User login
Home-based HPV cervical cancer screening ‘cost effective’
For women who are overdue for cervical cancer screening, mailing self-sampling kits for high-risk human papillomavirus (HPV) is a cost-effective means of increasing screening uptake, reveals an analysis of a large U.S. trial.
The finding comes from a randomized trial in almost 20,000 women, which compared women who received a mailed HPV testing kit with those who did not. The results show that mailing was most cost-effective in women aged 50-64 years and in those who were only recently overdue for cervical screening.
The study was published by JAMA Network Open.
“These results support mailing HPV kits as an efficient outreach strategy for increasing screening rates in U.S. health care systems,” say the authors, led by Rachel L. Winer, PhD, MPH, department of epidemiology, University of Washington, Seattle.
They note that their results are consistent with those from previous studies in other health care contexts, but their analysis “benefited from the randomized clinical trial design and a large sample size,” they write.
However, they point out that the trial was conducted “before the beginning of the COVID-19 pandemic,” and it is “well established” that cancer screening rates “decreased substantially during the pandemic.”
They suggest that mailed HPV self-sampling kits could nevertheless be a “means of overcoming screening barriers among underscreened women,” which may have been exacerbated by the “societal consequences of the pandemic.”
Reducing barriers to screening
Cervical screening is associated with “substantial global reductions” in the incidence and mortality of cervical cancer, the authors point out. Because most cases of the disease are consequently preventable, it now occurs “predominantly in individuals who have never or rarely received screening.”
Home-based HPV-only testing reduces the need for office visits and reduces barriers to screening, such as discomfort, embarrassment, and difficulties with scheduling or attending appointments.
Previous studies have shown that the direct mailing of home-based HPV self-collection kits is associated with increased uptake of screening among underscreened women and is cost-effective, although the researchers point out that these previous studies were conducted in countries with “organized national screening programs.”
For their own study, they focused on home-based HPV screening among underscreened individuals in the United States. The team examined data from the Home-based Options to Make cervical cancer screening Easy trial, which has previously showed that mailing kits to women increased screening uptake, compared with usual care alone.
For the current analysis, they conducted an economic evaluation of data on 19,851 trial participants, who were randomized to receive home-based screening or usual care between February 2014 and August 2016 and were followed up to February 2018.
All of the women were aged 30-64 years and had been enrolled in a health plan from Kaiser Permanente Washington (KPW) for at least 3 years and 5 months. They were also required not have undergone a hysterectomy.
Participant-level economic data were collected between June 2019 and March 2021, with intervention delivery costs calculated from the perspective of both the KPW and Medicare health systems and based on the cost of either a wellness visit or Papanicolaou (Pap) test–only visit.
The mean age of the participants was 50.1 years, and the majority (76.7%) were White; 9.7% were Asian and 4.7% were Black or African American.
There were no significant differences in baseline characteristics between the group assigned to usual care, which comprised patient reminders and ad hoc screening outreach, and those in the intervention group, who received usual care and a mailed HPV self-sampling kit.
The researchers report that 1,206 women in the intervention group sent back a mailed HPV kit, with 1,178 (97.7%) meeting the criteria for completed screening uptake.
Overall, screening uptake was higher in the intervention group than in control participants, at 26.3% vs. 17.4%, respectively (relative risk, 1.51).
Intervention participants were also more likely than controls to have a positive test result (relative risk, 1.49) and to receive treatment (relative risk, 1.70).
The incremental cost-effectiveness ratio for increased screening uptake, defined as the incremental difference in cost between the study groups divided by the difference in the number of participants completing screening within 6 months, ranged from $85.84 per additional completed screening to $146.29, depending on the health system and test considered.
In terms of willingness-to-pay (WTP) thresholds for each additional completed screening, the team found that home-based screening achieved a 90% probability of cost-effectiveness, at a WTP of just $148 if the participant’s last Pap test was between 3.4 and 5.0 years before randomization.
A 90% probability of cost-effectiveness was also achieved at a WTP of $198 among participants aged 50-64 years, a threshold that was lower than that among other age groups.
At a WTP threshold of over $350, the intervention was considered to have 100% probability of being cost-effective in all age groups.
The study was supported by a grant from the National Cancer Institute of the National Institutes of Health. Dr. Winer reports a relationship with the National Cancer Institute outside of the submitted work, as do several other authors.
A version of this article first appeared on Medscape.com.
For women who are overdue for cervical cancer screening, mailing self-sampling kits for high-risk human papillomavirus (HPV) is a cost-effective means of increasing screening uptake, reveals an analysis of a large U.S. trial.
The finding comes from a randomized trial in almost 20,000 women, which compared women who received a mailed HPV testing kit with those who did not. The results show that mailing was most cost-effective in women aged 50-64 years and in those who were only recently overdue for cervical screening.
The study was published by JAMA Network Open.
“These results support mailing HPV kits as an efficient outreach strategy for increasing screening rates in U.S. health care systems,” say the authors, led by Rachel L. Winer, PhD, MPH, department of epidemiology, University of Washington, Seattle.
They note that their results are consistent with those from previous studies in other health care contexts, but their analysis “benefited from the randomized clinical trial design and a large sample size,” they write.
However, they point out that the trial was conducted “before the beginning of the COVID-19 pandemic,” and it is “well established” that cancer screening rates “decreased substantially during the pandemic.”
They suggest that mailed HPV self-sampling kits could nevertheless be a “means of overcoming screening barriers among underscreened women,” which may have been exacerbated by the “societal consequences of the pandemic.”
Reducing barriers to screening
Cervical screening is associated with “substantial global reductions” in the incidence and mortality of cervical cancer, the authors point out. Because most cases of the disease are consequently preventable, it now occurs “predominantly in individuals who have never or rarely received screening.”
Home-based HPV-only testing reduces the need for office visits and reduces barriers to screening, such as discomfort, embarrassment, and difficulties with scheduling or attending appointments.
Previous studies have shown that the direct mailing of home-based HPV self-collection kits is associated with increased uptake of screening among underscreened women and is cost-effective, although the researchers point out that these previous studies were conducted in countries with “organized national screening programs.”
For their own study, they focused on home-based HPV screening among underscreened individuals in the United States. The team examined data from the Home-based Options to Make cervical cancer screening Easy trial, which has previously showed that mailing kits to women increased screening uptake, compared with usual care alone.
For the current analysis, they conducted an economic evaluation of data on 19,851 trial participants, who were randomized to receive home-based screening or usual care between February 2014 and August 2016 and were followed up to February 2018.
All of the women were aged 30-64 years and had been enrolled in a health plan from Kaiser Permanente Washington (KPW) for at least 3 years and 5 months. They were also required not have undergone a hysterectomy.
Participant-level economic data were collected between June 2019 and March 2021, with intervention delivery costs calculated from the perspective of both the KPW and Medicare health systems and based on the cost of either a wellness visit or Papanicolaou (Pap) test–only visit.
The mean age of the participants was 50.1 years, and the majority (76.7%) were White; 9.7% were Asian and 4.7% were Black or African American.
There were no significant differences in baseline characteristics between the group assigned to usual care, which comprised patient reminders and ad hoc screening outreach, and those in the intervention group, who received usual care and a mailed HPV self-sampling kit.
The researchers report that 1,206 women in the intervention group sent back a mailed HPV kit, with 1,178 (97.7%) meeting the criteria for completed screening uptake.
Overall, screening uptake was higher in the intervention group than in control participants, at 26.3% vs. 17.4%, respectively (relative risk, 1.51).
Intervention participants were also more likely than controls to have a positive test result (relative risk, 1.49) and to receive treatment (relative risk, 1.70).
The incremental cost-effectiveness ratio for increased screening uptake, defined as the incremental difference in cost between the study groups divided by the difference in the number of participants completing screening within 6 months, ranged from $85.84 per additional completed screening to $146.29, depending on the health system and test considered.
In terms of willingness-to-pay (WTP) thresholds for each additional completed screening, the team found that home-based screening achieved a 90% probability of cost-effectiveness, at a WTP of just $148 if the participant’s last Pap test was between 3.4 and 5.0 years before randomization.
A 90% probability of cost-effectiveness was also achieved at a WTP of $198 among participants aged 50-64 years, a threshold that was lower than that among other age groups.
At a WTP threshold of over $350, the intervention was considered to have 100% probability of being cost-effective in all age groups.
The study was supported by a grant from the National Cancer Institute of the National Institutes of Health. Dr. Winer reports a relationship with the National Cancer Institute outside of the submitted work, as do several other authors.
A version of this article first appeared on Medscape.com.
For women who are overdue for cervical cancer screening, mailing self-sampling kits for high-risk human papillomavirus (HPV) is a cost-effective means of increasing screening uptake, reveals an analysis of a large U.S. trial.
The finding comes from a randomized trial in almost 20,000 women, which compared women who received a mailed HPV testing kit with those who did not. The results show that mailing was most cost-effective in women aged 50-64 years and in those who were only recently overdue for cervical screening.
The study was published by JAMA Network Open.
“These results support mailing HPV kits as an efficient outreach strategy for increasing screening rates in U.S. health care systems,” say the authors, led by Rachel L. Winer, PhD, MPH, department of epidemiology, University of Washington, Seattle.
They note that their results are consistent with those from previous studies in other health care contexts, but their analysis “benefited from the randomized clinical trial design and a large sample size,” they write.
However, they point out that the trial was conducted “before the beginning of the COVID-19 pandemic,” and it is “well established” that cancer screening rates “decreased substantially during the pandemic.”
They suggest that mailed HPV self-sampling kits could nevertheless be a “means of overcoming screening barriers among underscreened women,” which may have been exacerbated by the “societal consequences of the pandemic.”
Reducing barriers to screening
Cervical screening is associated with “substantial global reductions” in the incidence and mortality of cervical cancer, the authors point out. Because most cases of the disease are consequently preventable, it now occurs “predominantly in individuals who have never or rarely received screening.”
Home-based HPV-only testing reduces the need for office visits and reduces barriers to screening, such as discomfort, embarrassment, and difficulties with scheduling or attending appointments.
Previous studies have shown that the direct mailing of home-based HPV self-collection kits is associated with increased uptake of screening among underscreened women and is cost-effective, although the researchers point out that these previous studies were conducted in countries with “organized national screening programs.”
For their own study, they focused on home-based HPV screening among underscreened individuals in the United States. The team examined data from the Home-based Options to Make cervical cancer screening Easy trial, which has previously showed that mailing kits to women increased screening uptake, compared with usual care alone.
For the current analysis, they conducted an economic evaluation of data on 19,851 trial participants, who were randomized to receive home-based screening or usual care between February 2014 and August 2016 and were followed up to February 2018.
All of the women were aged 30-64 years and had been enrolled in a health plan from Kaiser Permanente Washington (KPW) for at least 3 years and 5 months. They were also required not have undergone a hysterectomy.
Participant-level economic data were collected between June 2019 and March 2021, with intervention delivery costs calculated from the perspective of both the KPW and Medicare health systems and based on the cost of either a wellness visit or Papanicolaou (Pap) test–only visit.
The mean age of the participants was 50.1 years, and the majority (76.7%) were White; 9.7% were Asian and 4.7% were Black or African American.
There were no significant differences in baseline characteristics between the group assigned to usual care, which comprised patient reminders and ad hoc screening outreach, and those in the intervention group, who received usual care and a mailed HPV self-sampling kit.
The researchers report that 1,206 women in the intervention group sent back a mailed HPV kit, with 1,178 (97.7%) meeting the criteria for completed screening uptake.
Overall, screening uptake was higher in the intervention group than in control participants, at 26.3% vs. 17.4%, respectively (relative risk, 1.51).
Intervention participants were also more likely than controls to have a positive test result (relative risk, 1.49) and to receive treatment (relative risk, 1.70).
The incremental cost-effectiveness ratio for increased screening uptake, defined as the incremental difference in cost between the study groups divided by the difference in the number of participants completing screening within 6 months, ranged from $85.84 per additional completed screening to $146.29, depending on the health system and test considered.
In terms of willingness-to-pay (WTP) thresholds for each additional completed screening, the team found that home-based screening achieved a 90% probability of cost-effectiveness, at a WTP of just $148 if the participant’s last Pap test was between 3.4 and 5.0 years before randomization.
A 90% probability of cost-effectiveness was also achieved at a WTP of $198 among participants aged 50-64 years, a threshold that was lower than that among other age groups.
At a WTP threshold of over $350, the intervention was considered to have 100% probability of being cost-effective in all age groups.
The study was supported by a grant from the National Cancer Institute of the National Institutes of Health. Dr. Winer reports a relationship with the National Cancer Institute outside of the submitted work, as do several other authors.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Current approaches and challenges to cervical cancer prevention in the United States
CASE Intervention approaches for decreasing the risk of cervical cancer
A 25-year-old woman presents to your practice for routine examination. She has never undergone cervical cancer screening or received the human papillomavirus (HPV) vaccine series. The patient has had 3 lifetime sexual partners and currently uses condoms as contraception. What interventions are appropriate to offer this patient to decrease her risk of cervical cancer? Choose as many that may apply:
1. cervical cytology with reflex HPV testing
2. cervical cytology with HPV cotesting
3. primary HPV testing
4. HPV vaccine series (3 doses)
5. all of the above
The answer is number 5, all of the above.
Choices 1, 2, and 3 are acceptable methods of cervical cancer screening for this patient. Catch-up HPV vaccination should be offered as well.
Equitable preventive care is needed
Cervical cancer is a unique cancer because it has a known preventative strategy. HPV vaccination, paired with cervical screening and management of abnormal results, has contributed to decreased rates of cervical cancer in the United States, from 13,914 cases in 1999 to 12,795 cases in 2019.1 In less-developed countries, however, cervical cancer continues to be a leading cause of mortality, with 90% of cervical cancer deaths in 2020 occurring in low- and middle-income countries.2
Disparate outcomes in cervical cancer are often a reflection of disparities in health access. Within the United States, Black women have a higher incidence of cervical cancer, advanced-stage disease, and mortality from cervical cancer than White women.3,4 Furthermore, the incidence of cervical cancer increased among American Indian and Alaska Native people between 2000 and 2019.5 The rate for patients who are overdue for cervical cancer screening is higher among Asian and Hispanic patients compared with non-Hispanic White patients (31.4% vs 20.1%; P=.01) and among patients who identify as LGBTQ+ compared with patients who identify as heterosexual (32.0% vs 22.2%; P<.001).6 Younger patients have a significantly higher rate for overdue screening compared with their older counterparts (29.1% vs 21.1%; P<.001), as do uninsured patients compared with those who are privately insured (41.7% vs 18.1%; P<.001). Overall, the proportion of women without up-to-date screening increased significantly from 2005 to 2019 (14.4% vs 23.0%; P<.001).6
Unfortunately, despite a known strategy to eliminate cervical cancer, we are not accomplishing equitable preventative care. Barriers to care can include patient-centered issues, such as fear of cancer or of painful evaluations, lack of trust in the health care system, and inadequate understanding of the benefits of cancer prevention, in addition to systemic and structural barriers. As we assess new technologies, one of our most important goals is to consider how such innovations can increase health access—whether through increasing ease and acceptability of testing or by creating more effective screening tests.
Updates to cervical screening guidance
In 2020, the American Cancer Society (ACS) updated its cervical screening guidelines to start screening at age 25 years with the “preferred” strategy of HPV primary testing every 5 years.7 By contrast, the US Preventive Services Task Force (USPSTF) continues to recommend 1 of 3 methods: cytology alone every 3 years; cytology alone every 3 years between ages 21 and 29 followed by cytology and HPV cotesting every 5 years at age 30 or older; or high-risk HPV testing alone every 5 years (TABLE).8
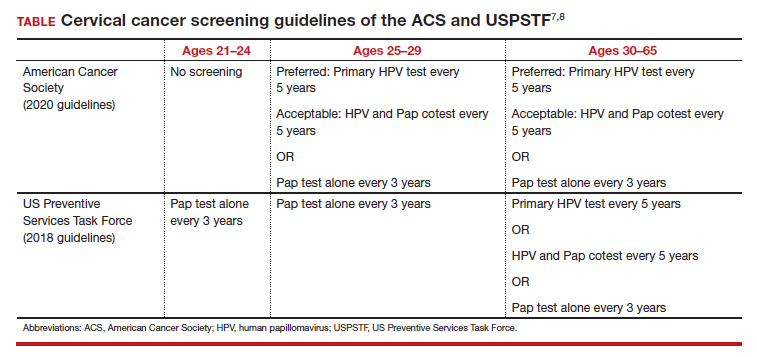
To successfully prevent cervical cancer, abnormal results are managed by performing either colposcopy with biopsy, immediate treatment, or close surveillance based on the risk of developing cervical intraepithelial neoplasia (CIN) 3 or worse. A patient’s risk is determined based on both current and prior test results. The ASCCP (American Society for Colposcopy and Cervical Pathology) transitioned to risk-based management guidelines in 2019 and has both an app and a web-based risk assessment tool available for clinicians (https://www.asccp.org).9
All organizations recommend stopping screening after age 65 provided there has been a history of adequate screening in the prior 10 years (defined as 2 normal cotests or 3 normal cytology tests, with the most recent test within 5 years) and no history of CIN 2 or worse within the prior 25 years.10,11 Recent studies that examined the rate of cervical cancer diagnosed in patients older than 65 years have questioned whether patients should continue screening beyond 65.10 In the United States, 20% of cervical cancer still occurs in women older than age 65.11 One reason may be that many women have not met the requirement for adequate and normal prior screening and may still need ongoing testing.12
Continue to: Primary HPV screening...
Primary HPV screening
Primary HPV testing means that an HPV test is performed first, and if it is positive for high-risk HPV, further testing is performed to determine next steps. This contrasts with the currently used method of obtaining cytology (Pap) first with either concurrent HPV testing or reflex HPV testing. The first HPV primary screening test was approved by the US Food and Drug Administration (FDA) in 2014.13
Multiple randomized controlled trials in Europe have demonstrated the accuracy of HPV-based screening compared with cytology in the detection of cervical cancer and its precursors.14-17 The HPV FOCAL trial demonstrated increased efficacy of primary HPV screening in the detection of CIN 2+ lesions.18 This trial recruited a total of 19,000 women, ages 25 to 65, in Canada and randomly assigned them to receive primary HPV testing or liquid-based cytology. If primary HPV testing was negative, participants would return in 48 months for cytology and HPV cotesting. If primary liquid-based cytology testing was negative, participants would return at 24 months for cytology testing alone and at 48 months for cytology and HPV cotesting. Both groups had similar incidences of CIN 2+ over the study period. HPV testing was shown to detect CIN 2+ at higher rates at the time of initial screen (risk ratio [RR], 1.61; 95% confidence interval [CI], 1.24–2.09) and then significantly lower rates at the time of exit screening at 48 months (RR, 0.36; 95% CI, 0.24–0.54).18 These results demonstrated that primary HPV testing detects CIN 2+ earlier than cytology alone. In follow-up analyses, primary HPV screening missed fewer CIN 2+ diagnoses than cytology screening.19
While not as many studies have compared primary HPV testing to cytology with an HPV cotest, the current most common practice in the United States, one study performed in the United States found that a negative cytology result did not further decrease the risk of CIN 3 for HPV-negative patients (risk of CIN 3+ at 5 years: 0.16% vs 0.17%; P=0.8) and concluded that a negative HPV test was enough reassurance for a low risk of CIN 3+.20
Another study, the ATHENA trial, evaluated more than 42,000 women who were 25 years and older over a 3-year period.21 Patients underwent either primary HPV testing or combination cytology and reflex HPV (if ages 25–29) or HPV cotesting (if age 30 or older). Primary HPV testing was found to have a sensitivity and specificity of 76.1% and 93.5%, respectively, compared with 61.7% and 94.6% for cytology with HPV cotesting, but it also increased the total number of colposcopies performed.21
Subsequent management of a primary HPV-positive result can be triaged using genotyping, cytology, or a combination of both. FDA-approved HPV screening tests provide genotyping and current management guidelines use genotyping to triage positive HPV results into HPV 16, 18, or 1 of 12 other high-risk HPV genotypes.
In the ATHENA trial, the 3-year incidence of CIN 3+ for HPV 16/18-positive results was 21.16% (95% CI, 18.39%–24.01%) compared with 5.4% (95% CI, 4.5%–6.4%) among patients with an HPV test positive for 1 of the other HPV genotypes.21 While a patient with an HPV result positive for HPV 16/18 should directly undergo colposcopy, clinical guidance for an HPV-positive result for one of the other genotypes suggests using reflex cytology to triage patients. The ASCCP recommended management of primary HPV testing is included in the FIGURE.22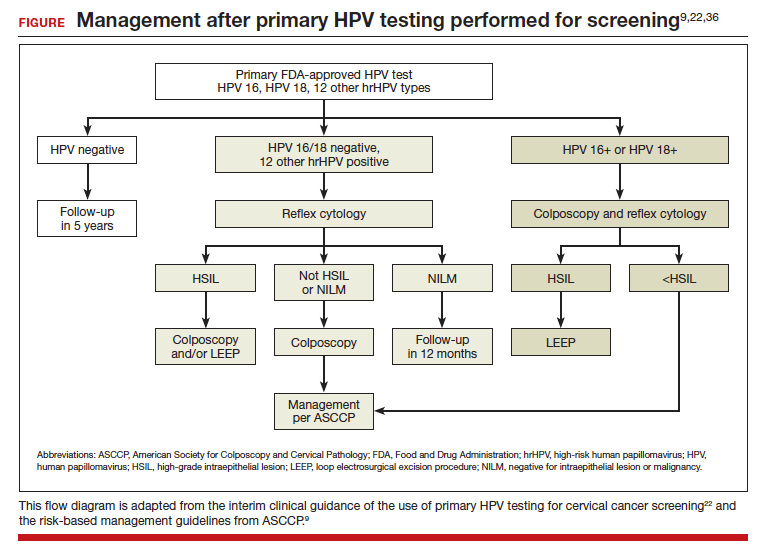
Many barriers remain to transitioning to primary HPV testing, including laboratory test availability as well as patient and provider acceptance. At present, 2 FDA-approved primary HPV screening tests are available: the Cobas HPV test (Roche Molecular Systems, Inc) and the BD Onclarity HPV assay (Becton, Dickinson and Company). Changes to screening recommendations need to be accompanied by patient and provider outreach and education.
In a survey of more than 500 US women in 2015 after guidelines allowed for increased screening intervals after negative results, a majority of women (55.6%; 95% CI, 51.4%–59.8%) were aware that screening recommendations had changed; however, 74.1% (95% CI, 70.3%–77.7%) still believed that women should be screened annually.23 By contrast, participants in the HPV FOCAL trial, who were able to learn more about HPV-based screening, were surveyed about their willingness to undergo primary HPV testing rather than Pap testing at the conclusion of the trial.24 Of the participants, 63% were comfortable with primary HPV testing, and 54% were accepting of an extended screening interval of 4 to 5 years.24
Continue to: p16/Ki-67 dual-stain cytology...
p16/Ki-67 dual-stain cytology
An additional tool for triaging HPV-positive patients is the p16/Ki-67 dual stain test (CINtec Plus Cytology; Roche), which was FDA approved in March 2020. A tumor suppressor protein, p16 is found to be overexpressed by HPV oncogenic activity, and Ki-67 is a marker of cellular proliferation. Coexpression of p16 and Ki-67 indicates a loss of cell cycle regulation and is a hallmark of neoplastic transformation. When positive, this test is supportive of active HPV infection and of a high-grade lesion. While the dual stain test is not yet formally incorporated into triage algorithms by national guidelines, it has demonstrated efficacy in detecting CIN 3+
In the IMPACT trial, nearly 5,000 HPV-positive patients underwent p16/Ki-67 dual stain testing compared with cytology and HPV genotyping.25 The sensitivity of dual stain for CIN 3+ was 91.9% (95% CI, 86.1%–95.4%) in HPV 16/18–positive and 86.0% (95% CI, 77.5%–91.6%) in the 12 other genotypes. Using dual stain testing alone to triage HPV-positive results showed significantly higher sensitivity but lower specificity than using cytology alone to triage HPV-positive results. Importantly, triage with dual stain testing alone would have referred significantly fewer women to colposcopy than HPV 16/18 genotyping with cytology triage for the 12 other genotypes (48.6% vs 56.0%; P< .0001).
Self-sampling methods: An approach for potentially improving access to screening
One technology that may help bridge gaps in access to cervical cancer screening is self-collected HPV testing, which would preclude the need for a clinician-performed pelvic exam. At present, no self-sampling method is approved by the FDA. However, many studies have examined the efficacy and safety of various self-sampling kits.26
One randomized controlled trial in the Netherlands compared sensitivity and specificity of CIN 2+ detection in patient-collected versus clinician-collected swabs.27 After a median follow-up of 20 months, the sensitivity and specificity of HPV testing did not differ between the patient-collected and the clinician-collected groups (specificity 100%; 95% CI, 0.91–1.08; sensitivity 96%; 95% CI, 0.90–1.03).27 This analysis did not include patients who did not return their self-collected sample, which leaves the question of whether self-sampling may exacerbate issues with patients who are lost to follow-up.
In a study performed in the United States, 16,590 patients who were overdue for cervical cancer screening were randomly assigned to usual care reminders (annual mailed reminders and phone calls from clinics) or to the addition of a mailed HPV self-sampling test kit.28 While the study did not demonstrate significant difference in the detection of overall CIN 2+ between the 2 groups, screening uptake was higher in the self-sampling kit group than in the usual care reminders group (RR, 1.51; 95% CI, 1.43–1.60), and the number of abnormal screens that warranted colposcopy referral was similar between the 2 groups (36.4% vs 36.8%).28 In qualitative interviews of the participants of this trial, patients who were sent at-home self-sampling kits found that the convenience of at-home testing lowered barriers to scheduling an in-office appointment.29 The hope is that self-sampling methods will expand access of cervical cancer screening to vulnerable populations that face significant barriers to having an in-office pelvic exam.
It is important to note that self-collection and self-sample testing requires multidisciplinary systems for processing results and assuring necessary patient follow-up. Implementing and disseminating such a program has been well tested only in developed countries27,30 with universal health care systems or within an integrated care delivery system. Bringing such technology broadly to the United States and less developed countries will require continued commitment to increasing laboratory capacity, a central electronic health record or system for monitoring results, educational materials for clinicians and patients, and expanding insurance reimbursement for such testing.
HPV vaccination rates must increase
While we continue to investigate which screening methods will most improve our secondary prevention of cervical cancer, our path to increasing primary prevention of cervical cancer is clear: We must increase rates of HPV vaccination. The 9-valent HPV vaccine is FDA approved for use in all patients aged 9 to 45 years.
The American College of Obstetricians and Gynecologists and other organizations recommend HPV vaccination between the ages of 9 and 13, and a “catch-up period” from ages 13 to 26 in which patients previously not vaccinated should receive the vaccine.31 Initiation of the vaccine course earlier (ages 9–10) compared with later (ages 11–12) is correlated with higher overall completion rates by age 15 and has been suggested to be associated with a stronger immune response.32
A study from Sweden found that HPV vaccination before age 17 was most strongly correlated with the lowest rates of cervical cancer, although vaccination between ages 17 and 30 still significantly decreased the risk of cervical cancer compared with those who were unvaccinated.33
Overall HPV vaccination rates in the United States continue to improve, with 58.6%34 of US adolescents having completed vaccination in 2020. However, these rates still are significantly lower than those in many other developed countries, including Australia, which had a complete vaccination rate of 80.5% in 2020.35 Continued disparities in vaccination rates could be contributing to the rise in cervical cancer among certain groups, such as American Indian and Alaska Native populations.5
Work—and innovations—must continue
In conclusion, the incidence of cervical cancer in the United States continues to decrease, although at disparate rates among marginalized populations. To ensure that we are working toward eliminating cervical cancer for all patients, we must continue efforts to eliminate disparities in health access. Continued innovations, including primary HPV testing and self-collection samples, may contribute to lowering barriers to all patients being able to access the preventative care they need. ●
- Centers for Disease Control and Prevention. United States Cancer Statistics: data visualizations. Trends: changes over time: cervix. Accessed January 8, 2023. https://gis.cdc.gov /Cancer/USCS/#/Trends/
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249. doi:10.3322/caac.21660.
- Francoeur AA, Liao CI, Casear MA, et al. The increasing incidence of stage IV cervical cancer in the USA: what factors are related? Int J Gynecol Cancer. 2022;32:ijgc-2022-003728. doi:10.1136/ijgc-2022-003728.
- Abdalla E, Habtemariam T, Fall S, et al. A comparative study of health disparities in cervical cancer mortality rates through time between Black and Caucasian women in Alabama and the US. Int J Stud Nurs. 2021;6:9-23. doi:10.20849/ijsn. v6i1.864.
- Bruegl AS, Emerson J, Tirumala K. Persistent disparities of cervical cancer among American Indians/Alaska natives: are we maximizing prevention tools? Gynecol Oncol. 2023;168:5661. doi:10.1016/j.ygyno.2022.11.007.
- Suk R, Hong YR, Rajan SS, et al. Assessment of US Preventive Services Task Force Guideline–Concordant cervical cancer screening rates and reasons for underscreening by age, race and ethnicity, sexual orientation, rurality, and insurance, 2005 to 2019. JAMA Netw Open. 2022;5:e2143582. doi:10.1001/ jamanetworkopen.2021.43582.
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation statement. JAMA. 2018;320:674-686. doi:10.1001/jama.2018.10897.
- Nayar R, Chhieng DC, Crothers B, et al. Moving forward—the 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors and beyond: implications and suggestions for laboratories. J Am Soc Cytopathol. 2020;9:291-303. doi:10.1016/j.jasc.2020.05.002.
- Cooley JJP, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
- National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Cervical Cancer. Accessed February 21, 2023. https://seer.cancer.gov /statfacts/html/cervix.html
- Feldman S. Screening options for preventing cervical cancer. JAMA Intern Med. 2019;179:879-880. doi:10.1001/ jamainternmed.2019.0298.
- ASCO Post Staff. FDA approves first HPV test for primary cervical cancer screening. ASCO Post. May 15, 2014. Accessed January 8, 2023. https://ascopost.com/issues/may-15-2014 /fda-approves-first-hpv-test-for-primary-cervical-cancer -screening/
- Rijkaart DC, Berkhof J, Rozendaal L, et al. Human papillomavirus testing for the detection of high-grade cervical intraepithelial neoplasia and cancer: final results of the POBASCAM randomised controlled trial. Lancet Oncol. 2012;13:78-88. doi:10.1016/S1470-2045(11)70296-0.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249-257. doi:10.1016/S1470-2045(09)70360-2.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol. 2009;10:672-682. doi:10.1016/S1470-2045(09)70156-1.
- Bulkmans NWJ, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year followup of a randomised controlled implementation trial. Lancet. 2007;370:1764-1772. doi:10.1016/S0140-6736(07)61450-0.
- Ogilvie GS, Van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52. doi:10.1001/jama.2018.7464.
- Gottschlich A, Gondara L, Smith LW, et al. Human papillomavirus‐based screening at extended intervals missed fewer cervical precancers than cytology in the HPV For Cervical Cancer (HPV FOCAL) trial. Int J Cancer. 2022;151:897-905. doi:10.1002/ijc.34039.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12:663672. doi:10.1016/S1470-2045(11)70145-0.
- Wright TC, Stoler MH, Behrens CM, et al. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136:189-197. doi:10.1016/j.ygyno.2014.11.076
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125:330-337. doi:10.1097/AOG.0000000000000669.
- Silver MI, Rositch AF, Burke AE, et al. Patient concerns about human papillomavirus testing and 5-year intervals in routine cervical cancer screening. Obstet Gynecol. 2015;125:317-329. doi:10.1097/AOG.0000000000000638.
- Smith LW, Racey CS, Gondara L, et al. Women’s acceptability of and experience with primary human papillomavirus testing for cervical screening: HPV FOCAL trial cross-sectional online survey results. BMJ Open. 2021;11:e052084. doi:10.1136/bmjopen-2021-052084.
- Wright TC, Stoler MH, Ranger-Moore J, et al. Clinical validation of p16/Ki-67 dual-stained cytology triage of HPV-positive women: results from the IMPACT trial. Int J Cancer. 2022;150:461-471. doi:10.1002/ijc.33812.
- Yeh PT, Kennedy CE, De Vuyst H, et al. Self-sampling for human papillomavirus (HPV) testing: a systematic review and meta-analysis. BMJ Global Health. 2019;4:e001351. doi:10.1136/bmjgh-2018-001351.
- Polman NJ, Ebisch RMF, Heideman DAM, et al. Performance of human papillomavirus testing on self-collected versus clinician-collected samples for the detection of cervical intraepithelial neoplasia of grade 2 or worse: a randomised, paired screen-positive, non-inferiority trial. Lancet Oncol. 2019;20:229-238. doi:10.1016/S1470-2045(18)30763-0.
- Winer RL, Lin J, Tiro JA, et al. Effect of mailed human papillomavirus test kits vs usual care reminders on cervical cancer screening uptake, precancer detection, and treatment: a randomized clinical trial. JAMA Netw Open. 2019;2:e1914729. doi:10.1001/jamanetworkopen.2019.14729.
- Tiro JA, Betts AC, Kimbel K, et al. Understanding patients’ perspectives and information needs following a positive home human papillomavirus self-sampling kit result. J Womens Health (Larchmt). 2019;28:384-392. doi:10.1089/ jwh.2018.7070.
- Knauss T, Hansen BT, Pedersen K, et al. The cost-effectiveness of opt-in and send-to-all HPV self-sampling among long-term non-attenders to cervical cancer screening in Norway: the Equalscreen randomized controlled trial. Gynecol Oncol. 2023;168:39-47. doi:10.1016/j.ygyno.2022.10.027.
- ACOG committee opinion no. 809. Human papillomavirus vaccination: correction. Obstet Gynecol. 2022;139:345. doi:10.1097/AOG.0000000000004680.
- St Sauver JL, Finney Rutten LJF, Ebbert JO, et al. Younger age at initiation of the human papillomavirus (HPV) vaccination series is associated with higher rates of on-time completion. Prev Med. 2016;89:327-333. doi:10.1016/j.ypmed.2016.02.039.
- Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:13401348. doi:10.1056/NEJMoa1917338.
- Pingali C, Yankey D, Elam-Evans LD, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years — United States, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:1183-1190. doi:10.15585/ mmwr.mm7035a1.
- National Centre for Immunisation Research and Surveillance Australia. Annual Immunisation Coverage Report 2020. November 29, 2021. Accessed March 1, 2023. https://ncirs .org.au/sites/default/files/2021-11/NCIRS%20Annual%20 Immunisation%20Coverage%20Report%202020_FINAL.pdf
- Leung SOA, Feldman S. 2022 Update on cervical disease. OBG Manag. 2022;34(5):16-17, 22-24, 26, 28. doi:10.12788/ obgm.0197.
CASE Intervention approaches for decreasing the risk of cervical cancer
A 25-year-old woman presents to your practice for routine examination. She has never undergone cervical cancer screening or received the human papillomavirus (HPV) vaccine series. The patient has had 3 lifetime sexual partners and currently uses condoms as contraception. What interventions are appropriate to offer this patient to decrease her risk of cervical cancer? Choose as many that may apply:
1. cervical cytology with reflex HPV testing
2. cervical cytology with HPV cotesting
3. primary HPV testing
4. HPV vaccine series (3 doses)
5. all of the above
The answer is number 5, all of the above.
Choices 1, 2, and 3 are acceptable methods of cervical cancer screening for this patient. Catch-up HPV vaccination should be offered as well.
Equitable preventive care is needed
Cervical cancer is a unique cancer because it has a known preventative strategy. HPV vaccination, paired with cervical screening and management of abnormal results, has contributed to decreased rates of cervical cancer in the United States, from 13,914 cases in 1999 to 12,795 cases in 2019.1 In less-developed countries, however, cervical cancer continues to be a leading cause of mortality, with 90% of cervical cancer deaths in 2020 occurring in low- and middle-income countries.2
Disparate outcomes in cervical cancer are often a reflection of disparities in health access. Within the United States, Black women have a higher incidence of cervical cancer, advanced-stage disease, and mortality from cervical cancer than White women.3,4 Furthermore, the incidence of cervical cancer increased among American Indian and Alaska Native people between 2000 and 2019.5 The rate for patients who are overdue for cervical cancer screening is higher among Asian and Hispanic patients compared with non-Hispanic White patients (31.4% vs 20.1%; P=.01) and among patients who identify as LGBTQ+ compared with patients who identify as heterosexual (32.0% vs 22.2%; P<.001).6 Younger patients have a significantly higher rate for overdue screening compared with their older counterparts (29.1% vs 21.1%; P<.001), as do uninsured patients compared with those who are privately insured (41.7% vs 18.1%; P<.001). Overall, the proportion of women without up-to-date screening increased significantly from 2005 to 2019 (14.4% vs 23.0%; P<.001).6
Unfortunately, despite a known strategy to eliminate cervical cancer, we are not accomplishing equitable preventative care. Barriers to care can include patient-centered issues, such as fear of cancer or of painful evaluations, lack of trust in the health care system, and inadequate understanding of the benefits of cancer prevention, in addition to systemic and structural barriers. As we assess new technologies, one of our most important goals is to consider how such innovations can increase health access—whether through increasing ease and acceptability of testing or by creating more effective screening tests.
Updates to cervical screening guidance
In 2020, the American Cancer Society (ACS) updated its cervical screening guidelines to start screening at age 25 years with the “preferred” strategy of HPV primary testing every 5 years.7 By contrast, the US Preventive Services Task Force (USPSTF) continues to recommend 1 of 3 methods: cytology alone every 3 years; cytology alone every 3 years between ages 21 and 29 followed by cytology and HPV cotesting every 5 years at age 30 or older; or high-risk HPV testing alone every 5 years (TABLE).8

To successfully prevent cervical cancer, abnormal results are managed by performing either colposcopy with biopsy, immediate treatment, or close surveillance based on the risk of developing cervical intraepithelial neoplasia (CIN) 3 or worse. A patient’s risk is determined based on both current and prior test results. The ASCCP (American Society for Colposcopy and Cervical Pathology) transitioned to risk-based management guidelines in 2019 and has both an app and a web-based risk assessment tool available for clinicians (https://www.asccp.org).9
All organizations recommend stopping screening after age 65 provided there has been a history of adequate screening in the prior 10 years (defined as 2 normal cotests or 3 normal cytology tests, with the most recent test within 5 years) and no history of CIN 2 or worse within the prior 25 years.10,11 Recent studies that examined the rate of cervical cancer diagnosed in patients older than 65 years have questioned whether patients should continue screening beyond 65.10 In the United States, 20% of cervical cancer still occurs in women older than age 65.11 One reason may be that many women have not met the requirement for adequate and normal prior screening and may still need ongoing testing.12

Continue to: Primary HPV screening...
Primary HPV screening
Primary HPV testing means that an HPV test is performed first, and if it is positive for high-risk HPV, further testing is performed to determine next steps. This contrasts with the currently used method of obtaining cytology (Pap) first with either concurrent HPV testing or reflex HPV testing. The first HPV primary screening test was approved by the US Food and Drug Administration (FDA) in 2014.13
Multiple randomized controlled trials in Europe have demonstrated the accuracy of HPV-based screening compared with cytology in the detection of cervical cancer and its precursors.14-17 The HPV FOCAL trial demonstrated increased efficacy of primary HPV screening in the detection of CIN 2+ lesions.18 This trial recruited a total of 19,000 women, ages 25 to 65, in Canada and randomly assigned them to receive primary HPV testing or liquid-based cytology. If primary HPV testing was negative, participants would return in 48 months for cytology and HPV cotesting. If primary liquid-based cytology testing was negative, participants would return at 24 months for cytology testing alone and at 48 months for cytology and HPV cotesting. Both groups had similar incidences of CIN 2+ over the study period. HPV testing was shown to detect CIN 2+ at higher rates at the time of initial screen (risk ratio [RR], 1.61; 95% confidence interval [CI], 1.24–2.09) and then significantly lower rates at the time of exit screening at 48 months (RR, 0.36; 95% CI, 0.24–0.54).18 These results demonstrated that primary HPV testing detects CIN 2+ earlier than cytology alone. In follow-up analyses, primary HPV screening missed fewer CIN 2+ diagnoses than cytology screening.19
While not as many studies have compared primary HPV testing to cytology with an HPV cotest, the current most common practice in the United States, one study performed in the United States found that a negative cytology result did not further decrease the risk of CIN 3 for HPV-negative patients (risk of CIN 3+ at 5 years: 0.16% vs 0.17%; P=0.8) and concluded that a negative HPV test was enough reassurance for a low risk of CIN 3+.20
Another study, the ATHENA trial, evaluated more than 42,000 women who were 25 years and older over a 3-year period.21 Patients underwent either primary HPV testing or combination cytology and reflex HPV (if ages 25–29) or HPV cotesting (if age 30 or older). Primary HPV testing was found to have a sensitivity and specificity of 76.1% and 93.5%, respectively, compared with 61.7% and 94.6% for cytology with HPV cotesting, but it also increased the total number of colposcopies performed.21
Subsequent management of a primary HPV-positive result can be triaged using genotyping, cytology, or a combination of both. FDA-approved HPV screening tests provide genotyping and current management guidelines use genotyping to triage positive HPV results into HPV 16, 18, or 1 of 12 other high-risk HPV genotypes.
In the ATHENA trial, the 3-year incidence of CIN 3+ for HPV 16/18-positive results was 21.16% (95% CI, 18.39%–24.01%) compared with 5.4% (95% CI, 4.5%–6.4%) among patients with an HPV test positive for 1 of the other HPV genotypes.21 While a patient with an HPV result positive for HPV 16/18 should directly undergo colposcopy, clinical guidance for an HPV-positive result for one of the other genotypes suggests using reflex cytology to triage patients. The ASCCP recommended management of primary HPV testing is included in the FIGURE.22
Many barriers remain to transitioning to primary HPV testing, including laboratory test availability as well as patient and provider acceptance. At present, 2 FDA-approved primary HPV screening tests are available: the Cobas HPV test (Roche Molecular Systems, Inc) and the BD Onclarity HPV assay (Becton, Dickinson and Company). Changes to screening recommendations need to be accompanied by patient and provider outreach and education.
In a survey of more than 500 US women in 2015 after guidelines allowed for increased screening intervals after negative results, a majority of women (55.6%; 95% CI, 51.4%–59.8%) were aware that screening recommendations had changed; however, 74.1% (95% CI, 70.3%–77.7%) still believed that women should be screened annually.23 By contrast, participants in the HPV FOCAL trial, who were able to learn more about HPV-based screening, were surveyed about their willingness to undergo primary HPV testing rather than Pap testing at the conclusion of the trial.24 Of the participants, 63% were comfortable with primary HPV testing, and 54% were accepting of an extended screening interval of 4 to 5 years.24
Continue to: p16/Ki-67 dual-stain cytology...
p16/Ki-67 dual-stain cytology
An additional tool for triaging HPV-positive patients is the p16/Ki-67 dual stain test (CINtec Plus Cytology; Roche), which was FDA approved in March 2020. A tumor suppressor protein, p16 is found to be overexpressed by HPV oncogenic activity, and Ki-67 is a marker of cellular proliferation. Coexpression of p16 and Ki-67 indicates a loss of cell cycle regulation and is a hallmark of neoplastic transformation. When positive, this test is supportive of active HPV infection and of a high-grade lesion. While the dual stain test is not yet formally incorporated into triage algorithms by national guidelines, it has demonstrated efficacy in detecting CIN 3+
In the IMPACT trial, nearly 5,000 HPV-positive patients underwent p16/Ki-67 dual stain testing compared with cytology and HPV genotyping.25 The sensitivity of dual stain for CIN 3+ was 91.9% (95% CI, 86.1%–95.4%) in HPV 16/18–positive and 86.0% (95% CI, 77.5%–91.6%) in the 12 other genotypes. Using dual stain testing alone to triage HPV-positive results showed significantly higher sensitivity but lower specificity than using cytology alone to triage HPV-positive results. Importantly, triage with dual stain testing alone would have referred significantly fewer women to colposcopy than HPV 16/18 genotyping with cytology triage for the 12 other genotypes (48.6% vs 56.0%; P< .0001).
Self-sampling methods: An approach for potentially improving access to screening
One technology that may help bridge gaps in access to cervical cancer screening is self-collected HPV testing, which would preclude the need for a clinician-performed pelvic exam. At present, no self-sampling method is approved by the FDA. However, many studies have examined the efficacy and safety of various self-sampling kits.26
One randomized controlled trial in the Netherlands compared sensitivity and specificity of CIN 2+ detection in patient-collected versus clinician-collected swabs.27 After a median follow-up of 20 months, the sensitivity and specificity of HPV testing did not differ between the patient-collected and the clinician-collected groups (specificity 100%; 95% CI, 0.91–1.08; sensitivity 96%; 95% CI, 0.90–1.03).27 This analysis did not include patients who did not return their self-collected sample, which leaves the question of whether self-sampling may exacerbate issues with patients who are lost to follow-up.
In a study performed in the United States, 16,590 patients who were overdue for cervical cancer screening were randomly assigned to usual care reminders (annual mailed reminders and phone calls from clinics) or to the addition of a mailed HPV self-sampling test kit.28 While the study did not demonstrate significant difference in the detection of overall CIN 2+ between the 2 groups, screening uptake was higher in the self-sampling kit group than in the usual care reminders group (RR, 1.51; 95% CI, 1.43–1.60), and the number of abnormal screens that warranted colposcopy referral was similar between the 2 groups (36.4% vs 36.8%).28 In qualitative interviews of the participants of this trial, patients who were sent at-home self-sampling kits found that the convenience of at-home testing lowered barriers to scheduling an in-office appointment.29 The hope is that self-sampling methods will expand access of cervical cancer screening to vulnerable populations that face significant barriers to having an in-office pelvic exam.
It is important to note that self-collection and self-sample testing requires multidisciplinary systems for processing results and assuring necessary patient follow-up. Implementing and disseminating such a program has been well tested only in developed countries27,30 with universal health care systems or within an integrated care delivery system. Bringing such technology broadly to the United States and less developed countries will require continued commitment to increasing laboratory capacity, a central electronic health record or system for monitoring results, educational materials for clinicians and patients, and expanding insurance reimbursement for such testing.
HPV vaccination rates must increase
While we continue to investigate which screening methods will most improve our secondary prevention of cervical cancer, our path to increasing primary prevention of cervical cancer is clear: We must increase rates of HPV vaccination. The 9-valent HPV vaccine is FDA approved for use in all patients aged 9 to 45 years.
The American College of Obstetricians and Gynecologists and other organizations recommend HPV vaccination between the ages of 9 and 13, and a “catch-up period” from ages 13 to 26 in which patients previously not vaccinated should receive the vaccine.31 Initiation of the vaccine course earlier (ages 9–10) compared with later (ages 11–12) is correlated with higher overall completion rates by age 15 and has been suggested to be associated with a stronger immune response.32
A study from Sweden found that HPV vaccination before age 17 was most strongly correlated with the lowest rates of cervical cancer, although vaccination between ages 17 and 30 still significantly decreased the risk of cervical cancer compared with those who were unvaccinated.33
Overall HPV vaccination rates in the United States continue to improve, with 58.6%34 of US adolescents having completed vaccination in 2020. However, these rates still are significantly lower than those in many other developed countries, including Australia, which had a complete vaccination rate of 80.5% in 2020.35 Continued disparities in vaccination rates could be contributing to the rise in cervical cancer among certain groups, such as American Indian and Alaska Native populations.5
Work—and innovations—must continue
In conclusion, the incidence of cervical cancer in the United States continues to decrease, although at disparate rates among marginalized populations. To ensure that we are working toward eliminating cervical cancer for all patients, we must continue efforts to eliminate disparities in health access. Continued innovations, including primary HPV testing and self-collection samples, may contribute to lowering barriers to all patients being able to access the preventative care they need. ●
CASE Intervention approaches for decreasing the risk of cervical cancer
A 25-year-old woman presents to your practice for routine examination. She has never undergone cervical cancer screening or received the human papillomavirus (HPV) vaccine series. The patient has had 3 lifetime sexual partners and currently uses condoms as contraception. What interventions are appropriate to offer this patient to decrease her risk of cervical cancer? Choose as many that may apply:
1. cervical cytology with reflex HPV testing
2. cervical cytology with HPV cotesting
3. primary HPV testing
4. HPV vaccine series (3 doses)
5. all of the above
The answer is number 5, all of the above.
Choices 1, 2, and 3 are acceptable methods of cervical cancer screening for this patient. Catch-up HPV vaccination should be offered as well.
Equitable preventive care is needed
Cervical cancer is a unique cancer because it has a known preventative strategy. HPV vaccination, paired with cervical screening and management of abnormal results, has contributed to decreased rates of cervical cancer in the United States, from 13,914 cases in 1999 to 12,795 cases in 2019.1 In less-developed countries, however, cervical cancer continues to be a leading cause of mortality, with 90% of cervical cancer deaths in 2020 occurring in low- and middle-income countries.2
Disparate outcomes in cervical cancer are often a reflection of disparities in health access. Within the United States, Black women have a higher incidence of cervical cancer, advanced-stage disease, and mortality from cervical cancer than White women.3,4 Furthermore, the incidence of cervical cancer increased among American Indian and Alaska Native people between 2000 and 2019.5 The rate for patients who are overdue for cervical cancer screening is higher among Asian and Hispanic patients compared with non-Hispanic White patients (31.4% vs 20.1%; P=.01) and among patients who identify as LGBTQ+ compared with patients who identify as heterosexual (32.0% vs 22.2%; P<.001).6 Younger patients have a significantly higher rate for overdue screening compared with their older counterparts (29.1% vs 21.1%; P<.001), as do uninsured patients compared with those who are privately insured (41.7% vs 18.1%; P<.001). Overall, the proportion of women without up-to-date screening increased significantly from 2005 to 2019 (14.4% vs 23.0%; P<.001).6
Unfortunately, despite a known strategy to eliminate cervical cancer, we are not accomplishing equitable preventative care. Barriers to care can include patient-centered issues, such as fear of cancer or of painful evaluations, lack of trust in the health care system, and inadequate understanding of the benefits of cancer prevention, in addition to systemic and structural barriers. As we assess new technologies, one of our most important goals is to consider how such innovations can increase health access—whether through increasing ease and acceptability of testing or by creating more effective screening tests.
Updates to cervical screening guidance
In 2020, the American Cancer Society (ACS) updated its cervical screening guidelines to start screening at age 25 years with the “preferred” strategy of HPV primary testing every 5 years.7 By contrast, the US Preventive Services Task Force (USPSTF) continues to recommend 1 of 3 methods: cytology alone every 3 years; cytology alone every 3 years between ages 21 and 29 followed by cytology and HPV cotesting every 5 years at age 30 or older; or high-risk HPV testing alone every 5 years (TABLE).8

To successfully prevent cervical cancer, abnormal results are managed by performing either colposcopy with biopsy, immediate treatment, or close surveillance based on the risk of developing cervical intraepithelial neoplasia (CIN) 3 or worse. A patient’s risk is determined based on both current and prior test results. The ASCCP (American Society for Colposcopy and Cervical Pathology) transitioned to risk-based management guidelines in 2019 and has both an app and a web-based risk assessment tool available for clinicians (https://www.asccp.org).9
All organizations recommend stopping screening after age 65 provided there has been a history of adequate screening in the prior 10 years (defined as 2 normal cotests or 3 normal cytology tests, with the most recent test within 5 years) and no history of CIN 2 or worse within the prior 25 years.10,11 Recent studies that examined the rate of cervical cancer diagnosed in patients older than 65 years have questioned whether patients should continue screening beyond 65.10 In the United States, 20% of cervical cancer still occurs in women older than age 65.11 One reason may be that many women have not met the requirement for adequate and normal prior screening and may still need ongoing testing.12

Continue to: Primary HPV screening...
Primary HPV screening
Primary HPV testing means that an HPV test is performed first, and if it is positive for high-risk HPV, further testing is performed to determine next steps. This contrasts with the currently used method of obtaining cytology (Pap) first with either concurrent HPV testing or reflex HPV testing. The first HPV primary screening test was approved by the US Food and Drug Administration (FDA) in 2014.13
Multiple randomized controlled trials in Europe have demonstrated the accuracy of HPV-based screening compared with cytology in the detection of cervical cancer and its precursors.14-17 The HPV FOCAL trial demonstrated increased efficacy of primary HPV screening in the detection of CIN 2+ lesions.18 This trial recruited a total of 19,000 women, ages 25 to 65, in Canada and randomly assigned them to receive primary HPV testing or liquid-based cytology. If primary HPV testing was negative, participants would return in 48 months for cytology and HPV cotesting. If primary liquid-based cytology testing was negative, participants would return at 24 months for cytology testing alone and at 48 months for cytology and HPV cotesting. Both groups had similar incidences of CIN 2+ over the study period. HPV testing was shown to detect CIN 2+ at higher rates at the time of initial screen (risk ratio [RR], 1.61; 95% confidence interval [CI], 1.24–2.09) and then significantly lower rates at the time of exit screening at 48 months (RR, 0.36; 95% CI, 0.24–0.54).18 These results demonstrated that primary HPV testing detects CIN 2+ earlier than cytology alone. In follow-up analyses, primary HPV screening missed fewer CIN 2+ diagnoses than cytology screening.19
While not as many studies have compared primary HPV testing to cytology with an HPV cotest, the current most common practice in the United States, one study performed in the United States found that a negative cytology result did not further decrease the risk of CIN 3 for HPV-negative patients (risk of CIN 3+ at 5 years: 0.16% vs 0.17%; P=0.8) and concluded that a negative HPV test was enough reassurance for a low risk of CIN 3+.20
Another study, the ATHENA trial, evaluated more than 42,000 women who were 25 years and older over a 3-year period.21 Patients underwent either primary HPV testing or combination cytology and reflex HPV (if ages 25–29) or HPV cotesting (if age 30 or older). Primary HPV testing was found to have a sensitivity and specificity of 76.1% and 93.5%, respectively, compared with 61.7% and 94.6% for cytology with HPV cotesting, but it also increased the total number of colposcopies performed.21
Subsequent management of a primary HPV-positive result can be triaged using genotyping, cytology, or a combination of both. FDA-approved HPV screening tests provide genotyping and current management guidelines use genotyping to triage positive HPV results into HPV 16, 18, or 1 of 12 other high-risk HPV genotypes.
In the ATHENA trial, the 3-year incidence of CIN 3+ for HPV 16/18-positive results was 21.16% (95% CI, 18.39%–24.01%) compared with 5.4% (95% CI, 4.5%–6.4%) among patients with an HPV test positive for 1 of the other HPV genotypes.21 While a patient with an HPV result positive for HPV 16/18 should directly undergo colposcopy, clinical guidance for an HPV-positive result for one of the other genotypes suggests using reflex cytology to triage patients. The ASCCP recommended management of primary HPV testing is included in the FIGURE.22
Many barriers remain to transitioning to primary HPV testing, including laboratory test availability as well as patient and provider acceptance. At present, 2 FDA-approved primary HPV screening tests are available: the Cobas HPV test (Roche Molecular Systems, Inc) and the BD Onclarity HPV assay (Becton, Dickinson and Company). Changes to screening recommendations need to be accompanied by patient and provider outreach and education.
In a survey of more than 500 US women in 2015 after guidelines allowed for increased screening intervals after negative results, a majority of women (55.6%; 95% CI, 51.4%–59.8%) were aware that screening recommendations had changed; however, 74.1% (95% CI, 70.3%–77.7%) still believed that women should be screened annually.23 By contrast, participants in the HPV FOCAL trial, who were able to learn more about HPV-based screening, were surveyed about their willingness to undergo primary HPV testing rather than Pap testing at the conclusion of the trial.24 Of the participants, 63% were comfortable with primary HPV testing, and 54% were accepting of an extended screening interval of 4 to 5 years.24
Continue to: p16/Ki-67 dual-stain cytology...
p16/Ki-67 dual-stain cytology
An additional tool for triaging HPV-positive patients is the p16/Ki-67 dual stain test (CINtec Plus Cytology; Roche), which was FDA approved in March 2020. A tumor suppressor protein, p16 is found to be overexpressed by HPV oncogenic activity, and Ki-67 is a marker of cellular proliferation. Coexpression of p16 and Ki-67 indicates a loss of cell cycle regulation and is a hallmark of neoplastic transformation. When positive, this test is supportive of active HPV infection and of a high-grade lesion. While the dual stain test is not yet formally incorporated into triage algorithms by national guidelines, it has demonstrated efficacy in detecting CIN 3+
In the IMPACT trial, nearly 5,000 HPV-positive patients underwent p16/Ki-67 dual stain testing compared with cytology and HPV genotyping.25 The sensitivity of dual stain for CIN 3+ was 91.9% (95% CI, 86.1%–95.4%) in HPV 16/18–positive and 86.0% (95% CI, 77.5%–91.6%) in the 12 other genotypes. Using dual stain testing alone to triage HPV-positive results showed significantly higher sensitivity but lower specificity than using cytology alone to triage HPV-positive results. Importantly, triage with dual stain testing alone would have referred significantly fewer women to colposcopy than HPV 16/18 genotyping with cytology triage for the 12 other genotypes (48.6% vs 56.0%; P< .0001).
Self-sampling methods: An approach for potentially improving access to screening
One technology that may help bridge gaps in access to cervical cancer screening is self-collected HPV testing, which would preclude the need for a clinician-performed pelvic exam. At present, no self-sampling method is approved by the FDA. However, many studies have examined the efficacy and safety of various self-sampling kits.26
One randomized controlled trial in the Netherlands compared sensitivity and specificity of CIN 2+ detection in patient-collected versus clinician-collected swabs.27 After a median follow-up of 20 months, the sensitivity and specificity of HPV testing did not differ between the patient-collected and the clinician-collected groups (specificity 100%; 95% CI, 0.91–1.08; sensitivity 96%; 95% CI, 0.90–1.03).27 This analysis did not include patients who did not return their self-collected sample, which leaves the question of whether self-sampling may exacerbate issues with patients who are lost to follow-up.
In a study performed in the United States, 16,590 patients who were overdue for cervical cancer screening were randomly assigned to usual care reminders (annual mailed reminders and phone calls from clinics) or to the addition of a mailed HPV self-sampling test kit.28 While the study did not demonstrate significant difference in the detection of overall CIN 2+ between the 2 groups, screening uptake was higher in the self-sampling kit group than in the usual care reminders group (RR, 1.51; 95% CI, 1.43–1.60), and the number of abnormal screens that warranted colposcopy referral was similar between the 2 groups (36.4% vs 36.8%).28 In qualitative interviews of the participants of this trial, patients who were sent at-home self-sampling kits found that the convenience of at-home testing lowered barriers to scheduling an in-office appointment.29 The hope is that self-sampling methods will expand access of cervical cancer screening to vulnerable populations that face significant barriers to having an in-office pelvic exam.
It is important to note that self-collection and self-sample testing requires multidisciplinary systems for processing results and assuring necessary patient follow-up. Implementing and disseminating such a program has been well tested only in developed countries27,30 with universal health care systems or within an integrated care delivery system. Bringing such technology broadly to the United States and less developed countries will require continued commitment to increasing laboratory capacity, a central electronic health record or system for monitoring results, educational materials for clinicians and patients, and expanding insurance reimbursement for such testing.
HPV vaccination rates must increase
While we continue to investigate which screening methods will most improve our secondary prevention of cervical cancer, our path to increasing primary prevention of cervical cancer is clear: We must increase rates of HPV vaccination. The 9-valent HPV vaccine is FDA approved for use in all patients aged 9 to 45 years.
The American College of Obstetricians and Gynecologists and other organizations recommend HPV vaccination between the ages of 9 and 13, and a “catch-up period” from ages 13 to 26 in which patients previously not vaccinated should receive the vaccine.31 Initiation of the vaccine course earlier (ages 9–10) compared with later (ages 11–12) is correlated with higher overall completion rates by age 15 and has been suggested to be associated with a stronger immune response.32
A study from Sweden found that HPV vaccination before age 17 was most strongly correlated with the lowest rates of cervical cancer, although vaccination between ages 17 and 30 still significantly decreased the risk of cervical cancer compared with those who were unvaccinated.33
Overall HPV vaccination rates in the United States continue to improve, with 58.6%34 of US adolescents having completed vaccination in 2020. However, these rates still are significantly lower than those in many other developed countries, including Australia, which had a complete vaccination rate of 80.5% in 2020.35 Continued disparities in vaccination rates could be contributing to the rise in cervical cancer among certain groups, such as American Indian and Alaska Native populations.5
Work—and innovations—must continue
In conclusion, the incidence of cervical cancer in the United States continues to decrease, although at disparate rates among marginalized populations. To ensure that we are working toward eliminating cervical cancer for all patients, we must continue efforts to eliminate disparities in health access. Continued innovations, including primary HPV testing and self-collection samples, may contribute to lowering barriers to all patients being able to access the preventative care they need. ●
- Centers for Disease Control and Prevention. United States Cancer Statistics: data visualizations. Trends: changes over time: cervix. Accessed January 8, 2023. https://gis.cdc.gov /Cancer/USCS/#/Trends/
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249. doi:10.3322/caac.21660.
- Francoeur AA, Liao CI, Casear MA, et al. The increasing incidence of stage IV cervical cancer in the USA: what factors are related? Int J Gynecol Cancer. 2022;32:ijgc-2022-003728. doi:10.1136/ijgc-2022-003728.
- Abdalla E, Habtemariam T, Fall S, et al. A comparative study of health disparities in cervical cancer mortality rates through time between Black and Caucasian women in Alabama and the US. Int J Stud Nurs. 2021;6:9-23. doi:10.20849/ijsn. v6i1.864.
- Bruegl AS, Emerson J, Tirumala K. Persistent disparities of cervical cancer among American Indians/Alaska natives: are we maximizing prevention tools? Gynecol Oncol. 2023;168:5661. doi:10.1016/j.ygyno.2022.11.007.
- Suk R, Hong YR, Rajan SS, et al. Assessment of US Preventive Services Task Force Guideline–Concordant cervical cancer screening rates and reasons for underscreening by age, race and ethnicity, sexual orientation, rurality, and insurance, 2005 to 2019. JAMA Netw Open. 2022;5:e2143582. doi:10.1001/ jamanetworkopen.2021.43582.
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation statement. JAMA. 2018;320:674-686. doi:10.1001/jama.2018.10897.
- Nayar R, Chhieng DC, Crothers B, et al. Moving forward—the 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors and beyond: implications and suggestions for laboratories. J Am Soc Cytopathol. 2020;9:291-303. doi:10.1016/j.jasc.2020.05.002.
- Cooley JJP, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
- National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Cervical Cancer. Accessed February 21, 2023. https://seer.cancer.gov /statfacts/html/cervix.html
- Feldman S. Screening options for preventing cervical cancer. JAMA Intern Med. 2019;179:879-880. doi:10.1001/ jamainternmed.2019.0298.
- ASCO Post Staff. FDA approves first HPV test for primary cervical cancer screening. ASCO Post. May 15, 2014. Accessed January 8, 2023. https://ascopost.com/issues/may-15-2014 /fda-approves-first-hpv-test-for-primary-cervical-cancer -screening/
- Rijkaart DC, Berkhof J, Rozendaal L, et al. Human papillomavirus testing for the detection of high-grade cervical intraepithelial neoplasia and cancer: final results of the POBASCAM randomised controlled trial. Lancet Oncol. 2012;13:78-88. doi:10.1016/S1470-2045(11)70296-0.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249-257. doi:10.1016/S1470-2045(09)70360-2.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol. 2009;10:672-682. doi:10.1016/S1470-2045(09)70156-1.
- Bulkmans NWJ, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year followup of a randomised controlled implementation trial. Lancet. 2007;370:1764-1772. doi:10.1016/S0140-6736(07)61450-0.
- Ogilvie GS, Van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52. doi:10.1001/jama.2018.7464.
- Gottschlich A, Gondara L, Smith LW, et al. Human papillomavirus‐based screening at extended intervals missed fewer cervical precancers than cytology in the HPV For Cervical Cancer (HPV FOCAL) trial. Int J Cancer. 2022;151:897-905. doi:10.1002/ijc.34039.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12:663672. doi:10.1016/S1470-2045(11)70145-0.
- Wright TC, Stoler MH, Behrens CM, et al. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136:189-197. doi:10.1016/j.ygyno.2014.11.076
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125:330-337. doi:10.1097/AOG.0000000000000669.
- Silver MI, Rositch AF, Burke AE, et al. Patient concerns about human papillomavirus testing and 5-year intervals in routine cervical cancer screening. Obstet Gynecol. 2015;125:317-329. doi:10.1097/AOG.0000000000000638.
- Smith LW, Racey CS, Gondara L, et al. Women’s acceptability of and experience with primary human papillomavirus testing for cervical screening: HPV FOCAL trial cross-sectional online survey results. BMJ Open. 2021;11:e052084. doi:10.1136/bmjopen-2021-052084.
- Wright TC, Stoler MH, Ranger-Moore J, et al. Clinical validation of p16/Ki-67 dual-stained cytology triage of HPV-positive women: results from the IMPACT trial. Int J Cancer. 2022;150:461-471. doi:10.1002/ijc.33812.
- Yeh PT, Kennedy CE, De Vuyst H, et al. Self-sampling for human papillomavirus (HPV) testing: a systematic review and meta-analysis. BMJ Global Health. 2019;4:e001351. doi:10.1136/bmjgh-2018-001351.
- Polman NJ, Ebisch RMF, Heideman DAM, et al. Performance of human papillomavirus testing on self-collected versus clinician-collected samples for the detection of cervical intraepithelial neoplasia of grade 2 or worse: a randomised, paired screen-positive, non-inferiority trial. Lancet Oncol. 2019;20:229-238. doi:10.1016/S1470-2045(18)30763-0.
- Winer RL, Lin J, Tiro JA, et al. Effect of mailed human papillomavirus test kits vs usual care reminders on cervical cancer screening uptake, precancer detection, and treatment: a randomized clinical trial. JAMA Netw Open. 2019;2:e1914729. doi:10.1001/jamanetworkopen.2019.14729.
- Tiro JA, Betts AC, Kimbel K, et al. Understanding patients’ perspectives and information needs following a positive home human papillomavirus self-sampling kit result. J Womens Health (Larchmt). 2019;28:384-392. doi:10.1089/ jwh.2018.7070.
- Knauss T, Hansen BT, Pedersen K, et al. The cost-effectiveness of opt-in and send-to-all HPV self-sampling among long-term non-attenders to cervical cancer screening in Norway: the Equalscreen randomized controlled trial. Gynecol Oncol. 2023;168:39-47. doi:10.1016/j.ygyno.2022.10.027.
- ACOG committee opinion no. 809. Human papillomavirus vaccination: correction. Obstet Gynecol. 2022;139:345. doi:10.1097/AOG.0000000000004680.
- St Sauver JL, Finney Rutten LJF, Ebbert JO, et al. Younger age at initiation of the human papillomavirus (HPV) vaccination series is associated with higher rates of on-time completion. Prev Med. 2016;89:327-333. doi:10.1016/j.ypmed.2016.02.039.
- Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:13401348. doi:10.1056/NEJMoa1917338.
- Pingali C, Yankey D, Elam-Evans LD, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years — United States, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:1183-1190. doi:10.15585/ mmwr.mm7035a1.
- National Centre for Immunisation Research and Surveillance Australia. Annual Immunisation Coverage Report 2020. November 29, 2021. Accessed March 1, 2023. https://ncirs .org.au/sites/default/files/2021-11/NCIRS%20Annual%20 Immunisation%20Coverage%20Report%202020_FINAL.pdf
- Leung SOA, Feldman S. 2022 Update on cervical disease. OBG Manag. 2022;34(5):16-17, 22-24, 26, 28. doi:10.12788/ obgm.0197.
- Centers for Disease Control and Prevention. United States Cancer Statistics: data visualizations. Trends: changes over time: cervix. Accessed January 8, 2023. https://gis.cdc.gov /Cancer/USCS/#/Trends/
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249. doi:10.3322/caac.21660.
- Francoeur AA, Liao CI, Casear MA, et al. The increasing incidence of stage IV cervical cancer in the USA: what factors are related? Int J Gynecol Cancer. 2022;32:ijgc-2022-003728. doi:10.1136/ijgc-2022-003728.
- Abdalla E, Habtemariam T, Fall S, et al. A comparative study of health disparities in cervical cancer mortality rates through time between Black and Caucasian women in Alabama and the US. Int J Stud Nurs. 2021;6:9-23. doi:10.20849/ijsn. v6i1.864.
- Bruegl AS, Emerson J, Tirumala K. Persistent disparities of cervical cancer among American Indians/Alaska natives: are we maximizing prevention tools? Gynecol Oncol. 2023;168:5661. doi:10.1016/j.ygyno.2022.11.007.
- Suk R, Hong YR, Rajan SS, et al. Assessment of US Preventive Services Task Force Guideline–Concordant cervical cancer screening rates and reasons for underscreening by age, race and ethnicity, sexual orientation, rurality, and insurance, 2005 to 2019. JAMA Netw Open. 2022;5:e2143582. doi:10.1001/ jamanetworkopen.2021.43582.
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation statement. JAMA. 2018;320:674-686. doi:10.1001/jama.2018.10897.
- Nayar R, Chhieng DC, Crothers B, et al. Moving forward—the 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors and beyond: implications and suggestions for laboratories. J Am Soc Cytopathol. 2020;9:291-303. doi:10.1016/j.jasc.2020.05.002.
- Cooley JJP, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
- National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Cervical Cancer. Accessed February 21, 2023. https://seer.cancer.gov /statfacts/html/cervix.html
- Feldman S. Screening options for preventing cervical cancer. JAMA Intern Med. 2019;179:879-880. doi:10.1001/ jamainternmed.2019.0298.
- ASCO Post Staff. FDA approves first HPV test for primary cervical cancer screening. ASCO Post. May 15, 2014. Accessed January 8, 2023. https://ascopost.com/issues/may-15-2014 /fda-approves-first-hpv-test-for-primary-cervical-cancer -screening/
- Rijkaart DC, Berkhof J, Rozendaal L, et al. Human papillomavirus testing for the detection of high-grade cervical intraepithelial neoplasia and cancer: final results of the POBASCAM randomised controlled trial. Lancet Oncol. 2012;13:78-88. doi:10.1016/S1470-2045(11)70296-0.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249-257. doi:10.1016/S1470-2045(09)70360-2.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol. 2009;10:672-682. doi:10.1016/S1470-2045(09)70156-1.
- Bulkmans NWJ, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year followup of a randomised controlled implementation trial. Lancet. 2007;370:1764-1772. doi:10.1016/S0140-6736(07)61450-0.
- Ogilvie GS, Van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52. doi:10.1001/jama.2018.7464.
- Gottschlich A, Gondara L, Smith LW, et al. Human papillomavirus‐based screening at extended intervals missed fewer cervical precancers than cytology in the HPV For Cervical Cancer (HPV FOCAL) trial. Int J Cancer. 2022;151:897-905. doi:10.1002/ijc.34039.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12:663672. doi:10.1016/S1470-2045(11)70145-0.
- Wright TC, Stoler MH, Behrens CM, et al. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136:189-197. doi:10.1016/j.ygyno.2014.11.076
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125:330-337. doi:10.1097/AOG.0000000000000669.
- Silver MI, Rositch AF, Burke AE, et al. Patient concerns about human papillomavirus testing and 5-year intervals in routine cervical cancer screening. Obstet Gynecol. 2015;125:317-329. doi:10.1097/AOG.0000000000000638.
- Smith LW, Racey CS, Gondara L, et al. Women’s acceptability of and experience with primary human papillomavirus testing for cervical screening: HPV FOCAL trial cross-sectional online survey results. BMJ Open. 2021;11:e052084. doi:10.1136/bmjopen-2021-052084.
- Wright TC, Stoler MH, Ranger-Moore J, et al. Clinical validation of p16/Ki-67 dual-stained cytology triage of HPV-positive women: results from the IMPACT trial. Int J Cancer. 2022;150:461-471. doi:10.1002/ijc.33812.
- Yeh PT, Kennedy CE, De Vuyst H, et al. Self-sampling for human papillomavirus (HPV) testing: a systematic review and meta-analysis. BMJ Global Health. 2019;4:e001351. doi:10.1136/bmjgh-2018-001351.
- Polman NJ, Ebisch RMF, Heideman DAM, et al. Performance of human papillomavirus testing on self-collected versus clinician-collected samples for the detection of cervical intraepithelial neoplasia of grade 2 or worse: a randomised, paired screen-positive, non-inferiority trial. Lancet Oncol. 2019;20:229-238. doi:10.1016/S1470-2045(18)30763-0.
- Winer RL, Lin J, Tiro JA, et al. Effect of mailed human papillomavirus test kits vs usual care reminders on cervical cancer screening uptake, precancer detection, and treatment: a randomized clinical trial. JAMA Netw Open. 2019;2:e1914729. doi:10.1001/jamanetworkopen.2019.14729.
- Tiro JA, Betts AC, Kimbel K, et al. Understanding patients’ perspectives and information needs following a positive home human papillomavirus self-sampling kit result. J Womens Health (Larchmt). 2019;28:384-392. doi:10.1089/ jwh.2018.7070.
- Knauss T, Hansen BT, Pedersen K, et al. The cost-effectiveness of opt-in and send-to-all HPV self-sampling among long-term non-attenders to cervical cancer screening in Norway: the Equalscreen randomized controlled trial. Gynecol Oncol. 2023;168:39-47. doi:10.1016/j.ygyno.2022.10.027.
- ACOG committee opinion no. 809. Human papillomavirus vaccination: correction. Obstet Gynecol. 2022;139:345. doi:10.1097/AOG.0000000000004680.
- St Sauver JL, Finney Rutten LJF, Ebbert JO, et al. Younger age at initiation of the human papillomavirus (HPV) vaccination series is associated with higher rates of on-time completion. Prev Med. 2016;89:327-333. doi:10.1016/j.ypmed.2016.02.039.
- Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:13401348. doi:10.1056/NEJMoa1917338.
- Pingali C, Yankey D, Elam-Evans LD, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years — United States, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:1183-1190. doi:10.15585/ mmwr.mm7035a1.
- National Centre for Immunisation Research and Surveillance Australia. Annual Immunisation Coverage Report 2020. November 29, 2021. Accessed March 1, 2023. https://ncirs .org.au/sites/default/files/2021-11/NCIRS%20Annual%20 Immunisation%20Coverage%20Report%202020_FINAL.pdf
- Leung SOA, Feldman S. 2022 Update on cervical disease. OBG Manag. 2022;34(5):16-17, 22-24, 26, 28. doi:10.12788/ obgm.0197.
Increased cancer in military pilots and ground crew: Pentagon
“Military aircrew and ground crew were overall more likely to be diagnosed with cancer, but less likely to die from cancer compared to the U.S. population,” the report concludes.
The study involved 156,050 aircrew and 737,891 ground crew. Participants were followed between 1992 and 2017. Both groups were predominantly male and non-Hispanic.
Data on cancer incidence and mortality for these two groups were compared with data from groups of similar age in the general population through use of the Surveillance, Epidemiology, and End Results (SEER) Database of the National Cancer Institute.
For aircrew, the study found an 87% higher rate of melanoma, a 39% higher rate of thyroid cancer, a 16% higher rate of prostate cancer, and a 24% higher rate of cancer for all sites combined.
A higher rate of melanoma and prostate cancer among aircrew has been reported previously, but the increased rate of thyroid cancer is a new finding, the authors note.
The uptick in melanoma has also been reported in studies of civilian pilots and cabin crew. It has been attributed to exposure to hazardous ultraviolet and cosmic radiation.
For ground crew members, the analysis found a 19% higher rate of cancers of the brain and nervous system, a 15% higher rate of thyroid cancer, a 9% higher rate of melanoma and of kidney and renal pelvis cancers, and a 3% higher rate of cancer for all sites combined.
There is little to compare these findings with: This is the first time that cancer risk has been evaluated in such a large population of military ground crew.
Lower rates of cancer mortality
In contrast to the increase in cancer incidence, the report found a decrease in cancer mortality.
When compared with a demographically similar U.S. population, the mortality rate among aircrew was 56% lower for all cancer sites; for ground crew, the mortality rate was 35% lower.
However, the report authors emphasize that “it is important to note that the military study population was relatively young.”
The median age at the end of follow-up for the cancer incidence analysis was 41 years for aircrew and 26 years for ground crew. The median age at the end of follow-up for the cancer mortality analysis was 48 years for aircrew and 41 years for ground crew.
“Results may have differed if additional older former Service members had been included in the study, since cancer risk and mortality rates increase with age,” the authors comment.
Other studies have found an increase in deaths from melanoma as well as an increase in the incidence of melanoma. A meta-analysis published in 2019 in the British Journal of Dermatology found that airline pilots and cabin crew have about twice the risk of melanoma and other skin cancers than the general population. Pilots are also more likely to die from melanoma.
Further study underway
The findings on military air and ground crew come from phase 1 of a study that was required by Congress in the 2021 defense bill. Because the investigators found an increase in the incidence of cancer, phase 2 of the study is now necessary.
The report authors explain that phase 2 will consist of identifying the carcinogenic toxicants or hazardous materials associated with military flight operations; identifying operating environments that could be associated with increased amounts of ionizing and nonionizing radiation; identifying specific duties, dates of service, and types of aircraft flown that could have increased the risk for cancer; identifying duty locations associated with a higher incidence of cancers; identifying potential exposures through military service that are not related to aviation; and determining the appropriate age to begin screening military aircrew and ground crew for cancers.
A version of this article first appeared on Medscape.com.
“Military aircrew and ground crew were overall more likely to be diagnosed with cancer, but less likely to die from cancer compared to the U.S. population,” the report concludes.
The study involved 156,050 aircrew and 737,891 ground crew. Participants were followed between 1992 and 2017. Both groups were predominantly male and non-Hispanic.
Data on cancer incidence and mortality for these two groups were compared with data from groups of similar age in the general population through use of the Surveillance, Epidemiology, and End Results (SEER) Database of the National Cancer Institute.
For aircrew, the study found an 87% higher rate of melanoma, a 39% higher rate of thyroid cancer, a 16% higher rate of prostate cancer, and a 24% higher rate of cancer for all sites combined.
A higher rate of melanoma and prostate cancer among aircrew has been reported previously, but the increased rate of thyroid cancer is a new finding, the authors note.
The uptick in melanoma has also been reported in studies of civilian pilots and cabin crew. It has been attributed to exposure to hazardous ultraviolet and cosmic radiation.
For ground crew members, the analysis found a 19% higher rate of cancers of the brain and nervous system, a 15% higher rate of thyroid cancer, a 9% higher rate of melanoma and of kidney and renal pelvis cancers, and a 3% higher rate of cancer for all sites combined.
There is little to compare these findings with: This is the first time that cancer risk has been evaluated in such a large population of military ground crew.
Lower rates of cancer mortality
In contrast to the increase in cancer incidence, the report found a decrease in cancer mortality.
When compared with a demographically similar U.S. population, the mortality rate among aircrew was 56% lower for all cancer sites; for ground crew, the mortality rate was 35% lower.
However, the report authors emphasize that “it is important to note that the military study population was relatively young.”
The median age at the end of follow-up for the cancer incidence analysis was 41 years for aircrew and 26 years for ground crew. The median age at the end of follow-up for the cancer mortality analysis was 48 years for aircrew and 41 years for ground crew.
“Results may have differed if additional older former Service members had been included in the study, since cancer risk and mortality rates increase with age,” the authors comment.
Other studies have found an increase in deaths from melanoma as well as an increase in the incidence of melanoma. A meta-analysis published in 2019 in the British Journal of Dermatology found that airline pilots and cabin crew have about twice the risk of melanoma and other skin cancers than the general population. Pilots are also more likely to die from melanoma.
Further study underway
The findings on military air and ground crew come from phase 1 of a study that was required by Congress in the 2021 defense bill. Because the investigators found an increase in the incidence of cancer, phase 2 of the study is now necessary.
The report authors explain that phase 2 will consist of identifying the carcinogenic toxicants or hazardous materials associated with military flight operations; identifying operating environments that could be associated with increased amounts of ionizing and nonionizing radiation; identifying specific duties, dates of service, and types of aircraft flown that could have increased the risk for cancer; identifying duty locations associated with a higher incidence of cancers; identifying potential exposures through military service that are not related to aviation; and determining the appropriate age to begin screening military aircrew and ground crew for cancers.
A version of this article first appeared on Medscape.com.
“Military aircrew and ground crew were overall more likely to be diagnosed with cancer, but less likely to die from cancer compared to the U.S. population,” the report concludes.
The study involved 156,050 aircrew and 737,891 ground crew. Participants were followed between 1992 and 2017. Both groups were predominantly male and non-Hispanic.
Data on cancer incidence and mortality for these two groups were compared with data from groups of similar age in the general population through use of the Surveillance, Epidemiology, and End Results (SEER) Database of the National Cancer Institute.
For aircrew, the study found an 87% higher rate of melanoma, a 39% higher rate of thyroid cancer, a 16% higher rate of prostate cancer, and a 24% higher rate of cancer for all sites combined.
A higher rate of melanoma and prostate cancer among aircrew has been reported previously, but the increased rate of thyroid cancer is a new finding, the authors note.
The uptick in melanoma has also been reported in studies of civilian pilots and cabin crew. It has been attributed to exposure to hazardous ultraviolet and cosmic radiation.
For ground crew members, the analysis found a 19% higher rate of cancers of the brain and nervous system, a 15% higher rate of thyroid cancer, a 9% higher rate of melanoma and of kidney and renal pelvis cancers, and a 3% higher rate of cancer for all sites combined.
There is little to compare these findings with: This is the first time that cancer risk has been evaluated in such a large population of military ground crew.
Lower rates of cancer mortality
In contrast to the increase in cancer incidence, the report found a decrease in cancer mortality.
When compared with a demographically similar U.S. population, the mortality rate among aircrew was 56% lower for all cancer sites; for ground crew, the mortality rate was 35% lower.
However, the report authors emphasize that “it is important to note that the military study population was relatively young.”
The median age at the end of follow-up for the cancer incidence analysis was 41 years for aircrew and 26 years for ground crew. The median age at the end of follow-up for the cancer mortality analysis was 48 years for aircrew and 41 years for ground crew.
“Results may have differed if additional older former Service members had been included in the study, since cancer risk and mortality rates increase with age,” the authors comment.
Other studies have found an increase in deaths from melanoma as well as an increase in the incidence of melanoma. A meta-analysis published in 2019 in the British Journal of Dermatology found that airline pilots and cabin crew have about twice the risk of melanoma and other skin cancers than the general population. Pilots are also more likely to die from melanoma.
Further study underway
The findings on military air and ground crew come from phase 1 of a study that was required by Congress in the 2021 defense bill. Because the investigators found an increase in the incidence of cancer, phase 2 of the study is now necessary.
The report authors explain that phase 2 will consist of identifying the carcinogenic toxicants or hazardous materials associated with military flight operations; identifying operating environments that could be associated with increased amounts of ionizing and nonionizing radiation; identifying specific duties, dates of service, and types of aircraft flown that could have increased the risk for cancer; identifying duty locations associated with a higher incidence of cancers; identifying potential exposures through military service that are not related to aviation; and determining the appropriate age to begin screening military aircrew and ground crew for cancers.
A version of this article first appeared on Medscape.com.
Ovarian cancer risk lower with daily aspirin, despite genetics
new research suggests.
The study found that daily or almost daily aspirin use was associated with a 13% reduction in ovarian cancer risk, which was not modified by an individual’s polygenic score (PGS).
“Our findings suggest that frequent use of aspirin is associated with reduced ovarian cancer risk, regardless of whether a woman has lower or higher genetic susceptibility to ovarian cancer, as predicted by a set of known, common risk variants,” said lead author Lauren M. Hurwitz, PhD, MHS, division of cancer epidemiology and genetics at the National Cancer Institute, Rockville, Md.
The study was published online in JAMA Network Open.
Patients diagnosed with ovarian cancer face difficult survival odds, which make preventive strategies especially important. Evidence suggests that frequent aspirin use can reduce the risk for ovarian cancer by about 13%, but it’s unclear whether genetic factors change those odds.
Although promising for chemoprevention, aspirin use can also come with downsides, including gastric ulcer and hemorrhagic stroke, which is why identifying and targeting individuals at higher risk for ovarian cancer who may benefit from frequent aspirin use is important.
In the current analysis, Dr. Hurwitz and colleagues used a PGS to determine whether the protective effects of daily or near-daily aspirin use for 6 months or more could be modified by genetics.
The study was a pooled analysis of eight case-controlled studies from the Ovarian Cancer Association Consortium conducted in the United Kingdom, the United States, and Australia over a 14-year period. The researchers looked at genetic data and data on frequent aspirin use among 4,476 case patients with nonmucinous ovarian cancer (average age, 57) and 6,659 control participants (average age, 58). Overall, 575 patients (13%) and 1,030 controls (15%) reported frequent aspirin use.
The authors used a PGS previously developed using 22 single-nucleotide variants. Because this PGS was developed for nonmucinous epithelial ovarian cancer, only these patients were included in the analysis.
Consistent with previous evidence, the authors found that frequent aspirin use was associated with a 13% lower risk for nonmucinous ovarian cancer (odds ratio, 0.87). And, notably, this association did not differ by PGS. Risk reductions were greatest for high-grade serous (OR, 0.83) and endometrioid tumors (OR, 0.73), with no evidence that PGS modified this association.
Overall, “we observed consistent protective associations between frequent aspirin use and nonmucinous ovarian cancer across strata of genetic susceptibility to ovarian cancer,” the authors conclude. “This work expands on the evidence base to suggest that chemoprevention programs could target individuals at higher risk of ovarian cancer.”
However, the authors noted that they were unable to test for effect modifications by specific pathogenic variants, such as BRCA1 or BRCA2.
“Our study did not address whether aspirin use is associated with reduced ovarian cancer risk among BRCA or other pathogenic variant carriers, and so our findings should not be used to inform discussions around aspirin use for these specific high-risk groups,” Dr. Hurwitz said. “Women with higher genetic susceptibility to ovarian cancer based on these common risk variants should discuss with their doctor the benefits and harms of taking aspirin for disease prevention.”
This study was supported by a grant from the DoD Ovarian Cancer Research Program. Dr. Hurwitz reports no relevant financial relationships, but several coauthors did report funding and support.
A version of this article first appeared on Medscape.com.
new research suggests.
The study found that daily or almost daily aspirin use was associated with a 13% reduction in ovarian cancer risk, which was not modified by an individual’s polygenic score (PGS).
“Our findings suggest that frequent use of aspirin is associated with reduced ovarian cancer risk, regardless of whether a woman has lower or higher genetic susceptibility to ovarian cancer, as predicted by a set of known, common risk variants,” said lead author Lauren M. Hurwitz, PhD, MHS, division of cancer epidemiology and genetics at the National Cancer Institute, Rockville, Md.
The study was published online in JAMA Network Open.
Patients diagnosed with ovarian cancer face difficult survival odds, which make preventive strategies especially important. Evidence suggests that frequent aspirin use can reduce the risk for ovarian cancer by about 13%, but it’s unclear whether genetic factors change those odds.
Although promising for chemoprevention, aspirin use can also come with downsides, including gastric ulcer and hemorrhagic stroke, which is why identifying and targeting individuals at higher risk for ovarian cancer who may benefit from frequent aspirin use is important.
In the current analysis, Dr. Hurwitz and colleagues used a PGS to determine whether the protective effects of daily or near-daily aspirin use for 6 months or more could be modified by genetics.
The study was a pooled analysis of eight case-controlled studies from the Ovarian Cancer Association Consortium conducted in the United Kingdom, the United States, and Australia over a 14-year period. The researchers looked at genetic data and data on frequent aspirin use among 4,476 case patients with nonmucinous ovarian cancer (average age, 57) and 6,659 control participants (average age, 58). Overall, 575 patients (13%) and 1,030 controls (15%) reported frequent aspirin use.
The authors used a PGS previously developed using 22 single-nucleotide variants. Because this PGS was developed for nonmucinous epithelial ovarian cancer, only these patients were included in the analysis.
Consistent with previous evidence, the authors found that frequent aspirin use was associated with a 13% lower risk for nonmucinous ovarian cancer (odds ratio, 0.87). And, notably, this association did not differ by PGS. Risk reductions were greatest for high-grade serous (OR, 0.83) and endometrioid tumors (OR, 0.73), with no evidence that PGS modified this association.
Overall, “we observed consistent protective associations between frequent aspirin use and nonmucinous ovarian cancer across strata of genetic susceptibility to ovarian cancer,” the authors conclude. “This work expands on the evidence base to suggest that chemoprevention programs could target individuals at higher risk of ovarian cancer.”
However, the authors noted that they were unable to test for effect modifications by specific pathogenic variants, such as BRCA1 or BRCA2.
“Our study did not address whether aspirin use is associated with reduced ovarian cancer risk among BRCA or other pathogenic variant carriers, and so our findings should not be used to inform discussions around aspirin use for these specific high-risk groups,” Dr. Hurwitz said. “Women with higher genetic susceptibility to ovarian cancer based on these common risk variants should discuss with their doctor the benefits and harms of taking aspirin for disease prevention.”
This study was supported by a grant from the DoD Ovarian Cancer Research Program. Dr. Hurwitz reports no relevant financial relationships, but several coauthors did report funding and support.
A version of this article first appeared on Medscape.com.
new research suggests.
The study found that daily or almost daily aspirin use was associated with a 13% reduction in ovarian cancer risk, which was not modified by an individual’s polygenic score (PGS).
“Our findings suggest that frequent use of aspirin is associated with reduced ovarian cancer risk, regardless of whether a woman has lower or higher genetic susceptibility to ovarian cancer, as predicted by a set of known, common risk variants,” said lead author Lauren M. Hurwitz, PhD, MHS, division of cancer epidemiology and genetics at the National Cancer Institute, Rockville, Md.
The study was published online in JAMA Network Open.
Patients diagnosed with ovarian cancer face difficult survival odds, which make preventive strategies especially important. Evidence suggests that frequent aspirin use can reduce the risk for ovarian cancer by about 13%, but it’s unclear whether genetic factors change those odds.
Although promising for chemoprevention, aspirin use can also come with downsides, including gastric ulcer and hemorrhagic stroke, which is why identifying and targeting individuals at higher risk for ovarian cancer who may benefit from frequent aspirin use is important.
In the current analysis, Dr. Hurwitz and colleagues used a PGS to determine whether the protective effects of daily or near-daily aspirin use for 6 months or more could be modified by genetics.
The study was a pooled analysis of eight case-controlled studies from the Ovarian Cancer Association Consortium conducted in the United Kingdom, the United States, and Australia over a 14-year period. The researchers looked at genetic data and data on frequent aspirin use among 4,476 case patients with nonmucinous ovarian cancer (average age, 57) and 6,659 control participants (average age, 58). Overall, 575 patients (13%) and 1,030 controls (15%) reported frequent aspirin use.
The authors used a PGS previously developed using 22 single-nucleotide variants. Because this PGS was developed for nonmucinous epithelial ovarian cancer, only these patients were included in the analysis.
Consistent with previous evidence, the authors found that frequent aspirin use was associated with a 13% lower risk for nonmucinous ovarian cancer (odds ratio, 0.87). And, notably, this association did not differ by PGS. Risk reductions were greatest for high-grade serous (OR, 0.83) and endometrioid tumors (OR, 0.73), with no evidence that PGS modified this association.
Overall, “we observed consistent protective associations between frequent aspirin use and nonmucinous ovarian cancer across strata of genetic susceptibility to ovarian cancer,” the authors conclude. “This work expands on the evidence base to suggest that chemoprevention programs could target individuals at higher risk of ovarian cancer.”
However, the authors noted that they were unable to test for effect modifications by specific pathogenic variants, such as BRCA1 or BRCA2.
“Our study did not address whether aspirin use is associated with reduced ovarian cancer risk among BRCA or other pathogenic variant carriers, and so our findings should not be used to inform discussions around aspirin use for these specific high-risk groups,” Dr. Hurwitz said. “Women with higher genetic susceptibility to ovarian cancer based on these common risk variants should discuss with their doctor the benefits and harms of taking aspirin for disease prevention.”
This study was supported by a grant from the DoD Ovarian Cancer Research Program. Dr. Hurwitz reports no relevant financial relationships, but several coauthors did report funding and support.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Scientists create ‘vagina on a chip’: What to know
For years, women’s health advocates have argued that far more research is needed on women’s bodies and health. The world’s first-ever “vagina on a chip,” recently developed at Harvard’s Wyss Institute for Biologically Inspired Engineering in Boston, could go a long way to making that happen.
“Women’s health has not received the attention it deserves,” says Don Ingber, MD, PhD, who led the team that created the vagina chip. The advance quickly drew media attention after it was reported in the journal Microbiome. But researchers hope for more than headlines. They see the chip as a way to facilitate vaginal health research and open the door to vital new treatments.
By now, you may have heard of “organs on chips”: tiny devices about the size of a flash drive that are designed to mimic the biological activity of human organs. These glass chips contain living human cells within grooves that allow the passage of fluid, to either maintain or disrupt the cells’ function. So far, Dr. Ingber and his team at the Wyss Institute have developed more than 15 organ chip models, including chips that mimic the lung, intestine, kidney, and bone marrow.
The idea to develop a vagina chip grew out of research, funded by the Gates Foundation, on a childhood disease called environmental enteric dysfunction, an intestinal disease most commonly found in low-resource nations that is the second leading cause of death in children under 5. That’s when Dr. Ingber discovered just how much the child’s microbiome influences this disease.
Stemming from that work, the Gates Foundation turned its attention to newborn health – in particular, the impact of bacterial vaginosis, an imbalance in the vagina’s bacterial makeup. Bacterial vaginosis occurs in one out of four women worldwide and has been linked to premature birth as well as HIV, HPV persistence, and cervical cancer.
The goal was to test “live biotherapeutic products,” or living microbes like probiotics, that might restore the vagina’s microbiome to health.
No other preclinical model exists to perform tests like that, says Dr. Ingber.
“The vagina chip is a way to help make some advances,” he says.
The Gates Foundation recognized that women’s reproductive health is a major issue, not only in low-income nations, but everywhere around the world. As the project evolved, Dr. Ingber began to hear from female colleagues about how neglected women’s reproductive health is in medical science.
“It is something I became sensitive to and realized this is just the starting point,” Dr. Ingber says.
Take bacterial vaginosis, for example. Since 1982, treatment has revolved around the same two antibiotics. That’s partly because there is no animal model to study. No other species has the same vaginal bacterial community as humans do.
That makes developing any new therapy “incredibly challenging,” explains Caroline Mitchell, MD, MPH, an ob.gyn. at Massachusetts General Hospital, Boston, and a member of the consortium.
It turns out, replicating the vagina in a lab dish is, to use the technical term, very hard.
“That’s where a vagina chip offers an opportunity,” Dr. Mitchell says. “It’s not super-high throughput, but it’s way more high throughput than a [human] clinical trial.”
As such, the vagina chip could help scientists find new treatments much faster.
Like Dr. Ingber, Dr. Mitchell also sees the chip as a way to bring more attention to the largely unmet needs in female reproductive medicine.
“Women’s reproductive health has been under-resourced, under-prioritized, and largely disregarded for decades,” she says. And the time may be ripe for change: Dr. Mitchell says she was encouraged by the National Institutes of Health’s Advancing NIH Research on the Health of Women conference, held in 2021 in response to a congressional request to address women’s health research efforts.
Beyond bacterial vaginosis, Dr. Mitchell imagines the chip could help scientists find new treatments for vaginal yeast infection (candidiasis), chlamydia, and endometriosis. As with bacterial vaginosis, medicines for vaginal yeast infections have not advanced in decades, Dr. Mitchell says. Efforts to develop a vaccine for chlamydia – which can cause permanent damage to a woman’s reproductive system – have dragged on for many years. And endometriosis, an often painful condition in which the tissue that makes up the uterine lining grows outside the uterus, remains under-researched despite affecting 10% of childbearing-age women.
While some mouse models are used in chlamydia research, it’s hard to say if they’ll translate to humans, given the vaginal and cervical bacterial differences.
“Our understanding of the basic physiology of the environment of the vagina and cervix is another area where we’re woefully ignorant,” Dr. Mitchell says.
To that end, Dr. Ingber’s team is developing more complex chips mimicking the vagina and the cervix. One of his team members wants to use the chips to study infertility. The researchers have already used the chips to see how bacterial vaginosis and mucous changes impact the way sperm migrates up the reproductive tract.
The lab is now linking vagina and cervix chips together to study viral infections of the cervix, like HPV, and all types of bacterial diseases of the vaginal tract. By applying cervical mucus to the vagina chip, they hope to learn more about how female reproductive tissues respond to infection and inflammation.
“I always say that organ chips are like synthetic biology at the cell tissue and organ level,” says Dr. Ingber. “You start simple and see if you [can] mimic a clinical situation.”
As they make the chips more complex – perhaps by adding blood vessel cells and female hormones – Dr. Ingber foresees being able to study the response to hormonal changes during the menstrual cycle.
“We can begin to explore the effects of cycling over time as well as other types of hormonal effects,” he says.
Dr. Ingber also envisions linking the vagina chip to other organ chips – he’s already succeeded in linking eight different organ types together. But for now, the team hopes the vagina chip will enhance our understanding of basic female reproductive biology and speed up the process of developing new treatments for women’s health.
A version of this article first appeared on WebMD.com.
For years, women’s health advocates have argued that far more research is needed on women’s bodies and health. The world’s first-ever “vagina on a chip,” recently developed at Harvard’s Wyss Institute for Biologically Inspired Engineering in Boston, could go a long way to making that happen.
“Women’s health has not received the attention it deserves,” says Don Ingber, MD, PhD, who led the team that created the vagina chip. The advance quickly drew media attention after it was reported in the journal Microbiome. But researchers hope for more than headlines. They see the chip as a way to facilitate vaginal health research and open the door to vital new treatments.
By now, you may have heard of “organs on chips”: tiny devices about the size of a flash drive that are designed to mimic the biological activity of human organs. These glass chips contain living human cells within grooves that allow the passage of fluid, to either maintain or disrupt the cells’ function. So far, Dr. Ingber and his team at the Wyss Institute have developed more than 15 organ chip models, including chips that mimic the lung, intestine, kidney, and bone marrow.
The idea to develop a vagina chip grew out of research, funded by the Gates Foundation, on a childhood disease called environmental enteric dysfunction, an intestinal disease most commonly found in low-resource nations that is the second leading cause of death in children under 5. That’s when Dr. Ingber discovered just how much the child’s microbiome influences this disease.
Stemming from that work, the Gates Foundation turned its attention to newborn health – in particular, the impact of bacterial vaginosis, an imbalance in the vagina’s bacterial makeup. Bacterial vaginosis occurs in one out of four women worldwide and has been linked to premature birth as well as HIV, HPV persistence, and cervical cancer.
The goal was to test “live biotherapeutic products,” or living microbes like probiotics, that might restore the vagina’s microbiome to health.
No other preclinical model exists to perform tests like that, says Dr. Ingber.
“The vagina chip is a way to help make some advances,” he says.
The Gates Foundation recognized that women’s reproductive health is a major issue, not only in low-income nations, but everywhere around the world. As the project evolved, Dr. Ingber began to hear from female colleagues about how neglected women’s reproductive health is in medical science.
“It is something I became sensitive to and realized this is just the starting point,” Dr. Ingber says.
Take bacterial vaginosis, for example. Since 1982, treatment has revolved around the same two antibiotics. That’s partly because there is no animal model to study. No other species has the same vaginal bacterial community as humans do.
That makes developing any new therapy “incredibly challenging,” explains Caroline Mitchell, MD, MPH, an ob.gyn. at Massachusetts General Hospital, Boston, and a member of the consortium.
It turns out, replicating the vagina in a lab dish is, to use the technical term, very hard.
“That’s where a vagina chip offers an opportunity,” Dr. Mitchell says. “It’s not super-high throughput, but it’s way more high throughput than a [human] clinical trial.”
As such, the vagina chip could help scientists find new treatments much faster.
Like Dr. Ingber, Dr. Mitchell also sees the chip as a way to bring more attention to the largely unmet needs in female reproductive medicine.
“Women’s reproductive health has been under-resourced, under-prioritized, and largely disregarded for decades,” she says. And the time may be ripe for change: Dr. Mitchell says she was encouraged by the National Institutes of Health’s Advancing NIH Research on the Health of Women conference, held in 2021 in response to a congressional request to address women’s health research efforts.
Beyond bacterial vaginosis, Dr. Mitchell imagines the chip could help scientists find new treatments for vaginal yeast infection (candidiasis), chlamydia, and endometriosis. As with bacterial vaginosis, medicines for vaginal yeast infections have not advanced in decades, Dr. Mitchell says. Efforts to develop a vaccine for chlamydia – which can cause permanent damage to a woman’s reproductive system – have dragged on for many years. And endometriosis, an often painful condition in which the tissue that makes up the uterine lining grows outside the uterus, remains under-researched despite affecting 10% of childbearing-age women.
While some mouse models are used in chlamydia research, it’s hard to say if they’ll translate to humans, given the vaginal and cervical bacterial differences.
“Our understanding of the basic physiology of the environment of the vagina and cervix is another area where we’re woefully ignorant,” Dr. Mitchell says.
To that end, Dr. Ingber’s team is developing more complex chips mimicking the vagina and the cervix. One of his team members wants to use the chips to study infertility. The researchers have already used the chips to see how bacterial vaginosis and mucous changes impact the way sperm migrates up the reproductive tract.
The lab is now linking vagina and cervix chips together to study viral infections of the cervix, like HPV, and all types of bacterial diseases of the vaginal tract. By applying cervical mucus to the vagina chip, they hope to learn more about how female reproductive tissues respond to infection and inflammation.
“I always say that organ chips are like synthetic biology at the cell tissue and organ level,” says Dr. Ingber. “You start simple and see if you [can] mimic a clinical situation.”
As they make the chips more complex – perhaps by adding blood vessel cells and female hormones – Dr. Ingber foresees being able to study the response to hormonal changes during the menstrual cycle.
“We can begin to explore the effects of cycling over time as well as other types of hormonal effects,” he says.
Dr. Ingber also envisions linking the vagina chip to other organ chips – he’s already succeeded in linking eight different organ types together. But for now, the team hopes the vagina chip will enhance our understanding of basic female reproductive biology and speed up the process of developing new treatments for women’s health.
A version of this article first appeared on WebMD.com.
For years, women’s health advocates have argued that far more research is needed on women’s bodies and health. The world’s first-ever “vagina on a chip,” recently developed at Harvard’s Wyss Institute for Biologically Inspired Engineering in Boston, could go a long way to making that happen.
“Women’s health has not received the attention it deserves,” says Don Ingber, MD, PhD, who led the team that created the vagina chip. The advance quickly drew media attention after it was reported in the journal Microbiome. But researchers hope for more than headlines. They see the chip as a way to facilitate vaginal health research and open the door to vital new treatments.
By now, you may have heard of “organs on chips”: tiny devices about the size of a flash drive that are designed to mimic the biological activity of human organs. These glass chips contain living human cells within grooves that allow the passage of fluid, to either maintain or disrupt the cells’ function. So far, Dr. Ingber and his team at the Wyss Institute have developed more than 15 organ chip models, including chips that mimic the lung, intestine, kidney, and bone marrow.
The idea to develop a vagina chip grew out of research, funded by the Gates Foundation, on a childhood disease called environmental enteric dysfunction, an intestinal disease most commonly found in low-resource nations that is the second leading cause of death in children under 5. That’s when Dr. Ingber discovered just how much the child’s microbiome influences this disease.
Stemming from that work, the Gates Foundation turned its attention to newborn health – in particular, the impact of bacterial vaginosis, an imbalance in the vagina’s bacterial makeup. Bacterial vaginosis occurs in one out of four women worldwide and has been linked to premature birth as well as HIV, HPV persistence, and cervical cancer.
The goal was to test “live biotherapeutic products,” or living microbes like probiotics, that might restore the vagina’s microbiome to health.
No other preclinical model exists to perform tests like that, says Dr. Ingber.
“The vagina chip is a way to help make some advances,” he says.
The Gates Foundation recognized that women’s reproductive health is a major issue, not only in low-income nations, but everywhere around the world. As the project evolved, Dr. Ingber began to hear from female colleagues about how neglected women’s reproductive health is in medical science.
“It is something I became sensitive to and realized this is just the starting point,” Dr. Ingber says.
Take bacterial vaginosis, for example. Since 1982, treatment has revolved around the same two antibiotics. That’s partly because there is no animal model to study. No other species has the same vaginal bacterial community as humans do.
That makes developing any new therapy “incredibly challenging,” explains Caroline Mitchell, MD, MPH, an ob.gyn. at Massachusetts General Hospital, Boston, and a member of the consortium.
It turns out, replicating the vagina in a lab dish is, to use the technical term, very hard.
“That’s where a vagina chip offers an opportunity,” Dr. Mitchell says. “It’s not super-high throughput, but it’s way more high throughput than a [human] clinical trial.”
As such, the vagina chip could help scientists find new treatments much faster.
Like Dr. Ingber, Dr. Mitchell also sees the chip as a way to bring more attention to the largely unmet needs in female reproductive medicine.
“Women’s reproductive health has been under-resourced, under-prioritized, and largely disregarded for decades,” she says. And the time may be ripe for change: Dr. Mitchell says she was encouraged by the National Institutes of Health’s Advancing NIH Research on the Health of Women conference, held in 2021 in response to a congressional request to address women’s health research efforts.
Beyond bacterial vaginosis, Dr. Mitchell imagines the chip could help scientists find new treatments for vaginal yeast infection (candidiasis), chlamydia, and endometriosis. As with bacterial vaginosis, medicines for vaginal yeast infections have not advanced in decades, Dr. Mitchell says. Efforts to develop a vaccine for chlamydia – which can cause permanent damage to a woman’s reproductive system – have dragged on for many years. And endometriosis, an often painful condition in which the tissue that makes up the uterine lining grows outside the uterus, remains under-researched despite affecting 10% of childbearing-age women.
While some mouse models are used in chlamydia research, it’s hard to say if they’ll translate to humans, given the vaginal and cervical bacterial differences.
“Our understanding of the basic physiology of the environment of the vagina and cervix is another area where we’re woefully ignorant,” Dr. Mitchell says.
To that end, Dr. Ingber’s team is developing more complex chips mimicking the vagina and the cervix. One of his team members wants to use the chips to study infertility. The researchers have already used the chips to see how bacterial vaginosis and mucous changes impact the way sperm migrates up the reproductive tract.
The lab is now linking vagina and cervix chips together to study viral infections of the cervix, like HPV, and all types of bacterial diseases of the vaginal tract. By applying cervical mucus to the vagina chip, they hope to learn more about how female reproductive tissues respond to infection and inflammation.
“I always say that organ chips are like synthetic biology at the cell tissue and organ level,” says Dr. Ingber. “You start simple and see if you [can] mimic a clinical situation.”
As they make the chips more complex – perhaps by adding blood vessel cells and female hormones – Dr. Ingber foresees being able to study the response to hormonal changes during the menstrual cycle.
“We can begin to explore the effects of cycling over time as well as other types of hormonal effects,” he says.
Dr. Ingber also envisions linking the vagina chip to other organ chips – he’s already succeeded in linking eight different organ types together. But for now, the team hopes the vagina chip will enhance our understanding of basic female reproductive biology and speed up the process of developing new treatments for women’s health.
A version of this article first appeared on WebMD.com.
FROM MICROBIOME
Renewed calls for fallopian tube removal to avoid ovarian cancer
All women, regardless of their risk profile, should consider prophylactic removal of the fallopian tubes at the same time as other pelvic surgery once they are finished having children, the Ovarian Cancer Research Alliance has advised.
The recommendation, announced Feb. 1, replaces the decades-old focus on symptom awareness and early detection and follows “sobering and deeply disappointing” results from a large U.K. study published 2 years ago, the organization said.
That was the UK Collaborative Trial of Ovarian Cancer Screening published in The Lancet in 2021, which followed more than 200,000 women for a median 16 years. It showed that screening average-risk women with a CA-125 blood test and ultrasound does not reduce deaths from the disease, as reported at the time by this news organization.
“We all hoped that the trial would show that early detection was effective in changing mortality rates. When the results came out, it was very hard to accept,” Audra Moran, OCRA president and CEO, said in an interview.
“We have an obligation to let people know that symptom awareness and early detection will not save lives” but considering opportunistic salpingectomy “absolutely will,” said Ms. Moran. Hence the renewed call for women to consider having their fallopian tubes removed.
What sounds new about this call is that the group is directing fallopian tube removal to all women “who are undergoing pelvic surgeries for benign conditions,” irrespective of what perceived risk they have of developing ovarian cancer (for example, based on family history).
But this advice has been in place for years for women who are known to be at higher risk for the disease.
For instance, women at high risk for ovarian cancer based on Hereditary Breast and Ovarian Cancer Syndrome (HBOC) have long been recommended to undergo surgery to remove ovaries and fallopian tubes (risk-reducing bilateral salpingo-oophorectomy or RRBSO) once there is no longer a desire for pregnancy.
Approached for comment about the new messaging, Stephanie V. Blank, MD, president of the Society of Gynecologic Oncology, says that the new recommendation – that all women who are finished childbearing consider opportunistic salpingectomy at the time of other pelvic surgery for benign conditions – is “not aggressive.”
“It’s reasonable and makes sense,” Dr. Blank said in an interview.
And she pointed out that it’s actually not “new”; it is, however, getting “new attention” based on the disappointing U.K. screening study, said Dr. Blank, director of gynecologic oncology for the Mount Sinai Health System in New York and professor of gynecologic oncology at Icahn School of Medicine at Mount Sinai.
She noted that the procedure of opportunistic salpingectomy has been endorsed by SGO since 2013 and by the American College of Obstetricians and Gynecologists since 2015.
There is increasing evidence that most high-grade serous ovarian cancers arise from cells in the fallopian tubes, William Dahut, MD, chief scientific officer for the American Cancer Society, told this news organization.
“Indirect evidence suggests a fairly strong degree of risk reduction associated with opportunistic salpingectomy for the most prevalent type of ovarian cancer (serous), and some risk reduction of epithelial ovarian cancer. At this time, these discussions seem warranted,” Dr. Dahut said.
At this point, however, the fact that leading organizations advise “consideration” means that the evidence base has “not been judged to be sufficiently strong (in terms of what we can say about benefits and harms) to advise a direct recommendation for opportunistic salpingectomy,” Dr. Dahut added.
There is no current recommendation to have fallopian tubes removed as a stand-alone procedure, he pointed out. However, he commented that “the occasion of scheduled gynecologic surgery presents an opportunity to possibly reduce the risk of ovarian cancer without known adverse effects in women who have completed childbearing. Having the discussion seems to be justified by the current evidence,” Dr. Dahut said.
Deanna Gerber, MD, a gynecologic oncologist at NYU Langone Perlmutter Cancer Center-Long Island, agrees. “In women who are scheduled to have a gynecologic or pelvic procedure, clinicians should discuss the possibility of removing the fallopian tubes at that time. A salpingectomy is a relatively low-risk procedure and adds little time to the surgery,” Dr. Gerber said in an interview.
“Women should understand that there is still ongoing research on this topic, but this low-risk procedure may reduce their risk of developing an ovarian or fallopian tube cancer,” Dr. Gerber said.
OCRA also encourages all women (or anyone born with ovaries) to know their risk for ovarian cancer. To that end, the organization has launched a pilot program offering free, at-home genetic testing kits to people with a personal or family history of breast, ovarian, uterine, or colorectal cancer.
Ms. Moran, Dr. Blank, Dr. Dahut, and Dr. Gerber report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
All women, regardless of their risk profile, should consider prophylactic removal of the fallopian tubes at the same time as other pelvic surgery once they are finished having children, the Ovarian Cancer Research Alliance has advised.
The recommendation, announced Feb. 1, replaces the decades-old focus on symptom awareness and early detection and follows “sobering and deeply disappointing” results from a large U.K. study published 2 years ago, the organization said.
That was the UK Collaborative Trial of Ovarian Cancer Screening published in The Lancet in 2021, which followed more than 200,000 women for a median 16 years. It showed that screening average-risk women with a CA-125 blood test and ultrasound does not reduce deaths from the disease, as reported at the time by this news organization.
“We all hoped that the trial would show that early detection was effective in changing mortality rates. When the results came out, it was very hard to accept,” Audra Moran, OCRA president and CEO, said in an interview.
“We have an obligation to let people know that symptom awareness and early detection will not save lives” but considering opportunistic salpingectomy “absolutely will,” said Ms. Moran. Hence the renewed call for women to consider having their fallopian tubes removed.
What sounds new about this call is that the group is directing fallopian tube removal to all women “who are undergoing pelvic surgeries for benign conditions,” irrespective of what perceived risk they have of developing ovarian cancer (for example, based on family history).
But this advice has been in place for years for women who are known to be at higher risk for the disease.
For instance, women at high risk for ovarian cancer based on Hereditary Breast and Ovarian Cancer Syndrome (HBOC) have long been recommended to undergo surgery to remove ovaries and fallopian tubes (risk-reducing bilateral salpingo-oophorectomy or RRBSO) once there is no longer a desire for pregnancy.
Approached for comment about the new messaging, Stephanie V. Blank, MD, president of the Society of Gynecologic Oncology, says that the new recommendation – that all women who are finished childbearing consider opportunistic salpingectomy at the time of other pelvic surgery for benign conditions – is “not aggressive.”
“It’s reasonable and makes sense,” Dr. Blank said in an interview.
And she pointed out that it’s actually not “new”; it is, however, getting “new attention” based on the disappointing U.K. screening study, said Dr. Blank, director of gynecologic oncology for the Mount Sinai Health System in New York and professor of gynecologic oncology at Icahn School of Medicine at Mount Sinai.
She noted that the procedure of opportunistic salpingectomy has been endorsed by SGO since 2013 and by the American College of Obstetricians and Gynecologists since 2015.
There is increasing evidence that most high-grade serous ovarian cancers arise from cells in the fallopian tubes, William Dahut, MD, chief scientific officer for the American Cancer Society, told this news organization.
“Indirect evidence suggests a fairly strong degree of risk reduction associated with opportunistic salpingectomy for the most prevalent type of ovarian cancer (serous), and some risk reduction of epithelial ovarian cancer. At this time, these discussions seem warranted,” Dr. Dahut said.
At this point, however, the fact that leading organizations advise “consideration” means that the evidence base has “not been judged to be sufficiently strong (in terms of what we can say about benefits and harms) to advise a direct recommendation for opportunistic salpingectomy,” Dr. Dahut added.
There is no current recommendation to have fallopian tubes removed as a stand-alone procedure, he pointed out. However, he commented that “the occasion of scheduled gynecologic surgery presents an opportunity to possibly reduce the risk of ovarian cancer without known adverse effects in women who have completed childbearing. Having the discussion seems to be justified by the current evidence,” Dr. Dahut said.
Deanna Gerber, MD, a gynecologic oncologist at NYU Langone Perlmutter Cancer Center-Long Island, agrees. “In women who are scheduled to have a gynecologic or pelvic procedure, clinicians should discuss the possibility of removing the fallopian tubes at that time. A salpingectomy is a relatively low-risk procedure and adds little time to the surgery,” Dr. Gerber said in an interview.
“Women should understand that there is still ongoing research on this topic, but this low-risk procedure may reduce their risk of developing an ovarian or fallopian tube cancer,” Dr. Gerber said.
OCRA also encourages all women (or anyone born with ovaries) to know their risk for ovarian cancer. To that end, the organization has launched a pilot program offering free, at-home genetic testing kits to people with a personal or family history of breast, ovarian, uterine, or colorectal cancer.
Ms. Moran, Dr. Blank, Dr. Dahut, and Dr. Gerber report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
All women, regardless of their risk profile, should consider prophylactic removal of the fallopian tubes at the same time as other pelvic surgery once they are finished having children, the Ovarian Cancer Research Alliance has advised.
The recommendation, announced Feb. 1, replaces the decades-old focus on symptom awareness and early detection and follows “sobering and deeply disappointing” results from a large U.K. study published 2 years ago, the organization said.
That was the UK Collaborative Trial of Ovarian Cancer Screening published in The Lancet in 2021, which followed more than 200,000 women for a median 16 years. It showed that screening average-risk women with a CA-125 blood test and ultrasound does not reduce deaths from the disease, as reported at the time by this news organization.
“We all hoped that the trial would show that early detection was effective in changing mortality rates. When the results came out, it was very hard to accept,” Audra Moran, OCRA president and CEO, said in an interview.
“We have an obligation to let people know that symptom awareness and early detection will not save lives” but considering opportunistic salpingectomy “absolutely will,” said Ms. Moran. Hence the renewed call for women to consider having their fallopian tubes removed.
What sounds new about this call is that the group is directing fallopian tube removal to all women “who are undergoing pelvic surgeries for benign conditions,” irrespective of what perceived risk they have of developing ovarian cancer (for example, based on family history).
But this advice has been in place for years for women who are known to be at higher risk for the disease.
For instance, women at high risk for ovarian cancer based on Hereditary Breast and Ovarian Cancer Syndrome (HBOC) have long been recommended to undergo surgery to remove ovaries and fallopian tubes (risk-reducing bilateral salpingo-oophorectomy or RRBSO) once there is no longer a desire for pregnancy.
Approached for comment about the new messaging, Stephanie V. Blank, MD, president of the Society of Gynecologic Oncology, says that the new recommendation – that all women who are finished childbearing consider opportunistic salpingectomy at the time of other pelvic surgery for benign conditions – is “not aggressive.”
“It’s reasonable and makes sense,” Dr. Blank said in an interview.
And she pointed out that it’s actually not “new”; it is, however, getting “new attention” based on the disappointing U.K. screening study, said Dr. Blank, director of gynecologic oncology for the Mount Sinai Health System in New York and professor of gynecologic oncology at Icahn School of Medicine at Mount Sinai.
She noted that the procedure of opportunistic salpingectomy has been endorsed by SGO since 2013 and by the American College of Obstetricians and Gynecologists since 2015.
There is increasing evidence that most high-grade serous ovarian cancers arise from cells in the fallopian tubes, William Dahut, MD, chief scientific officer for the American Cancer Society, told this news organization.
“Indirect evidence suggests a fairly strong degree of risk reduction associated with opportunistic salpingectomy for the most prevalent type of ovarian cancer (serous), and some risk reduction of epithelial ovarian cancer. At this time, these discussions seem warranted,” Dr. Dahut said.
At this point, however, the fact that leading organizations advise “consideration” means that the evidence base has “not been judged to be sufficiently strong (in terms of what we can say about benefits and harms) to advise a direct recommendation for opportunistic salpingectomy,” Dr. Dahut added.
There is no current recommendation to have fallopian tubes removed as a stand-alone procedure, he pointed out. However, he commented that “the occasion of scheduled gynecologic surgery presents an opportunity to possibly reduce the risk of ovarian cancer without known adverse effects in women who have completed childbearing. Having the discussion seems to be justified by the current evidence,” Dr. Dahut said.
Deanna Gerber, MD, a gynecologic oncologist at NYU Langone Perlmutter Cancer Center-Long Island, agrees. “In women who are scheduled to have a gynecologic or pelvic procedure, clinicians should discuss the possibility of removing the fallopian tubes at that time. A salpingectomy is a relatively low-risk procedure and adds little time to the surgery,” Dr. Gerber said in an interview.
“Women should understand that there is still ongoing research on this topic, but this low-risk procedure may reduce their risk of developing an ovarian or fallopian tube cancer,” Dr. Gerber said.
OCRA also encourages all women (or anyone born with ovaries) to know their risk for ovarian cancer. To that end, the organization has launched a pilot program offering free, at-home genetic testing kits to people with a personal or family history of breast, ovarian, uterine, or colorectal cancer.
Ms. Moran, Dr. Blank, Dr. Dahut, and Dr. Gerber report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Which populations should be screened for cervical cancer?
Montrouge, France – Whether you are a cisgender woman or a transgender man who has kept his uterus, regardless of the sex of your partner, and even if you are a woman who is no longer sexually active, you must take part in cervical cancer screening. This is the reminder issued by Julia Maruani, MD, a medical gynecologist in Marseille, France, at a press conference ahead of the 46th meeting of the French Colposcopy and Cervical-Vaginal Diseases Society (SFCPCV).
Cervical screening currently targets asymptomatic, immunocompetent, and sexually active women between ages 25 and 65 years.
Sex between women
There is a widely held belief that only men can transmit human papillomavirus (HPV). “If you are in a sexual relationship with a man, then yes, you can get HPV from him. But it’s also possible for HPV to be transmitted in a sexual relationship between two women via touch, bodily fluids, or sex toys,” said Dr. Maruani, who pointed out that 20% of lesbians and 30% of bisexual women are HPV carriers.
Because women who have sexual relationships with other women have the mistaken view that their demographic is less affected, they are less likely to take part in cervical screening. They also present more often with advanced lesions and with cancer because of the lack of screening in this group.
Transgender men
Dr. Maruani defines transgender men as “women who have changed gender and who have become men.” Why are they affected by cervical screening? Not all of them are. Those who’ve had their uterus removed no longer have a cervix, so this screening doesn’t affect them. But hysterectomies are rarely performed, as they’re not required in most European countries to legally change gender.
The figures are concerning: 27% of transgender men are screened versus 60% of cisgender females.
“For this demographic, specialist gynecology appointments are hard to come by. Sitting in a women’s waiting room is not easy,” said Dr. Maruani, recalling that often discussion about the transition phase takes up the entire appointment time. It’s also usually the case that any medical problems or health care prevention issues not related to the topic of transitioning are not discussed.
Moreover, the online appointment-booking software doesn’t allow transgender men who have kept their cervix and legally identify as men to make an appointment. “Gynecologists must disable this default option,” said Dr. Maruani.
Likewise, transgender men will not receive an invitation to take part in cervical or breast cancer screening, as they are identified as male by social security services and screening sites. Furthermore, in what Dr. Maruani referred to as an “administrative head-scratcher that needs to change,” some medical procedures are not funded for men.
Yet the risk of contracting HPV is higher among transgender men than in the rest of the population because of different sexual practices in this demographic, as well as the propensity to have multiple sexual partners. The risk of finding abnormalities on cytology screening is greater.
Although data regarding cancer are lacking, “if screening is inadequate but the risk of infection with HPV is great, logic tells us that there will be more lesions, more cancer” in this demographic, said Dr. Maruani.
Celibate women
Nowadays, screening drops with age in women, especially after menopause. This is especially true for women who are no longer sexually active. Another preconceived notion to be addressed is that women who are no longer sexually active no longer need screening. But this concept completely goes against the natural history of HPV infection. “There are years, at least 5, between infection and the development of precancerous lesions. There is a further 5 years between a precancerous lesion and cancer,” said Dr. Maruani.
A woman could still be at risk even 20 years after contracting HPV. Approximately 80% of women are exposed to HPV, and 5%-10% have a persistent infection that could lead to the development of precancerous lesions.
“So, a woman who is no longer sexually active can’t stop participating in cervical screening, especially since there aren’t any symptoms until a fairly advanced stage of cancer.” No longer having sex does not mean that screening can be stopped.
What treatment is appropriate for partners of a woman who is no longer sexually active? None. During the press conference, the specialists agreed that a positive HPV test would be of importance to her partner. Even so, they recalled that the infection would generally be an old one and that the woman’s partner (whether male or female) would therefore have probably already been exposed to it. Patients should also be reminded that, in the past, cytology testing did not look for HPV, so the virus could already have been there. According to these specialists, you don’t need to change your sexual habits, just continue to monitor yourself.
This article was translated from the Medscape French edition and a version first appeared on Medscape.com.
Montrouge, France – Whether you are a cisgender woman or a transgender man who has kept his uterus, regardless of the sex of your partner, and even if you are a woman who is no longer sexually active, you must take part in cervical cancer screening. This is the reminder issued by Julia Maruani, MD, a medical gynecologist in Marseille, France, at a press conference ahead of the 46th meeting of the French Colposcopy and Cervical-Vaginal Diseases Society (SFCPCV).
Cervical screening currently targets asymptomatic, immunocompetent, and sexually active women between ages 25 and 65 years.
Sex between women
There is a widely held belief that only men can transmit human papillomavirus (HPV). “If you are in a sexual relationship with a man, then yes, you can get HPV from him. But it’s also possible for HPV to be transmitted in a sexual relationship between two women via touch, bodily fluids, or sex toys,” said Dr. Maruani, who pointed out that 20% of lesbians and 30% of bisexual women are HPV carriers.
Because women who have sexual relationships with other women have the mistaken view that their demographic is less affected, they are less likely to take part in cervical screening. They also present more often with advanced lesions and with cancer because of the lack of screening in this group.
Transgender men
Dr. Maruani defines transgender men as “women who have changed gender and who have become men.” Why are they affected by cervical screening? Not all of them are. Those who’ve had their uterus removed no longer have a cervix, so this screening doesn’t affect them. But hysterectomies are rarely performed, as they’re not required in most European countries to legally change gender.
The figures are concerning: 27% of transgender men are screened versus 60% of cisgender females.
“For this demographic, specialist gynecology appointments are hard to come by. Sitting in a women’s waiting room is not easy,” said Dr. Maruani, recalling that often discussion about the transition phase takes up the entire appointment time. It’s also usually the case that any medical problems or health care prevention issues not related to the topic of transitioning are not discussed.
Moreover, the online appointment-booking software doesn’t allow transgender men who have kept their cervix and legally identify as men to make an appointment. “Gynecologists must disable this default option,” said Dr. Maruani.
Likewise, transgender men will not receive an invitation to take part in cervical or breast cancer screening, as they are identified as male by social security services and screening sites. Furthermore, in what Dr. Maruani referred to as an “administrative head-scratcher that needs to change,” some medical procedures are not funded for men.
Yet the risk of contracting HPV is higher among transgender men than in the rest of the population because of different sexual practices in this demographic, as well as the propensity to have multiple sexual partners. The risk of finding abnormalities on cytology screening is greater.
Although data regarding cancer are lacking, “if screening is inadequate but the risk of infection with HPV is great, logic tells us that there will be more lesions, more cancer” in this demographic, said Dr. Maruani.
Celibate women
Nowadays, screening drops with age in women, especially after menopause. This is especially true for women who are no longer sexually active. Another preconceived notion to be addressed is that women who are no longer sexually active no longer need screening. But this concept completely goes against the natural history of HPV infection. “There are years, at least 5, between infection and the development of precancerous lesions. There is a further 5 years between a precancerous lesion and cancer,” said Dr. Maruani.
A woman could still be at risk even 20 years after contracting HPV. Approximately 80% of women are exposed to HPV, and 5%-10% have a persistent infection that could lead to the development of precancerous lesions.
“So, a woman who is no longer sexually active can’t stop participating in cervical screening, especially since there aren’t any symptoms until a fairly advanced stage of cancer.” No longer having sex does not mean that screening can be stopped.
What treatment is appropriate for partners of a woman who is no longer sexually active? None. During the press conference, the specialists agreed that a positive HPV test would be of importance to her partner. Even so, they recalled that the infection would generally be an old one and that the woman’s partner (whether male or female) would therefore have probably already been exposed to it. Patients should also be reminded that, in the past, cytology testing did not look for HPV, so the virus could already have been there. According to these specialists, you don’t need to change your sexual habits, just continue to monitor yourself.
This article was translated from the Medscape French edition and a version first appeared on Medscape.com.
Montrouge, France – Whether you are a cisgender woman or a transgender man who has kept his uterus, regardless of the sex of your partner, and even if you are a woman who is no longer sexually active, you must take part in cervical cancer screening. This is the reminder issued by Julia Maruani, MD, a medical gynecologist in Marseille, France, at a press conference ahead of the 46th meeting of the French Colposcopy and Cervical-Vaginal Diseases Society (SFCPCV).
Cervical screening currently targets asymptomatic, immunocompetent, and sexually active women between ages 25 and 65 years.
Sex between women
There is a widely held belief that only men can transmit human papillomavirus (HPV). “If you are in a sexual relationship with a man, then yes, you can get HPV from him. But it’s also possible for HPV to be transmitted in a sexual relationship between two women via touch, bodily fluids, or sex toys,” said Dr. Maruani, who pointed out that 20% of lesbians and 30% of bisexual women are HPV carriers.
Because women who have sexual relationships with other women have the mistaken view that their demographic is less affected, they are less likely to take part in cervical screening. They also present more often with advanced lesions and with cancer because of the lack of screening in this group.
Transgender men
Dr. Maruani defines transgender men as “women who have changed gender and who have become men.” Why are they affected by cervical screening? Not all of them are. Those who’ve had their uterus removed no longer have a cervix, so this screening doesn’t affect them. But hysterectomies are rarely performed, as they’re not required in most European countries to legally change gender.
The figures are concerning: 27% of transgender men are screened versus 60% of cisgender females.
“For this demographic, specialist gynecology appointments are hard to come by. Sitting in a women’s waiting room is not easy,” said Dr. Maruani, recalling that often discussion about the transition phase takes up the entire appointment time. It’s also usually the case that any medical problems or health care prevention issues not related to the topic of transitioning are not discussed.
Moreover, the online appointment-booking software doesn’t allow transgender men who have kept their cervix and legally identify as men to make an appointment. “Gynecologists must disable this default option,” said Dr. Maruani.
Likewise, transgender men will not receive an invitation to take part in cervical or breast cancer screening, as they are identified as male by social security services and screening sites. Furthermore, in what Dr. Maruani referred to as an “administrative head-scratcher that needs to change,” some medical procedures are not funded for men.
Yet the risk of contracting HPV is higher among transgender men than in the rest of the population because of different sexual practices in this demographic, as well as the propensity to have multiple sexual partners. The risk of finding abnormalities on cytology screening is greater.
Although data regarding cancer are lacking, “if screening is inadequate but the risk of infection with HPV is great, logic tells us that there will be more lesions, more cancer” in this demographic, said Dr. Maruani.
Celibate women
Nowadays, screening drops with age in women, especially after menopause. This is especially true for women who are no longer sexually active. Another preconceived notion to be addressed is that women who are no longer sexually active no longer need screening. But this concept completely goes against the natural history of HPV infection. “There are years, at least 5, between infection and the development of precancerous lesions. There is a further 5 years between a precancerous lesion and cancer,” said Dr. Maruani.
A woman could still be at risk even 20 years after contracting HPV. Approximately 80% of women are exposed to HPV, and 5%-10% have a persistent infection that could lead to the development of precancerous lesions.
“So, a woman who is no longer sexually active can’t stop participating in cervical screening, especially since there aren’t any symptoms until a fairly advanced stage of cancer.” No longer having sex does not mean that screening can be stopped.
What treatment is appropriate for partners of a woman who is no longer sexually active? None. During the press conference, the specialists agreed that a positive HPV test would be of importance to her partner. Even so, they recalled that the infection would generally be an old one and that the woman’s partner (whether male or female) would therefore have probably already been exposed to it. Patients should also be reminded that, in the past, cytology testing did not look for HPV, so the virus could already have been there. According to these specialists, you don’t need to change your sexual habits, just continue to monitor yourself.
This article was translated from the Medscape French edition and a version first appeared on Medscape.com.
Update on secondary cytoreduction in recurrent ovarian cancer
Recurrent ovarian cancer is difficult to treat; it has high recurrence rates and poor targeted treatment options. Between 60% and 75% of patients initially diagnosed with advanced-stage ovarian cancer will relapse within 2-3 years.1 Survival for these patients is poor, with an average overall survival (OS) of 30-40 months from the time of recurrence.2 Historically, immunotherapy has shown poor efficacy for recurrent ovarian malignancy, leaving few options for patients and their providers. Given the lack of effective treatment options, secondary cytoreductive surgery (surgery at the time of recurrence) has been heavily studied as a potential therapeutic option.
The initial rationale for cytoreductive surgery (CRS) in patients with advanced ovarian cancer focused on palliation of symptoms from large, bulky disease that frequently caused obstructive symptoms and pain. Now, cytoreduction is a critical part of therapy. It decreases chemotherapy-resistant tumor cells, improves the immune response, and is thought to optimize perfusion of the residual cancer for systemic therapy. The survival benefit of surgery in the frontline setting, either with primary or interval debulking, is well established, and much of the data now demonstrate that complete resection of all macroscopic disease (also known as an R0 resection) has the greatest survival benefit.3 Given the benefits of an initial debulking surgery, secondary cytoreduction has been studied since the 1980s with mixed results. These data have demonstrated that the largest barrier to care has been appropriate patient selection for this often complex surgical procedure.
The 2020 National Comprehensive Cancer Network guidelines list secondary CRS as a treatment option; however, the procedure should only be considered in patients who have platinum sensitive disease, a performance status of 0-1, no ascites, and an isolated focus or limited focus of disease that is amenable to complete resection. Numerous retrospective studies have suggested that secondary CRS is beneficial to patients with recurrent ovarian cancer, especially if complete cytoreduction can be accomplished. Many of these studies have similarly concluded that there are benefits, such as less ascites at the time of recurrence, smaller disease burden, and a longer disease-free interval. From that foundation, multiple groups used retrospective data to investigate prognostic models to determine who would benefit most from secondary cytoreduction.
The DESKTOP Group initially published their retrospective study in 2006 and created a scoring system assessing who would benefit from secondary CRS.4 Data demonstrated that a performance status of 0, FIGO stage of I/II at the time of initial diagnosis, no residual tumor after primary surgery, and ascites less than 500 mL were associated with improved survival after secondary cytoreduction. They created the AGO score out of these data, which is positive only if three criteria are met: a performance status of 0, R0 after primary debulk, and ascites less than 500 mL at the time of recurrence.
They prospectively tested this score in DESKTOP II, which validated their findings and showed that complete secondary CRS could be achieved in 76% of those with a positive AGO score.5 Many believed that the AGO score was too restrictive, and a second retrospective study performed by a group at Memorial Sloan Kettering showed that optimal secondary cytoreduction could be achieved to prolong survival by a median of 30 months in patients with a longer disease-free interval, a single site of recurrence, and residual disease measuring less than 5 mm at time of initial/first-line surgery.6 Many individuals now use this scoring system to determine candidacy for secondary debulking: disease-free interval, number of sites of recurrence (ideally oligometastatic disease), and residual disease less than 5 mm at the time of primary debulking.
Finally, the iMODEL was developed by a group from China and found that complete R0 secondary CRS was associated with a low initial FIGO stage, no residual disease after primary surgery, longer platinum-free interval, better Eastern Cooperative Oncology Group performance status, lower CA-125 levels, as well as no ascites at the time of recurrence. Based on these criteria, individuals received either high or low iMODEL scores, and those with a low score were said to be candidates for secondary CRS. Overall, these models demonstrate that the strongest predictive factor that suggests a survival benefit from secondary CRS is the ability to achieve a complete R0 resection at the time of surgery.
Secondary debulking surgery has been tested in three large randomized controlled trials. The DESKTOP investigators and the SOC-1 trial have been the most successful groups to publish on this topic with positive results. Both groups use prognostic models for their inclusion criteria to select candidates in whom an R0 resection is believed to be most feasible. The first randomized controlled trial to publish on this topic was GOG-213,7 which did not use prognostic modeling for their inclusion criteria. Patients were randomized to secondary cytoreduction followed by platinum-based chemotherapy with or without bevacizumab versus chemotherapy alone. The median OS was 50.6 months in the surgery group and 64.7 months in the no-surgery group (P = .08), suggesting no survival benefit to secondary cytoreduction; however, an ad hoc exploratory analysis of the surgery arm showed that both overall and progression-free survival were significantly improved in the complete cytoreduction group, compared with those with residual disease at time of surgery.
The results from the GOG-213 group suggested that improved survival from secondary debulking might be achieved when prognostic modeling is used to select optimal surgical candidates. The SOC-1 trial, published in 2021, was a phase 3, randomized, controlled trial that used the iMODEL scoring system combined with PET/CT imaging for patient selection.8 Patients were again randomized to surgery followed by platinum-based chemotherapy versus chemotherapy alone. Complete cytoreduction was achieved in 73% of patients with a low iMODEL score, and these data showed improved OS in the surgery group of 58.1 months versus 53.9 months (P < .05) in the no-surgery group. Lastly, the DESKTOP group most recently published results on this topic in a large randomized, controlled trial.9 Patients were again randomized to surgery followed by platinum-based chemotherapy versus chemotherapy alone. Inclusion criteria were only met in patients with a positive AGO score. An improved OS of 7.7 months (53.7 vs. 46 months; P < .05) was demonstrated in patients that underwent surgery versus those exposed to only chemotherapy. Again, this group showed that overall survival was further improved when complete cytoreduction was achieved.
Given the results of these three trials, the Society for Gynecologic Oncology has released a statement on secondary cytoreduction in recurrent ovarian cancer (see Table).10 While it is important to use caution when comparing the three studies as study populations differed substantially, the most important takeaway the difference in survival outcomes in patients in whom complete gross resection was achieved versus no complete gross resection versus no surgery. This comparison highlights the benefit of complete cytoreduction as well as the potential harms of secondary debulking when an R0 resection cannot be achieved. Although not yet evaluated in this clinical setting, laparoscopic exploration may be useful to augment assessment of disease extent and possibility of disease resection, just as it is in frontline ovarian cancer surgery.
The importance of bevacizumab use in recurrent ovarian cancer is also highlighted in the SGO statement. In GOG-213, 84% of the total study population (in both the surgery and no surgery cohort) were treated with concurrent followed by maintenance bevacizumab with an improved survival outcome, which may suggest that this trial generalizes better than the others to contemporary management of platinum-sensitive recurrent ovarian cancer.
Overall, given the mixed data, the recommendation is for surgeons to consider all available data to guide them in treatment planning with a strong emphasis on using all available technology to assess whether complete cytoreduction can be achieved in the setting of recurrence so as to not delay the patient’s ability to receive chemotherapy.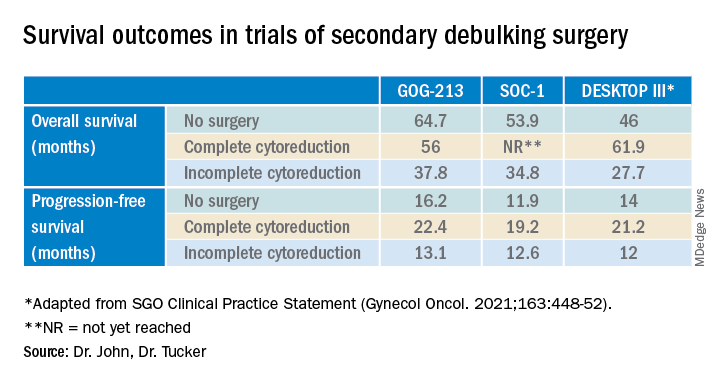
Dr. John is a gynecologic oncology fellow at the University of North Carolina at Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the university.
References
1. du Bois A et al. J Natl Cancer Inst. 2003;95:1320-9.
2. Wagner U et al. Br J Cancer. 2012;107:588-91.
3. Vergote I et al. N Engl J Med. 2010;363:943-53.
4. Harter P et al. Ann Surg Oncol. 2006;13:1702-10.
5. Harter P et al. Int J Gynecol Cancer. 2011;21:289-95.
6. Chi DS et al. Cancer. 2006 106:1933-9.
7. Coleman RL et al. Lancet Oncol. 2017;18:779-1.
8. Shi T et al. Lancet Oncol. 2021;22:439-49.
9. Harter P et al. N Engl J Med 2021;385:2123-31.
10. Harrison R, et al. Gynecol Oncol. 2021;163:448-52.
Recurrent ovarian cancer is difficult to treat; it has high recurrence rates and poor targeted treatment options. Between 60% and 75% of patients initially diagnosed with advanced-stage ovarian cancer will relapse within 2-3 years.1 Survival for these patients is poor, with an average overall survival (OS) of 30-40 months from the time of recurrence.2 Historically, immunotherapy has shown poor efficacy for recurrent ovarian malignancy, leaving few options for patients and their providers. Given the lack of effective treatment options, secondary cytoreductive surgery (surgery at the time of recurrence) has been heavily studied as a potential therapeutic option.
The initial rationale for cytoreductive surgery (CRS) in patients with advanced ovarian cancer focused on palliation of symptoms from large, bulky disease that frequently caused obstructive symptoms and pain. Now, cytoreduction is a critical part of therapy. It decreases chemotherapy-resistant tumor cells, improves the immune response, and is thought to optimize perfusion of the residual cancer for systemic therapy. The survival benefit of surgery in the frontline setting, either with primary or interval debulking, is well established, and much of the data now demonstrate that complete resection of all macroscopic disease (also known as an R0 resection) has the greatest survival benefit.3 Given the benefits of an initial debulking surgery, secondary cytoreduction has been studied since the 1980s with mixed results. These data have demonstrated that the largest barrier to care has been appropriate patient selection for this often complex surgical procedure.
The 2020 National Comprehensive Cancer Network guidelines list secondary CRS as a treatment option; however, the procedure should only be considered in patients who have platinum sensitive disease, a performance status of 0-1, no ascites, and an isolated focus or limited focus of disease that is amenable to complete resection. Numerous retrospective studies have suggested that secondary CRS is beneficial to patients with recurrent ovarian cancer, especially if complete cytoreduction can be accomplished. Many of these studies have similarly concluded that there are benefits, such as less ascites at the time of recurrence, smaller disease burden, and a longer disease-free interval. From that foundation, multiple groups used retrospective data to investigate prognostic models to determine who would benefit most from secondary cytoreduction.
The DESKTOP Group initially published their retrospective study in 2006 and created a scoring system assessing who would benefit from secondary CRS.4 Data demonstrated that a performance status of 0, FIGO stage of I/II at the time of initial diagnosis, no residual tumor after primary surgery, and ascites less than 500 mL were associated with improved survival after secondary cytoreduction. They created the AGO score out of these data, which is positive only if three criteria are met: a performance status of 0, R0 after primary debulk, and ascites less than 500 mL at the time of recurrence.
They prospectively tested this score in DESKTOP II, which validated their findings and showed that complete secondary CRS could be achieved in 76% of those with a positive AGO score.5 Many believed that the AGO score was too restrictive, and a second retrospective study performed by a group at Memorial Sloan Kettering showed that optimal secondary cytoreduction could be achieved to prolong survival by a median of 30 months in patients with a longer disease-free interval, a single site of recurrence, and residual disease measuring less than 5 mm at time of initial/first-line surgery.6 Many individuals now use this scoring system to determine candidacy for secondary debulking: disease-free interval, number of sites of recurrence (ideally oligometastatic disease), and residual disease less than 5 mm at the time of primary debulking.
Finally, the iMODEL was developed by a group from China and found that complete R0 secondary CRS was associated with a low initial FIGO stage, no residual disease after primary surgery, longer platinum-free interval, better Eastern Cooperative Oncology Group performance status, lower CA-125 levels, as well as no ascites at the time of recurrence. Based on these criteria, individuals received either high or low iMODEL scores, and those with a low score were said to be candidates for secondary CRS. Overall, these models demonstrate that the strongest predictive factor that suggests a survival benefit from secondary CRS is the ability to achieve a complete R0 resection at the time of surgery.
Secondary debulking surgery has been tested in three large randomized controlled trials. The DESKTOP investigators and the SOC-1 trial have been the most successful groups to publish on this topic with positive results. Both groups use prognostic models for their inclusion criteria to select candidates in whom an R0 resection is believed to be most feasible. The first randomized controlled trial to publish on this topic was GOG-213,7 which did not use prognostic modeling for their inclusion criteria. Patients were randomized to secondary cytoreduction followed by platinum-based chemotherapy with or without bevacizumab versus chemotherapy alone. The median OS was 50.6 months in the surgery group and 64.7 months in the no-surgery group (P = .08), suggesting no survival benefit to secondary cytoreduction; however, an ad hoc exploratory analysis of the surgery arm showed that both overall and progression-free survival were significantly improved in the complete cytoreduction group, compared with those with residual disease at time of surgery.
The results from the GOG-213 group suggested that improved survival from secondary debulking might be achieved when prognostic modeling is used to select optimal surgical candidates. The SOC-1 trial, published in 2021, was a phase 3, randomized, controlled trial that used the iMODEL scoring system combined with PET/CT imaging for patient selection.8 Patients were again randomized to surgery followed by platinum-based chemotherapy versus chemotherapy alone. Complete cytoreduction was achieved in 73% of patients with a low iMODEL score, and these data showed improved OS in the surgery group of 58.1 months versus 53.9 months (P < .05) in the no-surgery group. Lastly, the DESKTOP group most recently published results on this topic in a large randomized, controlled trial.9 Patients were again randomized to surgery followed by platinum-based chemotherapy versus chemotherapy alone. Inclusion criteria were only met in patients with a positive AGO score. An improved OS of 7.7 months (53.7 vs. 46 months; P < .05) was demonstrated in patients that underwent surgery versus those exposed to only chemotherapy. Again, this group showed that overall survival was further improved when complete cytoreduction was achieved.
Given the results of these three trials, the Society for Gynecologic Oncology has released a statement on secondary cytoreduction in recurrent ovarian cancer (see Table).10 While it is important to use caution when comparing the three studies as study populations differed substantially, the most important takeaway the difference in survival outcomes in patients in whom complete gross resection was achieved versus no complete gross resection versus no surgery. This comparison highlights the benefit of complete cytoreduction as well as the potential harms of secondary debulking when an R0 resection cannot be achieved. Although not yet evaluated in this clinical setting, laparoscopic exploration may be useful to augment assessment of disease extent and possibility of disease resection, just as it is in frontline ovarian cancer surgery.
The importance of bevacizumab use in recurrent ovarian cancer is also highlighted in the SGO statement. In GOG-213, 84% of the total study population (in both the surgery and no surgery cohort) were treated with concurrent followed by maintenance bevacizumab with an improved survival outcome, which may suggest that this trial generalizes better than the others to contemporary management of platinum-sensitive recurrent ovarian cancer.
Overall, given the mixed data, the recommendation is for surgeons to consider all available data to guide them in treatment planning with a strong emphasis on using all available technology to assess whether complete cytoreduction can be achieved in the setting of recurrence so as to not delay the patient’s ability to receive chemotherapy.
Dr. John is a gynecologic oncology fellow at the University of North Carolina at Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the university.
References
1. du Bois A et al. J Natl Cancer Inst. 2003;95:1320-9.
2. Wagner U et al. Br J Cancer. 2012;107:588-91.
3. Vergote I et al. N Engl J Med. 2010;363:943-53.
4. Harter P et al. Ann Surg Oncol. 2006;13:1702-10.
5. Harter P et al. Int J Gynecol Cancer. 2011;21:289-95.
6. Chi DS et al. Cancer. 2006 106:1933-9.
7. Coleman RL et al. Lancet Oncol. 2017;18:779-1.
8. Shi T et al. Lancet Oncol. 2021;22:439-49.
9. Harter P et al. N Engl J Med 2021;385:2123-31.
10. Harrison R, et al. Gynecol Oncol. 2021;163:448-52.
Recurrent ovarian cancer is difficult to treat; it has high recurrence rates and poor targeted treatment options. Between 60% and 75% of patients initially diagnosed with advanced-stage ovarian cancer will relapse within 2-3 years.1 Survival for these patients is poor, with an average overall survival (OS) of 30-40 months from the time of recurrence.2 Historically, immunotherapy has shown poor efficacy for recurrent ovarian malignancy, leaving few options for patients and their providers. Given the lack of effective treatment options, secondary cytoreductive surgery (surgery at the time of recurrence) has been heavily studied as a potential therapeutic option.
The initial rationale for cytoreductive surgery (CRS) in patients with advanced ovarian cancer focused on palliation of symptoms from large, bulky disease that frequently caused obstructive symptoms and pain. Now, cytoreduction is a critical part of therapy. It decreases chemotherapy-resistant tumor cells, improves the immune response, and is thought to optimize perfusion of the residual cancer for systemic therapy. The survival benefit of surgery in the frontline setting, either with primary or interval debulking, is well established, and much of the data now demonstrate that complete resection of all macroscopic disease (also known as an R0 resection) has the greatest survival benefit.3 Given the benefits of an initial debulking surgery, secondary cytoreduction has been studied since the 1980s with mixed results. These data have demonstrated that the largest barrier to care has been appropriate patient selection for this often complex surgical procedure.
The 2020 National Comprehensive Cancer Network guidelines list secondary CRS as a treatment option; however, the procedure should only be considered in patients who have platinum sensitive disease, a performance status of 0-1, no ascites, and an isolated focus or limited focus of disease that is amenable to complete resection. Numerous retrospective studies have suggested that secondary CRS is beneficial to patients with recurrent ovarian cancer, especially if complete cytoreduction can be accomplished. Many of these studies have similarly concluded that there are benefits, such as less ascites at the time of recurrence, smaller disease burden, and a longer disease-free interval. From that foundation, multiple groups used retrospective data to investigate prognostic models to determine who would benefit most from secondary cytoreduction.
The DESKTOP Group initially published their retrospective study in 2006 and created a scoring system assessing who would benefit from secondary CRS.4 Data demonstrated that a performance status of 0, FIGO stage of I/II at the time of initial diagnosis, no residual tumor after primary surgery, and ascites less than 500 mL were associated with improved survival after secondary cytoreduction. They created the AGO score out of these data, which is positive only if three criteria are met: a performance status of 0, R0 after primary debulk, and ascites less than 500 mL at the time of recurrence.
They prospectively tested this score in DESKTOP II, which validated their findings and showed that complete secondary CRS could be achieved in 76% of those with a positive AGO score.5 Many believed that the AGO score was too restrictive, and a second retrospective study performed by a group at Memorial Sloan Kettering showed that optimal secondary cytoreduction could be achieved to prolong survival by a median of 30 months in patients with a longer disease-free interval, a single site of recurrence, and residual disease measuring less than 5 mm at time of initial/first-line surgery.6 Many individuals now use this scoring system to determine candidacy for secondary debulking: disease-free interval, number of sites of recurrence (ideally oligometastatic disease), and residual disease less than 5 mm at the time of primary debulking.
Finally, the iMODEL was developed by a group from China and found that complete R0 secondary CRS was associated with a low initial FIGO stage, no residual disease after primary surgery, longer platinum-free interval, better Eastern Cooperative Oncology Group performance status, lower CA-125 levels, as well as no ascites at the time of recurrence. Based on these criteria, individuals received either high or low iMODEL scores, and those with a low score were said to be candidates for secondary CRS. Overall, these models demonstrate that the strongest predictive factor that suggests a survival benefit from secondary CRS is the ability to achieve a complete R0 resection at the time of surgery.
Secondary debulking surgery has been tested in three large randomized controlled trials. The DESKTOP investigators and the SOC-1 trial have been the most successful groups to publish on this topic with positive results. Both groups use prognostic models for their inclusion criteria to select candidates in whom an R0 resection is believed to be most feasible. The first randomized controlled trial to publish on this topic was GOG-213,7 which did not use prognostic modeling for their inclusion criteria. Patients were randomized to secondary cytoreduction followed by platinum-based chemotherapy with or without bevacizumab versus chemotherapy alone. The median OS was 50.6 months in the surgery group and 64.7 months in the no-surgery group (P = .08), suggesting no survival benefit to secondary cytoreduction; however, an ad hoc exploratory analysis of the surgery arm showed that both overall and progression-free survival were significantly improved in the complete cytoreduction group, compared with those with residual disease at time of surgery.
The results from the GOG-213 group suggested that improved survival from secondary debulking might be achieved when prognostic modeling is used to select optimal surgical candidates. The SOC-1 trial, published in 2021, was a phase 3, randomized, controlled trial that used the iMODEL scoring system combined with PET/CT imaging for patient selection.8 Patients were again randomized to surgery followed by platinum-based chemotherapy versus chemotherapy alone. Complete cytoreduction was achieved in 73% of patients with a low iMODEL score, and these data showed improved OS in the surgery group of 58.1 months versus 53.9 months (P < .05) in the no-surgery group. Lastly, the DESKTOP group most recently published results on this topic in a large randomized, controlled trial.9 Patients were again randomized to surgery followed by platinum-based chemotherapy versus chemotherapy alone. Inclusion criteria were only met in patients with a positive AGO score. An improved OS of 7.7 months (53.7 vs. 46 months; P < .05) was demonstrated in patients that underwent surgery versus those exposed to only chemotherapy. Again, this group showed that overall survival was further improved when complete cytoreduction was achieved.
Given the results of these three trials, the Society for Gynecologic Oncology has released a statement on secondary cytoreduction in recurrent ovarian cancer (see Table).10 While it is important to use caution when comparing the three studies as study populations differed substantially, the most important takeaway the difference in survival outcomes in patients in whom complete gross resection was achieved versus no complete gross resection versus no surgery. This comparison highlights the benefit of complete cytoreduction as well as the potential harms of secondary debulking when an R0 resection cannot be achieved. Although not yet evaluated in this clinical setting, laparoscopic exploration may be useful to augment assessment of disease extent and possibility of disease resection, just as it is in frontline ovarian cancer surgery.
The importance of bevacizumab use in recurrent ovarian cancer is also highlighted in the SGO statement. In GOG-213, 84% of the total study population (in both the surgery and no surgery cohort) were treated with concurrent followed by maintenance bevacizumab with an improved survival outcome, which may suggest that this trial generalizes better than the others to contemporary management of platinum-sensitive recurrent ovarian cancer.
Overall, given the mixed data, the recommendation is for surgeons to consider all available data to guide them in treatment planning with a strong emphasis on using all available technology to assess whether complete cytoreduction can be achieved in the setting of recurrence so as to not delay the patient’s ability to receive chemotherapy.
Dr. John is a gynecologic oncology fellow at the University of North Carolina at Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the university.
References
1. du Bois A et al. J Natl Cancer Inst. 2003;95:1320-9.
2. Wagner U et al. Br J Cancer. 2012;107:588-91.
3. Vergote I et al. N Engl J Med. 2010;363:943-53.
4. Harter P et al. Ann Surg Oncol. 2006;13:1702-10.
5. Harter P et al. Int J Gynecol Cancer. 2011;21:289-95.
6. Chi DS et al. Cancer. 2006 106:1933-9.
7. Coleman RL et al. Lancet Oncol. 2017;18:779-1.
8. Shi T et al. Lancet Oncol. 2021;22:439-49.
9. Harter P et al. N Engl J Med 2021;385:2123-31.
10. Harrison R, et al. Gynecol Oncol. 2021;163:448-52.
3D-printed tumor models could advance new cancer therapies
Scientists have made big strides in the fight against cancer. A person’s risk of dying of cancer in the U.S. fell by 27% in the past 2 decades, thanks in large part to researchers who continue to uncover the complex details of how cancer works and to make advances in treatment.
Now the by enabling scientists to develop 3D tumor models that better represent samples from patients.
The impact could be “huge,” says Y. Shrike Zhang, PhD, an assistant professor of medicine at Harvard Medical School and associate bioengineer at Brigham and Women’s Hospital, both in Boston, who studies 3D bioprinting. “It is not the only technology that may allow modeling of tumors in vitro, but it certainly is one of the most capable.”
Why does that matter? Because the 2D cell cultures that scientists often use now may not capture all the complexities of how cancer grows, spreads, and responds to treatment. It’s one reason why so few potential new cancer drugs – 3.4%, according to one estimate – can pass all clinical trials. Results may not carry over from the culture dish to the patient.
Researchers say these 3D-printed blood vessels may treat certain dangerous health problems that affect your veins, arteries, or capillaries.
A 3D-bioprinted model, on the other hand, may be better at copying a tumor’s “microenvironment” – all the parts (cells, molecules, blood vessels) that surround a tumor.
“The tumor microenvironment plays an integral role in defining how cancer progresses,” says Madhuri Dey, a PhD candidate and researcher at Penn State University. “In vitro 3D models are an attempt at reconstituting a [cancer] microenvironment, which sheds light on how tumors respond to chemo or immunotherapeutic treatments when they are present in a native-like microenvironment.”
Ms. Dey is the lead author of a study funded by the National Science Foundation in which breast cancer tumors were 3D-bioprinted and successfully treated. Unlike some previous 3D models of cancer cells, this model did a better job of imitating that microenvironment, she explains.
So far, “3D bioprinting of cancer models has been limited to bioprinting of individual cancer cells laden in hydrogels,” she says. But she and her colleagues developed a technique called aspiration-assisted bioprinting that lets them control where blood vessels are located relative to the tumor. “This model lays the foundation for studying these nuances of cancer,” Ms. Dey says.
“This is a quite cool work,” Dr. Zhang says of the Penn State study (which he was not involved in). “Vascularization is always a key component in [a] majority of the tumor types.” A model that incorporates blood vessels provides a “critical niche” to help tumor models reach their full potential in cancer research.
A 3D printer for your body
Chances are you’ve heard of 3D printing and may even own (or know someone who owns) a 3D printer. The concept is like regular printing, but instead of spewing ink onto paper, a 3D printer releases layers of plastic or other materials, hundreds or thousands of times, to build an object from the ground up.
Three-dimensional bioprinting works much the same way, except those layers are made of living cells to create biological structures like skin, vessels, organs, or bone.
Bioprinting has been around since 1988. So far, it’s mainly used in research settings, such as in the field of regenerative medicine. Research is underway for ear reconstruction, nerve regeneration, and skin regeneration. The technology was also recently used to create eye tissue to help researchers study eye diseases.
The technology’s potential for use in cancer research has yet to be fully realized, Ms. Dey says. But that may be changing.
“The use of 3D-bioprinted tumor models is getting close to translations in cancer research,” says Dr. Zhang. “They are being increasingly adopted by the research field, and [the technology] has started to be explored by the pharma industry for use towards cancer drug development.”
Because bioprinting can be automated, it could allow researchers to create high-quality, complex tumor models at scale, Dr. Zhang says.
Such 3D models also have the potential to replace or reduce the use of animals in tumor drug testing, Ms. Dey notes. They “are expected to provide a more accurate drug response, compared [with] animal models, as animal physiology does not match humans’.”
The FDA Modernization Act 2.0, a new U.S. law eliminating the requirement that drugs be tested in animals before humans, has “further paved the way for such technologies in the drug development pipeline,” Dr. Zhang says.
What if we could build a custom tumor model for each patient?
Possible uses for bioprinting go beyond the lab, Ms. Dey says. Imagine if we could customize 3D tumor models based on biopsies from individual patients. Doctors could test many treatments on these patient-specific models, letting them more accurately predict how each patient would respond to different therapies. This would help doctors decide which course of treatment is best.
In Ms. Dey’s study, the 3D model was treated with chemotherapy and with immunotherapy, and it responded to both. This highlights the potential for such 3D models to reveal the body’s immune response and be used to screen therapies, she says. “We hope is that in the future, this technique can be adapted in the hospital, which would speed up the course of cancer treatment.”
To that end, she and her colleagues are now working with real breast cancer tumors removed from patients, re-creating them in the lab in 3D to use for chemo and immunotherapy screening.
A version of this article first appeared on WebMD.com.
Scientists have made big strides in the fight against cancer. A person’s risk of dying of cancer in the U.S. fell by 27% in the past 2 decades, thanks in large part to researchers who continue to uncover the complex details of how cancer works and to make advances in treatment.
Now the by enabling scientists to develop 3D tumor models that better represent samples from patients.
The impact could be “huge,” says Y. Shrike Zhang, PhD, an assistant professor of medicine at Harvard Medical School and associate bioengineer at Brigham and Women’s Hospital, both in Boston, who studies 3D bioprinting. “It is not the only technology that may allow modeling of tumors in vitro, but it certainly is one of the most capable.”
Why does that matter? Because the 2D cell cultures that scientists often use now may not capture all the complexities of how cancer grows, spreads, and responds to treatment. It’s one reason why so few potential new cancer drugs – 3.4%, according to one estimate – can pass all clinical trials. Results may not carry over from the culture dish to the patient.
Researchers say these 3D-printed blood vessels may treat certain dangerous health problems that affect your veins, arteries, or capillaries.
A 3D-bioprinted model, on the other hand, may be better at copying a tumor’s “microenvironment” – all the parts (cells, molecules, blood vessels) that surround a tumor.
“The tumor microenvironment plays an integral role in defining how cancer progresses,” says Madhuri Dey, a PhD candidate and researcher at Penn State University. “In vitro 3D models are an attempt at reconstituting a [cancer] microenvironment, which sheds light on how tumors respond to chemo or immunotherapeutic treatments when they are present in a native-like microenvironment.”
Ms. Dey is the lead author of a study funded by the National Science Foundation in which breast cancer tumors were 3D-bioprinted and successfully treated. Unlike some previous 3D models of cancer cells, this model did a better job of imitating that microenvironment, she explains.
So far, “3D bioprinting of cancer models has been limited to bioprinting of individual cancer cells laden in hydrogels,” she says. But she and her colleagues developed a technique called aspiration-assisted bioprinting that lets them control where blood vessels are located relative to the tumor. “This model lays the foundation for studying these nuances of cancer,” Ms. Dey says.
“This is a quite cool work,” Dr. Zhang says of the Penn State study (which he was not involved in). “Vascularization is always a key component in [a] majority of the tumor types.” A model that incorporates blood vessels provides a “critical niche” to help tumor models reach their full potential in cancer research.
A 3D printer for your body
Chances are you’ve heard of 3D printing and may even own (or know someone who owns) a 3D printer. The concept is like regular printing, but instead of spewing ink onto paper, a 3D printer releases layers of plastic or other materials, hundreds or thousands of times, to build an object from the ground up.
Three-dimensional bioprinting works much the same way, except those layers are made of living cells to create biological structures like skin, vessels, organs, or bone.
Bioprinting has been around since 1988. So far, it’s mainly used in research settings, such as in the field of regenerative medicine. Research is underway for ear reconstruction, nerve regeneration, and skin regeneration. The technology was also recently used to create eye tissue to help researchers study eye diseases.
The technology’s potential for use in cancer research has yet to be fully realized, Ms. Dey says. But that may be changing.
“The use of 3D-bioprinted tumor models is getting close to translations in cancer research,” says Dr. Zhang. “They are being increasingly adopted by the research field, and [the technology] has started to be explored by the pharma industry for use towards cancer drug development.”
Because bioprinting can be automated, it could allow researchers to create high-quality, complex tumor models at scale, Dr. Zhang says.
Such 3D models also have the potential to replace or reduce the use of animals in tumor drug testing, Ms. Dey notes. They “are expected to provide a more accurate drug response, compared [with] animal models, as animal physiology does not match humans’.”
The FDA Modernization Act 2.0, a new U.S. law eliminating the requirement that drugs be tested in animals before humans, has “further paved the way for such technologies in the drug development pipeline,” Dr. Zhang says.
What if we could build a custom tumor model for each patient?
Possible uses for bioprinting go beyond the lab, Ms. Dey says. Imagine if we could customize 3D tumor models based on biopsies from individual patients. Doctors could test many treatments on these patient-specific models, letting them more accurately predict how each patient would respond to different therapies. This would help doctors decide which course of treatment is best.
In Ms. Dey’s study, the 3D model was treated with chemotherapy and with immunotherapy, and it responded to both. This highlights the potential for such 3D models to reveal the body’s immune response and be used to screen therapies, she says. “We hope is that in the future, this technique can be adapted in the hospital, which would speed up the course of cancer treatment.”
To that end, she and her colleagues are now working with real breast cancer tumors removed from patients, re-creating them in the lab in 3D to use for chemo and immunotherapy screening.
A version of this article first appeared on WebMD.com.
Scientists have made big strides in the fight against cancer. A person’s risk of dying of cancer in the U.S. fell by 27% in the past 2 decades, thanks in large part to researchers who continue to uncover the complex details of how cancer works and to make advances in treatment.
Now the by enabling scientists to develop 3D tumor models that better represent samples from patients.
The impact could be “huge,” says Y. Shrike Zhang, PhD, an assistant professor of medicine at Harvard Medical School and associate bioengineer at Brigham and Women’s Hospital, both in Boston, who studies 3D bioprinting. “It is not the only technology that may allow modeling of tumors in vitro, but it certainly is one of the most capable.”
Why does that matter? Because the 2D cell cultures that scientists often use now may not capture all the complexities of how cancer grows, spreads, and responds to treatment. It’s one reason why so few potential new cancer drugs – 3.4%, according to one estimate – can pass all clinical trials. Results may not carry over from the culture dish to the patient.
Researchers say these 3D-printed blood vessels may treat certain dangerous health problems that affect your veins, arteries, or capillaries.
A 3D-bioprinted model, on the other hand, may be better at copying a tumor’s “microenvironment” – all the parts (cells, molecules, blood vessels) that surround a tumor.
“The tumor microenvironment plays an integral role in defining how cancer progresses,” says Madhuri Dey, a PhD candidate and researcher at Penn State University. “In vitro 3D models are an attempt at reconstituting a [cancer] microenvironment, which sheds light on how tumors respond to chemo or immunotherapeutic treatments when they are present in a native-like microenvironment.”
Ms. Dey is the lead author of a study funded by the National Science Foundation in which breast cancer tumors were 3D-bioprinted and successfully treated. Unlike some previous 3D models of cancer cells, this model did a better job of imitating that microenvironment, she explains.
So far, “3D bioprinting of cancer models has been limited to bioprinting of individual cancer cells laden in hydrogels,” she says. But she and her colleagues developed a technique called aspiration-assisted bioprinting that lets them control where blood vessels are located relative to the tumor. “This model lays the foundation for studying these nuances of cancer,” Ms. Dey says.
“This is a quite cool work,” Dr. Zhang says of the Penn State study (which he was not involved in). “Vascularization is always a key component in [a] majority of the tumor types.” A model that incorporates blood vessels provides a “critical niche” to help tumor models reach their full potential in cancer research.
A 3D printer for your body
Chances are you’ve heard of 3D printing and may even own (or know someone who owns) a 3D printer. The concept is like regular printing, but instead of spewing ink onto paper, a 3D printer releases layers of plastic or other materials, hundreds or thousands of times, to build an object from the ground up.
Three-dimensional bioprinting works much the same way, except those layers are made of living cells to create biological structures like skin, vessels, organs, or bone.
Bioprinting has been around since 1988. So far, it’s mainly used in research settings, such as in the field of regenerative medicine. Research is underway for ear reconstruction, nerve regeneration, and skin regeneration. The technology was also recently used to create eye tissue to help researchers study eye diseases.
The technology’s potential for use in cancer research has yet to be fully realized, Ms. Dey says. But that may be changing.
“The use of 3D-bioprinted tumor models is getting close to translations in cancer research,” says Dr. Zhang. “They are being increasingly adopted by the research field, and [the technology] has started to be explored by the pharma industry for use towards cancer drug development.”
Because bioprinting can be automated, it could allow researchers to create high-quality, complex tumor models at scale, Dr. Zhang says.
Such 3D models also have the potential to replace or reduce the use of animals in tumor drug testing, Ms. Dey notes. They “are expected to provide a more accurate drug response, compared [with] animal models, as animal physiology does not match humans’.”
The FDA Modernization Act 2.0, a new U.S. law eliminating the requirement that drugs be tested in animals before humans, has “further paved the way for such technologies in the drug development pipeline,” Dr. Zhang says.
What if we could build a custom tumor model for each patient?
Possible uses for bioprinting go beyond the lab, Ms. Dey says. Imagine if we could customize 3D tumor models based on biopsies from individual patients. Doctors could test many treatments on these patient-specific models, letting them more accurately predict how each patient would respond to different therapies. This would help doctors decide which course of treatment is best.
In Ms. Dey’s study, the 3D model was treated with chemotherapy and with immunotherapy, and it responded to both. This highlights the potential for such 3D models to reveal the body’s immune response and be used to screen therapies, she says. “We hope is that in the future, this technique can be adapted in the hospital, which would speed up the course of cancer treatment.”
To that end, she and her colleagues are now working with real breast cancer tumors removed from patients, re-creating them in the lab in 3D to use for chemo and immunotherapy screening.
A version of this article first appeared on WebMD.com.
People with cancer should be wary of taking dietary supplements
Cancer dietitian Lisa Cianciotta often finds herself sitting across from a patient who suddenly fishes a bottle of antioxidant supplements from their bag and says, “My friend told me this works really well,” or “I read on the Internet that this is supposed to be really good for cancer.”
Although taking an antioxidant pill sounds harmless, Ms. Cianciotta, a clinical dietitian who works with cancer patients at New York–Presbyterian Hospital, knows well that this popular dietary supplement can interfere with a patient’s radiation or chemotherapy.
But many patients with cancer believe these over-the-counter vitamins, minerals, or herbal remedies will help them, and most use at least one dietary supplement alongside their cancer treatment.
And that leaves Ms. Cianciotta with a delicate conversation ahead of her.
. Popular dietary supplements may, for instance, cancel the effects of a cancer treatment, making it less effective, or increase serious side effects, such as liver toxicity. But in other cases, supplementation, such as vitamin D for patients who lack the vitamin, may be beneficial, Ms. Cianciotta said.
These drug-supplement interactions can be hard to pinpoint, given that more than two-thirds of doctors don’t know their patients are using supplements.
Here’s what patients need to know about the potential risks of supplement use during treatment, and how oncologists can address this thorny, often poorly understood topic with patients.
The complex drug-supplement landscape
The list of dietary supplements and how they can interact with different treatments and cancer types is long and nuanced.
But certain supplements appear to affect cancer treatments regardless of other things and should be avoided. Any supplement that strongly alters the body’s levels of the protein cytochromes P450 is one example. This group of enzymes plays a key role in metabolizing drugs, including chemotherapy and immunotherapy agents.
Certain supplements – most notably St. John’s wort extract – may decrease or increase the activity of cytochrome P450, which can then affect the concentrations of anticancer drugs in the blood, said William Figg, PharmD, an associate director of the Center for Cancer Research at the National Cancer Institute in Bethesda, Md. Studies show, for instance, that this common herbal supplement can increase the activity of cytochrome P450, resulting in lower levels of cancer drugs.
Outside of drug metabolism, patients with hormone-related cancers, such as breast and prostate cancers, should steer clear of dietary supplements that can alter levels of testosterone or estrogen, Dr. Figg said. The evergreen shrub ashwagandha, for example, is marketed to reduce stress and fatigue, but can also increase testosterone levels – a potential problem for those with prostate cancer receiving androgen deprivation therapy, which lowers testosterone levels.
Many oncologists counsel patients against using antioxidant-based dietary supplements – particularly turmeric and green tea extract – while they have radiation therapy and certain chemotherapies. These therapies work by creating an abundance of highly reactive molecules called free radicals in tumor cells, which increase stress within these cells, ultimately killing them off. Antioxidants, in theory, can neutralize this effect, said Skyler Johnson, MD, a radiation oncologist at Huntsman Cancer Institute at the University of Utah, Salt Lake City. Some studies suggest that antioxidant supplements may lessen the effects of radiation and chemotherapy, although the evidence is mixed.
Some dietary supplements, including high-dose green tea extract and vitamin A, can cause kidney or liver toxicity, and “many cancer patients already have compromised kidney or liver function,” said Jun J. Mao, MD, chief of integrative medicine at Memorial Sloan Kettering Cancer Center in New York. Even herbs that don’t interfere with how well a cancer drug works, such as stevia, can increase treatment-related side effects, such as nausea and vomiting.
Another potential problem with dietary supplements: It’s nearly impossible to know exactly what’s in them. For instance, just last year, the Food and Drug Administration sent nearly 50 warning letters to companies marketing dietary supplements. The issue is that federal regulations governing production are less strict for supplements than for medications. As a result, some supplements contain ingredients not listed on the label.
One historical example was the supplement PC-SPES, a mix of eight herbs, marketed to men with prostate cancer. The supplement was recalled in 2002 after certain batches were found to contain traces of prescription drugs, including diethylstilbestrol, ethinyl estradiol, warfarin, and alprazolam.
To further complicate matters, some dietary supplements can be helpful. Most patients with cancer “are malnourished and missing out on nutrients they could be getting from food,” said Ms. Cianciotta.
Patients are tested routinely for vitamin deficiencies and receive supplements as needed, she said. Vitamin D and folic acid are two of the most common deficiencies in this patient population. Vitamin D supplementation can improve outcomes in patients who have received a stem cell transplant by aiding engraftment and rebuilding the immune system, while folic acid supplementation can help to raise low red blood cell counts and hemoglobin levels.
Although she rarely sees vitamin toxicity, Ms. Cianciotta stressed that more is not always better and supplement use, even when it seems safe or warranted due to a deficiency, should be taken under supervision, and monitored carefully by the patient’s care team.
Bringing supplement use into the light
Too often, providers are unaware of a patient’s supplement use.
A core reason: Dietary supplements are often touted as natural, which many patients equate with safety, said Samantha Heller, a senior clinical nutritionist at New York (N.Y.) University Langone Health.
That means patients may not know a supplement can act like a drug and interfere with their cancer treatment, and thus may not see the importance of telling their doctors.
Still, the promise of herbs, vitamins, and minerals can be alluring, and there are many reasons patients decide to partake. One major appeal: Dietary supplements can help some patients feel empowered.
“Cancer is a disease that takes away a lot of control from the individual. Taking supplements or herbs is a way to regain some sense of control,” said Dr. Mao.
The phenomenon can also be cultural, he said. Some people grow up taking herbs and supplements to stay healthy or combat health woes.
Pressure or advice from family or friends who may think they are helping a loved one with their dietary recommendations may play a role as well. Friends and family “cannot prescribe chemo, but they can buy herbs and supplements,” Dr. Mao said.
Patients seeking greater control over their health or who feel high levels of anxiety may be more likely to take suggestions from friends and family or more likely to believe false or misleading claims about the efficacy or safety of supplements, explained medical oncologist William Dahut, MD, chief scientific officer for the American Cancer Society.
Plus, social media often amplifies and normalizes this misinformation, noted Dr. Johnson. In a 2021 study published in the Journal of the National Cancer Institute, he and colleagues found that one-third of the most popular articles on cancer treatment posted to social media in 2018 and 2019 contained false, inaccurate, or misleading information that was often harmful.
Some of the false claims centered on unproven, potentially unsafe herbal remedies, according to Dr. Johnson. These included “lung cancer can be cured with cannabis oil” and “golden berries cure and prevent cancer.”
Given exaggerated claims of “cures,” some patients may keep their supplement use under the radar out of fear they will be judged or criticized.
“Clinicians should avoid making patients feel judged or telling people not to go online to do their own research,” Dr. Johnson said.
Guiding patients, instead, to accurate sources of online information may be one way to help patients feel empowered, he said. Cancer.gov and the Memorial Sloan Kettering Cancer Center’s About Herbs database provide accessible and accurate information on dietary supplements and cancer treatment for both health care professionals and patients, he noted.
If a particular supplement is not safe during treatment, providers should be able to explain why, said Ms. Cianciotta. In a recent study, 80% of health care providers surveyed believed that interactions between herbals and medications could be problematic, but only 15% could explain why.
“Being able to explain why we are discouraging a particular supplement right now tends to be much better received than just telling a patient not to take something, because it is bad,” she said.
Another key is listening closely to patients to understand why they may be taking a particular supplement. Does the patient feel out of control? Is nausea a problem?
“Allowing patients to tell you why they are using a particular supplement will often reveal unmet needs or psychosocial challenges,” Dr. Mao said. This information can allow providers to suggest an evidence-based alternative, such as mindfulness meditation or acupuncture, to manage stress.
And if a patient has received a dietary supplement from well-meaning family and friends?
“Simply telling a patient that a given supplement is useless or harmful could create family tension,” said Dr. Mao.
Instead, he recommends reframing the issue.
“We want to have a better understanding of how patients are tolerating chemo or immunotherapy before throwing other things on top of it. Let them know that now may just not be the right time to add a supplement to the mix,” Dr. Mao said.
The bottom line: “Patients want to play an active role in their own care, and we want to help them do that in a safe way,” he said.
A version of this article first appeared on WebMD.com.
Cancer dietitian Lisa Cianciotta often finds herself sitting across from a patient who suddenly fishes a bottle of antioxidant supplements from their bag and says, “My friend told me this works really well,” or “I read on the Internet that this is supposed to be really good for cancer.”
Although taking an antioxidant pill sounds harmless, Ms. Cianciotta, a clinical dietitian who works with cancer patients at New York–Presbyterian Hospital, knows well that this popular dietary supplement can interfere with a patient’s radiation or chemotherapy.
But many patients with cancer believe these over-the-counter vitamins, minerals, or herbal remedies will help them, and most use at least one dietary supplement alongside their cancer treatment.
And that leaves Ms. Cianciotta with a delicate conversation ahead of her.
. Popular dietary supplements may, for instance, cancel the effects of a cancer treatment, making it less effective, or increase serious side effects, such as liver toxicity. But in other cases, supplementation, such as vitamin D for patients who lack the vitamin, may be beneficial, Ms. Cianciotta said.
These drug-supplement interactions can be hard to pinpoint, given that more than two-thirds of doctors don’t know their patients are using supplements.
Here’s what patients need to know about the potential risks of supplement use during treatment, and how oncologists can address this thorny, often poorly understood topic with patients.
The complex drug-supplement landscape
The list of dietary supplements and how they can interact with different treatments and cancer types is long and nuanced.
But certain supplements appear to affect cancer treatments regardless of other things and should be avoided. Any supplement that strongly alters the body’s levels of the protein cytochromes P450 is one example. This group of enzymes plays a key role in metabolizing drugs, including chemotherapy and immunotherapy agents.
Certain supplements – most notably St. John’s wort extract – may decrease or increase the activity of cytochrome P450, which can then affect the concentrations of anticancer drugs in the blood, said William Figg, PharmD, an associate director of the Center for Cancer Research at the National Cancer Institute in Bethesda, Md. Studies show, for instance, that this common herbal supplement can increase the activity of cytochrome P450, resulting in lower levels of cancer drugs.
Outside of drug metabolism, patients with hormone-related cancers, such as breast and prostate cancers, should steer clear of dietary supplements that can alter levels of testosterone or estrogen, Dr. Figg said. The evergreen shrub ashwagandha, for example, is marketed to reduce stress and fatigue, but can also increase testosterone levels – a potential problem for those with prostate cancer receiving androgen deprivation therapy, which lowers testosterone levels.
Many oncologists counsel patients against using antioxidant-based dietary supplements – particularly turmeric and green tea extract – while they have radiation therapy and certain chemotherapies. These therapies work by creating an abundance of highly reactive molecules called free radicals in tumor cells, which increase stress within these cells, ultimately killing them off. Antioxidants, in theory, can neutralize this effect, said Skyler Johnson, MD, a radiation oncologist at Huntsman Cancer Institute at the University of Utah, Salt Lake City. Some studies suggest that antioxidant supplements may lessen the effects of radiation and chemotherapy, although the evidence is mixed.
Some dietary supplements, including high-dose green tea extract and vitamin A, can cause kidney or liver toxicity, and “many cancer patients already have compromised kidney or liver function,” said Jun J. Mao, MD, chief of integrative medicine at Memorial Sloan Kettering Cancer Center in New York. Even herbs that don’t interfere with how well a cancer drug works, such as stevia, can increase treatment-related side effects, such as nausea and vomiting.
Another potential problem with dietary supplements: It’s nearly impossible to know exactly what’s in them. For instance, just last year, the Food and Drug Administration sent nearly 50 warning letters to companies marketing dietary supplements. The issue is that federal regulations governing production are less strict for supplements than for medications. As a result, some supplements contain ingredients not listed on the label.
One historical example was the supplement PC-SPES, a mix of eight herbs, marketed to men with prostate cancer. The supplement was recalled in 2002 after certain batches were found to contain traces of prescription drugs, including diethylstilbestrol, ethinyl estradiol, warfarin, and alprazolam.
To further complicate matters, some dietary supplements can be helpful. Most patients with cancer “are malnourished and missing out on nutrients they could be getting from food,” said Ms. Cianciotta.
Patients are tested routinely for vitamin deficiencies and receive supplements as needed, she said. Vitamin D and folic acid are two of the most common deficiencies in this patient population. Vitamin D supplementation can improve outcomes in patients who have received a stem cell transplant by aiding engraftment and rebuilding the immune system, while folic acid supplementation can help to raise low red blood cell counts and hemoglobin levels.
Although she rarely sees vitamin toxicity, Ms. Cianciotta stressed that more is not always better and supplement use, even when it seems safe or warranted due to a deficiency, should be taken under supervision, and monitored carefully by the patient’s care team.
Bringing supplement use into the light
Too often, providers are unaware of a patient’s supplement use.
A core reason: Dietary supplements are often touted as natural, which many patients equate with safety, said Samantha Heller, a senior clinical nutritionist at New York (N.Y.) University Langone Health.
That means patients may not know a supplement can act like a drug and interfere with their cancer treatment, and thus may not see the importance of telling their doctors.
Still, the promise of herbs, vitamins, and minerals can be alluring, and there are many reasons patients decide to partake. One major appeal: Dietary supplements can help some patients feel empowered.
“Cancer is a disease that takes away a lot of control from the individual. Taking supplements or herbs is a way to regain some sense of control,” said Dr. Mao.
The phenomenon can also be cultural, he said. Some people grow up taking herbs and supplements to stay healthy or combat health woes.
Pressure or advice from family or friends who may think they are helping a loved one with their dietary recommendations may play a role as well. Friends and family “cannot prescribe chemo, but they can buy herbs and supplements,” Dr. Mao said.
Patients seeking greater control over their health or who feel high levels of anxiety may be more likely to take suggestions from friends and family or more likely to believe false or misleading claims about the efficacy or safety of supplements, explained medical oncologist William Dahut, MD, chief scientific officer for the American Cancer Society.
Plus, social media often amplifies and normalizes this misinformation, noted Dr. Johnson. In a 2021 study published in the Journal of the National Cancer Institute, he and colleagues found that one-third of the most popular articles on cancer treatment posted to social media in 2018 and 2019 contained false, inaccurate, or misleading information that was often harmful.
Some of the false claims centered on unproven, potentially unsafe herbal remedies, according to Dr. Johnson. These included “lung cancer can be cured with cannabis oil” and “golden berries cure and prevent cancer.”
Given exaggerated claims of “cures,” some patients may keep their supplement use under the radar out of fear they will be judged or criticized.
“Clinicians should avoid making patients feel judged or telling people not to go online to do their own research,” Dr. Johnson said.
Guiding patients, instead, to accurate sources of online information may be one way to help patients feel empowered, he said. Cancer.gov and the Memorial Sloan Kettering Cancer Center’s About Herbs database provide accessible and accurate information on dietary supplements and cancer treatment for both health care professionals and patients, he noted.
If a particular supplement is not safe during treatment, providers should be able to explain why, said Ms. Cianciotta. In a recent study, 80% of health care providers surveyed believed that interactions between herbals and medications could be problematic, but only 15% could explain why.
“Being able to explain why we are discouraging a particular supplement right now tends to be much better received than just telling a patient not to take something, because it is bad,” she said.
Another key is listening closely to patients to understand why they may be taking a particular supplement. Does the patient feel out of control? Is nausea a problem?
“Allowing patients to tell you why they are using a particular supplement will often reveal unmet needs or psychosocial challenges,” Dr. Mao said. This information can allow providers to suggest an evidence-based alternative, such as mindfulness meditation or acupuncture, to manage stress.
And if a patient has received a dietary supplement from well-meaning family and friends?
“Simply telling a patient that a given supplement is useless or harmful could create family tension,” said Dr. Mao.
Instead, he recommends reframing the issue.
“We want to have a better understanding of how patients are tolerating chemo or immunotherapy before throwing other things on top of it. Let them know that now may just not be the right time to add a supplement to the mix,” Dr. Mao said.
The bottom line: “Patients want to play an active role in their own care, and we want to help them do that in a safe way,” he said.
A version of this article first appeared on WebMD.com.
Cancer dietitian Lisa Cianciotta often finds herself sitting across from a patient who suddenly fishes a bottle of antioxidant supplements from their bag and says, “My friend told me this works really well,” or “I read on the Internet that this is supposed to be really good for cancer.”
Although taking an antioxidant pill sounds harmless, Ms. Cianciotta, a clinical dietitian who works with cancer patients at New York–Presbyterian Hospital, knows well that this popular dietary supplement can interfere with a patient’s radiation or chemotherapy.
But many patients with cancer believe these over-the-counter vitamins, minerals, or herbal remedies will help them, and most use at least one dietary supplement alongside their cancer treatment.
And that leaves Ms. Cianciotta with a delicate conversation ahead of her.
. Popular dietary supplements may, for instance, cancel the effects of a cancer treatment, making it less effective, or increase serious side effects, such as liver toxicity. But in other cases, supplementation, such as vitamin D for patients who lack the vitamin, may be beneficial, Ms. Cianciotta said.
These drug-supplement interactions can be hard to pinpoint, given that more than two-thirds of doctors don’t know their patients are using supplements.
Here’s what patients need to know about the potential risks of supplement use during treatment, and how oncologists can address this thorny, often poorly understood topic with patients.
The complex drug-supplement landscape
The list of dietary supplements and how they can interact with different treatments and cancer types is long and nuanced.
But certain supplements appear to affect cancer treatments regardless of other things and should be avoided. Any supplement that strongly alters the body’s levels of the protein cytochromes P450 is one example. This group of enzymes plays a key role in metabolizing drugs, including chemotherapy and immunotherapy agents.
Certain supplements – most notably St. John’s wort extract – may decrease or increase the activity of cytochrome P450, which can then affect the concentrations of anticancer drugs in the blood, said William Figg, PharmD, an associate director of the Center for Cancer Research at the National Cancer Institute in Bethesda, Md. Studies show, for instance, that this common herbal supplement can increase the activity of cytochrome P450, resulting in lower levels of cancer drugs.
Outside of drug metabolism, patients with hormone-related cancers, such as breast and prostate cancers, should steer clear of dietary supplements that can alter levels of testosterone or estrogen, Dr. Figg said. The evergreen shrub ashwagandha, for example, is marketed to reduce stress and fatigue, but can also increase testosterone levels – a potential problem for those with prostate cancer receiving androgen deprivation therapy, which lowers testosterone levels.
Many oncologists counsel patients against using antioxidant-based dietary supplements – particularly turmeric and green tea extract – while they have radiation therapy and certain chemotherapies. These therapies work by creating an abundance of highly reactive molecules called free radicals in tumor cells, which increase stress within these cells, ultimately killing them off. Antioxidants, in theory, can neutralize this effect, said Skyler Johnson, MD, a radiation oncologist at Huntsman Cancer Institute at the University of Utah, Salt Lake City. Some studies suggest that antioxidant supplements may lessen the effects of radiation and chemotherapy, although the evidence is mixed.
Some dietary supplements, including high-dose green tea extract and vitamin A, can cause kidney or liver toxicity, and “many cancer patients already have compromised kidney or liver function,” said Jun J. Mao, MD, chief of integrative medicine at Memorial Sloan Kettering Cancer Center in New York. Even herbs that don’t interfere with how well a cancer drug works, such as stevia, can increase treatment-related side effects, such as nausea and vomiting.
Another potential problem with dietary supplements: It’s nearly impossible to know exactly what’s in them. For instance, just last year, the Food and Drug Administration sent nearly 50 warning letters to companies marketing dietary supplements. The issue is that federal regulations governing production are less strict for supplements than for medications. As a result, some supplements contain ingredients not listed on the label.
One historical example was the supplement PC-SPES, a mix of eight herbs, marketed to men with prostate cancer. The supplement was recalled in 2002 after certain batches were found to contain traces of prescription drugs, including diethylstilbestrol, ethinyl estradiol, warfarin, and alprazolam.
To further complicate matters, some dietary supplements can be helpful. Most patients with cancer “are malnourished and missing out on nutrients they could be getting from food,” said Ms. Cianciotta.
Patients are tested routinely for vitamin deficiencies and receive supplements as needed, she said. Vitamin D and folic acid are two of the most common deficiencies in this patient population. Vitamin D supplementation can improve outcomes in patients who have received a stem cell transplant by aiding engraftment and rebuilding the immune system, while folic acid supplementation can help to raise low red blood cell counts and hemoglobin levels.
Although she rarely sees vitamin toxicity, Ms. Cianciotta stressed that more is not always better and supplement use, even when it seems safe or warranted due to a deficiency, should be taken under supervision, and monitored carefully by the patient’s care team.
Bringing supplement use into the light
Too often, providers are unaware of a patient’s supplement use.
A core reason: Dietary supplements are often touted as natural, which many patients equate with safety, said Samantha Heller, a senior clinical nutritionist at New York (N.Y.) University Langone Health.
That means patients may not know a supplement can act like a drug and interfere with their cancer treatment, and thus may not see the importance of telling their doctors.
Still, the promise of herbs, vitamins, and minerals can be alluring, and there are many reasons patients decide to partake. One major appeal: Dietary supplements can help some patients feel empowered.
“Cancer is a disease that takes away a lot of control from the individual. Taking supplements or herbs is a way to regain some sense of control,” said Dr. Mao.
The phenomenon can also be cultural, he said. Some people grow up taking herbs and supplements to stay healthy or combat health woes.
Pressure or advice from family or friends who may think they are helping a loved one with their dietary recommendations may play a role as well. Friends and family “cannot prescribe chemo, but they can buy herbs and supplements,” Dr. Mao said.
Patients seeking greater control over their health or who feel high levels of anxiety may be more likely to take suggestions from friends and family or more likely to believe false or misleading claims about the efficacy or safety of supplements, explained medical oncologist William Dahut, MD, chief scientific officer for the American Cancer Society.
Plus, social media often amplifies and normalizes this misinformation, noted Dr. Johnson. In a 2021 study published in the Journal of the National Cancer Institute, he and colleagues found that one-third of the most popular articles on cancer treatment posted to social media in 2018 and 2019 contained false, inaccurate, or misleading information that was often harmful.
Some of the false claims centered on unproven, potentially unsafe herbal remedies, according to Dr. Johnson. These included “lung cancer can be cured with cannabis oil” and “golden berries cure and prevent cancer.”
Given exaggerated claims of “cures,” some patients may keep their supplement use under the radar out of fear they will be judged or criticized.
“Clinicians should avoid making patients feel judged or telling people not to go online to do their own research,” Dr. Johnson said.
Guiding patients, instead, to accurate sources of online information may be one way to help patients feel empowered, he said. Cancer.gov and the Memorial Sloan Kettering Cancer Center’s About Herbs database provide accessible and accurate information on dietary supplements and cancer treatment for both health care professionals and patients, he noted.
If a particular supplement is not safe during treatment, providers should be able to explain why, said Ms. Cianciotta. In a recent study, 80% of health care providers surveyed believed that interactions between herbals and medications could be problematic, but only 15% could explain why.
“Being able to explain why we are discouraging a particular supplement right now tends to be much better received than just telling a patient not to take something, because it is bad,” she said.
Another key is listening closely to patients to understand why they may be taking a particular supplement. Does the patient feel out of control? Is nausea a problem?
“Allowing patients to tell you why they are using a particular supplement will often reveal unmet needs or psychosocial challenges,” Dr. Mao said. This information can allow providers to suggest an evidence-based alternative, such as mindfulness meditation or acupuncture, to manage stress.
And if a patient has received a dietary supplement from well-meaning family and friends?
“Simply telling a patient that a given supplement is useless or harmful could create family tension,” said Dr. Mao.
Instead, he recommends reframing the issue.
“We want to have a better understanding of how patients are tolerating chemo or immunotherapy before throwing other things on top of it. Let them know that now may just not be the right time to add a supplement to the mix,” Dr. Mao said.
The bottom line: “Patients want to play an active role in their own care, and we want to help them do that in a safe way,” he said.
A version of this article first appeared on WebMD.com.




