User login
Perioperative interruption of dual antiplatelet therapy
To the Editor: We read with great interest the article by Munyon et al1 addressing recent developments in perioperative medicine. We would like to comment on the perioperative interruption of dual antiplatelet therapy, a common clinical problem.
Several registry analyses have shown that, with second-generation drug-eluting stents, interruption of 1 antiplatelet agent after the first month is safe.2,3 These registries included a substantial proportion of patients whose index stenting procedure was performed for acute coronary syndrome (up to 60%).2 On average, antiplatelet therapy interruption was brief (about 6 to 7 days).
Additional registry analyses have shown that surgery may be safely performed beyond the first month after drug-eluting stent placement.4,5 Specifically, a large Danish analysis of patients with a drug-eluting stent who underwent noncardiac surgery, matched to control patients without ischemic heart disease, showed that the risk of perioperative myocardial infarction and death was not increased beyond the first month after drug-eluting stent implantation. Specifically, the risk was not increased at the 1- to 2-month and 2- to 12-month postimplantation intervals. Acute coronary syndrome was the indication for stenting in 56% of the patients.
Therefore, while surgery is preferably delayed 6 months after drug-eluting stent implantation (class I recommendation in the European Society of Cardiology guidelines), surgery may be selectively performed 1 to 6 months after drug-eluting stent implantation with an acceptable risk. This is particularly so if the index stenting was performed in the setting of stable coronary arterial disease (class IIa recommendation if stenting was performed in the setting of stable coronary arterial disease without complex procedural features; class IIb recommendation if stenting was performed in the setting of acute coronary syndrome or complex procedural features).6 After drug-eluting stent implantation, the earliest cutpoint for considering surgery is 1 month rather than 3 months.
When surgery is performed within this 1- to 6-month interval, thienopyridine interruption should be kept brief and dual antiplatelet therapy reinitiated as soon as possible postoperatively. In fact, when thienopyridine therapy is interrupted 1 to 6 months after drug-eluting stent implantation, stent thrombosis typically occurs more than 6 or 7 days after interruption.7
- Munyon R, Cohn SL, Slawski B, Smetana GW, Pfeifer K. 2017 update in perioperative medicine: 6 questions answered. Cleve Clin J Med 2017; 84(11):863–872. doi:10.3949/ccjm.84a.17068
- Ferreira-Gonzáles, Marsal JR, Ribera A, et al. Double antiplatelet therapy after drug-eluting stent implantation: risk associated with discontinuation within the first year. J Am Coll Cardiol 2012; 60(15):1333–1339. doi:10.1016/j.jacc.2012.04.057
- Naidu SS, Krucoff MW, Rutledge DR, et al. Contemporary incidence and predictors of stent thrombosis and other major adverse cardiac events in the year after XIENCE V implantation: results from the 8,061-patient XIENCE V United States study. JACC Cardiovasc Interv 2012; 5(5):626–635. doi:10.1016/j.jcin.2012.02.014
- Egholm G, Kristensen SD, Thim T, et al. Risk associated with surgery within 12 months after coronary drug-eluting stent implantation. J Am Coll Cardiol 2016; 68(24):2622–2632. doi:10.1016/j.jacc.2016.09.967
- Singla S, Sachdeva R, Uretsky BF. The risk of adverse cardiac and bleeding events following noncardiac surgery relative to antiplatelet therapy in patients with prior percutaneous coronary intervention. J Am Coll Cardiol 2012; 60(20):2005–2016. doi:10.1016/j.jacc.2012.04.062
- Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018; 39(3):213–260. doi:10.1093/eurheartj/ehx419
- Airoldi F, Colombo A, Morici N, et al. Incidence and predictors of drug-eluting stent thrombosis during and after discontinuation of thienopyridine treatment. Circulation 2007; 116(7):745–754. doi:10.1161/CIRCULATIONAHA.106.686048
To the Editor: We read with great interest the article by Munyon et al1 addressing recent developments in perioperative medicine. We would like to comment on the perioperative interruption of dual antiplatelet therapy, a common clinical problem.
Several registry analyses have shown that, with second-generation drug-eluting stents, interruption of 1 antiplatelet agent after the first month is safe.2,3 These registries included a substantial proportion of patients whose index stenting procedure was performed for acute coronary syndrome (up to 60%).2 On average, antiplatelet therapy interruption was brief (about 6 to 7 days).
Additional registry analyses have shown that surgery may be safely performed beyond the first month after drug-eluting stent placement.4,5 Specifically, a large Danish analysis of patients with a drug-eluting stent who underwent noncardiac surgery, matched to control patients without ischemic heart disease, showed that the risk of perioperative myocardial infarction and death was not increased beyond the first month after drug-eluting stent implantation. Specifically, the risk was not increased at the 1- to 2-month and 2- to 12-month postimplantation intervals. Acute coronary syndrome was the indication for stenting in 56% of the patients.
Therefore, while surgery is preferably delayed 6 months after drug-eluting stent implantation (class I recommendation in the European Society of Cardiology guidelines), surgery may be selectively performed 1 to 6 months after drug-eluting stent implantation with an acceptable risk. This is particularly so if the index stenting was performed in the setting of stable coronary arterial disease (class IIa recommendation if stenting was performed in the setting of stable coronary arterial disease without complex procedural features; class IIb recommendation if stenting was performed in the setting of acute coronary syndrome or complex procedural features).6 After drug-eluting stent implantation, the earliest cutpoint for considering surgery is 1 month rather than 3 months.
When surgery is performed within this 1- to 6-month interval, thienopyridine interruption should be kept brief and dual antiplatelet therapy reinitiated as soon as possible postoperatively. In fact, when thienopyridine therapy is interrupted 1 to 6 months after drug-eluting stent implantation, stent thrombosis typically occurs more than 6 or 7 days after interruption.7
To the Editor: We read with great interest the article by Munyon et al1 addressing recent developments in perioperative medicine. We would like to comment on the perioperative interruption of dual antiplatelet therapy, a common clinical problem.
Several registry analyses have shown that, with second-generation drug-eluting stents, interruption of 1 antiplatelet agent after the first month is safe.2,3 These registries included a substantial proportion of patients whose index stenting procedure was performed for acute coronary syndrome (up to 60%).2 On average, antiplatelet therapy interruption was brief (about 6 to 7 days).
Additional registry analyses have shown that surgery may be safely performed beyond the first month after drug-eluting stent placement.4,5 Specifically, a large Danish analysis of patients with a drug-eluting stent who underwent noncardiac surgery, matched to control patients without ischemic heart disease, showed that the risk of perioperative myocardial infarction and death was not increased beyond the first month after drug-eluting stent implantation. Specifically, the risk was not increased at the 1- to 2-month and 2- to 12-month postimplantation intervals. Acute coronary syndrome was the indication for stenting in 56% of the patients.
Therefore, while surgery is preferably delayed 6 months after drug-eluting stent implantation (class I recommendation in the European Society of Cardiology guidelines), surgery may be selectively performed 1 to 6 months after drug-eluting stent implantation with an acceptable risk. This is particularly so if the index stenting was performed in the setting of stable coronary arterial disease (class IIa recommendation if stenting was performed in the setting of stable coronary arterial disease without complex procedural features; class IIb recommendation if stenting was performed in the setting of acute coronary syndrome or complex procedural features).6 After drug-eluting stent implantation, the earliest cutpoint for considering surgery is 1 month rather than 3 months.
When surgery is performed within this 1- to 6-month interval, thienopyridine interruption should be kept brief and dual antiplatelet therapy reinitiated as soon as possible postoperatively. In fact, when thienopyridine therapy is interrupted 1 to 6 months after drug-eluting stent implantation, stent thrombosis typically occurs more than 6 or 7 days after interruption.7
- Munyon R, Cohn SL, Slawski B, Smetana GW, Pfeifer K. 2017 update in perioperative medicine: 6 questions answered. Cleve Clin J Med 2017; 84(11):863–872. doi:10.3949/ccjm.84a.17068
- Ferreira-Gonzáles, Marsal JR, Ribera A, et al. Double antiplatelet therapy after drug-eluting stent implantation: risk associated with discontinuation within the first year. J Am Coll Cardiol 2012; 60(15):1333–1339. doi:10.1016/j.jacc.2012.04.057
- Naidu SS, Krucoff MW, Rutledge DR, et al. Contemporary incidence and predictors of stent thrombosis and other major adverse cardiac events in the year after XIENCE V implantation: results from the 8,061-patient XIENCE V United States study. JACC Cardiovasc Interv 2012; 5(5):626–635. doi:10.1016/j.jcin.2012.02.014
- Egholm G, Kristensen SD, Thim T, et al. Risk associated with surgery within 12 months after coronary drug-eluting stent implantation. J Am Coll Cardiol 2016; 68(24):2622–2632. doi:10.1016/j.jacc.2016.09.967
- Singla S, Sachdeva R, Uretsky BF. The risk of adverse cardiac and bleeding events following noncardiac surgery relative to antiplatelet therapy in patients with prior percutaneous coronary intervention. J Am Coll Cardiol 2012; 60(20):2005–2016. doi:10.1016/j.jacc.2012.04.062
- Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018; 39(3):213–260. doi:10.1093/eurheartj/ehx419
- Airoldi F, Colombo A, Morici N, et al. Incidence and predictors of drug-eluting stent thrombosis during and after discontinuation of thienopyridine treatment. Circulation 2007; 116(7):745–754. doi:10.1161/CIRCULATIONAHA.106.686048
- Munyon R, Cohn SL, Slawski B, Smetana GW, Pfeifer K. 2017 update in perioperative medicine: 6 questions answered. Cleve Clin J Med 2017; 84(11):863–872. doi:10.3949/ccjm.84a.17068
- Ferreira-Gonzáles, Marsal JR, Ribera A, et al. Double antiplatelet therapy after drug-eluting stent implantation: risk associated with discontinuation within the first year. J Am Coll Cardiol 2012; 60(15):1333–1339. doi:10.1016/j.jacc.2012.04.057
- Naidu SS, Krucoff MW, Rutledge DR, et al. Contemporary incidence and predictors of stent thrombosis and other major adverse cardiac events in the year after XIENCE V implantation: results from the 8,061-patient XIENCE V United States study. JACC Cardiovasc Interv 2012; 5(5):626–635. doi:10.1016/j.jcin.2012.02.014
- Egholm G, Kristensen SD, Thim T, et al. Risk associated with surgery within 12 months after coronary drug-eluting stent implantation. J Am Coll Cardiol 2016; 68(24):2622–2632. doi:10.1016/j.jacc.2016.09.967
- Singla S, Sachdeva R, Uretsky BF. The risk of adverse cardiac and bleeding events following noncardiac surgery relative to antiplatelet therapy in patients with prior percutaneous coronary intervention. J Am Coll Cardiol 2012; 60(20):2005–2016. doi:10.1016/j.jacc.2012.04.062
- Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018; 39(3):213–260. doi:10.1093/eurheartj/ehx419
- Airoldi F, Colombo A, Morici N, et al. Incidence and predictors of drug-eluting stent thrombosis during and after discontinuation of thienopyridine treatment. Circulation 2007; 116(7):745–754. doi:10.1161/CIRCULATIONAHA.106.686048
In reply: Perioperative interruption of dual antiplatelet therapy
In Reply: We reported on publications from 2016–2017 and, unfortunately, at the time we were writing our paper, the European Society of Cardiology (ESC) update on dual antiplatelet therapy1 had not yet been published. We presented the recommendations from the American College of Cardiology (ACC) and American Heart Association (AHA),2 which differ from the recently published ESC guidelines. The ESC suggests that the minimum waiting period after drug-eluting stent placement before noncardiac surgery should be 1 month rather than 3 months but acknowledges that in the setting of complex stenting or recent acute coronary syndrome, 6 months is preferred. The recommendation in this latter scenario is a class IIb C recommendation—essentially expert consensus opinion.
Further, in the study by Egholm et al,3 the event rates in patients undergoing noncardiac surgery in the 1- to 2-month period were numerically higher than in the control group, and no adjusted odds ratios were given. The numbers of events were very low, and a change of only 1 or 2 events in the other direction in the groups would likely make it statistically significant.
All of these recommendations are based on observational studies and registry data, as there are no randomized controlled trials to address this issue. There are many complexities to be accounted for including the type of stent, timing, circumstances surrounding stenting, anatomy, number of stents, patient comorbidities (particularly age, diabetes mellitus, cardiac disease), type of surgery and anesthesia, and perioperative management of antiplatelet therapy. While we acknowledge the ESC recommendation, we would urge caution in the recommendation to wait only 1 month, and in the United States most would prefer to wait 3 months if possible.
- Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018; 39(3):213–260. doi:10.1093/eurheartj/ehx419
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016; 134(10):e123–e155. doi:10.1161/CIR.0000000000000404
- Egholm G, Kristensen SD, Thim T, et al. Risk associated with surgery within 12 months after coronary drug-eluting stent implantation. J Am Coll Cardiol 2016; 68(24):2622–2632. doi:10.1016/j.jacc.2016.09.967
In Reply: We reported on publications from 2016–2017 and, unfortunately, at the time we were writing our paper, the European Society of Cardiology (ESC) update on dual antiplatelet therapy1 had not yet been published. We presented the recommendations from the American College of Cardiology (ACC) and American Heart Association (AHA),2 which differ from the recently published ESC guidelines. The ESC suggests that the minimum waiting period after drug-eluting stent placement before noncardiac surgery should be 1 month rather than 3 months but acknowledges that in the setting of complex stenting or recent acute coronary syndrome, 6 months is preferred. The recommendation in this latter scenario is a class IIb C recommendation—essentially expert consensus opinion.
Further, in the study by Egholm et al,3 the event rates in patients undergoing noncardiac surgery in the 1- to 2-month period were numerically higher than in the control group, and no adjusted odds ratios were given. The numbers of events were very low, and a change of only 1 or 2 events in the other direction in the groups would likely make it statistically significant.
All of these recommendations are based on observational studies and registry data, as there are no randomized controlled trials to address this issue. There are many complexities to be accounted for including the type of stent, timing, circumstances surrounding stenting, anatomy, number of stents, patient comorbidities (particularly age, diabetes mellitus, cardiac disease), type of surgery and anesthesia, and perioperative management of antiplatelet therapy. While we acknowledge the ESC recommendation, we would urge caution in the recommendation to wait only 1 month, and in the United States most would prefer to wait 3 months if possible.
In Reply: We reported on publications from 2016–2017 and, unfortunately, at the time we were writing our paper, the European Society of Cardiology (ESC) update on dual antiplatelet therapy1 had not yet been published. We presented the recommendations from the American College of Cardiology (ACC) and American Heart Association (AHA),2 which differ from the recently published ESC guidelines. The ESC suggests that the minimum waiting period after drug-eluting stent placement before noncardiac surgery should be 1 month rather than 3 months but acknowledges that in the setting of complex stenting or recent acute coronary syndrome, 6 months is preferred. The recommendation in this latter scenario is a class IIb C recommendation—essentially expert consensus opinion.
Further, in the study by Egholm et al,3 the event rates in patients undergoing noncardiac surgery in the 1- to 2-month period were numerically higher than in the control group, and no adjusted odds ratios were given. The numbers of events were very low, and a change of only 1 or 2 events in the other direction in the groups would likely make it statistically significant.
All of these recommendations are based on observational studies and registry data, as there are no randomized controlled trials to address this issue. There are many complexities to be accounted for including the type of stent, timing, circumstances surrounding stenting, anatomy, number of stents, patient comorbidities (particularly age, diabetes mellitus, cardiac disease), type of surgery and anesthesia, and perioperative management of antiplatelet therapy. While we acknowledge the ESC recommendation, we would urge caution in the recommendation to wait only 1 month, and in the United States most would prefer to wait 3 months if possible.
- Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018; 39(3):213–260. doi:10.1093/eurheartj/ehx419
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016; 134(10):e123–e155. doi:10.1161/CIR.0000000000000404
- Egholm G, Kristensen SD, Thim T, et al. Risk associated with surgery within 12 months after coronary drug-eluting stent implantation. J Am Coll Cardiol 2016; 68(24):2622–2632. doi:10.1016/j.jacc.2016.09.967
- Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018; 39(3):213–260. doi:10.1093/eurheartj/ehx419
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016; 134(10):e123–e155. doi:10.1161/CIR.0000000000000404
- Egholm G, Kristensen SD, Thim T, et al. Risk associated with surgery within 12 months after coronary drug-eluting stent implantation. J Am Coll Cardiol 2016; 68(24):2622–2632. doi:10.1016/j.jacc.2016.09.967
Effect of Romosozumab vs. Alendronate on Osteoporosis Fracture Risk
Study Overview
Objective. To determine if romosuzumab, an antisclerostin antibody, is superior to alendronate in reducing the incidence of fracture in postmenopausal women with osteoporosis at high-risk for fracture.
Design. Multicenter, international, double-blind, randomized clinical trial.
Setting and participants. 4093 postmenopausal women with osteoporosis and a previous fragility fracture were enrolled from over 40 countries worldwide. Patients were eligible for the study if they were 55 to 90 years old and were deemed at high risk for future fracture based on bone mineral density (BMD) T score at the total hip or femoral neck and fracture history. This included T score ≤ –2.5 and ≥ 1 moderate or severe vertebral fractures or ≥ 2 mild vertebral fractures; T score ≤ –2.0 and either ≥ 2 moderate or severe vertebral fractures or proximal femur fracture within 3 to 24 months before randomization. Subjects with a history of prior use of medications that affect bone metabolism were excluded, as were those with other metabolic bone disease, vitamin D deficiency, uncontrolled metabolic disease, malabsorption syndromes, history of transplant, severe renal insufficiency, malignancy or severe illness.
Intervention. Patients were randomized to either subcutaneous romosuzumab 210 mg monthly or oral alendronate 70 mg weekly for 12 months. Following the 12-month double-blind period, all patients received open-label weekly alendronate until the end of the trial, with maintenance of blinding to the initial treatment assignment. Primary analysis occurred when all subjects had completed the 24-month visit and clinical fractures had been confirmed in at least 330 patients. All patients received daily calcium and vitamin D. Lateral radiographs of the thoracic and lumbar spine were obtained at screening and months 12 and 24. The BMD at the lumbar spine and proximal femur was evaluated by dual-energy x-ray absorptiometry at baseline and every 12 months thereafter. Serum concentrations of bone-turnover markers were measured in a subgroup of patients.
Main outcome measures. The primary outcomes were the incidence of new vertebral fracture and the incidence of clinical fracture at 24 months. Clinical fractures included symptomatic vertebral fracture and nonvertebral fractures. The secondary outcomes were the BMD at the lumbar spine, total hip, and femoral neck at 12 and 24 months, the incidence of nonvertebral fracture, and fracture category. Safety outcomes included the incidence of adjudicated clinical events, including serious cardiovascular adverse events, osteonecrosis of the jaw, and atypical femoral fracture. Serious cardiovascular events were defined as cardiac ischemic event, cerebrovascular event, heart failure, death, non-coronary revascularization and peripheral vascular ischemic event not requiring revascularization.
Analysis. An intention to treat approach was used for data analysis. For the incidence of fractures, the treatment groups were compared using a Cox proportional-hazards model and the Mantel-Haenszel method with adjustment for age (< 75 vs ≥ 75 years), the presence or absence of severe vertebral fracture at baseline, and baseline BMD T score at the total hip. Between-group comparisons of the percentage change in BMD from baseline were analyzed by means of a repeated-measures model with adjustment for treatment, age category, baseline severe vertebral fracture, visit, treatment-by-visit interaction, and baseline BMD. Percentage changes from baseline in bone turnover were assessed using a Wilcoxon rank-sum test. The safety analysis included cumulated incidence rates of adverse outcomes. Odds ratios and confidence intervals were estimated for serious cardiovascular adverse events with the use of a logistic regression model.
Main results. 2046 participants were randomized to the romosozumab group and 2047 to the alendronate group. A total of 3654 participants from both groups (89.3%) completed 12 months of the trial, and 3150 (77.0%) completed the primary analysis period. The treatment groups were similar in baseline age, ethnicity, and fracture history. The majority of patients in both groups were non-Hispanic (> 60%) and ≥ 75 years old (> 50%). The mean age of the patients was 74.3 years. Baseline mean bone mineral density T scores were –2.96 at the lumbar spine, –2.8 at the total hip, and –2.9 at the femoral neck.
After 24 months of treatment, 6.2% of patients in the romosozumab-alendronate group had a new vertebral fracture as compared to 11.9% in the alendronate-alendronate group. This represents a 48% lower risk (risk ratio 0.52, 95% confidence interval [CI] 0.4–0.66; P < 0.001) of new vertebral fractures with romosozumab. At the time of the primary analysis, romosozumab followed by alendronate resulted in a 27% lower risk of clinical fracture than alendronate alone (hazard ratio 0.73, 95% CI 0.61–0.88; P < 0.001). 8.7% of the romosozumab-alendronate group had a nonvertebral fracture versus 10.6% in the alendronate-alendronate group, representing a 19% lower risk with romosozumab (hazard ratio 0.81, 95% CI 0.66–0.99; P = 0.04). Hip fractures occurred in 2.0% of the romosozumab-alendronate group as compared with 3.2% in the alendronate-alendronate group, representing a 38% lower risk with romosozumab (hazard ratio 0.62, 95% CI 0.42–0.92; P
Patients in the romosozumab-alendronate group had greater gains in BMD from baseline at the lumbar spine (14.9% vs 8.5%) and total hip (7% vs 3.6%) compared to the alendronate-alendronate group. (P < 0.001 for all comparisons). At 12 months, romosozumab treatment resulted in decreased levels of bone resorption marker β-CTX and increased levels of bone formation marker P1NP. β-CTX and P1NP decreased and remained below baseline levels after transitioning to alendronate. In the alendronate-alendronate group, P1NP and β-CTX decreased within 1 month and remained below baseline levels at 36 months.
Overall, the adverse events and serious event rates were similar between the 2 treatment groups during the double-blind period with 2 exceptions. In the first 12 months, injection-site reactions were reported in 4.4% of patients receiving romosozumab compared to 2.6% in those receiving alendronate. Patients in the romosozumab group had an increased incidence of adjudicated serious cardiovascular outcomes during the double-blind period, 2.5% (50 of 2040 patients) compared to 1.9% (38 of 2014 patients) in the alendronate group. During the open-label period, osteonecrosis of the jaw occurred in one patient in each group. Two atypical femoral fractures occurred in the romosozumab-alendronate group, compared to 4 in the alendronate-alendronate group. During the first 18 months of the study, binding anti-romosozumab antibodies were observed in 15.3% of the romosozumab group, with neutralizing antibodies in 0.6%.
Conclusion. In postmenopausal woman with osteoporosis and high fracture risk, 12 months of romosozumab treatment followed by alendronate resulted in significantly lower risk of fracture than use of alendronate alone.
Commentary
Osteoporosis-related fragility fractures carry a substantial risk of morbidity and mortality [1]. The goal of osteoporosis treatment is to ameliorate this risk. The current FDA-approved medications for osteoporosis can be divided into anabolic (teriparatide, abaloparatide) and anti-resorptive (bisphosphonate, denosumab, selective estrogen receptor modulators) categories. Sclerostin is a glycoprotein produced by osteocytes that inhibits the Wnt signaling pathway, thereby impeding osteoblast proliferation and activity. Romosozumab is a monoclonal antisclerostin antibody that results in both increased bone formation and decreased bone resorption [1]. By apparently uncoupling bone formation and resorption to increase bone mass, this medication holds promise to become the ideal osteoporosis drug.
Initial studies have shown that 12 months of romosozumab treatment significantly increased BMD at the lumbar spine (+11.3%), as compared to placebo (–0.1%), alendronate (+4.1%), and teriparatide (+7.1%) [2]. The Fracture Study in Postmenopausal Women with Osteoporosis (FRAME) was a large (7180 patients) randomized controlled trial that demonstrated that 12 months of romosozumab resulted in a 73% lower risk of vertebral fracture and 36% lower risk of clinical fracture compared to placebo [3]. However, there was no significant reduction in non-vertebral facture [3]. This may be due to the fact that FRAME excluded women at the highest risk for fracture. That is, exclusion criteria included history of hip fracture, any severe vertebral facture, or more than 2 moderate vertebral fractures. The current phase 3 ARCH trial (Active-Controlled Fracture Study in Postmenopausal Women with Osteoporosis at High Risk) attempts to clarify the potential benefit of romosozumab treatment in this very high-risk patient population, compared to a common first-line osteoporosis treatment, alendronate.
Indeed, ARCH demonstrates that sequential therapy with romosozumab followed by alendronate is superior to alendronate alone in improving BMD at all sites and preventing new vertebral, clinical, and non-vertebral fractures in postmenopausal women with osteoporosis and a history of fragility fracture. While ARCH was not designed as a cardiovascular outcomes trial, the higher rate of serious cardiovascular adverse events in the romosozumab group raises concern that romosozumab may have a negative effect on vascular tissue. Sclerostin is expressed in vascular smooth muscle [4] and upregulated at sites of vascular calcification [5]. It is possible that inhibiting sclerostin activity could alter vascular remodeling or increase vascular calcification. However, it is interesting that in the larger FRAME trial, no increase in adverse cardiovascular events was seen in the romosozumab group compared to placebo. This may be due to the fact that the average age of patients in FRAME was lower than ARCH. However, it also raises the hypothesis that alendronate itself may be protective in terms of cardiovascular risk. It has been postulated that bisphosphonates may have cardiovascular protective effects, given animal studies have demonstrated that alendronate downregulates monocyte chemoattractant protein 1 and macrophage inflammatory protein 1 [6]. However no cardioprotective benefit was seen in meta-analysis [7].
ARCH has several strengths, including its design as an international, double-blind, and randomized clinical trial. The primary outcome of cumulative fracture incidence is a hard endpoint and is clinically relevant. The intervention is simple and the results are clearly defined. The statistical assessment yields significant results. However, there are some limitations to the study. The lead author has received research support from Amgen and UCB Pharma, the makers of romosuzumab. Amgen and UCB Pharma designed the trial, and Amgen was responsible for trial oversight and data analyses per a pre-specified statistical analysis plan. An external independent data monitoring committee monitored unblinded safety data. Because there was no placebo-controlled arm, it is difficult to determine whether the unexpected cardiovascular signal was due to romosuzumab itself or a protective effect of alendronate. In addition, the majority of study participants were non-Hispanic from Central or Eastern Europe and Latin America, with only ~2% of patients from North America. As a result, ARCH findings may not be generalizable to other regional or ethnic populations. Furthermore, the majority of the patients were ≥ 75 years of age and were at very high fracture risk. It is unclear if younger patients or those with lower risk of fracture would see the same fracture prevention and BMD gain. In addition, because of the relatively short length of the trial, the durability of the metabolic bone benefit and cardiovascular risk is unknown. While the authors reported the increased anti-romosozumab antibodies in the romosozumab group had no detectable effect on efficacy or safety, given the short duration of the trial, this has not been proven.
Applications for Clinical Practice
The dual anti-resorptive and anabolic effect of romosozumab makes it an attractive and promising new osteoporosis therapy. ARCH suggests that sequential therapy with romosuzumab and alendronate is superior in terms of fracture prevention to alendronate alone in elderly postmenopausal women with osteoporosis and a history of fragility fractures, although longer term studies are needed to define the durability of this effect. While the absolute number of serious adjudicated cardiovascular events was low, the increased incidence in the romosuzumab group will likely prevent the FDA from approving this medication for widespread use at this time. Additional studies are needed to clarify the cause and magnitude of this cardiovascular risk and to determine whether prevention of fracture-associated morbidity and mortality is enough to mitigate it.
—Simona Frunza-Stefan, MD, and Hillary B. Whitlach, MD, University of Maryland School of Medicine, Baltimore, MD
1. Cummings SR, Melton IJ. Epidemiology and outcomes of osteoporotic fractures. Lancet 2002; 359:176107.
2. McClung MR, Grauer A, Boonen S, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med 2014;370:412–20.
3. Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med 2016;375:1532–43.
4. Zhu D, Mackenzie NCW, Millán JL, et al. The appearance and modulation of osteocyte marker expres- sion during calcification of vascular smooth muscle cells. PLoS One 2011;6:e19595.
5. Evenepoel P, Goffin E, Meijers B, et al. Sclerostin serum levels and vascular calcification progression in prevalent renal transplant recipients. J Clin Endocrinol Metab 2015;100:4669–76.
6. Masuda T, Deng X, Tamai R. Mouse macrophages primed with alendronate down-regulate monocyte chemoattractant protein-1 (MCP-1) and macrophage inflammatory protein-1alpha (MIP-1alpha) production in response to Toll-like receptor (TLR) 2 and TLR4 agonist via Smad3 activation. Int Immunopharmacol 2009;9:1115–21.
7. Kim DH, Rogers JR, Fulchino LA, et al. Bisphosphonates and risk of cardiovascular events: a meta-analysis. PLoS One 2015;10:e0122646.
Study Overview
Objective. To determine if romosuzumab, an antisclerostin antibody, is superior to alendronate in reducing the incidence of fracture in postmenopausal women with osteoporosis at high-risk for fracture.
Design. Multicenter, international, double-blind, randomized clinical trial.
Setting and participants. 4093 postmenopausal women with osteoporosis and a previous fragility fracture were enrolled from over 40 countries worldwide. Patients were eligible for the study if they were 55 to 90 years old and were deemed at high risk for future fracture based on bone mineral density (BMD) T score at the total hip or femoral neck and fracture history. This included T score ≤ –2.5 and ≥ 1 moderate or severe vertebral fractures or ≥ 2 mild vertebral fractures; T score ≤ –2.0 and either ≥ 2 moderate or severe vertebral fractures or proximal femur fracture within 3 to 24 months before randomization. Subjects with a history of prior use of medications that affect bone metabolism were excluded, as were those with other metabolic bone disease, vitamin D deficiency, uncontrolled metabolic disease, malabsorption syndromes, history of transplant, severe renal insufficiency, malignancy or severe illness.
Intervention. Patients were randomized to either subcutaneous romosuzumab 210 mg monthly or oral alendronate 70 mg weekly for 12 months. Following the 12-month double-blind period, all patients received open-label weekly alendronate until the end of the trial, with maintenance of blinding to the initial treatment assignment. Primary analysis occurred when all subjects had completed the 24-month visit and clinical fractures had been confirmed in at least 330 patients. All patients received daily calcium and vitamin D. Lateral radiographs of the thoracic and lumbar spine were obtained at screening and months 12 and 24. The BMD at the lumbar spine and proximal femur was evaluated by dual-energy x-ray absorptiometry at baseline and every 12 months thereafter. Serum concentrations of bone-turnover markers were measured in a subgroup of patients.
Main outcome measures. The primary outcomes were the incidence of new vertebral fracture and the incidence of clinical fracture at 24 months. Clinical fractures included symptomatic vertebral fracture and nonvertebral fractures. The secondary outcomes were the BMD at the lumbar spine, total hip, and femoral neck at 12 and 24 months, the incidence of nonvertebral fracture, and fracture category. Safety outcomes included the incidence of adjudicated clinical events, including serious cardiovascular adverse events, osteonecrosis of the jaw, and atypical femoral fracture. Serious cardiovascular events were defined as cardiac ischemic event, cerebrovascular event, heart failure, death, non-coronary revascularization and peripheral vascular ischemic event not requiring revascularization.
Analysis. An intention to treat approach was used for data analysis. For the incidence of fractures, the treatment groups were compared using a Cox proportional-hazards model and the Mantel-Haenszel method with adjustment for age (< 75 vs ≥ 75 years), the presence or absence of severe vertebral fracture at baseline, and baseline BMD T score at the total hip. Between-group comparisons of the percentage change in BMD from baseline were analyzed by means of a repeated-measures model with adjustment for treatment, age category, baseline severe vertebral fracture, visit, treatment-by-visit interaction, and baseline BMD. Percentage changes from baseline in bone turnover were assessed using a Wilcoxon rank-sum test. The safety analysis included cumulated incidence rates of adverse outcomes. Odds ratios and confidence intervals were estimated for serious cardiovascular adverse events with the use of a logistic regression model.
Main results. 2046 participants were randomized to the romosozumab group and 2047 to the alendronate group. A total of 3654 participants from both groups (89.3%) completed 12 months of the trial, and 3150 (77.0%) completed the primary analysis period. The treatment groups were similar in baseline age, ethnicity, and fracture history. The majority of patients in both groups were non-Hispanic (> 60%) and ≥ 75 years old (> 50%). The mean age of the patients was 74.3 years. Baseline mean bone mineral density T scores were –2.96 at the lumbar spine, –2.8 at the total hip, and –2.9 at the femoral neck.
After 24 months of treatment, 6.2% of patients in the romosozumab-alendronate group had a new vertebral fracture as compared to 11.9% in the alendronate-alendronate group. This represents a 48% lower risk (risk ratio 0.52, 95% confidence interval [CI] 0.4–0.66; P < 0.001) of new vertebral fractures with romosozumab. At the time of the primary analysis, romosozumab followed by alendronate resulted in a 27% lower risk of clinical fracture than alendronate alone (hazard ratio 0.73, 95% CI 0.61–0.88; P < 0.001). 8.7% of the romosozumab-alendronate group had a nonvertebral fracture versus 10.6% in the alendronate-alendronate group, representing a 19% lower risk with romosozumab (hazard ratio 0.81, 95% CI 0.66–0.99; P = 0.04). Hip fractures occurred in 2.0% of the romosozumab-alendronate group as compared with 3.2% in the alendronate-alendronate group, representing a 38% lower risk with romosozumab (hazard ratio 0.62, 95% CI 0.42–0.92; P
Patients in the romosozumab-alendronate group had greater gains in BMD from baseline at the lumbar spine (14.9% vs 8.5%) and total hip (7% vs 3.6%) compared to the alendronate-alendronate group. (P < 0.001 for all comparisons). At 12 months, romosozumab treatment resulted in decreased levels of bone resorption marker β-CTX and increased levels of bone formation marker P1NP. β-CTX and P1NP decreased and remained below baseline levels after transitioning to alendronate. In the alendronate-alendronate group, P1NP and β-CTX decreased within 1 month and remained below baseline levels at 36 months.
Overall, the adverse events and serious event rates were similar between the 2 treatment groups during the double-blind period with 2 exceptions. In the first 12 months, injection-site reactions were reported in 4.4% of patients receiving romosozumab compared to 2.6% in those receiving alendronate. Patients in the romosozumab group had an increased incidence of adjudicated serious cardiovascular outcomes during the double-blind period, 2.5% (50 of 2040 patients) compared to 1.9% (38 of 2014 patients) in the alendronate group. During the open-label period, osteonecrosis of the jaw occurred in one patient in each group. Two atypical femoral fractures occurred in the romosozumab-alendronate group, compared to 4 in the alendronate-alendronate group. During the first 18 months of the study, binding anti-romosozumab antibodies were observed in 15.3% of the romosozumab group, with neutralizing antibodies in 0.6%.
Conclusion. In postmenopausal woman with osteoporosis and high fracture risk, 12 months of romosozumab treatment followed by alendronate resulted in significantly lower risk of fracture than use of alendronate alone.
Commentary
Osteoporosis-related fragility fractures carry a substantial risk of morbidity and mortality [1]. The goal of osteoporosis treatment is to ameliorate this risk. The current FDA-approved medications for osteoporosis can be divided into anabolic (teriparatide, abaloparatide) and anti-resorptive (bisphosphonate, denosumab, selective estrogen receptor modulators) categories. Sclerostin is a glycoprotein produced by osteocytes that inhibits the Wnt signaling pathway, thereby impeding osteoblast proliferation and activity. Romosozumab is a monoclonal antisclerostin antibody that results in both increased bone formation and decreased bone resorption [1]. By apparently uncoupling bone formation and resorption to increase bone mass, this medication holds promise to become the ideal osteoporosis drug.
Initial studies have shown that 12 months of romosozumab treatment significantly increased BMD at the lumbar spine (+11.3%), as compared to placebo (–0.1%), alendronate (+4.1%), and teriparatide (+7.1%) [2]. The Fracture Study in Postmenopausal Women with Osteoporosis (FRAME) was a large (7180 patients) randomized controlled trial that demonstrated that 12 months of romosozumab resulted in a 73% lower risk of vertebral fracture and 36% lower risk of clinical fracture compared to placebo [3]. However, there was no significant reduction in non-vertebral facture [3]. This may be due to the fact that FRAME excluded women at the highest risk for fracture. That is, exclusion criteria included history of hip fracture, any severe vertebral facture, or more than 2 moderate vertebral fractures. The current phase 3 ARCH trial (Active-Controlled Fracture Study in Postmenopausal Women with Osteoporosis at High Risk) attempts to clarify the potential benefit of romosozumab treatment in this very high-risk patient population, compared to a common first-line osteoporosis treatment, alendronate.
Indeed, ARCH demonstrates that sequential therapy with romosozumab followed by alendronate is superior to alendronate alone in improving BMD at all sites and preventing new vertebral, clinical, and non-vertebral fractures in postmenopausal women with osteoporosis and a history of fragility fracture. While ARCH was not designed as a cardiovascular outcomes trial, the higher rate of serious cardiovascular adverse events in the romosozumab group raises concern that romosozumab may have a negative effect on vascular tissue. Sclerostin is expressed in vascular smooth muscle [4] and upregulated at sites of vascular calcification [5]. It is possible that inhibiting sclerostin activity could alter vascular remodeling or increase vascular calcification. However, it is interesting that in the larger FRAME trial, no increase in adverse cardiovascular events was seen in the romosozumab group compared to placebo. This may be due to the fact that the average age of patients in FRAME was lower than ARCH. However, it also raises the hypothesis that alendronate itself may be protective in terms of cardiovascular risk. It has been postulated that bisphosphonates may have cardiovascular protective effects, given animal studies have demonstrated that alendronate downregulates monocyte chemoattractant protein 1 and macrophage inflammatory protein 1 [6]. However no cardioprotective benefit was seen in meta-analysis [7].
ARCH has several strengths, including its design as an international, double-blind, and randomized clinical trial. The primary outcome of cumulative fracture incidence is a hard endpoint and is clinically relevant. The intervention is simple and the results are clearly defined. The statistical assessment yields significant results. However, there are some limitations to the study. The lead author has received research support from Amgen and UCB Pharma, the makers of romosuzumab. Amgen and UCB Pharma designed the trial, and Amgen was responsible for trial oversight and data analyses per a pre-specified statistical analysis plan. An external independent data monitoring committee monitored unblinded safety data. Because there was no placebo-controlled arm, it is difficult to determine whether the unexpected cardiovascular signal was due to romosuzumab itself or a protective effect of alendronate. In addition, the majority of study participants were non-Hispanic from Central or Eastern Europe and Latin America, with only ~2% of patients from North America. As a result, ARCH findings may not be generalizable to other regional or ethnic populations. Furthermore, the majority of the patients were ≥ 75 years of age and were at very high fracture risk. It is unclear if younger patients or those with lower risk of fracture would see the same fracture prevention and BMD gain. In addition, because of the relatively short length of the trial, the durability of the metabolic bone benefit and cardiovascular risk is unknown. While the authors reported the increased anti-romosozumab antibodies in the romosozumab group had no detectable effect on efficacy or safety, given the short duration of the trial, this has not been proven.
Applications for Clinical Practice
The dual anti-resorptive and anabolic effect of romosozumab makes it an attractive and promising new osteoporosis therapy. ARCH suggests that sequential therapy with romosuzumab and alendronate is superior in terms of fracture prevention to alendronate alone in elderly postmenopausal women with osteoporosis and a history of fragility fractures, although longer term studies are needed to define the durability of this effect. While the absolute number of serious adjudicated cardiovascular events was low, the increased incidence in the romosuzumab group will likely prevent the FDA from approving this medication for widespread use at this time. Additional studies are needed to clarify the cause and magnitude of this cardiovascular risk and to determine whether prevention of fracture-associated morbidity and mortality is enough to mitigate it.
—Simona Frunza-Stefan, MD, and Hillary B. Whitlach, MD, University of Maryland School of Medicine, Baltimore, MD
Study Overview
Objective. To determine if romosuzumab, an antisclerostin antibody, is superior to alendronate in reducing the incidence of fracture in postmenopausal women with osteoporosis at high-risk for fracture.
Design. Multicenter, international, double-blind, randomized clinical trial.
Setting and participants. 4093 postmenopausal women with osteoporosis and a previous fragility fracture were enrolled from over 40 countries worldwide. Patients were eligible for the study if they were 55 to 90 years old and were deemed at high risk for future fracture based on bone mineral density (BMD) T score at the total hip or femoral neck and fracture history. This included T score ≤ –2.5 and ≥ 1 moderate or severe vertebral fractures or ≥ 2 mild vertebral fractures; T score ≤ –2.0 and either ≥ 2 moderate or severe vertebral fractures or proximal femur fracture within 3 to 24 months before randomization. Subjects with a history of prior use of medications that affect bone metabolism were excluded, as were those with other metabolic bone disease, vitamin D deficiency, uncontrolled metabolic disease, malabsorption syndromes, history of transplant, severe renal insufficiency, malignancy or severe illness.
Intervention. Patients were randomized to either subcutaneous romosuzumab 210 mg monthly or oral alendronate 70 mg weekly for 12 months. Following the 12-month double-blind period, all patients received open-label weekly alendronate until the end of the trial, with maintenance of blinding to the initial treatment assignment. Primary analysis occurred when all subjects had completed the 24-month visit and clinical fractures had been confirmed in at least 330 patients. All patients received daily calcium and vitamin D. Lateral radiographs of the thoracic and lumbar spine were obtained at screening and months 12 and 24. The BMD at the lumbar spine and proximal femur was evaluated by dual-energy x-ray absorptiometry at baseline and every 12 months thereafter. Serum concentrations of bone-turnover markers were measured in a subgroup of patients.
Main outcome measures. The primary outcomes were the incidence of new vertebral fracture and the incidence of clinical fracture at 24 months. Clinical fractures included symptomatic vertebral fracture and nonvertebral fractures. The secondary outcomes were the BMD at the lumbar spine, total hip, and femoral neck at 12 and 24 months, the incidence of nonvertebral fracture, and fracture category. Safety outcomes included the incidence of adjudicated clinical events, including serious cardiovascular adverse events, osteonecrosis of the jaw, and atypical femoral fracture. Serious cardiovascular events were defined as cardiac ischemic event, cerebrovascular event, heart failure, death, non-coronary revascularization and peripheral vascular ischemic event not requiring revascularization.
Analysis. An intention to treat approach was used for data analysis. For the incidence of fractures, the treatment groups were compared using a Cox proportional-hazards model and the Mantel-Haenszel method with adjustment for age (< 75 vs ≥ 75 years), the presence or absence of severe vertebral fracture at baseline, and baseline BMD T score at the total hip. Between-group comparisons of the percentage change in BMD from baseline were analyzed by means of a repeated-measures model with adjustment for treatment, age category, baseline severe vertebral fracture, visit, treatment-by-visit interaction, and baseline BMD. Percentage changes from baseline in bone turnover were assessed using a Wilcoxon rank-sum test. The safety analysis included cumulated incidence rates of adverse outcomes. Odds ratios and confidence intervals were estimated for serious cardiovascular adverse events with the use of a logistic regression model.
Main results. 2046 participants were randomized to the romosozumab group and 2047 to the alendronate group. A total of 3654 participants from both groups (89.3%) completed 12 months of the trial, and 3150 (77.0%) completed the primary analysis period. The treatment groups were similar in baseline age, ethnicity, and fracture history. The majority of patients in both groups were non-Hispanic (> 60%) and ≥ 75 years old (> 50%). The mean age of the patients was 74.3 years. Baseline mean bone mineral density T scores were –2.96 at the lumbar spine, –2.8 at the total hip, and –2.9 at the femoral neck.
After 24 months of treatment, 6.2% of patients in the romosozumab-alendronate group had a new vertebral fracture as compared to 11.9% in the alendronate-alendronate group. This represents a 48% lower risk (risk ratio 0.52, 95% confidence interval [CI] 0.4–0.66; P < 0.001) of new vertebral fractures with romosozumab. At the time of the primary analysis, romosozumab followed by alendronate resulted in a 27% lower risk of clinical fracture than alendronate alone (hazard ratio 0.73, 95% CI 0.61–0.88; P < 0.001). 8.7% of the romosozumab-alendronate group had a nonvertebral fracture versus 10.6% in the alendronate-alendronate group, representing a 19% lower risk with romosozumab (hazard ratio 0.81, 95% CI 0.66–0.99; P = 0.04). Hip fractures occurred in 2.0% of the romosozumab-alendronate group as compared with 3.2% in the alendronate-alendronate group, representing a 38% lower risk with romosozumab (hazard ratio 0.62, 95% CI 0.42–0.92; P
Patients in the romosozumab-alendronate group had greater gains in BMD from baseline at the lumbar spine (14.9% vs 8.5%) and total hip (7% vs 3.6%) compared to the alendronate-alendronate group. (P < 0.001 for all comparisons). At 12 months, romosozumab treatment resulted in decreased levels of bone resorption marker β-CTX and increased levels of bone formation marker P1NP. β-CTX and P1NP decreased and remained below baseline levels after transitioning to alendronate. In the alendronate-alendronate group, P1NP and β-CTX decreased within 1 month and remained below baseline levels at 36 months.
Overall, the adverse events and serious event rates were similar between the 2 treatment groups during the double-blind period with 2 exceptions. In the first 12 months, injection-site reactions were reported in 4.4% of patients receiving romosozumab compared to 2.6% in those receiving alendronate. Patients in the romosozumab group had an increased incidence of adjudicated serious cardiovascular outcomes during the double-blind period, 2.5% (50 of 2040 patients) compared to 1.9% (38 of 2014 patients) in the alendronate group. During the open-label period, osteonecrosis of the jaw occurred in one patient in each group. Two atypical femoral fractures occurred in the romosozumab-alendronate group, compared to 4 in the alendronate-alendronate group. During the first 18 months of the study, binding anti-romosozumab antibodies were observed in 15.3% of the romosozumab group, with neutralizing antibodies in 0.6%.
Conclusion. In postmenopausal woman with osteoporosis and high fracture risk, 12 months of romosozumab treatment followed by alendronate resulted in significantly lower risk of fracture than use of alendronate alone.
Commentary
Osteoporosis-related fragility fractures carry a substantial risk of morbidity and mortality [1]. The goal of osteoporosis treatment is to ameliorate this risk. The current FDA-approved medications for osteoporosis can be divided into anabolic (teriparatide, abaloparatide) and anti-resorptive (bisphosphonate, denosumab, selective estrogen receptor modulators) categories. Sclerostin is a glycoprotein produced by osteocytes that inhibits the Wnt signaling pathway, thereby impeding osteoblast proliferation and activity. Romosozumab is a monoclonal antisclerostin antibody that results in both increased bone formation and decreased bone resorption [1]. By apparently uncoupling bone formation and resorption to increase bone mass, this medication holds promise to become the ideal osteoporosis drug.
Initial studies have shown that 12 months of romosozumab treatment significantly increased BMD at the lumbar spine (+11.3%), as compared to placebo (–0.1%), alendronate (+4.1%), and teriparatide (+7.1%) [2]. The Fracture Study in Postmenopausal Women with Osteoporosis (FRAME) was a large (7180 patients) randomized controlled trial that demonstrated that 12 months of romosozumab resulted in a 73% lower risk of vertebral fracture and 36% lower risk of clinical fracture compared to placebo [3]. However, there was no significant reduction in non-vertebral facture [3]. This may be due to the fact that FRAME excluded women at the highest risk for fracture. That is, exclusion criteria included history of hip fracture, any severe vertebral facture, or more than 2 moderate vertebral fractures. The current phase 3 ARCH trial (Active-Controlled Fracture Study in Postmenopausal Women with Osteoporosis at High Risk) attempts to clarify the potential benefit of romosozumab treatment in this very high-risk patient population, compared to a common first-line osteoporosis treatment, alendronate.
Indeed, ARCH demonstrates that sequential therapy with romosozumab followed by alendronate is superior to alendronate alone in improving BMD at all sites and preventing new vertebral, clinical, and non-vertebral fractures in postmenopausal women with osteoporosis and a history of fragility fracture. While ARCH was not designed as a cardiovascular outcomes trial, the higher rate of serious cardiovascular adverse events in the romosozumab group raises concern that romosozumab may have a negative effect on vascular tissue. Sclerostin is expressed in vascular smooth muscle [4] and upregulated at sites of vascular calcification [5]. It is possible that inhibiting sclerostin activity could alter vascular remodeling or increase vascular calcification. However, it is interesting that in the larger FRAME trial, no increase in adverse cardiovascular events was seen in the romosozumab group compared to placebo. This may be due to the fact that the average age of patients in FRAME was lower than ARCH. However, it also raises the hypothesis that alendronate itself may be protective in terms of cardiovascular risk. It has been postulated that bisphosphonates may have cardiovascular protective effects, given animal studies have demonstrated that alendronate downregulates monocyte chemoattractant protein 1 and macrophage inflammatory protein 1 [6]. However no cardioprotective benefit was seen in meta-analysis [7].
ARCH has several strengths, including its design as an international, double-blind, and randomized clinical trial. The primary outcome of cumulative fracture incidence is a hard endpoint and is clinically relevant. The intervention is simple and the results are clearly defined. The statistical assessment yields significant results. However, there are some limitations to the study. The lead author has received research support from Amgen and UCB Pharma, the makers of romosuzumab. Amgen and UCB Pharma designed the trial, and Amgen was responsible for trial oversight and data analyses per a pre-specified statistical analysis plan. An external independent data monitoring committee monitored unblinded safety data. Because there was no placebo-controlled arm, it is difficult to determine whether the unexpected cardiovascular signal was due to romosuzumab itself or a protective effect of alendronate. In addition, the majority of study participants were non-Hispanic from Central or Eastern Europe and Latin America, with only ~2% of patients from North America. As a result, ARCH findings may not be generalizable to other regional or ethnic populations. Furthermore, the majority of the patients were ≥ 75 years of age and were at very high fracture risk. It is unclear if younger patients or those with lower risk of fracture would see the same fracture prevention and BMD gain. In addition, because of the relatively short length of the trial, the durability of the metabolic bone benefit and cardiovascular risk is unknown. While the authors reported the increased anti-romosozumab antibodies in the romosozumab group had no detectable effect on efficacy or safety, given the short duration of the trial, this has not been proven.
Applications for Clinical Practice
The dual anti-resorptive and anabolic effect of romosozumab makes it an attractive and promising new osteoporosis therapy. ARCH suggests that sequential therapy with romosuzumab and alendronate is superior in terms of fracture prevention to alendronate alone in elderly postmenopausal women with osteoporosis and a history of fragility fractures, although longer term studies are needed to define the durability of this effect. While the absolute number of serious adjudicated cardiovascular events was low, the increased incidence in the romosuzumab group will likely prevent the FDA from approving this medication for widespread use at this time. Additional studies are needed to clarify the cause and magnitude of this cardiovascular risk and to determine whether prevention of fracture-associated morbidity and mortality is enough to mitigate it.
—Simona Frunza-Stefan, MD, and Hillary B. Whitlach, MD, University of Maryland School of Medicine, Baltimore, MD
1. Cummings SR, Melton IJ. Epidemiology and outcomes of osteoporotic fractures. Lancet 2002; 359:176107.
2. McClung MR, Grauer A, Boonen S, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med 2014;370:412–20.
3. Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med 2016;375:1532–43.
4. Zhu D, Mackenzie NCW, Millán JL, et al. The appearance and modulation of osteocyte marker expres- sion during calcification of vascular smooth muscle cells. PLoS One 2011;6:e19595.
5. Evenepoel P, Goffin E, Meijers B, et al. Sclerostin serum levels and vascular calcification progression in prevalent renal transplant recipients. J Clin Endocrinol Metab 2015;100:4669–76.
6. Masuda T, Deng X, Tamai R. Mouse macrophages primed with alendronate down-regulate monocyte chemoattractant protein-1 (MCP-1) and macrophage inflammatory protein-1alpha (MIP-1alpha) production in response to Toll-like receptor (TLR) 2 and TLR4 agonist via Smad3 activation. Int Immunopharmacol 2009;9:1115–21.
7. Kim DH, Rogers JR, Fulchino LA, et al. Bisphosphonates and risk of cardiovascular events: a meta-analysis. PLoS One 2015;10:e0122646.
1. Cummings SR, Melton IJ. Epidemiology and outcomes of osteoporotic fractures. Lancet 2002; 359:176107.
2. McClung MR, Grauer A, Boonen S, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med 2014;370:412–20.
3. Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med 2016;375:1532–43.
4. Zhu D, Mackenzie NCW, Millán JL, et al. The appearance and modulation of osteocyte marker expres- sion during calcification of vascular smooth muscle cells. PLoS One 2011;6:e19595.
5. Evenepoel P, Goffin E, Meijers B, et al. Sclerostin serum levels and vascular calcification progression in prevalent renal transplant recipients. J Clin Endocrinol Metab 2015;100:4669–76.
6. Masuda T, Deng X, Tamai R. Mouse macrophages primed with alendronate down-regulate monocyte chemoattractant protein-1 (MCP-1) and macrophage inflammatory protein-1alpha (MIP-1alpha) production in response to Toll-like receptor (TLR) 2 and TLR4 agonist via Smad3 activation. Int Immunopharmacol 2009;9:1115–21.
7. Kim DH, Rogers JR, Fulchino LA, et al. Bisphosphonates and risk of cardiovascular events: a meta-analysis. PLoS One 2015;10:e0122646.
Alzheimer dementia: Starting, stopping drug therapy
Alzheimer disease is the most common form of dementia. In 2016, an estimated 5.2 million Americans age 65 and older had Alzheimer disease. The prevalence is projected to increase to 13.8 million by 2050, including 7 million people age 85 and older.1
Although no cure for dementia exists, several cognition-enhancing drugs have been approved by the US Food and Drug Administration (FDA) to treat the symptoms of Alzheimer dementia. The purpose of these drugs is to stabilize cognitive and functional status, with a secondary benefit of potentially reducing behavioral problems associated with dementia.
CURRENTLY APPROVED DRUGS
Two classes of drugs are approved to treat Alzheimer disease: cholinesterase inhibitors and an N-methyl-d-aspartate (NMDA) receptor antagonist (Table 1).
Cholinesterase inhibitors
The cholinesterase inhibitors act by reversibly binding and inactivating acetylcholinesterase, consequently increasing the time the neurotransmitter acetylcholine remains in the synaptic cleft. The 3 FDA-approved cholinesterase inhibitors are donepezil, galantamine, and rivastigmine. Tacrine, the first approved cholinesterase inhibitor, was removed from the US market after reports of severe hepatic toxicity.2
The clinical efficacy of cholinesterase inhibitors in improving cognitive function has been shown in several randomized controlled trials.3–10 However, benefits were generally modest, and some trials used questionable methodology, leading experts to challenge the overall efficacy of these agents.
All 3 drugs are approved for mild to moderate Alzheimer disease (stages 4–6 on the Global Deterioration Scale; Table 2)11,12; only donepezil is approved for severe Alzheimer disease. Rivastigmine has an added indication for treating mild to moderate dementia associated with Parkinson disease. Cholinesterase inhibitors are often used off-label to treat other forms of dementia such as vascular dementia, mixed dementia, and dementia with Lewy bodies.13
NMDA receptor antagonist
Memantine, currently the only FDA-approved NMDA receptor antagonist, acts by reducing neuronal calcium ion influx and its associated excitation and toxicity. Memantine is approved for moderate to severe Alzheimer disease.
Combination therapy
Often, these 2 classes of medications are prescribed in combination. In a randomized controlled trial that added memantine to stable doses of donepezil, patients had significantly better clinical response on combination therapy than on cholinesterase inhibitor monotherapy.14
In December 2014, the FDA approved a capsule formulation combining donepezil and memantine to treat symptoms of Alzheimer dementia. However, no novel pharmacologic treatment for Alzheimer disease has been approved since 2003. Furthermore, recently Pfizer announced a plan to eliminate 300 research positions aimed at finding new drugs to treat Alzheimer disease and Parkinson disease.15
CONSIDERATIONS WHEN STARTING COGNITIVE ENHANCERS
Cholinesterase inhibitors
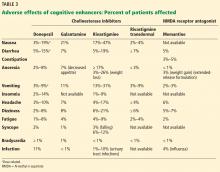
Adverse effects of cholinesterase inhibitors are generally mild and well tolerated and subside within 1 to 2 weeks. Gastrointestinal effects are common, primarily diarrhea, nausea, and vomiting. They are transient but can occur in about 20% of patients (Table 3).
Other potential adverse effects include bradycardia, syncope, rhabdomyolysis, neuroleptic malignant syndrome, and esophageal rupture. Often, the side-effect profile helps determine which patients are appropriate candidates for these medications.
As expected, higher doses of donepezil (23 mg vs 5–10 mg) are associated with higher rates of nausea, diarrhea, and vomiting.
Dosing. The cholinesterase inhibitors should be slowly titrated to minimize side effects. Starting at the lowest dose and maintaining it for 4 weeks allows sufficient time for transient side effects to abate. Some patients may require a longer titration period.
As the dose is escalated, the probability of side effects may increase. If they do not subside, dose reduction with maintenance at the next lower dose is appropriate.
Gastrointestinal effects. Given the adverse gastrointestinal effects associated with this class of medications, patients experiencing significant anorexia and weight loss should generally avoid cholinesterase inhibitors. However, the rivastigmine patch, a transdermal formulation, is an alternative for patients who experience gastrointestinal side effects.
Bradycardia risk. Patients with significant bradycardia or who are taking medications that lower the heart rate may experience a worsening of their bradycardia or associated symptoms if they take a cholinesterase inhibitor. Syncope from bradycardia is a significant concern, especially in patients already at risk of falls or fracture due to osteoporosis.
NMDA receptor antagonist
The side-effect profile of memantine is generally more favorable than that of cholinesterase inhibitors. In clinical trials, it has been better tolerated with fewer adverse effects than placebo, with the exception of an increased incidence of dizziness, confusion, and delusions.16,17
Caution is required when treating patients with renal impairment. In patients with a creatinine clearance of 5 to 29 mL/min, the recommended maximum total daily dose is 10 mg (twice-daily formulation) or 14 mg (once-daily formulation).
Off-label use to treat behavioral problems
These medications have been used off-label to treat behavioral problems associated with dementia. A systematic review and meta-analysis showed cholinesterase inhibitor therapy had a statistically significant effect in reducing the severity of behavioral problems.18 Unfortunately, the number of dropouts increased in the active-treatment groups.
Patients with behavioral problems associated with dementia with Lewy bodies may experience a greater response to cholinesterase inhibitors than those with Alzheimer disease.19 Published post hoc analyses suggest that patients with moderate to severe Alzheimer disease receiving memantine therapy have less severe agitation, aggression, irritability, and other behavioral disturbances compared with those on placebo.20,21 However, systematic reviews have not found that memantine has a clinically significant effect on neuropsychiatric symptoms of dementia.18,22,23
Combination therapy
In early randomized controlled trials, adding memantine to a cholinesterase inhibitor provided additional cognitive benefit in patients with Alzheimer disease.15,24 However, a more recent randomized controlled trial did not show significant benefits for combined memantine and donepezil vs donepezil alone in moderate to severe dementia.25
In patients who had mild to moderate Alzheimer disease at 14 Veterans Affairs medical centers who were already on cholinesterase inhibitor treatment, adding memantine did not show benefit. However, the group receiving alpha-tocopherol (vitamin E) showed slower functional decline than those on placebo.26 Cognition and function are not expected to improve with memantine.
CONSIDERATIONS WHEN STOPPING COGNITIVE ENHANCERS
The cholinesterase inhibitors are usually prescribed early in the course of dementia, and some patients take these drugs for years, although no studies have investigated benefit or risk beyond 1 year. It is generally recommended that cholinesterase inhibitor therapy be assessed periodically, eg, every 3 to 6 months, for perceived cognitive benefits and adverse gastrointestinal effects.
These medications should be stopped if the desired effects—stabilizing cognitive and functional status—are not perceived within a reasonable time, such as 12 weeks. In some cases, stopping cholinesterase inhibitor therapy may cause negative effects on cognition and neuropsychiatric symptoms.27
Deciding whether benefit has occurred during a trial of cholinesterase inhibitors often requires input and observations from the family and caregivers. Soliciting this information is key for practitioners to determine the correct treatment approach for each patient.
Although some patients with moderately severe disease experience clinical benefits from cholinesterase inhibitor therapy, it is reasonable to consider discontinuing therapy when a patient has progressed to advanced dementia with loss of functional independence, thus making the use of the therapy—ie, to preserve functional status—less relevant. Results from a randomized discontinuation trial of cholinesterase inhibitors in institutionalized patients with moderate to severe dementia suggest that discontinuation is safe and well tolerated in most of these patients.28
Abruptly stopping high-dose cholinesterase inhibitors is not recommended. Most clinical trials tapered these medications over 2 to 4 weeks. Patients taking the maximum dose of a cholinesterase inhibitor should have the dose reduced to the next lowest dose for 2 weeks before the dose is reduced further or stopped completely.
CONSIDERATIONS FOR OTHER DEMENTIA THERAPY
Behavioral and psychiatric problems often accompany dementia; however, no drugs are approved to treat these symptoms in patients with Alzheimer disease. Nonpharmacologic interventions are recommended as the initial treatment.29 Some practitioners prescribe psychotropic drugs off-label for Alzheimer disease, but most clinical trials have not found these therapies to be very effective for psychiatric symptoms associated with Alzheimer disease.30,31
Recently, a randomized controlled trial of dextromethorphan-quinidine showed mild reduction in agitation in patients with Alzheimer disease, but there were significant increases in falls, dizziness, and diarrhea.32
Patients prescribed medications for behavioral and psychological symptoms of dementia should be assessed every 3 to 6 months to determine if the medications have been effective in reducing the symptoms they were meant to reduce. If there has been no clear reduction in the target behaviors, a trial off the drug should be initiated, with careful monitoring to see if the target behavior changes. Dementia-related behaviors may worsen off the medication, but a lower dose may be found to be as effective as a higher dose. As dementia advances, behaviors initially encountered during one stage may diminish or abate.
In a long-term care setting, a gradual dose-reduction trial of psychotropic medications should be conducted every year to determine if the medications are still necessary.33 This should be considered during routine management and follow-up of patients with dementia-associated behavioral problems.
REASONABLE TO TRY
Cognitive enhancers have been around for more than 10 years and are reasonable to try in patients with Alzheimer disease. All the available drugs are FDA-approved for reducing dementia symptoms associated with mild to moderate Alzheimer disease; donepezil and memantine are also approved for severe Alzheimer disease, either in combination or as monotherapy.
When selecting a cognitive enhancer, practitioners need to consider the potential for adverse effects. And if a cholinesterase inhibitor is prescribed, it is important to periodically assess for perceived cognitive benefits and adverse gastrointestinal effects. The NMDA receptor antagonist has a more favorable side effect profile. Combining the drugs is also an option.
Similarly, patients prescribed psychotropic medications for behavioral problems related to dementia should be reassessed to determine if the dose could be reduced or eliminated, particularly if targeted behaviors have not responded to the treatment or the dementia has advanced.
For patients on cognitive enhancers, discontinuation should be considered when the dementia advances to the point where the patient is totally dependent for all basic activities of daily living, and the initial intended purpose of these medications—preservation of cognitive and functional status—is no longer achievable.
- Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 2013; 80:1778–1783.
- Watkins PB, Zimmerman HJ, Knapp MJ, et al. Hepatotoxic effects of tacrine administration in patients with Alzheimer’s disease. JAMA 1994; 271:992–998.
- Courtney C, Farrell D, Gray R, et al. Long-term donepezil treatment in 565 patients with Alzheimer’s disease (AD2000): randomised double-blind trial. Lancet 2004; 363:2105–2115.
- Wang J, Yu JT, Wang HF, et al. Pharmacological treatment of neuropsychiatric symptoms in Alzheimer’s disease: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2015; 86:101–109.
- Raina P, Santaguida P, Ismaila A, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med 2008; 148:379–397.
- Lanctot KL, Hermann N, Yau KK, et al. Efficacy and safety of cholinesterase inhibitors in Alzheimer’s disease: a meta-analysis. CMAJ 2003; 169:557–564.
- Qaseem A, Snow V, Cross JT Jr, et al. Current pharmacologic treatment of dementia: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med 2008; 148:370–378.
- Trinh NH, Hoblyn J, Mohanty S, Yaffe K. Efficacy of cholinesterase inhibitors in the treatment of neuropsychiatric symptoms and functional impairment in Alzheimer disease: a meta-analysis. JAMA 2003; 289:210–216.
- Kaduszkiewicz H, Zimmermann T, Beck-Bornholdt HP, van den Bussche H. Cholinesterase inhibitors for patients with Alzheimer’s disease: systematic review of randomised clinical trials. BMJ 2005; 331:321–327.
- Birks J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev 2006; 1:CD005593.
- Reisberg B, Ferris SH, de Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry 1982; 139:1136–1139.
- Mitchell SL. Advanced dementia. N Engl J Med 2015; 372:2533–2540.
- Rolinski M, Fox C, Maidment I, McShane R. Cholinesterase inhibitors for dementia with Lewy bodies, Parkinson’s disease dementia and cognitive impairment in Parkinson’s disease. Cochrane Database Syst Rev 2012; 3:CD006504.
- Tariot PN, Farlow MR, Grossberg GT, et al. Memantine treatment in patients with moderate to severe Alzheimer’s disease already receiving donepezil: a randomized controlled trial. JAMA 2004; 291:317–324.
- Reuters Staff. Pfizer ends research for new Alzheimer’s, Parkinson’s drugs. January 7, 2018. https://www.reuters.com/article/us-pfizer-alzheimers/pfizer-ends-research-for-new-alzheimers-parkinsons-drugs-idUSKBN1EW0TN. Accessed February 2, 2018.
- Aerosa SA, Sherriff F, McShane R. Memantine for dementia. Cochrane Database Syst Rev 2005 Jul 20;(3):CD003154.
- Rossom R, Adityanjee, Dysken M. Efficacy and tolerability of memantine in the treatment of dementia. Am J Geriatr Pharmacother 2004; 2:303–312.
- Wang J, Yu JT, Wang HF, et al. Pharmacological treatment of neuropsychiatric symptoms in Alzheimer’s disease: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2015; 86:101–109.
- McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomized, double-blind, placebo-controlled international study. Lancet 2000; 356:2031–2036.
- Cummings JL, Schneider E, Tariot PN, Graham SM, Memantine MEM-MD-02 Study Group. Behavioral effects of memantine in Alzheimer disease patients receiving donepezil treatment. Neurology 2006; 67:57–63.
- Wilcock GK, Ballard CG, Cooper JA, Loft H. Memantine for agitation/aggression and psychosis in moderately severe to severe Alzheimer’s disease: a pooled analysis of 3 studies. J Clin Psychiatry 2008; 69:341–348.
- Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA 2005; 293:596–608.
- McShane R, Areosa Sastre A, Minakaran N. Memantine for dementia. Cochrane Database Syst Rev 2006; 2:CD003154.
- Reisberg B, Doody R, Stoffler A, et al. Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med 2003; 348:1333–1341.
- Howard R, McShane R, Lindesay J, et al. Donepezil and memantine for moderate-to-severe Alzheimer’s disease. N Engl J Med 2012; 366:893–903.
- Dysken MW, Sano M, Asthana S, et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: the TEAM-AD VA cooperative randomized trial. JAMA 2014; 311:33–44.
- O’Regan J, Lanctot KL, Mazereeuw G, Herrmann N. Cholinesterase inhibitor discontinuation in patients with Alzheimer’s disease: a meta-analysis of randomized controlled trials. J Clin Psychiatry 2015; 76:e1424–e1431.
- Herrmann N, O’Reagan J, Ruthirahukhan M, et al. A randomized placebo-controlled discontinuation study of cholinesterase inhibitors in institutionalized patients with moderate to severe Alzheimer disease. J Am Med Dir Assoc 2016; 17:142–174.
- Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacological management of behavioral symptoms in dementia. JAMA 2012; 308:2020–2029.
- Schwab W, Messinger-Rapport B, Franco K. Psychiatric symptoms of dementia: treatable, but no silver bullet. Cleve Clin J Med 2009; 76:167–174.
- Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA 2005; 293:596–608.
- Cummings JL, Lyketsos CG, Peskind ER, et al. Effects of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA 2015; 314:1242–1254.
- Centers for Medicare and Medicaid Services. Dementia care in nursing homes: clarification to Appendix P State Operations Manual (SOM) and Appendix PP in the SOM for F309—quality of care and F329—unnecessary drugs. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertificationGenInfo/Downloads/Survey-and-Cert-Letter-13-35.pdf. Accessed February 1, 2018.
Alzheimer disease is the most common form of dementia. In 2016, an estimated 5.2 million Americans age 65 and older had Alzheimer disease. The prevalence is projected to increase to 13.8 million by 2050, including 7 million people age 85 and older.1
Although no cure for dementia exists, several cognition-enhancing drugs have been approved by the US Food and Drug Administration (FDA) to treat the symptoms of Alzheimer dementia. The purpose of these drugs is to stabilize cognitive and functional status, with a secondary benefit of potentially reducing behavioral problems associated with dementia.
CURRENTLY APPROVED DRUGS
Two classes of drugs are approved to treat Alzheimer disease: cholinesterase inhibitors and an N-methyl-d-aspartate (NMDA) receptor antagonist (Table 1).
Cholinesterase inhibitors
The cholinesterase inhibitors act by reversibly binding and inactivating acetylcholinesterase, consequently increasing the time the neurotransmitter acetylcholine remains in the synaptic cleft. The 3 FDA-approved cholinesterase inhibitors are donepezil, galantamine, and rivastigmine. Tacrine, the first approved cholinesterase inhibitor, was removed from the US market after reports of severe hepatic toxicity.2
The clinical efficacy of cholinesterase inhibitors in improving cognitive function has been shown in several randomized controlled trials.3–10 However, benefits were generally modest, and some trials used questionable methodology, leading experts to challenge the overall efficacy of these agents.
All 3 drugs are approved for mild to moderate Alzheimer disease (stages 4–6 on the Global Deterioration Scale; Table 2)11,12; only donepezil is approved for severe Alzheimer disease. Rivastigmine has an added indication for treating mild to moderate dementia associated with Parkinson disease. Cholinesterase inhibitors are often used off-label to treat other forms of dementia such as vascular dementia, mixed dementia, and dementia with Lewy bodies.13
NMDA receptor antagonist
Memantine, currently the only FDA-approved NMDA receptor antagonist, acts by reducing neuronal calcium ion influx and its associated excitation and toxicity. Memantine is approved for moderate to severe Alzheimer disease.
Combination therapy
Often, these 2 classes of medications are prescribed in combination. In a randomized controlled trial that added memantine to stable doses of donepezil, patients had significantly better clinical response on combination therapy than on cholinesterase inhibitor monotherapy.14
In December 2014, the FDA approved a capsule formulation combining donepezil and memantine to treat symptoms of Alzheimer dementia. However, no novel pharmacologic treatment for Alzheimer disease has been approved since 2003. Furthermore, recently Pfizer announced a plan to eliminate 300 research positions aimed at finding new drugs to treat Alzheimer disease and Parkinson disease.15
CONSIDERATIONS WHEN STARTING COGNITIVE ENHANCERS
Cholinesterase inhibitors
Adverse effects of cholinesterase inhibitors are generally mild and well tolerated and subside within 1 to 2 weeks. Gastrointestinal effects are common, primarily diarrhea, nausea, and vomiting. They are transient but can occur in about 20% of patients (Table 3).
Other potential adverse effects include bradycardia, syncope, rhabdomyolysis, neuroleptic malignant syndrome, and esophageal rupture. Often, the side-effect profile helps determine which patients are appropriate candidates for these medications.
As expected, higher doses of donepezil (23 mg vs 5–10 mg) are associated with higher rates of nausea, diarrhea, and vomiting.
Dosing. The cholinesterase inhibitors should be slowly titrated to minimize side effects. Starting at the lowest dose and maintaining it for 4 weeks allows sufficient time for transient side effects to abate. Some patients may require a longer titration period.
As the dose is escalated, the probability of side effects may increase. If they do not subside, dose reduction with maintenance at the next lower dose is appropriate.
Gastrointestinal effects. Given the adverse gastrointestinal effects associated with this class of medications, patients experiencing significant anorexia and weight loss should generally avoid cholinesterase inhibitors. However, the rivastigmine patch, a transdermal formulation, is an alternative for patients who experience gastrointestinal side effects.
Bradycardia risk. Patients with significant bradycardia or who are taking medications that lower the heart rate may experience a worsening of their bradycardia or associated symptoms if they take a cholinesterase inhibitor. Syncope from bradycardia is a significant concern, especially in patients already at risk of falls or fracture due to osteoporosis.
NMDA receptor antagonist
The side-effect profile of memantine is generally more favorable than that of cholinesterase inhibitors. In clinical trials, it has been better tolerated with fewer adverse effects than placebo, with the exception of an increased incidence of dizziness, confusion, and delusions.16,17
Caution is required when treating patients with renal impairment. In patients with a creatinine clearance of 5 to 29 mL/min, the recommended maximum total daily dose is 10 mg (twice-daily formulation) or 14 mg (once-daily formulation).
Off-label use to treat behavioral problems
These medications have been used off-label to treat behavioral problems associated with dementia. A systematic review and meta-analysis showed cholinesterase inhibitor therapy had a statistically significant effect in reducing the severity of behavioral problems.18 Unfortunately, the number of dropouts increased in the active-treatment groups.
Patients with behavioral problems associated with dementia with Lewy bodies may experience a greater response to cholinesterase inhibitors than those with Alzheimer disease.19 Published post hoc analyses suggest that patients with moderate to severe Alzheimer disease receiving memantine therapy have less severe agitation, aggression, irritability, and other behavioral disturbances compared with those on placebo.20,21 However, systematic reviews have not found that memantine has a clinically significant effect on neuropsychiatric symptoms of dementia.18,22,23
Combination therapy
In early randomized controlled trials, adding memantine to a cholinesterase inhibitor provided additional cognitive benefit in patients with Alzheimer disease.15,24 However, a more recent randomized controlled trial did not show significant benefits for combined memantine and donepezil vs donepezil alone in moderate to severe dementia.25
In patients who had mild to moderate Alzheimer disease at 14 Veterans Affairs medical centers who were already on cholinesterase inhibitor treatment, adding memantine did not show benefit. However, the group receiving alpha-tocopherol (vitamin E) showed slower functional decline than those on placebo.26 Cognition and function are not expected to improve with memantine.
CONSIDERATIONS WHEN STOPPING COGNITIVE ENHANCERS
The cholinesterase inhibitors are usually prescribed early in the course of dementia, and some patients take these drugs for years, although no studies have investigated benefit or risk beyond 1 year. It is generally recommended that cholinesterase inhibitor therapy be assessed periodically, eg, every 3 to 6 months, for perceived cognitive benefits and adverse gastrointestinal effects.
These medications should be stopped if the desired effects—stabilizing cognitive and functional status—are not perceived within a reasonable time, such as 12 weeks. In some cases, stopping cholinesterase inhibitor therapy may cause negative effects on cognition and neuropsychiatric symptoms.27
Deciding whether benefit has occurred during a trial of cholinesterase inhibitors often requires input and observations from the family and caregivers. Soliciting this information is key for practitioners to determine the correct treatment approach for each patient.
Although some patients with moderately severe disease experience clinical benefits from cholinesterase inhibitor therapy, it is reasonable to consider discontinuing therapy when a patient has progressed to advanced dementia with loss of functional independence, thus making the use of the therapy—ie, to preserve functional status—less relevant. Results from a randomized discontinuation trial of cholinesterase inhibitors in institutionalized patients with moderate to severe dementia suggest that discontinuation is safe and well tolerated in most of these patients.28
Abruptly stopping high-dose cholinesterase inhibitors is not recommended. Most clinical trials tapered these medications over 2 to 4 weeks. Patients taking the maximum dose of a cholinesterase inhibitor should have the dose reduced to the next lowest dose for 2 weeks before the dose is reduced further or stopped completely.
CONSIDERATIONS FOR OTHER DEMENTIA THERAPY
Behavioral and psychiatric problems often accompany dementia; however, no drugs are approved to treat these symptoms in patients with Alzheimer disease. Nonpharmacologic interventions are recommended as the initial treatment.29 Some practitioners prescribe psychotropic drugs off-label for Alzheimer disease, but most clinical trials have not found these therapies to be very effective for psychiatric symptoms associated with Alzheimer disease.30,31
Recently, a randomized controlled trial of dextromethorphan-quinidine showed mild reduction in agitation in patients with Alzheimer disease, but there were significant increases in falls, dizziness, and diarrhea.32
Patients prescribed medications for behavioral and psychological symptoms of dementia should be assessed every 3 to 6 months to determine if the medications have been effective in reducing the symptoms they were meant to reduce. If there has been no clear reduction in the target behaviors, a trial off the drug should be initiated, with careful monitoring to see if the target behavior changes. Dementia-related behaviors may worsen off the medication, but a lower dose may be found to be as effective as a higher dose. As dementia advances, behaviors initially encountered during one stage may diminish or abate.
In a long-term care setting, a gradual dose-reduction trial of psychotropic medications should be conducted every year to determine if the medications are still necessary.33 This should be considered during routine management and follow-up of patients with dementia-associated behavioral problems.
REASONABLE TO TRY
Cognitive enhancers have been around for more than 10 years and are reasonable to try in patients with Alzheimer disease. All the available drugs are FDA-approved for reducing dementia symptoms associated with mild to moderate Alzheimer disease; donepezil and memantine are also approved for severe Alzheimer disease, either in combination or as monotherapy.
When selecting a cognitive enhancer, practitioners need to consider the potential for adverse effects. And if a cholinesterase inhibitor is prescribed, it is important to periodically assess for perceived cognitive benefits and adverse gastrointestinal effects. The NMDA receptor antagonist has a more favorable side effect profile. Combining the drugs is also an option.
Similarly, patients prescribed psychotropic medications for behavioral problems related to dementia should be reassessed to determine if the dose could be reduced or eliminated, particularly if targeted behaviors have not responded to the treatment or the dementia has advanced.
For patients on cognitive enhancers, discontinuation should be considered when the dementia advances to the point where the patient is totally dependent for all basic activities of daily living, and the initial intended purpose of these medications—preservation of cognitive and functional status—is no longer achievable.
Alzheimer disease is the most common form of dementia. In 2016, an estimated 5.2 million Americans age 65 and older had Alzheimer disease. The prevalence is projected to increase to 13.8 million by 2050, including 7 million people age 85 and older.1
Although no cure for dementia exists, several cognition-enhancing drugs have been approved by the US Food and Drug Administration (FDA) to treat the symptoms of Alzheimer dementia. The purpose of these drugs is to stabilize cognitive and functional status, with a secondary benefit of potentially reducing behavioral problems associated with dementia.
CURRENTLY APPROVED DRUGS
Two classes of drugs are approved to treat Alzheimer disease: cholinesterase inhibitors and an N-methyl-d-aspartate (NMDA) receptor antagonist (Table 1).
Cholinesterase inhibitors
The cholinesterase inhibitors act by reversibly binding and inactivating acetylcholinesterase, consequently increasing the time the neurotransmitter acetylcholine remains in the synaptic cleft. The 3 FDA-approved cholinesterase inhibitors are donepezil, galantamine, and rivastigmine. Tacrine, the first approved cholinesterase inhibitor, was removed from the US market after reports of severe hepatic toxicity.2
The clinical efficacy of cholinesterase inhibitors in improving cognitive function has been shown in several randomized controlled trials.3–10 However, benefits were generally modest, and some trials used questionable methodology, leading experts to challenge the overall efficacy of these agents.
All 3 drugs are approved for mild to moderate Alzheimer disease (stages 4–6 on the Global Deterioration Scale; Table 2)11,12; only donepezil is approved for severe Alzheimer disease. Rivastigmine has an added indication for treating mild to moderate dementia associated with Parkinson disease. Cholinesterase inhibitors are often used off-label to treat other forms of dementia such as vascular dementia, mixed dementia, and dementia with Lewy bodies.13
NMDA receptor antagonist
Memantine, currently the only FDA-approved NMDA receptor antagonist, acts by reducing neuronal calcium ion influx and its associated excitation and toxicity. Memantine is approved for moderate to severe Alzheimer disease.
Combination therapy
Often, these 2 classes of medications are prescribed in combination. In a randomized controlled trial that added memantine to stable doses of donepezil, patients had significantly better clinical response on combination therapy than on cholinesterase inhibitor monotherapy.14
In December 2014, the FDA approved a capsule formulation combining donepezil and memantine to treat symptoms of Alzheimer dementia. However, no novel pharmacologic treatment for Alzheimer disease has been approved since 2003. Furthermore, recently Pfizer announced a plan to eliminate 300 research positions aimed at finding new drugs to treat Alzheimer disease and Parkinson disease.15
CONSIDERATIONS WHEN STARTING COGNITIVE ENHANCERS
Cholinesterase inhibitors
Adverse effects of cholinesterase inhibitors are generally mild and well tolerated and subside within 1 to 2 weeks. Gastrointestinal effects are common, primarily diarrhea, nausea, and vomiting. They are transient but can occur in about 20% of patients (Table 3).
Other potential adverse effects include bradycardia, syncope, rhabdomyolysis, neuroleptic malignant syndrome, and esophageal rupture. Often, the side-effect profile helps determine which patients are appropriate candidates for these medications.
As expected, higher doses of donepezil (23 mg vs 5–10 mg) are associated with higher rates of nausea, diarrhea, and vomiting.
Dosing. The cholinesterase inhibitors should be slowly titrated to minimize side effects. Starting at the lowest dose and maintaining it for 4 weeks allows sufficient time for transient side effects to abate. Some patients may require a longer titration period.
As the dose is escalated, the probability of side effects may increase. If they do not subside, dose reduction with maintenance at the next lower dose is appropriate.
Gastrointestinal effects. Given the adverse gastrointestinal effects associated with this class of medications, patients experiencing significant anorexia and weight loss should generally avoid cholinesterase inhibitors. However, the rivastigmine patch, a transdermal formulation, is an alternative for patients who experience gastrointestinal side effects.
Bradycardia risk. Patients with significant bradycardia or who are taking medications that lower the heart rate may experience a worsening of their bradycardia or associated symptoms if they take a cholinesterase inhibitor. Syncope from bradycardia is a significant concern, especially in patients already at risk of falls or fracture due to osteoporosis.
NMDA receptor antagonist
The side-effect profile of memantine is generally more favorable than that of cholinesterase inhibitors. In clinical trials, it has been better tolerated with fewer adverse effects than placebo, with the exception of an increased incidence of dizziness, confusion, and delusions.16,17
Caution is required when treating patients with renal impairment. In patients with a creatinine clearance of 5 to 29 mL/min, the recommended maximum total daily dose is 10 mg (twice-daily formulation) or 14 mg (once-daily formulation).
Off-label use to treat behavioral problems
These medications have been used off-label to treat behavioral problems associated with dementia. A systematic review and meta-analysis showed cholinesterase inhibitor therapy had a statistically significant effect in reducing the severity of behavioral problems.18 Unfortunately, the number of dropouts increased in the active-treatment groups.
Patients with behavioral problems associated with dementia with Lewy bodies may experience a greater response to cholinesterase inhibitors than those with Alzheimer disease.19 Published post hoc analyses suggest that patients with moderate to severe Alzheimer disease receiving memantine therapy have less severe agitation, aggression, irritability, and other behavioral disturbances compared with those on placebo.20,21 However, systematic reviews have not found that memantine has a clinically significant effect on neuropsychiatric symptoms of dementia.18,22,23
Combination therapy
In early randomized controlled trials, adding memantine to a cholinesterase inhibitor provided additional cognitive benefit in patients with Alzheimer disease.15,24 However, a more recent randomized controlled trial did not show significant benefits for combined memantine and donepezil vs donepezil alone in moderate to severe dementia.25
In patients who had mild to moderate Alzheimer disease at 14 Veterans Affairs medical centers who were already on cholinesterase inhibitor treatment, adding memantine did not show benefit. However, the group receiving alpha-tocopherol (vitamin E) showed slower functional decline than those on placebo.26 Cognition and function are not expected to improve with memantine.
CONSIDERATIONS WHEN STOPPING COGNITIVE ENHANCERS
The cholinesterase inhibitors are usually prescribed early in the course of dementia, and some patients take these drugs for years, although no studies have investigated benefit or risk beyond 1 year. It is generally recommended that cholinesterase inhibitor therapy be assessed periodically, eg, every 3 to 6 months, for perceived cognitive benefits and adverse gastrointestinal effects.
These medications should be stopped if the desired effects—stabilizing cognitive and functional status—are not perceived within a reasonable time, such as 12 weeks. In some cases, stopping cholinesterase inhibitor therapy may cause negative effects on cognition and neuropsychiatric symptoms.27
Deciding whether benefit has occurred during a trial of cholinesterase inhibitors often requires input and observations from the family and caregivers. Soliciting this information is key for practitioners to determine the correct treatment approach for each patient.
Although some patients with moderately severe disease experience clinical benefits from cholinesterase inhibitor therapy, it is reasonable to consider discontinuing therapy when a patient has progressed to advanced dementia with loss of functional independence, thus making the use of the therapy—ie, to preserve functional status—less relevant. Results from a randomized discontinuation trial of cholinesterase inhibitors in institutionalized patients with moderate to severe dementia suggest that discontinuation is safe and well tolerated in most of these patients.28
Abruptly stopping high-dose cholinesterase inhibitors is not recommended. Most clinical trials tapered these medications over 2 to 4 weeks. Patients taking the maximum dose of a cholinesterase inhibitor should have the dose reduced to the next lowest dose for 2 weeks before the dose is reduced further or stopped completely.
CONSIDERATIONS FOR OTHER DEMENTIA THERAPY
Behavioral and psychiatric problems often accompany dementia; however, no drugs are approved to treat these symptoms in patients with Alzheimer disease. Nonpharmacologic interventions are recommended as the initial treatment.29 Some practitioners prescribe psychotropic drugs off-label for Alzheimer disease, but most clinical trials have not found these therapies to be very effective for psychiatric symptoms associated with Alzheimer disease.30,31
Recently, a randomized controlled trial of dextromethorphan-quinidine showed mild reduction in agitation in patients with Alzheimer disease, but there were significant increases in falls, dizziness, and diarrhea.32
Patients prescribed medications for behavioral and psychological symptoms of dementia should be assessed every 3 to 6 months to determine if the medications have been effective in reducing the symptoms they were meant to reduce. If there has been no clear reduction in the target behaviors, a trial off the drug should be initiated, with careful monitoring to see if the target behavior changes. Dementia-related behaviors may worsen off the medication, but a lower dose may be found to be as effective as a higher dose. As dementia advances, behaviors initially encountered during one stage may diminish or abate.
In a long-term care setting, a gradual dose-reduction trial of psychotropic medications should be conducted every year to determine if the medications are still necessary.33 This should be considered during routine management and follow-up of patients with dementia-associated behavioral problems.
REASONABLE TO TRY
Cognitive enhancers have been around for more than 10 years and are reasonable to try in patients with Alzheimer disease. All the available drugs are FDA-approved for reducing dementia symptoms associated with mild to moderate Alzheimer disease; donepezil and memantine are also approved for severe Alzheimer disease, either in combination or as monotherapy.
When selecting a cognitive enhancer, practitioners need to consider the potential for adverse effects. And if a cholinesterase inhibitor is prescribed, it is important to periodically assess for perceived cognitive benefits and adverse gastrointestinal effects. The NMDA receptor antagonist has a more favorable side effect profile. Combining the drugs is also an option.
Similarly, patients prescribed psychotropic medications for behavioral problems related to dementia should be reassessed to determine if the dose could be reduced or eliminated, particularly if targeted behaviors have not responded to the treatment or the dementia has advanced.
For patients on cognitive enhancers, discontinuation should be considered when the dementia advances to the point where the patient is totally dependent for all basic activities of daily living, and the initial intended purpose of these medications—preservation of cognitive and functional status—is no longer achievable.
- Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 2013; 80:1778–1783.
- Watkins PB, Zimmerman HJ, Knapp MJ, et al. Hepatotoxic effects of tacrine administration in patients with Alzheimer’s disease. JAMA 1994; 271:992–998.
- Courtney C, Farrell D, Gray R, et al. Long-term donepezil treatment in 565 patients with Alzheimer’s disease (AD2000): randomised double-blind trial. Lancet 2004; 363:2105–2115.
- Wang J, Yu JT, Wang HF, et al. Pharmacological treatment of neuropsychiatric symptoms in Alzheimer’s disease: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2015; 86:101–109.
- Raina P, Santaguida P, Ismaila A, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med 2008; 148:379–397.
- Lanctot KL, Hermann N, Yau KK, et al. Efficacy and safety of cholinesterase inhibitors in Alzheimer’s disease: a meta-analysis. CMAJ 2003; 169:557–564.
- Qaseem A, Snow V, Cross JT Jr, et al. Current pharmacologic treatment of dementia: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med 2008; 148:370–378.
- Trinh NH, Hoblyn J, Mohanty S, Yaffe K. Efficacy of cholinesterase inhibitors in the treatment of neuropsychiatric symptoms and functional impairment in Alzheimer disease: a meta-analysis. JAMA 2003; 289:210–216.
- Kaduszkiewicz H, Zimmermann T, Beck-Bornholdt HP, van den Bussche H. Cholinesterase inhibitors for patients with Alzheimer’s disease: systematic review of randomised clinical trials. BMJ 2005; 331:321–327.
- Birks J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev 2006; 1:CD005593.
- Reisberg B, Ferris SH, de Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry 1982; 139:1136–1139.
- Mitchell SL. Advanced dementia. N Engl J Med 2015; 372:2533–2540.
- Rolinski M, Fox C, Maidment I, McShane R. Cholinesterase inhibitors for dementia with Lewy bodies, Parkinson’s disease dementia and cognitive impairment in Parkinson’s disease. Cochrane Database Syst Rev 2012; 3:CD006504.
- Tariot PN, Farlow MR, Grossberg GT, et al. Memantine treatment in patients with moderate to severe Alzheimer’s disease already receiving donepezil: a randomized controlled trial. JAMA 2004; 291:317–324.
- Reuters Staff. Pfizer ends research for new Alzheimer’s, Parkinson’s drugs. January 7, 2018. https://www.reuters.com/article/us-pfizer-alzheimers/pfizer-ends-research-for-new-alzheimers-parkinsons-drugs-idUSKBN1EW0TN. Accessed February 2, 2018.
- Aerosa SA, Sherriff F, McShane R. Memantine for dementia. Cochrane Database Syst Rev 2005 Jul 20;(3):CD003154.
- Rossom R, Adityanjee, Dysken M. Efficacy and tolerability of memantine in the treatment of dementia. Am J Geriatr Pharmacother 2004; 2:303–312.
- Wang J, Yu JT, Wang HF, et al. Pharmacological treatment of neuropsychiatric symptoms in Alzheimer’s disease: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2015; 86:101–109.
- McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomized, double-blind, placebo-controlled international study. Lancet 2000; 356:2031–2036.
- Cummings JL, Schneider E, Tariot PN, Graham SM, Memantine MEM-MD-02 Study Group. Behavioral effects of memantine in Alzheimer disease patients receiving donepezil treatment. Neurology 2006; 67:57–63.
- Wilcock GK, Ballard CG, Cooper JA, Loft H. Memantine for agitation/aggression and psychosis in moderately severe to severe Alzheimer’s disease: a pooled analysis of 3 studies. J Clin Psychiatry 2008; 69:341–348.
- Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA 2005; 293:596–608.
- McShane R, Areosa Sastre A, Minakaran N. Memantine for dementia. Cochrane Database Syst Rev 2006; 2:CD003154.
- Reisberg B, Doody R, Stoffler A, et al. Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med 2003; 348:1333–1341.
- Howard R, McShane R, Lindesay J, et al. Donepezil and memantine for moderate-to-severe Alzheimer’s disease. N Engl J Med 2012; 366:893–903.
- Dysken MW, Sano M, Asthana S, et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: the TEAM-AD VA cooperative randomized trial. JAMA 2014; 311:33–44.
- O’Regan J, Lanctot KL, Mazereeuw G, Herrmann N. Cholinesterase inhibitor discontinuation in patients with Alzheimer’s disease: a meta-analysis of randomized controlled trials. J Clin Psychiatry 2015; 76:e1424–e1431.
- Herrmann N, O’Reagan J, Ruthirahukhan M, et al. A randomized placebo-controlled discontinuation study of cholinesterase inhibitors in institutionalized patients with moderate to severe Alzheimer disease. J Am Med Dir Assoc 2016; 17:142–174.
- Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacological management of behavioral symptoms in dementia. JAMA 2012; 308:2020–2029.
- Schwab W, Messinger-Rapport B, Franco K. Psychiatric symptoms of dementia: treatable, but no silver bullet. Cleve Clin J Med 2009; 76:167–174.
- Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA 2005; 293:596–608.
- Cummings JL, Lyketsos CG, Peskind ER, et al. Effects of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA 2015; 314:1242–1254.
- Centers for Medicare and Medicaid Services. Dementia care in nursing homes: clarification to Appendix P State Operations Manual (SOM) and Appendix PP in the SOM for F309—quality of care and F329—unnecessary drugs. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertificationGenInfo/Downloads/Survey-and-Cert-Letter-13-35.pdf. Accessed February 1, 2018.
- Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 2013; 80:1778–1783.
- Watkins PB, Zimmerman HJ, Knapp MJ, et al. Hepatotoxic effects of tacrine administration in patients with Alzheimer’s disease. JAMA 1994; 271:992–998.
- Courtney C, Farrell D, Gray R, et al. Long-term donepezil treatment in 565 patients with Alzheimer’s disease (AD2000): randomised double-blind trial. Lancet 2004; 363:2105–2115.
- Wang J, Yu JT, Wang HF, et al. Pharmacological treatment of neuropsychiatric symptoms in Alzheimer’s disease: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2015; 86:101–109.
- Raina P, Santaguida P, Ismaila A, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med 2008; 148:379–397.
- Lanctot KL, Hermann N, Yau KK, et al. Efficacy and safety of cholinesterase inhibitors in Alzheimer’s disease: a meta-analysis. CMAJ 2003; 169:557–564.
- Qaseem A, Snow V, Cross JT Jr, et al. Current pharmacologic treatment of dementia: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med 2008; 148:370–378.
- Trinh NH, Hoblyn J, Mohanty S, Yaffe K. Efficacy of cholinesterase inhibitors in the treatment of neuropsychiatric symptoms and functional impairment in Alzheimer disease: a meta-analysis. JAMA 2003; 289:210–216.
- Kaduszkiewicz H, Zimmermann T, Beck-Bornholdt HP, van den Bussche H. Cholinesterase inhibitors for patients with Alzheimer’s disease: systematic review of randomised clinical trials. BMJ 2005; 331:321–327.
- Birks J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev 2006; 1:CD005593.
- Reisberg B, Ferris SH, de Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry 1982; 139:1136–1139.
- Mitchell SL. Advanced dementia. N Engl J Med 2015; 372:2533–2540.
- Rolinski M, Fox C, Maidment I, McShane R. Cholinesterase inhibitors for dementia with Lewy bodies, Parkinson’s disease dementia and cognitive impairment in Parkinson’s disease. Cochrane Database Syst Rev 2012; 3:CD006504.
- Tariot PN, Farlow MR, Grossberg GT, et al. Memantine treatment in patients with moderate to severe Alzheimer’s disease already receiving donepezil: a randomized controlled trial. JAMA 2004; 291:317–324.
- Reuters Staff. Pfizer ends research for new Alzheimer’s, Parkinson’s drugs. January 7, 2018. https://www.reuters.com/article/us-pfizer-alzheimers/pfizer-ends-research-for-new-alzheimers-parkinsons-drugs-idUSKBN1EW0TN. Accessed February 2, 2018.
- Aerosa SA, Sherriff F, McShane R. Memantine for dementia. Cochrane Database Syst Rev 2005 Jul 20;(3):CD003154.
- Rossom R, Adityanjee, Dysken M. Efficacy and tolerability of memantine in the treatment of dementia. Am J Geriatr Pharmacother 2004; 2:303–312.
- Wang J, Yu JT, Wang HF, et al. Pharmacological treatment of neuropsychiatric symptoms in Alzheimer’s disease: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2015; 86:101–109.
- McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomized, double-blind, placebo-controlled international study. Lancet 2000; 356:2031–2036.
- Cummings JL, Schneider E, Tariot PN, Graham SM, Memantine MEM-MD-02 Study Group. Behavioral effects of memantine in Alzheimer disease patients receiving donepezil treatment. Neurology 2006; 67:57–63.
- Wilcock GK, Ballard CG, Cooper JA, Loft H. Memantine for agitation/aggression and psychosis in moderately severe to severe Alzheimer’s disease: a pooled analysis of 3 studies. J Clin Psychiatry 2008; 69:341–348.
- Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA 2005; 293:596–608.
- McShane R, Areosa Sastre A, Minakaran N. Memantine for dementia. Cochrane Database Syst Rev 2006; 2:CD003154.
- Reisberg B, Doody R, Stoffler A, et al. Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med 2003; 348:1333–1341.
- Howard R, McShane R, Lindesay J, et al. Donepezil and memantine for moderate-to-severe Alzheimer’s disease. N Engl J Med 2012; 366:893–903.
- Dysken MW, Sano M, Asthana S, et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: the TEAM-AD VA cooperative randomized trial. JAMA 2014; 311:33–44.
- O’Regan J, Lanctot KL, Mazereeuw G, Herrmann N. Cholinesterase inhibitor discontinuation in patients with Alzheimer’s disease: a meta-analysis of randomized controlled trials. J Clin Psychiatry 2015; 76:e1424–e1431.
- Herrmann N, O’Reagan J, Ruthirahukhan M, et al. A randomized placebo-controlled discontinuation study of cholinesterase inhibitors in institutionalized patients with moderate to severe Alzheimer disease. J Am Med Dir Assoc 2016; 17:142–174.
- Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacological management of behavioral symptoms in dementia. JAMA 2012; 308:2020–2029.
- Schwab W, Messinger-Rapport B, Franco K. Psychiatric symptoms of dementia: treatable, but no silver bullet. Cleve Clin J Med 2009; 76:167–174.
- Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA 2005; 293:596–608.
- Cummings JL, Lyketsos CG, Peskind ER, et al. Effects of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA 2015; 314:1242–1254.
- Centers for Medicare and Medicaid Services. Dementia care in nursing homes: clarification to Appendix P State Operations Manual (SOM) and Appendix PP in the SOM for F309—quality of care and F329—unnecessary drugs. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertificationGenInfo/Downloads/Survey-and-Cert-Letter-13-35.pdf. Accessed February 1, 2018.
KEY POINTS
- In 2016, an estimated 5.2 million Americans age 65 and older had Alzheimer disease; by 2050, the prevalence is expected to be 13.8 million.
- Cognitive enhancers (cholinesterase inhibitors and an N-methyl-d-aspartate receptor antagonist) have shown modest efficacy in preserving cognitive function.
- When evaluating therapy with a cognitive enhancer, practitioners need to consider the potential adverse effects, especially gastrointestinal effects with cholinesterase inhibitors.
- Discontinuation should be considered when the dementia reaches the advanced stage and the initial intended purpose of these drugs is no longer achievable.
Methemoglobinemia in an HIV patient
A 45-year-old man with known human immunodeficiency virus infection presented with a 5-day history of dyspnea. When his dyspnea had become symptomatic, he had restarted his home dapsone prophylaxis, but his dyspnea had progressively worsened, and his urine became dark.
Based on these test results, the patient’s dapsone was stopped and replaced with atovaquone. Intravenous infusion of methylene blue was started, with subsequent improvement of the hypoxia and cyanosis (Figure 1). His urine became green, but it returned to a normal color in a matter of hours. He was ultimately diagnosed with P jirovecii pneumonia and completed a course of atovaquone with total resolution of his symptoms.
THE MECHANISMS BEHIND METHEMOGLOBINEMIA
Heme iron is normally in the ferrous state (Fe2+), which allows for hemoglobin to carry oxygen and release it to tissues.1 Exposure to an oxidative stress can lead to methemoglobinemia from an increase in abnormal hemoglobin that contains iron in a ferric state (Fe3+).1,2
Methemoglobin reduces oxygen-carrying capacity in two ways: it is unable to carry oxygen, and its presence shifts the oxygen dissociation curve to the left, causing any remaining normal hemoglobin to be unable to release oxygen to the tissues.1,2
Causes of acquired methemoglobinemia include topical anesthetics (eg, benzocaine, lidocaine) and antibiotics (eg, dapsone).2,3 Signs and symptoms include cyanosis, headache, fatigue, dyspnea, lethargy, respiratory distress, and dark-colored urine.1,2
MANAGEMENT
Treatment consists of intravenous methylene blue, which reduces the hemoglobin from a ferric state to a ferrous state.1–4 Methylene blue is a water-soluble dye excreted primarily in the urine, and common side effects include dizziness, nausea, and green urine.5–7 The blue pigments from methylene blue combine with urobilin (a yellow pigment in the urine), producing a green color.7 This is not pathological and requires no treatment, as the urine returns to normal color after the body fully excretes the dye.5–7
If intravenous methylene blue fails to produce a response, other treatments to consider include hemodialysis, blood transfusion, exchange transfusion, and hyperbaric oxygen therapy.2
- Umbreit J. Methemoglobin—it’s not just blue: a concise review. Am J Hematol 2007; 82:134–144.
- Ash-Bernal R, Wise R, Wright SM. Acquired methemoglobinemia: a retrospective series of 138 cases at 2 teaching hospitals. Medicine (Baltimore) 2004; 83:265–273.
- Coleman MD, Coleman NA. Drug-induced methaemoglobinaemia. Treatment issues. Drug Saf 1996; 14:394–405.
- Sikka P, Bindra VK, Kapoor S, Jain V, Saxena KK. Blue cures blue but be cautious. J Pharm Bioallied Sci 2011; 3:543–545.
- Stratta P, Barbe MC. Images in clinical medicine. Green urine. N Engl J Med 2008; 358:e12.
- Miri-Aliabad G. Green urine secondary to methylene blue. Indian J Pediatr 2014; 81:1255–1256.
- Prakash S, Saini S, Mullick P, Pawar M. Green urine: a cause for concern? J Anaesthesiol Clin Pharmacol 2017; 33:128–130.
A 45-year-old man with known human immunodeficiency virus infection presented with a 5-day history of dyspnea. When his dyspnea had become symptomatic, he had restarted his home dapsone prophylaxis, but his dyspnea had progressively worsened, and his urine became dark.
Based on these test results, the patient’s dapsone was stopped and replaced with atovaquone. Intravenous infusion of methylene blue was started, with subsequent improvement of the hypoxia and cyanosis (Figure 1). His urine became green, but it returned to a normal color in a matter of hours. He was ultimately diagnosed with P jirovecii pneumonia and completed a course of atovaquone with total resolution of his symptoms.
THE MECHANISMS BEHIND METHEMOGLOBINEMIA
Heme iron is normally in the ferrous state (Fe2+), which allows for hemoglobin to carry oxygen and release it to tissues.1 Exposure to an oxidative stress can lead to methemoglobinemia from an increase in abnormal hemoglobin that contains iron in a ferric state (Fe3+).1,2
Methemoglobin reduces oxygen-carrying capacity in two ways: it is unable to carry oxygen, and its presence shifts the oxygen dissociation curve to the left, causing any remaining normal hemoglobin to be unable to release oxygen to the tissues.1,2
Causes of acquired methemoglobinemia include topical anesthetics (eg, benzocaine, lidocaine) and antibiotics (eg, dapsone).2,3 Signs and symptoms include cyanosis, headache, fatigue, dyspnea, lethargy, respiratory distress, and dark-colored urine.1,2
MANAGEMENT
Treatment consists of intravenous methylene blue, which reduces the hemoglobin from a ferric state to a ferrous state.1–4 Methylene blue is a water-soluble dye excreted primarily in the urine, and common side effects include dizziness, nausea, and green urine.5–7 The blue pigments from methylene blue combine with urobilin (a yellow pigment in the urine), producing a green color.7 This is not pathological and requires no treatment, as the urine returns to normal color after the body fully excretes the dye.5–7
If intravenous methylene blue fails to produce a response, other treatments to consider include hemodialysis, blood transfusion, exchange transfusion, and hyperbaric oxygen therapy.2
A 45-year-old man with known human immunodeficiency virus infection presented with a 5-day history of dyspnea. When his dyspnea had become symptomatic, he had restarted his home dapsone prophylaxis, but his dyspnea had progressively worsened, and his urine became dark.
Based on these test results, the patient’s dapsone was stopped and replaced with atovaquone. Intravenous infusion of methylene blue was started, with subsequent improvement of the hypoxia and cyanosis (Figure 1). His urine became green, but it returned to a normal color in a matter of hours. He was ultimately diagnosed with P jirovecii pneumonia and completed a course of atovaquone with total resolution of his symptoms.
THE MECHANISMS BEHIND METHEMOGLOBINEMIA
Heme iron is normally in the ferrous state (Fe2+), which allows for hemoglobin to carry oxygen and release it to tissues.1 Exposure to an oxidative stress can lead to methemoglobinemia from an increase in abnormal hemoglobin that contains iron in a ferric state (Fe3+).1,2
Methemoglobin reduces oxygen-carrying capacity in two ways: it is unable to carry oxygen, and its presence shifts the oxygen dissociation curve to the left, causing any remaining normal hemoglobin to be unable to release oxygen to the tissues.1,2
Causes of acquired methemoglobinemia include topical anesthetics (eg, benzocaine, lidocaine) and antibiotics (eg, dapsone).2,3 Signs and symptoms include cyanosis, headache, fatigue, dyspnea, lethargy, respiratory distress, and dark-colored urine.1,2
MANAGEMENT
Treatment consists of intravenous methylene blue, which reduces the hemoglobin from a ferric state to a ferrous state.1–4 Methylene blue is a water-soluble dye excreted primarily in the urine, and common side effects include dizziness, nausea, and green urine.5–7 The blue pigments from methylene blue combine with urobilin (a yellow pigment in the urine), producing a green color.7 This is not pathological and requires no treatment, as the urine returns to normal color after the body fully excretes the dye.5–7
If intravenous methylene blue fails to produce a response, other treatments to consider include hemodialysis, blood transfusion, exchange transfusion, and hyperbaric oxygen therapy.2
- Umbreit J. Methemoglobin—it’s not just blue: a concise review. Am J Hematol 2007; 82:134–144.
- Ash-Bernal R, Wise R, Wright SM. Acquired methemoglobinemia: a retrospective series of 138 cases at 2 teaching hospitals. Medicine (Baltimore) 2004; 83:265–273.
- Coleman MD, Coleman NA. Drug-induced methaemoglobinaemia. Treatment issues. Drug Saf 1996; 14:394–405.
- Sikka P, Bindra VK, Kapoor S, Jain V, Saxena KK. Blue cures blue but be cautious. J Pharm Bioallied Sci 2011; 3:543–545.
- Stratta P, Barbe MC. Images in clinical medicine. Green urine. N Engl J Med 2008; 358:e12.
- Miri-Aliabad G. Green urine secondary to methylene blue. Indian J Pediatr 2014; 81:1255–1256.
- Prakash S, Saini S, Mullick P, Pawar M. Green urine: a cause for concern? J Anaesthesiol Clin Pharmacol 2017; 33:128–130.
- Umbreit J. Methemoglobin—it’s not just blue: a concise review. Am J Hematol 2007; 82:134–144.
- Ash-Bernal R, Wise R, Wright SM. Acquired methemoglobinemia: a retrospective series of 138 cases at 2 teaching hospitals. Medicine (Baltimore) 2004; 83:265–273.
- Coleman MD, Coleman NA. Drug-induced methaemoglobinaemia. Treatment issues. Drug Saf 1996; 14:394–405.
- Sikka P, Bindra VK, Kapoor S, Jain V, Saxena KK. Blue cures blue but be cautious. J Pharm Bioallied Sci 2011; 3:543–545.
- Stratta P, Barbe MC. Images in clinical medicine. Green urine. N Engl J Med 2008; 358:e12.
- Miri-Aliabad G. Green urine secondary to methylene blue. Indian J Pediatr 2014; 81:1255–1256.
- Prakash S, Saini S, Mullick P, Pawar M. Green urine: a cause for concern? J Anaesthesiol Clin Pharmacol 2017; 33:128–130.
Deprescribing: When trying for less is more
Sometimes the answer is straightforward—the pills were started to reduce knee pain, but the pain is still there. But sometimes if I suggest stopping a drug, I get surprising pushback from the patient: “But the pain may be worse without those pills.” And it can be hard to assess whether a medication has attained its therapeutic goal, such as when a drug is given to prevent or reduce the occurrence of intermittent events (eg, hydroxychloroquine to reduce flares in lupus, aspirin to prevent transient ischemic attacks). Unless a medication has been given sufficient time to have its effect and has clearly failed, we often have to trust its efficacy because those events might be more frequent if the drug were stopped.
In this issue of the Journal, Kim and Factora discuss a difficult scenario—discontinuing cognitive-enhancing therapy in patients with Alzheimer dementia. The stakes, hopes, and anxiety are high for the patient and caregivers. These drugs have only modest efficacy, and it is often difficult to know if they are working. To complicate matters, dementia is progressive, but the rate of progression differs among individuals, making it harder to be convinced that the drugs have lost their efficacy and that discontinuation is warranted in order to reduce the side effects, cost, and pill burden. Similar challenges arise for similar reasons when considering discontinuation of antipsychotics or other drugs given for behavioral reasons to elderly patients with dementia.1
The issues surrounding downsizing medication lists—or deprescribing—extend far beyond patients with Alzheimer dementia. A disease may have progressed beyond the point where the drug can make a significant impact. Patients often develop tachyphylaxis to the newer protein drugs (biologics for rheumatoid arthritis, inflammatory bowel disease, psoriasis, or gout) due to the generation of neutralizing antidrug antibodies. At some point the patient’s life expectancy becomes a factor when considering medications directed at preventing long-term complications of a disease and the increased likelihood of significant adverse effects in patients as they age (eg, from aggressively prescribed antihypertensive and antidiabetic drugs). For drugs such as bisphosphonates, alkylating agents, and metoclopramide, complications of cumulative dosing over time should be considered a reason to discontinue therapy.
The take-home message from this article is to create opportunities to periodically revisit the rationale for all of a patient’s prescriptions, and to make sure patients are comfortable knowing why they should keep taking each of their medications. While revisiting a patient’s prescriptions may indeed reduce the medication burden, I believe it also enhances adherence to the remaining prescribed medications. The medication reconciliation process requires more than simply checking off the box in the EMR to indicate that the medications were “reviewed.”
- Bjerre LM, Farrell B, Hogel M, et al. Deprescribing antipsychotics for behavioural and psychological symptoms of dementia and insomnia: evidence-based clinical practice guideline. Can Fam Physician 2018; 64(1):17–27.
Sometimes the answer is straightforward—the pills were started to reduce knee pain, but the pain is still there. But sometimes if I suggest stopping a drug, I get surprising pushback from the patient: “But the pain may be worse without those pills.” And it can be hard to assess whether a medication has attained its therapeutic goal, such as when a drug is given to prevent or reduce the occurrence of intermittent events (eg, hydroxychloroquine to reduce flares in lupus, aspirin to prevent transient ischemic attacks). Unless a medication has been given sufficient time to have its effect and has clearly failed, we often have to trust its efficacy because those events might be more frequent if the drug were stopped.
In this issue of the Journal, Kim and Factora discuss a difficult scenario—discontinuing cognitive-enhancing therapy in patients with Alzheimer dementia. The stakes, hopes, and anxiety are high for the patient and caregivers. These drugs have only modest efficacy, and it is often difficult to know if they are working. To complicate matters, dementia is progressive, but the rate of progression differs among individuals, making it harder to be convinced that the drugs have lost their efficacy and that discontinuation is warranted in order to reduce the side effects, cost, and pill burden. Similar challenges arise for similar reasons when considering discontinuation of antipsychotics or other drugs given for behavioral reasons to elderly patients with dementia.1
The issues surrounding downsizing medication lists—or deprescribing—extend far beyond patients with Alzheimer dementia. A disease may have progressed beyond the point where the drug can make a significant impact. Patients often develop tachyphylaxis to the newer protein drugs (biologics for rheumatoid arthritis, inflammatory bowel disease, psoriasis, or gout) due to the generation of neutralizing antidrug antibodies. At some point the patient’s life expectancy becomes a factor when considering medications directed at preventing long-term complications of a disease and the increased likelihood of significant adverse effects in patients as they age (eg, from aggressively prescribed antihypertensive and antidiabetic drugs). For drugs such as bisphosphonates, alkylating agents, and metoclopramide, complications of cumulative dosing over time should be considered a reason to discontinue therapy.
The take-home message from this article is to create opportunities to periodically revisit the rationale for all of a patient’s prescriptions, and to make sure patients are comfortable knowing why they should keep taking each of their medications. While revisiting a patient’s prescriptions may indeed reduce the medication burden, I believe it also enhances adherence to the remaining prescribed medications. The medication reconciliation process requires more than simply checking off the box in the EMR to indicate that the medications were “reviewed.”
Sometimes the answer is straightforward—the pills were started to reduce knee pain, but the pain is still there. But sometimes if I suggest stopping a drug, I get surprising pushback from the patient: “But the pain may be worse without those pills.” And it can be hard to assess whether a medication has attained its therapeutic goal, such as when a drug is given to prevent or reduce the occurrence of intermittent events (eg, hydroxychloroquine to reduce flares in lupus, aspirin to prevent transient ischemic attacks). Unless a medication has been given sufficient time to have its effect and has clearly failed, we often have to trust its efficacy because those events might be more frequent if the drug were stopped.
In this issue of the Journal, Kim and Factora discuss a difficult scenario—discontinuing cognitive-enhancing therapy in patients with Alzheimer dementia. The stakes, hopes, and anxiety are high for the patient and caregivers. These drugs have only modest efficacy, and it is often difficult to know if they are working. To complicate matters, dementia is progressive, but the rate of progression differs among individuals, making it harder to be convinced that the drugs have lost their efficacy and that discontinuation is warranted in order to reduce the side effects, cost, and pill burden. Similar challenges arise for similar reasons when considering discontinuation of antipsychotics or other drugs given for behavioral reasons to elderly patients with dementia.1
The issues surrounding downsizing medication lists—or deprescribing—extend far beyond patients with Alzheimer dementia. A disease may have progressed beyond the point where the drug can make a significant impact. Patients often develop tachyphylaxis to the newer protein drugs (biologics for rheumatoid arthritis, inflammatory bowel disease, psoriasis, or gout) due to the generation of neutralizing antidrug antibodies. At some point the patient’s life expectancy becomes a factor when considering medications directed at preventing long-term complications of a disease and the increased likelihood of significant adverse effects in patients as they age (eg, from aggressively prescribed antihypertensive and antidiabetic drugs). For drugs such as bisphosphonates, alkylating agents, and metoclopramide, complications of cumulative dosing over time should be considered a reason to discontinue therapy.
The take-home message from this article is to create opportunities to periodically revisit the rationale for all of a patient’s prescriptions, and to make sure patients are comfortable knowing why they should keep taking each of their medications. While revisiting a patient’s prescriptions may indeed reduce the medication burden, I believe it also enhances adherence to the remaining prescribed medications. The medication reconciliation process requires more than simply checking off the box in the EMR to indicate that the medications were “reviewed.”
- Bjerre LM, Farrell B, Hogel M, et al. Deprescribing antipsychotics for behavioural and psychological symptoms of dementia and insomnia: evidence-based clinical practice guideline. Can Fam Physician 2018; 64(1):17–27.
- Bjerre LM, Farrell B, Hogel M, et al. Deprescribing antipsychotics for behavioural and psychological symptoms of dementia and insomnia: evidence-based clinical practice guideline. Can Fam Physician 2018; 64(1):17–27.
Medication management in older adults
Medications started for appropriate indications in middle age may need to be monitored more closely as the patient ages. Some drugs may become unnecessary or even dangerous as the patient ages, functional status and renal function decline, and goals of care change.
Older adults tend to have multiple illnesses and therefore take more drugs, and polypharmacy increases the risk of poor outcomes. The number of medications a person uses is a risk factor for adverse drug reactions, nonadherence, financial burden, drug-drug interactions, and worse outcomes.1
The prevalence of polypharmacy increased from an estimated 8.2% to 15% from 1999 to 2011 based on the National Health and Nutrition Examination Survey.2 Guideline-based therapy for specific diseases may lead to the addition of more medications to reach disease targets.3 Most older adults in the United States compound the risk of prescribed medications by also taking over-the-counter medications and dietary supplements.4
In addition, medications are often used in older adults based on studies of younger persons without significant comorbidities. Applying clinical guidelines based on these studies to older adults with comorbidity and functional impairment is challenging.5 Age-related pharmacokinetic and pharmacodynamic changes increase the risk of adverse drug reactions.6
In this article, we review commonly used medications that are potentially inappropriate based on clinical practice. We also review tools to evaluate appropriate drug therapy in older adults.
DRUGS THAT ARE COMMONLY USED, BUT POTENTIALLY INAPPROPRIATE
Statins
Statins are effective when used as secondary prevention in older adults,7 but their efficacy when used as primary prevention of atherosclerotic cardiovascular disease in people age 75 and older is questionable.8 Nevertheless, they are widely used for this purpose. For example, before the 2013 joint guidelines of the American College of Cardiology and the American Heart Association (ACC/AHA) were released, 22% of patients age 80 and older in the Geisinger health system were taking a statin for primary prevention.9
The 2013 ACC/AHA guidelines included a limited recommendation for statins for primary prevention of atherosclerotic cardiovascular disease in adults age 75 and older.10 The guideline noted, however, that few data were available to support this recommendation.10
In a systematic review of 18 randomized clinical trials of statins for primary prevention of atherosclerotic cardiovascular disease, the mean age was 57, yet conclusions were extrapolated to an older patient population.11 The estimated 10-year risk of atherosclerotic cardiovascular disease based on pooled cohort risk equations of adults age 75 and older always exceeds the 7.5% treatment threshold recommended by the guidelines.8
Myopathy is a common adverse effect of statins. In addition, statins interact with other drugs that inhibit the cytochrome P450 3A4 isoenzyme, such as amlodipine, amiodarone, and diltiazem.8,12 If statin therapy caused no functional limitation due to muscle pain or weakness, statins for primary prevention would be cost-effective, but even a small increase in adverse effects in an elderly patient can offset the cardiovascular benefit.13 A recent post hoc secondary analysis found no benefit of pravastatin for primary prevention in adults age 75 and older.14
Thus, statin treatment for primary prevention in older patients should be individualized, based on life expectancy, function, and cardiovascular risk. Statin therapy does not replace modification of other risk factors.
Anticholinergics
Drugs with anticholinergic properties are commonly prescribed in the elderly for conditions such as muscle spasm, overactive bladder, psychiatric disorders, insomnia, extrapyramidal symptoms, vertigo, pruritus, peptic ulcer disease, seasonal allergies, and even the common cold,15 and many of the drugs often prescribed have strong anticholinergic properties (Table 1). Taking multiple medications with anticholinergic properties results in a high “anticholinergic burden,” which is associated with falls, impulsive behavior, poor physical performance, loss of independence, dementia, delirium, and brain atrophy.15–18
The 2014 American College of Physicians guideline on nonsurgical management of urinary incontinence in women recommends pharmacologic treatment for urgency and stress urinary incontinence after failure of nonpharmacologic therapy,19 and many drugs for these urinary symptoms have anticholinergic properties. If an anticholinergic is necessary, an agent that results in a lower anticholinergic burden should be considered in older patients.
A pharmacist-initiated medication review and intervention may be another way to adjust medications to reduce the patient’s anticholinergic burden.20,21 The common use of anticholinergic drugs in older adults reminds us to monitor their use closely.22
Benzodiazepines and nonbenzodiazepines
Benzodiazepines are among the most commonly prescribed psychotropics in developed countries and are prescribed mainly by primary care physicians rather than psychiatrists.23
In 2008, 5.2% of US adults ages 18 to 80 used a benzodiazepine, and long-term use was more prevalent in older patients (ages 65–80).23
Benzodiazepines are prescribed for anxiety,24 insomnia,25 and agitation. They can cause withdrawal26 and have potential for abuse.27 Benzodiazepines are associated with cognitive decline,28 impaired driving,29 falls,30 and hip fractures31 in older adults.
In addition, use of nonbenzodiazepine hypnotics (eg, zolpidem) is on the rise,32 and these drugs are known to increase the risk of hip fracture in nursing home residents.33
The American Geriatrics Society, through the American Board of Internal Medicine’s Choosing Wisely campaign, recommends avoiding benzodiazepines as a first-line treatment for insomnia, agitation, or delirium in older adults.34 Yet prescribing practices with these drugs in primary care settings conflict with guidelines, partly due to lack of training in constructive strategies regarding appropriate use of benzodiazepines.35 Educating patients on the risks and benefits of benzodiazepine treatment, especially long-term use, has been shown to reduce the rate of benzodiazepine-associated secondary events.36
Antipsychotics
Off-label use of antipsychotics is common and is increasing in the United States. In 2008, off-label use of antipsychotic drugs accounted for an estimated $6 billion.37 A common off-label use is to manage behavioral symptoms of dementia, despite a black-box warning about an increased risk of death in patients with dementia who are treated with antipsychotics.38,39 The Choosing Wisely campaign recommends against prescribing antipsychotics as a first-line treatment of behavioral and psychological symptoms of dementia.34
Antipsychotic drugs are associated with risk of acute kidney injury,40 as well as increased risk of falls and fractures (eg, a 52% higher risk of a serious fall, and a 50% higher risk of a nonvertebral osteoporotic fracture).41
Patients with dementia often exhibit aggression, resistance to care, and other challenging or disruptive behaviors. In such instances, antipsychotic drugs are often prescribed, but they provide limited and inconsistent benefits, while causing oversedation and worsening of cognitive function and increasing the likelihood of falling, stroke, and death.38,39,41
Because pharmacologic treatments for dementia are only modestly effective, have notable risks, and do not treat some of the behaviors that family members and caregivers find most distressing, nonpharmacologic measures are recommended as first-line treatment.42 These include caregiver education and support, training in problem-solving, and targeted therapy directed at the underlying causes of specific behaviors (eg, implementing nighttime routines to address sleep disturbances).42 Nonpharmacologic management of behavioral symptoms in dementia can significantly improve quality of life for patients and caregivers.42 Use of antipsychotic drugs in patients with dementia should be limited to cases in which nonpharmacologic measures have failed and patients pose an imminent threat to themselves or others.43
Proton pump inhibitors
Proton pump inhibitors are among the most commonly prescribed medications in the United States, and their use has increased significantly over the decade. It has been estimated that between 25% and 70% of these prescriptions have no appropriate indication.44
There is considerable excess use of acid suppressants in both inpatient and outpatient settings.45,46 In one study, at discharge from an internal medicine service, almost half of patients were taking a proton pump inhibitor.47
Evidence-based guidelines recommend these drugs to treat gastroesophageal reflux disease, nonerosive reflux disease, erosive esophagitis, dyspepsia, and peptic ulcer disease. However, long-term use (ie, beyond 8 weeks) is recommended only for patients with erosive esophagitis, Barrett esophagus, a pathologic hypersecretory condition, or a demonstrated need for maintenance treatment for reflux disease.48
Although proton pump inhibitors are highly effective and have low toxicity, there are reports of an association with Clostridium difficile infection,49 community-acquired pneumonia,50 hip fracture,51 vitamin B12 deficiency,52 atrophic gastritis,53 kidney disease,54 and dementia.55
Nondrug therapies such as weight loss and elevation of the head of the bed may improve esophageal pH levels and reflux symptoms.56
Deprescribing.org has practical advice for healthcare providers, patients, and caregivers on how to discontinue proton pump inhibitors, including videos, algorithms, and guidelines.
TOOLS TO EVALUATE APPROPRIATE DRUG THERAPY
Beers criteria
The Beers criteria (Table 2), developed in 1991 by a geriatrician as an approach to safer, more effective drug therapy in frail elderly nursing home patients,57 were updated by the American Geriatrics Society in 2015 for use in any clinical setting.58 (The criteria are also available as a smartphone application through the American Geriatrics Society at www.americangeriatrics.org.)
The Beers criteria offer evidence-based recommendations on drugs to avoid in the elderly, along with the rationale for use, the quality of evidence behind the recommendation, and the graded strength of the recommendation. The Beers criteria should be viewed through the lens of clinical judgment to offer safer nonpharmacologic and pharmacologic treatments.
The Joint Commission recommends medication reconciliation at every transition of care.59 The Beers criteria are a good starting point for a comprehensive medication review.
STOPP/START criteria
Another tool to aid safe prescribing in older adults is the Screening Tool of Older Persons’ Potentially Inappropriate Prescriptions (STOPP), used in conjuction with the Screening Tool to Alert Doctors to Right Treatment (START). The STOPP/START criteria60,61 are based on an up-to-date literature review and consensus (Table 3).
THE BOTTOM LINE
Physicians caring for older adults need to diligently weigh the benefits of drug therapy and consider the patient’s care goals, current level of functioning, life expectancy, values, and preferences. Statin therapy for primary prevention, anticholinergics, benzodiazepines, antipsychotics, and proton pump inhibitors are widely used without proper indications, pointing to the need for a periodic comprehensive review of medications to reevaluate the risks vs the benefits of the patient’s medications. The Beers criteria and the STOPP/ START criteria can be useful tools for this purpose.
- Steinman MA. Polypharmacy—time to get beyond numbers. JAMA Intern Med 2016; 176:482–483.
- Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999–2012. JAMA 2015; 314:1818–1831.
- Tinetti ME, Bogardus ST Jr, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med 2004; 351:2870–2874.
- Qato DM, Wilder J, Schumm LP, Gillet V, Alexander GC. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med 2016; 176:473–482.
- Boyd CM, Darer J, Boult C, et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA 2005; 294:716–724.
- Atkin PA, Veitch PC, Veitch EM, Ogle SJ. The epidemiology of serious adverse drug reactions among the elderly. Drugs Aging 1999; 14:141–152.
- Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016; 338:2532–2561.
- Gurwitz JH, Go AS, Fortman SP. Statins for primary prevention in older adults: uncertainty and the need for more evidence. JAMA 2016; 316:1971–1972.
- Chokshi NP, Messerli FH, Sutin D, Supariwala AA, Shah NR. Appropriateness of statins in patients aged ≥ 80 years and comparison to other age groups. Am J Cardiol 2012; 110:1477–1481.
- Stone NJ, Robinson J, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(suppl 2):S1–S45.
- Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2013; 1:CD004816.
- Chatzizisis YS, Koskinas KC, Misirli G, Vaklavas C, Hatzitolios A, Giannoglou GD. Risk factors and drug interactions predisposing to statin-induced myopathy: implications for risk assessment, prevention and treatment. Drug Saf 2010; 33:171–187.
- Odden MC, Pletcher MJ, Coxson PG, et al. Cost-effectiveness and population impact of statins for primary prevention in adults aged 75 years or older in the United States. Ann Intern Med 2015; 162:533–541.
- Han BH, Sutin D, Williamson JD, et al. Effect of statin treatment vs usual care on primary cardiovascular prevention among older adults. The ALLHAT-LLT randomized clinical trial. JAMA Intern Med 2017; 177:955–965.
- Gray SL, Anderson ML, Dublin S, et al. Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern Med 2015; 175:401–407.
- Rudolph JL, Salow MJ, Angelini MC, McGlinchey RE. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med 2008; 168:508–513.
- Hilmer SN, Mager DE, Simonsick EM, et al. A drug burden index to define the functional burden of medications in older people. Arch Intern Med 2007; 167:781–787.
- Risacher SL, McDonald BC, Tallman EF, et al; Alzheimer’s Disease Neuroimaging Initiative. Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurol 2016; 73:721–732.
- Qaseem A, Dallas P, Forciea MA, Starkey M, Denberg TD, Shekelle P; Clinical Guidelines Committee of the American College of Physicians. Nonsurgical management of urinary incontinence in women: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2014; 161:429–440.
- Efjestad AS, Molden E, Oksengard AR. Pharmacist-initiated management of antagonistic interactions between anticholinergic drugs and acetyl cholinesterase inhibitors in individuals with dementia. J Am Geriatr Soc 2013; 61:1624–1625.
- Kersten H, Molden E, Tolo IK, Skovlund E, Engedal K, Wyller TB. Cognitive effects of reducing anticholinergic drug burden in a frail elderly population: a randomized controlled trial. J Gerontol A Biol Sci Med Sci 2013; 68:271–278.
- Curtis LH, Østbye T, Sendersky V, et al. Inappropriate prescribing for elderly Americans in a large outpatient population. Arch Intern Med 2004; 164:1621–1625.
- Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry 2015; 72:136–142.
- Martin JL, Sainz-Pardo M, Furukawa TA, Martín-Sánchez E, Seoane T, Galán C. Benzodiazepines in generalized anxiety disorder: heterogeneity of outcomes based on a systematic review and meta-analysis of clinical trials. J Psychopharmacol 2007; 21:774–782.
- Buscemi N, Vandermeer B, Friesen C, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen Intern Med 2007; 22:1335–1350.
- Rickels K, Schweizer E, Case WG, Greenblatt DJ. Long-term therapeutic use of benzodiazepines, I. Effects of abrupt discontinuation. Arch Gen Psychiatry 1990; 47:899–907.
- Fenton MC, Keyes KM, Martins SS, Hasin DS. The role of a prescription in anxiety medication use, abuse, and dependence. Am J Psychiatry 2010; 167:1247–1253.
- Billoti de Gage S, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case-control study. BMJ 2014; 349:g5205.
- Smink BE, Egberts AC, Lusthof KJ, Uges DR, de Gier JJ. The relationship between benzodiazepine use and traffic accidents: a systemic literature review. CNS Drugs 2010; 24:639–653.
- Tinett, ME, Speechley M, Ginter S. Risk factors for falls among elderly persons living in the community. N Engl J Med 1988; 319:1701–1707.
- Zint K, Haefeli WE, Glynn RJ, Mogun H, Avorn J, Stürmer T. Impact of drug interactions, dosage, and duration of therapy on the risk of hip fracture associated with benzodiazepine use in older adults. Pharmacoepidemiol Drug Saf 2010; 19:1248–1255.
- Briesacher BA, Soumerai SB, Field TS, Fouayzi H, Gurwitz JH. Medicare Part D’s exclusion of benzodiazepines and fracture risk in nursing homes. Arch Intern Med 2010; 170:693–698.
- Berry SD, Lee Y, Cai S, Dore DD. Nonbenzodiazepine sleep medication use and hip fractures in nursing home residents. JAMA Intern Med 2013; 173:754–761.
- American Geriatrics Society. Choosing Wisely. Ten things clinicians and patients should question. www.choosingwisely.org/societies/american-geriatrics-society/. Accessed December 3, 2017.
- Cook JM, Marshall R, Masci C, Coyne JC. Physicians’ perspectives on prescribing benzodiazepines for older adults: a qualitative study. J Gen Intern Med 2007; 22:303–307.
- Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med 2014; 174:890–898.
- Alexander GC, Gallagher SA, Mascola A, Moloney RM, Stafford RS. Increasing off-label use of antipsychotic medications in the United States, 1995–2008. Phamacoepidemiol Drug Saf 2011; 20:177–184.
- Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med 2007; 146:775–786.
- US Food and Drug Administration (FDA). Public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm053171.htm. Accessed December 4, 2017.
- Hwang YJ, Dixon SN, Reiss JP, et al. Atypical antipsychotic drugs and the risk for acute kidney injury and other adverse outcomes in older adults. Ann Intern Med 2014; 161:242–248.
- Fraser L, Liu K, Naylor KL, et al. Falls and fractures with atypical antipsychotic medication use: a population-based cohort study. JAMA Intern Med 2015; 175:450–452.
- Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA 2012; 308:2020–2029.
- Schneider LS, Tariot PN, Dagerman KS, et al; CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med 2006; 355:1525–1538.
- Forgacs I, Loganayagam A. Overprescribing proton pump inhibitors. BMJ 2008; 336:2–3.
- Mazer-Amirshahi M, Mullins PM, van den Anker J, Meltzer A, Pines JM. Rising rates of proton pump inhibitor prescribing in US emergency departments. Am J Emerg Med 2014; 32:618–622.
- Heidelbaugh JJ, Goldberg KL, Inadomi JM. Magnitude and economic effect of overuse of antisecretory therapy in the ambulatory care setting. Am J Manag Care 2010; 16:e228–e324.
- Pham CQ, Regal RE, Bostwich TR, Knauf KS. Acid suppressive therapy used on an inpatient internal medicine service. Ann Pharmacother 2006; 40:1261–1266.
- Kahrilas PJ, Shaheen NJ, Vaezi MF, et al; American Gastroenterological Association. American Gastroenterological Association medical position statement on the management of gastroesophageal reflux disease. Gastroenterology 2008; 135:1383–1391.e1–e5.
- Howell MD, Novack V, Grgurich P, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med 2010; 170:784–790.
- Gulmez SE, Holm A, Frederiksen H, Jensen TG, Pedersen C, Hallas J. Use of proton pump inhibitors and the risk of community-acquired pneumonia: a population-based case-control study. Arch Intern Med 2007; 167:950–955.
- Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 2006; 296:2947–2953.
- Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA 2013; 310:2435–2442.
- Kuipers EJ, Lundell L, Klinkenberg-Knol EC, et al. Atrophic gastritis and Helicobacter pylori infection in patients with reflux esophagitis treated with omeprazole or fundoplication. N Engl J Med 1996; 334:1018–1022.
- Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med 2016; 176:238–246.
- Gomm W, von Holt K, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol 2016; 73:410–416.
- Kaltenbach T, Crockett S, Gerson LB. Are lifestyle measures effective in patients with gastroesophageal reflux disease? An evidence-based approach. Arch Intern Med 2006; 166:965–971.
- Beers MH, Ouslander JG, Rollingher I, Reuben DB, Brooks J, Beck JC. Explicit criteria for determining inappropriate medication use in nursing home residents. Arch Intern Med 1991; 151:1825–1832.
- American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015; 63:2227–2246.
- Joint Commission. Sentinel event alert, Issue 35: using medication reconciliation to prevent errors. www.jointcommission.org/sentinel_event_alert_issue_35_using_medication_reconciliation_to_prevent_errors/. Accessed August 18, 2017.
- Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther 2008; 46:72–83.
- O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 2015; 44:213–218.
Medications started for appropriate indications in middle age may need to be monitored more closely as the patient ages. Some drugs may become unnecessary or even dangerous as the patient ages, functional status and renal function decline, and goals of care change.
Older adults tend to have multiple illnesses and therefore take more drugs, and polypharmacy increases the risk of poor outcomes. The number of medications a person uses is a risk factor for adverse drug reactions, nonadherence, financial burden, drug-drug interactions, and worse outcomes.1
The prevalence of polypharmacy increased from an estimated 8.2% to 15% from 1999 to 2011 based on the National Health and Nutrition Examination Survey.2 Guideline-based therapy for specific diseases may lead to the addition of more medications to reach disease targets.3 Most older adults in the United States compound the risk of prescribed medications by also taking over-the-counter medications and dietary supplements.4
In addition, medications are often used in older adults based on studies of younger persons without significant comorbidities. Applying clinical guidelines based on these studies to older adults with comorbidity and functional impairment is challenging.5 Age-related pharmacokinetic and pharmacodynamic changes increase the risk of adverse drug reactions.6
In this article, we review commonly used medications that are potentially inappropriate based on clinical practice. We also review tools to evaluate appropriate drug therapy in older adults.
DRUGS THAT ARE COMMONLY USED, BUT POTENTIALLY INAPPROPRIATE
Statins
Statins are effective when used as secondary prevention in older adults,7 but their efficacy when used as primary prevention of atherosclerotic cardiovascular disease in people age 75 and older is questionable.8 Nevertheless, they are widely used for this purpose. For example, before the 2013 joint guidelines of the American College of Cardiology and the American Heart Association (ACC/AHA) were released, 22% of patients age 80 and older in the Geisinger health system were taking a statin for primary prevention.9
The 2013 ACC/AHA guidelines included a limited recommendation for statins for primary prevention of atherosclerotic cardiovascular disease in adults age 75 and older.10 The guideline noted, however, that few data were available to support this recommendation.10
In a systematic review of 18 randomized clinical trials of statins for primary prevention of atherosclerotic cardiovascular disease, the mean age was 57, yet conclusions were extrapolated to an older patient population.11 The estimated 10-year risk of atherosclerotic cardiovascular disease based on pooled cohort risk equations of adults age 75 and older always exceeds the 7.5% treatment threshold recommended by the guidelines.8
Myopathy is a common adverse effect of statins. In addition, statins interact with other drugs that inhibit the cytochrome P450 3A4 isoenzyme, such as amlodipine, amiodarone, and diltiazem.8,12 If statin therapy caused no functional limitation due to muscle pain or weakness, statins for primary prevention would be cost-effective, but even a small increase in adverse effects in an elderly patient can offset the cardiovascular benefit.13 A recent post hoc secondary analysis found no benefit of pravastatin for primary prevention in adults age 75 and older.14
Thus, statin treatment for primary prevention in older patients should be individualized, based on life expectancy, function, and cardiovascular risk. Statin therapy does not replace modification of other risk factors.
Anticholinergics
Drugs with anticholinergic properties are commonly prescribed in the elderly for conditions such as muscle spasm, overactive bladder, psychiatric disorders, insomnia, extrapyramidal symptoms, vertigo, pruritus, peptic ulcer disease, seasonal allergies, and even the common cold,15 and many of the drugs often prescribed have strong anticholinergic properties (Table 1). Taking multiple medications with anticholinergic properties results in a high “anticholinergic burden,” which is associated with falls, impulsive behavior, poor physical performance, loss of independence, dementia, delirium, and brain atrophy.15–18
The 2014 American College of Physicians guideline on nonsurgical management of urinary incontinence in women recommends pharmacologic treatment for urgency and stress urinary incontinence after failure of nonpharmacologic therapy,19 and many drugs for these urinary symptoms have anticholinergic properties. If an anticholinergic is necessary, an agent that results in a lower anticholinergic burden should be considered in older patients.
A pharmacist-initiated medication review and intervention may be another way to adjust medications to reduce the patient’s anticholinergic burden.20,21 The common use of anticholinergic drugs in older adults reminds us to monitor their use closely.22
Benzodiazepines and nonbenzodiazepines
Benzodiazepines are among the most commonly prescribed psychotropics in developed countries and are prescribed mainly by primary care physicians rather than psychiatrists.23
In 2008, 5.2% of US adults ages 18 to 80 used a benzodiazepine, and long-term use was more prevalent in older patients (ages 65–80).23
Benzodiazepines are prescribed for anxiety,24 insomnia,25 and agitation. They can cause withdrawal26 and have potential for abuse.27 Benzodiazepines are associated with cognitive decline,28 impaired driving,29 falls,30 and hip fractures31 in older adults.
In addition, use of nonbenzodiazepine hypnotics (eg, zolpidem) is on the rise,32 and these drugs are known to increase the risk of hip fracture in nursing home residents.33
The American Geriatrics Society, through the American Board of Internal Medicine’s Choosing Wisely campaign, recommends avoiding benzodiazepines as a first-line treatment for insomnia, agitation, or delirium in older adults.34 Yet prescribing practices with these drugs in primary care settings conflict with guidelines, partly due to lack of training in constructive strategies regarding appropriate use of benzodiazepines.35 Educating patients on the risks and benefits of benzodiazepine treatment, especially long-term use, has been shown to reduce the rate of benzodiazepine-associated secondary events.36
Antipsychotics
Off-label use of antipsychotics is common and is increasing in the United States. In 2008, off-label use of antipsychotic drugs accounted for an estimated $6 billion.37 A common off-label use is to manage behavioral symptoms of dementia, despite a black-box warning about an increased risk of death in patients with dementia who are treated with antipsychotics.38,39 The Choosing Wisely campaign recommends against prescribing antipsychotics as a first-line treatment of behavioral and psychological symptoms of dementia.34
Antipsychotic drugs are associated with risk of acute kidney injury,40 as well as increased risk of falls and fractures (eg, a 52% higher risk of a serious fall, and a 50% higher risk of a nonvertebral osteoporotic fracture).41
Patients with dementia often exhibit aggression, resistance to care, and other challenging or disruptive behaviors. In such instances, antipsychotic drugs are often prescribed, but they provide limited and inconsistent benefits, while causing oversedation and worsening of cognitive function and increasing the likelihood of falling, stroke, and death.38,39,41
Because pharmacologic treatments for dementia are only modestly effective, have notable risks, and do not treat some of the behaviors that family members and caregivers find most distressing, nonpharmacologic measures are recommended as first-line treatment.42 These include caregiver education and support, training in problem-solving, and targeted therapy directed at the underlying causes of specific behaviors (eg, implementing nighttime routines to address sleep disturbances).42 Nonpharmacologic management of behavioral symptoms in dementia can significantly improve quality of life for patients and caregivers.42 Use of antipsychotic drugs in patients with dementia should be limited to cases in which nonpharmacologic measures have failed and patients pose an imminent threat to themselves or others.43
Proton pump inhibitors
Proton pump inhibitors are among the most commonly prescribed medications in the United States, and their use has increased significantly over the decade. It has been estimated that between 25% and 70% of these prescriptions have no appropriate indication.44
There is considerable excess use of acid suppressants in both inpatient and outpatient settings.45,46 In one study, at discharge from an internal medicine service, almost half of patients were taking a proton pump inhibitor.47
Evidence-based guidelines recommend these drugs to treat gastroesophageal reflux disease, nonerosive reflux disease, erosive esophagitis, dyspepsia, and peptic ulcer disease. However, long-term use (ie, beyond 8 weeks) is recommended only for patients with erosive esophagitis, Barrett esophagus, a pathologic hypersecretory condition, or a demonstrated need for maintenance treatment for reflux disease.48
Although proton pump inhibitors are highly effective and have low toxicity, there are reports of an association with Clostridium difficile infection,49 community-acquired pneumonia,50 hip fracture,51 vitamin B12 deficiency,52 atrophic gastritis,53 kidney disease,54 and dementia.55
Nondrug therapies such as weight loss and elevation of the head of the bed may improve esophageal pH levels and reflux symptoms.56
Deprescribing.org has practical advice for healthcare providers, patients, and caregivers on how to discontinue proton pump inhibitors, including videos, algorithms, and guidelines.
TOOLS TO EVALUATE APPROPRIATE DRUG THERAPY
Beers criteria
The Beers criteria (Table 2), developed in 1991 by a geriatrician as an approach to safer, more effective drug therapy in frail elderly nursing home patients,57 were updated by the American Geriatrics Society in 2015 for use in any clinical setting.58 (The criteria are also available as a smartphone application through the American Geriatrics Society at www.americangeriatrics.org.)
The Beers criteria offer evidence-based recommendations on drugs to avoid in the elderly, along with the rationale for use, the quality of evidence behind the recommendation, and the graded strength of the recommendation. The Beers criteria should be viewed through the lens of clinical judgment to offer safer nonpharmacologic and pharmacologic treatments.
The Joint Commission recommends medication reconciliation at every transition of care.59 The Beers criteria are a good starting point for a comprehensive medication review.
STOPP/START criteria
Another tool to aid safe prescribing in older adults is the Screening Tool of Older Persons’ Potentially Inappropriate Prescriptions (STOPP), used in conjuction with the Screening Tool to Alert Doctors to Right Treatment (START). The STOPP/START criteria60,61 are based on an up-to-date literature review and consensus (Table 3).
THE BOTTOM LINE
Physicians caring for older adults need to diligently weigh the benefits of drug therapy and consider the patient’s care goals, current level of functioning, life expectancy, values, and preferences. Statin therapy for primary prevention, anticholinergics, benzodiazepines, antipsychotics, and proton pump inhibitors are widely used without proper indications, pointing to the need for a periodic comprehensive review of medications to reevaluate the risks vs the benefits of the patient’s medications. The Beers criteria and the STOPP/ START criteria can be useful tools for this purpose.
Medications started for appropriate indications in middle age may need to be monitored more closely as the patient ages. Some drugs may become unnecessary or even dangerous as the patient ages, functional status and renal function decline, and goals of care change.
Older adults tend to have multiple illnesses and therefore take more drugs, and polypharmacy increases the risk of poor outcomes. The number of medications a person uses is a risk factor for adverse drug reactions, nonadherence, financial burden, drug-drug interactions, and worse outcomes.1
The prevalence of polypharmacy increased from an estimated 8.2% to 15% from 1999 to 2011 based on the National Health and Nutrition Examination Survey.2 Guideline-based therapy for specific diseases may lead to the addition of more medications to reach disease targets.3 Most older adults in the United States compound the risk of prescribed medications by also taking over-the-counter medications and dietary supplements.4
In addition, medications are often used in older adults based on studies of younger persons without significant comorbidities. Applying clinical guidelines based on these studies to older adults with comorbidity and functional impairment is challenging.5 Age-related pharmacokinetic and pharmacodynamic changes increase the risk of adverse drug reactions.6
In this article, we review commonly used medications that are potentially inappropriate based on clinical practice. We also review tools to evaluate appropriate drug therapy in older adults.
DRUGS THAT ARE COMMONLY USED, BUT POTENTIALLY INAPPROPRIATE
Statins
Statins are effective when used as secondary prevention in older adults,7 but their efficacy when used as primary prevention of atherosclerotic cardiovascular disease in people age 75 and older is questionable.8 Nevertheless, they are widely used for this purpose. For example, before the 2013 joint guidelines of the American College of Cardiology and the American Heart Association (ACC/AHA) were released, 22% of patients age 80 and older in the Geisinger health system were taking a statin for primary prevention.9
The 2013 ACC/AHA guidelines included a limited recommendation for statins for primary prevention of atherosclerotic cardiovascular disease in adults age 75 and older.10 The guideline noted, however, that few data were available to support this recommendation.10
In a systematic review of 18 randomized clinical trials of statins for primary prevention of atherosclerotic cardiovascular disease, the mean age was 57, yet conclusions were extrapolated to an older patient population.11 The estimated 10-year risk of atherosclerotic cardiovascular disease based on pooled cohort risk equations of adults age 75 and older always exceeds the 7.5% treatment threshold recommended by the guidelines.8
Myopathy is a common adverse effect of statins. In addition, statins interact with other drugs that inhibit the cytochrome P450 3A4 isoenzyme, such as amlodipine, amiodarone, and diltiazem.8,12 If statin therapy caused no functional limitation due to muscle pain or weakness, statins for primary prevention would be cost-effective, but even a small increase in adverse effects in an elderly patient can offset the cardiovascular benefit.13 A recent post hoc secondary analysis found no benefit of pravastatin for primary prevention in adults age 75 and older.14
Thus, statin treatment for primary prevention in older patients should be individualized, based on life expectancy, function, and cardiovascular risk. Statin therapy does not replace modification of other risk factors.
Anticholinergics
Drugs with anticholinergic properties are commonly prescribed in the elderly for conditions such as muscle spasm, overactive bladder, psychiatric disorders, insomnia, extrapyramidal symptoms, vertigo, pruritus, peptic ulcer disease, seasonal allergies, and even the common cold,15 and many of the drugs often prescribed have strong anticholinergic properties (Table 1). Taking multiple medications with anticholinergic properties results in a high “anticholinergic burden,” which is associated with falls, impulsive behavior, poor physical performance, loss of independence, dementia, delirium, and brain atrophy.15–18
The 2014 American College of Physicians guideline on nonsurgical management of urinary incontinence in women recommends pharmacologic treatment for urgency and stress urinary incontinence after failure of nonpharmacologic therapy,19 and many drugs for these urinary symptoms have anticholinergic properties. If an anticholinergic is necessary, an agent that results in a lower anticholinergic burden should be considered in older patients.
A pharmacist-initiated medication review and intervention may be another way to adjust medications to reduce the patient’s anticholinergic burden.20,21 The common use of anticholinergic drugs in older adults reminds us to monitor their use closely.22
Benzodiazepines and nonbenzodiazepines
Benzodiazepines are among the most commonly prescribed psychotropics in developed countries and are prescribed mainly by primary care physicians rather than psychiatrists.23
In 2008, 5.2% of US adults ages 18 to 80 used a benzodiazepine, and long-term use was more prevalent in older patients (ages 65–80).23
Benzodiazepines are prescribed for anxiety,24 insomnia,25 and agitation. They can cause withdrawal26 and have potential for abuse.27 Benzodiazepines are associated with cognitive decline,28 impaired driving,29 falls,30 and hip fractures31 in older adults.
In addition, use of nonbenzodiazepine hypnotics (eg, zolpidem) is on the rise,32 and these drugs are known to increase the risk of hip fracture in nursing home residents.33
The American Geriatrics Society, through the American Board of Internal Medicine’s Choosing Wisely campaign, recommends avoiding benzodiazepines as a first-line treatment for insomnia, agitation, or delirium in older adults.34 Yet prescribing practices with these drugs in primary care settings conflict with guidelines, partly due to lack of training in constructive strategies regarding appropriate use of benzodiazepines.35 Educating patients on the risks and benefits of benzodiazepine treatment, especially long-term use, has been shown to reduce the rate of benzodiazepine-associated secondary events.36
Antipsychotics
Off-label use of antipsychotics is common and is increasing in the United States. In 2008, off-label use of antipsychotic drugs accounted for an estimated $6 billion.37 A common off-label use is to manage behavioral symptoms of dementia, despite a black-box warning about an increased risk of death in patients with dementia who are treated with antipsychotics.38,39 The Choosing Wisely campaign recommends against prescribing antipsychotics as a first-line treatment of behavioral and psychological symptoms of dementia.34
Antipsychotic drugs are associated with risk of acute kidney injury,40 as well as increased risk of falls and fractures (eg, a 52% higher risk of a serious fall, and a 50% higher risk of a nonvertebral osteoporotic fracture).41
Patients with dementia often exhibit aggression, resistance to care, and other challenging or disruptive behaviors. In such instances, antipsychotic drugs are often prescribed, but they provide limited and inconsistent benefits, while causing oversedation and worsening of cognitive function and increasing the likelihood of falling, stroke, and death.38,39,41
Because pharmacologic treatments for dementia are only modestly effective, have notable risks, and do not treat some of the behaviors that family members and caregivers find most distressing, nonpharmacologic measures are recommended as first-line treatment.42 These include caregiver education and support, training in problem-solving, and targeted therapy directed at the underlying causes of specific behaviors (eg, implementing nighttime routines to address sleep disturbances).42 Nonpharmacologic management of behavioral symptoms in dementia can significantly improve quality of life for patients and caregivers.42 Use of antipsychotic drugs in patients with dementia should be limited to cases in which nonpharmacologic measures have failed and patients pose an imminent threat to themselves or others.43
Proton pump inhibitors
Proton pump inhibitors are among the most commonly prescribed medications in the United States, and their use has increased significantly over the decade. It has been estimated that between 25% and 70% of these prescriptions have no appropriate indication.44
There is considerable excess use of acid suppressants in both inpatient and outpatient settings.45,46 In one study, at discharge from an internal medicine service, almost half of patients were taking a proton pump inhibitor.47
Evidence-based guidelines recommend these drugs to treat gastroesophageal reflux disease, nonerosive reflux disease, erosive esophagitis, dyspepsia, and peptic ulcer disease. However, long-term use (ie, beyond 8 weeks) is recommended only for patients with erosive esophagitis, Barrett esophagus, a pathologic hypersecretory condition, or a demonstrated need for maintenance treatment for reflux disease.48
Although proton pump inhibitors are highly effective and have low toxicity, there are reports of an association with Clostridium difficile infection,49 community-acquired pneumonia,50 hip fracture,51 vitamin B12 deficiency,52 atrophic gastritis,53 kidney disease,54 and dementia.55
Nondrug therapies such as weight loss and elevation of the head of the bed may improve esophageal pH levels and reflux symptoms.56
Deprescribing.org has practical advice for healthcare providers, patients, and caregivers on how to discontinue proton pump inhibitors, including videos, algorithms, and guidelines.
TOOLS TO EVALUATE APPROPRIATE DRUG THERAPY
Beers criteria
The Beers criteria (Table 2), developed in 1991 by a geriatrician as an approach to safer, more effective drug therapy in frail elderly nursing home patients,57 were updated by the American Geriatrics Society in 2015 for use in any clinical setting.58 (The criteria are also available as a smartphone application through the American Geriatrics Society at www.americangeriatrics.org.)
The Beers criteria offer evidence-based recommendations on drugs to avoid in the elderly, along with the rationale for use, the quality of evidence behind the recommendation, and the graded strength of the recommendation. The Beers criteria should be viewed through the lens of clinical judgment to offer safer nonpharmacologic and pharmacologic treatments.
The Joint Commission recommends medication reconciliation at every transition of care.59 The Beers criteria are a good starting point for a comprehensive medication review.
STOPP/START criteria
Another tool to aid safe prescribing in older adults is the Screening Tool of Older Persons’ Potentially Inappropriate Prescriptions (STOPP), used in conjuction with the Screening Tool to Alert Doctors to Right Treatment (START). The STOPP/START criteria60,61 are based on an up-to-date literature review and consensus (Table 3).
THE BOTTOM LINE
Physicians caring for older adults need to diligently weigh the benefits of drug therapy and consider the patient’s care goals, current level of functioning, life expectancy, values, and preferences. Statin therapy for primary prevention, anticholinergics, benzodiazepines, antipsychotics, and proton pump inhibitors are widely used without proper indications, pointing to the need for a periodic comprehensive review of medications to reevaluate the risks vs the benefits of the patient’s medications. The Beers criteria and the STOPP/ START criteria can be useful tools for this purpose.
- Steinman MA. Polypharmacy—time to get beyond numbers. JAMA Intern Med 2016; 176:482–483.
- Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999–2012. JAMA 2015; 314:1818–1831.
- Tinetti ME, Bogardus ST Jr, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med 2004; 351:2870–2874.
- Qato DM, Wilder J, Schumm LP, Gillet V, Alexander GC. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med 2016; 176:473–482.
- Boyd CM, Darer J, Boult C, et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA 2005; 294:716–724.
- Atkin PA, Veitch PC, Veitch EM, Ogle SJ. The epidemiology of serious adverse drug reactions among the elderly. Drugs Aging 1999; 14:141–152.
- Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016; 338:2532–2561.
- Gurwitz JH, Go AS, Fortman SP. Statins for primary prevention in older adults: uncertainty and the need for more evidence. JAMA 2016; 316:1971–1972.
- Chokshi NP, Messerli FH, Sutin D, Supariwala AA, Shah NR. Appropriateness of statins in patients aged ≥ 80 years and comparison to other age groups. Am J Cardiol 2012; 110:1477–1481.
- Stone NJ, Robinson J, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(suppl 2):S1–S45.
- Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2013; 1:CD004816.
- Chatzizisis YS, Koskinas KC, Misirli G, Vaklavas C, Hatzitolios A, Giannoglou GD. Risk factors and drug interactions predisposing to statin-induced myopathy: implications for risk assessment, prevention and treatment. Drug Saf 2010; 33:171–187.
- Odden MC, Pletcher MJ, Coxson PG, et al. Cost-effectiveness and population impact of statins for primary prevention in adults aged 75 years or older in the United States. Ann Intern Med 2015; 162:533–541.
- Han BH, Sutin D, Williamson JD, et al. Effect of statin treatment vs usual care on primary cardiovascular prevention among older adults. The ALLHAT-LLT randomized clinical trial. JAMA Intern Med 2017; 177:955–965.
- Gray SL, Anderson ML, Dublin S, et al. Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern Med 2015; 175:401–407.
- Rudolph JL, Salow MJ, Angelini MC, McGlinchey RE. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med 2008; 168:508–513.
- Hilmer SN, Mager DE, Simonsick EM, et al. A drug burden index to define the functional burden of medications in older people. Arch Intern Med 2007; 167:781–787.
- Risacher SL, McDonald BC, Tallman EF, et al; Alzheimer’s Disease Neuroimaging Initiative. Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurol 2016; 73:721–732.
- Qaseem A, Dallas P, Forciea MA, Starkey M, Denberg TD, Shekelle P; Clinical Guidelines Committee of the American College of Physicians. Nonsurgical management of urinary incontinence in women: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2014; 161:429–440.
- Efjestad AS, Molden E, Oksengard AR. Pharmacist-initiated management of antagonistic interactions between anticholinergic drugs and acetyl cholinesterase inhibitors in individuals with dementia. J Am Geriatr Soc 2013; 61:1624–1625.
- Kersten H, Molden E, Tolo IK, Skovlund E, Engedal K, Wyller TB. Cognitive effects of reducing anticholinergic drug burden in a frail elderly population: a randomized controlled trial. J Gerontol A Biol Sci Med Sci 2013; 68:271–278.
- Curtis LH, Østbye T, Sendersky V, et al. Inappropriate prescribing for elderly Americans in a large outpatient population. Arch Intern Med 2004; 164:1621–1625.
- Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry 2015; 72:136–142.
- Martin JL, Sainz-Pardo M, Furukawa TA, Martín-Sánchez E, Seoane T, Galán C. Benzodiazepines in generalized anxiety disorder: heterogeneity of outcomes based on a systematic review and meta-analysis of clinical trials. J Psychopharmacol 2007; 21:774–782.
- Buscemi N, Vandermeer B, Friesen C, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen Intern Med 2007; 22:1335–1350.
- Rickels K, Schweizer E, Case WG, Greenblatt DJ. Long-term therapeutic use of benzodiazepines, I. Effects of abrupt discontinuation. Arch Gen Psychiatry 1990; 47:899–907.
- Fenton MC, Keyes KM, Martins SS, Hasin DS. The role of a prescription in anxiety medication use, abuse, and dependence. Am J Psychiatry 2010; 167:1247–1253.
- Billoti de Gage S, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case-control study. BMJ 2014; 349:g5205.
- Smink BE, Egberts AC, Lusthof KJ, Uges DR, de Gier JJ. The relationship between benzodiazepine use and traffic accidents: a systemic literature review. CNS Drugs 2010; 24:639–653.
- Tinett, ME, Speechley M, Ginter S. Risk factors for falls among elderly persons living in the community. N Engl J Med 1988; 319:1701–1707.
- Zint K, Haefeli WE, Glynn RJ, Mogun H, Avorn J, Stürmer T. Impact of drug interactions, dosage, and duration of therapy on the risk of hip fracture associated with benzodiazepine use in older adults. Pharmacoepidemiol Drug Saf 2010; 19:1248–1255.
- Briesacher BA, Soumerai SB, Field TS, Fouayzi H, Gurwitz JH. Medicare Part D’s exclusion of benzodiazepines and fracture risk in nursing homes. Arch Intern Med 2010; 170:693–698.
- Berry SD, Lee Y, Cai S, Dore DD. Nonbenzodiazepine sleep medication use and hip fractures in nursing home residents. JAMA Intern Med 2013; 173:754–761.
- American Geriatrics Society. Choosing Wisely. Ten things clinicians and patients should question. www.choosingwisely.org/societies/american-geriatrics-society/. Accessed December 3, 2017.
- Cook JM, Marshall R, Masci C, Coyne JC. Physicians’ perspectives on prescribing benzodiazepines for older adults: a qualitative study. J Gen Intern Med 2007; 22:303–307.
- Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med 2014; 174:890–898.
- Alexander GC, Gallagher SA, Mascola A, Moloney RM, Stafford RS. Increasing off-label use of antipsychotic medications in the United States, 1995–2008. Phamacoepidemiol Drug Saf 2011; 20:177–184.
- Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med 2007; 146:775–786.
- US Food and Drug Administration (FDA). Public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm053171.htm. Accessed December 4, 2017.
- Hwang YJ, Dixon SN, Reiss JP, et al. Atypical antipsychotic drugs and the risk for acute kidney injury and other adverse outcomes in older adults. Ann Intern Med 2014; 161:242–248.
- Fraser L, Liu K, Naylor KL, et al. Falls and fractures with atypical antipsychotic medication use: a population-based cohort study. JAMA Intern Med 2015; 175:450–452.
- Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA 2012; 308:2020–2029.
- Schneider LS, Tariot PN, Dagerman KS, et al; CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med 2006; 355:1525–1538.
- Forgacs I, Loganayagam A. Overprescribing proton pump inhibitors. BMJ 2008; 336:2–3.
- Mazer-Amirshahi M, Mullins PM, van den Anker J, Meltzer A, Pines JM. Rising rates of proton pump inhibitor prescribing in US emergency departments. Am J Emerg Med 2014; 32:618–622.
- Heidelbaugh JJ, Goldberg KL, Inadomi JM. Magnitude and economic effect of overuse of antisecretory therapy in the ambulatory care setting. Am J Manag Care 2010; 16:e228–e324.
- Pham CQ, Regal RE, Bostwich TR, Knauf KS. Acid suppressive therapy used on an inpatient internal medicine service. Ann Pharmacother 2006; 40:1261–1266.
- Kahrilas PJ, Shaheen NJ, Vaezi MF, et al; American Gastroenterological Association. American Gastroenterological Association medical position statement on the management of gastroesophageal reflux disease. Gastroenterology 2008; 135:1383–1391.e1–e5.
- Howell MD, Novack V, Grgurich P, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med 2010; 170:784–790.
- Gulmez SE, Holm A, Frederiksen H, Jensen TG, Pedersen C, Hallas J. Use of proton pump inhibitors and the risk of community-acquired pneumonia: a population-based case-control study. Arch Intern Med 2007; 167:950–955.
- Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 2006; 296:2947–2953.
- Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA 2013; 310:2435–2442.
- Kuipers EJ, Lundell L, Klinkenberg-Knol EC, et al. Atrophic gastritis and Helicobacter pylori infection in patients with reflux esophagitis treated with omeprazole or fundoplication. N Engl J Med 1996; 334:1018–1022.
- Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med 2016; 176:238–246.
- Gomm W, von Holt K, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol 2016; 73:410–416.
- Kaltenbach T, Crockett S, Gerson LB. Are lifestyle measures effective in patients with gastroesophageal reflux disease? An evidence-based approach. Arch Intern Med 2006; 166:965–971.
- Beers MH, Ouslander JG, Rollingher I, Reuben DB, Brooks J, Beck JC. Explicit criteria for determining inappropriate medication use in nursing home residents. Arch Intern Med 1991; 151:1825–1832.
- American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015; 63:2227–2246.
- Joint Commission. Sentinel event alert, Issue 35: using medication reconciliation to prevent errors. www.jointcommission.org/sentinel_event_alert_issue_35_using_medication_reconciliation_to_prevent_errors/. Accessed August 18, 2017.
- Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther 2008; 46:72–83.
- O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 2015; 44:213–218.
- Steinman MA. Polypharmacy—time to get beyond numbers. JAMA Intern Med 2016; 176:482–483.
- Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999–2012. JAMA 2015; 314:1818–1831.
- Tinetti ME, Bogardus ST Jr, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med 2004; 351:2870–2874.
- Qato DM, Wilder J, Schumm LP, Gillet V, Alexander GC. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med 2016; 176:473–482.
- Boyd CM, Darer J, Boult C, et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA 2005; 294:716–724.
- Atkin PA, Veitch PC, Veitch EM, Ogle SJ. The epidemiology of serious adverse drug reactions among the elderly. Drugs Aging 1999; 14:141–152.
- Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016; 338:2532–2561.
- Gurwitz JH, Go AS, Fortman SP. Statins for primary prevention in older adults: uncertainty and the need for more evidence. JAMA 2016; 316:1971–1972.
- Chokshi NP, Messerli FH, Sutin D, Supariwala AA, Shah NR. Appropriateness of statins in patients aged ≥ 80 years and comparison to other age groups. Am J Cardiol 2012; 110:1477–1481.
- Stone NJ, Robinson J, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(suppl 2):S1–S45.
- Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2013; 1:CD004816.
- Chatzizisis YS, Koskinas KC, Misirli G, Vaklavas C, Hatzitolios A, Giannoglou GD. Risk factors and drug interactions predisposing to statin-induced myopathy: implications for risk assessment, prevention and treatment. Drug Saf 2010; 33:171–187.
- Odden MC, Pletcher MJ, Coxson PG, et al. Cost-effectiveness and population impact of statins for primary prevention in adults aged 75 years or older in the United States. Ann Intern Med 2015; 162:533–541.
- Han BH, Sutin D, Williamson JD, et al. Effect of statin treatment vs usual care on primary cardiovascular prevention among older adults. The ALLHAT-LLT randomized clinical trial. JAMA Intern Med 2017; 177:955–965.
- Gray SL, Anderson ML, Dublin S, et al. Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern Med 2015; 175:401–407.
- Rudolph JL, Salow MJ, Angelini MC, McGlinchey RE. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med 2008; 168:508–513.
- Hilmer SN, Mager DE, Simonsick EM, et al. A drug burden index to define the functional burden of medications in older people. Arch Intern Med 2007; 167:781–787.
- Risacher SL, McDonald BC, Tallman EF, et al; Alzheimer’s Disease Neuroimaging Initiative. Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurol 2016; 73:721–732.
- Qaseem A, Dallas P, Forciea MA, Starkey M, Denberg TD, Shekelle P; Clinical Guidelines Committee of the American College of Physicians. Nonsurgical management of urinary incontinence in women: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2014; 161:429–440.
- Efjestad AS, Molden E, Oksengard AR. Pharmacist-initiated management of antagonistic interactions between anticholinergic drugs and acetyl cholinesterase inhibitors in individuals with dementia. J Am Geriatr Soc 2013; 61:1624–1625.
- Kersten H, Molden E, Tolo IK, Skovlund E, Engedal K, Wyller TB. Cognitive effects of reducing anticholinergic drug burden in a frail elderly population: a randomized controlled trial. J Gerontol A Biol Sci Med Sci 2013; 68:271–278.
- Curtis LH, Østbye T, Sendersky V, et al. Inappropriate prescribing for elderly Americans in a large outpatient population. Arch Intern Med 2004; 164:1621–1625.
- Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry 2015; 72:136–142.
- Martin JL, Sainz-Pardo M, Furukawa TA, Martín-Sánchez E, Seoane T, Galán C. Benzodiazepines in generalized anxiety disorder: heterogeneity of outcomes based on a systematic review and meta-analysis of clinical trials. J Psychopharmacol 2007; 21:774–782.
- Buscemi N, Vandermeer B, Friesen C, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen Intern Med 2007; 22:1335–1350.
- Rickels K, Schweizer E, Case WG, Greenblatt DJ. Long-term therapeutic use of benzodiazepines, I. Effects of abrupt discontinuation. Arch Gen Psychiatry 1990; 47:899–907.
- Fenton MC, Keyes KM, Martins SS, Hasin DS. The role of a prescription in anxiety medication use, abuse, and dependence. Am J Psychiatry 2010; 167:1247–1253.
- Billoti de Gage S, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case-control study. BMJ 2014; 349:g5205.
- Smink BE, Egberts AC, Lusthof KJ, Uges DR, de Gier JJ. The relationship between benzodiazepine use and traffic accidents: a systemic literature review. CNS Drugs 2010; 24:639–653.
- Tinett, ME, Speechley M, Ginter S. Risk factors for falls among elderly persons living in the community. N Engl J Med 1988; 319:1701–1707.
- Zint K, Haefeli WE, Glynn RJ, Mogun H, Avorn J, Stürmer T. Impact of drug interactions, dosage, and duration of therapy on the risk of hip fracture associated with benzodiazepine use in older adults. Pharmacoepidemiol Drug Saf 2010; 19:1248–1255.
- Briesacher BA, Soumerai SB, Field TS, Fouayzi H, Gurwitz JH. Medicare Part D’s exclusion of benzodiazepines and fracture risk in nursing homes. Arch Intern Med 2010; 170:693–698.
- Berry SD, Lee Y, Cai S, Dore DD. Nonbenzodiazepine sleep medication use and hip fractures in nursing home residents. JAMA Intern Med 2013; 173:754–761.
- American Geriatrics Society. Choosing Wisely. Ten things clinicians and patients should question. www.choosingwisely.org/societies/american-geriatrics-society/. Accessed December 3, 2017.
- Cook JM, Marshall R, Masci C, Coyne JC. Physicians’ perspectives on prescribing benzodiazepines for older adults: a qualitative study. J Gen Intern Med 2007; 22:303–307.
- Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med 2014; 174:890–898.
- Alexander GC, Gallagher SA, Mascola A, Moloney RM, Stafford RS. Increasing off-label use of antipsychotic medications in the United States, 1995–2008. Phamacoepidemiol Drug Saf 2011; 20:177–184.
- Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med 2007; 146:775–786.
- US Food and Drug Administration (FDA). Public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm053171.htm. Accessed December 4, 2017.
- Hwang YJ, Dixon SN, Reiss JP, et al. Atypical antipsychotic drugs and the risk for acute kidney injury and other adverse outcomes in older adults. Ann Intern Med 2014; 161:242–248.
- Fraser L, Liu K, Naylor KL, et al. Falls and fractures with atypical antipsychotic medication use: a population-based cohort study. JAMA Intern Med 2015; 175:450–452.
- Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA 2012; 308:2020–2029.
- Schneider LS, Tariot PN, Dagerman KS, et al; CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med 2006; 355:1525–1538.
- Forgacs I, Loganayagam A. Overprescribing proton pump inhibitors. BMJ 2008; 336:2–3.
- Mazer-Amirshahi M, Mullins PM, van den Anker J, Meltzer A, Pines JM. Rising rates of proton pump inhibitor prescribing in US emergency departments. Am J Emerg Med 2014; 32:618–622.
- Heidelbaugh JJ, Goldberg KL, Inadomi JM. Magnitude and economic effect of overuse of antisecretory therapy in the ambulatory care setting. Am J Manag Care 2010; 16:e228–e324.
- Pham CQ, Regal RE, Bostwich TR, Knauf KS. Acid suppressive therapy used on an inpatient internal medicine service. Ann Pharmacother 2006; 40:1261–1266.
- Kahrilas PJ, Shaheen NJ, Vaezi MF, et al; American Gastroenterological Association. American Gastroenterological Association medical position statement on the management of gastroesophageal reflux disease. Gastroenterology 2008; 135:1383–1391.e1–e5.
- Howell MD, Novack V, Grgurich P, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med 2010; 170:784–790.
- Gulmez SE, Holm A, Frederiksen H, Jensen TG, Pedersen C, Hallas J. Use of proton pump inhibitors and the risk of community-acquired pneumonia: a population-based case-control study. Arch Intern Med 2007; 167:950–955.
- Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 2006; 296:2947–2953.
- Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA 2013; 310:2435–2442.
- Kuipers EJ, Lundell L, Klinkenberg-Knol EC, et al. Atrophic gastritis and Helicobacter pylori infection in patients with reflux esophagitis treated with omeprazole or fundoplication. N Engl J Med 1996; 334:1018–1022.
- Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med 2016; 176:238–246.
- Gomm W, von Holt K, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol 2016; 73:410–416.
- Kaltenbach T, Crockett S, Gerson LB. Are lifestyle measures effective in patients with gastroesophageal reflux disease? An evidence-based approach. Arch Intern Med 2006; 166:965–971.
- Beers MH, Ouslander JG, Rollingher I, Reuben DB, Brooks J, Beck JC. Explicit criteria for determining inappropriate medication use in nursing home residents. Arch Intern Med 1991; 151:1825–1832.
- American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015; 63:2227–2246.
- Joint Commission. Sentinel event alert, Issue 35: using medication reconciliation to prevent errors. www.jointcommission.org/sentinel_event_alert_issue_35_using_medication_reconciliation_to_prevent_errors/. Accessed August 18, 2017.
- Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther 2008; 46:72–83.
- O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 2015; 44:213–218.
KEY POINTS
- Statins, anticholinergics, benzodiazepines, antipsychotics, and proton pump inhibitors are widely prescribed.
- In older patients, a periodic comprehensive medication review is needed to reevaluate the risks and the benefits of current medications in light of goals of care, life expectancy, and the patient’s preferences.
- The Beers criteria and the Screening Tool of Older Persons’ Potentially Inappropriate Prescriptions provide valuable guidance for safe prescribing in older adults.
Finding balance: Optimizing medication prescribing in older patients
According to a 2016 study, more than one-third of older adults in the United States take 5 or more medications.1 This is a growing problem. Not only do older patients take more drugs than younger patients, they are also at higher risk of adverse drug events, drug-drug interactions, geriatric syndromes, and lower adherence.2
Many drugs that older patients are given are potentially inappropriate, ie, their risks outweigh the expected benefits, particularly when effective and safer alternative therapies exist. Although many clinicians are aware of the risks of polypharmacy, they may not be confident in discontinuing potentially inappropriate medications. The process of deliberately tapering, stopping, or reducing doses of medications with the goal of reducing harm and improving patient outcomes is known as deprescribing.3
In this issue, Kim et al4 review several medications that are overused or often used inappropriately in older adults: statins for primary prevention of atherosclerotic cardiovascular disease, anticholinergic drugs, benzodiazepines, antipsychotics, and proton pump inhibitors. They offer guidance about the situations in which these drugs may be inappropriate as well as alternative drug and nondrug treatments. Further, they suggest that, when prescribing or deprescribing drugs in older adults, clinicians consult tools such as the Beers criteria and the STOPP/START criteria (the Screening Tool of Older Persons’ Potentially Inappropriate Prescriptions, and the Screening Tool to Alert Doctors to Right Treatment).
The issues Kim et al review are highly relevant and may increase awareness of specific potentially inappropriate medications. They also remind us that nonpharmacologic treatments are first-line for many medical conditions. In an era of a pill for every ill and a quick-fix mentality among both patients and providers, lifestyle changes and other nonpharmacologic treatments may be overlooked. Similarly, the STOPP/START criteria, which are concrete, evidence-based recommendations that can be applied to patient care, are likely underused in clinical practice.
Although necessary and valuable, simply arming clinicians with knowledge is insufficient to tackle the problems of polypharmacy and inappropriate prescribing. As the authors note in their discussion of benzodiazepines, practice guidelines exist regarding prescribing these agents, and data from randomized trials support specific interventions to deprescribe them.5 Nevertheless, clinicians report feeling inadequately prepared to discontinue benzodiazepines, particularly when patients perceive benefit from them. As such, user-friendly tools and specific strategies for weighing risks vs benefits are critical for clinicians.
PUTTING KNOWLEDGE INTO PRACTICE
How do we translate knowledge into practice with regard to deprescribing potentially inappropriate medications in older patients—or prescribing drugs only if appropriate in the first place?
An opportunity arises when patients are in the hospital. Taking a medication history on admission and matching medications with indications are key starting points. Clinical pharmacists can help screen for side effects and potential interactions and can provide deprescribing recommendations. Meticulous discharge medication reconciliation, patient education, and communication of the updated medication list to the outpatient provider are central to ensuring that patients adhere to medication adjustments after they go home.
A MATTER OF BALANCE
Another factor to consider is the patient’s physiologic age compared with his or her chronologic age. If a patient has multiple comorbidities, frailty, limited life expectancy, or poor renal function, we may consider her older than her chronologic age. In this case, a drug’s risks may outweigh its benefit, which is something to be discussed. On the other hand, a high-functioning and relatively healthy elderly patient may be a candidate for medications known to reduce the risk of death or control a chronic disease better. Incorporating a patient’s goals of care and using shared decision-making are also likely to yield an optimal medication regimen.
Smartphone apps and resources embedded in electronic health records provide additional decision support. Used when prescribing or reconciling medications, these supplemental brains offer instant feedback and information on dose adjustments, drug interactions, clinical guidelines, and even potentially inappropriate medications. While the impacts of these electronic tools on prescribing patterns and outcomes in geriatric populations remain unclear, new ones are being developed and studied.6 This may be the most promising way to translate knowledge into practice, as it is more easily integrated with existing clinician workflows.
AN OPPORTUNITY TO IMPROVE
There is significant opportunity to reduce polypharmacy and optimize medication prescribing practices for older adults. Awareness of potentially inappropriate medications and clinical situations in which the use of certain classes of medications should be minimized is the first step in addressing this problem. Using tools such as the STOPP/START criteria, reviewing medications at critical transition points, prioritizing patient function and goals, and using electronic clinical decision support should aid prescribing decisions.
Whenever possible, collaborating with other care team members such as pharmacists may increase efficiency and effectiveness of medication management. Ultimately, inclusion of more older adults in clinical trials may provide data-driven guidance for weighing risks and benefits. Finally, further study of the effects of deprescribing on clinical outcomes may be the missing piece to help clinicians and patients find balance in prescription management.
- Qato DM, Wilder J, Schumm LP, Gillet V, Alexander GC. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med 2016; 176:473–482.
- Saraf AA, Petersen AW, Simmons SF, et al. Medications associated with geriatric syndromes and their prevalence in older hospitalized adults discharged to skilled nursing facilities. J Hosp Med 2016; 11:694–700.
- Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med 2015; 175:827–834.
- Kim LD, Koncilja K, Nielsen C. Medication management in older adults. Cleve Clin J Med 2018; 85:129–135.
- Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med 2014; 174:890–898.
- Alagiakrishnan K, Wilson P, Sadowski CA, et al. Physicians’ use of computerized clinical decision supports to improve medication management in the elderly—the Seniors Medication Alert and Review Technology intervention. Clin Interv Aging 2016; 11:73–81.
According to a 2016 study, more than one-third of older adults in the United States take 5 or more medications.1 This is a growing problem. Not only do older patients take more drugs than younger patients, they are also at higher risk of adverse drug events, drug-drug interactions, geriatric syndromes, and lower adherence.2
Many drugs that older patients are given are potentially inappropriate, ie, their risks outweigh the expected benefits, particularly when effective and safer alternative therapies exist. Although many clinicians are aware of the risks of polypharmacy, they may not be confident in discontinuing potentially inappropriate medications. The process of deliberately tapering, stopping, or reducing doses of medications with the goal of reducing harm and improving patient outcomes is known as deprescribing.3
In this issue, Kim et al4 review several medications that are overused or often used inappropriately in older adults: statins for primary prevention of atherosclerotic cardiovascular disease, anticholinergic drugs, benzodiazepines, antipsychotics, and proton pump inhibitors. They offer guidance about the situations in which these drugs may be inappropriate as well as alternative drug and nondrug treatments. Further, they suggest that, when prescribing or deprescribing drugs in older adults, clinicians consult tools such as the Beers criteria and the STOPP/START criteria (the Screening Tool of Older Persons’ Potentially Inappropriate Prescriptions, and the Screening Tool to Alert Doctors to Right Treatment).
The issues Kim et al review are highly relevant and may increase awareness of specific potentially inappropriate medications. They also remind us that nonpharmacologic treatments are first-line for many medical conditions. In an era of a pill for every ill and a quick-fix mentality among both patients and providers, lifestyle changes and other nonpharmacologic treatments may be overlooked. Similarly, the STOPP/START criteria, which are concrete, evidence-based recommendations that can be applied to patient care, are likely underused in clinical practice.
Although necessary and valuable, simply arming clinicians with knowledge is insufficient to tackle the problems of polypharmacy and inappropriate prescribing. As the authors note in their discussion of benzodiazepines, practice guidelines exist regarding prescribing these agents, and data from randomized trials support specific interventions to deprescribe them.5 Nevertheless, clinicians report feeling inadequately prepared to discontinue benzodiazepines, particularly when patients perceive benefit from them. As such, user-friendly tools and specific strategies for weighing risks vs benefits are critical for clinicians.
PUTTING KNOWLEDGE INTO PRACTICE
How do we translate knowledge into practice with regard to deprescribing potentially inappropriate medications in older patients—or prescribing drugs only if appropriate in the first place?
An opportunity arises when patients are in the hospital. Taking a medication history on admission and matching medications with indications are key starting points. Clinical pharmacists can help screen for side effects and potential interactions and can provide deprescribing recommendations. Meticulous discharge medication reconciliation, patient education, and communication of the updated medication list to the outpatient provider are central to ensuring that patients adhere to medication adjustments after they go home.
A MATTER OF BALANCE
Another factor to consider is the patient’s physiologic age compared with his or her chronologic age. If a patient has multiple comorbidities, frailty, limited life expectancy, or poor renal function, we may consider her older than her chronologic age. In this case, a drug’s risks may outweigh its benefit, which is something to be discussed. On the other hand, a high-functioning and relatively healthy elderly patient may be a candidate for medications known to reduce the risk of death or control a chronic disease better. Incorporating a patient’s goals of care and using shared decision-making are also likely to yield an optimal medication regimen.
Smartphone apps and resources embedded in electronic health records provide additional decision support. Used when prescribing or reconciling medications, these supplemental brains offer instant feedback and information on dose adjustments, drug interactions, clinical guidelines, and even potentially inappropriate medications. While the impacts of these electronic tools on prescribing patterns and outcomes in geriatric populations remain unclear, new ones are being developed and studied.6 This may be the most promising way to translate knowledge into practice, as it is more easily integrated with existing clinician workflows.
AN OPPORTUNITY TO IMPROVE
There is significant opportunity to reduce polypharmacy and optimize medication prescribing practices for older adults. Awareness of potentially inappropriate medications and clinical situations in which the use of certain classes of medications should be minimized is the first step in addressing this problem. Using tools such as the STOPP/START criteria, reviewing medications at critical transition points, prioritizing patient function and goals, and using electronic clinical decision support should aid prescribing decisions.
Whenever possible, collaborating with other care team members such as pharmacists may increase efficiency and effectiveness of medication management. Ultimately, inclusion of more older adults in clinical trials may provide data-driven guidance for weighing risks and benefits. Finally, further study of the effects of deprescribing on clinical outcomes may be the missing piece to help clinicians and patients find balance in prescription management.
According to a 2016 study, more than one-third of older adults in the United States take 5 or more medications.1 This is a growing problem. Not only do older patients take more drugs than younger patients, they are also at higher risk of adverse drug events, drug-drug interactions, geriatric syndromes, and lower adherence.2
Many drugs that older patients are given are potentially inappropriate, ie, their risks outweigh the expected benefits, particularly when effective and safer alternative therapies exist. Although many clinicians are aware of the risks of polypharmacy, they may not be confident in discontinuing potentially inappropriate medications. The process of deliberately tapering, stopping, or reducing doses of medications with the goal of reducing harm and improving patient outcomes is known as deprescribing.3
In this issue, Kim et al4 review several medications that are overused or often used inappropriately in older adults: statins for primary prevention of atherosclerotic cardiovascular disease, anticholinergic drugs, benzodiazepines, antipsychotics, and proton pump inhibitors. They offer guidance about the situations in which these drugs may be inappropriate as well as alternative drug and nondrug treatments. Further, they suggest that, when prescribing or deprescribing drugs in older adults, clinicians consult tools such as the Beers criteria and the STOPP/START criteria (the Screening Tool of Older Persons’ Potentially Inappropriate Prescriptions, and the Screening Tool to Alert Doctors to Right Treatment).
The issues Kim et al review are highly relevant and may increase awareness of specific potentially inappropriate medications. They also remind us that nonpharmacologic treatments are first-line for many medical conditions. In an era of a pill for every ill and a quick-fix mentality among both patients and providers, lifestyle changes and other nonpharmacologic treatments may be overlooked. Similarly, the STOPP/START criteria, which are concrete, evidence-based recommendations that can be applied to patient care, are likely underused in clinical practice.
Although necessary and valuable, simply arming clinicians with knowledge is insufficient to tackle the problems of polypharmacy and inappropriate prescribing. As the authors note in their discussion of benzodiazepines, practice guidelines exist regarding prescribing these agents, and data from randomized trials support specific interventions to deprescribe them.5 Nevertheless, clinicians report feeling inadequately prepared to discontinue benzodiazepines, particularly when patients perceive benefit from them. As such, user-friendly tools and specific strategies for weighing risks vs benefits are critical for clinicians.
PUTTING KNOWLEDGE INTO PRACTICE
How do we translate knowledge into practice with regard to deprescribing potentially inappropriate medications in older patients—or prescribing drugs only if appropriate in the first place?
An opportunity arises when patients are in the hospital. Taking a medication history on admission and matching medications with indications are key starting points. Clinical pharmacists can help screen for side effects and potential interactions and can provide deprescribing recommendations. Meticulous discharge medication reconciliation, patient education, and communication of the updated medication list to the outpatient provider are central to ensuring that patients adhere to medication adjustments after they go home.
A MATTER OF BALANCE
Another factor to consider is the patient’s physiologic age compared with his or her chronologic age. If a patient has multiple comorbidities, frailty, limited life expectancy, or poor renal function, we may consider her older than her chronologic age. In this case, a drug’s risks may outweigh its benefit, which is something to be discussed. On the other hand, a high-functioning and relatively healthy elderly patient may be a candidate for medications known to reduce the risk of death or control a chronic disease better. Incorporating a patient’s goals of care and using shared decision-making are also likely to yield an optimal medication regimen.
Smartphone apps and resources embedded in electronic health records provide additional decision support. Used when prescribing or reconciling medications, these supplemental brains offer instant feedback and information on dose adjustments, drug interactions, clinical guidelines, and even potentially inappropriate medications. While the impacts of these electronic tools on prescribing patterns and outcomes in geriatric populations remain unclear, new ones are being developed and studied.6 This may be the most promising way to translate knowledge into practice, as it is more easily integrated with existing clinician workflows.
AN OPPORTUNITY TO IMPROVE
There is significant opportunity to reduce polypharmacy and optimize medication prescribing practices for older adults. Awareness of potentially inappropriate medications and clinical situations in which the use of certain classes of medications should be minimized is the first step in addressing this problem. Using tools such as the STOPP/START criteria, reviewing medications at critical transition points, prioritizing patient function and goals, and using electronic clinical decision support should aid prescribing decisions.
Whenever possible, collaborating with other care team members such as pharmacists may increase efficiency and effectiveness of medication management. Ultimately, inclusion of more older adults in clinical trials may provide data-driven guidance for weighing risks and benefits. Finally, further study of the effects of deprescribing on clinical outcomes may be the missing piece to help clinicians and patients find balance in prescription management.
- Qato DM, Wilder J, Schumm LP, Gillet V, Alexander GC. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med 2016; 176:473–482.
- Saraf AA, Petersen AW, Simmons SF, et al. Medications associated with geriatric syndromes and their prevalence in older hospitalized adults discharged to skilled nursing facilities. J Hosp Med 2016; 11:694–700.
- Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med 2015; 175:827–834.
- Kim LD, Koncilja K, Nielsen C. Medication management in older adults. Cleve Clin J Med 2018; 85:129–135.
- Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med 2014; 174:890–898.
- Alagiakrishnan K, Wilson P, Sadowski CA, et al. Physicians’ use of computerized clinical decision supports to improve medication management in the elderly—the Seniors Medication Alert and Review Technology intervention. Clin Interv Aging 2016; 11:73–81.
- Qato DM, Wilder J, Schumm LP, Gillet V, Alexander GC. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med 2016; 176:473–482.
- Saraf AA, Petersen AW, Simmons SF, et al. Medications associated with geriatric syndromes and their prevalence in older hospitalized adults discharged to skilled nursing facilities. J Hosp Med 2016; 11:694–700.
- Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med 2015; 175:827–834.
- Kim LD, Koncilja K, Nielsen C. Medication management in older adults. Cleve Clin J Med 2018; 85:129–135.
- Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med 2014; 174:890–898.
- Alagiakrishnan K, Wilson P, Sadowski CA, et al. Physicians’ use of computerized clinical decision supports to improve medication management in the elderly—the Seniors Medication Alert and Review Technology intervention. Clin Interv Aging 2016; 11:73–81.
Preventing cardiovascular disease in older adults: One size does not fit all
When assessing and attempting to modify the risk of cardiovascular disease in older patients, physicians should consider incorporating the concept of frailty. The balance of risk and benefit may differ considerably for 2 patients of the same age if one is fit and the other is frail. Because the aging population is a diverse group, a one-size-fits-all approach to cardiovascular disease prevention and risk-factor management is not appropriate.
A GROWING, DIVERSE GROUP
The number of older adults with multiple cardiovascular risk factors is increasing as life expectancy improves. US residents who are age 65 today can expect to live to an average age of 84 (men) or 87 (women).1
However, the range of life expectancy for people reaching these advanced ages is wide, and chronologic age is no longer sufficient to determine a patient’s risk profile. Furthermore, the prevalence of cardiovascular disease rises with age, and age itself is the strongest predictor of cardiovascular risk.2
Current risk calculators have not been validated in people over age 80,2 making them inadequate for use in older patients. Age alone cannot identify who will benefit from preventive strategies, except in situations when a dominant disease such as metastatic cancer, end-stage renal disease, end-stage dementia, or end-stage heart failure is expected to lead to mortality within a year. Guidelines for treating common risk factors such as elevated cholesterol3 in the general population have generally not focused on adults over 75 or recognized their diversity in health status.4 In order to generate an individualized prescription for cardiovascular disease prevention for older adults, issues such as frailty, cognitive and functional status, disability, and comorbidity must be considered.
WHAT IS FRAILTY?
Clinicians have recognized frailty for decades, but to date there remains a debate on how to define it.
Clegg et al5 described frailty as “a state of increased vulnerability to poor resolution of homeostasis after a stressor event,”5 a definition generally agreed upon, as frailty predicts both poor health outcomes and death.
Indeed, in a prospective study of 5,317 men and women ranging in age from 65 to 101, those identified as frail at baseline were 6 times more likely to have died 3 years later (mortality rates 18% vs 3%), and the difference persisted at 7 years.6 After adjusting for comorbidities, those identified as frail were also more likely to fall, develop limitations in mobility or activities of daily living, or be hospitalized.
The two current leading theories of frailty were defined by Fried et al6 and by Rockwood and Mitnitski.7
Fried et al6 have operationalized frailty as a “physical phenotype,” defined as 3 or more of the following:
- Unintentional weight loss of 10 pounds in the past year
- Self-reported exhaustion
- Weakness as measured by grip strength
- Slow walking speed
- Decreased physical activity.6
Rockwood and Mitnitski7 define frailty as an accumulation of health-related deficits over time. They recommend that 30 to 40 possible deficits that cover a variety of health systems be included such as cognition, mood, function, and comorbidity. These are added and divided by the total possible number of variables to generate a score between 0 and 1.8
The difficulty in defining frailty has led to varying estimates of its prevalence, ranging from 25% to 50% in adults over 65 who have cardiovascular disease.9
CAUSE AND CONSEQUENCE OF CARDIOVASCULAR DISEASE
Studies have highlighted the bidirectional connection between frailty and cardiovascular disease.10 Frailty may predict cardiovascular disease, while cardiovascular disease is associated with an increased risk of incident frailty.9,11
Frail adults with cardiovascular disease have a higher risk of poor outcomes, even after correcting for age, comorbidities, disability, and disease severity. For example, frailty is associated with a twofold higher mortality rate in individuals with cardiovascular disease.9
A prospective cohort study12 of 3,895 middle-aged men and women demonstrated that those with an elevated cardiovascular risk score were at increased risk of frailty over 10 years (odds ratio [OR] 1.35, 95% confidence interval [CI] 1.21–1.51) and incident cardiovascular events (OR 1.36, 95% CI 1.15–1.61). This suggests that modification of cardiovascular risk factors earlier in life may reduce the risk of subsequently becoming frail.
Biologic mechanisms that may explain the connection between frailty and cardiovascular disease include derangements in inflammatory, hematologic, and endocrine pathways. People who are found to be clinically frail are more likely to have insulin resistance and elevated biomarkers such as C-reactive protein, D-dimer, and factor VIII.13 The inflammatory cytokine interleukin 6 is suggested as a common link between inflammation and thrombosis, perhaps contributing to the connection between cardiovascular disease and frailty. Many of these biomarkers have been linked to the pathophysiologic changes of aging, so-called “inflamm-aging” or immunosenescence, including sarcopenia, osteoporosis, and cardiovascular disease.14
ASSESSING FRAILTY IN THE CLINIC
For adults over age 70, frailty assessment is an important first step in managing cardiovascular disease risk.15 Frailty status will better identify those at risk of adverse outcomes in the short term and those who are most likely to benefit from long-term cardiovascular preventive strategies. Additionally, incorporating frailty assessment into traditional risk factor evaluation may permit appropriate intervention and prevention of a potentially modifiable risk factor.
Gait speed is a quick, easy, inexpensive, and sensitive way to assess frailty status, with excellent inter-rater and test-retest reliability, even in those with cognitive impairment.16 Slow gait speed predicts limitations in mobility, limitations in activities of daily living, and death.8,17
In a prospective study18 of 1,567 men and women, mean age 74, slow gait speed was the strongest predictor of subsequent cardiovascular events.18
Gait speed is usually measured over a distance of 4 meters (13.1 feet),17 and the patient is asked to walk comfortably in an unobstructed, marked area. An assistive walking device can be used if needed. If possible, this is repeated once after a brief recovery period, and the average is recorded.
The FRAIL scale19,20 is a simple, validated questionnaire that combines the Fried and Rockwood concepts of frailty and can be given over the phone or to patients in a waiting room. One point is given for each of the following, and people who have 3 or more are considered frail:
- Fatigue
- Resistance (inability to climb 1 flight of stairs)
- Ambulation (inability to walk 1 block)
- Illnesses (having more than 5)
- Loss of more than 5% of body weight.
Other measures of physical function such as grip strength (using a dynamometer), the Timed Up and Go test (assessing the ability to get up from a chair and walk a short distance), and Short Physical Performance Battery (assessing balance, chair stands, and walking speed) can be used to screen for frailty, but are more time-intensive than gait speed alone, and so are not always practical to use in a busy clinic.21
MANAGEMENT OF RISK FACTORS
Management of cardiovascular risk factors is best individualized as outlined below.
LOWERING HIGH BLOOD PRESSURE
The incidence of ischemic heart disease and stroke increases with age across all levels of elevated systolic and diastolic blood pressure.22 Hypertension is also associated with increased risk of cognitive decline. However, a J-shaped relationship has been observed in older adults, with increased cardiovascular events for both low and elevated blood pressure, although the clinical relevance remains controversial.23
Odden et al24 performed an observational study and found that high blood pressure was associated with an increased mortality rate in older adults with normal gait speed, while in those with slow gait speed, high blood pressure neither harmed nor helped. Those who could not walk 6 meters appeared to benefit from higher blood pressure.
HYVET (the Hypertension in the Very Elderly Trial),25 a randomized controlled trial in 3,845 community-dwelling people age 80 or older with sustained systolic blood pressure higher than 160 mm Hg, found a significant reduction in rates of stroke and all-cause mortality (relative risk [RR] 0.76, P = .007) in the treatment arm using indapamide with perindopril if necessary to reach a target blood pressure of 150/80 mm Hg.
Frailty was not assessed during the trial; however, in a reanalysis, the results did not change in those identified as frail using a Rockwood frailty index (a count of health-related deficits accumulated over the lifespan).26
SPRINT (the Systolic Blood Pressure Intervention Trial)27 randomized participants age 50 and older with systolic blood pressure of 130 to 180 mm Hg and at increased risk of cardiovascular disease to intensive treatment (goal systolic blood pressure ≤ 120 mm Hg) or standard treatment (goal systolic blood pressure ≤ 140 mm Hg). In a prespecified subgroup of 2,636 participants over age 75 (mean age 80), hazard ratios and 95% confidence intervals for adverse outcomes with intensive treatment were:
- Major cardiovascular events: HR 0.66, 95% CI 0.51–0.85
- Death: HR 0.67, 95% CI 0.49–0.91.
Over 3 years of treatment this translated into a number needed to treat of 27 to prevent 1 cardiovascular event and 41 to prevent 1 death.
Within this subgroup, the benefit was similar regardless of level of frailty (measured both by a Rockwood frailty index and by gait speed).
However, the incidence of serious adverse treatment effects such as hypotension, orthostasis, electrolyte abnormalities, and acute kidney injury was higher with intensive treatment in the frail group. Although the difference was not statistically significant, it is cause for caution. Further, the exclusion criteria (history of diabetes, heart failure, dementia, stroke, weight loss of > 10%, nursing home residence) make it difficult to generalize the SPRINT findings to the general aging population.27
Tinetti et al28 performed an observational study using a nationally representative sample of older adults. They found that receiving any antihypertensive therapy was associated with an increased risk of falls with serious adverse outcomes. The risks of adverse events related to antihypertensive therapy increased with age.
Recommendations on hypertension
Managing hypertension in frail patients at risk of cardiovascular disease requires balancing the benefits vs the risks of treatment, such as polypharmacy, falls, and orthostatic hypotension.
The Eighth Joint National Committee suggests a blood pressure goal of less than 150/90 mm Hg for all adults over age 60, and less than 140/90 mm Hg for those with a history of cardiovascular disease or diabetes.29
The American College of Cardiology/American Heart Association (ACC/AHA) guidelines on hypertension, recently released, recommend a new blood pressure target of <120/<80 as normal, with 120–129/<80 considered elevated, 130–139/80–89 stage 1 hypertension, and ≥140/≥90 as stage 2 hypertension.30 An important caveat to these guidelines is the recommendation to measure blood pressure accurately and with accurate technique, which is often not possible in many busy clinics. These guidelines are intended to apply to older adults as well, with a note that those with multiple morbidities and limited life expectancy will benefit from a shared decision that incorporates patient preferences and clinical judgment. Little guidance is given on how to incorporate frailty, although note is made that older adults who reside in assisted living facilities and nursing homes have not been represented in randomized controlled trials.30
American Diabetes Association guidelines on hypertension in patients with diabetes recommend considering functional status, frailty, and life expectancy to decide on a blood pressure goal of either 140/90 mm Hg (if fit) or 150/90 mm Hg (if frail). They do not specify how to diagnose frailty.31
Canadian guidelines say that in those with advanced frailty (ie, entirely dependent for personal care and activities of daily living) and short life expectancy (months), it is reasonable to liberalize the systolic blood pressure goal to 160 to 190 mm Hg.32
Our recommendations. In both frail and nonfrail individuals without a limited life expectancy, it is reasonable to aim for a blood pressure of at least less than 140/90 mm Hg. For those at increased risk of cardiovascular disease and able to tolerate treatment, careful lowering to 130/80 mm Hg may be considered, with close attention to side effects.
Treatment should start with the lowest possible dose, be titrated slowly, and may need to be tailored to standing blood pressure to avoid orthostatic hypotension.
Home blood pressure measurements may be beneficial in monitoring treatment.
MANAGING LIPIDS
For those over age 75, data on efficacy of statins are mixed due to the small number of older adults enrolled in randomized controlled trials of these drugs. To our knowledge, no statin trial has examined the role of frailty.
The PROSPER trial (Prospective Study of Pravastatin in the Elderly at Risk)33 randomized 5,804 patients ages 70 to 82 to receive either pravastatin or placebo. Overall, the incidence of a composite end point of major cardiovascular events was 15% lower with active treatment (P = .014). However, the mean age was 75, which does little to address the paucity of evidence for those over age 75; follow-up time was only 3 years, and subgroup analysis did not show benefit in those who did not have a history of cardiovascular disease or in women.
The JUPITER trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin)34 randomized 5,695 people over age 70 without cardiovascular disease to receive either rosuvastatin or placebo. Exploratory analysis showed a significant 39% reduction in all-cause mortality and major cardiovascular events with active treatment (HR 0.61, 95% CI 0.46–0.82). Over 5 years of treatment, this translates to a number needed to treat of 19 to prevent 1 major cardiovascular event and 29 to prevent 1 cardiovascular death.
The benefit of statins for primary prevention in these trials began to be apparent 2 years after treatment was initiated.
The Women’s Health Initiative,35 an observational study, found no difference in incident frailty in women older than 65 taking statins for 3 years compared with those who did not take statins
Odden et al36 found that although statin use is generally well tolerated, the risks of statin-associated functional and cognitive decline may outweigh the benefits in those older than 75. The ongoing Statin in Reducing Events in the Elderly (STAREE) trial may shed light on this issue.
Recommendations on lipid management
The ACC/AHA,3 in their 2013 guidelines, do not recommend routine statin treatment for primary prevention in those over age 75, given a lack of evidence from randomized controlled trials. For secondary prevention, ie, for those who have a history of atherosclerotic cardiovascular disease, they recommend moderate-intensity statin therapy in this age group.
Our recommendations. For patients over age 75 without cardiovascular disease or frailty and with a life expectancy of at least 2 years, consider offering a statin for primary prevention of cardiovascular disease as part of shared decision-making.
In those with known cardiovascular disease, it is reasonable to continue statin therapy except in situations where the life expectancy is less than 6 months.37
Although moderate- or high-intensity statin therapy is recommended in current guidelines, for many older adults it is prudent to consider the lowest tolerable dose to improve adherence, with close monitoring for side effects such as myalgia and weakness.
TYPE 2 DIABETES
Evidence suggests that tight glycemic control in type 2 diabetes is harmful for adults ages 55 to 79 and does not provide clear benefits for cardiovascular risk reduction, and controlling hemoglobin A1c to less than 6.0% is associated with increased mortality in older adults.38
The American Diabetes Association31 and the American Geriatrics Society39 recommend hemoglobin A1c goals of:
- 7.5% or less for older adults with 3 or more coexisting chronic illnesses requiring medical intervention (eg, arthritis, hypertension, and heart failure) and with intact cognition and function
- 8.0% or less for those identified as frail, or with multiple chronic illnesses or moderate cognitive or functional impairment
- 8.5% or 9.0% or less for those with very complex comorbidities, in long-term care, or with end-stage chronic illnesses (eg, end-stage heart failure), or with moderate to severe cognitive or functional limitation.
These guidelines do not endorse a specific frailty assessment, although the references allude to the Fried phenotype criteria, which include gait speed. An update from the American Diabetes Association provides a patient-centered approach to tailoring treatment regimens, taking into consideration the risk of hypoglycemia for each class of drugs, side effects, and cost.40
Our recommendations. Hyperglycemia remains a risk factor for cardiovascular disease in older adults and increases the risk of many geriatric conditions including delirium, dementia, frailty, and functional decline. The goal in individualizing hemoglobin A1c goals should be to avoid both hyper- and hypoglycemia.
Sulfonylureas and insulins should be used with caution, as they have the highest associated incidence of hypoglycemia of the diabetes medications.
ASPIRIN
For secondary prevention in older adults with a history of cardiovascular disease, pooled trials have consistently demonstrated a long-term benefit for aspirin use that exceeds bleeding risks, although age and frailty status were not considered.41
Aspirin for primary prevention?
The evidence for aspirin for primary prevention in older adults is mixed. Meta-analysis suggests a modest decrease in risk of nonfatal myocardial infarction but no appreciable effects on nonfatal stroke and cardiovascular death.42
The Japanese Primary Prevention Project,43 a randomized trial of low-dose aspirin for primary prevention of cardiovascular disease in adults ages 60 to 85, showed no reduction in major cardiovascular events. However, the event rate was lower than expected, the crossover rates were high, the incidence of hemorrhagic strokes was higher than in Western studies, and the trial may have been underpowered to detect the benefits of aspirin.
The US Preventive Services Task Force44 in 2016 noted that among individuals with a 10-year cardiovascular disease risk of 10% or higher based on the ACC/AHA pooled cohort equation,3 the greatest benefit of aspirin was in those ages 50 to 59. In this age group, 225 nonfatal myocardial infarctions and 84 nonfatal strokes were prevented per 10,000 men treated, with a net gain of 333 life-years. Similar findings were noted in women.
However, in those ages 60 to 69, the risks of harm begin to rise and the benefit of starting daily aspirin necessitates individualized clinical decision-making, with particular attention to bleeding risk and life expectancy.44
In those age 70 and older, data on benefit and harm are mixed. The bleeding risk of aspirin increases with age, predominantly due to gastrointestinal bleeding.44
The ongoing Aspirin in Reducing Events in Elderly trial will add to the evidence.
Aspirin recommendations for primary prevention
The American Geriatrics Society Beers Criteria do not routinely recommend aspirin use for primary prevention in those over age 80, even in those with diabetes.45
Our recommendations. In adults over age 75 who are not frail but are identified as being at moderate to high risk of cardiovascular disease using either the ACC/AHA calculator or any other risk estimator, and without a limited life expectancy, we believe it is reasonable to consider low-dose aspirin (75–100 mg daily) for primary prevention. However, there must be careful consideration particularly for those at risk of major bleeding. One approach to consider would be the addition of a proton pump inhibitor along with aspirin, though this requires further study.46
For those who have been on aspirin for primary prevention and are now older than age 80 without an adverse bleeding event, it is reasonable to stop aspirin, although risks and benefits of discontinuing aspirin should be discussed with the patient as part of shared decision-making.
In frail individuals the risks of aspirin therapy likely outweigh any benefit for primary prevention, and aspirin cannot be routinely recommended.
EXERCISE AND WEIGHT MANAGEMENT
A low body mass index is often associated with frailty, and weight loss may be a marker of underlying illness, which increases the risk of poor outcomes. However, those with an elevated body mass index and increased adiposity are in fact more likely to be frail (using the Fried physical phenotype definition) than those with a low body mass index,47 due in part to unrecognized sarcopenic obesity, ie, replacement of lean muscle with fat.
Physical activity is currently the only intervention known to improve frailty.5
Physical activity and a balanced diet are just as important in older adults, including those with reduced functional ability and multiple comorbid conditions, as in younger individuals.
A trial in frail long-term care residents (mean age 87) found that high-intensity resistance training improved muscle strength and mobility.48 The addition of a nutritional supplement with or without exercise did not affect frailty status. In community-dwelling older adults, physical activity has also been shown to improve sarcopenia and reduce falls and hip fractures.49
Progressive resistance training has been shown to improve strength and gait speed even in those with dementia.50
Tai chi has shown promising results in reducing falls and improving balance and function in both community-dwelling older adults and those in assisted living.51,52
Exercise recommendations
The US Department of Health and Human Services53 issued physical activity guidelines in 2008 with specific recommendations for older adults that include flexibility and balance training, which have been shown to reduce falls, in addition to aerobic activities and strength training.
Our recommendations. For all older adults, particularly those who are frail, we recommend a regimen of general daily activity, balance training such as tai chi, moderate-intensity aerobics such as cycling, resistance training such as using light weights, and stretching. Sessions lasting as little as 10 minutes are beneficial.
Gait speed can be monitored in the clinic to assess improvement in function over time.
SMOKING CESSATION
Although rates of smoking are decreasing, smoking remains one of the most important cardiovascular risk factors. Smoking has been associated with increased risk of frailty and significantly increased risk of death compared with never smoking.54 Smoking cessation is beneficial even for those who quit later in life.
The US Department of Health and Human Services in 2008 released an update on tobacco use and dependence,55 with specific attention to the benefit of smoking cessation for older adults.
All counseling interventions have been shown to be effective in older adults, as has nicotine replacement. Newer medications such as varenicline should be used with caution, as the risk of side effects is higher in older patients.
NUTRITION
Samieri et al,56 in an observational study of 10,670 nurses, found that those adhering to Mediterranean-style diets during midlife had 46% increased odds of healthy aging.
The PREDIMED study (Primary Prevention of Cardiovascular Disease With a Mediterranean Diet)57 in adults ages 55 to 80 showed the Mediterranean diet supplemented with olive oil and nuts reduced the incidence of major cardiovascular disease.
Leon-Munoz et al.58 A prospective study of 1,815 community-dwelling older adults followed for 3.5 years in Spain demonstrated that adhering to a Mediterranean diet was associated with a lower incidence of frailty (P = .002) and a lower risk of slow gait speed (OR 0.53, 95% CI 0.35–0.79). Interestingly, this study also found a protective association between fish and fruit consumption and frailty.
Our recommendations. A well-balanced, diverse diet rich in whole grains, fruits, vegetables, nuts, fish, and healthy fats (polyunsaturated fatty acids), with a moderate amount of lean meats, is recommended to prevent heart disease. However, poor dental health may limit the ability of older individuals to adhere to such diets, and modifications may be needed. Additionally, age-related changes in taste and smell may contribute to poor nutrition and unintended weight loss.59 Involving a nutritionist and social worker in the patient care team should be considered especially as poor nutrition may be a sign of cognitive impairment, functional decline, and frailty.
SPECIAL CONSIDERATIONS
Special considerations when managing cardiovascular risk in the older adult include polypharmacy, multimorbidity, quality of life, and the patient’s personal preferences.
Polypharmacy, defined as taking more than 5 medications, is associated with an increased risk of adverse drug events, falls, fractures, decreased adherence, and “prescribing cascade”— prescribing more drugs to treat side effects of the first drug (eg, adding hypertensive medications to treat hypertension induced by nonsteroidal anti-inflammatory drugs).60 This is particularly important when considering adding additional medications. If a statin will be the 20th pill, it may be less beneficial and more likely to lead to additional adverse effects than if it is the fifth medication.
Patient preferences are critically important, particularly when adding or removing medications. Interventions should include a detailed medication review for appropriate prescribing and deprescribing, referral to a pharmacist, and engaging the patient’s support system.
Multimorbidity. Many older individuals have multiple chronic illnesses. The interaction of multiple conditions must be considered in creating a comprehensive plan, including prognosis, patient preference, available evidence, treatment interactions, and risks and benefits.
Quality of life. Outlook on life and choices made regarding prolongation vs quality of life may be different for the older patient than the younger patient.
Personal preferences. Although interventions such as high-intensity statins for a robust 85-year-old may be appropriate, the individual can choose to forgo any treatment. It is important to explore the patient’s goals of care and advanced directives as part of shared decision-making when building a patient-centered prevention plan.61
ONE SIZE DOES NOT FIT ALL
The heterogeneity of aging rules out a one-size-fits-all recommendation for cardiovascular disease prevention and management of cardiovascular risk factors in older adults.
There is significant overlap between cardiovascular risk status and frailty.
Incorporating frailty into the creation of a cardiovascular risk prescription can aid in the development of an individualized care plan for the prevention of cardiovascular disease in the aging population.
- Social Security Administration (SSA). Calculators: life expectancy. www.ssa.gov/planners/lifeexpectancy.html. Accessed December 8, 2017.
- Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 2017; 135:e146–e603.
- Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:2889–2934.
- Rich MW, Chyun DA, Skolnick AH, et al; American Heart Association Older Populations Committee of the Council on Clinical Cardiology, Council on Cardiovascular and Stroke Nursing, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council; American College of Cardiology; and American Geriatrics Society. Knowledge gaps in cardiovascular care of the older adult population: a scientific statement from the American Heart Association, American College of Cardiology, and American Geriatrics Society. Circulation 2016; 133:2103–2122.
- Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013; 381:752–762.
- Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci 2007; 62:722–727.
- Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA 2011; 305:50–58.
- Afilalo J, Alexander KP, Mack MJ, et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol 2014; 63:747–762.
- Afilalo J, Karunananthan S, Eisenberg MJ, Alexander KP, Bergman H. Role of frailty in patients with cardiovascular disease. Am J Cardiol 2009; 103:1616–1621.
- Woods NF, LaCroix AZ, Gray SL, et al; Women’s Health Initiative. Frailty: emergence and consequences in women aged 65 and older in the Women's Health Initiative Observational Study. J Am Geriatr Soc 2005; 53:1321–1330.
- Bouillon K, Batty GD, Hamer M, et al. Cardiovascular disease risk scores in identifying future frailty: the Whitehall II prospective cohort study. Heart 2013; 99:737–742.
- Walston J, McBurnie MA, Newman A, et al; Cardiovascular Health Study. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med 2002; 162:2333–2341.
- De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflammation markers predicting frailty and mortality in the elderly. Exp Mol Pathol 2006; 80:219–227.
- Morley JE. Frailty fantasia. J Am Med Dir Assoc 2017; 18:813–815.
- Munoz-Mendoza CL, Cabanero-Martinez MJ, Millan-Calenti JC, Cabrero-Garcia J, Lopez-Sanchez R, Maseda-Rodriguez A. Reliability of 4-m and 6-m walking speed tests in elderly people with cognitive impairment. Arch Gerontol Geriatr 2011; 52:e67–e70.
- Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging 2009; 13:881–889.
- Sergi G, Veronese N, Fontana L, et al. Pre-frailty and risk of cardiovascular disease in elderly men and women: the Pro.V.A. study. J Am Coll Cardiol 2015; 65:976–983.
- Abellan van Kan G, Rolland Y, Bergman H, Morley JE, Kritchevsky SB, Vellas B. The I.A.N.A Task Force on frailty assessment of older people in clinical practice. J Nutr Health Aging 2008; 12:29–37.
- Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle-aged African Americans. J Nutr Health Aging 2012;16:601–608.
- Forman DE, Arena R, Boxer R, et al; American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Stroke Council. Prioritizing functional capacity as a principal end point for therapies oriented to older adults with cardiovascular disease: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2017; 135:e894–e918.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913.
- Mancia G, Grassi G. Aggressive blood pressure lowering is dangerous: the J-curve: pro side of the argument. Hypertension 2014; 63:29–36.
- Odden MC, Peralta CA, Haan MN, Covinsky KE. Rethinking the association of high blood pressure with mortality in elderly adults: the impact of frailty. Arch Intern Med 2012; 172:1162–1168.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- Warwick J, Falaschetti E, Rockwood K, et al. No evidence that frailty modifies the positive impact of antihypertensive treatment in very elderly people: an investigation of the impact of frailty upon treatment effect in the HYpertension in the Very Elderly Trial (HYVET) study, a double-blind, placebo-controlled study of antihypertensives in people with hypertension aged 80 and over. BMC Med 2015 9;13:78.
- Williamson JD, Supiano MA, Applegate WB, et al; SPRINT Research Group. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥ 75 years: a randomized clinical trial. JAMA 2016; 315:2673–2682.
- Tinetti ME, Han L, Lee DS, et al. Antihypertensive medications and serious fall injuries in a nationally representative sample of older adults. JAMA Intern Med 2014; 174:588–595.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2017. Nov 13 [Epub ahead of print].)
- American Diabetes Association. 11. Older adults. Diabetes Care 2017; 40(suppl 1):S99–S104.
- Mallery LH, Allen M, Fleming I, et al. Promoting higher blood pressure targets for frail older adults: a consensus guideline from Canada. Cleve Clin J Med 2014; 81:427–437.
- Shepherd J, Blauw GJ, Murphy MB, et al; PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002; 360:1623–1630.
- Glynn RJ, Koenig W, Nordestgaard BG, Shepherd J, Ridker PM. Rosuvastatin for primary prevention in older persons with elevated C-reactive protein and low to average low-density lipoprotein cholesterol levels: exploratory analysis of a randomized trial. Ann Intern Med 2010; 152:488–496, W174.
- LaCroix AZ, Gray SL, Aragaki A, et al; Women’s Health Initiative. Statin use and incident frailty in women aged 65 years or older: prospective findings from the Women’s Health Initiative Observational Study. J Gerontol A Biol Sci Med Sci 2008; 63:369–375.
- Odden MC, Pletcher MJ, Coxson PG, et al. Cost-effectiveness and population impact of statins for primary prevention in adults aged 75 years or older in the United States. Ann Intern Med 2015; 162:533–541.
- Kutner JS, Blatchford PJ, Taylor DH Jr, et al. Safety and benefit of discontinuing statin therapy in the setting of advanced, life-limiting illness: a randomized clinical trial. JAMA Intern Med 2015; 175:691–700.
- Huang ES, Liu JY, Moffet HH, John PM, Karter AJ. Glycemic control, complications, and death in older diabetic patients: the diabetes and aging study. Diabetes Care 2011; 34:1329–1336.
- Kirkman MS, Briscoe VJ, Clark N, et al; Consensus Development Conference on Diabetes and Older Adults. Diabetes in older adults: a consensus report. J Am Geriatr Soc 2012; 60:2342–2356.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015; 38:140–149.
- Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ (Clinical research ed) 2002; 324:71–86.
- Antithrombotic Trialists’ (ATT) Collaboration; Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009; 373:1849–1860.
- Ikeda Y, Shimada K, Teramoto T, et al. Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomized clinical trial. JAMA 2014; 312:2510–2520.
- Bibbins-Domingo K; US Preventive Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: US Preventive Services Task Force Recommendation Statement. Ann Intern Med 2016; 164:836–845.
- American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2012; 60:616–631.
- Li L, Geraghty OC, Mehta Z, Rothwell PM. Age-specific risks, severity, time course, and outcome of bleeding on long-term antiplatelet treatment after vascular events: a population-based cohort study. Lancet 2017; 390:490–499.
- Barzilay JI, Blaum C, Moore T, et al. Insulin resistance and inflammation as precursors of frailty: the Cardiovascular Health Study. Arch Intern Med 2007; 167:635–641.
- Fiatarone MA, O’Neill EF, Ryan ND, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med 1994; 330:1769–1775.
- Uusi-Rasi K, Patil R, Karinkanta S, et al. Exercise and vitamin D in fall prevention among older women: a randomized clinical trial. JAMA Intern Med 2015; 175:703–711.
- Hauer K, Schwenk M, Zieschang T, Essig M, Becker C, Oster P. Physical training improves motor performance in people with dementia: a randomized controlled trial. J Am Geriatr Soc 2012; 60:8–15.
- Li F, Harmer P, Fitzgerald K. Implementing an evidence-based fall prevention intervention in community senior centers. Am J Public Health 2016; 106:2026–2031.
- Manor B, Lough M, Gagnon MM, Cupples A, Wayne PM, Lipsitz LA. Functional benefits of tai chi training in senior housing facilities. J Am Geriatr Soc 2014; 62:1484–1489.
- Physical Activity Guidelines Advisory Committee report, 2008. To the Secretary of Health and Human Services. Part A: executive summary. Nutr Rev 2009; 67:114–120.
- Hubbard RE, Searle SD, Mitnitski A, Rockwood K. Effect of smoking on the accumulation of deficits, frailty and survival in older adults: a secondary analysis from the Canadian Study of Health and Aging. J Nutr Health Aging 2009; 13:468–472.
- Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A US Public Health Service report. Am J Prev Med 2008; 35:158–176.
- Samieri C, Sun Q, Townsend MK, et al. The association between dietary patterns at midlife and health in aging: an observational study. Ann Intern Med 2013; 159:584–591.
- Estruch R, Ros E, Martinez-Gonzalez MA. Mediterranean diet for primary prevention of cardiovascular disease. N Engl J Med 2013; 369:676–677.
- Leon-Munoz LM, Guallar-Castillon P, Lopez-Garcia E, Rodriguez-Artalejo F. Mediterranean diet and risk of frailty in community-dwelling older adults. J Am Med Dir Assoc 2014; 15:899–903.
- Doty RL, Shaman P, Applebaum SL, Giberson R, Siksorski L, Rosenberg L. Smell identification ability: changes with age. Science 1984; 226:1441–1443.
- Merel SE, Paauw DS. Common drug side effects and drug-drug interactions in elderly adults in primary care. J Am Geriatr Soc 2017 Mar 21. Epub ahead of print.
- Epstein RM, Peters E. Beyond information: exploring patients’ preferences. JAMA 2009; 302:195–197.
When assessing and attempting to modify the risk of cardiovascular disease in older patients, physicians should consider incorporating the concept of frailty. The balance of risk and benefit may differ considerably for 2 patients of the same age if one is fit and the other is frail. Because the aging population is a diverse group, a one-size-fits-all approach to cardiovascular disease prevention and risk-factor management is not appropriate.
A GROWING, DIVERSE GROUP
The number of older adults with multiple cardiovascular risk factors is increasing as life expectancy improves. US residents who are age 65 today can expect to live to an average age of 84 (men) or 87 (women).1
However, the range of life expectancy for people reaching these advanced ages is wide, and chronologic age is no longer sufficient to determine a patient’s risk profile. Furthermore, the prevalence of cardiovascular disease rises with age, and age itself is the strongest predictor of cardiovascular risk.2
Current risk calculators have not been validated in people over age 80,2 making them inadequate for use in older patients. Age alone cannot identify who will benefit from preventive strategies, except in situations when a dominant disease such as metastatic cancer, end-stage renal disease, end-stage dementia, or end-stage heart failure is expected to lead to mortality within a year. Guidelines for treating common risk factors such as elevated cholesterol3 in the general population have generally not focused on adults over 75 or recognized their diversity in health status.4 In order to generate an individualized prescription for cardiovascular disease prevention for older adults, issues such as frailty, cognitive and functional status, disability, and comorbidity must be considered.
WHAT IS FRAILTY?
Clinicians have recognized frailty for decades, but to date there remains a debate on how to define it.
Clegg et al5 described frailty as “a state of increased vulnerability to poor resolution of homeostasis after a stressor event,”5 a definition generally agreed upon, as frailty predicts both poor health outcomes and death.
Indeed, in a prospective study of 5,317 men and women ranging in age from 65 to 101, those identified as frail at baseline were 6 times more likely to have died 3 years later (mortality rates 18% vs 3%), and the difference persisted at 7 years.6 After adjusting for comorbidities, those identified as frail were also more likely to fall, develop limitations in mobility or activities of daily living, or be hospitalized.
The two current leading theories of frailty were defined by Fried et al6 and by Rockwood and Mitnitski.7
Fried et al6 have operationalized frailty as a “physical phenotype,” defined as 3 or more of the following:
- Unintentional weight loss of 10 pounds in the past year
- Self-reported exhaustion
- Weakness as measured by grip strength
- Slow walking speed
- Decreased physical activity.6
Rockwood and Mitnitski7 define frailty as an accumulation of health-related deficits over time. They recommend that 30 to 40 possible deficits that cover a variety of health systems be included such as cognition, mood, function, and comorbidity. These are added and divided by the total possible number of variables to generate a score between 0 and 1.8
The difficulty in defining frailty has led to varying estimates of its prevalence, ranging from 25% to 50% in adults over 65 who have cardiovascular disease.9
CAUSE AND CONSEQUENCE OF CARDIOVASCULAR DISEASE
Studies have highlighted the bidirectional connection between frailty and cardiovascular disease.10 Frailty may predict cardiovascular disease, while cardiovascular disease is associated with an increased risk of incident frailty.9,11
Frail adults with cardiovascular disease have a higher risk of poor outcomes, even after correcting for age, comorbidities, disability, and disease severity. For example, frailty is associated with a twofold higher mortality rate in individuals with cardiovascular disease.9
A prospective cohort study12 of 3,895 middle-aged men and women demonstrated that those with an elevated cardiovascular risk score were at increased risk of frailty over 10 years (odds ratio [OR] 1.35, 95% confidence interval [CI] 1.21–1.51) and incident cardiovascular events (OR 1.36, 95% CI 1.15–1.61). This suggests that modification of cardiovascular risk factors earlier in life may reduce the risk of subsequently becoming frail.
Biologic mechanisms that may explain the connection between frailty and cardiovascular disease include derangements in inflammatory, hematologic, and endocrine pathways. People who are found to be clinically frail are more likely to have insulin resistance and elevated biomarkers such as C-reactive protein, D-dimer, and factor VIII.13 The inflammatory cytokine interleukin 6 is suggested as a common link between inflammation and thrombosis, perhaps contributing to the connection between cardiovascular disease and frailty. Many of these biomarkers have been linked to the pathophysiologic changes of aging, so-called “inflamm-aging” or immunosenescence, including sarcopenia, osteoporosis, and cardiovascular disease.14
ASSESSING FRAILTY IN THE CLINIC
For adults over age 70, frailty assessment is an important first step in managing cardiovascular disease risk.15 Frailty status will better identify those at risk of adverse outcomes in the short term and those who are most likely to benefit from long-term cardiovascular preventive strategies. Additionally, incorporating frailty assessment into traditional risk factor evaluation may permit appropriate intervention and prevention of a potentially modifiable risk factor.
Gait speed is a quick, easy, inexpensive, and sensitive way to assess frailty status, with excellent inter-rater and test-retest reliability, even in those with cognitive impairment.16 Slow gait speed predicts limitations in mobility, limitations in activities of daily living, and death.8,17
In a prospective study18 of 1,567 men and women, mean age 74, slow gait speed was the strongest predictor of subsequent cardiovascular events.18
Gait speed is usually measured over a distance of 4 meters (13.1 feet),17 and the patient is asked to walk comfortably in an unobstructed, marked area. An assistive walking device can be used if needed. If possible, this is repeated once after a brief recovery period, and the average is recorded.
The FRAIL scale19,20 is a simple, validated questionnaire that combines the Fried and Rockwood concepts of frailty and can be given over the phone or to patients in a waiting room. One point is given for each of the following, and people who have 3 or more are considered frail:
- Fatigue
- Resistance (inability to climb 1 flight of stairs)
- Ambulation (inability to walk 1 block)
- Illnesses (having more than 5)
- Loss of more than 5% of body weight.
Other measures of physical function such as grip strength (using a dynamometer), the Timed Up and Go test (assessing the ability to get up from a chair and walk a short distance), and Short Physical Performance Battery (assessing balance, chair stands, and walking speed) can be used to screen for frailty, but are more time-intensive than gait speed alone, and so are not always practical to use in a busy clinic.21
MANAGEMENT OF RISK FACTORS
Management of cardiovascular risk factors is best individualized as outlined below.
LOWERING HIGH BLOOD PRESSURE
The incidence of ischemic heart disease and stroke increases with age across all levels of elevated systolic and diastolic blood pressure.22 Hypertension is also associated with increased risk of cognitive decline. However, a J-shaped relationship has been observed in older adults, with increased cardiovascular events for both low and elevated blood pressure, although the clinical relevance remains controversial.23
Odden et al24 performed an observational study and found that high blood pressure was associated with an increased mortality rate in older adults with normal gait speed, while in those with slow gait speed, high blood pressure neither harmed nor helped. Those who could not walk 6 meters appeared to benefit from higher blood pressure.
HYVET (the Hypertension in the Very Elderly Trial),25 a randomized controlled trial in 3,845 community-dwelling people age 80 or older with sustained systolic blood pressure higher than 160 mm Hg, found a significant reduction in rates of stroke and all-cause mortality (relative risk [RR] 0.76, P = .007) in the treatment arm using indapamide with perindopril if necessary to reach a target blood pressure of 150/80 mm Hg.
Frailty was not assessed during the trial; however, in a reanalysis, the results did not change in those identified as frail using a Rockwood frailty index (a count of health-related deficits accumulated over the lifespan).26
SPRINT (the Systolic Blood Pressure Intervention Trial)27 randomized participants age 50 and older with systolic blood pressure of 130 to 180 mm Hg and at increased risk of cardiovascular disease to intensive treatment (goal systolic blood pressure ≤ 120 mm Hg) or standard treatment (goal systolic blood pressure ≤ 140 mm Hg). In a prespecified subgroup of 2,636 participants over age 75 (mean age 80), hazard ratios and 95% confidence intervals for adverse outcomes with intensive treatment were:
- Major cardiovascular events: HR 0.66, 95% CI 0.51–0.85
- Death: HR 0.67, 95% CI 0.49–0.91.
Over 3 years of treatment this translated into a number needed to treat of 27 to prevent 1 cardiovascular event and 41 to prevent 1 death.
Within this subgroup, the benefit was similar regardless of level of frailty (measured both by a Rockwood frailty index and by gait speed).
However, the incidence of serious adverse treatment effects such as hypotension, orthostasis, electrolyte abnormalities, and acute kidney injury was higher with intensive treatment in the frail group. Although the difference was not statistically significant, it is cause for caution. Further, the exclusion criteria (history of diabetes, heart failure, dementia, stroke, weight loss of > 10%, nursing home residence) make it difficult to generalize the SPRINT findings to the general aging population.27
Tinetti et al28 performed an observational study using a nationally representative sample of older adults. They found that receiving any antihypertensive therapy was associated with an increased risk of falls with serious adverse outcomes. The risks of adverse events related to antihypertensive therapy increased with age.
Recommendations on hypertension
Managing hypertension in frail patients at risk of cardiovascular disease requires balancing the benefits vs the risks of treatment, such as polypharmacy, falls, and orthostatic hypotension.
The Eighth Joint National Committee suggests a blood pressure goal of less than 150/90 mm Hg for all adults over age 60, and less than 140/90 mm Hg for those with a history of cardiovascular disease or diabetes.29
The American College of Cardiology/American Heart Association (ACC/AHA) guidelines on hypertension, recently released, recommend a new blood pressure target of <120/<80 as normal, with 120–129/<80 considered elevated, 130–139/80–89 stage 1 hypertension, and ≥140/≥90 as stage 2 hypertension.30 An important caveat to these guidelines is the recommendation to measure blood pressure accurately and with accurate technique, which is often not possible in many busy clinics. These guidelines are intended to apply to older adults as well, with a note that those with multiple morbidities and limited life expectancy will benefit from a shared decision that incorporates patient preferences and clinical judgment. Little guidance is given on how to incorporate frailty, although note is made that older adults who reside in assisted living facilities and nursing homes have not been represented in randomized controlled trials.30
American Diabetes Association guidelines on hypertension in patients with diabetes recommend considering functional status, frailty, and life expectancy to decide on a blood pressure goal of either 140/90 mm Hg (if fit) or 150/90 mm Hg (if frail). They do not specify how to diagnose frailty.31
Canadian guidelines say that in those with advanced frailty (ie, entirely dependent for personal care and activities of daily living) and short life expectancy (months), it is reasonable to liberalize the systolic blood pressure goal to 160 to 190 mm Hg.32
Our recommendations. In both frail and nonfrail individuals without a limited life expectancy, it is reasonable to aim for a blood pressure of at least less than 140/90 mm Hg. For those at increased risk of cardiovascular disease and able to tolerate treatment, careful lowering to 130/80 mm Hg may be considered, with close attention to side effects.
Treatment should start with the lowest possible dose, be titrated slowly, and may need to be tailored to standing blood pressure to avoid orthostatic hypotension.
Home blood pressure measurements may be beneficial in monitoring treatment.
MANAGING LIPIDS
For those over age 75, data on efficacy of statins are mixed due to the small number of older adults enrolled in randomized controlled trials of these drugs. To our knowledge, no statin trial has examined the role of frailty.
The PROSPER trial (Prospective Study of Pravastatin in the Elderly at Risk)33 randomized 5,804 patients ages 70 to 82 to receive either pravastatin or placebo. Overall, the incidence of a composite end point of major cardiovascular events was 15% lower with active treatment (P = .014). However, the mean age was 75, which does little to address the paucity of evidence for those over age 75; follow-up time was only 3 years, and subgroup analysis did not show benefit in those who did not have a history of cardiovascular disease or in women.
The JUPITER trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin)34 randomized 5,695 people over age 70 without cardiovascular disease to receive either rosuvastatin or placebo. Exploratory analysis showed a significant 39% reduction in all-cause mortality and major cardiovascular events with active treatment (HR 0.61, 95% CI 0.46–0.82). Over 5 years of treatment, this translates to a number needed to treat of 19 to prevent 1 major cardiovascular event and 29 to prevent 1 cardiovascular death.
The benefit of statins for primary prevention in these trials began to be apparent 2 years after treatment was initiated.
The Women’s Health Initiative,35 an observational study, found no difference in incident frailty in women older than 65 taking statins for 3 years compared with those who did not take statins
Odden et al36 found that although statin use is generally well tolerated, the risks of statin-associated functional and cognitive decline may outweigh the benefits in those older than 75. The ongoing Statin in Reducing Events in the Elderly (STAREE) trial may shed light on this issue.
Recommendations on lipid management
The ACC/AHA,3 in their 2013 guidelines, do not recommend routine statin treatment for primary prevention in those over age 75, given a lack of evidence from randomized controlled trials. For secondary prevention, ie, for those who have a history of atherosclerotic cardiovascular disease, they recommend moderate-intensity statin therapy in this age group.
Our recommendations. For patients over age 75 without cardiovascular disease or frailty and with a life expectancy of at least 2 years, consider offering a statin for primary prevention of cardiovascular disease as part of shared decision-making.
In those with known cardiovascular disease, it is reasonable to continue statin therapy except in situations where the life expectancy is less than 6 months.37
Although moderate- or high-intensity statin therapy is recommended in current guidelines, for many older adults it is prudent to consider the lowest tolerable dose to improve adherence, with close monitoring for side effects such as myalgia and weakness.
TYPE 2 DIABETES
Evidence suggests that tight glycemic control in type 2 diabetes is harmful for adults ages 55 to 79 and does not provide clear benefits for cardiovascular risk reduction, and controlling hemoglobin A1c to less than 6.0% is associated with increased mortality in older adults.38
The American Diabetes Association31 and the American Geriatrics Society39 recommend hemoglobin A1c goals of:
- 7.5% or less for older adults with 3 or more coexisting chronic illnesses requiring medical intervention (eg, arthritis, hypertension, and heart failure) and with intact cognition and function
- 8.0% or less for those identified as frail, or with multiple chronic illnesses or moderate cognitive or functional impairment
- 8.5% or 9.0% or less for those with very complex comorbidities, in long-term care, or with end-stage chronic illnesses (eg, end-stage heart failure), or with moderate to severe cognitive or functional limitation.
These guidelines do not endorse a specific frailty assessment, although the references allude to the Fried phenotype criteria, which include gait speed. An update from the American Diabetes Association provides a patient-centered approach to tailoring treatment regimens, taking into consideration the risk of hypoglycemia for each class of drugs, side effects, and cost.40
Our recommendations. Hyperglycemia remains a risk factor for cardiovascular disease in older adults and increases the risk of many geriatric conditions including delirium, dementia, frailty, and functional decline. The goal in individualizing hemoglobin A1c goals should be to avoid both hyper- and hypoglycemia.
Sulfonylureas and insulins should be used with caution, as they have the highest associated incidence of hypoglycemia of the diabetes medications.
ASPIRIN
For secondary prevention in older adults with a history of cardiovascular disease, pooled trials have consistently demonstrated a long-term benefit for aspirin use that exceeds bleeding risks, although age and frailty status were not considered.41
Aspirin for primary prevention?
The evidence for aspirin for primary prevention in older adults is mixed. Meta-analysis suggests a modest decrease in risk of nonfatal myocardial infarction but no appreciable effects on nonfatal stroke and cardiovascular death.42
The Japanese Primary Prevention Project,43 a randomized trial of low-dose aspirin for primary prevention of cardiovascular disease in adults ages 60 to 85, showed no reduction in major cardiovascular events. However, the event rate was lower than expected, the crossover rates were high, the incidence of hemorrhagic strokes was higher than in Western studies, and the trial may have been underpowered to detect the benefits of aspirin.
The US Preventive Services Task Force44 in 2016 noted that among individuals with a 10-year cardiovascular disease risk of 10% or higher based on the ACC/AHA pooled cohort equation,3 the greatest benefit of aspirin was in those ages 50 to 59. In this age group, 225 nonfatal myocardial infarctions and 84 nonfatal strokes were prevented per 10,000 men treated, with a net gain of 333 life-years. Similar findings were noted in women.
However, in those ages 60 to 69, the risks of harm begin to rise and the benefit of starting daily aspirin necessitates individualized clinical decision-making, with particular attention to bleeding risk and life expectancy.44
In those age 70 and older, data on benefit and harm are mixed. The bleeding risk of aspirin increases with age, predominantly due to gastrointestinal bleeding.44
The ongoing Aspirin in Reducing Events in Elderly trial will add to the evidence.
Aspirin recommendations for primary prevention
The American Geriatrics Society Beers Criteria do not routinely recommend aspirin use for primary prevention in those over age 80, even in those with diabetes.45
Our recommendations. In adults over age 75 who are not frail but are identified as being at moderate to high risk of cardiovascular disease using either the ACC/AHA calculator or any other risk estimator, and without a limited life expectancy, we believe it is reasonable to consider low-dose aspirin (75–100 mg daily) for primary prevention. However, there must be careful consideration particularly for those at risk of major bleeding. One approach to consider would be the addition of a proton pump inhibitor along with aspirin, though this requires further study.46
For those who have been on aspirin for primary prevention and are now older than age 80 without an adverse bleeding event, it is reasonable to stop aspirin, although risks and benefits of discontinuing aspirin should be discussed with the patient as part of shared decision-making.
In frail individuals the risks of aspirin therapy likely outweigh any benefit for primary prevention, and aspirin cannot be routinely recommended.
EXERCISE AND WEIGHT MANAGEMENT
A low body mass index is often associated with frailty, and weight loss may be a marker of underlying illness, which increases the risk of poor outcomes. However, those with an elevated body mass index and increased adiposity are in fact more likely to be frail (using the Fried physical phenotype definition) than those with a low body mass index,47 due in part to unrecognized sarcopenic obesity, ie, replacement of lean muscle with fat.
Physical activity is currently the only intervention known to improve frailty.5
Physical activity and a balanced diet are just as important in older adults, including those with reduced functional ability and multiple comorbid conditions, as in younger individuals.
A trial in frail long-term care residents (mean age 87) found that high-intensity resistance training improved muscle strength and mobility.48 The addition of a nutritional supplement with or without exercise did not affect frailty status. In community-dwelling older adults, physical activity has also been shown to improve sarcopenia and reduce falls and hip fractures.49
Progressive resistance training has been shown to improve strength and gait speed even in those with dementia.50
Tai chi has shown promising results in reducing falls and improving balance and function in both community-dwelling older adults and those in assisted living.51,52
Exercise recommendations
The US Department of Health and Human Services53 issued physical activity guidelines in 2008 with specific recommendations for older adults that include flexibility and balance training, which have been shown to reduce falls, in addition to aerobic activities and strength training.
Our recommendations. For all older adults, particularly those who are frail, we recommend a regimen of general daily activity, balance training such as tai chi, moderate-intensity aerobics such as cycling, resistance training such as using light weights, and stretching. Sessions lasting as little as 10 minutes are beneficial.
Gait speed can be monitored in the clinic to assess improvement in function over time.
SMOKING CESSATION
Although rates of smoking are decreasing, smoking remains one of the most important cardiovascular risk factors. Smoking has been associated with increased risk of frailty and significantly increased risk of death compared with never smoking.54 Smoking cessation is beneficial even for those who quit later in life.
The US Department of Health and Human Services in 2008 released an update on tobacco use and dependence,55 with specific attention to the benefit of smoking cessation for older adults.
All counseling interventions have been shown to be effective in older adults, as has nicotine replacement. Newer medications such as varenicline should be used with caution, as the risk of side effects is higher in older patients.
NUTRITION
Samieri et al,56 in an observational study of 10,670 nurses, found that those adhering to Mediterranean-style diets during midlife had 46% increased odds of healthy aging.
The PREDIMED study (Primary Prevention of Cardiovascular Disease With a Mediterranean Diet)57 in adults ages 55 to 80 showed the Mediterranean diet supplemented with olive oil and nuts reduced the incidence of major cardiovascular disease.
Leon-Munoz et al.58 A prospective study of 1,815 community-dwelling older adults followed for 3.5 years in Spain demonstrated that adhering to a Mediterranean diet was associated with a lower incidence of frailty (P = .002) and a lower risk of slow gait speed (OR 0.53, 95% CI 0.35–0.79). Interestingly, this study also found a protective association between fish and fruit consumption and frailty.
Our recommendations. A well-balanced, diverse diet rich in whole grains, fruits, vegetables, nuts, fish, and healthy fats (polyunsaturated fatty acids), with a moderate amount of lean meats, is recommended to prevent heart disease. However, poor dental health may limit the ability of older individuals to adhere to such diets, and modifications may be needed. Additionally, age-related changes in taste and smell may contribute to poor nutrition and unintended weight loss.59 Involving a nutritionist and social worker in the patient care team should be considered especially as poor nutrition may be a sign of cognitive impairment, functional decline, and frailty.
SPECIAL CONSIDERATIONS
Special considerations when managing cardiovascular risk in the older adult include polypharmacy, multimorbidity, quality of life, and the patient’s personal preferences.
Polypharmacy, defined as taking more than 5 medications, is associated with an increased risk of adverse drug events, falls, fractures, decreased adherence, and “prescribing cascade”— prescribing more drugs to treat side effects of the first drug (eg, adding hypertensive medications to treat hypertension induced by nonsteroidal anti-inflammatory drugs).60 This is particularly important when considering adding additional medications. If a statin will be the 20th pill, it may be less beneficial and more likely to lead to additional adverse effects than if it is the fifth medication.
Patient preferences are critically important, particularly when adding or removing medications. Interventions should include a detailed medication review for appropriate prescribing and deprescribing, referral to a pharmacist, and engaging the patient’s support system.
Multimorbidity. Many older individuals have multiple chronic illnesses. The interaction of multiple conditions must be considered in creating a comprehensive plan, including prognosis, patient preference, available evidence, treatment interactions, and risks and benefits.
Quality of life. Outlook on life and choices made regarding prolongation vs quality of life may be different for the older patient than the younger patient.
Personal preferences. Although interventions such as high-intensity statins for a robust 85-year-old may be appropriate, the individual can choose to forgo any treatment. It is important to explore the patient’s goals of care and advanced directives as part of shared decision-making when building a patient-centered prevention plan.61
ONE SIZE DOES NOT FIT ALL
The heterogeneity of aging rules out a one-size-fits-all recommendation for cardiovascular disease prevention and management of cardiovascular risk factors in older adults.
There is significant overlap between cardiovascular risk status and frailty.
Incorporating frailty into the creation of a cardiovascular risk prescription can aid in the development of an individualized care plan for the prevention of cardiovascular disease in the aging population.
When assessing and attempting to modify the risk of cardiovascular disease in older patients, physicians should consider incorporating the concept of frailty. The balance of risk and benefit may differ considerably for 2 patients of the same age if one is fit and the other is frail. Because the aging population is a diverse group, a one-size-fits-all approach to cardiovascular disease prevention and risk-factor management is not appropriate.
A GROWING, DIVERSE GROUP
The number of older adults with multiple cardiovascular risk factors is increasing as life expectancy improves. US residents who are age 65 today can expect to live to an average age of 84 (men) or 87 (women).1
However, the range of life expectancy for people reaching these advanced ages is wide, and chronologic age is no longer sufficient to determine a patient’s risk profile. Furthermore, the prevalence of cardiovascular disease rises with age, and age itself is the strongest predictor of cardiovascular risk.2
Current risk calculators have not been validated in people over age 80,2 making them inadequate for use in older patients. Age alone cannot identify who will benefit from preventive strategies, except in situations when a dominant disease such as metastatic cancer, end-stage renal disease, end-stage dementia, or end-stage heart failure is expected to lead to mortality within a year. Guidelines for treating common risk factors such as elevated cholesterol3 in the general population have generally not focused on adults over 75 or recognized their diversity in health status.4 In order to generate an individualized prescription for cardiovascular disease prevention for older adults, issues such as frailty, cognitive and functional status, disability, and comorbidity must be considered.
WHAT IS FRAILTY?
Clinicians have recognized frailty for decades, but to date there remains a debate on how to define it.
Clegg et al5 described frailty as “a state of increased vulnerability to poor resolution of homeostasis after a stressor event,”5 a definition generally agreed upon, as frailty predicts both poor health outcomes and death.
Indeed, in a prospective study of 5,317 men and women ranging in age from 65 to 101, those identified as frail at baseline were 6 times more likely to have died 3 years later (mortality rates 18% vs 3%), and the difference persisted at 7 years.6 After adjusting for comorbidities, those identified as frail were also more likely to fall, develop limitations in mobility or activities of daily living, or be hospitalized.
The two current leading theories of frailty were defined by Fried et al6 and by Rockwood and Mitnitski.7
Fried et al6 have operationalized frailty as a “physical phenotype,” defined as 3 or more of the following:
- Unintentional weight loss of 10 pounds in the past year
- Self-reported exhaustion
- Weakness as measured by grip strength
- Slow walking speed
- Decreased physical activity.6
Rockwood and Mitnitski7 define frailty as an accumulation of health-related deficits over time. They recommend that 30 to 40 possible deficits that cover a variety of health systems be included such as cognition, mood, function, and comorbidity. These are added and divided by the total possible number of variables to generate a score between 0 and 1.8
The difficulty in defining frailty has led to varying estimates of its prevalence, ranging from 25% to 50% in adults over 65 who have cardiovascular disease.9
CAUSE AND CONSEQUENCE OF CARDIOVASCULAR DISEASE
Studies have highlighted the bidirectional connection between frailty and cardiovascular disease.10 Frailty may predict cardiovascular disease, while cardiovascular disease is associated with an increased risk of incident frailty.9,11
Frail adults with cardiovascular disease have a higher risk of poor outcomes, even after correcting for age, comorbidities, disability, and disease severity. For example, frailty is associated with a twofold higher mortality rate in individuals with cardiovascular disease.9
A prospective cohort study12 of 3,895 middle-aged men and women demonstrated that those with an elevated cardiovascular risk score were at increased risk of frailty over 10 years (odds ratio [OR] 1.35, 95% confidence interval [CI] 1.21–1.51) and incident cardiovascular events (OR 1.36, 95% CI 1.15–1.61). This suggests that modification of cardiovascular risk factors earlier in life may reduce the risk of subsequently becoming frail.
Biologic mechanisms that may explain the connection between frailty and cardiovascular disease include derangements in inflammatory, hematologic, and endocrine pathways. People who are found to be clinically frail are more likely to have insulin resistance and elevated biomarkers such as C-reactive protein, D-dimer, and factor VIII.13 The inflammatory cytokine interleukin 6 is suggested as a common link between inflammation and thrombosis, perhaps contributing to the connection between cardiovascular disease and frailty. Many of these biomarkers have been linked to the pathophysiologic changes of aging, so-called “inflamm-aging” or immunosenescence, including sarcopenia, osteoporosis, and cardiovascular disease.14
ASSESSING FRAILTY IN THE CLINIC
For adults over age 70, frailty assessment is an important first step in managing cardiovascular disease risk.15 Frailty status will better identify those at risk of adverse outcomes in the short term and those who are most likely to benefit from long-term cardiovascular preventive strategies. Additionally, incorporating frailty assessment into traditional risk factor evaluation may permit appropriate intervention and prevention of a potentially modifiable risk factor.
Gait speed is a quick, easy, inexpensive, and sensitive way to assess frailty status, with excellent inter-rater and test-retest reliability, even in those with cognitive impairment.16 Slow gait speed predicts limitations in mobility, limitations in activities of daily living, and death.8,17
In a prospective study18 of 1,567 men and women, mean age 74, slow gait speed was the strongest predictor of subsequent cardiovascular events.18
Gait speed is usually measured over a distance of 4 meters (13.1 feet),17 and the patient is asked to walk comfortably in an unobstructed, marked area. An assistive walking device can be used if needed. If possible, this is repeated once after a brief recovery period, and the average is recorded.
The FRAIL scale19,20 is a simple, validated questionnaire that combines the Fried and Rockwood concepts of frailty and can be given over the phone or to patients in a waiting room. One point is given for each of the following, and people who have 3 or more are considered frail:
- Fatigue
- Resistance (inability to climb 1 flight of stairs)
- Ambulation (inability to walk 1 block)
- Illnesses (having more than 5)
- Loss of more than 5% of body weight.
Other measures of physical function such as grip strength (using a dynamometer), the Timed Up and Go test (assessing the ability to get up from a chair and walk a short distance), and Short Physical Performance Battery (assessing balance, chair stands, and walking speed) can be used to screen for frailty, but are more time-intensive than gait speed alone, and so are not always practical to use in a busy clinic.21
MANAGEMENT OF RISK FACTORS
Management of cardiovascular risk factors is best individualized as outlined below.
LOWERING HIGH BLOOD PRESSURE
The incidence of ischemic heart disease and stroke increases with age across all levels of elevated systolic and diastolic blood pressure.22 Hypertension is also associated with increased risk of cognitive decline. However, a J-shaped relationship has been observed in older adults, with increased cardiovascular events for both low and elevated blood pressure, although the clinical relevance remains controversial.23
Odden et al24 performed an observational study and found that high blood pressure was associated with an increased mortality rate in older adults with normal gait speed, while in those with slow gait speed, high blood pressure neither harmed nor helped. Those who could not walk 6 meters appeared to benefit from higher blood pressure.
HYVET (the Hypertension in the Very Elderly Trial),25 a randomized controlled trial in 3,845 community-dwelling people age 80 or older with sustained systolic blood pressure higher than 160 mm Hg, found a significant reduction in rates of stroke and all-cause mortality (relative risk [RR] 0.76, P = .007) in the treatment arm using indapamide with perindopril if necessary to reach a target blood pressure of 150/80 mm Hg.
Frailty was not assessed during the trial; however, in a reanalysis, the results did not change in those identified as frail using a Rockwood frailty index (a count of health-related deficits accumulated over the lifespan).26
SPRINT (the Systolic Blood Pressure Intervention Trial)27 randomized participants age 50 and older with systolic blood pressure of 130 to 180 mm Hg and at increased risk of cardiovascular disease to intensive treatment (goal systolic blood pressure ≤ 120 mm Hg) or standard treatment (goal systolic blood pressure ≤ 140 mm Hg). In a prespecified subgroup of 2,636 participants over age 75 (mean age 80), hazard ratios and 95% confidence intervals for adverse outcomes with intensive treatment were:
- Major cardiovascular events: HR 0.66, 95% CI 0.51–0.85
- Death: HR 0.67, 95% CI 0.49–0.91.
Over 3 years of treatment this translated into a number needed to treat of 27 to prevent 1 cardiovascular event and 41 to prevent 1 death.
Within this subgroup, the benefit was similar regardless of level of frailty (measured both by a Rockwood frailty index and by gait speed).
However, the incidence of serious adverse treatment effects such as hypotension, orthostasis, electrolyte abnormalities, and acute kidney injury was higher with intensive treatment in the frail group. Although the difference was not statistically significant, it is cause for caution. Further, the exclusion criteria (history of diabetes, heart failure, dementia, stroke, weight loss of > 10%, nursing home residence) make it difficult to generalize the SPRINT findings to the general aging population.27
Tinetti et al28 performed an observational study using a nationally representative sample of older adults. They found that receiving any antihypertensive therapy was associated with an increased risk of falls with serious adverse outcomes. The risks of adverse events related to antihypertensive therapy increased with age.
Recommendations on hypertension
Managing hypertension in frail patients at risk of cardiovascular disease requires balancing the benefits vs the risks of treatment, such as polypharmacy, falls, and orthostatic hypotension.
The Eighth Joint National Committee suggests a blood pressure goal of less than 150/90 mm Hg for all adults over age 60, and less than 140/90 mm Hg for those with a history of cardiovascular disease or diabetes.29
The American College of Cardiology/American Heart Association (ACC/AHA) guidelines on hypertension, recently released, recommend a new blood pressure target of <120/<80 as normal, with 120–129/<80 considered elevated, 130–139/80–89 stage 1 hypertension, and ≥140/≥90 as stage 2 hypertension.30 An important caveat to these guidelines is the recommendation to measure blood pressure accurately and with accurate technique, which is often not possible in many busy clinics. These guidelines are intended to apply to older adults as well, with a note that those with multiple morbidities and limited life expectancy will benefit from a shared decision that incorporates patient preferences and clinical judgment. Little guidance is given on how to incorporate frailty, although note is made that older adults who reside in assisted living facilities and nursing homes have not been represented in randomized controlled trials.30
American Diabetes Association guidelines on hypertension in patients with diabetes recommend considering functional status, frailty, and life expectancy to decide on a blood pressure goal of either 140/90 mm Hg (if fit) or 150/90 mm Hg (if frail). They do not specify how to diagnose frailty.31
Canadian guidelines say that in those with advanced frailty (ie, entirely dependent for personal care and activities of daily living) and short life expectancy (months), it is reasonable to liberalize the systolic blood pressure goal to 160 to 190 mm Hg.32
Our recommendations. In both frail and nonfrail individuals without a limited life expectancy, it is reasonable to aim for a blood pressure of at least less than 140/90 mm Hg. For those at increased risk of cardiovascular disease and able to tolerate treatment, careful lowering to 130/80 mm Hg may be considered, with close attention to side effects.
Treatment should start with the lowest possible dose, be titrated slowly, and may need to be tailored to standing blood pressure to avoid orthostatic hypotension.
Home blood pressure measurements may be beneficial in monitoring treatment.
MANAGING LIPIDS
For those over age 75, data on efficacy of statins are mixed due to the small number of older adults enrolled in randomized controlled trials of these drugs. To our knowledge, no statin trial has examined the role of frailty.
The PROSPER trial (Prospective Study of Pravastatin in the Elderly at Risk)33 randomized 5,804 patients ages 70 to 82 to receive either pravastatin or placebo. Overall, the incidence of a composite end point of major cardiovascular events was 15% lower with active treatment (P = .014). However, the mean age was 75, which does little to address the paucity of evidence for those over age 75; follow-up time was only 3 years, and subgroup analysis did not show benefit in those who did not have a history of cardiovascular disease or in women.
The JUPITER trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin)34 randomized 5,695 people over age 70 without cardiovascular disease to receive either rosuvastatin or placebo. Exploratory analysis showed a significant 39% reduction in all-cause mortality and major cardiovascular events with active treatment (HR 0.61, 95% CI 0.46–0.82). Over 5 years of treatment, this translates to a number needed to treat of 19 to prevent 1 major cardiovascular event and 29 to prevent 1 cardiovascular death.
The benefit of statins for primary prevention in these trials began to be apparent 2 years after treatment was initiated.
The Women’s Health Initiative,35 an observational study, found no difference in incident frailty in women older than 65 taking statins for 3 years compared with those who did not take statins
Odden et al36 found that although statin use is generally well tolerated, the risks of statin-associated functional and cognitive decline may outweigh the benefits in those older than 75. The ongoing Statin in Reducing Events in the Elderly (STAREE) trial may shed light on this issue.
Recommendations on lipid management
The ACC/AHA,3 in their 2013 guidelines, do not recommend routine statin treatment for primary prevention in those over age 75, given a lack of evidence from randomized controlled trials. For secondary prevention, ie, for those who have a history of atherosclerotic cardiovascular disease, they recommend moderate-intensity statin therapy in this age group.
Our recommendations. For patients over age 75 without cardiovascular disease or frailty and with a life expectancy of at least 2 years, consider offering a statin for primary prevention of cardiovascular disease as part of shared decision-making.
In those with known cardiovascular disease, it is reasonable to continue statin therapy except in situations where the life expectancy is less than 6 months.37
Although moderate- or high-intensity statin therapy is recommended in current guidelines, for many older adults it is prudent to consider the lowest tolerable dose to improve adherence, with close monitoring for side effects such as myalgia and weakness.
TYPE 2 DIABETES
Evidence suggests that tight glycemic control in type 2 diabetes is harmful for adults ages 55 to 79 and does not provide clear benefits for cardiovascular risk reduction, and controlling hemoglobin A1c to less than 6.0% is associated with increased mortality in older adults.38
The American Diabetes Association31 and the American Geriatrics Society39 recommend hemoglobin A1c goals of:
- 7.5% or less for older adults with 3 or more coexisting chronic illnesses requiring medical intervention (eg, arthritis, hypertension, and heart failure) and with intact cognition and function
- 8.0% or less for those identified as frail, or with multiple chronic illnesses or moderate cognitive or functional impairment
- 8.5% or 9.0% or less for those with very complex comorbidities, in long-term care, or with end-stage chronic illnesses (eg, end-stage heart failure), or with moderate to severe cognitive or functional limitation.
These guidelines do not endorse a specific frailty assessment, although the references allude to the Fried phenotype criteria, which include gait speed. An update from the American Diabetes Association provides a patient-centered approach to tailoring treatment regimens, taking into consideration the risk of hypoglycemia for each class of drugs, side effects, and cost.40
Our recommendations. Hyperglycemia remains a risk factor for cardiovascular disease in older adults and increases the risk of many geriatric conditions including delirium, dementia, frailty, and functional decline. The goal in individualizing hemoglobin A1c goals should be to avoid both hyper- and hypoglycemia.
Sulfonylureas and insulins should be used with caution, as they have the highest associated incidence of hypoglycemia of the diabetes medications.
ASPIRIN
For secondary prevention in older adults with a history of cardiovascular disease, pooled trials have consistently demonstrated a long-term benefit for aspirin use that exceeds bleeding risks, although age and frailty status were not considered.41
Aspirin for primary prevention?
The evidence for aspirin for primary prevention in older adults is mixed. Meta-analysis suggests a modest decrease in risk of nonfatal myocardial infarction but no appreciable effects on nonfatal stroke and cardiovascular death.42
The Japanese Primary Prevention Project,43 a randomized trial of low-dose aspirin for primary prevention of cardiovascular disease in adults ages 60 to 85, showed no reduction in major cardiovascular events. However, the event rate was lower than expected, the crossover rates were high, the incidence of hemorrhagic strokes was higher than in Western studies, and the trial may have been underpowered to detect the benefits of aspirin.
The US Preventive Services Task Force44 in 2016 noted that among individuals with a 10-year cardiovascular disease risk of 10% or higher based on the ACC/AHA pooled cohort equation,3 the greatest benefit of aspirin was in those ages 50 to 59. In this age group, 225 nonfatal myocardial infarctions and 84 nonfatal strokes were prevented per 10,000 men treated, with a net gain of 333 life-years. Similar findings were noted in women.
However, in those ages 60 to 69, the risks of harm begin to rise and the benefit of starting daily aspirin necessitates individualized clinical decision-making, with particular attention to bleeding risk and life expectancy.44
In those age 70 and older, data on benefit and harm are mixed. The bleeding risk of aspirin increases with age, predominantly due to gastrointestinal bleeding.44
The ongoing Aspirin in Reducing Events in Elderly trial will add to the evidence.
Aspirin recommendations for primary prevention
The American Geriatrics Society Beers Criteria do not routinely recommend aspirin use for primary prevention in those over age 80, even in those with diabetes.45
Our recommendations. In adults over age 75 who are not frail but are identified as being at moderate to high risk of cardiovascular disease using either the ACC/AHA calculator or any other risk estimator, and without a limited life expectancy, we believe it is reasonable to consider low-dose aspirin (75–100 mg daily) for primary prevention. However, there must be careful consideration particularly for those at risk of major bleeding. One approach to consider would be the addition of a proton pump inhibitor along with aspirin, though this requires further study.46
For those who have been on aspirin for primary prevention and are now older than age 80 without an adverse bleeding event, it is reasonable to stop aspirin, although risks and benefits of discontinuing aspirin should be discussed with the patient as part of shared decision-making.
In frail individuals the risks of aspirin therapy likely outweigh any benefit for primary prevention, and aspirin cannot be routinely recommended.
EXERCISE AND WEIGHT MANAGEMENT
A low body mass index is often associated with frailty, and weight loss may be a marker of underlying illness, which increases the risk of poor outcomes. However, those with an elevated body mass index and increased adiposity are in fact more likely to be frail (using the Fried physical phenotype definition) than those with a low body mass index,47 due in part to unrecognized sarcopenic obesity, ie, replacement of lean muscle with fat.
Physical activity is currently the only intervention known to improve frailty.5
Physical activity and a balanced diet are just as important in older adults, including those with reduced functional ability and multiple comorbid conditions, as in younger individuals.
A trial in frail long-term care residents (mean age 87) found that high-intensity resistance training improved muscle strength and mobility.48 The addition of a nutritional supplement with or without exercise did not affect frailty status. In community-dwelling older adults, physical activity has also been shown to improve sarcopenia and reduce falls and hip fractures.49
Progressive resistance training has been shown to improve strength and gait speed even in those with dementia.50
Tai chi has shown promising results in reducing falls and improving balance and function in both community-dwelling older adults and those in assisted living.51,52
Exercise recommendations
The US Department of Health and Human Services53 issued physical activity guidelines in 2008 with specific recommendations for older adults that include flexibility and balance training, which have been shown to reduce falls, in addition to aerobic activities and strength training.
Our recommendations. For all older adults, particularly those who are frail, we recommend a regimen of general daily activity, balance training such as tai chi, moderate-intensity aerobics such as cycling, resistance training such as using light weights, and stretching. Sessions lasting as little as 10 minutes are beneficial.
Gait speed can be monitored in the clinic to assess improvement in function over time.
SMOKING CESSATION
Although rates of smoking are decreasing, smoking remains one of the most important cardiovascular risk factors. Smoking has been associated with increased risk of frailty and significantly increased risk of death compared with never smoking.54 Smoking cessation is beneficial even for those who quit later in life.
The US Department of Health and Human Services in 2008 released an update on tobacco use and dependence,55 with specific attention to the benefit of smoking cessation for older adults.
All counseling interventions have been shown to be effective in older adults, as has nicotine replacement. Newer medications such as varenicline should be used with caution, as the risk of side effects is higher in older patients.
NUTRITION
Samieri et al,56 in an observational study of 10,670 nurses, found that those adhering to Mediterranean-style diets during midlife had 46% increased odds of healthy aging.
The PREDIMED study (Primary Prevention of Cardiovascular Disease With a Mediterranean Diet)57 in adults ages 55 to 80 showed the Mediterranean diet supplemented with olive oil and nuts reduced the incidence of major cardiovascular disease.
Leon-Munoz et al.58 A prospective study of 1,815 community-dwelling older adults followed for 3.5 years in Spain demonstrated that adhering to a Mediterranean diet was associated with a lower incidence of frailty (P = .002) and a lower risk of slow gait speed (OR 0.53, 95% CI 0.35–0.79). Interestingly, this study also found a protective association between fish and fruit consumption and frailty.
Our recommendations. A well-balanced, diverse diet rich in whole grains, fruits, vegetables, nuts, fish, and healthy fats (polyunsaturated fatty acids), with a moderate amount of lean meats, is recommended to prevent heart disease. However, poor dental health may limit the ability of older individuals to adhere to such diets, and modifications may be needed. Additionally, age-related changes in taste and smell may contribute to poor nutrition and unintended weight loss.59 Involving a nutritionist and social worker in the patient care team should be considered especially as poor nutrition may be a sign of cognitive impairment, functional decline, and frailty.
SPECIAL CONSIDERATIONS
Special considerations when managing cardiovascular risk in the older adult include polypharmacy, multimorbidity, quality of life, and the patient’s personal preferences.
Polypharmacy, defined as taking more than 5 medications, is associated with an increased risk of adverse drug events, falls, fractures, decreased adherence, and “prescribing cascade”— prescribing more drugs to treat side effects of the first drug (eg, adding hypertensive medications to treat hypertension induced by nonsteroidal anti-inflammatory drugs).60 This is particularly important when considering adding additional medications. If a statin will be the 20th pill, it may be less beneficial and more likely to lead to additional adverse effects than if it is the fifth medication.
Patient preferences are critically important, particularly when adding or removing medications. Interventions should include a detailed medication review for appropriate prescribing and deprescribing, referral to a pharmacist, and engaging the patient’s support system.
Multimorbidity. Many older individuals have multiple chronic illnesses. The interaction of multiple conditions must be considered in creating a comprehensive plan, including prognosis, patient preference, available evidence, treatment interactions, and risks and benefits.
Quality of life. Outlook on life and choices made regarding prolongation vs quality of life may be different for the older patient than the younger patient.
Personal preferences. Although interventions such as high-intensity statins for a robust 85-year-old may be appropriate, the individual can choose to forgo any treatment. It is important to explore the patient’s goals of care and advanced directives as part of shared decision-making when building a patient-centered prevention plan.61
ONE SIZE DOES NOT FIT ALL
The heterogeneity of aging rules out a one-size-fits-all recommendation for cardiovascular disease prevention and management of cardiovascular risk factors in older adults.
There is significant overlap between cardiovascular risk status and frailty.
Incorporating frailty into the creation of a cardiovascular risk prescription can aid in the development of an individualized care plan for the prevention of cardiovascular disease in the aging population.
- Social Security Administration (SSA). Calculators: life expectancy. www.ssa.gov/planners/lifeexpectancy.html. Accessed December 8, 2017.
- Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 2017; 135:e146–e603.
- Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:2889–2934.
- Rich MW, Chyun DA, Skolnick AH, et al; American Heart Association Older Populations Committee of the Council on Clinical Cardiology, Council on Cardiovascular and Stroke Nursing, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council; American College of Cardiology; and American Geriatrics Society. Knowledge gaps in cardiovascular care of the older adult population: a scientific statement from the American Heart Association, American College of Cardiology, and American Geriatrics Society. Circulation 2016; 133:2103–2122.
- Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013; 381:752–762.
- Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci 2007; 62:722–727.
- Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA 2011; 305:50–58.
- Afilalo J, Alexander KP, Mack MJ, et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol 2014; 63:747–762.
- Afilalo J, Karunananthan S, Eisenberg MJ, Alexander KP, Bergman H. Role of frailty in patients with cardiovascular disease. Am J Cardiol 2009; 103:1616–1621.
- Woods NF, LaCroix AZ, Gray SL, et al; Women’s Health Initiative. Frailty: emergence and consequences in women aged 65 and older in the Women's Health Initiative Observational Study. J Am Geriatr Soc 2005; 53:1321–1330.
- Bouillon K, Batty GD, Hamer M, et al. Cardiovascular disease risk scores in identifying future frailty: the Whitehall II prospective cohort study. Heart 2013; 99:737–742.
- Walston J, McBurnie MA, Newman A, et al; Cardiovascular Health Study. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med 2002; 162:2333–2341.
- De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflammation markers predicting frailty and mortality in the elderly. Exp Mol Pathol 2006; 80:219–227.
- Morley JE. Frailty fantasia. J Am Med Dir Assoc 2017; 18:813–815.
- Munoz-Mendoza CL, Cabanero-Martinez MJ, Millan-Calenti JC, Cabrero-Garcia J, Lopez-Sanchez R, Maseda-Rodriguez A. Reliability of 4-m and 6-m walking speed tests in elderly people with cognitive impairment. Arch Gerontol Geriatr 2011; 52:e67–e70.
- Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging 2009; 13:881–889.
- Sergi G, Veronese N, Fontana L, et al. Pre-frailty and risk of cardiovascular disease in elderly men and women: the Pro.V.A. study. J Am Coll Cardiol 2015; 65:976–983.
- Abellan van Kan G, Rolland Y, Bergman H, Morley JE, Kritchevsky SB, Vellas B. The I.A.N.A Task Force on frailty assessment of older people in clinical practice. J Nutr Health Aging 2008; 12:29–37.
- Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle-aged African Americans. J Nutr Health Aging 2012;16:601–608.
- Forman DE, Arena R, Boxer R, et al; American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Stroke Council. Prioritizing functional capacity as a principal end point for therapies oriented to older adults with cardiovascular disease: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2017; 135:e894–e918.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913.
- Mancia G, Grassi G. Aggressive blood pressure lowering is dangerous: the J-curve: pro side of the argument. Hypertension 2014; 63:29–36.
- Odden MC, Peralta CA, Haan MN, Covinsky KE. Rethinking the association of high blood pressure with mortality in elderly adults: the impact of frailty. Arch Intern Med 2012; 172:1162–1168.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- Warwick J, Falaschetti E, Rockwood K, et al. No evidence that frailty modifies the positive impact of antihypertensive treatment in very elderly people: an investigation of the impact of frailty upon treatment effect in the HYpertension in the Very Elderly Trial (HYVET) study, a double-blind, placebo-controlled study of antihypertensives in people with hypertension aged 80 and over. BMC Med 2015 9;13:78.
- Williamson JD, Supiano MA, Applegate WB, et al; SPRINT Research Group. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥ 75 years: a randomized clinical trial. JAMA 2016; 315:2673–2682.
- Tinetti ME, Han L, Lee DS, et al. Antihypertensive medications and serious fall injuries in a nationally representative sample of older adults. JAMA Intern Med 2014; 174:588–595.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2017. Nov 13 [Epub ahead of print].)
- American Diabetes Association. 11. Older adults. Diabetes Care 2017; 40(suppl 1):S99–S104.
- Mallery LH, Allen M, Fleming I, et al. Promoting higher blood pressure targets for frail older adults: a consensus guideline from Canada. Cleve Clin J Med 2014; 81:427–437.
- Shepherd J, Blauw GJ, Murphy MB, et al; PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002; 360:1623–1630.
- Glynn RJ, Koenig W, Nordestgaard BG, Shepherd J, Ridker PM. Rosuvastatin for primary prevention in older persons with elevated C-reactive protein and low to average low-density lipoprotein cholesterol levels: exploratory analysis of a randomized trial. Ann Intern Med 2010; 152:488–496, W174.
- LaCroix AZ, Gray SL, Aragaki A, et al; Women’s Health Initiative. Statin use and incident frailty in women aged 65 years or older: prospective findings from the Women’s Health Initiative Observational Study. J Gerontol A Biol Sci Med Sci 2008; 63:369–375.
- Odden MC, Pletcher MJ, Coxson PG, et al. Cost-effectiveness and population impact of statins for primary prevention in adults aged 75 years or older in the United States. Ann Intern Med 2015; 162:533–541.
- Kutner JS, Blatchford PJ, Taylor DH Jr, et al. Safety and benefit of discontinuing statin therapy in the setting of advanced, life-limiting illness: a randomized clinical trial. JAMA Intern Med 2015; 175:691–700.
- Huang ES, Liu JY, Moffet HH, John PM, Karter AJ. Glycemic control, complications, and death in older diabetic patients: the diabetes and aging study. Diabetes Care 2011; 34:1329–1336.
- Kirkman MS, Briscoe VJ, Clark N, et al; Consensus Development Conference on Diabetes and Older Adults. Diabetes in older adults: a consensus report. J Am Geriatr Soc 2012; 60:2342–2356.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015; 38:140–149.
- Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ (Clinical research ed) 2002; 324:71–86.
- Antithrombotic Trialists’ (ATT) Collaboration; Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009; 373:1849–1860.
- Ikeda Y, Shimada K, Teramoto T, et al. Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomized clinical trial. JAMA 2014; 312:2510–2520.
- Bibbins-Domingo K; US Preventive Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: US Preventive Services Task Force Recommendation Statement. Ann Intern Med 2016; 164:836–845.
- American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2012; 60:616–631.
- Li L, Geraghty OC, Mehta Z, Rothwell PM. Age-specific risks, severity, time course, and outcome of bleeding on long-term antiplatelet treatment after vascular events: a population-based cohort study. Lancet 2017; 390:490–499.
- Barzilay JI, Blaum C, Moore T, et al. Insulin resistance and inflammation as precursors of frailty: the Cardiovascular Health Study. Arch Intern Med 2007; 167:635–641.
- Fiatarone MA, O’Neill EF, Ryan ND, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med 1994; 330:1769–1775.
- Uusi-Rasi K, Patil R, Karinkanta S, et al. Exercise and vitamin D in fall prevention among older women: a randomized clinical trial. JAMA Intern Med 2015; 175:703–711.
- Hauer K, Schwenk M, Zieschang T, Essig M, Becker C, Oster P. Physical training improves motor performance in people with dementia: a randomized controlled trial. J Am Geriatr Soc 2012; 60:8–15.
- Li F, Harmer P, Fitzgerald K. Implementing an evidence-based fall prevention intervention in community senior centers. Am J Public Health 2016; 106:2026–2031.
- Manor B, Lough M, Gagnon MM, Cupples A, Wayne PM, Lipsitz LA. Functional benefits of tai chi training in senior housing facilities. J Am Geriatr Soc 2014; 62:1484–1489.
- Physical Activity Guidelines Advisory Committee report, 2008. To the Secretary of Health and Human Services. Part A: executive summary. Nutr Rev 2009; 67:114–120.
- Hubbard RE, Searle SD, Mitnitski A, Rockwood K. Effect of smoking on the accumulation of deficits, frailty and survival in older adults: a secondary analysis from the Canadian Study of Health and Aging. J Nutr Health Aging 2009; 13:468–472.
- Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A US Public Health Service report. Am J Prev Med 2008; 35:158–176.
- Samieri C, Sun Q, Townsend MK, et al. The association between dietary patterns at midlife and health in aging: an observational study. Ann Intern Med 2013; 159:584–591.
- Estruch R, Ros E, Martinez-Gonzalez MA. Mediterranean diet for primary prevention of cardiovascular disease. N Engl J Med 2013; 369:676–677.
- Leon-Munoz LM, Guallar-Castillon P, Lopez-Garcia E, Rodriguez-Artalejo F. Mediterranean diet and risk of frailty in community-dwelling older adults. J Am Med Dir Assoc 2014; 15:899–903.
- Doty RL, Shaman P, Applebaum SL, Giberson R, Siksorski L, Rosenberg L. Smell identification ability: changes with age. Science 1984; 226:1441–1443.
- Merel SE, Paauw DS. Common drug side effects and drug-drug interactions in elderly adults in primary care. J Am Geriatr Soc 2017 Mar 21. Epub ahead of print.
- Epstein RM, Peters E. Beyond information: exploring patients’ preferences. JAMA 2009; 302:195–197.
- Social Security Administration (SSA). Calculators: life expectancy. www.ssa.gov/planners/lifeexpectancy.html. Accessed December 8, 2017.
- Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 2017; 135:e146–e603.
- Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:2889–2934.
- Rich MW, Chyun DA, Skolnick AH, et al; American Heart Association Older Populations Committee of the Council on Clinical Cardiology, Council on Cardiovascular and Stroke Nursing, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council; American College of Cardiology; and American Geriatrics Society. Knowledge gaps in cardiovascular care of the older adult population: a scientific statement from the American Heart Association, American College of Cardiology, and American Geriatrics Society. Circulation 2016; 133:2103–2122.
- Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013; 381:752–762.
- Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci 2007; 62:722–727.
- Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA 2011; 305:50–58.
- Afilalo J, Alexander KP, Mack MJ, et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol 2014; 63:747–762.
- Afilalo J, Karunananthan S, Eisenberg MJ, Alexander KP, Bergman H. Role of frailty in patients with cardiovascular disease. Am J Cardiol 2009; 103:1616–1621.
- Woods NF, LaCroix AZ, Gray SL, et al; Women’s Health Initiative. Frailty: emergence and consequences in women aged 65 and older in the Women's Health Initiative Observational Study. J Am Geriatr Soc 2005; 53:1321–1330.
- Bouillon K, Batty GD, Hamer M, et al. Cardiovascular disease risk scores in identifying future frailty: the Whitehall II prospective cohort study. Heart 2013; 99:737–742.
- Walston J, McBurnie MA, Newman A, et al; Cardiovascular Health Study. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med 2002; 162:2333–2341.
- De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflammation markers predicting frailty and mortality in the elderly. Exp Mol Pathol 2006; 80:219–227.
- Morley JE. Frailty fantasia. J Am Med Dir Assoc 2017; 18:813–815.
- Munoz-Mendoza CL, Cabanero-Martinez MJ, Millan-Calenti JC, Cabrero-Garcia J, Lopez-Sanchez R, Maseda-Rodriguez A. Reliability of 4-m and 6-m walking speed tests in elderly people with cognitive impairment. Arch Gerontol Geriatr 2011; 52:e67–e70.
- Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging 2009; 13:881–889.
- Sergi G, Veronese N, Fontana L, et al. Pre-frailty and risk of cardiovascular disease in elderly men and women: the Pro.V.A. study. J Am Coll Cardiol 2015; 65:976–983.
- Abellan van Kan G, Rolland Y, Bergman H, Morley JE, Kritchevsky SB, Vellas B. The I.A.N.A Task Force on frailty assessment of older people in clinical practice. J Nutr Health Aging 2008; 12:29–37.
- Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle-aged African Americans. J Nutr Health Aging 2012;16:601–608.
- Forman DE, Arena R, Boxer R, et al; American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Stroke Council. Prioritizing functional capacity as a principal end point for therapies oriented to older adults with cardiovascular disease: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2017; 135:e894–e918.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913.
- Mancia G, Grassi G. Aggressive blood pressure lowering is dangerous: the J-curve: pro side of the argument. Hypertension 2014; 63:29–36.
- Odden MC, Peralta CA, Haan MN, Covinsky KE. Rethinking the association of high blood pressure with mortality in elderly adults: the impact of frailty. Arch Intern Med 2012; 172:1162–1168.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- Warwick J, Falaschetti E, Rockwood K, et al. No evidence that frailty modifies the positive impact of antihypertensive treatment in very elderly people: an investigation of the impact of frailty upon treatment effect in the HYpertension in the Very Elderly Trial (HYVET) study, a double-blind, placebo-controlled study of antihypertensives in people with hypertension aged 80 and over. BMC Med 2015 9;13:78.
- Williamson JD, Supiano MA, Applegate WB, et al; SPRINT Research Group. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥ 75 years: a randomized clinical trial. JAMA 2016; 315:2673–2682.
- Tinetti ME, Han L, Lee DS, et al. Antihypertensive medications and serious fall injuries in a nationally representative sample of older adults. JAMA Intern Med 2014; 174:588–595.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2017. Nov 13 [Epub ahead of print].)
- American Diabetes Association. 11. Older adults. Diabetes Care 2017; 40(suppl 1):S99–S104.
- Mallery LH, Allen M, Fleming I, et al. Promoting higher blood pressure targets for frail older adults: a consensus guideline from Canada. Cleve Clin J Med 2014; 81:427–437.
- Shepherd J, Blauw GJ, Murphy MB, et al; PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002; 360:1623–1630.
- Glynn RJ, Koenig W, Nordestgaard BG, Shepherd J, Ridker PM. Rosuvastatin for primary prevention in older persons with elevated C-reactive protein and low to average low-density lipoprotein cholesterol levels: exploratory analysis of a randomized trial. Ann Intern Med 2010; 152:488–496, W174.
- LaCroix AZ, Gray SL, Aragaki A, et al; Women’s Health Initiative. Statin use and incident frailty in women aged 65 years or older: prospective findings from the Women’s Health Initiative Observational Study. J Gerontol A Biol Sci Med Sci 2008; 63:369–375.
- Odden MC, Pletcher MJ, Coxson PG, et al. Cost-effectiveness and population impact of statins for primary prevention in adults aged 75 years or older in the United States. Ann Intern Med 2015; 162:533–541.
- Kutner JS, Blatchford PJ, Taylor DH Jr, et al. Safety and benefit of discontinuing statin therapy in the setting of advanced, life-limiting illness: a randomized clinical trial. JAMA Intern Med 2015; 175:691–700.
- Huang ES, Liu JY, Moffet HH, John PM, Karter AJ. Glycemic control, complications, and death in older diabetic patients: the diabetes and aging study. Diabetes Care 2011; 34:1329–1336.
- Kirkman MS, Briscoe VJ, Clark N, et al; Consensus Development Conference on Diabetes and Older Adults. Diabetes in older adults: a consensus report. J Am Geriatr Soc 2012; 60:2342–2356.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015; 38:140–149.
- Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ (Clinical research ed) 2002; 324:71–86.
- Antithrombotic Trialists’ (ATT) Collaboration; Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009; 373:1849–1860.
- Ikeda Y, Shimada K, Teramoto T, et al. Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomized clinical trial. JAMA 2014; 312:2510–2520.
- Bibbins-Domingo K; US Preventive Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: US Preventive Services Task Force Recommendation Statement. Ann Intern Med 2016; 164:836–845.
- American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2012; 60:616–631.
- Li L, Geraghty OC, Mehta Z, Rothwell PM. Age-specific risks, severity, time course, and outcome of bleeding on long-term antiplatelet treatment after vascular events: a population-based cohort study. Lancet 2017; 390:490–499.
- Barzilay JI, Blaum C, Moore T, et al. Insulin resistance and inflammation as precursors of frailty: the Cardiovascular Health Study. Arch Intern Med 2007; 167:635–641.
- Fiatarone MA, O’Neill EF, Ryan ND, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med 1994; 330:1769–1775.
- Uusi-Rasi K, Patil R, Karinkanta S, et al. Exercise and vitamin D in fall prevention among older women: a randomized clinical trial. JAMA Intern Med 2015; 175:703–711.
- Hauer K, Schwenk M, Zieschang T, Essig M, Becker C, Oster P. Physical training improves motor performance in people with dementia: a randomized controlled trial. J Am Geriatr Soc 2012; 60:8–15.
- Li F, Harmer P, Fitzgerald K. Implementing an evidence-based fall prevention intervention in community senior centers. Am J Public Health 2016; 106:2026–2031.
- Manor B, Lough M, Gagnon MM, Cupples A, Wayne PM, Lipsitz LA. Functional benefits of tai chi training in senior housing facilities. J Am Geriatr Soc 2014; 62:1484–1489.
- Physical Activity Guidelines Advisory Committee report, 2008. To the Secretary of Health and Human Services. Part A: executive summary. Nutr Rev 2009; 67:114–120.
- Hubbard RE, Searle SD, Mitnitski A, Rockwood K. Effect of smoking on the accumulation of deficits, frailty and survival in older adults: a secondary analysis from the Canadian Study of Health and Aging. J Nutr Health Aging 2009; 13:468–472.
- Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A US Public Health Service report. Am J Prev Med 2008; 35:158–176.
- Samieri C, Sun Q, Townsend MK, et al. The association between dietary patterns at midlife and health in aging: an observational study. Ann Intern Med 2013; 159:584–591.
- Estruch R, Ros E, Martinez-Gonzalez MA. Mediterranean diet for primary prevention of cardiovascular disease. N Engl J Med 2013; 369:676–677.
- Leon-Munoz LM, Guallar-Castillon P, Lopez-Garcia E, Rodriguez-Artalejo F. Mediterranean diet and risk of frailty in community-dwelling older adults. J Am Med Dir Assoc 2014; 15:899–903.
- Doty RL, Shaman P, Applebaum SL, Giberson R, Siksorski L, Rosenberg L. Smell identification ability: changes with age. Science 1984; 226:1441–1443.
- Merel SE, Paauw DS. Common drug side effects and drug-drug interactions in elderly adults in primary care. J Am Geriatr Soc 2017 Mar 21. Epub ahead of print.
- Epstein RM, Peters E. Beyond information: exploring patients’ preferences. JAMA 2009; 302:195–197.
KEY POINTS
- With the aging of the population, individualized prevention strategies must incorporate geriatric syndromes such as frailty.
- However, current guidelines and available evidence for cardiovascular disease prevention strategies have not incorporated frailty or make no recommendation at all for those over age 75.
- Four-meter gait speed, a simple measure of physical function and a proxy for frailty, can be used clinically to diagnose frailty.
Frailty and cardiovascular disease: A two-way street?
Despite a marked increase in awareness in recent years surrounding the prevalence and prognosis of frailty in our aging population and its association with cardiovascular disease, itself highly prevalent in elderly cohorts, the exact pathobiological links between the 2 conditions have not been fully elucidated. As a consequence, this has led to difficulty not only in accurately defining cardiovascular risk in vulnerable elderly patients, but also in adequately mitigating against it.
It is well accepted that cardiovascular disease, whether clinical or subclinical, is associated with an increased risk of developing the frail phenotype.1,2 Frailty, in turn, has been consistently identified as a universal marker of adverse outcomes in patients at risk of, and in patients with already manifest, cardiovascular disease.2,3 However, whether or not frailty is its own unique risk factor for cardiovascular disease, independent of co-associated risk markers, or is merely a downstream byproduct indicating a more advanced disease state, has yet to be determined. Furthermore, the question of whether modification of frail status may impact the development and progression of cardiovascular disease has not yet been established.
The article by Orkaby et al4 in this issue delves deeper into this question by looking specifically at the interaction between frailty and standard risk factors as they relate to the prevention of cardiovascular disease.
NEEDED: A UNIVERSAL DEFINITION OF FRAILTY
It is important to acknowledge up front that before we can truly examine frailty as a novel risk entity in the assessment and management of cardiovascular risk in older-age patients, we need to agree on an accepted, validated definition of the phenotype as it relates to this population. As acknowledged by Orkaby et al,4 lack of such a standardized definition has resulted in highly variable estimates of the prevalence of frailty, ranging from 6.9% in a community-dwelling population in the original Cardiovascular Health Study to as high as 50% in older adults with manifest cardiovascular disease.1,2
The ideal frailty assessment tool should be a simple, quantitative, objective, and universally accepted method, capable of providing a consistent, valid, reproducible definition that can then be used in real time by the clinician to determine the absolute presence or absence of the phenotype, much like hypertension or diabetes. Whether this optimal tool will turn out to be the traditional or modified version of the Fried Scale,1 an alternative multicomponent measure such as the Deficit Index,5 or even the increasingly popular single-item measures such as gait speed or grip strength, remains to be determined.
Exact choice of tool is perhaps less important than the singular adoption of a universal method that can then be rigorously tried and tested in multicenter studies. Given the bulk of data to date for the original Fried phenotype and its development in an older-age community setting with a typical prevalence of cardiovascular risk factors, the Fried Scale appears a particularly suitable tool to use for this domain of disease prevention. Single-item spin-off measures from this phenotype, including gait speed, may also be useful for their increased feasibility and practicality in certain situations.
A TWO-WAY STREET
Given what we know about the pathophysiological, immunological, and inflammatory processes underlying advancing age that have also been implicated in both frailty and cardiovascular disease syndromes, how can we determine if frailty truly is an independent risk factor for cardiovascular disease or merely an epiphenomenon of the aging process?
We do know that older age is not a prerequisite for frailty, as is evident in studies of the phenotype in middle-aged (and younger) patients with advanced heart failure.6 We also know not only that frail populations have a higher age-adjusted prevalence of cardiovascular risk factors including diabetes and hypertension,1 but also that community-dwellers with prefrailty (as defined in studies using the Fried criteria as 1 or 2 vs 3 present criteria) at baseline have a significantly increased risk of developing incident cardiovascular disease compared with those defined as nonfrail, even after adjustment for traditional risk factors and other biomarkers.3 Exploring the differences between these subgroups at baseline revealed that prefrailty was significantly associated with several subclinical insults that may serve as adverse vascular mediators, including insulin resistance, elevated inflammatory markers, and central adiposity.3
A substudy of the Cardiovascular Health Study also found that in over 1,200 participants without a prior history of a cardiovascular event, the presence of frailty was associated with multiple noninvasive measures of subclinical cardiovascular disease, including electrocardiographic and echocardiographic markers of left ventricular hypertrophy, carotid stenosis, and silent cerebrovascular infarcts on magnetic resonance imaging.7
These findings support a mechanistic link between evolving stages of frailty and a gradient of progressive cardiovascular risk, with a multifaceted dysregulation of metabolic processes known to underpin the pathogenesis of the frailty phenotype likely also triggering risk pathways (altered insulin metabolism, inflammation) involved in incident cardiovascular disease. Although the exact pathobiological pathways underlying these complex interlinked relationships between aging, frailty, and cardiovascular disease have yet to be fully elucidated, awareness of the bidirectional relationship between both morbid conditions highlights the absolute importance of modifying risk factors and subclinical conditions that are common to both.
CAN RISK BE MODIFIED IN FRAIL ADULTS?
Orkaby et al4 nicely lay out the guidelines for standard cardiovascular risk factor modification viewed in light of what is currently known—or not known—about how these recommendations should be interpreted for the older, frail, at-risk population. It is important to note at the outset that clinical trial data both inclusive of this population and incorporating the up-front assessment of frailty to predefine frail-or-not subgroups are sparse, and thereby evidence for how to optimize cardiovascular disease prevention in this important cohort is largely based on smaller observational studies and expert consensus.
Hypertension
However, important subanalyses derived from 2 large randomized controlled trials (Hypertension in the Very Elderly Trial [HYVET] and Systolic Blood Pressure Intervention Trial [SPRINT]) looking specifically at the impact of frail status on blood pressure treatment targets and related outcomes in elderly adults have recently been published.8,9 Notably, both studies showed the beneficial outcomes of more intensive treatment (to 150/80 mm Hg or 120 mm Hg systolic, respectively) persisted in those characterized as frail (via Rockwood frailty index or slow gait speed).8,9 Importantly, in the SPRINT analysis, higher event rates were seen with increasing frailty in both treatment groups; across each frailty stratum, absolute event rates were lower for the intensive treatment arm.9 These results were evident without a significant difference in the overall rate of serious adverse events9 or withdrawal rates8 between treatment groups.
Hypertension is the primary domain in which up-to-date clinical trial data have shown benefit for continued aggressive treatment for cardiovascular disease prevention regardless of the presence of frailty. Despite these data, in the real world, the “eyeball” frailty test often leads us to err on the side of caution regarding blood pressure management in the frail older adult. Certainly, the use of antihypertensive therapy in this population requires balanced consideration of the risk for adverse effects; the SPRINT analysis also found higher absolute rates of hypotension, falls, and acute kidney injury in the more intensively treated group.9 These adverse effects may be ameliorated not necessarily by modifying the target goal that is required, but by employing alternative strategies in achieving this goal, such as starting with lower doses, uptitrating more slowly, and monitoring with more frequent laboratory testing.
Currently, consensus guidelines in Canada have recommended liberalizing blood pressure treatment goals in those with “advanced frailty” associated with a shorter life expectancy.10
Dyslipidemia
Regarding the other major vascular risk factors, trials looking at the role of frailty in the targeted treatment of hyperlipidemia with statins in older patients for primary prevention of cardiovascular disease are lacking, although the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial showed a significant positive benefit for statin therapy in adults over age 70 (number needed to treat of 19 to prevent 1 major cardiovascular event, and 29 to prevent 1 cardiovascular death).11 This again may be counterbalanced by the purported increased risk of cognitive and potential adverse functional effects of statins in this age group; however, trial data specific to frail status or not is required to truly assess the benefit-risk ratio in this population.
Hyperglycemia
Meanwhile, recent clinical trials looking at the impact of age, functional impairment, and burden of comorbidities (rather than specific frailty measures) on glucose-lowering targets and cardiovascular outcomes have failed to show a benefit from intensive glycemic control strategies, leading guideline societies to endorse less-stringent hemoglobin A1c goals in this population.12 Given the well-documented association between hyperglycemia and cardiovascular disease, as well as the purported dysregulated glucose metabolism underlying the frail phenotype, it is important that future trials looking at optimal hemoglobin A1c targets incorporate the presence or absence of frailty to better inform specific recommendations for this population.
ONE SIZE MAY NOT FIT ALL
Overall, if both prefrailty and frailty are independent risk factors for, and a consequence of, clinical cardiovascular disease, it is worth bearing in mind that the modification of “intensive” or best practice therapies based on qualitatively assessed frailty may actually contribute to the problem. With best intentions, the negative impact of frailty on cardiovascular outcomes may be augmented by automatically assuming it to reflect a need for “therapy-light.” The adverse downstream consequences of inadequately treated cardiovascular risk factors are not in doubt, and it is important as the role of frailty becomes an increasingly recognized cofactor in the management of older adults with these risk factors that the vicious cycle underlying both syndromes is kept in mind, in order to avoid frailty becoming a harbinger of undertreatment in older, geriatric populations.
What is clear is that more prospective clinical trial data in this population are urgently needed in order to better delineate the exact interactions between frail status and these risk factors and the potential downstream consequences, using prespecified and robust frailty assessment methods.
Perhaps frailty should be seen as a series of stages rather than simply as a binary “there or not there” biomarker; through initial and established stages of the syndrome, which have been independently associated with both clinical events and subclinical surrogates of cardiovascular disease, risk factors should continue to be treated aggressively and according to best available evidence. However, as guideline societies are already beginning to endorse as highlighted above, once the phenotype becomes tethered with a certain threshold burden of comorbidity, cognitive or functional impairment, or more end-stage disease status, then goals for cardiovascular disease prevention may need to be readdressed and modified. If frailty is truly confirmed as a cardiovascular disease equivalent, not only appropriately treating associated cardiovascular risk factors but also seeking therapies that actively target the frailty syndrome itself should be an important goal of future studies seeking to impact the development of both clinical and subclinical cardiovascular disease in this population.
- Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Afilalo J, Alexander KP, Mack MJ, et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol 2014; 63:747–762.
- Sergi G, Veronese N, Fontana L, et al. Pre-frailty and risk of cardiovascular disease in elderly men and women: the Pro.V.A. study. J Am Coll Cardiol 2015; 65:976–983.
- Orkaby AR, Onuma O, Qazi S, Gaziano JM, Driver JA. Preventing cardiovascular disease in older adults: one size does not fit all. Cleve Clin J Med 2018; 85:55–64.
- Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr 2008;8:24.
- Joyce E. Frailty in advanced heart failure. Heart Fail Clin 2016; 12:363–374.
- Newman AB, Gottdiener JS, McBurnie MA, et al; Cardiovascular Health Study Research Group. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci 2001; 56:M158–M166.
- Warwick J, Falaschetti E, Rockwood K, et al. No evidence that frailty modifies the positive impact of antihypertensive treatment in very elderly people: an investigation of the impact of frailty upon treatment effect in the Hypertension in the Very Elderly Trial (HYVET) study, a double-blind, placebo-controlled study of antihypertensives in people with hypertension aged 80 and over. BMC Med 2015; 13:78.
- Williamson JD, Supiano MA, Applegate WB, et al; SPRINT Research Group. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥ 75 years: a randomized clinical trial. JAMA 2016; 315:2673–2682.
- Mallery LH, Allen M, Fleming I, et al. Promoting higher blood pressure targets for frail older adults: a consensus guideline from Canada. Cleve Clin J Med 2014; 81:427–437.
- Glynn RJ, Koenig W, Nordestgaard BG, Shepherd J, Ridker PM. Rosuvastatin for primary prevention in older persons with elevated C-reactive protein and low to average low-density lipoprotein cholesterol levels: exploratory analysis of a randomized trial. Ann Intern Med 2010; 152:488–496.
- Ismail-Beigi F, Moghissi E, Tiktin M, Hirsch IB, Inzucchi SE, Genuth S. Individualizing glycemic targets in type 2 diabetes mellitus: implications of recent clinical trials. Ann Intern Med 2011; 154:554–559.
Despite a marked increase in awareness in recent years surrounding the prevalence and prognosis of frailty in our aging population and its association with cardiovascular disease, itself highly prevalent in elderly cohorts, the exact pathobiological links between the 2 conditions have not been fully elucidated. As a consequence, this has led to difficulty not only in accurately defining cardiovascular risk in vulnerable elderly patients, but also in adequately mitigating against it.
It is well accepted that cardiovascular disease, whether clinical or subclinical, is associated with an increased risk of developing the frail phenotype.1,2 Frailty, in turn, has been consistently identified as a universal marker of adverse outcomes in patients at risk of, and in patients with already manifest, cardiovascular disease.2,3 However, whether or not frailty is its own unique risk factor for cardiovascular disease, independent of co-associated risk markers, or is merely a downstream byproduct indicating a more advanced disease state, has yet to be determined. Furthermore, the question of whether modification of frail status may impact the development and progression of cardiovascular disease has not yet been established.
The article by Orkaby et al4 in this issue delves deeper into this question by looking specifically at the interaction between frailty and standard risk factors as they relate to the prevention of cardiovascular disease.
NEEDED: A UNIVERSAL DEFINITION OF FRAILTY
It is important to acknowledge up front that before we can truly examine frailty as a novel risk entity in the assessment and management of cardiovascular risk in older-age patients, we need to agree on an accepted, validated definition of the phenotype as it relates to this population. As acknowledged by Orkaby et al,4 lack of such a standardized definition has resulted in highly variable estimates of the prevalence of frailty, ranging from 6.9% in a community-dwelling population in the original Cardiovascular Health Study to as high as 50% in older adults with manifest cardiovascular disease.1,2
The ideal frailty assessment tool should be a simple, quantitative, objective, and universally accepted method, capable of providing a consistent, valid, reproducible definition that can then be used in real time by the clinician to determine the absolute presence or absence of the phenotype, much like hypertension or diabetes. Whether this optimal tool will turn out to be the traditional or modified version of the Fried Scale,1 an alternative multicomponent measure such as the Deficit Index,5 or even the increasingly popular single-item measures such as gait speed or grip strength, remains to be determined.
Exact choice of tool is perhaps less important than the singular adoption of a universal method that can then be rigorously tried and tested in multicenter studies. Given the bulk of data to date for the original Fried phenotype and its development in an older-age community setting with a typical prevalence of cardiovascular risk factors, the Fried Scale appears a particularly suitable tool to use for this domain of disease prevention. Single-item spin-off measures from this phenotype, including gait speed, may also be useful for their increased feasibility and practicality in certain situations.
A TWO-WAY STREET
Given what we know about the pathophysiological, immunological, and inflammatory processes underlying advancing age that have also been implicated in both frailty and cardiovascular disease syndromes, how can we determine if frailty truly is an independent risk factor for cardiovascular disease or merely an epiphenomenon of the aging process?
We do know that older age is not a prerequisite for frailty, as is evident in studies of the phenotype in middle-aged (and younger) patients with advanced heart failure.6 We also know not only that frail populations have a higher age-adjusted prevalence of cardiovascular risk factors including diabetes and hypertension,1 but also that community-dwellers with prefrailty (as defined in studies using the Fried criteria as 1 or 2 vs 3 present criteria) at baseline have a significantly increased risk of developing incident cardiovascular disease compared with those defined as nonfrail, even after adjustment for traditional risk factors and other biomarkers.3 Exploring the differences between these subgroups at baseline revealed that prefrailty was significantly associated with several subclinical insults that may serve as adverse vascular mediators, including insulin resistance, elevated inflammatory markers, and central adiposity.3
A substudy of the Cardiovascular Health Study also found that in over 1,200 participants without a prior history of a cardiovascular event, the presence of frailty was associated with multiple noninvasive measures of subclinical cardiovascular disease, including electrocardiographic and echocardiographic markers of left ventricular hypertrophy, carotid stenosis, and silent cerebrovascular infarcts on magnetic resonance imaging.7
These findings support a mechanistic link between evolving stages of frailty and a gradient of progressive cardiovascular risk, with a multifaceted dysregulation of metabolic processes known to underpin the pathogenesis of the frailty phenotype likely also triggering risk pathways (altered insulin metabolism, inflammation) involved in incident cardiovascular disease. Although the exact pathobiological pathways underlying these complex interlinked relationships between aging, frailty, and cardiovascular disease have yet to be fully elucidated, awareness of the bidirectional relationship between both morbid conditions highlights the absolute importance of modifying risk factors and subclinical conditions that are common to both.
CAN RISK BE MODIFIED IN FRAIL ADULTS?
Orkaby et al4 nicely lay out the guidelines for standard cardiovascular risk factor modification viewed in light of what is currently known—or not known—about how these recommendations should be interpreted for the older, frail, at-risk population. It is important to note at the outset that clinical trial data both inclusive of this population and incorporating the up-front assessment of frailty to predefine frail-or-not subgroups are sparse, and thereby evidence for how to optimize cardiovascular disease prevention in this important cohort is largely based on smaller observational studies and expert consensus.
Hypertension
However, important subanalyses derived from 2 large randomized controlled trials (Hypertension in the Very Elderly Trial [HYVET] and Systolic Blood Pressure Intervention Trial [SPRINT]) looking specifically at the impact of frail status on blood pressure treatment targets and related outcomes in elderly adults have recently been published.8,9 Notably, both studies showed the beneficial outcomes of more intensive treatment (to 150/80 mm Hg or 120 mm Hg systolic, respectively) persisted in those characterized as frail (via Rockwood frailty index or slow gait speed).8,9 Importantly, in the SPRINT analysis, higher event rates were seen with increasing frailty in both treatment groups; across each frailty stratum, absolute event rates were lower for the intensive treatment arm.9 These results were evident without a significant difference in the overall rate of serious adverse events9 or withdrawal rates8 between treatment groups.
Hypertension is the primary domain in which up-to-date clinical trial data have shown benefit for continued aggressive treatment for cardiovascular disease prevention regardless of the presence of frailty. Despite these data, in the real world, the “eyeball” frailty test often leads us to err on the side of caution regarding blood pressure management in the frail older adult. Certainly, the use of antihypertensive therapy in this population requires balanced consideration of the risk for adverse effects; the SPRINT analysis also found higher absolute rates of hypotension, falls, and acute kidney injury in the more intensively treated group.9 These adverse effects may be ameliorated not necessarily by modifying the target goal that is required, but by employing alternative strategies in achieving this goal, such as starting with lower doses, uptitrating more slowly, and monitoring with more frequent laboratory testing.
Currently, consensus guidelines in Canada have recommended liberalizing blood pressure treatment goals in those with “advanced frailty” associated with a shorter life expectancy.10
Dyslipidemia
Regarding the other major vascular risk factors, trials looking at the role of frailty in the targeted treatment of hyperlipidemia with statins in older patients for primary prevention of cardiovascular disease are lacking, although the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial showed a significant positive benefit for statin therapy in adults over age 70 (number needed to treat of 19 to prevent 1 major cardiovascular event, and 29 to prevent 1 cardiovascular death).11 This again may be counterbalanced by the purported increased risk of cognitive and potential adverse functional effects of statins in this age group; however, trial data specific to frail status or not is required to truly assess the benefit-risk ratio in this population.
Hyperglycemia
Meanwhile, recent clinical trials looking at the impact of age, functional impairment, and burden of comorbidities (rather than specific frailty measures) on glucose-lowering targets and cardiovascular outcomes have failed to show a benefit from intensive glycemic control strategies, leading guideline societies to endorse less-stringent hemoglobin A1c goals in this population.12 Given the well-documented association between hyperglycemia and cardiovascular disease, as well as the purported dysregulated glucose metabolism underlying the frail phenotype, it is important that future trials looking at optimal hemoglobin A1c targets incorporate the presence or absence of frailty to better inform specific recommendations for this population.
ONE SIZE MAY NOT FIT ALL
Overall, if both prefrailty and frailty are independent risk factors for, and a consequence of, clinical cardiovascular disease, it is worth bearing in mind that the modification of “intensive” or best practice therapies based on qualitatively assessed frailty may actually contribute to the problem. With best intentions, the negative impact of frailty on cardiovascular outcomes may be augmented by automatically assuming it to reflect a need for “therapy-light.” The adverse downstream consequences of inadequately treated cardiovascular risk factors are not in doubt, and it is important as the role of frailty becomes an increasingly recognized cofactor in the management of older adults with these risk factors that the vicious cycle underlying both syndromes is kept in mind, in order to avoid frailty becoming a harbinger of undertreatment in older, geriatric populations.
What is clear is that more prospective clinical trial data in this population are urgently needed in order to better delineate the exact interactions between frail status and these risk factors and the potential downstream consequences, using prespecified and robust frailty assessment methods.
Perhaps frailty should be seen as a series of stages rather than simply as a binary “there or not there” biomarker; through initial and established stages of the syndrome, which have been independently associated with both clinical events and subclinical surrogates of cardiovascular disease, risk factors should continue to be treated aggressively and according to best available evidence. However, as guideline societies are already beginning to endorse as highlighted above, once the phenotype becomes tethered with a certain threshold burden of comorbidity, cognitive or functional impairment, or more end-stage disease status, then goals for cardiovascular disease prevention may need to be readdressed and modified. If frailty is truly confirmed as a cardiovascular disease equivalent, not only appropriately treating associated cardiovascular risk factors but also seeking therapies that actively target the frailty syndrome itself should be an important goal of future studies seeking to impact the development of both clinical and subclinical cardiovascular disease in this population.
Despite a marked increase in awareness in recent years surrounding the prevalence and prognosis of frailty in our aging population and its association with cardiovascular disease, itself highly prevalent in elderly cohorts, the exact pathobiological links between the 2 conditions have not been fully elucidated. As a consequence, this has led to difficulty not only in accurately defining cardiovascular risk in vulnerable elderly patients, but also in adequately mitigating against it.
It is well accepted that cardiovascular disease, whether clinical or subclinical, is associated with an increased risk of developing the frail phenotype.1,2 Frailty, in turn, has been consistently identified as a universal marker of adverse outcomes in patients at risk of, and in patients with already manifest, cardiovascular disease.2,3 However, whether or not frailty is its own unique risk factor for cardiovascular disease, independent of co-associated risk markers, or is merely a downstream byproduct indicating a more advanced disease state, has yet to be determined. Furthermore, the question of whether modification of frail status may impact the development and progression of cardiovascular disease has not yet been established.
The article by Orkaby et al4 in this issue delves deeper into this question by looking specifically at the interaction between frailty and standard risk factors as they relate to the prevention of cardiovascular disease.
NEEDED: A UNIVERSAL DEFINITION OF FRAILTY
It is important to acknowledge up front that before we can truly examine frailty as a novel risk entity in the assessment and management of cardiovascular risk in older-age patients, we need to agree on an accepted, validated definition of the phenotype as it relates to this population. As acknowledged by Orkaby et al,4 lack of such a standardized definition has resulted in highly variable estimates of the prevalence of frailty, ranging from 6.9% in a community-dwelling population in the original Cardiovascular Health Study to as high as 50% in older adults with manifest cardiovascular disease.1,2
The ideal frailty assessment tool should be a simple, quantitative, objective, and universally accepted method, capable of providing a consistent, valid, reproducible definition that can then be used in real time by the clinician to determine the absolute presence or absence of the phenotype, much like hypertension or diabetes. Whether this optimal tool will turn out to be the traditional or modified version of the Fried Scale,1 an alternative multicomponent measure such as the Deficit Index,5 or even the increasingly popular single-item measures such as gait speed or grip strength, remains to be determined.
Exact choice of tool is perhaps less important than the singular adoption of a universal method that can then be rigorously tried and tested in multicenter studies. Given the bulk of data to date for the original Fried phenotype and its development in an older-age community setting with a typical prevalence of cardiovascular risk factors, the Fried Scale appears a particularly suitable tool to use for this domain of disease prevention. Single-item spin-off measures from this phenotype, including gait speed, may also be useful for their increased feasibility and practicality in certain situations.
A TWO-WAY STREET
Given what we know about the pathophysiological, immunological, and inflammatory processes underlying advancing age that have also been implicated in both frailty and cardiovascular disease syndromes, how can we determine if frailty truly is an independent risk factor for cardiovascular disease or merely an epiphenomenon of the aging process?
We do know that older age is not a prerequisite for frailty, as is evident in studies of the phenotype in middle-aged (and younger) patients with advanced heart failure.6 We also know not only that frail populations have a higher age-adjusted prevalence of cardiovascular risk factors including diabetes and hypertension,1 but also that community-dwellers with prefrailty (as defined in studies using the Fried criteria as 1 or 2 vs 3 present criteria) at baseline have a significantly increased risk of developing incident cardiovascular disease compared with those defined as nonfrail, even after adjustment for traditional risk factors and other biomarkers.3 Exploring the differences between these subgroups at baseline revealed that prefrailty was significantly associated with several subclinical insults that may serve as adverse vascular mediators, including insulin resistance, elevated inflammatory markers, and central adiposity.3
A substudy of the Cardiovascular Health Study also found that in over 1,200 participants without a prior history of a cardiovascular event, the presence of frailty was associated with multiple noninvasive measures of subclinical cardiovascular disease, including electrocardiographic and echocardiographic markers of left ventricular hypertrophy, carotid stenosis, and silent cerebrovascular infarcts on magnetic resonance imaging.7
These findings support a mechanistic link between evolving stages of frailty and a gradient of progressive cardiovascular risk, with a multifaceted dysregulation of metabolic processes known to underpin the pathogenesis of the frailty phenotype likely also triggering risk pathways (altered insulin metabolism, inflammation) involved in incident cardiovascular disease. Although the exact pathobiological pathways underlying these complex interlinked relationships between aging, frailty, and cardiovascular disease have yet to be fully elucidated, awareness of the bidirectional relationship between both morbid conditions highlights the absolute importance of modifying risk factors and subclinical conditions that are common to both.
CAN RISK BE MODIFIED IN FRAIL ADULTS?
Orkaby et al4 nicely lay out the guidelines for standard cardiovascular risk factor modification viewed in light of what is currently known—or not known—about how these recommendations should be interpreted for the older, frail, at-risk population. It is important to note at the outset that clinical trial data both inclusive of this population and incorporating the up-front assessment of frailty to predefine frail-or-not subgroups are sparse, and thereby evidence for how to optimize cardiovascular disease prevention in this important cohort is largely based on smaller observational studies and expert consensus.
Hypertension
However, important subanalyses derived from 2 large randomized controlled trials (Hypertension in the Very Elderly Trial [HYVET] and Systolic Blood Pressure Intervention Trial [SPRINT]) looking specifically at the impact of frail status on blood pressure treatment targets and related outcomes in elderly adults have recently been published.8,9 Notably, both studies showed the beneficial outcomes of more intensive treatment (to 150/80 mm Hg or 120 mm Hg systolic, respectively) persisted in those characterized as frail (via Rockwood frailty index or slow gait speed).8,9 Importantly, in the SPRINT analysis, higher event rates were seen with increasing frailty in both treatment groups; across each frailty stratum, absolute event rates were lower for the intensive treatment arm.9 These results were evident without a significant difference in the overall rate of serious adverse events9 or withdrawal rates8 between treatment groups.
Hypertension is the primary domain in which up-to-date clinical trial data have shown benefit for continued aggressive treatment for cardiovascular disease prevention regardless of the presence of frailty. Despite these data, in the real world, the “eyeball” frailty test often leads us to err on the side of caution regarding blood pressure management in the frail older adult. Certainly, the use of antihypertensive therapy in this population requires balanced consideration of the risk for adverse effects; the SPRINT analysis also found higher absolute rates of hypotension, falls, and acute kidney injury in the more intensively treated group.9 These adverse effects may be ameliorated not necessarily by modifying the target goal that is required, but by employing alternative strategies in achieving this goal, such as starting with lower doses, uptitrating more slowly, and monitoring with more frequent laboratory testing.
Currently, consensus guidelines in Canada have recommended liberalizing blood pressure treatment goals in those with “advanced frailty” associated with a shorter life expectancy.10
Dyslipidemia
Regarding the other major vascular risk factors, trials looking at the role of frailty in the targeted treatment of hyperlipidemia with statins in older patients for primary prevention of cardiovascular disease are lacking, although the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial showed a significant positive benefit for statin therapy in adults over age 70 (number needed to treat of 19 to prevent 1 major cardiovascular event, and 29 to prevent 1 cardiovascular death).11 This again may be counterbalanced by the purported increased risk of cognitive and potential adverse functional effects of statins in this age group; however, trial data specific to frail status or not is required to truly assess the benefit-risk ratio in this population.
Hyperglycemia
Meanwhile, recent clinical trials looking at the impact of age, functional impairment, and burden of comorbidities (rather than specific frailty measures) on glucose-lowering targets and cardiovascular outcomes have failed to show a benefit from intensive glycemic control strategies, leading guideline societies to endorse less-stringent hemoglobin A1c goals in this population.12 Given the well-documented association between hyperglycemia and cardiovascular disease, as well as the purported dysregulated glucose metabolism underlying the frail phenotype, it is important that future trials looking at optimal hemoglobin A1c targets incorporate the presence or absence of frailty to better inform specific recommendations for this population.
ONE SIZE MAY NOT FIT ALL
Overall, if both prefrailty and frailty are independent risk factors for, and a consequence of, clinical cardiovascular disease, it is worth bearing in mind that the modification of “intensive” or best practice therapies based on qualitatively assessed frailty may actually contribute to the problem. With best intentions, the negative impact of frailty on cardiovascular outcomes may be augmented by automatically assuming it to reflect a need for “therapy-light.” The adverse downstream consequences of inadequately treated cardiovascular risk factors are not in doubt, and it is important as the role of frailty becomes an increasingly recognized cofactor in the management of older adults with these risk factors that the vicious cycle underlying both syndromes is kept in mind, in order to avoid frailty becoming a harbinger of undertreatment in older, geriatric populations.
What is clear is that more prospective clinical trial data in this population are urgently needed in order to better delineate the exact interactions between frail status and these risk factors and the potential downstream consequences, using prespecified and robust frailty assessment methods.
Perhaps frailty should be seen as a series of stages rather than simply as a binary “there or not there” biomarker; through initial and established stages of the syndrome, which have been independently associated with both clinical events and subclinical surrogates of cardiovascular disease, risk factors should continue to be treated aggressively and according to best available evidence. However, as guideline societies are already beginning to endorse as highlighted above, once the phenotype becomes tethered with a certain threshold burden of comorbidity, cognitive or functional impairment, or more end-stage disease status, then goals for cardiovascular disease prevention may need to be readdressed and modified. If frailty is truly confirmed as a cardiovascular disease equivalent, not only appropriately treating associated cardiovascular risk factors but also seeking therapies that actively target the frailty syndrome itself should be an important goal of future studies seeking to impact the development of both clinical and subclinical cardiovascular disease in this population.
- Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Afilalo J, Alexander KP, Mack MJ, et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol 2014; 63:747–762.
- Sergi G, Veronese N, Fontana L, et al. Pre-frailty and risk of cardiovascular disease in elderly men and women: the Pro.V.A. study. J Am Coll Cardiol 2015; 65:976–983.
- Orkaby AR, Onuma O, Qazi S, Gaziano JM, Driver JA. Preventing cardiovascular disease in older adults: one size does not fit all. Cleve Clin J Med 2018; 85:55–64.
- Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr 2008;8:24.
- Joyce E. Frailty in advanced heart failure. Heart Fail Clin 2016; 12:363–374.
- Newman AB, Gottdiener JS, McBurnie MA, et al; Cardiovascular Health Study Research Group. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci 2001; 56:M158–M166.
- Warwick J, Falaschetti E, Rockwood K, et al. No evidence that frailty modifies the positive impact of antihypertensive treatment in very elderly people: an investigation of the impact of frailty upon treatment effect in the Hypertension in the Very Elderly Trial (HYVET) study, a double-blind, placebo-controlled study of antihypertensives in people with hypertension aged 80 and over. BMC Med 2015; 13:78.
- Williamson JD, Supiano MA, Applegate WB, et al; SPRINT Research Group. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥ 75 years: a randomized clinical trial. JAMA 2016; 315:2673–2682.
- Mallery LH, Allen M, Fleming I, et al. Promoting higher blood pressure targets for frail older adults: a consensus guideline from Canada. Cleve Clin J Med 2014; 81:427–437.
- Glynn RJ, Koenig W, Nordestgaard BG, Shepherd J, Ridker PM. Rosuvastatin for primary prevention in older persons with elevated C-reactive protein and low to average low-density lipoprotein cholesterol levels: exploratory analysis of a randomized trial. Ann Intern Med 2010; 152:488–496.
- Ismail-Beigi F, Moghissi E, Tiktin M, Hirsch IB, Inzucchi SE, Genuth S. Individualizing glycemic targets in type 2 diabetes mellitus: implications of recent clinical trials. Ann Intern Med 2011; 154:554–559.
- Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Afilalo J, Alexander KP, Mack MJ, et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol 2014; 63:747–762.
- Sergi G, Veronese N, Fontana L, et al. Pre-frailty and risk of cardiovascular disease in elderly men and women: the Pro.V.A. study. J Am Coll Cardiol 2015; 65:976–983.
- Orkaby AR, Onuma O, Qazi S, Gaziano JM, Driver JA. Preventing cardiovascular disease in older adults: one size does not fit all. Cleve Clin J Med 2018; 85:55–64.
- Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr 2008;8:24.
- Joyce E. Frailty in advanced heart failure. Heart Fail Clin 2016; 12:363–374.
- Newman AB, Gottdiener JS, McBurnie MA, et al; Cardiovascular Health Study Research Group. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci 2001; 56:M158–M166.
- Warwick J, Falaschetti E, Rockwood K, et al. No evidence that frailty modifies the positive impact of antihypertensive treatment in very elderly people: an investigation of the impact of frailty upon treatment effect in the Hypertension in the Very Elderly Trial (HYVET) study, a double-blind, placebo-controlled study of antihypertensives in people with hypertension aged 80 and over. BMC Med 2015; 13:78.
- Williamson JD, Supiano MA, Applegate WB, et al; SPRINT Research Group. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥ 75 years: a randomized clinical trial. JAMA 2016; 315:2673–2682.
- Mallery LH, Allen M, Fleming I, et al. Promoting higher blood pressure targets for frail older adults: a consensus guideline from Canada. Cleve Clin J Med 2014; 81:427–437.
- Glynn RJ, Koenig W, Nordestgaard BG, Shepherd J, Ridker PM. Rosuvastatin for primary prevention in older persons with elevated C-reactive protein and low to average low-density lipoprotein cholesterol levels: exploratory analysis of a randomized trial. Ann Intern Med 2010; 152:488–496.
- Ismail-Beigi F, Moghissi E, Tiktin M, Hirsch IB, Inzucchi SE, Genuth S. Individualizing glycemic targets in type 2 diabetes mellitus: implications of recent clinical trials. Ann Intern Med 2011; 154:554–559.