User login
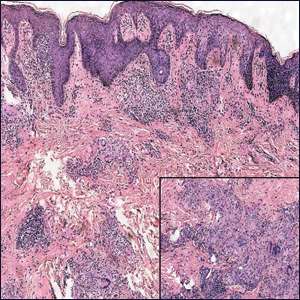
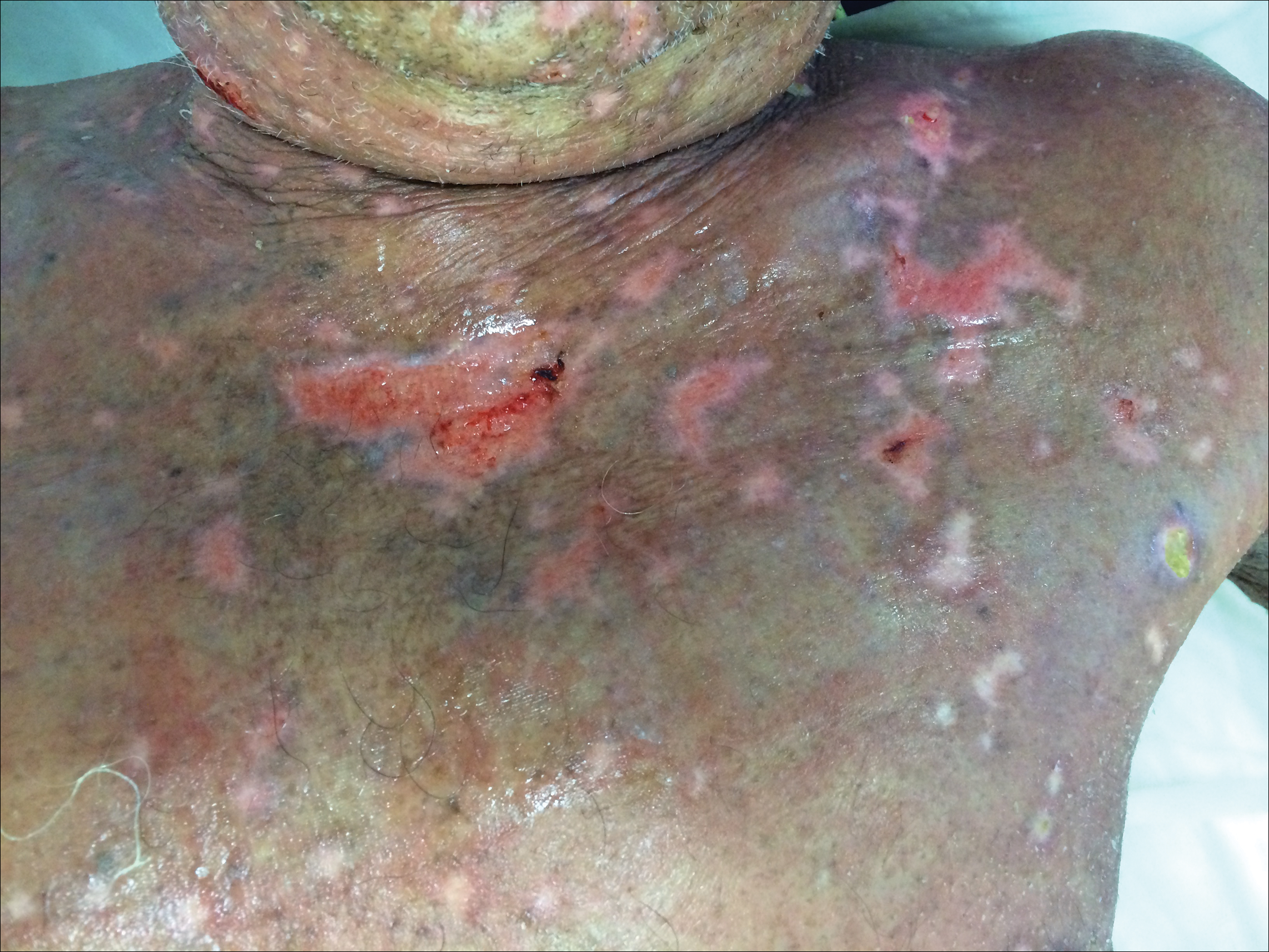
Perianal Condyloma Acuminatum-like Plaque
The Diagnosis: Metastatic Crohn Disease
Crohn disease (CD), a chronic inflammatory granulomatous disease of the gastrointestinal tract, has a wide spectrum of presentations.1 The condition may affect the vulva, perineum, or perianal skin by direct extension from the gastrointestinal tract or may appear as a separate and distinct cutaneous focus of disease referred to as metastatic Crohn disease (MCD).2
Cutaneous lesions of MCD include ulcers, fissures, sinus tracts, abscesses, and vegetative plaques, which typically extend in continuity with sites of intra-abdominal disease to the perineum, buttocks, or abdominal wall, as well as ostomy sites or incisional scars. Erythema nodosum and pyoderma gangrenosum are the most common nonspecific cutaneous manifestations. Other cutaneous lesions described in CD include polyarteritis nodosa, psoriasis, erythema multiforme, erythema elevatum diutinum, epidermolysis bullosa acquisita, acne fulminans, pyoderma faciale, neutrophilic lobular panniculitis, granulomatous vasculitis, and porokeratosis.3
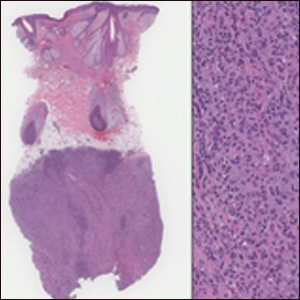
Perianal skin is the most common site of cutaneous involvement in individuals with CD. It is a marker of more severe disease and is associated with multiple surgical interventions and frequent relapses and has been reported in 22% of patients with CD.4 Most already had an existing diagnosis of gastrointestinal CD, which was active in one-third of individuals; however, 20% presented with disease at nongastrointestinal sites 2 months to 4 years prior to developing the gastrointestinal CD manifestations.5 Our patient presented with lesions on the perianal skin of 2 years' duration and a 6-month history of diarrhea. A colonoscopy demonstrated shallow ulcers involving the ileocecal portion of the gut, colon, and rectum. A biopsy from intestinal mucosal tissue showed acute and chronic inflammation with necrosis mixed with granulomatous inflammation, suggestive of CD.
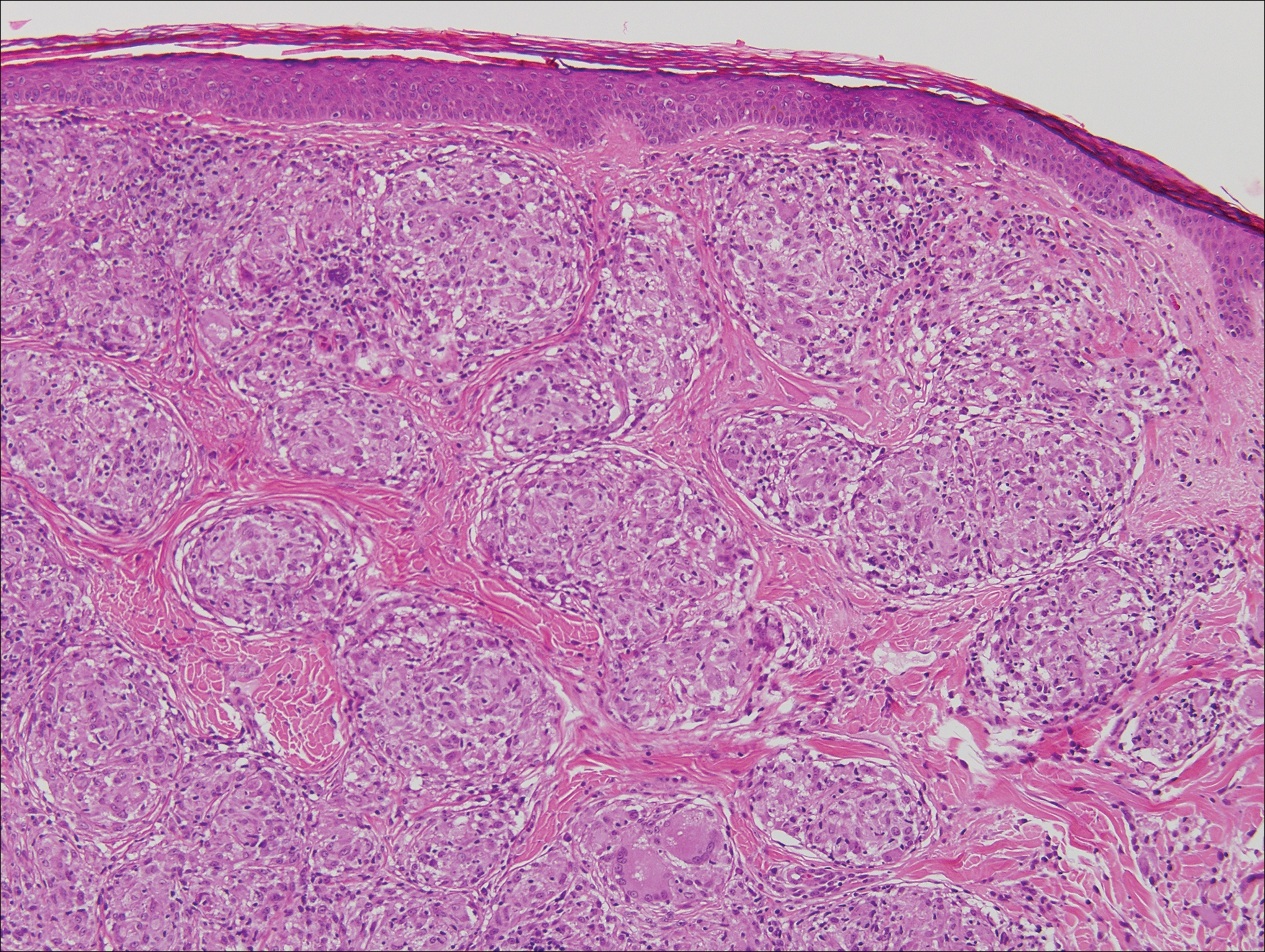
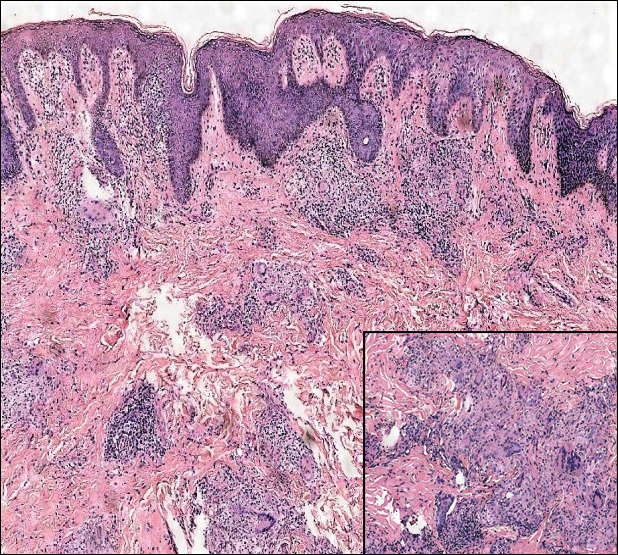
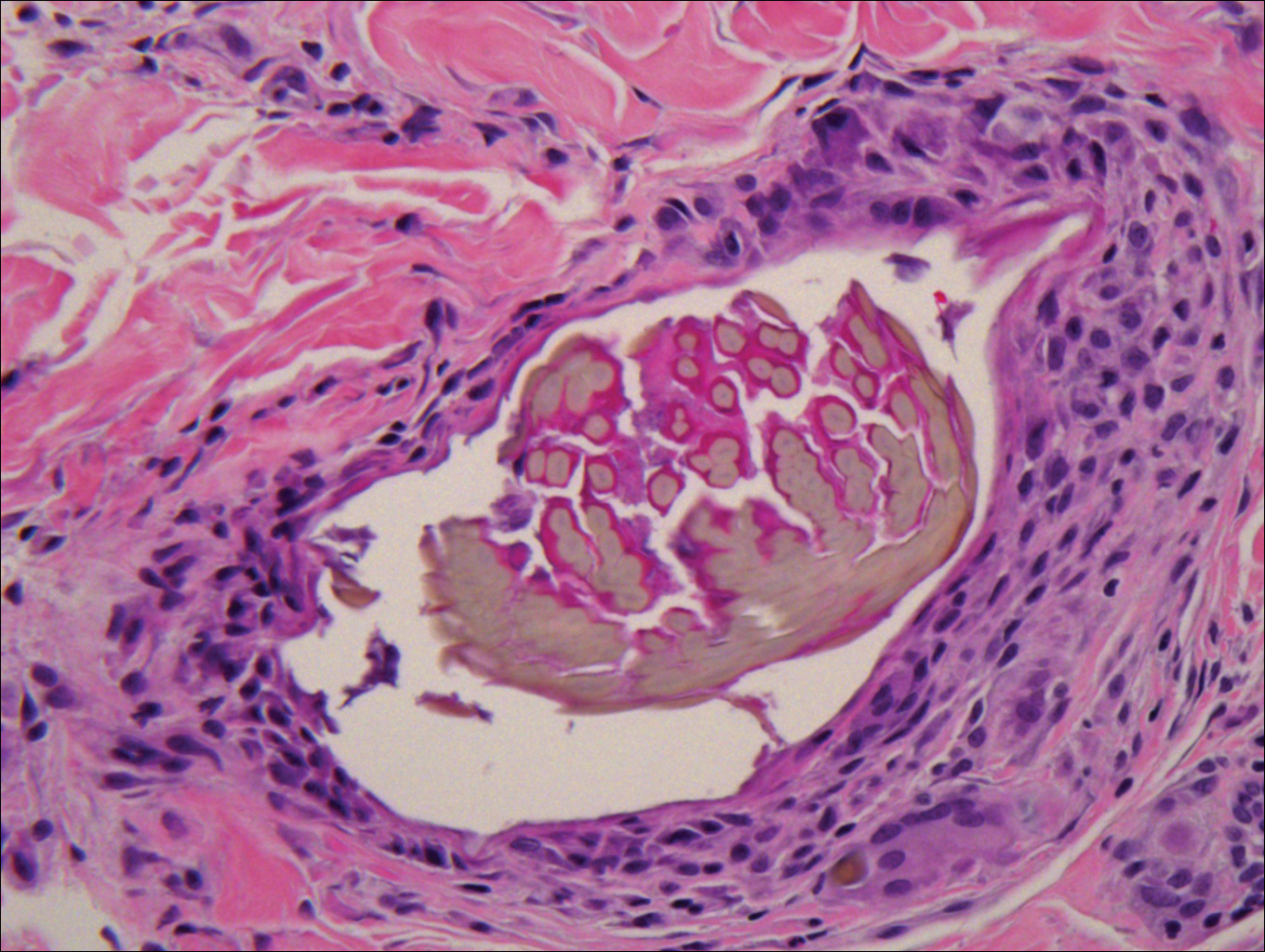
Microscopically, the dominant histologic features of MCD are similar to those of bowel lesions, including an inflammatory infiltrate commonly consisting of sterile noncaseating sarcoidal granulomas, foreign body and Langhans giant cells, epithelioid histiocytes, and plasma cells surrounded by numerous mononuclear cells within the dermis with occasional extension into the subcutis (quiz image). Less common features include collagen degeneration, an infiltrate rich in eosinophils, dermal edema, and mixed lichenoid and granulomatous dermatitis.6
Metastatic CD often is misdiagnosed. A detailed history and physical examination may help narrow the differential; however, biopsy is necessary to establish a diagnosis of MCD. The histologic differential diagnosis of sarcoidal granulomatous inflammation of genital skin includes sarcoidosis, rheumatoid arthritis, leprosy or other mycobacterial and parasitic infection, granulomatosis with polyangiitis (GPA), and granulomatous infiltrate associated with certain exogenous material (eg, silica, zirconium, beryllium, tattoo pigment).
Sarcoidosis is a multiorgan disease that most frequently affects the lungs, skin, and lymph nodes. Its etiopathogenesis has not been clearly elucidated.7 Cutaneous lesions are present in 20% to 35% of patients.8 Given the wide variability of clinical manifestations, cutaneous sarcoidosis is another one of the great imitators. Cutaneous lesions are classified as specific and nonspecific depending on the presence of noncaseating granulomas on histologic studies and include maculopapules, plaques, nodules, lupus pernio, scar infiltration, alopecia, ulcerative lesions, and hypopigmentation. The most common nonspecific lesion of cutaneous sarcoidosis is erythema nodosum. Other manifestations include calcifications, prurigo, erythema multiforme, nail clubbing, and Sweet syndrome.9
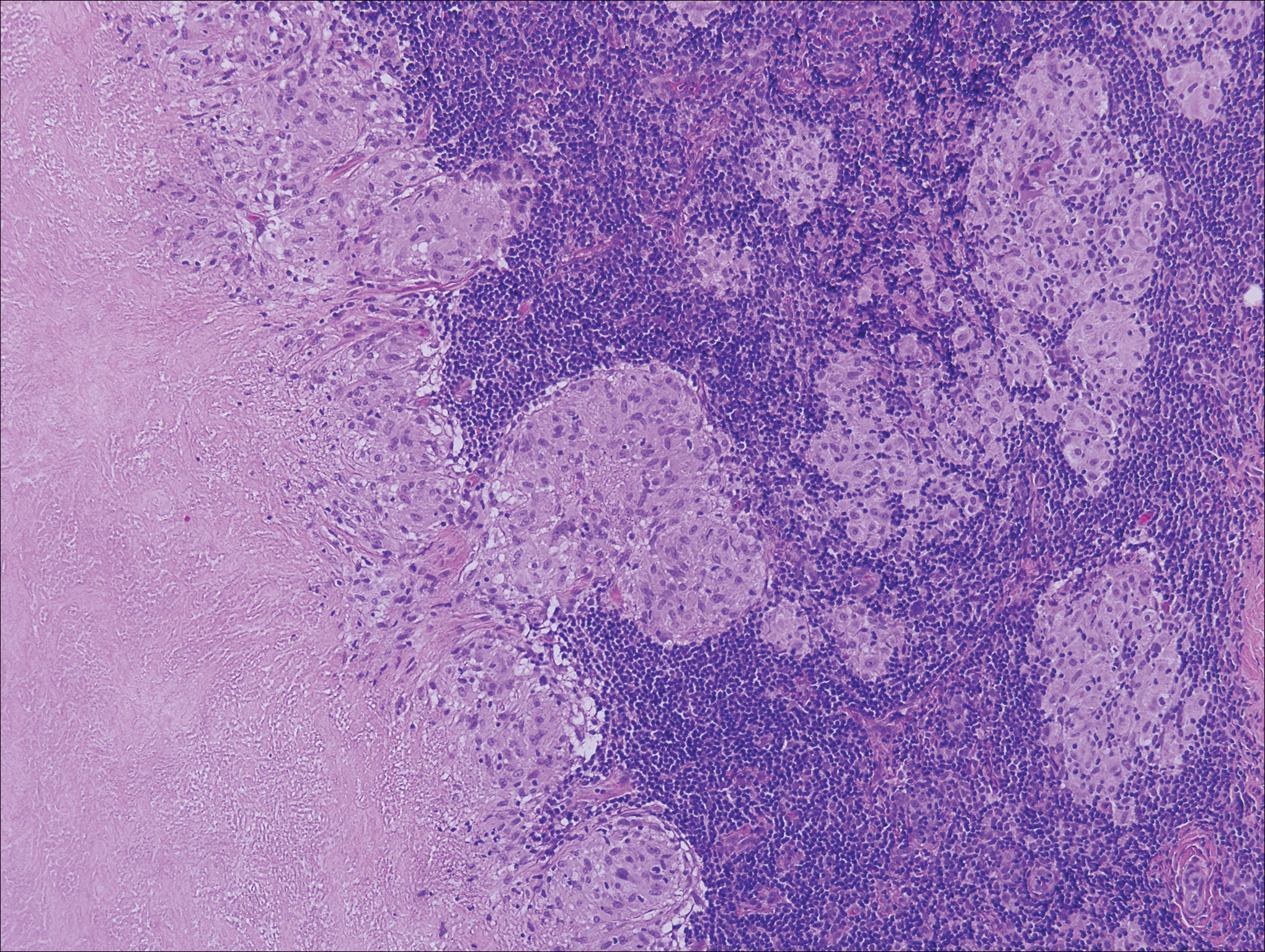
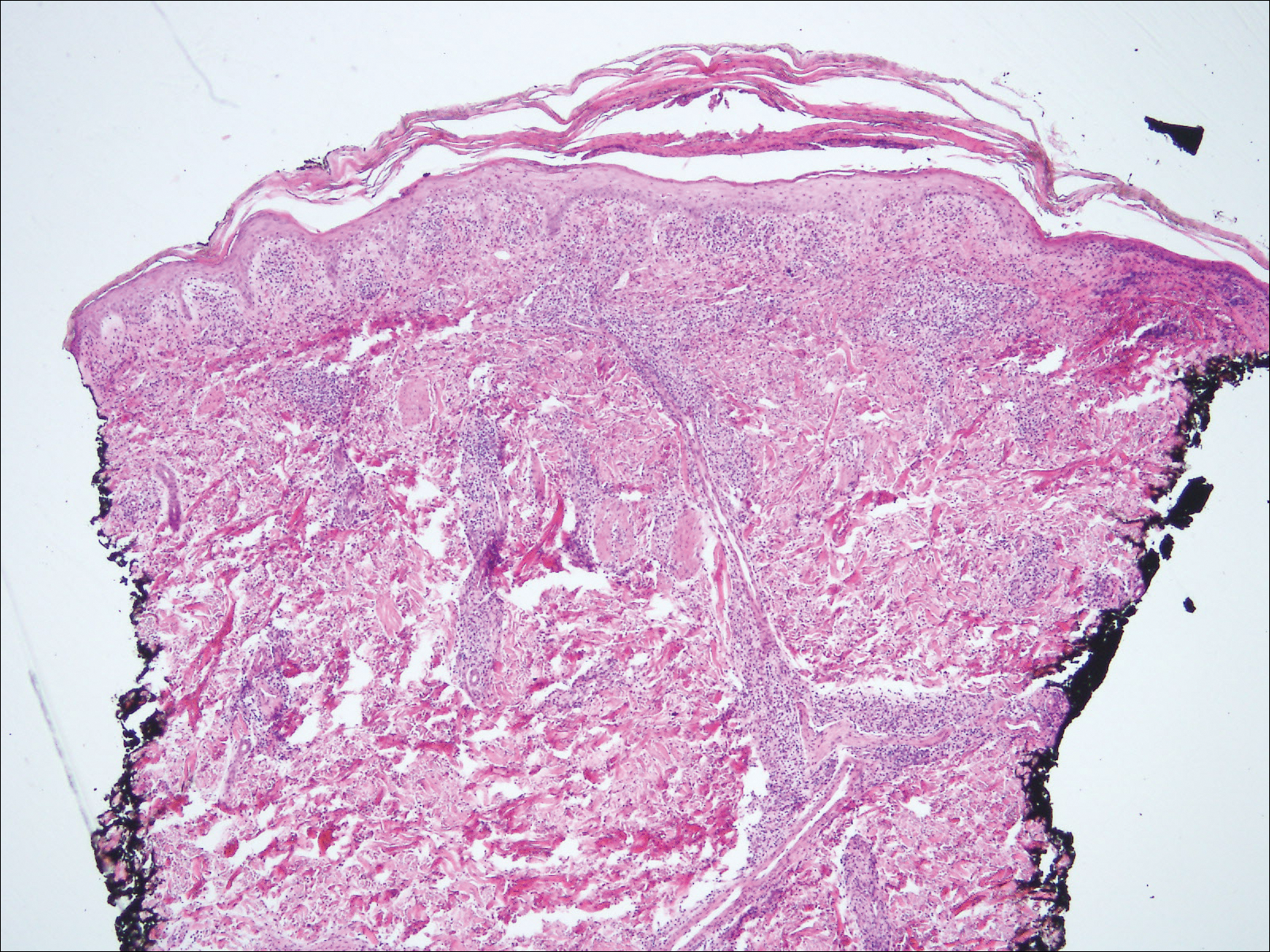
Histologic findings in sarcoidosis generally are independent of the respective organ and clinical disease presentation. The epidermis usually remains unchanged, whereas the dermis shows a superficial and deep nodular granulomatous infiltrate. Granulomas consist of epithelioid cells with only few giant cells and no surrounding lymphocytes or a very sparse lymphocytic infiltrate ("naked" granuloma)(Figure 1). Foreign bodies, including silica, are known to be able to induce sarcoid granulomas, especially in patients with sarcoidosis. A sarcoidal reaction in long-standing scar tissue points to a diagnosis of sarcoidosis.10
Cutaneous tuberculosis primarily is caused by Mycobacterium tuberculosis and less frequently Mycobacterium bovis.11,12 The manifestations of cutaneous tuberculosis depends on various factors such as the type of infection, mode of dissemination, host immunity, and whether it is a first-time infection or a recurrence. In Europe, the head and neck regions are most frequently affected.13 Lesions present as red-brown papules coalescing into a plaque. The tissue, especially in central parts of the lesion, is fragile (probe phenomenon). Diascopy shows the typical apple jelly-like color.
Histologically, cutaneous tuberculosis is characterized by typical tuberculoid granulomas with epithelioid cells and Langhans giant cells at the center surrounded by lymphocytes (Figure 2). Caseous necrosis as well as fibrosis may occur,14,15 and the granulomas tend to coalesce.
Granulomatosis with polyangiitis, formerly known as Wegener granulomatosis, is a complex, multisystemic disease with varying manifestations. The condition has been defined as a necrotizing granulomatous inflammation usually involving the upper and lower respiratory tracts and necrotizing vasculitis affecting predominantly small- to medium-sized vessels.16 The etiology of GPA is thought to be linked to environmental and infectious triggers inciting onset of disease in genetically predisposed individuals. Antineutrophil cytoplasmic antibodies play an important role in the pathogenesis of this disease. Cutaneous vasculitis secondary to GPA can present as papules, nodules, palpable purpura, ulcers resembling pyoderma gangrenosum, or necrotizing lesions leading to gangrene.17
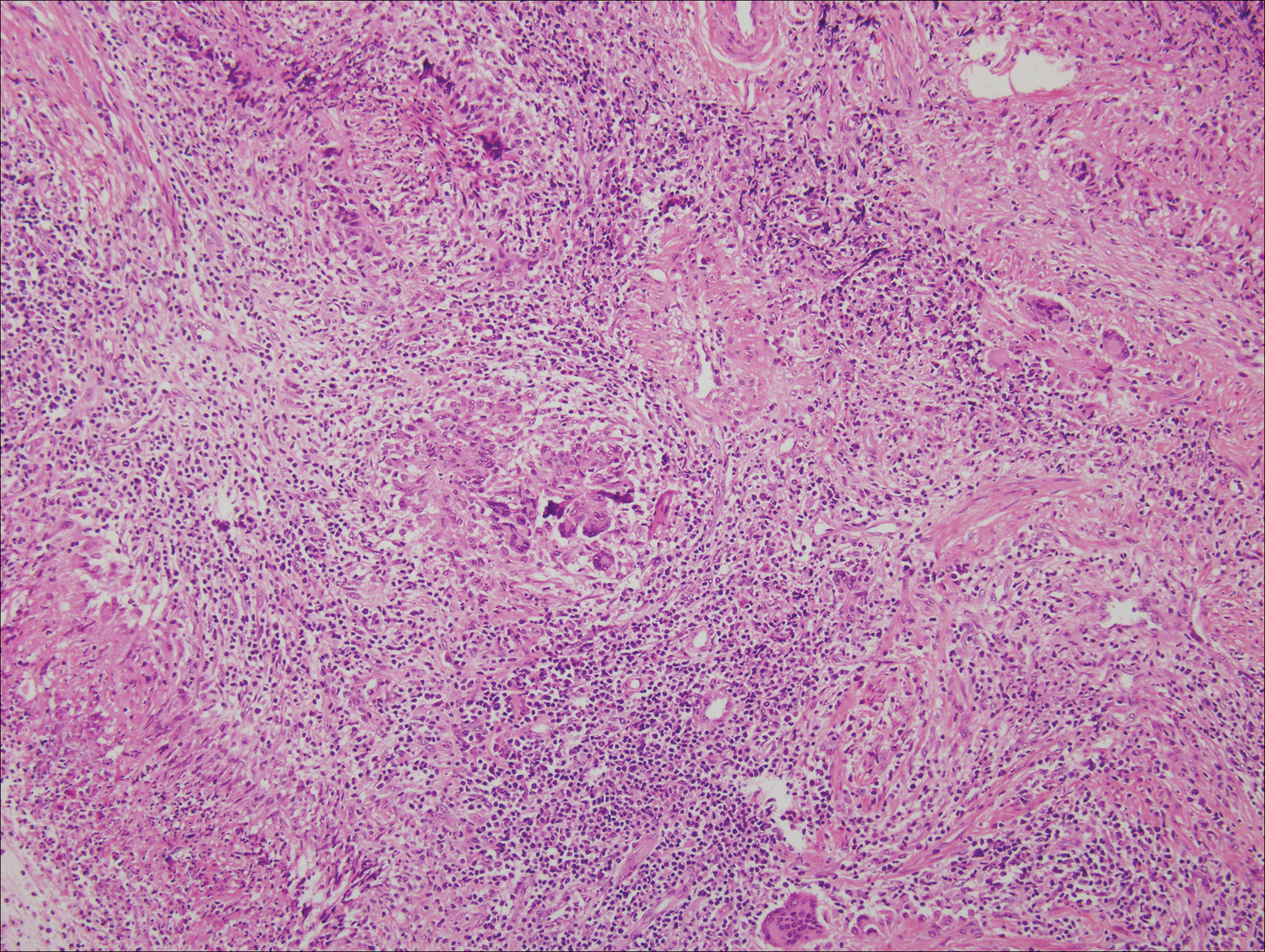
The predominant histopathologic pattern in cutaneous lesions of GPA is leukocytoclastic vasculitis, which is present in up to 50% of biopsies.18 Characteristic findings that aid in establishing the diagnosis include histologic evidence of focal necrosis, fibrinoid degeneration, palisading granuloma surrounding neutrophils (Figure 3), and granulomatous vasculitis involving muscular vessel walls.19 Nonpalisading foci of necrosis or fibrinoid degeneration may precede the development of the typical palisading granuloma.20
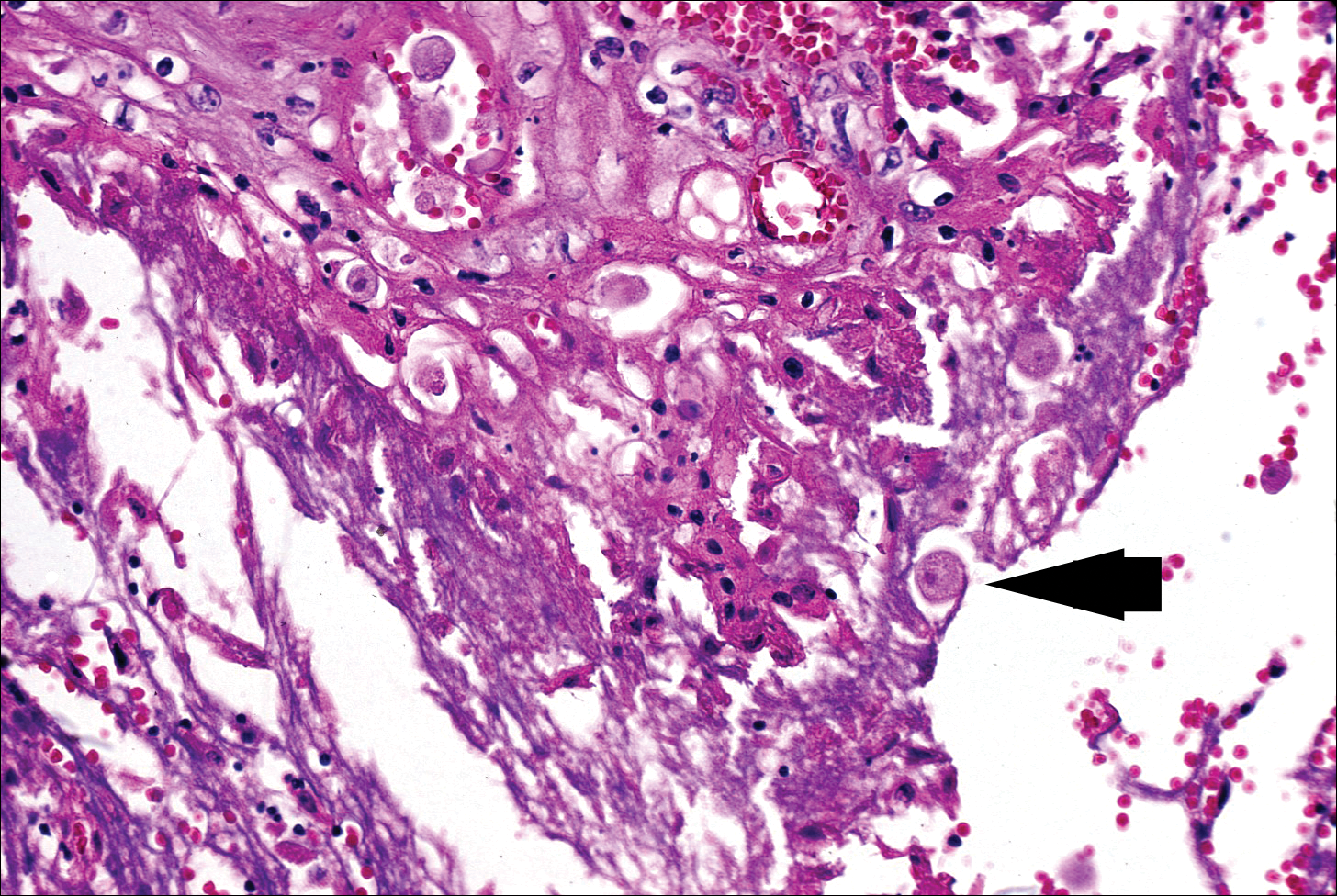
The typical histopathologic pattern of cutaneous amebiasis is ulceration with vascular necrosis (Figure 4).21 The organisms have prominent round nuclei and nucleoli and the cytoplasm may have a scalloped border.
- Crohn BB, Ginzburg L, Oppenheimer GD. Landmark article Oct 25, 1932. regional ileitis. a pathologic and clinical entity. by Burril B. Crohn, Leon Gonzburg and Gordon D. Oppenheimer. JAMA. 1984;251:73-79.
- Parks AG, Morson BC, Pegum JS. Crohn's disease with cutaneous involvement. Proc R Soc Med. 1965;58:241-242.
- Weedon D. Miscellaneous conditions. Skin Pathology. 2nd ed. London, England: Churchill Livingstone; 2002:554.
- Samitz MH, Dana Jr AS, Rosenberg P. Cutaneous vasculitis in association with Crohn's disease. Cutis. 1970;6:51-56.
- Palamaras I, El-Jabbour J, Pietropaolo N, et al. Metastatic Crohn's disease: a review. J Eur Acad Dermatol Venereol. 2008;22:1033-1043.
- Aberumand B, Howard J, Howard J. Metastatic Crohn's disease: an approach to an uncommon but important cutaneous disorder: a review [published online January 3, 2017]. BioMed Res Int. 2017;2017:8192150.
- Mahony J, Helms SE, Brodell RT. The sarcoidal granuloma: a unifying hypothesis for an enigmatic response. Clin Dermatol. 2014;32:654-659.
- Freedberg IM, Eisen AZ, Wolf K, et al. Fitzpatrick's Dermatology in General Medicine. 6th ed. New York, NY: McGraw Hill; 2003.
- Fernandez-Faith E, McDonnell J. Cutaneous sarcoidosis: differential diagnosis. Clin Dermatol. 2007;25:276-287.
- Walsh NM, Hanly JG, Tremaine R, et al. Cutaneous sarcoidosis and foreign bodies. Am J Dermatopathol. 1993;15:203-207.
- Semaan R, Traboulsi R, Kanj S. Primary Mycobacterium tuberculosis complex cutaneous infection: report of two cases and literature review. Int J Infect Dis. 2008;12:472-477.
- Lai-Cheong JE, Perez A, Tang V, et al. Cutaneous manifestations of tuberculosis. Clin Exp Dermatol. 2007;32:461-466.
- Marcoval J, Servitje O, Moreno A, et al. Lupus vulgaris. clinical, histopathologic, and bacteriologic study of 10 cases. J Am Acad Dermatol. 1992;26:404-407.
- Tronnier M, Wolff H. Dermatosen mit granulomatöser Entzündung. Histopathologie der Haut. In: Kerl H, Garbe C, Cerroni L, et al, eds. New York, NY: Springer; 2003.
- Min KW, Ko JY, Park CK. Histopathological spectrum of cutaneous tuberculosis and non-tuberculous mycobacterial infections. J Cutan Pathol. 2012;39:582-595.
- Jennette JC, Falk RJ, Bacon PA, et al. 2012 Revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Arthritis Rheum. 2013;65:1-11.
- Comfere NI, Macaron NC, Gibson LE. Cutaneous manifestations of Wegener's granulomatosis: a clinicopathologic study of 17 patients and correlation to antineutrophil cytoplasmic antibody status. J Cutan Pathol. 2007;34:739-747.
- Marzano AV, Vezzoli P, Berti E. Skin involvement in cutaneous and systemic vasculitis. Autoimmun Rev. 2012;12:467-476.
- Bramsiepe I, Danz B, Heine R, et al. Primary cutaneous manifestation of Wegener's granulomatosis [in German]. Dtsch Med Wochenschr. 2008;27:1429-1432.
- Daoud MS, Gibson LE, DeRemee RA, et al. Cutaneous Wegener's granulomatosis: clinical, histopathologic, and immunopathologic features of thirty patients. J Am Acad Dermatol. 1994;31:605-612.
- Guidry JA, Downing C, Tyring SK. Deep fungal infections, blastomycosis-like pyoderma, and granulomatous sexually transmitted infections. Dermatol Clin. 2015;33:595-607.
The Diagnosis: Metastatic Crohn Disease
Crohn disease (CD), a chronic inflammatory granulomatous disease of the gastrointestinal tract, has a wide spectrum of presentations.1 The condition may affect the vulva, perineum, or perianal skin by direct extension from the gastrointestinal tract or may appear as a separate and distinct cutaneous focus of disease referred to as metastatic Crohn disease (MCD).2
Cutaneous lesions of MCD include ulcers, fissures, sinus tracts, abscesses, and vegetative plaques, which typically extend in continuity with sites of intra-abdominal disease to the perineum, buttocks, or abdominal wall, as well as ostomy sites or incisional scars. Erythema nodosum and pyoderma gangrenosum are the most common nonspecific cutaneous manifestations. Other cutaneous lesions described in CD include polyarteritis nodosa, psoriasis, erythema multiforme, erythema elevatum diutinum, epidermolysis bullosa acquisita, acne fulminans, pyoderma faciale, neutrophilic lobular panniculitis, granulomatous vasculitis, and porokeratosis.3
Perianal skin is the most common site of cutaneous involvement in individuals with CD. It is a marker of more severe disease and is associated with multiple surgical interventions and frequent relapses and has been reported in 22% of patients with CD.4 Most already had an existing diagnosis of gastrointestinal CD, which was active in one-third of individuals; however, 20% presented with disease at nongastrointestinal sites 2 months to 4 years prior to developing the gastrointestinal CD manifestations.5 Our patient presented with lesions on the perianal skin of 2 years' duration and a 6-month history of diarrhea. A colonoscopy demonstrated shallow ulcers involving the ileocecal portion of the gut, colon, and rectum. A biopsy from intestinal mucosal tissue showed acute and chronic inflammation with necrosis mixed with granulomatous inflammation, suggestive of CD.
Microscopically, the dominant histologic features of MCD are similar to those of bowel lesions, including an inflammatory infiltrate commonly consisting of sterile noncaseating sarcoidal granulomas, foreign body and Langhans giant cells, epithelioid histiocytes, and plasma cells surrounded by numerous mononuclear cells within the dermis with occasional extension into the subcutis (quiz image). Less common features include collagen degeneration, an infiltrate rich in eosinophils, dermal edema, and mixed lichenoid and granulomatous dermatitis.6
Metastatic CD often is misdiagnosed. A detailed history and physical examination may help narrow the differential; however, biopsy is necessary to establish a diagnosis of MCD. The histologic differential diagnosis of sarcoidal granulomatous inflammation of genital skin includes sarcoidosis, rheumatoid arthritis, leprosy or other mycobacterial and parasitic infection, granulomatosis with polyangiitis (GPA), and granulomatous infiltrate associated with certain exogenous material (eg, silica, zirconium, beryllium, tattoo pigment).
Sarcoidosis is a multiorgan disease that most frequently affects the lungs, skin, and lymph nodes. Its etiopathogenesis has not been clearly elucidated.7 Cutaneous lesions are present in 20% to 35% of patients.8 Given the wide variability of clinical manifestations, cutaneous sarcoidosis is another one of the great imitators. Cutaneous lesions are classified as specific and nonspecific depending on the presence of noncaseating granulomas on histologic studies and include maculopapules, plaques, nodules, lupus pernio, scar infiltration, alopecia, ulcerative lesions, and hypopigmentation. The most common nonspecific lesion of cutaneous sarcoidosis is erythema nodosum. Other manifestations include calcifications, prurigo, erythema multiforme, nail clubbing, and Sweet syndrome.9
Histologic findings in sarcoidosis generally are independent of the respective organ and clinical disease presentation. The epidermis usually remains unchanged, whereas the dermis shows a superficial and deep nodular granulomatous infiltrate. Granulomas consist of epithelioid cells with only few giant cells and no surrounding lymphocytes or a very sparse lymphocytic infiltrate ("naked" granuloma)(Figure 1). Foreign bodies, including silica, are known to be able to induce sarcoid granulomas, especially in patients with sarcoidosis. A sarcoidal reaction in long-standing scar tissue points to a diagnosis of sarcoidosis.10

Cutaneous tuberculosis primarily is caused by Mycobacterium tuberculosis and less frequently Mycobacterium bovis.11,12 The manifestations of cutaneous tuberculosis depends on various factors such as the type of infection, mode of dissemination, host immunity, and whether it is a first-time infection or a recurrence. In Europe, the head and neck regions are most frequently affected.13 Lesions present as red-brown papules coalescing into a plaque. The tissue, especially in central parts of the lesion, is fragile (probe phenomenon). Diascopy shows the typical apple jelly-like color.
Histologically, cutaneous tuberculosis is characterized by typical tuberculoid granulomas with epithelioid cells and Langhans giant cells at the center surrounded by lymphocytes (Figure 2). Caseous necrosis as well as fibrosis may occur,14,15 and the granulomas tend to coalesce.

Granulomatosis with polyangiitis, formerly known as Wegener granulomatosis, is a complex, multisystemic disease with varying manifestations. The condition has been defined as a necrotizing granulomatous inflammation usually involving the upper and lower respiratory tracts and necrotizing vasculitis affecting predominantly small- to medium-sized vessels.16 The etiology of GPA is thought to be linked to environmental and infectious triggers inciting onset of disease in genetically predisposed individuals. Antineutrophil cytoplasmic antibodies play an important role in the pathogenesis of this disease. Cutaneous vasculitis secondary to GPA can present as papules, nodules, palpable purpura, ulcers resembling pyoderma gangrenosum, or necrotizing lesions leading to gangrene.17
The predominant histopathologic pattern in cutaneous lesions of GPA is leukocytoclastic vasculitis, which is present in up to 50% of biopsies.18 Characteristic findings that aid in establishing the diagnosis include histologic evidence of focal necrosis, fibrinoid degeneration, palisading granuloma surrounding neutrophils (Figure 3), and granulomatous vasculitis involving muscular vessel walls.19 Nonpalisading foci of necrosis or fibrinoid degeneration may precede the development of the typical palisading granuloma.20

The typical histopathologic pattern of cutaneous amebiasis is ulceration with vascular necrosis (Figure 4).21 The organisms have prominent round nuclei and nucleoli and the cytoplasm may have a scalloped border.

The Diagnosis: Metastatic Crohn Disease
Crohn disease (CD), a chronic inflammatory granulomatous disease of the gastrointestinal tract, has a wide spectrum of presentations.1 The condition may affect the vulva, perineum, or perianal skin by direct extension from the gastrointestinal tract or may appear as a separate and distinct cutaneous focus of disease referred to as metastatic Crohn disease (MCD).2
Cutaneous lesions of MCD include ulcers, fissures, sinus tracts, abscesses, and vegetative plaques, which typically extend in continuity with sites of intra-abdominal disease to the perineum, buttocks, or abdominal wall, as well as ostomy sites or incisional scars. Erythema nodosum and pyoderma gangrenosum are the most common nonspecific cutaneous manifestations. Other cutaneous lesions described in CD include polyarteritis nodosa, psoriasis, erythema multiforme, erythema elevatum diutinum, epidermolysis bullosa acquisita, acne fulminans, pyoderma faciale, neutrophilic lobular panniculitis, granulomatous vasculitis, and porokeratosis.3
Perianal skin is the most common site of cutaneous involvement in individuals with CD. It is a marker of more severe disease and is associated with multiple surgical interventions and frequent relapses and has been reported in 22% of patients with CD.4 Most already had an existing diagnosis of gastrointestinal CD, which was active in one-third of individuals; however, 20% presented with disease at nongastrointestinal sites 2 months to 4 years prior to developing the gastrointestinal CD manifestations.5 Our patient presented with lesions on the perianal skin of 2 years' duration and a 6-month history of diarrhea. A colonoscopy demonstrated shallow ulcers involving the ileocecal portion of the gut, colon, and rectum. A biopsy from intestinal mucosal tissue showed acute and chronic inflammation with necrosis mixed with granulomatous inflammation, suggestive of CD.
Microscopically, the dominant histologic features of MCD are similar to those of bowel lesions, including an inflammatory infiltrate commonly consisting of sterile noncaseating sarcoidal granulomas, foreign body and Langhans giant cells, epithelioid histiocytes, and plasma cells surrounded by numerous mononuclear cells within the dermis with occasional extension into the subcutis (quiz image). Less common features include collagen degeneration, an infiltrate rich in eosinophils, dermal edema, and mixed lichenoid and granulomatous dermatitis.6
Metastatic CD often is misdiagnosed. A detailed history and physical examination may help narrow the differential; however, biopsy is necessary to establish a diagnosis of MCD. The histologic differential diagnosis of sarcoidal granulomatous inflammation of genital skin includes sarcoidosis, rheumatoid arthritis, leprosy or other mycobacterial and parasitic infection, granulomatosis with polyangiitis (GPA), and granulomatous infiltrate associated with certain exogenous material (eg, silica, zirconium, beryllium, tattoo pigment).
Sarcoidosis is a multiorgan disease that most frequently affects the lungs, skin, and lymph nodes. Its etiopathogenesis has not been clearly elucidated.7 Cutaneous lesions are present in 20% to 35% of patients.8 Given the wide variability of clinical manifestations, cutaneous sarcoidosis is another one of the great imitators. Cutaneous lesions are classified as specific and nonspecific depending on the presence of noncaseating granulomas on histologic studies and include maculopapules, plaques, nodules, lupus pernio, scar infiltration, alopecia, ulcerative lesions, and hypopigmentation. The most common nonspecific lesion of cutaneous sarcoidosis is erythema nodosum. Other manifestations include calcifications, prurigo, erythema multiforme, nail clubbing, and Sweet syndrome.9
Histologic findings in sarcoidosis generally are independent of the respective organ and clinical disease presentation. The epidermis usually remains unchanged, whereas the dermis shows a superficial and deep nodular granulomatous infiltrate. Granulomas consist of epithelioid cells with only few giant cells and no surrounding lymphocytes or a very sparse lymphocytic infiltrate ("naked" granuloma)(Figure 1). Foreign bodies, including silica, are known to be able to induce sarcoid granulomas, especially in patients with sarcoidosis. A sarcoidal reaction in long-standing scar tissue points to a diagnosis of sarcoidosis.10

Cutaneous tuberculosis primarily is caused by Mycobacterium tuberculosis and less frequently Mycobacterium bovis.11,12 The manifestations of cutaneous tuberculosis depends on various factors such as the type of infection, mode of dissemination, host immunity, and whether it is a first-time infection or a recurrence. In Europe, the head and neck regions are most frequently affected.13 Lesions present as red-brown papules coalescing into a plaque. The tissue, especially in central parts of the lesion, is fragile (probe phenomenon). Diascopy shows the typical apple jelly-like color.
Histologically, cutaneous tuberculosis is characterized by typical tuberculoid granulomas with epithelioid cells and Langhans giant cells at the center surrounded by lymphocytes (Figure 2). Caseous necrosis as well as fibrosis may occur,14,15 and the granulomas tend to coalesce.

Granulomatosis with polyangiitis, formerly known as Wegener granulomatosis, is a complex, multisystemic disease with varying manifestations. The condition has been defined as a necrotizing granulomatous inflammation usually involving the upper and lower respiratory tracts and necrotizing vasculitis affecting predominantly small- to medium-sized vessels.16 The etiology of GPA is thought to be linked to environmental and infectious triggers inciting onset of disease in genetically predisposed individuals. Antineutrophil cytoplasmic antibodies play an important role in the pathogenesis of this disease. Cutaneous vasculitis secondary to GPA can present as papules, nodules, palpable purpura, ulcers resembling pyoderma gangrenosum, or necrotizing lesions leading to gangrene.17
The predominant histopathologic pattern in cutaneous lesions of GPA is leukocytoclastic vasculitis, which is present in up to 50% of biopsies.18 Characteristic findings that aid in establishing the diagnosis include histologic evidence of focal necrosis, fibrinoid degeneration, palisading granuloma surrounding neutrophils (Figure 3), and granulomatous vasculitis involving muscular vessel walls.19 Nonpalisading foci of necrosis or fibrinoid degeneration may precede the development of the typical palisading granuloma.20

The typical histopathologic pattern of cutaneous amebiasis is ulceration with vascular necrosis (Figure 4).21 The organisms have prominent round nuclei and nucleoli and the cytoplasm may have a scalloped border.

- Crohn BB, Ginzburg L, Oppenheimer GD. Landmark article Oct 25, 1932. regional ileitis. a pathologic and clinical entity. by Burril B. Crohn, Leon Gonzburg and Gordon D. Oppenheimer. JAMA. 1984;251:73-79.
- Parks AG, Morson BC, Pegum JS. Crohn's disease with cutaneous involvement. Proc R Soc Med. 1965;58:241-242.
- Weedon D. Miscellaneous conditions. Skin Pathology. 2nd ed. London, England: Churchill Livingstone; 2002:554.
- Samitz MH, Dana Jr AS, Rosenberg P. Cutaneous vasculitis in association with Crohn's disease. Cutis. 1970;6:51-56.
- Palamaras I, El-Jabbour J, Pietropaolo N, et al. Metastatic Crohn's disease: a review. J Eur Acad Dermatol Venereol. 2008;22:1033-1043.
- Aberumand B, Howard J, Howard J. Metastatic Crohn's disease: an approach to an uncommon but important cutaneous disorder: a review [published online January 3, 2017]. BioMed Res Int. 2017;2017:8192150.
- Mahony J, Helms SE, Brodell RT. The sarcoidal granuloma: a unifying hypothesis for an enigmatic response. Clin Dermatol. 2014;32:654-659.
- Freedberg IM, Eisen AZ, Wolf K, et al. Fitzpatrick's Dermatology in General Medicine. 6th ed. New York, NY: McGraw Hill; 2003.
- Fernandez-Faith E, McDonnell J. Cutaneous sarcoidosis: differential diagnosis. Clin Dermatol. 2007;25:276-287.
- Walsh NM, Hanly JG, Tremaine R, et al. Cutaneous sarcoidosis and foreign bodies. Am J Dermatopathol. 1993;15:203-207.
- Semaan R, Traboulsi R, Kanj S. Primary Mycobacterium tuberculosis complex cutaneous infection: report of two cases and literature review. Int J Infect Dis. 2008;12:472-477.
- Lai-Cheong JE, Perez A, Tang V, et al. Cutaneous manifestations of tuberculosis. Clin Exp Dermatol. 2007;32:461-466.
- Marcoval J, Servitje O, Moreno A, et al. Lupus vulgaris. clinical, histopathologic, and bacteriologic study of 10 cases. J Am Acad Dermatol. 1992;26:404-407.
- Tronnier M, Wolff H. Dermatosen mit granulomatöser Entzündung. Histopathologie der Haut. In: Kerl H, Garbe C, Cerroni L, et al, eds. New York, NY: Springer; 2003.
- Min KW, Ko JY, Park CK. Histopathological spectrum of cutaneous tuberculosis and non-tuberculous mycobacterial infections. J Cutan Pathol. 2012;39:582-595.
- Jennette JC, Falk RJ, Bacon PA, et al. 2012 Revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Arthritis Rheum. 2013;65:1-11.
- Comfere NI, Macaron NC, Gibson LE. Cutaneous manifestations of Wegener's granulomatosis: a clinicopathologic study of 17 patients and correlation to antineutrophil cytoplasmic antibody status. J Cutan Pathol. 2007;34:739-747.
- Marzano AV, Vezzoli P, Berti E. Skin involvement in cutaneous and systemic vasculitis. Autoimmun Rev. 2012;12:467-476.
- Bramsiepe I, Danz B, Heine R, et al. Primary cutaneous manifestation of Wegener's granulomatosis [in German]. Dtsch Med Wochenschr. 2008;27:1429-1432.
- Daoud MS, Gibson LE, DeRemee RA, et al. Cutaneous Wegener's granulomatosis: clinical, histopathologic, and immunopathologic features of thirty patients. J Am Acad Dermatol. 1994;31:605-612.
- Guidry JA, Downing C, Tyring SK. Deep fungal infections, blastomycosis-like pyoderma, and granulomatous sexually transmitted infections. Dermatol Clin. 2015;33:595-607.
- Crohn BB, Ginzburg L, Oppenheimer GD. Landmark article Oct 25, 1932. regional ileitis. a pathologic and clinical entity. by Burril B. Crohn, Leon Gonzburg and Gordon D. Oppenheimer. JAMA. 1984;251:73-79.
- Parks AG, Morson BC, Pegum JS. Crohn's disease with cutaneous involvement. Proc R Soc Med. 1965;58:241-242.
- Weedon D. Miscellaneous conditions. Skin Pathology. 2nd ed. London, England: Churchill Livingstone; 2002:554.
- Samitz MH, Dana Jr AS, Rosenberg P. Cutaneous vasculitis in association with Crohn's disease. Cutis. 1970;6:51-56.
- Palamaras I, El-Jabbour J, Pietropaolo N, et al. Metastatic Crohn's disease: a review. J Eur Acad Dermatol Venereol. 2008;22:1033-1043.
- Aberumand B, Howard J, Howard J. Metastatic Crohn's disease: an approach to an uncommon but important cutaneous disorder: a review [published online January 3, 2017]. BioMed Res Int. 2017;2017:8192150.
- Mahony J, Helms SE, Brodell RT. The sarcoidal granuloma: a unifying hypothesis for an enigmatic response. Clin Dermatol. 2014;32:654-659.
- Freedberg IM, Eisen AZ, Wolf K, et al. Fitzpatrick's Dermatology in General Medicine. 6th ed. New York, NY: McGraw Hill; 2003.
- Fernandez-Faith E, McDonnell J. Cutaneous sarcoidosis: differential diagnosis. Clin Dermatol. 2007;25:276-287.
- Walsh NM, Hanly JG, Tremaine R, et al. Cutaneous sarcoidosis and foreign bodies. Am J Dermatopathol. 1993;15:203-207.
- Semaan R, Traboulsi R, Kanj S. Primary Mycobacterium tuberculosis complex cutaneous infection: report of two cases and literature review. Int J Infect Dis. 2008;12:472-477.
- Lai-Cheong JE, Perez A, Tang V, et al. Cutaneous manifestations of tuberculosis. Clin Exp Dermatol. 2007;32:461-466.
- Marcoval J, Servitje O, Moreno A, et al. Lupus vulgaris. clinical, histopathologic, and bacteriologic study of 10 cases. J Am Acad Dermatol. 1992;26:404-407.
- Tronnier M, Wolff H. Dermatosen mit granulomatöser Entzündung. Histopathologie der Haut. In: Kerl H, Garbe C, Cerroni L, et al, eds. New York, NY: Springer; 2003.
- Min KW, Ko JY, Park CK. Histopathological spectrum of cutaneous tuberculosis and non-tuberculous mycobacterial infections. J Cutan Pathol. 2012;39:582-595.
- Jennette JC, Falk RJ, Bacon PA, et al. 2012 Revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Arthritis Rheum. 2013;65:1-11.
- Comfere NI, Macaron NC, Gibson LE. Cutaneous manifestations of Wegener's granulomatosis: a clinicopathologic study of 17 patients and correlation to antineutrophil cytoplasmic antibody status. J Cutan Pathol. 2007;34:739-747.
- Marzano AV, Vezzoli P, Berti E. Skin involvement in cutaneous and systemic vasculitis. Autoimmun Rev. 2012;12:467-476.
- Bramsiepe I, Danz B, Heine R, et al. Primary cutaneous manifestation of Wegener's granulomatosis [in German]. Dtsch Med Wochenschr. 2008;27:1429-1432.
- Daoud MS, Gibson LE, DeRemee RA, et al. Cutaneous Wegener's granulomatosis: clinical, histopathologic, and immunopathologic features of thirty patients. J Am Acad Dermatol. 1994;31:605-612.
- Guidry JA, Downing C, Tyring SK. Deep fungal infections, blastomycosis-like pyoderma, and granulomatous sexually transmitted infections. Dermatol Clin. 2015;33:595-607.
A 19-year-old man presented with a perianal condyloma acuminatum-like plaque of 2 years' duration and a 6-month history of diarrhea.
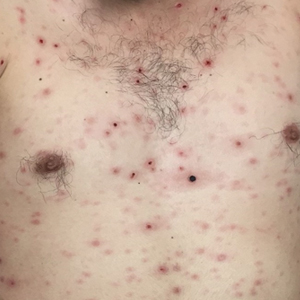
Disseminated Vesicles and Necrotic Papules
The Diagnosis: Lues Maligna
Laboratory evaluation demonstrated a total CD4 count of 26 cells/μL (reference range, 443-1471 cells/μL) with a viral load of 1,770,111 copies/mL (reference range, 0 copies/mL), as well as a positive rapid plasma reagin (RPR) test with a titer of 1:8 (reference range, nonreactive). A reactive treponemal antibody test confirmed a true positive RPR test result. Viral culture as well as direct fluorescence antibodies for varicella-zoster virus and an active vesicle of herpes simplex virus (HSV) were negative. Serum immunoglobulin titers for varicella-zoster virus demonstrated low IgM with a positive IgG demonstrating immunity without recent infection. Blood and lesional skin tissue cultures were negative for additional infectious etiologies including bacterial and fungal elements. A lumbar puncture was not performed.
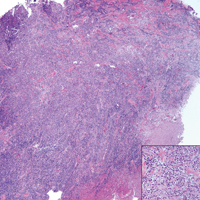
Biopsy of a papulonodule on the left arm demonstrated a lichenoid lymphohistiocytic infiltrate with superficial and deep inflammation (Figure 1). Neutrophils also were noted within a follicle with ballooning and acantholysis within the follicular epithelium. Additional staining for Mycobacterium, HSV-1, HSV-2, and Treponema were negative. In the clinical setting, this histologic pattern was most consistent with secondary syphilis. Pityriasis lichenoides et varioliformis acuta also was included in the histopathologic differential diagnosis by a dermatopathologist (M.C.).
Based on the clinical, microbiologic, and histopathologic findings, a diagnosis of lues maligna (cutaneous secondary syphilis) with a vesiculonecrotic presentation was made. The patient's low RPR titer was attributed to profound immunosuppression, while a confirmation of syphilis infection was made with treponemal antibody testing. Histopathologic examination was consistent with lues maligna and did not demonstrate evidence of any other infectious etiologies.
Following 7 days of intravenous penicillin, the patient demonstrated dramatic improvement of all skin lesions and was discharged receiving once-weekly intramuscular penicillin for 4 weeks. In accordance with the diagnosis, the patient demonstrated rapid improvement of the lesions following appropriate antibiotic therapy.
After the diagnosis of lues maligna was made, the patient disclosed a sexual encounter with a male partner 6 weeks prior to the current presentation, after which he developed a self-resolving genital ulcer suspicious for a primary chancre.
Increasing rates of syphilis transmission have been attributed to males aged 15 to 44 years who have sexual encounters with other males.1 Although syphilis commonly is known as the great mimicker, syphilology texts state that lesions are not associated with syphilis if vesicles are part of the cutaneous eruption in an adult.2 However, rare reports of secondary syphilis presenting as vesicles, pustules, bullae, and pityriasis lichenoides et varioliformis acuta-like eruptions also have been documented.2-4
Initial screening for suspected syphilis involves sensitive, but not specific, nontreponemal RPR testing reported in the form of a titer. Nontreponemal titers in human immunodeficiency virus-positive individuals can be unusually high or low, fluctuate rapidly, and/or be unresponsive to antibiotic therapy.1
Lues maligna is a rare form of malignant secondary syphilis that most commonly presents in human immunodeficiency virus-positive hosts.5 Although lues maligna often presents with ulceronodular lesions, 2 cases presenting with vesiculonecrotic lesions also have been reported.6 Patients often experience systemic symptoms including fever, fatigue, and joint pain. Rapid plasma reagin titers can range from 1:8 to 1:128 in affected individuals.6 Diagnosis is dependent on serologic and histologic confirmation while ruling out viral, fungal, and bacterial etiologies. Characteristic red-brown lesions of secondary syphilis involving the palms and soles (Figure 2) alsoaid in diagnosis.1 Additionally, identification of the Jarisch-Herxheimer reaction following treatment and rapid response to antibiotic therapy are helpful diagnostic findings.6,7 While histopathologic examination of lues maligna typically does not reveal evidence of spirochetes, it also is important to rule out other infectious etiologies.7

Our case emphasizes the importance of early recognition and treatment of the variable clinical, laboratory, and histologic presentations of lues maligna.
- Syphilis fact sheet. Centers for Disease Control and Prevention website. https://www.cdc.gov/std/syphilis/stdfact-syphilis.htm. Updated June 13, 2017. Accessed March 22, 2018.
- Lawrence P, Saxe N. Bullous secondary syphilis. Clin Exp Dermatol. 1992;17:44-46.
- Pastuszczak M, Woz´niak W, Jaworek AK, et al. Pityriasis lichenoides-like secondary syphilis and neurosyphilis in a HIV-infected patient. Postepy Dermatol Alergol. 2013;30:127-130.
- Schnirring-Judge M, Gustaferro C, Terol C. Vesiculobullous syphilis: a case involving an unusual cutaneous manifestation of secondary syphilis [published online November 24, 2010]. J Foot Ankle Surg. 2011;50:96-101.
- Pföhler C, Koerner R, von Müller L, et al. Lues maligna in a patient with unknown HIV infection. BMJ Case Rep. 2011. pii: bcr0520114221. doi: 10.1136/bcr.05.2011.4221.
- Don PC, Rubinstein R, Christie S. Malignant syphilis (lues maligna) and concurrent infection with HIV. Int J Dermatol. 1995;34:403-407.
- Tucker JD, Shah S, Jarell AD, et al. Lues maligna in early HIV infection case report and review of the literature. Sex Transm Dis. 2009;36:512-514.
The Diagnosis: Lues Maligna
Laboratory evaluation demonstrated a total CD4 count of 26 cells/μL (reference range, 443-1471 cells/μL) with a viral load of 1,770,111 copies/mL (reference range, 0 copies/mL), as well as a positive rapid plasma reagin (RPR) test with a titer of 1:8 (reference range, nonreactive). A reactive treponemal antibody test confirmed a true positive RPR test result. Viral culture as well as direct fluorescence antibodies for varicella-zoster virus and an active vesicle of herpes simplex virus (HSV) were negative. Serum immunoglobulin titers for varicella-zoster virus demonstrated low IgM with a positive IgG demonstrating immunity without recent infection. Blood and lesional skin tissue cultures were negative for additional infectious etiologies including bacterial and fungal elements. A lumbar puncture was not performed.
Biopsy of a papulonodule on the left arm demonstrated a lichenoid lymphohistiocytic infiltrate with superficial and deep inflammation (Figure 1). Neutrophils also were noted within a follicle with ballooning and acantholysis within the follicular epithelium. Additional staining for Mycobacterium, HSV-1, HSV-2, and Treponema were negative. In the clinical setting, this histologic pattern was most consistent with secondary syphilis. Pityriasis lichenoides et varioliformis acuta also was included in the histopathologic differential diagnosis by a dermatopathologist (M.C.).

Based on the clinical, microbiologic, and histopathologic findings, a diagnosis of lues maligna (cutaneous secondary syphilis) with a vesiculonecrotic presentation was made. The patient's low RPR titer was attributed to profound immunosuppression, while a confirmation of syphilis infection was made with treponemal antibody testing. Histopathologic examination was consistent with lues maligna and did not demonstrate evidence of any other infectious etiologies.
Following 7 days of intravenous penicillin, the patient demonstrated dramatic improvement of all skin lesions and was discharged receiving once-weekly intramuscular penicillin for 4 weeks. In accordance with the diagnosis, the patient demonstrated rapid improvement of the lesions following appropriate antibiotic therapy.
After the diagnosis of lues maligna was made, the patient disclosed a sexual encounter with a male partner 6 weeks prior to the current presentation, after which he developed a self-resolving genital ulcer suspicious for a primary chancre.
Increasing rates of syphilis transmission have been attributed to males aged 15 to 44 years who have sexual encounters with other males.1 Although syphilis commonly is known as the great mimicker, syphilology texts state that lesions are not associated with syphilis if vesicles are part of the cutaneous eruption in an adult.2 However, rare reports of secondary syphilis presenting as vesicles, pustules, bullae, and pityriasis lichenoides et varioliformis acuta-like eruptions also have been documented.2-4
Initial screening for suspected syphilis involves sensitive, but not specific, nontreponemal RPR testing reported in the form of a titer. Nontreponemal titers in human immunodeficiency virus-positive individuals can be unusually high or low, fluctuate rapidly, and/or be unresponsive to antibiotic therapy.1
Lues maligna is a rare form of malignant secondary syphilis that most commonly presents in human immunodeficiency virus-positive hosts.5 Although lues maligna often presents with ulceronodular lesions, 2 cases presenting with vesiculonecrotic lesions also have been reported.6 Patients often experience systemic symptoms including fever, fatigue, and joint pain. Rapid plasma reagin titers can range from 1:8 to 1:128 in affected individuals.6 Diagnosis is dependent on serologic and histologic confirmation while ruling out viral, fungal, and bacterial etiologies. Characteristic red-brown lesions of secondary syphilis involving the palms and soles (Figure 2) alsoaid in diagnosis.1 Additionally, identification of the Jarisch-Herxheimer reaction following treatment and rapid response to antibiotic therapy are helpful diagnostic findings.6,7 While histopathologic examination of lues maligna typically does not reveal evidence of spirochetes, it also is important to rule out other infectious etiologies.7

Our case emphasizes the importance of early recognition and treatment of the variable clinical, laboratory, and histologic presentations of lues maligna.
The Diagnosis: Lues Maligna
Laboratory evaluation demonstrated a total CD4 count of 26 cells/μL (reference range, 443-1471 cells/μL) with a viral load of 1,770,111 copies/mL (reference range, 0 copies/mL), as well as a positive rapid plasma reagin (RPR) test with a titer of 1:8 (reference range, nonreactive). A reactive treponemal antibody test confirmed a true positive RPR test result. Viral culture as well as direct fluorescence antibodies for varicella-zoster virus and an active vesicle of herpes simplex virus (HSV) were negative. Serum immunoglobulin titers for varicella-zoster virus demonstrated low IgM with a positive IgG demonstrating immunity without recent infection. Blood and lesional skin tissue cultures were negative for additional infectious etiologies including bacterial and fungal elements. A lumbar puncture was not performed.
Biopsy of a papulonodule on the left arm demonstrated a lichenoid lymphohistiocytic infiltrate with superficial and deep inflammation (Figure 1). Neutrophils also were noted within a follicle with ballooning and acantholysis within the follicular epithelium. Additional staining for Mycobacterium, HSV-1, HSV-2, and Treponema were negative. In the clinical setting, this histologic pattern was most consistent with secondary syphilis. Pityriasis lichenoides et varioliformis acuta also was included in the histopathologic differential diagnosis by a dermatopathologist (M.C.).

Based on the clinical, microbiologic, and histopathologic findings, a diagnosis of lues maligna (cutaneous secondary syphilis) with a vesiculonecrotic presentation was made. The patient's low RPR titer was attributed to profound immunosuppression, while a confirmation of syphilis infection was made with treponemal antibody testing. Histopathologic examination was consistent with lues maligna and did not demonstrate evidence of any other infectious etiologies.
Following 7 days of intravenous penicillin, the patient demonstrated dramatic improvement of all skin lesions and was discharged receiving once-weekly intramuscular penicillin for 4 weeks. In accordance with the diagnosis, the patient demonstrated rapid improvement of the lesions following appropriate antibiotic therapy.
After the diagnosis of lues maligna was made, the patient disclosed a sexual encounter with a male partner 6 weeks prior to the current presentation, after which he developed a self-resolving genital ulcer suspicious for a primary chancre.
Increasing rates of syphilis transmission have been attributed to males aged 15 to 44 years who have sexual encounters with other males.1 Although syphilis commonly is known as the great mimicker, syphilology texts state that lesions are not associated with syphilis if vesicles are part of the cutaneous eruption in an adult.2 However, rare reports of secondary syphilis presenting as vesicles, pustules, bullae, and pityriasis lichenoides et varioliformis acuta-like eruptions also have been documented.2-4
Initial screening for suspected syphilis involves sensitive, but not specific, nontreponemal RPR testing reported in the form of a titer. Nontreponemal titers in human immunodeficiency virus-positive individuals can be unusually high or low, fluctuate rapidly, and/or be unresponsive to antibiotic therapy.1
Lues maligna is a rare form of malignant secondary syphilis that most commonly presents in human immunodeficiency virus-positive hosts.5 Although lues maligna often presents with ulceronodular lesions, 2 cases presenting with vesiculonecrotic lesions also have been reported.6 Patients often experience systemic symptoms including fever, fatigue, and joint pain. Rapid plasma reagin titers can range from 1:8 to 1:128 in affected individuals.6 Diagnosis is dependent on serologic and histologic confirmation while ruling out viral, fungal, and bacterial etiologies. Characteristic red-brown lesions of secondary syphilis involving the palms and soles (Figure 2) alsoaid in diagnosis.1 Additionally, identification of the Jarisch-Herxheimer reaction following treatment and rapid response to antibiotic therapy are helpful diagnostic findings.6,7 While histopathologic examination of lues maligna typically does not reveal evidence of spirochetes, it also is important to rule out other infectious etiologies.7

Our case emphasizes the importance of early recognition and treatment of the variable clinical, laboratory, and histologic presentations of lues maligna.
- Syphilis fact sheet. Centers for Disease Control and Prevention website. https://www.cdc.gov/std/syphilis/stdfact-syphilis.htm. Updated June 13, 2017. Accessed March 22, 2018.
- Lawrence P, Saxe N. Bullous secondary syphilis. Clin Exp Dermatol. 1992;17:44-46.
- Pastuszczak M, Woz´niak W, Jaworek AK, et al. Pityriasis lichenoides-like secondary syphilis and neurosyphilis in a HIV-infected patient. Postepy Dermatol Alergol. 2013;30:127-130.
- Schnirring-Judge M, Gustaferro C, Terol C. Vesiculobullous syphilis: a case involving an unusual cutaneous manifestation of secondary syphilis [published online November 24, 2010]. J Foot Ankle Surg. 2011;50:96-101.
- Pföhler C, Koerner R, von Müller L, et al. Lues maligna in a patient with unknown HIV infection. BMJ Case Rep. 2011. pii: bcr0520114221. doi: 10.1136/bcr.05.2011.4221.
- Don PC, Rubinstein R, Christie S. Malignant syphilis (lues maligna) and concurrent infection with HIV. Int J Dermatol. 1995;34:403-407.
- Tucker JD, Shah S, Jarell AD, et al. Lues maligna in early HIV infection case report and review of the literature. Sex Transm Dis. 2009;36:512-514.
- Syphilis fact sheet. Centers for Disease Control and Prevention website. https://www.cdc.gov/std/syphilis/stdfact-syphilis.htm. Updated June 13, 2017. Accessed March 22, 2018.
- Lawrence P, Saxe N. Bullous secondary syphilis. Clin Exp Dermatol. 1992;17:44-46.
- Pastuszczak M, Woz´niak W, Jaworek AK, et al. Pityriasis lichenoides-like secondary syphilis and neurosyphilis in a HIV-infected patient. Postepy Dermatol Alergol. 2013;30:127-130.
- Schnirring-Judge M, Gustaferro C, Terol C. Vesiculobullous syphilis: a case involving an unusual cutaneous manifestation of secondary syphilis [published online November 24, 2010]. J Foot Ankle Surg. 2011;50:96-101.
- Pföhler C, Koerner R, von Müller L, et al. Lues maligna in a patient with unknown HIV infection. BMJ Case Rep. 2011. pii: bcr0520114221. doi: 10.1136/bcr.05.2011.4221.
- Don PC, Rubinstein R, Christie S. Malignant syphilis (lues maligna) and concurrent infection with HIV. Int J Dermatol. 1995;34:403-407.
- Tucker JD, Shah S, Jarell AD, et al. Lues maligna in early HIV infection case report and review of the literature. Sex Transm Dis. 2009;36:512-514.

A 30-year-old man who had contracted human immunodeficiency virus from a male sexual partner 4 years prior presented to the emergency department with fevers, chills, night sweats, and rhinorrhea of 2 weeks' duration. He reported that he had been off highly active antiretroviral therapy for 2 years. Physical examination revealed numerous erythematous, papulonecrotic, crusted lesions on the face, neck, chest, back, arms, and legs that had developed over the past 4 days. Fluid-filled vesicles also were noted on the arms and legs, while erythematous, indurated nodules with overlying scaling were noted on the bilateral palms and soles. The patient reported that he had been vaccinated for varicella-zoster virus as a child without primary infection.
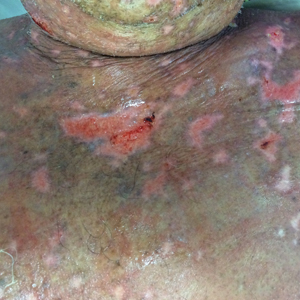
A Recalcitrant Case of Toxic Epidermal Necrolysis
One of the most severe complications of systemic medications is the development of a life-threatening rash, especially toxic epidermal necrolysis (TEN). Most patients can expect a full recovery if the complicating medication is discontinued early on in its course.1 When suspected TEN does not improve despite discontinuation of the detrimental medication, other diseases must be considered, particularly immunobullous and infectious etiologies. Treatment of these diseases differs substantially; therefore, a quick diagnosis is crucial. We present a case of a patient with an acute blistering eruption that was initially diagnosed and managed as TEN but physical examination and histopathologic confirmed another diagnosis. We review key examination findings that can help differentiate the causes of an acute blistering eruption with mucosal involvement, allowing for earlier diagnosis and treatment of these patients.
Case Report
An 85-year-old immunocompetent man was admitted to an outside hospital with a pruritic blistering eruption associated with myalgia, weakness, and fatigue of 3 weeks’ duration. The eruption initiated on the scalp and face and then spread down to the trunk and proximal arms and legs, with oral erosions also reported. An outside dermatologist was consulted on admission and performed a skin biopsy; the initial pathology was read as TEN. The patient was admitted to our institution on the same day, and all potentially complicating medications were stopped. He was treated with intravenous (IV)
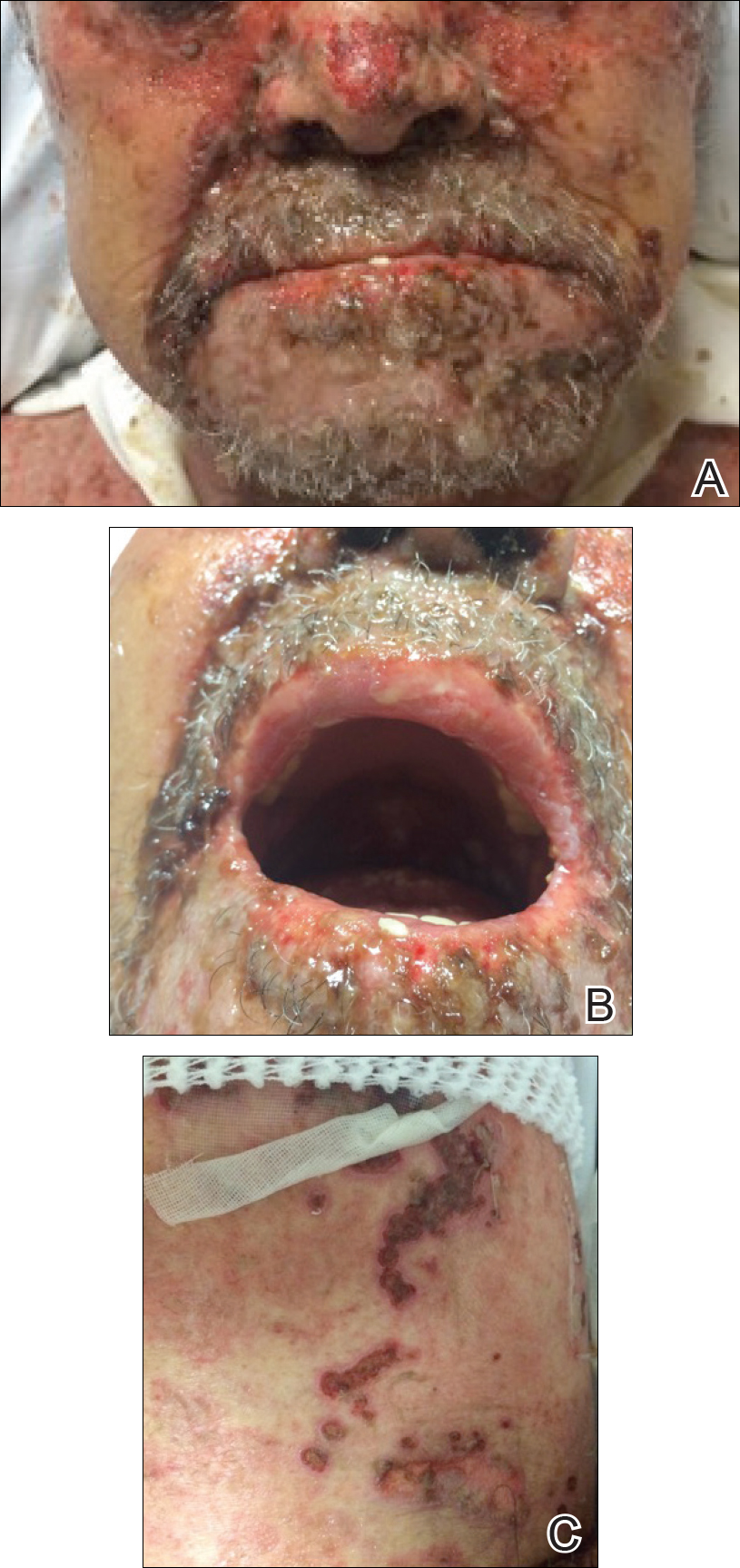
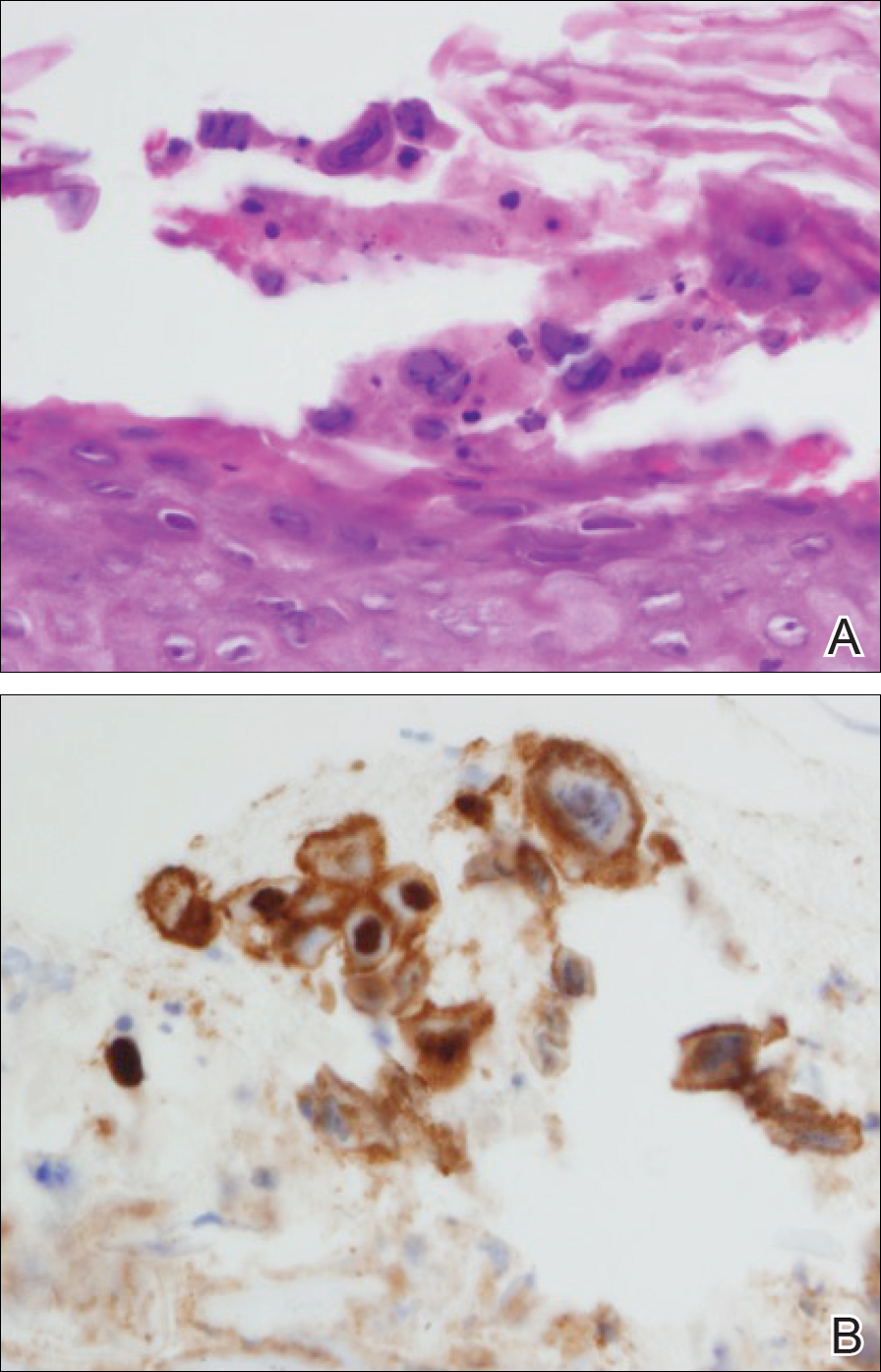
At that time, physical examination revealed numerous confluent erosions with honey-colored crust involving the entire face (Figure 1A) and sharp demarcation at the cutaneous lip (Figure 1B). There was a large erosion on the dorsal aspect of the tongue, but the rest of the oral mucosa was spared. The trunk and proximal extremities showed numerous grouped, punched-out erosions with scalloped borders (Figure 1C). A repeat skin biopsy showed an ulcer with viral cytopathic changes. Immunoperoxidase studies demonstrated positive staining for herpes simplex virus (HSV) type 1 (Figure 2). The original slides were a frozen section from an outside facility and could not be obtained. A tissue culture and direct fluorescent antibody also confirmed HSV-1, and the patient was diagnosed with disseminated herpes. He was rapidly tapered off of the steroids and started on IV acyclovir 10 mg/kg every 8 hours for 21 days. All prior erosions reepithelialized within 7 days of treatment (Figure 3). The patient had an otherwise uncomplicated hospital course and was discharged on hospital day 21.
Comment
A patient with an acute generalized blistering eruption requires urgent workup and treatment given the potentially devastating sequelae. Toxic epidermal necrolysis and immunobullous diseases often are the first diagnoses to be ruled out. Certainly infections such as HSV can cause a vesicular and erosive eruption, especially in the setting of a poorly controlled dermatitis, but they typically are not in the same differential as the other diagnoses.
Clinical Presentation
This case highlights 2 key physical examination findings that can alert the clinician to a possible underlying herpetic infection. First, the distribution of this patient’s oral lesions was telling. In most cases of TEN or pemphigus vulgaris, there is notable involvement of the oral mucosa, particularly the buccal and labial mucosa. Although herpes can involve any mucocutaneous surface, it does have a predilection for keratinized tissue, with the tongue and cutaneous lip commonly involved.2,3 Our patient had a solitary linear erosion on the dorsal aspect of the tongue, but the rest of the oral cavity was strikingly spared. In addition, the erosions around the mouth stopped right at the cutaneous lip, sparing the labial mucosa (Figure 1B).
Second, the configuration of the erosions on the trunk, arms, and legs was diagnostic. Herpes classically presents as a cluster of vesicles overlying an erythematous base. When these vesicles rupture, punched-out erosions are left behind. Because these vesicles often are grouped, they can develop a scalloped border, which is a helpful indicator of HSV (Figure 1C). When these erosions become more confluent and irregular, the distinction from other conditions may not be as clear. A careful skin examination often can show areas that have preserved this herpetiform configuration.
Immune Compromise
Additionally, this case is illustrative of how immunosuppression and immunocompromise can affect the clinical presentation of HSV infection. Herpetic infections in the immunocompromised host tend to have a more protracted course, with chronic enlarging ulcers involving multiple sites.
Conclusion
This case is a good reminder that not everything that blisters and involves the mucosa is due to a hypersensitivity state such as TEN and Stevens-Johnson syndrome or an immunobullous disorder such as pemphigus vulgaris and pemphigus vegetans. The fact that this patient was worsening despite drug cessation, high-dose steroids, and IV immunoglobulin should have indicated a misdiagnosis. This case also shows that the early histopathologic findings of disseminated HSV and TEN can be nonspecific, and viral cytopathic changes may not always be obvious early in the disease.
Disseminated HSV should be considered in the differential diagnosis of a patient with an acute blistering eruption with mucosal involvement, and careful history and physical examination should be taken to rule out a viral etiology.
- Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: part I. introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J Am Acad Dermatol. 2013;69:173.e1-173.e13.
- Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. New York, NY: Mosby; 2008.
- Woo SB, Lee SF. Oral recrudescent herpes simplex virus infection. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:239-243.
One of the most severe complications of systemic medications is the development of a life-threatening rash, especially toxic epidermal necrolysis (TEN). Most patients can expect a full recovery if the complicating medication is discontinued early on in its course.1 When suspected TEN does not improve despite discontinuation of the detrimental medication, other diseases must be considered, particularly immunobullous and infectious etiologies. Treatment of these diseases differs substantially; therefore, a quick diagnosis is crucial. We present a case of a patient with an acute blistering eruption that was initially diagnosed and managed as TEN but physical examination and histopathologic confirmed another diagnosis. We review key examination findings that can help differentiate the causes of an acute blistering eruption with mucosal involvement, allowing for earlier diagnosis and treatment of these patients.
Case Report
An 85-year-old immunocompetent man was admitted to an outside hospital with a pruritic blistering eruption associated with myalgia, weakness, and fatigue of 3 weeks’ duration. The eruption initiated on the scalp and face and then spread down to the trunk and proximal arms and legs, with oral erosions also reported. An outside dermatologist was consulted on admission and performed a skin biopsy; the initial pathology was read as TEN. The patient was admitted to our institution on the same day, and all potentially complicating medications were stopped. He was treated with intravenous (IV)
At that time, physical examination revealed numerous confluent erosions with honey-colored crust involving the entire face (Figure 1A) and sharp demarcation at the cutaneous lip (Figure 1B). There was a large erosion on the dorsal aspect of the tongue, but the rest of the oral mucosa was spared. The trunk and proximal extremities showed numerous grouped, punched-out erosions with scalloped borders (Figure 1C). A repeat skin biopsy showed an ulcer with viral cytopathic changes. Immunoperoxidase studies demonstrated positive staining for herpes simplex virus (HSV) type 1 (Figure 2). The original slides were a frozen section from an outside facility and could not be obtained. A tissue culture and direct fluorescent antibody also confirmed HSV-1, and the patient was diagnosed with disseminated herpes. He was rapidly tapered off of the steroids and started on IV acyclovir 10 mg/kg every 8 hours for 21 days. All prior erosions reepithelialized within 7 days of treatment (Figure 3). The patient had an otherwise uncomplicated hospital course and was discharged on hospital day 21.



Comment
A patient with an acute generalized blistering eruption requires urgent workup and treatment given the potentially devastating sequelae. Toxic epidermal necrolysis and immunobullous diseases often are the first diagnoses to be ruled out. Certainly infections such as HSV can cause a vesicular and erosive eruption, especially in the setting of a poorly controlled dermatitis, but they typically are not in the same differential as the other diagnoses.
Clinical Presentation
This case highlights 2 key physical examination findings that can alert the clinician to a possible underlying herpetic infection. First, the distribution of this patient’s oral lesions was telling. In most cases of TEN or pemphigus vulgaris, there is notable involvement of the oral mucosa, particularly the buccal and labial mucosa. Although herpes can involve any mucocutaneous surface, it does have a predilection for keratinized tissue, with the tongue and cutaneous lip commonly involved.2,3 Our patient had a solitary linear erosion on the dorsal aspect of the tongue, but the rest of the oral cavity was strikingly spared. In addition, the erosions around the mouth stopped right at the cutaneous lip, sparing the labial mucosa (Figure 1B).
Second, the configuration of the erosions on the trunk, arms, and legs was diagnostic. Herpes classically presents as a cluster of vesicles overlying an erythematous base. When these vesicles rupture, punched-out erosions are left behind. Because these vesicles often are grouped, they can develop a scalloped border, which is a helpful indicator of HSV (Figure 1C). When these erosions become more confluent and irregular, the distinction from other conditions may not be as clear. A careful skin examination often can show areas that have preserved this herpetiform configuration.
Immune Compromise
Additionally, this case is illustrative of how immunosuppression and immunocompromise can affect the clinical presentation of HSV infection. Herpetic infections in the immunocompromised host tend to have a more protracted course, with chronic enlarging ulcers involving multiple sites.
Conclusion
This case is a good reminder that not everything that blisters and involves the mucosa is due to a hypersensitivity state such as TEN and Stevens-Johnson syndrome or an immunobullous disorder such as pemphigus vulgaris and pemphigus vegetans. The fact that this patient was worsening despite drug cessation, high-dose steroids, and IV immunoglobulin should have indicated a misdiagnosis. This case also shows that the early histopathologic findings of disseminated HSV and TEN can be nonspecific, and viral cytopathic changes may not always be obvious early in the disease.
Disseminated HSV should be considered in the differential diagnosis of a patient with an acute blistering eruption with mucosal involvement, and careful history and physical examination should be taken to rule out a viral etiology.
One of the most severe complications of systemic medications is the development of a life-threatening rash, especially toxic epidermal necrolysis (TEN). Most patients can expect a full recovery if the complicating medication is discontinued early on in its course.1 When suspected TEN does not improve despite discontinuation of the detrimental medication, other diseases must be considered, particularly immunobullous and infectious etiologies. Treatment of these diseases differs substantially; therefore, a quick diagnosis is crucial. We present a case of a patient with an acute blistering eruption that was initially diagnosed and managed as TEN but physical examination and histopathologic confirmed another diagnosis. We review key examination findings that can help differentiate the causes of an acute blistering eruption with mucosal involvement, allowing for earlier diagnosis and treatment of these patients.
Case Report
An 85-year-old immunocompetent man was admitted to an outside hospital with a pruritic blistering eruption associated with myalgia, weakness, and fatigue of 3 weeks’ duration. The eruption initiated on the scalp and face and then spread down to the trunk and proximal arms and legs, with oral erosions also reported. An outside dermatologist was consulted on admission and performed a skin biopsy; the initial pathology was read as TEN. The patient was admitted to our institution on the same day, and all potentially complicating medications were stopped. He was treated with intravenous (IV)
At that time, physical examination revealed numerous confluent erosions with honey-colored crust involving the entire face (Figure 1A) and sharp demarcation at the cutaneous lip (Figure 1B). There was a large erosion on the dorsal aspect of the tongue, but the rest of the oral mucosa was spared. The trunk and proximal extremities showed numerous grouped, punched-out erosions with scalloped borders (Figure 1C). A repeat skin biopsy showed an ulcer with viral cytopathic changes. Immunoperoxidase studies demonstrated positive staining for herpes simplex virus (HSV) type 1 (Figure 2). The original slides were a frozen section from an outside facility and could not be obtained. A tissue culture and direct fluorescent antibody also confirmed HSV-1, and the patient was diagnosed with disseminated herpes. He was rapidly tapered off of the steroids and started on IV acyclovir 10 mg/kg every 8 hours for 21 days. All prior erosions reepithelialized within 7 days of treatment (Figure 3). The patient had an otherwise uncomplicated hospital course and was discharged on hospital day 21.



Comment
A patient with an acute generalized blistering eruption requires urgent workup and treatment given the potentially devastating sequelae. Toxic epidermal necrolysis and immunobullous diseases often are the first diagnoses to be ruled out. Certainly infections such as HSV can cause a vesicular and erosive eruption, especially in the setting of a poorly controlled dermatitis, but they typically are not in the same differential as the other diagnoses.
Clinical Presentation
This case highlights 2 key physical examination findings that can alert the clinician to a possible underlying herpetic infection. First, the distribution of this patient’s oral lesions was telling. In most cases of TEN or pemphigus vulgaris, there is notable involvement of the oral mucosa, particularly the buccal and labial mucosa. Although herpes can involve any mucocutaneous surface, it does have a predilection for keratinized tissue, with the tongue and cutaneous lip commonly involved.2,3 Our patient had a solitary linear erosion on the dorsal aspect of the tongue, but the rest of the oral cavity was strikingly spared. In addition, the erosions around the mouth stopped right at the cutaneous lip, sparing the labial mucosa (Figure 1B).
Second, the configuration of the erosions on the trunk, arms, and legs was diagnostic. Herpes classically presents as a cluster of vesicles overlying an erythematous base. When these vesicles rupture, punched-out erosions are left behind. Because these vesicles often are grouped, they can develop a scalloped border, which is a helpful indicator of HSV (Figure 1C). When these erosions become more confluent and irregular, the distinction from other conditions may not be as clear. A careful skin examination often can show areas that have preserved this herpetiform configuration.
Immune Compromise
Additionally, this case is illustrative of how immunosuppression and immunocompromise can affect the clinical presentation of HSV infection. Herpetic infections in the immunocompromised host tend to have a more protracted course, with chronic enlarging ulcers involving multiple sites.
Conclusion
This case is a good reminder that not everything that blisters and involves the mucosa is due to a hypersensitivity state such as TEN and Stevens-Johnson syndrome or an immunobullous disorder such as pemphigus vulgaris and pemphigus vegetans. The fact that this patient was worsening despite drug cessation, high-dose steroids, and IV immunoglobulin should have indicated a misdiagnosis. This case also shows that the early histopathologic findings of disseminated HSV and TEN can be nonspecific, and viral cytopathic changes may not always be obvious early in the disease.
Disseminated HSV should be considered in the differential diagnosis of a patient with an acute blistering eruption with mucosal involvement, and careful history and physical examination should be taken to rule out a viral etiology.
- Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: part I. introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J Am Acad Dermatol. 2013;69:173.e1-173.e13.
- Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. New York, NY: Mosby; 2008.
- Woo SB, Lee SF. Oral recrudescent herpes simplex virus infection. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:239-243.
- Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: part I. introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J Am Acad Dermatol. 2013;69:173.e1-173.e13.
- Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. New York, NY: Mosby; 2008.
- Woo SB, Lee SF. Oral recrudescent herpes simplex virus infection. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:239-243.
Practice Points
- Toxic epidermal necrolysis can be difficult to diagnose and treat.
- Patients who are refractory to treatment should prompt further management considerations.
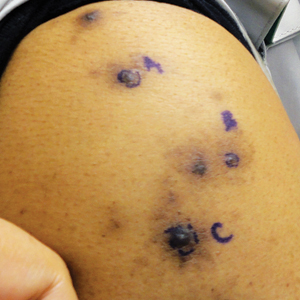
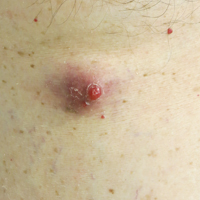
Brown-Black Papulonodules on the Arm
The Diagnosis: Glochid Dermatitis
Biopsy of a nodule on the upper right arm showed chronic granulomatous inflammation and polarizable foreign material consistent with plant cellulose (Figure). A diagnosis of glochid dermatitis was made. The treatment plan included follow-up skin evaluation and punch excision of persistent papules 1 month after the initial presentation. The patient reported the rash began after he fell on a cactus plant while chasing his grandson. He was seen by various clinicians and was given hydrocortisone and clobetasol, which helped with pruritis but did not resolve the rash. His grandson developed a similar rash at the site of contact with the cactus plant. The patient and his grandson did not detect the presence of any cactus spines.
Injuries from cactus glochids most often occur due to accidental falls on cactus plants, but glochids also may be transferred from clothing to other individuals. The thin, hairlike glochids easily detach from the stem of the cactus and can become deeply embedded with virtually no pressure.1
Glochid implantation from the prickly pear cactus commonly presents as a pruritic papular eruption known as glochid dermatitis. These penetrating injuries can lead to inoculation of Clostridium tetani and Staphylococcus aureus. Additionally, unrecognized and unremoved cactus spines may be highly inflammatory and may cause chronic granulomatous inflammation.2
Initially, acute glochid dermatitis occurs due to mechanical damage caused by the detatched cactus spine and may not resolve for up to 4 months. Granuloma formation has been reported several weeks after exposure and may persist for more than 8 months.3 Although an immune mechanism has been suggested, the literature has indicated that delayed hypersensitivity reactions are a more probable cause of the granulomatous inflammation after glochid exposure.3 Madkan et al4 reported that relatively few patients developed granulomas after implantation of glochids in the skin, thus suggesting that granuloma formation is an allergic response.
With regard to the pathogenesis of glochid dermatitis, the initial response to foreign plant matter in the dermis involves a neutrophilic infiltrate, which later is replaced by histiocytes; however, the foreign material remains undegraded in the macrophage cytoplasm.5 Activated macrophages secrete cytokines that intensify the inflammatory response, resulting in formation of a granuloma around the foreign body. The granuloma acts as a wall to isolate the foreign matter from the rest of the body.5
Regarding treatment of chronic granulomas, Madkan et al4 reported a case that showed some improvement with clobetasol ointment; however, clinical lesions resolved only after punch biopsies were performed to confirm the diagnosis of cactus spine granuloma. In a controlled study in rabbits, glochids were successfully removed by first detaching the larger clumps with tweezers then applying glue and gauze to the affected area.6 After the glue dried, the gauze was peeled off, resulting in the removal of 95% of the implanted glochids. Overall, removal of embedded spines is difficult because the glochids typically radiate in several directions.7 Treatment of foreign body granulomas caused by cactus spines can be achieved by expulsion of plant matter remnants and symptomatic treatment using midpotency topical steroids twice daily.4 Uncovering and performing punch biopsies of papules also can result in rapid healing of the lesions. Without manual removal of the glochid, lesions can persist for 2 to 8 months until gradual resolution with possible postinflammatory hyperpigmentation.4
- Suzuki H, Baba S. Cactus granuloma of the skin. J Dermatol. 1993;20:424-427.
- Suárez A, Freeman S, Puls L, et al. Unusual presentation of cactus spines in the flank of an elderly man: a case report. J Med Case Rep. 2010;4:152.
- Spoerke DG, Spoerke SE. Granuloma formation induced by spines of the cactus, Opuntia acanthocarpa. Vet Hum Toxicol. 1991;33:342-344.
- Madkan VK, Abraham T, Lesher JL Jr. Cactus spine granuloma. Cutis. 2007;79:208-210.
- Molina-Ruiz AM, Requena L. Foreign body granulomas. Dermatol Clin. 2015;33:497-523.
- McGovern TW, Barkley TM. Botanical dermatology. Int J Dermatol. 1998;37:321-334.
- Lindsey D, Lindsey WE. Cactus spine injuries. Am J Emerg Med. 1988;6:362-369.
The Diagnosis: Glochid Dermatitis
Biopsy of a nodule on the upper right arm showed chronic granulomatous inflammation and polarizable foreign material consistent with plant cellulose (Figure). A diagnosis of glochid dermatitis was made. The treatment plan included follow-up skin evaluation and punch excision of persistent papules 1 month after the initial presentation. The patient reported the rash began after he fell on a cactus plant while chasing his grandson. He was seen by various clinicians and was given hydrocortisone and clobetasol, which helped with pruritis but did not resolve the rash. His grandson developed a similar rash at the site of contact with the cactus plant. The patient and his grandson did not detect the presence of any cactus spines.

Injuries from cactus glochids most often occur due to accidental falls on cactus plants, but glochids also may be transferred from clothing to other individuals. The thin, hairlike glochids easily detach from the stem of the cactus and can become deeply embedded with virtually no pressure.1
Glochid implantation from the prickly pear cactus commonly presents as a pruritic papular eruption known as glochid dermatitis. These penetrating injuries can lead to inoculation of Clostridium tetani and Staphylococcus aureus. Additionally, unrecognized and unremoved cactus spines may be highly inflammatory and may cause chronic granulomatous inflammation.2
Initially, acute glochid dermatitis occurs due to mechanical damage caused by the detatched cactus spine and may not resolve for up to 4 months. Granuloma formation has been reported several weeks after exposure and may persist for more than 8 months.3 Although an immune mechanism has been suggested, the literature has indicated that delayed hypersensitivity reactions are a more probable cause of the granulomatous inflammation after glochid exposure.3 Madkan et al4 reported that relatively few patients developed granulomas after implantation of glochids in the skin, thus suggesting that granuloma formation is an allergic response.
With regard to the pathogenesis of glochid dermatitis, the initial response to foreign plant matter in the dermis involves a neutrophilic infiltrate, which later is replaced by histiocytes; however, the foreign material remains undegraded in the macrophage cytoplasm.5 Activated macrophages secrete cytokines that intensify the inflammatory response, resulting in formation of a granuloma around the foreign body. The granuloma acts as a wall to isolate the foreign matter from the rest of the body.5
Regarding treatment of chronic granulomas, Madkan et al4 reported a case that showed some improvement with clobetasol ointment; however, clinical lesions resolved only after punch biopsies were performed to confirm the diagnosis of cactus spine granuloma. In a controlled study in rabbits, glochids were successfully removed by first detaching the larger clumps with tweezers then applying glue and gauze to the affected area.6 After the glue dried, the gauze was peeled off, resulting in the removal of 95% of the implanted glochids. Overall, removal of embedded spines is difficult because the glochids typically radiate in several directions.7 Treatment of foreign body granulomas caused by cactus spines can be achieved by expulsion of plant matter remnants and symptomatic treatment using midpotency topical steroids twice daily.4 Uncovering and performing punch biopsies of papules also can result in rapid healing of the lesions. Without manual removal of the glochid, lesions can persist for 2 to 8 months until gradual resolution with possible postinflammatory hyperpigmentation.4
The Diagnosis: Glochid Dermatitis
Biopsy of a nodule on the upper right arm showed chronic granulomatous inflammation and polarizable foreign material consistent with plant cellulose (Figure). A diagnosis of glochid dermatitis was made. The treatment plan included follow-up skin evaluation and punch excision of persistent papules 1 month after the initial presentation. The patient reported the rash began after he fell on a cactus plant while chasing his grandson. He was seen by various clinicians and was given hydrocortisone and clobetasol, which helped with pruritis but did not resolve the rash. His grandson developed a similar rash at the site of contact with the cactus plant. The patient and his grandson did not detect the presence of any cactus spines.

Injuries from cactus glochids most often occur due to accidental falls on cactus plants, but glochids also may be transferred from clothing to other individuals. The thin, hairlike glochids easily detach from the stem of the cactus and can become deeply embedded with virtually no pressure.1
Glochid implantation from the prickly pear cactus commonly presents as a pruritic papular eruption known as glochid dermatitis. These penetrating injuries can lead to inoculation of Clostridium tetani and Staphylococcus aureus. Additionally, unrecognized and unremoved cactus spines may be highly inflammatory and may cause chronic granulomatous inflammation.2
Initially, acute glochid dermatitis occurs due to mechanical damage caused by the detatched cactus spine and may not resolve for up to 4 months. Granuloma formation has been reported several weeks after exposure and may persist for more than 8 months.3 Although an immune mechanism has been suggested, the literature has indicated that delayed hypersensitivity reactions are a more probable cause of the granulomatous inflammation after glochid exposure.3 Madkan et al4 reported that relatively few patients developed granulomas after implantation of glochids in the skin, thus suggesting that granuloma formation is an allergic response.
With regard to the pathogenesis of glochid dermatitis, the initial response to foreign plant matter in the dermis involves a neutrophilic infiltrate, which later is replaced by histiocytes; however, the foreign material remains undegraded in the macrophage cytoplasm.5 Activated macrophages secrete cytokines that intensify the inflammatory response, resulting in formation of a granuloma around the foreign body. The granuloma acts as a wall to isolate the foreign matter from the rest of the body.5
Regarding treatment of chronic granulomas, Madkan et al4 reported a case that showed some improvement with clobetasol ointment; however, clinical lesions resolved only after punch biopsies were performed to confirm the diagnosis of cactus spine granuloma. In a controlled study in rabbits, glochids were successfully removed by first detaching the larger clumps with tweezers then applying glue and gauze to the affected area.6 After the glue dried, the gauze was peeled off, resulting in the removal of 95% of the implanted glochids. Overall, removal of embedded spines is difficult because the glochids typically radiate in several directions.7 Treatment of foreign body granulomas caused by cactus spines can be achieved by expulsion of plant matter remnants and symptomatic treatment using midpotency topical steroids twice daily.4 Uncovering and performing punch biopsies of papules also can result in rapid healing of the lesions. Without manual removal of the glochid, lesions can persist for 2 to 8 months until gradual resolution with possible postinflammatory hyperpigmentation.4
- Suzuki H, Baba S. Cactus granuloma of the skin. J Dermatol. 1993;20:424-427.
- Suárez A, Freeman S, Puls L, et al. Unusual presentation of cactus spines in the flank of an elderly man: a case report. J Med Case Rep. 2010;4:152.
- Spoerke DG, Spoerke SE. Granuloma formation induced by spines of the cactus, Opuntia acanthocarpa. Vet Hum Toxicol. 1991;33:342-344.
- Madkan VK, Abraham T, Lesher JL Jr. Cactus spine granuloma. Cutis. 2007;79:208-210.
- Molina-Ruiz AM, Requena L. Foreign body granulomas. Dermatol Clin. 2015;33:497-523.
- McGovern TW, Barkley TM. Botanical dermatology. Int J Dermatol. 1998;37:321-334.
- Lindsey D, Lindsey WE. Cactus spine injuries. Am J Emerg Med. 1988;6:362-369.
- Suzuki H, Baba S. Cactus granuloma of the skin. J Dermatol. 1993;20:424-427.
- Suárez A, Freeman S, Puls L, et al. Unusual presentation of cactus spines in the flank of an elderly man: a case report. J Med Case Rep. 2010;4:152.
- Spoerke DG, Spoerke SE. Granuloma formation induced by spines of the cactus, Opuntia acanthocarpa. Vet Hum Toxicol. 1991;33:342-344.
- Madkan VK, Abraham T, Lesher JL Jr. Cactus spine granuloma. Cutis. 2007;79:208-210.
- Molina-Ruiz AM, Requena L. Foreign body granulomas. Dermatol Clin. 2015;33:497-523.
- McGovern TW, Barkley TM. Botanical dermatology. Int J Dermatol. 1998;37:321-334.
- Lindsey D, Lindsey WE. Cactus spine injuries. Am J Emerg Med. 1988;6:362-369.
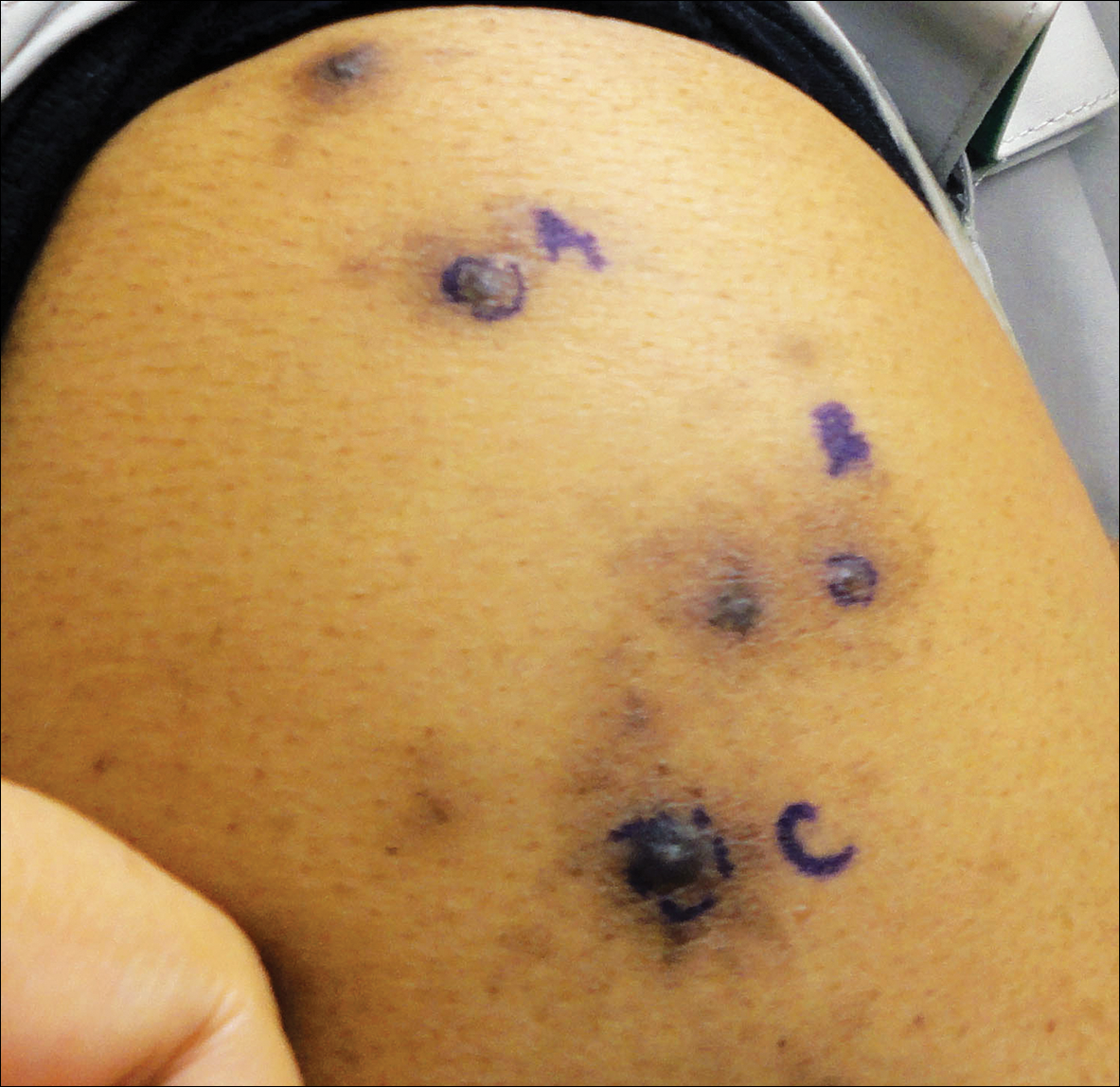
A 63-year-old man presented with a pruritic rash on the right arm of approximately 3 months' duration. On physical examination, several discrete, 4- to 5-mm, brown-black papulonodules with a central punctum were identified along the extensor aspects of the upper and lower right arm. No foreign bodies were appreciated. Biopsies of nodules on the right upper arm were performed (sites marked with letters).
Asymptomatic Subcutaneous Nodule on the Cheek
The Diagnosis: Lymphoepitheliomalike Carcinoma of the Skin
The term lymphoepitheliomalike carcinoma of the skin (LELCS) initially was proposed by Swanson et al1 in 1988 when they described 5 patients with cutaneous neoplasms histologically resembling nasopharyngeal carcinoma, also known as lymphoepithelioma. A PubMed search of articles indexed for MEDLINE using the term lymphoepitheliomalike carcinoma of the skin revealed over 60 cases of LELCS since 1988. However, unlike nasopharyngeal carcinoma, LELCS has not been associated with Epstein-Barr virus, with the exception of 1 known reported case.2 The clinical appearance of LELCS is nonspecific but usually presents as a flesh-colored to erythematous nodule, as was seen in the current case. Lesions commonly are found on the head and neck in middle-aged to elderly patients with a slight male predominance.2
On histology, LELCS is characterized by aggregations of large, atypical epithelioid cells surrounded by a dense lymphoplasmocytic infiltrate (right quiz image). The neoplasm tends to reside within the deep dermis and/or subcutis1 without appreciable epidermal involvement (left quiz image). The atypical epithelioid cells demonstrate positive immunoreactivity for cytokeratins (right quiz image inset), p40/p63, and epithelial membrane antigen,3 and the surrounding lymphocytic infiltrate stains positively for leukocyte common antigen. The tumor histogenesis still is unknown, although an epidermal origin has been suggested given its staining pattern.2 Other investigators have postulated on an adnexal origin, citing the tumor's dermal location along with case reports describing possible glandular, sebaceous, or follicular differentiation.2,4
Treatment for LELCS can include either standard surgical excision or Mohs micrographic surgery, with radiation reserved for lymph node involvement, tumor recurrence, or poor surgical candidates.2,3,5 With appropriate therapy, prognosis may be considered favorable. Data from 49 LELCS patients presenting from 1988 and 2008 showed that 36 (73.5%) had no evidence of recurrence after treatment with standard surgical excision, 4 (8.2%) had local recurrence, and 6 (12.2%) developed lymph node metastasis, which led to death in 1 (2.0%) patient.2
Given the histologic similarity of LELCS to nasopharyngeal carcinoma, it is important to rule out the possibility of cutaneous metastasis, which can be done by testing for Epstein-Barr virus and performing either computed tomography imaging or comprehensive laryngoscopic examination of the head and neck region. In the current case, the patient was referred for laryngoscopy, at which time no suspicious lesions were identified. He subsequently underwent treatment with Mohs micrographic surgery, and the tumor was cleared after 2 surgical stages. At 5-month follow-up, the patient continued to do well with no signs of clinical recurrence.
Cutaneous lymphadenoma may be included in the differential diagnosis for LELCS on histopathology. This neoplasm is characterized by a well-circumscribed dermal proliferation of basaloid tumor islands within a fibrotic stroma (Figure 1). The basaloid cells may display peripheral palisading, and lymphocytes often are seen infiltrating the tumor lobules and the surrounding stroma (Figure 1 inset). Clinically, cutaneous lymphadenomas are slowly growing nodules that typically occur in young to middle-aged patients,4,6 unlike LELCS, which is more commonly observed in middle-aged to elderly patients.2
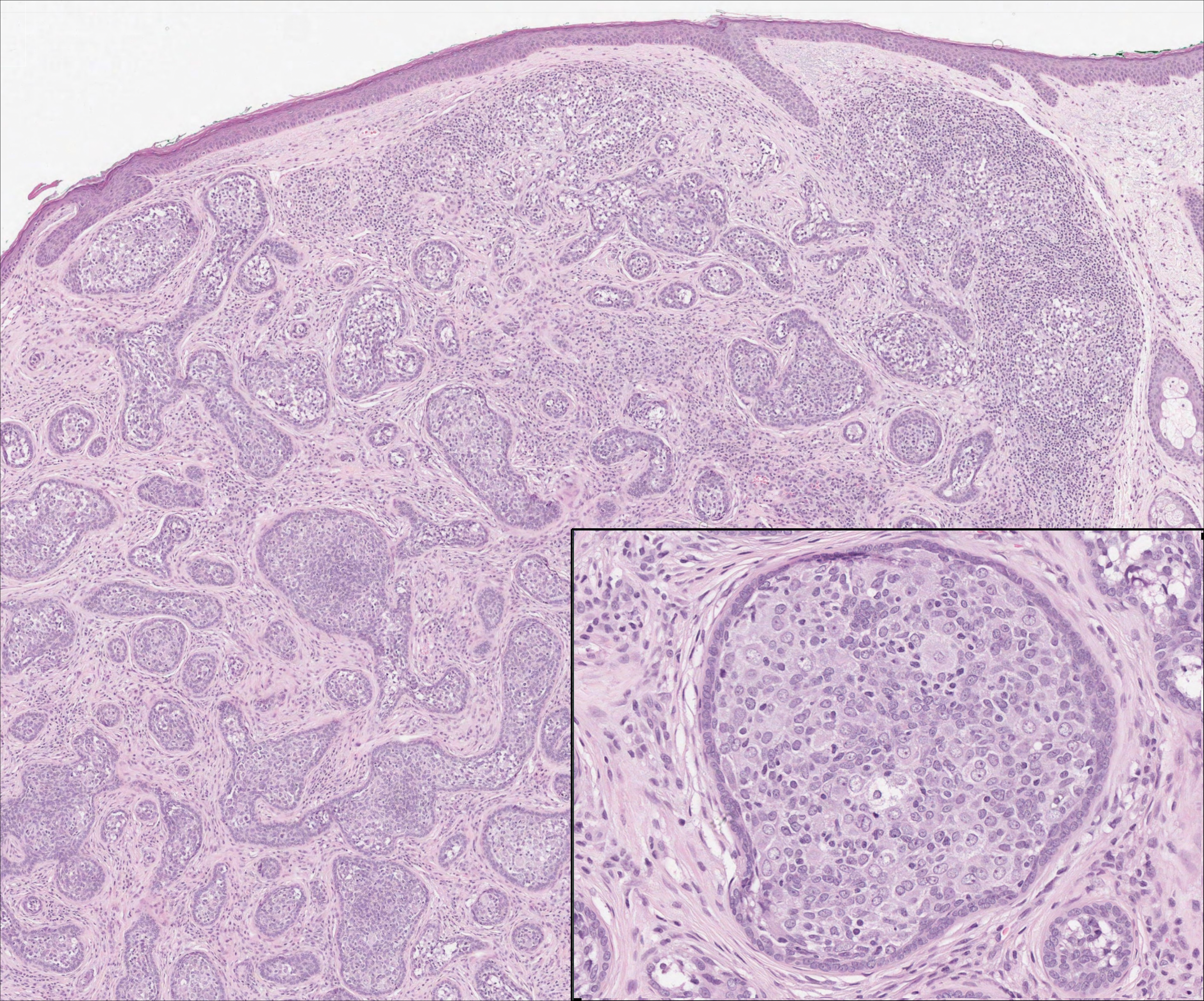
The dense lymphocytic infiltrate seen in LELCS may obscure the neoplastic epithelioid cells and in doing so may mimic a lymphoproliferative disorder, such as lymphomatoid papulosis (LyP). Lymphomatoid papulosis is a chronic CD30+ lymphoproliferative disorder consisting of recurrent crops of self-resolving papulonodules occurring on the trunk, arms, and legs. The average age of onset is in the third to fourth decades of life. Histology is dependent on the subtype; type A, the most common subtype, displays a wedge-shaped dermal infiltrate consisting of small lymphocytes (Figure 2) admixed with larger CD30+ atypical lymphocytes with prominent nucleoli (Figure 2 inset).7 Bizarre, binucleated forms resembling Reed-Sternberg cells also may be observed along with hallmark cells, which contain a horseshoe-shaped nucleus. The presence of admixed neutrophils and eosinophils also are common in type A LyP, a feature that is not characteristic of LELCS. Moreover, the atypical cells in LyP would not stain positively for epithelial markers as they would in LELCS.
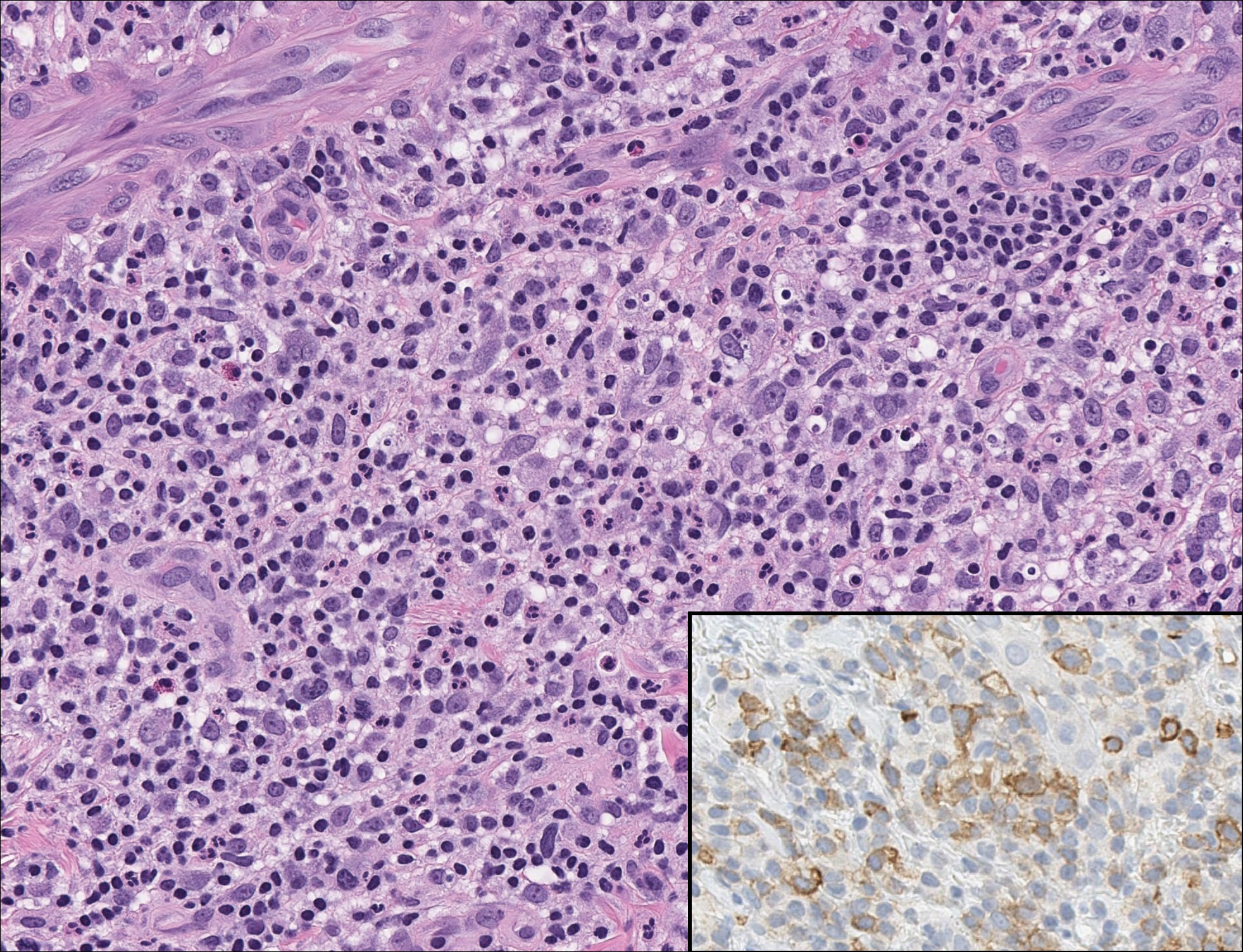
Rosai-Dorfman disease is a rare condition that usually presents with painless cervical lymphadenopathy, typically in the first and second decades of life. Skin involvement can be seen in a small subset of extranodal cases, but cutaneous involvement alone is uncommon. On histopathology, cutaneous lesions are characterized by a dense dermal infiltrate of atypical histiocytes with vesicular nuclei and pale cytoplasm admixed with inflammatory cells, including lymphocytes, neutrophils, and plasma cells (Figure 3). Intracytoplasmic inflammatory cells or emperipolesis often is appreciated (Figure 3 inset).8,9 The atypical histiocytes stain positively for S100 and negatively for CD1a.
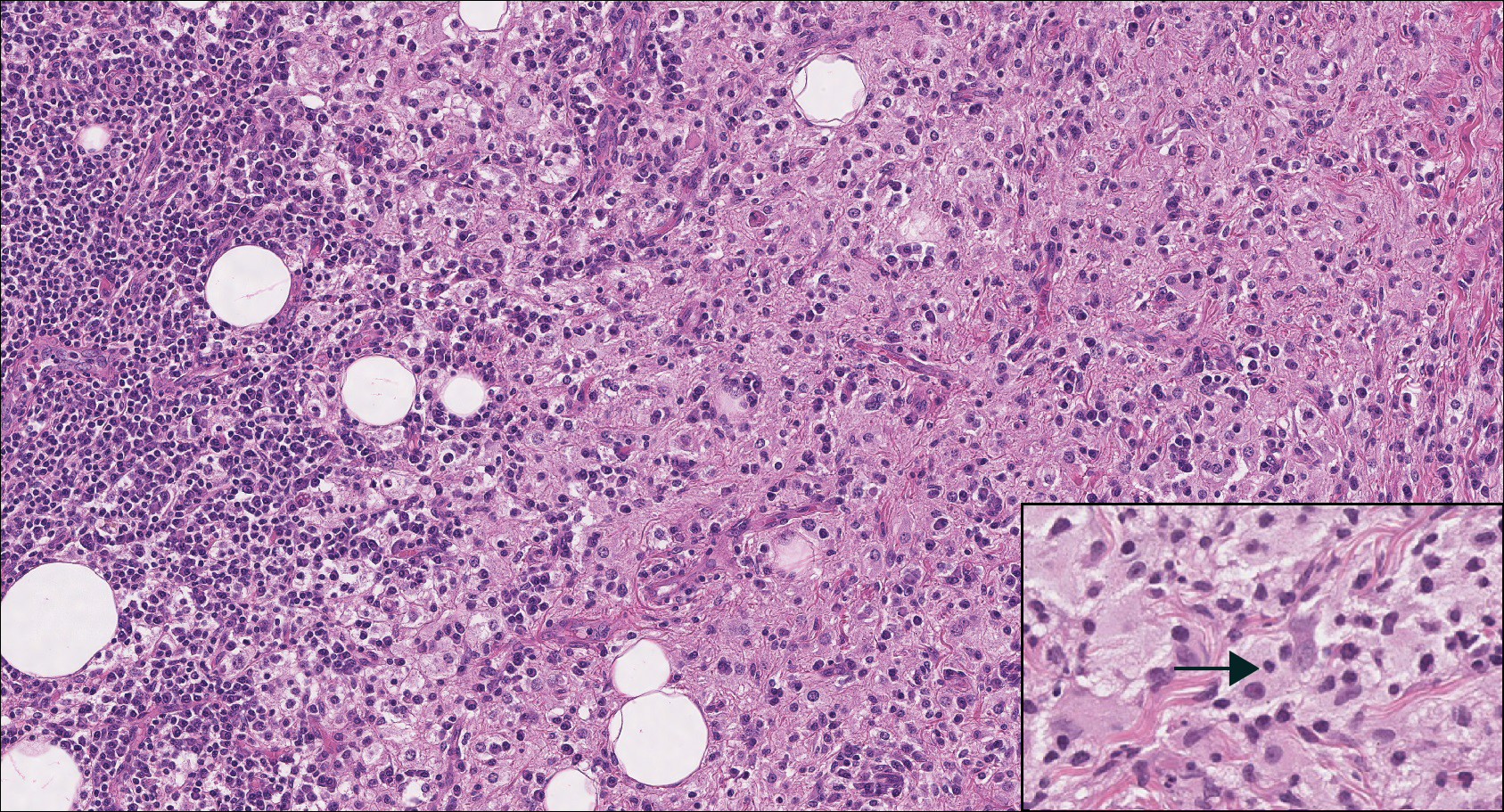
Lymphoepitheliomalike carcinoma of the skin sometimes is considered to be a poorly differentiated, inflamed variant of squamous cell carcinoma (SCC).10 A number of features may allow distinction of a primary cutaneous SCC from LELCS; for instance, SCC is more likely to have an epidermal connection and at least focal signs of squamous differentiation,11 which can include the presence of poorly differentiated epithelial cells with mitoses (Figure 4), keratin pearls, dyskeratotic cells, or intercellular bridges.12 Moreover, SCCs have a more variable surrounding inflammatory infiltrate compared to LELCS.
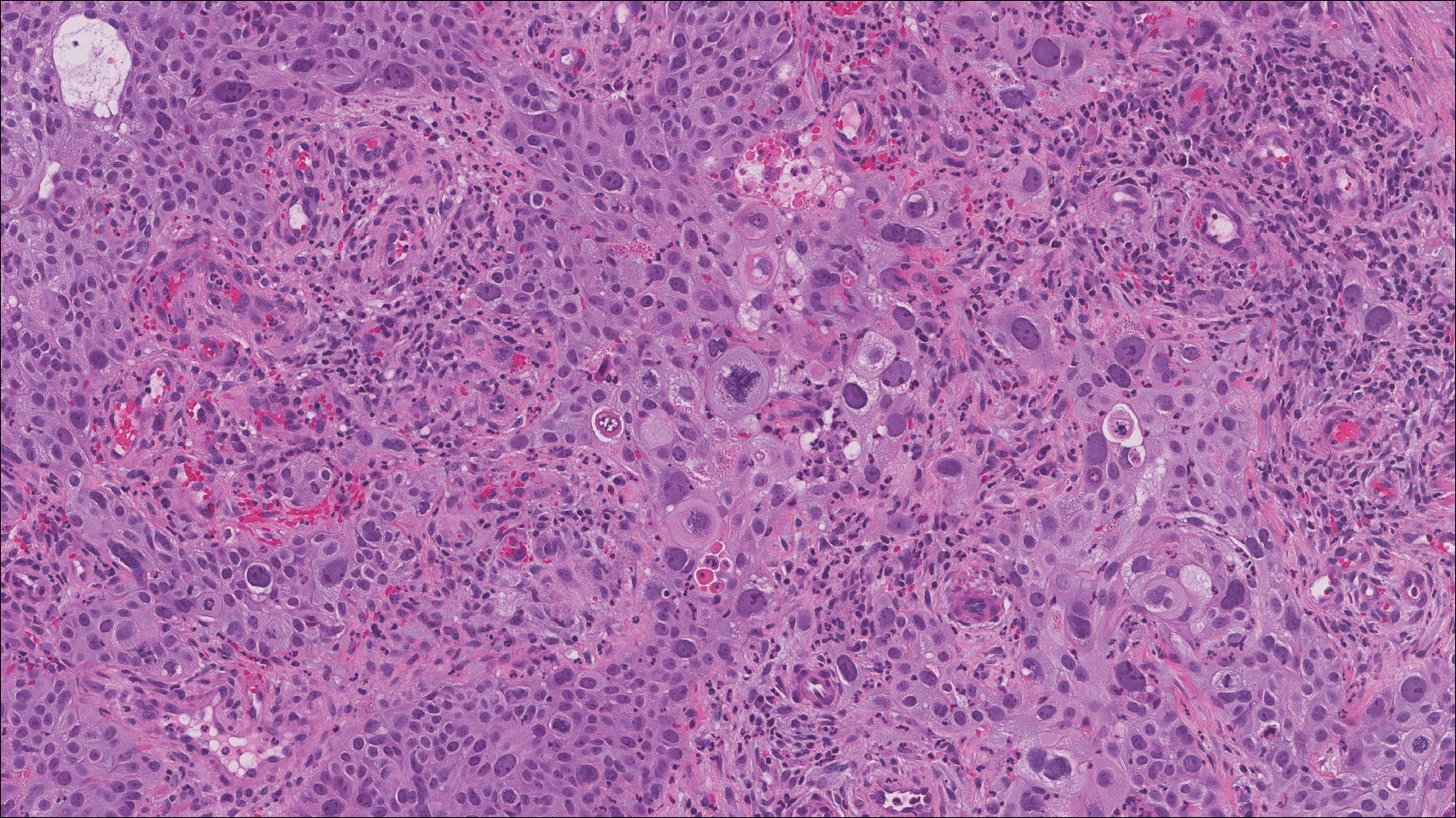
- Swanson SA, Cooper PH, Mills SE, et al. Lymphoepithelioma-like carcinoma of the skin. Mod Pathol. 1988;1:359-365.
- Aoki R, Mitsui H, Harada K, et al. A case of lymphoepithelioma-like carcinoma of the skin associated with Epstein-Barr virus infection. J Am Acad Dermatol. 2010;62:681-684.
- Morteza Abedi S, Salama S, Alowami S. Lymphoepithelioma-like carcinoma of the skin: case report and approach to surgical pathology sign out. Rare Tumors. 2013;5:E47.
- Requena L, Sánchez Yus E, Jiménez E, et al. Lymphoepithelioma-like carcinoma of the skin: a light-microscopic and immunohistochemical study. J Cutan Pathol. 1994;21:541-548.
- Welch PQ, Williams SB, Foss RD, et al. Lymphoepithelioma-like carcinoma of head and neck skin: a systematic analysis of 11 cases and review of literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:78-86.
- Santa Cruz DJ, Barr RJ, Headington JT. Cutaneous lymphadenoma. Am J Surg Pathol. 1991;15:101-110.
- Patterson JW. Cutaneous infiltrates--lymphomatous and leukemic. In: Patterson JW, Hosler GA, eds. Weedon's Skin Pathology. 4th ed. London, United Kingdom: Churchill Livingstone; 2016:1186-1189.
- Patterson JW. Cutaneous infiltrates--nonlymphoid. In: Patterson JW, Hosler GA, eds. Weedon's Skin Pathology. 4th ed. London, United Kingdom: Churchill Livingstone; 2016:1158.
- Skiljo M, Garcia-Lora E, Tercedor J, et al. Purely cutaneous Rosai-Dorfman disease. Dermatology. 1995;191:49-51.
- Wang G, Bordeaux JS, Rowe DJ, et al. Lymphoepithelioma-like carcinoma vs inflamed squamous cell carcinoma of the skin. JAMA Dermatol. 2014;150:1367-1368.
- Hall G, Duncan A, Azurdia R, et al. Lymphoepithelioma-like carcinoma of the skin: a case with lymph node metastases at presentation. Am J Dermatopathol. 2006;28:211-215.
- Lind AC, Breer WA, Wick MR. Lymphoepithelioma-like carcinoma of the skin with apparent origin in the epidermis--a pattern or an entity? a case report. Cancer. 1999;85:884-890.
The Diagnosis: Lymphoepitheliomalike Carcinoma of the Skin
The term lymphoepitheliomalike carcinoma of the skin (LELCS) initially was proposed by Swanson et al1 in 1988 when they described 5 patients with cutaneous neoplasms histologically resembling nasopharyngeal carcinoma, also known as lymphoepithelioma. A PubMed search of articles indexed for MEDLINE using the term lymphoepitheliomalike carcinoma of the skin revealed over 60 cases of LELCS since 1988. However, unlike nasopharyngeal carcinoma, LELCS has not been associated with Epstein-Barr virus, with the exception of 1 known reported case.2 The clinical appearance of LELCS is nonspecific but usually presents as a flesh-colored to erythematous nodule, as was seen in the current case. Lesions commonly are found on the head and neck in middle-aged to elderly patients with a slight male predominance.2
On histology, LELCS is characterized by aggregations of large, atypical epithelioid cells surrounded by a dense lymphoplasmocytic infiltrate (right quiz image). The neoplasm tends to reside within the deep dermis and/or subcutis1 without appreciable epidermal involvement (left quiz image). The atypical epithelioid cells demonstrate positive immunoreactivity for cytokeratins (right quiz image inset), p40/p63, and epithelial membrane antigen,3 and the surrounding lymphocytic infiltrate stains positively for leukocyte common antigen. The tumor histogenesis still is unknown, although an epidermal origin has been suggested given its staining pattern.2 Other investigators have postulated on an adnexal origin, citing the tumor's dermal location along with case reports describing possible glandular, sebaceous, or follicular differentiation.2,4
Treatment for LELCS can include either standard surgical excision or Mohs micrographic surgery, with radiation reserved for lymph node involvement, tumor recurrence, or poor surgical candidates.2,3,5 With appropriate therapy, prognosis may be considered favorable. Data from 49 LELCS patients presenting from 1988 and 2008 showed that 36 (73.5%) had no evidence of recurrence after treatment with standard surgical excision, 4 (8.2%) had local recurrence, and 6 (12.2%) developed lymph node metastasis, which led to death in 1 (2.0%) patient.2
Given the histologic similarity of LELCS to nasopharyngeal carcinoma, it is important to rule out the possibility of cutaneous metastasis, which can be done by testing for Epstein-Barr virus and performing either computed tomography imaging or comprehensive laryngoscopic examination of the head and neck region. In the current case, the patient was referred for laryngoscopy, at which time no suspicious lesions were identified. He subsequently underwent treatment with Mohs micrographic surgery, and the tumor was cleared after 2 surgical stages. At 5-month follow-up, the patient continued to do well with no signs of clinical recurrence.
Cutaneous lymphadenoma may be included in the differential diagnosis for LELCS on histopathology. This neoplasm is characterized by a well-circumscribed dermal proliferation of basaloid tumor islands within a fibrotic stroma (Figure 1). The basaloid cells may display peripheral palisading, and lymphocytes often are seen infiltrating the tumor lobules and the surrounding stroma (Figure 1 inset). Clinically, cutaneous lymphadenomas are slowly growing nodules that typically occur in young to middle-aged patients,4,6 unlike LELCS, which is more commonly observed in middle-aged to elderly patients.2

The dense lymphocytic infiltrate seen in LELCS may obscure the neoplastic epithelioid cells and in doing so may mimic a lymphoproliferative disorder, such as lymphomatoid papulosis (LyP). Lymphomatoid papulosis is a chronic CD30+ lymphoproliferative disorder consisting of recurrent crops of self-resolving papulonodules occurring on the trunk, arms, and legs. The average age of onset is in the third to fourth decades of life. Histology is dependent on the subtype; type A, the most common subtype, displays a wedge-shaped dermal infiltrate consisting of small lymphocytes (Figure 2) admixed with larger CD30+ atypical lymphocytes with prominent nucleoli (Figure 2 inset).7 Bizarre, binucleated forms resembling Reed-Sternberg cells also may be observed along with hallmark cells, which contain a horseshoe-shaped nucleus. The presence of admixed neutrophils and eosinophils also are common in type A LyP, a feature that is not characteristic of LELCS. Moreover, the atypical cells in LyP would not stain positively for epithelial markers as they would in LELCS.

Rosai-Dorfman disease is a rare condition that usually presents with painless cervical lymphadenopathy, typically in the first and second decades of life. Skin involvement can be seen in a small subset of extranodal cases, but cutaneous involvement alone is uncommon. On histopathology, cutaneous lesions are characterized by a dense dermal infiltrate of atypical histiocytes with vesicular nuclei and pale cytoplasm admixed with inflammatory cells, including lymphocytes, neutrophils, and plasma cells (Figure 3). Intracytoplasmic inflammatory cells or emperipolesis often is appreciated (Figure 3 inset).8,9 The atypical histiocytes stain positively for S100 and negatively for CD1a.

Lymphoepitheliomalike carcinoma of the skin sometimes is considered to be a poorly differentiated, inflamed variant of squamous cell carcinoma (SCC).10 A number of features may allow distinction of a primary cutaneous SCC from LELCS; for instance, SCC is more likely to have an epidermal connection and at least focal signs of squamous differentiation,11 which can include the presence of poorly differentiated epithelial cells with mitoses (Figure 4), keratin pearls, dyskeratotic cells, or intercellular bridges.12 Moreover, SCCs have a more variable surrounding inflammatory infiltrate compared to LELCS.

The Diagnosis: Lymphoepitheliomalike Carcinoma of the Skin
The term lymphoepitheliomalike carcinoma of the skin (LELCS) initially was proposed by Swanson et al1 in 1988 when they described 5 patients with cutaneous neoplasms histologically resembling nasopharyngeal carcinoma, also known as lymphoepithelioma. A PubMed search of articles indexed for MEDLINE using the term lymphoepitheliomalike carcinoma of the skin revealed over 60 cases of LELCS since 1988. However, unlike nasopharyngeal carcinoma, LELCS has not been associated with Epstein-Barr virus, with the exception of 1 known reported case.2 The clinical appearance of LELCS is nonspecific but usually presents as a flesh-colored to erythematous nodule, as was seen in the current case. Lesions commonly are found on the head and neck in middle-aged to elderly patients with a slight male predominance.2
On histology, LELCS is characterized by aggregations of large, atypical epithelioid cells surrounded by a dense lymphoplasmocytic infiltrate (right quiz image). The neoplasm tends to reside within the deep dermis and/or subcutis1 without appreciable epidermal involvement (left quiz image). The atypical epithelioid cells demonstrate positive immunoreactivity for cytokeratins (right quiz image inset), p40/p63, and epithelial membrane antigen,3 and the surrounding lymphocytic infiltrate stains positively for leukocyte common antigen. The tumor histogenesis still is unknown, although an epidermal origin has been suggested given its staining pattern.2 Other investigators have postulated on an adnexal origin, citing the tumor's dermal location along with case reports describing possible glandular, sebaceous, or follicular differentiation.2,4
Treatment for LELCS can include either standard surgical excision or Mohs micrographic surgery, with radiation reserved for lymph node involvement, tumor recurrence, or poor surgical candidates.2,3,5 With appropriate therapy, prognosis may be considered favorable. Data from 49 LELCS patients presenting from 1988 and 2008 showed that 36 (73.5%) had no evidence of recurrence after treatment with standard surgical excision, 4 (8.2%) had local recurrence, and 6 (12.2%) developed lymph node metastasis, which led to death in 1 (2.0%) patient.2
Given the histologic similarity of LELCS to nasopharyngeal carcinoma, it is important to rule out the possibility of cutaneous metastasis, which can be done by testing for Epstein-Barr virus and performing either computed tomography imaging or comprehensive laryngoscopic examination of the head and neck region. In the current case, the patient was referred for laryngoscopy, at which time no suspicious lesions were identified. He subsequently underwent treatment with Mohs micrographic surgery, and the tumor was cleared after 2 surgical stages. At 5-month follow-up, the patient continued to do well with no signs of clinical recurrence.
Cutaneous lymphadenoma may be included in the differential diagnosis for LELCS on histopathology. This neoplasm is characterized by a well-circumscribed dermal proliferation of basaloid tumor islands within a fibrotic stroma (Figure 1). The basaloid cells may display peripheral palisading, and lymphocytes often are seen infiltrating the tumor lobules and the surrounding stroma (Figure 1 inset). Clinically, cutaneous lymphadenomas are slowly growing nodules that typically occur in young to middle-aged patients,4,6 unlike LELCS, which is more commonly observed in middle-aged to elderly patients.2

The dense lymphocytic infiltrate seen in LELCS may obscure the neoplastic epithelioid cells and in doing so may mimic a lymphoproliferative disorder, such as lymphomatoid papulosis (LyP). Lymphomatoid papulosis is a chronic CD30+ lymphoproliferative disorder consisting of recurrent crops of self-resolving papulonodules occurring on the trunk, arms, and legs. The average age of onset is in the third to fourth decades of life. Histology is dependent on the subtype; type A, the most common subtype, displays a wedge-shaped dermal infiltrate consisting of small lymphocytes (Figure 2) admixed with larger CD30+ atypical lymphocytes with prominent nucleoli (Figure 2 inset).7 Bizarre, binucleated forms resembling Reed-Sternberg cells also may be observed along with hallmark cells, which contain a horseshoe-shaped nucleus. The presence of admixed neutrophils and eosinophils also are common in type A LyP, a feature that is not characteristic of LELCS. Moreover, the atypical cells in LyP would not stain positively for epithelial markers as they would in LELCS.

Rosai-Dorfman disease is a rare condition that usually presents with painless cervical lymphadenopathy, typically in the first and second decades of life. Skin involvement can be seen in a small subset of extranodal cases, but cutaneous involvement alone is uncommon. On histopathology, cutaneous lesions are characterized by a dense dermal infiltrate of atypical histiocytes with vesicular nuclei and pale cytoplasm admixed with inflammatory cells, including lymphocytes, neutrophils, and plasma cells (Figure 3). Intracytoplasmic inflammatory cells or emperipolesis often is appreciated (Figure 3 inset).8,9 The atypical histiocytes stain positively for S100 and negatively for CD1a.

Lymphoepitheliomalike carcinoma of the skin sometimes is considered to be a poorly differentiated, inflamed variant of squamous cell carcinoma (SCC).10 A number of features may allow distinction of a primary cutaneous SCC from LELCS; for instance, SCC is more likely to have an epidermal connection and at least focal signs of squamous differentiation,11 which can include the presence of poorly differentiated epithelial cells with mitoses (Figure 4), keratin pearls, dyskeratotic cells, or intercellular bridges.12 Moreover, SCCs have a more variable surrounding inflammatory infiltrate compared to LELCS.

- Swanson SA, Cooper PH, Mills SE, et al. Lymphoepithelioma-like carcinoma of the skin. Mod Pathol. 1988;1:359-365.
- Aoki R, Mitsui H, Harada K, et al. A case of lymphoepithelioma-like carcinoma of the skin associated with Epstein-Barr virus infection. J Am Acad Dermatol. 2010;62:681-684.
- Morteza Abedi S, Salama S, Alowami S. Lymphoepithelioma-like carcinoma of the skin: case report and approach to surgical pathology sign out. Rare Tumors. 2013;5:E47.
- Requena L, Sánchez Yus E, Jiménez E, et al. Lymphoepithelioma-like carcinoma of the skin: a light-microscopic and immunohistochemical study. J Cutan Pathol. 1994;21:541-548.
- Welch PQ, Williams SB, Foss RD, et al. Lymphoepithelioma-like carcinoma of head and neck skin: a systematic analysis of 11 cases and review of literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:78-86.
- Santa Cruz DJ, Barr RJ, Headington JT. Cutaneous lymphadenoma. Am J Surg Pathol. 1991;15:101-110.
- Patterson JW. Cutaneous infiltrates--lymphomatous and leukemic. In: Patterson JW, Hosler GA, eds. Weedon's Skin Pathology. 4th ed. London, United Kingdom: Churchill Livingstone; 2016:1186-1189.
- Patterson JW. Cutaneous infiltrates--nonlymphoid. In: Patterson JW, Hosler GA, eds. Weedon's Skin Pathology. 4th ed. London, United Kingdom: Churchill Livingstone; 2016:1158.
- Skiljo M, Garcia-Lora E, Tercedor J, et al. Purely cutaneous Rosai-Dorfman disease. Dermatology. 1995;191:49-51.
- Wang G, Bordeaux JS, Rowe DJ, et al. Lymphoepithelioma-like carcinoma vs inflamed squamous cell carcinoma of the skin. JAMA Dermatol. 2014;150:1367-1368.
- Hall G, Duncan A, Azurdia R, et al. Lymphoepithelioma-like carcinoma of the skin: a case with lymph node metastases at presentation. Am J Dermatopathol. 2006;28:211-215.
- Lind AC, Breer WA, Wick MR. Lymphoepithelioma-like carcinoma of the skin with apparent origin in the epidermis--a pattern or an entity? a case report. Cancer. 1999;85:884-890.
- Swanson SA, Cooper PH, Mills SE, et al. Lymphoepithelioma-like carcinoma of the skin. Mod Pathol. 1988;1:359-365.
- Aoki R, Mitsui H, Harada K, et al. A case of lymphoepithelioma-like carcinoma of the skin associated with Epstein-Barr virus infection. J Am Acad Dermatol. 2010;62:681-684.
- Morteza Abedi S, Salama S, Alowami S. Lymphoepithelioma-like carcinoma of the skin: case report and approach to surgical pathology sign out. Rare Tumors. 2013;5:E47.
- Requena L, Sánchez Yus E, Jiménez E, et al. Lymphoepithelioma-like carcinoma of the skin: a light-microscopic and immunohistochemical study. J Cutan Pathol. 1994;21:541-548.
- Welch PQ, Williams SB, Foss RD, et al. Lymphoepithelioma-like carcinoma of head and neck skin: a systematic analysis of 11 cases and review of literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:78-86.
- Santa Cruz DJ, Barr RJ, Headington JT. Cutaneous lymphadenoma. Am J Surg Pathol. 1991;15:101-110.
- Patterson JW. Cutaneous infiltrates--lymphomatous and leukemic. In: Patterson JW, Hosler GA, eds. Weedon's Skin Pathology. 4th ed. London, United Kingdom: Churchill Livingstone; 2016:1186-1189.
- Patterson JW. Cutaneous infiltrates--nonlymphoid. In: Patterson JW, Hosler GA, eds. Weedon's Skin Pathology. 4th ed. London, United Kingdom: Churchill Livingstone; 2016:1158.
- Skiljo M, Garcia-Lora E, Tercedor J, et al. Purely cutaneous Rosai-Dorfman disease. Dermatology. 1995;191:49-51.
- Wang G, Bordeaux JS, Rowe DJ, et al. Lymphoepithelioma-like carcinoma vs inflamed squamous cell carcinoma of the skin. JAMA Dermatol. 2014;150:1367-1368.
- Hall G, Duncan A, Azurdia R, et al. Lymphoepithelioma-like carcinoma of the skin: a case with lymph node metastases at presentation. Am J Dermatopathol. 2006;28:211-215.
- Lind AC, Breer WA, Wick MR. Lymphoepithelioma-like carcinoma of the skin with apparent origin in the epidermis--a pattern or an entity? a case report. Cancer. 1999;85:884-890.
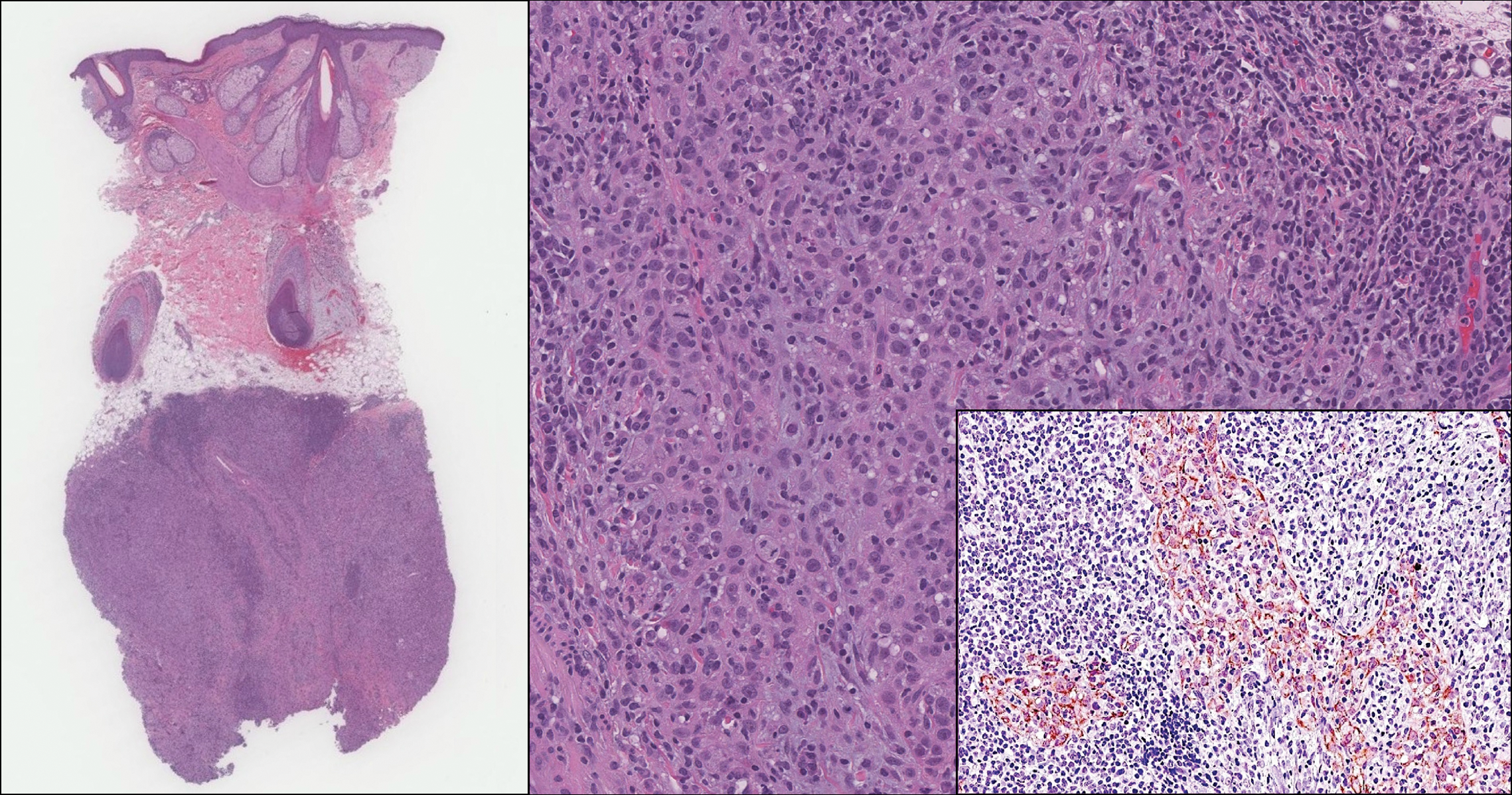
An 81-year-old man with history of melanoma and nonmelanoma skin cancer presented with a subcutaneous nodule on the left cheek of 3 months' duration. The lesion was reportedly asymptomatic and measured 2.6×2.9 cm. A punch biopsy of the lesion was obtained for histopathologic evaluation.
Asymptomatic Erythematous Plaques on the Scalp and Face
The Diagnosis: Granuloma Faciale
A biopsy from a scalp lesion showed an intense mixed inflammatory infiltrate mainly consisting of eosinophils, but lymphocytes, histiocytes, neutrophils, and plasma cells also were present. A grenz zone was observed between the dermal infiltrate and epidermis. Perivascular infiltrates were penetrating vessel walls, and hyalinization of the vessel walls also was seen (Figure 1). Direct immunofluorescence demonstrated IgG positivity on vessel walls (Figure 2). A diagnosis of granuloma faciale with extrafacial lesions was made. Twice daily application of tacrolimus ointment 0.1% was started, but after a 10-month course of treatment, there was no notable difference in the lesions.
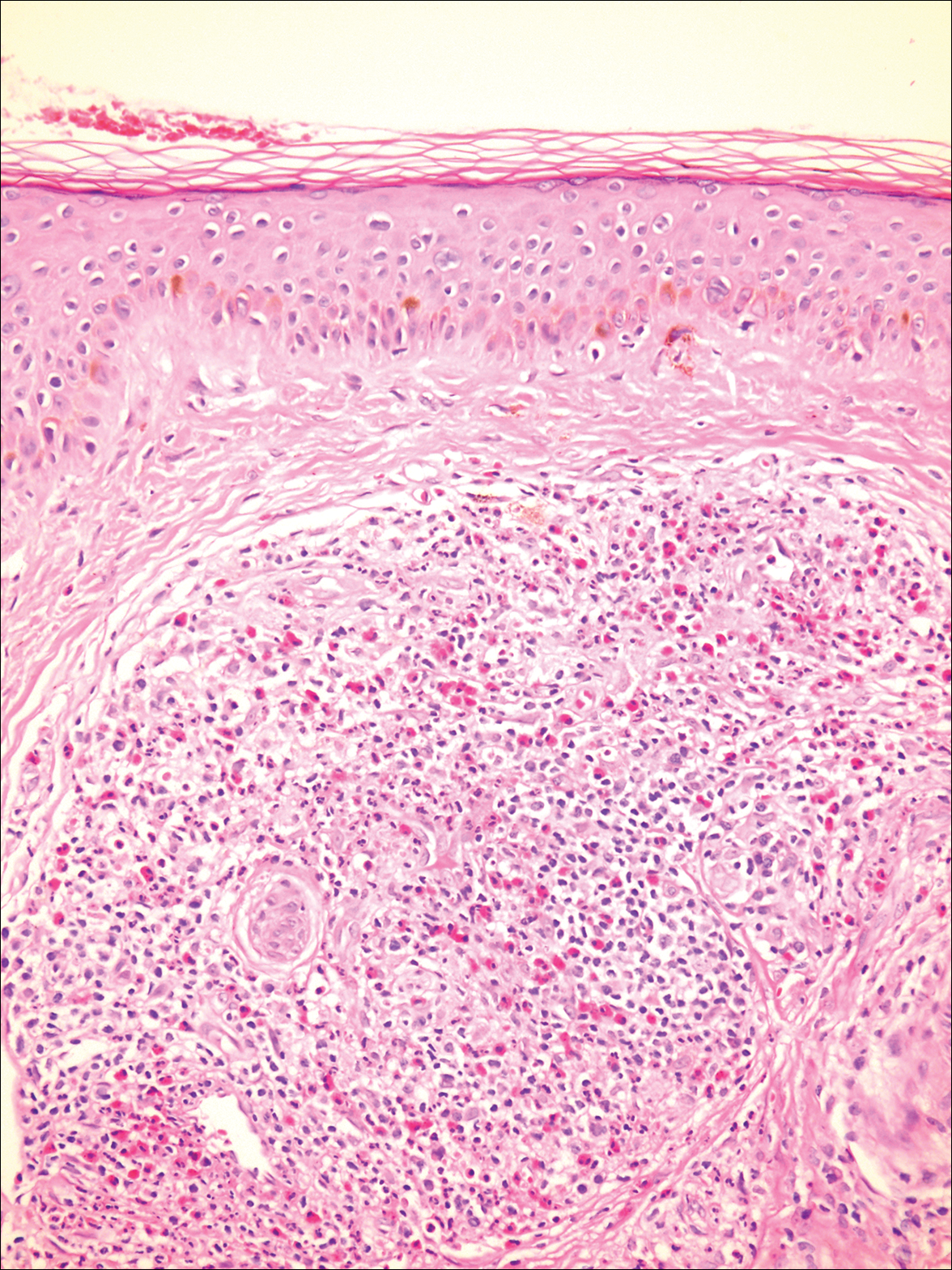
Granuloma faciale (GF) is an uncommon benign dermatosis of unknown pathogenesis characterized by erythematous, brown, or violaceous papules, plaques, or nodules. Granuloma faciale lesions can be solitary or multiple as well as disseminated and most often occur on the face. Predilection sites include the nose, periauricular area, cheeks, forehead, eyelids, and ears; however, lesions also have been reported to occur in extrafacial areas such as the trunk, arms, and legs.1-4 In our patient, multiple plaques were seen on the scalp. Facial lesions usually precede extrafacial lesions, which may present months to several years after the appearance of facial disease; however, according to our patient's history his scalp lesions appeared before the facial lesions.
The differential diagnoses for GF mainly include erythema elevatum diutinum, cutaneous sarcoidosis, cutaneous lymphoma, lupus, basal cell carcinoma, and cutaneous pseudolymphoma.5 Diagnosis may be established based on a combination of clinical features and skin biopsy results. On histopathologic examination, small-vessel vasculitis usually is present with an infiltrate predominantly consisting of neutrophils and eosinophils.6
It has been suggested that actinic damage plays a role in the etiology of GF.7 The pathogenesis is uncertain, but it is thought that immunophenotypic and molecular analysis of the dermal infiltrate in GF reveals that most lymphocytes are clonally expanded and the process is mediated by interferon gamma.7 Tacrolimus acts by binding and inactivating calcineurin and thus blocking T-cell activation and proliferation, so it is not surprising that topical tacrolimus has been shown to be useful in the management of this condition.8
- Leite I, Moreira A, Guedes R, et al. Granuloma faciale of the scalp. Dermatol Online J. 2011;17:6.
- De D, Kanwar AJ, Radotra BD, et al. Extrafacial granuloma faciale: report of a case. J Eur Acad Dermatol Venereol. 2007;21:1284-1286.
- Castellano-Howard L, Fairbee SI, Hogan DJ, et al. Extrafacial granuloma faciale: report of a case and response to treatment. Cutis. 2001;67:413-415.
- Inanir I, Alvur Y. Granuloma faciale with extrafacial lesions. Br J Dermatol. 2001;145:360-362.
- Ortonne N, Wechsler J, Bagot M, et al. Granuloma faciale: a clinicopathologic study of 66 patients. J Am Acad Dermatol. 2005;53:1002-1009.
- LeBoit PE. Granuloma faciale: a diagnosis deserving of dignity. Am J Dermatopathol. 2002;24:440-443.
- Koplon BS, Wood MG. Granuloma faciale. first reported case in a Negro. Arch Dermatol. 1967;96:188-192.
- Ludwig E, Allam JP, Bieber T, et al. New treatment modalities for granuloma faciale. Br J Dermatol. 2003;149:634-637.
The Diagnosis: Granuloma Faciale
A biopsy from a scalp lesion showed an intense mixed inflammatory infiltrate mainly consisting of eosinophils, but lymphocytes, histiocytes, neutrophils, and plasma cells also were present. A grenz zone was observed between the dermal infiltrate and epidermis. Perivascular infiltrates were penetrating vessel walls, and hyalinization of the vessel walls also was seen (Figure 1). Direct immunofluorescence demonstrated IgG positivity on vessel walls (Figure 2). A diagnosis of granuloma faciale with extrafacial lesions was made. Twice daily application of tacrolimus ointment 0.1% was started, but after a 10-month course of treatment, there was no notable difference in the lesions.


Granuloma faciale (GF) is an uncommon benign dermatosis of unknown pathogenesis characterized by erythematous, brown, or violaceous papules, plaques, or nodules. Granuloma faciale lesions can be solitary or multiple as well as disseminated and most often occur on the face. Predilection sites include the nose, periauricular area, cheeks, forehead, eyelids, and ears; however, lesions also have been reported to occur in extrafacial areas such as the trunk, arms, and legs.1-4 In our patient, multiple plaques were seen on the scalp. Facial lesions usually precede extrafacial lesions, which may present months to several years after the appearance of facial disease; however, according to our patient's history his scalp lesions appeared before the facial lesions.
The differential diagnoses for GF mainly include erythema elevatum diutinum, cutaneous sarcoidosis, cutaneous lymphoma, lupus, basal cell carcinoma, and cutaneous pseudolymphoma.5 Diagnosis may be established based on a combination of clinical features and skin biopsy results. On histopathologic examination, small-vessel vasculitis usually is present with an infiltrate predominantly consisting of neutrophils and eosinophils.6
It has been suggested that actinic damage plays a role in the etiology of GF.7 The pathogenesis is uncertain, but it is thought that immunophenotypic and molecular analysis of the dermal infiltrate in GF reveals that most lymphocytes are clonally expanded and the process is mediated by interferon gamma.7 Tacrolimus acts by binding and inactivating calcineurin and thus blocking T-cell activation and proliferation, so it is not surprising that topical tacrolimus has been shown to be useful in the management of this condition.8
The Diagnosis: Granuloma Faciale
A biopsy from a scalp lesion showed an intense mixed inflammatory infiltrate mainly consisting of eosinophils, but lymphocytes, histiocytes, neutrophils, and plasma cells also were present. A grenz zone was observed between the dermal infiltrate and epidermis. Perivascular infiltrates were penetrating vessel walls, and hyalinization of the vessel walls also was seen (Figure 1). Direct immunofluorescence demonstrated IgG positivity on vessel walls (Figure 2). A diagnosis of granuloma faciale with extrafacial lesions was made. Twice daily application of tacrolimus ointment 0.1% was started, but after a 10-month course of treatment, there was no notable difference in the lesions.


Granuloma faciale (GF) is an uncommon benign dermatosis of unknown pathogenesis characterized by erythematous, brown, or violaceous papules, plaques, or nodules. Granuloma faciale lesions can be solitary or multiple as well as disseminated and most often occur on the face. Predilection sites include the nose, periauricular area, cheeks, forehead, eyelids, and ears; however, lesions also have been reported to occur in extrafacial areas such as the trunk, arms, and legs.1-4 In our patient, multiple plaques were seen on the scalp. Facial lesions usually precede extrafacial lesions, which may present months to several years after the appearance of facial disease; however, according to our patient's history his scalp lesions appeared before the facial lesions.
The differential diagnoses for GF mainly include erythema elevatum diutinum, cutaneous sarcoidosis, cutaneous lymphoma, lupus, basal cell carcinoma, and cutaneous pseudolymphoma.5 Diagnosis may be established based on a combination of clinical features and skin biopsy results. On histopathologic examination, small-vessel vasculitis usually is present with an infiltrate predominantly consisting of neutrophils and eosinophils.6
It has been suggested that actinic damage plays a role in the etiology of GF.7 The pathogenesis is uncertain, but it is thought that immunophenotypic and molecular analysis of the dermal infiltrate in GF reveals that most lymphocytes are clonally expanded and the process is mediated by interferon gamma.7 Tacrolimus acts by binding and inactivating calcineurin and thus blocking T-cell activation and proliferation, so it is not surprising that topical tacrolimus has been shown to be useful in the management of this condition.8
- Leite I, Moreira A, Guedes R, et al. Granuloma faciale of the scalp. Dermatol Online J. 2011;17:6.
- De D, Kanwar AJ, Radotra BD, et al. Extrafacial granuloma faciale: report of a case. J Eur Acad Dermatol Venereol. 2007;21:1284-1286.
- Castellano-Howard L, Fairbee SI, Hogan DJ, et al. Extrafacial granuloma faciale: report of a case and response to treatment. Cutis. 2001;67:413-415.
- Inanir I, Alvur Y. Granuloma faciale with extrafacial lesions. Br J Dermatol. 2001;145:360-362.
- Ortonne N, Wechsler J, Bagot M, et al. Granuloma faciale: a clinicopathologic study of 66 patients. J Am Acad Dermatol. 2005;53:1002-1009.
- LeBoit PE. Granuloma faciale: a diagnosis deserving of dignity. Am J Dermatopathol. 2002;24:440-443.
- Koplon BS, Wood MG. Granuloma faciale. first reported case in a Negro. Arch Dermatol. 1967;96:188-192.
- Ludwig E, Allam JP, Bieber T, et al. New treatment modalities for granuloma faciale. Br J Dermatol. 2003;149:634-637.
- Leite I, Moreira A, Guedes R, et al. Granuloma faciale of the scalp. Dermatol Online J. 2011;17:6.
- De D, Kanwar AJ, Radotra BD, et al. Extrafacial granuloma faciale: report of a case. J Eur Acad Dermatol Venereol. 2007;21:1284-1286.
- Castellano-Howard L, Fairbee SI, Hogan DJ, et al. Extrafacial granuloma faciale: report of a case and response to treatment. Cutis. 2001;67:413-415.
- Inanir I, Alvur Y. Granuloma faciale with extrafacial lesions. Br J Dermatol. 2001;145:360-362.
- Ortonne N, Wechsler J, Bagot M, et al. Granuloma faciale: a clinicopathologic study of 66 patients. J Am Acad Dermatol. 2005;53:1002-1009.
- LeBoit PE. Granuloma faciale: a diagnosis deserving of dignity. Am J Dermatopathol. 2002;24:440-443.
- Koplon BS, Wood MG. Granuloma faciale. first reported case in a Negro. Arch Dermatol. 1967;96:188-192.
- Ludwig E, Allam JP, Bieber T, et al. New treatment modalities for granuloma faciale. Br J Dermatol. 2003;149:634-637.
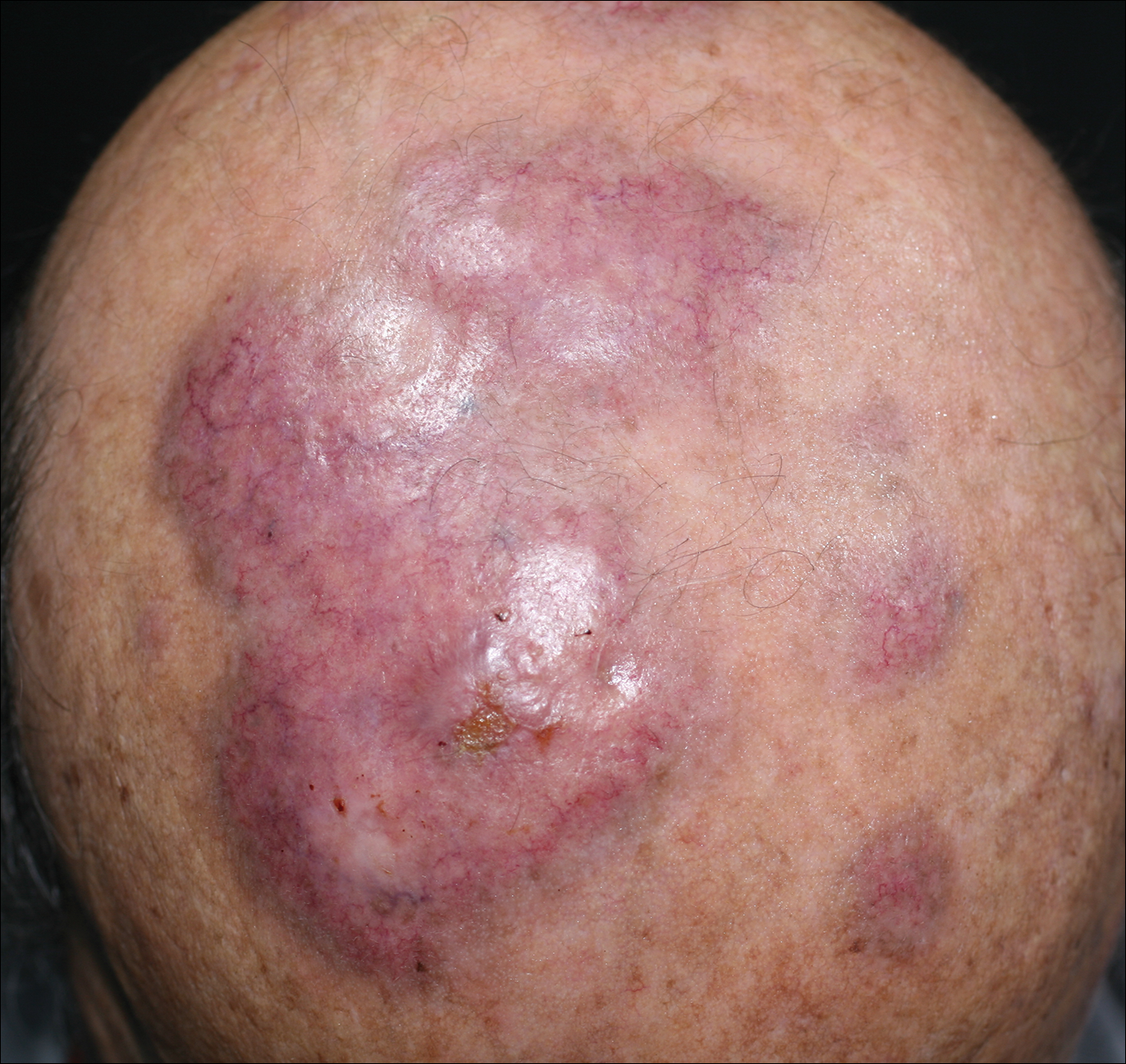
An 84-year-old man presented with gradually enlarging, asymptomatic, erythematous to violaceous plaques on the face and scalp of 11 years' duration ranging in size from 0.5×0.5 cm to 10×8 cm. The plaques were unresponsive to treatment with topical steroids. The lesions were nontender with no associated bleeding, burning, or pruritus. The patient denied any trauma to the sites or systemic symptoms. He had a history of essential hypertension and benign prostatic hyperplasia and had been taking ramipril, tamsulosin, and dutasteride for 5 years. His medical history was otherwise unremarkable, and routine laboratory findings were within normal range.
Enlarging Red Papulonodule on the Chest
The Diagnosis: Metastatic Renal Cell Carcinoma
Histopathologic examination of the punch biopsy demonstrated epithelioid cells with abundant clear cytoplasm and numerous chicken wire-like vascular channels consistent with a diagnosis of cutaneous metastasis of renal cell carcinoma (RCC)(Figure). Collateral history revealed that 8 years prior, the patient had been diagnosed with clear cell RCC, stage III (T3aN0M0). At that time, he was treated with radical nephrectomy, which was considered curative. He remained disease free until several months prior to the development of the cutaneous lesion when he was found to have pulmonary and cerebral metastases with biopsies showing metastatic RCC. He was treated with lobectomy and Gamma Knife radiation for the lung and cerebral metastases, respectively. His oncologist planned to initiate therapy with the multikinase inhibitor sunitinib, which inhibits vascular endothelial growth factor (VEGF) signaling. Unfortunately, the patient died prior to treatment due to overwhelming tumor burden.
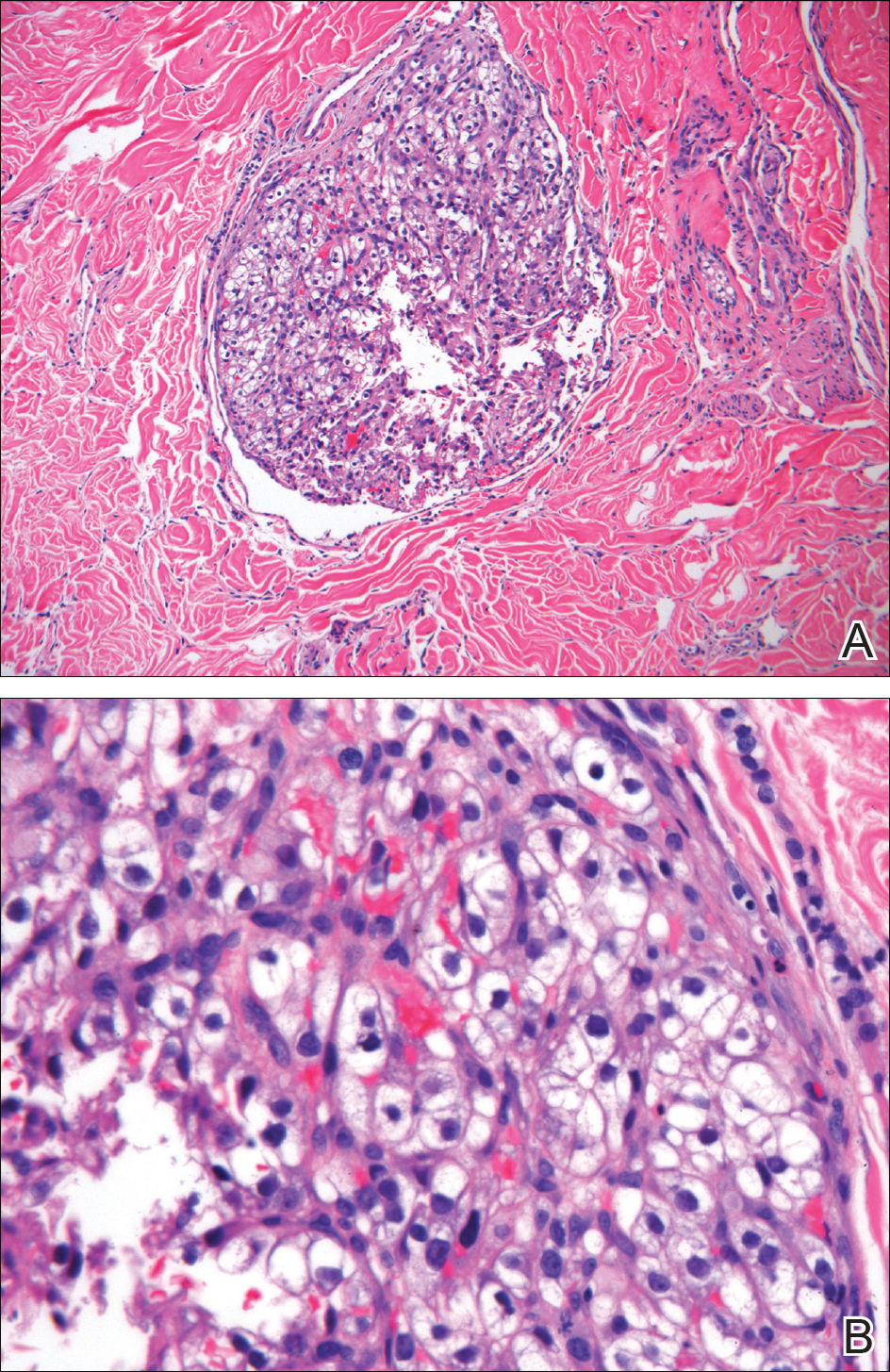
Clear cell RCC, the most common renal malignancy, presents with metastatic disease at the time of diagnosis in 21% of patients.1 An additional 20% of patients with localized disease develop metastases within several years of receiving a nephrectomy without adjuvant therapy, which is standard treatment for stage I to stage III disease.1,2 Metastatic RCC most frequently targets the lungs, bone, liver, and brain, though virtually any organ can be involved. Cutaneous involvement is estimated to occur in 3.3% of RCC cases,3 accounting for only 1.4% of cutaneous metastases overall.4 The risk for developing cutaneous metastases is greatest within 3 years following nephrectomy.3 However, our patient demonstrates that metastasis of RCC to skin can be long delayed (>5 years) despite an initial diagnosis of localized disease.
Cutaneous RCC classically presents as a painless firm papulonodule with a deep red or purple color due to its high vascularity.4 Several retrospective studies have identified the scalp as the most frequent site of cutaneous involvement, followed by the chest, abdomen, and nephrectomy scar.3,4 The differential diagnosis includes other vascular lesions such as pyogenic granuloma, hemangioma, angiosarcoma, bacillary angiomatosis, and Kaposi sarcoma. Diagnosis usually is easily confirmed histologically. Proliferative nests of epithelioid cells with clear cell morphology are surrounded by delicately branching vessels referred to as chicken wire-like vasculature. Immunohistochemical studies demonstrate positivity for pan-cytokeratin, vimentin, and CD-10, and negativity for p63 and cytokeratins 5 and 6, helping to confirm the diagnosis in more challenging cases, especially when there is no known history of primary RCC.5
If cutaneous metastasis of RCC is diagnosed, a chest and abdominal computed tomography scan as well as serum alkaline phosphatase test are warranted, as up to 90% of patients with RCC in the skin have additional lesions in at least 1 other site such as the lungs, bones, or liver.3 Management of metastatic RCC includes surgical excision if a single metastasis is found and either immunotherapy with high-dose IL-2 or an anti-programmed cell death inhibitor. Patients with progressive disease also may receive targeted anti-VEGF inhibitors (eg, axitinib, pazopanib, sunitinib), which have been shown to increase progression-free survival in metastatic RCC.6-8 Interestingly, some evidence suggests severely delayed recurrence of RCC (>5 years following nephrectomy) may predict better response to systemic therapy.9
This case of severely delayed metastasis of RCC 8 years after nephrectomy raises the question of whether routine surveillance for RCC recurrence should continue beyond 5 years. It also underscores the need for further studies to determine the utility of postsurgical adjuvant therapy for localized disease (stages I-III). A randomized clinical trial showed no significant difference in disease-free survival when the multikinase inhibitors sunitinib and sorafenib were used as adjuvant therapy.10 The randomized, placebo-controlled PROTECT trial showed no significant difference in disease-free survival between the VEGF inhibitor pazopanib and placebo when used as adjuvant therapy.11 However, trials are ongoing to investigate a potential survival advantage of adjuvant therapy with the VEGF receptor inhibitor axitinib and the mammalian target of rapamycin inhibitor everolimus.
- Dabestani S, Thorstenson A, Lindblad P, et al. Renal cell carcinoma recurrences and metastases in primary non-metastatic patients: a population-based study. World J Urol. 2016;34:1081-1086.
- Ljungberg B, Campbell SC, Choi HY, et al. The epidemiology of renal cell carcinoma. Eur Urol. 2011;60:615-621.
- Dorairajan LN, Hemal AK, Aron M, et al. Cutaneous metastases in renal cell carcinoma. Urol Int. 1999;63:164-167.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2, pt 1):228-236.
- Sariya D, Ruth K, Adams-McDonnell R, et al. Clinicopathologic correlation of cutaneous metastases: experience from a cancer center. Arch Dermatol. 2007;143:613-620.
- Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061-1068.
- Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584-3590.
- Rini BI, Grunwald V, Fishman MN, et al. Axitinib for first-line metastatic renal cell carcinoma (mRCC): overall efficacy and pharmacokinetic (PK) analyses from a randomized phase II study. J Clin Oncol. 2012;30(suppl). doi:10.1200/jco.2012.30.15_suppl.4503.
- Ficarra V, Novara G. Characterizing late recurrence of renal cell carcinoma. Nat Rev Urol. 2013;10:687-689.
- Haas NB, Manola J, Uzzo RG, et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial [published online March 9, 2016]. Lancet. 2016;387:2008-2016.
- Motzer RJ, Haas NB, Donskov F, et al; PROTECT investigators. Randomized phase III trial of adjuvant pazopanib versus placebo after nephrectomy in patients with localized or locally advanced renal cell carcinoma [published online September 13, 2017]. J Clin Oncol. 2017;35:3916-3923.
The Diagnosis: Metastatic Renal Cell Carcinoma
Histopathologic examination of the punch biopsy demonstrated epithelioid cells with abundant clear cytoplasm and numerous chicken wire-like vascular channels consistent with a diagnosis of cutaneous metastasis of renal cell carcinoma (RCC)(Figure). Collateral history revealed that 8 years prior, the patient had been diagnosed with clear cell RCC, stage III (T3aN0M0). At that time, he was treated with radical nephrectomy, which was considered curative. He remained disease free until several months prior to the development of the cutaneous lesion when he was found to have pulmonary and cerebral metastases with biopsies showing metastatic RCC. He was treated with lobectomy and Gamma Knife radiation for the lung and cerebral metastases, respectively. His oncologist planned to initiate therapy with the multikinase inhibitor sunitinib, which inhibits vascular endothelial growth factor (VEGF) signaling. Unfortunately, the patient died prior to treatment due to overwhelming tumor burden.

Clear cell RCC, the most common renal malignancy, presents with metastatic disease at the time of diagnosis in 21% of patients.1 An additional 20% of patients with localized disease develop metastases within several years of receiving a nephrectomy without adjuvant therapy, which is standard treatment for stage I to stage III disease.1,2 Metastatic RCC most frequently targets the lungs, bone, liver, and brain, though virtually any organ can be involved. Cutaneous involvement is estimated to occur in 3.3% of RCC cases,3 accounting for only 1.4% of cutaneous metastases overall.4 The risk for developing cutaneous metastases is greatest within 3 years following nephrectomy.3 However, our patient demonstrates that metastasis of RCC to skin can be long delayed (>5 years) despite an initial diagnosis of localized disease.
Cutaneous RCC classically presents as a painless firm papulonodule with a deep red or purple color due to its high vascularity.4 Several retrospective studies have identified the scalp as the most frequent site of cutaneous involvement, followed by the chest, abdomen, and nephrectomy scar.3,4 The differential diagnosis includes other vascular lesions such as pyogenic granuloma, hemangioma, angiosarcoma, bacillary angiomatosis, and Kaposi sarcoma. Diagnosis usually is easily confirmed histologically. Proliferative nests of epithelioid cells with clear cell morphology are surrounded by delicately branching vessels referred to as chicken wire-like vasculature. Immunohistochemical studies demonstrate positivity for pan-cytokeratin, vimentin, and CD-10, and negativity for p63 and cytokeratins 5 and 6, helping to confirm the diagnosis in more challenging cases, especially when there is no known history of primary RCC.5
If cutaneous metastasis of RCC is diagnosed, a chest and abdominal computed tomography scan as well as serum alkaline phosphatase test are warranted, as up to 90% of patients with RCC in the skin have additional lesions in at least 1 other site such as the lungs, bones, or liver.3 Management of metastatic RCC includes surgical excision if a single metastasis is found and either immunotherapy with high-dose IL-2 or an anti-programmed cell death inhibitor. Patients with progressive disease also may receive targeted anti-VEGF inhibitors (eg, axitinib, pazopanib, sunitinib), which have been shown to increase progression-free survival in metastatic RCC.6-8 Interestingly, some evidence suggests severely delayed recurrence of RCC (>5 years following nephrectomy) may predict better response to systemic therapy.9
This case of severely delayed metastasis of RCC 8 years after nephrectomy raises the question of whether routine surveillance for RCC recurrence should continue beyond 5 years. It also underscores the need for further studies to determine the utility of postsurgical adjuvant therapy for localized disease (stages I-III). A randomized clinical trial showed no significant difference in disease-free survival when the multikinase inhibitors sunitinib and sorafenib were used as adjuvant therapy.10 The randomized, placebo-controlled PROTECT trial showed no significant difference in disease-free survival between the VEGF inhibitor pazopanib and placebo when used as adjuvant therapy.11 However, trials are ongoing to investigate a potential survival advantage of adjuvant therapy with the VEGF receptor inhibitor axitinib and the mammalian target of rapamycin inhibitor everolimus.
The Diagnosis: Metastatic Renal Cell Carcinoma
Histopathologic examination of the punch biopsy demonstrated epithelioid cells with abundant clear cytoplasm and numerous chicken wire-like vascular channels consistent with a diagnosis of cutaneous metastasis of renal cell carcinoma (RCC)(Figure). Collateral history revealed that 8 years prior, the patient had been diagnosed with clear cell RCC, stage III (T3aN0M0). At that time, he was treated with radical nephrectomy, which was considered curative. He remained disease free until several months prior to the development of the cutaneous lesion when he was found to have pulmonary and cerebral metastases with biopsies showing metastatic RCC. He was treated with lobectomy and Gamma Knife radiation for the lung and cerebral metastases, respectively. His oncologist planned to initiate therapy with the multikinase inhibitor sunitinib, which inhibits vascular endothelial growth factor (VEGF) signaling. Unfortunately, the patient died prior to treatment due to overwhelming tumor burden.

Clear cell RCC, the most common renal malignancy, presents with metastatic disease at the time of diagnosis in 21% of patients.1 An additional 20% of patients with localized disease develop metastases within several years of receiving a nephrectomy without adjuvant therapy, which is standard treatment for stage I to stage III disease.1,2 Metastatic RCC most frequently targets the lungs, bone, liver, and brain, though virtually any organ can be involved. Cutaneous involvement is estimated to occur in 3.3% of RCC cases,3 accounting for only 1.4% of cutaneous metastases overall.4 The risk for developing cutaneous metastases is greatest within 3 years following nephrectomy.3 However, our patient demonstrates that metastasis of RCC to skin can be long delayed (>5 years) despite an initial diagnosis of localized disease.
Cutaneous RCC classically presents as a painless firm papulonodule with a deep red or purple color due to its high vascularity.4 Several retrospective studies have identified the scalp as the most frequent site of cutaneous involvement, followed by the chest, abdomen, and nephrectomy scar.3,4 The differential diagnosis includes other vascular lesions such as pyogenic granuloma, hemangioma, angiosarcoma, bacillary angiomatosis, and Kaposi sarcoma. Diagnosis usually is easily confirmed histologically. Proliferative nests of epithelioid cells with clear cell morphology are surrounded by delicately branching vessels referred to as chicken wire-like vasculature. Immunohistochemical studies demonstrate positivity for pan-cytokeratin, vimentin, and CD-10, and negativity for p63 and cytokeratins 5 and 6, helping to confirm the diagnosis in more challenging cases, especially when there is no known history of primary RCC.5
If cutaneous metastasis of RCC is diagnosed, a chest and abdominal computed tomography scan as well as serum alkaline phosphatase test are warranted, as up to 90% of patients with RCC in the skin have additional lesions in at least 1 other site such as the lungs, bones, or liver.3 Management of metastatic RCC includes surgical excision if a single metastasis is found and either immunotherapy with high-dose IL-2 or an anti-programmed cell death inhibitor. Patients with progressive disease also may receive targeted anti-VEGF inhibitors (eg, axitinib, pazopanib, sunitinib), which have been shown to increase progression-free survival in metastatic RCC.6-8 Interestingly, some evidence suggests severely delayed recurrence of RCC (>5 years following nephrectomy) may predict better response to systemic therapy.9
This case of severely delayed metastasis of RCC 8 years after nephrectomy raises the question of whether routine surveillance for RCC recurrence should continue beyond 5 years. It also underscores the need for further studies to determine the utility of postsurgical adjuvant therapy for localized disease (stages I-III). A randomized clinical trial showed no significant difference in disease-free survival when the multikinase inhibitors sunitinib and sorafenib were used as adjuvant therapy.10 The randomized, placebo-controlled PROTECT trial showed no significant difference in disease-free survival between the VEGF inhibitor pazopanib and placebo when used as adjuvant therapy.11 However, trials are ongoing to investigate a potential survival advantage of adjuvant therapy with the VEGF receptor inhibitor axitinib and the mammalian target of rapamycin inhibitor everolimus.
- Dabestani S, Thorstenson A, Lindblad P, et al. Renal cell carcinoma recurrences and metastases in primary non-metastatic patients: a population-based study. World J Urol. 2016;34:1081-1086.
- Ljungberg B, Campbell SC, Choi HY, et al. The epidemiology of renal cell carcinoma. Eur Urol. 2011;60:615-621.
- Dorairajan LN, Hemal AK, Aron M, et al. Cutaneous metastases in renal cell carcinoma. Urol Int. 1999;63:164-167.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2, pt 1):228-236.
- Sariya D, Ruth K, Adams-McDonnell R, et al. Clinicopathologic correlation of cutaneous metastases: experience from a cancer center. Arch Dermatol. 2007;143:613-620.
- Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061-1068.
- Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584-3590.
- Rini BI, Grunwald V, Fishman MN, et al. Axitinib for first-line metastatic renal cell carcinoma (mRCC): overall efficacy and pharmacokinetic (PK) analyses from a randomized phase II study. J Clin Oncol. 2012;30(suppl). doi:10.1200/jco.2012.30.15_suppl.4503.
- Ficarra V, Novara G. Characterizing late recurrence of renal cell carcinoma. Nat Rev Urol. 2013;10:687-689.
- Haas NB, Manola J, Uzzo RG, et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial [published online March 9, 2016]. Lancet. 2016;387:2008-2016.
- Motzer RJ, Haas NB, Donskov F, et al; PROTECT investigators. Randomized phase III trial of adjuvant pazopanib versus placebo after nephrectomy in patients with localized or locally advanced renal cell carcinoma [published online September 13, 2017]. J Clin Oncol. 2017;35:3916-3923.
- Dabestani S, Thorstenson A, Lindblad P, et al. Renal cell carcinoma recurrences and metastases in primary non-metastatic patients: a population-based study. World J Urol. 2016;34:1081-1086.
- Ljungberg B, Campbell SC, Choi HY, et al. The epidemiology of renal cell carcinoma. Eur Urol. 2011;60:615-621.
- Dorairajan LN, Hemal AK, Aron M, et al. Cutaneous metastases in renal cell carcinoma. Urol Int. 1999;63:164-167.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2, pt 1):228-236.
- Sariya D, Ruth K, Adams-McDonnell R, et al. Clinicopathologic correlation of cutaneous metastases: experience from a cancer center. Arch Dermatol. 2007;143:613-620.
- Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061-1068.
- Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584-3590.
- Rini BI, Grunwald V, Fishman MN, et al. Axitinib for first-line metastatic renal cell carcinoma (mRCC): overall efficacy and pharmacokinetic (PK) analyses from a randomized phase II study. J Clin Oncol. 2012;30(suppl). doi:10.1200/jco.2012.30.15_suppl.4503.
- Ficarra V, Novara G. Characterizing late recurrence of renal cell carcinoma. Nat Rev Urol. 2013;10:687-689.
- Haas NB, Manola J, Uzzo RG, et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial [published online March 9, 2016]. Lancet. 2016;387:2008-2016.
- Motzer RJ, Haas NB, Donskov F, et al; PROTECT investigators. Randomized phase III trial of adjuvant pazopanib versus placebo after nephrectomy in patients with localized or locally advanced renal cell carcinoma [published online September 13, 2017]. J Clin Oncol. 2017;35:3916-3923.
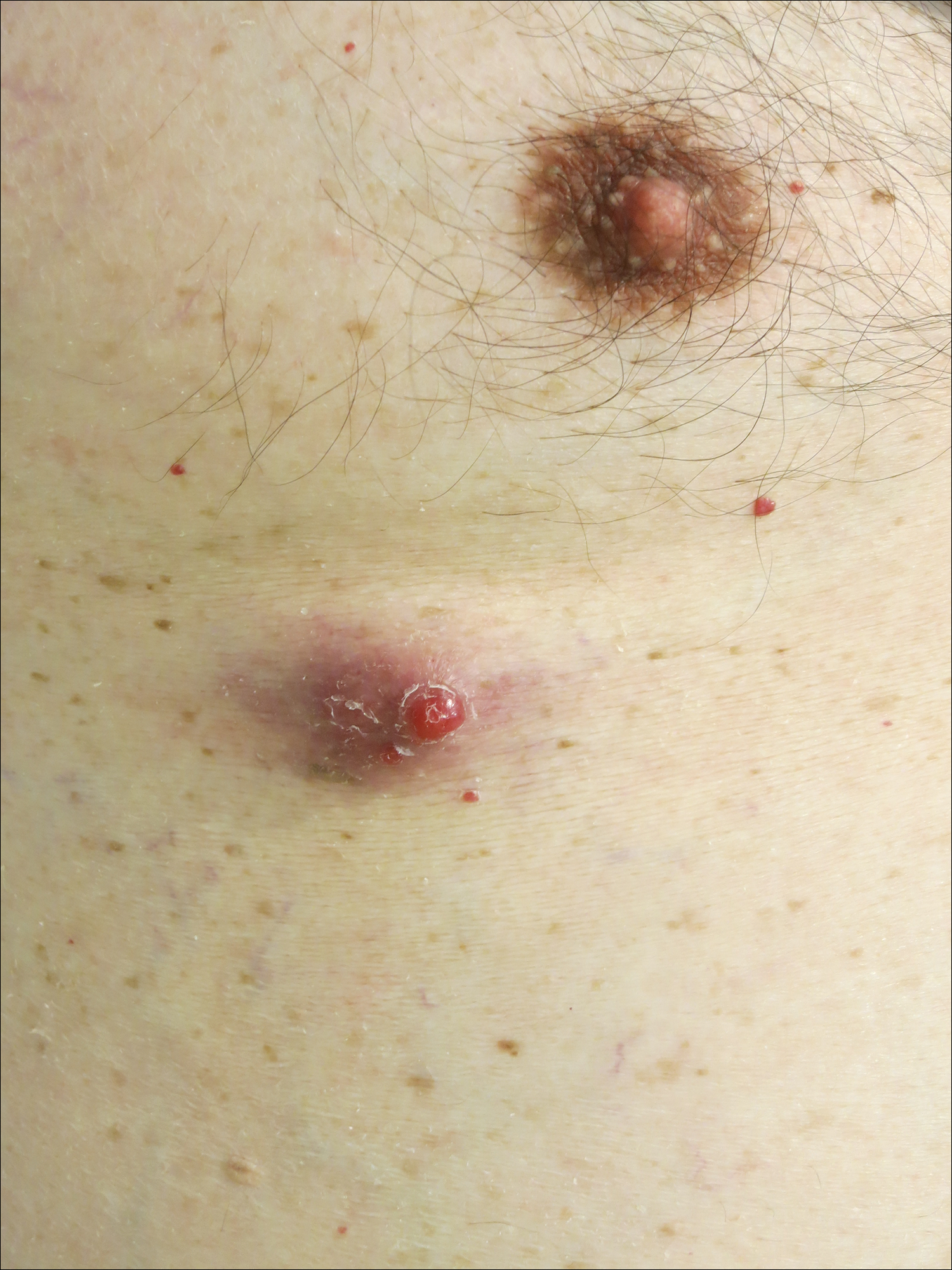
A man in his 60s presented with a subcutaneous nodule on the right side of the chest. Due to impaired mental status, he was unable to describe the precise age of the lesion, but his wife reported it had been present at least several weeks. She recently noted a new, bright red growth on top of the nodule. The lesion was asymptomatic but seemed to be growing in size. Physical examination revealed a 3-cm firm fixed nodule on the right side of the chest with an overlying, exophytic bright red papule. No similar lesions were found elsewhere on physical examination. A punch biopsy of the lesion was performed.
Growing Nodule on the Arm
The Diagnosis: Primary Cutaneous Anaplastic Large Cell Lymphoma
Primary cutaneous CD30+ lymphoproliferative disorders encompass lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma (PCALCL) as well as borderline cases. Primary cutaneous anaplastic large cell lymphoma is a rare disease that is more common in white patients with slight male predominance and median age at diagnosis of 61 years.1 Prognosis is excellent, with a 90% survival rate at 10 years. Although lesions spontaneously regress in 6% to 22% of cases, complete resolution is rare.2 Clinically, the classic presentation is a solitary, rapidly growing, flesh-colored, erythematous nodule or plaque on the arms and legs or trunk, often with ulceration. Proper diagnosis requires clinical, histopathologic, and immunophenotypic correlation.
Histopathologic examination of PCALCL typically reveals large, atypical, Reed-Sternberg-like cells most commonly with anaplastic cytomorphology, but pleomorphic or immunoblastic morphology is not uncommon. Cells are in sheets or nodules, diffusely occupying the dermis and often the subcutaneous fat, with more than 75% of large cells expressing CD30.3 In addition to CD30 positivity, immunophenotype is classically CD4+, cutaneous lymphocyte-associated antigen positive, epithelial membrane antigen negative, and anaplastic lymphoma kinase negative; CD2, CD5, and CD3 expression is variable. Interestingly, in our case, there was a minor population of CD8+ cells. CD8 expression is seen in less than 5% of PCALCL cases; this phenotype is associated with an indolent disease with favorable prognosis.3 Of note, anaplastic lymphoma kinase positivity corresponding to a t(2;5) translocation is more suggestive of systemic anaplastic large cell lymphoma with secondary skin involvement and more commonly is seen in children. For reasons possibly related to mediators such as epidermal growth factor or transforming growth factor α from CD30+ cells, epidermal hyperplasia can be seen in PCALCL.4 The subsequent hyperkeratosis, crusting, and ulceration can be difficult to distinguish from lesions such as pyoderma gangrenosum, squamous cell carcinoma, arthropod bite, leukemia cutis, Merkel cell carcinoma (MCC), and metastatic breast cancer.
Skin involvement with leukemia is rare but most commonly is seen in acute myelogenous leukemia, specifically more mature forms such as acute myelomonocytic leukemia and acute monocytic leukemia. Approximately 10% to 20% of acute myelomonocytic leukemia cases have cutaneous involvement.5 Although there is a variety of potential skin lesions, the most common is a red-purple papule or nodule, sometimes with hemorrhage or ulceration, on the head, neck, and trunk. Leukemic infiltrates may arise from sites of prior trauma. Histopathology depends on the type of leukemia; however, general features include a normal epidermis without epidermotropism and perivascular, nodular, or diffuse infiltrate of neoplastic cells in the dermis, often with a Grenz zone (Figure 1). Compared to PCALCL, leukemia cutis shows sparing of the papillary dermis (Grenz zone), and the cells have more cytoplasm and show a different immunophenotype. The cells often are fragile and show crush artifact. Acute myelogenous leukemia often will show cytoplasmic granules; however, immature precursor cells may not have granules. The myeloid cells will stain with myeloperoxidase and chloroacetate. Positivity is seen for CD13, CD33, and CD68. Clinical correlation is important because other diseases with nodular or diffuse infiltrates of small cell infiltrates, such as extramedullary hematopoiesis and lymphoma, appear similar. Acute myelogenous leukemia is associated with neutrophilic dermatoses such as Sweet syndrome and pyoderma gangrenosum. Cutaneous eruption resolves with successful treatment of the leukemia.
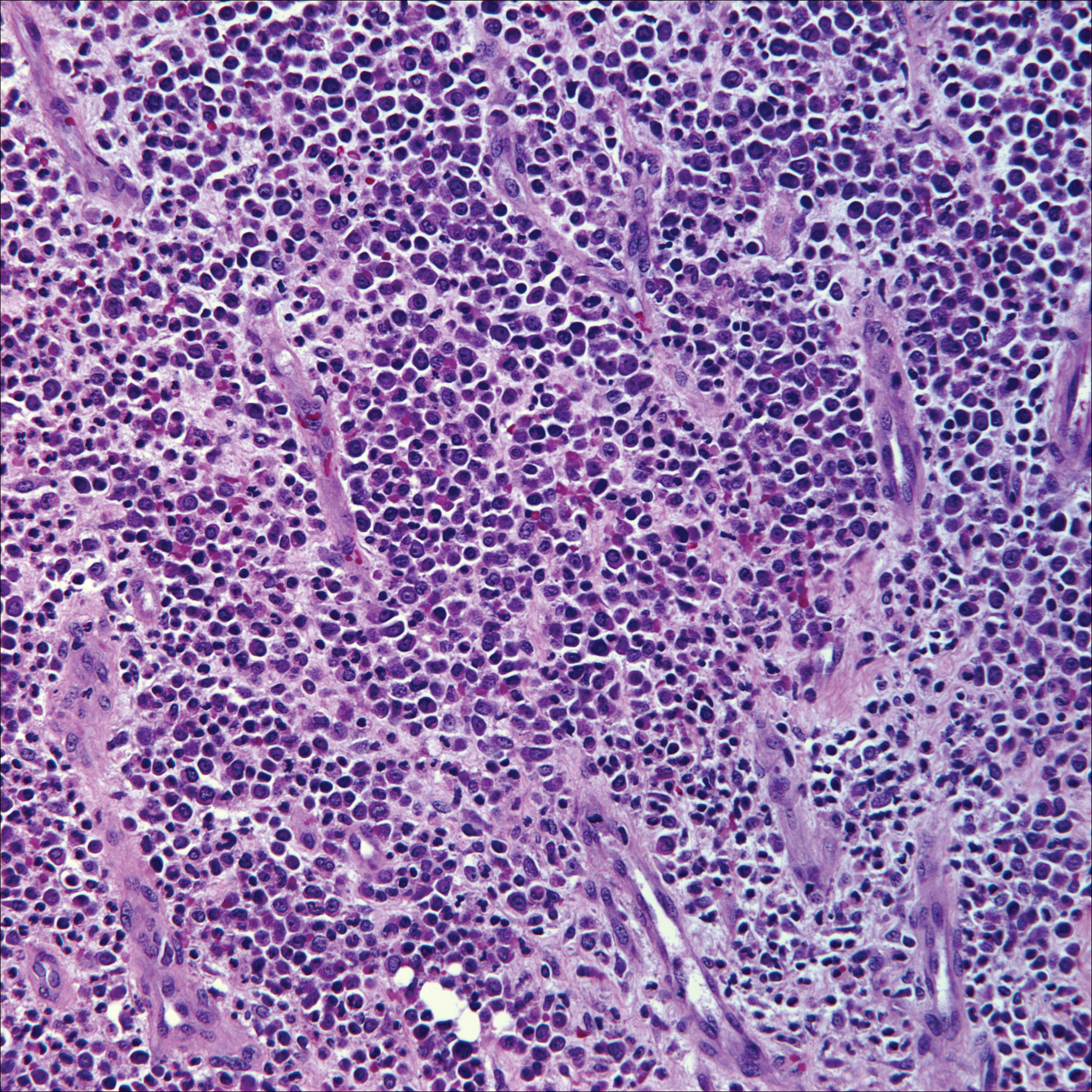
Breast cancer is the most common cancer to metastasize to the skin in women, accounting for 73% of cutaneous metastases, followed by melanoma, which is responsible for 11%.5 The classic presentation is an erythematous patch with spreading borders or a nodule on the trunk. Many cases of metastatic breast cancer with skin involvement may represent direct extension of the cancer into the skin. General histologic clues to cutaneous metastasis include well-circumscribed dermal or subcutaneous nodules of atypical cells with an increase in mitotic activity without connection to the epidermis. Tumor cells may show diffuse, nodular, or single file pattern and may exhibit areas of necrosis. Ductal carcinoma additionally may show ductal or glandular differentiation with surrounding desmoplasia (Figure 2). Immunohistochemistry typically is positive for cytokeratin (CK) 7, estrogen receptor/progesterone receptor, mammaglobin, and gross cystic disease fluid protein-15, and negative for CK20, CK5/6, and thyroid transcription factor-1.
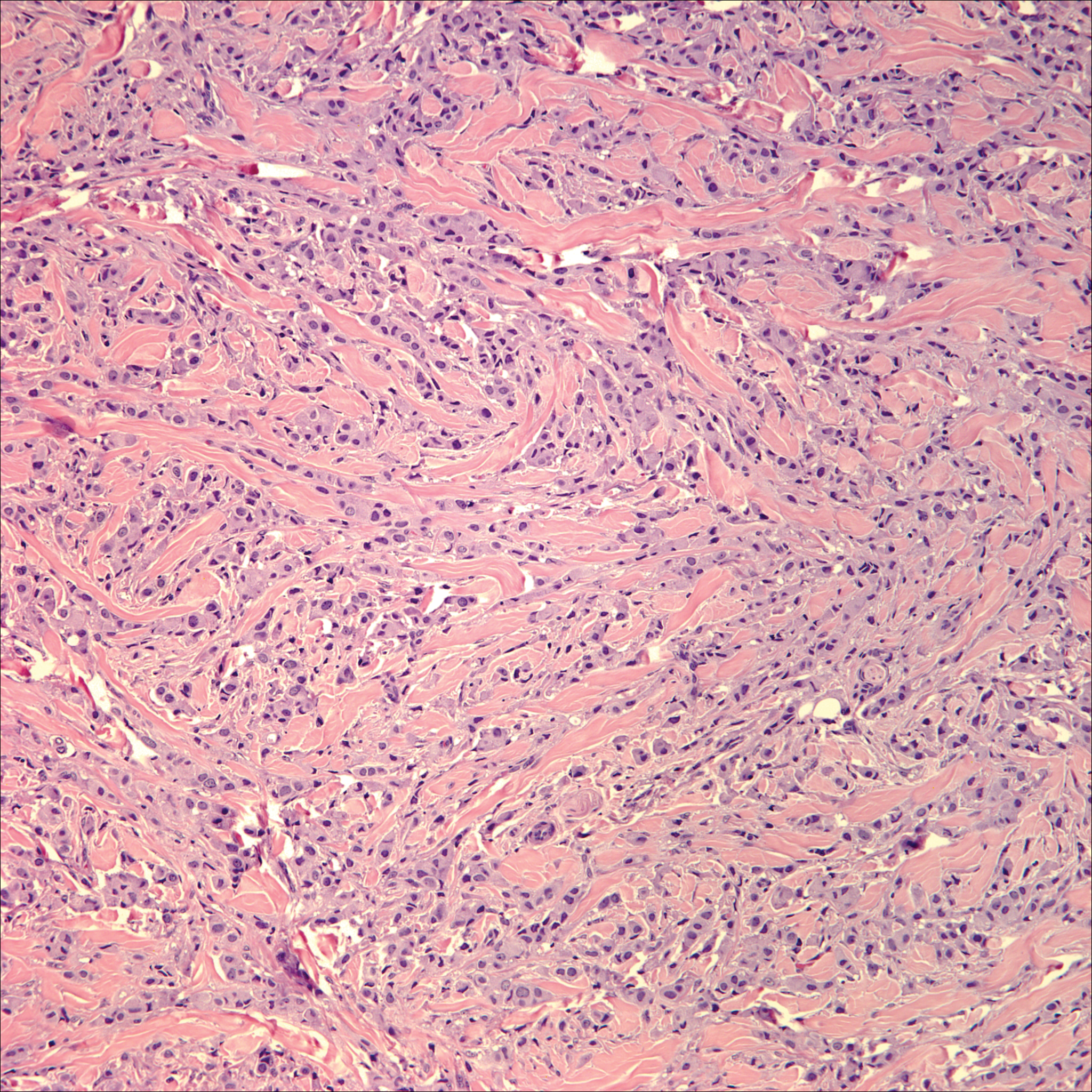
Papulovesicular and nodular lesions appearing as an arthropod bite have been noted in hematologic malignancies, underscoring the importance of histopathology and clinical correlation. Arthropod bites commonly present as red papules, nodules, vesicles, or pustules at the site of the bite. Pseudolymphomatous nodules occasionally develop. Excoriations and further progression to persistent prurigo also may occur. Histopathology shows variable epidermal features including spongiosis, acanthosis, parakeratosis, dermal edema, and superficial and deep perivascular neutrophils (Figure 3). Additionally, lymphocytes sometimes with CD30 positivity may be seen. The presence of eosinophils in interstitial areas, especially in the deep dermis, is a useful clue.
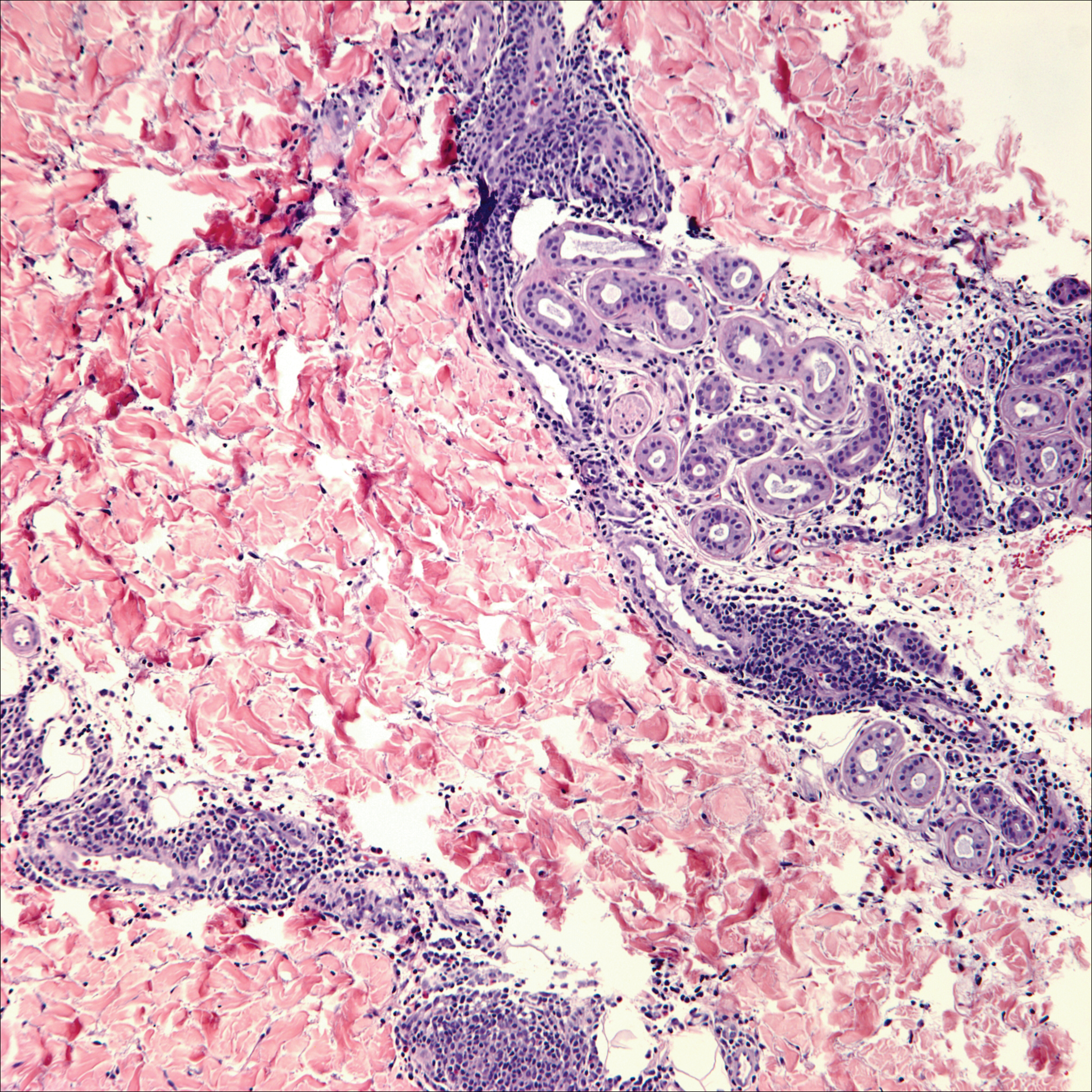
Lack of staining for epithelial and neuroendocrine markers differentiates PCALCL from MCC; specifically CK20, an epithelial marker positive in more than 90% of MCC cases, excludes lymphoma.6 Merkel cell carcinoma presents as a solitary, quickly growing, red and often ulcerated nodule or plaque on the head, neck, or legs of elderly patients. The lesions often are in areas of sun damage. Histopathology classically shows a diffuse dermal infiltrate of monotonous round blue cells with a scant cytoplasmic rim and multiple inconspicuous nucleoli in nests, rosettes, or strands in the dermis. There are frequent mitotic figures. The cells are uniform and 2 to 3 times larger than mature lymphocytes. Single-cell necrosis and crush artifact is common. Epidermotropism or coexisting Bowenoid change also may be observed (Figure 4). The term primary neuroendocrine carcinoma of the skin is preferred over Merkel cell carcinoma because the tumor cells share similar morphology to the specialized touch receptor of the basal layer (Merkel cell), but no direct histogenetic relationship has been established.7,8
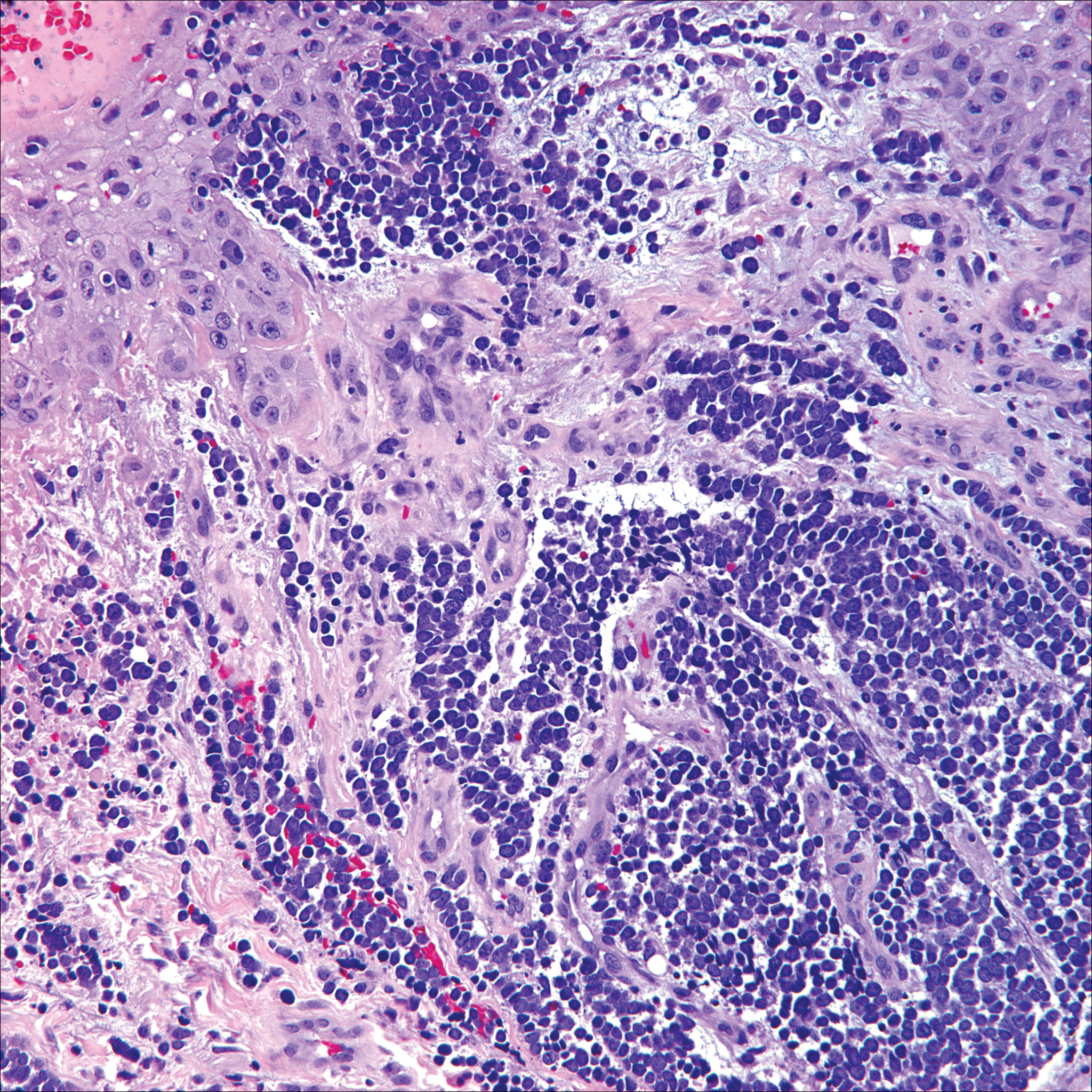
Immunohistochemistry is key to diagnosis because MCC stains for both epithelial and neuroendocrine markers. Positivity is seen for neuron-specific enolase, epithelial membrane antigen, neurofilament, synaptophysin, and chromogranin. Because the histology of MCC may resemble small cell carcinoma of the lung, staining for low-molecular-weight keratin such as CK20 and CK7 help to distinguish MCC. Merkel cell carcinoma typically is CK20+ and CK7-, while small cell carcinoma of the lung is the opposite.9 The tumor grows aggressively and metastasis is common, thus surgery is the primary approach, but adjuvant chemotherapy and radiation often are given in addition.
- Yu J, Blitzblau R, Decker R, et al. Analysis of primary CD30+ cutaneous lymphoproliferative disease and survival from the Surveillance, Epidemiology, and End Results database. J Clin Oncol. 2008;26:1483-1488.
- Liu HL, Hoppe RT, Kohler S, et al. CD30+ cutaneous lymphoproliferative disorders: the Stanford experience in lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma. J Am Acad Dermatol. 2003;49:1049-1058.
- Nasit JG, Patel SC. Primary cutaneous CD8(+) CD30(+) anaplastic large cell lymphoma: an unusual case with a high Ki-67 index--a short review. Indian J Dermatol. 2015;60:373-377.
- Park J, Lee J, Lim Y, et al. Synchronous occurrence of primary cutaneous anaplastic large cell lymphoma and squamous cell carcinoma. Ann Dermatol. 2016;28:491-494.
- Marks JG Jr, Miller JJ. Lookingbill and Marks' Principles of Dermatology. 5th ed. Philadelphia, PA: Elsevier Saunders; 2013.
- Kudchadkar R, Gonzalez R, Lewis K, et al. A case of Merkel cell carcinoma. Oncology. 2008;22:322-328.
- Ratner D, Nelson BR, Brown MD, et al. Merkel cell carcinoma. J Am Acad Dermatol. 1993;29:143-156.
- Zur Hausen A, Rennspiess D, Winnepenninckx V, et al. Early B-cell differentiation in Merkel cell carcinomas: clues to cellular ancestry [published online April 10, 2013]. Cancer Res. 2013;73:4982-4987.
- Sidiropoulos M, Hanna W, Raphael SJ, et al. Expression of TdT in Merkel cell carcinoma and small cell lung carcinoma. Am J Clin Pathol. 2011;135:831-838.
The Diagnosis: Primary Cutaneous Anaplastic Large Cell Lymphoma
Primary cutaneous CD30+ lymphoproliferative disorders encompass lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma (PCALCL) as well as borderline cases. Primary cutaneous anaplastic large cell lymphoma is a rare disease that is more common in white patients with slight male predominance and median age at diagnosis of 61 years.1 Prognosis is excellent, with a 90% survival rate at 10 years. Although lesions spontaneously regress in 6% to 22% of cases, complete resolution is rare.2 Clinically, the classic presentation is a solitary, rapidly growing, flesh-colored, erythematous nodule or plaque on the arms and legs or trunk, often with ulceration. Proper diagnosis requires clinical, histopathologic, and immunophenotypic correlation.
Histopathologic examination of PCALCL typically reveals large, atypical, Reed-Sternberg-like cells most commonly with anaplastic cytomorphology, but pleomorphic or immunoblastic morphology is not uncommon. Cells are in sheets or nodules, diffusely occupying the dermis and often the subcutaneous fat, with more than 75% of large cells expressing CD30.3 In addition to CD30 positivity, immunophenotype is classically CD4+, cutaneous lymphocyte-associated antigen positive, epithelial membrane antigen negative, and anaplastic lymphoma kinase negative; CD2, CD5, and CD3 expression is variable. Interestingly, in our case, there was a minor population of CD8+ cells. CD8 expression is seen in less than 5% of PCALCL cases; this phenotype is associated with an indolent disease with favorable prognosis.3 Of note, anaplastic lymphoma kinase positivity corresponding to a t(2;5) translocation is more suggestive of systemic anaplastic large cell lymphoma with secondary skin involvement and more commonly is seen in children. For reasons possibly related to mediators such as epidermal growth factor or transforming growth factor α from CD30+ cells, epidermal hyperplasia can be seen in PCALCL.4 The subsequent hyperkeratosis, crusting, and ulceration can be difficult to distinguish from lesions such as pyoderma gangrenosum, squamous cell carcinoma, arthropod bite, leukemia cutis, Merkel cell carcinoma (MCC), and metastatic breast cancer.
Skin involvement with leukemia is rare but most commonly is seen in acute myelogenous leukemia, specifically more mature forms such as acute myelomonocytic leukemia and acute monocytic leukemia. Approximately 10% to 20% of acute myelomonocytic leukemia cases have cutaneous involvement.5 Although there is a variety of potential skin lesions, the most common is a red-purple papule or nodule, sometimes with hemorrhage or ulceration, on the head, neck, and trunk. Leukemic infiltrates may arise from sites of prior trauma. Histopathology depends on the type of leukemia; however, general features include a normal epidermis without epidermotropism and perivascular, nodular, or diffuse infiltrate of neoplastic cells in the dermis, often with a Grenz zone (Figure 1). Compared to PCALCL, leukemia cutis shows sparing of the papillary dermis (Grenz zone), and the cells have more cytoplasm and show a different immunophenotype. The cells often are fragile and show crush artifact. Acute myelogenous leukemia often will show cytoplasmic granules; however, immature precursor cells may not have granules. The myeloid cells will stain with myeloperoxidase and chloroacetate. Positivity is seen for CD13, CD33, and CD68. Clinical correlation is important because other diseases with nodular or diffuse infiltrates of small cell infiltrates, such as extramedullary hematopoiesis and lymphoma, appear similar. Acute myelogenous leukemia is associated with neutrophilic dermatoses such as Sweet syndrome and pyoderma gangrenosum. Cutaneous eruption resolves with successful treatment of the leukemia.

Breast cancer is the most common cancer to metastasize to the skin in women, accounting for 73% of cutaneous metastases, followed by melanoma, which is responsible for 11%.5 The classic presentation is an erythematous patch with spreading borders or a nodule on the trunk. Many cases of metastatic breast cancer with skin involvement may represent direct extension of the cancer into the skin. General histologic clues to cutaneous metastasis include well-circumscribed dermal or subcutaneous nodules of atypical cells with an increase in mitotic activity without connection to the epidermis. Tumor cells may show diffuse, nodular, or single file pattern and may exhibit areas of necrosis. Ductal carcinoma additionally may show ductal or glandular differentiation with surrounding desmoplasia (Figure 2). Immunohistochemistry typically is positive for cytokeratin (CK) 7, estrogen receptor/progesterone receptor, mammaglobin, and gross cystic disease fluid protein-15, and negative for CK20, CK5/6, and thyroid transcription factor-1.

Papulovesicular and nodular lesions appearing as an arthropod bite have been noted in hematologic malignancies, underscoring the importance of histopathology and clinical correlation. Arthropod bites commonly present as red papules, nodules, vesicles, or pustules at the site of the bite. Pseudolymphomatous nodules occasionally develop. Excoriations and further progression to persistent prurigo also may occur. Histopathology shows variable epidermal features including spongiosis, acanthosis, parakeratosis, dermal edema, and superficial and deep perivascular neutrophils (Figure 3). Additionally, lymphocytes sometimes with CD30 positivity may be seen. The presence of eosinophils in interstitial areas, especially in the deep dermis, is a useful clue.

Lack of staining for epithelial and neuroendocrine markers differentiates PCALCL from MCC; specifically CK20, an epithelial marker positive in more than 90% of MCC cases, excludes lymphoma.6 Merkel cell carcinoma presents as a solitary, quickly growing, red and often ulcerated nodule or plaque on the head, neck, or legs of elderly patients. The lesions often are in areas of sun damage. Histopathology classically shows a diffuse dermal infiltrate of monotonous round blue cells with a scant cytoplasmic rim and multiple inconspicuous nucleoli in nests, rosettes, or strands in the dermis. There are frequent mitotic figures. The cells are uniform and 2 to 3 times larger than mature lymphocytes. Single-cell necrosis and crush artifact is common. Epidermotropism or coexisting Bowenoid change also may be observed (Figure 4). The term primary neuroendocrine carcinoma of the skin is preferred over Merkel cell carcinoma because the tumor cells share similar morphology to the specialized touch receptor of the basal layer (Merkel cell), but no direct histogenetic relationship has been established.7,8

Immunohistochemistry is key to diagnosis because MCC stains for both epithelial and neuroendocrine markers. Positivity is seen for neuron-specific enolase, epithelial membrane antigen, neurofilament, synaptophysin, and chromogranin. Because the histology of MCC may resemble small cell carcinoma of the lung, staining for low-molecular-weight keratin such as CK20 and CK7 help to distinguish MCC. Merkel cell carcinoma typically is CK20+ and CK7-, while small cell carcinoma of the lung is the opposite.9 The tumor grows aggressively and metastasis is common, thus surgery is the primary approach, but adjuvant chemotherapy and radiation often are given in addition.
The Diagnosis: Primary Cutaneous Anaplastic Large Cell Lymphoma
Primary cutaneous CD30+ lymphoproliferative disorders encompass lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma (PCALCL) as well as borderline cases. Primary cutaneous anaplastic large cell lymphoma is a rare disease that is more common in white patients with slight male predominance and median age at diagnosis of 61 years.1 Prognosis is excellent, with a 90% survival rate at 10 years. Although lesions spontaneously regress in 6% to 22% of cases, complete resolution is rare.2 Clinically, the classic presentation is a solitary, rapidly growing, flesh-colored, erythematous nodule or plaque on the arms and legs or trunk, often with ulceration. Proper diagnosis requires clinical, histopathologic, and immunophenotypic correlation.
Histopathologic examination of PCALCL typically reveals large, atypical, Reed-Sternberg-like cells most commonly with anaplastic cytomorphology, but pleomorphic or immunoblastic morphology is not uncommon. Cells are in sheets or nodules, diffusely occupying the dermis and often the subcutaneous fat, with more than 75% of large cells expressing CD30.3 In addition to CD30 positivity, immunophenotype is classically CD4+, cutaneous lymphocyte-associated antigen positive, epithelial membrane antigen negative, and anaplastic lymphoma kinase negative; CD2, CD5, and CD3 expression is variable. Interestingly, in our case, there was a minor population of CD8+ cells. CD8 expression is seen in less than 5% of PCALCL cases; this phenotype is associated with an indolent disease with favorable prognosis.3 Of note, anaplastic lymphoma kinase positivity corresponding to a t(2;5) translocation is more suggestive of systemic anaplastic large cell lymphoma with secondary skin involvement and more commonly is seen in children. For reasons possibly related to mediators such as epidermal growth factor or transforming growth factor α from CD30+ cells, epidermal hyperplasia can be seen in PCALCL.4 The subsequent hyperkeratosis, crusting, and ulceration can be difficult to distinguish from lesions such as pyoderma gangrenosum, squamous cell carcinoma, arthropod bite, leukemia cutis, Merkel cell carcinoma (MCC), and metastatic breast cancer.
Skin involvement with leukemia is rare but most commonly is seen in acute myelogenous leukemia, specifically more mature forms such as acute myelomonocytic leukemia and acute monocytic leukemia. Approximately 10% to 20% of acute myelomonocytic leukemia cases have cutaneous involvement.5 Although there is a variety of potential skin lesions, the most common is a red-purple papule or nodule, sometimes with hemorrhage or ulceration, on the head, neck, and trunk. Leukemic infiltrates may arise from sites of prior trauma. Histopathology depends on the type of leukemia; however, general features include a normal epidermis without epidermotropism and perivascular, nodular, or diffuse infiltrate of neoplastic cells in the dermis, often with a Grenz zone (Figure 1). Compared to PCALCL, leukemia cutis shows sparing of the papillary dermis (Grenz zone), and the cells have more cytoplasm and show a different immunophenotype. The cells often are fragile and show crush artifact. Acute myelogenous leukemia often will show cytoplasmic granules; however, immature precursor cells may not have granules. The myeloid cells will stain with myeloperoxidase and chloroacetate. Positivity is seen for CD13, CD33, and CD68. Clinical correlation is important because other diseases with nodular or diffuse infiltrates of small cell infiltrates, such as extramedullary hematopoiesis and lymphoma, appear similar. Acute myelogenous leukemia is associated with neutrophilic dermatoses such as Sweet syndrome and pyoderma gangrenosum. Cutaneous eruption resolves with successful treatment of the leukemia.

Breast cancer is the most common cancer to metastasize to the skin in women, accounting for 73% of cutaneous metastases, followed by melanoma, which is responsible for 11%.5 The classic presentation is an erythematous patch with spreading borders or a nodule on the trunk. Many cases of metastatic breast cancer with skin involvement may represent direct extension of the cancer into the skin. General histologic clues to cutaneous metastasis include well-circumscribed dermal or subcutaneous nodules of atypical cells with an increase in mitotic activity without connection to the epidermis. Tumor cells may show diffuse, nodular, or single file pattern and may exhibit areas of necrosis. Ductal carcinoma additionally may show ductal or glandular differentiation with surrounding desmoplasia (Figure 2). Immunohistochemistry typically is positive for cytokeratin (CK) 7, estrogen receptor/progesterone receptor, mammaglobin, and gross cystic disease fluid protein-15, and negative for CK20, CK5/6, and thyroid transcription factor-1.

Papulovesicular and nodular lesions appearing as an arthropod bite have been noted in hematologic malignancies, underscoring the importance of histopathology and clinical correlation. Arthropod bites commonly present as red papules, nodules, vesicles, or pustules at the site of the bite. Pseudolymphomatous nodules occasionally develop. Excoriations and further progression to persistent prurigo also may occur. Histopathology shows variable epidermal features including spongiosis, acanthosis, parakeratosis, dermal edema, and superficial and deep perivascular neutrophils (Figure 3). Additionally, lymphocytes sometimes with CD30 positivity may be seen. The presence of eosinophils in interstitial areas, especially in the deep dermis, is a useful clue.

Lack of staining for epithelial and neuroendocrine markers differentiates PCALCL from MCC; specifically CK20, an epithelial marker positive in more than 90% of MCC cases, excludes lymphoma.6 Merkel cell carcinoma presents as a solitary, quickly growing, red and often ulcerated nodule or plaque on the head, neck, or legs of elderly patients. The lesions often are in areas of sun damage. Histopathology classically shows a diffuse dermal infiltrate of monotonous round blue cells with a scant cytoplasmic rim and multiple inconspicuous nucleoli in nests, rosettes, or strands in the dermis. There are frequent mitotic figures. The cells are uniform and 2 to 3 times larger than mature lymphocytes. Single-cell necrosis and crush artifact is common. Epidermotropism or coexisting Bowenoid change also may be observed (Figure 4). The term primary neuroendocrine carcinoma of the skin is preferred over Merkel cell carcinoma because the tumor cells share similar morphology to the specialized touch receptor of the basal layer (Merkel cell), but no direct histogenetic relationship has been established.7,8

Immunohistochemistry is key to diagnosis because MCC stains for both epithelial and neuroendocrine markers. Positivity is seen for neuron-specific enolase, epithelial membrane antigen, neurofilament, synaptophysin, and chromogranin. Because the histology of MCC may resemble small cell carcinoma of the lung, staining for low-molecular-weight keratin such as CK20 and CK7 help to distinguish MCC. Merkel cell carcinoma typically is CK20+ and CK7-, while small cell carcinoma of the lung is the opposite.9 The tumor grows aggressively and metastasis is common, thus surgery is the primary approach, but adjuvant chemotherapy and radiation often are given in addition.
- Yu J, Blitzblau R, Decker R, et al. Analysis of primary CD30+ cutaneous lymphoproliferative disease and survival from the Surveillance, Epidemiology, and End Results database. J Clin Oncol. 2008;26:1483-1488.
- Liu HL, Hoppe RT, Kohler S, et al. CD30+ cutaneous lymphoproliferative disorders: the Stanford experience in lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma. J Am Acad Dermatol. 2003;49:1049-1058.
- Nasit JG, Patel SC. Primary cutaneous CD8(+) CD30(+) anaplastic large cell lymphoma: an unusual case with a high Ki-67 index--a short review. Indian J Dermatol. 2015;60:373-377.
- Park J, Lee J, Lim Y, et al. Synchronous occurrence of primary cutaneous anaplastic large cell lymphoma and squamous cell carcinoma. Ann Dermatol. 2016;28:491-494.
- Marks JG Jr, Miller JJ. Lookingbill and Marks' Principles of Dermatology. 5th ed. Philadelphia, PA: Elsevier Saunders; 2013.
- Kudchadkar R, Gonzalez R, Lewis K, et al. A case of Merkel cell carcinoma. Oncology. 2008;22:322-328.
- Ratner D, Nelson BR, Brown MD, et al. Merkel cell carcinoma. J Am Acad Dermatol. 1993;29:143-156.
- Zur Hausen A, Rennspiess D, Winnepenninckx V, et al. Early B-cell differentiation in Merkel cell carcinomas: clues to cellular ancestry [published online April 10, 2013]. Cancer Res. 2013;73:4982-4987.
- Sidiropoulos M, Hanna W, Raphael SJ, et al. Expression of TdT in Merkel cell carcinoma and small cell lung carcinoma. Am J Clin Pathol. 2011;135:831-838.
- Yu J, Blitzblau R, Decker R, et al. Analysis of primary CD30+ cutaneous lymphoproliferative disease and survival from the Surveillance, Epidemiology, and End Results database. J Clin Oncol. 2008;26:1483-1488.
- Liu HL, Hoppe RT, Kohler S, et al. CD30+ cutaneous lymphoproliferative disorders: the Stanford experience in lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma. J Am Acad Dermatol. 2003;49:1049-1058.
- Nasit JG, Patel SC. Primary cutaneous CD8(+) CD30(+) anaplastic large cell lymphoma: an unusual case with a high Ki-67 index--a short review. Indian J Dermatol. 2015;60:373-377.
- Park J, Lee J, Lim Y, et al. Synchronous occurrence of primary cutaneous anaplastic large cell lymphoma and squamous cell carcinoma. Ann Dermatol. 2016;28:491-494.
- Marks JG Jr, Miller JJ. Lookingbill and Marks' Principles of Dermatology. 5th ed. Philadelphia, PA: Elsevier Saunders; 2013.
- Kudchadkar R, Gonzalez R, Lewis K, et al. A case of Merkel cell carcinoma. Oncology. 2008;22:322-328.
- Ratner D, Nelson BR, Brown MD, et al. Merkel cell carcinoma. J Am Acad Dermatol. 1993;29:143-156.
- Zur Hausen A, Rennspiess D, Winnepenninckx V, et al. Early B-cell differentiation in Merkel cell carcinomas: clues to cellular ancestry [published online April 10, 2013]. Cancer Res. 2013;73:4982-4987.
- Sidiropoulos M, Hanna W, Raphael SJ, et al. Expression of TdT in Merkel cell carcinoma and small cell lung carcinoma. Am J Clin Pathol. 2011;135:831-838.
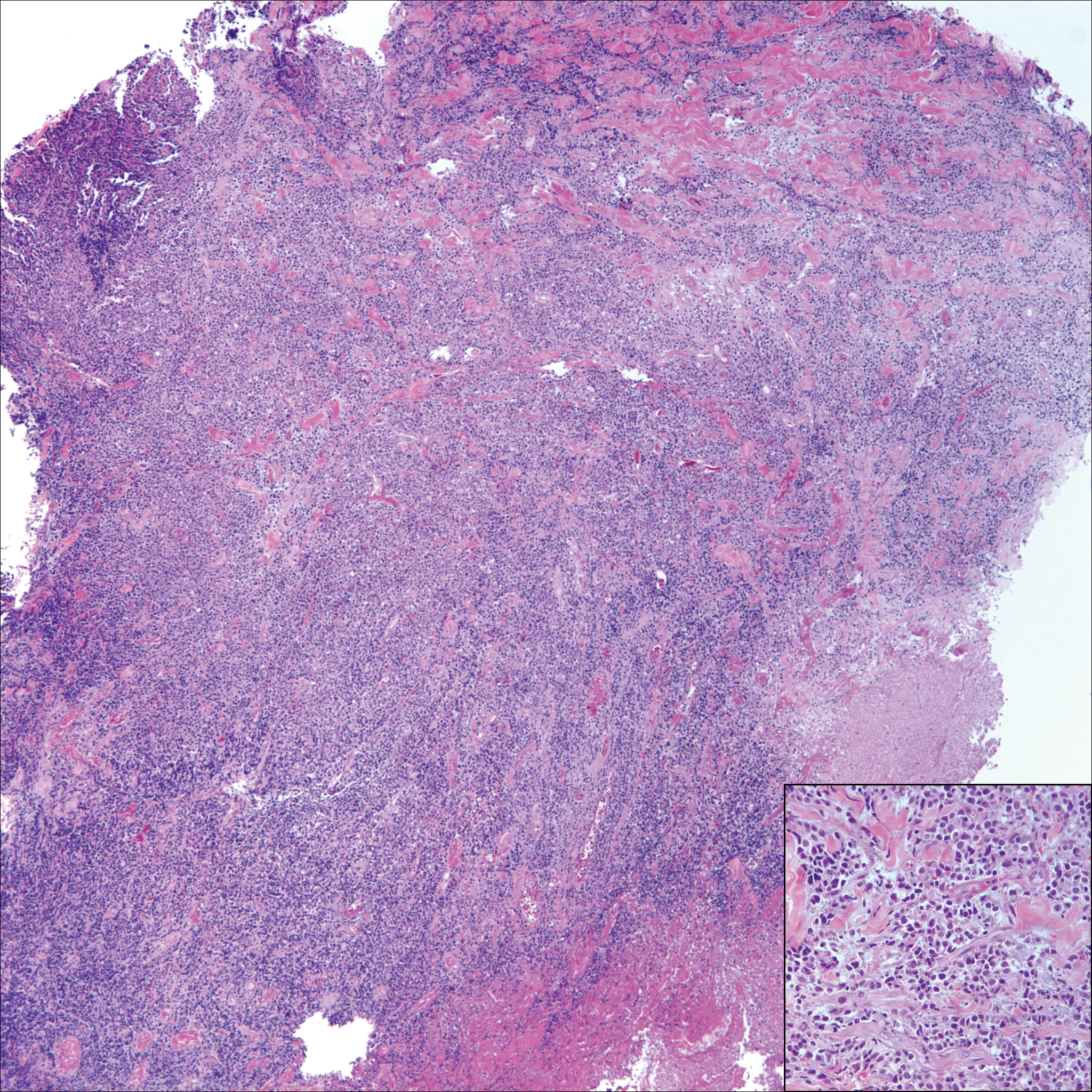
A 65-year-old white woman presented with an asymptomatic bump on the left upper arm of 4 months' duration that arose following a cat scratch. Physical examination was notable for a 35×30-mm, firm, ulcerated, exophytic nodule. Histologic examination demonstrated an ulcerated epidermis and a dense basophilic infiltrate occupying the entire dermis and extending to the subcutaneous tissue. Higher magnification (inset) demonstrated a pleomorphic population of medium- to large-sized discohesive round cells containing variable amounts of slightly eosinophilic cytoplasm, irregular nuclear contours, and prominent nucleoli. Scattered atypical mitotic figures were identified. CD30, CD4, leukocyte common antigen, and Ki-67 immunostains were strongly and diffusely positive. Notable negative stains included anaplastic lymphoma kinase, synaptophysin, epithelial membrane antigen, neuron-specific enolase, CD20, and S-100.
Primary Cutaneous Follicle Center Lymphoma Mimicking Folliculitis
The 2008 World Health Organization and European Organization for Treatment of Cancer joint classification has distinguished 3 categories of primary cutaneous B-cell lymphomas: primary cutaneous follicle center lymphoma (PCFCL), primary cutaneous diffuse large B-cell lymphoma, and primary cutaneous marginal zone lymphoma.1-3 Primary cutaneous follicle center lymphoma is the most common type of cutaneous B-cell lymphoma, accounting for approximately 60% of cases worldwide.4 The median age at diagnosis is 60 years, and most lesions are located on the scalp, forehead, neck, and trunk.5 Histologically, PCFCL is characterized by dermal proliferation of centrocytes and centroblasts derived from germinal center B cells that are arranged in either a follicular, diffuse, or mixed growth pattern.1 The cutaneous manifestations of PCFCL include solitary erythematous or violaceous plaques, nodules, or tumors of varying sizes.4 Grouped lesions also may be observed, but multifocal disease is rare.1 We report a rare presentation of PCFCL mimicking folliculitis with multiple multifocal papules on the back.
Case Report
A 54-year-old woman presented with fever and leukocytosis of 4 days’ duration and was admitted to the hospital for presumed sepsis. She had a history of mastectomy for treatment of ductal carcinoma in situ of the right breast 5 years prior to the current presentation and endocrine therapy with tamoxifen. Her symptoms were thought to be a complication from a surgery for implantation of a tissue expander in the right breast 5 years prior to presentation.
During her hospital admission, she developed a papular and cystic eruption on the back that was clinically suggestive of folliculitis, transient acantholytic dermatosis (Grover disease), or miliaria rubra (Figure 1). This papular and cystic eruption initially was managed conservatively with observation as she recovered from an occult infection. Due to the persistent nature of the eruption on the back, an excisional biopsy of the cystic component was performed 2 months after her discharge from the hospital. Histologic studies showed a dense infiltrate of lymphocytes, which expanded into the deep dermis in a nodular and diffuse growth pattern that was accentuated in the periadnexal areas. The B lymphocytes were small and hyperchromatic with few scattered centroblasts (Figure 2). Further immunohistochemical studies demonstrated that the neoplastic cells were positive for CD20, CD79a, BCL-2, and BCL-6; CD3, CD5, and cyclin D1 were negative. Staining for antigen Ki-67 revealed a proliferation index of 15% to 20% among the neoplastic cells (Figure 3). These findings were consistent with either PCFCL or secondary cutaneous follicle center lymphoma.
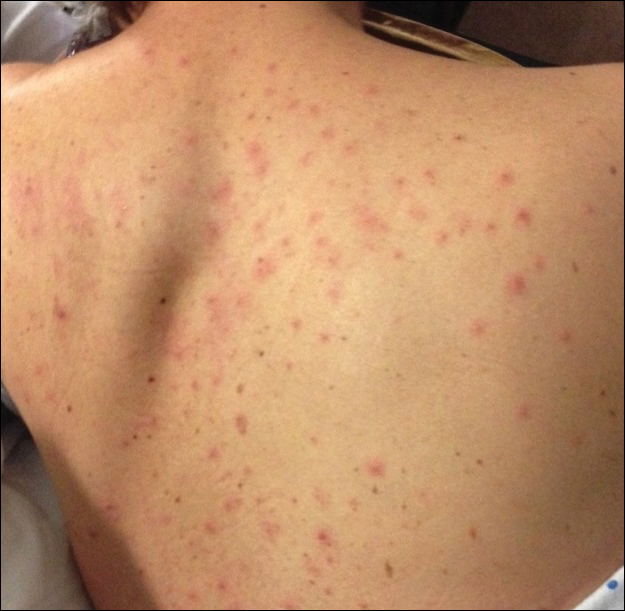
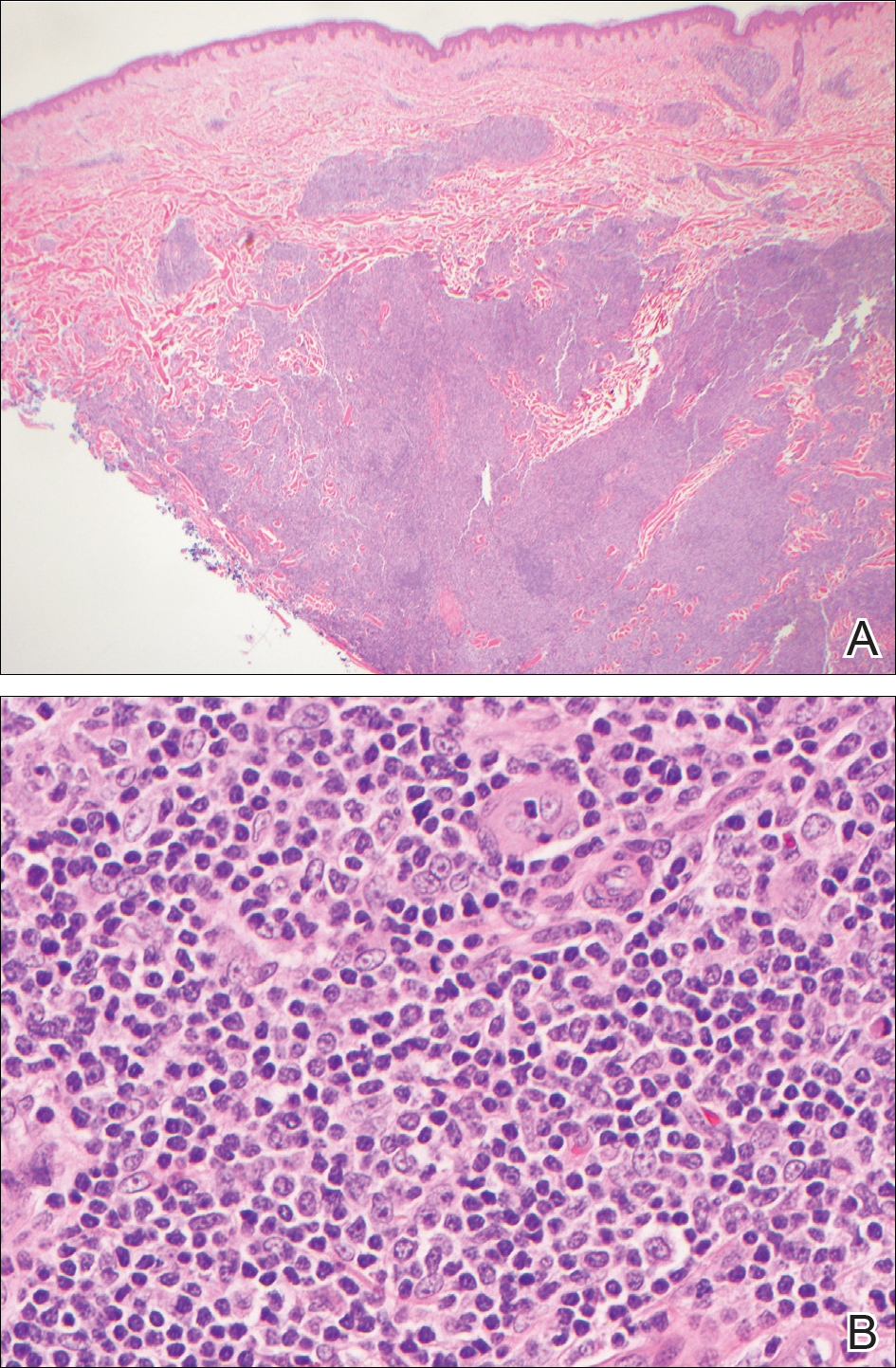
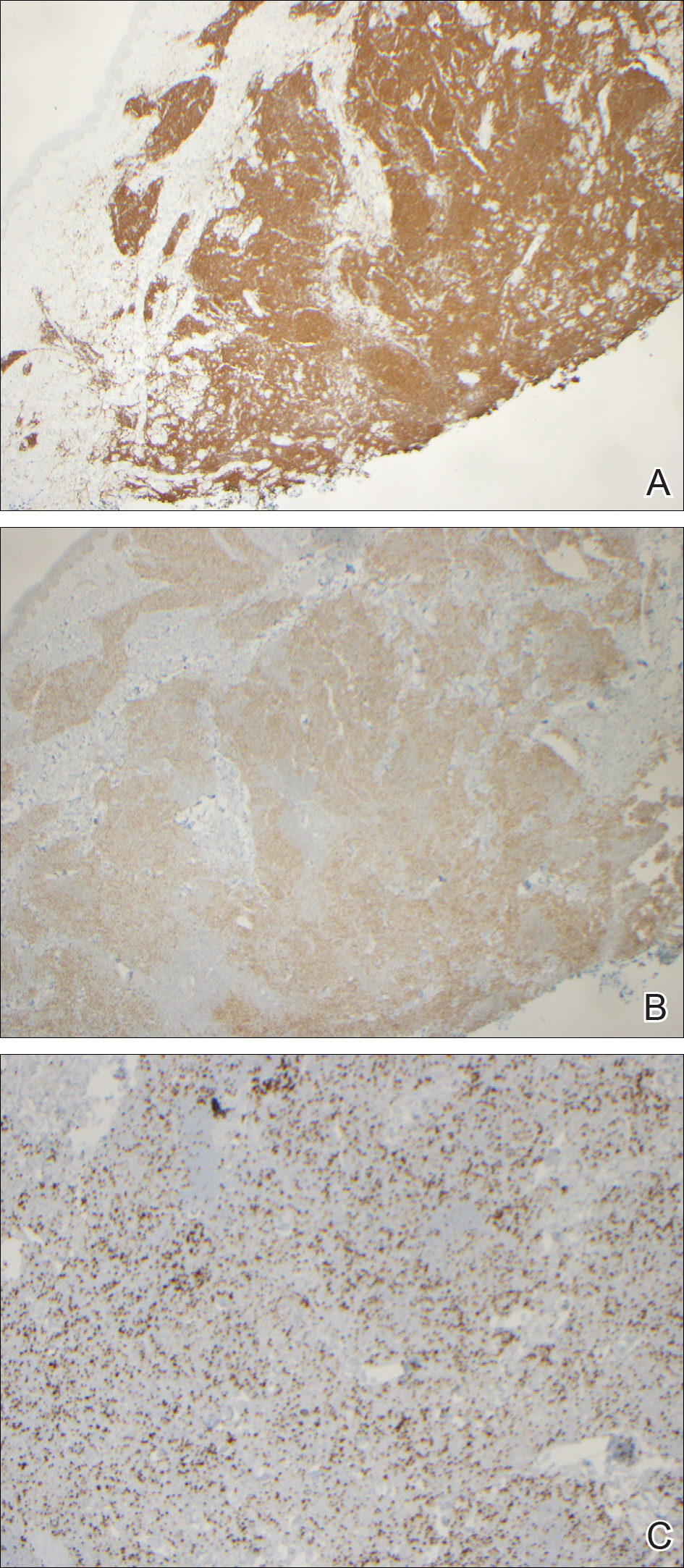
Further evaluation for systemic disease was unremarkable. Positron emission tomography–computed tomography revealed no evidence of nodal lymphoma, and a bone marrow biopsy was negative. Other laboratory studies including lactate dehydrogenase were within reference range, which conferred a diagnosis of PCFCL. The patient was treated with localized electron beam radiation therapy to the skin of the mid back for a total dose of 24 Gy in 12 fractions at 2 Gy per fraction once daily over a 12-day period. She tolerated the treatment well and has remained clinically and radiographically without evidence of disease for more than 3 years.
Comment
Because the incidence of cutaneous B-cell lymphomas has been increasing, especially among males, non-Hispanic whites, and adults older than 50 years,1 it is important for clinicians to have a high index of suspicion for this entity. In our patient, the clinical findings of a papular, largely asymptomatic eruption on the back with acute onset were initially thought to be consistent with folliculitis; the differential diagnosis included transient acantholytic dermatosis and miliaria rubra. Lymphoma was not in the initial clinical differential, and we only arrived at this diagnosis based on histopathologic evaluation.
The neoplastic cells typically are positive for CD20, CD79a, and BCL-6, and negative for BCL-2.4 Most cases of PCFCL do not express the t(14;18) translocation involving the BCL-2 locus, in contrast to systemic follicular lymphoma.1 Systemic imaging and evaluation is needed to definitively differentiate PCFCL from systemic lymphoma with cutaneous involvement. Our patient was unusual in that BCL-2 was strongly staining in the setting of a negative systemic workup.
With regard to treatment of PCFCL, electron beam radiation therapy is highly effective and safe in patients with solitary lesions, as the remission rate is close to 100%.1 For patients with multiple lesions confined to one area, electron beam radiation therapy also can be helpful, as in our patient. In patients with more extensive skin involvement, rituximab therapy may be preferable. Relapse following treatment with either radiation or rituximab occurs in approximately one-third of patients, but these relapses generally are limited to the skin.1 The International Extranodal Lymphoma Study Group has noted that elevated lactate dehydrogenase, presence of more than 2 skin lesions, and presence of nodular lesions are negative prognostic factors in patients with PCFCL6; however, PCFCL has an excellent prognosis overall with a 5-year survival rate of 95%.1
Other rare heterogeneous presentations of PCFCL have been reported in the literature. A large multinodular mass on the scalp with multifocal facial lesions has been described in a patient with essential thrombocytopenia.7 Another report identified a variant of PCFCL characterized by multiple erythematous firm papules that were distributed in a miliary pattern, predominantly on the forehead and cheeks.8 Barzilai et al9 described 4 patients with PCFCL who developed lesions that were clinically similar to rosacea or rhinophyma, including papulonodular eruptions on the cheeks; infiltrated erythematous nasal plaques; and small flesh-colored to erythematous papules on the cheeks, nose, helices, and upper back. Hodak et al10 identified 2 cases of PCFCL that manifested as anetoderma, a condition characterized by the focal loss of elastic tissue. In the setting of chronic lymphocytic leukemia, PCFCL has been observed as a red or violaceous nodule with a centrally depressed scar on the legs.11 In one case, PCFCL manifested as recurrent episodes of extraorbital swelling and a multifocal red-blue macular lesion that extended from the inferior orbital rim to the nasojugal fold.12 An interesting presentation of PCFCL was noted as a small, recurring, blood-filled blister on the cheek with perineural spread of the tumor along cranial nerves V2, V3, VII, and VIII.13 In the pediatric literature, PCFCL has been reported to present as an erythematous nodule with a smooth surface and a hard elastic consistency that appeared on the nose and nasolabial fold and spread to the ipsilateral cheek, maxillary sinus, and soft palate.14 In many of these unusual cases, the diagnosis of PCFCL was made after treatment with topical or systemic anti-inflammatory therapies failed.
Increased recognition of anomalous presentations of PCFCL among dermatologists can lead to more timely diagnoses and treatment. Based on our experience with this patient, we recommend considering biopsy for histopathologic evaluation when treating patients with presumed folliculitis or transient acantholytic dermatosis that does not improve with routine treatment or is accompanied by systemic symptoms.
- Wilcox RA. Cutaneous B-cell lymphomas: 2015 update on diagnosis, risk-stratification, and management. Am J Hematol. 2015;90:73-76.
- Kim YH, Willemze R, Pimpinelli N, et al. TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sézary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:479-484.
- World Health Organization. WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. Lyon, France: World Health Organization; 2008: 227.
- Dilly M, Ben-Rejeb H, Vergier B, et al. Primary cutaneous follicle center lymphoma with Hodgkin and Reed-Sternberg-like cells: a new histopathologic variant. J Cutan Pathol. 2014;41:797-801.
- Suárez AL, Pulitzer M, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part I. clinical features, diagnosis, and classification. J Am Acad Dermatol. 2013;69:329.e1-13; quiz 341-342.
- Mian M, Marcheselli L, Luminari S, et al. CLIPI: a new prognostic index for indolent cutaneous B cell lymphoma proposed by the International Extranodal Lymphoma Study Group (IELSG 11) [published online September 25, 2010]. Ann Hematol. 2011;90:401-408.
- Tirefort Y, Pham XC, Ibrahim YL, et al. A rare case of primary cutaneous follicle centre lymphoma presenting as a giant tumour of the scalp and combined with JAK2V617F positive essential thrombocythaemia. Biomark Res. 2014;2:7.
- Massone C, Fink-Puches R, Laimer M, et al. Miliary and agminated-type primary cutaneous follicle center lymphoma: report of 18 cases.J Am Acad Dermatol. 2011;65:749-755.
- Barzilai A, Feuerman H, Quaglino P, et al. Cutaneous B-cell neoplasms mimicking granulomatous rosacea or rhinophyma. Arch Dermatol. 2012;148:824-831.
- Hodak E, Feuerman H, Barzilai A, et al. Anetodermic primary cutaneous B-cell lymphoma: a unique clinicopathological presentation of lymphoma possibly associated with antiphospholipid antibodies. Arch Dermatol. 2010;146:175-182.
- Konda S, Beckford A, Demierre MF, et al. Primary cutaneous follicle center lymphoma in the setting of chronic lymphocytic leukemia. Indian J Dermatol Venereol Leprol. 2011;77:314-317.
- Pandya VB, Conway RM, Taylor SF. Primary cutaneous B cell lymphoma presenting as recurrent eyelid swelling. Clin Exp Ophthalmol. 2008;36:672-674.
- Buda-Okreglak EM, Walden MJ, Brissette MD. Perineural CNS invasion in primary cutaneous follicular center lymphoma. J Clin Oncol. 2007;25:4684-4686.
- Ghislanzoni M, Gambini D, Perrone T, et al. Primary cutaneous follicular center cell lymphoma of the nose with maxillary sinus involvement in a pediatric patient. J Am Acad Dermatol. 2005;52(5 suppl 1):S73-S75.
The 2008 World Health Organization and European Organization for Treatment of Cancer joint classification has distinguished 3 categories of primary cutaneous B-cell lymphomas: primary cutaneous follicle center lymphoma (PCFCL), primary cutaneous diffuse large B-cell lymphoma, and primary cutaneous marginal zone lymphoma.1-3 Primary cutaneous follicle center lymphoma is the most common type of cutaneous B-cell lymphoma, accounting for approximately 60% of cases worldwide.4 The median age at diagnosis is 60 years, and most lesions are located on the scalp, forehead, neck, and trunk.5 Histologically, PCFCL is characterized by dermal proliferation of centrocytes and centroblasts derived from germinal center B cells that are arranged in either a follicular, diffuse, or mixed growth pattern.1 The cutaneous manifestations of PCFCL include solitary erythematous or violaceous plaques, nodules, or tumors of varying sizes.4 Grouped lesions also may be observed, but multifocal disease is rare.1 We report a rare presentation of PCFCL mimicking folliculitis with multiple multifocal papules on the back.
Case Report
A 54-year-old woman presented with fever and leukocytosis of 4 days’ duration and was admitted to the hospital for presumed sepsis. She had a history of mastectomy for treatment of ductal carcinoma in situ of the right breast 5 years prior to the current presentation and endocrine therapy with tamoxifen. Her symptoms were thought to be a complication from a surgery for implantation of a tissue expander in the right breast 5 years prior to presentation.
During her hospital admission, she developed a papular and cystic eruption on the back that was clinically suggestive of folliculitis, transient acantholytic dermatosis (Grover disease), or miliaria rubra (Figure 1). This papular and cystic eruption initially was managed conservatively with observation as she recovered from an occult infection. Due to the persistent nature of the eruption on the back, an excisional biopsy of the cystic component was performed 2 months after her discharge from the hospital. Histologic studies showed a dense infiltrate of lymphocytes, which expanded into the deep dermis in a nodular and diffuse growth pattern that was accentuated in the periadnexal areas. The B lymphocytes were small and hyperchromatic with few scattered centroblasts (Figure 2). Further immunohistochemical studies demonstrated that the neoplastic cells were positive for CD20, CD79a, BCL-2, and BCL-6; CD3, CD5, and cyclin D1 were negative. Staining for antigen Ki-67 revealed a proliferation index of 15% to 20% among the neoplastic cells (Figure 3). These findings were consistent with either PCFCL or secondary cutaneous follicle center lymphoma.



Further evaluation for systemic disease was unremarkable. Positron emission tomography–computed tomography revealed no evidence of nodal lymphoma, and a bone marrow biopsy was negative. Other laboratory studies including lactate dehydrogenase were within reference range, which conferred a diagnosis of PCFCL. The patient was treated with localized electron beam radiation therapy to the skin of the mid back for a total dose of 24 Gy in 12 fractions at 2 Gy per fraction once daily over a 12-day period. She tolerated the treatment well and has remained clinically and radiographically without evidence of disease for more than 3 years.
Comment
Because the incidence of cutaneous B-cell lymphomas has been increasing, especially among males, non-Hispanic whites, and adults older than 50 years,1 it is important for clinicians to have a high index of suspicion for this entity. In our patient, the clinical findings of a papular, largely asymptomatic eruption on the back with acute onset were initially thought to be consistent with folliculitis; the differential diagnosis included transient acantholytic dermatosis and miliaria rubra. Lymphoma was not in the initial clinical differential, and we only arrived at this diagnosis based on histopathologic evaluation.
The neoplastic cells typically are positive for CD20, CD79a, and BCL-6, and negative for BCL-2.4 Most cases of PCFCL do not express the t(14;18) translocation involving the BCL-2 locus, in contrast to systemic follicular lymphoma.1 Systemic imaging and evaluation is needed to definitively differentiate PCFCL from systemic lymphoma with cutaneous involvement. Our patient was unusual in that BCL-2 was strongly staining in the setting of a negative systemic workup.
With regard to treatment of PCFCL, electron beam radiation therapy is highly effective and safe in patients with solitary lesions, as the remission rate is close to 100%.1 For patients with multiple lesions confined to one area, electron beam radiation therapy also can be helpful, as in our patient. In patients with more extensive skin involvement, rituximab therapy may be preferable. Relapse following treatment with either radiation or rituximab occurs in approximately one-third of patients, but these relapses generally are limited to the skin.1 The International Extranodal Lymphoma Study Group has noted that elevated lactate dehydrogenase, presence of more than 2 skin lesions, and presence of nodular lesions are negative prognostic factors in patients with PCFCL6; however, PCFCL has an excellent prognosis overall with a 5-year survival rate of 95%.1
Other rare heterogeneous presentations of PCFCL have been reported in the literature. A large multinodular mass on the scalp with multifocal facial lesions has been described in a patient with essential thrombocytopenia.7 Another report identified a variant of PCFCL characterized by multiple erythematous firm papules that were distributed in a miliary pattern, predominantly on the forehead and cheeks.8 Barzilai et al9 described 4 patients with PCFCL who developed lesions that were clinically similar to rosacea or rhinophyma, including papulonodular eruptions on the cheeks; infiltrated erythematous nasal plaques; and small flesh-colored to erythematous papules on the cheeks, nose, helices, and upper back. Hodak et al10 identified 2 cases of PCFCL that manifested as anetoderma, a condition characterized by the focal loss of elastic tissue. In the setting of chronic lymphocytic leukemia, PCFCL has been observed as a red or violaceous nodule with a centrally depressed scar on the legs.11 In one case, PCFCL manifested as recurrent episodes of extraorbital swelling and a multifocal red-blue macular lesion that extended from the inferior orbital rim to the nasojugal fold.12 An interesting presentation of PCFCL was noted as a small, recurring, blood-filled blister on the cheek with perineural spread of the tumor along cranial nerves V2, V3, VII, and VIII.13 In the pediatric literature, PCFCL has been reported to present as an erythematous nodule with a smooth surface and a hard elastic consistency that appeared on the nose and nasolabial fold and spread to the ipsilateral cheek, maxillary sinus, and soft palate.14 In many of these unusual cases, the diagnosis of PCFCL was made after treatment with topical or systemic anti-inflammatory therapies failed.
Increased recognition of anomalous presentations of PCFCL among dermatologists can lead to more timely diagnoses and treatment. Based on our experience with this patient, we recommend considering biopsy for histopathologic evaluation when treating patients with presumed folliculitis or transient acantholytic dermatosis that does not improve with routine treatment or is accompanied by systemic symptoms.
The 2008 World Health Organization and European Organization for Treatment of Cancer joint classification has distinguished 3 categories of primary cutaneous B-cell lymphomas: primary cutaneous follicle center lymphoma (PCFCL), primary cutaneous diffuse large B-cell lymphoma, and primary cutaneous marginal zone lymphoma.1-3 Primary cutaneous follicle center lymphoma is the most common type of cutaneous B-cell lymphoma, accounting for approximately 60% of cases worldwide.4 The median age at diagnosis is 60 years, and most lesions are located on the scalp, forehead, neck, and trunk.5 Histologically, PCFCL is characterized by dermal proliferation of centrocytes and centroblasts derived from germinal center B cells that are arranged in either a follicular, diffuse, or mixed growth pattern.1 The cutaneous manifestations of PCFCL include solitary erythematous or violaceous plaques, nodules, or tumors of varying sizes.4 Grouped lesions also may be observed, but multifocal disease is rare.1 We report a rare presentation of PCFCL mimicking folliculitis with multiple multifocal papules on the back.
Case Report
A 54-year-old woman presented with fever and leukocytosis of 4 days’ duration and was admitted to the hospital for presumed sepsis. She had a history of mastectomy for treatment of ductal carcinoma in situ of the right breast 5 years prior to the current presentation and endocrine therapy with tamoxifen. Her symptoms were thought to be a complication from a surgery for implantation of a tissue expander in the right breast 5 years prior to presentation.
During her hospital admission, she developed a papular and cystic eruption on the back that was clinically suggestive of folliculitis, transient acantholytic dermatosis (Grover disease), or miliaria rubra (Figure 1). This papular and cystic eruption initially was managed conservatively with observation as she recovered from an occult infection. Due to the persistent nature of the eruption on the back, an excisional biopsy of the cystic component was performed 2 months after her discharge from the hospital. Histologic studies showed a dense infiltrate of lymphocytes, which expanded into the deep dermis in a nodular and diffuse growth pattern that was accentuated in the periadnexal areas. The B lymphocytes were small and hyperchromatic with few scattered centroblasts (Figure 2). Further immunohistochemical studies demonstrated that the neoplastic cells were positive for CD20, CD79a, BCL-2, and BCL-6; CD3, CD5, and cyclin D1 were negative. Staining for antigen Ki-67 revealed a proliferation index of 15% to 20% among the neoplastic cells (Figure 3). These findings were consistent with either PCFCL or secondary cutaneous follicle center lymphoma.



Further evaluation for systemic disease was unremarkable. Positron emission tomography–computed tomography revealed no evidence of nodal lymphoma, and a bone marrow biopsy was negative. Other laboratory studies including lactate dehydrogenase were within reference range, which conferred a diagnosis of PCFCL. The patient was treated with localized electron beam radiation therapy to the skin of the mid back for a total dose of 24 Gy in 12 fractions at 2 Gy per fraction once daily over a 12-day period. She tolerated the treatment well and has remained clinically and radiographically without evidence of disease for more than 3 years.
Comment
Because the incidence of cutaneous B-cell lymphomas has been increasing, especially among males, non-Hispanic whites, and adults older than 50 years,1 it is important for clinicians to have a high index of suspicion for this entity. In our patient, the clinical findings of a papular, largely asymptomatic eruption on the back with acute onset were initially thought to be consistent with folliculitis; the differential diagnosis included transient acantholytic dermatosis and miliaria rubra. Lymphoma was not in the initial clinical differential, and we only arrived at this diagnosis based on histopathologic evaluation.
The neoplastic cells typically are positive for CD20, CD79a, and BCL-6, and negative for BCL-2.4 Most cases of PCFCL do not express the t(14;18) translocation involving the BCL-2 locus, in contrast to systemic follicular lymphoma.1 Systemic imaging and evaluation is needed to definitively differentiate PCFCL from systemic lymphoma with cutaneous involvement. Our patient was unusual in that BCL-2 was strongly staining in the setting of a negative systemic workup.
With regard to treatment of PCFCL, electron beam radiation therapy is highly effective and safe in patients with solitary lesions, as the remission rate is close to 100%.1 For patients with multiple lesions confined to one area, electron beam radiation therapy also can be helpful, as in our patient. In patients with more extensive skin involvement, rituximab therapy may be preferable. Relapse following treatment with either radiation or rituximab occurs in approximately one-third of patients, but these relapses generally are limited to the skin.1 The International Extranodal Lymphoma Study Group has noted that elevated lactate dehydrogenase, presence of more than 2 skin lesions, and presence of nodular lesions are negative prognostic factors in patients with PCFCL6; however, PCFCL has an excellent prognosis overall with a 5-year survival rate of 95%.1
Other rare heterogeneous presentations of PCFCL have been reported in the literature. A large multinodular mass on the scalp with multifocal facial lesions has been described in a patient with essential thrombocytopenia.7 Another report identified a variant of PCFCL characterized by multiple erythematous firm papules that were distributed in a miliary pattern, predominantly on the forehead and cheeks.8 Barzilai et al9 described 4 patients with PCFCL who developed lesions that were clinically similar to rosacea or rhinophyma, including papulonodular eruptions on the cheeks; infiltrated erythematous nasal plaques; and small flesh-colored to erythematous papules on the cheeks, nose, helices, and upper back. Hodak et al10 identified 2 cases of PCFCL that manifested as anetoderma, a condition characterized by the focal loss of elastic tissue. In the setting of chronic lymphocytic leukemia, PCFCL has been observed as a red or violaceous nodule with a centrally depressed scar on the legs.11 In one case, PCFCL manifested as recurrent episodes of extraorbital swelling and a multifocal red-blue macular lesion that extended from the inferior orbital rim to the nasojugal fold.12 An interesting presentation of PCFCL was noted as a small, recurring, blood-filled blister on the cheek with perineural spread of the tumor along cranial nerves V2, V3, VII, and VIII.13 In the pediatric literature, PCFCL has been reported to present as an erythematous nodule with a smooth surface and a hard elastic consistency that appeared on the nose and nasolabial fold and spread to the ipsilateral cheek, maxillary sinus, and soft palate.14 In many of these unusual cases, the diagnosis of PCFCL was made after treatment with topical or systemic anti-inflammatory therapies failed.
Increased recognition of anomalous presentations of PCFCL among dermatologists can lead to more timely diagnoses and treatment. Based on our experience with this patient, we recommend considering biopsy for histopathologic evaluation when treating patients with presumed folliculitis or transient acantholytic dermatosis that does not improve with routine treatment or is accompanied by systemic symptoms.
- Wilcox RA. Cutaneous B-cell lymphomas: 2015 update on diagnosis, risk-stratification, and management. Am J Hematol. 2015;90:73-76.
- Kim YH, Willemze R, Pimpinelli N, et al. TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sézary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:479-484.
- World Health Organization. WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. Lyon, France: World Health Organization; 2008: 227.
- Dilly M, Ben-Rejeb H, Vergier B, et al. Primary cutaneous follicle center lymphoma with Hodgkin and Reed-Sternberg-like cells: a new histopathologic variant. J Cutan Pathol. 2014;41:797-801.
- Suárez AL, Pulitzer M, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part I. clinical features, diagnosis, and classification. J Am Acad Dermatol. 2013;69:329.e1-13; quiz 341-342.
- Mian M, Marcheselli L, Luminari S, et al. CLIPI: a new prognostic index for indolent cutaneous B cell lymphoma proposed by the International Extranodal Lymphoma Study Group (IELSG 11) [published online September 25, 2010]. Ann Hematol. 2011;90:401-408.
- Tirefort Y, Pham XC, Ibrahim YL, et al. A rare case of primary cutaneous follicle centre lymphoma presenting as a giant tumour of the scalp and combined with JAK2V617F positive essential thrombocythaemia. Biomark Res. 2014;2:7.
- Massone C, Fink-Puches R, Laimer M, et al. Miliary and agminated-type primary cutaneous follicle center lymphoma: report of 18 cases.J Am Acad Dermatol. 2011;65:749-755.
- Barzilai A, Feuerman H, Quaglino P, et al. Cutaneous B-cell neoplasms mimicking granulomatous rosacea or rhinophyma. Arch Dermatol. 2012;148:824-831.
- Hodak E, Feuerman H, Barzilai A, et al. Anetodermic primary cutaneous B-cell lymphoma: a unique clinicopathological presentation of lymphoma possibly associated with antiphospholipid antibodies. Arch Dermatol. 2010;146:175-182.
- Konda S, Beckford A, Demierre MF, et al. Primary cutaneous follicle center lymphoma in the setting of chronic lymphocytic leukemia. Indian J Dermatol Venereol Leprol. 2011;77:314-317.
- Pandya VB, Conway RM, Taylor SF. Primary cutaneous B cell lymphoma presenting as recurrent eyelid swelling. Clin Exp Ophthalmol. 2008;36:672-674.
- Buda-Okreglak EM, Walden MJ, Brissette MD. Perineural CNS invasion in primary cutaneous follicular center lymphoma. J Clin Oncol. 2007;25:4684-4686.
- Ghislanzoni M, Gambini D, Perrone T, et al. Primary cutaneous follicular center cell lymphoma of the nose with maxillary sinus involvement in a pediatric patient. J Am Acad Dermatol. 2005;52(5 suppl 1):S73-S75.
- Wilcox RA. Cutaneous B-cell lymphomas: 2015 update on diagnosis, risk-stratification, and management. Am J Hematol. 2015;90:73-76.
- Kim YH, Willemze R, Pimpinelli N, et al. TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sézary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:479-484.
- World Health Organization. WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. Lyon, France: World Health Organization; 2008: 227.
- Dilly M, Ben-Rejeb H, Vergier B, et al. Primary cutaneous follicle center lymphoma with Hodgkin and Reed-Sternberg-like cells: a new histopathologic variant. J Cutan Pathol. 2014;41:797-801.
- Suárez AL, Pulitzer M, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part I. clinical features, diagnosis, and classification. J Am Acad Dermatol. 2013;69:329.e1-13; quiz 341-342.
- Mian M, Marcheselli L, Luminari S, et al. CLIPI: a new prognostic index for indolent cutaneous B cell lymphoma proposed by the International Extranodal Lymphoma Study Group (IELSG 11) [published online September 25, 2010]. Ann Hematol. 2011;90:401-408.
- Tirefort Y, Pham XC, Ibrahim YL, et al. A rare case of primary cutaneous follicle centre lymphoma presenting as a giant tumour of the scalp and combined with JAK2V617F positive essential thrombocythaemia. Biomark Res. 2014;2:7.
- Massone C, Fink-Puches R, Laimer M, et al. Miliary and agminated-type primary cutaneous follicle center lymphoma: report of 18 cases.J Am Acad Dermatol. 2011;65:749-755.
- Barzilai A, Feuerman H, Quaglino P, et al. Cutaneous B-cell neoplasms mimicking granulomatous rosacea or rhinophyma. Arch Dermatol. 2012;148:824-831.
- Hodak E, Feuerman H, Barzilai A, et al. Anetodermic primary cutaneous B-cell lymphoma: a unique clinicopathological presentation of lymphoma possibly associated with antiphospholipid antibodies. Arch Dermatol. 2010;146:175-182.
- Konda S, Beckford A, Demierre MF, et al. Primary cutaneous follicle center lymphoma in the setting of chronic lymphocytic leukemia. Indian J Dermatol Venereol Leprol. 2011;77:314-317.
- Pandya VB, Conway RM, Taylor SF. Primary cutaneous B cell lymphoma presenting as recurrent eyelid swelling. Clin Exp Ophthalmol. 2008;36:672-674.
- Buda-Okreglak EM, Walden MJ, Brissette MD. Perineural CNS invasion in primary cutaneous follicular center lymphoma. J Clin Oncol. 2007;25:4684-4686.
- Ghislanzoni M, Gambini D, Perrone T, et al. Primary cutaneous follicular center cell lymphoma of the nose with maxillary sinus involvement in a pediatric patient. J Am Acad Dermatol. 2005;52(5 suppl 1):S73-S75.
Practice Points
- Atypical or unresponsive folliculitis should be biopsied.
- Primary cutaneous follicle center lymphoma can mimic folliculitis or Grover disease.
Nonmalignant Cutaneous Findings Associated With Vemurafenib
To the Editor:
A 53-year-old woman was referred by her oncologist to our dermatology office with lesions on the face and body that presented 8 days after starting vemurafenib 960 mg twice daily for metastatic melanoma. The patient denied any symptoms from the lesions but was concerned they would spread to cover her entire face and body.
The patient's medical history included a diagnosis of metastatic melanoma 6 years prior to presentation. She stated that the primary cutaneous melanoma site was unknown. The patient had endured numerous surgeries to excise lymph node tumors, with some lesions up to 3 cm. The patient recently started vemurafenib, a treatment for BRAF V600E mutation-positive metastatic melanoma. The patient's personal history was notable for hepatitis A, B, and C, and her family history revealed her mother had metastatic lung cancer.
Physical examination revealed numerous 2- to 3-mm, round-oval, flesh-colored to light-brown papules on the cheeks, chest, abdomen (Figure 1), back, and both arms and legs. Some papules were inflamed and some had a stuck-on appearance. Lesions on the chest between the breasts and inframammary region were slightly inflamed. Two skin biopsies were performed. Biopsy of the lesion on the right lateral back revealed solar lentigo, early macular seborrheic keratosis, and a focus of inflamed mild solar keratosis. The dermis showed a mild superficial perivascular and interstitial inflammatory infiltrate composed mostly of lymphocytes, histiocytes, and eosinophils. There were occasional melanophages present (Figure 2). Biopsy of the lesion between the breasts revealed inflamed verrucous seborrheic keratosis (Figure 3).


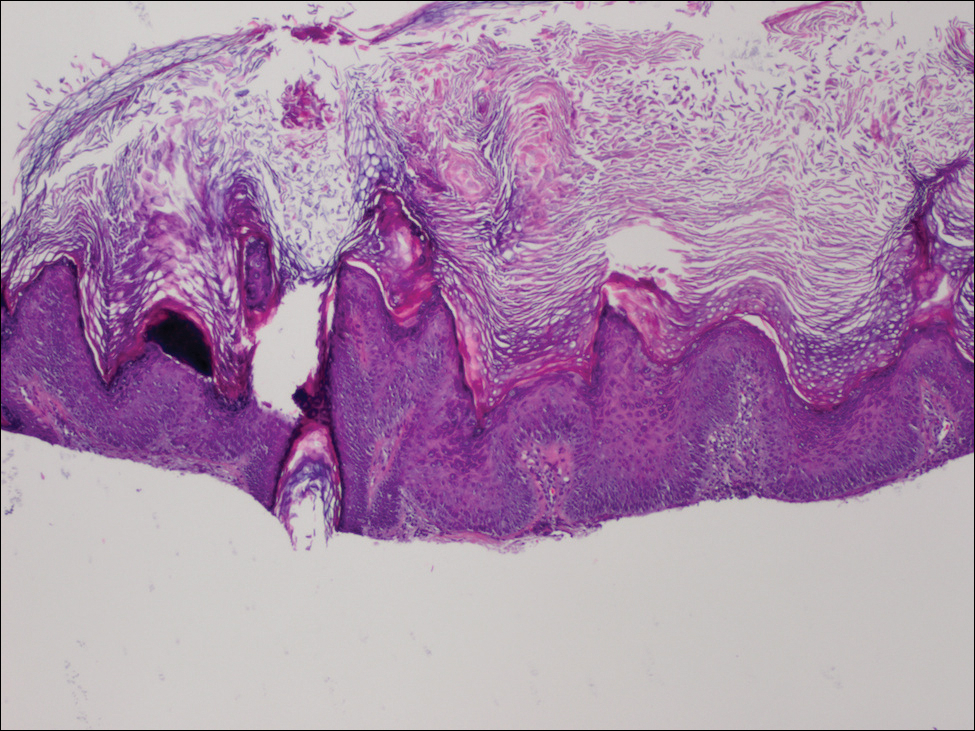
We treated the lesion on the right lateral back with cycles of cryotherapy and explained to the patient that the lesion between the breasts was benign. We also reiterated to the patient the importance of wearing sun-protective clothing and UVA/UVB sunblock with a sun protection factor of 30 or higher.
Our patient was diagnosed with pneumonia and subsequently had to discontinue vemurafenib. During the period of nontreatment, the keratotic lesions cleared with postinflammatory hyperpigmentation and no epidermal changes, which showed a possible inference of a direct relationship between the vemurafenib and the appearance of the nonmalignant cutaneous lesions. Although this report only represents 1 patient, other patients possibly can benefit from a modified dose of vemurafenib, which either would resolve or lessen the quantity of these lesions.
Vemurafenib is the first US Food and Drug Administration-approved treatment for nonresectable metastatic melanoma with the BRAF V600E mutation as detected by a US Food and Drug Administration-approved test.1,2 Mutated BRAF is present in approximately 60% of cutaneous melanomas.3 Vemurafenib targets the oncogenic BRAF V600E making the protein inactive, thus inhibiting cell proliferation and leading to apoptosis and shrinkage of the metastatic tumors.3-5 Vemurafenib has a response rate of more than 50% and is associated with rapid improvement in quality of life.3
Cutaneous side effects include increased incidence of squamous cell carcinoma and keratoacanthomas, appearing approximately 7 to 8 weeks after starting vemurafenib.4 The incidence of these lesions increases in patients 65 years and older and in patients with prior skin cancer and chronic sun exposure. The paradoxical activation of the mitogen-activated protein kinase pathway by mutant BRAF-selective inhibitors provides an explanation of the induction of squamous cell carcinomas.4 Prior to the initiation of vemurafenib, all patients should receive a total-body skin examination and every 2 months thereafter while on treatment. After discontinuation of the medicine, the patient should continue to receive total-body skin evaluations every 6 months indefinitely.
Patients should be aware of the potential for mild to severe photosensitivity reactions. They should be advised to limit their sun exposure time and to wear sun-protective clothing when outdoors. The use of broad-spectrum UVA/UVB sunscreen and lip protectant with a sun protection factor of 30 or higher also should be stressed.6,7 Patients should be aware that UVA rays penetrate glass; therefore, UV-protective clothing should be worn throughout the day and during all seasons.7
In clinical trials of vemurafenib, Stevens-Johnson syndrome and toxic epidermal necrolysis was reported in 2 patients.8,9 Clinical trials also reported patients developing new primary malignant melanoma lesions.10 These findings further emphasize the need for patients to undergo total-body skin examinations during and after treatment.
Other possible dermatologic reactions include a generalized rash, erythema, alopecia, and pruritus.2,3 The development of benign growths associated with patients on vemurafenib include follicular plugging seen in keratosis pilaris, palmar and plantar hyperkeratosis, seborrheic dermatitis-like rashes, verrucous keratosis, and acantholytic dyskeratosis.8,11,12
We report a case of nonmalignant growths occurring 8 days after starting vemurafenib. This case illustrates potential cutaneous adverse reactions that were benign yet still of great concern to our patient. Many of these nonmalignant cutaneous findings are associated with abnormal follicular keratinization thought to be secondary to abnormal signaling of the mitogen-activated protein kinase pathway that occurs with the use of BRAF inhibitors.8 Although in this case malignant lesions were not discovered, the need for total-body skin examinations exists during all stages of treatment. Supportive care and reassurance should be given to patients along with local treatments including topical therapies (steroids, retinoids), cryotherapy, and biopsies or excisions when necessary.13,14
- Holstein S, Hohl R. Therapeutic additions and possible deletions in oncology in 2011. Clin Pharmacol Ther. 2011;91:15-17.
- Zambon A, Niculescu-Dovaz I, Niculescu-Dovaz D, et al. Small molecule inhibitors of BRAF in clinical trials. Bioorg Med Chem Lett. 2012;22:789-792.
- Luke JJ, Hodi FS. Vemurafenib and BRAF inhibition: a new class of treatment for metastatic melanoma [published online November 14, 2011]. Clin Cancer Res. 2012;18:9-14.
- Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010; 363:809-819.
- Tsai J, Lee JT, Wang W, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci USA. 2008;105:3041-3046.
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516.
- Dummer R, Rinderknecht J, Goldinger SM. Ultraviolet A and photosensitivity during vemuranefib therapy. N Engl J Med. 2012;366:480-481.
- Bovd KP, Vincent B, Andrea A, et al. Nonmalignant cutaneous findings associated with vemurafenib use in patients with metastatic melanoma. J Am Acad Dermatol. 2012;67:1375-1379.
- Wang CM, Fleming KF Hsu S. A case of vemurafenib-induced keratosis pilaris-like eruption. Dermatol Online J. 2012;18:7.
- Zimmer L, Hillen U, Livingstone E, et al. Atypical melanocytic proliferations and new primary melanomas in patients with advanced melanoma undergoing selective BRAF inhibition. J Clin Oncol. 2012;30:2375-2383.
- Huang V, Hepper D, Anadkat M, et al. Cutaneous toxic effects associated with vemurafenib and inhibition of the BRAF pathway. Arch Dermatol. 2012;148:628-633.
- Gupta M, Huang V, Linette G, et al. Unusual complication of vemurafenib treatment of metastatic melanoma: exacerbation of acantholytic dyskeratosis complicated by Kaposi varicelliform eruption. Arch Dermatol. 2012;148:966-968;
- Sinha R, Edmonds K, Newton-Bishop JA, et al. Cutaneous adverse events associated with vemurafenib in patients with metastatic melanoma: practical advice on diagnosis, preventions and management of the main treatment related skin toxicities. Br J Dermatol. 2012;167:987-994.
- Boussemart L, Routier E, Mateus C, et al. Prospective study of cutaneous side effects associated with the BRAF inhibitor vemurafenib: a study of 42 patients. Ann Oncol. 2013;24:1691-1697.
To the Editor:
A 53-year-old woman was referred by her oncologist to our dermatology office with lesions on the face and body that presented 8 days after starting vemurafenib 960 mg twice daily for metastatic melanoma. The patient denied any symptoms from the lesions but was concerned they would spread to cover her entire face and body.
The patient's medical history included a diagnosis of metastatic melanoma 6 years prior to presentation. She stated that the primary cutaneous melanoma site was unknown. The patient had endured numerous surgeries to excise lymph node tumors, with some lesions up to 3 cm. The patient recently started vemurafenib, a treatment for BRAF V600E mutation-positive metastatic melanoma. The patient's personal history was notable for hepatitis A, B, and C, and her family history revealed her mother had metastatic lung cancer.
Physical examination revealed numerous 2- to 3-mm, round-oval, flesh-colored to light-brown papules on the cheeks, chest, abdomen (Figure 1), back, and both arms and legs. Some papules were inflamed and some had a stuck-on appearance. Lesions on the chest between the breasts and inframammary region were slightly inflamed. Two skin biopsies were performed. Biopsy of the lesion on the right lateral back revealed solar lentigo, early macular seborrheic keratosis, and a focus of inflamed mild solar keratosis. The dermis showed a mild superficial perivascular and interstitial inflammatory infiltrate composed mostly of lymphocytes, histiocytes, and eosinophils. There were occasional melanophages present (Figure 2). Biopsy of the lesion between the breasts revealed inflamed verrucous seborrheic keratosis (Figure 3).



We treated the lesion on the right lateral back with cycles of cryotherapy and explained to the patient that the lesion between the breasts was benign. We also reiterated to the patient the importance of wearing sun-protective clothing and UVA/UVB sunblock with a sun protection factor of 30 or higher.
Our patient was diagnosed with pneumonia and subsequently had to discontinue vemurafenib. During the period of nontreatment, the keratotic lesions cleared with postinflammatory hyperpigmentation and no epidermal changes, which showed a possible inference of a direct relationship between the vemurafenib and the appearance of the nonmalignant cutaneous lesions. Although this report only represents 1 patient, other patients possibly can benefit from a modified dose of vemurafenib, which either would resolve or lessen the quantity of these lesions.
Vemurafenib is the first US Food and Drug Administration-approved treatment for nonresectable metastatic melanoma with the BRAF V600E mutation as detected by a US Food and Drug Administration-approved test.1,2 Mutated BRAF is present in approximately 60% of cutaneous melanomas.3 Vemurafenib targets the oncogenic BRAF V600E making the protein inactive, thus inhibiting cell proliferation and leading to apoptosis and shrinkage of the metastatic tumors.3-5 Vemurafenib has a response rate of more than 50% and is associated with rapid improvement in quality of life.3
Cutaneous side effects include increased incidence of squamous cell carcinoma and keratoacanthomas, appearing approximately 7 to 8 weeks after starting vemurafenib.4 The incidence of these lesions increases in patients 65 years and older and in patients with prior skin cancer and chronic sun exposure. The paradoxical activation of the mitogen-activated protein kinase pathway by mutant BRAF-selective inhibitors provides an explanation of the induction of squamous cell carcinomas.4 Prior to the initiation of vemurafenib, all patients should receive a total-body skin examination and every 2 months thereafter while on treatment. After discontinuation of the medicine, the patient should continue to receive total-body skin evaluations every 6 months indefinitely.
Patients should be aware of the potential for mild to severe photosensitivity reactions. They should be advised to limit their sun exposure time and to wear sun-protective clothing when outdoors. The use of broad-spectrum UVA/UVB sunscreen and lip protectant with a sun protection factor of 30 or higher also should be stressed.6,7 Patients should be aware that UVA rays penetrate glass; therefore, UV-protective clothing should be worn throughout the day and during all seasons.7
In clinical trials of vemurafenib, Stevens-Johnson syndrome and toxic epidermal necrolysis was reported in 2 patients.8,9 Clinical trials also reported patients developing new primary malignant melanoma lesions.10 These findings further emphasize the need for patients to undergo total-body skin examinations during and after treatment.
Other possible dermatologic reactions include a generalized rash, erythema, alopecia, and pruritus.2,3 The development of benign growths associated with patients on vemurafenib include follicular plugging seen in keratosis pilaris, palmar and plantar hyperkeratosis, seborrheic dermatitis-like rashes, verrucous keratosis, and acantholytic dyskeratosis.8,11,12
We report a case of nonmalignant growths occurring 8 days after starting vemurafenib. This case illustrates potential cutaneous adverse reactions that were benign yet still of great concern to our patient. Many of these nonmalignant cutaneous findings are associated with abnormal follicular keratinization thought to be secondary to abnormal signaling of the mitogen-activated protein kinase pathway that occurs with the use of BRAF inhibitors.8 Although in this case malignant lesions were not discovered, the need for total-body skin examinations exists during all stages of treatment. Supportive care and reassurance should be given to patients along with local treatments including topical therapies (steroids, retinoids), cryotherapy, and biopsies or excisions when necessary.13,14
To the Editor:
A 53-year-old woman was referred by her oncologist to our dermatology office with lesions on the face and body that presented 8 days after starting vemurafenib 960 mg twice daily for metastatic melanoma. The patient denied any symptoms from the lesions but was concerned they would spread to cover her entire face and body.
The patient's medical history included a diagnosis of metastatic melanoma 6 years prior to presentation. She stated that the primary cutaneous melanoma site was unknown. The patient had endured numerous surgeries to excise lymph node tumors, with some lesions up to 3 cm. The patient recently started vemurafenib, a treatment for BRAF V600E mutation-positive metastatic melanoma. The patient's personal history was notable for hepatitis A, B, and C, and her family history revealed her mother had metastatic lung cancer.
Physical examination revealed numerous 2- to 3-mm, round-oval, flesh-colored to light-brown papules on the cheeks, chest, abdomen (Figure 1), back, and both arms and legs. Some papules were inflamed and some had a stuck-on appearance. Lesions on the chest between the breasts and inframammary region were slightly inflamed. Two skin biopsies were performed. Biopsy of the lesion on the right lateral back revealed solar lentigo, early macular seborrheic keratosis, and a focus of inflamed mild solar keratosis. The dermis showed a mild superficial perivascular and interstitial inflammatory infiltrate composed mostly of lymphocytes, histiocytes, and eosinophils. There were occasional melanophages present (Figure 2). Biopsy of the lesion between the breasts revealed inflamed verrucous seborrheic keratosis (Figure 3).



We treated the lesion on the right lateral back with cycles of cryotherapy and explained to the patient that the lesion between the breasts was benign. We also reiterated to the patient the importance of wearing sun-protective clothing and UVA/UVB sunblock with a sun protection factor of 30 or higher.
Our patient was diagnosed with pneumonia and subsequently had to discontinue vemurafenib. During the period of nontreatment, the keratotic lesions cleared with postinflammatory hyperpigmentation and no epidermal changes, which showed a possible inference of a direct relationship between the vemurafenib and the appearance of the nonmalignant cutaneous lesions. Although this report only represents 1 patient, other patients possibly can benefit from a modified dose of vemurafenib, which either would resolve or lessen the quantity of these lesions.
Vemurafenib is the first US Food and Drug Administration-approved treatment for nonresectable metastatic melanoma with the BRAF V600E mutation as detected by a US Food and Drug Administration-approved test.1,2 Mutated BRAF is present in approximately 60% of cutaneous melanomas.3 Vemurafenib targets the oncogenic BRAF V600E making the protein inactive, thus inhibiting cell proliferation and leading to apoptosis and shrinkage of the metastatic tumors.3-5 Vemurafenib has a response rate of more than 50% and is associated with rapid improvement in quality of life.3
Cutaneous side effects include increased incidence of squamous cell carcinoma and keratoacanthomas, appearing approximately 7 to 8 weeks after starting vemurafenib.4 The incidence of these lesions increases in patients 65 years and older and in patients with prior skin cancer and chronic sun exposure. The paradoxical activation of the mitogen-activated protein kinase pathway by mutant BRAF-selective inhibitors provides an explanation of the induction of squamous cell carcinomas.4 Prior to the initiation of vemurafenib, all patients should receive a total-body skin examination and every 2 months thereafter while on treatment. After discontinuation of the medicine, the patient should continue to receive total-body skin evaluations every 6 months indefinitely.
Patients should be aware of the potential for mild to severe photosensitivity reactions. They should be advised to limit their sun exposure time and to wear sun-protective clothing when outdoors. The use of broad-spectrum UVA/UVB sunscreen and lip protectant with a sun protection factor of 30 or higher also should be stressed.6,7 Patients should be aware that UVA rays penetrate glass; therefore, UV-protective clothing should be worn throughout the day and during all seasons.7
In clinical trials of vemurafenib, Stevens-Johnson syndrome and toxic epidermal necrolysis was reported in 2 patients.8,9 Clinical trials also reported patients developing new primary malignant melanoma lesions.10 These findings further emphasize the need for patients to undergo total-body skin examinations during and after treatment.
Other possible dermatologic reactions include a generalized rash, erythema, alopecia, and pruritus.2,3 The development of benign growths associated with patients on vemurafenib include follicular plugging seen in keratosis pilaris, palmar and plantar hyperkeratosis, seborrheic dermatitis-like rashes, verrucous keratosis, and acantholytic dyskeratosis.8,11,12
We report a case of nonmalignant growths occurring 8 days after starting vemurafenib. This case illustrates potential cutaneous adverse reactions that were benign yet still of great concern to our patient. Many of these nonmalignant cutaneous findings are associated with abnormal follicular keratinization thought to be secondary to abnormal signaling of the mitogen-activated protein kinase pathway that occurs with the use of BRAF inhibitors.8 Although in this case malignant lesions were not discovered, the need for total-body skin examinations exists during all stages of treatment. Supportive care and reassurance should be given to patients along with local treatments including topical therapies (steroids, retinoids), cryotherapy, and biopsies or excisions when necessary.13,14
- Holstein S, Hohl R. Therapeutic additions and possible deletions in oncology in 2011. Clin Pharmacol Ther. 2011;91:15-17.
- Zambon A, Niculescu-Dovaz I, Niculescu-Dovaz D, et al. Small molecule inhibitors of BRAF in clinical trials. Bioorg Med Chem Lett. 2012;22:789-792.
- Luke JJ, Hodi FS. Vemurafenib and BRAF inhibition: a new class of treatment for metastatic melanoma [published online November 14, 2011]. Clin Cancer Res. 2012;18:9-14.
- Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010; 363:809-819.
- Tsai J, Lee JT, Wang W, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci USA. 2008;105:3041-3046.
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516.
- Dummer R, Rinderknecht J, Goldinger SM. Ultraviolet A and photosensitivity during vemuranefib therapy. N Engl J Med. 2012;366:480-481.
- Bovd KP, Vincent B, Andrea A, et al. Nonmalignant cutaneous findings associated with vemurafenib use in patients with metastatic melanoma. J Am Acad Dermatol. 2012;67:1375-1379.
- Wang CM, Fleming KF Hsu S. A case of vemurafenib-induced keratosis pilaris-like eruption. Dermatol Online J. 2012;18:7.
- Zimmer L, Hillen U, Livingstone E, et al. Atypical melanocytic proliferations and new primary melanomas in patients with advanced melanoma undergoing selective BRAF inhibition. J Clin Oncol. 2012;30:2375-2383.
- Huang V, Hepper D, Anadkat M, et al. Cutaneous toxic effects associated with vemurafenib and inhibition of the BRAF pathway. Arch Dermatol. 2012;148:628-633.
- Gupta M, Huang V, Linette G, et al. Unusual complication of vemurafenib treatment of metastatic melanoma: exacerbation of acantholytic dyskeratosis complicated by Kaposi varicelliform eruption. Arch Dermatol. 2012;148:966-968;
- Sinha R, Edmonds K, Newton-Bishop JA, et al. Cutaneous adverse events associated with vemurafenib in patients with metastatic melanoma: practical advice on diagnosis, preventions and management of the main treatment related skin toxicities. Br J Dermatol. 2012;167:987-994.
- Boussemart L, Routier E, Mateus C, et al. Prospective study of cutaneous side effects associated with the BRAF inhibitor vemurafenib: a study of 42 patients. Ann Oncol. 2013;24:1691-1697.
- Holstein S, Hohl R. Therapeutic additions and possible deletions in oncology in 2011. Clin Pharmacol Ther. 2011;91:15-17.
- Zambon A, Niculescu-Dovaz I, Niculescu-Dovaz D, et al. Small molecule inhibitors of BRAF in clinical trials. Bioorg Med Chem Lett. 2012;22:789-792.
- Luke JJ, Hodi FS. Vemurafenib and BRAF inhibition: a new class of treatment for metastatic melanoma [published online November 14, 2011]. Clin Cancer Res. 2012;18:9-14.
- Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010; 363:809-819.
- Tsai J, Lee JT, Wang W, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci USA. 2008;105:3041-3046.
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516.
- Dummer R, Rinderknecht J, Goldinger SM. Ultraviolet A and photosensitivity during vemuranefib therapy. N Engl J Med. 2012;366:480-481.
- Bovd KP, Vincent B, Andrea A, et al. Nonmalignant cutaneous findings associated with vemurafenib use in patients with metastatic melanoma. J Am Acad Dermatol. 2012;67:1375-1379.
- Wang CM, Fleming KF Hsu S. A case of vemurafenib-induced keratosis pilaris-like eruption. Dermatol Online J. 2012;18:7.
- Zimmer L, Hillen U, Livingstone E, et al. Atypical melanocytic proliferations and new primary melanomas in patients with advanced melanoma undergoing selective BRAF inhibition. J Clin Oncol. 2012;30:2375-2383.
- Huang V, Hepper D, Anadkat M, et al. Cutaneous toxic effects associated with vemurafenib and inhibition of the BRAF pathway. Arch Dermatol. 2012;148:628-633.
- Gupta M, Huang V, Linette G, et al. Unusual complication of vemurafenib treatment of metastatic melanoma: exacerbation of acantholytic dyskeratosis complicated by Kaposi varicelliform eruption. Arch Dermatol. 2012;148:966-968;
- Sinha R, Edmonds K, Newton-Bishop JA, et al. Cutaneous adverse events associated with vemurafenib in patients with metastatic melanoma: practical advice on diagnosis, preventions and management of the main treatment related skin toxicities. Br J Dermatol. 2012;167:987-994.
- Boussemart L, Routier E, Mateus C, et al. Prospective study of cutaneous side effects associated with the BRAF inhibitor vemurafenib: a study of 42 patients. Ann Oncol. 2013;24:1691-1697.
Practice Points
- Prior to starting a BRAF inhibitor, clinicians should perform a baseline total-body skin examination and follow-up every 2 months.
- Take photographs of the patient's entire body on initial total-body skin examination.
- Encourage sun protection for exposed areas on the body in all seasons.