User login
Spherical heart may predict cardiomyopathy, AFib
A round heart, or left ventricle sphericity, predicted cardiomyopathy and atrial fibrillation (AFib) in a deep learning analysis of MRI images from close to 39,000 participants in the UK Biobank, a new study shows.
An increase of 1 standard deviation in the sphericity index (short axis length/long axis length) was associated with a 47% increased incidence of cardiomyopathy and a 20% increased incidence of AFib, independent of clinical factors and traditional MRI measures.
Furthermore, a genetic analysis suggested a shared architecture between sphericity and nonischemic cardiomyopathy, pointing to NICM as a possible causal factor for left ventricle sphericity among individuals with normal LV size and function.
“Physicians have known the heart gets rounder after heart attacks and as we get older,” David Ouyang, MD, a cardiologist in the Smidt Heart Institute at Cedars-Sinai Medical Center, Los Angeles, and a researcher in the division of artificial intelligence in medicine, said in an interview. “We wanted to see if this sphericity is prognostic of future disease even in healthy individuals.”
Although it is too early to recommend heart shape assessment in healthy asymptomatic people, he said, “physicians should be extra careful and think about treatments when they notice a patient’s heart is particularly round.”
The study was published online March 29 in the journal Med.
Sphericity index key
The investigators hypothesized that there is variation in LV sphericity within the spectrum of normal LV chamber size and systolic function, and that such variation might be a marker of cardiac risk with genetic influences.
To test this hypothesis, they used automated deep-learning segmentation of cardiac MRI data to estimate and analyze the sphericity index in a cohort of 38,897 individuals participating in the UK Biobank.
After adjustment for age at MRI and sex, an increased sphericity index was associated with an increased risk for cardiomyopathy (hazard ratio, 1.57), AFib (HR, 1.35), and heart failure (HR, 1.37).
No significant association was seen with cardiac arrest.
The team then stratified the cohort into quintiles and compared the top 20%, middle 60%, and bottom 20%. The relationship between the sphericity index and risk extended across the distribution; individuals with higher than median sphericity had increased disease incidence, and those with lower than median sphericity had decreased incidence.
Overall, a single standard deviation in the sphericity index was associated with increased risk of cardiomyopathy (HR, 1.47) and of AFib (HR, 1.20), independent of clinical factors and usual MRI measurements.
In a minimally adjusted model, the sphericity index was a predictor of incident cardiomyopathy, AFib, and heart failure.
Adjustment for clinical factors partially attenuated the heart failure association; additional adjustment for MRI measurements fully attenuated that association and partially attenuated the association with AFib.
However, in all adjusted models, the association with cardiomyopathy showed little attenuation.
Furthermore, the team identified four loci associated with sphericity at genomewide significance – PLN, ANGPT1, PDZRN3, and HLA DR/DQ – and Mendelian randomization supported NICM as a cause of LV sphericity.
Looking ahead
“While conventional imaging metrics have significant diagnostic and prognostic value, some of these measurements have been adopted out of convenience or tradition,” the authors noted. “By representing a specific multidimensional remodeling phenotype, sphericity has emerged as a distinct morphologic trait with features not adequately captured by conventional measurements.
“We expect that the search space of potential imaging measurements is vast, and we have only begun to scratch at the surface of disease associations.”
Indeed, Dr. Ouyang said his group is “trying to evaluate the sphericity in echocardiograms or heart ultrasounds, which are more common and cheaper than MRI.”
“The main caveat is translating the information directly to patient care,” Richard C. Becker, MD, director and physician-in-chief of the University of Cincinnati Heart, Lung, and Vascular Institute, said in an interview. “Near-term yield could include using the spherical calculation in routine MRI of the heart, and based on the findings, following patients more closely if there is an abnormal shape. Or performing an MRI and targeted gene testing if there is a family history of cardiomyopathy or [of] an abnormal shape of the heart.”
“Validation of the findings and large-scale evaluation of the genes identified, and how they interact with patient and environmental factors, will be very important,” he added.
Nevertheless, “the study was well done and may serve as a foundation for future research,” Dr. Becker said. “The investigators used several powerful tools, including MRI, genomics, and [artificial intelligence] to draw their conclusions. This is precisely the way that ‘big data’ should be used – in a complementary fashion.”
The study authors and Dr. Becker reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A round heart, or left ventricle sphericity, predicted cardiomyopathy and atrial fibrillation (AFib) in a deep learning analysis of MRI images from close to 39,000 participants in the UK Biobank, a new study shows.
An increase of 1 standard deviation in the sphericity index (short axis length/long axis length) was associated with a 47% increased incidence of cardiomyopathy and a 20% increased incidence of AFib, independent of clinical factors and traditional MRI measures.
Furthermore, a genetic analysis suggested a shared architecture between sphericity and nonischemic cardiomyopathy, pointing to NICM as a possible causal factor for left ventricle sphericity among individuals with normal LV size and function.
“Physicians have known the heart gets rounder after heart attacks and as we get older,” David Ouyang, MD, a cardiologist in the Smidt Heart Institute at Cedars-Sinai Medical Center, Los Angeles, and a researcher in the division of artificial intelligence in medicine, said in an interview. “We wanted to see if this sphericity is prognostic of future disease even in healthy individuals.”
Although it is too early to recommend heart shape assessment in healthy asymptomatic people, he said, “physicians should be extra careful and think about treatments when they notice a patient’s heart is particularly round.”
The study was published online March 29 in the journal Med.
Sphericity index key
The investigators hypothesized that there is variation in LV sphericity within the spectrum of normal LV chamber size and systolic function, and that such variation might be a marker of cardiac risk with genetic influences.
To test this hypothesis, they used automated deep-learning segmentation of cardiac MRI data to estimate and analyze the sphericity index in a cohort of 38,897 individuals participating in the UK Biobank.
After adjustment for age at MRI and sex, an increased sphericity index was associated with an increased risk for cardiomyopathy (hazard ratio, 1.57), AFib (HR, 1.35), and heart failure (HR, 1.37).
No significant association was seen with cardiac arrest.
The team then stratified the cohort into quintiles and compared the top 20%, middle 60%, and bottom 20%. The relationship between the sphericity index and risk extended across the distribution; individuals with higher than median sphericity had increased disease incidence, and those with lower than median sphericity had decreased incidence.
Overall, a single standard deviation in the sphericity index was associated with increased risk of cardiomyopathy (HR, 1.47) and of AFib (HR, 1.20), independent of clinical factors and usual MRI measurements.
In a minimally adjusted model, the sphericity index was a predictor of incident cardiomyopathy, AFib, and heart failure.
Adjustment for clinical factors partially attenuated the heart failure association; additional adjustment for MRI measurements fully attenuated that association and partially attenuated the association with AFib.
However, in all adjusted models, the association with cardiomyopathy showed little attenuation.
Furthermore, the team identified four loci associated with sphericity at genomewide significance – PLN, ANGPT1, PDZRN3, and HLA DR/DQ – and Mendelian randomization supported NICM as a cause of LV sphericity.
Looking ahead
“While conventional imaging metrics have significant diagnostic and prognostic value, some of these measurements have been adopted out of convenience or tradition,” the authors noted. “By representing a specific multidimensional remodeling phenotype, sphericity has emerged as a distinct morphologic trait with features not adequately captured by conventional measurements.
“We expect that the search space of potential imaging measurements is vast, and we have only begun to scratch at the surface of disease associations.”
Indeed, Dr. Ouyang said his group is “trying to evaluate the sphericity in echocardiograms or heart ultrasounds, which are more common and cheaper than MRI.”
“The main caveat is translating the information directly to patient care,” Richard C. Becker, MD, director and physician-in-chief of the University of Cincinnati Heart, Lung, and Vascular Institute, said in an interview. “Near-term yield could include using the spherical calculation in routine MRI of the heart, and based on the findings, following patients more closely if there is an abnormal shape. Or performing an MRI and targeted gene testing if there is a family history of cardiomyopathy or [of] an abnormal shape of the heart.”
“Validation of the findings and large-scale evaluation of the genes identified, and how they interact with patient and environmental factors, will be very important,” he added.
Nevertheless, “the study was well done and may serve as a foundation for future research,” Dr. Becker said. “The investigators used several powerful tools, including MRI, genomics, and [artificial intelligence] to draw their conclusions. This is precisely the way that ‘big data’ should be used – in a complementary fashion.”
The study authors and Dr. Becker reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A round heart, or left ventricle sphericity, predicted cardiomyopathy and atrial fibrillation (AFib) in a deep learning analysis of MRI images from close to 39,000 participants in the UK Biobank, a new study shows.
An increase of 1 standard deviation in the sphericity index (short axis length/long axis length) was associated with a 47% increased incidence of cardiomyopathy and a 20% increased incidence of AFib, independent of clinical factors and traditional MRI measures.
Furthermore, a genetic analysis suggested a shared architecture between sphericity and nonischemic cardiomyopathy, pointing to NICM as a possible causal factor for left ventricle sphericity among individuals with normal LV size and function.
“Physicians have known the heart gets rounder after heart attacks and as we get older,” David Ouyang, MD, a cardiologist in the Smidt Heart Institute at Cedars-Sinai Medical Center, Los Angeles, and a researcher in the division of artificial intelligence in medicine, said in an interview. “We wanted to see if this sphericity is prognostic of future disease even in healthy individuals.”
Although it is too early to recommend heart shape assessment in healthy asymptomatic people, he said, “physicians should be extra careful and think about treatments when they notice a patient’s heart is particularly round.”
The study was published online March 29 in the journal Med.
Sphericity index key
The investigators hypothesized that there is variation in LV sphericity within the spectrum of normal LV chamber size and systolic function, and that such variation might be a marker of cardiac risk with genetic influences.
To test this hypothesis, they used automated deep-learning segmentation of cardiac MRI data to estimate and analyze the sphericity index in a cohort of 38,897 individuals participating in the UK Biobank.
After adjustment for age at MRI and sex, an increased sphericity index was associated with an increased risk for cardiomyopathy (hazard ratio, 1.57), AFib (HR, 1.35), and heart failure (HR, 1.37).
No significant association was seen with cardiac arrest.
The team then stratified the cohort into quintiles and compared the top 20%, middle 60%, and bottom 20%. The relationship between the sphericity index and risk extended across the distribution; individuals with higher than median sphericity had increased disease incidence, and those with lower than median sphericity had decreased incidence.
Overall, a single standard deviation in the sphericity index was associated with increased risk of cardiomyopathy (HR, 1.47) and of AFib (HR, 1.20), independent of clinical factors and usual MRI measurements.
In a minimally adjusted model, the sphericity index was a predictor of incident cardiomyopathy, AFib, and heart failure.
Adjustment for clinical factors partially attenuated the heart failure association; additional adjustment for MRI measurements fully attenuated that association and partially attenuated the association with AFib.
However, in all adjusted models, the association with cardiomyopathy showed little attenuation.
Furthermore, the team identified four loci associated with sphericity at genomewide significance – PLN, ANGPT1, PDZRN3, and HLA DR/DQ – and Mendelian randomization supported NICM as a cause of LV sphericity.
Looking ahead
“While conventional imaging metrics have significant diagnostic and prognostic value, some of these measurements have been adopted out of convenience or tradition,” the authors noted. “By representing a specific multidimensional remodeling phenotype, sphericity has emerged as a distinct morphologic trait with features not adequately captured by conventional measurements.
“We expect that the search space of potential imaging measurements is vast, and we have only begun to scratch at the surface of disease associations.”
Indeed, Dr. Ouyang said his group is “trying to evaluate the sphericity in echocardiograms or heart ultrasounds, which are more common and cheaper than MRI.”
“The main caveat is translating the information directly to patient care,” Richard C. Becker, MD, director and physician-in-chief of the University of Cincinnati Heart, Lung, and Vascular Institute, said in an interview. “Near-term yield could include using the spherical calculation in routine MRI of the heart, and based on the findings, following patients more closely if there is an abnormal shape. Or performing an MRI and targeted gene testing if there is a family history of cardiomyopathy or [of] an abnormal shape of the heart.”
“Validation of the findings and large-scale evaluation of the genes identified, and how they interact with patient and environmental factors, will be very important,” he added.
Nevertheless, “the study was well done and may serve as a foundation for future research,” Dr. Becker said. “The investigators used several powerful tools, including MRI, genomics, and [artificial intelligence] to draw their conclusions. This is precisely the way that ‘big data’ should be used – in a complementary fashion.”
The study authors and Dr. Becker reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM MED
Statins don’t worsen muscle injury from moderately intense exercise
People who are physically active and on statins may have one less potential concern about the drugs. Despite their reputation for causing muscle injury, a study suggests statins won’t worsen the toll that sustained, moderately intensive exercise already takes on patients’ muscles.
Statin therapy in this prospective, controlled study wasn’t seen to aggravate normal muscle fatigue or pain from sustained exercise or adversely affect enzymes or other biomarkers associated with muscle injury.
The findings come from 100 individuals, of whom about two-thirds were on statins, participating in a public, 4-day, long-distance walking event held annually in the Netherlands. Results were published in the Journal of the American College of Cardiology with Neeltje A.E. Allard, MD, Radboud University Medical Center, Nijmegen, the Netherlands, as lead author.
For all of statins’ common use in adults with cardiovascular (CV) risk factors, the drugs are often blamed for causing excessive muscle pain or injury as a side effect. Yet there is a predominance of evidence to the contrary based on meta-analyses and clinical trials, suggesting that the drugs are taking the rap for many entirely unrelated muscle symptoms.
The new findings, from people ranging widely in fitness levels, suggest that “exercise of moderate intensity is feasible and safe” in statin users, that the drugs won’t exacerbate normal muscle symptoms from exercise, Dr. Allard told this news organization.
And that exercise doesn’t have to be on an unusual scale. Regular exercise in statin users can simply be consistent with broader guidelines, say 30 minutes of walking per day, she noted.
The study has such broad applicability, Dr. Allard said, because participants represented the spectrum of the thousands who signed up for the walking event, who varied in age, level of physical fitness, and number of CV risk factors. They included CV patients, the physically fit, “recreational walkers who didn’t really exercise regularly,” and “habitual nonexercisers.”
It enrolled three groups of participants in the Four Days Marches in Nijmegen, which in a typical year attracts tens of thousands of participants who walk up to 30 km, 40 km, or 50 km per day for 4 consecutive days.
They included 35 statin users who walked the event despite muscle symptoms, 34 on statins but without such symptoms, and 31 non–statin-using controls. Their mean ages ranged from 65 to 68 years.
Statin users were overwhelmingly on simvastatin or atorvastatin. The average statin therapy durations were 60 months and 96 months for those with and without symptoms, respectively.
Assessments were performed several days before the event, at baseline, and after the end of walking on days 1, 2, and 3.
Scores for muscle pain on the Brief Pain Inventory were higher at baseline for the symptomatic-on-statins group (P < .001) compared with the other two groups, and went up (P < .001) similarly across the three groups during each of the 3 days, the report notes. Fatigue scores on the Brief Fatigue Inventory followed the same pattern.
All biomarkers of muscle injury or stress were at comparable levels at baseline in the three groups and went up similarly (P < .001) with no significant differences at the end of day 3. Biomarkers included lactate dehydrogenase, creatine kinase, myoglobin, cardiac troponin I, and N-terminal pro-brain natriuretic peptide.
Statin-related reductions in levels of coenzyme Q 10 (CoQ10) have been thought to exacerbate muscle injury, the authors note. But levels of CoQ10 weren’t significantly different across the three groups at any point in the study, and they did not show any significant associations with measures of muscle injury, symptoms, or fatigue.
Patients with statin-associated muscle symptoms (SAMS) often limit physical activity because of muscle pain or weakness, but also “concerns that exercise will exacerbate muscle injury,” an accompanying editorial notes. “Therefore, exercise, a foundation of improving and maintaining cardiometabolic health, is often avoided or limited.”
But the current study, writes Robert S. Rosenson, MD, of Mount Sinai Heart, New York, indeed suggests that “many patients who develop SAMS may engage in a moderately intensive walking program without concern for worsened muscle biomarkers or performance.”
The exercise didn’t seem to improve muscle function in symptomatic statin users, compared with the other groups over the study’s very short follow-up, Dr. Rosenson observes. But “it remains uncertain from this study whether sustained exercise in SAMS patients will effectuate improved metabolic biomarkers or exercise capacity in the long term.”
Dr. Allard is supported by a grant from the Radboud Institute for Health Sciences; the other authors have disclosed no relevant financial relationships. Dr. Rosenson disclosed receiving research funding to his institution from Amgen, Arrowhead, Lilly, Novartis, and Regeneron; consulting fees from Amgen, Arrowhead, Lilly, Lipigon, Novartis, CRISPR Therapeutics, Precision BioSciences, Verve, Ultragenyx Pharmaceutical, and Regeneron; speaking fees from Amgen, Kowa, and Regeneron; and royalties from Wolters Kluwer (UpToDate); and that he holds stock in MediMergent.
A version of this article first appeared on Medscape.com.
People who are physically active and on statins may have one less potential concern about the drugs. Despite their reputation for causing muscle injury, a study suggests statins won’t worsen the toll that sustained, moderately intensive exercise already takes on patients’ muscles.
Statin therapy in this prospective, controlled study wasn’t seen to aggravate normal muscle fatigue or pain from sustained exercise or adversely affect enzymes or other biomarkers associated with muscle injury.
The findings come from 100 individuals, of whom about two-thirds were on statins, participating in a public, 4-day, long-distance walking event held annually in the Netherlands. Results were published in the Journal of the American College of Cardiology with Neeltje A.E. Allard, MD, Radboud University Medical Center, Nijmegen, the Netherlands, as lead author.
For all of statins’ common use in adults with cardiovascular (CV) risk factors, the drugs are often blamed for causing excessive muscle pain or injury as a side effect. Yet there is a predominance of evidence to the contrary based on meta-analyses and clinical trials, suggesting that the drugs are taking the rap for many entirely unrelated muscle symptoms.
The new findings, from people ranging widely in fitness levels, suggest that “exercise of moderate intensity is feasible and safe” in statin users, that the drugs won’t exacerbate normal muscle symptoms from exercise, Dr. Allard told this news organization.
And that exercise doesn’t have to be on an unusual scale. Regular exercise in statin users can simply be consistent with broader guidelines, say 30 minutes of walking per day, she noted.
The study has such broad applicability, Dr. Allard said, because participants represented the spectrum of the thousands who signed up for the walking event, who varied in age, level of physical fitness, and number of CV risk factors. They included CV patients, the physically fit, “recreational walkers who didn’t really exercise regularly,” and “habitual nonexercisers.”
It enrolled three groups of participants in the Four Days Marches in Nijmegen, which in a typical year attracts tens of thousands of participants who walk up to 30 km, 40 km, or 50 km per day for 4 consecutive days.
They included 35 statin users who walked the event despite muscle symptoms, 34 on statins but without such symptoms, and 31 non–statin-using controls. Their mean ages ranged from 65 to 68 years.
Statin users were overwhelmingly on simvastatin or atorvastatin. The average statin therapy durations were 60 months and 96 months for those with and without symptoms, respectively.
Assessments were performed several days before the event, at baseline, and after the end of walking on days 1, 2, and 3.
Scores for muscle pain on the Brief Pain Inventory were higher at baseline for the symptomatic-on-statins group (P < .001) compared with the other two groups, and went up (P < .001) similarly across the three groups during each of the 3 days, the report notes. Fatigue scores on the Brief Fatigue Inventory followed the same pattern.
All biomarkers of muscle injury or stress were at comparable levels at baseline in the three groups and went up similarly (P < .001) with no significant differences at the end of day 3. Biomarkers included lactate dehydrogenase, creatine kinase, myoglobin, cardiac troponin I, and N-terminal pro-brain natriuretic peptide.
Statin-related reductions in levels of coenzyme Q 10 (CoQ10) have been thought to exacerbate muscle injury, the authors note. But levels of CoQ10 weren’t significantly different across the three groups at any point in the study, and they did not show any significant associations with measures of muscle injury, symptoms, or fatigue.
Patients with statin-associated muscle symptoms (SAMS) often limit physical activity because of muscle pain or weakness, but also “concerns that exercise will exacerbate muscle injury,” an accompanying editorial notes. “Therefore, exercise, a foundation of improving and maintaining cardiometabolic health, is often avoided or limited.”
But the current study, writes Robert S. Rosenson, MD, of Mount Sinai Heart, New York, indeed suggests that “many patients who develop SAMS may engage in a moderately intensive walking program without concern for worsened muscle biomarkers or performance.”
The exercise didn’t seem to improve muscle function in symptomatic statin users, compared with the other groups over the study’s very short follow-up, Dr. Rosenson observes. But “it remains uncertain from this study whether sustained exercise in SAMS patients will effectuate improved metabolic biomarkers or exercise capacity in the long term.”
Dr. Allard is supported by a grant from the Radboud Institute for Health Sciences; the other authors have disclosed no relevant financial relationships. Dr. Rosenson disclosed receiving research funding to his institution from Amgen, Arrowhead, Lilly, Novartis, and Regeneron; consulting fees from Amgen, Arrowhead, Lilly, Lipigon, Novartis, CRISPR Therapeutics, Precision BioSciences, Verve, Ultragenyx Pharmaceutical, and Regeneron; speaking fees from Amgen, Kowa, and Regeneron; and royalties from Wolters Kluwer (UpToDate); and that he holds stock in MediMergent.
A version of this article first appeared on Medscape.com.
People who are physically active and on statins may have one less potential concern about the drugs. Despite their reputation for causing muscle injury, a study suggests statins won’t worsen the toll that sustained, moderately intensive exercise already takes on patients’ muscles.
Statin therapy in this prospective, controlled study wasn’t seen to aggravate normal muscle fatigue or pain from sustained exercise or adversely affect enzymes or other biomarkers associated with muscle injury.
The findings come from 100 individuals, of whom about two-thirds were on statins, participating in a public, 4-day, long-distance walking event held annually in the Netherlands. Results were published in the Journal of the American College of Cardiology with Neeltje A.E. Allard, MD, Radboud University Medical Center, Nijmegen, the Netherlands, as lead author.
For all of statins’ common use in adults with cardiovascular (CV) risk factors, the drugs are often blamed for causing excessive muscle pain or injury as a side effect. Yet there is a predominance of evidence to the contrary based on meta-analyses and clinical trials, suggesting that the drugs are taking the rap for many entirely unrelated muscle symptoms.
The new findings, from people ranging widely in fitness levels, suggest that “exercise of moderate intensity is feasible and safe” in statin users, that the drugs won’t exacerbate normal muscle symptoms from exercise, Dr. Allard told this news organization.
And that exercise doesn’t have to be on an unusual scale. Regular exercise in statin users can simply be consistent with broader guidelines, say 30 minutes of walking per day, she noted.
The study has such broad applicability, Dr. Allard said, because participants represented the spectrum of the thousands who signed up for the walking event, who varied in age, level of physical fitness, and number of CV risk factors. They included CV patients, the physically fit, “recreational walkers who didn’t really exercise regularly,” and “habitual nonexercisers.”
It enrolled three groups of participants in the Four Days Marches in Nijmegen, which in a typical year attracts tens of thousands of participants who walk up to 30 km, 40 km, or 50 km per day for 4 consecutive days.
They included 35 statin users who walked the event despite muscle symptoms, 34 on statins but without such symptoms, and 31 non–statin-using controls. Their mean ages ranged from 65 to 68 years.
Statin users were overwhelmingly on simvastatin or atorvastatin. The average statin therapy durations were 60 months and 96 months for those with and without symptoms, respectively.
Assessments were performed several days before the event, at baseline, and after the end of walking on days 1, 2, and 3.
Scores for muscle pain on the Brief Pain Inventory were higher at baseline for the symptomatic-on-statins group (P < .001) compared with the other two groups, and went up (P < .001) similarly across the three groups during each of the 3 days, the report notes. Fatigue scores on the Brief Fatigue Inventory followed the same pattern.
All biomarkers of muscle injury or stress were at comparable levels at baseline in the three groups and went up similarly (P < .001) with no significant differences at the end of day 3. Biomarkers included lactate dehydrogenase, creatine kinase, myoglobin, cardiac troponin I, and N-terminal pro-brain natriuretic peptide.
Statin-related reductions in levels of coenzyme Q 10 (CoQ10) have been thought to exacerbate muscle injury, the authors note. But levels of CoQ10 weren’t significantly different across the three groups at any point in the study, and they did not show any significant associations with measures of muscle injury, symptoms, or fatigue.
Patients with statin-associated muscle symptoms (SAMS) often limit physical activity because of muscle pain or weakness, but also “concerns that exercise will exacerbate muscle injury,” an accompanying editorial notes. “Therefore, exercise, a foundation of improving and maintaining cardiometabolic health, is often avoided or limited.”
But the current study, writes Robert S. Rosenson, MD, of Mount Sinai Heart, New York, indeed suggests that “many patients who develop SAMS may engage in a moderately intensive walking program without concern for worsened muscle biomarkers or performance.”
The exercise didn’t seem to improve muscle function in symptomatic statin users, compared with the other groups over the study’s very short follow-up, Dr. Rosenson observes. But “it remains uncertain from this study whether sustained exercise in SAMS patients will effectuate improved metabolic biomarkers or exercise capacity in the long term.”
Dr. Allard is supported by a grant from the Radboud Institute for Health Sciences; the other authors have disclosed no relevant financial relationships. Dr. Rosenson disclosed receiving research funding to his institution from Amgen, Arrowhead, Lilly, Novartis, and Regeneron; consulting fees from Amgen, Arrowhead, Lilly, Lipigon, Novartis, CRISPR Therapeutics, Precision BioSciences, Verve, Ultragenyx Pharmaceutical, and Regeneron; speaking fees from Amgen, Kowa, and Regeneron; and royalties from Wolters Kluwer (UpToDate); and that he holds stock in MediMergent.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
High salt intake linked to atherosclerosis even with normal BP
A high salt intake is an important risk factor for atherosclerosis, even in the absence of hypertension, a large study from Sweden concludes.
The study, including more than 10,000 individuals between the ages of 50 and 64 years from the Swedish Cardiopulmonary bioImage Study, showed a significant link between dietary salt intake and the risk for atherosclerotic lesions in the coronary and carotid arteries, even in participants with normal blood pressure and without known cardiovascular disease.
The finding suggests that salt could be a damaging factor in its own right before the development of hypertension, the authors write. The results were published online in European Heart Journal Open.
It has been known for a long time that salt is linked to hypertension, but the role that salt plays in atherosclerosis has not been examined, first author Jonas Wuopio, MD, Karolinska Institutet, Huddinge, and Clinical Research Center, Falun, Uppsala University, both in Sweden, told this news organization.
“Hardly anyone looks at changes in the arteries’ calcification, the atherosclerotic plaques and the association with salt intake,” Dr. Wuopio said. “We had this exclusive data from our cohort, so we wanted to use it to close this knowledge gap.”
The analysis included 10,788 adults aged 50-64 years, (average age, 58 years; 52% women) who underwent a coronary computed tomography angiography (CCTA) scan. The estimated 24-hour sodium excretion was used to measure sodium intake.
CCTA was used to obtain 3-D images of the coronary arteries to measure the degree of coronary artery calcium as well as detect stenosis in the coronary arteries. Participants also had an ultrasound of the carotid arteries.
After adjusting for age, sex, and study site (the study was done at Uppsala and Malmö, Sweden), the researchers found that rising salt consumption was linked with increasing atherosclerosis in a linear fashion in both the coronary and carotid arteries.
Each 1,000 mg rise in sodium excretion was associated with a 9% increased occurrence of carotid plaque (odds ratio, 1.09; P < .001; confidence interval, 1.06-1.12), a higher coronary artery calcium score (OR, 1.16; P < .001; CI, 1.12-1.19), and a 17% increased occurrence of coronary artery stenosis (OR, 1.17; P < .001; CI, 1.13-1.20).
The association was abolished, though, after adjusting for blood pressure, they note. Their “interpretation is that the increase in blood pressure from sodium intake, even below the level that currently defines arterial hypertension, is an important factor that mediates the interplay between salt intake and the atherosclerotic process,” they write. “As we observed an association in individuals with normal blood pressure, one possible explanation for these findings is that the detrimental pathological processes begin already prior to the development of hypertension,” they note, although they caution that no causal relationships can be gleaned from this cross-sectional study.
They also reported no sign of a “J-curve”; participants with the lowest levels of sodium excretion had the lowest occurrence of both coronary and carotid atherosclerosis, which contradicts findings in some studies that found very low sodium linked to increased cardiovascular disease–related events.
“There have been some controversies among researchers regarding very low intake, where some say very low salt intake can increase the risk of cardiovascular disease, but we could not find this in this study,” Dr. Wuopio said.
“Our study is confirming that excess salt is not a good thing, but the fact that it is linked to atherosclerosis, even in the absence of hypertension, was a bit of a surprise,” he said.
“I will be telling my patients to follow the advice given by the World Health Organization and other medical societies, to limit your intake of salt to approximately 1 teaspoon, even if your blood pressure is normal.”
Time to scrutinize salt’s role in atherosclerosis
In an accompanying editorial, Maciej Banach, MD, Medical University of Lodz, and Stanislaw Surma, MD, Faculty of Medical Sciences in Katowice, both in Poland, write that excessive dietary salt intake is a well-documented cardiovascular risk factor, and that the association is explained in most studies by increased blood pressure.
“We should look more extensively on the role of dietary salt, as it affects many pathological mechanisms, by which, especially with the coexistence of other risk factors, atherosclerosis may progress very fast,” they write.
“The results of the study shed new light on the direct relationship between excessive dietary salt intake and the risk of ASCVD [atherosclerotic cardiovascular disease], indicating that salt intake might be a risk factor for atherosclerosis even prior to the development of hypertension,” they conclude.
Confirmatory and novel
“Nobody questions the fact that high blood pressure is a powerful risk factor for atherosclerotic disease, but not all studies have suggested that, at least at significantly higher levels of sodium intake, that high salt intake tracks with risk for atherosclerotic disease,” Alon Gitig, MD, assistant professor and director of cardiology, Mount Sinai Doctors-Westchester, Yonkers, New York, told this news organization.
Most of the studies of salt intake in the diet are based on patient self-reports via food frequency questionnaires, which can give a general idea of salt intake, but are often not totally accurate, Dr. Gitig said.
“Here, they measured sodium in the urine and estimated the 24-hour salt intake from that, which is slightly novel,” he said.
Everybody knows that high blood pressure is associated with future cardiovascular disease risk, but what many don’t realize is that that risk starts to increase slightly but significantly above a blood pressure that is already in the range of 115 mm Hg/75 mm Hg, he said.
“The lower you can get your blood pressure down, to around 115-120, the lower your risk for cardiovascular disease,” Dr. Gitig said.
It is possible for most people to lower blood pressure through attention to diet, restricting sodium, performing cardio and weight training exercises, and maintaining a healthy weight, he said.
An example of a cardiovascular health diet is the Dietary Approaches to Stop Hypertension (DASH) diet.
“The DASH diet, consisting of 9 servings of fruits and vegetables a day with few refined carbs, flour and sugar, has been shown in a randomized trial to dramatically reduce blood pressure. There are two reasons for that. One is that the fruits and vegetables have many phytonutrients that are good for arteries. The other is that a large proportion of U.S. adults have insulin resistance, which leads to high blood pressure.
“The more fruits and vegetables and healthy animal products, and less sugar and flour, the more you are going to improve your insulin resistance, so you can bring your blood pressure down that way,” Dr. Gitig said.
The study was funded by the Swedish Heart-Lung Foundation, the Knut and Alice Wallenberg Foundation, the Swedish Research Council and Vinnova (Sweden’s Innovation agency), the University of Gothenburg and Sahlgrenska University Hospital, the Karolinska Institutet and Stockholm County Council, the Linköping University and University Hospital, the Lund University and Skane University Hospital, the Umea University and University Hospital, and the Uppsala University and University Hospital. Dr. Wuopio and Dr. Gitig report no relevant financial relationships. Dr. Banach reports financial relationships with Adamed, Amgen, Daichii Sankyo, Esperion, KrKa, NewAmsterdam, Polpharma, Novartis, Pfizer, Sanofi, Teva, Viatris, and CMDO at Longevity Group (LU). Dr. Surma reports a financial relationship with Sanofi and Novartis.
A version of this article first appeared on Medscape.com.
A high salt intake is an important risk factor for atherosclerosis, even in the absence of hypertension, a large study from Sweden concludes.
The study, including more than 10,000 individuals between the ages of 50 and 64 years from the Swedish Cardiopulmonary bioImage Study, showed a significant link between dietary salt intake and the risk for atherosclerotic lesions in the coronary and carotid arteries, even in participants with normal blood pressure and without known cardiovascular disease.
The finding suggests that salt could be a damaging factor in its own right before the development of hypertension, the authors write. The results were published online in European Heart Journal Open.
It has been known for a long time that salt is linked to hypertension, but the role that salt plays in atherosclerosis has not been examined, first author Jonas Wuopio, MD, Karolinska Institutet, Huddinge, and Clinical Research Center, Falun, Uppsala University, both in Sweden, told this news organization.
“Hardly anyone looks at changes in the arteries’ calcification, the atherosclerotic plaques and the association with salt intake,” Dr. Wuopio said. “We had this exclusive data from our cohort, so we wanted to use it to close this knowledge gap.”
The analysis included 10,788 adults aged 50-64 years, (average age, 58 years; 52% women) who underwent a coronary computed tomography angiography (CCTA) scan. The estimated 24-hour sodium excretion was used to measure sodium intake.
CCTA was used to obtain 3-D images of the coronary arteries to measure the degree of coronary artery calcium as well as detect stenosis in the coronary arteries. Participants also had an ultrasound of the carotid arteries.
After adjusting for age, sex, and study site (the study was done at Uppsala and Malmö, Sweden), the researchers found that rising salt consumption was linked with increasing atherosclerosis in a linear fashion in both the coronary and carotid arteries.
Each 1,000 mg rise in sodium excretion was associated with a 9% increased occurrence of carotid plaque (odds ratio, 1.09; P < .001; confidence interval, 1.06-1.12), a higher coronary artery calcium score (OR, 1.16; P < .001; CI, 1.12-1.19), and a 17% increased occurrence of coronary artery stenosis (OR, 1.17; P < .001; CI, 1.13-1.20).
The association was abolished, though, after adjusting for blood pressure, they note. Their “interpretation is that the increase in blood pressure from sodium intake, even below the level that currently defines arterial hypertension, is an important factor that mediates the interplay between salt intake and the atherosclerotic process,” they write. “As we observed an association in individuals with normal blood pressure, one possible explanation for these findings is that the detrimental pathological processes begin already prior to the development of hypertension,” they note, although they caution that no causal relationships can be gleaned from this cross-sectional study.
They also reported no sign of a “J-curve”; participants with the lowest levels of sodium excretion had the lowest occurrence of both coronary and carotid atherosclerosis, which contradicts findings in some studies that found very low sodium linked to increased cardiovascular disease–related events.
“There have been some controversies among researchers regarding very low intake, where some say very low salt intake can increase the risk of cardiovascular disease, but we could not find this in this study,” Dr. Wuopio said.
“Our study is confirming that excess salt is not a good thing, but the fact that it is linked to atherosclerosis, even in the absence of hypertension, was a bit of a surprise,” he said.
“I will be telling my patients to follow the advice given by the World Health Organization and other medical societies, to limit your intake of salt to approximately 1 teaspoon, even if your blood pressure is normal.”
Time to scrutinize salt’s role in atherosclerosis
In an accompanying editorial, Maciej Banach, MD, Medical University of Lodz, and Stanislaw Surma, MD, Faculty of Medical Sciences in Katowice, both in Poland, write that excessive dietary salt intake is a well-documented cardiovascular risk factor, and that the association is explained in most studies by increased blood pressure.
“We should look more extensively on the role of dietary salt, as it affects many pathological mechanisms, by which, especially with the coexistence of other risk factors, atherosclerosis may progress very fast,” they write.
“The results of the study shed new light on the direct relationship between excessive dietary salt intake and the risk of ASCVD [atherosclerotic cardiovascular disease], indicating that salt intake might be a risk factor for atherosclerosis even prior to the development of hypertension,” they conclude.
Confirmatory and novel
“Nobody questions the fact that high blood pressure is a powerful risk factor for atherosclerotic disease, but not all studies have suggested that, at least at significantly higher levels of sodium intake, that high salt intake tracks with risk for atherosclerotic disease,” Alon Gitig, MD, assistant professor and director of cardiology, Mount Sinai Doctors-Westchester, Yonkers, New York, told this news organization.
Most of the studies of salt intake in the diet are based on patient self-reports via food frequency questionnaires, which can give a general idea of salt intake, but are often not totally accurate, Dr. Gitig said.
“Here, they measured sodium in the urine and estimated the 24-hour salt intake from that, which is slightly novel,” he said.
Everybody knows that high blood pressure is associated with future cardiovascular disease risk, but what many don’t realize is that that risk starts to increase slightly but significantly above a blood pressure that is already in the range of 115 mm Hg/75 mm Hg, he said.
“The lower you can get your blood pressure down, to around 115-120, the lower your risk for cardiovascular disease,” Dr. Gitig said.
It is possible for most people to lower blood pressure through attention to diet, restricting sodium, performing cardio and weight training exercises, and maintaining a healthy weight, he said.
An example of a cardiovascular health diet is the Dietary Approaches to Stop Hypertension (DASH) diet.
“The DASH diet, consisting of 9 servings of fruits and vegetables a day with few refined carbs, flour and sugar, has been shown in a randomized trial to dramatically reduce blood pressure. There are two reasons for that. One is that the fruits and vegetables have many phytonutrients that are good for arteries. The other is that a large proportion of U.S. adults have insulin resistance, which leads to high blood pressure.
“The more fruits and vegetables and healthy animal products, and less sugar and flour, the more you are going to improve your insulin resistance, so you can bring your blood pressure down that way,” Dr. Gitig said.
The study was funded by the Swedish Heart-Lung Foundation, the Knut and Alice Wallenberg Foundation, the Swedish Research Council and Vinnova (Sweden’s Innovation agency), the University of Gothenburg and Sahlgrenska University Hospital, the Karolinska Institutet and Stockholm County Council, the Linköping University and University Hospital, the Lund University and Skane University Hospital, the Umea University and University Hospital, and the Uppsala University and University Hospital. Dr. Wuopio and Dr. Gitig report no relevant financial relationships. Dr. Banach reports financial relationships with Adamed, Amgen, Daichii Sankyo, Esperion, KrKa, NewAmsterdam, Polpharma, Novartis, Pfizer, Sanofi, Teva, Viatris, and CMDO at Longevity Group (LU). Dr. Surma reports a financial relationship with Sanofi and Novartis.
A version of this article first appeared on Medscape.com.
A high salt intake is an important risk factor for atherosclerosis, even in the absence of hypertension, a large study from Sweden concludes.
The study, including more than 10,000 individuals between the ages of 50 and 64 years from the Swedish Cardiopulmonary bioImage Study, showed a significant link between dietary salt intake and the risk for atherosclerotic lesions in the coronary and carotid arteries, even in participants with normal blood pressure and without known cardiovascular disease.
The finding suggests that salt could be a damaging factor in its own right before the development of hypertension, the authors write. The results were published online in European Heart Journal Open.
It has been known for a long time that salt is linked to hypertension, but the role that salt plays in atherosclerosis has not been examined, first author Jonas Wuopio, MD, Karolinska Institutet, Huddinge, and Clinical Research Center, Falun, Uppsala University, both in Sweden, told this news organization.
“Hardly anyone looks at changes in the arteries’ calcification, the atherosclerotic plaques and the association with salt intake,” Dr. Wuopio said. “We had this exclusive data from our cohort, so we wanted to use it to close this knowledge gap.”
The analysis included 10,788 adults aged 50-64 years, (average age, 58 years; 52% women) who underwent a coronary computed tomography angiography (CCTA) scan. The estimated 24-hour sodium excretion was used to measure sodium intake.
CCTA was used to obtain 3-D images of the coronary arteries to measure the degree of coronary artery calcium as well as detect stenosis in the coronary arteries. Participants also had an ultrasound of the carotid arteries.
After adjusting for age, sex, and study site (the study was done at Uppsala and Malmö, Sweden), the researchers found that rising salt consumption was linked with increasing atherosclerosis in a linear fashion in both the coronary and carotid arteries.
Each 1,000 mg rise in sodium excretion was associated with a 9% increased occurrence of carotid plaque (odds ratio, 1.09; P < .001; confidence interval, 1.06-1.12), a higher coronary artery calcium score (OR, 1.16; P < .001; CI, 1.12-1.19), and a 17% increased occurrence of coronary artery stenosis (OR, 1.17; P < .001; CI, 1.13-1.20).
The association was abolished, though, after adjusting for blood pressure, they note. Their “interpretation is that the increase in blood pressure from sodium intake, even below the level that currently defines arterial hypertension, is an important factor that mediates the interplay between salt intake and the atherosclerotic process,” they write. “As we observed an association in individuals with normal blood pressure, one possible explanation for these findings is that the detrimental pathological processes begin already prior to the development of hypertension,” they note, although they caution that no causal relationships can be gleaned from this cross-sectional study.
They also reported no sign of a “J-curve”; participants with the lowest levels of sodium excretion had the lowest occurrence of both coronary and carotid atherosclerosis, which contradicts findings in some studies that found very low sodium linked to increased cardiovascular disease–related events.
“There have been some controversies among researchers regarding very low intake, where some say very low salt intake can increase the risk of cardiovascular disease, but we could not find this in this study,” Dr. Wuopio said.
“Our study is confirming that excess salt is not a good thing, but the fact that it is linked to atherosclerosis, even in the absence of hypertension, was a bit of a surprise,” he said.
“I will be telling my patients to follow the advice given by the World Health Organization and other medical societies, to limit your intake of salt to approximately 1 teaspoon, even if your blood pressure is normal.”
Time to scrutinize salt’s role in atherosclerosis
In an accompanying editorial, Maciej Banach, MD, Medical University of Lodz, and Stanislaw Surma, MD, Faculty of Medical Sciences in Katowice, both in Poland, write that excessive dietary salt intake is a well-documented cardiovascular risk factor, and that the association is explained in most studies by increased blood pressure.
“We should look more extensively on the role of dietary salt, as it affects many pathological mechanisms, by which, especially with the coexistence of other risk factors, atherosclerosis may progress very fast,” they write.
“The results of the study shed new light on the direct relationship between excessive dietary salt intake and the risk of ASCVD [atherosclerotic cardiovascular disease], indicating that salt intake might be a risk factor for atherosclerosis even prior to the development of hypertension,” they conclude.
Confirmatory and novel
“Nobody questions the fact that high blood pressure is a powerful risk factor for atherosclerotic disease, but not all studies have suggested that, at least at significantly higher levels of sodium intake, that high salt intake tracks with risk for atherosclerotic disease,” Alon Gitig, MD, assistant professor and director of cardiology, Mount Sinai Doctors-Westchester, Yonkers, New York, told this news organization.
Most of the studies of salt intake in the diet are based on patient self-reports via food frequency questionnaires, which can give a general idea of salt intake, but are often not totally accurate, Dr. Gitig said.
“Here, they measured sodium in the urine and estimated the 24-hour salt intake from that, which is slightly novel,” he said.
Everybody knows that high blood pressure is associated with future cardiovascular disease risk, but what many don’t realize is that that risk starts to increase slightly but significantly above a blood pressure that is already in the range of 115 mm Hg/75 mm Hg, he said.
“The lower you can get your blood pressure down, to around 115-120, the lower your risk for cardiovascular disease,” Dr. Gitig said.
It is possible for most people to lower blood pressure through attention to diet, restricting sodium, performing cardio and weight training exercises, and maintaining a healthy weight, he said.
An example of a cardiovascular health diet is the Dietary Approaches to Stop Hypertension (DASH) diet.
“The DASH diet, consisting of 9 servings of fruits and vegetables a day with few refined carbs, flour and sugar, has been shown in a randomized trial to dramatically reduce blood pressure. There are two reasons for that. One is that the fruits and vegetables have many phytonutrients that are good for arteries. The other is that a large proportion of U.S. adults have insulin resistance, which leads to high blood pressure.
“The more fruits and vegetables and healthy animal products, and less sugar and flour, the more you are going to improve your insulin resistance, so you can bring your blood pressure down that way,” Dr. Gitig said.
The study was funded by the Swedish Heart-Lung Foundation, the Knut and Alice Wallenberg Foundation, the Swedish Research Council and Vinnova (Sweden’s Innovation agency), the University of Gothenburg and Sahlgrenska University Hospital, the Karolinska Institutet and Stockholm County Council, the Linköping University and University Hospital, the Lund University and Skane University Hospital, the Umea University and University Hospital, and the Uppsala University and University Hospital. Dr. Wuopio and Dr. Gitig report no relevant financial relationships. Dr. Banach reports financial relationships with Adamed, Amgen, Daichii Sankyo, Esperion, KrKa, NewAmsterdam, Polpharma, Novartis, Pfizer, Sanofi, Teva, Viatris, and CMDO at Longevity Group (LU). Dr. Surma reports a financial relationship with Sanofi and Novartis.
A version of this article first appeared on Medscape.com.
New update on left atrial appendage closure recommendations
An updated consensus statement on transcatheter left atrial appendage closure (LAAC) has put a newfound focus on patient selection for the procedure, specifically recommending that the procedure is appropriate for patients with nonvalvular atrial fibrillation who have risk for thromboembolism, aren’t well suited for direct oral anticoagulants (DOACs) and have a good chance of living for at least another year.
The statement, published online in the Journal of the Society for Cardiovascular Angiography & Interventions, also makes recommendations for how much experience operators should have, how many procedures they should perform to keep their skills up, and when and how to use imaging and prescribe DOACs, among other suggestions.
The statement represents the first updated guidance for LAAC since 2015. “Since then this field has really expanded and evolved,” writing group chair Jacqueline Saw, MD, said in an interview. “For instance, the indications are more matured and specific, and the procedural technical steps have matured. Imaging has also advanced, there’s more understanding about postprocedural care and there are also new devices that have been approved.”
Dr. Saw, an interventional cardiologist at Vancouver General Hospital and St. Paul’s Hospital, and a professor at the University of British Columbia in Vancouver, called the statement “a piece that puts everything together.”
“This document really summarizes the whole practice for doing transcatheter procedures,” she added, “so it’s all-in-one document in terms of recommendation of who we do the procedure for, how we should do it, how we should image and guide the procedure, and what complications to look out for and how to manage patients post procedure, be it with antithrombotic therapy and/or device surveillance.”
13 recommendations
In all, the statement carries 13 recommendations for LAAC. The Society for Cardiovascular Angiography & Interventions and the Heart Rhythm Society commissioned the writing group. The American College of Cardiology and Society of Cardiovascular Computed Tomography have endorsed the statement. The following are among the recommendations:
- Transcatheter LAAC is appropriate for patients with nonvalvular atrial fibrillation with high thromboembolic risk but for whom long-term oral anticoagulation may be contraindicated and who have at least 1 year’s life expectancy.
- Operators should have performed at least 50 prior left-sided ablations or structural procedures and at least 25 transseptal punctures (TSPs). Interventional-imaging physicians should have experience in guiding 25 or more TSPs before supporting LAAC procedures independently.
- To maintain skills, operators should do 25 or more TSPs and at least 12 LAACs over each 2-year period.
- On-site cardiovascular surgery backup should be available for new programs and for operators early in their learning curve.
- Baseline imaging with transesophageal echocardiography (TEE) or cardiac computed tomography should be performed before LAAC.
- Intraprocedural imaging guidance with TEE or intracardiac echocardiography.
- Follow labeling of each specific LAAC device for technical aspects of the procedure.
- Familiarity with avoiding, recognizing, and managing LAAC complications.
- Predischarge 2-dimensional TEE to rule out pericardial effusion and device embolization.
- Anticoagulation for device-related thrombus.
- Make all efforts to minimize peridevice leaks during implantation because their clinical impact and management isn’t well understood.
- Antithrombotic therapy with warfarin, DOAC, or dual-antiplatelet therapy after LAAC based on the studied regimen and instructions for each specific device, tailored to the bleeding risks for each patient.
- TEE or cardiac computed tomography at 45-90 days after LAAC for device surveillance to assess for peridevice leak and device-related thrombus.
The statement also includes precautionary recommendations. It advises against using routine closure of LAAC-associated iatrogenic atrial septal defects and states that combined procedures with LAAC, such as structural interventions and pulmonary vein isolation, should be avoided because randomized controlled trial data are pending.
“These recommendations are based upon data from updated publications and randomized trial data as well as large registries, including the National Cardiovascular Data Registry, so I think this is a very practical statement that puts all these pieces together for any budding interventionalist doing this procedure and even experienced operations,” Dr. Saw said.
Authors of an accompanying editorial agreed that the “rigorous standards” set out in the statement will help maintain “a high level of procedural safety in the setting of rapid expansion.”
The editorialists, Faisal M. Merchant, MD, of Emory University, Atlanta, and Mohamad Alkhouli, MD, professor of medicine at Mayo Clinic School of Medicine, Rochester, Minn., point out that the incidence of pericardial effusion has decreased from more than 5% in the pivotal Watchman trials to less than 1.5% in the most recent report from the National Cardiovascular Data Registry, which shows that more than 100,000 procedures have been performed in the United States.
But most important as the field moves forward, they stress, is patient selection. The recommendation of limiting patients to those with a life expectancy of 1 year “is a tacit recognition of the fact that the benefits of LAAC take time to accrue, and many older and frail patients are unlikely to derive meaningful benefit.”
Dr. Merchant and Dr. Alkhouli also note that there remains a conundrum in patient selection that remains from the original LAAC trials, which enrolled patients who were eligible for anticoagulation. “Somewhat paradoxically, after its approval, LAAC is mostly prescribed to patients who are not felt to be good anticoagulation candidates.” This leaves physicians “in the precarious position of extrapolating data to patients who were excluded from the original clinical trials.”
Therefore, the consensus statement “is right to put patient selection front and center in its recommendations, but as the field of LAAC comes of age, better evidence to support patient selection will be the real sign of maturity.”
Dr. Saw said she envisions another update over the next 2 years or so as ongoing clinical trials comparing DOAC and LAAC, namely the CHAMPION-AF and OPTION trials, report results.
Dr. Saw and Dr. Merchant, reported no conflicts of interest. Dr. Alkhouli has financial ties to Boston Scientific, Abbott, and Philips.
An updated consensus statement on transcatheter left atrial appendage closure (LAAC) has put a newfound focus on patient selection for the procedure, specifically recommending that the procedure is appropriate for patients with nonvalvular atrial fibrillation who have risk for thromboembolism, aren’t well suited for direct oral anticoagulants (DOACs) and have a good chance of living for at least another year.
The statement, published online in the Journal of the Society for Cardiovascular Angiography & Interventions, also makes recommendations for how much experience operators should have, how many procedures they should perform to keep their skills up, and when and how to use imaging and prescribe DOACs, among other suggestions.
The statement represents the first updated guidance for LAAC since 2015. “Since then this field has really expanded and evolved,” writing group chair Jacqueline Saw, MD, said in an interview. “For instance, the indications are more matured and specific, and the procedural technical steps have matured. Imaging has also advanced, there’s more understanding about postprocedural care and there are also new devices that have been approved.”
Dr. Saw, an interventional cardiologist at Vancouver General Hospital and St. Paul’s Hospital, and a professor at the University of British Columbia in Vancouver, called the statement “a piece that puts everything together.”
“This document really summarizes the whole practice for doing transcatheter procedures,” she added, “so it’s all-in-one document in terms of recommendation of who we do the procedure for, how we should do it, how we should image and guide the procedure, and what complications to look out for and how to manage patients post procedure, be it with antithrombotic therapy and/or device surveillance.”
13 recommendations
In all, the statement carries 13 recommendations for LAAC. The Society for Cardiovascular Angiography & Interventions and the Heart Rhythm Society commissioned the writing group. The American College of Cardiology and Society of Cardiovascular Computed Tomography have endorsed the statement. The following are among the recommendations:
- Transcatheter LAAC is appropriate for patients with nonvalvular atrial fibrillation with high thromboembolic risk but for whom long-term oral anticoagulation may be contraindicated and who have at least 1 year’s life expectancy.
- Operators should have performed at least 50 prior left-sided ablations or structural procedures and at least 25 transseptal punctures (TSPs). Interventional-imaging physicians should have experience in guiding 25 or more TSPs before supporting LAAC procedures independently.
- To maintain skills, operators should do 25 or more TSPs and at least 12 LAACs over each 2-year period.
- On-site cardiovascular surgery backup should be available for new programs and for operators early in their learning curve.
- Baseline imaging with transesophageal echocardiography (TEE) or cardiac computed tomography should be performed before LAAC.
- Intraprocedural imaging guidance with TEE or intracardiac echocardiography.
- Follow labeling of each specific LAAC device for technical aspects of the procedure.
- Familiarity with avoiding, recognizing, and managing LAAC complications.
- Predischarge 2-dimensional TEE to rule out pericardial effusion and device embolization.
- Anticoagulation for device-related thrombus.
- Make all efforts to minimize peridevice leaks during implantation because their clinical impact and management isn’t well understood.
- Antithrombotic therapy with warfarin, DOAC, or dual-antiplatelet therapy after LAAC based on the studied regimen and instructions for each specific device, tailored to the bleeding risks for each patient.
- TEE or cardiac computed tomography at 45-90 days after LAAC for device surveillance to assess for peridevice leak and device-related thrombus.
The statement also includes precautionary recommendations. It advises against using routine closure of LAAC-associated iatrogenic atrial septal defects and states that combined procedures with LAAC, such as structural interventions and pulmonary vein isolation, should be avoided because randomized controlled trial data are pending.
“These recommendations are based upon data from updated publications and randomized trial data as well as large registries, including the National Cardiovascular Data Registry, so I think this is a very practical statement that puts all these pieces together for any budding interventionalist doing this procedure and even experienced operations,” Dr. Saw said.
Authors of an accompanying editorial agreed that the “rigorous standards” set out in the statement will help maintain “a high level of procedural safety in the setting of rapid expansion.”
The editorialists, Faisal M. Merchant, MD, of Emory University, Atlanta, and Mohamad Alkhouli, MD, professor of medicine at Mayo Clinic School of Medicine, Rochester, Minn., point out that the incidence of pericardial effusion has decreased from more than 5% in the pivotal Watchman trials to less than 1.5% in the most recent report from the National Cardiovascular Data Registry, which shows that more than 100,000 procedures have been performed in the United States.
But most important as the field moves forward, they stress, is patient selection. The recommendation of limiting patients to those with a life expectancy of 1 year “is a tacit recognition of the fact that the benefits of LAAC take time to accrue, and many older and frail patients are unlikely to derive meaningful benefit.”
Dr. Merchant and Dr. Alkhouli also note that there remains a conundrum in patient selection that remains from the original LAAC trials, which enrolled patients who were eligible for anticoagulation. “Somewhat paradoxically, after its approval, LAAC is mostly prescribed to patients who are not felt to be good anticoagulation candidates.” This leaves physicians “in the precarious position of extrapolating data to patients who were excluded from the original clinical trials.”
Therefore, the consensus statement “is right to put patient selection front and center in its recommendations, but as the field of LAAC comes of age, better evidence to support patient selection will be the real sign of maturity.”
Dr. Saw said she envisions another update over the next 2 years or so as ongoing clinical trials comparing DOAC and LAAC, namely the CHAMPION-AF and OPTION trials, report results.
Dr. Saw and Dr. Merchant, reported no conflicts of interest. Dr. Alkhouli has financial ties to Boston Scientific, Abbott, and Philips.
An updated consensus statement on transcatheter left atrial appendage closure (LAAC) has put a newfound focus on patient selection for the procedure, specifically recommending that the procedure is appropriate for patients with nonvalvular atrial fibrillation who have risk for thromboembolism, aren’t well suited for direct oral anticoagulants (DOACs) and have a good chance of living for at least another year.
The statement, published online in the Journal of the Society for Cardiovascular Angiography & Interventions, also makes recommendations for how much experience operators should have, how many procedures they should perform to keep their skills up, and when and how to use imaging and prescribe DOACs, among other suggestions.
The statement represents the first updated guidance for LAAC since 2015. “Since then this field has really expanded and evolved,” writing group chair Jacqueline Saw, MD, said in an interview. “For instance, the indications are more matured and specific, and the procedural technical steps have matured. Imaging has also advanced, there’s more understanding about postprocedural care and there are also new devices that have been approved.”
Dr. Saw, an interventional cardiologist at Vancouver General Hospital and St. Paul’s Hospital, and a professor at the University of British Columbia in Vancouver, called the statement “a piece that puts everything together.”
“This document really summarizes the whole practice for doing transcatheter procedures,” she added, “so it’s all-in-one document in terms of recommendation of who we do the procedure for, how we should do it, how we should image and guide the procedure, and what complications to look out for and how to manage patients post procedure, be it with antithrombotic therapy and/or device surveillance.”
13 recommendations
In all, the statement carries 13 recommendations for LAAC. The Society for Cardiovascular Angiography & Interventions and the Heart Rhythm Society commissioned the writing group. The American College of Cardiology and Society of Cardiovascular Computed Tomography have endorsed the statement. The following are among the recommendations:
- Transcatheter LAAC is appropriate for patients with nonvalvular atrial fibrillation with high thromboembolic risk but for whom long-term oral anticoagulation may be contraindicated and who have at least 1 year’s life expectancy.
- Operators should have performed at least 50 prior left-sided ablations or structural procedures and at least 25 transseptal punctures (TSPs). Interventional-imaging physicians should have experience in guiding 25 or more TSPs before supporting LAAC procedures independently.
- To maintain skills, operators should do 25 or more TSPs and at least 12 LAACs over each 2-year period.
- On-site cardiovascular surgery backup should be available for new programs and for operators early in their learning curve.
- Baseline imaging with transesophageal echocardiography (TEE) or cardiac computed tomography should be performed before LAAC.
- Intraprocedural imaging guidance with TEE or intracardiac echocardiography.
- Follow labeling of each specific LAAC device for technical aspects of the procedure.
- Familiarity with avoiding, recognizing, and managing LAAC complications.
- Predischarge 2-dimensional TEE to rule out pericardial effusion and device embolization.
- Anticoagulation for device-related thrombus.
- Make all efforts to minimize peridevice leaks during implantation because their clinical impact and management isn’t well understood.
- Antithrombotic therapy with warfarin, DOAC, or dual-antiplatelet therapy after LAAC based on the studied regimen and instructions for each specific device, tailored to the bleeding risks for each patient.
- TEE or cardiac computed tomography at 45-90 days after LAAC for device surveillance to assess for peridevice leak and device-related thrombus.
The statement also includes precautionary recommendations. It advises against using routine closure of LAAC-associated iatrogenic atrial septal defects and states that combined procedures with LAAC, such as structural interventions and pulmonary vein isolation, should be avoided because randomized controlled trial data are pending.
“These recommendations are based upon data from updated publications and randomized trial data as well as large registries, including the National Cardiovascular Data Registry, so I think this is a very practical statement that puts all these pieces together for any budding interventionalist doing this procedure and even experienced operations,” Dr. Saw said.
Authors of an accompanying editorial agreed that the “rigorous standards” set out in the statement will help maintain “a high level of procedural safety in the setting of rapid expansion.”
The editorialists, Faisal M. Merchant, MD, of Emory University, Atlanta, and Mohamad Alkhouli, MD, professor of medicine at Mayo Clinic School of Medicine, Rochester, Minn., point out that the incidence of pericardial effusion has decreased from more than 5% in the pivotal Watchman trials to less than 1.5% in the most recent report from the National Cardiovascular Data Registry, which shows that more than 100,000 procedures have been performed in the United States.
But most important as the field moves forward, they stress, is patient selection. The recommendation of limiting patients to those with a life expectancy of 1 year “is a tacit recognition of the fact that the benefits of LAAC take time to accrue, and many older and frail patients are unlikely to derive meaningful benefit.”
Dr. Merchant and Dr. Alkhouli also note that there remains a conundrum in patient selection that remains from the original LAAC trials, which enrolled patients who were eligible for anticoagulation. “Somewhat paradoxically, after its approval, LAAC is mostly prescribed to patients who are not felt to be good anticoagulation candidates.” This leaves physicians “in the precarious position of extrapolating data to patients who were excluded from the original clinical trials.”
Therefore, the consensus statement “is right to put patient selection front and center in its recommendations, but as the field of LAAC comes of age, better evidence to support patient selection will be the real sign of maturity.”
Dr. Saw said she envisions another update over the next 2 years or so as ongoing clinical trials comparing DOAC and LAAC, namely the CHAMPION-AF and OPTION trials, report results.
Dr. Saw and Dr. Merchant, reported no conflicts of interest. Dr. Alkhouli has financial ties to Boston Scientific, Abbott, and Philips.
FROM THE JOURNAL OF THE SOCIETY FOR CARDIOVASCULAR ANGIOGRAPHY & INTERVENTIONS
New AHA statement on pediatric primary hypertension issued
the American Heart Association said in a new scientific statement.
“Children can have secondary hypertension that is caused by an underlying condition such as chronic kidney disease, endocrine disorders, cardiac anomalies, and some syndromes. However, primary hypertension is now recognized as the most common type of hypertension in childhood,” Bonita Falkner, MD, chair of the writing group and emeritus professor of medicine and pediatrics, Thomas Jefferson University, Philadelphia, said in an interview.
And hypertensive children are “highly likely” to become hypertensive adults and to have measurable target organ injury, particularly left ventricular hypertrophy and vascular stiffening, the writing group noted.
The AHA statement on primary pediatric hypertension was published online in Hypertension.
Primary or essential hypertension occurs in up to 5% of children and adolescents in the United States and other countries.
The American Academy of Pediatrics (AAP), European Society of Hypertension and Hypertension Canada all define hypertension as repeated BP readings at or above the 95th percentile for children, but the thresholds differ by age.
The AAP adopts 130/80 mm Hg starting at age 13 years; the European Society of Hypertension adopts 140/90 mm Hg starting at age 16 years; and Hypertension Canada adopts 120/80 mm Hg for those aged 6-11 years and 130/85 mm Hg for those aged 12-17 years.
Adolescents entering adulthood with a BP < 120/80 mm Hg is an optimal goal, the writing group advised.
They recommend that health care professionals be trained on evidence-based methods to obtain accurate and reliable BP values with either auscultatory or oscillometric methods.
When the initial BP measurement is abnormal, repeat measurement by auscultation is recommended, within the same visit if possible, and then within weeks if the screening BP is hypertensive, or months if the screening BP is elevated.
Because BP levels are variable, even within a single visit, “best practice” is to obtain up to three BP measurements and to record the average of the latter two measurements unless the first measurement is normal, the writing group said. Further confirmation of diagnosis of hypertension can be obtained with 24-hour ambulatory BP monitoring (ABPM).
“Primary hypertension in youth is difficult to recognize in asymptomatic, otherwise healthy youth. There is now evidence that children and adolescents with primary hypertension may also have cardiac and vascular injury due to the hypertension,” Dr. Falkner told this news organization.
“If not identified and treated, the condition can progress to hypertension in young adulthood with heightened risk of premature cardiovascular events,” Dr. Falkner said.
The writing group said “primordial prevention” is an important public health goal because a population with lower BP will have fewer comorbidities related to hypertension and CVD.
Modifiable risk factors for primary hypertension in childhood include obesity, physical inactivity and poor diet/nutrition, disturbed sleep patterns, and environmental stress.
A healthy lifestyle in childhood – including eating healthy food, encouraging physical activity that leads to improved physical fitness and healthy sleep, and avoiding the development of obesity – may help mitigate the risk of hypertension in childhood, the writing group noted.
Looking ahead, they said efforts to improve recognition and diagnosis of high BP in children, as well as clinical trials to evaluate medical treatment and recommend public health initiatives, are all vital to combat rising rates of primary hypertension in children.
This scientific statement was prepared by the volunteer writing group on behalf of the American Heart Association’s Council on Hypertension, the Council on Lifelong Congenital Heart Disease and Heart Health in the Young, the Council on Kidney in Cardiovascular Disease, the Council on Lifestyle and Cardiometabolic Health, and the Council on Cardiovascular and Stroke Nursing.
A version of this article first appeared on Medscape.com.
the American Heart Association said in a new scientific statement.
“Children can have secondary hypertension that is caused by an underlying condition such as chronic kidney disease, endocrine disorders, cardiac anomalies, and some syndromes. However, primary hypertension is now recognized as the most common type of hypertension in childhood,” Bonita Falkner, MD, chair of the writing group and emeritus professor of medicine and pediatrics, Thomas Jefferson University, Philadelphia, said in an interview.
And hypertensive children are “highly likely” to become hypertensive adults and to have measurable target organ injury, particularly left ventricular hypertrophy and vascular stiffening, the writing group noted.
The AHA statement on primary pediatric hypertension was published online in Hypertension.
Primary or essential hypertension occurs in up to 5% of children and adolescents in the United States and other countries.
The American Academy of Pediatrics (AAP), European Society of Hypertension and Hypertension Canada all define hypertension as repeated BP readings at or above the 95th percentile for children, but the thresholds differ by age.
The AAP adopts 130/80 mm Hg starting at age 13 years; the European Society of Hypertension adopts 140/90 mm Hg starting at age 16 years; and Hypertension Canada adopts 120/80 mm Hg for those aged 6-11 years and 130/85 mm Hg for those aged 12-17 years.
Adolescents entering adulthood with a BP < 120/80 mm Hg is an optimal goal, the writing group advised.
They recommend that health care professionals be trained on evidence-based methods to obtain accurate and reliable BP values with either auscultatory or oscillometric methods.
When the initial BP measurement is abnormal, repeat measurement by auscultation is recommended, within the same visit if possible, and then within weeks if the screening BP is hypertensive, or months if the screening BP is elevated.
Because BP levels are variable, even within a single visit, “best practice” is to obtain up to three BP measurements and to record the average of the latter two measurements unless the first measurement is normal, the writing group said. Further confirmation of diagnosis of hypertension can be obtained with 24-hour ambulatory BP monitoring (ABPM).
“Primary hypertension in youth is difficult to recognize in asymptomatic, otherwise healthy youth. There is now evidence that children and adolescents with primary hypertension may also have cardiac and vascular injury due to the hypertension,” Dr. Falkner told this news organization.
“If not identified and treated, the condition can progress to hypertension in young adulthood with heightened risk of premature cardiovascular events,” Dr. Falkner said.
The writing group said “primordial prevention” is an important public health goal because a population with lower BP will have fewer comorbidities related to hypertension and CVD.
Modifiable risk factors for primary hypertension in childhood include obesity, physical inactivity and poor diet/nutrition, disturbed sleep patterns, and environmental stress.
A healthy lifestyle in childhood – including eating healthy food, encouraging physical activity that leads to improved physical fitness and healthy sleep, and avoiding the development of obesity – may help mitigate the risk of hypertension in childhood, the writing group noted.
Looking ahead, they said efforts to improve recognition and diagnosis of high BP in children, as well as clinical trials to evaluate medical treatment and recommend public health initiatives, are all vital to combat rising rates of primary hypertension in children.
This scientific statement was prepared by the volunteer writing group on behalf of the American Heart Association’s Council on Hypertension, the Council on Lifelong Congenital Heart Disease and Heart Health in the Young, the Council on Kidney in Cardiovascular Disease, the Council on Lifestyle and Cardiometabolic Health, and the Council on Cardiovascular and Stroke Nursing.
A version of this article first appeared on Medscape.com.
the American Heart Association said in a new scientific statement.
“Children can have secondary hypertension that is caused by an underlying condition such as chronic kidney disease, endocrine disorders, cardiac anomalies, and some syndromes. However, primary hypertension is now recognized as the most common type of hypertension in childhood,” Bonita Falkner, MD, chair of the writing group and emeritus professor of medicine and pediatrics, Thomas Jefferson University, Philadelphia, said in an interview.
And hypertensive children are “highly likely” to become hypertensive adults and to have measurable target organ injury, particularly left ventricular hypertrophy and vascular stiffening, the writing group noted.
The AHA statement on primary pediatric hypertension was published online in Hypertension.
Primary or essential hypertension occurs in up to 5% of children and adolescents in the United States and other countries.
The American Academy of Pediatrics (AAP), European Society of Hypertension and Hypertension Canada all define hypertension as repeated BP readings at or above the 95th percentile for children, but the thresholds differ by age.
The AAP adopts 130/80 mm Hg starting at age 13 years; the European Society of Hypertension adopts 140/90 mm Hg starting at age 16 years; and Hypertension Canada adopts 120/80 mm Hg for those aged 6-11 years and 130/85 mm Hg for those aged 12-17 years.
Adolescents entering adulthood with a BP < 120/80 mm Hg is an optimal goal, the writing group advised.
They recommend that health care professionals be trained on evidence-based methods to obtain accurate and reliable BP values with either auscultatory or oscillometric methods.
When the initial BP measurement is abnormal, repeat measurement by auscultation is recommended, within the same visit if possible, and then within weeks if the screening BP is hypertensive, or months if the screening BP is elevated.
Because BP levels are variable, even within a single visit, “best practice” is to obtain up to three BP measurements and to record the average of the latter two measurements unless the first measurement is normal, the writing group said. Further confirmation of diagnosis of hypertension can be obtained with 24-hour ambulatory BP monitoring (ABPM).
“Primary hypertension in youth is difficult to recognize in asymptomatic, otherwise healthy youth. There is now evidence that children and adolescents with primary hypertension may also have cardiac and vascular injury due to the hypertension,” Dr. Falkner told this news organization.
“If not identified and treated, the condition can progress to hypertension in young adulthood with heightened risk of premature cardiovascular events,” Dr. Falkner said.
The writing group said “primordial prevention” is an important public health goal because a population with lower BP will have fewer comorbidities related to hypertension and CVD.
Modifiable risk factors for primary hypertension in childhood include obesity, physical inactivity and poor diet/nutrition, disturbed sleep patterns, and environmental stress.
A healthy lifestyle in childhood – including eating healthy food, encouraging physical activity that leads to improved physical fitness and healthy sleep, and avoiding the development of obesity – may help mitigate the risk of hypertension in childhood, the writing group noted.
Looking ahead, they said efforts to improve recognition and diagnosis of high BP in children, as well as clinical trials to evaluate medical treatment and recommend public health initiatives, are all vital to combat rising rates of primary hypertension in children.
This scientific statement was prepared by the volunteer writing group on behalf of the American Heart Association’s Council on Hypertension, the Council on Lifelong Congenital Heart Disease and Heart Health in the Young, the Council on Kidney in Cardiovascular Disease, the Council on Lifestyle and Cardiometabolic Health, and the Council on Cardiovascular and Stroke Nursing.
A version of this article first appeared on Medscape.com.
FROM HYPERTENSION
Specific brain damage links hypertension to cognitive impairment
Researchers have identified specific regions of the brain that appear to be damaged by high blood pressure. The finding may explain the link between hypertension and cognitive impairment.
They used genetic information from genome-wide association studies (GWASs) and MRI scans of the brain to study the relationship between hypertension, changes in brain structures, and cognitive impairment. Using Mendelian randomization techniques, they identified nine brain structures related to cognitive impairment that are affected by blood pressure.
“We knew before that raised blood pressure was related to changes in the brain, but our research has narrowed down the changes to those that appear to be potentially causally related to cognitive impairment,” senior author Tomasz Guzik, professor of cardiovascular medicine, at the University of Edinburgh and of the Jagiellonian University, Krakow, Poland, told this news organization.
“Our study confirms a potentially causal relationship between raised blood pressure and cognitive impairment, emphasizing the importance of preventing and treating hypertension,” Prof. Guzik noted.
“But it also identifies the brain culprits of this relationship,” he added.
In the future, it may be possible to assess these nine brain structures in people with high blood pressure to identify those at increased risk of developing cognitive impairment, he said. “These patients may need more intensive care for their blood pressure. We can also investigate these brain structures for potential signaling pathways and molecular changes to see if we can find new targets for treatment to prevent cognitive impairment.”
For this report, the investigators married together different research datasets to identify brain structures potentially responsible for the effects of blood pressure on cognitive function, using results from previous GWASs and observational data from 39,000 people in the UK Biobank registry for whom brain MRI data were available.
First, they mapped brain structures potentially influenced by blood pressure in midlife using MRI scans from people in the UK Biobank registry. Then they examined the relationship between blood pressure and cognitive function in the UK Biobank registry. Next, of the brain structures affected by blood pressure, they identified those that are causally linked to cognitive impairment.
This was possible thanks to genetic markers coding for increased blood pressure, brain structure imaging phenotypes, and those coding for cognitive impairment that could be used in Mendelian randomization studies.
“We looked at 3935 brain magnetic resonance imaging–derived phenotypes in the brain and cognitive function defined by fluid intelligence score to identify genetically predicted causal relationships,” Prof. Guzik said.
They identified 200 brain structures that were causally affected by systolic blood pressure. Of these, nine were also causally related to cognitive impairment. The results were validated in a second prospective cohort of patients with hypertension.
“Some of these structures, including putamen and the white matter regions spanning between the anterior corona radiata, anterior thalamic radiation, and anterior limb of the internal capsule, may represent the target brain regions at which systolic blood pressure acts on cognitive function,” the authors comment.
In an accompanying editorial, Ernesto Schiffrin, MD, and James Engert, PhD, McGill University, Montreal, say that further mechanistic studies of the effects of blood pressure on cognitive function are required to determine precise causal pathways and the roles of relevant brain regions.
“Eventually, biomarkers could be developed to inform antihypertensive trials. Whether clinical trials targeting the specific brain structures will be feasible or if specific antihypertensives could be found that target specific structures remains to be demonstrated,” they write.
“Thus, these new studies could lead to an understanding of the signaling pathways that explain how these structures relate vascular damage to cognitive impairment in hypertension, and contribute to the development of novel interventions to more successfully address the scourge of cognitive decline and dementia in the future,” the editorialists conclude.
The study was funded by the European Research Council, the British Heart Foundation, and the Italian Ministry of Health.
A version of this article first appeared on Medscape.com.
Researchers have identified specific regions of the brain that appear to be damaged by high blood pressure. The finding may explain the link between hypertension and cognitive impairment.
They used genetic information from genome-wide association studies (GWASs) and MRI scans of the brain to study the relationship between hypertension, changes in brain structures, and cognitive impairment. Using Mendelian randomization techniques, they identified nine brain structures related to cognitive impairment that are affected by blood pressure.
“We knew before that raised blood pressure was related to changes in the brain, but our research has narrowed down the changes to those that appear to be potentially causally related to cognitive impairment,” senior author Tomasz Guzik, professor of cardiovascular medicine, at the University of Edinburgh and of the Jagiellonian University, Krakow, Poland, told this news organization.
“Our study confirms a potentially causal relationship between raised blood pressure and cognitive impairment, emphasizing the importance of preventing and treating hypertension,” Prof. Guzik noted.
“But it also identifies the brain culprits of this relationship,” he added.
In the future, it may be possible to assess these nine brain structures in people with high blood pressure to identify those at increased risk of developing cognitive impairment, he said. “These patients may need more intensive care for their blood pressure. We can also investigate these brain structures for potential signaling pathways and molecular changes to see if we can find new targets for treatment to prevent cognitive impairment.”
For this report, the investigators married together different research datasets to identify brain structures potentially responsible for the effects of blood pressure on cognitive function, using results from previous GWASs and observational data from 39,000 people in the UK Biobank registry for whom brain MRI data were available.
First, they mapped brain structures potentially influenced by blood pressure in midlife using MRI scans from people in the UK Biobank registry. Then they examined the relationship between blood pressure and cognitive function in the UK Biobank registry. Next, of the brain structures affected by blood pressure, they identified those that are causally linked to cognitive impairment.
This was possible thanks to genetic markers coding for increased blood pressure, brain structure imaging phenotypes, and those coding for cognitive impairment that could be used in Mendelian randomization studies.
“We looked at 3935 brain magnetic resonance imaging–derived phenotypes in the brain and cognitive function defined by fluid intelligence score to identify genetically predicted causal relationships,” Prof. Guzik said.
They identified 200 brain structures that were causally affected by systolic blood pressure. Of these, nine were also causally related to cognitive impairment. The results were validated in a second prospective cohort of patients with hypertension.
“Some of these structures, including putamen and the white matter regions spanning between the anterior corona radiata, anterior thalamic radiation, and anterior limb of the internal capsule, may represent the target brain regions at which systolic blood pressure acts on cognitive function,” the authors comment.
In an accompanying editorial, Ernesto Schiffrin, MD, and James Engert, PhD, McGill University, Montreal, say that further mechanistic studies of the effects of blood pressure on cognitive function are required to determine precise causal pathways and the roles of relevant brain regions.
“Eventually, biomarkers could be developed to inform antihypertensive trials. Whether clinical trials targeting the specific brain structures will be feasible or if specific antihypertensives could be found that target specific structures remains to be demonstrated,” they write.
“Thus, these new studies could lead to an understanding of the signaling pathways that explain how these structures relate vascular damage to cognitive impairment in hypertension, and contribute to the development of novel interventions to more successfully address the scourge of cognitive decline and dementia in the future,” the editorialists conclude.
The study was funded by the European Research Council, the British Heart Foundation, and the Italian Ministry of Health.
A version of this article first appeared on Medscape.com.
Researchers have identified specific regions of the brain that appear to be damaged by high blood pressure. The finding may explain the link between hypertension and cognitive impairment.
They used genetic information from genome-wide association studies (GWASs) and MRI scans of the brain to study the relationship between hypertension, changes in brain structures, and cognitive impairment. Using Mendelian randomization techniques, they identified nine brain structures related to cognitive impairment that are affected by blood pressure.
“We knew before that raised blood pressure was related to changes in the brain, but our research has narrowed down the changes to those that appear to be potentially causally related to cognitive impairment,” senior author Tomasz Guzik, professor of cardiovascular medicine, at the University of Edinburgh and of the Jagiellonian University, Krakow, Poland, told this news organization.
“Our study confirms a potentially causal relationship between raised blood pressure and cognitive impairment, emphasizing the importance of preventing and treating hypertension,” Prof. Guzik noted.
“But it also identifies the brain culprits of this relationship,” he added.
In the future, it may be possible to assess these nine brain structures in people with high blood pressure to identify those at increased risk of developing cognitive impairment, he said. “These patients may need more intensive care for their blood pressure. We can also investigate these brain structures for potential signaling pathways and molecular changes to see if we can find new targets for treatment to prevent cognitive impairment.”
For this report, the investigators married together different research datasets to identify brain structures potentially responsible for the effects of blood pressure on cognitive function, using results from previous GWASs and observational data from 39,000 people in the UK Biobank registry for whom brain MRI data were available.
First, they mapped brain structures potentially influenced by blood pressure in midlife using MRI scans from people in the UK Biobank registry. Then they examined the relationship between blood pressure and cognitive function in the UK Biobank registry. Next, of the brain structures affected by blood pressure, they identified those that are causally linked to cognitive impairment.
This was possible thanks to genetic markers coding for increased blood pressure, brain structure imaging phenotypes, and those coding for cognitive impairment that could be used in Mendelian randomization studies.
“We looked at 3935 brain magnetic resonance imaging–derived phenotypes in the brain and cognitive function defined by fluid intelligence score to identify genetically predicted causal relationships,” Prof. Guzik said.
They identified 200 brain structures that were causally affected by systolic blood pressure. Of these, nine were also causally related to cognitive impairment. The results were validated in a second prospective cohort of patients with hypertension.
“Some of these structures, including putamen and the white matter regions spanning between the anterior corona radiata, anterior thalamic radiation, and anterior limb of the internal capsule, may represent the target brain regions at which systolic blood pressure acts on cognitive function,” the authors comment.
In an accompanying editorial, Ernesto Schiffrin, MD, and James Engert, PhD, McGill University, Montreal, say that further mechanistic studies of the effects of blood pressure on cognitive function are required to determine precise causal pathways and the roles of relevant brain regions.
“Eventually, biomarkers could be developed to inform antihypertensive trials. Whether clinical trials targeting the specific brain structures will be feasible or if specific antihypertensives could be found that target specific structures remains to be demonstrated,” they write.
“Thus, these new studies could lead to an understanding of the signaling pathways that explain how these structures relate vascular damage to cognitive impairment in hypertension, and contribute to the development of novel interventions to more successfully address the scourge of cognitive decline and dementia in the future,” the editorialists conclude.
The study was funded by the European Research Council, the British Heart Foundation, and the Italian Ministry of Health.
A version of this article first appeared on Medscape.com.
AHA, ACC push supervised exercise training for HFpEF
A statement released by the American Heart Association and the American College of Cardiology advocates use of supervised exercise training in patients with heart failure with preserved ejection fraction (HFpEF), as well as coverage for these services by third-party payers.
The authors hope to boost the stature of supervised exercise training (SET) in HFpEF among practitioners and show Medicare and insurers that it deserves reimbursement. Currently, they noted, clinicians tend to recognize exercise as therapy more in HF with reduced ejection fraction (HFrEF). And Medicare covers exercise training within broader cardiac rehabilitation programs for patients with HFrEF but not HFpEF.
Yet exercise has been broadly effective in HFpEF clinical trials, as outlined in the document. And there are good mechanistic reasons to believe that patients with the disorder can gain as much or more from SET than those with HFrEF.
“The signals for improvement from exercise training, in symptoms and objective measures of exercise capacity, are considerably larger for HFpEF than for HFrEF,” Dalane W. Kitzman, MD, Wake Forest University, Winston-Salem, N.C., said in an interview.
So, it’s a bit of a paradox that clinicians don’t prescribe it as often in HFpEF, probably because of the lack of reimbursement but also from less “awareness” and understanding of the disease itself, he proposed.
Dr. Kitzman is senior author on the statement sponsored by the AHA and the ACC. It was published in the societies’ flagship journals Circulation and the Journal of the American College of Cardiology. The statement was also endorsed by the Heart Failure Society of America, the American Association of Cardiovascular and Pulmonary Rehabilitation, and the American Association of Heart Failure Nurses.
Carefully chosen words
The statement makes its case in HFpEF specifically for SET rather than cardiac rehabilitation, the latter typically a comprehensive program that goes beyond exercise, Dr. Kitzman noted. And SET is closer to the exercise interventions used in the supportive HFpEF trials.
“Also, Medicare in recent years has approved something called ‘supervised exercise training’ for other disorders, such as peripheral artery disease.” So, the document specifies SET “to be fully aligned with the evidence base,” he said, as well as “align it with a type of treatment that Medicare has a precedent for approving for other disorders.”
Data and physiologic basis
Core features of the AHA/ACC statement is its review of HFpEF exercise physiology, survey of randomized trials supporting SET in the disease, and characterization of exercise as an especially suitable pleiotropic therapy.
Increasingly, “HFpEF is now accepted as a systemic disorder that affects and impacts all organs,” Dr. Kitzman observed. “With a systemic multiorgan disorder, it would make sense that a broad treatment like exercise might be just the right thing. We think that’s the reason that its benefits are really quite large in magnitude.”
The document notes that exercise seems “potentially well suited for the treatment of both the cardiac and, in particular, the extracardiac abnormalities that contribute to exercise intolerance in HFpEF.”
Its effects in the disorder are “anti-inflammatory, rheological, lipid lowering, antihypertensive, positive inotropic, positive lusitropic, negative chronotropic, vasodilation, diuretic, weight-reducing, hypoglycemic, hypnotic, and antidepressive,” the statement notes. It achieves them via multiple pathways involving the heart, lungs, vasculature and, notably, the skeletal muscles.
“It’s been widely overlooked that at least 50% of low exercise capacity and symptoms in HFpEF are due to skeletal muscle dysfunction,” said Dr. Kitzman, an authority on exercise physiology in heart failure.
“But we’ve spent about 95% of our attention trying to modify and understand the cardiac component.” Skeletal muscles, he said, “are not an innocent bystander. They’re part of the problem. And that’s why we should really spend more time focusing on them.”
Dr. Kitzman disclosed receiving consulting fees from Bayer, Medtronic, Corvia Medical, Boehringer Ingelheim, Keyto, Rivus, NovoNordisk, AstraZeneca, and Pfizer; holding stock in Gilead; and receiving grants to his institution from Bayer, Novo Nordisk, AstraZeneca, Rivus, and Pfizer.
A version of this article first appeared on Medscape.com.
A statement released by the American Heart Association and the American College of Cardiology advocates use of supervised exercise training in patients with heart failure with preserved ejection fraction (HFpEF), as well as coverage for these services by third-party payers.
The authors hope to boost the stature of supervised exercise training (SET) in HFpEF among practitioners and show Medicare and insurers that it deserves reimbursement. Currently, they noted, clinicians tend to recognize exercise as therapy more in HF with reduced ejection fraction (HFrEF). And Medicare covers exercise training within broader cardiac rehabilitation programs for patients with HFrEF but not HFpEF.
Yet exercise has been broadly effective in HFpEF clinical trials, as outlined in the document. And there are good mechanistic reasons to believe that patients with the disorder can gain as much or more from SET than those with HFrEF.
“The signals for improvement from exercise training, in symptoms and objective measures of exercise capacity, are considerably larger for HFpEF than for HFrEF,” Dalane W. Kitzman, MD, Wake Forest University, Winston-Salem, N.C., said in an interview.
So, it’s a bit of a paradox that clinicians don’t prescribe it as often in HFpEF, probably because of the lack of reimbursement but also from less “awareness” and understanding of the disease itself, he proposed.
Dr. Kitzman is senior author on the statement sponsored by the AHA and the ACC. It was published in the societies’ flagship journals Circulation and the Journal of the American College of Cardiology. The statement was also endorsed by the Heart Failure Society of America, the American Association of Cardiovascular and Pulmonary Rehabilitation, and the American Association of Heart Failure Nurses.
Carefully chosen words
The statement makes its case in HFpEF specifically for SET rather than cardiac rehabilitation, the latter typically a comprehensive program that goes beyond exercise, Dr. Kitzman noted. And SET is closer to the exercise interventions used in the supportive HFpEF trials.
“Also, Medicare in recent years has approved something called ‘supervised exercise training’ for other disorders, such as peripheral artery disease.” So, the document specifies SET “to be fully aligned with the evidence base,” he said, as well as “align it with a type of treatment that Medicare has a precedent for approving for other disorders.”
Data and physiologic basis
Core features of the AHA/ACC statement is its review of HFpEF exercise physiology, survey of randomized trials supporting SET in the disease, and characterization of exercise as an especially suitable pleiotropic therapy.
Increasingly, “HFpEF is now accepted as a systemic disorder that affects and impacts all organs,” Dr. Kitzman observed. “With a systemic multiorgan disorder, it would make sense that a broad treatment like exercise might be just the right thing. We think that’s the reason that its benefits are really quite large in magnitude.”
The document notes that exercise seems “potentially well suited for the treatment of both the cardiac and, in particular, the extracardiac abnormalities that contribute to exercise intolerance in HFpEF.”
Its effects in the disorder are “anti-inflammatory, rheological, lipid lowering, antihypertensive, positive inotropic, positive lusitropic, negative chronotropic, vasodilation, diuretic, weight-reducing, hypoglycemic, hypnotic, and antidepressive,” the statement notes. It achieves them via multiple pathways involving the heart, lungs, vasculature and, notably, the skeletal muscles.
“It’s been widely overlooked that at least 50% of low exercise capacity and symptoms in HFpEF are due to skeletal muscle dysfunction,” said Dr. Kitzman, an authority on exercise physiology in heart failure.
“But we’ve spent about 95% of our attention trying to modify and understand the cardiac component.” Skeletal muscles, he said, “are not an innocent bystander. They’re part of the problem. And that’s why we should really spend more time focusing on them.”
Dr. Kitzman disclosed receiving consulting fees from Bayer, Medtronic, Corvia Medical, Boehringer Ingelheim, Keyto, Rivus, NovoNordisk, AstraZeneca, and Pfizer; holding stock in Gilead; and receiving grants to his institution from Bayer, Novo Nordisk, AstraZeneca, Rivus, and Pfizer.
A version of this article first appeared on Medscape.com.
A statement released by the American Heart Association and the American College of Cardiology advocates use of supervised exercise training in patients with heart failure with preserved ejection fraction (HFpEF), as well as coverage for these services by third-party payers.
The authors hope to boost the stature of supervised exercise training (SET) in HFpEF among practitioners and show Medicare and insurers that it deserves reimbursement. Currently, they noted, clinicians tend to recognize exercise as therapy more in HF with reduced ejection fraction (HFrEF). And Medicare covers exercise training within broader cardiac rehabilitation programs for patients with HFrEF but not HFpEF.
Yet exercise has been broadly effective in HFpEF clinical trials, as outlined in the document. And there are good mechanistic reasons to believe that patients with the disorder can gain as much or more from SET than those with HFrEF.
“The signals for improvement from exercise training, in symptoms and objective measures of exercise capacity, are considerably larger for HFpEF than for HFrEF,” Dalane W. Kitzman, MD, Wake Forest University, Winston-Salem, N.C., said in an interview.
So, it’s a bit of a paradox that clinicians don’t prescribe it as often in HFpEF, probably because of the lack of reimbursement but also from less “awareness” and understanding of the disease itself, he proposed.
Dr. Kitzman is senior author on the statement sponsored by the AHA and the ACC. It was published in the societies’ flagship journals Circulation and the Journal of the American College of Cardiology. The statement was also endorsed by the Heart Failure Society of America, the American Association of Cardiovascular and Pulmonary Rehabilitation, and the American Association of Heart Failure Nurses.
Carefully chosen words
The statement makes its case in HFpEF specifically for SET rather than cardiac rehabilitation, the latter typically a comprehensive program that goes beyond exercise, Dr. Kitzman noted. And SET is closer to the exercise interventions used in the supportive HFpEF trials.
“Also, Medicare in recent years has approved something called ‘supervised exercise training’ for other disorders, such as peripheral artery disease.” So, the document specifies SET “to be fully aligned with the evidence base,” he said, as well as “align it with a type of treatment that Medicare has a precedent for approving for other disorders.”
Data and physiologic basis
Core features of the AHA/ACC statement is its review of HFpEF exercise physiology, survey of randomized trials supporting SET in the disease, and characterization of exercise as an especially suitable pleiotropic therapy.
Increasingly, “HFpEF is now accepted as a systemic disorder that affects and impacts all organs,” Dr. Kitzman observed. “With a systemic multiorgan disorder, it would make sense that a broad treatment like exercise might be just the right thing. We think that’s the reason that its benefits are really quite large in magnitude.”
The document notes that exercise seems “potentially well suited for the treatment of both the cardiac and, in particular, the extracardiac abnormalities that contribute to exercise intolerance in HFpEF.”
Its effects in the disorder are “anti-inflammatory, rheological, lipid lowering, antihypertensive, positive inotropic, positive lusitropic, negative chronotropic, vasodilation, diuretic, weight-reducing, hypoglycemic, hypnotic, and antidepressive,” the statement notes. It achieves them via multiple pathways involving the heart, lungs, vasculature and, notably, the skeletal muscles.
“It’s been widely overlooked that at least 50% of low exercise capacity and symptoms in HFpEF are due to skeletal muscle dysfunction,” said Dr. Kitzman, an authority on exercise physiology in heart failure.
“But we’ve spent about 95% of our attention trying to modify and understand the cardiac component.” Skeletal muscles, he said, “are not an innocent bystander. They’re part of the problem. And that’s why we should really spend more time focusing on them.”
Dr. Kitzman disclosed receiving consulting fees from Bayer, Medtronic, Corvia Medical, Boehringer Ingelheim, Keyto, Rivus, NovoNordisk, AstraZeneca, and Pfizer; holding stock in Gilead; and receiving grants to his institution from Bayer, Novo Nordisk, AstraZeneca, Rivus, and Pfizer.
A version of this article first appeared on Medscape.com.
Analysis identifies gaps in CV risk screening of patients with psoriasis
Just , according to an analysis of 10 years of national survey data.
From 2007 to 2016, national screening rates for four CV risk factors at 14.8 million psoriasis-related visits to dermatology providers were 11% (body-mass index), 7.4% (blood pressure), 2.9% (cholesterol), and 1.7% (glucose). Data from the National Ambulatory Medical Care Survey showed that at least one of the four factors was screened at 16% of dermatology visits, said William B. Song, BS, of the department of dermatology, University of Pennsylvania, Philadelphia, and associates.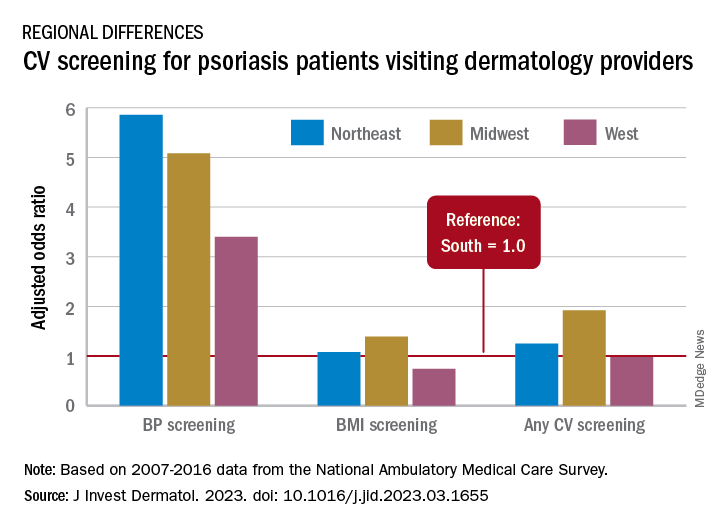
The main focus of their study, however, was regional differences. “CV risk factor screening by dermatology providers for patients with psoriasis is low across all regions of the United States and lowest in the South, the region that experiences the highest CVD burden in the United States,” they wrote in a letter to the editor.
Compared with the South, the adjusted odds of any CV screening were 0.98 in the West, 1.25 in the Northeast, and 1.92 in the Midwest. Blood pressure screening was significantly higher in all three regions, compared with the South, while BMI screening was actually lower in the West (0.74), the investigators reported. Odds ratios were not available for cholesterol and glucose screening because of sample size limitations.
The regional variation in screening rates “is not explained by patient demographics or disease severity,” they noted, adding that 2.8 million visits with BP screening would have been added over the 10-year study period “if providers in the South screened patients with psoriasis for high blood pressure at the same rate as providers in the Northeast.”
Guidelines published in 2019 by the American Academy of Dermatology and the National Psoriasis Foundation – which were cowritten by Joel M. Gelfand, MD, senior author of the current study – noted that dermatologists “play an important role in evidence-based screening of CV risk factors in patients with psoriasis,” the investigators wrote. But the regional variations suggest “that some regions experience barriers to appropriate screening or challenges in adhering to guidelines for managing psoriasis and CV risk.”
While the lack of data from after 2016 is one of the study limitations, they added, “continued efforts to develop effective interventions to improve CV screening and care for people with psoriasis in all regions of the U.S. are needed to more effectively address the burden of CV disease experienced by people with psoriasis.”
The study was partly funded by the National Psoriasis Foundation. Three of the seven investigators disclosed earnings from private companies in the form of consultant fees, research support, and honoraria. Dr. Gelfand is a deputy editor for the Journal of Investigative Dermatology.
Just , according to an analysis of 10 years of national survey data.
From 2007 to 2016, national screening rates for four CV risk factors at 14.8 million psoriasis-related visits to dermatology providers were 11% (body-mass index), 7.4% (blood pressure), 2.9% (cholesterol), and 1.7% (glucose). Data from the National Ambulatory Medical Care Survey showed that at least one of the four factors was screened at 16% of dermatology visits, said William B. Song, BS, of the department of dermatology, University of Pennsylvania, Philadelphia, and associates.
The main focus of their study, however, was regional differences. “CV risk factor screening by dermatology providers for patients with psoriasis is low across all regions of the United States and lowest in the South, the region that experiences the highest CVD burden in the United States,” they wrote in a letter to the editor.
Compared with the South, the adjusted odds of any CV screening were 0.98 in the West, 1.25 in the Northeast, and 1.92 in the Midwest. Blood pressure screening was significantly higher in all three regions, compared with the South, while BMI screening was actually lower in the West (0.74), the investigators reported. Odds ratios were not available for cholesterol and glucose screening because of sample size limitations.
The regional variation in screening rates “is not explained by patient demographics or disease severity,” they noted, adding that 2.8 million visits with BP screening would have been added over the 10-year study period “if providers in the South screened patients with psoriasis for high blood pressure at the same rate as providers in the Northeast.”
Guidelines published in 2019 by the American Academy of Dermatology and the National Psoriasis Foundation – which were cowritten by Joel M. Gelfand, MD, senior author of the current study – noted that dermatologists “play an important role in evidence-based screening of CV risk factors in patients with psoriasis,” the investigators wrote. But the regional variations suggest “that some regions experience barriers to appropriate screening or challenges in adhering to guidelines for managing psoriasis and CV risk.”
While the lack of data from after 2016 is one of the study limitations, they added, “continued efforts to develop effective interventions to improve CV screening and care for people with psoriasis in all regions of the U.S. are needed to more effectively address the burden of CV disease experienced by people with psoriasis.”
The study was partly funded by the National Psoriasis Foundation. Three of the seven investigators disclosed earnings from private companies in the form of consultant fees, research support, and honoraria. Dr. Gelfand is a deputy editor for the Journal of Investigative Dermatology.
Just , according to an analysis of 10 years of national survey data.
From 2007 to 2016, national screening rates for four CV risk factors at 14.8 million psoriasis-related visits to dermatology providers were 11% (body-mass index), 7.4% (blood pressure), 2.9% (cholesterol), and 1.7% (glucose). Data from the National Ambulatory Medical Care Survey showed that at least one of the four factors was screened at 16% of dermatology visits, said William B. Song, BS, of the department of dermatology, University of Pennsylvania, Philadelphia, and associates.
The main focus of their study, however, was regional differences. “CV risk factor screening by dermatology providers for patients with psoriasis is low across all regions of the United States and lowest in the South, the region that experiences the highest CVD burden in the United States,” they wrote in a letter to the editor.
Compared with the South, the adjusted odds of any CV screening were 0.98 in the West, 1.25 in the Northeast, and 1.92 in the Midwest. Blood pressure screening was significantly higher in all three regions, compared with the South, while BMI screening was actually lower in the West (0.74), the investigators reported. Odds ratios were not available for cholesterol and glucose screening because of sample size limitations.
The regional variation in screening rates “is not explained by patient demographics or disease severity,” they noted, adding that 2.8 million visits with BP screening would have been added over the 10-year study period “if providers in the South screened patients with psoriasis for high blood pressure at the same rate as providers in the Northeast.”
Guidelines published in 2019 by the American Academy of Dermatology and the National Psoriasis Foundation – which were cowritten by Joel M. Gelfand, MD, senior author of the current study – noted that dermatologists “play an important role in evidence-based screening of CV risk factors in patients with psoriasis,” the investigators wrote. But the regional variations suggest “that some regions experience barriers to appropriate screening or challenges in adhering to guidelines for managing psoriasis and CV risk.”
While the lack of data from after 2016 is one of the study limitations, they added, “continued efforts to develop effective interventions to improve CV screening and care for people with psoriasis in all regions of the U.S. are needed to more effectively address the burden of CV disease experienced by people with psoriasis.”
The study was partly funded by the National Psoriasis Foundation. Three of the seven investigators disclosed earnings from private companies in the form of consultant fees, research support, and honoraria. Dr. Gelfand is a deputy editor for the Journal of Investigative Dermatology.
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
Some diets better than others for heart protection
In an analysis of randomized trials, the Mediterranean diet and low-fat diets were linked to reduced risks of all-cause mortality and nonfatal MI over 3 years in adults at increased risk for cardiovascular disease (CVD), while the Mediterranean diet also showed lower risk of stroke.
Five other popular diets appeared to have little or no benefit with regard to these outcomes.
“These findings with data presentations are extremely important for patients who are skeptical about the desirability of diet change,” wrote the authors, led by Giorgio Karam, a medical student at the University of Manitoba, Winnipeg.
The results were published online in The BMJ.
Dietary guidelines recommend various diets along with physical activity or other cointerventions for adults at increased CVD risk, but they are often based on low-certainty evidence from nonrandomized studies and on surrogate outcomes.
Several meta-analyses of randomized controlled trials with mortality and major CV outcomes have reported benefits of some dietary programs, but those studies did not use network meta-analysis to give absolute estimates and certainty of estimates for adults at intermediate and high risk, the authors noted.
For this study, Mr. Karam and colleagues conducted a comprehensive systematic review and network meta-analysis in which they compared the effects of seven popular structured diets on mortality and CVD events for adults with CVD or CVD risk factors.
The seven diet plans were the Mediterranean, low fat, very low fat, modified fat, combined low fat and low sodium, Ornish, and Pritikin diets. Data for the analysis came from 40 randomized controlled trials that involved 35,548 participants who were followed for an average of 3 years.
There was evidence of “moderate” certainty that the Mediterranean diet was superior to minimal intervention for all-cause mortality (odds ratio [OR], 0.72), CV mortality (OR, 0.55), stroke (OR, 0.65), and nonfatal MI (OR, 0.48).
On an absolute basis (per 1,000 over 5 years), the Mediterranean diet let to 17 fewer deaths from any cause, 13 fewer CV deaths, seven fewer strokes, and 17 fewer nonfatal MIs.
There was evidence of moderate certainty that a low-fat diet was superior to minimal intervention for prevention of all-cause mortality (OR, 0.84; nine fewer deaths per 1,000) and nonfatal MI (OR, 0.77; seven fewer deaths per 1,000). The low-fat diet had little to no benefit with regard to stroke reduction.
The Mediterranean diet was not “convincingly” superior to a low-fat diet for mortality or nonfatal MI, the authors noted.
The absolute effects for the Mediterranean and low-fat diets were more pronounced in adults at high CVD risk. With the Mediterranean diet, there were 36 fewer all-cause deaths and 39 fewer CV deaths per 1,000 over 5 years.
The five other dietary programs generally had “little or no benefit” compared with minimal intervention. The evidence was of low to moderate certainty.
The studies did not provide enough data to gauge the impact of the diets on angina, heart failure, peripheral vascular events, and atrial fibrillation.
The researchers say that strengths of their analysis include a comprehensive review and thorough literature search and a rigorous assessment of study bias. In addition, the researchers adhered to recognized GRADE methods for assessing the certainty of estimates.
Limitations of their work include not being able to measure adherence to dietary programs and the possibility that some of the benefits may have been due to other factors, such as drug treatment and support for quitting smoking.
The study had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In an analysis of randomized trials, the Mediterranean diet and low-fat diets were linked to reduced risks of all-cause mortality and nonfatal MI over 3 years in adults at increased risk for cardiovascular disease (CVD), while the Mediterranean diet also showed lower risk of stroke.
Five other popular diets appeared to have little or no benefit with regard to these outcomes.
“These findings with data presentations are extremely important for patients who are skeptical about the desirability of diet change,” wrote the authors, led by Giorgio Karam, a medical student at the University of Manitoba, Winnipeg.
The results were published online in The BMJ.
Dietary guidelines recommend various diets along with physical activity or other cointerventions for adults at increased CVD risk, but they are often based on low-certainty evidence from nonrandomized studies and on surrogate outcomes.
Several meta-analyses of randomized controlled trials with mortality and major CV outcomes have reported benefits of some dietary programs, but those studies did not use network meta-analysis to give absolute estimates and certainty of estimates for adults at intermediate and high risk, the authors noted.
For this study, Mr. Karam and colleagues conducted a comprehensive systematic review and network meta-analysis in which they compared the effects of seven popular structured diets on mortality and CVD events for adults with CVD or CVD risk factors.
The seven diet plans were the Mediterranean, low fat, very low fat, modified fat, combined low fat and low sodium, Ornish, and Pritikin diets. Data for the analysis came from 40 randomized controlled trials that involved 35,548 participants who were followed for an average of 3 years.
There was evidence of “moderate” certainty that the Mediterranean diet was superior to minimal intervention for all-cause mortality (odds ratio [OR], 0.72), CV mortality (OR, 0.55), stroke (OR, 0.65), and nonfatal MI (OR, 0.48).
On an absolute basis (per 1,000 over 5 years), the Mediterranean diet let to 17 fewer deaths from any cause, 13 fewer CV deaths, seven fewer strokes, and 17 fewer nonfatal MIs.
There was evidence of moderate certainty that a low-fat diet was superior to minimal intervention for prevention of all-cause mortality (OR, 0.84; nine fewer deaths per 1,000) and nonfatal MI (OR, 0.77; seven fewer deaths per 1,000). The low-fat diet had little to no benefit with regard to stroke reduction.
The Mediterranean diet was not “convincingly” superior to a low-fat diet for mortality or nonfatal MI, the authors noted.
The absolute effects for the Mediterranean and low-fat diets were more pronounced in adults at high CVD risk. With the Mediterranean diet, there were 36 fewer all-cause deaths and 39 fewer CV deaths per 1,000 over 5 years.
The five other dietary programs generally had “little or no benefit” compared with minimal intervention. The evidence was of low to moderate certainty.
The studies did not provide enough data to gauge the impact of the diets on angina, heart failure, peripheral vascular events, and atrial fibrillation.
The researchers say that strengths of their analysis include a comprehensive review and thorough literature search and a rigorous assessment of study bias. In addition, the researchers adhered to recognized GRADE methods for assessing the certainty of estimates.
Limitations of their work include not being able to measure adherence to dietary programs and the possibility that some of the benefits may have been due to other factors, such as drug treatment and support for quitting smoking.
The study had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In an analysis of randomized trials, the Mediterranean diet and low-fat diets were linked to reduced risks of all-cause mortality and nonfatal MI over 3 years in adults at increased risk for cardiovascular disease (CVD), while the Mediterranean diet also showed lower risk of stroke.
Five other popular diets appeared to have little or no benefit with regard to these outcomes.
“These findings with data presentations are extremely important for patients who are skeptical about the desirability of diet change,” wrote the authors, led by Giorgio Karam, a medical student at the University of Manitoba, Winnipeg.
The results were published online in The BMJ.
Dietary guidelines recommend various diets along with physical activity or other cointerventions for adults at increased CVD risk, but they are often based on low-certainty evidence from nonrandomized studies and on surrogate outcomes.
Several meta-analyses of randomized controlled trials with mortality and major CV outcomes have reported benefits of some dietary programs, but those studies did not use network meta-analysis to give absolute estimates and certainty of estimates for adults at intermediate and high risk, the authors noted.
For this study, Mr. Karam and colleagues conducted a comprehensive systematic review and network meta-analysis in which they compared the effects of seven popular structured diets on mortality and CVD events for adults with CVD or CVD risk factors.
The seven diet plans were the Mediterranean, low fat, very low fat, modified fat, combined low fat and low sodium, Ornish, and Pritikin diets. Data for the analysis came from 40 randomized controlled trials that involved 35,548 participants who were followed for an average of 3 years.
There was evidence of “moderate” certainty that the Mediterranean diet was superior to minimal intervention for all-cause mortality (odds ratio [OR], 0.72), CV mortality (OR, 0.55), stroke (OR, 0.65), and nonfatal MI (OR, 0.48).
On an absolute basis (per 1,000 over 5 years), the Mediterranean diet let to 17 fewer deaths from any cause, 13 fewer CV deaths, seven fewer strokes, and 17 fewer nonfatal MIs.
There was evidence of moderate certainty that a low-fat diet was superior to minimal intervention for prevention of all-cause mortality (OR, 0.84; nine fewer deaths per 1,000) and nonfatal MI (OR, 0.77; seven fewer deaths per 1,000). The low-fat diet had little to no benefit with regard to stroke reduction.
The Mediterranean diet was not “convincingly” superior to a low-fat diet for mortality or nonfatal MI, the authors noted.
The absolute effects for the Mediterranean and low-fat diets were more pronounced in adults at high CVD risk. With the Mediterranean diet, there were 36 fewer all-cause deaths and 39 fewer CV deaths per 1,000 over 5 years.
The five other dietary programs generally had “little or no benefit” compared with minimal intervention. The evidence was of low to moderate certainty.
The studies did not provide enough data to gauge the impact of the diets on angina, heart failure, peripheral vascular events, and atrial fibrillation.
The researchers say that strengths of their analysis include a comprehensive review and thorough literature search and a rigorous assessment of study bias. In addition, the researchers adhered to recognized GRADE methods for assessing the certainty of estimates.
Limitations of their work include not being able to measure adherence to dietary programs and the possibility that some of the benefits may have been due to other factors, such as drug treatment and support for quitting smoking.
The study had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Subclinical CAD by CT predicts MI risk, with or without stenoses
About half of middle-aged adults in the community without cardiovascular (CV) symptoms have coronary atherosclerosis by CT angiography (CTA) that puts them at substantial risk for myocardial infarction (MI), suggests a prospective cohort study.
The 10% of participants who had subclinical disease considered obstructive at CTA showed a ninefold increased risk for MI over several years. Obstructive disease seemed to elevate risk more than subclinical disease that wasn’t obstructive but still considered extensive within the coronary arteries.
The findings, based on a Copenhagen General Population Study cohort, are new for CTA but consistent with research based on coronary artery calcium (CAC) scores and other ways to assess CV risk, say researchers.
Although all participants underwent CTA, such imaging isn’t used in the general population for atherosclerosis screening. But the findings may have implications for “opportunistic screening” for subclinical coronary disease at CTA conducted for other reasons, notes the study’s report, published online in the Annals of Internal Medicine.
“Identification of luminal obstructive or extensive subclinical coronary atherosclerosis” could potentially provide “clinically relevant, incremental risk assessment” in nonischemic patients who undergo cardiac CT or electrocardiogram-gated chest CT before procedures such as arrhythmia ablation or valve repair, it states.
Such patients found with subclinical coronary atherosclerosis might potentially “benefit from referral to intensified cardiovascular primary prevention therapy,” write the authors, led by Andreas Fuchs, MD, PhD, Copenhagen University Hospital-Rigshospitalet.
The group acknowledges the findings may not entirely apply to a non-Danish population.
A screening role for CTA?
Whether CTA has a role to play in adults without symptoms “is a big, open question in the field right now,” observed Ron Blankstein, MD, not associated with the current analysis, for this news organization.
Most population studies of CV risk prediction, such as MESA, have looked at CAC scores, not CTA, and have shown that “the more plaque individuals have, the higher the risk.” The current findings are similar but novel in coming from coronary CTA in a large asymptomatic community population, said Dr. Blankstein, who is director of cardiac CT at Brigham and Women’s Hospital, Boston.
“It’s possible that patients who have obstructive plaque in general tend to have a larger amount of plaque as well,” he said. So, while the study suggests that “the more plaque individuals have, the worse their overall risk,” it also shows that the risk “is enhanced even more if they have obstructive disease.”
The Danish cohort analysis “provides a unique opportunity to study the contemporary natural history of coronary artery disease in the absence of intervention,” notes an accompanying editorial.
For example, both patients and clinicians were blinded to CTA results, and CV preventive therapies weren’t common, observe Michael McDermott, MBChB, and David E. Newby, DM, PhD, of the BHF Centre for Cardiovascular Science, University of Edinburgh.
The analysis suggests that subclinical coronary disease that is obstructive predicts MI risk more strongly than extensive coronary disease, they note, and may be present in two-thirds of MI patients. “This contrasts with symptomatic populations, where nonobstructive disease accounts for most future myocardial infarctions, presumably from plaque rupture.”
It also points to “strong associations between nonobstructive extensive disease and adverse plaque characteristics,” write Dr. McDermott and Dr. Newby. “This underscores the major importance of plaque burden” for the prediction of coronary events.
Graded risk
The analysis included 9,533 persons aged 40 and older without known ischemic heart disease or symptoms with available CTA assessments.
Obstructive disease, defined as presence of a luminal stenosis of at least 50%, was seen in 10% and nonobstructive disease in 36% of the total cohort, the report states.
Disease occupying more than one-third of the coronary tree was considered extensive and less than one-third of the coronaries nonextensive, occurring in 10.5% and 35.8% of the cohort, respectively.
There were 71 MIs and 193 deaths over a median of 3.5 years. The adjusted relative risk for MI, compared with those without coronary atherosclerosis, was:
- 7.65 (95% confidence interval, 3.53-16.57) overall in patients with extensive disease.
- 8.28 (95% CI, 3.75-18.32) in those with obstructive but nonextensive disease.
- 9.19 (95% CI, 4.49-18.82) overall in those with obstructive disease.
- 12.48 (95% CI, 5.50-28.12) in those with or obstructive and extensive disease.
The adjusted RR for the composite of death or MI was also elevated in persons with extensive disease:
- 2.70 (95% CI, 1.72-4.25) in those with extensive but nonobstructive disease.
- 3.15 (95% CI, 2.05-4.83) in those with extensive and obstructive disease.
“It’s one thing to show that the more plaque, the higher the risk,” Dr. Blankstein said. But “does the information ultimately lead to better outcomes? Do patients have fewer MIs or fewer deaths?” Several ongoing randomized trials are exploring these questions.
They include DANE-HEART (Computed Tomography Coronary Angiography for Primary Prevention), projected to enroll about 6,000 participants from the Copenhagen General Population Study cohort who have at least one CV risk factor, and SCOT-HEART 2 (second Computed Tomography Coronary Angiography for the Prevention of Myocardial Infarction), enrolling a similar cohort in Scotland.
The study was supported by grants from AP Møller og Hustru Chastine Mc-Kinney Møllers Fond, the Research Council of Rigshospitalet, and Danish Heart Foundation. Dr. Fuchs reports no relevant financial relationships. Disclosures for the other authors can be found here. Dr. Blankstein recently disclosed serving as a consultant to Amgen, Caristo Diagnostics, Novartis, and Silence Therapeutics. Disclosures for Dr. McDermott and Dr. Newby, who are SCOT-HEART 2 investigators, can be found here.
A version of this article originally appeared on Medscape.com.
About half of middle-aged adults in the community without cardiovascular (CV) symptoms have coronary atherosclerosis by CT angiography (CTA) that puts them at substantial risk for myocardial infarction (MI), suggests a prospective cohort study.
The 10% of participants who had subclinical disease considered obstructive at CTA showed a ninefold increased risk for MI over several years. Obstructive disease seemed to elevate risk more than subclinical disease that wasn’t obstructive but still considered extensive within the coronary arteries.
The findings, based on a Copenhagen General Population Study cohort, are new for CTA but consistent with research based on coronary artery calcium (CAC) scores and other ways to assess CV risk, say researchers.
Although all participants underwent CTA, such imaging isn’t used in the general population for atherosclerosis screening. But the findings may have implications for “opportunistic screening” for subclinical coronary disease at CTA conducted for other reasons, notes the study’s report, published online in the Annals of Internal Medicine.
“Identification of luminal obstructive or extensive subclinical coronary atherosclerosis” could potentially provide “clinically relevant, incremental risk assessment” in nonischemic patients who undergo cardiac CT or electrocardiogram-gated chest CT before procedures such as arrhythmia ablation or valve repair, it states.
Such patients found with subclinical coronary atherosclerosis might potentially “benefit from referral to intensified cardiovascular primary prevention therapy,” write the authors, led by Andreas Fuchs, MD, PhD, Copenhagen University Hospital-Rigshospitalet.
The group acknowledges the findings may not entirely apply to a non-Danish population.
A screening role for CTA?
Whether CTA has a role to play in adults without symptoms “is a big, open question in the field right now,” observed Ron Blankstein, MD, not associated with the current analysis, for this news organization.
Most population studies of CV risk prediction, such as MESA, have looked at CAC scores, not CTA, and have shown that “the more plaque individuals have, the higher the risk.” The current findings are similar but novel in coming from coronary CTA in a large asymptomatic community population, said Dr. Blankstein, who is director of cardiac CT at Brigham and Women’s Hospital, Boston.
“It’s possible that patients who have obstructive plaque in general tend to have a larger amount of plaque as well,” he said. So, while the study suggests that “the more plaque individuals have, the worse their overall risk,” it also shows that the risk “is enhanced even more if they have obstructive disease.”
The Danish cohort analysis “provides a unique opportunity to study the contemporary natural history of coronary artery disease in the absence of intervention,” notes an accompanying editorial.
For example, both patients and clinicians were blinded to CTA results, and CV preventive therapies weren’t common, observe Michael McDermott, MBChB, and David E. Newby, DM, PhD, of the BHF Centre for Cardiovascular Science, University of Edinburgh.
The analysis suggests that subclinical coronary disease that is obstructive predicts MI risk more strongly than extensive coronary disease, they note, and may be present in two-thirds of MI patients. “This contrasts with symptomatic populations, where nonobstructive disease accounts for most future myocardial infarctions, presumably from plaque rupture.”
It also points to “strong associations between nonobstructive extensive disease and adverse plaque characteristics,” write Dr. McDermott and Dr. Newby. “This underscores the major importance of plaque burden” for the prediction of coronary events.
Graded risk
The analysis included 9,533 persons aged 40 and older without known ischemic heart disease or symptoms with available CTA assessments.
Obstructive disease, defined as presence of a luminal stenosis of at least 50%, was seen in 10% and nonobstructive disease in 36% of the total cohort, the report states.
Disease occupying more than one-third of the coronary tree was considered extensive and less than one-third of the coronaries nonextensive, occurring in 10.5% and 35.8% of the cohort, respectively.
There were 71 MIs and 193 deaths over a median of 3.5 years. The adjusted relative risk for MI, compared with those without coronary atherosclerosis, was:
- 7.65 (95% confidence interval, 3.53-16.57) overall in patients with extensive disease.
- 8.28 (95% CI, 3.75-18.32) in those with obstructive but nonextensive disease.
- 9.19 (95% CI, 4.49-18.82) overall in those with obstructive disease.
- 12.48 (95% CI, 5.50-28.12) in those with or obstructive and extensive disease.
The adjusted RR for the composite of death or MI was also elevated in persons with extensive disease:
- 2.70 (95% CI, 1.72-4.25) in those with extensive but nonobstructive disease.
- 3.15 (95% CI, 2.05-4.83) in those with extensive and obstructive disease.
“It’s one thing to show that the more plaque, the higher the risk,” Dr. Blankstein said. But “does the information ultimately lead to better outcomes? Do patients have fewer MIs or fewer deaths?” Several ongoing randomized trials are exploring these questions.
They include DANE-HEART (Computed Tomography Coronary Angiography for Primary Prevention), projected to enroll about 6,000 participants from the Copenhagen General Population Study cohort who have at least one CV risk factor, and SCOT-HEART 2 (second Computed Tomography Coronary Angiography for the Prevention of Myocardial Infarction), enrolling a similar cohort in Scotland.
The study was supported by grants from AP Møller og Hustru Chastine Mc-Kinney Møllers Fond, the Research Council of Rigshospitalet, and Danish Heart Foundation. Dr. Fuchs reports no relevant financial relationships. Disclosures for the other authors can be found here. Dr. Blankstein recently disclosed serving as a consultant to Amgen, Caristo Diagnostics, Novartis, and Silence Therapeutics. Disclosures for Dr. McDermott and Dr. Newby, who are SCOT-HEART 2 investigators, can be found here.
A version of this article originally appeared on Medscape.com.
About half of middle-aged adults in the community without cardiovascular (CV) symptoms have coronary atherosclerosis by CT angiography (CTA) that puts them at substantial risk for myocardial infarction (MI), suggests a prospective cohort study.
The 10% of participants who had subclinical disease considered obstructive at CTA showed a ninefold increased risk for MI over several years. Obstructive disease seemed to elevate risk more than subclinical disease that wasn’t obstructive but still considered extensive within the coronary arteries.
The findings, based on a Copenhagen General Population Study cohort, are new for CTA but consistent with research based on coronary artery calcium (CAC) scores and other ways to assess CV risk, say researchers.
Although all participants underwent CTA, such imaging isn’t used in the general population for atherosclerosis screening. But the findings may have implications for “opportunistic screening” for subclinical coronary disease at CTA conducted for other reasons, notes the study’s report, published online in the Annals of Internal Medicine.
“Identification of luminal obstructive or extensive subclinical coronary atherosclerosis” could potentially provide “clinically relevant, incremental risk assessment” in nonischemic patients who undergo cardiac CT or electrocardiogram-gated chest CT before procedures such as arrhythmia ablation or valve repair, it states.
Such patients found with subclinical coronary atherosclerosis might potentially “benefit from referral to intensified cardiovascular primary prevention therapy,” write the authors, led by Andreas Fuchs, MD, PhD, Copenhagen University Hospital-Rigshospitalet.
The group acknowledges the findings may not entirely apply to a non-Danish population.
A screening role for CTA?
Whether CTA has a role to play in adults without symptoms “is a big, open question in the field right now,” observed Ron Blankstein, MD, not associated with the current analysis, for this news organization.
Most population studies of CV risk prediction, such as MESA, have looked at CAC scores, not CTA, and have shown that “the more plaque individuals have, the higher the risk.” The current findings are similar but novel in coming from coronary CTA in a large asymptomatic community population, said Dr. Blankstein, who is director of cardiac CT at Brigham and Women’s Hospital, Boston.
“It’s possible that patients who have obstructive plaque in general tend to have a larger amount of plaque as well,” he said. So, while the study suggests that “the more plaque individuals have, the worse their overall risk,” it also shows that the risk “is enhanced even more if they have obstructive disease.”
The Danish cohort analysis “provides a unique opportunity to study the contemporary natural history of coronary artery disease in the absence of intervention,” notes an accompanying editorial.
For example, both patients and clinicians were blinded to CTA results, and CV preventive therapies weren’t common, observe Michael McDermott, MBChB, and David E. Newby, DM, PhD, of the BHF Centre for Cardiovascular Science, University of Edinburgh.
The analysis suggests that subclinical coronary disease that is obstructive predicts MI risk more strongly than extensive coronary disease, they note, and may be present in two-thirds of MI patients. “This contrasts with symptomatic populations, where nonobstructive disease accounts for most future myocardial infarctions, presumably from plaque rupture.”
It also points to “strong associations between nonobstructive extensive disease and adverse plaque characteristics,” write Dr. McDermott and Dr. Newby. “This underscores the major importance of plaque burden” for the prediction of coronary events.
Graded risk
The analysis included 9,533 persons aged 40 and older without known ischemic heart disease or symptoms with available CTA assessments.
Obstructive disease, defined as presence of a luminal stenosis of at least 50%, was seen in 10% and nonobstructive disease in 36% of the total cohort, the report states.
Disease occupying more than one-third of the coronary tree was considered extensive and less than one-third of the coronaries nonextensive, occurring in 10.5% and 35.8% of the cohort, respectively.
There were 71 MIs and 193 deaths over a median of 3.5 years. The adjusted relative risk for MI, compared with those without coronary atherosclerosis, was:
- 7.65 (95% confidence interval, 3.53-16.57) overall in patients with extensive disease.
- 8.28 (95% CI, 3.75-18.32) in those with obstructive but nonextensive disease.
- 9.19 (95% CI, 4.49-18.82) overall in those with obstructive disease.
- 12.48 (95% CI, 5.50-28.12) in those with or obstructive and extensive disease.
The adjusted RR for the composite of death or MI was also elevated in persons with extensive disease:
- 2.70 (95% CI, 1.72-4.25) in those with extensive but nonobstructive disease.
- 3.15 (95% CI, 2.05-4.83) in those with extensive and obstructive disease.
“It’s one thing to show that the more plaque, the higher the risk,” Dr. Blankstein said. But “does the information ultimately lead to better outcomes? Do patients have fewer MIs or fewer deaths?” Several ongoing randomized trials are exploring these questions.
They include DANE-HEART (Computed Tomography Coronary Angiography for Primary Prevention), projected to enroll about 6,000 participants from the Copenhagen General Population Study cohort who have at least one CV risk factor, and SCOT-HEART 2 (second Computed Tomography Coronary Angiography for the Prevention of Myocardial Infarction), enrolling a similar cohort in Scotland.
The study was supported by grants from AP Møller og Hustru Chastine Mc-Kinney Møllers Fond, the Research Council of Rigshospitalet, and Danish Heart Foundation. Dr. Fuchs reports no relevant financial relationships. Disclosures for the other authors can be found here. Dr. Blankstein recently disclosed serving as a consultant to Amgen, Caristo Diagnostics, Novartis, and Silence Therapeutics. Disclosures for Dr. McDermott and Dr. Newby, who are SCOT-HEART 2 investigators, can be found here.
A version of this article originally appeared on Medscape.com.