User login
Sustained response at 2 years reported for newly approved oral psoriasis agent
MILAN – The day after deucravacitinib became the first TYK2 inhibitor approved for the treatment of moderate to severe psoriasis, long-term data were presented at the annual congress of the European Academy of Dermatology and Venereology, suggesting that a high degree of benefit persists for at least 2 years, making this oral drug a potential competitor for biologics.
,” said Mark G. Lebwohl, MD, professor of dermatology and dean of clinical therapeutics, Icahn School of Medicine at Mount Sinai, New York.
Just 2 months after the 52-week data from the phase 3 POETYK PSO-1 trial were published online in the Journal of the American Academy of Dermatology, a long-term extension study found essentially no loss of benefit at 112 weeks, according to Dr. Lebwohl.
One of the two co-primary endpoints was a 75% clearance on the Psoriasis and Severity Index (PASI75) score. At 52 weeks, 80.2% of patients on deucravacitinib had met this criterion of benefit. At 112 weeks, the proportion was 84.4%.
The other primary endpoint was a static Physician’s Global Assessment (sPGA) score of clear or almost clear skin. The proportion of patients meeting this criterion at weeks 52 and 112 weeks were 65.6% and 67.6%, respectively.
When assessed by Treatment Failure Rule (TFR) or modified nonresponder imputation (mNRI), results were similar. For both, the primary endpoints at every time interval were just one or two percentage points lower but not clinically meaningfully different, according to Dr. Lebwohl.
The same type of sustained response out to 112 weeks was observed in multiple analyses. When the researchers isolated the subgroup of patients who had achieved a PASI 75 response at 16 weeks (100%), there was a modest decline in the PASI 75 rate at week 52 (90.2%) but then no additional decline at week 112 (91.3%).
There were essentially no changes in the PASI 90 rates at week 16 (63%), week 52 (65.3%), and week 112 (63.1%), Dr. Lebwohl reported. PASI 100 rates, once achieved, were sustained long term.
The target, TYK2, is one of four Janus kinase (JAK) inhibitors. Until now, almost all JAK inhibitors have had greater relative specificity for JAK 1, JAK 2, and JAK 3, but several inhibitors of TYK2 inhibitors other than deucravacitinib are in development for inflammatory diseases. Deucravacitinib (Sotyktu), approved by the Food and Drug Administration on Sept. 9, is the only TYK2 inhibitor with regulatory approval for plaque psoriasis.
In the POETYK PSO-1 trial, 666 patients were initially randomized in a 2:1:1 ratio to 6 mg deucravacitinib (now the approved dose), placebo, or the oral phosphodiesterase 4 inhibitor apremilast. At week 16, patients on placebo were switched over to deucravacitinib. At week 24, patients who did not achieve a PASI 50 on apremilast (which had been titrated to 10 mg daily to 30 mg twice a day over the first 5 days of dosing) were switched to deucravacitinib.
In the previously reported data, deucravacitinib was superior for all efficacy endpoints at week 16, including an analysis of quality of life when compared with placebo (P < .0001) or apremilast (P = .0088). At week 52, after having been switched to deucravacitinib at week 16, patients on placebo achieved comparable responses on the efficacy measures in this study, including PASI75.
Relative to JAK inhibitors commonly used in rheumatoid arthritis and other inflammatory diseases, the greater specificity of deucravacitinib for TYK2 appears to have meaningful safety advantages, according to Dr. Lebwohl. Targeted mostly on the TYK2 regulatory domain, deucravacitinib largely avoids inhibition of the JAK 1, 2, and 3 subtypes. Dr. Lebwohl said this explains why deucravacitinib labeling does not share the boxed warnings about off-target effects, such as those on the cardiovascular system, that can be found in the labeling of other JAK inhibitors.
In the published 52-week data, the discontinuation rate for adverse events was lower in the group randomized to deucravacitinib arm than in the placebo arm. In the extended follow-up, there were no new signals for adverse events, including those involving the CV system or immune function.
The key message so far from the long-term follow-up, which is ongoing, is that “continuous treatment with deucravacitinib is associated with durable efficacy,” Dr. Lebwohl said. It is this combination of sustained efficacy and safety that led Dr. Lebwohl to suggest it as a reasonable oral competitor to injectable biologics.
“Patients now have a choice,” he said.
Jashin J. Wu, MD, a board member of the National Psoriasis Foundation and an associate professor in the department of dermatology, University of Miami, has been following the development of deucravacitinib. He said that the recent FDA approval validates the clinical evidence of benefit and safety, while the long-term data presented at the EADV congress support its role in expanding treatment options.
“Deucravacitinib is a very effective oral agent for moderate to severe plaque psoriasis with strong maintenance of effect through week 112,” he said. Differentiating it from other JAK inhibitors, the FDA approval “confirms the safety of this agent as there is no boxed warning,” he added.
Dr. Lebwohl reports financial relationships with more than 30 pharmaceutical companies, including Bristol-Myers Squibb, the manufacturer of deucravacitinib. Dr. Wu has financial relationships with 14 pharmaceutical companies, including Bristol-Myers Squibb, but he was not an investigator for the phase 3 trials of deucravacitinib.
MILAN – The day after deucravacitinib became the first TYK2 inhibitor approved for the treatment of moderate to severe psoriasis, long-term data were presented at the annual congress of the European Academy of Dermatology and Venereology, suggesting that a high degree of benefit persists for at least 2 years, making this oral drug a potential competitor for biologics.
,” said Mark G. Lebwohl, MD, professor of dermatology and dean of clinical therapeutics, Icahn School of Medicine at Mount Sinai, New York.
Just 2 months after the 52-week data from the phase 3 POETYK PSO-1 trial were published online in the Journal of the American Academy of Dermatology, a long-term extension study found essentially no loss of benefit at 112 weeks, according to Dr. Lebwohl.
One of the two co-primary endpoints was a 75% clearance on the Psoriasis and Severity Index (PASI75) score. At 52 weeks, 80.2% of patients on deucravacitinib had met this criterion of benefit. At 112 weeks, the proportion was 84.4%.
The other primary endpoint was a static Physician’s Global Assessment (sPGA) score of clear or almost clear skin. The proportion of patients meeting this criterion at weeks 52 and 112 weeks were 65.6% and 67.6%, respectively.
When assessed by Treatment Failure Rule (TFR) or modified nonresponder imputation (mNRI), results were similar. For both, the primary endpoints at every time interval were just one or two percentage points lower but not clinically meaningfully different, according to Dr. Lebwohl.
The same type of sustained response out to 112 weeks was observed in multiple analyses. When the researchers isolated the subgroup of patients who had achieved a PASI 75 response at 16 weeks (100%), there was a modest decline in the PASI 75 rate at week 52 (90.2%) but then no additional decline at week 112 (91.3%).
There were essentially no changes in the PASI 90 rates at week 16 (63%), week 52 (65.3%), and week 112 (63.1%), Dr. Lebwohl reported. PASI 100 rates, once achieved, were sustained long term.
The target, TYK2, is one of four Janus kinase (JAK) inhibitors. Until now, almost all JAK inhibitors have had greater relative specificity for JAK 1, JAK 2, and JAK 3, but several inhibitors of TYK2 inhibitors other than deucravacitinib are in development for inflammatory diseases. Deucravacitinib (Sotyktu), approved by the Food and Drug Administration on Sept. 9, is the only TYK2 inhibitor with regulatory approval for plaque psoriasis.
In the POETYK PSO-1 trial, 666 patients were initially randomized in a 2:1:1 ratio to 6 mg deucravacitinib (now the approved dose), placebo, or the oral phosphodiesterase 4 inhibitor apremilast. At week 16, patients on placebo were switched over to deucravacitinib. At week 24, patients who did not achieve a PASI 50 on apremilast (which had been titrated to 10 mg daily to 30 mg twice a day over the first 5 days of dosing) were switched to deucravacitinib.
In the previously reported data, deucravacitinib was superior for all efficacy endpoints at week 16, including an analysis of quality of life when compared with placebo (P < .0001) or apremilast (P = .0088). At week 52, after having been switched to deucravacitinib at week 16, patients on placebo achieved comparable responses on the efficacy measures in this study, including PASI75.
Relative to JAK inhibitors commonly used in rheumatoid arthritis and other inflammatory diseases, the greater specificity of deucravacitinib for TYK2 appears to have meaningful safety advantages, according to Dr. Lebwohl. Targeted mostly on the TYK2 regulatory domain, deucravacitinib largely avoids inhibition of the JAK 1, 2, and 3 subtypes. Dr. Lebwohl said this explains why deucravacitinib labeling does not share the boxed warnings about off-target effects, such as those on the cardiovascular system, that can be found in the labeling of other JAK inhibitors.
In the published 52-week data, the discontinuation rate for adverse events was lower in the group randomized to deucravacitinib arm than in the placebo arm. In the extended follow-up, there were no new signals for adverse events, including those involving the CV system or immune function.
The key message so far from the long-term follow-up, which is ongoing, is that “continuous treatment with deucravacitinib is associated with durable efficacy,” Dr. Lebwohl said. It is this combination of sustained efficacy and safety that led Dr. Lebwohl to suggest it as a reasonable oral competitor to injectable biologics.
“Patients now have a choice,” he said.
Jashin J. Wu, MD, a board member of the National Psoriasis Foundation and an associate professor in the department of dermatology, University of Miami, has been following the development of deucravacitinib. He said that the recent FDA approval validates the clinical evidence of benefit and safety, while the long-term data presented at the EADV congress support its role in expanding treatment options.
“Deucravacitinib is a very effective oral agent for moderate to severe plaque psoriasis with strong maintenance of effect through week 112,” he said. Differentiating it from other JAK inhibitors, the FDA approval “confirms the safety of this agent as there is no boxed warning,” he added.
Dr. Lebwohl reports financial relationships with more than 30 pharmaceutical companies, including Bristol-Myers Squibb, the manufacturer of deucravacitinib. Dr. Wu has financial relationships with 14 pharmaceutical companies, including Bristol-Myers Squibb, but he was not an investigator for the phase 3 trials of deucravacitinib.
MILAN – The day after deucravacitinib became the first TYK2 inhibitor approved for the treatment of moderate to severe psoriasis, long-term data were presented at the annual congress of the European Academy of Dermatology and Venereology, suggesting that a high degree of benefit persists for at least 2 years, making this oral drug a potential competitor for biologics.
,” said Mark G. Lebwohl, MD, professor of dermatology and dean of clinical therapeutics, Icahn School of Medicine at Mount Sinai, New York.
Just 2 months after the 52-week data from the phase 3 POETYK PSO-1 trial were published online in the Journal of the American Academy of Dermatology, a long-term extension study found essentially no loss of benefit at 112 weeks, according to Dr. Lebwohl.
One of the two co-primary endpoints was a 75% clearance on the Psoriasis and Severity Index (PASI75) score. At 52 weeks, 80.2% of patients on deucravacitinib had met this criterion of benefit. At 112 weeks, the proportion was 84.4%.
The other primary endpoint was a static Physician’s Global Assessment (sPGA) score of clear or almost clear skin. The proportion of patients meeting this criterion at weeks 52 and 112 weeks were 65.6% and 67.6%, respectively.
When assessed by Treatment Failure Rule (TFR) or modified nonresponder imputation (mNRI), results were similar. For both, the primary endpoints at every time interval were just one or two percentage points lower but not clinically meaningfully different, according to Dr. Lebwohl.
The same type of sustained response out to 112 weeks was observed in multiple analyses. When the researchers isolated the subgroup of patients who had achieved a PASI 75 response at 16 weeks (100%), there was a modest decline in the PASI 75 rate at week 52 (90.2%) but then no additional decline at week 112 (91.3%).
There were essentially no changes in the PASI 90 rates at week 16 (63%), week 52 (65.3%), and week 112 (63.1%), Dr. Lebwohl reported. PASI 100 rates, once achieved, were sustained long term.
The target, TYK2, is one of four Janus kinase (JAK) inhibitors. Until now, almost all JAK inhibitors have had greater relative specificity for JAK 1, JAK 2, and JAK 3, but several inhibitors of TYK2 inhibitors other than deucravacitinib are in development for inflammatory diseases. Deucravacitinib (Sotyktu), approved by the Food and Drug Administration on Sept. 9, is the only TYK2 inhibitor with regulatory approval for plaque psoriasis.
In the POETYK PSO-1 trial, 666 patients were initially randomized in a 2:1:1 ratio to 6 mg deucravacitinib (now the approved dose), placebo, or the oral phosphodiesterase 4 inhibitor apremilast. At week 16, patients on placebo were switched over to deucravacitinib. At week 24, patients who did not achieve a PASI 50 on apremilast (which had been titrated to 10 mg daily to 30 mg twice a day over the first 5 days of dosing) were switched to deucravacitinib.
In the previously reported data, deucravacitinib was superior for all efficacy endpoints at week 16, including an analysis of quality of life when compared with placebo (P < .0001) or apremilast (P = .0088). At week 52, after having been switched to deucravacitinib at week 16, patients on placebo achieved comparable responses on the efficacy measures in this study, including PASI75.
Relative to JAK inhibitors commonly used in rheumatoid arthritis and other inflammatory diseases, the greater specificity of deucravacitinib for TYK2 appears to have meaningful safety advantages, according to Dr. Lebwohl. Targeted mostly on the TYK2 regulatory domain, deucravacitinib largely avoids inhibition of the JAK 1, 2, and 3 subtypes. Dr. Lebwohl said this explains why deucravacitinib labeling does not share the boxed warnings about off-target effects, such as those on the cardiovascular system, that can be found in the labeling of other JAK inhibitors.
In the published 52-week data, the discontinuation rate for adverse events was lower in the group randomized to deucravacitinib arm than in the placebo arm. In the extended follow-up, there were no new signals for adverse events, including those involving the CV system or immune function.
The key message so far from the long-term follow-up, which is ongoing, is that “continuous treatment with deucravacitinib is associated with durable efficacy,” Dr. Lebwohl said. It is this combination of sustained efficacy and safety that led Dr. Lebwohl to suggest it as a reasonable oral competitor to injectable biologics.
“Patients now have a choice,” he said.
Jashin J. Wu, MD, a board member of the National Psoriasis Foundation and an associate professor in the department of dermatology, University of Miami, has been following the development of deucravacitinib. He said that the recent FDA approval validates the clinical evidence of benefit and safety, while the long-term data presented at the EADV congress support its role in expanding treatment options.
“Deucravacitinib is a very effective oral agent for moderate to severe plaque psoriasis with strong maintenance of effect through week 112,” he said. Differentiating it from other JAK inhibitors, the FDA approval “confirms the safety of this agent as there is no boxed warning,” he added.
Dr. Lebwohl reports financial relationships with more than 30 pharmaceutical companies, including Bristol-Myers Squibb, the manufacturer of deucravacitinib. Dr. Wu has financial relationships with 14 pharmaceutical companies, including Bristol-Myers Squibb, but he was not an investigator for the phase 3 trials of deucravacitinib.
AT THE EADV CONGRESS
Targeted anti-IgE therapy found safe and effective for chronic urticaria
MILAN – The therapeutic .
Both doses of ligelizumab evaluated met the primary endpoint of superiority to placebo for a complete response at 16 weeks of therapy, reported Marcus Maurer, MD, director of the Urticaria Center for Reference and Excellence at the Charité Hospital, Berlin.
The data from the two identically designed trials, PEARL 1 and PEARL 2, were presented at the annual congress of the European Academy of Dermatology and Venereology. The two ligelizumab experimental arms (72 mg or 120 mg administered subcutaneously every 4 weeks) and the active comparative arm of omalizumab (300 mg administered subcutaneously every 4 weeks) demonstrated similar efficacy, all three of which were highly superior to placebo.
The data show that “another anti-IgE therapy – ligelizumab – is effective in CSU,” Dr. Maurer said.
“While the benefit was not different from omalizumab, ligelizumab showed remarkable results in disease activity and by demonstrating just how many patients achieved what we want them to achieve, which is to have no more signs and symptoms,” he added.
Majority of participants with severe urticaria
All of the patients entered into the two trials had severe (about 65%) or moderate (about 35%) symptoms at baseline. The results of the two trials were almost identical. In the randomization arms, a weekly Urticaria Activity Score (UAS7) of 0, which was the primary endpoint, was achieved at week 16 by 31.0% of those receiving 72-mg ligelizumab, 38.3% of those receiving 120-mg ligelizumab, and 34.1% of those receiving omalizumab (Xolair). The placebo response was 5.7%.
The UAS7 score is drawn from two components, wheals and itch. The range is 0 (no symptoms) to 42 (most severe). At baseline, the average patients’ scores were about 30, which correlates with a substantial symptom burden, according to Dr. Maurer.
The mean reduction in the UAS7 score in PEARL 2, which differed from PEARL 1 by no more than 0.4 points for any treatment group, was 19.2 points in the 72-mg ligelizumab group, 19.3 points in the 120-mg ligelizumab group, 19.6 points in the omalizumab group, and 9.2 points in the placebo group. There were no significant differences between any active treatment arm.
Complete symptom relief, meaning a UAS7 score of 0, was selected as the primary endpoint, because Dr. Maurer said that this is the goal of treatment. Although he admitted that a UAS7 score of 0 is analogous to a PASI score in psoriasis of 100 (complete clearing), he said, “Chronic urticaria is a debilitating disease, and we want to eliminate the symptoms. Gone is gone.”
Combined, the two phase 3 trials represent “the biggest chronic urticaria program ever,” according to Dr. Maurer. The 1,034 patients enrolled in PEARL 1 and the 1,023 enrolled in PEARL 2 were randomized in a 3:3:3:1 ratio with placebo representing the smaller group.
The planned follow-up is 52 weeks, but the placebo group will be switched to 120 mg ligelizumab every 4 weeks at the end of 24 weeks. The switch is required because “you cannot maintain patients with this disease on placebo over a long period,” Dr. Maurer said.
Ligelizumab associated with low discontinuation rate
Adverse events overall and stratified by severity have been similar across treatment arms, including placebo. The possible exception was a lower rate of moderate events (16.5%) in the placebo arm relative to the 72-mg ligelizumab arm (19.8%), the 120-mg ligelizumab arm (21.6%), and the omalizumab arm (22.3%). Discontinuations because of an adverse event were under 4% in every treatment arm.
Although Dr. Maurer did not present outcomes at 52 weeks, he did note that “only 15% of those who enrolled in these trials have discontinued treatment.” He considered this remarkable in that the study was conducted in the midst of the COVID-19 pandemic, and it appears that at least some of those left the trial did so because of concern for clinic visits.
Despite the similar benefit provided by ligelizumab and omalizumab, Dr. Maurer said that subgroup analyses will be coming. The possibility that some patients benefit more from one than the another cannot yet be ruled out. There are also, as of yet, no data to determine whether at least some patients respond to one after an inadequate response to the other.
Still, given the efficacy and the safety of ligelizumab, Dr. Maurer indicated that the drug is likely to find a role in routine management of CSU if approved.
“We only have two options for chronic spontaneous urticaria. There are antihistamines, which do not usually work, and omalizumab,” he said. “It is very important we develop more treatment options.”
Adam Friedman, MD, professor and chair of dermatology, George Washington University, Washington, agreed.
“More therapeutic options, especially for disease states that have a small armament – even if equivalent in efficacy to established therapies – is always a win for patients as it almost always increases access to treatment,” Dr. Friedman said in an interview.
“Furthermore, the heterogeneous nature of inflammatory skin diseases is often not captured in even phase 3 studies. Therefore, having additional options could offer relief where previous therapies have failed,” he added.
Dr. Maurer reports financial relationships with more than 10 pharmaceutical companies, including Novartis, which is developing ligelizumab. Dr. Friedman has a financial relationship with more than 20 pharmaceutical companies but has no current financial association with Novartis and was not involved in the PEARL 1 and 2 trials.
MILAN – The therapeutic .
Both doses of ligelizumab evaluated met the primary endpoint of superiority to placebo for a complete response at 16 weeks of therapy, reported Marcus Maurer, MD, director of the Urticaria Center for Reference and Excellence at the Charité Hospital, Berlin.
The data from the two identically designed trials, PEARL 1 and PEARL 2, were presented at the annual congress of the European Academy of Dermatology and Venereology. The two ligelizumab experimental arms (72 mg or 120 mg administered subcutaneously every 4 weeks) and the active comparative arm of omalizumab (300 mg administered subcutaneously every 4 weeks) demonstrated similar efficacy, all three of which were highly superior to placebo.
The data show that “another anti-IgE therapy – ligelizumab – is effective in CSU,” Dr. Maurer said.
“While the benefit was not different from omalizumab, ligelizumab showed remarkable results in disease activity and by demonstrating just how many patients achieved what we want them to achieve, which is to have no more signs and symptoms,” he added.
Majority of participants with severe urticaria
All of the patients entered into the two trials had severe (about 65%) or moderate (about 35%) symptoms at baseline. The results of the two trials were almost identical. In the randomization arms, a weekly Urticaria Activity Score (UAS7) of 0, which was the primary endpoint, was achieved at week 16 by 31.0% of those receiving 72-mg ligelizumab, 38.3% of those receiving 120-mg ligelizumab, and 34.1% of those receiving omalizumab (Xolair). The placebo response was 5.7%.
The UAS7 score is drawn from two components, wheals and itch. The range is 0 (no symptoms) to 42 (most severe). At baseline, the average patients’ scores were about 30, which correlates with a substantial symptom burden, according to Dr. Maurer.
The mean reduction in the UAS7 score in PEARL 2, which differed from PEARL 1 by no more than 0.4 points for any treatment group, was 19.2 points in the 72-mg ligelizumab group, 19.3 points in the 120-mg ligelizumab group, 19.6 points in the omalizumab group, and 9.2 points in the placebo group. There were no significant differences between any active treatment arm.
Complete symptom relief, meaning a UAS7 score of 0, was selected as the primary endpoint, because Dr. Maurer said that this is the goal of treatment. Although he admitted that a UAS7 score of 0 is analogous to a PASI score in psoriasis of 100 (complete clearing), he said, “Chronic urticaria is a debilitating disease, and we want to eliminate the symptoms. Gone is gone.”
Combined, the two phase 3 trials represent “the biggest chronic urticaria program ever,” according to Dr. Maurer. The 1,034 patients enrolled in PEARL 1 and the 1,023 enrolled in PEARL 2 were randomized in a 3:3:3:1 ratio with placebo representing the smaller group.
The planned follow-up is 52 weeks, but the placebo group will be switched to 120 mg ligelizumab every 4 weeks at the end of 24 weeks. The switch is required because “you cannot maintain patients with this disease on placebo over a long period,” Dr. Maurer said.
Ligelizumab associated with low discontinuation rate
Adverse events overall and stratified by severity have been similar across treatment arms, including placebo. The possible exception was a lower rate of moderate events (16.5%) in the placebo arm relative to the 72-mg ligelizumab arm (19.8%), the 120-mg ligelizumab arm (21.6%), and the omalizumab arm (22.3%). Discontinuations because of an adverse event were under 4% in every treatment arm.
Although Dr. Maurer did not present outcomes at 52 weeks, he did note that “only 15% of those who enrolled in these trials have discontinued treatment.” He considered this remarkable in that the study was conducted in the midst of the COVID-19 pandemic, and it appears that at least some of those left the trial did so because of concern for clinic visits.
Despite the similar benefit provided by ligelizumab and omalizumab, Dr. Maurer said that subgroup analyses will be coming. The possibility that some patients benefit more from one than the another cannot yet be ruled out. There are also, as of yet, no data to determine whether at least some patients respond to one after an inadequate response to the other.
Still, given the efficacy and the safety of ligelizumab, Dr. Maurer indicated that the drug is likely to find a role in routine management of CSU if approved.
“We only have two options for chronic spontaneous urticaria. There are antihistamines, which do not usually work, and omalizumab,” he said. “It is very important we develop more treatment options.”
Adam Friedman, MD, professor and chair of dermatology, George Washington University, Washington, agreed.
“More therapeutic options, especially for disease states that have a small armament – even if equivalent in efficacy to established therapies – is always a win for patients as it almost always increases access to treatment,” Dr. Friedman said in an interview.
“Furthermore, the heterogeneous nature of inflammatory skin diseases is often not captured in even phase 3 studies. Therefore, having additional options could offer relief where previous therapies have failed,” he added.
Dr. Maurer reports financial relationships with more than 10 pharmaceutical companies, including Novartis, which is developing ligelizumab. Dr. Friedman has a financial relationship with more than 20 pharmaceutical companies but has no current financial association with Novartis and was not involved in the PEARL 1 and 2 trials.
MILAN – The therapeutic .
Both doses of ligelizumab evaluated met the primary endpoint of superiority to placebo for a complete response at 16 weeks of therapy, reported Marcus Maurer, MD, director of the Urticaria Center for Reference and Excellence at the Charité Hospital, Berlin.
The data from the two identically designed trials, PEARL 1 and PEARL 2, were presented at the annual congress of the European Academy of Dermatology and Venereology. The two ligelizumab experimental arms (72 mg or 120 mg administered subcutaneously every 4 weeks) and the active comparative arm of omalizumab (300 mg administered subcutaneously every 4 weeks) demonstrated similar efficacy, all three of which were highly superior to placebo.
The data show that “another anti-IgE therapy – ligelizumab – is effective in CSU,” Dr. Maurer said.
“While the benefit was not different from omalizumab, ligelizumab showed remarkable results in disease activity and by demonstrating just how many patients achieved what we want them to achieve, which is to have no more signs and symptoms,” he added.
Majority of participants with severe urticaria
All of the patients entered into the two trials had severe (about 65%) or moderate (about 35%) symptoms at baseline. The results of the two trials were almost identical. In the randomization arms, a weekly Urticaria Activity Score (UAS7) of 0, which was the primary endpoint, was achieved at week 16 by 31.0% of those receiving 72-mg ligelizumab, 38.3% of those receiving 120-mg ligelizumab, and 34.1% of those receiving omalizumab (Xolair). The placebo response was 5.7%.
The UAS7 score is drawn from two components, wheals and itch. The range is 0 (no symptoms) to 42 (most severe). At baseline, the average patients’ scores were about 30, which correlates with a substantial symptom burden, according to Dr. Maurer.
The mean reduction in the UAS7 score in PEARL 2, which differed from PEARL 1 by no more than 0.4 points for any treatment group, was 19.2 points in the 72-mg ligelizumab group, 19.3 points in the 120-mg ligelizumab group, 19.6 points in the omalizumab group, and 9.2 points in the placebo group. There were no significant differences between any active treatment arm.
Complete symptom relief, meaning a UAS7 score of 0, was selected as the primary endpoint, because Dr. Maurer said that this is the goal of treatment. Although he admitted that a UAS7 score of 0 is analogous to a PASI score in psoriasis of 100 (complete clearing), he said, “Chronic urticaria is a debilitating disease, and we want to eliminate the symptoms. Gone is gone.”
Combined, the two phase 3 trials represent “the biggest chronic urticaria program ever,” according to Dr. Maurer. The 1,034 patients enrolled in PEARL 1 and the 1,023 enrolled in PEARL 2 were randomized in a 3:3:3:1 ratio with placebo representing the smaller group.
The planned follow-up is 52 weeks, but the placebo group will be switched to 120 mg ligelizumab every 4 weeks at the end of 24 weeks. The switch is required because “you cannot maintain patients with this disease on placebo over a long period,” Dr. Maurer said.
Ligelizumab associated with low discontinuation rate
Adverse events overall and stratified by severity have been similar across treatment arms, including placebo. The possible exception was a lower rate of moderate events (16.5%) in the placebo arm relative to the 72-mg ligelizumab arm (19.8%), the 120-mg ligelizumab arm (21.6%), and the omalizumab arm (22.3%). Discontinuations because of an adverse event were under 4% in every treatment arm.
Although Dr. Maurer did not present outcomes at 52 weeks, he did note that “only 15% of those who enrolled in these trials have discontinued treatment.” He considered this remarkable in that the study was conducted in the midst of the COVID-19 pandemic, and it appears that at least some of those left the trial did so because of concern for clinic visits.
Despite the similar benefit provided by ligelizumab and omalizumab, Dr. Maurer said that subgroup analyses will be coming. The possibility that some patients benefit more from one than the another cannot yet be ruled out. There are also, as of yet, no data to determine whether at least some patients respond to one after an inadequate response to the other.
Still, given the efficacy and the safety of ligelizumab, Dr. Maurer indicated that the drug is likely to find a role in routine management of CSU if approved.
“We only have two options for chronic spontaneous urticaria. There are antihistamines, which do not usually work, and omalizumab,” he said. “It is very important we develop more treatment options.”
Adam Friedman, MD, professor and chair of dermatology, George Washington University, Washington, agreed.
“More therapeutic options, especially for disease states that have a small armament – even if equivalent in efficacy to established therapies – is always a win for patients as it almost always increases access to treatment,” Dr. Friedman said in an interview.
“Furthermore, the heterogeneous nature of inflammatory skin diseases is often not captured in even phase 3 studies. Therefore, having additional options could offer relief where previous therapies have failed,” he added.
Dr. Maurer reports financial relationships with more than 10 pharmaceutical companies, including Novartis, which is developing ligelizumab. Dr. Friedman has a financial relationship with more than 20 pharmaceutical companies but has no current financial association with Novartis and was not involved in the PEARL 1 and 2 trials.
AT THE EADV CONGRESS
FDA warns of cancer risk in scar tissue around breast implants
.
The FDA safety communication is based on several dozen reports of these cancers occurring in the capsule or scar tissue around breast implants. This issue differs from breast implant–associated anaplastic large-cell lymphoma (BIA-ALCL) – a known risk among implant recipients.
“After preliminary review of published literature as part of our ongoing monitoring of the safety of breast implants, the FDA is aware of less than 20 cases of SCC and less than 30 cases of various lymphomas in the capsule around the breast implant,” the agency’s alert explains.
One avenue through which the FDA has identified cases is via medical device reports. As of Sept. 1, the FDA has received 10 medical device reports about SCC related to breast implants and 12 about various lymphomas.
The incidence rate and risk factors for these events are currently unknown, but reports of SCC and various lymphomas in the capsule around the breast implants have been reported for both textured and smooth breast implants, as well as for both saline and silicone breast implants. In some cases, the cancers were diagnosed years after breast implant surgery.
Reported signs and symptoms included swelling, pain, lumps, or skin changes.
Although the risks of SCC and lymphomas in the tissue around breast implants appears rare, “when safety risks with medical devices are identified, we wanted to provide clear and understandable information to the public as quickly as possible,” Binita Ashar, MD, director of the Office of Surgical and Infection Control Devices, FDA Center for Devices and Radiological Health, explained in a press release.
Patients and providers are strongly encouraged to report breast implant–related problems and cases of SCC or lymphoma of the breast implant capsule to MedWatch, the FDA’s adverse event reporting program.
The FDA plans to complete “a thorough literature review” as well as “identify ways to collect more detailed information regarding patient cases.”
A version of this article first appeared on Medscape.com.
.
The FDA safety communication is based on several dozen reports of these cancers occurring in the capsule or scar tissue around breast implants. This issue differs from breast implant–associated anaplastic large-cell lymphoma (BIA-ALCL) – a known risk among implant recipients.
“After preliminary review of published literature as part of our ongoing monitoring of the safety of breast implants, the FDA is aware of less than 20 cases of SCC and less than 30 cases of various lymphomas in the capsule around the breast implant,” the agency’s alert explains.
One avenue through which the FDA has identified cases is via medical device reports. As of Sept. 1, the FDA has received 10 medical device reports about SCC related to breast implants and 12 about various lymphomas.
The incidence rate and risk factors for these events are currently unknown, but reports of SCC and various lymphomas in the capsule around the breast implants have been reported for both textured and smooth breast implants, as well as for both saline and silicone breast implants. In some cases, the cancers were diagnosed years after breast implant surgery.
Reported signs and symptoms included swelling, pain, lumps, or skin changes.
Although the risks of SCC and lymphomas in the tissue around breast implants appears rare, “when safety risks with medical devices are identified, we wanted to provide clear and understandable information to the public as quickly as possible,” Binita Ashar, MD, director of the Office of Surgical and Infection Control Devices, FDA Center for Devices and Radiological Health, explained in a press release.
Patients and providers are strongly encouraged to report breast implant–related problems and cases of SCC or lymphoma of the breast implant capsule to MedWatch, the FDA’s adverse event reporting program.
The FDA plans to complete “a thorough literature review” as well as “identify ways to collect more detailed information regarding patient cases.”
A version of this article first appeared on Medscape.com.
.
The FDA safety communication is based on several dozen reports of these cancers occurring in the capsule or scar tissue around breast implants. This issue differs from breast implant–associated anaplastic large-cell lymphoma (BIA-ALCL) – a known risk among implant recipients.
“After preliminary review of published literature as part of our ongoing monitoring of the safety of breast implants, the FDA is aware of less than 20 cases of SCC and less than 30 cases of various lymphomas in the capsule around the breast implant,” the agency’s alert explains.
One avenue through which the FDA has identified cases is via medical device reports. As of Sept. 1, the FDA has received 10 medical device reports about SCC related to breast implants and 12 about various lymphomas.
The incidence rate and risk factors for these events are currently unknown, but reports of SCC and various lymphomas in the capsule around the breast implants have been reported for both textured and smooth breast implants, as well as for both saline and silicone breast implants. In some cases, the cancers were diagnosed years after breast implant surgery.
Reported signs and symptoms included swelling, pain, lumps, or skin changes.
Although the risks of SCC and lymphomas in the tissue around breast implants appears rare, “when safety risks with medical devices are identified, we wanted to provide clear and understandable information to the public as quickly as possible,” Binita Ashar, MD, director of the Office of Surgical and Infection Control Devices, FDA Center for Devices and Radiological Health, explained in a press release.
Patients and providers are strongly encouraged to report breast implant–related problems and cases of SCC or lymphoma of the breast implant capsule to MedWatch, the FDA’s adverse event reporting program.
The FDA plans to complete “a thorough literature review” as well as “identify ways to collect more detailed information regarding patient cases.”
A version of this article first appeared on Medscape.com.
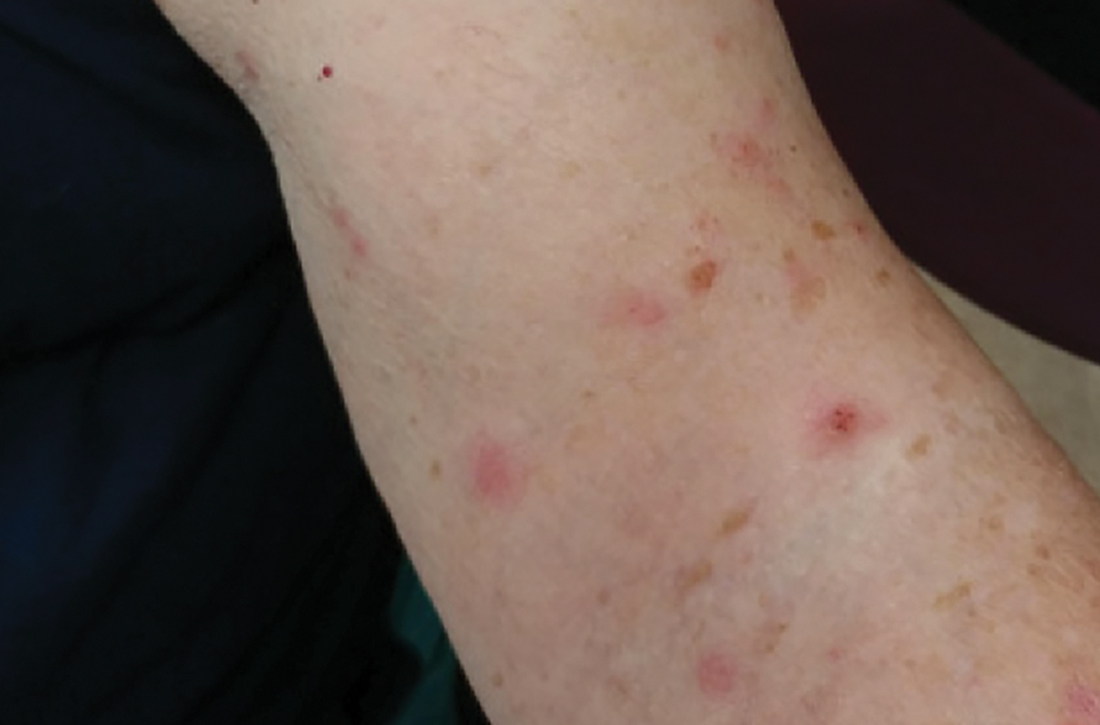
An asymptomatic rash
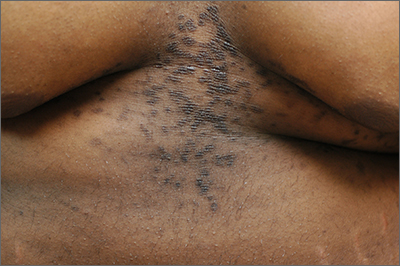
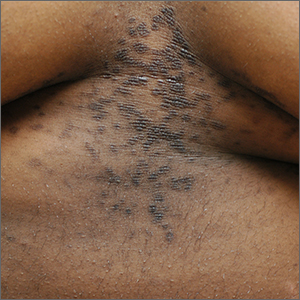
This patient was given a diagnosis of confluent and reticulated papillomatosis (CRP) based on the clinical presentation.
CRP is characterized by centrally confluent and peripherally reticulated scaly brown plaques and papules that are cosmetically disfiguring.1 CRP is usually asymptomatic and primarily impacts young adults—especially teenagers.2,3 It affects both males and females and commonly occurs on the trunk.1-3 CRP is believed to be a disorder of keratinization. Malassezia furfur may induce CRP’s hyperproliferative epidermal changes, but systemic treatment that eliminates this organism does not clear CRP.3
A CRP diagnosis is made based on clinical presentation. The eruption usually begins as verrucous papules in the inframammary or epigastric region that enlarge to 4 to 5 mm in diameter and coalesce to form a confluent plaque with a peripheral reticulated pattern. CRP can extend over the back, chest, and abdomen to the neck, shoulders, and gluteal cleft. CRP does not affect the oral mucosa, and rarely involves flexural areas, which differentiates it from the similar looking acanthosis nigricans.2 Although most cases are asymptomatic, mild pruritus may occur.1,2
A skin biopsy is rarely necessary for making a CRP diagnosis, but histopathologic findings include papillomatosis, hyperkeratosis, variable acanthosis, follicular plugging, and sparse dermal inflammation.1,3
Systemic antibiotics, most commonly minocycline 100 mg bid for 30 days or doxycycline 100 mg bid for 30 days, are safe and effective for CRP.1,4 Sometimes treatment is extended for as long as 6 months. Although CRP usually responds to minocycline or doxycycline, it is believed that this is the result of these drugs’ anti-inflammatory—rather than antibiotic—properties.1,2,4 Azithromycin is an effective alternative therapy.2,4 There is a high rate of recurrence of CRP in patients after systemic antibiotics are discontinued.2 Uniform responses to treatment and retreatment of flares have solidified the belief that antibiotics are an effective suppressive (if not curative) therapy despite a lack of randomized controlled trials.4
This patient was treated with minocycline 100 mg bid. After 1 month, the rash had improved by 70%. At 3 months, it was completely clear, and treatment was discontinued.
This case was adapted from: Sessums MT, Ward KMH, Brodell R. Cutaneous eruption on chest and back. J Fam Pract. 2014;63:467-468.
1. Davis MD, Weenig RH, Camilleri MJ. Confluent and reticulated papillomatosis (Gougerot-Carteaud syndrome): a minocycline-responsive dermatosis without evidence for yeast in pathogenesis. A study of 39 patients and a proposal of diagnostic criteria. Br J Dermatol. 2006;154:287-293. doi: 10.1111/j.1365-2133.2005.06955.x
2. Scheinfeld N. Confluent and reticulated papillomatosis: a review of the literature. Am J Clin Dermatol. 2006;7:305-313. doi: 10.2165/00128071-200607050-00004
3. Tamraz H, Raffoul M, Kurban M, et al. Confluent and reticulated papillomatosis: clinical and histopathological study of 10 cases from Lebanon. J Eur Acad Dermatol Venereol. 2013;27:e119-e123. doi: 10.1111/j.1468-3083.2011.04328.x
4. Jang HS, Oh CK, Cha JH, et al. Six cases of confluent and reticulated papillomatosis alleviated by various antibiotics. J Am Acad Dermatol. 2001;44:652-655. doi: 10.1067/mjd.2001.112577

This patient was given a diagnosis of confluent and reticulated papillomatosis (CRP) based on the clinical presentation.
CRP is characterized by centrally confluent and peripherally reticulated scaly brown plaques and papules that are cosmetically disfiguring.1 CRP is usually asymptomatic and primarily impacts young adults—especially teenagers.2,3 It affects both males and females and commonly occurs on the trunk.1-3 CRP is believed to be a disorder of keratinization. Malassezia furfur may induce CRP’s hyperproliferative epidermal changes, but systemic treatment that eliminates this organism does not clear CRP.3
A CRP diagnosis is made based on clinical presentation. The eruption usually begins as verrucous papules in the inframammary or epigastric region that enlarge to 4 to 5 mm in diameter and coalesce to form a confluent plaque with a peripheral reticulated pattern. CRP can extend over the back, chest, and abdomen to the neck, shoulders, and gluteal cleft. CRP does not affect the oral mucosa, and rarely involves flexural areas, which differentiates it from the similar looking acanthosis nigricans.2 Although most cases are asymptomatic, mild pruritus may occur.1,2
A skin biopsy is rarely necessary for making a CRP diagnosis, but histopathologic findings include papillomatosis, hyperkeratosis, variable acanthosis, follicular plugging, and sparse dermal inflammation.1,3
Systemic antibiotics, most commonly minocycline 100 mg bid for 30 days or doxycycline 100 mg bid for 30 days, are safe and effective for CRP.1,4 Sometimes treatment is extended for as long as 6 months. Although CRP usually responds to minocycline or doxycycline, it is believed that this is the result of these drugs’ anti-inflammatory—rather than antibiotic—properties.1,2,4 Azithromycin is an effective alternative therapy.2,4 There is a high rate of recurrence of CRP in patients after systemic antibiotics are discontinued.2 Uniform responses to treatment and retreatment of flares have solidified the belief that antibiotics are an effective suppressive (if not curative) therapy despite a lack of randomized controlled trials.4
This patient was treated with minocycline 100 mg bid. After 1 month, the rash had improved by 70%. At 3 months, it was completely clear, and treatment was discontinued.
This case was adapted from: Sessums MT, Ward KMH, Brodell R. Cutaneous eruption on chest and back. J Fam Pract. 2014;63:467-468.

This patient was given a diagnosis of confluent and reticulated papillomatosis (CRP) based on the clinical presentation.
CRP is characterized by centrally confluent and peripherally reticulated scaly brown plaques and papules that are cosmetically disfiguring.1 CRP is usually asymptomatic and primarily impacts young adults—especially teenagers.2,3 It affects both males and females and commonly occurs on the trunk.1-3 CRP is believed to be a disorder of keratinization. Malassezia furfur may induce CRP’s hyperproliferative epidermal changes, but systemic treatment that eliminates this organism does not clear CRP.3
A CRP diagnosis is made based on clinical presentation. The eruption usually begins as verrucous papules in the inframammary or epigastric region that enlarge to 4 to 5 mm in diameter and coalesce to form a confluent plaque with a peripheral reticulated pattern. CRP can extend over the back, chest, and abdomen to the neck, shoulders, and gluteal cleft. CRP does not affect the oral mucosa, and rarely involves flexural areas, which differentiates it from the similar looking acanthosis nigricans.2 Although most cases are asymptomatic, mild pruritus may occur.1,2
A skin biopsy is rarely necessary for making a CRP diagnosis, but histopathologic findings include papillomatosis, hyperkeratosis, variable acanthosis, follicular plugging, and sparse dermal inflammation.1,3
Systemic antibiotics, most commonly minocycline 100 mg bid for 30 days or doxycycline 100 mg bid for 30 days, are safe and effective for CRP.1,4 Sometimes treatment is extended for as long as 6 months. Although CRP usually responds to minocycline or doxycycline, it is believed that this is the result of these drugs’ anti-inflammatory—rather than antibiotic—properties.1,2,4 Azithromycin is an effective alternative therapy.2,4 There is a high rate of recurrence of CRP in patients after systemic antibiotics are discontinued.2 Uniform responses to treatment and retreatment of flares have solidified the belief that antibiotics are an effective suppressive (if not curative) therapy despite a lack of randomized controlled trials.4
This patient was treated with minocycline 100 mg bid. After 1 month, the rash had improved by 70%. At 3 months, it was completely clear, and treatment was discontinued.
This case was adapted from: Sessums MT, Ward KMH, Brodell R. Cutaneous eruption on chest and back. J Fam Pract. 2014;63:467-468.
1. Davis MD, Weenig RH, Camilleri MJ. Confluent and reticulated papillomatosis (Gougerot-Carteaud syndrome): a minocycline-responsive dermatosis without evidence for yeast in pathogenesis. A study of 39 patients and a proposal of diagnostic criteria. Br J Dermatol. 2006;154:287-293. doi: 10.1111/j.1365-2133.2005.06955.x
2. Scheinfeld N. Confluent and reticulated papillomatosis: a review of the literature. Am J Clin Dermatol. 2006;7:305-313. doi: 10.2165/00128071-200607050-00004
3. Tamraz H, Raffoul M, Kurban M, et al. Confluent and reticulated papillomatosis: clinical and histopathological study of 10 cases from Lebanon. J Eur Acad Dermatol Venereol. 2013;27:e119-e123. doi: 10.1111/j.1468-3083.2011.04328.x
4. Jang HS, Oh CK, Cha JH, et al. Six cases of confluent and reticulated papillomatosis alleviated by various antibiotics. J Am Acad Dermatol. 2001;44:652-655. doi: 10.1067/mjd.2001.112577
1. Davis MD, Weenig RH, Camilleri MJ. Confluent and reticulated papillomatosis (Gougerot-Carteaud syndrome): a minocycline-responsive dermatosis without evidence for yeast in pathogenesis. A study of 39 patients and a proposal of diagnostic criteria. Br J Dermatol. 2006;154:287-293. doi: 10.1111/j.1365-2133.2005.06955.x
2. Scheinfeld N. Confluent and reticulated papillomatosis: a review of the literature. Am J Clin Dermatol. 2006;7:305-313. doi: 10.2165/00128071-200607050-00004
3. Tamraz H, Raffoul M, Kurban M, et al. Confluent and reticulated papillomatosis: clinical and histopathological study of 10 cases from Lebanon. J Eur Acad Dermatol Venereol. 2013;27:e119-e123. doi: 10.1111/j.1468-3083.2011.04328.x
4. Jang HS, Oh CK, Cha JH, et al. Six cases of confluent and reticulated papillomatosis alleviated by various antibiotics. J Am Acad Dermatol. 2001;44:652-655. doi: 10.1067/mjd.2001.112577
Roflumilast foam effectively eases seborrheic dermatitis
.
More than half experienced clearance of their symptoms, and three out of five achieved a significant improvement in pruritus, it was revealed during a late-breaking session at the annual congress of the European Academy of Dermatology and Venereology.
Common condition led to rapid recruitment
“Seborrheic dermatitis is a disease that’s very common, yet in my opinion, undertreated in dermatology,” said Andrew Blauvelt, MD, MBA, who presented the findings.
“It’s so common that when we did this trial, I was very surprised to see how easy it was to recruit,” said Dr. Blauvelt, a dermatologist who is president of the Oregon Medical Research Center, Portland. “Patients came in rapidly, out of the woodwork – they were desperate.”
While there are several tried and tested treatments for the condition, such as topical steroids and antifungal agents, he noted that they have their limitations: “Sometimes efficacy, sometimes the ability to be used on hair-bearing areas.”
Roflumilast is a phosphodiesterase 4 (PDE4) inhibitor that is available for topical use in a 0.3% cream formulation (Zoryve). This formulation gained FDA approval for plaque psoriasis for patients ages 12 and older this summer and is also under investigation as a treatment for atopic dermatitis.
It’s the same product in both preparations, Dr. Blauvelt said during the discussion period. “The only major difference between the cream and the foam is the propellant used to make it into a foam. Otherwise, they have the exact same list of ingredients.”
Dr. Blauvelt reported that just over 450 patients had been recruited at 53 U.S. centers into the 8-week, double-blind, placebo-controlled trial.
For inclusion, patients had to have moderate seborrheic dermatitis, defined as an Investigator’s Global Assessment (IGA) score of three or more. Dr. Blauvelt noted that patients as young as 9 years old could be recruited, and there was no upper age limit. The average age of participating patients, however, was around 42 years.
Multiple improvements seen in ‘happy trial’
The primary endpoint was an IGA score of 0 or 1 with at least a 2-grade improvement (IGA success) after 8 weeks of treatment. This was achieved by 80% of patients who were treated with roflumilast 0.3% foam, compared with 60% of those who were treated with the vehicle (P less than .0001).
Dr. Blauvelt pointed out that significant improvements had also been seen after 2 weeks (about 42% vs. about 26%; P = .0003) and 4 weeks (about 72% vs. about 49%; P less than .0001) of treatment.
“Now if we raise the bar a little higher” and ask how many patients were completely clear of their seborrheic dermatitis, Dr. Blauvelt said, it was 50% at 8 weeks, more than a third at 4 weeks, over 15% at 2 weeks with the foam, and significantly lower at just under 30%, 15%, and 7% in the vehicle group.
A 4-point or more improvement in the Worst Itch Numeric Rating Scale (WI-NRS) – accepted as the minimally clinically important difference – was achieved by more than 60% of patients treated with the foam at week 8, just under 50% at week 4, and just over 30% at week 2. Corresponding rates in the vehicle group were around 40%, 30%, and 15%.
“Many patients responded in this trial. So much so that when I was doing it, I called it the ‘happy trial.’ Every time I saw patients in this trial, they seemed to be happy,” Dr. Blauvelt said anecdotally.
“In terms of adverse events, the drug turned out to be very safe, and there didn’t seem to be any issues with any things that we see with, for example, oral phosphodiesterase inhibitors,” he added.
The tolerability findings suggest that the foam vehicle “was an excellent vehicle to be used for this particular drug,” with no signs of skin irritation, as rated by patients or investigators.
Lesson for practice: Advise patients to moisturize?
“It seems like the vehicle would be a good skincare product for patients,” observed the session’s cochair, Jo Lambert, MD, PhD, professor and academic head of the department of dermatology at Ghent University Hospital, Belgium.
It was “a pretty dramatic vehicle response, right?” Dr. Blauvelt responded. “We normally don’t think of telling seborrheic dermatitis patients to moisturize,” he added.
“I think one of the interesting findings is perhaps we should be telling them to moisturize their scalp or moisturize their face, or it could be something unique to this particular foam.”
The study was funded by Arcutis Biotherapeutics. Dr. Blauvelt disclosed that he was an investigator for the trial and acted as consultant to the company, receiving grants/research funding and/or honoraria. Several of the study’s co-investigators are employees of Arcutis. Dr. Lambert was not involved in the study and cochaired the late-breaking session during which the STRATUM trial findings were reported.
A version of this article first appeared on Medscape.com.
.
More than half experienced clearance of their symptoms, and three out of five achieved a significant improvement in pruritus, it was revealed during a late-breaking session at the annual congress of the European Academy of Dermatology and Venereology.
Common condition led to rapid recruitment
“Seborrheic dermatitis is a disease that’s very common, yet in my opinion, undertreated in dermatology,” said Andrew Blauvelt, MD, MBA, who presented the findings.
“It’s so common that when we did this trial, I was very surprised to see how easy it was to recruit,” said Dr. Blauvelt, a dermatologist who is president of the Oregon Medical Research Center, Portland. “Patients came in rapidly, out of the woodwork – they were desperate.”
While there are several tried and tested treatments for the condition, such as topical steroids and antifungal agents, he noted that they have their limitations: “Sometimes efficacy, sometimes the ability to be used on hair-bearing areas.”
Roflumilast is a phosphodiesterase 4 (PDE4) inhibitor that is available for topical use in a 0.3% cream formulation (Zoryve). This formulation gained FDA approval for plaque psoriasis for patients ages 12 and older this summer and is also under investigation as a treatment for atopic dermatitis.
It’s the same product in both preparations, Dr. Blauvelt said during the discussion period. “The only major difference between the cream and the foam is the propellant used to make it into a foam. Otherwise, they have the exact same list of ingredients.”
Dr. Blauvelt reported that just over 450 patients had been recruited at 53 U.S. centers into the 8-week, double-blind, placebo-controlled trial.
For inclusion, patients had to have moderate seborrheic dermatitis, defined as an Investigator’s Global Assessment (IGA) score of three or more. Dr. Blauvelt noted that patients as young as 9 years old could be recruited, and there was no upper age limit. The average age of participating patients, however, was around 42 years.
Multiple improvements seen in ‘happy trial’
The primary endpoint was an IGA score of 0 or 1 with at least a 2-grade improvement (IGA success) after 8 weeks of treatment. This was achieved by 80% of patients who were treated with roflumilast 0.3% foam, compared with 60% of those who were treated with the vehicle (P less than .0001).
Dr. Blauvelt pointed out that significant improvements had also been seen after 2 weeks (about 42% vs. about 26%; P = .0003) and 4 weeks (about 72% vs. about 49%; P less than .0001) of treatment.
“Now if we raise the bar a little higher” and ask how many patients were completely clear of their seborrheic dermatitis, Dr. Blauvelt said, it was 50% at 8 weeks, more than a third at 4 weeks, over 15% at 2 weeks with the foam, and significantly lower at just under 30%, 15%, and 7% in the vehicle group.
A 4-point or more improvement in the Worst Itch Numeric Rating Scale (WI-NRS) – accepted as the minimally clinically important difference – was achieved by more than 60% of patients treated with the foam at week 8, just under 50% at week 4, and just over 30% at week 2. Corresponding rates in the vehicle group were around 40%, 30%, and 15%.
“Many patients responded in this trial. So much so that when I was doing it, I called it the ‘happy trial.’ Every time I saw patients in this trial, they seemed to be happy,” Dr. Blauvelt said anecdotally.
“In terms of adverse events, the drug turned out to be very safe, and there didn’t seem to be any issues with any things that we see with, for example, oral phosphodiesterase inhibitors,” he added.
The tolerability findings suggest that the foam vehicle “was an excellent vehicle to be used for this particular drug,” with no signs of skin irritation, as rated by patients or investigators.
Lesson for practice: Advise patients to moisturize?
“It seems like the vehicle would be a good skincare product for patients,” observed the session’s cochair, Jo Lambert, MD, PhD, professor and academic head of the department of dermatology at Ghent University Hospital, Belgium.
It was “a pretty dramatic vehicle response, right?” Dr. Blauvelt responded. “We normally don’t think of telling seborrheic dermatitis patients to moisturize,” he added.
“I think one of the interesting findings is perhaps we should be telling them to moisturize their scalp or moisturize their face, or it could be something unique to this particular foam.”
The study was funded by Arcutis Biotherapeutics. Dr. Blauvelt disclosed that he was an investigator for the trial and acted as consultant to the company, receiving grants/research funding and/or honoraria. Several of the study’s co-investigators are employees of Arcutis. Dr. Lambert was not involved in the study and cochaired the late-breaking session during which the STRATUM trial findings were reported.
A version of this article first appeared on Medscape.com.
.
More than half experienced clearance of their symptoms, and three out of five achieved a significant improvement in pruritus, it was revealed during a late-breaking session at the annual congress of the European Academy of Dermatology and Venereology.
Common condition led to rapid recruitment
“Seborrheic dermatitis is a disease that’s very common, yet in my opinion, undertreated in dermatology,” said Andrew Blauvelt, MD, MBA, who presented the findings.
“It’s so common that when we did this trial, I was very surprised to see how easy it was to recruit,” said Dr. Blauvelt, a dermatologist who is president of the Oregon Medical Research Center, Portland. “Patients came in rapidly, out of the woodwork – they were desperate.”
While there are several tried and tested treatments for the condition, such as topical steroids and antifungal agents, he noted that they have their limitations: “Sometimes efficacy, sometimes the ability to be used on hair-bearing areas.”
Roflumilast is a phosphodiesterase 4 (PDE4) inhibitor that is available for topical use in a 0.3% cream formulation (Zoryve). This formulation gained FDA approval for plaque psoriasis for patients ages 12 and older this summer and is also under investigation as a treatment for atopic dermatitis.
It’s the same product in both preparations, Dr. Blauvelt said during the discussion period. “The only major difference between the cream and the foam is the propellant used to make it into a foam. Otherwise, they have the exact same list of ingredients.”
Dr. Blauvelt reported that just over 450 patients had been recruited at 53 U.S. centers into the 8-week, double-blind, placebo-controlled trial.
For inclusion, patients had to have moderate seborrheic dermatitis, defined as an Investigator’s Global Assessment (IGA) score of three or more. Dr. Blauvelt noted that patients as young as 9 years old could be recruited, and there was no upper age limit. The average age of participating patients, however, was around 42 years.
Multiple improvements seen in ‘happy trial’
The primary endpoint was an IGA score of 0 or 1 with at least a 2-grade improvement (IGA success) after 8 weeks of treatment. This was achieved by 80% of patients who were treated with roflumilast 0.3% foam, compared with 60% of those who were treated with the vehicle (P less than .0001).
Dr. Blauvelt pointed out that significant improvements had also been seen after 2 weeks (about 42% vs. about 26%; P = .0003) and 4 weeks (about 72% vs. about 49%; P less than .0001) of treatment.
“Now if we raise the bar a little higher” and ask how many patients were completely clear of their seborrheic dermatitis, Dr. Blauvelt said, it was 50% at 8 weeks, more than a third at 4 weeks, over 15% at 2 weeks with the foam, and significantly lower at just under 30%, 15%, and 7% in the vehicle group.
A 4-point or more improvement in the Worst Itch Numeric Rating Scale (WI-NRS) – accepted as the minimally clinically important difference – was achieved by more than 60% of patients treated with the foam at week 8, just under 50% at week 4, and just over 30% at week 2. Corresponding rates in the vehicle group were around 40%, 30%, and 15%.
“Many patients responded in this trial. So much so that when I was doing it, I called it the ‘happy trial.’ Every time I saw patients in this trial, they seemed to be happy,” Dr. Blauvelt said anecdotally.
“In terms of adverse events, the drug turned out to be very safe, and there didn’t seem to be any issues with any things that we see with, for example, oral phosphodiesterase inhibitors,” he added.
The tolerability findings suggest that the foam vehicle “was an excellent vehicle to be used for this particular drug,” with no signs of skin irritation, as rated by patients or investigators.
Lesson for practice: Advise patients to moisturize?
“It seems like the vehicle would be a good skincare product for patients,” observed the session’s cochair, Jo Lambert, MD, PhD, professor and academic head of the department of dermatology at Ghent University Hospital, Belgium.
It was “a pretty dramatic vehicle response, right?” Dr. Blauvelt responded. “We normally don’t think of telling seborrheic dermatitis patients to moisturize,” he added.
“I think one of the interesting findings is perhaps we should be telling them to moisturize their scalp or moisturize their face, or it could be something unique to this particular foam.”
The study was funded by Arcutis Biotherapeutics. Dr. Blauvelt disclosed that he was an investigator for the trial and acted as consultant to the company, receiving grants/research funding and/or honoraria. Several of the study’s co-investigators are employees of Arcutis. Dr. Lambert was not involved in the study and cochaired the late-breaking session during which the STRATUM trial findings were reported.
A version of this article first appeared on Medscape.com.
FROM THE EADV CONGRESS
Dupilumab offers ‘clinically meaningful’ improvements in prurigo nodularis
(Dupixent), indicate results from the phase 2 LIBERTY-PN PRIME trial.
The research was presented at the annual Congress of the European Academy of Dermatology and Venereology.
More than 150 patients with severe PN whose quality of life was impaired were randomly assigned to receive dupilumab (Dupixent) or placebo for 24 weeks. Use of the monoclonal antibody was associated with significant improvements in itch scores.
The researchers also found that the percentage of patients who had no or few PN lesions increased substantially with use of dupilumab, and there were no new safety signals, confirming results from previous studies. Dupilumab, an interleukin-4 receptor alpha antagonist administered by injection, was initially approved by the U.S. Food and Drug Administration for treating atopic dermatitis in 2022.
Study presenter Gil Yosipovitch, MD, professor of dermatology at the University of Miami, emphasized that the improvements in itch and skin lesions seen in these patients were “clinically meaningful.”
In the discussion after the presentation, Dr. Yosipovitch was asked whether the presence or absence of atopy had any bearing on the results.
He replied that although there were too few patients with atopy in the current study to answer that question, other data indicate that there is no overall difference between patients with atopy and those without atopy.
Asked whether dupilumab should be used for only 24 weeks, Dr. Yosipovitch said his that “impression” is that there can be a “honeymoon period” during which the medication is stopped and the treating clinician sees “what happens.”
“It would be interesting in the future” to find out, he added, but he noted that whatever the result, patients would need treatment “for the rest of their life.”
Dr. Yosipovitch, director of the Miami Itch Center and the study’s principal investigator, began his presentation by noting that currently, no systemic therapies have been approved by the FDA or the European Medicines Agency for PN.
Although treatments such as topical medications, ultraviolet light therapy, immunosuppressive agents, and systemic neuromodulators are used off label, for many patients with moderate to severe PN, disease control is inadequate, and the patients are “miserable.”
Recently, the phase 3 LIBERTY-PN PRIME2 trial showed that dupilumab significantly reduced itch and skin lesions for patients with PN, and the safety profile was consistent with that seen in approved indications for the drug.
Dr. Yosipovitch explained that LIBERTY-PN PRIME was a phase 2 study in which, after a screening period, patients with PN were randomly assigned in a 1:1 ratio to receive dupilumab as a 600-mg loading dose followed by 300 mg twice weekly or a matched placebo. Treatment was given for 24 weeks, after which there was a post treatment 12-week follow-up period.
Participants were aged 18-80 years and had been diagnosed with PN for a period of at least 3 months. To be included in the trial, patients had to have an average Worst Itch Numerical Rating Scale (WI-NRS) score of at least 7 and at least 20 lesions, among other criteria. (Patients were allowed to continue treatment with mid- to low-potency topical steroids or topical calcineurin inhibitors if they had been taking them at baseline.)
Among 151 patients in the study, the mean age was 50.1 years, and 66.2% were women. The majority (53.0%) were White; 7.3% were Black; and 35.8% were Asian; 40.4% of patients had a history of atopy. The mean WI-NRS was 8.5, and the mean skin pain score on a 10-point scale was 7.2.
The Investigator’s Global Assessment for PN stage of disease (IGA PN-S) was also employed in the trial. That measure uses a 5-point scale to assess disease severity, with 0 indicating no lesions and 4 indicating more than 100 lesions. At baseline, 28.7% of patients had a score of 4, and the remainder had a score of 3, indicating the presence of 20-100 PN lesions.
Dr. Yosipovitch said that quality of life for these patients was “low” and that scores on the Hospital Anxiety and Depression scale indicated that the participants, many of whom had previously received topical and systemic medications for their PN, indicated they were depressed.
He showed that at week 24, the proportion of patients who had experienced an improvement in the WI-NRS score of greater than or equal to 4 (the study’s primary endpoint) was significantly greater with dupilumab, at 60.0% versus 18.4% among patients given placebo (P < .0001).
Moreover, the proportion of patients at week 24 with an IGA PN-S score of 0 or 1 (the secondary endpoint) was 48.0% in the active treatment group, versus 18.4% with placebo (P =.0004).
With regard to safety, rates of any treatment-emergent adverse events were similar between the groups, at 70.7% for dupilumab and 62.7% for placebo, as were rates for severe treatment-emergent adverse events, at 6.7% and 10.7%, respectively.
Rates of treatment-emergent adverse events of interest, such as skin infections, conjunctivitis, herpes viral infections, and injection site reactions, also suggested that there was no increased risk with active treatment.
Dupilumab is currently under review at the FDA and in Europe for the treatment of PN, according to dupilumab manufacturers Regeneron and Sanofi.
The study was sponsored by Sanofi in collaboration with Regeneron Pharmaceuticals. Dr. Yosipovitch has relationships with Arcutis Biotherapeutics, Bellus Health, Eli Lilly, Galderma, GSK, Kiniksa Pharmaceuticals, LEO Pharma, Novartis, Pfizer, Regeneron Pharmaceuticals, Sanofi, and Trevi Therapeutics.
A version of this article first appeared on Medscape.com.
(Dupixent), indicate results from the phase 2 LIBERTY-PN PRIME trial.
The research was presented at the annual Congress of the European Academy of Dermatology and Venereology.
More than 150 patients with severe PN whose quality of life was impaired were randomly assigned to receive dupilumab (Dupixent) or placebo for 24 weeks. Use of the monoclonal antibody was associated with significant improvements in itch scores.
The researchers also found that the percentage of patients who had no or few PN lesions increased substantially with use of dupilumab, and there were no new safety signals, confirming results from previous studies. Dupilumab, an interleukin-4 receptor alpha antagonist administered by injection, was initially approved by the U.S. Food and Drug Administration for treating atopic dermatitis in 2022.
Study presenter Gil Yosipovitch, MD, professor of dermatology at the University of Miami, emphasized that the improvements in itch and skin lesions seen in these patients were “clinically meaningful.”
In the discussion after the presentation, Dr. Yosipovitch was asked whether the presence or absence of atopy had any bearing on the results.
He replied that although there were too few patients with atopy in the current study to answer that question, other data indicate that there is no overall difference between patients with atopy and those without atopy.
Asked whether dupilumab should be used for only 24 weeks, Dr. Yosipovitch said his that “impression” is that there can be a “honeymoon period” during which the medication is stopped and the treating clinician sees “what happens.”
“It would be interesting in the future” to find out, he added, but he noted that whatever the result, patients would need treatment “for the rest of their life.”
Dr. Yosipovitch, director of the Miami Itch Center and the study’s principal investigator, began his presentation by noting that currently, no systemic therapies have been approved by the FDA or the European Medicines Agency for PN.
Although treatments such as topical medications, ultraviolet light therapy, immunosuppressive agents, and systemic neuromodulators are used off label, for many patients with moderate to severe PN, disease control is inadequate, and the patients are “miserable.”
Recently, the phase 3 LIBERTY-PN PRIME2 trial showed that dupilumab significantly reduced itch and skin lesions for patients with PN, and the safety profile was consistent with that seen in approved indications for the drug.
Dr. Yosipovitch explained that LIBERTY-PN PRIME was a phase 2 study in which, after a screening period, patients with PN were randomly assigned in a 1:1 ratio to receive dupilumab as a 600-mg loading dose followed by 300 mg twice weekly or a matched placebo. Treatment was given for 24 weeks, after which there was a post treatment 12-week follow-up period.
Participants were aged 18-80 years and had been diagnosed with PN for a period of at least 3 months. To be included in the trial, patients had to have an average Worst Itch Numerical Rating Scale (WI-NRS) score of at least 7 and at least 20 lesions, among other criteria. (Patients were allowed to continue treatment with mid- to low-potency topical steroids or topical calcineurin inhibitors if they had been taking them at baseline.)
Among 151 patients in the study, the mean age was 50.1 years, and 66.2% were women. The majority (53.0%) were White; 7.3% were Black; and 35.8% were Asian; 40.4% of patients had a history of atopy. The mean WI-NRS was 8.5, and the mean skin pain score on a 10-point scale was 7.2.
The Investigator’s Global Assessment for PN stage of disease (IGA PN-S) was also employed in the trial. That measure uses a 5-point scale to assess disease severity, with 0 indicating no lesions and 4 indicating more than 100 lesions. At baseline, 28.7% of patients had a score of 4, and the remainder had a score of 3, indicating the presence of 20-100 PN lesions.
Dr. Yosipovitch said that quality of life for these patients was “low” and that scores on the Hospital Anxiety and Depression scale indicated that the participants, many of whom had previously received topical and systemic medications for their PN, indicated they were depressed.
He showed that at week 24, the proportion of patients who had experienced an improvement in the WI-NRS score of greater than or equal to 4 (the study’s primary endpoint) was significantly greater with dupilumab, at 60.0% versus 18.4% among patients given placebo (P < .0001).
Moreover, the proportion of patients at week 24 with an IGA PN-S score of 0 or 1 (the secondary endpoint) was 48.0% in the active treatment group, versus 18.4% with placebo (P =.0004).
With regard to safety, rates of any treatment-emergent adverse events were similar between the groups, at 70.7% for dupilumab and 62.7% for placebo, as were rates for severe treatment-emergent adverse events, at 6.7% and 10.7%, respectively.
Rates of treatment-emergent adverse events of interest, such as skin infections, conjunctivitis, herpes viral infections, and injection site reactions, also suggested that there was no increased risk with active treatment.
Dupilumab is currently under review at the FDA and in Europe for the treatment of PN, according to dupilumab manufacturers Regeneron and Sanofi.
The study was sponsored by Sanofi in collaboration with Regeneron Pharmaceuticals. Dr. Yosipovitch has relationships with Arcutis Biotherapeutics, Bellus Health, Eli Lilly, Galderma, GSK, Kiniksa Pharmaceuticals, LEO Pharma, Novartis, Pfizer, Regeneron Pharmaceuticals, Sanofi, and Trevi Therapeutics.
A version of this article first appeared on Medscape.com.
(Dupixent), indicate results from the phase 2 LIBERTY-PN PRIME trial.
The research was presented at the annual Congress of the European Academy of Dermatology and Venereology.
More than 150 patients with severe PN whose quality of life was impaired were randomly assigned to receive dupilumab (Dupixent) or placebo for 24 weeks. Use of the monoclonal antibody was associated with significant improvements in itch scores.
The researchers also found that the percentage of patients who had no or few PN lesions increased substantially with use of dupilumab, and there were no new safety signals, confirming results from previous studies. Dupilumab, an interleukin-4 receptor alpha antagonist administered by injection, was initially approved by the U.S. Food and Drug Administration for treating atopic dermatitis in 2022.
Study presenter Gil Yosipovitch, MD, professor of dermatology at the University of Miami, emphasized that the improvements in itch and skin lesions seen in these patients were “clinically meaningful.”
In the discussion after the presentation, Dr. Yosipovitch was asked whether the presence or absence of atopy had any bearing on the results.
He replied that although there were too few patients with atopy in the current study to answer that question, other data indicate that there is no overall difference between patients with atopy and those without atopy.
Asked whether dupilumab should be used for only 24 weeks, Dr. Yosipovitch said his that “impression” is that there can be a “honeymoon period” during which the medication is stopped and the treating clinician sees “what happens.”
“It would be interesting in the future” to find out, he added, but he noted that whatever the result, patients would need treatment “for the rest of their life.”
Dr. Yosipovitch, director of the Miami Itch Center and the study’s principal investigator, began his presentation by noting that currently, no systemic therapies have been approved by the FDA or the European Medicines Agency for PN.
Although treatments such as topical medications, ultraviolet light therapy, immunosuppressive agents, and systemic neuromodulators are used off label, for many patients with moderate to severe PN, disease control is inadequate, and the patients are “miserable.”
Recently, the phase 3 LIBERTY-PN PRIME2 trial showed that dupilumab significantly reduced itch and skin lesions for patients with PN, and the safety profile was consistent with that seen in approved indications for the drug.
Dr. Yosipovitch explained that LIBERTY-PN PRIME was a phase 2 study in which, after a screening period, patients with PN were randomly assigned in a 1:1 ratio to receive dupilumab as a 600-mg loading dose followed by 300 mg twice weekly or a matched placebo. Treatment was given for 24 weeks, after which there was a post treatment 12-week follow-up period.
Participants were aged 18-80 years and had been diagnosed with PN for a period of at least 3 months. To be included in the trial, patients had to have an average Worst Itch Numerical Rating Scale (WI-NRS) score of at least 7 and at least 20 lesions, among other criteria. (Patients were allowed to continue treatment with mid- to low-potency topical steroids or topical calcineurin inhibitors if they had been taking them at baseline.)
Among 151 patients in the study, the mean age was 50.1 years, and 66.2% were women. The majority (53.0%) were White; 7.3% were Black; and 35.8% were Asian; 40.4% of patients had a history of atopy. The mean WI-NRS was 8.5, and the mean skin pain score on a 10-point scale was 7.2.
The Investigator’s Global Assessment for PN stage of disease (IGA PN-S) was also employed in the trial. That measure uses a 5-point scale to assess disease severity, with 0 indicating no lesions and 4 indicating more than 100 lesions. At baseline, 28.7% of patients had a score of 4, and the remainder had a score of 3, indicating the presence of 20-100 PN lesions.
Dr. Yosipovitch said that quality of life for these patients was “low” and that scores on the Hospital Anxiety and Depression scale indicated that the participants, many of whom had previously received topical and systemic medications for their PN, indicated they were depressed.
He showed that at week 24, the proportion of patients who had experienced an improvement in the WI-NRS score of greater than or equal to 4 (the study’s primary endpoint) was significantly greater with dupilumab, at 60.0% versus 18.4% among patients given placebo (P < .0001).
Moreover, the proportion of patients at week 24 with an IGA PN-S score of 0 or 1 (the secondary endpoint) was 48.0% in the active treatment group, versus 18.4% with placebo (P =.0004).
With regard to safety, rates of any treatment-emergent adverse events were similar between the groups, at 70.7% for dupilumab and 62.7% for placebo, as were rates for severe treatment-emergent adverse events, at 6.7% and 10.7%, respectively.
Rates of treatment-emergent adverse events of interest, such as skin infections, conjunctivitis, herpes viral infections, and injection site reactions, also suggested that there was no increased risk with active treatment.
Dupilumab is currently under review at the FDA and in Europe for the treatment of PN, according to dupilumab manufacturers Regeneron and Sanofi.
The study was sponsored by Sanofi in collaboration with Regeneron Pharmaceuticals. Dr. Yosipovitch has relationships with Arcutis Biotherapeutics, Bellus Health, Eli Lilly, Galderma, GSK, Kiniksa Pharmaceuticals, LEO Pharma, Novartis, Pfizer, Regeneron Pharmaceuticals, Sanofi, and Trevi Therapeutics.
A version of this article first appeared on Medscape.com.
FROM THE EADV CONGRESS
Dermatoses often occur in people who wear face masks
according to a recently published systematic review and meta-analysis.
“This report finds the most statistically significant risk factor for developing a facial dermatosis under a face mask is how long one wears the mask. Specifically, wearing a mask for more than 4 to 6 hours correlated most strongly with the development of a facial skin problem,” Jami L. Miller, MD, associate professor of dermatology, Vanderbilt University Medical Center, Nashville, Tenn., told this news organization. Dr. Miller was not involved in the study.
“The type of mask and the environment were of less significance,” she added.
Mask wearing for infection control has been common during the COVID-19 pandemic and will likely continue for some time, study coauthors Lim Yi Shen Justin, MBBS, and Yik Weng Yew*, MBBS, MPH, PhD, Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore, write in Contact Dermatitis. And cross-sectional studies have suggested a link between mask wearing and various facial dermatoses.
To evaluate this link, as well as potential risk factors for facial dermatoses, the researchers reviewed 37 studies published between 2004 and 2022 involving 29,557 adult participants self-reporting regular use of any face mask type across 17 countries in Europe and Asia. The mask types commonly studied in the papers they analyzed included surgical masks and respirators.
Facial dermatoses were self-reported in 30 studies (81.1%) and were diagnosed by trained dermatologists in seven studies (18.9%).
Dr. Justin and Dr. Yew found that:
- The overall prevalence of facial dermatoses was 55%
- Individually, facial dermatitis, itch, acne, and pressure injuries were consistently reported as facial dermatoses, with pooled prevalence rates of 24%, 30%, 31%, and 31%, respectively
- The duration of mask wearing was the most significant risk factor for facial dermatoses (P < .001)
- Respirators, including N95 masks, were not more likely than surgical masks to be linked with facial dermatoses
“Understanding risk factors of mask wearing, including situation, duration, and type of mask, may allow for targeted interventions to mitigate problems,” Dr. Yew told this news organization.
He advised taking a break from mask wearing after 4 to 6 hours to improve outcomes.
Dr. Yew acknowledged limitations, including that most of the reviewed studies relied on self-reported symptoms.
“Patient factors were not investigated in most studies; therefore, we were not able to ascertain their contributory role in the development of facial dermatoses from mask wearing,” he said. “We were also unable to prove causation between risk factors and outcome.”
Four dermatologists welcome the findings
Dr. Miller called this an “interesting, and certainly relevant” study, now that mask wearing is common and facial skin problems are fairly common complaints in medical visits.
“As the authors say, irritants or contact allergens with longer exposures can be expected to cause a more severe dermatitis than short contact,” she said. “Longer duration also can cause occlusion of pores and hair follicles, which can be expected to worsen acne and folliculitis.”
“I was surprised that the type of mask did not seem to matter significantly,” she added. “Patients wearing N95 masks may be relieved to know N95s do not cause more skin problems than lighter masks.”
Still, Dr. Miller had several questions, including if the materials and chemical finishes that vary by manufacturer may affect skin conditions.
Olga Bunimovich, MD, assistant professor, department of dermatology, University of Pittsburgh School of Medicine, Pennsylvania, called this study “an excellent step towards characterizing the role masks play in facial dermatoses.”
“The study provides a window into the prevalence of these conditions, as well as some understanding of the factors that may be contributing to it,” Dr. Bunimovich, who was not part of the study, added. But “we can also utilize this information to alter behavior in the work environment, allowing ‘mask-free’ breaks to decrease the risk of facial dermatoses.”
Elma Baron, MD, professor and director, Skin Study Center, department of dermatology, Case Western Reserve University School of Medicine, Cleveland, expected skin problems to be linked with mask wearing but didn’t expect the prevalence to be as high as 55%, which she called “very significant.”
“Mask wearing is an important means to prevent transmission of communicable infections, and the practice will most likely continue,” she said.
“Given the data, it is reasonable to advise patients who are already prone to these specific dermatoses to be proactive,” she added. “Early intervention with proper topical medications, preferably prescribed by a dermatologist or other health care provider, and changing masks frequently before they get soaked with moisture, will hopefully lessen the severity of skin rashes and minimize the negative impact on quality of life.”
Also commenting on the study, Susan Massick, MD, dermatologist and clinical associate professor of internal medicine, The Ohio State University Wexner Medical Center, Westerville, said in an interview that she urges people to wear masks, despite these risks.
“The majority of concerns are straightforward, manageable, and overall benign,” she said. “We have a multitude of treatments that can help control, address, or improve symptoms.”
“Masks are an effective and easy way to protect yourself from infection, and they remain one of the most reliable preventions we have,” Dr. Massick noted. “The findings in this article should not preclude anyone from wearing a mask, nor should facial dermatoses be a cause for people to stop wearing their masks.”
The study received no funding. The authors, as well as Dr. Baron, Dr. Miller, Dr. Bunimovich, and Dr. Massick, who were not involved in the study, reported no relevant financial relationships. All experts commented by email.
A version of this article first appeared on Medscape.com.
Correction, 9/22/22: An earlier version of this article misstated the name of Dr. Yik Weng Yew.
according to a recently published systematic review and meta-analysis.
“This report finds the most statistically significant risk factor for developing a facial dermatosis under a face mask is how long one wears the mask. Specifically, wearing a mask for more than 4 to 6 hours correlated most strongly with the development of a facial skin problem,” Jami L. Miller, MD, associate professor of dermatology, Vanderbilt University Medical Center, Nashville, Tenn., told this news organization. Dr. Miller was not involved in the study.
“The type of mask and the environment were of less significance,” she added.
Mask wearing for infection control has been common during the COVID-19 pandemic and will likely continue for some time, study coauthors Lim Yi Shen Justin, MBBS, and Yik Weng Yew*, MBBS, MPH, PhD, Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore, write in Contact Dermatitis. And cross-sectional studies have suggested a link between mask wearing and various facial dermatoses.
To evaluate this link, as well as potential risk factors for facial dermatoses, the researchers reviewed 37 studies published between 2004 and 2022 involving 29,557 adult participants self-reporting regular use of any face mask type across 17 countries in Europe and Asia. The mask types commonly studied in the papers they analyzed included surgical masks and respirators.
Facial dermatoses were self-reported in 30 studies (81.1%) and were diagnosed by trained dermatologists in seven studies (18.9%).
Dr. Justin and Dr. Yew found that:
- The overall prevalence of facial dermatoses was 55%
- Individually, facial dermatitis, itch, acne, and pressure injuries were consistently reported as facial dermatoses, with pooled prevalence rates of 24%, 30%, 31%, and 31%, respectively
- The duration of mask wearing was the most significant risk factor for facial dermatoses (P < .001)
- Respirators, including N95 masks, were not more likely than surgical masks to be linked with facial dermatoses
“Understanding risk factors of mask wearing, including situation, duration, and type of mask, may allow for targeted interventions to mitigate problems,” Dr. Yew told this news organization.
He advised taking a break from mask wearing after 4 to 6 hours to improve outcomes.
Dr. Yew acknowledged limitations, including that most of the reviewed studies relied on self-reported symptoms.
“Patient factors were not investigated in most studies; therefore, we were not able to ascertain their contributory role in the development of facial dermatoses from mask wearing,” he said. “We were also unable to prove causation between risk factors and outcome.”
Four dermatologists welcome the findings
Dr. Miller called this an “interesting, and certainly relevant” study, now that mask wearing is common and facial skin problems are fairly common complaints in medical visits.
“As the authors say, irritants or contact allergens with longer exposures can be expected to cause a more severe dermatitis than short contact,” she said. “Longer duration also can cause occlusion of pores and hair follicles, which can be expected to worsen acne and folliculitis.”
“I was surprised that the type of mask did not seem to matter significantly,” she added. “Patients wearing N95 masks may be relieved to know N95s do not cause more skin problems than lighter masks.”
Still, Dr. Miller had several questions, including if the materials and chemical finishes that vary by manufacturer may affect skin conditions.
Olga Bunimovich, MD, assistant professor, department of dermatology, University of Pittsburgh School of Medicine, Pennsylvania, called this study “an excellent step towards characterizing the role masks play in facial dermatoses.”
“The study provides a window into the prevalence of these conditions, as well as some understanding of the factors that may be contributing to it,” Dr. Bunimovich, who was not part of the study, added. But “we can also utilize this information to alter behavior in the work environment, allowing ‘mask-free’ breaks to decrease the risk of facial dermatoses.”
Elma Baron, MD, professor and director, Skin Study Center, department of dermatology, Case Western Reserve University School of Medicine, Cleveland, expected skin problems to be linked with mask wearing but didn’t expect the prevalence to be as high as 55%, which she called “very significant.”
“Mask wearing is an important means to prevent transmission of communicable infections, and the practice will most likely continue,” she said.
“Given the data, it is reasonable to advise patients who are already prone to these specific dermatoses to be proactive,” she added. “Early intervention with proper topical medications, preferably prescribed by a dermatologist or other health care provider, and changing masks frequently before they get soaked with moisture, will hopefully lessen the severity of skin rashes and minimize the negative impact on quality of life.”
Also commenting on the study, Susan Massick, MD, dermatologist and clinical associate professor of internal medicine, The Ohio State University Wexner Medical Center, Westerville, said in an interview that she urges people to wear masks, despite these risks.
“The majority of concerns are straightforward, manageable, and overall benign,” she said. “We have a multitude of treatments that can help control, address, or improve symptoms.”
“Masks are an effective and easy way to protect yourself from infection, and they remain one of the most reliable preventions we have,” Dr. Massick noted. “The findings in this article should not preclude anyone from wearing a mask, nor should facial dermatoses be a cause for people to stop wearing their masks.”
The study received no funding. The authors, as well as Dr. Baron, Dr. Miller, Dr. Bunimovich, and Dr. Massick, who were not involved in the study, reported no relevant financial relationships. All experts commented by email.
A version of this article first appeared on Medscape.com.
Correction, 9/22/22: An earlier version of this article misstated the name of Dr. Yik Weng Yew.
according to a recently published systematic review and meta-analysis.
“This report finds the most statistically significant risk factor for developing a facial dermatosis under a face mask is how long one wears the mask. Specifically, wearing a mask for more than 4 to 6 hours correlated most strongly with the development of a facial skin problem,” Jami L. Miller, MD, associate professor of dermatology, Vanderbilt University Medical Center, Nashville, Tenn., told this news organization. Dr. Miller was not involved in the study.
“The type of mask and the environment were of less significance,” she added.
Mask wearing for infection control has been common during the COVID-19 pandemic and will likely continue for some time, study coauthors Lim Yi Shen Justin, MBBS, and Yik Weng Yew*, MBBS, MPH, PhD, Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore, write in Contact Dermatitis. And cross-sectional studies have suggested a link between mask wearing and various facial dermatoses.
To evaluate this link, as well as potential risk factors for facial dermatoses, the researchers reviewed 37 studies published between 2004 and 2022 involving 29,557 adult participants self-reporting regular use of any face mask type across 17 countries in Europe and Asia. The mask types commonly studied in the papers they analyzed included surgical masks and respirators.
Facial dermatoses were self-reported in 30 studies (81.1%) and were diagnosed by trained dermatologists in seven studies (18.9%).
Dr. Justin and Dr. Yew found that:
- The overall prevalence of facial dermatoses was 55%
- Individually, facial dermatitis, itch, acne, and pressure injuries were consistently reported as facial dermatoses, with pooled prevalence rates of 24%, 30%, 31%, and 31%, respectively
- The duration of mask wearing was the most significant risk factor for facial dermatoses (P < .001)
- Respirators, including N95 masks, were not more likely than surgical masks to be linked with facial dermatoses
“Understanding risk factors of mask wearing, including situation, duration, and type of mask, may allow for targeted interventions to mitigate problems,” Dr. Yew told this news organization.
He advised taking a break from mask wearing after 4 to 6 hours to improve outcomes.
Dr. Yew acknowledged limitations, including that most of the reviewed studies relied on self-reported symptoms.
“Patient factors were not investigated in most studies; therefore, we were not able to ascertain their contributory role in the development of facial dermatoses from mask wearing,” he said. “We were also unable to prove causation between risk factors and outcome.”
Four dermatologists welcome the findings
Dr. Miller called this an “interesting, and certainly relevant” study, now that mask wearing is common and facial skin problems are fairly common complaints in medical visits.
“As the authors say, irritants or contact allergens with longer exposures can be expected to cause a more severe dermatitis than short contact,” she said. “Longer duration also can cause occlusion of pores and hair follicles, which can be expected to worsen acne and folliculitis.”
“I was surprised that the type of mask did not seem to matter significantly,” she added. “Patients wearing N95 masks may be relieved to know N95s do not cause more skin problems than lighter masks.”
Still, Dr. Miller had several questions, including if the materials and chemical finishes that vary by manufacturer may affect skin conditions.
Olga Bunimovich, MD, assistant professor, department of dermatology, University of Pittsburgh School of Medicine, Pennsylvania, called this study “an excellent step towards characterizing the role masks play in facial dermatoses.”
“The study provides a window into the prevalence of these conditions, as well as some understanding of the factors that may be contributing to it,” Dr. Bunimovich, who was not part of the study, added. But “we can also utilize this information to alter behavior in the work environment, allowing ‘mask-free’ breaks to decrease the risk of facial dermatoses.”
Elma Baron, MD, professor and director, Skin Study Center, department of dermatology, Case Western Reserve University School of Medicine, Cleveland, expected skin problems to be linked with mask wearing but didn’t expect the prevalence to be as high as 55%, which she called “very significant.”
“Mask wearing is an important means to prevent transmission of communicable infections, and the practice will most likely continue,” she said.
“Given the data, it is reasonable to advise patients who are already prone to these specific dermatoses to be proactive,” she added. “Early intervention with proper topical medications, preferably prescribed by a dermatologist or other health care provider, and changing masks frequently before they get soaked with moisture, will hopefully lessen the severity of skin rashes and minimize the negative impact on quality of life.”
Also commenting on the study, Susan Massick, MD, dermatologist and clinical associate professor of internal medicine, The Ohio State University Wexner Medical Center, Westerville, said in an interview that she urges people to wear masks, despite these risks.
“The majority of concerns are straightforward, manageable, and overall benign,” she said. “We have a multitude of treatments that can help control, address, or improve symptoms.”
“Masks are an effective and easy way to protect yourself from infection, and they remain one of the most reliable preventions we have,” Dr. Massick noted. “The findings in this article should not preclude anyone from wearing a mask, nor should facial dermatoses be a cause for people to stop wearing their masks.”
The study received no funding. The authors, as well as Dr. Baron, Dr. Miller, Dr. Bunimovich, and Dr. Massick, who were not involved in the study, reported no relevant financial relationships. All experts commented by email.
A version of this article first appeared on Medscape.com.
Correction, 9/22/22: An earlier version of this article misstated the name of Dr. Yik Weng Yew.
FDA approves oral TYK2 inhibitor deucravacitinib for treating psoriasis
the manufacturer announced on Sept. 9.
Deucravacitinib targets TYK2, which inhibits signaling of interleukin-23, interleukin-12, and type 1 interferons, key cytokines involved in the pathogenesis of multiple immune-mediated diseases, according to Bristol Myers Squibb (BMS). This is the first approval for deucravacitinib, which will be marketed as Sotyktu, and the first drug in this class to be approved.
It is also currently under review for the same indication in Europe and Japan, and elsewhere, and for treating pustular psoriasis and erythrodermic psoriasis in Japan.
FDA approval was based on the results of POETYK PSO-1 and POETYK PSO-2, phase 3 trials of almost 1,700 adults with moderate to severe plaque psoriasis. In these studies, treatment with once-daily deucravacitinib showed significant and clinically meaningful improvements in skin clearance and symptoms, compared with placebo and with apremilast (Otezla), according to the company.
In the two studies, patients were randomly assigned to receive 6 mg daily of deucravacitinib, placebo, or a 30-mg twice-daily dose of apremilast, the oral phosphodiesterase 4 inhibitor approved for psoriasis. The primary endpoints were the percentage of patients who achieved a Psoriasis Area and Severity Index (PASI) 75 response and a static Physician’s Global Assessment (sPGA) score of 0 or 1 (clear or almost clear) at 16 weeks.
At 16 weeks, 58% and 53% of patients receiving deucravacitinib in the POETYK PSO-1 and POETYK PSO-2 studies, respectively, achieved PASI 75 response, compared with 13% and 9% of those receiving placebo (P < .0001 for both) and 35% and 40% receiving apremilast (P < .0001, P = .0004, respectively), according to the company’s announcement of the approval. PASI 75 responses were maintained through 52 weeks among the patients who remained on treatment, in both studies, according to BMS.
In the POETYK PSO-1 and PSO-2 studies, respectively, 54% and 50% of those on deucravacitinib achieved an sPGA of 0/1 at 16 weeks, compared with 7% and 9% of those receiving placebo (P < .0001 for both) and 32% and 34% of those receiving apremilast (P < .0001 for both).
Across the two studies, at 16 weeks, the most common adverse events that affected at least 1% of patients on deucravacitinib and that occurred at higher rates than in the placebo group were upper respiratory infections (19.2%), increases in serum creatine phosphokinase (2.7%), herpes simplex (2%), mouth ulcers (1.9%), folliculitis (1.7%), and acne (1.4%). Adverse events resulting in discontinuation of treatment were reported in 2.4% of persons receiving deucravacitinib and 5.2% of those receiving apremilast, compared with 3.8% of those receiving placebo.
Up to 16 weeks, according to the BMS statement, 28% of persons receiving deucravacitinib had infections, most of which were mild to moderate and not serious and did not result in stopping treatment, compared with 22% of those receiving placebo. In addition, five patients treated with deucravacitinib and five patients receiving placebo had serious infections, and three patients receiving deucravacitinib had cancer (not including nonmelanoma skin cancer).
Deucravacitinib is also being evaluated in clinical trials for psoriatic arthritis, lupus, and inflammatory bowel disease. It is not recommended for use in combination with other potent immunosuppressants, according to BMS.
The prescribing information and patient medication guide are available online.
The POETYK PSO-1 and POETYK PSO-2 studies were funded by Bristol Myers Squibb.
A version of this article first appeared on Medscape.com.
the manufacturer announced on Sept. 9.
Deucravacitinib targets TYK2, which inhibits signaling of interleukin-23, interleukin-12, and type 1 interferons, key cytokines involved in the pathogenesis of multiple immune-mediated diseases, according to Bristol Myers Squibb (BMS). This is the first approval for deucravacitinib, which will be marketed as Sotyktu, and the first drug in this class to be approved.
It is also currently under review for the same indication in Europe and Japan, and elsewhere, and for treating pustular psoriasis and erythrodermic psoriasis in Japan.
FDA approval was based on the results of POETYK PSO-1 and POETYK PSO-2, phase 3 trials of almost 1,700 adults with moderate to severe plaque psoriasis. In these studies, treatment with once-daily deucravacitinib showed significant and clinically meaningful improvements in skin clearance and symptoms, compared with placebo and with apremilast (Otezla), according to the company.
In the two studies, patients were randomly assigned to receive 6 mg daily of deucravacitinib, placebo, or a 30-mg twice-daily dose of apremilast, the oral phosphodiesterase 4 inhibitor approved for psoriasis. The primary endpoints were the percentage of patients who achieved a Psoriasis Area and Severity Index (PASI) 75 response and a static Physician’s Global Assessment (sPGA) score of 0 or 1 (clear or almost clear) at 16 weeks.
At 16 weeks, 58% and 53% of patients receiving deucravacitinib in the POETYK PSO-1 and POETYK PSO-2 studies, respectively, achieved PASI 75 response, compared with 13% and 9% of those receiving placebo (P < .0001 for both) and 35% and 40% receiving apremilast (P < .0001, P = .0004, respectively), according to the company’s announcement of the approval. PASI 75 responses were maintained through 52 weeks among the patients who remained on treatment, in both studies, according to BMS.
In the POETYK PSO-1 and PSO-2 studies, respectively, 54% and 50% of those on deucravacitinib achieved an sPGA of 0/1 at 16 weeks, compared with 7% and 9% of those receiving placebo (P < .0001 for both) and 32% and 34% of those receiving apremilast (P < .0001 for both).
Across the two studies, at 16 weeks, the most common adverse events that affected at least 1% of patients on deucravacitinib and that occurred at higher rates than in the placebo group were upper respiratory infections (19.2%), increases in serum creatine phosphokinase (2.7%), herpes simplex (2%), mouth ulcers (1.9%), folliculitis (1.7%), and acne (1.4%). Adverse events resulting in discontinuation of treatment were reported in 2.4% of persons receiving deucravacitinib and 5.2% of those receiving apremilast, compared with 3.8% of those receiving placebo.
Up to 16 weeks, according to the BMS statement, 28% of persons receiving deucravacitinib had infections, most of which were mild to moderate and not serious and did not result in stopping treatment, compared with 22% of those receiving placebo. In addition, five patients treated with deucravacitinib and five patients receiving placebo had serious infections, and three patients receiving deucravacitinib had cancer (not including nonmelanoma skin cancer).
Deucravacitinib is also being evaluated in clinical trials for psoriatic arthritis, lupus, and inflammatory bowel disease. It is not recommended for use in combination with other potent immunosuppressants, according to BMS.
The prescribing information and patient medication guide are available online.
The POETYK PSO-1 and POETYK PSO-2 studies were funded by Bristol Myers Squibb.
A version of this article first appeared on Medscape.com.
the manufacturer announced on Sept. 9.
Deucravacitinib targets TYK2, which inhibits signaling of interleukin-23, interleukin-12, and type 1 interferons, key cytokines involved in the pathogenesis of multiple immune-mediated diseases, according to Bristol Myers Squibb (BMS). This is the first approval for deucravacitinib, which will be marketed as Sotyktu, and the first drug in this class to be approved.
It is also currently under review for the same indication in Europe and Japan, and elsewhere, and for treating pustular psoriasis and erythrodermic psoriasis in Japan.
FDA approval was based on the results of POETYK PSO-1 and POETYK PSO-2, phase 3 trials of almost 1,700 adults with moderate to severe plaque psoriasis. In these studies, treatment with once-daily deucravacitinib showed significant and clinically meaningful improvements in skin clearance and symptoms, compared with placebo and with apremilast (Otezla), according to the company.
In the two studies, patients were randomly assigned to receive 6 mg daily of deucravacitinib, placebo, or a 30-mg twice-daily dose of apremilast, the oral phosphodiesterase 4 inhibitor approved for psoriasis. The primary endpoints were the percentage of patients who achieved a Psoriasis Area and Severity Index (PASI) 75 response and a static Physician’s Global Assessment (sPGA) score of 0 or 1 (clear or almost clear) at 16 weeks.
At 16 weeks, 58% and 53% of patients receiving deucravacitinib in the POETYK PSO-1 and POETYK PSO-2 studies, respectively, achieved PASI 75 response, compared with 13% and 9% of those receiving placebo (P < .0001 for both) and 35% and 40% receiving apremilast (P < .0001, P = .0004, respectively), according to the company’s announcement of the approval. PASI 75 responses were maintained through 52 weeks among the patients who remained on treatment, in both studies, according to BMS.
In the POETYK PSO-1 and PSO-2 studies, respectively, 54% and 50% of those on deucravacitinib achieved an sPGA of 0/1 at 16 weeks, compared with 7% and 9% of those receiving placebo (P < .0001 for both) and 32% and 34% of those receiving apremilast (P < .0001 for both).
Across the two studies, at 16 weeks, the most common adverse events that affected at least 1% of patients on deucravacitinib and that occurred at higher rates than in the placebo group were upper respiratory infections (19.2%), increases in serum creatine phosphokinase (2.7%), herpes simplex (2%), mouth ulcers (1.9%), folliculitis (1.7%), and acne (1.4%). Adverse events resulting in discontinuation of treatment were reported in 2.4% of persons receiving deucravacitinib and 5.2% of those receiving apremilast, compared with 3.8% of those receiving placebo.
Up to 16 weeks, according to the BMS statement, 28% of persons receiving deucravacitinib had infections, most of which were mild to moderate and not serious and did not result in stopping treatment, compared with 22% of those receiving placebo. In addition, five patients treated with deucravacitinib and five patients receiving placebo had serious infections, and three patients receiving deucravacitinib had cancer (not including nonmelanoma skin cancer).
Deucravacitinib is also being evaluated in clinical trials for psoriatic arthritis, lupus, and inflammatory bowel disease. It is not recommended for use in combination with other potent immunosuppressants, according to BMS.
The prescribing information and patient medication guide are available online.
The POETYK PSO-1 and POETYK PSO-2 studies were funded by Bristol Myers Squibb.
A version of this article first appeared on Medscape.com.
Overall survival dips with vitamin D deficiency in melanoma
, according to research presented at the annual congress of the European Academy of Dermatology and Venereology.
Whereas the 5-year overall survival was 90% when vitamin D serum levels were above a 10 ng/mL threshold, it was 84% when levels fell below it. Notably, the gap in overall survival between those above and below the threshold appeared to widen as time went on.
The research adds to existing evidence that “vitamin D levels can play an important and independent role in patients’ survival outcomes,” study investigator Inés Gracia-Darder, MD, told this news organization. “The important application in clinical practice would be to know if vitamin D supplementation influences the survival of melanoma patients,” said Dr. Gracia-Darder, a clinical specialist in dermatology at the Hospital Universitari Son Espases, Mallorca, Spain.
Known association, but not much data
“It is not a new finding,” but there are limited data, especially in melanoma, said Julie De Smedt, MD, of KU Leuven, Belgium, who was asked to comment on the results. Other groups have shown, certainly for cancer in general, that vitamin D can have an effect on overall survival.
“Low levels of vitamin D are associated with the pathological parameters of the melanoma, such as the thickness of the tumor,” Dr. De Smedt said in an interview, indicating that it’s not just overall survival that might be affected.
“So we assume that also has an effect on melanoma-specific survival,” she added.
That assumption, however, is not supported by the data Dr. Gracia-Darder presented, as there was no difference in melanoma-specific survival among the two groups of patients that had been studied.
Retrospective cohort analysis
Vitamin D levels had been studied in 264 patients who were included in the retrospective cohort analysis. All had invasive melanomas, and all had been seen at the Hospital Clinic of Barcelona between January 1998 and June 2021. Their mean age was 57 years, and the median follow-up was 6.7 years.
For inclusion, all patients had to have had their vitamin D levels measured after being diagnosed with melanoma; those with a 25-hydroxyvitamin D3 serum level of less than 10 ng/mL were deemed to be vitamin D deficient, whereas those with levels of 10 ng/mL and above were deemed normal or insufficient.
A measurement less than 10 ng/mL is considered vitamin D deficiency, Dr. De Smedt said. “But there is a difference between countries, and there’s also a difference between societies,” noting the cut-off used in the lab where she works is 20 ng/mL. This makes it difficult to compare studies, she said.
Independent association with overall survival
Seasonal variation in vitamin D levels were considered as a possible confounding factor, but Dr. Gracia-Darder noted that there was a similar distribution of measurements taken between October to March and April to September.
Univariate and multivariate analyses established vitamin D deficiency as being independently associated with overall survival with hazard ratios of 2.34 and 2.45, respectively.
Other predictive factors were having a higher Breslow index, as well as older age and gender.
Time to recommend vitamin D supplementation?
So should patients with melanoma have their vitamin D levels routinely checked? And what about advising them to take vitamin D supplements?
“In our practice, we analyze the vitamin D levels of our patients,” Dr. Gracia-Darder said. Patients are told to limit their exposure to the sun because of their skin cancer, so they are very likely to become vitamin D deficient.
While dietary changes or supplements might be suggested, there’s no real evidence to support upping vitamin D levels to date, so “future prospective studies are needed,” Dr. Gracia-Darder added.
Such studies have already started, including one in Italy, one in Australia, and another study that Dr. De Smedt has been involved with for the past few years.
Called the ViDMe study, it’s a multicenter, randomized, double-blind trial in which patients are being given a high-dose oral vitamin D supplement or placebo once a month for at least 1 year. About 430 patients with a first cutaneous malignant melanoma have been included in the trial, which started in December 2012.
It is hoped that the results will show that the supplementation will have had a protective effect on the risk of relapse and that there will be a correlation between vitamin D levels in the blood and vitamin D receptor immunoreactivity in the tumor.
“The study is still blinded,” Dr. De Smedt said. “We will unblind in the coming months and then at the end of the year, maybe next year, we will have the results.”
The study reported by Dr. Gracia-Darder did not receive any specific funding. Dr. Gracia-Darder disclosed that the melanoma unit where the study was performed receives many grants and funds to carry out research. She reported no other relevant financial relationships. Dr. De Smedt had no relevant financial relationships. The ViDMe study is sponsored by the Universitaire Ziekenhuizen Leuven.
A version of this article first appeared on Medscape.com.
, according to research presented at the annual congress of the European Academy of Dermatology and Venereology.
Whereas the 5-year overall survival was 90% when vitamin D serum levels were above a 10 ng/mL threshold, it was 84% when levels fell below it. Notably, the gap in overall survival between those above and below the threshold appeared to widen as time went on.
The research adds to existing evidence that “vitamin D levels can play an important and independent role in patients’ survival outcomes,” study investigator Inés Gracia-Darder, MD, told this news organization. “The important application in clinical practice would be to know if vitamin D supplementation influences the survival of melanoma patients,” said Dr. Gracia-Darder, a clinical specialist in dermatology at the Hospital Universitari Son Espases, Mallorca, Spain.
Known association, but not much data
“It is not a new finding,” but there are limited data, especially in melanoma, said Julie De Smedt, MD, of KU Leuven, Belgium, who was asked to comment on the results. Other groups have shown, certainly for cancer in general, that vitamin D can have an effect on overall survival.
“Low levels of vitamin D are associated with the pathological parameters of the melanoma, such as the thickness of the tumor,” Dr. De Smedt said in an interview, indicating that it’s not just overall survival that might be affected.
“So we assume that also has an effect on melanoma-specific survival,” she added.
That assumption, however, is not supported by the data Dr. Gracia-Darder presented, as there was no difference in melanoma-specific survival among the two groups of patients that had been studied.
Retrospective cohort analysis
Vitamin D levels had been studied in 264 patients who were included in the retrospective cohort analysis. All had invasive melanomas, and all had been seen at the Hospital Clinic of Barcelona between January 1998 and June 2021. Their mean age was 57 years, and the median follow-up was 6.7 years.
For inclusion, all patients had to have had their vitamin D levels measured after being diagnosed with melanoma; those with a 25-hydroxyvitamin D3 serum level of less than 10 ng/mL were deemed to be vitamin D deficient, whereas those with levels of 10 ng/mL and above were deemed normal or insufficient.
A measurement less than 10 ng/mL is considered vitamin D deficiency, Dr. De Smedt said. “But there is a difference between countries, and there’s also a difference between societies,” noting the cut-off used in the lab where she works is 20 ng/mL. This makes it difficult to compare studies, she said.
Independent association with overall survival
Seasonal variation in vitamin D levels were considered as a possible confounding factor, but Dr. Gracia-Darder noted that there was a similar distribution of measurements taken between October to March and April to September.
Univariate and multivariate analyses established vitamin D deficiency as being independently associated with overall survival with hazard ratios of 2.34 and 2.45, respectively.
Other predictive factors were having a higher Breslow index, as well as older age and gender.
Time to recommend vitamin D supplementation?
So should patients with melanoma have their vitamin D levels routinely checked? And what about advising them to take vitamin D supplements?
“In our practice, we analyze the vitamin D levels of our patients,” Dr. Gracia-Darder said. Patients are told to limit their exposure to the sun because of their skin cancer, so they are very likely to become vitamin D deficient.
While dietary changes or supplements might be suggested, there’s no real evidence to support upping vitamin D levels to date, so “future prospective studies are needed,” Dr. Gracia-Darder added.
Such studies have already started, including one in Italy, one in Australia, and another study that Dr. De Smedt has been involved with for the past few years.
Called the ViDMe study, it’s a multicenter, randomized, double-blind trial in which patients are being given a high-dose oral vitamin D supplement or placebo once a month for at least 1 year. About 430 patients with a first cutaneous malignant melanoma have been included in the trial, which started in December 2012.
It is hoped that the results will show that the supplementation will have had a protective effect on the risk of relapse and that there will be a correlation between vitamin D levels in the blood and vitamin D receptor immunoreactivity in the tumor.
“The study is still blinded,” Dr. De Smedt said. “We will unblind in the coming months and then at the end of the year, maybe next year, we will have the results.”
The study reported by Dr. Gracia-Darder did not receive any specific funding. Dr. Gracia-Darder disclosed that the melanoma unit where the study was performed receives many grants and funds to carry out research. She reported no other relevant financial relationships. Dr. De Smedt had no relevant financial relationships. The ViDMe study is sponsored by the Universitaire Ziekenhuizen Leuven.
A version of this article first appeared on Medscape.com.
, according to research presented at the annual congress of the European Academy of Dermatology and Venereology.
Whereas the 5-year overall survival was 90% when vitamin D serum levels were above a 10 ng/mL threshold, it was 84% when levels fell below it. Notably, the gap in overall survival between those above and below the threshold appeared to widen as time went on.
The research adds to existing evidence that “vitamin D levels can play an important and independent role in patients’ survival outcomes,” study investigator Inés Gracia-Darder, MD, told this news organization. “The important application in clinical practice would be to know if vitamin D supplementation influences the survival of melanoma patients,” said Dr. Gracia-Darder, a clinical specialist in dermatology at the Hospital Universitari Son Espases, Mallorca, Spain.
Known association, but not much data
“It is not a new finding,” but there are limited data, especially in melanoma, said Julie De Smedt, MD, of KU Leuven, Belgium, who was asked to comment on the results. Other groups have shown, certainly for cancer in general, that vitamin D can have an effect on overall survival.
“Low levels of vitamin D are associated with the pathological parameters of the melanoma, such as the thickness of the tumor,” Dr. De Smedt said in an interview, indicating that it’s not just overall survival that might be affected.
“So we assume that also has an effect on melanoma-specific survival,” she added.
That assumption, however, is not supported by the data Dr. Gracia-Darder presented, as there was no difference in melanoma-specific survival among the two groups of patients that had been studied.
Retrospective cohort analysis
Vitamin D levels had been studied in 264 patients who were included in the retrospective cohort analysis. All had invasive melanomas, and all had been seen at the Hospital Clinic of Barcelona between January 1998 and June 2021. Their mean age was 57 years, and the median follow-up was 6.7 years.
For inclusion, all patients had to have had their vitamin D levels measured after being diagnosed with melanoma; those with a 25-hydroxyvitamin D3 serum level of less than 10 ng/mL were deemed to be vitamin D deficient, whereas those with levels of 10 ng/mL and above were deemed normal or insufficient.
A measurement less than 10 ng/mL is considered vitamin D deficiency, Dr. De Smedt said. “But there is a difference between countries, and there’s also a difference between societies,” noting the cut-off used in the lab where she works is 20 ng/mL. This makes it difficult to compare studies, she said.
Independent association with overall survival
Seasonal variation in vitamin D levels were considered as a possible confounding factor, but Dr. Gracia-Darder noted that there was a similar distribution of measurements taken between October to March and April to September.
Univariate and multivariate analyses established vitamin D deficiency as being independently associated with overall survival with hazard ratios of 2.34 and 2.45, respectively.
Other predictive factors were having a higher Breslow index, as well as older age and gender.
Time to recommend vitamin D supplementation?
So should patients with melanoma have their vitamin D levels routinely checked? And what about advising them to take vitamin D supplements?
“In our practice, we analyze the vitamin D levels of our patients,” Dr. Gracia-Darder said. Patients are told to limit their exposure to the sun because of their skin cancer, so they are very likely to become vitamin D deficient.
While dietary changes or supplements might be suggested, there’s no real evidence to support upping vitamin D levels to date, so “future prospective studies are needed,” Dr. Gracia-Darder added.
Such studies have already started, including one in Italy, one in Australia, and another study that Dr. De Smedt has been involved with for the past few years.
Called the ViDMe study, it’s a multicenter, randomized, double-blind trial in which patients are being given a high-dose oral vitamin D supplement or placebo once a month for at least 1 year. About 430 patients with a first cutaneous malignant melanoma have been included in the trial, which started in December 2012.
It is hoped that the results will show that the supplementation will have had a protective effect on the risk of relapse and that there will be a correlation between vitamin D levels in the blood and vitamin D receptor immunoreactivity in the tumor.
“The study is still blinded,” Dr. De Smedt said. “We will unblind in the coming months and then at the end of the year, maybe next year, we will have the results.”
The study reported by Dr. Gracia-Darder did not receive any specific funding. Dr. Gracia-Darder disclosed that the melanoma unit where the study was performed receives many grants and funds to carry out research. She reported no other relevant financial relationships. Dr. De Smedt had no relevant financial relationships. The ViDMe study is sponsored by the Universitaire Ziekenhuizen Leuven.
A version of this article first appeared on Medscape.com.
FROM THE EADV CONGRESS
Nocturnally pruritic rash
A 74-YEAR-OLD WOMAN presented with a 3-day history of an intensely pruritic rash that was localized to her upper arms, upper chest between her breasts, and upper back. The pruritus was much worse at night while the patient was in bed. Symptoms did not improve with over-the-counter topical corticosteroids.
The patient had a history of atrial fibrillation (for which she was receiving chronic anticoagulation therapy), hypertension, an implanted pacemaker, depression, and Parkinson disease. Her medications included carbidopa-levodopa, fluoxetine, hydrochlorothiazide, metoprolol tartrate, naproxen, and warfarin. She had no known allergies. She reported that she was a nonsmoker and drank 1 glass of wine per week.
There were no recent changes in soaps, detergents, lotions, or makeup, nor did the patient have any bug bites or plant exposure. She shared a home with her spouse and several pets: a dog, a cat, and a Bantam-breed chicken. The patient’s husband, who slept in a different bedroom, had no rash. Recently, the cat had been bringing its captured prey of rabbits into the home.
Review of systems was negative for fever, chills, shortness of breath, cough, throat swelling, and rhinorrhea. Physical examination revealed red/pink macules and papules scattered over the upper arms (FIGURE 1), chest, and upper back. Many lesions were excoriated but had no active bleeding or vesicles. Under dermatoscope, no burrowing was found; however, a small (< 1 mm) creature was seen moving rapidly across the skin surface. The physician (CTW) captured and isolated the creature using a sterile lab cup.
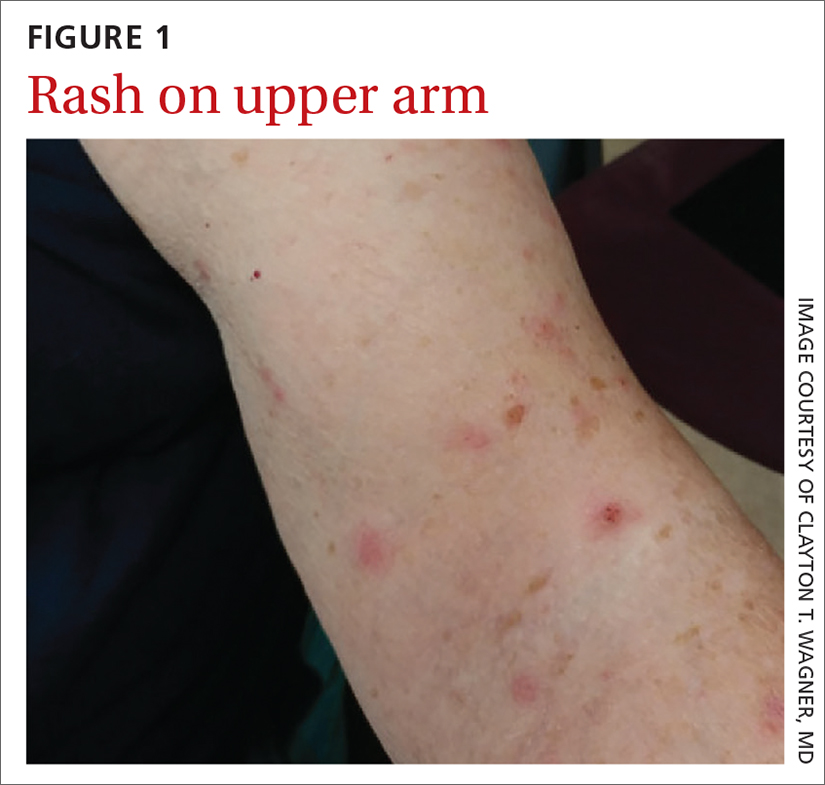
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Gamasoidosis
The collected sample (FIGURE 2) was examined and identified as an avian mite by a colleague who specializes in entomology, confirming the diagnosis of gamasoidosis.

Two genera of avian mites are responsible: Dermanyssus and Ornithonyssus. The most common culprits are the red poultry mite (D gallinae) and the northern fowl mite (O bursa). These small mites parasitize birds, such as poultry livestock, domesticated birds, and wild game birds. When unfed, the mite appears translucent brown and measures 0.3 to 0.7 mm in length, but after a blood meal, it appears red and increases in size to 1 mm. The mites tend to be active and feed at night and hide during the day.2 This explained the severe nighttime pruritus in this case.
Human infestation, although infrequent, can be a concern for those who work with poultry, or during the spring and summer seasons when young birds leave their nests and the mites migrate to find alternative hosts.3 The 1- to 2-mm erythematous maculopapules are often found with excoriations in covered areas.3,4 Unlike scabies, the genitalia and interdigital areas are spared.3,5
Differential for arthropod dermatoses
The differential diagnosis includes cimicosis, pulicosis, pediculosis corporis, and scabies.
Cimicosis is caused by bed bugs (from the insect Cimex genus). Bed bugs are oval and reddish brown, have 6 legs, and range in size from 1 to 7 mm. Most bed bugs hide in cracks or crevices of furniture and other surfaces (eg, bed frames, headboards, seams or holes of box springs or mattresses, or behind wallpaper, switch plates, and picture frames) by day and come out at night to feed on a sleeping host. Commonly, bed bugs will leave a series of bites grouped in rows (described as “breakfast, lunch, and dinner”). The bites can mimic urticaria, and bullous reactions may also occur.2
Continue to: Pulicosis
Pulicosis results from bites caused by a variety of flea species including, but not limited to, human, dog, oriental rat, sticktight, mouse, and chicken fleas. Fleas are small brown insects measuring about 2.5 mm in length, with flat sides and long hind legs. Their bites are most often arranged in a zigzag pattern around a host’s legs and waist. Hypersensitivity reactions may appear as papular urticaria, nodules, or bullae.2
Pediculosis corporis is caused by body lice. The adult louse is 2.5 to 3.5 mm in size, has 6 legs, and is a tan to greyish white color.6 Lice live in clothing, lay their eggs within the seams, and obtain blood meals from the host. Symptoms include generalized itching. The erythematous blue- and copper-colored macules, wheals, and lichenification can occur throughout the body, but spare the hands and feet. Secondary impetigo and furunculosis commonly occur.2
Scabies is caused by an oval mite that is ventrally flat, with dorsal spines. The mite is < 0.5 mm in size, appearing as a pinpoint of white. It burrows into its host’s skin, where it lives and lays eggs, causing pruritic papular lesions and ensuing excoriations. The mite burrows with a predilection for the finger web spaces, wrists, axillae, areolae, umbilicus, lower abdomen, genitals, and buttocks.2
Treatment involves a 3-step process
The mainstay of treatment is removal of the infested bird, decontamination of bedding and clothing, and use of oral antihistamines and topical corticosteroids.1,3,5 Bedding and clothing should be washed. Carpets, rugs, and curtains should be vacuumed and the vacuum bag placed in a sealed bag in the freezer for several hours before it can be thrown away. Eggs, larvae, nymphs, and adults are killed at 55 to 60 °F. Because humans are only incidental hosts and mites do not reproduce on them, the use of scabicidal agents, such as permethrin, is controversial.
Our patient was treated with permethrin cream before definitive identification of the mite. Once the mite was identified, the chicken was removed from the home and the patient’s bedding and clothing were decontaminated. The patient continued to apply over-the-counter topical steroids and take oral antihistamines for several more days after the chicken was removed from the home.
ACKNOWLEDGEMENT
The authors would like to acknowledge Patrick Liesch of the University of Wisconsin-Madison’s Department of Entomology, Insect Diagnostic Lab, for his help in identifying the avian mite.
1. Leib AE, Anderson BE. Pruritic dermatitis caused by bird mite infestation. Cutis. 2016;97:E6-E8.
2. Collgros H, Iglesias-Sancho M, Aldunce MJ, et al. Dermanyssus gallinae (chicken mite): an underdiagnosed environmental infestation. Clin Exp Dermatol. 2013;38:374-377. doi: 10.1111/j.1365-2230.2012.04434.x
3. Baselga E, Drolet BA, Esterly NB. Avian mite dermatitis. Pediatrics. 1996;97:743-745.
4. James WD, Elston DM, Treat J, et al, eds. Andrews Diseases of the Skin: Clinical Dermatology. 13th ed. Elsevier; 2020.
Dermanyssus gallinae infestation: an unusual cause of scalp pruritus treated with permethrin shampoo. J Dermatolog Treat. 2010;21:319-321. doi: 10.3109/09546630903287437
6. Centers for Disease Control and Prevention. Parasites. Reviewed September 12, 2019. Accessed August 4, 2022. www.cdc.gov/parasites/lice/body/biology.html
A 74-YEAR-OLD WOMAN presented with a 3-day history of an intensely pruritic rash that was localized to her upper arms, upper chest between her breasts, and upper back. The pruritus was much worse at night while the patient was in bed. Symptoms did not improve with over-the-counter topical corticosteroids.
The patient had a history of atrial fibrillation (for which she was receiving chronic anticoagulation therapy), hypertension, an implanted pacemaker, depression, and Parkinson disease. Her medications included carbidopa-levodopa, fluoxetine, hydrochlorothiazide, metoprolol tartrate, naproxen, and warfarin. She had no known allergies. She reported that she was a nonsmoker and drank 1 glass of wine per week.
There were no recent changes in soaps, detergents, lotions, or makeup, nor did the patient have any bug bites or plant exposure. She shared a home with her spouse and several pets: a dog, a cat, and a Bantam-breed chicken. The patient’s husband, who slept in a different bedroom, had no rash. Recently, the cat had been bringing its captured prey of rabbits into the home.
Review of systems was negative for fever, chills, shortness of breath, cough, throat swelling, and rhinorrhea. Physical examination revealed red/pink macules and papules scattered over the upper arms (FIGURE 1), chest, and upper back. Many lesions were excoriated but had no active bleeding or vesicles. Under dermatoscope, no burrowing was found; however, a small (< 1 mm) creature was seen moving rapidly across the skin surface. The physician (CTW) captured and isolated the creature using a sterile lab cup.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Gamasoidosis
The collected sample (FIGURE 2) was examined and identified as an avian mite by a colleague who specializes in entomology, confirming the diagnosis of gamasoidosis.

Two genera of avian mites are responsible: Dermanyssus and Ornithonyssus. The most common culprits are the red poultry mite (D gallinae) and the northern fowl mite (O bursa). These small mites parasitize birds, such as poultry livestock, domesticated birds, and wild game birds. When unfed, the mite appears translucent brown and measures 0.3 to 0.7 mm in length, but after a blood meal, it appears red and increases in size to 1 mm. The mites tend to be active and feed at night and hide during the day.2 This explained the severe nighttime pruritus in this case.
Human infestation, although infrequent, can be a concern for those who work with poultry, or during the spring and summer seasons when young birds leave their nests and the mites migrate to find alternative hosts.3 The 1- to 2-mm erythematous maculopapules are often found with excoriations in covered areas.3,4 Unlike scabies, the genitalia and interdigital areas are spared.3,5
Differential for arthropod dermatoses
The differential diagnosis includes cimicosis, pulicosis, pediculosis corporis, and scabies.
Cimicosis is caused by bed bugs (from the insect Cimex genus). Bed bugs are oval and reddish brown, have 6 legs, and range in size from 1 to 7 mm. Most bed bugs hide in cracks or crevices of furniture and other surfaces (eg, bed frames, headboards, seams or holes of box springs or mattresses, or behind wallpaper, switch plates, and picture frames) by day and come out at night to feed on a sleeping host. Commonly, bed bugs will leave a series of bites grouped in rows (described as “breakfast, lunch, and dinner”). The bites can mimic urticaria, and bullous reactions may also occur.2
Continue to: Pulicosis
Pulicosis results from bites caused by a variety of flea species including, but not limited to, human, dog, oriental rat, sticktight, mouse, and chicken fleas. Fleas are small brown insects measuring about 2.5 mm in length, with flat sides and long hind legs. Their bites are most often arranged in a zigzag pattern around a host’s legs and waist. Hypersensitivity reactions may appear as papular urticaria, nodules, or bullae.2
Pediculosis corporis is caused by body lice. The adult louse is 2.5 to 3.5 mm in size, has 6 legs, and is a tan to greyish white color.6 Lice live in clothing, lay their eggs within the seams, and obtain blood meals from the host. Symptoms include generalized itching. The erythematous blue- and copper-colored macules, wheals, and lichenification can occur throughout the body, but spare the hands and feet. Secondary impetigo and furunculosis commonly occur.2
Scabies is caused by an oval mite that is ventrally flat, with dorsal spines. The mite is < 0.5 mm in size, appearing as a pinpoint of white. It burrows into its host’s skin, where it lives and lays eggs, causing pruritic papular lesions and ensuing excoriations. The mite burrows with a predilection for the finger web spaces, wrists, axillae, areolae, umbilicus, lower abdomen, genitals, and buttocks.2
Treatment involves a 3-step process
The mainstay of treatment is removal of the infested bird, decontamination of bedding and clothing, and use of oral antihistamines and topical corticosteroids.1,3,5 Bedding and clothing should be washed. Carpets, rugs, and curtains should be vacuumed and the vacuum bag placed in a sealed bag in the freezer for several hours before it can be thrown away. Eggs, larvae, nymphs, and adults are killed at 55 to 60 °F. Because humans are only incidental hosts and mites do not reproduce on them, the use of scabicidal agents, such as permethrin, is controversial.
Our patient was treated with permethrin cream before definitive identification of the mite. Once the mite was identified, the chicken was removed from the home and the patient’s bedding and clothing were decontaminated. The patient continued to apply over-the-counter topical steroids and take oral antihistamines for several more days after the chicken was removed from the home.
ACKNOWLEDGEMENT
The authors would like to acknowledge Patrick Liesch of the University of Wisconsin-Madison’s Department of Entomology, Insect Diagnostic Lab, for his help in identifying the avian mite.
A 74-YEAR-OLD WOMAN presented with a 3-day history of an intensely pruritic rash that was localized to her upper arms, upper chest between her breasts, and upper back. The pruritus was much worse at night while the patient was in bed. Symptoms did not improve with over-the-counter topical corticosteroids.
The patient had a history of atrial fibrillation (for which she was receiving chronic anticoagulation therapy), hypertension, an implanted pacemaker, depression, and Parkinson disease. Her medications included carbidopa-levodopa, fluoxetine, hydrochlorothiazide, metoprolol tartrate, naproxen, and warfarin. She had no known allergies. She reported that she was a nonsmoker and drank 1 glass of wine per week.
There were no recent changes in soaps, detergents, lotions, or makeup, nor did the patient have any bug bites or plant exposure. She shared a home with her spouse and several pets: a dog, a cat, and a Bantam-breed chicken. The patient’s husband, who slept in a different bedroom, had no rash. Recently, the cat had been bringing its captured prey of rabbits into the home.
Review of systems was negative for fever, chills, shortness of breath, cough, throat swelling, and rhinorrhea. Physical examination revealed red/pink macules and papules scattered over the upper arms (FIGURE 1), chest, and upper back. Many lesions were excoriated but had no active bleeding or vesicles. Under dermatoscope, no burrowing was found; however, a small (< 1 mm) creature was seen moving rapidly across the skin surface. The physician (CTW) captured and isolated the creature using a sterile lab cup.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Gamasoidosis
The collected sample (FIGURE 2) was examined and identified as an avian mite by a colleague who specializes in entomology, confirming the diagnosis of gamasoidosis.

Two genera of avian mites are responsible: Dermanyssus and Ornithonyssus. The most common culprits are the red poultry mite (D gallinae) and the northern fowl mite (O bursa). These small mites parasitize birds, such as poultry livestock, domesticated birds, and wild game birds. When unfed, the mite appears translucent brown and measures 0.3 to 0.7 mm in length, but after a blood meal, it appears red and increases in size to 1 mm. The mites tend to be active and feed at night and hide during the day.2 This explained the severe nighttime pruritus in this case.
Human infestation, although infrequent, can be a concern for those who work with poultry, or during the spring and summer seasons when young birds leave their nests and the mites migrate to find alternative hosts.3 The 1- to 2-mm erythematous maculopapules are often found with excoriations in covered areas.3,4 Unlike scabies, the genitalia and interdigital areas are spared.3,5
Differential for arthropod dermatoses
The differential diagnosis includes cimicosis, pulicosis, pediculosis corporis, and scabies.
Cimicosis is caused by bed bugs (from the insect Cimex genus). Bed bugs are oval and reddish brown, have 6 legs, and range in size from 1 to 7 mm. Most bed bugs hide in cracks or crevices of furniture and other surfaces (eg, bed frames, headboards, seams or holes of box springs or mattresses, or behind wallpaper, switch plates, and picture frames) by day and come out at night to feed on a sleeping host. Commonly, bed bugs will leave a series of bites grouped in rows (described as “breakfast, lunch, and dinner”). The bites can mimic urticaria, and bullous reactions may also occur.2
Continue to: Pulicosis
Pulicosis results from bites caused by a variety of flea species including, but not limited to, human, dog, oriental rat, sticktight, mouse, and chicken fleas. Fleas are small brown insects measuring about 2.5 mm in length, with flat sides and long hind legs. Their bites are most often arranged in a zigzag pattern around a host’s legs and waist. Hypersensitivity reactions may appear as papular urticaria, nodules, or bullae.2
Pediculosis corporis is caused by body lice. The adult louse is 2.5 to 3.5 mm in size, has 6 legs, and is a tan to greyish white color.6 Lice live in clothing, lay their eggs within the seams, and obtain blood meals from the host. Symptoms include generalized itching. The erythematous blue- and copper-colored macules, wheals, and lichenification can occur throughout the body, but spare the hands and feet. Secondary impetigo and furunculosis commonly occur.2
Scabies is caused by an oval mite that is ventrally flat, with dorsal spines. The mite is < 0.5 mm in size, appearing as a pinpoint of white. It burrows into its host’s skin, where it lives and lays eggs, causing pruritic papular lesions and ensuing excoriations. The mite burrows with a predilection for the finger web spaces, wrists, axillae, areolae, umbilicus, lower abdomen, genitals, and buttocks.2
Treatment involves a 3-step process
The mainstay of treatment is removal of the infested bird, decontamination of bedding and clothing, and use of oral antihistamines and topical corticosteroids.1,3,5 Bedding and clothing should be washed. Carpets, rugs, and curtains should be vacuumed and the vacuum bag placed in a sealed bag in the freezer for several hours before it can be thrown away. Eggs, larvae, nymphs, and adults are killed at 55 to 60 °F. Because humans are only incidental hosts and mites do not reproduce on them, the use of scabicidal agents, such as permethrin, is controversial.
Our patient was treated with permethrin cream before definitive identification of the mite. Once the mite was identified, the chicken was removed from the home and the patient’s bedding and clothing were decontaminated. The patient continued to apply over-the-counter topical steroids and take oral antihistamines for several more days after the chicken was removed from the home.
ACKNOWLEDGEMENT
The authors would like to acknowledge Patrick Liesch of the University of Wisconsin-Madison’s Department of Entomology, Insect Diagnostic Lab, for his help in identifying the avian mite.
1. Leib AE, Anderson BE. Pruritic dermatitis caused by bird mite infestation. Cutis. 2016;97:E6-E8.
2. Collgros H, Iglesias-Sancho M, Aldunce MJ, et al. Dermanyssus gallinae (chicken mite): an underdiagnosed environmental infestation. Clin Exp Dermatol. 2013;38:374-377. doi: 10.1111/j.1365-2230.2012.04434.x
3. Baselga E, Drolet BA, Esterly NB. Avian mite dermatitis. Pediatrics. 1996;97:743-745.
4. James WD, Elston DM, Treat J, et al, eds. Andrews Diseases of the Skin: Clinical Dermatology. 13th ed. Elsevier; 2020.
Dermanyssus gallinae infestation: an unusual cause of scalp pruritus treated with permethrin shampoo. J Dermatolog Treat. 2010;21:319-321. doi: 10.3109/09546630903287437
6. Centers for Disease Control and Prevention. Parasites. Reviewed September 12, 2019. Accessed August 4, 2022. www.cdc.gov/parasites/lice/body/biology.html
1. Leib AE, Anderson BE. Pruritic dermatitis caused by bird mite infestation. Cutis. 2016;97:E6-E8.
2. Collgros H, Iglesias-Sancho M, Aldunce MJ, et al. Dermanyssus gallinae (chicken mite): an underdiagnosed environmental infestation. Clin Exp Dermatol. 2013;38:374-377. doi: 10.1111/j.1365-2230.2012.04434.x
3. Baselga E, Drolet BA, Esterly NB. Avian mite dermatitis. Pediatrics. 1996;97:743-745.
4. James WD, Elston DM, Treat J, et al, eds. Andrews Diseases of the Skin: Clinical Dermatology. 13th ed. Elsevier; 2020.
Dermanyssus gallinae infestation: an unusual cause of scalp pruritus treated with permethrin shampoo. J Dermatolog Treat. 2010;21:319-321. doi: 10.3109/09546630903287437
6. Centers for Disease Control and Prevention. Parasites. Reviewed September 12, 2019. Accessed August 4, 2022. www.cdc.gov/parasites/lice/body/biology.html