conducted shortly after, the start of treatment,” the authors wrote. “Our study reaffirms that these drugs should only be prescribed for the shortest period possible.”
‘Serious Harms’
In an accompanying editorial, Raya Elfadel Kheirbek, MD, and Cristina LaFont, Department of Medicine, University of Maryland School of Medicine, Baltimore, Maryland, said the findings “highlight the need for careful justification of antipsychotic use in dementia care, including a comprehensive assessment of the benefits weighed against a broader range of serious harms than previously acknowledged.”
“Using antipsychotics for the management of dementia-related behaviors requires nuanced decision-making after careful assessment, informed by a personalized approach,” they continued. “Dr. Mok and colleagues call for a critical re-evaluation of antipsychotic use in this clinical setting.”
While the findings add to and expand what was already known, “we need to be clear that they don’t show antipsychotics cause all the adverse outcomes reported,” Masud Husain, DPhil, professor of neurology, University of Oxford, England, said in a statement.
While investigators attempted to use matched controls with dementia who had not received antipsychotics, “the people who were prescribed the drugs may simply have been more vulnerable to some of the conditions that occurred more frequently in them, such as pneumonia and cardiovascular disorders,” said Dr. Husain, who was not part of the research.
Although the study was not designed to explore reverse causality, the findings are important for clinicians who prescribe antipsychotics for patients with dementia, Robert Howard, professor of old age psychiatry, at the University of College London, London, England said in a statement.
“Initiation of these drugs in people with dementia should only ever be under specialist supervision, with involvement of patients and family members in informed discussion and review,” said Dr. Howard, who was not involved in the study.
The study was funded by the National Institute for Health and Care Research. Dr. Mok reported no relevant conflicts. Other authors’ disclosures are included in the original article. Dr. Hussain, Dr. Howard, Dr. Kheirbek, and Dr. LeFon reported no relevant conflicts.
A version of this article appeared on Medscape.com.
Antipsychotic use in older adults with dementia is associated with a significant increased risk for stroke, myocardial infarction, heart failure, pneumonia, fracture, acute kidney injury, and a range of other health problems compared with nonuse, new research showed.
The adverse events are far broader and pose more severe health risks than previously reported, investigators noted, and suggested greater caution is needed when prescribing antipsychotics to treat psychological symptoms of dementia.
The matched cohort study used patient registry data on nearly 174,000 people with dementia and compared those who were prescribed an antipsychotic on or after their dementia diagnosis with those who had not received a prescription for the drugs.
Any antipsychotic use was associated with double the risk for pneumonia, a 1.7-fold increased risk for acute kidney injury, and 1.6-fold higher odds of venous thromboembolism compared to nonuse.
Investigators found an increased risk for all outcomes studied, except for ventricular arrythmia, and risk was highest for most within the first week of treatment.
“Any potential benefits of antipsychotic treatment therefore need to be weighed against the risk of serious harm across multiple outcomes. Although there may be times when an antipsychotic prescription is the least bad option, clinicians should actively consider the risks, considering patients’ pre-existing comorbidities and living support,” lead investigator Pearl Mok, research fellow at the Centre for Pharmacoepidemiology and Drug Safety, The University of Manchester, Manchester, England, and colleagues wrote.
The findings were published online in The BMJ.
High Risk
Depression, aggression, anxiety, psychosis, and other behavioral and psychological symptoms are common in people with dementia. Despite earlier reports of increased risk for stroke and mortality with antipsychotic use, the drugs are frequently prescribed to treat these symptoms.
While some preliminary studies identified other adverse outcomes from antipsychotic use, results are limited and inconsistent.
Investigators used primary and secondary care data from the Clinical Practice Research Datalink in England. A total of 173,910 adults (63% women) had a dementia diagnosis between January 1998 and May 2018.
Of the total cohort, 35,339 patients were prescribed an antipsychotic on, or after, a dementia diagnosis. Each was matched with up to 15 patients with dementia with no history of antipsychotic use following diagnosis.
Almost 80% of antipsychotic prescriptions were for risperidone, quetiapine, haloperidol, and olanzapine.
Any antipsychotic use was associated with significantly higher risks for pneumonia (hazard ratio [HR], 2.03; 95% CI, 1.96-2.10), acute kidney injury (HR, 1.57; 95% CI, 1.48-1.66), stroke (HR, 1.54; 95% CI, 1.46-1.63), venous thromboembolism (HR, 1.52; 95% CI, 1.38-1.67), fracture (HR, 1.36; 95% CI, 1.30-1.44), myocardial infarction (HR, 1.22; 95% CI, 1.12-1.34), and heart failure (HR, 1.16; 95% CI, 1.09-1.24).
The risk for all conditions was highest within the first 3 months of treatment, with a cumulative incidence of pneumonia among antipsychotic users of 4.48% vs 1.49% among nonusers. At 1 year, this increased to 10.41% for users vs 5.63% for nonusers.
“Given the higher risks of adverse events in the early days after drug initiation, clinical examinations should be taken before, and clinical reviews conducted shortly after, the start of treatment,” the authors wrote. “Our study reaffirms that these drugs should only be prescribed for the shortest period possible.”
‘Serious Harms’
In an accompanying editorial, Raya Elfadel Kheirbek, MD, and Cristina LaFont, Department of Medicine, University of Maryland School of Medicine, Baltimore, Maryland, said the findings “highlight the need for careful justification of antipsychotic use in dementia care, including a comprehensive assessment of the benefits weighed against a broader range of serious harms than previously acknowledged.”
“Using antipsychotics for the management of dementia-related behaviors requires nuanced decision-making after careful assessment, informed by a personalized approach,” they continued. “Dr. Mok and colleagues call for a critical re-evaluation of antipsychotic use in this clinical setting.”
While the findings add to and expand what was already known, “we need to be clear that they don’t show antipsychotics cause all the adverse outcomes reported,” Masud Husain, DPhil, professor of neurology, University of Oxford, England, said in a statement.
While investigators attempted to use matched controls with dementia who had not received antipsychotics, “the people who were prescribed the drugs may simply have been more vulnerable to some of the conditions that occurred more frequently in them, such as pneumonia and cardiovascular disorders,” said Dr. Husain, who was not part of the research.
Although the study was not designed to explore reverse causality, the findings are important for clinicians who prescribe antipsychotics for patients with dementia, Robert Howard, professor of old age psychiatry, at the University of College London, London, England said in a statement.
“Initiation of these drugs in people with dementia should only ever be under specialist supervision, with involvement of patients and family members in informed discussion and review,” said Dr. Howard, who was not involved in the study.
The study was funded by the National Institute for Health and Care Research. Dr. Mok reported no relevant conflicts. Other authors’ disclosures are included in the original article. Dr. Hussain, Dr. Howard, Dr. Kheirbek, and Dr. LeFon reported no relevant conflicts.
A version of this article appeared on Medscape.com.
FROM THE BMJ
In Lecanemab Alzheimer Extension Study, Placebo Roll-Over Group Does Not Catch Up
DENVER — , according to a first report of 6-month OLE data.
Due to the steady disease progression observed after the switch of placebo to active therapy, the message of these data is that “early initiation of lecanemab is important,” according to Michael Irizarry, MD, the senior vice president of clinical research at Eisai Ltd, which markets lecanemab.
The 6-month OLE data along with data from a tau PET substudy were presented by Dr. Irizarry at the 2024 annual meeting of the American Academy of Neurology.
From the start of the OLE through the 6-month follow-up, the downward trajectory of cognitive function, as measured with the Clinical Dementia Rating – Sum of Boxes (CDR-SB), has been parallel for the lecanemab-start and switch arms. As a result, the degree of separation between active and placebo groups over the course of the OLE has remained unchanged from the end of the randomized trial.
This does not rule out any benefit in the switch arm, according to Dr. Irizarry. Although there was no discernible change in the trajectory of decline among placebo patients after they were switched to lecanemab, Dr. Irizarry postulated that this might overlook the greater likely decline over time with no treatment.
“There was no placebo group in the OLE to compare with those on active treatment,” he pointed out. He then juxtaposed data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Over the same 6-month timeframe, these data show a hypothetical separation of the curves if no treatment had been received.
The 6-month OLE data provide a preliminary look at outcomes in a planned 4-year follow-up. At the end of the randomized CLARITY trial, the mean decline from the baseline CDR-SB score of 3.2, was 1.21 in the lecanemab group, translating into a 38% decline, and 1.66 in the placebo group, translating into about a 50% decline. Over the 6 months of OLE, there has been a further mean CDR-SB reduction of approximately 0.6 in both arms, suggesting a further 18% decline from baseline.
Additional Data
In the pivotal CLARITY trial, which was published a few months prior to regulatory approval early last year, 1785 patients were randomized to 10 mg/kg lecanemab or placebo infused every 2 weeks. At the end of 18 months, the superiority of lecanemab for the primary endpoint of adverse change in CDR-SB was highly significant (P < .001) as were the differences in key secondary endpoints, such as Alzheimer’s Disease Composite Score (P < .001).
Of those who participated in CLARITY, 1385 patients entered the OLE. Placebo patients were switched to lecanemab which is being maintained in all patients on the trial schedule of 10 mg/kg administered by intravenous infusion every 2 weeks.
In addition to the overall OLE 6-month data, which has not raised any new safety signals, Dr. Irizarry provided a new look at the PET TAU substudy with a focus on patients who entered the study with a low relative tau burden. Of the three classifications, which also included medium and high tau, as measured with positron-emission tomography (PET), the low tau group represented 41.2% of the 342 tau PET substudy participants. With only 2.9% entering the study with a high tau burden, almost all the others fell in the medium stratification.
Due to the potential for a lower therapeutic response, “patients with low Tau are often excluded from trials,” Dr. Irizarry said. But the sizable proportion of low tau patients has permitted an assessment of relative response with lecanemab, which turned out to be substantial.
“Consistent rates of clinical stability or improvements were observed regardless of baseline tau levels with the highest rates of improvements observed for the low tau group after 24 months of follow-up,” Dr. Irizarry reported.
In previously reported results from the tau PET substudy, lecanemab was shown to slow tau spread at least numerically in every section of the brain evaluated, including the frontal, cingulate, parietal, and whole cortical gray matter areas. The reductions reached significance for the medial temporal (P = .0024), meta temporal (P = .012), and temporal (P = .16) portions.
When most recently evaluated in the OLE, the CDR-SB score declined 38% less among those treated with lecanemab than those treated with placebo in the subgroup enrolled in the tau PET substudy.
Relative to those with intermediate or high tau, patients in the low tau had an even greater reduction in cognitive decline than those with higher tau burdens. Although Dr. Irizarry cautioned that greater baseline CDR-SB scores exaggerated the treatment effect in the low tau group, the message is that “a lecanemab treatment effect is seen even when baseline tau levels are low.”
Now, with the recent market withdrawal of aducanumab, another anti-amyloid monoclonal antibody that was previously approved for Alzheimer’s disease, lecanemab is the only therapy currently available for the goal of changing disease progression, not just modifying symptoms.
Looking Long Term
Both sets of data provide important messages for clinicians, according to Marcelo Matiello, MD, a physician investigator at Mass General Hospital and associate professor of neurology at Harvard Medical School, Boston.
“Clinicians are really looking for more data because this remains a relatively new drug,” he said. Both sets of findings presented by Dr. Irizarry “look good but the follow-up is still short, so I think everyone is still looking closely at long-term safety and efficacy.”
The need for continuous indefinite therapy is one concern that Dr. Matiello expressed. As moderator of the session in which these data were presented, Dr. Matiello specifically asked Dr. Irizarry if there are plans to explore whether periods without treatment might be a means to reduce the cost and burden of frequent infusions while preserving cognitive gains.
In response, Dr. Irizarry said that earlier studies showed rapid progression when lecanemab was stopped. On this basis, he thinks therapy must be maintained, but he did say that there are plans to look at less frequent dosing, such as once per month. He also said that a subcutaneous formulation in development might also reduce the burden of prolonged treatment.
Dr. Irizarry is an employee of Eisai Ltd., which manufacturers lecanemab. Dr. Matiello reports no potential conflicts of interest.
DENVER — , according to a first report of 6-month OLE data.
Due to the steady disease progression observed after the switch of placebo to active therapy, the message of these data is that “early initiation of lecanemab is important,” according to Michael Irizarry, MD, the senior vice president of clinical research at Eisai Ltd, which markets lecanemab.
The 6-month OLE data along with data from a tau PET substudy were presented by Dr. Irizarry at the 2024 annual meeting of the American Academy of Neurology.
From the start of the OLE through the 6-month follow-up, the downward trajectory of cognitive function, as measured with the Clinical Dementia Rating – Sum of Boxes (CDR-SB), has been parallel for the lecanemab-start and switch arms. As a result, the degree of separation between active and placebo groups over the course of the OLE has remained unchanged from the end of the randomized trial.
This does not rule out any benefit in the switch arm, according to Dr. Irizarry. Although there was no discernible change in the trajectory of decline among placebo patients after they were switched to lecanemab, Dr. Irizarry postulated that this might overlook the greater likely decline over time with no treatment.
“There was no placebo group in the OLE to compare with those on active treatment,” he pointed out. He then juxtaposed data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Over the same 6-month timeframe, these data show a hypothetical separation of the curves if no treatment had been received.
The 6-month OLE data provide a preliminary look at outcomes in a planned 4-year follow-up. At the end of the randomized CLARITY trial, the mean decline from the baseline CDR-SB score of 3.2, was 1.21 in the lecanemab group, translating into a 38% decline, and 1.66 in the placebo group, translating into about a 50% decline. Over the 6 months of OLE, there has been a further mean CDR-SB reduction of approximately 0.6 in both arms, suggesting a further 18% decline from baseline.
Additional Data
In the pivotal CLARITY trial, which was published a few months prior to regulatory approval early last year, 1785 patients were randomized to 10 mg/kg lecanemab or placebo infused every 2 weeks. At the end of 18 months, the superiority of lecanemab for the primary endpoint of adverse change in CDR-SB was highly significant (P < .001) as were the differences in key secondary endpoints, such as Alzheimer’s Disease Composite Score (P < .001).
Of those who participated in CLARITY, 1385 patients entered the OLE. Placebo patients were switched to lecanemab which is being maintained in all patients on the trial schedule of 10 mg/kg administered by intravenous infusion every 2 weeks.
In addition to the overall OLE 6-month data, which has not raised any new safety signals, Dr. Irizarry provided a new look at the PET TAU substudy with a focus on patients who entered the study with a low relative tau burden. Of the three classifications, which also included medium and high tau, as measured with positron-emission tomography (PET), the low tau group represented 41.2% of the 342 tau PET substudy participants. With only 2.9% entering the study with a high tau burden, almost all the others fell in the medium stratification.
Due to the potential for a lower therapeutic response, “patients with low Tau are often excluded from trials,” Dr. Irizarry said. But the sizable proportion of low tau patients has permitted an assessment of relative response with lecanemab, which turned out to be substantial.
“Consistent rates of clinical stability or improvements were observed regardless of baseline tau levels with the highest rates of improvements observed for the low tau group after 24 months of follow-up,” Dr. Irizarry reported.
In previously reported results from the tau PET substudy, lecanemab was shown to slow tau spread at least numerically in every section of the brain evaluated, including the frontal, cingulate, parietal, and whole cortical gray matter areas. The reductions reached significance for the medial temporal (P = .0024), meta temporal (P = .012), and temporal (P = .16) portions.
When most recently evaluated in the OLE, the CDR-SB score declined 38% less among those treated with lecanemab than those treated with placebo in the subgroup enrolled in the tau PET substudy.
Relative to those with intermediate or high tau, patients in the low tau had an even greater reduction in cognitive decline than those with higher tau burdens. Although Dr. Irizarry cautioned that greater baseline CDR-SB scores exaggerated the treatment effect in the low tau group, the message is that “a lecanemab treatment effect is seen even when baseline tau levels are low.”
Now, with the recent market withdrawal of aducanumab, another anti-amyloid monoclonal antibody that was previously approved for Alzheimer’s disease, lecanemab is the only therapy currently available for the goal of changing disease progression, not just modifying symptoms.
Looking Long Term
Both sets of data provide important messages for clinicians, according to Marcelo Matiello, MD, a physician investigator at Mass General Hospital and associate professor of neurology at Harvard Medical School, Boston.
“Clinicians are really looking for more data because this remains a relatively new drug,” he said. Both sets of findings presented by Dr. Irizarry “look good but the follow-up is still short, so I think everyone is still looking closely at long-term safety and efficacy.”
The need for continuous indefinite therapy is one concern that Dr. Matiello expressed. As moderator of the session in which these data were presented, Dr. Matiello specifically asked Dr. Irizarry if there are plans to explore whether periods without treatment might be a means to reduce the cost and burden of frequent infusions while preserving cognitive gains.
In response, Dr. Irizarry said that earlier studies showed rapid progression when lecanemab was stopped. On this basis, he thinks therapy must be maintained, but he did say that there are plans to look at less frequent dosing, such as once per month. He also said that a subcutaneous formulation in development might also reduce the burden of prolonged treatment.
Dr. Irizarry is an employee of Eisai Ltd., which manufacturers lecanemab. Dr. Matiello reports no potential conflicts of interest.
DENVER — , according to a first report of 6-month OLE data.
Due to the steady disease progression observed after the switch of placebo to active therapy, the message of these data is that “early initiation of lecanemab is important,” according to Michael Irizarry, MD, the senior vice president of clinical research at Eisai Ltd, which markets lecanemab.
The 6-month OLE data along with data from a tau PET substudy were presented by Dr. Irizarry at the 2024 annual meeting of the American Academy of Neurology.
From the start of the OLE through the 6-month follow-up, the downward trajectory of cognitive function, as measured with the Clinical Dementia Rating – Sum of Boxes (CDR-SB), has been parallel for the lecanemab-start and switch arms. As a result, the degree of separation between active and placebo groups over the course of the OLE has remained unchanged from the end of the randomized trial.
This does not rule out any benefit in the switch arm, according to Dr. Irizarry. Although there was no discernible change in the trajectory of decline among placebo patients after they were switched to lecanemab, Dr. Irizarry postulated that this might overlook the greater likely decline over time with no treatment.
“There was no placebo group in the OLE to compare with those on active treatment,” he pointed out. He then juxtaposed data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Over the same 6-month timeframe, these data show a hypothetical separation of the curves if no treatment had been received.
The 6-month OLE data provide a preliminary look at outcomes in a planned 4-year follow-up. At the end of the randomized CLARITY trial, the mean decline from the baseline CDR-SB score of 3.2, was 1.21 in the lecanemab group, translating into a 38% decline, and 1.66 in the placebo group, translating into about a 50% decline. Over the 6 months of OLE, there has been a further mean CDR-SB reduction of approximately 0.6 in both arms, suggesting a further 18% decline from baseline.
Additional Data
In the pivotal CLARITY trial, which was published a few months prior to regulatory approval early last year, 1785 patients were randomized to 10 mg/kg lecanemab or placebo infused every 2 weeks. At the end of 18 months, the superiority of lecanemab for the primary endpoint of adverse change in CDR-SB was highly significant (P < .001) as were the differences in key secondary endpoints, such as Alzheimer’s Disease Composite Score (P < .001).
Of those who participated in CLARITY, 1385 patients entered the OLE. Placebo patients were switched to lecanemab which is being maintained in all patients on the trial schedule of 10 mg/kg administered by intravenous infusion every 2 weeks.
In addition to the overall OLE 6-month data, which has not raised any new safety signals, Dr. Irizarry provided a new look at the PET TAU substudy with a focus on patients who entered the study with a low relative tau burden. Of the three classifications, which also included medium and high tau, as measured with positron-emission tomography (PET), the low tau group represented 41.2% of the 342 tau PET substudy participants. With only 2.9% entering the study with a high tau burden, almost all the others fell in the medium stratification.
Due to the potential for a lower therapeutic response, “patients with low Tau are often excluded from trials,” Dr. Irizarry said. But the sizable proportion of low tau patients has permitted an assessment of relative response with lecanemab, which turned out to be substantial.
“Consistent rates of clinical stability or improvements were observed regardless of baseline tau levels with the highest rates of improvements observed for the low tau group after 24 months of follow-up,” Dr. Irizarry reported.
In previously reported results from the tau PET substudy, lecanemab was shown to slow tau spread at least numerically in every section of the brain evaluated, including the frontal, cingulate, parietal, and whole cortical gray matter areas. The reductions reached significance for the medial temporal (P = .0024), meta temporal (P = .012), and temporal (P = .16) portions.
When most recently evaluated in the OLE, the CDR-SB score declined 38% less among those treated with lecanemab than those treated with placebo in the subgroup enrolled in the tau PET substudy.
Relative to those with intermediate or high tau, patients in the low tau had an even greater reduction in cognitive decline than those with higher tau burdens. Although Dr. Irizarry cautioned that greater baseline CDR-SB scores exaggerated the treatment effect in the low tau group, the message is that “a lecanemab treatment effect is seen even when baseline tau levels are low.”
Now, with the recent market withdrawal of aducanumab, another anti-amyloid monoclonal antibody that was previously approved for Alzheimer’s disease, lecanemab is the only therapy currently available for the goal of changing disease progression, not just modifying symptoms.
Looking Long Term
Both sets of data provide important messages for clinicians, according to Marcelo Matiello, MD, a physician investigator at Mass General Hospital and associate professor of neurology at Harvard Medical School, Boston.
“Clinicians are really looking for more data because this remains a relatively new drug,” he said. Both sets of findings presented by Dr. Irizarry “look good but the follow-up is still short, so I think everyone is still looking closely at long-term safety and efficacy.”
The need for continuous indefinite therapy is one concern that Dr. Matiello expressed. As moderator of the session in which these data were presented, Dr. Matiello specifically asked Dr. Irizarry if there are plans to explore whether periods without treatment might be a means to reduce the cost and burden of frequent infusions while preserving cognitive gains.
In response, Dr. Irizarry said that earlier studies showed rapid progression when lecanemab was stopped. On this basis, he thinks therapy must be maintained, but he did say that there are plans to look at less frequent dosing, such as once per month. He also said that a subcutaneous formulation in development might also reduce the burden of prolonged treatment.
Dr. Irizarry is an employee of Eisai Ltd., which manufacturers lecanemab. Dr. Matiello reports no potential conflicts of interest.
FROM AAN 2024
Time Wasted to Avoid Penalties
Depression is a serious issue. I want to say that off the top, because nothing below is intended to minimize it.
But does everyone need to be tested for it?
A lot of general practices test for it with every patient and every visit. After all, mandates say you have to or you’ll get penalized a few bucks. Since no one wants to leave any money on the table in the razor-thin margins of running a medical practice, they ask these questions (I don’t blame them for that).
I can see where this might be useful, but does it really do much? Or is it just a mandatory waste of time?
Good question.
A recent review by the American College of Physicians found it was mostly a waste of time (which surprises no one). Only one of the eight measures involved in depression screening (suicide risk assessment) turned out to be useful. So, basically, 88% of the time spent on these questions contributed absolutely nothing of clinical relevance.
Of course, this isn’t unique to family medicine. Every time I see a Medicare or Medicare Advantage patient I have to document whether they’ve had flu and pneumonia vaccines. While there are occasional cases where asking about recent vaccines is critical to the history, for most it’s not. But I do it so I don’t get penalized, even though the answer changes nothing. It’s not like I give vaccines in my practice.
A fair number of people come to me for hospital follow-ups, so I go into the system and review the chart. The notes inevitably contain questions of sexual activity, fear of violence, fear of domestic abuse, food security, recent travel patterns, and so on. Some of them are useful in certain situations, but not in all, or even most. All they do is increase the length of the note until anything of relevance is obscured, and allow someone in coding to check the boxes to raise the billing level. Realistically, the ER staff involved probably didn’t ask any of them, and just clicked “no.”
Once this probably seemed like a good idea, but clearly most of it is now a waste of time. These “quality measures” have turned the art of taking a good history into a session of mouse and box clicking.
Does that really improve care?
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
Depression is a serious issue. I want to say that off the top, because nothing below is intended to minimize it.
But does everyone need to be tested for it?
A lot of general practices test for it with every patient and every visit. After all, mandates say you have to or you’ll get penalized a few bucks. Since no one wants to leave any money on the table in the razor-thin margins of running a medical practice, they ask these questions (I don’t blame them for that).
I can see where this might be useful, but does it really do much? Or is it just a mandatory waste of time?
Good question.
A recent review by the American College of Physicians found it was mostly a waste of time (which surprises no one). Only one of the eight measures involved in depression screening (suicide risk assessment) turned out to be useful. So, basically, 88% of the time spent on these questions contributed absolutely nothing of clinical relevance.
Of course, this isn’t unique to family medicine. Every time I see a Medicare or Medicare Advantage patient I have to document whether they’ve had flu and pneumonia vaccines. While there are occasional cases where asking about recent vaccines is critical to the history, for most it’s not. But I do it so I don’t get penalized, even though the answer changes nothing. It’s not like I give vaccines in my practice.
A fair number of people come to me for hospital follow-ups, so I go into the system and review the chart. The notes inevitably contain questions of sexual activity, fear of violence, fear of domestic abuse, food security, recent travel patterns, and so on. Some of them are useful in certain situations, but not in all, or even most. All they do is increase the length of the note until anything of relevance is obscured, and allow someone in coding to check the boxes to raise the billing level. Realistically, the ER staff involved probably didn’t ask any of them, and just clicked “no.”
Once this probably seemed like a good idea, but clearly most of it is now a waste of time. These “quality measures” have turned the art of taking a good history into a session of mouse and box clicking.
Does that really improve care?
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
Depression is a serious issue. I want to say that off the top, because nothing below is intended to minimize it.
But does everyone need to be tested for it?
A lot of general practices test for it with every patient and every visit. After all, mandates say you have to or you’ll get penalized a few bucks. Since no one wants to leave any money on the table in the razor-thin margins of running a medical practice, they ask these questions (I don’t blame them for that).
I can see where this might be useful, but does it really do much? Or is it just a mandatory waste of time?
Good question.
A recent review by the American College of Physicians found it was mostly a waste of time (which surprises no one). Only one of the eight measures involved in depression screening (suicide risk assessment) turned out to be useful. So, basically, 88% of the time spent on these questions contributed absolutely nothing of clinical relevance.
Of course, this isn’t unique to family medicine. Every time I see a Medicare or Medicare Advantage patient I have to document whether they’ve had flu and pneumonia vaccines. While there are occasional cases where asking about recent vaccines is critical to the history, for most it’s not. But I do it so I don’t get penalized, even though the answer changes nothing. It’s not like I give vaccines in my practice.
A fair number of people come to me for hospital follow-ups, so I go into the system and review the chart. The notes inevitably contain questions of sexual activity, fear of violence, fear of domestic abuse, food security, recent travel patterns, and so on. Some of them are useful in certain situations, but not in all, or even most. All they do is increase the length of the note until anything of relevance is obscured, and allow someone in coding to check the boxes to raise the billing level. Realistically, the ER staff involved probably didn’t ask any of them, and just clicked “no.”
Once this probably seemed like a good idea, but clearly most of it is now a waste of time. These “quality measures” have turned the art of taking a good history into a session of mouse and box clicking.
Does that really improve care?
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
One-Minute Speech Test Could Help Assess Dementia Risk
BUDAPEST, HUNGARY — , suggests research.
János Kálmán, MD, PhD, and colleagues at the University of Szeged in Hungary have developed an automated speech analysis approach called the Speech-Gap Test (S-GAP Test) that is unique because it focuses on the temporal changes made when someone talks. This means it does not overcomplicate matters by also assessing the phonetics and semantics of speech, Dr. Kálmán said.
Dr. Kálmán presented his findings at the 32nd European Congress of Psychiatry.
Temporal Speech Parameters
The test analyzes parameters such as how quickly someone speaks, whether they hesitate when they talk, how long the hesitation lasts, and how many silent pauses they make. This can be done with a mere 60-second sample of speech, Dr. Kálmán said, noting that other automated speech and language tools currently in development need much longer audio samples.
“We tried different approaches and we finally ended up with the temporal speech parameters because these are not culture-dependent, not education-dependent, and could be more reliable than the semantic parts of [speech] analysis,” he explained.
The analysis of temporal speech parameters is also not language-dependent. Although the S-GAP Test was developed using audio samples from native Hungarian speakers, Dr. Kálmán and his collaborators have shown that it works just as well with samples from native English and German speakers. They now plan to validate the test further using samples from native Spanish speakers.
For Screening, Not Diagnosis
Currently, “the only purpose of this tool would be initial screening,” Dr. Kálmán said at the congress. It is not for diagnosis, and there is no intention to get it registered as a medical device.
A national survey of primary care physicians conducted by Dr. Kálmán and collaborators showed that there was little time for performing standard cognitive tests during the average consultation. Thus, the original idea was that the S-GAP Test would be an aid to help primary care physicians quickly flag whether a patient might have cognitive problems that needed further assessment at a memory clinic or by more specialist neurology services.
The goalposts have since been moved, from developing a pure telemedicine solution to a more widespread application that perhaps anyone could buy and download from the internet or using a smartphone.
Dr. Kálmán doesn’t discount developing a more sophisticated version of the S-GAP Test in the future that combines temporal speech parameters with biomarkers for mild cognitive impairment or Alzheimer’s disease and could be used in memory clinics and by neurologists for the purpose of diagnosis.
Detect to Prevent?
The big question is: What happens to all the people that could be flagged as needing further assessment using tools such as the S-GAP Test?
Tackling risk factors for dementia will probably be key, said Robert Perneckzy, MD, MBA, professor of translational dementia research at Ludwig Maximilian University of Munich, Imperial College London, and the University of Sheffield.
According to the Lancet Commission on dementia, there are 12 potentially modifiable risk factors for dementia. Their influence varies throughout the life course, but certain early events, such as the level of education attained, can’t be modified in an older patient.
That said, there are many risk factors that might still be influenced later in life, such as adequately treating comorbid conditions such as diabetes, and addressing alcohol consumption and smoking practices.
“We can do things in terms of personal risk, risk mitigation, which have a huge effect on dementia risk much later in life,” said Dr. Perneckzy.
“The speech-based assessments are another opportunity to save our time as doctors to do assessments before someone comes to the memory clinic,” he said.
The S-GAP Test is under development by the University of Szeged. Dr. Kálmán is a co-inventor. Dr. Perneckzy had no relevant conflicts of interest but has helped validate the S-GAP Test in the German language.
A version of this article appeared on Medscape.com.
BUDAPEST, HUNGARY — , suggests research.
János Kálmán, MD, PhD, and colleagues at the University of Szeged in Hungary have developed an automated speech analysis approach called the Speech-Gap Test (S-GAP Test) that is unique because it focuses on the temporal changes made when someone talks. This means it does not overcomplicate matters by also assessing the phonetics and semantics of speech, Dr. Kálmán said.
Dr. Kálmán presented his findings at the 32nd European Congress of Psychiatry.
Temporal Speech Parameters
The test analyzes parameters such as how quickly someone speaks, whether they hesitate when they talk, how long the hesitation lasts, and how many silent pauses they make. This can be done with a mere 60-second sample of speech, Dr. Kálmán said, noting that other automated speech and language tools currently in development need much longer audio samples.
“We tried different approaches and we finally ended up with the temporal speech parameters because these are not culture-dependent, not education-dependent, and could be more reliable than the semantic parts of [speech] analysis,” he explained.
The analysis of temporal speech parameters is also not language-dependent. Although the S-GAP Test was developed using audio samples from native Hungarian speakers, Dr. Kálmán and his collaborators have shown that it works just as well with samples from native English and German speakers. They now plan to validate the test further using samples from native Spanish speakers.
For Screening, Not Diagnosis
Currently, “the only purpose of this tool would be initial screening,” Dr. Kálmán said at the congress. It is not for diagnosis, and there is no intention to get it registered as a medical device.
A national survey of primary care physicians conducted by Dr. Kálmán and collaborators showed that there was little time for performing standard cognitive tests during the average consultation. Thus, the original idea was that the S-GAP Test would be an aid to help primary care physicians quickly flag whether a patient might have cognitive problems that needed further assessment at a memory clinic or by more specialist neurology services.
The goalposts have since been moved, from developing a pure telemedicine solution to a more widespread application that perhaps anyone could buy and download from the internet or using a smartphone.
Dr. Kálmán doesn’t discount developing a more sophisticated version of the S-GAP Test in the future that combines temporal speech parameters with biomarkers for mild cognitive impairment or Alzheimer’s disease and could be used in memory clinics and by neurologists for the purpose of diagnosis.
Detect to Prevent?
The big question is: What happens to all the people that could be flagged as needing further assessment using tools such as the S-GAP Test?
Tackling risk factors for dementia will probably be key, said Robert Perneckzy, MD, MBA, professor of translational dementia research at Ludwig Maximilian University of Munich, Imperial College London, and the University of Sheffield.
According to the Lancet Commission on dementia, there are 12 potentially modifiable risk factors for dementia. Their influence varies throughout the life course, but certain early events, such as the level of education attained, can’t be modified in an older patient.
That said, there are many risk factors that might still be influenced later in life, such as adequately treating comorbid conditions such as diabetes, and addressing alcohol consumption and smoking practices.
“We can do things in terms of personal risk, risk mitigation, which have a huge effect on dementia risk much later in life,” said Dr. Perneckzy.
“The speech-based assessments are another opportunity to save our time as doctors to do assessments before someone comes to the memory clinic,” he said.
The S-GAP Test is under development by the University of Szeged. Dr. Kálmán is a co-inventor. Dr. Perneckzy had no relevant conflicts of interest but has helped validate the S-GAP Test in the German language.
A version of this article appeared on Medscape.com.
BUDAPEST, HUNGARY — , suggests research.
János Kálmán, MD, PhD, and colleagues at the University of Szeged in Hungary have developed an automated speech analysis approach called the Speech-Gap Test (S-GAP Test) that is unique because it focuses on the temporal changes made when someone talks. This means it does not overcomplicate matters by also assessing the phonetics and semantics of speech, Dr. Kálmán said.
Dr. Kálmán presented his findings at the 32nd European Congress of Psychiatry.
Temporal Speech Parameters
The test analyzes parameters such as how quickly someone speaks, whether they hesitate when they talk, how long the hesitation lasts, and how many silent pauses they make. This can be done with a mere 60-second sample of speech, Dr. Kálmán said, noting that other automated speech and language tools currently in development need much longer audio samples.
“We tried different approaches and we finally ended up with the temporal speech parameters because these are not culture-dependent, not education-dependent, and could be more reliable than the semantic parts of [speech] analysis,” he explained.
The analysis of temporal speech parameters is also not language-dependent. Although the S-GAP Test was developed using audio samples from native Hungarian speakers, Dr. Kálmán and his collaborators have shown that it works just as well with samples from native English and German speakers. They now plan to validate the test further using samples from native Spanish speakers.
For Screening, Not Diagnosis
Currently, “the only purpose of this tool would be initial screening,” Dr. Kálmán said at the congress. It is not for diagnosis, and there is no intention to get it registered as a medical device.
A national survey of primary care physicians conducted by Dr. Kálmán and collaborators showed that there was little time for performing standard cognitive tests during the average consultation. Thus, the original idea was that the S-GAP Test would be an aid to help primary care physicians quickly flag whether a patient might have cognitive problems that needed further assessment at a memory clinic or by more specialist neurology services.
The goalposts have since been moved, from developing a pure telemedicine solution to a more widespread application that perhaps anyone could buy and download from the internet or using a smartphone.
Dr. Kálmán doesn’t discount developing a more sophisticated version of the S-GAP Test in the future that combines temporal speech parameters with biomarkers for mild cognitive impairment or Alzheimer’s disease and could be used in memory clinics and by neurologists for the purpose of diagnosis.
Detect to Prevent?
The big question is: What happens to all the people that could be flagged as needing further assessment using tools such as the S-GAP Test?
Tackling risk factors for dementia will probably be key, said Robert Perneckzy, MD, MBA, professor of translational dementia research at Ludwig Maximilian University of Munich, Imperial College London, and the University of Sheffield.
According to the Lancet Commission on dementia, there are 12 potentially modifiable risk factors for dementia. Their influence varies throughout the life course, but certain early events, such as the level of education attained, can’t be modified in an older patient.
That said, there are many risk factors that might still be influenced later in life, such as adequately treating comorbid conditions such as diabetes, and addressing alcohol consumption and smoking practices.
“We can do things in terms of personal risk, risk mitigation, which have a huge effect on dementia risk much later in life,” said Dr. Perneckzy.
“The speech-based assessments are another opportunity to save our time as doctors to do assessments before someone comes to the memory clinic,” he said.
The S-GAP Test is under development by the University of Szeged. Dr. Kálmán is a co-inventor. Dr. Perneckzy had no relevant conflicts of interest but has helped validate the S-GAP Test in the German language.
A version of this article appeared on Medscape.com.
FROM THE EUROPEAN CONGRESS OF PSYCHIATRY
Delirium Linked to a Threefold Increased Risk for Dementia
, new research showed.
Incident dementia risk was more than three times higher in those who experienced just one episode of delirium, with each additional episode linked to a further 20% increase in dementia risk. The association was strongest in men.
Patients with delirium also had a 39% higher mortality risk than those with no history of delirium.
“We have known for a long time that delirium is dangerous, and this provides evidence that it’s even more dangerous than perhaps we had appreciated,” said study investigator Emily H. Gordon, PhD, MBBS, a geriatrician and senior lecturer at the University of Queensland, Brisbane, Australia.
“But we also know delirium is preventable. There are no excuses anymore; we really need to work together to improve the hospital system, to implement what are known to be effective interventions,” she added.
The findings were published online in The BMJ.
Close Matching
Prior studies that suggested an association between delirium and dementia were relatively small with short follow-up and varied in their adjustment for confounders. They also didn’t account for the competing risk for death, researchers noted.
Investigators used a linked New South Wales (NSW) statewide dataset that includes records of care episodes from all NSW hospitals as well as personal, administrative, clinical, and death information.
The study included an eligible sample of 626,467 older adults without dementia at baseline with at least one hospital admission between 2009 and 2014. For these patients, researchers calculated a hospital frailty risk score and collected other information including primary diagnosis and mean length of hospital stay and stay in the intensive care unit. From diagnostic codes, they categorized patients into no delirium and delirium groups and determined the number of delirium episodes.
Investigators matched patients in the delirium group to patients with no delirium according to characteristics with potential to confound the association between delirium and risk for dementia, including age, gender, frailty, reason for hospitalization, and length of stay in hospital and intensive care.
The matched study sample included 55,211 (mean age, 83 years) each in the delirium and the no delirium groups. Despite matching, the length of hospital stay for the index episode was longer for the delirium group than the no delirium group (mean, 9 days vs 6 days).
The primary outcomes were death and incident dementia, determined via diagnostic codes. During a follow-up of 5.25 years, 58% of patients died, and 17% had a new dementia diagnosis.
Among patients with at least one episode of delirium, the rate of incident dementia was 3.4 times higher than in those without delirium. After accounting for the competing risk for death, incident dementia risk remained three times higher among the delirium group (hazard ratio [HR], 3.00; 95% CI, 2.91-3.10).
This association was stronger for men than women (HR, 3.17 and 2.88, respectively; P = .004).
Sex Differences
The study is thought to be the first to identify a difference between sexes in dementia risk and delirium, Dr. Gordon said. It’s possible delirium in men is more severe in intensity or lasts longer than in women, or the male brain is, for whatever reason, more vulnerable to the effects of delirium, said Dr. Gordon. But she stressed these are only theories.
Investigators also found a mortality rate 1.4 times higher in the delirium group versus those without delirium, equating to a 39% increased risk for death (HR, 1.39; 95% CI, 1.37-1.41). The risk was similar for men and women (interaction P = .62).
When researchers categorized delirium by number of episodes, they found each additional episode was associated with a 10% increased risk for death (HR, 1.10; 95% CI, 1.09-1.12).
In addition to its large size, long follow-up, and close matching, what sets this new study apart from previous research is it accounted for the competing risk for death, said Dr. Gordon.
“This is really important because you’re not going to get dementia if you die, and in this population, the rate of death is incredibly high,” she said. “If we just assume people who died didn’t get dementia, then that screws the results.”
Causal Link?
For those who experienced at least one episode of delirium within the first 12 months, each additional episode of delirium was associated with a 20% increased risk for dementia (HR, 1.20; 95% CI, 1.18-1.23).
That dose-response association suggests a causal link between the two, Dr. Gordon said.
“The number one way to prove causality is to do a randomized controlled trial,” which isn’t feasible with delirium, she said. “By demonstrating a dose-response relationship suggests that it could be a causal pathway.”
Exact mechanisms linking delirium with dementia are unclear. Delirium might uncover preexisting or preclinical dementia, or it might cause dementia by accelerating underlying neuropathologic processes or de novo mechanisms, the authors noted.
Study limitations included the potential for residual confounding from unmeasured variables in the matching criteria. Delirium and dementia diagnoses depended on clinical coding of medical information recorded in the administrative dataset, and under-coding of dementia during hospitalization is well-recognized.
Although the study controlled for length of stay in hospital and in intensive care, this may not have fully captured differences in severity of medical conditions. Data about the duration and severity of delirium episodes were also unavailable, which limited the dose-response analysis.
Commenting on the findings, Christopher Weber, PhD, Alzheimer’s Association as director of Global Science Initiatives, said the results are consistent with other research on the association between delirium and incidents of dementia.
The increased risk for dementia following delirium in males is “an interesting finding,” said Dr. Weber. “This suggests a need for more research to understand the impact of sex differences in delirium, as well as research to see if preventing incidents of delirium could ultimately reduce rates of dementia.”
The study received support from the National Health and Medical Research Council: Partnership Centre for Health System Sustainability. Dr. Gordon and Dr. Weber reported no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
, new research showed.
Incident dementia risk was more than three times higher in those who experienced just one episode of delirium, with each additional episode linked to a further 20% increase in dementia risk. The association was strongest in men.
Patients with delirium also had a 39% higher mortality risk than those with no history of delirium.
“We have known for a long time that delirium is dangerous, and this provides evidence that it’s even more dangerous than perhaps we had appreciated,” said study investigator Emily H. Gordon, PhD, MBBS, a geriatrician and senior lecturer at the University of Queensland, Brisbane, Australia.
“But we also know delirium is preventable. There are no excuses anymore; we really need to work together to improve the hospital system, to implement what are known to be effective interventions,” she added.
The findings were published online in The BMJ.
Close Matching
Prior studies that suggested an association between delirium and dementia were relatively small with short follow-up and varied in their adjustment for confounders. They also didn’t account for the competing risk for death, researchers noted.
Investigators used a linked New South Wales (NSW) statewide dataset that includes records of care episodes from all NSW hospitals as well as personal, administrative, clinical, and death information.
The study included an eligible sample of 626,467 older adults without dementia at baseline with at least one hospital admission between 2009 and 2014. For these patients, researchers calculated a hospital frailty risk score and collected other information including primary diagnosis and mean length of hospital stay and stay in the intensive care unit. From diagnostic codes, they categorized patients into no delirium and delirium groups and determined the number of delirium episodes.
Investigators matched patients in the delirium group to patients with no delirium according to characteristics with potential to confound the association between delirium and risk for dementia, including age, gender, frailty, reason for hospitalization, and length of stay in hospital and intensive care.
The matched study sample included 55,211 (mean age, 83 years) each in the delirium and the no delirium groups. Despite matching, the length of hospital stay for the index episode was longer for the delirium group than the no delirium group (mean, 9 days vs 6 days).
The primary outcomes were death and incident dementia, determined via diagnostic codes. During a follow-up of 5.25 years, 58% of patients died, and 17% had a new dementia diagnosis.
Among patients with at least one episode of delirium, the rate of incident dementia was 3.4 times higher than in those without delirium. After accounting for the competing risk for death, incident dementia risk remained three times higher among the delirium group (hazard ratio [HR], 3.00; 95% CI, 2.91-3.10).
This association was stronger for men than women (HR, 3.17 and 2.88, respectively; P = .004).
Sex Differences
The study is thought to be the first to identify a difference between sexes in dementia risk and delirium, Dr. Gordon said. It’s possible delirium in men is more severe in intensity or lasts longer than in women, or the male brain is, for whatever reason, more vulnerable to the effects of delirium, said Dr. Gordon. But she stressed these are only theories.
Investigators also found a mortality rate 1.4 times higher in the delirium group versus those without delirium, equating to a 39% increased risk for death (HR, 1.39; 95% CI, 1.37-1.41). The risk was similar for men and women (interaction P = .62).
When researchers categorized delirium by number of episodes, they found each additional episode was associated with a 10% increased risk for death (HR, 1.10; 95% CI, 1.09-1.12).
In addition to its large size, long follow-up, and close matching, what sets this new study apart from previous research is it accounted for the competing risk for death, said Dr. Gordon.
“This is really important because you’re not going to get dementia if you die, and in this population, the rate of death is incredibly high,” she said. “If we just assume people who died didn’t get dementia, then that screws the results.”
Causal Link?
For those who experienced at least one episode of delirium within the first 12 months, each additional episode of delirium was associated with a 20% increased risk for dementia (HR, 1.20; 95% CI, 1.18-1.23).
That dose-response association suggests a causal link between the two, Dr. Gordon said.
“The number one way to prove causality is to do a randomized controlled trial,” which isn’t feasible with delirium, she said. “By demonstrating a dose-response relationship suggests that it could be a causal pathway.”
Exact mechanisms linking delirium with dementia are unclear. Delirium might uncover preexisting or preclinical dementia, or it might cause dementia by accelerating underlying neuropathologic processes or de novo mechanisms, the authors noted.
Study limitations included the potential for residual confounding from unmeasured variables in the matching criteria. Delirium and dementia diagnoses depended on clinical coding of medical information recorded in the administrative dataset, and under-coding of dementia during hospitalization is well-recognized.
Although the study controlled for length of stay in hospital and in intensive care, this may not have fully captured differences in severity of medical conditions. Data about the duration and severity of delirium episodes were also unavailable, which limited the dose-response analysis.
Commenting on the findings, Christopher Weber, PhD, Alzheimer’s Association as director of Global Science Initiatives, said the results are consistent with other research on the association between delirium and incidents of dementia.
The increased risk for dementia following delirium in males is “an interesting finding,” said Dr. Weber. “This suggests a need for more research to understand the impact of sex differences in delirium, as well as research to see if preventing incidents of delirium could ultimately reduce rates of dementia.”
The study received support from the National Health and Medical Research Council: Partnership Centre for Health System Sustainability. Dr. Gordon and Dr. Weber reported no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
, new research showed.
Incident dementia risk was more than three times higher in those who experienced just one episode of delirium, with each additional episode linked to a further 20% increase in dementia risk. The association was strongest in men.
Patients with delirium also had a 39% higher mortality risk than those with no history of delirium.
“We have known for a long time that delirium is dangerous, and this provides evidence that it’s even more dangerous than perhaps we had appreciated,” said study investigator Emily H. Gordon, PhD, MBBS, a geriatrician and senior lecturer at the University of Queensland, Brisbane, Australia.
“But we also know delirium is preventable. There are no excuses anymore; we really need to work together to improve the hospital system, to implement what are known to be effective interventions,” she added.
The findings were published online in The BMJ.
Close Matching
Prior studies that suggested an association between delirium and dementia were relatively small with short follow-up and varied in their adjustment for confounders. They also didn’t account for the competing risk for death, researchers noted.
Investigators used a linked New South Wales (NSW) statewide dataset that includes records of care episodes from all NSW hospitals as well as personal, administrative, clinical, and death information.
The study included an eligible sample of 626,467 older adults without dementia at baseline with at least one hospital admission between 2009 and 2014. For these patients, researchers calculated a hospital frailty risk score and collected other information including primary diagnosis and mean length of hospital stay and stay in the intensive care unit. From diagnostic codes, they categorized patients into no delirium and delirium groups and determined the number of delirium episodes.
Investigators matched patients in the delirium group to patients with no delirium according to characteristics with potential to confound the association between delirium and risk for dementia, including age, gender, frailty, reason for hospitalization, and length of stay in hospital and intensive care.
The matched study sample included 55,211 (mean age, 83 years) each in the delirium and the no delirium groups. Despite matching, the length of hospital stay for the index episode was longer for the delirium group than the no delirium group (mean, 9 days vs 6 days).
The primary outcomes were death and incident dementia, determined via diagnostic codes. During a follow-up of 5.25 years, 58% of patients died, and 17% had a new dementia diagnosis.
Among patients with at least one episode of delirium, the rate of incident dementia was 3.4 times higher than in those without delirium. After accounting for the competing risk for death, incident dementia risk remained three times higher among the delirium group (hazard ratio [HR], 3.00; 95% CI, 2.91-3.10).
This association was stronger for men than women (HR, 3.17 and 2.88, respectively; P = .004).
Sex Differences
The study is thought to be the first to identify a difference between sexes in dementia risk and delirium, Dr. Gordon said. It’s possible delirium in men is more severe in intensity or lasts longer than in women, or the male brain is, for whatever reason, more vulnerable to the effects of delirium, said Dr. Gordon. But she stressed these are only theories.
Investigators also found a mortality rate 1.4 times higher in the delirium group versus those without delirium, equating to a 39% increased risk for death (HR, 1.39; 95% CI, 1.37-1.41). The risk was similar for men and women (interaction P = .62).
When researchers categorized delirium by number of episodes, they found each additional episode was associated with a 10% increased risk for death (HR, 1.10; 95% CI, 1.09-1.12).
In addition to its large size, long follow-up, and close matching, what sets this new study apart from previous research is it accounted for the competing risk for death, said Dr. Gordon.
“This is really important because you’re not going to get dementia if you die, and in this population, the rate of death is incredibly high,” she said. “If we just assume people who died didn’t get dementia, then that screws the results.”
Causal Link?
For those who experienced at least one episode of delirium within the first 12 months, each additional episode of delirium was associated with a 20% increased risk for dementia (HR, 1.20; 95% CI, 1.18-1.23).
That dose-response association suggests a causal link between the two, Dr. Gordon said.
“The number one way to prove causality is to do a randomized controlled trial,” which isn’t feasible with delirium, she said. “By demonstrating a dose-response relationship suggests that it could be a causal pathway.”
Exact mechanisms linking delirium with dementia are unclear. Delirium might uncover preexisting or preclinical dementia, or it might cause dementia by accelerating underlying neuropathologic processes or de novo mechanisms, the authors noted.
Study limitations included the potential for residual confounding from unmeasured variables in the matching criteria. Delirium and dementia diagnoses depended on clinical coding of medical information recorded in the administrative dataset, and under-coding of dementia during hospitalization is well-recognized.
Although the study controlled for length of stay in hospital and in intensive care, this may not have fully captured differences in severity of medical conditions. Data about the duration and severity of delirium episodes were also unavailable, which limited the dose-response analysis.
Commenting on the findings, Christopher Weber, PhD, Alzheimer’s Association as director of Global Science Initiatives, said the results are consistent with other research on the association between delirium and incidents of dementia.
The increased risk for dementia following delirium in males is “an interesting finding,” said Dr. Weber. “This suggests a need for more research to understand the impact of sex differences in delirium, as well as research to see if preventing incidents of delirium could ultimately reduce rates of dementia.”
The study received support from the National Health and Medical Research Council: Partnership Centre for Health System Sustainability. Dr. Gordon and Dr. Weber reported no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
FROM THE BMJ
Chronic Pain Linked to Accelerated Brain Aging
, new research showed.
Using structural MRI data from more than 9000 adults with knee osteoarthritis (KOA) from the UK Biobank, investigators developed a brain age model to compare an individual’s brain age with their chronological age. Those with KOA showed a much faster rate of brain aging than healthy individuals.
The acceleration in brain aging was largely driven by the hippocampus and predicted memory decline and incident dementia during follow-up. Researchers identified a gene highly expressed in glial cells as a possible genetic factor for accelerated brain aging.
“We demonstrate the accelerated brain aging and cognitive decline in chronic musculoskeletal pain, in particular knee osteoarthritis, and provide a neural marker for early detection and intervention,” said co-first author Jiao Liu, PhD candidate, Chinese Academy of Sciences, Beijing.
“We are interested to know how to slow down the aging brain in chronic musculoskeletal pain patients. Proper exercise and lifestyle may reduce the risk,” Dr. Liu said.
The study was published online in Nature Mental Health.
Common Condition
CMP affects more than 40% of the world’s population and has been shown to have a harmful impact on cognitive function, although the exact mechanisms remain unclear. Prior research suggests that inflammatory markers associated with brain aging are higher in patients with CMP, suggesting a link between brain aging and CMP.
To investigate further, researchers explored patterns of brain aging in healthy cohorts and cohorts with four common types of CMP — chronic knee pain, chronic back pain, chronic neck pain, and chronic hip pain.
Using their brain age model, investigators observed significantly increased brain aging, or “predicted age difference,” only in individuals with KOA (P < .001). The observation was validated in an independent dataset (P = .020), suggesting a pattern of brain aging acceleration specific to KOA.
This acceleration was primarily driven by key brain regions involved in cognitive processing, including hippocampus and orbitofrontal cortex, and was correlated with longitudinal memory decline and dementia risk.
These data also suggest that the SLC39A8 gene, which is highly expressed in glial cells, might be a key genetic factor underpinning this acceleration.
“We not only revealed the specificity of accelerated brain aging in knee osteoarthritis patients, but importantly, we also provided longitudinal evidence suggesting the ability of our brain aging marker to predict future memory decline and increased dementia risk,” corresponding author Yiheng Tu, PhD, also with Chinese Academy of Sciences, Beijing, said in a news release.
A Future Treatment Target?
Commenting on this research, Shaheen Lakhan, MD, PhD, a neurologist and researcher based in Miami, noted that in this study, people with KOA showed signs of “faster brain aging on scans. Think of it as your brain wearing a disguise, appearing older than its actual years,” Dr. Lakhan said.
“Inflammation, a key player in osteoarthritis, might be playing a double agent, wreaking havoc not just on your joints but potentially on your memory too. Researchers even identified a specific gene linked to both knee pain and faster brain aging, hinting at a potential target for future treatments,” he added.
“Importantly, the increased risk of cognitive decline and dementia associated with chronic pain is likely one of many factors, and probably not a very high one on its own,” Dr. Lakhan noted.
The “good news,” he said, is that there are many “well-established ways to keep your brain sharp. Regular exercise, a healthy diet, and staying mentally stimulated are all proven strategies to reduce dementia risk. Think of chronic pain management as another tool you can add to your brain health toolbox.”
Support for the study was provided by the STI-2030 Major Project, the National Natural Science Foundation of China, the Scientific Foundation of the Institute of Psychology, Chinese Academy of Sciences, and the Young Elite Scientist Sponsorship Program by the China Association for Science and Technology. Dr. Liu and Dr. Lakhan had no relevant disclosures.
A version of this article appeared on Medscape.com.
, new research showed.
Using structural MRI data from more than 9000 adults with knee osteoarthritis (KOA) from the UK Biobank, investigators developed a brain age model to compare an individual’s brain age with their chronological age. Those with KOA showed a much faster rate of brain aging than healthy individuals.
The acceleration in brain aging was largely driven by the hippocampus and predicted memory decline and incident dementia during follow-up. Researchers identified a gene highly expressed in glial cells as a possible genetic factor for accelerated brain aging.
“We demonstrate the accelerated brain aging and cognitive decline in chronic musculoskeletal pain, in particular knee osteoarthritis, and provide a neural marker for early detection and intervention,” said co-first author Jiao Liu, PhD candidate, Chinese Academy of Sciences, Beijing.
“We are interested to know how to slow down the aging brain in chronic musculoskeletal pain patients. Proper exercise and lifestyle may reduce the risk,” Dr. Liu said.
The study was published online in Nature Mental Health.
Common Condition
CMP affects more than 40% of the world’s population and has been shown to have a harmful impact on cognitive function, although the exact mechanisms remain unclear. Prior research suggests that inflammatory markers associated with brain aging are higher in patients with CMP, suggesting a link between brain aging and CMP.
To investigate further, researchers explored patterns of brain aging in healthy cohorts and cohorts with four common types of CMP — chronic knee pain, chronic back pain, chronic neck pain, and chronic hip pain.
Using their brain age model, investigators observed significantly increased brain aging, or “predicted age difference,” only in individuals with KOA (P < .001). The observation was validated in an independent dataset (P = .020), suggesting a pattern of brain aging acceleration specific to KOA.
This acceleration was primarily driven by key brain regions involved in cognitive processing, including hippocampus and orbitofrontal cortex, and was correlated with longitudinal memory decline and dementia risk.
These data also suggest that the SLC39A8 gene, which is highly expressed in glial cells, might be a key genetic factor underpinning this acceleration.
“We not only revealed the specificity of accelerated brain aging in knee osteoarthritis patients, but importantly, we also provided longitudinal evidence suggesting the ability of our brain aging marker to predict future memory decline and increased dementia risk,” corresponding author Yiheng Tu, PhD, also with Chinese Academy of Sciences, Beijing, said in a news release.
A Future Treatment Target?
Commenting on this research, Shaheen Lakhan, MD, PhD, a neurologist and researcher based in Miami, noted that in this study, people with KOA showed signs of “faster brain aging on scans. Think of it as your brain wearing a disguise, appearing older than its actual years,” Dr. Lakhan said.
“Inflammation, a key player in osteoarthritis, might be playing a double agent, wreaking havoc not just on your joints but potentially on your memory too. Researchers even identified a specific gene linked to both knee pain and faster brain aging, hinting at a potential target for future treatments,” he added.
“Importantly, the increased risk of cognitive decline and dementia associated with chronic pain is likely one of many factors, and probably not a very high one on its own,” Dr. Lakhan noted.
The “good news,” he said, is that there are many “well-established ways to keep your brain sharp. Regular exercise, a healthy diet, and staying mentally stimulated are all proven strategies to reduce dementia risk. Think of chronic pain management as another tool you can add to your brain health toolbox.”
Support for the study was provided by the STI-2030 Major Project, the National Natural Science Foundation of China, the Scientific Foundation of the Institute of Psychology, Chinese Academy of Sciences, and the Young Elite Scientist Sponsorship Program by the China Association for Science and Technology. Dr. Liu and Dr. Lakhan had no relevant disclosures.
A version of this article appeared on Medscape.com.
, new research showed.
Using structural MRI data from more than 9000 adults with knee osteoarthritis (KOA) from the UK Biobank, investigators developed a brain age model to compare an individual’s brain age with their chronological age. Those with KOA showed a much faster rate of brain aging than healthy individuals.
The acceleration in brain aging was largely driven by the hippocampus and predicted memory decline and incident dementia during follow-up. Researchers identified a gene highly expressed in glial cells as a possible genetic factor for accelerated brain aging.
“We demonstrate the accelerated brain aging and cognitive decline in chronic musculoskeletal pain, in particular knee osteoarthritis, and provide a neural marker for early detection and intervention,” said co-first author Jiao Liu, PhD candidate, Chinese Academy of Sciences, Beijing.
“We are interested to know how to slow down the aging brain in chronic musculoskeletal pain patients. Proper exercise and lifestyle may reduce the risk,” Dr. Liu said.
The study was published online in Nature Mental Health.
Common Condition
CMP affects more than 40% of the world’s population and has been shown to have a harmful impact on cognitive function, although the exact mechanisms remain unclear. Prior research suggests that inflammatory markers associated with brain aging are higher in patients with CMP, suggesting a link between brain aging and CMP.
To investigate further, researchers explored patterns of brain aging in healthy cohorts and cohorts with four common types of CMP — chronic knee pain, chronic back pain, chronic neck pain, and chronic hip pain.
Using their brain age model, investigators observed significantly increased brain aging, or “predicted age difference,” only in individuals with KOA (P < .001). The observation was validated in an independent dataset (P = .020), suggesting a pattern of brain aging acceleration specific to KOA.
This acceleration was primarily driven by key brain regions involved in cognitive processing, including hippocampus and orbitofrontal cortex, and was correlated with longitudinal memory decline and dementia risk.
These data also suggest that the SLC39A8 gene, which is highly expressed in glial cells, might be a key genetic factor underpinning this acceleration.
“We not only revealed the specificity of accelerated brain aging in knee osteoarthritis patients, but importantly, we also provided longitudinal evidence suggesting the ability of our brain aging marker to predict future memory decline and increased dementia risk,” corresponding author Yiheng Tu, PhD, also with Chinese Academy of Sciences, Beijing, said in a news release.
A Future Treatment Target?
Commenting on this research, Shaheen Lakhan, MD, PhD, a neurologist and researcher based in Miami, noted that in this study, people with KOA showed signs of “faster brain aging on scans. Think of it as your brain wearing a disguise, appearing older than its actual years,” Dr. Lakhan said.
“Inflammation, a key player in osteoarthritis, might be playing a double agent, wreaking havoc not just on your joints but potentially on your memory too. Researchers even identified a specific gene linked to both knee pain and faster brain aging, hinting at a potential target for future treatments,” he added.
“Importantly, the increased risk of cognitive decline and dementia associated with chronic pain is likely one of many factors, and probably not a very high one on its own,” Dr. Lakhan noted.
The “good news,” he said, is that there are many “well-established ways to keep your brain sharp. Regular exercise, a healthy diet, and staying mentally stimulated are all proven strategies to reduce dementia risk. Think of chronic pain management as another tool you can add to your brain health toolbox.”
Support for the study was provided by the STI-2030 Major Project, the National Natural Science Foundation of China, the Scientific Foundation of the Institute of Psychology, Chinese Academy of Sciences, and the Young Elite Scientist Sponsorship Program by the China Association for Science and Technology. Dr. Liu and Dr. Lakhan had no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM NATURE MENTAL HEALTH

