User login
Atopic Dermatitis: Upadacitinib Effectiveness Maintained Through 76 weeks Among Adolescents
TOPLINE:
across three phase 3 trials.
METHODOLOGY:
- Researchers conducted three double-blind, placebo-controlled phase 3 randomized clinical trials (Measure Up 1, Measure Up 2, and AD Up) involving 542 adolescents aged 12-17 years with moderate to severe AD.
- Participants were randomized to receive the oral Janus kinase inhibitor upadacitinib (15 mg or 30 mg once daily) or placebo, with or without topical corticosteroids, for 16 weeks, followed by rerandomization of patients in the placebo group to upadacitinib for up to 76 weeks.
- Study endpoints were at least a 75%, 90%, or 100% reduction in the Eczema Area and Severity Index (EASI-75, EASI-90, and EASI-100, respectively), Validated Investigator Global Assessment for AD (vIGA-AD) score of 0 or 1, and a ≥ 4-point improvement in the Worst Pruritus Numerical Rating Scale (WP-NRS).
- Adverse events were monitored, including serious infections, herpes zoster, and creatine kinase elevation.
TAKEAWAY:
- Among those who continued treatment on upadacitinib, 15 mg and 30 mg, EASI-75 response rates were maintained or improved through week 76 in all three studies. Patients who switched from placebo to upadacitinib also experienced improvements in EASI-75 through week 76.
- The proportion of patients who achieved EASI-90 and EASI-100 responses increased, and in general, were maintained from week 16 through week 76 in all three studies; the proportion was numerically higher among patients on 30 mg for all three studies.
- The proportion of adolescents achieving vIGA-AD score of 0 or 1 and WP-NRS improvement of ≥ 4 points was sustained or improved through 76 weeks.
- Serious infections were reported in five patients or fewer in each treatment group for all three studies. All opportunistic infections were eczema herpeticum; most cases were not serious, or were mild or moderate, and in general, did not require stopping treatment.
IN PRACTICE:
“These results through 76 weeks demonstrated that upadacitinib, with a favorable benefit-risk profile, was an effective long-term treatment option for adolescents with moderate to severe AD,” the authors wrote.
SOURCE:
The study was led by Amy S. Paller, MD, professor and chair of dermatology, Northwestern University, Chicago, and was published online on October 23 in JAMA Dermatology.
LIMITATIONS:
The study limitations included a small sample size, and the findings did not extend to patients under 12 years or those weighing < 40 kg.
DISCLOSURES:
This study was supported by AbbVie. Paller received grants and personal fees from pharmaceutical companies including AbbVie during the conduct of the study. Several authors reported financial ties with various sources, including AbbVie.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
across three phase 3 trials.
METHODOLOGY:
- Researchers conducted three double-blind, placebo-controlled phase 3 randomized clinical trials (Measure Up 1, Measure Up 2, and AD Up) involving 542 adolescents aged 12-17 years with moderate to severe AD.
- Participants were randomized to receive the oral Janus kinase inhibitor upadacitinib (15 mg or 30 mg once daily) or placebo, with or without topical corticosteroids, for 16 weeks, followed by rerandomization of patients in the placebo group to upadacitinib for up to 76 weeks.
- Study endpoints were at least a 75%, 90%, or 100% reduction in the Eczema Area and Severity Index (EASI-75, EASI-90, and EASI-100, respectively), Validated Investigator Global Assessment for AD (vIGA-AD) score of 0 or 1, and a ≥ 4-point improvement in the Worst Pruritus Numerical Rating Scale (WP-NRS).
- Adverse events were monitored, including serious infections, herpes zoster, and creatine kinase elevation.
TAKEAWAY:
- Among those who continued treatment on upadacitinib, 15 mg and 30 mg, EASI-75 response rates were maintained or improved through week 76 in all three studies. Patients who switched from placebo to upadacitinib also experienced improvements in EASI-75 through week 76.
- The proportion of patients who achieved EASI-90 and EASI-100 responses increased, and in general, were maintained from week 16 through week 76 in all three studies; the proportion was numerically higher among patients on 30 mg for all three studies.
- The proportion of adolescents achieving vIGA-AD score of 0 or 1 and WP-NRS improvement of ≥ 4 points was sustained or improved through 76 weeks.
- Serious infections were reported in five patients or fewer in each treatment group for all three studies. All opportunistic infections were eczema herpeticum; most cases were not serious, or were mild or moderate, and in general, did not require stopping treatment.
IN PRACTICE:
“These results through 76 weeks demonstrated that upadacitinib, with a favorable benefit-risk profile, was an effective long-term treatment option for adolescents with moderate to severe AD,” the authors wrote.
SOURCE:
The study was led by Amy S. Paller, MD, professor and chair of dermatology, Northwestern University, Chicago, and was published online on October 23 in JAMA Dermatology.
LIMITATIONS:
The study limitations included a small sample size, and the findings did not extend to patients under 12 years or those weighing < 40 kg.
DISCLOSURES:
This study was supported by AbbVie. Paller received grants and personal fees from pharmaceutical companies including AbbVie during the conduct of the study. Several authors reported financial ties with various sources, including AbbVie.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
across three phase 3 trials.
METHODOLOGY:
- Researchers conducted three double-blind, placebo-controlled phase 3 randomized clinical trials (Measure Up 1, Measure Up 2, and AD Up) involving 542 adolescents aged 12-17 years with moderate to severe AD.
- Participants were randomized to receive the oral Janus kinase inhibitor upadacitinib (15 mg or 30 mg once daily) or placebo, with or without topical corticosteroids, for 16 weeks, followed by rerandomization of patients in the placebo group to upadacitinib for up to 76 weeks.
- Study endpoints were at least a 75%, 90%, or 100% reduction in the Eczema Area and Severity Index (EASI-75, EASI-90, and EASI-100, respectively), Validated Investigator Global Assessment for AD (vIGA-AD) score of 0 or 1, and a ≥ 4-point improvement in the Worst Pruritus Numerical Rating Scale (WP-NRS).
- Adverse events were monitored, including serious infections, herpes zoster, and creatine kinase elevation.
TAKEAWAY:
- Among those who continued treatment on upadacitinib, 15 mg and 30 mg, EASI-75 response rates were maintained or improved through week 76 in all three studies. Patients who switched from placebo to upadacitinib also experienced improvements in EASI-75 through week 76.
- The proportion of patients who achieved EASI-90 and EASI-100 responses increased, and in general, were maintained from week 16 through week 76 in all three studies; the proportion was numerically higher among patients on 30 mg for all three studies.
- The proportion of adolescents achieving vIGA-AD score of 0 or 1 and WP-NRS improvement of ≥ 4 points was sustained or improved through 76 weeks.
- Serious infections were reported in five patients or fewer in each treatment group for all three studies. All opportunistic infections were eczema herpeticum; most cases were not serious, or were mild or moderate, and in general, did not require stopping treatment.
IN PRACTICE:
“These results through 76 weeks demonstrated that upadacitinib, with a favorable benefit-risk profile, was an effective long-term treatment option for adolescents with moderate to severe AD,” the authors wrote.
SOURCE:
The study was led by Amy S. Paller, MD, professor and chair of dermatology, Northwestern University, Chicago, and was published online on October 23 in JAMA Dermatology.
LIMITATIONS:
The study limitations included a small sample size, and the findings did not extend to patients under 12 years or those weighing < 40 kg.
DISCLOSURES:
This study was supported by AbbVie. Paller received grants and personal fees from pharmaceutical companies including AbbVie during the conduct of the study. Several authors reported financial ties with various sources, including AbbVie.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Phenytoin-Induced DRESS Syndrome: Clinical and Laboratory Characteristics
To the Editor:
Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome—a severe cutaneous adverse drug reaction—is characterized by a cutaneous rash and systemic upset in the form of various internal organ and hematologic disturbances. This delayed and idiosyncratic syndrome went by several names, including anticonvulsant hypersensitivity syndrome, before Bocquet et al1 proposed the term DRESS syndrome.
Phenytoin, a hydantoin derivative used in neurology, was implicated in 41% of cases of DRESS syndrome in a study of 100 patients conducted in southern India.2,3 While DRESS syndrome is a newer name, the clinical picture of DRESS secondary to phenytoin use remains similar in that it manifests with a morbilliform rash and systemic upset. We sought to describe the clinical and laboratory characteristics of phenytoin-induced DRESS syndrome in this case series.
The analysis included 23 patients with DRESS syndrome secondary to phenytoin use who presented to a tertiary care institution in North India between July 2021 and December 2022, satisfied the European Registry of Severe Cutaneous Adverse Reaction (RegiSCAR) criteria,4 and achieved a DRESS diagnostic score of more than 1. The mean age of the patients was 44 years (range, 14–74 years). There was a slight female predominance with a male to female ratio of 0.9:1. More than half of the patients (52.2% [12/23]) presented directly to the dermatology outpatient department; the remaining patients were referred from other departments (47.8% [11/23]). Patients primarily were receiving phenytoin for neurologic indications. Specific reasons included antiseizure prophylaxis following a traffic accident (34.8% [8/23]); epilepsy (26.1% [6/23]); and neoplastic (17.4% [4/23]), vascular (17.4% [4/23]), and infectious (4.3% [1/23]) causes. The mean latency period from drug intake to symptom onset was 29 days (range, 6–62 days), and the mean illness duration was 9 days (range, 1–45 days).
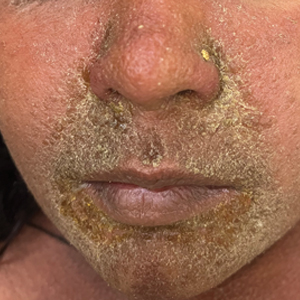
The majority of patients experienced pruritus (91.3% [21/23]) and fever (74.0% [17/23]), and all initially had a rash. Maculopapular morphology was seen in all patients. Erythema multiforme–like (17.4% [4/23]), erythrodermic (17.4% [4/23]), and vesicular (13.0% [3/23]) rashes also were documented (Figure 1). The trunk (100% [23/23]) and extremities (95.7% [22/23]) were involved most often, followed by the palms and soles (56.5% [13/23]). The mean total body surface area affected was 73.65%. Only 7 patients (30.4%) had mucosal involvement; nonhemorrhagic cheilitis was the most common manifestation.
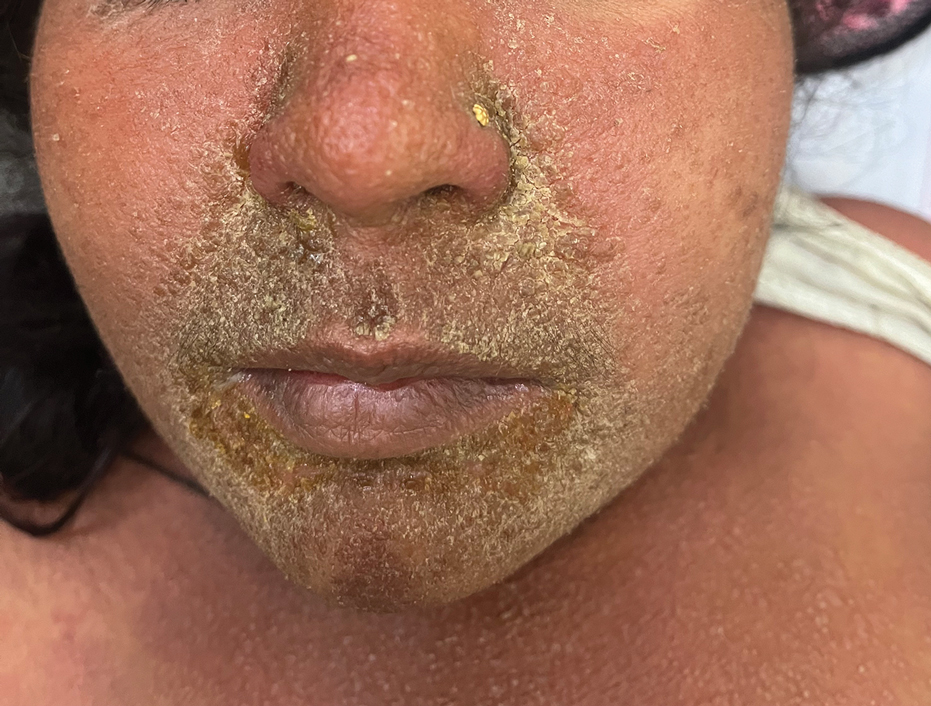
Facial edema, a hallmark feature of DRESS syndrome, was noted in 69.6% (16/23) of patients (Figure 2). Lymphadenopathy was present in 43.5% (10/23) of patients; of those cases, the inguinal (40.0%; n=4) and cervical (30%; n=3) nodes most commonly were involved. Although DRESS syndrome can affect internal organs, this was an issue for only 2 (8.7%) patients who experienced mild hepatomegaly.
Laboratory investigations revealed a mean differential eosinophil percentage of 10.3% (reference range, 1%–4%), while the mean absolute eosinophil count was 1.0634×109/L (reference range, 0.02–0.5×109/L). Other hematologic findings included the mean percentages of neutrophils (60%; reference range, 50%–60%), lymphocytes (19.95%; reference range, 20%–50%), and monocytes (8.70%; reference range, 2%–8%).
Liver function tests revealed transaminitis5 as the most common finding, with mean aspartate aminotransferase levels of 109 U/L (reference range, 8–33 U/L), mean alanine aminotransferase of 97.9 U/L (reference range, 7–56 U/L), and mean alkaline phosphatase levels of 211.35 U/L (reference range, 44–147 U/L). Half of the patients had notable (>2 times the upper limit of normal) transaminitis.
Renal blood workup revealed slightly elevated blood urea nitrogen levels with a mean value of 28.4 mg/dL (reference range, 6–24 mg/dL), and mean serum creatinine was 0.78 mg/dL (reference range for men, 0.7–1.3 mg/dL; for women, 0.6–1.1 mg/dL).
All patients were treated with oral steroids (prednisolone 1 mg/kg/d) before tapering slowly over the following 6 to 8 weeks. The culprit drug (phenytoin) was stopped on the day of presentation. Resolution of rash and itching was seen in all patients by 3 weeks after presentation without any relapse by follow-up at 6 weeks from presentation to the hospital.
Our case series seeks to discuss the clinical and laboratory features of phenytoin-induced DRESS syndrome. Our patients had more erythrodermic and erythema multiforme–like morphologies, less mucosal involvement, more hepatic involvement, and earlier resolution.
- Bocquet H, Bagot M, Roujeau JC. Drug-induced pseudolymphoma and drug hypersensitivity syndrome (drug rash with eosinophilia and systemic symptoms: DRESS). Semin Cutan Med Surg. 1996;15:250-257. doi:10.1016/s1085-5629(96)80038-1
- Patocka J, Wu Q, Nepovimova E, et al. Phenytoin—an anti-seizure drug: overview of its chemistry, pharmacology and toxicology. Food Chem Toxicol. 2020;142:111393. doi:10.1016/j.fct.2020.111393
- Sasidharanpillai S, Chathoth AT, Khader A, et al. Predictors of disease severity in drug reaction with eosinophilia and systemic symptoms. Indian J Dermatol Venereol Leprol. 2019;85:266-275. doi:10.4103/ijdvl.IJDVL_482_17
- Kardaun SH, Sekula P, Valeyrie-Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Brit J Dermatol. 2013;169:1071-1080.
- Morán-Mariños C, Alva-Diaz C, De la Cruz Ramirez W, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS) induced by phenytoin re-exposure: case report and systematic review. Acta Clin Belg. 2022;77:177-185. doi:10.1080/17843286.2020.1767459
To the Editor:
Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome—a severe cutaneous adverse drug reaction—is characterized by a cutaneous rash and systemic upset in the form of various internal organ and hematologic disturbances. This delayed and idiosyncratic syndrome went by several names, including anticonvulsant hypersensitivity syndrome, before Bocquet et al1 proposed the term DRESS syndrome.
Phenytoin, a hydantoin derivative used in neurology, was implicated in 41% of cases of DRESS syndrome in a study of 100 patients conducted in southern India.2,3 While DRESS syndrome is a newer name, the clinical picture of DRESS secondary to phenytoin use remains similar in that it manifests with a morbilliform rash and systemic upset. We sought to describe the clinical and laboratory characteristics of phenytoin-induced DRESS syndrome in this case series.
The analysis included 23 patients with DRESS syndrome secondary to phenytoin use who presented to a tertiary care institution in North India between July 2021 and December 2022, satisfied the European Registry of Severe Cutaneous Adverse Reaction (RegiSCAR) criteria,4 and achieved a DRESS diagnostic score of more than 1. The mean age of the patients was 44 years (range, 14–74 years). There was a slight female predominance with a male to female ratio of 0.9:1. More than half of the patients (52.2% [12/23]) presented directly to the dermatology outpatient department; the remaining patients were referred from other departments (47.8% [11/23]). Patients primarily were receiving phenytoin for neurologic indications. Specific reasons included antiseizure prophylaxis following a traffic accident (34.8% [8/23]); epilepsy (26.1% [6/23]); and neoplastic (17.4% [4/23]), vascular (17.4% [4/23]), and infectious (4.3% [1/23]) causes. The mean latency period from drug intake to symptom onset was 29 days (range, 6–62 days), and the mean illness duration was 9 days (range, 1–45 days).
The majority of patients experienced pruritus (91.3% [21/23]) and fever (74.0% [17/23]), and all initially had a rash. Maculopapular morphology was seen in all patients. Erythema multiforme–like (17.4% [4/23]), erythrodermic (17.4% [4/23]), and vesicular (13.0% [3/23]) rashes also were documented (Figure 1). The trunk (100% [23/23]) and extremities (95.7% [22/23]) were involved most often, followed by the palms and soles (56.5% [13/23]). The mean total body surface area affected was 73.65%. Only 7 patients (30.4%) had mucosal involvement; nonhemorrhagic cheilitis was the most common manifestation.

Facial edema, a hallmark feature of DRESS syndrome, was noted in 69.6% (16/23) of patients (Figure 2). Lymphadenopathy was present in 43.5% (10/23) of patients; of those cases, the inguinal (40.0%; n=4) and cervical (30%; n=3) nodes most commonly were involved. Although DRESS syndrome can affect internal organs, this was an issue for only 2 (8.7%) patients who experienced mild hepatomegaly.

Laboratory investigations revealed a mean differential eosinophil percentage of 10.3% (reference range, 1%–4%), while the mean absolute eosinophil count was 1.0634×109/L (reference range, 0.02–0.5×109/L). Other hematologic findings included the mean percentages of neutrophils (60%; reference range, 50%–60%), lymphocytes (19.95%; reference range, 20%–50%), and monocytes (8.70%; reference range, 2%–8%).
Liver function tests revealed transaminitis5 as the most common finding, with mean aspartate aminotransferase levels of 109 U/L (reference range, 8–33 U/L), mean alanine aminotransferase of 97.9 U/L (reference range, 7–56 U/L), and mean alkaline phosphatase levels of 211.35 U/L (reference range, 44–147 U/L). Half of the patients had notable (>2 times the upper limit of normal) transaminitis.
Renal blood workup revealed slightly elevated blood urea nitrogen levels with a mean value of 28.4 mg/dL (reference range, 6–24 mg/dL), and mean serum creatinine was 0.78 mg/dL (reference range for men, 0.7–1.3 mg/dL; for women, 0.6–1.1 mg/dL).
All patients were treated with oral steroids (prednisolone 1 mg/kg/d) before tapering slowly over the following 6 to 8 weeks. The culprit drug (phenytoin) was stopped on the day of presentation. Resolution of rash and itching was seen in all patients by 3 weeks after presentation without any relapse by follow-up at 6 weeks from presentation to the hospital.
Our case series seeks to discuss the clinical and laboratory features of phenytoin-induced DRESS syndrome. Our patients had more erythrodermic and erythema multiforme–like morphologies, less mucosal involvement, more hepatic involvement, and earlier resolution.
To the Editor:
Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome—a severe cutaneous adverse drug reaction—is characterized by a cutaneous rash and systemic upset in the form of various internal organ and hematologic disturbances. This delayed and idiosyncratic syndrome went by several names, including anticonvulsant hypersensitivity syndrome, before Bocquet et al1 proposed the term DRESS syndrome.
Phenytoin, a hydantoin derivative used in neurology, was implicated in 41% of cases of DRESS syndrome in a study of 100 patients conducted in southern India.2,3 While DRESS syndrome is a newer name, the clinical picture of DRESS secondary to phenytoin use remains similar in that it manifests with a morbilliform rash and systemic upset. We sought to describe the clinical and laboratory characteristics of phenytoin-induced DRESS syndrome in this case series.
The analysis included 23 patients with DRESS syndrome secondary to phenytoin use who presented to a tertiary care institution in North India between July 2021 and December 2022, satisfied the European Registry of Severe Cutaneous Adverse Reaction (RegiSCAR) criteria,4 and achieved a DRESS diagnostic score of more than 1. The mean age of the patients was 44 years (range, 14–74 years). There was a slight female predominance with a male to female ratio of 0.9:1. More than half of the patients (52.2% [12/23]) presented directly to the dermatology outpatient department; the remaining patients were referred from other departments (47.8% [11/23]). Patients primarily were receiving phenytoin for neurologic indications. Specific reasons included antiseizure prophylaxis following a traffic accident (34.8% [8/23]); epilepsy (26.1% [6/23]); and neoplastic (17.4% [4/23]), vascular (17.4% [4/23]), and infectious (4.3% [1/23]) causes. The mean latency period from drug intake to symptom onset was 29 days (range, 6–62 days), and the mean illness duration was 9 days (range, 1–45 days).
The majority of patients experienced pruritus (91.3% [21/23]) and fever (74.0% [17/23]), and all initially had a rash. Maculopapular morphology was seen in all patients. Erythema multiforme–like (17.4% [4/23]), erythrodermic (17.4% [4/23]), and vesicular (13.0% [3/23]) rashes also were documented (Figure 1). The trunk (100% [23/23]) and extremities (95.7% [22/23]) were involved most often, followed by the palms and soles (56.5% [13/23]). The mean total body surface area affected was 73.65%. Only 7 patients (30.4%) had mucosal involvement; nonhemorrhagic cheilitis was the most common manifestation.

Facial edema, a hallmark feature of DRESS syndrome, was noted in 69.6% (16/23) of patients (Figure 2). Lymphadenopathy was present in 43.5% (10/23) of patients; of those cases, the inguinal (40.0%; n=4) and cervical (30%; n=3) nodes most commonly were involved. Although DRESS syndrome can affect internal organs, this was an issue for only 2 (8.7%) patients who experienced mild hepatomegaly.

Laboratory investigations revealed a mean differential eosinophil percentage of 10.3% (reference range, 1%–4%), while the mean absolute eosinophil count was 1.0634×109/L (reference range, 0.02–0.5×109/L). Other hematologic findings included the mean percentages of neutrophils (60%; reference range, 50%–60%), lymphocytes (19.95%; reference range, 20%–50%), and monocytes (8.70%; reference range, 2%–8%).
Liver function tests revealed transaminitis5 as the most common finding, with mean aspartate aminotransferase levels of 109 U/L (reference range, 8–33 U/L), mean alanine aminotransferase of 97.9 U/L (reference range, 7–56 U/L), and mean alkaline phosphatase levels of 211.35 U/L (reference range, 44–147 U/L). Half of the patients had notable (>2 times the upper limit of normal) transaminitis.
Renal blood workup revealed slightly elevated blood urea nitrogen levels with a mean value of 28.4 mg/dL (reference range, 6–24 mg/dL), and mean serum creatinine was 0.78 mg/dL (reference range for men, 0.7–1.3 mg/dL; for women, 0.6–1.1 mg/dL).
All patients were treated with oral steroids (prednisolone 1 mg/kg/d) before tapering slowly over the following 6 to 8 weeks. The culprit drug (phenytoin) was stopped on the day of presentation. Resolution of rash and itching was seen in all patients by 3 weeks after presentation without any relapse by follow-up at 6 weeks from presentation to the hospital.
Our case series seeks to discuss the clinical and laboratory features of phenytoin-induced DRESS syndrome. Our patients had more erythrodermic and erythema multiforme–like morphologies, less mucosal involvement, more hepatic involvement, and earlier resolution.
- Bocquet H, Bagot M, Roujeau JC. Drug-induced pseudolymphoma and drug hypersensitivity syndrome (drug rash with eosinophilia and systemic symptoms: DRESS). Semin Cutan Med Surg. 1996;15:250-257. doi:10.1016/s1085-5629(96)80038-1
- Patocka J, Wu Q, Nepovimova E, et al. Phenytoin—an anti-seizure drug: overview of its chemistry, pharmacology and toxicology. Food Chem Toxicol. 2020;142:111393. doi:10.1016/j.fct.2020.111393
- Sasidharanpillai S, Chathoth AT, Khader A, et al. Predictors of disease severity in drug reaction with eosinophilia and systemic symptoms. Indian J Dermatol Venereol Leprol. 2019;85:266-275. doi:10.4103/ijdvl.IJDVL_482_17
- Kardaun SH, Sekula P, Valeyrie-Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Brit J Dermatol. 2013;169:1071-1080.
- Morán-Mariños C, Alva-Diaz C, De la Cruz Ramirez W, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS) induced by phenytoin re-exposure: case report and systematic review. Acta Clin Belg. 2022;77:177-185. doi:10.1080/17843286.2020.1767459
- Bocquet H, Bagot M, Roujeau JC. Drug-induced pseudolymphoma and drug hypersensitivity syndrome (drug rash with eosinophilia and systemic symptoms: DRESS). Semin Cutan Med Surg. 1996;15:250-257. doi:10.1016/s1085-5629(96)80038-1
- Patocka J, Wu Q, Nepovimova E, et al. Phenytoin—an anti-seizure drug: overview of its chemistry, pharmacology and toxicology. Food Chem Toxicol. 2020;142:111393. doi:10.1016/j.fct.2020.111393
- Sasidharanpillai S, Chathoth AT, Khader A, et al. Predictors of disease severity in drug reaction with eosinophilia and systemic symptoms. Indian J Dermatol Venereol Leprol. 2019;85:266-275. doi:10.4103/ijdvl.IJDVL_482_17
- Kardaun SH, Sekula P, Valeyrie-Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Brit J Dermatol. 2013;169:1071-1080.
- Morán-Mariños C, Alva-Diaz C, De la Cruz Ramirez W, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS) induced by phenytoin re-exposure: case report and systematic review. Acta Clin Belg. 2022;77:177-185. doi:10.1080/17843286.2020.1767459
Practice Points
- Phenytoin has been implicated in drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, and common symptoms include rash, pruritus, and fever.
- Transaminitis may occur in patients with DRESS syndrome secondary to phenytoin use.
Over 3 Years, Atopic Dermatitis Well-Controlled with Lebrikizumab
AMSTERDAM — among those followed up for an additional 2 years, according to the latest data from an extension study.
At the end of the maintenance phase of the pivotal trials at 12 months, 84% of the patients enrolled into the extension had clear or almost clear skin, as per the Investigator Global Assessment (IGA). This overall figure as well as the proportion with even better responses have persisted unchanged, reported Diamant Thaçi, MD, PhD, professor and head of the Comprehensive Center for Inflammatory Medicine, University of Lübeck in Germany.
Responses at 3 Years Maintained
“This is really quite remarkable,” Dr. Thaçi said. “Roughly all the patients maintained their response.” These results became even more remarkable when patients were assessed for their use of adjunctive therapy to control flares.
“Over the whole follow-up, 90% had no need for topical corticosteroids or any other rescue therapy,” Dr. Thaçi reported, providing data from the ADjoin lebrikizumab extension study during a late-breaking news session at the annual meeting of the European Academy of Dermatology and Venereology.
The patients in ADjoin were enrolled from the pivotal phase 3 ADvocate 1 and 2 trials completed almost 2 years ago and published together in March 2023. Lebrikizumab was approved in the United States in September 2024 for moderate to severe AD in patients aged ≥ 12 years, following previous approvals in Europe in 2023 and in Japan in January 2024.
In these two identical trials with a total of 564 patients, the primary endpoint was an IGA of 0 or 1, signifying clear or almost clear skin. At nearly 40%, the proportion of patients reaching this outcome at 16 weeks was about threefold greater (P < .001) on lebrikizumab than on placebo. The benefit was similar on secondary endpoints, such as 75% improvement in the Eczema Area and Severity Index (EASI75) score.
At the end of the double-blind, placebo-controlled 16-week phase of the ADvocate 1 and 2 trials, which enrolled adults and adolescents aged ≥ 12 years, responders were enrolled into a maintenance phase in which they were rerandomized to 250 mg lebrikizumab every 2 weeks (Q2W) or every 4 weeks (Q4W). The latter is the approved maintenance dose.
At the end of the maintenance phase, which lasted another 32 weeks (total exposure of 52 weeks for those initially randomized to lebrikizumab), patients were invited into the ADjoin extension. The only exclusions from the extension were serious adverse events related to lebrikizumab and noncompliance.
Response Curves Appear as Straight Lines
Over the next 2 years of ADjoin, response curves appeared as straight lines not only for the overall response but when patients were stratified for different levels of response at the extension study entry. Specifically, 81.5% and 83.3% had an IGA score of 0 or 1 in the Q2W and Q4W arms at completion of the ADvocate 16-week double-blind phase. At 3 years, the rates were 84.0% and 82.9%, respectively.
For the subgroup who entered ADjoin with an EASI75 or an EASI90 response, the persistence of this level of response over 2 years was similar, although there was some gain observed among those who entered the trial with an EASI75 response.
“Not only did these patients maintain their response, but the response on average slowly improved, so that there were more patients with an EASI90 response at the 3-year timepoint,” Dr. Thaçi said.
Of the 181 patients in the ADjoin extension, 82 patients were maintained on Q2W dosing and 99 were maintained on Q4W lebrikizumab. Their mean age was about 35 years, more than half were women, and nearly 40% had severe AD at the time they enrolled in the ADvocate trials. There was essentially no difference in response rates among those in the Q2W and Q4W arms over time in ADjoin.
Side Effect Profile Essentially Unchanged
The side effect and tolerability profiles, which were favorable in the original 16-week placebo-controlled study, have remained unchanged over the subsequent maintenance phase and through the additional 2 years of the ADjoin extension.
“There continued to be reports of conjunctivitis, which is very specific for anti–IL-13 therapies,” Dr. Thaçi said. However, he said that the incidence did not increase over time, and because it was easy to treat, “most patients do not discontinue lebrikizumab for this reason.” Moreover, he said the impression was that “the number of patients experiencing adverse effects has been decreasing over time.”
Calling these long-term results “very exciting,” Dr. Thaçi called lebrikizumab “a very valuable option for long-term AD care.”
Asked for his perspective on the results, Jonathan I. Silverberg, MD, PhD, Director of Clinical Research, Department of Dermatology, George Washington University, Washington, DC, said that it is important to study long-term efficacy, and these results are positive. Without direct comparisons to other biologics available for AD, nothing can be implied about the relative efficacy of monoclonal antibodies approved for AD.
“These data are important both from an efficacy and safety perspective” for those advising patients who need chronic AD treatment, said Dr. Silverberg, who was the principal investigator of the ADvocate trials.
Earlier this year, 5-year follow-up data were published for dupilumab. Of 326 patients who remained on therapy this long, 220 (67%) maintained an IGA of 0 or 1 at the end of the study. There were no unexpected adverse events, which were generally stable or declined throughout the study.
Dr. Thaçi has financial relationships with AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Celltrion, Galderma, Leo Pharma, L’Oreal, Janssen-Cilag, New Bridge, Novartis, Pfizer, Regeneron, Roche, Sanofi, Sun Pharma, UCB, and Vichy. Dr. Silverberg reported financial relationships with more than 40 pharmaceutical companies including those that make drugs for AD.
A version of this article appeared on Medscape.com.
AMSTERDAM — among those followed up for an additional 2 years, according to the latest data from an extension study.
At the end of the maintenance phase of the pivotal trials at 12 months, 84% of the patients enrolled into the extension had clear or almost clear skin, as per the Investigator Global Assessment (IGA). This overall figure as well as the proportion with even better responses have persisted unchanged, reported Diamant Thaçi, MD, PhD, professor and head of the Comprehensive Center for Inflammatory Medicine, University of Lübeck in Germany.
Responses at 3 Years Maintained
“This is really quite remarkable,” Dr. Thaçi said. “Roughly all the patients maintained their response.” These results became even more remarkable when patients were assessed for their use of adjunctive therapy to control flares.
“Over the whole follow-up, 90% had no need for topical corticosteroids or any other rescue therapy,” Dr. Thaçi reported, providing data from the ADjoin lebrikizumab extension study during a late-breaking news session at the annual meeting of the European Academy of Dermatology and Venereology.
The patients in ADjoin were enrolled from the pivotal phase 3 ADvocate 1 and 2 trials completed almost 2 years ago and published together in March 2023. Lebrikizumab was approved in the United States in September 2024 for moderate to severe AD in patients aged ≥ 12 years, following previous approvals in Europe in 2023 and in Japan in January 2024.
In these two identical trials with a total of 564 patients, the primary endpoint was an IGA of 0 or 1, signifying clear or almost clear skin. At nearly 40%, the proportion of patients reaching this outcome at 16 weeks was about threefold greater (P < .001) on lebrikizumab than on placebo. The benefit was similar on secondary endpoints, such as 75% improvement in the Eczema Area and Severity Index (EASI75) score.
At the end of the double-blind, placebo-controlled 16-week phase of the ADvocate 1 and 2 trials, which enrolled adults and adolescents aged ≥ 12 years, responders were enrolled into a maintenance phase in which they were rerandomized to 250 mg lebrikizumab every 2 weeks (Q2W) or every 4 weeks (Q4W). The latter is the approved maintenance dose.
At the end of the maintenance phase, which lasted another 32 weeks (total exposure of 52 weeks for those initially randomized to lebrikizumab), patients were invited into the ADjoin extension. The only exclusions from the extension were serious adverse events related to lebrikizumab and noncompliance.
Response Curves Appear as Straight Lines
Over the next 2 years of ADjoin, response curves appeared as straight lines not only for the overall response but when patients were stratified for different levels of response at the extension study entry. Specifically, 81.5% and 83.3% had an IGA score of 0 or 1 in the Q2W and Q4W arms at completion of the ADvocate 16-week double-blind phase. At 3 years, the rates were 84.0% and 82.9%, respectively.
For the subgroup who entered ADjoin with an EASI75 or an EASI90 response, the persistence of this level of response over 2 years was similar, although there was some gain observed among those who entered the trial with an EASI75 response.
“Not only did these patients maintain their response, but the response on average slowly improved, so that there were more patients with an EASI90 response at the 3-year timepoint,” Dr. Thaçi said.
Of the 181 patients in the ADjoin extension, 82 patients were maintained on Q2W dosing and 99 were maintained on Q4W lebrikizumab. Their mean age was about 35 years, more than half were women, and nearly 40% had severe AD at the time they enrolled in the ADvocate trials. There was essentially no difference in response rates among those in the Q2W and Q4W arms over time in ADjoin.
Side Effect Profile Essentially Unchanged
The side effect and tolerability profiles, which were favorable in the original 16-week placebo-controlled study, have remained unchanged over the subsequent maintenance phase and through the additional 2 years of the ADjoin extension.
“There continued to be reports of conjunctivitis, which is very specific for anti–IL-13 therapies,” Dr. Thaçi said. However, he said that the incidence did not increase over time, and because it was easy to treat, “most patients do not discontinue lebrikizumab for this reason.” Moreover, he said the impression was that “the number of patients experiencing adverse effects has been decreasing over time.”
Calling these long-term results “very exciting,” Dr. Thaçi called lebrikizumab “a very valuable option for long-term AD care.”
Asked for his perspective on the results, Jonathan I. Silverberg, MD, PhD, Director of Clinical Research, Department of Dermatology, George Washington University, Washington, DC, said that it is important to study long-term efficacy, and these results are positive. Without direct comparisons to other biologics available for AD, nothing can be implied about the relative efficacy of monoclonal antibodies approved for AD.
“These data are important both from an efficacy and safety perspective” for those advising patients who need chronic AD treatment, said Dr. Silverberg, who was the principal investigator of the ADvocate trials.
Earlier this year, 5-year follow-up data were published for dupilumab. Of 326 patients who remained on therapy this long, 220 (67%) maintained an IGA of 0 or 1 at the end of the study. There were no unexpected adverse events, which were generally stable or declined throughout the study.
Dr. Thaçi has financial relationships with AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Celltrion, Galderma, Leo Pharma, L’Oreal, Janssen-Cilag, New Bridge, Novartis, Pfizer, Regeneron, Roche, Sanofi, Sun Pharma, UCB, and Vichy. Dr. Silverberg reported financial relationships with more than 40 pharmaceutical companies including those that make drugs for AD.
A version of this article appeared on Medscape.com.
AMSTERDAM — among those followed up for an additional 2 years, according to the latest data from an extension study.
At the end of the maintenance phase of the pivotal trials at 12 months, 84% of the patients enrolled into the extension had clear or almost clear skin, as per the Investigator Global Assessment (IGA). This overall figure as well as the proportion with even better responses have persisted unchanged, reported Diamant Thaçi, MD, PhD, professor and head of the Comprehensive Center for Inflammatory Medicine, University of Lübeck in Germany.
Responses at 3 Years Maintained
“This is really quite remarkable,” Dr. Thaçi said. “Roughly all the patients maintained their response.” These results became even more remarkable when patients were assessed for their use of adjunctive therapy to control flares.
“Over the whole follow-up, 90% had no need for topical corticosteroids or any other rescue therapy,” Dr. Thaçi reported, providing data from the ADjoin lebrikizumab extension study during a late-breaking news session at the annual meeting of the European Academy of Dermatology and Venereology.
The patients in ADjoin were enrolled from the pivotal phase 3 ADvocate 1 and 2 trials completed almost 2 years ago and published together in March 2023. Lebrikizumab was approved in the United States in September 2024 for moderate to severe AD in patients aged ≥ 12 years, following previous approvals in Europe in 2023 and in Japan in January 2024.
In these two identical trials with a total of 564 patients, the primary endpoint was an IGA of 0 or 1, signifying clear or almost clear skin. At nearly 40%, the proportion of patients reaching this outcome at 16 weeks was about threefold greater (P < .001) on lebrikizumab than on placebo. The benefit was similar on secondary endpoints, such as 75% improvement in the Eczema Area and Severity Index (EASI75) score.
At the end of the double-blind, placebo-controlled 16-week phase of the ADvocate 1 and 2 trials, which enrolled adults and adolescents aged ≥ 12 years, responders were enrolled into a maintenance phase in which they were rerandomized to 250 mg lebrikizumab every 2 weeks (Q2W) or every 4 weeks (Q4W). The latter is the approved maintenance dose.
At the end of the maintenance phase, which lasted another 32 weeks (total exposure of 52 weeks for those initially randomized to lebrikizumab), patients were invited into the ADjoin extension. The only exclusions from the extension were serious adverse events related to lebrikizumab and noncompliance.
Response Curves Appear as Straight Lines
Over the next 2 years of ADjoin, response curves appeared as straight lines not only for the overall response but when patients were stratified for different levels of response at the extension study entry. Specifically, 81.5% and 83.3% had an IGA score of 0 or 1 in the Q2W and Q4W arms at completion of the ADvocate 16-week double-blind phase. At 3 years, the rates were 84.0% and 82.9%, respectively.
For the subgroup who entered ADjoin with an EASI75 or an EASI90 response, the persistence of this level of response over 2 years was similar, although there was some gain observed among those who entered the trial with an EASI75 response.
“Not only did these patients maintain their response, but the response on average slowly improved, so that there were more patients with an EASI90 response at the 3-year timepoint,” Dr. Thaçi said.
Of the 181 patients in the ADjoin extension, 82 patients were maintained on Q2W dosing and 99 were maintained on Q4W lebrikizumab. Their mean age was about 35 years, more than half were women, and nearly 40% had severe AD at the time they enrolled in the ADvocate trials. There was essentially no difference in response rates among those in the Q2W and Q4W arms over time in ADjoin.
Side Effect Profile Essentially Unchanged
The side effect and tolerability profiles, which were favorable in the original 16-week placebo-controlled study, have remained unchanged over the subsequent maintenance phase and through the additional 2 years of the ADjoin extension.
“There continued to be reports of conjunctivitis, which is very specific for anti–IL-13 therapies,” Dr. Thaçi said. However, he said that the incidence did not increase over time, and because it was easy to treat, “most patients do not discontinue lebrikizumab for this reason.” Moreover, he said the impression was that “the number of patients experiencing adverse effects has been decreasing over time.”
Calling these long-term results “very exciting,” Dr. Thaçi called lebrikizumab “a very valuable option for long-term AD care.”
Asked for his perspective on the results, Jonathan I. Silverberg, MD, PhD, Director of Clinical Research, Department of Dermatology, George Washington University, Washington, DC, said that it is important to study long-term efficacy, and these results are positive. Without direct comparisons to other biologics available for AD, nothing can be implied about the relative efficacy of monoclonal antibodies approved for AD.
“These data are important both from an efficacy and safety perspective” for those advising patients who need chronic AD treatment, said Dr. Silverberg, who was the principal investigator of the ADvocate trials.
Earlier this year, 5-year follow-up data were published for dupilumab. Of 326 patients who remained on therapy this long, 220 (67%) maintained an IGA of 0 or 1 at the end of the study. There were no unexpected adverse events, which were generally stable or declined throughout the study.
Dr. Thaçi has financial relationships with AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Celltrion, Galderma, Leo Pharma, L’Oreal, Janssen-Cilag, New Bridge, Novartis, Pfizer, Regeneron, Roche, Sanofi, Sun Pharma, UCB, and Vichy. Dr. Silverberg reported financial relationships with more than 40 pharmaceutical companies including those that make drugs for AD.
A version of this article appeared on Medscape.com.
FROM EADV 2024
Different Biomarker Profiles Identified in Study of Late Dupilumab Responders
AMSTERDAM — A proteomics study designed to determine why some patients with atopic dermatitis (AD) respond quickly to dupilumab, others respond more slowly, and the remainder do not respond at all demonstrated that molecular responses in these three groups are very different.
A discovery that could lead to personalizing therapies, the data identified “distinct systemic biomarker profiles,” according to Ester Del Duca, MD, an instructor in the Laboratory of Inflammatory Skin Diseases at the Icahn School of Medicine at Mount Sinai, New York City.
The study was conducted with 67 patients with AD and 16 healthy controls. Serum was collected at two timepoints: An average of 20 weeks after starting dupilumab, then at a mean interval of about 9 months later. At these timepoints, called follow-up 1 and 2, a panel of more than 600 proteins, including unique markers for immunologic, cardiovascular, and neurologic activity, were evaluated.
The criterion for differentiating the three response groups was an Investigator Global Assessment (IGA) score of 0 or 1, signifying clear or almost clear skin (or at least a 2-point IGA reduction from baseline). Early responders were those who met the criterion at both follow-ups, late responders were those who met this criterion only at the second follow-up, and nonresponders never met the criterion.
“There were no significant differences at baseline in clinical severity, past medical history, or patient characteristics,” said Del Duca, who presented these data in a late breaking news session at the European Academy of Dermatology and Venereology (EADV) 2024 Congress.
For early responders, there was an early normalization of the proteome, reported Del Duca, illustrating the differences in the proteome of the three groups with a color-coded chart of protein up-regulation and down-regulation relative to healthy controls. The normalization of the proteome persisted in early responders when assessed at the second follow-up.
In the late responders, the proteome dysregulation was substantial relative to healthy controls at the first follow-up, but there was considerable improvement by the second follow-up. Although the change at the second follow-up was still not as robust as that seen in the early responders at either follow-up, Del Duca described the proteomic profile as a 45% improvement from the first follow-up.
In contrast, nonresponders showed worsening in their blood proteome from follow-up 1 to 2. Nonresponders at first follow-up showed up-regulation relative to healthy controls for many proteins associated with the Th1 response, such as interferon gamma, CXCL9, and CXCL10, and Th2 response, such as interleukin-4 and Th17/22, and these did not normalize or worsen by the second follow-up.
“Uniquely to nonresponders, key Th1 biomarkers remained significantly up-regulated relative to controls at both follow-up 1 and 2,” with a P value < .05, Del Duca reported.
To achieve normalization of the proteome as defined by healthy controls, both up-regulation and down-regulation of protein activity were required, although more up-regulations than down-regulations were observed.
When evaluating the proteome changes most implicated in immunoregulation, the investigators were able to show a correlation between worsening in the proteome and greater severity of AD as defined by IGA, Eczema Area and Severity Index, and body surface area involvement.
“Spearman analysis revealed strong and positive correlations between improvements in biomarkers at follow-up 1 and 2 with improvements in clinical markers,” Del Duca said. As examples, she noted favorable changes in biomarkers specifically associated with T cells, dendritic cells, and natural killer cells as clinical outcomes improved.
Conversely, the worsening in T-cell activation among nonresponders, particularly Th1 biomarkers, also tracked with increasing AD symptoms over time.
The implications of the research are broad, and most importantly, it shows that therapeutic targets are likely to differ between patients with AD, according to Del Duca. Although proteomic studies have not yet been conducted with other treatments, these might provide further insight about how patients with AD differ in response across other drugs.
This is important work, according to Brigitte Dréno, MD, PhD, head of the Department of Dermatology, Nantes University Hospital in France. As moderator of the late-breaking news session, she suggested that there are many potential messages from these data, not least that treatment of AD likely involves targeting cytokines beyond those affected by dupilumab in at least some patients.
When Dréno asked Del Duca about what could be surmised about changes from baseline before treatment to the first follow-up, Del Duca said that the study was retrospective, so baseline data were not available.
This is an important missing piece of this investigation, according to Dréno.
“As you move this work forward,” she said that it would be “very important” to determine “if there are predictive markers for evaluating which patients will respond.”
This is a small study with many additional variables to consider in order to develop a clinically useful tool, Del Duca noted. However, this work not only has the potential to guide treatment selection but the biomarkers up-regulated in nonresponders are already “suggesting potential targets for refining therapeutic strategies,” she said.
The study received funding from Bristol-Myers Squibb. Del Duca reported no financial relationships with industry. Dréno reported financial relationships with La Roche–Posay, Pierre Fabré, and Galderma.
A version of this article appeared on Medscape.com.
AMSTERDAM — A proteomics study designed to determine why some patients with atopic dermatitis (AD) respond quickly to dupilumab, others respond more slowly, and the remainder do not respond at all demonstrated that molecular responses in these three groups are very different.
A discovery that could lead to personalizing therapies, the data identified “distinct systemic biomarker profiles,” according to Ester Del Duca, MD, an instructor in the Laboratory of Inflammatory Skin Diseases at the Icahn School of Medicine at Mount Sinai, New York City.
The study was conducted with 67 patients with AD and 16 healthy controls. Serum was collected at two timepoints: An average of 20 weeks after starting dupilumab, then at a mean interval of about 9 months later. At these timepoints, called follow-up 1 and 2, a panel of more than 600 proteins, including unique markers for immunologic, cardiovascular, and neurologic activity, were evaluated.
The criterion for differentiating the three response groups was an Investigator Global Assessment (IGA) score of 0 or 1, signifying clear or almost clear skin (or at least a 2-point IGA reduction from baseline). Early responders were those who met the criterion at both follow-ups, late responders were those who met this criterion only at the second follow-up, and nonresponders never met the criterion.
“There were no significant differences at baseline in clinical severity, past medical history, or patient characteristics,” said Del Duca, who presented these data in a late breaking news session at the European Academy of Dermatology and Venereology (EADV) 2024 Congress.
For early responders, there was an early normalization of the proteome, reported Del Duca, illustrating the differences in the proteome of the three groups with a color-coded chart of protein up-regulation and down-regulation relative to healthy controls. The normalization of the proteome persisted in early responders when assessed at the second follow-up.
In the late responders, the proteome dysregulation was substantial relative to healthy controls at the first follow-up, but there was considerable improvement by the second follow-up. Although the change at the second follow-up was still not as robust as that seen in the early responders at either follow-up, Del Duca described the proteomic profile as a 45% improvement from the first follow-up.
In contrast, nonresponders showed worsening in their blood proteome from follow-up 1 to 2. Nonresponders at first follow-up showed up-regulation relative to healthy controls for many proteins associated with the Th1 response, such as interferon gamma, CXCL9, and CXCL10, and Th2 response, such as interleukin-4 and Th17/22, and these did not normalize or worsen by the second follow-up.
“Uniquely to nonresponders, key Th1 biomarkers remained significantly up-regulated relative to controls at both follow-up 1 and 2,” with a P value < .05, Del Duca reported.
To achieve normalization of the proteome as defined by healthy controls, both up-regulation and down-regulation of protein activity were required, although more up-regulations than down-regulations were observed.
When evaluating the proteome changes most implicated in immunoregulation, the investigators were able to show a correlation between worsening in the proteome and greater severity of AD as defined by IGA, Eczema Area and Severity Index, and body surface area involvement.
“Spearman analysis revealed strong and positive correlations between improvements in biomarkers at follow-up 1 and 2 with improvements in clinical markers,” Del Duca said. As examples, she noted favorable changes in biomarkers specifically associated with T cells, dendritic cells, and natural killer cells as clinical outcomes improved.
Conversely, the worsening in T-cell activation among nonresponders, particularly Th1 biomarkers, also tracked with increasing AD symptoms over time.
The implications of the research are broad, and most importantly, it shows that therapeutic targets are likely to differ between patients with AD, according to Del Duca. Although proteomic studies have not yet been conducted with other treatments, these might provide further insight about how patients with AD differ in response across other drugs.
This is important work, according to Brigitte Dréno, MD, PhD, head of the Department of Dermatology, Nantes University Hospital in France. As moderator of the late-breaking news session, she suggested that there are many potential messages from these data, not least that treatment of AD likely involves targeting cytokines beyond those affected by dupilumab in at least some patients.
When Dréno asked Del Duca about what could be surmised about changes from baseline before treatment to the first follow-up, Del Duca said that the study was retrospective, so baseline data were not available.
This is an important missing piece of this investigation, according to Dréno.
“As you move this work forward,” she said that it would be “very important” to determine “if there are predictive markers for evaluating which patients will respond.”
This is a small study with many additional variables to consider in order to develop a clinically useful tool, Del Duca noted. However, this work not only has the potential to guide treatment selection but the biomarkers up-regulated in nonresponders are already “suggesting potential targets for refining therapeutic strategies,” she said.
The study received funding from Bristol-Myers Squibb. Del Duca reported no financial relationships with industry. Dréno reported financial relationships with La Roche–Posay, Pierre Fabré, and Galderma.
A version of this article appeared on Medscape.com.
AMSTERDAM — A proteomics study designed to determine why some patients with atopic dermatitis (AD) respond quickly to dupilumab, others respond more slowly, and the remainder do not respond at all demonstrated that molecular responses in these three groups are very different.
A discovery that could lead to personalizing therapies, the data identified “distinct systemic biomarker profiles,” according to Ester Del Duca, MD, an instructor in the Laboratory of Inflammatory Skin Diseases at the Icahn School of Medicine at Mount Sinai, New York City.
The study was conducted with 67 patients with AD and 16 healthy controls. Serum was collected at two timepoints: An average of 20 weeks after starting dupilumab, then at a mean interval of about 9 months later. At these timepoints, called follow-up 1 and 2, a panel of more than 600 proteins, including unique markers for immunologic, cardiovascular, and neurologic activity, were evaluated.
The criterion for differentiating the three response groups was an Investigator Global Assessment (IGA) score of 0 or 1, signifying clear or almost clear skin (or at least a 2-point IGA reduction from baseline). Early responders were those who met the criterion at both follow-ups, late responders were those who met this criterion only at the second follow-up, and nonresponders never met the criterion.
“There were no significant differences at baseline in clinical severity, past medical history, or patient characteristics,” said Del Duca, who presented these data in a late breaking news session at the European Academy of Dermatology and Venereology (EADV) 2024 Congress.
For early responders, there was an early normalization of the proteome, reported Del Duca, illustrating the differences in the proteome of the three groups with a color-coded chart of protein up-regulation and down-regulation relative to healthy controls. The normalization of the proteome persisted in early responders when assessed at the second follow-up.
In the late responders, the proteome dysregulation was substantial relative to healthy controls at the first follow-up, but there was considerable improvement by the second follow-up. Although the change at the second follow-up was still not as robust as that seen in the early responders at either follow-up, Del Duca described the proteomic profile as a 45% improvement from the first follow-up.
In contrast, nonresponders showed worsening in their blood proteome from follow-up 1 to 2. Nonresponders at first follow-up showed up-regulation relative to healthy controls for many proteins associated with the Th1 response, such as interferon gamma, CXCL9, and CXCL10, and Th2 response, such as interleukin-4 and Th17/22, and these did not normalize or worsen by the second follow-up.
“Uniquely to nonresponders, key Th1 biomarkers remained significantly up-regulated relative to controls at both follow-up 1 and 2,” with a P value < .05, Del Duca reported.
To achieve normalization of the proteome as defined by healthy controls, both up-regulation and down-regulation of protein activity were required, although more up-regulations than down-regulations were observed.
When evaluating the proteome changes most implicated in immunoregulation, the investigators were able to show a correlation between worsening in the proteome and greater severity of AD as defined by IGA, Eczema Area and Severity Index, and body surface area involvement.
“Spearman analysis revealed strong and positive correlations between improvements in biomarkers at follow-up 1 and 2 with improvements in clinical markers,” Del Duca said. As examples, she noted favorable changes in biomarkers specifically associated with T cells, dendritic cells, and natural killer cells as clinical outcomes improved.
Conversely, the worsening in T-cell activation among nonresponders, particularly Th1 biomarkers, also tracked with increasing AD symptoms over time.
The implications of the research are broad, and most importantly, it shows that therapeutic targets are likely to differ between patients with AD, according to Del Duca. Although proteomic studies have not yet been conducted with other treatments, these might provide further insight about how patients with AD differ in response across other drugs.
This is important work, according to Brigitte Dréno, MD, PhD, head of the Department of Dermatology, Nantes University Hospital in France. As moderator of the late-breaking news session, she suggested that there are many potential messages from these data, not least that treatment of AD likely involves targeting cytokines beyond those affected by dupilumab in at least some patients.
When Dréno asked Del Duca about what could be surmised about changes from baseline before treatment to the first follow-up, Del Duca said that the study was retrospective, so baseline data were not available.
This is an important missing piece of this investigation, according to Dréno.
“As you move this work forward,” she said that it would be “very important” to determine “if there are predictive markers for evaluating which patients will respond.”
This is a small study with many additional variables to consider in order to develop a clinically useful tool, Del Duca noted. However, this work not only has the potential to guide treatment selection but the biomarkers up-regulated in nonresponders are already “suggesting potential targets for refining therapeutic strategies,” she said.
The study received funding from Bristol-Myers Squibb. Del Duca reported no financial relationships with industry. Dréno reported financial relationships with La Roche–Posay, Pierre Fabré, and Galderma.
A version of this article appeared on Medscape.com.
FROM EADV 2024
PCPs Play a Key Role in Managing and Preventing the Atopic March in Children
Primary care physicians (PCPs) play a key role in treating young patients as they progress through the “atopic march” from atopic dermatitis through food allergy, asthma, and allergic rhinitis. They can also help prevent the process from starting.
“The PCP is usually the first clinician a family with concerns about atopic conditions sees, unless they first visit urgent care or an emergency department after an allergic reaction to food. Either way, families rely on their PCP for ongoing guidance,” said Terri F. Brown-Whitehorn, MD, attending physician in the Division of Allergy and Immunology at the Center for Pediatric Eosinophilic Disorders and the Integrative Health Program at Children’s Hospital of Philadelphia.
“The most important thing PCPs can do is know that the atopic march exists, how it progresses over time, and what signs and symptoms to look for,” she told this news organization.
The Atopic March
The atopic march describes the progression of allergic diseases in a child over time, with atopic dermatitis and food allergy in infancy tending to be followed by allergic rhinitis and asthma into later childhood and adulthood.
Although the pathophysiology of the inflammation that precedes atopic dermatitis is unclear, two main hypotheses have been proposed. The first suggests a primary immune dysfunction leads to immunoglobulin E (IgE) sensitization, allergic inflammation, and a secondary disturbance of the epithelial barrier; the second starts with a primary defect in the epithelial barrier that leads to secondary immunologic dysregulation and results in inflammation.
Genetics, infection, hygiene, extreme climate, food allergens, probiotics, aeroallergens, and tobacco smoke are thought to play roles in atopic dermatitis. An estimated 10%-12% of children and 1% of adults in the United States have been reported to have the condition, and the prevalence appears to be increasing. An estimated 85% of cases occur during the first year of life and 95% before the age of 5 years.
“Atopy often, though not always, runs in families, so PCPs should inquire about the history of atopic dermatitis, IgE-mediated food allergies, allergic rhinitis, and asthma in the patient’s siblings, parents, and grandparents,” Brown-Whitehorn said.
Key Educators
PCPs treat the full gamut of atopic conditions and are key educators on ways families can help mitigate their children’s atopic march or stop it before it begins, said Gerald Bell Lee, MD, an allergist and immunologist at Children’s Healthcare of Atlanta and an associate professor in the Division of Allergy and Immunology at Emory University School of Medicine, Atlanta.
“Most parents who bring their infants with eczema to the PCP assume their child ate something that caused their rash. But the relationship between atopic dermatitis, a type of eczema, and food allergy is more complicated,” he added.
Lee said PCPs should explain to their patients what atopic dermatitis is, how it starts and progresses, and how families can help prevent the condition by, for example, introducing allergenic foods to infants at around 4-6 months of age.
Atopic Dermatitis
PCPs should inform parents and other caregivers to wash their hands before moisturizing their child, take care not to contaminate the moisturizer, and bathe their child only when the child is dirty.
“Soap removes protective natural skin oils and increases moisture loss, and exposure to soap and bathing is a main contributor to eczema,” said Lee. “Dry skin loses its protective barrier, allowing outside agents to penetrate and be identified by the immune system.”
“According to one hypothesis, parents may eat food, not wash their hands afterwards, then moisturize their baby. This unhygienic practice spreads food proteins from the adult’s meal, and possibly from contaminants present in the moisturizer, all over the baby’s body,” he added.
Lee said he and his colleagues discourage overbathing babies to minimize the risk for skin injury that begins the atopic march: “New parents are inundated with infant skincare messaging and products. But we need to weigh societal pressures against practicality and ask, ‘Is the child’s skin actually dirty?’ ”
Atopic dermatitis tends to appear on the extensor surfaces, face, and scalp in infants and around arm and leg creases in toddlers and older children. Severe forms of the condition can be more widely distributed on the body, said Aarti P. Pandya, MD, medical director of the Food Allergy Center at Children’s Mercy Kansas City and clinical assistant professor of pediatrics at the University of Missouri-Kansas City School of Medicine, Kansas City, Missouri.
Avoid Triggers, Minimize Flares
Triggers of eczema are varied and common. To help minimize flares, PCPs can encourage caregivers to avoid products with fragrances or dyes, minimize the use of soaps, and completely rinse laundry detergent from clothing and household items. “Advise them to keep fingernails short and control dander, pollen, mold, household chemicals, and tobacco smoke, as well as the child’s stress and anxiety, which can also be a trigger,” Lee said.
“Skin infections from organisms such as staph, herpes, or coxsackie can also exacerbate symptoms,” Brown-Whitehorn added. “PCPs can educate caregivers to avoid all known triggers and give them an ‘action plan’ to carry out when skin flares.”
Food Allergies
Parents may be unaware food allergens can travel far beyond the plate, Lee said. Researchers vacuuming household bedding, carpets, furniture, and other surfaces have detected unnoticeably tiny quantities of allergenic food proteins in ordinary house dust. Touching this dust appears to provide the main exposure to those allergens.
“According to the dual exposure to allergen hypothesis, an infant’s tolerance to antigens occurs through high-dose exposure by mouth, and allergic sensitization occurs through low-dose exposure through the skin,” he said. “As young as four to six months of age, even before eating solid food, a child develops eczema, has a leaky skin barrier, comes in contact with food, and develops a food allergy.”
IgE-mediated food allergies can begin at any age. “Symptoms occur when a food is ingested and the patient develops symptoms including but not limited to urticaria, angioedema, pruritus, flushing, vomiting, diarrhea, coughing, wheezing, difficulty breathing, presyncope, or syncope,” Pandya noted.
In the case of eosinophilic esophagitis, which may also be part of the atopic march, infants and toddlers often have challenging-to-treat symptoms of reflux, while school-age children have reflux and abdominal pain, and adolescents and adults may experience difficulty swallowing and impactions of food or pills, Brown-Whitehorn said.
To differentiate between food allergy and contact dermatitis, Lee suggested providers ask, “ ’Is the rash hives? If yes, is the rash generalized or in a limited area?’ Then consider the statistical probabilities. Skin problems after milk, egg, wheat, soy, peanut, tree nut, fish, shellfish, or sesame are likely due to IgE-mediated food allergy, but after ketchup or strawberry are probably from skin contact.”
Allergic Rhinitis and Asthma
“For asthma, ask about frequency of night cough and symptoms with exercise, laughing, or crying. For allergic rhinitis, look for runny nose, itchy eyes, or sneezing,” Brown-Whitehorn said.
Testing and Monitoring
Assessing the extent of eczema with the Eczema Area and Severity Index or the SCORing Atopic Dermatitis index takes time but may be necessary to obtain insurance coverage for treatments such as biologics.
Avoid ordering IgE food panels, which can result in false positives that can lead to loss of tolerance and nutritional deficiencies; psychological harm from bullying, anxiety, and decreased quality of life; and higher food and healthcare costs, Pandya said.
Treatments
Caregivers may be wary about treatments, and all the three experts this news organization spoke with stressed the importance of educating caregivers about how treatments work and what to expect from them.
“Early and aggressive atopic dermatitis treatment could prevent sensitization to food or aeroallergens, which could help prevent additional atopic diseases, including those on the atopic march,” Pandya said. “Topical steroids are considered first line at any age. Topical phosphodiesterase inhibitors are approved at 3 months of age and above. Topical calcineurin inhibitors are approved at 2 years of age and above. Wet wrap therapy and bleach baths can be effective. Other options include biologic therapy, allergen immunotherapy, and UV therapy.”
“Epinephrine auto-injectors can counteract food reactions. For allergic rhinitis, non-sedating antihistamines, steroidal nasal sprays, and nasal antihistamines help. Asthma treatments include various inhaled medications,” Brown-Whitehorn added.
When to Refer to Specialists
Involving an allergist, dermatologist, pulmonologist, or ear nose throat specialist to the patient’s care team is advisable in more challenging cases.
If a child is younger than 3 months and has moderate to severe atopic dermatitis, an underlying immune defect may be to blame, so an allergy and immunology assessment is warranted, Brown-Whitehorn said. “An allergist can help any child who has recurrent coughing or wheezing avoid the emergency room or hospitalization.”
“In pediatrics, we always try to find the medication, regimen, and avoidance strategies that use the least treatment to provide the best care for each patient,” Brown-Whitehorn added. “Children eat, play, learn, and sleep, and every stage of the atopic march affects each of these activities. As clinicians, we need to be sure that we are helping children make the best of all these activities.”
Brown-Whitehorn reported financial relationships with DBV Technologies and Regeneron Pharmaceuticals. Lee reported financial relationships with Novartis. Pandya reported financial relationships with DBV Technologies, Thermo Fisher Scientific, and Sanofi.
A version of this article first appeared on Medscape.com.
Primary care physicians (PCPs) play a key role in treating young patients as they progress through the “atopic march” from atopic dermatitis through food allergy, asthma, and allergic rhinitis. They can also help prevent the process from starting.
“The PCP is usually the first clinician a family with concerns about atopic conditions sees, unless they first visit urgent care or an emergency department after an allergic reaction to food. Either way, families rely on their PCP for ongoing guidance,” said Terri F. Brown-Whitehorn, MD, attending physician in the Division of Allergy and Immunology at the Center for Pediatric Eosinophilic Disorders and the Integrative Health Program at Children’s Hospital of Philadelphia.
“The most important thing PCPs can do is know that the atopic march exists, how it progresses over time, and what signs and symptoms to look for,” she told this news organization.
The Atopic March
The atopic march describes the progression of allergic diseases in a child over time, with atopic dermatitis and food allergy in infancy tending to be followed by allergic rhinitis and asthma into later childhood and adulthood.
Although the pathophysiology of the inflammation that precedes atopic dermatitis is unclear, two main hypotheses have been proposed. The first suggests a primary immune dysfunction leads to immunoglobulin E (IgE) sensitization, allergic inflammation, and a secondary disturbance of the epithelial barrier; the second starts with a primary defect in the epithelial barrier that leads to secondary immunologic dysregulation and results in inflammation.
Genetics, infection, hygiene, extreme climate, food allergens, probiotics, aeroallergens, and tobacco smoke are thought to play roles in atopic dermatitis. An estimated 10%-12% of children and 1% of adults in the United States have been reported to have the condition, and the prevalence appears to be increasing. An estimated 85% of cases occur during the first year of life and 95% before the age of 5 years.
“Atopy often, though not always, runs in families, so PCPs should inquire about the history of atopic dermatitis, IgE-mediated food allergies, allergic rhinitis, and asthma in the patient’s siblings, parents, and grandparents,” Brown-Whitehorn said.
Key Educators
PCPs treat the full gamut of atopic conditions and are key educators on ways families can help mitigate their children’s atopic march or stop it before it begins, said Gerald Bell Lee, MD, an allergist and immunologist at Children’s Healthcare of Atlanta and an associate professor in the Division of Allergy and Immunology at Emory University School of Medicine, Atlanta.
“Most parents who bring their infants with eczema to the PCP assume their child ate something that caused their rash. But the relationship between atopic dermatitis, a type of eczema, and food allergy is more complicated,” he added.
Lee said PCPs should explain to their patients what atopic dermatitis is, how it starts and progresses, and how families can help prevent the condition by, for example, introducing allergenic foods to infants at around 4-6 months of age.
Atopic Dermatitis
PCPs should inform parents and other caregivers to wash their hands before moisturizing their child, take care not to contaminate the moisturizer, and bathe their child only when the child is dirty.
“Soap removes protective natural skin oils and increases moisture loss, and exposure to soap and bathing is a main contributor to eczema,” said Lee. “Dry skin loses its protective barrier, allowing outside agents to penetrate and be identified by the immune system.”
“According to one hypothesis, parents may eat food, not wash their hands afterwards, then moisturize their baby. This unhygienic practice spreads food proteins from the adult’s meal, and possibly from contaminants present in the moisturizer, all over the baby’s body,” he added.
Lee said he and his colleagues discourage overbathing babies to minimize the risk for skin injury that begins the atopic march: “New parents are inundated with infant skincare messaging and products. But we need to weigh societal pressures against practicality and ask, ‘Is the child’s skin actually dirty?’ ”
Atopic dermatitis tends to appear on the extensor surfaces, face, and scalp in infants and around arm and leg creases in toddlers and older children. Severe forms of the condition can be more widely distributed on the body, said Aarti P. Pandya, MD, medical director of the Food Allergy Center at Children’s Mercy Kansas City and clinical assistant professor of pediatrics at the University of Missouri-Kansas City School of Medicine, Kansas City, Missouri.
Avoid Triggers, Minimize Flares
Triggers of eczema are varied and common. To help minimize flares, PCPs can encourage caregivers to avoid products with fragrances or dyes, minimize the use of soaps, and completely rinse laundry detergent from clothing and household items. “Advise them to keep fingernails short and control dander, pollen, mold, household chemicals, and tobacco smoke, as well as the child’s stress and anxiety, which can also be a trigger,” Lee said.
“Skin infections from organisms such as staph, herpes, or coxsackie can also exacerbate symptoms,” Brown-Whitehorn added. “PCPs can educate caregivers to avoid all known triggers and give them an ‘action plan’ to carry out when skin flares.”
Food Allergies
Parents may be unaware food allergens can travel far beyond the plate, Lee said. Researchers vacuuming household bedding, carpets, furniture, and other surfaces have detected unnoticeably tiny quantities of allergenic food proteins in ordinary house dust. Touching this dust appears to provide the main exposure to those allergens.
“According to the dual exposure to allergen hypothesis, an infant’s tolerance to antigens occurs through high-dose exposure by mouth, and allergic sensitization occurs through low-dose exposure through the skin,” he said. “As young as four to six months of age, even before eating solid food, a child develops eczema, has a leaky skin barrier, comes in contact with food, and develops a food allergy.”
IgE-mediated food allergies can begin at any age. “Symptoms occur when a food is ingested and the patient develops symptoms including but not limited to urticaria, angioedema, pruritus, flushing, vomiting, diarrhea, coughing, wheezing, difficulty breathing, presyncope, or syncope,” Pandya noted.
In the case of eosinophilic esophagitis, which may also be part of the atopic march, infants and toddlers often have challenging-to-treat symptoms of reflux, while school-age children have reflux and abdominal pain, and adolescents and adults may experience difficulty swallowing and impactions of food or pills, Brown-Whitehorn said.
To differentiate between food allergy and contact dermatitis, Lee suggested providers ask, “ ’Is the rash hives? If yes, is the rash generalized or in a limited area?’ Then consider the statistical probabilities. Skin problems after milk, egg, wheat, soy, peanut, tree nut, fish, shellfish, or sesame are likely due to IgE-mediated food allergy, but after ketchup or strawberry are probably from skin contact.”
Allergic Rhinitis and Asthma
“For asthma, ask about frequency of night cough and symptoms with exercise, laughing, or crying. For allergic rhinitis, look for runny nose, itchy eyes, or sneezing,” Brown-Whitehorn said.
Testing and Monitoring
Assessing the extent of eczema with the Eczema Area and Severity Index or the SCORing Atopic Dermatitis index takes time but may be necessary to obtain insurance coverage for treatments such as biologics.
Avoid ordering IgE food panels, which can result in false positives that can lead to loss of tolerance and nutritional deficiencies; psychological harm from bullying, anxiety, and decreased quality of life; and higher food and healthcare costs, Pandya said.
Treatments
Caregivers may be wary about treatments, and all the three experts this news organization spoke with stressed the importance of educating caregivers about how treatments work and what to expect from them.
“Early and aggressive atopic dermatitis treatment could prevent sensitization to food or aeroallergens, which could help prevent additional atopic diseases, including those on the atopic march,” Pandya said. “Topical steroids are considered first line at any age. Topical phosphodiesterase inhibitors are approved at 3 months of age and above. Topical calcineurin inhibitors are approved at 2 years of age and above. Wet wrap therapy and bleach baths can be effective. Other options include biologic therapy, allergen immunotherapy, and UV therapy.”
“Epinephrine auto-injectors can counteract food reactions. For allergic rhinitis, non-sedating antihistamines, steroidal nasal sprays, and nasal antihistamines help. Asthma treatments include various inhaled medications,” Brown-Whitehorn added.
When to Refer to Specialists
Involving an allergist, dermatologist, pulmonologist, or ear nose throat specialist to the patient’s care team is advisable in more challenging cases.
If a child is younger than 3 months and has moderate to severe atopic dermatitis, an underlying immune defect may be to blame, so an allergy and immunology assessment is warranted, Brown-Whitehorn said. “An allergist can help any child who has recurrent coughing or wheezing avoid the emergency room or hospitalization.”
“In pediatrics, we always try to find the medication, regimen, and avoidance strategies that use the least treatment to provide the best care for each patient,” Brown-Whitehorn added. “Children eat, play, learn, and sleep, and every stage of the atopic march affects each of these activities. As clinicians, we need to be sure that we are helping children make the best of all these activities.”
Brown-Whitehorn reported financial relationships with DBV Technologies and Regeneron Pharmaceuticals. Lee reported financial relationships with Novartis. Pandya reported financial relationships with DBV Technologies, Thermo Fisher Scientific, and Sanofi.
A version of this article first appeared on Medscape.com.
Primary care physicians (PCPs) play a key role in treating young patients as they progress through the “atopic march” from atopic dermatitis through food allergy, asthma, and allergic rhinitis. They can also help prevent the process from starting.
“The PCP is usually the first clinician a family with concerns about atopic conditions sees, unless they first visit urgent care or an emergency department after an allergic reaction to food. Either way, families rely on their PCP for ongoing guidance,” said Terri F. Brown-Whitehorn, MD, attending physician in the Division of Allergy and Immunology at the Center for Pediatric Eosinophilic Disorders and the Integrative Health Program at Children’s Hospital of Philadelphia.
“The most important thing PCPs can do is know that the atopic march exists, how it progresses over time, and what signs and symptoms to look for,” she told this news organization.
The Atopic March
The atopic march describes the progression of allergic diseases in a child over time, with atopic dermatitis and food allergy in infancy tending to be followed by allergic rhinitis and asthma into later childhood and adulthood.
Although the pathophysiology of the inflammation that precedes atopic dermatitis is unclear, two main hypotheses have been proposed. The first suggests a primary immune dysfunction leads to immunoglobulin E (IgE) sensitization, allergic inflammation, and a secondary disturbance of the epithelial barrier; the second starts with a primary defect in the epithelial barrier that leads to secondary immunologic dysregulation and results in inflammation.
Genetics, infection, hygiene, extreme climate, food allergens, probiotics, aeroallergens, and tobacco smoke are thought to play roles in atopic dermatitis. An estimated 10%-12% of children and 1% of adults in the United States have been reported to have the condition, and the prevalence appears to be increasing. An estimated 85% of cases occur during the first year of life and 95% before the age of 5 years.
“Atopy often, though not always, runs in families, so PCPs should inquire about the history of atopic dermatitis, IgE-mediated food allergies, allergic rhinitis, and asthma in the patient’s siblings, parents, and grandparents,” Brown-Whitehorn said.
Key Educators
PCPs treat the full gamut of atopic conditions and are key educators on ways families can help mitigate their children’s atopic march or stop it before it begins, said Gerald Bell Lee, MD, an allergist and immunologist at Children’s Healthcare of Atlanta and an associate professor in the Division of Allergy and Immunology at Emory University School of Medicine, Atlanta.
“Most parents who bring their infants with eczema to the PCP assume their child ate something that caused their rash. But the relationship between atopic dermatitis, a type of eczema, and food allergy is more complicated,” he added.
Lee said PCPs should explain to their patients what atopic dermatitis is, how it starts and progresses, and how families can help prevent the condition by, for example, introducing allergenic foods to infants at around 4-6 months of age.
Atopic Dermatitis
PCPs should inform parents and other caregivers to wash their hands before moisturizing their child, take care not to contaminate the moisturizer, and bathe their child only when the child is dirty.
“Soap removes protective natural skin oils and increases moisture loss, and exposure to soap and bathing is a main contributor to eczema,” said Lee. “Dry skin loses its protective barrier, allowing outside agents to penetrate and be identified by the immune system.”
“According to one hypothesis, parents may eat food, not wash their hands afterwards, then moisturize their baby. This unhygienic practice spreads food proteins from the adult’s meal, and possibly from contaminants present in the moisturizer, all over the baby’s body,” he added.
Lee said he and his colleagues discourage overbathing babies to minimize the risk for skin injury that begins the atopic march: “New parents are inundated with infant skincare messaging and products. But we need to weigh societal pressures against practicality and ask, ‘Is the child’s skin actually dirty?’ ”
Atopic dermatitis tends to appear on the extensor surfaces, face, and scalp in infants and around arm and leg creases in toddlers and older children. Severe forms of the condition can be more widely distributed on the body, said Aarti P. Pandya, MD, medical director of the Food Allergy Center at Children’s Mercy Kansas City and clinical assistant professor of pediatrics at the University of Missouri-Kansas City School of Medicine, Kansas City, Missouri.
Avoid Triggers, Minimize Flares
Triggers of eczema are varied and common. To help minimize flares, PCPs can encourage caregivers to avoid products with fragrances or dyes, minimize the use of soaps, and completely rinse laundry detergent from clothing and household items. “Advise them to keep fingernails short and control dander, pollen, mold, household chemicals, and tobacco smoke, as well as the child’s stress and anxiety, which can also be a trigger,” Lee said.
“Skin infections from organisms such as staph, herpes, or coxsackie can also exacerbate symptoms,” Brown-Whitehorn added. “PCPs can educate caregivers to avoid all known triggers and give them an ‘action plan’ to carry out when skin flares.”
Food Allergies
Parents may be unaware food allergens can travel far beyond the plate, Lee said. Researchers vacuuming household bedding, carpets, furniture, and other surfaces have detected unnoticeably tiny quantities of allergenic food proteins in ordinary house dust. Touching this dust appears to provide the main exposure to those allergens.
“According to the dual exposure to allergen hypothesis, an infant’s tolerance to antigens occurs through high-dose exposure by mouth, and allergic sensitization occurs through low-dose exposure through the skin,” he said. “As young as four to six months of age, even before eating solid food, a child develops eczema, has a leaky skin barrier, comes in contact with food, and develops a food allergy.”
IgE-mediated food allergies can begin at any age. “Symptoms occur when a food is ingested and the patient develops symptoms including but not limited to urticaria, angioedema, pruritus, flushing, vomiting, diarrhea, coughing, wheezing, difficulty breathing, presyncope, or syncope,” Pandya noted.
In the case of eosinophilic esophagitis, which may also be part of the atopic march, infants and toddlers often have challenging-to-treat symptoms of reflux, while school-age children have reflux and abdominal pain, and adolescents and adults may experience difficulty swallowing and impactions of food or pills, Brown-Whitehorn said.
To differentiate between food allergy and contact dermatitis, Lee suggested providers ask, “ ’Is the rash hives? If yes, is the rash generalized or in a limited area?’ Then consider the statistical probabilities. Skin problems after milk, egg, wheat, soy, peanut, tree nut, fish, shellfish, or sesame are likely due to IgE-mediated food allergy, but after ketchup or strawberry are probably from skin contact.”
Allergic Rhinitis and Asthma
“For asthma, ask about frequency of night cough and symptoms with exercise, laughing, or crying. For allergic rhinitis, look for runny nose, itchy eyes, or sneezing,” Brown-Whitehorn said.
Testing and Monitoring
Assessing the extent of eczema with the Eczema Area and Severity Index or the SCORing Atopic Dermatitis index takes time but may be necessary to obtain insurance coverage for treatments such as biologics.
Avoid ordering IgE food panels, which can result in false positives that can lead to loss of tolerance and nutritional deficiencies; psychological harm from bullying, anxiety, and decreased quality of life; and higher food and healthcare costs, Pandya said.
Treatments
Caregivers may be wary about treatments, and all the three experts this news organization spoke with stressed the importance of educating caregivers about how treatments work and what to expect from them.
“Early and aggressive atopic dermatitis treatment could prevent sensitization to food or aeroallergens, which could help prevent additional atopic diseases, including those on the atopic march,” Pandya said. “Topical steroids are considered first line at any age. Topical phosphodiesterase inhibitors are approved at 3 months of age and above. Topical calcineurin inhibitors are approved at 2 years of age and above. Wet wrap therapy and bleach baths can be effective. Other options include biologic therapy, allergen immunotherapy, and UV therapy.”
“Epinephrine auto-injectors can counteract food reactions. For allergic rhinitis, non-sedating antihistamines, steroidal nasal sprays, and nasal antihistamines help. Asthma treatments include various inhaled medications,” Brown-Whitehorn added.
When to Refer to Specialists
Involving an allergist, dermatologist, pulmonologist, or ear nose throat specialist to the patient’s care team is advisable in more challenging cases.
If a child is younger than 3 months and has moderate to severe atopic dermatitis, an underlying immune defect may be to blame, so an allergy and immunology assessment is warranted, Brown-Whitehorn said. “An allergist can help any child who has recurrent coughing or wheezing avoid the emergency room or hospitalization.”
“In pediatrics, we always try to find the medication, regimen, and avoidance strategies that use the least treatment to provide the best care for each patient,” Brown-Whitehorn added. “Children eat, play, learn, and sleep, and every stage of the atopic march affects each of these activities. As clinicians, we need to be sure that we are helping children make the best of all these activities.”
Brown-Whitehorn reported financial relationships with DBV Technologies and Regeneron Pharmaceuticals. Lee reported financial relationships with Novartis. Pandya reported financial relationships with DBV Technologies, Thermo Fisher Scientific, and Sanofi.
A version of this article first appeared on Medscape.com.
Down Syndrome: Several Cutaneous Conditions Common, Study Finds
TOPLINE:
(DS) in a 10-year retrospective study.
METHODOLOGY:
- Researchers conducted a multicenter retrospective study of 1529 patients with DS from eight outpatient dermatology clinics in the United States and Canada between 2011 and 2021.
- In total, 50.8% of patients were children (0-12 years), 25.2% were adolescents (13-17 years), and 24% were adults (≥ 18 years).
- The researchers evaluated skin conditions in the patients.
TAKEAWAY:
- Eczematous dermatitis was the most common diagnosis, affecting 26% of patients, followed by folliculitis (19.3%) and seborrheic dermatitis (15.6%). Dermatophyte infections were diagnosed in 13%.
- Alopecia areata was the most common autoimmune skin condition, diagnosed in 178 patients (11.6%); 135 (75.8%) were children. Vitiligo was diagnosed in 66 patients (4.3%).
- The most common cutaneous infections were onychomycosis (5.9%), tinea pedis (5%), and verruca vulgaris/other viral warts (5%).
- High-risk medication use was reported in 4.3% of patients; acne vulgaris, hidradenitis suppurativa, and eczematous dermatitis were the most common associated conditions with such medications.
IN PRACTICE:
“Children, adolescents, and adults with DS are most often found to have eczematous, adnexal, and autoimmune skin conditions at outpatient dermatology visits,” the authors wrote. Their findings, they added, “offer valuable insights for clinicians and researchers, aiding in the improved prioritization of screening, diagnosis, and management, as well as facilitating both basic science and clinical research into prevalent skin conditions in individuals with DS.”
SOURCE:
The study was led by Tasya Rakasiwi, of the Department of Dermatology, Dartmouth Health, Manchester, New Hampshire, and was published online in Pediatric Dermatology.
LIMITATIONS:
Over 50% of the patients were children, potentially resulting in bias toward pediatric diagnoses and younger ages of presentation. Race, ethnicity, and sociodemographic factors were not captured, limiting the generalizability of the findings. Medical codes often do not capture disease phenotype or severity, and the manual conversion of International Classification of Diseases (ICD) 9 to ICD-10 codes may introduce potential conversion errors.
DISCLOSURES:
The study was supported by the Pediatric Dermatology Research Alliance. The authors declared no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
(DS) in a 10-year retrospective study.
METHODOLOGY:
- Researchers conducted a multicenter retrospective study of 1529 patients with DS from eight outpatient dermatology clinics in the United States and Canada between 2011 and 2021.
- In total, 50.8% of patients were children (0-12 years), 25.2% were adolescents (13-17 years), and 24% were adults (≥ 18 years).
- The researchers evaluated skin conditions in the patients.
TAKEAWAY:
- Eczematous dermatitis was the most common diagnosis, affecting 26% of patients, followed by folliculitis (19.3%) and seborrheic dermatitis (15.6%). Dermatophyte infections were diagnosed in 13%.
- Alopecia areata was the most common autoimmune skin condition, diagnosed in 178 patients (11.6%); 135 (75.8%) were children. Vitiligo was diagnosed in 66 patients (4.3%).
- The most common cutaneous infections were onychomycosis (5.9%), tinea pedis (5%), and verruca vulgaris/other viral warts (5%).
- High-risk medication use was reported in 4.3% of patients; acne vulgaris, hidradenitis suppurativa, and eczematous dermatitis were the most common associated conditions with such medications.
IN PRACTICE:
“Children, adolescents, and adults with DS are most often found to have eczematous, adnexal, and autoimmune skin conditions at outpatient dermatology visits,” the authors wrote. Their findings, they added, “offer valuable insights for clinicians and researchers, aiding in the improved prioritization of screening, diagnosis, and management, as well as facilitating both basic science and clinical research into prevalent skin conditions in individuals with DS.”
SOURCE:
The study was led by Tasya Rakasiwi, of the Department of Dermatology, Dartmouth Health, Manchester, New Hampshire, and was published online in Pediatric Dermatology.
LIMITATIONS:
Over 50% of the patients were children, potentially resulting in bias toward pediatric diagnoses and younger ages of presentation. Race, ethnicity, and sociodemographic factors were not captured, limiting the generalizability of the findings. Medical codes often do not capture disease phenotype or severity, and the manual conversion of International Classification of Diseases (ICD) 9 to ICD-10 codes may introduce potential conversion errors.
DISCLOSURES:
The study was supported by the Pediatric Dermatology Research Alliance. The authors declared no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
(DS) in a 10-year retrospective study.
METHODOLOGY:
- Researchers conducted a multicenter retrospective study of 1529 patients with DS from eight outpatient dermatology clinics in the United States and Canada between 2011 and 2021.
- In total, 50.8% of patients were children (0-12 years), 25.2% were adolescents (13-17 years), and 24% were adults (≥ 18 years).
- The researchers evaluated skin conditions in the patients.
TAKEAWAY:
- Eczematous dermatitis was the most common diagnosis, affecting 26% of patients, followed by folliculitis (19.3%) and seborrheic dermatitis (15.6%). Dermatophyte infections were diagnosed in 13%.
- Alopecia areata was the most common autoimmune skin condition, diagnosed in 178 patients (11.6%); 135 (75.8%) were children. Vitiligo was diagnosed in 66 patients (4.3%).
- The most common cutaneous infections were onychomycosis (5.9%), tinea pedis (5%), and verruca vulgaris/other viral warts (5%).
- High-risk medication use was reported in 4.3% of patients; acne vulgaris, hidradenitis suppurativa, and eczematous dermatitis were the most common associated conditions with such medications.
IN PRACTICE:
“Children, adolescents, and adults with DS are most often found to have eczematous, adnexal, and autoimmune skin conditions at outpatient dermatology visits,” the authors wrote. Their findings, they added, “offer valuable insights for clinicians and researchers, aiding in the improved prioritization of screening, diagnosis, and management, as well as facilitating both basic science and clinical research into prevalent skin conditions in individuals with DS.”
SOURCE:
The study was led by Tasya Rakasiwi, of the Department of Dermatology, Dartmouth Health, Manchester, New Hampshire, and was published online in Pediatric Dermatology.
LIMITATIONS:
Over 50% of the patients were children, potentially resulting in bias toward pediatric diagnoses and younger ages of presentation. Race, ethnicity, and sociodemographic factors were not captured, limiting the generalizability of the findings. Medical codes often do not capture disease phenotype or severity, and the manual conversion of International Classification of Diseases (ICD) 9 to ICD-10 codes may introduce potential conversion errors.
DISCLOSURES:
The study was supported by the Pediatric Dermatology Research Alliance. The authors declared no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Considerations for the Use of Biologics in Pregnancy
Biologics have revolutionized dermatologic treatment, offering substantial relief from chronic and debilitating skin conditions such as psoriasis,
Biologics for Cutaneous Conditions
Biologics—tumor necrosis factor (TNF) α inhibitors; IL-17, IL-23, IL-12, and IL-36 inhibitors; and agents such as omalizumab and dupilumab—have shown remarkable efficacy in controlling severe or recalcitrant dermatologic conditions and typically are more effective than traditional systemic therapies.1 For instance, randomized clinical trials (RCTs) and real-world data have shown that patients with psoriasis can achieve considerable skin clearance with biologics, greatly enhancing QOL.2 Adalimumab and secukinumab, which have been approved for use in moderate to severe cases of hidradenitis suppurativa, reduce the frequency of painful nodules and abscesses, thereby decreasing pain and improving QOL. Dupilumab, an IL-4/13 receptor antagonist, has revolutionized the treatment of AD by drastically reducing itch and skin lesions and improving QOL.3 For chronic urticaria, the anti-IgE antibody omalizumab has effectively reduced the incidence of hives and itching, providing pronounced symptom relief when traditional antihistamines fail.4 Use of rituximab, an anti-CD20 monoclonal antibody, has led to remission in severe cases of pemphigus vulgaris and bullous pemphigoid.5
Impact of Untreated Cutaneous Conditions in Pregnancy
When treating patients who are pregnant, dermatologists must consider the health of both the expectant mother and the developing fetus. This dual focus complicates decision-making, particularly with the use of biologics. Untreated cutaneous conditions can profoundly impact a pregnant patient’s health and QOL as well as lead to pregnancy complications affecting the fetus, such as preterm birth or low birth weight. In some studies, moderate to severe psoriasis has been associated with increased risk for complications during pregnancy, including preeclampsia and intrauterine growth restriction.6 Although specific data on hidradenitis suppurativa are lacking, the highly inflammatory nature of the condition suggests similar adverse effects on pregnancy.7 Atopic dermatitis can be exacerbated during pregnancy due to a shift in the immune system to become more allergic dominant.8 Generalized pustular psoriasis manifests with widespread pustules, fever, and systemic inflammation, posing serious risks to both the mother and the fetus if left untreated9; in such a life-threatening scenario, the use of potent treatments such as spesolimab, an IL-36 receptor antagonist, may be warranted. Therefore, managing these conditions effectively is crucial not only for the mother’s health but also for fetal well-being.
Which Biologics Can Dermatologists Safely Prescribe?
Despite the benefits, many dermatologists are hesitant to prescribe biologics to pregnant patients due to the lack of understanding and definitive safety data.10,11 Although there are no RCTs that involve pregnant patients, current evidence suggests that several biologics are not teratogenic and do not cause fetal malformations. Extensive postexposure data support the safety of TNF-α inhibitors during pregnancy.12 Research has shown that children exposed to these agents in utero have normal development, infection rates, and vaccination outcomes comparable to nonexposed children. For example, a systematic review and meta-analysis found no significant increase in the risk for major congenital malformations, spontaneous abortions, or preterm births among patients exposed to anti–TNF-α agents during pregnancy.2 The Organization of Teratology Information Specialists Autoimmune Diseases in Pregnancy Project has provided valuable real-world data indicating that the use of TNF-α inhibitors in pregnancy, particularly during the first trimester, does not substantially elevate the risk for adverse outcomes.13 These findings have been corroborated by several other registry studies and RCTs, providing a robust safety profile for these agents during pregnancy.14
Similarly, postexposure data on IL-17 and IL-12/23 inhibitors indicate a favorable safety profile, though the sample sizes are smaller than those for anti–TNF-α agents.12,14 Studies of drugs such as secukinumab (IL-17 inhibitor), guselkumab (IL-23 inhibitor), or ustekinumab (IL-12/23 inhibitor) have shown no association with teratogenic effects or increased risk for miscarriage.14 However, agents such as spesolimab (IL-36 inhibitor) are relatively new, and ongoing studies are expected to provide more comprehensive safety data.15 Similarly, omalizumab and dupilumab have not been associated with increased risk for fetal malformations or adverse pregnancy outcomes. Omalizumab, indicated for chronic urticaria, has a good safety profile in pregnancy, with no significant increase in adverse outcomes reported in studies and registries.16 Dupilumab, used for AD, has demonstrated safety in pregnancy, with ongoing studies continuing to monitor outcomes.17
Conversely, rituximab (an anti-CD20 antibody for autoimmune bullous diseases) has shown evidence of adverse pregnancy outcomes, including fetal harm.18 Its use generally is discouraged unless deemed absolutely necessary, and no safer alternatives are available. Rituximab can cross the placenta, especially in the second and third trimesters, and has been associated with B-cell depletion in the fetus, leading to potential immunosuppression and increased risk for infections.5
Although the data on the safety of biologics in pregnancy are largely reassuring, it is essential to recognize that potential risks have not been ruled out entirely. There are extensive safety data for anti–TNF-α inhibitors, which provides a level of confidence; although newer agents such as IL-17 and IL-23 inhibitors have shown promising early results, further research is required to solidify their safety profiles during pregnancy.
Dermatologists must balance the risks and benefits of using biologics in pregnant patients. This decision-making process involves careful consideration of the severity of the mother’s condition, the potential risks to the fetus, and the availability of alternative treatments. For many severe dermatologic conditions, the benefits of biologics in controlling disease activity and improving QOL may outweigh the potential risks, especially when other treatments have failed or are not suitable.
Final Thoughts
The increasing use of biologics in dermatology has undoubtedly improved the management of severe skin conditions, substantially enhancing patients’ QOL. As more data become available and clinical guidelines evolve, health care providers will be better equipped to make informed decisions about the use of biologics, particularly in pregnant patients. Collaborative efforts between dermatologists, obstetricians, and researchers will help refine treatment guidelines and ensure that pregnant patients with severe dermatologic conditions receive the best possible care.
For now, although the current evidence supports the safety of many biologics during pregnancy,10,11 individualized care and informed decision-making remain paramount. Careful management and adherence to current guidelines make it possible to navigate the complexities of treating severe dermatologic conditions in pregnant patients, ensuring the best outcomes for both mother and child.
- Sehgal VN, Pandhi D, Khurana A. Biologics in dermatology: an integrated review. Indian J Dermatol. 2014; 59:425-441. doi:10.4103/0019-5154.139859
- Mahadevan U, Wolf DC, Dubinsky M, et al. Placental transfer of anti-tumor necrosis factor agents in pregnant patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11:286-292. doi:10.1016/j.cgh.2012.11.011
- Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335-2348.
- Saini SS, Bindslev-Jensen C, Maurer M, et al. Efficacy and safety of omalizumab in patients with chronic idiopathic/spontaneous urticaria who remain symptomatic on H1 antihistamines: a randomized, placebo-controlled study. J Invest Dermatol. 2015;135:67-75. doi:10.1038/jid.2014.306
- Mariette X, Forger F, Abraham B, et al. Lack of placental transfer of certolizumab pegol during pregnancy: results from CRIB, a prospective, postmarketing, pharmacokinetic study. Ann Rheum Dis. 2018;77:228-233. doi:10.1136/annrheumdis-2017-212196
- Yang Y-W, Chen C-S, Chen Y-H, et al. Psoriasis and pregnancy outcomes: a nationwide population-based study. J Am Acad Dermatol. 2011;64:71-77.
- Zouboulis CC, Del Marmol V, Mrowietz U, et al. Hidradenitis suppurativa/acne inversa: criteria for diagnosis, severity assessment, classification and disease evaluation. Dermatology. 2015;231:184-190.
- Balakirski G, Novak N. Atopic dermatitis and pregnancy. J Allergy Clin Immunol. 2022;149:1185-1194. doi:10.1016/j.jaci.2022.01.010
- Bachelez H, Choon S-E, Marrakchi S, et al. Inhibition of the interleukin-36 pathway for the treatment of generalized pustular psoriasis. N Engl J Med. 2019;380:981-983.
- McMullan P, Yaghi M, Truong TM, et al. Safety of dermatologic medications in pregnancy and lactation: an update—part I: pregnancy. J Am Acad Dermatol. Published online January 25, 2024. doi:10.1016/j.jaad.2023.10.072
- Yaghi M, McMullan P, Truong TM, et al. Safety of dermatologic medications in pregnancy and lactation: an update—part II: lactation. J Am Acad Dermatol. Published online January 25, 2024. doi:10.1016/j.jaad.2023.10.071
- Owczarek W, Walecka I, Lesiak A, et al. The use of biological drugs in psoriasis patients prior to pregnancy, during pregnancy and lactation: a review of current clinical guidelines. Postepy Dermatol Alergol. 2020;37:821-830. doi:10.5114/ada.2020.102089
- Organization of Teratology Information Services (OTIS) Autoimmune Diseases in Pregnancy Project. ClinicalTrials.gov identifier: NCT00116272. Updated October 6, 2023. Accessed August 29, 2024. https://clinicaltrials.gov/study/NCT00116272
- Sanchez-Garcia V, Hernandez-Quiles R, de-Miguel-Balsa E, et al. Exposure to biologic therapy before and during pregnancy in patients with psoriasis: systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2023;37:1971-1990. doi:10.1111/jdv.19238
- Silverberg JI, Boguniewicz M, Hanifin J, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis is efficacious regardless of age of disease onset: a post hoc analysis of two phase 3 clinical trials. Dermatol Ther (Heidelb). 2022;12:2731-2746. doi:10.1007/s13555-022-00822-x
- Levi-Schaffer F, Mankuta D. Omalizumab safety in pregnancy. J Allergy Clin Immunol. 2020;145:481-483. doi:10.1016/j.jaci.2019.11.018
- Thaci D, Simpson EL, Beck LA, et al. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: a randomised, placebo-controlled, dose-ranging phase 2b trial. Lancet. 2016;387:40-52.
- Chakravarty EF, Murray ER, Kelman A, et al. Pregnancy outcomes after maternal exposure to rituximab. Blood. 2011;117:1499-1506. doi:10.1182/blood-2010-07-295444
Biologics have revolutionized dermatologic treatment, offering substantial relief from chronic and debilitating skin conditions such as psoriasis,
Biologics for Cutaneous Conditions
Biologics—tumor necrosis factor (TNF) α inhibitors; IL-17, IL-23, IL-12, and IL-36 inhibitors; and agents such as omalizumab and dupilumab—have shown remarkable efficacy in controlling severe or recalcitrant dermatologic conditions and typically are more effective than traditional systemic therapies.1 For instance, randomized clinical trials (RCTs) and real-world data have shown that patients with psoriasis can achieve considerable skin clearance with biologics, greatly enhancing QOL.2 Adalimumab and secukinumab, which have been approved for use in moderate to severe cases of hidradenitis suppurativa, reduce the frequency of painful nodules and abscesses, thereby decreasing pain and improving QOL. Dupilumab, an IL-4/13 receptor antagonist, has revolutionized the treatment of AD by drastically reducing itch and skin lesions and improving QOL.3 For chronic urticaria, the anti-IgE antibody omalizumab has effectively reduced the incidence of hives and itching, providing pronounced symptom relief when traditional antihistamines fail.4 Use of rituximab, an anti-CD20 monoclonal antibody, has led to remission in severe cases of pemphigus vulgaris and bullous pemphigoid.5
Impact of Untreated Cutaneous Conditions in Pregnancy
When treating patients who are pregnant, dermatologists must consider the health of both the expectant mother and the developing fetus. This dual focus complicates decision-making, particularly with the use of biologics. Untreated cutaneous conditions can profoundly impact a pregnant patient’s health and QOL as well as lead to pregnancy complications affecting the fetus, such as preterm birth or low birth weight. In some studies, moderate to severe psoriasis has been associated with increased risk for complications during pregnancy, including preeclampsia and intrauterine growth restriction.6 Although specific data on hidradenitis suppurativa are lacking, the highly inflammatory nature of the condition suggests similar adverse effects on pregnancy.7 Atopic dermatitis can be exacerbated during pregnancy due to a shift in the immune system to become more allergic dominant.8 Generalized pustular psoriasis manifests with widespread pustules, fever, and systemic inflammation, posing serious risks to both the mother and the fetus if left untreated9; in such a life-threatening scenario, the use of potent treatments such as spesolimab, an IL-36 receptor antagonist, may be warranted. Therefore, managing these conditions effectively is crucial not only for the mother’s health but also for fetal well-being.
Which Biologics Can Dermatologists Safely Prescribe?
Despite the benefits, many dermatologists are hesitant to prescribe biologics to pregnant patients due to the lack of understanding and definitive safety data.10,11 Although there are no RCTs that involve pregnant patients, current evidence suggests that several biologics are not teratogenic and do not cause fetal malformations. Extensive postexposure data support the safety of TNF-α inhibitors during pregnancy.12 Research has shown that children exposed to these agents in utero have normal development, infection rates, and vaccination outcomes comparable to nonexposed children. For example, a systematic review and meta-analysis found no significant increase in the risk for major congenital malformations, spontaneous abortions, or preterm births among patients exposed to anti–TNF-α agents during pregnancy.2 The Organization of Teratology Information Specialists Autoimmune Diseases in Pregnancy Project has provided valuable real-world data indicating that the use of TNF-α inhibitors in pregnancy, particularly during the first trimester, does not substantially elevate the risk for adverse outcomes.13 These findings have been corroborated by several other registry studies and RCTs, providing a robust safety profile for these agents during pregnancy.14
Similarly, postexposure data on IL-17 and IL-12/23 inhibitors indicate a favorable safety profile, though the sample sizes are smaller than those for anti–TNF-α agents.12,14 Studies of drugs such as secukinumab (IL-17 inhibitor), guselkumab (IL-23 inhibitor), or ustekinumab (IL-12/23 inhibitor) have shown no association with teratogenic effects or increased risk for miscarriage.14 However, agents such as spesolimab (IL-36 inhibitor) are relatively new, and ongoing studies are expected to provide more comprehensive safety data.15 Similarly, omalizumab and dupilumab have not been associated with increased risk for fetal malformations or adverse pregnancy outcomes. Omalizumab, indicated for chronic urticaria, has a good safety profile in pregnancy, with no significant increase in adverse outcomes reported in studies and registries.16 Dupilumab, used for AD, has demonstrated safety in pregnancy, with ongoing studies continuing to monitor outcomes.17
Conversely, rituximab (an anti-CD20 antibody for autoimmune bullous diseases) has shown evidence of adverse pregnancy outcomes, including fetal harm.18 Its use generally is discouraged unless deemed absolutely necessary, and no safer alternatives are available. Rituximab can cross the placenta, especially in the second and third trimesters, and has been associated with B-cell depletion in the fetus, leading to potential immunosuppression and increased risk for infections.5
Although the data on the safety of biologics in pregnancy are largely reassuring, it is essential to recognize that potential risks have not been ruled out entirely. There are extensive safety data for anti–TNF-α inhibitors, which provides a level of confidence; although newer agents such as IL-17 and IL-23 inhibitors have shown promising early results, further research is required to solidify their safety profiles during pregnancy.
Dermatologists must balance the risks and benefits of using biologics in pregnant patients. This decision-making process involves careful consideration of the severity of the mother’s condition, the potential risks to the fetus, and the availability of alternative treatments. For many severe dermatologic conditions, the benefits of biologics in controlling disease activity and improving QOL may outweigh the potential risks, especially when other treatments have failed or are not suitable.
Final Thoughts
The increasing use of biologics in dermatology has undoubtedly improved the management of severe skin conditions, substantially enhancing patients’ QOL. As more data become available and clinical guidelines evolve, health care providers will be better equipped to make informed decisions about the use of biologics, particularly in pregnant patients. Collaborative efforts between dermatologists, obstetricians, and researchers will help refine treatment guidelines and ensure that pregnant patients with severe dermatologic conditions receive the best possible care.
For now, although the current evidence supports the safety of many biologics during pregnancy,10,11 individualized care and informed decision-making remain paramount. Careful management and adherence to current guidelines make it possible to navigate the complexities of treating severe dermatologic conditions in pregnant patients, ensuring the best outcomes for both mother and child.
Biologics have revolutionized dermatologic treatment, offering substantial relief from chronic and debilitating skin conditions such as psoriasis,
Biologics for Cutaneous Conditions
Biologics—tumor necrosis factor (TNF) α inhibitors; IL-17, IL-23, IL-12, and IL-36 inhibitors; and agents such as omalizumab and dupilumab—have shown remarkable efficacy in controlling severe or recalcitrant dermatologic conditions and typically are more effective than traditional systemic therapies.1 For instance, randomized clinical trials (RCTs) and real-world data have shown that patients with psoriasis can achieve considerable skin clearance with biologics, greatly enhancing QOL.2 Adalimumab and secukinumab, which have been approved for use in moderate to severe cases of hidradenitis suppurativa, reduce the frequency of painful nodules and abscesses, thereby decreasing pain and improving QOL. Dupilumab, an IL-4/13 receptor antagonist, has revolutionized the treatment of AD by drastically reducing itch and skin lesions and improving QOL.3 For chronic urticaria, the anti-IgE antibody omalizumab has effectively reduced the incidence of hives and itching, providing pronounced symptom relief when traditional antihistamines fail.4 Use of rituximab, an anti-CD20 monoclonal antibody, has led to remission in severe cases of pemphigus vulgaris and bullous pemphigoid.5
Impact of Untreated Cutaneous Conditions in Pregnancy
When treating patients who are pregnant, dermatologists must consider the health of both the expectant mother and the developing fetus. This dual focus complicates decision-making, particularly with the use of biologics. Untreated cutaneous conditions can profoundly impact a pregnant patient’s health and QOL as well as lead to pregnancy complications affecting the fetus, such as preterm birth or low birth weight. In some studies, moderate to severe psoriasis has been associated with increased risk for complications during pregnancy, including preeclampsia and intrauterine growth restriction.6 Although specific data on hidradenitis suppurativa are lacking, the highly inflammatory nature of the condition suggests similar adverse effects on pregnancy.7 Atopic dermatitis can be exacerbated during pregnancy due to a shift in the immune system to become more allergic dominant.8 Generalized pustular psoriasis manifests with widespread pustules, fever, and systemic inflammation, posing serious risks to both the mother and the fetus if left untreated9; in such a life-threatening scenario, the use of potent treatments such as spesolimab, an IL-36 receptor antagonist, may be warranted. Therefore, managing these conditions effectively is crucial not only for the mother’s health but also for fetal well-being.
Which Biologics Can Dermatologists Safely Prescribe?
Despite the benefits, many dermatologists are hesitant to prescribe biologics to pregnant patients due to the lack of understanding and definitive safety data.10,11 Although there are no RCTs that involve pregnant patients, current evidence suggests that several biologics are not teratogenic and do not cause fetal malformations. Extensive postexposure data support the safety of TNF-α inhibitors during pregnancy.12 Research has shown that children exposed to these agents in utero have normal development, infection rates, and vaccination outcomes comparable to nonexposed children. For example, a systematic review and meta-analysis found no significant increase in the risk for major congenital malformations, spontaneous abortions, or preterm births among patients exposed to anti–TNF-α agents during pregnancy.2 The Organization of Teratology Information Specialists Autoimmune Diseases in Pregnancy Project has provided valuable real-world data indicating that the use of TNF-α inhibitors in pregnancy, particularly during the first trimester, does not substantially elevate the risk for adverse outcomes.13 These findings have been corroborated by several other registry studies and RCTs, providing a robust safety profile for these agents during pregnancy.14
Similarly, postexposure data on IL-17 and IL-12/23 inhibitors indicate a favorable safety profile, though the sample sizes are smaller than those for anti–TNF-α agents.12,14 Studies of drugs such as secukinumab (IL-17 inhibitor), guselkumab (IL-23 inhibitor), or ustekinumab (IL-12/23 inhibitor) have shown no association with teratogenic effects or increased risk for miscarriage.14 However, agents such as spesolimab (IL-36 inhibitor) are relatively new, and ongoing studies are expected to provide more comprehensive safety data.15 Similarly, omalizumab and dupilumab have not been associated with increased risk for fetal malformations or adverse pregnancy outcomes. Omalizumab, indicated for chronic urticaria, has a good safety profile in pregnancy, with no significant increase in adverse outcomes reported in studies and registries.16 Dupilumab, used for AD, has demonstrated safety in pregnancy, with ongoing studies continuing to monitor outcomes.17
Conversely, rituximab (an anti-CD20 antibody for autoimmune bullous diseases) has shown evidence of adverse pregnancy outcomes, including fetal harm.18 Its use generally is discouraged unless deemed absolutely necessary, and no safer alternatives are available. Rituximab can cross the placenta, especially in the second and third trimesters, and has been associated with B-cell depletion in the fetus, leading to potential immunosuppression and increased risk for infections.5
Although the data on the safety of biologics in pregnancy are largely reassuring, it is essential to recognize that potential risks have not been ruled out entirely. There are extensive safety data for anti–TNF-α inhibitors, which provides a level of confidence; although newer agents such as IL-17 and IL-23 inhibitors have shown promising early results, further research is required to solidify their safety profiles during pregnancy.
Dermatologists must balance the risks and benefits of using biologics in pregnant patients. This decision-making process involves careful consideration of the severity of the mother’s condition, the potential risks to the fetus, and the availability of alternative treatments. For many severe dermatologic conditions, the benefits of biologics in controlling disease activity and improving QOL may outweigh the potential risks, especially when other treatments have failed or are not suitable.
Final Thoughts
The increasing use of biologics in dermatology has undoubtedly improved the management of severe skin conditions, substantially enhancing patients’ QOL. As more data become available and clinical guidelines evolve, health care providers will be better equipped to make informed decisions about the use of biologics, particularly in pregnant patients. Collaborative efforts between dermatologists, obstetricians, and researchers will help refine treatment guidelines and ensure that pregnant patients with severe dermatologic conditions receive the best possible care.
For now, although the current evidence supports the safety of many biologics during pregnancy,10,11 individualized care and informed decision-making remain paramount. Careful management and adherence to current guidelines make it possible to navigate the complexities of treating severe dermatologic conditions in pregnant patients, ensuring the best outcomes for both mother and child.
- Sehgal VN, Pandhi D, Khurana A. Biologics in dermatology: an integrated review. Indian J Dermatol. 2014; 59:425-441. doi:10.4103/0019-5154.139859
- Mahadevan U, Wolf DC, Dubinsky M, et al. Placental transfer of anti-tumor necrosis factor agents in pregnant patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11:286-292. doi:10.1016/j.cgh.2012.11.011
- Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335-2348.
- Saini SS, Bindslev-Jensen C, Maurer M, et al. Efficacy and safety of omalizumab in patients with chronic idiopathic/spontaneous urticaria who remain symptomatic on H1 antihistamines: a randomized, placebo-controlled study. J Invest Dermatol. 2015;135:67-75. doi:10.1038/jid.2014.306
- Mariette X, Forger F, Abraham B, et al. Lack of placental transfer of certolizumab pegol during pregnancy: results from CRIB, a prospective, postmarketing, pharmacokinetic study. Ann Rheum Dis. 2018;77:228-233. doi:10.1136/annrheumdis-2017-212196
- Yang Y-W, Chen C-S, Chen Y-H, et al. Psoriasis and pregnancy outcomes: a nationwide population-based study. J Am Acad Dermatol. 2011;64:71-77.
- Zouboulis CC, Del Marmol V, Mrowietz U, et al. Hidradenitis suppurativa/acne inversa: criteria for diagnosis, severity assessment, classification and disease evaluation. Dermatology. 2015;231:184-190.
- Balakirski G, Novak N. Atopic dermatitis and pregnancy. J Allergy Clin Immunol. 2022;149:1185-1194. doi:10.1016/j.jaci.2022.01.010
- Bachelez H, Choon S-E, Marrakchi S, et al. Inhibition of the interleukin-36 pathway for the treatment of generalized pustular psoriasis. N Engl J Med. 2019;380:981-983.
- McMullan P, Yaghi M, Truong TM, et al. Safety of dermatologic medications in pregnancy and lactation: an update—part I: pregnancy. J Am Acad Dermatol. Published online January 25, 2024. doi:10.1016/j.jaad.2023.10.072
- Yaghi M, McMullan P, Truong TM, et al. Safety of dermatologic medications in pregnancy and lactation: an update—part II: lactation. J Am Acad Dermatol. Published online January 25, 2024. doi:10.1016/j.jaad.2023.10.071
- Owczarek W, Walecka I, Lesiak A, et al. The use of biological drugs in psoriasis patients prior to pregnancy, during pregnancy and lactation: a review of current clinical guidelines. Postepy Dermatol Alergol. 2020;37:821-830. doi:10.5114/ada.2020.102089
- Organization of Teratology Information Services (OTIS) Autoimmune Diseases in Pregnancy Project. ClinicalTrials.gov identifier: NCT00116272. Updated October 6, 2023. Accessed August 29, 2024. https://clinicaltrials.gov/study/NCT00116272
- Sanchez-Garcia V, Hernandez-Quiles R, de-Miguel-Balsa E, et al. Exposure to biologic therapy before and during pregnancy in patients with psoriasis: systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2023;37:1971-1990. doi:10.1111/jdv.19238
- Silverberg JI, Boguniewicz M, Hanifin J, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis is efficacious regardless of age of disease onset: a post hoc analysis of two phase 3 clinical trials. Dermatol Ther (Heidelb). 2022;12:2731-2746. doi:10.1007/s13555-022-00822-x
- Levi-Schaffer F, Mankuta D. Omalizumab safety in pregnancy. J Allergy Clin Immunol. 2020;145:481-483. doi:10.1016/j.jaci.2019.11.018
- Thaci D, Simpson EL, Beck LA, et al. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: a randomised, placebo-controlled, dose-ranging phase 2b trial. Lancet. 2016;387:40-52.
- Chakravarty EF, Murray ER, Kelman A, et al. Pregnancy outcomes after maternal exposure to rituximab. Blood. 2011;117:1499-1506. doi:10.1182/blood-2010-07-295444
- Sehgal VN, Pandhi D, Khurana A. Biologics in dermatology: an integrated review. Indian J Dermatol. 2014; 59:425-441. doi:10.4103/0019-5154.139859
- Mahadevan U, Wolf DC, Dubinsky M, et al. Placental transfer of anti-tumor necrosis factor agents in pregnant patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11:286-292. doi:10.1016/j.cgh.2012.11.011
- Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335-2348.
- Saini SS, Bindslev-Jensen C, Maurer M, et al. Efficacy and safety of omalizumab in patients with chronic idiopathic/spontaneous urticaria who remain symptomatic on H1 antihistamines: a randomized, placebo-controlled study. J Invest Dermatol. 2015;135:67-75. doi:10.1038/jid.2014.306
- Mariette X, Forger F, Abraham B, et al. Lack of placental transfer of certolizumab pegol during pregnancy: results from CRIB, a prospective, postmarketing, pharmacokinetic study. Ann Rheum Dis. 2018;77:228-233. doi:10.1136/annrheumdis-2017-212196
- Yang Y-W, Chen C-S, Chen Y-H, et al. Psoriasis and pregnancy outcomes: a nationwide population-based study. J Am Acad Dermatol. 2011;64:71-77.
- Zouboulis CC, Del Marmol V, Mrowietz U, et al. Hidradenitis suppurativa/acne inversa: criteria for diagnosis, severity assessment, classification and disease evaluation. Dermatology. 2015;231:184-190.
- Balakirski G, Novak N. Atopic dermatitis and pregnancy. J Allergy Clin Immunol. 2022;149:1185-1194. doi:10.1016/j.jaci.2022.01.010
- Bachelez H, Choon S-E, Marrakchi S, et al. Inhibition of the interleukin-36 pathway for the treatment of generalized pustular psoriasis. N Engl J Med. 2019;380:981-983.
- McMullan P, Yaghi M, Truong TM, et al. Safety of dermatologic medications in pregnancy and lactation: an update—part I: pregnancy. J Am Acad Dermatol. Published online January 25, 2024. doi:10.1016/j.jaad.2023.10.072
- Yaghi M, McMullan P, Truong TM, et al. Safety of dermatologic medications in pregnancy and lactation: an update—part II: lactation. J Am Acad Dermatol. Published online January 25, 2024. doi:10.1016/j.jaad.2023.10.071
- Owczarek W, Walecka I, Lesiak A, et al. The use of biological drugs in psoriasis patients prior to pregnancy, during pregnancy and lactation: a review of current clinical guidelines. Postepy Dermatol Alergol. 2020;37:821-830. doi:10.5114/ada.2020.102089
- Organization of Teratology Information Services (OTIS) Autoimmune Diseases in Pregnancy Project. ClinicalTrials.gov identifier: NCT00116272. Updated October 6, 2023. Accessed August 29, 2024. https://clinicaltrials.gov/study/NCT00116272
- Sanchez-Garcia V, Hernandez-Quiles R, de-Miguel-Balsa E, et al. Exposure to biologic therapy before and during pregnancy in patients with psoriasis: systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2023;37:1971-1990. doi:10.1111/jdv.19238
- Silverberg JI, Boguniewicz M, Hanifin J, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis is efficacious regardless of age of disease onset: a post hoc analysis of two phase 3 clinical trials. Dermatol Ther (Heidelb). 2022;12:2731-2746. doi:10.1007/s13555-022-00822-x
- Levi-Schaffer F, Mankuta D. Omalizumab safety in pregnancy. J Allergy Clin Immunol. 2020;145:481-483. doi:10.1016/j.jaci.2019.11.018
- Thaci D, Simpson EL, Beck LA, et al. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: a randomised, placebo-controlled, dose-ranging phase 2b trial. Lancet. 2016;387:40-52.
- Chakravarty EF, Murray ER, Kelman A, et al. Pregnancy outcomes after maternal exposure to rituximab. Blood. 2011;117:1499-1506. doi:10.1182/blood-2010-07-295444
Aspects of the Skin Microbiome Remain Elusive
SAN DIEGO — Although it has been known for several years that
In one review of the topic, researchers from the National Institutes of Health wrote that the skin is composed of 1.8 million diverse habitats with an abundance of folds, invaginations, and specialized niches that support a wide range of microorganisms. “Many of these microorganisms are harmless and, in some cases, provide vital functions for us to live and they have not evolved over time,” Jill S. Waibel, MD, medical director of the Miami Dermatology and Laser Institute, said at the annual Masters of Aesthetics Symposium.
“This is complex ecosystem that we don’t really talk about,” she said. “There is wide topographical distribution of bacteria on skin sites. The bacteria we have on our head and neck area is different from that on our feet. There is also a lot of interpersonal variation of the skin microbiome, so one person may have a lot of one type of bacteria and not as much of another.”
A Shield From Foreign Pathogens
At its core, Dr. Waibel continued, the skin microbiome functions as an interface between the human body and the environment, a physical barrier that prevents the invasion of foreign pathogens. The skin also provides a home to commensal microbiota. She likened the skin’s landscape to that of the tundra: “It’s desiccated, has poor nutrients, and it’s very acidic, thus pathogens have a hard time living on it,” she said. “However, our skin microorganisms have adapted to utilize the sparse nutrients available on the skin. That’s why I tell my patients, ‘don’t use a sugar scrub because you’re potentially feeding these bad bacteria.’ ”
According to more recent research, the skin microbiota in healthy adults remains stable over time, despite environmental perturbations, and they have important roles in educating the innate and adaptive arms of the cutaneous immune system. “Some skin diseases are associated with an altered microbial state: dysbiosis,” said Dr. Waibel, subsection chief of dermatology at Baptist Health South Florida, Miami Beach. “Reversion of this may help prevent or treat the disease.”
She cited the following factors that influence the skin microbiome:
- Genetics affects the skin microbiome considerably. Individuals with autoimmune predispositions have different microbiota compared with those who don’t.
- Climate, pollution, and hygiene practices the other influencing factors. “Even clothing can impact the microbiome, by causing the transfer of microorganisms,” she said.
- Age and hormonal changes (particularly during puberty) and senescence alter the microbial landscape.
- Systemic health conditions such as diabetes mellitus and irritable bowel disease, as well as cutaneous conditions like psoriasis and atopic dermatitis can also disrupt the skin microbiome.
Ingredients contained in soaps, antibiotics, and cosmetics can also cause skin dysbiosis, Dr. Waibel said. However, the integrity of the skin’s microbiome following dermatological procedures such as excisions, dermabrasion, laser therapy, and other physical procedures is less understood, according to a recent review of the topic. Phototherapy appears to be the most extensively studied, “and shows an increase in microbial diversity post-treatment,” she said. “Light treatments have been found to kill bacteria by inducing DNA damage. More studies need to be performed on specific wavelengths of light used, conditions being treated and individual patient differences.”
According to the review’s authors, no change in the microbiome was observed in studies of debridement. “That was surprising, as it is a method to remove unhealthy tissue that often contains pathogenic bacteria,” Dr. Waibel said. “The big take-home message is that we need more research.”
Dr. Waibel disclosed that she has conducted clinical trials for several device and pharmaceutical companies.
A version of this article first appeared on Medscape.com.
SAN DIEGO — Although it has been known for several years that
In one review of the topic, researchers from the National Institutes of Health wrote that the skin is composed of 1.8 million diverse habitats with an abundance of folds, invaginations, and specialized niches that support a wide range of microorganisms. “Many of these microorganisms are harmless and, in some cases, provide vital functions for us to live and they have not evolved over time,” Jill S. Waibel, MD, medical director of the Miami Dermatology and Laser Institute, said at the annual Masters of Aesthetics Symposium.
“This is complex ecosystem that we don’t really talk about,” she said. “There is wide topographical distribution of bacteria on skin sites. The bacteria we have on our head and neck area is different from that on our feet. There is also a lot of interpersonal variation of the skin microbiome, so one person may have a lot of one type of bacteria and not as much of another.”
A Shield From Foreign Pathogens
At its core, Dr. Waibel continued, the skin microbiome functions as an interface between the human body and the environment, a physical barrier that prevents the invasion of foreign pathogens. The skin also provides a home to commensal microbiota. She likened the skin’s landscape to that of the tundra: “It’s desiccated, has poor nutrients, and it’s very acidic, thus pathogens have a hard time living on it,” she said. “However, our skin microorganisms have adapted to utilize the sparse nutrients available on the skin. That’s why I tell my patients, ‘don’t use a sugar scrub because you’re potentially feeding these bad bacteria.’ ”
According to more recent research, the skin microbiota in healthy adults remains stable over time, despite environmental perturbations, and they have important roles in educating the innate and adaptive arms of the cutaneous immune system. “Some skin diseases are associated with an altered microbial state: dysbiosis,” said Dr. Waibel, subsection chief of dermatology at Baptist Health South Florida, Miami Beach. “Reversion of this may help prevent or treat the disease.”
She cited the following factors that influence the skin microbiome:
- Genetics affects the skin microbiome considerably. Individuals with autoimmune predispositions have different microbiota compared with those who don’t.
- Climate, pollution, and hygiene practices the other influencing factors. “Even clothing can impact the microbiome, by causing the transfer of microorganisms,” she said.
- Age and hormonal changes (particularly during puberty) and senescence alter the microbial landscape.
- Systemic health conditions such as diabetes mellitus and irritable bowel disease, as well as cutaneous conditions like psoriasis and atopic dermatitis can also disrupt the skin microbiome.
Ingredients contained in soaps, antibiotics, and cosmetics can also cause skin dysbiosis, Dr. Waibel said. However, the integrity of the skin’s microbiome following dermatological procedures such as excisions, dermabrasion, laser therapy, and other physical procedures is less understood, according to a recent review of the topic. Phototherapy appears to be the most extensively studied, “and shows an increase in microbial diversity post-treatment,” she said. “Light treatments have been found to kill bacteria by inducing DNA damage. More studies need to be performed on specific wavelengths of light used, conditions being treated and individual patient differences.”
According to the review’s authors, no change in the microbiome was observed in studies of debridement. “That was surprising, as it is a method to remove unhealthy tissue that often contains pathogenic bacteria,” Dr. Waibel said. “The big take-home message is that we need more research.”
Dr. Waibel disclosed that she has conducted clinical trials for several device and pharmaceutical companies.
A version of this article first appeared on Medscape.com.
SAN DIEGO — Although it has been known for several years that
In one review of the topic, researchers from the National Institutes of Health wrote that the skin is composed of 1.8 million diverse habitats with an abundance of folds, invaginations, and specialized niches that support a wide range of microorganisms. “Many of these microorganisms are harmless and, in some cases, provide vital functions for us to live and they have not evolved over time,” Jill S. Waibel, MD, medical director of the Miami Dermatology and Laser Institute, said at the annual Masters of Aesthetics Symposium.
“This is complex ecosystem that we don’t really talk about,” she said. “There is wide topographical distribution of bacteria on skin sites. The bacteria we have on our head and neck area is different from that on our feet. There is also a lot of interpersonal variation of the skin microbiome, so one person may have a lot of one type of bacteria and not as much of another.”
A Shield From Foreign Pathogens
At its core, Dr. Waibel continued, the skin microbiome functions as an interface between the human body and the environment, a physical barrier that prevents the invasion of foreign pathogens. The skin also provides a home to commensal microbiota. She likened the skin’s landscape to that of the tundra: “It’s desiccated, has poor nutrients, and it’s very acidic, thus pathogens have a hard time living on it,” she said. “However, our skin microorganisms have adapted to utilize the sparse nutrients available on the skin. That’s why I tell my patients, ‘don’t use a sugar scrub because you’re potentially feeding these bad bacteria.’ ”
According to more recent research, the skin microbiota in healthy adults remains stable over time, despite environmental perturbations, and they have important roles in educating the innate and adaptive arms of the cutaneous immune system. “Some skin diseases are associated with an altered microbial state: dysbiosis,” said Dr. Waibel, subsection chief of dermatology at Baptist Health South Florida, Miami Beach. “Reversion of this may help prevent or treat the disease.”
She cited the following factors that influence the skin microbiome:
- Genetics affects the skin microbiome considerably. Individuals with autoimmune predispositions have different microbiota compared with those who don’t.
- Climate, pollution, and hygiene practices the other influencing factors. “Even clothing can impact the microbiome, by causing the transfer of microorganisms,” she said.
- Age and hormonal changes (particularly during puberty) and senescence alter the microbial landscape.
- Systemic health conditions such as diabetes mellitus and irritable bowel disease, as well as cutaneous conditions like psoriasis and atopic dermatitis can also disrupt the skin microbiome.
Ingredients contained in soaps, antibiotics, and cosmetics can also cause skin dysbiosis, Dr. Waibel said. However, the integrity of the skin’s microbiome following dermatological procedures such as excisions, dermabrasion, laser therapy, and other physical procedures is less understood, according to a recent review of the topic. Phototherapy appears to be the most extensively studied, “and shows an increase in microbial diversity post-treatment,” she said. “Light treatments have been found to kill bacteria by inducing DNA damage. More studies need to be performed on specific wavelengths of light used, conditions being treated and individual patient differences.”
According to the review’s authors, no change in the microbiome was observed in studies of debridement. “That was surprising, as it is a method to remove unhealthy tissue that often contains pathogenic bacteria,” Dr. Waibel said. “The big take-home message is that we need more research.”
Dr. Waibel disclosed that she has conducted clinical trials for several device and pharmaceutical companies.
A version of this article first appeared on Medscape.com.
FROM THE 2024 MASTERS OF AESTHETICS SYMPOSIUM
Childhood-Onset Atopic Dermatitis Adds Burden in Adulthood
AMSTERDAM — There is a mountain of evidence that atopic dermatitis (AD) exerts a large negative impact on quality of life, but a unique study with
These data, drawn from the ambitious Scars of Life (SOL) project, “suggest that childhood AD persisting into adulthood is its own phenotype,” reported Jonathan I. Silverberg, MD, PhD, director of clinical research, Department of Dermatology, George Washington University, Washington, DC.
One reasonable message from these data is that the failure to achieve adequate control of AD in children, whether by a late start of systemic agents or other reasons, results in a greater lifetime burden of disease when the burden beyond physical symptoms is measured, according to Dr. Silverberg.
More Than 30,000 From Five Continents Participated
In the SOL project, which was designed to analyze how the age of AD onset affects the severity of symptoms and quality of life, completed questionnaires were collected from 30,801 individuals in 27 countries on five continents. The questions, which elicited data to measure the burden of AD, were developed in association with several professional and patient associations with an interest in AD, including the National Eczema Association.
The SOL project has produced an enormous amount of data in four distinct groups, but Dr. Silverberg, speaking in a late-breaking news session at the annual congress of the European Academy of Dermatology and Venereology, focused on a comparison between the 2875 participants who had AD in childhood that has persisted into adulthood and the 7383 adults with adult-onset AD. Data from the other two subsets in SOL — AD in childhood but not in adulthood and no AD in either phase of life — are expected to fuel an extended series of publications.
In the two groups, baseline characteristics were similar with about 60% reporting moderate to severe symptoms and a median age of about 37 years. The proportion of women was 61% in both groups.
Using the PUSH-D questionnaire, which Dr. Silverberg described as a validated tool for gauging a sense of stigmatization, the greater burden of AD was remarkably consistent for those with childhood-onset AD vs adult-onset AD. With higher scores representing a greater sense of stigmatization, the differences in the overall score (23.0 vs 18.1; P < .0001) were highly significant as was every other domain evaluated.
For all five social behavior domains, such as avoiding contact in public and wariness of approaching people spontaneously, having AD onset in childhood persisting into adulthood produced significantly higher scores than having AD onset in adulthood, with no exceptions (P < .001 for all).
AD From Childhood Consistently Results in Worse Outcomes
Providing examples for some of the other 12 domains, Dr. Silverberg maintained that feelings of shame and psychological discomfort were always greater in adults with AD persistent since childhood vs AD starting in adulthood. The P values for these outcomes, such as experiencing bias at work or reporting a sense that others avoided them, were typically highly significant (P < .001).
Compared with those whose AD started in adulthood, “adults with atopic eczema that started during childhood have significantly more difficulties in their life, including occupational relationships, daily life, personal life, and partner or family relationships,” Dr. Silverberg reported.
He said that the data were controlled for multiple confounders, particularly greater severity of AD. He acknowledged that childhood onset might be considered a surrogate for more severe disease, but the data were controlled for this possibility.
Despite the fact that there are “thousands of studies across all age groups showing the burden of AD,” Dr. Silverberg considers these data to be unique by emphasizing the burden of chronicity rather than the impact of AD in any single moment in time.
For those with chronic AD from childhood, “the effect is not just on physical health but a deep negative influence on psychological and social aspects of life,” Dr. Silverberg said, suggesting that the independent effects of chronicity might be worth studying across other dermatologic diseases.
“Regulatory agencies focus on what you can do in that moment of time, losing the bigger picture of how patients are affected chronically,” he said, adding that this is an area of clinical research that should be further explored.
What the data further suggest “is that the earlier we intervene, the more likely patients will do better long term,” he said.
Data Provide Evidence of Systemic Therapy in Kids
For Gudrun Ratzinger, MD, of the Department of Dermatology and Venerology at the Medical University of Innsbruck in Austria, these are valuable data.
“When I prescribe systemic therapies to children, I often get resistance from the healthcare system and even other colleagues,” said Dr. Ratzinger, who was asked to comment on the results. “We are at a teaching hospital, but I often find that when patients return to their home physician, the systemic therapies are stopped.”
In her own practice, she believes the most effective therapies should be introduced in children and adults when complete control is not achieved on first-line drugs. “These data are very helpful for me in explaining to others the importance of effective treatment of atopic dermatitis in children,” she said.
Dr. Silverberg reported financial relationships with more than 40 pharmaceutical companies, including those that make drugs for AD. Dr. Ratzinger reported financial relationships with AbbVie, Almirall, Boehringer Ingelheim, Eli Lilly, Janssen, Leo Pharma, Novartis, Pelpharma, Pfizer, and UCB.
A version of this article first appeared on Medscape.com.
AMSTERDAM — There is a mountain of evidence that atopic dermatitis (AD) exerts a large negative impact on quality of life, but a unique study with
These data, drawn from the ambitious Scars of Life (SOL) project, “suggest that childhood AD persisting into adulthood is its own phenotype,” reported Jonathan I. Silverberg, MD, PhD, director of clinical research, Department of Dermatology, George Washington University, Washington, DC.
One reasonable message from these data is that the failure to achieve adequate control of AD in children, whether by a late start of systemic agents or other reasons, results in a greater lifetime burden of disease when the burden beyond physical symptoms is measured, according to Dr. Silverberg.
More Than 30,000 From Five Continents Participated
In the SOL project, which was designed to analyze how the age of AD onset affects the severity of symptoms and quality of life, completed questionnaires were collected from 30,801 individuals in 27 countries on five continents. The questions, which elicited data to measure the burden of AD, were developed in association with several professional and patient associations with an interest in AD, including the National Eczema Association.
The SOL project has produced an enormous amount of data in four distinct groups, but Dr. Silverberg, speaking in a late-breaking news session at the annual congress of the European Academy of Dermatology and Venereology, focused on a comparison between the 2875 participants who had AD in childhood that has persisted into adulthood and the 7383 adults with adult-onset AD. Data from the other two subsets in SOL — AD in childhood but not in adulthood and no AD in either phase of life — are expected to fuel an extended series of publications.
In the two groups, baseline characteristics were similar with about 60% reporting moderate to severe symptoms and a median age of about 37 years. The proportion of women was 61% in both groups.
Using the PUSH-D questionnaire, which Dr. Silverberg described as a validated tool for gauging a sense of stigmatization, the greater burden of AD was remarkably consistent for those with childhood-onset AD vs adult-onset AD. With higher scores representing a greater sense of stigmatization, the differences in the overall score (23.0 vs 18.1; P < .0001) were highly significant as was every other domain evaluated.
For all five social behavior domains, such as avoiding contact in public and wariness of approaching people spontaneously, having AD onset in childhood persisting into adulthood produced significantly higher scores than having AD onset in adulthood, with no exceptions (P < .001 for all).
AD From Childhood Consistently Results in Worse Outcomes
Providing examples for some of the other 12 domains, Dr. Silverberg maintained that feelings of shame and psychological discomfort were always greater in adults with AD persistent since childhood vs AD starting in adulthood. The P values for these outcomes, such as experiencing bias at work or reporting a sense that others avoided them, were typically highly significant (P < .001).
Compared with those whose AD started in adulthood, “adults with atopic eczema that started during childhood have significantly more difficulties in their life, including occupational relationships, daily life, personal life, and partner or family relationships,” Dr. Silverberg reported.
He said that the data were controlled for multiple confounders, particularly greater severity of AD. He acknowledged that childhood onset might be considered a surrogate for more severe disease, but the data were controlled for this possibility.
Despite the fact that there are “thousands of studies across all age groups showing the burden of AD,” Dr. Silverberg considers these data to be unique by emphasizing the burden of chronicity rather than the impact of AD in any single moment in time.
For those with chronic AD from childhood, “the effect is not just on physical health but a deep negative influence on psychological and social aspects of life,” Dr. Silverberg said, suggesting that the independent effects of chronicity might be worth studying across other dermatologic diseases.
“Regulatory agencies focus on what you can do in that moment of time, losing the bigger picture of how patients are affected chronically,” he said, adding that this is an area of clinical research that should be further explored.
What the data further suggest “is that the earlier we intervene, the more likely patients will do better long term,” he said.
Data Provide Evidence of Systemic Therapy in Kids
For Gudrun Ratzinger, MD, of the Department of Dermatology and Venerology at the Medical University of Innsbruck in Austria, these are valuable data.
“When I prescribe systemic therapies to children, I often get resistance from the healthcare system and even other colleagues,” said Dr. Ratzinger, who was asked to comment on the results. “We are at a teaching hospital, but I often find that when patients return to their home physician, the systemic therapies are stopped.”
In her own practice, she believes the most effective therapies should be introduced in children and adults when complete control is not achieved on first-line drugs. “These data are very helpful for me in explaining to others the importance of effective treatment of atopic dermatitis in children,” she said.
Dr. Silverberg reported financial relationships with more than 40 pharmaceutical companies, including those that make drugs for AD. Dr. Ratzinger reported financial relationships with AbbVie, Almirall, Boehringer Ingelheim, Eli Lilly, Janssen, Leo Pharma, Novartis, Pelpharma, Pfizer, and UCB.
A version of this article first appeared on Medscape.com.
AMSTERDAM — There is a mountain of evidence that atopic dermatitis (AD) exerts a large negative impact on quality of life, but a unique study with
These data, drawn from the ambitious Scars of Life (SOL) project, “suggest that childhood AD persisting into adulthood is its own phenotype,” reported Jonathan I. Silverberg, MD, PhD, director of clinical research, Department of Dermatology, George Washington University, Washington, DC.
One reasonable message from these data is that the failure to achieve adequate control of AD in children, whether by a late start of systemic agents or other reasons, results in a greater lifetime burden of disease when the burden beyond physical symptoms is measured, according to Dr. Silverberg.
More Than 30,000 From Five Continents Participated
In the SOL project, which was designed to analyze how the age of AD onset affects the severity of symptoms and quality of life, completed questionnaires were collected from 30,801 individuals in 27 countries on five continents. The questions, which elicited data to measure the burden of AD, were developed in association with several professional and patient associations with an interest in AD, including the National Eczema Association.
The SOL project has produced an enormous amount of data in four distinct groups, but Dr. Silverberg, speaking in a late-breaking news session at the annual congress of the European Academy of Dermatology and Venereology, focused on a comparison between the 2875 participants who had AD in childhood that has persisted into adulthood and the 7383 adults with adult-onset AD. Data from the other two subsets in SOL — AD in childhood but not in adulthood and no AD in either phase of life — are expected to fuel an extended series of publications.
In the two groups, baseline characteristics were similar with about 60% reporting moderate to severe symptoms and a median age of about 37 years. The proportion of women was 61% in both groups.
Using the PUSH-D questionnaire, which Dr. Silverberg described as a validated tool for gauging a sense of stigmatization, the greater burden of AD was remarkably consistent for those with childhood-onset AD vs adult-onset AD. With higher scores representing a greater sense of stigmatization, the differences in the overall score (23.0 vs 18.1; P < .0001) were highly significant as was every other domain evaluated.
For all five social behavior domains, such as avoiding contact in public and wariness of approaching people spontaneously, having AD onset in childhood persisting into adulthood produced significantly higher scores than having AD onset in adulthood, with no exceptions (P < .001 for all).
AD From Childhood Consistently Results in Worse Outcomes
Providing examples for some of the other 12 domains, Dr. Silverberg maintained that feelings of shame and psychological discomfort were always greater in adults with AD persistent since childhood vs AD starting in adulthood. The P values for these outcomes, such as experiencing bias at work or reporting a sense that others avoided them, were typically highly significant (P < .001).
Compared with those whose AD started in adulthood, “adults with atopic eczema that started during childhood have significantly more difficulties in their life, including occupational relationships, daily life, personal life, and partner or family relationships,” Dr. Silverberg reported.
He said that the data were controlled for multiple confounders, particularly greater severity of AD. He acknowledged that childhood onset might be considered a surrogate for more severe disease, but the data were controlled for this possibility.
Despite the fact that there are “thousands of studies across all age groups showing the burden of AD,” Dr. Silverberg considers these data to be unique by emphasizing the burden of chronicity rather than the impact of AD in any single moment in time.
For those with chronic AD from childhood, “the effect is not just on physical health but a deep negative influence on psychological and social aspects of life,” Dr. Silverberg said, suggesting that the independent effects of chronicity might be worth studying across other dermatologic diseases.
“Regulatory agencies focus on what you can do in that moment of time, losing the bigger picture of how patients are affected chronically,” he said, adding that this is an area of clinical research that should be further explored.
What the data further suggest “is that the earlier we intervene, the more likely patients will do better long term,” he said.
Data Provide Evidence of Systemic Therapy in Kids
For Gudrun Ratzinger, MD, of the Department of Dermatology and Venerology at the Medical University of Innsbruck in Austria, these are valuable data.
“When I prescribe systemic therapies to children, I often get resistance from the healthcare system and even other colleagues,” said Dr. Ratzinger, who was asked to comment on the results. “We are at a teaching hospital, but I often find that when patients return to their home physician, the systemic therapies are stopped.”
In her own practice, she believes the most effective therapies should be introduced in children and adults when complete control is not achieved on first-line drugs. “These data are very helpful for me in explaining to others the importance of effective treatment of atopic dermatitis in children,” she said.
Dr. Silverberg reported financial relationships with more than 40 pharmaceutical companies, including those that make drugs for AD. Dr. Ratzinger reported financial relationships with AbbVie, Almirall, Boehringer Ingelheim, Eli Lilly, Janssen, Leo Pharma, Novartis, Pelpharma, Pfizer, and UCB.
A version of this article first appeared on Medscape.com.
FROM EADV 2024
Phase3 Data: Atopic Dermatitis Symptoms Improved with Topical Roflumilast
TOPLINE:
(AD) in two phase 3 trials.
METHODOLOGY:
- Two randomized, parallel-group, double-blind, vehicle-controlled phase 3 trials, INTEGUMENT-1 (n = 654) and INTEGUMENT-2 (n = 683), enrolled patients aged ≥ 6 years with mild to moderate AD who were randomly assigned in a 2:1 ratio to roflumilast cream 0.15%, a phosphodiesterase 4 inhibitor, or vehicle cream once daily for 4 weeks.
- The primary efficacy endpoint was the Validated Investigator Global Assessment for AD (vIGA-AD) success at week 4, defined as a score of 0 (clear) or 1 (almost clear) plus improvement of at least two grades from baseline.
- Secondary endpoints included vIGA-AD success at week 4 in patients with a baseline score of 3, at least a four-point reduction in the Worst Itch Numeric Rating Scale (WI-NRS), and at least a 75% reduction in the Eczema Area and Severity Index (EASI-75) at weeks 1, 2, and 4.
TAKEAWAY:
- Significantly more patients receiving roflumilast achieved vIGA-AD success at week 4 vs those in the vehicle group in INTEGUMENT-1 (32.0% vs 15.2%; P < .001) and INTEGUMENT-2 (28.9% vs 12.0%; P < .001), which was consistent across all age groups and in those with a baseline score of 3.
- Similarly, a greater proportion of patients treated with roflumilast vs vehicle achieved at least a four-point reduction in WI-NRS at weeks 1, 2, and 4, with improvements noted as early as 24 hours after the first application (P < .05 at all subsequent timepoints).
- The number of patients achieving EASI-75 and vIGA-AD scores of 0 or 1 was significantly higher with roflumilast than with vehicle at weeks 1, 2, and 4.
- Most treatment-emergent adverse events (TEAEs) were mild to moderate, with only 0.9% of the patients experiencing serious TEAEs in each trial. More than 95% of the patients showed no signs of irritation, and over 90% reported no or mild sensation at the application site.
IN PRACTICE:
The two phase 3 randomized clinical trials of patients with AD treated with roflumilast cream 0.15% “demonstrated improvement across multiple efficacy endpoints, including reducing pruritus within 24 hours after application, with favorable safety and tolerability,” the authors wrote. “Additional research, including subgroup analyses, will provide more data regarding the efficacy and safety of roflumilast cream 0.15%, in patients with AD,” they added.
SOURCE:
The study was led by Eric L. Simpson, MD, of the Department of Dermatology, Oregon Health & Science University, Portland, Oregon, and was published online on September 18 in JAMA Dermatology.
LIMITATIONS:
A short duration, a minimum age limit of 6 years, and the lack of an active comparator may influence the interpretation and generalizability of the results.
DISCLOSURES:
The study was sponsored by Arcutis Biotherapeutics. Simpson received grants and personal fees from Arcutis during this study. Three authors reported being employees and/or stockholders of Arcutis, two other authors reported patents for Arcutis, and several authors declared having various ties with various sources, including Arcutis.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
(AD) in two phase 3 trials.
METHODOLOGY:
- Two randomized, parallel-group, double-blind, vehicle-controlled phase 3 trials, INTEGUMENT-1 (n = 654) and INTEGUMENT-2 (n = 683), enrolled patients aged ≥ 6 years with mild to moderate AD who were randomly assigned in a 2:1 ratio to roflumilast cream 0.15%, a phosphodiesterase 4 inhibitor, or vehicle cream once daily for 4 weeks.
- The primary efficacy endpoint was the Validated Investigator Global Assessment for AD (vIGA-AD) success at week 4, defined as a score of 0 (clear) or 1 (almost clear) plus improvement of at least two grades from baseline.
- Secondary endpoints included vIGA-AD success at week 4 in patients with a baseline score of 3, at least a four-point reduction in the Worst Itch Numeric Rating Scale (WI-NRS), and at least a 75% reduction in the Eczema Area and Severity Index (EASI-75) at weeks 1, 2, and 4.
TAKEAWAY:
- Significantly more patients receiving roflumilast achieved vIGA-AD success at week 4 vs those in the vehicle group in INTEGUMENT-1 (32.0% vs 15.2%; P < .001) and INTEGUMENT-2 (28.9% vs 12.0%; P < .001), which was consistent across all age groups and in those with a baseline score of 3.
- Similarly, a greater proportion of patients treated with roflumilast vs vehicle achieved at least a four-point reduction in WI-NRS at weeks 1, 2, and 4, with improvements noted as early as 24 hours after the first application (P < .05 at all subsequent timepoints).
- The number of patients achieving EASI-75 and vIGA-AD scores of 0 or 1 was significantly higher with roflumilast than with vehicle at weeks 1, 2, and 4.
- Most treatment-emergent adverse events (TEAEs) were mild to moderate, with only 0.9% of the patients experiencing serious TEAEs in each trial. More than 95% of the patients showed no signs of irritation, and over 90% reported no or mild sensation at the application site.
IN PRACTICE:
The two phase 3 randomized clinical trials of patients with AD treated with roflumilast cream 0.15% “demonstrated improvement across multiple efficacy endpoints, including reducing pruritus within 24 hours after application, with favorable safety and tolerability,” the authors wrote. “Additional research, including subgroup analyses, will provide more data regarding the efficacy and safety of roflumilast cream 0.15%, in patients with AD,” they added.
SOURCE:
The study was led by Eric L. Simpson, MD, of the Department of Dermatology, Oregon Health & Science University, Portland, Oregon, and was published online on September 18 in JAMA Dermatology.
LIMITATIONS:
A short duration, a minimum age limit of 6 years, and the lack of an active comparator may influence the interpretation and generalizability of the results.
DISCLOSURES:
The study was sponsored by Arcutis Biotherapeutics. Simpson received grants and personal fees from Arcutis during this study. Three authors reported being employees and/or stockholders of Arcutis, two other authors reported patents for Arcutis, and several authors declared having various ties with various sources, including Arcutis.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
(AD) in two phase 3 trials.
METHODOLOGY:
- Two randomized, parallel-group, double-blind, vehicle-controlled phase 3 trials, INTEGUMENT-1 (n = 654) and INTEGUMENT-2 (n = 683), enrolled patients aged ≥ 6 years with mild to moderate AD who were randomly assigned in a 2:1 ratio to roflumilast cream 0.15%, a phosphodiesterase 4 inhibitor, or vehicle cream once daily for 4 weeks.
- The primary efficacy endpoint was the Validated Investigator Global Assessment for AD (vIGA-AD) success at week 4, defined as a score of 0 (clear) or 1 (almost clear) plus improvement of at least two grades from baseline.
- Secondary endpoints included vIGA-AD success at week 4 in patients with a baseline score of 3, at least a four-point reduction in the Worst Itch Numeric Rating Scale (WI-NRS), and at least a 75% reduction in the Eczema Area and Severity Index (EASI-75) at weeks 1, 2, and 4.
TAKEAWAY:
- Significantly more patients receiving roflumilast achieved vIGA-AD success at week 4 vs those in the vehicle group in INTEGUMENT-1 (32.0% vs 15.2%; P < .001) and INTEGUMENT-2 (28.9% vs 12.0%; P < .001), which was consistent across all age groups and in those with a baseline score of 3.
- Similarly, a greater proportion of patients treated with roflumilast vs vehicle achieved at least a four-point reduction in WI-NRS at weeks 1, 2, and 4, with improvements noted as early as 24 hours after the first application (P < .05 at all subsequent timepoints).
- The number of patients achieving EASI-75 and vIGA-AD scores of 0 or 1 was significantly higher with roflumilast than with vehicle at weeks 1, 2, and 4.
- Most treatment-emergent adverse events (TEAEs) were mild to moderate, with only 0.9% of the patients experiencing serious TEAEs in each trial. More than 95% of the patients showed no signs of irritation, and over 90% reported no or mild sensation at the application site.
IN PRACTICE:
The two phase 3 randomized clinical trials of patients with AD treated with roflumilast cream 0.15% “demonstrated improvement across multiple efficacy endpoints, including reducing pruritus within 24 hours after application, with favorable safety and tolerability,” the authors wrote. “Additional research, including subgroup analyses, will provide more data regarding the efficacy and safety of roflumilast cream 0.15%, in patients with AD,” they added.
SOURCE:
The study was led by Eric L. Simpson, MD, of the Department of Dermatology, Oregon Health & Science University, Portland, Oregon, and was published online on September 18 in JAMA Dermatology.
LIMITATIONS:
A short duration, a minimum age limit of 6 years, and the lack of an active comparator may influence the interpretation and generalizability of the results.
DISCLOSURES:
The study was sponsored by Arcutis Biotherapeutics. Simpson received grants and personal fees from Arcutis during this study. Three authors reported being employees and/or stockholders of Arcutis, two other authors reported patents for Arcutis, and several authors declared having various ties with various sources, including Arcutis.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.