User login
Best diets in 2023: Mediterranean diet wins again
After all, weight loss usually lands one of the top spots on New Year’s resolution surveys.
And just in time, there’s guidance to pick the best plan, as U.S. News & World Report’s annual rankings of the best diet plans were released on Jan. 3.
Once again, the Mediterranean diet, which emphasizes fruits, vegetables, olive oil, and fish, got the top spot, as best diet overall. It’s the sixth consecutive year for that win. But many other diets got top marks as well.
In 2023, U.S. News, with the help of more than 30 nutritionists, doctors, and epidemiologists, ranked 24 diets in several categories to help people find a plan that meets their goals, whether it’s finding the best weight loss diet, easiest one to follow, or plans for other goals, such as managing diabetes or heart disease. Two new categories were added: Best Diets for Bone & Joint Health and Best Family-Friendly Diets.
In previous years, the publication ranked 40 diets. Even if a diet is no longer ranked, its profile with detailed information remains on the site.
“Each year we ask ourselves what we can do better or differently next time,” said Gretel Schueller, managing editor of health for U.S. News. When the publication got feedback from their experts this year, they had requests to consider sustainability of diets and whether they meet a busy family’s needs, in addition to considering many other factors.
This year’s report ranks plans in 11 categories.
The winners and the categories:
Best diets overall
After the Mediterranean diet, two others tied for second place:
- DASH (Dietary Approaches to Stop Hypertension) diet, which fights high blood pressure and emphasizes fruits, vegetables, whole grains, lean protein, and low-fat dairy.
- Flexitarian diet, which focuses on fruits, vegetables, and other healthy foods but also allows occasional meat.
Best weight-loss diets
WW, formerly known as Weight Watchers, got first place. The plan emphasizes not only weight loss but healthier eating and regular activity. The Points program, which assigns specific points to foods, with a daily Points budget, is more personalized than in the past.
- DASH got second place.
- Mayo Clinic Diet and TLC diet tied for third place. The Mayo Clinic Diet focuses on fruits, vegetables, and whole grains. It helps people improve their eating habits. The TLC diet (Therapeutic Lifestyle Changes) focuses on vegetables, fruit, lean protein, and reducing cholesterol levels.
Best fast weight-loss diets
The keto diet got first place. It’s a high-fat, low-carb diet that aims to achieve weight loss through fat burning. Four others tied for second place:
- Atkins, a diet created by the cardiologist Robert Atkins, which begins with very few carbs and then recommends progressively eating more until the weight loss goal is achieved
- Nutrisystem, a commercial program that includes prepackaged meals and focuses on high-protein, lower-glycemic foods to stabilize blood sugar levels
- Optavia, a plan focused on low-carb, low-calorie foods and including fortified meal replacements
- SlimFast Diet, a plan of shakes, smoothies, and meal bars to replace two of three meals a day
Best diets for healthy eating
- Mediterranean
- DASH
- Flexitarian
Best heart-healthy diets
- DASH
- Mediterranean
- Flexitarian and Ornish tied for third. The Ornish Diet focuses on plant-based and whole foods and limiting animal products. It recommends daily exercise and stress reduction.
Best diets for diabetes
- DASH
- Mediterranean
- Flexitarian
Best diets for bone and joint health
DASH and Mediterranean are in a first-place tie, followed by the flexitarian diet.
Best family-friendly diets
This category has a three-way tie: the flexitarian, Mediterranean, and TLC diets.
Best plant-based diets
Mediterranean was first, then flexitarian and the MIND diet. The MIND diet combines the DASH and Mediterranean diets and focuses on “brain-healthy” foods.
Easiest diets to follow
Flexitarian and TLC tied for first, followed by a tie between DASH and Mediterranean.
Best diet programs (formerly called commercial plans)
- WW
- There was a tie for second place between Jenny Craig and Noom, the latter of which focuses on low-calorie foods, with personalized calorie ranges and coaching to help meet goals.
Methodology
A variety of factors were considered, such as whether a diet includes all food groups, how easy it is to follow, whether it can be customized to meet cultural and personal preferences, and if it has a realistic timeline for weight loss.
Response from diet plans
Representatives from two plans that received mixed reviews in the rankings responded.
Jenny Craig was ranked second for best diet program but much lower for family friendly, landing at 22nd place of 24.
“Our program is designed to address the needs of the individual through personalized experiences,” Jenny Craig CEO Mandy Dowson said. “We have many families that participate in our program together but are still evaluated separately to determine appropriate individual goals.”
Its high ranking for best diet program reflects feedback from satisfied members, she said. Among advances will be the new Jenny Fresh program, a line of entrées prepared fresh and delivered to customers’ doors.
Atkins got second place for best fast weight loss but ranked near the bottom for best overall, best weight loss, diabetes, healthy eating, and heart health. In response, Colette Heimowitz, vice president of nutrition and education for Simply Good Foods, which makes Atkins’s food products, said that low-carb eating approaches are a viable option for anyone today.
“There are more than 130 independent, peer-reviewed published studies that show the efficacy and safety of low-carb eating,” she said. “The studies have been conducted for several decades and counting.”
Expert perspective
Samantha Cassetty, a registered dietitian, nutritionist, and wellness expert in New York and author of Sugar Shock, reviewed the report for this news organization. She was not involved in the rankings.
“I think what this shows you is, the best diet overall is also the best for various conditions,” she said. For instance, the Mediterranean, the No. 1 overall, also got high ranking for diabetes, heart health, and bone and joint health.
For consumers trying to lose weight: “If you see fast weight loss, that should be a red flag. A healthy diet for weight loss is one you can sustain,” she said.
She’s not a fan of the programs with prepackaged foods. “It takes the guesswork out, but the portion sizes tend to be unsatisfying. They don’t teach you how to deal with some of the challenges [such as realizing an ‘ideal’ portion size].”
How to use the report
Ms. Schueller’s advice: “Recognize that no diet fits everyone.” When considering which plan to choose, she suggests thinking long-term.
“Whatever we choose has to work in the long run,” she said.
Consumers should consider expenses, meal prep time, and whether the diet fits their lifestyle.
Ideally, she said, the best diet “teaches you smart food preparation and how to make healthy choices, allows the flexibility to be social and eat with groups, whether family or friends.”
Before choosing a diet to follow, consult a medical professional for input on the decision, U.S. News cautioned.
A version of this article first appeared on Medscape.com.
After all, weight loss usually lands one of the top spots on New Year’s resolution surveys.
And just in time, there’s guidance to pick the best plan, as U.S. News & World Report’s annual rankings of the best diet plans were released on Jan. 3.
Once again, the Mediterranean diet, which emphasizes fruits, vegetables, olive oil, and fish, got the top spot, as best diet overall. It’s the sixth consecutive year for that win. But many other diets got top marks as well.
In 2023, U.S. News, with the help of more than 30 nutritionists, doctors, and epidemiologists, ranked 24 diets in several categories to help people find a plan that meets their goals, whether it’s finding the best weight loss diet, easiest one to follow, or plans for other goals, such as managing diabetes or heart disease. Two new categories were added: Best Diets for Bone & Joint Health and Best Family-Friendly Diets.
In previous years, the publication ranked 40 diets. Even if a diet is no longer ranked, its profile with detailed information remains on the site.
“Each year we ask ourselves what we can do better or differently next time,” said Gretel Schueller, managing editor of health for U.S. News. When the publication got feedback from their experts this year, they had requests to consider sustainability of diets and whether they meet a busy family’s needs, in addition to considering many other factors.
This year’s report ranks plans in 11 categories.
The winners and the categories:
Best diets overall
After the Mediterranean diet, two others tied for second place:
- DASH (Dietary Approaches to Stop Hypertension) diet, which fights high blood pressure and emphasizes fruits, vegetables, whole grains, lean protein, and low-fat dairy.
- Flexitarian diet, which focuses on fruits, vegetables, and other healthy foods but also allows occasional meat.
Best weight-loss diets
WW, formerly known as Weight Watchers, got first place. The plan emphasizes not only weight loss but healthier eating and regular activity. The Points program, which assigns specific points to foods, with a daily Points budget, is more personalized than in the past.
- DASH got second place.
- Mayo Clinic Diet and TLC diet tied for third place. The Mayo Clinic Diet focuses on fruits, vegetables, and whole grains. It helps people improve their eating habits. The TLC diet (Therapeutic Lifestyle Changes) focuses on vegetables, fruit, lean protein, and reducing cholesterol levels.
Best fast weight-loss diets
The keto diet got first place. It’s a high-fat, low-carb diet that aims to achieve weight loss through fat burning. Four others tied for second place:
- Atkins, a diet created by the cardiologist Robert Atkins, which begins with very few carbs and then recommends progressively eating more until the weight loss goal is achieved
- Nutrisystem, a commercial program that includes prepackaged meals and focuses on high-protein, lower-glycemic foods to stabilize blood sugar levels
- Optavia, a plan focused on low-carb, low-calorie foods and including fortified meal replacements
- SlimFast Diet, a plan of shakes, smoothies, and meal bars to replace two of three meals a day
Best diets for healthy eating
- Mediterranean
- DASH
- Flexitarian
Best heart-healthy diets
- DASH
- Mediterranean
- Flexitarian and Ornish tied for third. The Ornish Diet focuses on plant-based and whole foods and limiting animal products. It recommends daily exercise and stress reduction.
Best diets for diabetes
- DASH
- Mediterranean
- Flexitarian
Best diets for bone and joint health
DASH and Mediterranean are in a first-place tie, followed by the flexitarian diet.
Best family-friendly diets
This category has a three-way tie: the flexitarian, Mediterranean, and TLC diets.
Best plant-based diets
Mediterranean was first, then flexitarian and the MIND diet. The MIND diet combines the DASH and Mediterranean diets and focuses on “brain-healthy” foods.
Easiest diets to follow
Flexitarian and TLC tied for first, followed by a tie between DASH and Mediterranean.
Best diet programs (formerly called commercial plans)
- WW
- There was a tie for second place between Jenny Craig and Noom, the latter of which focuses on low-calorie foods, with personalized calorie ranges and coaching to help meet goals.
Methodology
A variety of factors were considered, such as whether a diet includes all food groups, how easy it is to follow, whether it can be customized to meet cultural and personal preferences, and if it has a realistic timeline for weight loss.
Response from diet plans
Representatives from two plans that received mixed reviews in the rankings responded.
Jenny Craig was ranked second for best diet program but much lower for family friendly, landing at 22nd place of 24.
“Our program is designed to address the needs of the individual through personalized experiences,” Jenny Craig CEO Mandy Dowson said. “We have many families that participate in our program together but are still evaluated separately to determine appropriate individual goals.”
Its high ranking for best diet program reflects feedback from satisfied members, she said. Among advances will be the new Jenny Fresh program, a line of entrées prepared fresh and delivered to customers’ doors.
Atkins got second place for best fast weight loss but ranked near the bottom for best overall, best weight loss, diabetes, healthy eating, and heart health. In response, Colette Heimowitz, vice president of nutrition and education for Simply Good Foods, which makes Atkins’s food products, said that low-carb eating approaches are a viable option for anyone today.
“There are more than 130 independent, peer-reviewed published studies that show the efficacy and safety of low-carb eating,” she said. “The studies have been conducted for several decades and counting.”
Expert perspective
Samantha Cassetty, a registered dietitian, nutritionist, and wellness expert in New York and author of Sugar Shock, reviewed the report for this news organization. She was not involved in the rankings.
“I think what this shows you is, the best diet overall is also the best for various conditions,” she said. For instance, the Mediterranean, the No. 1 overall, also got high ranking for diabetes, heart health, and bone and joint health.
For consumers trying to lose weight: “If you see fast weight loss, that should be a red flag. A healthy diet for weight loss is one you can sustain,” she said.
She’s not a fan of the programs with prepackaged foods. “It takes the guesswork out, but the portion sizes tend to be unsatisfying. They don’t teach you how to deal with some of the challenges [such as realizing an ‘ideal’ portion size].”
How to use the report
Ms. Schueller’s advice: “Recognize that no diet fits everyone.” When considering which plan to choose, she suggests thinking long-term.
“Whatever we choose has to work in the long run,” she said.
Consumers should consider expenses, meal prep time, and whether the diet fits their lifestyle.
Ideally, she said, the best diet “teaches you smart food preparation and how to make healthy choices, allows the flexibility to be social and eat with groups, whether family or friends.”
Before choosing a diet to follow, consult a medical professional for input on the decision, U.S. News cautioned.
A version of this article first appeared on Medscape.com.
After all, weight loss usually lands one of the top spots on New Year’s resolution surveys.
And just in time, there’s guidance to pick the best plan, as U.S. News & World Report’s annual rankings of the best diet plans were released on Jan. 3.
Once again, the Mediterranean diet, which emphasizes fruits, vegetables, olive oil, and fish, got the top spot, as best diet overall. It’s the sixth consecutive year for that win. But many other diets got top marks as well.
In 2023, U.S. News, with the help of more than 30 nutritionists, doctors, and epidemiologists, ranked 24 diets in several categories to help people find a plan that meets their goals, whether it’s finding the best weight loss diet, easiest one to follow, or plans for other goals, such as managing diabetes or heart disease. Two new categories were added: Best Diets for Bone & Joint Health and Best Family-Friendly Diets.
In previous years, the publication ranked 40 diets. Even if a diet is no longer ranked, its profile with detailed information remains on the site.
“Each year we ask ourselves what we can do better or differently next time,” said Gretel Schueller, managing editor of health for U.S. News. When the publication got feedback from their experts this year, they had requests to consider sustainability of diets and whether they meet a busy family’s needs, in addition to considering many other factors.
This year’s report ranks plans in 11 categories.
The winners and the categories:
Best diets overall
After the Mediterranean diet, two others tied for second place:
- DASH (Dietary Approaches to Stop Hypertension) diet, which fights high blood pressure and emphasizes fruits, vegetables, whole grains, lean protein, and low-fat dairy.
- Flexitarian diet, which focuses on fruits, vegetables, and other healthy foods but also allows occasional meat.
Best weight-loss diets
WW, formerly known as Weight Watchers, got first place. The plan emphasizes not only weight loss but healthier eating and regular activity. The Points program, which assigns specific points to foods, with a daily Points budget, is more personalized than in the past.
- DASH got second place.
- Mayo Clinic Diet and TLC diet tied for third place. The Mayo Clinic Diet focuses on fruits, vegetables, and whole grains. It helps people improve their eating habits. The TLC diet (Therapeutic Lifestyle Changes) focuses on vegetables, fruit, lean protein, and reducing cholesterol levels.
Best fast weight-loss diets
The keto diet got first place. It’s a high-fat, low-carb diet that aims to achieve weight loss through fat burning. Four others tied for second place:
- Atkins, a diet created by the cardiologist Robert Atkins, which begins with very few carbs and then recommends progressively eating more until the weight loss goal is achieved
- Nutrisystem, a commercial program that includes prepackaged meals and focuses on high-protein, lower-glycemic foods to stabilize blood sugar levels
- Optavia, a plan focused on low-carb, low-calorie foods and including fortified meal replacements
- SlimFast Diet, a plan of shakes, smoothies, and meal bars to replace two of three meals a day
Best diets for healthy eating
- Mediterranean
- DASH
- Flexitarian
Best heart-healthy diets
- DASH
- Mediterranean
- Flexitarian and Ornish tied for third. The Ornish Diet focuses on plant-based and whole foods and limiting animal products. It recommends daily exercise and stress reduction.
Best diets for diabetes
- DASH
- Mediterranean
- Flexitarian
Best diets for bone and joint health
DASH and Mediterranean are in a first-place tie, followed by the flexitarian diet.
Best family-friendly diets
This category has a three-way tie: the flexitarian, Mediterranean, and TLC diets.
Best plant-based diets
Mediterranean was first, then flexitarian and the MIND diet. The MIND diet combines the DASH and Mediterranean diets and focuses on “brain-healthy” foods.
Easiest diets to follow
Flexitarian and TLC tied for first, followed by a tie between DASH and Mediterranean.
Best diet programs (formerly called commercial plans)
- WW
- There was a tie for second place between Jenny Craig and Noom, the latter of which focuses on low-calorie foods, with personalized calorie ranges and coaching to help meet goals.
Methodology
A variety of factors were considered, such as whether a diet includes all food groups, how easy it is to follow, whether it can be customized to meet cultural and personal preferences, and if it has a realistic timeline for weight loss.
Response from diet plans
Representatives from two plans that received mixed reviews in the rankings responded.
Jenny Craig was ranked second for best diet program but much lower for family friendly, landing at 22nd place of 24.
“Our program is designed to address the needs of the individual through personalized experiences,” Jenny Craig CEO Mandy Dowson said. “We have many families that participate in our program together but are still evaluated separately to determine appropriate individual goals.”
Its high ranking for best diet program reflects feedback from satisfied members, she said. Among advances will be the new Jenny Fresh program, a line of entrées prepared fresh and delivered to customers’ doors.
Atkins got second place for best fast weight loss but ranked near the bottom for best overall, best weight loss, diabetes, healthy eating, and heart health. In response, Colette Heimowitz, vice president of nutrition and education for Simply Good Foods, which makes Atkins’s food products, said that low-carb eating approaches are a viable option for anyone today.
“There are more than 130 independent, peer-reviewed published studies that show the efficacy and safety of low-carb eating,” she said. “The studies have been conducted for several decades and counting.”
Expert perspective
Samantha Cassetty, a registered dietitian, nutritionist, and wellness expert in New York and author of Sugar Shock, reviewed the report for this news organization. She was not involved in the rankings.
“I think what this shows you is, the best diet overall is also the best for various conditions,” she said. For instance, the Mediterranean, the No. 1 overall, also got high ranking for diabetes, heart health, and bone and joint health.
For consumers trying to lose weight: “If you see fast weight loss, that should be a red flag. A healthy diet for weight loss is one you can sustain,” she said.
She’s not a fan of the programs with prepackaged foods. “It takes the guesswork out, but the portion sizes tend to be unsatisfying. They don’t teach you how to deal with some of the challenges [such as realizing an ‘ideal’ portion size].”
How to use the report
Ms. Schueller’s advice: “Recognize that no diet fits everyone.” When considering which plan to choose, she suggests thinking long-term.
“Whatever we choose has to work in the long run,” she said.
Consumers should consider expenses, meal prep time, and whether the diet fits their lifestyle.
Ideally, she said, the best diet “teaches you smart food preparation and how to make healthy choices, allows the flexibility to be social and eat with groups, whether family or friends.”
Before choosing a diet to follow, consult a medical professional for input on the decision, U.S. News cautioned.
A version of this article first appeared on Medscape.com.
FDA considers regulating CBD products
The products can have drug-like effects on the body and contain CBD (cannabidiol) and THC (tetrahydrocannabinol). Both CBD and THC can be derived from hemp, which was legalized by Congress in 2018.
“Given what we know about the safety of CBD so far, it raises concerns for FDA about whether these existing regulatory pathways for food and dietary supplements are appropriate for this substance,” FDA Principal Deputy Commissioner Janet Woodcock, MD, told The Wall Street Journal.
A 2021 FDA report valued the CBD market at $4.6 billion and projected it to quadruple by 2026. The only FDA-approved CBD product is an oil called Epidiolex, which can be prescribed for the seizure-associated disease epilepsy. Research on CBD to treat other diseases is ongoing.
Food, beverage, and beauty products containing CBD are sold in stores and online in many forms, including oils, vaporized liquids, and oil-based capsules, but “research supporting the drug’s benefits is still limited,” the Mayo Clinic said.
Recently, investigations have found that many CBD products also contain THC, which can be derived from legal hemp in a form that is referred to as Delta 8 and produces a psychoactive high. The CDC warned in 2022 that people “mistook” THC products for CBD products, which are often sold at the same stores, and experienced “adverse events.”
The Centers for Disease Control and Prevention and FDA warn that much is unknown about CBD and delta-8 products. The CDC says known CBD risks include liver damage; interference with other drugs you are taking, which may lead to injury or serious side effects; drowsiness or sleepiness; diarrhea or changes in appetite; changes in mood, such as crankiness; potential negative effects on fetuses during pregnancy or on babies during breastfeeding; or unintentional poisoning of children when mistaking THC products for CBD products or due to containing other ingredients such as THC or pesticides.
“I don’t think that we can have the perfect be the enemy of the good when we’re looking at such a vast market that is so available and utilized,” Norman Birenbaum, a senior FDA adviser who is working on the regulatory issue, told the Journal. “You’ve got a widely unregulated market.”
A version of this article first appeared on WebMD.com.
The products can have drug-like effects on the body and contain CBD (cannabidiol) and THC (tetrahydrocannabinol). Both CBD and THC can be derived from hemp, which was legalized by Congress in 2018.
“Given what we know about the safety of CBD so far, it raises concerns for FDA about whether these existing regulatory pathways for food and dietary supplements are appropriate for this substance,” FDA Principal Deputy Commissioner Janet Woodcock, MD, told The Wall Street Journal.
A 2021 FDA report valued the CBD market at $4.6 billion and projected it to quadruple by 2026. The only FDA-approved CBD product is an oil called Epidiolex, which can be prescribed for the seizure-associated disease epilepsy. Research on CBD to treat other diseases is ongoing.
Food, beverage, and beauty products containing CBD are sold in stores and online in many forms, including oils, vaporized liquids, and oil-based capsules, but “research supporting the drug’s benefits is still limited,” the Mayo Clinic said.
Recently, investigations have found that many CBD products also contain THC, which can be derived from legal hemp in a form that is referred to as Delta 8 and produces a psychoactive high. The CDC warned in 2022 that people “mistook” THC products for CBD products, which are often sold at the same stores, and experienced “adverse events.”
The Centers for Disease Control and Prevention and FDA warn that much is unknown about CBD and delta-8 products. The CDC says known CBD risks include liver damage; interference with other drugs you are taking, which may lead to injury or serious side effects; drowsiness or sleepiness; diarrhea or changes in appetite; changes in mood, such as crankiness; potential negative effects on fetuses during pregnancy or on babies during breastfeeding; or unintentional poisoning of children when mistaking THC products for CBD products or due to containing other ingredients such as THC or pesticides.
“I don’t think that we can have the perfect be the enemy of the good when we’re looking at such a vast market that is so available and utilized,” Norman Birenbaum, a senior FDA adviser who is working on the regulatory issue, told the Journal. “You’ve got a widely unregulated market.”
A version of this article first appeared on WebMD.com.
The products can have drug-like effects on the body and contain CBD (cannabidiol) and THC (tetrahydrocannabinol). Both CBD and THC can be derived from hemp, which was legalized by Congress in 2018.
“Given what we know about the safety of CBD so far, it raises concerns for FDA about whether these existing regulatory pathways for food and dietary supplements are appropriate for this substance,” FDA Principal Deputy Commissioner Janet Woodcock, MD, told The Wall Street Journal.
A 2021 FDA report valued the CBD market at $4.6 billion and projected it to quadruple by 2026. The only FDA-approved CBD product is an oil called Epidiolex, which can be prescribed for the seizure-associated disease epilepsy. Research on CBD to treat other diseases is ongoing.
Food, beverage, and beauty products containing CBD are sold in stores and online in many forms, including oils, vaporized liquids, and oil-based capsules, but “research supporting the drug’s benefits is still limited,” the Mayo Clinic said.
Recently, investigations have found that many CBD products also contain THC, which can be derived from legal hemp in a form that is referred to as Delta 8 and produces a psychoactive high. The CDC warned in 2022 that people “mistook” THC products for CBD products, which are often sold at the same stores, and experienced “adverse events.”
The Centers for Disease Control and Prevention and FDA warn that much is unknown about CBD and delta-8 products. The CDC says known CBD risks include liver damage; interference with other drugs you are taking, which may lead to injury or serious side effects; drowsiness or sleepiness; diarrhea or changes in appetite; changes in mood, such as crankiness; potential negative effects on fetuses during pregnancy or on babies during breastfeeding; or unintentional poisoning of children when mistaking THC products for CBD products or due to containing other ingredients such as THC or pesticides.
“I don’t think that we can have the perfect be the enemy of the good when we’re looking at such a vast market that is so available and utilized,” Norman Birenbaum, a senior FDA adviser who is working on the regulatory issue, told the Journal. “You’ve got a widely unregulated market.”
A version of this article first appeared on WebMD.com.
Why it’s important to offer cosmeceuticals in a cosmetic practice
SAN DIEGO – , advised Ava Shamban, MD.
It’s important to provide patients with high-quality products to take home with them and cosmeceuticals contain biologically active ingredients that enhance skin care efficacy, Dr. Shamban, a dermatologist who practices in Santa Monica, Calif., said at the annual Masters of Aesthetics Symposium. “You can do all the lasers, injectables, and peels that you want, but if you’re not giving your patients high-quality products to take home with them, you’re not doing your job,” she commented.
“Look for brands that are formulated and tested for effectiveness,” she added. “In my office, we like to have products that are designed for specific issues to accompany prescription products, everything from rosacea, acne, melasma, and eczema to psoriasis.”
Dr. Shamban, author of the 2011 book, “Heal Your Skin: The Breakthrough Plan for Renewal,” recommends that dermatologists devise a questionnaire for patients asking them to list their skin-related concerns and use the responses to create a list of products for them to use at home. Provide clear instructions on use, including proper layering of products, how often to use them, and the correct amount to apply. “If you’re not going to do this, someone else will,” she said. Next, instruct them that cosmeceuticals must be used routinely to achieve optimal benefit. “Nothing happens overnight, and be wary of anyone that promises you otherwise,” Dr. Shamban said. “Offering cosmeceuticals helps bridge the gap between at-home routines and in-office treatments. If in-office procedures are a marathon, view the consistent use of the right products at home as your training.”
During her presentation, she showed a photo of the “beauty bar,” the dedicated space with a counter and shelves for displaying skin care products in her Santa Monica office. “It’s good to set something up like this in your office, even if it’s just a little corner, because it gives it authority,” Dr. Shamban said. “Encourage clients to explore the beauty bar after their appointment with you.” She emphasized the importance of offering a wide range of products to accommodate different lifestyles, budgets, skin types, ages, and specific skin concerns, and training staff about the products. “There is never a one-size-fits-all approach to skincare; it’s all about the individual,” she said. “It’s never about pushing product; it’s always about educating patients.”
Dr. Shamban disclosed that she conducts clinical trials for many pharmaceutical and device companies.
SAN DIEGO – , advised Ava Shamban, MD.
It’s important to provide patients with high-quality products to take home with them and cosmeceuticals contain biologically active ingredients that enhance skin care efficacy, Dr. Shamban, a dermatologist who practices in Santa Monica, Calif., said at the annual Masters of Aesthetics Symposium. “You can do all the lasers, injectables, and peels that you want, but if you’re not giving your patients high-quality products to take home with them, you’re not doing your job,” she commented.
“Look for brands that are formulated and tested for effectiveness,” she added. “In my office, we like to have products that are designed for specific issues to accompany prescription products, everything from rosacea, acne, melasma, and eczema to psoriasis.”
Dr. Shamban, author of the 2011 book, “Heal Your Skin: The Breakthrough Plan for Renewal,” recommends that dermatologists devise a questionnaire for patients asking them to list their skin-related concerns and use the responses to create a list of products for them to use at home. Provide clear instructions on use, including proper layering of products, how often to use them, and the correct amount to apply. “If you’re not going to do this, someone else will,” she said. Next, instruct them that cosmeceuticals must be used routinely to achieve optimal benefit. “Nothing happens overnight, and be wary of anyone that promises you otherwise,” Dr. Shamban said. “Offering cosmeceuticals helps bridge the gap between at-home routines and in-office treatments. If in-office procedures are a marathon, view the consistent use of the right products at home as your training.”
During her presentation, she showed a photo of the “beauty bar,” the dedicated space with a counter and shelves for displaying skin care products in her Santa Monica office. “It’s good to set something up like this in your office, even if it’s just a little corner, because it gives it authority,” Dr. Shamban said. “Encourage clients to explore the beauty bar after their appointment with you.” She emphasized the importance of offering a wide range of products to accommodate different lifestyles, budgets, skin types, ages, and specific skin concerns, and training staff about the products. “There is never a one-size-fits-all approach to skincare; it’s all about the individual,” she said. “It’s never about pushing product; it’s always about educating patients.”
Dr. Shamban disclosed that she conducts clinical trials for many pharmaceutical and device companies.
SAN DIEGO – , advised Ava Shamban, MD.
It’s important to provide patients with high-quality products to take home with them and cosmeceuticals contain biologically active ingredients that enhance skin care efficacy, Dr. Shamban, a dermatologist who practices in Santa Monica, Calif., said at the annual Masters of Aesthetics Symposium. “You can do all the lasers, injectables, and peels that you want, but if you’re not giving your patients high-quality products to take home with them, you’re not doing your job,” she commented.
“Look for brands that are formulated and tested for effectiveness,” she added. “In my office, we like to have products that are designed for specific issues to accompany prescription products, everything from rosacea, acne, melasma, and eczema to psoriasis.”
Dr. Shamban, author of the 2011 book, “Heal Your Skin: The Breakthrough Plan for Renewal,” recommends that dermatologists devise a questionnaire for patients asking them to list their skin-related concerns and use the responses to create a list of products for them to use at home. Provide clear instructions on use, including proper layering of products, how often to use them, and the correct amount to apply. “If you’re not going to do this, someone else will,” she said. Next, instruct them that cosmeceuticals must be used routinely to achieve optimal benefit. “Nothing happens overnight, and be wary of anyone that promises you otherwise,” Dr. Shamban said. “Offering cosmeceuticals helps bridge the gap between at-home routines and in-office treatments. If in-office procedures are a marathon, view the consistent use of the right products at home as your training.”
During her presentation, she showed a photo of the “beauty bar,” the dedicated space with a counter and shelves for displaying skin care products in her Santa Monica office. “It’s good to set something up like this in your office, even if it’s just a little corner, because it gives it authority,” Dr. Shamban said. “Encourage clients to explore the beauty bar after their appointment with you.” She emphasized the importance of offering a wide range of products to accommodate different lifestyles, budgets, skin types, ages, and specific skin concerns, and training staff about the products. “There is never a one-size-fits-all approach to skincare; it’s all about the individual,” she said. “It’s never about pushing product; it’s always about educating patients.”
Dr. Shamban disclosed that she conducts clinical trials for many pharmaceutical and device companies.
AT MOAS 2022
STEMI times to treatment usually miss established goals
Therapy initiated within national treatment-time goals set a decade ago for patients with ST-segment elevation myocardial infarction (STEMI) remains associated with improved survival in recent years. But for many such patients, time from first symptoms to initiation of reperfusion therapy still fails to meet those goals, suggests a cross-sectional registry analysis.
For example, patients initially transported to centers with percutaneous coronary intervention (PCI) capability had a median treatment time of 148 minutes, in the analysis spanning the second quarter (Q2) of 2018 to the third quarter (Q3) of 2021. But the goal for centers called for treatment initiation within 90 minutes for at least 75% of such STEMI patients.
Moreover, overall STEMI treatment times and in-hospital mortality rose in tandem significantly from Q2 2018 through the first quarter (Q1) of 2021, which included the first year of the COVID-19 pandemic. Median time to treatment went from 86 minutes to 91 minutes during that period. Meanwhile, in-hospital mortality went from 5.6% to 8.7%, report the study authors led by James G. Jollis, MD, Duke University, Durham, N.C.
Their report, based on 114,871 STEMI patients at 601 US hospitals contributing to the Get With The Guidelines – Coronary Artery Disease registry, was published online in JAMA.
Of those patients, 25,085 had been transferred from non-PCI hospitals, 32,483 were walk-ins, and 57,303 arrived via emergency medical services (EMS). Their median times from symptom onset to PCI were 240, 195, and 148 minutes, respectively.
In-hospital mortality was significantly reduced in an adjusted analysis for patients treated within target times, compared with those whose treatment missed the time goals, regardless of whether they were transported by EMS, walked into a hospital with on-site PCI, or were transferred from a non-PCI center (Table 1).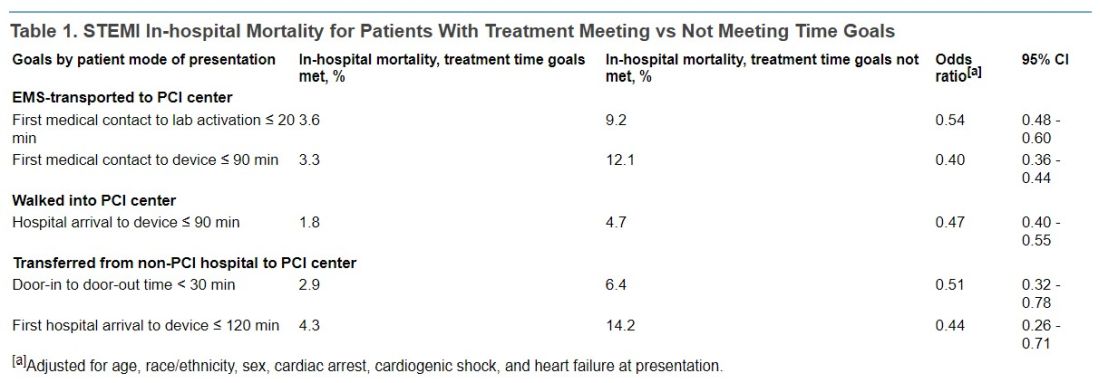
Regardless of mode of patient presentation, treatment time goals were not met most of the time, the group reports. Patients who required interhospital transfer experienced the longest system delays; only 17% were treated within 120 minutes.
Among patients who received primary PCI, 20% had a registry-defined hospital-specified reason for delay, including cardiac arrest and/or need for intubation in 6.8%, “difficulty crossing the culprit lesion” in 3.8%, and “other reasons” in 5.8%, the group reports.
“In 2020, a new reason for delay was added to the registry, ‘need for additional personal protective equipment for suspected/confirmed infectious disease.’ This reason was most commonly used in the second quarter of 2020 (6%) and then declined over time to 1% in the final 2 quarters,” they write.
“Thus, active SARS-CoV-2 infection appeared to have a smaller direct role in longer treatment times or worse outcomes.” Rather, they continue, “the pandemic potentially had a significant indirect role as hospitals were overwhelmed with patients, EMS and hospitals were challenged in maintaining paramedic and nurse staffing and intensive care bed availability, and patients experienced delayed care due to barriers to access or perceived fear of becoming entangled in an overwhelmed medical system.”
Still an important quality metric
STEMI treatment times remain an important quality metric to which hospitals should continue to pay attention because shorter times improve patient care, Deepak Bhatt, MD, MPH, told this news organization.
“Having said that, as with all metrics, one needs to be thoughtful and realize that a difference of a couple of minutes is probably not a crucial thing,” said Dr. Bhatt, Brigham and Women’s Hospital and Harvard Medical School, Boston, who was not involved with the current study.
Interhospital transfers indeed involve longer delays, he observed, suggesting that regional integrated health systems should develop methods for optimizing STEMI care – even, for example, if they involve bypassing non-PCI centers or stopping patients briefly for stabilization followed by rapid transport to a PCI-capable facility.
“That, of course, requires cooperation among hospitals. Sometimes that requires hospitals putting aside economic considerations and just focusing on doing the right thing for that individual patient,” Dr. Bhatt said.
Transfer delays are common for patients presenting with STEMI at hospitals without PCI capability, he noted. “Having clear protocols in place that expedite that type of transfer, I think, could go a long way in reducing the time to treatment in patients that are presenting to the hospital without cath labs. That’s an important message that these data provide.”
The onset of COVID-19 led to widespread delays in STEMI time to treatment early in the pandemic. There were concerns about exposing cath lab personnel to SARS-CoV-2 and potential adverse consequences of sick personnel being unable to provide patient care in the subsequent weeks and months, Dr. Bhatt observed.
However, “All of that seems to have quieted down, and I don’t think COVID is impacting time to treatment right now.”
‘Suboptimal compliance’ with standards
The current findings of “suboptimal compliance with national targets underscore why reassessing quality metrics, in light of changing practice patterns and other secular trends, is critical,” write Andrew S. Oseran, MD, MBA, and Robert W. Yeh, MD, both of Harvard Medical School, in an accompanying editorial.
“While the importance of coordinated and expeditious care for this high-risk patient population is undeniable, the specific actions that hospitals can – or should – take to further improve overall STEMI outcomes are less clear,” they say.
“As physicians contemplate the optimal path forward in managing the care of STEMI patients, they must recognize the clinical and operational nuance that exists in caring for this diverse population and acknowledge the trade-offs associated with uniform quality metrics,” write the editorialists.
“Global reductions in time to treatment for STEMI patients has been one of health care’s great success stories. As we move forward, it may be time to consider whether efforts to achieve additional improvement in target treatment times will result in substantive benefits, or whether we have reached the point of diminishing returns.”
A version of this article first appeared on Medscape.com.
Therapy initiated within national treatment-time goals set a decade ago for patients with ST-segment elevation myocardial infarction (STEMI) remains associated with improved survival in recent years. But for many such patients, time from first symptoms to initiation of reperfusion therapy still fails to meet those goals, suggests a cross-sectional registry analysis.
For example, patients initially transported to centers with percutaneous coronary intervention (PCI) capability had a median treatment time of 148 minutes, in the analysis spanning the second quarter (Q2) of 2018 to the third quarter (Q3) of 2021. But the goal for centers called for treatment initiation within 90 minutes for at least 75% of such STEMI patients.
Moreover, overall STEMI treatment times and in-hospital mortality rose in tandem significantly from Q2 2018 through the first quarter (Q1) of 2021, which included the first year of the COVID-19 pandemic. Median time to treatment went from 86 minutes to 91 minutes during that period. Meanwhile, in-hospital mortality went from 5.6% to 8.7%, report the study authors led by James G. Jollis, MD, Duke University, Durham, N.C.
Their report, based on 114,871 STEMI patients at 601 US hospitals contributing to the Get With The Guidelines – Coronary Artery Disease registry, was published online in JAMA.
Of those patients, 25,085 had been transferred from non-PCI hospitals, 32,483 were walk-ins, and 57,303 arrived via emergency medical services (EMS). Their median times from symptom onset to PCI were 240, 195, and 148 minutes, respectively.
In-hospital mortality was significantly reduced in an adjusted analysis for patients treated within target times, compared with those whose treatment missed the time goals, regardless of whether they were transported by EMS, walked into a hospital with on-site PCI, or were transferred from a non-PCI center (Table 1).
Regardless of mode of patient presentation, treatment time goals were not met most of the time, the group reports. Patients who required interhospital transfer experienced the longest system delays; only 17% were treated within 120 minutes.
Among patients who received primary PCI, 20% had a registry-defined hospital-specified reason for delay, including cardiac arrest and/or need for intubation in 6.8%, “difficulty crossing the culprit lesion” in 3.8%, and “other reasons” in 5.8%, the group reports.
“In 2020, a new reason for delay was added to the registry, ‘need for additional personal protective equipment for suspected/confirmed infectious disease.’ This reason was most commonly used in the second quarter of 2020 (6%) and then declined over time to 1% in the final 2 quarters,” they write.
“Thus, active SARS-CoV-2 infection appeared to have a smaller direct role in longer treatment times or worse outcomes.” Rather, they continue, “the pandemic potentially had a significant indirect role as hospitals were overwhelmed with patients, EMS and hospitals were challenged in maintaining paramedic and nurse staffing and intensive care bed availability, and patients experienced delayed care due to barriers to access or perceived fear of becoming entangled in an overwhelmed medical system.”
Still an important quality metric
STEMI treatment times remain an important quality metric to which hospitals should continue to pay attention because shorter times improve patient care, Deepak Bhatt, MD, MPH, told this news organization.
“Having said that, as with all metrics, one needs to be thoughtful and realize that a difference of a couple of minutes is probably not a crucial thing,” said Dr. Bhatt, Brigham and Women’s Hospital and Harvard Medical School, Boston, who was not involved with the current study.
Interhospital transfers indeed involve longer delays, he observed, suggesting that regional integrated health systems should develop methods for optimizing STEMI care – even, for example, if they involve bypassing non-PCI centers or stopping patients briefly for stabilization followed by rapid transport to a PCI-capable facility.
“That, of course, requires cooperation among hospitals. Sometimes that requires hospitals putting aside economic considerations and just focusing on doing the right thing for that individual patient,” Dr. Bhatt said.
Transfer delays are common for patients presenting with STEMI at hospitals without PCI capability, he noted. “Having clear protocols in place that expedite that type of transfer, I think, could go a long way in reducing the time to treatment in patients that are presenting to the hospital without cath labs. That’s an important message that these data provide.”
The onset of COVID-19 led to widespread delays in STEMI time to treatment early in the pandemic. There were concerns about exposing cath lab personnel to SARS-CoV-2 and potential adverse consequences of sick personnel being unable to provide patient care in the subsequent weeks and months, Dr. Bhatt observed.
However, “All of that seems to have quieted down, and I don’t think COVID is impacting time to treatment right now.”
‘Suboptimal compliance’ with standards
The current findings of “suboptimal compliance with national targets underscore why reassessing quality metrics, in light of changing practice patterns and other secular trends, is critical,” write Andrew S. Oseran, MD, MBA, and Robert W. Yeh, MD, both of Harvard Medical School, in an accompanying editorial.
“While the importance of coordinated and expeditious care for this high-risk patient population is undeniable, the specific actions that hospitals can – or should – take to further improve overall STEMI outcomes are less clear,” they say.
“As physicians contemplate the optimal path forward in managing the care of STEMI patients, they must recognize the clinical and operational nuance that exists in caring for this diverse population and acknowledge the trade-offs associated with uniform quality metrics,” write the editorialists.
“Global reductions in time to treatment for STEMI patients has been one of health care’s great success stories. As we move forward, it may be time to consider whether efforts to achieve additional improvement in target treatment times will result in substantive benefits, or whether we have reached the point of diminishing returns.”
A version of this article first appeared on Medscape.com.
Therapy initiated within national treatment-time goals set a decade ago for patients with ST-segment elevation myocardial infarction (STEMI) remains associated with improved survival in recent years. But for many such patients, time from first symptoms to initiation of reperfusion therapy still fails to meet those goals, suggests a cross-sectional registry analysis.
For example, patients initially transported to centers with percutaneous coronary intervention (PCI) capability had a median treatment time of 148 minutes, in the analysis spanning the second quarter (Q2) of 2018 to the third quarter (Q3) of 2021. But the goal for centers called for treatment initiation within 90 minutes for at least 75% of such STEMI patients.
Moreover, overall STEMI treatment times and in-hospital mortality rose in tandem significantly from Q2 2018 through the first quarter (Q1) of 2021, which included the first year of the COVID-19 pandemic. Median time to treatment went from 86 minutes to 91 minutes during that period. Meanwhile, in-hospital mortality went from 5.6% to 8.7%, report the study authors led by James G. Jollis, MD, Duke University, Durham, N.C.
Their report, based on 114,871 STEMI patients at 601 US hospitals contributing to the Get With The Guidelines – Coronary Artery Disease registry, was published online in JAMA.
Of those patients, 25,085 had been transferred from non-PCI hospitals, 32,483 were walk-ins, and 57,303 arrived via emergency medical services (EMS). Their median times from symptom onset to PCI were 240, 195, and 148 minutes, respectively.
In-hospital mortality was significantly reduced in an adjusted analysis for patients treated within target times, compared with those whose treatment missed the time goals, regardless of whether they were transported by EMS, walked into a hospital with on-site PCI, or were transferred from a non-PCI center (Table 1).
Regardless of mode of patient presentation, treatment time goals were not met most of the time, the group reports. Patients who required interhospital transfer experienced the longest system delays; only 17% were treated within 120 minutes.
Among patients who received primary PCI, 20% had a registry-defined hospital-specified reason for delay, including cardiac arrest and/or need for intubation in 6.8%, “difficulty crossing the culprit lesion” in 3.8%, and “other reasons” in 5.8%, the group reports.
“In 2020, a new reason for delay was added to the registry, ‘need for additional personal protective equipment for suspected/confirmed infectious disease.’ This reason was most commonly used in the second quarter of 2020 (6%) and then declined over time to 1% in the final 2 quarters,” they write.
“Thus, active SARS-CoV-2 infection appeared to have a smaller direct role in longer treatment times or worse outcomes.” Rather, they continue, “the pandemic potentially had a significant indirect role as hospitals were overwhelmed with patients, EMS and hospitals were challenged in maintaining paramedic and nurse staffing and intensive care bed availability, and patients experienced delayed care due to barriers to access or perceived fear of becoming entangled in an overwhelmed medical system.”
Still an important quality metric
STEMI treatment times remain an important quality metric to which hospitals should continue to pay attention because shorter times improve patient care, Deepak Bhatt, MD, MPH, told this news organization.
“Having said that, as with all metrics, one needs to be thoughtful and realize that a difference of a couple of minutes is probably not a crucial thing,” said Dr. Bhatt, Brigham and Women’s Hospital and Harvard Medical School, Boston, who was not involved with the current study.
Interhospital transfers indeed involve longer delays, he observed, suggesting that regional integrated health systems should develop methods for optimizing STEMI care – even, for example, if they involve bypassing non-PCI centers or stopping patients briefly for stabilization followed by rapid transport to a PCI-capable facility.
“That, of course, requires cooperation among hospitals. Sometimes that requires hospitals putting aside economic considerations and just focusing on doing the right thing for that individual patient,” Dr. Bhatt said.
Transfer delays are common for patients presenting with STEMI at hospitals without PCI capability, he noted. “Having clear protocols in place that expedite that type of transfer, I think, could go a long way in reducing the time to treatment in patients that are presenting to the hospital without cath labs. That’s an important message that these data provide.”
The onset of COVID-19 led to widespread delays in STEMI time to treatment early in the pandemic. There were concerns about exposing cath lab personnel to SARS-CoV-2 and potential adverse consequences of sick personnel being unable to provide patient care in the subsequent weeks and months, Dr. Bhatt observed.
However, “All of that seems to have quieted down, and I don’t think COVID is impacting time to treatment right now.”
‘Suboptimal compliance’ with standards
The current findings of “suboptimal compliance with national targets underscore why reassessing quality metrics, in light of changing practice patterns and other secular trends, is critical,” write Andrew S. Oseran, MD, MBA, and Robert W. Yeh, MD, both of Harvard Medical School, in an accompanying editorial.
“While the importance of coordinated and expeditious care for this high-risk patient population is undeniable, the specific actions that hospitals can – or should – take to further improve overall STEMI outcomes are less clear,” they say.
“As physicians contemplate the optimal path forward in managing the care of STEMI patients, they must recognize the clinical and operational nuance that exists in caring for this diverse population and acknowledge the trade-offs associated with uniform quality metrics,” write the editorialists.
“Global reductions in time to treatment for STEMI patients has been one of health care’s great success stories. As we move forward, it may be time to consider whether efforts to achieve additional improvement in target treatment times will result in substantive benefits, or whether we have reached the point of diminishing returns.”
A version of this article first appeared on Medscape.com.
FROM JAMA
Nearly 1,400% rise in young children ingesting cannabis edibles
according to a new analysis of data from poison control centers.
In 2017, centers received 207 reports of children aged 5 years and younger who ingested edible cannabis. In 2021, 3,054 such cases were reported, according to the study, which was published online in Pediatrics.
Many of the children experienced clinical effects, such as depression of the central nervous system, impaired coordination, confusion, agitation, an increase in heart rate, or dilated pupils. No deaths were reported.
“These exposures can cause significant toxicity and are responsible for an increasing number of hospitalizations,” study coauthor Marit S. Tweet, MD, of Southern Illinois University, Springfield, and colleagues wrote.
About 97% of the exposures occurred in residences – 90% at the child’s own home – and about half of the cases involved 2- and 3-year-olds, they noted.
Examining national trends
Twenty-one states have approved recreational cannabis for people aged 21 years and older.
Prior research has shown that calls to poison centers and visits to emergency departments for pediatric cannabis consumption increased in certain states after the drug became legal in those jurisdictions.
To assess national trends, Dr. Tweet’s group analyzed cases in the National Poison Data System, which tracks potentially toxic exposures reported to poison control centers in the United States.
During the 5-year period, they identified 7,043 exposures to edible cannabis by children younger than age 6. In 2.2% of the cases, the drug had a major effect, defined as being either life-threatening or causing residual disability. In 21.9% of cases, the effect was considered to be moderate, with symptoms that were more pronounced, prolonged, or systemic than minor effects.
About 8% of the children were admitted to critical care units; 14.6% were admitted to non–critical care units.
Of 4,827 cases for which there was information about the clinical effects of the exposure and therapies used, 70% involved CNS depression, including 1.9% with “more severe CNS effects, including major CNS depression or coma,” according to the report.
Patients also experienced ataxia (7.4%), agitation (7.1%), confusion (6.1%), tremor (2%), and seizures (1.6%). Other common symptoms included tachycardia (11.4%), vomiting (9.5%), mydriasis (5.9%), and respiratory depression (3.1%).
Treatments for the exposures included intravenous fluids (20.7%), food or snacks (10.3%), and oxygen therapy (4%). Some patients also received naloxone (1.4%) or charcoal (2.1%).
“The total number of children requiring intubation during the study period was 35, or approximately 1 in 140,” the researchers reported. “Although this was a relatively rare occurrence, it is important for clinicians to be aware that life-threatening sequelae can develop and may necessitate invasive supportive care measures.”
Tempting and toxic
For toddlers, edible cannabis may be especially tempting and toxic. Edibles can “resemble common treats such as candies, chocolates, cookies, or other baked goods,” the researchers wrote. Children would not recognize, for example, that one chocolate bar might contain multiple 10-mg servings of tetrahydrocannabinol intended for adults.
Poison centers have been fielding more calls about edible cannabis use by older children, as well.
Adrienne Hughes, MD, assistant professor of emergency medicine at Oregon Health and Science University, Portland, recently found that many cases of intentional misuse and abuse by adolescents involve edible forms of cannabis.
“While marijuana carries a low risk for severe toxicity, it can be inebriating to the point of poor judgment, risk of falls or other injury, and occasionally a panic reaction in the novice user and unsuspecting children who accidentally ingest these products,” Dr. Hughes said in an interview.
Measures to keep edibles away from children could include changing how the products are packaged, limiting the maximum dose of drug per package, and educating the public about the risks to children, Dr. Tweet’s group wrote. They highlighted a 2019 position statement from the American College of Medical Toxicology that includes recommendations for responsible storage habits.
Dr. Hughes echoed one suggestion that is mentioned in the position statement: Parents should consider keeping their cannabis products locked up.
The researchers disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a new analysis of data from poison control centers.
In 2017, centers received 207 reports of children aged 5 years and younger who ingested edible cannabis. In 2021, 3,054 such cases were reported, according to the study, which was published online in Pediatrics.
Many of the children experienced clinical effects, such as depression of the central nervous system, impaired coordination, confusion, agitation, an increase in heart rate, or dilated pupils. No deaths were reported.
“These exposures can cause significant toxicity and are responsible for an increasing number of hospitalizations,” study coauthor Marit S. Tweet, MD, of Southern Illinois University, Springfield, and colleagues wrote.
About 97% of the exposures occurred in residences – 90% at the child’s own home – and about half of the cases involved 2- and 3-year-olds, they noted.
Examining national trends
Twenty-one states have approved recreational cannabis for people aged 21 years and older.
Prior research has shown that calls to poison centers and visits to emergency departments for pediatric cannabis consumption increased in certain states after the drug became legal in those jurisdictions.
To assess national trends, Dr. Tweet’s group analyzed cases in the National Poison Data System, which tracks potentially toxic exposures reported to poison control centers in the United States.
During the 5-year period, they identified 7,043 exposures to edible cannabis by children younger than age 6. In 2.2% of the cases, the drug had a major effect, defined as being either life-threatening or causing residual disability. In 21.9% of cases, the effect was considered to be moderate, with symptoms that were more pronounced, prolonged, or systemic than minor effects.
About 8% of the children were admitted to critical care units; 14.6% were admitted to non–critical care units.
Of 4,827 cases for which there was information about the clinical effects of the exposure and therapies used, 70% involved CNS depression, including 1.9% with “more severe CNS effects, including major CNS depression or coma,” according to the report.
Patients also experienced ataxia (7.4%), agitation (7.1%), confusion (6.1%), tremor (2%), and seizures (1.6%). Other common symptoms included tachycardia (11.4%), vomiting (9.5%), mydriasis (5.9%), and respiratory depression (3.1%).
Treatments for the exposures included intravenous fluids (20.7%), food or snacks (10.3%), and oxygen therapy (4%). Some patients also received naloxone (1.4%) or charcoal (2.1%).
“The total number of children requiring intubation during the study period was 35, or approximately 1 in 140,” the researchers reported. “Although this was a relatively rare occurrence, it is important for clinicians to be aware that life-threatening sequelae can develop and may necessitate invasive supportive care measures.”
Tempting and toxic
For toddlers, edible cannabis may be especially tempting and toxic. Edibles can “resemble common treats such as candies, chocolates, cookies, or other baked goods,” the researchers wrote. Children would not recognize, for example, that one chocolate bar might contain multiple 10-mg servings of tetrahydrocannabinol intended for adults.
Poison centers have been fielding more calls about edible cannabis use by older children, as well.
Adrienne Hughes, MD, assistant professor of emergency medicine at Oregon Health and Science University, Portland, recently found that many cases of intentional misuse and abuse by adolescents involve edible forms of cannabis.
“While marijuana carries a low risk for severe toxicity, it can be inebriating to the point of poor judgment, risk of falls or other injury, and occasionally a panic reaction in the novice user and unsuspecting children who accidentally ingest these products,” Dr. Hughes said in an interview.
Measures to keep edibles away from children could include changing how the products are packaged, limiting the maximum dose of drug per package, and educating the public about the risks to children, Dr. Tweet’s group wrote. They highlighted a 2019 position statement from the American College of Medical Toxicology that includes recommendations for responsible storage habits.
Dr. Hughes echoed one suggestion that is mentioned in the position statement: Parents should consider keeping their cannabis products locked up.
The researchers disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a new analysis of data from poison control centers.
In 2017, centers received 207 reports of children aged 5 years and younger who ingested edible cannabis. In 2021, 3,054 such cases were reported, according to the study, which was published online in Pediatrics.
Many of the children experienced clinical effects, such as depression of the central nervous system, impaired coordination, confusion, agitation, an increase in heart rate, or dilated pupils. No deaths were reported.
“These exposures can cause significant toxicity and are responsible for an increasing number of hospitalizations,” study coauthor Marit S. Tweet, MD, of Southern Illinois University, Springfield, and colleagues wrote.
About 97% of the exposures occurred in residences – 90% at the child’s own home – and about half of the cases involved 2- and 3-year-olds, they noted.
Examining national trends
Twenty-one states have approved recreational cannabis for people aged 21 years and older.
Prior research has shown that calls to poison centers and visits to emergency departments for pediatric cannabis consumption increased in certain states after the drug became legal in those jurisdictions.
To assess national trends, Dr. Tweet’s group analyzed cases in the National Poison Data System, which tracks potentially toxic exposures reported to poison control centers in the United States.
During the 5-year period, they identified 7,043 exposures to edible cannabis by children younger than age 6. In 2.2% of the cases, the drug had a major effect, defined as being either life-threatening or causing residual disability. In 21.9% of cases, the effect was considered to be moderate, with symptoms that were more pronounced, prolonged, or systemic than minor effects.
About 8% of the children were admitted to critical care units; 14.6% were admitted to non–critical care units.
Of 4,827 cases for which there was information about the clinical effects of the exposure and therapies used, 70% involved CNS depression, including 1.9% with “more severe CNS effects, including major CNS depression or coma,” according to the report.
Patients also experienced ataxia (7.4%), agitation (7.1%), confusion (6.1%), tremor (2%), and seizures (1.6%). Other common symptoms included tachycardia (11.4%), vomiting (9.5%), mydriasis (5.9%), and respiratory depression (3.1%).
Treatments for the exposures included intravenous fluids (20.7%), food or snacks (10.3%), and oxygen therapy (4%). Some patients also received naloxone (1.4%) or charcoal (2.1%).
“The total number of children requiring intubation during the study period was 35, or approximately 1 in 140,” the researchers reported. “Although this was a relatively rare occurrence, it is important for clinicians to be aware that life-threatening sequelae can develop and may necessitate invasive supportive care measures.”
Tempting and toxic
For toddlers, edible cannabis may be especially tempting and toxic. Edibles can “resemble common treats such as candies, chocolates, cookies, or other baked goods,” the researchers wrote. Children would not recognize, for example, that one chocolate bar might contain multiple 10-mg servings of tetrahydrocannabinol intended for adults.
Poison centers have been fielding more calls about edible cannabis use by older children, as well.
Adrienne Hughes, MD, assistant professor of emergency medicine at Oregon Health and Science University, Portland, recently found that many cases of intentional misuse and abuse by adolescents involve edible forms of cannabis.
“While marijuana carries a low risk for severe toxicity, it can be inebriating to the point of poor judgment, risk of falls or other injury, and occasionally a panic reaction in the novice user and unsuspecting children who accidentally ingest these products,” Dr. Hughes said in an interview.
Measures to keep edibles away from children could include changing how the products are packaged, limiting the maximum dose of drug per package, and educating the public about the risks to children, Dr. Tweet’s group wrote. They highlighted a 2019 position statement from the American College of Medical Toxicology that includes recommendations for responsible storage habits.
Dr. Hughes echoed one suggestion that is mentioned in the position statement: Parents should consider keeping their cannabis products locked up.
The researchers disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PEDIATRICS
Study of beliefs about what causes cancer sparks debate
The study, entitled, “Everything Causes Cancer? Beliefs and Attitudes Towards Cancer Prevention Among Anti-Vaxxers, Flat Earthers, and Reptilian Conspiracists: Online Cross Sectional Survey,” was published in the Christmas 2022 issue of The British Medical Journal (BMJ).
The authors explain that they set out to evaluate “the patterns of beliefs about cancer among people who believed in conspiracies, rejected the COVID-19 vaccine, or preferred alternative medicine.”
They sought such people on social media and online chat platforms and asked them questions about real and mythical causes of cancer.
Almost half of survey participants agreed with the statement, “It seems like everything causes cancer.”
Overall, among all participants, awareness of the actual causes of cancer was greater than awareness of the mythical causes of cancer, the authors report. However, awareness of the actual causes of cancer was lower among the unvaccinated and members of conspiracy groups than among their counterparts.
The authors are concerned that their findings suggest “a direct connection between digital misinformation and consequent potential erroneous health decisions, which may represent a further preventable fraction of cancer.”
Backlash and criticism
The study “highlights the difficulty society encounters in distinguishing the actual causes of cancer from mythical causes,” The BMJ commented on Twitter.
However, both the study and the journal received some backlash.
This is a “horrible article seeking to smear people with concerns about COVID vaccines,” commented Clare Craig, a British consultant pathologist who specializes in cancer diagnostics.
The study and its methodology were also harshly criticized on Twitter by Normal Fenton, professor of risk information management at the Queen Mary University of London.
The senior author of the study, Laura Costas, a medical epidemiologist with the Catalan Institute of Oncology, Barcelona, told this news organization that the naysayers on social media, many of whom focused their comments on the COVID-19 vaccine, prove the purpose of the study – that misinformation spreads widely on the internet.
“Most comments focused on spreading COVID-19 myths, which were not the direct subject of the study, and questioned the motivations of BMJ authors and the scientific community, assuming they had a common malevolent hidden agenda,” Ms. Costas said.
“They stated the need of having critical thinking, a trait in common with the scientific method, but dogmatically dismissed any information that comes from official sources,” she added.
Ms. Costas commented that “society encounters difficulty in differentiating actual from mythical causes of cancer owing to mass information. We therefore planned this study with a certain satire, which is in line with the essence of The BMJ Christmas issue.”
The BMJ has a long history of publishing a lighthearted Christmas edition full of original, satirical, and nontraditional studies. Previous years have seen studies that explored potential harms from holly and ivy, survival time of chocolates on hospital wards, and the question, “Were James Bond’s drinks shaken because of alcohol induced tremor?”
Study details
Ms. Costas and colleagues sought participants for their survey from online forums that included 4chan and Reddit, which are known for their controversial content posted by anonymous users. Data were also collected from ForoCoches and HispaChan, well-known Spanish online forums. These online sites were intentionally chosen because researchers thought “conspiracy beliefs would be more prevalent,” according to Ms. Costas.
Across the multiple forums, there were 1,494 participants. Of these, 209 participants were unvaccinated against COVID-19, 112 preferred alternatives rather than conventional medicine, and 62 reported that they believed the earth was flat or believed that humanoids take reptilian forms to manipulate human societies.
The team then sought to assess beliefs about actual and mythical (nonestablished) causes of cancer by presenting the participants with the closed risk factor questions on two validated scales – the Cancer Awareness Measure (CAM) and CAM–Mythical Causes Scale (CAM-MYCS).
Responses to both were recorded on a five-point scale; answers ranged from “strongly disagree” to “strongly agree.”
The CAM assesses cancer risk perceptions of 11 established risk factors for cancer: smoking actively or passively, consuming alcohol, low levels of physical activity, consuming red or processed meat, getting sunburnt as a child, family history of cancer, human papillomavirus infection, being overweight, age greater than or equal to 70 years, and low vegetable and fruit consumption.
The CAM-MYCS measure includes 12 questions on risk perceptions of mythical causes of cancer – nonestablished causes that are commonly believed to cause cancer but for which there is no supporting scientific evidence, the authors explain. These items include drinking from plastic bottles; eating food containing artificial sweeteners or additives and genetically modified food; using microwave ovens, aerosol containers, mobile phones, and cleaning products; living near power lines; feeling stressed; experiencing physical trauma; and being exposed to electromagnetic frequencies/non-ionizing radiation, such as wi-fi networks, radio, and television.
The most endorsed mythical causes of cancer were eating food containing additives (63.9%) or sweeteners (50.7%), feeling stressed (59.7%), and eating genetically modified foods (38.4%).
A version of this article first appeared on Medscape.com.
The study, entitled, “Everything Causes Cancer? Beliefs and Attitudes Towards Cancer Prevention Among Anti-Vaxxers, Flat Earthers, and Reptilian Conspiracists: Online Cross Sectional Survey,” was published in the Christmas 2022 issue of The British Medical Journal (BMJ).
The authors explain that they set out to evaluate “the patterns of beliefs about cancer among people who believed in conspiracies, rejected the COVID-19 vaccine, or preferred alternative medicine.”
They sought such people on social media and online chat platforms and asked them questions about real and mythical causes of cancer.
Almost half of survey participants agreed with the statement, “It seems like everything causes cancer.”
Overall, among all participants, awareness of the actual causes of cancer was greater than awareness of the mythical causes of cancer, the authors report. However, awareness of the actual causes of cancer was lower among the unvaccinated and members of conspiracy groups than among their counterparts.
The authors are concerned that their findings suggest “a direct connection between digital misinformation and consequent potential erroneous health decisions, which may represent a further preventable fraction of cancer.”
Backlash and criticism
The study “highlights the difficulty society encounters in distinguishing the actual causes of cancer from mythical causes,” The BMJ commented on Twitter.
However, both the study and the journal received some backlash.
This is a “horrible article seeking to smear people with concerns about COVID vaccines,” commented Clare Craig, a British consultant pathologist who specializes in cancer diagnostics.
The study and its methodology were also harshly criticized on Twitter by Normal Fenton, professor of risk information management at the Queen Mary University of London.
The senior author of the study, Laura Costas, a medical epidemiologist with the Catalan Institute of Oncology, Barcelona, told this news organization that the naysayers on social media, many of whom focused their comments on the COVID-19 vaccine, prove the purpose of the study – that misinformation spreads widely on the internet.
“Most comments focused on spreading COVID-19 myths, which were not the direct subject of the study, and questioned the motivations of BMJ authors and the scientific community, assuming they had a common malevolent hidden agenda,” Ms. Costas said.
“They stated the need of having critical thinking, a trait in common with the scientific method, but dogmatically dismissed any information that comes from official sources,” she added.
Ms. Costas commented that “society encounters difficulty in differentiating actual from mythical causes of cancer owing to mass information. We therefore planned this study with a certain satire, which is in line with the essence of The BMJ Christmas issue.”
The BMJ has a long history of publishing a lighthearted Christmas edition full of original, satirical, and nontraditional studies. Previous years have seen studies that explored potential harms from holly and ivy, survival time of chocolates on hospital wards, and the question, “Were James Bond’s drinks shaken because of alcohol induced tremor?”
Study details
Ms. Costas and colleagues sought participants for their survey from online forums that included 4chan and Reddit, which are known for their controversial content posted by anonymous users. Data were also collected from ForoCoches and HispaChan, well-known Spanish online forums. These online sites were intentionally chosen because researchers thought “conspiracy beliefs would be more prevalent,” according to Ms. Costas.
Across the multiple forums, there were 1,494 participants. Of these, 209 participants were unvaccinated against COVID-19, 112 preferred alternatives rather than conventional medicine, and 62 reported that they believed the earth was flat or believed that humanoids take reptilian forms to manipulate human societies.
The team then sought to assess beliefs about actual and mythical (nonestablished) causes of cancer by presenting the participants with the closed risk factor questions on two validated scales – the Cancer Awareness Measure (CAM) and CAM–Mythical Causes Scale (CAM-MYCS).
Responses to both were recorded on a five-point scale; answers ranged from “strongly disagree” to “strongly agree.”
The CAM assesses cancer risk perceptions of 11 established risk factors for cancer: smoking actively or passively, consuming alcohol, low levels of physical activity, consuming red or processed meat, getting sunburnt as a child, family history of cancer, human papillomavirus infection, being overweight, age greater than or equal to 70 years, and low vegetable and fruit consumption.
The CAM-MYCS measure includes 12 questions on risk perceptions of mythical causes of cancer – nonestablished causes that are commonly believed to cause cancer but for which there is no supporting scientific evidence, the authors explain. These items include drinking from plastic bottles; eating food containing artificial sweeteners or additives and genetically modified food; using microwave ovens, aerosol containers, mobile phones, and cleaning products; living near power lines; feeling stressed; experiencing physical trauma; and being exposed to electromagnetic frequencies/non-ionizing radiation, such as wi-fi networks, radio, and television.
The most endorsed mythical causes of cancer were eating food containing additives (63.9%) or sweeteners (50.7%), feeling stressed (59.7%), and eating genetically modified foods (38.4%).
A version of this article first appeared on Medscape.com.
The study, entitled, “Everything Causes Cancer? Beliefs and Attitudes Towards Cancer Prevention Among Anti-Vaxxers, Flat Earthers, and Reptilian Conspiracists: Online Cross Sectional Survey,” was published in the Christmas 2022 issue of The British Medical Journal (BMJ).
The authors explain that they set out to evaluate “the patterns of beliefs about cancer among people who believed in conspiracies, rejected the COVID-19 vaccine, or preferred alternative medicine.”
They sought such people on social media and online chat platforms and asked them questions about real and mythical causes of cancer.
Almost half of survey participants agreed with the statement, “It seems like everything causes cancer.”
Overall, among all participants, awareness of the actual causes of cancer was greater than awareness of the mythical causes of cancer, the authors report. However, awareness of the actual causes of cancer was lower among the unvaccinated and members of conspiracy groups than among their counterparts.
The authors are concerned that their findings suggest “a direct connection between digital misinformation and consequent potential erroneous health decisions, which may represent a further preventable fraction of cancer.”
Backlash and criticism
The study “highlights the difficulty society encounters in distinguishing the actual causes of cancer from mythical causes,” The BMJ commented on Twitter.
However, both the study and the journal received some backlash.
This is a “horrible article seeking to smear people with concerns about COVID vaccines,” commented Clare Craig, a British consultant pathologist who specializes in cancer diagnostics.
The study and its methodology were also harshly criticized on Twitter by Normal Fenton, professor of risk information management at the Queen Mary University of London.
The senior author of the study, Laura Costas, a medical epidemiologist with the Catalan Institute of Oncology, Barcelona, told this news organization that the naysayers on social media, many of whom focused their comments on the COVID-19 vaccine, prove the purpose of the study – that misinformation spreads widely on the internet.
“Most comments focused on spreading COVID-19 myths, which were not the direct subject of the study, and questioned the motivations of BMJ authors and the scientific community, assuming they had a common malevolent hidden agenda,” Ms. Costas said.
“They stated the need of having critical thinking, a trait in common with the scientific method, but dogmatically dismissed any information that comes from official sources,” she added.
Ms. Costas commented that “society encounters difficulty in differentiating actual from mythical causes of cancer owing to mass information. We therefore planned this study with a certain satire, which is in line with the essence of The BMJ Christmas issue.”
The BMJ has a long history of publishing a lighthearted Christmas edition full of original, satirical, and nontraditional studies. Previous years have seen studies that explored potential harms from holly and ivy, survival time of chocolates on hospital wards, and the question, “Were James Bond’s drinks shaken because of alcohol induced tremor?”
Study details
Ms. Costas and colleagues sought participants for their survey from online forums that included 4chan and Reddit, which are known for their controversial content posted by anonymous users. Data were also collected from ForoCoches and HispaChan, well-known Spanish online forums. These online sites were intentionally chosen because researchers thought “conspiracy beliefs would be more prevalent,” according to Ms. Costas.
Across the multiple forums, there were 1,494 participants. Of these, 209 participants were unvaccinated against COVID-19, 112 preferred alternatives rather than conventional medicine, and 62 reported that they believed the earth was flat or believed that humanoids take reptilian forms to manipulate human societies.
The team then sought to assess beliefs about actual and mythical (nonestablished) causes of cancer by presenting the participants with the closed risk factor questions on two validated scales – the Cancer Awareness Measure (CAM) and CAM–Mythical Causes Scale (CAM-MYCS).
Responses to both were recorded on a five-point scale; answers ranged from “strongly disagree” to “strongly agree.”
The CAM assesses cancer risk perceptions of 11 established risk factors for cancer: smoking actively or passively, consuming alcohol, low levels of physical activity, consuming red or processed meat, getting sunburnt as a child, family history of cancer, human papillomavirus infection, being overweight, age greater than or equal to 70 years, and low vegetable and fruit consumption.
The CAM-MYCS measure includes 12 questions on risk perceptions of mythical causes of cancer – nonestablished causes that are commonly believed to cause cancer but for which there is no supporting scientific evidence, the authors explain. These items include drinking from plastic bottles; eating food containing artificial sweeteners or additives and genetically modified food; using microwave ovens, aerosol containers, mobile phones, and cleaning products; living near power lines; feeling stressed; experiencing physical trauma; and being exposed to electromagnetic frequencies/non-ionizing radiation, such as wi-fi networks, radio, and television.
The most endorsed mythical causes of cancer were eating food containing additives (63.9%) or sweeteners (50.7%), feeling stressed (59.7%), and eating genetically modified foods (38.4%).
A version of this article first appeared on Medscape.com.
Top cardiology societies call for revamp of clinical trials
Leading cardiology societies have issued a “call for action” on a global scale to reinvent randomized clinical trials fit for the 21st century.
“Randomized trials are an essential tool for reliably assessing the effects of treatments, but they have become too costly and too burdensome,” first author Louise Bowman, University of Oxford, England, told this news organization. “We urgently need to modernize our approach to clinical trials in order to continue to improve patient care.”
The joint opinion is from the European Society of Cardiology, the American Heart Association, the American College of Cardiology, and the World Heart Federation. It was simultaneously published online in the European Heart Journal, Circulation, Journal of the American College of Cardiology, and Global Heart.
The authors note that the availability of large-scale “real-world” data is increasingly being touted as a way to bypass the challenges of conducting randomized trials. Yet, observational analyses of real-world data “are not a suitable alternative to randomization,” Prof. Bowman said.
Cardiology has historically led the way in transforming clinical practice with groundbreaking “mega-trials,” such as the International Study of Infarct Survival (ISIS), Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto (GISSI), and Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO).
But over the past 25 years, there has been a huge increase in the rules and related bureaucracy governing clinical trials, which hinders the ability to conduct trials swiftly and affordably, the authors point out.
The COVID-19 pandemic has shown that important clinical trials can be performed quickly and efficiently in busy hospitals, they note.
“The RECOVERY trial in COVID-19 has been an excellent example of this, with results that are estimated to have saved around 1 million lives worldwide within just 1 year,” Prof. Bowman told this news organization.
A Good Clinical Trials Collaborative made up of key stakeholders recently developed new guidelines designed to promote better, more efficient randomized controlled trials.
“If widely adopted and used alongside valuable 21st century electronic health records, we could transform the clinical trials landscape and do many more high-quality trials very cost-effectively,” Prof. Bowman said.
“Widespread adoption and implementation of the revised guidelines will require collaboration with a wide range of national and international organizations, including patient, professional, academic, and industry groups, funders and government organizations, and ethics, health policy, and regulatory bodies,” Prof. Bowman acknowledged.
“This is work that the Good Clinical Trials Collaborative is leading. It is hoped that this endorsement by the joint cardiovascular societies will increase awareness and provide valuable support to his important work,” she added.
No commercial funding was received. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Leading cardiology societies have issued a “call for action” on a global scale to reinvent randomized clinical trials fit for the 21st century.
“Randomized trials are an essential tool for reliably assessing the effects of treatments, but they have become too costly and too burdensome,” first author Louise Bowman, University of Oxford, England, told this news organization. “We urgently need to modernize our approach to clinical trials in order to continue to improve patient care.”
The joint opinion is from the European Society of Cardiology, the American Heart Association, the American College of Cardiology, and the World Heart Federation. It was simultaneously published online in the European Heart Journal, Circulation, Journal of the American College of Cardiology, and Global Heart.
The authors note that the availability of large-scale “real-world” data is increasingly being touted as a way to bypass the challenges of conducting randomized trials. Yet, observational analyses of real-world data “are not a suitable alternative to randomization,” Prof. Bowman said.
Cardiology has historically led the way in transforming clinical practice with groundbreaking “mega-trials,” such as the International Study of Infarct Survival (ISIS), Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto (GISSI), and Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO).
But over the past 25 years, there has been a huge increase in the rules and related bureaucracy governing clinical trials, which hinders the ability to conduct trials swiftly and affordably, the authors point out.
The COVID-19 pandemic has shown that important clinical trials can be performed quickly and efficiently in busy hospitals, they note.
“The RECOVERY trial in COVID-19 has been an excellent example of this, with results that are estimated to have saved around 1 million lives worldwide within just 1 year,” Prof. Bowman told this news organization.
A Good Clinical Trials Collaborative made up of key stakeholders recently developed new guidelines designed to promote better, more efficient randomized controlled trials.
“If widely adopted and used alongside valuable 21st century electronic health records, we could transform the clinical trials landscape and do many more high-quality trials very cost-effectively,” Prof. Bowman said.
“Widespread adoption and implementation of the revised guidelines will require collaboration with a wide range of national and international organizations, including patient, professional, academic, and industry groups, funders and government organizations, and ethics, health policy, and regulatory bodies,” Prof. Bowman acknowledged.
“This is work that the Good Clinical Trials Collaborative is leading. It is hoped that this endorsement by the joint cardiovascular societies will increase awareness and provide valuable support to his important work,” she added.
No commercial funding was received. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Leading cardiology societies have issued a “call for action” on a global scale to reinvent randomized clinical trials fit for the 21st century.
“Randomized trials are an essential tool for reliably assessing the effects of treatments, but they have become too costly and too burdensome,” first author Louise Bowman, University of Oxford, England, told this news organization. “We urgently need to modernize our approach to clinical trials in order to continue to improve patient care.”
The joint opinion is from the European Society of Cardiology, the American Heart Association, the American College of Cardiology, and the World Heart Federation. It was simultaneously published online in the European Heart Journal, Circulation, Journal of the American College of Cardiology, and Global Heart.
The authors note that the availability of large-scale “real-world” data is increasingly being touted as a way to bypass the challenges of conducting randomized trials. Yet, observational analyses of real-world data “are not a suitable alternative to randomization,” Prof. Bowman said.
Cardiology has historically led the way in transforming clinical practice with groundbreaking “mega-trials,” such as the International Study of Infarct Survival (ISIS), Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto (GISSI), and Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO).
But over the past 25 years, there has been a huge increase in the rules and related bureaucracy governing clinical trials, which hinders the ability to conduct trials swiftly and affordably, the authors point out.
The COVID-19 pandemic has shown that important clinical trials can be performed quickly and efficiently in busy hospitals, they note.
“The RECOVERY trial in COVID-19 has been an excellent example of this, with results that are estimated to have saved around 1 million lives worldwide within just 1 year,” Prof. Bowman told this news organization.
A Good Clinical Trials Collaborative made up of key stakeholders recently developed new guidelines designed to promote better, more efficient randomized controlled trials.
“If widely adopted and used alongside valuable 21st century electronic health records, we could transform the clinical trials landscape and do many more high-quality trials very cost-effectively,” Prof. Bowman said.
“Widespread adoption and implementation of the revised guidelines will require collaboration with a wide range of national and international organizations, including patient, professional, academic, and industry groups, funders and government organizations, and ethics, health policy, and regulatory bodies,” Prof. Bowman acknowledged.
“This is work that the Good Clinical Trials Collaborative is leading. It is hoped that this endorsement by the joint cardiovascular societies will increase awareness and provide valuable support to his important work,” she added.
No commercial funding was received. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Time for a rest
“More than Jews have kept Shabbat, Shabbat has kept the Jews.” – Ahad Ha’am
You should all be well rested by now. After all, we’ve just come through the festive shutdown of the holiday season where all of your pumpkin/peppermint/marshmallow flavored coffees were sipped while walking around in your jimjams at 10 a.m. It was the time of year for you to take time off to get a proper rest and be energized to get back to work. Yet, I’m not feeling it from you.
So let’s talk about burnout – just kidding, that would only make it worse. “Burned-out’’ is a hackneyed and defective phrase to describe what many of us are feeling. We are not “destroyed, gutted by fire or by overheating.” No, we are, as one of our docs put it to me: “Just tired.” Ah, a much better Old English word! “Tired” captures it. It means to feel “in need of rest.” We are not ruined, we are just depleted. We don’t need discarding. We need some rest.
I asked some docs when they thought this feeling of exhaustion first began. We agreed that the pandemic, doubledemic, tripledemic, backlog have taken a toll. But The consumerization of medicine? All factors, but not the beginning. No, the beginning was before paper charts. Well, actually it was before paper. We have to go back to the 5th or 6th century BCE. That is when scholars believe the book of Genesis originated from the Yahwist source. In it, it is written that the 7th day be set aside as a day of rest from labor. It is not written that burnout would ensue if sabbath wasn’t observed; however, if you failed to keep it, then you might have been killed. They took rest seriously back then.
This innovation of setting aside a day each week to rest, reflect, and worship was such a good idea that it was codified as one of the 10 commandments. It spread widely. Early Christians kept the Jewish tradition of observing Shabbat from Friday sundown to Saturday until the ever practical Romans decided that Sunday would be a better day. Sunday was already the day to worship the sun god. The newly-converted Christian Emperor Constantine issued an edict on March 7th, 321 CE that all “city people and craftsmen shall rest from labor upon the venerable day of the sun.” And so Sunday it was.
Protestant Seventh-day denomination churches later shifted sabbath back to Saturday believing that Sunday must have been the Pope’s idea. The best deal seems to have been around 1273 when the Ethiopian Orthodox leader Ewostatewos decreed that both Saturday AND Sunday would be days of rest. (But when would one go to Costco?!) In Islam, there is Jumu’ah on Friday. Buddhists have Uposatha, a day of rest and observance every 7 or 8 days. Bah’ai keep Friday as a day of rest and worship. So vital are days of respite to the health of our communities that the state has made working on certain days a violation of the law, “blue laws” they are called. We’ve had blue laws on the books since the time of the Jamestown Colony in 1619 where the first Virginia Assembly required taking Sunday off for worship. Most of these laws have been repealed, although a few states, such as Rhode Island, still have blue laws prohibiting retail and grocery stores from opening on Thanksgiving or Christmas. So there – enjoy your two days off this year!
Ironically, this column, like most of mine, comes to you after my having written it on a Saturday and Sunday. I also just logged on to my EMR and checked results, renewed a few prescriptions, and answered a couple messages. If I didn’t, my Monday’s work would be crushingly heavy.
Maybe I need to be more efficient and finish my work during the week. Or maybe I need to realize that work has not let up since about 600 BCE and taking one day off each week to rest is an obligation to myself, my family and my community.
I wonder if I can choose Mondays.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
“More than Jews have kept Shabbat, Shabbat has kept the Jews.” – Ahad Ha’am
You should all be well rested by now. After all, we’ve just come through the festive shutdown of the holiday season where all of your pumpkin/peppermint/marshmallow flavored coffees were sipped while walking around in your jimjams at 10 a.m. It was the time of year for you to take time off to get a proper rest and be energized to get back to work. Yet, I’m not feeling it from you.
So let’s talk about burnout – just kidding, that would only make it worse. “Burned-out’’ is a hackneyed and defective phrase to describe what many of us are feeling. We are not “destroyed, gutted by fire or by overheating.” No, we are, as one of our docs put it to me: “Just tired.” Ah, a much better Old English word! “Tired” captures it. It means to feel “in need of rest.” We are not ruined, we are just depleted. We don’t need discarding. We need some rest.
I asked some docs when they thought this feeling of exhaustion first began. We agreed that the pandemic, doubledemic, tripledemic, backlog have taken a toll. But The consumerization of medicine? All factors, but not the beginning. No, the beginning was before paper charts. Well, actually it was before paper. We have to go back to the 5th or 6th century BCE. That is when scholars believe the book of Genesis originated from the Yahwist source. In it, it is written that the 7th day be set aside as a day of rest from labor. It is not written that burnout would ensue if sabbath wasn’t observed; however, if you failed to keep it, then you might have been killed. They took rest seriously back then.
This innovation of setting aside a day each week to rest, reflect, and worship was such a good idea that it was codified as one of the 10 commandments. It spread widely. Early Christians kept the Jewish tradition of observing Shabbat from Friday sundown to Saturday until the ever practical Romans decided that Sunday would be a better day. Sunday was already the day to worship the sun god. The newly-converted Christian Emperor Constantine issued an edict on March 7th, 321 CE that all “city people and craftsmen shall rest from labor upon the venerable day of the sun.” And so Sunday it was.
Protestant Seventh-day denomination churches later shifted sabbath back to Saturday believing that Sunday must have been the Pope’s idea. The best deal seems to have been around 1273 when the Ethiopian Orthodox leader Ewostatewos decreed that both Saturday AND Sunday would be days of rest. (But when would one go to Costco?!) In Islam, there is Jumu’ah on Friday. Buddhists have Uposatha, a day of rest and observance every 7 or 8 days. Bah’ai keep Friday as a day of rest and worship. So vital are days of respite to the health of our communities that the state has made working on certain days a violation of the law, “blue laws” they are called. We’ve had blue laws on the books since the time of the Jamestown Colony in 1619 where the first Virginia Assembly required taking Sunday off for worship. Most of these laws have been repealed, although a few states, such as Rhode Island, still have blue laws prohibiting retail and grocery stores from opening on Thanksgiving or Christmas. So there – enjoy your two days off this year!
Ironically, this column, like most of mine, comes to you after my having written it on a Saturday and Sunday. I also just logged on to my EMR and checked results, renewed a few prescriptions, and answered a couple messages. If I didn’t, my Monday’s work would be crushingly heavy.
Maybe I need to be more efficient and finish my work during the week. Or maybe I need to realize that work has not let up since about 600 BCE and taking one day off each week to rest is an obligation to myself, my family and my community.
I wonder if I can choose Mondays.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
“More than Jews have kept Shabbat, Shabbat has kept the Jews.” – Ahad Ha’am
You should all be well rested by now. After all, we’ve just come through the festive shutdown of the holiday season where all of your pumpkin/peppermint/marshmallow flavored coffees were sipped while walking around in your jimjams at 10 a.m. It was the time of year for you to take time off to get a proper rest and be energized to get back to work. Yet, I’m not feeling it from you.
So let’s talk about burnout – just kidding, that would only make it worse. “Burned-out’’ is a hackneyed and defective phrase to describe what many of us are feeling. We are not “destroyed, gutted by fire or by overheating.” No, we are, as one of our docs put it to me: “Just tired.” Ah, a much better Old English word! “Tired” captures it. It means to feel “in need of rest.” We are not ruined, we are just depleted. We don’t need discarding. We need some rest.
I asked some docs when they thought this feeling of exhaustion first began. We agreed that the pandemic, doubledemic, tripledemic, backlog have taken a toll. But The consumerization of medicine? All factors, but not the beginning. No, the beginning was before paper charts. Well, actually it was before paper. We have to go back to the 5th or 6th century BCE. That is when scholars believe the book of Genesis originated from the Yahwist source. In it, it is written that the 7th day be set aside as a day of rest from labor. It is not written that burnout would ensue if sabbath wasn’t observed; however, if you failed to keep it, then you might have been killed. They took rest seriously back then.
This innovation of setting aside a day each week to rest, reflect, and worship was such a good idea that it was codified as one of the 10 commandments. It spread widely. Early Christians kept the Jewish tradition of observing Shabbat from Friday sundown to Saturday until the ever practical Romans decided that Sunday would be a better day. Sunday was already the day to worship the sun god. The newly-converted Christian Emperor Constantine issued an edict on March 7th, 321 CE that all “city people and craftsmen shall rest from labor upon the venerable day of the sun.” And so Sunday it was.
Protestant Seventh-day denomination churches later shifted sabbath back to Saturday believing that Sunday must have been the Pope’s idea. The best deal seems to have been around 1273 when the Ethiopian Orthodox leader Ewostatewos decreed that both Saturday AND Sunday would be days of rest. (But when would one go to Costco?!) In Islam, there is Jumu’ah on Friday. Buddhists have Uposatha, a day of rest and observance every 7 or 8 days. Bah’ai keep Friday as a day of rest and worship. So vital are days of respite to the health of our communities that the state has made working on certain days a violation of the law, “blue laws” they are called. We’ve had blue laws on the books since the time of the Jamestown Colony in 1619 where the first Virginia Assembly required taking Sunday off for worship. Most of these laws have been repealed, although a few states, such as Rhode Island, still have blue laws prohibiting retail and grocery stores from opening on Thanksgiving or Christmas. So there – enjoy your two days off this year!
Ironically, this column, like most of mine, comes to you after my having written it on a Saturday and Sunday. I also just logged on to my EMR and checked results, renewed a few prescriptions, and answered a couple messages. If I didn’t, my Monday’s work would be crushingly heavy.
Maybe I need to be more efficient and finish my work during the week. Or maybe I need to realize that work has not let up since about 600 BCE and taking one day off each week to rest is an obligation to myself, my family and my community.
I wonder if I can choose Mondays.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Nursing exam failure rates spark review of test results
Nursing oversight groups in the United States and Canada are holding the line on testing standards as more would-be nurses fail entry exams. As a result, pressure is growing to make tests easier to pass given widespread nursing shortages, and some critics wonder whether the exams accurately assess students’ true abilities.
When it comes to training more nurses to keep up with growing demand, the U.S. organization that oversees the main licensing exams for nurses decided earlier this month not to change the passing standards for entry-level tests. Meanwhile,
A similar scenario also is unfolding in Quebec, where the agency overseeing nurse licensing exams announced last month it is holding the line on its passing rates despite an outcry from nurses after more than half of those taking the exam in September failed. Quebec’s commissioner for professional admissions is investigating dozens of complaints from nurses about the failure rate. Nurses who failed the test can sign up to retake it in March.
Joseph Oujeil, DESS, DEF, has been teaching in Canada for 4 years, now at two Quebec nursing schools. “This is surprising and very shocking to our students as well as nurses from outside Quebec who were [completing] an integration program to adjust their practice to Quebec guidelines,” Mr. Oujeil told this news organization. Students from outside the province failed the licensing exam at a higher rate than their Quebec-native peers, he explained.
Quebec’s professional Order of Nurses of Quebec (OIIQ) responded to the nurses’ outcry in a press release last month, saying that the pandemic may be partly to blame for the lower passing rate because it made it more difficult to access internships, labs, and face-to-face teaching. Some students weren’t able to demonstrate their ability to practice during the exam as in previous years, OIIQ reported.
Mr. Oujeil agreed. “I’m sure the pandemic has an impact on the situation as well as some students did less training in hospitals” because of restrictions caused by the pandemic, he said. But students also told Mr. Oujeil some questions seemed ambiguous.
OIIQ stated in its release that it doesn’t want to lower the standard. The goal is to protect the public “by and with nurses,” to “ensure the competence and integrity of nurses in Quebec,” and “promote quality nursing practice,” the release noted.
Similarly in the United States, the National Council of State Boards of Nursing (NCSBN) announced Dec. 8 that it would uphold the current passing standards for its entry-level NCLEX tests for registered nurses and practical nurses. NCSBN analyzes the passing standard every 3 years “to keep the test plan and passing standard current,” a press release explains.
NCLEX pass rates have dropped from about 73% for all candidates and 88% for first-time U.S.-educated candidates to 69% and 82% respectively in 2021, the last full year for which results are available, NCSBN spokesperson Dawn Kappel told this news organization.
Over the past 3 years, including during the pandemic, the board decided “that the current passing standard is appropriate as a measure of safe and effective entry-level nurse practice,” after reviewing national nurse surveys and the findings of panels of nurses representing NCSBN’s geographic areas in the United States and Canada, board president Jay Douglas, MSM, RN, CSAC, said in a press release.
Still, NCSBN is not blind to the larger issues facing nursing, Ms. Kappel told this news organization. “There is a huge nursing shortage in the U.S. and Canada. We want as many nurses in the workforce as possible, but we want to ensure safe practice,” she said.
“Everyone has access to the same test, regardless of which state, province, or country they take it in,” she said. Some international students may not perform as well as U.S.-educated students because of their command of English and the nursing education standards in their home countries, Ms. Kappel added.
“Obviously COVID and the challenge of education in general” affected the results, she said.
Mr. Oujeil, the nursing school professor, said he is frustrated by the test results because the majority of students who failed maintained good grades and passed all of their trainings. Yet they scored just below the passing rate of 55%. He said students are proposing the passing rate be lowered to 50%. The current test doesn’t reflect what students are learning in the classroom or during clinical trainings, Mr. Oujeil added. “I don’t know of any students who scored more than 60%.”
He said he understands that the mission of the OIIQ is protecting the population, but he doesn’t believe lowering the passing rate to 50% will put the population at risk – and it will help offset the staffing shortage.
“I’m especially frustrated by those who were doing integration programs – mothers and fathers with children with family lives and financial responsibilities. Many of them are good, hard workers and were shocked they should have to pass the exam another time.”
Since 2018, the pass rate on the first attempt at the Quebec test has generally been between 71% and 96%, compared with 51.4% during the exam in September, according to the OIIQ press release. Meanwhile, graduates from 30 of the 55 schools and universities in Quebec teaching nurses performed above the average on the recent professional exam, OIIQ reported.
The professional licensing organization pointed out that nursing candidates have three attempts to pass their exam. “In order to better prepare for their next attempt, all those who failed received an individual response detailing the difficulties encountered. The OIIQ offers all the tools necessary to pass the exam; a detailed guide and preparatory workshops are available online.
“In the run up to the next exams, we will continue to support students by working with educational institutions to provide the optimal conditions for passing the exam. This exam is usually successful and we are convinced that the return to face-to-face teaching, as well as support for students, will be factors of success,” OIIQ President Luc Mathieu said in a press release.
Nursing candidates “who have not passed the exam will be put to work in the network, with the possibility of practicing under the supervision of a nurse. ... In addition, we will contact health establishments in order to support them in their supervision activities” of the candidates, he said.
A version of this article first appeared on Medscape.com.
Nursing oversight groups in the United States and Canada are holding the line on testing standards as more would-be nurses fail entry exams. As a result, pressure is growing to make tests easier to pass given widespread nursing shortages, and some critics wonder whether the exams accurately assess students’ true abilities.
When it comes to training more nurses to keep up with growing demand, the U.S. organization that oversees the main licensing exams for nurses decided earlier this month not to change the passing standards for entry-level tests. Meanwhile,
A similar scenario also is unfolding in Quebec, where the agency overseeing nurse licensing exams announced last month it is holding the line on its passing rates despite an outcry from nurses after more than half of those taking the exam in September failed. Quebec’s commissioner for professional admissions is investigating dozens of complaints from nurses about the failure rate. Nurses who failed the test can sign up to retake it in March.
Joseph Oujeil, DESS, DEF, has been teaching in Canada for 4 years, now at two Quebec nursing schools. “This is surprising and very shocking to our students as well as nurses from outside Quebec who were [completing] an integration program to adjust their practice to Quebec guidelines,” Mr. Oujeil told this news organization. Students from outside the province failed the licensing exam at a higher rate than their Quebec-native peers, he explained.
Quebec’s professional Order of Nurses of Quebec (OIIQ) responded to the nurses’ outcry in a press release last month, saying that the pandemic may be partly to blame for the lower passing rate because it made it more difficult to access internships, labs, and face-to-face teaching. Some students weren’t able to demonstrate their ability to practice during the exam as in previous years, OIIQ reported.
Mr. Oujeil agreed. “I’m sure the pandemic has an impact on the situation as well as some students did less training in hospitals” because of restrictions caused by the pandemic, he said. But students also told Mr. Oujeil some questions seemed ambiguous.
OIIQ stated in its release that it doesn’t want to lower the standard. The goal is to protect the public “by and with nurses,” to “ensure the competence and integrity of nurses in Quebec,” and “promote quality nursing practice,” the release noted.
Similarly in the United States, the National Council of State Boards of Nursing (NCSBN) announced Dec. 8 that it would uphold the current passing standards for its entry-level NCLEX tests for registered nurses and practical nurses. NCSBN analyzes the passing standard every 3 years “to keep the test plan and passing standard current,” a press release explains.
NCLEX pass rates have dropped from about 73% for all candidates and 88% for first-time U.S.-educated candidates to 69% and 82% respectively in 2021, the last full year for which results are available, NCSBN spokesperson Dawn Kappel told this news organization.
Over the past 3 years, including during the pandemic, the board decided “that the current passing standard is appropriate as a measure of safe and effective entry-level nurse practice,” after reviewing national nurse surveys and the findings of panels of nurses representing NCSBN’s geographic areas in the United States and Canada, board president Jay Douglas, MSM, RN, CSAC, said in a press release.
Still, NCSBN is not blind to the larger issues facing nursing, Ms. Kappel told this news organization. “There is a huge nursing shortage in the U.S. and Canada. We want as many nurses in the workforce as possible, but we want to ensure safe practice,” she said.
“Everyone has access to the same test, regardless of which state, province, or country they take it in,” she said. Some international students may not perform as well as U.S.-educated students because of their command of English and the nursing education standards in their home countries, Ms. Kappel added.
“Obviously COVID and the challenge of education in general” affected the results, she said.
Mr. Oujeil, the nursing school professor, said he is frustrated by the test results because the majority of students who failed maintained good grades and passed all of their trainings. Yet they scored just below the passing rate of 55%. He said students are proposing the passing rate be lowered to 50%. The current test doesn’t reflect what students are learning in the classroom or during clinical trainings, Mr. Oujeil added. “I don’t know of any students who scored more than 60%.”
He said he understands that the mission of the OIIQ is protecting the population, but he doesn’t believe lowering the passing rate to 50% will put the population at risk – and it will help offset the staffing shortage.
“I’m especially frustrated by those who were doing integration programs – mothers and fathers with children with family lives and financial responsibilities. Many of them are good, hard workers and were shocked they should have to pass the exam another time.”
Since 2018, the pass rate on the first attempt at the Quebec test has generally been between 71% and 96%, compared with 51.4% during the exam in September, according to the OIIQ press release. Meanwhile, graduates from 30 of the 55 schools and universities in Quebec teaching nurses performed above the average on the recent professional exam, OIIQ reported.
The professional licensing organization pointed out that nursing candidates have three attempts to pass their exam. “In order to better prepare for their next attempt, all those who failed received an individual response detailing the difficulties encountered. The OIIQ offers all the tools necessary to pass the exam; a detailed guide and preparatory workshops are available online.
“In the run up to the next exams, we will continue to support students by working with educational institutions to provide the optimal conditions for passing the exam. This exam is usually successful and we are convinced that the return to face-to-face teaching, as well as support for students, will be factors of success,” OIIQ President Luc Mathieu said in a press release.
Nursing candidates “who have not passed the exam will be put to work in the network, with the possibility of practicing under the supervision of a nurse. ... In addition, we will contact health establishments in order to support them in their supervision activities” of the candidates, he said.
A version of this article first appeared on Medscape.com.
Nursing oversight groups in the United States and Canada are holding the line on testing standards as more would-be nurses fail entry exams. As a result, pressure is growing to make tests easier to pass given widespread nursing shortages, and some critics wonder whether the exams accurately assess students’ true abilities.
When it comes to training more nurses to keep up with growing demand, the U.S. organization that oversees the main licensing exams for nurses decided earlier this month not to change the passing standards for entry-level tests. Meanwhile,
A similar scenario also is unfolding in Quebec, where the agency overseeing nurse licensing exams announced last month it is holding the line on its passing rates despite an outcry from nurses after more than half of those taking the exam in September failed. Quebec’s commissioner for professional admissions is investigating dozens of complaints from nurses about the failure rate. Nurses who failed the test can sign up to retake it in March.
Joseph Oujeil, DESS, DEF, has been teaching in Canada for 4 years, now at two Quebec nursing schools. “This is surprising and very shocking to our students as well as nurses from outside Quebec who were [completing] an integration program to adjust their practice to Quebec guidelines,” Mr. Oujeil told this news organization. Students from outside the province failed the licensing exam at a higher rate than their Quebec-native peers, he explained.
Quebec’s professional Order of Nurses of Quebec (OIIQ) responded to the nurses’ outcry in a press release last month, saying that the pandemic may be partly to blame for the lower passing rate because it made it more difficult to access internships, labs, and face-to-face teaching. Some students weren’t able to demonstrate their ability to practice during the exam as in previous years, OIIQ reported.
Mr. Oujeil agreed. “I’m sure the pandemic has an impact on the situation as well as some students did less training in hospitals” because of restrictions caused by the pandemic, he said. But students also told Mr. Oujeil some questions seemed ambiguous.
OIIQ stated in its release that it doesn’t want to lower the standard. The goal is to protect the public “by and with nurses,” to “ensure the competence and integrity of nurses in Quebec,” and “promote quality nursing practice,” the release noted.
Similarly in the United States, the National Council of State Boards of Nursing (NCSBN) announced Dec. 8 that it would uphold the current passing standards for its entry-level NCLEX tests for registered nurses and practical nurses. NCSBN analyzes the passing standard every 3 years “to keep the test plan and passing standard current,” a press release explains.
NCLEX pass rates have dropped from about 73% for all candidates and 88% for first-time U.S.-educated candidates to 69% and 82% respectively in 2021, the last full year for which results are available, NCSBN spokesperson Dawn Kappel told this news organization.
Over the past 3 years, including during the pandemic, the board decided “that the current passing standard is appropriate as a measure of safe and effective entry-level nurse practice,” after reviewing national nurse surveys and the findings of panels of nurses representing NCSBN’s geographic areas in the United States and Canada, board president Jay Douglas, MSM, RN, CSAC, said in a press release.
Still, NCSBN is not blind to the larger issues facing nursing, Ms. Kappel told this news organization. “There is a huge nursing shortage in the U.S. and Canada. We want as many nurses in the workforce as possible, but we want to ensure safe practice,” she said.
“Everyone has access to the same test, regardless of which state, province, or country they take it in,” she said. Some international students may not perform as well as U.S.-educated students because of their command of English and the nursing education standards in their home countries, Ms. Kappel added.
“Obviously COVID and the challenge of education in general” affected the results, she said.
Mr. Oujeil, the nursing school professor, said he is frustrated by the test results because the majority of students who failed maintained good grades and passed all of their trainings. Yet they scored just below the passing rate of 55%. He said students are proposing the passing rate be lowered to 50%. The current test doesn’t reflect what students are learning in the classroom or during clinical trainings, Mr. Oujeil added. “I don’t know of any students who scored more than 60%.”
He said he understands that the mission of the OIIQ is protecting the population, but he doesn’t believe lowering the passing rate to 50% will put the population at risk – and it will help offset the staffing shortage.
“I’m especially frustrated by those who were doing integration programs – mothers and fathers with children with family lives and financial responsibilities. Many of them are good, hard workers and were shocked they should have to pass the exam another time.”
Since 2018, the pass rate on the first attempt at the Quebec test has generally been between 71% and 96%, compared with 51.4% during the exam in September, according to the OIIQ press release. Meanwhile, graduates from 30 of the 55 schools and universities in Quebec teaching nurses performed above the average on the recent professional exam, OIIQ reported.
The professional licensing organization pointed out that nursing candidates have three attempts to pass their exam. “In order to better prepare for their next attempt, all those who failed received an individual response detailing the difficulties encountered. The OIIQ offers all the tools necessary to pass the exam; a detailed guide and preparatory workshops are available online.
“In the run up to the next exams, we will continue to support students by working with educational institutions to provide the optimal conditions for passing the exam. This exam is usually successful and we are convinced that the return to face-to-face teaching, as well as support for students, will be factors of success,” OIIQ President Luc Mathieu said in a press release.
Nursing candidates “who have not passed the exam will be put to work in the network, with the possibility of practicing under the supervision of a nurse. ... In addition, we will contact health establishments in order to support them in their supervision activities” of the candidates, he said.
A version of this article first appeared on Medscape.com.
Medicare pay cuts partly averted in massive budget bill
Congress averted bigger reductions in Medicare’s future payments for clinicians in its massive, year-end spending bill, but physicians will still see a 2% cut in a key payment variable in 2023.
The bill also authorizes new policies regarding accelerated drug approvals and substance use disorder treatment.
The House voted 225-201 to clear a wide-ranging legislative package, known as an omnibus, for President Joe Biden’s signature. The Senate voted 68-29 to approve the measure.
Clinicians had been facing as much as 8.5% in cuts to certain factors that set their Medicare payment. The American Medical Association credited an advocacy campaign it joined with more than 150 organizations with fending off the much-feared reimbursement cuts. The 2% trim for 2023 will decline to 1.25% for 2024.
These reductions will hit as many clinicians face the toll on rising costs for running their practices, as , the AMA said.
“Congress must immediately begin the work of long-overdue Medicare physician payment reform that will lead to the program stability that beneficiaries and physicians need,” AMA President Jack Resneck, MD, said in a statement.
While the omnibus bill blocks 6.5% of Medicare payment cuts originally slated to take effect in 2023, it still puts “untenable strain” on primary care clinicians, said Tochi Iroku-Malize, MD, MPH, president of the American Academy of Family Physicians, in a statement.
“However, we’re pleased to see several provisions that will improve access to care, including bolstering mental health services, extending telehealth, and expanding Medicaid and CHIP coverage,” Dr. Iroku-Malize added.
New health care policies in omnibus
Lawmakers adopted many health care policy changes in the omnibus package, which contained 12 overdue spending bills for fiscal year 2023. (Much of the federal government has been funded through stop-gap measures since this budget year began on Oct. 1.) The final measure runs to more than 4,100 pages in PDF form.
House Energy and Commerce Chairman Frank Pallone Jr. (D-NJ) said the health care provisions will:
- Expand patient access to opioid addiction treatment by making it easier for clinicians to dispense buprenorphine for opioid use disorder maintenance or detoxification treatment
- Require health care providers to complete a training requirement on identifying and treating patients with substance use disorders
- Guarantee 12 months of continuous Medicaid coverage for 40 million children
- Provide 2 years of additional Children’s Health Insurance Program (CHIP) funding
- Permanently extend the option for states to offer 12 months of Medicaid coverage to new mothers
- Continue Medicare’s expanded access to telehealth by extending COVID-19 telehealth flexibilities through Dec. 31, 2024.
FDA’s accelerated approval
The omnibus also will shorten the period of uncertainty patients and clinicians face with medicines cleared under the accelerated approval pathway.
The Food and Drug Administration uses accelerated approvals to give conditional clearances to medicines for fatal and serious conditions based on limited evidence signaling a potential benefit. Companies are expected to continue research needed to prove whether promising signals, such as stemming tumor growth, benefits patients.
Concerns have mounted when companies delay confirmatory trials or try to maintain accelerated approvals for drugs that fail those trials.
Mr. Pallone said the omnibus contains provisions that:
- Require the FDA to specify conditions for required post-approval studies
- Authorize the FDA to require post-approval studies to be underway at the time of approval or within a specified time period following approval.
- Clarify and streamline current FDA authority to withdraw approvals when sponsors fail to conduct studies with due diligence.
Reshma Ramachandran, MD, MPP, MHS, who serves as the chair of the Doctors for America’s FDA Task Force, told this news organization that she was pleased to see these provisions pass. She had been disappointed they were not included earlier this year in the latest Prescription Drug User Fee Act reauthorization.
The provisions in the omnibus make “clear what steps the FDA can take to remove an unproven drug off the market should manufacturers fail to complete these studies or demonstrate meaningful clinical benefit,” Dr. Ramachandran wrote in an email.
Dr. Ramachandran said she hopes lawmakers build on these steps in the future. She suggested Congress add a mandate to require drug labels to clearly state when the FDA is still waiting for evidence needed to confirm benefits of medicines cleared by accelerated approval.
“Nevertheless, Congress in including and, hopefully, passing these reforms has made it clear that drug companies need to provide meaningful evidence that their accelerated approval drugs work in patients and FDA can take action to protect patients should this not occur,” Dr. Ramachandran wrote.
A version of this article first appeared on Medscape.com.
Congress averted bigger reductions in Medicare’s future payments for clinicians in its massive, year-end spending bill, but physicians will still see a 2% cut in a key payment variable in 2023.
The bill also authorizes new policies regarding accelerated drug approvals and substance use disorder treatment.
The House voted 225-201 to clear a wide-ranging legislative package, known as an omnibus, for President Joe Biden’s signature. The Senate voted 68-29 to approve the measure.
Clinicians had been facing as much as 8.5% in cuts to certain factors that set their Medicare payment. The American Medical Association credited an advocacy campaign it joined with more than 150 organizations with fending off the much-feared reimbursement cuts. The 2% trim for 2023 will decline to 1.25% for 2024.
These reductions will hit as many clinicians face the toll on rising costs for running their practices, as , the AMA said.
“Congress must immediately begin the work of long-overdue Medicare physician payment reform that will lead to the program stability that beneficiaries and physicians need,” AMA President Jack Resneck, MD, said in a statement.
While the omnibus bill blocks 6.5% of Medicare payment cuts originally slated to take effect in 2023, it still puts “untenable strain” on primary care clinicians, said Tochi Iroku-Malize, MD, MPH, president of the American Academy of Family Physicians, in a statement.
“However, we’re pleased to see several provisions that will improve access to care, including bolstering mental health services, extending telehealth, and expanding Medicaid and CHIP coverage,” Dr. Iroku-Malize added.
New health care policies in omnibus
Lawmakers adopted many health care policy changes in the omnibus package, which contained 12 overdue spending bills for fiscal year 2023. (Much of the federal government has been funded through stop-gap measures since this budget year began on Oct. 1.) The final measure runs to more than 4,100 pages in PDF form.
House Energy and Commerce Chairman Frank Pallone Jr. (D-NJ) said the health care provisions will:
- Expand patient access to opioid addiction treatment by making it easier for clinicians to dispense buprenorphine for opioid use disorder maintenance or detoxification treatment
- Require health care providers to complete a training requirement on identifying and treating patients with substance use disorders
- Guarantee 12 months of continuous Medicaid coverage for 40 million children
- Provide 2 years of additional Children’s Health Insurance Program (CHIP) funding
- Permanently extend the option for states to offer 12 months of Medicaid coverage to new mothers
- Continue Medicare’s expanded access to telehealth by extending COVID-19 telehealth flexibilities through Dec. 31, 2024.
FDA’s accelerated approval
The omnibus also will shorten the period of uncertainty patients and clinicians face with medicines cleared under the accelerated approval pathway.
The Food and Drug Administration uses accelerated approvals to give conditional clearances to medicines for fatal and serious conditions based on limited evidence signaling a potential benefit. Companies are expected to continue research needed to prove whether promising signals, such as stemming tumor growth, benefits patients.
Concerns have mounted when companies delay confirmatory trials or try to maintain accelerated approvals for drugs that fail those trials.
Mr. Pallone said the omnibus contains provisions that:
- Require the FDA to specify conditions for required post-approval studies
- Authorize the FDA to require post-approval studies to be underway at the time of approval or within a specified time period following approval.
- Clarify and streamline current FDA authority to withdraw approvals when sponsors fail to conduct studies with due diligence.
Reshma Ramachandran, MD, MPP, MHS, who serves as the chair of the Doctors for America’s FDA Task Force, told this news organization that she was pleased to see these provisions pass. She had been disappointed they were not included earlier this year in the latest Prescription Drug User Fee Act reauthorization.
The provisions in the omnibus make “clear what steps the FDA can take to remove an unproven drug off the market should manufacturers fail to complete these studies or demonstrate meaningful clinical benefit,” Dr. Ramachandran wrote in an email.
Dr. Ramachandran said she hopes lawmakers build on these steps in the future. She suggested Congress add a mandate to require drug labels to clearly state when the FDA is still waiting for evidence needed to confirm benefits of medicines cleared by accelerated approval.
“Nevertheless, Congress in including and, hopefully, passing these reforms has made it clear that drug companies need to provide meaningful evidence that their accelerated approval drugs work in patients and FDA can take action to protect patients should this not occur,” Dr. Ramachandran wrote.
A version of this article first appeared on Medscape.com.
Congress averted bigger reductions in Medicare’s future payments for clinicians in its massive, year-end spending bill, but physicians will still see a 2% cut in a key payment variable in 2023.
The bill also authorizes new policies regarding accelerated drug approvals and substance use disorder treatment.
The House voted 225-201 to clear a wide-ranging legislative package, known as an omnibus, for President Joe Biden’s signature. The Senate voted 68-29 to approve the measure.
Clinicians had been facing as much as 8.5% in cuts to certain factors that set their Medicare payment. The American Medical Association credited an advocacy campaign it joined with more than 150 organizations with fending off the much-feared reimbursement cuts. The 2% trim for 2023 will decline to 1.25% for 2024.
These reductions will hit as many clinicians face the toll on rising costs for running their practices, as , the AMA said.
“Congress must immediately begin the work of long-overdue Medicare physician payment reform that will lead to the program stability that beneficiaries and physicians need,” AMA President Jack Resneck, MD, said in a statement.
While the omnibus bill blocks 6.5% of Medicare payment cuts originally slated to take effect in 2023, it still puts “untenable strain” on primary care clinicians, said Tochi Iroku-Malize, MD, MPH, president of the American Academy of Family Physicians, in a statement.
“However, we’re pleased to see several provisions that will improve access to care, including bolstering mental health services, extending telehealth, and expanding Medicaid and CHIP coverage,” Dr. Iroku-Malize added.
New health care policies in omnibus
Lawmakers adopted many health care policy changes in the omnibus package, which contained 12 overdue spending bills for fiscal year 2023. (Much of the federal government has been funded through stop-gap measures since this budget year began on Oct. 1.) The final measure runs to more than 4,100 pages in PDF form.
House Energy and Commerce Chairman Frank Pallone Jr. (D-NJ) said the health care provisions will:
- Expand patient access to opioid addiction treatment by making it easier for clinicians to dispense buprenorphine for opioid use disorder maintenance or detoxification treatment
- Require health care providers to complete a training requirement on identifying and treating patients with substance use disorders
- Guarantee 12 months of continuous Medicaid coverage for 40 million children
- Provide 2 years of additional Children’s Health Insurance Program (CHIP) funding
- Permanently extend the option for states to offer 12 months of Medicaid coverage to new mothers
- Continue Medicare’s expanded access to telehealth by extending COVID-19 telehealth flexibilities through Dec. 31, 2024.
FDA’s accelerated approval
The omnibus also will shorten the period of uncertainty patients and clinicians face with medicines cleared under the accelerated approval pathway.
The Food and Drug Administration uses accelerated approvals to give conditional clearances to medicines for fatal and serious conditions based on limited evidence signaling a potential benefit. Companies are expected to continue research needed to prove whether promising signals, such as stemming tumor growth, benefits patients.
Concerns have mounted when companies delay confirmatory trials or try to maintain accelerated approvals for drugs that fail those trials.
Mr. Pallone said the omnibus contains provisions that:
- Require the FDA to specify conditions for required post-approval studies
- Authorize the FDA to require post-approval studies to be underway at the time of approval or within a specified time period following approval.
- Clarify and streamline current FDA authority to withdraw approvals when sponsors fail to conduct studies with due diligence.
Reshma Ramachandran, MD, MPP, MHS, who serves as the chair of the Doctors for America’s FDA Task Force, told this news organization that she was pleased to see these provisions pass. She had been disappointed they were not included earlier this year in the latest Prescription Drug User Fee Act reauthorization.
The provisions in the omnibus make “clear what steps the FDA can take to remove an unproven drug off the market should manufacturers fail to complete these studies or demonstrate meaningful clinical benefit,” Dr. Ramachandran wrote in an email.
Dr. Ramachandran said she hopes lawmakers build on these steps in the future. She suggested Congress add a mandate to require drug labels to clearly state when the FDA is still waiting for evidence needed to confirm benefits of medicines cleared by accelerated approval.
“Nevertheless, Congress in including and, hopefully, passing these reforms has made it clear that drug companies need to provide meaningful evidence that their accelerated approval drugs work in patients and FDA can take action to protect patients should this not occur,” Dr. Ramachandran wrote.
A version of this article first appeared on Medscape.com.




