User login
Antipsychotic safe, effective for resistant depression in phase 3 trial
, new results from a phase 3 study show.
Already approved by the U.S. Food and Drug Administration to treat adults with schizophrenia and manic, mixed, or depressive episodes of bipolar I disorder, cariprazine is under investigation as an add-on therapy for MDD.
“Even patients who appear to be nonresponsive to standard antidepressant drugs have a very good chance of responding” to cariprazine, lead study author Gary Sachs, MD, associate clinical professor of psychiatry at Massachusetts General Hospital, Boston, told this news organization.
He noted that cariprazine, which is a partial agonist at D2 and D3, as well as 5-HT1A, “is an entirely different class” of drugs.
“It’s worth understanding how to use drugs like cariprazine and expanding our nomenclature; instead of referring to these drugs as atypical antipsychotics, perhaps referring to them as atypical antidepressants makes more sense,” Dr. Sachs said.
The findings were presented at the annual meeting of the American Psychiatric Association.
More options critical
MDD is among the most common psychiatric disorders in the United States. In 2020, an estimated 21 million adults had at least one major depressive episode.
Previous research has shown almost half of patients with MDD do not experience satisfactory results from their current treatment regimen. Therefore, research on more options for patients is critical, Dr. Sachs said.
Results from a previously published placebo-controlled study showed adjunctive treatment with cariprazine at 2-mg to 4.5-mg per day doses was more effective than placebo in improving depressive symptoms in adults with MDD.
The new analysis included patients with MDD and an inadequate response to antidepressant therapy, including selective serotonin reuptake inhibitors, serotonin and norepinephrine reuptake inhibitors (SNRIs), or tricyclic antidepressants. They were recruited from 116 centers in the United States and Europe.
Dr. Sachs noted that a nonresponse to an adequate dose of an antidepressant typically means having less than a 50% improvement over 6 weeks or more.
Researchers randomly assigned the patients to oral cariprazine 1.5 mg/day, cariprazine 3 mg/day, or placebo. All continued to take their antidepressant monotherapy.
The analysis included 757 mostly White participants (mean age, 44.8 years; 73.4% women). All had experienced depression for a “huge” part of their life (average, about 14 years), “not to mention their adult life,” said Dr. Sachs.
In addition, at the start of the study, the participants had been depressed for almost 8 months on average.
The primary endpoint was change at week 6 in Montgomery-Åsberg Depression Rating Scale (MADRS) total score. The mean baseline MADRS total score was 32.5.
Less is sometimes more
Results showed a significantly greater mean reduction in MADRS total score for cariprazine 1.5 mg/day vs. placebo at week 6 (P = .005). Significant differences from placebo were observed as early as week 2 and were maintained at week 4, as well as week 6.
“I can say with great confidence that the 1.5-mg dose met all the standards for efficacy,” Dr. Sachs said.
However, this was not the case for the 3-mg/day dose. Although there was a numerically greater reduction in MADRS total score for this dosage of the drug vs. placebo at week 6, the difference was not statistically significant (P = .07).
At week 6, more patients taking the active drug at 1.5 mg/day than placebo responded to treatment, defined as 50% or greater reduction in MADRS total score (44% vs. 34.9%, respectively; P < .05).
Researchers also assessed scores on the Clinical Global Impressions, finding significantly greater score improvement for both the 1.5-mg/day (P = .0026) and 3-mg/day (P =.0076) groups vs. the placebo group.
Improvement at week 6 in mean total score on the Hamilton Depression Rating Scale (HAM-17) reached nominal significance for cariprazine 1.5 mg/day vs. placebo – but not for 3 mg/day.
The results of this “high-quality” double-blind, randomized, controlled, parallel group study provide “what I regard as proven efficacy,” Dr. Sachs said.
He added that the investigational drug was also relatively safe. “The vast majority of patients tolerated it quite well,” he stressed. In addition, the drop-out rate because of adverse events was “quite low overall.”
The only adverse events (AEs) that occurred with the active treatment at a frequency of 5% or more and double that of placebo were akathisia and nausea. Changes in weight were relatively small, at less than 1 kg, in all treatment groups.
There was one serious AE in each active drug group, one of which was a kidney infection. There were two serious AEs reported in the placebo group, including one patient with multiple sclerosis. There were no deaths.
Dr. Sachs noted an advantage of cariprazine is its long half-life, which makes it more user-friendly because “it forgives you if you miss a dose or two.”
Drug manufacturer AbbVie’s supplemental New Drug Application for cariprazine is currently under review by the FDA for expanded use as adjunctive treatment of MDD. A decision by the agency is expected by the end of this year.
Another potential treatment option
Commenting on the findings, James Murrough, MD, PhD, associate professor of psychiatry and of neuroscience and director of the Depression and Anxiety Center for Discovery and Treatment at the Icahn School of Medicine at Mount Sinai, New York, said he welcomes research into additional treatments for MDD.
“Each medicine in a particular class has a unique pharmacology, so a larger number of medication options may help the clinician find a good match for a particular patient,” said Dr. Murrough, who was not involved with the research.
He noted cariprazine is “somewhat unique” among the dopamine modulators in “preferring interactions with the D3 receptor, one of many types of dopamine receptors.”
Although the study results showed cariprazine was effective in MDD, it “does not entirely break new ground” because previous research has already established the drug’s efficacy as adjunctive therapy for patients with depression not responding to a standard antidepressant, said Dr. Murrough.
He also noted that the lower dose, but not the higher dose, of the drug was found to be significantly beneficial for patients, compared with placebo.
“This is a good reminder that higher doses of a medication are not always better,” Dr. Murrough said.
The study was funded by AbbVie. Dr. Sachs is a full-time employee of Signant Health, which conducted the training and quality control for this study. Dr. Murrough has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new results from a phase 3 study show.
Already approved by the U.S. Food and Drug Administration to treat adults with schizophrenia and manic, mixed, or depressive episodes of bipolar I disorder, cariprazine is under investigation as an add-on therapy for MDD.
“Even patients who appear to be nonresponsive to standard antidepressant drugs have a very good chance of responding” to cariprazine, lead study author Gary Sachs, MD, associate clinical professor of psychiatry at Massachusetts General Hospital, Boston, told this news organization.
He noted that cariprazine, which is a partial agonist at D2 and D3, as well as 5-HT1A, “is an entirely different class” of drugs.
“It’s worth understanding how to use drugs like cariprazine and expanding our nomenclature; instead of referring to these drugs as atypical antipsychotics, perhaps referring to them as atypical antidepressants makes more sense,” Dr. Sachs said.
The findings were presented at the annual meeting of the American Psychiatric Association.
More options critical
MDD is among the most common psychiatric disorders in the United States. In 2020, an estimated 21 million adults had at least one major depressive episode.
Previous research has shown almost half of patients with MDD do not experience satisfactory results from their current treatment regimen. Therefore, research on more options for patients is critical, Dr. Sachs said.
Results from a previously published placebo-controlled study showed adjunctive treatment with cariprazine at 2-mg to 4.5-mg per day doses was more effective than placebo in improving depressive symptoms in adults with MDD.
The new analysis included patients with MDD and an inadequate response to antidepressant therapy, including selective serotonin reuptake inhibitors, serotonin and norepinephrine reuptake inhibitors (SNRIs), or tricyclic antidepressants. They were recruited from 116 centers in the United States and Europe.
Dr. Sachs noted that a nonresponse to an adequate dose of an antidepressant typically means having less than a 50% improvement over 6 weeks or more.
Researchers randomly assigned the patients to oral cariprazine 1.5 mg/day, cariprazine 3 mg/day, or placebo. All continued to take their antidepressant monotherapy.
The analysis included 757 mostly White participants (mean age, 44.8 years; 73.4% women). All had experienced depression for a “huge” part of their life (average, about 14 years), “not to mention their adult life,” said Dr. Sachs.
In addition, at the start of the study, the participants had been depressed for almost 8 months on average.
The primary endpoint was change at week 6 in Montgomery-Åsberg Depression Rating Scale (MADRS) total score. The mean baseline MADRS total score was 32.5.
Less is sometimes more
Results showed a significantly greater mean reduction in MADRS total score for cariprazine 1.5 mg/day vs. placebo at week 6 (P = .005). Significant differences from placebo were observed as early as week 2 and were maintained at week 4, as well as week 6.
“I can say with great confidence that the 1.5-mg dose met all the standards for efficacy,” Dr. Sachs said.
However, this was not the case for the 3-mg/day dose. Although there was a numerically greater reduction in MADRS total score for this dosage of the drug vs. placebo at week 6, the difference was not statistically significant (P = .07).
At week 6, more patients taking the active drug at 1.5 mg/day than placebo responded to treatment, defined as 50% or greater reduction in MADRS total score (44% vs. 34.9%, respectively; P < .05).
Researchers also assessed scores on the Clinical Global Impressions, finding significantly greater score improvement for both the 1.5-mg/day (P = .0026) and 3-mg/day (P =.0076) groups vs. the placebo group.
Improvement at week 6 in mean total score on the Hamilton Depression Rating Scale (HAM-17) reached nominal significance for cariprazine 1.5 mg/day vs. placebo – but not for 3 mg/day.
The results of this “high-quality” double-blind, randomized, controlled, parallel group study provide “what I regard as proven efficacy,” Dr. Sachs said.
He added that the investigational drug was also relatively safe. “The vast majority of patients tolerated it quite well,” he stressed. In addition, the drop-out rate because of adverse events was “quite low overall.”
The only adverse events (AEs) that occurred with the active treatment at a frequency of 5% or more and double that of placebo were akathisia and nausea. Changes in weight were relatively small, at less than 1 kg, in all treatment groups.
There was one serious AE in each active drug group, one of which was a kidney infection. There were two serious AEs reported in the placebo group, including one patient with multiple sclerosis. There were no deaths.
Dr. Sachs noted an advantage of cariprazine is its long half-life, which makes it more user-friendly because “it forgives you if you miss a dose or two.”
Drug manufacturer AbbVie’s supplemental New Drug Application for cariprazine is currently under review by the FDA for expanded use as adjunctive treatment of MDD. A decision by the agency is expected by the end of this year.
Another potential treatment option
Commenting on the findings, James Murrough, MD, PhD, associate professor of psychiatry and of neuroscience and director of the Depression and Anxiety Center for Discovery and Treatment at the Icahn School of Medicine at Mount Sinai, New York, said he welcomes research into additional treatments for MDD.
“Each medicine in a particular class has a unique pharmacology, so a larger number of medication options may help the clinician find a good match for a particular patient,” said Dr. Murrough, who was not involved with the research.
He noted cariprazine is “somewhat unique” among the dopamine modulators in “preferring interactions with the D3 receptor, one of many types of dopamine receptors.”
Although the study results showed cariprazine was effective in MDD, it “does not entirely break new ground” because previous research has already established the drug’s efficacy as adjunctive therapy for patients with depression not responding to a standard antidepressant, said Dr. Murrough.
He also noted that the lower dose, but not the higher dose, of the drug was found to be significantly beneficial for patients, compared with placebo.
“This is a good reminder that higher doses of a medication are not always better,” Dr. Murrough said.
The study was funded by AbbVie. Dr. Sachs is a full-time employee of Signant Health, which conducted the training and quality control for this study. Dr. Murrough has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new results from a phase 3 study show.
Already approved by the U.S. Food and Drug Administration to treat adults with schizophrenia and manic, mixed, or depressive episodes of bipolar I disorder, cariprazine is under investigation as an add-on therapy for MDD.
“Even patients who appear to be nonresponsive to standard antidepressant drugs have a very good chance of responding” to cariprazine, lead study author Gary Sachs, MD, associate clinical professor of psychiatry at Massachusetts General Hospital, Boston, told this news organization.
He noted that cariprazine, which is a partial agonist at D2 and D3, as well as 5-HT1A, “is an entirely different class” of drugs.
“It’s worth understanding how to use drugs like cariprazine and expanding our nomenclature; instead of referring to these drugs as atypical antipsychotics, perhaps referring to them as atypical antidepressants makes more sense,” Dr. Sachs said.
The findings were presented at the annual meeting of the American Psychiatric Association.
More options critical
MDD is among the most common psychiatric disorders in the United States. In 2020, an estimated 21 million adults had at least one major depressive episode.
Previous research has shown almost half of patients with MDD do not experience satisfactory results from their current treatment regimen. Therefore, research on more options for patients is critical, Dr. Sachs said.
Results from a previously published placebo-controlled study showed adjunctive treatment with cariprazine at 2-mg to 4.5-mg per day doses was more effective than placebo in improving depressive symptoms in adults with MDD.
The new analysis included patients with MDD and an inadequate response to antidepressant therapy, including selective serotonin reuptake inhibitors, serotonin and norepinephrine reuptake inhibitors (SNRIs), or tricyclic antidepressants. They were recruited from 116 centers in the United States and Europe.
Dr. Sachs noted that a nonresponse to an adequate dose of an antidepressant typically means having less than a 50% improvement over 6 weeks or more.
Researchers randomly assigned the patients to oral cariprazine 1.5 mg/day, cariprazine 3 mg/day, or placebo. All continued to take their antidepressant monotherapy.
The analysis included 757 mostly White participants (mean age, 44.8 years; 73.4% women). All had experienced depression for a “huge” part of their life (average, about 14 years), “not to mention their adult life,” said Dr. Sachs.
In addition, at the start of the study, the participants had been depressed for almost 8 months on average.
The primary endpoint was change at week 6 in Montgomery-Åsberg Depression Rating Scale (MADRS) total score. The mean baseline MADRS total score was 32.5.
Less is sometimes more
Results showed a significantly greater mean reduction in MADRS total score for cariprazine 1.5 mg/day vs. placebo at week 6 (P = .005). Significant differences from placebo were observed as early as week 2 and were maintained at week 4, as well as week 6.
“I can say with great confidence that the 1.5-mg dose met all the standards for efficacy,” Dr. Sachs said.
However, this was not the case for the 3-mg/day dose. Although there was a numerically greater reduction in MADRS total score for this dosage of the drug vs. placebo at week 6, the difference was not statistically significant (P = .07).
At week 6, more patients taking the active drug at 1.5 mg/day than placebo responded to treatment, defined as 50% or greater reduction in MADRS total score (44% vs. 34.9%, respectively; P < .05).
Researchers also assessed scores on the Clinical Global Impressions, finding significantly greater score improvement for both the 1.5-mg/day (P = .0026) and 3-mg/day (P =.0076) groups vs. the placebo group.
Improvement at week 6 in mean total score on the Hamilton Depression Rating Scale (HAM-17) reached nominal significance for cariprazine 1.5 mg/day vs. placebo – but not for 3 mg/day.
The results of this “high-quality” double-blind, randomized, controlled, parallel group study provide “what I regard as proven efficacy,” Dr. Sachs said.
He added that the investigational drug was also relatively safe. “The vast majority of patients tolerated it quite well,” he stressed. In addition, the drop-out rate because of adverse events was “quite low overall.”
The only adverse events (AEs) that occurred with the active treatment at a frequency of 5% or more and double that of placebo were akathisia and nausea. Changes in weight were relatively small, at less than 1 kg, in all treatment groups.
There was one serious AE in each active drug group, one of which was a kidney infection. There were two serious AEs reported in the placebo group, including one patient with multiple sclerosis. There were no deaths.
Dr. Sachs noted an advantage of cariprazine is its long half-life, which makes it more user-friendly because “it forgives you if you miss a dose or two.”
Drug manufacturer AbbVie’s supplemental New Drug Application for cariprazine is currently under review by the FDA for expanded use as adjunctive treatment of MDD. A decision by the agency is expected by the end of this year.
Another potential treatment option
Commenting on the findings, James Murrough, MD, PhD, associate professor of psychiatry and of neuroscience and director of the Depression and Anxiety Center for Discovery and Treatment at the Icahn School of Medicine at Mount Sinai, New York, said he welcomes research into additional treatments for MDD.
“Each medicine in a particular class has a unique pharmacology, so a larger number of medication options may help the clinician find a good match for a particular patient,” said Dr. Murrough, who was not involved with the research.
He noted cariprazine is “somewhat unique” among the dopamine modulators in “preferring interactions with the D3 receptor, one of many types of dopamine receptors.”
Although the study results showed cariprazine was effective in MDD, it “does not entirely break new ground” because previous research has already established the drug’s efficacy as adjunctive therapy for patients with depression not responding to a standard antidepressant, said Dr. Murrough.
He also noted that the lower dose, but not the higher dose, of the drug was found to be significantly beneficial for patients, compared with placebo.
“This is a good reminder that higher doses of a medication are not always better,” Dr. Murrough said.
The study was funded by AbbVie. Dr. Sachs is a full-time employee of Signant Health, which conducted the training and quality control for this study. Dr. Murrough has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM APA 2022
Depressed patients respond faster to IV ketamine than intranasal ketamine
NEW ORLEANS – New research reveals that patients with treatment-resistant depression who were treated with repeated intravenous ketamine show no significant differences in achieving response or remission, compared with those receiving the intranasal formulation of the drug, esketamine – although fewer treatments appear necessary with the intravenous formulation.
“ although at the end, the responses are similar,” said first author Balwinder Singh, MD, of the department of psychiatry and psychology, Mayo Clinic, in Rochester, Minn.
The findings were presented at the annual meeting of the American Psychiatric Association.
Commenting on the study, Roger S. McIntyre, MD, underscored that “this is an important study that addresses the priority questions that everyone wants to know – not only for clinical reasons, but economic reasons.” Dr. McIntyre, a professor of psychiatry and pharmacology at the University of Toronto, and head of the university’s mood disorders psychopharmacology unit, said that “there are implications not only for clinical outcomes and cost, but also implementation because IV is obviously more demanding and complicated.”
As intravenous ketamine increasingly gained interest as a rapid-acting treatment for patients with severe, treatment-resistant depression, the introduction of a more convenient intranasal formulation was seen as a welcome improvement and received approval from the Food and Drug Administration in 2019. However, while the approval ushered in more coverage by insurance companies, the treatment can still be expensive. Intravenous ketamine does not have FDA approval.
With a lack of studies in the real-world setting comparing efficacy of the two formulations, Dr. Singh and his colleagues conducted the observational study, evaluating the responses of 62 adults with treatment-resistant depression who had received either up to six IV ketamine infusions of 0.5 mg/kg, infused over 40 minutes, or up to eight intranasal esketamine treatments of 56/84 mg, as approved by the FDA, at the Mayo Clinic Depression Center.
Of the patients, who had a mean age of 47 years, 59 had major depression and 3 had bipolar depression. Among them, 76% (47) received intravenous ketamine and 24% (15) received esketamine, which Dr. Singh noted reflected the higher number of patients included before esketamine received FDA approval. The patients had similar comorbidity profiles, with the intravenous ketamine group having a higher body mass index at baseline.
Overall, the patients all had significant improvement in their depression at the end of the acute phase of 4 weeks, with a mean change in on the 16-Item Quick Inventory of Depressive Symptomatology (QIDS-SR) scale of –8.6 from baseline (P < .001).
The overall remission rate was 38.7% and overall response rate was 58.1%. Those receiving intravenous ketamine had response and remission rates of 57.4% and 42.6%, versus response and remission rates of 60.0% and 26.7% among the esketamine group, which Dr. Singh said were not significant differences (P > .05).
However, the mean number of treatments necessary to achieve response in the intravenous ketamine group was just 2.3 versus 4.6 with esketamine, and the mean number of treatments to achieve remission were 2.5 versus 6.3, respectively (P = .008).
After a multivariate adjustment, the time to response was determined to be faster with intravenous ketamine versus esketamine (hazard ratio, 2.61; P = .05) and the time to remission was also faster (HR, 5.0; P = .02).
“What this means is you would need fewer treatments to achieve a response or remission with IV ketamine, so there could be an acceleration of patients’ antidepressant response,” Dr. Singh explained.
There were no significant differences between the groups in terms of side effects, and most patients tolerated the treatments well.
Dr. Singh noted the limitation of the study is that it was observational and included a small sample size. Nevertheless, when asked which he would choose if starting treatment when insurance was not an issue, Dr. Singh replied: “I would take patient preference into account, but certainly IV seems to have an advantage.”
Dr. McIntyre noted that, though small, the study’s setting in a real world clinical environment is important.
“Obviously this is observational and not controlled, but the strength is that this involved a real-world cohort of patients and real world applications,” he said. “It’s difficult to have a true comparator head-to-head trial, so that makes this all the more important because it takes into consideration all of the complexities of real world patients.”
Dr. McIntyre emphasized that the study is not “the last word on the story because we need to see a larger sample and replication. But certainly they make an argument that IV ketamine may have an advantage over the speed of onset with intranasal ketamine, which will need to be either replicated or refuted, but it’s a great starting point in the conversation.”
Navigating patient preference
Robert Meisner, MD, founding medical director of the McLean Ketamine Service, Division of Psychiatric Neurotherapeutics, McLean Hospital, Harvard Medical School, in Boston, noted that wide-ranging factors may influence patient as well as clinician decisions about which ketamine treatment approach to use.
“When a patient appears to be equally well-suited for both interventions, I continue to be surprised by why one patient will indicate a preference for intranasal esketamine, while another will lean toward IV racemic ketamine,” he said in an interview.
“Some patients find esketamine’s clear and consistent protocol optimal for scheduling and navigating the logistics of daily life; others value the flexibility offered by certain evidence-based, racemic (IV) protocols,” he said. “Predicting who will prefer each treatment, even with the apparent temporal advantage with IV ketamine, is extremely difficult.”
Likewise, in terms of clinician preference, Dr. Meisner notes that key concerns may sway decisions.
“If I’m concerned with labile pressures or hypertension, for example, or if I have a patient with, say, Erlos Danlos Syndrome without a clear subtype, and hence, some risk of undiscovered aneurysmal vascular disease, I may lean toward racemic IV ketamine.”
On the other hand, “some patients find the simplicity and predictability of the maintenance esketamine protocol comforting and psychologically stabilizing,” he added. “Yet others find that their work or family’s erratic demands on their time make one of the evidence-based racemic regimens preferable – inasmuch as it integrates more flexibility and allows them to remain more fully engaged in the basic activities or work and family.”
Dr. Meisner noted the caveat that efforts to decide which method to use are often complicated by substantial misinformation.
“I can’t emphasize how much misinformation continues to abound regarding appropriate (evidence-based) and safe use of ketamine and esketamine,” he said. “Especially on the IV racemic side, there simply is no substantive evidence base for many of the claims that some providers are preaching.”
The confusion, driven in part by social media, “has diffused into sectors of the field and industry that one might assume are relatively immune (i.e., allied physicians, sophisticated payers, etc),” he added.
“In short, two mantra continue to apply,” Dr. Meisner said. “One – if it sounds too good to be true, it probably is; and two – in pharmacology and interventional psychiatry, we see remarkable progress and potential, but there simply is no such thing as a magic bullet.”
Dr. Singh and Dr. Meisner had no disclosures to report. Dr. McIntyre has received research grant support from Canadian Institutes of Health Research/Global Alliance for Chronic Diseases/National Natural Science Foundation of China, and speaker/consultation fees from Lundbeck, Janssen, Alkermes,Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Abbvie, and Atai Life Sciences. Dr. McIntyre is a CEO of Braxia Scientific.
NEW ORLEANS – New research reveals that patients with treatment-resistant depression who were treated with repeated intravenous ketamine show no significant differences in achieving response or remission, compared with those receiving the intranasal formulation of the drug, esketamine – although fewer treatments appear necessary with the intravenous formulation.
“ although at the end, the responses are similar,” said first author Balwinder Singh, MD, of the department of psychiatry and psychology, Mayo Clinic, in Rochester, Minn.
The findings were presented at the annual meeting of the American Psychiatric Association.
Commenting on the study, Roger S. McIntyre, MD, underscored that “this is an important study that addresses the priority questions that everyone wants to know – not only for clinical reasons, but economic reasons.” Dr. McIntyre, a professor of psychiatry and pharmacology at the University of Toronto, and head of the university’s mood disorders psychopharmacology unit, said that “there are implications not only for clinical outcomes and cost, but also implementation because IV is obviously more demanding and complicated.”
As intravenous ketamine increasingly gained interest as a rapid-acting treatment for patients with severe, treatment-resistant depression, the introduction of a more convenient intranasal formulation was seen as a welcome improvement and received approval from the Food and Drug Administration in 2019. However, while the approval ushered in more coverage by insurance companies, the treatment can still be expensive. Intravenous ketamine does not have FDA approval.
With a lack of studies in the real-world setting comparing efficacy of the two formulations, Dr. Singh and his colleagues conducted the observational study, evaluating the responses of 62 adults with treatment-resistant depression who had received either up to six IV ketamine infusions of 0.5 mg/kg, infused over 40 minutes, or up to eight intranasal esketamine treatments of 56/84 mg, as approved by the FDA, at the Mayo Clinic Depression Center.
Of the patients, who had a mean age of 47 years, 59 had major depression and 3 had bipolar depression. Among them, 76% (47) received intravenous ketamine and 24% (15) received esketamine, which Dr. Singh noted reflected the higher number of patients included before esketamine received FDA approval. The patients had similar comorbidity profiles, with the intravenous ketamine group having a higher body mass index at baseline.
Overall, the patients all had significant improvement in their depression at the end of the acute phase of 4 weeks, with a mean change in on the 16-Item Quick Inventory of Depressive Symptomatology (QIDS-SR) scale of –8.6 from baseline (P < .001).
The overall remission rate was 38.7% and overall response rate was 58.1%. Those receiving intravenous ketamine had response and remission rates of 57.4% and 42.6%, versus response and remission rates of 60.0% and 26.7% among the esketamine group, which Dr. Singh said were not significant differences (P > .05).
However, the mean number of treatments necessary to achieve response in the intravenous ketamine group was just 2.3 versus 4.6 with esketamine, and the mean number of treatments to achieve remission were 2.5 versus 6.3, respectively (P = .008).
After a multivariate adjustment, the time to response was determined to be faster with intravenous ketamine versus esketamine (hazard ratio, 2.61; P = .05) and the time to remission was also faster (HR, 5.0; P = .02).
“What this means is you would need fewer treatments to achieve a response or remission with IV ketamine, so there could be an acceleration of patients’ antidepressant response,” Dr. Singh explained.
There were no significant differences between the groups in terms of side effects, and most patients tolerated the treatments well.
Dr. Singh noted the limitation of the study is that it was observational and included a small sample size. Nevertheless, when asked which he would choose if starting treatment when insurance was not an issue, Dr. Singh replied: “I would take patient preference into account, but certainly IV seems to have an advantage.”
Dr. McIntyre noted that, though small, the study’s setting in a real world clinical environment is important.
“Obviously this is observational and not controlled, but the strength is that this involved a real-world cohort of patients and real world applications,” he said. “It’s difficult to have a true comparator head-to-head trial, so that makes this all the more important because it takes into consideration all of the complexities of real world patients.”
Dr. McIntyre emphasized that the study is not “the last word on the story because we need to see a larger sample and replication. But certainly they make an argument that IV ketamine may have an advantage over the speed of onset with intranasal ketamine, which will need to be either replicated or refuted, but it’s a great starting point in the conversation.”
Navigating patient preference
Robert Meisner, MD, founding medical director of the McLean Ketamine Service, Division of Psychiatric Neurotherapeutics, McLean Hospital, Harvard Medical School, in Boston, noted that wide-ranging factors may influence patient as well as clinician decisions about which ketamine treatment approach to use.
“When a patient appears to be equally well-suited for both interventions, I continue to be surprised by why one patient will indicate a preference for intranasal esketamine, while another will lean toward IV racemic ketamine,” he said in an interview.
“Some patients find esketamine’s clear and consistent protocol optimal for scheduling and navigating the logistics of daily life; others value the flexibility offered by certain evidence-based, racemic (IV) protocols,” he said. “Predicting who will prefer each treatment, even with the apparent temporal advantage with IV ketamine, is extremely difficult.”
Likewise, in terms of clinician preference, Dr. Meisner notes that key concerns may sway decisions.
“If I’m concerned with labile pressures or hypertension, for example, or if I have a patient with, say, Erlos Danlos Syndrome without a clear subtype, and hence, some risk of undiscovered aneurysmal vascular disease, I may lean toward racemic IV ketamine.”
On the other hand, “some patients find the simplicity and predictability of the maintenance esketamine protocol comforting and psychologically stabilizing,” he added. “Yet others find that their work or family’s erratic demands on their time make one of the evidence-based racemic regimens preferable – inasmuch as it integrates more flexibility and allows them to remain more fully engaged in the basic activities or work and family.”
Dr. Meisner noted the caveat that efforts to decide which method to use are often complicated by substantial misinformation.
“I can’t emphasize how much misinformation continues to abound regarding appropriate (evidence-based) and safe use of ketamine and esketamine,” he said. “Especially on the IV racemic side, there simply is no substantive evidence base for many of the claims that some providers are preaching.”
The confusion, driven in part by social media, “has diffused into sectors of the field and industry that one might assume are relatively immune (i.e., allied physicians, sophisticated payers, etc),” he added.
“In short, two mantra continue to apply,” Dr. Meisner said. “One – if it sounds too good to be true, it probably is; and two – in pharmacology and interventional psychiatry, we see remarkable progress and potential, but there simply is no such thing as a magic bullet.”
Dr. Singh and Dr. Meisner had no disclosures to report. Dr. McIntyre has received research grant support from Canadian Institutes of Health Research/Global Alliance for Chronic Diseases/National Natural Science Foundation of China, and speaker/consultation fees from Lundbeck, Janssen, Alkermes,Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Abbvie, and Atai Life Sciences. Dr. McIntyre is a CEO of Braxia Scientific.
NEW ORLEANS – New research reveals that patients with treatment-resistant depression who were treated with repeated intravenous ketamine show no significant differences in achieving response or remission, compared with those receiving the intranasal formulation of the drug, esketamine – although fewer treatments appear necessary with the intravenous formulation.
“ although at the end, the responses are similar,” said first author Balwinder Singh, MD, of the department of psychiatry and psychology, Mayo Clinic, in Rochester, Minn.
The findings were presented at the annual meeting of the American Psychiatric Association.
Commenting on the study, Roger S. McIntyre, MD, underscored that “this is an important study that addresses the priority questions that everyone wants to know – not only for clinical reasons, but economic reasons.” Dr. McIntyre, a professor of psychiatry and pharmacology at the University of Toronto, and head of the university’s mood disorders psychopharmacology unit, said that “there are implications not only for clinical outcomes and cost, but also implementation because IV is obviously more demanding and complicated.”
As intravenous ketamine increasingly gained interest as a rapid-acting treatment for patients with severe, treatment-resistant depression, the introduction of a more convenient intranasal formulation was seen as a welcome improvement and received approval from the Food and Drug Administration in 2019. However, while the approval ushered in more coverage by insurance companies, the treatment can still be expensive. Intravenous ketamine does not have FDA approval.
With a lack of studies in the real-world setting comparing efficacy of the two formulations, Dr. Singh and his colleagues conducted the observational study, evaluating the responses of 62 adults with treatment-resistant depression who had received either up to six IV ketamine infusions of 0.5 mg/kg, infused over 40 minutes, or up to eight intranasal esketamine treatments of 56/84 mg, as approved by the FDA, at the Mayo Clinic Depression Center.
Of the patients, who had a mean age of 47 years, 59 had major depression and 3 had bipolar depression. Among them, 76% (47) received intravenous ketamine and 24% (15) received esketamine, which Dr. Singh noted reflected the higher number of patients included before esketamine received FDA approval. The patients had similar comorbidity profiles, with the intravenous ketamine group having a higher body mass index at baseline.
Overall, the patients all had significant improvement in their depression at the end of the acute phase of 4 weeks, with a mean change in on the 16-Item Quick Inventory of Depressive Symptomatology (QIDS-SR) scale of –8.6 from baseline (P < .001).
The overall remission rate was 38.7% and overall response rate was 58.1%. Those receiving intravenous ketamine had response and remission rates of 57.4% and 42.6%, versus response and remission rates of 60.0% and 26.7% among the esketamine group, which Dr. Singh said were not significant differences (P > .05).
However, the mean number of treatments necessary to achieve response in the intravenous ketamine group was just 2.3 versus 4.6 with esketamine, and the mean number of treatments to achieve remission were 2.5 versus 6.3, respectively (P = .008).
After a multivariate adjustment, the time to response was determined to be faster with intravenous ketamine versus esketamine (hazard ratio, 2.61; P = .05) and the time to remission was also faster (HR, 5.0; P = .02).
“What this means is you would need fewer treatments to achieve a response or remission with IV ketamine, so there could be an acceleration of patients’ antidepressant response,” Dr. Singh explained.
There were no significant differences between the groups in terms of side effects, and most patients tolerated the treatments well.
Dr. Singh noted the limitation of the study is that it was observational and included a small sample size. Nevertheless, when asked which he would choose if starting treatment when insurance was not an issue, Dr. Singh replied: “I would take patient preference into account, but certainly IV seems to have an advantage.”
Dr. McIntyre noted that, though small, the study’s setting in a real world clinical environment is important.
“Obviously this is observational and not controlled, but the strength is that this involved a real-world cohort of patients and real world applications,” he said. “It’s difficult to have a true comparator head-to-head trial, so that makes this all the more important because it takes into consideration all of the complexities of real world patients.”
Dr. McIntyre emphasized that the study is not “the last word on the story because we need to see a larger sample and replication. But certainly they make an argument that IV ketamine may have an advantage over the speed of onset with intranasal ketamine, which will need to be either replicated or refuted, but it’s a great starting point in the conversation.”
Navigating patient preference
Robert Meisner, MD, founding medical director of the McLean Ketamine Service, Division of Psychiatric Neurotherapeutics, McLean Hospital, Harvard Medical School, in Boston, noted that wide-ranging factors may influence patient as well as clinician decisions about which ketamine treatment approach to use.
“When a patient appears to be equally well-suited for both interventions, I continue to be surprised by why one patient will indicate a preference for intranasal esketamine, while another will lean toward IV racemic ketamine,” he said in an interview.
“Some patients find esketamine’s clear and consistent protocol optimal for scheduling and navigating the logistics of daily life; others value the flexibility offered by certain evidence-based, racemic (IV) protocols,” he said. “Predicting who will prefer each treatment, even with the apparent temporal advantage with IV ketamine, is extremely difficult.”
Likewise, in terms of clinician preference, Dr. Meisner notes that key concerns may sway decisions.
“If I’m concerned with labile pressures or hypertension, for example, or if I have a patient with, say, Erlos Danlos Syndrome without a clear subtype, and hence, some risk of undiscovered aneurysmal vascular disease, I may lean toward racemic IV ketamine.”
On the other hand, “some patients find the simplicity and predictability of the maintenance esketamine protocol comforting and psychologically stabilizing,” he added. “Yet others find that their work or family’s erratic demands on their time make one of the evidence-based racemic regimens preferable – inasmuch as it integrates more flexibility and allows them to remain more fully engaged in the basic activities or work and family.”
Dr. Meisner noted the caveat that efforts to decide which method to use are often complicated by substantial misinformation.
“I can’t emphasize how much misinformation continues to abound regarding appropriate (evidence-based) and safe use of ketamine and esketamine,” he said. “Especially on the IV racemic side, there simply is no substantive evidence base for many of the claims that some providers are preaching.”
The confusion, driven in part by social media, “has diffused into sectors of the field and industry that one might assume are relatively immune (i.e., allied physicians, sophisticated payers, etc),” he added.
“In short, two mantra continue to apply,” Dr. Meisner said. “One – if it sounds too good to be true, it probably is; and two – in pharmacology and interventional psychiatry, we see remarkable progress and potential, but there simply is no such thing as a magic bullet.”
Dr. Singh and Dr. Meisner had no disclosures to report. Dr. McIntyre has received research grant support from Canadian Institutes of Health Research/Global Alliance for Chronic Diseases/National Natural Science Foundation of China, and speaker/consultation fees from Lundbeck, Janssen, Alkermes,Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Abbvie, and Atai Life Sciences. Dr. McIntyre is a CEO of Braxia Scientific.
AT APA 2022
Disasters abroad a major trigger for mental illness in expats
The 2020 explosion that rocked Beirut, killing more than 200, injuring more than 7,000 and causing millions of dollars in damage had a significant impact on the mental health of Lebanese expatriates, leaving many grappling with anxiety, depression, and posttraumatic stress disorder, results of a new survey show.
The findings highlight the importance of considering the well-being of expatriates dealing with adverse events in their home countries, the investigators say.
“Everyone, including doctors, should be more sensitive to expatriates around them; we should look out for them especially when their home country is going through a traumatic event,” study investigator Gaëlle Rached, MD, MSc, research postdoctoral fellow, Northwestern University, Chicago, told this news organization.
The findings were presented at the annual meeting of the American Psychiatric Association.
A historic explosion
It is estimated that approximately 14 million Lebanese citizens live outside their home country, which is more than double the population of Lebanon. However, the trauma-related mental health of these and other expatriate communities is understudied, said Dr. Rached.
“If you look at the literature, next to no one has examined expatriates’ mental health, and more so in the context of trauma.”
Dr. Rached has personal experience with the event. She was in Beirut on Aug. 4, 2020, when the Lebanese capital was rocked by an explosion attributed to ammonium nitrate stored at the city’s port. It was one of the biggest nonnuclear explosions in history and left hundreds homeless, killed, or injured. Dr. Rached watched as her father was injured and her house destroyed.
She heard anecdotes of Lebanese expatriates, experiencing trauma as a result of the blast. Many were unable to contact friends and loved ones in the wake of the tragedy.
“That prompted us to look at expatriate mental health following this traumatic incident,” she said.
She and her colleagues used various social media platforms to advertise the survey. They also reached out to the International Lebanese Medical Association, which has “a strong base” in the United States, said Dr. Rached.
She was “shocked” at how many expatriates responded. “People really wanted to speak up and express themselves” – whether because of survivor’s guilt or for some other reason, she said.
The survey included 670 adults with Lebanese nationality or who were first generation Lebanese living abroad. The study population had a median age 31 years and 62.2% female, most living in North America or Europe. Over one-third of respondents (270) had been living abroad from 1-5 years but many had been away for more than 20 years.
Study participants completed the Hopkins Symptoms Checklist (HSCL), which screens for anxiety and depression. On this checklist, a score of 1.75 is a typical cutoff value for symptomatic cases.
The investigators found 41.2% of participants scored higher than this threshold. Being younger, female and visiting Lebanon at the time of the blast, were factors associated with higher HSCL scores.
No tincture of time
Interestingly, the amount of time since emigrating from Lebanon was unrelated to the score. “Our results show that, no matter how long you’ve been away, you’re prone to the same negative outcome,” said Dr. Rached.
Of the total study population, 268 personally experienced the explosion and/or had close friends or family physically affected by it. These expatriates completed the Post-traumatic Checklist for DSM-5 (PCL-5).
Here, the analysis showed that many of these respondents (57.5%) scored above 33, which is higher than the threshold for probable PTSD. Being female was linked to higher PCL-5 scores.
The results may be especially timely as many countries are taking in a flood of refugees fleeing war in Ukraine. However, Dr. Rached said, the findings from her research may not apply to Ukrainians.
“I don’t think the results can be extrapolated, given that the nature of the trauma is a little bit different,” she said, adding that the Beirut blast was “monumental” but it was over quickly. In contrast, there’s no end in sight for the Russian invasion of Ukraine.
Dr. Rached noted the study data are preliminary and limited because there’s no way to determine whether respondents had mental health issues before the blast.
Global psychiatrist shortage
Commenting on the study, Howard Liu, MD, chair of the University of Nebraska Medical Center department of psychiatry in Omaha, and incoming chair of the APA’s Council on Communications, said he found the presentation “fascinating on several levels.”
It’s increasingly important for psychiatrists to be “trauma informed,” Dr. Liu told a press briefing highlighting the study. “It’s not just about looking at the biological correlates of illness,” meaning looking at genetic markers etc, “but also looking at the environment in which people live, work, and/or are in therapy or in treatment.”
In a later interview, Dr. Liu said he was impressed by the fact that Dr. Rached, who has “a very deep personal connection to this community,” is using her own personal trauma to help identify others are at risk who may need future care.
Dr. Liu, whose own family sponsors Afghan refugees, said the research underlines the need to ensure training for psychiatrists everywhere to help manage the expatriate population. As it stands, there’s “a huge shortage of psychiatrists around the world,” particularly in countries that have been affected by trauma, said Dr. Liu.
The researchers and Dr. Liu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The 2020 explosion that rocked Beirut, killing more than 200, injuring more than 7,000 and causing millions of dollars in damage had a significant impact on the mental health of Lebanese expatriates, leaving many grappling with anxiety, depression, and posttraumatic stress disorder, results of a new survey show.
The findings highlight the importance of considering the well-being of expatriates dealing with adverse events in their home countries, the investigators say.
“Everyone, including doctors, should be more sensitive to expatriates around them; we should look out for them especially when their home country is going through a traumatic event,” study investigator Gaëlle Rached, MD, MSc, research postdoctoral fellow, Northwestern University, Chicago, told this news organization.
The findings were presented at the annual meeting of the American Psychiatric Association.
A historic explosion
It is estimated that approximately 14 million Lebanese citizens live outside their home country, which is more than double the population of Lebanon. However, the trauma-related mental health of these and other expatriate communities is understudied, said Dr. Rached.
“If you look at the literature, next to no one has examined expatriates’ mental health, and more so in the context of trauma.”
Dr. Rached has personal experience with the event. She was in Beirut on Aug. 4, 2020, when the Lebanese capital was rocked by an explosion attributed to ammonium nitrate stored at the city’s port. It was one of the biggest nonnuclear explosions in history and left hundreds homeless, killed, or injured. Dr. Rached watched as her father was injured and her house destroyed.
She heard anecdotes of Lebanese expatriates, experiencing trauma as a result of the blast. Many were unable to contact friends and loved ones in the wake of the tragedy.
“That prompted us to look at expatriate mental health following this traumatic incident,” she said.
She and her colleagues used various social media platforms to advertise the survey. They also reached out to the International Lebanese Medical Association, which has “a strong base” in the United States, said Dr. Rached.
She was “shocked” at how many expatriates responded. “People really wanted to speak up and express themselves” – whether because of survivor’s guilt or for some other reason, she said.
The survey included 670 adults with Lebanese nationality or who were first generation Lebanese living abroad. The study population had a median age 31 years and 62.2% female, most living in North America or Europe. Over one-third of respondents (270) had been living abroad from 1-5 years but many had been away for more than 20 years.
Study participants completed the Hopkins Symptoms Checklist (HSCL), which screens for anxiety and depression. On this checklist, a score of 1.75 is a typical cutoff value for symptomatic cases.
The investigators found 41.2% of participants scored higher than this threshold. Being younger, female and visiting Lebanon at the time of the blast, were factors associated with higher HSCL scores.
No tincture of time
Interestingly, the amount of time since emigrating from Lebanon was unrelated to the score. “Our results show that, no matter how long you’ve been away, you’re prone to the same negative outcome,” said Dr. Rached.
Of the total study population, 268 personally experienced the explosion and/or had close friends or family physically affected by it. These expatriates completed the Post-traumatic Checklist for DSM-5 (PCL-5).
Here, the analysis showed that many of these respondents (57.5%) scored above 33, which is higher than the threshold for probable PTSD. Being female was linked to higher PCL-5 scores.
The results may be especially timely as many countries are taking in a flood of refugees fleeing war in Ukraine. However, Dr. Rached said, the findings from her research may not apply to Ukrainians.
“I don’t think the results can be extrapolated, given that the nature of the trauma is a little bit different,” she said, adding that the Beirut blast was “monumental” but it was over quickly. In contrast, there’s no end in sight for the Russian invasion of Ukraine.
Dr. Rached noted the study data are preliminary and limited because there’s no way to determine whether respondents had mental health issues before the blast.
Global psychiatrist shortage
Commenting on the study, Howard Liu, MD, chair of the University of Nebraska Medical Center department of psychiatry in Omaha, and incoming chair of the APA’s Council on Communications, said he found the presentation “fascinating on several levels.”
It’s increasingly important for psychiatrists to be “trauma informed,” Dr. Liu told a press briefing highlighting the study. “It’s not just about looking at the biological correlates of illness,” meaning looking at genetic markers etc, “but also looking at the environment in which people live, work, and/or are in therapy or in treatment.”
In a later interview, Dr. Liu said he was impressed by the fact that Dr. Rached, who has “a very deep personal connection to this community,” is using her own personal trauma to help identify others are at risk who may need future care.
Dr. Liu, whose own family sponsors Afghan refugees, said the research underlines the need to ensure training for psychiatrists everywhere to help manage the expatriate population. As it stands, there’s “a huge shortage of psychiatrists around the world,” particularly in countries that have been affected by trauma, said Dr. Liu.
The researchers and Dr. Liu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The 2020 explosion that rocked Beirut, killing more than 200, injuring more than 7,000 and causing millions of dollars in damage had a significant impact on the mental health of Lebanese expatriates, leaving many grappling with anxiety, depression, and posttraumatic stress disorder, results of a new survey show.
The findings highlight the importance of considering the well-being of expatriates dealing with adverse events in their home countries, the investigators say.
“Everyone, including doctors, should be more sensitive to expatriates around them; we should look out for them especially when their home country is going through a traumatic event,” study investigator Gaëlle Rached, MD, MSc, research postdoctoral fellow, Northwestern University, Chicago, told this news organization.
The findings were presented at the annual meeting of the American Psychiatric Association.
A historic explosion
It is estimated that approximately 14 million Lebanese citizens live outside their home country, which is more than double the population of Lebanon. However, the trauma-related mental health of these and other expatriate communities is understudied, said Dr. Rached.
“If you look at the literature, next to no one has examined expatriates’ mental health, and more so in the context of trauma.”
Dr. Rached has personal experience with the event. She was in Beirut on Aug. 4, 2020, when the Lebanese capital was rocked by an explosion attributed to ammonium nitrate stored at the city’s port. It was one of the biggest nonnuclear explosions in history and left hundreds homeless, killed, or injured. Dr. Rached watched as her father was injured and her house destroyed.
She heard anecdotes of Lebanese expatriates, experiencing trauma as a result of the blast. Many were unable to contact friends and loved ones in the wake of the tragedy.
“That prompted us to look at expatriate mental health following this traumatic incident,” she said.
She and her colleagues used various social media platforms to advertise the survey. They also reached out to the International Lebanese Medical Association, which has “a strong base” in the United States, said Dr. Rached.
She was “shocked” at how many expatriates responded. “People really wanted to speak up and express themselves” – whether because of survivor’s guilt or for some other reason, she said.
The survey included 670 adults with Lebanese nationality or who were first generation Lebanese living abroad. The study population had a median age 31 years and 62.2% female, most living in North America or Europe. Over one-third of respondents (270) had been living abroad from 1-5 years but many had been away for more than 20 years.
Study participants completed the Hopkins Symptoms Checklist (HSCL), which screens for anxiety and depression. On this checklist, a score of 1.75 is a typical cutoff value for symptomatic cases.
The investigators found 41.2% of participants scored higher than this threshold. Being younger, female and visiting Lebanon at the time of the blast, were factors associated with higher HSCL scores.
No tincture of time
Interestingly, the amount of time since emigrating from Lebanon was unrelated to the score. “Our results show that, no matter how long you’ve been away, you’re prone to the same negative outcome,” said Dr. Rached.
Of the total study population, 268 personally experienced the explosion and/or had close friends or family physically affected by it. These expatriates completed the Post-traumatic Checklist for DSM-5 (PCL-5).
Here, the analysis showed that many of these respondents (57.5%) scored above 33, which is higher than the threshold for probable PTSD. Being female was linked to higher PCL-5 scores.
The results may be especially timely as many countries are taking in a flood of refugees fleeing war in Ukraine. However, Dr. Rached said, the findings from her research may not apply to Ukrainians.
“I don’t think the results can be extrapolated, given that the nature of the trauma is a little bit different,” she said, adding that the Beirut blast was “monumental” but it was over quickly. In contrast, there’s no end in sight for the Russian invasion of Ukraine.
Dr. Rached noted the study data are preliminary and limited because there’s no way to determine whether respondents had mental health issues before the blast.
Global psychiatrist shortage
Commenting on the study, Howard Liu, MD, chair of the University of Nebraska Medical Center department of psychiatry in Omaha, and incoming chair of the APA’s Council on Communications, said he found the presentation “fascinating on several levels.”
It’s increasingly important for psychiatrists to be “trauma informed,” Dr. Liu told a press briefing highlighting the study. “It’s not just about looking at the biological correlates of illness,” meaning looking at genetic markers etc, “but also looking at the environment in which people live, work, and/or are in therapy or in treatment.”
In a later interview, Dr. Liu said he was impressed by the fact that Dr. Rached, who has “a very deep personal connection to this community,” is using her own personal trauma to help identify others are at risk who may need future care.
Dr. Liu, whose own family sponsors Afghan refugees, said the research underlines the need to ensure training for psychiatrists everywhere to help manage the expatriate population. As it stands, there’s “a huge shortage of psychiatrists around the world,” particularly in countries that have been affected by trauma, said Dr. Liu.
The researchers and Dr. Liu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM APA 2022
Multiple mental health woes? Blame it on genetics
Investigators conducted a genetic analysis of 11 major psychiatric disorders, including schizophrenia and bipolar disorder.
“Our findings confirm that high comorbidity across some disorders in part reflects overlapping pathways of genetic risk,” lead author Andrew Grotzinger, PhD, department of psychology and neuroscience, University of Colorado at Boulder, said in a press release.
The results could lead to the development of treatments that address multiple psychiatric disorders at once and help reshape the way diagnoses are established, the researchers note.
The findings were published online in Nature Genetics.
Common genetic patterns
Using the massive UK Biobank and the Psychiatric Genomics Consortium, the researchers applied novel statistical genetic methods to identify common patterns across 11 major psychiatric disorders: schizophrenia, bipolar disorder, major depressive disorder, anxiety disorder, anorexia nervosa, obsessive-compulsive disorder (OCD), Tourette syndrome, post traumatic stress disorder, problematic alcohol use, attention deficit hyperactive disorder, and autism.
The average total sample size per disorder was 156,771 participants, with a range of 9,725 to 802,939 participants.
In all, the investigators identified 152 genetic variants shared across multiple disorders, including those already known to influence certain types of brain cells.
For example, they found that 70% of the genetic signal associated with schizophrenia was also associated with bipolar disorder.
Results also showed that anorexia nervosa and OCD have a strong, shared genetic architecture and that individuals with a genetic predisposition to low body mass index also tend to have a genetic predisposition to these two disorders.
Not surprisingly, the researchers note, there was a large genetic overlap between anxiety disorder and major depressive disorder.
They also observed that psychiatric disorders that tend to cluster together also tend to share genes that influence how and when individuals are physically active during the day.
For example, patients with internalizing disorders such as anxiety and depression tend to have a genetic architecture associated with low movement throughout the day. On the other hand, those with OCD and anorexia tend to have genes associated with higher movement throughout the day.
“When you think about it, it makes sense,” said Dr. Grotzinger. Depressed individuals often experience fatigue or low energy while those with compulsive disorders may have a tough time sitting still, he noted.
One treatment for multiple disorders?
“Collectively, these results offer key insights into the shared and disorder-specific mechanisms of genetic risk for psychiatric disease,” the investigators write.
Their research is also a first step toward developing therapies that can address multiple disorders with one treatment, they add.
“People are more likely today to be prescribed multiple medications intended to treat multiple diagnoses, and in some instances those medicines can have side effects,” Dr. Grotzinger said.
“By identifying what is shared across these issues, we can hopefully come up with ways to target them in a different way that doesn’t require four separate pills or four separate psychotherapy interventions,” he added.
Dr. Grotzinger noted that, for now, the knowledge that genetics are underlying their disorders may provide comfort to some patients.
“It’s important for people to know that they didn’t just get a terrible roll of the dice in life – that they are not facing multiple different issues but rather one set of risk factors bleeding into them all,” he said.
This research had no commercial funding. Dr. Grotzinger reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Investigators conducted a genetic analysis of 11 major psychiatric disorders, including schizophrenia and bipolar disorder.
“Our findings confirm that high comorbidity across some disorders in part reflects overlapping pathways of genetic risk,” lead author Andrew Grotzinger, PhD, department of psychology and neuroscience, University of Colorado at Boulder, said in a press release.
The results could lead to the development of treatments that address multiple psychiatric disorders at once and help reshape the way diagnoses are established, the researchers note.
The findings were published online in Nature Genetics.
Common genetic patterns
Using the massive UK Biobank and the Psychiatric Genomics Consortium, the researchers applied novel statistical genetic methods to identify common patterns across 11 major psychiatric disorders: schizophrenia, bipolar disorder, major depressive disorder, anxiety disorder, anorexia nervosa, obsessive-compulsive disorder (OCD), Tourette syndrome, post traumatic stress disorder, problematic alcohol use, attention deficit hyperactive disorder, and autism.
The average total sample size per disorder was 156,771 participants, with a range of 9,725 to 802,939 participants.
In all, the investigators identified 152 genetic variants shared across multiple disorders, including those already known to influence certain types of brain cells.
For example, they found that 70% of the genetic signal associated with schizophrenia was also associated with bipolar disorder.
Results also showed that anorexia nervosa and OCD have a strong, shared genetic architecture and that individuals with a genetic predisposition to low body mass index also tend to have a genetic predisposition to these two disorders.
Not surprisingly, the researchers note, there was a large genetic overlap between anxiety disorder and major depressive disorder.
They also observed that psychiatric disorders that tend to cluster together also tend to share genes that influence how and when individuals are physically active during the day.
For example, patients with internalizing disorders such as anxiety and depression tend to have a genetic architecture associated with low movement throughout the day. On the other hand, those with OCD and anorexia tend to have genes associated with higher movement throughout the day.
“When you think about it, it makes sense,” said Dr. Grotzinger. Depressed individuals often experience fatigue or low energy while those with compulsive disorders may have a tough time sitting still, he noted.
One treatment for multiple disorders?
“Collectively, these results offer key insights into the shared and disorder-specific mechanisms of genetic risk for psychiatric disease,” the investigators write.
Their research is also a first step toward developing therapies that can address multiple disorders with one treatment, they add.
“People are more likely today to be prescribed multiple medications intended to treat multiple diagnoses, and in some instances those medicines can have side effects,” Dr. Grotzinger said.
“By identifying what is shared across these issues, we can hopefully come up with ways to target them in a different way that doesn’t require four separate pills or four separate psychotherapy interventions,” he added.
Dr. Grotzinger noted that, for now, the knowledge that genetics are underlying their disorders may provide comfort to some patients.
“It’s important for people to know that they didn’t just get a terrible roll of the dice in life – that they are not facing multiple different issues but rather one set of risk factors bleeding into them all,” he said.
This research had no commercial funding. Dr. Grotzinger reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Investigators conducted a genetic analysis of 11 major psychiatric disorders, including schizophrenia and bipolar disorder.
“Our findings confirm that high comorbidity across some disorders in part reflects overlapping pathways of genetic risk,” lead author Andrew Grotzinger, PhD, department of psychology and neuroscience, University of Colorado at Boulder, said in a press release.
The results could lead to the development of treatments that address multiple psychiatric disorders at once and help reshape the way diagnoses are established, the researchers note.
The findings were published online in Nature Genetics.
Common genetic patterns
Using the massive UK Biobank and the Psychiatric Genomics Consortium, the researchers applied novel statistical genetic methods to identify common patterns across 11 major psychiatric disorders: schizophrenia, bipolar disorder, major depressive disorder, anxiety disorder, anorexia nervosa, obsessive-compulsive disorder (OCD), Tourette syndrome, post traumatic stress disorder, problematic alcohol use, attention deficit hyperactive disorder, and autism.
The average total sample size per disorder was 156,771 participants, with a range of 9,725 to 802,939 participants.
In all, the investigators identified 152 genetic variants shared across multiple disorders, including those already known to influence certain types of brain cells.
For example, they found that 70% of the genetic signal associated with schizophrenia was also associated with bipolar disorder.
Results also showed that anorexia nervosa and OCD have a strong, shared genetic architecture and that individuals with a genetic predisposition to low body mass index also tend to have a genetic predisposition to these two disorders.
Not surprisingly, the researchers note, there was a large genetic overlap between anxiety disorder and major depressive disorder.
They also observed that psychiatric disorders that tend to cluster together also tend to share genes that influence how and when individuals are physically active during the day.
For example, patients with internalizing disorders such as anxiety and depression tend to have a genetic architecture associated with low movement throughout the day. On the other hand, those with OCD and anorexia tend to have genes associated with higher movement throughout the day.
“When you think about it, it makes sense,” said Dr. Grotzinger. Depressed individuals often experience fatigue or low energy while those with compulsive disorders may have a tough time sitting still, he noted.
One treatment for multiple disorders?
“Collectively, these results offer key insights into the shared and disorder-specific mechanisms of genetic risk for psychiatric disease,” the investigators write.
Their research is also a first step toward developing therapies that can address multiple disorders with one treatment, they add.
“People are more likely today to be prescribed multiple medications intended to treat multiple diagnoses, and in some instances those medicines can have side effects,” Dr. Grotzinger said.
“By identifying what is shared across these issues, we can hopefully come up with ways to target them in a different way that doesn’t require four separate pills or four separate psychotherapy interventions,” he added.
Dr. Grotzinger noted that, for now, the knowledge that genetics are underlying their disorders may provide comfort to some patients.
“It’s important for people to know that they didn’t just get a terrible roll of the dice in life – that they are not facing multiple different issues but rather one set of risk factors bleeding into them all,” he said.
This research had no commercial funding. Dr. Grotzinger reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM NATURE GENETICS
New insight into how brain stimulation eases major depression
For the first time, researchers understand what happens to the brain when patients with treatment-resistant depression receive repetitive transcranial magnetic stimulation (rTMS).
Using functional magnetic resonance imaging (fMRI), they showed that rTMS induces widespread alterations in functional connectivity in brain regions involved in emotion and motor control.
“‘How does rTMS work?’ is one of the most frequent questions I get in clinic. Providing an accurate explanation and narrative to patients is critical,” senior investigator Fidel Vila-Rodriguez, MD, PhD, director of the Non-Invasive Neurostimulation Therapies Laboratory, University of British Columbia, Vancouver, told this news organization.
“Our findings suggest that rTMS might rely on the brain’s capacity for change (neuroplasticity) to exert its effects and that rTMS effects on the brain are widespread beyond the focal area stimulated (functional network effects),” Dr. Vila-Rodriguez added.
The study was published online in the American Journal of Psychiatry.
Mechanistic insights
Although rTMS has proven efficacy for treatment-resistant depression, the mechanisms behind how it affects the brain are not well understood.
In the current study, researchers used fMRI to assess changes in functional connectivity induced by a single rTMS session in 26 women and 12 men with treatment-resistant depression.
They found that – from managing emotional responses to memory and motor control.
Following a 4-week course of rTMS, these connectivity changes predicted about 30% of the variance of improvement in scores on the Montgomery-Åsberg Depression Rating Scale after rTMS treatment.
The most robust predictive associations involved connections between prefrontal regions and motor, parietal, and insular cortices and between bilateral regions of the thalamus.
“By demonstrating this principle and identifying regions of the brain that are activated by rTMS, we can now try to understand whether this pattern can be used as a biomarker,” Dr. Vila-Rodriguez said in a news release.
“This work provides a mechanistic explanation of what rTMS does to treat depression and supports the notion that for rTMS to treat depressive symptoms a distributed change in brain activity (network or circuit base) is necessary,” he told this news organization.
With funding from the Canadian Institutes of Health Research (CIHR), the team will next see if they can use fMRI to guide rTMS at the individual level, with the ultimate goal of “personalizing” rTMS using individualized functional targets, Dr. Vila-Rodriguez said.
New generation of tms researchers
Reached for comment, Jonathan Downar, MD, PhD, department of psychiatry, University of Toronto, noted that TMS can be “very effective” for treatment-resistant depression, and it has a “very clean side effect profile compared to medications.”
What the field is trying to figure out now is “who it works for and how we can predict more effectively who’s going to benefit from it,” Dr. Downar said in an interview.
He noted that the study’s investigators are part of a “new generation of TMS researchers who are bringing new ideas into the fold and figuring out how to use brain imaging to personalize the treatment.” This study represents “a step” in that direction.
“A challenge for the field is that it’s often pretty easy to demonstrate a change at the group level, but the question is whether we can use that at the individual level. That’s a higher bar to meet, and we’re still not there yet,” Dr. Downar added.
Support for the study was provided by Brain Canada, the Michael Smith Foundation for Health Research and the Vancouver Coastal Health Research Institute. Dr. Vila-Rodriguez has received research support from CIHR, Brain Canada, the Michael Smith Foundation for Health Research, the Vancouver Coastal Health Research Institute, and the Weston Brain Institute for investigator-initiated research and philanthropic support from the Seedlings Foundation; he received in-kind equipment support from MagVenture for this investigator-initiated trial; and he has received honoraria for participation on an advisory board for Janssen. Dr. Downar has served as an adviser for BrainCheck, NeuroStim TMS, and Salience Neuro Health; received research grant from CIHR, National Institute for Mental Health, Brain Canada, Canadian Biomarker Integration Network in Depression, Ontario Brain Institute, Klarman Family Foundation, Arrell Family Foundation and the Edgestone Foundation; received travel stipends from Lundbeck and ANT Neuro; and received in-kind equipment support for investigator-initiated trials from MagVenture.
A version of this article first appeared on Medscape.com.
For the first time, researchers understand what happens to the brain when patients with treatment-resistant depression receive repetitive transcranial magnetic stimulation (rTMS).
Using functional magnetic resonance imaging (fMRI), they showed that rTMS induces widespread alterations in functional connectivity in brain regions involved in emotion and motor control.
“‘How does rTMS work?’ is one of the most frequent questions I get in clinic. Providing an accurate explanation and narrative to patients is critical,” senior investigator Fidel Vila-Rodriguez, MD, PhD, director of the Non-Invasive Neurostimulation Therapies Laboratory, University of British Columbia, Vancouver, told this news organization.
“Our findings suggest that rTMS might rely on the brain’s capacity for change (neuroplasticity) to exert its effects and that rTMS effects on the brain are widespread beyond the focal area stimulated (functional network effects),” Dr. Vila-Rodriguez added.
The study was published online in the American Journal of Psychiatry.
Mechanistic insights
Although rTMS has proven efficacy for treatment-resistant depression, the mechanisms behind how it affects the brain are not well understood.
In the current study, researchers used fMRI to assess changes in functional connectivity induced by a single rTMS session in 26 women and 12 men with treatment-resistant depression.
They found that – from managing emotional responses to memory and motor control.
Following a 4-week course of rTMS, these connectivity changes predicted about 30% of the variance of improvement in scores on the Montgomery-Åsberg Depression Rating Scale after rTMS treatment.
The most robust predictive associations involved connections between prefrontal regions and motor, parietal, and insular cortices and between bilateral regions of the thalamus.
“By demonstrating this principle and identifying regions of the brain that are activated by rTMS, we can now try to understand whether this pattern can be used as a biomarker,” Dr. Vila-Rodriguez said in a news release.
“This work provides a mechanistic explanation of what rTMS does to treat depression and supports the notion that for rTMS to treat depressive symptoms a distributed change in brain activity (network or circuit base) is necessary,” he told this news organization.
With funding from the Canadian Institutes of Health Research (CIHR), the team will next see if they can use fMRI to guide rTMS at the individual level, with the ultimate goal of “personalizing” rTMS using individualized functional targets, Dr. Vila-Rodriguez said.
New generation of tms researchers
Reached for comment, Jonathan Downar, MD, PhD, department of psychiatry, University of Toronto, noted that TMS can be “very effective” for treatment-resistant depression, and it has a “very clean side effect profile compared to medications.”
What the field is trying to figure out now is “who it works for and how we can predict more effectively who’s going to benefit from it,” Dr. Downar said in an interview.
He noted that the study’s investigators are part of a “new generation of TMS researchers who are bringing new ideas into the fold and figuring out how to use brain imaging to personalize the treatment.” This study represents “a step” in that direction.
“A challenge for the field is that it’s often pretty easy to demonstrate a change at the group level, but the question is whether we can use that at the individual level. That’s a higher bar to meet, and we’re still not there yet,” Dr. Downar added.
Support for the study was provided by Brain Canada, the Michael Smith Foundation for Health Research and the Vancouver Coastal Health Research Institute. Dr. Vila-Rodriguez has received research support from CIHR, Brain Canada, the Michael Smith Foundation for Health Research, the Vancouver Coastal Health Research Institute, and the Weston Brain Institute for investigator-initiated research and philanthropic support from the Seedlings Foundation; he received in-kind equipment support from MagVenture for this investigator-initiated trial; and he has received honoraria for participation on an advisory board for Janssen. Dr. Downar has served as an adviser for BrainCheck, NeuroStim TMS, and Salience Neuro Health; received research grant from CIHR, National Institute for Mental Health, Brain Canada, Canadian Biomarker Integration Network in Depression, Ontario Brain Institute, Klarman Family Foundation, Arrell Family Foundation and the Edgestone Foundation; received travel stipends from Lundbeck and ANT Neuro; and received in-kind equipment support for investigator-initiated trials from MagVenture.
A version of this article first appeared on Medscape.com.
For the first time, researchers understand what happens to the brain when patients with treatment-resistant depression receive repetitive transcranial magnetic stimulation (rTMS).
Using functional magnetic resonance imaging (fMRI), they showed that rTMS induces widespread alterations in functional connectivity in brain regions involved in emotion and motor control.
“‘How does rTMS work?’ is one of the most frequent questions I get in clinic. Providing an accurate explanation and narrative to patients is critical,” senior investigator Fidel Vila-Rodriguez, MD, PhD, director of the Non-Invasive Neurostimulation Therapies Laboratory, University of British Columbia, Vancouver, told this news organization.
“Our findings suggest that rTMS might rely on the brain’s capacity for change (neuroplasticity) to exert its effects and that rTMS effects on the brain are widespread beyond the focal area stimulated (functional network effects),” Dr. Vila-Rodriguez added.
The study was published online in the American Journal of Psychiatry.
Mechanistic insights
Although rTMS has proven efficacy for treatment-resistant depression, the mechanisms behind how it affects the brain are not well understood.
In the current study, researchers used fMRI to assess changes in functional connectivity induced by a single rTMS session in 26 women and 12 men with treatment-resistant depression.
They found that – from managing emotional responses to memory and motor control.
Following a 4-week course of rTMS, these connectivity changes predicted about 30% of the variance of improvement in scores on the Montgomery-Åsberg Depression Rating Scale after rTMS treatment.
The most robust predictive associations involved connections between prefrontal regions and motor, parietal, and insular cortices and between bilateral regions of the thalamus.
“By demonstrating this principle and identifying regions of the brain that are activated by rTMS, we can now try to understand whether this pattern can be used as a biomarker,” Dr. Vila-Rodriguez said in a news release.
“This work provides a mechanistic explanation of what rTMS does to treat depression and supports the notion that for rTMS to treat depressive symptoms a distributed change in brain activity (network or circuit base) is necessary,” he told this news organization.
With funding from the Canadian Institutes of Health Research (CIHR), the team will next see if they can use fMRI to guide rTMS at the individual level, with the ultimate goal of “personalizing” rTMS using individualized functional targets, Dr. Vila-Rodriguez said.
New generation of tms researchers
Reached for comment, Jonathan Downar, MD, PhD, department of psychiatry, University of Toronto, noted that TMS can be “very effective” for treatment-resistant depression, and it has a “very clean side effect profile compared to medications.”
What the field is trying to figure out now is “who it works for and how we can predict more effectively who’s going to benefit from it,” Dr. Downar said in an interview.
He noted that the study’s investigators are part of a “new generation of TMS researchers who are bringing new ideas into the fold and figuring out how to use brain imaging to personalize the treatment.” This study represents “a step” in that direction.
“A challenge for the field is that it’s often pretty easy to demonstrate a change at the group level, but the question is whether we can use that at the individual level. That’s a higher bar to meet, and we’re still not there yet,” Dr. Downar added.
Support for the study was provided by Brain Canada, the Michael Smith Foundation for Health Research and the Vancouver Coastal Health Research Institute. Dr. Vila-Rodriguez has received research support from CIHR, Brain Canada, the Michael Smith Foundation for Health Research, the Vancouver Coastal Health Research Institute, and the Weston Brain Institute for investigator-initiated research and philanthropic support from the Seedlings Foundation; he received in-kind equipment support from MagVenture for this investigator-initiated trial; and he has received honoraria for participation on an advisory board for Janssen. Dr. Downar has served as an adviser for BrainCheck, NeuroStim TMS, and Salience Neuro Health; received research grant from CIHR, National Institute for Mental Health, Brain Canada, Canadian Biomarker Integration Network in Depression, Ontario Brain Institute, Klarman Family Foundation, Arrell Family Foundation and the Edgestone Foundation; received travel stipends from Lundbeck and ANT Neuro; and received in-kind equipment support for investigator-initiated trials from MagVenture.
A version of this article first appeared on Medscape.com.
Anxiety in America: COVID ‘takes a backseat’ to global events
NEW ORLEANS – With 2 years of COVID-19 in the rearview mirror, anxiety among U.S. adults has turned instead toward global events, results from the annual Healthy Minds Poll from the American Psychiatric Association show.
“It’s not surprising that recent events, such as the war in Ukraine, racially motivated mass shootings, or the impacts of climate change, are weighing heavily on Americans’ minds,” APA president Vivian Pender, MD, said in a news release.
“COVID-19 in a way has taken a back seat, but the pandemic and its mental health effects are very much still with us. It’s important that we are cognizant of that and continue to work to ensure people who need psychiatric care, whether the causes are tied to the pandemic or to other issues, can access it,” Dr. Pender added.
Results from the 2022’s poll were released May 22 during the annual meeting of the APA.
Record low COVID anxiety
The poll was conducted by Morning Consult between April 23-24 and included 2,210 adult participants.
Results showed that This was down from 65% in 2021 and from 75% in 2020.
Instead, nearly three-quarters (73%) of adults are somewhat or extremely anxious about current events happening around the world, 64% are anxious about keeping themselves or their families safe, and 60% worry about their health in general.
Overall, about one-third (32%) reported being more anxious now than in 2021, 46% reported no change in their anxiety level, and 18% were less anxious.
About one-quarter (26%) have spoken with a mental health care professional in the past few years, which is down from 34% in 2021. In addition, Hispanic (36%) and Black (35%) adults were more likely to have reached out for help than White (25%) adults.
Despite the U.S. Surgeon General’s recent advisory on the mental health crisis among children, the poll results also showed that Americans are less concerned about their children’s mental health than in 2021. A total of 41% of parents expressed concern about this topic, which was down from 53% in 2021.
Still, 40% of parents said their children had received help from a mental health professional since the pandemic hit. Of that group, 36% sought help before the pandemic, whereas half said the pandemic had caused mental health issues for their children.
“While the overall level of concern has dropped, still 4 in 10 parents are worried about how their children are doing, and a third are having issues with access to care,” Saul Levin, MD, CEO and medical director of the APA, said in the release.
“This is unacceptable and as a nation, we need to invest in the kind of systems that will ensure any parent who’s worried about their child has access to lifesaving treatment,” Dr. Levin added.
Workplace mental health
In addition, the poll showed employees often have a tough time getting mental health support from employers, or are hesitant to ask for help.
“What’s troubling about the results of this poll is that, even as the pandemic has continued and its mental health effects wear on, fewer employees are reporting that they have access to mental health services,” Dr. Pender said.
“Workplaces need to ensure that they are paying attention to what their employees need, particularly now, and moving away from mental health benefits isn’t the right move,” she added.
About half (48%) of those polled said they can discuss mental health openly and honestly with their supervisor, down from 56% in 2021 and 62% in 2020.
Only about half (52%) said they feel comfortable using mental health services with their current employer, compared with 64% in 2021 and 67% in 2020.
In addition, fewer workers felt their employer is offering sufficient mental health resources and benefits. In 2022, 53% of workers thought resources and benefits were adequate, which was down from 65% in 2021 and 68% in 2020.
“It’s quite concerning to see that fewer people feel comfortable discussing mental health with a supervisor, at a time when people experiencing symptoms of anxiety, depression, and other conditions are on the rise and impact nearly every aspect of work, including productivity, performance, retention, and overall health care costs,” said Darcy Gruttadaro, JD, director of the APA Foundation’s Center for Workplace Mental Health.
“As rates of these conditions rise, we should see more employees knowing about available workplace mental health resources, not less,” Ms. Gruttadaro said.
Strong bipartisan support
Perhaps unexpectedly, the poll shows strong support among Democrats, Republicans, and Independents for three APA-backed approaches to improve timely access to mental health care and treatment.
Specifically, about three-quarters of those polled supported making it easier to see a mental health professional via telehealth, allowing patients to receive mental health care through a primary care provider, and funding mental health care professionals to work in rural or urban communities that are traditionally underserved.
“We’re in a moment when mental health is a big part of the national conversation, and clearly political party doesn’t matter as much on this issue,” Dr. Pender noted.
“It’s a rare thing in Washington these days to see such a resounding endorsement, but there is strong support for these practical workable solutions that mean more access to mental health care,” she said.
“What you see in this poll is agreement: It’s hard to access mental [health care] but we do have great solutions that could work across party lines,” Dr. Levin added.
“Many policy makers, in the administration and in Congress, are already putting these ideas into action, and they should feel encouraged that the public wants to see Congress act on them,” he said.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – With 2 years of COVID-19 in the rearview mirror, anxiety among U.S. adults has turned instead toward global events, results from the annual Healthy Minds Poll from the American Psychiatric Association show.
“It’s not surprising that recent events, such as the war in Ukraine, racially motivated mass shootings, or the impacts of climate change, are weighing heavily on Americans’ minds,” APA president Vivian Pender, MD, said in a news release.
“COVID-19 in a way has taken a back seat, but the pandemic and its mental health effects are very much still with us. It’s important that we are cognizant of that and continue to work to ensure people who need psychiatric care, whether the causes are tied to the pandemic or to other issues, can access it,” Dr. Pender added.
Results from the 2022’s poll were released May 22 during the annual meeting of the APA.
Record low COVID anxiety
The poll was conducted by Morning Consult between April 23-24 and included 2,210 adult participants.
Results showed that This was down from 65% in 2021 and from 75% in 2020.
Instead, nearly three-quarters (73%) of adults are somewhat or extremely anxious about current events happening around the world, 64% are anxious about keeping themselves or their families safe, and 60% worry about their health in general.
Overall, about one-third (32%) reported being more anxious now than in 2021, 46% reported no change in their anxiety level, and 18% were less anxious.
About one-quarter (26%) have spoken with a mental health care professional in the past few years, which is down from 34% in 2021. In addition, Hispanic (36%) and Black (35%) adults were more likely to have reached out for help than White (25%) adults.
Despite the U.S. Surgeon General’s recent advisory on the mental health crisis among children, the poll results also showed that Americans are less concerned about their children’s mental health than in 2021. A total of 41% of parents expressed concern about this topic, which was down from 53% in 2021.
Still, 40% of parents said their children had received help from a mental health professional since the pandemic hit. Of that group, 36% sought help before the pandemic, whereas half said the pandemic had caused mental health issues for their children.
“While the overall level of concern has dropped, still 4 in 10 parents are worried about how their children are doing, and a third are having issues with access to care,” Saul Levin, MD, CEO and medical director of the APA, said in the release.
“This is unacceptable and as a nation, we need to invest in the kind of systems that will ensure any parent who’s worried about their child has access to lifesaving treatment,” Dr. Levin added.
Workplace mental health
In addition, the poll showed employees often have a tough time getting mental health support from employers, or are hesitant to ask for help.
“What’s troubling about the results of this poll is that, even as the pandemic has continued and its mental health effects wear on, fewer employees are reporting that they have access to mental health services,” Dr. Pender said.
“Workplaces need to ensure that they are paying attention to what their employees need, particularly now, and moving away from mental health benefits isn’t the right move,” she added.
About half (48%) of those polled said they can discuss mental health openly and honestly with their supervisor, down from 56% in 2021 and 62% in 2020.
Only about half (52%) said they feel comfortable using mental health services with their current employer, compared with 64% in 2021 and 67% in 2020.
In addition, fewer workers felt their employer is offering sufficient mental health resources and benefits. In 2022, 53% of workers thought resources and benefits were adequate, which was down from 65% in 2021 and 68% in 2020.
“It’s quite concerning to see that fewer people feel comfortable discussing mental health with a supervisor, at a time when people experiencing symptoms of anxiety, depression, and other conditions are on the rise and impact nearly every aspect of work, including productivity, performance, retention, and overall health care costs,” said Darcy Gruttadaro, JD, director of the APA Foundation’s Center for Workplace Mental Health.
“As rates of these conditions rise, we should see more employees knowing about available workplace mental health resources, not less,” Ms. Gruttadaro said.
Strong bipartisan support
Perhaps unexpectedly, the poll shows strong support among Democrats, Republicans, and Independents for three APA-backed approaches to improve timely access to mental health care and treatment.
Specifically, about three-quarters of those polled supported making it easier to see a mental health professional via telehealth, allowing patients to receive mental health care through a primary care provider, and funding mental health care professionals to work in rural or urban communities that are traditionally underserved.
“We’re in a moment when mental health is a big part of the national conversation, and clearly political party doesn’t matter as much on this issue,” Dr. Pender noted.
“It’s a rare thing in Washington these days to see such a resounding endorsement, but there is strong support for these practical workable solutions that mean more access to mental health care,” she said.
“What you see in this poll is agreement: It’s hard to access mental [health care] but we do have great solutions that could work across party lines,” Dr. Levin added.
“Many policy makers, in the administration and in Congress, are already putting these ideas into action, and they should feel encouraged that the public wants to see Congress act on them,” he said.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – With 2 years of COVID-19 in the rearview mirror, anxiety among U.S. adults has turned instead toward global events, results from the annual Healthy Minds Poll from the American Psychiatric Association show.
“It’s not surprising that recent events, such as the war in Ukraine, racially motivated mass shootings, or the impacts of climate change, are weighing heavily on Americans’ minds,” APA president Vivian Pender, MD, said in a news release.
“COVID-19 in a way has taken a back seat, but the pandemic and its mental health effects are very much still with us. It’s important that we are cognizant of that and continue to work to ensure people who need psychiatric care, whether the causes are tied to the pandemic or to other issues, can access it,” Dr. Pender added.
Results from the 2022’s poll were released May 22 during the annual meeting of the APA.
Record low COVID anxiety
The poll was conducted by Morning Consult between April 23-24 and included 2,210 adult participants.
Results showed that This was down from 65% in 2021 and from 75% in 2020.
Instead, nearly three-quarters (73%) of adults are somewhat or extremely anxious about current events happening around the world, 64% are anxious about keeping themselves or their families safe, and 60% worry about their health in general.
Overall, about one-third (32%) reported being more anxious now than in 2021, 46% reported no change in their anxiety level, and 18% were less anxious.
About one-quarter (26%) have spoken with a mental health care professional in the past few years, which is down from 34% in 2021. In addition, Hispanic (36%) and Black (35%) adults were more likely to have reached out for help than White (25%) adults.
Despite the U.S. Surgeon General’s recent advisory on the mental health crisis among children, the poll results also showed that Americans are less concerned about their children’s mental health than in 2021. A total of 41% of parents expressed concern about this topic, which was down from 53% in 2021.
Still, 40% of parents said their children had received help from a mental health professional since the pandemic hit. Of that group, 36% sought help before the pandemic, whereas half said the pandemic had caused mental health issues for their children.
“While the overall level of concern has dropped, still 4 in 10 parents are worried about how their children are doing, and a third are having issues with access to care,” Saul Levin, MD, CEO and medical director of the APA, said in the release.
“This is unacceptable and as a nation, we need to invest in the kind of systems that will ensure any parent who’s worried about their child has access to lifesaving treatment,” Dr. Levin added.
Workplace mental health
In addition, the poll showed employees often have a tough time getting mental health support from employers, or are hesitant to ask for help.
“What’s troubling about the results of this poll is that, even as the pandemic has continued and its mental health effects wear on, fewer employees are reporting that they have access to mental health services,” Dr. Pender said.
“Workplaces need to ensure that they are paying attention to what their employees need, particularly now, and moving away from mental health benefits isn’t the right move,” she added.
About half (48%) of those polled said they can discuss mental health openly and honestly with their supervisor, down from 56% in 2021 and 62% in 2020.
Only about half (52%) said they feel comfortable using mental health services with their current employer, compared with 64% in 2021 and 67% in 2020.
In addition, fewer workers felt their employer is offering sufficient mental health resources and benefits. In 2022, 53% of workers thought resources and benefits were adequate, which was down from 65% in 2021 and 68% in 2020.
“It’s quite concerning to see that fewer people feel comfortable discussing mental health with a supervisor, at a time when people experiencing symptoms of anxiety, depression, and other conditions are on the rise and impact nearly every aspect of work, including productivity, performance, retention, and overall health care costs,” said Darcy Gruttadaro, JD, director of the APA Foundation’s Center for Workplace Mental Health.
“As rates of these conditions rise, we should see more employees knowing about available workplace mental health resources, not less,” Ms. Gruttadaro said.
Strong bipartisan support
Perhaps unexpectedly, the poll shows strong support among Democrats, Republicans, and Independents for three APA-backed approaches to improve timely access to mental health care and treatment.
Specifically, about three-quarters of those polled supported making it easier to see a mental health professional via telehealth, allowing patients to receive mental health care through a primary care provider, and funding mental health care professionals to work in rural or urban communities that are traditionally underserved.
“We’re in a moment when mental health is a big part of the national conversation, and clearly political party doesn’t matter as much on this issue,” Dr. Pender noted.
“It’s a rare thing in Washington these days to see such a resounding endorsement, but there is strong support for these practical workable solutions that mean more access to mental health care,” she said.
“What you see in this poll is agreement: It’s hard to access mental [health care] but we do have great solutions that could work across party lines,” Dr. Levin added.
“Many policy makers, in the administration and in Congress, are already putting these ideas into action, and they should feel encouraged that the public wants to see Congress act on them,” he said.
A version of this article first appeared on Medscape.com.
FROM APA 2022
Does suicide risk show up in the blood?
Investigators found patients with MDD who died by suicide had a gene expression signature in blood distinct from patients with MDD who died by other means.
The signature included genes involved in stress response changes, including polyamine metabolism, circadian rhythm, immune dysregulation, and telomere maintenance.
“These blood biomarkers are an important step toward developing blood tests to identify patients with imminent risk of ending their lives,” study investigator Adolfo Sequeira, PhD, associate researcher in the department of psychiatry and human behavior, University of California, Irvine, said in a news release.
“To our knowledge, this is the first study to analyze blood and brain samples in a well-defined population of MDDs demonstrating significant differences in gene expression associated with completed suicide,” Dr. Sequeira added.
The findings were published online in Translational Psychiatry.
A pressing challenge
Suicide rates in the United States have jumped by more than 35% over the past 2 decades, with more than 48,000 deaths by suicide occurring just last year. MDD is the most common diagnosis among completed suicides, and identifying individuals at the highest risk for suicide remains a “pressing challenge,” the researchers noted.
They looked for changes in gene expression associated with suicide in archived postmortem blood and brain samples from adults with MDD who died by suicide (MDD-S) or by other means (MDD-NS), as well as a group of controls with no psychiatric illness.
In total, there were blood and brain samples for 45 adults, including 53 blood samples and 69 dorsolateral prefrontal cortex (DLPFC) tissue samples.
In blood, investigators identified 14 genes that significantly differentiated MDD-S from MDD-NS. The top six genes differentially expressed in blood were PER3, MTPAP, SLC25A26, CD19, SOX9, and GAR1.
In addition, four genes showed significant changes in brain and blood between the MDD-S and MDD-NS groups. SOX9 was decreased and PER3 was increased in MDD-S in both blood and brain samples, while CD19 and TERF1 were increased in blood but decreased in DLPFC.
SOX9, an astrocytic marker in the brain and B-cell marker in blood, has been shown to be decreased in MDD-S compared with controls in the prefrontal cortex.
In the current study, researchers found that SOX9 expression was significantly reduced both in blood and brain in MDD-S compared with MDD-NS, “suggesting similar immune/astrocytic dysregulations in suicide that could be further investigated.”
Potential signatures, potential targets
PER3 is a circadian rhythm gene implicated in sleep disorders associated with shifts in circadian rhythms and is thought to increase susceptibility to MDD.
Mutations in PER3 have been shown previously to alter multiple systems, including response to antidepressants; and increased blood expression of PER1 has been linked to suicidality in women, the researchers noted.
There also were significantly higher levels of two inflammatory markers (CD19 and CD6 genes) in blood of MDD-S patients compared to MDD-NS patients.
Another “significant” finding was the involvement of several mitochondrial genes in suicide, the researchers said.
Two nuclear genes coding for mitochondria-located proteins MTPAP (a mitochondrial poly(A) polymerase) and the mitochondrial polyamine transporter SLC25A26 were increased in blood in MDD-S compared with MDD-NS and controls, suggesting that “mitochondrial alterations could be used as potential signatures to differentiate MDD-S from MDD-NS patients and also from controls.”
The researchers added that the genes found to be dysregulated in suicide represent potential targets for future drug therapies to prevent suicide and could also be used to develop a molecular test to identify individuals at high risk for suicide.
The study was funded by the National Institute of Mental Health, the American Society for Suicide Prevention, and the Pritzker Family Philanthropic Fund. The investigators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators found patients with MDD who died by suicide had a gene expression signature in blood distinct from patients with MDD who died by other means.
The signature included genes involved in stress response changes, including polyamine metabolism, circadian rhythm, immune dysregulation, and telomere maintenance.
“These blood biomarkers are an important step toward developing blood tests to identify patients with imminent risk of ending their lives,” study investigator Adolfo Sequeira, PhD, associate researcher in the department of psychiatry and human behavior, University of California, Irvine, said in a news release.
“To our knowledge, this is the first study to analyze blood and brain samples in a well-defined population of MDDs demonstrating significant differences in gene expression associated with completed suicide,” Dr. Sequeira added.
The findings were published online in Translational Psychiatry.
A pressing challenge
Suicide rates in the United States have jumped by more than 35% over the past 2 decades, with more than 48,000 deaths by suicide occurring just last year. MDD is the most common diagnosis among completed suicides, and identifying individuals at the highest risk for suicide remains a “pressing challenge,” the researchers noted.
They looked for changes in gene expression associated with suicide in archived postmortem blood and brain samples from adults with MDD who died by suicide (MDD-S) or by other means (MDD-NS), as well as a group of controls with no psychiatric illness.
In total, there were blood and brain samples for 45 adults, including 53 blood samples and 69 dorsolateral prefrontal cortex (DLPFC) tissue samples.
In blood, investigators identified 14 genes that significantly differentiated MDD-S from MDD-NS. The top six genes differentially expressed in blood were PER3, MTPAP, SLC25A26, CD19, SOX9, and GAR1.
In addition, four genes showed significant changes in brain and blood between the MDD-S and MDD-NS groups. SOX9 was decreased and PER3 was increased in MDD-S in both blood and brain samples, while CD19 and TERF1 were increased in blood but decreased in DLPFC.
SOX9, an astrocytic marker in the brain and B-cell marker in blood, has been shown to be decreased in MDD-S compared with controls in the prefrontal cortex.
In the current study, researchers found that SOX9 expression was significantly reduced both in blood and brain in MDD-S compared with MDD-NS, “suggesting similar immune/astrocytic dysregulations in suicide that could be further investigated.”
Potential signatures, potential targets
PER3 is a circadian rhythm gene implicated in sleep disorders associated with shifts in circadian rhythms and is thought to increase susceptibility to MDD.
Mutations in PER3 have been shown previously to alter multiple systems, including response to antidepressants; and increased blood expression of PER1 has been linked to suicidality in women, the researchers noted.
There also were significantly higher levels of two inflammatory markers (CD19 and CD6 genes) in blood of MDD-S patients compared to MDD-NS patients.
Another “significant” finding was the involvement of several mitochondrial genes in suicide, the researchers said.
Two nuclear genes coding for mitochondria-located proteins MTPAP (a mitochondrial poly(A) polymerase) and the mitochondrial polyamine transporter SLC25A26 were increased in blood in MDD-S compared with MDD-NS and controls, suggesting that “mitochondrial alterations could be used as potential signatures to differentiate MDD-S from MDD-NS patients and also from controls.”
The researchers added that the genes found to be dysregulated in suicide represent potential targets for future drug therapies to prevent suicide and could also be used to develop a molecular test to identify individuals at high risk for suicide.
The study was funded by the National Institute of Mental Health, the American Society for Suicide Prevention, and the Pritzker Family Philanthropic Fund. The investigators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators found patients with MDD who died by suicide had a gene expression signature in blood distinct from patients with MDD who died by other means.
The signature included genes involved in stress response changes, including polyamine metabolism, circadian rhythm, immune dysregulation, and telomere maintenance.
“These blood biomarkers are an important step toward developing blood tests to identify patients with imminent risk of ending their lives,” study investigator Adolfo Sequeira, PhD, associate researcher in the department of psychiatry and human behavior, University of California, Irvine, said in a news release.
“To our knowledge, this is the first study to analyze blood and brain samples in a well-defined population of MDDs demonstrating significant differences in gene expression associated with completed suicide,” Dr. Sequeira added.
The findings were published online in Translational Psychiatry.
A pressing challenge
Suicide rates in the United States have jumped by more than 35% over the past 2 decades, with more than 48,000 deaths by suicide occurring just last year. MDD is the most common diagnosis among completed suicides, and identifying individuals at the highest risk for suicide remains a “pressing challenge,” the researchers noted.
They looked for changes in gene expression associated with suicide in archived postmortem blood and brain samples from adults with MDD who died by suicide (MDD-S) or by other means (MDD-NS), as well as a group of controls with no psychiatric illness.
In total, there were blood and brain samples for 45 adults, including 53 blood samples and 69 dorsolateral prefrontal cortex (DLPFC) tissue samples.
In blood, investigators identified 14 genes that significantly differentiated MDD-S from MDD-NS. The top six genes differentially expressed in blood were PER3, MTPAP, SLC25A26, CD19, SOX9, and GAR1.
In addition, four genes showed significant changes in brain and blood between the MDD-S and MDD-NS groups. SOX9 was decreased and PER3 was increased in MDD-S in both blood and brain samples, while CD19 and TERF1 were increased in blood but decreased in DLPFC.
SOX9, an astrocytic marker in the brain and B-cell marker in blood, has been shown to be decreased in MDD-S compared with controls in the prefrontal cortex.
In the current study, researchers found that SOX9 expression was significantly reduced both in blood and brain in MDD-S compared with MDD-NS, “suggesting similar immune/astrocytic dysregulations in suicide that could be further investigated.”
Potential signatures, potential targets
PER3 is a circadian rhythm gene implicated in sleep disorders associated with shifts in circadian rhythms and is thought to increase susceptibility to MDD.
Mutations in PER3 have been shown previously to alter multiple systems, including response to antidepressants; and increased blood expression of PER1 has been linked to suicidality in women, the researchers noted.
There also were significantly higher levels of two inflammatory markers (CD19 and CD6 genes) in blood of MDD-S patients compared to MDD-NS patients.
Another “significant” finding was the involvement of several mitochondrial genes in suicide, the researchers said.
Two nuclear genes coding for mitochondria-located proteins MTPAP (a mitochondrial poly(A) polymerase) and the mitochondrial polyamine transporter SLC25A26 were increased in blood in MDD-S compared with MDD-NS and controls, suggesting that “mitochondrial alterations could be used as potential signatures to differentiate MDD-S from MDD-NS patients and also from controls.”
The researchers added that the genes found to be dysregulated in suicide represent potential targets for future drug therapies to prevent suicide and could also be used to develop a molecular test to identify individuals at high risk for suicide.
The study was funded by the National Institute of Mental Health, the American Society for Suicide Prevention, and the Pritzker Family Philanthropic Fund. The investigators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM TRANSLATIONAL PSYCHIATRY
When burnout is moral injury
Several years have passed since I stood among a cohort of eager medical students wearing regalia that signaled a new beginning. Four years of grueling study culminated in a cacophony of unified voices, each reciting a pledge that I had longed to take since early adolescence. Together we celebrated, triumphant despite innumerable exams and various iterations of the Socratic method – all under the guise of assessing knowledge while in truth seeking to insidiously erode the crowd of prospective physicians. Yet our anxiety and uncertainty melted away as names were called, hands firmly clasped, and tassels transposed. For a moment in time, we stood on the precipice of victory, enthusiastic albeit oblivious of the tremendous obstacles that loomed ahead.
Wistfully I reminisce about the unequivocal joy that abounds within the protective shield of naiveté. Specifically, I think about that time when the edict of medicine and the art of being a physician felt congruent. Yet, reality is fickle and often supersedes expectation. Occasionally my thoughts drift to the early days of residency – a time during which the emotional weight of caring for vulnerable patients while learning to master my chosen specialty felt woefully insurmountable. I recall wading blindly through each rotation attempting to emulate the competent and compassionate care so effortlessly demonstrated by senior physicians as they moved through the health care system with apparent ease. They stepped fluidly, as I watched in awe through rose-tinted glasses.
As months passed into years, my perception cleared. What I initially viewed as graceful patient care belied a complex tapestry of health care workers often pressured into arduous decisions, not necessarily in service of a well-constructed treatment plan. Gradually, formidable barriers emerged, guidelines and restrictions embedded within a confining path that suffocated those who dared to cross it. As a result, a field built on the foundations of autonomy, benevolence, and nonmaleficence was slowly engulfed by a system fraught with contrivances. Amid such stressors, physical and psychological health grows tenuous. Classically, this overwhelming feeling of distress is recognized as burnout. Studies reformulated this malady to that which was first described in Vietnam war veterans, a condition known as “moral injury.”
The impact of burnout
To explain the development – and explore the complexities – of moral injury, we must return to 1975 when the term burnout was initially formulated by Herbert Freudenberger, PhD, a psychologist renowned for his work in substance use disorders, psychoanalysis, and clinical education.1 Dr. Freudenberger’s studies noted incidences of heightened emotional and physical distress in his colleagues working in substance abuse and other clinics. He sought to define these experiences as well as understand his own battle with malaise, apathy, and frustration.1 Ultimately, Dr. Freudenberger described burnout as “Becoming exhausted by making excessive demands on energy, strength, or resources in the workplace.”2 Although it characteristically overlaps with depression and anxiety, burnout is conceptualized as a separate entity specifically forged within a context of perfectionism, integrity, and self-sacrifice.2 Such qualities are integral in health care and, as a result, physicians are particularly vulnerable.
Since Dr. Freudenberger published “Burnout: The High Cost of Achievement” in 1980, immense research has assisted in not only identifying critical factors that contribute to its development but also the detrimental effects it has on physiological health.3 These include exhaustion from poor work conditions and extreme commitment to employee responsibilities that in turn precipitate mood destabilization and impaired work performance.3 Furthermore, research has also demonstrated that burnout triggers alterations in neural circuitry via the prefrontal cortex and the amygdala, structures critical for emotional regulation.4 To combat the ill effects of burnout while maintaining productivity and maximizing profit, several high-profile corporations instituted changes focusing on self-care, wellness, benefits, and incentives. Although these modifications are effective in decreasing the rate of employee turnover, such strategies are not easily transferable to health care. In fact, the rate of physician burnout has steadily increased over the past two decades as the business of medicine shifts towards longer hours, decreased reimbursement rates, and inexhaustible insurance stipulations.2,5 Consequently, occupational dissatisfaction increases the risk of cynicism, frustration with patients, internalization of failure, and likelihood of early retirement.5 Moreover, burnout may also fracture interpersonal relationships as well as precipitate errors, negative patient outcomes, malpractice, and development of severe mental health conditions associated with high morbidity and mortality.5,8
Although the concept of burnout is critical in understanding the side effects of stereotypical workplace culture, critics of the concept bemoan a suggestion of individual blame.6,8 In essence, they argue that burnout is explained as a side effect of toxic workplace conditions, but covertly represents a lack of resilience, motivation, and ambition to thrive in a physically or emotionally taxing occupational setting.6,8 Thus, the responsibility of acclimation lies upon the impacted individuals rather than the employer. For this reason, many strategies to ameliorate burnout are focused on the individual, including meditation, wellness retreats, creating or adjusting self-care regimens, or in some cases psychotherapy and psychopharmacology.6 Whereas burnout may respond (at least partially) to such interventions, without altering the causal factors, it is unlikely to remit. This is especially the case in health care, where systemic constraints lie beyond the control of an individual physician. Rather than promoting or specifically relying upon personal improvement and recovery, amendments are needed on multiple levels to affect meaningful change.
Moral injury
Similar to burnout, moral injury was not initially conceived within the scope of health care. In the 1990s Jonathan Shay, MD, PhD, identified veterans presenting with symptoms mimicking PTSD that failed to respond to standard, well established and efficacious treatments.9-11 With further analysis he determined that veterans who demonstrated minimal improvement reported similar histories of guilt, shame, and disgust following perceived injustices enacted or abetted by immoral leaders.10,11 Ultimately Shay identified three components of moral injury: 1. A betrayal of what is morally right; 2. By someone who holds legitimate priority; 3. In a high stakes situation.10
This definition was further modified in 2007 by Brett Linz, PhD, and colleagues as: “Perpetuating, failing to prevent, or bearing witness to acts that transgress deeply held moral beliefs and expectations.”10,11 By expanding this description to include distress experienced by physicians and health care workers, Wendy Dean and Simon Talbot (in 2018 and 2019 respectively) explored how the health care system leads practitioners to deliver what they identify as substandard treatment.6-8 This results in disillusionment and lays the foundation for ethical and moral dilemmas in clinicians.
Themes of moral injury are repeatedly cited in various surveys and studies as a cause for occupational dissatisfaction. As physicians and other health care professionals reel from the aftermath of COVID-19, the effects of reconfiguring medicine into a business-oriented framework are glaringly conspicuous. Vast hospital nursing shortages, high patient census exacerbated by the political misuse and polarization of science, and insufficient availability of psychiatric beds, have culminated in a deluge of psychological strain in emergency medical physicians. Furthermore, pressure from administrators, mandated patient satisfaction measures, tedious electronic medical record systems, and copious licensing and certification requirements, contribute to physician distress as they attempt to navigate a system that challenges the vows which they swore to uphold.8 Because the cost of pursuing a medical degree frequently necessitates acquisition of loans that, without a physician income, may be difficult to repay,9 many doctors feel trapped within a seemingly endless cycle of misgiving that contributes to emotional exhaustion, pessimism, and low morale.
In my next series of The Myth of the Superdoctor columns, we will explore various factors that potentiate risk of moral injury. From medical school and residency training to corporate infrastructure and insurance obstacles, I will seek to discern and deliberate strategies for repair and rehabilitation. It is my hope that together we will illuminate the myriad complexities within the business of medicine, and become advocates and harbingers of change not only for physicians and health care workers but also for the sake of our patients and their families.
Dr. Thomas is a board-certified adult psychiatrist with interests in chronic illness, women’s behavioral health, and minority mental health. She currently practices in North Kingstown and East Providence, R.I. Dr. Thomas has no conflicts of interest.
References
1. King N. When a Psychologist Succumbed to Stress, He Coined The Term Burnout. 2016 Dec 8. NPR: All Things Considered.
2. Maslach C and Leiter MP. World Psychiatry. 2016 Jun;15(2):103-11. doi: 10.1002/wps.20311.
3. InformedHealth.org and Institute for Quality and Efficiency in Health Care. Depression: What is burnout?. https://www.ncbi.nlm.nih.gov/books/NBK279286/.
4. Michel A. Burnout and the Brain. Observer. 2016 Jan 29. https://www.psychologicalscience.org/observer/burnout-and-the-brain.
5. Patel RS et al. Behav Sci. 2018;8(11):98. doi:10.3390/bs8110098.
6. Dean W and Talbot S. Physicians aren’t ‘burning out.’ They’re suffering from moral injury. Stat. 2018 Jul 26. https://www.statnews.com/2018/07/26/physicians-not-burning-out-they-are-suffering-moral-injury/.
7. Dean W and Talbot S. Moral injury and burnout in medicine: A year of lessons learned. Stat. 2019 Jul 26. https://www.statnews.com/2019/07/26/moral-injury-burnout-medicine-lessons-learned/.
8. Dean W et al. Reframing Clinician Distress: Moral Injury Not Burnout. Fed Pract. 2019 Sep; 36(9):400-2. https://www.mdedge.com/fedprac/article/207458/mental-health/reframing-clinician-distress-moral-injury-not-burnout.
9. Bailey M. Beyond Burnout: Docs Decry ‘Moral Injury’ From Financial Pressures of Health Care. KHN. 2020 Feb 4. https://khn.org/news/beyond-burnout-docs-decry-moral-injury-from-financial-pressures-of-health-care/.
10. Litz B et al. Clin Psychol Rev. 2009 Dec;29(8):695-706. doi: 10.1016/j.cpr.2009.07.003.
11. Norman S and Maguen S. Moral Injury. PTSD: National Center for PTSD. https://www.ptsd.va.gov/professional/treat/cooccurring/moral_injury.asp.
Several years have passed since I stood among a cohort of eager medical students wearing regalia that signaled a new beginning. Four years of grueling study culminated in a cacophony of unified voices, each reciting a pledge that I had longed to take since early adolescence. Together we celebrated, triumphant despite innumerable exams and various iterations of the Socratic method – all under the guise of assessing knowledge while in truth seeking to insidiously erode the crowd of prospective physicians. Yet our anxiety and uncertainty melted away as names were called, hands firmly clasped, and tassels transposed. For a moment in time, we stood on the precipice of victory, enthusiastic albeit oblivious of the tremendous obstacles that loomed ahead.
Wistfully I reminisce about the unequivocal joy that abounds within the protective shield of naiveté. Specifically, I think about that time when the edict of medicine and the art of being a physician felt congruent. Yet, reality is fickle and often supersedes expectation. Occasionally my thoughts drift to the early days of residency – a time during which the emotional weight of caring for vulnerable patients while learning to master my chosen specialty felt woefully insurmountable. I recall wading blindly through each rotation attempting to emulate the competent and compassionate care so effortlessly demonstrated by senior physicians as they moved through the health care system with apparent ease. They stepped fluidly, as I watched in awe through rose-tinted glasses.
As months passed into years, my perception cleared. What I initially viewed as graceful patient care belied a complex tapestry of health care workers often pressured into arduous decisions, not necessarily in service of a well-constructed treatment plan. Gradually, formidable barriers emerged, guidelines and restrictions embedded within a confining path that suffocated those who dared to cross it. As a result, a field built on the foundations of autonomy, benevolence, and nonmaleficence was slowly engulfed by a system fraught with contrivances. Amid such stressors, physical and psychological health grows tenuous. Classically, this overwhelming feeling of distress is recognized as burnout. Studies reformulated this malady to that which was first described in Vietnam war veterans, a condition known as “moral injury.”
The impact of burnout
To explain the development – and explore the complexities – of moral injury, we must return to 1975 when the term burnout was initially formulated by Herbert Freudenberger, PhD, a psychologist renowned for his work in substance use disorders, psychoanalysis, and clinical education.1 Dr. Freudenberger’s studies noted incidences of heightened emotional and physical distress in his colleagues working in substance abuse and other clinics. He sought to define these experiences as well as understand his own battle with malaise, apathy, and frustration.1 Ultimately, Dr. Freudenberger described burnout as “Becoming exhausted by making excessive demands on energy, strength, or resources in the workplace.”2 Although it characteristically overlaps with depression and anxiety, burnout is conceptualized as a separate entity specifically forged within a context of perfectionism, integrity, and self-sacrifice.2 Such qualities are integral in health care and, as a result, physicians are particularly vulnerable.
Since Dr. Freudenberger published “Burnout: The High Cost of Achievement” in 1980, immense research has assisted in not only identifying critical factors that contribute to its development but also the detrimental effects it has on physiological health.3 These include exhaustion from poor work conditions and extreme commitment to employee responsibilities that in turn precipitate mood destabilization and impaired work performance.3 Furthermore, research has also demonstrated that burnout triggers alterations in neural circuitry via the prefrontal cortex and the amygdala, structures critical for emotional regulation.4 To combat the ill effects of burnout while maintaining productivity and maximizing profit, several high-profile corporations instituted changes focusing on self-care, wellness, benefits, and incentives. Although these modifications are effective in decreasing the rate of employee turnover, such strategies are not easily transferable to health care. In fact, the rate of physician burnout has steadily increased over the past two decades as the business of medicine shifts towards longer hours, decreased reimbursement rates, and inexhaustible insurance stipulations.2,5 Consequently, occupational dissatisfaction increases the risk of cynicism, frustration with patients, internalization of failure, and likelihood of early retirement.5 Moreover, burnout may also fracture interpersonal relationships as well as precipitate errors, negative patient outcomes, malpractice, and development of severe mental health conditions associated with high morbidity and mortality.5,8
Although the concept of burnout is critical in understanding the side effects of stereotypical workplace culture, critics of the concept bemoan a suggestion of individual blame.6,8 In essence, they argue that burnout is explained as a side effect of toxic workplace conditions, but covertly represents a lack of resilience, motivation, and ambition to thrive in a physically or emotionally taxing occupational setting.6,8 Thus, the responsibility of acclimation lies upon the impacted individuals rather than the employer. For this reason, many strategies to ameliorate burnout are focused on the individual, including meditation, wellness retreats, creating or adjusting self-care regimens, or in some cases psychotherapy and psychopharmacology.6 Whereas burnout may respond (at least partially) to such interventions, without altering the causal factors, it is unlikely to remit. This is especially the case in health care, where systemic constraints lie beyond the control of an individual physician. Rather than promoting or specifically relying upon personal improvement and recovery, amendments are needed on multiple levels to affect meaningful change.
Moral injury
Similar to burnout, moral injury was not initially conceived within the scope of health care. In the 1990s Jonathan Shay, MD, PhD, identified veterans presenting with symptoms mimicking PTSD that failed to respond to standard, well established and efficacious treatments.9-11 With further analysis he determined that veterans who demonstrated minimal improvement reported similar histories of guilt, shame, and disgust following perceived injustices enacted or abetted by immoral leaders.10,11 Ultimately Shay identified three components of moral injury: 1. A betrayal of what is morally right; 2. By someone who holds legitimate priority; 3. In a high stakes situation.10
This definition was further modified in 2007 by Brett Linz, PhD, and colleagues as: “Perpetuating, failing to prevent, or bearing witness to acts that transgress deeply held moral beliefs and expectations.”10,11 By expanding this description to include distress experienced by physicians and health care workers, Wendy Dean and Simon Talbot (in 2018 and 2019 respectively) explored how the health care system leads practitioners to deliver what they identify as substandard treatment.6-8 This results in disillusionment and lays the foundation for ethical and moral dilemmas in clinicians.
Themes of moral injury are repeatedly cited in various surveys and studies as a cause for occupational dissatisfaction. As physicians and other health care professionals reel from the aftermath of COVID-19, the effects of reconfiguring medicine into a business-oriented framework are glaringly conspicuous. Vast hospital nursing shortages, high patient census exacerbated by the political misuse and polarization of science, and insufficient availability of psychiatric beds, have culminated in a deluge of psychological strain in emergency medical physicians. Furthermore, pressure from administrators, mandated patient satisfaction measures, tedious electronic medical record systems, and copious licensing and certification requirements, contribute to physician distress as they attempt to navigate a system that challenges the vows which they swore to uphold.8 Because the cost of pursuing a medical degree frequently necessitates acquisition of loans that, without a physician income, may be difficult to repay,9 many doctors feel trapped within a seemingly endless cycle of misgiving that contributes to emotional exhaustion, pessimism, and low morale.
In my next series of The Myth of the Superdoctor columns, we will explore various factors that potentiate risk of moral injury. From medical school and residency training to corporate infrastructure and insurance obstacles, I will seek to discern and deliberate strategies for repair and rehabilitation. It is my hope that together we will illuminate the myriad complexities within the business of medicine, and become advocates and harbingers of change not only for physicians and health care workers but also for the sake of our patients and their families.
Dr. Thomas is a board-certified adult psychiatrist with interests in chronic illness, women’s behavioral health, and minority mental health. She currently practices in North Kingstown and East Providence, R.I. Dr. Thomas has no conflicts of interest.
References
1. King N. When a Psychologist Succumbed to Stress, He Coined The Term Burnout. 2016 Dec 8. NPR: All Things Considered.
2. Maslach C and Leiter MP. World Psychiatry. 2016 Jun;15(2):103-11. doi: 10.1002/wps.20311.
3. InformedHealth.org and Institute for Quality and Efficiency in Health Care. Depression: What is burnout?. https://www.ncbi.nlm.nih.gov/books/NBK279286/.
4. Michel A. Burnout and the Brain. Observer. 2016 Jan 29. https://www.psychologicalscience.org/observer/burnout-and-the-brain.
5. Patel RS et al. Behav Sci. 2018;8(11):98. doi:10.3390/bs8110098.
6. Dean W and Talbot S. Physicians aren’t ‘burning out.’ They’re suffering from moral injury. Stat. 2018 Jul 26. https://www.statnews.com/2018/07/26/physicians-not-burning-out-they-are-suffering-moral-injury/.
7. Dean W and Talbot S. Moral injury and burnout in medicine: A year of lessons learned. Stat. 2019 Jul 26. https://www.statnews.com/2019/07/26/moral-injury-burnout-medicine-lessons-learned/.
8. Dean W et al. Reframing Clinician Distress: Moral Injury Not Burnout. Fed Pract. 2019 Sep; 36(9):400-2. https://www.mdedge.com/fedprac/article/207458/mental-health/reframing-clinician-distress-moral-injury-not-burnout.
9. Bailey M. Beyond Burnout: Docs Decry ‘Moral Injury’ From Financial Pressures of Health Care. KHN. 2020 Feb 4. https://khn.org/news/beyond-burnout-docs-decry-moral-injury-from-financial-pressures-of-health-care/.
10. Litz B et al. Clin Psychol Rev. 2009 Dec;29(8):695-706. doi: 10.1016/j.cpr.2009.07.003.
11. Norman S and Maguen S. Moral Injury. PTSD: National Center for PTSD. https://www.ptsd.va.gov/professional/treat/cooccurring/moral_injury.asp.
Several years have passed since I stood among a cohort of eager medical students wearing regalia that signaled a new beginning. Four years of grueling study culminated in a cacophony of unified voices, each reciting a pledge that I had longed to take since early adolescence. Together we celebrated, triumphant despite innumerable exams and various iterations of the Socratic method – all under the guise of assessing knowledge while in truth seeking to insidiously erode the crowd of prospective physicians. Yet our anxiety and uncertainty melted away as names were called, hands firmly clasped, and tassels transposed. For a moment in time, we stood on the precipice of victory, enthusiastic albeit oblivious of the tremendous obstacles that loomed ahead.
Wistfully I reminisce about the unequivocal joy that abounds within the protective shield of naiveté. Specifically, I think about that time when the edict of medicine and the art of being a physician felt congruent. Yet, reality is fickle and often supersedes expectation. Occasionally my thoughts drift to the early days of residency – a time during which the emotional weight of caring for vulnerable patients while learning to master my chosen specialty felt woefully insurmountable. I recall wading blindly through each rotation attempting to emulate the competent and compassionate care so effortlessly demonstrated by senior physicians as they moved through the health care system with apparent ease. They stepped fluidly, as I watched in awe through rose-tinted glasses.
As months passed into years, my perception cleared. What I initially viewed as graceful patient care belied a complex tapestry of health care workers often pressured into arduous decisions, not necessarily in service of a well-constructed treatment plan. Gradually, formidable barriers emerged, guidelines and restrictions embedded within a confining path that suffocated those who dared to cross it. As a result, a field built on the foundations of autonomy, benevolence, and nonmaleficence was slowly engulfed by a system fraught with contrivances. Amid such stressors, physical and psychological health grows tenuous. Classically, this overwhelming feeling of distress is recognized as burnout. Studies reformulated this malady to that which was first described in Vietnam war veterans, a condition known as “moral injury.”
The impact of burnout
To explain the development – and explore the complexities – of moral injury, we must return to 1975 when the term burnout was initially formulated by Herbert Freudenberger, PhD, a psychologist renowned for his work in substance use disorders, psychoanalysis, and clinical education.1 Dr. Freudenberger’s studies noted incidences of heightened emotional and physical distress in his colleagues working in substance abuse and other clinics. He sought to define these experiences as well as understand his own battle with malaise, apathy, and frustration.1 Ultimately, Dr. Freudenberger described burnout as “Becoming exhausted by making excessive demands on energy, strength, or resources in the workplace.”2 Although it characteristically overlaps with depression and anxiety, burnout is conceptualized as a separate entity specifically forged within a context of perfectionism, integrity, and self-sacrifice.2 Such qualities are integral in health care and, as a result, physicians are particularly vulnerable.
Since Dr. Freudenberger published “Burnout: The High Cost of Achievement” in 1980, immense research has assisted in not only identifying critical factors that contribute to its development but also the detrimental effects it has on physiological health.3 These include exhaustion from poor work conditions and extreme commitment to employee responsibilities that in turn precipitate mood destabilization and impaired work performance.3 Furthermore, research has also demonstrated that burnout triggers alterations in neural circuitry via the prefrontal cortex and the amygdala, structures critical for emotional regulation.4 To combat the ill effects of burnout while maintaining productivity and maximizing profit, several high-profile corporations instituted changes focusing on self-care, wellness, benefits, and incentives. Although these modifications are effective in decreasing the rate of employee turnover, such strategies are not easily transferable to health care. In fact, the rate of physician burnout has steadily increased over the past two decades as the business of medicine shifts towards longer hours, decreased reimbursement rates, and inexhaustible insurance stipulations.2,5 Consequently, occupational dissatisfaction increases the risk of cynicism, frustration with patients, internalization of failure, and likelihood of early retirement.5 Moreover, burnout may also fracture interpersonal relationships as well as precipitate errors, negative patient outcomes, malpractice, and development of severe mental health conditions associated with high morbidity and mortality.5,8
Although the concept of burnout is critical in understanding the side effects of stereotypical workplace culture, critics of the concept bemoan a suggestion of individual blame.6,8 In essence, they argue that burnout is explained as a side effect of toxic workplace conditions, but covertly represents a lack of resilience, motivation, and ambition to thrive in a physically or emotionally taxing occupational setting.6,8 Thus, the responsibility of acclimation lies upon the impacted individuals rather than the employer. For this reason, many strategies to ameliorate burnout are focused on the individual, including meditation, wellness retreats, creating or adjusting self-care regimens, or in some cases psychotherapy and psychopharmacology.6 Whereas burnout may respond (at least partially) to such interventions, without altering the causal factors, it is unlikely to remit. This is especially the case in health care, where systemic constraints lie beyond the control of an individual physician. Rather than promoting or specifically relying upon personal improvement and recovery, amendments are needed on multiple levels to affect meaningful change.
Moral injury
Similar to burnout, moral injury was not initially conceived within the scope of health care. In the 1990s Jonathan Shay, MD, PhD, identified veterans presenting with symptoms mimicking PTSD that failed to respond to standard, well established and efficacious treatments.9-11 With further analysis he determined that veterans who demonstrated minimal improvement reported similar histories of guilt, shame, and disgust following perceived injustices enacted or abetted by immoral leaders.10,11 Ultimately Shay identified three components of moral injury: 1. A betrayal of what is morally right; 2. By someone who holds legitimate priority; 3. In a high stakes situation.10
This definition was further modified in 2007 by Brett Linz, PhD, and colleagues as: “Perpetuating, failing to prevent, or bearing witness to acts that transgress deeply held moral beliefs and expectations.”10,11 By expanding this description to include distress experienced by physicians and health care workers, Wendy Dean and Simon Talbot (in 2018 and 2019 respectively) explored how the health care system leads practitioners to deliver what they identify as substandard treatment.6-8 This results in disillusionment and lays the foundation for ethical and moral dilemmas in clinicians.
Themes of moral injury are repeatedly cited in various surveys and studies as a cause for occupational dissatisfaction. As physicians and other health care professionals reel from the aftermath of COVID-19, the effects of reconfiguring medicine into a business-oriented framework are glaringly conspicuous. Vast hospital nursing shortages, high patient census exacerbated by the political misuse and polarization of science, and insufficient availability of psychiatric beds, have culminated in a deluge of psychological strain in emergency medical physicians. Furthermore, pressure from administrators, mandated patient satisfaction measures, tedious electronic medical record systems, and copious licensing and certification requirements, contribute to physician distress as they attempt to navigate a system that challenges the vows which they swore to uphold.8 Because the cost of pursuing a medical degree frequently necessitates acquisition of loans that, without a physician income, may be difficult to repay,9 many doctors feel trapped within a seemingly endless cycle of misgiving that contributes to emotional exhaustion, pessimism, and low morale.
In my next series of The Myth of the Superdoctor columns, we will explore various factors that potentiate risk of moral injury. From medical school and residency training to corporate infrastructure and insurance obstacles, I will seek to discern and deliberate strategies for repair and rehabilitation. It is my hope that together we will illuminate the myriad complexities within the business of medicine, and become advocates and harbingers of change not only for physicians and health care workers but also for the sake of our patients and their families.
Dr. Thomas is a board-certified adult psychiatrist with interests in chronic illness, women’s behavioral health, and minority mental health. She currently practices in North Kingstown and East Providence, R.I. Dr. Thomas has no conflicts of interest.
References
1. King N. When a Psychologist Succumbed to Stress, He Coined The Term Burnout. 2016 Dec 8. NPR: All Things Considered.
2. Maslach C and Leiter MP. World Psychiatry. 2016 Jun;15(2):103-11. doi: 10.1002/wps.20311.
3. InformedHealth.org and Institute for Quality and Efficiency in Health Care. Depression: What is burnout?. https://www.ncbi.nlm.nih.gov/books/NBK279286/.
4. Michel A. Burnout and the Brain. Observer. 2016 Jan 29. https://www.psychologicalscience.org/observer/burnout-and-the-brain.
5. Patel RS et al. Behav Sci. 2018;8(11):98. doi:10.3390/bs8110098.
6. Dean W and Talbot S. Physicians aren’t ‘burning out.’ They’re suffering from moral injury. Stat. 2018 Jul 26. https://www.statnews.com/2018/07/26/physicians-not-burning-out-they-are-suffering-moral-injury/.
7. Dean W and Talbot S. Moral injury and burnout in medicine: A year of lessons learned. Stat. 2019 Jul 26. https://www.statnews.com/2019/07/26/moral-injury-burnout-medicine-lessons-learned/.
8. Dean W et al. Reframing Clinician Distress: Moral Injury Not Burnout. Fed Pract. 2019 Sep; 36(9):400-2. https://www.mdedge.com/fedprac/article/207458/mental-health/reframing-clinician-distress-moral-injury-not-burnout.
9. Bailey M. Beyond Burnout: Docs Decry ‘Moral Injury’ From Financial Pressures of Health Care. KHN. 2020 Feb 4. https://khn.org/news/beyond-burnout-docs-decry-moral-injury-from-financial-pressures-of-health-care/.
10. Litz B et al. Clin Psychol Rev. 2009 Dec;29(8):695-706. doi: 10.1016/j.cpr.2009.07.003.
11. Norman S and Maguen S. Moral Injury. PTSD: National Center for PTSD. https://www.ptsd.va.gov/professional/treat/cooccurring/moral_injury.asp.
Four mental health trajectories in youth: Predicting persistent psychopathology
A study that tracked psychopathology in 13,000 children and adolescents found that
Investigators also found a strong correlation between new incidence of high psychopathology and externalizing problems such as hyperactivity. “It is of paramount importance to identify factors that distinguish those with persisting problems and escalating trajectories so that resources can be appropriately directed,” wrote the authors of the study published online in JAMA Network Open.
Recent studies have shown that concurrent and sequential comorbidity of psychiatric disorders are very common in adult populations, lead author Colm Healy, PhD, a postdoctoral researcher for psychiatry with the University of Medicine and Health Sciences, Ireland, said in an interview.
The speculation is that this occurs in early life when psychiatry symptoms experience high fluidity. “This presents a complex scenario to model, where young people’s mental health appears to shift and change across development. Few investigations to date have had the data available to examine these trajectories over the full range of child development,” said Dr. Healy.
He and his colleagues attempted to map the profiles and trajectories of psychopathology in children and adolescents, using latent profile transition analysis (LPTA), a person-centered method, to assess comorbidity and movement in the various phases of childhood development.
“The idea behind person-centered methods such as LTPA is that it identifies unobserved subgroups of participants who respond similarly to specific variables – in this case responses to a broad measure of psychopathology,” explained Dr. Healy.
The study included 7,507 children from the child sample (ages 3, 5, and 9 years) and 6,039 children from the adolescent sample (ages 9, 13, and 17 or 18 years). Data analysis took place from October 2020 to September 2021.
Dr. Healy and colleagues in a supplementary investigation compared cohorts at age 9 years to look for sex and generational differences.
Four developmental profiles
Researchers identified 4 distinct developmental profies for person-centered psychopathological trajectories: no psychopathology (incidence range, 60%-70%), high psychopathology (incidence range, 3%-5%), externalizing problems (incidence range, 15%-25%), and internalizing problems (incidence range, 7%-12%).
Internalizing problems reflect issues with peers and emotional problems whereas externalizing problems more closely associate with hyperactivity and conduct.
Less than 5% of the youth studied experienced persistent symptoms. However, 48.6% in the child cohort and 44.1% in the adolescent cohort moved into one of the 3 psychopathology profiles (high psychopathology, externalizing, internalizing problems) at some point in development.
The spread of trajectories was more diverse in the child cohort, said Dr. Healy. “Children ebbed and flowed between the different profiles over time with a large proportion falling into one of the psychopathology categories and then switching between these profiles.” Switching was also evident in the adolescent cohort but to a lesser extent, he said.
Externalizing problems link to high psychopathology
Rates of remittance were higher among individuals in both cohorts for internalizing problems, compared with externalizing problems.
It’s possible that for some of these young people, internalizing problems are a reaction to environmental stressors such as bullying,” said Dr. Healy. “When that stress is relieved, the internalizing problems may dissipate.”
In a clinically relevant finding, children with externalizing problems (age 5, 129 [61.3%] and age 9, 95 [74.3%]) were more likely to present with new incidents of high psychopathology. This was also true in the adolescent group (age 13, 129 [91.1%] and age 17, 146 [89.9%]).
This suggests that a proportion of youth with externalizing problems have an escalating trajectory of psychopathology. “Thus, it may be possible to distinguish those with an escalating trajectory from a stable or remitting trajectory. The specific distinguishing factors require further investigation, but it has been observed before that some of those reporting externalizing problems in early life continue to have difficulties into later life,” noted Dr. Healy.
A combination of environmental or biological factors may explain this escalation, which could respond to early intervention, he said.
Overall, few children in the study transitioned directly from no psychopathology to high psychopathology.
Differences between boys, girls
In both cohorts, investigators noticed significant differences between the sexes.
Boys in childhood made up a larger proportion of the three psychopathology profiles. But by late adolescence, girls made up a larger proportion of the internalizing profile whereas boys made up a larger proportion of the externalizing profile. “These differences were in line with our expectations,” said Dr. Healy.
Trajectories also differed among boys and girls. In childhood, girls had a higher percentage of de-escalating trajectories relative to boys. “More girls than boys in the psychopathology profiles switched to a non or less severe profile. In adolescence, differences in trajectories were less obvious, with the exception that girls were more likely than boys to transition to internalizing problems from all of the other profiles at age 17,” said Dr. Healy.
Most young people who experience psychopathology will eventually see an improvement in symptoms, noted Dr. Healy. Next steps are to identify markers that distinguish individuals with persistent trajectories from remitting trajectories at the different phases of development, he said.
Study draws mixed reviews
Clinical psychiatrists not involved in the study had varying reactions to the results.
“This study is notable for its data-driven and powerful illustration of how childhood and adolescence are dynamic periods during which psychiatric symptoms can emerge and evolve,” said Sunny X. Tang, MD, a psychiatrist and an assistant professor at the Institute of Behavioral Science and the Feinstein Institutes for Medical Research, Manhasset, New York.
The clinical call for action is for person-centered mental health screening to be a routine part of pediatric and adolescent primary care or school-based services, noted Dr. Tang.
Paul S. Nestadt, MD, an assistant professor and public mental health researcher at Johns Hopkins University, Baltimore, did not think the study would have a significant impact on clinical practice.
He noted that Dr. Healy and coauthors found that some children stayed true to type, but many fluctuated between the four profile groups. The finding that fluctuation occurred more frequently in younger children is not surprising “and is consistent with what we know about the ‘moving targets’ that make diagnosing children so difficult,” said Dr. Nestadt.
“It would have been helpful to have identified clinical indicators of likely persistence in psychopathology, but the measure employed here did not allow that. It is also frustrating to not have any information on treatment, such that we cannot know whether the children who shifted to ‘no psychopathology’ did so because of treatment or spontaneously,” he added.
Victor M. Fornari, MD, MS, director of the Division of Child & Adolescent Psychiatry at The Zucker Hillside Hospital and Cohen’s Children’s Medical Center, New York, said the study is an important contribution to understanding the development of psychopathology during childhood.
“Generally, it is felt that nearly one in five youth will meet criteria for at least one psychiatric disorder by the age of 18. It is well known that externalizing disorders like ADHD manifest earlier in childhood and that depression often manifests later in adolescence,” he said.
No disclosures were reported.
A study that tracked psychopathology in 13,000 children and adolescents found that
Investigators also found a strong correlation between new incidence of high psychopathology and externalizing problems such as hyperactivity. “It is of paramount importance to identify factors that distinguish those with persisting problems and escalating trajectories so that resources can be appropriately directed,” wrote the authors of the study published online in JAMA Network Open.
Recent studies have shown that concurrent and sequential comorbidity of psychiatric disorders are very common in adult populations, lead author Colm Healy, PhD, a postdoctoral researcher for psychiatry with the University of Medicine and Health Sciences, Ireland, said in an interview.
The speculation is that this occurs in early life when psychiatry symptoms experience high fluidity. “This presents a complex scenario to model, where young people’s mental health appears to shift and change across development. Few investigations to date have had the data available to examine these trajectories over the full range of child development,” said Dr. Healy.
He and his colleagues attempted to map the profiles and trajectories of psychopathology in children and adolescents, using latent profile transition analysis (LPTA), a person-centered method, to assess comorbidity and movement in the various phases of childhood development.
“The idea behind person-centered methods such as LTPA is that it identifies unobserved subgroups of participants who respond similarly to specific variables – in this case responses to a broad measure of psychopathology,” explained Dr. Healy.
The study included 7,507 children from the child sample (ages 3, 5, and 9 years) and 6,039 children from the adolescent sample (ages 9, 13, and 17 or 18 years). Data analysis took place from October 2020 to September 2021.
Dr. Healy and colleagues in a supplementary investigation compared cohorts at age 9 years to look for sex and generational differences.
Four developmental profiles
Researchers identified 4 distinct developmental profies for person-centered psychopathological trajectories: no psychopathology (incidence range, 60%-70%), high psychopathology (incidence range, 3%-5%), externalizing problems (incidence range, 15%-25%), and internalizing problems (incidence range, 7%-12%).
Internalizing problems reflect issues with peers and emotional problems whereas externalizing problems more closely associate with hyperactivity and conduct.
Less than 5% of the youth studied experienced persistent symptoms. However, 48.6% in the child cohort and 44.1% in the adolescent cohort moved into one of the 3 psychopathology profiles (high psychopathology, externalizing, internalizing problems) at some point in development.
The spread of trajectories was more diverse in the child cohort, said Dr. Healy. “Children ebbed and flowed between the different profiles over time with a large proportion falling into one of the psychopathology categories and then switching between these profiles.” Switching was also evident in the adolescent cohort but to a lesser extent, he said.
Externalizing problems link to high psychopathology
Rates of remittance were higher among individuals in both cohorts for internalizing problems, compared with externalizing problems.
It’s possible that for some of these young people, internalizing problems are a reaction to environmental stressors such as bullying,” said Dr. Healy. “When that stress is relieved, the internalizing problems may dissipate.”
In a clinically relevant finding, children with externalizing problems (age 5, 129 [61.3%] and age 9, 95 [74.3%]) were more likely to present with new incidents of high psychopathology. This was also true in the adolescent group (age 13, 129 [91.1%] and age 17, 146 [89.9%]).
This suggests that a proportion of youth with externalizing problems have an escalating trajectory of psychopathology. “Thus, it may be possible to distinguish those with an escalating trajectory from a stable or remitting trajectory. The specific distinguishing factors require further investigation, but it has been observed before that some of those reporting externalizing problems in early life continue to have difficulties into later life,” noted Dr. Healy.
A combination of environmental or biological factors may explain this escalation, which could respond to early intervention, he said.
Overall, few children in the study transitioned directly from no psychopathology to high psychopathology.
Differences between boys, girls
In both cohorts, investigators noticed significant differences between the sexes.
Boys in childhood made up a larger proportion of the three psychopathology profiles. But by late adolescence, girls made up a larger proportion of the internalizing profile whereas boys made up a larger proportion of the externalizing profile. “These differences were in line with our expectations,” said Dr. Healy.
Trajectories also differed among boys and girls. In childhood, girls had a higher percentage of de-escalating trajectories relative to boys. “More girls than boys in the psychopathology profiles switched to a non or less severe profile. In adolescence, differences in trajectories were less obvious, with the exception that girls were more likely than boys to transition to internalizing problems from all of the other profiles at age 17,” said Dr. Healy.
Most young people who experience psychopathology will eventually see an improvement in symptoms, noted Dr. Healy. Next steps are to identify markers that distinguish individuals with persistent trajectories from remitting trajectories at the different phases of development, he said.
Study draws mixed reviews
Clinical psychiatrists not involved in the study had varying reactions to the results.
“This study is notable for its data-driven and powerful illustration of how childhood and adolescence are dynamic periods during which psychiatric symptoms can emerge and evolve,” said Sunny X. Tang, MD, a psychiatrist and an assistant professor at the Institute of Behavioral Science and the Feinstein Institutes for Medical Research, Manhasset, New York.
The clinical call for action is for person-centered mental health screening to be a routine part of pediatric and adolescent primary care or school-based services, noted Dr. Tang.
Paul S. Nestadt, MD, an assistant professor and public mental health researcher at Johns Hopkins University, Baltimore, did not think the study would have a significant impact on clinical practice.
He noted that Dr. Healy and coauthors found that some children stayed true to type, but many fluctuated between the four profile groups. The finding that fluctuation occurred more frequently in younger children is not surprising “and is consistent with what we know about the ‘moving targets’ that make diagnosing children so difficult,” said Dr. Nestadt.
“It would have been helpful to have identified clinical indicators of likely persistence in psychopathology, but the measure employed here did not allow that. It is also frustrating to not have any information on treatment, such that we cannot know whether the children who shifted to ‘no psychopathology’ did so because of treatment or spontaneously,” he added.
Victor M. Fornari, MD, MS, director of the Division of Child & Adolescent Psychiatry at The Zucker Hillside Hospital and Cohen’s Children’s Medical Center, New York, said the study is an important contribution to understanding the development of psychopathology during childhood.
“Generally, it is felt that nearly one in five youth will meet criteria for at least one psychiatric disorder by the age of 18. It is well known that externalizing disorders like ADHD manifest earlier in childhood and that depression often manifests later in adolescence,” he said.
No disclosures were reported.
A study that tracked psychopathology in 13,000 children and adolescents found that
Investigators also found a strong correlation between new incidence of high psychopathology and externalizing problems such as hyperactivity. “It is of paramount importance to identify factors that distinguish those with persisting problems and escalating trajectories so that resources can be appropriately directed,” wrote the authors of the study published online in JAMA Network Open.
Recent studies have shown that concurrent and sequential comorbidity of psychiatric disorders are very common in adult populations, lead author Colm Healy, PhD, a postdoctoral researcher for psychiatry with the University of Medicine and Health Sciences, Ireland, said in an interview.
The speculation is that this occurs in early life when psychiatry symptoms experience high fluidity. “This presents a complex scenario to model, where young people’s mental health appears to shift and change across development. Few investigations to date have had the data available to examine these trajectories over the full range of child development,” said Dr. Healy.
He and his colleagues attempted to map the profiles and trajectories of psychopathology in children and adolescents, using latent profile transition analysis (LPTA), a person-centered method, to assess comorbidity and movement in the various phases of childhood development.
“The idea behind person-centered methods such as LTPA is that it identifies unobserved subgroups of participants who respond similarly to specific variables – in this case responses to a broad measure of psychopathology,” explained Dr. Healy.
The study included 7,507 children from the child sample (ages 3, 5, and 9 years) and 6,039 children from the adolescent sample (ages 9, 13, and 17 or 18 years). Data analysis took place from October 2020 to September 2021.
Dr. Healy and colleagues in a supplementary investigation compared cohorts at age 9 years to look for sex and generational differences.
Four developmental profiles
Researchers identified 4 distinct developmental profies for person-centered psychopathological trajectories: no psychopathology (incidence range, 60%-70%), high psychopathology (incidence range, 3%-5%), externalizing problems (incidence range, 15%-25%), and internalizing problems (incidence range, 7%-12%).
Internalizing problems reflect issues with peers and emotional problems whereas externalizing problems more closely associate with hyperactivity and conduct.
Less than 5% of the youth studied experienced persistent symptoms. However, 48.6% in the child cohort and 44.1% in the adolescent cohort moved into one of the 3 psychopathology profiles (high psychopathology, externalizing, internalizing problems) at some point in development.
The spread of trajectories was more diverse in the child cohort, said Dr. Healy. “Children ebbed and flowed between the different profiles over time with a large proportion falling into one of the psychopathology categories and then switching between these profiles.” Switching was also evident in the adolescent cohort but to a lesser extent, he said.
Externalizing problems link to high psychopathology
Rates of remittance were higher among individuals in both cohorts for internalizing problems, compared with externalizing problems.
It’s possible that for some of these young people, internalizing problems are a reaction to environmental stressors such as bullying,” said Dr. Healy. “When that stress is relieved, the internalizing problems may dissipate.”
In a clinically relevant finding, children with externalizing problems (age 5, 129 [61.3%] and age 9, 95 [74.3%]) were more likely to present with new incidents of high psychopathology. This was also true in the adolescent group (age 13, 129 [91.1%] and age 17, 146 [89.9%]).
This suggests that a proportion of youth with externalizing problems have an escalating trajectory of psychopathology. “Thus, it may be possible to distinguish those with an escalating trajectory from a stable or remitting trajectory. The specific distinguishing factors require further investigation, but it has been observed before that some of those reporting externalizing problems in early life continue to have difficulties into later life,” noted Dr. Healy.
A combination of environmental or biological factors may explain this escalation, which could respond to early intervention, he said.
Overall, few children in the study transitioned directly from no psychopathology to high psychopathology.
Differences between boys, girls
In both cohorts, investigators noticed significant differences between the sexes.
Boys in childhood made up a larger proportion of the three psychopathology profiles. But by late adolescence, girls made up a larger proportion of the internalizing profile whereas boys made up a larger proportion of the externalizing profile. “These differences were in line with our expectations,” said Dr. Healy.
Trajectories also differed among boys and girls. In childhood, girls had a higher percentage of de-escalating trajectories relative to boys. “More girls than boys in the psychopathology profiles switched to a non or less severe profile. In adolescence, differences in trajectories were less obvious, with the exception that girls were more likely than boys to transition to internalizing problems from all of the other profiles at age 17,” said Dr. Healy.
Most young people who experience psychopathology will eventually see an improvement in symptoms, noted Dr. Healy. Next steps are to identify markers that distinguish individuals with persistent trajectories from remitting trajectories at the different phases of development, he said.
Study draws mixed reviews
Clinical psychiatrists not involved in the study had varying reactions to the results.
“This study is notable for its data-driven and powerful illustration of how childhood and adolescence are dynamic periods during which psychiatric symptoms can emerge and evolve,” said Sunny X. Tang, MD, a psychiatrist and an assistant professor at the Institute of Behavioral Science and the Feinstein Institutes for Medical Research, Manhasset, New York.
The clinical call for action is for person-centered mental health screening to be a routine part of pediatric and adolescent primary care or school-based services, noted Dr. Tang.
Paul S. Nestadt, MD, an assistant professor and public mental health researcher at Johns Hopkins University, Baltimore, did not think the study would have a significant impact on clinical practice.
He noted that Dr. Healy and coauthors found that some children stayed true to type, but many fluctuated between the four profile groups. The finding that fluctuation occurred more frequently in younger children is not surprising “and is consistent with what we know about the ‘moving targets’ that make diagnosing children so difficult,” said Dr. Nestadt.
“It would have been helpful to have identified clinical indicators of likely persistence in psychopathology, but the measure employed here did not allow that. It is also frustrating to not have any information on treatment, such that we cannot know whether the children who shifted to ‘no psychopathology’ did so because of treatment or spontaneously,” he added.
Victor M. Fornari, MD, MS, director of the Division of Child & Adolescent Psychiatry at The Zucker Hillside Hospital and Cohen’s Children’s Medical Center, New York, said the study is an important contribution to understanding the development of psychopathology during childhood.
“Generally, it is felt that nearly one in five youth will meet criteria for at least one psychiatric disorder by the age of 18. It is well known that externalizing disorders like ADHD manifest earlier in childhood and that depression often manifests later in adolescence,” he said.
No disclosures were reported.
FROM JAMA NETWORK OPEN
Neuropsychiatric risks of COVID-19: New data
The neuropsychiatric ramifications of severe COVID-19 infection appear to be no different than for other severe acute respiratory infections (SARI).
This suggests that disease severity, rather than pathogen, is the most relevant factor in new-onset neuropsychiatric illness, the investigators note.
The risk of new-onset neuropsychological illness after severe COVID-19 infection are “substantial, but similar to those after other severe respiratory infections,” study investigator Peter Watkinson, MD, Nuffield Department of Clinical Neurosciences, University of Oxford, and John Radcliffe Hospital, Oxford, England, told this news organization.
The study was published online in JAMA Psychiatry.
Significant mental health burden
Research has shown a significant burden of neuropsychological illness after severe COVID-19 infection. However, it’s unclear how this risk compares to SARI.
To investigate, Dr. Watkinson and colleagues evaluated electronic health record data on more than 8.3 million adults, including 16,679 (0.02%) who survived a hospital admission for SARI and 32,525 (0.03%) who survived a hospital stay for COVID-19.
Compared with the remaining population, risks of new anxiety disorder, dementia, psychotic disorder, depression, and bipolar disorder diagnoses were significantly and similarly increased in adults surviving hospitalization for either COVID-19 or SARI.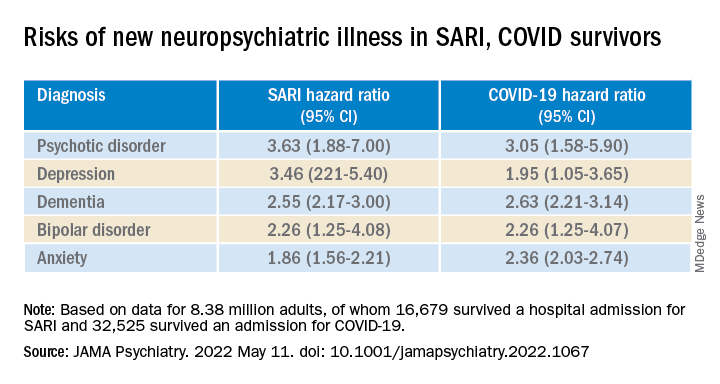
Compared with the wider population, survivors of severe SARI or COVID-19 were also at increased risk of starting treatment with antidepressants, hypnotics/anxiolytics, or antipsychotics.
When comparing survivors of SARI hospitalization to survivors of COVID-19 hospitalization, no significant differences were observed in the postdischarge rates of new-onset anxiety disorder, dementia, depression, or bipolar affective disorder.
The SARI and COVID groups also did not differ in terms of their postdischarge risks of antidepressant or hypnotic/anxiolytic use, but the COVID survivors had a 20% lower risk of starting an antipsychotic.
“In this cohort study, SARI were found to be associated with significant postacute neuropsychiatric morbidity, for which COVID-19 is not distinctly different,” Dr. Watkinson and colleagues write.
“These results may help refine our understanding of the post–severe COVID-19 phenotype and may inform post-discharge support for patients requiring hospital-based and intensive care for SARI regardless of causative pathogen,” they write.
Caveats, cautionary notes
Kevin McConway, PhD, emeritus professor of applied statistics at the Open University in Milton Keynes, England, described the study as “impressive.” However, he pointed out that the study’s observational design is a limitation.
“One can never be absolutely certain about the interpretation of findings of an observational study. What the research can’t tell us is what caused the increased psychiatric risks for people hospitalized with COVID-19 or some other serious respiratory disease,” Dr. McConway said.
“It can’t tell us what might happen in the future, when, we all hope, many fewer are being hospitalized with COVID-19 than was the case in those first two waves, and the current backlog of provision of some health services has decreased,” he added.
“So we can’t just say that, in general, serious COVID-19 has much the same neuropsychiatric consequences as other very serious respiratory illness. Maybe it does, maybe it doesn’t,” Dr. McConway cautioned.
Max Taquet, PhD, with the University of Oxford, noted that the study is limited to hospitalized adult patients, leaving open the question of risk in nonhospitalized individuals – which is the overwhelming majority of patients with COVID-19 – or in children.
Whether the neuropsychiatric risks have remained the same since the emergence of the Omicron variant also remains “an open question since all patients in this study were diagnosed before July 2021,” Dr. Taquet said in statement.
The study was funded by the Wellcome Trust, the John Fell Oxford University Press Research Fund, the Oxford Wellcome Institutional Strategic Support Fund and Cancer Research UK, through the Cancer Research UK Oxford Centre. Dr. Watkinson disclosed grants from the National Institute for Health Research and Sensyne Health outside the submitted work; and serving as chief medical officer for Sensyne Health prior to this work, as well as holding shares in the company. Dr. McConway is a trustee of the UK Science Media Centre and a member of its advisory committee. His comments were provided in his capacity as an independent professional statistician. Dr. Taquet has worked on similar studies trying to identify, quantify, and specify the neurological and psychiatric consequences of COVID-19.
A version of this article first appeared on Medscape.com.
The neuropsychiatric ramifications of severe COVID-19 infection appear to be no different than for other severe acute respiratory infections (SARI).
This suggests that disease severity, rather than pathogen, is the most relevant factor in new-onset neuropsychiatric illness, the investigators note.
The risk of new-onset neuropsychological illness after severe COVID-19 infection are “substantial, but similar to those after other severe respiratory infections,” study investigator Peter Watkinson, MD, Nuffield Department of Clinical Neurosciences, University of Oxford, and John Radcliffe Hospital, Oxford, England, told this news organization.
The study was published online in JAMA Psychiatry.
Significant mental health burden
Research has shown a significant burden of neuropsychological illness after severe COVID-19 infection. However, it’s unclear how this risk compares to SARI.
To investigate, Dr. Watkinson and colleagues evaluated electronic health record data on more than 8.3 million adults, including 16,679 (0.02%) who survived a hospital admission for SARI and 32,525 (0.03%) who survived a hospital stay for COVID-19.
Compared with the remaining population, risks of new anxiety disorder, dementia, psychotic disorder, depression, and bipolar disorder diagnoses were significantly and similarly increased in adults surviving hospitalization for either COVID-19 or SARI.
Compared with the wider population, survivors of severe SARI or COVID-19 were also at increased risk of starting treatment with antidepressants, hypnotics/anxiolytics, or antipsychotics.
When comparing survivors of SARI hospitalization to survivors of COVID-19 hospitalization, no significant differences were observed in the postdischarge rates of new-onset anxiety disorder, dementia, depression, or bipolar affective disorder.
The SARI and COVID groups also did not differ in terms of their postdischarge risks of antidepressant or hypnotic/anxiolytic use, but the COVID survivors had a 20% lower risk of starting an antipsychotic.
“In this cohort study, SARI were found to be associated with significant postacute neuropsychiatric morbidity, for which COVID-19 is not distinctly different,” Dr. Watkinson and colleagues write.
“These results may help refine our understanding of the post–severe COVID-19 phenotype and may inform post-discharge support for patients requiring hospital-based and intensive care for SARI regardless of causative pathogen,” they write.
Caveats, cautionary notes
Kevin McConway, PhD, emeritus professor of applied statistics at the Open University in Milton Keynes, England, described the study as “impressive.” However, he pointed out that the study’s observational design is a limitation.
“One can never be absolutely certain about the interpretation of findings of an observational study. What the research can’t tell us is what caused the increased psychiatric risks for people hospitalized with COVID-19 or some other serious respiratory disease,” Dr. McConway said.
“It can’t tell us what might happen in the future, when, we all hope, many fewer are being hospitalized with COVID-19 than was the case in those first two waves, and the current backlog of provision of some health services has decreased,” he added.
“So we can’t just say that, in general, serious COVID-19 has much the same neuropsychiatric consequences as other very serious respiratory illness. Maybe it does, maybe it doesn’t,” Dr. McConway cautioned.
Max Taquet, PhD, with the University of Oxford, noted that the study is limited to hospitalized adult patients, leaving open the question of risk in nonhospitalized individuals – which is the overwhelming majority of patients with COVID-19 – or in children.
Whether the neuropsychiatric risks have remained the same since the emergence of the Omicron variant also remains “an open question since all patients in this study were diagnosed before July 2021,” Dr. Taquet said in statement.
The study was funded by the Wellcome Trust, the John Fell Oxford University Press Research Fund, the Oxford Wellcome Institutional Strategic Support Fund and Cancer Research UK, through the Cancer Research UK Oxford Centre. Dr. Watkinson disclosed grants from the National Institute for Health Research and Sensyne Health outside the submitted work; and serving as chief medical officer for Sensyne Health prior to this work, as well as holding shares in the company. Dr. McConway is a trustee of the UK Science Media Centre and a member of its advisory committee. His comments were provided in his capacity as an independent professional statistician. Dr. Taquet has worked on similar studies trying to identify, quantify, and specify the neurological and psychiatric consequences of COVID-19.
A version of this article first appeared on Medscape.com.
The neuropsychiatric ramifications of severe COVID-19 infection appear to be no different than for other severe acute respiratory infections (SARI).
This suggests that disease severity, rather than pathogen, is the most relevant factor in new-onset neuropsychiatric illness, the investigators note.
The risk of new-onset neuropsychological illness after severe COVID-19 infection are “substantial, but similar to those after other severe respiratory infections,” study investigator Peter Watkinson, MD, Nuffield Department of Clinical Neurosciences, University of Oxford, and John Radcliffe Hospital, Oxford, England, told this news organization.
The study was published online in JAMA Psychiatry.
Significant mental health burden
Research has shown a significant burden of neuropsychological illness after severe COVID-19 infection. However, it’s unclear how this risk compares to SARI.
To investigate, Dr. Watkinson and colleagues evaluated electronic health record data on more than 8.3 million adults, including 16,679 (0.02%) who survived a hospital admission for SARI and 32,525 (0.03%) who survived a hospital stay for COVID-19.
Compared with the remaining population, risks of new anxiety disorder, dementia, psychotic disorder, depression, and bipolar disorder diagnoses were significantly and similarly increased in adults surviving hospitalization for either COVID-19 or SARI.
Compared with the wider population, survivors of severe SARI or COVID-19 were also at increased risk of starting treatment with antidepressants, hypnotics/anxiolytics, or antipsychotics.
When comparing survivors of SARI hospitalization to survivors of COVID-19 hospitalization, no significant differences were observed in the postdischarge rates of new-onset anxiety disorder, dementia, depression, or bipolar affective disorder.
The SARI and COVID groups also did not differ in terms of their postdischarge risks of antidepressant or hypnotic/anxiolytic use, but the COVID survivors had a 20% lower risk of starting an antipsychotic.
“In this cohort study, SARI were found to be associated with significant postacute neuropsychiatric morbidity, for which COVID-19 is not distinctly different,” Dr. Watkinson and colleagues write.
“These results may help refine our understanding of the post–severe COVID-19 phenotype and may inform post-discharge support for patients requiring hospital-based and intensive care for SARI regardless of causative pathogen,” they write.
Caveats, cautionary notes
Kevin McConway, PhD, emeritus professor of applied statistics at the Open University in Milton Keynes, England, described the study as “impressive.” However, he pointed out that the study’s observational design is a limitation.
“One can never be absolutely certain about the interpretation of findings of an observational study. What the research can’t tell us is what caused the increased psychiatric risks for people hospitalized with COVID-19 or some other serious respiratory disease,” Dr. McConway said.
“It can’t tell us what might happen in the future, when, we all hope, many fewer are being hospitalized with COVID-19 than was the case in those first two waves, and the current backlog of provision of some health services has decreased,” he added.
“So we can’t just say that, in general, serious COVID-19 has much the same neuropsychiatric consequences as other very serious respiratory illness. Maybe it does, maybe it doesn’t,” Dr. McConway cautioned.
Max Taquet, PhD, with the University of Oxford, noted that the study is limited to hospitalized adult patients, leaving open the question of risk in nonhospitalized individuals – which is the overwhelming majority of patients with COVID-19 – or in children.
Whether the neuropsychiatric risks have remained the same since the emergence of the Omicron variant also remains “an open question since all patients in this study were diagnosed before July 2021,” Dr. Taquet said in statement.
The study was funded by the Wellcome Trust, the John Fell Oxford University Press Research Fund, the Oxford Wellcome Institutional Strategic Support Fund and Cancer Research UK, through the Cancer Research UK Oxford Centre. Dr. Watkinson disclosed grants from the National Institute for Health Research and Sensyne Health outside the submitted work; and serving as chief medical officer for Sensyne Health prior to this work, as well as holding shares in the company. Dr. McConway is a trustee of the UK Science Media Centre and a member of its advisory committee. His comments were provided in his capacity as an independent professional statistician. Dr. Taquet has worked on similar studies trying to identify, quantify, and specify the neurological and psychiatric consequences of COVID-19.
A version of this article first appeared on Medscape.com.

















