User login
Polypodium leucotomos found to reverse AK skin damage
MILAN – Application of topical or both treated over 12 months, in a randomized, blinded study presented at the annual congress of the European Academy of Dermatology and Venereology.
At 12 months, the percentage of patients with a normal or almost normal honeycomb pattern when evaluated blindly with reflectance confocal microscopy (RCM) was about twice as great in either of the two groups that received PLE relative to those treated with topical photoprotection alone, according to Giovanni Pellacani, MD, PhD, chair of dermatology, University of Sapienza, Rome.
“In patients with severe actinic keratosis, the 12-month use of a PLE-based topical or oral photoprotection is associated with positive clinical and anatomical outcomes,” Dr. Pellacani said.
PLE, which is already commonly used in sun protection products, is derived from a South American species of fern and has been proposed for a broad array of dermatologic diseases. According to Dr. Pellacani, in vivo studies associating PLE with immune photoprotection make this agent particularly promising for severe AKs.
In this study involving two clinical research centers in Italy, 131 patients with photoaging and at least three AKs were randomized to one of three treatment arms. The control arm received topical photoprotection with an SPF of 100 or higher applied twice daily to all sun-exposed areas. The two treatment arms received the same topical photoprotection plus either a PLE-containing topical cream alone or a PLE-containing topical cream plus PLE in an oral form (240 mg) once daily
Patients were evaluated at 3 months, 6 months, and 1 year with several measures, including the Actinic Keratosis Area Score Index (AKASI) and the AK Field Assessment Scale Area (AK-FAS). They were also assessed with RCM. All clinical assessments and RCM evaluations, which assessed seven different parameters, such as honeycomb pattern, mottled pigmentation, and reticulated collagen, were performed by dermatologists blinded to the treatment assignment.
Complete data were available for 116 patients who completed all three evaluations over the 12 months of follow-up. On RCM, 50% of those receiving the oral and topical forms of PLE and 45% of those receiving topical PLE had normalization of the honeycomb pattern. These responses were significantly greater (P = .04 for both) than the 26% with normalization in the control group.
Although there were no significant differences in any of the other parameters evaluated by RCM, the improvement in the honeycomb pattern was accompanied by a 7% improvement in the AKASI score in patients taking PLE, either topically or orally and topically, while there was a 6% worsening (P < .001) among controls.
The AK-FAS score improved at 12 months by 26% in the group on oral/topical PLE and by 4% in the group on topical PLE. The score worsened by 13% among controls.
Over the course of the study, patients were permitted to take an appropriate therapy, such as imiquimod, cryotherapy, or 5-flourouracil if there was worsening of the AK-FAS score or if new lesions appeared.
On this measure, 38% of controls and 11% of those randomized to topical PLE had progressive disease versus only 2% of those randomized to take both topical and oral PLE, Dr. Pellacani reported.
The lower rate of new lesions or a start of a new drug over the course of the study in the group receiving both the topical and the oral formulations of PLE relative to those receiving topical PLE alone did not reach statistical significance, but Dr. Pellacani concluded that the addition of PLE to topical photoprotection without PLE seemed to provide a potentially clinically meaningful advantage.
Larger studies and longer term studies are needed, according to Dr. Pellacani, who noted that the substantial body of clinical studies associating PLE with benefit in a variety of dermatologic disorders has been weakened by the absence of well-designed studies that are adequately powered to guide clinical use.
Salvador González, MD, PhD, a dermatology specialist at Alcalá University, Madrid, also believes that PLE deserves further evaluation not just for photoprotection but for reinvigorating damaged skin due to its antioxidant and anti-inflammatory properties. He was the senior author of a 2020 paper in Photochemical and Photobiological Sciences that summarized the potential benefits of PLE in preventing damage related to sun exposure.
Among its mechanism, PLE generates reactive oxygen species (ROS) and prevents depletion of Langerhans cells induced by ultraviolet (UV) light, Dr. González explained in an interview. “At the cellular level, PLE activates tumor suppression p53, inhibits UV-induced COX-2 expression, reduces inflammation, and preventions immunosuppression,” he continued. In addition, he said PLE also prevents UV-A-induced common deletions related to mitochondrial damage and MMP1 expression induced by various UV wavelengths.
“These molecular and cellular effects may translate into long-term inhibition of carcinogenesis including actinic keratosis,” he said, noting that all of these findings “justify the work by Pellacani and collaborators.”
Dr. Pellacani reports no potential conflicts of interest. Dr. González has a financial relationship with Cantabria Laboratories.
MILAN – Application of topical or both treated over 12 months, in a randomized, blinded study presented at the annual congress of the European Academy of Dermatology and Venereology.
At 12 months, the percentage of patients with a normal or almost normal honeycomb pattern when evaluated blindly with reflectance confocal microscopy (RCM) was about twice as great in either of the two groups that received PLE relative to those treated with topical photoprotection alone, according to Giovanni Pellacani, MD, PhD, chair of dermatology, University of Sapienza, Rome.
“In patients with severe actinic keratosis, the 12-month use of a PLE-based topical or oral photoprotection is associated with positive clinical and anatomical outcomes,” Dr. Pellacani said.
PLE, which is already commonly used in sun protection products, is derived from a South American species of fern and has been proposed for a broad array of dermatologic diseases. According to Dr. Pellacani, in vivo studies associating PLE with immune photoprotection make this agent particularly promising for severe AKs.
In this study involving two clinical research centers in Italy, 131 patients with photoaging and at least three AKs were randomized to one of three treatment arms. The control arm received topical photoprotection with an SPF of 100 or higher applied twice daily to all sun-exposed areas. The two treatment arms received the same topical photoprotection plus either a PLE-containing topical cream alone or a PLE-containing topical cream plus PLE in an oral form (240 mg) once daily
Patients were evaluated at 3 months, 6 months, and 1 year with several measures, including the Actinic Keratosis Area Score Index (AKASI) and the AK Field Assessment Scale Area (AK-FAS). They were also assessed with RCM. All clinical assessments and RCM evaluations, which assessed seven different parameters, such as honeycomb pattern, mottled pigmentation, and reticulated collagen, were performed by dermatologists blinded to the treatment assignment.
Complete data were available for 116 patients who completed all three evaluations over the 12 months of follow-up. On RCM, 50% of those receiving the oral and topical forms of PLE and 45% of those receiving topical PLE had normalization of the honeycomb pattern. These responses were significantly greater (P = .04 for both) than the 26% with normalization in the control group.
Although there were no significant differences in any of the other parameters evaluated by RCM, the improvement in the honeycomb pattern was accompanied by a 7% improvement in the AKASI score in patients taking PLE, either topically or orally and topically, while there was a 6% worsening (P < .001) among controls.
The AK-FAS score improved at 12 months by 26% in the group on oral/topical PLE and by 4% in the group on topical PLE. The score worsened by 13% among controls.
Over the course of the study, patients were permitted to take an appropriate therapy, such as imiquimod, cryotherapy, or 5-flourouracil if there was worsening of the AK-FAS score or if new lesions appeared.
On this measure, 38% of controls and 11% of those randomized to topical PLE had progressive disease versus only 2% of those randomized to take both topical and oral PLE, Dr. Pellacani reported.
The lower rate of new lesions or a start of a new drug over the course of the study in the group receiving both the topical and the oral formulations of PLE relative to those receiving topical PLE alone did not reach statistical significance, but Dr. Pellacani concluded that the addition of PLE to topical photoprotection without PLE seemed to provide a potentially clinically meaningful advantage.
Larger studies and longer term studies are needed, according to Dr. Pellacani, who noted that the substantial body of clinical studies associating PLE with benefit in a variety of dermatologic disorders has been weakened by the absence of well-designed studies that are adequately powered to guide clinical use.
Salvador González, MD, PhD, a dermatology specialist at Alcalá University, Madrid, also believes that PLE deserves further evaluation not just for photoprotection but for reinvigorating damaged skin due to its antioxidant and anti-inflammatory properties. He was the senior author of a 2020 paper in Photochemical and Photobiological Sciences that summarized the potential benefits of PLE in preventing damage related to sun exposure.
Among its mechanism, PLE generates reactive oxygen species (ROS) and prevents depletion of Langerhans cells induced by ultraviolet (UV) light, Dr. González explained in an interview. “At the cellular level, PLE activates tumor suppression p53, inhibits UV-induced COX-2 expression, reduces inflammation, and preventions immunosuppression,” he continued. In addition, he said PLE also prevents UV-A-induced common deletions related to mitochondrial damage and MMP1 expression induced by various UV wavelengths.
“These molecular and cellular effects may translate into long-term inhibition of carcinogenesis including actinic keratosis,” he said, noting that all of these findings “justify the work by Pellacani and collaborators.”
Dr. Pellacani reports no potential conflicts of interest. Dr. González has a financial relationship with Cantabria Laboratories.
MILAN – Application of topical or both treated over 12 months, in a randomized, blinded study presented at the annual congress of the European Academy of Dermatology and Venereology.
At 12 months, the percentage of patients with a normal or almost normal honeycomb pattern when evaluated blindly with reflectance confocal microscopy (RCM) was about twice as great in either of the two groups that received PLE relative to those treated with topical photoprotection alone, according to Giovanni Pellacani, MD, PhD, chair of dermatology, University of Sapienza, Rome.
“In patients with severe actinic keratosis, the 12-month use of a PLE-based topical or oral photoprotection is associated with positive clinical and anatomical outcomes,” Dr. Pellacani said.
PLE, which is already commonly used in sun protection products, is derived from a South American species of fern and has been proposed for a broad array of dermatologic diseases. According to Dr. Pellacani, in vivo studies associating PLE with immune photoprotection make this agent particularly promising for severe AKs.
In this study involving two clinical research centers in Italy, 131 patients with photoaging and at least three AKs were randomized to one of three treatment arms. The control arm received topical photoprotection with an SPF of 100 or higher applied twice daily to all sun-exposed areas. The two treatment arms received the same topical photoprotection plus either a PLE-containing topical cream alone or a PLE-containing topical cream plus PLE in an oral form (240 mg) once daily
Patients were evaluated at 3 months, 6 months, and 1 year with several measures, including the Actinic Keratosis Area Score Index (AKASI) and the AK Field Assessment Scale Area (AK-FAS). They were also assessed with RCM. All clinical assessments and RCM evaluations, which assessed seven different parameters, such as honeycomb pattern, mottled pigmentation, and reticulated collagen, were performed by dermatologists blinded to the treatment assignment.
Complete data were available for 116 patients who completed all three evaluations over the 12 months of follow-up. On RCM, 50% of those receiving the oral and topical forms of PLE and 45% of those receiving topical PLE had normalization of the honeycomb pattern. These responses were significantly greater (P = .04 for both) than the 26% with normalization in the control group.
Although there were no significant differences in any of the other parameters evaluated by RCM, the improvement in the honeycomb pattern was accompanied by a 7% improvement in the AKASI score in patients taking PLE, either topically or orally and topically, while there was a 6% worsening (P < .001) among controls.
The AK-FAS score improved at 12 months by 26% in the group on oral/topical PLE and by 4% in the group on topical PLE. The score worsened by 13% among controls.
Over the course of the study, patients were permitted to take an appropriate therapy, such as imiquimod, cryotherapy, or 5-flourouracil if there was worsening of the AK-FAS score or if new lesions appeared.
On this measure, 38% of controls and 11% of those randomized to topical PLE had progressive disease versus only 2% of those randomized to take both topical and oral PLE, Dr. Pellacani reported.
The lower rate of new lesions or a start of a new drug over the course of the study in the group receiving both the topical and the oral formulations of PLE relative to those receiving topical PLE alone did not reach statistical significance, but Dr. Pellacani concluded that the addition of PLE to topical photoprotection without PLE seemed to provide a potentially clinically meaningful advantage.
Larger studies and longer term studies are needed, according to Dr. Pellacani, who noted that the substantial body of clinical studies associating PLE with benefit in a variety of dermatologic disorders has been weakened by the absence of well-designed studies that are adequately powered to guide clinical use.
Salvador González, MD, PhD, a dermatology specialist at Alcalá University, Madrid, also believes that PLE deserves further evaluation not just for photoprotection but for reinvigorating damaged skin due to its antioxidant and anti-inflammatory properties. He was the senior author of a 2020 paper in Photochemical and Photobiological Sciences that summarized the potential benefits of PLE in preventing damage related to sun exposure.
Among its mechanism, PLE generates reactive oxygen species (ROS) and prevents depletion of Langerhans cells induced by ultraviolet (UV) light, Dr. González explained in an interview. “At the cellular level, PLE activates tumor suppression p53, inhibits UV-induced COX-2 expression, reduces inflammation, and preventions immunosuppression,” he continued. In addition, he said PLE also prevents UV-A-induced common deletions related to mitochondrial damage and MMP1 expression induced by various UV wavelengths.
“These molecular and cellular effects may translate into long-term inhibition of carcinogenesis including actinic keratosis,” he said, noting that all of these findings “justify the work by Pellacani and collaborators.”
Dr. Pellacani reports no potential conflicts of interest. Dr. González has a financial relationship with Cantabria Laboratories.
AT THE EADV CONGRESS
Isotretinoin prescribers need better education on emergency contraception
, in a survey of 57 clinicians.
Pregnancies among patients on isotretinoin have declined since the iPLEDGE risk management program was introduced in 2005, but from 2011 to 2017, 210 to 310 pregnancies were reported to the Food and Drug Administration every year, wrote Catherine E. Smiley of Penn State University, Hershey, Pa., and coauthors Melissa Butt, DrPH, and Andrea L. Zaenglein, MD, of Penn State.
For patients on isotretinoin, EC “becomes critical when abstinence fails or contraception is not used properly,” but EC merits only a brief mention in iPLEDGE materials for patients and providers, they noted.
Patients on isotretinoin who choose abstinence as their form of birth control are the group at greatest risk for pregnancy, Dr. Zaenglein, professor of dermatology and pediatric dermatology, Penn State University, said in an interview. “However, the iPLEDGE program fails to educate patients adequately on emergency contraception,” she explained.
To assess pediatric dermatologists’ understanding of EC and their contraception counseling practices for isotretinoin patients, the researchers surveyed 57 pediatric dermatologists who prescribed isotretinoin as part of their practices. The findings were published in Pediatric Dermatology.Respondents included 53 practicing dermatologists, 2 residents, and 2 fellows. Approximately one-third (31.6%) had been in practice for 6-10 years, almost 23% had been in practice for 3-5 years, and almost 20% had been in practice for 21 or more years. Almost two-thirds practiced pediatric dermatology only.
Overall, 58% of the respondents strongly agreed that they provided contraception counseling to patients at their initial visit for isotretinoin, but only 7% and 3.5% reported providing EC counseling at initial and follow-up visits, respectively. More than half (58%) said they did not counsel patients on the side effects of EC.
As for provider education, 7.1% of respondents said they had received formal education on EC counseling, 25% reported receiving informal education on EC counseling, and 68% said they received no education on EC counseling.
A total of 32% of respondents said they were at least somewhat confident in how to obtain EC in their state.
EC is an effective form of contraception if used after unprotected intercourse, and discounts can reduce the price to as low as $9.69, the researchers wrote in their discussion. “Given that most providers in this study did not receive formal education on EC, and most do not provide EC counseling to their patients of reproductive potential on isotretinoin, EC education should be a core competency in dermatology residency education on isotretinoin prescribing,” the researchers noted. In addition, EC counseling in the iPLEDGE program should be improved by including more information in education materials and reminding patients that EC is an option, they said.
The study findings were limited by several factors including the small sample size and the multiple-choice format that prevented respondents to share rationales for their responses, the researchers noted.
However, the results highlight the need to improve EC education among pediatric dermatologists to better inform patients considering isotretinoin, especially those choosing abstinence as a method of birth control, they emphasized.
“This study is very important at this specific time for two reasons,” Dr. Zaenglein said in an interview. “The first is that with the recent disastrous rollout of the new iPLEDGE changes, there have been many calls to reform the REMS program. For the first time in the 22-year history of the program, the isotretinoin manufacturers, who manage the iPLEDGE program as an unidentified group (the IPMG), have been forced by the FDA to meet with the AAD iPLEDGE Task Force,” said Dr. Zaenglein, a member of the task force.
“The task force is currently advocating for common sense changes to iPLEDGE and I think enhancing education on emergency contraception is vital to the goal of the program, stated as ‘to manage the risk of isotretinoin’s teratogenicity and to minimize fetal exposure,’ ” she added. For many patients who previously became pregnant on isotretinoin, Plan B, an over-the-counter, FDA-approved form of contraception, might have prevented that pregnancy if the patients received adequate education on EC, she said.
The current study is especially relevant now, said Dr. Zaenglein. “With the reversal of Roe v. Wade, access to abortion is restricted or completely banned in many states, which makes educating our patients on how to prevent pregnancy even more important.”
Dr. Zaenglein said she was “somewhat surprised” by how many respondents were not educating their isotretinoin patients on EC. “However, these results follow a known trend among dermatologists. Only 50% of dermatologists prescribe oral contraceptives for acne, despite its being an FDA-approved treatment for the most common dermatologic condition we see in adolescents and young adults,” she noted.
“In general, dermatologists, and subsequently dermatology residents, are poorly educated on issues of reproductive health and how they are relevant to dermatologic care,” she added.
Dr. Zaenglein’s take home message: “Dermatologists should educate all patients of childbearing potential taking isotretinoin on how to acquire and use emergency contraception at every visit.” As for additional research, she said that since the study was conducted with pediatric dermatologists, “it would be very interesting to see if general dermatologists had the same lack of comfort in educating patients on emergency contraception and what their standard counseling practices are.”
The study received no outside funding. Dr. Zaenglein is a member of the AAD’s iPLEDGE Work Group and serves as an editor-in-chief of Pediatric Dermatology.
, in a survey of 57 clinicians.
Pregnancies among patients on isotretinoin have declined since the iPLEDGE risk management program was introduced in 2005, but from 2011 to 2017, 210 to 310 pregnancies were reported to the Food and Drug Administration every year, wrote Catherine E. Smiley of Penn State University, Hershey, Pa., and coauthors Melissa Butt, DrPH, and Andrea L. Zaenglein, MD, of Penn State.
For patients on isotretinoin, EC “becomes critical when abstinence fails or contraception is not used properly,” but EC merits only a brief mention in iPLEDGE materials for patients and providers, they noted.
Patients on isotretinoin who choose abstinence as their form of birth control are the group at greatest risk for pregnancy, Dr. Zaenglein, professor of dermatology and pediatric dermatology, Penn State University, said in an interview. “However, the iPLEDGE program fails to educate patients adequately on emergency contraception,” she explained.
To assess pediatric dermatologists’ understanding of EC and their contraception counseling practices for isotretinoin patients, the researchers surveyed 57 pediatric dermatologists who prescribed isotretinoin as part of their practices. The findings were published in Pediatric Dermatology.Respondents included 53 practicing dermatologists, 2 residents, and 2 fellows. Approximately one-third (31.6%) had been in practice for 6-10 years, almost 23% had been in practice for 3-5 years, and almost 20% had been in practice for 21 or more years. Almost two-thirds practiced pediatric dermatology only.
Overall, 58% of the respondents strongly agreed that they provided contraception counseling to patients at their initial visit for isotretinoin, but only 7% and 3.5% reported providing EC counseling at initial and follow-up visits, respectively. More than half (58%) said they did not counsel patients on the side effects of EC.
As for provider education, 7.1% of respondents said they had received formal education on EC counseling, 25% reported receiving informal education on EC counseling, and 68% said they received no education on EC counseling.
A total of 32% of respondents said they were at least somewhat confident in how to obtain EC in their state.
EC is an effective form of contraception if used after unprotected intercourse, and discounts can reduce the price to as low as $9.69, the researchers wrote in their discussion. “Given that most providers in this study did not receive formal education on EC, and most do not provide EC counseling to their patients of reproductive potential on isotretinoin, EC education should be a core competency in dermatology residency education on isotretinoin prescribing,” the researchers noted. In addition, EC counseling in the iPLEDGE program should be improved by including more information in education materials and reminding patients that EC is an option, they said.
The study findings were limited by several factors including the small sample size and the multiple-choice format that prevented respondents to share rationales for their responses, the researchers noted.
However, the results highlight the need to improve EC education among pediatric dermatologists to better inform patients considering isotretinoin, especially those choosing abstinence as a method of birth control, they emphasized.
“This study is very important at this specific time for two reasons,” Dr. Zaenglein said in an interview. “The first is that with the recent disastrous rollout of the new iPLEDGE changes, there have been many calls to reform the REMS program. For the first time in the 22-year history of the program, the isotretinoin manufacturers, who manage the iPLEDGE program as an unidentified group (the IPMG), have been forced by the FDA to meet with the AAD iPLEDGE Task Force,” said Dr. Zaenglein, a member of the task force.
“The task force is currently advocating for common sense changes to iPLEDGE and I think enhancing education on emergency contraception is vital to the goal of the program, stated as ‘to manage the risk of isotretinoin’s teratogenicity and to minimize fetal exposure,’ ” she added. For many patients who previously became pregnant on isotretinoin, Plan B, an over-the-counter, FDA-approved form of contraception, might have prevented that pregnancy if the patients received adequate education on EC, she said.
The current study is especially relevant now, said Dr. Zaenglein. “With the reversal of Roe v. Wade, access to abortion is restricted or completely banned in many states, which makes educating our patients on how to prevent pregnancy even more important.”
Dr. Zaenglein said she was “somewhat surprised” by how many respondents were not educating their isotretinoin patients on EC. “However, these results follow a known trend among dermatologists. Only 50% of dermatologists prescribe oral contraceptives for acne, despite its being an FDA-approved treatment for the most common dermatologic condition we see in adolescents and young adults,” she noted.
“In general, dermatologists, and subsequently dermatology residents, are poorly educated on issues of reproductive health and how they are relevant to dermatologic care,” she added.
Dr. Zaenglein’s take home message: “Dermatologists should educate all patients of childbearing potential taking isotretinoin on how to acquire and use emergency contraception at every visit.” As for additional research, she said that since the study was conducted with pediatric dermatologists, “it would be very interesting to see if general dermatologists had the same lack of comfort in educating patients on emergency contraception and what their standard counseling practices are.”
The study received no outside funding. Dr. Zaenglein is a member of the AAD’s iPLEDGE Work Group and serves as an editor-in-chief of Pediatric Dermatology.
, in a survey of 57 clinicians.
Pregnancies among patients on isotretinoin have declined since the iPLEDGE risk management program was introduced in 2005, but from 2011 to 2017, 210 to 310 pregnancies were reported to the Food and Drug Administration every year, wrote Catherine E. Smiley of Penn State University, Hershey, Pa., and coauthors Melissa Butt, DrPH, and Andrea L. Zaenglein, MD, of Penn State.
For patients on isotretinoin, EC “becomes critical when abstinence fails or contraception is not used properly,” but EC merits only a brief mention in iPLEDGE materials for patients and providers, they noted.
Patients on isotretinoin who choose abstinence as their form of birth control are the group at greatest risk for pregnancy, Dr. Zaenglein, professor of dermatology and pediatric dermatology, Penn State University, said in an interview. “However, the iPLEDGE program fails to educate patients adequately on emergency contraception,” she explained.
To assess pediatric dermatologists’ understanding of EC and their contraception counseling practices for isotretinoin patients, the researchers surveyed 57 pediatric dermatologists who prescribed isotretinoin as part of their practices. The findings were published in Pediatric Dermatology.Respondents included 53 practicing dermatologists, 2 residents, and 2 fellows. Approximately one-third (31.6%) had been in practice for 6-10 years, almost 23% had been in practice for 3-5 years, and almost 20% had been in practice for 21 or more years. Almost two-thirds practiced pediatric dermatology only.
Overall, 58% of the respondents strongly agreed that they provided contraception counseling to patients at their initial visit for isotretinoin, but only 7% and 3.5% reported providing EC counseling at initial and follow-up visits, respectively. More than half (58%) said they did not counsel patients on the side effects of EC.
As for provider education, 7.1% of respondents said they had received formal education on EC counseling, 25% reported receiving informal education on EC counseling, and 68% said they received no education on EC counseling.
A total of 32% of respondents said they were at least somewhat confident in how to obtain EC in their state.
EC is an effective form of contraception if used after unprotected intercourse, and discounts can reduce the price to as low as $9.69, the researchers wrote in their discussion. “Given that most providers in this study did not receive formal education on EC, and most do not provide EC counseling to their patients of reproductive potential on isotretinoin, EC education should be a core competency in dermatology residency education on isotretinoin prescribing,” the researchers noted. In addition, EC counseling in the iPLEDGE program should be improved by including more information in education materials and reminding patients that EC is an option, they said.
The study findings were limited by several factors including the small sample size and the multiple-choice format that prevented respondents to share rationales for their responses, the researchers noted.
However, the results highlight the need to improve EC education among pediatric dermatologists to better inform patients considering isotretinoin, especially those choosing abstinence as a method of birth control, they emphasized.
“This study is very important at this specific time for two reasons,” Dr. Zaenglein said in an interview. “The first is that with the recent disastrous rollout of the new iPLEDGE changes, there have been many calls to reform the REMS program. For the first time in the 22-year history of the program, the isotretinoin manufacturers, who manage the iPLEDGE program as an unidentified group (the IPMG), have been forced by the FDA to meet with the AAD iPLEDGE Task Force,” said Dr. Zaenglein, a member of the task force.
“The task force is currently advocating for common sense changes to iPLEDGE and I think enhancing education on emergency contraception is vital to the goal of the program, stated as ‘to manage the risk of isotretinoin’s teratogenicity and to minimize fetal exposure,’ ” she added. For many patients who previously became pregnant on isotretinoin, Plan B, an over-the-counter, FDA-approved form of contraception, might have prevented that pregnancy if the patients received adequate education on EC, she said.
The current study is especially relevant now, said Dr. Zaenglein. “With the reversal of Roe v. Wade, access to abortion is restricted or completely banned in many states, which makes educating our patients on how to prevent pregnancy even more important.”
Dr. Zaenglein said she was “somewhat surprised” by how many respondents were not educating their isotretinoin patients on EC. “However, these results follow a known trend among dermatologists. Only 50% of dermatologists prescribe oral contraceptives for acne, despite its being an FDA-approved treatment for the most common dermatologic condition we see in adolescents and young adults,” she noted.
“In general, dermatologists, and subsequently dermatology residents, are poorly educated on issues of reproductive health and how they are relevant to dermatologic care,” she added.
Dr. Zaenglein’s take home message: “Dermatologists should educate all patients of childbearing potential taking isotretinoin on how to acquire and use emergency contraception at every visit.” As for additional research, she said that since the study was conducted with pediatric dermatologists, “it would be very interesting to see if general dermatologists had the same lack of comfort in educating patients on emergency contraception and what their standard counseling practices are.”
The study received no outside funding. Dr. Zaenglein is a member of the AAD’s iPLEDGE Work Group and serves as an editor-in-chief of Pediatric Dermatology.
FROM PEDIATRIC DERMATOLOGY
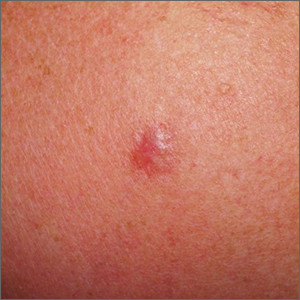
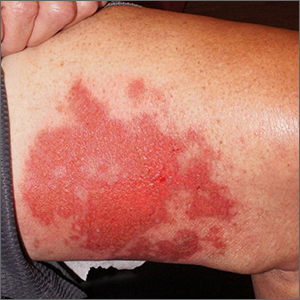
Shoulder lesion
A punch biopsy of the lesion was performed and the results were consistent with a dermatofibroma, which is a benign growth.
Dermatofibromas may manifest as a pink papule on fair-skinned individuals or a darker brown papule in patients of color. Clinically, the texture can be helpful to discern an etiology—dermatofibromas may dimple when pinched laterally, while melanocytic nevi or melanomas tend to be somewhat softer on palpation. Cutaneous sarcoma, while exceedingly rare, may be firmer and chaotic, and varied with multiple colors and topographical changes.
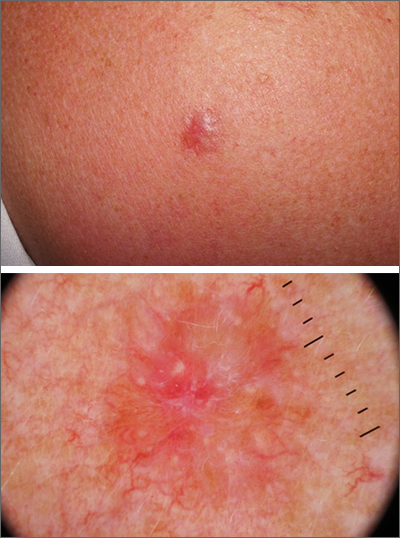
The dermoscopic pattern of a dermatofibroma includes central scar-like areas, a peripheral pigment network, occasional shiny white lines, and confluent circular brown macules. Other less frequent dermoscopic structures may also be seen. A prospective study of the dermoscopic morphology of 412 dermatofibromas found 10 distinct dermoscopic patterns, but also noted that 25% of the dermatofibromas exhibited an atypical pattern.1 Atypical pigment, multiple scar-like areas, and dotted vessels can occur in a dermatofibroma, as well as in a Spitz nevus, and melanoma. Thus, such findings should prompt a biopsy.
Dermatofibromas are safe to observe, but they can be surgically excised if they cause pain or cosmetic concerns.
This patient was reassured to know that the lesion would not require surgical intervention and was unlikely to enlarge or change significantly over time.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Zaballos P, Puig S, Llambrich A, Malvehy J. Dermoscopy of dermatofibromas: a prospective morphological study of 412 cases. Arch Dermatol. 2008;144:75-83. doi: 10.1001/archdermatol.2007.8

A punch biopsy of the lesion was performed and the results were consistent with a dermatofibroma, which is a benign growth.
Dermatofibromas may manifest as a pink papule on fair-skinned individuals or a darker brown papule in patients of color. Clinically, the texture can be helpful to discern an etiology—dermatofibromas may dimple when pinched laterally, while melanocytic nevi or melanomas tend to be somewhat softer on palpation. Cutaneous sarcoma, while exceedingly rare, may be firmer and chaotic, and varied with multiple colors and topographical changes.
The dermoscopic pattern of a dermatofibroma includes central scar-like areas, a peripheral pigment network, occasional shiny white lines, and confluent circular brown macules. Other less frequent dermoscopic structures may also be seen. A prospective study of the dermoscopic morphology of 412 dermatofibromas found 10 distinct dermoscopic patterns, but also noted that 25% of the dermatofibromas exhibited an atypical pattern.1 Atypical pigment, multiple scar-like areas, and dotted vessels can occur in a dermatofibroma, as well as in a Spitz nevus, and melanoma. Thus, such findings should prompt a biopsy.
Dermatofibromas are safe to observe, but they can be surgically excised if they cause pain or cosmetic concerns.
This patient was reassured to know that the lesion would not require surgical intervention and was unlikely to enlarge or change significantly over time.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

A punch biopsy of the lesion was performed and the results were consistent with a dermatofibroma, which is a benign growth.
Dermatofibromas may manifest as a pink papule on fair-skinned individuals or a darker brown papule in patients of color. Clinically, the texture can be helpful to discern an etiology—dermatofibromas may dimple when pinched laterally, while melanocytic nevi or melanomas tend to be somewhat softer on palpation. Cutaneous sarcoma, while exceedingly rare, may be firmer and chaotic, and varied with multiple colors and topographical changes.
The dermoscopic pattern of a dermatofibroma includes central scar-like areas, a peripheral pigment network, occasional shiny white lines, and confluent circular brown macules. Other less frequent dermoscopic structures may also be seen. A prospective study of the dermoscopic morphology of 412 dermatofibromas found 10 distinct dermoscopic patterns, but also noted that 25% of the dermatofibromas exhibited an atypical pattern.1 Atypical pigment, multiple scar-like areas, and dotted vessels can occur in a dermatofibroma, as well as in a Spitz nevus, and melanoma. Thus, such findings should prompt a biopsy.
Dermatofibromas are safe to observe, but they can be surgically excised if they cause pain or cosmetic concerns.
This patient was reassured to know that the lesion would not require surgical intervention and was unlikely to enlarge or change significantly over time.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Zaballos P, Puig S, Llambrich A, Malvehy J. Dermoscopy of dermatofibromas: a prospective morphological study of 412 cases. Arch Dermatol. 2008;144:75-83. doi: 10.1001/archdermatol.2007.8
1. Zaballos P, Puig S, Llambrich A, Malvehy J. Dermoscopy of dermatofibromas: a prospective morphological study of 412 cases. Arch Dermatol. 2008;144:75-83. doi: 10.1001/archdermatol.2007.8
FDA okays spesolimab, first treatment for generalized pustular psoriasis
The U.S. Food and Drug Administration has approved the biologic agent spesolimab (Spevigo) for the treatment of flares in adults with generalized pustular psoriasis (GPP), the company that manufactures the drug has announced.
Until this approval, “there were no FDA-approved options to treat patients experiencing a GPP flare,” Mark Lebwohl, MD, principal investigator in the pivotal spesolimab trial, told this news organization. The approval “is a turning point for dermatologists and clinicians who treat patients living with this devastating and debilitating disease,” he said. Treatment with spesolimab “rapidly improves the clinical symptoms of GPP flares and will greatly improve our ability to help our patients manage painful flares,” noted Dr. Lebwohl, dean of clinical therapeutics and professor of dermatology, Icahn School of Medicine at Mount Sinai, New York.
Spesolimab, manufactured by Boehringer Ingelheim, is a novel, selective monoclonal antibody that blocks interleukin-36 signaling known to be involved in GPP. It received priority review and had orphan drug and breakthrough therapy designation.
GPP affects an estimated 1 of every 10,000 people in the United States.
Though rare, GPP is a potentially life-threatening disease that is distinct from plaque psoriasis. GPP is caused by the accumulation of neutrophils in the skin. Throughout the course of the disease, patients may suffer recurring episodes of widespread eruptions of painful, sterile pustules across all parts of the body.
Spesolimab was evaluated in a global, 12-week, placebo-controlled clinical trial that involved 53 adults experiencing a GPP flare. After 1 week, significantly more patients treated with spesolimab than placebo showed no visible pustules (54% vs 6%), according to the company.
The most common adverse reactions, seen in at least 5% of patients treated with spesolimab, were asthenia and fatigue; nausea and vomiting; headache; pruritus and prurigo; hematoma and bruising at the infusion site; and urinary tract infection.
Dr. Lebwohl is a paid consultant to Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
This article was updated 9/6/22.
The U.S. Food and Drug Administration has approved the biologic agent spesolimab (Spevigo) for the treatment of flares in adults with generalized pustular psoriasis (GPP), the company that manufactures the drug has announced.
Until this approval, “there were no FDA-approved options to treat patients experiencing a GPP flare,” Mark Lebwohl, MD, principal investigator in the pivotal spesolimab trial, told this news organization. The approval “is a turning point for dermatologists and clinicians who treat patients living with this devastating and debilitating disease,” he said. Treatment with spesolimab “rapidly improves the clinical symptoms of GPP flares and will greatly improve our ability to help our patients manage painful flares,” noted Dr. Lebwohl, dean of clinical therapeutics and professor of dermatology, Icahn School of Medicine at Mount Sinai, New York.
Spesolimab, manufactured by Boehringer Ingelheim, is a novel, selective monoclonal antibody that blocks interleukin-36 signaling known to be involved in GPP. It received priority review and had orphan drug and breakthrough therapy designation.
GPP affects an estimated 1 of every 10,000 people in the United States.
Though rare, GPP is a potentially life-threatening disease that is distinct from plaque psoriasis. GPP is caused by the accumulation of neutrophils in the skin. Throughout the course of the disease, patients may suffer recurring episodes of widespread eruptions of painful, sterile pustules across all parts of the body.
Spesolimab was evaluated in a global, 12-week, placebo-controlled clinical trial that involved 53 adults experiencing a GPP flare. After 1 week, significantly more patients treated with spesolimab than placebo showed no visible pustules (54% vs 6%), according to the company.
The most common adverse reactions, seen in at least 5% of patients treated with spesolimab, were asthenia and fatigue; nausea and vomiting; headache; pruritus and prurigo; hematoma and bruising at the infusion site; and urinary tract infection.
Dr. Lebwohl is a paid consultant to Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
This article was updated 9/6/22.
The U.S. Food and Drug Administration has approved the biologic agent spesolimab (Spevigo) for the treatment of flares in adults with generalized pustular psoriasis (GPP), the company that manufactures the drug has announced.
Until this approval, “there were no FDA-approved options to treat patients experiencing a GPP flare,” Mark Lebwohl, MD, principal investigator in the pivotal spesolimab trial, told this news organization. The approval “is a turning point for dermatologists and clinicians who treat patients living with this devastating and debilitating disease,” he said. Treatment with spesolimab “rapidly improves the clinical symptoms of GPP flares and will greatly improve our ability to help our patients manage painful flares,” noted Dr. Lebwohl, dean of clinical therapeutics and professor of dermatology, Icahn School of Medicine at Mount Sinai, New York.
Spesolimab, manufactured by Boehringer Ingelheim, is a novel, selective monoclonal antibody that blocks interleukin-36 signaling known to be involved in GPP. It received priority review and had orphan drug and breakthrough therapy designation.
GPP affects an estimated 1 of every 10,000 people in the United States.
Though rare, GPP is a potentially life-threatening disease that is distinct from plaque psoriasis. GPP is caused by the accumulation of neutrophils in the skin. Throughout the course of the disease, patients may suffer recurring episodes of widespread eruptions of painful, sterile pustules across all parts of the body.
Spesolimab was evaluated in a global, 12-week, placebo-controlled clinical trial that involved 53 adults experiencing a GPP flare. After 1 week, significantly more patients treated with spesolimab than placebo showed no visible pustules (54% vs 6%), according to the company.
The most common adverse reactions, seen in at least 5% of patients treated with spesolimab, were asthenia and fatigue; nausea and vomiting; headache; pruritus and prurigo; hematoma and bruising at the infusion site; and urinary tract infection.
Dr. Lebwohl is a paid consultant to Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
This article was updated 9/6/22.
Expert shares tips on hair disorders and photoprotection for patients of color
PORTLAND, ORE. – , but sometimes their doctors fall short.
“Many times, you may not have race concordant visits with patients of color,” Janiene Luke, MD, said at the annual meeting of the Pacific Dermatologic Association. She referred to a survey of 200 Black women aged 21-83 years, which found that 28% had visited a physician to discuss hair or scalp issues. Of those, 68% felt like their dermatologists did not understand African American hair.
“I recommend trying the best you can to familiarize yourself with various common cultural hair styling methods and practices in patients of color. It’s important to understand what your patients are engaging in and the types of styles they’re using,” said Dr. Luke, associate professor of dermatology at Loma Linda (Calif.) University. “Approach all patients with cultural humility. We know from studies that patients value dermatologists who take time to listen to their concerns, involve them in the decision-making process, and educate them about their conditions,” she added.
National efforts to educate clinicians on treating skin of color have emerged in recent years, including textbooks, CME courses at dermatology conferences, and the American Academy of Dermatology’s Skin of Color Curriculum, which consists of 15-minute modules that can be viewed online.
At the meeting, Dr. Luke, shared her approach to assessing hair and scalp disorders in skin of color. She begins by taking a thorough history, “because not all things that are associated with hair styling will be the reason why your patient comes in,” she said. “Patients of color can have telogen effluvium and seborrheic dermatitis just like anyone else. I ask about the hair styling practices they use. I also ask how often they wash their hair, because sometimes our recommendations for treatment are not realistic based on their current routine.”
Next, she examines the scalp with her hands – which sometimes surprises patients. “I’ve had so many patients come in and say, ‘the dermatologist never touched my scalp,’ or ‘they never even looked at my hair,’ ” said Dr. Luke, who directs the university’s dermatology residency program. She asks patients to remove any hair extensions or weaves prior to the office visit and to remove wigs prior to the exam itself. The lab tests she customarily orders include CBC, TSH, iron, total iron binding capacity, ferritin, vitamin D, and zinc. If there are signs of androgen excess, she may check testosterone, sex hormone binding globulin, and dehydroepiandrosterone sulfate (DHEA-S). She routinely incorporates a dermoscopy-directed biopsy into the evaluation.
Dr. Luke examines the patient from above, the sides, and the back to assess the pattern/distribution of hair loss. A visible scalp at the vertex indicates a 50% reduction in normal hair density. “I’m looking at the hairline, their part width, and the length of their hair,” she said. “I also look at the eyebrows and eyelashes, because these can be involved in alopecia areata, frontal fibrosing alopecia, or congenital hair shaft disorders.”
On closeup examination, she looks for scarring versus non-scarring types of hair loss, and for the presence or absence of follicular ostia. “I also look at hair changes,” she said. “Is the texture of their hair different? Are there signs of breakage or fragility? It’s been noted in studies that breakage can be an early sign of central centrifugal cicatricial alopecia.” (For more tips on examining tightly coiled hair among patients with hair loss in race discordant patient-physician interactions, she recommended a 2021 article in JAMA Dermatology)..
Trichoscopy allows for magnified observation of the hair shafts, hair follicle openings, perifollicular dermis, and blood vessels. Normal trichoscopy findings in skin of color reveal a perifollicular pigment network (honeycomb pattern) and pinpoint white dots that are regularly distributed between follicular units.
Common abnormalities seen on trichoscopy include central centrifugal cicatricial alopecia (CCCA), with one or two hairs emerging together, surrounded by a gray halo; lichen planopilaris/frontal fibrosing alopecia, characterized by hair with peripilar casts and absence of vellus hairs; discoid lupus erythematosus, characterized by keratotic plugs; and traction, characterized by hair casts.
Once a diagnosis is confirmed, Dr. Luke provides other general advice for optimal skin health, including a balanced (whole food) diet to ensure adequate nutrition. “I tend to find a lot of nutrient deficiencies that contribute to and compound their condition,” she said. Other recommendations include avoiding excess tension on the hair, such as hair styles with tight ponytails, buns, braids, and weaves; avoiding or limiting chemical treatments with hair color, relaxers, and permanents; and avoiding or limiting excessive heat styling with blow dryers, flat irons, and curling irons.
Photoprotection misconceptions
At the meeting, Dr. Luke also discussed three misconceptions of photoprotection in skin of color, drawn from an article on the topic published in 2021.
- Myth No. 1: Endogenous melanin provides complete photoprotection for Fitzpatrick skin types IV-V. Many people with skin of color may believe sunscreen is not needed given the melanin already present in their skin, but research has shown that the epidermis of dark skin has an intrinsic sun protection factor (SPF) of 13.4, compared with an SPF of 3.3 in light skin. “That may not provide them with full protection,” Dr. Luke said. “Many dermatologists are not counseling their skin of color patients about photoprotection.”
- Myth No. 2: Individuals with skin of color have negligible risks associated with skin cancer. Skin cancer prevalence in patients with skin of color is significantly lower compared with those with light skin. However, people with skin of color tend to be diagnosed with cancers at a more advanced stage, and cancers associated with a worse prognosis and poorer survival rate. An analysis of ethnic differences among patients with cutaneous melanoma that drew from the Surveillance, Epidemiology, and End Results (SEER) program found that Hispanic individuals (odds ratio [OR], 3.6), Black individuals (OR, 4.2), and Asian individuals (OR, 2.4), were more likely than were White individuals to have stage IV melanoma at the time of presentation. “For melanoma in skin of color, UV radiation does not seem to be a major risk factor, as melanoma tends to occur on palmar/plantar and subungual skin as well as mucous membranes,” Dr. Luke said. “For squamous cell carcinoma in skin of color, lesions are more likely to be present in areas that are not sun exposed. The risk factors for this tend to be chronic wounds, nonhealing ulcers, and people with chronic inflammatory conditions.” For basal cell carcinoma, she added, UV radiation seems to play more of a role and tends to occur in sun-exposed areas in patients with lighter Fitzpatrick skin types. Patients are more likely to present with pigmented BCCs.
- Myth No. 3: Broad-spectrum sunscreens provide photoprotection against all wavelengths of light that cause skin damage. To be labeled “broad-spectrum” the Food and Drug Administration requires that sunscreens have a critical wavelength of 370 nm or below, but Dr. Luke noted that broad-spectrum sunscreens do not necessarily protect against visible light (VL) and UV-A1. Research has demonstrated that VL exposure induces both transient and long-term cutaneous pigmentation in a dose-dependent manner.
“This induces free radicals and reactive oxygen species, leading to a cascade of events including the induction of pro-inflammatory cytokines, matrix metalloproteinases, and melanogenesis,” she said. “More intense and persistent VL-induced pigmentation occurs in subjects with darker skin. However, there is increasing evidence that antioxidants may help to mitigate these negative effects, so we are starting to see the addition of antioxidants into sunscreens.”
Dr. Luke recommends a broad-spectrum sunscreen with an SPF of 30 or higher for skin of color patients. Tinted sunscreens, which contain iron oxide pigments, are recommended for the prevention and treatment of pigmentary disorders in patients with Fitzpatrick skin types IV-VI skin. “What about adding antioxidants to prevent formation of reactive oxygen species?” she asked. “It’s possible but we don’t have a lot of research yet. You also want a sunscreen that’s aesthetically elegant, meaning it doesn’t leave a white cast.”
Dr. Luke reported having no relevant disclosures.
PORTLAND, ORE. – , but sometimes their doctors fall short.
“Many times, you may not have race concordant visits with patients of color,” Janiene Luke, MD, said at the annual meeting of the Pacific Dermatologic Association. She referred to a survey of 200 Black women aged 21-83 years, which found that 28% had visited a physician to discuss hair or scalp issues. Of those, 68% felt like their dermatologists did not understand African American hair.
“I recommend trying the best you can to familiarize yourself with various common cultural hair styling methods and practices in patients of color. It’s important to understand what your patients are engaging in and the types of styles they’re using,” said Dr. Luke, associate professor of dermatology at Loma Linda (Calif.) University. “Approach all patients with cultural humility. We know from studies that patients value dermatologists who take time to listen to their concerns, involve them in the decision-making process, and educate them about their conditions,” she added.
National efforts to educate clinicians on treating skin of color have emerged in recent years, including textbooks, CME courses at dermatology conferences, and the American Academy of Dermatology’s Skin of Color Curriculum, which consists of 15-minute modules that can be viewed online.
At the meeting, Dr. Luke, shared her approach to assessing hair and scalp disorders in skin of color. She begins by taking a thorough history, “because not all things that are associated with hair styling will be the reason why your patient comes in,” she said. “Patients of color can have telogen effluvium and seborrheic dermatitis just like anyone else. I ask about the hair styling practices they use. I also ask how often they wash their hair, because sometimes our recommendations for treatment are not realistic based on their current routine.”
Next, she examines the scalp with her hands – which sometimes surprises patients. “I’ve had so many patients come in and say, ‘the dermatologist never touched my scalp,’ or ‘they never even looked at my hair,’ ” said Dr. Luke, who directs the university’s dermatology residency program. She asks patients to remove any hair extensions or weaves prior to the office visit and to remove wigs prior to the exam itself. The lab tests she customarily orders include CBC, TSH, iron, total iron binding capacity, ferritin, vitamin D, and zinc. If there are signs of androgen excess, she may check testosterone, sex hormone binding globulin, and dehydroepiandrosterone sulfate (DHEA-S). She routinely incorporates a dermoscopy-directed biopsy into the evaluation.
Dr. Luke examines the patient from above, the sides, and the back to assess the pattern/distribution of hair loss. A visible scalp at the vertex indicates a 50% reduction in normal hair density. “I’m looking at the hairline, their part width, and the length of their hair,” she said. “I also look at the eyebrows and eyelashes, because these can be involved in alopecia areata, frontal fibrosing alopecia, or congenital hair shaft disorders.”
On closeup examination, she looks for scarring versus non-scarring types of hair loss, and for the presence or absence of follicular ostia. “I also look at hair changes,” she said. “Is the texture of their hair different? Are there signs of breakage or fragility? It’s been noted in studies that breakage can be an early sign of central centrifugal cicatricial alopecia.” (For more tips on examining tightly coiled hair among patients with hair loss in race discordant patient-physician interactions, she recommended a 2021 article in JAMA Dermatology)..
Trichoscopy allows for magnified observation of the hair shafts, hair follicle openings, perifollicular dermis, and blood vessels. Normal trichoscopy findings in skin of color reveal a perifollicular pigment network (honeycomb pattern) and pinpoint white dots that are regularly distributed between follicular units.
Common abnormalities seen on trichoscopy include central centrifugal cicatricial alopecia (CCCA), with one or two hairs emerging together, surrounded by a gray halo; lichen planopilaris/frontal fibrosing alopecia, characterized by hair with peripilar casts and absence of vellus hairs; discoid lupus erythematosus, characterized by keratotic plugs; and traction, characterized by hair casts.
Once a diagnosis is confirmed, Dr. Luke provides other general advice for optimal skin health, including a balanced (whole food) diet to ensure adequate nutrition. “I tend to find a lot of nutrient deficiencies that contribute to and compound their condition,” she said. Other recommendations include avoiding excess tension on the hair, such as hair styles with tight ponytails, buns, braids, and weaves; avoiding or limiting chemical treatments with hair color, relaxers, and permanents; and avoiding or limiting excessive heat styling with blow dryers, flat irons, and curling irons.
Photoprotection misconceptions
At the meeting, Dr. Luke also discussed three misconceptions of photoprotection in skin of color, drawn from an article on the topic published in 2021.
- Myth No. 1: Endogenous melanin provides complete photoprotection for Fitzpatrick skin types IV-V. Many people with skin of color may believe sunscreen is not needed given the melanin already present in their skin, but research has shown that the epidermis of dark skin has an intrinsic sun protection factor (SPF) of 13.4, compared with an SPF of 3.3 in light skin. “That may not provide them with full protection,” Dr. Luke said. “Many dermatologists are not counseling their skin of color patients about photoprotection.”
- Myth No. 2: Individuals with skin of color have negligible risks associated with skin cancer. Skin cancer prevalence in patients with skin of color is significantly lower compared with those with light skin. However, people with skin of color tend to be diagnosed with cancers at a more advanced stage, and cancers associated with a worse prognosis and poorer survival rate. An analysis of ethnic differences among patients with cutaneous melanoma that drew from the Surveillance, Epidemiology, and End Results (SEER) program found that Hispanic individuals (odds ratio [OR], 3.6), Black individuals (OR, 4.2), and Asian individuals (OR, 2.4), were more likely than were White individuals to have stage IV melanoma at the time of presentation. “For melanoma in skin of color, UV radiation does not seem to be a major risk factor, as melanoma tends to occur on palmar/plantar and subungual skin as well as mucous membranes,” Dr. Luke said. “For squamous cell carcinoma in skin of color, lesions are more likely to be present in areas that are not sun exposed. The risk factors for this tend to be chronic wounds, nonhealing ulcers, and people with chronic inflammatory conditions.” For basal cell carcinoma, she added, UV radiation seems to play more of a role and tends to occur in sun-exposed areas in patients with lighter Fitzpatrick skin types. Patients are more likely to present with pigmented BCCs.
- Myth No. 3: Broad-spectrum sunscreens provide photoprotection against all wavelengths of light that cause skin damage. To be labeled “broad-spectrum” the Food and Drug Administration requires that sunscreens have a critical wavelength of 370 nm or below, but Dr. Luke noted that broad-spectrum sunscreens do not necessarily protect against visible light (VL) and UV-A1. Research has demonstrated that VL exposure induces both transient and long-term cutaneous pigmentation in a dose-dependent manner.
“This induces free radicals and reactive oxygen species, leading to a cascade of events including the induction of pro-inflammatory cytokines, matrix metalloproteinases, and melanogenesis,” she said. “More intense and persistent VL-induced pigmentation occurs in subjects with darker skin. However, there is increasing evidence that antioxidants may help to mitigate these negative effects, so we are starting to see the addition of antioxidants into sunscreens.”
Dr. Luke recommends a broad-spectrum sunscreen with an SPF of 30 or higher for skin of color patients. Tinted sunscreens, which contain iron oxide pigments, are recommended for the prevention and treatment of pigmentary disorders in patients with Fitzpatrick skin types IV-VI skin. “What about adding antioxidants to prevent formation of reactive oxygen species?” she asked. “It’s possible but we don’t have a lot of research yet. You also want a sunscreen that’s aesthetically elegant, meaning it doesn’t leave a white cast.”
Dr. Luke reported having no relevant disclosures.
PORTLAND, ORE. – , but sometimes their doctors fall short.
“Many times, you may not have race concordant visits with patients of color,” Janiene Luke, MD, said at the annual meeting of the Pacific Dermatologic Association. She referred to a survey of 200 Black women aged 21-83 years, which found that 28% had visited a physician to discuss hair or scalp issues. Of those, 68% felt like their dermatologists did not understand African American hair.
“I recommend trying the best you can to familiarize yourself with various common cultural hair styling methods and practices in patients of color. It’s important to understand what your patients are engaging in and the types of styles they’re using,” said Dr. Luke, associate professor of dermatology at Loma Linda (Calif.) University. “Approach all patients with cultural humility. We know from studies that patients value dermatologists who take time to listen to their concerns, involve them in the decision-making process, and educate them about their conditions,” she added.
National efforts to educate clinicians on treating skin of color have emerged in recent years, including textbooks, CME courses at dermatology conferences, and the American Academy of Dermatology’s Skin of Color Curriculum, which consists of 15-minute modules that can be viewed online.
At the meeting, Dr. Luke, shared her approach to assessing hair and scalp disorders in skin of color. She begins by taking a thorough history, “because not all things that are associated with hair styling will be the reason why your patient comes in,” she said. “Patients of color can have telogen effluvium and seborrheic dermatitis just like anyone else. I ask about the hair styling practices they use. I also ask how often they wash their hair, because sometimes our recommendations for treatment are not realistic based on their current routine.”
Next, she examines the scalp with her hands – which sometimes surprises patients. “I’ve had so many patients come in and say, ‘the dermatologist never touched my scalp,’ or ‘they never even looked at my hair,’ ” said Dr. Luke, who directs the university’s dermatology residency program. She asks patients to remove any hair extensions or weaves prior to the office visit and to remove wigs prior to the exam itself. The lab tests she customarily orders include CBC, TSH, iron, total iron binding capacity, ferritin, vitamin D, and zinc. If there are signs of androgen excess, she may check testosterone, sex hormone binding globulin, and dehydroepiandrosterone sulfate (DHEA-S). She routinely incorporates a dermoscopy-directed biopsy into the evaluation.
Dr. Luke examines the patient from above, the sides, and the back to assess the pattern/distribution of hair loss. A visible scalp at the vertex indicates a 50% reduction in normal hair density. “I’m looking at the hairline, their part width, and the length of their hair,” she said. “I also look at the eyebrows and eyelashes, because these can be involved in alopecia areata, frontal fibrosing alopecia, or congenital hair shaft disorders.”
On closeup examination, she looks for scarring versus non-scarring types of hair loss, and for the presence or absence of follicular ostia. “I also look at hair changes,” she said. “Is the texture of their hair different? Are there signs of breakage or fragility? It’s been noted in studies that breakage can be an early sign of central centrifugal cicatricial alopecia.” (For more tips on examining tightly coiled hair among patients with hair loss in race discordant patient-physician interactions, she recommended a 2021 article in JAMA Dermatology)..
Trichoscopy allows for magnified observation of the hair shafts, hair follicle openings, perifollicular dermis, and blood vessels. Normal trichoscopy findings in skin of color reveal a perifollicular pigment network (honeycomb pattern) and pinpoint white dots that are regularly distributed between follicular units.
Common abnormalities seen on trichoscopy include central centrifugal cicatricial alopecia (CCCA), with one or two hairs emerging together, surrounded by a gray halo; lichen planopilaris/frontal fibrosing alopecia, characterized by hair with peripilar casts and absence of vellus hairs; discoid lupus erythematosus, characterized by keratotic plugs; and traction, characterized by hair casts.
Once a diagnosis is confirmed, Dr. Luke provides other general advice for optimal skin health, including a balanced (whole food) diet to ensure adequate nutrition. “I tend to find a lot of nutrient deficiencies that contribute to and compound their condition,” she said. Other recommendations include avoiding excess tension on the hair, such as hair styles with tight ponytails, buns, braids, and weaves; avoiding or limiting chemical treatments with hair color, relaxers, and permanents; and avoiding or limiting excessive heat styling with blow dryers, flat irons, and curling irons.
Photoprotection misconceptions
At the meeting, Dr. Luke also discussed three misconceptions of photoprotection in skin of color, drawn from an article on the topic published in 2021.
- Myth No. 1: Endogenous melanin provides complete photoprotection for Fitzpatrick skin types IV-V. Many people with skin of color may believe sunscreen is not needed given the melanin already present in their skin, but research has shown that the epidermis of dark skin has an intrinsic sun protection factor (SPF) of 13.4, compared with an SPF of 3.3 in light skin. “That may not provide them with full protection,” Dr. Luke said. “Many dermatologists are not counseling their skin of color patients about photoprotection.”
- Myth No. 2: Individuals with skin of color have negligible risks associated with skin cancer. Skin cancer prevalence in patients with skin of color is significantly lower compared with those with light skin. However, people with skin of color tend to be diagnosed with cancers at a more advanced stage, and cancers associated with a worse prognosis and poorer survival rate. An analysis of ethnic differences among patients with cutaneous melanoma that drew from the Surveillance, Epidemiology, and End Results (SEER) program found that Hispanic individuals (odds ratio [OR], 3.6), Black individuals (OR, 4.2), and Asian individuals (OR, 2.4), were more likely than were White individuals to have stage IV melanoma at the time of presentation. “For melanoma in skin of color, UV radiation does not seem to be a major risk factor, as melanoma tends to occur on palmar/plantar and subungual skin as well as mucous membranes,” Dr. Luke said. “For squamous cell carcinoma in skin of color, lesions are more likely to be present in areas that are not sun exposed. The risk factors for this tend to be chronic wounds, nonhealing ulcers, and people with chronic inflammatory conditions.” For basal cell carcinoma, she added, UV radiation seems to play more of a role and tends to occur in sun-exposed areas in patients with lighter Fitzpatrick skin types. Patients are more likely to present with pigmented BCCs.
- Myth No. 3: Broad-spectrum sunscreens provide photoprotection against all wavelengths of light that cause skin damage. To be labeled “broad-spectrum” the Food and Drug Administration requires that sunscreens have a critical wavelength of 370 nm or below, but Dr. Luke noted that broad-spectrum sunscreens do not necessarily protect against visible light (VL) and UV-A1. Research has demonstrated that VL exposure induces both transient and long-term cutaneous pigmentation in a dose-dependent manner.
“This induces free radicals and reactive oxygen species, leading to a cascade of events including the induction of pro-inflammatory cytokines, matrix metalloproteinases, and melanogenesis,” she said. “More intense and persistent VL-induced pigmentation occurs in subjects with darker skin. However, there is increasing evidence that antioxidants may help to mitigate these negative effects, so we are starting to see the addition of antioxidants into sunscreens.”
Dr. Luke recommends a broad-spectrum sunscreen with an SPF of 30 or higher for skin of color patients. Tinted sunscreens, which contain iron oxide pigments, are recommended for the prevention and treatment of pigmentary disorders in patients with Fitzpatrick skin types IV-VI skin. “What about adding antioxidants to prevent formation of reactive oxygen species?” she asked. “It’s possible but we don’t have a lot of research yet. You also want a sunscreen that’s aesthetically elegant, meaning it doesn’t leave a white cast.”
Dr. Luke reported having no relevant disclosures.
AT PDA 2022
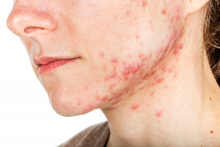
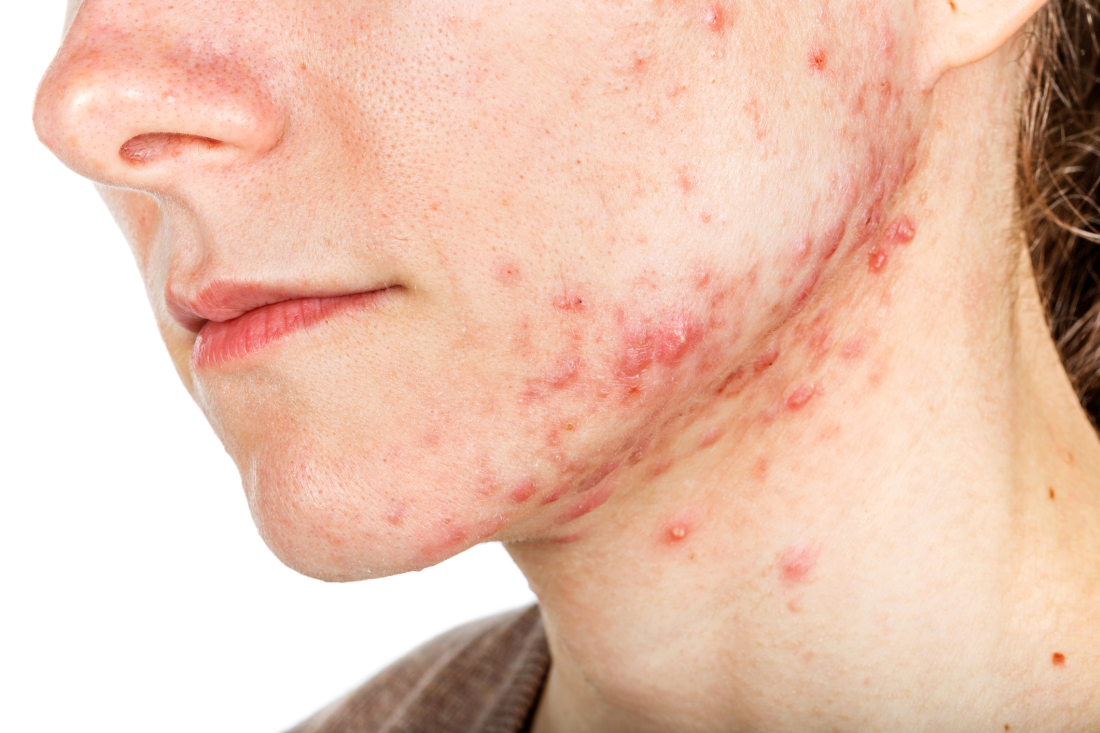
Hormonal therapy a safe, long term option for older women with recalcitrant acne
PORTLAND, ORE. – During her dermatology residency training at the University of California, Irvine, Medical Center, Jenny Murase, MD, remembers hearing a colleague say that her most angry patients of the day were adult women with recalcitrant acne who present to the clinic with questions like, “My skin has been clear my whole life! What’s going on?”
Such . In fact, 82% fail multiple courses of systemic antibiotics and 32% relapse after using isotretinoin, Dr. Murase, director of medical dermatology consultative services and patch testing at the Palo Alto Foundation Medical Group, said at the annual meeting of the Pacific Dermatologic Association.
In her clinical experience, hormonal therapy is a safe long-term option for recalcitrant acne in postmenarcheal females over the age of 14. “Although oral antibiotics are going to be superior to hormonal therapy in the first month or two, when you get to about six months, they have equivalent efficacy,” she said.
Telltale signs of acne associated with androgen excess include the development of nodulocystic papules along the jawline and small comedones over the forehead. Female patients with acne may request that labs be ordered to check their hormone levels, but that often is not necessary, according to Dr. Murase, who is also associate clinical professor of dermatology at the University of California, San Francisco. “There aren’t strict guidelines to indicate when you should perform hormonal testing, but warning signs that warrant further evaluation include hirsutism, androgenetic alopecia, virilization, infertility, oligomenorrhea or amenorrhea, and sudden onset of severe acne. The most common situation that warrants hormonal testing is polycystic ovary syndrome (PCOS).”
When there is a strong suspicion for hyperandrogenism, essential labs include free and total testosterone. Free testosterone is commonly elevated in patients with PCOS and total testosterone levels over 200 ng/dL is suggestive of an ovarian tumor. Other essential labs include 17-hyydroxyprogesterone (values greater than 200 ng/dL indicate congenital adrenal hyperplasia), and dehydroepiandrosterone sulfate (DHEA-S); levels over 8,000 mcg/dL indicate an adrenal tumor, while levels in the 4,000-8,000 mcg/dL range indicate congenital adrenal hyperplasia.
Helpful lab tests to consider include the ratio of luteinizing hormone to follicle-stimulating hormone; a 3:1 ratio or greater is suggestive for PCOS. “Ordering a prolactin level can also help, especially if patients are describing issues with headaches, which could indicate a pituitary tumor,” Dr. Murase added. Measuring sex hormone binding globulin (SHBG) levels can also be helpful. “If a patient has been on oral contraceptives for a long time, it increases their SHBG,” which, in older women, she said, “is inversely related to the development of type 2 diabetes.”
All labs for hyperandrogenism should be performed early in the morning on day 3 of the patient’s menstrual cycle. “If patients are on some kind of hormonal therapy, they need to be off of it for at least 6 weeks in order for you get a relevant test,” she said. Other relevant labs to consider include fasting glucose and lipids, cortisol, and thyroid-stimulating hormone.
Oral contraceptives
Estrogen contained in oral contraceptives (OCs) provides the most benefit to acne patients. “It reduces sebum production, decreases free testosterone and DHEA-S by stimulating SHBG synthesis in the liver, inhibits 5-alpha-reductase, which decreases peripheral testosterone conversion, and it decreases the production of ovarian and adrenal androgens,” Dr. Murase explained. “On average, you can get about 40%-70% reduction of lesion count, which is pretty good.”
Progestins with low androgenetic activity are the most helpful for acne, including norgestimate, desogestrel, and drospirenone. FDA-approved OC options include Ortho Tri-Cyclen, EstroStep, Yaz, and Beyaz. None has data showing superior efficacy.
No Pap smear or pelvic exam is required when prescribing OCs, but the risk of clotting should be discussed with patients. According to Dr. Murase, the risk of deep vein thrombosis (DVT) at baseline is about 1 per 10,000 woman-years, while the risk of DVT after 1 year on an OC is 3.4 per 10,000 years.
“This is a very mild increased risk that we’re talking about, but it is relevant in smokers, in those with hypertension, and in those who are diabetic,” she said. As for the risk of cancer associated with the use of OCs, a large collaborative study found a relative risk of 1.24 for developing breast cancer (not dose or duration related), but a risk reduction for endometrial, colorectal, and ovarian cancer.
The most common side effects associated with OCs are unscheduled bleeding, nausea, breast tenderness, and possible weight gain. Concomitant antibiotics can be used, with the exception of CYP3A4 inducers, such as rifampin. “That’s the main antibiotic we have to worry about that could affect the efficacy of the birth control pill,” she said. “It accounts for about three-quarters of pregnancies on antibiotics.”
Tetracyclines do not appear to increase the rate of birth defects with incidental first-trimester exposure, and data are reassuring but “tetracycline should be stopped within the first trimester as soon as the patient discovers she is pregnant,” Dr. Murase said.
Contraindications for OCs include being pregnant or breastfeeding; history of stroke, venous thromboembolism, or MI; history of smoking and being over age 35; uncontrolled hypertension; migraines with focal symptoms/aura; current or past breast cancer; hypercholesterolemia; diabetes with end-organ damage or having diabetes over age 35; liver issues such as a tumor, viral hepatitis, or cirrhosis; and a history of major surgery with prolonged immobilization.
Spironolactone
Another treatment option is spironolactone, a potassium-sparing diuretic that blocks aldosterone at a dose of 25 mg/day. At doses of 50-100 mg/day, it blocks androgen. “It can be used in combination with an oral contraceptive, with the rates of efficacy reported to range between 33% and 85%,” Dr. Murase said.
Spironolactone can also reduce hirsutism, improve androgenetic alopecia, and lower blood pressure by about 5 mm Hg systolic and 2.5 mm Hg diastolic. Dr. Murase usually checks blood pressure in patients, and “only if they’re really low I’ll talk about the potential for postural hypotension and the fact that you can get a little bit dizzy when going from a position of lying down to standing up.” Potassium levels should be checked at baseline and 4 weeks in patients older than age 46, in those with cardiac and/or renal disease, or in those on concomitant drospirenone or a third-generation progestin.
Spironolactone is classified as a pregnancy category D drug that could compromise the genital development of a male fetus. “So the onus is on us as providers to have the conversation with our patient,” she said. “If you’re putting a patient on spironolactone and they are of child-bearing age, you need to make sure that you’ve had the conversation with them about the fact that they should not get pregnant while on the medicine.”
Spironolactone also has a boxed warning citing the development of benign tumors in animal studies. That warning is based on studies in rats at doses of 10-150 mg/kg per day, “which is an extremely high dose and would never be given in humans,” said Dr. Murase, who has coauthored CME content regarding the safety of dermatologic medications in pregnancy and lactation.
In humans, there has been no evidence of the development of benign tumors associated with spironolactone therapy, and “there has been a decreased risk of prostate cancer and no association with its use and the development of breast, ovarian, bladder, kidney, gastric, or esophageal cancer,” she said.
Dr. Murase noted that during pregnancy, first-line oral antibiotics include amoxicillin for acne rosacea and cefadroxil for acne vulgaris. Macrolides are a second-line choice because of an increase in atrial/ventricular septal defects and pyloric stenosis that have been reported with first-trimester exposure.
“Erythromycin is the preferred choice over azithromycin and clarithromycin because it has the most data, [but] erythromycin estolate has been associated with increased AST levels in the second trimester,” she said. “It occurs in about 10% of cases and is reversible. Erythromycin base and erythromycin ethylsuccinate do not have this risk, and those are preferable.”
Dr. Murase disclosed that she has been a paid speaker of unbranded medical content for Regeneron and UCB. She is also a member of the advisory board for Leo Pharma, Eli Lilly, UCB, and Genzyme/Sanofi.
PORTLAND, ORE. – During her dermatology residency training at the University of California, Irvine, Medical Center, Jenny Murase, MD, remembers hearing a colleague say that her most angry patients of the day were adult women with recalcitrant acne who present to the clinic with questions like, “My skin has been clear my whole life! What’s going on?”
Such . In fact, 82% fail multiple courses of systemic antibiotics and 32% relapse after using isotretinoin, Dr. Murase, director of medical dermatology consultative services and patch testing at the Palo Alto Foundation Medical Group, said at the annual meeting of the Pacific Dermatologic Association.
In her clinical experience, hormonal therapy is a safe long-term option for recalcitrant acne in postmenarcheal females over the age of 14. “Although oral antibiotics are going to be superior to hormonal therapy in the first month or two, when you get to about six months, they have equivalent efficacy,” she said.
Telltale signs of acne associated with androgen excess include the development of nodulocystic papules along the jawline and small comedones over the forehead. Female patients with acne may request that labs be ordered to check their hormone levels, but that often is not necessary, according to Dr. Murase, who is also associate clinical professor of dermatology at the University of California, San Francisco. “There aren’t strict guidelines to indicate when you should perform hormonal testing, but warning signs that warrant further evaluation include hirsutism, androgenetic alopecia, virilization, infertility, oligomenorrhea or amenorrhea, and sudden onset of severe acne. The most common situation that warrants hormonal testing is polycystic ovary syndrome (PCOS).”
When there is a strong suspicion for hyperandrogenism, essential labs include free and total testosterone. Free testosterone is commonly elevated in patients with PCOS and total testosterone levels over 200 ng/dL is suggestive of an ovarian tumor. Other essential labs include 17-hyydroxyprogesterone (values greater than 200 ng/dL indicate congenital adrenal hyperplasia), and dehydroepiandrosterone sulfate (DHEA-S); levels over 8,000 mcg/dL indicate an adrenal tumor, while levels in the 4,000-8,000 mcg/dL range indicate congenital adrenal hyperplasia.
Helpful lab tests to consider include the ratio of luteinizing hormone to follicle-stimulating hormone; a 3:1 ratio or greater is suggestive for PCOS. “Ordering a prolactin level can also help, especially if patients are describing issues with headaches, which could indicate a pituitary tumor,” Dr. Murase added. Measuring sex hormone binding globulin (SHBG) levels can also be helpful. “If a patient has been on oral contraceptives for a long time, it increases their SHBG,” which, in older women, she said, “is inversely related to the development of type 2 diabetes.”
All labs for hyperandrogenism should be performed early in the morning on day 3 of the patient’s menstrual cycle. “If patients are on some kind of hormonal therapy, they need to be off of it for at least 6 weeks in order for you get a relevant test,” she said. Other relevant labs to consider include fasting glucose and lipids, cortisol, and thyroid-stimulating hormone.
Oral contraceptives
Estrogen contained in oral contraceptives (OCs) provides the most benefit to acne patients. “It reduces sebum production, decreases free testosterone and DHEA-S by stimulating SHBG synthesis in the liver, inhibits 5-alpha-reductase, which decreases peripheral testosterone conversion, and it decreases the production of ovarian and adrenal androgens,” Dr. Murase explained. “On average, you can get about 40%-70% reduction of lesion count, which is pretty good.”
Progestins with low androgenetic activity are the most helpful for acne, including norgestimate, desogestrel, and drospirenone. FDA-approved OC options include Ortho Tri-Cyclen, EstroStep, Yaz, and Beyaz. None has data showing superior efficacy.
No Pap smear or pelvic exam is required when prescribing OCs, but the risk of clotting should be discussed with patients. According to Dr. Murase, the risk of deep vein thrombosis (DVT) at baseline is about 1 per 10,000 woman-years, while the risk of DVT after 1 year on an OC is 3.4 per 10,000 years.
“This is a very mild increased risk that we’re talking about, but it is relevant in smokers, in those with hypertension, and in those who are diabetic,” she said. As for the risk of cancer associated with the use of OCs, a large collaborative study found a relative risk of 1.24 for developing breast cancer (not dose or duration related), but a risk reduction for endometrial, colorectal, and ovarian cancer.
The most common side effects associated with OCs are unscheduled bleeding, nausea, breast tenderness, and possible weight gain. Concomitant antibiotics can be used, with the exception of CYP3A4 inducers, such as rifampin. “That’s the main antibiotic we have to worry about that could affect the efficacy of the birth control pill,” she said. “It accounts for about three-quarters of pregnancies on antibiotics.”
Tetracyclines do not appear to increase the rate of birth defects with incidental first-trimester exposure, and data are reassuring but “tetracycline should be stopped within the first trimester as soon as the patient discovers she is pregnant,” Dr. Murase said.
Contraindications for OCs include being pregnant or breastfeeding; history of stroke, venous thromboembolism, or MI; history of smoking and being over age 35; uncontrolled hypertension; migraines with focal symptoms/aura; current or past breast cancer; hypercholesterolemia; diabetes with end-organ damage or having diabetes over age 35; liver issues such as a tumor, viral hepatitis, or cirrhosis; and a history of major surgery with prolonged immobilization.
Spironolactone
Another treatment option is spironolactone, a potassium-sparing diuretic that blocks aldosterone at a dose of 25 mg/day. At doses of 50-100 mg/day, it blocks androgen. “It can be used in combination with an oral contraceptive, with the rates of efficacy reported to range between 33% and 85%,” Dr. Murase said.
Spironolactone can also reduce hirsutism, improve androgenetic alopecia, and lower blood pressure by about 5 mm Hg systolic and 2.5 mm Hg diastolic. Dr. Murase usually checks blood pressure in patients, and “only if they’re really low I’ll talk about the potential for postural hypotension and the fact that you can get a little bit dizzy when going from a position of lying down to standing up.” Potassium levels should be checked at baseline and 4 weeks in patients older than age 46, in those with cardiac and/or renal disease, or in those on concomitant drospirenone or a third-generation progestin.
Spironolactone is classified as a pregnancy category D drug that could compromise the genital development of a male fetus. “So the onus is on us as providers to have the conversation with our patient,” she said. “If you’re putting a patient on spironolactone and they are of child-bearing age, you need to make sure that you’ve had the conversation with them about the fact that they should not get pregnant while on the medicine.”
Spironolactone also has a boxed warning citing the development of benign tumors in animal studies. That warning is based on studies in rats at doses of 10-150 mg/kg per day, “which is an extremely high dose and would never be given in humans,” said Dr. Murase, who has coauthored CME content regarding the safety of dermatologic medications in pregnancy and lactation.
In humans, there has been no evidence of the development of benign tumors associated with spironolactone therapy, and “there has been a decreased risk of prostate cancer and no association with its use and the development of breast, ovarian, bladder, kidney, gastric, or esophageal cancer,” she said.
Dr. Murase noted that during pregnancy, first-line oral antibiotics include amoxicillin for acne rosacea and cefadroxil for acne vulgaris. Macrolides are a second-line choice because of an increase in atrial/ventricular septal defects and pyloric stenosis that have been reported with first-trimester exposure.
“Erythromycin is the preferred choice over azithromycin and clarithromycin because it has the most data, [but] erythromycin estolate has been associated with increased AST levels in the second trimester,” she said. “It occurs in about 10% of cases and is reversible. Erythromycin base and erythromycin ethylsuccinate do not have this risk, and those are preferable.”
Dr. Murase disclosed that she has been a paid speaker of unbranded medical content for Regeneron and UCB. She is also a member of the advisory board for Leo Pharma, Eli Lilly, UCB, and Genzyme/Sanofi.
PORTLAND, ORE. – During her dermatology residency training at the University of California, Irvine, Medical Center, Jenny Murase, MD, remembers hearing a colleague say that her most angry patients of the day were adult women with recalcitrant acne who present to the clinic with questions like, “My skin has been clear my whole life! What’s going on?”
Such . In fact, 82% fail multiple courses of systemic antibiotics and 32% relapse after using isotretinoin, Dr. Murase, director of medical dermatology consultative services and patch testing at the Palo Alto Foundation Medical Group, said at the annual meeting of the Pacific Dermatologic Association.
In her clinical experience, hormonal therapy is a safe long-term option for recalcitrant acne in postmenarcheal females over the age of 14. “Although oral antibiotics are going to be superior to hormonal therapy in the first month or two, when you get to about six months, they have equivalent efficacy,” she said.
Telltale signs of acne associated with androgen excess include the development of nodulocystic papules along the jawline and small comedones over the forehead. Female patients with acne may request that labs be ordered to check their hormone levels, but that often is not necessary, according to Dr. Murase, who is also associate clinical professor of dermatology at the University of California, San Francisco. “There aren’t strict guidelines to indicate when you should perform hormonal testing, but warning signs that warrant further evaluation include hirsutism, androgenetic alopecia, virilization, infertility, oligomenorrhea or amenorrhea, and sudden onset of severe acne. The most common situation that warrants hormonal testing is polycystic ovary syndrome (PCOS).”
When there is a strong suspicion for hyperandrogenism, essential labs include free and total testosterone. Free testosterone is commonly elevated in patients with PCOS and total testosterone levels over 200 ng/dL is suggestive of an ovarian tumor. Other essential labs include 17-hyydroxyprogesterone (values greater than 200 ng/dL indicate congenital adrenal hyperplasia), and dehydroepiandrosterone sulfate (DHEA-S); levels over 8,000 mcg/dL indicate an adrenal tumor, while levels in the 4,000-8,000 mcg/dL range indicate congenital adrenal hyperplasia.
Helpful lab tests to consider include the ratio of luteinizing hormone to follicle-stimulating hormone; a 3:1 ratio or greater is suggestive for PCOS. “Ordering a prolactin level can also help, especially if patients are describing issues with headaches, which could indicate a pituitary tumor,” Dr. Murase added. Measuring sex hormone binding globulin (SHBG) levels can also be helpful. “If a patient has been on oral contraceptives for a long time, it increases their SHBG,” which, in older women, she said, “is inversely related to the development of type 2 diabetes.”
All labs for hyperandrogenism should be performed early in the morning on day 3 of the patient’s menstrual cycle. “If patients are on some kind of hormonal therapy, they need to be off of it for at least 6 weeks in order for you get a relevant test,” she said. Other relevant labs to consider include fasting glucose and lipids, cortisol, and thyroid-stimulating hormone.
Oral contraceptives
Estrogen contained in oral contraceptives (OCs) provides the most benefit to acne patients. “It reduces sebum production, decreases free testosterone and DHEA-S by stimulating SHBG synthesis in the liver, inhibits 5-alpha-reductase, which decreases peripheral testosterone conversion, and it decreases the production of ovarian and adrenal androgens,” Dr. Murase explained. “On average, you can get about 40%-70% reduction of lesion count, which is pretty good.”
Progestins with low androgenetic activity are the most helpful for acne, including norgestimate, desogestrel, and drospirenone. FDA-approved OC options include Ortho Tri-Cyclen, EstroStep, Yaz, and Beyaz. None has data showing superior efficacy.
No Pap smear or pelvic exam is required when prescribing OCs, but the risk of clotting should be discussed with patients. According to Dr. Murase, the risk of deep vein thrombosis (DVT) at baseline is about 1 per 10,000 woman-years, while the risk of DVT after 1 year on an OC is 3.4 per 10,000 years.
“This is a very mild increased risk that we’re talking about, but it is relevant in smokers, in those with hypertension, and in those who are diabetic,” she said. As for the risk of cancer associated with the use of OCs, a large collaborative study found a relative risk of 1.24 for developing breast cancer (not dose or duration related), but a risk reduction for endometrial, colorectal, and ovarian cancer.
The most common side effects associated with OCs are unscheduled bleeding, nausea, breast tenderness, and possible weight gain. Concomitant antibiotics can be used, with the exception of CYP3A4 inducers, such as rifampin. “That’s the main antibiotic we have to worry about that could affect the efficacy of the birth control pill,” she said. “It accounts for about three-quarters of pregnancies on antibiotics.”
Tetracyclines do not appear to increase the rate of birth defects with incidental first-trimester exposure, and data are reassuring but “tetracycline should be stopped within the first trimester as soon as the patient discovers she is pregnant,” Dr. Murase said.
Contraindications for OCs include being pregnant or breastfeeding; history of stroke, venous thromboembolism, or MI; history of smoking and being over age 35; uncontrolled hypertension; migraines with focal symptoms/aura; current or past breast cancer; hypercholesterolemia; diabetes with end-organ damage or having diabetes over age 35; liver issues such as a tumor, viral hepatitis, or cirrhosis; and a history of major surgery with prolonged immobilization.
Spironolactone
Another treatment option is spironolactone, a potassium-sparing diuretic that blocks aldosterone at a dose of 25 mg/day. At doses of 50-100 mg/day, it blocks androgen. “It can be used in combination with an oral contraceptive, with the rates of efficacy reported to range between 33% and 85%,” Dr. Murase said.
Spironolactone can also reduce hirsutism, improve androgenetic alopecia, and lower blood pressure by about 5 mm Hg systolic and 2.5 mm Hg diastolic. Dr. Murase usually checks blood pressure in patients, and “only if they’re really low I’ll talk about the potential for postural hypotension and the fact that you can get a little bit dizzy when going from a position of lying down to standing up.” Potassium levels should be checked at baseline and 4 weeks in patients older than age 46, in those with cardiac and/or renal disease, or in those on concomitant drospirenone or a third-generation progestin.
Spironolactone is classified as a pregnancy category D drug that could compromise the genital development of a male fetus. “So the onus is on us as providers to have the conversation with our patient,” she said. “If you’re putting a patient on spironolactone and they are of child-bearing age, you need to make sure that you’ve had the conversation with them about the fact that they should not get pregnant while on the medicine.”
Spironolactone also has a boxed warning citing the development of benign tumors in animal studies. That warning is based on studies in rats at doses of 10-150 mg/kg per day, “which is an extremely high dose and would never be given in humans,” said Dr. Murase, who has coauthored CME content regarding the safety of dermatologic medications in pregnancy and lactation.
In humans, there has been no evidence of the development of benign tumors associated with spironolactone therapy, and “there has been a decreased risk of prostate cancer and no association with its use and the development of breast, ovarian, bladder, kidney, gastric, or esophageal cancer,” she said.
Dr. Murase noted that during pregnancy, first-line oral antibiotics include amoxicillin for acne rosacea and cefadroxil for acne vulgaris. Macrolides are a second-line choice because of an increase in atrial/ventricular septal defects and pyloric stenosis that have been reported with first-trimester exposure.
“Erythromycin is the preferred choice over azithromycin and clarithromycin because it has the most data, [but] erythromycin estolate has been associated with increased AST levels in the second trimester,” she said. “It occurs in about 10% of cases and is reversible. Erythromycin base and erythromycin ethylsuccinate do not have this risk, and those are preferable.”
Dr. Murase disclosed that she has been a paid speaker of unbranded medical content for Regeneron and UCB. She is also a member of the advisory board for Leo Pharma, Eli Lilly, UCB, and Genzyme/Sanofi.
AT PDA 2022
Myeloid Neoplasm Masquerading as Hypereosinophilia and Sweet Syndrome
Introduction
Hypereosinophilia can be seen in many medical conditions, including myeloproliferative disorders, and can lead to serious complications if untreated. Sweet syndrome is a rare and painful cutaneous inflammatory condition that has been linked to underlying malignancies.
Case Presentation
A 72-year-old male presented with 6-month history of painful maculopapular rash, night sweats, fever, and weight loss. He was treated with antibiotics and steroids with no improvement. A skin biopsy demonstrated neutrophilic dermatosis consistent with sweet syndrome. Laboratory studies a showed hemoglobin 7.1g/dl, WBC 12.9x103/uL, 30% eosinophils, absolute eosinophil count 3x109/L, and normal platelets. Infectious and immunological work up was negative. CT scan revealed splenomegaly. Bone marrow biopsy showed 100% hypercellularity, trilineage atypia, eosinophils 43% (normal, 1-5%) and 3-4% blasts positive for CD34 and CD117. FISH studies detected loss of PDGFRB signal, cytogenetics revealed a complex karyotype. He was diagnosed with a high-risk (based on IPSS-R) MDS/MPN cross-over with peripheral eosinophilia and is planned to undergo HSCT.
Discussion
Hematologic malignancies are associated with several paraneoplastic syndromes including sweet syndrome, also known as acute febrile neutrophilic dermatosis. The literature describes sweet syndrome occurring mostly with AML but can also be seen with other malignancies like MDS and solid tumor. The distinction between sweet syndrome and infectious or immune-mediated rash can be challenging as it requires histopathologic evaluation and is usually mistreated. Hypereosinophilia is defined as persistent eosinophil count of at least 1.5x109/L. It can be idiopathic or associated with allergic, rheumatologic, infectious, or neoplastic conditions. Clonal hypereosinophilia is most frequently associated with chronic myeloid neoplasms such as myeloproliferative neoplasm (MPN) or overlapping MDS/MPN, and more less frequently with AML. Hypereosinophilia related to hematological malignancies has been linked to gene rearrangements involving PDGFRA, PDGFRB, FGFR1, and JAK2. Patients with documented rearrangements or mutations in PDGFRB are treated with imatinib, which is a potent kinase inhibitor. However, patients with high-risk MDS/MPN with associated eosinophilia are typically treated as MDS and should undergo allogenic HSCT if eligible.
Conclusions
Both hypereosinophlia and sweet syndrome have been linked to myeloid neoplasms. Early recognition of either phenomenon as a paraneoplastic syndrome is important for early diagnosis and treatment.
Introduction
Hypereosinophilia can be seen in many medical conditions, including myeloproliferative disorders, and can lead to serious complications if untreated. Sweet syndrome is a rare and painful cutaneous inflammatory condition that has been linked to underlying malignancies.
Case Presentation
A 72-year-old male presented with 6-month history of painful maculopapular rash, night sweats, fever, and weight loss. He was treated with antibiotics and steroids with no improvement. A skin biopsy demonstrated neutrophilic dermatosis consistent with sweet syndrome. Laboratory studies a showed hemoglobin 7.1g/dl, WBC 12.9x103/uL, 30% eosinophils, absolute eosinophil count 3x109/L, and normal platelets. Infectious and immunological work up was negative. CT scan revealed splenomegaly. Bone marrow biopsy showed 100% hypercellularity, trilineage atypia, eosinophils 43% (normal, 1-5%) and 3-4% blasts positive for CD34 and CD117. FISH studies detected loss of PDGFRB signal, cytogenetics revealed a complex karyotype. He was diagnosed with a high-risk (based on IPSS-R) MDS/MPN cross-over with peripheral eosinophilia and is planned to undergo HSCT.
Discussion
Hematologic malignancies are associated with several paraneoplastic syndromes including sweet syndrome, also known as acute febrile neutrophilic dermatosis. The literature describes sweet syndrome occurring mostly with AML but can also be seen with other malignancies like MDS and solid tumor. The distinction between sweet syndrome and infectious or immune-mediated rash can be challenging as it requires histopathologic evaluation and is usually mistreated. Hypereosinophilia is defined as persistent eosinophil count of at least 1.5x109/L. It can be idiopathic or associated with allergic, rheumatologic, infectious, or neoplastic conditions. Clonal hypereosinophilia is most frequently associated with chronic myeloid neoplasms such as myeloproliferative neoplasm (MPN) or overlapping MDS/MPN, and more less frequently with AML. Hypereosinophilia related to hematological malignancies has been linked to gene rearrangements involving PDGFRA, PDGFRB, FGFR1, and JAK2. Patients with documented rearrangements or mutations in PDGFRB are treated with imatinib, which is a potent kinase inhibitor. However, patients with high-risk MDS/MPN with associated eosinophilia are typically treated as MDS and should undergo allogenic HSCT if eligible.
Conclusions
Both hypereosinophlia and sweet syndrome have been linked to myeloid neoplasms. Early recognition of either phenomenon as a paraneoplastic syndrome is important for early diagnosis and treatment.
Introduction
Hypereosinophilia can be seen in many medical conditions, including myeloproliferative disorders, and can lead to serious complications if untreated. Sweet syndrome is a rare and painful cutaneous inflammatory condition that has been linked to underlying malignancies.
Case Presentation
A 72-year-old male presented with 6-month history of painful maculopapular rash, night sweats, fever, and weight loss. He was treated with antibiotics and steroids with no improvement. A skin biopsy demonstrated neutrophilic dermatosis consistent with sweet syndrome. Laboratory studies a showed hemoglobin 7.1g/dl, WBC 12.9x103/uL, 30% eosinophils, absolute eosinophil count 3x109/L, and normal platelets. Infectious and immunological work up was negative. CT scan revealed splenomegaly. Bone marrow biopsy showed 100% hypercellularity, trilineage atypia, eosinophils 43% (normal, 1-5%) and 3-4% blasts positive for CD34 and CD117. FISH studies detected loss of PDGFRB signal, cytogenetics revealed a complex karyotype. He was diagnosed with a high-risk (based on IPSS-R) MDS/MPN cross-over with peripheral eosinophilia and is planned to undergo HSCT.
Discussion
Hematologic malignancies are associated with several paraneoplastic syndromes including sweet syndrome, also known as acute febrile neutrophilic dermatosis. The literature describes sweet syndrome occurring mostly with AML but can also be seen with other malignancies like MDS and solid tumor. The distinction between sweet syndrome and infectious or immune-mediated rash can be challenging as it requires histopathologic evaluation and is usually mistreated. Hypereosinophilia is defined as persistent eosinophil count of at least 1.5x109/L. It can be idiopathic or associated with allergic, rheumatologic, infectious, or neoplastic conditions. Clonal hypereosinophilia is most frequently associated with chronic myeloid neoplasms such as myeloproliferative neoplasm (MPN) or overlapping MDS/MPN, and more less frequently with AML. Hypereosinophilia related to hematological malignancies has been linked to gene rearrangements involving PDGFRA, PDGFRB, FGFR1, and JAK2. Patients with documented rearrangements or mutations in PDGFRB are treated with imatinib, which is a potent kinase inhibitor. However, patients with high-risk MDS/MPN with associated eosinophilia are typically treated as MDS and should undergo allogenic HSCT if eligible.
Conclusions
Both hypereosinophlia and sweet syndrome have been linked to myeloid neoplasms. Early recognition of either phenomenon as a paraneoplastic syndrome is important for early diagnosis and treatment.
Hydroquinone, found in skin-lightening agents worldwide, linked with increased skin cancer risk
an analysis of records from a large research database suggests.
In the study, hydroquinone use was associated with an approximately threefold increase for skin cancer risk, coauthor Brittany Miles, a fourth-year medical student at the University of Texas Medical Branch at Galveston’s John Sealy School of Medicine, told this news organization. “The magnitude of the risk was surprising. Increased risk should be disclosed to patients considering hydroquinone treatment.”
The results of the study were presented in a poster at the annual meeting of the Society for Investigative Dermatology.
Hydroquinone (multiple brand names), a tyrosinase inhibitor used worldwide for skin lightening because of its inhibition of melanin production, was once considered “generally safe and effective” by the Food and Drug Administration, the authors wrote.
The compound’s use in over-the-counter products in the United States has been restricted based on suspicion of carcinogenicity, but few human studies have been conducted. In April, the FDA issued warning letters to 12 companies that sold hydroquinone in concentrations not generally recognized as safe and effective, because of other concerns including rashes, facial swelling, and ochronosis (skin discoloration).
Ms. Miles and her coauthor, Michael Wilkerson, MD, professor and chair of the department of dermatology at UTMB, analyzed data from TriNetX, the medical research database of anonymized medical record information from 61 million patients in 57 large health care organizations, almost all of them in the United States.
The researchers created two cohorts of patients aged 15 years and older with no prior diagnosis of skin cancer: one group had been treated with hydroquinone (medication code 5509 in the TriNetX system), and the other had not been exposed to the drug. Using ICD-10 codes for melanoma, nonmelanoma skin cancer, and all skin cancers, they investigated which groups of people were likely to develop these cancers.
They found that hydroquinone exposure was linked with a significant increase in melanoma (relative risk, 3.0; 95% confidence interval, 1.704-5.281; P < .0001), nonmelanoma skin cancers (RR, 3.6; 95%; CI, 2.815-4.561; P < .0001), and all reported skin cancers combined (relative risk, 3.4; 95% CI, 2.731-4.268; P < .0001)
While “the source of the data and the number of patients in the study are significant strengths,” Ms. Miles said, “the inability to determine how long and how consistently the patients used hydroquinone is likely the biggest weakness.”
Skin lightening is big business and more research is needed
“The U.S. market for skin-lightening agents was approximately 330 million dollars in 2021, and 330,000 prescriptions containing hydroquinone were dispensed in 2019,” Ms. Miles said.
Valencia D. Thomas, MD, professor in the department of dermatology of the University of Texas MD Anderson Cancer Center, Houston, said in an email that over-the-counter skin-lightening products containing low-concentration hydroquinone are in widespread use and are commonly used in populations of color.
“Hydroquinone preparations in higher concentrations are unfortunately also available in the United States,” added Dr. Thomas, who was not involved in the study and referred to the FDA warning letter issued in April.
Only one hydroquinone-containing medication – Tri-Luma at 4% concentration, used to treat melasma – is currently FDA-approved, she said.
The data in the study do not show an increased risk for skin cancer with hydroquinone exposure, but do show “an increased risk of cancer in the TriNetX medication code 5509 hydroquinone exposure group, which does not prove causation,” Dr. Thomas commented.
“Because ‘hydroquinone exposure’ is not defined, it is unclear how TriNetX identified the hydroquinone exposure cohort,” she noted. “Does ‘exposure’ count prescriptions written and potentially not used, the use of hydroquinone products of high concentration not approved by the FDA, or the use of over-the-counter hydroquinone products?
“The strength of this study is its size,” Dr. Thomas acknowledged. “This study is a wonderful starting point to further investigate the ‘hydroquinone exposure’ cohort to determine if hydroquinone is a driver of cancer, or if hydroquinone is itself a confounder.”
These results highlight the need to examine the social determinants of health that may explain increased risk for cancer, including race, geography, and poverty, she added.
“Given the global consumption of hydroquinone, multinational collaboration investigating hydroquinone and cancer data will likely be needed to provide insight into this continuing question,” Dr. Thomas advised.
Christiane Querfeld, MD, PhD, associate professor of dermatology and dermatopathology at City of Hope in Duarte, Calif., agreed that the occurrence of skin cancer following use of hydroquinone is largely understudied.
“The findings have a huge impact on how we counsel and monitor future patients,” Dr. Querfeld, who also was not involved in the study, said in an email. “There may be a trade-off at the start of treatment: Get rid of melasma but develop a skin cancer or melanoma with potentially severe outcomes.
“It remains to be seen if there is a higher incidence of skin cancer following use of hydroquinone or other voluntary bleaching and depigmentation remedies in ethnic groups such as African American or Hispanic patient populations, who have historically been at low risk of developing skin cancer,” she added. “It also remains to be seen if increased risk is due to direct effects or to indirect effects on already-photodamaged skin.
“These data are critical, and I am sure this will open further investigations to study effects in more detail,” Dr. Querfeld said.
The study authors, Dr. Thomas, and Dr. Querfeld reported no relevant financial relationships. The study did not receive external funding.
A version of this article first appeared on Medscape.com.
an analysis of records from a large research database suggests.
In the study, hydroquinone use was associated with an approximately threefold increase for skin cancer risk, coauthor Brittany Miles, a fourth-year medical student at the University of Texas Medical Branch at Galveston’s John Sealy School of Medicine, told this news organization. “The magnitude of the risk was surprising. Increased risk should be disclosed to patients considering hydroquinone treatment.”
The results of the study were presented in a poster at the annual meeting of the Society for Investigative Dermatology.
Hydroquinone (multiple brand names), a tyrosinase inhibitor used worldwide for skin lightening because of its inhibition of melanin production, was once considered “generally safe and effective” by the Food and Drug Administration, the authors wrote.
The compound’s use in over-the-counter products in the United States has been restricted based on suspicion of carcinogenicity, but few human studies have been conducted. In April, the FDA issued warning letters to 12 companies that sold hydroquinone in concentrations not generally recognized as safe and effective, because of other concerns including rashes, facial swelling, and ochronosis (skin discoloration).
Ms. Miles and her coauthor, Michael Wilkerson, MD, professor and chair of the department of dermatology at UTMB, analyzed data from TriNetX, the medical research database of anonymized medical record information from 61 million patients in 57 large health care organizations, almost all of them in the United States.
The researchers created two cohorts of patients aged 15 years and older with no prior diagnosis of skin cancer: one group had been treated with hydroquinone (medication code 5509 in the TriNetX system), and the other had not been exposed to the drug. Using ICD-10 codes for melanoma, nonmelanoma skin cancer, and all skin cancers, they investigated which groups of people were likely to develop these cancers.
They found that hydroquinone exposure was linked with a significant increase in melanoma (relative risk, 3.0; 95% confidence interval, 1.704-5.281; P < .0001), nonmelanoma skin cancers (RR, 3.6; 95%; CI, 2.815-4.561; P < .0001), and all reported skin cancers combined (relative risk, 3.4; 95% CI, 2.731-4.268; P < .0001)
While “the source of the data and the number of patients in the study are significant strengths,” Ms. Miles said, “the inability to determine how long and how consistently the patients used hydroquinone is likely the biggest weakness.”
Skin lightening is big business and more research is needed
“The U.S. market for skin-lightening agents was approximately 330 million dollars in 2021, and 330,000 prescriptions containing hydroquinone were dispensed in 2019,” Ms. Miles said.
Valencia D. Thomas, MD, professor in the department of dermatology of the University of Texas MD Anderson Cancer Center, Houston, said in an email that over-the-counter skin-lightening products containing low-concentration hydroquinone are in widespread use and are commonly used in populations of color.
“Hydroquinone preparations in higher concentrations are unfortunately also available in the United States,” added Dr. Thomas, who was not involved in the study and referred to the FDA warning letter issued in April.
Only one hydroquinone-containing medication – Tri-Luma at 4% concentration, used to treat melasma – is currently FDA-approved, she said.
The data in the study do not show an increased risk for skin cancer with hydroquinone exposure, but do show “an increased risk of cancer in the TriNetX medication code 5509 hydroquinone exposure group, which does not prove causation,” Dr. Thomas commented.
“Because ‘hydroquinone exposure’ is not defined, it is unclear how TriNetX identified the hydroquinone exposure cohort,” she noted. “Does ‘exposure’ count prescriptions written and potentially not used, the use of hydroquinone products of high concentration not approved by the FDA, or the use of over-the-counter hydroquinone products?
“The strength of this study is its size,” Dr. Thomas acknowledged. “This study is a wonderful starting point to further investigate the ‘hydroquinone exposure’ cohort to determine if hydroquinone is a driver of cancer, or if hydroquinone is itself a confounder.”
These results highlight the need to examine the social determinants of health that may explain increased risk for cancer, including race, geography, and poverty, she added.
“Given the global consumption of hydroquinone, multinational collaboration investigating hydroquinone and cancer data will likely be needed to provide insight into this continuing question,” Dr. Thomas advised.
Christiane Querfeld, MD, PhD, associate professor of dermatology and dermatopathology at City of Hope in Duarte, Calif., agreed that the occurrence of skin cancer following use of hydroquinone is largely understudied.
“The findings have a huge impact on how we counsel and monitor future patients,” Dr. Querfeld, who also was not involved in the study, said in an email. “There may be a trade-off at the start of treatment: Get rid of melasma but develop a skin cancer or melanoma with potentially severe outcomes.
“It remains to be seen if there is a higher incidence of skin cancer following use of hydroquinone or other voluntary bleaching and depigmentation remedies in ethnic groups such as African American or Hispanic patient populations, who have historically been at low risk of developing skin cancer,” she added. “It also remains to be seen if increased risk is due to direct effects or to indirect effects on already-photodamaged skin.
“These data are critical, and I am sure this will open further investigations to study effects in more detail,” Dr. Querfeld said.
The study authors, Dr. Thomas, and Dr. Querfeld reported no relevant financial relationships. The study did not receive external funding.
A version of this article first appeared on Medscape.com.
an analysis of records from a large research database suggests.
In the study, hydroquinone use was associated with an approximately threefold increase for skin cancer risk, coauthor Brittany Miles, a fourth-year medical student at the University of Texas Medical Branch at Galveston’s John Sealy School of Medicine, told this news organization. “The magnitude of the risk was surprising. Increased risk should be disclosed to patients considering hydroquinone treatment.”
The results of the study were presented in a poster at the annual meeting of the Society for Investigative Dermatology.
Hydroquinone (multiple brand names), a tyrosinase inhibitor used worldwide for skin lightening because of its inhibition of melanin production, was once considered “generally safe and effective” by the Food and Drug Administration, the authors wrote.
The compound’s use in over-the-counter products in the United States has been restricted based on suspicion of carcinogenicity, but few human studies have been conducted. In April, the FDA issued warning letters to 12 companies that sold hydroquinone in concentrations not generally recognized as safe and effective, because of other concerns including rashes, facial swelling, and ochronosis (skin discoloration).
Ms. Miles and her coauthor, Michael Wilkerson, MD, professor and chair of the department of dermatology at UTMB, analyzed data from TriNetX, the medical research database of anonymized medical record information from 61 million patients in 57 large health care organizations, almost all of them in the United States.
The researchers created two cohorts of patients aged 15 years and older with no prior diagnosis of skin cancer: one group had been treated with hydroquinone (medication code 5509 in the TriNetX system), and the other had not been exposed to the drug. Using ICD-10 codes for melanoma, nonmelanoma skin cancer, and all skin cancers, they investigated which groups of people were likely to develop these cancers.
They found that hydroquinone exposure was linked with a significant increase in melanoma (relative risk, 3.0; 95% confidence interval, 1.704-5.281; P < .0001), nonmelanoma skin cancers (RR, 3.6; 95%; CI, 2.815-4.561; P < .0001), and all reported skin cancers combined (relative risk, 3.4; 95% CI, 2.731-4.268; P < .0001)
While “the source of the data and the number of patients in the study are significant strengths,” Ms. Miles said, “the inability to determine how long and how consistently the patients used hydroquinone is likely the biggest weakness.”
Skin lightening is big business and more research is needed
“The U.S. market for skin-lightening agents was approximately 330 million dollars in 2021, and 330,000 prescriptions containing hydroquinone were dispensed in 2019,” Ms. Miles said.
Valencia D. Thomas, MD, professor in the department of dermatology of the University of Texas MD Anderson Cancer Center, Houston, said in an email that over-the-counter skin-lightening products containing low-concentration hydroquinone are in widespread use and are commonly used in populations of color.
“Hydroquinone preparations in higher concentrations are unfortunately also available in the United States,” added Dr. Thomas, who was not involved in the study and referred to the FDA warning letter issued in April.
Only one hydroquinone-containing medication – Tri-Luma at 4% concentration, used to treat melasma – is currently FDA-approved, she said.
The data in the study do not show an increased risk for skin cancer with hydroquinone exposure, but do show “an increased risk of cancer in the TriNetX medication code 5509 hydroquinone exposure group, which does not prove causation,” Dr. Thomas commented.
“Because ‘hydroquinone exposure’ is not defined, it is unclear how TriNetX identified the hydroquinone exposure cohort,” she noted. “Does ‘exposure’ count prescriptions written and potentially not used, the use of hydroquinone products of high concentration not approved by the FDA, or the use of over-the-counter hydroquinone products?
“The strength of this study is its size,” Dr. Thomas acknowledged. “This study is a wonderful starting point to further investigate the ‘hydroquinone exposure’ cohort to determine if hydroquinone is a driver of cancer, or if hydroquinone is itself a confounder.”
These results highlight the need to examine the social determinants of health that may explain increased risk for cancer, including race, geography, and poverty, she added.
“Given the global consumption of hydroquinone, multinational collaboration investigating hydroquinone and cancer data will likely be needed to provide insight into this continuing question,” Dr. Thomas advised.
Christiane Querfeld, MD, PhD, associate professor of dermatology and dermatopathology at City of Hope in Duarte, Calif., agreed that the occurrence of skin cancer following use of hydroquinone is largely understudied.
“The findings have a huge impact on how we counsel and monitor future patients,” Dr. Querfeld, who also was not involved in the study, said in an email. “There may be a trade-off at the start of treatment: Get rid of melasma but develop a skin cancer or melanoma with potentially severe outcomes.
“It remains to be seen if there is a higher incidence of skin cancer following use of hydroquinone or other voluntary bleaching and depigmentation remedies in ethnic groups such as African American or Hispanic patient populations, who have historically been at low risk of developing skin cancer,” she added. “It also remains to be seen if increased risk is due to direct effects or to indirect effects on already-photodamaged skin.
“These data are critical, and I am sure this will open further investigations to study effects in more detail,” Dr. Querfeld said.
The study authors, Dr. Thomas, and Dr. Querfeld reported no relevant financial relationships. The study did not receive external funding.
A version of this article first appeared on Medscape.com.
FROM SID 2022
Leg rash
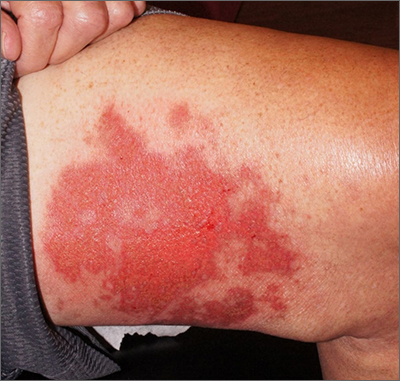
Punch biopsies for standard pathology and direct immunofluorescence were performed and ruled out vesiculobullous disease. Further conversation with the patient revealed that this was a phototoxic drug eruption that resulted from a medication mix-up. The patient had intended to treat an eczema flare with a topical steroid but had inadvertently applied 5-fluorouracil (5-FU), which he had left over from a previous bout of actinic keratosis. While selective to precancerous cells with rapid DNA replication, 5-FU can trigger a significant photodermatitis when applied to heavily sun-exposed skin.
Phototoxic skin reactions can be an adverse result of multiple systemic and topical therapies. Common systemic examples include amiodarone, chlorpromazine, doxycycline, hydrochlorothiazide, isotretinoin, nalidixic acid, naproxen, piroxicam, tetracycline, thioridazine, vemurafenib, and voriconazole.1 Topical examples include retinoids, levulinic acid, and 5-FU. Treatment requires that the patient stop the offending medication and use photoprotection. The patient followed this protocol and his erosions resolved over the course of a few weeks.
This case demonstrates that topical therapies, like systemic medications, can have chemical names that are confusing to patients. Further complicating matters can be the practice of folding metal tubes of cream over their life of use, thus obscuring the label.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Blakely KM, Drucker AM, Rosen CF. Drug-induced photosensitivity-an update: culprit drugs, prevention, and management. Drug Saf. 2019;42:827-847. doi: 10.1007/s40264-019-00806-5

Punch biopsies for standard pathology and direct immunofluorescence were performed and ruled out vesiculobullous disease. Further conversation with the patient revealed that this was a phototoxic drug eruption that resulted from a medication mix-up. The patient had intended to treat an eczema flare with a topical steroid but had inadvertently applied 5-fluorouracil (5-FU), which he had left over from a previous bout of actinic keratosis. While selective to precancerous cells with rapid DNA replication, 5-FU can trigger a significant photodermatitis when applied to heavily sun-exposed skin.
Phototoxic skin reactions can be an adverse result of multiple systemic and topical therapies. Common systemic examples include amiodarone, chlorpromazine, doxycycline, hydrochlorothiazide, isotretinoin, nalidixic acid, naproxen, piroxicam, tetracycline, thioridazine, vemurafenib, and voriconazole.1 Topical examples include retinoids, levulinic acid, and 5-FU. Treatment requires that the patient stop the offending medication and use photoprotection. The patient followed this protocol and his erosions resolved over the course of a few weeks.
This case demonstrates that topical therapies, like systemic medications, can have chemical names that are confusing to patients. Further complicating matters can be the practice of folding metal tubes of cream over their life of use, thus obscuring the label.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

Punch biopsies for standard pathology and direct immunofluorescence were performed and ruled out vesiculobullous disease. Further conversation with the patient revealed that this was a phototoxic drug eruption that resulted from a medication mix-up. The patient had intended to treat an eczema flare with a topical steroid but had inadvertently applied 5-fluorouracil (5-FU), which he had left over from a previous bout of actinic keratosis. While selective to precancerous cells with rapid DNA replication, 5-FU can trigger a significant photodermatitis when applied to heavily sun-exposed skin.
Phototoxic skin reactions can be an adverse result of multiple systemic and topical therapies. Common systemic examples include amiodarone, chlorpromazine, doxycycline, hydrochlorothiazide, isotretinoin, nalidixic acid, naproxen, piroxicam, tetracycline, thioridazine, vemurafenib, and voriconazole.1 Topical examples include retinoids, levulinic acid, and 5-FU. Treatment requires that the patient stop the offending medication and use photoprotection. The patient followed this protocol and his erosions resolved over the course of a few weeks.
This case demonstrates that topical therapies, like systemic medications, can have chemical names that are confusing to patients. Further complicating matters can be the practice of folding metal tubes of cream over their life of use, thus obscuring the label.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Blakely KM, Drucker AM, Rosen CF. Drug-induced photosensitivity-an update: culprit drugs, prevention, and management. Drug Saf. 2019;42:827-847. doi: 10.1007/s40264-019-00806-5
1. Blakely KM, Drucker AM, Rosen CF. Drug-induced photosensitivity-an update: culprit drugs, prevention, and management. Drug Saf. 2019;42:827-847. doi: 10.1007/s40264-019-00806-5
Chlorophyll water can trigger pseudoporphyria, expert warns
PORTLAND, ORE. – If a child presents with pseudoporphyria – a bullous photodermatosis with the clinical and histological features of porphyria cutanea tarda (PCT) but with normal porphyrins – chlorophyll water could be the culprit.
Commercially available, green pigment–infused chlorophyll water is marketed with claims that it supports cancer prevention and digestive health, facilitates weight loss, and improves skin complexion. “It also absorbs light, so lo and behold, if your patient is photosensitive, they might get pseudoporphyria,” Robert Sidbury, MD, MPH, chief of the division of dermatology at Seattle Children’s Hospital, said at the annual meeting of the Pacific Dermatologic Association.
This was one of the clinical pearls he shared during his presentation.
Dr. Sidbury added that the risk of photosensitivity increases in children who are taking other medications such as doxycycline, methotrexate, or even naproxen. At least two cases of pseudoporphyria following self-medication with chlorophyll have been described in the dermatology literature.
Is it SSSS or SJS?
Another clinical pearl that Dr. Sidbury shared at the meeting related to staphylococcal scalded skin syndrome (SSSS), which causes reddening and blistering of the skin that makes it appear scalded or burned. To rule out Stevens-Johnson Syndrome (SJS) in a child who presents with such skin manifestations, he routinely performs the unscientific lollipop test, which he learned from Bernard A. “Buddy” Cohen, MD, professor of dermatology and pediatrics at Johns Hopkins University, Baltimore.
“If they eat it, it’s Staph scalded skin,” said Dr. Sidbury, who is also professor of pediatrics at the University of Washington, Seattle. “If they don’t, it’s likely SJS. It’s not the most specific test, but it’s easy to do, because there’s no mucous membrane involvement in Staph scalded skin.”
In a poster presented during the 2022 annual meeting of the Society for Pediatric Dermatology, Sarah Cipriano, MD, MPH, and colleagues at the University of Utah, Salt Lake City, retrospectively study 85 patients aged younger than 18 years diagnosed with SSSS between Jan. 1, 2010, and Aug. 21, 2021. They found that ancillary blood cultures and CSF cultures did not improve diagnostic precision in SSSS patients.
“They don’t add anything unless there’s an indication beyond the Staph scalded skin,” said Dr. Sidbury, who was not involved in the study. “The researchers also found that clindamycin does not improve outcomes in these patients, so avoid using it.” Instead, a first-generation cephalosporin is indicated, and an alternate diagnosis should be considered if the patient does not improve within 48 hours.
Dr. Sidbury disclosed that he has conducted research for Regeneron, Galderma, and UCB. He is also an adviser for Leo Pharmaceuticals and a speaker for Biersdorf.
PORTLAND, ORE. – If a child presents with pseudoporphyria – a bullous photodermatosis with the clinical and histological features of porphyria cutanea tarda (PCT) but with normal porphyrins – chlorophyll water could be the culprit.
Commercially available, green pigment–infused chlorophyll water is marketed with claims that it supports cancer prevention and digestive health, facilitates weight loss, and improves skin complexion. “It also absorbs light, so lo and behold, if your patient is photosensitive, they might get pseudoporphyria,” Robert Sidbury, MD, MPH, chief of the division of dermatology at Seattle Children’s Hospital, said at the annual meeting of the Pacific Dermatologic Association.
This was one of the clinical pearls he shared during his presentation.
Dr. Sidbury added that the risk of photosensitivity increases in children who are taking other medications such as doxycycline, methotrexate, or even naproxen. At least two cases of pseudoporphyria following self-medication with chlorophyll have been described in the dermatology literature.
Is it SSSS or SJS?
Another clinical pearl that Dr. Sidbury shared at the meeting related to staphylococcal scalded skin syndrome (SSSS), which causes reddening and blistering of the skin that makes it appear scalded or burned. To rule out Stevens-Johnson Syndrome (SJS) in a child who presents with such skin manifestations, he routinely performs the unscientific lollipop test, which he learned from Bernard A. “Buddy” Cohen, MD, professor of dermatology and pediatrics at Johns Hopkins University, Baltimore.
“If they eat it, it’s Staph scalded skin,” said Dr. Sidbury, who is also professor of pediatrics at the University of Washington, Seattle. “If they don’t, it’s likely SJS. It’s not the most specific test, but it’s easy to do, because there’s no mucous membrane involvement in Staph scalded skin.”
In a poster presented during the 2022 annual meeting of the Society for Pediatric Dermatology, Sarah Cipriano, MD, MPH, and colleagues at the University of Utah, Salt Lake City, retrospectively study 85 patients aged younger than 18 years diagnosed with SSSS between Jan. 1, 2010, and Aug. 21, 2021. They found that ancillary blood cultures and CSF cultures did not improve diagnostic precision in SSSS patients.
“They don’t add anything unless there’s an indication beyond the Staph scalded skin,” said Dr. Sidbury, who was not involved in the study. “The researchers also found that clindamycin does not improve outcomes in these patients, so avoid using it.” Instead, a first-generation cephalosporin is indicated, and an alternate diagnosis should be considered if the patient does not improve within 48 hours.
Dr. Sidbury disclosed that he has conducted research for Regeneron, Galderma, and UCB. He is also an adviser for Leo Pharmaceuticals and a speaker for Biersdorf.
PORTLAND, ORE. – If a child presents with pseudoporphyria – a bullous photodermatosis with the clinical and histological features of porphyria cutanea tarda (PCT) but with normal porphyrins – chlorophyll water could be the culprit.
Commercially available, green pigment–infused chlorophyll water is marketed with claims that it supports cancer prevention and digestive health, facilitates weight loss, and improves skin complexion. “It also absorbs light, so lo and behold, if your patient is photosensitive, they might get pseudoporphyria,” Robert Sidbury, MD, MPH, chief of the division of dermatology at Seattle Children’s Hospital, said at the annual meeting of the Pacific Dermatologic Association.
This was one of the clinical pearls he shared during his presentation.
Dr. Sidbury added that the risk of photosensitivity increases in children who are taking other medications such as doxycycline, methotrexate, or even naproxen. At least two cases of pseudoporphyria following self-medication with chlorophyll have been described in the dermatology literature.
Is it SSSS or SJS?
Another clinical pearl that Dr. Sidbury shared at the meeting related to staphylococcal scalded skin syndrome (SSSS), which causes reddening and blistering of the skin that makes it appear scalded or burned. To rule out Stevens-Johnson Syndrome (SJS) in a child who presents with such skin manifestations, he routinely performs the unscientific lollipop test, which he learned from Bernard A. “Buddy” Cohen, MD, professor of dermatology and pediatrics at Johns Hopkins University, Baltimore.
“If they eat it, it’s Staph scalded skin,” said Dr. Sidbury, who is also professor of pediatrics at the University of Washington, Seattle. “If they don’t, it’s likely SJS. It’s not the most specific test, but it’s easy to do, because there’s no mucous membrane involvement in Staph scalded skin.”
In a poster presented during the 2022 annual meeting of the Society for Pediatric Dermatology, Sarah Cipriano, MD, MPH, and colleagues at the University of Utah, Salt Lake City, retrospectively study 85 patients aged younger than 18 years diagnosed with SSSS between Jan. 1, 2010, and Aug. 21, 2021. They found that ancillary blood cultures and CSF cultures did not improve diagnostic precision in SSSS patients.
“They don’t add anything unless there’s an indication beyond the Staph scalded skin,” said Dr. Sidbury, who was not involved in the study. “The researchers also found that clindamycin does not improve outcomes in these patients, so avoid using it.” Instead, a first-generation cephalosporin is indicated, and an alternate diagnosis should be considered if the patient does not improve within 48 hours.
Dr. Sidbury disclosed that he has conducted research for Regeneron, Galderma, and UCB. He is also an adviser for Leo Pharmaceuticals and a speaker for Biersdorf.
AT PDA 2022