User login
Early mobility in the ICU: Working with the TEAM
Critical Care Network
Nonrespiratory Critical Care Section
This is especially true for critically ill patients, in which weakness is more common and can result in worse outcomes (Kress JP, et al. N Engl J Med. 2014;370:1626). This advocacy is endorsed by major societies and guidelines, like the ABCDEF bundle (Balas MC, et al. Crit Care Med. 2013;41:S116), in which “E” stands for Early mobility and exercise. In fact, the PADIS guidelines, addressing Pain, Agitation, Delirium, Immobility, and Sleep in the ICU, added Immobility and Sleep (the “I” and “S” in PADIS) to the prior PAD guidelines in the latest update in 2018, to stress the importance of early mobility in the ICU (Devlin JW, et al. Crit Care Med. 2018;46[9]:e825). Multiple studies have shown a positive impact of early mobility in the ICU on patients’ outcomes (Tipping CJ, et al. Intensive Care Med. 2017;43:171).
The recent TEAM study examined an early mobility approach in mechanically ventilated patients and found no difference in the primary outcome of alive and out-of-hospital at 180 days (N Engl J Med. 2022;387:1747).
Before concluding, it is worth realizing that the usual care arm included mobilization that was otherwise normally provided. The intervention arm protocolized the early mobility to be done simultaneously with the minimization of sedation. Patients’ assessment occurred in 81% in the usual care arm vs 94% in the intervention arm; both numbers are much higher than reported data in the ICU (Jolley SE, et al. Crit Care Med. 2017;45:205).
Revisiting the question of early mobility in the ICU, more data are needed to clarify the best methodology, sedation, timing, amount, and type of patients who will benefit the most. Until then, it should remain a goal for ICUs and part of the daily discussion when caring for critically ill patients.
Mohammed J. Al-Jaghbeer, MBBS, FCCP
Section Member-at-Large
Salim Surani, MD, MPH, FCCP
Critical Care Network
Nonrespiratory Critical Care Section
This is especially true for critically ill patients, in which weakness is more common and can result in worse outcomes (Kress JP, et al. N Engl J Med. 2014;370:1626). This advocacy is endorsed by major societies and guidelines, like the ABCDEF bundle (Balas MC, et al. Crit Care Med. 2013;41:S116), in which “E” stands for Early mobility and exercise. In fact, the PADIS guidelines, addressing Pain, Agitation, Delirium, Immobility, and Sleep in the ICU, added Immobility and Sleep (the “I” and “S” in PADIS) to the prior PAD guidelines in the latest update in 2018, to stress the importance of early mobility in the ICU (Devlin JW, et al. Crit Care Med. 2018;46[9]:e825). Multiple studies have shown a positive impact of early mobility in the ICU on patients’ outcomes (Tipping CJ, et al. Intensive Care Med. 2017;43:171).
The recent TEAM study examined an early mobility approach in mechanically ventilated patients and found no difference in the primary outcome of alive and out-of-hospital at 180 days (N Engl J Med. 2022;387:1747).
Before concluding, it is worth realizing that the usual care arm included mobilization that was otherwise normally provided. The intervention arm protocolized the early mobility to be done simultaneously with the minimization of sedation. Patients’ assessment occurred in 81% in the usual care arm vs 94% in the intervention arm; both numbers are much higher than reported data in the ICU (Jolley SE, et al. Crit Care Med. 2017;45:205).
Revisiting the question of early mobility in the ICU, more data are needed to clarify the best methodology, sedation, timing, amount, and type of patients who will benefit the most. Until then, it should remain a goal for ICUs and part of the daily discussion when caring for critically ill patients.
Mohammed J. Al-Jaghbeer, MBBS, FCCP
Section Member-at-Large
Salim Surani, MD, MPH, FCCP
Critical Care Network
Nonrespiratory Critical Care Section
This is especially true for critically ill patients, in which weakness is more common and can result in worse outcomes (Kress JP, et al. N Engl J Med. 2014;370:1626). This advocacy is endorsed by major societies and guidelines, like the ABCDEF bundle (Balas MC, et al. Crit Care Med. 2013;41:S116), in which “E” stands for Early mobility and exercise. In fact, the PADIS guidelines, addressing Pain, Agitation, Delirium, Immobility, and Sleep in the ICU, added Immobility and Sleep (the “I” and “S” in PADIS) to the prior PAD guidelines in the latest update in 2018, to stress the importance of early mobility in the ICU (Devlin JW, et al. Crit Care Med. 2018;46[9]:e825). Multiple studies have shown a positive impact of early mobility in the ICU on patients’ outcomes (Tipping CJ, et al. Intensive Care Med. 2017;43:171).
The recent TEAM study examined an early mobility approach in mechanically ventilated patients and found no difference in the primary outcome of alive and out-of-hospital at 180 days (N Engl J Med. 2022;387:1747).
Before concluding, it is worth realizing that the usual care arm included mobilization that was otherwise normally provided. The intervention arm protocolized the early mobility to be done simultaneously with the minimization of sedation. Patients’ assessment occurred in 81% in the usual care arm vs 94% in the intervention arm; both numbers are much higher than reported data in the ICU (Jolley SE, et al. Crit Care Med. 2017;45:205).
Revisiting the question of early mobility in the ICU, more data are needed to clarify the best methodology, sedation, timing, amount, and type of patients who will benefit the most. Until then, it should remain a goal for ICUs and part of the daily discussion when caring for critically ill patients.
Mohammed J. Al-Jaghbeer, MBBS, FCCP
Section Member-at-Large
Salim Surani, MD, MPH, FCCP
COVID vs. flu: Which is deadlier?
a new study shows.
People who were hospitalized with Omicron COVID-19 infections were 54% more likely to die, compared with people who were hospitalized with the flu, Swiss researchers found.
The results of the study continue to debunk an earlier belief from the start of the pandemic that the flu was the more dangerous of the two respiratory viruses. The researchers noted that the deadliness of COVID-19, compared with flu, persisted “despite virus evolution and improved management strategies.”
The study was published in JAMA Network Open and included 5,212 patients in Switzerland hospitalized with COVID-19 or the flu. All the COVID patients were infected with the Omicron variant and hospitalized between Jan. 15, 2022, and March 15, 2022. Flu data included cases from January 2018 to March 15, 2022.
Overall, 7% of COVID-19 patients died, compared with 4.4% of flu patients. Researchers noted that the death rate for hospitalized COVID patients had declined since their previous study, which was conducted during the first COVID wave in the first half of 2020. At that time, the death rate of hospitalized COVID patients was 12.8%.
Since then, 98% of the Swiss population has been vaccinated. “Vaccination still plays a significant role regarding the main outcome,” the authors concluded, since a secondary analysis in this most recent study showed that unvaccinated COVID patients were twice as likely to die, compared with flu patients.
“Our results demonstrate that COVID-19 still cannot simply be compared with influenza,” they wrote.
While the death rate among COVID patients was significantly higher, there was no difference in the rate that COVID or flu patients were admitted to the ICU, which was around 8%.
A limitation of the study was that all the COVID cases did not have laboratory testing to confirm the Omicron variant. However, the study authors noted that Omicron accounted for at least 95% of cases during the time the patients were hospitalized. The authors were confident that their results were not biased by the potential for other variants being included in the data.
Four coauthors reported receiving grants and personal fees from various sources.
A version of this article first appeared on WebMD.com.
a new study shows.
People who were hospitalized with Omicron COVID-19 infections were 54% more likely to die, compared with people who were hospitalized with the flu, Swiss researchers found.
The results of the study continue to debunk an earlier belief from the start of the pandemic that the flu was the more dangerous of the two respiratory viruses. The researchers noted that the deadliness of COVID-19, compared with flu, persisted “despite virus evolution and improved management strategies.”
The study was published in JAMA Network Open and included 5,212 patients in Switzerland hospitalized with COVID-19 or the flu. All the COVID patients were infected with the Omicron variant and hospitalized between Jan. 15, 2022, and March 15, 2022. Flu data included cases from January 2018 to March 15, 2022.
Overall, 7% of COVID-19 patients died, compared with 4.4% of flu patients. Researchers noted that the death rate for hospitalized COVID patients had declined since their previous study, which was conducted during the first COVID wave in the first half of 2020. At that time, the death rate of hospitalized COVID patients was 12.8%.
Since then, 98% of the Swiss population has been vaccinated. “Vaccination still plays a significant role regarding the main outcome,” the authors concluded, since a secondary analysis in this most recent study showed that unvaccinated COVID patients were twice as likely to die, compared with flu patients.
“Our results demonstrate that COVID-19 still cannot simply be compared with influenza,” they wrote.
While the death rate among COVID patients was significantly higher, there was no difference in the rate that COVID or flu patients were admitted to the ICU, which was around 8%.
A limitation of the study was that all the COVID cases did not have laboratory testing to confirm the Omicron variant. However, the study authors noted that Omicron accounted for at least 95% of cases during the time the patients were hospitalized. The authors were confident that their results were not biased by the potential for other variants being included in the data.
Four coauthors reported receiving grants and personal fees from various sources.
A version of this article first appeared on WebMD.com.
a new study shows.
People who were hospitalized with Omicron COVID-19 infections were 54% more likely to die, compared with people who were hospitalized with the flu, Swiss researchers found.
The results of the study continue to debunk an earlier belief from the start of the pandemic that the flu was the more dangerous of the two respiratory viruses. The researchers noted that the deadliness of COVID-19, compared with flu, persisted “despite virus evolution and improved management strategies.”
The study was published in JAMA Network Open and included 5,212 patients in Switzerland hospitalized with COVID-19 or the flu. All the COVID patients were infected with the Omicron variant and hospitalized between Jan. 15, 2022, and March 15, 2022. Flu data included cases from January 2018 to March 15, 2022.
Overall, 7% of COVID-19 patients died, compared with 4.4% of flu patients. Researchers noted that the death rate for hospitalized COVID patients had declined since their previous study, which was conducted during the first COVID wave in the first half of 2020. At that time, the death rate of hospitalized COVID patients was 12.8%.
Since then, 98% of the Swiss population has been vaccinated. “Vaccination still plays a significant role regarding the main outcome,” the authors concluded, since a secondary analysis in this most recent study showed that unvaccinated COVID patients were twice as likely to die, compared with flu patients.
“Our results demonstrate that COVID-19 still cannot simply be compared with influenza,” they wrote.
While the death rate among COVID patients was significantly higher, there was no difference in the rate that COVID or flu patients were admitted to the ICU, which was around 8%.
A limitation of the study was that all the COVID cases did not have laboratory testing to confirm the Omicron variant. However, the study authors noted that Omicron accounted for at least 95% of cases during the time the patients were hospitalized. The authors were confident that their results were not biased by the potential for other variants being included in the data.
Four coauthors reported receiving grants and personal fees from various sources.
A version of this article first appeared on WebMD.com.
FROM JAMA NETWORK OPEN
Pulmonary embolism workup needed for any sudden onset of exertional dyspnea
A diagnostic workup for pulmonary embolism (PE) should be performed in all patients with recent onset of exertional dyspnea, according to the authors of an article published in the Journal of Thrombosis and Haemostasis. That conclusion emerged from an analysis of PE prevalence in 417 patients with recent marked exertional dyspnea performing previously well-tolerated physical activities.
Exertional dyspnea is a frequently encountered complaint in clinical practice. Missteps in both diagnosis and early management, however, have been found to be prevalent in emergency department practices. with a complicated clinical course or mortality as a consequence, stated the researchers. Also, failure to diagnose PE is a common malpractice allegation.
Noting that the prevalence of PE among patients with dyspnea on exertion has not been reported, the authors hypothesized: “PE might be a frequent underlying condition in patients presenting for care complaining of marked dyspnea on exertion of recent onset.”
In a multicenter prospective, cross-sectional study among 14 university or hospital centers in Italy, patients who were referred for outpatient evaluation with recent (< 1 month) dyspnea on exertion with a severity of 3 or 4 on the modified Medical Research Council dyspnea scale were potentially eligible for the study. Prior deep-vein thrombosis (DVT), PE, and use of therapeutic anticoagulation were among exclusion criteria. All patients aged 75 years or younger with recent (< 1 month) marked exertional dyspnea had a systematic workup for PE, irrespective of concomitant signs or symptoms of venous thromboembolism and alternative explanations for dyspnea. The main study outcome was prevalence of PE in the entire cohort of patients with recent marked dyspnea on exertion.
When about 400 patients had been enrolled after an interim analysis in which the preestablished stopping rule (if the lower limit of the 95% confidence interval of the prevalence of PE exceeds 20%) was met, the study was prematurely terminated. PE was found, after exclusion of 134 patients based on low PE clinical probability and normal D-dimer, in 134 (47.3%) of the remaining 283 patients. The overall PE prevalence was 32.1% (95% confidence interval, 27.8-36.8).
PE was present in 40 of 204 (19.6%) patients without other findings suspicious for PE and in 94 of 213 patients (44.1%) with PE-suspicious findings. PE involved a main pulmonary artery in 37% and multiple lobes in 87% of the patients.
The researchers pointed out that, while the prevalence of PE was highest (44%) in patients who had concomitant signs or symptoms suspicious of PE or underlying DVT, PE was detected in almost 20% of patients without concomitant PE signs and symptoms. Also, the detected pulmonary emboli were deemed significant.
“Our findings suggest that, regardless of the diagnostic algorithm in use, physicians should rule in or out PE in patients who solely report recent onset of marked dyspnea on exertion,” they concluded.
Agreeing with the authors’ conclusions, Mary Jo S. Farmer, MD, PhD, of the department of medicine at University of Massachusetts, Worcester, stated in an interview, “The results of the current study support a diagnostic workup for pulmonary embolus in all patients with recent onset of exertional dyspnea.” She added, “Pulmonary emboli detected were significant as almost all were segmental or more proximal emboli involving multiple lobes. The observed overall prevalence of pulmonary embolus of 32% may seem high when compared with the low prevalence of 7%-13% reported in other studies of patients with suspected pulmonary embolus. However, the prevalence of pulmonary embolus among emergency department cohorts in European countries is generally higher, as is the diagnostic yield from [CT pulmonary angiogram] compared to North American countries. This could be explained by differences in applied thresholds for suspicion of pulmonary embolus. The incidence of COVID-19 and association with thrombosis was not reported.
“It has been reported that nonspecific clinical manifestations and absence of typical signs and symptoms can result in delay in diagnosis of pulmonary embolus or result in pulmonary embolus being missed, an unfortunate situation that could result in malpractice allegation.” Dr. Farmer concluded.
Among limitations of the study, the authors noted that their results are not applicable to patients older than 75 years or patients with chronic (more than 1 month) symptoms of dyspnea or less severe dyspnea (modified Medical Research Council dyspnea score of 2 or lower). Also, no attempt to stratify the clinical relevance of PE was made.
The study was funded by the Arianna Foundation on Anticoagulation, Bologna, Italy. The authors reported that they had no potential conflicts. Dr. Farmer also declared she had no relevant conflicts.
A diagnostic workup for pulmonary embolism (PE) should be performed in all patients with recent onset of exertional dyspnea, according to the authors of an article published in the Journal of Thrombosis and Haemostasis. That conclusion emerged from an analysis of PE prevalence in 417 patients with recent marked exertional dyspnea performing previously well-tolerated physical activities.
Exertional dyspnea is a frequently encountered complaint in clinical practice. Missteps in both diagnosis and early management, however, have been found to be prevalent in emergency department practices. with a complicated clinical course or mortality as a consequence, stated the researchers. Also, failure to diagnose PE is a common malpractice allegation.
Noting that the prevalence of PE among patients with dyspnea on exertion has not been reported, the authors hypothesized: “PE might be a frequent underlying condition in patients presenting for care complaining of marked dyspnea on exertion of recent onset.”
In a multicenter prospective, cross-sectional study among 14 university or hospital centers in Italy, patients who were referred for outpatient evaluation with recent (< 1 month) dyspnea on exertion with a severity of 3 or 4 on the modified Medical Research Council dyspnea scale were potentially eligible for the study. Prior deep-vein thrombosis (DVT), PE, and use of therapeutic anticoagulation were among exclusion criteria. All patients aged 75 years or younger with recent (< 1 month) marked exertional dyspnea had a systematic workup for PE, irrespective of concomitant signs or symptoms of venous thromboembolism and alternative explanations for dyspnea. The main study outcome was prevalence of PE in the entire cohort of patients with recent marked dyspnea on exertion.
When about 400 patients had been enrolled after an interim analysis in which the preestablished stopping rule (if the lower limit of the 95% confidence interval of the prevalence of PE exceeds 20%) was met, the study was prematurely terminated. PE was found, after exclusion of 134 patients based on low PE clinical probability and normal D-dimer, in 134 (47.3%) of the remaining 283 patients. The overall PE prevalence was 32.1% (95% confidence interval, 27.8-36.8).
PE was present in 40 of 204 (19.6%) patients without other findings suspicious for PE and in 94 of 213 patients (44.1%) with PE-suspicious findings. PE involved a main pulmonary artery in 37% and multiple lobes in 87% of the patients.
The researchers pointed out that, while the prevalence of PE was highest (44%) in patients who had concomitant signs or symptoms suspicious of PE or underlying DVT, PE was detected in almost 20% of patients without concomitant PE signs and symptoms. Also, the detected pulmonary emboli were deemed significant.
“Our findings suggest that, regardless of the diagnostic algorithm in use, physicians should rule in or out PE in patients who solely report recent onset of marked dyspnea on exertion,” they concluded.
Agreeing with the authors’ conclusions, Mary Jo S. Farmer, MD, PhD, of the department of medicine at University of Massachusetts, Worcester, stated in an interview, “The results of the current study support a diagnostic workup for pulmonary embolus in all patients with recent onset of exertional dyspnea.” She added, “Pulmonary emboli detected were significant as almost all were segmental or more proximal emboli involving multiple lobes. The observed overall prevalence of pulmonary embolus of 32% may seem high when compared with the low prevalence of 7%-13% reported in other studies of patients with suspected pulmonary embolus. However, the prevalence of pulmonary embolus among emergency department cohorts in European countries is generally higher, as is the diagnostic yield from [CT pulmonary angiogram] compared to North American countries. This could be explained by differences in applied thresholds for suspicion of pulmonary embolus. The incidence of COVID-19 and association with thrombosis was not reported.
“It has been reported that nonspecific clinical manifestations and absence of typical signs and symptoms can result in delay in diagnosis of pulmonary embolus or result in pulmonary embolus being missed, an unfortunate situation that could result in malpractice allegation.” Dr. Farmer concluded.
Among limitations of the study, the authors noted that their results are not applicable to patients older than 75 years or patients with chronic (more than 1 month) symptoms of dyspnea or less severe dyspnea (modified Medical Research Council dyspnea score of 2 or lower). Also, no attempt to stratify the clinical relevance of PE was made.
The study was funded by the Arianna Foundation on Anticoagulation, Bologna, Italy. The authors reported that they had no potential conflicts. Dr. Farmer also declared she had no relevant conflicts.
A diagnostic workup for pulmonary embolism (PE) should be performed in all patients with recent onset of exertional dyspnea, according to the authors of an article published in the Journal of Thrombosis and Haemostasis. That conclusion emerged from an analysis of PE prevalence in 417 patients with recent marked exertional dyspnea performing previously well-tolerated physical activities.
Exertional dyspnea is a frequently encountered complaint in clinical practice. Missteps in both diagnosis and early management, however, have been found to be prevalent in emergency department practices. with a complicated clinical course or mortality as a consequence, stated the researchers. Also, failure to diagnose PE is a common malpractice allegation.
Noting that the prevalence of PE among patients with dyspnea on exertion has not been reported, the authors hypothesized: “PE might be a frequent underlying condition in patients presenting for care complaining of marked dyspnea on exertion of recent onset.”
In a multicenter prospective, cross-sectional study among 14 university or hospital centers in Italy, patients who were referred for outpatient evaluation with recent (< 1 month) dyspnea on exertion with a severity of 3 or 4 on the modified Medical Research Council dyspnea scale were potentially eligible for the study. Prior deep-vein thrombosis (DVT), PE, and use of therapeutic anticoagulation were among exclusion criteria. All patients aged 75 years or younger with recent (< 1 month) marked exertional dyspnea had a systematic workup for PE, irrespective of concomitant signs or symptoms of venous thromboembolism and alternative explanations for dyspnea. The main study outcome was prevalence of PE in the entire cohort of patients with recent marked dyspnea on exertion.
When about 400 patients had been enrolled after an interim analysis in which the preestablished stopping rule (if the lower limit of the 95% confidence interval of the prevalence of PE exceeds 20%) was met, the study was prematurely terminated. PE was found, after exclusion of 134 patients based on low PE clinical probability and normal D-dimer, in 134 (47.3%) of the remaining 283 patients. The overall PE prevalence was 32.1% (95% confidence interval, 27.8-36.8).
PE was present in 40 of 204 (19.6%) patients without other findings suspicious for PE and in 94 of 213 patients (44.1%) with PE-suspicious findings. PE involved a main pulmonary artery in 37% and multiple lobes in 87% of the patients.
The researchers pointed out that, while the prevalence of PE was highest (44%) in patients who had concomitant signs or symptoms suspicious of PE or underlying DVT, PE was detected in almost 20% of patients without concomitant PE signs and symptoms. Also, the detected pulmonary emboli were deemed significant.
“Our findings suggest that, regardless of the diagnostic algorithm in use, physicians should rule in or out PE in patients who solely report recent onset of marked dyspnea on exertion,” they concluded.
Agreeing with the authors’ conclusions, Mary Jo S. Farmer, MD, PhD, of the department of medicine at University of Massachusetts, Worcester, stated in an interview, “The results of the current study support a diagnostic workup for pulmonary embolus in all patients with recent onset of exertional dyspnea.” She added, “Pulmonary emboli detected were significant as almost all were segmental or more proximal emboli involving multiple lobes. The observed overall prevalence of pulmonary embolus of 32% may seem high when compared with the low prevalence of 7%-13% reported in other studies of patients with suspected pulmonary embolus. However, the prevalence of pulmonary embolus among emergency department cohorts in European countries is generally higher, as is the diagnostic yield from [CT pulmonary angiogram] compared to North American countries. This could be explained by differences in applied thresholds for suspicion of pulmonary embolus. The incidence of COVID-19 and association with thrombosis was not reported.
“It has been reported that nonspecific clinical manifestations and absence of typical signs and symptoms can result in delay in diagnosis of pulmonary embolus or result in pulmonary embolus being missed, an unfortunate situation that could result in malpractice allegation.” Dr. Farmer concluded.
Among limitations of the study, the authors noted that their results are not applicable to patients older than 75 years or patients with chronic (more than 1 month) symptoms of dyspnea or less severe dyspnea (modified Medical Research Council dyspnea score of 2 or lower). Also, no attempt to stratify the clinical relevance of PE was made.
The study was funded by the Arianna Foundation on Anticoagulation, Bologna, Italy. The authors reported that they had no potential conflicts. Dr. Farmer also declared she had no relevant conflicts.
FROM THE JOURNAL OF THROMBOSIS AND HAEMOSTASIS
A doctor must go to extremes to save a choking victim
Some time ago I was invited to join a bipartisan congressional task force on valley fever, also known as coccidioidomycosis. A large and diverse crowd attended the task force’s first meeting in Bakersfield, Calif. – a meeting for everyone: the medical profession, the public, it even included veterinarians.
The whole thing was a resounding success. Francis Collins was there, the just-retired director of the NIH. Tom Frieden, then-director of the Centers for Disease Control and Prevention was there, as were several congresspeople and also my college roommate, a retired Navy medical corps captain. I was enjoying it.
Afterward, we had a banquet dinner at a restaurant in downtown Bakersfield. One of the people there was a woman I knew well – her husband was a physician friend. The restaurant served steak and salmon, and this woman made the mistake of ordering the steak.
Not long after the entrees were served, I heard a commotion at the table just behind me. I turned around and saw that woman in distress. A piece of steak had wedged in her trachea and she couldn’t breathe.
Almost immediately, the chef showed up. I don’t know how he got there. The chef at this restaurant was a big guy. I mean, probably 6 feet, 5 inches tall and 275 pounds. He tried the Heimlich maneuver. It didn’t work.
At that point, I jumped up. I thought, “Well, maybe I know how to do this better than him.” Probably not, actually. I tried and couldn’t make it work either. So I knew we were going to have to do something.
Paul Krogstad, my friend and research partner who is a pediatric infectious disease physician, stepped up and tried to put his finger in her throat and dig it out. He couldn’t get it. The patient had lost consciousness.
So, I’m thinking, okay, there’s really only one choice. You have to get an airway surgically.
I said, “We have to put her down on the floor.” And then I said, “Knife!”
I was looking at the steak knives on the table and they weren’t to my liking for doing a procedure. My college roommate – the retired Navy man – whipped out this very good pocketknife.
I had never done this in my life.
While I was making the incision, somebody gave Paul a ballpoint pen and he broke it into pieces to make a tracheostomy tube. Once I’d made the little incision, I put the tube in. She wasn’t breathing, but she still had a pulse.
I leaned forward and blew into the tube and inflated her lungs. I could see her lungs balloon up. It was a nice feeling, because I knew I was clearly in the right place.
I can’t quite explain it, but while I was doing this, I was enormously calm and totally focused. I knew there was a crowd of people around me, all looking at me, but I wasn’t conscious of that.
It was really just the four of us: Paul and Tom and me and our patient. Those were the only people that I was really cognizant of. Paul and Tom were not panic stricken at all. I remember somebody shouting, “We have to start CPR!” and Frieden said, “No. We don’t.”
Moments later, she woke up, sat up, coughed, and shot the piece of steak across the room.
She was breathing on her own, but we still taped that tube into place. Somebody had already summoned an ambulance; they were there not very long after we completed this procedure. I got in the ambulance with her and we rode over to the emergency room at Mercy Truxtun.
She was stable and doing okay. I sat with her until a thoracic surgeon showed up. He checked out the situation and decided we didn’t need that tube and took it out. I didn’t want to take that out until I had a surgeon there who could do a formal tracheostomy.
They kept her in the hospital for 3 or 4 days. Now, this woman had always had difficulties swallowing, so steak may not have been the best choice. She still had trouble swallowing afterward but recovered.
I’ve known her and her husband a long time, so it was certainly rewarding to be able to provide this service. Years later, though, when her husband died, I spoke at his funeral. When she was speaking to the gathering, she said, “And oh, by the way, Royce, thanks for saving my life.”
That surprised me. I didn’t think we were going to go there.
I’d never tried to practice medicine “at the roadside” before. But that’s part of the career.
Royce Johnson, MD, is the chief of the division of infectious disease among other leadership positions at Kern Medical in Bakersfield, Calif., and the medical director of the Valley Fever Institute.
A version of this article first appeared on Medscape.com.
Some time ago I was invited to join a bipartisan congressional task force on valley fever, also known as coccidioidomycosis. A large and diverse crowd attended the task force’s first meeting in Bakersfield, Calif. – a meeting for everyone: the medical profession, the public, it even included veterinarians.
The whole thing was a resounding success. Francis Collins was there, the just-retired director of the NIH. Tom Frieden, then-director of the Centers for Disease Control and Prevention was there, as were several congresspeople and also my college roommate, a retired Navy medical corps captain. I was enjoying it.
Afterward, we had a banquet dinner at a restaurant in downtown Bakersfield. One of the people there was a woman I knew well – her husband was a physician friend. The restaurant served steak and salmon, and this woman made the mistake of ordering the steak.
Not long after the entrees were served, I heard a commotion at the table just behind me. I turned around and saw that woman in distress. A piece of steak had wedged in her trachea and she couldn’t breathe.
Almost immediately, the chef showed up. I don’t know how he got there. The chef at this restaurant was a big guy. I mean, probably 6 feet, 5 inches tall and 275 pounds. He tried the Heimlich maneuver. It didn’t work.
At that point, I jumped up. I thought, “Well, maybe I know how to do this better than him.” Probably not, actually. I tried and couldn’t make it work either. So I knew we were going to have to do something.
Paul Krogstad, my friend and research partner who is a pediatric infectious disease physician, stepped up and tried to put his finger in her throat and dig it out. He couldn’t get it. The patient had lost consciousness.
So, I’m thinking, okay, there’s really only one choice. You have to get an airway surgically.
I said, “We have to put her down on the floor.” And then I said, “Knife!”
I was looking at the steak knives on the table and they weren’t to my liking for doing a procedure. My college roommate – the retired Navy man – whipped out this very good pocketknife.
I had never done this in my life.
While I was making the incision, somebody gave Paul a ballpoint pen and he broke it into pieces to make a tracheostomy tube. Once I’d made the little incision, I put the tube in. She wasn’t breathing, but she still had a pulse.
I leaned forward and blew into the tube and inflated her lungs. I could see her lungs balloon up. It was a nice feeling, because I knew I was clearly in the right place.
I can’t quite explain it, but while I was doing this, I was enormously calm and totally focused. I knew there was a crowd of people around me, all looking at me, but I wasn’t conscious of that.
It was really just the four of us: Paul and Tom and me and our patient. Those were the only people that I was really cognizant of. Paul and Tom were not panic stricken at all. I remember somebody shouting, “We have to start CPR!” and Frieden said, “No. We don’t.”
Moments later, she woke up, sat up, coughed, and shot the piece of steak across the room.
She was breathing on her own, but we still taped that tube into place. Somebody had already summoned an ambulance; they were there not very long after we completed this procedure. I got in the ambulance with her and we rode over to the emergency room at Mercy Truxtun.
She was stable and doing okay. I sat with her until a thoracic surgeon showed up. He checked out the situation and decided we didn’t need that tube and took it out. I didn’t want to take that out until I had a surgeon there who could do a formal tracheostomy.
They kept her in the hospital for 3 or 4 days. Now, this woman had always had difficulties swallowing, so steak may not have been the best choice. She still had trouble swallowing afterward but recovered.
I’ve known her and her husband a long time, so it was certainly rewarding to be able to provide this service. Years later, though, when her husband died, I spoke at his funeral. When she was speaking to the gathering, she said, “And oh, by the way, Royce, thanks for saving my life.”
That surprised me. I didn’t think we were going to go there.
I’d never tried to practice medicine “at the roadside” before. But that’s part of the career.
Royce Johnson, MD, is the chief of the division of infectious disease among other leadership positions at Kern Medical in Bakersfield, Calif., and the medical director of the Valley Fever Institute.
A version of this article first appeared on Medscape.com.
Some time ago I was invited to join a bipartisan congressional task force on valley fever, also known as coccidioidomycosis. A large and diverse crowd attended the task force’s first meeting in Bakersfield, Calif. – a meeting for everyone: the medical profession, the public, it even included veterinarians.
The whole thing was a resounding success. Francis Collins was there, the just-retired director of the NIH. Tom Frieden, then-director of the Centers for Disease Control and Prevention was there, as were several congresspeople and also my college roommate, a retired Navy medical corps captain. I was enjoying it.
Afterward, we had a banquet dinner at a restaurant in downtown Bakersfield. One of the people there was a woman I knew well – her husband was a physician friend. The restaurant served steak and salmon, and this woman made the mistake of ordering the steak.
Not long after the entrees were served, I heard a commotion at the table just behind me. I turned around and saw that woman in distress. A piece of steak had wedged in her trachea and she couldn’t breathe.
Almost immediately, the chef showed up. I don’t know how he got there. The chef at this restaurant was a big guy. I mean, probably 6 feet, 5 inches tall and 275 pounds. He tried the Heimlich maneuver. It didn’t work.
At that point, I jumped up. I thought, “Well, maybe I know how to do this better than him.” Probably not, actually. I tried and couldn’t make it work either. So I knew we were going to have to do something.
Paul Krogstad, my friend and research partner who is a pediatric infectious disease physician, stepped up and tried to put his finger in her throat and dig it out. He couldn’t get it. The patient had lost consciousness.
So, I’m thinking, okay, there’s really only one choice. You have to get an airway surgically.
I said, “We have to put her down on the floor.” And then I said, “Knife!”
I was looking at the steak knives on the table and they weren’t to my liking for doing a procedure. My college roommate – the retired Navy man – whipped out this very good pocketknife.
I had never done this in my life.
While I was making the incision, somebody gave Paul a ballpoint pen and he broke it into pieces to make a tracheostomy tube. Once I’d made the little incision, I put the tube in. She wasn’t breathing, but she still had a pulse.
I leaned forward and blew into the tube and inflated her lungs. I could see her lungs balloon up. It was a nice feeling, because I knew I was clearly in the right place.
I can’t quite explain it, but while I was doing this, I was enormously calm and totally focused. I knew there was a crowd of people around me, all looking at me, but I wasn’t conscious of that.
It was really just the four of us: Paul and Tom and me and our patient. Those were the only people that I was really cognizant of. Paul and Tom were not panic stricken at all. I remember somebody shouting, “We have to start CPR!” and Frieden said, “No. We don’t.”
Moments later, she woke up, sat up, coughed, and shot the piece of steak across the room.
She was breathing on her own, but we still taped that tube into place. Somebody had already summoned an ambulance; they were there not very long after we completed this procedure. I got in the ambulance with her and we rode over to the emergency room at Mercy Truxtun.
She was stable and doing okay. I sat with her until a thoracic surgeon showed up. He checked out the situation and decided we didn’t need that tube and took it out. I didn’t want to take that out until I had a surgeon there who could do a formal tracheostomy.
They kept her in the hospital for 3 or 4 days. Now, this woman had always had difficulties swallowing, so steak may not have been the best choice. She still had trouble swallowing afterward but recovered.
I’ve known her and her husband a long time, so it was certainly rewarding to be able to provide this service. Years later, though, when her husband died, I spoke at his funeral. When she was speaking to the gathering, she said, “And oh, by the way, Royce, thanks for saving my life.”
That surprised me. I didn’t think we were going to go there.
I’d never tried to practice medicine “at the roadside” before. But that’s part of the career.
Royce Johnson, MD, is the chief of the division of infectious disease among other leadership positions at Kern Medical in Bakersfield, Calif., and the medical director of the Valley Fever Institute.
A version of this article first appeared on Medscape.com.
Doc never met patient who died from insect bite, but negligence suit moves forward; more
On-call specialist incurred a clear ‘duty of care,’ court rules
a state appeals court ruled late in January.
The appeals decision is the result of a case involving the late Dennis Blagden.
On July 26, 2017, Mr. Blagden arrived at the Graham Hospital ED, in Canton, Ill., complaining of neck pain and an insect bite that had resulted in a swollen elbow. His ED doctor, Matthew McMillin, MD, who worked for Coleman Medical Associates, ordered tests and prescribed an anti-inflammatory pain medication and a muscle relaxant.
Dr. McMillin consulted via telephone with Kenneth Krock, MD, an internal medicine specialist and pediatrician, who was on call that day and who enjoyed admitting privileges at Graham. (Krock was also an employee of Coleman Medical Associates, which provided clinical staffing for the hospital.)
Dr. Krock had final admitting authority in this instance. Court records show that Dr. McMillin and he agreed that the patient could be discharged from the ED, despite Krock’s differential diagnosis indicating a possible infection.
Three days later, now with “hypercapnic respiratory failure, sepsis, and an altered mental state,” Mr. Blagden was again seen at the Graham Hospital ED. Mr. Blagden underwent intubation by Dr. McMillin, his original ED doctor, and was airlifted to Methodist Medical Center, in Peoria, 30 miles away. There, an MRI showed that he’d developed a spinal epidural abscess. On Aug. 7, 2017, a little over a week after his admission to Methodist, Mr. Blagden died from complications of his infection.
In January 2019, Mr. Blagden’s wife, Judy, filed a suit against Dr. McMillin, his practice, and Graham Hospital, which is a part of Graham Health System. Her suit alleged medical negligence in the death of her husband.
About 6 months later, Mr.s Blagden amended her original complaint, adding a second count of medical negligence against Dr. Krock; his practice and employer, Coleman Medical Associates; and Graham Hospital. In her amended complaint, Mrs. Blagden alleged that although Krock hadn’t actually seen her husband Dennis, his consultation with Dr. McMillin was sufficient to establish a doctor-patient relationship and thus a legal duty of care. That duty, Mrs. Blagden further alleged, was breached when Dr. Krock failed both to rule out her husband’s “infectious process” and to admit him for proper follow-up monitoring.
In July 2021, after the case had been transferred from Peoria County to Fulton County, Dr. Krock cried foul. In a motion to the court for summary judgment – that is, a ruling prior to an actual trial – he and his practice put forth the following argument: As a mere on-call consultant that day in 2017, he had neither seen the patient nor established a relationship with him, thereby precluding his legal duty of care.
The trial court judge agreed and granted both Dr. Krock and Dr. Coleman the summary judgment they had sought.
Mrs. Blagden then appealed to the Appellate Court of Illinois, Fourth District, which is located in Springfield.
In its unanimous decision, the three-judge panel reversed the lower court’s ruling. Taking direct aim at Dr. Krock’s earlier motion, Justice Eugene Doherty, who wrote the panel’s opinion, said that state law had long established that “the special relationship giving rise to a duty of care may exist even in the absence of any meeting between the physician and the patient where the physician performs specific services for the benefit of the patient.”
As Justice Doherty explained, Dr. Krock’s status that day as both the on-call doctor and the one with final admitting authority undermined his argument for summary judgment. Also undermining it, Justice Doherty added, was the fact that the conversation between the two doctors that day in 2017 was a formal exchange “contemplated by hospital bylaws.”
“While public policy should encourage informal consultations between physicians,” the justice continued, “it must not ignore actual physician involvement in decisions that directly affect a patient’s care.”
Following the Fourth District decision, the suit against Dr. McMillin, Dr. Krock, and the other defendants has now been tossed back to the trial court for further proceedings. At press time, no trial date had been set.
Will this proposed damages cap help retain more physicians?
Fear of a doctor shortage, triggered in part by a recent history of large payouts, has prompted Iowa lawmakers to push for new state caps on medical malpractice awards, as a story in the Des Moines Register reports.
Currently, Iowa caps most noneconomic damages – including those for pain and suffering – at $250,000, which is among the lowest such caps in the nation.
Under existing Iowa law, however, the limit doesn’t apply in extraordinary cases – that is, those involving “substantial or permanent loss of body function, substantial disfigurement, or death.” It also isn’t applicable in cases in which a jury decides that a defendant acted with intentional malice.
Lawmakers and Iowa Gov. Kim Reynolds would like to change this.
Under a Senate bill that has now passed out of committee and is awaiting debate on the Senate floor, even plaintiffs involved in extreme cases would receive no more than $1 million to compensate for their pain, suffering, or emotional distress. (The bill also includes a 2.1% annual hike to compensate for inflation. A similar bill, which adds “loss of pregnancy” to the list of extreme cases, has advanced to the House floor.)
Supporters say the proposed cap would help to limit mega awards. In Johnson County in March 2022, for instance, a jury awarded $97.4 million to the parents of a young boy who sustained severe brain injuries during his delivery, causing the clinic that had been involved in the case to file for bankruptcy. This award was nearly three times the total payouts ($35 million) in the entire state of Iowa in all of 2021, a year in which there were 192 closed claims, including at least a dozen that resulted in payouts of $1 million or more.
Supporters also think the proposed cap will mitigate what they see as a looming doctor shortage, especially among ob.gyns. in eastern Iowa. “I just cannot overstate how much this is affecting our workforce, and that turns into effects for the women and the children, the babies, in our state,” Shannon Leveridge, MD, an obstetrician in Davenport said. “In order to keep these women and their babies safe, we need doctors.”
But critics of the bill, including some lawmakers and the trial bar, say it overreaches, even in the case of the $97.4 million award.
“They don’t want to talk about the actual damages that are caused by medical negligence,” explained a spokesman for the trial lawyers. “So, you don’t hear about the fact that, of the $50 million of economic damages ... most of that is going to go to the 24/7 care for this child for the rest of his life.”
A version of this article first appeared on Medscape.com.
On-call specialist incurred a clear ‘duty of care,’ court rules
a state appeals court ruled late in January.
The appeals decision is the result of a case involving the late Dennis Blagden.
On July 26, 2017, Mr. Blagden arrived at the Graham Hospital ED, in Canton, Ill., complaining of neck pain and an insect bite that had resulted in a swollen elbow. His ED doctor, Matthew McMillin, MD, who worked for Coleman Medical Associates, ordered tests and prescribed an anti-inflammatory pain medication and a muscle relaxant.
Dr. McMillin consulted via telephone with Kenneth Krock, MD, an internal medicine specialist and pediatrician, who was on call that day and who enjoyed admitting privileges at Graham. (Krock was also an employee of Coleman Medical Associates, which provided clinical staffing for the hospital.)
Dr. Krock had final admitting authority in this instance. Court records show that Dr. McMillin and he agreed that the patient could be discharged from the ED, despite Krock’s differential diagnosis indicating a possible infection.
Three days later, now with “hypercapnic respiratory failure, sepsis, and an altered mental state,” Mr. Blagden was again seen at the Graham Hospital ED. Mr. Blagden underwent intubation by Dr. McMillin, his original ED doctor, and was airlifted to Methodist Medical Center, in Peoria, 30 miles away. There, an MRI showed that he’d developed a spinal epidural abscess. On Aug. 7, 2017, a little over a week after his admission to Methodist, Mr. Blagden died from complications of his infection.
In January 2019, Mr. Blagden’s wife, Judy, filed a suit against Dr. McMillin, his practice, and Graham Hospital, which is a part of Graham Health System. Her suit alleged medical negligence in the death of her husband.
About 6 months later, Mr.s Blagden amended her original complaint, adding a second count of medical negligence against Dr. Krock; his practice and employer, Coleman Medical Associates; and Graham Hospital. In her amended complaint, Mrs. Blagden alleged that although Krock hadn’t actually seen her husband Dennis, his consultation with Dr. McMillin was sufficient to establish a doctor-patient relationship and thus a legal duty of care. That duty, Mrs. Blagden further alleged, was breached when Dr. Krock failed both to rule out her husband’s “infectious process” and to admit him for proper follow-up monitoring.
In July 2021, after the case had been transferred from Peoria County to Fulton County, Dr. Krock cried foul. In a motion to the court for summary judgment – that is, a ruling prior to an actual trial – he and his practice put forth the following argument: As a mere on-call consultant that day in 2017, he had neither seen the patient nor established a relationship with him, thereby precluding his legal duty of care.
The trial court judge agreed and granted both Dr. Krock and Dr. Coleman the summary judgment they had sought.
Mrs. Blagden then appealed to the Appellate Court of Illinois, Fourth District, which is located in Springfield.
In its unanimous decision, the three-judge panel reversed the lower court’s ruling. Taking direct aim at Dr. Krock’s earlier motion, Justice Eugene Doherty, who wrote the panel’s opinion, said that state law had long established that “the special relationship giving rise to a duty of care may exist even in the absence of any meeting between the physician and the patient where the physician performs specific services for the benefit of the patient.”
As Justice Doherty explained, Dr. Krock’s status that day as both the on-call doctor and the one with final admitting authority undermined his argument for summary judgment. Also undermining it, Justice Doherty added, was the fact that the conversation between the two doctors that day in 2017 was a formal exchange “contemplated by hospital bylaws.”
“While public policy should encourage informal consultations between physicians,” the justice continued, “it must not ignore actual physician involvement in decisions that directly affect a patient’s care.”
Following the Fourth District decision, the suit against Dr. McMillin, Dr. Krock, and the other defendants has now been tossed back to the trial court for further proceedings. At press time, no trial date had been set.
Will this proposed damages cap help retain more physicians?
Fear of a doctor shortage, triggered in part by a recent history of large payouts, has prompted Iowa lawmakers to push for new state caps on medical malpractice awards, as a story in the Des Moines Register reports.
Currently, Iowa caps most noneconomic damages – including those for pain and suffering – at $250,000, which is among the lowest such caps in the nation.
Under existing Iowa law, however, the limit doesn’t apply in extraordinary cases – that is, those involving “substantial or permanent loss of body function, substantial disfigurement, or death.” It also isn’t applicable in cases in which a jury decides that a defendant acted with intentional malice.
Lawmakers and Iowa Gov. Kim Reynolds would like to change this.
Under a Senate bill that has now passed out of committee and is awaiting debate on the Senate floor, even plaintiffs involved in extreme cases would receive no more than $1 million to compensate for their pain, suffering, or emotional distress. (The bill also includes a 2.1% annual hike to compensate for inflation. A similar bill, which adds “loss of pregnancy” to the list of extreme cases, has advanced to the House floor.)
Supporters say the proposed cap would help to limit mega awards. In Johnson County in March 2022, for instance, a jury awarded $97.4 million to the parents of a young boy who sustained severe brain injuries during his delivery, causing the clinic that had been involved in the case to file for bankruptcy. This award was nearly three times the total payouts ($35 million) in the entire state of Iowa in all of 2021, a year in which there were 192 closed claims, including at least a dozen that resulted in payouts of $1 million or more.
Supporters also think the proposed cap will mitigate what they see as a looming doctor shortage, especially among ob.gyns. in eastern Iowa. “I just cannot overstate how much this is affecting our workforce, and that turns into effects for the women and the children, the babies, in our state,” Shannon Leveridge, MD, an obstetrician in Davenport said. “In order to keep these women and their babies safe, we need doctors.”
But critics of the bill, including some lawmakers and the trial bar, say it overreaches, even in the case of the $97.4 million award.
“They don’t want to talk about the actual damages that are caused by medical negligence,” explained a spokesman for the trial lawyers. “So, you don’t hear about the fact that, of the $50 million of economic damages ... most of that is going to go to the 24/7 care for this child for the rest of his life.”
A version of this article first appeared on Medscape.com.
On-call specialist incurred a clear ‘duty of care,’ court rules
a state appeals court ruled late in January.
The appeals decision is the result of a case involving the late Dennis Blagden.
On July 26, 2017, Mr. Blagden arrived at the Graham Hospital ED, in Canton, Ill., complaining of neck pain and an insect bite that had resulted in a swollen elbow. His ED doctor, Matthew McMillin, MD, who worked for Coleman Medical Associates, ordered tests and prescribed an anti-inflammatory pain medication and a muscle relaxant.
Dr. McMillin consulted via telephone with Kenneth Krock, MD, an internal medicine specialist and pediatrician, who was on call that day and who enjoyed admitting privileges at Graham. (Krock was also an employee of Coleman Medical Associates, which provided clinical staffing for the hospital.)
Dr. Krock had final admitting authority in this instance. Court records show that Dr. McMillin and he agreed that the patient could be discharged from the ED, despite Krock’s differential diagnosis indicating a possible infection.
Three days later, now with “hypercapnic respiratory failure, sepsis, and an altered mental state,” Mr. Blagden was again seen at the Graham Hospital ED. Mr. Blagden underwent intubation by Dr. McMillin, his original ED doctor, and was airlifted to Methodist Medical Center, in Peoria, 30 miles away. There, an MRI showed that he’d developed a spinal epidural abscess. On Aug. 7, 2017, a little over a week after his admission to Methodist, Mr. Blagden died from complications of his infection.
In January 2019, Mr. Blagden’s wife, Judy, filed a suit against Dr. McMillin, his practice, and Graham Hospital, which is a part of Graham Health System. Her suit alleged medical negligence in the death of her husband.
About 6 months later, Mr.s Blagden amended her original complaint, adding a second count of medical negligence against Dr. Krock; his practice and employer, Coleman Medical Associates; and Graham Hospital. In her amended complaint, Mrs. Blagden alleged that although Krock hadn’t actually seen her husband Dennis, his consultation with Dr. McMillin was sufficient to establish a doctor-patient relationship and thus a legal duty of care. That duty, Mrs. Blagden further alleged, was breached when Dr. Krock failed both to rule out her husband’s “infectious process” and to admit him for proper follow-up monitoring.
In July 2021, after the case had been transferred from Peoria County to Fulton County, Dr. Krock cried foul. In a motion to the court for summary judgment – that is, a ruling prior to an actual trial – he and his practice put forth the following argument: As a mere on-call consultant that day in 2017, he had neither seen the patient nor established a relationship with him, thereby precluding his legal duty of care.
The trial court judge agreed and granted both Dr. Krock and Dr. Coleman the summary judgment they had sought.
Mrs. Blagden then appealed to the Appellate Court of Illinois, Fourth District, which is located in Springfield.
In its unanimous decision, the three-judge panel reversed the lower court’s ruling. Taking direct aim at Dr. Krock’s earlier motion, Justice Eugene Doherty, who wrote the panel’s opinion, said that state law had long established that “the special relationship giving rise to a duty of care may exist even in the absence of any meeting between the physician and the patient where the physician performs specific services for the benefit of the patient.”
As Justice Doherty explained, Dr. Krock’s status that day as both the on-call doctor and the one with final admitting authority undermined his argument for summary judgment. Also undermining it, Justice Doherty added, was the fact that the conversation between the two doctors that day in 2017 was a formal exchange “contemplated by hospital bylaws.”
“While public policy should encourage informal consultations between physicians,” the justice continued, “it must not ignore actual physician involvement in decisions that directly affect a patient’s care.”
Following the Fourth District decision, the suit against Dr. McMillin, Dr. Krock, and the other defendants has now been tossed back to the trial court for further proceedings. At press time, no trial date had been set.
Will this proposed damages cap help retain more physicians?
Fear of a doctor shortage, triggered in part by a recent history of large payouts, has prompted Iowa lawmakers to push for new state caps on medical malpractice awards, as a story in the Des Moines Register reports.
Currently, Iowa caps most noneconomic damages – including those for pain and suffering – at $250,000, which is among the lowest such caps in the nation.
Under existing Iowa law, however, the limit doesn’t apply in extraordinary cases – that is, those involving “substantial or permanent loss of body function, substantial disfigurement, or death.” It also isn’t applicable in cases in which a jury decides that a defendant acted with intentional malice.
Lawmakers and Iowa Gov. Kim Reynolds would like to change this.
Under a Senate bill that has now passed out of committee and is awaiting debate on the Senate floor, even plaintiffs involved in extreme cases would receive no more than $1 million to compensate for their pain, suffering, or emotional distress. (The bill also includes a 2.1% annual hike to compensate for inflation. A similar bill, which adds “loss of pregnancy” to the list of extreme cases, has advanced to the House floor.)
Supporters say the proposed cap would help to limit mega awards. In Johnson County in March 2022, for instance, a jury awarded $97.4 million to the parents of a young boy who sustained severe brain injuries during his delivery, causing the clinic that had been involved in the case to file for bankruptcy. This award was nearly three times the total payouts ($35 million) in the entire state of Iowa in all of 2021, a year in which there were 192 closed claims, including at least a dozen that resulted in payouts of $1 million or more.
Supporters also think the proposed cap will mitigate what they see as a looming doctor shortage, especially among ob.gyns. in eastern Iowa. “I just cannot overstate how much this is affecting our workforce, and that turns into effects for the women and the children, the babies, in our state,” Shannon Leveridge, MD, an obstetrician in Davenport said. “In order to keep these women and their babies safe, we need doctors.”
But critics of the bill, including some lawmakers and the trial bar, say it overreaches, even in the case of the $97.4 million award.
“They don’t want to talk about the actual damages that are caused by medical negligence,” explained a spokesman for the trial lawyers. “So, you don’t hear about the fact that, of the $50 million of economic damages ... most of that is going to go to the 24/7 care for this child for the rest of his life.”
A version of this article first appeared on Medscape.com.
Management strategies for patients with COVID-19 pneumonia/ARDS
Since the first SARS-CoV-2 (COVID-19) outbreak in Wuhan, China, in December 2019, more than 6.6 million deaths have occurred. . One of the strategies for those cases refractory to traditional ARDS treatments has been the use of extracorporeal membrane oxygenation (ECMO).
Before the COVID-19 pandemic, a substantial amount of data regarding the use of ECMO in ARDS was gathered during the H1N1 influenza outbreak in 2009. Mortality ranged from 8% to 65% (Zangrillo, et al. Crit Care. 2013;17[1]:R30). From these data, we learned the importance of patient selection. Young patients with few co-morbidities and less than 7 days supported by mechanical ventilation did remarkably better than elderly patients or those who had prolonged positive-pressure ventilation prior to ECMO.
To date, the mortality rate for COVID-19 patients with ARDS requiring ECMO is 48% based on data from ELSO. Interestingly though, using May 1, 2020, as a cutoff date, mortality rates for patients with COVID-19 receiving ECMO significantly increased from 37% to 52% (Barbaro, et al. Lancet. 2021;398[10307]:1230). This escalation in mortality engendered concern that ECMO may not be useful in treating patients with COVID-19 and ARDS.
Several factors can be cited for this increase in mortality. First, many new ECMO programs launched after May 1. These new programs had a higher mortality rate (59%) compared with established programs, suggesting that program and provider experience play a significant role in patient outcomes (Barbaro, et al. Lancet. 2021;398[10307]:1230). Second, patients in the latter part of 2020 experienced much longer intervals between the onset of symptoms and time of intubation. Clinicians had a tendency to delay intubation as long as possible. Subsequently, the number of days receiving high flow nasal oxygen or noninvasive ventilation (NIV) was significantly longer (Schmidt, et al. Crit Care. 2021;25[1]:355). These data suggest that prolonged NIV on high Fio2 may be a negative prognostic indicator and should be considered when assessing a patient’s candidacy for ECMO.
Early in the pandemic, clinicians realized that average ECMO run times for patients with COVID-19 and ARDS were significantly longer, 15 vs 9 days, respectively (Jacobs, et al. Ann Thorac Surg. 2022;113[5]:1452). With such long run times, beds were slow to turn over, and a shortage of ECMO beds resulted during the height of the pandemic. In a retrospective study, Gannon looked at 90 patients, all of whom were deemed medically appropriate for ECMO. Two groups were created: (1) no capacity for ECMO vs (2) ECMO provided. Mortality rates were staggering at 89% and 43%, respectively (P =.001) (Gannon, et al. Am J Respir Crit Care Med. 2022;205[11]:1354). This study demonstrated a profound point: during a pandemic, when demand overcomes supply, there is a unique opportunity to see the effect of lifesaving therapies, such as ECMO, on outcomes. This study was particularly poignant, as the average age of the patients was 40 years old.
It is now widely accepted that prone positioning has survival benefit in ARDS. Prone positioning while receiving ECMO has generally been avoided due to concern for potential complications associated with the cannula(s). However, it has been shown that prone positioning while receiving veno-venous (VV) -ECMO reduces mortality rates, 37% proned vs 50% supine positioning (P =.02) (Giani, et al. Ann Am Thorac Soc. 2021;18[3]:495). In this study, no major complications occurred, and minor complications occurred in 6% of the proning events. Prone positioning improves ventilation-perfusion mismatch and reduces hypoxic vasoconstriction, which is thought to be right-sided heart-protective.
Right-sided heart dysfunction (RHD) is common in ARDS, whether COVID-19-related or not. The pathogenesis includes hypoxic vasoconstriction, pulmonary fibrosis, and ventilator-induced lung injury. Pulmonary microthrombi and patient-specific characteristics, such as obesity, are additional factors leading to RHD in patients with COVID-19. During the pandemic, several articles described using right-sided heart protective cannulation strategies for patients with COVID-19 requiring ECMO with favorable results (Mustafa, et al. JAMA Surg. 2020;155[10]:990; Cain, et al. J Surg Res. 2021;264:81-89). This right-sided heart protective strategy involves inserting a single access dual lumen cannula into the right internal jugular vein, which is advanced into the pulmonary artery, effectively bypassing the right ventricle. This setup is more typical of right ventricle assist device (RVAD), rather than typical VV-ECMO, which returns blood to the right atrium. Unfortunately, these studies did not include echocardiographic information to evaluate the effects of this intervention on RVD, and this is an area for future research. However, this vein to pulmonary artery strategy was found to facilitate decreased sedation, earlier liberation from mechanical ventilation, reduced need for tracheostomy, improved mobilization out of bed, and ease in prone positioning (Mustafa, et al. JAMA Surg. 2020;155[10]:990).
In conclusion, there is evidence to support the use of ECMO in patients with COVID-19 patients and ARDS failing conventional mechanical ventilation. The success of ECMO therapy is highly dependent on patient selection. Prolonged use of NIV on high Fio2 may be a negative predictor of ECMO survival and should be considered when assessing a patient for ECMO candidacy. Prone positioning with ECMO has been shown to have survival benefit and should be considered in all patients receiving ECMO.
Dr. Gaillard, Dr. Staples, and Dr. Kapoor are with the Department of Anesthesiology, Section on Critical Care, at Wake Forest School of Medicine in Winston-Salem, N.C. Dr. Gaillard is also with the Department of Emergency Medicine and Department of Internal Medicine, Section on Pulmonary, Critical Care, Allergy, and Immunology at Wake Forest School of Medicine.
Since the first SARS-CoV-2 (COVID-19) outbreak in Wuhan, China, in December 2019, more than 6.6 million deaths have occurred. . One of the strategies for those cases refractory to traditional ARDS treatments has been the use of extracorporeal membrane oxygenation (ECMO).
Before the COVID-19 pandemic, a substantial amount of data regarding the use of ECMO in ARDS was gathered during the H1N1 influenza outbreak in 2009. Mortality ranged from 8% to 65% (Zangrillo, et al. Crit Care. 2013;17[1]:R30). From these data, we learned the importance of patient selection. Young patients with few co-morbidities and less than 7 days supported by mechanical ventilation did remarkably better than elderly patients or those who had prolonged positive-pressure ventilation prior to ECMO.
To date, the mortality rate for COVID-19 patients with ARDS requiring ECMO is 48% based on data from ELSO. Interestingly though, using May 1, 2020, as a cutoff date, mortality rates for patients with COVID-19 receiving ECMO significantly increased from 37% to 52% (Barbaro, et al. Lancet. 2021;398[10307]:1230). This escalation in mortality engendered concern that ECMO may not be useful in treating patients with COVID-19 and ARDS.
Several factors can be cited for this increase in mortality. First, many new ECMO programs launched after May 1. These new programs had a higher mortality rate (59%) compared with established programs, suggesting that program and provider experience play a significant role in patient outcomes (Barbaro, et al. Lancet. 2021;398[10307]:1230). Second, patients in the latter part of 2020 experienced much longer intervals between the onset of symptoms and time of intubation. Clinicians had a tendency to delay intubation as long as possible. Subsequently, the number of days receiving high flow nasal oxygen or noninvasive ventilation (NIV) was significantly longer (Schmidt, et al. Crit Care. 2021;25[1]:355). These data suggest that prolonged NIV on high Fio2 may be a negative prognostic indicator and should be considered when assessing a patient’s candidacy for ECMO.
Early in the pandemic, clinicians realized that average ECMO run times for patients with COVID-19 and ARDS were significantly longer, 15 vs 9 days, respectively (Jacobs, et al. Ann Thorac Surg. 2022;113[5]:1452). With such long run times, beds were slow to turn over, and a shortage of ECMO beds resulted during the height of the pandemic. In a retrospective study, Gannon looked at 90 patients, all of whom were deemed medically appropriate for ECMO. Two groups were created: (1) no capacity for ECMO vs (2) ECMO provided. Mortality rates were staggering at 89% and 43%, respectively (P =.001) (Gannon, et al. Am J Respir Crit Care Med. 2022;205[11]:1354). This study demonstrated a profound point: during a pandemic, when demand overcomes supply, there is a unique opportunity to see the effect of lifesaving therapies, such as ECMO, on outcomes. This study was particularly poignant, as the average age of the patients was 40 years old.
It is now widely accepted that prone positioning has survival benefit in ARDS. Prone positioning while receiving ECMO has generally been avoided due to concern for potential complications associated with the cannula(s). However, it has been shown that prone positioning while receiving veno-venous (VV) -ECMO reduces mortality rates, 37% proned vs 50% supine positioning (P =.02) (Giani, et al. Ann Am Thorac Soc. 2021;18[3]:495). In this study, no major complications occurred, and minor complications occurred in 6% of the proning events. Prone positioning improves ventilation-perfusion mismatch and reduces hypoxic vasoconstriction, which is thought to be right-sided heart-protective.
Right-sided heart dysfunction (RHD) is common in ARDS, whether COVID-19-related or not. The pathogenesis includes hypoxic vasoconstriction, pulmonary fibrosis, and ventilator-induced lung injury. Pulmonary microthrombi and patient-specific characteristics, such as obesity, are additional factors leading to RHD in patients with COVID-19. During the pandemic, several articles described using right-sided heart protective cannulation strategies for patients with COVID-19 requiring ECMO with favorable results (Mustafa, et al. JAMA Surg. 2020;155[10]:990; Cain, et al. J Surg Res. 2021;264:81-89). This right-sided heart protective strategy involves inserting a single access dual lumen cannula into the right internal jugular vein, which is advanced into the pulmonary artery, effectively bypassing the right ventricle. This setup is more typical of right ventricle assist device (RVAD), rather than typical VV-ECMO, which returns blood to the right atrium. Unfortunately, these studies did not include echocardiographic information to evaluate the effects of this intervention on RVD, and this is an area for future research. However, this vein to pulmonary artery strategy was found to facilitate decreased sedation, earlier liberation from mechanical ventilation, reduced need for tracheostomy, improved mobilization out of bed, and ease in prone positioning (Mustafa, et al. JAMA Surg. 2020;155[10]:990).
In conclusion, there is evidence to support the use of ECMO in patients with COVID-19 patients and ARDS failing conventional mechanical ventilation. The success of ECMO therapy is highly dependent on patient selection. Prolonged use of NIV on high Fio2 may be a negative predictor of ECMO survival and should be considered when assessing a patient for ECMO candidacy. Prone positioning with ECMO has been shown to have survival benefit and should be considered in all patients receiving ECMO.
Dr. Gaillard, Dr. Staples, and Dr. Kapoor are with the Department of Anesthesiology, Section on Critical Care, at Wake Forest School of Medicine in Winston-Salem, N.C. Dr. Gaillard is also with the Department of Emergency Medicine and Department of Internal Medicine, Section on Pulmonary, Critical Care, Allergy, and Immunology at Wake Forest School of Medicine.
Since the first SARS-CoV-2 (COVID-19) outbreak in Wuhan, China, in December 2019, more than 6.6 million deaths have occurred. . One of the strategies for those cases refractory to traditional ARDS treatments has been the use of extracorporeal membrane oxygenation (ECMO).
Before the COVID-19 pandemic, a substantial amount of data regarding the use of ECMO in ARDS was gathered during the H1N1 influenza outbreak in 2009. Mortality ranged from 8% to 65% (Zangrillo, et al. Crit Care. 2013;17[1]:R30). From these data, we learned the importance of patient selection. Young patients with few co-morbidities and less than 7 days supported by mechanical ventilation did remarkably better than elderly patients or those who had prolonged positive-pressure ventilation prior to ECMO.
To date, the mortality rate for COVID-19 patients with ARDS requiring ECMO is 48% based on data from ELSO. Interestingly though, using May 1, 2020, as a cutoff date, mortality rates for patients with COVID-19 receiving ECMO significantly increased from 37% to 52% (Barbaro, et al. Lancet. 2021;398[10307]:1230). This escalation in mortality engendered concern that ECMO may not be useful in treating patients with COVID-19 and ARDS.
Several factors can be cited for this increase in mortality. First, many new ECMO programs launched after May 1. These new programs had a higher mortality rate (59%) compared with established programs, suggesting that program and provider experience play a significant role in patient outcomes (Barbaro, et al. Lancet. 2021;398[10307]:1230). Second, patients in the latter part of 2020 experienced much longer intervals between the onset of symptoms and time of intubation. Clinicians had a tendency to delay intubation as long as possible. Subsequently, the number of days receiving high flow nasal oxygen or noninvasive ventilation (NIV) was significantly longer (Schmidt, et al. Crit Care. 2021;25[1]:355). These data suggest that prolonged NIV on high Fio2 may be a negative prognostic indicator and should be considered when assessing a patient’s candidacy for ECMO.
Early in the pandemic, clinicians realized that average ECMO run times for patients with COVID-19 and ARDS were significantly longer, 15 vs 9 days, respectively (Jacobs, et al. Ann Thorac Surg. 2022;113[5]:1452). With such long run times, beds were slow to turn over, and a shortage of ECMO beds resulted during the height of the pandemic. In a retrospective study, Gannon looked at 90 patients, all of whom were deemed medically appropriate for ECMO. Two groups were created: (1) no capacity for ECMO vs (2) ECMO provided. Mortality rates were staggering at 89% and 43%, respectively (P =.001) (Gannon, et al. Am J Respir Crit Care Med. 2022;205[11]:1354). This study demonstrated a profound point: during a pandemic, when demand overcomes supply, there is a unique opportunity to see the effect of lifesaving therapies, such as ECMO, on outcomes. This study was particularly poignant, as the average age of the patients was 40 years old.
It is now widely accepted that prone positioning has survival benefit in ARDS. Prone positioning while receiving ECMO has generally been avoided due to concern for potential complications associated with the cannula(s). However, it has been shown that prone positioning while receiving veno-venous (VV) -ECMO reduces mortality rates, 37% proned vs 50% supine positioning (P =.02) (Giani, et al. Ann Am Thorac Soc. 2021;18[3]:495). In this study, no major complications occurred, and minor complications occurred in 6% of the proning events. Prone positioning improves ventilation-perfusion mismatch and reduces hypoxic vasoconstriction, which is thought to be right-sided heart-protective.
Right-sided heart dysfunction (RHD) is common in ARDS, whether COVID-19-related or not. The pathogenesis includes hypoxic vasoconstriction, pulmonary fibrosis, and ventilator-induced lung injury. Pulmonary microthrombi and patient-specific characteristics, such as obesity, are additional factors leading to RHD in patients with COVID-19. During the pandemic, several articles described using right-sided heart protective cannulation strategies for patients with COVID-19 requiring ECMO with favorable results (Mustafa, et al. JAMA Surg. 2020;155[10]:990; Cain, et al. J Surg Res. 2021;264:81-89). This right-sided heart protective strategy involves inserting a single access dual lumen cannula into the right internal jugular vein, which is advanced into the pulmonary artery, effectively bypassing the right ventricle. This setup is more typical of right ventricle assist device (RVAD), rather than typical VV-ECMO, which returns blood to the right atrium. Unfortunately, these studies did not include echocardiographic information to evaluate the effects of this intervention on RVD, and this is an area for future research. However, this vein to pulmonary artery strategy was found to facilitate decreased sedation, earlier liberation from mechanical ventilation, reduced need for tracheostomy, improved mobilization out of bed, and ease in prone positioning (Mustafa, et al. JAMA Surg. 2020;155[10]:990).
In conclusion, there is evidence to support the use of ECMO in patients with COVID-19 patients and ARDS failing conventional mechanical ventilation. The success of ECMO therapy is highly dependent on patient selection. Prolonged use of NIV on high Fio2 may be a negative predictor of ECMO survival and should be considered when assessing a patient for ECMO candidacy. Prone positioning with ECMO has been shown to have survival benefit and should be considered in all patients receiving ECMO.
Dr. Gaillard, Dr. Staples, and Dr. Kapoor are with the Department of Anesthesiology, Section on Critical Care, at Wake Forest School of Medicine in Winston-Salem, N.C. Dr. Gaillard is also with the Department of Emergency Medicine and Department of Internal Medicine, Section on Pulmonary, Critical Care, Allergy, and Immunology at Wake Forest School of Medicine.
A doctor intervenes in a fiery car crash
Emergencies happen anywhere, anytime, and sometimes physicians find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a Medscape series telling these stories.
I was coming off a 48-hour shift plus a day of doing outpatient sedation at Sparrow Hospital in Lansing. It was December and Michigan-cold.
I drove on the side of the road where I wasn’t really supposed to and got closer. An SUV had crashed into one of the big concrete structures under the bridge. I saw people running around but wasn’t able to spot EMS or any health care workers. From where I was, I could identify four kids who had already been extricated and one adult still in the driver’s seat. I estimated the kids’ ages were around 7, 5, 3, and an infant who was a few months old. I left my car and went to help.
I was able to peg the ages correctly because I’m a pediatric critical care physician. As a specialty, we’re not commonly known. We oversee patient care in intensive care units, except the patients are children. Part of the job is that we’re experts at triaging. We recognize what’s life-threatening and less so.
The kids were with some adults who kept them warm with blankets. I examined each of them. The infant was asleep but arousable and acting like a normal baby. The 3-year-old boy was vomiting and appeared very fatigued. The 5-year-old boy had a forehead laceration and was in and out of consciousness. The 7-year-old girl was screaming because of different injuries.
While all of the children were concerning to me, I identified one in particular: the 5-year-old boy. It was obvious he needed serious medical attention and fast. So, I kept that little guy in mind. The others had sustained significant injuries, but my best guess was they could get to a hospital and be stabilized.
That said, I’m a trauma instructor, and one of the things I always tell trainees is: Trauma is a black box. On the outside, it may seem like a patient doesn’t have a lot of injuries. But underneath, there might be something worse, like a brain injury. Or the chest might have taken a blunt impact affecting the heart. There may be internal bleeding somewhere in the belly. It’s really hard to tease out what exactly is going on without equipment and testing.
I didn’t even have a pulse oximeter or heart rate monitor. I pretty much just went by the appearance of the child: pulse, heart rate, awareness, things like that.
After the kids, I moved to look at the man in the car. The front end had already caught fire. I could see the driver – the kids’ father, I guessed – unconscious and hunched over. I was wondering, Why hasn’t this guy been extricated?
I approached the car on the front passenger side. And then I just had this feeling. I knew I needed to step back. Immediately.
I did. And a few seconds later, the whole car exploded in flames.
I believe God is in control of everything. I tried to get to that man. But the scene was unsafe. Later I learned that several people, including a young nurse at the scene, had tried to get to him as well.
When EMS came, I identified myself. Obviously, these people do very, very important work. But they may be more used to the 60-year-old heart attack, the 25-year-old gunshot wound, the occasional ill child. I thought that four kids – each with possible critical poly-traumatic injuries – posed a challenge to anyone.
I told them, “This is what I do on a daily basis, and this is the kid I’m worried about the most. The other kids are definitely worrisome, but I would prioritize getting this kid to the hospital first. Can I ride with you?” They agreed.
We got that boy and his older sister into the first ambulance (she was in a lot of pain, the result of a femur fracture). The two other kids rode in the second ambulance. The hospital where I had just left was 10 minutes away. I called the other pediatric critical care doctor there, my partner. He thought I was calling for a routine issue – no such luck. I said, “I’m with four kids who are level-1 traumas in two ambulances and I’m heading to the hospital right now, ETA 10 minutes.”
En route, I thought the little boy might lose consciousness at any moment. He needed a breathing tube, and I debated whether it should be done in the ambulance vs. waiting until we got to the emergency room. Based on my judgment and his vital signs, I elected to wait to have it done it in a more controlled environment. Had I felt like he was in immediate need of an airway, I would’ve attempted it. But those are the tough calls that you must make.
My partner had alerted the trauma and emergency medicine teams at the hospital. By the time we arrived, my partner was down in the ER with the trauma team and ER staff. Everyone was ready. Then it was like divide and conquer. He attended to one of the kids. The ER team and I were with the little guy I was really worried about. We had his breathing tube in within minutes. The trauma team attended to the other two.
All the kids were stabilized and then admitted to the pediatric intensive care unit. I’m happy to say that all of them did well in the end. Even the little guy I was worried about the most.
I must say this incident gave me perspective on what EMS goes through. The field medicine we do in the United States is still in its infancy in a lot of ways. One of the things I would love to see in the future is a mobile ICU. After a critical illness hits, sometimes you only have seconds, minutes, maybe hours if you’re lucky. The earlier you can get patients the treatment they need, the better the outcomes.
I like taking care of critically ill children and their families. It fits my personality. And it’s a wonderful cause. But you have to be ready for tragic cases like this one. Yes, the children came out alive, but the accident claimed a life in a horrible way. And there was nothing I could do about it.
Critical care takes an emotional, psychological, and physical toll. It’s a roller coaster: Some kids do well; some kids don’t do well. All I can do is hold myself accountable. I keep my emotions in check, whether the outcome is positive or negative. And I do my best.
Mohamed Hani Farhat, MD, is a pediatric critical care physician at the University of Michigan C.S. Mott Children’s Hospital in Ann Arbor and Sparrow Hospital in Lansing, Mich. Are you a physician with a dramatic medical story outside the clinic? Medscape would love to consider your story for Is There a Doctor in the House? Please email your contact information and a short summary of your story to [email protected] . A version of this article appeared on Medscape.com.
Emergencies happen anywhere, anytime, and sometimes physicians find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a Medscape series telling these stories.
I was coming off a 48-hour shift plus a day of doing outpatient sedation at Sparrow Hospital in Lansing. It was December and Michigan-cold.
I drove on the side of the road where I wasn’t really supposed to and got closer. An SUV had crashed into one of the big concrete structures under the bridge. I saw people running around but wasn’t able to spot EMS or any health care workers. From where I was, I could identify four kids who had already been extricated and one adult still in the driver’s seat. I estimated the kids’ ages were around 7, 5, 3, and an infant who was a few months old. I left my car and went to help.
I was able to peg the ages correctly because I’m a pediatric critical care physician. As a specialty, we’re not commonly known. We oversee patient care in intensive care units, except the patients are children. Part of the job is that we’re experts at triaging. We recognize what’s life-threatening and less so.
The kids were with some adults who kept them warm with blankets. I examined each of them. The infant was asleep but arousable and acting like a normal baby. The 3-year-old boy was vomiting and appeared very fatigued. The 5-year-old boy had a forehead laceration and was in and out of consciousness. The 7-year-old girl was screaming because of different injuries.
While all of the children were concerning to me, I identified one in particular: the 5-year-old boy. It was obvious he needed serious medical attention and fast. So, I kept that little guy in mind. The others had sustained significant injuries, but my best guess was they could get to a hospital and be stabilized.
That said, I’m a trauma instructor, and one of the things I always tell trainees is: Trauma is a black box. On the outside, it may seem like a patient doesn’t have a lot of injuries. But underneath, there might be something worse, like a brain injury. Or the chest might have taken a blunt impact affecting the heart. There may be internal bleeding somewhere in the belly. It’s really hard to tease out what exactly is going on without equipment and testing.
I didn’t even have a pulse oximeter or heart rate monitor. I pretty much just went by the appearance of the child: pulse, heart rate, awareness, things like that.
After the kids, I moved to look at the man in the car. The front end had already caught fire. I could see the driver – the kids’ father, I guessed – unconscious and hunched over. I was wondering, Why hasn’t this guy been extricated?
I approached the car on the front passenger side. And then I just had this feeling. I knew I needed to step back. Immediately.
I did. And a few seconds later, the whole car exploded in flames.
I believe God is in control of everything. I tried to get to that man. But the scene was unsafe. Later I learned that several people, including a young nurse at the scene, had tried to get to him as well.
When EMS came, I identified myself. Obviously, these people do very, very important work. But they may be more used to the 60-year-old heart attack, the 25-year-old gunshot wound, the occasional ill child. I thought that four kids – each with possible critical poly-traumatic injuries – posed a challenge to anyone.
I told them, “This is what I do on a daily basis, and this is the kid I’m worried about the most. The other kids are definitely worrisome, but I would prioritize getting this kid to the hospital first. Can I ride with you?” They agreed.
We got that boy and his older sister into the first ambulance (she was in a lot of pain, the result of a femur fracture). The two other kids rode in the second ambulance. The hospital where I had just left was 10 minutes away. I called the other pediatric critical care doctor there, my partner. He thought I was calling for a routine issue – no such luck. I said, “I’m with four kids who are level-1 traumas in two ambulances and I’m heading to the hospital right now, ETA 10 minutes.”
En route, I thought the little boy might lose consciousness at any moment. He needed a breathing tube, and I debated whether it should be done in the ambulance vs. waiting until we got to the emergency room. Based on my judgment and his vital signs, I elected to wait to have it done it in a more controlled environment. Had I felt like he was in immediate need of an airway, I would’ve attempted it. But those are the tough calls that you must make.
My partner had alerted the trauma and emergency medicine teams at the hospital. By the time we arrived, my partner was down in the ER with the trauma team and ER staff. Everyone was ready. Then it was like divide and conquer. He attended to one of the kids. The ER team and I were with the little guy I was really worried about. We had his breathing tube in within minutes. The trauma team attended to the other two.
All the kids were stabilized and then admitted to the pediatric intensive care unit. I’m happy to say that all of them did well in the end. Even the little guy I was worried about the most.
I must say this incident gave me perspective on what EMS goes through. The field medicine we do in the United States is still in its infancy in a lot of ways. One of the things I would love to see in the future is a mobile ICU. After a critical illness hits, sometimes you only have seconds, minutes, maybe hours if you’re lucky. The earlier you can get patients the treatment they need, the better the outcomes.
I like taking care of critically ill children and their families. It fits my personality. And it’s a wonderful cause. But you have to be ready for tragic cases like this one. Yes, the children came out alive, but the accident claimed a life in a horrible way. And there was nothing I could do about it.
Critical care takes an emotional, psychological, and physical toll. It’s a roller coaster: Some kids do well; some kids don’t do well. All I can do is hold myself accountable. I keep my emotions in check, whether the outcome is positive or negative. And I do my best.
Mohamed Hani Farhat, MD, is a pediatric critical care physician at the University of Michigan C.S. Mott Children’s Hospital in Ann Arbor and Sparrow Hospital in Lansing, Mich. Are you a physician with a dramatic medical story outside the clinic? Medscape would love to consider your story for Is There a Doctor in the House? Please email your contact information and a short summary of your story to [email protected] . A version of this article appeared on Medscape.com.
Emergencies happen anywhere, anytime, and sometimes physicians find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a Medscape series telling these stories.
I was coming off a 48-hour shift plus a day of doing outpatient sedation at Sparrow Hospital in Lansing. It was December and Michigan-cold.
I drove on the side of the road where I wasn’t really supposed to and got closer. An SUV had crashed into one of the big concrete structures under the bridge. I saw people running around but wasn’t able to spot EMS or any health care workers. From where I was, I could identify four kids who had already been extricated and one adult still in the driver’s seat. I estimated the kids’ ages were around 7, 5, 3, and an infant who was a few months old. I left my car and went to help.
I was able to peg the ages correctly because I’m a pediatric critical care physician. As a specialty, we’re not commonly known. We oversee patient care in intensive care units, except the patients are children. Part of the job is that we’re experts at triaging. We recognize what’s life-threatening and less so.
The kids were with some adults who kept them warm with blankets. I examined each of them. The infant was asleep but arousable and acting like a normal baby. The 3-year-old boy was vomiting and appeared very fatigued. The 5-year-old boy had a forehead laceration and was in and out of consciousness. The 7-year-old girl was screaming because of different injuries.
While all of the children were concerning to me, I identified one in particular: the 5-year-old boy. It was obvious he needed serious medical attention and fast. So, I kept that little guy in mind. The others had sustained significant injuries, but my best guess was they could get to a hospital and be stabilized.
That said, I’m a trauma instructor, and one of the things I always tell trainees is: Trauma is a black box. On the outside, it may seem like a patient doesn’t have a lot of injuries. But underneath, there might be something worse, like a brain injury. Or the chest might have taken a blunt impact affecting the heart. There may be internal bleeding somewhere in the belly. It’s really hard to tease out what exactly is going on without equipment and testing.
I didn’t even have a pulse oximeter or heart rate monitor. I pretty much just went by the appearance of the child: pulse, heart rate, awareness, things like that.
After the kids, I moved to look at the man in the car. The front end had already caught fire. I could see the driver – the kids’ father, I guessed – unconscious and hunched over. I was wondering, Why hasn’t this guy been extricated?
I approached the car on the front passenger side. And then I just had this feeling. I knew I needed to step back. Immediately.
I did. And a few seconds later, the whole car exploded in flames.
I believe God is in control of everything. I tried to get to that man. But the scene was unsafe. Later I learned that several people, including a young nurse at the scene, had tried to get to him as well.
When EMS came, I identified myself. Obviously, these people do very, very important work. But they may be more used to the 60-year-old heart attack, the 25-year-old gunshot wound, the occasional ill child. I thought that four kids – each with possible critical poly-traumatic injuries – posed a challenge to anyone.
I told them, “This is what I do on a daily basis, and this is the kid I’m worried about the most. The other kids are definitely worrisome, but I would prioritize getting this kid to the hospital first. Can I ride with you?” They agreed.
We got that boy and his older sister into the first ambulance (she was in a lot of pain, the result of a femur fracture). The two other kids rode in the second ambulance. The hospital where I had just left was 10 minutes away. I called the other pediatric critical care doctor there, my partner. He thought I was calling for a routine issue – no such luck. I said, “I’m with four kids who are level-1 traumas in two ambulances and I’m heading to the hospital right now, ETA 10 minutes.”
En route, I thought the little boy might lose consciousness at any moment. He needed a breathing tube, and I debated whether it should be done in the ambulance vs. waiting until we got to the emergency room. Based on my judgment and his vital signs, I elected to wait to have it done it in a more controlled environment. Had I felt like he was in immediate need of an airway, I would’ve attempted it. But those are the tough calls that you must make.
My partner had alerted the trauma and emergency medicine teams at the hospital. By the time we arrived, my partner was down in the ER with the trauma team and ER staff. Everyone was ready. Then it was like divide and conquer. He attended to one of the kids. The ER team and I were with the little guy I was really worried about. We had his breathing tube in within minutes. The trauma team attended to the other two.
All the kids were stabilized and then admitted to the pediatric intensive care unit. I’m happy to say that all of them did well in the end. Even the little guy I was worried about the most.
I must say this incident gave me perspective on what EMS goes through. The field medicine we do in the United States is still in its infancy in a lot of ways. One of the things I would love to see in the future is a mobile ICU. After a critical illness hits, sometimes you only have seconds, minutes, maybe hours if you’re lucky. The earlier you can get patients the treatment they need, the better the outcomes.
I like taking care of critically ill children and their families. It fits my personality. And it’s a wonderful cause. But you have to be ready for tragic cases like this one. Yes, the children came out alive, but the accident claimed a life in a horrible way. And there was nothing I could do about it.
Critical care takes an emotional, psychological, and physical toll. It’s a roller coaster: Some kids do well; some kids don’t do well. All I can do is hold myself accountable. I keep my emotions in check, whether the outcome is positive or negative. And I do my best.
Mohamed Hani Farhat, MD, is a pediatric critical care physician at the University of Michigan C.S. Mott Children’s Hospital in Ann Arbor and Sparrow Hospital in Lansing, Mich. Are you a physician with a dramatic medical story outside the clinic? Medscape would love to consider your story for Is There a Doctor in the House? Please email your contact information and a short summary of your story to [email protected] . A version of this article appeared on Medscape.com.
Three wild technologies about to change health care
When I was a child, I watched syndicated episodes of the original “Star Trek.” I was dazzled by the space travel, sure, but also the medical technology.
A handheld “tricorder” detected diseases, while an intramuscular injector (“hypospray”) could treat them. Sickbay “biobeds” came with real-time health monitors that looked futuristic at the time but seem primitive today.
Such visions inspired a lot of us kids to pursue science. Little did we know the real-life advances many of us would see in our lifetimes.
Artificial intelligence helping to spot disease, robots performing surgery, even video calls between doctor and patient – all these once sounded fantastical but now happen in clinical care.
Now, in the 23rd year of the 21st century, you might not believe wht we’ll be capable of next. Three especially wild examples are moving closer to clinical reality.
Human hibernation
Captain America, Han Solo, and “Star Trek” villain Khan – all were preserved at low temperatures and then revived, waking up alive and well months, decades, or centuries later. These are fictional examples, to be sure, but the science they’re rooted in is real.
(In one extreme case, a climber survived after almost 9 hours of efforts to revive him.)
Useful for a space traveler? Maybe not. But it’s potentially huge for someone with life-threatening injuries from a car accident or a gunshot wound.
That’s the thinking behind a breakthrough procedure that came after decades of research on pigs and dogs, now in a clinical trial. The idea: A person with massive blood loss whose heart has stopped is injected with an ice-cold fluid, cooling them from the inside, down to about 50° F.
Doctors already induce more modest hypothermia to protect the brain and other organs after cardiac arrest and during surgery on the aortic arch (the main artery carrying blood from the heart).
But this experimental procedure – called emergency preservation and resuscitation (EPR) – goes far beyond that, dramatically “decreasing the body’s need for oxygen and blood flow,” says Samuel Tisherman, MD, a trauma surgeon at the University of Maryland Medical Center and the trial’s lead researcher. This puts the patient in a state of suspended animation that “could buy time for surgeons to stop the bleeding and save more of these patients.”
The technique has been done on at least six patients, though none were reported to survive. The trial is expected to include 20 people by the time it wraps up in December, according to the listing on the U.S. clinical trials database. Though given the strict requirements for candidates (emergency trauma victims who are not likely to survive), one can’t exactly rely on a set schedule.
Still, the technology is promising. Someday we may even use it to keep patients in suspended animation for months or years, experts predict, helping astronauts through decades-long spaceflights, or stalling death in sick patients awaiting a cure.
Artificial womb
Another sci-fi classic: growing human babies outside the womb. Think the fetus fields from “The Matrix,” or the frozen embryos in “Alien: Covenant.”
In 1923, British biologist J.B.S. Haldane coined a term for that – ectogenesis. He predicted that 70% of pregnancies would take place, from fertilization to birth, in artificial wombs by 2074. That many seems unlikely, but the timeline is on track.
Developing an embryo outside the womb is already routine in in vitro fertilization. And technology enables preterm babies to survive through much of the second half of gestation. Normal human pregnancy is 40 weeks, and the youngest preterm baby ever to survive was 21 weeks and 1 day old, just a few days younger than a smattering of others who lived.
The biggest obstacle for babies younger than that is lung viability. Mechanical ventilation can damage the lungs and lead to a chronic (sometimes fatal) lung disease known as bronchopulmonary dysplasia. Avoiding this would mean figuring out a way to maintain fetal circulation – the intricate system that delivers oxygenated blood from the placenta to the fetus via the umbilical cord. Researchers at Children’s Hospital of Philadelphia have done this using a fetal lamb.
The key to their invention is a substitute placenta: an oxygenator connected to the lamb’s umbilical cord. Tubes inserted through the umbilical vein and arteries carry oxygenated blood from the “placenta” to the fetus, and deoxygenated blood back out. The lamb resides in an artificial, fluid-filled amniotic sac until its lungs and other organs are developed.
Fertility treatment could benefit, too. “An artificial womb may substitute in situations in which a gestational carrier – surrogate – is indicated,” says Paula Amato, MD, a professor of obstetrics and gynecology at Oregon Health and Science University, Portland. (Dr. Amato is not involved in the CHOP research.) For example: when the mother is missing a uterus or can’t carry a pregnancy safely.
No date is set for clinical trials yet. But according to the research, the main difference between human and lamb may come down to size. A lamb’s umbilical vessels are larger, so feeding in a tube is easier. With today’s advances in miniaturizing surgical methods, that seems like a challenge scientists can overcome.
Messenger RNA therapeutics
Back to “Star Trek.” The hypospray injector’s contents could cure just about any disease, even one newly discovered on a strange planet. That’s not unlike messenger RNA (mRNA) technology, a breakthrough that enabled scientists to quickly develop some of the first COVID-19 vaccines.
But vaccines are just the beginning of what this technology can do.
A whole field of immunotherapy is emerging that uses mRNA to deliver instructions to produce chimeric antigen receptor–modified immune cells (CAR-modified immune cells). These cells are engineered to target diseased cells and tissues, like cancer cells and harmful fibroblasts (scar tissue) that promote fibrosis in, for example, the heart and lungs.
The field is bursting with rodent research, and clinical trials have started for treating some advanced-stage malignancies.
Actual clinical use may be years away, but if all goes well, these medicines could help treat or even cure the core medical problems facing humanity. We’re talking cancer, heart disease, neurodegenerative disease – transforming one therapy into another by simply changing the mRNA’s “nucleotide sequence,” the blueprint containing instructions telling it what to do, and what disease to attack.
As this technology matures, we may start to feel as if we’re really on “Star Trek,” where Dr. Leonard “Bones” McCoy pulls out the same device to treat just about every disease or injury.
A version of this article first appeared on WebMD.com.
When I was a child, I watched syndicated episodes of the original “Star Trek.” I was dazzled by the space travel, sure, but also the medical technology.
A handheld “tricorder” detected diseases, while an intramuscular injector (“hypospray”) could treat them. Sickbay “biobeds” came with real-time health monitors that looked futuristic at the time but seem primitive today.
Such visions inspired a lot of us kids to pursue science. Little did we know the real-life advances many of us would see in our lifetimes.
Artificial intelligence helping to spot disease, robots performing surgery, even video calls between doctor and patient – all these once sounded fantastical but now happen in clinical care.
Now, in the 23rd year of the 21st century, you might not believe wht we’ll be capable of next. Three especially wild examples are moving closer to clinical reality.
Human hibernation
Captain America, Han Solo, and “Star Trek” villain Khan – all were preserved at low temperatures and then revived, waking up alive and well months, decades, or centuries later. These are fictional examples, to be sure, but the science they’re rooted in is real.
(In one extreme case, a climber survived after almost 9 hours of efforts to revive him.)
Useful for a space traveler? Maybe not. But it’s potentially huge for someone with life-threatening injuries from a car accident or a gunshot wound.
That’s the thinking behind a breakthrough procedure that came after decades of research on pigs and dogs, now in a clinical trial. The idea: A person with massive blood loss whose heart has stopped is injected with an ice-cold fluid, cooling them from the inside, down to about 50° F.
Doctors already induce more modest hypothermia to protect the brain and other organs after cardiac arrest and during surgery on the aortic arch (the main artery carrying blood from the heart).
But this experimental procedure – called emergency preservation and resuscitation (EPR) – goes far beyond that, dramatically “decreasing the body’s need for oxygen and blood flow,” says Samuel Tisherman, MD, a trauma surgeon at the University of Maryland Medical Center and the trial’s lead researcher. This puts the patient in a state of suspended animation that “could buy time for surgeons to stop the bleeding and save more of these patients.”
The technique has been done on at least six patients, though none were reported to survive. The trial is expected to include 20 people by the time it wraps up in December, according to the listing on the U.S. clinical trials database. Though given the strict requirements for candidates (emergency trauma victims who are not likely to survive), one can’t exactly rely on a set schedule.
Still, the technology is promising. Someday we may even use it to keep patients in suspended animation for months or years, experts predict, helping astronauts through decades-long spaceflights, or stalling death in sick patients awaiting a cure.
Artificial womb
Another sci-fi classic: growing human babies outside the womb. Think the fetus fields from “The Matrix,” or the frozen embryos in “Alien: Covenant.”
In 1923, British biologist J.B.S. Haldane coined a term for that – ectogenesis. He predicted that 70% of pregnancies would take place, from fertilization to birth, in artificial wombs by 2074. That many seems unlikely, but the timeline is on track.
Developing an embryo outside the womb is already routine in in vitro fertilization. And technology enables preterm babies to survive through much of the second half of gestation. Normal human pregnancy is 40 weeks, and the youngest preterm baby ever to survive was 21 weeks and 1 day old, just a few days younger than a smattering of others who lived.
The biggest obstacle for babies younger than that is lung viability. Mechanical ventilation can damage the lungs and lead to a chronic (sometimes fatal) lung disease known as bronchopulmonary dysplasia. Avoiding this would mean figuring out a way to maintain fetal circulation – the intricate system that delivers oxygenated blood from the placenta to the fetus via the umbilical cord. Researchers at Children’s Hospital of Philadelphia have done this using a fetal lamb.
The key to their invention is a substitute placenta: an oxygenator connected to the lamb’s umbilical cord. Tubes inserted through the umbilical vein and arteries carry oxygenated blood from the “placenta” to the fetus, and deoxygenated blood back out. The lamb resides in an artificial, fluid-filled amniotic sac until its lungs and other organs are developed.
Fertility treatment could benefit, too. “An artificial womb may substitute in situations in which a gestational carrier – surrogate – is indicated,” says Paula Amato, MD, a professor of obstetrics and gynecology at Oregon Health and Science University, Portland. (Dr. Amato is not involved in the CHOP research.) For example: when the mother is missing a uterus or can’t carry a pregnancy safely.
No date is set for clinical trials yet. But according to the research, the main difference between human and lamb may come down to size. A lamb’s umbilical vessels are larger, so feeding in a tube is easier. With today’s advances in miniaturizing surgical methods, that seems like a challenge scientists can overcome.
Messenger RNA therapeutics
Back to “Star Trek.” The hypospray injector’s contents could cure just about any disease, even one newly discovered on a strange planet. That’s not unlike messenger RNA (mRNA) technology, a breakthrough that enabled scientists to quickly develop some of the first COVID-19 vaccines.
But vaccines are just the beginning of what this technology can do.
A whole field of immunotherapy is emerging that uses mRNA to deliver instructions to produce chimeric antigen receptor–modified immune cells (CAR-modified immune cells). These cells are engineered to target diseased cells and tissues, like cancer cells and harmful fibroblasts (scar tissue) that promote fibrosis in, for example, the heart and lungs.
The field is bursting with rodent research, and clinical trials have started for treating some advanced-stage malignancies.
Actual clinical use may be years away, but if all goes well, these medicines could help treat or even cure the core medical problems facing humanity. We’re talking cancer, heart disease, neurodegenerative disease – transforming one therapy into another by simply changing the mRNA’s “nucleotide sequence,” the blueprint containing instructions telling it what to do, and what disease to attack.
As this technology matures, we may start to feel as if we’re really on “Star Trek,” where Dr. Leonard “Bones” McCoy pulls out the same device to treat just about every disease or injury.
A version of this article first appeared on WebMD.com.
When I was a child, I watched syndicated episodes of the original “Star Trek.” I was dazzled by the space travel, sure, but also the medical technology.
A handheld “tricorder” detected diseases, while an intramuscular injector (“hypospray”) could treat them. Sickbay “biobeds” came with real-time health monitors that looked futuristic at the time but seem primitive today.
Such visions inspired a lot of us kids to pursue science. Little did we know the real-life advances many of us would see in our lifetimes.
Artificial intelligence helping to spot disease, robots performing surgery, even video calls between doctor and patient – all these once sounded fantastical but now happen in clinical care.
Now, in the 23rd year of the 21st century, you might not believe wht we’ll be capable of next. Three especially wild examples are moving closer to clinical reality.
Human hibernation
Captain America, Han Solo, and “Star Trek” villain Khan – all were preserved at low temperatures and then revived, waking up alive and well months, decades, or centuries later. These are fictional examples, to be sure, but the science they’re rooted in is real.
(In one extreme case, a climber survived after almost 9 hours of efforts to revive him.)
Useful for a space traveler? Maybe not. But it’s potentially huge for someone with life-threatening injuries from a car accident or a gunshot wound.
That’s the thinking behind a breakthrough procedure that came after decades of research on pigs and dogs, now in a clinical trial. The idea: A person with massive blood loss whose heart has stopped is injected with an ice-cold fluid, cooling them from the inside, down to about 50° F.
Doctors already induce more modest hypothermia to protect the brain and other organs after cardiac arrest and during surgery on the aortic arch (the main artery carrying blood from the heart).
But this experimental procedure – called emergency preservation and resuscitation (EPR) – goes far beyond that, dramatically “decreasing the body’s need for oxygen and blood flow,” says Samuel Tisherman, MD, a trauma surgeon at the University of Maryland Medical Center and the trial’s lead researcher. This puts the patient in a state of suspended animation that “could buy time for surgeons to stop the bleeding and save more of these patients.”
The technique has been done on at least six patients, though none were reported to survive. The trial is expected to include 20 people by the time it wraps up in December, according to the listing on the U.S. clinical trials database. Though given the strict requirements for candidates (emergency trauma victims who are not likely to survive), one can’t exactly rely on a set schedule.
Still, the technology is promising. Someday we may even use it to keep patients in suspended animation for months or years, experts predict, helping astronauts through decades-long spaceflights, or stalling death in sick patients awaiting a cure.
Artificial womb
Another sci-fi classic: growing human babies outside the womb. Think the fetus fields from “The Matrix,” or the frozen embryos in “Alien: Covenant.”
In 1923, British biologist J.B.S. Haldane coined a term for that – ectogenesis. He predicted that 70% of pregnancies would take place, from fertilization to birth, in artificial wombs by 2074. That many seems unlikely, but the timeline is on track.
Developing an embryo outside the womb is already routine in in vitro fertilization. And technology enables preterm babies to survive through much of the second half of gestation. Normal human pregnancy is 40 weeks, and the youngest preterm baby ever to survive was 21 weeks and 1 day old, just a few days younger than a smattering of others who lived.
The biggest obstacle for babies younger than that is lung viability. Mechanical ventilation can damage the lungs and lead to a chronic (sometimes fatal) lung disease known as bronchopulmonary dysplasia. Avoiding this would mean figuring out a way to maintain fetal circulation – the intricate system that delivers oxygenated blood from the placenta to the fetus via the umbilical cord. Researchers at Children’s Hospital of Philadelphia have done this using a fetal lamb.
The key to their invention is a substitute placenta: an oxygenator connected to the lamb’s umbilical cord. Tubes inserted through the umbilical vein and arteries carry oxygenated blood from the “placenta” to the fetus, and deoxygenated blood back out. The lamb resides in an artificial, fluid-filled amniotic sac until its lungs and other organs are developed.
Fertility treatment could benefit, too. “An artificial womb may substitute in situations in which a gestational carrier – surrogate – is indicated,” says Paula Amato, MD, a professor of obstetrics and gynecology at Oregon Health and Science University, Portland. (Dr. Amato is not involved in the CHOP research.) For example: when the mother is missing a uterus or can’t carry a pregnancy safely.
No date is set for clinical trials yet. But according to the research, the main difference between human and lamb may come down to size. A lamb’s umbilical vessels are larger, so feeding in a tube is easier. With today’s advances in miniaturizing surgical methods, that seems like a challenge scientists can overcome.
Messenger RNA therapeutics
Back to “Star Trek.” The hypospray injector’s contents could cure just about any disease, even one newly discovered on a strange planet. That’s not unlike messenger RNA (mRNA) technology, a breakthrough that enabled scientists to quickly develop some of the first COVID-19 vaccines.
But vaccines are just the beginning of what this technology can do.
A whole field of immunotherapy is emerging that uses mRNA to deliver instructions to produce chimeric antigen receptor–modified immune cells (CAR-modified immune cells). These cells are engineered to target diseased cells and tissues, like cancer cells and harmful fibroblasts (scar tissue) that promote fibrosis in, for example, the heart and lungs.
The field is bursting with rodent research, and clinical trials have started for treating some advanced-stage malignancies.
Actual clinical use may be years away, but if all goes well, these medicines could help treat or even cure the core medical problems facing humanity. We’re talking cancer, heart disease, neurodegenerative disease – transforming one therapy into another by simply changing the mRNA’s “nucleotide sequence,” the blueprint containing instructions telling it what to do, and what disease to attack.
As this technology matures, we may start to feel as if we’re really on “Star Trek,” where Dr. Leonard “Bones” McCoy pulls out the same device to treat just about every disease or injury.
A version of this article first appeared on WebMD.com.
A new (old) drug joins the COVID fray, and guess what? It works
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
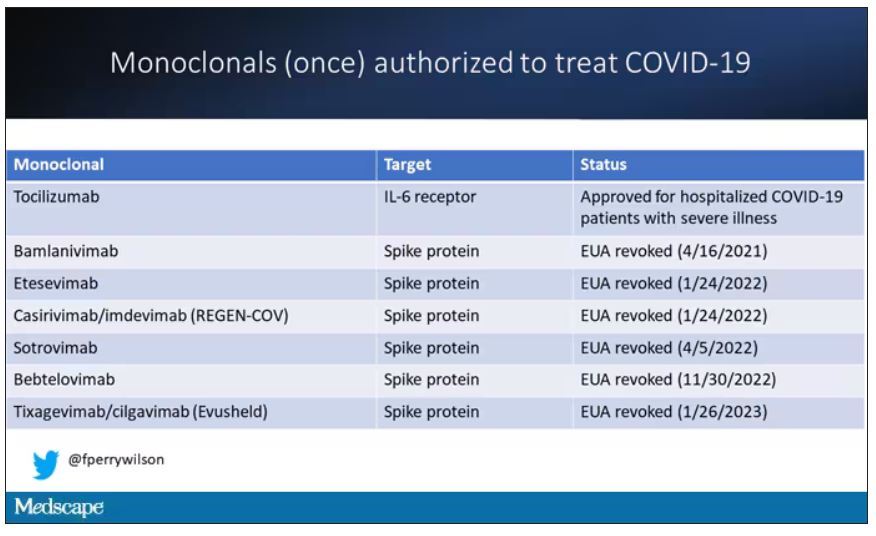
At this point, with the monoclonals found to be essentially useless, we are left with remdesivir with its modest efficacy and Paxlovid, which, for some reason, people don’t seem to be taking.
Part of the reason the monoclonals have failed lately is because of their specificity; they are homogeneous antibodies targeted toward a very specific epitope that may change from variant to variant. We need a broader therapeutic, one that has activity across all variants — maybe even one that has activity against all viruses? We’ve got one. Interferon.
The first mention of interferon as a potential COVID therapy was at the very start of the pandemic, so I’m sort of surprised that the first large, randomized trial is only being reported now in the New England Journal of Medicine.
Before we dig into the results, let’s talk mechanism. This is a trial of interferon-lambda, also known as interleukin-29.
The lambda interferons were only discovered in 2003. They differ from the more familiar interferons only in their cellular receptors; the downstream effects seem quite similar. As opposed to the cellular receptors for interferon alfa, which are widely expressed, the receptors for lambda are restricted to epithelial tissues. This makes it a good choice as a COVID treatment, since the virus also preferentially targets those epithelial cells.
In this study, 1,951 participants from Brazil and Canada, but mostly Brazil, with new COVID infections who were not yet hospitalized were randomized to receive 180 mcg of interferon lambda or placebo.
This was a relatively current COVID trial, as you can see from the participant characteristics. The majority had been vaccinated, and nearly half of the infections were during the Omicron phase of the pandemic.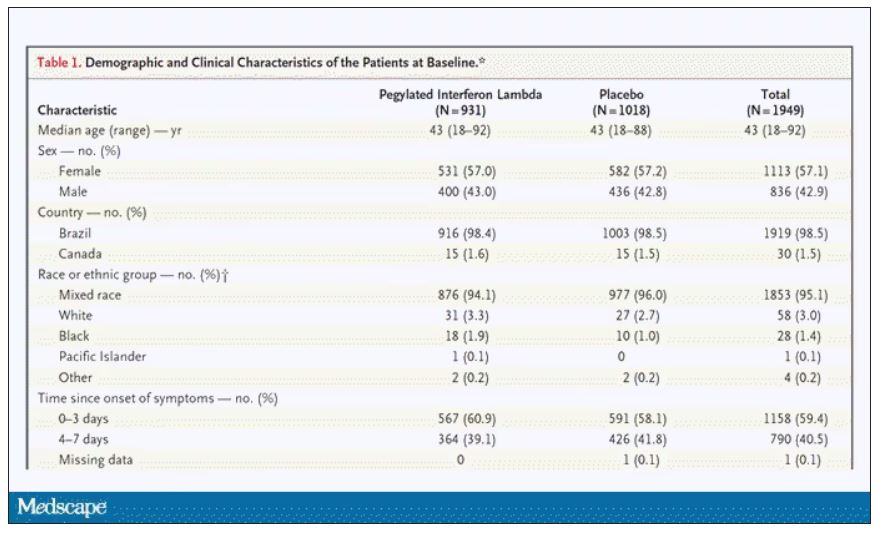
If you just want to cut to the chase, interferon worked.
The primary outcome – hospitalization or a prolonged emergency room visit for COVID – was 50% lower in the interferon group.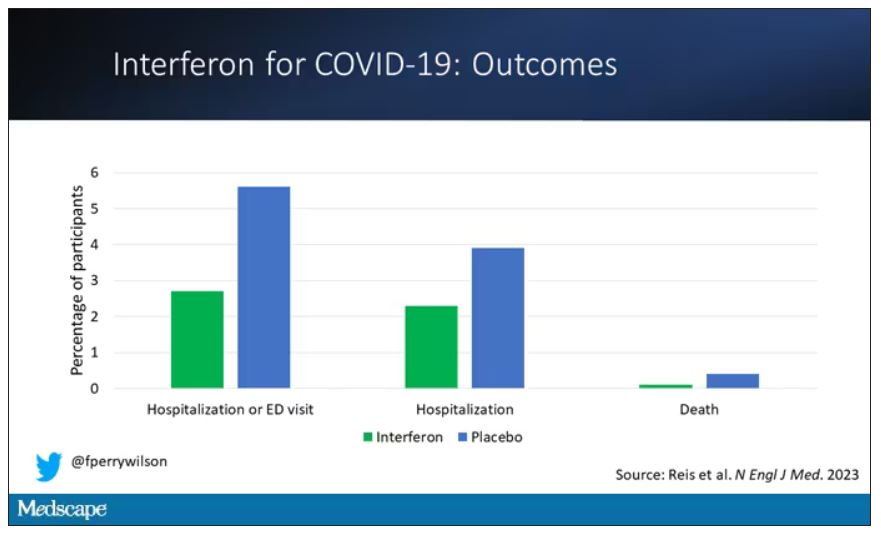
Key secondary outcomes, including death from COVID, were lower in the interferon group as well. These effects persisted across most of the subgroups I was looking out for.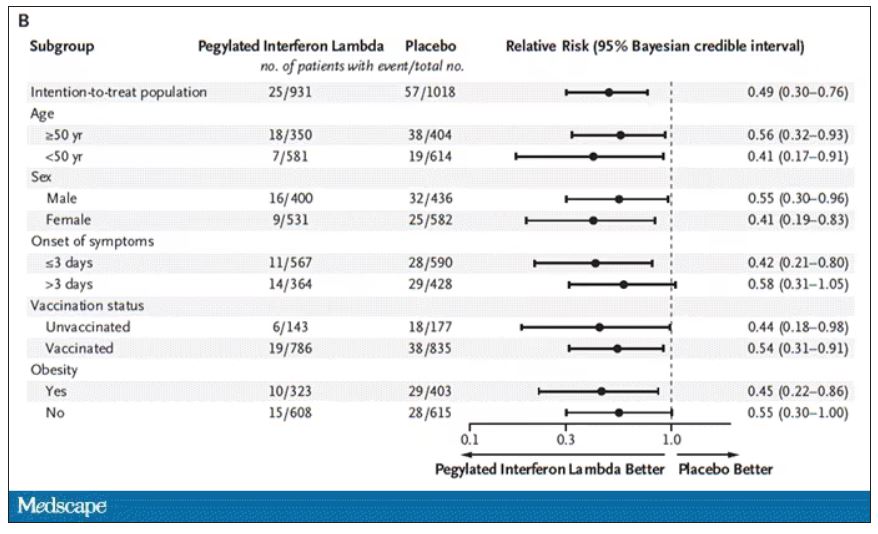
Interferon seemed to help those who were already vaccinated and those who were unvaccinated. There’s a hint that it works better within the first few days of symptoms, which isn’t surprising; we’ve seen this for many of the therapeutics, including Paxlovid. Time is of the essence. Encouragingly, the effect was a bit more pronounced among those infected with Omicron.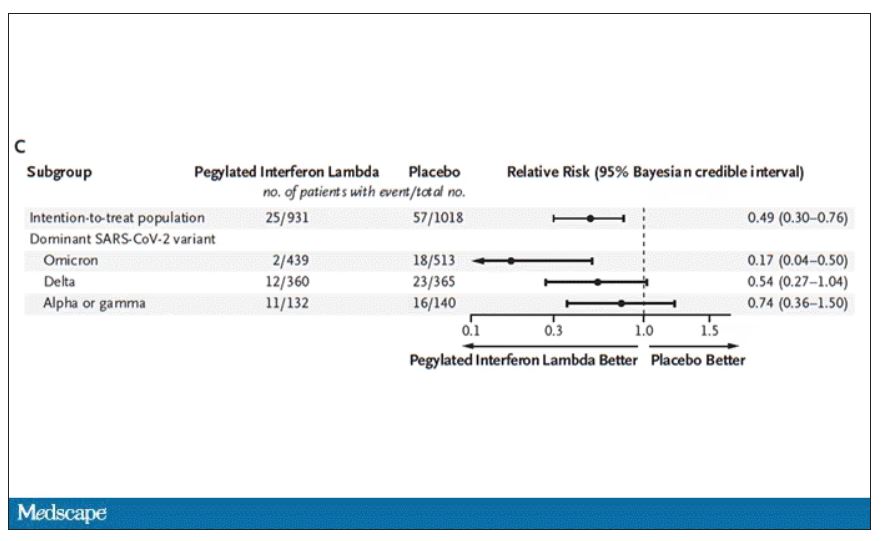
Of course, if you have any experience with interferon, you know that the side effects can be pretty rough. In the bad old days when we treated hepatitis C infection with interferon, patients would get their injections on Friday in anticipation of being essentially out of commission with flu-like symptoms through the weekend. But we don’t see much evidence of adverse events in this trial, maybe due to the greater specificity of interferon lambda.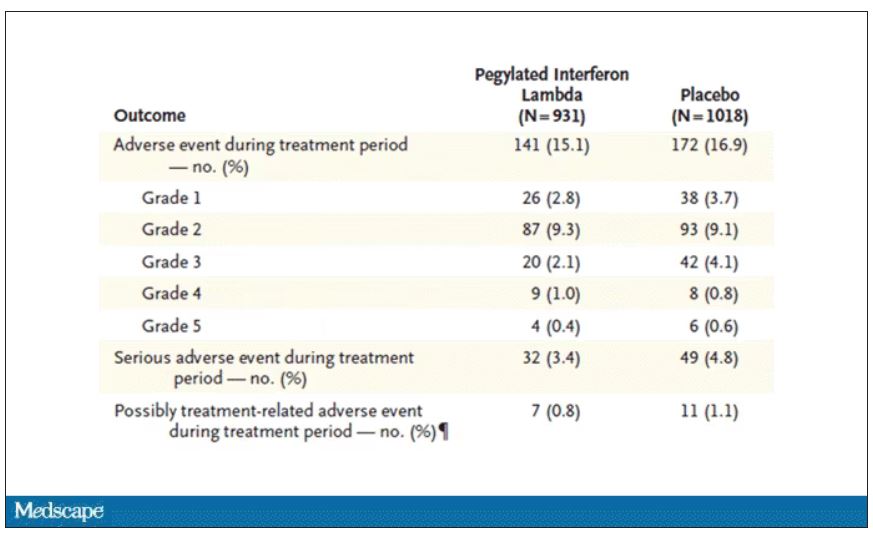
Putting it all together, the state of play for interferons in COVID may be changing. To date, the FDA has not recommended the use of interferon alfa or -beta for COVID-19, citing some data that they are ineffective or even harmful in hospitalized patients with COVID. Interferon lambda is not FDA approved and thus not even available in the United States. But the reason it has not been approved is that there has not been a large, well-conducted interferon lambda trial. Now there is. Will this study be enough to prompt an emergency use authorization? The elephant in the room, of course, is Paxlovid, which at this point has a longer safety track record and, importantly, is oral. I’d love to see a head-to-head trial. Short of that, I tend to be in favor of having more options on the table.
Dr. Perry Wilson is associate professor, department of medicine, and director, Clinical and Translational Research Accelerator, at Yale University, New Haven, Conn. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.

At this point, with the monoclonals found to be essentially useless, we are left with remdesivir with its modest efficacy and Paxlovid, which, for some reason, people don’t seem to be taking.
Part of the reason the monoclonals have failed lately is because of their specificity; they are homogeneous antibodies targeted toward a very specific epitope that may change from variant to variant. We need a broader therapeutic, one that has activity across all variants — maybe even one that has activity against all viruses? We’ve got one. Interferon.
The first mention of interferon as a potential COVID therapy was at the very start of the pandemic, so I’m sort of surprised that the first large, randomized trial is only being reported now in the New England Journal of Medicine.
Before we dig into the results, let’s talk mechanism. This is a trial of interferon-lambda, also known as interleukin-29.
The lambda interferons were only discovered in 2003. They differ from the more familiar interferons only in their cellular receptors; the downstream effects seem quite similar. As opposed to the cellular receptors for interferon alfa, which are widely expressed, the receptors for lambda are restricted to epithelial tissues. This makes it a good choice as a COVID treatment, since the virus also preferentially targets those epithelial cells.
In this study, 1,951 participants from Brazil and Canada, but mostly Brazil, with new COVID infections who were not yet hospitalized were randomized to receive 180 mcg of interferon lambda or placebo.
This was a relatively current COVID trial, as you can see from the participant characteristics. The majority had been vaccinated, and nearly half of the infections were during the Omicron phase of the pandemic.
If you just want to cut to the chase, interferon worked.
The primary outcome – hospitalization or a prolonged emergency room visit for COVID – was 50% lower in the interferon group.
Key secondary outcomes, including death from COVID, were lower in the interferon group as well. These effects persisted across most of the subgroups I was looking out for.
Interferon seemed to help those who were already vaccinated and those who were unvaccinated. There’s a hint that it works better within the first few days of symptoms, which isn’t surprising; we’ve seen this for many of the therapeutics, including Paxlovid. Time is of the essence. Encouragingly, the effect was a bit more pronounced among those infected with Omicron.
Of course, if you have any experience with interferon, you know that the side effects can be pretty rough. In the bad old days when we treated hepatitis C infection with interferon, patients would get their injections on Friday in anticipation of being essentially out of commission with flu-like symptoms through the weekend. But we don’t see much evidence of adverse events in this trial, maybe due to the greater specificity of interferon lambda.
Putting it all together, the state of play for interferons in COVID may be changing. To date, the FDA has not recommended the use of interferon alfa or -beta for COVID-19, citing some data that they are ineffective or even harmful in hospitalized patients with COVID. Interferon lambda is not FDA approved and thus not even available in the United States. But the reason it has not been approved is that there has not been a large, well-conducted interferon lambda trial. Now there is. Will this study be enough to prompt an emergency use authorization? The elephant in the room, of course, is Paxlovid, which at this point has a longer safety track record and, importantly, is oral. I’d love to see a head-to-head trial. Short of that, I tend to be in favor of having more options on the table.
Dr. Perry Wilson is associate professor, department of medicine, and director, Clinical and Translational Research Accelerator, at Yale University, New Haven, Conn. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.

At this point, with the monoclonals found to be essentially useless, we are left with remdesivir with its modest efficacy and Paxlovid, which, for some reason, people don’t seem to be taking.
Part of the reason the monoclonals have failed lately is because of their specificity; they are homogeneous antibodies targeted toward a very specific epitope that may change from variant to variant. We need a broader therapeutic, one that has activity across all variants — maybe even one that has activity against all viruses? We’ve got one. Interferon.
The first mention of interferon as a potential COVID therapy was at the very start of the pandemic, so I’m sort of surprised that the first large, randomized trial is only being reported now in the New England Journal of Medicine.
Before we dig into the results, let’s talk mechanism. This is a trial of interferon-lambda, also known as interleukin-29.
The lambda interferons were only discovered in 2003. They differ from the more familiar interferons only in their cellular receptors; the downstream effects seem quite similar. As opposed to the cellular receptors for interferon alfa, which are widely expressed, the receptors for lambda are restricted to epithelial tissues. This makes it a good choice as a COVID treatment, since the virus also preferentially targets those epithelial cells.
In this study, 1,951 participants from Brazil and Canada, but mostly Brazil, with new COVID infections who were not yet hospitalized were randomized to receive 180 mcg of interferon lambda or placebo.
This was a relatively current COVID trial, as you can see from the participant characteristics. The majority had been vaccinated, and nearly half of the infections were during the Omicron phase of the pandemic.
If you just want to cut to the chase, interferon worked.
The primary outcome – hospitalization or a prolonged emergency room visit for COVID – was 50% lower in the interferon group.
Key secondary outcomes, including death from COVID, were lower in the interferon group as well. These effects persisted across most of the subgroups I was looking out for.
Interferon seemed to help those who were already vaccinated and those who were unvaccinated. There’s a hint that it works better within the first few days of symptoms, which isn’t surprising; we’ve seen this for many of the therapeutics, including Paxlovid. Time is of the essence. Encouragingly, the effect was a bit more pronounced among those infected with Omicron.
Of course, if you have any experience with interferon, you know that the side effects can be pretty rough. In the bad old days when we treated hepatitis C infection with interferon, patients would get their injections on Friday in anticipation of being essentially out of commission with flu-like symptoms through the weekend. But we don’t see much evidence of adverse events in this trial, maybe due to the greater specificity of interferon lambda.
Putting it all together, the state of play for interferons in COVID may be changing. To date, the FDA has not recommended the use of interferon alfa or -beta for COVID-19, citing some data that they are ineffective or even harmful in hospitalized patients with COVID. Interferon lambda is not FDA approved and thus not even available in the United States. But the reason it has not been approved is that there has not been a large, well-conducted interferon lambda trial. Now there is. Will this study be enough to prompt an emergency use authorization? The elephant in the room, of course, is Paxlovid, which at this point has a longer safety track record and, importantly, is oral. I’d love to see a head-to-head trial. Short of that, I tend to be in favor of having more options on the table.
Dr. Perry Wilson is associate professor, department of medicine, and director, Clinical and Translational Research Accelerator, at Yale University, New Haven, Conn. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
What is the psychological cost of performing CPR?
One year ago, as the sun was setting on a late fall day, Andrés Snitcofsky, a 40-year-old designer from Buenos Aires, Argentina, heard harrowing cries for help. It was the niece and the wife of one of his neighbors: a man in his 60s who the women had found “passed out” in the bedroom. “I did CPR for 5 minutes straight until a friend of the victim came in and asked me to stop, telling me that the man had probably been dead for 2 or 3 hours already. But I had no idea because I’d never seen a dead body before,” Mr. Snitcofsky told this news organization. A few minutes later, the ambulance arrived. The doctor confirmed that there was nothing more that could be done.
Mr. Snitcofsky went home. Nobody had asked for his name or address or phone number. … And it wasn’t because they already knew who he was. In fact, there wasn’t any sort of relationship there. Mr. Snitcofsky had only known his neighbors by sight. His actions that day, however, “did not come without a cost. It took me weeks – months, actually – to put myself together again,” he said. The things he saw, the things he heard, everything about that night played over and over in his head. “I had trouble sleeping. I would play out different scenarios in my head. I questioned myself. I second-guessed myself, criticized myself. It’s like some taboo subject. There’s no one to share the experience with, no one who gets it. But with time, I was able to process the event.
“For 2 months, I talked to my psychologist about it all,” he continued. “That really helped me a lot. In addition to therapy, I reached out to a couple I know – they’re both physicians – and to a firefighter who teaches CPR. Their insight and guidance allowed me to get to a point where I was able to understand that what I did was a good thing and that what I did was all that could have been done. But anyone who finds themselves in the position of having to do CPR – they’re going to be affected in many, many ways. It goes beyond the euphoria of seeing a person come back to life. Of that, I’m quite certain.”
We’ve all seen campaigns encouraging people to learn CPR and to be prepared if the need arises. But in training the public (and even health care professionals), not much, if anything, is said about the “collateral damage”: the psychological and emotional consequences of carrying out the procedure. These especially come into play when you don’t know whether the person survived, when your efforts weren’t able to reverse the sudden cardiac arrest, or when the person you gave CPR to was a loved one – a case that may entail immediate therapeutic interventions to minimize or prevent the risk of suffering long-lasting trauma.
In May 2020, popular American activist and educator Kristin Flanary saw someone suffering cardiac arrest. She stepped in and started doing CPR. And she continued doing CPR … for 10 long minutes. The person she was trying to save was her 34-year-old husband, ophthalmologist and comedian Will Flanary. On Twitter, where she’s known as Lady Glaucomflecken, Ms. Flanary recently shared the following message, putting the topic of CPR and automated external defibrillator training front and center.
“Yes, everyone should learn #CPRandAED. But if we are going to ask people to perform such a brutal task, it’s imperative that we also provide them with the info and resources they need to process it mentally and emotionally. It’s traumatic and life changing. It’s irresponsible and unethical to ask people to help in such a brutal and traumatic way and then neglect to help them in return.” In less than a month, the tweet has racked up over 200,000 views.
Doing one’s duty
There are many people who work to promote CPR and strengthen the other links in the chain of survival for out-of-hospital sudden cardiac arrest, such as prompt access to and delivery of early defibrillation. According to them, any negative psychological impact of intervening is temporary and, when compared with the satisfaction of having done one’s duty, quite insignificant – even if the efforts to save a person’s life are not successful.
“In 99.9% of cases, people who have performed CPR feel a sense of satisfaction, even happiness, knowing that they’ve helped. The individuals I’ve spoken with, I’ve never heard any of them say that they felt worse after the event or that they needed to see a psychologist,” said Mario Fitz Maurice, MD, director of the Arrhythmia Council of the Argentine Society of Cardiology and head of Electrophysiology at Rivadavia Hospital in Buenos Aires. He went on to tell this news organization, “Of course, some degree of fear, sadness, or melancholy can remain afterward. But it seems to me, and there are reports saying as much, that, in the end, what stands out in the person’s mind is the fact that they tried to save a life. And for them, there’s joy in knowing this.”
Dr. Fitz Maurice, who is also the director of the National Arrhythmia Institute in Buenos Aires, pointed out that the kind of person who takes CPR classes “has a profile that’s going to allow them to be psychologically involved; they’re the caring person, the one who’s ready and willing to help people.” And he added that, at his hospital, if they can identify the individuals or first responders who have done CPR on a patient, the protocol is to always contact them to offer psychological care and assistance. “But in 99% of cases, they don’t even understand why we’re calling them, they’re extremely happy to have taken part.”
Some studies, though, paint a much different picture, one that shows that providing CPR can be emotionally challenging and have consequences in terms of one’s family and work life. A qualitative study published in 2016 looked into the experiences of 20 lay rescuers in Norway – five were health educated – who had provided CPR to 18 out-of-hospital cardiac arrest (OHCA) victims, 66% of whom survived. The time from experiencing the OHCA incident to participating in the interview ranged from 6 days to 13 years (median 5.5 years). Several participants reported the OHCA incident as a “shocking and terrifying” experience. Tiredness, exhaustion, confusion, and feeling alone about the OHCA experience were individual reactions that could vary in time from days to months. Anxiety and insomnia were also experienced following the incident.
Some lay rescuers described the influence on work and family life, and a few of them described deep sorrow, even several years after the incident. Overall, they reported repetitive self-criticism regarding whether they could have carried out anything else to achieve a better outcome for the cardiac arrest victim. All of them wanted to be informed about the outcome. And four of the lay rescuers needed professional counseling to process the OHCA experience.
In 2020, another qualitative study was conducted, this time in Taiwan. There were nine participants, none of whom were health professionals. Each had provided initial CPR and defibrillation with AED in public locations. Event-to-interview duration was within 1 year and 1-2 years. The major findings from the study were the following:
- The lay rescuers possessed helping traits and high motivation.
- The lay rescuers reported certain aspects of rescue reality that differed much from prior training and expectations, including difficulty in the depth of chest compression, and uncertainties in real emergency situations.
- The lay rescuers gained positive personal fulfillment in sharing their experience and receiving positive feedback from others, and were willing to help next time, although they experienced a short-term negative psychological impact from the event. “Measures should be taken to increase [a] layperson’s confidence and situation awareness, to reduce training-reality discrepancy, and to build up a support system to avoid negative psychological effects.” This was the conclusion of the study team, which was led by Matthew Huei-Ming Ma, MD, PhD. A professor in the department of emergency medicine at National Taiwan University in Taipei, he is also on the board of directors of the Resuscitation Council of Asia.
Potential trauma
In recalling his experience, Mr. Snitcofsky said, “The hardest part of it all was the moment that I stopped giving CPR, that moment of letting go. This became the image that kept coming back to me, the traumatic moment I hadn’t thought about.”
Psychiatrist Daniel Mosca, MD, is the founder and former president of the Argentine Society of Trauma Psychology. He is also the coordinator of the human factors team at the City of Buenos Aires Emergency Medical Care System. “Any event has the potential to be traumatic, all the more so when it’s an event where you come face to face with death and uncertainty. But how a rescuer reacts will depend on their psychological makeup.” Of the individuals who were held for months or years in the jungle as hostages of the Revolutionary Armed Forces of Colombia, “only” half developed symptoms of posttraumatic stress disorder.
Dr. Mosca believes that a comment by Frank Ochberg, MD, speaks to this finding. “In many cases, peritraumatic symptoms are a normal person’s normal response to an abnormal situation.” For a lot of people who have found themselves having to perform CPR, the symptoms associated with the initial acute stress reaction will resolve on their own in 30-90 days. “But if this doesn’t happen, and those symptoms persist, psychotherapeutic or pharmacological intervention will be necessary,” he noted.
“In CPR classes, it would be good for the instructors to talk about the warning signs that people should look out for in themselves and their fellow rescuers. So, for example, insomnia, anxiety, a heightened state of alertness, feeling disconnected from reality,” Dr. Mosca told this news organization.
“Another thing that can help rescuers is letting them know what happened to the person they gave CPR to. This way, they can get closure,” suggested Manlio Márquez Murillo, MD, a cardiologist and electrophysiologist in Mexico. He is also the coordinator of the Alliance Against Sudden Cardiac Death at the Interamerican Society of Cardiology.
“Medical and nursing societies would have to develop a brief protocol or performance standard. The goal would be to ensure that rescuers are asked for their contact information and that someone gets in touch to debrief them and to offer them care. Next would come the treatment part, to resolve any remaining aftereffects,” said in an interview.
For example, a three-stage Lay Responder Support Model (LRSM) was developed and implemented as part of a lay responder support program established in 2014 by the Peel Regional Council in Ontario. The LRSM identifies and engages individuals who witnessed or participated directly or indirectly in an OHCA, inviting them to participate in a debriefing session facilitated by a trained practitioner. Held 24-48 hours post event, the debriefing allows lay responders to contextualize their reaction to the event. The conversation also serves as an opportunity for them to fully articulate their concerns, questions, and thoughts. The facilitator can communicate stress reduction techniques and address psychological first aid needs as they emerge. Approximately 1 week post event, a secondary follow-up occurs. If the lay responder communicates a continuing struggle with symptoms impacting and interfering with everyday life, the facilitator offers a coordinated or facilitated referral for mental health support.
In an article published in the Journal of Cardiac Failure. Ms. Flanary speaks about the three kinds of language that anyone who was either forced to or inspired to perform CPR can use to help process their trauma: words that explain what happened, words that name (eg, “forgotten patients”), and words that validate the experience and allow the person to articulate their feelings. The tools and technologies that organizations and health care professionals provide can help the healing process. Empathy and compassion, too, have a place.
But there are virtually no standardized and proactive initiatives of this kind in much of the world, including Latin America. So, most people who just happened to be in the right place at the right time find that they have to navigate the “after” part all on their own.
Other obstacles
Dr. Márquez Murillo finds it unfortunate that countries in the region have yet to enact “Good Samaritan” laws. If individuals render aid to someone suffering cardiac arrest, then these laws would ensure that they will not be held liable in any way. This is the case in Argentina and Uruguay. So, the fear of things turning into a legal matter may be holding people back from taking action; that fear could also create additional stress for those who end up stepping in to help.
Even with the legal safeguards, exceptional circumstances may arise where rescuers find themselves facing unexpected emotional challenges. In Argentina, Virginia Pérez Antonelli, the 17-year-old who tried in vain to save the life of Fernando Báez Sosa, had to testify at the trial of the eight defendants accused of brutally beating him in January 2020. The press, the public – the attention of an entire country – was focused on her. She had to respond to the defense attorneys who were able to ask whether she was sure that she performed the CPR maneuvers correctly. And a few weeks ago, a medical examiner hired by the defense suggested that “the CPR may have made the situation worse” for the victim. An indignant Dr. Fitz Maurice responded on Twitter: “CPR SAVES LIVES!! Let’s not let a CHEAP AND BASELESS argument destroy all the work that’s been done…!”
Of course, there are consequences that are beyond our control and others that can, in fact, be anticipated and planned for. Dr. Fitz Maurice brought up a preventive approach: Make CPR second nature, teach it in schools, help people overcome their fears. “Cardiac deaths are 200 times more frequent than deaths resulting from fires – and we practice fire drills a lot more than we practice CPR,” he told this news organization. In a society where there is widespread training on the procedure, where people regularly practice the technique, those who have had the experience of giving someone CPR will feel less alone, will be better understood by others.
“On the other hand, beyond the initial impact and the lack of a formal support system, the medium- and long-term outcome for those who acted is also psychologically and emotionally favorable,” said Jorge Bombau, MD, an obstetrician/gynecologist in Buenos Aires. After Dr. Bombau’s 14-year-old son Beltrán suddenly died during a school sports tournament, Dr. Bombau became a prominent advocate spreading the word about CPR.
“I don’t know anyone who regrets doing CPR,” he told this news organization. “There may be a brief period when the person feels distressed or depressed, when they have trouble sleeping. But it’s been proven that doing a good deed improves one’s mood. And what better deed is there than trying to save someone’s life? Whether their efforts were successful or in vain, that person has, at the end of the day, done something meaningful and worthwhile.”
Mr. Snitcofsky shares this sentiment. For several months now, he’s been feeling he’s “in a good place.” And he’s been actively promoting CPR on social media. As he recently posted on Twitter, “I’m here to retweet everything that has to do with getting us all to become familiar with how to do CPR and working up the courage to do it. The training takes no more than a few hours.
“I want to know that, if I ever have an out-of-hospital sudden cardiac arrest, there will be neighbors, friends, or family members around who know how to do CPR. Every person who knows how to do CPR can persuade others, and those of us who’ve had to do CPR in real life are even better candidates for persuading others. And if one day a person ends up needing CPR, I want to step in again and make up for lost time. Here’s hoping it’ll do the job,” he concluded.
It’s the same for Matías Alonso, a journalist in Buenos Aires. On New Year’s Eve 15 years ago, he was at a family dinner when, a few minutes before midnight, he found himself giving CPR to his stepmother’s father. “Unfortunately, he passed away, but I continued doing CPR on him until the ambulance arrived. For some time, I felt a little guilty for not taking charge of the situation from the beginning, and because I had this idea in my head that more people pulled through and recovered. But afterwards, they really thanked me a lot. And that helped me realize that I’d done something. I didn’t stand still when faced with the inevitability of death. I understood that it was good to have tried,” Mr. Alonso told this news organization. “And next time … hopefully there won’t be a next time … but I’m more prepared, and I now know how I can do better.”
Mr. Alonso, Mr. Snitcofsky, Dr. Fitz Maurice, Dr. Mosca, Dr. Bombau, and Dr. Márquez Murillo disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com. This article was translated from Medscape Spanish.
One year ago, as the sun was setting on a late fall day, Andrés Snitcofsky, a 40-year-old designer from Buenos Aires, Argentina, heard harrowing cries for help. It was the niece and the wife of one of his neighbors: a man in his 60s who the women had found “passed out” in the bedroom. “I did CPR for 5 minutes straight until a friend of the victim came in and asked me to stop, telling me that the man had probably been dead for 2 or 3 hours already. But I had no idea because I’d never seen a dead body before,” Mr. Snitcofsky told this news organization. A few minutes later, the ambulance arrived. The doctor confirmed that there was nothing more that could be done.
Mr. Snitcofsky went home. Nobody had asked for his name or address or phone number. … And it wasn’t because they already knew who he was. In fact, there wasn’t any sort of relationship there. Mr. Snitcofsky had only known his neighbors by sight. His actions that day, however, “did not come without a cost. It took me weeks – months, actually – to put myself together again,” he said. The things he saw, the things he heard, everything about that night played over and over in his head. “I had trouble sleeping. I would play out different scenarios in my head. I questioned myself. I second-guessed myself, criticized myself. It’s like some taboo subject. There’s no one to share the experience with, no one who gets it. But with time, I was able to process the event.
“For 2 months, I talked to my psychologist about it all,” he continued. “That really helped me a lot. In addition to therapy, I reached out to a couple I know – they’re both physicians – and to a firefighter who teaches CPR. Their insight and guidance allowed me to get to a point where I was able to understand that what I did was a good thing and that what I did was all that could have been done. But anyone who finds themselves in the position of having to do CPR – they’re going to be affected in many, many ways. It goes beyond the euphoria of seeing a person come back to life. Of that, I’m quite certain.”
We’ve all seen campaigns encouraging people to learn CPR and to be prepared if the need arises. But in training the public (and even health care professionals), not much, if anything, is said about the “collateral damage”: the psychological and emotional consequences of carrying out the procedure. These especially come into play when you don’t know whether the person survived, when your efforts weren’t able to reverse the sudden cardiac arrest, or when the person you gave CPR to was a loved one – a case that may entail immediate therapeutic interventions to minimize or prevent the risk of suffering long-lasting trauma.
In May 2020, popular American activist and educator Kristin Flanary saw someone suffering cardiac arrest. She stepped in and started doing CPR. And she continued doing CPR … for 10 long minutes. The person she was trying to save was her 34-year-old husband, ophthalmologist and comedian Will Flanary. On Twitter, where she’s known as Lady Glaucomflecken, Ms. Flanary recently shared the following message, putting the topic of CPR and automated external defibrillator training front and center.
“Yes, everyone should learn #CPRandAED. But if we are going to ask people to perform such a brutal task, it’s imperative that we also provide them with the info and resources they need to process it mentally and emotionally. It’s traumatic and life changing. It’s irresponsible and unethical to ask people to help in such a brutal and traumatic way and then neglect to help them in return.” In less than a month, the tweet has racked up over 200,000 views.
Doing one’s duty
There are many people who work to promote CPR and strengthen the other links in the chain of survival for out-of-hospital sudden cardiac arrest, such as prompt access to and delivery of early defibrillation. According to them, any negative psychological impact of intervening is temporary and, when compared with the satisfaction of having done one’s duty, quite insignificant – even if the efforts to save a person’s life are not successful.
“In 99.9% of cases, people who have performed CPR feel a sense of satisfaction, even happiness, knowing that they’ve helped. The individuals I’ve spoken with, I’ve never heard any of them say that they felt worse after the event or that they needed to see a psychologist,” said Mario Fitz Maurice, MD, director of the Arrhythmia Council of the Argentine Society of Cardiology and head of Electrophysiology at Rivadavia Hospital in Buenos Aires. He went on to tell this news organization, “Of course, some degree of fear, sadness, or melancholy can remain afterward. But it seems to me, and there are reports saying as much, that, in the end, what stands out in the person’s mind is the fact that they tried to save a life. And for them, there’s joy in knowing this.”
Dr. Fitz Maurice, who is also the director of the National Arrhythmia Institute in Buenos Aires, pointed out that the kind of person who takes CPR classes “has a profile that’s going to allow them to be psychologically involved; they’re the caring person, the one who’s ready and willing to help people.” And he added that, at his hospital, if they can identify the individuals or first responders who have done CPR on a patient, the protocol is to always contact them to offer psychological care and assistance. “But in 99% of cases, they don’t even understand why we’re calling them, they’re extremely happy to have taken part.”
Some studies, though, paint a much different picture, one that shows that providing CPR can be emotionally challenging and have consequences in terms of one’s family and work life. A qualitative study published in 2016 looked into the experiences of 20 lay rescuers in Norway – five were health educated – who had provided CPR to 18 out-of-hospital cardiac arrest (OHCA) victims, 66% of whom survived. The time from experiencing the OHCA incident to participating in the interview ranged from 6 days to 13 years (median 5.5 years). Several participants reported the OHCA incident as a “shocking and terrifying” experience. Tiredness, exhaustion, confusion, and feeling alone about the OHCA experience were individual reactions that could vary in time from days to months. Anxiety and insomnia were also experienced following the incident.
Some lay rescuers described the influence on work and family life, and a few of them described deep sorrow, even several years after the incident. Overall, they reported repetitive self-criticism regarding whether they could have carried out anything else to achieve a better outcome for the cardiac arrest victim. All of them wanted to be informed about the outcome. And four of the lay rescuers needed professional counseling to process the OHCA experience.
In 2020, another qualitative study was conducted, this time in Taiwan. There were nine participants, none of whom were health professionals. Each had provided initial CPR and defibrillation with AED in public locations. Event-to-interview duration was within 1 year and 1-2 years. The major findings from the study were the following:
- The lay rescuers possessed helping traits and high motivation.
- The lay rescuers reported certain aspects of rescue reality that differed much from prior training and expectations, including difficulty in the depth of chest compression, and uncertainties in real emergency situations.
- The lay rescuers gained positive personal fulfillment in sharing their experience and receiving positive feedback from others, and were willing to help next time, although they experienced a short-term negative psychological impact from the event. “Measures should be taken to increase [a] layperson’s confidence and situation awareness, to reduce training-reality discrepancy, and to build up a support system to avoid negative psychological effects.” This was the conclusion of the study team, which was led by Matthew Huei-Ming Ma, MD, PhD. A professor in the department of emergency medicine at National Taiwan University in Taipei, he is also on the board of directors of the Resuscitation Council of Asia.
Potential trauma
In recalling his experience, Mr. Snitcofsky said, “The hardest part of it all was the moment that I stopped giving CPR, that moment of letting go. This became the image that kept coming back to me, the traumatic moment I hadn’t thought about.”
Psychiatrist Daniel Mosca, MD, is the founder and former president of the Argentine Society of Trauma Psychology. He is also the coordinator of the human factors team at the City of Buenos Aires Emergency Medical Care System. “Any event has the potential to be traumatic, all the more so when it’s an event where you come face to face with death and uncertainty. But how a rescuer reacts will depend on their psychological makeup.” Of the individuals who were held for months or years in the jungle as hostages of the Revolutionary Armed Forces of Colombia, “only” half developed symptoms of posttraumatic stress disorder.
Dr. Mosca believes that a comment by Frank Ochberg, MD, speaks to this finding. “In many cases, peritraumatic symptoms are a normal person’s normal response to an abnormal situation.” For a lot of people who have found themselves having to perform CPR, the symptoms associated with the initial acute stress reaction will resolve on their own in 30-90 days. “But if this doesn’t happen, and those symptoms persist, psychotherapeutic or pharmacological intervention will be necessary,” he noted.
“In CPR classes, it would be good for the instructors to talk about the warning signs that people should look out for in themselves and their fellow rescuers. So, for example, insomnia, anxiety, a heightened state of alertness, feeling disconnected from reality,” Dr. Mosca told this news organization.
“Another thing that can help rescuers is letting them know what happened to the person they gave CPR to. This way, they can get closure,” suggested Manlio Márquez Murillo, MD, a cardiologist and electrophysiologist in Mexico. He is also the coordinator of the Alliance Against Sudden Cardiac Death at the Interamerican Society of Cardiology.
“Medical and nursing societies would have to develop a brief protocol or performance standard. The goal would be to ensure that rescuers are asked for their contact information and that someone gets in touch to debrief them and to offer them care. Next would come the treatment part, to resolve any remaining aftereffects,” said in an interview.
For example, a three-stage Lay Responder Support Model (LRSM) was developed and implemented as part of a lay responder support program established in 2014 by the Peel Regional Council in Ontario. The LRSM identifies and engages individuals who witnessed or participated directly or indirectly in an OHCA, inviting them to participate in a debriefing session facilitated by a trained practitioner. Held 24-48 hours post event, the debriefing allows lay responders to contextualize their reaction to the event. The conversation also serves as an opportunity for them to fully articulate their concerns, questions, and thoughts. The facilitator can communicate stress reduction techniques and address psychological first aid needs as they emerge. Approximately 1 week post event, a secondary follow-up occurs. If the lay responder communicates a continuing struggle with symptoms impacting and interfering with everyday life, the facilitator offers a coordinated or facilitated referral for mental health support.
In an article published in the Journal of Cardiac Failure. Ms. Flanary speaks about the three kinds of language that anyone who was either forced to or inspired to perform CPR can use to help process their trauma: words that explain what happened, words that name (eg, “forgotten patients”), and words that validate the experience and allow the person to articulate their feelings. The tools and technologies that organizations and health care professionals provide can help the healing process. Empathy and compassion, too, have a place.
But there are virtually no standardized and proactive initiatives of this kind in much of the world, including Latin America. So, most people who just happened to be in the right place at the right time find that they have to navigate the “after” part all on their own.
Other obstacles
Dr. Márquez Murillo finds it unfortunate that countries in the region have yet to enact “Good Samaritan” laws. If individuals render aid to someone suffering cardiac arrest, then these laws would ensure that they will not be held liable in any way. This is the case in Argentina and Uruguay. So, the fear of things turning into a legal matter may be holding people back from taking action; that fear could also create additional stress for those who end up stepping in to help.
Even with the legal safeguards, exceptional circumstances may arise where rescuers find themselves facing unexpected emotional challenges. In Argentina, Virginia Pérez Antonelli, the 17-year-old who tried in vain to save the life of Fernando Báez Sosa, had to testify at the trial of the eight defendants accused of brutally beating him in January 2020. The press, the public – the attention of an entire country – was focused on her. She had to respond to the defense attorneys who were able to ask whether she was sure that she performed the CPR maneuvers correctly. And a few weeks ago, a medical examiner hired by the defense suggested that “the CPR may have made the situation worse” for the victim. An indignant Dr. Fitz Maurice responded on Twitter: “CPR SAVES LIVES!! Let’s not let a CHEAP AND BASELESS argument destroy all the work that’s been done…!”
Of course, there are consequences that are beyond our control and others that can, in fact, be anticipated and planned for. Dr. Fitz Maurice brought up a preventive approach: Make CPR second nature, teach it in schools, help people overcome their fears. “Cardiac deaths are 200 times more frequent than deaths resulting from fires – and we practice fire drills a lot more than we practice CPR,” he told this news organization. In a society where there is widespread training on the procedure, where people regularly practice the technique, those who have had the experience of giving someone CPR will feel less alone, will be better understood by others.
“On the other hand, beyond the initial impact and the lack of a formal support system, the medium- and long-term outcome for those who acted is also psychologically and emotionally favorable,” said Jorge Bombau, MD, an obstetrician/gynecologist in Buenos Aires. After Dr. Bombau’s 14-year-old son Beltrán suddenly died during a school sports tournament, Dr. Bombau became a prominent advocate spreading the word about CPR.
“I don’t know anyone who regrets doing CPR,” he told this news organization. “There may be a brief period when the person feels distressed or depressed, when they have trouble sleeping. But it’s been proven that doing a good deed improves one’s mood. And what better deed is there than trying to save someone’s life? Whether their efforts were successful or in vain, that person has, at the end of the day, done something meaningful and worthwhile.”
Mr. Snitcofsky shares this sentiment. For several months now, he’s been feeling he’s “in a good place.” And he’s been actively promoting CPR on social media. As he recently posted on Twitter, “I’m here to retweet everything that has to do with getting us all to become familiar with how to do CPR and working up the courage to do it. The training takes no more than a few hours.
“I want to know that, if I ever have an out-of-hospital sudden cardiac arrest, there will be neighbors, friends, or family members around who know how to do CPR. Every person who knows how to do CPR can persuade others, and those of us who’ve had to do CPR in real life are even better candidates for persuading others. And if one day a person ends up needing CPR, I want to step in again and make up for lost time. Here’s hoping it’ll do the job,” he concluded.
It’s the same for Matías Alonso, a journalist in Buenos Aires. On New Year’s Eve 15 years ago, he was at a family dinner when, a few minutes before midnight, he found himself giving CPR to his stepmother’s father. “Unfortunately, he passed away, but I continued doing CPR on him until the ambulance arrived. For some time, I felt a little guilty for not taking charge of the situation from the beginning, and because I had this idea in my head that more people pulled through and recovered. But afterwards, they really thanked me a lot. And that helped me realize that I’d done something. I didn’t stand still when faced with the inevitability of death. I understood that it was good to have tried,” Mr. Alonso told this news organization. “And next time … hopefully there won’t be a next time … but I’m more prepared, and I now know how I can do better.”
Mr. Alonso, Mr. Snitcofsky, Dr. Fitz Maurice, Dr. Mosca, Dr. Bombau, and Dr. Márquez Murillo disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com. This article was translated from Medscape Spanish.
One year ago, as the sun was setting on a late fall day, Andrés Snitcofsky, a 40-year-old designer from Buenos Aires, Argentina, heard harrowing cries for help. It was the niece and the wife of one of his neighbors: a man in his 60s who the women had found “passed out” in the bedroom. “I did CPR for 5 minutes straight until a friend of the victim came in and asked me to stop, telling me that the man had probably been dead for 2 or 3 hours already. But I had no idea because I’d never seen a dead body before,” Mr. Snitcofsky told this news organization. A few minutes later, the ambulance arrived. The doctor confirmed that there was nothing more that could be done.
Mr. Snitcofsky went home. Nobody had asked for his name or address or phone number. … And it wasn’t because they already knew who he was. In fact, there wasn’t any sort of relationship there. Mr. Snitcofsky had only known his neighbors by sight. His actions that day, however, “did not come without a cost. It took me weeks – months, actually – to put myself together again,” he said. The things he saw, the things he heard, everything about that night played over and over in his head. “I had trouble sleeping. I would play out different scenarios in my head. I questioned myself. I second-guessed myself, criticized myself. It’s like some taboo subject. There’s no one to share the experience with, no one who gets it. But with time, I was able to process the event.
“For 2 months, I talked to my psychologist about it all,” he continued. “That really helped me a lot. In addition to therapy, I reached out to a couple I know – they’re both physicians – and to a firefighter who teaches CPR. Their insight and guidance allowed me to get to a point where I was able to understand that what I did was a good thing and that what I did was all that could have been done. But anyone who finds themselves in the position of having to do CPR – they’re going to be affected in many, many ways. It goes beyond the euphoria of seeing a person come back to life. Of that, I’m quite certain.”
We’ve all seen campaigns encouraging people to learn CPR and to be prepared if the need arises. But in training the public (and even health care professionals), not much, if anything, is said about the “collateral damage”: the psychological and emotional consequences of carrying out the procedure. These especially come into play when you don’t know whether the person survived, when your efforts weren’t able to reverse the sudden cardiac arrest, or when the person you gave CPR to was a loved one – a case that may entail immediate therapeutic interventions to minimize or prevent the risk of suffering long-lasting trauma.
In May 2020, popular American activist and educator Kristin Flanary saw someone suffering cardiac arrest. She stepped in and started doing CPR. And she continued doing CPR … for 10 long minutes. The person she was trying to save was her 34-year-old husband, ophthalmologist and comedian Will Flanary. On Twitter, where she’s known as Lady Glaucomflecken, Ms. Flanary recently shared the following message, putting the topic of CPR and automated external defibrillator training front and center.
“Yes, everyone should learn #CPRandAED. But if we are going to ask people to perform such a brutal task, it’s imperative that we also provide them with the info and resources they need to process it mentally and emotionally. It’s traumatic and life changing. It’s irresponsible and unethical to ask people to help in such a brutal and traumatic way and then neglect to help them in return.” In less than a month, the tweet has racked up over 200,000 views.
Doing one’s duty
There are many people who work to promote CPR and strengthen the other links in the chain of survival for out-of-hospital sudden cardiac arrest, such as prompt access to and delivery of early defibrillation. According to them, any negative psychological impact of intervening is temporary and, when compared with the satisfaction of having done one’s duty, quite insignificant – even if the efforts to save a person’s life are not successful.
“In 99.9% of cases, people who have performed CPR feel a sense of satisfaction, even happiness, knowing that they’ve helped. The individuals I’ve spoken with, I’ve never heard any of them say that they felt worse after the event or that they needed to see a psychologist,” said Mario Fitz Maurice, MD, director of the Arrhythmia Council of the Argentine Society of Cardiology and head of Electrophysiology at Rivadavia Hospital in Buenos Aires. He went on to tell this news organization, “Of course, some degree of fear, sadness, or melancholy can remain afterward. But it seems to me, and there are reports saying as much, that, in the end, what stands out in the person’s mind is the fact that they tried to save a life. And for them, there’s joy in knowing this.”
Dr. Fitz Maurice, who is also the director of the National Arrhythmia Institute in Buenos Aires, pointed out that the kind of person who takes CPR classes “has a profile that’s going to allow them to be psychologically involved; they’re the caring person, the one who’s ready and willing to help people.” And he added that, at his hospital, if they can identify the individuals or first responders who have done CPR on a patient, the protocol is to always contact them to offer psychological care and assistance. “But in 99% of cases, they don’t even understand why we’re calling them, they’re extremely happy to have taken part.”
Some studies, though, paint a much different picture, one that shows that providing CPR can be emotionally challenging and have consequences in terms of one’s family and work life. A qualitative study published in 2016 looked into the experiences of 20 lay rescuers in Norway – five were health educated – who had provided CPR to 18 out-of-hospital cardiac arrest (OHCA) victims, 66% of whom survived. The time from experiencing the OHCA incident to participating in the interview ranged from 6 days to 13 years (median 5.5 years). Several participants reported the OHCA incident as a “shocking and terrifying” experience. Tiredness, exhaustion, confusion, and feeling alone about the OHCA experience were individual reactions that could vary in time from days to months. Anxiety and insomnia were also experienced following the incident.
Some lay rescuers described the influence on work and family life, and a few of them described deep sorrow, even several years after the incident. Overall, they reported repetitive self-criticism regarding whether they could have carried out anything else to achieve a better outcome for the cardiac arrest victim. All of them wanted to be informed about the outcome. And four of the lay rescuers needed professional counseling to process the OHCA experience.
In 2020, another qualitative study was conducted, this time in Taiwan. There were nine participants, none of whom were health professionals. Each had provided initial CPR and defibrillation with AED in public locations. Event-to-interview duration was within 1 year and 1-2 years. The major findings from the study were the following:
- The lay rescuers possessed helping traits and high motivation.
- The lay rescuers reported certain aspects of rescue reality that differed much from prior training and expectations, including difficulty in the depth of chest compression, and uncertainties in real emergency situations.
- The lay rescuers gained positive personal fulfillment in sharing their experience and receiving positive feedback from others, and were willing to help next time, although they experienced a short-term negative psychological impact from the event. “Measures should be taken to increase [a] layperson’s confidence and situation awareness, to reduce training-reality discrepancy, and to build up a support system to avoid negative psychological effects.” This was the conclusion of the study team, which was led by Matthew Huei-Ming Ma, MD, PhD. A professor in the department of emergency medicine at National Taiwan University in Taipei, he is also on the board of directors of the Resuscitation Council of Asia.
Potential trauma
In recalling his experience, Mr. Snitcofsky said, “The hardest part of it all was the moment that I stopped giving CPR, that moment of letting go. This became the image that kept coming back to me, the traumatic moment I hadn’t thought about.”
Psychiatrist Daniel Mosca, MD, is the founder and former president of the Argentine Society of Trauma Psychology. He is also the coordinator of the human factors team at the City of Buenos Aires Emergency Medical Care System. “Any event has the potential to be traumatic, all the more so when it’s an event where you come face to face with death and uncertainty. But how a rescuer reacts will depend on their psychological makeup.” Of the individuals who were held for months or years in the jungle as hostages of the Revolutionary Armed Forces of Colombia, “only” half developed symptoms of posttraumatic stress disorder.
Dr. Mosca believes that a comment by Frank Ochberg, MD, speaks to this finding. “In many cases, peritraumatic symptoms are a normal person’s normal response to an abnormal situation.” For a lot of people who have found themselves having to perform CPR, the symptoms associated with the initial acute stress reaction will resolve on their own in 30-90 days. “But if this doesn’t happen, and those symptoms persist, psychotherapeutic or pharmacological intervention will be necessary,” he noted.
“In CPR classes, it would be good for the instructors to talk about the warning signs that people should look out for in themselves and their fellow rescuers. So, for example, insomnia, anxiety, a heightened state of alertness, feeling disconnected from reality,” Dr. Mosca told this news organization.
“Another thing that can help rescuers is letting them know what happened to the person they gave CPR to. This way, they can get closure,” suggested Manlio Márquez Murillo, MD, a cardiologist and electrophysiologist in Mexico. He is also the coordinator of the Alliance Against Sudden Cardiac Death at the Interamerican Society of Cardiology.
“Medical and nursing societies would have to develop a brief protocol or performance standard. The goal would be to ensure that rescuers are asked for their contact information and that someone gets in touch to debrief them and to offer them care. Next would come the treatment part, to resolve any remaining aftereffects,” said in an interview.
For example, a three-stage Lay Responder Support Model (LRSM) was developed and implemented as part of a lay responder support program established in 2014 by the Peel Regional Council in Ontario. The LRSM identifies and engages individuals who witnessed or participated directly or indirectly in an OHCA, inviting them to participate in a debriefing session facilitated by a trained practitioner. Held 24-48 hours post event, the debriefing allows lay responders to contextualize their reaction to the event. The conversation also serves as an opportunity for them to fully articulate their concerns, questions, and thoughts. The facilitator can communicate stress reduction techniques and address psychological first aid needs as they emerge. Approximately 1 week post event, a secondary follow-up occurs. If the lay responder communicates a continuing struggle with symptoms impacting and interfering with everyday life, the facilitator offers a coordinated or facilitated referral for mental health support.
In an article published in the Journal of Cardiac Failure. Ms. Flanary speaks about the three kinds of language that anyone who was either forced to or inspired to perform CPR can use to help process their trauma: words that explain what happened, words that name (eg, “forgotten patients”), and words that validate the experience and allow the person to articulate their feelings. The tools and technologies that organizations and health care professionals provide can help the healing process. Empathy and compassion, too, have a place.
But there are virtually no standardized and proactive initiatives of this kind in much of the world, including Latin America. So, most people who just happened to be in the right place at the right time find that they have to navigate the “after” part all on their own.
Other obstacles
Dr. Márquez Murillo finds it unfortunate that countries in the region have yet to enact “Good Samaritan” laws. If individuals render aid to someone suffering cardiac arrest, then these laws would ensure that they will not be held liable in any way. This is the case in Argentina and Uruguay. So, the fear of things turning into a legal matter may be holding people back from taking action; that fear could also create additional stress for those who end up stepping in to help.
Even with the legal safeguards, exceptional circumstances may arise where rescuers find themselves facing unexpected emotional challenges. In Argentina, Virginia Pérez Antonelli, the 17-year-old who tried in vain to save the life of Fernando Báez Sosa, had to testify at the trial of the eight defendants accused of brutally beating him in January 2020. The press, the public – the attention of an entire country – was focused on her. She had to respond to the defense attorneys who were able to ask whether she was sure that she performed the CPR maneuvers correctly. And a few weeks ago, a medical examiner hired by the defense suggested that “the CPR may have made the situation worse” for the victim. An indignant Dr. Fitz Maurice responded on Twitter: “CPR SAVES LIVES!! Let’s not let a CHEAP AND BASELESS argument destroy all the work that’s been done…!”
Of course, there are consequences that are beyond our control and others that can, in fact, be anticipated and planned for. Dr. Fitz Maurice brought up a preventive approach: Make CPR second nature, teach it in schools, help people overcome their fears. “Cardiac deaths are 200 times more frequent than deaths resulting from fires – and we practice fire drills a lot more than we practice CPR,” he told this news organization. In a society where there is widespread training on the procedure, where people regularly practice the technique, those who have had the experience of giving someone CPR will feel less alone, will be better understood by others.
“On the other hand, beyond the initial impact and the lack of a formal support system, the medium- and long-term outcome for those who acted is also psychologically and emotionally favorable,” said Jorge Bombau, MD, an obstetrician/gynecologist in Buenos Aires. After Dr. Bombau’s 14-year-old son Beltrán suddenly died during a school sports tournament, Dr. Bombau became a prominent advocate spreading the word about CPR.
“I don’t know anyone who regrets doing CPR,” he told this news organization. “There may be a brief period when the person feels distressed or depressed, when they have trouble sleeping. But it’s been proven that doing a good deed improves one’s mood. And what better deed is there than trying to save someone’s life? Whether their efforts were successful or in vain, that person has, at the end of the day, done something meaningful and worthwhile.”
Mr. Snitcofsky shares this sentiment. For several months now, he’s been feeling he’s “in a good place.” And he’s been actively promoting CPR on social media. As he recently posted on Twitter, “I’m here to retweet everything that has to do with getting us all to become familiar with how to do CPR and working up the courage to do it. The training takes no more than a few hours.
“I want to know that, if I ever have an out-of-hospital sudden cardiac arrest, there will be neighbors, friends, or family members around who know how to do CPR. Every person who knows how to do CPR can persuade others, and those of us who’ve had to do CPR in real life are even better candidates for persuading others. And if one day a person ends up needing CPR, I want to step in again and make up for lost time. Here’s hoping it’ll do the job,” he concluded.
It’s the same for Matías Alonso, a journalist in Buenos Aires. On New Year’s Eve 15 years ago, he was at a family dinner when, a few minutes before midnight, he found himself giving CPR to his stepmother’s father. “Unfortunately, he passed away, but I continued doing CPR on him until the ambulance arrived. For some time, I felt a little guilty for not taking charge of the situation from the beginning, and because I had this idea in my head that more people pulled through and recovered. But afterwards, they really thanked me a lot. And that helped me realize that I’d done something. I didn’t stand still when faced with the inevitability of death. I understood that it was good to have tried,” Mr. Alonso told this news organization. “And next time … hopefully there won’t be a next time … but I’m more prepared, and I now know how I can do better.”
Mr. Alonso, Mr. Snitcofsky, Dr. Fitz Maurice, Dr. Mosca, Dr. Bombau, and Dr. Márquez Murillo disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com. This article was translated from Medscape Spanish.