User login
New CRC Risk Prediction Model Outperforms Polyp-Based Model
TOPLINE:
A comprehensive model considering patient age, diabetes, colonoscopy indications, and polyp findings can predict colorectal cancer (CRC) risk more accurately than the solely polyp-based model in patients with a first diagnosis of adenoma on colonoscopy.
METHODOLOGY:
- Because colonoscopy surveillance guidelines relying solely on previous polyp findings to assess CRC risk are imprecise, researchers developed and tested a comprehensive risk prediction model from a list of CRC-related predictors that included patient characteristics and clinical factors in addition to polyp findings.
- The comprehensive model included baseline colonoscopy indication, age group, diabetes diagnosis, and polyp findings (adenoma with advanced histology, polyp size ≥ 10 mm, and sessile serrated or traditional serrated adenoma).
- They randomly assigned 95,001 patients (mean age, 61.9 years; 45.5% women) who underwent colonoscopy with polypectomy to remove a conventional adenoma into two cohorts: Model development (66,500) and internal validation (28,501).
- In both cohorts, researchers compared the performance of the polyp findings-only method against the comprehensive model in predicting CRC, defined as an adenocarcinoma of the colon or rectum diagnosed a year after the baseline colonoscopy.
TAKEAWAY:
- During the follow-up period starting 1 year after colonoscopy, 495 patients were diagnosed with CRC; 354 were in the development cohort and 141 were in the validation cohort.
- The comprehensive model demonstrated better predictive performance than the traditional polyp-based model in the development cohort (area under the curve [AUC], 0.71 vs 0.61) and in the validation cohort (AUC, 0.7 vs 0.62).
- The difference in the Akaike Information Criterion values between the comprehensive and polyp models was 45.7, much above the threshold of 10, strongly indicating the superior performance of the comprehensive model.
IN PRACTICE:
“Improving the ability to accurately predict the patients at highest risk for CRC after polypectomy is critically important, given the considerable costs and resources associated with treating CRC and the better prognosis associated with early cancer detection. The current findings provide proof of concept that inclusion of CRC risk factors beyond prior polyp findings has the potential to improve post-colonoscopy risk stratification,” the authors wrote.
SOURCE:
The study, led by Jeffrey K. Lee, MD, MPH, Division of Research, Kaiser Permanente Northern California, Oakland, California, was published online in The American Journal of Gastroenterology.
LIMITATIONS:
External validation of the model’s performance is needed in different practice settings. The generalizability of the findings is limited because the study population did not include individuals without a prior adenoma or those with an isolated serrated polyp. Moreover, the examination of polyp size > 20 mm as a potential predictor of CRC was precluded due to incomplete data.
DISCLOSURES:
The study was conducted within the National Cancer Institute–funded Population-Based Research to Optimize the Screening Process II consortium and funded by a career development grant from the National Cancer Institute to Lee. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
A comprehensive model considering patient age, diabetes, colonoscopy indications, and polyp findings can predict colorectal cancer (CRC) risk more accurately than the solely polyp-based model in patients with a first diagnosis of adenoma on colonoscopy.
METHODOLOGY:
- Because colonoscopy surveillance guidelines relying solely on previous polyp findings to assess CRC risk are imprecise, researchers developed and tested a comprehensive risk prediction model from a list of CRC-related predictors that included patient characteristics and clinical factors in addition to polyp findings.
- The comprehensive model included baseline colonoscopy indication, age group, diabetes diagnosis, and polyp findings (adenoma with advanced histology, polyp size ≥ 10 mm, and sessile serrated or traditional serrated adenoma).
- They randomly assigned 95,001 patients (mean age, 61.9 years; 45.5% women) who underwent colonoscopy with polypectomy to remove a conventional adenoma into two cohorts: Model development (66,500) and internal validation (28,501).
- In both cohorts, researchers compared the performance of the polyp findings-only method against the comprehensive model in predicting CRC, defined as an adenocarcinoma of the colon or rectum diagnosed a year after the baseline colonoscopy.
TAKEAWAY:
- During the follow-up period starting 1 year after colonoscopy, 495 patients were diagnosed with CRC; 354 were in the development cohort and 141 were in the validation cohort.
- The comprehensive model demonstrated better predictive performance than the traditional polyp-based model in the development cohort (area under the curve [AUC], 0.71 vs 0.61) and in the validation cohort (AUC, 0.7 vs 0.62).
- The difference in the Akaike Information Criterion values between the comprehensive and polyp models was 45.7, much above the threshold of 10, strongly indicating the superior performance of the comprehensive model.
IN PRACTICE:
“Improving the ability to accurately predict the patients at highest risk for CRC after polypectomy is critically important, given the considerable costs and resources associated with treating CRC and the better prognosis associated with early cancer detection. The current findings provide proof of concept that inclusion of CRC risk factors beyond prior polyp findings has the potential to improve post-colonoscopy risk stratification,” the authors wrote.
SOURCE:
The study, led by Jeffrey K. Lee, MD, MPH, Division of Research, Kaiser Permanente Northern California, Oakland, California, was published online in The American Journal of Gastroenterology.
LIMITATIONS:
External validation of the model’s performance is needed in different practice settings. The generalizability of the findings is limited because the study population did not include individuals without a prior adenoma or those with an isolated serrated polyp. Moreover, the examination of polyp size > 20 mm as a potential predictor of CRC was precluded due to incomplete data.
DISCLOSURES:
The study was conducted within the National Cancer Institute–funded Population-Based Research to Optimize the Screening Process II consortium and funded by a career development grant from the National Cancer Institute to Lee. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
A comprehensive model considering patient age, diabetes, colonoscopy indications, and polyp findings can predict colorectal cancer (CRC) risk more accurately than the solely polyp-based model in patients with a first diagnosis of adenoma on colonoscopy.
METHODOLOGY:
- Because colonoscopy surveillance guidelines relying solely on previous polyp findings to assess CRC risk are imprecise, researchers developed and tested a comprehensive risk prediction model from a list of CRC-related predictors that included patient characteristics and clinical factors in addition to polyp findings.
- The comprehensive model included baseline colonoscopy indication, age group, diabetes diagnosis, and polyp findings (adenoma with advanced histology, polyp size ≥ 10 mm, and sessile serrated or traditional serrated adenoma).
- They randomly assigned 95,001 patients (mean age, 61.9 years; 45.5% women) who underwent colonoscopy with polypectomy to remove a conventional adenoma into two cohorts: Model development (66,500) and internal validation (28,501).
- In both cohorts, researchers compared the performance of the polyp findings-only method against the comprehensive model in predicting CRC, defined as an adenocarcinoma of the colon or rectum diagnosed a year after the baseline colonoscopy.
TAKEAWAY:
- During the follow-up period starting 1 year after colonoscopy, 495 patients were diagnosed with CRC; 354 were in the development cohort and 141 were in the validation cohort.
- The comprehensive model demonstrated better predictive performance than the traditional polyp-based model in the development cohort (area under the curve [AUC], 0.71 vs 0.61) and in the validation cohort (AUC, 0.7 vs 0.62).
- The difference in the Akaike Information Criterion values between the comprehensive and polyp models was 45.7, much above the threshold of 10, strongly indicating the superior performance of the comprehensive model.
IN PRACTICE:
“Improving the ability to accurately predict the patients at highest risk for CRC after polypectomy is critically important, given the considerable costs and resources associated with treating CRC and the better prognosis associated with early cancer detection. The current findings provide proof of concept that inclusion of CRC risk factors beyond prior polyp findings has the potential to improve post-colonoscopy risk stratification,” the authors wrote.
SOURCE:
The study, led by Jeffrey K. Lee, MD, MPH, Division of Research, Kaiser Permanente Northern California, Oakland, California, was published online in The American Journal of Gastroenterology.
LIMITATIONS:
External validation of the model’s performance is needed in different practice settings. The generalizability of the findings is limited because the study population did not include individuals without a prior adenoma or those with an isolated serrated polyp. Moreover, the examination of polyp size > 20 mm as a potential predictor of CRC was precluded due to incomplete data.
DISCLOSURES:
The study was conducted within the National Cancer Institute–funded Population-Based Research to Optimize the Screening Process II consortium and funded by a career development grant from the National Cancer Institute to Lee. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
New CRC stool test beats FIT for sensitivity but not specificity
, according to the large prospective BLUE-C study.
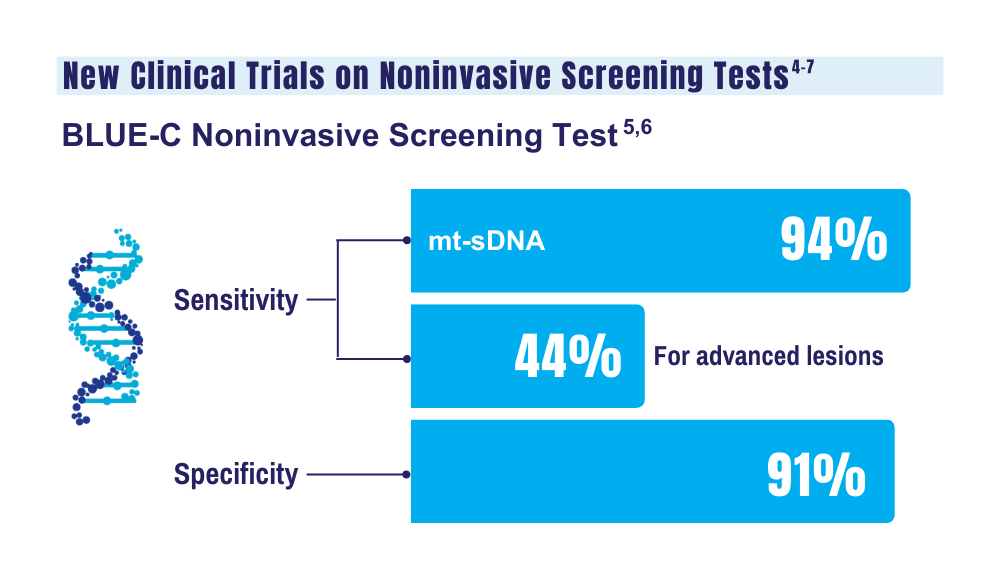
The multi-target assay by Exact Sciences Corporation, the makers of Cologuard, includes new biomarkers designed to increase specificity without decreasing sensitivity. It showed a sensitivity for CRC of almost 94%, with more than 43% sensitivity for advanced precancerous lesions and nearly 91% specificity for advanced neoplasia, according to the study results, which were published in The New England Journal of Medicine.
Adherence to CRC screening in the United States is well below the 80% national target, and the quest continues for noninvasive screening assays that might improve screening adherence, noted lead author Thomas F. Imperiale, MD, AGAF, a professor of medicine at Indiana University School of medicine in Indianapolis, and colleagues.
“The test’s manufacturer developed a new version of its existing Cologuard FIT/DNA test because it took to heart the feedback from primary care providers and gastroenterologists about the test’s low specificity,” Dr. Imperiale said in an interview. “The goal of the new test was to improve specificity without losing, and perhaps even gaining, some sensitivity — a goal that is not easily accomplished when you’re trying to improve on a sensitivity for colorectal cancer that was already 92.3% in the current version of Cologuard.”
Compared with the earlier version of Cologuard, he added, the new generation retained sensitivity for CRC and advanced precancerous lesions or polyps while improving specificity by 30% (90.6% vs 86.6%) for advanced neoplasia — a combination of CRC and advanced precancerous lesions, he said. “This with the caveat, however, that the two versions were not compared head-to-head in this new study,” Dr. Imperiale said.
The higher specificity for advanced lesions is expected to translate to a lower false positive rate. Lowering false positive rates is crucial because that reduces the need for costly, invasive, and unnecessary colonoscopies, said Aasma Shaukat, MD, MPH, AGAF, director of outcomes research in NYU Langone Health’s division of gastroenterology and hepatology in New York City.
“Many physicians felt there were too many false positives with the existing version, and that is anxiety-provoking in patients and providers,” said Dr. Shaukat, who was not involved in the study.
In her view, however, the test’s moderate improvements in detecting certain lesions does not make it demonstrably superior to its predecessor, and there is always the possibility of higher cost to consider.
While acknowledging that a higher sensitivity for all advanced precancerous lesions would have been welcome, Dr. Imperiale said the test detected 75% of the most worrisome of such lesions — “the ones containing high-grade dysplastic cells and suggesting near-term conversion to cancer. And its ability to detect other advanced lesions improved as the size of the lesions increased.”
Testing details
Almost 21,000 asymptomatic participants age 40 years and older undergoing screening colonoscopy were evaluated at 186 US sites during the period 2019 to 2023. Of the cohort, 98 had CRC, 2144 had advanced precancerous lesions, 6973 had nonadvanced adenomas, and 10,961 had nonneoplastic findings or negative colonoscopy.
Advanced precancerous lesions included one or more adenomas or sessile serrated lesions measuring at least 1 cm in the longest dimension, lesions with villous histologic features, and high-grade dysplasia. The new DNA test identified 92 of 98 participants with CRC and 76 of 82 participants with screening-relevant cancers. Among the findings for the new assay:
- Sensitivity for any-stage CRC was 93.9% (95% confidence interval [CI], 87.1- 97.7)
- Sensitivity for advanced precancerous lesions was 43.4% (95% CI, 41.3-45.6)
- Sensitivity for high-grade dysplasia was 74.6% (95% CI, 65.6-82.3)
- Specificity for advanced neoplasia was 90.6% (95% CI, 90.1- 91.0).
- Specificity for nonneoplastic findings or negative colonoscopy was 92.7% (95% CI, 92.2-93.1)
- Specificity for negative colonoscopy was 93.3 (95% CI, 92.8-93.9)
- No adverse events occurred.
In the comparator assay, OC-AUTO FIT by Polymedco, sensitivity was 67.3% (95% CI, 57.1-76.5) for CRC, 23.3% (95% CI, 21.5-25.2) for advanced precancerous lesions, and 47.4% (95% CI, 37.9-56.9) for high-grade dysplasia. In the comparator FIT, however, specificity was better across all age groups — at 94.8% (95% CI, 94.4-95.1) for advanced neoplasia, 95.7% (95% CI, 95.3- 96.1) for nonneoplastic findings, and 96.0% (95% CI, 95.5-96.4) for negative colonoscopy.
In another article in the same issue of NEJM, Guardant Health’s cell-free DNA blood-based test had 83% sensitivity for CRC, 90% specificity for advanced neoplasia, and 13% sensitivity for advanced precancerous lesions in an average-risk population.
An age-related decrease in specificity was observed with the new Cologuard test, but that did not concern Dr. Imperiale because the same observation was made with the current version. “In fact, the next-gen version appears to have less of an age-related decrease in specificity than the current version, although, again, the two versions were not tested head-to-head,” he noted.
The effect of age-related background methylation of DNA is well known, he explained. “Clinicians and older patients in the screening age range do need to be aware of this effect on specificity before ordering or agreeing to do the test. I do not see this as a stumbling block to implementation, but it does require discussion between patient and ordering provider.”
The new version of the DNA test is expected to be available in about a year.
According to Dr. Imperiale, further research is needed to ascertain the test’s acceptability and adherence rates and to quantify its yield in population-based screening. Determining its cost-effectiveness and making it easier to use are other goals. “And most importantly, the degree of reduction in the incidence and mortality from colorectal cancer,” he said.
Cost-effectiveness and the selection of the testing interval may play roles in adherence, particularly in populations with lower rates of screening adherence than the general population, John M. Carethers, MD, AGAF, of the University of California, San Diego, noted in a related editorial.
“Adherence to screening varies according to age group, including persons in the 45- to 49-year age group who are now eligible for average-risk screening,” he wrote. “It is hoped that these newer tests will increase use and adherence and elevate the percentage of the population undergoing screening in order to reduce deaths from colorectal cancer.”
This study was sponsored by Exact Sciences Corporation, which conducted the stool testing at its laboratories.
Dr. Imperiale had no competing interests to disclose. Several study co-authors reported employment with Exact Sciences, or stock and intellectual property ownership. Dr. Shaukat disclosed consulting for Freenome. Dr. Carethers reported ties to Avantor Inc. and Geneoscopy.
, according to the large prospective BLUE-C study.
The multi-target assay by Exact Sciences Corporation, the makers of Cologuard, includes new biomarkers designed to increase specificity without decreasing sensitivity. It showed a sensitivity for CRC of almost 94%, with more than 43% sensitivity for advanced precancerous lesions and nearly 91% specificity for advanced neoplasia, according to the study results, which were published in The New England Journal of Medicine.
Adherence to CRC screening in the United States is well below the 80% national target, and the quest continues for noninvasive screening assays that might improve screening adherence, noted lead author Thomas F. Imperiale, MD, AGAF, a professor of medicine at Indiana University School of medicine in Indianapolis, and colleagues.
“The test’s manufacturer developed a new version of its existing Cologuard FIT/DNA test because it took to heart the feedback from primary care providers and gastroenterologists about the test’s low specificity,” Dr. Imperiale said in an interview. “The goal of the new test was to improve specificity without losing, and perhaps even gaining, some sensitivity — a goal that is not easily accomplished when you’re trying to improve on a sensitivity for colorectal cancer that was already 92.3% in the current version of Cologuard.”
Compared with the earlier version of Cologuard, he added, the new generation retained sensitivity for CRC and advanced precancerous lesions or polyps while improving specificity by 30% (90.6% vs 86.6%) for advanced neoplasia — a combination of CRC and advanced precancerous lesions, he said. “This with the caveat, however, that the two versions were not compared head-to-head in this new study,” Dr. Imperiale said.
The higher specificity for advanced lesions is expected to translate to a lower false positive rate. Lowering false positive rates is crucial because that reduces the need for costly, invasive, and unnecessary colonoscopies, said Aasma Shaukat, MD, MPH, AGAF, director of outcomes research in NYU Langone Health’s division of gastroenterology and hepatology in New York City.
“Many physicians felt there were too many false positives with the existing version, and that is anxiety-provoking in patients and providers,” said Dr. Shaukat, who was not involved in the study.
In her view, however, the test’s moderate improvements in detecting certain lesions does not make it demonstrably superior to its predecessor, and there is always the possibility of higher cost to consider.
While acknowledging that a higher sensitivity for all advanced precancerous lesions would have been welcome, Dr. Imperiale said the test detected 75% of the most worrisome of such lesions — “the ones containing high-grade dysplastic cells and suggesting near-term conversion to cancer. And its ability to detect other advanced lesions improved as the size of the lesions increased.”
Testing details
Almost 21,000 asymptomatic participants age 40 years and older undergoing screening colonoscopy were evaluated at 186 US sites during the period 2019 to 2023. Of the cohort, 98 had CRC, 2144 had advanced precancerous lesions, 6973 had nonadvanced adenomas, and 10,961 had nonneoplastic findings or negative colonoscopy.
Advanced precancerous lesions included one or more adenomas or sessile serrated lesions measuring at least 1 cm in the longest dimension, lesions with villous histologic features, and high-grade dysplasia. The new DNA test identified 92 of 98 participants with CRC and 76 of 82 participants with screening-relevant cancers. Among the findings for the new assay:
- Sensitivity for any-stage CRC was 93.9% (95% confidence interval [CI], 87.1- 97.7)
- Sensitivity for advanced precancerous lesions was 43.4% (95% CI, 41.3-45.6)
- Sensitivity for high-grade dysplasia was 74.6% (95% CI, 65.6-82.3)
- Specificity for advanced neoplasia was 90.6% (95% CI, 90.1- 91.0).
- Specificity for nonneoplastic findings or negative colonoscopy was 92.7% (95% CI, 92.2-93.1)
- Specificity for negative colonoscopy was 93.3 (95% CI, 92.8-93.9)
- No adverse events occurred.
In the comparator assay, OC-AUTO FIT by Polymedco, sensitivity was 67.3% (95% CI, 57.1-76.5) for CRC, 23.3% (95% CI, 21.5-25.2) for advanced precancerous lesions, and 47.4% (95% CI, 37.9-56.9) for high-grade dysplasia. In the comparator FIT, however, specificity was better across all age groups — at 94.8% (95% CI, 94.4-95.1) for advanced neoplasia, 95.7% (95% CI, 95.3- 96.1) for nonneoplastic findings, and 96.0% (95% CI, 95.5-96.4) for negative colonoscopy.
In another article in the same issue of NEJM, Guardant Health’s cell-free DNA blood-based test had 83% sensitivity for CRC, 90% specificity for advanced neoplasia, and 13% sensitivity for advanced precancerous lesions in an average-risk population.
An age-related decrease in specificity was observed with the new Cologuard test, but that did not concern Dr. Imperiale because the same observation was made with the current version. “In fact, the next-gen version appears to have less of an age-related decrease in specificity than the current version, although, again, the two versions were not tested head-to-head,” he noted.
The effect of age-related background methylation of DNA is well known, he explained. “Clinicians and older patients in the screening age range do need to be aware of this effect on specificity before ordering or agreeing to do the test. I do not see this as a stumbling block to implementation, but it does require discussion between patient and ordering provider.”
The new version of the DNA test is expected to be available in about a year.
According to Dr. Imperiale, further research is needed to ascertain the test’s acceptability and adherence rates and to quantify its yield in population-based screening. Determining its cost-effectiveness and making it easier to use are other goals. “And most importantly, the degree of reduction in the incidence and mortality from colorectal cancer,” he said.
Cost-effectiveness and the selection of the testing interval may play roles in adherence, particularly in populations with lower rates of screening adherence than the general population, John M. Carethers, MD, AGAF, of the University of California, San Diego, noted in a related editorial.
“Adherence to screening varies according to age group, including persons in the 45- to 49-year age group who are now eligible for average-risk screening,” he wrote. “It is hoped that these newer tests will increase use and adherence and elevate the percentage of the population undergoing screening in order to reduce deaths from colorectal cancer.”
This study was sponsored by Exact Sciences Corporation, which conducted the stool testing at its laboratories.
Dr. Imperiale had no competing interests to disclose. Several study co-authors reported employment with Exact Sciences, or stock and intellectual property ownership. Dr. Shaukat disclosed consulting for Freenome. Dr. Carethers reported ties to Avantor Inc. and Geneoscopy.
, according to the large prospective BLUE-C study.
The multi-target assay by Exact Sciences Corporation, the makers of Cologuard, includes new biomarkers designed to increase specificity without decreasing sensitivity. It showed a sensitivity for CRC of almost 94%, with more than 43% sensitivity for advanced precancerous lesions and nearly 91% specificity for advanced neoplasia, according to the study results, which were published in The New England Journal of Medicine.
Adherence to CRC screening in the United States is well below the 80% national target, and the quest continues for noninvasive screening assays that might improve screening adherence, noted lead author Thomas F. Imperiale, MD, AGAF, a professor of medicine at Indiana University School of medicine in Indianapolis, and colleagues.
“The test’s manufacturer developed a new version of its existing Cologuard FIT/DNA test because it took to heart the feedback from primary care providers and gastroenterologists about the test’s low specificity,” Dr. Imperiale said in an interview. “The goal of the new test was to improve specificity without losing, and perhaps even gaining, some sensitivity — a goal that is not easily accomplished when you’re trying to improve on a sensitivity for colorectal cancer that was already 92.3% in the current version of Cologuard.”
Compared with the earlier version of Cologuard, he added, the new generation retained sensitivity for CRC and advanced precancerous lesions or polyps while improving specificity by 30% (90.6% vs 86.6%) for advanced neoplasia — a combination of CRC and advanced precancerous lesions, he said. “This with the caveat, however, that the two versions were not compared head-to-head in this new study,” Dr. Imperiale said.
The higher specificity for advanced lesions is expected to translate to a lower false positive rate. Lowering false positive rates is crucial because that reduces the need for costly, invasive, and unnecessary colonoscopies, said Aasma Shaukat, MD, MPH, AGAF, director of outcomes research in NYU Langone Health’s division of gastroenterology and hepatology in New York City.
“Many physicians felt there were too many false positives with the existing version, and that is anxiety-provoking in patients and providers,” said Dr. Shaukat, who was not involved in the study.
In her view, however, the test’s moderate improvements in detecting certain lesions does not make it demonstrably superior to its predecessor, and there is always the possibility of higher cost to consider.
While acknowledging that a higher sensitivity for all advanced precancerous lesions would have been welcome, Dr. Imperiale said the test detected 75% of the most worrisome of such lesions — “the ones containing high-grade dysplastic cells and suggesting near-term conversion to cancer. And its ability to detect other advanced lesions improved as the size of the lesions increased.”
Testing details
Almost 21,000 asymptomatic participants age 40 years and older undergoing screening colonoscopy were evaluated at 186 US sites during the period 2019 to 2023. Of the cohort, 98 had CRC, 2144 had advanced precancerous lesions, 6973 had nonadvanced adenomas, and 10,961 had nonneoplastic findings or negative colonoscopy.
Advanced precancerous lesions included one or more adenomas or sessile serrated lesions measuring at least 1 cm in the longest dimension, lesions with villous histologic features, and high-grade dysplasia. The new DNA test identified 92 of 98 participants with CRC and 76 of 82 participants with screening-relevant cancers. Among the findings for the new assay:
- Sensitivity for any-stage CRC was 93.9% (95% confidence interval [CI], 87.1- 97.7)
- Sensitivity for advanced precancerous lesions was 43.4% (95% CI, 41.3-45.6)
- Sensitivity for high-grade dysplasia was 74.6% (95% CI, 65.6-82.3)
- Specificity for advanced neoplasia was 90.6% (95% CI, 90.1- 91.0).
- Specificity for nonneoplastic findings or negative colonoscopy was 92.7% (95% CI, 92.2-93.1)
- Specificity for negative colonoscopy was 93.3 (95% CI, 92.8-93.9)
- No adverse events occurred.
In the comparator assay, OC-AUTO FIT by Polymedco, sensitivity was 67.3% (95% CI, 57.1-76.5) for CRC, 23.3% (95% CI, 21.5-25.2) for advanced precancerous lesions, and 47.4% (95% CI, 37.9-56.9) for high-grade dysplasia. In the comparator FIT, however, specificity was better across all age groups — at 94.8% (95% CI, 94.4-95.1) for advanced neoplasia, 95.7% (95% CI, 95.3- 96.1) for nonneoplastic findings, and 96.0% (95% CI, 95.5-96.4) for negative colonoscopy.
In another article in the same issue of NEJM, Guardant Health’s cell-free DNA blood-based test had 83% sensitivity for CRC, 90% specificity for advanced neoplasia, and 13% sensitivity for advanced precancerous lesions in an average-risk population.
An age-related decrease in specificity was observed with the new Cologuard test, but that did not concern Dr. Imperiale because the same observation was made with the current version. “In fact, the next-gen version appears to have less of an age-related decrease in specificity than the current version, although, again, the two versions were not tested head-to-head,” he noted.
The effect of age-related background methylation of DNA is well known, he explained. “Clinicians and older patients in the screening age range do need to be aware of this effect on specificity before ordering or agreeing to do the test. I do not see this as a stumbling block to implementation, but it does require discussion between patient and ordering provider.”
The new version of the DNA test is expected to be available in about a year.
According to Dr. Imperiale, further research is needed to ascertain the test’s acceptability and adherence rates and to quantify its yield in population-based screening. Determining its cost-effectiveness and making it easier to use are other goals. “And most importantly, the degree of reduction in the incidence and mortality from colorectal cancer,” he said.
Cost-effectiveness and the selection of the testing interval may play roles in adherence, particularly in populations with lower rates of screening adherence than the general population, John M. Carethers, MD, AGAF, of the University of California, San Diego, noted in a related editorial.
“Adherence to screening varies according to age group, including persons in the 45- to 49-year age group who are now eligible for average-risk screening,” he wrote. “It is hoped that these newer tests will increase use and adherence and elevate the percentage of the population undergoing screening in order to reduce deaths from colorectal cancer.”
This study was sponsored by Exact Sciences Corporation, which conducted the stool testing at its laboratories.
Dr. Imperiale had no competing interests to disclose. Several study co-authors reported employment with Exact Sciences, or stock and intellectual property ownership. Dr. Shaukat disclosed consulting for Freenome. Dr. Carethers reported ties to Avantor Inc. and Geneoscopy.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Cell-Free DNA Blood Test Has High Accuracy for Detecting Colorectal Cancer
, according to a new study.
The cfDNA blood test had 83% sensitivity for CRC, 90% specificity for advanced neoplasia, and 13% sensitivity for advanced precancerous lesions. Other noninvasive screening methods have sensitivity from 67% to 94% for CRC and 22% to 43% for advanced precancerous lesions.
“The results of the study are a promising step toward developing more convenient tools to detect colorectal cancer early while it is more easily treated,” said senior author William M. Grady, MD, AGAF, medical director of the Gastrointestinal Cancer Prevention Program at the Fred Hutchinson Cancer Center in Seattle.
“The test, which has an accuracy rate for colon cancer detection similar to stool tests used for early detection of cancer, could offer an alternative for patients who may otherwise decline current screening options,” he said.
The study was published online on March 14 in The New England Journal of Medicine.
Analyzing the Blood Test’s Accuracy
Dr. Grady and colleagues conducted a multisite clinical trial called ECLIPSE, which compared the sensitivity and specificity of a cfDNA blood test (Shield, Guardant Health) against that obtained with colonoscopy, the gold standard for CRC screening. Guardant led and funded the study.
Guardant’s Shield test is designed to detect CRC through genomic alterations, aberrant methylation status, and fragmentomic patterns, which show up as an “abnormal signal detected” result. Similar blood tests are being developed as “liquid biopsy” tests for other emerging cancer screenings as well.
The study included 7861 people with average CRC risk who underwent routine screening with colonoscopy at 265 sites in the United States, including primary care and endoscopy centers in academic and community-based institutions. Eligible people were aged 45-84 years (average age, 60 years), and 53.7% were women. The race and ethnicity characteristics of the participants closely mirrored the demographic distribution in the 2020 US Census.
Overall, 54 of 65 (83.1%) participants with colonoscopy-detected CRC had a positive cfDNA blood test. However, 11 participants (16.9%) with CRC had a negative test.
The cfDNA blood test identified 42 of 48 stage I, II, or III CRCs, indicating a sensitivity of 87.5%, including 65% for stage I cancers, 100% for stage II cancers, and 100% for stage III cancers. The test also identified all 10 of the stage IV CRC cases. There were no substantial differences in sensitivity for CRC based on primary tumor location, tumor histologic grade, or demographic characteristics.
Among participants without advanced colorectal neoplasia on colonoscopy, 89.6% had a negative cfDNA blood test, and 10.4% had a positive test.
Among those with a negative colonoscopy — with no CRC, advanced precancerous lesions, or nonadvanced precancerous lesions — specificity was 89.9%.
Among 1116 participants with advanced precancerous lesions identified as the most advanced lesion on colonoscopy, the cfDNA blood test was positive for 147, indicating a sensitivity for advanced precancerous lesions of 13.2%.
Although the blood test has sensitivity similar to stool-based tests for CRC, the accuracy is lower than it is with colonoscopy, which remains the current gold standard for CRC screening, Dr. Grady said.
“Colorectal cancer is common and very preventable with screening, but only about 50% to 60% of people who are eligible for screening actually take those tests,” he said. “Getting people to be screened for cancer works best when we offer them screening options and then let them choose what works best for them.”
Future Research
Colorectal cancer is the second leading cause of cancer-related death among US adults and is now the third most diagnosed cancer for people younger than 50 years, Dr. Grady said. Although overall CRC death rates have declined in recent years, the rates among those younger than 55 years have increased since the mid-2000s.
“When colorectal cancer is found earlier and the cancer has not yet spread throughout the body, patient outcomes are much better, as reflected in 5-year survival being much better. It makes sense that an effective blood-based test could have a potential role, in particular for those not getting screened yet,” said Joshua Melson, MD, AGAF, clinical professor of medicine and director of the High-Risk Clinic for Gastrointestinal Cancers at the University of Arizona Cancer Center in Tucson.
Dr. Melson, who wasn’t involved with this study, noted that blood-based testing shows promise for cancer detection but needs additional support for real-world implementation. For instance, the Shield blood test has difficulty detecting precancerous lesions, and it remains unclear what the optimal intervals for repeat testing would be after a negative test, he said. In addition, screening programs will need to ensure they have capacity to effectively deal with a positive test result.
“For a screening program to actually work, when a noninvasive test (whether blood-based or stool-based) is read as positive, those patients need to have a follow-up colonoscopy,” he said.
Proper communication with patients will be important as well, said Gloria Coronado, PhD, associate director of Population Sciences at the University of Arizona Cancer Center, Tucson. Dr. Coronado, who wasn’t involved with this study, has developed CRC screening messages for specific patient populations and studied patient reactions to CRC blood tests.
In a study by Dr. Coronado and colleagues, among more than 2000 patients who passively declined fecal testing and had an upcoming clinic visit, CRC screening proportions were 17.5 percentage points higher in the group offered the blood test vs those offered usual care. In qualitative interviews, one patient said of the blood-based testing option, “I was screaming hallelujah!”
“Patients believed that a blood test would be more accurate than a stool-based test. However, for the detection of advanced adenomas, the reverse is true,” she said. “It will be important to balance the high acceptance and enthusiasm for the blood test with the lower performance of the blood test compared to other tests already on the market.”
In a statement accompanying the study’s publication, the American Gastroenterological Association welcomed these results as an exciting development, but cautioned that a blood-based test was not interchangeable with colonoscopy.
“The Centers for Medicare and Medicaid Services (CMS) has determined it will cover a blood test for colorectal cancer screening every three years if the test achieves 74% sensitivity for CRC, 90% specificity, and FDA approval,” the statement reads. “However, a blood test that meets only the CMS criteria will be inferior to current recommended tests and should not be recommended to replace current tests. Such a test could be recommended for patients who decline all other recommended tests, since any screening is better than no screening at all.”
Dr. Grady is a paid member of Guardant’s scientific advisory board and advised on the design and procedure of the clinical trial and data analysis. Dr. Melson previously served as consultant for Guardant. Dr. Coronado reported no relevant disclosures.
A version of this article appeared on Medscape.com .
, according to a new study.
The cfDNA blood test had 83% sensitivity for CRC, 90% specificity for advanced neoplasia, and 13% sensitivity for advanced precancerous lesions. Other noninvasive screening methods have sensitivity from 67% to 94% for CRC and 22% to 43% for advanced precancerous lesions.
“The results of the study are a promising step toward developing more convenient tools to detect colorectal cancer early while it is more easily treated,” said senior author William M. Grady, MD, AGAF, medical director of the Gastrointestinal Cancer Prevention Program at the Fred Hutchinson Cancer Center in Seattle.
“The test, which has an accuracy rate for colon cancer detection similar to stool tests used for early detection of cancer, could offer an alternative for patients who may otherwise decline current screening options,” he said.
The study was published online on March 14 in The New England Journal of Medicine.
Analyzing the Blood Test’s Accuracy
Dr. Grady and colleagues conducted a multisite clinical trial called ECLIPSE, which compared the sensitivity and specificity of a cfDNA blood test (Shield, Guardant Health) against that obtained with colonoscopy, the gold standard for CRC screening. Guardant led and funded the study.
Guardant’s Shield test is designed to detect CRC through genomic alterations, aberrant methylation status, and fragmentomic patterns, which show up as an “abnormal signal detected” result. Similar blood tests are being developed as “liquid biopsy” tests for other emerging cancer screenings as well.
The study included 7861 people with average CRC risk who underwent routine screening with colonoscopy at 265 sites in the United States, including primary care and endoscopy centers in academic and community-based institutions. Eligible people were aged 45-84 years (average age, 60 years), and 53.7% were women. The race and ethnicity characteristics of the participants closely mirrored the demographic distribution in the 2020 US Census.
Overall, 54 of 65 (83.1%) participants with colonoscopy-detected CRC had a positive cfDNA blood test. However, 11 participants (16.9%) with CRC had a negative test.
The cfDNA blood test identified 42 of 48 stage I, II, or III CRCs, indicating a sensitivity of 87.5%, including 65% for stage I cancers, 100% for stage II cancers, and 100% for stage III cancers. The test also identified all 10 of the stage IV CRC cases. There were no substantial differences in sensitivity for CRC based on primary tumor location, tumor histologic grade, or demographic characteristics.
Among participants without advanced colorectal neoplasia on colonoscopy, 89.6% had a negative cfDNA blood test, and 10.4% had a positive test.
Among those with a negative colonoscopy — with no CRC, advanced precancerous lesions, or nonadvanced precancerous lesions — specificity was 89.9%.
Among 1116 participants with advanced precancerous lesions identified as the most advanced lesion on colonoscopy, the cfDNA blood test was positive for 147, indicating a sensitivity for advanced precancerous lesions of 13.2%.
Although the blood test has sensitivity similar to stool-based tests for CRC, the accuracy is lower than it is with colonoscopy, which remains the current gold standard for CRC screening, Dr. Grady said.
“Colorectal cancer is common and very preventable with screening, but only about 50% to 60% of people who are eligible for screening actually take those tests,” he said. “Getting people to be screened for cancer works best when we offer them screening options and then let them choose what works best for them.”
Future Research
Colorectal cancer is the second leading cause of cancer-related death among US adults and is now the third most diagnosed cancer for people younger than 50 years, Dr. Grady said. Although overall CRC death rates have declined in recent years, the rates among those younger than 55 years have increased since the mid-2000s.
“When colorectal cancer is found earlier and the cancer has not yet spread throughout the body, patient outcomes are much better, as reflected in 5-year survival being much better. It makes sense that an effective blood-based test could have a potential role, in particular for those not getting screened yet,” said Joshua Melson, MD, AGAF, clinical professor of medicine and director of the High-Risk Clinic for Gastrointestinal Cancers at the University of Arizona Cancer Center in Tucson.
Dr. Melson, who wasn’t involved with this study, noted that blood-based testing shows promise for cancer detection but needs additional support for real-world implementation. For instance, the Shield blood test has difficulty detecting precancerous lesions, and it remains unclear what the optimal intervals for repeat testing would be after a negative test, he said. In addition, screening programs will need to ensure they have capacity to effectively deal with a positive test result.
“For a screening program to actually work, when a noninvasive test (whether blood-based or stool-based) is read as positive, those patients need to have a follow-up colonoscopy,” he said.
Proper communication with patients will be important as well, said Gloria Coronado, PhD, associate director of Population Sciences at the University of Arizona Cancer Center, Tucson. Dr. Coronado, who wasn’t involved with this study, has developed CRC screening messages for specific patient populations and studied patient reactions to CRC blood tests.
In a study by Dr. Coronado and colleagues, among more than 2000 patients who passively declined fecal testing and had an upcoming clinic visit, CRC screening proportions were 17.5 percentage points higher in the group offered the blood test vs those offered usual care. In qualitative interviews, one patient said of the blood-based testing option, “I was screaming hallelujah!”
“Patients believed that a blood test would be more accurate than a stool-based test. However, for the detection of advanced adenomas, the reverse is true,” she said. “It will be important to balance the high acceptance and enthusiasm for the blood test with the lower performance of the blood test compared to other tests already on the market.”
In a statement accompanying the study’s publication, the American Gastroenterological Association welcomed these results as an exciting development, but cautioned that a blood-based test was not interchangeable with colonoscopy.
“The Centers for Medicare and Medicaid Services (CMS) has determined it will cover a blood test for colorectal cancer screening every three years if the test achieves 74% sensitivity for CRC, 90% specificity, and FDA approval,” the statement reads. “However, a blood test that meets only the CMS criteria will be inferior to current recommended tests and should not be recommended to replace current tests. Such a test could be recommended for patients who decline all other recommended tests, since any screening is better than no screening at all.”
Dr. Grady is a paid member of Guardant’s scientific advisory board and advised on the design and procedure of the clinical trial and data analysis. Dr. Melson previously served as consultant for Guardant. Dr. Coronado reported no relevant disclosures.
A version of this article appeared on Medscape.com .
, according to a new study.
The cfDNA blood test had 83% sensitivity for CRC, 90% specificity for advanced neoplasia, and 13% sensitivity for advanced precancerous lesions. Other noninvasive screening methods have sensitivity from 67% to 94% for CRC and 22% to 43% for advanced precancerous lesions.
“The results of the study are a promising step toward developing more convenient tools to detect colorectal cancer early while it is more easily treated,” said senior author William M. Grady, MD, AGAF, medical director of the Gastrointestinal Cancer Prevention Program at the Fred Hutchinson Cancer Center in Seattle.
“The test, which has an accuracy rate for colon cancer detection similar to stool tests used for early detection of cancer, could offer an alternative for patients who may otherwise decline current screening options,” he said.
The study was published online on March 14 in The New England Journal of Medicine.
Analyzing the Blood Test’s Accuracy
Dr. Grady and colleagues conducted a multisite clinical trial called ECLIPSE, which compared the sensitivity and specificity of a cfDNA blood test (Shield, Guardant Health) against that obtained with colonoscopy, the gold standard for CRC screening. Guardant led and funded the study.
Guardant’s Shield test is designed to detect CRC through genomic alterations, aberrant methylation status, and fragmentomic patterns, which show up as an “abnormal signal detected” result. Similar blood tests are being developed as “liquid biopsy” tests for other emerging cancer screenings as well.
The study included 7861 people with average CRC risk who underwent routine screening with colonoscopy at 265 sites in the United States, including primary care and endoscopy centers in academic and community-based institutions. Eligible people were aged 45-84 years (average age, 60 years), and 53.7% were women. The race and ethnicity characteristics of the participants closely mirrored the demographic distribution in the 2020 US Census.
Overall, 54 of 65 (83.1%) participants with colonoscopy-detected CRC had a positive cfDNA blood test. However, 11 participants (16.9%) with CRC had a negative test.
The cfDNA blood test identified 42 of 48 stage I, II, or III CRCs, indicating a sensitivity of 87.5%, including 65% for stage I cancers, 100% for stage II cancers, and 100% for stage III cancers. The test also identified all 10 of the stage IV CRC cases. There were no substantial differences in sensitivity for CRC based on primary tumor location, tumor histologic grade, or demographic characteristics.
Among participants without advanced colorectal neoplasia on colonoscopy, 89.6% had a negative cfDNA blood test, and 10.4% had a positive test.
Among those with a negative colonoscopy — with no CRC, advanced precancerous lesions, or nonadvanced precancerous lesions — specificity was 89.9%.
Among 1116 participants with advanced precancerous lesions identified as the most advanced lesion on colonoscopy, the cfDNA blood test was positive for 147, indicating a sensitivity for advanced precancerous lesions of 13.2%.
Although the blood test has sensitivity similar to stool-based tests for CRC, the accuracy is lower than it is with colonoscopy, which remains the current gold standard for CRC screening, Dr. Grady said.
“Colorectal cancer is common and very preventable with screening, but only about 50% to 60% of people who are eligible for screening actually take those tests,” he said. “Getting people to be screened for cancer works best when we offer them screening options and then let them choose what works best for them.”
Future Research
Colorectal cancer is the second leading cause of cancer-related death among US adults and is now the third most diagnosed cancer for people younger than 50 years, Dr. Grady said. Although overall CRC death rates have declined in recent years, the rates among those younger than 55 years have increased since the mid-2000s.
“When colorectal cancer is found earlier and the cancer has not yet spread throughout the body, patient outcomes are much better, as reflected in 5-year survival being much better. It makes sense that an effective blood-based test could have a potential role, in particular for those not getting screened yet,” said Joshua Melson, MD, AGAF, clinical professor of medicine and director of the High-Risk Clinic for Gastrointestinal Cancers at the University of Arizona Cancer Center in Tucson.
Dr. Melson, who wasn’t involved with this study, noted that blood-based testing shows promise for cancer detection but needs additional support for real-world implementation. For instance, the Shield blood test has difficulty detecting precancerous lesions, and it remains unclear what the optimal intervals for repeat testing would be after a negative test, he said. In addition, screening programs will need to ensure they have capacity to effectively deal with a positive test result.
“For a screening program to actually work, when a noninvasive test (whether blood-based or stool-based) is read as positive, those patients need to have a follow-up colonoscopy,” he said.
Proper communication with patients will be important as well, said Gloria Coronado, PhD, associate director of Population Sciences at the University of Arizona Cancer Center, Tucson. Dr. Coronado, who wasn’t involved with this study, has developed CRC screening messages for specific patient populations and studied patient reactions to CRC blood tests.
In a study by Dr. Coronado and colleagues, among more than 2000 patients who passively declined fecal testing and had an upcoming clinic visit, CRC screening proportions were 17.5 percentage points higher in the group offered the blood test vs those offered usual care. In qualitative interviews, one patient said of the blood-based testing option, “I was screaming hallelujah!”
“Patients believed that a blood test would be more accurate than a stool-based test. However, for the detection of advanced adenomas, the reverse is true,” she said. “It will be important to balance the high acceptance and enthusiasm for the blood test with the lower performance of the blood test compared to other tests already on the market.”
In a statement accompanying the study’s publication, the American Gastroenterological Association welcomed these results as an exciting development, but cautioned that a blood-based test was not interchangeable with colonoscopy.
“The Centers for Medicare and Medicaid Services (CMS) has determined it will cover a blood test for colorectal cancer screening every three years if the test achieves 74% sensitivity for CRC, 90% specificity, and FDA approval,” the statement reads. “However, a blood test that meets only the CMS criteria will be inferior to current recommended tests and should not be recommended to replace current tests. Such a test could be recommended for patients who decline all other recommended tests, since any screening is better than no screening at all.”
Dr. Grady is a paid member of Guardant’s scientific advisory board and advised on the design and procedure of the clinical trial and data analysis. Dr. Melson previously served as consultant for Guardant. Dr. Coronado reported no relevant disclosures.
A version of this article appeared on Medscape.com .
FROM NEJM
Cancer Data Trends 2024: Colorectal Cancer
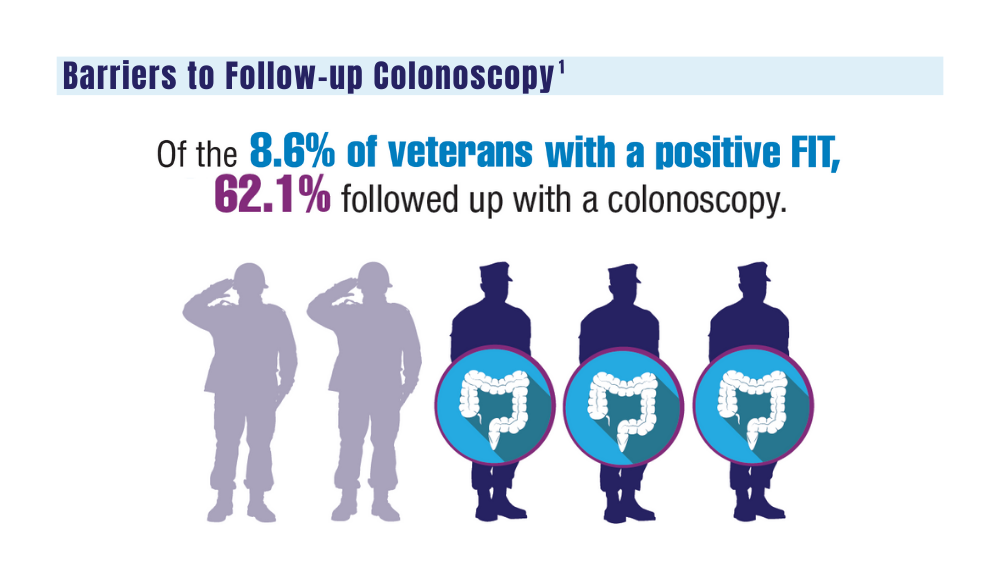
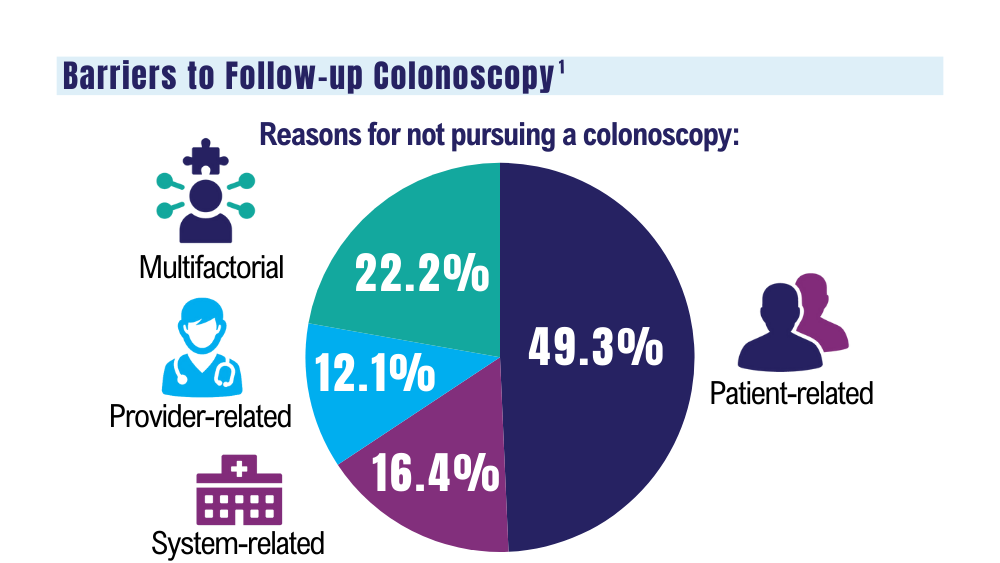
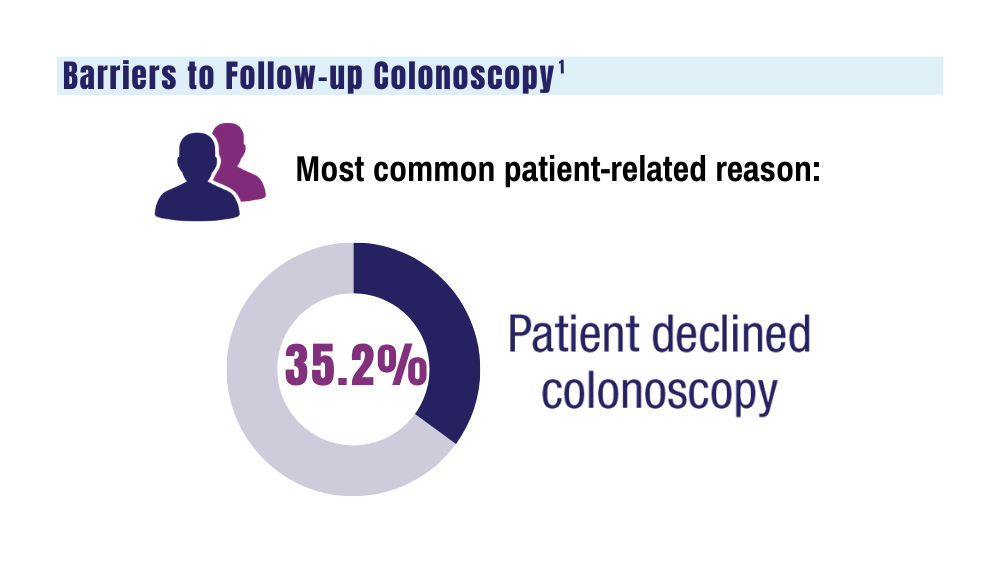
1. May FP, Yano EM, Provenzale D, et al. Barriers to follow-up colonoscopies for patients with positive results from fecal immunochemical tests during colorectal cancer screening. Clin Gastroenterol Hepatol. 2019;17(3):469-476. doi:10.1016/j.cgh.2018.05.022
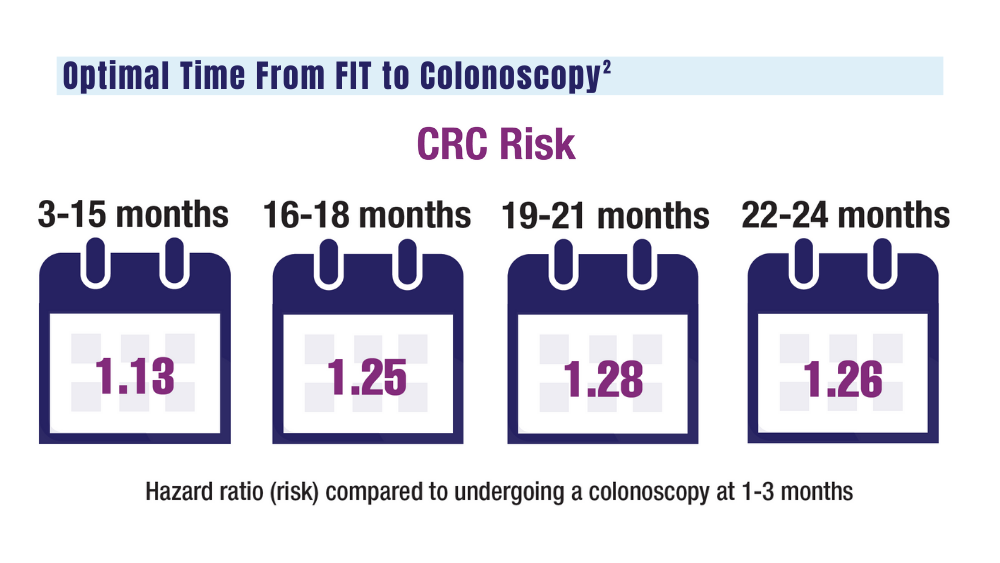
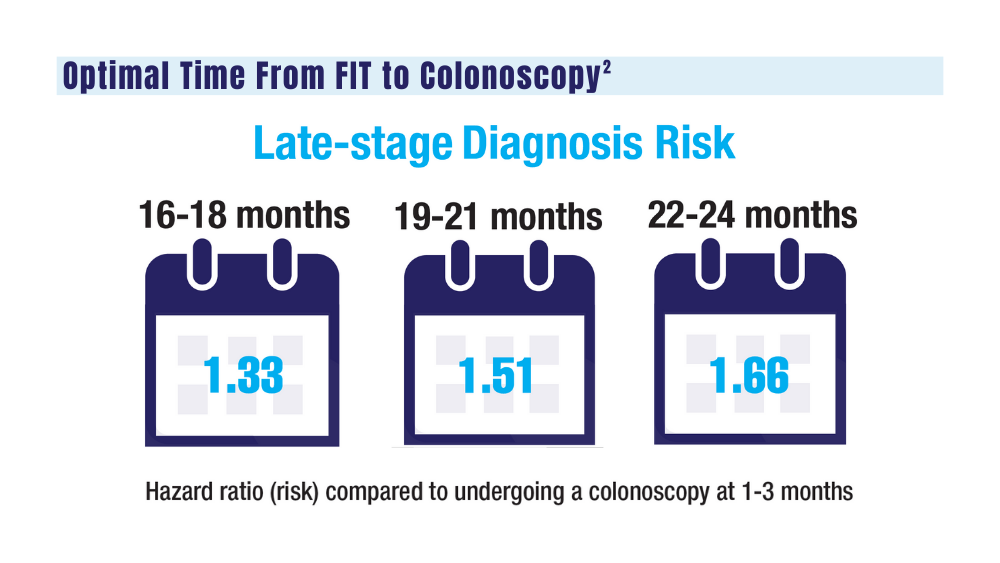
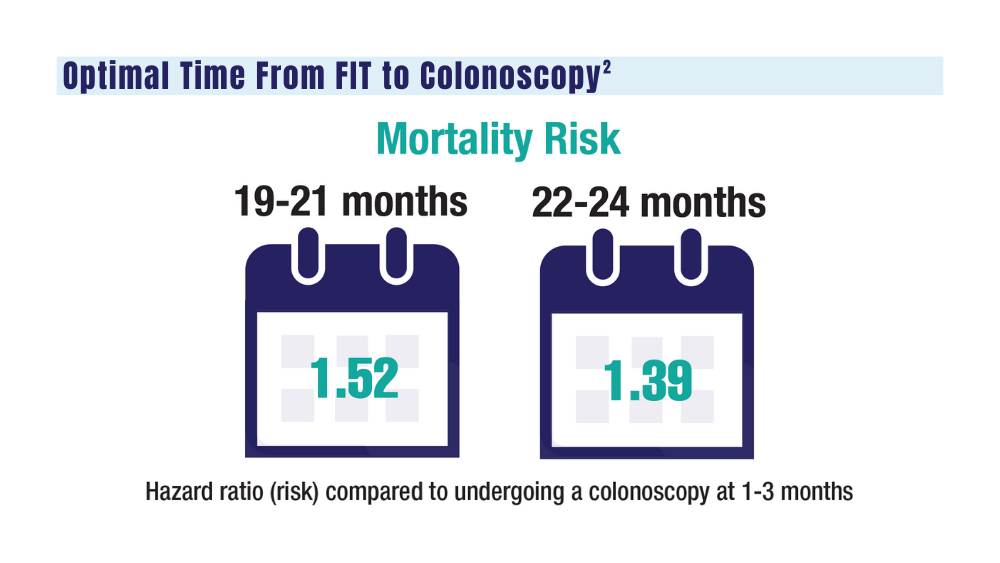
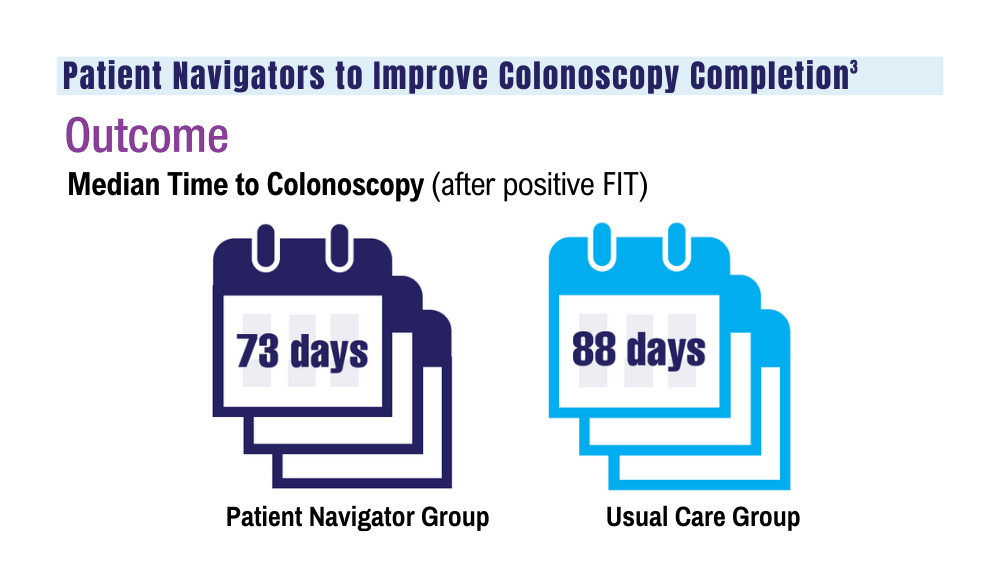
2. San Miguel Y, Demb J, Martinez ME, Gupta S, May FP. Time to colonoscopy after abnormal stool-based screening and risk for colorectal cancer incidence and mortality. Gastroenterology. 2021;160(6):1997-2005.e3. doi:10.1053/j.gastro.2021.01.219
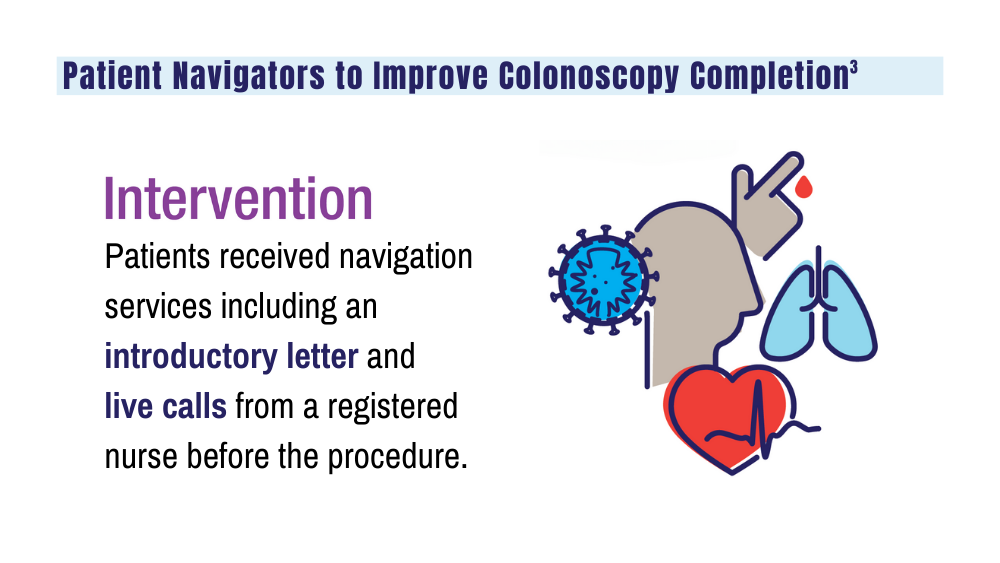
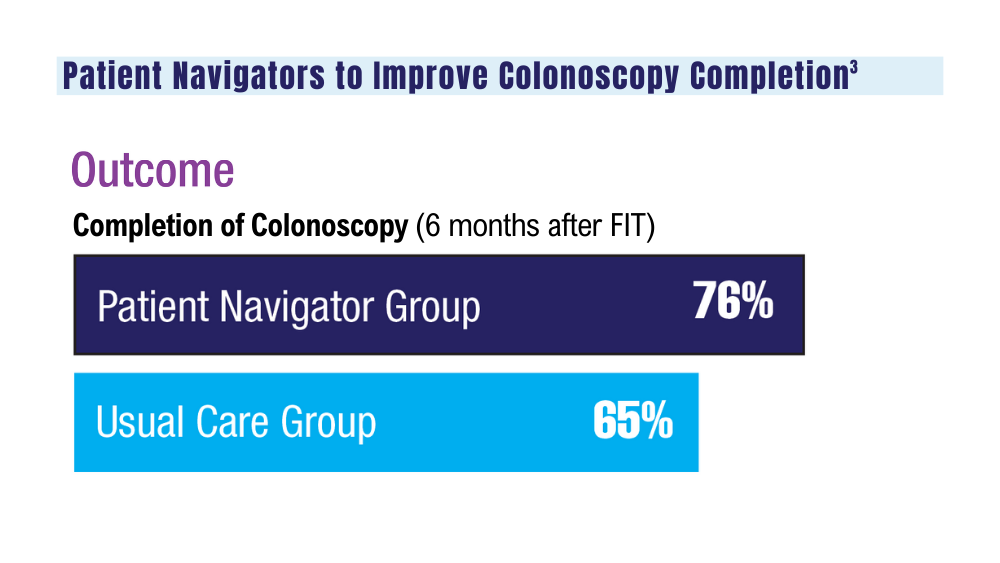
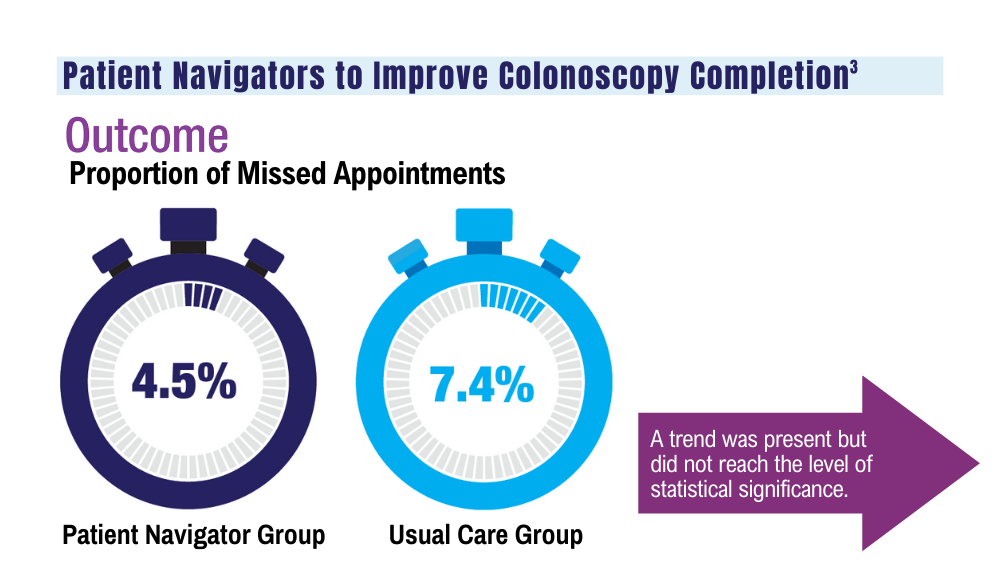
3. Coronado GD, Rawlings AM, Petrik AF, et al. Precision patient navigation to improve rates of follow-up colonoscopy, an individual randomized effectiveness trial. Cancer Epidemiol Biomarkers Prev. 2021;30(12):2327-2333. doi:10.1158/1055-9965.EPI-20-1793
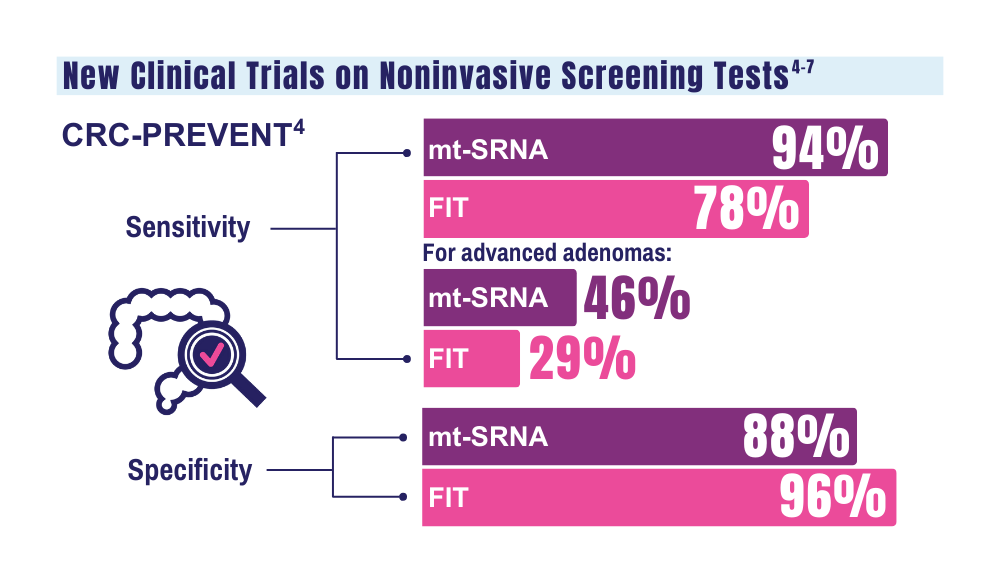
4. Barnell EK, Wurtzler EM, La Rocca J, et al. Multitarget stool RNA test for colorectal cancer screening. JAMA. 2023;330(18):1760- 1768. doi:10.1001/jama.2023.22231
5. McNamara D. Two multitarget stool tests show promise for CRC screening: studies. Medscape. Published October 22, 2023. Accessed December 20, 2023. https://www.medscape.com/viewarticle/997609#vp_1
6. Clinical validation of an optimized multi-target stool DNA (Mt-sDNA 2.0) test, for colorectal cancer screening “BLUE-C.” ClinicalTrials. gov identifier: NCT04144738. Updated September 1, 2023. Accessed December 20, 2023. https://www.clinicaltrials.gov/study/ NCT04144738
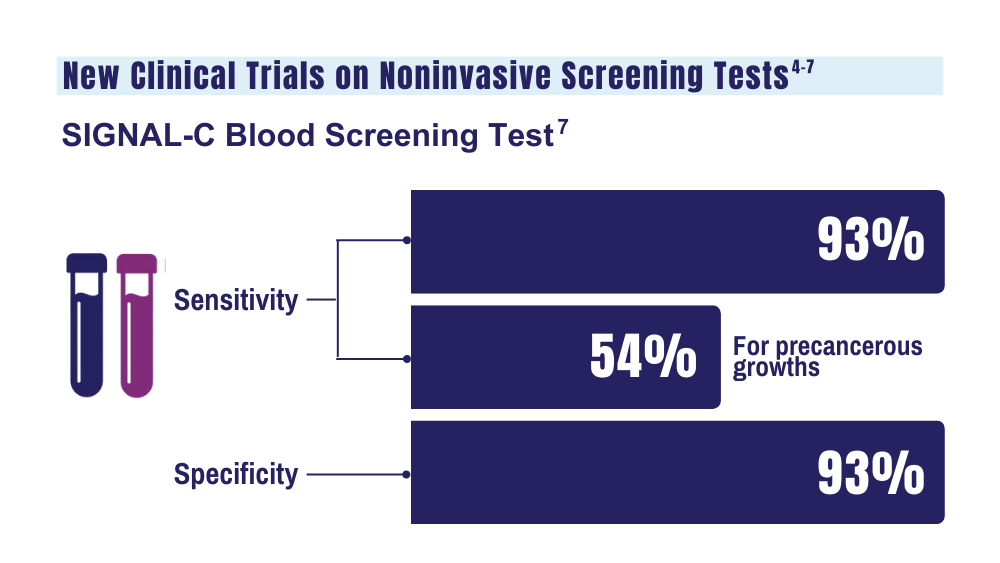
7. Universal Diagnostics. Universal DX Presents Data from Large, 1,000-patient, Multi-Cohort Study Proving 93% Sensitivity for Colorectal Cancer and 54% Sensitivity for Advanced Adenoma at 92% Specificity. Press Release. Published May 2023. Accessed January 26. 2024.
1. May FP, Yano EM, Provenzale D, et al. Barriers to follow-up colonoscopies for patients with positive results from fecal immunochemical tests during colorectal cancer screening. Clin Gastroenterol Hepatol. 2019;17(3):469-476. doi:10.1016/j.cgh.2018.05.022
2. San Miguel Y, Demb J, Martinez ME, Gupta S, May FP. Time to colonoscopy after abnormal stool-based screening and risk for colorectal cancer incidence and mortality. Gastroenterology. 2021;160(6):1997-2005.e3. doi:10.1053/j.gastro.2021.01.219
3. Coronado GD, Rawlings AM, Petrik AF, et al. Precision patient navigation to improve rates of follow-up colonoscopy, an individual randomized effectiveness trial. Cancer Epidemiol Biomarkers Prev. 2021;30(12):2327-2333. doi:10.1158/1055-9965.EPI-20-1793
4. Barnell EK, Wurtzler EM, La Rocca J, et al. Multitarget stool RNA test for colorectal cancer screening. JAMA. 2023;330(18):1760- 1768. doi:10.1001/jama.2023.22231
5. McNamara D. Two multitarget stool tests show promise for CRC screening: studies. Medscape. Published October 22, 2023. Accessed December 20, 2023. https://www.medscape.com/viewarticle/997609#vp_1
6. Clinical validation of an optimized multi-target stool DNA (Mt-sDNA 2.0) test, for colorectal cancer screening “BLUE-C.” ClinicalTrials. gov identifier: NCT04144738. Updated September 1, 2023. Accessed December 20, 2023. https://www.clinicaltrials.gov/study/ NCT04144738
7. Universal Diagnostics. Universal DX Presents Data from Large, 1,000-patient, Multi-Cohort Study Proving 93% Sensitivity for Colorectal Cancer and 54% Sensitivity for Advanced Adenoma at 92% Specificity. Press Release. Published May 2023. Accessed January 26. 2024.
1. May FP, Yano EM, Provenzale D, et al. Barriers to follow-up colonoscopies for patients with positive results from fecal immunochemical tests during colorectal cancer screening. Clin Gastroenterol Hepatol. 2019;17(3):469-476. doi:10.1016/j.cgh.2018.05.022
2. San Miguel Y, Demb J, Martinez ME, Gupta S, May FP. Time to colonoscopy after abnormal stool-based screening and risk for colorectal cancer incidence and mortality. Gastroenterology. 2021;160(6):1997-2005.e3. doi:10.1053/j.gastro.2021.01.219
3. Coronado GD, Rawlings AM, Petrik AF, et al. Precision patient navigation to improve rates of follow-up colonoscopy, an individual randomized effectiveness trial. Cancer Epidemiol Biomarkers Prev. 2021;30(12):2327-2333. doi:10.1158/1055-9965.EPI-20-1793
4. Barnell EK, Wurtzler EM, La Rocca J, et al. Multitarget stool RNA test for colorectal cancer screening. JAMA. 2023;330(18):1760- 1768. doi:10.1001/jama.2023.22231
5. McNamara D. Two multitarget stool tests show promise for CRC screening: studies. Medscape. Published October 22, 2023. Accessed December 20, 2023. https://www.medscape.com/viewarticle/997609#vp_1
6. Clinical validation of an optimized multi-target stool DNA (Mt-sDNA 2.0) test, for colorectal cancer screening “BLUE-C.” ClinicalTrials. gov identifier: NCT04144738. Updated September 1, 2023. Accessed December 20, 2023. https://www.clinicaltrials.gov/study/ NCT04144738
7. Universal Diagnostics. Universal DX Presents Data from Large, 1,000-patient, Multi-Cohort Study Proving 93% Sensitivity for Colorectal Cancer and 54% Sensitivity for Advanced Adenoma at 92% Specificity. Press Release. Published May 2023. Accessed January 26. 2024.
Cancer Data Trends 2024
The annual issue of Cancer Data Trends, produced in collaboration with the Association of VA Hematology/Oncology (AVAHO), highlights the latest research in some of the top cancers impacting US veterans.
Click to view the Digital Edition.
In this issue:
Hepatocellular Carcinoma
Special care for veterans, changes in staging, and biomarkers for early diagnosis
Lung Cancer
Guideline updates and racial disparities in veterans
Multiple Myeloma
Improving survival in the VA
Colorectal Cancer
Barriers to follow-up colonoscopies after FIT testing
B-Cell Lymphomas
Findings from the VA's National TeleOncology Program and recent therapy updates
Breast Cancer
A look at the VA's Risk Assessment Pipeline and incidence among veterans vs the general population
Genitourinary Cancers
Molecular testing in prostate cancer, improving survival for metastatic RCC, and links between bladder cancer and Agent Orange exposure
The annual issue of Cancer Data Trends, produced in collaboration with the Association of VA Hematology/Oncology (AVAHO), highlights the latest research in some of the top cancers impacting US veterans.
Click to view the Digital Edition.
In this issue:
Hepatocellular Carcinoma
Special care for veterans, changes in staging, and biomarkers for early diagnosis
Lung Cancer
Guideline updates and racial disparities in veterans
Multiple Myeloma
Improving survival in the VA
Colorectal Cancer
Barriers to follow-up colonoscopies after FIT testing
B-Cell Lymphomas
Findings from the VA's National TeleOncology Program and recent therapy updates
Breast Cancer
A look at the VA's Risk Assessment Pipeline and incidence among veterans vs the general population
Genitourinary Cancers
Molecular testing in prostate cancer, improving survival for metastatic RCC, and links between bladder cancer and Agent Orange exposure
The annual issue of Cancer Data Trends, produced in collaboration with the Association of VA Hematology/Oncology (AVAHO), highlights the latest research in some of the top cancers impacting US veterans.
Click to view the Digital Edition.
In this issue:
Hepatocellular Carcinoma
Special care for veterans, changes in staging, and biomarkers for early diagnosis
Lung Cancer
Guideline updates and racial disparities in veterans
Multiple Myeloma
Improving survival in the VA
Colorectal Cancer
Barriers to follow-up colonoscopies after FIT testing
B-Cell Lymphomas
Findings from the VA's National TeleOncology Program and recent therapy updates
Breast Cancer
A look at the VA's Risk Assessment Pipeline and incidence among veterans vs the general population
Genitourinary Cancers
Molecular testing in prostate cancer, improving survival for metastatic RCC, and links between bladder cancer and Agent Orange exposure
Is Primary Tumor Resection Beneficial in Stage IV CRC?
TOPLINE:
not amenable to curative therapy, new data showed.
METHODOLOGY:
- Chemotherapy is the primary treatment in patients with stage IV (CRC) and unresectable metastases. It’s unclear whether primary tumor resection before chemotherapy prolongs survival.
- Among 393 patients with stage IV colon cancer and unresectable metastases enrolled in the and trials, 187 were randomly allocated to undergo primary tumor resection and 206 to upfront chemotherapy.
- The chemotherapy regimen was left up to the treating physician. Overall survival was the primary endpoint. Median follow-up time was 36.7 months.
TAKEAWAY:
- Median overall survival was 16.7 months with primary tumor resection and 18.6 months with upfront chemotherapy (P = .191).
- Comparable overall survival between the study groups was further confirmed on multivariate analysis (hazard ratio, 0.944; P = .65) and across all subgroups.
- Serious adverse events were more common with upfront chemo than surgery (18% vs 10%; P = .027), due mainly to a significantly higher incidence of GI-related events (11% vs 5%; P = .031).
- Overall, 24% of the primary tumor resection group did not receive any chemotherapy.
IN PRACTICE:
“The results of our study provide compelling data that upfront primary tumor resection in treatment-naive stage IV CRC not amenable for curative treatment does not prolong [overall survival]. A relatively low incidence of serious adverse events in patients with an intact primary tumor together with a considerable number of patients who did not receive any chemotherapy in the primary tumor resection group provides further arguments against resection of the primary tumor in this group of patients,” the authors of the combined analysis concluded.
SOURCE:
The study, with first author Nuh N. Rahbari, MD, University of Ulm, Ulm, Germany, was published online in the Journal of Clinical Oncology.
LIMITATIONS:
Neither study completed their planned patient accrual. Although both trials are nearly identical, differences in the individual study cohorts and trial implementation could have introduced bias. Tumor molecular profiling was not performed.
DISCLOSURES:
The study had no commercial funding. Disclosures for authors are available with the original article.
A version of this article appeared on Medscape.com.
TOPLINE:
not amenable to curative therapy, new data showed.
METHODOLOGY:
- Chemotherapy is the primary treatment in patients with stage IV (CRC) and unresectable metastases. It’s unclear whether primary tumor resection before chemotherapy prolongs survival.
- Among 393 patients with stage IV colon cancer and unresectable metastases enrolled in the and trials, 187 were randomly allocated to undergo primary tumor resection and 206 to upfront chemotherapy.
- The chemotherapy regimen was left up to the treating physician. Overall survival was the primary endpoint. Median follow-up time was 36.7 months.
TAKEAWAY:
- Median overall survival was 16.7 months with primary tumor resection and 18.6 months with upfront chemotherapy (P = .191).
- Comparable overall survival between the study groups was further confirmed on multivariate analysis (hazard ratio, 0.944; P = .65) and across all subgroups.
- Serious adverse events were more common with upfront chemo than surgery (18% vs 10%; P = .027), due mainly to a significantly higher incidence of GI-related events (11% vs 5%; P = .031).
- Overall, 24% of the primary tumor resection group did not receive any chemotherapy.
IN PRACTICE:
“The results of our study provide compelling data that upfront primary tumor resection in treatment-naive stage IV CRC not amenable for curative treatment does not prolong [overall survival]. A relatively low incidence of serious adverse events in patients with an intact primary tumor together with a considerable number of patients who did not receive any chemotherapy in the primary tumor resection group provides further arguments against resection of the primary tumor in this group of patients,” the authors of the combined analysis concluded.
SOURCE:
The study, with first author Nuh N. Rahbari, MD, University of Ulm, Ulm, Germany, was published online in the Journal of Clinical Oncology.
LIMITATIONS:
Neither study completed their planned patient accrual. Although both trials are nearly identical, differences in the individual study cohorts and trial implementation could have introduced bias. Tumor molecular profiling was not performed.
DISCLOSURES:
The study had no commercial funding. Disclosures for authors are available with the original article.
A version of this article appeared on Medscape.com.
TOPLINE:
not amenable to curative therapy, new data showed.
METHODOLOGY:
- Chemotherapy is the primary treatment in patients with stage IV (CRC) and unresectable metastases. It’s unclear whether primary tumor resection before chemotherapy prolongs survival.
- Among 393 patients with stage IV colon cancer and unresectable metastases enrolled in the and trials, 187 were randomly allocated to undergo primary tumor resection and 206 to upfront chemotherapy.
- The chemotherapy regimen was left up to the treating physician. Overall survival was the primary endpoint. Median follow-up time was 36.7 months.
TAKEAWAY:
- Median overall survival was 16.7 months with primary tumor resection and 18.6 months with upfront chemotherapy (P = .191).
- Comparable overall survival between the study groups was further confirmed on multivariate analysis (hazard ratio, 0.944; P = .65) and across all subgroups.
- Serious adverse events were more common with upfront chemo than surgery (18% vs 10%; P = .027), due mainly to a significantly higher incidence of GI-related events (11% vs 5%; P = .031).
- Overall, 24% of the primary tumor resection group did not receive any chemotherapy.
IN PRACTICE:
“The results of our study provide compelling data that upfront primary tumor resection in treatment-naive stage IV CRC not amenable for curative treatment does not prolong [overall survival]. A relatively low incidence of serious adverse events in patients with an intact primary tumor together with a considerable number of patients who did not receive any chemotherapy in the primary tumor resection group provides further arguments against resection of the primary tumor in this group of patients,” the authors of the combined analysis concluded.
SOURCE:
The study, with first author Nuh N. Rahbari, MD, University of Ulm, Ulm, Germany, was published online in the Journal of Clinical Oncology.
LIMITATIONS:
Neither study completed their planned patient accrual. Although both trials are nearly identical, differences in the individual study cohorts and trial implementation could have introduced bias. Tumor molecular profiling was not performed.
DISCLOSURES:
The study had no commercial funding. Disclosures for authors are available with the original article.
A version of this article appeared on Medscape.com.
Low-Dose Aspirin Associated With Reduced CRC Risk
TOPLINE:
Low-dose aspirin use is associated with a reduced risk for colorectal cancer (CRC), confirms a large-scale cohort study, which also suggests that the risk reduction is greatest for metastatic disease and in individuals who take the drug for at least 5 years.
METHODOLOGY:
- Researchers used several population-based registries to identify individuals aged ≥ 50 years living in Norway between 2014 and 2018, excluding those with a prior history of invasive cancer or who had lived in Norway for less than 6 months before study commencement.
- Sociodemographic information was obtained, as well as low-dose aspirin prescription data to determine the prescription date, number of dispensed packages, and defined daily dose.
- Follow-up began 6 months after entering the cohort and continued until CRC diagnosis, another cancer diagnosis, death, emigration, or the end of follow-up on December 31, 2018.
- CRC cases were categorized by site as well as by clinical stage.
TAKEAWAY:
- Of 2,186,390 individuals included, 38,577 (1.8%) were diagnosed with CRC after a median follow-up of 10.9 years. Low-dose aspirin was used at least once by 579,196 (26.5%) individuals.
- Low-dose aspirin use was more common among males, older individuals, those with a lower education or lower income, those of Norwegian origin, and individuals using other medications, including those targeting cardiovascular conditions.
- Duration of current aspirin use was also associated with the degree of CRC risk, at HRs of 0.91 for < 3 years, 0.85 for ≥ 3 and < 5 years, and 0.84 for ≥ 5 years.
- It was estimated that aspirin use averted 1073 cases of CRC over the study period.
IN PRACTICE:
“We believe that new randomized controlled trials are urgently needed to confirm the potential protective effect of aspirin against CRC and to identify subgroups in the population who might benefit the most from the use of aspirin,” the authors wrote.
SOURCE:
The research, led by Edoardo Botteri, PhD, Department of Research, Cancer Registry of Norway, National Institute of Public Health, Oslo, Norway, was published online in The American Journal of Gastroenterology.
LIMITATIONS:
This study is limited by its observational nature. Users and nonusers are also “incomparable,” as aspirin is used for the primary prevention of cardiovascular events. Moreover, information was lacking in the registries about “several known risk factors for CRC,” and so the link between aspirin and CRC risk could have been over- or underestimated. Finally, the defined daily dose may not necessarily reflect the dose actually taken by the individual or how often it was taken.
DISCLOSURES:
No relevant financial relationships were declared. The study was funded by the Norwegian Research Council.
A version of this article appeared on Medscape.com.
TOPLINE:
Low-dose aspirin use is associated with a reduced risk for colorectal cancer (CRC), confirms a large-scale cohort study, which also suggests that the risk reduction is greatest for metastatic disease and in individuals who take the drug for at least 5 years.
METHODOLOGY:
- Researchers used several population-based registries to identify individuals aged ≥ 50 years living in Norway between 2014 and 2018, excluding those with a prior history of invasive cancer or who had lived in Norway for less than 6 months before study commencement.
- Sociodemographic information was obtained, as well as low-dose aspirin prescription data to determine the prescription date, number of dispensed packages, and defined daily dose.
- Follow-up began 6 months after entering the cohort and continued until CRC diagnosis, another cancer diagnosis, death, emigration, or the end of follow-up on December 31, 2018.
- CRC cases were categorized by site as well as by clinical stage.
TAKEAWAY:
- Of 2,186,390 individuals included, 38,577 (1.8%) were diagnosed with CRC after a median follow-up of 10.9 years. Low-dose aspirin was used at least once by 579,196 (26.5%) individuals.
- Low-dose aspirin use was more common among males, older individuals, those with a lower education or lower income, those of Norwegian origin, and individuals using other medications, including those targeting cardiovascular conditions.
- Duration of current aspirin use was also associated with the degree of CRC risk, at HRs of 0.91 for < 3 years, 0.85 for ≥ 3 and < 5 years, and 0.84 for ≥ 5 years.
- It was estimated that aspirin use averted 1073 cases of CRC over the study period.
IN PRACTICE:
“We believe that new randomized controlled trials are urgently needed to confirm the potential protective effect of aspirin against CRC and to identify subgroups in the population who might benefit the most from the use of aspirin,” the authors wrote.
SOURCE:
The research, led by Edoardo Botteri, PhD, Department of Research, Cancer Registry of Norway, National Institute of Public Health, Oslo, Norway, was published online in The American Journal of Gastroenterology.
LIMITATIONS:
This study is limited by its observational nature. Users and nonusers are also “incomparable,” as aspirin is used for the primary prevention of cardiovascular events. Moreover, information was lacking in the registries about “several known risk factors for CRC,” and so the link between aspirin and CRC risk could have been over- or underestimated. Finally, the defined daily dose may not necessarily reflect the dose actually taken by the individual or how often it was taken.
DISCLOSURES:
No relevant financial relationships were declared. The study was funded by the Norwegian Research Council.
A version of this article appeared on Medscape.com.
TOPLINE:
Low-dose aspirin use is associated with a reduced risk for colorectal cancer (CRC), confirms a large-scale cohort study, which also suggests that the risk reduction is greatest for metastatic disease and in individuals who take the drug for at least 5 years.
METHODOLOGY:
- Researchers used several population-based registries to identify individuals aged ≥ 50 years living in Norway between 2014 and 2018, excluding those with a prior history of invasive cancer or who had lived in Norway for less than 6 months before study commencement.
- Sociodemographic information was obtained, as well as low-dose aspirin prescription data to determine the prescription date, number of dispensed packages, and defined daily dose.
- Follow-up began 6 months after entering the cohort and continued until CRC diagnosis, another cancer diagnosis, death, emigration, or the end of follow-up on December 31, 2018.
- CRC cases were categorized by site as well as by clinical stage.
TAKEAWAY:
- Of 2,186,390 individuals included, 38,577 (1.8%) were diagnosed with CRC after a median follow-up of 10.9 years. Low-dose aspirin was used at least once by 579,196 (26.5%) individuals.
- Low-dose aspirin use was more common among males, older individuals, those with a lower education or lower income, those of Norwegian origin, and individuals using other medications, including those targeting cardiovascular conditions.
- Duration of current aspirin use was also associated with the degree of CRC risk, at HRs of 0.91 for < 3 years, 0.85 for ≥ 3 and < 5 years, and 0.84 for ≥ 5 years.
- It was estimated that aspirin use averted 1073 cases of CRC over the study period.
IN PRACTICE:
“We believe that new randomized controlled trials are urgently needed to confirm the potential protective effect of aspirin against CRC and to identify subgroups in the population who might benefit the most from the use of aspirin,” the authors wrote.
SOURCE:
The research, led by Edoardo Botteri, PhD, Department of Research, Cancer Registry of Norway, National Institute of Public Health, Oslo, Norway, was published online in The American Journal of Gastroenterology.
LIMITATIONS:
This study is limited by its observational nature. Users and nonusers are also “incomparable,” as aspirin is used for the primary prevention of cardiovascular events. Moreover, information was lacking in the registries about “several known risk factors for CRC,” and so the link between aspirin and CRC risk could have been over- or underestimated. Finally, the defined daily dose may not necessarily reflect the dose actually taken by the individual or how often it was taken.
DISCLOSURES:
No relevant financial relationships were declared. The study was funded by the Norwegian Research Council.
A version of this article appeared on Medscape.com.
Does Bariatric Surgery Increase or Decrease Cancer Risk? It Depends.
Bariatric surgery appears to decrease the risk for some cancers, but it may increase the risk for others, particularly colorectal cancer (CRC), according to a synthesis of current evidence.
“There has been a recent burst of studies examining the association between bariatric surgery and the longitudinal risks of developing cancer,” corresponding author Zhi Ven Fong, MD, MPH, DrPH, surgical oncologist, Mayo Clinic Arizona, Phoenix, said in an interview. “However, there has not been a rigorous and critical analysis of the data published to date.”
In evaluating research showing an association between bariatric surgery and longitudinal cancer risk, the investigators found that the quality of the studies and their findings are “heterogeneous and might be susceptible to bias,” Dr. Fong said.
Bariatric surgery appears to have the strongest and most consistent association with the reduction of breast, ovarian, and endometrial cancer risk, first author Pei-Wen Lim, MD, MS, bariatric surgeon at Mayo Clinic Arizona, Phoenix, told this news organization. “However, there have been concerning signals from preclinical and epidemiological studies that bariatric surgery may be associated with a higher risk of developing colorectal cancers,” she added.
The authors cautioned against certain changes in clinical management.
“First, cancer surveillance frequency should not be altered after bariatric surgery because of any assumed reduction in longitudinal cancer risk, and surveillance strategy should mirror that of an average-risk individual,” they wrote. “Secondly, the indications for bariatric surgery should not be expanded for the purpose of cancer-risk mitigation.”
The review was published online in JAMA Surgery.
Protection Against Hormone-Related Cancers
The authors pointed to several studies that appear to support the association between bariatric surgery and decreased risk for hormone-related cancers.
Among them is an observational study of 6781 patients in Canada that showed a significant reduction in breast cancer risk at a median follow-up of 5 years in those who had bariatric surgery vs those who did not (P = .01).
The largest study to date on risk for hormone-related cancer after bariatric surgery was conducted using New York State data for 302,883 women.
It showed a lower rate of breast, endometrial, and ovarian cancers after bariatric surgery (hazard ratio [HR], 0.78; P < .001), with Roux-en-Y gastric bypass conferring the greatest benefit compared with laparoscopic sleeve gastrectomy (HR, 0.66; P = .006) and laparoscopic adjustable gastric banding (HR, 0.83; P = .006).
Beyond the shared mechanisms explaining obesity and cancer risk, a proposed explanation for the strong, consistent association between bariatric surgery and hormone-sensitive cancers is the role obesity-related changes in estrogen stimulation play in development of such cancers, the authors noted.
Association With GI Cancers
The association between bariatric surgery and development of esophageal, gastric, liver, and pancreas cancers is less clear. The data are heterogeneous, with studies showing either no association or decreased longitudinal incidence, the authors reported.
The data are also mixed when it comes to CRC. Epidemiological studies have demonstrated decreased longitudinal incidence of colon and rectal cancer after bariatric surgery; however, two studies have suggested an increased CRC risk after bariatric surgery, the authors noted.
A 15-year study from England that matched 8794 patients with obesity who underwent bariatric surgery with 8794 patients with obesity who did not have the surgery showed that gastric bypass (but not gastric banding or sleeve gastrectomy) was associated with a greater than twofold increased risk of developing colon and rectal cancer (odds ratio, 2.63).
These findings were corroborated in a Swedish cohort study with more than 10 years of follow-up data.
One potential explanation for the heterogeneous findings is that “present studies do not discriminate the sub-types of colon and rectal cancer, with bariatric surgery possibly increasing the incidence of colitis-associated cancers but not hereditary cancers,” the authors wrote.
“The mechanism by which gastric bypass may increase the risk of colorectal cancer is through changes in the gut’s microbiome. These changes in gut flora may triumph the protective effect of weight loss on the development of colorectal cancers,” Dr. Fong said.
Prospective studies are necessary to better delineate CRC risk after bariatric surgery, the authors wrote.
Benefits Outweigh Risk
“Ultimately, it has been proven that bariatric surgery saves lives by improving the metabolic profile of patients with obesity through reduction in cardiovascular risk factors such as hypertension, diabetes, and nonalcoholic fatty liver disease,” Dr. Lim said.
“If patients qualify for bariatric surgery on the basis of their BMI or comorbidities, they should pursue it for its metabolic benefits, but perhaps consider timely or closer-interval screening colonoscopies to monitor for potential colorectal cancer development,” Dr. Lim added.
When asked to comment on the review, Marina Kurian, MD, president, American Society for Metabolic and Bariatric Surgery, also pointed to the advantages of bariatric surgery in reducing major adverse cardiovascular events and improving hypertension, hyperlipidemia, and diabetes.
Bariatric surgery reduces many types of cancers, although the data specific to CRC risk with bariatric surgery are mixed, she added.
“The jury is still out,” said Dr. Kurian, clinical professor of surgery at NYU Langone Health in New York, who was not involved in the review. “There are papers and meta-analyses that show benefit even in colorectal cancer, but then there are a couple of papers out there that suggest a risk that seems to be specific to men.
“It could just be a numbers game, where we may not have enough data. We need more granular data that will help address these nuances and really determine what is the actual risk,” Dr. Kurian said. “But overall, for cancer, bariatric surgery is a win.”
This research had no specific funding. Dr. Fong and Dr. Lim had no relevant disclosures. Dr. Kurian disclosed relationships with Allergan, Allurion, CineMed, CSATS, Ezisurg Medical, Hernon, Johnson & Johnson, Medtronic, Novo, Stryker, and Vivus.
A version of this article appeared on Medscape.com.
Bariatric surgery appears to decrease the risk for some cancers, but it may increase the risk for others, particularly colorectal cancer (CRC), according to a synthesis of current evidence.
“There has been a recent burst of studies examining the association between bariatric surgery and the longitudinal risks of developing cancer,” corresponding author Zhi Ven Fong, MD, MPH, DrPH, surgical oncologist, Mayo Clinic Arizona, Phoenix, said in an interview. “However, there has not been a rigorous and critical analysis of the data published to date.”
In evaluating research showing an association between bariatric surgery and longitudinal cancer risk, the investigators found that the quality of the studies and their findings are “heterogeneous and might be susceptible to bias,” Dr. Fong said.
Bariatric surgery appears to have the strongest and most consistent association with the reduction of breast, ovarian, and endometrial cancer risk, first author Pei-Wen Lim, MD, MS, bariatric surgeon at Mayo Clinic Arizona, Phoenix, told this news organization. “However, there have been concerning signals from preclinical and epidemiological studies that bariatric surgery may be associated with a higher risk of developing colorectal cancers,” she added.
The authors cautioned against certain changes in clinical management.
“First, cancer surveillance frequency should not be altered after bariatric surgery because of any assumed reduction in longitudinal cancer risk, and surveillance strategy should mirror that of an average-risk individual,” they wrote. “Secondly, the indications for bariatric surgery should not be expanded for the purpose of cancer-risk mitigation.”
The review was published online in JAMA Surgery.
Protection Against Hormone-Related Cancers
The authors pointed to several studies that appear to support the association between bariatric surgery and decreased risk for hormone-related cancers.
Among them is an observational study of 6781 patients in Canada that showed a significant reduction in breast cancer risk at a median follow-up of 5 years in those who had bariatric surgery vs those who did not (P = .01).
The largest study to date on risk for hormone-related cancer after bariatric surgery was conducted using New York State data for 302,883 women.
It showed a lower rate of breast, endometrial, and ovarian cancers after bariatric surgery (hazard ratio [HR], 0.78; P < .001), with Roux-en-Y gastric bypass conferring the greatest benefit compared with laparoscopic sleeve gastrectomy (HR, 0.66; P = .006) and laparoscopic adjustable gastric banding (HR, 0.83; P = .006).
Beyond the shared mechanisms explaining obesity and cancer risk, a proposed explanation for the strong, consistent association between bariatric surgery and hormone-sensitive cancers is the role obesity-related changes in estrogen stimulation play in development of such cancers, the authors noted.
Association With GI Cancers
The association between bariatric surgery and development of esophageal, gastric, liver, and pancreas cancers is less clear. The data are heterogeneous, with studies showing either no association or decreased longitudinal incidence, the authors reported.
The data are also mixed when it comes to CRC. Epidemiological studies have demonstrated decreased longitudinal incidence of colon and rectal cancer after bariatric surgery; however, two studies have suggested an increased CRC risk after bariatric surgery, the authors noted.
A 15-year study from England that matched 8794 patients with obesity who underwent bariatric surgery with 8794 patients with obesity who did not have the surgery showed that gastric bypass (but not gastric banding or sleeve gastrectomy) was associated with a greater than twofold increased risk of developing colon and rectal cancer (odds ratio, 2.63).
These findings were corroborated in a Swedish cohort study with more than 10 years of follow-up data.
One potential explanation for the heterogeneous findings is that “present studies do not discriminate the sub-types of colon and rectal cancer, with bariatric surgery possibly increasing the incidence of colitis-associated cancers but not hereditary cancers,” the authors wrote.
“The mechanism by which gastric bypass may increase the risk of colorectal cancer is through changes in the gut’s microbiome. These changes in gut flora may triumph the protective effect of weight loss on the development of colorectal cancers,” Dr. Fong said.
Prospective studies are necessary to better delineate CRC risk after bariatric surgery, the authors wrote.
Benefits Outweigh Risk
“Ultimately, it has been proven that bariatric surgery saves lives by improving the metabolic profile of patients with obesity through reduction in cardiovascular risk factors such as hypertension, diabetes, and nonalcoholic fatty liver disease,” Dr. Lim said.
“If patients qualify for bariatric surgery on the basis of their BMI or comorbidities, they should pursue it for its metabolic benefits, but perhaps consider timely or closer-interval screening colonoscopies to monitor for potential colorectal cancer development,” Dr. Lim added.
When asked to comment on the review, Marina Kurian, MD, president, American Society for Metabolic and Bariatric Surgery, also pointed to the advantages of bariatric surgery in reducing major adverse cardiovascular events and improving hypertension, hyperlipidemia, and diabetes.
Bariatric surgery reduces many types of cancers, although the data specific to CRC risk with bariatric surgery are mixed, she added.
“The jury is still out,” said Dr. Kurian, clinical professor of surgery at NYU Langone Health in New York, who was not involved in the review. “There are papers and meta-analyses that show benefit even in colorectal cancer, but then there are a couple of papers out there that suggest a risk that seems to be specific to men.
“It could just be a numbers game, where we may not have enough data. We need more granular data that will help address these nuances and really determine what is the actual risk,” Dr. Kurian said. “But overall, for cancer, bariatric surgery is a win.”
This research had no specific funding. Dr. Fong and Dr. Lim had no relevant disclosures. Dr. Kurian disclosed relationships with Allergan, Allurion, CineMed, CSATS, Ezisurg Medical, Hernon, Johnson & Johnson, Medtronic, Novo, Stryker, and Vivus.
A version of this article appeared on Medscape.com.
Bariatric surgery appears to decrease the risk for some cancers, but it may increase the risk for others, particularly colorectal cancer (CRC), according to a synthesis of current evidence.
“There has been a recent burst of studies examining the association between bariatric surgery and the longitudinal risks of developing cancer,” corresponding author Zhi Ven Fong, MD, MPH, DrPH, surgical oncologist, Mayo Clinic Arizona, Phoenix, said in an interview. “However, there has not been a rigorous and critical analysis of the data published to date.”
In evaluating research showing an association between bariatric surgery and longitudinal cancer risk, the investigators found that the quality of the studies and their findings are “heterogeneous and might be susceptible to bias,” Dr. Fong said.
Bariatric surgery appears to have the strongest and most consistent association with the reduction of breast, ovarian, and endometrial cancer risk, first author Pei-Wen Lim, MD, MS, bariatric surgeon at Mayo Clinic Arizona, Phoenix, told this news organization. “However, there have been concerning signals from preclinical and epidemiological studies that bariatric surgery may be associated with a higher risk of developing colorectal cancers,” she added.
The authors cautioned against certain changes in clinical management.
“First, cancer surveillance frequency should not be altered after bariatric surgery because of any assumed reduction in longitudinal cancer risk, and surveillance strategy should mirror that of an average-risk individual,” they wrote. “Secondly, the indications for bariatric surgery should not be expanded for the purpose of cancer-risk mitigation.”
The review was published online in JAMA Surgery.
Protection Against Hormone-Related Cancers
The authors pointed to several studies that appear to support the association between bariatric surgery and decreased risk for hormone-related cancers.
Among them is an observational study of 6781 patients in Canada that showed a significant reduction in breast cancer risk at a median follow-up of 5 years in those who had bariatric surgery vs those who did not (P = .01).
The largest study to date on risk for hormone-related cancer after bariatric surgery was conducted using New York State data for 302,883 women.
It showed a lower rate of breast, endometrial, and ovarian cancers after bariatric surgery (hazard ratio [HR], 0.78; P < .001), with Roux-en-Y gastric bypass conferring the greatest benefit compared with laparoscopic sleeve gastrectomy (HR, 0.66; P = .006) and laparoscopic adjustable gastric banding (HR, 0.83; P = .006).
Beyond the shared mechanisms explaining obesity and cancer risk, a proposed explanation for the strong, consistent association between bariatric surgery and hormone-sensitive cancers is the role obesity-related changes in estrogen stimulation play in development of such cancers, the authors noted.
Association With GI Cancers
The association between bariatric surgery and development of esophageal, gastric, liver, and pancreas cancers is less clear. The data are heterogeneous, with studies showing either no association or decreased longitudinal incidence, the authors reported.
The data are also mixed when it comes to CRC. Epidemiological studies have demonstrated decreased longitudinal incidence of colon and rectal cancer after bariatric surgery; however, two studies have suggested an increased CRC risk after bariatric surgery, the authors noted.
A 15-year study from England that matched 8794 patients with obesity who underwent bariatric surgery with 8794 patients with obesity who did not have the surgery showed that gastric bypass (but not gastric banding or sleeve gastrectomy) was associated with a greater than twofold increased risk of developing colon and rectal cancer (odds ratio, 2.63).
These findings were corroborated in a Swedish cohort study with more than 10 years of follow-up data.
One potential explanation for the heterogeneous findings is that “present studies do not discriminate the sub-types of colon and rectal cancer, with bariatric surgery possibly increasing the incidence of colitis-associated cancers but not hereditary cancers,” the authors wrote.
“The mechanism by which gastric bypass may increase the risk of colorectal cancer is through changes in the gut’s microbiome. These changes in gut flora may triumph the protective effect of weight loss on the development of colorectal cancers,” Dr. Fong said.
Prospective studies are necessary to better delineate CRC risk after bariatric surgery, the authors wrote.
Benefits Outweigh Risk
“Ultimately, it has been proven that bariatric surgery saves lives by improving the metabolic profile of patients with obesity through reduction in cardiovascular risk factors such as hypertension, diabetes, and nonalcoholic fatty liver disease,” Dr. Lim said.
“If patients qualify for bariatric surgery on the basis of their BMI or comorbidities, they should pursue it for its metabolic benefits, but perhaps consider timely or closer-interval screening colonoscopies to monitor for potential colorectal cancer development,” Dr. Lim added.
When asked to comment on the review, Marina Kurian, MD, president, American Society for Metabolic and Bariatric Surgery, also pointed to the advantages of bariatric surgery in reducing major adverse cardiovascular events and improving hypertension, hyperlipidemia, and diabetes.
Bariatric surgery reduces many types of cancers, although the data specific to CRC risk with bariatric surgery are mixed, she added.
“The jury is still out,” said Dr. Kurian, clinical professor of surgery at NYU Langone Health in New York, who was not involved in the review. “There are papers and meta-analyses that show benefit even in colorectal cancer, but then there are a couple of papers out there that suggest a risk that seems to be specific to men.
“It could just be a numbers game, where we may not have enough data. We need more granular data that will help address these nuances and really determine what is the actual risk,” Dr. Kurian said. “But overall, for cancer, bariatric surgery is a win.”
This research had no specific funding. Dr. Fong and Dr. Lim had no relevant disclosures. Dr. Kurian disclosed relationships with Allergan, Allurion, CineMed, CSATS, Ezisurg Medical, Hernon, Johnson & Johnson, Medtronic, Novo, Stryker, and Vivus.
A version of this article appeared on Medscape.com.
Oxaliplatin in Older Adults With Resected Colorectal Cancer: Is There a Benefit?
This transcript has been edited for clarity.
Our group, the QUASAR Group, made a very significant contribution in terms of trials and knowledge in this field. One of the things that we still need to discuss and think about in our wider community is the impact of adjuvant chemotherapy in older patients.
Colorectal cancer is a disease of the elderly, with the median age of presentation around 72. We know that, at presentation, more than 50% of patients are aged 65 or over and one third of patients are 75 or over. It’s a disease predominantly of the elderly. Are we justified in giving combination chemotherapy with oxaliplatin to high-risk resected colorectal cancer patients?
There’s a very nice report of a meta-analysis by Dottorini and colleagues that came out recently in the Journal of Clinical Oncology. It’s an excellent group. They did their meta-analytical work according to a strict, rational protocol. Their statistical analyses were on point, and they collected data from all the relevant trials.
According to the results of their study, it could be concluded that the addition of oxaliplatin to adjuvant therapy for resected high-risk colorectal cancer in older patients — patients older than 70 — doesn’t result in any statistically significant gain in terms of preventing recurrences or saving lives.
When we did our QUASAR trials, initially we were looking at control vs fluoropyrimidine chemotherapy. Although there was an overall impact on survival of the whole trial group (the 5000 patients in our study), when we looked by decile, there was a significant diminution of benefit even to fluoropyrimidine therapy in our trial in patients aged 70 or above. I think this careful meta-analysis must make us question the use of oxaliplatin in elderly patients.
What could the explanation be? Why could the well-known and described benefits of oxaliplatin, particularly for stage III disease, attenuate in older people? It may be to do with reduction in dose intensity. Older people have more side effects; therefore, the chemotherapy isn’t completed as planned. Although, increasingly these days, we tend only to be giving 3 months of treatment.
Is there something biologically in terms of the biology or the somatic mutational landscape of the tumor in older people? I don’t think so. Certainly, in terms of their capacity, in terms of stem cell reserve to be as resistant to the side effects of chemotherapy as younger people, we know that does attenuate with age.
Food for thought: The majority of patients I see in the clinic for the adjuvant treatment are elderly. The majority are coming these days with high-risk stage II or stage III disease. There is a real question mark about whether we should be using oxaliplatin at all.
Clearly, one would say that we need more trials of chemotherapy in older folk to see if the addition of drugs like oxaliplatin to a fluoropyrimidine backbone really does make a difference. I’ve said many times before that we, the medical community recommending adjuvant treatment, need to have better risk stratifiers. We need to have better prognostic markers. We need to have better indices that would allow us to perhaps consider these combination treatments in a more focused group of patients who may have a higher risk for recurrence.
Have a look at the paper and see what you think. I think it’s well done. It’s certainly given me pause for thought about the treatment that we will offer our elderly patients.
Have a think about it. Let me know if there are any comments that you’d like to make. For the time being, over and out.
Dr. Kerr is Professor, Nuffield Department of Clinical Laboratory Science, University of Oxford; Professor of Cancer Medicine, Oxford Cancer Centre, Oxford, United Kingdom. He disclosed ties with Celleron Therapeutics, Oxford Cancer Biomarkers, Afrox, GlaxoSmithKline, Bayer HealthCare Pharmaceuticals, Genomic Health, Merck Serono, and Roche.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Our group, the QUASAR Group, made a very significant contribution in terms of trials and knowledge in this field. One of the things that we still need to discuss and think about in our wider community is the impact of adjuvant chemotherapy in older patients.
Colorectal cancer is a disease of the elderly, with the median age of presentation around 72. We know that, at presentation, more than 50% of patients are aged 65 or over and one third of patients are 75 or over. It’s a disease predominantly of the elderly. Are we justified in giving combination chemotherapy with oxaliplatin to high-risk resected colorectal cancer patients?
There’s a very nice report of a meta-analysis by Dottorini and colleagues that came out recently in the Journal of Clinical Oncology. It’s an excellent group. They did their meta-analytical work according to a strict, rational protocol. Their statistical analyses were on point, and they collected data from all the relevant trials.
According to the results of their study, it could be concluded that the addition of oxaliplatin to adjuvant therapy for resected high-risk colorectal cancer in older patients — patients older than 70 — doesn’t result in any statistically significant gain in terms of preventing recurrences or saving lives.
When we did our QUASAR trials, initially we were looking at control vs fluoropyrimidine chemotherapy. Although there was an overall impact on survival of the whole trial group (the 5000 patients in our study), when we looked by decile, there was a significant diminution of benefit even to fluoropyrimidine therapy in our trial in patients aged 70 or above. I think this careful meta-analysis must make us question the use of oxaliplatin in elderly patients.
What could the explanation be? Why could the well-known and described benefits of oxaliplatin, particularly for stage III disease, attenuate in older people? It may be to do with reduction in dose intensity. Older people have more side effects; therefore, the chemotherapy isn’t completed as planned. Although, increasingly these days, we tend only to be giving 3 months of treatment.
Is there something biologically in terms of the biology or the somatic mutational landscape of the tumor in older people? I don’t think so. Certainly, in terms of their capacity, in terms of stem cell reserve to be as resistant to the side effects of chemotherapy as younger people, we know that does attenuate with age.
Food for thought: The majority of patients I see in the clinic for the adjuvant treatment are elderly. The majority are coming these days with high-risk stage II or stage III disease. There is a real question mark about whether we should be using oxaliplatin at all.
Clearly, one would say that we need more trials of chemotherapy in older folk to see if the addition of drugs like oxaliplatin to a fluoropyrimidine backbone really does make a difference. I’ve said many times before that we, the medical community recommending adjuvant treatment, need to have better risk stratifiers. We need to have better prognostic markers. We need to have better indices that would allow us to perhaps consider these combination treatments in a more focused group of patients who may have a higher risk for recurrence.
Have a look at the paper and see what you think. I think it’s well done. It’s certainly given me pause for thought about the treatment that we will offer our elderly patients.
Have a think about it. Let me know if there are any comments that you’d like to make. For the time being, over and out.
Dr. Kerr is Professor, Nuffield Department of Clinical Laboratory Science, University of Oxford; Professor of Cancer Medicine, Oxford Cancer Centre, Oxford, United Kingdom. He disclosed ties with Celleron Therapeutics, Oxford Cancer Biomarkers, Afrox, GlaxoSmithKline, Bayer HealthCare Pharmaceuticals, Genomic Health, Merck Serono, and Roche.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Our group, the QUASAR Group, made a very significant contribution in terms of trials and knowledge in this field. One of the things that we still need to discuss and think about in our wider community is the impact of adjuvant chemotherapy in older patients.
Colorectal cancer is a disease of the elderly, with the median age of presentation around 72. We know that, at presentation, more than 50% of patients are aged 65 or over and one third of patients are 75 or over. It’s a disease predominantly of the elderly. Are we justified in giving combination chemotherapy with oxaliplatin to high-risk resected colorectal cancer patients?
There’s a very nice report of a meta-analysis by Dottorini and colleagues that came out recently in the Journal of Clinical Oncology. It’s an excellent group. They did their meta-analytical work according to a strict, rational protocol. Their statistical analyses were on point, and they collected data from all the relevant trials.
According to the results of their study, it could be concluded that the addition of oxaliplatin to adjuvant therapy for resected high-risk colorectal cancer in older patients — patients older than 70 — doesn’t result in any statistically significant gain in terms of preventing recurrences or saving lives.
When we did our QUASAR trials, initially we were looking at control vs fluoropyrimidine chemotherapy. Although there was an overall impact on survival of the whole trial group (the 5000 patients in our study), when we looked by decile, there was a significant diminution of benefit even to fluoropyrimidine therapy in our trial in patients aged 70 or above. I think this careful meta-analysis must make us question the use of oxaliplatin in elderly patients.
What could the explanation be? Why could the well-known and described benefits of oxaliplatin, particularly for stage III disease, attenuate in older people? It may be to do with reduction in dose intensity. Older people have more side effects; therefore, the chemotherapy isn’t completed as planned. Although, increasingly these days, we tend only to be giving 3 months of treatment.
Is there something biologically in terms of the biology or the somatic mutational landscape of the tumor in older people? I don’t think so. Certainly, in terms of their capacity, in terms of stem cell reserve to be as resistant to the side effects of chemotherapy as younger people, we know that does attenuate with age.
Food for thought: The majority of patients I see in the clinic for the adjuvant treatment are elderly. The majority are coming these days with high-risk stage II or stage III disease. There is a real question mark about whether we should be using oxaliplatin at all.
Clearly, one would say that we need more trials of chemotherapy in older folk to see if the addition of drugs like oxaliplatin to a fluoropyrimidine backbone really does make a difference. I’ve said many times before that we, the medical community recommending adjuvant treatment, need to have better risk stratifiers. We need to have better prognostic markers. We need to have better indices that would allow us to perhaps consider these combination treatments in a more focused group of patients who may have a higher risk for recurrence.
Have a look at the paper and see what you think. I think it’s well done. It’s certainly given me pause for thought about the treatment that we will offer our elderly patients.
Have a think about it. Let me know if there are any comments that you’d like to make. For the time being, over and out.
Dr. Kerr is Professor, Nuffield Department of Clinical Laboratory Science, University of Oxford; Professor of Cancer Medicine, Oxford Cancer Centre, Oxford, United Kingdom. He disclosed ties with Celleron Therapeutics, Oxford Cancer Biomarkers, Afrox, GlaxoSmithKline, Bayer HealthCare Pharmaceuticals, Genomic Health, Merck Serono, and Roche.
A version of this article appeared on Medscape.com.
Transcatheter Arterial Chemoembolization for Rectal Tumors?
TOPLINE:
METHODOLOGY:
- The combination of neoadjuvant chemoradiotherapy, total mesorectal excision, and postoperative adjuvant chemotherapy is the current standard of care for locally advanced rectal cancer. But with pathological complete response rates of only 10%-15% and more than 30% of patients developing distant metastases within 3 years, outcomes remain suboptimal.
- Chinese investigators took a step to improve the situation, applying TACE — a standard treatment for colorectal liver metastases — to rectal tumors, dubbing the approach transcatheter rectal arterial chemoembolization (TRACE).
- As in TACE, TRACE uses precisely injected chemotherapeutic and vaso-occlusive agents to shut down blood flow to tumors, starving them of oxygen and nutrients.
- The research team tried the approach in 111 patients with stage II or III rectal tumors and performance status scores of 0-1.
- TRACE was delivered with oxaliplatin and followed by radiotherapy and S1 chemotherapy (tegafur, gimeracil, and potassium oteracil). Total mesorectal excisions were performed 4-8 weeks later, followed by mFOLFOX6 (5-fluorouracil, leucovorin, and oxaliplatin) or CAPOX (oxaliplatin and capecitabine) chemotherapy for 4-6 months.
TAKEAWAY:
- Overall, 20.7% of patients undergoing TRACE had a pathological complete response, and almost half (48.65%) had a major pathological response.
- Nearly 62% of patients were disease-free at 5 years, and almost 75% were alive at 5 years.
- No serious surgical complications occurred, but 21.6% of patients had postoperative complications. Overall, about 26% of patients (29 of 111) had grade 3/4 toxicities.
IN PRACTICE:
“The addition of transcatheter rectal arterial chemoembolisation to the neoadjuvant therapy can improve the pathological remission rate and prognosis in patients with locally advanced rectal cancer, without increasing the incidence of preoperative adverse events and postoperative complications,” the researchers concluded. “Given its promising effectiveness and safe profile, incorporating TRACE into the standard treatment strategy for patients with [locally advanced rectal cancer] should be considered.”
SOURCE:
The work, led by W. Yang of the Army Medical University in Chongqing, China, was published in Clinical Oncology.
LIMITATIONS:
The study was performed at a single center with no control arm in a Chinese population.
DISCLOSURES:
The work was funded by the Third Military Medical University in China. The investigators had no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- The combination of neoadjuvant chemoradiotherapy, total mesorectal excision, and postoperative adjuvant chemotherapy is the current standard of care for locally advanced rectal cancer. But with pathological complete response rates of only 10%-15% and more than 30% of patients developing distant metastases within 3 years, outcomes remain suboptimal.
- Chinese investigators took a step to improve the situation, applying TACE — a standard treatment for colorectal liver metastases — to rectal tumors, dubbing the approach transcatheter rectal arterial chemoembolization (TRACE).
- As in TACE, TRACE uses precisely injected chemotherapeutic and vaso-occlusive agents to shut down blood flow to tumors, starving them of oxygen and nutrients.
- The research team tried the approach in 111 patients with stage II or III rectal tumors and performance status scores of 0-1.
- TRACE was delivered with oxaliplatin and followed by radiotherapy and S1 chemotherapy (tegafur, gimeracil, and potassium oteracil). Total mesorectal excisions were performed 4-8 weeks later, followed by mFOLFOX6 (5-fluorouracil, leucovorin, and oxaliplatin) or CAPOX (oxaliplatin and capecitabine) chemotherapy for 4-6 months.
TAKEAWAY:
- Overall, 20.7% of patients undergoing TRACE had a pathological complete response, and almost half (48.65%) had a major pathological response.
- Nearly 62% of patients were disease-free at 5 years, and almost 75% were alive at 5 years.
- No serious surgical complications occurred, but 21.6% of patients had postoperative complications. Overall, about 26% of patients (29 of 111) had grade 3/4 toxicities.
IN PRACTICE:
“The addition of transcatheter rectal arterial chemoembolisation to the neoadjuvant therapy can improve the pathological remission rate and prognosis in patients with locally advanced rectal cancer, without increasing the incidence of preoperative adverse events and postoperative complications,” the researchers concluded. “Given its promising effectiveness and safe profile, incorporating TRACE into the standard treatment strategy for patients with [locally advanced rectal cancer] should be considered.”
SOURCE:
The work, led by W. Yang of the Army Medical University in Chongqing, China, was published in Clinical Oncology.
LIMITATIONS:
The study was performed at a single center with no control arm in a Chinese population.
DISCLOSURES:
The work was funded by the Third Military Medical University in China. The investigators had no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- The combination of neoadjuvant chemoradiotherapy, total mesorectal excision, and postoperative adjuvant chemotherapy is the current standard of care for locally advanced rectal cancer. But with pathological complete response rates of only 10%-15% and more than 30% of patients developing distant metastases within 3 years, outcomes remain suboptimal.
- Chinese investigators took a step to improve the situation, applying TACE — a standard treatment for colorectal liver metastases — to rectal tumors, dubbing the approach transcatheter rectal arterial chemoembolization (TRACE).
- As in TACE, TRACE uses precisely injected chemotherapeutic and vaso-occlusive agents to shut down blood flow to tumors, starving them of oxygen and nutrients.
- The research team tried the approach in 111 patients with stage II or III rectal tumors and performance status scores of 0-1.
- TRACE was delivered with oxaliplatin and followed by radiotherapy and S1 chemotherapy (tegafur, gimeracil, and potassium oteracil). Total mesorectal excisions were performed 4-8 weeks later, followed by mFOLFOX6 (5-fluorouracil, leucovorin, and oxaliplatin) or CAPOX (oxaliplatin and capecitabine) chemotherapy for 4-6 months.
TAKEAWAY:
- Overall, 20.7% of patients undergoing TRACE had a pathological complete response, and almost half (48.65%) had a major pathological response.
- Nearly 62% of patients were disease-free at 5 years, and almost 75% were alive at 5 years.
- No serious surgical complications occurred, but 21.6% of patients had postoperative complications. Overall, about 26% of patients (29 of 111) had grade 3/4 toxicities.
IN PRACTICE:
“The addition of transcatheter rectal arterial chemoembolisation to the neoadjuvant therapy can improve the pathological remission rate and prognosis in patients with locally advanced rectal cancer, without increasing the incidence of preoperative adverse events and postoperative complications,” the researchers concluded. “Given its promising effectiveness and safe profile, incorporating TRACE into the standard treatment strategy for patients with [locally advanced rectal cancer] should be considered.”
SOURCE:
The work, led by W. Yang of the Army Medical University in Chongqing, China, was published in Clinical Oncology.
LIMITATIONS:
The study was performed at a single center with no control arm in a Chinese population.
DISCLOSURES:
The work was funded by the Third Military Medical University in China. The investigators had no conflicts of interest.
A version of this article appeared on Medscape.com.