User login
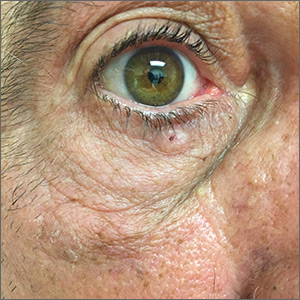
Pigmented lesion on face
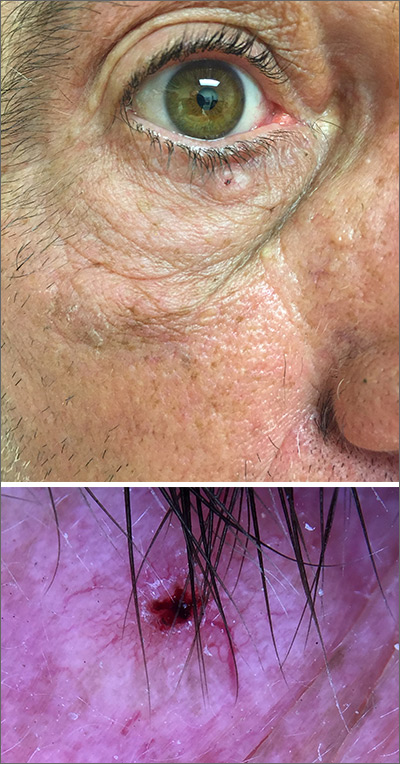
While the lesion’s proximity to the eyelashes and lid margin made dermoscopy difficult, the physician was able to use a dermatoscope to view the lesion and recognize it as nodular basal cell carcinoma (BCC). (If dermoscopy had not been an option, a hand magnifier or otoscope could have been used to help with magnification and diagnosis.)
Nodular BCCs usually present with a raised pearly border, a central ulceration, and telangiectasias. In this case, the central erosion was much more obvious with dermoscopy. Also visible were abnormal telangiectasias around the central erosion; they were especially dilated and tortuous (referred to as an arborizing pattern) at the 4:00 position. The diagnosis was confirmed by a small tangential shave biopsy of the inferior aspect of the lesion.
BCCs are referred for Mohs micrographic surgery (MMS) when they are any of the following: in high-risk locations such as the T-zone of the face (eyes, nose, and mouth); > 2 cm in diameter; a recurrence of a previous BCC; or a high-risk type including infiltrating, morpheaform, or basosquamous (based on pathology). Lower risk nodular BCCs are usually treated with excision or electrodesiccation and curettage.
In this case, the BCC was in a high-risk location and required MMS. The challenge was that the lesion was so close to the lid margin that resection of the cancer and subsequent repair could lead to ectropion/poor lid closure. The Mohs surgeon resected the lesion in 3 stages. The oculoplastic surgeon then closed the defect via a multilayered repair.
Images and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

While the lesion’s proximity to the eyelashes and lid margin made dermoscopy difficult, the physician was able to use a dermatoscope to view the lesion and recognize it as nodular basal cell carcinoma (BCC). (If dermoscopy had not been an option, a hand magnifier or otoscope could have been used to help with magnification and diagnosis.)
Nodular BCCs usually present with a raised pearly border, a central ulceration, and telangiectasias. In this case, the central erosion was much more obvious with dermoscopy. Also visible were abnormal telangiectasias around the central erosion; they were especially dilated and tortuous (referred to as an arborizing pattern) at the 4:00 position. The diagnosis was confirmed by a small tangential shave biopsy of the inferior aspect of the lesion.
BCCs are referred for Mohs micrographic surgery (MMS) when they are any of the following: in high-risk locations such as the T-zone of the face (eyes, nose, and mouth); > 2 cm in diameter; a recurrence of a previous BCC; or a high-risk type including infiltrating, morpheaform, or basosquamous (based on pathology). Lower risk nodular BCCs are usually treated with excision or electrodesiccation and curettage.
In this case, the BCC was in a high-risk location and required MMS. The challenge was that the lesion was so close to the lid margin that resection of the cancer and subsequent repair could lead to ectropion/poor lid closure. The Mohs surgeon resected the lesion in 3 stages. The oculoplastic surgeon then closed the defect via a multilayered repair.
Images and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

While the lesion’s proximity to the eyelashes and lid margin made dermoscopy difficult, the physician was able to use a dermatoscope to view the lesion and recognize it as nodular basal cell carcinoma (BCC). (If dermoscopy had not been an option, a hand magnifier or otoscope could have been used to help with magnification and diagnosis.)
Nodular BCCs usually present with a raised pearly border, a central ulceration, and telangiectasias. In this case, the central erosion was much more obvious with dermoscopy. Also visible were abnormal telangiectasias around the central erosion; they were especially dilated and tortuous (referred to as an arborizing pattern) at the 4:00 position. The diagnosis was confirmed by a small tangential shave biopsy of the inferior aspect of the lesion.
BCCs are referred for Mohs micrographic surgery (MMS) when they are any of the following: in high-risk locations such as the T-zone of the face (eyes, nose, and mouth); > 2 cm in diameter; a recurrence of a previous BCC; or a high-risk type including infiltrating, morpheaform, or basosquamous (based on pathology). Lower risk nodular BCCs are usually treated with excision or electrodesiccation and curettage.
In this case, the BCC was in a high-risk location and required MMS. The challenge was that the lesion was so close to the lid margin that resection of the cancer and subsequent repair could lead to ectropion/poor lid closure. The Mohs surgeon resected the lesion in 3 stages. The oculoplastic surgeon then closed the defect via a multilayered repair.
Images and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
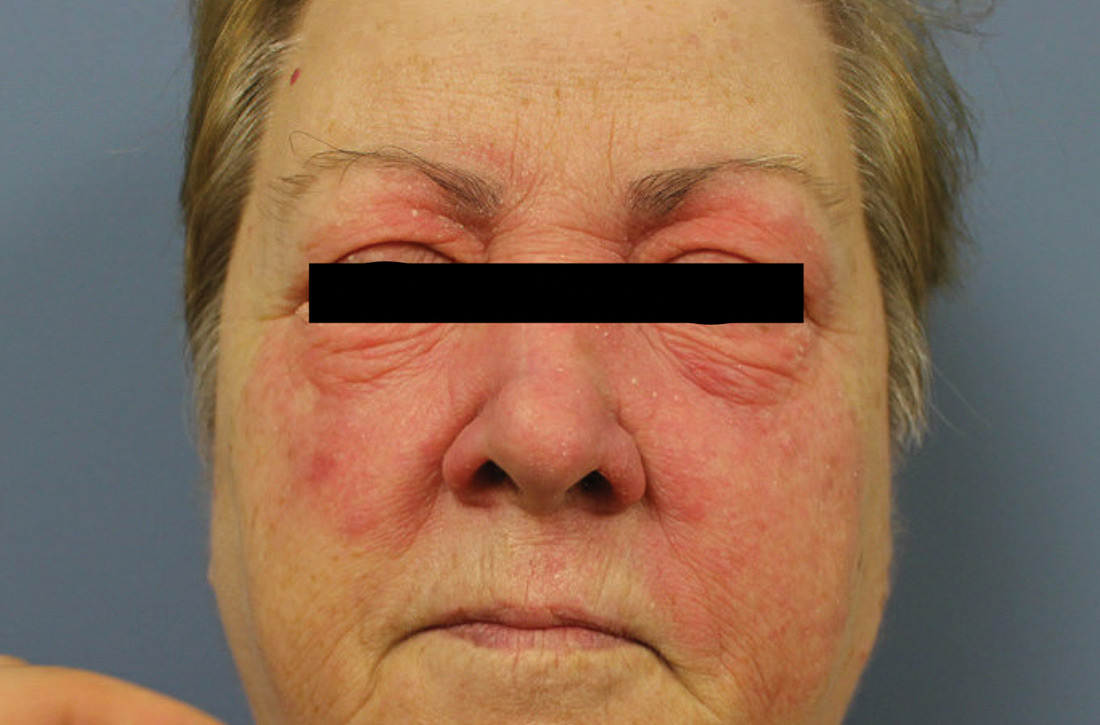
An erythematous facial rash
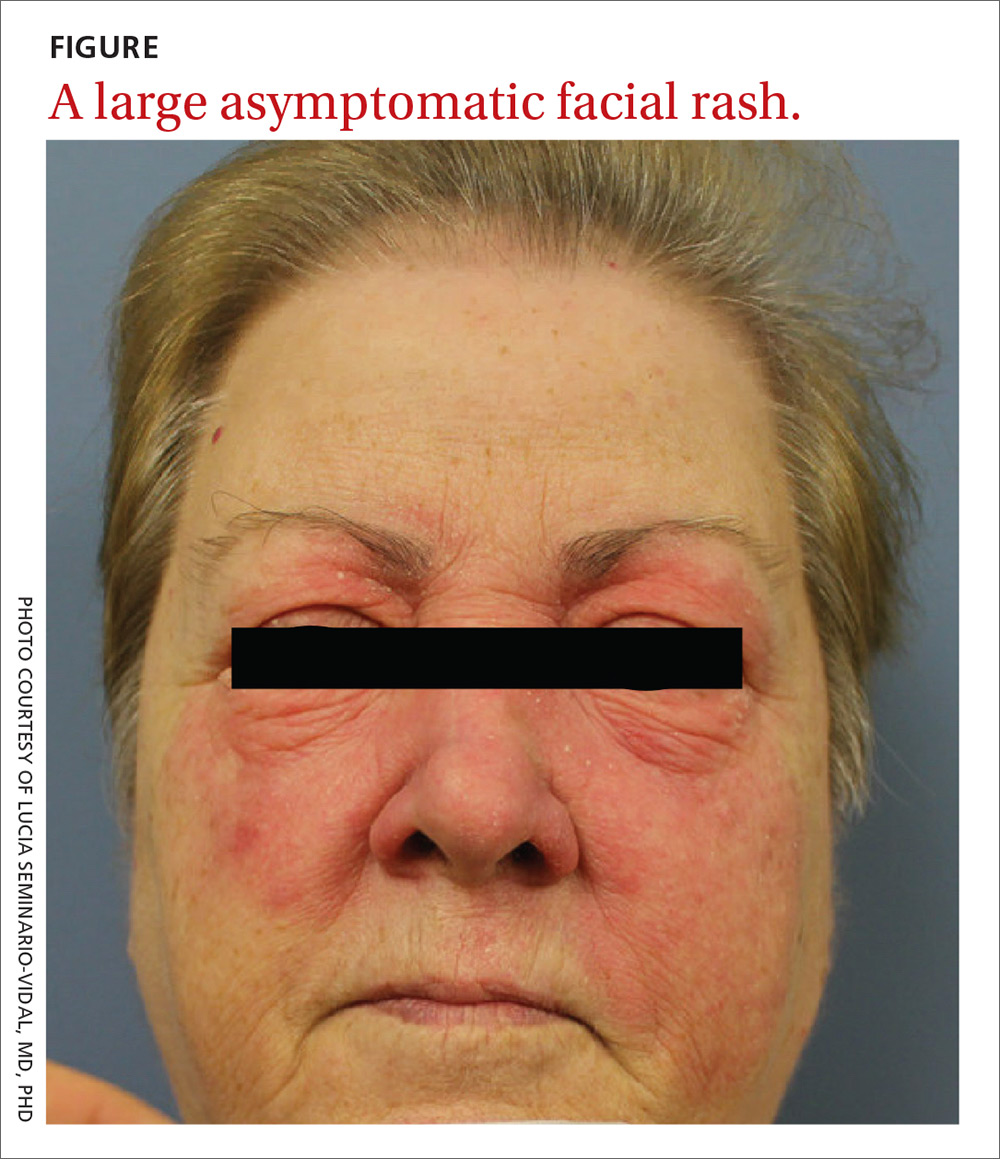
A 59-year-old woman presented to our clinic with a large asymptomatic facial rash that had developed several months earlier. The rash had been slowly growing but did not change day to day. Her past medical history was significant for hypertension, hyperlipidemia, and cutaneous lymphoma, which was localized to her arms. She denied the use of any new products, including hair or facial products, nail polish, or any new medications.
Initially, she was presumed (by an outside provider) to have rosacea, and she received treatment with doxycycline 100 mg/d for 2 months. However, the rash did not improve.
Physical examination revealed a large erythematous rash involving her cheeks, nose, and periocular area with no other significant findings (FIGURE).
A biopsy of her right cheek was performed.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Mycosis fungoides
Following the biopsy of her right cheek, a histopathologic analysis demonstrated an atypical lymphocytic infiltrate positive for CD3 and CD4. These histopathologic features led to a diagnosis of recurrent mycosis fungoides (MF), a type of cutaneous lymphoma. (Our patient’s cutaneous lymphoma had been in remission for a year following local radiotherapy.)
MF is the most common type of cutaneous lymphoma, with an incidence of 6.4 to 9.6 cases per million people in the United States.1 There are also 2 rare subtypes of MF: the psoriasiform and palmoplantar forms. Psoriasiform MF presents with psoriasis-like plaques, while palmoplantar MF initially presents on the palms and soles.
Patients with classic MF typically present with patches and plaques—with the late evolution of tumors—on non–sun-exposed areas.1 Our patient’s clinical presentation was atypical because the rash manifested on a sun-exposed area of her body.
MF and other cutaneous lymphomas should always be part of the differential diagnosis for an unexplained persistent rash, especially in a patient with a history of MF. The development of lymphomas is thought to be a stepwise process through which chronic antigenic stimulation results in an accumulation of genetic mutations that then cause cells to undergo clonal expansion and, ultimately, malignant transformation. Genetic, environmental, and immunologic factors that contribute to the disease pathogenesis have been identified.2
Once clinical features point toward MF, the diagnosis can be further differentiated from other benign inflammatory mimics with a biopsy demonstrating cerebriform lymphocytes homing toward the epidermis, monoclonal expansion of T cells, and defective apoptosis.3
Continue to: Differential includes rosacea and seborrheic dermatitis
Differential includes rosacea and seborrheic dermatitis
The diagnosis of MF can be difficult as it often imitates other benign inflammatory conditions.
Rosacea manifests as an erythematous facial rash but usually spares the nasolabial folds and eyelids. There are several forms, including ocular (featuring swollen and irritated conjunctiva), erythematotelangiectatic (with visible blood vessels), and papulopustular (with acneic lesions). Over time, the skin may develop a thickened, bumpy texture, referred to as phymatous rosacea.4 A history of acute worsening with exposure to certain hot or spicy foods, alcohol, or ultraviolet light suggests a diagnosis of rosacea.
Seborrheic dermatitis classically presents as yellow scaling on a mildly erythematous base and often involves nasolabial folds and eyebrows. Seborrheic dermatitis can be associated with human immunodeficiency virus, Parkinson’s disease, and other chronic medical conditions.
Allergic contact dermatitis can look identical to MF, but in our case, there was no new allergen in the history. A thorough history regarding new medications, creams, and household supplies is integral to differentiating this diagnosis.
Misdiagnosis can lead to advanced-stage disease
This case of persistent facial erythema, originally treated as rosacea, highlights the importance of having a low threshold of suspicion of MF, especially in a patient with a prior history of MF. A recent study by Kelati et al3 indicated that certain subtypes of MF are easily misdiagnosed and treated as psoriasis or eczema respectively for an average of 10.5 years.3 These years of misdiagnosis are significantly correlated with the development of advanced-stage MF, which is more difficult to treat.3
Continue to: Treatment with topical desonide and mechlorethamine
Treatment with topical desonide and mechlorethamine
There are multiple treatment options for MF, depending on the stage, starting with topical therapies and advancing to systemic therapies in more advanced stages. Topical treatments include steroids, nitrogen mustard, and retinoids.5 Our patient was referred to a multidisciplinary lymphoma clinic, where topical treatment was initiated with desonide cream .05% and mechlorethamine gel .016%. Our patient experienced a 50% improvement in skin involvement at 3 months.
As MF progresses to more advanced stages, treatment often combines skin-directed therapies with systemic immunomodulators, biologics, radiation, and total skin electron beam therapy.6 TSEBT is a low-dose full-body radiation treatment that targets the skin surface and therefore effectively treats cutaneous lymphoma. Although TSEBT is usually well tolerated, there have been documented acute and chronic adverse effects, including dermatitis, alopecia, peripheral edema, cutaneous malignancies, and infertility in men.7
While the use of topical desonide and mechlorethamine was initially favored over radiation due to eyelid involvement, our patient developed new patches on her legs 11 months after her initial visit. When biopsies indicated MF with large cell transformation, she received 1 course of low-dose TSEBT (12 Gy), with complete response noted at the 2 month follow-up.
CORRESPONDENCE
Lucia Seminario-Vidal, MD, PhD, Department of Dermatology and Cutaneous Surgery, 13330 USF Laurel Drive, Tampa, FL 33612; [email protected]
1. Jawed S, Myskowski P, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome). Part I. Diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-e16.
2. Wohl Y, Tur E. Environmental risk factors for mycosis fungoides. Curr Probl Dermatol. 2007;35:52-64.
3. Kelati A, Gallouj S, Tahiri L, et al. Defining the mimics and clinico-histological diagnosis criteria for mycosis fungoides to minimize misdiagnosis. Int J Womens Dermatol. 2017;3:100-106.
4. Two AM, Wu W, Gallo RL, et al. Rosacea. part I. Introduction, categorization, histology, pathogenesis, and risk factors. J AM Acad Dermatol. 2015;72:749-758.
5. Lessin SR, Duvic M, Guitart J, et al. Topical chemotherapy in cutaneous T-cell lymphoma positive results of a randomized, controlled, multicenter trial testing the efficacy and safety of a novel mechlorethamine, 0.02%, gel in mycosis fungoides. JAMA Dermatol. 2013;149:25-32.
6. Jawed S, Myskowski P, Horwitz S, et al. Continuing medical education: Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part II. Prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e1-e17.
7. De Moraes FY, Carvalho Hde A, Hanna SA, et al. Literature review of clinical results of total skin electron irradiation (TSEBT) of mycosis fungoides in adults. Rep Pract Oncol Radiother. 2014;19:92-98.
A 59-year-old woman presented to our clinic with a large asymptomatic facial rash that had developed several months earlier. The rash had been slowly growing but did not change day to day. Her past medical history was significant for hypertension, hyperlipidemia, and cutaneous lymphoma, which was localized to her arms. She denied the use of any new products, including hair or facial products, nail polish, or any new medications.
Initially, she was presumed (by an outside provider) to have rosacea, and she received treatment with doxycycline 100 mg/d for 2 months. However, the rash did not improve.
Physical examination revealed a large erythematous rash involving her cheeks, nose, and periocular area with no other significant findings (FIGURE).

A biopsy of her right cheek was performed.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Mycosis fungoides
Following the biopsy of her right cheek, a histopathologic analysis demonstrated an atypical lymphocytic infiltrate positive for CD3 and CD4. These histopathologic features led to a diagnosis of recurrent mycosis fungoides (MF), a type of cutaneous lymphoma. (Our patient’s cutaneous lymphoma had been in remission for a year following local radiotherapy.)
MF is the most common type of cutaneous lymphoma, with an incidence of 6.4 to 9.6 cases per million people in the United States.1 There are also 2 rare subtypes of MF: the psoriasiform and palmoplantar forms. Psoriasiform MF presents with psoriasis-like plaques, while palmoplantar MF initially presents on the palms and soles.
Patients with classic MF typically present with patches and plaques—with the late evolution of tumors—on non–sun-exposed areas.1 Our patient’s clinical presentation was atypical because the rash manifested on a sun-exposed area of her body.
MF and other cutaneous lymphomas should always be part of the differential diagnosis for an unexplained persistent rash, especially in a patient with a history of MF. The development of lymphomas is thought to be a stepwise process through which chronic antigenic stimulation results in an accumulation of genetic mutations that then cause cells to undergo clonal expansion and, ultimately, malignant transformation. Genetic, environmental, and immunologic factors that contribute to the disease pathogenesis have been identified.2
Once clinical features point toward MF, the diagnosis can be further differentiated from other benign inflammatory mimics with a biopsy demonstrating cerebriform lymphocytes homing toward the epidermis, monoclonal expansion of T cells, and defective apoptosis.3
Continue to: Differential includes rosacea and seborrheic dermatitis
Differential includes rosacea and seborrheic dermatitis
The diagnosis of MF can be difficult as it often imitates other benign inflammatory conditions.
Rosacea manifests as an erythematous facial rash but usually spares the nasolabial folds and eyelids. There are several forms, including ocular (featuring swollen and irritated conjunctiva), erythematotelangiectatic (with visible blood vessels), and papulopustular (with acneic lesions). Over time, the skin may develop a thickened, bumpy texture, referred to as phymatous rosacea.4 A history of acute worsening with exposure to certain hot or spicy foods, alcohol, or ultraviolet light suggests a diagnosis of rosacea.
Seborrheic dermatitis classically presents as yellow scaling on a mildly erythematous base and often involves nasolabial folds and eyebrows. Seborrheic dermatitis can be associated with human immunodeficiency virus, Parkinson’s disease, and other chronic medical conditions.
Allergic contact dermatitis can look identical to MF, but in our case, there was no new allergen in the history. A thorough history regarding new medications, creams, and household supplies is integral to differentiating this diagnosis.
Misdiagnosis can lead to advanced-stage disease
This case of persistent facial erythema, originally treated as rosacea, highlights the importance of having a low threshold of suspicion of MF, especially in a patient with a prior history of MF. A recent study by Kelati et al3 indicated that certain subtypes of MF are easily misdiagnosed and treated as psoriasis or eczema respectively for an average of 10.5 years.3 These years of misdiagnosis are significantly correlated with the development of advanced-stage MF, which is more difficult to treat.3
Continue to: Treatment with topical desonide and mechlorethamine
Treatment with topical desonide and mechlorethamine
There are multiple treatment options for MF, depending on the stage, starting with topical therapies and advancing to systemic therapies in more advanced stages. Topical treatments include steroids, nitrogen mustard, and retinoids.5 Our patient was referred to a multidisciplinary lymphoma clinic, where topical treatment was initiated with desonide cream .05% and mechlorethamine gel .016%. Our patient experienced a 50% improvement in skin involvement at 3 months.
As MF progresses to more advanced stages, treatment often combines skin-directed therapies with systemic immunomodulators, biologics, radiation, and total skin electron beam therapy.6 TSEBT is a low-dose full-body radiation treatment that targets the skin surface and therefore effectively treats cutaneous lymphoma. Although TSEBT is usually well tolerated, there have been documented acute and chronic adverse effects, including dermatitis, alopecia, peripheral edema, cutaneous malignancies, and infertility in men.7
While the use of topical desonide and mechlorethamine was initially favored over radiation due to eyelid involvement, our patient developed new patches on her legs 11 months after her initial visit. When biopsies indicated MF with large cell transformation, she received 1 course of low-dose TSEBT (12 Gy), with complete response noted at the 2 month follow-up.
CORRESPONDENCE
Lucia Seminario-Vidal, MD, PhD, Department of Dermatology and Cutaneous Surgery, 13330 USF Laurel Drive, Tampa, FL 33612; [email protected]
A 59-year-old woman presented to our clinic with a large asymptomatic facial rash that had developed several months earlier. The rash had been slowly growing but did not change day to day. Her past medical history was significant for hypertension, hyperlipidemia, and cutaneous lymphoma, which was localized to her arms. She denied the use of any new products, including hair or facial products, nail polish, or any new medications.
Initially, she was presumed (by an outside provider) to have rosacea, and she received treatment with doxycycline 100 mg/d for 2 months. However, the rash did not improve.
Physical examination revealed a large erythematous rash involving her cheeks, nose, and periocular area with no other significant findings (FIGURE).

A biopsy of her right cheek was performed.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Mycosis fungoides
Following the biopsy of her right cheek, a histopathologic analysis demonstrated an atypical lymphocytic infiltrate positive for CD3 and CD4. These histopathologic features led to a diagnosis of recurrent mycosis fungoides (MF), a type of cutaneous lymphoma. (Our patient’s cutaneous lymphoma had been in remission for a year following local radiotherapy.)
MF is the most common type of cutaneous lymphoma, with an incidence of 6.4 to 9.6 cases per million people in the United States.1 There are also 2 rare subtypes of MF: the psoriasiform and palmoplantar forms. Psoriasiform MF presents with psoriasis-like plaques, while palmoplantar MF initially presents on the palms and soles.
Patients with classic MF typically present with patches and plaques—with the late evolution of tumors—on non–sun-exposed areas.1 Our patient’s clinical presentation was atypical because the rash manifested on a sun-exposed area of her body.
MF and other cutaneous lymphomas should always be part of the differential diagnosis for an unexplained persistent rash, especially in a patient with a history of MF. The development of lymphomas is thought to be a stepwise process through which chronic antigenic stimulation results in an accumulation of genetic mutations that then cause cells to undergo clonal expansion and, ultimately, malignant transformation. Genetic, environmental, and immunologic factors that contribute to the disease pathogenesis have been identified.2
Once clinical features point toward MF, the diagnosis can be further differentiated from other benign inflammatory mimics with a biopsy demonstrating cerebriform lymphocytes homing toward the epidermis, monoclonal expansion of T cells, and defective apoptosis.3
Continue to: Differential includes rosacea and seborrheic dermatitis
Differential includes rosacea and seborrheic dermatitis
The diagnosis of MF can be difficult as it often imitates other benign inflammatory conditions.
Rosacea manifests as an erythematous facial rash but usually spares the nasolabial folds and eyelids. There are several forms, including ocular (featuring swollen and irritated conjunctiva), erythematotelangiectatic (with visible blood vessels), and papulopustular (with acneic lesions). Over time, the skin may develop a thickened, bumpy texture, referred to as phymatous rosacea.4 A history of acute worsening with exposure to certain hot or spicy foods, alcohol, or ultraviolet light suggests a diagnosis of rosacea.
Seborrheic dermatitis classically presents as yellow scaling on a mildly erythematous base and often involves nasolabial folds and eyebrows. Seborrheic dermatitis can be associated with human immunodeficiency virus, Parkinson’s disease, and other chronic medical conditions.
Allergic contact dermatitis can look identical to MF, but in our case, there was no new allergen in the history. A thorough history regarding new medications, creams, and household supplies is integral to differentiating this diagnosis.
Misdiagnosis can lead to advanced-stage disease
This case of persistent facial erythema, originally treated as rosacea, highlights the importance of having a low threshold of suspicion of MF, especially in a patient with a prior history of MF. A recent study by Kelati et al3 indicated that certain subtypes of MF are easily misdiagnosed and treated as psoriasis or eczema respectively for an average of 10.5 years.3 These years of misdiagnosis are significantly correlated with the development of advanced-stage MF, which is more difficult to treat.3
Continue to: Treatment with topical desonide and mechlorethamine
Treatment with topical desonide and mechlorethamine
There are multiple treatment options for MF, depending on the stage, starting with topical therapies and advancing to systemic therapies in more advanced stages. Topical treatments include steroids, nitrogen mustard, and retinoids.5 Our patient was referred to a multidisciplinary lymphoma clinic, where topical treatment was initiated with desonide cream .05% and mechlorethamine gel .016%. Our patient experienced a 50% improvement in skin involvement at 3 months.
As MF progresses to more advanced stages, treatment often combines skin-directed therapies with systemic immunomodulators, biologics, radiation, and total skin electron beam therapy.6 TSEBT is a low-dose full-body radiation treatment that targets the skin surface and therefore effectively treats cutaneous lymphoma. Although TSEBT is usually well tolerated, there have been documented acute and chronic adverse effects, including dermatitis, alopecia, peripheral edema, cutaneous malignancies, and infertility in men.7
While the use of topical desonide and mechlorethamine was initially favored over radiation due to eyelid involvement, our patient developed new patches on her legs 11 months after her initial visit. When biopsies indicated MF with large cell transformation, she received 1 course of low-dose TSEBT (12 Gy), with complete response noted at the 2 month follow-up.
CORRESPONDENCE
Lucia Seminario-Vidal, MD, PhD, Department of Dermatology and Cutaneous Surgery, 13330 USF Laurel Drive, Tampa, FL 33612; [email protected]
1. Jawed S, Myskowski P, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome). Part I. Diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-e16.
2. Wohl Y, Tur E. Environmental risk factors for mycosis fungoides. Curr Probl Dermatol. 2007;35:52-64.
3. Kelati A, Gallouj S, Tahiri L, et al. Defining the mimics and clinico-histological diagnosis criteria for mycosis fungoides to minimize misdiagnosis. Int J Womens Dermatol. 2017;3:100-106.
4. Two AM, Wu W, Gallo RL, et al. Rosacea. part I. Introduction, categorization, histology, pathogenesis, and risk factors. J AM Acad Dermatol. 2015;72:749-758.
5. Lessin SR, Duvic M, Guitart J, et al. Topical chemotherapy in cutaneous T-cell lymphoma positive results of a randomized, controlled, multicenter trial testing the efficacy and safety of a novel mechlorethamine, 0.02%, gel in mycosis fungoides. JAMA Dermatol. 2013;149:25-32.
6. Jawed S, Myskowski P, Horwitz S, et al. Continuing medical education: Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part II. Prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e1-e17.
7. De Moraes FY, Carvalho Hde A, Hanna SA, et al. Literature review of clinical results of total skin electron irradiation (TSEBT) of mycosis fungoides in adults. Rep Pract Oncol Radiother. 2014;19:92-98.
1. Jawed S, Myskowski P, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome). Part I. Diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-e16.
2. Wohl Y, Tur E. Environmental risk factors for mycosis fungoides. Curr Probl Dermatol. 2007;35:52-64.
3. Kelati A, Gallouj S, Tahiri L, et al. Defining the mimics and clinico-histological diagnosis criteria for mycosis fungoides to minimize misdiagnosis. Int J Womens Dermatol. 2017;3:100-106.
4. Two AM, Wu W, Gallo RL, et al. Rosacea. part I. Introduction, categorization, histology, pathogenesis, and risk factors. J AM Acad Dermatol. 2015;72:749-758.
5. Lessin SR, Duvic M, Guitart J, et al. Topical chemotherapy in cutaneous T-cell lymphoma positive results of a randomized, controlled, multicenter trial testing the efficacy and safety of a novel mechlorethamine, 0.02%, gel in mycosis fungoides. JAMA Dermatol. 2013;149:25-32.
6. Jawed S, Myskowski P, Horwitz S, et al. Continuing medical education: Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part II. Prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e1-e17.
7. De Moraes FY, Carvalho Hde A, Hanna SA, et al. Literature review of clinical results of total skin electron irradiation (TSEBT) of mycosis fungoides in adults. Rep Pract Oncol Radiother. 2014;19:92-98.
FDA approves infliximab-axxq for numerous indications
The Food and Drug Administration has approved the biosimilar infliximab-axxq (Avsola) for various indications, making it the fourth biosimilar of infliximab (Remicade) to be cleared for marketing by the agency.
The tumor necrosis factor inhibitor is indicated for patients with Crohn’s disease or ulcerative colitis who are aged 6 years and older, RA in combination with methotrexate, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis. The approval is based on numerous trials. The most common adverse reactions are infections, infusion-related reactions, headache, and abdominal pain.
Full prescribing information can be found on the FDA website, as can more information about biosimilars.
The Food and Drug Administration has approved the biosimilar infliximab-axxq (Avsola) for various indications, making it the fourth biosimilar of infliximab (Remicade) to be cleared for marketing by the agency.
The tumor necrosis factor inhibitor is indicated for patients with Crohn’s disease or ulcerative colitis who are aged 6 years and older, RA in combination with methotrexate, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis. The approval is based on numerous trials. The most common adverse reactions are infections, infusion-related reactions, headache, and abdominal pain.
Full prescribing information can be found on the FDA website, as can more information about biosimilars.
The Food and Drug Administration has approved the biosimilar infliximab-axxq (Avsola) for various indications, making it the fourth biosimilar of infliximab (Remicade) to be cleared for marketing by the agency.
The tumor necrosis factor inhibitor is indicated for patients with Crohn’s disease or ulcerative colitis who are aged 6 years and older, RA in combination with methotrexate, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis. The approval is based on numerous trials. The most common adverse reactions are infections, infusion-related reactions, headache, and abdominal pain.
Full prescribing information can be found on the FDA website, as can more information about biosimilars.
Recurrent Angiotensin-Converting Enzyme Inhibitor-Induced Angioedema Refractory to Fresh Frozen Plasma
Angioedema induced by angiotensin-converting enzyme inhibitors (ACEIs) is present in from 0.1% to 0.7% of treated patients and more often involves the head, neck, face, lips, tongue, and larynx.1 ACEI-induced angioedema results from inhibition of angiotensin-converting enzyme (ACE), which results in reduced degradation and resultant accumulation of bradykinin, a potent inflammatory mediator.2
The treatment of choice is discontinuing all ACEIs; however, the patient may be at increased risk of a subsequent angioedema attack for many weeks.3 Antihistamines (H1 and H2 receptor blockade), epinephrine, and glucocorticoids are effective in allergic/histaminergic angioedema but are usually ineffective for hereditary angioedema or ACEI angioedema and are not recommended for acute therapy.4 Kallikrein-bradykinin pathway targeted therapies are now approved by the Food and Drug Administration (FDA) for hereditary angioedema attacks and have been studied for ACEI-induced angioedema. Ecallantide and icatibant inhibit conversion of precursors to bradykinin. Multiple randomized trials of ecallantide have not shown any advantage over traditional therapies.5 On the other hand, icatibant has shown resolution of angioedema in several case reports and in a randomized trial.6 Icatibant for ACEI-induced angioedema continues to be off-label because the data are conflicting.
Case Presentation
A 67-year-old man presented with a medical history of arterial hypertension (diagnosed 17 years previously), hypercholesterolemia, type 2 diabetes mellitus, alcohol dependence, and obesity. His outpatient medications included simvastatin, aripiprazole, losartan/hydrochlorothiazide, and amlodipine. He was voluntarily admitted for inpatient detoxification. After evaluation by the internist, medication reconciliation was done, and the therapy was adjusted according to medication availability. He reported having no drug allergies, and the losartan was changed for lisinopril. About 24 hours after the first dose of lisinopril, the patient developed swelling of the lips. Antihistamine and IV steroids were administered, and the ACEI was discontinued. His baseline vital signs were temperature 98° F, heart rate 83 beats per minute, respiratory rate 19 breaths per minute, blood pressure 150/94, and oxygen saturation 98% by pulse oximeter.
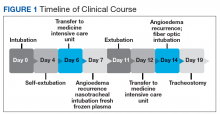
During the night shift the patient’s symptoms worsened, developing difficulty swallowing and shortness of breath. He was transferred to the medicine intensive care unit (MICU), intubated, and placed on mechanical ventilation to protect his airway. Laryngoscopic examination was notable for edematous tongue, uvula, and larynx. Also, the patient had mild stridor. His laboratory test results showed normal levels of complement, tryptase, and C1 esterase. On the fourth day after admission to MICU (Figure 1), the patient extubated himself. At that time, he did not present stridor or respiratory distress and remained at the MICU for 24 hours for close monitoring.
Thirty-six hours after self-extubation the patient developed stridor and shortness of breath at the general medicine ward. In view of his clinical presentation of recurrent ACEI-induced angioedema, the Anesthesiology Service was consulted. Direct visualization of the airways showed edema of the epiglottis and vocal cords, requiring nasotracheal intubation. Two units of fresh frozen plasma (FFP) were administered. Complete resolution of angioedema took at least 72 hours even after the administration of FFP. As part of the ventilator-associated pneumonia prevention bundle, the patient continued with daily spontaneous breathing trials. On the fourth day, he was he was extubated after a cuff-leak test was positive and his rapid shallow breathing index was adequate.
The cuff-leak test is usually done to predict postextubation stridor. It consists of deflating the endotracheal tube cuff to verify if gas can pass around the tube. Absence of cuff leak is suggestive of airway edema, a risk factor for postextubation stridor and failure of extubation. For example, if the patient has an endotracheal tube that is too large in relation to the patient’s airway, the leak test can result in a false negative. In this case, fiber optic visualization of the airway can confirm the endotracheal tube occluding all the airway even with the cuff deflated and without evidence of swelling of the vocal cords. The rapid shallow breathing index is a ratio of respiratory rate over tidal volume in liters and is used to predict successful extubation. Values < 105 have a high sensitivity for successful extubation.
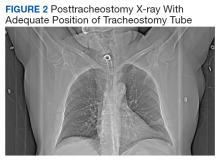
The patient remained under observation for 24 hours in the MICU and then was transferred to the general medicine ward. Unfortunately, 36 hours after, the patient had a new episode of angioedema requiring endotracheal intubation and placement on mechanical ventilation. This was his third episode of angioedema; he had a difficult airway classified as a Cormack-Lehane grade 3, requiring intubation with fiber-optic laryngoscope. In view of the recurrent events, a tracheostomy was done several days later. Figure 2 shows posttracheostomy X-ray with adequate position of the tracheostomy tube.
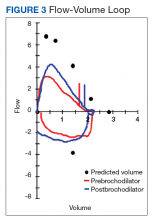
The patient was transferred to the Respiratory Care Unit and weaned off mechanical ventilation. He completed an intensive physical rehabilitation program and was discharged home. On discharge, he was followed by the Otorhinolaryngology Service and was decannulated about 5 months after. After tracheostomy decannulation, he developed asymptomatic stridor. A neck computer tomography scan revealed soft tissue thickening at the anterior and lateral aspects of the proximal tracheal likely representing granulation tissue/scarring. The findings were consistent with proximal tracheal stenosis sequelae of tracheostomy and intubation. In Figure 3, the upper portion of the curve represents the expiratory limb of the forced vital capacity and the lower portion represents inspiration. The flow-volume loop graph showed flattening of the inspiratory limb. There was a plateau in the inspiratory limb, suggestive of limitation of inspiratory flow as seen in variable extrathoracic lesions, such as glotticstricture, tumors, and vocal cord paralysis.7 The findings on the flow-volume loop were consistent with the subglottic stenosis identified by laryngoscopic examination. The patient was reluctant to undergo further interventions.
Discussion
The standard therapy for ACEI-inducedangioedema continues to be airway management and discontinuation of medication. However, life-threatening progression of symptoms have led to the use of off-label therapies, including FFP and bradykinin receptor antagonists, such as icatibant, which has been approved by the FDA for the treatment of hereditary angioedema. Icatibant is expensive and most hospitals do not have access to it. When considering the bradykinin pathway for therapy, FFP is commonly used. The cases described in the literature that have reported success with the use of FFP have used up to 2 units. There is no reported benefit of its use beyond 2 units. The initial randomized trials of icatibant for ACEI angioedema showed decreased time of resolution of angioedema.6 However, repeated trials showed conflicting results. At Veterans Affairs Caribbean Healthcare System, this medication was not available, and we decided to use FFP to improve the patient’s symptoms.
The administration of 2 units of FFP has been documented on case reports as a method to decrease the time of resolution of angioedema and the risk of recurrence. The mechanism of action thought to be involved includes the degradation of bradykinin by the enzyme ACE into inactive peptides and by supplying C1 inhibitor.8 No randomized clinical trial has investigated the use of FFP for the treatment of ACEI-induced angioedema. However, a retrospective cohort study report compared patients who presented with acute (nonhereditary) angioedema and airway compromise and received FFP with patients who were not treated with FFP.9 The study suggested a shorter ICU stay in the group treated with FFP, but the findings did not present statistical outcomes.
Nevertheless, our patient had recurrent ACEI-induced angioedema refractory to FFP. In addition to ACE or kininase II, FFP contains high-molecular weight-kininogen and kallikrein, the substrates that form bradykinin, which explained the mechanism of worsening angioedema.10 No randomized trials have investigated the use of FFP for the treatment of bradykinin-induced angioedema nor the appropriate dose.
Conclusion
In view of the emerging case reports of the effectiveness of FFP, this case of refractory angioedema raises concern for its true effectiveness and other possible factors involved in the mechanism of recurrence. Probably it would be unwise to conduct randomized studies in clinical situations such as the ones outlined. A collection of case series where FFP administration was done may be a more reasonable source of conclusions to be analyzed by a panel of experts.
1. Sánchez-Borges M, González-Aveledo LA. Angiotensin-converting enzyme inhibitors and angioedema. Allergy Asthma Immunol Res. 2010;2(3):195-198.
2. Kaplan AP. Angioedema. World Allergy Organ J. 2008;1(6):103-113.
3. Moellman JJ, Bernstein JA, Lindsell C, et al; American College of Allergy, Asthma & Immunology (ACAAI); Society for Academic Emergency Medicine (SAEM). A consensus parameter for the evaluation and management of angioedema in the emergency department. Acad Emerg Med. 2014;21(4):469-484.
4. LoVerde D, Files DC, Krishnaswamy G. Angioedema. Crit Care Med. 2017;45(4):725-735.
5. van den Elzen M, Go MFLC, Knulst AC, Blankestijn MA, van Os-Medendorp H, Otten HG. Efficacy of treatment of non-hereditary angioedema. Clinic Rev Allerg Immunol. 2018;54(3):412-431.
6. Bas M, Greve J, Stelter S, et al. A randomized trial of icatibant in ace-inhibitor–induced angioedema. N Engl J Med. 2015;372(5):418-425.
7. Diaz J, Casal J, Rodriguez W. Flow-volume loops: clinical correlation. PR Health Sci J. 2008;27(2):181-182.
8. Stewart M, McGlone R. Fresh frozen plasma in the treatment of ACE inhibitor-induced angioedema. BMJ Case Rep. 2012;2012:pii:bcr2012006849.
9. Saeb A, Hagglund KH, Cigolle CT. Using fresh frozen plasma for acute airway angioedema to prevent intubation in the emergency department: a retrospective cohort study. Emerg Med Int. 2016;2016:6091510.
10. Brown T, Gonzalez J, Monteleone C. Angiotensin-converting enzyme inhibitor-induced angioedema: a review of the literature. J Clin Hypertens (Greenwich). 2017;19(12):1377-1382.
Angioedema induced by angiotensin-converting enzyme inhibitors (ACEIs) is present in from 0.1% to 0.7% of treated patients and more often involves the head, neck, face, lips, tongue, and larynx.1 ACEI-induced angioedema results from inhibition of angiotensin-converting enzyme (ACE), which results in reduced degradation and resultant accumulation of bradykinin, a potent inflammatory mediator.2
The treatment of choice is discontinuing all ACEIs; however, the patient may be at increased risk of a subsequent angioedema attack for many weeks.3 Antihistamines (H1 and H2 receptor blockade), epinephrine, and glucocorticoids are effective in allergic/histaminergic angioedema but are usually ineffective for hereditary angioedema or ACEI angioedema and are not recommended for acute therapy.4 Kallikrein-bradykinin pathway targeted therapies are now approved by the Food and Drug Administration (FDA) for hereditary angioedema attacks and have been studied for ACEI-induced angioedema. Ecallantide and icatibant inhibit conversion of precursors to bradykinin. Multiple randomized trials of ecallantide have not shown any advantage over traditional therapies.5 On the other hand, icatibant has shown resolution of angioedema in several case reports and in a randomized trial.6 Icatibant for ACEI-induced angioedema continues to be off-label because the data are conflicting.
Case Presentation
A 67-year-old man presented with a medical history of arterial hypertension (diagnosed 17 years previously), hypercholesterolemia, type 2 diabetes mellitus, alcohol dependence, and obesity. His outpatient medications included simvastatin, aripiprazole, losartan/hydrochlorothiazide, and amlodipine. He was voluntarily admitted for inpatient detoxification. After evaluation by the internist, medication reconciliation was done, and the therapy was adjusted according to medication availability. He reported having no drug allergies, and the losartan was changed for lisinopril. About 24 hours after the first dose of lisinopril, the patient developed swelling of the lips. Antihistamine and IV steroids were administered, and the ACEI was discontinued. His baseline vital signs were temperature 98° F, heart rate 83 beats per minute, respiratory rate 19 breaths per minute, blood pressure 150/94, and oxygen saturation 98% by pulse oximeter.
During the night shift the patient’s symptoms worsened, developing difficulty swallowing and shortness of breath. He was transferred to the medicine intensive care unit (MICU), intubated, and placed on mechanical ventilation to protect his airway. Laryngoscopic examination was notable for edematous tongue, uvula, and larynx. Also, the patient had mild stridor. His laboratory test results showed normal levels of complement, tryptase, and C1 esterase. On the fourth day after admission to MICU (Figure 1), the patient extubated himself. At that time, he did not present stridor or respiratory distress and remained at the MICU for 24 hours for close monitoring.
Thirty-six hours after self-extubation the patient developed stridor and shortness of breath at the general medicine ward. In view of his clinical presentation of recurrent ACEI-induced angioedema, the Anesthesiology Service was consulted. Direct visualization of the airways showed edema of the epiglottis and vocal cords, requiring nasotracheal intubation. Two units of fresh frozen plasma (FFP) were administered. Complete resolution of angioedema took at least 72 hours even after the administration of FFP. As part of the ventilator-associated pneumonia prevention bundle, the patient continued with daily spontaneous breathing trials. On the fourth day, he was he was extubated after a cuff-leak test was positive and his rapid shallow breathing index was adequate.
The cuff-leak test is usually done to predict postextubation stridor. It consists of deflating the endotracheal tube cuff to verify if gas can pass around the tube. Absence of cuff leak is suggestive of airway edema, a risk factor for postextubation stridor and failure of extubation. For example, if the patient has an endotracheal tube that is too large in relation to the patient’s airway, the leak test can result in a false negative. In this case, fiber optic visualization of the airway can confirm the endotracheal tube occluding all the airway even with the cuff deflated and without evidence of swelling of the vocal cords. The rapid shallow breathing index is a ratio of respiratory rate over tidal volume in liters and is used to predict successful extubation. Values < 105 have a high sensitivity for successful extubation.
The patient remained under observation for 24 hours in the MICU and then was transferred to the general medicine ward. Unfortunately, 36 hours after, the patient had a new episode of angioedema requiring endotracheal intubation and placement on mechanical ventilation. This was his third episode of angioedema; he had a difficult airway classified as a Cormack-Lehane grade 3, requiring intubation with fiber-optic laryngoscope. In view of the recurrent events, a tracheostomy was done several days later. Figure 2 shows posttracheostomy X-ray with adequate position of the tracheostomy tube.
The patient was transferred to the Respiratory Care Unit and weaned off mechanical ventilation. He completed an intensive physical rehabilitation program and was discharged home. On discharge, he was followed by the Otorhinolaryngology Service and was decannulated about 5 months after. After tracheostomy decannulation, he developed asymptomatic stridor. A neck computer tomography scan revealed soft tissue thickening at the anterior and lateral aspects of the proximal tracheal likely representing granulation tissue/scarring. The findings were consistent with proximal tracheal stenosis sequelae of tracheostomy and intubation. In Figure 3, the upper portion of the curve represents the expiratory limb of the forced vital capacity and the lower portion represents inspiration. The flow-volume loop graph showed flattening of the inspiratory limb. There was a plateau in the inspiratory limb, suggestive of limitation of inspiratory flow as seen in variable extrathoracic lesions, such as glotticstricture, tumors, and vocal cord paralysis.7 The findings on the flow-volume loop were consistent with the subglottic stenosis identified by laryngoscopic examination. The patient was reluctant to undergo further interventions.
Discussion
The standard therapy for ACEI-inducedangioedema continues to be airway management and discontinuation of medication. However, life-threatening progression of symptoms have led to the use of off-label therapies, including FFP and bradykinin receptor antagonists, such as icatibant, which has been approved by the FDA for the treatment of hereditary angioedema. Icatibant is expensive and most hospitals do not have access to it. When considering the bradykinin pathway for therapy, FFP is commonly used. The cases described in the literature that have reported success with the use of FFP have used up to 2 units. There is no reported benefit of its use beyond 2 units. The initial randomized trials of icatibant for ACEI angioedema showed decreased time of resolution of angioedema.6 However, repeated trials showed conflicting results. At Veterans Affairs Caribbean Healthcare System, this medication was not available, and we decided to use FFP to improve the patient’s symptoms.
The administration of 2 units of FFP has been documented on case reports as a method to decrease the time of resolution of angioedema and the risk of recurrence. The mechanism of action thought to be involved includes the degradation of bradykinin by the enzyme ACE into inactive peptides and by supplying C1 inhibitor.8 No randomized clinical trial has investigated the use of FFP for the treatment of ACEI-induced angioedema. However, a retrospective cohort study report compared patients who presented with acute (nonhereditary) angioedema and airway compromise and received FFP with patients who were not treated with FFP.9 The study suggested a shorter ICU stay in the group treated with FFP, but the findings did not present statistical outcomes.
Nevertheless, our patient had recurrent ACEI-induced angioedema refractory to FFP. In addition to ACE or kininase II, FFP contains high-molecular weight-kininogen and kallikrein, the substrates that form bradykinin, which explained the mechanism of worsening angioedema.10 No randomized trials have investigated the use of FFP for the treatment of bradykinin-induced angioedema nor the appropriate dose.
Conclusion
In view of the emerging case reports of the effectiveness of FFP, this case of refractory angioedema raises concern for its true effectiveness and other possible factors involved in the mechanism of recurrence. Probably it would be unwise to conduct randomized studies in clinical situations such as the ones outlined. A collection of case series where FFP administration was done may be a more reasonable source of conclusions to be analyzed by a panel of experts.
Angioedema induced by angiotensin-converting enzyme inhibitors (ACEIs) is present in from 0.1% to 0.7% of treated patients and more often involves the head, neck, face, lips, tongue, and larynx.1 ACEI-induced angioedema results from inhibition of angiotensin-converting enzyme (ACE), which results in reduced degradation and resultant accumulation of bradykinin, a potent inflammatory mediator.2
The treatment of choice is discontinuing all ACEIs; however, the patient may be at increased risk of a subsequent angioedema attack for many weeks.3 Antihistamines (H1 and H2 receptor blockade), epinephrine, and glucocorticoids are effective in allergic/histaminergic angioedema but are usually ineffective for hereditary angioedema or ACEI angioedema and are not recommended for acute therapy.4 Kallikrein-bradykinin pathway targeted therapies are now approved by the Food and Drug Administration (FDA) for hereditary angioedema attacks and have been studied for ACEI-induced angioedema. Ecallantide and icatibant inhibit conversion of precursors to bradykinin. Multiple randomized trials of ecallantide have not shown any advantage over traditional therapies.5 On the other hand, icatibant has shown resolution of angioedema in several case reports and in a randomized trial.6 Icatibant for ACEI-induced angioedema continues to be off-label because the data are conflicting.
Case Presentation
A 67-year-old man presented with a medical history of arterial hypertension (diagnosed 17 years previously), hypercholesterolemia, type 2 diabetes mellitus, alcohol dependence, and obesity. His outpatient medications included simvastatin, aripiprazole, losartan/hydrochlorothiazide, and amlodipine. He was voluntarily admitted for inpatient detoxification. After evaluation by the internist, medication reconciliation was done, and the therapy was adjusted according to medication availability. He reported having no drug allergies, and the losartan was changed for lisinopril. About 24 hours after the first dose of lisinopril, the patient developed swelling of the lips. Antihistamine and IV steroids were administered, and the ACEI was discontinued. His baseline vital signs were temperature 98° F, heart rate 83 beats per minute, respiratory rate 19 breaths per minute, blood pressure 150/94, and oxygen saturation 98% by pulse oximeter.
During the night shift the patient’s symptoms worsened, developing difficulty swallowing and shortness of breath. He was transferred to the medicine intensive care unit (MICU), intubated, and placed on mechanical ventilation to protect his airway. Laryngoscopic examination was notable for edematous tongue, uvula, and larynx. Also, the patient had mild stridor. His laboratory test results showed normal levels of complement, tryptase, and C1 esterase. On the fourth day after admission to MICU (Figure 1), the patient extubated himself. At that time, he did not present stridor or respiratory distress and remained at the MICU for 24 hours for close monitoring.
Thirty-six hours after self-extubation the patient developed stridor and shortness of breath at the general medicine ward. In view of his clinical presentation of recurrent ACEI-induced angioedema, the Anesthesiology Service was consulted. Direct visualization of the airways showed edema of the epiglottis and vocal cords, requiring nasotracheal intubation. Two units of fresh frozen plasma (FFP) were administered. Complete resolution of angioedema took at least 72 hours even after the administration of FFP. As part of the ventilator-associated pneumonia prevention bundle, the patient continued with daily spontaneous breathing trials. On the fourth day, he was he was extubated after a cuff-leak test was positive and his rapid shallow breathing index was adequate.
The cuff-leak test is usually done to predict postextubation stridor. It consists of deflating the endotracheal tube cuff to verify if gas can pass around the tube. Absence of cuff leak is suggestive of airway edema, a risk factor for postextubation stridor and failure of extubation. For example, if the patient has an endotracheal tube that is too large in relation to the patient’s airway, the leak test can result in a false negative. In this case, fiber optic visualization of the airway can confirm the endotracheal tube occluding all the airway even with the cuff deflated and without evidence of swelling of the vocal cords. The rapid shallow breathing index is a ratio of respiratory rate over tidal volume in liters and is used to predict successful extubation. Values < 105 have a high sensitivity for successful extubation.
The patient remained under observation for 24 hours in the MICU and then was transferred to the general medicine ward. Unfortunately, 36 hours after, the patient had a new episode of angioedema requiring endotracheal intubation and placement on mechanical ventilation. This was his third episode of angioedema; he had a difficult airway classified as a Cormack-Lehane grade 3, requiring intubation with fiber-optic laryngoscope. In view of the recurrent events, a tracheostomy was done several days later. Figure 2 shows posttracheostomy X-ray with adequate position of the tracheostomy tube.
The patient was transferred to the Respiratory Care Unit and weaned off mechanical ventilation. He completed an intensive physical rehabilitation program and was discharged home. On discharge, he was followed by the Otorhinolaryngology Service and was decannulated about 5 months after. After tracheostomy decannulation, he developed asymptomatic stridor. A neck computer tomography scan revealed soft tissue thickening at the anterior and lateral aspects of the proximal tracheal likely representing granulation tissue/scarring. The findings were consistent with proximal tracheal stenosis sequelae of tracheostomy and intubation. In Figure 3, the upper portion of the curve represents the expiratory limb of the forced vital capacity and the lower portion represents inspiration. The flow-volume loop graph showed flattening of the inspiratory limb. There was a plateau in the inspiratory limb, suggestive of limitation of inspiratory flow as seen in variable extrathoracic lesions, such as glotticstricture, tumors, and vocal cord paralysis.7 The findings on the flow-volume loop were consistent with the subglottic stenosis identified by laryngoscopic examination. The patient was reluctant to undergo further interventions.
Discussion
The standard therapy for ACEI-inducedangioedema continues to be airway management and discontinuation of medication. However, life-threatening progression of symptoms have led to the use of off-label therapies, including FFP and bradykinin receptor antagonists, such as icatibant, which has been approved by the FDA for the treatment of hereditary angioedema. Icatibant is expensive and most hospitals do not have access to it. When considering the bradykinin pathway for therapy, FFP is commonly used. The cases described in the literature that have reported success with the use of FFP have used up to 2 units. There is no reported benefit of its use beyond 2 units. The initial randomized trials of icatibant for ACEI angioedema showed decreased time of resolution of angioedema.6 However, repeated trials showed conflicting results. At Veterans Affairs Caribbean Healthcare System, this medication was not available, and we decided to use FFP to improve the patient’s symptoms.
The administration of 2 units of FFP has been documented on case reports as a method to decrease the time of resolution of angioedema and the risk of recurrence. The mechanism of action thought to be involved includes the degradation of bradykinin by the enzyme ACE into inactive peptides and by supplying C1 inhibitor.8 No randomized clinical trial has investigated the use of FFP for the treatment of ACEI-induced angioedema. However, a retrospective cohort study report compared patients who presented with acute (nonhereditary) angioedema and airway compromise and received FFP with patients who were not treated with FFP.9 The study suggested a shorter ICU stay in the group treated with FFP, but the findings did not present statistical outcomes.
Nevertheless, our patient had recurrent ACEI-induced angioedema refractory to FFP. In addition to ACE or kininase II, FFP contains high-molecular weight-kininogen and kallikrein, the substrates that form bradykinin, which explained the mechanism of worsening angioedema.10 No randomized trials have investigated the use of FFP for the treatment of bradykinin-induced angioedema nor the appropriate dose.
Conclusion
In view of the emerging case reports of the effectiveness of FFP, this case of refractory angioedema raises concern for its true effectiveness and other possible factors involved in the mechanism of recurrence. Probably it would be unwise to conduct randomized studies in clinical situations such as the ones outlined. A collection of case series where FFP administration was done may be a more reasonable source of conclusions to be analyzed by a panel of experts.
1. Sánchez-Borges M, González-Aveledo LA. Angiotensin-converting enzyme inhibitors and angioedema. Allergy Asthma Immunol Res. 2010;2(3):195-198.
2. Kaplan AP. Angioedema. World Allergy Organ J. 2008;1(6):103-113.
3. Moellman JJ, Bernstein JA, Lindsell C, et al; American College of Allergy, Asthma & Immunology (ACAAI); Society for Academic Emergency Medicine (SAEM). A consensus parameter for the evaluation and management of angioedema in the emergency department. Acad Emerg Med. 2014;21(4):469-484.
4. LoVerde D, Files DC, Krishnaswamy G. Angioedema. Crit Care Med. 2017;45(4):725-735.
5. van den Elzen M, Go MFLC, Knulst AC, Blankestijn MA, van Os-Medendorp H, Otten HG. Efficacy of treatment of non-hereditary angioedema. Clinic Rev Allerg Immunol. 2018;54(3):412-431.
6. Bas M, Greve J, Stelter S, et al. A randomized trial of icatibant in ace-inhibitor–induced angioedema. N Engl J Med. 2015;372(5):418-425.
7. Diaz J, Casal J, Rodriguez W. Flow-volume loops: clinical correlation. PR Health Sci J. 2008;27(2):181-182.
8. Stewart M, McGlone R. Fresh frozen plasma in the treatment of ACE inhibitor-induced angioedema. BMJ Case Rep. 2012;2012:pii:bcr2012006849.
9. Saeb A, Hagglund KH, Cigolle CT. Using fresh frozen plasma for acute airway angioedema to prevent intubation in the emergency department: a retrospective cohort study. Emerg Med Int. 2016;2016:6091510.
10. Brown T, Gonzalez J, Monteleone C. Angiotensin-converting enzyme inhibitor-induced angioedema: a review of the literature. J Clin Hypertens (Greenwich). 2017;19(12):1377-1382.
1. Sánchez-Borges M, González-Aveledo LA. Angiotensin-converting enzyme inhibitors and angioedema. Allergy Asthma Immunol Res. 2010;2(3):195-198.
2. Kaplan AP. Angioedema. World Allergy Organ J. 2008;1(6):103-113.
3. Moellman JJ, Bernstein JA, Lindsell C, et al; American College of Allergy, Asthma & Immunology (ACAAI); Society for Academic Emergency Medicine (SAEM). A consensus parameter for the evaluation and management of angioedema in the emergency department. Acad Emerg Med. 2014;21(4):469-484.
4. LoVerde D, Files DC, Krishnaswamy G. Angioedema. Crit Care Med. 2017;45(4):725-735.
5. van den Elzen M, Go MFLC, Knulst AC, Blankestijn MA, van Os-Medendorp H, Otten HG. Efficacy of treatment of non-hereditary angioedema. Clinic Rev Allerg Immunol. 2018;54(3):412-431.
6. Bas M, Greve J, Stelter S, et al. A randomized trial of icatibant in ace-inhibitor–induced angioedema. N Engl J Med. 2015;372(5):418-425.
7. Diaz J, Casal J, Rodriguez W. Flow-volume loops: clinical correlation. PR Health Sci J. 2008;27(2):181-182.
8. Stewart M, McGlone R. Fresh frozen plasma in the treatment of ACE inhibitor-induced angioedema. BMJ Case Rep. 2012;2012:pii:bcr2012006849.
9. Saeb A, Hagglund KH, Cigolle CT. Using fresh frozen plasma for acute airway angioedema to prevent intubation in the emergency department: a retrospective cohort study. Emerg Med Int. 2016;2016:6091510.
10. Brown T, Gonzalez J, Monteleone C. Angiotensin-converting enzyme inhibitor-induced angioedema: a review of the literature. J Clin Hypertens (Greenwich). 2017;19(12):1377-1382.
New opioid recommendations: Pain from most dermatologic procedures should be managed with acetaminophen, ibuprofen
has recommended.
Rotation flaps, interpolation flaps, wedge resections, cartilage alar-batten grafts, and Mustarde flaps were among the 20 procedures that can be managed with up to 10 oral oxycodone 5-mg equivalents, according to the panel. Only the Abbe procedure might warrant dispensing up to 15 oxycodone 5-mg pills, Justin McLawhorn, MD, and colleagues wrote in the Journal of the American Academy of Dermatology. The recommended amount of opioids are in addition to nonopioid analgesics, the guidelines point out.
All the other procedures can – and should – be managed with a combination of acetaminophen and ibuprofen, either alone or in an alternating dose pattern, said Dr. McLawhorn, of the department of dermatology at the University of Oklahoma Health Sciences Center, Oklahoma City, and coauthors.
But limited opioid prescribing is an important part of healing for patients who undergo the most invasive procedures, they wrote. “The management of complications, including adequate pain control, should be tailored to each patient on a case-by-case basis. Moreover, any pain management plan should not strictly adhere to any single guideline, but rather should be formed with consideration of the expected pain from the procedure and/or closure and consider the patient’s expectations for pain control.”
The time is ripe for dermatologists to make a stand in combating the opioid crisis, according to a group email response to questions from Dr. McLawhorn, Thomas Stasko, MD, professor and chair of dermatology at the University of Oklahoma, Oklahoma City, and Lindsey Collins, MD, also of the University of Oklahoma.
“The opioid crisis has reached epidemic proportions. More than 70,000 Americans have died from an opioid overdose in 2017,” they wrote. “Moreover, recent data suggest that nearly 6% of postsurgical, opioid-naive patients become long-term users of opioids. The lack of specific evidence-based recommendations likely contributes to a wide variety in prescribing patterns and a steady supply of unused opioids. Countering the opioid crisis necessitates a restructuring of the opioid prescribing practices that addresses pain in a procedure-specific manner. These recommendations are one tool in the dermatologists’ arsenal that can be used as a reference to help guide opioid management and prevent excessive opioid prescriptions at discharge following dermatologic interventions.”
Unfortunately, they added, dermatologists have inadvertently fueled the opioid abuse fire.
“It is difficult to quantify which providers are responsible for the onslaught of opioids into our communities,” the authors wrote in the email interview. “However, we can deduce, based on recent opioid prescribing patterns, that dermatologists provide approximately 500,000 unused opioid pills to their communities on an annual basis. This is the result of a wide variation in practice patterns and narratives that have been previously circulated in an attempt to mitigate the providers’ perception of the addictive nature of opioid analgesics. Our hope is that by addressing pain in a procedure-specific manner, we can help to limit the excessive number of unused opioid pills that are provided by dermatologists and ultimately decrease the rate of opioid-related complications, including addiction and death.”
Still, patients need and deserve effective pain management after a procedure. In the guidelines, the investigators wrote that a “one-size-fits-all” approach “does not account for the mechanism of pain, the invasiveness of the procedure, or the anatomic structures that are manipulated. As a result, current guidelines cannot accurately predict the quantity of opioids that are necessary to manage postoperative pain.”
The panel brought together experts in general dermatology, dermatologic surgery, cosmetics, and phlebology to develop a consensus on opioid prescribing guidelines for 87 of the most common procedures. Everyone on the panel was a member of the American College of Mohs Surgery, American Academy of Dermatology, or the American Vein and Lymphatic Society. The panel conducted a literature review to determine which procedures might require opioids and which would not. At least 75% of the panel had to agree on a reasonable but effective opioid amount; they were then polled as to whether they might employ that recommendation in their own clinical practice.
The recommendations are aimed at patients who experienced no peri- or postoperative complications.
The panel agreed that acetaminophen and ibuprofen – alone, in combination, or with opioids – were reasonable choices for all the 87 procedures. In such instances, acetaminophen 1 g can be staggered with ibuprofen 400 mg every 4 or 8 hours.
“I think providers will encounter a mixed bag of preconceived notions regarding patients’ expectations for pain control,” Dr. McLawhorn and coauthors wrote in the interview. “The important point for providers to make is to emphasize the noninferiority of acetaminophen and/or ibuprofen in controlling acute pain for patients who are not dependent on opioids for the management of chronic pain. Our experience in caring for many surgical patients has shown that patients are usually receptive to the use of nonopioid analgesics as many are familiar with their addictive potential because of the uptick in the publicity of the opioid-related complications.”
In cases where opioids might be appropriate, the panel unanimously agreed that dose limits be imposed. For 15 of the 87 procedures, the panel recommend a maximum prescription of 10 oxycodone 5-mg equivalents. Only one other – the Abbe flap – might warrant more, with a maximum of 15 oxycodone 5-mg pills at discharge.
Sometimes called a “lip switch,” the Abbe flap is reconstruction for full-thickness lip defects. It is a composite flap that moves skin, muscle, mucosa, and blood supply from the lower lip to reconstruct a defect of the upper lip. This reconstruction attempts to respect the native anatomic landmarks of the lip and allow for a better functional outcome.
“Because of the extensive nature of the repair and the anatomic territories that are manipulated, including the suturing of the lower lip to the upper lip with delayed separation, adequate pain control may require opioid analgesics in the immediate postoperative period,” the team wrote in the interview.
The panel could not agree on pain management strategies for five other procedures: Karapandzic flaps, en bloc nail excisions, facial resurfacing with deep chemical peels, and small- or large-volume liposuction. This was partly because of a lack of personal experience. Only 8 of the 40 panelists performed Karapandzic flaps. The maximum number of 5-mg oxycodone tablets any panelist prescribed for Karapandzic flaps and en bloc nail excisions was 20.
Facial resurfacing was likewise an uncommon procedure for the panel, with just 11 members performing this using deep chemical peels. However, five of those panelists said that opioids were routinely needed for postoperative pain with a maximum of 15 oxycodone 5-mg equivalents. And just four panelists performed liposuction, for which they used a maximum of 15 oxycodone 5-mg equivalents.
“However,” they wrote in the guidelines, “these providers noted that the location where the procedure is performed strongly influences the need for opioid pain management, with small-volume removal in the neck, arms, or flanks being unlikely to require opioids for adequate pain control, whereas large-volume removal in the thighs, knees, and hips may routinely require opioids.”
Addressing patient expectations is a very important part of pain management, the panel noted. “Patients will invariably experience postoperative pain after cutaneous surgeries or other interventions, often peaking within 4 hours after surgery. Wound tension, size and type of repair, anatomical location/nerve innervation, and patient pain tolerance are all factors that contribute to postoperative discomfort and should be considered when developing a postoperative pain management plan.”
Ultimately, according to Dr. McLawhorn and coauthors, the decision to use opioids at discharge for postoperative pain control should be an individual one based on patients’ comorbidities and expectations.
“Admittedly, many of the procedures listed within the recommendations may result in a rather large or complex defect that requires an equally large or complex repair,” they wrote in the interview. “However, proper education of the patient and provider regarding the risks of addiction with the use of opioids even short term should be discussed as part of every preoperative consultation. Furthermore, the patient and the provider must discuss their expectations for postoperative pain interventions for adequate pain control.”
SOURCE: McLawhorn J et al. J Am Acad Dermatol. 2019 Nov 12. doi: 10.1016/j.jaad.2019.09.080.
has recommended.
Rotation flaps, interpolation flaps, wedge resections, cartilage alar-batten grafts, and Mustarde flaps were among the 20 procedures that can be managed with up to 10 oral oxycodone 5-mg equivalents, according to the panel. Only the Abbe procedure might warrant dispensing up to 15 oxycodone 5-mg pills, Justin McLawhorn, MD, and colleagues wrote in the Journal of the American Academy of Dermatology. The recommended amount of opioids are in addition to nonopioid analgesics, the guidelines point out.
All the other procedures can – and should – be managed with a combination of acetaminophen and ibuprofen, either alone or in an alternating dose pattern, said Dr. McLawhorn, of the department of dermatology at the University of Oklahoma Health Sciences Center, Oklahoma City, and coauthors.
But limited opioid prescribing is an important part of healing for patients who undergo the most invasive procedures, they wrote. “The management of complications, including adequate pain control, should be tailored to each patient on a case-by-case basis. Moreover, any pain management plan should not strictly adhere to any single guideline, but rather should be formed with consideration of the expected pain from the procedure and/or closure and consider the patient’s expectations for pain control.”
The time is ripe for dermatologists to make a stand in combating the opioid crisis, according to a group email response to questions from Dr. McLawhorn, Thomas Stasko, MD, professor and chair of dermatology at the University of Oklahoma, Oklahoma City, and Lindsey Collins, MD, also of the University of Oklahoma.
“The opioid crisis has reached epidemic proportions. More than 70,000 Americans have died from an opioid overdose in 2017,” they wrote. “Moreover, recent data suggest that nearly 6% of postsurgical, opioid-naive patients become long-term users of opioids. The lack of specific evidence-based recommendations likely contributes to a wide variety in prescribing patterns and a steady supply of unused opioids. Countering the opioid crisis necessitates a restructuring of the opioid prescribing practices that addresses pain in a procedure-specific manner. These recommendations are one tool in the dermatologists’ arsenal that can be used as a reference to help guide opioid management and prevent excessive opioid prescriptions at discharge following dermatologic interventions.”
Unfortunately, they added, dermatologists have inadvertently fueled the opioid abuse fire.
“It is difficult to quantify which providers are responsible for the onslaught of opioids into our communities,” the authors wrote in the email interview. “However, we can deduce, based on recent opioid prescribing patterns, that dermatologists provide approximately 500,000 unused opioid pills to their communities on an annual basis. This is the result of a wide variation in practice patterns and narratives that have been previously circulated in an attempt to mitigate the providers’ perception of the addictive nature of opioid analgesics. Our hope is that by addressing pain in a procedure-specific manner, we can help to limit the excessive number of unused opioid pills that are provided by dermatologists and ultimately decrease the rate of opioid-related complications, including addiction and death.”
Still, patients need and deserve effective pain management after a procedure. In the guidelines, the investigators wrote that a “one-size-fits-all” approach “does not account for the mechanism of pain, the invasiveness of the procedure, or the anatomic structures that are manipulated. As a result, current guidelines cannot accurately predict the quantity of opioids that are necessary to manage postoperative pain.”
The panel brought together experts in general dermatology, dermatologic surgery, cosmetics, and phlebology to develop a consensus on opioid prescribing guidelines for 87 of the most common procedures. Everyone on the panel was a member of the American College of Mohs Surgery, American Academy of Dermatology, or the American Vein and Lymphatic Society. The panel conducted a literature review to determine which procedures might require opioids and which would not. At least 75% of the panel had to agree on a reasonable but effective opioid amount; they were then polled as to whether they might employ that recommendation in their own clinical practice.
The recommendations are aimed at patients who experienced no peri- or postoperative complications.
The panel agreed that acetaminophen and ibuprofen – alone, in combination, or with opioids – were reasonable choices for all the 87 procedures. In such instances, acetaminophen 1 g can be staggered with ibuprofen 400 mg every 4 or 8 hours.
“I think providers will encounter a mixed bag of preconceived notions regarding patients’ expectations for pain control,” Dr. McLawhorn and coauthors wrote in the interview. “The important point for providers to make is to emphasize the noninferiority of acetaminophen and/or ibuprofen in controlling acute pain for patients who are not dependent on opioids for the management of chronic pain. Our experience in caring for many surgical patients has shown that patients are usually receptive to the use of nonopioid analgesics as many are familiar with their addictive potential because of the uptick in the publicity of the opioid-related complications.”
In cases where opioids might be appropriate, the panel unanimously agreed that dose limits be imposed. For 15 of the 87 procedures, the panel recommend a maximum prescription of 10 oxycodone 5-mg equivalents. Only one other – the Abbe flap – might warrant more, with a maximum of 15 oxycodone 5-mg pills at discharge.
Sometimes called a “lip switch,” the Abbe flap is reconstruction for full-thickness lip defects. It is a composite flap that moves skin, muscle, mucosa, and blood supply from the lower lip to reconstruct a defect of the upper lip. This reconstruction attempts to respect the native anatomic landmarks of the lip and allow for a better functional outcome.
“Because of the extensive nature of the repair and the anatomic territories that are manipulated, including the suturing of the lower lip to the upper lip with delayed separation, adequate pain control may require opioid analgesics in the immediate postoperative period,” the team wrote in the interview.
The panel could not agree on pain management strategies for five other procedures: Karapandzic flaps, en bloc nail excisions, facial resurfacing with deep chemical peels, and small- or large-volume liposuction. This was partly because of a lack of personal experience. Only 8 of the 40 panelists performed Karapandzic flaps. The maximum number of 5-mg oxycodone tablets any panelist prescribed for Karapandzic flaps and en bloc nail excisions was 20.
Facial resurfacing was likewise an uncommon procedure for the panel, with just 11 members performing this using deep chemical peels. However, five of those panelists said that opioids were routinely needed for postoperative pain with a maximum of 15 oxycodone 5-mg equivalents. And just four panelists performed liposuction, for which they used a maximum of 15 oxycodone 5-mg equivalents.
“However,” they wrote in the guidelines, “these providers noted that the location where the procedure is performed strongly influences the need for opioid pain management, with small-volume removal in the neck, arms, or flanks being unlikely to require opioids for adequate pain control, whereas large-volume removal in the thighs, knees, and hips may routinely require opioids.”
Addressing patient expectations is a very important part of pain management, the panel noted. “Patients will invariably experience postoperative pain after cutaneous surgeries or other interventions, often peaking within 4 hours after surgery. Wound tension, size and type of repair, anatomical location/nerve innervation, and patient pain tolerance are all factors that contribute to postoperative discomfort and should be considered when developing a postoperative pain management plan.”
Ultimately, according to Dr. McLawhorn and coauthors, the decision to use opioids at discharge for postoperative pain control should be an individual one based on patients’ comorbidities and expectations.
“Admittedly, many of the procedures listed within the recommendations may result in a rather large or complex defect that requires an equally large or complex repair,” they wrote in the interview. “However, proper education of the patient and provider regarding the risks of addiction with the use of opioids even short term should be discussed as part of every preoperative consultation. Furthermore, the patient and the provider must discuss their expectations for postoperative pain interventions for adequate pain control.”
SOURCE: McLawhorn J et al. J Am Acad Dermatol. 2019 Nov 12. doi: 10.1016/j.jaad.2019.09.080.
has recommended.
Rotation flaps, interpolation flaps, wedge resections, cartilage alar-batten grafts, and Mustarde flaps were among the 20 procedures that can be managed with up to 10 oral oxycodone 5-mg equivalents, according to the panel. Only the Abbe procedure might warrant dispensing up to 15 oxycodone 5-mg pills, Justin McLawhorn, MD, and colleagues wrote in the Journal of the American Academy of Dermatology. The recommended amount of opioids are in addition to nonopioid analgesics, the guidelines point out.
All the other procedures can – and should – be managed with a combination of acetaminophen and ibuprofen, either alone or in an alternating dose pattern, said Dr. McLawhorn, of the department of dermatology at the University of Oklahoma Health Sciences Center, Oklahoma City, and coauthors.
But limited opioid prescribing is an important part of healing for patients who undergo the most invasive procedures, they wrote. “The management of complications, including adequate pain control, should be tailored to each patient on a case-by-case basis. Moreover, any pain management plan should not strictly adhere to any single guideline, but rather should be formed with consideration of the expected pain from the procedure and/or closure and consider the patient’s expectations for pain control.”
The time is ripe for dermatologists to make a stand in combating the opioid crisis, according to a group email response to questions from Dr. McLawhorn, Thomas Stasko, MD, professor and chair of dermatology at the University of Oklahoma, Oklahoma City, and Lindsey Collins, MD, also of the University of Oklahoma.
“The opioid crisis has reached epidemic proportions. More than 70,000 Americans have died from an opioid overdose in 2017,” they wrote. “Moreover, recent data suggest that nearly 6% of postsurgical, opioid-naive patients become long-term users of opioids. The lack of specific evidence-based recommendations likely contributes to a wide variety in prescribing patterns and a steady supply of unused opioids. Countering the opioid crisis necessitates a restructuring of the opioid prescribing practices that addresses pain in a procedure-specific manner. These recommendations are one tool in the dermatologists’ arsenal that can be used as a reference to help guide opioid management and prevent excessive opioid prescriptions at discharge following dermatologic interventions.”
Unfortunately, they added, dermatologists have inadvertently fueled the opioid abuse fire.
“It is difficult to quantify which providers are responsible for the onslaught of opioids into our communities,” the authors wrote in the email interview. “However, we can deduce, based on recent opioid prescribing patterns, that dermatologists provide approximately 500,000 unused opioid pills to their communities on an annual basis. This is the result of a wide variation in practice patterns and narratives that have been previously circulated in an attempt to mitigate the providers’ perception of the addictive nature of opioid analgesics. Our hope is that by addressing pain in a procedure-specific manner, we can help to limit the excessive number of unused opioid pills that are provided by dermatologists and ultimately decrease the rate of opioid-related complications, including addiction and death.”
Still, patients need and deserve effective pain management after a procedure. In the guidelines, the investigators wrote that a “one-size-fits-all” approach “does not account for the mechanism of pain, the invasiveness of the procedure, or the anatomic structures that are manipulated. As a result, current guidelines cannot accurately predict the quantity of opioids that are necessary to manage postoperative pain.”
The panel brought together experts in general dermatology, dermatologic surgery, cosmetics, and phlebology to develop a consensus on opioid prescribing guidelines for 87 of the most common procedures. Everyone on the panel was a member of the American College of Mohs Surgery, American Academy of Dermatology, or the American Vein and Lymphatic Society. The panel conducted a literature review to determine which procedures might require opioids and which would not. At least 75% of the panel had to agree on a reasonable but effective opioid amount; they were then polled as to whether they might employ that recommendation in their own clinical practice.
The recommendations are aimed at patients who experienced no peri- or postoperative complications.
The panel agreed that acetaminophen and ibuprofen – alone, in combination, or with opioids – were reasonable choices for all the 87 procedures. In such instances, acetaminophen 1 g can be staggered with ibuprofen 400 mg every 4 or 8 hours.
“I think providers will encounter a mixed bag of preconceived notions regarding patients’ expectations for pain control,” Dr. McLawhorn and coauthors wrote in the interview. “The important point for providers to make is to emphasize the noninferiority of acetaminophen and/or ibuprofen in controlling acute pain for patients who are not dependent on opioids for the management of chronic pain. Our experience in caring for many surgical patients has shown that patients are usually receptive to the use of nonopioid analgesics as many are familiar with their addictive potential because of the uptick in the publicity of the opioid-related complications.”
In cases where opioids might be appropriate, the panel unanimously agreed that dose limits be imposed. For 15 of the 87 procedures, the panel recommend a maximum prescription of 10 oxycodone 5-mg equivalents. Only one other – the Abbe flap – might warrant more, with a maximum of 15 oxycodone 5-mg pills at discharge.
Sometimes called a “lip switch,” the Abbe flap is reconstruction for full-thickness lip defects. It is a composite flap that moves skin, muscle, mucosa, and blood supply from the lower lip to reconstruct a defect of the upper lip. This reconstruction attempts to respect the native anatomic landmarks of the lip and allow for a better functional outcome.
“Because of the extensive nature of the repair and the anatomic territories that are manipulated, including the suturing of the lower lip to the upper lip with delayed separation, adequate pain control may require opioid analgesics in the immediate postoperative period,” the team wrote in the interview.
The panel could not agree on pain management strategies for five other procedures: Karapandzic flaps, en bloc nail excisions, facial resurfacing with deep chemical peels, and small- or large-volume liposuction. This was partly because of a lack of personal experience. Only 8 of the 40 panelists performed Karapandzic flaps. The maximum number of 5-mg oxycodone tablets any panelist prescribed for Karapandzic flaps and en bloc nail excisions was 20.
Facial resurfacing was likewise an uncommon procedure for the panel, with just 11 members performing this using deep chemical peels. However, five of those panelists said that opioids were routinely needed for postoperative pain with a maximum of 15 oxycodone 5-mg equivalents. And just four panelists performed liposuction, for which they used a maximum of 15 oxycodone 5-mg equivalents.
“However,” they wrote in the guidelines, “these providers noted that the location where the procedure is performed strongly influences the need for opioid pain management, with small-volume removal in the neck, arms, or flanks being unlikely to require opioids for adequate pain control, whereas large-volume removal in the thighs, knees, and hips may routinely require opioids.”
Addressing patient expectations is a very important part of pain management, the panel noted. “Patients will invariably experience postoperative pain after cutaneous surgeries or other interventions, often peaking within 4 hours after surgery. Wound tension, size and type of repair, anatomical location/nerve innervation, and patient pain tolerance are all factors that contribute to postoperative discomfort and should be considered when developing a postoperative pain management plan.”
Ultimately, according to Dr. McLawhorn and coauthors, the decision to use opioids at discharge for postoperative pain control should be an individual one based on patients’ comorbidities and expectations.
“Admittedly, many of the procedures listed within the recommendations may result in a rather large or complex defect that requires an equally large or complex repair,” they wrote in the interview. “However, proper education of the patient and provider regarding the risks of addiction with the use of opioids even short term should be discussed as part of every preoperative consultation. Furthermore, the patient and the provider must discuss their expectations for postoperative pain interventions for adequate pain control.”
SOURCE: McLawhorn J et al. J Am Acad Dermatol. 2019 Nov 12. doi: 10.1016/j.jaad.2019.09.080.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Mole on back
The FP used a dermatoscope to get a better look at the lesion and recognized this as a halo nevus, which is characterized by a central nevus with a halo of depigmentation around it (arrow) and sometimes depigmentation within the nevus itself.
The halo is caused when, occasionally, the body develops an immune reaction to a nevus and its melanocytes. As cytotoxic T cells target those melanocytes, there is loss of pigment to the tissue surrounding the nevus.1
The appearance of the central nevus, rather than the hypopigmentation, determines management strategies. A globular or homogeneous pattern seen on dermoscopy is typically indicative of a benign lesion.2 An atypical pigment network or other melanoma specific structures should raise your suspicions for melanoma or atypical nevus and prompt a deep shave excision sent for pathology to rule out melanoma. A nevus without suspicious features, other than the surrounding hypopigmentation, can be managed conservatively with self-monitoring and re-evaluation.
In this case, the lesion displayed a homogeneous pattern, and the FP advised the patient to self-monitor.
Images and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque, NM.
1. Bayer-Garner IB, Ivan D, Schwartz MR, et al. The immunopathology of regression in benign lichenoid keratosis, keratoacanthoma and halo nevus. Clin Med Res. 2004;2:89-97.
2. Porto AC, Blumetti TP, de Paula Ramos Castro R, et al. Recurrent halo nevus: dermoscopy and confocal microscopy features. JAAD Case Rep. 2017;3:256-258.

The FP used a dermatoscope to get a better look at the lesion and recognized this as a halo nevus, which is characterized by a central nevus with a halo of depigmentation around it (arrow) and sometimes depigmentation within the nevus itself.
The halo is caused when, occasionally, the body develops an immune reaction to a nevus and its melanocytes. As cytotoxic T cells target those melanocytes, there is loss of pigment to the tissue surrounding the nevus.1
The appearance of the central nevus, rather than the hypopigmentation, determines management strategies. A globular or homogeneous pattern seen on dermoscopy is typically indicative of a benign lesion.2 An atypical pigment network or other melanoma specific structures should raise your suspicions for melanoma or atypical nevus and prompt a deep shave excision sent for pathology to rule out melanoma. A nevus without suspicious features, other than the surrounding hypopigmentation, can be managed conservatively with self-monitoring and re-evaluation.
In this case, the lesion displayed a homogeneous pattern, and the FP advised the patient to self-monitor.
Images and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque, NM.

The FP used a dermatoscope to get a better look at the lesion and recognized this as a halo nevus, which is characterized by a central nevus with a halo of depigmentation around it (arrow) and sometimes depigmentation within the nevus itself.
The halo is caused when, occasionally, the body develops an immune reaction to a nevus and its melanocytes. As cytotoxic T cells target those melanocytes, there is loss of pigment to the tissue surrounding the nevus.1
The appearance of the central nevus, rather than the hypopigmentation, determines management strategies. A globular or homogeneous pattern seen on dermoscopy is typically indicative of a benign lesion.2 An atypical pigment network or other melanoma specific structures should raise your suspicions for melanoma or atypical nevus and prompt a deep shave excision sent for pathology to rule out melanoma. A nevus without suspicious features, other than the surrounding hypopigmentation, can be managed conservatively with self-monitoring and re-evaluation.
In this case, the lesion displayed a homogeneous pattern, and the FP advised the patient to self-monitor.
Images and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque, NM.
1. Bayer-Garner IB, Ivan D, Schwartz MR, et al. The immunopathology of regression in benign lichenoid keratosis, keratoacanthoma and halo nevus. Clin Med Res. 2004;2:89-97.
2. Porto AC, Blumetti TP, de Paula Ramos Castro R, et al. Recurrent halo nevus: dermoscopy and confocal microscopy features. JAAD Case Rep. 2017;3:256-258.
1. Bayer-Garner IB, Ivan D, Schwartz MR, et al. The immunopathology of regression in benign lichenoid keratosis, keratoacanthoma and halo nevus. Clin Med Res. 2004;2:89-97.
2. Porto AC, Blumetti TP, de Paula Ramos Castro R, et al. Recurrent halo nevus: dermoscopy and confocal microscopy features. JAAD Case Rep. 2017;3:256-258.
Getting a Leg up on the Diagnosis
Three years ago, lesions began appearing on this now 68-year-old woman’s legs. They have grown in size and number, and their roughness disturbs the patient. She has been told the lesions are related to aging, but she has never seen anything like them on her friends or family—and she is worried about what they might mean for her health.
Her primary care provider diagnosed warts and performed cryotherapy on several of the lesions. However, the pain was intolerable and the treatment ineffective. To add insult to injury, each treated spot blistered and took more than a month to heal, leaving behind a pinkish brown blemish.
In all other respects, the patient’s health is excellent.
EXAMINATION
Both legs, from the upper thighs to the tops of the feet, are covered with thousands of uniformly distributed, tiny, keratotic, rough, dry papules. All the lesions are essentially identical: white, with no associated signs of inflammation. The patient’s skin is quite dry in general. Neither her palms nor soles are affected.
What’s the diagnosis?
DISCUSSION
The most common problem seen in dermatology offices worldwide is seborrheic keratosis (SK), a totally benign epidermal excrescence that appears to be related to aging and heredity. Most patients are in their 50s when they first notice an SK, and with a bit of luck, they will only see a few in their lifetime. But some patients develop hundreds of SKs, many of which become quite large (3-5 cm) and unsightly. In certain circumstances, SKs can herald the arrival of an occult carcinoma (the Leser-Trelat sign).
This patient has what some consider a variant of SK, called stucco keratosis. These lesions manifest almost exclusively on the lower legs and feet—perhaps due to the relative lack of sebaceous glands in those areas—and most often on men older than 60. Distressing as they are, stucco keratoses have no pathologic implications.
Grossly and histologically, stucco keratoses are different from ordinary SKs. Each stucco keratosis lesion is essentially identical to the others, with a spiculated surface, white color, and average diameter of 2 to 3 mm. Histologically, they demonstrate a thickened epidermis with focal exophytic upward projections that resemble church spires. The lesions do not extend into the dermis.
Treatment of stucco keratoses is, at best, tedious, painful, and futile. The modalities used are cryotherapy or electrodessication with curettage. For a degree of comfort, use of a loofah after bathing will remove or smooth down a few lesions, but this process must be followed by application of a heavy emollient. Alas, regrowth is a certainty.
TAKE-HOME LEARNING POINTS
- Stucco keratosis is considered a variant of seborrheic keratosis, although they differ in several significant ways.
- The lesions of stucco keratosis are fairly uniform in appearance: white, rough, spiculated, epidermal papules measuring 2 to 3 mm.
- Stucco keratoses affect about 10% of the population (men more often than women) and have no racial predilection or pathologic implications.
- The lesions are found almost exclusively on the legs, from the knees down to and including the dorsa of the feet.
- Treatment is far from satisfactory, for multiple reasons, including resultant pain and scarring.
Three years ago, lesions began appearing on this now 68-year-old woman’s legs. They have grown in size and number, and their roughness disturbs the patient. She has been told the lesions are related to aging, but she has never seen anything like them on her friends or family—and she is worried about what they might mean for her health.
Her primary care provider diagnosed warts and performed cryotherapy on several of the lesions. However, the pain was intolerable and the treatment ineffective. To add insult to injury, each treated spot blistered and took more than a month to heal, leaving behind a pinkish brown blemish.
In all other respects, the patient’s health is excellent.
EXAMINATION
Both legs, from the upper thighs to the tops of the feet, are covered with thousands of uniformly distributed, tiny, keratotic, rough, dry papules. All the lesions are essentially identical: white, with no associated signs of inflammation. The patient’s skin is quite dry in general. Neither her palms nor soles are affected.

What’s the diagnosis?
DISCUSSION
The most common problem seen in dermatology offices worldwide is seborrheic keratosis (SK), a totally benign epidermal excrescence that appears to be related to aging and heredity. Most patients are in their 50s when they first notice an SK, and with a bit of luck, they will only see a few in their lifetime. But some patients develop hundreds of SKs, many of which become quite large (3-5 cm) and unsightly. In certain circumstances, SKs can herald the arrival of an occult carcinoma (the Leser-Trelat sign).
This patient has what some consider a variant of SK, called stucco keratosis. These lesions manifest almost exclusively on the lower legs and feet—perhaps due to the relative lack of sebaceous glands in those areas—and most often on men older than 60. Distressing as they are, stucco keratoses have no pathologic implications.
Grossly and histologically, stucco keratoses are different from ordinary SKs. Each stucco keratosis lesion is essentially identical to the others, with a spiculated surface, white color, and average diameter of 2 to 3 mm. Histologically, they demonstrate a thickened epidermis with focal exophytic upward projections that resemble church spires. The lesions do not extend into the dermis.
Treatment of stucco keratoses is, at best, tedious, painful, and futile. The modalities used are cryotherapy or electrodessication with curettage. For a degree of comfort, use of a loofah after bathing will remove or smooth down a few lesions, but this process must be followed by application of a heavy emollient. Alas, regrowth is a certainty.
TAKE-HOME LEARNING POINTS
- Stucco keratosis is considered a variant of seborrheic keratosis, although they differ in several significant ways.
- The lesions of stucco keratosis are fairly uniform in appearance: white, rough, spiculated, epidermal papules measuring 2 to 3 mm.
- Stucco keratoses affect about 10% of the population (men more often than women) and have no racial predilection or pathologic implications.
- The lesions are found almost exclusively on the legs, from the knees down to and including the dorsa of the feet.
- Treatment is far from satisfactory, for multiple reasons, including resultant pain and scarring.
Three years ago, lesions began appearing on this now 68-year-old woman’s legs. They have grown in size and number, and their roughness disturbs the patient. She has been told the lesions are related to aging, but she has never seen anything like them on her friends or family—and she is worried about what they might mean for her health.
Her primary care provider diagnosed warts and performed cryotherapy on several of the lesions. However, the pain was intolerable and the treatment ineffective. To add insult to injury, each treated spot blistered and took more than a month to heal, leaving behind a pinkish brown blemish.
In all other respects, the patient’s health is excellent.
EXAMINATION
Both legs, from the upper thighs to the tops of the feet, are covered with thousands of uniformly distributed, tiny, keratotic, rough, dry papules. All the lesions are essentially identical: white, with no associated signs of inflammation. The patient’s skin is quite dry in general. Neither her palms nor soles are affected.

What’s the diagnosis?
DISCUSSION
The most common problem seen in dermatology offices worldwide is seborrheic keratosis (SK), a totally benign epidermal excrescence that appears to be related to aging and heredity. Most patients are in their 50s when they first notice an SK, and with a bit of luck, they will only see a few in their lifetime. But some patients develop hundreds of SKs, many of which become quite large (3-5 cm) and unsightly. In certain circumstances, SKs can herald the arrival of an occult carcinoma (the Leser-Trelat sign).
This patient has what some consider a variant of SK, called stucco keratosis. These lesions manifest almost exclusively on the lower legs and feet—perhaps due to the relative lack of sebaceous glands in those areas—and most often on men older than 60. Distressing as they are, stucco keratoses have no pathologic implications.
Grossly and histologically, stucco keratoses are different from ordinary SKs. Each stucco keratosis lesion is essentially identical to the others, with a spiculated surface, white color, and average diameter of 2 to 3 mm. Histologically, they demonstrate a thickened epidermis with focal exophytic upward projections that resemble church spires. The lesions do not extend into the dermis.
Treatment of stucco keratoses is, at best, tedious, painful, and futile. The modalities used are cryotherapy or electrodessication with curettage. For a degree of comfort, use of a loofah after bathing will remove or smooth down a few lesions, but this process must be followed by application of a heavy emollient. Alas, regrowth is a certainty.
TAKE-HOME LEARNING POINTS
- Stucco keratosis is considered a variant of seborrheic keratosis, although they differ in several significant ways.
- The lesions of stucco keratosis are fairly uniform in appearance: white, rough, spiculated, epidermal papules measuring 2 to 3 mm.
- Stucco keratoses affect about 10% of the population (men more often than women) and have no racial predilection or pathologic implications.
- The lesions are found almost exclusively on the legs, from the knees down to and including the dorsa of the feet.
- Treatment is far from satisfactory, for multiple reasons, including resultant pain and scarring.
Desquamating pustular rash
A 66-year-old man presented with burning pain and erythema over the left axilla, and pustules that had ruptured and crusted over. The rash also involved the right axilla, trunk, abdomen, and face.
He said the symptoms had developed 3 days after starting to use ciprofloxacin eye drops for eye redness and purulent discharge that had been diagnosed as bacterial conjunctivitis. He was taking no other new medications. He was afebrile.
The ciprofloxacin drops were stopped. The skin lesions were treated with emollients, topical steroids, and topical mupirocin. Improvement was noted 3 days into the hospitalization, as the lesions started to crust over and dry up and no new lesions were forming. The conjunctivitis improved with topical bacitracin ointment and prednisolone drops.
ACUTE GENERALIZED EXANTHEMATOUS PUSTULOSIS
The differential diagnosis of drug-related acute generalized exanthematous pustulosis includes Stevens-Johnson syndrome, pustular psoriasis, folliculitis, and varicella infection. The characteristic features of the rash and lesions and the temporal relationship between the start of ciprofloxacin eye drops and the development of symptoms, combined with rapid resolution of symptoms within days after discontinuing the drops, accompanied by skin biopsy study showing diffuse spongiosis with scattered eosinophils and a subcorneal pustule, confirmed the diagnosis of AGEP.
Key features of AGEP include numerous small, sterile, nonfollicular pustules on an erythematous background with associated fever and sometimes neutrophilia and eosinophilia.1 It usually begins on the face or in the intertriginous areas and then spreads to the trunk and lower limbs with rare mucosal involvement.1 It can be associated with viral infections, but most reported cases are related to drug reactions.2
Our patient’s case was unusual because AGEP triggered by topical medications is rarely reported, especially with ophthalmic medications.3 Drugs most commonly implicated are antibiotics including penicillins, sulfonamides, and quinolones, but other drugs such as terbinafine, diltiazem, and hydroxychloroquine have also been associated.2
AGEP may present with extensive skin desquamation, as in our patient, sometimes with bullae formation and skin sloughing manifesting as AGEP with overlapping toxic epidermal necrolysis.4
Diagnosis entails a careful review of medications, attention to lesion morphology, compatible disease course, and a high index of suspicion. Treatment is supportive and consists of stopping the offending agent, wound care, and antipyretics. Evidence for the use of steroids is weak.5
TAKE-HOME POINTS
AGEP should be considered in sudden-onset pustular desquamating erythematous rash related to use of a new medication. It is important to be aware that topical and ophthalmic medications are possible triggers. A thorough medication review should be done. Antibiotics are the most commonly implicated medications. An alternative medication should be tried. Treatment is supportive, as the condition is usually self-limiting once the offending medication is discontinued. Rarely, extensive desquamation and bullae formation may occur, which may be a manifestation of overlap features with toxic epidermal necrolysis.
- Speeckaert MM, Speeckaert R, Lambert J, Brochez L. Acute generalized exanthematous pustulosis: an overview of the clinical, immunological and diagnostic concepts. Eur J Dermatol 2010; 20(4):425–433. doi:10.1684/ejd.2010.0932
- Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)—results of a multinational case–control study (EuroSCAR). Br J Dermatol 2007; 157(5):989–996. doi:10.1111/j.1365-2133.2007.08156.x
- Beltran C, Vergier B, Doutre MS, Beylot C, Beylot-Barry M. Acute generalized exanthematous pustulosis induced by topical application of Algipan. Ann Dermatol Venereol 2009; 136(10):709–712. French. doi:10.1016/j.annder.2008.10.042
- Peermohamed S, Haber RM. Acute generalized exanthematous pustulosis simulating toxic epidermal necrolysis: a case report and review of the literature. Arch Dermatol 2011; 147(6):697–701. doi:10.1001/archdermatol.2011.147
- Feldmeyer L, Heidemeyer K, Yawalkar N. Acute generalized exanthematous pustulosis: pathogenesis, genetic background, clinical variants and therapy. Int J Mol Sci 2016; 17(8). pii:E1214. doi:10.3390/ijms17081214
A 66-year-old man presented with burning pain and erythema over the left axilla, and pustules that had ruptured and crusted over. The rash also involved the right axilla, trunk, abdomen, and face.
He said the symptoms had developed 3 days after starting to use ciprofloxacin eye drops for eye redness and purulent discharge that had been diagnosed as bacterial conjunctivitis. He was taking no other new medications. He was afebrile.
The ciprofloxacin drops were stopped. The skin lesions were treated with emollients, topical steroids, and topical mupirocin. Improvement was noted 3 days into the hospitalization, as the lesions started to crust over and dry up and no new lesions were forming. The conjunctivitis improved with topical bacitracin ointment and prednisolone drops.
ACUTE GENERALIZED EXANTHEMATOUS PUSTULOSIS
The differential diagnosis of drug-related acute generalized exanthematous pustulosis includes Stevens-Johnson syndrome, pustular psoriasis, folliculitis, and varicella infection. The characteristic features of the rash and lesions and the temporal relationship between the start of ciprofloxacin eye drops and the development of symptoms, combined with rapid resolution of symptoms within days after discontinuing the drops, accompanied by skin biopsy study showing diffuse spongiosis with scattered eosinophils and a subcorneal pustule, confirmed the diagnosis of AGEP.
Key features of AGEP include numerous small, sterile, nonfollicular pustules on an erythematous background with associated fever and sometimes neutrophilia and eosinophilia.1 It usually begins on the face or in the intertriginous areas and then spreads to the trunk and lower limbs with rare mucosal involvement.1 It can be associated with viral infections, but most reported cases are related to drug reactions.2
Our patient’s case was unusual because AGEP triggered by topical medications is rarely reported, especially with ophthalmic medications.3 Drugs most commonly implicated are antibiotics including penicillins, sulfonamides, and quinolones, but other drugs such as terbinafine, diltiazem, and hydroxychloroquine have also been associated.2
AGEP may present with extensive skin desquamation, as in our patient, sometimes with bullae formation and skin sloughing manifesting as AGEP with overlapping toxic epidermal necrolysis.4
Diagnosis entails a careful review of medications, attention to lesion morphology, compatible disease course, and a high index of suspicion. Treatment is supportive and consists of stopping the offending agent, wound care, and antipyretics. Evidence for the use of steroids is weak.5
TAKE-HOME POINTS
AGEP should be considered in sudden-onset pustular desquamating erythematous rash related to use of a new medication. It is important to be aware that topical and ophthalmic medications are possible triggers. A thorough medication review should be done. Antibiotics are the most commonly implicated medications. An alternative medication should be tried. Treatment is supportive, as the condition is usually self-limiting once the offending medication is discontinued. Rarely, extensive desquamation and bullae formation may occur, which may be a manifestation of overlap features with toxic epidermal necrolysis.
A 66-year-old man presented with burning pain and erythema over the left axilla, and pustules that had ruptured and crusted over. The rash also involved the right axilla, trunk, abdomen, and face.
He said the symptoms had developed 3 days after starting to use ciprofloxacin eye drops for eye redness and purulent discharge that had been diagnosed as bacterial conjunctivitis. He was taking no other new medications. He was afebrile.
The ciprofloxacin drops were stopped. The skin lesions were treated with emollients, topical steroids, and topical mupirocin. Improvement was noted 3 days into the hospitalization, as the lesions started to crust over and dry up and no new lesions were forming. The conjunctivitis improved with topical bacitracin ointment and prednisolone drops.
ACUTE GENERALIZED EXANTHEMATOUS PUSTULOSIS
The differential diagnosis of drug-related acute generalized exanthematous pustulosis includes Stevens-Johnson syndrome, pustular psoriasis, folliculitis, and varicella infection. The characteristic features of the rash and lesions and the temporal relationship between the start of ciprofloxacin eye drops and the development of symptoms, combined with rapid resolution of symptoms within days after discontinuing the drops, accompanied by skin biopsy study showing diffuse spongiosis with scattered eosinophils and a subcorneal pustule, confirmed the diagnosis of AGEP.
Key features of AGEP include numerous small, sterile, nonfollicular pustules on an erythematous background with associated fever and sometimes neutrophilia and eosinophilia.1 It usually begins on the face or in the intertriginous areas and then spreads to the trunk and lower limbs with rare mucosal involvement.1 It can be associated with viral infections, but most reported cases are related to drug reactions.2
Our patient’s case was unusual because AGEP triggered by topical medications is rarely reported, especially with ophthalmic medications.3 Drugs most commonly implicated are antibiotics including penicillins, sulfonamides, and quinolones, but other drugs such as terbinafine, diltiazem, and hydroxychloroquine have also been associated.2
AGEP may present with extensive skin desquamation, as in our patient, sometimes with bullae formation and skin sloughing manifesting as AGEP with overlapping toxic epidermal necrolysis.4
Diagnosis entails a careful review of medications, attention to lesion morphology, compatible disease course, and a high index of suspicion. Treatment is supportive and consists of stopping the offending agent, wound care, and antipyretics. Evidence for the use of steroids is weak.5
TAKE-HOME POINTS
AGEP should be considered in sudden-onset pustular desquamating erythematous rash related to use of a new medication. It is important to be aware that topical and ophthalmic medications are possible triggers. A thorough medication review should be done. Antibiotics are the most commonly implicated medications. An alternative medication should be tried. Treatment is supportive, as the condition is usually self-limiting once the offending medication is discontinued. Rarely, extensive desquamation and bullae formation may occur, which may be a manifestation of overlap features with toxic epidermal necrolysis.
- Speeckaert MM, Speeckaert R, Lambert J, Brochez L. Acute generalized exanthematous pustulosis: an overview of the clinical, immunological and diagnostic concepts. Eur J Dermatol 2010; 20(4):425–433. doi:10.1684/ejd.2010.0932
- Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)—results of a multinational case–control study (EuroSCAR). Br J Dermatol 2007; 157(5):989–996. doi:10.1111/j.1365-2133.2007.08156.x
- Beltran C, Vergier B, Doutre MS, Beylot C, Beylot-Barry M. Acute generalized exanthematous pustulosis induced by topical application of Algipan. Ann Dermatol Venereol 2009; 136(10):709–712. French. doi:10.1016/j.annder.2008.10.042
- Peermohamed S, Haber RM. Acute generalized exanthematous pustulosis simulating toxic epidermal necrolysis: a case report and review of the literature. Arch Dermatol 2011; 147(6):697–701. doi:10.1001/archdermatol.2011.147
- Feldmeyer L, Heidemeyer K, Yawalkar N. Acute generalized exanthematous pustulosis: pathogenesis, genetic background, clinical variants and therapy. Int J Mol Sci 2016; 17(8). pii:E1214. doi:10.3390/ijms17081214
- Speeckaert MM, Speeckaert R, Lambert J, Brochez L. Acute generalized exanthematous pustulosis: an overview of the clinical, immunological and diagnostic concepts. Eur J Dermatol 2010; 20(4):425–433. doi:10.1684/ejd.2010.0932
- Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)—results of a multinational case–control study (EuroSCAR). Br J Dermatol 2007; 157(5):989–996. doi:10.1111/j.1365-2133.2007.08156.x
- Beltran C, Vergier B, Doutre MS, Beylot C, Beylot-Barry M. Acute generalized exanthematous pustulosis induced by topical application of Algipan. Ann Dermatol Venereol 2009; 136(10):709–712. French. doi:10.1016/j.annder.2008.10.042
- Peermohamed S, Haber RM. Acute generalized exanthematous pustulosis simulating toxic epidermal necrolysis: a case report and review of the literature. Arch Dermatol 2011; 147(6):697–701. doi:10.1001/archdermatol.2011.147
- Feldmeyer L, Heidemeyer K, Yawalkar N. Acute generalized exanthematous pustulosis: pathogenesis, genetic background, clinical variants and therapy. Int J Mol Sci 2016; 17(8). pii:E1214. doi:10.3390/ijms17081214
Certolizumab safety profile varies widely across indications
MADRID – , Andrew Blauvelt, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
He presented a comprehensive analysis of safety data from all 49 clinical trials of the tumor necrosis factor inhibitor for its approved indications. The data set included 11,317 patients who received certolizumab for a collective 21,695 person-years in 27 trials in rheumatoid arthritis patients, 5 in psoriasis, 15 for Crohn’s disease, and one trial each for axial spondyloarthritis and psoriatic arthritis.
“It’s not real-world data, but it is a large group of patients [studied] over many years,” noted Dr. Blauvelt, a dermatologist and president of the Oregon Medical Research Center, Portland.
As a renowned authority on psoriasis, he was part of a multidisciplinary expert panel commissioned by UCB to analyze serious adverse events in the complete clinical trials experience involving the company’s tumor necrosis factor inhibitor certolizumab (Cimzia). The panel included experts from rheumatology, gastroenterology, epidemiology, and other disciplines.
The key takeaway: “When you think about the serious side effects of the drug, you have to think about what the indication is, whether the patients are on systemic corticosteroids, and whether they’re heavy or not,” Dr. Blauvelt said.
Take, for example, the risk of serious infections requiring treatment with intravenous antibiotics. The incidence rates ranged from a low of 1.5 per 100 patient-years in psoriasis patients on certolizumab to a high of 5.97 in those with Crohn’s disease, with rates of 3.44 cases per 100 patient-years among rheumatoid arthritis patients and 1.64-1.67 in those with psoriatic arthritis and ankylosing spondylitis, respectively. Patients with Crohn’s disease were 2.22-fold more likely than were those with rheumatoid arthritis to experience a serious infection during their clinical trial experience on certolizumab. In contrast, psoriasis patients had a 52% relative risk reduction and those with psoriatic arthritis were 31% less likely to develop a serious infection compared with those with rheumatoid arthritis.
The explanation for these highly variable serious infection rates lies in part on the huge differences in the concurrent use of systemic corticosteroids with certolizumab across indications. A mere 3.3% of psoriasis patients were also on steroids, compared with 46.2% of rheumatoid arthritis patients, 50.8% of those with ankylosing spondylitis, and about 25% of the Crohn’s disease and psoriatic arthritis patients, he noted.
Advanced age was independently associated with increased risk of serious infections. Patients aged 65 or older were 1.68-fold more likely to experience this event than were those under age 45. And patients whose disease duration was 10 years or more at baseline had a 1.36-fold increased serious infection risk compared with those who had less than a 1-year-long disease history, independent of which disease they had.
The prevalence of baseline obesity varied by indication. The mean body mass index was 30.1 kg/m2 in the psoriasis patients, 29.8 kg/m2 in those with psoriatic arthritis, lowest at 24 kg/m2 in Crohn’s disease patients, and a bit over 27 kg/m2 in those with rheumatoid arthritis or ankylosing spondylitis.
Obesity alone was not an independent risk factor for serious infection in certolizumab-treated patients; however, the combination of a BMI of 30 kg/m2 or more plus systemic corticosteroid use was associated with a greater risk than with steroids alone.
Based upon a multivariate regression analysis adjusted for age, sex, indication, disease duration, use of methotrexate, and prior use of other TNF inhibitors, the investigators calculated that in patients with Crohn’s disease 16.6% of serious infections in patients on certolizumab were attributable to systemic corticosteroid use.
Risks of major adverse cardiovascular events and cancer on certolizumab
The risk of major adverse cardiovascular events (MACE) while on certolizumab ranged from a high of 0.62 MACE events per 100 patient-years in the rheumatoid arthritis population to a low of 0.1 per 100 patient-years in patients treated for Crohn’s disease or ankylosing spondylitis. Psoriasis and psoriatic arthritis patients had MACE rates of 0.27 and 0.54, respectively.
Obesity was independently associated with increased risk of an acute MI and other MACEs. So was advanced age. No surprises there. The investigators calculated that 16.7% of MACEs in patients on certolizumab were attributable to obesity and another 20.9% were attributable to use of systemic corticosteroids.
The incidence rate for all malignancies, including nonmelanoma skin cancer, ranged from a low of 0.46 cases per 100 patient-years in the psoriatic arthritis cohort on certolizumab to a high of 0.93 in those with rheumatoid arthritis, with rates of 0.68, 0.73, and 0.51 in patients with psoriasis, Crohn’s disease, and ankylosing spondylitis, respectively.
Neither systemic corticosteroids, obesity, disease duration, or prior exposure to a TNF inhibitor was linked to increased risk of cancer in patients on certolizumab. The standout risk factor was age: Patients who were 65 or older at baseline were 11.4-fold more likely to develop cancer during participation in their clinical trial than were those younger than 45. Those who were 45 to 65 years old were 4.3-fold more likely to be diagnosed with a malignancy than were those younger than age 45.
Of note, concomitant use of methotrexate was associated with a statistically significant 28% reduction in malignancy risk.
Dr. Blauvelt reported serving as a consultant to and receiving research funding from UCB, the study sponsor, as well as more than two dozen other pharmaceutical companies.
SOURCE: Blauvelt A. EADV Congress, Abstract FC04.06.
MADRID – , Andrew Blauvelt, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
He presented a comprehensive analysis of safety data from all 49 clinical trials of the tumor necrosis factor inhibitor for its approved indications. The data set included 11,317 patients who received certolizumab for a collective 21,695 person-years in 27 trials in rheumatoid arthritis patients, 5 in psoriasis, 15 for Crohn’s disease, and one trial each for axial spondyloarthritis and psoriatic arthritis.
“It’s not real-world data, but it is a large group of patients [studied] over many years,” noted Dr. Blauvelt, a dermatologist and president of the Oregon Medical Research Center, Portland.
As a renowned authority on psoriasis, he was part of a multidisciplinary expert panel commissioned by UCB to analyze serious adverse events in the complete clinical trials experience involving the company’s tumor necrosis factor inhibitor certolizumab (Cimzia). The panel included experts from rheumatology, gastroenterology, epidemiology, and other disciplines.
The key takeaway: “When you think about the serious side effects of the drug, you have to think about what the indication is, whether the patients are on systemic corticosteroids, and whether they’re heavy or not,” Dr. Blauvelt said.
Take, for example, the risk of serious infections requiring treatment with intravenous antibiotics. The incidence rates ranged from a low of 1.5 per 100 patient-years in psoriasis patients on certolizumab to a high of 5.97 in those with Crohn’s disease, with rates of 3.44 cases per 100 patient-years among rheumatoid arthritis patients and 1.64-1.67 in those with psoriatic arthritis and ankylosing spondylitis, respectively. Patients with Crohn’s disease were 2.22-fold more likely than were those with rheumatoid arthritis to experience a serious infection during their clinical trial experience on certolizumab. In contrast, psoriasis patients had a 52% relative risk reduction and those with psoriatic arthritis were 31% less likely to develop a serious infection compared with those with rheumatoid arthritis.
The explanation for these highly variable serious infection rates lies in part on the huge differences in the concurrent use of systemic corticosteroids with certolizumab across indications. A mere 3.3% of psoriasis patients were also on steroids, compared with 46.2% of rheumatoid arthritis patients, 50.8% of those with ankylosing spondylitis, and about 25% of the Crohn’s disease and psoriatic arthritis patients, he noted.
Advanced age was independently associated with increased risk of serious infections. Patients aged 65 or older were 1.68-fold more likely to experience this event than were those under age 45. And patients whose disease duration was 10 years or more at baseline had a 1.36-fold increased serious infection risk compared with those who had less than a 1-year-long disease history, independent of which disease they had.
The prevalence of baseline obesity varied by indication. The mean body mass index was 30.1 kg/m2 in the psoriasis patients, 29.8 kg/m2 in those with psoriatic arthritis, lowest at 24 kg/m2 in Crohn’s disease patients, and a bit over 27 kg/m2 in those with rheumatoid arthritis or ankylosing spondylitis.
Obesity alone was not an independent risk factor for serious infection in certolizumab-treated patients; however, the combination of a BMI of 30 kg/m2 or more plus systemic corticosteroid use was associated with a greater risk than with steroids alone.
Based upon a multivariate regression analysis adjusted for age, sex, indication, disease duration, use of methotrexate, and prior use of other TNF inhibitors, the investigators calculated that in patients with Crohn’s disease 16.6% of serious infections in patients on certolizumab were attributable to systemic corticosteroid use.
Risks of major adverse cardiovascular events and cancer on certolizumab
The risk of major adverse cardiovascular events (MACE) while on certolizumab ranged from a high of 0.62 MACE events per 100 patient-years in the rheumatoid arthritis population to a low of 0.1 per 100 patient-years in patients treated for Crohn’s disease or ankylosing spondylitis. Psoriasis and psoriatic arthritis patients had MACE rates of 0.27 and 0.54, respectively.
Obesity was independently associated with increased risk of an acute MI and other MACEs. So was advanced age. No surprises there. The investigators calculated that 16.7% of MACEs in patients on certolizumab were attributable to obesity and another 20.9% were attributable to use of systemic corticosteroids.
The incidence rate for all malignancies, including nonmelanoma skin cancer, ranged from a low of 0.46 cases per 100 patient-years in the psoriatic arthritis cohort on certolizumab to a high of 0.93 in those with rheumatoid arthritis, with rates of 0.68, 0.73, and 0.51 in patients with psoriasis, Crohn’s disease, and ankylosing spondylitis, respectively.
Neither systemic corticosteroids, obesity, disease duration, or prior exposure to a TNF inhibitor was linked to increased risk of cancer in patients on certolizumab. The standout risk factor was age: Patients who were 65 or older at baseline were 11.4-fold more likely to develop cancer during participation in their clinical trial than were those younger than 45. Those who were 45 to 65 years old were 4.3-fold more likely to be diagnosed with a malignancy than were those younger than age 45.
Of note, concomitant use of methotrexate was associated with a statistically significant 28% reduction in malignancy risk.
Dr. Blauvelt reported serving as a consultant to and receiving research funding from UCB, the study sponsor, as well as more than two dozen other pharmaceutical companies.
SOURCE: Blauvelt A. EADV Congress, Abstract FC04.06.
MADRID – , Andrew Blauvelt, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
He presented a comprehensive analysis of safety data from all 49 clinical trials of the tumor necrosis factor inhibitor for its approved indications. The data set included 11,317 patients who received certolizumab for a collective 21,695 person-years in 27 trials in rheumatoid arthritis patients, 5 in psoriasis, 15 for Crohn’s disease, and one trial each for axial spondyloarthritis and psoriatic arthritis.
“It’s not real-world data, but it is a large group of patients [studied] over many years,” noted Dr. Blauvelt, a dermatologist and president of the Oregon Medical Research Center, Portland.
As a renowned authority on psoriasis, he was part of a multidisciplinary expert panel commissioned by UCB to analyze serious adverse events in the complete clinical trials experience involving the company’s tumor necrosis factor inhibitor certolizumab (Cimzia). The panel included experts from rheumatology, gastroenterology, epidemiology, and other disciplines.
The key takeaway: “When you think about the serious side effects of the drug, you have to think about what the indication is, whether the patients are on systemic corticosteroids, and whether they’re heavy or not,” Dr. Blauvelt said.
Take, for example, the risk of serious infections requiring treatment with intravenous antibiotics. The incidence rates ranged from a low of 1.5 per 100 patient-years in psoriasis patients on certolizumab to a high of 5.97 in those with Crohn’s disease, with rates of 3.44 cases per 100 patient-years among rheumatoid arthritis patients and 1.64-1.67 in those with psoriatic arthritis and ankylosing spondylitis, respectively. Patients with Crohn’s disease were 2.22-fold more likely than were those with rheumatoid arthritis to experience a serious infection during their clinical trial experience on certolizumab. In contrast, psoriasis patients had a 52% relative risk reduction and those with psoriatic arthritis were 31% less likely to develop a serious infection compared with those with rheumatoid arthritis.
The explanation for these highly variable serious infection rates lies in part on the huge differences in the concurrent use of systemic corticosteroids with certolizumab across indications. A mere 3.3% of psoriasis patients were also on steroids, compared with 46.2% of rheumatoid arthritis patients, 50.8% of those with ankylosing spondylitis, and about 25% of the Crohn’s disease and psoriatic arthritis patients, he noted.
Advanced age was independently associated with increased risk of serious infections. Patients aged 65 or older were 1.68-fold more likely to experience this event than were those under age 45. And patients whose disease duration was 10 years or more at baseline had a 1.36-fold increased serious infection risk compared with those who had less than a 1-year-long disease history, independent of which disease they had.
The prevalence of baseline obesity varied by indication. The mean body mass index was 30.1 kg/m2 in the psoriasis patients, 29.8 kg/m2 in those with psoriatic arthritis, lowest at 24 kg/m2 in Crohn’s disease patients, and a bit over 27 kg/m2 in those with rheumatoid arthritis or ankylosing spondylitis.
Obesity alone was not an independent risk factor for serious infection in certolizumab-treated patients; however, the combination of a BMI of 30 kg/m2 or more plus systemic corticosteroid use was associated with a greater risk than with steroids alone.
Based upon a multivariate regression analysis adjusted for age, sex, indication, disease duration, use of methotrexate, and prior use of other TNF inhibitors, the investigators calculated that in patients with Crohn’s disease 16.6% of serious infections in patients on certolizumab were attributable to systemic corticosteroid use.
Risks of major adverse cardiovascular events and cancer on certolizumab
The risk of major adverse cardiovascular events (MACE) while on certolizumab ranged from a high of 0.62 MACE events per 100 patient-years in the rheumatoid arthritis population to a low of 0.1 per 100 patient-years in patients treated for Crohn’s disease or ankylosing spondylitis. Psoriasis and psoriatic arthritis patients had MACE rates of 0.27 and 0.54, respectively.
Obesity was independently associated with increased risk of an acute MI and other MACEs. So was advanced age. No surprises there. The investigators calculated that 16.7% of MACEs in patients on certolizumab were attributable to obesity and another 20.9% were attributable to use of systemic corticosteroids.
The incidence rate for all malignancies, including nonmelanoma skin cancer, ranged from a low of 0.46 cases per 100 patient-years in the psoriatic arthritis cohort on certolizumab to a high of 0.93 in those with rheumatoid arthritis, with rates of 0.68, 0.73, and 0.51 in patients with psoriasis, Crohn’s disease, and ankylosing spondylitis, respectively.
Neither systemic corticosteroids, obesity, disease duration, or prior exposure to a TNF inhibitor was linked to increased risk of cancer in patients on certolizumab. The standout risk factor was age: Patients who were 65 or older at baseline were 11.4-fold more likely to develop cancer during participation in their clinical trial than were those younger than 45. Those who were 45 to 65 years old were 4.3-fold more likely to be diagnosed with a malignancy than were those younger than age 45.
Of note, concomitant use of methotrexate was associated with a statistically significant 28% reduction in malignancy risk.
Dr. Blauvelt reported serving as a consultant to and receiving research funding from UCB, the study sponsor, as well as more than two dozen other pharmaceutical companies.
SOURCE: Blauvelt A. EADV Congress, Abstract FC04.06.
REPORTING FROM THE EADV CONGRESS
Combo elicits lasting responses in metastatic melanoma
NATIONAL HARBOR, MD. – The combination of bempegaldesleukin and nivolumab produced durable responses in a phase 1/2 trial of patients with previously untreated metastatic melanoma.
The overall response rate was 53%, and most responders were still in response at a median follow-up of about 19 months. The median progression-free survival was not reached, and the combination was considered well tolerated.
Adi Diab, MD, of the University of Texas MD Anderson Cancer Center, Houston, presented these results from the PIVOT-02 study at the annual meeting of the Society for Immunotherapy of Cancer.
Dr. Diab explained that bempegaldesleukin (bempeg) is a CD122-preferential interleukin-2 pathway agonist, and earlier results from the PIVOT-02 trial showed that adding bempeg to nivolumab can convert baseline tumors from programmed death–ligand 1 (PD-L1) negative to PD-L1 positive (SITC 2018, Abstract O4).
Dr. Diab presented updated results from PIVOT-02 (NCT02983045) in 41 patients with metastatic melanoma who received bempeg plus nivolumab as first-line treatment. The patients had a median age of 63 years (range, 22-80 years) at baseline, and 58.5% were male. Most patients (58.5%) were PD-L1 positive, although PD-L1 status was unknown in 7.3% of patients.
Patients received bempeg at 0.006 mg/kg and nivolumab at 360 mg every 3 weeks. They received a median of nine cycles (range, 1-34), and the median follow-up was 18.6 months.
Efficacy
In the 38 patients who were evaluable for efficacy, the overall response rate was 53% (n = 20), and the complete response rate was 34% (n = 13). The median time to response was 2.0 months, and the median time to complete response was 7.9 months.
Dr. Diab noted that responses were seen regardless of PD-L1 expression at baseline. The response rate was 39% among PD-L1-negative patients, 64% among PD-L1-positive patients, and 33% among patients whose PD-L1 status was unknown.
Dr. Diab also pointed out that responses were durable and deepened over time. The median duration of response was not reached, and 17 of the 20 responders had ongoing responses at last follow-up. The median progression-free survival has not been reached.
Safety
“This combination is safe and tolerable, there’s no overlapping immune-related adverse events, and the most common side effects are grade 1/2 flu-like symptoms,” Dr. Diab said.
The most common grade 1/2 treatment-related adverse events (AEs) were flu-like symptoms (80.5%), rash (70.7%), fatigue (65.9%), pruritus (48.8%), nausea (46.3%), arthralgia (43.9%), decreased appetite (36.6%), and myalgia (36.6%).
Dr. Diab noted that cytokine-related AEs (flu-like symptoms, rash, and pruritus) were easily managed with NSAIDs; decreased with subsequent cycles of treatment; and did not necessitate dose delays, reductions, or discontinuations.
Grade 3/4 treatment-related AEs included two cases of acute kidney injury, two cases of atrial fibrillation, one case of dizziness, one case of dyspnea, one case of hypoxia, one case of hyperglycemia, and one case of hypernatremia.
Five patients discontinued treatment because of related AEs, including cerebrovascular accident, peripheral edema, blood creatinine increase, malaise, and pharyngitis. There were no treatment-related deaths.
Dr. Diab said these results were used to support the recent breakthrough therapy designation granted to bempeg in combination with nivolumab. The results have also prompted a phase 3 trial in which researchers are comparing the combination with nivolumab alone (NCT03635983).
The phase 1/2 trial is sponsored by Nektar Therapeutics in collaboration with Bristol-Myers Squibb. Dr. Diab reported relationships with Nektar, Celgene, CureVac, Idera, and Pfizer.
SOURCE: Diab A et al. SITC 2019, Abstract O35.
NATIONAL HARBOR, MD. – The combination of bempegaldesleukin and nivolumab produced durable responses in a phase 1/2 trial of patients with previously untreated metastatic melanoma.
The overall response rate was 53%, and most responders were still in response at a median follow-up of about 19 months. The median progression-free survival was not reached, and the combination was considered well tolerated.
Adi Diab, MD, of the University of Texas MD Anderson Cancer Center, Houston, presented these results from the PIVOT-02 study at the annual meeting of the Society for Immunotherapy of Cancer.
Dr. Diab explained that bempegaldesleukin (bempeg) is a CD122-preferential interleukin-2 pathway agonist, and earlier results from the PIVOT-02 trial showed that adding bempeg to nivolumab can convert baseline tumors from programmed death–ligand 1 (PD-L1) negative to PD-L1 positive (SITC 2018, Abstract O4).
Dr. Diab presented updated results from PIVOT-02 (NCT02983045) in 41 patients with metastatic melanoma who received bempeg plus nivolumab as first-line treatment. The patients had a median age of 63 years (range, 22-80 years) at baseline, and 58.5% were male. Most patients (58.5%) were PD-L1 positive, although PD-L1 status was unknown in 7.3% of patients.
Patients received bempeg at 0.006 mg/kg and nivolumab at 360 mg every 3 weeks. They received a median of nine cycles (range, 1-34), and the median follow-up was 18.6 months.
Efficacy
In the 38 patients who were evaluable for efficacy, the overall response rate was 53% (n = 20), and the complete response rate was 34% (n = 13). The median time to response was 2.0 months, and the median time to complete response was 7.9 months.
Dr. Diab noted that responses were seen regardless of PD-L1 expression at baseline. The response rate was 39% among PD-L1-negative patients, 64% among PD-L1-positive patients, and 33% among patients whose PD-L1 status was unknown.
Dr. Diab also pointed out that responses were durable and deepened over time. The median duration of response was not reached, and 17 of the 20 responders had ongoing responses at last follow-up. The median progression-free survival has not been reached.
Safety
“This combination is safe and tolerable, there’s no overlapping immune-related adverse events, and the most common side effects are grade 1/2 flu-like symptoms,” Dr. Diab said.
The most common grade 1/2 treatment-related adverse events (AEs) were flu-like symptoms (80.5%), rash (70.7%), fatigue (65.9%), pruritus (48.8%), nausea (46.3%), arthralgia (43.9%), decreased appetite (36.6%), and myalgia (36.6%).
Dr. Diab noted that cytokine-related AEs (flu-like symptoms, rash, and pruritus) were easily managed with NSAIDs; decreased with subsequent cycles of treatment; and did not necessitate dose delays, reductions, or discontinuations.
Grade 3/4 treatment-related AEs included two cases of acute kidney injury, two cases of atrial fibrillation, one case of dizziness, one case of dyspnea, one case of hypoxia, one case of hyperglycemia, and one case of hypernatremia.
Five patients discontinued treatment because of related AEs, including cerebrovascular accident, peripheral edema, blood creatinine increase, malaise, and pharyngitis. There were no treatment-related deaths.
Dr. Diab said these results were used to support the recent breakthrough therapy designation granted to bempeg in combination with nivolumab. The results have also prompted a phase 3 trial in which researchers are comparing the combination with nivolumab alone (NCT03635983).
The phase 1/2 trial is sponsored by Nektar Therapeutics in collaboration with Bristol-Myers Squibb. Dr. Diab reported relationships with Nektar, Celgene, CureVac, Idera, and Pfizer.
SOURCE: Diab A et al. SITC 2019, Abstract O35.
NATIONAL HARBOR, MD. – The combination of bempegaldesleukin and nivolumab produced durable responses in a phase 1/2 trial of patients with previously untreated metastatic melanoma.
The overall response rate was 53%, and most responders were still in response at a median follow-up of about 19 months. The median progression-free survival was not reached, and the combination was considered well tolerated.
Adi Diab, MD, of the University of Texas MD Anderson Cancer Center, Houston, presented these results from the PIVOT-02 study at the annual meeting of the Society for Immunotherapy of Cancer.
Dr. Diab explained that bempegaldesleukin (bempeg) is a CD122-preferential interleukin-2 pathway agonist, and earlier results from the PIVOT-02 trial showed that adding bempeg to nivolumab can convert baseline tumors from programmed death–ligand 1 (PD-L1) negative to PD-L1 positive (SITC 2018, Abstract O4).
Dr. Diab presented updated results from PIVOT-02 (NCT02983045) in 41 patients with metastatic melanoma who received bempeg plus nivolumab as first-line treatment. The patients had a median age of 63 years (range, 22-80 years) at baseline, and 58.5% were male. Most patients (58.5%) were PD-L1 positive, although PD-L1 status was unknown in 7.3% of patients.
Patients received bempeg at 0.006 mg/kg and nivolumab at 360 mg every 3 weeks. They received a median of nine cycles (range, 1-34), and the median follow-up was 18.6 months.
Efficacy
In the 38 patients who were evaluable for efficacy, the overall response rate was 53% (n = 20), and the complete response rate was 34% (n = 13). The median time to response was 2.0 months, and the median time to complete response was 7.9 months.
Dr. Diab noted that responses were seen regardless of PD-L1 expression at baseline. The response rate was 39% among PD-L1-negative patients, 64% among PD-L1-positive patients, and 33% among patients whose PD-L1 status was unknown.
Dr. Diab also pointed out that responses were durable and deepened over time. The median duration of response was not reached, and 17 of the 20 responders had ongoing responses at last follow-up. The median progression-free survival has not been reached.
Safety
“This combination is safe and tolerable, there’s no overlapping immune-related adverse events, and the most common side effects are grade 1/2 flu-like symptoms,” Dr. Diab said.
The most common grade 1/2 treatment-related adverse events (AEs) were flu-like symptoms (80.5%), rash (70.7%), fatigue (65.9%), pruritus (48.8%), nausea (46.3%), arthralgia (43.9%), decreased appetite (36.6%), and myalgia (36.6%).
Dr. Diab noted that cytokine-related AEs (flu-like symptoms, rash, and pruritus) were easily managed with NSAIDs; decreased with subsequent cycles of treatment; and did not necessitate dose delays, reductions, or discontinuations.
Grade 3/4 treatment-related AEs included two cases of acute kidney injury, two cases of atrial fibrillation, one case of dizziness, one case of dyspnea, one case of hypoxia, one case of hyperglycemia, and one case of hypernatremia.
Five patients discontinued treatment because of related AEs, including cerebrovascular accident, peripheral edema, blood creatinine increase, malaise, and pharyngitis. There were no treatment-related deaths.
Dr. Diab said these results were used to support the recent breakthrough therapy designation granted to bempeg in combination with nivolumab. The results have also prompted a phase 3 trial in which researchers are comparing the combination with nivolumab alone (NCT03635983).
The phase 1/2 trial is sponsored by Nektar Therapeutics in collaboration with Bristol-Myers Squibb. Dr. Diab reported relationships with Nektar, Celgene, CureVac, Idera, and Pfizer.
SOURCE: Diab A et al. SITC 2019, Abstract O35.
REPORTING FROM SITC 2019