User login
COVID-19 vaccination does not increase risk of flare in patients with lupus
COVID-19 vaccinations appear to be well tolerated in patients with systemic lupus erythematosus (SLE) and come with a low risk of flare, according to the results of a global, web-based survey.
“Disseminating these reassuring data might prove crucial to increasing vaccine coverage in patients with SLE,” wrote lead author Renaud Felten, MD, of Strasbourg (France) University Hospital. Their results were published as a comment in Lancet Rheumatology.
To assess vaccine tolerability among lupus patients, the cross-sectional Tolerance and Consequences of Vaccination Against COVID-19 in Lupus Patients (VACOLUP) study analyzed a 43-question survey of 696 participants with a self-reported, medically confirmed diagnosis of SLE from 30 countries between March 22, 2021, and May 17, 2021. The cohort was 96% women, and their median age was 42 (interquartile range, 34-51). Nearly 36% of respondents were from Italy, 27% were from Chile, 13% were from France, and just under 9% were Americans. All participants received at least one dose of COVID-19 vaccine, and 49% received a second dose. The most common vaccines were Pfizer-BioNTech (57%), Sinovac (22%), AstraZeneca (10%), and Moderna (8%).
Only 21 participants (3%) reported a medically confirmed SLE flare after a median of 3 days (IQR, 0-29) post COVID vaccination, with most experiencing musculoskeletal symptoms (90%) and fatigue (86%). Of the 21 cases, 15 reported a subsequent change in SLE treatment and 4 were admitted to the hospital. A previous flare that occurred within a year before vaccination was associated with an increased risk of flare post vaccination (relative risk, 5.52; 95% confidence interval, 2.17-14.03; P < .0001).
Side effects – including swelling, soreness, fever, chills, fatigue, joint and muscle pain, nausea, and headache – were reported in 45% of participants (n = 316) after their first dose and in 53% of the 343 participants who received a second dose. There was no notable difference in the likelihood of side effects across gender and age or in patients who received mRNA vaccines, compared with vaccines with other modes of action. Patients who reported side effects after the first dose were more likely to also report them after the second, compared with those who reported none (109 [81%] of 135 vs. 72 [35%] of 205; RR, 2.30; 95% CI, 1.88-2.82; P < .0001).
In the majority of cases (2,232 of 2,683), the side effects were of minor or moderate intensity and did not affect the participants’ ability to perform daily tasks. The study found no significant association between side effects and a SLE flare and SLE medications or previous SLE disease manifestations.
When asked to comment on the study, Amit Saxena, MD, of the Lupus Center at New York University Langone Health, said: “What we are seeing is pretty mild to moderate in terms of follow-up side effects or lupus-related activity. Several studies have shown this amongst our autoimmune rheumatology cohort, as well as what I’ve seen clinically in my own patients. More than anything else, numbers are the most important, and this is a large study.”
He acknowledged the benefits of going directly to patients to gauge their responses and reactions, giving them the opportunity to share concerns that physicians may not think about.
“As rheumatologists, we tend to focus on certain things that might not necessarily be what the patients themselves focus on,” he said. “I think the fact that this questionnaire dealt with a lot of what people complain about – fatigue, sore arm, things that we know are part of getting the vaccine – they aren’t necessarily things we capture with tools that screen for lupus flares, for example.”
More than anything, Dr. Saxena commended the study’s timeliness. “Patients are constantly asking us about the vaccine, and there’s so much misinformation,” he said. “People say, ‘Because I have lupus, I was told not to get vaccinated.’ I don’t know where they get that information from; we are telling everyone to get it, especially our lupus patients.”
The authors recognized their study’s main limitation as the self-reported and subjective nature of the survey, which they attempted to mitigate by asking for medically confirmed flares only. They noted, however, that the short median time between vaccination and flare onset could be caused by patients confusing expected side effects for something more serious, meaning the 3% figure “could be an overestimation of the actual flare rate.”
“Vaccination is recommended for patients with rheumatic and musculoskeletal diseases according to the American College of Rheumatology,” they added, “irrespective of disease activity and severity.”
Several authors reported potential conflicts of interest, including receiving consultancy fees and grants from Pfizer, GlaxoSmithKline, AbbVie, and Janssen, all unrelated to the study.
COVID-19 vaccinations appear to be well tolerated in patients with systemic lupus erythematosus (SLE) and come with a low risk of flare, according to the results of a global, web-based survey.
“Disseminating these reassuring data might prove crucial to increasing vaccine coverage in patients with SLE,” wrote lead author Renaud Felten, MD, of Strasbourg (France) University Hospital. Their results were published as a comment in Lancet Rheumatology.
To assess vaccine tolerability among lupus patients, the cross-sectional Tolerance and Consequences of Vaccination Against COVID-19 in Lupus Patients (VACOLUP) study analyzed a 43-question survey of 696 participants with a self-reported, medically confirmed diagnosis of SLE from 30 countries between March 22, 2021, and May 17, 2021. The cohort was 96% women, and their median age was 42 (interquartile range, 34-51). Nearly 36% of respondents were from Italy, 27% were from Chile, 13% were from France, and just under 9% were Americans. All participants received at least one dose of COVID-19 vaccine, and 49% received a second dose. The most common vaccines were Pfizer-BioNTech (57%), Sinovac (22%), AstraZeneca (10%), and Moderna (8%).
Only 21 participants (3%) reported a medically confirmed SLE flare after a median of 3 days (IQR, 0-29) post COVID vaccination, with most experiencing musculoskeletal symptoms (90%) and fatigue (86%). Of the 21 cases, 15 reported a subsequent change in SLE treatment and 4 were admitted to the hospital. A previous flare that occurred within a year before vaccination was associated with an increased risk of flare post vaccination (relative risk, 5.52; 95% confidence interval, 2.17-14.03; P < .0001).
Side effects – including swelling, soreness, fever, chills, fatigue, joint and muscle pain, nausea, and headache – were reported in 45% of participants (n = 316) after their first dose and in 53% of the 343 participants who received a second dose. There was no notable difference in the likelihood of side effects across gender and age or in patients who received mRNA vaccines, compared with vaccines with other modes of action. Patients who reported side effects after the first dose were more likely to also report them after the second, compared with those who reported none (109 [81%] of 135 vs. 72 [35%] of 205; RR, 2.30; 95% CI, 1.88-2.82; P < .0001).
In the majority of cases (2,232 of 2,683), the side effects were of minor or moderate intensity and did not affect the participants’ ability to perform daily tasks. The study found no significant association between side effects and a SLE flare and SLE medications or previous SLE disease manifestations.
When asked to comment on the study, Amit Saxena, MD, of the Lupus Center at New York University Langone Health, said: “What we are seeing is pretty mild to moderate in terms of follow-up side effects or lupus-related activity. Several studies have shown this amongst our autoimmune rheumatology cohort, as well as what I’ve seen clinically in my own patients. More than anything else, numbers are the most important, and this is a large study.”
He acknowledged the benefits of going directly to patients to gauge their responses and reactions, giving them the opportunity to share concerns that physicians may not think about.
“As rheumatologists, we tend to focus on certain things that might not necessarily be what the patients themselves focus on,” he said. “I think the fact that this questionnaire dealt with a lot of what people complain about – fatigue, sore arm, things that we know are part of getting the vaccine – they aren’t necessarily things we capture with tools that screen for lupus flares, for example.”
More than anything, Dr. Saxena commended the study’s timeliness. “Patients are constantly asking us about the vaccine, and there’s so much misinformation,” he said. “People say, ‘Because I have lupus, I was told not to get vaccinated.’ I don’t know where they get that information from; we are telling everyone to get it, especially our lupus patients.”
The authors recognized their study’s main limitation as the self-reported and subjective nature of the survey, which they attempted to mitigate by asking for medically confirmed flares only. They noted, however, that the short median time between vaccination and flare onset could be caused by patients confusing expected side effects for something more serious, meaning the 3% figure “could be an overestimation of the actual flare rate.”
“Vaccination is recommended for patients with rheumatic and musculoskeletal diseases according to the American College of Rheumatology,” they added, “irrespective of disease activity and severity.”
Several authors reported potential conflicts of interest, including receiving consultancy fees and grants from Pfizer, GlaxoSmithKline, AbbVie, and Janssen, all unrelated to the study.
COVID-19 vaccinations appear to be well tolerated in patients with systemic lupus erythematosus (SLE) and come with a low risk of flare, according to the results of a global, web-based survey.
“Disseminating these reassuring data might prove crucial to increasing vaccine coverage in patients with SLE,” wrote lead author Renaud Felten, MD, of Strasbourg (France) University Hospital. Their results were published as a comment in Lancet Rheumatology.
To assess vaccine tolerability among lupus patients, the cross-sectional Tolerance and Consequences of Vaccination Against COVID-19 in Lupus Patients (VACOLUP) study analyzed a 43-question survey of 696 participants with a self-reported, medically confirmed diagnosis of SLE from 30 countries between March 22, 2021, and May 17, 2021. The cohort was 96% women, and their median age was 42 (interquartile range, 34-51). Nearly 36% of respondents were from Italy, 27% were from Chile, 13% were from France, and just under 9% were Americans. All participants received at least one dose of COVID-19 vaccine, and 49% received a second dose. The most common vaccines were Pfizer-BioNTech (57%), Sinovac (22%), AstraZeneca (10%), and Moderna (8%).
Only 21 participants (3%) reported a medically confirmed SLE flare after a median of 3 days (IQR, 0-29) post COVID vaccination, with most experiencing musculoskeletal symptoms (90%) and fatigue (86%). Of the 21 cases, 15 reported a subsequent change in SLE treatment and 4 were admitted to the hospital. A previous flare that occurred within a year before vaccination was associated with an increased risk of flare post vaccination (relative risk, 5.52; 95% confidence interval, 2.17-14.03; P < .0001).
Side effects – including swelling, soreness, fever, chills, fatigue, joint and muscle pain, nausea, and headache – were reported in 45% of participants (n = 316) after their first dose and in 53% of the 343 participants who received a second dose. There was no notable difference in the likelihood of side effects across gender and age or in patients who received mRNA vaccines, compared with vaccines with other modes of action. Patients who reported side effects after the first dose were more likely to also report them after the second, compared with those who reported none (109 [81%] of 135 vs. 72 [35%] of 205; RR, 2.30; 95% CI, 1.88-2.82; P < .0001).
In the majority of cases (2,232 of 2,683), the side effects were of minor or moderate intensity and did not affect the participants’ ability to perform daily tasks. The study found no significant association between side effects and a SLE flare and SLE medications or previous SLE disease manifestations.
When asked to comment on the study, Amit Saxena, MD, of the Lupus Center at New York University Langone Health, said: “What we are seeing is pretty mild to moderate in terms of follow-up side effects or lupus-related activity. Several studies have shown this amongst our autoimmune rheumatology cohort, as well as what I’ve seen clinically in my own patients. More than anything else, numbers are the most important, and this is a large study.”
He acknowledged the benefits of going directly to patients to gauge their responses and reactions, giving them the opportunity to share concerns that physicians may not think about.
“As rheumatologists, we tend to focus on certain things that might not necessarily be what the patients themselves focus on,” he said. “I think the fact that this questionnaire dealt with a lot of what people complain about – fatigue, sore arm, things that we know are part of getting the vaccine – they aren’t necessarily things we capture with tools that screen for lupus flares, for example.”
More than anything, Dr. Saxena commended the study’s timeliness. “Patients are constantly asking us about the vaccine, and there’s so much misinformation,” he said. “People say, ‘Because I have lupus, I was told not to get vaccinated.’ I don’t know where they get that information from; we are telling everyone to get it, especially our lupus patients.”
The authors recognized their study’s main limitation as the self-reported and subjective nature of the survey, which they attempted to mitigate by asking for medically confirmed flares only. They noted, however, that the short median time between vaccination and flare onset could be caused by patients confusing expected side effects for something more serious, meaning the 3% figure “could be an overestimation of the actual flare rate.”
“Vaccination is recommended for patients with rheumatic and musculoskeletal diseases according to the American College of Rheumatology,” they added, “irrespective of disease activity and severity.”
Several authors reported potential conflicts of interest, including receiving consultancy fees and grants from Pfizer, GlaxoSmithKline, AbbVie, and Janssen, all unrelated to the study.
FROM THE LANCET RHEUMATOLOGY
Autoinflammatory diseases ‘not so rare after all,’ expert says
Not long ago, after all.
“Patients with autoinflammatory diseases are all around us, but many go several years without a diagnosis,” Dr. Dissanayake, a rheumatologist at the Autoinflammatory Disease Clinic at the Hospital for Sick Children, Toronto, said during the annual meeting of the Society for Pediatric Dermatology. “The median time to diagnosis has been estimated to be between 2.5 and 5 years. You can imagine that this type of delay can lead to significant issues, not only with quality of life but also morbidity due to unchecked inflammation that can cause organ damage, and in the most severe cases, can result in an early death.”
Effective treatment options such as biologic medications, however, can prevent these negative sequelae if the disease is recognized early. “Dermatologists are in a unique position because they will often be the first specialist to see these patients and therefore make the diagnosis early on and really alter the lives of these patients,” he said.
While it’s common to classify autoinflammatory diseases by presenting features, such as age of onset, associated symptoms, family history/ethnicity, and triggers/alleviating factors for episodes, Dr. Dissanayake prefers to classify them into one of three groups based on pathophysiology, the first being inflammasomopathies. “When activated, an inflammasome is responsible for processing cytokines from the [interleukin]-1 family from the pro form to the active form,” he explained. As a result, if there is dysregulation and overactivity of the inflammasome, there is excessive production of cytokines like IL-1 beta and IL-18 driving the disease.
Clinical characteristics include fevers and organ involvement, notably abdominal pain, nonvasculitic rashes, uveitis, arthritis, elevated white blood cell count/neutrophils, and highly elevated inflammatory markers. Potential treatments include IL-1 blockers.
The second category of autoinflammatory diseases are the interferonopathies, which are caused by overactivity of the antiviral side of the innate immune system. “For example, if you have overactivity of a sensor for a nucleic acid in your cytosol, the cell misinterprets this as a viral infection and will turn on type 1 interferon production,” said Dr. Dissanayake, who is also an assistant professor of pediatrics at the University of Toronto. “As a result, if you have dysregulation of these pathways, you will get excessive type 1 interferon that contributes to your disease manifestations.” Clinical characteristics include fevers and organ involvement, notably vasculitic rashes, interstitial lung disease, and intracranial calcifications. Inflammatory markers may not be as elevated, and autoantibodies may be present. Janus kinase inhibitors are a potential treatment, he said.
The third category of autoinflammatory diseases are the NF-kappaBopathies, which are caused by overactivity of the NF-kappaB signaling pathway. Clinical characteristics can include fevers with organ involvement that can be highly variable but may include mucocutaneous lesions or granulomatous disease as potential clues. Treatment options depend on the pathway that is involved but tumor necrosis factor blockers often play a role because of the importance of NF-KB in this signaling pathway.
From a skin perspective, most of the rashes Dr. Dissanayake and colleagues see in the rheumatology clinic consist of nonspecific dermohypodermatitis: macules, papules, patches, or plaques. The most common monogenic autoinflammatory disease is Familial Mediterranean Fever syndrome, which “commonly presents as an erysipelas-like rash of the lower extremities, typically below the knee, often over the malleolus,” he said.
Other monogenic autoinflammatory diseases with similar rashes include TNF receptor–associated periodic syndrome, Hyper-IgD syndrome, and systemic juvenile idiopathic arthritis.
Other patients present with urticarial rashes, most commonly cryopyrin-associated periodic syndrome (CAPS). “This is a neutrophilic urticaria, so it tends not to be pruritic and can actually sometimes be tender,” he said. “It also tends not to be as transient as your typical urticaria.” Urticarial rashes can also appear with NLRP12-associated autoinflammatory syndrome (familial cold autoinflammatory syndrome–2), PLCgamma2-associated antibody deficiency and immune dysregulation, and Schnitzler syndrome (monoclonal IgM gammopathy).
Patients can also present with pyogenic or pustular lesions, which can appear with pyoderma gangrenosum–related diseases, such as pyogenic arthritis, pyoderma gangrenosum, arthritis (PAPA) syndrome; pyrin-associated inflammation with neutrophilic dermatosis; deficiency of the IL-1 receptor antagonist; deficiency of IL-36 receptor antagonist; and Majeed syndrome, a mutation in the LPIN2 gene.
The mucocutaneous system can also be affected in autoinflammatory diseases, often presenting with symptoms such as periodic fever, aphthous stomatitis, and pharyngitis. Cervical adenitis syndrome is the most common autoinflammatory disease in childhood and can present with aphthous stomatitis, he said, while Behcet’s disease typically presents with oral and genital ulcers. “More recently, monogenic forms of Behcet’s disease have been described, with haploinsufficiency of A20 and RelA, which are both part of the NF-KB pathway,” he said.
Finally, the presence of vasculitic lesions often suggest interferonopathies such as STING-associated vasculopathy in infancy, proteasome-associated autoinflammatory syndrome and deficiency of adenosine deaminase 2.
Dr. Dissanayake noted that dermatologists should suspect an autoimmune disease if a patient has recurrent fevers, evidence of systemic inflammation on blood work, and if multiple organ systems are involved, especially the lungs, gut, joints, CNS system, and eyes. “Many of these patients have episodic and stereotypical attacks,” he said.
“One of the tools we use in the autoinflammatory clinic is to have patients and families keep a symptom diary where they track the dates of the various symptoms. We can review this during their appointment and try to come up with a diagnosis based on the pattern,” he said.
Since many of these diseases are due to a single gene defect, if there’s any evidence to suggest a monogenic cause, consider an autoinflammatory disease, he added. “If there’s a family history, if there’s consanguinity, or if there’s early age of onset – these may all lead you to think about monogenic autoinflammatory disease.”
During a question-and-answer session, a meeting attendee asked what type of workup he recommends when an autoinflammatory syndrome is suspected. “It partially depends on what organ systems you suspect to be involved,” Dr. Dissanayake said. “As a routine baseline, typically what we would check is CBC and differential, [erythrocyte sedimentation rate] and [C-reactive protein], and we screen for liver transaminases and creatinine to check for liver and kidney issues. A serum albumin will also tell you if the patient is hypoalbuminemic, that there’s been some chronic inflammation and they’re starting to leak the protein out. It’s good to check blood work during the flare and off the flare, to get a sense of the persistence of that inflammation.”
Dr. Dissanayake disclosed that he has received research finding from Gilead Sciences and speaker fees from Novartis.
*This story was updated on 9/20/2021.
Not long ago, after all.
“Patients with autoinflammatory diseases are all around us, but many go several years without a diagnosis,” Dr. Dissanayake, a rheumatologist at the Autoinflammatory Disease Clinic at the Hospital for Sick Children, Toronto, said during the annual meeting of the Society for Pediatric Dermatology. “The median time to diagnosis has been estimated to be between 2.5 and 5 years. You can imagine that this type of delay can lead to significant issues, not only with quality of life but also morbidity due to unchecked inflammation that can cause organ damage, and in the most severe cases, can result in an early death.”
Effective treatment options such as biologic medications, however, can prevent these negative sequelae if the disease is recognized early. “Dermatologists are in a unique position because they will often be the first specialist to see these patients and therefore make the diagnosis early on and really alter the lives of these patients,” he said.
While it’s common to classify autoinflammatory diseases by presenting features, such as age of onset, associated symptoms, family history/ethnicity, and triggers/alleviating factors for episodes, Dr. Dissanayake prefers to classify them into one of three groups based on pathophysiology, the first being inflammasomopathies. “When activated, an inflammasome is responsible for processing cytokines from the [interleukin]-1 family from the pro form to the active form,” he explained. As a result, if there is dysregulation and overactivity of the inflammasome, there is excessive production of cytokines like IL-1 beta and IL-18 driving the disease.
Clinical characteristics include fevers and organ involvement, notably abdominal pain, nonvasculitic rashes, uveitis, arthritis, elevated white blood cell count/neutrophils, and highly elevated inflammatory markers. Potential treatments include IL-1 blockers.
The second category of autoinflammatory diseases are the interferonopathies, which are caused by overactivity of the antiviral side of the innate immune system. “For example, if you have overactivity of a sensor for a nucleic acid in your cytosol, the cell misinterprets this as a viral infection and will turn on type 1 interferon production,” said Dr. Dissanayake, who is also an assistant professor of pediatrics at the University of Toronto. “As a result, if you have dysregulation of these pathways, you will get excessive type 1 interferon that contributes to your disease manifestations.” Clinical characteristics include fevers and organ involvement, notably vasculitic rashes, interstitial lung disease, and intracranial calcifications. Inflammatory markers may not be as elevated, and autoantibodies may be present. Janus kinase inhibitors are a potential treatment, he said.
The third category of autoinflammatory diseases are the NF-kappaBopathies, which are caused by overactivity of the NF-kappaB signaling pathway. Clinical characteristics can include fevers with organ involvement that can be highly variable but may include mucocutaneous lesions or granulomatous disease as potential clues. Treatment options depend on the pathway that is involved but tumor necrosis factor blockers often play a role because of the importance of NF-KB in this signaling pathway.
From a skin perspective, most of the rashes Dr. Dissanayake and colleagues see in the rheumatology clinic consist of nonspecific dermohypodermatitis: macules, papules, patches, or plaques. The most common monogenic autoinflammatory disease is Familial Mediterranean Fever syndrome, which “commonly presents as an erysipelas-like rash of the lower extremities, typically below the knee, often over the malleolus,” he said.
Other monogenic autoinflammatory diseases with similar rashes include TNF receptor–associated periodic syndrome, Hyper-IgD syndrome, and systemic juvenile idiopathic arthritis.
Other patients present with urticarial rashes, most commonly cryopyrin-associated periodic syndrome (CAPS). “This is a neutrophilic urticaria, so it tends not to be pruritic and can actually sometimes be tender,” he said. “It also tends not to be as transient as your typical urticaria.” Urticarial rashes can also appear with NLRP12-associated autoinflammatory syndrome (familial cold autoinflammatory syndrome–2), PLCgamma2-associated antibody deficiency and immune dysregulation, and Schnitzler syndrome (monoclonal IgM gammopathy).
Patients can also present with pyogenic or pustular lesions, which can appear with pyoderma gangrenosum–related diseases, such as pyogenic arthritis, pyoderma gangrenosum, arthritis (PAPA) syndrome; pyrin-associated inflammation with neutrophilic dermatosis; deficiency of the IL-1 receptor antagonist; deficiency of IL-36 receptor antagonist; and Majeed syndrome, a mutation in the LPIN2 gene.
The mucocutaneous system can also be affected in autoinflammatory diseases, often presenting with symptoms such as periodic fever, aphthous stomatitis, and pharyngitis. Cervical adenitis syndrome is the most common autoinflammatory disease in childhood and can present with aphthous stomatitis, he said, while Behcet’s disease typically presents with oral and genital ulcers. “More recently, monogenic forms of Behcet’s disease have been described, with haploinsufficiency of A20 and RelA, which are both part of the NF-KB pathway,” he said.
Finally, the presence of vasculitic lesions often suggest interferonopathies such as STING-associated vasculopathy in infancy, proteasome-associated autoinflammatory syndrome and deficiency of adenosine deaminase 2.
Dr. Dissanayake noted that dermatologists should suspect an autoimmune disease if a patient has recurrent fevers, evidence of systemic inflammation on blood work, and if multiple organ systems are involved, especially the lungs, gut, joints, CNS system, and eyes. “Many of these patients have episodic and stereotypical attacks,” he said.
“One of the tools we use in the autoinflammatory clinic is to have patients and families keep a symptom diary where they track the dates of the various symptoms. We can review this during their appointment and try to come up with a diagnosis based on the pattern,” he said.
Since many of these diseases are due to a single gene defect, if there’s any evidence to suggest a monogenic cause, consider an autoinflammatory disease, he added. “If there’s a family history, if there’s consanguinity, or if there’s early age of onset – these may all lead you to think about monogenic autoinflammatory disease.”
During a question-and-answer session, a meeting attendee asked what type of workup he recommends when an autoinflammatory syndrome is suspected. “It partially depends on what organ systems you suspect to be involved,” Dr. Dissanayake said. “As a routine baseline, typically what we would check is CBC and differential, [erythrocyte sedimentation rate] and [C-reactive protein], and we screen for liver transaminases and creatinine to check for liver and kidney issues. A serum albumin will also tell you if the patient is hypoalbuminemic, that there’s been some chronic inflammation and they’re starting to leak the protein out. It’s good to check blood work during the flare and off the flare, to get a sense of the persistence of that inflammation.”
Dr. Dissanayake disclosed that he has received research finding from Gilead Sciences and speaker fees from Novartis.
*This story was updated on 9/20/2021.
Not long ago, after all.
“Patients with autoinflammatory diseases are all around us, but many go several years without a diagnosis,” Dr. Dissanayake, a rheumatologist at the Autoinflammatory Disease Clinic at the Hospital for Sick Children, Toronto, said during the annual meeting of the Society for Pediatric Dermatology. “The median time to diagnosis has been estimated to be between 2.5 and 5 years. You can imagine that this type of delay can lead to significant issues, not only with quality of life but also morbidity due to unchecked inflammation that can cause organ damage, and in the most severe cases, can result in an early death.”
Effective treatment options such as biologic medications, however, can prevent these negative sequelae if the disease is recognized early. “Dermatologists are in a unique position because they will often be the first specialist to see these patients and therefore make the diagnosis early on and really alter the lives of these patients,” he said.
While it’s common to classify autoinflammatory diseases by presenting features, such as age of onset, associated symptoms, family history/ethnicity, and triggers/alleviating factors for episodes, Dr. Dissanayake prefers to classify them into one of three groups based on pathophysiology, the first being inflammasomopathies. “When activated, an inflammasome is responsible for processing cytokines from the [interleukin]-1 family from the pro form to the active form,” he explained. As a result, if there is dysregulation and overactivity of the inflammasome, there is excessive production of cytokines like IL-1 beta and IL-18 driving the disease.
Clinical characteristics include fevers and organ involvement, notably abdominal pain, nonvasculitic rashes, uveitis, arthritis, elevated white blood cell count/neutrophils, and highly elevated inflammatory markers. Potential treatments include IL-1 blockers.
The second category of autoinflammatory diseases are the interferonopathies, which are caused by overactivity of the antiviral side of the innate immune system. “For example, if you have overactivity of a sensor for a nucleic acid in your cytosol, the cell misinterprets this as a viral infection and will turn on type 1 interferon production,” said Dr. Dissanayake, who is also an assistant professor of pediatrics at the University of Toronto. “As a result, if you have dysregulation of these pathways, you will get excessive type 1 interferon that contributes to your disease manifestations.” Clinical characteristics include fevers and organ involvement, notably vasculitic rashes, interstitial lung disease, and intracranial calcifications. Inflammatory markers may not be as elevated, and autoantibodies may be present. Janus kinase inhibitors are a potential treatment, he said.
The third category of autoinflammatory diseases are the NF-kappaBopathies, which are caused by overactivity of the NF-kappaB signaling pathway. Clinical characteristics can include fevers with organ involvement that can be highly variable but may include mucocutaneous lesions or granulomatous disease as potential clues. Treatment options depend on the pathway that is involved but tumor necrosis factor blockers often play a role because of the importance of NF-KB in this signaling pathway.
From a skin perspective, most of the rashes Dr. Dissanayake and colleagues see in the rheumatology clinic consist of nonspecific dermohypodermatitis: macules, papules, patches, or plaques. The most common monogenic autoinflammatory disease is Familial Mediterranean Fever syndrome, which “commonly presents as an erysipelas-like rash of the lower extremities, typically below the knee, often over the malleolus,” he said.
Other monogenic autoinflammatory diseases with similar rashes include TNF receptor–associated periodic syndrome, Hyper-IgD syndrome, and systemic juvenile idiopathic arthritis.
Other patients present with urticarial rashes, most commonly cryopyrin-associated periodic syndrome (CAPS). “This is a neutrophilic urticaria, so it tends not to be pruritic and can actually sometimes be tender,” he said. “It also tends not to be as transient as your typical urticaria.” Urticarial rashes can also appear with NLRP12-associated autoinflammatory syndrome (familial cold autoinflammatory syndrome–2), PLCgamma2-associated antibody deficiency and immune dysregulation, and Schnitzler syndrome (monoclonal IgM gammopathy).
Patients can also present with pyogenic or pustular lesions, which can appear with pyoderma gangrenosum–related diseases, such as pyogenic arthritis, pyoderma gangrenosum, arthritis (PAPA) syndrome; pyrin-associated inflammation with neutrophilic dermatosis; deficiency of the IL-1 receptor antagonist; deficiency of IL-36 receptor antagonist; and Majeed syndrome, a mutation in the LPIN2 gene.
The mucocutaneous system can also be affected in autoinflammatory diseases, often presenting with symptoms such as periodic fever, aphthous stomatitis, and pharyngitis. Cervical adenitis syndrome is the most common autoinflammatory disease in childhood and can present with aphthous stomatitis, he said, while Behcet’s disease typically presents with oral and genital ulcers. “More recently, monogenic forms of Behcet’s disease have been described, with haploinsufficiency of A20 and RelA, which are both part of the NF-KB pathway,” he said.
Finally, the presence of vasculitic lesions often suggest interferonopathies such as STING-associated vasculopathy in infancy, proteasome-associated autoinflammatory syndrome and deficiency of adenosine deaminase 2.
Dr. Dissanayake noted that dermatologists should suspect an autoimmune disease if a patient has recurrent fevers, evidence of systemic inflammation on blood work, and if multiple organ systems are involved, especially the lungs, gut, joints, CNS system, and eyes. “Many of these patients have episodic and stereotypical attacks,” he said.
“One of the tools we use in the autoinflammatory clinic is to have patients and families keep a symptom diary where they track the dates of the various symptoms. We can review this during their appointment and try to come up with a diagnosis based on the pattern,” he said.
Since many of these diseases are due to a single gene defect, if there’s any evidence to suggest a monogenic cause, consider an autoinflammatory disease, he added. “If there’s a family history, if there’s consanguinity, or if there’s early age of onset – these may all lead you to think about monogenic autoinflammatory disease.”
During a question-and-answer session, a meeting attendee asked what type of workup he recommends when an autoinflammatory syndrome is suspected. “It partially depends on what organ systems you suspect to be involved,” Dr. Dissanayake said. “As a routine baseline, typically what we would check is CBC and differential, [erythrocyte sedimentation rate] and [C-reactive protein], and we screen for liver transaminases and creatinine to check for liver and kidney issues. A serum albumin will also tell you if the patient is hypoalbuminemic, that there’s been some chronic inflammation and they’re starting to leak the protein out. It’s good to check blood work during the flare and off the flare, to get a sense of the persistence of that inflammation.”
Dr. Dissanayake disclosed that he has received research finding from Gilead Sciences and speaker fees from Novartis.
*This story was updated on 9/20/2021.
FROM SPD 2021
FDA approves intravenous immunoglobulin for dermatomyositis
, according to a statement from manufacturer Octapharma USA.
Dermatomyositis is a rare, idiopathic autoimmune disorder that affects approximately 10 out of every million people in the United States, mainly adults in their late 40s to early 60s, according to the company, but children aged 5-15 years can be affected. The disease is characterized by skin rashes, chronic muscle inflammation, progressive muscle weakness, and risk for mortality that is three times higher than for the general population.
There are no previously approved treatments for dermatomyositis prior to Octagam 10%, which also is indicated for chronic immune thrombocytopenic purpura in adults.
The approval for dermatomyositis was based on the results of a phase 3 randomized, double-blind, placebo-controlled clinical trial (the ProDERM trial) that included 95 adult patients at 36 sites worldwide, with 17 sites in the United States. In the trial, 78.7% of patients with dermatomyositis who were randomized to receive 2 g/kg of Octagam 10% every 4 weeks showed response at 16 weeks, compared with 43.8% of patients who received placebo. Response was based on the 2016 American College of Rheumatology/European Alliance of Associations for Rheumatology myositis response criteria. Placebo patients who switched to intravenous immunoglobulin (IVIG) during a trial extension had response rates at week 40 similar to the original patients at week 16.
“The study gives clinicians much more confidence in the efficacy and safety of intravenous immunoglobulin and provides valuable information about what type of patient is best suited for the treatment,” Rohit Aggarwal, MD, medical director of the Arthritis and Autoimmunity Center at the University of Pittsburgh and a member of the ProDERM study Steering Committee, said in the Octapharma statement.
Safety and tolerability were similar to profiles seen with other IVIG medications, according to the statement. The medication does carry a boxed warning from its chronic ITP approval, cautioning about the potential for thrombosis, renal dysfunction, and acute renal failure.
The most common adverse reactions reported by dermatomyositis patients in the ProDERM trial were headache, fever, nausea, vomiting, increased blood pressure, chills, musculoskeletal pain, increased heart rate, dyspnea, and reactions at the infusion sites.
Read the full prescribing information here.
, according to a statement from manufacturer Octapharma USA.
Dermatomyositis is a rare, idiopathic autoimmune disorder that affects approximately 10 out of every million people in the United States, mainly adults in their late 40s to early 60s, according to the company, but children aged 5-15 years can be affected. The disease is characterized by skin rashes, chronic muscle inflammation, progressive muscle weakness, and risk for mortality that is three times higher than for the general population.
There are no previously approved treatments for dermatomyositis prior to Octagam 10%, which also is indicated for chronic immune thrombocytopenic purpura in adults.
The approval for dermatomyositis was based on the results of a phase 3 randomized, double-blind, placebo-controlled clinical trial (the ProDERM trial) that included 95 adult patients at 36 sites worldwide, with 17 sites in the United States. In the trial, 78.7% of patients with dermatomyositis who were randomized to receive 2 g/kg of Octagam 10% every 4 weeks showed response at 16 weeks, compared with 43.8% of patients who received placebo. Response was based on the 2016 American College of Rheumatology/European Alliance of Associations for Rheumatology myositis response criteria. Placebo patients who switched to intravenous immunoglobulin (IVIG) during a trial extension had response rates at week 40 similar to the original patients at week 16.
“The study gives clinicians much more confidence in the efficacy and safety of intravenous immunoglobulin and provides valuable information about what type of patient is best suited for the treatment,” Rohit Aggarwal, MD, medical director of the Arthritis and Autoimmunity Center at the University of Pittsburgh and a member of the ProDERM study Steering Committee, said in the Octapharma statement.
Safety and tolerability were similar to profiles seen with other IVIG medications, according to the statement. The medication does carry a boxed warning from its chronic ITP approval, cautioning about the potential for thrombosis, renal dysfunction, and acute renal failure.
The most common adverse reactions reported by dermatomyositis patients in the ProDERM trial were headache, fever, nausea, vomiting, increased blood pressure, chills, musculoskeletal pain, increased heart rate, dyspnea, and reactions at the infusion sites.
Read the full prescribing information here.
, according to a statement from manufacturer Octapharma USA.
Dermatomyositis is a rare, idiopathic autoimmune disorder that affects approximately 10 out of every million people in the United States, mainly adults in their late 40s to early 60s, according to the company, but children aged 5-15 years can be affected. The disease is characterized by skin rashes, chronic muscle inflammation, progressive muscle weakness, and risk for mortality that is three times higher than for the general population.
There are no previously approved treatments for dermatomyositis prior to Octagam 10%, which also is indicated for chronic immune thrombocytopenic purpura in adults.
The approval for dermatomyositis was based on the results of a phase 3 randomized, double-blind, placebo-controlled clinical trial (the ProDERM trial) that included 95 adult patients at 36 sites worldwide, with 17 sites in the United States. In the trial, 78.7% of patients with dermatomyositis who were randomized to receive 2 g/kg of Octagam 10% every 4 weeks showed response at 16 weeks, compared with 43.8% of patients who received placebo. Response was based on the 2016 American College of Rheumatology/European Alliance of Associations for Rheumatology myositis response criteria. Placebo patients who switched to intravenous immunoglobulin (IVIG) during a trial extension had response rates at week 40 similar to the original patients at week 16.
“The study gives clinicians much more confidence in the efficacy and safety of intravenous immunoglobulin and provides valuable information about what type of patient is best suited for the treatment,” Rohit Aggarwal, MD, medical director of the Arthritis and Autoimmunity Center at the University of Pittsburgh and a member of the ProDERM study Steering Committee, said in the Octapharma statement.
Safety and tolerability were similar to profiles seen with other IVIG medications, according to the statement. The medication does carry a boxed warning from its chronic ITP approval, cautioning about the potential for thrombosis, renal dysfunction, and acute renal failure.
The most common adverse reactions reported by dermatomyositis patients in the ProDERM trial were headache, fever, nausea, vomiting, increased blood pressure, chills, musculoskeletal pain, increased heart rate, dyspnea, and reactions at the infusion sites.
Read the full prescribing information here.
Neuropsychiatric event etiology in lupus helps define predictors, outcomes
Different kinds of neuropsychiatric (NP) events in patients with systemic lupus erythematosus (SLE) have substantial variability in their occurrence, resolution, and recurrence over time, as well as in their predictors, according to new research from a large, prospective, international, inception cohort study.
Because “multiple NP events due to different causes may present concurrently in individual patients, the findings emphasize the importance of recognizing attribution of NP events as a determinant of clinical outcome,” John G. Hanly, MD, of Queen Elizabeth II Health Sciences Centre and Dalhousie University, Halifax, N.S., and colleagues wrote in Arthritis & Rheumatology.
In a previous study of the same group of 1,827 patients with SLE, NP events occurred in about half and approximately one-third of these events were deemed disease related. They also “occurred most frequently around the diagnosis of SLE and had a significant negative impact on health-related quality of life,” the researchers wrote.
Researchers involved with the Systemic Lupus International Collaborating Clinics recruited the 1,827 adults with SLE over an 11-year period during 1999-2011 from a total of 31 sites in Europe, Asia, and North America. The average age of the patients at study enrollment was 35 years, 89% were women, and 49% were White. The mean disease duration was 5.6 months, and 70% of patients were taking corticosteroids at enrollment.
Over an average follow-up period of 7.6 years, 955 patients (52.3%) experienced a single neuropsychiatric event, and 493 (27.0%) experienced two or more events; the total number of unique NP events was 1,910. Most of these unique events (92%) involved the central nervous system, and 8.4% involved the peripheral nervous system.
The researchers used multistate models to attribute NP events to SLE based on factors that included the temporal onset of NP events in relation to SLE diagnosis, concurrent non-SLE factors, and NP events that are common in healthy controls. The four states in the multistate models were no NP events, no current NP event but a history of at least one event, new or ongoing NP events, and death. The results included a multivariate analysis of a model involving 492 observed transitions into new or ongoing NP events.
In the multivariate analysis, factors positively associated with SLE-attributed NP events included male sex (hazard ratio, 1.35; P = .028), concurrent non-SLE NP events excluding headache (HR, 1.83; P < .001), active SLE based on the Systemic Lupus Erythematosus Disease Activity Index 2000 (HR, 1.19; P = .012), and corticosteroid use (HR, 1.59; P = .008). The researchers also found that SLE-attributed NP events were negatively associated with Asian race/ethnicity, postsecondary education, and use of immunosuppressive drugs.
Another multivariate analysis found that non-SLE NP events were positively associated with only concurrent SLE-attributed NP events excluding headache (HR, 2.31; P < .001), but negative associations were seen with non-U.S. African race/ethnicity and Asian race/ethnicity.
The researchers found that SLE-attributed NP events had higher rates of resolution, compared with non-SLE NP events, with the exception of headache, which had similar resolution for both event groups.
“Resolution of SLE events was more likely in patients with Asian race/ethnicity and those with Central/Focal nervous system disease with no effect seen for age at diagnosis,” the researchers noted. “For non-SLE NP events, African race/ethnicity at non-U.S. sites and younger age at diagnosis was associated with a better outcome.”
The study findings were limited by several factors including the predominantly White patient population and the clustering of NP events into limited categories, which may have reduced the identification of more specific associations, the researchers noted. Also, the assessment of NP event outcomes did not include patient perceptions, and the relatively short follow-up period does not allow for assessment of later NP events such as cerebrovascular disease. However, “despite these limitations the current study provides valuable data on the presentation, outcome and predictors of NP disease in SLE patients enrolled in a long-term, international, disease inception cohort,” the researchers concluded.
The study received no outside funding. Dr. Hanly was supported by a grant from the Canadian Institutes of Health Research but had no financial conflicts to disclose. Several coauthors received grant support from various institutions, but not from industry, and had no financial conflicts to disclose.
Different kinds of neuropsychiatric (NP) events in patients with systemic lupus erythematosus (SLE) have substantial variability in their occurrence, resolution, and recurrence over time, as well as in their predictors, according to new research from a large, prospective, international, inception cohort study.
Because “multiple NP events due to different causes may present concurrently in individual patients, the findings emphasize the importance of recognizing attribution of NP events as a determinant of clinical outcome,” John G. Hanly, MD, of Queen Elizabeth II Health Sciences Centre and Dalhousie University, Halifax, N.S., and colleagues wrote in Arthritis & Rheumatology.
In a previous study of the same group of 1,827 patients with SLE, NP events occurred in about half and approximately one-third of these events were deemed disease related. They also “occurred most frequently around the diagnosis of SLE and had a significant negative impact on health-related quality of life,” the researchers wrote.
Researchers involved with the Systemic Lupus International Collaborating Clinics recruited the 1,827 adults with SLE over an 11-year period during 1999-2011 from a total of 31 sites in Europe, Asia, and North America. The average age of the patients at study enrollment was 35 years, 89% were women, and 49% were White. The mean disease duration was 5.6 months, and 70% of patients were taking corticosteroids at enrollment.
Over an average follow-up period of 7.6 years, 955 patients (52.3%) experienced a single neuropsychiatric event, and 493 (27.0%) experienced two or more events; the total number of unique NP events was 1,910. Most of these unique events (92%) involved the central nervous system, and 8.4% involved the peripheral nervous system.
The researchers used multistate models to attribute NP events to SLE based on factors that included the temporal onset of NP events in relation to SLE diagnosis, concurrent non-SLE factors, and NP events that are common in healthy controls. The four states in the multistate models were no NP events, no current NP event but a history of at least one event, new or ongoing NP events, and death. The results included a multivariate analysis of a model involving 492 observed transitions into new or ongoing NP events.
In the multivariate analysis, factors positively associated with SLE-attributed NP events included male sex (hazard ratio, 1.35; P = .028), concurrent non-SLE NP events excluding headache (HR, 1.83; P < .001), active SLE based on the Systemic Lupus Erythematosus Disease Activity Index 2000 (HR, 1.19; P = .012), and corticosteroid use (HR, 1.59; P = .008). The researchers also found that SLE-attributed NP events were negatively associated with Asian race/ethnicity, postsecondary education, and use of immunosuppressive drugs.
Another multivariate analysis found that non-SLE NP events were positively associated with only concurrent SLE-attributed NP events excluding headache (HR, 2.31; P < .001), but negative associations were seen with non-U.S. African race/ethnicity and Asian race/ethnicity.
The researchers found that SLE-attributed NP events had higher rates of resolution, compared with non-SLE NP events, with the exception of headache, which had similar resolution for both event groups.
“Resolution of SLE events was more likely in patients with Asian race/ethnicity and those with Central/Focal nervous system disease with no effect seen for age at diagnosis,” the researchers noted. “For non-SLE NP events, African race/ethnicity at non-U.S. sites and younger age at diagnosis was associated with a better outcome.”
The study findings were limited by several factors including the predominantly White patient population and the clustering of NP events into limited categories, which may have reduced the identification of more specific associations, the researchers noted. Also, the assessment of NP event outcomes did not include patient perceptions, and the relatively short follow-up period does not allow for assessment of later NP events such as cerebrovascular disease. However, “despite these limitations the current study provides valuable data on the presentation, outcome and predictors of NP disease in SLE patients enrolled in a long-term, international, disease inception cohort,” the researchers concluded.
The study received no outside funding. Dr. Hanly was supported by a grant from the Canadian Institutes of Health Research but had no financial conflicts to disclose. Several coauthors received grant support from various institutions, but not from industry, and had no financial conflicts to disclose.
Different kinds of neuropsychiatric (NP) events in patients with systemic lupus erythematosus (SLE) have substantial variability in their occurrence, resolution, and recurrence over time, as well as in their predictors, according to new research from a large, prospective, international, inception cohort study.
Because “multiple NP events due to different causes may present concurrently in individual patients, the findings emphasize the importance of recognizing attribution of NP events as a determinant of clinical outcome,” John G. Hanly, MD, of Queen Elizabeth II Health Sciences Centre and Dalhousie University, Halifax, N.S., and colleagues wrote in Arthritis & Rheumatology.
In a previous study of the same group of 1,827 patients with SLE, NP events occurred in about half and approximately one-third of these events were deemed disease related. They also “occurred most frequently around the diagnosis of SLE and had a significant negative impact on health-related quality of life,” the researchers wrote.
Researchers involved with the Systemic Lupus International Collaborating Clinics recruited the 1,827 adults with SLE over an 11-year period during 1999-2011 from a total of 31 sites in Europe, Asia, and North America. The average age of the patients at study enrollment was 35 years, 89% were women, and 49% were White. The mean disease duration was 5.6 months, and 70% of patients were taking corticosteroids at enrollment.
Over an average follow-up period of 7.6 years, 955 patients (52.3%) experienced a single neuropsychiatric event, and 493 (27.0%) experienced two or more events; the total number of unique NP events was 1,910. Most of these unique events (92%) involved the central nervous system, and 8.4% involved the peripheral nervous system.
The researchers used multistate models to attribute NP events to SLE based on factors that included the temporal onset of NP events in relation to SLE diagnosis, concurrent non-SLE factors, and NP events that are common in healthy controls. The four states in the multistate models were no NP events, no current NP event but a history of at least one event, new or ongoing NP events, and death. The results included a multivariate analysis of a model involving 492 observed transitions into new or ongoing NP events.
In the multivariate analysis, factors positively associated with SLE-attributed NP events included male sex (hazard ratio, 1.35; P = .028), concurrent non-SLE NP events excluding headache (HR, 1.83; P < .001), active SLE based on the Systemic Lupus Erythematosus Disease Activity Index 2000 (HR, 1.19; P = .012), and corticosteroid use (HR, 1.59; P = .008). The researchers also found that SLE-attributed NP events were negatively associated with Asian race/ethnicity, postsecondary education, and use of immunosuppressive drugs.
Another multivariate analysis found that non-SLE NP events were positively associated with only concurrent SLE-attributed NP events excluding headache (HR, 2.31; P < .001), but negative associations were seen with non-U.S. African race/ethnicity and Asian race/ethnicity.
The researchers found that SLE-attributed NP events had higher rates of resolution, compared with non-SLE NP events, with the exception of headache, which had similar resolution for both event groups.
“Resolution of SLE events was more likely in patients with Asian race/ethnicity and those with Central/Focal nervous system disease with no effect seen for age at diagnosis,” the researchers noted. “For non-SLE NP events, African race/ethnicity at non-U.S. sites and younger age at diagnosis was associated with a better outcome.”
The study findings were limited by several factors including the predominantly White patient population and the clustering of NP events into limited categories, which may have reduced the identification of more specific associations, the researchers noted. Also, the assessment of NP event outcomes did not include patient perceptions, and the relatively short follow-up period does not allow for assessment of later NP events such as cerebrovascular disease. However, “despite these limitations the current study provides valuable data on the presentation, outcome and predictors of NP disease in SLE patients enrolled in a long-term, international, disease inception cohort,” the researchers concluded.
The study received no outside funding. Dr. Hanly was supported by a grant from the Canadian Institutes of Health Research but had no financial conflicts to disclose. Several coauthors received grant support from various institutions, but not from industry, and had no financial conflicts to disclose.
FROM ARTHRITIS & RHEUMATOLOGY
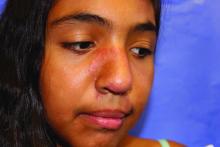
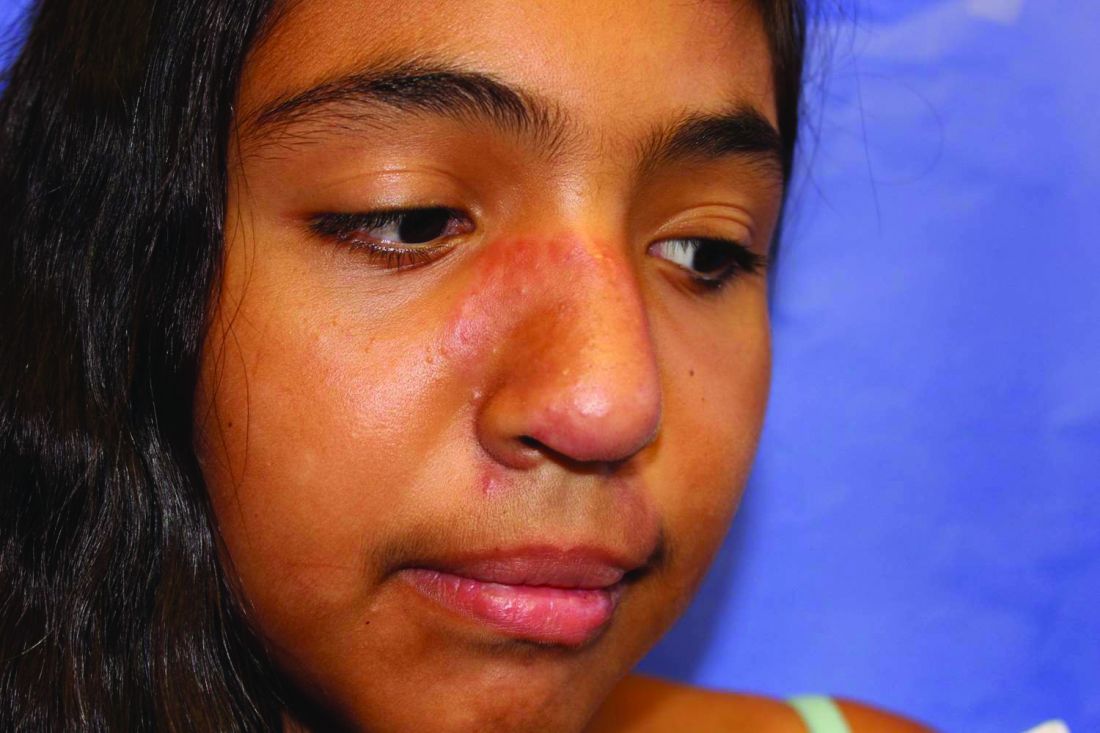
Lupus images fall short on diverse examples
Lupus images in medical resource materials underrepresent patients with skin of color, based on data from a review of more than 1,400 images published between 2014 and 2019 in materials from a university’s online medical library.
Patients with skin of color who develop lupus tend to present earlier and with more severe cases, and often experience worse outcomes, compared with other populations, wrote Amaad Rana, MD, of Washington University, St. Louis, and colleagues. Medical resources in general have historically underrepresented patients of color, and the researchers reviewed lupus materials for a similar publication bias.
In a study published in Arthritis Care & Research, the investigators identified 1,417 images in rheumatology, dermatology, and internal medicine resources, including 119 medical textbooks, 15 medical journals, 2 online image libraries, and the online image collections of Google and UpToDate. An additional 24 images came from skin of color atlases.
Excluding the skin of color atlases, 56.4% of the images represented light skin, 35.1% showed medium skin, and 8.5% showed dark skin. Overall, publishers were more than twice as likely to portray light skin tones and were significantly less likely to portray dark skin tones (odds ratios, 2.59 and 0.19, respectively), compared with an equal representation of skin tones; however, the difference was not significant for portrayal of medium skin tones (OR, 1.08).
By specialty, dermatology was more inclusive of skin of color images than rheumatology or internal medicine, although the internal medicine sample size was too small for comparable analysis, the researchers noted. Dermatology textbooks were 2.42 times more likely and rheumatology textbooks were 4.87 times more likely to depict light skin tones than an equal representation of light, medium, and dark skin tones.
The researchers rated the skin color in the images using the New Immigrant Survey Skin Color Scale and categorized the images as representing light (NISSCS scores, 1-2), medium (NISSCS scores, 3-5), or dark skin (NISSCS scores, 6-10). Medical journals had the most images of dark skin, excluding skin of color atlases. In a comparison of specialties, dermatology materials included the most images of medium and darker skin tones.
The underrepresentation of skin of color patients can contribute to a limited knowledge of lupus presentation that could lead to disparate health outcomes, the researchers noted.
The study findings were limited by several factors, including the review of only the online textbooks and journals available through the medical library of a single university, the researchers noted. In addition, definitions of light, medium, and dark skin tones were variable among studies, and the researchers did not distinguish among lupus pathologies.
“Further research is needed to quantitatively assess the influence these materials have on healthcare providers’ ability to care for patients with lupus and SOC, and new material and strategies will be required to correct this disparity and promote equitable representation,” the researchers emphasized. “Ultimately, this will arm practitioners with the resources to competently treat patients with any skin color and work towards reducing disparities in health outcomes.”
The study received no outside funding. The researchers had no financial conflicts to disclose.
Lupus images in medical resource materials underrepresent patients with skin of color, based on data from a review of more than 1,400 images published between 2014 and 2019 in materials from a university’s online medical library.
Patients with skin of color who develop lupus tend to present earlier and with more severe cases, and often experience worse outcomes, compared with other populations, wrote Amaad Rana, MD, of Washington University, St. Louis, and colleagues. Medical resources in general have historically underrepresented patients of color, and the researchers reviewed lupus materials for a similar publication bias.
In a study published in Arthritis Care & Research, the investigators identified 1,417 images in rheumatology, dermatology, and internal medicine resources, including 119 medical textbooks, 15 medical journals, 2 online image libraries, and the online image collections of Google and UpToDate. An additional 24 images came from skin of color atlases.
Excluding the skin of color atlases, 56.4% of the images represented light skin, 35.1% showed medium skin, and 8.5% showed dark skin. Overall, publishers were more than twice as likely to portray light skin tones and were significantly less likely to portray dark skin tones (odds ratios, 2.59 and 0.19, respectively), compared with an equal representation of skin tones; however, the difference was not significant for portrayal of medium skin tones (OR, 1.08).
By specialty, dermatology was more inclusive of skin of color images than rheumatology or internal medicine, although the internal medicine sample size was too small for comparable analysis, the researchers noted. Dermatology textbooks were 2.42 times more likely and rheumatology textbooks were 4.87 times more likely to depict light skin tones than an equal representation of light, medium, and dark skin tones.
The researchers rated the skin color in the images using the New Immigrant Survey Skin Color Scale and categorized the images as representing light (NISSCS scores, 1-2), medium (NISSCS scores, 3-5), or dark skin (NISSCS scores, 6-10). Medical journals had the most images of dark skin, excluding skin of color atlases. In a comparison of specialties, dermatology materials included the most images of medium and darker skin tones.
The underrepresentation of skin of color patients can contribute to a limited knowledge of lupus presentation that could lead to disparate health outcomes, the researchers noted.
The study findings were limited by several factors, including the review of only the online textbooks and journals available through the medical library of a single university, the researchers noted. In addition, definitions of light, medium, and dark skin tones were variable among studies, and the researchers did not distinguish among lupus pathologies.
“Further research is needed to quantitatively assess the influence these materials have on healthcare providers’ ability to care for patients with lupus and SOC, and new material and strategies will be required to correct this disparity and promote equitable representation,” the researchers emphasized. “Ultimately, this will arm practitioners with the resources to competently treat patients with any skin color and work towards reducing disparities in health outcomes.”
The study received no outside funding. The researchers had no financial conflicts to disclose.
Lupus images in medical resource materials underrepresent patients with skin of color, based on data from a review of more than 1,400 images published between 2014 and 2019 in materials from a university’s online medical library.
Patients with skin of color who develop lupus tend to present earlier and with more severe cases, and often experience worse outcomes, compared with other populations, wrote Amaad Rana, MD, of Washington University, St. Louis, and colleagues. Medical resources in general have historically underrepresented patients of color, and the researchers reviewed lupus materials for a similar publication bias.
In a study published in Arthritis Care & Research, the investigators identified 1,417 images in rheumatology, dermatology, and internal medicine resources, including 119 medical textbooks, 15 medical journals, 2 online image libraries, and the online image collections of Google and UpToDate. An additional 24 images came from skin of color atlases.
Excluding the skin of color atlases, 56.4% of the images represented light skin, 35.1% showed medium skin, and 8.5% showed dark skin. Overall, publishers were more than twice as likely to portray light skin tones and were significantly less likely to portray dark skin tones (odds ratios, 2.59 and 0.19, respectively), compared with an equal representation of skin tones; however, the difference was not significant for portrayal of medium skin tones (OR, 1.08).
By specialty, dermatology was more inclusive of skin of color images than rheumatology or internal medicine, although the internal medicine sample size was too small for comparable analysis, the researchers noted. Dermatology textbooks were 2.42 times more likely and rheumatology textbooks were 4.87 times more likely to depict light skin tones than an equal representation of light, medium, and dark skin tones.
The researchers rated the skin color in the images using the New Immigrant Survey Skin Color Scale and categorized the images as representing light (NISSCS scores, 1-2), medium (NISSCS scores, 3-5), or dark skin (NISSCS scores, 6-10). Medical journals had the most images of dark skin, excluding skin of color atlases. In a comparison of specialties, dermatology materials included the most images of medium and darker skin tones.
The underrepresentation of skin of color patients can contribute to a limited knowledge of lupus presentation that could lead to disparate health outcomes, the researchers noted.
The study findings were limited by several factors, including the review of only the online textbooks and journals available through the medical library of a single university, the researchers noted. In addition, definitions of light, medium, and dark skin tones were variable among studies, and the researchers did not distinguish among lupus pathologies.
“Further research is needed to quantitatively assess the influence these materials have on healthcare providers’ ability to care for patients with lupus and SOC, and new material and strategies will be required to correct this disparity and promote equitable representation,” the researchers emphasized. “Ultimately, this will arm practitioners with the resources to competently treat patients with any skin color and work towards reducing disparities in health outcomes.”
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM ARTHRITIS CARE & RESEARCH
New biomarkers may predict interstitial lung disease progression in patients with systemic sclerosis
Quantitative assessment of the extent of interstitial lung disease in patients with systemic sclerosis and levels of certain proteins in bronchoalveolar lavage samples have potential for predicting mortality and disease progression, according to two analyses of data from the Scleroderma Lung Study I and II.
The analyses, presented at the annual European Congress of Rheumatology, aim to improve current prognostic abilities in patients with systemic sclerosis–interstitial lung disease (SSc-ILD). Although forced vital capacity is commonly used as a biomarker for survival in many SSc-ILD trials, other factors can affect FVC, such as respiratory muscle weakness and skin fibrosis. Further, FVC correlates poorly with patient-reported outcomes, explained first author Elizabeth Volkmann, MD, director of the scleroderma program at the University of California, Los Angeles, and the founder and codirector of the UCLA connective tissue disease–related interstitial lung disease program.
Dr. Volkmann presented two studies that investigated the potential of radiographic and protein biomarkers for predicting mortality and identifying patients at risk for ILD progression. The biomarkers may also help to identify patients who would benefit most from immunosuppressive therapy.
The first study found that tracking the quantitative extent of ILD (QILD) over time with high-resolution CT (HRCT) predicted poorer outcomes and could therefore act as a surrogate endpoint for mortality among patients with SSc-ILD. The other study identified associations between specific proteins from bronchoalveolar lavage (BAL) and the likelihood of ILD progression, although some associations were treatment dependent.
Jacob M. van Laar, MD, PhD, professor of rheumatology at the University Medical Center Utrecht (the Netherlands), who was not involved in the study, found the results intriguing and noted the importance of further validation in research before these biomarkers are considered for clinical use.
“It would be wonderful if we can tailor therapy based on BAL biomarkers in the future, as clinicians often struggle to decide on selection, timing, and duration of immunosuppressive treatment,” Dr. van Laar told this news organization. “This has become even more relevant with the introduction of new drugs such as nintedanib.”
Extent of ILD progression as a surrogate for mortality
Scleroderma Lung Study I involved 158 patients with SSc-ILD who were randomly assigned to receive either cyclophosphamide or placebo for 12 months. Scleroderma Lung Study II included 142 patients with SSc-ILD who were randomly assigned to receive either mycophenolate for 24 months or cyclophosphamide for 12 months followed by placebo for 12 months.
The researchers calculated QILD in the whole lung at baseline, at 12 months in the first trial, and at 24 months in the second trial. However, only 82 participants from the first trial and 90 participants from the second trial underwent HRCT. Demographic and disease characteristics were similar between the two groups on follow-up scans.
Follow-up continued for 12 years for patients in the first trial and 8 years in the second. The researchers compared survival rates between the 41% of participants from the first study and 31% of participants from the second study who had poorer QILD scores (at least a 2% increase) with the participants who had stable or improved scores (less than 2% increase).
Participants from both trials had significantly poorer long-term survival if their QILD scores had increased by at least 2% at follow-up (P = .01 for I; P = .019 for II). The association was no longer significant after adjustment for baseline FVC, age, and modified Rodnan skin score in the first trial (hazard ratio, 1.98; P = .089), but it remained significant for participants of the second trial (HR, 3.86; P = .014).
“Data from two independent trial cohorts demonstrated that radiographic progression of SSc-ILD at 1 and 2 years is associated with worse long-term survival,” Dr. Volkmann told attendees.
However, FVC did not significantly predict risk of mortality in either trial.
“To me, the most striking finding from the first study was that change in QILD performed better as a predictor of survival than change in FVC,” Dr. van Laar said in an interview. “This indicates QILD is fit for purpose and worth including in future clinical trials.”
Limitations of the study included lack of HRCT for all participants in the trials and the difference in timing (1 year and 2 years) of HRCT assessment between the two trials. The greater hazard ratio for worsened QILD in the second trial may suggest that assessment at 2 years provides more reliable data as a biomarker, Dr. Volkmann said.
“QILD may represent a better proxy for how a patient feels, functions, and survives than FVC,” she said.
Treatment-dependent biomarkers for worsening lung fibrosis
In the second study, the researchers looked for any associations between changes in the radiographic extent of SSc-ILD and 68 proteins from BAL.
“Being able to risk-stratify patients with interstitial lung disease at the time of diagnosis and predict which patients are likely to have a stable versus progressive disease course is critical for making important treatment decisions for these patients,” Dr. Volkmann told attendees.
The second study she presented involved Scleroderma Lung Study I. Of the 158 participants, 144 underwent a bronchoscopy, yielding BAL protein samples from 103 participants. The researchers determined the extent of radiographic fibrosis in the whole lung with quantitative imaging analysis of HRCT of the chest at baseline and 12 months.
Although the researchers identified several statistically significant associations between certain proteins and changes in radiographic fibrosis, “baseline protein levels were differentially associated with the course of ILD based on treatment status,” she told attendees.
For example, increased levels of the following proteins were linked to poor radiographic fibrosis scores for patients who received placebo:
- Granulocyte-macrophage colony-stimulating factor
- Interleukin-1
- Monocyte chemoattractant protein–3
- Chemokine ligand–5
- Transforming growth factor–beta
- Hepatocyte growth factor
- Stem cell factor
- IL-4
- TGF-alpha
Yet increases in these proteins predicted improvement in radiographic fibrosis in patients who had taken cyclophosphamide.
Independently of treatment, the researchers also identified an association between higher levels of fractalkine and poorer radiographic fibrosis scores and between higher IL-7 levels and improved radiographic fibrosis scores.
After adjusting for treatment arm and baseline severity of ILD, significant associations remained between change in radiographic fibrosis score and IL-1, MCP-3, surfactant protein C, IL-7 and CCL-5 levels.
“Biomarker discovery is really central to our ability to risk stratify patients with SSc-ILD,” Dr. Volkmann told attendees. “Understanding how biomarkers predict outcomes in treated and untreated patients may improve personalized medicine to patients with SSc-ILD and could also reveal novel treatment targets.”
Dr. van Laar said in an interview that this study’s biggest strength lay in its large sample size and in the comprehensiveness of the biomarkers studied.
“The findings are interesting from a research perspective and potentially relevant for clinical practice, but the utility of measuring biomarkers in BAL should be further studied for predictive value on clinical endpoints,” Dr. van Laar said. “BAL is an invasive procedure [that] is not routinely done.”
The research was funded by the National Institutes of Health. Dr. Volkmann has consulted for Boehringer Ingelheim and received grant funding from Corbus, Forbius, and Kadmon. Dr. van Laar has received grant funding or personal fees from Arthrogen, Arxx Therapeutics, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Gesynta, Leadiant, Merck Sharp & Dohme, Roche, Sanofi, and Thermofisher.
A version of this article first appeared on Medscape.com.
Quantitative assessment of the extent of interstitial lung disease in patients with systemic sclerosis and levels of certain proteins in bronchoalveolar lavage samples have potential for predicting mortality and disease progression, according to two analyses of data from the Scleroderma Lung Study I and II.
The analyses, presented at the annual European Congress of Rheumatology, aim to improve current prognostic abilities in patients with systemic sclerosis–interstitial lung disease (SSc-ILD). Although forced vital capacity is commonly used as a biomarker for survival in many SSc-ILD trials, other factors can affect FVC, such as respiratory muscle weakness and skin fibrosis. Further, FVC correlates poorly with patient-reported outcomes, explained first author Elizabeth Volkmann, MD, director of the scleroderma program at the University of California, Los Angeles, and the founder and codirector of the UCLA connective tissue disease–related interstitial lung disease program.
Dr. Volkmann presented two studies that investigated the potential of radiographic and protein biomarkers for predicting mortality and identifying patients at risk for ILD progression. The biomarkers may also help to identify patients who would benefit most from immunosuppressive therapy.
The first study found that tracking the quantitative extent of ILD (QILD) over time with high-resolution CT (HRCT) predicted poorer outcomes and could therefore act as a surrogate endpoint for mortality among patients with SSc-ILD. The other study identified associations between specific proteins from bronchoalveolar lavage (BAL) and the likelihood of ILD progression, although some associations were treatment dependent.
Jacob M. van Laar, MD, PhD, professor of rheumatology at the University Medical Center Utrecht (the Netherlands), who was not involved in the study, found the results intriguing and noted the importance of further validation in research before these biomarkers are considered for clinical use.
“It would be wonderful if we can tailor therapy based on BAL biomarkers in the future, as clinicians often struggle to decide on selection, timing, and duration of immunosuppressive treatment,” Dr. van Laar told this news organization. “This has become even more relevant with the introduction of new drugs such as nintedanib.”
Extent of ILD progression as a surrogate for mortality
Scleroderma Lung Study I involved 158 patients with SSc-ILD who were randomly assigned to receive either cyclophosphamide or placebo for 12 months. Scleroderma Lung Study II included 142 patients with SSc-ILD who were randomly assigned to receive either mycophenolate for 24 months or cyclophosphamide for 12 months followed by placebo for 12 months.
The researchers calculated QILD in the whole lung at baseline, at 12 months in the first trial, and at 24 months in the second trial. However, only 82 participants from the first trial and 90 participants from the second trial underwent HRCT. Demographic and disease characteristics were similar between the two groups on follow-up scans.
Follow-up continued for 12 years for patients in the first trial and 8 years in the second. The researchers compared survival rates between the 41% of participants from the first study and 31% of participants from the second study who had poorer QILD scores (at least a 2% increase) with the participants who had stable or improved scores (less than 2% increase).
Participants from both trials had significantly poorer long-term survival if their QILD scores had increased by at least 2% at follow-up (P = .01 for I; P = .019 for II). The association was no longer significant after adjustment for baseline FVC, age, and modified Rodnan skin score in the first trial (hazard ratio, 1.98; P = .089), but it remained significant for participants of the second trial (HR, 3.86; P = .014).
“Data from two independent trial cohorts demonstrated that radiographic progression of SSc-ILD at 1 and 2 years is associated with worse long-term survival,” Dr. Volkmann told attendees.
However, FVC did not significantly predict risk of mortality in either trial.
“To me, the most striking finding from the first study was that change in QILD performed better as a predictor of survival than change in FVC,” Dr. van Laar said in an interview. “This indicates QILD is fit for purpose and worth including in future clinical trials.”
Limitations of the study included lack of HRCT for all participants in the trials and the difference in timing (1 year and 2 years) of HRCT assessment between the two trials. The greater hazard ratio for worsened QILD in the second trial may suggest that assessment at 2 years provides more reliable data as a biomarker, Dr. Volkmann said.
“QILD may represent a better proxy for how a patient feels, functions, and survives than FVC,” she said.
Treatment-dependent biomarkers for worsening lung fibrosis
In the second study, the researchers looked for any associations between changes in the radiographic extent of SSc-ILD and 68 proteins from BAL.
“Being able to risk-stratify patients with interstitial lung disease at the time of diagnosis and predict which patients are likely to have a stable versus progressive disease course is critical for making important treatment decisions for these patients,” Dr. Volkmann told attendees.
The second study she presented involved Scleroderma Lung Study I. Of the 158 participants, 144 underwent a bronchoscopy, yielding BAL protein samples from 103 participants. The researchers determined the extent of radiographic fibrosis in the whole lung with quantitative imaging analysis of HRCT of the chest at baseline and 12 months.
Although the researchers identified several statistically significant associations between certain proteins and changes in radiographic fibrosis, “baseline protein levels were differentially associated with the course of ILD based on treatment status,” she told attendees.
For example, increased levels of the following proteins were linked to poor radiographic fibrosis scores for patients who received placebo:
- Granulocyte-macrophage colony-stimulating factor
- Interleukin-1
- Monocyte chemoattractant protein–3
- Chemokine ligand–5
- Transforming growth factor–beta
- Hepatocyte growth factor
- Stem cell factor
- IL-4
- TGF-alpha
Yet increases in these proteins predicted improvement in radiographic fibrosis in patients who had taken cyclophosphamide.
Independently of treatment, the researchers also identified an association between higher levels of fractalkine and poorer radiographic fibrosis scores and between higher IL-7 levels and improved radiographic fibrosis scores.
After adjusting for treatment arm and baseline severity of ILD, significant associations remained between change in radiographic fibrosis score and IL-1, MCP-3, surfactant protein C, IL-7 and CCL-5 levels.
“Biomarker discovery is really central to our ability to risk stratify patients with SSc-ILD,” Dr. Volkmann told attendees. “Understanding how biomarkers predict outcomes in treated and untreated patients may improve personalized medicine to patients with SSc-ILD and could also reveal novel treatment targets.”
Dr. van Laar said in an interview that this study’s biggest strength lay in its large sample size and in the comprehensiveness of the biomarkers studied.
“The findings are interesting from a research perspective and potentially relevant for clinical practice, but the utility of measuring biomarkers in BAL should be further studied for predictive value on clinical endpoints,” Dr. van Laar said. “BAL is an invasive procedure [that] is not routinely done.”
The research was funded by the National Institutes of Health. Dr. Volkmann has consulted for Boehringer Ingelheim and received grant funding from Corbus, Forbius, and Kadmon. Dr. van Laar has received grant funding or personal fees from Arthrogen, Arxx Therapeutics, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Gesynta, Leadiant, Merck Sharp & Dohme, Roche, Sanofi, and Thermofisher.
A version of this article first appeared on Medscape.com.
Quantitative assessment of the extent of interstitial lung disease in patients with systemic sclerosis and levels of certain proteins in bronchoalveolar lavage samples have potential for predicting mortality and disease progression, according to two analyses of data from the Scleroderma Lung Study I and II.
The analyses, presented at the annual European Congress of Rheumatology, aim to improve current prognostic abilities in patients with systemic sclerosis–interstitial lung disease (SSc-ILD). Although forced vital capacity is commonly used as a biomarker for survival in many SSc-ILD trials, other factors can affect FVC, such as respiratory muscle weakness and skin fibrosis. Further, FVC correlates poorly with patient-reported outcomes, explained first author Elizabeth Volkmann, MD, director of the scleroderma program at the University of California, Los Angeles, and the founder and codirector of the UCLA connective tissue disease–related interstitial lung disease program.
Dr. Volkmann presented two studies that investigated the potential of radiographic and protein biomarkers for predicting mortality and identifying patients at risk for ILD progression. The biomarkers may also help to identify patients who would benefit most from immunosuppressive therapy.
The first study found that tracking the quantitative extent of ILD (QILD) over time with high-resolution CT (HRCT) predicted poorer outcomes and could therefore act as a surrogate endpoint for mortality among patients with SSc-ILD. The other study identified associations between specific proteins from bronchoalveolar lavage (BAL) and the likelihood of ILD progression, although some associations were treatment dependent.
Jacob M. van Laar, MD, PhD, professor of rheumatology at the University Medical Center Utrecht (the Netherlands), who was not involved in the study, found the results intriguing and noted the importance of further validation in research before these biomarkers are considered for clinical use.
“It would be wonderful if we can tailor therapy based on BAL biomarkers in the future, as clinicians often struggle to decide on selection, timing, and duration of immunosuppressive treatment,” Dr. van Laar told this news organization. “This has become even more relevant with the introduction of new drugs such as nintedanib.”
Extent of ILD progression as a surrogate for mortality
Scleroderma Lung Study I involved 158 patients with SSc-ILD who were randomly assigned to receive either cyclophosphamide or placebo for 12 months. Scleroderma Lung Study II included 142 patients with SSc-ILD who were randomly assigned to receive either mycophenolate for 24 months or cyclophosphamide for 12 months followed by placebo for 12 months.
The researchers calculated QILD in the whole lung at baseline, at 12 months in the first trial, and at 24 months in the second trial. However, only 82 participants from the first trial and 90 participants from the second trial underwent HRCT. Demographic and disease characteristics were similar between the two groups on follow-up scans.
Follow-up continued for 12 years for patients in the first trial and 8 years in the second. The researchers compared survival rates between the 41% of participants from the first study and 31% of participants from the second study who had poorer QILD scores (at least a 2% increase) with the participants who had stable or improved scores (less than 2% increase).
Participants from both trials had significantly poorer long-term survival if their QILD scores had increased by at least 2% at follow-up (P = .01 for I; P = .019 for II). The association was no longer significant after adjustment for baseline FVC, age, and modified Rodnan skin score in the first trial (hazard ratio, 1.98; P = .089), but it remained significant for participants of the second trial (HR, 3.86; P = .014).
“Data from two independent trial cohorts demonstrated that radiographic progression of SSc-ILD at 1 and 2 years is associated with worse long-term survival,” Dr. Volkmann told attendees.
However, FVC did not significantly predict risk of mortality in either trial.
“To me, the most striking finding from the first study was that change in QILD performed better as a predictor of survival than change in FVC,” Dr. van Laar said in an interview. “This indicates QILD is fit for purpose and worth including in future clinical trials.”
Limitations of the study included lack of HRCT for all participants in the trials and the difference in timing (1 year and 2 years) of HRCT assessment between the two trials. The greater hazard ratio for worsened QILD in the second trial may suggest that assessment at 2 years provides more reliable data as a biomarker, Dr. Volkmann said.
“QILD may represent a better proxy for how a patient feels, functions, and survives than FVC,” she said.
Treatment-dependent biomarkers for worsening lung fibrosis
In the second study, the researchers looked for any associations between changes in the radiographic extent of SSc-ILD and 68 proteins from BAL.
“Being able to risk-stratify patients with interstitial lung disease at the time of diagnosis and predict which patients are likely to have a stable versus progressive disease course is critical for making important treatment decisions for these patients,” Dr. Volkmann told attendees.
The second study she presented involved Scleroderma Lung Study I. Of the 158 participants, 144 underwent a bronchoscopy, yielding BAL protein samples from 103 participants. The researchers determined the extent of radiographic fibrosis in the whole lung with quantitative imaging analysis of HRCT of the chest at baseline and 12 months.
Although the researchers identified several statistically significant associations between certain proteins and changes in radiographic fibrosis, “baseline protein levels were differentially associated with the course of ILD based on treatment status,” she told attendees.
For example, increased levels of the following proteins were linked to poor radiographic fibrosis scores for patients who received placebo:
- Granulocyte-macrophage colony-stimulating factor
- Interleukin-1
- Monocyte chemoattractant protein–3
- Chemokine ligand–5
- Transforming growth factor–beta
- Hepatocyte growth factor
- Stem cell factor
- IL-4
- TGF-alpha
Yet increases in these proteins predicted improvement in radiographic fibrosis in patients who had taken cyclophosphamide.
Independently of treatment, the researchers also identified an association between higher levels of fractalkine and poorer radiographic fibrosis scores and between higher IL-7 levels and improved radiographic fibrosis scores.
After adjusting for treatment arm and baseline severity of ILD, significant associations remained between change in radiographic fibrosis score and IL-1, MCP-3, surfactant protein C, IL-7 and CCL-5 levels.
“Biomarker discovery is really central to our ability to risk stratify patients with SSc-ILD,” Dr. Volkmann told attendees. “Understanding how biomarkers predict outcomes in treated and untreated patients may improve personalized medicine to patients with SSc-ILD and could also reveal novel treatment targets.”
Dr. van Laar said in an interview that this study’s biggest strength lay in its large sample size and in the comprehensiveness of the biomarkers studied.
“The findings are interesting from a research perspective and potentially relevant for clinical practice, but the utility of measuring biomarkers in BAL should be further studied for predictive value on clinical endpoints,” Dr. van Laar said. “BAL is an invasive procedure [that] is not routinely done.”
The research was funded by the National Institutes of Health. Dr. Volkmann has consulted for Boehringer Ingelheim and received grant funding from Corbus, Forbius, and Kadmon. Dr. van Laar has received grant funding or personal fees from Arthrogen, Arxx Therapeutics, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Gesynta, Leadiant, Merck Sharp & Dohme, Roche, Sanofi, and Thermofisher.
A version of this article first appeared on Medscape.com.
Nivolumab-Induced Granuloma Annulare
Granuloma annulare (GA) is a benign, cutaneous, granulomatous disease of unclear etiology. Typically, GA presents in young adults as asymptomatic, annular, flesh-colored to pink papules and plaques, commonly on the upper and lower extremities. Histologically, GA is characterized by mucin deposition, palisading or an interstitial granulomatous pattern, and collagen and elastic fiber degeneration.1
Granuloma annulare has been associated with various medications and medical conditions, including diabetes mellitus, hyperlipidemia, thyroid disease, and HIV.1 More recently, immune-checkpoint inhibitors (ICIs) have been reported to trigger GA.2 We report a case of nivolumab-induced GA in a 54-year-old woman.
Case Report
A 54-year-old woman presented with an itchy rash on the upper extremities, face, and chest of 4 months’ duration. The patient noted that the rash started on the hands and progressed to include the arms, face, and chest. She also reported associated mild tenderness. She had a history of stage IV non–small-cell lung carcinoma with metastases to the ribs and adrenal glands. She had been started on biweekly intravenous infusions of the ICI nivolumab by her oncologist approximately 1 year prior to the current presentation after failing a course of conventional chemotherapy. The most recent positron emission tomography–computed tomography scan 1 month prior to presentation showed a stable lung mass with radiologic disappearance of metastases, indicating a favorable response to nivolumab. The patient also had a history of hypothyroidism and depression, which were treated with oral levothyroxine 75 μg once daily and oral sertraline 50 mg once daily, respectively, both for longer than 5 years.
Physical examination revealed annular, erythematous, flat-topped papules, some with surmounting fine scale, coalescing into larger plaques along the dorsal surface of the hands and arms (Figure 1) as well as the forehead and chest. A biopsy of a papule on the dorsal aspect of the left hand revealed nodules of histiocytes admixed with Langerhans giant cells within the dermis; mucin was noted centrally within some nodules (Figure 2). Periodic acid–Schiff staining was negative for fungal elements compared to control. Polarization of the specimen was negative for foreign bodies. The biopsy findings therefore were consistent with a diagnosis of GA.
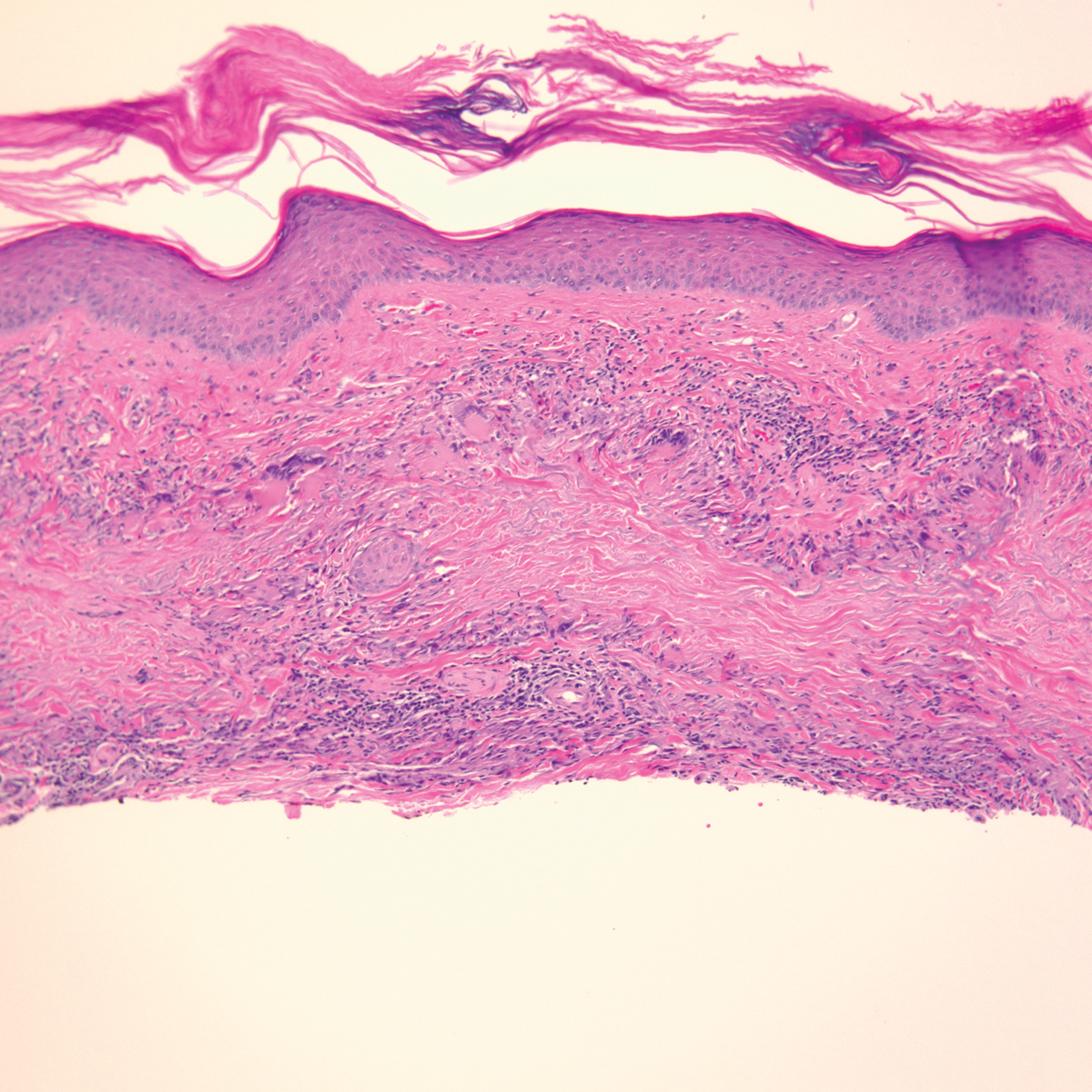
A 3-month treatment course of betamethasone dipropionate 0.05% cream twice daily failed. Narrowband UVB phototherapy was then initiated at 3 sessions weekly. The eruption of GA improved after 3 months of phototherapy. Subsequently, the patient was lost to follow-up.
Comment
Discovery of specific immune checkpoints in tumor-induced immunosuppression revolutionized oncologic therapy. An example is the programmed cell-death protein 1 (PD-1) receptor that is expressed on activated immune cells, including T cells and macrophages.3,4 Upon binding to the PD-1 ligand (PD-L1), T-cell proliferation is inhibited, resulting in downregulation of the immune response. As a result, tumor cells have evolved to overexpress PD-L1 to evade immunologic detection.3 Nivolumab, a fully human IgG4 antibody to PD-1, has emerged along with other ICIs as effective treatments for numerous cancers, including melanoma and non–small-cell lung cancer. By disrupting downregulation of T cells, ICIs improve immune-mediated antitumor activity.3
However, the resulting immunologic disturbance by ICIs has been reported to induce various cutaneous and systemic immune-mediated adverse reactions, including granulomatous reactions such as sarcoidosis, GA, and a cutaneous sarcoidlike granulomatous reaction.1,2,5,6 Our patient represents a rare case of nivolumab-induced GA.
Recent evidence suggests that GA might be caused in part by a cell-mediated hypersensitivity reaction that is regulated by a helper T cell subset 1 inflammatory reaction. Through release of cytokines by activated CD4+ T cells, macrophages are recruited, forming the granulomatous pattern and secreting enzymes that can degrade connective tissue. Nivolumab and other ICIs can thus trigger this reaction because their blockade of PD-1 enhances T cell–mediated immune reactions.2 In addition, because macrophages themselves also express PD-1, ICIs can directly enhance macrophage recruitment and proliferation, further increasing the risk of a granulomatous reaction.4
Interestingly, cutaneous adverse reactions to nivolumab have been associated with improved survival in melanoma patients.7 The nature of this association with granulomatous reactions in general and with GA specifically remains to be determined.
Conclusion
Since the approval of the first PD-1 inhibitors, pembrolizumab and nivolumab, in 2014, other ICIs targeting the immune checkpoint pathway have been developed. Newer agents targeting PD-L1 (avelumab, atezolizumab, and durvalumab) were recently approved. Additionally, cemiplimab, another PD-1 inhibitor, was approved by the US Food and Drug Administration in 2018 for the treatment of advanced cutaneous squamous cell carcinoma.8 Indications for all ICIs also have expanded considerably.3 Therefore, the incidence of immune-mediated adverse reactions, including GA, is bound to increase. Physicians should be cognizant of this association to accurately diagnose and effectively treat adverse reactions in patients who are taking ICIs.
- Piette EW, Rosenbach M. Granuloma annulare: pathogenesis, disease associations and triggers, and therapeutic options. J Am Acad Dermatol. 2016;75:467-479. doi:10.1016/j.jaad.2015.03.055
- Wu J, Kwong BY, Martires KJ, et al. Granuloma annulare associated with immune checkpoint inhibitors. J Eur Acad Dermatol. 2018;32:E124-E126. doi:10.1111/jdv.14617
- Gong J, Chehrazi-Raffle A, Reddi S, et al. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6:8. doi:10.1186/s40425-018-0316-z
- Gordon SR, Maute RL, Dulken BW, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495-499. doi:10.1038/nature22396
- Birnbaum MR, Ma MW, Fleisig S, et al. Nivolumab-related cutaneous sarcoidosis in a patient with lung adenocarcinoma. JAAD Case Rep. 2017;3:208-211. doi:10.1016/j.jdcr.2017.02.015
- Danlos F-X, Pagès C, Baroudjian B, et al. Nivolumab-induced sarcoid-like granulomatous reaction in a patient with advanced melanoma. Chest. 2016;149:E133-E136. doi:10.1016/j.chest.2015.10.082
- Freeman-Keller M, Kim Y, Cronin H, et al. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2016;22:886-894. doi:10.1158/1078-0432.CCR-15-1136
- Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379:341-351. doi:10.1056/NEJMoa1805131
Granuloma annulare (GA) is a benign, cutaneous, granulomatous disease of unclear etiology. Typically, GA presents in young adults as asymptomatic, annular, flesh-colored to pink papules and plaques, commonly on the upper and lower extremities. Histologically, GA is characterized by mucin deposition, palisading or an interstitial granulomatous pattern, and collagen and elastic fiber degeneration.1
Granuloma annulare has been associated with various medications and medical conditions, including diabetes mellitus, hyperlipidemia, thyroid disease, and HIV.1 More recently, immune-checkpoint inhibitors (ICIs) have been reported to trigger GA.2 We report a case of nivolumab-induced GA in a 54-year-old woman.
Case Report
A 54-year-old woman presented with an itchy rash on the upper extremities, face, and chest of 4 months’ duration. The patient noted that the rash started on the hands and progressed to include the arms, face, and chest. She also reported associated mild tenderness. She had a history of stage IV non–small-cell lung carcinoma with metastases to the ribs and adrenal glands. She had been started on biweekly intravenous infusions of the ICI nivolumab by her oncologist approximately 1 year prior to the current presentation after failing a course of conventional chemotherapy. The most recent positron emission tomography–computed tomography scan 1 month prior to presentation showed a stable lung mass with radiologic disappearance of metastases, indicating a favorable response to nivolumab. The patient also had a history of hypothyroidism and depression, which were treated with oral levothyroxine 75 μg once daily and oral sertraline 50 mg once daily, respectively, both for longer than 5 years.
Physical examination revealed annular, erythematous, flat-topped papules, some with surmounting fine scale, coalescing into larger plaques along the dorsal surface of the hands and arms (Figure 1) as well as the forehead and chest. A biopsy of a papule on the dorsal aspect of the left hand revealed nodules of histiocytes admixed with Langerhans giant cells within the dermis; mucin was noted centrally within some nodules (Figure 2). Periodic acid–Schiff staining was negative for fungal elements compared to control. Polarization of the specimen was negative for foreign bodies. The biopsy findings therefore were consistent with a diagnosis of GA.


A 3-month treatment course of betamethasone dipropionate 0.05% cream twice daily failed. Narrowband UVB phototherapy was then initiated at 3 sessions weekly. The eruption of GA improved after 3 months of phototherapy. Subsequently, the patient was lost to follow-up.
Comment
Discovery of specific immune checkpoints in tumor-induced immunosuppression revolutionized oncologic therapy. An example is the programmed cell-death protein 1 (PD-1) receptor that is expressed on activated immune cells, including T cells and macrophages.3,4 Upon binding to the PD-1 ligand (PD-L1), T-cell proliferation is inhibited, resulting in downregulation of the immune response. As a result, tumor cells have evolved to overexpress PD-L1 to evade immunologic detection.3 Nivolumab, a fully human IgG4 antibody to PD-1, has emerged along with other ICIs as effective treatments for numerous cancers, including melanoma and non–small-cell lung cancer. By disrupting downregulation of T cells, ICIs improve immune-mediated antitumor activity.3
However, the resulting immunologic disturbance by ICIs has been reported to induce various cutaneous and systemic immune-mediated adverse reactions, including granulomatous reactions such as sarcoidosis, GA, and a cutaneous sarcoidlike granulomatous reaction.1,2,5,6 Our patient represents a rare case of nivolumab-induced GA.
Recent evidence suggests that GA might be caused in part by a cell-mediated hypersensitivity reaction that is regulated by a helper T cell subset 1 inflammatory reaction. Through release of cytokines by activated CD4+ T cells, macrophages are recruited, forming the granulomatous pattern and secreting enzymes that can degrade connective tissue. Nivolumab and other ICIs can thus trigger this reaction because their blockade of PD-1 enhances T cell–mediated immune reactions.2 In addition, because macrophages themselves also express PD-1, ICIs can directly enhance macrophage recruitment and proliferation, further increasing the risk of a granulomatous reaction.4
Interestingly, cutaneous adverse reactions to nivolumab have been associated with improved survival in melanoma patients.7 The nature of this association with granulomatous reactions in general and with GA specifically remains to be determined.
Conclusion
Since the approval of the first PD-1 inhibitors, pembrolizumab and nivolumab, in 2014, other ICIs targeting the immune checkpoint pathway have been developed. Newer agents targeting PD-L1 (avelumab, atezolizumab, and durvalumab) were recently approved. Additionally, cemiplimab, another PD-1 inhibitor, was approved by the US Food and Drug Administration in 2018 for the treatment of advanced cutaneous squamous cell carcinoma.8 Indications for all ICIs also have expanded considerably.3 Therefore, the incidence of immune-mediated adverse reactions, including GA, is bound to increase. Physicians should be cognizant of this association to accurately diagnose and effectively treat adverse reactions in patients who are taking ICIs.
Granuloma annulare (GA) is a benign, cutaneous, granulomatous disease of unclear etiology. Typically, GA presents in young adults as asymptomatic, annular, flesh-colored to pink papules and plaques, commonly on the upper and lower extremities. Histologically, GA is characterized by mucin deposition, palisading or an interstitial granulomatous pattern, and collagen and elastic fiber degeneration.1
Granuloma annulare has been associated with various medications and medical conditions, including diabetes mellitus, hyperlipidemia, thyroid disease, and HIV.1 More recently, immune-checkpoint inhibitors (ICIs) have been reported to trigger GA.2 We report a case of nivolumab-induced GA in a 54-year-old woman.
Case Report
A 54-year-old woman presented with an itchy rash on the upper extremities, face, and chest of 4 months’ duration. The patient noted that the rash started on the hands and progressed to include the arms, face, and chest. She also reported associated mild tenderness. She had a history of stage IV non–small-cell lung carcinoma with metastases to the ribs and adrenal glands. She had been started on biweekly intravenous infusions of the ICI nivolumab by her oncologist approximately 1 year prior to the current presentation after failing a course of conventional chemotherapy. The most recent positron emission tomography–computed tomography scan 1 month prior to presentation showed a stable lung mass with radiologic disappearance of metastases, indicating a favorable response to nivolumab. The patient also had a history of hypothyroidism and depression, which were treated with oral levothyroxine 75 μg once daily and oral sertraline 50 mg once daily, respectively, both for longer than 5 years.
Physical examination revealed annular, erythematous, flat-topped papules, some with surmounting fine scale, coalescing into larger plaques along the dorsal surface of the hands and arms (Figure 1) as well as the forehead and chest. A biopsy of a papule on the dorsal aspect of the left hand revealed nodules of histiocytes admixed with Langerhans giant cells within the dermis; mucin was noted centrally within some nodules (Figure 2). Periodic acid–Schiff staining was negative for fungal elements compared to control. Polarization of the specimen was negative for foreign bodies. The biopsy findings therefore were consistent with a diagnosis of GA.


A 3-month treatment course of betamethasone dipropionate 0.05% cream twice daily failed. Narrowband UVB phototherapy was then initiated at 3 sessions weekly. The eruption of GA improved after 3 months of phototherapy. Subsequently, the patient was lost to follow-up.
Comment
Discovery of specific immune checkpoints in tumor-induced immunosuppression revolutionized oncologic therapy. An example is the programmed cell-death protein 1 (PD-1) receptor that is expressed on activated immune cells, including T cells and macrophages.3,4 Upon binding to the PD-1 ligand (PD-L1), T-cell proliferation is inhibited, resulting in downregulation of the immune response. As a result, tumor cells have evolved to overexpress PD-L1 to evade immunologic detection.3 Nivolumab, a fully human IgG4 antibody to PD-1, has emerged along with other ICIs as effective treatments for numerous cancers, including melanoma and non–small-cell lung cancer. By disrupting downregulation of T cells, ICIs improve immune-mediated antitumor activity.3
However, the resulting immunologic disturbance by ICIs has been reported to induce various cutaneous and systemic immune-mediated adverse reactions, including granulomatous reactions such as sarcoidosis, GA, and a cutaneous sarcoidlike granulomatous reaction.1,2,5,6 Our patient represents a rare case of nivolumab-induced GA.
Recent evidence suggests that GA might be caused in part by a cell-mediated hypersensitivity reaction that is regulated by a helper T cell subset 1 inflammatory reaction. Through release of cytokines by activated CD4+ T cells, macrophages are recruited, forming the granulomatous pattern and secreting enzymes that can degrade connective tissue. Nivolumab and other ICIs can thus trigger this reaction because their blockade of PD-1 enhances T cell–mediated immune reactions.2 In addition, because macrophages themselves also express PD-1, ICIs can directly enhance macrophage recruitment and proliferation, further increasing the risk of a granulomatous reaction.4
Interestingly, cutaneous adverse reactions to nivolumab have been associated with improved survival in melanoma patients.7 The nature of this association with granulomatous reactions in general and with GA specifically remains to be determined.
Conclusion
Since the approval of the first PD-1 inhibitors, pembrolizumab and nivolumab, in 2014, other ICIs targeting the immune checkpoint pathway have been developed. Newer agents targeting PD-L1 (avelumab, atezolizumab, and durvalumab) were recently approved. Additionally, cemiplimab, another PD-1 inhibitor, was approved by the US Food and Drug Administration in 2018 for the treatment of advanced cutaneous squamous cell carcinoma.8 Indications for all ICIs also have expanded considerably.3 Therefore, the incidence of immune-mediated adverse reactions, including GA, is bound to increase. Physicians should be cognizant of this association to accurately diagnose and effectively treat adverse reactions in patients who are taking ICIs.
- Piette EW, Rosenbach M. Granuloma annulare: pathogenesis, disease associations and triggers, and therapeutic options. J Am Acad Dermatol. 2016;75:467-479. doi:10.1016/j.jaad.2015.03.055
- Wu J, Kwong BY, Martires KJ, et al. Granuloma annulare associated with immune checkpoint inhibitors. J Eur Acad Dermatol. 2018;32:E124-E126. doi:10.1111/jdv.14617
- Gong J, Chehrazi-Raffle A, Reddi S, et al. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6:8. doi:10.1186/s40425-018-0316-z
- Gordon SR, Maute RL, Dulken BW, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495-499. doi:10.1038/nature22396
- Birnbaum MR, Ma MW, Fleisig S, et al. Nivolumab-related cutaneous sarcoidosis in a patient with lung adenocarcinoma. JAAD Case Rep. 2017;3:208-211. doi:10.1016/j.jdcr.2017.02.015
- Danlos F-X, Pagès C, Baroudjian B, et al. Nivolumab-induced sarcoid-like granulomatous reaction in a patient with advanced melanoma. Chest. 2016;149:E133-E136. doi:10.1016/j.chest.2015.10.082
- Freeman-Keller M, Kim Y, Cronin H, et al. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2016;22:886-894. doi:10.1158/1078-0432.CCR-15-1136
- Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379:341-351. doi:10.1056/NEJMoa1805131
- Piette EW, Rosenbach M. Granuloma annulare: pathogenesis, disease associations and triggers, and therapeutic options. J Am Acad Dermatol. 2016;75:467-479. doi:10.1016/j.jaad.2015.03.055
- Wu J, Kwong BY, Martires KJ, et al. Granuloma annulare associated with immune checkpoint inhibitors. J Eur Acad Dermatol. 2018;32:E124-E126. doi:10.1111/jdv.14617
- Gong J, Chehrazi-Raffle A, Reddi S, et al. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6:8. doi:10.1186/s40425-018-0316-z
- Gordon SR, Maute RL, Dulken BW, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495-499. doi:10.1038/nature22396
- Birnbaum MR, Ma MW, Fleisig S, et al. Nivolumab-related cutaneous sarcoidosis in a patient with lung adenocarcinoma. JAAD Case Rep. 2017;3:208-211. doi:10.1016/j.jdcr.2017.02.015
- Danlos F-X, Pagès C, Baroudjian B, et al. Nivolumab-induced sarcoid-like granulomatous reaction in a patient with advanced melanoma. Chest. 2016;149:E133-E136. doi:10.1016/j.chest.2015.10.082
- Freeman-Keller M, Kim Y, Cronin H, et al. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2016;22:886-894. doi:10.1158/1078-0432.CCR-15-1136
- Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379:341-351. doi:10.1056/NEJMoa1805131
Practice Points
- Immune-related adverse events (irAEs) frequently occur in patients on immunotherapy, with the skin representing the most common site of involvement.
- Although rare, granulomatous reactions such as granuloma annulare increasingly are recognized as potential irAEs.
- Clinicians should be aware of this novel association to accurately diagnose and effectively treat adverse reactions in patients receiving immunotherapy.
Lenabasum missed mark for systemic sclerosis but may show promise for adjunctive therapy
Although a phase 3 trial of lenabasum did not meet its primary endpoint for treatment of diffuse cutaneous systemic sclerosis (dcSSc), the drug led to more improvement in participants who were not receiving background immunosuppressant therapy during the trial than that seen in participants who received the placebo. Lenabasum also had a favorable safety profile, according to findings presented at the annual European Congress of Rheumatology.
The double-blind, randomized, placebo-controlled trial involved 363 adults who had had dcSSc for up to 6 years. One third of the participants received 5 mg of oral lenabasum, one third received 20 mg, and one third received a placebo. Patients already receiving immunosuppressant therapy could continue to receive it during the trial if the dose had been stable for at least 8 weeks before screening and corticosteroid therapy did not exceed 10 mg prednisone per day or the equivalent.
“The decision to allow background immunosuppressant therapies was made to reflect real-world clinical practice,” coprincipal investigator Robert Dr. Spiera, MD, director of the Vasculitis and Scleroderma Program at the Hospital for Special Surgery, New York, told attendees.
“It is surprising that we do not see any added efficacy of lenabasum in this trial, given the fact that the previous phase 2 trial in 42 patients did show a clear benefit of lenabasum over placebo in the same population,” Jeska K. de Vries-Bouwstra, MD, PhD, a rheumatologist at Leiden (the Netherlands) University Medical Center told this news organization. “Even more, the clinical response in the phase 2 study was supported by a greater change in gene expression in skin tissue of pathways involved in inflammation and fibrosis with lenabasum as compared to placebo.”
Background immunosuppressants contribute to unprecedented placebo responses
The researchers compared the ACR CRISS (Combined Response Index in Diffuse Cutaneous Systemic Sclerosis) score and several secondary endpoints at 52 weeks between the 123 participants who received the placebo and the 120 participants who received 20 mg of lenabasum. A total of 60% of the lenabasum group and 66% of the placebo group had a disease duration of 3 or fewer years, and the modified Rodnan skin score (mRSS) was 22 in the lenabasum group and 23.3 in the placebo group at baseline.
A large majority of participants in both groups – 89% in the lenabasum group and 84% in the placebo group – were receiving background immunosuppressant therapy during the trial. Specifically, 53% of each group was taking mycophenolate, and 23% of the lenabasum group and 32% of the placebo group were taking corticosteroids. In addition, 22% of the lenabasum group and 12% of the placebo group were on methotrexate, and 27% of the lenabasum group and 22% of the placebo group were on another immunosuppressant therapy.
Half of the placebo group and 58% of the lenabasum group were taking only one immunosuppressive therapy. About one-third of the lenabasum (32%) and placebo (34%) groups were taking two or more immunosuppressive therapies.
The primary endpoint at 52 weeks was not significantly different between the two groups: a CRISS score of 0.888 in the lenabasum group and 0.887 in the placebo group. A CRISS score of 0.6 or higher indicates likelihood that a patient improved on treatment. Patients with significant worsening of renal or cardiopulmonary involvement are classified as not improved (score of 0), regardless of improvements in other core items.
“We had very high CRISS scores in all three groups, and they were comparable in all three groups,” Dr. Spiera reported. Because improvement in placebo group far exceeded expectations, the researchers were unable to discern the treatment effect of lenabasum on top of the placebo effect.
The placebo group had better outcomes than expected because of the background immunosuppressant therapy, particularly the use of mycophenolate. When the researchers looked only at placebo participants, the CRISS score was 0.936 in the 97 patients receiving background immunosuppressant therapy of any kind and 0.935 in the 29 patients taking only mycophenolate with no other immunosuppressant therapy, compared with 0.417 in the 16 patients not receiving any background therapy.
In a prespecified analysis, the researchers investigated background immunosuppressive therapy as a mediator. The CRISS score for the 10 lenabasum participants not receiving background therapy was 0.811, compared with 0.417 seen in the placebo group patients not on background therapy.
Among the 173 participants taking mycophenolate in particular, the mycophenolate “had a statistically significant improvement on CRISS score that increased with each visit,” Dr. Spiera reported. The duration of mycophenolate therapy also affected efficacy results.
Patients who had been taking mycophenolate longer saw less improvement in their CRISS score over time. Those taking it more than 2 years at baseline had a CRISS score of 0.86, compared with 0.96 for those taking it for 1-2 years at baseline and 0.98 for those taking it from 6 months to 1 year at baseline. Those who had only been taking mycophenolate for up to 6 months at baseline had a CRISS score of 0.99. Meanwhile, patients not taking any background immunosuppressant therapies only had a CRISS score of about 0.35.
Changes in secondary endpoints followed same pattern as CRISS
The secondary endpoints similarly showed no statistically significant difference when comparing the lenabasum and placebo groups overall. These endpoints included change in mRSS score, change in forced vital capacity (FVC) percentage and volume, and change in the Health Assessment Questionnaire Disability Index (HAQ-DI) score.
However, the researchers again found that duration of background therapy affected FVC.
“You were more likely to have declined [in FVC] if you were on placebo and more likely to have improved or stayed stable if you were on lenabasum if you were a patient on more than 2 years of immunomodulatory therapy at baseline,” Dr. Spiera reported. “There was evidence for an effect of lenabasum on FVC suggested by post-hoc analyses that considered the effect of background immunosuppressive therapies on outcomes, but those results would require confirmation in additional studies to determine the potential of lenabasum for treating patients with diffuse cutaneous systemic sclerosis,” Dr. Spiera noted in his conclusions.
Serious adverse events occurred in 9.2% of the lenabasum group and 5.8% of the placebo group. Rates of severe adverse events were similar between the lenabasum (14.6%) and placebo (13%) groups.
Is there a subgroup for whom lenabasum would be efficacious?
Although De Vries-Bouwstra of Leiden University Medical Center acknowledged the role of mycophenolate in the trial, she does not think background therapy can totally explain the observation and speculated on other possibilities.
“For example, there were fewer males in the placebo group as compared to the phase 2 study. From previous cohort studies we know that males have higher risk of worsening of skin disease,” she said. “In addition, it could be worthwhile to evaluate antibody profiles of the population under study; some subpopulations defined by autoantibody have higher risk for skin progression, while others can show spontaneous improvement.”
Dr. De Vries-Bouwstra said that, although it’s not currently appropriate to advocate for lenabasum to treat dcSSc, it may eventually become an additional treatment in those who still show active skin or lung disease after 2 years of mycophenolate treatment if future research identifies a benefit from that application. She would also like to see an evaluation of lenabasum’s possible benefits in patients with very early and active inflammatory disease. “Ideally, one could stratify patients based on biomarkers reflecting activation in relevant pathways, for example by using gene expression analysis from skin tissue to stratify,” she said.
Jacob M. van Laar, MD, PhD, professor of rheumatology at University Medical Center Utrecht (the Netherlands), also commented on the potential differences in using the drug in early versus later disease.
“Based on ex vivo analyses of skin samples from systemic sclerosis patients, one would expect such a mechanism of action to be particularly relevant in very early disease, so the observation that it might also be effective at a later disease stage is interesting,” Dr. van Laar told this news organization. “We still have a lot to learn about this complex disease.”
Given that safety does not appear to be a major concern and that there may be a benefit in a subgroup of patients, Dr. van Laar also said he hoped “the company is not deterred by the seemingly negative result of the primary endpoint.”
Dr. Spiera expressed optimism about what this trial’s findings have revealed about management of dcSSc.
“Independent of what lenabasum did or didn’t do in this trial, I think there’s going to be a lot that we’re going to learn from this trial and that we’re already learning and analyzing right now about treating scleroderma,” he said in an interview.
He reiterated the value of allowing background therapy in the trial to ensure it better replicated real-world clinical practice.
“You’re not withholding therapies that we think are probably active from patients with active disease that, once you incur organ damage, is probably not going to be reversible,” Dr. Spiera said. “The downside is that it makes it harder to see an effect of a drug on top of the background therapy if that background therapy is effective. So what we saw in terms of this absence of benefit from lenabasum really may have been a ceiling effect.”
Nevertheless, Dr. Spiera said the findings still strongly suggest that lenabasum is an active compound.
“It’s not an enormously powerful effect, but it probably has a role as an adjunctive therapy in people on stable background therapy who have either plateaued or are getting worse,” he said. “The thing we have to keep in mind also is this was an incredibly safe therapy. It’s not immunosuppressive.”
The trial was funded by Corbus. Dr. Spiera has received grant support or consulting fees from Roche-Genentech, GlaxoSmithKline, Boehringer Ingelheim, Chemocentryx, Corbus, Formation Biologics, Inflarx, Kadmon, AstraZeneca, AbbVie, CSL Behring, Sanofi, and Janssen. Dr. De Vries-Bouwstra has received consulting fees from AbbVie and Boehringer Ingelheim and research grants from Galapagos and Janssen. Dr. Van Laar has received grant funding or personal fees from Arthrogen, Arxx Therapeutics, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Gesynta, Leadiant, Merck Sharp & Dohme, Roche, Sanofi, and Thermofisher.
A version of this article first appeared on Medscape.com.
Although a phase 3 trial of lenabasum did not meet its primary endpoint for treatment of diffuse cutaneous systemic sclerosis (dcSSc), the drug led to more improvement in participants who were not receiving background immunosuppressant therapy during the trial than that seen in participants who received the placebo. Lenabasum also had a favorable safety profile, according to findings presented at the annual European Congress of Rheumatology.
The double-blind, randomized, placebo-controlled trial involved 363 adults who had had dcSSc for up to 6 years. One third of the participants received 5 mg of oral lenabasum, one third received 20 mg, and one third received a placebo. Patients already receiving immunosuppressant therapy could continue to receive it during the trial if the dose had been stable for at least 8 weeks before screening and corticosteroid therapy did not exceed 10 mg prednisone per day or the equivalent.
“The decision to allow background immunosuppressant therapies was made to reflect real-world clinical practice,” coprincipal investigator Robert Dr. Spiera, MD, director of the Vasculitis and Scleroderma Program at the Hospital for Special Surgery, New York, told attendees.
“It is surprising that we do not see any added efficacy of lenabasum in this trial, given the fact that the previous phase 2 trial in 42 patients did show a clear benefit of lenabasum over placebo in the same population,” Jeska K. de Vries-Bouwstra, MD, PhD, a rheumatologist at Leiden (the Netherlands) University Medical Center told this news organization. “Even more, the clinical response in the phase 2 study was supported by a greater change in gene expression in skin tissue of pathways involved in inflammation and fibrosis with lenabasum as compared to placebo.”
Background immunosuppressants contribute to unprecedented placebo responses
The researchers compared the ACR CRISS (Combined Response Index in Diffuse Cutaneous Systemic Sclerosis) score and several secondary endpoints at 52 weeks between the 123 participants who received the placebo and the 120 participants who received 20 mg of lenabasum. A total of 60% of the lenabasum group and 66% of the placebo group had a disease duration of 3 or fewer years, and the modified Rodnan skin score (mRSS) was 22 in the lenabasum group and 23.3 in the placebo group at baseline.
A large majority of participants in both groups – 89% in the lenabasum group and 84% in the placebo group – were receiving background immunosuppressant therapy during the trial. Specifically, 53% of each group was taking mycophenolate, and 23% of the lenabasum group and 32% of the placebo group were taking corticosteroids. In addition, 22% of the lenabasum group and 12% of the placebo group were on methotrexate, and 27% of the lenabasum group and 22% of the placebo group were on another immunosuppressant therapy.
Half of the placebo group and 58% of the lenabasum group were taking only one immunosuppressive therapy. About one-third of the lenabasum (32%) and placebo (34%) groups were taking two or more immunosuppressive therapies.
The primary endpoint at 52 weeks was not significantly different between the two groups: a CRISS score of 0.888 in the lenabasum group and 0.887 in the placebo group. A CRISS score of 0.6 or higher indicates likelihood that a patient improved on treatment. Patients with significant worsening of renal or cardiopulmonary involvement are classified as not improved (score of 0), regardless of improvements in other core items.
“We had very high CRISS scores in all three groups, and they were comparable in all three groups,” Dr. Spiera reported. Because improvement in placebo group far exceeded expectations, the researchers were unable to discern the treatment effect of lenabasum on top of the placebo effect.
The placebo group had better outcomes than expected because of the background immunosuppressant therapy, particularly the use of mycophenolate. When the researchers looked only at placebo participants, the CRISS score was 0.936 in the 97 patients receiving background immunosuppressant therapy of any kind and 0.935 in the 29 patients taking only mycophenolate with no other immunosuppressant therapy, compared with 0.417 in the 16 patients not receiving any background therapy.
In a prespecified analysis, the researchers investigated background immunosuppressive therapy as a mediator. The CRISS score for the 10 lenabasum participants not receiving background therapy was 0.811, compared with 0.417 seen in the placebo group patients not on background therapy.
Among the 173 participants taking mycophenolate in particular, the mycophenolate “had a statistically significant improvement on CRISS score that increased with each visit,” Dr. Spiera reported. The duration of mycophenolate therapy also affected efficacy results.
Patients who had been taking mycophenolate longer saw less improvement in their CRISS score over time. Those taking it more than 2 years at baseline had a CRISS score of 0.86, compared with 0.96 for those taking it for 1-2 years at baseline and 0.98 for those taking it from 6 months to 1 year at baseline. Those who had only been taking mycophenolate for up to 6 months at baseline had a CRISS score of 0.99. Meanwhile, patients not taking any background immunosuppressant therapies only had a CRISS score of about 0.35.
Changes in secondary endpoints followed same pattern as CRISS
The secondary endpoints similarly showed no statistically significant difference when comparing the lenabasum and placebo groups overall. These endpoints included change in mRSS score, change in forced vital capacity (FVC) percentage and volume, and change in the Health Assessment Questionnaire Disability Index (HAQ-DI) score.
However, the researchers again found that duration of background therapy affected FVC.
“You were more likely to have declined [in FVC] if you were on placebo and more likely to have improved or stayed stable if you were on lenabasum if you were a patient on more than 2 years of immunomodulatory therapy at baseline,” Dr. Spiera reported. “There was evidence for an effect of lenabasum on FVC suggested by post-hoc analyses that considered the effect of background immunosuppressive therapies on outcomes, but those results would require confirmation in additional studies to determine the potential of lenabasum for treating patients with diffuse cutaneous systemic sclerosis,” Dr. Spiera noted in his conclusions.
Serious adverse events occurred in 9.2% of the lenabasum group and 5.8% of the placebo group. Rates of severe adverse events were similar between the lenabasum (14.6%) and placebo (13%) groups.
Is there a subgroup for whom lenabasum would be efficacious?
Although De Vries-Bouwstra of Leiden University Medical Center acknowledged the role of mycophenolate in the trial, she does not think background therapy can totally explain the observation and speculated on other possibilities.
“For example, there were fewer males in the placebo group as compared to the phase 2 study. From previous cohort studies we know that males have higher risk of worsening of skin disease,” she said. “In addition, it could be worthwhile to evaluate antibody profiles of the population under study; some subpopulations defined by autoantibody have higher risk for skin progression, while others can show spontaneous improvement.”
Dr. De Vries-Bouwstra said that, although it’s not currently appropriate to advocate for lenabasum to treat dcSSc, it may eventually become an additional treatment in those who still show active skin or lung disease after 2 years of mycophenolate treatment if future research identifies a benefit from that application. She would also like to see an evaluation of lenabasum’s possible benefits in patients with very early and active inflammatory disease. “Ideally, one could stratify patients based on biomarkers reflecting activation in relevant pathways, for example by using gene expression analysis from skin tissue to stratify,” she said.
Jacob M. van Laar, MD, PhD, professor of rheumatology at University Medical Center Utrecht (the Netherlands), also commented on the potential differences in using the drug in early versus later disease.
“Based on ex vivo analyses of skin samples from systemic sclerosis patients, one would expect such a mechanism of action to be particularly relevant in very early disease, so the observation that it might also be effective at a later disease stage is interesting,” Dr. van Laar told this news organization. “We still have a lot to learn about this complex disease.”
Given that safety does not appear to be a major concern and that there may be a benefit in a subgroup of patients, Dr. van Laar also said he hoped “the company is not deterred by the seemingly negative result of the primary endpoint.”
Dr. Spiera expressed optimism about what this trial’s findings have revealed about management of dcSSc.
“Independent of what lenabasum did or didn’t do in this trial, I think there’s going to be a lot that we’re going to learn from this trial and that we’re already learning and analyzing right now about treating scleroderma,” he said in an interview.
He reiterated the value of allowing background therapy in the trial to ensure it better replicated real-world clinical practice.
“You’re not withholding therapies that we think are probably active from patients with active disease that, once you incur organ damage, is probably not going to be reversible,” Dr. Spiera said. “The downside is that it makes it harder to see an effect of a drug on top of the background therapy if that background therapy is effective. So what we saw in terms of this absence of benefit from lenabasum really may have been a ceiling effect.”
Nevertheless, Dr. Spiera said the findings still strongly suggest that lenabasum is an active compound.
“It’s not an enormously powerful effect, but it probably has a role as an adjunctive therapy in people on stable background therapy who have either plateaued or are getting worse,” he said. “The thing we have to keep in mind also is this was an incredibly safe therapy. It’s not immunosuppressive.”
The trial was funded by Corbus. Dr. Spiera has received grant support or consulting fees from Roche-Genentech, GlaxoSmithKline, Boehringer Ingelheim, Chemocentryx, Corbus, Formation Biologics, Inflarx, Kadmon, AstraZeneca, AbbVie, CSL Behring, Sanofi, and Janssen. Dr. De Vries-Bouwstra has received consulting fees from AbbVie and Boehringer Ingelheim and research grants from Galapagos and Janssen. Dr. Van Laar has received grant funding or personal fees from Arthrogen, Arxx Therapeutics, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Gesynta, Leadiant, Merck Sharp & Dohme, Roche, Sanofi, and Thermofisher.
A version of this article first appeared on Medscape.com.
Although a phase 3 trial of lenabasum did not meet its primary endpoint for treatment of diffuse cutaneous systemic sclerosis (dcSSc), the drug led to more improvement in participants who were not receiving background immunosuppressant therapy during the trial than that seen in participants who received the placebo. Lenabasum also had a favorable safety profile, according to findings presented at the annual European Congress of Rheumatology.
The double-blind, randomized, placebo-controlled trial involved 363 adults who had had dcSSc for up to 6 years. One third of the participants received 5 mg of oral lenabasum, one third received 20 mg, and one third received a placebo. Patients already receiving immunosuppressant therapy could continue to receive it during the trial if the dose had been stable for at least 8 weeks before screening and corticosteroid therapy did not exceed 10 mg prednisone per day or the equivalent.
“The decision to allow background immunosuppressant therapies was made to reflect real-world clinical practice,” coprincipal investigator Robert Dr. Spiera, MD, director of the Vasculitis and Scleroderma Program at the Hospital for Special Surgery, New York, told attendees.
“It is surprising that we do not see any added efficacy of lenabasum in this trial, given the fact that the previous phase 2 trial in 42 patients did show a clear benefit of lenabasum over placebo in the same population,” Jeska K. de Vries-Bouwstra, MD, PhD, a rheumatologist at Leiden (the Netherlands) University Medical Center told this news organization. “Even more, the clinical response in the phase 2 study was supported by a greater change in gene expression in skin tissue of pathways involved in inflammation and fibrosis with lenabasum as compared to placebo.”
Background immunosuppressants contribute to unprecedented placebo responses
The researchers compared the ACR CRISS (Combined Response Index in Diffuse Cutaneous Systemic Sclerosis) score and several secondary endpoints at 52 weeks between the 123 participants who received the placebo and the 120 participants who received 20 mg of lenabasum. A total of 60% of the lenabasum group and 66% of the placebo group had a disease duration of 3 or fewer years, and the modified Rodnan skin score (mRSS) was 22 in the lenabasum group and 23.3 in the placebo group at baseline.
A large majority of participants in both groups – 89% in the lenabasum group and 84% in the placebo group – were receiving background immunosuppressant therapy during the trial. Specifically, 53% of each group was taking mycophenolate, and 23% of the lenabasum group and 32% of the placebo group were taking corticosteroids. In addition, 22% of the lenabasum group and 12% of the placebo group were on methotrexate, and 27% of the lenabasum group and 22% of the placebo group were on another immunosuppressant therapy.
Half of the placebo group and 58% of the lenabasum group were taking only one immunosuppressive therapy. About one-third of the lenabasum (32%) and placebo (34%) groups were taking two or more immunosuppressive therapies.
The primary endpoint at 52 weeks was not significantly different between the two groups: a CRISS score of 0.888 in the lenabasum group and 0.887 in the placebo group. A CRISS score of 0.6 or higher indicates likelihood that a patient improved on treatment. Patients with significant worsening of renal or cardiopulmonary involvement are classified as not improved (score of 0), regardless of improvements in other core items.
“We had very high CRISS scores in all three groups, and they were comparable in all three groups,” Dr. Spiera reported. Because improvement in placebo group far exceeded expectations, the researchers were unable to discern the treatment effect of lenabasum on top of the placebo effect.
The placebo group had better outcomes than expected because of the background immunosuppressant therapy, particularly the use of mycophenolate. When the researchers looked only at placebo participants, the CRISS score was 0.936 in the 97 patients receiving background immunosuppressant therapy of any kind and 0.935 in the 29 patients taking only mycophenolate with no other immunosuppressant therapy, compared with 0.417 in the 16 patients not receiving any background therapy.
In a prespecified analysis, the researchers investigated background immunosuppressive therapy as a mediator. The CRISS score for the 10 lenabasum participants not receiving background therapy was 0.811, compared with 0.417 seen in the placebo group patients not on background therapy.
Among the 173 participants taking mycophenolate in particular, the mycophenolate “had a statistically significant improvement on CRISS score that increased with each visit,” Dr. Spiera reported. The duration of mycophenolate therapy also affected efficacy results.
Patients who had been taking mycophenolate longer saw less improvement in their CRISS score over time. Those taking it more than 2 years at baseline had a CRISS score of 0.86, compared with 0.96 for those taking it for 1-2 years at baseline and 0.98 for those taking it from 6 months to 1 year at baseline. Those who had only been taking mycophenolate for up to 6 months at baseline had a CRISS score of 0.99. Meanwhile, patients not taking any background immunosuppressant therapies only had a CRISS score of about 0.35.
Changes in secondary endpoints followed same pattern as CRISS
The secondary endpoints similarly showed no statistically significant difference when comparing the lenabasum and placebo groups overall. These endpoints included change in mRSS score, change in forced vital capacity (FVC) percentage and volume, and change in the Health Assessment Questionnaire Disability Index (HAQ-DI) score.
However, the researchers again found that duration of background therapy affected FVC.
“You were more likely to have declined [in FVC] if you were on placebo and more likely to have improved or stayed stable if you were on lenabasum if you were a patient on more than 2 years of immunomodulatory therapy at baseline,” Dr. Spiera reported. “There was evidence for an effect of lenabasum on FVC suggested by post-hoc analyses that considered the effect of background immunosuppressive therapies on outcomes, but those results would require confirmation in additional studies to determine the potential of lenabasum for treating patients with diffuse cutaneous systemic sclerosis,” Dr. Spiera noted in his conclusions.
Serious adverse events occurred in 9.2% of the lenabasum group and 5.8% of the placebo group. Rates of severe adverse events were similar between the lenabasum (14.6%) and placebo (13%) groups.
Is there a subgroup for whom lenabasum would be efficacious?
Although De Vries-Bouwstra of Leiden University Medical Center acknowledged the role of mycophenolate in the trial, she does not think background therapy can totally explain the observation and speculated on other possibilities.
“For example, there were fewer males in the placebo group as compared to the phase 2 study. From previous cohort studies we know that males have higher risk of worsening of skin disease,” she said. “In addition, it could be worthwhile to evaluate antibody profiles of the population under study; some subpopulations defined by autoantibody have higher risk for skin progression, while others can show spontaneous improvement.”
Dr. De Vries-Bouwstra said that, although it’s not currently appropriate to advocate for lenabasum to treat dcSSc, it may eventually become an additional treatment in those who still show active skin or lung disease after 2 years of mycophenolate treatment if future research identifies a benefit from that application. She would also like to see an evaluation of lenabasum’s possible benefits in patients with very early and active inflammatory disease. “Ideally, one could stratify patients based on biomarkers reflecting activation in relevant pathways, for example by using gene expression analysis from skin tissue to stratify,” she said.
Jacob M. van Laar, MD, PhD, professor of rheumatology at University Medical Center Utrecht (the Netherlands), also commented on the potential differences in using the drug in early versus later disease.
“Based on ex vivo analyses of skin samples from systemic sclerosis patients, one would expect such a mechanism of action to be particularly relevant in very early disease, so the observation that it might also be effective at a later disease stage is interesting,” Dr. van Laar told this news organization. “We still have a lot to learn about this complex disease.”
Given that safety does not appear to be a major concern and that there may be a benefit in a subgroup of patients, Dr. van Laar also said he hoped “the company is not deterred by the seemingly negative result of the primary endpoint.”
Dr. Spiera expressed optimism about what this trial’s findings have revealed about management of dcSSc.
“Independent of what lenabasum did or didn’t do in this trial, I think there’s going to be a lot that we’re going to learn from this trial and that we’re already learning and analyzing right now about treating scleroderma,” he said in an interview.
He reiterated the value of allowing background therapy in the trial to ensure it better replicated real-world clinical practice.
“You’re not withholding therapies that we think are probably active from patients with active disease that, once you incur organ damage, is probably not going to be reversible,” Dr. Spiera said. “The downside is that it makes it harder to see an effect of a drug on top of the background therapy if that background therapy is effective. So what we saw in terms of this absence of benefit from lenabasum really may have been a ceiling effect.”
Nevertheless, Dr. Spiera said the findings still strongly suggest that lenabasum is an active compound.
“It’s not an enormously powerful effect, but it probably has a role as an adjunctive therapy in people on stable background therapy who have either plateaued or are getting worse,” he said. “The thing we have to keep in mind also is this was an incredibly safe therapy. It’s not immunosuppressive.”
The trial was funded by Corbus. Dr. Spiera has received grant support or consulting fees from Roche-Genentech, GlaxoSmithKline, Boehringer Ingelheim, Chemocentryx, Corbus, Formation Biologics, Inflarx, Kadmon, AstraZeneca, AbbVie, CSL Behring, Sanofi, and Janssen. Dr. De Vries-Bouwstra has received consulting fees from AbbVie and Boehringer Ingelheim and research grants from Galapagos and Janssen. Dr. Van Laar has received grant funding or personal fees from Arthrogen, Arxx Therapeutics, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Gesynta, Leadiant, Merck Sharp & Dohme, Roche, Sanofi, and Thermofisher.
A version of this article first appeared on Medscape.com.
Reexamining the Role of Diet in Dermatology
Within the last decade, almost 3000 articles have been published on the role of diet in the prevention and management of dermatologic conditions. Patients are increasingly interested in—and employing—dietary modifications that may influence skin appearance and aid in the treatment of cutaneous disease.1 It is essential that dermatologists are familiar with existing evidence on the role of diet in dermatology to counsel patients appropriately. Herein, we discuss the compositions of several popular diets and their proposed utility for dermatologic purposes. We highlight the limited literature that exists surrounding this topic and emphasize the need for future, well-designed clinical trials that study the impact of diet on skin disease.
Ketogenic Diet
The ketogenic diet has a macronutrient profile composed of high fat, low to moderate protein, and very low carbohydrates. Nutritional ketosis occurs as the body begins to use free fatty acids (via beta oxidation) as the primary metabolite driving cellular metabolism. It has been suggested that the ketogenic diet may impart beneficial effects on skin disease; however, limited literature exists on the role of nutritional ketosis in the treatment of dermatologic conditions.
Mechanistically, the ketogenic diet decreases the secretion of insulin and insulinlike growth factor 1, resulting in a reduction of circulating androgens and increased activity of the retinoid X receptor.2 In acne vulgaris, it has been suggested that the ketogenic diet may be beneficial in decreasing androgen-induced sebum production and the overproliferation of keratinocytes.2-7 The ketogenic diet is one of the most rapidly effective dietary strategies for normalizing both insulin and androgens, thus it may theoretically be useful for other metabolic and hormone-dependent skin diseases, such as hidradenitis suppurativa.8,9
The cutaneous manifestations associated with chronic hyperinsulinemia and hyperglycemia are numerous and include acanthosis nigricans, acrochordons, diabetic dermopathy, scleredema diabeticorum, bullosis diabeticorum, keratosis pilaris, and generalized granuloma annulare. There also is an increased risk for bacterial and fungal skin infections associated with hyperglycemic states.10 The ketogenic diet is an effective nonpharmacologic tool for normalizing serum insulin and glucose levels in most patients and may have utility in the aforementioned conditions.11,12 In addition to improving insulin sensitivity, it has been used as a dietary strategy for weight loss.11-15 Because obesity and metabolic syndrome are highly correlated with common skin conditions such as psoriasis, hidradenitis suppurativa, and androgenetic alopecia, there may be a role for employing the ketogenic diet in these patient populations.16,17
Although robust clinical studies on ketogenic diets in skin disease are lacking, a recent single-arm, open-label clinical trial observed benefit in all 37 drug-naïve, overweight patients with chronic plaque psoriasis who underwent a ketogenic weight loss protocol. Significant reductions in psoriasis area and severity index (PASI) score and dermatology life quality index score were reported (P<.001).18 Another study of 30 patients with psoriasis found that a 4-week, low-calorie, ketogenic diet resulted in 50% improvement of PASI scores, 10% weight loss, and a reduction in the proinflammatory cytokines IL-1β and IL-2.19 Despite these results, it is a challenge to tease out if the specific dietary intervention or its associated weight loss was the main driver in these reported improvements in skin disease.
There is mixed evidence on the anti-inflammatory nature of the ketogenic diet, likely due to wide variation in the composition of foods included in individual diets. In many instances, the ketogenic diet is thought to possess considerable antioxidant and anti-inflammatory capabilities. Ketones are known activators of the nuclear factor erythroid 2–related factor 2 pathway, which upregulates the production of glutathione, a major endogenous intracellular antioxidant.20 Additionally, dietary compounds from foods that are encouraged while on the ketogenic diet, such as sulforaphane from broccoli, also are independent activators of nuclear factor erythroid 2–related factor 2.21 Ketones are efficiently utilized by mitochondria, which also may result in the decreased production of reactive oxygen species and lower oxidative stress.22 Moreover, the ketone body β-hydroxybutyrate has demonstrated the ability to reduce proinflammatory IL-1β levels via suppression of nucleotide-binding domain-like receptor protein 3 inflammasome activity.23,24 The activity of IL-1β is known to be elevated in many dermatologic conditions, including juvenile idiopathic arthritis, relapsing polychondritis, Schnitzler syndrome, hidradenitis suppurativa, Behçet disease, and other autoinflammatory syndromes.25 Ketones also have been shown to inhibit the nuclear factor–κB proinflammatory signaling pathway.22,26,27 Overexpression of IL-1β and aberrant activation of nuclear factor–κB are implicated in a variety of inflammatory, autoimmune, and oncologic cutaneous pathologies. The ketogenic diet may prove to be an effective adjunctive treatment for dermatologists to consider in select patient populations.23,24,28-30
For patients with keratinocyte carcinomas, the ketogenic diet may offer the aforementioned anti-inflammatory and antioxidant effects, as well as suppression of the mechanistic target of rapamycin, a major regulator of cell metabolism and proliferation.31,32 Inhibition of mechanistic target of rapamycin activity has been shown to slow tumor growth and reduce the development of squamous cell carcinoma.25,33,34 The ketogenic diet also may exploit the preferential utilization of glucose exhibited by many types of cancer cells, thereby “starving” the tumor of its primary fuel source.35,36 In vitro and animal studies in a variety of cancer types have demonstrated that a ketogenic metabolic state—achieved through the ketogenic diet or fasting—can sensitize tumor cells to chemotherapy and radiation while conferring a protective effect to normal cells.37-40 This recently described phenomenon is known as differential stress resistance, but it has not been studied in keratinocyte malignancies or melanoma to date. Importantly, some basal cell carcinomas and BRAF V600E–mutated melanomas have worsened while on the ketogenic diet, suggesting more data is needed before it can be recommended for all cancer patients.41,42 Furthermore, other skin conditions such as prurigo pigmentosa have been associated with initiation of the ketogenic diet.43
Low FODMAP Diet
Fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) are short-chain carbohydrates that are poorly absorbed, osmotically active, and rapidly fermented by intestinal bacteria.44 The low FODMAP diet has been shown to be efficacious for treatment of irritable bowel syndrome, small intestinal bacterial overgrowth (SIBO), and some cases of inflammatory bowel disease (IBD).44-49 A low FODMAP diet may have potential implications for several dermatologic conditions.
Rosacea has been associated with various gastrointestinal tract disorders including irritable bowel syndrome, SIBO, and IBD.50-54 A single study found that patients with rosacea had a 13-fold increased risk for SIBO.55,56 Treatment of 40 patients with SIBO using rifaximin resulted in complete resolution of rosacea in all patients, with no relapse after a 3-year follow-up period.55 Psoriasis also has been associated with SIBO and IBD.57,58 One small study found that eradication of SIBO in psoriatic patients resulted in improved PASI scores and colorimetric values.59
Although the long-term health consequences of the low FODMAP diet are unknown, further research on such dietary interventions for inflammatory skin conditions is warranted given the mounting evidence of a gut-skin connection and the role of the intestinal microbiome in skin health.50,51
Gluten-Free Diet
Gluten is a protein found in a variety of grains. Although the role of gluten in the pathogenesis of celiac disease and dermatitis herpetiformis is indisputable, the deleterious effects of gluten outside of the context of these diseases remain controversial. There may be a compelling case for eliminating gluten in psoriasis patients with seropositivity for celiac disease. A recent systematic review found a 2.2-fold increased risk for celiac disease in psoriasis patients.60 Antigliadin antibody titers also were found to be positively correlated with psoriatic disease severity.61 In addition, one open-label study found a reduction in PASI scores in 73% of patients with antigliadin antibodies after 3 months on a gluten-free diet compared to those without antibodies; however, the study only included 22 patients.62 Several other small studies have yielded similar results63,64; however, antigliadin antibodies are neither the most sensitive nor specific markers of celiac disease, and additional testing should be completed in any patient who may carry this diagnosis. A survey study by the National Psoriasis Foundation found that the dietary change associated with the greatest skin improvement was removal of gluten and nightshade vegetables in approximately 50% of the 1200 psoriasis patients that responded.65 Case reports of various dermatologic conditions including sarcoidosis, vitiligo, alopecia areata, lichen planus, dermatomyositis, pyoderma gangrenosum, erythema nodosum, leukocytoclastic vasculitis, linear IgA bullous dermatosis, and aphthous ulcerations have reportedly improved with a gluten-free diet; however, this should not be used as primary therapy in patients without celiac disease.66-71 Because gluten-free diets can be expensive and challenging to follow, a formal assessment for celiac disease should be considered before recommendation of this dietary intervention.
Low Histamine Diet
Histamine is a biogenic amine produced by the decarboxylation of the amino acid histidine.72 It is found in several foods in varying amounts. Because bacteria can convert histidine into histamine, many fermented and aged foods such as kimchi, sauerkraut, cheese, and red wine contain high levels of histamine. Individuals who have decreased activity of diamine oxidase (DAO), an enzyme that degrades histamine, may be more susceptible to histamine intolerance.72 The symptoms of histamine intolerance are numerous and include gastrointestinal tract distress, rhinorrhea and nasal congestion, headache, urticaria, flushing, and pruritus. Histamine intolerance can mimic an IgE-mediated food allergy; however, allergy testing is negative in these patients. Unfortunately, there is no laboratory test for histamine intolerance; a double-blind, placebo-controlled food challenge is considered the gold-standard test.72
As it pertains to dermatology, a low histamine diet may play a role in the treatment of certain patients with atopic dermatitis and chronic spontaneous urticaria. One study reported that 17 of 54 (31.5%) atopic patients had higher basal levels of serum histamine compared to controls.73 Another study found that a histamine-free diet led to improvement in both histamine intolerance symptoms and atopic dermatitis disease severity (SCORing atopic dermatitis) in patients with low DAO activity.74 In chronic spontaneous urticaria, a recent systematic review found that in 223 patients placed on a low histamine diet for 3 to 4 weeks, 12% and 44% achieved complete and partial remission, respectively.75 Although treatment response based on a patient’s DAO activity level has not been correlated, a diet low in histamine may prove useful for patients with persistent atopic dermatitis and chronic spontaneous urticaria who have negative food allergy tests and report exacerbation of symptoms after ingestion of histamine-rich foods.76,77
Mediterranean Diet
The Mediterranean diet has been touted as one of the healthiest diets to date, and large randomized clinical trials have demonstrated its effectiveness in weight loss, improving insulin sensitivity, and reducing inflammatory cytokine profiles.78,79 A major criticism of the Mediterranean diet is that it has considerable ambiguity and lacks a precise definition due to the variability of what is consumed in different Mediterranean regions. Generally, the diet emphasizes high consumption of colorful fruits and vegetables, aromatic herbs and spices, olive oil, nuts, and seafood, as well as modest amounts of dairy, eggs, and red meat.80 The anti-inflammatory effects of this diet largely have been attributed to its abundance of polyphenols, carotenoids, monounsaturated fatty acids, and omega-3 polyunsaturated fatty acids (PUFAs).80,81 Examples of polyphenols include resveratrol in red grapes, quercetin in apples and red onions, and curcumin in turmeric, while examples of carotenoids include lycopene in tomatoes and zeaxanthin in dark leafy greens. Oleic acid is a monounsaturated fatty acid present in high concentrations in olive oil, while eicosapentaenoic acid and docosahexaenoic acid are omega-3 PUFAs predominantly found in fish.82
Unfortunately, rigorous clinical trials regarding the Mediterranean diet as it pertains to dermatology have not been undertaken. Numerous observational studies in patients with psoriasis have suggested that close adherence to the Mediterranean diet was associated with improvement in PASI scores.83-86 The National Psoriasis Foundation now recommends a trial of the Mediterranean diet in some patients with psoriasis, emphasizing increased dietary intake of olive oil, fish, and vegetables.87 Adherence to a Mediterranean diet also has been inversely correlated to the severity of acne vulgaris and hidradenitis suppurativa88,89; however, these studies failed to account for the multifactorial risk factors associated with these conditions. Mediterranean diets also may impart a chemopreventive effect, supported by a number of in vivo and in vitro studies demonstrating the inhibition and/or reversal of cutaneous DNA damage induced by UV radiation through supplementation with various phytonutrients and omega-3 PUFAs.81,90-92 Although small case-control studies have found a decreased risk of basal cell carcinoma in those who closely adhered to a Mediterranean diet, more rigorous clinical research is needed.93
Whole-Food, Plant-Based Diet
A whole-food, plant-based (WFPB) diet is another popular dietary approach that consists of eating fruits, vegetables, legumes, nuts, seeds, and grains in their whole natural form.94 This diet discourages all animal products, including red meat, seafood, dairy, and eggs. It is similar to a vegan diet except that it eliminates all highly refined carbohydrates, vegetable oils, and other processed foods.94 Randomized clinical studies have demonstrated the WFPB diet to be effective in the treatment of obesity and metabolic syndrome.95,96
A WFPB diet has been shown to increase the antioxidant capacity of cells, lengthen telomeres, and reduce formation of advanced glycation end products.94,97,98 These benefits may help combat accelerated skin aging, including increased skin permeability, reduced elasticity and hydration, decreased angiogenesis, impaired immune function, and decreased vitamin D synthesis. Accelerated skin aging can result in delayed wound healing and susceptibility to skin tears and ecchymoses and also may promote the development of cutaneous malignancies.99 There remains a lack of clinical data studying a properly formulated WFPB diet in the dermatologic setting.
Paleolithic Diet
The paleolithic (Paleo) diet is an increasingly popular way of eating that attempts to mirror what our ancestors may have consumed between 10,000 and 2.5 million years ago.100 It is similar to the Mediterranean diet but excludes grains, dairy, legumes, and nightshade vegetables. It also calls for elimination of highly processed sugars and oils as well as chemical food additives and preservatives. There is a strict variation of the diet for individuals with autoimmune disease that also excludes eggs, nuts, and seeds, as these can be inflammatory or immunogenic in some patients.100-106 Other variations of the diet exist, including the ketogenic Paleo diet, pegan (Paleo vegan) diet, and lacto-Paleo diet.100 An often cited criticism of the Paleo diet is the low intake of calcium and risk for osteoporosis; however, consumption of calcium-rich foods or a calcium supplement can address this concern.107
Although small clinical studies have found the Paleo diet to be beneficial for various autoimmune diseases, clinical data evaluating the utility of the diet for cutaneous disease is lacking.108,109 Numerous randomized trials have demonstrated the Paleo diet to be effective for weight loss and improving insulin sensitivity and lipid levels.110-116 Thus, the Paleo diet may theoretically serve as a viable adjunct dietary approach to the treatment of cutaneous diseases associated with obesity and metabolic derangement.117
Carnivore Diet
Arguably the most controversial and radical diet is the carnivore diet. As the name implies, the carnivore diet is based on consuming solely animal products. A properly structured carnivore diet emphasizes a “nose-to-tail” eating approach where all parts of the animal including the muscle meats, organs, and fat are consumed. Proponents of the diet cite anthropologic evidence from fossil-stable carbon-13/carbon-12 isotope analyses, craniodental features, and numerous other adaptations that indicate increased consumption of meat during human evolution.118-122 Notably, many early humans ate a carnivore diet, but life span was very short at this time, suggesting the diet may not be as beneficial as has been suggested.
Despite the abundance of anecdotal evidence supporting its use for a variety of chronic conditions, including cutaneous autoimmune disease, there is a virtual absence of high-quality research on the carnivore diet.123-125
The purported benefits of the carnivore diet may be attributed to the consumption of organ meats that contain highly bioavailable essential vitamins and minerals, such as iron, zinc, copper, selenium, thiamine, niacin, folate, vitamin B6, vitamin B12, vitamin A, vitamin D, vitamin K, and choline.126-128 Other dietary compounds that have demonstrated benefit for skin health and are predominantly found in animal foods include carnosine, carnitine, creatine, taurine, coenzyme Q10, and collagen.129-134 Nevertheless, there is no data to recommend the elimination of antioxidant- and micronutrient-dense plant-based foods. Rigorous clinical research evaluating the efficacy and safety of the carnivore diet in dermatologic patients is needed. A carnivore diet should not be undertaken without the assistance of a dietician who can ensure adequate micronutrient and macronutrient support.
Final Thoughts
The adjunctive role of diet in the treatment of skin disease is expanding and becoming more widely accepted among dermatologists. Unfortunately, there remains a lack of randomized controlled trials confirming the efficacy of various dietary interventions in the dermatologic setting. Although evidence-based dietary recommendations currently are limited, it is important for dermatologists to be aware of the varied and nuanced dietary interventions employed by patients.
Ultimately, dietary recommendations must be personalized, considering a patient’s comorbidities, personal beliefs and preferences, and nutrigenetics. The emerging field of dermatonutrigenomics—the study of how dietary compounds interact with one’s genes to influence skin health—may allow for precise dietary recommendations to be made in dermatologic practice. Direct-to-consumer genetic tests targeted toward dermatology patients are already on the market, but their clinical utility awaits validation.1 Because nutritional science is a constantly evolving field, becoming familiar with these popular diets will serve both dermatologists and their patients well.
- Jaros J, Katta R, Shi VY. Dermatonutrigenomics: past, present, and future. Dermatology. 2019;235:164-166.
- Paoli A, Grimaldi K, Toniolo L, et al. Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- Melnik BC, Schmitz G. Role of insulin, insulin-like growth factor-1, hyperglycaemic food and milk consumption in the pathogenesis of acne vulgaris. Exp Dermatol. 2009;18:833-841.
- Smith RN, Mann NJ, Braue A, et al. The effect of a high-protein, low glycemic-load diet versus a conventional, high glycemic-load diet on biochemical parameters associated with acne vulgaris: a randomized, investigator-masked, controlled trial. J Am Acad Dermatol. 2007;57:247-256.
- Smith R, Mann N, Mäkeläinen H, et al. A pilot study to determine the short-term effects of a low glycemic load diet on hormonal markers of acne: a nonrandomized, parallel, controlled feeding trial. Mol Nutr Food Res. 2008;52:718-726.
- Smith RN, Braue A, Varigos GA, et al. The effect of a low glycemic load diet on acne vulgaris and the fatty acid composition of skin surface triglycerides. J Dermatol Sci. 2008;50:41-52.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Khandalavala BN, Do MV. Finasteride in hidradenitis suppurativa: a "male" therapy for a predominantly "female" disease. J Clin Aesthet Dermatol. 2016;9:44.
- Nikolakis G, Karagiannidis I, Vaiopoulos AG, et al. Endocrinological mechanisms in the pathophysiology of hidradenitis suppurativa [in German]. Hautarzt. 2020;71:762-771.
- Karadag AS, Ozlu E, Lavery MJ. Cutaneous manifestations of diabetes mellitus and the metabolic syndrome. Clin Dermatology. 2018;36:89-93.
- Gardner CD, Kiazand A, Alhassan S, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA. 2007;297:969-977.
- Anton SD, Hida A, Heekin K, et al. Effects of popular diets without specific calorie targets on weight loss outcomes: systematic review of findings from clinical trials. Nutrients. 2017;9:822.
- Castellana M, Conte E, Cignarelli A, et al. Efficacy and safety of very low calorie ketogenic diet (VLCKD) in patients with overweight and obesity: a systematic review and meta-analysis. Rev Endocr Metab Disord. 2020;21:5-16.
- Paoli A, Mancin L, Giacona MC, et al. Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. J Transl Med. 2020;18:104.
- Dashti HM, Mathew TC, Hussein T, et al. Long-term effects of a ketogenic diet in obese patients. Exp Clin Cardiol. 2004;9:200-205.
- Lian N, Chen M. Metabolic syndrome and skin disease: potential connection and risk. Int J Dermatol Venereol. 2019;2:89-93.
- Hu Y, Zhu Y, Lian N, et al. Metabolic syndrome and skin diseases. Front Endocrinol (Lausanne). 2019;10:788.
- Castaldo G, Rastrelli L, Galdo G, et al. Aggressive weight-loss program with a ketogenic induction phase for the treatment of chronic plaque psoriasis: a proof-of-concept, single-arm, open-label clinical trial. Nutrition. 2020;74:110757.
- Castaldo G, Pagano I, Grimaldi M, et al. Effect of very-low-calorie ketogenic diet on psoriasis patients: a nuclear magnetic resonance-based metabolomic study. J Proteome Res. 2021;20:1509-1521.
- Milder J, Liang L-P, Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis. 2010;40:238-244.
- Kubo E, Chhunchha B, Singh P, et al. Sulforaphane reactivates cellular antioxidant defense by inducing Nrf2/ARE/Prdx6 activity during aging and oxidative stress. Sci Rep. 2017;7:14130.
- Pinto A, Bonucci A, Maggi E, et al. Anti-oxidant and anti-inflammatory activity of ketogenic diet: new perspectives for neuroprotection in Alzheimer's disease. Antioxidants (Basel). 2018;7:63.
- Youm Y-H, Nguyen KY, Grant RW, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Kelley N, Jeltema D, Duan Y, et al. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20:3328.
- Fomin DA, McDaniel B, Crane J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatol Treat. 2017;28:484-487.
- Rahman M, Muhammad S, Khan MA, et al. The β-hydroxybutyrate receptor HCA 2 activates a neuroprotective subset of macrophages. Nat Commun. 2014;5:1-11.
- Lu Y, Yang YY, Zhou MW, et al. Ketogenic diet attenuates oxidative stress and inflammation after spinal cord injury by activating Nrf2 and suppressing the NF-κB signaling pathways. Neurosci Lett. 2018;683:13-18.
- Hamarsheh S, Zeiser R. NLRP3 inflammasome activation in cancer: a double-edged sword. Front Immunol. 2020;11:1444.
- Bell S, Degitz K, Quirling M, et al. Involvement of NF-κB signalling in skin physiology and disease. Cell Signal. 2003;15:1-7.
- Goldminz AM, Au SC, Kim N, et al. NF-κB: an essential transcription factor in psoriasis. J Dermatol Sci. 2013;69:89-94.
- Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589.
- McDaniel S, Rensing N, Yamada K, et al. The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia. 2011;52:E7-E11.
- Alter M, Satzger I, Schrem H, et al. Non-melanoma skin cancer is reduced after switch of immunosuppression to mTOR-inhibitors in organ transplant recipients. J Dtsch Dermatol Ges. 2014;12:480-488.
- Feldmeyer L, Hofbauer GF, Böni T, et al. Mammalian target of rapamycin (mTOR) inhibitors slow skin carcinogenesis, but impair wound healing. Br J Dermatol. 2012;166:422-424.
- Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41:211-218.
- Li W. "Warburg effect" and mitochondrial metabolism in skin cancer.J Carcinogene Mutagene. 2012:S4.
- Naveed S, Aslam M, Ahmad A. Starvation based differential chemotherapy: a novel approach for cancer treatment. Oman Med J. 2014;29:391-398.
- Raffaghello L, Lee C, Safdie FM, et al. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Natl Acad Sci U S A. 2008;105:8215-8220.
- Buono R, Longo VD. Starvation, stress resistance, and cancer. Trends Endocrinol Metab. 2018;29:271-280.
- de Groot S, Pijl H, van der Hoeven JJM, et al. Effects of short-term fasting on cancer treatment. J Exp Clin Cancer Res. 2019;38:209.
- Hosseini M, Kasraian Z, Rezvani HR. Energy metabolism in skin cancers: a therapeutic perspective. Biochim Biophys Acta Bioenerg. 2017;1858:712-722.
- Feichtinger RG, Lang R, Geilberger R, et al. Melanoma tumors exhibit a variable but distinct metabolic signature. Exp Dermatol. 2018;27:204-207.
- Alshaya MA, Turkmani MG, Alissa AM. Prurigo pigmentosa following ketogenic diet and bariatric surgery: a growing association. JAAD Case Rep. 2019;5:504-507.
- Bellini M, Tonarelli S, Nagy AG, et al. Low FODMAP diet: evidence, doubts, and hopes. Nutrients. 2020;12:148.
- Kwiatkowski L, Rice E, Langland J. Integrative treatment of chronic abdominal bloating and pain associated with overgrowth of small intestinal bacteria: a case report. Altern Ther Health Med. 2017;23:56-61.
- Hubkova T. No more pain in the gut: lifestyle medicine approach to irritable bowel syndrome. Am J Lifestyle Med. 2017;11:223-226.
- Schumann D, Klose P, Lauche R, et al. Low fermentable, oligo-, di-, mono-saccharides and polyol diet in the treatment of irritable bowel syndrome: a systematic review and meta-analysis. Nutrition. 2018;45:24-31.
- Cox SR, Prince AC, Myers CE, et al. Fermentable carbohydrates [FODMAPs] exacerbate functional gastrointestinal symptoms in patients with inflammatory bowel disease: a randomised, double-blind, placebo-controlled, cross-over, re-challenge trial. J Crohns Colitis. 2017;11:1420-1429.
- Damas OM, Garces L, Abreu MT. Diet as adjunctive treatment for inflammatory bowel disease: review and update of the latest literature. Curr Treat Options Gastroenterol. 2019;17:313-325.
- Wang FY, Chi CC. Rosacea, germs, and bowels: a review on gastrointestinal comorbidities and gut-skin axis of rosacea. Adv Ther. 2021;38:1415-1424.
- Daou H, Paradiso M, Hennessy K, et al. Rosacea and the microbiome: a systematic review. Dermatol Ther (Heidelb). 2021;11:1-12.
- Weinstock LB, Steinhoff M. Rosacea and small intestinal bacterial overgrowth: prevalence and response to rifaximin. J Am Acad Dermatol. 2013;68:875-876.
- Wu CY, Chang YT, Juan CK, et al. Risk of inflammatory bowel disease in patients with rosacea: results from a nationwide cohort study in Taiwan. J Am Acad Dermatol. 2017;76:911-917.
- Egeberg A, Weinstock LB, Thyssen EP, et al. Rosacea and gastrointestinal disorders: a population-based cohort study. Br J Dermatol. 2017;176:100-106.
- Drago F, De Col E, Agnoletti AF, et al. The role of small intestinal bacterial overgrowth in rosacea: a 3-year follow-up. J Am Acad Dermatol. 2016;75:E113-E115.
- Parodi A, Paolino S, Greco A, et al. Small intestinal bacterial overgrowth in rosacea: clinical effectiveness of its eradication. Clin Gastroenterol Hepatol. 2008;6:759-764.
- Ojetti V, De Simone C, Aguilar Sanchez J, et al. Malabsorption in psoriatic patients: cause or consequence? Scand J Gastroenterol. 2006;41:1267-1271.
- Kim M, Choi KH, Hwang SW, et al. Inflammatory bowel disease is associated with an increased risk of inflammatory skin diseases: a population-based cross-sectional study. J Am Acad Dermatol. 2017;76:40-48.
- Drago F, Ciccarese G, Indemini E, et al. Psoriasis and small intestine bacterial overgrowth. Int J Dermatol. 2018;57:112-113.
- Acharya P, Mathur M. Association between psoriasis and celiac disease: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:1376-1385.
- Bhatia BK, Millsop JW, Debbaneh M, et al. Diet and psoriasis, part II: celiac disease and role of a gluten-free diet. J Am Acad Dermatol. 2014;71:350-358.
- Michaëlsson G, Gerdén B, Hagforsen E, et al. Psoriasis patients with antibodies to gliadin can be improved by a gluten-free diet. Br J Dermatol. 2000;142:44-51.
- Kolchak NA, Tetarnikova MK, Theodoropoulou MS, et al. Prevalence of antigliadin IgA antibodies in psoriasis vulgaris and response of seropositive patients to a gluten-free diet. J Multidiscip Healthc. 2018;11:13-19.
- De Bastiani R, Gabrielli M, Lora L, et al. Association between coeliac disease and psoriasis: Italian primary care multicentre study. Dermatology. 2015;230:156-160.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Loche F, Bazex J. Celiac disease associated with cutaneous sarcoidosic granuloma [in French]. Rev Med Interne. 1997;18:975-978.
- Rodríguez-García C, González-Hernández S, Pérez-Robayna N, et al. Repigmentation of vitiligo lesions in a child with celiac disease after a gluten-free diet. Pediatr Dermatol. 2011;28:209-210.
- Wijarnpreecha K, Panjawatanan P, Corral JE, et al. Celiac disease and risk of sarcoidosis: a systematic review and meta-analysis. J Evid Based Med. 2019;12:194-199.
- Rodrigo L, Beteta-Gorriti V, Alvarez N, et al. Cutaneous and mucosal manifestations associated with celiac disease. Nutrients. 2018;10:800.
- Song MS, Farber D, Bitton A, et al. Dermatomyositis associated with celiac disease: response to a gluten-free diet. Can J Gastroenterol. 2006;20:433-435.
- Egan CA, Smith EP, Taylor TB, et al. Linear IgA bullous dermatosis responsive to a gluten-free diet. Am J Gastroenterol. 2001;96:1927-1929.
- Comas-Basté O, Sánchez-Pérez S, Veciana-Nogués MT, et al. Histamine intolerance: the current state of the art. Biomolecules. 2020;10:1181.
- Ring J. Plasma histamine concentrations in atopic eczema. Clin Allergy. 1983;13:545-552.
- Maintz L, Benfadal S, Allam JP, et al. Evidence for a reduced histamine degradation capacity in a subgroup of patients with atopic eczema. J Allergy Clin Immunol. 2006;117:1106-1112.
- Cornillier H, Giraudeau B, Samimi M, et al. Effect of diet in chronic spontaneous urticaria: a systematic review. Acta Derm Venereol. 2019;99:127-132.
- Son JH, Chung BY, Kim HO, et al. A histamine-free diet is helpful for treatment of adult patients with chronic spontaneous urticaria. Ann Dermatol. 2018;30:164-172.
- Wagner N, Dirk D, Peveling-Oberhag A, et al. A popular myth - low-histamine diet improves chronic spontaneous urticaria - fact or fiction? J Eur Acad Dermatol Venereol. 2017;31:650-655.
- Esposito K, Marfella R, Ciotola M, et al. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292:1440-1446.
- Steffen LM, Van Horn L, Daviglus ML, et al. A modified Mediterranean diet score is associated with a lower risk of incident metabolic syndrome over 25 years among young adults: the CARDIA (coronary artery risk development in young adults) study. Br J Nutr. 2014;112:1654-1661.
- Bower A, Marquez S, de Mejia EG. The health benefits of selected culinary herbs and spices found in the traditional Mediterranean diet. Crit Rev Food Sci Nutr. 2016;56:2728-2746.
- Bosch R, Philips N, Suárez-Pérez JA, et al. Mechanisms of photoaging and cutaneous photocarcinogenesis, and photoprotective strategies with phytochemicals. Antioxidants (Basel). 2015;4:248-268.
- Katsimbri P, Korakas E, Kountouri A, et al. The effect of antioxidant and anti-inflammatory capacity of diet on psoriasis and psoriatic arthritis phenotype: nutrition as therapeutic tool? Antioxidants. 2021;10:157.
- Molina-Leyva A, Cuenca-Barrales C, Vega-Castillo JJ, et al. Adherence to Mediterranean diet in Spanish patients with psoriasis: cardiovascular benefits? Dermatol Ther. 2019;32:E12810.
- Barrea L, Balato N, Di Somma C, et al. Nutrition and psoriasis: is there any association between the severity of the disease and adherence to the Mediterranean diet? J Transl Med. 2015;13:1-10.
- Phan C, Touvier M, Kesse-Guyot E, et al. Association between Mediterranean anti-inflammatory dietary profile and severity of psoriasis: results from the NutriNet-Santé cohort. JAMA Dermatol. 2018;154:1017-1024.
- Korovesi A, Dalamaga M, Kotopouli M, et al. Adherence to the Mediterranean diet is independently associated with psoriasis risk, severity, and quality of life: a cross-sectional observational study. Int J Dermatol. 2019;58:E164-E165.
- Ford AR, Siegel M, Bagel J, et al. Dietary recommendations for adults with psoriasis or psoriatic arthritis from the medical board of the National Psoriasis Foundation: a systematic review. JAMA Dermatol. 2018;154:934-950.
- Skroza N, Tolino E, Semyonov L, et al. Mediterranean diet and familial dysmetabolism as factors influencing the development of acne. Scand J Public Health. 2012;40:466-474.
- Barrea L, Fabbrocini G, Annunziata G, et al. Role of nutrition and adherence to the Mediterranean diet in the multidisciplinary approach of hidradenitis suppurativa: evaluation of nutritional status and its association with severity of disease. Nutrients. 2018;11:57.
- Nichols JA, Katiyar SK. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch Dermatol Res. 2010;302:71-83.
- Huang T-H, Wang P-W, Yang S-C, et al. Cosmetic and therapeutic applications of fish oil's fatty acids on the skin. Mar Drugs. 2018;16:256.
- Rizwan M, Rodriguez-Blanco I, Harbottle A, et al. Tomato paste rich in lycopene protects against cutaneous photodamage in humans in vivo: a randomized controlled trial. Br J Dermatol. 2011;164:154-162.
- Leone A, Martínez-González M, Martin-Gorgojo A, et al. Mediterranean diet, dietary approaches to stop hypertension, and pro-vegetarian dietary pattern in relation to the risk of basal cell carcinoma: a nested case-control study within the Seguimiento Universidad de Navarra (SUN) cohort. Am J Clin Nutr. 2020;112:364-372.
- Solway J, McBride M, Haq F, et al. Diet and dermatology: the role of a whole-food, plant-based diet in preventing and reversing skin aging--a review. J Clin Aesthet Dermatol. 2020;13:38-43.
- Greger M. A whole food plant-based diet is effective for weight loss: the evidence. Am J Lifestyle Med. 2020;14:500-510.
- Wright N, Wilson L, Smith M, et al. The BROAD study: a randomised controlled trial using a whole food plant-based diet in the community for obesity, ischaemic heart disease or diabetes. Nutr Diabetes. 2017;7:E256.
- Ornish D, Lin J, Chan JM, et al. Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. Lancet Oncol. 2013;14:1112-1120.
- Ornish D, Lin J, Daubenmier J, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008;9:1048-1057.
- Zouboulis CC, Makrantonaki E. Clinical aspects and molecular diagnostics of skin aging. Clin Dermatol. 2011;29:3-14.
- Gupta L, Khandelwal D, Lal PR, et al. Palaeolithic diet in diabesity and endocrinopathies--a vegan's perspective. Eur Endocrinol. 2019;15:77-82.
- Chassaing B, Van de Wiele T, De Bodt J, et al. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut. 2017;66:1414-1427.
- Thorburn Alison N, Macia L, Mackay Charles R. Diet, metabolites, and "Western lifestyle" inflammatory diseases. Immunity. 2014;40:833-842.
- Katta R, Schlichte M. Diet and dermatitis: food triggers. J Clin Aesthet Dermatol. 2014;7:30-36.
- Dhar S, Srinivas SM. Food allergy in atopic dermatitis. Indian J Dermatol. 2016;61:645-648.
- Birmingham N, Thanesvorakul S, Gangur V. Relative immunogenicity of commonly allergenic foods versus rarely allergenic and nonallergenic foods in mice. J Food Prot. 2002;65:1988-1991.
- Yu W, Freeland DMH, Nadeau KC. Food allergy: immune mechanisms, diagnosis and immunotherapy. Nat Rev Immunol. 2016;16:751-765.
- Kowalski LM, Bujko J. Evaluation of biological and clinical potential of paleolithic diet [in Polish]. Rocz Panstw Zakl Hig. 2012;63:9-15.
- Lee JE, Titcomb TJ, Bisht B, et al. A modified MCT-based ketogenic diet increases plasma β-hydroxybutyrate but has less effect on fatigue and quality of life in people with multiple sclerosis compared to a modified paleolithic diet: a waitlist-controlled, randomized pilot study. J Am Coll Nutr. 2021;40:13-25.
- Abbott RD, Sadowski A, Alt AG. Efficacy of the autoimmune protocol diet as part of a multi-disciplinary, supported lifestyle intervention for Hashimoto's thyroiditis. Cureus. 2019;11:E4556.
- Lindeberg S, Jönsson T, Granfeldt Y, et al. A palaeolithic diet improves glucose tolerance more than a Mediterranean-like diet in individuals with ischaemic heart disease. Diabetologia. 2007;50:1795-1807.
- Jönsson T, Granfeldt Y, Ahrén B, et al. Beneficial effects of a paleolithic diet on cardiovascular risk factors in type 2 diabetes: a randomized cross-over pilot study. Cardiovasc Diabetol. 2009;8:35.
- Boers I, Muskiet FAJ, Berkelaar E, et al. Favourable effects of consuming a palaeolithic-type diet on characteristics of the metabolic syndrome: a randomized controlled pilot-study. Lipids Health Dis. 2014;13:160.
- Ghaedi E, Mohammadi M, Mohammadi H, et al. Effects of a paleolithic diet on cardiovascular disease risk factors: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2019;10:634-646.
- Mellberg C, Sandberg S, Ryberg M, et al. Long-term effects of a palaeolithic-type diet in obese postmenopausal women: a 2-year randomized trial. Eur J Clin Nutr. 2014;68:350-357.
- Pastore RL, Brooks JT, Carbone JW. Paleolithic nutrition improves plasma lipid concentrations of hypercholesterolemic adults to a greater extent than traditional heart-healthy dietary recommendations. Nutr Res. 2015;35:474-479.
- Otten J, Stomby A, Waling M, et al. Benefits of a paleolithic diet with and without supervised exercise on fat mass, insulin sensitivity, and glycemic control: a randomized controlled trial in individuals with type 2 diabetes. Diabetes Metab Res Rev. 2017;33:E2828.
- Stefanadi EC, Dimitrakakis G, Antoniou C-K, et al. Metabolic syndrome and the skin: a more than superficial association. reviewing the association between skin diseases and metabolic syndrome and a clinical decision algorithm for high risk patients. Diabetol Metab Syndr. 2018;10:9.
- Mann N. Meat in the human diet: an anthropological perspective. Nutr Dietetics. 2007;64(suppl 4):S102-S107.
- Bramble DM, Lieberman DE. Endurance running and the evolution of Homo. Nature. 2004;432:345-352.
- Kuhn JE. Throwing, the shoulder, and human evolution. Am J Orthop (Belle Mead NJ). 2016;45:110-114.
- Kobayashi H, Kohshima S. Unique morphology of the human eye and its adaptive meaning: comparative studies on external morphology of the primate eye. J Hum Evol. 2001;40:419-435.
- Cordain L, Eaton SB, Miller JB, et al. The paradoxical nature of hunter-gatherer diets: meat-based, yet non-atherogenic. Eur J Clin Nutr. 2002;56(suppl 1):S42-S52.
- McClellan WS, Du Bois EF. Clinical calorimetry: XLV. prolonged meat diets with a study of kidney function and ketosis. J Biol Chem. 1930;87:651-668.
- O'Hearn A. Can a carnivore diet provide all essential nutrients? Curr Opin Endocrinol Diabetes Obes. 2020;27:312-316.
- O'Hearn LA. A survey of improvements experienced on a carnivore diet compared to only carbohydrate restriction. Open Science Forum website. Published February 12, 2019. Accessed May 17, 2021. doi:10.17605/OSF.IO/5FU4D
- Williams P. Nutritional composition of red meat. Nutrition & Dietetics. 2007;64(suppl 4):S113-S119.
- Biel W, Czerniawska-Piątkowska E, Kowalczyk A. Offal chemical composition from veal, beef, and lamb maintained in organic production systems. Animals (Basel). 2019;9:489.
- Elmadfa I, Meyer AL. The role of the status of selected micronutrients in shaping the immune function. Endocr Metab Immune Disord Drug Targets. 2019;19:1100-1115.
- Babizhayev M. Treatment of skin aging and photoaging with innovative oral dosage forms of nonhydrolized carnosine and carcinine. Int J Clin Derm Res. 2017;5:116-143.
- Danby FW. Nutrition and aging skin: sugar and glycation. Clin Dermatol. 2010;28:409-411.
- Siefken W, Carstensen S, Springmann G, et al. Role of taurine accumulation in keratinocyte hydration. J Invest Dermatol. 2003;121:354-361.
- Vollmer DL, West VA, Lephart ED. Enhancing skin health: by oral administration of natural compounds and minerals with implications to the dermal microbiome. Int J Mol Sci. 2018;19:3059.
- Fischer F, Achterberg V, März A, et al. Folic acid and creatineimprove the firmness of human skin in vivo. J Cosmet Dermatol. 2011;10:15-23.
- Blatt T, Lenz H, Weber T. Topical application of creatine is multibeneficial for human skin. J Am Acad Dermatol. 2005;52:P32.
Within the last decade, almost 3000 articles have been published on the role of diet in the prevention and management of dermatologic conditions. Patients are increasingly interested in—and employing—dietary modifications that may influence skin appearance and aid in the treatment of cutaneous disease.1 It is essential that dermatologists are familiar with existing evidence on the role of diet in dermatology to counsel patients appropriately. Herein, we discuss the compositions of several popular diets and their proposed utility for dermatologic purposes. We highlight the limited literature that exists surrounding this topic and emphasize the need for future, well-designed clinical trials that study the impact of diet on skin disease.
Ketogenic Diet
The ketogenic diet has a macronutrient profile composed of high fat, low to moderate protein, and very low carbohydrates. Nutritional ketosis occurs as the body begins to use free fatty acids (via beta oxidation) as the primary metabolite driving cellular metabolism. It has been suggested that the ketogenic diet may impart beneficial effects on skin disease; however, limited literature exists on the role of nutritional ketosis in the treatment of dermatologic conditions.
Mechanistically, the ketogenic diet decreases the secretion of insulin and insulinlike growth factor 1, resulting in a reduction of circulating androgens and increased activity of the retinoid X receptor.2 In acne vulgaris, it has been suggested that the ketogenic diet may be beneficial in decreasing androgen-induced sebum production and the overproliferation of keratinocytes.2-7 The ketogenic diet is one of the most rapidly effective dietary strategies for normalizing both insulin and androgens, thus it may theoretically be useful for other metabolic and hormone-dependent skin diseases, such as hidradenitis suppurativa.8,9
The cutaneous manifestations associated with chronic hyperinsulinemia and hyperglycemia are numerous and include acanthosis nigricans, acrochordons, diabetic dermopathy, scleredema diabeticorum, bullosis diabeticorum, keratosis pilaris, and generalized granuloma annulare. There also is an increased risk for bacterial and fungal skin infections associated with hyperglycemic states.10 The ketogenic diet is an effective nonpharmacologic tool for normalizing serum insulin and glucose levels in most patients and may have utility in the aforementioned conditions.11,12 In addition to improving insulin sensitivity, it has been used as a dietary strategy for weight loss.11-15 Because obesity and metabolic syndrome are highly correlated with common skin conditions such as psoriasis, hidradenitis suppurativa, and androgenetic alopecia, there may be a role for employing the ketogenic diet in these patient populations.16,17
Although robust clinical studies on ketogenic diets in skin disease are lacking, a recent single-arm, open-label clinical trial observed benefit in all 37 drug-naïve, overweight patients with chronic plaque psoriasis who underwent a ketogenic weight loss protocol. Significant reductions in psoriasis area and severity index (PASI) score and dermatology life quality index score were reported (P<.001).18 Another study of 30 patients with psoriasis found that a 4-week, low-calorie, ketogenic diet resulted in 50% improvement of PASI scores, 10% weight loss, and a reduction in the proinflammatory cytokines IL-1β and IL-2.19 Despite these results, it is a challenge to tease out if the specific dietary intervention or its associated weight loss was the main driver in these reported improvements in skin disease.
There is mixed evidence on the anti-inflammatory nature of the ketogenic diet, likely due to wide variation in the composition of foods included in individual diets. In many instances, the ketogenic diet is thought to possess considerable antioxidant and anti-inflammatory capabilities. Ketones are known activators of the nuclear factor erythroid 2–related factor 2 pathway, which upregulates the production of glutathione, a major endogenous intracellular antioxidant.20 Additionally, dietary compounds from foods that are encouraged while on the ketogenic diet, such as sulforaphane from broccoli, also are independent activators of nuclear factor erythroid 2–related factor 2.21 Ketones are efficiently utilized by mitochondria, which also may result in the decreased production of reactive oxygen species and lower oxidative stress.22 Moreover, the ketone body β-hydroxybutyrate has demonstrated the ability to reduce proinflammatory IL-1β levels via suppression of nucleotide-binding domain-like receptor protein 3 inflammasome activity.23,24 The activity of IL-1β is known to be elevated in many dermatologic conditions, including juvenile idiopathic arthritis, relapsing polychondritis, Schnitzler syndrome, hidradenitis suppurativa, Behçet disease, and other autoinflammatory syndromes.25 Ketones also have been shown to inhibit the nuclear factor–κB proinflammatory signaling pathway.22,26,27 Overexpression of IL-1β and aberrant activation of nuclear factor–κB are implicated in a variety of inflammatory, autoimmune, and oncologic cutaneous pathologies. The ketogenic diet may prove to be an effective adjunctive treatment for dermatologists to consider in select patient populations.23,24,28-30
For patients with keratinocyte carcinomas, the ketogenic diet may offer the aforementioned anti-inflammatory and antioxidant effects, as well as suppression of the mechanistic target of rapamycin, a major regulator of cell metabolism and proliferation.31,32 Inhibition of mechanistic target of rapamycin activity has been shown to slow tumor growth and reduce the development of squamous cell carcinoma.25,33,34 The ketogenic diet also may exploit the preferential utilization of glucose exhibited by many types of cancer cells, thereby “starving” the tumor of its primary fuel source.35,36 In vitro and animal studies in a variety of cancer types have demonstrated that a ketogenic metabolic state—achieved through the ketogenic diet or fasting—can sensitize tumor cells to chemotherapy and radiation while conferring a protective effect to normal cells.37-40 This recently described phenomenon is known as differential stress resistance, but it has not been studied in keratinocyte malignancies or melanoma to date. Importantly, some basal cell carcinomas and BRAF V600E–mutated melanomas have worsened while on the ketogenic diet, suggesting more data is needed before it can be recommended for all cancer patients.41,42 Furthermore, other skin conditions such as prurigo pigmentosa have been associated with initiation of the ketogenic diet.43
Low FODMAP Diet
Fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) are short-chain carbohydrates that are poorly absorbed, osmotically active, and rapidly fermented by intestinal bacteria.44 The low FODMAP diet has been shown to be efficacious for treatment of irritable bowel syndrome, small intestinal bacterial overgrowth (SIBO), and some cases of inflammatory bowel disease (IBD).44-49 A low FODMAP diet may have potential implications for several dermatologic conditions.
Rosacea has been associated with various gastrointestinal tract disorders including irritable bowel syndrome, SIBO, and IBD.50-54 A single study found that patients with rosacea had a 13-fold increased risk for SIBO.55,56 Treatment of 40 patients with SIBO using rifaximin resulted in complete resolution of rosacea in all patients, with no relapse after a 3-year follow-up period.55 Psoriasis also has been associated with SIBO and IBD.57,58 One small study found that eradication of SIBO in psoriatic patients resulted in improved PASI scores and colorimetric values.59
Although the long-term health consequences of the low FODMAP diet are unknown, further research on such dietary interventions for inflammatory skin conditions is warranted given the mounting evidence of a gut-skin connection and the role of the intestinal microbiome in skin health.50,51
Gluten-Free Diet
Gluten is a protein found in a variety of grains. Although the role of gluten in the pathogenesis of celiac disease and dermatitis herpetiformis is indisputable, the deleterious effects of gluten outside of the context of these diseases remain controversial. There may be a compelling case for eliminating gluten in psoriasis patients with seropositivity for celiac disease. A recent systematic review found a 2.2-fold increased risk for celiac disease in psoriasis patients.60 Antigliadin antibody titers also were found to be positively correlated with psoriatic disease severity.61 In addition, one open-label study found a reduction in PASI scores in 73% of patients with antigliadin antibodies after 3 months on a gluten-free diet compared to those without antibodies; however, the study only included 22 patients.62 Several other small studies have yielded similar results63,64; however, antigliadin antibodies are neither the most sensitive nor specific markers of celiac disease, and additional testing should be completed in any patient who may carry this diagnosis. A survey study by the National Psoriasis Foundation found that the dietary change associated with the greatest skin improvement was removal of gluten and nightshade vegetables in approximately 50% of the 1200 psoriasis patients that responded.65 Case reports of various dermatologic conditions including sarcoidosis, vitiligo, alopecia areata, lichen planus, dermatomyositis, pyoderma gangrenosum, erythema nodosum, leukocytoclastic vasculitis, linear IgA bullous dermatosis, and aphthous ulcerations have reportedly improved with a gluten-free diet; however, this should not be used as primary therapy in patients without celiac disease.66-71 Because gluten-free diets can be expensive and challenging to follow, a formal assessment for celiac disease should be considered before recommendation of this dietary intervention.
Low Histamine Diet
Histamine is a biogenic amine produced by the decarboxylation of the amino acid histidine.72 It is found in several foods in varying amounts. Because bacteria can convert histidine into histamine, many fermented and aged foods such as kimchi, sauerkraut, cheese, and red wine contain high levels of histamine. Individuals who have decreased activity of diamine oxidase (DAO), an enzyme that degrades histamine, may be more susceptible to histamine intolerance.72 The symptoms of histamine intolerance are numerous and include gastrointestinal tract distress, rhinorrhea and nasal congestion, headache, urticaria, flushing, and pruritus. Histamine intolerance can mimic an IgE-mediated food allergy; however, allergy testing is negative in these patients. Unfortunately, there is no laboratory test for histamine intolerance; a double-blind, placebo-controlled food challenge is considered the gold-standard test.72
As it pertains to dermatology, a low histamine diet may play a role in the treatment of certain patients with atopic dermatitis and chronic spontaneous urticaria. One study reported that 17 of 54 (31.5%) atopic patients had higher basal levels of serum histamine compared to controls.73 Another study found that a histamine-free diet led to improvement in both histamine intolerance symptoms and atopic dermatitis disease severity (SCORing atopic dermatitis) in patients with low DAO activity.74 In chronic spontaneous urticaria, a recent systematic review found that in 223 patients placed on a low histamine diet for 3 to 4 weeks, 12% and 44% achieved complete and partial remission, respectively.75 Although treatment response based on a patient’s DAO activity level has not been correlated, a diet low in histamine may prove useful for patients with persistent atopic dermatitis and chronic spontaneous urticaria who have negative food allergy tests and report exacerbation of symptoms after ingestion of histamine-rich foods.76,77
Mediterranean Diet
The Mediterranean diet has been touted as one of the healthiest diets to date, and large randomized clinical trials have demonstrated its effectiveness in weight loss, improving insulin sensitivity, and reducing inflammatory cytokine profiles.78,79 A major criticism of the Mediterranean diet is that it has considerable ambiguity and lacks a precise definition due to the variability of what is consumed in different Mediterranean regions. Generally, the diet emphasizes high consumption of colorful fruits and vegetables, aromatic herbs and spices, olive oil, nuts, and seafood, as well as modest amounts of dairy, eggs, and red meat.80 The anti-inflammatory effects of this diet largely have been attributed to its abundance of polyphenols, carotenoids, monounsaturated fatty acids, and omega-3 polyunsaturated fatty acids (PUFAs).80,81 Examples of polyphenols include resveratrol in red grapes, quercetin in apples and red onions, and curcumin in turmeric, while examples of carotenoids include lycopene in tomatoes and zeaxanthin in dark leafy greens. Oleic acid is a monounsaturated fatty acid present in high concentrations in olive oil, while eicosapentaenoic acid and docosahexaenoic acid are omega-3 PUFAs predominantly found in fish.82
Unfortunately, rigorous clinical trials regarding the Mediterranean diet as it pertains to dermatology have not been undertaken. Numerous observational studies in patients with psoriasis have suggested that close adherence to the Mediterranean diet was associated with improvement in PASI scores.83-86 The National Psoriasis Foundation now recommends a trial of the Mediterranean diet in some patients with psoriasis, emphasizing increased dietary intake of olive oil, fish, and vegetables.87 Adherence to a Mediterranean diet also has been inversely correlated to the severity of acne vulgaris and hidradenitis suppurativa88,89; however, these studies failed to account for the multifactorial risk factors associated with these conditions. Mediterranean diets also may impart a chemopreventive effect, supported by a number of in vivo and in vitro studies demonstrating the inhibition and/or reversal of cutaneous DNA damage induced by UV radiation through supplementation with various phytonutrients and omega-3 PUFAs.81,90-92 Although small case-control studies have found a decreased risk of basal cell carcinoma in those who closely adhered to a Mediterranean diet, more rigorous clinical research is needed.93
Whole-Food, Plant-Based Diet
A whole-food, plant-based (WFPB) diet is another popular dietary approach that consists of eating fruits, vegetables, legumes, nuts, seeds, and grains in their whole natural form.94 This diet discourages all animal products, including red meat, seafood, dairy, and eggs. It is similar to a vegan diet except that it eliminates all highly refined carbohydrates, vegetable oils, and other processed foods.94 Randomized clinical studies have demonstrated the WFPB diet to be effective in the treatment of obesity and metabolic syndrome.95,96
A WFPB diet has been shown to increase the antioxidant capacity of cells, lengthen telomeres, and reduce formation of advanced glycation end products.94,97,98 These benefits may help combat accelerated skin aging, including increased skin permeability, reduced elasticity and hydration, decreased angiogenesis, impaired immune function, and decreased vitamin D synthesis. Accelerated skin aging can result in delayed wound healing and susceptibility to skin tears and ecchymoses and also may promote the development of cutaneous malignancies.99 There remains a lack of clinical data studying a properly formulated WFPB diet in the dermatologic setting.
Paleolithic Diet
The paleolithic (Paleo) diet is an increasingly popular way of eating that attempts to mirror what our ancestors may have consumed between 10,000 and 2.5 million years ago.100 It is similar to the Mediterranean diet but excludes grains, dairy, legumes, and nightshade vegetables. It also calls for elimination of highly processed sugars and oils as well as chemical food additives and preservatives. There is a strict variation of the diet for individuals with autoimmune disease that also excludes eggs, nuts, and seeds, as these can be inflammatory or immunogenic in some patients.100-106 Other variations of the diet exist, including the ketogenic Paleo diet, pegan (Paleo vegan) diet, and lacto-Paleo diet.100 An often cited criticism of the Paleo diet is the low intake of calcium and risk for osteoporosis; however, consumption of calcium-rich foods or a calcium supplement can address this concern.107
Although small clinical studies have found the Paleo diet to be beneficial for various autoimmune diseases, clinical data evaluating the utility of the diet for cutaneous disease is lacking.108,109 Numerous randomized trials have demonstrated the Paleo diet to be effective for weight loss and improving insulin sensitivity and lipid levels.110-116 Thus, the Paleo diet may theoretically serve as a viable adjunct dietary approach to the treatment of cutaneous diseases associated with obesity and metabolic derangement.117
Carnivore Diet
Arguably the most controversial and radical diet is the carnivore diet. As the name implies, the carnivore diet is based on consuming solely animal products. A properly structured carnivore diet emphasizes a “nose-to-tail” eating approach where all parts of the animal including the muscle meats, organs, and fat are consumed. Proponents of the diet cite anthropologic evidence from fossil-stable carbon-13/carbon-12 isotope analyses, craniodental features, and numerous other adaptations that indicate increased consumption of meat during human evolution.118-122 Notably, many early humans ate a carnivore diet, but life span was very short at this time, suggesting the diet may not be as beneficial as has been suggested.
Despite the abundance of anecdotal evidence supporting its use for a variety of chronic conditions, including cutaneous autoimmune disease, there is a virtual absence of high-quality research on the carnivore diet.123-125
The purported benefits of the carnivore diet may be attributed to the consumption of organ meats that contain highly bioavailable essential vitamins and minerals, such as iron, zinc, copper, selenium, thiamine, niacin, folate, vitamin B6, vitamin B12, vitamin A, vitamin D, vitamin K, and choline.126-128 Other dietary compounds that have demonstrated benefit for skin health and are predominantly found in animal foods include carnosine, carnitine, creatine, taurine, coenzyme Q10, and collagen.129-134 Nevertheless, there is no data to recommend the elimination of antioxidant- and micronutrient-dense plant-based foods. Rigorous clinical research evaluating the efficacy and safety of the carnivore diet in dermatologic patients is needed. A carnivore diet should not be undertaken without the assistance of a dietician who can ensure adequate micronutrient and macronutrient support.
Final Thoughts
The adjunctive role of diet in the treatment of skin disease is expanding and becoming more widely accepted among dermatologists. Unfortunately, there remains a lack of randomized controlled trials confirming the efficacy of various dietary interventions in the dermatologic setting. Although evidence-based dietary recommendations currently are limited, it is important for dermatologists to be aware of the varied and nuanced dietary interventions employed by patients.
Ultimately, dietary recommendations must be personalized, considering a patient’s comorbidities, personal beliefs and preferences, and nutrigenetics. The emerging field of dermatonutrigenomics—the study of how dietary compounds interact with one’s genes to influence skin health—may allow for precise dietary recommendations to be made in dermatologic practice. Direct-to-consumer genetic tests targeted toward dermatology patients are already on the market, but their clinical utility awaits validation.1 Because nutritional science is a constantly evolving field, becoming familiar with these popular diets will serve both dermatologists and their patients well.
Within the last decade, almost 3000 articles have been published on the role of diet in the prevention and management of dermatologic conditions. Patients are increasingly interested in—and employing—dietary modifications that may influence skin appearance and aid in the treatment of cutaneous disease.1 It is essential that dermatologists are familiar with existing evidence on the role of diet in dermatology to counsel patients appropriately. Herein, we discuss the compositions of several popular diets and their proposed utility for dermatologic purposes. We highlight the limited literature that exists surrounding this topic and emphasize the need for future, well-designed clinical trials that study the impact of diet on skin disease.
Ketogenic Diet
The ketogenic diet has a macronutrient profile composed of high fat, low to moderate protein, and very low carbohydrates. Nutritional ketosis occurs as the body begins to use free fatty acids (via beta oxidation) as the primary metabolite driving cellular metabolism. It has been suggested that the ketogenic diet may impart beneficial effects on skin disease; however, limited literature exists on the role of nutritional ketosis in the treatment of dermatologic conditions.
Mechanistically, the ketogenic diet decreases the secretion of insulin and insulinlike growth factor 1, resulting in a reduction of circulating androgens and increased activity of the retinoid X receptor.2 In acne vulgaris, it has been suggested that the ketogenic diet may be beneficial in decreasing androgen-induced sebum production and the overproliferation of keratinocytes.2-7 The ketogenic diet is one of the most rapidly effective dietary strategies for normalizing both insulin and androgens, thus it may theoretically be useful for other metabolic and hormone-dependent skin diseases, such as hidradenitis suppurativa.8,9
The cutaneous manifestations associated with chronic hyperinsulinemia and hyperglycemia are numerous and include acanthosis nigricans, acrochordons, diabetic dermopathy, scleredema diabeticorum, bullosis diabeticorum, keratosis pilaris, and generalized granuloma annulare. There also is an increased risk for bacterial and fungal skin infections associated with hyperglycemic states.10 The ketogenic diet is an effective nonpharmacologic tool for normalizing serum insulin and glucose levels in most patients and may have utility in the aforementioned conditions.11,12 In addition to improving insulin sensitivity, it has been used as a dietary strategy for weight loss.11-15 Because obesity and metabolic syndrome are highly correlated with common skin conditions such as psoriasis, hidradenitis suppurativa, and androgenetic alopecia, there may be a role for employing the ketogenic diet in these patient populations.16,17
Although robust clinical studies on ketogenic diets in skin disease are lacking, a recent single-arm, open-label clinical trial observed benefit in all 37 drug-naïve, overweight patients with chronic plaque psoriasis who underwent a ketogenic weight loss protocol. Significant reductions in psoriasis area and severity index (PASI) score and dermatology life quality index score were reported (P<.001).18 Another study of 30 patients with psoriasis found that a 4-week, low-calorie, ketogenic diet resulted in 50% improvement of PASI scores, 10% weight loss, and a reduction in the proinflammatory cytokines IL-1β and IL-2.19 Despite these results, it is a challenge to tease out if the specific dietary intervention or its associated weight loss was the main driver in these reported improvements in skin disease.
There is mixed evidence on the anti-inflammatory nature of the ketogenic diet, likely due to wide variation in the composition of foods included in individual diets. In many instances, the ketogenic diet is thought to possess considerable antioxidant and anti-inflammatory capabilities. Ketones are known activators of the nuclear factor erythroid 2–related factor 2 pathway, which upregulates the production of glutathione, a major endogenous intracellular antioxidant.20 Additionally, dietary compounds from foods that are encouraged while on the ketogenic diet, such as sulforaphane from broccoli, also are independent activators of nuclear factor erythroid 2–related factor 2.21 Ketones are efficiently utilized by mitochondria, which also may result in the decreased production of reactive oxygen species and lower oxidative stress.22 Moreover, the ketone body β-hydroxybutyrate has demonstrated the ability to reduce proinflammatory IL-1β levels via suppression of nucleotide-binding domain-like receptor protein 3 inflammasome activity.23,24 The activity of IL-1β is known to be elevated in many dermatologic conditions, including juvenile idiopathic arthritis, relapsing polychondritis, Schnitzler syndrome, hidradenitis suppurativa, Behçet disease, and other autoinflammatory syndromes.25 Ketones also have been shown to inhibit the nuclear factor–κB proinflammatory signaling pathway.22,26,27 Overexpression of IL-1β and aberrant activation of nuclear factor–κB are implicated in a variety of inflammatory, autoimmune, and oncologic cutaneous pathologies. The ketogenic diet may prove to be an effective adjunctive treatment for dermatologists to consider in select patient populations.23,24,28-30
For patients with keratinocyte carcinomas, the ketogenic diet may offer the aforementioned anti-inflammatory and antioxidant effects, as well as suppression of the mechanistic target of rapamycin, a major regulator of cell metabolism and proliferation.31,32 Inhibition of mechanistic target of rapamycin activity has been shown to slow tumor growth and reduce the development of squamous cell carcinoma.25,33,34 The ketogenic diet also may exploit the preferential utilization of glucose exhibited by many types of cancer cells, thereby “starving” the tumor of its primary fuel source.35,36 In vitro and animal studies in a variety of cancer types have demonstrated that a ketogenic metabolic state—achieved through the ketogenic diet or fasting—can sensitize tumor cells to chemotherapy and radiation while conferring a protective effect to normal cells.37-40 This recently described phenomenon is known as differential stress resistance, but it has not been studied in keratinocyte malignancies or melanoma to date. Importantly, some basal cell carcinomas and BRAF V600E–mutated melanomas have worsened while on the ketogenic diet, suggesting more data is needed before it can be recommended for all cancer patients.41,42 Furthermore, other skin conditions such as prurigo pigmentosa have been associated with initiation of the ketogenic diet.43
Low FODMAP Diet
Fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) are short-chain carbohydrates that are poorly absorbed, osmotically active, and rapidly fermented by intestinal bacteria.44 The low FODMAP diet has been shown to be efficacious for treatment of irritable bowel syndrome, small intestinal bacterial overgrowth (SIBO), and some cases of inflammatory bowel disease (IBD).44-49 A low FODMAP diet may have potential implications for several dermatologic conditions.
Rosacea has been associated with various gastrointestinal tract disorders including irritable bowel syndrome, SIBO, and IBD.50-54 A single study found that patients with rosacea had a 13-fold increased risk for SIBO.55,56 Treatment of 40 patients with SIBO using rifaximin resulted in complete resolution of rosacea in all patients, with no relapse after a 3-year follow-up period.55 Psoriasis also has been associated with SIBO and IBD.57,58 One small study found that eradication of SIBO in psoriatic patients resulted in improved PASI scores and colorimetric values.59
Although the long-term health consequences of the low FODMAP diet are unknown, further research on such dietary interventions for inflammatory skin conditions is warranted given the mounting evidence of a gut-skin connection and the role of the intestinal microbiome in skin health.50,51
Gluten-Free Diet
Gluten is a protein found in a variety of grains. Although the role of gluten in the pathogenesis of celiac disease and dermatitis herpetiformis is indisputable, the deleterious effects of gluten outside of the context of these diseases remain controversial. There may be a compelling case for eliminating gluten in psoriasis patients with seropositivity for celiac disease. A recent systematic review found a 2.2-fold increased risk for celiac disease in psoriasis patients.60 Antigliadin antibody titers also were found to be positively correlated with psoriatic disease severity.61 In addition, one open-label study found a reduction in PASI scores in 73% of patients with antigliadin antibodies after 3 months on a gluten-free diet compared to those without antibodies; however, the study only included 22 patients.62 Several other small studies have yielded similar results63,64; however, antigliadin antibodies are neither the most sensitive nor specific markers of celiac disease, and additional testing should be completed in any patient who may carry this diagnosis. A survey study by the National Psoriasis Foundation found that the dietary change associated with the greatest skin improvement was removal of gluten and nightshade vegetables in approximately 50% of the 1200 psoriasis patients that responded.65 Case reports of various dermatologic conditions including sarcoidosis, vitiligo, alopecia areata, lichen planus, dermatomyositis, pyoderma gangrenosum, erythema nodosum, leukocytoclastic vasculitis, linear IgA bullous dermatosis, and aphthous ulcerations have reportedly improved with a gluten-free diet; however, this should not be used as primary therapy in patients without celiac disease.66-71 Because gluten-free diets can be expensive and challenging to follow, a formal assessment for celiac disease should be considered before recommendation of this dietary intervention.
Low Histamine Diet
Histamine is a biogenic amine produced by the decarboxylation of the amino acid histidine.72 It is found in several foods in varying amounts. Because bacteria can convert histidine into histamine, many fermented and aged foods such as kimchi, sauerkraut, cheese, and red wine contain high levels of histamine. Individuals who have decreased activity of diamine oxidase (DAO), an enzyme that degrades histamine, may be more susceptible to histamine intolerance.72 The symptoms of histamine intolerance are numerous and include gastrointestinal tract distress, rhinorrhea and nasal congestion, headache, urticaria, flushing, and pruritus. Histamine intolerance can mimic an IgE-mediated food allergy; however, allergy testing is negative in these patients. Unfortunately, there is no laboratory test for histamine intolerance; a double-blind, placebo-controlled food challenge is considered the gold-standard test.72
As it pertains to dermatology, a low histamine diet may play a role in the treatment of certain patients with atopic dermatitis and chronic spontaneous urticaria. One study reported that 17 of 54 (31.5%) atopic patients had higher basal levels of serum histamine compared to controls.73 Another study found that a histamine-free diet led to improvement in both histamine intolerance symptoms and atopic dermatitis disease severity (SCORing atopic dermatitis) in patients with low DAO activity.74 In chronic spontaneous urticaria, a recent systematic review found that in 223 patients placed on a low histamine diet for 3 to 4 weeks, 12% and 44% achieved complete and partial remission, respectively.75 Although treatment response based on a patient’s DAO activity level has not been correlated, a diet low in histamine may prove useful for patients with persistent atopic dermatitis and chronic spontaneous urticaria who have negative food allergy tests and report exacerbation of symptoms after ingestion of histamine-rich foods.76,77
Mediterranean Diet
The Mediterranean diet has been touted as one of the healthiest diets to date, and large randomized clinical trials have demonstrated its effectiveness in weight loss, improving insulin sensitivity, and reducing inflammatory cytokine profiles.78,79 A major criticism of the Mediterranean diet is that it has considerable ambiguity and lacks a precise definition due to the variability of what is consumed in different Mediterranean regions. Generally, the diet emphasizes high consumption of colorful fruits and vegetables, aromatic herbs and spices, olive oil, nuts, and seafood, as well as modest amounts of dairy, eggs, and red meat.80 The anti-inflammatory effects of this diet largely have been attributed to its abundance of polyphenols, carotenoids, monounsaturated fatty acids, and omega-3 polyunsaturated fatty acids (PUFAs).80,81 Examples of polyphenols include resveratrol in red grapes, quercetin in apples and red onions, and curcumin in turmeric, while examples of carotenoids include lycopene in tomatoes and zeaxanthin in dark leafy greens. Oleic acid is a monounsaturated fatty acid present in high concentrations in olive oil, while eicosapentaenoic acid and docosahexaenoic acid are omega-3 PUFAs predominantly found in fish.82
Unfortunately, rigorous clinical trials regarding the Mediterranean diet as it pertains to dermatology have not been undertaken. Numerous observational studies in patients with psoriasis have suggested that close adherence to the Mediterranean diet was associated with improvement in PASI scores.83-86 The National Psoriasis Foundation now recommends a trial of the Mediterranean diet in some patients with psoriasis, emphasizing increased dietary intake of olive oil, fish, and vegetables.87 Adherence to a Mediterranean diet also has been inversely correlated to the severity of acne vulgaris and hidradenitis suppurativa88,89; however, these studies failed to account for the multifactorial risk factors associated with these conditions. Mediterranean diets also may impart a chemopreventive effect, supported by a number of in vivo and in vitro studies demonstrating the inhibition and/or reversal of cutaneous DNA damage induced by UV radiation through supplementation with various phytonutrients and omega-3 PUFAs.81,90-92 Although small case-control studies have found a decreased risk of basal cell carcinoma in those who closely adhered to a Mediterranean diet, more rigorous clinical research is needed.93
Whole-Food, Plant-Based Diet
A whole-food, plant-based (WFPB) diet is another popular dietary approach that consists of eating fruits, vegetables, legumes, nuts, seeds, and grains in their whole natural form.94 This diet discourages all animal products, including red meat, seafood, dairy, and eggs. It is similar to a vegan diet except that it eliminates all highly refined carbohydrates, vegetable oils, and other processed foods.94 Randomized clinical studies have demonstrated the WFPB diet to be effective in the treatment of obesity and metabolic syndrome.95,96
A WFPB diet has been shown to increase the antioxidant capacity of cells, lengthen telomeres, and reduce formation of advanced glycation end products.94,97,98 These benefits may help combat accelerated skin aging, including increased skin permeability, reduced elasticity and hydration, decreased angiogenesis, impaired immune function, and decreased vitamin D synthesis. Accelerated skin aging can result in delayed wound healing and susceptibility to skin tears and ecchymoses and also may promote the development of cutaneous malignancies.99 There remains a lack of clinical data studying a properly formulated WFPB diet in the dermatologic setting.
Paleolithic Diet
The paleolithic (Paleo) diet is an increasingly popular way of eating that attempts to mirror what our ancestors may have consumed between 10,000 and 2.5 million years ago.100 It is similar to the Mediterranean diet but excludes grains, dairy, legumes, and nightshade vegetables. It also calls for elimination of highly processed sugars and oils as well as chemical food additives and preservatives. There is a strict variation of the diet for individuals with autoimmune disease that also excludes eggs, nuts, and seeds, as these can be inflammatory or immunogenic in some patients.100-106 Other variations of the diet exist, including the ketogenic Paleo diet, pegan (Paleo vegan) diet, and lacto-Paleo diet.100 An often cited criticism of the Paleo diet is the low intake of calcium and risk for osteoporosis; however, consumption of calcium-rich foods or a calcium supplement can address this concern.107
Although small clinical studies have found the Paleo diet to be beneficial for various autoimmune diseases, clinical data evaluating the utility of the diet for cutaneous disease is lacking.108,109 Numerous randomized trials have demonstrated the Paleo diet to be effective for weight loss and improving insulin sensitivity and lipid levels.110-116 Thus, the Paleo diet may theoretically serve as a viable adjunct dietary approach to the treatment of cutaneous diseases associated with obesity and metabolic derangement.117
Carnivore Diet
Arguably the most controversial and radical diet is the carnivore diet. As the name implies, the carnivore diet is based on consuming solely animal products. A properly structured carnivore diet emphasizes a “nose-to-tail” eating approach where all parts of the animal including the muscle meats, organs, and fat are consumed. Proponents of the diet cite anthropologic evidence from fossil-stable carbon-13/carbon-12 isotope analyses, craniodental features, and numerous other adaptations that indicate increased consumption of meat during human evolution.118-122 Notably, many early humans ate a carnivore diet, but life span was very short at this time, suggesting the diet may not be as beneficial as has been suggested.
Despite the abundance of anecdotal evidence supporting its use for a variety of chronic conditions, including cutaneous autoimmune disease, there is a virtual absence of high-quality research on the carnivore diet.123-125
The purported benefits of the carnivore diet may be attributed to the consumption of organ meats that contain highly bioavailable essential vitamins and minerals, such as iron, zinc, copper, selenium, thiamine, niacin, folate, vitamin B6, vitamin B12, vitamin A, vitamin D, vitamin K, and choline.126-128 Other dietary compounds that have demonstrated benefit for skin health and are predominantly found in animal foods include carnosine, carnitine, creatine, taurine, coenzyme Q10, and collagen.129-134 Nevertheless, there is no data to recommend the elimination of antioxidant- and micronutrient-dense plant-based foods. Rigorous clinical research evaluating the efficacy and safety of the carnivore diet in dermatologic patients is needed. A carnivore diet should not be undertaken without the assistance of a dietician who can ensure adequate micronutrient and macronutrient support.
Final Thoughts
The adjunctive role of diet in the treatment of skin disease is expanding and becoming more widely accepted among dermatologists. Unfortunately, there remains a lack of randomized controlled trials confirming the efficacy of various dietary interventions in the dermatologic setting. Although evidence-based dietary recommendations currently are limited, it is important for dermatologists to be aware of the varied and nuanced dietary interventions employed by patients.
Ultimately, dietary recommendations must be personalized, considering a patient’s comorbidities, personal beliefs and preferences, and nutrigenetics. The emerging field of dermatonutrigenomics—the study of how dietary compounds interact with one’s genes to influence skin health—may allow for precise dietary recommendations to be made in dermatologic practice. Direct-to-consumer genetic tests targeted toward dermatology patients are already on the market, but their clinical utility awaits validation.1 Because nutritional science is a constantly evolving field, becoming familiar with these popular diets will serve both dermatologists and their patients well.
- Jaros J, Katta R, Shi VY. Dermatonutrigenomics: past, present, and future. Dermatology. 2019;235:164-166.
- Paoli A, Grimaldi K, Toniolo L, et al. Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- Melnik BC, Schmitz G. Role of insulin, insulin-like growth factor-1, hyperglycaemic food and milk consumption in the pathogenesis of acne vulgaris. Exp Dermatol. 2009;18:833-841.
- Smith RN, Mann NJ, Braue A, et al. The effect of a high-protein, low glycemic-load diet versus a conventional, high glycemic-load diet on biochemical parameters associated with acne vulgaris: a randomized, investigator-masked, controlled trial. J Am Acad Dermatol. 2007;57:247-256.
- Smith R, Mann N, Mäkeläinen H, et al. A pilot study to determine the short-term effects of a low glycemic load diet on hormonal markers of acne: a nonrandomized, parallel, controlled feeding trial. Mol Nutr Food Res. 2008;52:718-726.
- Smith RN, Braue A, Varigos GA, et al. The effect of a low glycemic load diet on acne vulgaris and the fatty acid composition of skin surface triglycerides. J Dermatol Sci. 2008;50:41-52.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Khandalavala BN, Do MV. Finasteride in hidradenitis suppurativa: a "male" therapy for a predominantly "female" disease. J Clin Aesthet Dermatol. 2016;9:44.
- Nikolakis G, Karagiannidis I, Vaiopoulos AG, et al. Endocrinological mechanisms in the pathophysiology of hidradenitis suppurativa [in German]. Hautarzt. 2020;71:762-771.
- Karadag AS, Ozlu E, Lavery MJ. Cutaneous manifestations of diabetes mellitus and the metabolic syndrome. Clin Dermatology. 2018;36:89-93.
- Gardner CD, Kiazand A, Alhassan S, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA. 2007;297:969-977.
- Anton SD, Hida A, Heekin K, et al. Effects of popular diets without specific calorie targets on weight loss outcomes: systematic review of findings from clinical trials. Nutrients. 2017;9:822.
- Castellana M, Conte E, Cignarelli A, et al. Efficacy and safety of very low calorie ketogenic diet (VLCKD) in patients with overweight and obesity: a systematic review and meta-analysis. Rev Endocr Metab Disord. 2020;21:5-16.
- Paoli A, Mancin L, Giacona MC, et al. Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. J Transl Med. 2020;18:104.
- Dashti HM, Mathew TC, Hussein T, et al. Long-term effects of a ketogenic diet in obese patients. Exp Clin Cardiol. 2004;9:200-205.
- Lian N, Chen M. Metabolic syndrome and skin disease: potential connection and risk. Int J Dermatol Venereol. 2019;2:89-93.
- Hu Y, Zhu Y, Lian N, et al. Metabolic syndrome and skin diseases. Front Endocrinol (Lausanne). 2019;10:788.
- Castaldo G, Rastrelli L, Galdo G, et al. Aggressive weight-loss program with a ketogenic induction phase for the treatment of chronic plaque psoriasis: a proof-of-concept, single-arm, open-label clinical trial. Nutrition. 2020;74:110757.
- Castaldo G, Pagano I, Grimaldi M, et al. Effect of very-low-calorie ketogenic diet on psoriasis patients: a nuclear magnetic resonance-based metabolomic study. J Proteome Res. 2021;20:1509-1521.
- Milder J, Liang L-P, Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis. 2010;40:238-244.
- Kubo E, Chhunchha B, Singh P, et al. Sulforaphane reactivates cellular antioxidant defense by inducing Nrf2/ARE/Prdx6 activity during aging and oxidative stress. Sci Rep. 2017;7:14130.
- Pinto A, Bonucci A, Maggi E, et al. Anti-oxidant and anti-inflammatory activity of ketogenic diet: new perspectives for neuroprotection in Alzheimer's disease. Antioxidants (Basel). 2018;7:63.
- Youm Y-H, Nguyen KY, Grant RW, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Kelley N, Jeltema D, Duan Y, et al. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20:3328.
- Fomin DA, McDaniel B, Crane J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatol Treat. 2017;28:484-487.
- Rahman M, Muhammad S, Khan MA, et al. The β-hydroxybutyrate receptor HCA 2 activates a neuroprotective subset of macrophages. Nat Commun. 2014;5:1-11.
- Lu Y, Yang YY, Zhou MW, et al. Ketogenic diet attenuates oxidative stress and inflammation after spinal cord injury by activating Nrf2 and suppressing the NF-κB signaling pathways. Neurosci Lett. 2018;683:13-18.
- Hamarsheh S, Zeiser R. NLRP3 inflammasome activation in cancer: a double-edged sword. Front Immunol. 2020;11:1444.
- Bell S, Degitz K, Quirling M, et al. Involvement of NF-κB signalling in skin physiology and disease. Cell Signal. 2003;15:1-7.
- Goldminz AM, Au SC, Kim N, et al. NF-κB: an essential transcription factor in psoriasis. J Dermatol Sci. 2013;69:89-94.
- Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589.
- McDaniel S, Rensing N, Yamada K, et al. The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia. 2011;52:E7-E11.
- Alter M, Satzger I, Schrem H, et al. Non-melanoma skin cancer is reduced after switch of immunosuppression to mTOR-inhibitors in organ transplant recipients. J Dtsch Dermatol Ges. 2014;12:480-488.
- Feldmeyer L, Hofbauer GF, Böni T, et al. Mammalian target of rapamycin (mTOR) inhibitors slow skin carcinogenesis, but impair wound healing. Br J Dermatol. 2012;166:422-424.
- Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41:211-218.
- Li W. "Warburg effect" and mitochondrial metabolism in skin cancer.J Carcinogene Mutagene. 2012:S4.
- Naveed S, Aslam M, Ahmad A. Starvation based differential chemotherapy: a novel approach for cancer treatment. Oman Med J. 2014;29:391-398.
- Raffaghello L, Lee C, Safdie FM, et al. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Natl Acad Sci U S A. 2008;105:8215-8220.
- Buono R, Longo VD. Starvation, stress resistance, and cancer. Trends Endocrinol Metab. 2018;29:271-280.
- de Groot S, Pijl H, van der Hoeven JJM, et al. Effects of short-term fasting on cancer treatment. J Exp Clin Cancer Res. 2019;38:209.
- Hosseini M, Kasraian Z, Rezvani HR. Energy metabolism in skin cancers: a therapeutic perspective. Biochim Biophys Acta Bioenerg. 2017;1858:712-722.
- Feichtinger RG, Lang R, Geilberger R, et al. Melanoma tumors exhibit a variable but distinct metabolic signature. Exp Dermatol. 2018;27:204-207.
- Alshaya MA, Turkmani MG, Alissa AM. Prurigo pigmentosa following ketogenic diet and bariatric surgery: a growing association. JAAD Case Rep. 2019;5:504-507.
- Bellini M, Tonarelli S, Nagy AG, et al. Low FODMAP diet: evidence, doubts, and hopes. Nutrients. 2020;12:148.
- Kwiatkowski L, Rice E, Langland J. Integrative treatment of chronic abdominal bloating and pain associated with overgrowth of small intestinal bacteria: a case report. Altern Ther Health Med. 2017;23:56-61.
- Hubkova T. No more pain in the gut: lifestyle medicine approach to irritable bowel syndrome. Am J Lifestyle Med. 2017;11:223-226.
- Schumann D, Klose P, Lauche R, et al. Low fermentable, oligo-, di-, mono-saccharides and polyol diet in the treatment of irritable bowel syndrome: a systematic review and meta-analysis. Nutrition. 2018;45:24-31.
- Cox SR, Prince AC, Myers CE, et al. Fermentable carbohydrates [FODMAPs] exacerbate functional gastrointestinal symptoms in patients with inflammatory bowel disease: a randomised, double-blind, placebo-controlled, cross-over, re-challenge trial. J Crohns Colitis. 2017;11:1420-1429.
- Damas OM, Garces L, Abreu MT. Diet as adjunctive treatment for inflammatory bowel disease: review and update of the latest literature. Curr Treat Options Gastroenterol. 2019;17:313-325.
- Wang FY, Chi CC. Rosacea, germs, and bowels: a review on gastrointestinal comorbidities and gut-skin axis of rosacea. Adv Ther. 2021;38:1415-1424.
- Daou H, Paradiso M, Hennessy K, et al. Rosacea and the microbiome: a systematic review. Dermatol Ther (Heidelb). 2021;11:1-12.
- Weinstock LB, Steinhoff M. Rosacea and small intestinal bacterial overgrowth: prevalence and response to rifaximin. J Am Acad Dermatol. 2013;68:875-876.
- Wu CY, Chang YT, Juan CK, et al. Risk of inflammatory bowel disease in patients with rosacea: results from a nationwide cohort study in Taiwan. J Am Acad Dermatol. 2017;76:911-917.
- Egeberg A, Weinstock LB, Thyssen EP, et al. Rosacea and gastrointestinal disorders: a population-based cohort study. Br J Dermatol. 2017;176:100-106.
- Drago F, De Col E, Agnoletti AF, et al. The role of small intestinal bacterial overgrowth in rosacea: a 3-year follow-up. J Am Acad Dermatol. 2016;75:E113-E115.
- Parodi A, Paolino S, Greco A, et al. Small intestinal bacterial overgrowth in rosacea: clinical effectiveness of its eradication. Clin Gastroenterol Hepatol. 2008;6:759-764.
- Ojetti V, De Simone C, Aguilar Sanchez J, et al. Malabsorption in psoriatic patients: cause or consequence? Scand J Gastroenterol. 2006;41:1267-1271.
- Kim M, Choi KH, Hwang SW, et al. Inflammatory bowel disease is associated with an increased risk of inflammatory skin diseases: a population-based cross-sectional study. J Am Acad Dermatol. 2017;76:40-48.
- Drago F, Ciccarese G, Indemini E, et al. Psoriasis and small intestine bacterial overgrowth. Int J Dermatol. 2018;57:112-113.
- Acharya P, Mathur M. Association between psoriasis and celiac disease: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:1376-1385.
- Bhatia BK, Millsop JW, Debbaneh M, et al. Diet and psoriasis, part II: celiac disease and role of a gluten-free diet. J Am Acad Dermatol. 2014;71:350-358.
- Michaëlsson G, Gerdén B, Hagforsen E, et al. Psoriasis patients with antibodies to gliadin can be improved by a gluten-free diet. Br J Dermatol. 2000;142:44-51.
- Kolchak NA, Tetarnikova MK, Theodoropoulou MS, et al. Prevalence of antigliadin IgA antibodies in psoriasis vulgaris and response of seropositive patients to a gluten-free diet. J Multidiscip Healthc. 2018;11:13-19.
- De Bastiani R, Gabrielli M, Lora L, et al. Association between coeliac disease and psoriasis: Italian primary care multicentre study. Dermatology. 2015;230:156-160.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Loche F, Bazex J. Celiac disease associated with cutaneous sarcoidosic granuloma [in French]. Rev Med Interne. 1997;18:975-978.
- Rodríguez-García C, González-Hernández S, Pérez-Robayna N, et al. Repigmentation of vitiligo lesions in a child with celiac disease after a gluten-free diet. Pediatr Dermatol. 2011;28:209-210.
- Wijarnpreecha K, Panjawatanan P, Corral JE, et al. Celiac disease and risk of sarcoidosis: a systematic review and meta-analysis. J Evid Based Med. 2019;12:194-199.
- Rodrigo L, Beteta-Gorriti V, Alvarez N, et al. Cutaneous and mucosal manifestations associated with celiac disease. Nutrients. 2018;10:800.
- Song MS, Farber D, Bitton A, et al. Dermatomyositis associated with celiac disease: response to a gluten-free diet. Can J Gastroenterol. 2006;20:433-435.
- Egan CA, Smith EP, Taylor TB, et al. Linear IgA bullous dermatosis responsive to a gluten-free diet. Am J Gastroenterol. 2001;96:1927-1929.
- Comas-Basté O, Sánchez-Pérez S, Veciana-Nogués MT, et al. Histamine intolerance: the current state of the art. Biomolecules. 2020;10:1181.
- Ring J. Plasma histamine concentrations in atopic eczema. Clin Allergy. 1983;13:545-552.
- Maintz L, Benfadal S, Allam JP, et al. Evidence for a reduced histamine degradation capacity in a subgroup of patients with atopic eczema. J Allergy Clin Immunol. 2006;117:1106-1112.
- Cornillier H, Giraudeau B, Samimi M, et al. Effect of diet in chronic spontaneous urticaria: a systematic review. Acta Derm Venereol. 2019;99:127-132.
- Son JH, Chung BY, Kim HO, et al. A histamine-free diet is helpful for treatment of adult patients with chronic spontaneous urticaria. Ann Dermatol. 2018;30:164-172.
- Wagner N, Dirk D, Peveling-Oberhag A, et al. A popular myth - low-histamine diet improves chronic spontaneous urticaria - fact or fiction? J Eur Acad Dermatol Venereol. 2017;31:650-655.
- Esposito K, Marfella R, Ciotola M, et al. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292:1440-1446.
- Steffen LM, Van Horn L, Daviglus ML, et al. A modified Mediterranean diet score is associated with a lower risk of incident metabolic syndrome over 25 years among young adults: the CARDIA (coronary artery risk development in young adults) study. Br J Nutr. 2014;112:1654-1661.
- Bower A, Marquez S, de Mejia EG. The health benefits of selected culinary herbs and spices found in the traditional Mediterranean diet. Crit Rev Food Sci Nutr. 2016;56:2728-2746.
- Bosch R, Philips N, Suárez-Pérez JA, et al. Mechanisms of photoaging and cutaneous photocarcinogenesis, and photoprotective strategies with phytochemicals. Antioxidants (Basel). 2015;4:248-268.
- Katsimbri P, Korakas E, Kountouri A, et al. The effect of antioxidant and anti-inflammatory capacity of diet on psoriasis and psoriatic arthritis phenotype: nutrition as therapeutic tool? Antioxidants. 2021;10:157.
- Molina-Leyva A, Cuenca-Barrales C, Vega-Castillo JJ, et al. Adherence to Mediterranean diet in Spanish patients with psoriasis: cardiovascular benefits? Dermatol Ther. 2019;32:E12810.
- Barrea L, Balato N, Di Somma C, et al. Nutrition and psoriasis: is there any association between the severity of the disease and adherence to the Mediterranean diet? J Transl Med. 2015;13:1-10.
- Phan C, Touvier M, Kesse-Guyot E, et al. Association between Mediterranean anti-inflammatory dietary profile and severity of psoriasis: results from the NutriNet-Santé cohort. JAMA Dermatol. 2018;154:1017-1024.
- Korovesi A, Dalamaga M, Kotopouli M, et al. Adherence to the Mediterranean diet is independently associated with psoriasis risk, severity, and quality of life: a cross-sectional observational study. Int J Dermatol. 2019;58:E164-E165.
- Ford AR, Siegel M, Bagel J, et al. Dietary recommendations for adults with psoriasis or psoriatic arthritis from the medical board of the National Psoriasis Foundation: a systematic review. JAMA Dermatol. 2018;154:934-950.
- Skroza N, Tolino E, Semyonov L, et al. Mediterranean diet and familial dysmetabolism as factors influencing the development of acne. Scand J Public Health. 2012;40:466-474.
- Barrea L, Fabbrocini G, Annunziata G, et al. Role of nutrition and adherence to the Mediterranean diet in the multidisciplinary approach of hidradenitis suppurativa: evaluation of nutritional status and its association with severity of disease. Nutrients. 2018;11:57.
- Nichols JA, Katiyar SK. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch Dermatol Res. 2010;302:71-83.
- Huang T-H, Wang P-W, Yang S-C, et al. Cosmetic and therapeutic applications of fish oil's fatty acids on the skin. Mar Drugs. 2018;16:256.
- Rizwan M, Rodriguez-Blanco I, Harbottle A, et al. Tomato paste rich in lycopene protects against cutaneous photodamage in humans in vivo: a randomized controlled trial. Br J Dermatol. 2011;164:154-162.
- Leone A, Martínez-González M, Martin-Gorgojo A, et al. Mediterranean diet, dietary approaches to stop hypertension, and pro-vegetarian dietary pattern in relation to the risk of basal cell carcinoma: a nested case-control study within the Seguimiento Universidad de Navarra (SUN) cohort. Am J Clin Nutr. 2020;112:364-372.
- Solway J, McBride M, Haq F, et al. Diet and dermatology: the role of a whole-food, plant-based diet in preventing and reversing skin aging--a review. J Clin Aesthet Dermatol. 2020;13:38-43.
- Greger M. A whole food plant-based diet is effective for weight loss: the evidence. Am J Lifestyle Med. 2020;14:500-510.
- Wright N, Wilson L, Smith M, et al. The BROAD study: a randomised controlled trial using a whole food plant-based diet in the community for obesity, ischaemic heart disease or diabetes. Nutr Diabetes. 2017;7:E256.
- Ornish D, Lin J, Chan JM, et al. Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. Lancet Oncol. 2013;14:1112-1120.
- Ornish D, Lin J, Daubenmier J, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008;9:1048-1057.
- Zouboulis CC, Makrantonaki E. Clinical aspects and molecular diagnostics of skin aging. Clin Dermatol. 2011;29:3-14.
- Gupta L, Khandelwal D, Lal PR, et al. Palaeolithic diet in diabesity and endocrinopathies--a vegan's perspective. Eur Endocrinol. 2019;15:77-82.
- Chassaing B, Van de Wiele T, De Bodt J, et al. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut. 2017;66:1414-1427.
- Thorburn Alison N, Macia L, Mackay Charles R. Diet, metabolites, and "Western lifestyle" inflammatory diseases. Immunity. 2014;40:833-842.
- Katta R, Schlichte M. Diet and dermatitis: food triggers. J Clin Aesthet Dermatol. 2014;7:30-36.
- Dhar S, Srinivas SM. Food allergy in atopic dermatitis. Indian J Dermatol. 2016;61:645-648.
- Birmingham N, Thanesvorakul S, Gangur V. Relative immunogenicity of commonly allergenic foods versus rarely allergenic and nonallergenic foods in mice. J Food Prot. 2002;65:1988-1991.
- Yu W, Freeland DMH, Nadeau KC. Food allergy: immune mechanisms, diagnosis and immunotherapy. Nat Rev Immunol. 2016;16:751-765.
- Kowalski LM, Bujko J. Evaluation of biological and clinical potential of paleolithic diet [in Polish]. Rocz Panstw Zakl Hig. 2012;63:9-15.
- Lee JE, Titcomb TJ, Bisht B, et al. A modified MCT-based ketogenic diet increases plasma β-hydroxybutyrate but has less effect on fatigue and quality of life in people with multiple sclerosis compared to a modified paleolithic diet: a waitlist-controlled, randomized pilot study. J Am Coll Nutr. 2021;40:13-25.
- Abbott RD, Sadowski A, Alt AG. Efficacy of the autoimmune protocol diet as part of a multi-disciplinary, supported lifestyle intervention for Hashimoto's thyroiditis. Cureus. 2019;11:E4556.
- Lindeberg S, Jönsson T, Granfeldt Y, et al. A palaeolithic diet improves glucose tolerance more than a Mediterranean-like diet in individuals with ischaemic heart disease. Diabetologia. 2007;50:1795-1807.
- Jönsson T, Granfeldt Y, Ahrén B, et al. Beneficial effects of a paleolithic diet on cardiovascular risk factors in type 2 diabetes: a randomized cross-over pilot study. Cardiovasc Diabetol. 2009;8:35.
- Boers I, Muskiet FAJ, Berkelaar E, et al. Favourable effects of consuming a palaeolithic-type diet on characteristics of the metabolic syndrome: a randomized controlled pilot-study. Lipids Health Dis. 2014;13:160.
- Ghaedi E, Mohammadi M, Mohammadi H, et al. Effects of a paleolithic diet on cardiovascular disease risk factors: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2019;10:634-646.
- Mellberg C, Sandberg S, Ryberg M, et al. Long-term effects of a palaeolithic-type diet in obese postmenopausal women: a 2-year randomized trial. Eur J Clin Nutr. 2014;68:350-357.
- Pastore RL, Brooks JT, Carbone JW. Paleolithic nutrition improves plasma lipid concentrations of hypercholesterolemic adults to a greater extent than traditional heart-healthy dietary recommendations. Nutr Res. 2015;35:474-479.
- Otten J, Stomby A, Waling M, et al. Benefits of a paleolithic diet with and without supervised exercise on fat mass, insulin sensitivity, and glycemic control: a randomized controlled trial in individuals with type 2 diabetes. Diabetes Metab Res Rev. 2017;33:E2828.
- Stefanadi EC, Dimitrakakis G, Antoniou C-K, et al. Metabolic syndrome and the skin: a more than superficial association. reviewing the association between skin diseases and metabolic syndrome and a clinical decision algorithm for high risk patients. Diabetol Metab Syndr. 2018;10:9.
- Mann N. Meat in the human diet: an anthropological perspective. Nutr Dietetics. 2007;64(suppl 4):S102-S107.
- Bramble DM, Lieberman DE. Endurance running and the evolution of Homo. Nature. 2004;432:345-352.
- Kuhn JE. Throwing, the shoulder, and human evolution. Am J Orthop (Belle Mead NJ). 2016;45:110-114.
- Kobayashi H, Kohshima S. Unique morphology of the human eye and its adaptive meaning: comparative studies on external morphology of the primate eye. J Hum Evol. 2001;40:419-435.
- Cordain L, Eaton SB, Miller JB, et al. The paradoxical nature of hunter-gatherer diets: meat-based, yet non-atherogenic. Eur J Clin Nutr. 2002;56(suppl 1):S42-S52.
- McClellan WS, Du Bois EF. Clinical calorimetry: XLV. prolonged meat diets with a study of kidney function and ketosis. J Biol Chem. 1930;87:651-668.
- O'Hearn A. Can a carnivore diet provide all essential nutrients? Curr Opin Endocrinol Diabetes Obes. 2020;27:312-316.
- O'Hearn LA. A survey of improvements experienced on a carnivore diet compared to only carbohydrate restriction. Open Science Forum website. Published February 12, 2019. Accessed May 17, 2021. doi:10.17605/OSF.IO/5FU4D
- Williams P. Nutritional composition of red meat. Nutrition & Dietetics. 2007;64(suppl 4):S113-S119.
- Biel W, Czerniawska-Piątkowska E, Kowalczyk A. Offal chemical composition from veal, beef, and lamb maintained in organic production systems. Animals (Basel). 2019;9:489.
- Elmadfa I, Meyer AL. The role of the status of selected micronutrients in shaping the immune function. Endocr Metab Immune Disord Drug Targets. 2019;19:1100-1115.
- Babizhayev M. Treatment of skin aging and photoaging with innovative oral dosage forms of nonhydrolized carnosine and carcinine. Int J Clin Derm Res. 2017;5:116-143.
- Danby FW. Nutrition and aging skin: sugar and glycation. Clin Dermatol. 2010;28:409-411.
- Siefken W, Carstensen S, Springmann G, et al. Role of taurine accumulation in keratinocyte hydration. J Invest Dermatol. 2003;121:354-361.
- Vollmer DL, West VA, Lephart ED. Enhancing skin health: by oral administration of natural compounds and minerals with implications to the dermal microbiome. Int J Mol Sci. 2018;19:3059.
- Fischer F, Achterberg V, März A, et al. Folic acid and creatineimprove the firmness of human skin in vivo. J Cosmet Dermatol. 2011;10:15-23.
- Blatt T, Lenz H, Weber T. Topical application of creatine is multibeneficial for human skin. J Am Acad Dermatol. 2005;52:P32.
- Jaros J, Katta R, Shi VY. Dermatonutrigenomics: past, present, and future. Dermatology. 2019;235:164-166.
- Paoli A, Grimaldi K, Toniolo L, et al. Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- Melnik BC, Schmitz G. Role of insulin, insulin-like growth factor-1, hyperglycaemic food and milk consumption in the pathogenesis of acne vulgaris. Exp Dermatol. 2009;18:833-841.
- Smith RN, Mann NJ, Braue A, et al. The effect of a high-protein, low glycemic-load diet versus a conventional, high glycemic-load diet on biochemical parameters associated with acne vulgaris: a randomized, investigator-masked, controlled trial. J Am Acad Dermatol. 2007;57:247-256.
- Smith R, Mann N, Mäkeläinen H, et al. A pilot study to determine the short-term effects of a low glycemic load diet on hormonal markers of acne: a nonrandomized, parallel, controlled feeding trial. Mol Nutr Food Res. 2008;52:718-726.
- Smith RN, Braue A, Varigos GA, et al. The effect of a low glycemic load diet on acne vulgaris and the fatty acid composition of skin surface triglycerides. J Dermatol Sci. 2008;50:41-52.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Khandalavala BN, Do MV. Finasteride in hidradenitis suppurativa: a "male" therapy for a predominantly "female" disease. J Clin Aesthet Dermatol. 2016;9:44.
- Nikolakis G, Karagiannidis I, Vaiopoulos AG, et al. Endocrinological mechanisms in the pathophysiology of hidradenitis suppurativa [in German]. Hautarzt. 2020;71:762-771.
- Karadag AS, Ozlu E, Lavery MJ. Cutaneous manifestations of diabetes mellitus and the metabolic syndrome. Clin Dermatology. 2018;36:89-93.
- Gardner CD, Kiazand A, Alhassan S, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA. 2007;297:969-977.
- Anton SD, Hida A, Heekin K, et al. Effects of popular diets without specific calorie targets on weight loss outcomes: systematic review of findings from clinical trials. Nutrients. 2017;9:822.
- Castellana M, Conte E, Cignarelli A, et al. Efficacy and safety of very low calorie ketogenic diet (VLCKD) in patients with overweight and obesity: a systematic review and meta-analysis. Rev Endocr Metab Disord. 2020;21:5-16.
- Paoli A, Mancin L, Giacona MC, et al. Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. J Transl Med. 2020;18:104.
- Dashti HM, Mathew TC, Hussein T, et al. Long-term effects of a ketogenic diet in obese patients. Exp Clin Cardiol. 2004;9:200-205.
- Lian N, Chen M. Metabolic syndrome and skin disease: potential connection and risk. Int J Dermatol Venereol. 2019;2:89-93.
- Hu Y, Zhu Y, Lian N, et al. Metabolic syndrome and skin diseases. Front Endocrinol (Lausanne). 2019;10:788.
- Castaldo G, Rastrelli L, Galdo G, et al. Aggressive weight-loss program with a ketogenic induction phase for the treatment of chronic plaque psoriasis: a proof-of-concept, single-arm, open-label clinical trial. Nutrition. 2020;74:110757.
- Castaldo G, Pagano I, Grimaldi M, et al. Effect of very-low-calorie ketogenic diet on psoriasis patients: a nuclear magnetic resonance-based metabolomic study. J Proteome Res. 2021;20:1509-1521.
- Milder J, Liang L-P, Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis. 2010;40:238-244.
- Kubo E, Chhunchha B, Singh P, et al. Sulforaphane reactivates cellular antioxidant defense by inducing Nrf2/ARE/Prdx6 activity during aging and oxidative stress. Sci Rep. 2017;7:14130.
- Pinto A, Bonucci A, Maggi E, et al. Anti-oxidant and anti-inflammatory activity of ketogenic diet: new perspectives for neuroprotection in Alzheimer's disease. Antioxidants (Basel). 2018;7:63.
- Youm Y-H, Nguyen KY, Grant RW, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Kelley N, Jeltema D, Duan Y, et al. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20:3328.
- Fomin DA, McDaniel B, Crane J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatol Treat. 2017;28:484-487.
- Rahman M, Muhammad S, Khan MA, et al. The β-hydroxybutyrate receptor HCA 2 activates a neuroprotective subset of macrophages. Nat Commun. 2014;5:1-11.
- Lu Y, Yang YY, Zhou MW, et al. Ketogenic diet attenuates oxidative stress and inflammation after spinal cord injury by activating Nrf2 and suppressing the NF-κB signaling pathways. Neurosci Lett. 2018;683:13-18.
- Hamarsheh S, Zeiser R. NLRP3 inflammasome activation in cancer: a double-edged sword. Front Immunol. 2020;11:1444.
- Bell S, Degitz K, Quirling M, et al. Involvement of NF-κB signalling in skin physiology and disease. Cell Signal. 2003;15:1-7.
- Goldminz AM, Au SC, Kim N, et al. NF-κB: an essential transcription factor in psoriasis. J Dermatol Sci. 2013;69:89-94.
- Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589.
- McDaniel S, Rensing N, Yamada K, et al. The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia. 2011;52:E7-E11.
- Alter M, Satzger I, Schrem H, et al. Non-melanoma skin cancer is reduced after switch of immunosuppression to mTOR-inhibitors in organ transplant recipients. J Dtsch Dermatol Ges. 2014;12:480-488.
- Feldmeyer L, Hofbauer GF, Böni T, et al. Mammalian target of rapamycin (mTOR) inhibitors slow skin carcinogenesis, but impair wound healing. Br J Dermatol. 2012;166:422-424.
- Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41:211-218.
- Li W. "Warburg effect" and mitochondrial metabolism in skin cancer.J Carcinogene Mutagene. 2012:S4.
- Naveed S, Aslam M, Ahmad A. Starvation based differential chemotherapy: a novel approach for cancer treatment. Oman Med J. 2014;29:391-398.
- Raffaghello L, Lee C, Safdie FM, et al. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Natl Acad Sci U S A. 2008;105:8215-8220.
- Buono R, Longo VD. Starvation, stress resistance, and cancer. Trends Endocrinol Metab. 2018;29:271-280.
- de Groot S, Pijl H, van der Hoeven JJM, et al. Effects of short-term fasting on cancer treatment. J Exp Clin Cancer Res. 2019;38:209.
- Hosseini M, Kasraian Z, Rezvani HR. Energy metabolism in skin cancers: a therapeutic perspective. Biochim Biophys Acta Bioenerg. 2017;1858:712-722.
- Feichtinger RG, Lang R, Geilberger R, et al. Melanoma tumors exhibit a variable but distinct metabolic signature. Exp Dermatol. 2018;27:204-207.
- Alshaya MA, Turkmani MG, Alissa AM. Prurigo pigmentosa following ketogenic diet and bariatric surgery: a growing association. JAAD Case Rep. 2019;5:504-507.
- Bellini M, Tonarelli S, Nagy AG, et al. Low FODMAP diet: evidence, doubts, and hopes. Nutrients. 2020;12:148.
- Kwiatkowski L, Rice E, Langland J. Integrative treatment of chronic abdominal bloating and pain associated with overgrowth of small intestinal bacteria: a case report. Altern Ther Health Med. 2017;23:56-61.
- Hubkova T. No more pain in the gut: lifestyle medicine approach to irritable bowel syndrome. Am J Lifestyle Med. 2017;11:223-226.
- Schumann D, Klose P, Lauche R, et al. Low fermentable, oligo-, di-, mono-saccharides and polyol diet in the treatment of irritable bowel syndrome: a systematic review and meta-analysis. Nutrition. 2018;45:24-31.
- Cox SR, Prince AC, Myers CE, et al. Fermentable carbohydrates [FODMAPs] exacerbate functional gastrointestinal symptoms in patients with inflammatory bowel disease: a randomised, double-blind, placebo-controlled, cross-over, re-challenge trial. J Crohns Colitis. 2017;11:1420-1429.
- Damas OM, Garces L, Abreu MT. Diet as adjunctive treatment for inflammatory bowel disease: review and update of the latest literature. Curr Treat Options Gastroenterol. 2019;17:313-325.
- Wang FY, Chi CC. Rosacea, germs, and bowels: a review on gastrointestinal comorbidities and gut-skin axis of rosacea. Adv Ther. 2021;38:1415-1424.
- Daou H, Paradiso M, Hennessy K, et al. Rosacea and the microbiome: a systematic review. Dermatol Ther (Heidelb). 2021;11:1-12.
- Weinstock LB, Steinhoff M. Rosacea and small intestinal bacterial overgrowth: prevalence and response to rifaximin. J Am Acad Dermatol. 2013;68:875-876.
- Wu CY, Chang YT, Juan CK, et al. Risk of inflammatory bowel disease in patients with rosacea: results from a nationwide cohort study in Taiwan. J Am Acad Dermatol. 2017;76:911-917.
- Egeberg A, Weinstock LB, Thyssen EP, et al. Rosacea and gastrointestinal disorders: a population-based cohort study. Br J Dermatol. 2017;176:100-106.
- Drago F, De Col E, Agnoletti AF, et al. The role of small intestinal bacterial overgrowth in rosacea: a 3-year follow-up. J Am Acad Dermatol. 2016;75:E113-E115.
- Parodi A, Paolino S, Greco A, et al. Small intestinal bacterial overgrowth in rosacea: clinical effectiveness of its eradication. Clin Gastroenterol Hepatol. 2008;6:759-764.
- Ojetti V, De Simone C, Aguilar Sanchez J, et al. Malabsorption in psoriatic patients: cause or consequence? Scand J Gastroenterol. 2006;41:1267-1271.
- Kim M, Choi KH, Hwang SW, et al. Inflammatory bowel disease is associated with an increased risk of inflammatory skin diseases: a population-based cross-sectional study. J Am Acad Dermatol. 2017;76:40-48.
- Drago F, Ciccarese G, Indemini E, et al. Psoriasis and small intestine bacterial overgrowth. Int J Dermatol. 2018;57:112-113.
- Acharya P, Mathur M. Association between psoriasis and celiac disease: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:1376-1385.
- Bhatia BK, Millsop JW, Debbaneh M, et al. Diet and psoriasis, part II: celiac disease and role of a gluten-free diet. J Am Acad Dermatol. 2014;71:350-358.
- Michaëlsson G, Gerdén B, Hagforsen E, et al. Psoriasis patients with antibodies to gliadin can be improved by a gluten-free diet. Br J Dermatol. 2000;142:44-51.
- Kolchak NA, Tetarnikova MK, Theodoropoulou MS, et al. Prevalence of antigliadin IgA antibodies in psoriasis vulgaris and response of seropositive patients to a gluten-free diet. J Multidiscip Healthc. 2018;11:13-19.
- De Bastiani R, Gabrielli M, Lora L, et al. Association between coeliac disease and psoriasis: Italian primary care multicentre study. Dermatology. 2015;230:156-160.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Loche F, Bazex J. Celiac disease associated with cutaneous sarcoidosic granuloma [in French]. Rev Med Interne. 1997;18:975-978.
- Rodríguez-García C, González-Hernández S, Pérez-Robayna N, et al. Repigmentation of vitiligo lesions in a child with celiac disease after a gluten-free diet. Pediatr Dermatol. 2011;28:209-210.
- Wijarnpreecha K, Panjawatanan P, Corral JE, et al. Celiac disease and risk of sarcoidosis: a systematic review and meta-analysis. J Evid Based Med. 2019;12:194-199.
- Rodrigo L, Beteta-Gorriti V, Alvarez N, et al. Cutaneous and mucosal manifestations associated with celiac disease. Nutrients. 2018;10:800.
- Song MS, Farber D, Bitton A, et al. Dermatomyositis associated with celiac disease: response to a gluten-free diet. Can J Gastroenterol. 2006;20:433-435.
- Egan CA, Smith EP, Taylor TB, et al. Linear IgA bullous dermatosis responsive to a gluten-free diet. Am J Gastroenterol. 2001;96:1927-1929.
- Comas-Basté O, Sánchez-Pérez S, Veciana-Nogués MT, et al. Histamine intolerance: the current state of the art. Biomolecules. 2020;10:1181.
- Ring J. Plasma histamine concentrations in atopic eczema. Clin Allergy. 1983;13:545-552.
- Maintz L, Benfadal S, Allam JP, et al. Evidence for a reduced histamine degradation capacity in a subgroup of patients with atopic eczema. J Allergy Clin Immunol. 2006;117:1106-1112.
- Cornillier H, Giraudeau B, Samimi M, et al. Effect of diet in chronic spontaneous urticaria: a systematic review. Acta Derm Venereol. 2019;99:127-132.
- Son JH, Chung BY, Kim HO, et al. A histamine-free diet is helpful for treatment of adult patients with chronic spontaneous urticaria. Ann Dermatol. 2018;30:164-172.
- Wagner N, Dirk D, Peveling-Oberhag A, et al. A popular myth - low-histamine diet improves chronic spontaneous urticaria - fact or fiction? J Eur Acad Dermatol Venereol. 2017;31:650-655.
- Esposito K, Marfella R, Ciotola M, et al. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292:1440-1446.
- Steffen LM, Van Horn L, Daviglus ML, et al. A modified Mediterranean diet score is associated with a lower risk of incident metabolic syndrome over 25 years among young adults: the CARDIA (coronary artery risk development in young adults) study. Br J Nutr. 2014;112:1654-1661.
- Bower A, Marquez S, de Mejia EG. The health benefits of selected culinary herbs and spices found in the traditional Mediterranean diet. Crit Rev Food Sci Nutr. 2016;56:2728-2746.
- Bosch R, Philips N, Suárez-Pérez JA, et al. Mechanisms of photoaging and cutaneous photocarcinogenesis, and photoprotective strategies with phytochemicals. Antioxidants (Basel). 2015;4:248-268.
- Katsimbri P, Korakas E, Kountouri A, et al. The effect of antioxidant and anti-inflammatory capacity of diet on psoriasis and psoriatic arthritis phenotype: nutrition as therapeutic tool? Antioxidants. 2021;10:157.
- Molina-Leyva A, Cuenca-Barrales C, Vega-Castillo JJ, et al. Adherence to Mediterranean diet in Spanish patients with psoriasis: cardiovascular benefits? Dermatol Ther. 2019;32:E12810.
- Barrea L, Balato N, Di Somma C, et al. Nutrition and psoriasis: is there any association between the severity of the disease and adherence to the Mediterranean diet? J Transl Med. 2015;13:1-10.
- Phan C, Touvier M, Kesse-Guyot E, et al. Association between Mediterranean anti-inflammatory dietary profile and severity of psoriasis: results from the NutriNet-Santé cohort. JAMA Dermatol. 2018;154:1017-1024.
- Korovesi A, Dalamaga M, Kotopouli M, et al. Adherence to the Mediterranean diet is independently associated with psoriasis risk, severity, and quality of life: a cross-sectional observational study. Int J Dermatol. 2019;58:E164-E165.
- Ford AR, Siegel M, Bagel J, et al. Dietary recommendations for adults with psoriasis or psoriatic arthritis from the medical board of the National Psoriasis Foundation: a systematic review. JAMA Dermatol. 2018;154:934-950.
- Skroza N, Tolino E, Semyonov L, et al. Mediterranean diet and familial dysmetabolism as factors influencing the development of acne. Scand J Public Health. 2012;40:466-474.
- Barrea L, Fabbrocini G, Annunziata G, et al. Role of nutrition and adherence to the Mediterranean diet in the multidisciplinary approach of hidradenitis suppurativa: evaluation of nutritional status and its association with severity of disease. Nutrients. 2018;11:57.
- Nichols JA, Katiyar SK. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch Dermatol Res. 2010;302:71-83.
- Huang T-H, Wang P-W, Yang S-C, et al. Cosmetic and therapeutic applications of fish oil's fatty acids on the skin. Mar Drugs. 2018;16:256.
- Rizwan M, Rodriguez-Blanco I, Harbottle A, et al. Tomato paste rich in lycopene protects against cutaneous photodamage in humans in vivo: a randomized controlled trial. Br J Dermatol. 2011;164:154-162.
- Leone A, Martínez-González M, Martin-Gorgojo A, et al. Mediterranean diet, dietary approaches to stop hypertension, and pro-vegetarian dietary pattern in relation to the risk of basal cell carcinoma: a nested case-control study within the Seguimiento Universidad de Navarra (SUN) cohort. Am J Clin Nutr. 2020;112:364-372.
- Solway J, McBride M, Haq F, et al. Diet and dermatology: the role of a whole-food, plant-based diet in preventing and reversing skin aging--a review. J Clin Aesthet Dermatol. 2020;13:38-43.
- Greger M. A whole food plant-based diet is effective for weight loss: the evidence. Am J Lifestyle Med. 2020;14:500-510.
- Wright N, Wilson L, Smith M, et al. The BROAD study: a randomised controlled trial using a whole food plant-based diet in the community for obesity, ischaemic heart disease or diabetes. Nutr Diabetes. 2017;7:E256.
- Ornish D, Lin J, Chan JM, et al. Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. Lancet Oncol. 2013;14:1112-1120.
- Ornish D, Lin J, Daubenmier J, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008;9:1048-1057.
- Zouboulis CC, Makrantonaki E. Clinical aspects and molecular diagnostics of skin aging. Clin Dermatol. 2011;29:3-14.
- Gupta L, Khandelwal D, Lal PR, et al. Palaeolithic diet in diabesity and endocrinopathies--a vegan's perspective. Eur Endocrinol. 2019;15:77-82.
- Chassaing B, Van de Wiele T, De Bodt J, et al. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut. 2017;66:1414-1427.
- Thorburn Alison N, Macia L, Mackay Charles R. Diet, metabolites, and "Western lifestyle" inflammatory diseases. Immunity. 2014;40:833-842.
- Katta R, Schlichte M. Diet and dermatitis: food triggers. J Clin Aesthet Dermatol. 2014;7:30-36.
- Dhar S, Srinivas SM. Food allergy in atopic dermatitis. Indian J Dermatol. 2016;61:645-648.
- Birmingham N, Thanesvorakul S, Gangur V. Relative immunogenicity of commonly allergenic foods versus rarely allergenic and nonallergenic foods in mice. J Food Prot. 2002;65:1988-1991.
- Yu W, Freeland DMH, Nadeau KC. Food allergy: immune mechanisms, diagnosis and immunotherapy. Nat Rev Immunol. 2016;16:751-765.
- Kowalski LM, Bujko J. Evaluation of biological and clinical potential of paleolithic diet [in Polish]. Rocz Panstw Zakl Hig. 2012;63:9-15.
- Lee JE, Titcomb TJ, Bisht B, et al. A modified MCT-based ketogenic diet increases plasma β-hydroxybutyrate but has less effect on fatigue and quality of life in people with multiple sclerosis compared to a modified paleolithic diet: a waitlist-controlled, randomized pilot study. J Am Coll Nutr. 2021;40:13-25.
- Abbott RD, Sadowski A, Alt AG. Efficacy of the autoimmune protocol diet as part of a multi-disciplinary, supported lifestyle intervention for Hashimoto's thyroiditis. Cureus. 2019;11:E4556.
- Lindeberg S, Jönsson T, Granfeldt Y, et al. A palaeolithic diet improves glucose tolerance more than a Mediterranean-like diet in individuals with ischaemic heart disease. Diabetologia. 2007;50:1795-1807.
- Jönsson T, Granfeldt Y, Ahrén B, et al. Beneficial effects of a paleolithic diet on cardiovascular risk factors in type 2 diabetes: a randomized cross-over pilot study. Cardiovasc Diabetol. 2009;8:35.
- Boers I, Muskiet FAJ, Berkelaar E, et al. Favourable effects of consuming a palaeolithic-type diet on characteristics of the metabolic syndrome: a randomized controlled pilot-study. Lipids Health Dis. 2014;13:160.
- Ghaedi E, Mohammadi M, Mohammadi H, et al. Effects of a paleolithic diet on cardiovascular disease risk factors: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2019;10:634-646.
- Mellberg C, Sandberg S, Ryberg M, et al. Long-term effects of a palaeolithic-type diet in obese postmenopausal women: a 2-year randomized trial. Eur J Clin Nutr. 2014;68:350-357.
- Pastore RL, Brooks JT, Carbone JW. Paleolithic nutrition improves plasma lipid concentrations of hypercholesterolemic adults to a greater extent than traditional heart-healthy dietary recommendations. Nutr Res. 2015;35:474-479.
- Otten J, Stomby A, Waling M, et al. Benefits of a paleolithic diet with and without supervised exercise on fat mass, insulin sensitivity, and glycemic control: a randomized controlled trial in individuals with type 2 diabetes. Diabetes Metab Res Rev. 2017;33:E2828.
- Stefanadi EC, Dimitrakakis G, Antoniou C-K, et al. Metabolic syndrome and the skin: a more than superficial association. reviewing the association between skin diseases and metabolic syndrome and a clinical decision algorithm for high risk patients. Diabetol Metab Syndr. 2018;10:9.
- Mann N. Meat in the human diet: an anthropological perspective. Nutr Dietetics. 2007;64(suppl 4):S102-S107.
- Bramble DM, Lieberman DE. Endurance running and the evolution of Homo. Nature. 2004;432:345-352.
- Kuhn JE. Throwing, the shoulder, and human evolution. Am J Orthop (Belle Mead NJ). 2016;45:110-114.
- Kobayashi H, Kohshima S. Unique morphology of the human eye and its adaptive meaning: comparative studies on external morphology of the primate eye. J Hum Evol. 2001;40:419-435.
- Cordain L, Eaton SB, Miller JB, et al. The paradoxical nature of hunter-gatherer diets: meat-based, yet non-atherogenic. Eur J Clin Nutr. 2002;56(suppl 1):S42-S52.
- McClellan WS, Du Bois EF. Clinical calorimetry: XLV. prolonged meat diets with a study of kidney function and ketosis. J Biol Chem. 1930;87:651-668.
- O'Hearn A. Can a carnivore diet provide all essential nutrients? Curr Opin Endocrinol Diabetes Obes. 2020;27:312-316.
- O'Hearn LA. A survey of improvements experienced on a carnivore diet compared to only carbohydrate restriction. Open Science Forum website. Published February 12, 2019. Accessed May 17, 2021. doi:10.17605/OSF.IO/5FU4D
- Williams P. Nutritional composition of red meat. Nutrition & Dietetics. 2007;64(suppl 4):S113-S119.
- Biel W, Czerniawska-Piątkowska E, Kowalczyk A. Offal chemical composition from veal, beef, and lamb maintained in organic production systems. Animals (Basel). 2019;9:489.
- Elmadfa I, Meyer AL. The role of the status of selected micronutrients in shaping the immune function. Endocr Metab Immune Disord Drug Targets. 2019;19:1100-1115.
- Babizhayev M. Treatment of skin aging and photoaging with innovative oral dosage forms of nonhydrolized carnosine and carcinine. Int J Clin Derm Res. 2017;5:116-143.
- Danby FW. Nutrition and aging skin: sugar and glycation. Clin Dermatol. 2010;28:409-411.
- Siefken W, Carstensen S, Springmann G, et al. Role of taurine accumulation in keratinocyte hydration. J Invest Dermatol. 2003;121:354-361.
- Vollmer DL, West VA, Lephart ED. Enhancing skin health: by oral administration of natural compounds and minerals with implications to the dermal microbiome. Int J Mol Sci. 2018;19:3059.
- Fischer F, Achterberg V, März A, et al. Folic acid and creatineimprove the firmness of human skin in vivo. J Cosmet Dermatol. 2011;10:15-23.
- Blatt T, Lenz H, Weber T. Topical application of creatine is multibeneficial for human skin. J Am Acad Dermatol. 2005;52:P32.
Practice Points
- Patients are increasingly interested in dietary modifications that may influence skin appearance and aid in the treatment of cutaneous disease.
- Although evidence-based dietary recommendations currently are limited, it is important for dermatologists to be aware of the varied and nuanced dietary interventions employed by patients.
- There remains a lack of randomized controlled trials assessing the efficacy of various dietary interventions in the dermatologic setting.
BEAT-LUPUS: Belimumab after rituximab delays severe flares
Using belimumab after rituximab to treat patients with systemic lupus erythematosus (SLE) refractory to conventional therapy not only significantly decreased levels of serum IgG anti-dsDNA antibody levels but also prolonged the time before severe flares of disease occurred in the phase 2b BEAT-LUPUS (Belimumab after B cell depletion in SLE) study.
The trial’s primary outcome of serum IgG anti-dsDNA antibody levels showed a decline from a geometric mean of 162 IU/mL at baseline to 69 IU/mL at 24 weeks and 47 IU/mL at 1 year in patients treated with belimumab (Benlysta) after rituximab (Rituxan and biosimilars). These reductions were significantly lower than the values seen in the placebo after rituximab arm (a respective 121 IU/mL, 99 IU/mL, and 103 IU/mL; P < .001).
Just 3 patients who received belimumab versus 10 who received placebo after rituximab experienced a severe BILAG (British Isles Lupus Assessment Group) index A flare by the end of the study at 52 weeks. The hazard ratio (HR) for the flare reduction was 0.27 (P = .03), indicating a 73% reduction.
The use of belimumab rather than a placebo also led to a small reduction in total serum IgG, and significantly suppressed B-cell repopulation (P = .03).
These results need confirming in a larger, phase 3 trial, the trial’s principal investigator, Michael Ehrenstein, PhD, said at the annual European Congress of Rheumatology. They are “clearly encouraging” and “support the hypothesis that BAFF [B-cell–activating factor] can drive flares after rituximab,” he said.
Although B-cell depletion with rituximab is recommended by national and international guidelines to treat some patients with SLE who are refractory to conventional therapy, its use is not licensed.
“Certainly, rituximab is a controversial drug in lupus,” Dr. Ehrenstein, a consultant rheumatologist based at University College London, said in an interview. Although there is real-world evidence from registries and open-label studies suggesting that it is widely used and effective in some patients, the randomized, controlled trials conducted with rituximab about 10 years ago failed to meet their primary endpoints.
“A lot has been written about why that was, but probably the biggest reason was the high dose of steroids in both groups,” Dr. Ehrenstein said. To try to avoid muddying the waters of the BEAT-LUPUS trial findings, the maximum dose of prednisolone allowed to be used as background therapy was 20 mg/day. The trial’s investigators were also encouraged to reduce the baseline steroid dose to at least 50% by the trial’s 6-month halfway point.
“We tried to reflect what was going on in the U.K.,” Dr. Ehrenstein said, noting that the inspiration for the trial was a patient who had received sequential rituximab treatment. Although she got better with each cycle of rituximab, when her disease flared it would be worse than the time before, with increasingly higher anti-dsDNA levels recorded. The reason for this seemed to be because of increasing BAFF levels, and so the hypothesis was that if rituximab was associated with increased BAFF levels, then co-targeting BAFF with belimumab should be able to prevent those flares from happening.
The BEAT-LUPUS trial has been a huge collaborative effort and was conducted across 16 U.K. centers. From initial funding to the data analysis, it has taken 6 years to complete and was made possible by a unique partnership between Versus Arthritis, University College London Hospitals Biomedical Research Center, the National Institute for Health Research UK Musculoskeletal Translational Research Collaboration, and GlaxoSmithKline (GSK). GSK provided belimumab free of charge, as well as additional funding, but had no role in the design of the study and will not have any role going forward.
From an initial 172 patients assessed for eligibility, 52 patients were finally enrolled into the trial and received rituximab as two infusions given 2 weeks apart. Patients were then randomized in a double-blind manner to receive either belimumab (n = 26) or placebo (n = 26) 4-8 weeks after their first dose of rituximab. The intention-to-treat population consisted of 43 patients.
The use of belimumab after rituximab did not increase the risk for infection – serious or otherwise – or adverse effects, Dr. Ehrenstein reported. Serious adverse events were reported in six (23%) patients in each arm, and serious infections were seen in two (8%) of the belimumab- and four (15%) of the placebo-treated patients.
“I think the take-home message is that it seems that belimumab can reduce the number of severe flares that occur after rituximab therapy,” Dr. Ehrenstein said. “It’s promising, but not definitive,” he added. The next step is of course to publish these data and to perform a phase 3 trial.
In the discussion time following the presentation, session moderator Xavier Mariette, MD, PhD, of Bicêtre Hospital, Paris-Saclay University, asked why not give belimumab first before rituximab if using belimumab afterward works?
“Our strategy was driven by the observation that BAFF levels surged after rituximab, and therefore it’s logical to give the belimumab to block that BAFF surge,” he answered.
“Certainly, there are ideas that belimumab releases mature B cells into the circulation and rituximab can target that,” he added. That strategy is under investigation in the BLISS-BELIEVE trial, which should also report by the end of this year. It’s a much larger, phase 3 trial, involving nearly 300 patients and is sponsored by GSK.
“Clearly, this is a combination treatment [but] whether you give one before the other is uncertain,” Dr. Ehrenstein observed.
Another member of the viewing audience asked whether it would have been a fairer comparison if another dose of rituximab had been given to patients at week 24 instead of no treatment. Dr. Ehrenstein noted that it was a “good point” to make, but the investigators mainly wanted to answer whether giving belimumab after rituximab would target BAFF and thereby drop serum anti-dsDNA antibody levels. He said that a full trial of rituximab for patients with SLE, perhaps adding this extra dose, needs to be conducted.
Dr. Ehrenstein disclosed receiving research funding and educational grants from GSK and participating in advisory panels for the company.
Using belimumab after rituximab to treat patients with systemic lupus erythematosus (SLE) refractory to conventional therapy not only significantly decreased levels of serum IgG anti-dsDNA antibody levels but also prolonged the time before severe flares of disease occurred in the phase 2b BEAT-LUPUS (Belimumab after B cell depletion in SLE) study.
The trial’s primary outcome of serum IgG anti-dsDNA antibody levels showed a decline from a geometric mean of 162 IU/mL at baseline to 69 IU/mL at 24 weeks and 47 IU/mL at 1 year in patients treated with belimumab (Benlysta) after rituximab (Rituxan and biosimilars). These reductions were significantly lower than the values seen in the placebo after rituximab arm (a respective 121 IU/mL, 99 IU/mL, and 103 IU/mL; P < .001).
Just 3 patients who received belimumab versus 10 who received placebo after rituximab experienced a severe BILAG (British Isles Lupus Assessment Group) index A flare by the end of the study at 52 weeks. The hazard ratio (HR) for the flare reduction was 0.27 (P = .03), indicating a 73% reduction.
The use of belimumab rather than a placebo also led to a small reduction in total serum IgG, and significantly suppressed B-cell repopulation (P = .03).
These results need confirming in a larger, phase 3 trial, the trial’s principal investigator, Michael Ehrenstein, PhD, said at the annual European Congress of Rheumatology. They are “clearly encouraging” and “support the hypothesis that BAFF [B-cell–activating factor] can drive flares after rituximab,” he said.
Although B-cell depletion with rituximab is recommended by national and international guidelines to treat some patients with SLE who are refractory to conventional therapy, its use is not licensed.
“Certainly, rituximab is a controversial drug in lupus,” Dr. Ehrenstein, a consultant rheumatologist based at University College London, said in an interview. Although there is real-world evidence from registries and open-label studies suggesting that it is widely used and effective in some patients, the randomized, controlled trials conducted with rituximab about 10 years ago failed to meet their primary endpoints.
“A lot has been written about why that was, but probably the biggest reason was the high dose of steroids in both groups,” Dr. Ehrenstein said. To try to avoid muddying the waters of the BEAT-LUPUS trial findings, the maximum dose of prednisolone allowed to be used as background therapy was 20 mg/day. The trial’s investigators were also encouraged to reduce the baseline steroid dose to at least 50% by the trial’s 6-month halfway point.
“We tried to reflect what was going on in the U.K.,” Dr. Ehrenstein said, noting that the inspiration for the trial was a patient who had received sequential rituximab treatment. Although she got better with each cycle of rituximab, when her disease flared it would be worse than the time before, with increasingly higher anti-dsDNA levels recorded. The reason for this seemed to be because of increasing BAFF levels, and so the hypothesis was that if rituximab was associated with increased BAFF levels, then co-targeting BAFF with belimumab should be able to prevent those flares from happening.
The BEAT-LUPUS trial has been a huge collaborative effort and was conducted across 16 U.K. centers. From initial funding to the data analysis, it has taken 6 years to complete and was made possible by a unique partnership between Versus Arthritis, University College London Hospitals Biomedical Research Center, the National Institute for Health Research UK Musculoskeletal Translational Research Collaboration, and GlaxoSmithKline (GSK). GSK provided belimumab free of charge, as well as additional funding, but had no role in the design of the study and will not have any role going forward.
From an initial 172 patients assessed for eligibility, 52 patients were finally enrolled into the trial and received rituximab as two infusions given 2 weeks apart. Patients were then randomized in a double-blind manner to receive either belimumab (n = 26) or placebo (n = 26) 4-8 weeks after their first dose of rituximab. The intention-to-treat population consisted of 43 patients.
The use of belimumab after rituximab did not increase the risk for infection – serious or otherwise – or adverse effects, Dr. Ehrenstein reported. Serious adverse events were reported in six (23%) patients in each arm, and serious infections were seen in two (8%) of the belimumab- and four (15%) of the placebo-treated patients.
“I think the take-home message is that it seems that belimumab can reduce the number of severe flares that occur after rituximab therapy,” Dr. Ehrenstein said. “It’s promising, but not definitive,” he added. The next step is of course to publish these data and to perform a phase 3 trial.
In the discussion time following the presentation, session moderator Xavier Mariette, MD, PhD, of Bicêtre Hospital, Paris-Saclay University, asked why not give belimumab first before rituximab if using belimumab afterward works?
“Our strategy was driven by the observation that BAFF levels surged after rituximab, and therefore it’s logical to give the belimumab to block that BAFF surge,” he answered.
“Certainly, there are ideas that belimumab releases mature B cells into the circulation and rituximab can target that,” he added. That strategy is under investigation in the BLISS-BELIEVE trial, which should also report by the end of this year. It’s a much larger, phase 3 trial, involving nearly 300 patients and is sponsored by GSK.
“Clearly, this is a combination treatment [but] whether you give one before the other is uncertain,” Dr. Ehrenstein observed.
Another member of the viewing audience asked whether it would have been a fairer comparison if another dose of rituximab had been given to patients at week 24 instead of no treatment. Dr. Ehrenstein noted that it was a “good point” to make, but the investigators mainly wanted to answer whether giving belimumab after rituximab would target BAFF and thereby drop serum anti-dsDNA antibody levels. He said that a full trial of rituximab for patients with SLE, perhaps adding this extra dose, needs to be conducted.
Dr. Ehrenstein disclosed receiving research funding and educational grants from GSK and participating in advisory panels for the company.
Using belimumab after rituximab to treat patients with systemic lupus erythematosus (SLE) refractory to conventional therapy not only significantly decreased levels of serum IgG anti-dsDNA antibody levels but also prolonged the time before severe flares of disease occurred in the phase 2b BEAT-LUPUS (Belimumab after B cell depletion in SLE) study.
The trial’s primary outcome of serum IgG anti-dsDNA antibody levels showed a decline from a geometric mean of 162 IU/mL at baseline to 69 IU/mL at 24 weeks and 47 IU/mL at 1 year in patients treated with belimumab (Benlysta) after rituximab (Rituxan and biosimilars). These reductions were significantly lower than the values seen in the placebo after rituximab arm (a respective 121 IU/mL, 99 IU/mL, and 103 IU/mL; P < .001).
Just 3 patients who received belimumab versus 10 who received placebo after rituximab experienced a severe BILAG (British Isles Lupus Assessment Group) index A flare by the end of the study at 52 weeks. The hazard ratio (HR) for the flare reduction was 0.27 (P = .03), indicating a 73% reduction.
The use of belimumab rather than a placebo also led to a small reduction in total serum IgG, and significantly suppressed B-cell repopulation (P = .03).
These results need confirming in a larger, phase 3 trial, the trial’s principal investigator, Michael Ehrenstein, PhD, said at the annual European Congress of Rheumatology. They are “clearly encouraging” and “support the hypothesis that BAFF [B-cell–activating factor] can drive flares after rituximab,” he said.
Although B-cell depletion with rituximab is recommended by national and international guidelines to treat some patients with SLE who are refractory to conventional therapy, its use is not licensed.
“Certainly, rituximab is a controversial drug in lupus,” Dr. Ehrenstein, a consultant rheumatologist based at University College London, said in an interview. Although there is real-world evidence from registries and open-label studies suggesting that it is widely used and effective in some patients, the randomized, controlled trials conducted with rituximab about 10 years ago failed to meet their primary endpoints.
“A lot has been written about why that was, but probably the biggest reason was the high dose of steroids in both groups,” Dr. Ehrenstein said. To try to avoid muddying the waters of the BEAT-LUPUS trial findings, the maximum dose of prednisolone allowed to be used as background therapy was 20 mg/day. The trial’s investigators were also encouraged to reduce the baseline steroid dose to at least 50% by the trial’s 6-month halfway point.
“We tried to reflect what was going on in the U.K.,” Dr. Ehrenstein said, noting that the inspiration for the trial was a patient who had received sequential rituximab treatment. Although she got better with each cycle of rituximab, when her disease flared it would be worse than the time before, with increasingly higher anti-dsDNA levels recorded. The reason for this seemed to be because of increasing BAFF levels, and so the hypothesis was that if rituximab was associated with increased BAFF levels, then co-targeting BAFF with belimumab should be able to prevent those flares from happening.
The BEAT-LUPUS trial has been a huge collaborative effort and was conducted across 16 U.K. centers. From initial funding to the data analysis, it has taken 6 years to complete and was made possible by a unique partnership between Versus Arthritis, University College London Hospitals Biomedical Research Center, the National Institute for Health Research UK Musculoskeletal Translational Research Collaboration, and GlaxoSmithKline (GSK). GSK provided belimumab free of charge, as well as additional funding, but had no role in the design of the study and will not have any role going forward.
From an initial 172 patients assessed for eligibility, 52 patients were finally enrolled into the trial and received rituximab as two infusions given 2 weeks apart. Patients were then randomized in a double-blind manner to receive either belimumab (n = 26) or placebo (n = 26) 4-8 weeks after their first dose of rituximab. The intention-to-treat population consisted of 43 patients.
The use of belimumab after rituximab did not increase the risk for infection – serious or otherwise – or adverse effects, Dr. Ehrenstein reported. Serious adverse events were reported in six (23%) patients in each arm, and serious infections were seen in two (8%) of the belimumab- and four (15%) of the placebo-treated patients.
“I think the take-home message is that it seems that belimumab can reduce the number of severe flares that occur after rituximab therapy,” Dr. Ehrenstein said. “It’s promising, but not definitive,” he added. The next step is of course to publish these data and to perform a phase 3 trial.
In the discussion time following the presentation, session moderator Xavier Mariette, MD, PhD, of Bicêtre Hospital, Paris-Saclay University, asked why not give belimumab first before rituximab if using belimumab afterward works?
“Our strategy was driven by the observation that BAFF levels surged after rituximab, and therefore it’s logical to give the belimumab to block that BAFF surge,” he answered.
“Certainly, there are ideas that belimumab releases mature B cells into the circulation and rituximab can target that,” he added. That strategy is under investigation in the BLISS-BELIEVE trial, which should also report by the end of this year. It’s a much larger, phase 3 trial, involving nearly 300 patients and is sponsored by GSK.
“Clearly, this is a combination treatment [but] whether you give one before the other is uncertain,” Dr. Ehrenstein observed.
Another member of the viewing audience asked whether it would have been a fairer comparison if another dose of rituximab had been given to patients at week 24 instead of no treatment. Dr. Ehrenstein noted that it was a “good point” to make, but the investigators mainly wanted to answer whether giving belimumab after rituximab would target BAFF and thereby drop serum anti-dsDNA antibody levels. He said that a full trial of rituximab for patients with SLE, perhaps adding this extra dose, needs to be conducted.
Dr. Ehrenstein disclosed receiving research funding and educational grants from GSK and participating in advisory panels for the company.
FROM THE EULAR 2021 CONGRESS