User login
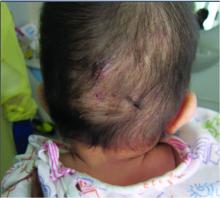
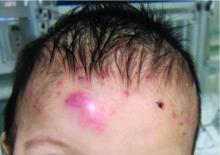
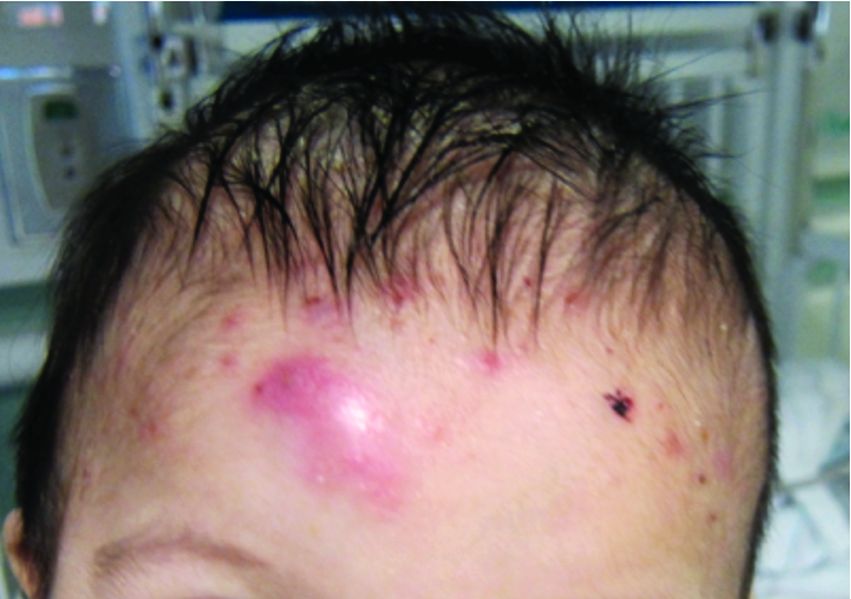
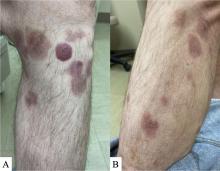
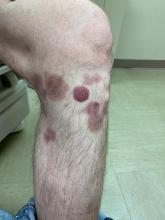
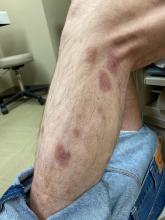
A 7-month-old male presents with pustules and inflamed papules on the scalp and extremities
The bacterial, fungal, and atypical mycobacterial cultures from the lesions performed at the emergency department were all negative.
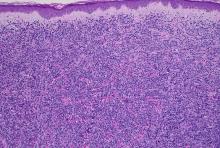
Pediatric dermatology was consulted and a punch biopsy of one of the lesions was done. Histopathologic examination showed a mixed perifollicular infiltrate of predominantly eosinophils with some neutrophils and associated microabscesses. Periodic acid Schiff and Fite stains failed to reveal any organisms. CD1 immunostain was negative. Fresh tissue cultures for bacteria, fungi, and atypical mycobacteria were negative.
Given the clinical presentation of chronic recurrent sterile pustules on an infant with associated eosinophilia and the reported histopathologic findings, the patient was diagnosed with eosinophilic pustular folliculitis of infancy (EPFI).
EPFI is a rare and idiopathic cutaneous disorder present in children. About 70% of the cases reported occur in the first 6 month of life and rarely present past 3 years of age. EPF encompasses a group of conditions including the classic adult form, or Ofuji disease. EPF is seen in immunosuppressed patients, mainly HIV positive, and EPF is also seen in infants and children.
In EPFI, males are most commonly affected. The condition presents, as it did in our patient, with recurrent crops of sterile papules and pustules mainly on the scalp, but they can occur in other parts of the body. The lesions go away within a few weeks to months without leaving any scars but it can take months to years to resolve. Histopathologic analysis of the lesions show an eosinophilic infiltrate which can be follicular, perifollicular, or periadnexal with associated flame figures in about 26% of cases.
Aggressive treatment is usually not needed as lesions are self-limited. Lesions can be treated with topical corticosteroids and oral antihistamine medications like cetirizine if symptomatic.
If the lesions start to present during the neonatal period, one may consider in the differential diagnosis, neonatal rashes like transient neonatal pustular melanosis and erythema toxicum neonatorum. Both of these neonatal conditions tend to resolve in the first month of life, compared with EPFI where lesions can come and go for months to years. EPFI lesions can be described as pustules and inflammatory papules, as well as furuncles and vesicles. All of the lesions may be seen in one patient at one time, which will not be typical for transient neonatal pustular melanosis or erythema toxicum. Eosinophils can be seen in erythema toxicum but folliculitis is not present. The inflammatory infiltrate seen in transient neonatal pustular melanosis is polymorphonuclear, not eosinophilic.
Early in the presentation, infectious conditions like staphylococcal or streptococcal folliculitis, cellulitis and furunculosis, tinea capitis, atypical mycobacterial infections, herpes simplex, and parasitic infections like scabies should be considered. In young infants, empiric antibiotic treatment may be started until cultures are finalized. If there is a family history of pruritic papules and pustules, scabies should be considered. A scabies prep can be done to rule out this entity.
Langerhans cell histiocytosis can also present with pustules and papules in early infancy and also has a predilection for the scalp. When this condition is in question, a skin biopsy should be performed which shows a CD1 positive histiocytic infiltrate.
In conclusion, EPFI is a benign rare condition that can present in infants as recurrent pustules and papules, mainly on the scalp, which are self-limited and if symptomatic can be treated with topical corticosteroids and antihistamines.
References
Alonso-Castro L et al. Dermatol Online J. 2012 Oct 15;18(10):6.
Frølunde AS et al. Clin Case Rep. 2021 May 11;9(5):e04167.
Hernández-Martín Á et al. J Am Acad Dermatol. 2013 Jan;68(1):150-5.
The bacterial, fungal, and atypical mycobacterial cultures from the lesions performed at the emergency department were all negative.
Pediatric dermatology was consulted and a punch biopsy of one of the lesions was done. Histopathologic examination showed a mixed perifollicular infiltrate of predominantly eosinophils with some neutrophils and associated microabscesses. Periodic acid Schiff and Fite stains failed to reveal any organisms. CD1 immunostain was negative. Fresh tissue cultures for bacteria, fungi, and atypical mycobacteria were negative.
Given the clinical presentation of chronic recurrent sterile pustules on an infant with associated eosinophilia and the reported histopathologic findings, the patient was diagnosed with eosinophilic pustular folliculitis of infancy (EPFI).
EPFI is a rare and idiopathic cutaneous disorder present in children. About 70% of the cases reported occur in the first 6 month of life and rarely present past 3 years of age. EPF encompasses a group of conditions including the classic adult form, or Ofuji disease. EPF is seen in immunosuppressed patients, mainly HIV positive, and EPF is also seen in infants and children.
In EPFI, males are most commonly affected. The condition presents, as it did in our patient, with recurrent crops of sterile papules and pustules mainly on the scalp, but they can occur in other parts of the body. The lesions go away within a few weeks to months without leaving any scars but it can take months to years to resolve. Histopathologic analysis of the lesions show an eosinophilic infiltrate which can be follicular, perifollicular, or periadnexal with associated flame figures in about 26% of cases.
Aggressive treatment is usually not needed as lesions are self-limited. Lesions can be treated with topical corticosteroids and oral antihistamine medications like cetirizine if symptomatic.
If the lesions start to present during the neonatal period, one may consider in the differential diagnosis, neonatal rashes like transient neonatal pustular melanosis and erythema toxicum neonatorum. Both of these neonatal conditions tend to resolve in the first month of life, compared with EPFI where lesions can come and go for months to years. EPFI lesions can be described as pustules and inflammatory papules, as well as furuncles and vesicles. All of the lesions may be seen in one patient at one time, which will not be typical for transient neonatal pustular melanosis or erythema toxicum. Eosinophils can be seen in erythema toxicum but folliculitis is not present. The inflammatory infiltrate seen in transient neonatal pustular melanosis is polymorphonuclear, not eosinophilic.
Early in the presentation, infectious conditions like staphylococcal or streptococcal folliculitis, cellulitis and furunculosis, tinea capitis, atypical mycobacterial infections, herpes simplex, and parasitic infections like scabies should be considered. In young infants, empiric antibiotic treatment may be started until cultures are finalized. If there is a family history of pruritic papules and pustules, scabies should be considered. A scabies prep can be done to rule out this entity.
Langerhans cell histiocytosis can also present with pustules and papules in early infancy and also has a predilection for the scalp. When this condition is in question, a skin biopsy should be performed which shows a CD1 positive histiocytic infiltrate.
In conclusion, EPFI is a benign rare condition that can present in infants as recurrent pustules and papules, mainly on the scalp, which are self-limited and if symptomatic can be treated with topical corticosteroids and antihistamines.
References
Alonso-Castro L et al. Dermatol Online J. 2012 Oct 15;18(10):6.
Frølunde AS et al. Clin Case Rep. 2021 May 11;9(5):e04167.
Hernández-Martín Á et al. J Am Acad Dermatol. 2013 Jan;68(1):150-5.
The bacterial, fungal, and atypical mycobacterial cultures from the lesions performed at the emergency department were all negative.
Pediatric dermatology was consulted and a punch biopsy of one of the lesions was done. Histopathologic examination showed a mixed perifollicular infiltrate of predominantly eosinophils with some neutrophils and associated microabscesses. Periodic acid Schiff and Fite stains failed to reveal any organisms. CD1 immunostain was negative. Fresh tissue cultures for bacteria, fungi, and atypical mycobacteria were negative.
Given the clinical presentation of chronic recurrent sterile pustules on an infant with associated eosinophilia and the reported histopathologic findings, the patient was diagnosed with eosinophilic pustular folliculitis of infancy (EPFI).
EPFI is a rare and idiopathic cutaneous disorder present in children. About 70% of the cases reported occur in the first 6 month of life and rarely present past 3 years of age. EPF encompasses a group of conditions including the classic adult form, or Ofuji disease. EPF is seen in immunosuppressed patients, mainly HIV positive, and EPF is also seen in infants and children.
In EPFI, males are most commonly affected. The condition presents, as it did in our patient, with recurrent crops of sterile papules and pustules mainly on the scalp, but they can occur in other parts of the body. The lesions go away within a few weeks to months without leaving any scars but it can take months to years to resolve. Histopathologic analysis of the lesions show an eosinophilic infiltrate which can be follicular, perifollicular, or periadnexal with associated flame figures in about 26% of cases.
Aggressive treatment is usually not needed as lesions are self-limited. Lesions can be treated with topical corticosteroids and oral antihistamine medications like cetirizine if symptomatic.
If the lesions start to present during the neonatal period, one may consider in the differential diagnosis, neonatal rashes like transient neonatal pustular melanosis and erythema toxicum neonatorum. Both of these neonatal conditions tend to resolve in the first month of life, compared with EPFI where lesions can come and go for months to years. EPFI lesions can be described as pustules and inflammatory papules, as well as furuncles and vesicles. All of the lesions may be seen in one patient at one time, which will not be typical for transient neonatal pustular melanosis or erythema toxicum. Eosinophils can be seen in erythema toxicum but folliculitis is not present. The inflammatory infiltrate seen in transient neonatal pustular melanosis is polymorphonuclear, not eosinophilic.
Early in the presentation, infectious conditions like staphylococcal or streptococcal folliculitis, cellulitis and furunculosis, tinea capitis, atypical mycobacterial infections, herpes simplex, and parasitic infections like scabies should be considered. In young infants, empiric antibiotic treatment may be started until cultures are finalized. If there is a family history of pruritic papules and pustules, scabies should be considered. A scabies prep can be done to rule out this entity.
Langerhans cell histiocytosis can also present with pustules and papules in early infancy and also has a predilection for the scalp. When this condition is in question, a skin biopsy should be performed which shows a CD1 positive histiocytic infiltrate.
In conclusion, EPFI is a benign rare condition that can present in infants as recurrent pustules and papules, mainly on the scalp, which are self-limited and if symptomatic can be treated with topical corticosteroids and antihistamines.
References
Alonso-Castro L et al. Dermatol Online J. 2012 Oct 15;18(10):6.
Frølunde AS et al. Clin Case Rep. 2021 May 11;9(5):e04167.
Hernández-Martín Á et al. J Am Acad Dermatol. 2013 Jan;68(1):150-5.
A 7-month-old male is brought to the emergency department for evaluation of pustules and inflamed papules on the scalp and extremities for several weeks of duration. The parents report the lesions started about a month prior and he has already been treated with cephalexin, clindamycin, and sulfamethoxazole without any improvement. Cultures sent prior by the child's pediatrician did not reveal any fungus or bacteria. The parents report a low-grade fever for about 3 days.
He was born via natural vaginal delivery with no instrumentation or external monitoring. Mom had prenatal care. Besides the skin lesions, the baby has been healthy and growing well. He has no history of eczema or severe infections. He has not been hospitalized before.
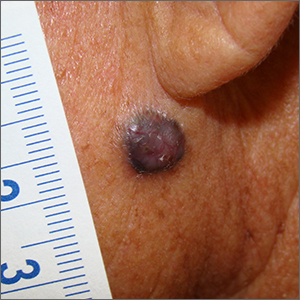
On physical examination the baby was not febrile. On the scalp and forehead, he had diffusely distributed pustules, erythematous papules, and nodules. He also presented with scattered, fine, small, crusted 1-2-mm pink papules on the trunk and extremities. He had no adenopathy or hepatosplenomegaly.
At the emergency department, samples from one of the pustules were sent for bacterial, fungal, and atypical mycobacteria cultures. Laboratory test showed a normal blood count with associated eosinophilia (2.8 x 109 L), and normal liver and kidney function. A head ultrasound showed three ill-defined hypoechoic foci within the scalp.
The patient was admitted for treatment with broad-spectrum antibiotics and dermatology was consulted.
USPSTF releases updated recommendations on skin cancer screening
.
This final recommendation applies to the general public and is not meant for those at higher risk, such as people with a family history of skin cancer or who have any signs or symptoms, such as irregular moles.
“The new recommendations are consistent with those from 2016, and we are unable to balance benefits and harms,” said Task Force member Katrina Donahue, MD, MPH, professor and vice chair of research in the department of family medicine at the University of North Carolina, Chapel Hill. “Unfortunately, there is not enough evidence to recommend for or against screening, and health care professionals should use their judgment when deciding whether or not to screen.”
Dr. Donahue told this news organization that this is a call for more research: “Our recommendations are for patients who present to primary care without symptoms, and after a careful assessment of benefit and harms, we didn’t have evidence to push us towards screening as a benefit. We did look at data from two large screening programs, but they were from Europe and not representative of the U.S. population. They also did not show a benefit for reducing melanoma-related mortality.”
The USPSTF final recommendation statement and corresponding evidence summary have been published online in JAMA, as well as on the USPSTF website.
Skin cancer is the most commonly diagnosed cancer in the United States, but there are different types that vary in their incidence and severity. Basal and squamous cell carcinomas are the most common types of skin cancer, but they infrequently lead to death or substantial morbidity, notes the USPTSF. Melanomas represent about 1% of skin cancer and cause the most skin cancer deaths. An estimated 8,000 individuals in the United States will die of melanoma in 2023.
There are racial differences in melanoma incidence; it is about 30 times more common in White versus Black persons, but disease in persons with darker skin color tends to be diagnosed at a later stage. These disparities may be due to differences in risk factors, access to care, and clinical presentation.
In an accompanying editorial, Maryam M. Asgari, MD, MPH, of the department of dermatology, Massachusetts General Hospital, Boston, and Lori A. Crane, PhD, MPH, of the Colorado School of Public Health, University of Colorado, Aurora, point out that people with darker skin phenotypes also tend to be affected by skin cancers that are not associated with UV radiation, such as acral melanoma, which arises on the palms and soles, and skin cancers that arise in areas of chronic inflammation, such as wounds.
Thus, differences in anatomical distribution of skin cancers in in the various subpopulations needs to be considered when performing skin screening, they write. “Furthermore, while skin cancer risk is lower among people with darker skin pigmentation, survival is often worse for cancers like melanoma, highlighting the potential need for screening.”
“More data are needed, particularly regarding genetic and environmental risk factors for skin cancer in people with darker pigmentation, to help inform guidelines that can be broadly applied to the U.S. population,” add Dr. Asgari and Dr. Crane. “The diversity of the U.S. population extends also to geography, culture, and socioeconomic status, all of which affect skin cancer risk.”
Review of evidence
The USPSTF commissioned a systematic review to evaluate the benefits and harms of screening for skin cancer in asymptomatic adolescents and adults, including evidence for both keratinocyte carcinoma (basal cell carcinoma and squamous cell carcinoma) and cutaneous melanoma.
Foundational evidence showed that the sensitivity of visual skin examination by a clinician to detect melanoma ranged from 40% to 70% and specificity ranged from 86% to 98%. Evidence that evaluated the diagnostic accuracy of visual skin examination to detect keratinocyte carcinoma was limited and inconsistent. There were no new studies reporting on diagnostic accuracy for an asymptomatic screening population.
The USPSTF also reviewed 20 studies in 29 articles (n = 6,053,411). This included three nonrandomized studies evaluating two skin cancer screening programs in Germany, but results were inconsistent. In addition, the ecological and nonrandomized design of the studies limited the conclusions that could be drawn and the applicability to a U.S. population was difficult to assess because of differences in population diversity and health care delivery in the United States.
Other nonrandomized studies that looked at various outcomes, such as harms and stage at diagnosis and melanoma or all-cause mortality, also did not provide sufficient evidence to support screening.
Research is needed
In a second accompanying editorial published in JAMA Dermatology, Adewole S. Adamson, MD, MPP, of the division of dermatology and dermatologic surgery at the University of Texas, Austin, pointed out that unlike other cancer screening programs, such as those for breast, colon, and prostate cancer, skin cancer screening programs are somewhat less organized.
The other programs focus on defined groups of the population, generally with easily identifiable characteristics such as age, sex, and family history, and importantly, there are always defined ages for initiation and halting of screening and intervals for screening frequency. None of these basic screening parameters have been widely adopted among dermatologists in the United States, he wrote. “One important reason why skin cancer screening has remained inconsistent is that it is not covered by Medicare or by many commercial insurance companies,” Dr. Adamson told this news organization. “The test, in this case the skin exam, is often performed as part of a routine dermatology visit.”
Dermatologists should take the lead on this, he said. “Dermatologists should push for a high quality prospective clinical trial of skin cancer screening, preferably in a high-risk population.”
Dr. Donahue agrees that research is needed, as noted in the recommendation. For example, studies are needed demonstrating consistent data of the effects of screening on morbidity and mortality or early detection of skin cancer, and clearer descriptions of skin color and inclusion of a full spectrum of skin colors in study participants. Clinical research is also needed on outcomes in participants that reflect the diversity of the U.S. population.
“I hope funding agencies will be interested in this area of study,” she said. “We put out the whole systematic review and point out the gaps. We need consistent evidence in detecting cancer early and reducing complications from skin cancer.”
The U.S. Congress mandates that the Agency for Healthcare Research and Quality support the operations of the USPSTF.
None of the USPSTF authors report any disclosures. Dr. Asgari reported receiving royalties from UpToDate. Dr. Crane did not make any disclosures. Dr. Adamson reported serving as an expert reviewer for the U.S. Preventive Services Task Force skin cancer screening report, as well as support from the Robert Wood Johnson Foundation, the Dermatology Foundation Public Health Career Development Award, the National Institutes of Health, the American Cancer Society, and Meredith’s Mission for Melanoma.
A version of this article originally appeared on Medscape.com.
.
This final recommendation applies to the general public and is not meant for those at higher risk, such as people with a family history of skin cancer or who have any signs or symptoms, such as irregular moles.
“The new recommendations are consistent with those from 2016, and we are unable to balance benefits and harms,” said Task Force member Katrina Donahue, MD, MPH, professor and vice chair of research in the department of family medicine at the University of North Carolina, Chapel Hill. “Unfortunately, there is not enough evidence to recommend for or against screening, and health care professionals should use their judgment when deciding whether or not to screen.”
Dr. Donahue told this news organization that this is a call for more research: “Our recommendations are for patients who present to primary care without symptoms, and after a careful assessment of benefit and harms, we didn’t have evidence to push us towards screening as a benefit. We did look at data from two large screening programs, but they were from Europe and not representative of the U.S. population. They also did not show a benefit for reducing melanoma-related mortality.”
The USPSTF final recommendation statement and corresponding evidence summary have been published online in JAMA, as well as on the USPSTF website.
Skin cancer is the most commonly diagnosed cancer in the United States, but there are different types that vary in their incidence and severity. Basal and squamous cell carcinomas are the most common types of skin cancer, but they infrequently lead to death or substantial morbidity, notes the USPTSF. Melanomas represent about 1% of skin cancer and cause the most skin cancer deaths. An estimated 8,000 individuals in the United States will die of melanoma in 2023.
There are racial differences in melanoma incidence; it is about 30 times more common in White versus Black persons, but disease in persons with darker skin color tends to be diagnosed at a later stage. These disparities may be due to differences in risk factors, access to care, and clinical presentation.
In an accompanying editorial, Maryam M. Asgari, MD, MPH, of the department of dermatology, Massachusetts General Hospital, Boston, and Lori A. Crane, PhD, MPH, of the Colorado School of Public Health, University of Colorado, Aurora, point out that people with darker skin phenotypes also tend to be affected by skin cancers that are not associated with UV radiation, such as acral melanoma, which arises on the palms and soles, and skin cancers that arise in areas of chronic inflammation, such as wounds.
Thus, differences in anatomical distribution of skin cancers in in the various subpopulations needs to be considered when performing skin screening, they write. “Furthermore, while skin cancer risk is lower among people with darker skin pigmentation, survival is often worse for cancers like melanoma, highlighting the potential need for screening.”
“More data are needed, particularly regarding genetic and environmental risk factors for skin cancer in people with darker pigmentation, to help inform guidelines that can be broadly applied to the U.S. population,” add Dr. Asgari and Dr. Crane. “The diversity of the U.S. population extends also to geography, culture, and socioeconomic status, all of which affect skin cancer risk.”
Review of evidence
The USPSTF commissioned a systematic review to evaluate the benefits and harms of screening for skin cancer in asymptomatic adolescents and adults, including evidence for both keratinocyte carcinoma (basal cell carcinoma and squamous cell carcinoma) and cutaneous melanoma.
Foundational evidence showed that the sensitivity of visual skin examination by a clinician to detect melanoma ranged from 40% to 70% and specificity ranged from 86% to 98%. Evidence that evaluated the diagnostic accuracy of visual skin examination to detect keratinocyte carcinoma was limited and inconsistent. There were no new studies reporting on diagnostic accuracy for an asymptomatic screening population.
The USPSTF also reviewed 20 studies in 29 articles (n = 6,053,411). This included three nonrandomized studies evaluating two skin cancer screening programs in Germany, but results were inconsistent. In addition, the ecological and nonrandomized design of the studies limited the conclusions that could be drawn and the applicability to a U.S. population was difficult to assess because of differences in population diversity and health care delivery in the United States.
Other nonrandomized studies that looked at various outcomes, such as harms and stage at diagnosis and melanoma or all-cause mortality, also did not provide sufficient evidence to support screening.
Research is needed
In a second accompanying editorial published in JAMA Dermatology, Adewole S. Adamson, MD, MPP, of the division of dermatology and dermatologic surgery at the University of Texas, Austin, pointed out that unlike other cancer screening programs, such as those for breast, colon, and prostate cancer, skin cancer screening programs are somewhat less organized.
The other programs focus on defined groups of the population, generally with easily identifiable characteristics such as age, sex, and family history, and importantly, there are always defined ages for initiation and halting of screening and intervals for screening frequency. None of these basic screening parameters have been widely adopted among dermatologists in the United States, he wrote. “One important reason why skin cancer screening has remained inconsistent is that it is not covered by Medicare or by many commercial insurance companies,” Dr. Adamson told this news organization. “The test, in this case the skin exam, is often performed as part of a routine dermatology visit.”
Dermatologists should take the lead on this, he said. “Dermatologists should push for a high quality prospective clinical trial of skin cancer screening, preferably in a high-risk population.”
Dr. Donahue agrees that research is needed, as noted in the recommendation. For example, studies are needed demonstrating consistent data of the effects of screening on morbidity and mortality or early detection of skin cancer, and clearer descriptions of skin color and inclusion of a full spectrum of skin colors in study participants. Clinical research is also needed on outcomes in participants that reflect the diversity of the U.S. population.
“I hope funding agencies will be interested in this area of study,” she said. “We put out the whole systematic review and point out the gaps. We need consistent evidence in detecting cancer early and reducing complications from skin cancer.”
The U.S. Congress mandates that the Agency for Healthcare Research and Quality support the operations of the USPSTF.
None of the USPSTF authors report any disclosures. Dr. Asgari reported receiving royalties from UpToDate. Dr. Crane did not make any disclosures. Dr. Adamson reported serving as an expert reviewer for the U.S. Preventive Services Task Force skin cancer screening report, as well as support from the Robert Wood Johnson Foundation, the Dermatology Foundation Public Health Career Development Award, the National Institutes of Health, the American Cancer Society, and Meredith’s Mission for Melanoma.
A version of this article originally appeared on Medscape.com.
.
This final recommendation applies to the general public and is not meant for those at higher risk, such as people with a family history of skin cancer or who have any signs or symptoms, such as irregular moles.
“The new recommendations are consistent with those from 2016, and we are unable to balance benefits and harms,” said Task Force member Katrina Donahue, MD, MPH, professor and vice chair of research in the department of family medicine at the University of North Carolina, Chapel Hill. “Unfortunately, there is not enough evidence to recommend for or against screening, and health care professionals should use their judgment when deciding whether or not to screen.”
Dr. Donahue told this news organization that this is a call for more research: “Our recommendations are for patients who present to primary care without symptoms, and after a careful assessment of benefit and harms, we didn’t have evidence to push us towards screening as a benefit. We did look at data from two large screening programs, but they were from Europe and not representative of the U.S. population. They also did not show a benefit for reducing melanoma-related mortality.”
The USPSTF final recommendation statement and corresponding evidence summary have been published online in JAMA, as well as on the USPSTF website.
Skin cancer is the most commonly diagnosed cancer in the United States, but there are different types that vary in their incidence and severity. Basal and squamous cell carcinomas are the most common types of skin cancer, but they infrequently lead to death or substantial morbidity, notes the USPTSF. Melanomas represent about 1% of skin cancer and cause the most skin cancer deaths. An estimated 8,000 individuals in the United States will die of melanoma in 2023.
There are racial differences in melanoma incidence; it is about 30 times more common in White versus Black persons, but disease in persons with darker skin color tends to be diagnosed at a later stage. These disparities may be due to differences in risk factors, access to care, and clinical presentation.
In an accompanying editorial, Maryam M. Asgari, MD, MPH, of the department of dermatology, Massachusetts General Hospital, Boston, and Lori A. Crane, PhD, MPH, of the Colorado School of Public Health, University of Colorado, Aurora, point out that people with darker skin phenotypes also tend to be affected by skin cancers that are not associated with UV radiation, such as acral melanoma, which arises on the palms and soles, and skin cancers that arise in areas of chronic inflammation, such as wounds.
Thus, differences in anatomical distribution of skin cancers in in the various subpopulations needs to be considered when performing skin screening, they write. “Furthermore, while skin cancer risk is lower among people with darker skin pigmentation, survival is often worse for cancers like melanoma, highlighting the potential need for screening.”
“More data are needed, particularly regarding genetic and environmental risk factors for skin cancer in people with darker pigmentation, to help inform guidelines that can be broadly applied to the U.S. population,” add Dr. Asgari and Dr. Crane. “The diversity of the U.S. population extends also to geography, culture, and socioeconomic status, all of which affect skin cancer risk.”
Review of evidence
The USPSTF commissioned a systematic review to evaluate the benefits and harms of screening for skin cancer in asymptomatic adolescents and adults, including evidence for both keratinocyte carcinoma (basal cell carcinoma and squamous cell carcinoma) and cutaneous melanoma.
Foundational evidence showed that the sensitivity of visual skin examination by a clinician to detect melanoma ranged from 40% to 70% and specificity ranged from 86% to 98%. Evidence that evaluated the diagnostic accuracy of visual skin examination to detect keratinocyte carcinoma was limited and inconsistent. There were no new studies reporting on diagnostic accuracy for an asymptomatic screening population.
The USPSTF also reviewed 20 studies in 29 articles (n = 6,053,411). This included three nonrandomized studies evaluating two skin cancer screening programs in Germany, but results were inconsistent. In addition, the ecological and nonrandomized design of the studies limited the conclusions that could be drawn and the applicability to a U.S. population was difficult to assess because of differences in population diversity and health care delivery in the United States.
Other nonrandomized studies that looked at various outcomes, such as harms and stage at diagnosis and melanoma or all-cause mortality, also did not provide sufficient evidence to support screening.
Research is needed
In a second accompanying editorial published in JAMA Dermatology, Adewole S. Adamson, MD, MPP, of the division of dermatology and dermatologic surgery at the University of Texas, Austin, pointed out that unlike other cancer screening programs, such as those for breast, colon, and prostate cancer, skin cancer screening programs are somewhat less organized.
The other programs focus on defined groups of the population, generally with easily identifiable characteristics such as age, sex, and family history, and importantly, there are always defined ages for initiation and halting of screening and intervals for screening frequency. None of these basic screening parameters have been widely adopted among dermatologists in the United States, he wrote. “One important reason why skin cancer screening has remained inconsistent is that it is not covered by Medicare or by many commercial insurance companies,” Dr. Adamson told this news organization. “The test, in this case the skin exam, is often performed as part of a routine dermatology visit.”
Dermatologists should take the lead on this, he said. “Dermatologists should push for a high quality prospective clinical trial of skin cancer screening, preferably in a high-risk population.”
Dr. Donahue agrees that research is needed, as noted in the recommendation. For example, studies are needed demonstrating consistent data of the effects of screening on morbidity and mortality or early detection of skin cancer, and clearer descriptions of skin color and inclusion of a full spectrum of skin colors in study participants. Clinical research is also needed on outcomes in participants that reflect the diversity of the U.S. population.
“I hope funding agencies will be interested in this area of study,” she said. “We put out the whole systematic review and point out the gaps. We need consistent evidence in detecting cancer early and reducing complications from skin cancer.”
The U.S. Congress mandates that the Agency for Healthcare Research and Quality support the operations of the USPSTF.
None of the USPSTF authors report any disclosures. Dr. Asgari reported receiving royalties from UpToDate. Dr. Crane did not make any disclosures. Dr. Adamson reported serving as an expert reviewer for the U.S. Preventive Services Task Force skin cancer screening report, as well as support from the Robert Wood Johnson Foundation, the Dermatology Foundation Public Health Career Development Award, the National Institutes of Health, the American Cancer Society, and Meredith’s Mission for Melanoma.
A version of this article originally appeared on Medscape.com.
Study compares noninvasive treatments of cutaneous neurofibromas
PHOENIX – after only one treatment, according to preliminary results of an ongoing prospective trial that compared several treatment modalities.
“Neurofibromatosis type 1 is the most common single-gene disease of mankind, but there is so much we have yet to learn about it,” study author Patricia Richey, MD, who practices Mohs surgery and cosmetic dermatology in Washington, D.C., said in an interview in advance of the annual conference of the American Society for Laser Medicine and Surgery, where she presented the results during an abstract session. Dr. Richey also conducts research for the Wellman Center for Photomedicine and the Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston, and is working with R. Rox Anderson, MD, director of the Wellman Center, on this project. In his words, she said, “the lack of better treatments for cNF is a ‘problem worth solving.’ ”
“The accepted and widely available treatments for cNF result in scars and hypopigmentation. Our treatments do not,” she added. Since the epidermis overlying cNF is normal, “there is no reason to use nonselective or surgical methods and destroy a perfectly good epidermis when you don’t need to.”
Four treatments vs. controls
For the study, Dr. Richey and colleagues enrolled 19 adults with a total of 307 cNFs measuring 2-4 mm in size to receive one of four treatments: electrocautery with an insulated radiofrequency needle; 755-nm alexandrite laser with negative pressure (8-mm spot size, 100 J/cm2 fluence, 3-ms pulse duration); 980-nm diode laser (delivered via 8-mm sapphire skin-contact window), and intratumoral injection of 10 mg/mL deoxycholic acid at a volume approximately equal to that of the tumor. The average age of the participants was 49 years and 15 were female.
The investigators applied 5% lidocaine/prilocaine for 40 minutes to treatment sites before randomizing the tumors to treatment or to the control arm (no treatment). They compared safety, tolerability (including pain scores), and efficacy of each modality as measured by the change in cNF volume/height via three-dimensional imaging and clinical improvement via physician assessment at 6 months. All 19 participants have completed the 6-month assessment.
All modalities reduced or eliminated some of the cNFs by 6 months after treatment, with statistically significant reductions in height and volume across all four treatments. A wide variation of responses was observed. Specifically, the mean tumor volume changes for each modality, compared with controls, were –33.4% versus –5.1% among those treated with the 755-nm alexandrite laser; –24.9% versus –9.2% among those treated with the 980-nm diode laser, –23.3% versus –0.8% among those treated with insulated-needle radiofrequency coagulation, and –29.4% versus –3.7% among those treated with deoxycholic acid.
The variation in responses “may be due to histologic diversity of cNF or may indicate a need for more fine-tuned dosimetry, or a combination,” Dr. Richey said. “Our future trials will address this. We will also be treating all skin types in our upcoming trials.”
No adverse events categorized as higher than grade 2 occurred in any of the treatment groups, and no signs of regrowth or growth stimulation have been observed to date.
Tolerability of treatments
As for general tolerability, the 980-nm laser treatment caused moderate to severe pain; the alexandrite laser caused mild pain; insulated-needle radiofrequency coagulation caused mild pain, though more than deoxycholic acid injections or alexandrite laser, and pain associated with the deoxycholic acid injections was minimal.
When residual neurofibroma tumor was present histologically, its appearance was similar to that of untreated tumors in controls. There was no evidence of atypia, mitosis, or tumor inflammation, and mild fibrosis was present at the sites of prior tumor.
“It was surprising that all four modalities did work to some extent,” Dr. Richey said, noting that the lack of ulceration with deoxycholic acid injection “was pleasantly surprising.” Treatment with the 980-nm diode laser “was a bit more painful than we anticipated.”
The positive results of this trial has raised “more questions for us to answer. We have three additional trials in the works to fine tune these treatments and optimize dose/delivery, with the end goal of treating younger people.”
Dr. Richey said that she was “amazed” by how motivated the enrollees were to participate in the trial, noting that many patients with cNF undergo general anesthesia to have dozens of tumors surgically removed at once. “They pay $10,000-$20,000 on average out of pocket, as this surgery is considered cosmetic,” she said.
“This very important study could lead to effective, relatively noninvasive, therapy for small neurofibromas,” said Jeffrey S. Dover, MD, codirector of SkinCare Physicians in Chestnut Hill, Mass., who was not involved with the study and was asked to comment on the results.
“Remarkably, all four treatments worked to varying degrees, but of all the treatments, the selective alexandrite laser appeared to achieve the best results. Further study will be needed to see just how effective these treatments are, and to determine the best and safest treatment parameters. Given how common this autosomal dominant disease is, and how disfiguring neurofibromas become as they enlarge, a well-tolerated noninvasive nonsurgical treatment with limited side effects is highly sought after.”
The study, which was named the best clinical abstract at the meeting, was supported by the Neurofibromatosis Therapeutic Acceleration Program. Dr. Anderson is supported in part as the Lancer Endowed Chair in Dermatology at MGH. Dr. Dover reported having no relevant disclosures.
PHOENIX – after only one treatment, according to preliminary results of an ongoing prospective trial that compared several treatment modalities.
“Neurofibromatosis type 1 is the most common single-gene disease of mankind, but there is so much we have yet to learn about it,” study author Patricia Richey, MD, who practices Mohs surgery and cosmetic dermatology in Washington, D.C., said in an interview in advance of the annual conference of the American Society for Laser Medicine and Surgery, where she presented the results during an abstract session. Dr. Richey also conducts research for the Wellman Center for Photomedicine and the Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston, and is working with R. Rox Anderson, MD, director of the Wellman Center, on this project. In his words, she said, “the lack of better treatments for cNF is a ‘problem worth solving.’ ”
“The accepted and widely available treatments for cNF result in scars and hypopigmentation. Our treatments do not,” she added. Since the epidermis overlying cNF is normal, “there is no reason to use nonselective or surgical methods and destroy a perfectly good epidermis when you don’t need to.”
Four treatments vs. controls
For the study, Dr. Richey and colleagues enrolled 19 adults with a total of 307 cNFs measuring 2-4 mm in size to receive one of four treatments: electrocautery with an insulated radiofrequency needle; 755-nm alexandrite laser with negative pressure (8-mm spot size, 100 J/cm2 fluence, 3-ms pulse duration); 980-nm diode laser (delivered via 8-mm sapphire skin-contact window), and intratumoral injection of 10 mg/mL deoxycholic acid at a volume approximately equal to that of the tumor. The average age of the participants was 49 years and 15 were female.
The investigators applied 5% lidocaine/prilocaine for 40 minutes to treatment sites before randomizing the tumors to treatment or to the control arm (no treatment). They compared safety, tolerability (including pain scores), and efficacy of each modality as measured by the change in cNF volume/height via three-dimensional imaging and clinical improvement via physician assessment at 6 months. All 19 participants have completed the 6-month assessment.
All modalities reduced or eliminated some of the cNFs by 6 months after treatment, with statistically significant reductions in height and volume across all four treatments. A wide variation of responses was observed. Specifically, the mean tumor volume changes for each modality, compared with controls, were –33.4% versus –5.1% among those treated with the 755-nm alexandrite laser; –24.9% versus –9.2% among those treated with the 980-nm diode laser, –23.3% versus –0.8% among those treated with insulated-needle radiofrequency coagulation, and –29.4% versus –3.7% among those treated with deoxycholic acid.
The variation in responses “may be due to histologic diversity of cNF or may indicate a need for more fine-tuned dosimetry, or a combination,” Dr. Richey said. “Our future trials will address this. We will also be treating all skin types in our upcoming trials.”
No adverse events categorized as higher than grade 2 occurred in any of the treatment groups, and no signs of regrowth or growth stimulation have been observed to date.
Tolerability of treatments
As for general tolerability, the 980-nm laser treatment caused moderate to severe pain; the alexandrite laser caused mild pain; insulated-needle radiofrequency coagulation caused mild pain, though more than deoxycholic acid injections or alexandrite laser, and pain associated with the deoxycholic acid injections was minimal.
When residual neurofibroma tumor was present histologically, its appearance was similar to that of untreated tumors in controls. There was no evidence of atypia, mitosis, or tumor inflammation, and mild fibrosis was present at the sites of prior tumor.
“It was surprising that all four modalities did work to some extent,” Dr. Richey said, noting that the lack of ulceration with deoxycholic acid injection “was pleasantly surprising.” Treatment with the 980-nm diode laser “was a bit more painful than we anticipated.”
The positive results of this trial has raised “more questions for us to answer. We have three additional trials in the works to fine tune these treatments and optimize dose/delivery, with the end goal of treating younger people.”
Dr. Richey said that she was “amazed” by how motivated the enrollees were to participate in the trial, noting that many patients with cNF undergo general anesthesia to have dozens of tumors surgically removed at once. “They pay $10,000-$20,000 on average out of pocket, as this surgery is considered cosmetic,” she said.
“This very important study could lead to effective, relatively noninvasive, therapy for small neurofibromas,” said Jeffrey S. Dover, MD, codirector of SkinCare Physicians in Chestnut Hill, Mass., who was not involved with the study and was asked to comment on the results.
“Remarkably, all four treatments worked to varying degrees, but of all the treatments, the selective alexandrite laser appeared to achieve the best results. Further study will be needed to see just how effective these treatments are, and to determine the best and safest treatment parameters. Given how common this autosomal dominant disease is, and how disfiguring neurofibromas become as they enlarge, a well-tolerated noninvasive nonsurgical treatment with limited side effects is highly sought after.”
The study, which was named the best clinical abstract at the meeting, was supported by the Neurofibromatosis Therapeutic Acceleration Program. Dr. Anderson is supported in part as the Lancer Endowed Chair in Dermatology at MGH. Dr. Dover reported having no relevant disclosures.
PHOENIX – after only one treatment, according to preliminary results of an ongoing prospective trial that compared several treatment modalities.
“Neurofibromatosis type 1 is the most common single-gene disease of mankind, but there is so much we have yet to learn about it,” study author Patricia Richey, MD, who practices Mohs surgery and cosmetic dermatology in Washington, D.C., said in an interview in advance of the annual conference of the American Society for Laser Medicine and Surgery, where she presented the results during an abstract session. Dr. Richey also conducts research for the Wellman Center for Photomedicine and the Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston, and is working with R. Rox Anderson, MD, director of the Wellman Center, on this project. In his words, she said, “the lack of better treatments for cNF is a ‘problem worth solving.’ ”
“The accepted and widely available treatments for cNF result in scars and hypopigmentation. Our treatments do not,” she added. Since the epidermis overlying cNF is normal, “there is no reason to use nonselective or surgical methods and destroy a perfectly good epidermis when you don’t need to.”
Four treatments vs. controls
For the study, Dr. Richey and colleagues enrolled 19 adults with a total of 307 cNFs measuring 2-4 mm in size to receive one of four treatments: electrocautery with an insulated radiofrequency needle; 755-nm alexandrite laser with negative pressure (8-mm spot size, 100 J/cm2 fluence, 3-ms pulse duration); 980-nm diode laser (delivered via 8-mm sapphire skin-contact window), and intratumoral injection of 10 mg/mL deoxycholic acid at a volume approximately equal to that of the tumor. The average age of the participants was 49 years and 15 were female.
The investigators applied 5% lidocaine/prilocaine for 40 minutes to treatment sites before randomizing the tumors to treatment or to the control arm (no treatment). They compared safety, tolerability (including pain scores), and efficacy of each modality as measured by the change in cNF volume/height via three-dimensional imaging and clinical improvement via physician assessment at 6 months. All 19 participants have completed the 6-month assessment.
All modalities reduced or eliminated some of the cNFs by 6 months after treatment, with statistically significant reductions in height and volume across all four treatments. A wide variation of responses was observed. Specifically, the mean tumor volume changes for each modality, compared with controls, were –33.4% versus –5.1% among those treated with the 755-nm alexandrite laser; –24.9% versus –9.2% among those treated with the 980-nm diode laser, –23.3% versus –0.8% among those treated with insulated-needle radiofrequency coagulation, and –29.4% versus –3.7% among those treated with deoxycholic acid.
The variation in responses “may be due to histologic diversity of cNF or may indicate a need for more fine-tuned dosimetry, or a combination,” Dr. Richey said. “Our future trials will address this. We will also be treating all skin types in our upcoming trials.”
No adverse events categorized as higher than grade 2 occurred in any of the treatment groups, and no signs of regrowth or growth stimulation have been observed to date.
Tolerability of treatments
As for general tolerability, the 980-nm laser treatment caused moderate to severe pain; the alexandrite laser caused mild pain; insulated-needle radiofrequency coagulation caused mild pain, though more than deoxycholic acid injections or alexandrite laser, and pain associated with the deoxycholic acid injections was minimal.
When residual neurofibroma tumor was present histologically, its appearance was similar to that of untreated tumors in controls. There was no evidence of atypia, mitosis, or tumor inflammation, and mild fibrosis was present at the sites of prior tumor.
“It was surprising that all four modalities did work to some extent,” Dr. Richey said, noting that the lack of ulceration with deoxycholic acid injection “was pleasantly surprising.” Treatment with the 980-nm diode laser “was a bit more painful than we anticipated.”
The positive results of this trial has raised “more questions for us to answer. We have three additional trials in the works to fine tune these treatments and optimize dose/delivery, with the end goal of treating younger people.”
Dr. Richey said that she was “amazed” by how motivated the enrollees were to participate in the trial, noting that many patients with cNF undergo general anesthesia to have dozens of tumors surgically removed at once. “They pay $10,000-$20,000 on average out of pocket, as this surgery is considered cosmetic,” she said.
“This very important study could lead to effective, relatively noninvasive, therapy for small neurofibromas,” said Jeffrey S. Dover, MD, codirector of SkinCare Physicians in Chestnut Hill, Mass., who was not involved with the study and was asked to comment on the results.
“Remarkably, all four treatments worked to varying degrees, but of all the treatments, the selective alexandrite laser appeared to achieve the best results. Further study will be needed to see just how effective these treatments are, and to determine the best and safest treatment parameters. Given how common this autosomal dominant disease is, and how disfiguring neurofibromas become as they enlarge, a well-tolerated noninvasive nonsurgical treatment with limited side effects is highly sought after.”
The study, which was named the best clinical abstract at the meeting, was supported by the Neurofibromatosis Therapeutic Acceleration Program. Dr. Anderson is supported in part as the Lancer Endowed Chair in Dermatology at MGH. Dr. Dover reported having no relevant disclosures.
AT ASLMS 2023
Study suggests narrow excision margins safe in early melanoma resection
Current U.S., European, and Australian or melanoma-specific mortality (MSM), results of a retrospective study suggest.
Among 1,179 patients with stage T1a melanomas near the face, scalp, external genitalia, or other critical areas, the weighted 10-year local recurrence rate for patients who underwent resection with 10-mm margins was 5.7%, compared with 6.7% for those who had resections with 5-mm margins, a nonsignificant difference.
Weighted 10-year melanoma-specific mortality was 1.8% for patients treated with wide margins, vs. 4.2% for those treated with narrow margins, also a nonsignificant difference. Patients treated with narrow margins did have significantly fewer reconstructive surgeries than patients treated with wide margins, reported Andrea Maurichi, MD, and colleagues at the National Cancer Institute of Italy in Milan.
“Because this association was found in melanomas of the head and neck, acral, and genital sites, there is no plausible reason why it could not be extrapolated to other locations. The findings also support the need for prospective randomized clinical trials to definitively answer the important question about appropriate excision margins for T1a melanoma,” they wrote in the study, published online in JAMA Dermatology.
The authors also found, however, that Breslow thickness greater than 0.4 mm and mitotic rate greater than 1/mm2 were associated with worse MSM, and that acral lentiginous melanoma, lentigo maligna melanoma, and increasing Breslow thickness were associated with a higher incidence of local recurrence.
A melanoma expert who was not involved in the study said that despite these findings, wider margins are always preferable.
“There is always a conversation around these general [critical] areas, but as a rule we try to get larger margins,” said Ryan J. Sullivan, MD, of Mass General Cancer Center in Boston.
In an interview, Dr. Sullivan said that the finding about lower frequency of reconstructive procedures in the narrow margins groups may be more of a concern for younger patients than for the elderly.
Study design
The investigators conducted a retrospective cohort study of consecutive patients aged 18 or older at the National Cancer Institute of Milan who were diagnosed with T1a cutaneous melanoma close to critical areas from 2001 through 2020.
Patients with primary cutaneous melanoma of the head and face areas with functional or cosmetic considerations, acral areas (plantar, palmar, digital and interdigital areas), external genitalia, or periumbilical and perineal areas were eligible for inclusion.
The cohort comprised 1,179 patients with a median age of 50 and equal sex distribution. Of these patients, 626 (53%) had a wide excision, of whom 434 had a linear repair, and 192 had a flap of graft reconstruction. The remaining 553 patients had narrow excisions, 491 with linear repair, and 62 with flap or graft reconstruction.
Analyses were adjusted to account for imbalances between the surgical groups.
The study was supported by the nonprofit foundation Emme Rouge. The authors and Dr. Sullivan reported having no relevant conflicts of interest to disclose.
Current U.S., European, and Australian or melanoma-specific mortality (MSM), results of a retrospective study suggest.
Among 1,179 patients with stage T1a melanomas near the face, scalp, external genitalia, or other critical areas, the weighted 10-year local recurrence rate for patients who underwent resection with 10-mm margins was 5.7%, compared with 6.7% for those who had resections with 5-mm margins, a nonsignificant difference.
Weighted 10-year melanoma-specific mortality was 1.8% for patients treated with wide margins, vs. 4.2% for those treated with narrow margins, also a nonsignificant difference. Patients treated with narrow margins did have significantly fewer reconstructive surgeries than patients treated with wide margins, reported Andrea Maurichi, MD, and colleagues at the National Cancer Institute of Italy in Milan.
“Because this association was found in melanomas of the head and neck, acral, and genital sites, there is no plausible reason why it could not be extrapolated to other locations. The findings also support the need for prospective randomized clinical trials to definitively answer the important question about appropriate excision margins for T1a melanoma,” they wrote in the study, published online in JAMA Dermatology.
The authors also found, however, that Breslow thickness greater than 0.4 mm and mitotic rate greater than 1/mm2 were associated with worse MSM, and that acral lentiginous melanoma, lentigo maligna melanoma, and increasing Breslow thickness were associated with a higher incidence of local recurrence.
A melanoma expert who was not involved in the study said that despite these findings, wider margins are always preferable.
“There is always a conversation around these general [critical] areas, but as a rule we try to get larger margins,” said Ryan J. Sullivan, MD, of Mass General Cancer Center in Boston.
In an interview, Dr. Sullivan said that the finding about lower frequency of reconstructive procedures in the narrow margins groups may be more of a concern for younger patients than for the elderly.
Study design
The investigators conducted a retrospective cohort study of consecutive patients aged 18 or older at the National Cancer Institute of Milan who were diagnosed with T1a cutaneous melanoma close to critical areas from 2001 through 2020.
Patients with primary cutaneous melanoma of the head and face areas with functional or cosmetic considerations, acral areas (plantar, palmar, digital and interdigital areas), external genitalia, or periumbilical and perineal areas were eligible for inclusion.
The cohort comprised 1,179 patients with a median age of 50 and equal sex distribution. Of these patients, 626 (53%) had a wide excision, of whom 434 had a linear repair, and 192 had a flap of graft reconstruction. The remaining 553 patients had narrow excisions, 491 with linear repair, and 62 with flap or graft reconstruction.
Analyses were adjusted to account for imbalances between the surgical groups.
The study was supported by the nonprofit foundation Emme Rouge. The authors and Dr. Sullivan reported having no relevant conflicts of interest to disclose.
Current U.S., European, and Australian or melanoma-specific mortality (MSM), results of a retrospective study suggest.
Among 1,179 patients with stage T1a melanomas near the face, scalp, external genitalia, or other critical areas, the weighted 10-year local recurrence rate for patients who underwent resection with 10-mm margins was 5.7%, compared with 6.7% for those who had resections with 5-mm margins, a nonsignificant difference.
Weighted 10-year melanoma-specific mortality was 1.8% for patients treated with wide margins, vs. 4.2% for those treated with narrow margins, also a nonsignificant difference. Patients treated with narrow margins did have significantly fewer reconstructive surgeries than patients treated with wide margins, reported Andrea Maurichi, MD, and colleagues at the National Cancer Institute of Italy in Milan.
“Because this association was found in melanomas of the head and neck, acral, and genital sites, there is no plausible reason why it could not be extrapolated to other locations. The findings also support the need for prospective randomized clinical trials to definitively answer the important question about appropriate excision margins for T1a melanoma,” they wrote in the study, published online in JAMA Dermatology.
The authors also found, however, that Breslow thickness greater than 0.4 mm and mitotic rate greater than 1/mm2 were associated with worse MSM, and that acral lentiginous melanoma, lentigo maligna melanoma, and increasing Breslow thickness were associated with a higher incidence of local recurrence.
A melanoma expert who was not involved in the study said that despite these findings, wider margins are always preferable.
“There is always a conversation around these general [critical] areas, but as a rule we try to get larger margins,” said Ryan J. Sullivan, MD, of Mass General Cancer Center in Boston.
In an interview, Dr. Sullivan said that the finding about lower frequency of reconstructive procedures in the narrow margins groups may be more of a concern for younger patients than for the elderly.
Study design
The investigators conducted a retrospective cohort study of consecutive patients aged 18 or older at the National Cancer Institute of Milan who were diagnosed with T1a cutaneous melanoma close to critical areas from 2001 through 2020.
Patients with primary cutaneous melanoma of the head and face areas with functional or cosmetic considerations, acral areas (plantar, palmar, digital and interdigital areas), external genitalia, or periumbilical and perineal areas were eligible for inclusion.
The cohort comprised 1,179 patients with a median age of 50 and equal sex distribution. Of these patients, 626 (53%) had a wide excision, of whom 434 had a linear repair, and 192 had a flap of graft reconstruction. The remaining 553 patients had narrow excisions, 491 with linear repair, and 62 with flap or graft reconstruction.
Analyses were adjusted to account for imbalances between the surgical groups.
The study was supported by the nonprofit foundation Emme Rouge. The authors and Dr. Sullivan reported having no relevant conflicts of interest to disclose.
FROM JAMA DERMATOLOGY
What happens to melanocytic nevi during laser hair removal?
PHOENIX – , while common histologic changes include mild atypia and thermal damage, according to results from a systematic review of literature on the topic. To date, no severe cases of severe dysplasia or melanoma have been reported.
“That’s reassuring,” study author Ahuva Cices, MD, said in an interview at the annual conference of the American Society for Laser Medicine and Surgery, where she presented the results during an abstract session. “But, with that in mind, we want to avoid treating nevi with laser hair removal to avoid changes that could be concerning. We also recommend baseline skin exams so we know what we’re looking at before we start treating with lasers, and any changes can be recognized from that baseline status. It’s important to keep an eye out for changes and always be evaluating.”
In December of 2022, Dr. Cices, chief dermatology resident at Mount Sinai Health System, New York, searched PubMed for articles that evaluated changes in melanocytic nevi after laser hair removal procedures. She used the search terms “nevi laser hair removal,” “nevi diode,” “nevi long pulse alexandrite,” “nevi long pulse neodymium doped yttrium aluminum garnet,” and “melanoma laser hair removal,” and limited the analysis to English language patient-based reports that discussed incidental treatment of melanocytic nevi while undergoing hair removal with a laser.
Reports excluded from the analysis were those that focused on changes following hair removal with nonlaser devices such as intense pulsed light (IPL), those evaluating nonmelanocytic nevi such as Becker’s nevus or nevus of Ota, and those evaluating the intentional ablation or removal of melanocytic lesions.
The search yielded 10 relevant studies for systematic review: seven case reports or series and three observational trials, two of which were prospective and one retrospective.
The results of the review, according to Dr. Cices, revealed that clinical and dermoscopic changes were noted to present as early as 15 days after treatment and persist to the maximum follow up time, at 3 years. Commonly reported changes included regression, decreased size, laser-induced asymmetry, bleaching, darkening, and altered pattern on dermoscopy. Histologic changes included mild atypia, thermal damage, scar formation, and regression.
“Although some of the clinical and dermoscopic alterations may be concerning for malignancy, to our knowledge, there are no documented cases of malignant transformation of nevi following treatment with laser hair removal,” she wrote in the abstract.
Dr. Cices acknowledged certain limitations of the systematic review, including the low number of relevant reports and their generally small sample size, many of which were limited to single cases.
Omar A. Ibrahimi, MD, PhD, medical director of the Connecticut Skin Institute, Stamford, who was asked to comment on the review, characterized the findings as important because laser hair removal is such a commonly performed procedure.
While the study is limited by the small number of studies on the subject matter, “it brings up an important discussion,” Dr. Ibrahimi said in an interview. “Generally speaking, we know that most hair removal lasers do indeed target melanin pigment and can be absorbed by melanocytes. While the wavelengths used for LHR [laser hair removal] will not result in DNA damage or cause mutations that can lead to melanoma, they can sometimes alter the appearance of pigmented lesions and that may change the dermatologist’s ability to monitor them for atypia,” he noted.
“For that reason, I would recommend all patients see a dermatologist for evaluation of their nevi prior to any treatments and they consider very carefully where they get their laser treatments. If they have any atypical pigmented lesions, then that information should be disclosed with the person performing the laser hair removal procedure particularly if there are lesions that are being specifically monitored.”
Dr. Cices reported having no disclosures. Dr. Ibrahimi disclosed that he is a member of the advisory board for Accure Acne, AbbVie, Cutera, Lutronic, Blueberry Therapeutics, Cytrellis, and Quthero. He also holds stock in many device and pharmaceutical companies.
PHOENIX – , while common histologic changes include mild atypia and thermal damage, according to results from a systematic review of literature on the topic. To date, no severe cases of severe dysplasia or melanoma have been reported.
“That’s reassuring,” study author Ahuva Cices, MD, said in an interview at the annual conference of the American Society for Laser Medicine and Surgery, where she presented the results during an abstract session. “But, with that in mind, we want to avoid treating nevi with laser hair removal to avoid changes that could be concerning. We also recommend baseline skin exams so we know what we’re looking at before we start treating with lasers, and any changes can be recognized from that baseline status. It’s important to keep an eye out for changes and always be evaluating.”
In December of 2022, Dr. Cices, chief dermatology resident at Mount Sinai Health System, New York, searched PubMed for articles that evaluated changes in melanocytic nevi after laser hair removal procedures. She used the search terms “nevi laser hair removal,” “nevi diode,” “nevi long pulse alexandrite,” “nevi long pulse neodymium doped yttrium aluminum garnet,” and “melanoma laser hair removal,” and limited the analysis to English language patient-based reports that discussed incidental treatment of melanocytic nevi while undergoing hair removal with a laser.
Reports excluded from the analysis were those that focused on changes following hair removal with nonlaser devices such as intense pulsed light (IPL), those evaluating nonmelanocytic nevi such as Becker’s nevus or nevus of Ota, and those evaluating the intentional ablation or removal of melanocytic lesions.
The search yielded 10 relevant studies for systematic review: seven case reports or series and three observational trials, two of which were prospective and one retrospective.
The results of the review, according to Dr. Cices, revealed that clinical and dermoscopic changes were noted to present as early as 15 days after treatment and persist to the maximum follow up time, at 3 years. Commonly reported changes included regression, decreased size, laser-induced asymmetry, bleaching, darkening, and altered pattern on dermoscopy. Histologic changes included mild atypia, thermal damage, scar formation, and regression.
“Although some of the clinical and dermoscopic alterations may be concerning for malignancy, to our knowledge, there are no documented cases of malignant transformation of nevi following treatment with laser hair removal,” she wrote in the abstract.
Dr. Cices acknowledged certain limitations of the systematic review, including the low number of relevant reports and their generally small sample size, many of which were limited to single cases.
Omar A. Ibrahimi, MD, PhD, medical director of the Connecticut Skin Institute, Stamford, who was asked to comment on the review, characterized the findings as important because laser hair removal is such a commonly performed procedure.
While the study is limited by the small number of studies on the subject matter, “it brings up an important discussion,” Dr. Ibrahimi said in an interview. “Generally speaking, we know that most hair removal lasers do indeed target melanin pigment and can be absorbed by melanocytes. While the wavelengths used for LHR [laser hair removal] will not result in DNA damage or cause mutations that can lead to melanoma, they can sometimes alter the appearance of pigmented lesions and that may change the dermatologist’s ability to monitor them for atypia,” he noted.
“For that reason, I would recommend all patients see a dermatologist for evaluation of their nevi prior to any treatments and they consider very carefully where they get their laser treatments. If they have any atypical pigmented lesions, then that information should be disclosed with the person performing the laser hair removal procedure particularly if there are lesions that are being specifically monitored.”
Dr. Cices reported having no disclosures. Dr. Ibrahimi disclosed that he is a member of the advisory board for Accure Acne, AbbVie, Cutera, Lutronic, Blueberry Therapeutics, Cytrellis, and Quthero. He also holds stock in many device and pharmaceutical companies.
PHOENIX – , while common histologic changes include mild atypia and thermal damage, according to results from a systematic review of literature on the topic. To date, no severe cases of severe dysplasia or melanoma have been reported.
“That’s reassuring,” study author Ahuva Cices, MD, said in an interview at the annual conference of the American Society for Laser Medicine and Surgery, where she presented the results during an abstract session. “But, with that in mind, we want to avoid treating nevi with laser hair removal to avoid changes that could be concerning. We also recommend baseline skin exams so we know what we’re looking at before we start treating with lasers, and any changes can be recognized from that baseline status. It’s important to keep an eye out for changes and always be evaluating.”
In December of 2022, Dr. Cices, chief dermatology resident at Mount Sinai Health System, New York, searched PubMed for articles that evaluated changes in melanocytic nevi after laser hair removal procedures. She used the search terms “nevi laser hair removal,” “nevi diode,” “nevi long pulse alexandrite,” “nevi long pulse neodymium doped yttrium aluminum garnet,” and “melanoma laser hair removal,” and limited the analysis to English language patient-based reports that discussed incidental treatment of melanocytic nevi while undergoing hair removal with a laser.
Reports excluded from the analysis were those that focused on changes following hair removal with nonlaser devices such as intense pulsed light (IPL), those evaluating nonmelanocytic nevi such as Becker’s nevus or nevus of Ota, and those evaluating the intentional ablation or removal of melanocytic lesions.
The search yielded 10 relevant studies for systematic review: seven case reports or series and three observational trials, two of which were prospective and one retrospective.
The results of the review, according to Dr. Cices, revealed that clinical and dermoscopic changes were noted to present as early as 15 days after treatment and persist to the maximum follow up time, at 3 years. Commonly reported changes included regression, decreased size, laser-induced asymmetry, bleaching, darkening, and altered pattern on dermoscopy. Histologic changes included mild atypia, thermal damage, scar formation, and regression.
“Although some of the clinical and dermoscopic alterations may be concerning for malignancy, to our knowledge, there are no documented cases of malignant transformation of nevi following treatment with laser hair removal,” she wrote in the abstract.
Dr. Cices acknowledged certain limitations of the systematic review, including the low number of relevant reports and their generally small sample size, many of which were limited to single cases.
Omar A. Ibrahimi, MD, PhD, medical director of the Connecticut Skin Institute, Stamford, who was asked to comment on the review, characterized the findings as important because laser hair removal is such a commonly performed procedure.
While the study is limited by the small number of studies on the subject matter, “it brings up an important discussion,” Dr. Ibrahimi said in an interview. “Generally speaking, we know that most hair removal lasers do indeed target melanin pigment and can be absorbed by melanocytes. While the wavelengths used for LHR [laser hair removal] will not result in DNA damage or cause mutations that can lead to melanoma, they can sometimes alter the appearance of pigmented lesions and that may change the dermatologist’s ability to monitor them for atypia,” he noted.
“For that reason, I would recommend all patients see a dermatologist for evaluation of their nevi prior to any treatments and they consider very carefully where they get their laser treatments. If they have any atypical pigmented lesions, then that information should be disclosed with the person performing the laser hair removal procedure particularly if there are lesions that are being specifically monitored.”
Dr. Cices reported having no disclosures. Dr. Ibrahimi disclosed that he is a member of the advisory board for Accure Acne, AbbVie, Cutera, Lutronic, Blueberry Therapeutics, Cytrellis, and Quthero. He also holds stock in many device and pharmaceutical companies.
AT ASLMS 2023
Alzheimer’s drug may ease hair pulling, skin-picking disorders
Results from the double-blind, placebo-controlled trial showed that 61% of participants who received memantine were “much or very much improved,” versus 8% in the placebo group.
“Memantine was far more effective than placebo,” lead investigator Jon Grant, MD, MPH, professor of psychiatry and behavioral neuroscience at the University of Chicago, said in an interview. “However, while subjects responded favorably, that didn’t necessarily mean there were no symptoms.”
The study was published online in the American Journal of Psychiatry.
Underrecognized, disabling
The investigators noted that trichotillomania and skin-picking disorder are underrecognized and are often disabling conditions. However, the researchers pointed out that with prevalence rates of 1.7% for trichotillomania and 2.1% for skin-picking disorder, they are not uncommon.
Behavioral therapy that attempts to reverse these habits is considered first-line treatment, but trained therapists are difficult to find. In addition, the investigators wrote that currently, there are no Food and Drug Administration–approved medications for either disorder, and pharmacologic clinical trials are relatively uncommon.
The existing data from double-blind, placebo-controlled studies support the use of the antipsychotic olanzapine, the tricyclic antidepressant clomipramine, and the supplement N-acetyl-L-cysteine (NAC). Dr. Grant also noted that previous drug trials involving patients with trichotillomania have been very short in duration.
Prior research has implicated the glutamate system in repetitive motor habits and the urges that drive them. Memantine, a glutamate receptor antagonist, targets excessive glutamatergic drive. To investigate whether this medication may be beneficial for patients with trichotillomania and skin-picking disorders, the investigators conducted a randomized placebo-controlled trial.
The study included 100 adults (86 women; mean age, 31.4) with trichotillomania, skin-picking disorder, or both; participants received memantine (n = 55) or placebo (n = 45) for 8 weeks; they received memantine 10 mg or placebo for the first 2 weeks, then 20 mg for the next 6 weeks.
The researchers, who were blinded to assignment, assessed participants every 2 weeks using the National Institute of Mental Health Trichotillomania Symptom Severity Scale, which was modified to include questions for skin-picking disorder.
The team also tracked symptoms and behaviors using additional scales, including the Sheehan Disability Scale and the Clinical Global Impressions severity scale.
At the study’s conclusion, 79 patients remained. Of those, 26 of the 43 participants in the memantine group were “very much” or “much” improved (61%), versus 3 of 36 (8%) in the placebo group. (P < .0001)
Six participants in the memantine group experienced complete remission of symptoms, compared with one in the placebo group. There were no differences between the study groups in terms of adverse events.
Study limitations included the relatively short length of the trial for what should be considered a chronic disease, as well as the inclusion of only mildly to moderately symptomatic participants.
Dr. Grant said that he would like to study how memantine works in combination with behavioral therapy.
‘Two great options’
Katharine Phillips, MD, professor of psychiatry at Weill Cornell Medicine, New York, said she has been using memantine for “quite some time” to treat her patients with skin-picking disorder, adding that she uses higher doses of the drug than were tested in the study.
She noted that both NAC and memantine affect glutamate, an amino acid in the brain that is likely involved in repetitive physical or motor habits, such as hair pulling and skin picking.
“The good news is that we have two great options” for the treatment of trichotillomania and skin-picking disorder, said Dr. Phillips, and that both are easy to tolerate.
Future research should focus on longer trials of memantine and at higher doses, as well as other glutamate modulators, she said.
The study was funded by departmental research funds at the University of Chicago. Dr. Grant reported receiving research funding from Biohaven Pharmaceuticals and Janssen, as well as yearly compensation from Springer Publishing for his role as editor-in-chief of the Journal of Gambling Studies. He has also received royalties from American Psychiatric Publishing, McGraw Hill, Oxford University Press, and WW Norton. Dr. Phillips reported receiving royalties from American Psychiatric Publishing and an honorarium from the Merck Manual.
A version of this article first appeared on Medscape.com.
Results from the double-blind, placebo-controlled trial showed that 61% of participants who received memantine were “much or very much improved,” versus 8% in the placebo group.
“Memantine was far more effective than placebo,” lead investigator Jon Grant, MD, MPH, professor of psychiatry and behavioral neuroscience at the University of Chicago, said in an interview. “However, while subjects responded favorably, that didn’t necessarily mean there were no symptoms.”
The study was published online in the American Journal of Psychiatry.
Underrecognized, disabling
The investigators noted that trichotillomania and skin-picking disorder are underrecognized and are often disabling conditions. However, the researchers pointed out that with prevalence rates of 1.7% for trichotillomania and 2.1% for skin-picking disorder, they are not uncommon.
Behavioral therapy that attempts to reverse these habits is considered first-line treatment, but trained therapists are difficult to find. In addition, the investigators wrote that currently, there are no Food and Drug Administration–approved medications for either disorder, and pharmacologic clinical trials are relatively uncommon.
The existing data from double-blind, placebo-controlled studies support the use of the antipsychotic olanzapine, the tricyclic antidepressant clomipramine, and the supplement N-acetyl-L-cysteine (NAC). Dr. Grant also noted that previous drug trials involving patients with trichotillomania have been very short in duration.
Prior research has implicated the glutamate system in repetitive motor habits and the urges that drive them. Memantine, a glutamate receptor antagonist, targets excessive glutamatergic drive. To investigate whether this medication may be beneficial for patients with trichotillomania and skin-picking disorders, the investigators conducted a randomized placebo-controlled trial.
The study included 100 adults (86 women; mean age, 31.4) with trichotillomania, skin-picking disorder, or both; participants received memantine (n = 55) or placebo (n = 45) for 8 weeks; they received memantine 10 mg or placebo for the first 2 weeks, then 20 mg for the next 6 weeks.
The researchers, who were blinded to assignment, assessed participants every 2 weeks using the National Institute of Mental Health Trichotillomania Symptom Severity Scale, which was modified to include questions for skin-picking disorder.
The team also tracked symptoms and behaviors using additional scales, including the Sheehan Disability Scale and the Clinical Global Impressions severity scale.
At the study’s conclusion, 79 patients remained. Of those, 26 of the 43 participants in the memantine group were “very much” or “much” improved (61%), versus 3 of 36 (8%) in the placebo group. (P < .0001)
Six participants in the memantine group experienced complete remission of symptoms, compared with one in the placebo group. There were no differences between the study groups in terms of adverse events.
Study limitations included the relatively short length of the trial for what should be considered a chronic disease, as well as the inclusion of only mildly to moderately symptomatic participants.
Dr. Grant said that he would like to study how memantine works in combination with behavioral therapy.
‘Two great options’
Katharine Phillips, MD, professor of psychiatry at Weill Cornell Medicine, New York, said she has been using memantine for “quite some time” to treat her patients with skin-picking disorder, adding that she uses higher doses of the drug than were tested in the study.
She noted that both NAC and memantine affect glutamate, an amino acid in the brain that is likely involved in repetitive physical or motor habits, such as hair pulling and skin picking.
“The good news is that we have two great options” for the treatment of trichotillomania and skin-picking disorder, said Dr. Phillips, and that both are easy to tolerate.
Future research should focus on longer trials of memantine and at higher doses, as well as other glutamate modulators, she said.
The study was funded by departmental research funds at the University of Chicago. Dr. Grant reported receiving research funding from Biohaven Pharmaceuticals and Janssen, as well as yearly compensation from Springer Publishing for his role as editor-in-chief of the Journal of Gambling Studies. He has also received royalties from American Psychiatric Publishing, McGraw Hill, Oxford University Press, and WW Norton. Dr. Phillips reported receiving royalties from American Psychiatric Publishing and an honorarium from the Merck Manual.
A version of this article first appeared on Medscape.com.
Results from the double-blind, placebo-controlled trial showed that 61% of participants who received memantine were “much or very much improved,” versus 8% in the placebo group.
“Memantine was far more effective than placebo,” lead investigator Jon Grant, MD, MPH, professor of psychiatry and behavioral neuroscience at the University of Chicago, said in an interview. “However, while subjects responded favorably, that didn’t necessarily mean there were no symptoms.”
The study was published online in the American Journal of Psychiatry.
Underrecognized, disabling
The investigators noted that trichotillomania and skin-picking disorder are underrecognized and are often disabling conditions. However, the researchers pointed out that with prevalence rates of 1.7% for trichotillomania and 2.1% for skin-picking disorder, they are not uncommon.
Behavioral therapy that attempts to reverse these habits is considered first-line treatment, but trained therapists are difficult to find. In addition, the investigators wrote that currently, there are no Food and Drug Administration–approved medications for either disorder, and pharmacologic clinical trials are relatively uncommon.
The existing data from double-blind, placebo-controlled studies support the use of the antipsychotic olanzapine, the tricyclic antidepressant clomipramine, and the supplement N-acetyl-L-cysteine (NAC). Dr. Grant also noted that previous drug trials involving patients with trichotillomania have been very short in duration.
Prior research has implicated the glutamate system in repetitive motor habits and the urges that drive them. Memantine, a glutamate receptor antagonist, targets excessive glutamatergic drive. To investigate whether this medication may be beneficial for patients with trichotillomania and skin-picking disorders, the investigators conducted a randomized placebo-controlled trial.
The study included 100 adults (86 women; mean age, 31.4) with trichotillomania, skin-picking disorder, or both; participants received memantine (n = 55) or placebo (n = 45) for 8 weeks; they received memantine 10 mg or placebo for the first 2 weeks, then 20 mg for the next 6 weeks.
The researchers, who were blinded to assignment, assessed participants every 2 weeks using the National Institute of Mental Health Trichotillomania Symptom Severity Scale, which was modified to include questions for skin-picking disorder.
The team also tracked symptoms and behaviors using additional scales, including the Sheehan Disability Scale and the Clinical Global Impressions severity scale.
At the study’s conclusion, 79 patients remained. Of those, 26 of the 43 participants in the memantine group were “very much” or “much” improved (61%), versus 3 of 36 (8%) in the placebo group. (P < .0001)
Six participants in the memantine group experienced complete remission of symptoms, compared with one in the placebo group. There were no differences between the study groups in terms of adverse events.
Study limitations included the relatively short length of the trial for what should be considered a chronic disease, as well as the inclusion of only mildly to moderately symptomatic participants.
Dr. Grant said that he would like to study how memantine works in combination with behavioral therapy.
‘Two great options’
Katharine Phillips, MD, professor of psychiatry at Weill Cornell Medicine, New York, said she has been using memantine for “quite some time” to treat her patients with skin-picking disorder, adding that she uses higher doses of the drug than were tested in the study.
She noted that both NAC and memantine affect glutamate, an amino acid in the brain that is likely involved in repetitive physical or motor habits, such as hair pulling and skin picking.
“The good news is that we have two great options” for the treatment of trichotillomania and skin-picking disorder, said Dr. Phillips, and that both are easy to tolerate.
Future research should focus on longer trials of memantine and at higher doses, as well as other glutamate modulators, she said.
The study was funded by departmental research funds at the University of Chicago. Dr. Grant reported receiving research funding from Biohaven Pharmaceuticals and Janssen, as well as yearly compensation from Springer Publishing for his role as editor-in-chief of the Journal of Gambling Studies. He has also received royalties from American Psychiatric Publishing, McGraw Hill, Oxford University Press, and WW Norton. Dr. Phillips reported receiving royalties from American Psychiatric Publishing and an honorarium from the Merck Manual.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF PSYCHIATRY
FDA clears first patch to treat axillary hyperhidrosis
The Food and Drug Administration on April 13 cleared the first patch to reduce excessive underarm sweating for adults with primary axillary hyperhidrosis.
The single-use, disposable, prescription-only patch will be marketed as Brella. It consists of a sodium sheet with an adhesive overlay. A health care provider applies it to the patient’s underarm for up to 3 minutes and then repeats the process on the other underarm.
The developer, Candesant Biomedical, says the patch uses the company’s patented targeted alkali thermolysis (TAT) technology, which was built on the principle that heat is generated when sodium reacts with water in sweat. “The thermal energy created by the sodium sheet is precisely localized, microtargeting sweat glands to significantly reduce sweat production,” according to the company’s press release announcing the FDA decision.
FDA clearance was based on data from the pivotal randomized, double-blind, multicenter SAHARA study, which indicated that the product is effective and well tolerated.
Patients experienced a reduction in sweat that was maintained for 3 months or longer, according to trial results.
The SAHARA trial results were reported in a late-breaking abstract at the annual meeting of the American Academy of Dermatology in March.
The trial enrolled 110 individuals with Hyperhidrosis Disease Severity Scale (HDSS) scores of 3 or 4 (indicating frequent sweating or sweating that always interferes with daily activities). Trial participants were randomly assigned to receive either an active TAT or a sham patch, which was applied for up to 3 minutes.
At the meeting, lead investigator David M. Pariser, MD, a dermatologist practicing in Norfolk, Va., reported that at 4 weeks, 63.6% of patients in the active patch group achieved an HDSS score of 1 or 2, compared with 44.2% of those in the sham treatment group (P = .0332). Also, 43.2% of those in the active-patch group achieved an improvement of 2 points or greater on the HDSS, as compared with 16.3% of those in the sham treatment group (P = .0107) .
In addition, 9.1% of those in the active-patch group achieved a 3-point improvement on the HDSS, compared with none in the sham group. “That’s an amazing improvement; you’re basically going from moderate or severe to none,” Dr. Pariser said at the meeting.
As for adverse events (AEs), 13 patients in the active-patch group experienced AEs at the treatment site. Six patients experienced erythema; four experienced erosion; two experienced burning, itching, or stinging; and one had underarm odor.
“The two procedure-related AEs in the TAT-treated group were compensatory sweating and irritant contact dermatitis due to the adhesive,” Dr. Pariser said. He noted that most AEs resolved in fewer than 2 weeks, and all AEs were mild to moderate.
According to the International Hyperhidrosis Society, about 1.3 million people in the United States have axillary hyperhidrosis, and about a third report that sweating is barely tolerable and frequently interferes with daily activities or is intolerable and always interferes with daily activities.
The patch will be available within months in select U.S. markets beginning in late summer. The company says the markets will be listed on its website.
A company representative told this news organization that because it is an in-office procedure, pricing will vary, depending on the practice. “With that said, Candesant expects doctors will charge about the same for one session of the Brella SweatControl Patch as they would for a high-end, in-office facial or chemical peel,” the representative said.
Dr. Pariser is a consultant or investigator for Bickel Biotechnology, Biofrontera AG, Bristol-Myers Squibb, the Celgene Corporation, Novartis Pharmaceuticals, Pfizer, Regeneron, and Sanofi.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration on April 13 cleared the first patch to reduce excessive underarm sweating for adults with primary axillary hyperhidrosis.
The single-use, disposable, prescription-only patch will be marketed as Brella. It consists of a sodium sheet with an adhesive overlay. A health care provider applies it to the patient’s underarm for up to 3 minutes and then repeats the process on the other underarm.
The developer, Candesant Biomedical, says the patch uses the company’s patented targeted alkali thermolysis (TAT) technology, which was built on the principle that heat is generated when sodium reacts with water in sweat. “The thermal energy created by the sodium sheet is precisely localized, microtargeting sweat glands to significantly reduce sweat production,” according to the company’s press release announcing the FDA decision.
FDA clearance was based on data from the pivotal randomized, double-blind, multicenter SAHARA study, which indicated that the product is effective and well tolerated.
Patients experienced a reduction in sweat that was maintained for 3 months or longer, according to trial results.
The SAHARA trial results were reported in a late-breaking abstract at the annual meeting of the American Academy of Dermatology in March.
The trial enrolled 110 individuals with Hyperhidrosis Disease Severity Scale (HDSS) scores of 3 or 4 (indicating frequent sweating or sweating that always interferes with daily activities). Trial participants were randomly assigned to receive either an active TAT or a sham patch, which was applied for up to 3 minutes.
At the meeting, lead investigator David M. Pariser, MD, a dermatologist practicing in Norfolk, Va., reported that at 4 weeks, 63.6% of patients in the active patch group achieved an HDSS score of 1 or 2, compared with 44.2% of those in the sham treatment group (P = .0332). Also, 43.2% of those in the active-patch group achieved an improvement of 2 points or greater on the HDSS, as compared with 16.3% of those in the sham treatment group (P = .0107) .
In addition, 9.1% of those in the active-patch group achieved a 3-point improvement on the HDSS, compared with none in the sham group. “That’s an amazing improvement; you’re basically going from moderate or severe to none,” Dr. Pariser said at the meeting.
As for adverse events (AEs), 13 patients in the active-patch group experienced AEs at the treatment site. Six patients experienced erythema; four experienced erosion; two experienced burning, itching, or stinging; and one had underarm odor.
“The two procedure-related AEs in the TAT-treated group were compensatory sweating and irritant contact dermatitis due to the adhesive,” Dr. Pariser said. He noted that most AEs resolved in fewer than 2 weeks, and all AEs were mild to moderate.
According to the International Hyperhidrosis Society, about 1.3 million people in the United States have axillary hyperhidrosis, and about a third report that sweating is barely tolerable and frequently interferes with daily activities or is intolerable and always interferes with daily activities.
The patch will be available within months in select U.S. markets beginning in late summer. The company says the markets will be listed on its website.
A company representative told this news organization that because it is an in-office procedure, pricing will vary, depending on the practice. “With that said, Candesant expects doctors will charge about the same for one session of the Brella SweatControl Patch as they would for a high-end, in-office facial or chemical peel,” the representative said.
Dr. Pariser is a consultant or investigator for Bickel Biotechnology, Biofrontera AG, Bristol-Myers Squibb, the Celgene Corporation, Novartis Pharmaceuticals, Pfizer, Regeneron, and Sanofi.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration on April 13 cleared the first patch to reduce excessive underarm sweating for adults with primary axillary hyperhidrosis.
The single-use, disposable, prescription-only patch will be marketed as Brella. It consists of a sodium sheet with an adhesive overlay. A health care provider applies it to the patient’s underarm for up to 3 minutes and then repeats the process on the other underarm.
The developer, Candesant Biomedical, says the patch uses the company’s patented targeted alkali thermolysis (TAT) technology, which was built on the principle that heat is generated when sodium reacts with water in sweat. “The thermal energy created by the sodium sheet is precisely localized, microtargeting sweat glands to significantly reduce sweat production,” according to the company’s press release announcing the FDA decision.
FDA clearance was based on data from the pivotal randomized, double-blind, multicenter SAHARA study, which indicated that the product is effective and well tolerated.
Patients experienced a reduction in sweat that was maintained for 3 months or longer, according to trial results.
The SAHARA trial results were reported in a late-breaking abstract at the annual meeting of the American Academy of Dermatology in March.
The trial enrolled 110 individuals with Hyperhidrosis Disease Severity Scale (HDSS) scores of 3 or 4 (indicating frequent sweating or sweating that always interferes with daily activities). Trial participants were randomly assigned to receive either an active TAT or a sham patch, which was applied for up to 3 minutes.
At the meeting, lead investigator David M. Pariser, MD, a dermatologist practicing in Norfolk, Va., reported that at 4 weeks, 63.6% of patients in the active patch group achieved an HDSS score of 1 or 2, compared with 44.2% of those in the sham treatment group (P = .0332). Also, 43.2% of those in the active-patch group achieved an improvement of 2 points or greater on the HDSS, as compared with 16.3% of those in the sham treatment group (P = .0107) .
In addition, 9.1% of those in the active-patch group achieved a 3-point improvement on the HDSS, compared with none in the sham group. “That’s an amazing improvement; you’re basically going from moderate or severe to none,” Dr. Pariser said at the meeting.
As for adverse events (AEs), 13 patients in the active-patch group experienced AEs at the treatment site. Six patients experienced erythema; four experienced erosion; two experienced burning, itching, or stinging; and one had underarm odor.
“The two procedure-related AEs in the TAT-treated group were compensatory sweating and irritant contact dermatitis due to the adhesive,” Dr. Pariser said. He noted that most AEs resolved in fewer than 2 weeks, and all AEs were mild to moderate.
According to the International Hyperhidrosis Society, about 1.3 million people in the United States have axillary hyperhidrosis, and about a third report that sweating is barely tolerable and frequently interferes with daily activities or is intolerable and always interferes with daily activities.
The patch will be available within months in select U.S. markets beginning in late summer. The company says the markets will be listed on its website.
A company representative told this news organization that because it is an in-office procedure, pricing will vary, depending on the practice. “With that said, Candesant expects doctors will charge about the same for one session of the Brella SweatControl Patch as they would for a high-end, in-office facial or chemical peel,” the representative said.
Dr. Pariser is a consultant or investigator for Bickel Biotechnology, Biofrontera AG, Bristol-Myers Squibb, the Celgene Corporation, Novartis Pharmaceuticals, Pfizer, Regeneron, and Sanofi.
A version of this article originally appeared on Medscape.com.
Brittle fingernails
The abnormal upward curve to the fingernails was consistent with a diagnosis of koilonychia—otherwise known as spoon nails.
Koilonychia is an abnormal nail growth pattern where the distal nail matrix is depressed below its normal level, resulting in the spoon shape. The reverse, where the distal nail matrix is elevated in contrast to the proximal nail matrix, results in clubbing.1
There are multiple factors and diseases that result in koilonychia, including lichen planus, psoriasis, nutritional deficiencies (including iron deficiency anemia), and endocrinopathies.1 Lichen planus, which can cause koilonychia, often affects multiple nails and can also cause an associated central ridge pattern. Psoriasis may display a range of nail abnormalities; these include koilonychia, pitting onycholysis, and oil staining.
This patient did not have any signs or symptoms of psoriasis or lichen planus of her nails or skin. A review of her laboratory tests on file made no mention of anemia. Her chemistry profile—including liver tests, renal function tests, and protein levels—were all normal except for glucose levels, which was consistent with her prediabetes. Her thyroid function was also normal. No additional testing was performed since she had no symptoms, physical exam findings, or laboratory clues that pointed to other diseases or systemic processes.
The patient was advised to pick up over-the-counter nail strengtheners and to keep her fingernails trimmed short to minimize the likelihood of painful distal splitting that often occurs with brittle nails. Her physician advised her to follow up with the primary care team if she developed any new signs or symptoms.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Walker J, Baran R, Vélez N, et al. Koilonychia: an update on pathophysiology, differential diagnosis and clinical relevance. J Eur Acad Dermatol Venereol. 2016;30:1985-1991. doi: 10.1111/jdv.13610

The abnormal upward curve to the fingernails was consistent with a diagnosis of koilonychia—otherwise known as spoon nails.
Koilonychia is an abnormal nail growth pattern where the distal nail matrix is depressed below its normal level, resulting in the spoon shape. The reverse, where the distal nail matrix is elevated in contrast to the proximal nail matrix, results in clubbing.1
There are multiple factors and diseases that result in koilonychia, including lichen planus, psoriasis, nutritional deficiencies (including iron deficiency anemia), and endocrinopathies.1 Lichen planus, which can cause koilonychia, often affects multiple nails and can also cause an associated central ridge pattern. Psoriasis may display a range of nail abnormalities; these include koilonychia, pitting onycholysis, and oil staining.
This patient did not have any signs or symptoms of psoriasis or lichen planus of her nails or skin. A review of her laboratory tests on file made no mention of anemia. Her chemistry profile—including liver tests, renal function tests, and protein levels—were all normal except for glucose levels, which was consistent with her prediabetes. Her thyroid function was also normal. No additional testing was performed since she had no symptoms, physical exam findings, or laboratory clues that pointed to other diseases or systemic processes.
The patient was advised to pick up over-the-counter nail strengtheners and to keep her fingernails trimmed short to minimize the likelihood of painful distal splitting that often occurs with brittle nails. Her physician advised her to follow up with the primary care team if she developed any new signs or symptoms.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

The abnormal upward curve to the fingernails was consistent with a diagnosis of koilonychia—otherwise known as spoon nails.
Koilonychia is an abnormal nail growth pattern where the distal nail matrix is depressed below its normal level, resulting in the spoon shape. The reverse, where the distal nail matrix is elevated in contrast to the proximal nail matrix, results in clubbing.1
There are multiple factors and diseases that result in koilonychia, including lichen planus, psoriasis, nutritional deficiencies (including iron deficiency anemia), and endocrinopathies.1 Lichen planus, which can cause koilonychia, often affects multiple nails and can also cause an associated central ridge pattern. Psoriasis may display a range of nail abnormalities; these include koilonychia, pitting onycholysis, and oil staining.
This patient did not have any signs or symptoms of psoriasis or lichen planus of her nails or skin. A review of her laboratory tests on file made no mention of anemia. Her chemistry profile—including liver tests, renal function tests, and protein levels—were all normal except for glucose levels, which was consistent with her prediabetes. Her thyroid function was also normal. No additional testing was performed since she had no symptoms, physical exam findings, or laboratory clues that pointed to other diseases or systemic processes.
The patient was advised to pick up over-the-counter nail strengtheners and to keep her fingernails trimmed short to minimize the likelihood of painful distal splitting that often occurs with brittle nails. Her physician advised her to follow up with the primary care team if she developed any new signs or symptoms.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Walker J, Baran R, Vélez N, et al. Koilonychia: an update on pathophysiology, differential diagnosis and clinical relevance. J Eur Acad Dermatol Venereol. 2016;30:1985-1991. doi: 10.1111/jdv.13610
1. Walker J, Baran R, Vélez N, et al. Koilonychia: an update on pathophysiology, differential diagnosis and clinical relevance. J Eur Acad Dermatol Venereol. 2016;30:1985-1991. doi: 10.1111/jdv.13610
Dark facial lesion
Although an elevated and pigmented lesion should be considered for possible melanoma, this one had prominent telangiectasias and was proven to be a basal cell carcinoma (BCC) on biopsy.
While the literature often focuses on light-colored skin types and the high risk of skin cancers, individuals with darker skin can also get melanoma and nonmelanoma skin cancer. Half of the BCCs in African American people are pigmented BCCs, compared to less than 10% for Caucasian individuals. Individuals who are Hispanic have twice the likelihood of pigmented BCCs as those who are Caucasian.1 Pigmented BCCs manifest as darker lesions, as occurred in this individual. Nonpigmented BCCs tend to be pink or pale in color.
Typically, superficial and very small, nodular BCCs can be successfully treated with 2 cycles of electrodesiccation and curettage. EDC should, however, be avoided in low-risk BCCs when these lesions occur in areas of secondary hair growth, such as the beard or scalp. This is because the epidermis follows the hair follicle, and in sites with deep hair follicles, EDC would have to get down to the subcutis to effectively clear the tumor.
For larger, nodular BCCs, full-thickness excision with adequate margins is warranted. For high-risk types, and those in high-risk areas near the nose, eyes, mouth, and ears, Mohs micrographic surgery is recommended to maximize the likelihood of complete excision while minimizing the loss of normal tissue.
Since the physician suspected this was a pigmented BCC, he performed a superficial shave biopsy on a small representative area of the lesion for diagnosis. This patient’s biopsy confirmed a nodular-type pigmented BCC. The lesion was removed in the office with 5-mm margins oriented along the resting skin tension lines with good closure and cosmetic results.
The patient was advised to have routine skin evaluations every 6 months due to the high risk of additional cancers. He was also advised to take oral niacinamide 500 mg twice daily, which can reduce the risk of actinic keratoses and nonmelanoma skin cancers by 15% and 23%, respectively, in those who have had lesions.2
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Higgins S, Nazemi A, Chow M, et al. Review of nonmelanoma skin cancer in African Americans, Hispanics, and Asians. Dermatol Surg. 2018;44:903-910. doi: 10.1097/DSS.0000000000001547
2. Starr P. Oral nicotinamide prevents common skin cancers in high-risk patients, reduces costs. Am Health Drug Benefits. 2015;8(spec issue):13-14.

Although an elevated and pigmented lesion should be considered for possible melanoma, this one had prominent telangiectasias and was proven to be a basal cell carcinoma (BCC) on biopsy.
While the literature often focuses on light-colored skin types and the high risk of skin cancers, individuals with darker skin can also get melanoma and nonmelanoma skin cancer. Half of the BCCs in African American people are pigmented BCCs, compared to less than 10% for Caucasian individuals. Individuals who are Hispanic have twice the likelihood of pigmented BCCs as those who are Caucasian.1 Pigmented BCCs manifest as darker lesions, as occurred in this individual. Nonpigmented BCCs tend to be pink or pale in color.
Typically, superficial and very small, nodular BCCs can be successfully treated with 2 cycles of electrodesiccation and curettage. EDC should, however, be avoided in low-risk BCCs when these lesions occur in areas of secondary hair growth, such as the beard or scalp. This is because the epidermis follows the hair follicle, and in sites with deep hair follicles, EDC would have to get down to the subcutis to effectively clear the tumor.
For larger, nodular BCCs, full-thickness excision with adequate margins is warranted. For high-risk types, and those in high-risk areas near the nose, eyes, mouth, and ears, Mohs micrographic surgery is recommended to maximize the likelihood of complete excision while minimizing the loss of normal tissue.
Since the physician suspected this was a pigmented BCC, he performed a superficial shave biopsy on a small representative area of the lesion for diagnosis. This patient’s biopsy confirmed a nodular-type pigmented BCC. The lesion was removed in the office with 5-mm margins oriented along the resting skin tension lines with good closure and cosmetic results.
The patient was advised to have routine skin evaluations every 6 months due to the high risk of additional cancers. He was also advised to take oral niacinamide 500 mg twice daily, which can reduce the risk of actinic keratoses and nonmelanoma skin cancers by 15% and 23%, respectively, in those who have had lesions.2
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

Although an elevated and pigmented lesion should be considered for possible melanoma, this one had prominent telangiectasias and was proven to be a basal cell carcinoma (BCC) on biopsy.
While the literature often focuses on light-colored skin types and the high risk of skin cancers, individuals with darker skin can also get melanoma and nonmelanoma skin cancer. Half of the BCCs in African American people are pigmented BCCs, compared to less than 10% for Caucasian individuals. Individuals who are Hispanic have twice the likelihood of pigmented BCCs as those who are Caucasian.1 Pigmented BCCs manifest as darker lesions, as occurred in this individual. Nonpigmented BCCs tend to be pink or pale in color.
Typically, superficial and very small, nodular BCCs can be successfully treated with 2 cycles of electrodesiccation and curettage. EDC should, however, be avoided in low-risk BCCs when these lesions occur in areas of secondary hair growth, such as the beard or scalp. This is because the epidermis follows the hair follicle, and in sites with deep hair follicles, EDC would have to get down to the subcutis to effectively clear the tumor.
For larger, nodular BCCs, full-thickness excision with adequate margins is warranted. For high-risk types, and those in high-risk areas near the nose, eyes, mouth, and ears, Mohs micrographic surgery is recommended to maximize the likelihood of complete excision while minimizing the loss of normal tissue.
Since the physician suspected this was a pigmented BCC, he performed a superficial shave biopsy on a small representative area of the lesion for diagnosis. This patient’s biopsy confirmed a nodular-type pigmented BCC. The lesion was removed in the office with 5-mm margins oriented along the resting skin tension lines with good closure and cosmetic results.
The patient was advised to have routine skin evaluations every 6 months due to the high risk of additional cancers. He was also advised to take oral niacinamide 500 mg twice daily, which can reduce the risk of actinic keratoses and nonmelanoma skin cancers by 15% and 23%, respectively, in those who have had lesions.2
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Higgins S, Nazemi A, Chow M, et al. Review of nonmelanoma skin cancer in African Americans, Hispanics, and Asians. Dermatol Surg. 2018;44:903-910. doi: 10.1097/DSS.0000000000001547
2. Starr P. Oral nicotinamide prevents common skin cancers in high-risk patients, reduces costs. Am Health Drug Benefits. 2015;8(spec issue):13-14.
1. Higgins S, Nazemi A, Chow M, et al. Review of nonmelanoma skin cancer in African Americans, Hispanics, and Asians. Dermatol Surg. 2018;44:903-910. doi: 10.1097/DSS.0000000000001547
2. Starr P. Oral nicotinamide prevents common skin cancers in high-risk patients, reduces costs. Am Health Drug Benefits. 2015;8(spec issue):13-14.
A 50-year-old White male presented with a 4- to 5-year history of progressively growing violaceous lesions on his left lower extremity
with scarce T-cells, classically presenting as rapidly progressive, plum-colored lesions on the lower extremities.1,2 CBCLs, with PCDLBCL-LT accounting for 4%, make up the minority of cutaneous lymphomas in the Western world.1-3 The leg type variant, typically demonstrating a female predominance and median age of onset in the 70s, is clinically aggressive and associated with a poorer prognosis, increased recurrence rate, and 40%-60% 5-year survival rate.1-5
Histologically, this variant demonstrates a diffuse sheet-like growth of enlarged atypical B-cells distinctively separated from the epidermis by a prominent grenz zone. Classic PCDLBCL-LT immunophenotype includes B-cell markers CD20 and IgM; triple expressor phenotype indicating c-MYC, BCL-2, and BCL-6 positivity; as well as CD10 negativity, lack of BCL-2 rearrangement, and presence of a positive MYD-88 molecular result.
Other characteristic histopathological findings include positivity for post-germinal markers IRF4/MUM-1 and FOXP-1, positivity for additional B-cell markers, including CD79 and PAX5, and negativity of t(14;18) (q32;21).1,3-5
This case is of significant interest as it falls within the approximately 10% of PCDLBCL-LT cases demonstrating weak to negative MUM-1 staining, in addition to its presentation in a younger male individual.
While MUM-1 positivity is common in this subtype, its presence, or lack thereof, should not be looked at in isolation when evaluating diagnostic criteria, nor has it been shown to have a statistically significant effect on survival rate – in contrast to factors like lesion location on the leg versus non-leg lesions, multiple lesions at diagnosis, and dissemination to other sites.2,6
PCDLBCL-LT can uncommonly present in non-leg locations and only 10% depict associated B-symptoms, such as fatigue, night sweats, weight loss, or lymphadenopathy.2,6 First-line treatment is with the R-CHOP chemotherapy regimen – consisting of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone – although radiotherapy is sometimes considered in patients with a single small lesion.1,2
Because of possible cutaneous involvement beyond the legs, common lack of systemic symptoms, and variable immunophenotypes, this case of MUM-1 negative PCDLBCL-LT highlights the importance of a clinicopathological approach to differentiate the subtypes of CBCLs, allowing for proper and individualized stratification of risk, prognosis, and treatment.
This case was submitted and written by Marlee Hill, BS, Michael Franzetti, MD, Jeffrey McBride, MD, and Allison Hood, MD, of the University of Oklahoma, Oklahoma City. They also provided the photos. Donna Bilu Martin, MD, edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Willemze R et al. Blood. 2019;133(16):1703-14.
2. Willemze R et al. Blood. 2005;105(10):3768-85.
3. Sukswai N et al. Pathology. 2020;52(1):53-67.
4. Hristov AC. Arch Pathol Lab Med. 2012;136(8):876-81.
5. Sokol L et al. Cancer Control. 2012;19(3):236-44.
6. Grange F et al. Arch Dermatol. 2007;143(9):1144-50.
with scarce T-cells, classically presenting as rapidly progressive, plum-colored lesions on the lower extremities.1,2 CBCLs, with PCDLBCL-LT accounting for 4%, make up the minority of cutaneous lymphomas in the Western world.1-3 The leg type variant, typically demonstrating a female predominance and median age of onset in the 70s, is clinically aggressive and associated with a poorer prognosis, increased recurrence rate, and 40%-60% 5-year survival rate.1-5
Histologically, this variant demonstrates a diffuse sheet-like growth of enlarged atypical B-cells distinctively separated from the epidermis by a prominent grenz zone. Classic PCDLBCL-LT immunophenotype includes B-cell markers CD20 and IgM; triple expressor phenotype indicating c-MYC, BCL-2, and BCL-6 positivity; as well as CD10 negativity, lack of BCL-2 rearrangement, and presence of a positive MYD-88 molecular result.
Other characteristic histopathological findings include positivity for post-germinal markers IRF4/MUM-1 and FOXP-1, positivity for additional B-cell markers, including CD79 and PAX5, and negativity of t(14;18) (q32;21).1,3-5
This case is of significant interest as it falls within the approximately 10% of PCDLBCL-LT cases demonstrating weak to negative MUM-1 staining, in addition to its presentation in a younger male individual.
While MUM-1 positivity is common in this subtype, its presence, or lack thereof, should not be looked at in isolation when evaluating diagnostic criteria, nor has it been shown to have a statistically significant effect on survival rate – in contrast to factors like lesion location on the leg versus non-leg lesions, multiple lesions at diagnosis, and dissemination to other sites.2,6
PCDLBCL-LT can uncommonly present in non-leg locations and only 10% depict associated B-symptoms, such as fatigue, night sweats, weight loss, or lymphadenopathy.2,6 First-line treatment is with the R-CHOP chemotherapy regimen – consisting of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone – although radiotherapy is sometimes considered in patients with a single small lesion.1,2
Because of possible cutaneous involvement beyond the legs, common lack of systemic symptoms, and variable immunophenotypes, this case of MUM-1 negative PCDLBCL-LT highlights the importance of a clinicopathological approach to differentiate the subtypes of CBCLs, allowing for proper and individualized stratification of risk, prognosis, and treatment.
This case was submitted and written by Marlee Hill, BS, Michael Franzetti, MD, Jeffrey McBride, MD, and Allison Hood, MD, of the University of Oklahoma, Oklahoma City. They also provided the photos. Donna Bilu Martin, MD, edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Willemze R et al. Blood. 2019;133(16):1703-14.
2. Willemze R et al. Blood. 2005;105(10):3768-85.
3. Sukswai N et al. Pathology. 2020;52(1):53-67.
4. Hristov AC. Arch Pathol Lab Med. 2012;136(8):876-81.
5. Sokol L et al. Cancer Control. 2012;19(3):236-44.
6. Grange F et al. Arch Dermatol. 2007;143(9):1144-50.
with scarce T-cells, classically presenting as rapidly progressive, plum-colored lesions on the lower extremities.1,2 CBCLs, with PCDLBCL-LT accounting for 4%, make up the minority of cutaneous lymphomas in the Western world.1-3 The leg type variant, typically demonstrating a female predominance and median age of onset in the 70s, is clinically aggressive and associated with a poorer prognosis, increased recurrence rate, and 40%-60% 5-year survival rate.1-5
Histologically, this variant demonstrates a diffuse sheet-like growth of enlarged atypical B-cells distinctively separated from the epidermis by a prominent grenz zone. Classic PCDLBCL-LT immunophenotype includes B-cell markers CD20 and IgM; triple expressor phenotype indicating c-MYC, BCL-2, and BCL-6 positivity; as well as CD10 negativity, lack of BCL-2 rearrangement, and presence of a positive MYD-88 molecular result.
Other characteristic histopathological findings include positivity for post-germinal markers IRF4/MUM-1 and FOXP-1, positivity for additional B-cell markers, including CD79 and PAX5, and negativity of t(14;18) (q32;21).1,3-5
This case is of significant interest as it falls within the approximately 10% of PCDLBCL-LT cases demonstrating weak to negative MUM-1 staining, in addition to its presentation in a younger male individual.
While MUM-1 positivity is common in this subtype, its presence, or lack thereof, should not be looked at in isolation when evaluating diagnostic criteria, nor has it been shown to have a statistically significant effect on survival rate – in contrast to factors like lesion location on the leg versus non-leg lesions, multiple lesions at diagnosis, and dissemination to other sites.2,6
PCDLBCL-LT can uncommonly present in non-leg locations and only 10% depict associated B-symptoms, such as fatigue, night sweats, weight loss, or lymphadenopathy.2,6 First-line treatment is with the R-CHOP chemotherapy regimen – consisting of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone – although radiotherapy is sometimes considered in patients with a single small lesion.1,2
Because of possible cutaneous involvement beyond the legs, common lack of systemic symptoms, and variable immunophenotypes, this case of MUM-1 negative PCDLBCL-LT highlights the importance of a clinicopathological approach to differentiate the subtypes of CBCLs, allowing for proper and individualized stratification of risk, prognosis, and treatment.
This case was submitted and written by Marlee Hill, BS, Michael Franzetti, MD, Jeffrey McBride, MD, and Allison Hood, MD, of the University of Oklahoma, Oklahoma City. They also provided the photos. Donna Bilu Martin, MD, edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Willemze R et al. Blood. 2019;133(16):1703-14.
2. Willemze R et al. Blood. 2005;105(10):3768-85.
3. Sukswai N et al. Pathology. 2020;52(1):53-67.
4. Hristov AC. Arch Pathol Lab Med. 2012;136(8):876-81.
5. Sokol L et al. Cancer Control. 2012;19(3):236-44.
6. Grange F et al. Arch Dermatol. 2007;143(9):1144-50.
There was no cervical, axillary, or inguinal lymphadenopathy.