User login
Sleep disorders in older adults
As humans live longer, a renewed focus on quality of life has made the prompt diagnosis and treatment of sleep-related disorders in older adults increasingly necessary.1 Normative aging results in multiple changes in sleep architecture, including decreased total sleep time, decreased sleep efficiency, decreased slow-wave sleep (SWS), and increased awakenings after sleep onset.2 Sleep disturbances in older adults are increasingly recognized as multifactorial health conditions requiring comprehensive modification of risk factors, diagnosis, and treatment.3
In this article, we discuss the effects of aging on sleep architecture and provide an overview of primary sleep disorders in older adults. We also summarize strategies for diagnosing and treating sleep disorders in these patients.
Elements of the sleep cycle
The human sleep cycle begins with light sleep (sleep stages 1 and 2), progresses into SWS (sleep stage 3), and culminates in rapid eye movement (REM) sleep. The first 3 stages are referred to as non-rapid eye movement sleep (NREM). Throughout the night, this coupling of NREM and REM cycles occurs 4 to 6 times, with each successive cycle decreasing in length until awakening.4
Two complex neurologic pathways intersect to regulate the timing of sleep and wakefulness on arousal. The first pathway, the circadian system, is located within the suprachiasmatic nucleus of the hypothalamus and is highly dependent on external stimuli (light, food, etc.) to synchronize sleep/wake cycles. The suprachiasmatic nucleus regulates melatonin secretion by the pineal gland, which signals day-night transitions. The other pathway, the homeostatic system, modifies the amount of sleep needed daily. When multiple days of poor sleep occur, homeostatic sleep pressure (colloquially described as sleep debt) compensates by increasing the amount of sleep required in the following days. Together, the circadian and homeostatic systems work in conjunction to regulate sleep quantity to approximately one-third of the total sleep-wake cycle.2,5
Age-related dysfunction of the regulatory sleep pathways leads to blunting of the ability to initiate and sustain high-quality sleep.6 Dysregulation of homeostatic sleep pressure decreases time spent in SWS, and failure of the circadian signaling apparatus results in delays in sleep/wake timing.2 While research into the underlying neurobiology of sleep reveals that some of these changes are inherent to aging (Box7-14), significant underdiagnosed pathologies may adversely affect sleep architecture, including polypharmacy, comorbid neuropathology (eg, synucleinopathies, tauopathies, etc.), and primary sleep disorders (insomnias, hypersomnias, and parasomnias).15
Box
It has long been known that sleep architecture changes significantly with age. One of the largest meta-analyses of sleep changes in healthy individuals throughout childhood into old age found that total sleep time, sleep efficiency, percentage of slow-wave sleep, percentage of rapid eye movement sleep (REM), and REM latency all decreased with normative aging.7 Other studies have also found a decreased ability to maintain sleep (increased frequency of awakenings and prolonged nocturnal awakenings).8
Based on several meta-analyses, the average total sleep time at night in the adult population decreases by approximately 10 minutes per decade in both men and women.7,9-11 However, this pattern is not observed after age 60, when the total sleep time plateaus.7 Similarly, the duration of wake after sleep onset increases by approximately 10 minutes every decade for adults age 30 to 60, and plateaus after that.7,8
Epidemiologic studies have suggested that the prevalence of daytime napping increases with age.8 This trend continues into older age without a noticeable plateau.
A study of a nationally representative sample of >7,000 Japanese participants found that a significantly higher proportion of older adults take daytime naps (27.4%) compared with middle-age adults (14.4%).12 Older adults nap more frequently because of both lifestyle and biologic changes that accompany normative aging. Polls in the United States have shown a correlation between frequent napping and an increase in excessive daytime sleepiness, depression, pain, and nocturia.13
While sleep latency steadily increases after age 50, recent studies have shown that in healthy individuals, these changes are modest at best,7,9,14 which suggests that other pathologic factors may be contributing to this problem. Although healthy older people were found to have more frequent arousals throughout the night, they retained the ability to reinitiate sleep as rapidly as younger adults.7,9
Primary sleep disorders
Obstructive sleep apnea (OSA) is one of the most common, yet frequently underdiagnosed reversible causes of sleep disturbances. It is characterized by partial or complete airway obstruction culminating in periods of involuntary cessation of respirations during sleep. The resultant fragmentation in sleep leads to significant downstream effects over time, including excessive daytime sleepiness and fatigue, poor occupational and social performance, and substantial cognitive impairment.3 While it is well known that OSA increases in prevalence throughout middle age, this relationship plateaus after age 60.16 An estimated 40% to 60% of Americans age >60 are affected by OSA.17 The hypoxemia and fragmented sleep caused by unrecognized OSA are associated with a significant decline in activities of daily living (ADL).18 Untreated OSA is strongly linked to the development and progression of several major health conditions, including cardiovascular disease, diabetes mellitus, hypertension, stroke, and depression.19 In studies of long-term care facility residents—many of whom may have comorbid cognitive decline—researchers found that unrecognized OSA often mimics the progressive cognitive decline seen in major neurocognitive disorders.20 However, classic symptoms of OSA may not always be present in these patients, and their daytime sleepiness is often attributed to old age rather than to a pathological etiology.16 Screening for OSA and prompt initiation of the appropriate treatment may reverse OSA-induced cognitive changes in these patients.21
The primary presenting symptom of OSA is snoring, which is correlated with pauses in breathing. Risk factors include increased body mass index (BMI), thick neck circumference, male sex, and advanced age. In older adults, BMI has a lower impact on the Apnea-Hypopnea Index, an indicator of the number of pauses in breathing per hour, when compared with young and middle-age adults.16 Validated screening questionnaires for OSA include the STOP-Bang Questionnaire (Table 122), OSA50, Berlin Questionnaire, and Epworth Sleepiness Scale, each of which is used in different subpopulations. The current diagnostic standard for OSA is nocturnal polysomnography in a sleep laboratory, but recent advances in home sleep apnea testing have made it a viable, low-cost alternative for patients who do not have significant medical comorbidities.23 Standard utilized cutoffs for diagnosis are ≥5 events/hour (hypopneas associated with at least 4% oxygen desaturations) in conjunction with clinical symptoms of OSA.24

Continue to: Treatment
Treatment. First-line treatment for OSA is continuous positive airway pressure therapy, but adherence rates vary widely with patient education and regular follow-up.25 Adjunctive therapy includes weight loss, oral appliances, and uvulopalatopharyngoplasty, a procedure in which tissue in the throat is remodeled or removed.
Central sleep apnea (CSA) is a pause in breathing without evidence of associated respiratory effort. In adults, the development of CSA is indicative of underlying lower brainstem dysfunction, due to intermittent failures in the pontomedullary centers responsible for regulation of rhythmic breathing.26 This can occur as a consequence of multiple diseases, including congestive heart failure, stroke, renal failure, chronic medication use (opioids), and brain tumors.
The Sleep Heart Health Study—the largest community-based cohort study to date examining CSA—estimated that the prevalence of CSA among adults age >65 was 1.1% (compared with 0.4% in those age <65).27 Subgroup analysis revealed that men had significantly higher rates of CSA compared with women (2.7% vs 0.2%, respectively).
CSA may present similarly to OSA (excessive daytime somnolence, insomnia, poor sleep quality, difficulties with attention and concentration). Symptoms may also mimic those of coexisting medical conditions in older adults, such as nocturnal angina or paroxysmal nocturnal dyspnea.27 Any older patient with daytime sleepiness and risk factors for CSA should be referred for in-laboratory nocturnal polysomnography, the gold standard diagnostic test. Unlike in OSA, ambulatory diagnostic measures (home sleep apnea testing) have not been validated for this disorder.27
Treatment. The primary treatment for CSA is to address the underlying medical problem. Positive pressure ventilation has been attempted with mixed results. Supplemental oxygen and medical management (acetazolamide or theophylline) can help stimulate breathing. Newer studies have shown favorable outcomes with transvenous neurostimulation or adaptive servoventilation.28-30
Continue to: Insomnia
Insomnia. For a primary diagnosis of insomnia, DSM-5 requires at least 3 nights per week of sleep disturbances that induce distress or functional impairment for at least 3 months.31 The International Classification of Disease, 10th Edition requires at least 1 month of symptoms (lying awake for a long time before falling asleep, sleeping for short periods, being awake for most of the night, feeling lack of sleep, waking up early) after ruling out other sleep disorders, substance use, or other medical conditions.4 Clinically, insomnia tends to present in older adults as a subjective complaint of dissatisfaction with the quality and/or quantity of their sleep. Insomnia has been consistently shown to be a significant risk factor for both the development or exacerbation of depression in older adults.32-34
While the diagnosis of insomnia is mainly clinical via a thorough sleep and medication history, assistive ancillary testing can include wrist actigraphy and screening questionnaires (the Insomnia Severity Index and the Pittsburgh Sleep Quality Index).4 Because population studies of older adults have found discrepancies between objective and subjective methods of assessing sleep quality, relying on the accuracy of self-reported symptoms alone is questionable.35
Treatment. Given that drug elimination half-life increases with age, and the risks of adverse effects are increased in older adults, the preferred treatment modalities for insomnia are nonpharmacologic.4 Sleep hygiene education (Table 2) and cognitive-behavioral therapy (CBT) for insomnia are often the first-line therapies.4,36,37 It is crucial to manage comorbidities such as heart disease and obesity, as well as sources of discomfort from conditions such as arthritic pain.38,39 If nonpharmacologic therapies are not effective, pharmacologic options can be considered.4 Before prescribing sleep medications, it may be more fruitful to treat underlying psychiatric disorders such as depression and anxiety with antidepressants.4 Although benzodiazepines are helpful for their sedative effects, they are not recommended for older adults because of an increased risk of falls, rebound insomnia, potential tolerance, and associated cognitive impairment.40 Benzodiazepine receptor agonists (eg, zolpidem, eszopiclone, zaleplon) were initially developed as a first-line treatment for insomnia to replace the reliance on benzodiazepines, but these medications have a “black-box” warning of a serious risk of complex sleep behaviors, including life-threatening parasomnias.41 As a result, guidelines suggest a shorter duration of treatment with a benzodiazepine receptor agonist may still provide benefit while limiting the risk of adverse effects.42
Doxepin is the only antidepressant FDA-approved for insomnia; it improves sleep latency (time taken to initiate sleep after lying down), duration, and quality in adults age >65.43 Melatonin receptor agonists such as ramelteon and melatonin have shown positive results in older patients with insomnia. In clinical trials of patients age ≥65, ramelteon, which is FDA-approved for insomnia, produced no rebound insomnia, withdrawal effects, memory impairment, or gait instability.44-46 Suvorexant, an orexin receptor antagonist, decreases sleep latency and increases total sleep time equally in both young and older adults.47-49Table 340-51 provides a list of medications used to treat insomnia (including off-label agents) and their common adverse effects in older adults.
Parasomnias are undesirable behaviors that occur during sleep, commonly associated with the sleep-wake transition period. These behaviors can occur during REM sleep (nightmare disorder, sleep paralysis, REM sleep behavior disorder) or NREM sleep (somnambulism [sleepwalking], confusional arousals, sleep terrors). According to a cross-sectional Norwegian study of parasomnias, the estimated lifetime prevalence of sleep walking is 22.4%; sleep talking, 66.8%; confusional arousal, 18.5%; and sleep terror, 10.4%.52
Continue to: When evaluating a patient...
When evaluating a patient with parasomnias, it is important to review their drug and substance use as well as coexisting medical conditions. Drugs and substances that can affect sleep include prescription medications (second-generation antidepressants, stimulants, dopamine agonists), excessive caffeine, alcohol, certain foods (coffee, chocolate milk, black tea, caffeinated soft drinks), environmental exposures (smoking, pesticides), and recreational drugs (amphetamines).53-56 Certain medical conditions are correlated with specific parasomnias (eg, sleep paralysis and narcolepsy, REM sleep behavior disorder and Parkinson’s disease [PD], etc.).54 Diagnosis of parasomnias is mainly clinical but supporting evidence can be obtained through in-lab polysomnography.
Treatment. For parasomnias, treatment is primarily supportive and includes creating a safe sleeping environment to reduce the risk of self-harm. Recommendations include sleeping in a room on the ground floor, minimizing furniture in the bedroom, padding any bedside furniture, child-proofing doorknobs, and locking up weapons and other dangerous household items.54
REM sleep behavior disorder (RBD). This disorder is characterized by a loss of the typical REM sleep-associated atonia and the presence of motor activity during dreaming (dream-enacted behaviors). While the estimated incidence of RBD in the general adult population is approximately 0.5%, it increases to 7.7% among those age >60.57 RBD occurs most commonly in the setting of the alpha-synucleinopathies (PD, Lewy body dementia, multisystem atrophy), but can also be found in patients with cerebral ischemia, demyelinating disorders, or alcohol misuse, or can be medication-induced (primarily antidepressants and antipsychotics).58 In patients with PD, the presence of RBD is associated with a more impaired cognitive profile, suggestive of widespread neurodegeneration.59 Recent studies revealed that RBD may also be a prodromal state of neurodegenerative diseases such as PD, which should prompt close monitoring and long-term follow up.60 Similar to other parasomnias, the diagnosis of RBD is primarily clinical, but polysomnography plays an important role in demonstrating loss of REM-related atonia.54
Treatment. Clonazepam and melatonin have been shown to be effective in treating the symptoms of RBD.54
Depression, anxiety, and sleep disturbances
Major depressive disorder (MDD) and generalized anxiety disorder (GAD) affect sleep in patients of all ages, but are underreported in older adults. According to national epidemiologic surveys, the estimated prevalence of MDD and GAD among older adults is 13% and 11.4%, respectively.61,62 Rates as high as 42% and 39% have been reported in meta-regression analyses among patients with Alzheimer’s dementia.63
Continue to: Depression and anxiety
Depression and anxiety may have additive effects and manifest as poor sleep satisfaction, increased sleep latency, insomnia, and daytime sleepiness.64 However, they may also have independent effects. Studies showed that patients with depression alone reported overall poor sleep satisfaction, whereas patients with anxiety alone reported problems with sleep latency, daytime drowsiness, and waking up at night in addition to their overall poor sleep satisfaction.65-67 Both depression and anxiety are risk factors for developing cognitive decline, and may be an early sign/prodrome of neurodegenerative diseases (dementias).68 The bidirectional relationship between depression/anxiety and sleep is complex and needs further investigation.
Treatment. Pharmacologic treatments for patients with depression/anxiety and sleep disturbances include selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, tricyclic antidepressants, and other serotonin receptor agonists.69-72 Nonpharmacologic treatments include CBT for both depression and anxiety, and problem-solving therapy for patients with mild cognitive impairment and depression.73,74 For severe depression and/or anxiety, electroconvulsive therapy is effective.75
Bottom Line
Sleep disorders in older adults are common but often underdiagnosed. Timely recognition of obstructive sleep apnea, central sleep apnea, insomnia, parasomnias, and other sleep disturbances can facilitate effective treatment and greatly improve older adults’ quality of life.
Related Resources
- American Academy of Sleep Medicine. International Classification of Sleep Disorders—Third Edition. https://aasm.org
- SleepFoundation.org. Sleep hygiene. https://www.sleepfoundation.org/articles/sleep-hygiene
Drug Brand Names
Acetazolamide • Diamox
Clonazepam • Klonopin
Doxepin • Silenor
Eszopiclone • Lunesta
Gabapentin • Neurontin
Mirtazapine • Remeron
Pramipexole • Mirapex
Quetiapine • Seroquel
Ramelteon • Rozerem
Suvorexant • Belsomra
Temazepam • Restoril
Theophylline • Elixophyllin
Tiagabine • Gabitril
Trazadone • Desyrel
Triazolam • Halcion
Zaleplon • Sonata
Zolpidem • Ambien
1. Centers for Disease Control and Prevention. The state of aging and health in America. 2013. Accessed January 27, 2021. https://www.cdc.gov/aging/pdf/state-aging-health-in-america-2013.pdf
2. Suzuki K, Miyamoto M, Hirata K. Sleep disorders in the elderly: diagnosis and management. J Gen Fam Med. 2017;18(2):61-71.
3. Inouye SK, Studenski S, Tinetti ME, et al. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55(5):780-791.
4. Patel D, Steinberg J, Patel P. Insomnia in the elderly: a review. J Clin Sleep Med. 2018;14(6):1017-1024.
5. Neubauer DN. A review of ramelteon in the treatment of sleep disorders. Neuropsychiatr Dis Treat. 2008;4(1):69-79.
6. Mander BA, Winer JR, Walker MP. Sleep and human aging. Neuron. 2017;94(1):19-36.
7. Ohayon MM, Carskadon MA, Guilleminault C, et al. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255-1273.
8. Li J, Vitiello MV, Gooneratne NS. Sleep in normal aging. Sleep Med Clin. 2018;13(1):1-11.
9. Floyd JA, Medler SM, Ager JW, et al. Age-related changes in initiation and maintenance of sleep: a meta-analysis. Res Nurs Health. 2000;23(2):106-117.
10. Floyd JA, Janisse JJ, Jenuwine ES, et al. Changes in REM-sleep percentage over the adult lifespan. Sleep. 2007;30(7):829-836.
11. Dorffner G, Vitr M, Anderer P. The effects of aging on sleep architecture in healthy subjects. Adv Exp Med Biol. 2015;821:93-100.
12. Furihata R, Kaneita Y, Jike M, et al. Napping and associated factors: a Japanese nationwide general population survey. Sleep Med. 2016;20:72-79.
13. Foley DJ, Vitiello MV, Bliwise DL, et al. Frequent napping is associated with excessive daytime sleepiness, depression, pain, and nocturia in older adults: findings from the National Sleep Foundation ‘2003 Sleep in America’ Poll. Am J Geriatr Psychiatry. 2007;15(4):344-350.
14. Floyd JA, Janisse JJ, Marshall Medler S, et al. Nonlinear components of age-related change in sleep initiation. Nurs Res. 2000;49(5):290-294.
15. Miner B, Kryger MH. Sleep in the aging population. Sleep Med Clin. 2017;12(1):31-38.
16. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217-1239.
17. Ancoli-Israel S, Klauber MR, Butters N, et al. Dementia in institutionalized elderly: relation to sleep apnea. J Am Geriatr Soc. 1991;39(3):258-263.
18. Spira AP, Stone KL, Rebok GW, et al. Sleep-disordered breathing and functional decline in older women. J Am Geriatr Soc. 2014;62(11):2040-2046.
19. Vijayan VK. Morbidities associated with obstructive sleep apnea. Expert Rev Respir Med. 2012;6(5):557-566.
20. Kerner NA, Roose SP. Obstructive sleep apnea is linked to depression and cognitive impairment: evidence and potential mechanisms. Am J Geriatr Psychiatry. 2016;24(6):496-508.
21. Dalmases M, Solé-Padullés C, Torres M, et al. Effect of CPAP on cognition, brain function, and structure among elderly patients with OSA: a randomized pilot study. Chest. 2015;148(5):1214-1223.
22. Toronto Western Hospital, University Health Network. University of Toronto. STOP-Bang Questionnaire. 2012. Accessed January 26, 2021. www.stopbang.ca
23. Freedman N. Doing it better for less: incorporating OSA management into alternative payment models. Chest. 2019;155(1):227-233.
24. Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479-504.
25. Semelka M, Wilson J, Floyd R. Diagnosis and treatment of obstructive sleep apnea in adults. Am Fam Physician. 2016;94(5):355-360.
26. Javaheri S, Dempsey JA. Central sleep apnea. Compr Physiol. 2013;3(1):141-163.
27. Donovan LM, Kapur VK. Prevalence and characteristics of central compared to obstructive sleep apnea: analyses from the Sleep Heart Health Study cohort. Sleep. 2016;39(7):1353-1359.
28. Cao M, Cardell CY, Willes L, et al. A novel adaptive servoventilation (ASVAuto) for the treatment of central sleep apnea associated with chronic use of opioids. J Clin Sleep Med. 2014;10(8):855-861.
29. Oldenburg O, Spießhöfer J, Fox H, et al. Performance of conventional and enhanced adaptive servoventilation (ASV) in heart failure patients with central sleep apnea who have adapted to conventional ASV. Sleep Breath. 2015;19(3):795-800.
30. Costanzo MR, Ponikowski P, Javaheri S, et al. Transvenous neurostimulation for central sleep apnoea: a randomised controlled trial. Lancet. 2016;388(10048):974-982.
31. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013:362.
32. Perlis ML, Smith LJ, Lyness JM, et al. Insomnia as a risk factor for onset of depression in the elderly. Behav Sleep Med. 2006;4(2):104-113.
33. Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am J Psychiatry. 2003;160(6):1147-1156.
34. Pigeon WR, Hegel M, Unützer J, et al. Is insomnia a perpetuating factor for late-life depression in the IMPACT cohort? Sleep. 2008;31(4):481-488.
35. Hughes JM, Song Y, Fung CH, et al. Measuring sleep in vulnerable older adults: a comparison of subjective and objective sleep measures. Clin Gerontol. 2018;41(2):145-157.
36. Irish LA, Kline CE, Gunn HE, et al. The role of sleep hygiene in promoting public health: a review of empirical evidence. Sleep Med Rev. 2015;22:23-36.
37. Sleep Foundation. Sleep hygiene. Accessed January 27, 2021. https://www.sleepfoundation.org/articles/sleep-hygiene
38. Foley D, Ancoli-Israel S, Britz P, et al. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56(5):497-502.
39. Eslami V, Zimmerman ME, Grewal T, et al. Pain grade and sleep disturbance in older adults: evaluation the role of pain, and stress for depressed and non-depressed individuals. Int J Geriatr Psychiatry. 2016;31(5):450-457.
40. American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
41. United States Food & Drug Administration. FDA adds Boxed Warning for risk of serious injuries caused by sleepwalking with certain prescription insomnia medicines. 2019. Accessed January 27, 2021. https://www.fda.gov/drugs/drug-safety-and-availability/fda-adds-boxed-warning-risk-serious-injuries-caused-sleepwalking-certain-prescription-insomnia
42. Schroeck JL, Ford J, Conway EL, et al. Review of safety and efficacy of sleep medicines in older adults. Clin Ther. 2016;38(11):2340-2372.
43. Krystal AD, Lankford A, Durrence HH, et al. Efficacy and safety of doxepin 3 and 6 mg in a 35-day sleep laboratory trial in adults with chronic primary insomnia. Sleep. 2011;34(10):1433-1442.
44. Roth T, Seiden D, Sainati S, et al. Effects of ramelteon on patient-reported sleep latency in older adults with chronic insomnia. Sleep Med. 2006;7(4):312-318.
45. Zammit G, Wang-Weigand S, Rosenthal M, et al. Effect of ramelteon on middle-of-the-night balance in older adults with chronic insomnia. J Clin Sleep Med. 2009;5(1):34-40.
46. Mets MAJ, de Vries JM, de Senerpont Domis LM, et al. Next-day effects of ramelteon (8 mg), zopiclone (7.5 mg), and placebo on highway driving performance, memory functioning, psychomotor performance, and mood in healthy adult subjects. Sleep. 2011;34(10):1327-1334.
47. Rhyne DN, Anderson SL. Suvorexant in insomnia: efficacy, safety and place in therapy. Ther Adv Drug Saf. 2015;6(5):189-195.
48. Norman JL, Anderson SL. Novel class of medications, orexin receptor antagonists, in the treatment of insomnia - critical appraisal of suvorexant. Nat Sci Sleep. 2016;8:239-247.
49. Owen RT. Suvorexant: efficacy and safety profile of a dual orexin receptor antagonist in treating insomnia. Drugs Today (Barc). 2016;52(1):29-40.
50. Shannon S, Lewis N, Lee H, et al. Cannabidiol in anxiety and sleep: a large case series. Perm J. 2019;23:18-041. doi: 10.7812/TPP/18-041
51. Yunusa I, Alsumali A, Garba AE, et al. Assessment of reported comparative effectiveness and safety of atypical antipsychotics in the treatment of behavioral and psychological symptoms of dementia: a network meta-analysis. JAMA Netw Open. 2019;2(3):e190828.
52. Bjorvatn B, Gronli J, Pallesen S. Prevalence of different parasomnias in the general population. Sleep Med. 2010;11(10):1031-1034.
53. Postuma RB, Montplaisir JY, Pelletier A, et al. Environmental risk factors for REM sleep behavior disorder: a multicenter case-control study. Neurology. 2012;79(5):428-434.
54. Fleetham JA, Fleming JA. Parasomnias. CMAJ. 2014;186(8):E273-E280.
55. Dinis-Oliveira RJ, Caldas I, Carvalho F, et al. Bruxism after 3,4-methylenedioxymethamphetamine (ecstasy) abuse. Clin Toxicol (Phila.) 2010;48(8):863-864.
56. Irfan MH, Howell MJ. Rapid eye movement sleep behavior disorder: overview and current perspective. Curr Sleep Medicine Rep. 2016;2:64-73.
57. Mahlknecht P, Seppi K, Frauscher B, et al. Probable RBD and association with neurodegenerative disease markers: a population-based study. Mov Disord. 2015;30(10):1417-1421.
58. Oertel WH, Depboylu C, Krenzer M, et al. [REM sleep behavior disorder as a prodromal stage of α-synucleinopathies: symptoms, epidemiology, pathophysiology, diagnosis and therapy]. Nervenarzt. 2014;85:19-25. German.
59. Jozwiak N, Postuma RB, Montplaisir J, et al. REM sleep behavior disorder and cognitive impairment in Parkinson’s disease. Sleep. 2017;40(8):zsx101. doi: 10.1093/sleep/zsx101
60. Claassen DO, Josephs KA, Ahlskog JE, et al. REM sleep behavior disorder preceding other aspects of synucleinopathies by up to half a century. Neurology 2010;75(6):494-499.
61. Reynolds K, Pietrzak RH, El-Gabalawy R, et al. Prevalence of psychiatric disorders in U.S. older adults: findings from a nationally representative survey. World Psychiatry. 2015;14(1):74-81.
62. Lohman MC, Mezuk B, Dumenci L. Depression and frailty: concurrent risks for adverse health outcomes. Aging Ment Health. 2017;21(4):399-408.
63. Zhao QF, Tan L, Wang HF, et al. The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: systematic review and meta-analysis. J Affect Disord. 2016;190:264-271.
64. Furihata R, Hall MH, Stone KL, et al. An aggregate measure of sleep health is associated with prevalent and incident clinically significant depression symptoms among community-dwelling older women. Sleep. 2017;40(3):zsw075. doi: 10.1093/sleep/zsw075
65. Spira AP, Stone K, Beaudreau SA, et al. Anxiety symptoms and objectively measured sleep quality in older women. Am J Geriatr Psychiatry. 2009;17(2):136-143.
66. Press Y, Punchik B, Freud T. The association between subjectively impaired sleep and symptoms of depression and anxiety in a frail elderly population. Aging Clin Exp Res. 2018;30(7):755-765.
67. Gould CE, Spira AP, Liou-Johnson V, et al. Association of anxiety symptom clusters with sleep quality and daytime sleepiness. J Gerontol B Psychol Sci Soc Sci. 2018;73(3):413-420.
68. Kassem AM, Ganguli M, Yaffe K, et al. Anxiety symptoms and risk of cognitive decline in older community-dwelling men. Int Psychogeriatr. 2017;29(7):1137-1145.
69. Frank C. Pharmacologic treatment of depression in the elderly. Can Fam Physician. 2014;60(2):121-126.
70. Subramanyam AA, Kedare J, Singh OP, et al. Clinical practice guidelines for geriatric anxiety disorders. Indian J Psychiatry. 2018;60(suppl 3):S371-S382.
71. Emsley R, Ahokas A, Suarez A, et al. Efficacy of tianeptine 25-50 mg in elderly patients with recurrent major depressive disorder: an 8-week placebo- and escitalopram-controlled study. J Clin Psychiatry. 2018;79(4):17m11741. doi: 10.4088/JCP.17m11741
72. Semel D, Murphy TK, Zlateva G, et al. Evaluation of the safety and efficacy of pregabalin in older patients with neuropathic pain: results from a pooled analysis of 11 clinical studies. BMC Fam Pract. 2010;11:85.
73. Orgeta V, Qazi A, Spector A, et al. Psychological treatments for depression and anxiety in dementia and mild cognitive impairment: systematic review and meta-analysis. Br J Psychiatry. 2015;207(4):293-298.
74. Morimoto SS, Kanellopoulos D, Manning KJ, et al. Diagnosis and treatment of depression and cognitive impairment in late life. Ann N Y Acad Sci. 2015;1345(1):36-46.
75. Casey DA. Depression in older adults: a treatable medical condition. Prim Care. 2017;44(3):499-510.
As humans live longer, a renewed focus on quality of life has made the prompt diagnosis and treatment of sleep-related disorders in older adults increasingly necessary.1 Normative aging results in multiple changes in sleep architecture, including decreased total sleep time, decreased sleep efficiency, decreased slow-wave sleep (SWS), and increased awakenings after sleep onset.2 Sleep disturbances in older adults are increasingly recognized as multifactorial health conditions requiring comprehensive modification of risk factors, diagnosis, and treatment.3
In this article, we discuss the effects of aging on sleep architecture and provide an overview of primary sleep disorders in older adults. We also summarize strategies for diagnosing and treating sleep disorders in these patients.
Elements of the sleep cycle
The human sleep cycle begins with light sleep (sleep stages 1 and 2), progresses into SWS (sleep stage 3), and culminates in rapid eye movement (REM) sleep. The first 3 stages are referred to as non-rapid eye movement sleep (NREM). Throughout the night, this coupling of NREM and REM cycles occurs 4 to 6 times, with each successive cycle decreasing in length until awakening.4
Two complex neurologic pathways intersect to regulate the timing of sleep and wakefulness on arousal. The first pathway, the circadian system, is located within the suprachiasmatic nucleus of the hypothalamus and is highly dependent on external stimuli (light, food, etc.) to synchronize sleep/wake cycles. The suprachiasmatic nucleus regulates melatonin secretion by the pineal gland, which signals day-night transitions. The other pathway, the homeostatic system, modifies the amount of sleep needed daily. When multiple days of poor sleep occur, homeostatic sleep pressure (colloquially described as sleep debt) compensates by increasing the amount of sleep required in the following days. Together, the circadian and homeostatic systems work in conjunction to regulate sleep quantity to approximately one-third of the total sleep-wake cycle.2,5
Age-related dysfunction of the regulatory sleep pathways leads to blunting of the ability to initiate and sustain high-quality sleep.6 Dysregulation of homeostatic sleep pressure decreases time spent in SWS, and failure of the circadian signaling apparatus results in delays in sleep/wake timing.2 While research into the underlying neurobiology of sleep reveals that some of these changes are inherent to aging (Box7-14), significant underdiagnosed pathologies may adversely affect sleep architecture, including polypharmacy, comorbid neuropathology (eg, synucleinopathies, tauopathies, etc.), and primary sleep disorders (insomnias, hypersomnias, and parasomnias).15
Box
It has long been known that sleep architecture changes significantly with age. One of the largest meta-analyses of sleep changes in healthy individuals throughout childhood into old age found that total sleep time, sleep efficiency, percentage of slow-wave sleep, percentage of rapid eye movement sleep (REM), and REM latency all decreased with normative aging.7 Other studies have also found a decreased ability to maintain sleep (increased frequency of awakenings and prolonged nocturnal awakenings).8
Based on several meta-analyses, the average total sleep time at night in the adult population decreases by approximately 10 minutes per decade in both men and women.7,9-11 However, this pattern is not observed after age 60, when the total sleep time plateaus.7 Similarly, the duration of wake after sleep onset increases by approximately 10 minutes every decade for adults age 30 to 60, and plateaus after that.7,8
Epidemiologic studies have suggested that the prevalence of daytime napping increases with age.8 This trend continues into older age without a noticeable plateau.
A study of a nationally representative sample of >7,000 Japanese participants found that a significantly higher proportion of older adults take daytime naps (27.4%) compared with middle-age adults (14.4%).12 Older adults nap more frequently because of both lifestyle and biologic changes that accompany normative aging. Polls in the United States have shown a correlation between frequent napping and an increase in excessive daytime sleepiness, depression, pain, and nocturia.13
While sleep latency steadily increases after age 50, recent studies have shown that in healthy individuals, these changes are modest at best,7,9,14 which suggests that other pathologic factors may be contributing to this problem. Although healthy older people were found to have more frequent arousals throughout the night, they retained the ability to reinitiate sleep as rapidly as younger adults.7,9
Primary sleep disorders
Obstructive sleep apnea (OSA) is one of the most common, yet frequently underdiagnosed reversible causes of sleep disturbances. It is characterized by partial or complete airway obstruction culminating in periods of involuntary cessation of respirations during sleep. The resultant fragmentation in sleep leads to significant downstream effects over time, including excessive daytime sleepiness and fatigue, poor occupational and social performance, and substantial cognitive impairment.3 While it is well known that OSA increases in prevalence throughout middle age, this relationship plateaus after age 60.16 An estimated 40% to 60% of Americans age >60 are affected by OSA.17 The hypoxemia and fragmented sleep caused by unrecognized OSA are associated with a significant decline in activities of daily living (ADL).18 Untreated OSA is strongly linked to the development and progression of several major health conditions, including cardiovascular disease, diabetes mellitus, hypertension, stroke, and depression.19 In studies of long-term care facility residents—many of whom may have comorbid cognitive decline—researchers found that unrecognized OSA often mimics the progressive cognitive decline seen in major neurocognitive disorders.20 However, classic symptoms of OSA may not always be present in these patients, and their daytime sleepiness is often attributed to old age rather than to a pathological etiology.16 Screening for OSA and prompt initiation of the appropriate treatment may reverse OSA-induced cognitive changes in these patients.21
The primary presenting symptom of OSA is snoring, which is correlated with pauses in breathing. Risk factors include increased body mass index (BMI), thick neck circumference, male sex, and advanced age. In older adults, BMI has a lower impact on the Apnea-Hypopnea Index, an indicator of the number of pauses in breathing per hour, when compared with young and middle-age adults.16 Validated screening questionnaires for OSA include the STOP-Bang Questionnaire (Table 122), OSA50, Berlin Questionnaire, and Epworth Sleepiness Scale, each of which is used in different subpopulations. The current diagnostic standard for OSA is nocturnal polysomnography in a sleep laboratory, but recent advances in home sleep apnea testing have made it a viable, low-cost alternative for patients who do not have significant medical comorbidities.23 Standard utilized cutoffs for diagnosis are ≥5 events/hour (hypopneas associated with at least 4% oxygen desaturations) in conjunction with clinical symptoms of OSA.24

Continue to: Treatment
Treatment. First-line treatment for OSA is continuous positive airway pressure therapy, but adherence rates vary widely with patient education and regular follow-up.25 Adjunctive therapy includes weight loss, oral appliances, and uvulopalatopharyngoplasty, a procedure in which tissue in the throat is remodeled or removed.
Central sleep apnea (CSA) is a pause in breathing without evidence of associated respiratory effort. In adults, the development of CSA is indicative of underlying lower brainstem dysfunction, due to intermittent failures in the pontomedullary centers responsible for regulation of rhythmic breathing.26 This can occur as a consequence of multiple diseases, including congestive heart failure, stroke, renal failure, chronic medication use (opioids), and brain tumors.
The Sleep Heart Health Study—the largest community-based cohort study to date examining CSA—estimated that the prevalence of CSA among adults age >65 was 1.1% (compared with 0.4% in those age <65).27 Subgroup analysis revealed that men had significantly higher rates of CSA compared with women (2.7% vs 0.2%, respectively).
CSA may present similarly to OSA (excessive daytime somnolence, insomnia, poor sleep quality, difficulties with attention and concentration). Symptoms may also mimic those of coexisting medical conditions in older adults, such as nocturnal angina or paroxysmal nocturnal dyspnea.27 Any older patient with daytime sleepiness and risk factors for CSA should be referred for in-laboratory nocturnal polysomnography, the gold standard diagnostic test. Unlike in OSA, ambulatory diagnostic measures (home sleep apnea testing) have not been validated for this disorder.27
Treatment. The primary treatment for CSA is to address the underlying medical problem. Positive pressure ventilation has been attempted with mixed results. Supplemental oxygen and medical management (acetazolamide or theophylline) can help stimulate breathing. Newer studies have shown favorable outcomes with transvenous neurostimulation or adaptive servoventilation.28-30
Continue to: Insomnia
Insomnia. For a primary diagnosis of insomnia, DSM-5 requires at least 3 nights per week of sleep disturbances that induce distress or functional impairment for at least 3 months.31 The International Classification of Disease, 10th Edition requires at least 1 month of symptoms (lying awake for a long time before falling asleep, sleeping for short periods, being awake for most of the night, feeling lack of sleep, waking up early) after ruling out other sleep disorders, substance use, or other medical conditions.4 Clinically, insomnia tends to present in older adults as a subjective complaint of dissatisfaction with the quality and/or quantity of their sleep. Insomnia has been consistently shown to be a significant risk factor for both the development or exacerbation of depression in older adults.32-34
While the diagnosis of insomnia is mainly clinical via a thorough sleep and medication history, assistive ancillary testing can include wrist actigraphy and screening questionnaires (the Insomnia Severity Index and the Pittsburgh Sleep Quality Index).4 Because population studies of older adults have found discrepancies between objective and subjective methods of assessing sleep quality, relying on the accuracy of self-reported symptoms alone is questionable.35
Treatment. Given that drug elimination half-life increases with age, and the risks of adverse effects are increased in older adults, the preferred treatment modalities for insomnia are nonpharmacologic.4 Sleep hygiene education (Table 2) and cognitive-behavioral therapy (CBT) for insomnia are often the first-line therapies.4,36,37 It is crucial to manage comorbidities such as heart disease and obesity, as well as sources of discomfort from conditions such as arthritic pain.38,39 If nonpharmacologic therapies are not effective, pharmacologic options can be considered.4 Before prescribing sleep medications, it may be more fruitful to treat underlying psychiatric disorders such as depression and anxiety with antidepressants.4 Although benzodiazepines are helpful for their sedative effects, they are not recommended for older adults because of an increased risk of falls, rebound insomnia, potential tolerance, and associated cognitive impairment.40 Benzodiazepine receptor agonists (eg, zolpidem, eszopiclone, zaleplon) were initially developed as a first-line treatment for insomnia to replace the reliance on benzodiazepines, but these medications have a “black-box” warning of a serious risk of complex sleep behaviors, including life-threatening parasomnias.41 As a result, guidelines suggest a shorter duration of treatment with a benzodiazepine receptor agonist may still provide benefit while limiting the risk of adverse effects.42
Doxepin is the only antidepressant FDA-approved for insomnia; it improves sleep latency (time taken to initiate sleep after lying down), duration, and quality in adults age >65.43 Melatonin receptor agonists such as ramelteon and melatonin have shown positive results in older patients with insomnia. In clinical trials of patients age ≥65, ramelteon, which is FDA-approved for insomnia, produced no rebound insomnia, withdrawal effects, memory impairment, or gait instability.44-46 Suvorexant, an orexin receptor antagonist, decreases sleep latency and increases total sleep time equally in both young and older adults.47-49Table 340-51 provides a list of medications used to treat insomnia (including off-label agents) and their common adverse effects in older adults.

Parasomnias are undesirable behaviors that occur during sleep, commonly associated with the sleep-wake transition period. These behaviors can occur during REM sleep (nightmare disorder, sleep paralysis, REM sleep behavior disorder) or NREM sleep (somnambulism [sleepwalking], confusional arousals, sleep terrors). According to a cross-sectional Norwegian study of parasomnias, the estimated lifetime prevalence of sleep walking is 22.4%; sleep talking, 66.8%; confusional arousal, 18.5%; and sleep terror, 10.4%.52
Continue to: When evaluating a patient...
When evaluating a patient with parasomnias, it is important to review their drug and substance use as well as coexisting medical conditions. Drugs and substances that can affect sleep include prescription medications (second-generation antidepressants, stimulants, dopamine agonists), excessive caffeine, alcohol, certain foods (coffee, chocolate milk, black tea, caffeinated soft drinks), environmental exposures (smoking, pesticides), and recreational drugs (amphetamines).53-56 Certain medical conditions are correlated with specific parasomnias (eg, sleep paralysis and narcolepsy, REM sleep behavior disorder and Parkinson’s disease [PD], etc.).54 Diagnosis of parasomnias is mainly clinical but supporting evidence can be obtained through in-lab polysomnography.
Treatment. For parasomnias, treatment is primarily supportive and includes creating a safe sleeping environment to reduce the risk of self-harm. Recommendations include sleeping in a room on the ground floor, minimizing furniture in the bedroom, padding any bedside furniture, child-proofing doorknobs, and locking up weapons and other dangerous household items.54
REM sleep behavior disorder (RBD). This disorder is characterized by a loss of the typical REM sleep-associated atonia and the presence of motor activity during dreaming (dream-enacted behaviors). While the estimated incidence of RBD in the general adult population is approximately 0.5%, it increases to 7.7% among those age >60.57 RBD occurs most commonly in the setting of the alpha-synucleinopathies (PD, Lewy body dementia, multisystem atrophy), but can also be found in patients with cerebral ischemia, demyelinating disorders, or alcohol misuse, or can be medication-induced (primarily antidepressants and antipsychotics).58 In patients with PD, the presence of RBD is associated with a more impaired cognitive profile, suggestive of widespread neurodegeneration.59 Recent studies revealed that RBD may also be a prodromal state of neurodegenerative diseases such as PD, which should prompt close monitoring and long-term follow up.60 Similar to other parasomnias, the diagnosis of RBD is primarily clinical, but polysomnography plays an important role in demonstrating loss of REM-related atonia.54
Treatment. Clonazepam and melatonin have been shown to be effective in treating the symptoms of RBD.54
Depression, anxiety, and sleep disturbances
Major depressive disorder (MDD) and generalized anxiety disorder (GAD) affect sleep in patients of all ages, but are underreported in older adults. According to national epidemiologic surveys, the estimated prevalence of MDD and GAD among older adults is 13% and 11.4%, respectively.61,62 Rates as high as 42% and 39% have been reported in meta-regression analyses among patients with Alzheimer’s dementia.63
Continue to: Depression and anxiety
Depression and anxiety may have additive effects and manifest as poor sleep satisfaction, increased sleep latency, insomnia, and daytime sleepiness.64 However, they may also have independent effects. Studies showed that patients with depression alone reported overall poor sleep satisfaction, whereas patients with anxiety alone reported problems with sleep latency, daytime drowsiness, and waking up at night in addition to their overall poor sleep satisfaction.65-67 Both depression and anxiety are risk factors for developing cognitive decline, and may be an early sign/prodrome of neurodegenerative diseases (dementias).68 The bidirectional relationship between depression/anxiety and sleep is complex and needs further investigation.
Treatment. Pharmacologic treatments for patients with depression/anxiety and sleep disturbances include selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, tricyclic antidepressants, and other serotonin receptor agonists.69-72 Nonpharmacologic treatments include CBT for both depression and anxiety, and problem-solving therapy for patients with mild cognitive impairment and depression.73,74 For severe depression and/or anxiety, electroconvulsive therapy is effective.75
Bottom Line
Sleep disorders in older adults are common but often underdiagnosed. Timely recognition of obstructive sleep apnea, central sleep apnea, insomnia, parasomnias, and other sleep disturbances can facilitate effective treatment and greatly improve older adults’ quality of life.
Related Resources
- American Academy of Sleep Medicine. International Classification of Sleep Disorders—Third Edition. https://aasm.org
- SleepFoundation.org. Sleep hygiene. https://www.sleepfoundation.org/articles/sleep-hygiene
Drug Brand Names
Acetazolamide • Diamox
Clonazepam • Klonopin
Doxepin • Silenor
Eszopiclone • Lunesta
Gabapentin • Neurontin
Mirtazapine • Remeron
Pramipexole • Mirapex
Quetiapine • Seroquel
Ramelteon • Rozerem
Suvorexant • Belsomra
Temazepam • Restoril
Theophylline • Elixophyllin
Tiagabine • Gabitril
Trazadone • Desyrel
Triazolam • Halcion
Zaleplon • Sonata
Zolpidem • Ambien
As humans live longer, a renewed focus on quality of life has made the prompt diagnosis and treatment of sleep-related disorders in older adults increasingly necessary.1 Normative aging results in multiple changes in sleep architecture, including decreased total sleep time, decreased sleep efficiency, decreased slow-wave sleep (SWS), and increased awakenings after sleep onset.2 Sleep disturbances in older adults are increasingly recognized as multifactorial health conditions requiring comprehensive modification of risk factors, diagnosis, and treatment.3
In this article, we discuss the effects of aging on sleep architecture and provide an overview of primary sleep disorders in older adults. We also summarize strategies for diagnosing and treating sleep disorders in these patients.
Elements of the sleep cycle
The human sleep cycle begins with light sleep (sleep stages 1 and 2), progresses into SWS (sleep stage 3), and culminates in rapid eye movement (REM) sleep. The first 3 stages are referred to as non-rapid eye movement sleep (NREM). Throughout the night, this coupling of NREM and REM cycles occurs 4 to 6 times, with each successive cycle decreasing in length until awakening.4
Two complex neurologic pathways intersect to regulate the timing of sleep and wakefulness on arousal. The first pathway, the circadian system, is located within the suprachiasmatic nucleus of the hypothalamus and is highly dependent on external stimuli (light, food, etc.) to synchronize sleep/wake cycles. The suprachiasmatic nucleus regulates melatonin secretion by the pineal gland, which signals day-night transitions. The other pathway, the homeostatic system, modifies the amount of sleep needed daily. When multiple days of poor sleep occur, homeostatic sleep pressure (colloquially described as sleep debt) compensates by increasing the amount of sleep required in the following days. Together, the circadian and homeostatic systems work in conjunction to regulate sleep quantity to approximately one-third of the total sleep-wake cycle.2,5
Age-related dysfunction of the regulatory sleep pathways leads to blunting of the ability to initiate and sustain high-quality sleep.6 Dysregulation of homeostatic sleep pressure decreases time spent in SWS, and failure of the circadian signaling apparatus results in delays in sleep/wake timing.2 While research into the underlying neurobiology of sleep reveals that some of these changes are inherent to aging (Box7-14), significant underdiagnosed pathologies may adversely affect sleep architecture, including polypharmacy, comorbid neuropathology (eg, synucleinopathies, tauopathies, etc.), and primary sleep disorders (insomnias, hypersomnias, and parasomnias).15
Box
It has long been known that sleep architecture changes significantly with age. One of the largest meta-analyses of sleep changes in healthy individuals throughout childhood into old age found that total sleep time, sleep efficiency, percentage of slow-wave sleep, percentage of rapid eye movement sleep (REM), and REM latency all decreased with normative aging.7 Other studies have also found a decreased ability to maintain sleep (increased frequency of awakenings and prolonged nocturnal awakenings).8
Based on several meta-analyses, the average total sleep time at night in the adult population decreases by approximately 10 minutes per decade in both men and women.7,9-11 However, this pattern is not observed after age 60, when the total sleep time plateaus.7 Similarly, the duration of wake after sleep onset increases by approximately 10 minutes every decade for adults age 30 to 60, and plateaus after that.7,8
Epidemiologic studies have suggested that the prevalence of daytime napping increases with age.8 This trend continues into older age without a noticeable plateau.
A study of a nationally representative sample of >7,000 Japanese participants found that a significantly higher proportion of older adults take daytime naps (27.4%) compared with middle-age adults (14.4%).12 Older adults nap more frequently because of both lifestyle and biologic changes that accompany normative aging. Polls in the United States have shown a correlation between frequent napping and an increase in excessive daytime sleepiness, depression, pain, and nocturia.13
While sleep latency steadily increases after age 50, recent studies have shown that in healthy individuals, these changes are modest at best,7,9,14 which suggests that other pathologic factors may be contributing to this problem. Although healthy older people were found to have more frequent arousals throughout the night, they retained the ability to reinitiate sleep as rapidly as younger adults.7,9
Primary sleep disorders
Obstructive sleep apnea (OSA) is one of the most common, yet frequently underdiagnosed reversible causes of sleep disturbances. It is characterized by partial or complete airway obstruction culminating in periods of involuntary cessation of respirations during sleep. The resultant fragmentation in sleep leads to significant downstream effects over time, including excessive daytime sleepiness and fatigue, poor occupational and social performance, and substantial cognitive impairment.3 While it is well known that OSA increases in prevalence throughout middle age, this relationship plateaus after age 60.16 An estimated 40% to 60% of Americans age >60 are affected by OSA.17 The hypoxemia and fragmented sleep caused by unrecognized OSA are associated with a significant decline in activities of daily living (ADL).18 Untreated OSA is strongly linked to the development and progression of several major health conditions, including cardiovascular disease, diabetes mellitus, hypertension, stroke, and depression.19 In studies of long-term care facility residents—many of whom may have comorbid cognitive decline—researchers found that unrecognized OSA often mimics the progressive cognitive decline seen in major neurocognitive disorders.20 However, classic symptoms of OSA may not always be present in these patients, and their daytime sleepiness is often attributed to old age rather than to a pathological etiology.16 Screening for OSA and prompt initiation of the appropriate treatment may reverse OSA-induced cognitive changes in these patients.21
The primary presenting symptom of OSA is snoring, which is correlated with pauses in breathing. Risk factors include increased body mass index (BMI), thick neck circumference, male sex, and advanced age. In older adults, BMI has a lower impact on the Apnea-Hypopnea Index, an indicator of the number of pauses in breathing per hour, when compared with young and middle-age adults.16 Validated screening questionnaires for OSA include the STOP-Bang Questionnaire (Table 122), OSA50, Berlin Questionnaire, and Epworth Sleepiness Scale, each of which is used in different subpopulations. The current diagnostic standard for OSA is nocturnal polysomnography in a sleep laboratory, but recent advances in home sleep apnea testing have made it a viable, low-cost alternative for patients who do not have significant medical comorbidities.23 Standard utilized cutoffs for diagnosis are ≥5 events/hour (hypopneas associated with at least 4% oxygen desaturations) in conjunction with clinical symptoms of OSA.24

Continue to: Treatment
Treatment. First-line treatment for OSA is continuous positive airway pressure therapy, but adherence rates vary widely with patient education and regular follow-up.25 Adjunctive therapy includes weight loss, oral appliances, and uvulopalatopharyngoplasty, a procedure in which tissue in the throat is remodeled or removed.
Central sleep apnea (CSA) is a pause in breathing without evidence of associated respiratory effort. In adults, the development of CSA is indicative of underlying lower brainstem dysfunction, due to intermittent failures in the pontomedullary centers responsible for regulation of rhythmic breathing.26 This can occur as a consequence of multiple diseases, including congestive heart failure, stroke, renal failure, chronic medication use (opioids), and brain tumors.
The Sleep Heart Health Study—the largest community-based cohort study to date examining CSA—estimated that the prevalence of CSA among adults age >65 was 1.1% (compared with 0.4% in those age <65).27 Subgroup analysis revealed that men had significantly higher rates of CSA compared with women (2.7% vs 0.2%, respectively).
CSA may present similarly to OSA (excessive daytime somnolence, insomnia, poor sleep quality, difficulties with attention and concentration). Symptoms may also mimic those of coexisting medical conditions in older adults, such as nocturnal angina or paroxysmal nocturnal dyspnea.27 Any older patient with daytime sleepiness and risk factors for CSA should be referred for in-laboratory nocturnal polysomnography, the gold standard diagnostic test. Unlike in OSA, ambulatory diagnostic measures (home sleep apnea testing) have not been validated for this disorder.27
Treatment. The primary treatment for CSA is to address the underlying medical problem. Positive pressure ventilation has been attempted with mixed results. Supplemental oxygen and medical management (acetazolamide or theophylline) can help stimulate breathing. Newer studies have shown favorable outcomes with transvenous neurostimulation or adaptive servoventilation.28-30
Continue to: Insomnia
Insomnia. For a primary diagnosis of insomnia, DSM-5 requires at least 3 nights per week of sleep disturbances that induce distress or functional impairment for at least 3 months.31 The International Classification of Disease, 10th Edition requires at least 1 month of symptoms (lying awake for a long time before falling asleep, sleeping for short periods, being awake for most of the night, feeling lack of sleep, waking up early) after ruling out other sleep disorders, substance use, or other medical conditions.4 Clinically, insomnia tends to present in older adults as a subjective complaint of dissatisfaction with the quality and/or quantity of their sleep. Insomnia has been consistently shown to be a significant risk factor for both the development or exacerbation of depression in older adults.32-34
While the diagnosis of insomnia is mainly clinical via a thorough sleep and medication history, assistive ancillary testing can include wrist actigraphy and screening questionnaires (the Insomnia Severity Index and the Pittsburgh Sleep Quality Index).4 Because population studies of older adults have found discrepancies between objective and subjective methods of assessing sleep quality, relying on the accuracy of self-reported symptoms alone is questionable.35
Treatment. Given that drug elimination half-life increases with age, and the risks of adverse effects are increased in older adults, the preferred treatment modalities for insomnia are nonpharmacologic.4 Sleep hygiene education (Table 2) and cognitive-behavioral therapy (CBT) for insomnia are often the first-line therapies.4,36,37 It is crucial to manage comorbidities such as heart disease and obesity, as well as sources of discomfort from conditions such as arthritic pain.38,39 If nonpharmacologic therapies are not effective, pharmacologic options can be considered.4 Before prescribing sleep medications, it may be more fruitful to treat underlying psychiatric disorders such as depression and anxiety with antidepressants.4 Although benzodiazepines are helpful for their sedative effects, they are not recommended for older adults because of an increased risk of falls, rebound insomnia, potential tolerance, and associated cognitive impairment.40 Benzodiazepine receptor agonists (eg, zolpidem, eszopiclone, zaleplon) were initially developed as a first-line treatment for insomnia to replace the reliance on benzodiazepines, but these medications have a “black-box” warning of a serious risk of complex sleep behaviors, including life-threatening parasomnias.41 As a result, guidelines suggest a shorter duration of treatment with a benzodiazepine receptor agonist may still provide benefit while limiting the risk of adverse effects.42
Doxepin is the only antidepressant FDA-approved for insomnia; it improves sleep latency (time taken to initiate sleep after lying down), duration, and quality in adults age >65.43 Melatonin receptor agonists such as ramelteon and melatonin have shown positive results in older patients with insomnia. In clinical trials of patients age ≥65, ramelteon, which is FDA-approved for insomnia, produced no rebound insomnia, withdrawal effects, memory impairment, or gait instability.44-46 Suvorexant, an orexin receptor antagonist, decreases sleep latency and increases total sleep time equally in both young and older adults.47-49Table 340-51 provides a list of medications used to treat insomnia (including off-label agents) and their common adverse effects in older adults.

Parasomnias are undesirable behaviors that occur during sleep, commonly associated with the sleep-wake transition period. These behaviors can occur during REM sleep (nightmare disorder, sleep paralysis, REM sleep behavior disorder) or NREM sleep (somnambulism [sleepwalking], confusional arousals, sleep terrors). According to a cross-sectional Norwegian study of parasomnias, the estimated lifetime prevalence of sleep walking is 22.4%; sleep talking, 66.8%; confusional arousal, 18.5%; and sleep terror, 10.4%.52
Continue to: When evaluating a patient...
When evaluating a patient with parasomnias, it is important to review their drug and substance use as well as coexisting medical conditions. Drugs and substances that can affect sleep include prescription medications (second-generation antidepressants, stimulants, dopamine agonists), excessive caffeine, alcohol, certain foods (coffee, chocolate milk, black tea, caffeinated soft drinks), environmental exposures (smoking, pesticides), and recreational drugs (amphetamines).53-56 Certain medical conditions are correlated with specific parasomnias (eg, sleep paralysis and narcolepsy, REM sleep behavior disorder and Parkinson’s disease [PD], etc.).54 Diagnosis of parasomnias is mainly clinical but supporting evidence can be obtained through in-lab polysomnography.
Treatment. For parasomnias, treatment is primarily supportive and includes creating a safe sleeping environment to reduce the risk of self-harm. Recommendations include sleeping in a room on the ground floor, minimizing furniture in the bedroom, padding any bedside furniture, child-proofing doorknobs, and locking up weapons and other dangerous household items.54
REM sleep behavior disorder (RBD). This disorder is characterized by a loss of the typical REM sleep-associated atonia and the presence of motor activity during dreaming (dream-enacted behaviors). While the estimated incidence of RBD in the general adult population is approximately 0.5%, it increases to 7.7% among those age >60.57 RBD occurs most commonly in the setting of the alpha-synucleinopathies (PD, Lewy body dementia, multisystem atrophy), but can also be found in patients with cerebral ischemia, demyelinating disorders, or alcohol misuse, or can be medication-induced (primarily antidepressants and antipsychotics).58 In patients with PD, the presence of RBD is associated with a more impaired cognitive profile, suggestive of widespread neurodegeneration.59 Recent studies revealed that RBD may also be a prodromal state of neurodegenerative diseases such as PD, which should prompt close monitoring and long-term follow up.60 Similar to other parasomnias, the diagnosis of RBD is primarily clinical, but polysomnography plays an important role in demonstrating loss of REM-related atonia.54
Treatment. Clonazepam and melatonin have been shown to be effective in treating the symptoms of RBD.54
Depression, anxiety, and sleep disturbances
Major depressive disorder (MDD) and generalized anxiety disorder (GAD) affect sleep in patients of all ages, but are underreported in older adults. According to national epidemiologic surveys, the estimated prevalence of MDD and GAD among older adults is 13% and 11.4%, respectively.61,62 Rates as high as 42% and 39% have been reported in meta-regression analyses among patients with Alzheimer’s dementia.63
Continue to: Depression and anxiety
Depression and anxiety may have additive effects and manifest as poor sleep satisfaction, increased sleep latency, insomnia, and daytime sleepiness.64 However, they may also have independent effects. Studies showed that patients with depression alone reported overall poor sleep satisfaction, whereas patients with anxiety alone reported problems with sleep latency, daytime drowsiness, and waking up at night in addition to their overall poor sleep satisfaction.65-67 Both depression and anxiety are risk factors for developing cognitive decline, and may be an early sign/prodrome of neurodegenerative diseases (dementias).68 The bidirectional relationship between depression/anxiety and sleep is complex and needs further investigation.
Treatment. Pharmacologic treatments for patients with depression/anxiety and sleep disturbances include selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, tricyclic antidepressants, and other serotonin receptor agonists.69-72 Nonpharmacologic treatments include CBT for both depression and anxiety, and problem-solving therapy for patients with mild cognitive impairment and depression.73,74 For severe depression and/or anxiety, electroconvulsive therapy is effective.75
Bottom Line
Sleep disorders in older adults are common but often underdiagnosed. Timely recognition of obstructive sleep apnea, central sleep apnea, insomnia, parasomnias, and other sleep disturbances can facilitate effective treatment and greatly improve older adults’ quality of life.
Related Resources
- American Academy of Sleep Medicine. International Classification of Sleep Disorders—Third Edition. https://aasm.org
- SleepFoundation.org. Sleep hygiene. https://www.sleepfoundation.org/articles/sleep-hygiene
Drug Brand Names
Acetazolamide • Diamox
Clonazepam • Klonopin
Doxepin • Silenor
Eszopiclone • Lunesta
Gabapentin • Neurontin
Mirtazapine • Remeron
Pramipexole • Mirapex
Quetiapine • Seroquel
Ramelteon • Rozerem
Suvorexant • Belsomra
Temazepam • Restoril
Theophylline • Elixophyllin
Tiagabine • Gabitril
Trazadone • Desyrel
Triazolam • Halcion
Zaleplon • Sonata
Zolpidem • Ambien
1. Centers for Disease Control and Prevention. The state of aging and health in America. 2013. Accessed January 27, 2021. https://www.cdc.gov/aging/pdf/state-aging-health-in-america-2013.pdf
2. Suzuki K, Miyamoto M, Hirata K. Sleep disorders in the elderly: diagnosis and management. J Gen Fam Med. 2017;18(2):61-71.
3. Inouye SK, Studenski S, Tinetti ME, et al. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55(5):780-791.
4. Patel D, Steinberg J, Patel P. Insomnia in the elderly: a review. J Clin Sleep Med. 2018;14(6):1017-1024.
5. Neubauer DN. A review of ramelteon in the treatment of sleep disorders. Neuropsychiatr Dis Treat. 2008;4(1):69-79.
6. Mander BA, Winer JR, Walker MP. Sleep and human aging. Neuron. 2017;94(1):19-36.
7. Ohayon MM, Carskadon MA, Guilleminault C, et al. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255-1273.
8. Li J, Vitiello MV, Gooneratne NS. Sleep in normal aging. Sleep Med Clin. 2018;13(1):1-11.
9. Floyd JA, Medler SM, Ager JW, et al. Age-related changes in initiation and maintenance of sleep: a meta-analysis. Res Nurs Health. 2000;23(2):106-117.
10. Floyd JA, Janisse JJ, Jenuwine ES, et al. Changes in REM-sleep percentage over the adult lifespan. Sleep. 2007;30(7):829-836.
11. Dorffner G, Vitr M, Anderer P. The effects of aging on sleep architecture in healthy subjects. Adv Exp Med Biol. 2015;821:93-100.
12. Furihata R, Kaneita Y, Jike M, et al. Napping and associated factors: a Japanese nationwide general population survey. Sleep Med. 2016;20:72-79.
13. Foley DJ, Vitiello MV, Bliwise DL, et al. Frequent napping is associated with excessive daytime sleepiness, depression, pain, and nocturia in older adults: findings from the National Sleep Foundation ‘2003 Sleep in America’ Poll. Am J Geriatr Psychiatry. 2007;15(4):344-350.
14. Floyd JA, Janisse JJ, Marshall Medler S, et al. Nonlinear components of age-related change in sleep initiation. Nurs Res. 2000;49(5):290-294.
15. Miner B, Kryger MH. Sleep in the aging population. Sleep Med Clin. 2017;12(1):31-38.
16. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217-1239.
17. Ancoli-Israel S, Klauber MR, Butters N, et al. Dementia in institutionalized elderly: relation to sleep apnea. J Am Geriatr Soc. 1991;39(3):258-263.
18. Spira AP, Stone KL, Rebok GW, et al. Sleep-disordered breathing and functional decline in older women. J Am Geriatr Soc. 2014;62(11):2040-2046.
19. Vijayan VK. Morbidities associated with obstructive sleep apnea. Expert Rev Respir Med. 2012;6(5):557-566.
20. Kerner NA, Roose SP. Obstructive sleep apnea is linked to depression and cognitive impairment: evidence and potential mechanisms. Am J Geriatr Psychiatry. 2016;24(6):496-508.
21. Dalmases M, Solé-Padullés C, Torres M, et al. Effect of CPAP on cognition, brain function, and structure among elderly patients with OSA: a randomized pilot study. Chest. 2015;148(5):1214-1223.
22. Toronto Western Hospital, University Health Network. University of Toronto. STOP-Bang Questionnaire. 2012. Accessed January 26, 2021. www.stopbang.ca
23. Freedman N. Doing it better for less: incorporating OSA management into alternative payment models. Chest. 2019;155(1):227-233.
24. Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479-504.
25. Semelka M, Wilson J, Floyd R. Diagnosis and treatment of obstructive sleep apnea in adults. Am Fam Physician. 2016;94(5):355-360.
26. Javaheri S, Dempsey JA. Central sleep apnea. Compr Physiol. 2013;3(1):141-163.
27. Donovan LM, Kapur VK. Prevalence and characteristics of central compared to obstructive sleep apnea: analyses from the Sleep Heart Health Study cohort. Sleep. 2016;39(7):1353-1359.
28. Cao M, Cardell CY, Willes L, et al. A novel adaptive servoventilation (ASVAuto) for the treatment of central sleep apnea associated with chronic use of opioids. J Clin Sleep Med. 2014;10(8):855-861.
29. Oldenburg O, Spießhöfer J, Fox H, et al. Performance of conventional and enhanced adaptive servoventilation (ASV) in heart failure patients with central sleep apnea who have adapted to conventional ASV. Sleep Breath. 2015;19(3):795-800.
30. Costanzo MR, Ponikowski P, Javaheri S, et al. Transvenous neurostimulation for central sleep apnoea: a randomised controlled trial. Lancet. 2016;388(10048):974-982.
31. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013:362.
32. Perlis ML, Smith LJ, Lyness JM, et al. Insomnia as a risk factor for onset of depression in the elderly. Behav Sleep Med. 2006;4(2):104-113.
33. Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am J Psychiatry. 2003;160(6):1147-1156.
34. Pigeon WR, Hegel M, Unützer J, et al. Is insomnia a perpetuating factor for late-life depression in the IMPACT cohort? Sleep. 2008;31(4):481-488.
35. Hughes JM, Song Y, Fung CH, et al. Measuring sleep in vulnerable older adults: a comparison of subjective and objective sleep measures. Clin Gerontol. 2018;41(2):145-157.
36. Irish LA, Kline CE, Gunn HE, et al. The role of sleep hygiene in promoting public health: a review of empirical evidence. Sleep Med Rev. 2015;22:23-36.
37. Sleep Foundation. Sleep hygiene. Accessed January 27, 2021. https://www.sleepfoundation.org/articles/sleep-hygiene
38. Foley D, Ancoli-Israel S, Britz P, et al. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56(5):497-502.
39. Eslami V, Zimmerman ME, Grewal T, et al. Pain grade and sleep disturbance in older adults: evaluation the role of pain, and stress for depressed and non-depressed individuals. Int J Geriatr Psychiatry. 2016;31(5):450-457.
40. American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
41. United States Food & Drug Administration. FDA adds Boxed Warning for risk of serious injuries caused by sleepwalking with certain prescription insomnia medicines. 2019. Accessed January 27, 2021. https://www.fda.gov/drugs/drug-safety-and-availability/fda-adds-boxed-warning-risk-serious-injuries-caused-sleepwalking-certain-prescription-insomnia
42. Schroeck JL, Ford J, Conway EL, et al. Review of safety and efficacy of sleep medicines in older adults. Clin Ther. 2016;38(11):2340-2372.
43. Krystal AD, Lankford A, Durrence HH, et al. Efficacy and safety of doxepin 3 and 6 mg in a 35-day sleep laboratory trial in adults with chronic primary insomnia. Sleep. 2011;34(10):1433-1442.
44. Roth T, Seiden D, Sainati S, et al. Effects of ramelteon on patient-reported sleep latency in older adults with chronic insomnia. Sleep Med. 2006;7(4):312-318.
45. Zammit G, Wang-Weigand S, Rosenthal M, et al. Effect of ramelteon on middle-of-the-night balance in older adults with chronic insomnia. J Clin Sleep Med. 2009;5(1):34-40.
46. Mets MAJ, de Vries JM, de Senerpont Domis LM, et al. Next-day effects of ramelteon (8 mg), zopiclone (7.5 mg), and placebo on highway driving performance, memory functioning, psychomotor performance, and mood in healthy adult subjects. Sleep. 2011;34(10):1327-1334.
47. Rhyne DN, Anderson SL. Suvorexant in insomnia: efficacy, safety and place in therapy. Ther Adv Drug Saf. 2015;6(5):189-195.
48. Norman JL, Anderson SL. Novel class of medications, orexin receptor antagonists, in the treatment of insomnia - critical appraisal of suvorexant. Nat Sci Sleep. 2016;8:239-247.
49. Owen RT. Suvorexant: efficacy and safety profile of a dual orexin receptor antagonist in treating insomnia. Drugs Today (Barc). 2016;52(1):29-40.
50. Shannon S, Lewis N, Lee H, et al. Cannabidiol in anxiety and sleep: a large case series. Perm J. 2019;23:18-041. doi: 10.7812/TPP/18-041
51. Yunusa I, Alsumali A, Garba AE, et al. Assessment of reported comparative effectiveness and safety of atypical antipsychotics in the treatment of behavioral and psychological symptoms of dementia: a network meta-analysis. JAMA Netw Open. 2019;2(3):e190828.
52. Bjorvatn B, Gronli J, Pallesen S. Prevalence of different parasomnias in the general population. Sleep Med. 2010;11(10):1031-1034.
53. Postuma RB, Montplaisir JY, Pelletier A, et al. Environmental risk factors for REM sleep behavior disorder: a multicenter case-control study. Neurology. 2012;79(5):428-434.
54. Fleetham JA, Fleming JA. Parasomnias. CMAJ. 2014;186(8):E273-E280.
55. Dinis-Oliveira RJ, Caldas I, Carvalho F, et al. Bruxism after 3,4-methylenedioxymethamphetamine (ecstasy) abuse. Clin Toxicol (Phila.) 2010;48(8):863-864.
56. Irfan MH, Howell MJ. Rapid eye movement sleep behavior disorder: overview and current perspective. Curr Sleep Medicine Rep. 2016;2:64-73.
57. Mahlknecht P, Seppi K, Frauscher B, et al. Probable RBD and association with neurodegenerative disease markers: a population-based study. Mov Disord. 2015;30(10):1417-1421.
58. Oertel WH, Depboylu C, Krenzer M, et al. [REM sleep behavior disorder as a prodromal stage of α-synucleinopathies: symptoms, epidemiology, pathophysiology, diagnosis and therapy]. Nervenarzt. 2014;85:19-25. German.
59. Jozwiak N, Postuma RB, Montplaisir J, et al. REM sleep behavior disorder and cognitive impairment in Parkinson’s disease. Sleep. 2017;40(8):zsx101. doi: 10.1093/sleep/zsx101
60. Claassen DO, Josephs KA, Ahlskog JE, et al. REM sleep behavior disorder preceding other aspects of synucleinopathies by up to half a century. Neurology 2010;75(6):494-499.
61. Reynolds K, Pietrzak RH, El-Gabalawy R, et al. Prevalence of psychiatric disorders in U.S. older adults: findings from a nationally representative survey. World Psychiatry. 2015;14(1):74-81.
62. Lohman MC, Mezuk B, Dumenci L. Depression and frailty: concurrent risks for adverse health outcomes. Aging Ment Health. 2017;21(4):399-408.
63. Zhao QF, Tan L, Wang HF, et al. The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: systematic review and meta-analysis. J Affect Disord. 2016;190:264-271.
64. Furihata R, Hall MH, Stone KL, et al. An aggregate measure of sleep health is associated with prevalent and incident clinically significant depression symptoms among community-dwelling older women. Sleep. 2017;40(3):zsw075. doi: 10.1093/sleep/zsw075
65. Spira AP, Stone K, Beaudreau SA, et al. Anxiety symptoms and objectively measured sleep quality in older women. Am J Geriatr Psychiatry. 2009;17(2):136-143.
66. Press Y, Punchik B, Freud T. The association between subjectively impaired sleep and symptoms of depression and anxiety in a frail elderly population. Aging Clin Exp Res. 2018;30(7):755-765.
67. Gould CE, Spira AP, Liou-Johnson V, et al. Association of anxiety symptom clusters with sleep quality and daytime sleepiness. J Gerontol B Psychol Sci Soc Sci. 2018;73(3):413-420.
68. Kassem AM, Ganguli M, Yaffe K, et al. Anxiety symptoms and risk of cognitive decline in older community-dwelling men. Int Psychogeriatr. 2017;29(7):1137-1145.
69. Frank C. Pharmacologic treatment of depression in the elderly. Can Fam Physician. 2014;60(2):121-126.
70. Subramanyam AA, Kedare J, Singh OP, et al. Clinical practice guidelines for geriatric anxiety disorders. Indian J Psychiatry. 2018;60(suppl 3):S371-S382.
71. Emsley R, Ahokas A, Suarez A, et al. Efficacy of tianeptine 25-50 mg in elderly patients with recurrent major depressive disorder: an 8-week placebo- and escitalopram-controlled study. J Clin Psychiatry. 2018;79(4):17m11741. doi: 10.4088/JCP.17m11741
72. Semel D, Murphy TK, Zlateva G, et al. Evaluation of the safety and efficacy of pregabalin in older patients with neuropathic pain: results from a pooled analysis of 11 clinical studies. BMC Fam Pract. 2010;11:85.
73. Orgeta V, Qazi A, Spector A, et al. Psychological treatments for depression and anxiety in dementia and mild cognitive impairment: systematic review and meta-analysis. Br J Psychiatry. 2015;207(4):293-298.
74. Morimoto SS, Kanellopoulos D, Manning KJ, et al. Diagnosis and treatment of depression and cognitive impairment in late life. Ann N Y Acad Sci. 2015;1345(1):36-46.
75. Casey DA. Depression in older adults: a treatable medical condition. Prim Care. 2017;44(3):499-510.
1. Centers for Disease Control and Prevention. The state of aging and health in America. 2013. Accessed January 27, 2021. https://www.cdc.gov/aging/pdf/state-aging-health-in-america-2013.pdf
2. Suzuki K, Miyamoto M, Hirata K. Sleep disorders in the elderly: diagnosis and management. J Gen Fam Med. 2017;18(2):61-71.
3. Inouye SK, Studenski S, Tinetti ME, et al. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55(5):780-791.
4. Patel D, Steinberg J, Patel P. Insomnia in the elderly: a review. J Clin Sleep Med. 2018;14(6):1017-1024.
5. Neubauer DN. A review of ramelteon in the treatment of sleep disorders. Neuropsychiatr Dis Treat. 2008;4(1):69-79.
6. Mander BA, Winer JR, Walker MP. Sleep and human aging. Neuron. 2017;94(1):19-36.
7. Ohayon MM, Carskadon MA, Guilleminault C, et al. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255-1273.
8. Li J, Vitiello MV, Gooneratne NS. Sleep in normal aging. Sleep Med Clin. 2018;13(1):1-11.
9. Floyd JA, Medler SM, Ager JW, et al. Age-related changes in initiation and maintenance of sleep: a meta-analysis. Res Nurs Health. 2000;23(2):106-117.
10. Floyd JA, Janisse JJ, Jenuwine ES, et al. Changes in REM-sleep percentage over the adult lifespan. Sleep. 2007;30(7):829-836.
11. Dorffner G, Vitr M, Anderer P. The effects of aging on sleep architecture in healthy subjects. Adv Exp Med Biol. 2015;821:93-100.
12. Furihata R, Kaneita Y, Jike M, et al. Napping and associated factors: a Japanese nationwide general population survey. Sleep Med. 2016;20:72-79.
13. Foley DJ, Vitiello MV, Bliwise DL, et al. Frequent napping is associated with excessive daytime sleepiness, depression, pain, and nocturia in older adults: findings from the National Sleep Foundation ‘2003 Sleep in America’ Poll. Am J Geriatr Psychiatry. 2007;15(4):344-350.
14. Floyd JA, Janisse JJ, Marshall Medler S, et al. Nonlinear components of age-related change in sleep initiation. Nurs Res. 2000;49(5):290-294.
15. Miner B, Kryger MH. Sleep in the aging population. Sleep Med Clin. 2017;12(1):31-38.
16. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217-1239.
17. Ancoli-Israel S, Klauber MR, Butters N, et al. Dementia in institutionalized elderly: relation to sleep apnea. J Am Geriatr Soc. 1991;39(3):258-263.
18. Spira AP, Stone KL, Rebok GW, et al. Sleep-disordered breathing and functional decline in older women. J Am Geriatr Soc. 2014;62(11):2040-2046.
19. Vijayan VK. Morbidities associated with obstructive sleep apnea. Expert Rev Respir Med. 2012;6(5):557-566.
20. Kerner NA, Roose SP. Obstructive sleep apnea is linked to depression and cognitive impairment: evidence and potential mechanisms. Am J Geriatr Psychiatry. 2016;24(6):496-508.
21. Dalmases M, Solé-Padullés C, Torres M, et al. Effect of CPAP on cognition, brain function, and structure among elderly patients with OSA: a randomized pilot study. Chest. 2015;148(5):1214-1223.
22. Toronto Western Hospital, University Health Network. University of Toronto. STOP-Bang Questionnaire. 2012. Accessed January 26, 2021. www.stopbang.ca
23. Freedman N. Doing it better for less: incorporating OSA management into alternative payment models. Chest. 2019;155(1):227-233.
24. Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479-504.
25. Semelka M, Wilson J, Floyd R. Diagnosis and treatment of obstructive sleep apnea in adults. Am Fam Physician. 2016;94(5):355-360.
26. Javaheri S, Dempsey JA. Central sleep apnea. Compr Physiol. 2013;3(1):141-163.
27. Donovan LM, Kapur VK. Prevalence and characteristics of central compared to obstructive sleep apnea: analyses from the Sleep Heart Health Study cohort. Sleep. 2016;39(7):1353-1359.
28. Cao M, Cardell CY, Willes L, et al. A novel adaptive servoventilation (ASVAuto) for the treatment of central sleep apnea associated with chronic use of opioids. J Clin Sleep Med. 2014;10(8):855-861.
29. Oldenburg O, Spießhöfer J, Fox H, et al. Performance of conventional and enhanced adaptive servoventilation (ASV) in heart failure patients with central sleep apnea who have adapted to conventional ASV. Sleep Breath. 2015;19(3):795-800.
30. Costanzo MR, Ponikowski P, Javaheri S, et al. Transvenous neurostimulation for central sleep apnoea: a randomised controlled trial. Lancet. 2016;388(10048):974-982.
31. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013:362.
32. Perlis ML, Smith LJ, Lyness JM, et al. Insomnia as a risk factor for onset of depression in the elderly. Behav Sleep Med. 2006;4(2):104-113.
33. Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am J Psychiatry. 2003;160(6):1147-1156.
34. Pigeon WR, Hegel M, Unützer J, et al. Is insomnia a perpetuating factor for late-life depression in the IMPACT cohort? Sleep. 2008;31(4):481-488.
35. Hughes JM, Song Y, Fung CH, et al. Measuring sleep in vulnerable older adults: a comparison of subjective and objective sleep measures. Clin Gerontol. 2018;41(2):145-157.
36. Irish LA, Kline CE, Gunn HE, et al. The role of sleep hygiene in promoting public health: a review of empirical evidence. Sleep Med Rev. 2015;22:23-36.
37. Sleep Foundation. Sleep hygiene. Accessed January 27, 2021. https://www.sleepfoundation.org/articles/sleep-hygiene
38. Foley D, Ancoli-Israel S, Britz P, et al. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56(5):497-502.
39. Eslami V, Zimmerman ME, Grewal T, et al. Pain grade and sleep disturbance in older adults: evaluation the role of pain, and stress for depressed and non-depressed individuals. Int J Geriatr Psychiatry. 2016;31(5):450-457.
40. American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
41. United States Food & Drug Administration. FDA adds Boxed Warning for risk of serious injuries caused by sleepwalking with certain prescription insomnia medicines. 2019. Accessed January 27, 2021. https://www.fda.gov/drugs/drug-safety-and-availability/fda-adds-boxed-warning-risk-serious-injuries-caused-sleepwalking-certain-prescription-insomnia
42. Schroeck JL, Ford J, Conway EL, et al. Review of safety and efficacy of sleep medicines in older adults. Clin Ther. 2016;38(11):2340-2372.
43. Krystal AD, Lankford A, Durrence HH, et al. Efficacy and safety of doxepin 3 and 6 mg in a 35-day sleep laboratory trial in adults with chronic primary insomnia. Sleep. 2011;34(10):1433-1442.
44. Roth T, Seiden D, Sainati S, et al. Effects of ramelteon on patient-reported sleep latency in older adults with chronic insomnia. Sleep Med. 2006;7(4):312-318.
45. Zammit G, Wang-Weigand S, Rosenthal M, et al. Effect of ramelteon on middle-of-the-night balance in older adults with chronic insomnia. J Clin Sleep Med. 2009;5(1):34-40.
46. Mets MAJ, de Vries JM, de Senerpont Domis LM, et al. Next-day effects of ramelteon (8 mg), zopiclone (7.5 mg), and placebo on highway driving performance, memory functioning, psychomotor performance, and mood in healthy adult subjects. Sleep. 2011;34(10):1327-1334.
47. Rhyne DN, Anderson SL. Suvorexant in insomnia: efficacy, safety and place in therapy. Ther Adv Drug Saf. 2015;6(5):189-195.
48. Norman JL, Anderson SL. Novel class of medications, orexin receptor antagonists, in the treatment of insomnia - critical appraisal of suvorexant. Nat Sci Sleep. 2016;8:239-247.
49. Owen RT. Suvorexant: efficacy and safety profile of a dual orexin receptor antagonist in treating insomnia. Drugs Today (Barc). 2016;52(1):29-40.
50. Shannon S, Lewis N, Lee H, et al. Cannabidiol in anxiety and sleep: a large case series. Perm J. 2019;23:18-041. doi: 10.7812/TPP/18-041
51. Yunusa I, Alsumali A, Garba AE, et al. Assessment of reported comparative effectiveness and safety of atypical antipsychotics in the treatment of behavioral and psychological symptoms of dementia: a network meta-analysis. JAMA Netw Open. 2019;2(3):e190828.
52. Bjorvatn B, Gronli J, Pallesen S. Prevalence of different parasomnias in the general population. Sleep Med. 2010;11(10):1031-1034.
53. Postuma RB, Montplaisir JY, Pelletier A, et al. Environmental risk factors for REM sleep behavior disorder: a multicenter case-control study. Neurology. 2012;79(5):428-434.
54. Fleetham JA, Fleming JA. Parasomnias. CMAJ. 2014;186(8):E273-E280.
55. Dinis-Oliveira RJ, Caldas I, Carvalho F, et al. Bruxism after 3,4-methylenedioxymethamphetamine (ecstasy) abuse. Clin Toxicol (Phila.) 2010;48(8):863-864.
56. Irfan MH, Howell MJ. Rapid eye movement sleep behavior disorder: overview and current perspective. Curr Sleep Medicine Rep. 2016;2:64-73.
57. Mahlknecht P, Seppi K, Frauscher B, et al. Probable RBD and association with neurodegenerative disease markers: a population-based study. Mov Disord. 2015;30(10):1417-1421.
58. Oertel WH, Depboylu C, Krenzer M, et al. [REM sleep behavior disorder as a prodromal stage of α-synucleinopathies: symptoms, epidemiology, pathophysiology, diagnosis and therapy]. Nervenarzt. 2014;85:19-25. German.
59. Jozwiak N, Postuma RB, Montplaisir J, et al. REM sleep behavior disorder and cognitive impairment in Parkinson’s disease. Sleep. 2017;40(8):zsx101. doi: 10.1093/sleep/zsx101
60. Claassen DO, Josephs KA, Ahlskog JE, et al. REM sleep behavior disorder preceding other aspects of synucleinopathies by up to half a century. Neurology 2010;75(6):494-499.
61. Reynolds K, Pietrzak RH, El-Gabalawy R, et al. Prevalence of psychiatric disorders in U.S. older adults: findings from a nationally representative survey. World Psychiatry. 2015;14(1):74-81.
62. Lohman MC, Mezuk B, Dumenci L. Depression and frailty: concurrent risks for adverse health outcomes. Aging Ment Health. 2017;21(4):399-408.
63. Zhao QF, Tan L, Wang HF, et al. The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: systematic review and meta-analysis. J Affect Disord. 2016;190:264-271.
64. Furihata R, Hall MH, Stone KL, et al. An aggregate measure of sleep health is associated with prevalent and incident clinically significant depression symptoms among community-dwelling older women. Sleep. 2017;40(3):zsw075. doi: 10.1093/sleep/zsw075
65. Spira AP, Stone K, Beaudreau SA, et al. Anxiety symptoms and objectively measured sleep quality in older women. Am J Geriatr Psychiatry. 2009;17(2):136-143.
66. Press Y, Punchik B, Freud T. The association between subjectively impaired sleep and symptoms of depression and anxiety in a frail elderly population. Aging Clin Exp Res. 2018;30(7):755-765.
67. Gould CE, Spira AP, Liou-Johnson V, et al. Association of anxiety symptom clusters with sleep quality and daytime sleepiness. J Gerontol B Psychol Sci Soc Sci. 2018;73(3):413-420.
68. Kassem AM, Ganguli M, Yaffe K, et al. Anxiety symptoms and risk of cognitive decline in older community-dwelling men. Int Psychogeriatr. 2017;29(7):1137-1145.
69. Frank C. Pharmacologic treatment of depression in the elderly. Can Fam Physician. 2014;60(2):121-126.
70. Subramanyam AA, Kedare J, Singh OP, et al. Clinical practice guidelines for geriatric anxiety disorders. Indian J Psychiatry. 2018;60(suppl 3):S371-S382.
71. Emsley R, Ahokas A, Suarez A, et al. Efficacy of tianeptine 25-50 mg in elderly patients with recurrent major depressive disorder: an 8-week placebo- and escitalopram-controlled study. J Clin Psychiatry. 2018;79(4):17m11741. doi: 10.4088/JCP.17m11741
72. Semel D, Murphy TK, Zlateva G, et al. Evaluation of the safety and efficacy of pregabalin in older patients with neuropathic pain: results from a pooled analysis of 11 clinical studies. BMC Fam Pract. 2010;11:85.
73. Orgeta V, Qazi A, Spector A, et al. Psychological treatments for depression and anxiety in dementia and mild cognitive impairment: systematic review and meta-analysis. Br J Psychiatry. 2015;207(4):293-298.
74. Morimoto SS, Kanellopoulos D, Manning KJ, et al. Diagnosis and treatment of depression and cognitive impairment in late life. Ann N Y Acad Sci. 2015;1345(1):36-46.
75. Casey DA. Depression in older adults: a treatable medical condition. Prim Care. 2017;44(3):499-510.
Romosozumab may not increase cardiovascular risk after all
The potent anabolic, antiosteoporosis agent romosozumab has been saddled with an Food and Drug Administration–mandated black-box warning for increased cardiovascular risk that may not be warranted, Glenn Haugeberg, MD, PhD, asserted at the 2021 Rheumatology Winter Clinical Symposium.
The black-box warning states that romosozumab (Evenity), a monoclonal antibody approved in 2019 for fracture prevention in patients with osteoporosis, may increase the risk of MI, stroke, and cardiovascular death. The warning arose from FDA concerns raised by the results of the phase 3 ARCH trial in which 4,093 postmenopausal women at high fracture risk were randomized to monthly subcutaneous injections of romosozumab or weekly dosing of the oral bisphosphonate alendronate (Fosamax) for 1 year, followed by 12 months of open-label alendronate for all. Alarm bells went off at the FDA because during year 1, the incidence of adjudicated major adverse cardiovascular events was 2.5% in the romosozumab arm, compared with 1.9% with alendronate.
Could a cardioprotective effect of bisphosphonates explain cardiovascular concerns?
However, evidence from multiple animal and human studies suggests that bisphosphonates actually have a cardioprotective effect. For example, a Taiwanese population-based cohort study of 1,548 patients on bisphosphonate therapy for osteoporotic fractures and 4,644 individuals with hip or vertebral fractures who were not on a bisphosphonate showed a 65% reduction in the risk of acute MI during 2 years of follow-up in those who received a bisphosphonate.
“That may explain the ARCH finding. It may – I say may – be that this concern in the ARCH study can be explained by the positive effect of the bisphosphonates on cardiovascular events,” according to Dr. Haugeberg, head of the division of rheumatology at the Southern Norway Hospital Trust, Kristiansand, and professor of medicine at the Norwegian University of Science and Technology, Trondheim.
He noted that, in the FRAME trial, another pivotal phase 3 trial of romosozumab, there was no signal of increased cardiovascular risk, compared with placebo. In FRAME, which included 7,180 osteoporotic postmenopausal women, rates of major adverse cardiovascular events and other adverse events were balanced between the two study arms at 12 months. Indeed, the incidence of adjudicated serious cardiovascular events was 0.5% with romosozumab and 0.4% with placebo injections. After 12 months, all participants were transitioned to denosumab (Prolia) for another 12 months. At 24 months, there remained no significant between-group difference in cardiovascular events, cancer, osteoarthritis, hyperostosis, or other major adverse events.
Potency of romosozumab
Romosozumab’s efficacy for fracture prevention in these two pivotal trials was striking. The risk of new vertebral fractures was reduced by 73% with romosozumab, compared with placebo at 12 months in FRAME, and by 75% at 24 months in the romosozumab-to-denosumab group.
“FRAME was a 12-month study for the primary endpoint. The bisphosphonate studies typically had a 3-year design in order to show benefit, but here you see only 12-month follow-up. This illustrates the potency of this drug. We saw rapid increase in bone density and a huge decrease in new vertebral fractures versus placebo in the first 12 months, then during follow-up with denosumab the reduction in fractures was maintained,” the rheumatologist commented.
In the ARCH trial, where romosozumab went head to head with a very effective oral bisphosphonate, the risk of new vertebral fractures was 48% lower at 24 months in the romosozumab-to-alendronate group than in women on alendronate for the full 24 months, while the risk of hip fractures was reduced by 38%.
Romosozumab is a humanized monoclonal antibody with a novel mechanism of anabolic action: This agent binds to sclerostin, which is produced in osteocytes. When sclerostin binds to receptors on osteoblasts it reduces their activity, thereby inhibiting bone formation. Romosozumab takes away this inhibition of osteoblasts, boosting their activity. The result is increased bone formation accompanied by decreased bone resorption. This allows for a logical treatment approach: first using an anabolic agent – in this instance, subcutaneously injected romosozumab at 210 mg once monthly for 12 months – then switching to an antiresorptive agent in order to maintain the gain in bone mineral density and decrease fracture risk. This is the same treatment strategy recommended when using the anabolic agents teriparatide (Forteo) and abaloparatide (Tymlos); however, those parathyroid hormone and parathyroid hormone–related protein analogs are seldom used in Norway because their cost is substantially greater than for romosozumab, he explained.
Updated Endocrine Society guidelines
Dr. Haugeberg called romosozumab “a new and wonderful drug.” The Endocrine Society also considers romosozumab an important new drug, as evidenced by the release of an 8-page update of the group’s clinical practice guideline on the pharmacologic management of osteoporosis in postmenopausal women; the update was devoted specifically to the use of romosozumab. The update, published in response to the biologic’s recent approval by U.S., Canadian, and European regulatory agencies, came just 10 months after release of the Endocrine Society’s comprehensive 28-page clinical practice guideline.
Dr. Haugeberg is a fan of the Endocrine Society guideline, which recommends romosozumab as a first-line therapy in postmenopausal women at very high risk of osteoporotic fracture, defined as those with a history of multiple vertebral fractures or severe osteoporosis with a T score of –2.5 or less at the hip or spine plus fractures. The updated guideline also recommends consideration of the antisclerostin biologic in high-risk patients who have failed on antiresorptive treatments.
The practice guideline states that the issue of a possible cardioprotective effect of alendronate in the ARCH trial “remains uncertain at this time.”
“Women at high risk of cardiovascular disease and stroke should not be considered for romosozumab pending further studies on cardiovascular risk associated with this treatment,” according to the Endocrine Society.
Dr. Haugeberg reported receiving research grants from Pfizer and Biogen and serving as a consultant to and/or on speakers’ bureaus for Amgen, which markets romosozumab, and more than a dozen other pharmaceutical companies.
The potent anabolic, antiosteoporosis agent romosozumab has been saddled with an Food and Drug Administration–mandated black-box warning for increased cardiovascular risk that may not be warranted, Glenn Haugeberg, MD, PhD, asserted at the 2021 Rheumatology Winter Clinical Symposium.
The black-box warning states that romosozumab (Evenity), a monoclonal antibody approved in 2019 for fracture prevention in patients with osteoporosis, may increase the risk of MI, stroke, and cardiovascular death. The warning arose from FDA concerns raised by the results of the phase 3 ARCH trial in which 4,093 postmenopausal women at high fracture risk were randomized to monthly subcutaneous injections of romosozumab or weekly dosing of the oral bisphosphonate alendronate (Fosamax) for 1 year, followed by 12 months of open-label alendronate for all. Alarm bells went off at the FDA because during year 1, the incidence of adjudicated major adverse cardiovascular events was 2.5% in the romosozumab arm, compared with 1.9% with alendronate.
Could a cardioprotective effect of bisphosphonates explain cardiovascular concerns?
However, evidence from multiple animal and human studies suggests that bisphosphonates actually have a cardioprotective effect. For example, a Taiwanese population-based cohort study of 1,548 patients on bisphosphonate therapy for osteoporotic fractures and 4,644 individuals with hip or vertebral fractures who were not on a bisphosphonate showed a 65% reduction in the risk of acute MI during 2 years of follow-up in those who received a bisphosphonate.
“That may explain the ARCH finding. It may – I say may – be that this concern in the ARCH study can be explained by the positive effect of the bisphosphonates on cardiovascular events,” according to Dr. Haugeberg, head of the division of rheumatology at the Southern Norway Hospital Trust, Kristiansand, and professor of medicine at the Norwegian University of Science and Technology, Trondheim.
He noted that, in the FRAME trial, another pivotal phase 3 trial of romosozumab, there was no signal of increased cardiovascular risk, compared with placebo. In FRAME, which included 7,180 osteoporotic postmenopausal women, rates of major adverse cardiovascular events and other adverse events were balanced between the two study arms at 12 months. Indeed, the incidence of adjudicated serious cardiovascular events was 0.5% with romosozumab and 0.4% with placebo injections. After 12 months, all participants were transitioned to denosumab (Prolia) for another 12 months. At 24 months, there remained no significant between-group difference in cardiovascular events, cancer, osteoarthritis, hyperostosis, or other major adverse events.
Potency of romosozumab
Romosozumab’s efficacy for fracture prevention in these two pivotal trials was striking. The risk of new vertebral fractures was reduced by 73% with romosozumab, compared with placebo at 12 months in FRAME, and by 75% at 24 months in the romosozumab-to-denosumab group.
“FRAME was a 12-month study for the primary endpoint. The bisphosphonate studies typically had a 3-year design in order to show benefit, but here you see only 12-month follow-up. This illustrates the potency of this drug. We saw rapid increase in bone density and a huge decrease in new vertebral fractures versus placebo in the first 12 months, then during follow-up with denosumab the reduction in fractures was maintained,” the rheumatologist commented.
In the ARCH trial, where romosozumab went head to head with a very effective oral bisphosphonate, the risk of new vertebral fractures was 48% lower at 24 months in the romosozumab-to-alendronate group than in women on alendronate for the full 24 months, while the risk of hip fractures was reduced by 38%.
Romosozumab is a humanized monoclonal antibody with a novel mechanism of anabolic action: This agent binds to sclerostin, which is produced in osteocytes. When sclerostin binds to receptors on osteoblasts it reduces their activity, thereby inhibiting bone formation. Romosozumab takes away this inhibition of osteoblasts, boosting their activity. The result is increased bone formation accompanied by decreased bone resorption. This allows for a logical treatment approach: first using an anabolic agent – in this instance, subcutaneously injected romosozumab at 210 mg once monthly for 12 months – then switching to an antiresorptive agent in order to maintain the gain in bone mineral density and decrease fracture risk. This is the same treatment strategy recommended when using the anabolic agents teriparatide (Forteo) and abaloparatide (Tymlos); however, those parathyroid hormone and parathyroid hormone–related protein analogs are seldom used in Norway because their cost is substantially greater than for romosozumab, he explained.
Updated Endocrine Society guidelines
Dr. Haugeberg called romosozumab “a new and wonderful drug.” The Endocrine Society also considers romosozumab an important new drug, as evidenced by the release of an 8-page update of the group’s clinical practice guideline on the pharmacologic management of osteoporosis in postmenopausal women; the update was devoted specifically to the use of romosozumab. The update, published in response to the biologic’s recent approval by U.S., Canadian, and European regulatory agencies, came just 10 months after release of the Endocrine Society’s comprehensive 28-page clinical practice guideline.
Dr. Haugeberg is a fan of the Endocrine Society guideline, which recommends romosozumab as a first-line therapy in postmenopausal women at very high risk of osteoporotic fracture, defined as those with a history of multiple vertebral fractures or severe osteoporosis with a T score of –2.5 or less at the hip or spine plus fractures. The updated guideline also recommends consideration of the antisclerostin biologic in high-risk patients who have failed on antiresorptive treatments.
The practice guideline states that the issue of a possible cardioprotective effect of alendronate in the ARCH trial “remains uncertain at this time.”
“Women at high risk of cardiovascular disease and stroke should not be considered for romosozumab pending further studies on cardiovascular risk associated with this treatment,” according to the Endocrine Society.
Dr. Haugeberg reported receiving research grants from Pfizer and Biogen and serving as a consultant to and/or on speakers’ bureaus for Amgen, which markets romosozumab, and more than a dozen other pharmaceutical companies.
The potent anabolic, antiosteoporosis agent romosozumab has been saddled with an Food and Drug Administration–mandated black-box warning for increased cardiovascular risk that may not be warranted, Glenn Haugeberg, MD, PhD, asserted at the 2021 Rheumatology Winter Clinical Symposium.
The black-box warning states that romosozumab (Evenity), a monoclonal antibody approved in 2019 for fracture prevention in patients with osteoporosis, may increase the risk of MI, stroke, and cardiovascular death. The warning arose from FDA concerns raised by the results of the phase 3 ARCH trial in which 4,093 postmenopausal women at high fracture risk were randomized to monthly subcutaneous injections of romosozumab or weekly dosing of the oral bisphosphonate alendronate (Fosamax) for 1 year, followed by 12 months of open-label alendronate for all. Alarm bells went off at the FDA because during year 1, the incidence of adjudicated major adverse cardiovascular events was 2.5% in the romosozumab arm, compared with 1.9% with alendronate.
Could a cardioprotective effect of bisphosphonates explain cardiovascular concerns?
However, evidence from multiple animal and human studies suggests that bisphosphonates actually have a cardioprotective effect. For example, a Taiwanese population-based cohort study of 1,548 patients on bisphosphonate therapy for osteoporotic fractures and 4,644 individuals with hip or vertebral fractures who were not on a bisphosphonate showed a 65% reduction in the risk of acute MI during 2 years of follow-up in those who received a bisphosphonate.
“That may explain the ARCH finding. It may – I say may – be that this concern in the ARCH study can be explained by the positive effect of the bisphosphonates on cardiovascular events,” according to Dr. Haugeberg, head of the division of rheumatology at the Southern Norway Hospital Trust, Kristiansand, and professor of medicine at the Norwegian University of Science and Technology, Trondheim.
He noted that, in the FRAME trial, another pivotal phase 3 trial of romosozumab, there was no signal of increased cardiovascular risk, compared with placebo. In FRAME, which included 7,180 osteoporotic postmenopausal women, rates of major adverse cardiovascular events and other adverse events were balanced between the two study arms at 12 months. Indeed, the incidence of adjudicated serious cardiovascular events was 0.5% with romosozumab and 0.4% with placebo injections. After 12 months, all participants were transitioned to denosumab (Prolia) for another 12 months. At 24 months, there remained no significant between-group difference in cardiovascular events, cancer, osteoarthritis, hyperostosis, or other major adverse events.
Potency of romosozumab
Romosozumab’s efficacy for fracture prevention in these two pivotal trials was striking. The risk of new vertebral fractures was reduced by 73% with romosozumab, compared with placebo at 12 months in FRAME, and by 75% at 24 months in the romosozumab-to-denosumab group.
“FRAME was a 12-month study for the primary endpoint. The bisphosphonate studies typically had a 3-year design in order to show benefit, but here you see only 12-month follow-up. This illustrates the potency of this drug. We saw rapid increase in bone density and a huge decrease in new vertebral fractures versus placebo in the first 12 months, then during follow-up with denosumab the reduction in fractures was maintained,” the rheumatologist commented.
In the ARCH trial, where romosozumab went head to head with a very effective oral bisphosphonate, the risk of new vertebral fractures was 48% lower at 24 months in the romosozumab-to-alendronate group than in women on alendronate for the full 24 months, while the risk of hip fractures was reduced by 38%.
Romosozumab is a humanized monoclonal antibody with a novel mechanism of anabolic action: This agent binds to sclerostin, which is produced in osteocytes. When sclerostin binds to receptors on osteoblasts it reduces their activity, thereby inhibiting bone formation. Romosozumab takes away this inhibition of osteoblasts, boosting their activity. The result is increased bone formation accompanied by decreased bone resorption. This allows for a logical treatment approach: first using an anabolic agent – in this instance, subcutaneously injected romosozumab at 210 mg once monthly for 12 months – then switching to an antiresorptive agent in order to maintain the gain in bone mineral density and decrease fracture risk. This is the same treatment strategy recommended when using the anabolic agents teriparatide (Forteo) and abaloparatide (Tymlos); however, those parathyroid hormone and parathyroid hormone–related protein analogs are seldom used in Norway because their cost is substantially greater than for romosozumab, he explained.
Updated Endocrine Society guidelines
Dr. Haugeberg called romosozumab “a new and wonderful drug.” The Endocrine Society also considers romosozumab an important new drug, as evidenced by the release of an 8-page update of the group’s clinical practice guideline on the pharmacologic management of osteoporosis in postmenopausal women; the update was devoted specifically to the use of romosozumab. The update, published in response to the biologic’s recent approval by U.S., Canadian, and European regulatory agencies, came just 10 months after release of the Endocrine Society’s comprehensive 28-page clinical practice guideline.
Dr. Haugeberg is a fan of the Endocrine Society guideline, which recommends romosozumab as a first-line therapy in postmenopausal women at very high risk of osteoporotic fracture, defined as those with a history of multiple vertebral fractures or severe osteoporosis with a T score of –2.5 or less at the hip or spine plus fractures. The updated guideline also recommends consideration of the antisclerostin biologic in high-risk patients who have failed on antiresorptive treatments.
The practice guideline states that the issue of a possible cardioprotective effect of alendronate in the ARCH trial “remains uncertain at this time.”
“Women at high risk of cardiovascular disease and stroke should not be considered for romosozumab pending further studies on cardiovascular risk associated with this treatment,” according to the Endocrine Society.
Dr. Haugeberg reported receiving research grants from Pfizer and Biogen and serving as a consultant to and/or on speakers’ bureaus for Amgen, which markets romosozumab, and more than a dozen other pharmaceutical companies.
FROM RWCS 2021
Cumulative exposure to high-potency topical steroid doses drives osteoporosis fractures
In support of previously published case reports, in a dose-response relationship.
In a stepwise manner, the hazard ratios for major osteoporotic fracture (MOF) were found to start climbing incrementally for those with a cumulative topical steroid dose equivalent of more than 500 g of mometasone furoate when compared with exposure of 200-499 g, according to the team of investigators from the University of Copenhagen.
“Use of these drugs is very common, and we found an estimated population-attributable risk of as much as 4.3%,” the investigators reported in the study, published in JAMA Dermatology.
The retrospective cohort study drew data from the Danish National Patient Registry, which covers 99% of the country’s population. It was linked to the Danish National Prescription Registry, which captures data on pharmacy-dispensed medications. Data collected from the beginning of 2003 to the end of 2017 were evaluated.
Exposures to potent or very potent topical corticosteroids were converted into a single standard with potency equivalent to 1 mg/g of mometasone furoate. Four strata of exposure were compared to a reference exposure of 200-499 g. These were 500-999 g, 1,000-1,999 g, 2,000-9,999 g, and 10,000 g or greater.
For the first strata, the small increased risk for MOF did not reach significance (HR, 1.01; 95% confidence interval, 0.99-1.03), but each of the others did. These climbed from a 5% greater risk (HR 1.05 95% CI 1.02-1.08) for a cumulative exposure of 1,000 to 1,999 g, to a 10% greater risk (HR, 1.10; 95% CI, 1.07-1.13) for a cumulative exposure of 2,000-9,999 g, and finally to a 27% greater risk (HR, 1.27; 95% CI, 1.19-1.35) for a cumulative exposure of 10,000 g or higher.
The study included more than 700,000 individuals exposed to topical mometasone at a potency equivalent of 200 g or more over the study period. The reference group (200-499 g) was the largest (317,907 individuals). The first strata (500-999 g) included 186,359 patients; the second (1,000-1,999 g), 111,203 patients; the third (2,000-9,999 g), 94,334 patients; and the fifth (10,000 g or more), 13,448 patients.
“A 3% increase in the relative risk of osteoporosis and MOF was observed per doubling of the TCS dose,” according to the investigators.
Patients exposed to doses of high-potency topical steroids that put them at risk of MOF is limited but substantial, according to the senior author, Alexander Egeberg, MD, PhD, of the department of dermatology and allergy at Herlev and Gentofte Hospital, Copenhagen.
“It is true that the risk is modest for the average user of topical steroids,” Dr. Egeberg said in an interview. However, despite the fact that topical steroids are intended for short-term use, “2% of all our users had been exposed to the equivalent of 10,000 g of mometasone, which mean 100 tubes of 100 g.”
If the other two strata at significantly increased risk of MOF (greater than 1,000 g) are included, an additional 28% of all users are facing the potential for clinically significant osteoporosis, according to the Danish data.
The adverse effect of steroids on bone metabolism has been established previously, and several studies have linked systemic corticosteroid exposure, including inhaled corticosteroids, with increased risk of osteoporotic fracture. For example, one study showed that patients with chronic obstructive pulmonary disease on daily inhaled doses of the equivalent of fluticasone at or above 1,000 mcg for more than 4 years had about a 10% increased risk of MOF relative to those not exposed.
The data associate topical steroids with increased risk of osteoporotic fracture, but Dr. Egeberg said osteoporosis is not the only reason to use topical steroids prudently.
“It is important to keep in mind that osteoporosis and fractures are at the extreme end of the side-effect profile and that other side effects, such as striae formation, skin thinning, and dysregulated diabetes, can occur with much lower quantities of topical steroids,” Dr. Egeberg said
For avoiding this risk, “there are no specific cutoffs” recommended for topical steroids in current guidelines, but dermatologists should be aware that many of the indications for topical steroids, such as psoriasis and atopic dermatitis, involve skin with an impaired barrier function, exposing patients to an increased likelihood of absorption, according to Dr. Egeberg.
“A general rule of thumb that we use is that, if a patient with persistent disease activity requires a new prescription of the equivalent of 100 g mometasone every 1-2 months, it might be worth considering if there is a suitable alternative,” Dr. Egeberg said.
In an accompanying editorial, Rebecca D. Jackson, MD, of the division of endocrinology, diabetes, and metabolism in the department of internal medicine at Ohio State University, Columbus, agreed that no guidelines specific to avoiding the risks of topical corticosteroids are currently available, but she advised clinicians to be considering these risks nonetheless. In general, she suggested that topical steroids, like oral steroids, should be used at “the lowest dose for the shortest duration necessary to manage the underlying medical condition.”
The correlation between topical corticosteroids and increased risk of osteoporotic fracture, although not established previously in a large study, is not surprising, according to Victoria Werth, MD, chief of dermatology at the Philadelphia Veterans Affairs Hospital and professor of dermatology at the University of Pennsylvania, also in Philadelphia.
“Systemic absorption of potent topical steroids has previously been demonstrated with a rapid decrease in serum cortisol levels,” Dr. Werth said in an interview. She indicated that concern about the risk of osteoporosis imposed by use of potent steroids over large body surface areas is appropriate.
To minimize this risk, “it is reasonable to use the lowest dose of steroid possible and to try to substitute other medications when possible,” she said.
Dr. Egeberg reported financial relationships with Abbvie, Almirall, Bristol-Myers Squibb, Dermavant Sciences, Galderma, Janssen Pharmaceuticals, Eli Lilly, Novartis, Pfizer, Samsung, Bioepis, and UCB. Five authors had disclosures related to some of those pharmaceutical companies and/or others. Dr. Jackson had no disclosures.
In support of previously published case reports, in a dose-response relationship.
In a stepwise manner, the hazard ratios for major osteoporotic fracture (MOF) were found to start climbing incrementally for those with a cumulative topical steroid dose equivalent of more than 500 g of mometasone furoate when compared with exposure of 200-499 g, according to the team of investigators from the University of Copenhagen.
“Use of these drugs is very common, and we found an estimated population-attributable risk of as much as 4.3%,” the investigators reported in the study, published in JAMA Dermatology.
The retrospective cohort study drew data from the Danish National Patient Registry, which covers 99% of the country’s population. It was linked to the Danish National Prescription Registry, which captures data on pharmacy-dispensed medications. Data collected from the beginning of 2003 to the end of 2017 were evaluated.
Exposures to potent or very potent topical corticosteroids were converted into a single standard with potency equivalent to 1 mg/g of mometasone furoate. Four strata of exposure were compared to a reference exposure of 200-499 g. These were 500-999 g, 1,000-1,999 g, 2,000-9,999 g, and 10,000 g or greater.
For the first strata, the small increased risk for MOF did not reach significance (HR, 1.01; 95% confidence interval, 0.99-1.03), but each of the others did. These climbed from a 5% greater risk (HR 1.05 95% CI 1.02-1.08) for a cumulative exposure of 1,000 to 1,999 g, to a 10% greater risk (HR, 1.10; 95% CI, 1.07-1.13) for a cumulative exposure of 2,000-9,999 g, and finally to a 27% greater risk (HR, 1.27; 95% CI, 1.19-1.35) for a cumulative exposure of 10,000 g or higher.
The study included more than 700,000 individuals exposed to topical mometasone at a potency equivalent of 200 g or more over the study period. The reference group (200-499 g) was the largest (317,907 individuals). The first strata (500-999 g) included 186,359 patients; the second (1,000-1,999 g), 111,203 patients; the third (2,000-9,999 g), 94,334 patients; and the fifth (10,000 g or more), 13,448 patients.
“A 3% increase in the relative risk of osteoporosis and MOF was observed per doubling of the TCS dose,” according to the investigators.
Patients exposed to doses of high-potency topical steroids that put them at risk of MOF is limited but substantial, according to the senior author, Alexander Egeberg, MD, PhD, of the department of dermatology and allergy at Herlev and Gentofte Hospital, Copenhagen.
“It is true that the risk is modest for the average user of topical steroids,” Dr. Egeberg said in an interview. However, despite the fact that topical steroids are intended for short-term use, “2% of all our users had been exposed to the equivalent of 10,000 g of mometasone, which mean 100 tubes of 100 g.”
If the other two strata at significantly increased risk of MOF (greater than 1,000 g) are included, an additional 28% of all users are facing the potential for clinically significant osteoporosis, according to the Danish data.
The adverse effect of steroids on bone metabolism has been established previously, and several studies have linked systemic corticosteroid exposure, including inhaled corticosteroids, with increased risk of osteoporotic fracture. For example, one study showed that patients with chronic obstructive pulmonary disease on daily inhaled doses of the equivalent of fluticasone at or above 1,000 mcg for more than 4 years had about a 10% increased risk of MOF relative to those not exposed.
The data associate topical steroids with increased risk of osteoporotic fracture, but Dr. Egeberg said osteoporosis is not the only reason to use topical steroids prudently.
“It is important to keep in mind that osteoporosis and fractures are at the extreme end of the side-effect profile and that other side effects, such as striae formation, skin thinning, and dysregulated diabetes, can occur with much lower quantities of topical steroids,” Dr. Egeberg said
For avoiding this risk, “there are no specific cutoffs” recommended for topical steroids in current guidelines, but dermatologists should be aware that many of the indications for topical steroids, such as psoriasis and atopic dermatitis, involve skin with an impaired barrier function, exposing patients to an increased likelihood of absorption, according to Dr. Egeberg.
“A general rule of thumb that we use is that, if a patient with persistent disease activity requires a new prescription of the equivalent of 100 g mometasone every 1-2 months, it might be worth considering if there is a suitable alternative,” Dr. Egeberg said.
In an accompanying editorial, Rebecca D. Jackson, MD, of the division of endocrinology, diabetes, and metabolism in the department of internal medicine at Ohio State University, Columbus, agreed that no guidelines specific to avoiding the risks of topical corticosteroids are currently available, but she advised clinicians to be considering these risks nonetheless. In general, she suggested that topical steroids, like oral steroids, should be used at “the lowest dose for the shortest duration necessary to manage the underlying medical condition.”
The correlation between topical corticosteroids and increased risk of osteoporotic fracture, although not established previously in a large study, is not surprising, according to Victoria Werth, MD, chief of dermatology at the Philadelphia Veterans Affairs Hospital and professor of dermatology at the University of Pennsylvania, also in Philadelphia.
“Systemic absorption of potent topical steroids has previously been demonstrated with a rapid decrease in serum cortisol levels,” Dr. Werth said in an interview. She indicated that concern about the risk of osteoporosis imposed by use of potent steroids over large body surface areas is appropriate.
To minimize this risk, “it is reasonable to use the lowest dose of steroid possible and to try to substitute other medications when possible,” she said.
Dr. Egeberg reported financial relationships with Abbvie, Almirall, Bristol-Myers Squibb, Dermavant Sciences, Galderma, Janssen Pharmaceuticals, Eli Lilly, Novartis, Pfizer, Samsung, Bioepis, and UCB. Five authors had disclosures related to some of those pharmaceutical companies and/or others. Dr. Jackson had no disclosures.
In support of previously published case reports, in a dose-response relationship.
In a stepwise manner, the hazard ratios for major osteoporotic fracture (MOF) were found to start climbing incrementally for those with a cumulative topical steroid dose equivalent of more than 500 g of mometasone furoate when compared with exposure of 200-499 g, according to the team of investigators from the University of Copenhagen.
“Use of these drugs is very common, and we found an estimated population-attributable risk of as much as 4.3%,” the investigators reported in the study, published in JAMA Dermatology.
The retrospective cohort study drew data from the Danish National Patient Registry, which covers 99% of the country’s population. It was linked to the Danish National Prescription Registry, which captures data on pharmacy-dispensed medications. Data collected from the beginning of 2003 to the end of 2017 were evaluated.
Exposures to potent or very potent topical corticosteroids were converted into a single standard with potency equivalent to 1 mg/g of mometasone furoate. Four strata of exposure were compared to a reference exposure of 200-499 g. These were 500-999 g, 1,000-1,999 g, 2,000-9,999 g, and 10,000 g or greater.
For the first strata, the small increased risk for MOF did not reach significance (HR, 1.01; 95% confidence interval, 0.99-1.03), but each of the others did. These climbed from a 5% greater risk (HR 1.05 95% CI 1.02-1.08) for a cumulative exposure of 1,000 to 1,999 g, to a 10% greater risk (HR, 1.10; 95% CI, 1.07-1.13) for a cumulative exposure of 2,000-9,999 g, and finally to a 27% greater risk (HR, 1.27; 95% CI, 1.19-1.35) for a cumulative exposure of 10,000 g or higher.
The study included more than 700,000 individuals exposed to topical mometasone at a potency equivalent of 200 g or more over the study period. The reference group (200-499 g) was the largest (317,907 individuals). The first strata (500-999 g) included 186,359 patients; the second (1,000-1,999 g), 111,203 patients; the third (2,000-9,999 g), 94,334 patients; and the fifth (10,000 g or more), 13,448 patients.
“A 3% increase in the relative risk of osteoporosis and MOF was observed per doubling of the TCS dose,” according to the investigators.
Patients exposed to doses of high-potency topical steroids that put them at risk of MOF is limited but substantial, according to the senior author, Alexander Egeberg, MD, PhD, of the department of dermatology and allergy at Herlev and Gentofte Hospital, Copenhagen.
“It is true that the risk is modest for the average user of topical steroids,” Dr. Egeberg said in an interview. However, despite the fact that topical steroids are intended for short-term use, “2% of all our users had been exposed to the equivalent of 10,000 g of mometasone, which mean 100 tubes of 100 g.”
If the other two strata at significantly increased risk of MOF (greater than 1,000 g) are included, an additional 28% of all users are facing the potential for clinically significant osteoporosis, according to the Danish data.
The adverse effect of steroids on bone metabolism has been established previously, and several studies have linked systemic corticosteroid exposure, including inhaled corticosteroids, with increased risk of osteoporotic fracture. For example, one study showed that patients with chronic obstructive pulmonary disease on daily inhaled doses of the equivalent of fluticasone at or above 1,000 mcg for more than 4 years had about a 10% increased risk of MOF relative to those not exposed.
The data associate topical steroids with increased risk of osteoporotic fracture, but Dr. Egeberg said osteoporosis is not the only reason to use topical steroids prudently.
“It is important to keep in mind that osteoporosis and fractures are at the extreme end of the side-effect profile and that other side effects, such as striae formation, skin thinning, and dysregulated diabetes, can occur with much lower quantities of topical steroids,” Dr. Egeberg said
For avoiding this risk, “there are no specific cutoffs” recommended for topical steroids in current guidelines, but dermatologists should be aware that many of the indications for topical steroids, such as psoriasis and atopic dermatitis, involve skin with an impaired barrier function, exposing patients to an increased likelihood of absorption, according to Dr. Egeberg.
“A general rule of thumb that we use is that, if a patient with persistent disease activity requires a new prescription of the equivalent of 100 g mometasone every 1-2 months, it might be worth considering if there is a suitable alternative,” Dr. Egeberg said.
In an accompanying editorial, Rebecca D. Jackson, MD, of the division of endocrinology, diabetes, and metabolism in the department of internal medicine at Ohio State University, Columbus, agreed that no guidelines specific to avoiding the risks of topical corticosteroids are currently available, but she advised clinicians to be considering these risks nonetheless. In general, she suggested that topical steroids, like oral steroids, should be used at “the lowest dose for the shortest duration necessary to manage the underlying medical condition.”
The correlation between topical corticosteroids and increased risk of osteoporotic fracture, although not established previously in a large study, is not surprising, according to Victoria Werth, MD, chief of dermatology at the Philadelphia Veterans Affairs Hospital and professor of dermatology at the University of Pennsylvania, also in Philadelphia.
“Systemic absorption of potent topical steroids has previously been demonstrated with a rapid decrease in serum cortisol levels,” Dr. Werth said in an interview. She indicated that concern about the risk of osteoporosis imposed by use of potent steroids over large body surface areas is appropriate.
To minimize this risk, “it is reasonable to use the lowest dose of steroid possible and to try to substitute other medications when possible,” she said.
Dr. Egeberg reported financial relationships with Abbvie, Almirall, Bristol-Myers Squibb, Dermavant Sciences, Galderma, Janssen Pharmaceuticals, Eli Lilly, Novartis, Pfizer, Samsung, Bioepis, and UCB. Five authors had disclosures related to some of those pharmaceutical companies and/or others. Dr. Jackson had no disclosures.
FROM JAMA DERMATOLOGY
Afternoon napping associated with better cognition in elderly, study shows
according to a new study in General Psychiatry.
The findings add to those seen in other observational studies showing afternoon napping promotes cognitive function, said the authors of the paper, published in General Psychiatry.
“The prevalence of afternoon napping has been increasing in older adults much more than in younger individuals,” wrote Han Cai, MS, of the department of geriatrics at The Fourth People’s Hospital of Wuhu, Anhui, China, and coauthors. “The elderly individuals who took afternoon naps showed significantly higher cognitive performance compared with those who did not nap.”
The researchers enrolled 2,214 people in the study – all Han Chinese and aged 60 or older. Afternoon napping was considered any period of inactivity of at least 5 minutes but less than 2 hours after lunch and outside of the person’s main sleep schedule. Those who reported ever napping – 1,534 subjects – were included in the napping group, and the others – 680 – in the nonnapping group. Patients with major physical conditions were excluded.
The Montreal Cognitive Assessment (MoCA), the Mini-Mental State Examination (MMSE), and the Neuropsychological Test Battery (NTB) were used to measure cognitive function, and 739 patients agreed to blood tests for lipid values.
The average total MMSE score was higher for the napping group at 25.3 points out of 30, than for the nonnapping group, at 24.56 (P = .003). Those in the napping group also had significantly higher scores in the orientation portion of the MoCA test, at 5.55 out of 6 points, compared with 5.41 for the nonnapping group (P = .006).
Those in the napping group scored significantly higher on the digit span and language fluency parts of the Neuropsychological Test Battery (P = .009 and .020, respectively).
Dementia was assessed with face-to-face visits with clinicians, but diagnoses of dementia were not different between the groups.
Triglycerides were found to be higher – though still in the normal range – in the napping group compared with the nonnapping group, 1.80 mmol/L to 1.75 mmol/L, the researchers found (P = .001). No differences were seen for HDL or LDL cholesterol levels, or in hypertension or diabetes, the researchers reported.
The authors noted that inflammation is likely an important feature in the relationship between napping and cognitive function. Inflammatory cytokines have been found to play a role in sleep disorders, and strong inflammatory responses can lead to adverse events, including cognitive impairment.
“Sleep is known to be a regulator of the immune response that counters these inflammatory mediators, whereas napping, in particular, is thought to be an evolved response to inflammation,” they said.
The average age of patients in the napping group was 72.8 years, slightly older than those in the nonnapping group at 71.3 years, and this was a significant difference (P = .016).
The researchers acknowledged that the study “could not show direct causality of napping, whether beneficial or harmful,” and that “a lack of detailed information regarding napping duration ... also limited the description of napping status.”
Junxin Li, PhD, RN, assistant professor at Johns Hopkins School of Nursing, Baltimore, who has studied napping and cognition, said that previous research generally supports a U-shaped relationship between napping and mental acuity, with shorter or medium-length naps benefiting cognition and no naps or naps that are too long being detrimental.
“This study looked at no nap versus naps of less than 2 hours and may not be able to capture this potential U-shaped association,” she said.
For clinicians, the duration, timing, frequency, and purpose of naps are important factors in making recommendations to patients, she said.
“For example, timing – napping in the early evening close to older adult’s bedtime may delay their bedtime and interfere with their nighttime sleep quality. Taking naps after lunchtime is hypothesized to provide the most therapeutic values to the health and usually recommended,” she said. Regular napping is better than “randomly dozing off,” Dr. Li added.
There are also cultural considerations – in east Asia, napping tends to be considered part of a healthy lifestyle, while in western countries it is not – and this could impact napping behaviors and how these behaviors affect cognition, she said.
Phyllis C. Zee, MD, PhD, director of the Center for Circadian and Sleep Medicine at the Northwestern University, Chicago, said the results are consistent with early cross-sectional studies that showed that regular, scheduled naps in the afternoon were associated with positive cognitive performance and lower cardiometabolic disease risk.
Dr. Zee noted that it’s important to recognize that the positive data are associated with naps that are planned, while older adults napping because of excess sleepiness are at a higher risk for cognitive impairment and other health issues.
The study authors, Dr. Li, and Dr. Zee reported no relevant financial disclosures.
according to a new study in General Psychiatry.
The findings add to those seen in other observational studies showing afternoon napping promotes cognitive function, said the authors of the paper, published in General Psychiatry.
“The prevalence of afternoon napping has been increasing in older adults much more than in younger individuals,” wrote Han Cai, MS, of the department of geriatrics at The Fourth People’s Hospital of Wuhu, Anhui, China, and coauthors. “The elderly individuals who took afternoon naps showed significantly higher cognitive performance compared with those who did not nap.”
The researchers enrolled 2,214 people in the study – all Han Chinese and aged 60 or older. Afternoon napping was considered any period of inactivity of at least 5 minutes but less than 2 hours after lunch and outside of the person’s main sleep schedule. Those who reported ever napping – 1,534 subjects – were included in the napping group, and the others – 680 – in the nonnapping group. Patients with major physical conditions were excluded.
The Montreal Cognitive Assessment (MoCA), the Mini-Mental State Examination (MMSE), and the Neuropsychological Test Battery (NTB) were used to measure cognitive function, and 739 patients agreed to blood tests for lipid values.
The average total MMSE score was higher for the napping group at 25.3 points out of 30, than for the nonnapping group, at 24.56 (P = .003). Those in the napping group also had significantly higher scores in the orientation portion of the MoCA test, at 5.55 out of 6 points, compared with 5.41 for the nonnapping group (P = .006).
Those in the napping group scored significantly higher on the digit span and language fluency parts of the Neuropsychological Test Battery (P = .009 and .020, respectively).
Dementia was assessed with face-to-face visits with clinicians, but diagnoses of dementia were not different between the groups.
Triglycerides were found to be higher – though still in the normal range – in the napping group compared with the nonnapping group, 1.80 mmol/L to 1.75 mmol/L, the researchers found (P = .001). No differences were seen for HDL or LDL cholesterol levels, or in hypertension or diabetes, the researchers reported.
The authors noted that inflammation is likely an important feature in the relationship between napping and cognitive function. Inflammatory cytokines have been found to play a role in sleep disorders, and strong inflammatory responses can lead to adverse events, including cognitive impairment.
“Sleep is known to be a regulator of the immune response that counters these inflammatory mediators, whereas napping, in particular, is thought to be an evolved response to inflammation,” they said.
The average age of patients in the napping group was 72.8 years, slightly older than those in the nonnapping group at 71.3 years, and this was a significant difference (P = .016).
The researchers acknowledged that the study “could not show direct causality of napping, whether beneficial or harmful,” and that “a lack of detailed information regarding napping duration ... also limited the description of napping status.”
Junxin Li, PhD, RN, assistant professor at Johns Hopkins School of Nursing, Baltimore, who has studied napping and cognition, said that previous research generally supports a U-shaped relationship between napping and mental acuity, with shorter or medium-length naps benefiting cognition and no naps or naps that are too long being detrimental.
“This study looked at no nap versus naps of less than 2 hours and may not be able to capture this potential U-shaped association,” she said.
For clinicians, the duration, timing, frequency, and purpose of naps are important factors in making recommendations to patients, she said.
“For example, timing – napping in the early evening close to older adult’s bedtime may delay their bedtime and interfere with their nighttime sleep quality. Taking naps after lunchtime is hypothesized to provide the most therapeutic values to the health and usually recommended,” she said. Regular napping is better than “randomly dozing off,” Dr. Li added.
There are also cultural considerations – in east Asia, napping tends to be considered part of a healthy lifestyle, while in western countries it is not – and this could impact napping behaviors and how these behaviors affect cognition, she said.
Phyllis C. Zee, MD, PhD, director of the Center for Circadian and Sleep Medicine at the Northwestern University, Chicago, said the results are consistent with early cross-sectional studies that showed that regular, scheduled naps in the afternoon were associated with positive cognitive performance and lower cardiometabolic disease risk.
Dr. Zee noted that it’s important to recognize that the positive data are associated with naps that are planned, while older adults napping because of excess sleepiness are at a higher risk for cognitive impairment and other health issues.
The study authors, Dr. Li, and Dr. Zee reported no relevant financial disclosures.
according to a new study in General Psychiatry.
The findings add to those seen in other observational studies showing afternoon napping promotes cognitive function, said the authors of the paper, published in General Psychiatry.
“The prevalence of afternoon napping has been increasing in older adults much more than in younger individuals,” wrote Han Cai, MS, of the department of geriatrics at The Fourth People’s Hospital of Wuhu, Anhui, China, and coauthors. “The elderly individuals who took afternoon naps showed significantly higher cognitive performance compared with those who did not nap.”
The researchers enrolled 2,214 people in the study – all Han Chinese and aged 60 or older. Afternoon napping was considered any period of inactivity of at least 5 minutes but less than 2 hours after lunch and outside of the person’s main sleep schedule. Those who reported ever napping – 1,534 subjects – were included in the napping group, and the others – 680 – in the nonnapping group. Patients with major physical conditions were excluded.
The Montreal Cognitive Assessment (MoCA), the Mini-Mental State Examination (MMSE), and the Neuropsychological Test Battery (NTB) were used to measure cognitive function, and 739 patients agreed to blood tests for lipid values.
The average total MMSE score was higher for the napping group at 25.3 points out of 30, than for the nonnapping group, at 24.56 (P = .003). Those in the napping group also had significantly higher scores in the orientation portion of the MoCA test, at 5.55 out of 6 points, compared with 5.41 for the nonnapping group (P = .006).
Those in the napping group scored significantly higher on the digit span and language fluency parts of the Neuropsychological Test Battery (P = .009 and .020, respectively).
Dementia was assessed with face-to-face visits with clinicians, but diagnoses of dementia were not different between the groups.
Triglycerides were found to be higher – though still in the normal range – in the napping group compared with the nonnapping group, 1.80 mmol/L to 1.75 mmol/L, the researchers found (P = .001). No differences were seen for HDL or LDL cholesterol levels, or in hypertension or diabetes, the researchers reported.
The authors noted that inflammation is likely an important feature in the relationship between napping and cognitive function. Inflammatory cytokines have been found to play a role in sleep disorders, and strong inflammatory responses can lead to adverse events, including cognitive impairment.
“Sleep is known to be a regulator of the immune response that counters these inflammatory mediators, whereas napping, in particular, is thought to be an evolved response to inflammation,” they said.
The average age of patients in the napping group was 72.8 years, slightly older than those in the nonnapping group at 71.3 years, and this was a significant difference (P = .016).
The researchers acknowledged that the study “could not show direct causality of napping, whether beneficial or harmful,” and that “a lack of detailed information regarding napping duration ... also limited the description of napping status.”
Junxin Li, PhD, RN, assistant professor at Johns Hopkins School of Nursing, Baltimore, who has studied napping and cognition, said that previous research generally supports a U-shaped relationship between napping and mental acuity, with shorter or medium-length naps benefiting cognition and no naps or naps that are too long being detrimental.
“This study looked at no nap versus naps of less than 2 hours and may not be able to capture this potential U-shaped association,” she said.
For clinicians, the duration, timing, frequency, and purpose of naps are important factors in making recommendations to patients, she said.
“For example, timing – napping in the early evening close to older adult’s bedtime may delay their bedtime and interfere with their nighttime sleep quality. Taking naps after lunchtime is hypothesized to provide the most therapeutic values to the health and usually recommended,” she said. Regular napping is better than “randomly dozing off,” Dr. Li added.
There are also cultural considerations – in east Asia, napping tends to be considered part of a healthy lifestyle, while in western countries it is not – and this could impact napping behaviors and how these behaviors affect cognition, she said.
Phyllis C. Zee, MD, PhD, director of the Center for Circadian and Sleep Medicine at the Northwestern University, Chicago, said the results are consistent with early cross-sectional studies that showed that regular, scheduled naps in the afternoon were associated with positive cognitive performance and lower cardiometabolic disease risk.
Dr. Zee noted that it’s important to recognize that the positive data are associated with naps that are planned, while older adults napping because of excess sleepiness are at a higher risk for cognitive impairment and other health issues.
The study authors, Dr. Li, and Dr. Zee reported no relevant financial disclosures.
COVID-19 and the risk of homicide-suicide among older adults
On March 25, 2020, in Cambridge, United Kingdom, a 71-year-old man stabbed his 71-year-old wife before suffocating himself to death. The couple was reportedly anxious about the coronavirus disease 2019 (COVID-19) pandemic lockdown measures and were on the verge of running out of food and medicine.1
One week later, in Chicago, Illinois, a 54-year-old man shot and killed his female partner, age 54, before killing himself. The couple was tested for COVID-19 2 days earlier and the man believed they had contracted the virus; however, the test results for both of them had come back negative.2
Intimate partner homicide-suicide is the most dramatic domestic abuse outcome.3 Homicide-suicide is defined as “homicide committed by a person who subsequently commits suicide within one week of the homicide. In most cases the subsequent suicide occurs within a 24-hour period.”4 Approximately one-quarter of all homicide-suicides are committed by persons age ≥55 years.5,6 We believe that during the COVID-19 pandemic, the risk of homicide-suicide among older adults may be increased due to several factors, including:
- physical distancing and quarantine measures. Protocols established to slow the spread of the virus may be associated with increased rates of depression and anxiety7 and an increased risk of suicide among older adults8
- increased intimate partner violence9
- increased firearm ownership rates in the United States.10
In this article, we review studies that identified risk factors for homicide-suicide among older adults, discuss the impact the COVID-19 pandemic has had on these risks, and describe steps clinicians can take to intervene.
A review of the literature
To better characterize the perpetrators of older adult homicide-suicide, we conducted a literature search of relevant terms. We identified 9 original research publications that examined homicide-suicide in older adults.
Bourget et al11 (2010) reviewed coroners’ charts of individuals killed by an older (age ≥65) spouse or family member from 1992 through 2007 in Quebec, Canada. They identified 19 cases of homicide-suicide, 17 (90%) of which were perpetrated by men. Perpetrators and victims were married (63%), in common-law relationships (16%), or separated/divorced (16%). A history of domestic violence was documented in 4 (21%) cases. The authors found that 13 of 15 perpetrators (87%) had “major depression” and 2 perpetrators had a psychotic disorder. Substance use at the time of the event was confirmed in 6 (32%) cases. Firearms and strangulation were the top methods used to carry out the homicide-suicide.11
Cheung et al12 (2016) conducted a review of coroners’ records of homicide-suicide cases among individuals age ≥65 in New Zealand from 2007 through 2012. In all 4 cases, the perpetrators were men, and their victims were predominantly female, live-in family members. Two cases involved men with a history of domestic violence who were undergoing significant changes in their home and social lives. Both men had a history suggestive of depression and used a firearm to carry out the homicide-suicide.12
Continue to: Cohen et al
Cohen et al13 (1998) conducted a review of coroners’ records from 1988 through 1994 in 2 regions in Florida. They found 48 intimate partner homicide-suicide cases among “old couples” (age ≥55). All were perpetrated by men. The authors identified sociocultural differences in risk factors between the 2 regions. In west-central Florida, perpetrators and victims were predominantly white and in a spousal relationship. Domestic violence was documented in <4% of cases. Approximately 55% of the couples were reported to be ill, and a substantial proportion were documented to be declining in health. One-quarter of the perpetrators and one-third of the victims had “pain and suffering.” More than one-third of perpetrators were reported to have “depression,” 15% were reported to have talked about suicide, and 4% had a history of a suicide attempt. Only 11% of perpetrators were described as abusing substances.
The authors noted several differences in cases in southeastern Florida. Approximately two-thirds of the couples were Hispanic, and 14% had a history of domestic violence. A minority of the couples were in a live-in relationship. Less than 15% of the perpetrators and victims were described as having a decline in health. Additionally, only 19% of perpetrators were reported to have “depression,” and none of the perpetrators had a documented history of attempted suicide or substance abuse. No information was provided regarding the methods used to carry out the homicide-suicide in the southeastern region.13 Financial stress was not a factor in either region.
Malphurs et al14 (2001) used the same database described in the Cohen et al13 study to compare 27 perpetrators of homicide-suicide to 36 age-matched suicide decedents in west central Florida. They found that homicide-suicide perpetrators were significantly less likely to have health problems and were 3 times more likely to be caregivers to their spouses. Approximately 52% of perpetrators had at least 1 documented psychiatric symptom (“depression” and/or substance abuse or other), but only 5% were seeking mental health services at the time of death.14
De Koning and Piette15 (2014) conducted a retrospective medicolegal chart review from 1935 to 2010 to identify homicide-suicide cases in West and East Flanders, Belgium. They found 19 cases of intimate partner homicide-suicide committed by offenders age ≥55 years. Ninety-five percent of the perpetrators were men who killed their female partners. In one-quarter of the cases, either the perpetrator or the victim had a health issue; 21% of the perpetrators were documented as having depression and 27% had alcohol intoxication at the time of death. A motive was documented in 14 out of 19 cases; “mercy killing” was determined as the motive in 6 (43%) cases and “amorous jealousy” in 5 cases (36%).15 Starting in the 1970s, firearms were the most prevalent method used to kill a partner.
Logan et al16 (2019) used data from the National Violent Death Reporting System between 2003 and 2015 to identify characteristics that differentiated male suicide decedents from male perpetrators of intimate partner homicide-suicide. They found that men age 50 to 64 years were 3 times more likely than men age 18 to 34 years to commit intimate partner homicide-suicide, and that men age ≥65 years were approximately 5 times more likely than men age 18 to 34 years to commit intimate partner homicide-suicide. The authors found that approximately 22% of all perpetrators had a documented history of physical domestic violence, and close to 17% had a prior interaction with the criminal justice system. Furthermore, one-third of perpetrators had relationship difficulties and were in the process of a breakup. Health issues were prevalent in 34% of the victims and 26% of the perpetrators. Perpetrator-caregiver burden was reported as a contributing factor for homicide-suicide in 16% of cases. In 27% of cases, multiple health-related contributing factors were mentioned.16
Continue to: Malphurs and Cohen
Malphurs and Cohen5 (2002) reviewed American newspapers from 1997 through 1999 and identified 673 homicide-suicide events, of which 152 (27%) were committed by individuals age ≥55 years. The victims and perpetrators (95% of which were men) were intimate partners in three-quarters of cases. In nearly one-third of cases, caregiving was a contributing factor for the homicide-suicide. A history of or a pending divorce was reported in nearly 14% of cases. Substance use history was rarely recorded. Firearms were used in 88% of the homicide-suicide cases.5
Malphurs and Cohen17 (2005) reviewed coroner records between 1998 and 1999 in Florida and compared 20 cases of intimate partner homicide-suicide involving perpetrators age ≥55 years with matched suicide decedents. They found that 60% of homicide-suicide perpetrators had documented health issues. The authors reported that a “recent change in health status” was more prevalent among perpetrators compared with decedents. Perpetrators were also more likely to be caregivers to their spouses. The authors found that 65% of perpetrators were reported to have a “depressed mood” and 15% of perpetrators had reportedly threatened suicide prior to the incident. However, none of the perpetrators tested positive for antidepressants as documented on post-mortem toxicology reports. Firearms were used in 100% of homicide-suicide cases.17
Salari3 (2007) reviewed multiple American media sources and published police reports between 1999 and 2005 to retrieve data about intimate partner homicide-suicide events in the United States. There were 225 events identified where the perpetrator and/or the victim were age ≥60 years. Ninety-six percent of the perpetrators were men and most homicide-suicide events were committed at the home. A history of domestic violence was reported in 14% of homicide-suicide cases. Thirteen percent of couples were separated or divorced. The perpetrator and/or victim had health issues in 124 (55%) events. Dementia was reported in 7.5% of cases, but overwhelmingly among the victims. Substance abuse was rarely mentioned as a contributing factor. In three-quarters of cases where a motive was described, the perpetrator was “suicidal”; however, a “suicide pact” was mentioned in only 4% of cases. Firearms were used in 87% of cases.3
Focus on common risk factors
The scarcity and heterogeneity of research regarding older adult homicide-suicide were major limitations to our review. Because most of the studies we identified had a small sample size and limited information regarding the mental health of victims and perpetrators, it would be an overreach to claim to have identified a typical profile of an older perpetrator of homicide-suicide. However, the literature has repeatedly identified several common characteristics of such perpetrators. They are significantly more likely to be men who use firearms to murder their intimate partners and then die by suicide in their home (Table3,5,11-17). Health issues afflicting 1 or both individuals in the couple appear to be a contributing factor, particularly when the perpetrator is in a caregiving role. Relational discord, with or without a history of domestic violence, increases the risk of homicide-suicide. Finally, older perpetrators are highly likely to be depressed and have suicidal ideations.
How COVID-19 affects these risks
Although it is too early to determine if there is a causal relationship between the COVID-19 pandemic and an increase in homicide-suicide, the pandemic is likely to promote risk factors for these events, especially among older adults. Confinement measures put into place during the pandemic context have already been shown to increase rates of domestic violence18 and depression and anxiety among older individuals.7 Furthermore,
Continue to: Late-life psychiatric disorders
Late-life psychiatric disorders
Early recognition and effective treatment of late-life psychiatric disorders is crucial. Unfortunately, depression in geriatric patients is often underdiagnosed and undertreated.20 Older adults have more frequent contact with their primary care physicians, and rarely consult mental health professionals.21,22 Several models of integrated depression care within primary care settings have shown the positive impact of this collaborative approach in treating late-life depression and preventing suicide in older individuals.23 Additionally, because alcohol abuse is also a risk factor for domestic violence and breaking the law in this population,24,25 older adults should be screened for alcohol use disorders, and referred to treatment when necessary.
Take steps to keep patients safe
In the context of the COVID-19 pandemic, there are several steps clinicians need to keep in mind when interacting with older patients:
- Screen for depressive symptoms, suicidality, and alcohol and substance use disorders. Individuals who have tested positive for COVID-19 or who have been in contact with a carrier are a particularly vulnerable population.
- Screen for domestic violence and access to weapons at home.4 Any older adult who has a psychiatric disorder and/or suicide ideation should receive immediate intervention through a social worker that includes providing gun-risk education to other family members or contacting law-enforcement officials.26
- Refer patients with a suspected psychiatric disorder to specialized mental health clinicians. Telemental health services can provide rapid access to subspecialists, allowing patients to be treated from their homes.27 These services need to be promoted among older adults during this critical period and reimbursed by public and private insurance systems to ensure accessibility and affordability.28
- Create psychiatric inpatient units specifically designed for suicidal and/or homicidal patients with COVID-19.
Additionally, informing the public about these major health issues is crucial. The media can raise awareness about domestic violence and depression among older adults; however, this should be done responsibly and with accuracy to prevent the spread of misinformation, confusion, fear, and panic.29
Bottom Line
The mental health burden of the coronavirus disease 2019 pandemic has significantly impacted individuals who are older and most vulnerable. Reducing the incidence of homicide-suicide among older adults requires timely screening and interventions by primary care providers, mental health specialists, social workers, media, and governmental agencies.
Related Resources
- Saeed SA, Hebishi K. The psychiatric consequences of COVID-19: 8 studies. Current Psychiatry. 2020;19(11):22-24,28-30,32-35.
- Schwab-Reese LM, Murfree L, Coppola EC, et al. Homicidesuicide across the lifespan: a mixed methods examination of factors contributing to older adult perpetration. Aging Ment Health. 2020;20:1-9.
1. Christodoulou H. LOCKDOWN ‘MURDER-SUICIDE’ OAP, 71, ‘stabbed wife to death then killed himself as he worried about coping with coronavirus lockdown.’ The Sun. Updated April 4, 2020. Accessed December 22, 2020. https://www.thesun.co.uk/news/11327095/coronavirus-lockdown-murder-suicide-cambridge/
2. Farberov S. Illinois man, 54, shoots dead his wife then kills himself in murder-suicide because he feared they had coronavirus - but tests later show the couple were NOT ill. Updated April 6, 2020. Accessed December 22, 2020. https://www.dailymail.co.uk/news/article-8191933/Man-kills-wife-feared-coronavirus.html
3. Salari S. Patterns of intimate partner homicide suicide in later life: strategies for prevention. Clin Interv Aging. 2007;2(3):441-452.
4. Kotzé C, Roos JL. Homicide–suicide: practical implications for risk reduction and support services at primary care level. South African Family Practice. 2019;61(4):165-169.
5. Malphurs JE, Cohen D. A newspaper surveillance study of homicide-suicide in the United States. Am J Forensic Med Pathol. 2002;23(2):142-148.
6. Eliason S. Murder-suicide: a review of the recent literature. J Am Acad Psychiatry Law. 2009;37(3):371-376.
7. Armitage R, Nellums LB. COVID-19 and the consequences of isolating the elderly. Lancet Public Health. 2020;5(5):e256. doi: 10.1016/S2468-2667(20)30061-X
8. Gunnell D, Appleby L, Arensman E, et al. Suicide risk and prevention during the COVID-19 pandemic. Lancet Psychiatry 2020;7(6):468-471.
9. Gosangi B, Park H, Thomas R, et al. Exacerbation of physical intimate partner violence during COVID-19 pandemic. Radiology. 2021;298(1):E38-E45.
10. Mannix R, Lee LK, Fleegler EW. Coronavirus disease 2019 (COVID-19) and firearms in the United States: will an epidemic of suicide follow? Ann Intern Med. 2020;173(3):228-229.
11. Bourget D, Gagne P, Whitehurst L. Domestic homicide and homicide-suicide: the older offender. J Am Acad Psychiatry Law. 2010;38(3):305-311.
12. Cheung G, Hatters Friedman S, Sundram F. Late-life homicide-suicide: a national case series in New Zealand. Psychogeriatrics. 2016;16(1):76-81.
13. Cohen D, Llorente M, Eisdorfer C. Homicide-suicide in older persons. Am J Psychiatry. 1998;155(3):390-396.
14. Malphurs JE, Eisdorfer C, Cohen D. A comparison of antecedents of homicide-suicide and suicide in older married men. Am J Geriatr Psychiatry. 2001;9(1):49-57.
15. De Koning E, Piette MHA. A retrospective study of murder–suicide at the Forensic Institute of Ghent University, Belgium: 1935–2010. Med Sci Law. 2014;54(2):88-98.
16. Logan JE, Ertl A, Bossarte R. Correlates of intimate partner homicide among male suicide decedents with known intimate partner problems. Suicide Life Threat Behav. 2019;49(6):1693-1706.
17. Malphurs JE, Cohen D. A statewide case-control study of spousal homicide-suicide in older persons. Am J Geriatr Psychiatry. 2005;13(3):211-217.
18. Sanford A. ‘Horrifying surge in domestic violence’ against women amid coronavirus-lockdowns, UN chief warns. Euronews. Published June 4, 2020. Accessed December 22, 2020. https://www.euronews.com/2020/04/06/horrifying-surge-in-domestic-violence-against-women-amid-coronavirus-lockdowns-un-chief-w
19. Appel JM. Intimate partner homicide in elderly populations. In: Friedman SH, ed. Family murder: pathologies of love and hate. American Psychiatric Association Publishing; 2019:131-142.
20. Hall CA, Reynolds-III CF. Late-life depression in the primary care setting: challenges, collaborative care, and prevention. Maturitas. 2014;79(2):147-152.
21. Unützer J. Diagnosis and treatment of older adults with depression in primary care. Biological Psychiatry. 2002;52(3):285-292.
22. Byers AL, Arean PA, Yaffe K. Low use of mental health services among older Americans with mood and anxiety disorders. Psychiatr Serv. 2012;63(1):66-72.
23. Bruce ML, Sirey JA. Integrated care for depression in older primary care patients. Can J Psychiatry. 2018;63(7):439-446.
24. Rao R, Roche A. Substance misuse in older people. BMJ. 2017;358:j3885. doi: 10.1136/bmj.j3885
25. Ghossoub E, Khoury R. Prevalence and correlates of criminal behavior among the non-institutionalized elderly: results from the National Survey on Drug Use and Health. J Geriatr Psychiatry Neurol. 2018;31(4):211-222.
26. Slater MAG. Older adults at risk for suicide. In: Berkman B. Handbook of social work in health and aging. Oxford University Press; 2006:149-161.
27. Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679-1681.
28. Centers for Medicare & Medicaid Services. President Trump expands telehealth benefits for Medicare beneficiaries during COVID-19 outbreak. Published March 17, 2020. Accessed December 23, 2020. https://www.cms.gov/newsroom/press-releases/president-trump-expands-telehealth-benefits-medicare-beneficiaries-during-covid-19-outbreak
29. Mian A, Khan S. Coronavirus: the spread of misinformation. BMC Med. 2020;18(1):89.
On March 25, 2020, in Cambridge, United Kingdom, a 71-year-old man stabbed his 71-year-old wife before suffocating himself to death. The couple was reportedly anxious about the coronavirus disease 2019 (COVID-19) pandemic lockdown measures and were on the verge of running out of food and medicine.1
One week later, in Chicago, Illinois, a 54-year-old man shot and killed his female partner, age 54, before killing himself. The couple was tested for COVID-19 2 days earlier and the man believed they had contracted the virus; however, the test results for both of them had come back negative.2
Intimate partner homicide-suicide is the most dramatic domestic abuse outcome.3 Homicide-suicide is defined as “homicide committed by a person who subsequently commits suicide within one week of the homicide. In most cases the subsequent suicide occurs within a 24-hour period.”4 Approximately one-quarter of all homicide-suicides are committed by persons age ≥55 years.5,6 We believe that during the COVID-19 pandemic, the risk of homicide-suicide among older adults may be increased due to several factors, including:
- physical distancing and quarantine measures. Protocols established to slow the spread of the virus may be associated with increased rates of depression and anxiety7 and an increased risk of suicide among older adults8
- increased intimate partner violence9
- increased firearm ownership rates in the United States.10
In this article, we review studies that identified risk factors for homicide-suicide among older adults, discuss the impact the COVID-19 pandemic has had on these risks, and describe steps clinicians can take to intervene.
A review of the literature
To better characterize the perpetrators of older adult homicide-suicide, we conducted a literature search of relevant terms. We identified 9 original research publications that examined homicide-suicide in older adults.
Bourget et al11 (2010) reviewed coroners’ charts of individuals killed by an older (age ≥65) spouse or family member from 1992 through 2007 in Quebec, Canada. They identified 19 cases of homicide-suicide, 17 (90%) of which were perpetrated by men. Perpetrators and victims were married (63%), in common-law relationships (16%), or separated/divorced (16%). A history of domestic violence was documented in 4 (21%) cases. The authors found that 13 of 15 perpetrators (87%) had “major depression” and 2 perpetrators had a psychotic disorder. Substance use at the time of the event was confirmed in 6 (32%) cases. Firearms and strangulation were the top methods used to carry out the homicide-suicide.11
Cheung et al12 (2016) conducted a review of coroners’ records of homicide-suicide cases among individuals age ≥65 in New Zealand from 2007 through 2012. In all 4 cases, the perpetrators were men, and their victims were predominantly female, live-in family members. Two cases involved men with a history of domestic violence who were undergoing significant changes in their home and social lives. Both men had a history suggestive of depression and used a firearm to carry out the homicide-suicide.12
Continue to: Cohen et al
Cohen et al13 (1998) conducted a review of coroners’ records from 1988 through 1994 in 2 regions in Florida. They found 48 intimate partner homicide-suicide cases among “old couples” (age ≥55). All were perpetrated by men. The authors identified sociocultural differences in risk factors between the 2 regions. In west-central Florida, perpetrators and victims were predominantly white and in a spousal relationship. Domestic violence was documented in <4% of cases. Approximately 55% of the couples were reported to be ill, and a substantial proportion were documented to be declining in health. One-quarter of the perpetrators and one-third of the victims had “pain and suffering.” More than one-third of perpetrators were reported to have “depression,” 15% were reported to have talked about suicide, and 4% had a history of a suicide attempt. Only 11% of perpetrators were described as abusing substances.
The authors noted several differences in cases in southeastern Florida. Approximately two-thirds of the couples were Hispanic, and 14% had a history of domestic violence. A minority of the couples were in a live-in relationship. Less than 15% of the perpetrators and victims were described as having a decline in health. Additionally, only 19% of perpetrators were reported to have “depression,” and none of the perpetrators had a documented history of attempted suicide or substance abuse. No information was provided regarding the methods used to carry out the homicide-suicide in the southeastern region.13 Financial stress was not a factor in either region.
Malphurs et al14 (2001) used the same database described in the Cohen et al13 study to compare 27 perpetrators of homicide-suicide to 36 age-matched suicide decedents in west central Florida. They found that homicide-suicide perpetrators were significantly less likely to have health problems and were 3 times more likely to be caregivers to their spouses. Approximately 52% of perpetrators had at least 1 documented psychiatric symptom (“depression” and/or substance abuse or other), but only 5% were seeking mental health services at the time of death.14
De Koning and Piette15 (2014) conducted a retrospective medicolegal chart review from 1935 to 2010 to identify homicide-suicide cases in West and East Flanders, Belgium. They found 19 cases of intimate partner homicide-suicide committed by offenders age ≥55 years. Ninety-five percent of the perpetrators were men who killed their female partners. In one-quarter of the cases, either the perpetrator or the victim had a health issue; 21% of the perpetrators were documented as having depression and 27% had alcohol intoxication at the time of death. A motive was documented in 14 out of 19 cases; “mercy killing” was determined as the motive in 6 (43%) cases and “amorous jealousy” in 5 cases (36%).15 Starting in the 1970s, firearms were the most prevalent method used to kill a partner.
Logan et al16 (2019) used data from the National Violent Death Reporting System between 2003 and 2015 to identify characteristics that differentiated male suicide decedents from male perpetrators of intimate partner homicide-suicide. They found that men age 50 to 64 years were 3 times more likely than men age 18 to 34 years to commit intimate partner homicide-suicide, and that men age ≥65 years were approximately 5 times more likely than men age 18 to 34 years to commit intimate partner homicide-suicide. The authors found that approximately 22% of all perpetrators had a documented history of physical domestic violence, and close to 17% had a prior interaction with the criminal justice system. Furthermore, one-third of perpetrators had relationship difficulties and were in the process of a breakup. Health issues were prevalent in 34% of the victims and 26% of the perpetrators. Perpetrator-caregiver burden was reported as a contributing factor for homicide-suicide in 16% of cases. In 27% of cases, multiple health-related contributing factors were mentioned.16
Continue to: Malphurs and Cohen
Malphurs and Cohen5 (2002) reviewed American newspapers from 1997 through 1999 and identified 673 homicide-suicide events, of which 152 (27%) were committed by individuals age ≥55 years. The victims and perpetrators (95% of which were men) were intimate partners in three-quarters of cases. In nearly one-third of cases, caregiving was a contributing factor for the homicide-suicide. A history of or a pending divorce was reported in nearly 14% of cases. Substance use history was rarely recorded. Firearms were used in 88% of the homicide-suicide cases.5
Malphurs and Cohen17 (2005) reviewed coroner records between 1998 and 1999 in Florida and compared 20 cases of intimate partner homicide-suicide involving perpetrators age ≥55 years with matched suicide decedents. They found that 60% of homicide-suicide perpetrators had documented health issues. The authors reported that a “recent change in health status” was more prevalent among perpetrators compared with decedents. Perpetrators were also more likely to be caregivers to their spouses. The authors found that 65% of perpetrators were reported to have a “depressed mood” and 15% of perpetrators had reportedly threatened suicide prior to the incident. However, none of the perpetrators tested positive for antidepressants as documented on post-mortem toxicology reports. Firearms were used in 100% of homicide-suicide cases.17
Salari3 (2007) reviewed multiple American media sources and published police reports between 1999 and 2005 to retrieve data about intimate partner homicide-suicide events in the United States. There were 225 events identified where the perpetrator and/or the victim were age ≥60 years. Ninety-six percent of the perpetrators were men and most homicide-suicide events were committed at the home. A history of domestic violence was reported in 14% of homicide-suicide cases. Thirteen percent of couples were separated or divorced. The perpetrator and/or victim had health issues in 124 (55%) events. Dementia was reported in 7.5% of cases, but overwhelmingly among the victims. Substance abuse was rarely mentioned as a contributing factor. In three-quarters of cases where a motive was described, the perpetrator was “suicidal”; however, a “suicide pact” was mentioned in only 4% of cases. Firearms were used in 87% of cases.3
Focus on common risk factors
The scarcity and heterogeneity of research regarding older adult homicide-suicide were major limitations to our review. Because most of the studies we identified had a small sample size and limited information regarding the mental health of victims and perpetrators, it would be an overreach to claim to have identified a typical profile of an older perpetrator of homicide-suicide. However, the literature has repeatedly identified several common characteristics of such perpetrators. They are significantly more likely to be men who use firearms to murder their intimate partners and then die by suicide in their home (Table3,5,11-17). Health issues afflicting 1 or both individuals in the couple appear to be a contributing factor, particularly when the perpetrator is in a caregiving role. Relational discord, with or without a history of domestic violence, increases the risk of homicide-suicide. Finally, older perpetrators are highly likely to be depressed and have suicidal ideations.
How COVID-19 affects these risks
Although it is too early to determine if there is a causal relationship between the COVID-19 pandemic and an increase in homicide-suicide, the pandemic is likely to promote risk factors for these events, especially among older adults. Confinement measures put into place during the pandemic context have already been shown to increase rates of domestic violence18 and depression and anxiety among older individuals.7 Furthermore,
Continue to: Late-life psychiatric disorders
Late-life psychiatric disorders
Early recognition and effective treatment of late-life psychiatric disorders is crucial. Unfortunately, depression in geriatric patients is often underdiagnosed and undertreated.20 Older adults have more frequent contact with their primary care physicians, and rarely consult mental health professionals.21,22 Several models of integrated depression care within primary care settings have shown the positive impact of this collaborative approach in treating late-life depression and preventing suicide in older individuals.23 Additionally, because alcohol abuse is also a risk factor for domestic violence and breaking the law in this population,24,25 older adults should be screened for alcohol use disorders, and referred to treatment when necessary.
Take steps to keep patients safe
In the context of the COVID-19 pandemic, there are several steps clinicians need to keep in mind when interacting with older patients:
- Screen for depressive symptoms, suicidality, and alcohol and substance use disorders. Individuals who have tested positive for COVID-19 or who have been in contact with a carrier are a particularly vulnerable population.
- Screen for domestic violence and access to weapons at home.4 Any older adult who has a psychiatric disorder and/or suicide ideation should receive immediate intervention through a social worker that includes providing gun-risk education to other family members or contacting law-enforcement officials.26
- Refer patients with a suspected psychiatric disorder to specialized mental health clinicians. Telemental health services can provide rapid access to subspecialists, allowing patients to be treated from their homes.27 These services need to be promoted among older adults during this critical period and reimbursed by public and private insurance systems to ensure accessibility and affordability.28
- Create psychiatric inpatient units specifically designed for suicidal and/or homicidal patients with COVID-19.
Additionally, informing the public about these major health issues is crucial. The media can raise awareness about domestic violence and depression among older adults; however, this should be done responsibly and with accuracy to prevent the spread of misinformation, confusion, fear, and panic.29
Bottom Line
The mental health burden of the coronavirus disease 2019 pandemic has significantly impacted individuals who are older and most vulnerable. Reducing the incidence of homicide-suicide among older adults requires timely screening and interventions by primary care providers, mental health specialists, social workers, media, and governmental agencies.
Related Resources
- Saeed SA, Hebishi K. The psychiatric consequences of COVID-19: 8 studies. Current Psychiatry. 2020;19(11):22-24,28-30,32-35.
- Schwab-Reese LM, Murfree L, Coppola EC, et al. Homicidesuicide across the lifespan: a mixed methods examination of factors contributing to older adult perpetration. Aging Ment Health. 2020;20:1-9.
On March 25, 2020, in Cambridge, United Kingdom, a 71-year-old man stabbed his 71-year-old wife before suffocating himself to death. The couple was reportedly anxious about the coronavirus disease 2019 (COVID-19) pandemic lockdown measures and were on the verge of running out of food and medicine.1
One week later, in Chicago, Illinois, a 54-year-old man shot and killed his female partner, age 54, before killing himself. The couple was tested for COVID-19 2 days earlier and the man believed they had contracted the virus; however, the test results for both of them had come back negative.2
Intimate partner homicide-suicide is the most dramatic domestic abuse outcome.3 Homicide-suicide is defined as “homicide committed by a person who subsequently commits suicide within one week of the homicide. In most cases the subsequent suicide occurs within a 24-hour period.”4 Approximately one-quarter of all homicide-suicides are committed by persons age ≥55 years.5,6 We believe that during the COVID-19 pandemic, the risk of homicide-suicide among older adults may be increased due to several factors, including:
- physical distancing and quarantine measures. Protocols established to slow the spread of the virus may be associated with increased rates of depression and anxiety7 and an increased risk of suicide among older adults8
- increased intimate partner violence9
- increased firearm ownership rates in the United States.10
In this article, we review studies that identified risk factors for homicide-suicide among older adults, discuss the impact the COVID-19 pandemic has had on these risks, and describe steps clinicians can take to intervene.
A review of the literature
To better characterize the perpetrators of older adult homicide-suicide, we conducted a literature search of relevant terms. We identified 9 original research publications that examined homicide-suicide in older adults.
Bourget et al11 (2010) reviewed coroners’ charts of individuals killed by an older (age ≥65) spouse or family member from 1992 through 2007 in Quebec, Canada. They identified 19 cases of homicide-suicide, 17 (90%) of which were perpetrated by men. Perpetrators and victims were married (63%), in common-law relationships (16%), or separated/divorced (16%). A history of domestic violence was documented in 4 (21%) cases. The authors found that 13 of 15 perpetrators (87%) had “major depression” and 2 perpetrators had a psychotic disorder. Substance use at the time of the event was confirmed in 6 (32%) cases. Firearms and strangulation were the top methods used to carry out the homicide-suicide.11
Cheung et al12 (2016) conducted a review of coroners’ records of homicide-suicide cases among individuals age ≥65 in New Zealand from 2007 through 2012. In all 4 cases, the perpetrators were men, and their victims were predominantly female, live-in family members. Two cases involved men with a history of domestic violence who were undergoing significant changes in their home and social lives. Both men had a history suggestive of depression and used a firearm to carry out the homicide-suicide.12
Continue to: Cohen et al
Cohen et al13 (1998) conducted a review of coroners’ records from 1988 through 1994 in 2 regions in Florida. They found 48 intimate partner homicide-suicide cases among “old couples” (age ≥55). All were perpetrated by men. The authors identified sociocultural differences in risk factors between the 2 regions. In west-central Florida, perpetrators and victims were predominantly white and in a spousal relationship. Domestic violence was documented in <4% of cases. Approximately 55% of the couples were reported to be ill, and a substantial proportion were documented to be declining in health. One-quarter of the perpetrators and one-third of the victims had “pain and suffering.” More than one-third of perpetrators were reported to have “depression,” 15% were reported to have talked about suicide, and 4% had a history of a suicide attempt. Only 11% of perpetrators were described as abusing substances.
The authors noted several differences in cases in southeastern Florida. Approximately two-thirds of the couples were Hispanic, and 14% had a history of domestic violence. A minority of the couples were in a live-in relationship. Less than 15% of the perpetrators and victims were described as having a decline in health. Additionally, only 19% of perpetrators were reported to have “depression,” and none of the perpetrators had a documented history of attempted suicide or substance abuse. No information was provided regarding the methods used to carry out the homicide-suicide in the southeastern region.13 Financial stress was not a factor in either region.
Malphurs et al14 (2001) used the same database described in the Cohen et al13 study to compare 27 perpetrators of homicide-suicide to 36 age-matched suicide decedents in west central Florida. They found that homicide-suicide perpetrators were significantly less likely to have health problems and were 3 times more likely to be caregivers to their spouses. Approximately 52% of perpetrators had at least 1 documented psychiatric symptom (“depression” and/or substance abuse or other), but only 5% were seeking mental health services at the time of death.14
De Koning and Piette15 (2014) conducted a retrospective medicolegal chart review from 1935 to 2010 to identify homicide-suicide cases in West and East Flanders, Belgium. They found 19 cases of intimate partner homicide-suicide committed by offenders age ≥55 years. Ninety-five percent of the perpetrators were men who killed their female partners. In one-quarter of the cases, either the perpetrator or the victim had a health issue; 21% of the perpetrators were documented as having depression and 27% had alcohol intoxication at the time of death. A motive was documented in 14 out of 19 cases; “mercy killing” was determined as the motive in 6 (43%) cases and “amorous jealousy” in 5 cases (36%).15 Starting in the 1970s, firearms were the most prevalent method used to kill a partner.
Logan et al16 (2019) used data from the National Violent Death Reporting System between 2003 and 2015 to identify characteristics that differentiated male suicide decedents from male perpetrators of intimate partner homicide-suicide. They found that men age 50 to 64 years were 3 times more likely than men age 18 to 34 years to commit intimate partner homicide-suicide, and that men age ≥65 years were approximately 5 times more likely than men age 18 to 34 years to commit intimate partner homicide-suicide. The authors found that approximately 22% of all perpetrators had a documented history of physical domestic violence, and close to 17% had a prior interaction with the criminal justice system. Furthermore, one-third of perpetrators had relationship difficulties and were in the process of a breakup. Health issues were prevalent in 34% of the victims and 26% of the perpetrators. Perpetrator-caregiver burden was reported as a contributing factor for homicide-suicide in 16% of cases. In 27% of cases, multiple health-related contributing factors were mentioned.16
Continue to: Malphurs and Cohen
Malphurs and Cohen5 (2002) reviewed American newspapers from 1997 through 1999 and identified 673 homicide-suicide events, of which 152 (27%) were committed by individuals age ≥55 years. The victims and perpetrators (95% of which were men) were intimate partners in three-quarters of cases. In nearly one-third of cases, caregiving was a contributing factor for the homicide-suicide. A history of or a pending divorce was reported in nearly 14% of cases. Substance use history was rarely recorded. Firearms were used in 88% of the homicide-suicide cases.5
Malphurs and Cohen17 (2005) reviewed coroner records between 1998 and 1999 in Florida and compared 20 cases of intimate partner homicide-suicide involving perpetrators age ≥55 years with matched suicide decedents. They found that 60% of homicide-suicide perpetrators had documented health issues. The authors reported that a “recent change in health status” was more prevalent among perpetrators compared with decedents. Perpetrators were also more likely to be caregivers to their spouses. The authors found that 65% of perpetrators were reported to have a “depressed mood” and 15% of perpetrators had reportedly threatened suicide prior to the incident. However, none of the perpetrators tested positive for antidepressants as documented on post-mortem toxicology reports. Firearms were used in 100% of homicide-suicide cases.17
Salari3 (2007) reviewed multiple American media sources and published police reports between 1999 and 2005 to retrieve data about intimate partner homicide-suicide events in the United States. There were 225 events identified where the perpetrator and/or the victim were age ≥60 years. Ninety-six percent of the perpetrators were men and most homicide-suicide events were committed at the home. A history of domestic violence was reported in 14% of homicide-suicide cases. Thirteen percent of couples were separated or divorced. The perpetrator and/or victim had health issues in 124 (55%) events. Dementia was reported in 7.5% of cases, but overwhelmingly among the victims. Substance abuse was rarely mentioned as a contributing factor. In three-quarters of cases where a motive was described, the perpetrator was “suicidal”; however, a “suicide pact” was mentioned in only 4% of cases. Firearms were used in 87% of cases.3
Focus on common risk factors
The scarcity and heterogeneity of research regarding older adult homicide-suicide were major limitations to our review. Because most of the studies we identified had a small sample size and limited information regarding the mental health of victims and perpetrators, it would be an overreach to claim to have identified a typical profile of an older perpetrator of homicide-suicide. However, the literature has repeatedly identified several common characteristics of such perpetrators. They are significantly more likely to be men who use firearms to murder their intimate partners and then die by suicide in their home (Table3,5,11-17). Health issues afflicting 1 or both individuals in the couple appear to be a contributing factor, particularly when the perpetrator is in a caregiving role. Relational discord, with or without a history of domestic violence, increases the risk of homicide-suicide. Finally, older perpetrators are highly likely to be depressed and have suicidal ideations.
How COVID-19 affects these risks
Although it is too early to determine if there is a causal relationship between the COVID-19 pandemic and an increase in homicide-suicide, the pandemic is likely to promote risk factors for these events, especially among older adults. Confinement measures put into place during the pandemic context have already been shown to increase rates of domestic violence18 and depression and anxiety among older individuals.7 Furthermore,
Continue to: Late-life psychiatric disorders
Late-life psychiatric disorders
Early recognition and effective treatment of late-life psychiatric disorders is crucial. Unfortunately, depression in geriatric patients is often underdiagnosed and undertreated.20 Older adults have more frequent contact with their primary care physicians, and rarely consult mental health professionals.21,22 Several models of integrated depression care within primary care settings have shown the positive impact of this collaborative approach in treating late-life depression and preventing suicide in older individuals.23 Additionally, because alcohol abuse is also a risk factor for domestic violence and breaking the law in this population,24,25 older adults should be screened for alcohol use disorders, and referred to treatment when necessary.
Take steps to keep patients safe
In the context of the COVID-19 pandemic, there are several steps clinicians need to keep in mind when interacting with older patients:
- Screen for depressive symptoms, suicidality, and alcohol and substance use disorders. Individuals who have tested positive for COVID-19 or who have been in contact with a carrier are a particularly vulnerable population.
- Screen for domestic violence and access to weapons at home.4 Any older adult who has a psychiatric disorder and/or suicide ideation should receive immediate intervention through a social worker that includes providing gun-risk education to other family members or contacting law-enforcement officials.26
- Refer patients with a suspected psychiatric disorder to specialized mental health clinicians. Telemental health services can provide rapid access to subspecialists, allowing patients to be treated from their homes.27 These services need to be promoted among older adults during this critical period and reimbursed by public and private insurance systems to ensure accessibility and affordability.28
- Create psychiatric inpatient units specifically designed for suicidal and/or homicidal patients with COVID-19.
Additionally, informing the public about these major health issues is crucial. The media can raise awareness about domestic violence and depression among older adults; however, this should be done responsibly and with accuracy to prevent the spread of misinformation, confusion, fear, and panic.29
Bottom Line
The mental health burden of the coronavirus disease 2019 pandemic has significantly impacted individuals who are older and most vulnerable. Reducing the incidence of homicide-suicide among older adults requires timely screening and interventions by primary care providers, mental health specialists, social workers, media, and governmental agencies.
Related Resources
- Saeed SA, Hebishi K. The psychiatric consequences of COVID-19: 8 studies. Current Psychiatry. 2020;19(11):22-24,28-30,32-35.
- Schwab-Reese LM, Murfree L, Coppola EC, et al. Homicidesuicide across the lifespan: a mixed methods examination of factors contributing to older adult perpetration. Aging Ment Health. 2020;20:1-9.
1. Christodoulou H. LOCKDOWN ‘MURDER-SUICIDE’ OAP, 71, ‘stabbed wife to death then killed himself as he worried about coping with coronavirus lockdown.’ The Sun. Updated April 4, 2020. Accessed December 22, 2020. https://www.thesun.co.uk/news/11327095/coronavirus-lockdown-murder-suicide-cambridge/
2. Farberov S. Illinois man, 54, shoots dead his wife then kills himself in murder-suicide because he feared they had coronavirus - but tests later show the couple were NOT ill. Updated April 6, 2020. Accessed December 22, 2020. https://www.dailymail.co.uk/news/article-8191933/Man-kills-wife-feared-coronavirus.html
3. Salari S. Patterns of intimate partner homicide suicide in later life: strategies for prevention. Clin Interv Aging. 2007;2(3):441-452.
4. Kotzé C, Roos JL. Homicide–suicide: practical implications for risk reduction and support services at primary care level. South African Family Practice. 2019;61(4):165-169.
5. Malphurs JE, Cohen D. A newspaper surveillance study of homicide-suicide in the United States. Am J Forensic Med Pathol. 2002;23(2):142-148.
6. Eliason S. Murder-suicide: a review of the recent literature. J Am Acad Psychiatry Law. 2009;37(3):371-376.
7. Armitage R, Nellums LB. COVID-19 and the consequences of isolating the elderly. Lancet Public Health. 2020;5(5):e256. doi: 10.1016/S2468-2667(20)30061-X
8. Gunnell D, Appleby L, Arensman E, et al. Suicide risk and prevention during the COVID-19 pandemic. Lancet Psychiatry 2020;7(6):468-471.
9. Gosangi B, Park H, Thomas R, et al. Exacerbation of physical intimate partner violence during COVID-19 pandemic. Radiology. 2021;298(1):E38-E45.
10. Mannix R, Lee LK, Fleegler EW. Coronavirus disease 2019 (COVID-19) and firearms in the United States: will an epidemic of suicide follow? Ann Intern Med. 2020;173(3):228-229.
11. Bourget D, Gagne P, Whitehurst L. Domestic homicide and homicide-suicide: the older offender. J Am Acad Psychiatry Law. 2010;38(3):305-311.
12. Cheung G, Hatters Friedman S, Sundram F. Late-life homicide-suicide: a national case series in New Zealand. Psychogeriatrics. 2016;16(1):76-81.
13. Cohen D, Llorente M, Eisdorfer C. Homicide-suicide in older persons. Am J Psychiatry. 1998;155(3):390-396.
14. Malphurs JE, Eisdorfer C, Cohen D. A comparison of antecedents of homicide-suicide and suicide in older married men. Am J Geriatr Psychiatry. 2001;9(1):49-57.
15. De Koning E, Piette MHA. A retrospective study of murder–suicide at the Forensic Institute of Ghent University, Belgium: 1935–2010. Med Sci Law. 2014;54(2):88-98.
16. Logan JE, Ertl A, Bossarte R. Correlates of intimate partner homicide among male suicide decedents with known intimate partner problems. Suicide Life Threat Behav. 2019;49(6):1693-1706.
17. Malphurs JE, Cohen D. A statewide case-control study of spousal homicide-suicide in older persons. Am J Geriatr Psychiatry. 2005;13(3):211-217.
18. Sanford A. ‘Horrifying surge in domestic violence’ against women amid coronavirus-lockdowns, UN chief warns. Euronews. Published June 4, 2020. Accessed December 22, 2020. https://www.euronews.com/2020/04/06/horrifying-surge-in-domestic-violence-against-women-amid-coronavirus-lockdowns-un-chief-w
19. Appel JM. Intimate partner homicide in elderly populations. In: Friedman SH, ed. Family murder: pathologies of love and hate. American Psychiatric Association Publishing; 2019:131-142.
20. Hall CA, Reynolds-III CF. Late-life depression in the primary care setting: challenges, collaborative care, and prevention. Maturitas. 2014;79(2):147-152.
21. Unützer J. Diagnosis and treatment of older adults with depression in primary care. Biological Psychiatry. 2002;52(3):285-292.
22. Byers AL, Arean PA, Yaffe K. Low use of mental health services among older Americans with mood and anxiety disorders. Psychiatr Serv. 2012;63(1):66-72.
23. Bruce ML, Sirey JA. Integrated care for depression in older primary care patients. Can J Psychiatry. 2018;63(7):439-446.
24. Rao R, Roche A. Substance misuse in older people. BMJ. 2017;358:j3885. doi: 10.1136/bmj.j3885
25. Ghossoub E, Khoury R. Prevalence and correlates of criminal behavior among the non-institutionalized elderly: results from the National Survey on Drug Use and Health. J Geriatr Psychiatry Neurol. 2018;31(4):211-222.
26. Slater MAG. Older adults at risk for suicide. In: Berkman B. Handbook of social work in health and aging. Oxford University Press; 2006:149-161.
27. Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679-1681.
28. Centers for Medicare & Medicaid Services. President Trump expands telehealth benefits for Medicare beneficiaries during COVID-19 outbreak. Published March 17, 2020. Accessed December 23, 2020. https://www.cms.gov/newsroom/press-releases/president-trump-expands-telehealth-benefits-medicare-beneficiaries-during-covid-19-outbreak
29. Mian A, Khan S. Coronavirus: the spread of misinformation. BMC Med. 2020;18(1):89.
1. Christodoulou H. LOCKDOWN ‘MURDER-SUICIDE’ OAP, 71, ‘stabbed wife to death then killed himself as he worried about coping with coronavirus lockdown.’ The Sun. Updated April 4, 2020. Accessed December 22, 2020. https://www.thesun.co.uk/news/11327095/coronavirus-lockdown-murder-suicide-cambridge/
2. Farberov S. Illinois man, 54, shoots dead his wife then kills himself in murder-suicide because he feared they had coronavirus - but tests later show the couple were NOT ill. Updated April 6, 2020. Accessed December 22, 2020. https://www.dailymail.co.uk/news/article-8191933/Man-kills-wife-feared-coronavirus.html
3. Salari S. Patterns of intimate partner homicide suicide in later life: strategies for prevention. Clin Interv Aging. 2007;2(3):441-452.
4. Kotzé C, Roos JL. Homicide–suicide: practical implications for risk reduction and support services at primary care level. South African Family Practice. 2019;61(4):165-169.
5. Malphurs JE, Cohen D. A newspaper surveillance study of homicide-suicide in the United States. Am J Forensic Med Pathol. 2002;23(2):142-148.
6. Eliason S. Murder-suicide: a review of the recent literature. J Am Acad Psychiatry Law. 2009;37(3):371-376.
7. Armitage R, Nellums LB. COVID-19 and the consequences of isolating the elderly. Lancet Public Health. 2020;5(5):e256. doi: 10.1016/S2468-2667(20)30061-X
8. Gunnell D, Appleby L, Arensman E, et al. Suicide risk and prevention during the COVID-19 pandemic. Lancet Psychiatry 2020;7(6):468-471.
9. Gosangi B, Park H, Thomas R, et al. Exacerbation of physical intimate partner violence during COVID-19 pandemic. Radiology. 2021;298(1):E38-E45.
10. Mannix R, Lee LK, Fleegler EW. Coronavirus disease 2019 (COVID-19) and firearms in the United States: will an epidemic of suicide follow? Ann Intern Med. 2020;173(3):228-229.
11. Bourget D, Gagne P, Whitehurst L. Domestic homicide and homicide-suicide: the older offender. J Am Acad Psychiatry Law. 2010;38(3):305-311.
12. Cheung G, Hatters Friedman S, Sundram F. Late-life homicide-suicide: a national case series in New Zealand. Psychogeriatrics. 2016;16(1):76-81.
13. Cohen D, Llorente M, Eisdorfer C. Homicide-suicide in older persons. Am J Psychiatry. 1998;155(3):390-396.
14. Malphurs JE, Eisdorfer C, Cohen D. A comparison of antecedents of homicide-suicide and suicide in older married men. Am J Geriatr Psychiatry. 2001;9(1):49-57.
15. De Koning E, Piette MHA. A retrospective study of murder–suicide at the Forensic Institute of Ghent University, Belgium: 1935–2010. Med Sci Law. 2014;54(2):88-98.
16. Logan JE, Ertl A, Bossarte R. Correlates of intimate partner homicide among male suicide decedents with known intimate partner problems. Suicide Life Threat Behav. 2019;49(6):1693-1706.
17. Malphurs JE, Cohen D. A statewide case-control study of spousal homicide-suicide in older persons. Am J Geriatr Psychiatry. 2005;13(3):211-217.
18. Sanford A. ‘Horrifying surge in domestic violence’ against women amid coronavirus-lockdowns, UN chief warns. Euronews. Published June 4, 2020. Accessed December 22, 2020. https://www.euronews.com/2020/04/06/horrifying-surge-in-domestic-violence-against-women-amid-coronavirus-lockdowns-un-chief-w
19. Appel JM. Intimate partner homicide in elderly populations. In: Friedman SH, ed. Family murder: pathologies of love and hate. American Psychiatric Association Publishing; 2019:131-142.
20. Hall CA, Reynolds-III CF. Late-life depression in the primary care setting: challenges, collaborative care, and prevention. Maturitas. 2014;79(2):147-152.
21. Unützer J. Diagnosis and treatment of older adults with depression in primary care. Biological Psychiatry. 2002;52(3):285-292.
22. Byers AL, Arean PA, Yaffe K. Low use of mental health services among older Americans with mood and anxiety disorders. Psychiatr Serv. 2012;63(1):66-72.
23. Bruce ML, Sirey JA. Integrated care for depression in older primary care patients. Can J Psychiatry. 2018;63(7):439-446.
24. Rao R, Roche A. Substance misuse in older people. BMJ. 2017;358:j3885. doi: 10.1136/bmj.j3885
25. Ghossoub E, Khoury R. Prevalence and correlates of criminal behavior among the non-institutionalized elderly: results from the National Survey on Drug Use and Health. J Geriatr Psychiatry Neurol. 2018;31(4):211-222.
26. Slater MAG. Older adults at risk for suicide. In: Berkman B. Handbook of social work in health and aging. Oxford University Press; 2006:149-161.
27. Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679-1681.
28. Centers for Medicare & Medicaid Services. President Trump expands telehealth benefits for Medicare beneficiaries during COVID-19 outbreak. Published March 17, 2020. Accessed December 23, 2020. https://www.cms.gov/newsroom/press-releases/president-trump-expands-telehealth-benefits-medicare-beneficiaries-during-covid-19-outbreak
29. Mian A, Khan S. Coronavirus: the spread of misinformation. BMC Med. 2020;18(1):89.
Suvorexant: An option for preventing delirium?
Delirium is characterized by a disturbance of consciousness or cognition that typically has a rapid onset and fluctuating course.1 Up to 42% of hospitalized geriatric patients experience delirium.1 Approximately 10% to 31% of these patients have the condition upon admission, and the remainder develop it during their hospitalization.1 Unfortunately, options for preventing or treating delirium are limited. Benzodiazepines and antipsychotic medications have been used to treat problematic behaviors associated with delirium, but they do not effectively reduce the occurrence, duration, or severity of this condition.2,3
Recent evidence suggests that suvorexant, which is FDA-approved for insomnia, may be useful for preventing delirium. Suvorexant—a dual orexin receptor (OX1R, OX2R) antagonist—promotes sleep onset and maintenance, and is associated with normal measures of sleep activity such as rapid eye movement (REM) sleep, non-REM sleep, and sleep stage–specific electroencephalographic profiles.4 Here we review 3 studies that evaluated suvorexant for preventing delirium.
Hatta et al.5 In this randomized, placebo-controlled, blinded, multicenter study, 72 patients (age 65 to 89) newly admitted to an ICU were randomized to suvorexant, 15 mg/d, (n = 36) or placebo (n = 36) for 3 days.5 None of the patients taking suvorexant developed delirium, whereas 17% (6 patients) in the placebo group did (P = .025).5
Azuma et al.6 In this 7-day, blinded, randomized study of 70 adult patients (age ≥20) admitted to an ICU, 34 participants received suvorexant (15 mg nightly for age <65, 20 mg nightly for age ≥65) and the rest received treatment as usual (TAU). Suvorexant was associated with a lower incidence of delirium symptoms (n = 6, 17.6%) compared with TAU (n = 17, 47.2%) (P = .011).6 The onset of delirium was earlier in the TAU group (P < .05).6
Hatta et al.7 In this large prospective, observational study of adults (age >65), 526 patients with significant risk factors for delirium were prescribed suvorexant and/or ramelteon. Approximately 16% of the patients who received either or both of these medications met DSM-5 criteria for delirium, compared with 24% who did not receive these medications (P = .005).7
Acknowledgment
The authors thank Jakob Evans, BS, for compiling much of the research for this article.
1. Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing. 2006;35(4):350-364.
2. Lonergan E, Luxenberg J, Areosa Sastre A. Benzodiazepines for delirium. Cochrane Database Syst Rev. 2009;2009(4):CD006379.
3. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev. 2018;6(6):CD005594.
4. Coleman PJ, Gotter AL, Herring WJ, et al. The discovery of suvorexant, the first orexin receptor drug for insomnia. Annu Rev Pharmacol Toxicol. 2017;57:509-533.
5. Hatta K, Kishi Y, Wada K, et al. Preventive effects of suvorexant on delirium: a randomized placebo-controlled trial. J Clin Psychiatry. 2017;78(8):e970-e979.
6. Azuma K, Takaesu Y, Soeda H, et al. Ability of suvorexant to prevent delirium in patients in the intensive care unit: a randomized controlled trial. Acute Med Surg. 2018;5(4):362-368.
7. Hatta K, Kishi Y, Wada K, et al. Real-world effectiveness of ramelteon and suvorexant for delirium prevention in 948 patients with delirium risk factors. J Clin Psychiatry. 2019;81(1):19m12865. doi: 10.4088/JCP.19m12865
Delirium is characterized by a disturbance of consciousness or cognition that typically has a rapid onset and fluctuating course.1 Up to 42% of hospitalized geriatric patients experience delirium.1 Approximately 10% to 31% of these patients have the condition upon admission, and the remainder develop it during their hospitalization.1 Unfortunately, options for preventing or treating delirium are limited. Benzodiazepines and antipsychotic medications have been used to treat problematic behaviors associated with delirium, but they do not effectively reduce the occurrence, duration, or severity of this condition.2,3
Recent evidence suggests that suvorexant, which is FDA-approved for insomnia, may be useful for preventing delirium. Suvorexant—a dual orexin receptor (OX1R, OX2R) antagonist—promotes sleep onset and maintenance, and is associated with normal measures of sleep activity such as rapid eye movement (REM) sleep, non-REM sleep, and sleep stage–specific electroencephalographic profiles.4 Here we review 3 studies that evaluated suvorexant for preventing delirium.
Hatta et al.5 In this randomized, placebo-controlled, blinded, multicenter study, 72 patients (age 65 to 89) newly admitted to an ICU were randomized to suvorexant, 15 mg/d, (n = 36) or placebo (n = 36) for 3 days.5 None of the patients taking suvorexant developed delirium, whereas 17% (6 patients) in the placebo group did (P = .025).5
Azuma et al.6 In this 7-day, blinded, randomized study of 70 adult patients (age ≥20) admitted to an ICU, 34 participants received suvorexant (15 mg nightly for age <65, 20 mg nightly for age ≥65) and the rest received treatment as usual (TAU). Suvorexant was associated with a lower incidence of delirium symptoms (n = 6, 17.6%) compared with TAU (n = 17, 47.2%) (P = .011).6 The onset of delirium was earlier in the TAU group (P < .05).6
Hatta et al.7 In this large prospective, observational study of adults (age >65), 526 patients with significant risk factors for delirium were prescribed suvorexant and/or ramelteon. Approximately 16% of the patients who received either or both of these medications met DSM-5 criteria for delirium, compared with 24% who did not receive these medications (P = .005).7
Acknowledgment
The authors thank Jakob Evans, BS, for compiling much of the research for this article.
Delirium is characterized by a disturbance of consciousness or cognition that typically has a rapid onset and fluctuating course.1 Up to 42% of hospitalized geriatric patients experience delirium.1 Approximately 10% to 31% of these patients have the condition upon admission, and the remainder develop it during their hospitalization.1 Unfortunately, options for preventing or treating delirium are limited. Benzodiazepines and antipsychotic medications have been used to treat problematic behaviors associated with delirium, but they do not effectively reduce the occurrence, duration, or severity of this condition.2,3
Recent evidence suggests that suvorexant, which is FDA-approved for insomnia, may be useful for preventing delirium. Suvorexant—a dual orexin receptor (OX1R, OX2R) antagonist—promotes sleep onset and maintenance, and is associated with normal measures of sleep activity such as rapid eye movement (REM) sleep, non-REM sleep, and sleep stage–specific electroencephalographic profiles.4 Here we review 3 studies that evaluated suvorexant for preventing delirium.
Hatta et al.5 In this randomized, placebo-controlled, blinded, multicenter study, 72 patients (age 65 to 89) newly admitted to an ICU were randomized to suvorexant, 15 mg/d, (n = 36) or placebo (n = 36) for 3 days.5 None of the patients taking suvorexant developed delirium, whereas 17% (6 patients) in the placebo group did (P = .025).5
Azuma et al.6 In this 7-day, blinded, randomized study of 70 adult patients (age ≥20) admitted to an ICU, 34 participants received suvorexant (15 mg nightly for age <65, 20 mg nightly for age ≥65) and the rest received treatment as usual (TAU). Suvorexant was associated with a lower incidence of delirium symptoms (n = 6, 17.6%) compared with TAU (n = 17, 47.2%) (P = .011).6 The onset of delirium was earlier in the TAU group (P < .05).6
Hatta et al.7 In this large prospective, observational study of adults (age >65), 526 patients with significant risk factors for delirium were prescribed suvorexant and/or ramelteon. Approximately 16% of the patients who received either or both of these medications met DSM-5 criteria for delirium, compared with 24% who did not receive these medications (P = .005).7
Acknowledgment
The authors thank Jakob Evans, BS, for compiling much of the research for this article.
1. Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing. 2006;35(4):350-364.
2. Lonergan E, Luxenberg J, Areosa Sastre A. Benzodiazepines for delirium. Cochrane Database Syst Rev. 2009;2009(4):CD006379.
3. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev. 2018;6(6):CD005594.
4. Coleman PJ, Gotter AL, Herring WJ, et al. The discovery of suvorexant, the first orexin receptor drug for insomnia. Annu Rev Pharmacol Toxicol. 2017;57:509-533.
5. Hatta K, Kishi Y, Wada K, et al. Preventive effects of suvorexant on delirium: a randomized placebo-controlled trial. J Clin Psychiatry. 2017;78(8):e970-e979.
6. Azuma K, Takaesu Y, Soeda H, et al. Ability of suvorexant to prevent delirium in patients in the intensive care unit: a randomized controlled trial. Acute Med Surg. 2018;5(4):362-368.
7. Hatta K, Kishi Y, Wada K, et al. Real-world effectiveness of ramelteon and suvorexant for delirium prevention in 948 patients with delirium risk factors. J Clin Psychiatry. 2019;81(1):19m12865. doi: 10.4088/JCP.19m12865
1. Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing. 2006;35(4):350-364.
2. Lonergan E, Luxenberg J, Areosa Sastre A. Benzodiazepines for delirium. Cochrane Database Syst Rev. 2009;2009(4):CD006379.
3. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev. 2018;6(6):CD005594.
4. Coleman PJ, Gotter AL, Herring WJ, et al. The discovery of suvorexant, the first orexin receptor drug for insomnia. Annu Rev Pharmacol Toxicol. 2017;57:509-533.
5. Hatta K, Kishi Y, Wada K, et al. Preventive effects of suvorexant on delirium: a randomized placebo-controlled trial. J Clin Psychiatry. 2017;78(8):e970-e979.
6. Azuma K, Takaesu Y, Soeda H, et al. Ability of suvorexant to prevent delirium in patients in the intensive care unit: a randomized controlled trial. Acute Med Surg. 2018;5(4):362-368.
7. Hatta K, Kishi Y, Wada K, et al. Real-world effectiveness of ramelteon and suvorexant for delirium prevention in 948 patients with delirium risk factors. J Clin Psychiatry. 2019;81(1):19m12865. doi: 10.4088/JCP.19m12865
First mammography guidelines for older breast cancer survivors
For women who have a life expectancy of 5-10 years, the guidelines recommend that consideration be given to discontinuing mammography.
Overall, the guidelines encourage shared decision-making that is individualized for each woman after weighing the benefits and harms associated with surveillance mammography and patient preferences.
The panel also recommended that patients with clinical findings and symptoms receive ongoing clinical breast examinations and diagnostic mammography and that patients be reassured that these practices will continue.
Guidelines on breast cancer screening for healthy women already “acknowledge the limitations of mammograms and the need to consider one’s health status and preferences when making decisions on how and when to stop routine mammograms,” said the article’s first author, Rachel A. Freedman, MD, MPH, of the Dana-Farber Cancer Institute, Boston.
However, “we don’t have this kind of consensus for women with a history of breast cancer,” she continued. “Current follow-up care guidelines simply state that women with a history of breast cancer with intact breasts should have annual mammography without any guidance.
“In practice, the use of mammograms is highly variable, with less than 50% of breast cancer survivors who have limited life expectancy having annual mammograms, according to survey data we have from prior work,” Dr. Freedman said in an interview.
The guidelines were published online Jan. 28 in JAMA Oncology.
Clinicians discuss how to have these discussions
As part of the process of developing these expert consensus guidelines, the researchers held several clinical focus groups that involved primary care physicians from Brigham and Women’s Hospital and oncology clinicians (including breast surgeons and medical oncologists) from the Dana-Farber Cancer Institute.
All clinicians felt that having expert guidelines and talking points to guide discussions would be helpful, the researchers report.
“However, some oncology clinicians felt that 75 years is often ‘too young’ to stop surveillance mammography and that 80 years may be a more comfortable age to stop routine testing,” they write. “Most clinicians felt that estimations of life expectancy, more than age, should inform the timing of this discussion.”
In contrast to primary and geriatric care clinicians, oncology clinicians reported discomfort with such discussions. They appreciated having the information but “felt it was easier to communicate findings indirectly, without specifically revealing life expectancy to patients. One oncology clinician, however, felt it would be ‘sneaky’ to calculate life expectancy without communicating this to patients, supporting more open discussions,” the authors report.
“All clinicians acknowledged that framing the conversation around patients’ low risk for in-breast cancer events and how mammography will not benefit them was more appealing than discussing life expectancy,” the researchers continue. Their literature review found that the risk of these individuals developing second breast cancers was similar to that of a healthy woman developing a first breast cancer, leading one clinician to comment: “If their risk is really equivalent to the general population – that is very powerful.”
“Some clinicians reported that they ‘focus on the risks’ or frame such discussions by asking: ‘If you were to find something on [a] mammogram, would you do anything about it?’ If a patient answered no, clinicians felt this was a signal to stop mammography,” they noted.
Literature review finds very low risk
Dr. Freedman and colleagues conducted a literature review of the risk for ipsilateral and contralateral breast cancer events among survivors and of the harms and benefits associated with mammography. Following the literature review, a multidisciplinary expert panel, which included patients and patient advocates, was convened to develop consensus guidelines.
The literature review confirmed that there was a low risk for in-breast cancer events in this population and that the risk was particularly low among patients who undergo treatment with endocrine therapy. Among those who did not receive systemic therapy for ERBB2-positive or triple-negative cancers, the rates of ipsilateral recurrence were estimated to be higher.
On the basis of the literature review, the estimated 10-year risk for in-breast cancer events ranged from 1% to 15% for ipsilateral breast cancers and from 1% to 5% for contralateral cancers. Among women in the same age group who did not have a history of breast cancer, the 5-year risk of developing the disease (average risk) was 2.2%.
The authors note that these findings mirror their estimates for new breast cancers among survivors who had low-risk disease. The findings are also similar to those cited in a large-scale mammography study, in which breast cancer survivors aged 70-80 years had a 1.1% annual risk for in-breast cancers. The risk was 0.7%-0.9% for similarly aged patients who did not have a history of breast cancer.
The benefits associated with mammography for older women are not well defined, but the literature suggests that mammography offers little to modest clinical benefit for patients in this age group. The limited benefits are likely because of the more than 10-year time lag that is needed to detect the small improvements in breast cancer mortality; slow-growing tumors generally do not affect the life expectancy of older women, they point out.
“Through our expert consensus process and after iterative feedback from clinicians, we created guidelines to support patients and clinicians in making individualized decisions on how and when to stop mammography,” said Dr. Freedman. “These guidelines are based on the risk of a breast cancer returning in the breast, one’s underlying health, and one’s preferences.”
The guidelines are also intended to provide information to patients on the benefits and harms of mammography in this setting, in addition to “how much we anticipate a mammogram may or may not continue to help a woman over time,” she said.
A companion guide for patients on these guidelines will be published in the coming months.
Dr. Freedman has received institutional clinical trial funding from Eisai and Puma Biotechnology outside the submitted work.
A version of this article first appeared on Medscape.com.
For women who have a life expectancy of 5-10 years, the guidelines recommend that consideration be given to discontinuing mammography.
Overall, the guidelines encourage shared decision-making that is individualized for each woman after weighing the benefits and harms associated with surveillance mammography and patient preferences.
The panel also recommended that patients with clinical findings and symptoms receive ongoing clinical breast examinations and diagnostic mammography and that patients be reassured that these practices will continue.
Guidelines on breast cancer screening for healthy women already “acknowledge the limitations of mammograms and the need to consider one’s health status and preferences when making decisions on how and when to stop routine mammograms,” said the article’s first author, Rachel A. Freedman, MD, MPH, of the Dana-Farber Cancer Institute, Boston.
However, “we don’t have this kind of consensus for women with a history of breast cancer,” she continued. “Current follow-up care guidelines simply state that women with a history of breast cancer with intact breasts should have annual mammography without any guidance.
“In practice, the use of mammograms is highly variable, with less than 50% of breast cancer survivors who have limited life expectancy having annual mammograms, according to survey data we have from prior work,” Dr. Freedman said in an interview.
The guidelines were published online Jan. 28 in JAMA Oncology.
Clinicians discuss how to have these discussions
As part of the process of developing these expert consensus guidelines, the researchers held several clinical focus groups that involved primary care physicians from Brigham and Women’s Hospital and oncology clinicians (including breast surgeons and medical oncologists) from the Dana-Farber Cancer Institute.
All clinicians felt that having expert guidelines and talking points to guide discussions would be helpful, the researchers report.
“However, some oncology clinicians felt that 75 years is often ‘too young’ to stop surveillance mammography and that 80 years may be a more comfortable age to stop routine testing,” they write. “Most clinicians felt that estimations of life expectancy, more than age, should inform the timing of this discussion.”
In contrast to primary and geriatric care clinicians, oncology clinicians reported discomfort with such discussions. They appreciated having the information but “felt it was easier to communicate findings indirectly, without specifically revealing life expectancy to patients. One oncology clinician, however, felt it would be ‘sneaky’ to calculate life expectancy without communicating this to patients, supporting more open discussions,” the authors report.
“All clinicians acknowledged that framing the conversation around patients’ low risk for in-breast cancer events and how mammography will not benefit them was more appealing than discussing life expectancy,” the researchers continue. Their literature review found that the risk of these individuals developing second breast cancers was similar to that of a healthy woman developing a first breast cancer, leading one clinician to comment: “If their risk is really equivalent to the general population – that is very powerful.”
“Some clinicians reported that they ‘focus on the risks’ or frame such discussions by asking: ‘If you were to find something on [a] mammogram, would you do anything about it?’ If a patient answered no, clinicians felt this was a signal to stop mammography,” they noted.
Literature review finds very low risk
Dr. Freedman and colleagues conducted a literature review of the risk for ipsilateral and contralateral breast cancer events among survivors and of the harms and benefits associated with mammography. Following the literature review, a multidisciplinary expert panel, which included patients and patient advocates, was convened to develop consensus guidelines.
The literature review confirmed that there was a low risk for in-breast cancer events in this population and that the risk was particularly low among patients who undergo treatment with endocrine therapy. Among those who did not receive systemic therapy for ERBB2-positive or triple-negative cancers, the rates of ipsilateral recurrence were estimated to be higher.
On the basis of the literature review, the estimated 10-year risk for in-breast cancer events ranged from 1% to 15% for ipsilateral breast cancers and from 1% to 5% for contralateral cancers. Among women in the same age group who did not have a history of breast cancer, the 5-year risk of developing the disease (average risk) was 2.2%.
The authors note that these findings mirror their estimates for new breast cancers among survivors who had low-risk disease. The findings are also similar to those cited in a large-scale mammography study, in which breast cancer survivors aged 70-80 years had a 1.1% annual risk for in-breast cancers. The risk was 0.7%-0.9% for similarly aged patients who did not have a history of breast cancer.
The benefits associated with mammography for older women are not well defined, but the literature suggests that mammography offers little to modest clinical benefit for patients in this age group. The limited benefits are likely because of the more than 10-year time lag that is needed to detect the small improvements in breast cancer mortality; slow-growing tumors generally do not affect the life expectancy of older women, they point out.
“Through our expert consensus process and after iterative feedback from clinicians, we created guidelines to support patients and clinicians in making individualized decisions on how and when to stop mammography,” said Dr. Freedman. “These guidelines are based on the risk of a breast cancer returning in the breast, one’s underlying health, and one’s preferences.”
The guidelines are also intended to provide information to patients on the benefits and harms of mammography in this setting, in addition to “how much we anticipate a mammogram may or may not continue to help a woman over time,” she said.
A companion guide for patients on these guidelines will be published in the coming months.
Dr. Freedman has received institutional clinical trial funding from Eisai and Puma Biotechnology outside the submitted work.
A version of this article first appeared on Medscape.com.
For women who have a life expectancy of 5-10 years, the guidelines recommend that consideration be given to discontinuing mammography.
Overall, the guidelines encourage shared decision-making that is individualized for each woman after weighing the benefits and harms associated with surveillance mammography and patient preferences.
The panel also recommended that patients with clinical findings and symptoms receive ongoing clinical breast examinations and diagnostic mammography and that patients be reassured that these practices will continue.
Guidelines on breast cancer screening for healthy women already “acknowledge the limitations of mammograms and the need to consider one’s health status and preferences when making decisions on how and when to stop routine mammograms,” said the article’s first author, Rachel A. Freedman, MD, MPH, of the Dana-Farber Cancer Institute, Boston.
However, “we don’t have this kind of consensus for women with a history of breast cancer,” she continued. “Current follow-up care guidelines simply state that women with a history of breast cancer with intact breasts should have annual mammography without any guidance.
“In practice, the use of mammograms is highly variable, with less than 50% of breast cancer survivors who have limited life expectancy having annual mammograms, according to survey data we have from prior work,” Dr. Freedman said in an interview.
The guidelines were published online Jan. 28 in JAMA Oncology.
Clinicians discuss how to have these discussions
As part of the process of developing these expert consensus guidelines, the researchers held several clinical focus groups that involved primary care physicians from Brigham and Women’s Hospital and oncology clinicians (including breast surgeons and medical oncologists) from the Dana-Farber Cancer Institute.
All clinicians felt that having expert guidelines and talking points to guide discussions would be helpful, the researchers report.
“However, some oncology clinicians felt that 75 years is often ‘too young’ to stop surveillance mammography and that 80 years may be a more comfortable age to stop routine testing,” they write. “Most clinicians felt that estimations of life expectancy, more than age, should inform the timing of this discussion.”
In contrast to primary and geriatric care clinicians, oncology clinicians reported discomfort with such discussions. They appreciated having the information but “felt it was easier to communicate findings indirectly, without specifically revealing life expectancy to patients. One oncology clinician, however, felt it would be ‘sneaky’ to calculate life expectancy without communicating this to patients, supporting more open discussions,” the authors report.
“All clinicians acknowledged that framing the conversation around patients’ low risk for in-breast cancer events and how mammography will not benefit them was more appealing than discussing life expectancy,” the researchers continue. Their literature review found that the risk of these individuals developing second breast cancers was similar to that of a healthy woman developing a first breast cancer, leading one clinician to comment: “If their risk is really equivalent to the general population – that is very powerful.”
“Some clinicians reported that they ‘focus on the risks’ or frame such discussions by asking: ‘If you were to find something on [a] mammogram, would you do anything about it?’ If a patient answered no, clinicians felt this was a signal to stop mammography,” they noted.
Literature review finds very low risk
Dr. Freedman and colleagues conducted a literature review of the risk for ipsilateral and contralateral breast cancer events among survivors and of the harms and benefits associated with mammography. Following the literature review, a multidisciplinary expert panel, which included patients and patient advocates, was convened to develop consensus guidelines.
The literature review confirmed that there was a low risk for in-breast cancer events in this population and that the risk was particularly low among patients who undergo treatment with endocrine therapy. Among those who did not receive systemic therapy for ERBB2-positive or triple-negative cancers, the rates of ipsilateral recurrence were estimated to be higher.
On the basis of the literature review, the estimated 10-year risk for in-breast cancer events ranged from 1% to 15% for ipsilateral breast cancers and from 1% to 5% for contralateral cancers. Among women in the same age group who did not have a history of breast cancer, the 5-year risk of developing the disease (average risk) was 2.2%.
The authors note that these findings mirror their estimates for new breast cancers among survivors who had low-risk disease. The findings are also similar to those cited in a large-scale mammography study, in which breast cancer survivors aged 70-80 years had a 1.1% annual risk for in-breast cancers. The risk was 0.7%-0.9% for similarly aged patients who did not have a history of breast cancer.
The benefits associated with mammography for older women are not well defined, but the literature suggests that mammography offers little to modest clinical benefit for patients in this age group. The limited benefits are likely because of the more than 10-year time lag that is needed to detect the small improvements in breast cancer mortality; slow-growing tumors generally do not affect the life expectancy of older women, they point out.
“Through our expert consensus process and after iterative feedback from clinicians, we created guidelines to support patients and clinicians in making individualized decisions on how and when to stop mammography,” said Dr. Freedman. “These guidelines are based on the risk of a breast cancer returning in the breast, one’s underlying health, and one’s preferences.”
The guidelines are also intended to provide information to patients on the benefits and harms of mammography in this setting, in addition to “how much we anticipate a mammogram may or may not continue to help a woman over time,” she said.
A companion guide for patients on these guidelines will be published in the coming months.
Dr. Freedman has received institutional clinical trial funding from Eisai and Puma Biotechnology outside the submitted work.
A version of this article first appeared on Medscape.com.
Menopause, not aging, may influence brain volume
Postmenopausal women not only have larger brain volume than women who are premenopausal, but they also experience larger reductions in brain volume over time, reported Ananthan Ambikairajah of the Centre for Research on Ageing, Health and Wellbeing, Australian National University, Canberra, and associates. Their report was published in Menopause.
In this large population-based cohort of 5,072 women aged 37-73 years, the goal of the study was to look at links between brain volume and measures of menstruation history, such as menopausal status, age at menopause, age at menarche, and the duration of a woman’s reproductive stage, but to do so within the context of how it relates to dementia prevalence. Citing a study in The Lancet Neurology, the authors noted that the age-standardized prevalence for dementia is 17% higher in women than in men, and they speculated that it may be important to look beyond age for answers.
What about menstrual history and Alzheimer’s disease?
According to the Framingham Study in Neurology, the remaining lifetime risk of Alzheimer’s disease (AD) is nearly double for a 65-year-old woman (12%) compared with a 65-year-old man (6.3%), leading Mr. Ambikairajah and associates to conclude that “menstruation history may also be particularly relevant, given that it is unique to female aging.” They further speculated, citing several related studies, that because AD pathology is initiated decades prior to the onset of clinical signs, menstruation history and its effects on brain health may, in fact, be reflected in brain volume.
Postmenopausal women had 0.82% and 1.33% larger total brain and hippocampal volume, respectively, compared with premenopausal women. Postmenopausal women had a 23% greater decrease in total brain volume but not in hippocampal volume over time, compared with premenopausal women.
As Braak and Braak illustrated in Acta Neuropathologica, chronic inflammation has been linked to brain shrinkage “consistent with the pattern of results in the present study,” Mr. Ambikairajah and colleagues noted, adding that longitudinal neuroimaging/biomarker studies are needed to explore this further.
What made this study unique was its ability to match pre- and postmenopausal women for age, a critically important attribute “given that aging and menopause both progress concurrently, which can make it difficult to determine the individual contribution of each for measures of brain health,” the authors explained.
In an interview, Constance Bohon, MD, an ob.gyn. in private practice and assistant clinical professor, George Washington University, Washington, observed: “The conclusion [in this study] is that an early age of menarche, delayed age of menopause and increased duration of the reproductive stage is negatively associated with brain volume.”
What of the neuroprotective effects of endogenous estrogen?
“Their findings are not consistent with a neuroprotective effect of endogenous estrogen exposure on brain volume,” she noted, adding that the study “did not assess the effect of exogenous estrogen on brain volume. Neither was the effect of exogenous or endogenous estrogen on cerebral blood flow assessed. In a study published in Obstetrics & Gynecology, the conclusion was that oophorectomy before the age of natural menopause is associated with a decrease in cognitive impairment and dementia. There was no assessment of brain volume or cerebral blood flow. Likewise in a report published in Neurobiology of Aging, Maki P and Resnick S M. concluded that estrogen helps maintain hippocampal and prefrontal function as women age,” observed Dr. Bohon, noting that the study did not assess brain volume.
“It is unclear whether the most predictive assessment for worsening cognition and dementia is the finding of decreased total brain volume, decreased hippocampal volume, or decreased cerebral blood flow. The effect of both endogenous and exogenous estrogen on the risk for dementia needs further evaluation,” she cautioned.
Mr. Ambikairajah cited one financial disclosure; the remaining contributors had no relevant disclosures.
Postmenopausal women not only have larger brain volume than women who are premenopausal, but they also experience larger reductions in brain volume over time, reported Ananthan Ambikairajah of the Centre for Research on Ageing, Health and Wellbeing, Australian National University, Canberra, and associates. Their report was published in Menopause.
In this large population-based cohort of 5,072 women aged 37-73 years, the goal of the study was to look at links between brain volume and measures of menstruation history, such as menopausal status, age at menopause, age at menarche, and the duration of a woman’s reproductive stage, but to do so within the context of how it relates to dementia prevalence. Citing a study in The Lancet Neurology, the authors noted that the age-standardized prevalence for dementia is 17% higher in women than in men, and they speculated that it may be important to look beyond age for answers.
What about menstrual history and Alzheimer’s disease?
According to the Framingham Study in Neurology, the remaining lifetime risk of Alzheimer’s disease (AD) is nearly double for a 65-year-old woman (12%) compared with a 65-year-old man (6.3%), leading Mr. Ambikairajah and associates to conclude that “menstruation history may also be particularly relevant, given that it is unique to female aging.” They further speculated, citing several related studies, that because AD pathology is initiated decades prior to the onset of clinical signs, menstruation history and its effects on brain health may, in fact, be reflected in brain volume.
Postmenopausal women had 0.82% and 1.33% larger total brain and hippocampal volume, respectively, compared with premenopausal women. Postmenopausal women had a 23% greater decrease in total brain volume but not in hippocampal volume over time, compared with premenopausal women.
As Braak and Braak illustrated in Acta Neuropathologica, chronic inflammation has been linked to brain shrinkage “consistent with the pattern of results in the present study,” Mr. Ambikairajah and colleagues noted, adding that longitudinal neuroimaging/biomarker studies are needed to explore this further.
What made this study unique was its ability to match pre- and postmenopausal women for age, a critically important attribute “given that aging and menopause both progress concurrently, which can make it difficult to determine the individual contribution of each for measures of brain health,” the authors explained.
In an interview, Constance Bohon, MD, an ob.gyn. in private practice and assistant clinical professor, George Washington University, Washington, observed: “The conclusion [in this study] is that an early age of menarche, delayed age of menopause and increased duration of the reproductive stage is negatively associated with brain volume.”
What of the neuroprotective effects of endogenous estrogen?
“Their findings are not consistent with a neuroprotective effect of endogenous estrogen exposure on brain volume,” she noted, adding that the study “did not assess the effect of exogenous estrogen on brain volume. Neither was the effect of exogenous or endogenous estrogen on cerebral blood flow assessed. In a study published in Obstetrics & Gynecology, the conclusion was that oophorectomy before the age of natural menopause is associated with a decrease in cognitive impairment and dementia. There was no assessment of brain volume or cerebral blood flow. Likewise in a report published in Neurobiology of Aging, Maki P and Resnick S M. concluded that estrogen helps maintain hippocampal and prefrontal function as women age,” observed Dr. Bohon, noting that the study did not assess brain volume.
“It is unclear whether the most predictive assessment for worsening cognition and dementia is the finding of decreased total brain volume, decreased hippocampal volume, or decreased cerebral blood flow. The effect of both endogenous and exogenous estrogen on the risk for dementia needs further evaluation,” she cautioned.
Mr. Ambikairajah cited one financial disclosure; the remaining contributors had no relevant disclosures.
Postmenopausal women not only have larger brain volume than women who are premenopausal, but they also experience larger reductions in brain volume over time, reported Ananthan Ambikairajah of the Centre for Research on Ageing, Health and Wellbeing, Australian National University, Canberra, and associates. Their report was published in Menopause.
In this large population-based cohort of 5,072 women aged 37-73 years, the goal of the study was to look at links between brain volume and measures of menstruation history, such as menopausal status, age at menopause, age at menarche, and the duration of a woman’s reproductive stage, but to do so within the context of how it relates to dementia prevalence. Citing a study in The Lancet Neurology, the authors noted that the age-standardized prevalence for dementia is 17% higher in women than in men, and they speculated that it may be important to look beyond age for answers.
What about menstrual history and Alzheimer’s disease?
According to the Framingham Study in Neurology, the remaining lifetime risk of Alzheimer’s disease (AD) is nearly double for a 65-year-old woman (12%) compared with a 65-year-old man (6.3%), leading Mr. Ambikairajah and associates to conclude that “menstruation history may also be particularly relevant, given that it is unique to female aging.” They further speculated, citing several related studies, that because AD pathology is initiated decades prior to the onset of clinical signs, menstruation history and its effects on brain health may, in fact, be reflected in brain volume.
Postmenopausal women had 0.82% and 1.33% larger total brain and hippocampal volume, respectively, compared with premenopausal women. Postmenopausal women had a 23% greater decrease in total brain volume but not in hippocampal volume over time, compared with premenopausal women.
As Braak and Braak illustrated in Acta Neuropathologica, chronic inflammation has been linked to brain shrinkage “consistent with the pattern of results in the present study,” Mr. Ambikairajah and colleagues noted, adding that longitudinal neuroimaging/biomarker studies are needed to explore this further.
What made this study unique was its ability to match pre- and postmenopausal women for age, a critically important attribute “given that aging and menopause both progress concurrently, which can make it difficult to determine the individual contribution of each for measures of brain health,” the authors explained.
In an interview, Constance Bohon, MD, an ob.gyn. in private practice and assistant clinical professor, George Washington University, Washington, observed: “The conclusion [in this study] is that an early age of menarche, delayed age of menopause and increased duration of the reproductive stage is negatively associated with brain volume.”
What of the neuroprotective effects of endogenous estrogen?
“Their findings are not consistent with a neuroprotective effect of endogenous estrogen exposure on brain volume,” she noted, adding that the study “did not assess the effect of exogenous estrogen on brain volume. Neither was the effect of exogenous or endogenous estrogen on cerebral blood flow assessed. In a study published in Obstetrics & Gynecology, the conclusion was that oophorectomy before the age of natural menopause is associated with a decrease in cognitive impairment and dementia. There was no assessment of brain volume or cerebral blood flow. Likewise in a report published in Neurobiology of Aging, Maki P and Resnick S M. concluded that estrogen helps maintain hippocampal and prefrontal function as women age,” observed Dr. Bohon, noting that the study did not assess brain volume.
“It is unclear whether the most predictive assessment for worsening cognition and dementia is the finding of decreased total brain volume, decreased hippocampal volume, or decreased cerebral blood flow. The effect of both endogenous and exogenous estrogen on the risk for dementia needs further evaluation,” she cautioned.
Mr. Ambikairajah cited one financial disclosure; the remaining contributors had no relevant disclosures.
FROM MENOPAUSE
Lung disease raises mortality risk in older RA patients
Patients with rheumatoid arthritis–associated interstitial lung disease showed increases in overall mortality, respiratory mortality, and cancer mortality, compared with RA patients without interstitial lung disease, based on data from more than 500,000 patients in a nationwide cohort study.
RA-associated interstitial lung disease (RA-ILD) has been associated with worse survival rates as well as reduced quality of life, functional impairment, and increased health care use and costs, wrote Jeffrey A. Sparks, MD, of Brigham and Women’s Hospital, Boston, and colleagues. However, data on the incidence and prevalence of RA-ILD have been inconsistent and large studies are lacking.
In a study published online in Rheumatology, the researchers identified 509,787 RA patients aged 65 years and older from Medicare claims data. The average age of the patients was 72.6 years, and 76.2% were women.
At baseline, 10,306 (2%) of the study population had RA-ILD, and 13,372 (2.7%) developed RA-ILD over an average of 3.8 years’ follow-up per person (total of 1,873,127 person-years of follow-up). The overall incidence of RA-ILD was 7.14 per 1,000 person-years.
Overall mortality was significantly higher among RA-ILD patients than in those with RA alone in a multivariate analysis (38.7% vs. 20.7%; hazard ratio, 1.66).
In addition, RA-ILD was associated with an increased risk of respiratory mortality (HR, 4.39) and cancer mortality (HR, 1.56), compared with RA without ILD. For these hazard regression analyses, the researchers used Fine and Gray subdistribution HRs “to handle competing risks of alternative causes of mortality. For example, the risk of respiratory mortality for patients with RA-ILD, compared with RA without ILD also accounted for the competing risk of cardiovascular, cancer, infection and other types of mortality.”
In another multivariate analysis, male gender, smoking, asthma, chronic obstructive pulmonary disorder, and medication use (specifically biologic disease-modifying antirheumatic drugs, targeted synthetic DMARDs, and glucocorticoids) were independently associated with increased incident RA-ILD at baseline. However, “the associations of RA-related medications with incident RA-ILD risk should be interpreted with caution since they may be explained by unmeasured factors, including RA disease activity, severity, comorbidities, and prior or concomitant medication use,” the researchers noted.
The study findings were limited by several factors, including the lack of data on disease activity, disease duration, disease severity, and RA-related autoantibodies, the researchers noted. However, the results support data from previous studies and were strengthened by the large sample size and data on demographics and health care use.
“Ours is the first to study the epidemiology and mortality outcomes of RA-ILD using a validated claims algorithm to identify RA and RA-ILD,” and “to quantify the mortality burden of RA-ILD and to identify a potentially novel association of RA-ILD with cancer mortality,” they noted.
The study was supported by an investigator-initiated grant from Bristol-Myers Squibb. Lead author Dr. Sparks disclosed support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Rheumatology Research Foundation, the Brigham Research Institute, and the R. Bruce and Joan M. Mickey Research Scholar Fund. Dr. Sparks also disclosed serving as a consultant to Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer for work unrelated to the current study. Other authors reported research funding from Bristol-Myers Squibb, involvement in a clinical trial funded by Genentech and Bristol-Myers Squibb, and receiving research support to Brigham and Women’s Hospital for other studies from AbbVie, Bayer, Bristol-Myers Squibb, Novartis, Pfizer, Roche, and Vertex.
Patients with rheumatoid arthritis–associated interstitial lung disease showed increases in overall mortality, respiratory mortality, and cancer mortality, compared with RA patients without interstitial lung disease, based on data from more than 500,000 patients in a nationwide cohort study.
RA-associated interstitial lung disease (RA-ILD) has been associated with worse survival rates as well as reduced quality of life, functional impairment, and increased health care use and costs, wrote Jeffrey A. Sparks, MD, of Brigham and Women’s Hospital, Boston, and colleagues. However, data on the incidence and prevalence of RA-ILD have been inconsistent and large studies are lacking.
In a study published online in Rheumatology, the researchers identified 509,787 RA patients aged 65 years and older from Medicare claims data. The average age of the patients was 72.6 years, and 76.2% were women.
At baseline, 10,306 (2%) of the study population had RA-ILD, and 13,372 (2.7%) developed RA-ILD over an average of 3.8 years’ follow-up per person (total of 1,873,127 person-years of follow-up). The overall incidence of RA-ILD was 7.14 per 1,000 person-years.
Overall mortality was significantly higher among RA-ILD patients than in those with RA alone in a multivariate analysis (38.7% vs. 20.7%; hazard ratio, 1.66).
In addition, RA-ILD was associated with an increased risk of respiratory mortality (HR, 4.39) and cancer mortality (HR, 1.56), compared with RA without ILD. For these hazard regression analyses, the researchers used Fine and Gray subdistribution HRs “to handle competing risks of alternative causes of mortality. For example, the risk of respiratory mortality for patients with RA-ILD, compared with RA without ILD also accounted for the competing risk of cardiovascular, cancer, infection and other types of mortality.”
In another multivariate analysis, male gender, smoking, asthma, chronic obstructive pulmonary disorder, and medication use (specifically biologic disease-modifying antirheumatic drugs, targeted synthetic DMARDs, and glucocorticoids) were independently associated with increased incident RA-ILD at baseline. However, “the associations of RA-related medications with incident RA-ILD risk should be interpreted with caution since they may be explained by unmeasured factors, including RA disease activity, severity, comorbidities, and prior or concomitant medication use,” the researchers noted.
The study findings were limited by several factors, including the lack of data on disease activity, disease duration, disease severity, and RA-related autoantibodies, the researchers noted. However, the results support data from previous studies and were strengthened by the large sample size and data on demographics and health care use.
“Ours is the first to study the epidemiology and mortality outcomes of RA-ILD using a validated claims algorithm to identify RA and RA-ILD,” and “to quantify the mortality burden of RA-ILD and to identify a potentially novel association of RA-ILD with cancer mortality,” they noted.
The study was supported by an investigator-initiated grant from Bristol-Myers Squibb. Lead author Dr. Sparks disclosed support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Rheumatology Research Foundation, the Brigham Research Institute, and the R. Bruce and Joan M. Mickey Research Scholar Fund. Dr. Sparks also disclosed serving as a consultant to Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer for work unrelated to the current study. Other authors reported research funding from Bristol-Myers Squibb, involvement in a clinical trial funded by Genentech and Bristol-Myers Squibb, and receiving research support to Brigham and Women’s Hospital for other studies from AbbVie, Bayer, Bristol-Myers Squibb, Novartis, Pfizer, Roche, and Vertex.
Patients with rheumatoid arthritis–associated interstitial lung disease showed increases in overall mortality, respiratory mortality, and cancer mortality, compared with RA patients without interstitial lung disease, based on data from more than 500,000 patients in a nationwide cohort study.
RA-associated interstitial lung disease (RA-ILD) has been associated with worse survival rates as well as reduced quality of life, functional impairment, and increased health care use and costs, wrote Jeffrey A. Sparks, MD, of Brigham and Women’s Hospital, Boston, and colleagues. However, data on the incidence and prevalence of RA-ILD have been inconsistent and large studies are lacking.
In a study published online in Rheumatology, the researchers identified 509,787 RA patients aged 65 years and older from Medicare claims data. The average age of the patients was 72.6 years, and 76.2% were women.
At baseline, 10,306 (2%) of the study population had RA-ILD, and 13,372 (2.7%) developed RA-ILD over an average of 3.8 years’ follow-up per person (total of 1,873,127 person-years of follow-up). The overall incidence of RA-ILD was 7.14 per 1,000 person-years.
Overall mortality was significantly higher among RA-ILD patients than in those with RA alone in a multivariate analysis (38.7% vs. 20.7%; hazard ratio, 1.66).
In addition, RA-ILD was associated with an increased risk of respiratory mortality (HR, 4.39) and cancer mortality (HR, 1.56), compared with RA without ILD. For these hazard regression analyses, the researchers used Fine and Gray subdistribution HRs “to handle competing risks of alternative causes of mortality. For example, the risk of respiratory mortality for patients with RA-ILD, compared with RA without ILD also accounted for the competing risk of cardiovascular, cancer, infection and other types of mortality.”
In another multivariate analysis, male gender, smoking, asthma, chronic obstructive pulmonary disorder, and medication use (specifically biologic disease-modifying antirheumatic drugs, targeted synthetic DMARDs, and glucocorticoids) were independently associated with increased incident RA-ILD at baseline. However, “the associations of RA-related medications with incident RA-ILD risk should be interpreted with caution since they may be explained by unmeasured factors, including RA disease activity, severity, comorbidities, and prior or concomitant medication use,” the researchers noted.
The study findings were limited by several factors, including the lack of data on disease activity, disease duration, disease severity, and RA-related autoantibodies, the researchers noted. However, the results support data from previous studies and were strengthened by the large sample size and data on demographics and health care use.
“Ours is the first to study the epidemiology and mortality outcomes of RA-ILD using a validated claims algorithm to identify RA and RA-ILD,” and “to quantify the mortality burden of RA-ILD and to identify a potentially novel association of RA-ILD with cancer mortality,” they noted.
The study was supported by an investigator-initiated grant from Bristol-Myers Squibb. Lead author Dr. Sparks disclosed support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Rheumatology Research Foundation, the Brigham Research Institute, and the R. Bruce and Joan M. Mickey Research Scholar Fund. Dr. Sparks also disclosed serving as a consultant to Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer for work unrelated to the current study. Other authors reported research funding from Bristol-Myers Squibb, involvement in a clinical trial funded by Genentech and Bristol-Myers Squibb, and receiving research support to Brigham and Women’s Hospital for other studies from AbbVie, Bayer, Bristol-Myers Squibb, Novartis, Pfizer, Roche, and Vertex.
FROM RHEUMATOLOGY
A Preoperative Transthoracic Echocardiography Protocol to Reduce Time to Hip Fracture Surgery
From Dignity Health Methodist Hospital of Sacramento Family Medicine Residency Program, Sacramento, CA (Dr. Oldach); Nationwide Children’s Hospital, Columbus, OH (Dr. Irwin); OhioHealth Research Institute, Columbus, OH (Dr. Pershing); Department of Clinical Transformation, OhioHealth, Columbus, OH (Dr. Zigmont and Dr. Gascon); and Department of Geriatrics, OhioHealth, Columbus, OH (Dr. Skully).
Abstract
Objective: An interdisciplinary committee was formed to identify factors contributing to surgical delays in urgent hip fracture repair at an urban, level 1 trauma center, with the goal of reducing preoperative time to less than 24 hours. Surgical optimization was identified as a primary, modifiable factor, as surgeons were reluctant to clear patients for surgery without cardiac consultation. Preoperative transthoracic echocardiogram (TTE) was recommended as a safe alternative to cardiac consultation in most patients.
Methods: A retrospective review was conducted for patients who underwent urgent hip fracture repair between January 2010 and April 2014 (n = 316). Time to medical optimization, time to surgery, hospital length of stay, and anesthesia induction were compared for 3 patient groups of interest: those who received (1) neither TTE nor cardiology consultation (ie, direct to surgery); (2) a preoperative TTE; or (3) preoperative cardiac consultation.
Results: There were significant between-group differences in medical optimization time (P = 0.001) and mean time to surgery (P < 0.001) when comparing the 3 groups of interest. Patients in the preoperative cardiac consult group had the longest times, followed by the TTE and direct-to-surgery groups. There were no differences in the type of induction agent used across treatment groups when stratifying by ejection fraction.
Conclusion: Preoperative TTE allows for decreased preoperative time compared to a cardiology consultation. It provides an easily implemented inter-departmental, intra-institutional intervention to decrease preoperative time in patients presenting with hip fractures.
Keywords: surgical delay; preoperative risk stratification; process improvement.
Hip fractures are common, expensive, and associated with poor outcomes.1,2 Ample literature suggests that morbidity, mortality, and cost of care may be reduced by minimizing surgical delays.3-5 While individual reports indicate mixed evidence, in a 2010 meta-analysis, surgery within 72 hours was associated with significant reductions in pneumonia and pressure sores, as well as a 19% reduction in all-cause mortality through 1 year.6 Additional reviews suggest evidence of improved patient outcomes (pain, length of stay, non-union, and/or mortality) when surgery occurs early, within 12 to 72 hours after injury.4,6,7 Regardless of the definition of “early surgery” used, surgical delay remains a challenge, often due to organizational factors, including admission day of the week and hospital staffing, and patient characteristics, such as comorbidities, echocardiographic findings, age, and insurance status.7-9
Among factors that contribute to surgical delays, the need for preoperative cardiovascular risk stratification is significantly modifiable.10 The American College of Cardiology (ACC)/American Heart Association (AHA) Task Force risk stratification framework for preoperative cardiac testing assists clinicians in determining surgical urgency, active cardiac conditions, cardiovascular risk factors, and functional capacity of each patient, and is well established for low- or intermediate-risk patients.11 Specifically, metabolic equivalents (METs) measurements are used to identify medically stable patients with good or excellent functional capacity versus poor or unknown functional status. Patients with ≥ 4 METs may proceed to surgery without further testing; patients with < 4 METs may either proceed with planned surgery or undergo additional testing. Patients with a perceived increased risk profile who require urgent or semi-urgent hip fracture repair may be confounded by disagreement about required preoperative cardiac testing.
At OhioHealth Grant Medical Center (GMC), an urban, level 1 trauma center, the consideration of further preoperative noninvasive testing frequently contributed to surgical delays. In 2009, hip fracture patients arriving to the emergency department (ED) waited an average of 51 hours before being transferred to the operating room (OR) for surgery. Presuming prompt surgery is both desirable and feasible, the Grant Hip Fracture Management Committee (GHFMC) was developed in order to expedite surgeries in hip fracture patients. The GHFMC recommended a preoperative hip fracture protocol, and the outcomes from protocol implementation are described in this article.
Methods
This study was approved by the OhioHealth Institutional Review Board, with a waiver of the informed consent requirement. Medical records from patients treated at GMC during the time period between January 2010 and April 2014 (ie, following implementation of GHFMC recommendations) were retrospectively reviewed to identify the extent to which the use of preoperative transthoracic echocardiography (TTE) reduced average time to surgery and total length of stay, compared to cardiac consultation. This chart review included 316 participants and was used to identify primary induction agent utilized, time to medical optimization, time to surgery, and total length of hospital stay.
Intervention
The GHFMC conducted a 9-month quality improvement project to decrease ED-to-OR time to less than 24 hours for hip fracture patients. The multidisciplinary committee consisted of physicians from orthopedic surgery, anesthesia, hospital medicine, and geriatrics, along with key administrators and nurse outcomes managers. While there is lack of complete clarity surrounding optimal surgical timing, the committee decided that surgery within 24 hours would be beneficial for the majority of patients and therefore was considered a prudent goal.
Based on identified barriers that contributed to surgical delays, several process improvement strategies were implemented, including admitting patients to the hospitalist service, engaging the orthopedic trauma team, and implementing pre- and postoperative protocols and order sets (eg, ED and pain management order sets). Specific emphasis was placed on establishing guidelines for determining medical optimization. In the absence of established guidelines, medical optimization was determined at the discretion of the attending physician. The necessity of preoperative cardiac assessment was based, in part, on physician concerns about determining safe anesthesia protocols and hemodynamically managing patients who may have occult heart disease, specifically those patients with low functional capacity (< 4 METs) and/or inability to accurately communicate their medical history.
Many hip fractures result from a fall, and it may be unclear whether the fall causing a fracture was purely mechanical or indicative of a distinct acute or chronic illness. As a result, many patients received cardiac consultations, with or without pharmacologic stress testing, adding another 24 to 36 hours to preoperative time. As invasive preoperative cardiac procedures generally result in surgical delays without improving outcomes,11 the committee recommended that clinicians reserve preoperative cardiac consultation for patients with active cardiac conditions.
In lieu of cardiac consultation, the committee suggested preoperative TTE. While use of TTE has not been shown to improve preoperative risk stratification in routine noncardiac surgeries, it has been shown to provide clinically useful information in patients at high risk for cardiac complications.11 There was consensus for incorporating preoperative TTE for several reasons: (1) the patients with hip fractures were not “routine,” and often did not have a reliable medical history; (2) a large percentage of patients had cardiac risk factors; (3) patients with undiagnosed aortic stenosis, severe left ventricular dysfunction, or severe pulmonary hypertension would likely have altered intraoperative fluid management; and (4) in supplanting cardiac consultations, TTE would likely expedite patients’ ED-to-OR times. Therefore, the GHFMC created a recommendation of ordering urgent TTE for patients who were unable to exercise at ≥ 4 METs but needed urgent hip fracture surgery.
In order to evaluate the success of the new protocol, the ED-to-OR times were calculated for a cohort of patients who underwent surgery for hip fracture following algorithm implementation.
Participants
A chart review was conducted for patients admitted to GMC between January 2010 and April 2014 for operative treatment of a hip fracture. Exclusion criteria included lack of radiologist-diagnosed hip fracture, periprosthetic hip fracture, or multiple traumas. Electronic patient charts were reviewed by investigators (KI and BO) using a standardized, electronic abstraction form for 3 groups of patients who (1) proceeded directly to planned surgery without TTE or cardiac consultation (direct-to-surgery group); (2) received preoperative TTE but not a cardiac consultation (TTE-only group); or (3) received preoperative cardiac consultation (cardiac consult group).
Measures
Demographics, comorbid conditions, MET score, anesthesia protocol, and in-hospital morbidity and mortality were extracted from medical charts. Medical optimization time was determined by the latest time stamp of 1 of the following: time that the final consulting specialist stated that the patient was stable for surgery; time that the hospitalist described the patient as being ready for surgery; time that the TTE report was certified by the reading cardiologist; or time that the hospitalist described the outcome of completed preoperative risk stratification. Time elapsed prior to medical optimization, surgery, and discharge were calculated using differences between the patient’s arrival date and time at the ED, first recorded time of medical optimization, surgical start time (from the surgical report), and discharge time, respectively.
To assess whether the TTE protocol may have affected anesthesia selection, the induction agent (etomidate or propofol) was abstracted from anesthesia reports and stratified by the ejection fraction of each patient: very low (≤ 35%), low (36%–50%), or normal (> 50%). Patients without an echocardiogram report were assumed to have a normal ejection fraction for this analysis.
Analysis
Descriptive statistics were produced using mean and standard deviation (SD) for continuous variables and frequency and percentage for categorical variables. To determine whether statistically significant differences existed between the 3 groups, the Kruskal-Wallis test was used to compare skewed continuous variables, and Pearson’s chi-square test was used to compare categorical variables. Due to differences in baseline patient characteristics across the 3 treatment groups, inverse probability weights were used to adjust for group differences (using a multinomial logit treatment model) while comparing differences in outcome variables. This modeling strategy does not rely on any assumptions for the distribution of the outcome variable. Covariates were considered for inclusion in the treatment or outcome model if they were significantly associated (P < 0.05) with the group variable. Additionally, anesthetic agent (etomidate or propofol) was compared across the treatment groups after stratifying by ejection fraction to identify whether any differences existed in anesthesia regimen. Patients who were prescribed more than 1 anesthetic agent (n = 2) or an agent that was not of interest were removed from the analysis (n = 13). Stata (version 14) was used for analysis. All other missing data with respect to the tested variables were omitted in the analysis for that variable. Any disagreements about abstraction were resolved through consensus between the investigators.
Results
A total of 316 cases met inclusion criteria, including 108 direct-to-surgery patients, 143 preoperative TTE patients, and 65 cardiac consult patients. Patient demographics and preoperative characteristics are shown in Table 1. The average age for all patients was 76.5 years of age (SD, 12.89; IQR, 34-97); however, direct-to-surgery patients were significantly (P < 0.001) younger (71.2 years; SD, 14.2; interquartile range [IQR], 34-95 years) than TTE-only patients (79.0 years; SD, 11.5; IQR, 35-97 years) and cardiac consult patients (79.57 years; SD, 10.63; IQR, 49-97 years). The majority of patients were female (69.9%) and experienced a fall prior to admission (94%). Almost three-fourths of patients had 1 or more cardiac risk factors (73.7%), including history of congestive heart failure (CHF; 19%), coronary artery disease (CAD; 26.3%), chronic obstructive pulmonary disease (COPD; 19.3%), or aortic stenosis (AS; 3.5%). Due to between-group differences in these comorbid conditions, confounding factors were adjusted for in subsequent analyses.

As shown in Table 2, before adjustment for confounding factors, there were significant between-group differences in medical optimization time for patients in all 3 groups. After adjustment for treatment differences using age and number of comorbid diseases, and medical optimization time differences using age and COPD, fewer between-group differences were statistically significant. Patients who received a cardiac consult had an 18.44-hour longer medical optimization time compared to patients who went directly to surgery (29.136 vs 10.696 hours; P = 0.001). Optimization remained approximately 5 hours longer for the TTE-only group than for the direct-to-surgery group; however, this difference was not significant (P = 0.075).
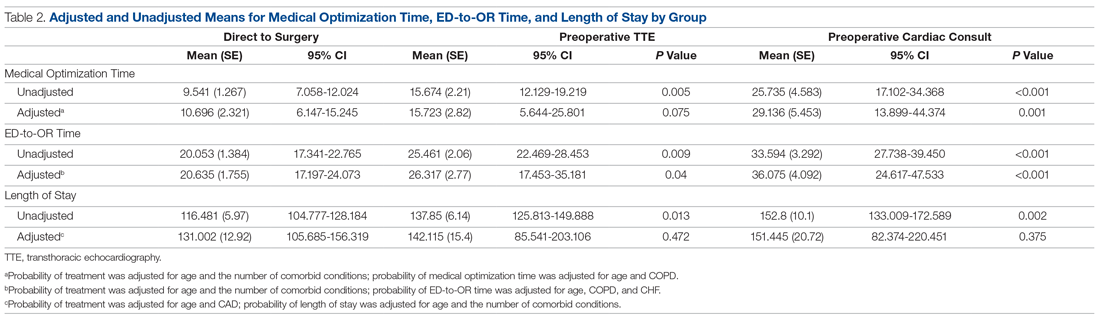
When comparing differences in ED-to-OR time for the 3 groups after adjusting the probability of treatment for age and the number of comorbid conditions, and adjusting the probability of ED-to-OR time for age, COPD, and CHF, significant differences remained in ED-to-OR times across all groups. Specifically, patients in the direct-to-surgery group experienced the shortest time (mean, 20.64 hours), compared to patients in the TTE-only group (mean, 26.32; P = 0.04) or patients in the cardiac consult group (mean, 36.08; P < 0.001). TTE-only patients had a longer time of 5.68 hours, compared to the direct-to-surgery group, and patients in the preoperative cardiac consult group were on average 15.44 hours longer than the direct-to-surgery group.
When comparing differences in the length of stay for the 3 groups before statistical adjustments, differences were observed; however, after removing the confounding factors related to treatment (age and CAD) and the outcome (age and the number of comorbid conditions), there were no statistically significant differences in the length of stay for the 3 groups. Average length of stay was 131 hours for direct-to-surgery patients, 142 hours for TTE-only patients, and 141 hours for cardiac consult patients.
The use of different anesthetic agents was compared for patients in the 3 groups. The majority of patients in the study (87.7%) were given propofol, and there were no differences after stratifying by ejection fraction (Table 3).
Discussion
The GHFMC was created to reduce surgical delays for hip fracture. Medical optimization was considered a primary, modifiable factor given that surgeons were reluctant to proceed without a cardiac consult. To address this gap, the committee recommended a preoperative TTE for patients with low or unknown functional status. This threshold provides a quick and easy method for stratifying patients who previously required risk stratification by a cardiologist, which often resulted in surgery delays.
In their recommendations for implementation of hip fracture quality improvement projects, the Geriatric Fracture Center emphasizes the importance of multidisciplinary physician leadership along with standardization of approach across patients.12 This recommendation is supported by increasing evidence that orthogeriatric collaborations are associated with decreased mortality and length of stay.13 The GHFMC and subsequent interventions reflect this approach, allowing for collaboration to identify cross-disciplinary procedural barriers to care. In our institution, addressing identified procedural barriers to care was associated with a reduction in the average time to surgery from 51 hours to 25.3 hours.
Multiple approaches have been attempted to decrease presurgical time in hip fracture patients in various settings. Prehospital interventions, such as providing ambulances with checklists and ability to bypass the ED, have not been shown to decrease time to surgery for hip fracture patients, though similar strategies have been successful in other conditions, such as stroke.14,15 In-hospital procedures, such as implementation of a hip fracture protocol and reduction of preoperative interventions, have more consistently been found to decrease time to surgery and in-hospital mortality.16,17 However, reduced delays have not been found universally. Luttrell and Nana found that preoperative TTE resulted in approximately 30.8-hour delays from the ED to OR, compared to patients who did not receive a preoperative TTE.18 However, in that study hospitalists used TTE at their own discretion, and there may have been confounding factors contributing to delays. When used as part of a protocol targeting patients with poor or unknown functional capacity, we believe that preoperative TTE results in modest surgical delays yet provides clinically useful information about each patient.
ACC/AHA preoperative guidelines were updated after we implemented our intervention and now recommend that patients with poor or unknown functional capacity in whom stress testing will not influence care proceed to surgery “according to guideline-directed medical care.”11 While routine use of preoperative evaluation of left ventricular function is not recommended, assessing left ventricular function may be reasonable for patients with heart failure with a change in clinical status. Guidelines also recommend that patients with clinically suspected valvular stenosis undergo preoperative echocardiography.11
Limitations
This study has several limitations. First, due to resource limitations, a substantial period of time elapsed between implementation of the new protocol and the analysis of the data set. That is, the hip fracture protocol assessed in this paper occurred from January 2010 through April 2014, and final analysis of the data set occurred in April 2020. This limitation precludes our ability to formally assess any pre- or post-protocol changes in patient outcomes. Second, randomization was not used to create groups that were balanced in differing health characteristics (ie, patients with noncardiac-related surgeries, patients in different age groups); however, the use of inverse probability treatment regression analysis was a way to statistically address these between-group differences. Moreover, this study is limited by the factors that were measured; unmeasured factors cannot be accounted for. Third, health care providers working at the hospital during this time were aware of the goal to decrease presurgical time, possibly creating exaggerated effects compared to a blinded trial. Finally, although this intervention is likely translatable to other centers, these results represent the experiences of a single level 1 trauma center and may not be replicable elsewhere.
Conclusion
Preoperative TTE in lieu of cardiac consultation has several advantages. First, it requires interdepartmental collaboration for implementation, but can be implemented through a single hospital or hospital system. Unlike prehospital interventions, preoperative urgent TTE for patients with low functional capacity does not require the support of emergency medical technicians, ambulance services, or other hospitals in the region. Second, while costs are associated with TTE, they are offset by a reduction in expensive consultations with specialists, surgical delays, and longer lengths of stay. Third, despite likely increased ED-to-OR times compared to no intervention, urgent TTE decreases time to surgery compared with cardiology consultation. Prior to the GHFMC, the ED-to-OR time at our institution was 51 hours. In contrast, the mean time following the GHFMC-led protocol was less than half that, at 25.3 hours (SD, 19.1 hours). In fact, nearly two-thirds (65.2%) of the patients evaluated in this study underwent surgery within 24 hours of admission. This improvement in presurgical time was attributed, in part, to the implementation of preoperative TTE over cardiology consultations.
Acknowledgments: The authors thank Jenny Williams, RN, who was instrumental in obtaining the data set for analysis, and Shauna Ayres, MPH, from the OhioHealth Research Institute, who provided writing and technical assistance.
Corresponding author: Robert Skully, MD, OhioHealth Family Medicine Grant, 290 East Town St., Columbus, OH 43215; [email protected].
Funding: This work was supported by the OhioHealth Summer Research Externship Program.
Financial disclosures: None.
1. Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302:1573-1579.
2. Lewiecki EM, Wright NC, Curtis JR, et al. Hip fracture trends in the United States 2002 to 2015. Osteoporos Int. 2018;29:717-722.
3. Colais P, Di Martino M, Fusco D, et al. The effect of early surgery after hip fracture on 1-year mortality. BMC Geriatr. 2015;15:141.
4. Nyholm AM, Gromov K, Palm H, et al. Time to surgery is associated with thirty-day and ninety-day mortality after proximal femoral fracture: a retrospective observational study on prospectively collected data from the Danish Fracture Database Collaborators. J Bone Joint Surg Am. 2015;97:1333-1339.
5. Judd KT, Christianson E. Expedited operative care of hip fractures results in significantly lower cost of treatment. Iowa Orthop J. 2015;35:62-64.
6. Simunovic N, Devereaux PJ, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182:1609-1616.
7. Ryan DJ, Yoshihara H, Yoneoka D, et al. Delay in hip fracture surgery: an analysis of patient-specific and hospital-specific risk factors. J Orthop Trauma. 2015;29:343-348.
8. Ricci WM, Brandt A, McAndrew C, Gardner MJ. Factors affecting delay to surgery and length of stay for patients with hip fracture. J Orthop Trauma. 2015;29:e109-e114.
9. Hagino T, Ochiai S, Senga S, et al. Efficacy of early surgery and causes of surgical delay in patients with hip fracture. J Orthop. 2015;12:142-146.
10. Rafiq A, Sklyar E, Bella JN. Cardiac evaluation and monitoring of patients undergoing noncardiac surgery. Health Serv Insights. 2017;9:1178632916686074.
11. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e77-e137.
12. Basu N, Natour M, Mounasamy V, Kates SL. Geriatric hip fracture management: keys to providing a successful program. Eur J Trauma Emerg Surg. 2016;42:565-569.
13. Grigoryan KV, Javedan H, Rudolph JL. Orthogeriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma. 2014;28:e49-e55.
14. Tai YJ, Yan B. Minimising time to treatment: targeted strategies to minimise time to thrombolysis for acute ischaemic stroke. Intern Med J. 2013;43:1176-1182.
15. Larsson G, Stromberg RU, Rogmark C, Nilsdotter A. Prehospital fast track care for patients with hip fracture: Impact on time to surgery, hospital stay, post-operative complications and mortality a randomised, controlled trial. Injury. 2016;47:881-886.
16. Bohm E, Loucks L, Wittmeier K, et al. Reduced time to surgery improves mortality and length of stay following hip fracture: results from an intervention study in a Canadian health authority. Can J Surg. 2015;58:257-263.
17. Ventura C, Trombetti S, Pioli G, et al. Impact of multidisciplinary hip fracture program on timing of surgery in elderly patients. Osteoporos Int J. 2014;25:2591-2597.
18. Luttrell K, Nana A. Effect of preoperative transthoracic echocardiogram on mortality and surgical timing in elderly adults with hip fracture. J Am Geriatr Soc. 2015;63:2505-2509.
From Dignity Health Methodist Hospital of Sacramento Family Medicine Residency Program, Sacramento, CA (Dr. Oldach); Nationwide Children’s Hospital, Columbus, OH (Dr. Irwin); OhioHealth Research Institute, Columbus, OH (Dr. Pershing); Department of Clinical Transformation, OhioHealth, Columbus, OH (Dr. Zigmont and Dr. Gascon); and Department of Geriatrics, OhioHealth, Columbus, OH (Dr. Skully).
Abstract
Objective: An interdisciplinary committee was formed to identify factors contributing to surgical delays in urgent hip fracture repair at an urban, level 1 trauma center, with the goal of reducing preoperative time to less than 24 hours. Surgical optimization was identified as a primary, modifiable factor, as surgeons were reluctant to clear patients for surgery without cardiac consultation. Preoperative transthoracic echocardiogram (TTE) was recommended as a safe alternative to cardiac consultation in most patients.
Methods: A retrospective review was conducted for patients who underwent urgent hip fracture repair between January 2010 and April 2014 (n = 316). Time to medical optimization, time to surgery, hospital length of stay, and anesthesia induction were compared for 3 patient groups of interest: those who received (1) neither TTE nor cardiology consultation (ie, direct to surgery); (2) a preoperative TTE; or (3) preoperative cardiac consultation.
Results: There were significant between-group differences in medical optimization time (P = 0.001) and mean time to surgery (P < 0.001) when comparing the 3 groups of interest. Patients in the preoperative cardiac consult group had the longest times, followed by the TTE and direct-to-surgery groups. There were no differences in the type of induction agent used across treatment groups when stratifying by ejection fraction.
Conclusion: Preoperative TTE allows for decreased preoperative time compared to a cardiology consultation. It provides an easily implemented inter-departmental, intra-institutional intervention to decrease preoperative time in patients presenting with hip fractures.
Keywords: surgical delay; preoperative risk stratification; process improvement.
Hip fractures are common, expensive, and associated with poor outcomes.1,2 Ample literature suggests that morbidity, mortality, and cost of care may be reduced by minimizing surgical delays.3-5 While individual reports indicate mixed evidence, in a 2010 meta-analysis, surgery within 72 hours was associated with significant reductions in pneumonia and pressure sores, as well as a 19% reduction in all-cause mortality through 1 year.6 Additional reviews suggest evidence of improved patient outcomes (pain, length of stay, non-union, and/or mortality) when surgery occurs early, within 12 to 72 hours after injury.4,6,7 Regardless of the definition of “early surgery” used, surgical delay remains a challenge, often due to organizational factors, including admission day of the week and hospital staffing, and patient characteristics, such as comorbidities, echocardiographic findings, age, and insurance status.7-9
Among factors that contribute to surgical delays, the need for preoperative cardiovascular risk stratification is significantly modifiable.10 The American College of Cardiology (ACC)/American Heart Association (AHA) Task Force risk stratification framework for preoperative cardiac testing assists clinicians in determining surgical urgency, active cardiac conditions, cardiovascular risk factors, and functional capacity of each patient, and is well established for low- or intermediate-risk patients.11 Specifically, metabolic equivalents (METs) measurements are used to identify medically stable patients with good or excellent functional capacity versus poor or unknown functional status. Patients with ≥ 4 METs may proceed to surgery without further testing; patients with < 4 METs may either proceed with planned surgery or undergo additional testing. Patients with a perceived increased risk profile who require urgent or semi-urgent hip fracture repair may be confounded by disagreement about required preoperative cardiac testing.
At OhioHealth Grant Medical Center (GMC), an urban, level 1 trauma center, the consideration of further preoperative noninvasive testing frequently contributed to surgical delays. In 2009, hip fracture patients arriving to the emergency department (ED) waited an average of 51 hours before being transferred to the operating room (OR) for surgery. Presuming prompt surgery is both desirable and feasible, the Grant Hip Fracture Management Committee (GHFMC) was developed in order to expedite surgeries in hip fracture patients. The GHFMC recommended a preoperative hip fracture protocol, and the outcomes from protocol implementation are described in this article.
Methods
This study was approved by the OhioHealth Institutional Review Board, with a waiver of the informed consent requirement. Medical records from patients treated at GMC during the time period between January 2010 and April 2014 (ie, following implementation of GHFMC recommendations) were retrospectively reviewed to identify the extent to which the use of preoperative transthoracic echocardiography (TTE) reduced average time to surgery and total length of stay, compared to cardiac consultation. This chart review included 316 participants and was used to identify primary induction agent utilized, time to medical optimization, time to surgery, and total length of hospital stay.
Intervention
The GHFMC conducted a 9-month quality improvement project to decrease ED-to-OR time to less than 24 hours for hip fracture patients. The multidisciplinary committee consisted of physicians from orthopedic surgery, anesthesia, hospital medicine, and geriatrics, along with key administrators and nurse outcomes managers. While there is lack of complete clarity surrounding optimal surgical timing, the committee decided that surgery within 24 hours would be beneficial for the majority of patients and therefore was considered a prudent goal.
Based on identified barriers that contributed to surgical delays, several process improvement strategies were implemented, including admitting patients to the hospitalist service, engaging the orthopedic trauma team, and implementing pre- and postoperative protocols and order sets (eg, ED and pain management order sets). Specific emphasis was placed on establishing guidelines for determining medical optimization. In the absence of established guidelines, medical optimization was determined at the discretion of the attending physician. The necessity of preoperative cardiac assessment was based, in part, on physician concerns about determining safe anesthesia protocols and hemodynamically managing patients who may have occult heart disease, specifically those patients with low functional capacity (< 4 METs) and/or inability to accurately communicate their medical history.
Many hip fractures result from a fall, and it may be unclear whether the fall causing a fracture was purely mechanical or indicative of a distinct acute or chronic illness. As a result, many patients received cardiac consultations, with or without pharmacologic stress testing, adding another 24 to 36 hours to preoperative time. As invasive preoperative cardiac procedures generally result in surgical delays without improving outcomes,11 the committee recommended that clinicians reserve preoperative cardiac consultation for patients with active cardiac conditions.
In lieu of cardiac consultation, the committee suggested preoperative TTE. While use of TTE has not been shown to improve preoperative risk stratification in routine noncardiac surgeries, it has been shown to provide clinically useful information in patients at high risk for cardiac complications.11 There was consensus for incorporating preoperative TTE for several reasons: (1) the patients with hip fractures were not “routine,” and often did not have a reliable medical history; (2) a large percentage of patients had cardiac risk factors; (3) patients with undiagnosed aortic stenosis, severe left ventricular dysfunction, or severe pulmonary hypertension would likely have altered intraoperative fluid management; and (4) in supplanting cardiac consultations, TTE would likely expedite patients’ ED-to-OR times. Therefore, the GHFMC created a recommendation of ordering urgent TTE for patients who were unable to exercise at ≥ 4 METs but needed urgent hip fracture surgery.
In order to evaluate the success of the new protocol, the ED-to-OR times were calculated for a cohort of patients who underwent surgery for hip fracture following algorithm implementation.
Participants
A chart review was conducted for patients admitted to GMC between January 2010 and April 2014 for operative treatment of a hip fracture. Exclusion criteria included lack of radiologist-diagnosed hip fracture, periprosthetic hip fracture, or multiple traumas. Electronic patient charts were reviewed by investigators (KI and BO) using a standardized, electronic abstraction form for 3 groups of patients who (1) proceeded directly to planned surgery without TTE or cardiac consultation (direct-to-surgery group); (2) received preoperative TTE but not a cardiac consultation (TTE-only group); or (3) received preoperative cardiac consultation (cardiac consult group).
Measures
Demographics, comorbid conditions, MET score, anesthesia protocol, and in-hospital morbidity and mortality were extracted from medical charts. Medical optimization time was determined by the latest time stamp of 1 of the following: time that the final consulting specialist stated that the patient was stable for surgery; time that the hospitalist described the patient as being ready for surgery; time that the TTE report was certified by the reading cardiologist; or time that the hospitalist described the outcome of completed preoperative risk stratification. Time elapsed prior to medical optimization, surgery, and discharge were calculated using differences between the patient’s arrival date and time at the ED, first recorded time of medical optimization, surgical start time (from the surgical report), and discharge time, respectively.
To assess whether the TTE protocol may have affected anesthesia selection, the induction agent (etomidate or propofol) was abstracted from anesthesia reports and stratified by the ejection fraction of each patient: very low (≤ 35%), low (36%–50%), or normal (> 50%). Patients without an echocardiogram report were assumed to have a normal ejection fraction for this analysis.
Analysis
Descriptive statistics were produced using mean and standard deviation (SD) for continuous variables and frequency and percentage for categorical variables. To determine whether statistically significant differences existed between the 3 groups, the Kruskal-Wallis test was used to compare skewed continuous variables, and Pearson’s chi-square test was used to compare categorical variables. Due to differences in baseline patient characteristics across the 3 treatment groups, inverse probability weights were used to adjust for group differences (using a multinomial logit treatment model) while comparing differences in outcome variables. This modeling strategy does not rely on any assumptions for the distribution of the outcome variable. Covariates were considered for inclusion in the treatment or outcome model if they were significantly associated (P < 0.05) with the group variable. Additionally, anesthetic agent (etomidate or propofol) was compared across the treatment groups after stratifying by ejection fraction to identify whether any differences existed in anesthesia regimen. Patients who were prescribed more than 1 anesthetic agent (n = 2) or an agent that was not of interest were removed from the analysis (n = 13). Stata (version 14) was used for analysis. All other missing data with respect to the tested variables were omitted in the analysis for that variable. Any disagreements about abstraction were resolved through consensus between the investigators.
Results
A total of 316 cases met inclusion criteria, including 108 direct-to-surgery patients, 143 preoperative TTE patients, and 65 cardiac consult patients. Patient demographics and preoperative characteristics are shown in Table 1. The average age for all patients was 76.5 years of age (SD, 12.89; IQR, 34-97); however, direct-to-surgery patients were significantly (P < 0.001) younger (71.2 years; SD, 14.2; interquartile range [IQR], 34-95 years) than TTE-only patients (79.0 years; SD, 11.5; IQR, 35-97 years) and cardiac consult patients (79.57 years; SD, 10.63; IQR, 49-97 years). The majority of patients were female (69.9%) and experienced a fall prior to admission (94%). Almost three-fourths of patients had 1 or more cardiac risk factors (73.7%), including history of congestive heart failure (CHF; 19%), coronary artery disease (CAD; 26.3%), chronic obstructive pulmonary disease (COPD; 19.3%), or aortic stenosis (AS; 3.5%). Due to between-group differences in these comorbid conditions, confounding factors were adjusted for in subsequent analyses.

As shown in Table 2, before adjustment for confounding factors, there were significant between-group differences in medical optimization time for patients in all 3 groups. After adjustment for treatment differences using age and number of comorbid diseases, and medical optimization time differences using age and COPD, fewer between-group differences were statistically significant. Patients who received a cardiac consult had an 18.44-hour longer medical optimization time compared to patients who went directly to surgery (29.136 vs 10.696 hours; P = 0.001). Optimization remained approximately 5 hours longer for the TTE-only group than for the direct-to-surgery group; however, this difference was not significant (P = 0.075).

When comparing differences in ED-to-OR time for the 3 groups after adjusting the probability of treatment for age and the number of comorbid conditions, and adjusting the probability of ED-to-OR time for age, COPD, and CHF, significant differences remained in ED-to-OR times across all groups. Specifically, patients in the direct-to-surgery group experienced the shortest time (mean, 20.64 hours), compared to patients in the TTE-only group (mean, 26.32; P = 0.04) or patients in the cardiac consult group (mean, 36.08; P < 0.001). TTE-only patients had a longer time of 5.68 hours, compared to the direct-to-surgery group, and patients in the preoperative cardiac consult group were on average 15.44 hours longer than the direct-to-surgery group.
When comparing differences in the length of stay for the 3 groups before statistical adjustments, differences were observed; however, after removing the confounding factors related to treatment (age and CAD) and the outcome (age and the number of comorbid conditions), there were no statistically significant differences in the length of stay for the 3 groups. Average length of stay was 131 hours for direct-to-surgery patients, 142 hours for TTE-only patients, and 141 hours for cardiac consult patients.
The use of different anesthetic agents was compared for patients in the 3 groups. The majority of patients in the study (87.7%) were given propofol, and there were no differences after stratifying by ejection fraction (Table 3).

Discussion
The GHFMC was created to reduce surgical delays for hip fracture. Medical optimization was considered a primary, modifiable factor given that surgeons were reluctant to proceed without a cardiac consult. To address this gap, the committee recommended a preoperative TTE for patients with low or unknown functional status. This threshold provides a quick and easy method for stratifying patients who previously required risk stratification by a cardiologist, which often resulted in surgery delays.
In their recommendations for implementation of hip fracture quality improvement projects, the Geriatric Fracture Center emphasizes the importance of multidisciplinary physician leadership along with standardization of approach across patients.12 This recommendation is supported by increasing evidence that orthogeriatric collaborations are associated with decreased mortality and length of stay.13 The GHFMC and subsequent interventions reflect this approach, allowing for collaboration to identify cross-disciplinary procedural barriers to care. In our institution, addressing identified procedural barriers to care was associated with a reduction in the average time to surgery from 51 hours to 25.3 hours.
Multiple approaches have been attempted to decrease presurgical time in hip fracture patients in various settings. Prehospital interventions, such as providing ambulances with checklists and ability to bypass the ED, have not been shown to decrease time to surgery for hip fracture patients, though similar strategies have been successful in other conditions, such as stroke.14,15 In-hospital procedures, such as implementation of a hip fracture protocol and reduction of preoperative interventions, have more consistently been found to decrease time to surgery and in-hospital mortality.16,17 However, reduced delays have not been found universally. Luttrell and Nana found that preoperative TTE resulted in approximately 30.8-hour delays from the ED to OR, compared to patients who did not receive a preoperative TTE.18 However, in that study hospitalists used TTE at their own discretion, and there may have been confounding factors contributing to delays. When used as part of a protocol targeting patients with poor or unknown functional capacity, we believe that preoperative TTE results in modest surgical delays yet provides clinically useful information about each patient.
ACC/AHA preoperative guidelines were updated after we implemented our intervention and now recommend that patients with poor or unknown functional capacity in whom stress testing will not influence care proceed to surgery “according to guideline-directed medical care.”11 While routine use of preoperative evaluation of left ventricular function is not recommended, assessing left ventricular function may be reasonable for patients with heart failure with a change in clinical status. Guidelines also recommend that patients with clinically suspected valvular stenosis undergo preoperative echocardiography.11
Limitations
This study has several limitations. First, due to resource limitations, a substantial period of time elapsed between implementation of the new protocol and the analysis of the data set. That is, the hip fracture protocol assessed in this paper occurred from January 2010 through April 2014, and final analysis of the data set occurred in April 2020. This limitation precludes our ability to formally assess any pre- or post-protocol changes in patient outcomes. Second, randomization was not used to create groups that were balanced in differing health characteristics (ie, patients with noncardiac-related surgeries, patients in different age groups); however, the use of inverse probability treatment regression analysis was a way to statistically address these between-group differences. Moreover, this study is limited by the factors that were measured; unmeasured factors cannot be accounted for. Third, health care providers working at the hospital during this time were aware of the goal to decrease presurgical time, possibly creating exaggerated effects compared to a blinded trial. Finally, although this intervention is likely translatable to other centers, these results represent the experiences of a single level 1 trauma center and may not be replicable elsewhere.
Conclusion
Preoperative TTE in lieu of cardiac consultation has several advantages. First, it requires interdepartmental collaboration for implementation, but can be implemented through a single hospital or hospital system. Unlike prehospital interventions, preoperative urgent TTE for patients with low functional capacity does not require the support of emergency medical technicians, ambulance services, or other hospitals in the region. Second, while costs are associated with TTE, they are offset by a reduction in expensive consultations with specialists, surgical delays, and longer lengths of stay. Third, despite likely increased ED-to-OR times compared to no intervention, urgent TTE decreases time to surgery compared with cardiology consultation. Prior to the GHFMC, the ED-to-OR time at our institution was 51 hours. In contrast, the mean time following the GHFMC-led protocol was less than half that, at 25.3 hours (SD, 19.1 hours). In fact, nearly two-thirds (65.2%) of the patients evaluated in this study underwent surgery within 24 hours of admission. This improvement in presurgical time was attributed, in part, to the implementation of preoperative TTE over cardiology consultations.
Acknowledgments: The authors thank Jenny Williams, RN, who was instrumental in obtaining the data set for analysis, and Shauna Ayres, MPH, from the OhioHealth Research Institute, who provided writing and technical assistance.
Corresponding author: Robert Skully, MD, OhioHealth Family Medicine Grant, 290 East Town St., Columbus, OH 43215; [email protected].
Funding: This work was supported by the OhioHealth Summer Research Externship Program.
Financial disclosures: None.
From Dignity Health Methodist Hospital of Sacramento Family Medicine Residency Program, Sacramento, CA (Dr. Oldach); Nationwide Children’s Hospital, Columbus, OH (Dr. Irwin); OhioHealth Research Institute, Columbus, OH (Dr. Pershing); Department of Clinical Transformation, OhioHealth, Columbus, OH (Dr. Zigmont and Dr. Gascon); and Department of Geriatrics, OhioHealth, Columbus, OH (Dr. Skully).
Abstract
Objective: An interdisciplinary committee was formed to identify factors contributing to surgical delays in urgent hip fracture repair at an urban, level 1 trauma center, with the goal of reducing preoperative time to less than 24 hours. Surgical optimization was identified as a primary, modifiable factor, as surgeons were reluctant to clear patients for surgery without cardiac consultation. Preoperative transthoracic echocardiogram (TTE) was recommended as a safe alternative to cardiac consultation in most patients.
Methods: A retrospective review was conducted for patients who underwent urgent hip fracture repair between January 2010 and April 2014 (n = 316). Time to medical optimization, time to surgery, hospital length of stay, and anesthesia induction were compared for 3 patient groups of interest: those who received (1) neither TTE nor cardiology consultation (ie, direct to surgery); (2) a preoperative TTE; or (3) preoperative cardiac consultation.
Results: There were significant between-group differences in medical optimization time (P = 0.001) and mean time to surgery (P < 0.001) when comparing the 3 groups of interest. Patients in the preoperative cardiac consult group had the longest times, followed by the TTE and direct-to-surgery groups. There were no differences in the type of induction agent used across treatment groups when stratifying by ejection fraction.
Conclusion: Preoperative TTE allows for decreased preoperative time compared to a cardiology consultation. It provides an easily implemented inter-departmental, intra-institutional intervention to decrease preoperative time in patients presenting with hip fractures.
Keywords: surgical delay; preoperative risk stratification; process improvement.
Hip fractures are common, expensive, and associated with poor outcomes.1,2 Ample literature suggests that morbidity, mortality, and cost of care may be reduced by minimizing surgical delays.3-5 While individual reports indicate mixed evidence, in a 2010 meta-analysis, surgery within 72 hours was associated with significant reductions in pneumonia and pressure sores, as well as a 19% reduction in all-cause mortality through 1 year.6 Additional reviews suggest evidence of improved patient outcomes (pain, length of stay, non-union, and/or mortality) when surgery occurs early, within 12 to 72 hours after injury.4,6,7 Regardless of the definition of “early surgery” used, surgical delay remains a challenge, often due to organizational factors, including admission day of the week and hospital staffing, and patient characteristics, such as comorbidities, echocardiographic findings, age, and insurance status.7-9
Among factors that contribute to surgical delays, the need for preoperative cardiovascular risk stratification is significantly modifiable.10 The American College of Cardiology (ACC)/American Heart Association (AHA) Task Force risk stratification framework for preoperative cardiac testing assists clinicians in determining surgical urgency, active cardiac conditions, cardiovascular risk factors, and functional capacity of each patient, and is well established for low- or intermediate-risk patients.11 Specifically, metabolic equivalents (METs) measurements are used to identify medically stable patients with good or excellent functional capacity versus poor or unknown functional status. Patients with ≥ 4 METs may proceed to surgery without further testing; patients with < 4 METs may either proceed with planned surgery or undergo additional testing. Patients with a perceived increased risk profile who require urgent or semi-urgent hip fracture repair may be confounded by disagreement about required preoperative cardiac testing.
At OhioHealth Grant Medical Center (GMC), an urban, level 1 trauma center, the consideration of further preoperative noninvasive testing frequently contributed to surgical delays. In 2009, hip fracture patients arriving to the emergency department (ED) waited an average of 51 hours before being transferred to the operating room (OR) for surgery. Presuming prompt surgery is both desirable and feasible, the Grant Hip Fracture Management Committee (GHFMC) was developed in order to expedite surgeries in hip fracture patients. The GHFMC recommended a preoperative hip fracture protocol, and the outcomes from protocol implementation are described in this article.
Methods
This study was approved by the OhioHealth Institutional Review Board, with a waiver of the informed consent requirement. Medical records from patients treated at GMC during the time period between January 2010 and April 2014 (ie, following implementation of GHFMC recommendations) were retrospectively reviewed to identify the extent to which the use of preoperative transthoracic echocardiography (TTE) reduced average time to surgery and total length of stay, compared to cardiac consultation. This chart review included 316 participants and was used to identify primary induction agent utilized, time to medical optimization, time to surgery, and total length of hospital stay.
Intervention
The GHFMC conducted a 9-month quality improvement project to decrease ED-to-OR time to less than 24 hours for hip fracture patients. The multidisciplinary committee consisted of physicians from orthopedic surgery, anesthesia, hospital medicine, and geriatrics, along with key administrators and nurse outcomes managers. While there is lack of complete clarity surrounding optimal surgical timing, the committee decided that surgery within 24 hours would be beneficial for the majority of patients and therefore was considered a prudent goal.
Based on identified barriers that contributed to surgical delays, several process improvement strategies were implemented, including admitting patients to the hospitalist service, engaging the orthopedic trauma team, and implementing pre- and postoperative protocols and order sets (eg, ED and pain management order sets). Specific emphasis was placed on establishing guidelines for determining medical optimization. In the absence of established guidelines, medical optimization was determined at the discretion of the attending physician. The necessity of preoperative cardiac assessment was based, in part, on physician concerns about determining safe anesthesia protocols and hemodynamically managing patients who may have occult heart disease, specifically those patients with low functional capacity (< 4 METs) and/or inability to accurately communicate their medical history.
Many hip fractures result from a fall, and it may be unclear whether the fall causing a fracture was purely mechanical or indicative of a distinct acute or chronic illness. As a result, many patients received cardiac consultations, with or without pharmacologic stress testing, adding another 24 to 36 hours to preoperative time. As invasive preoperative cardiac procedures generally result in surgical delays without improving outcomes,11 the committee recommended that clinicians reserve preoperative cardiac consultation for patients with active cardiac conditions.
In lieu of cardiac consultation, the committee suggested preoperative TTE. While use of TTE has not been shown to improve preoperative risk stratification in routine noncardiac surgeries, it has been shown to provide clinically useful information in patients at high risk for cardiac complications.11 There was consensus for incorporating preoperative TTE for several reasons: (1) the patients with hip fractures were not “routine,” and often did not have a reliable medical history; (2) a large percentage of patients had cardiac risk factors; (3) patients with undiagnosed aortic stenosis, severe left ventricular dysfunction, or severe pulmonary hypertension would likely have altered intraoperative fluid management; and (4) in supplanting cardiac consultations, TTE would likely expedite patients’ ED-to-OR times. Therefore, the GHFMC created a recommendation of ordering urgent TTE for patients who were unable to exercise at ≥ 4 METs but needed urgent hip fracture surgery.
In order to evaluate the success of the new protocol, the ED-to-OR times were calculated for a cohort of patients who underwent surgery for hip fracture following algorithm implementation.
Participants
A chart review was conducted for patients admitted to GMC between January 2010 and April 2014 for operative treatment of a hip fracture. Exclusion criteria included lack of radiologist-diagnosed hip fracture, periprosthetic hip fracture, or multiple traumas. Electronic patient charts were reviewed by investigators (KI and BO) using a standardized, electronic abstraction form for 3 groups of patients who (1) proceeded directly to planned surgery without TTE or cardiac consultation (direct-to-surgery group); (2) received preoperative TTE but not a cardiac consultation (TTE-only group); or (3) received preoperative cardiac consultation (cardiac consult group).
Measures
Demographics, comorbid conditions, MET score, anesthesia protocol, and in-hospital morbidity and mortality were extracted from medical charts. Medical optimization time was determined by the latest time stamp of 1 of the following: time that the final consulting specialist stated that the patient was stable for surgery; time that the hospitalist described the patient as being ready for surgery; time that the TTE report was certified by the reading cardiologist; or time that the hospitalist described the outcome of completed preoperative risk stratification. Time elapsed prior to medical optimization, surgery, and discharge were calculated using differences between the patient’s arrival date and time at the ED, first recorded time of medical optimization, surgical start time (from the surgical report), and discharge time, respectively.
To assess whether the TTE protocol may have affected anesthesia selection, the induction agent (etomidate or propofol) was abstracted from anesthesia reports and stratified by the ejection fraction of each patient: very low (≤ 35%), low (36%–50%), or normal (> 50%). Patients without an echocardiogram report were assumed to have a normal ejection fraction for this analysis.
Analysis
Descriptive statistics were produced using mean and standard deviation (SD) for continuous variables and frequency and percentage for categorical variables. To determine whether statistically significant differences existed between the 3 groups, the Kruskal-Wallis test was used to compare skewed continuous variables, and Pearson’s chi-square test was used to compare categorical variables. Due to differences in baseline patient characteristics across the 3 treatment groups, inverse probability weights were used to adjust for group differences (using a multinomial logit treatment model) while comparing differences in outcome variables. This modeling strategy does not rely on any assumptions for the distribution of the outcome variable. Covariates were considered for inclusion in the treatment or outcome model if they were significantly associated (P < 0.05) with the group variable. Additionally, anesthetic agent (etomidate or propofol) was compared across the treatment groups after stratifying by ejection fraction to identify whether any differences existed in anesthesia regimen. Patients who were prescribed more than 1 anesthetic agent (n = 2) or an agent that was not of interest were removed from the analysis (n = 13). Stata (version 14) was used for analysis. All other missing data with respect to the tested variables were omitted in the analysis for that variable. Any disagreements about abstraction were resolved through consensus between the investigators.
Results
A total of 316 cases met inclusion criteria, including 108 direct-to-surgery patients, 143 preoperative TTE patients, and 65 cardiac consult patients. Patient demographics and preoperative characteristics are shown in Table 1. The average age for all patients was 76.5 years of age (SD, 12.89; IQR, 34-97); however, direct-to-surgery patients were significantly (P < 0.001) younger (71.2 years; SD, 14.2; interquartile range [IQR], 34-95 years) than TTE-only patients (79.0 years; SD, 11.5; IQR, 35-97 years) and cardiac consult patients (79.57 years; SD, 10.63; IQR, 49-97 years). The majority of patients were female (69.9%) and experienced a fall prior to admission (94%). Almost three-fourths of patients had 1 or more cardiac risk factors (73.7%), including history of congestive heart failure (CHF; 19%), coronary artery disease (CAD; 26.3%), chronic obstructive pulmonary disease (COPD; 19.3%), or aortic stenosis (AS; 3.5%). Due to between-group differences in these comorbid conditions, confounding factors were adjusted for in subsequent analyses.

As shown in Table 2, before adjustment for confounding factors, there were significant between-group differences in medical optimization time for patients in all 3 groups. After adjustment for treatment differences using age and number of comorbid diseases, and medical optimization time differences using age and COPD, fewer between-group differences were statistically significant. Patients who received a cardiac consult had an 18.44-hour longer medical optimization time compared to patients who went directly to surgery (29.136 vs 10.696 hours; P = 0.001). Optimization remained approximately 5 hours longer for the TTE-only group than for the direct-to-surgery group; however, this difference was not significant (P = 0.075).

When comparing differences in ED-to-OR time for the 3 groups after adjusting the probability of treatment for age and the number of comorbid conditions, and adjusting the probability of ED-to-OR time for age, COPD, and CHF, significant differences remained in ED-to-OR times across all groups. Specifically, patients in the direct-to-surgery group experienced the shortest time (mean, 20.64 hours), compared to patients in the TTE-only group (mean, 26.32; P = 0.04) or patients in the cardiac consult group (mean, 36.08; P < 0.001). TTE-only patients had a longer time of 5.68 hours, compared to the direct-to-surgery group, and patients in the preoperative cardiac consult group were on average 15.44 hours longer than the direct-to-surgery group.
When comparing differences in the length of stay for the 3 groups before statistical adjustments, differences were observed; however, after removing the confounding factors related to treatment (age and CAD) and the outcome (age and the number of comorbid conditions), there were no statistically significant differences in the length of stay for the 3 groups. Average length of stay was 131 hours for direct-to-surgery patients, 142 hours for TTE-only patients, and 141 hours for cardiac consult patients.
The use of different anesthetic agents was compared for patients in the 3 groups. The majority of patients in the study (87.7%) were given propofol, and there were no differences after stratifying by ejection fraction (Table 3).

Discussion
The GHFMC was created to reduce surgical delays for hip fracture. Medical optimization was considered a primary, modifiable factor given that surgeons were reluctant to proceed without a cardiac consult. To address this gap, the committee recommended a preoperative TTE for patients with low or unknown functional status. This threshold provides a quick and easy method for stratifying patients who previously required risk stratification by a cardiologist, which often resulted in surgery delays.
In their recommendations for implementation of hip fracture quality improvement projects, the Geriatric Fracture Center emphasizes the importance of multidisciplinary physician leadership along with standardization of approach across patients.12 This recommendation is supported by increasing evidence that orthogeriatric collaborations are associated with decreased mortality and length of stay.13 The GHFMC and subsequent interventions reflect this approach, allowing for collaboration to identify cross-disciplinary procedural barriers to care. In our institution, addressing identified procedural barriers to care was associated with a reduction in the average time to surgery from 51 hours to 25.3 hours.
Multiple approaches have been attempted to decrease presurgical time in hip fracture patients in various settings. Prehospital interventions, such as providing ambulances with checklists and ability to bypass the ED, have not been shown to decrease time to surgery for hip fracture patients, though similar strategies have been successful in other conditions, such as stroke.14,15 In-hospital procedures, such as implementation of a hip fracture protocol and reduction of preoperative interventions, have more consistently been found to decrease time to surgery and in-hospital mortality.16,17 However, reduced delays have not been found universally. Luttrell and Nana found that preoperative TTE resulted in approximately 30.8-hour delays from the ED to OR, compared to patients who did not receive a preoperative TTE.18 However, in that study hospitalists used TTE at their own discretion, and there may have been confounding factors contributing to delays. When used as part of a protocol targeting patients with poor or unknown functional capacity, we believe that preoperative TTE results in modest surgical delays yet provides clinically useful information about each patient.
ACC/AHA preoperative guidelines were updated after we implemented our intervention and now recommend that patients with poor or unknown functional capacity in whom stress testing will not influence care proceed to surgery “according to guideline-directed medical care.”11 While routine use of preoperative evaluation of left ventricular function is not recommended, assessing left ventricular function may be reasonable for patients with heart failure with a change in clinical status. Guidelines also recommend that patients with clinically suspected valvular stenosis undergo preoperative echocardiography.11
Limitations
This study has several limitations. First, due to resource limitations, a substantial period of time elapsed between implementation of the new protocol and the analysis of the data set. That is, the hip fracture protocol assessed in this paper occurred from January 2010 through April 2014, and final analysis of the data set occurred in April 2020. This limitation precludes our ability to formally assess any pre- or post-protocol changes in patient outcomes. Second, randomization was not used to create groups that were balanced in differing health characteristics (ie, patients with noncardiac-related surgeries, patients in different age groups); however, the use of inverse probability treatment regression analysis was a way to statistically address these between-group differences. Moreover, this study is limited by the factors that were measured; unmeasured factors cannot be accounted for. Third, health care providers working at the hospital during this time were aware of the goal to decrease presurgical time, possibly creating exaggerated effects compared to a blinded trial. Finally, although this intervention is likely translatable to other centers, these results represent the experiences of a single level 1 trauma center and may not be replicable elsewhere.
Conclusion
Preoperative TTE in lieu of cardiac consultation has several advantages. First, it requires interdepartmental collaboration for implementation, but can be implemented through a single hospital or hospital system. Unlike prehospital interventions, preoperative urgent TTE for patients with low functional capacity does not require the support of emergency medical technicians, ambulance services, or other hospitals in the region. Second, while costs are associated with TTE, they are offset by a reduction in expensive consultations with specialists, surgical delays, and longer lengths of stay. Third, despite likely increased ED-to-OR times compared to no intervention, urgent TTE decreases time to surgery compared with cardiology consultation. Prior to the GHFMC, the ED-to-OR time at our institution was 51 hours. In contrast, the mean time following the GHFMC-led protocol was less than half that, at 25.3 hours (SD, 19.1 hours). In fact, nearly two-thirds (65.2%) of the patients evaluated in this study underwent surgery within 24 hours of admission. This improvement in presurgical time was attributed, in part, to the implementation of preoperative TTE over cardiology consultations.
Acknowledgments: The authors thank Jenny Williams, RN, who was instrumental in obtaining the data set for analysis, and Shauna Ayres, MPH, from the OhioHealth Research Institute, who provided writing and technical assistance.
Corresponding author: Robert Skully, MD, OhioHealth Family Medicine Grant, 290 East Town St., Columbus, OH 43215; [email protected].
Funding: This work was supported by the OhioHealth Summer Research Externship Program.
Financial disclosures: None.
1. Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302:1573-1579.
2. Lewiecki EM, Wright NC, Curtis JR, et al. Hip fracture trends in the United States 2002 to 2015. Osteoporos Int. 2018;29:717-722.
3. Colais P, Di Martino M, Fusco D, et al. The effect of early surgery after hip fracture on 1-year mortality. BMC Geriatr. 2015;15:141.
4. Nyholm AM, Gromov K, Palm H, et al. Time to surgery is associated with thirty-day and ninety-day mortality after proximal femoral fracture: a retrospective observational study on prospectively collected data from the Danish Fracture Database Collaborators. J Bone Joint Surg Am. 2015;97:1333-1339.
5. Judd KT, Christianson E. Expedited operative care of hip fractures results in significantly lower cost of treatment. Iowa Orthop J. 2015;35:62-64.
6. Simunovic N, Devereaux PJ, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182:1609-1616.
7. Ryan DJ, Yoshihara H, Yoneoka D, et al. Delay in hip fracture surgery: an analysis of patient-specific and hospital-specific risk factors. J Orthop Trauma. 2015;29:343-348.
8. Ricci WM, Brandt A, McAndrew C, Gardner MJ. Factors affecting delay to surgery and length of stay for patients with hip fracture. J Orthop Trauma. 2015;29:e109-e114.
9. Hagino T, Ochiai S, Senga S, et al. Efficacy of early surgery and causes of surgical delay in patients with hip fracture. J Orthop. 2015;12:142-146.
10. Rafiq A, Sklyar E, Bella JN. Cardiac evaluation and monitoring of patients undergoing noncardiac surgery. Health Serv Insights. 2017;9:1178632916686074.
11. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e77-e137.
12. Basu N, Natour M, Mounasamy V, Kates SL. Geriatric hip fracture management: keys to providing a successful program. Eur J Trauma Emerg Surg. 2016;42:565-569.
13. Grigoryan KV, Javedan H, Rudolph JL. Orthogeriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma. 2014;28:e49-e55.
14. Tai YJ, Yan B. Minimising time to treatment: targeted strategies to minimise time to thrombolysis for acute ischaemic stroke. Intern Med J. 2013;43:1176-1182.
15. Larsson G, Stromberg RU, Rogmark C, Nilsdotter A. Prehospital fast track care for patients with hip fracture: Impact on time to surgery, hospital stay, post-operative complications and mortality a randomised, controlled trial. Injury. 2016;47:881-886.
16. Bohm E, Loucks L, Wittmeier K, et al. Reduced time to surgery improves mortality and length of stay following hip fracture: results from an intervention study in a Canadian health authority. Can J Surg. 2015;58:257-263.
17. Ventura C, Trombetti S, Pioli G, et al. Impact of multidisciplinary hip fracture program on timing of surgery in elderly patients. Osteoporos Int J. 2014;25:2591-2597.
18. Luttrell K, Nana A. Effect of preoperative transthoracic echocardiogram on mortality and surgical timing in elderly adults with hip fracture. J Am Geriatr Soc. 2015;63:2505-2509.
1. Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302:1573-1579.
2. Lewiecki EM, Wright NC, Curtis JR, et al. Hip fracture trends in the United States 2002 to 2015. Osteoporos Int. 2018;29:717-722.
3. Colais P, Di Martino M, Fusco D, et al. The effect of early surgery after hip fracture on 1-year mortality. BMC Geriatr. 2015;15:141.
4. Nyholm AM, Gromov K, Palm H, et al. Time to surgery is associated with thirty-day and ninety-day mortality after proximal femoral fracture: a retrospective observational study on prospectively collected data from the Danish Fracture Database Collaborators. J Bone Joint Surg Am. 2015;97:1333-1339.
5. Judd KT, Christianson E. Expedited operative care of hip fractures results in significantly lower cost of treatment. Iowa Orthop J. 2015;35:62-64.
6. Simunovic N, Devereaux PJ, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182:1609-1616.
7. Ryan DJ, Yoshihara H, Yoneoka D, et al. Delay in hip fracture surgery: an analysis of patient-specific and hospital-specific risk factors. J Orthop Trauma. 2015;29:343-348.
8. Ricci WM, Brandt A, McAndrew C, Gardner MJ. Factors affecting delay to surgery and length of stay for patients with hip fracture. J Orthop Trauma. 2015;29:e109-e114.
9. Hagino T, Ochiai S, Senga S, et al. Efficacy of early surgery and causes of surgical delay in patients with hip fracture. J Orthop. 2015;12:142-146.
10. Rafiq A, Sklyar E, Bella JN. Cardiac evaluation and monitoring of patients undergoing noncardiac surgery. Health Serv Insights. 2017;9:1178632916686074.
11. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e77-e137.
12. Basu N, Natour M, Mounasamy V, Kates SL. Geriatric hip fracture management: keys to providing a successful program. Eur J Trauma Emerg Surg. 2016;42:565-569.
13. Grigoryan KV, Javedan H, Rudolph JL. Orthogeriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma. 2014;28:e49-e55.
14. Tai YJ, Yan B. Minimising time to treatment: targeted strategies to minimise time to thrombolysis for acute ischaemic stroke. Intern Med J. 2013;43:1176-1182.
15. Larsson G, Stromberg RU, Rogmark C, Nilsdotter A. Prehospital fast track care for patients with hip fracture: Impact on time to surgery, hospital stay, post-operative complications and mortality a randomised, controlled trial. Injury. 2016;47:881-886.
16. Bohm E, Loucks L, Wittmeier K, et al. Reduced time to surgery improves mortality and length of stay following hip fracture: results from an intervention study in a Canadian health authority. Can J Surg. 2015;58:257-263.
17. Ventura C, Trombetti S, Pioli G, et al. Impact of multidisciplinary hip fracture program on timing of surgery in elderly patients. Osteoporos Int J. 2014;25:2591-2597.
18. Luttrell K, Nana A. Effect of preoperative transthoracic echocardiogram on mortality and surgical timing in elderly adults with hip fracture. J Am Geriatr Soc. 2015;63:2505-2509.