User login
Pill not enough for ‘sexual problems’ female cancer patients face
The antidepressant bupropion failed to improve sexual dysfunction in female cancer survivors, according to new findings published online in the Journal of Clinical Oncology.
Using the Female Sexual Function Index (FSFI) as a measurement tool, investigators found that desire scores were not significantly different for participants who received bupropion versus a placebo over the 9-week study period.
“Sexual health is a complex phenomenon and [our results suggest that] no one intervention is going to solve the broader issue,” lead author Debra Barton, RN, PhD, FAAN, professor in the School of Nursing at the University of Michigan, Ann Arbor, told this news organization.
Sexual dysfunction is common among cancer survivors and experienced across multiple cancer types and stages of disease. Research shows that as many as 70% of female cancer survivors report loss of desire, compared with up to one-third of the general population.
Common sexual concerns among female cancer survivors include low desire, arousal issues, lack of appropriate lubrication, difficulty in achieving orgasm, and pain with penetrative sexual activity. Additionally, these women may experience significant overlap of symptoms, and often encounter multiple sexual issues that are exacerbated by a range of cancer treatments.
“It’s a huge problem,” Maryam B. Lustberg, MD, MPH, from Yale Cancer Center, New Haven, Conn., and colleagues wrote in an accompanying editorial.
Despite the prevalence of sexual dysfunction among cancer survivors, effective treatments remain elusive. Preliminary evidence suggests that bupropion, already approved for seasonal affective disorder, major depressive disorder, and smoking cessation, may also enhance libido.
Dr. Barton and colleagues conducted this phase 2 trial to determine whether bupropion can improve sexual desire in female cancer survivors without undesirable side effects.
In the study, Dr. Barton and colleagues compared two dose levels of extended-release bupropion in a cohort of 230 postmenopausal women diagnosed with breast or gynecologic cancer and low baseline FSFI desire scores (<3.3), who had completed definitive cancer therapy.
Participants were randomized to receive either 150 mg (79 patients) or 300 mg (74 patients) once daily of extended-release bupropion, or placebo (77 patients).
Barton and colleagues then evaluated whether sexual desire significantly improved over the 9-week study period comparing the bupropion arms and the placebo group.
Overall, the authors found no significant differences (mean between-arm change for 150 mg once daily and placebo of 0.02; P = .93; mean between-arm change for 300 mg once daily and placebo of –0.02; P = .92). Mean scores at 9 weeks on the desire subscale were 2.17, 2.27, and 2.30 for 150 mg, 300 mg, and the placebo group, respectively.
In addition, none of the subscales – which included arousal, lubrication, and orgasm – or the total score showed a significant difference between arms at either 5 or 9 weeks.
Bupropion did, however, appear to be well tolerated. No grade 4-5 treatment-related adverse events occurred. In the 150-mg bupropion arm, two patients (2.6%) experienced a grade 3 event (insomnia and headache) and one patient in the 300-mg bupropion arm (1.4%) and placebo arm (1.3%) experienced a grade 3 event related to treatment (hypertension and headache, respectively).
In the accompanying editorial, Dr. Lustberg and colleagues “applaud the authors for conducting a study in this population of cancer survivors,” noting that “evidenced-based approaches have not been extensively studied.”
Dr. Lustberg and colleagues also commented that other randomized controlled clinical trials evaluating sexual desire disorder assessed outcomes using additional metrics, such as the Female Sexual Distress Scale–Revised questionnaire, which measures distress related to sexual dysfunction and low desire, in particular.
“The use of specific validated instruments for libido in place of the FSFI might have helped determine the effect of the study intervention in this reported trial,” they wrote.
Overall, according to Dr. Lustberg and colleagues, the negative results of this study indicate that a multidisciplinary clinical approach may be needed.
“As much as we would like to have one intervention that addresses this prominent issue, the evidence strongly suggests that cancer-related sexual problems may need an integrative biopsychosocial model that intervenes on biologic, psychologic, interpersonal, and social-cultural factors, not just on one factor, such as libido,” they wrote. “Such work may require access to multidisciplinary care with specialists in women’s health, pelvic floor rehabilitation, and psychosocial oncology.”
Dr. Barton said she has been developing a multicomponent approach to addressing sexual health in female cancer survivors.
However, she noted, “there is still much we do not fully understand about the broader impact of the degree of hormone deprivation in the population of female cancer survivors. A better understanding would provide clearer targets for interventions.”
The study was supported by the National Cancer Institute and Breast Cancer Research Foundation. Dr. Barton has disclosed research funding from Merck. Dr. Lustberg reported receiving honoraria from Novartis and Biotheranostics; consulting or advising with PledPharma, Disarm Therapeutics, Pfizer; and other relationships with Cynosure/Hologic.
A version of this article first appeared on Medscape.com.
The antidepressant bupropion failed to improve sexual dysfunction in female cancer survivors, according to new findings published online in the Journal of Clinical Oncology.
Using the Female Sexual Function Index (FSFI) as a measurement tool, investigators found that desire scores were not significantly different for participants who received bupropion versus a placebo over the 9-week study period.
“Sexual health is a complex phenomenon and [our results suggest that] no one intervention is going to solve the broader issue,” lead author Debra Barton, RN, PhD, FAAN, professor in the School of Nursing at the University of Michigan, Ann Arbor, told this news organization.
Sexual dysfunction is common among cancer survivors and experienced across multiple cancer types and stages of disease. Research shows that as many as 70% of female cancer survivors report loss of desire, compared with up to one-third of the general population.
Common sexual concerns among female cancer survivors include low desire, arousal issues, lack of appropriate lubrication, difficulty in achieving orgasm, and pain with penetrative sexual activity. Additionally, these women may experience significant overlap of symptoms, and often encounter multiple sexual issues that are exacerbated by a range of cancer treatments.
“It’s a huge problem,” Maryam B. Lustberg, MD, MPH, from Yale Cancer Center, New Haven, Conn., and colleagues wrote in an accompanying editorial.
Despite the prevalence of sexual dysfunction among cancer survivors, effective treatments remain elusive. Preliminary evidence suggests that bupropion, already approved for seasonal affective disorder, major depressive disorder, and smoking cessation, may also enhance libido.
Dr. Barton and colleagues conducted this phase 2 trial to determine whether bupropion can improve sexual desire in female cancer survivors without undesirable side effects.
In the study, Dr. Barton and colleagues compared two dose levels of extended-release bupropion in a cohort of 230 postmenopausal women diagnosed with breast or gynecologic cancer and low baseline FSFI desire scores (<3.3), who had completed definitive cancer therapy.
Participants were randomized to receive either 150 mg (79 patients) or 300 mg (74 patients) once daily of extended-release bupropion, or placebo (77 patients).
Barton and colleagues then evaluated whether sexual desire significantly improved over the 9-week study period comparing the bupropion arms and the placebo group.
Overall, the authors found no significant differences (mean between-arm change for 150 mg once daily and placebo of 0.02; P = .93; mean between-arm change for 300 mg once daily and placebo of –0.02; P = .92). Mean scores at 9 weeks on the desire subscale were 2.17, 2.27, and 2.30 for 150 mg, 300 mg, and the placebo group, respectively.
In addition, none of the subscales – which included arousal, lubrication, and orgasm – or the total score showed a significant difference between arms at either 5 or 9 weeks.
Bupropion did, however, appear to be well tolerated. No grade 4-5 treatment-related adverse events occurred. In the 150-mg bupropion arm, two patients (2.6%) experienced a grade 3 event (insomnia and headache) and one patient in the 300-mg bupropion arm (1.4%) and placebo arm (1.3%) experienced a grade 3 event related to treatment (hypertension and headache, respectively).
In the accompanying editorial, Dr. Lustberg and colleagues “applaud the authors for conducting a study in this population of cancer survivors,” noting that “evidenced-based approaches have not been extensively studied.”
Dr. Lustberg and colleagues also commented that other randomized controlled clinical trials evaluating sexual desire disorder assessed outcomes using additional metrics, such as the Female Sexual Distress Scale–Revised questionnaire, which measures distress related to sexual dysfunction and low desire, in particular.
“The use of specific validated instruments for libido in place of the FSFI might have helped determine the effect of the study intervention in this reported trial,” they wrote.
Overall, according to Dr. Lustberg and colleagues, the negative results of this study indicate that a multidisciplinary clinical approach may be needed.
“As much as we would like to have one intervention that addresses this prominent issue, the evidence strongly suggests that cancer-related sexual problems may need an integrative biopsychosocial model that intervenes on biologic, psychologic, interpersonal, and social-cultural factors, not just on one factor, such as libido,” they wrote. “Such work may require access to multidisciplinary care with specialists in women’s health, pelvic floor rehabilitation, and psychosocial oncology.”
Dr. Barton said she has been developing a multicomponent approach to addressing sexual health in female cancer survivors.
However, she noted, “there is still much we do not fully understand about the broader impact of the degree of hormone deprivation in the population of female cancer survivors. A better understanding would provide clearer targets for interventions.”
The study was supported by the National Cancer Institute and Breast Cancer Research Foundation. Dr. Barton has disclosed research funding from Merck. Dr. Lustberg reported receiving honoraria from Novartis and Biotheranostics; consulting or advising with PledPharma, Disarm Therapeutics, Pfizer; and other relationships with Cynosure/Hologic.
A version of this article first appeared on Medscape.com.
The antidepressant bupropion failed to improve sexual dysfunction in female cancer survivors, according to new findings published online in the Journal of Clinical Oncology.
Using the Female Sexual Function Index (FSFI) as a measurement tool, investigators found that desire scores were not significantly different for participants who received bupropion versus a placebo over the 9-week study period.
“Sexual health is a complex phenomenon and [our results suggest that] no one intervention is going to solve the broader issue,” lead author Debra Barton, RN, PhD, FAAN, professor in the School of Nursing at the University of Michigan, Ann Arbor, told this news organization.
Sexual dysfunction is common among cancer survivors and experienced across multiple cancer types and stages of disease. Research shows that as many as 70% of female cancer survivors report loss of desire, compared with up to one-third of the general population.
Common sexual concerns among female cancer survivors include low desire, arousal issues, lack of appropriate lubrication, difficulty in achieving orgasm, and pain with penetrative sexual activity. Additionally, these women may experience significant overlap of symptoms, and often encounter multiple sexual issues that are exacerbated by a range of cancer treatments.
“It’s a huge problem,” Maryam B. Lustberg, MD, MPH, from Yale Cancer Center, New Haven, Conn., and colleagues wrote in an accompanying editorial.
Despite the prevalence of sexual dysfunction among cancer survivors, effective treatments remain elusive. Preliminary evidence suggests that bupropion, already approved for seasonal affective disorder, major depressive disorder, and smoking cessation, may also enhance libido.
Dr. Barton and colleagues conducted this phase 2 trial to determine whether bupropion can improve sexual desire in female cancer survivors without undesirable side effects.
In the study, Dr. Barton and colleagues compared two dose levels of extended-release bupropion in a cohort of 230 postmenopausal women diagnosed with breast or gynecologic cancer and low baseline FSFI desire scores (<3.3), who had completed definitive cancer therapy.
Participants were randomized to receive either 150 mg (79 patients) or 300 mg (74 patients) once daily of extended-release bupropion, or placebo (77 patients).
Barton and colleagues then evaluated whether sexual desire significantly improved over the 9-week study period comparing the bupropion arms and the placebo group.
Overall, the authors found no significant differences (mean between-arm change for 150 mg once daily and placebo of 0.02; P = .93; mean between-arm change for 300 mg once daily and placebo of –0.02; P = .92). Mean scores at 9 weeks on the desire subscale were 2.17, 2.27, and 2.30 for 150 mg, 300 mg, and the placebo group, respectively.
In addition, none of the subscales – which included arousal, lubrication, and orgasm – or the total score showed a significant difference between arms at either 5 or 9 weeks.
Bupropion did, however, appear to be well tolerated. No grade 4-5 treatment-related adverse events occurred. In the 150-mg bupropion arm, two patients (2.6%) experienced a grade 3 event (insomnia and headache) and one patient in the 300-mg bupropion arm (1.4%) and placebo arm (1.3%) experienced a grade 3 event related to treatment (hypertension and headache, respectively).
In the accompanying editorial, Dr. Lustberg and colleagues “applaud the authors for conducting a study in this population of cancer survivors,” noting that “evidenced-based approaches have not been extensively studied.”
Dr. Lustberg and colleagues also commented that other randomized controlled clinical trials evaluating sexual desire disorder assessed outcomes using additional metrics, such as the Female Sexual Distress Scale–Revised questionnaire, which measures distress related to sexual dysfunction and low desire, in particular.
“The use of specific validated instruments for libido in place of the FSFI might have helped determine the effect of the study intervention in this reported trial,” they wrote.
Overall, according to Dr. Lustberg and colleagues, the negative results of this study indicate that a multidisciplinary clinical approach may be needed.
“As much as we would like to have one intervention that addresses this prominent issue, the evidence strongly suggests that cancer-related sexual problems may need an integrative biopsychosocial model that intervenes on biologic, psychologic, interpersonal, and social-cultural factors, not just on one factor, such as libido,” they wrote. “Such work may require access to multidisciplinary care with specialists in women’s health, pelvic floor rehabilitation, and psychosocial oncology.”
Dr. Barton said she has been developing a multicomponent approach to addressing sexual health in female cancer survivors.
However, she noted, “there is still much we do not fully understand about the broader impact of the degree of hormone deprivation in the population of female cancer survivors. A better understanding would provide clearer targets for interventions.”
The study was supported by the National Cancer Institute and Breast Cancer Research Foundation. Dr. Barton has disclosed research funding from Merck. Dr. Lustberg reported receiving honoraria from Novartis and Biotheranostics; consulting or advising with PledPharma, Disarm Therapeutics, Pfizer; and other relationships with Cynosure/Hologic.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Average-risk women with dense breasts—What breast screening is appropriate?
Text copyright DenseBreast-info.org.
Answer
A. For women with extremely dense breasts who are not otherwise at increased risk for breast cancer, screening magnetic resonance imaging (MRI) is preferred, plus her mammogram or tomosynthesis. If MRI is not an option, consider ultrasonography or contrast-enhanced mammography.
The same screening considerations apply to women with heterogeneously dense breasts; however, there is limited capacity for MRI or even ultrasound screening at many facilities. Research supports MRI in dense breasts, and abbreviated, lower-cost protocols have been validated that address some of the barriers to MRI.1 Although not yet widely available, abbreviated MRI will likely have a greater role in screening women with dense breasts who are not high risk. It is important to note that preauthorization from insurance may be required for screening MRI, and in most US states, deductibles and copays apply.
The exam
Contrast-enhanced MRI requires IV injection of gadolinium-based contrast to look at the anatomy and blood flow patterns of the breast tissue. The patient lies face down with the breasts placed in two rectangular openings, or “coils.” The exam takes place inside the tunnel of the scanner, with the head facing out.After initial images are obtained, the contrast agent is injected into a vein in the arm, and additional images are taken, which will show areas of enhancement. The exam takes about 20 to 40 minutes. An “abbreviated” MRI can be performed for screening in some centers, which uses fewer sequences and takes about 10 minutes.
Benefits
At least 40% of cancers are missed on mammography in women with dense breasts.2 MRI is the most widely studied technique using a contrast agent, and it produces the highest additional cancer detection of all the supplemental technologies to date, yielding, in the first year, 10-16 additional cancers per 1,000 women screened after mammography/tomosynthesis (reviewed in Berg et al.3). The cancer-detection benefit is seen across all breast density categories, even among average-risk women.4 There is no ionizing radiation, and it has been shown to reduce the rate of interval cancers (those detected due to symptoms after a negative screening mammogram), as well as the rate of late-stage disease. Axillary lymph nodes can be examined at the same screening exam.
While tomosynthesis improves cancer detection in women with fatty breasts, scattered fibroglandular breast tissue, and heterogeneously dense breasts, it does not significantly improve cancer detection in women with extremely dense breasts.5,6 Current American Cancer Society and National Comprehensive Cancer Network guidelines recommend annual screening MRI for women at high risk for breast cancer (regardless of breast density); however, increasingly, research supports the effectiveness of MRI in women with dense breasts who are otherwise considered average risk. A large randomized controlled trial in the Netherlands compared outcomes in women with extremely dense breasts invited to have screening MRI after negative mammography to those assigned to continue receiving screening mammography only. The incremental cancer detection rate was 16.5 per 1,000 (79/4,783) women screened with MRI in the first round7 and 6 per 1,000 women screened in the second round 2 years later.8 The interval cancer rate was 0.8 per 1,000 (4/4,783) women screened with MRI, compared with 4.9 per 1,000 (16/3,278) women who declined MRI and received mammography only.7
Screening ultrasound will show up to 3 additional cancers per 1,000 women screened after mammography/tomosynthesis (reviewed in Vourtsis and Berg9 and Berg and Vourtsis10), far lower than the added cancer-detection rate of MRI. Consider screening ultrasound for women who cannot tolerate or access screening MRI.11 Contrast-enhanced mammography (CEM) uses iodinated contrast (as in computed tomography). CEM is not widely available but appears to show cancer-detection similar to MRI. For further discussion, see Berg et al’s 2021 review.3
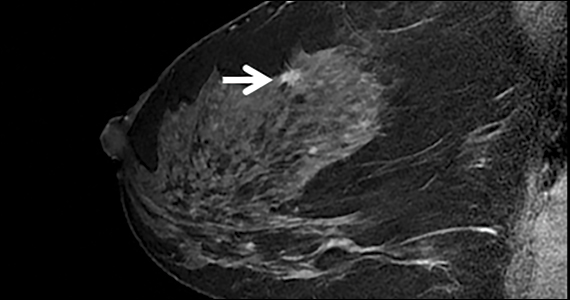
The FIGURE shows an example of an invasive cancer depicted on contrast-enhanced MRI in a 53-year-old woman with dense breasts and a family history of breast cancer that was not visible on tomosynthesis, even in retrospect, due to masking by dense tissue.

Considerations
Breast MRI increases callbacks even after mammography and ultrasound; however, such false alarms are reduced in subsequent screening rounds. MRI cannot be performed in women who have certain metal implants— some pacemakers or spinal fixation rods—and is not recommended for pregnant women. Claustrophobia may be an issue for some women. MRI is expensive and requires IV contrast. Gadolinium is known to accumulate in the brain, although the long-term effects of this are unknown and no harm has been shown.●
For more information, visit medically sourced DenseBreast-info.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
- Comstock CE, Gatsonis C, Newstead GM, et al. Comparison of abbreviated breast MRI vs digital breast tomosynthesis for breast cancer detection among women with dense breasts undergoing screening. JAMA. 2020;323:746-756. doi: 10.1001 /jama.2020.0572
- Kerlikowske K, Zhu W, Tosteson AN, et al. Identifying women with dense breasts at high risk for interval cancer: a cohort study. Ann Intern Med. 2015;162:673-681. doi: 10.7326/M14-1465.
- Berg WA, Rafferty EA, Friedewald SM, Hruska CB, Rahbar H. Screening Algorithms in Dense Breasts: AJR Expert Panel Narrative Review. AJR Am J Roentgenol. 2021;216:275-294. doi: 10.2214/AJR.20.24436.
- Kuhl CK, Strobel K, Bieling H, et al. Supplemental breast MR imaging screening of women with average risk of breast cancer. Radiology. 2017;283:361-370. doi: 10.1148/radiol.2016161444.
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315:1784-1786. doi: 10.1001/jama.2016.1708.
- Osteras BH, Martinsen ACT, Gullien R, et al. Digital mammography versus breast tomosynthesis: impact of breast density on diagnostic performance in population-based screening. Radiology. 2019;293:60-68. doi: 10.1148 /radiol.2019190425.
- Bakker MF, de Lange SV, Pijnappel RM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102. doi: 10.1056/NEJMoa1903986.
- Veenhuizen SGA, de Lange SV, Bakker MF, et al. Supplemental breast MRI for women with extremely dense breasts: results of the second screening round of the DENSE trial. Radiology. 2021;299:278-286. doi: 10.1148/radiol.2021203633.
- Vourtsis A, Berg WA. Breast density implications and supplemental screening. Eur Radiol. 2019;29:1762-1777. doi: 10.1007/s00330-018-5668-8.
- Berg WA, Vourtsis A. Screening ultrasound using handheld or automated technique in women with dense breasts. J Breast Imaging. 2019;1:283-296.
- National Comprehensive Cancer Network. Breast Cancer Screening and Diagnosis (Version 1.2021). https://www.nccn. org/professionals/physician_gls/pdf/breast-screening.pdf. Accessed November 18, 2021.
Text copyright DenseBreast-info.org.
Answer
A. For women with extremely dense breasts who are not otherwise at increased risk for breast cancer, screening magnetic resonance imaging (MRI) is preferred, plus her mammogram or tomosynthesis. If MRI is not an option, consider ultrasonography or contrast-enhanced mammography.
The same screening considerations apply to women with heterogeneously dense breasts; however, there is limited capacity for MRI or even ultrasound screening at many facilities. Research supports MRI in dense breasts, and abbreviated, lower-cost protocols have been validated that address some of the barriers to MRI.1 Although not yet widely available, abbreviated MRI will likely have a greater role in screening women with dense breasts who are not high risk. It is important to note that preauthorization from insurance may be required for screening MRI, and in most US states, deductibles and copays apply.
The exam
Contrast-enhanced MRI requires IV injection of gadolinium-based contrast to look at the anatomy and blood flow patterns of the breast tissue. The patient lies face down with the breasts placed in two rectangular openings, or “coils.” The exam takes place inside the tunnel of the scanner, with the head facing out.After initial images are obtained, the contrast agent is injected into a vein in the arm, and additional images are taken, which will show areas of enhancement. The exam takes about 20 to 40 minutes. An “abbreviated” MRI can be performed for screening in some centers, which uses fewer sequences and takes about 10 minutes.
Benefits
At least 40% of cancers are missed on mammography in women with dense breasts.2 MRI is the most widely studied technique using a contrast agent, and it produces the highest additional cancer detection of all the supplemental technologies to date, yielding, in the first year, 10-16 additional cancers per 1,000 women screened after mammography/tomosynthesis (reviewed in Berg et al.3). The cancer-detection benefit is seen across all breast density categories, even among average-risk women.4 There is no ionizing radiation, and it has been shown to reduce the rate of interval cancers (those detected due to symptoms after a negative screening mammogram), as well as the rate of late-stage disease. Axillary lymph nodes can be examined at the same screening exam.
While tomosynthesis improves cancer detection in women with fatty breasts, scattered fibroglandular breast tissue, and heterogeneously dense breasts, it does not significantly improve cancer detection in women with extremely dense breasts.5,6 Current American Cancer Society and National Comprehensive Cancer Network guidelines recommend annual screening MRI for women at high risk for breast cancer (regardless of breast density); however, increasingly, research supports the effectiveness of MRI in women with dense breasts who are otherwise considered average risk. A large randomized controlled trial in the Netherlands compared outcomes in women with extremely dense breasts invited to have screening MRI after negative mammography to those assigned to continue receiving screening mammography only. The incremental cancer detection rate was 16.5 per 1,000 (79/4,783) women screened with MRI in the first round7 and 6 per 1,000 women screened in the second round 2 years later.8 The interval cancer rate was 0.8 per 1,000 (4/4,783) women screened with MRI, compared with 4.9 per 1,000 (16/3,278) women who declined MRI and received mammography only.7
Screening ultrasound will show up to 3 additional cancers per 1,000 women screened after mammography/tomosynthesis (reviewed in Vourtsis and Berg9 and Berg and Vourtsis10), far lower than the added cancer-detection rate of MRI. Consider screening ultrasound for women who cannot tolerate or access screening MRI.11 Contrast-enhanced mammography (CEM) uses iodinated contrast (as in computed tomography). CEM is not widely available but appears to show cancer-detection similar to MRI. For further discussion, see Berg et al’s 2021 review.3
The FIGURE shows an example of an invasive cancer depicted on contrast-enhanced MRI in a 53-year-old woman with dense breasts and a family history of breast cancer that was not visible on tomosynthesis, even in retrospect, due to masking by dense tissue.

Considerations
Breast MRI increases callbacks even after mammography and ultrasound; however, such false alarms are reduced in subsequent screening rounds. MRI cannot be performed in women who have certain metal implants— some pacemakers or spinal fixation rods—and is not recommended for pregnant women. Claustrophobia may be an issue for some women. MRI is expensive and requires IV contrast. Gadolinium is known to accumulate in the brain, although the long-term effects of this are unknown and no harm has been shown.●
For more information, visit medically sourced DenseBreast-info.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
Text copyright DenseBreast-info.org.
Answer
A. For women with extremely dense breasts who are not otherwise at increased risk for breast cancer, screening magnetic resonance imaging (MRI) is preferred, plus her mammogram or tomosynthesis. If MRI is not an option, consider ultrasonography or contrast-enhanced mammography.
The same screening considerations apply to women with heterogeneously dense breasts; however, there is limited capacity for MRI or even ultrasound screening at many facilities. Research supports MRI in dense breasts, and abbreviated, lower-cost protocols have been validated that address some of the barriers to MRI.1 Although not yet widely available, abbreviated MRI will likely have a greater role in screening women with dense breasts who are not high risk. It is important to note that preauthorization from insurance may be required for screening MRI, and in most US states, deductibles and copays apply.
The exam
Contrast-enhanced MRI requires IV injection of gadolinium-based contrast to look at the anatomy and blood flow patterns of the breast tissue. The patient lies face down with the breasts placed in two rectangular openings, or “coils.” The exam takes place inside the tunnel of the scanner, with the head facing out.After initial images are obtained, the contrast agent is injected into a vein in the arm, and additional images are taken, which will show areas of enhancement. The exam takes about 20 to 40 minutes. An “abbreviated” MRI can be performed for screening in some centers, which uses fewer sequences and takes about 10 minutes.
Benefits
At least 40% of cancers are missed on mammography in women with dense breasts.2 MRI is the most widely studied technique using a contrast agent, and it produces the highest additional cancer detection of all the supplemental technologies to date, yielding, in the first year, 10-16 additional cancers per 1,000 women screened after mammography/tomosynthesis (reviewed in Berg et al.3). The cancer-detection benefit is seen across all breast density categories, even among average-risk women.4 There is no ionizing radiation, and it has been shown to reduce the rate of interval cancers (those detected due to symptoms after a negative screening mammogram), as well as the rate of late-stage disease. Axillary lymph nodes can be examined at the same screening exam.
While tomosynthesis improves cancer detection in women with fatty breasts, scattered fibroglandular breast tissue, and heterogeneously dense breasts, it does not significantly improve cancer detection in women with extremely dense breasts.5,6 Current American Cancer Society and National Comprehensive Cancer Network guidelines recommend annual screening MRI for women at high risk for breast cancer (regardless of breast density); however, increasingly, research supports the effectiveness of MRI in women with dense breasts who are otherwise considered average risk. A large randomized controlled trial in the Netherlands compared outcomes in women with extremely dense breasts invited to have screening MRI after negative mammography to those assigned to continue receiving screening mammography only. The incremental cancer detection rate was 16.5 per 1,000 (79/4,783) women screened with MRI in the first round7 and 6 per 1,000 women screened in the second round 2 years later.8 The interval cancer rate was 0.8 per 1,000 (4/4,783) women screened with MRI, compared with 4.9 per 1,000 (16/3,278) women who declined MRI and received mammography only.7
Screening ultrasound will show up to 3 additional cancers per 1,000 women screened after mammography/tomosynthesis (reviewed in Vourtsis and Berg9 and Berg and Vourtsis10), far lower than the added cancer-detection rate of MRI. Consider screening ultrasound for women who cannot tolerate or access screening MRI.11 Contrast-enhanced mammography (CEM) uses iodinated contrast (as in computed tomography). CEM is not widely available but appears to show cancer-detection similar to MRI. For further discussion, see Berg et al’s 2021 review.3
The FIGURE shows an example of an invasive cancer depicted on contrast-enhanced MRI in a 53-year-old woman with dense breasts and a family history of breast cancer that was not visible on tomosynthesis, even in retrospect, due to masking by dense tissue.

Considerations
Breast MRI increases callbacks even after mammography and ultrasound; however, such false alarms are reduced in subsequent screening rounds. MRI cannot be performed in women who have certain metal implants— some pacemakers or spinal fixation rods—and is not recommended for pregnant women. Claustrophobia may be an issue for some women. MRI is expensive and requires IV contrast. Gadolinium is known to accumulate in the brain, although the long-term effects of this are unknown and no harm has been shown.●
For more information, visit medically sourced DenseBreast-info.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
- Comstock CE, Gatsonis C, Newstead GM, et al. Comparison of abbreviated breast MRI vs digital breast tomosynthesis for breast cancer detection among women with dense breasts undergoing screening. JAMA. 2020;323:746-756. doi: 10.1001 /jama.2020.0572
- Kerlikowske K, Zhu W, Tosteson AN, et al. Identifying women with dense breasts at high risk for interval cancer: a cohort study. Ann Intern Med. 2015;162:673-681. doi: 10.7326/M14-1465.
- Berg WA, Rafferty EA, Friedewald SM, Hruska CB, Rahbar H. Screening Algorithms in Dense Breasts: AJR Expert Panel Narrative Review. AJR Am J Roentgenol. 2021;216:275-294. doi: 10.2214/AJR.20.24436.
- Kuhl CK, Strobel K, Bieling H, et al. Supplemental breast MR imaging screening of women with average risk of breast cancer. Radiology. 2017;283:361-370. doi: 10.1148/radiol.2016161444.
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315:1784-1786. doi: 10.1001/jama.2016.1708.
- Osteras BH, Martinsen ACT, Gullien R, et al. Digital mammography versus breast tomosynthesis: impact of breast density on diagnostic performance in population-based screening. Radiology. 2019;293:60-68. doi: 10.1148 /radiol.2019190425.
- Bakker MF, de Lange SV, Pijnappel RM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102. doi: 10.1056/NEJMoa1903986.
- Veenhuizen SGA, de Lange SV, Bakker MF, et al. Supplemental breast MRI for women with extremely dense breasts: results of the second screening round of the DENSE trial. Radiology. 2021;299:278-286. doi: 10.1148/radiol.2021203633.
- Vourtsis A, Berg WA. Breast density implications and supplemental screening. Eur Radiol. 2019;29:1762-1777. doi: 10.1007/s00330-018-5668-8.
- Berg WA, Vourtsis A. Screening ultrasound using handheld or automated technique in women with dense breasts. J Breast Imaging. 2019;1:283-296.
- National Comprehensive Cancer Network. Breast Cancer Screening and Diagnosis (Version 1.2021). https://www.nccn. org/professionals/physician_gls/pdf/breast-screening.pdf. Accessed November 18, 2021.
- Comstock CE, Gatsonis C, Newstead GM, et al. Comparison of abbreviated breast MRI vs digital breast tomosynthesis for breast cancer detection among women with dense breasts undergoing screening. JAMA. 2020;323:746-756. doi: 10.1001 /jama.2020.0572
- Kerlikowske K, Zhu W, Tosteson AN, et al. Identifying women with dense breasts at high risk for interval cancer: a cohort study. Ann Intern Med. 2015;162:673-681. doi: 10.7326/M14-1465.
- Berg WA, Rafferty EA, Friedewald SM, Hruska CB, Rahbar H. Screening Algorithms in Dense Breasts: AJR Expert Panel Narrative Review. AJR Am J Roentgenol. 2021;216:275-294. doi: 10.2214/AJR.20.24436.
- Kuhl CK, Strobel K, Bieling H, et al. Supplemental breast MR imaging screening of women with average risk of breast cancer. Radiology. 2017;283:361-370. doi: 10.1148/radiol.2016161444.
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315:1784-1786. doi: 10.1001/jama.2016.1708.
- Osteras BH, Martinsen ACT, Gullien R, et al. Digital mammography versus breast tomosynthesis: impact of breast density on diagnostic performance in population-based screening. Radiology. 2019;293:60-68. doi: 10.1148 /radiol.2019190425.
- Bakker MF, de Lange SV, Pijnappel RM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102. doi: 10.1056/NEJMoa1903986.
- Veenhuizen SGA, de Lange SV, Bakker MF, et al. Supplemental breast MRI for women with extremely dense breasts: results of the second screening round of the DENSE trial. Radiology. 2021;299:278-286. doi: 10.1148/radiol.2021203633.
- Vourtsis A, Berg WA. Breast density implications and supplemental screening. Eur Radiol. 2019;29:1762-1777. doi: 10.1007/s00330-018-5668-8.
- Berg WA, Vourtsis A. Screening ultrasound using handheld or automated technique in women with dense breasts. J Breast Imaging. 2019;1:283-296.
- National Comprehensive Cancer Network. Breast Cancer Screening and Diagnosis (Version 1.2021). https://www.nccn. org/professionals/physician_gls/pdf/breast-screening.pdf. Accessed November 18, 2021.
Quiz developed in collaboration with 
More evidence ties some antipsychotics to increased breast cancer risk
New research provides more evidence that antipsychotics that raise prolactin levels are tied to a significantly increased risk for breast cancer.
The relative risk for breast cancer was 62% higher in women who took category 1 antipsychotic medications associated with high prolactin levels. These include haloperidol (Haldol), paliperidone (Invega), and risperidone (Risperdal). Additionally, the risk was 54% higher in those taking category 2 antipsychotics that have mid-range effects on prolactin. These include iloperidone (Fanapt), lurasidone (Latuda), and olanzapine (Zyprexa).
In contrast, category 3 antipsychotics which have a lesser effect on prolactin levels were not associated with any increase in breast cancer risk. These drugs include aripiprazole (Abilify), asenapine (Saphris), brexpiprazole (Rexulti), cariprazine (Vraylar), clozapine (multiple brands), quetiapine (Seroquel), and ziprasidone (Geodon).
While the “absolute” breast cancer risk for these drugs is unclear, “we can make the case that high circulating prolactin levels are associated with breast cancer risk. This follows what is already known about prolactin from prior studies, notably the nurses’ health studies,” Tahir Rahman, MD, associate professor of psychiatry, Washington University School of Medicine, St. Louis, told this news organization.
“We don’t want to alarm patients taking antipsychotic drugs for life-threatening mental health problems, but we also think it is time for doctors to track prolactin levels and vigilantly monitor their patients who are being treated with antipsychotics,” Dr. Rahman added in a news release.
The study was published online Dec. 3 in the Journal of Clinical Psychopharmacology.
Test prolactin levels
Using administrative claims data, the researchers evaluated breast cancer risk in women aged 18-64 exposed to antipsychotic medications compared with anticonvulsants and/or lithium.
They identified 914 cases of invasive breast cancer among 540,737 women.
Roughly 52% of the study population filled at least one prescription for a category 3 antipsychotic agent, whereas 15% filled at least one prescription for a category 1 agent; 49% of women filled at least one prescription for an anticonvulsant medication during the study period.
Exposure to all antipsychotics was independently associated with a 35% increased risk for breast cancer (adjusted hazard ratio, 1.35; 95% CI, 1.14-1.61), the study team found.
Compared with anticonvulsants or lithium, the risk for breast cancer was significantly increased for high prolactin (category 1) antipsychotics (adjusted hazard ratio, 1.62; 95% CI, 1.30-2.03) and for mid-prolactin (category 2) drugs (aHR 1.54; 95% CI, 1.19-1.99), with no increased risk for category 3 antipsychotics.
“Our research is obviously of interest for preventing breast cancer in antipsychotic-treated patients. Checking a blood prolactin level is cheap and easy [and a high level is] fairly simple to mitigate,” said Dr. Rahman.
A matter of debate
Reached for comment, Christoph Correll, MD, professor of psychiatry and molecular medicine, Zucker School of Medicine at Hofstra/Northwell, Hempstead, New York, said, “The potential elevation of breast cancer risk depending on the dose and time of treatment with antipsychotic medications with varying degrees of prolactin-raising properties has been a topic of research and matter of debate.”
This new study “adds another data point indicating that antipsychotics that are associated on average with a higher prolactin-raising effect than other antipsychotics may increase the risk of breast cancer in women to some degree,” said Dr. Correll, who was not involved with the study.
However, he cautioned that “naturalistic data are always vulnerable to residual confounding, for example, unmeasured effects that could also at least partially explain the results, and the follow-up time of only 4 years (maximum 6 years) in this study was relatively short.
“Nevertheless, given availability of many different antipsychotics with varying degrees of prolactin-raising potential, in women requiring antipsychotic treatment, less prolactin-raising antipsychotics may be preferable,” Dr. Correll said.
“In women receiving prolactin-raising antipsychotics for medium- and longer-term maintenance therapy, prolactin levels should be monitored,” he added.
When an elevated prolactin level is detected, this should be addressed “either via dose reduction, a switch to an alternative antipsychotic that does not raise prolactin levels significantly, or the addition of a partial or full D2 agonist when the prolactin-raising antipsychotic should be continued based on individualized risk assessment,” Dr. Correll advised.
This work was supported by an award from the Alvin J. Siteman Cancer Center; the National Cancer Institute and the National Center for Advancing Translational Sciences of the National Institutes of Health; the Taylor Family Institute for Innovative Psychiatric Research; and the Center for Brain Research in Mood Disorders. The authors have disclosed no relevant financial relationships. Dr. Correll has received royalties from UpToDate and is a stock option holder of LB Pharma.
A version of this article first appeared on Medscape.com.
New research provides more evidence that antipsychotics that raise prolactin levels are tied to a significantly increased risk for breast cancer.
The relative risk for breast cancer was 62% higher in women who took category 1 antipsychotic medications associated with high prolactin levels. These include haloperidol (Haldol), paliperidone (Invega), and risperidone (Risperdal). Additionally, the risk was 54% higher in those taking category 2 antipsychotics that have mid-range effects on prolactin. These include iloperidone (Fanapt), lurasidone (Latuda), and olanzapine (Zyprexa).
In contrast, category 3 antipsychotics which have a lesser effect on prolactin levels were not associated with any increase in breast cancer risk. These drugs include aripiprazole (Abilify), asenapine (Saphris), brexpiprazole (Rexulti), cariprazine (Vraylar), clozapine (multiple brands), quetiapine (Seroquel), and ziprasidone (Geodon).
While the “absolute” breast cancer risk for these drugs is unclear, “we can make the case that high circulating prolactin levels are associated with breast cancer risk. This follows what is already known about prolactin from prior studies, notably the nurses’ health studies,” Tahir Rahman, MD, associate professor of psychiatry, Washington University School of Medicine, St. Louis, told this news organization.
“We don’t want to alarm patients taking antipsychotic drugs for life-threatening mental health problems, but we also think it is time for doctors to track prolactin levels and vigilantly monitor their patients who are being treated with antipsychotics,” Dr. Rahman added in a news release.
The study was published online Dec. 3 in the Journal of Clinical Psychopharmacology.
Test prolactin levels
Using administrative claims data, the researchers evaluated breast cancer risk in women aged 18-64 exposed to antipsychotic medications compared with anticonvulsants and/or lithium.
They identified 914 cases of invasive breast cancer among 540,737 women.
Roughly 52% of the study population filled at least one prescription for a category 3 antipsychotic agent, whereas 15% filled at least one prescription for a category 1 agent; 49% of women filled at least one prescription for an anticonvulsant medication during the study period.
Exposure to all antipsychotics was independently associated with a 35% increased risk for breast cancer (adjusted hazard ratio, 1.35; 95% CI, 1.14-1.61), the study team found.
Compared with anticonvulsants or lithium, the risk for breast cancer was significantly increased for high prolactin (category 1) antipsychotics (adjusted hazard ratio, 1.62; 95% CI, 1.30-2.03) and for mid-prolactin (category 2) drugs (aHR 1.54; 95% CI, 1.19-1.99), with no increased risk for category 3 antipsychotics.
“Our research is obviously of interest for preventing breast cancer in antipsychotic-treated patients. Checking a blood prolactin level is cheap and easy [and a high level is] fairly simple to mitigate,” said Dr. Rahman.
A matter of debate
Reached for comment, Christoph Correll, MD, professor of psychiatry and molecular medicine, Zucker School of Medicine at Hofstra/Northwell, Hempstead, New York, said, “The potential elevation of breast cancer risk depending on the dose and time of treatment with antipsychotic medications with varying degrees of prolactin-raising properties has been a topic of research and matter of debate.”
This new study “adds another data point indicating that antipsychotics that are associated on average with a higher prolactin-raising effect than other antipsychotics may increase the risk of breast cancer in women to some degree,” said Dr. Correll, who was not involved with the study.
However, he cautioned that “naturalistic data are always vulnerable to residual confounding, for example, unmeasured effects that could also at least partially explain the results, and the follow-up time of only 4 years (maximum 6 years) in this study was relatively short.
“Nevertheless, given availability of many different antipsychotics with varying degrees of prolactin-raising potential, in women requiring antipsychotic treatment, less prolactin-raising antipsychotics may be preferable,” Dr. Correll said.
“In women receiving prolactin-raising antipsychotics for medium- and longer-term maintenance therapy, prolactin levels should be monitored,” he added.
When an elevated prolactin level is detected, this should be addressed “either via dose reduction, a switch to an alternative antipsychotic that does not raise prolactin levels significantly, or the addition of a partial or full D2 agonist when the prolactin-raising antipsychotic should be continued based on individualized risk assessment,” Dr. Correll advised.
This work was supported by an award from the Alvin J. Siteman Cancer Center; the National Cancer Institute and the National Center for Advancing Translational Sciences of the National Institutes of Health; the Taylor Family Institute for Innovative Psychiatric Research; and the Center for Brain Research in Mood Disorders. The authors have disclosed no relevant financial relationships. Dr. Correll has received royalties from UpToDate and is a stock option holder of LB Pharma.
A version of this article first appeared on Medscape.com.
New research provides more evidence that antipsychotics that raise prolactin levels are tied to a significantly increased risk for breast cancer.
The relative risk for breast cancer was 62% higher in women who took category 1 antipsychotic medications associated with high prolactin levels. These include haloperidol (Haldol), paliperidone (Invega), and risperidone (Risperdal). Additionally, the risk was 54% higher in those taking category 2 antipsychotics that have mid-range effects on prolactin. These include iloperidone (Fanapt), lurasidone (Latuda), and olanzapine (Zyprexa).
In contrast, category 3 antipsychotics which have a lesser effect on prolactin levels were not associated with any increase in breast cancer risk. These drugs include aripiprazole (Abilify), asenapine (Saphris), brexpiprazole (Rexulti), cariprazine (Vraylar), clozapine (multiple brands), quetiapine (Seroquel), and ziprasidone (Geodon).
While the “absolute” breast cancer risk for these drugs is unclear, “we can make the case that high circulating prolactin levels are associated with breast cancer risk. This follows what is already known about prolactin from prior studies, notably the nurses’ health studies,” Tahir Rahman, MD, associate professor of psychiatry, Washington University School of Medicine, St. Louis, told this news organization.
“We don’t want to alarm patients taking antipsychotic drugs for life-threatening mental health problems, but we also think it is time for doctors to track prolactin levels and vigilantly monitor their patients who are being treated with antipsychotics,” Dr. Rahman added in a news release.
The study was published online Dec. 3 in the Journal of Clinical Psychopharmacology.
Test prolactin levels
Using administrative claims data, the researchers evaluated breast cancer risk in women aged 18-64 exposed to antipsychotic medications compared with anticonvulsants and/or lithium.
They identified 914 cases of invasive breast cancer among 540,737 women.
Roughly 52% of the study population filled at least one prescription for a category 3 antipsychotic agent, whereas 15% filled at least one prescription for a category 1 agent; 49% of women filled at least one prescription for an anticonvulsant medication during the study period.
Exposure to all antipsychotics was independently associated with a 35% increased risk for breast cancer (adjusted hazard ratio, 1.35; 95% CI, 1.14-1.61), the study team found.
Compared with anticonvulsants or lithium, the risk for breast cancer was significantly increased for high prolactin (category 1) antipsychotics (adjusted hazard ratio, 1.62; 95% CI, 1.30-2.03) and for mid-prolactin (category 2) drugs (aHR 1.54; 95% CI, 1.19-1.99), with no increased risk for category 3 antipsychotics.
“Our research is obviously of interest for preventing breast cancer in antipsychotic-treated patients. Checking a blood prolactin level is cheap and easy [and a high level is] fairly simple to mitigate,” said Dr. Rahman.
A matter of debate
Reached for comment, Christoph Correll, MD, professor of psychiatry and molecular medicine, Zucker School of Medicine at Hofstra/Northwell, Hempstead, New York, said, “The potential elevation of breast cancer risk depending on the dose and time of treatment with antipsychotic medications with varying degrees of prolactin-raising properties has been a topic of research and matter of debate.”
This new study “adds another data point indicating that antipsychotics that are associated on average with a higher prolactin-raising effect than other antipsychotics may increase the risk of breast cancer in women to some degree,” said Dr. Correll, who was not involved with the study.
However, he cautioned that “naturalistic data are always vulnerable to residual confounding, for example, unmeasured effects that could also at least partially explain the results, and the follow-up time of only 4 years (maximum 6 years) in this study was relatively short.
“Nevertheless, given availability of many different antipsychotics with varying degrees of prolactin-raising potential, in women requiring antipsychotic treatment, less prolactin-raising antipsychotics may be preferable,” Dr. Correll said.
“In women receiving prolactin-raising antipsychotics for medium- and longer-term maintenance therapy, prolactin levels should be monitored,” he added.
When an elevated prolactin level is detected, this should be addressed “either via dose reduction, a switch to an alternative antipsychotic that does not raise prolactin levels significantly, or the addition of a partial or full D2 agonist when the prolactin-raising antipsychotic should be continued based on individualized risk assessment,” Dr. Correll advised.
This work was supported by an award from the Alvin J. Siteman Cancer Center; the National Cancer Institute and the National Center for Advancing Translational Sciences of the National Institutes of Health; the Taylor Family Institute for Innovative Psychiatric Research; and the Center for Brain Research in Mood Disorders. The authors have disclosed no relevant financial relationships. Dr. Correll has received royalties from UpToDate and is a stock option holder of LB Pharma.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHOPHARMACOLOGY
LGBTQ health care: There is reason to be hopeful
I write a lot about watershed moments in my career, things that proved to be moments of tremendous growth, as a person and as a doctor.
One of these occurred early in my career when I met a new patient with ovarian cancer. When I walked into the exam room, I made eye contact with the woman who was accompanied by a man. I assumed they were married, so I went to her first. I introduced myself, stating that I was here to talk about how best to treat her cancer. She stopped me quickly. “Doctor, I am not the patient,” she said. “He is.”
It was the first time I had cared for a transgender man with ovarian cancer. I recall how awkward the following moments were – for all of us. It was the first time I realized that cancer does not have a gender. Men can get breast cancer. Trans women can get prostate cancer. Trans men can get ovarian cancer.
But even many years later, we are not much further along in how prepared we are as a medical community to care for LGBTQ persons. Lesbian, gay, bisexual, transgender, and queer people are not part of the normal medical school curriculum. For most medical students, LGBTQ health is still approached as an aside – perhaps during an infectious disease clerkship, while learning about STDs and HIV. Students do not learn how to approach the male couple seeking to become parents, STD risk reduction for gays and lesbians, or the trans man with ovarian cancer.
But they should, particularly in light of a 2015 study evaluating bias among U.S. medical students. The analysis found that about 45% of medical students exhibited explicit bias against LGBTQ individuals and 8 in 10 held an implicit bias. Fewer than 20% showed no evidence of bias. This lack of preparedness to treat LGBTQ individuals against a backdrop of bias in the medical community often leads patients to mistrust medicine.
To gain perspective outside of oncology, I spoke to Michelle Forcier (she/they), MD, MPH, assistant dean of admissions and professor of pediatrics at Brown University, Providence, R.I. Dr. Forcier agreed that “LGBTQ/rainbow health has been harmfully treated by the system, by both intention and by ignorance.”
“I have had patients who report that EMTs have tried to look under their clothes to determine their gender and transgender patients who have asked point blank to show a provider the results of gender reassignment surgery, not because it was relevant to the issue at hand, but purely out of curiosity,” Dr. Forcier continued. “Then there are the patients who are addressed by the name on their legal record rather than the name that reflects their actual lived experience and identity. These experiences foster this anticipation that is pervasive in this community, that something will be said or done that doesn’t fit who they are, and that ultimately will out them as ‘other.’ ”
I have also felt this sense of being “other” – something I thought I would be immune to as a physician. I have been asked on multiple occasions what my wife does for a living. Moments like this are always awkward. I’m either forced to come out of the closet yet again, or answer vaguely, as if I should be ashamed of my sexuality.
So, how can we move toward equity? Dr. Forcier explained how she lays the groundwork early. “I love pediatrics because kids know when you are being authentic,” she said. “I say who I am, I use she/they pronouns. I also teach by example. If there are more than just my patient in a room, I say, ‘Let’s go around the room and introduce ourselves’ so all have a chance to tell me who they are and how they have come together. If it’s not clear to me, sometimes I prod: ‘How are you here to support [the patient]?’ ”
The point, according to Dr. Forcier: Don’t make assumptions about relationships when you walk into a room with more than one person. Don’t even make assumptions about who the patient is.
But bringing up gender and sexuality can be awkward. Even I sometimes have a hard time. In oncology, patients are there to talk about their cancer and what can be done about it.
“I think it’s really about how it’s framed,” Dr. Forcier said. “In pediatrics, I might start by prefacing it with ‘I am going to ask you some personal questions, and it might seem invasive, but it’s important for your health care. How do you see yourself in the world? What gender identity fits you the best? Who are you attracted to?’ And then I shut up. Doctors need to learn how to stop and wait, provide the space to answer.”
I can see why understanding our patients more deeply is important. We treat people with cancer, not cancer people. As such, understanding someone more fully includes being cognizant of how they identify.
“I am continuously inspired by my LGBTQ patients who have fought to realize who they are and become their truer selves,” Dr. Forcier said. “They know who they are, and they know what they need. They have learned to demand it, to demand that their rights be respected – both civil and human rights.”
As we look toward a future in medicine where diversity, equity, and inclusion have gained prominence and urgency, I think there is reason to be hopeful. In oncology, one institutional study published in 2017 found that, although only about a third of practicing clinicians surveyed were comfortable treating LGBTQ patients, 92% of them acknowledged our unique needs, 78% wanted more education on how to appropriately care for our community, and 64% wanted to be listed as an LGBTQ-friendly provider.
“As an optimist, I believe that those struggling with homophobia/transphobia are open to doing things better,” Dr. Forcier said. “After all, we all strive to be better doctors. Whether explicit or implicit bias is at play, turning moments where colleagues are being inappropriate and showing them an alternative, more inclusive way to handle things is one mechanism to educate, rather than to shame. The bottom line is simple: You don’t have to be perfect. You just have to try.”
Dr. Dizon is the director of women’s cancers at Lifespan Cancer Institute and director of medical oncology at Rhode Island Hospital, both in Providence. He is also a professor of medicine at Brown University. His research interests are in novel treatments of women’s cancers and issues related to survivorship, particularly as they relate to sexual health after cancer for both men and women.
A version of this article first appeared on Medscape.com.
I write a lot about watershed moments in my career, things that proved to be moments of tremendous growth, as a person and as a doctor.
One of these occurred early in my career when I met a new patient with ovarian cancer. When I walked into the exam room, I made eye contact with the woman who was accompanied by a man. I assumed they were married, so I went to her first. I introduced myself, stating that I was here to talk about how best to treat her cancer. She stopped me quickly. “Doctor, I am not the patient,” she said. “He is.”
It was the first time I had cared for a transgender man with ovarian cancer. I recall how awkward the following moments were – for all of us. It was the first time I realized that cancer does not have a gender. Men can get breast cancer. Trans women can get prostate cancer. Trans men can get ovarian cancer.
But even many years later, we are not much further along in how prepared we are as a medical community to care for LGBTQ persons. Lesbian, gay, bisexual, transgender, and queer people are not part of the normal medical school curriculum. For most medical students, LGBTQ health is still approached as an aside – perhaps during an infectious disease clerkship, while learning about STDs and HIV. Students do not learn how to approach the male couple seeking to become parents, STD risk reduction for gays and lesbians, or the trans man with ovarian cancer.
But they should, particularly in light of a 2015 study evaluating bias among U.S. medical students. The analysis found that about 45% of medical students exhibited explicit bias against LGBTQ individuals and 8 in 10 held an implicit bias. Fewer than 20% showed no evidence of bias. This lack of preparedness to treat LGBTQ individuals against a backdrop of bias in the medical community often leads patients to mistrust medicine.
To gain perspective outside of oncology, I spoke to Michelle Forcier (she/they), MD, MPH, assistant dean of admissions and professor of pediatrics at Brown University, Providence, R.I. Dr. Forcier agreed that “LGBTQ/rainbow health has been harmfully treated by the system, by both intention and by ignorance.”
“I have had patients who report that EMTs have tried to look under their clothes to determine their gender and transgender patients who have asked point blank to show a provider the results of gender reassignment surgery, not because it was relevant to the issue at hand, but purely out of curiosity,” Dr. Forcier continued. “Then there are the patients who are addressed by the name on their legal record rather than the name that reflects their actual lived experience and identity. These experiences foster this anticipation that is pervasive in this community, that something will be said or done that doesn’t fit who they are, and that ultimately will out them as ‘other.’ ”
I have also felt this sense of being “other” – something I thought I would be immune to as a physician. I have been asked on multiple occasions what my wife does for a living. Moments like this are always awkward. I’m either forced to come out of the closet yet again, or answer vaguely, as if I should be ashamed of my sexuality.
So, how can we move toward equity? Dr. Forcier explained how she lays the groundwork early. “I love pediatrics because kids know when you are being authentic,” she said. “I say who I am, I use she/they pronouns. I also teach by example. If there are more than just my patient in a room, I say, ‘Let’s go around the room and introduce ourselves’ so all have a chance to tell me who they are and how they have come together. If it’s not clear to me, sometimes I prod: ‘How are you here to support [the patient]?’ ”
The point, according to Dr. Forcier: Don’t make assumptions about relationships when you walk into a room with more than one person. Don’t even make assumptions about who the patient is.
But bringing up gender and sexuality can be awkward. Even I sometimes have a hard time. In oncology, patients are there to talk about their cancer and what can be done about it.
“I think it’s really about how it’s framed,” Dr. Forcier said. “In pediatrics, I might start by prefacing it with ‘I am going to ask you some personal questions, and it might seem invasive, but it’s important for your health care. How do you see yourself in the world? What gender identity fits you the best? Who are you attracted to?’ And then I shut up. Doctors need to learn how to stop and wait, provide the space to answer.”
I can see why understanding our patients more deeply is important. We treat people with cancer, not cancer people. As such, understanding someone more fully includes being cognizant of how they identify.
“I am continuously inspired by my LGBTQ patients who have fought to realize who they are and become their truer selves,” Dr. Forcier said. “They know who they are, and they know what they need. They have learned to demand it, to demand that their rights be respected – both civil and human rights.”
As we look toward a future in medicine where diversity, equity, and inclusion have gained prominence and urgency, I think there is reason to be hopeful. In oncology, one institutional study published in 2017 found that, although only about a third of practicing clinicians surveyed were comfortable treating LGBTQ patients, 92% of them acknowledged our unique needs, 78% wanted more education on how to appropriately care for our community, and 64% wanted to be listed as an LGBTQ-friendly provider.
“As an optimist, I believe that those struggling with homophobia/transphobia are open to doing things better,” Dr. Forcier said. “After all, we all strive to be better doctors. Whether explicit or implicit bias is at play, turning moments where colleagues are being inappropriate and showing them an alternative, more inclusive way to handle things is one mechanism to educate, rather than to shame. The bottom line is simple: You don’t have to be perfect. You just have to try.”
Dr. Dizon is the director of women’s cancers at Lifespan Cancer Institute and director of medical oncology at Rhode Island Hospital, both in Providence. He is also a professor of medicine at Brown University. His research interests are in novel treatments of women’s cancers and issues related to survivorship, particularly as they relate to sexual health after cancer for both men and women.
A version of this article first appeared on Medscape.com.
I write a lot about watershed moments in my career, things that proved to be moments of tremendous growth, as a person and as a doctor.
One of these occurred early in my career when I met a new patient with ovarian cancer. When I walked into the exam room, I made eye contact with the woman who was accompanied by a man. I assumed they were married, so I went to her first. I introduced myself, stating that I was here to talk about how best to treat her cancer. She stopped me quickly. “Doctor, I am not the patient,” she said. “He is.”
It was the first time I had cared for a transgender man with ovarian cancer. I recall how awkward the following moments were – for all of us. It was the first time I realized that cancer does not have a gender. Men can get breast cancer. Trans women can get prostate cancer. Trans men can get ovarian cancer.
But even many years later, we are not much further along in how prepared we are as a medical community to care for LGBTQ persons. Lesbian, gay, bisexual, transgender, and queer people are not part of the normal medical school curriculum. For most medical students, LGBTQ health is still approached as an aside – perhaps during an infectious disease clerkship, while learning about STDs and HIV. Students do not learn how to approach the male couple seeking to become parents, STD risk reduction for gays and lesbians, or the trans man with ovarian cancer.
But they should, particularly in light of a 2015 study evaluating bias among U.S. medical students. The analysis found that about 45% of medical students exhibited explicit bias against LGBTQ individuals and 8 in 10 held an implicit bias. Fewer than 20% showed no evidence of bias. This lack of preparedness to treat LGBTQ individuals against a backdrop of bias in the medical community often leads patients to mistrust medicine.
To gain perspective outside of oncology, I spoke to Michelle Forcier (she/they), MD, MPH, assistant dean of admissions and professor of pediatrics at Brown University, Providence, R.I. Dr. Forcier agreed that “LGBTQ/rainbow health has been harmfully treated by the system, by both intention and by ignorance.”
“I have had patients who report that EMTs have tried to look under their clothes to determine their gender and transgender patients who have asked point blank to show a provider the results of gender reassignment surgery, not because it was relevant to the issue at hand, but purely out of curiosity,” Dr. Forcier continued. “Then there are the patients who are addressed by the name on their legal record rather than the name that reflects their actual lived experience and identity. These experiences foster this anticipation that is pervasive in this community, that something will be said or done that doesn’t fit who they are, and that ultimately will out them as ‘other.’ ”
I have also felt this sense of being “other” – something I thought I would be immune to as a physician. I have been asked on multiple occasions what my wife does for a living. Moments like this are always awkward. I’m either forced to come out of the closet yet again, or answer vaguely, as if I should be ashamed of my sexuality.
So, how can we move toward equity? Dr. Forcier explained how she lays the groundwork early. “I love pediatrics because kids know when you are being authentic,” she said. “I say who I am, I use she/they pronouns. I also teach by example. If there are more than just my patient in a room, I say, ‘Let’s go around the room and introduce ourselves’ so all have a chance to tell me who they are and how they have come together. If it’s not clear to me, sometimes I prod: ‘How are you here to support [the patient]?’ ”
The point, according to Dr. Forcier: Don’t make assumptions about relationships when you walk into a room with more than one person. Don’t even make assumptions about who the patient is.
But bringing up gender and sexuality can be awkward. Even I sometimes have a hard time. In oncology, patients are there to talk about their cancer and what can be done about it.
“I think it’s really about how it’s framed,” Dr. Forcier said. “In pediatrics, I might start by prefacing it with ‘I am going to ask you some personal questions, and it might seem invasive, but it’s important for your health care. How do you see yourself in the world? What gender identity fits you the best? Who are you attracted to?’ And then I shut up. Doctors need to learn how to stop and wait, provide the space to answer.”
I can see why understanding our patients more deeply is important. We treat people with cancer, not cancer people. As such, understanding someone more fully includes being cognizant of how they identify.
“I am continuously inspired by my LGBTQ patients who have fought to realize who they are and become their truer selves,” Dr. Forcier said. “They know who they are, and they know what they need. They have learned to demand it, to demand that their rights be respected – both civil and human rights.”
As we look toward a future in medicine where diversity, equity, and inclusion have gained prominence and urgency, I think there is reason to be hopeful. In oncology, one institutional study published in 2017 found that, although only about a third of practicing clinicians surveyed were comfortable treating LGBTQ patients, 92% of them acknowledged our unique needs, 78% wanted more education on how to appropriately care for our community, and 64% wanted to be listed as an LGBTQ-friendly provider.
“As an optimist, I believe that those struggling with homophobia/transphobia are open to doing things better,” Dr. Forcier said. “After all, we all strive to be better doctors. Whether explicit or implicit bias is at play, turning moments where colleagues are being inappropriate and showing them an alternative, more inclusive way to handle things is one mechanism to educate, rather than to shame. The bottom line is simple: You don’t have to be perfect. You just have to try.”
Dr. Dizon is the director of women’s cancers at Lifespan Cancer Institute and director of medical oncology at Rhode Island Hospital, both in Providence. He is also a professor of medicine at Brown University. His research interests are in novel treatments of women’s cancers and issues related to survivorship, particularly as they relate to sexual health after cancer for both men and women.
A version of this article first appeared on Medscape.com.
Single-dose HPV vaccination highly effective
A single dose of human papillomavirus (HPV) vaccine was highly effective at preventing oncogenic infection, rivaling the protection offered by multidose regimens, according to results from the KEN SHE trial, based in Kenya.
The findings, published on the preprint server Research Square and presented Nov. 17 at the 34th International Papillomavirus Conference in Toronto, bring “renewed energy to the push to make cervical cancer the first cancer to be wiped out globally,” according to co–principal investigator Ruanne V. Barnabas, PhD, a professor of global health at the University of Washington, Seattle.
Decision-makers will consider these findings, which have not yet been peer-reviewed, along with other evidence to determine if dosing-schedule changes are warranted, she told this news organization.
In a press release, Samuel Kariuki, PhD, acting director general, Kenya Medical Research Institute, who was not involved in the research, called the findings a “game changer” that could “substantially reduce the incidence of HPV-attributable cervical cancer.”
Between 2018 and 2019, Dr. Barnabas and her colleagues enrolled 2,275 sexually active, HPV-vaccine–naive women in Kenya in their study. The women, 15-20 years of age, were randomly assigned to receive a bivalent vaccine (HPV 16/18), a nonavalent vaccine (HPV 16/18/31/33/45/52/58/6/11), or a vaccine against meningococcal meningitis.
Most participants (57%) were between 15 and 17 years of age, and 61% reported one lifetime sexual partner. The women underwent genital and cervical swabs at enrollment to test for HPV DNA and had blood drawn to test for antibodies. During 18 months of follow-up, they had cervical swabs every 6 months and a vaginal swab at 3 months to test for HPV DNA.
The researchers detected 38 persistent HPV 16/18 infections in women who had tested negative for HPV 16/18 antibodies at enrollment and for HPV 16/18 DNA at enrollment and month 3 – one in each of the HPV-vaccine groups and 36 in the meningococcal group. This infection rate corresponded to a vaccine efficacy of 97.5% (P < .001) against HPV 16/18 for both the bivalent and nonavalent vaccines, which is “comparable to that seen in multidose vaccine trials,” the researchers write.
Among women negative for HPV 16/18/31/33/45/52/58 at the beginning of the trial, 33 had persistent infections: four in the nonavalent vaccine group and 29 in the meningococcal group, demonstrating an efficacy of 89% (P < .001) against all seven oncogenic strains contained in the vaccine.
Even if women tested positive for one strain of HPV, the vaccine protected them from other strains of the virus, the investigators noted.
Serious adverse events occurred in 4.5%-5.2% of participants across the study arms.
The KEN SHE trial comes 15 years after the U.S. Food and Drug Administration approved the first HPV vaccine – Merck’s Gardasil. Two others, Cervarix and Gardasil-9, have since been approved, but cost and supply issues have inhibited coverage, particularly in areas where the cervical cancer burden is high, the researchers noted.
Recent data indicate that just 15% of girls globally are vaccinated against HPV, but a single-dose vaccine would “simplify logistics and decrease costs,” thereby improving the chances of reaching the World Health Organization goal of vaccinating 90% of 15-year-old girls against HPV by 2030, Dr. Barnabas said in a press release about the trial.
Co–principal investigator Nelly Mugo, MBChB, MPH, senior principal clinical research scientist with the Center for Clinical Research at the Kenya Medical Research Institute in Nairobi, further emphasized the importance of the findings, noting in the press release that the “trial brings new energy to the elimination of cervical cancer. It brings great hope to the women living in countries like Kenya, who have a high burden of the disease.”
Dr. Mugo is also an associate research professor of global health at the University of Washington, Seattle.
Dr. Barnabas said women have been given multiple doses of the HPV vaccine because of “gaps in evidence for the effectiveness of a single-dose vaccine and concerns about clinically meaningful differences in efficacy.
“Observational data suggested that the single-dose HPV vaccine could have good efficacy, but because the data were not from randomized trials, that could have been from chance,” she explained, noting, however, that “sufficient evidence supported the decrease in doses from three to two doses for girls 15 years of age and younger.”
Going forward, the researchers will conduct immunobridging studies to other populations and will continue follow-up to assess the durability of single-dose efficacy, Dr. Barnabas said.
“The results from the KEN SHE trial support the use of single-dose HPV vaccination to increase access and coverage,” she concluded.
The KEN SHE trial was funded by the Bill & Melinda Gates Foundation (BMGF). Dr. Barnabas reports grants from BMGF and grants from King K. Holmes Professorship in STDs and AIDS during the conduct of the study, and grants from BMGF, National Institutes of Health, and manuscript and abstract writing support from Regeneron Pharmaceuticals outside the submitted work.
A version of this article first appeared on Medscape.com.
A single dose of human papillomavirus (HPV) vaccine was highly effective at preventing oncogenic infection, rivaling the protection offered by multidose regimens, according to results from the KEN SHE trial, based in Kenya.
The findings, published on the preprint server Research Square and presented Nov. 17 at the 34th International Papillomavirus Conference in Toronto, bring “renewed energy to the push to make cervical cancer the first cancer to be wiped out globally,” according to co–principal investigator Ruanne V. Barnabas, PhD, a professor of global health at the University of Washington, Seattle.
Decision-makers will consider these findings, which have not yet been peer-reviewed, along with other evidence to determine if dosing-schedule changes are warranted, she told this news organization.
In a press release, Samuel Kariuki, PhD, acting director general, Kenya Medical Research Institute, who was not involved in the research, called the findings a “game changer” that could “substantially reduce the incidence of HPV-attributable cervical cancer.”
Between 2018 and 2019, Dr. Barnabas and her colleagues enrolled 2,275 sexually active, HPV-vaccine–naive women in Kenya in their study. The women, 15-20 years of age, were randomly assigned to receive a bivalent vaccine (HPV 16/18), a nonavalent vaccine (HPV 16/18/31/33/45/52/58/6/11), or a vaccine against meningococcal meningitis.
Most participants (57%) were between 15 and 17 years of age, and 61% reported one lifetime sexual partner. The women underwent genital and cervical swabs at enrollment to test for HPV DNA and had blood drawn to test for antibodies. During 18 months of follow-up, they had cervical swabs every 6 months and a vaginal swab at 3 months to test for HPV DNA.
The researchers detected 38 persistent HPV 16/18 infections in women who had tested negative for HPV 16/18 antibodies at enrollment and for HPV 16/18 DNA at enrollment and month 3 – one in each of the HPV-vaccine groups and 36 in the meningococcal group. This infection rate corresponded to a vaccine efficacy of 97.5% (P < .001) against HPV 16/18 for both the bivalent and nonavalent vaccines, which is “comparable to that seen in multidose vaccine trials,” the researchers write.
Among women negative for HPV 16/18/31/33/45/52/58 at the beginning of the trial, 33 had persistent infections: four in the nonavalent vaccine group and 29 in the meningococcal group, demonstrating an efficacy of 89% (P < .001) against all seven oncogenic strains contained in the vaccine.
Even if women tested positive for one strain of HPV, the vaccine protected them from other strains of the virus, the investigators noted.
Serious adverse events occurred in 4.5%-5.2% of participants across the study arms.
The KEN SHE trial comes 15 years after the U.S. Food and Drug Administration approved the first HPV vaccine – Merck’s Gardasil. Two others, Cervarix and Gardasil-9, have since been approved, but cost and supply issues have inhibited coverage, particularly in areas where the cervical cancer burden is high, the researchers noted.
Recent data indicate that just 15% of girls globally are vaccinated against HPV, but a single-dose vaccine would “simplify logistics and decrease costs,” thereby improving the chances of reaching the World Health Organization goal of vaccinating 90% of 15-year-old girls against HPV by 2030, Dr. Barnabas said in a press release about the trial.
Co–principal investigator Nelly Mugo, MBChB, MPH, senior principal clinical research scientist with the Center for Clinical Research at the Kenya Medical Research Institute in Nairobi, further emphasized the importance of the findings, noting in the press release that the “trial brings new energy to the elimination of cervical cancer. It brings great hope to the women living in countries like Kenya, who have a high burden of the disease.”
Dr. Mugo is also an associate research professor of global health at the University of Washington, Seattle.
Dr. Barnabas said women have been given multiple doses of the HPV vaccine because of “gaps in evidence for the effectiveness of a single-dose vaccine and concerns about clinically meaningful differences in efficacy.
“Observational data suggested that the single-dose HPV vaccine could have good efficacy, but because the data were not from randomized trials, that could have been from chance,” she explained, noting, however, that “sufficient evidence supported the decrease in doses from three to two doses for girls 15 years of age and younger.”
Going forward, the researchers will conduct immunobridging studies to other populations and will continue follow-up to assess the durability of single-dose efficacy, Dr. Barnabas said.
“The results from the KEN SHE trial support the use of single-dose HPV vaccination to increase access and coverage,” she concluded.
The KEN SHE trial was funded by the Bill & Melinda Gates Foundation (BMGF). Dr. Barnabas reports grants from BMGF and grants from King K. Holmes Professorship in STDs and AIDS during the conduct of the study, and grants from BMGF, National Institutes of Health, and manuscript and abstract writing support from Regeneron Pharmaceuticals outside the submitted work.
A version of this article first appeared on Medscape.com.
A single dose of human papillomavirus (HPV) vaccine was highly effective at preventing oncogenic infection, rivaling the protection offered by multidose regimens, according to results from the KEN SHE trial, based in Kenya.
The findings, published on the preprint server Research Square and presented Nov. 17 at the 34th International Papillomavirus Conference in Toronto, bring “renewed energy to the push to make cervical cancer the first cancer to be wiped out globally,” according to co–principal investigator Ruanne V. Barnabas, PhD, a professor of global health at the University of Washington, Seattle.
Decision-makers will consider these findings, which have not yet been peer-reviewed, along with other evidence to determine if dosing-schedule changes are warranted, she told this news organization.
In a press release, Samuel Kariuki, PhD, acting director general, Kenya Medical Research Institute, who was not involved in the research, called the findings a “game changer” that could “substantially reduce the incidence of HPV-attributable cervical cancer.”
Between 2018 and 2019, Dr. Barnabas and her colleagues enrolled 2,275 sexually active, HPV-vaccine–naive women in Kenya in their study. The women, 15-20 years of age, were randomly assigned to receive a bivalent vaccine (HPV 16/18), a nonavalent vaccine (HPV 16/18/31/33/45/52/58/6/11), or a vaccine against meningococcal meningitis.
Most participants (57%) were between 15 and 17 years of age, and 61% reported one lifetime sexual partner. The women underwent genital and cervical swabs at enrollment to test for HPV DNA and had blood drawn to test for antibodies. During 18 months of follow-up, they had cervical swabs every 6 months and a vaginal swab at 3 months to test for HPV DNA.
The researchers detected 38 persistent HPV 16/18 infections in women who had tested negative for HPV 16/18 antibodies at enrollment and for HPV 16/18 DNA at enrollment and month 3 – one in each of the HPV-vaccine groups and 36 in the meningococcal group. This infection rate corresponded to a vaccine efficacy of 97.5% (P < .001) against HPV 16/18 for both the bivalent and nonavalent vaccines, which is “comparable to that seen in multidose vaccine trials,” the researchers write.
Among women negative for HPV 16/18/31/33/45/52/58 at the beginning of the trial, 33 had persistent infections: four in the nonavalent vaccine group and 29 in the meningococcal group, demonstrating an efficacy of 89% (P < .001) against all seven oncogenic strains contained in the vaccine.
Even if women tested positive for one strain of HPV, the vaccine protected them from other strains of the virus, the investigators noted.
Serious adverse events occurred in 4.5%-5.2% of participants across the study arms.
The KEN SHE trial comes 15 years after the U.S. Food and Drug Administration approved the first HPV vaccine – Merck’s Gardasil. Two others, Cervarix and Gardasil-9, have since been approved, but cost and supply issues have inhibited coverage, particularly in areas where the cervical cancer burden is high, the researchers noted.
Recent data indicate that just 15% of girls globally are vaccinated against HPV, but a single-dose vaccine would “simplify logistics and decrease costs,” thereby improving the chances of reaching the World Health Organization goal of vaccinating 90% of 15-year-old girls against HPV by 2030, Dr. Barnabas said in a press release about the trial.
Co–principal investigator Nelly Mugo, MBChB, MPH, senior principal clinical research scientist with the Center for Clinical Research at the Kenya Medical Research Institute in Nairobi, further emphasized the importance of the findings, noting in the press release that the “trial brings new energy to the elimination of cervical cancer. It brings great hope to the women living in countries like Kenya, who have a high burden of the disease.”
Dr. Mugo is also an associate research professor of global health at the University of Washington, Seattle.
Dr. Barnabas said women have been given multiple doses of the HPV vaccine because of “gaps in evidence for the effectiveness of a single-dose vaccine and concerns about clinically meaningful differences in efficacy.
“Observational data suggested that the single-dose HPV vaccine could have good efficacy, but because the data were not from randomized trials, that could have been from chance,” she explained, noting, however, that “sufficient evidence supported the decrease in doses from three to two doses for girls 15 years of age and younger.”
Going forward, the researchers will conduct immunobridging studies to other populations and will continue follow-up to assess the durability of single-dose efficacy, Dr. Barnabas said.
“The results from the KEN SHE trial support the use of single-dose HPV vaccination to increase access and coverage,” she concluded.
The KEN SHE trial was funded by the Bill & Melinda Gates Foundation (BMGF). Dr. Barnabas reports grants from BMGF and grants from King K. Holmes Professorship in STDs and AIDS during the conduct of the study, and grants from BMGF, National Institutes of Health, and manuscript and abstract writing support from Regeneron Pharmaceuticals outside the submitted work.
A version of this article first appeared on Medscape.com.
HPV vaccines reduce cervical cancer rates in young females
Two different studies have found that, provided young females are immunized with the human papilloma virus (HPV) vaccine at a young enough age, both the incidence of and mortality from cervical cancer can be dramatically curtailed, data from the United Kingdom and to a lesser extent, the United States indicate.
In the U.K. study, published online in The Lancet, researchers showed that the national vaccination program against HPV, initiated in England in 2008, has all but eradicated cervical cancer and cervical intraepithelial neoplasia (CIN3) in young girls who received the vaccine at the age of 12 and 13 years (school year 8) prior to their sexual debut.
In this age group, cervical cancer rates were 87% lower than rates among previously nonvaccinated generations, while CIN3 rates were reduced by 97%, as researchers report. “It’s been incredible to see the impact of HPV vaccination, and now we can prove it prevented hundreds of women from developing cancer in England,” senior author Peter Sasieni, MD, King’s College London, said in a statement. “To see the real-life impact of the vaccine has been truly rewarding,” he added.
“This study provides the first direct evidence of the impact of the UK HPV vaccination campaign on cervical cancer incidence, showing a large reduction in cervical cancer rates in vaccinated cohorts,” Kate Soldan, MD, UK Health Security Agency, London, said in the same statement.
“This represents an important step forward in cervical cancer prevention, and we hope that these new results encourage uptake as the success of the vaccination programme relies not only on the efficacy of the vaccine but also the proportion of the population vaccinated,” she added.
Vanessa Saliba, MD, a consultant epidemiologist for the UK Health Security Agency, agreed, adding that “these remarkable findings confirm that the HPV vaccine saves lives by dramatically reducing cervical cancer rates among women.”
“This reminds us that vaccines are one of the most important tools we have to help us live longer, healthier lives,” she reemphasized.
British HPV program
When initiated in 2008, the national HPV vaccination program used the bivalent, Cervarix vaccine against HPV 16 and 18. As researchers noted, these two HPV types are responsible for 70%-80% of all cervical cancers in England.
However, in 2012, the program switched to the quadrivalent HPV vaccine (Gardasil) which is also effective against two additional HPV types, 6 and 11, both of which cause genital warts. The program also originally recommended the three-dose regimen for both HPV vaccines.
Now, only two doses of the vaccine are given to girls under the age of 15 even though it has been shown that a single dose of the HPV vaccine provides good protection against persistent infection, with efficacy rates that are similar to that of three doses, as the authors point out.
Among the cohort eligible for vaccination at 12 or 13 years of age, 89% received at least one dose of the HPV vaccine while 85% of the same age group received all three shots.
Cancer registry
Data from a population-based cancer registry was used to estimate the early effect of the bivalent HPV program on the incidence of cervical cancer and CIN3 in England between January 2006 and June 2019. During the study interval, there were 27,946 diagnoses of cervical cancer and 318,058 diagnoses of CIN3, lead author Milena Falcaro, MD, King’s College London, and colleagues report. Participants were then analyzed separately according to their age at the time of vaccination and the incidence rates calculated for both cervical cancer and CIN3 in the three separate groups.
For slightly older girls who received the vaccine between 14 and 16 years of age (school year 10-11), cervical cancer was reduced by 62% while CIN3 rates were reduced by 75%. For those who received the vaccine between 16 and 18 years of age (school year 12-13), cervical cancer rates were reduced by 34% while CIN3 rates were reduced by 39%, study authors add.
Indeed, the authors estimate that by June 2019 there were approximately 450 fewer cases of cervical cancer and 17,200 fewer cases of CIN3 than would otherwise have been expected in the vaccinated population in England.
The authors acknowledge that cervical cancer is rare in young women and vaccinated populations are still young. For example, the youngest recipients would have been immunized at the age of 12 in 2008 and would still be only 23 years old in 2019 when the study ended.
Thus, the authors emphasize that, because the vaccinated populations are still young, it’s too early to assess the full effect of HPV vaccination on cervical cancer rates.
Asked to comment on the study, Maurice Markman, MD, president, Medicine and Science Cancer Treatment Centers of America, pointed out that results from the British study are very similar to those from a Swedish study assessing the effect of the quadrivalent vaccine alone.
“You can put any superlatives you want in here, but these are stunningly positive results,” Dr. Markman said in an interview. As an oncologist who has been treating cervical cancer for 40 years – particularly advanced cervical cancer – “I can tell you this is one of the most devastating diseases to women, and the ability to eliminate this cancer with something as simple as a vaccine is the goal of cancer therapy, and it’s been remarkably successful,” he stressed.
Editorial commentary
Commenting on the findings, editorialists Maggie Cruickshank, MD, University of Aberdeen (Scotland), and Mihaela Grigore, MD, University of Medicine and Pharmacy, Lasi, Romania, point out that published reports evaluating the effect of HPV vaccination on cervical cancer rates have been scarce until now.
“The most important issue, besides the availability of the vaccine ... is the education of the population to accept vaccination because a high rate of immunization is a key element of success,” they emphasize. “Even in a wealthy country such as England with free access to HPV immunization, uptake has not reached the 90% vaccination target of girls aged 15 years set by the WHO [World Health Organization],” the editorialists add.
Dr. Cruickshank and Dr. Grigore also suggest that the effect HPV vaccination is having on cervical cancer rates as shown in this study should also stimulate vaccination programs in low- and middle-income countries where cervical cancer is a far greater public health issue than it is in countries with established systems of vaccination and screening.
HPV vaccination in the United States
The HPV vaccination program is similarly reducing the incidence of and mortality from cervical cancer among younger women in the United States who are most likely to have received the vaccine. As reported by lead author, Justin Barnes, MD, Washington University, St. Louis, the incidence of cervical cancer dropped by 37.7% from 2001 through 2005 to 2010 through 2017 in girls and young women between 15 and 24 years of age.
The U.S. study was published online in JAMA Pediatrics.
“HPV vaccine coverage in the U.S. has improved over the last few years although it was quite poor for many years,” senior author of the U.K. study, Peter Sasieni, MD, King’s College London, said in an interview. “Thus, one would anticipate a lower impact on the population in the U.S., because vaccine uptake, particularly in those aged 11-14 years was so much lower than it was in the U.K.,” he noted.
SEER databases
National age-adjusted cervical cancer incidence and mortality data from January 2001 through December 2017 for women and girls between 15 and 39 years of age were obtained from the combined Surveillance, Epidemiology, and End Results as well as the National Program of Cancer Registries databases. Mortality data was obtained from the National Center for Health Statistics.
Investigators then compared percentage changes in the incidence of and mortality from cervical cancer from January 2001 through December 2005 during the prevaccination years to that observed between January 2010 through December 2017 during the postvaccination years. They also compared incidence and mortality rates in three different cohorts: females between 15 and 24 years of age, those between 25 and 29 years of age, and those between 30 and 39 years of age.
“The older two groups were included as comparison, given their low vaccination rates,” the authors explained. Results showed that, during the same study interval from 2001 through 2005 to 2010 through 2017, the incidence of cervical cancer dropped by only 16.1% in women between 25 and 29 years of age and by only 8% for women between 30 and 39 years of age, the investigators report.
Reductions in mortality from cervical cancer were only strikingly so in the youngest age group of females between 15 and 24 years of age, among whom there was a 43.3% reduction in mortality from 2001-2005 to 2010-2017, as Dr. Barnes and colleagues note.
This pattern changed substantially in women between the ages of 25 and 29, among whom there was a 4.3% increase in mortality from cervical cancer during the same study interval and a small, 4.7% reduction among women between 30 and 39 years of age, investigators add. In actual numbers, mortality rates from cervical cancer were very low at only 0.6 per 100,000 in females between 15 and 24 years of age.
This compared to a mortality rate of 0.57 per 100,000 in women between 25 and 29 years of age and 1.89 per 100,000 in the oldest age group. “These nationwide data showed decreased cervical cancer incidence and mortality among women and girls aged 15-24 years after HPV vaccine introduction,” Dr. Barnes notes.
“Thus, the current study adds to knowledge by quantitatively comparing changes in cervical cancer incidence by age-based vaccine eligibility and providing suggestive evidence for vaccine-associated decreases in cervical cancer mortality,” investigators add.
However, as the authors also point out, while the reduction in mortality from cervical cancer associated with HPV vaccination may translate to older age groups as HPV-vaccinated cohorts age, “the number of deaths and hence the number of potentially averted deaths in young women and girls was small,” they caution, “and efforts to further improve vaccination uptake remain important.”
None of the authors or the editorialists had any conflicts of interest to declare.
Two different studies have found that, provided young females are immunized with the human papilloma virus (HPV) vaccine at a young enough age, both the incidence of and mortality from cervical cancer can be dramatically curtailed, data from the United Kingdom and to a lesser extent, the United States indicate.
In the U.K. study, published online in The Lancet, researchers showed that the national vaccination program against HPV, initiated in England in 2008, has all but eradicated cervical cancer and cervical intraepithelial neoplasia (CIN3) in young girls who received the vaccine at the age of 12 and 13 years (school year 8) prior to their sexual debut.
In this age group, cervical cancer rates were 87% lower than rates among previously nonvaccinated generations, while CIN3 rates were reduced by 97%, as researchers report. “It’s been incredible to see the impact of HPV vaccination, and now we can prove it prevented hundreds of women from developing cancer in England,” senior author Peter Sasieni, MD, King’s College London, said in a statement. “To see the real-life impact of the vaccine has been truly rewarding,” he added.
“This study provides the first direct evidence of the impact of the UK HPV vaccination campaign on cervical cancer incidence, showing a large reduction in cervical cancer rates in vaccinated cohorts,” Kate Soldan, MD, UK Health Security Agency, London, said in the same statement.
“This represents an important step forward in cervical cancer prevention, and we hope that these new results encourage uptake as the success of the vaccination programme relies not only on the efficacy of the vaccine but also the proportion of the population vaccinated,” she added.
Vanessa Saliba, MD, a consultant epidemiologist for the UK Health Security Agency, agreed, adding that “these remarkable findings confirm that the HPV vaccine saves lives by dramatically reducing cervical cancer rates among women.”
“This reminds us that vaccines are one of the most important tools we have to help us live longer, healthier lives,” she reemphasized.
British HPV program
When initiated in 2008, the national HPV vaccination program used the bivalent, Cervarix vaccine against HPV 16 and 18. As researchers noted, these two HPV types are responsible for 70%-80% of all cervical cancers in England.
However, in 2012, the program switched to the quadrivalent HPV vaccine (Gardasil) which is also effective against two additional HPV types, 6 and 11, both of which cause genital warts. The program also originally recommended the three-dose regimen for both HPV vaccines.
Now, only two doses of the vaccine are given to girls under the age of 15 even though it has been shown that a single dose of the HPV vaccine provides good protection against persistent infection, with efficacy rates that are similar to that of three doses, as the authors point out.
Among the cohort eligible for vaccination at 12 or 13 years of age, 89% received at least one dose of the HPV vaccine while 85% of the same age group received all three shots.
Cancer registry
Data from a population-based cancer registry was used to estimate the early effect of the bivalent HPV program on the incidence of cervical cancer and CIN3 in England between January 2006 and June 2019. During the study interval, there were 27,946 diagnoses of cervical cancer and 318,058 diagnoses of CIN3, lead author Milena Falcaro, MD, King’s College London, and colleagues report. Participants were then analyzed separately according to their age at the time of vaccination and the incidence rates calculated for both cervical cancer and CIN3 in the three separate groups.
For slightly older girls who received the vaccine between 14 and 16 years of age (school year 10-11), cervical cancer was reduced by 62% while CIN3 rates were reduced by 75%. For those who received the vaccine between 16 and 18 years of age (school year 12-13), cervical cancer rates were reduced by 34% while CIN3 rates were reduced by 39%, study authors add.
Indeed, the authors estimate that by June 2019 there were approximately 450 fewer cases of cervical cancer and 17,200 fewer cases of CIN3 than would otherwise have been expected in the vaccinated population in England.
The authors acknowledge that cervical cancer is rare in young women and vaccinated populations are still young. For example, the youngest recipients would have been immunized at the age of 12 in 2008 and would still be only 23 years old in 2019 when the study ended.
Thus, the authors emphasize that, because the vaccinated populations are still young, it’s too early to assess the full effect of HPV vaccination on cervical cancer rates.
Asked to comment on the study, Maurice Markman, MD, president, Medicine and Science Cancer Treatment Centers of America, pointed out that results from the British study are very similar to those from a Swedish study assessing the effect of the quadrivalent vaccine alone.
“You can put any superlatives you want in here, but these are stunningly positive results,” Dr. Markman said in an interview. As an oncologist who has been treating cervical cancer for 40 years – particularly advanced cervical cancer – “I can tell you this is one of the most devastating diseases to women, and the ability to eliminate this cancer with something as simple as a vaccine is the goal of cancer therapy, and it’s been remarkably successful,” he stressed.
Editorial commentary
Commenting on the findings, editorialists Maggie Cruickshank, MD, University of Aberdeen (Scotland), and Mihaela Grigore, MD, University of Medicine and Pharmacy, Lasi, Romania, point out that published reports evaluating the effect of HPV vaccination on cervical cancer rates have been scarce until now.
“The most important issue, besides the availability of the vaccine ... is the education of the population to accept vaccination because a high rate of immunization is a key element of success,” they emphasize. “Even in a wealthy country such as England with free access to HPV immunization, uptake has not reached the 90% vaccination target of girls aged 15 years set by the WHO [World Health Organization],” the editorialists add.
Dr. Cruickshank and Dr. Grigore also suggest that the effect HPV vaccination is having on cervical cancer rates as shown in this study should also stimulate vaccination programs in low- and middle-income countries where cervical cancer is a far greater public health issue than it is in countries with established systems of vaccination and screening.
HPV vaccination in the United States
The HPV vaccination program is similarly reducing the incidence of and mortality from cervical cancer among younger women in the United States who are most likely to have received the vaccine. As reported by lead author, Justin Barnes, MD, Washington University, St. Louis, the incidence of cervical cancer dropped by 37.7% from 2001 through 2005 to 2010 through 2017 in girls and young women between 15 and 24 years of age.
The U.S. study was published online in JAMA Pediatrics.
“HPV vaccine coverage in the U.S. has improved over the last few years although it was quite poor for many years,” senior author of the U.K. study, Peter Sasieni, MD, King’s College London, said in an interview. “Thus, one would anticipate a lower impact on the population in the U.S., because vaccine uptake, particularly in those aged 11-14 years was so much lower than it was in the U.K.,” he noted.
SEER databases
National age-adjusted cervical cancer incidence and mortality data from January 2001 through December 2017 for women and girls between 15 and 39 years of age were obtained from the combined Surveillance, Epidemiology, and End Results as well as the National Program of Cancer Registries databases. Mortality data was obtained from the National Center for Health Statistics.
Investigators then compared percentage changes in the incidence of and mortality from cervical cancer from January 2001 through December 2005 during the prevaccination years to that observed between January 2010 through December 2017 during the postvaccination years. They also compared incidence and mortality rates in three different cohorts: females between 15 and 24 years of age, those between 25 and 29 years of age, and those between 30 and 39 years of age.
“The older two groups were included as comparison, given their low vaccination rates,” the authors explained. Results showed that, during the same study interval from 2001 through 2005 to 2010 through 2017, the incidence of cervical cancer dropped by only 16.1% in women between 25 and 29 years of age and by only 8% for women between 30 and 39 years of age, the investigators report.
Reductions in mortality from cervical cancer were only strikingly so in the youngest age group of females between 15 and 24 years of age, among whom there was a 43.3% reduction in mortality from 2001-2005 to 2010-2017, as Dr. Barnes and colleagues note.
This pattern changed substantially in women between the ages of 25 and 29, among whom there was a 4.3% increase in mortality from cervical cancer during the same study interval and a small, 4.7% reduction among women between 30 and 39 years of age, investigators add. In actual numbers, mortality rates from cervical cancer were very low at only 0.6 per 100,000 in females between 15 and 24 years of age.
This compared to a mortality rate of 0.57 per 100,000 in women between 25 and 29 years of age and 1.89 per 100,000 in the oldest age group. “These nationwide data showed decreased cervical cancer incidence and mortality among women and girls aged 15-24 years after HPV vaccine introduction,” Dr. Barnes notes.
“Thus, the current study adds to knowledge by quantitatively comparing changes in cervical cancer incidence by age-based vaccine eligibility and providing suggestive evidence for vaccine-associated decreases in cervical cancer mortality,” investigators add.
However, as the authors also point out, while the reduction in mortality from cervical cancer associated with HPV vaccination may translate to older age groups as HPV-vaccinated cohorts age, “the number of deaths and hence the number of potentially averted deaths in young women and girls was small,” they caution, “and efforts to further improve vaccination uptake remain important.”
None of the authors or the editorialists had any conflicts of interest to declare.
Two different studies have found that, provided young females are immunized with the human papilloma virus (HPV) vaccine at a young enough age, both the incidence of and mortality from cervical cancer can be dramatically curtailed, data from the United Kingdom and to a lesser extent, the United States indicate.
In the U.K. study, published online in The Lancet, researchers showed that the national vaccination program against HPV, initiated in England in 2008, has all but eradicated cervical cancer and cervical intraepithelial neoplasia (CIN3) in young girls who received the vaccine at the age of 12 and 13 years (school year 8) prior to their sexual debut.
In this age group, cervical cancer rates were 87% lower than rates among previously nonvaccinated generations, while CIN3 rates were reduced by 97%, as researchers report. “It’s been incredible to see the impact of HPV vaccination, and now we can prove it prevented hundreds of women from developing cancer in England,” senior author Peter Sasieni, MD, King’s College London, said in a statement. “To see the real-life impact of the vaccine has been truly rewarding,” he added.
“This study provides the first direct evidence of the impact of the UK HPV vaccination campaign on cervical cancer incidence, showing a large reduction in cervical cancer rates in vaccinated cohorts,” Kate Soldan, MD, UK Health Security Agency, London, said in the same statement.
“This represents an important step forward in cervical cancer prevention, and we hope that these new results encourage uptake as the success of the vaccination programme relies not only on the efficacy of the vaccine but also the proportion of the population vaccinated,” she added.
Vanessa Saliba, MD, a consultant epidemiologist for the UK Health Security Agency, agreed, adding that “these remarkable findings confirm that the HPV vaccine saves lives by dramatically reducing cervical cancer rates among women.”
“This reminds us that vaccines are one of the most important tools we have to help us live longer, healthier lives,” she reemphasized.
British HPV program
When initiated in 2008, the national HPV vaccination program used the bivalent, Cervarix vaccine against HPV 16 and 18. As researchers noted, these two HPV types are responsible for 70%-80% of all cervical cancers in England.
However, in 2012, the program switched to the quadrivalent HPV vaccine (Gardasil) which is also effective against two additional HPV types, 6 and 11, both of which cause genital warts. The program also originally recommended the three-dose regimen for both HPV vaccines.
Now, only two doses of the vaccine are given to girls under the age of 15 even though it has been shown that a single dose of the HPV vaccine provides good protection against persistent infection, with efficacy rates that are similar to that of three doses, as the authors point out.
Among the cohort eligible for vaccination at 12 or 13 years of age, 89% received at least one dose of the HPV vaccine while 85% of the same age group received all three shots.
Cancer registry
Data from a population-based cancer registry was used to estimate the early effect of the bivalent HPV program on the incidence of cervical cancer and CIN3 in England between January 2006 and June 2019. During the study interval, there were 27,946 diagnoses of cervical cancer and 318,058 diagnoses of CIN3, lead author Milena Falcaro, MD, King’s College London, and colleagues report. Participants were then analyzed separately according to their age at the time of vaccination and the incidence rates calculated for both cervical cancer and CIN3 in the three separate groups.
For slightly older girls who received the vaccine between 14 and 16 years of age (school year 10-11), cervical cancer was reduced by 62% while CIN3 rates were reduced by 75%. For those who received the vaccine between 16 and 18 years of age (school year 12-13), cervical cancer rates were reduced by 34% while CIN3 rates were reduced by 39%, study authors add.
Indeed, the authors estimate that by June 2019 there were approximately 450 fewer cases of cervical cancer and 17,200 fewer cases of CIN3 than would otherwise have been expected in the vaccinated population in England.
The authors acknowledge that cervical cancer is rare in young women and vaccinated populations are still young. For example, the youngest recipients would have been immunized at the age of 12 in 2008 and would still be only 23 years old in 2019 when the study ended.
Thus, the authors emphasize that, because the vaccinated populations are still young, it’s too early to assess the full effect of HPV vaccination on cervical cancer rates.
Asked to comment on the study, Maurice Markman, MD, president, Medicine and Science Cancer Treatment Centers of America, pointed out that results from the British study are very similar to those from a Swedish study assessing the effect of the quadrivalent vaccine alone.
“You can put any superlatives you want in here, but these are stunningly positive results,” Dr. Markman said in an interview. As an oncologist who has been treating cervical cancer for 40 years – particularly advanced cervical cancer – “I can tell you this is one of the most devastating diseases to women, and the ability to eliminate this cancer with something as simple as a vaccine is the goal of cancer therapy, and it’s been remarkably successful,” he stressed.
Editorial commentary
Commenting on the findings, editorialists Maggie Cruickshank, MD, University of Aberdeen (Scotland), and Mihaela Grigore, MD, University of Medicine and Pharmacy, Lasi, Romania, point out that published reports evaluating the effect of HPV vaccination on cervical cancer rates have been scarce until now.
“The most important issue, besides the availability of the vaccine ... is the education of the population to accept vaccination because a high rate of immunization is a key element of success,” they emphasize. “Even in a wealthy country such as England with free access to HPV immunization, uptake has not reached the 90% vaccination target of girls aged 15 years set by the WHO [World Health Organization],” the editorialists add.
Dr. Cruickshank and Dr. Grigore also suggest that the effect HPV vaccination is having on cervical cancer rates as shown in this study should also stimulate vaccination programs in low- and middle-income countries where cervical cancer is a far greater public health issue than it is in countries with established systems of vaccination and screening.
HPV vaccination in the United States
The HPV vaccination program is similarly reducing the incidence of and mortality from cervical cancer among younger women in the United States who are most likely to have received the vaccine. As reported by lead author, Justin Barnes, MD, Washington University, St. Louis, the incidence of cervical cancer dropped by 37.7% from 2001 through 2005 to 2010 through 2017 in girls and young women between 15 and 24 years of age.
The U.S. study was published online in JAMA Pediatrics.
“HPV vaccine coverage in the U.S. has improved over the last few years although it was quite poor for many years,” senior author of the U.K. study, Peter Sasieni, MD, King’s College London, said in an interview. “Thus, one would anticipate a lower impact on the population in the U.S., because vaccine uptake, particularly in those aged 11-14 years was so much lower than it was in the U.K.,” he noted.
SEER databases
National age-adjusted cervical cancer incidence and mortality data from January 2001 through December 2017 for women and girls between 15 and 39 years of age were obtained from the combined Surveillance, Epidemiology, and End Results as well as the National Program of Cancer Registries databases. Mortality data was obtained from the National Center for Health Statistics.
Investigators then compared percentage changes in the incidence of and mortality from cervical cancer from January 2001 through December 2005 during the prevaccination years to that observed between January 2010 through December 2017 during the postvaccination years. They also compared incidence and mortality rates in three different cohorts: females between 15 and 24 years of age, those between 25 and 29 years of age, and those between 30 and 39 years of age.
“The older two groups were included as comparison, given their low vaccination rates,” the authors explained. Results showed that, during the same study interval from 2001 through 2005 to 2010 through 2017, the incidence of cervical cancer dropped by only 16.1% in women between 25 and 29 years of age and by only 8% for women between 30 and 39 years of age, the investigators report.
Reductions in mortality from cervical cancer were only strikingly so in the youngest age group of females between 15 and 24 years of age, among whom there was a 43.3% reduction in mortality from 2001-2005 to 2010-2017, as Dr. Barnes and colleagues note.
This pattern changed substantially in women between the ages of 25 and 29, among whom there was a 4.3% increase in mortality from cervical cancer during the same study interval and a small, 4.7% reduction among women between 30 and 39 years of age, investigators add. In actual numbers, mortality rates from cervical cancer were very low at only 0.6 per 100,000 in females between 15 and 24 years of age.
This compared to a mortality rate of 0.57 per 100,000 in women between 25 and 29 years of age and 1.89 per 100,000 in the oldest age group. “These nationwide data showed decreased cervical cancer incidence and mortality among women and girls aged 15-24 years after HPV vaccine introduction,” Dr. Barnes notes.
“Thus, the current study adds to knowledge by quantitatively comparing changes in cervical cancer incidence by age-based vaccine eligibility and providing suggestive evidence for vaccine-associated decreases in cervical cancer mortality,” investigators add.
However, as the authors also point out, while the reduction in mortality from cervical cancer associated with HPV vaccination may translate to older age groups as HPV-vaccinated cohorts age, “the number of deaths and hence the number of potentially averted deaths in young women and girls was small,” they caution, “and efforts to further improve vaccination uptake remain important.”
None of the authors or the editorialists had any conflicts of interest to declare.
FDA approves imaging drug for detecting ovarian cancer lesions
The new drug “is designed to improve the ability to locate additional ovarian cancerous tissue that is normally difficult to detect during surgery,” according to the agency.
Pafolacianine, administered via intravenous injection prior to surgery, is the first FDA-approved tumor-targeted fluorescent agent for ovarian cancer.
In a press statement, drug inventor Philip Low, PhD, of Purdue University in West Lafayette, Ind., said the agent causes ovarian cancer lesions to “light up like stars against a night sky.”
Improving detection of ovarian cancer lesions is critical given that ovarian cancer is one of the “deadliest of all female reproductive system cancers,” according to the American Cancer Society. The organization estimates that there will be more than 21,000 new cases and more than 13,000 deaths in 2021.
Currently, surgeons use preoperative imaging as well as visual inspection of tumors under normal light and examination by touch to identify ovarian cancer lesions.
Pafolacianine offers a new tool to enhance surgeons’ ability “to identify deadly ovarian tumors that may otherwise go undetected,” Alex Gorovets, MD, deputy director of the office of specialty medicine in the FDA’s Center for Drug Evaluation and Research, said in a press statement.
Ovarian cancer often causes the body to overproduce the folate receptor protein in cell membranes. Pafolacianine, employed with a near-infrared fluorescence imaging system cleared by the FDA for use alongside the drug, binds to and illuminates these proteins under fluorescent light, “boosting surgeons’ ability to identify the cancerous tissue,” the agency in a statement.
The safety and effectiveness of pafolacianine was evaluated in a randomized, multi-center, open-label study of women diagnosed with ovarian cancer or with high clinical suspicion of ovarian cancer. Of the 134 women undergoing surgery who received a dose of pafolacianine and were evaluated under both normal and fluorescent light, 26.9% had at least one cancerous lesion detected that was not observed by standard visual or tactile inspection.
The most common side effects of pafolacianine were infusion-related reactions, including nausea, vomiting, abdominal pain, flushing, dyspepsia, chest discomfort, itching, and hypersensitivity.
Pafolacianine may cause fetal harm when administered to a pregnant woman. The use of folate, folic acid, or folate-containing supplements should be avoided within 48 hours before administration of pafolacianine.
The FDA also cautioned about the possible risk of image interpretation errors, including false negatives and false positives, with the use of the new drug and near-infrared fluorescence imaging system.
The FDA previously granted pafolacianine orphan-drug, priority, and fast track designations.
A version of this article first appeared on Medscape.com.
The new drug “is designed to improve the ability to locate additional ovarian cancerous tissue that is normally difficult to detect during surgery,” according to the agency.
Pafolacianine, administered via intravenous injection prior to surgery, is the first FDA-approved tumor-targeted fluorescent agent for ovarian cancer.
In a press statement, drug inventor Philip Low, PhD, of Purdue University in West Lafayette, Ind., said the agent causes ovarian cancer lesions to “light up like stars against a night sky.”
Improving detection of ovarian cancer lesions is critical given that ovarian cancer is one of the “deadliest of all female reproductive system cancers,” according to the American Cancer Society. The organization estimates that there will be more than 21,000 new cases and more than 13,000 deaths in 2021.
Currently, surgeons use preoperative imaging as well as visual inspection of tumors under normal light and examination by touch to identify ovarian cancer lesions.
Pafolacianine offers a new tool to enhance surgeons’ ability “to identify deadly ovarian tumors that may otherwise go undetected,” Alex Gorovets, MD, deputy director of the office of specialty medicine in the FDA’s Center for Drug Evaluation and Research, said in a press statement.
Ovarian cancer often causes the body to overproduce the folate receptor protein in cell membranes. Pafolacianine, employed with a near-infrared fluorescence imaging system cleared by the FDA for use alongside the drug, binds to and illuminates these proteins under fluorescent light, “boosting surgeons’ ability to identify the cancerous tissue,” the agency in a statement.
The safety and effectiveness of pafolacianine was evaluated in a randomized, multi-center, open-label study of women diagnosed with ovarian cancer or with high clinical suspicion of ovarian cancer. Of the 134 women undergoing surgery who received a dose of pafolacianine and were evaluated under both normal and fluorescent light, 26.9% had at least one cancerous lesion detected that was not observed by standard visual or tactile inspection.
The most common side effects of pafolacianine were infusion-related reactions, including nausea, vomiting, abdominal pain, flushing, dyspepsia, chest discomfort, itching, and hypersensitivity.
Pafolacianine may cause fetal harm when administered to a pregnant woman. The use of folate, folic acid, or folate-containing supplements should be avoided within 48 hours before administration of pafolacianine.
The FDA also cautioned about the possible risk of image interpretation errors, including false negatives and false positives, with the use of the new drug and near-infrared fluorescence imaging system.
The FDA previously granted pafolacianine orphan-drug, priority, and fast track designations.
A version of this article first appeared on Medscape.com.
The new drug “is designed to improve the ability to locate additional ovarian cancerous tissue that is normally difficult to detect during surgery,” according to the agency.
Pafolacianine, administered via intravenous injection prior to surgery, is the first FDA-approved tumor-targeted fluorescent agent for ovarian cancer.
In a press statement, drug inventor Philip Low, PhD, of Purdue University in West Lafayette, Ind., said the agent causes ovarian cancer lesions to “light up like stars against a night sky.”
Improving detection of ovarian cancer lesions is critical given that ovarian cancer is one of the “deadliest of all female reproductive system cancers,” according to the American Cancer Society. The organization estimates that there will be more than 21,000 new cases and more than 13,000 deaths in 2021.
Currently, surgeons use preoperative imaging as well as visual inspection of tumors under normal light and examination by touch to identify ovarian cancer lesions.
Pafolacianine offers a new tool to enhance surgeons’ ability “to identify deadly ovarian tumors that may otherwise go undetected,” Alex Gorovets, MD, deputy director of the office of specialty medicine in the FDA’s Center for Drug Evaluation and Research, said in a press statement.
Ovarian cancer often causes the body to overproduce the folate receptor protein in cell membranes. Pafolacianine, employed with a near-infrared fluorescence imaging system cleared by the FDA for use alongside the drug, binds to and illuminates these proteins under fluorescent light, “boosting surgeons’ ability to identify the cancerous tissue,” the agency in a statement.
The safety and effectiveness of pafolacianine was evaluated in a randomized, multi-center, open-label study of women diagnosed with ovarian cancer or with high clinical suspicion of ovarian cancer. Of the 134 women undergoing surgery who received a dose of pafolacianine and were evaluated under both normal and fluorescent light, 26.9% had at least one cancerous lesion detected that was not observed by standard visual or tactile inspection.
The most common side effects of pafolacianine were infusion-related reactions, including nausea, vomiting, abdominal pain, flushing, dyspepsia, chest discomfort, itching, and hypersensitivity.
Pafolacianine may cause fetal harm when administered to a pregnant woman. The use of folate, folic acid, or folate-containing supplements should be avoided within 48 hours before administration of pafolacianine.
The FDA also cautioned about the possible risk of image interpretation errors, including false negatives and false positives, with the use of the new drug and near-infrared fluorescence imaging system.
The FDA previously granted pafolacianine orphan-drug, priority, and fast track designations.
A version of this article first appeared on Medscape.com.
Big drop in U.S. cervical cancer rates, mortality in younger women
The analysis adds to a growing body of evidence demonstrating vaccine-associated changes in cervical cancer incidence and mortality.
Previous data from the United Kingdom, published earlier in November, showed that cervical cancer rates were 87% lower among girls who received the HPV vaccine compared to previously unvaccinated generations. Based on the analysis, the authors concluded that the UK’s HPV immunization program “almost eliminated cervical cancer” in women born since September 1995.
The latest study, published Nov. 29 in JAMA Pediatrics , reports a 38% drop in cervical cancer incidence and a 43% decline in mortality among young women and girls after HPV vaccination was introduced in the United States.
“These results are encouraging,” Peter Sasieni, MD, of King’s College London, and senior author on the U.K. study, told this news organization in an email.
The difference in incidence rates between the U.K. and U.S. studies, Dr. Sasieni explained, is likely due to HPV vaccine coverage not expanding as significantly in the United States as it has in the United Kingdom, and “thus one would anticipate a lower impact on the population in the U.S.”
In the U.S. analysis, Justin Barnes, MD, a radiation oncology resident at Washington University, St. Louis, and colleagues examined cervical cancer incidence between January 2001 and December 2017 using Surveillance, Epidemiology, and End Results and National Program of Cancer Registries data as well as mortality data from the National Center for Health Statistics.
Dr. Barnes and colleagues then compared changes in cervical cancer incidence and mortality between prevaccination years (January 2001 to December 2005) and postvaccination years (January 2010 to December 2017) among three age cohorts – 15-24 years, 25-29 years, and 30-39 years.
“The older 2 groups were included as comparison, given their low vaccination rates,” Dr. Barnes and colleagues explained.
Results show that between the prevaccination and postvaccination periods, the incidence of cervical cancer dropped by 38% in the youngest cohort and by only 16% in the middle-aged group and 8% in the oldest cohort.
Women and girls in the youngest group saw a striking drop in mortality: a 43% decline, which translated to a mortality rate of 0.6 per 100,000.
On the other hand, the authors report a 4.7% decline in mortality in the oldest group and a 4.3% increase in mortality in the middle-aged group – translating to a mortality rate of 1.89 per 100,000 and 0.57 per 100,000, respectively.
Overall, “these nationwide data showed decreased cervical cancer incidence and mortality among women and girls aged 15-24 years after HPV vaccine introduction,” Dr. Barnes and colleagues wrote. The changes in cervical cancer incidence and mortality observed in the youngest age group “were greater than changes in those aged 25 to 29 years and 30 to 39 years, suggesting possible associations with HPV vaccination.”
This analysis lines up with previous evidence from U.S. epidemiologic data, which “have shown decreased cervical cancer incidence after vaccine implementation in women and girls aged 15 to 24 years but not older women.”
Although “the number of deaths and hence the number of potentially averted deaths in young women and girls was small,” the study adds to the current literature by “providing suggestive evidence for vaccine-associated decreases in cervical cancer mortality,” investigators concluded.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The analysis adds to a growing body of evidence demonstrating vaccine-associated changes in cervical cancer incidence and mortality.
Previous data from the United Kingdom, published earlier in November, showed that cervical cancer rates were 87% lower among girls who received the HPV vaccine compared to previously unvaccinated generations. Based on the analysis, the authors concluded that the UK’s HPV immunization program “almost eliminated cervical cancer” in women born since September 1995.
The latest study, published Nov. 29 in JAMA Pediatrics , reports a 38% drop in cervical cancer incidence and a 43% decline in mortality among young women and girls after HPV vaccination was introduced in the United States.
“These results are encouraging,” Peter Sasieni, MD, of King’s College London, and senior author on the U.K. study, told this news organization in an email.
The difference in incidence rates between the U.K. and U.S. studies, Dr. Sasieni explained, is likely due to HPV vaccine coverage not expanding as significantly in the United States as it has in the United Kingdom, and “thus one would anticipate a lower impact on the population in the U.S.”
In the U.S. analysis, Justin Barnes, MD, a radiation oncology resident at Washington University, St. Louis, and colleagues examined cervical cancer incidence between January 2001 and December 2017 using Surveillance, Epidemiology, and End Results and National Program of Cancer Registries data as well as mortality data from the National Center for Health Statistics.
Dr. Barnes and colleagues then compared changes in cervical cancer incidence and mortality between prevaccination years (January 2001 to December 2005) and postvaccination years (January 2010 to December 2017) among three age cohorts – 15-24 years, 25-29 years, and 30-39 years.
“The older 2 groups were included as comparison, given their low vaccination rates,” Dr. Barnes and colleagues explained.
Results show that between the prevaccination and postvaccination periods, the incidence of cervical cancer dropped by 38% in the youngest cohort and by only 16% in the middle-aged group and 8% in the oldest cohort.
Women and girls in the youngest group saw a striking drop in mortality: a 43% decline, which translated to a mortality rate of 0.6 per 100,000.
On the other hand, the authors report a 4.7% decline in mortality in the oldest group and a 4.3% increase in mortality in the middle-aged group – translating to a mortality rate of 1.89 per 100,000 and 0.57 per 100,000, respectively.
Overall, “these nationwide data showed decreased cervical cancer incidence and mortality among women and girls aged 15-24 years after HPV vaccine introduction,” Dr. Barnes and colleagues wrote. The changes in cervical cancer incidence and mortality observed in the youngest age group “were greater than changes in those aged 25 to 29 years and 30 to 39 years, suggesting possible associations with HPV vaccination.”
This analysis lines up with previous evidence from U.S. epidemiologic data, which “have shown decreased cervical cancer incidence after vaccine implementation in women and girls aged 15 to 24 years but not older women.”
Although “the number of deaths and hence the number of potentially averted deaths in young women and girls was small,” the study adds to the current literature by “providing suggestive evidence for vaccine-associated decreases in cervical cancer mortality,” investigators concluded.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The analysis adds to a growing body of evidence demonstrating vaccine-associated changes in cervical cancer incidence and mortality.
Previous data from the United Kingdom, published earlier in November, showed that cervical cancer rates were 87% lower among girls who received the HPV vaccine compared to previously unvaccinated generations. Based on the analysis, the authors concluded that the UK’s HPV immunization program “almost eliminated cervical cancer” in women born since September 1995.
The latest study, published Nov. 29 in JAMA Pediatrics , reports a 38% drop in cervical cancer incidence and a 43% decline in mortality among young women and girls after HPV vaccination was introduced in the United States.
“These results are encouraging,” Peter Sasieni, MD, of King’s College London, and senior author on the U.K. study, told this news organization in an email.
The difference in incidence rates between the U.K. and U.S. studies, Dr. Sasieni explained, is likely due to HPV vaccine coverage not expanding as significantly in the United States as it has in the United Kingdom, and “thus one would anticipate a lower impact on the population in the U.S.”
In the U.S. analysis, Justin Barnes, MD, a radiation oncology resident at Washington University, St. Louis, and colleagues examined cervical cancer incidence between January 2001 and December 2017 using Surveillance, Epidemiology, and End Results and National Program of Cancer Registries data as well as mortality data from the National Center for Health Statistics.
Dr. Barnes and colleagues then compared changes in cervical cancer incidence and mortality between prevaccination years (January 2001 to December 2005) and postvaccination years (January 2010 to December 2017) among three age cohorts – 15-24 years, 25-29 years, and 30-39 years.
“The older 2 groups were included as comparison, given their low vaccination rates,” Dr. Barnes and colleagues explained.
Results show that between the prevaccination and postvaccination periods, the incidence of cervical cancer dropped by 38% in the youngest cohort and by only 16% in the middle-aged group and 8% in the oldest cohort.
Women and girls in the youngest group saw a striking drop in mortality: a 43% decline, which translated to a mortality rate of 0.6 per 100,000.
On the other hand, the authors report a 4.7% decline in mortality in the oldest group and a 4.3% increase in mortality in the middle-aged group – translating to a mortality rate of 1.89 per 100,000 and 0.57 per 100,000, respectively.
Overall, “these nationwide data showed decreased cervical cancer incidence and mortality among women and girls aged 15-24 years after HPV vaccine introduction,” Dr. Barnes and colleagues wrote. The changes in cervical cancer incidence and mortality observed in the youngest age group “were greater than changes in those aged 25 to 29 years and 30 to 39 years, suggesting possible associations with HPV vaccination.”
This analysis lines up with previous evidence from U.S. epidemiologic data, which “have shown decreased cervical cancer incidence after vaccine implementation in women and girls aged 15 to 24 years but not older women.”
Although “the number of deaths and hence the number of potentially averted deaths in young women and girls was small,” the study adds to the current literature by “providing suggestive evidence for vaccine-associated decreases in cervical cancer mortality,” investigators concluded.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA PEDIATRICS
Should gynecologists receive the HPV vaccine?
Gynecologists have experience managing human papillomavirus–associated diseases of the lower genital tract. However, HPV also causes warty disease, dysplasia, and carcinoma of the head and neck. Risk factors for head and neck cancer include smoking and smokeless tobacco use, alcohol consumption, periodontal disease, radiation exposure, and HPV. The incidence of HPV-associated head and neck cancer is rising, particularly among men, at a rate of 2.7% per year.1 The incidence of HPV-associated squamous cell carcinoma of the oropharynx now surpasses that of cervical cancer. Concerns exist regarding occupational exposure to HPV by health care providers (HCP) who perform smoke-generating procedures on HPV-infected tissues, and the potential for them to develop head and neck pathology.
In March of 2020, the American Society for Colposcopy and Cervical Pathology made the recommendation that clinicians who are routinely exposed to HPV should protect themselves against the sequela of occupationally acquired HPV by receiving the HPV vaccine.2 They advocate for the “complete provider team” including physicians, advanced practice providers, nurses, operative technicians, and residents and fellows to be considered for protective vaccination.
Similar to disease patterns in the genital tract, different strains of HPV have differing propensity to cause benign, premalignant, and malignant disease states. HPV 6 and 11 are more commonly associated with warty disease in the nares, pharynx, and tonsillar tissues. HPV 16, 18, 31, and 33 (most commonly 16) are considered high risk for carcinoma formation, particularly of the tonsils and base of the tongue.
The procedures most implicated in occupational HPV exposure include ablative procedures for anogenital warts, laser ablation of vaginal and vulvar dysplasia, and electrosurgical excisional procedures for cervical dysplasia. Smoke plumes from HPV-associated procedures are known to contain HPV for both laser and electrocautery sources.3 A study of 134 patients undergoing surgical procedures for laser ablation of HPV-infected tissues detected concordant strains of HPV in approximately 30% of smoke plumes and approximately 1.5% of surgeons’ nares.4 Not all procedures appear to carry the same risk. Electrocoagulation procedures appear to yield fewer postprocedural positive mucosal swabs for HPV, compared with those taken after CO2 laser.5
Animal studies have shown that papilloma virus procured from smoke plume has the capacity to generate disease. When 10 calves were inoculated with bovine papillary virus obtained from smoke plumes from laser ablation of bovine papillomavirus lesions, all calves manifested BPV fibropapilloma lesions at the sites of inoculation.6
There appears to be an increased incidence of HPV-associated head and neck disease among surgeons who perform procedures on HPV tissues, and there have been multiple case reports that have cited examples of HPV-associated benign and malignant disease among HCPs with frequent occupational exposure to HPV anogenital ablative and excisional procedures.7 While these observations are not proof of causation, they are cause for concern.
While the ASCCP guidelines advocate for HPV vaccination as a strategy for prevention of occupationally related HPV-associated disease, there are other strategies in place to minimize risk. The CDC guidelines for environmental infection control in health care facilities include the following recommendations:
- In settings where surgical lasers are used, wear appropriate personnel protective equipment (PPE), including N95 or N100 respirators to minimize exposure to laser plumes.
- Use central wall suction units with in-line filters to evacuate minimal laser plumes.
- Use a mechanical smoke evaluation system with a high efficiency filter to manage the generation of large amounts of laser plume, when ablating tissue infected with HPV.
- Use local exhaust ventilation (LEV).8
When closely adhered to, these methods appear to provide high-level protection. Data suggest that, when HCPs can access appropriate protective equipment, risks for HPV exposure are low. However, this is more feasible for larger hospital facilities, and may be more limited in outpatient settings. This has led to the consideration of background protection in the form of HPV vaccination for at-risk HCPs. This is analogous to mandates for HCPs to receive hepatitis B vaccination despite the concomitant practice of universal precautions in health care settings. Preventative strategies are typically most efficacious when performed in concert.
After nearly 2 decades of widespread use, we have confidence in the safety of the HPV vaccination. Its benefit through age 45 has been established, leading to the 2018 FDA approval for the 9-valent HPV vaccine, Guardisil-9, for this expanded age group. It would seem logical that systematic administration of the HPV vaccine for at-risk HCPs would be both feasible and safe. There are well-established systems for administering vaccines for HCPs in all health care systems. Perhaps health system administrators should consider routinely offering HPV vaccination for at-risk employees as part of their occupational health care responsibilities. One important caveat being the cost and efficacy of HPV vaccination in this group has not been not established.
In the meantime, it is critical that gynecology providers be aware of their risk for occupational exposure to HPV when using laser and electrocautery techniques on HPV-infected tissues and the potential for them developing head and neck pathology. They should strictly adhere to preventative measures such as use of fit-tested N-95 respirators, mechanical smoke evacuators with high-efficiency filters and work in environments with adequate room ventilation. We all should individually evaluate what role HPV vaccination may play for us in augmenting our own safety.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Van Dyne EA et al. MMWR Morb Mortal Wkly Rep. 2018 Aug 24;67(33):918-24.
2. ASCCP. ASCCP recommends HPV vaccination for providers.
3. Fox-Lewis A et al. Occup Environ Med. 2020 Dec;77(12):809-17.
4. Zhou Q et al. Cancer Manag Res. 2019;11:3643-54
5. Bergbrant I et al. Acta Derm Venereol. 1994 Sep;74(5):393-5.
6. Garden J et al. Arch Dermatol. 2002 Oct;138(10):1303-7.
7. Harrison R, Huh W. Obstet Gynecol. 2020;136:663-5.
8. CDC. 1996. DHHS (NIOSH) Publication Number 96-128.
Gynecologists have experience managing human papillomavirus–associated diseases of the lower genital tract. However, HPV also causes warty disease, dysplasia, and carcinoma of the head and neck. Risk factors for head and neck cancer include smoking and smokeless tobacco use, alcohol consumption, periodontal disease, radiation exposure, and HPV. The incidence of HPV-associated head and neck cancer is rising, particularly among men, at a rate of 2.7% per year.1 The incidence of HPV-associated squamous cell carcinoma of the oropharynx now surpasses that of cervical cancer. Concerns exist regarding occupational exposure to HPV by health care providers (HCP) who perform smoke-generating procedures on HPV-infected tissues, and the potential for them to develop head and neck pathology.
In March of 2020, the American Society for Colposcopy and Cervical Pathology made the recommendation that clinicians who are routinely exposed to HPV should protect themselves against the sequela of occupationally acquired HPV by receiving the HPV vaccine.2 They advocate for the “complete provider team” including physicians, advanced practice providers, nurses, operative technicians, and residents and fellows to be considered for protective vaccination.
Similar to disease patterns in the genital tract, different strains of HPV have differing propensity to cause benign, premalignant, and malignant disease states. HPV 6 and 11 are more commonly associated with warty disease in the nares, pharynx, and tonsillar tissues. HPV 16, 18, 31, and 33 (most commonly 16) are considered high risk for carcinoma formation, particularly of the tonsils and base of the tongue.
The procedures most implicated in occupational HPV exposure include ablative procedures for anogenital warts, laser ablation of vaginal and vulvar dysplasia, and electrosurgical excisional procedures for cervical dysplasia. Smoke plumes from HPV-associated procedures are known to contain HPV for both laser and electrocautery sources.3 A study of 134 patients undergoing surgical procedures for laser ablation of HPV-infected tissues detected concordant strains of HPV in approximately 30% of smoke plumes and approximately 1.5% of surgeons’ nares.4 Not all procedures appear to carry the same risk. Electrocoagulation procedures appear to yield fewer postprocedural positive mucosal swabs for HPV, compared with those taken after CO2 laser.5
Animal studies have shown that papilloma virus procured from smoke plume has the capacity to generate disease. When 10 calves were inoculated with bovine papillary virus obtained from smoke plumes from laser ablation of bovine papillomavirus lesions, all calves manifested BPV fibropapilloma lesions at the sites of inoculation.6
There appears to be an increased incidence of HPV-associated head and neck disease among surgeons who perform procedures on HPV tissues, and there have been multiple case reports that have cited examples of HPV-associated benign and malignant disease among HCPs with frequent occupational exposure to HPV anogenital ablative and excisional procedures.7 While these observations are not proof of causation, they are cause for concern.
While the ASCCP guidelines advocate for HPV vaccination as a strategy for prevention of occupationally related HPV-associated disease, there are other strategies in place to minimize risk. The CDC guidelines for environmental infection control in health care facilities include the following recommendations:
- In settings where surgical lasers are used, wear appropriate personnel protective equipment (PPE), including N95 or N100 respirators to minimize exposure to laser plumes.
- Use central wall suction units with in-line filters to evacuate minimal laser plumes.
- Use a mechanical smoke evaluation system with a high efficiency filter to manage the generation of large amounts of laser plume, when ablating tissue infected with HPV.
- Use local exhaust ventilation (LEV).8
When closely adhered to, these methods appear to provide high-level protection. Data suggest that, when HCPs can access appropriate protective equipment, risks for HPV exposure are low. However, this is more feasible for larger hospital facilities, and may be more limited in outpatient settings. This has led to the consideration of background protection in the form of HPV vaccination for at-risk HCPs. This is analogous to mandates for HCPs to receive hepatitis B vaccination despite the concomitant practice of universal precautions in health care settings. Preventative strategies are typically most efficacious when performed in concert.
After nearly 2 decades of widespread use, we have confidence in the safety of the HPV vaccination. Its benefit through age 45 has been established, leading to the 2018 FDA approval for the 9-valent HPV vaccine, Guardisil-9, for this expanded age group. It would seem logical that systematic administration of the HPV vaccine for at-risk HCPs would be both feasible and safe. There are well-established systems for administering vaccines for HCPs in all health care systems. Perhaps health system administrators should consider routinely offering HPV vaccination for at-risk employees as part of their occupational health care responsibilities. One important caveat being the cost and efficacy of HPV vaccination in this group has not been not established.
In the meantime, it is critical that gynecology providers be aware of their risk for occupational exposure to HPV when using laser and electrocautery techniques on HPV-infected tissues and the potential for them developing head and neck pathology. They should strictly adhere to preventative measures such as use of fit-tested N-95 respirators, mechanical smoke evacuators with high-efficiency filters and work in environments with adequate room ventilation. We all should individually evaluate what role HPV vaccination may play for us in augmenting our own safety.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Van Dyne EA et al. MMWR Morb Mortal Wkly Rep. 2018 Aug 24;67(33):918-24.
2. ASCCP. ASCCP recommends HPV vaccination for providers.
3. Fox-Lewis A et al. Occup Environ Med. 2020 Dec;77(12):809-17.
4. Zhou Q et al. Cancer Manag Res. 2019;11:3643-54
5. Bergbrant I et al. Acta Derm Venereol. 1994 Sep;74(5):393-5.
6. Garden J et al. Arch Dermatol. 2002 Oct;138(10):1303-7.
7. Harrison R, Huh W. Obstet Gynecol. 2020;136:663-5.
8. CDC. 1996. DHHS (NIOSH) Publication Number 96-128.
Gynecologists have experience managing human papillomavirus–associated diseases of the lower genital tract. However, HPV also causes warty disease, dysplasia, and carcinoma of the head and neck. Risk factors for head and neck cancer include smoking and smokeless tobacco use, alcohol consumption, periodontal disease, radiation exposure, and HPV. The incidence of HPV-associated head and neck cancer is rising, particularly among men, at a rate of 2.7% per year.1 The incidence of HPV-associated squamous cell carcinoma of the oropharynx now surpasses that of cervical cancer. Concerns exist regarding occupational exposure to HPV by health care providers (HCP) who perform smoke-generating procedures on HPV-infected tissues, and the potential for them to develop head and neck pathology.
In March of 2020, the American Society for Colposcopy and Cervical Pathology made the recommendation that clinicians who are routinely exposed to HPV should protect themselves against the sequela of occupationally acquired HPV by receiving the HPV vaccine.2 They advocate for the “complete provider team” including physicians, advanced practice providers, nurses, operative technicians, and residents and fellows to be considered for protective vaccination.
Similar to disease patterns in the genital tract, different strains of HPV have differing propensity to cause benign, premalignant, and malignant disease states. HPV 6 and 11 are more commonly associated with warty disease in the nares, pharynx, and tonsillar tissues. HPV 16, 18, 31, and 33 (most commonly 16) are considered high risk for carcinoma formation, particularly of the tonsils and base of the tongue.
The procedures most implicated in occupational HPV exposure include ablative procedures for anogenital warts, laser ablation of vaginal and vulvar dysplasia, and electrosurgical excisional procedures for cervical dysplasia. Smoke plumes from HPV-associated procedures are known to contain HPV for both laser and electrocautery sources.3 A study of 134 patients undergoing surgical procedures for laser ablation of HPV-infected tissues detected concordant strains of HPV in approximately 30% of smoke plumes and approximately 1.5% of surgeons’ nares.4 Not all procedures appear to carry the same risk. Electrocoagulation procedures appear to yield fewer postprocedural positive mucosal swabs for HPV, compared with those taken after CO2 laser.5
Animal studies have shown that papilloma virus procured from smoke plume has the capacity to generate disease. When 10 calves were inoculated with bovine papillary virus obtained from smoke plumes from laser ablation of bovine papillomavirus lesions, all calves manifested BPV fibropapilloma lesions at the sites of inoculation.6
There appears to be an increased incidence of HPV-associated head and neck disease among surgeons who perform procedures on HPV tissues, and there have been multiple case reports that have cited examples of HPV-associated benign and malignant disease among HCPs with frequent occupational exposure to HPV anogenital ablative and excisional procedures.7 While these observations are not proof of causation, they are cause for concern.
While the ASCCP guidelines advocate for HPV vaccination as a strategy for prevention of occupationally related HPV-associated disease, there are other strategies in place to minimize risk. The CDC guidelines for environmental infection control in health care facilities include the following recommendations:
- In settings where surgical lasers are used, wear appropriate personnel protective equipment (PPE), including N95 or N100 respirators to minimize exposure to laser plumes.
- Use central wall suction units with in-line filters to evacuate minimal laser plumes.
- Use a mechanical smoke evaluation system with a high efficiency filter to manage the generation of large amounts of laser plume, when ablating tissue infected with HPV.
- Use local exhaust ventilation (LEV).8
When closely adhered to, these methods appear to provide high-level protection. Data suggest that, when HCPs can access appropriate protective equipment, risks for HPV exposure are low. However, this is more feasible for larger hospital facilities, and may be more limited in outpatient settings. This has led to the consideration of background protection in the form of HPV vaccination for at-risk HCPs. This is analogous to mandates for HCPs to receive hepatitis B vaccination despite the concomitant practice of universal precautions in health care settings. Preventative strategies are typically most efficacious when performed in concert.
After nearly 2 decades of widespread use, we have confidence in the safety of the HPV vaccination. Its benefit through age 45 has been established, leading to the 2018 FDA approval for the 9-valent HPV vaccine, Guardisil-9, for this expanded age group. It would seem logical that systematic administration of the HPV vaccine for at-risk HCPs would be both feasible and safe. There are well-established systems for administering vaccines for HCPs in all health care systems. Perhaps health system administrators should consider routinely offering HPV vaccination for at-risk employees as part of their occupational health care responsibilities. One important caveat being the cost and efficacy of HPV vaccination in this group has not been not established.
In the meantime, it is critical that gynecology providers be aware of their risk for occupational exposure to HPV when using laser and electrocautery techniques on HPV-infected tissues and the potential for them developing head and neck pathology. They should strictly adhere to preventative measures such as use of fit-tested N-95 respirators, mechanical smoke evacuators with high-efficiency filters and work in environments with adequate room ventilation. We all should individually evaluate what role HPV vaccination may play for us in augmenting our own safety.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Van Dyne EA et al. MMWR Morb Mortal Wkly Rep. 2018 Aug 24;67(33):918-24.
2. ASCCP. ASCCP recommends HPV vaccination for providers.
3. Fox-Lewis A et al. Occup Environ Med. 2020 Dec;77(12):809-17.
4. Zhou Q et al. Cancer Manag Res. 2019;11:3643-54
5. Bergbrant I et al. Acta Derm Venereol. 1994 Sep;74(5):393-5.
6. Garden J et al. Arch Dermatol. 2002 Oct;138(10):1303-7.
7. Harrison R, Huh W. Obstet Gynecol. 2020;136:663-5.
8. CDC. 1996. DHHS (NIOSH) Publication Number 96-128.
New trials in gynecologic cancers: Could your patient benefit?
A number of clinical trials in gynecologic cancers have opened in recent months. Maybe one of your patients could benefit from being enrolled.
Uterine precancer (endometrial intraepithelial neoplasia). A phase 2 study sponsored by the National Cancer Institute is seeking adults with endometrial intraepithelial neoplasia (also called complex atypical hyperplasia or atypical hyperplasia) who are scheduled for hysterectomy within 3 months. Researchers are using the window of opportunity before an already-scheduled hysterectomy to see whether adding metformin to megestrol acetate, a treatment standard for nonsurgical patients, increases the effectiveness of megestrol in slowing this type of neoplasia. Participants will receive twice-daily oral medication for 4 weeks then undergo hysterectomy. The trial aims to enroll 50 participants. It began recruiting on Sept. 21 at its Northwestern University site in Evanston, Ill.; sites in California, Colorado, and North Carolina are also planned. The primary outcome is the change in endometrial cell proliferation. Overall survival (OS) and quality of life (QoL) will not be measured. .
Maurie Markman, MD, president of medicine and science at Cancer Treatment Centers of America, who is not involved in this trial, was approached for comment. “This is an interesting study concept, and patients with endometrial atypical hyperplasia may certainly wish to consider participation,” Dr. Markman said. He noted the “limited sample size” of the study.
Advanced, recurrent or refractory ovarian, fallopian, and endometrial cancers overexpressing folate receptor (FR)–alpha. Patients with these types of cancers are eligible for a phase 1/2 study of a new-concept targeted therapy called ELU-001. The molecular structure of ELU001 – called a C’Dot drug conjugate – consists of a drug “payload” riding with an FR-alpha–targeting molecule. For the first 28 days of the study, patients will receive escalating intravenous doses of ELU001 to determine the highest tolerated dose of the drug. All participants will then receive the selected dose for up to 12 months until lesions disappear (complete response) or there is a 30% decrease in the sum of the tumors’ longest diameter (partial response). This “basket” study – a trial involving a “basket” of different cancers with one genetic target – is hosted by New Experimental Therapeutics of San Antonio, which started recruitment for 166 patients on Sept. 13. Neither OS nor QoL will be tracked. .
Dr. Markman commented: “There is considerable interest in examining antineoplastic agents directed to tumor antigens overexpressed in ovarian cancer.”
Locally recurrent, unresectable, or metastatic cervical or endometrial cancer positive for PD-L1. Adults with these types of cancers are invited to join another basket trial, this time testing MK-7684A, a new coformulation of pembrolizumab (Keytruda) and the investigational drug vibostolimab. Most participants will receive intravenous infusions of either MK-7684A, MK-7684A plus chemotherapy, or pembrolizumab alone every 3 weeks for up to 3 years. One group will be given MK-7684A every 3 weeks plus paclitaxel weekly. People with endometrial cancer will also take a daily capsule of lenvatinib (Lenvima). The primary outcomes are response rate and progression-free survival; OS and QoL are secondary outcomes. The study opened on Sept. 16 and hopes to recruit 480 participants in eight countries with several different cancers, including cervical and endometrial cancers. U.S. patients can join at the City of Hope National Medical Center, Duarte, Calif. .
Persistent or recurrent rare epithelial tumors of the ovary, fallopian tube, or peritoneum. Adults with these cancers are sought for a phase 2 trial comparing four targeted-therapy regimens. Participants will receive either ipatasertib plus paclitaxel (Taxol); cobimetinib (Cotellic); trastuzumab emtansine (Kadcyla); or atezolizumab (Tecentriq) plus bevacizumab (Avastin) for up to 5 years until disease progression or unacceptable toxicity. Ipatasertib and cobimetinib are oral medications; all the others are administered intravenously on schedules that vary from once every 3 weeks (atezolizumab, trastuzumab) to weekly infusions 3 weeks out of 4 (paclitaxel). The study opened on Oct. 7 and hopes to enroll 200 participants at sites in Arizona, California, Minnesota, Missouri, Texas, Virginia, and worldwide. OS will be tracked, QoL will not.
Dr. Markman commented: “This is an interesting early study of the potential efficacy of a novel AKT inhibitor [ipatasertib] in rare gynecologic cancers.”
Platinum-resistant or refractory high-grade serous ovarian cancer. Adult women whose high-grade serous ovarian cancer is platinum resistant or refractory and who do not have germline BRCA mutations are sought for a phase 3 study comparing alpelisib (Piqray) plus olaparib (Lynparza) to investigator’s choice of chemotherapy. Alpelisib is approved for breast cancer in combination with fulvestrant; olaparib is approved for advanced ovarian cancer in platinum-responsive patients and/or those with BRCA- or HRD-positive tumors, so this study could lead to labeling changes for these drugs. Participants will either take daily oral doses of alpelisib plus olaparib or receive intravenous chemo on the appropriate schedules for approximately 2 years. Progression-free survival is the primary outcome measure; OS and QoL are secondary outcomes. The trial opened on July 2 and hopes to recruit 358 individuals in Singapore, Australia, Europe, and the United States (Arizona, Illinois, and Texas). .
All trial information is from the National Institutes of Health U.S. National Library of Medicine (online at clinicaltrials.gov).
Dr. Markman is not involved with any of these trials. He is a regular contributor to Medscape Oncology. He has received income in an amount equal to or greater than $250 from Genentech, AstraZeneca Celgene, Clovis, Amgen.
A version of this article first appeared on Medscape.com.
A number of clinical trials in gynecologic cancers have opened in recent months. Maybe one of your patients could benefit from being enrolled.
Uterine precancer (endometrial intraepithelial neoplasia). A phase 2 study sponsored by the National Cancer Institute is seeking adults with endometrial intraepithelial neoplasia (also called complex atypical hyperplasia or atypical hyperplasia) who are scheduled for hysterectomy within 3 months. Researchers are using the window of opportunity before an already-scheduled hysterectomy to see whether adding metformin to megestrol acetate, a treatment standard for nonsurgical patients, increases the effectiveness of megestrol in slowing this type of neoplasia. Participants will receive twice-daily oral medication for 4 weeks then undergo hysterectomy. The trial aims to enroll 50 participants. It began recruiting on Sept. 21 at its Northwestern University site in Evanston, Ill.; sites in California, Colorado, and North Carolina are also planned. The primary outcome is the change in endometrial cell proliferation. Overall survival (OS) and quality of life (QoL) will not be measured. .
Maurie Markman, MD, president of medicine and science at Cancer Treatment Centers of America, who is not involved in this trial, was approached for comment. “This is an interesting study concept, and patients with endometrial atypical hyperplasia may certainly wish to consider participation,” Dr. Markman said. He noted the “limited sample size” of the study.
Advanced, recurrent or refractory ovarian, fallopian, and endometrial cancers overexpressing folate receptor (FR)–alpha. Patients with these types of cancers are eligible for a phase 1/2 study of a new-concept targeted therapy called ELU-001. The molecular structure of ELU001 – called a C’Dot drug conjugate – consists of a drug “payload” riding with an FR-alpha–targeting molecule. For the first 28 days of the study, patients will receive escalating intravenous doses of ELU001 to determine the highest tolerated dose of the drug. All participants will then receive the selected dose for up to 12 months until lesions disappear (complete response) or there is a 30% decrease in the sum of the tumors’ longest diameter (partial response). This “basket” study – a trial involving a “basket” of different cancers with one genetic target – is hosted by New Experimental Therapeutics of San Antonio, which started recruitment for 166 patients on Sept. 13. Neither OS nor QoL will be tracked. .
Dr. Markman commented: “There is considerable interest in examining antineoplastic agents directed to tumor antigens overexpressed in ovarian cancer.”
Locally recurrent, unresectable, or metastatic cervical or endometrial cancer positive for PD-L1. Adults with these types of cancers are invited to join another basket trial, this time testing MK-7684A, a new coformulation of pembrolizumab (Keytruda) and the investigational drug vibostolimab. Most participants will receive intravenous infusions of either MK-7684A, MK-7684A plus chemotherapy, or pembrolizumab alone every 3 weeks for up to 3 years. One group will be given MK-7684A every 3 weeks plus paclitaxel weekly. People with endometrial cancer will also take a daily capsule of lenvatinib (Lenvima). The primary outcomes are response rate and progression-free survival; OS and QoL are secondary outcomes. The study opened on Sept. 16 and hopes to recruit 480 participants in eight countries with several different cancers, including cervical and endometrial cancers. U.S. patients can join at the City of Hope National Medical Center, Duarte, Calif. .
Persistent or recurrent rare epithelial tumors of the ovary, fallopian tube, or peritoneum. Adults with these cancers are sought for a phase 2 trial comparing four targeted-therapy regimens. Participants will receive either ipatasertib plus paclitaxel (Taxol); cobimetinib (Cotellic); trastuzumab emtansine (Kadcyla); or atezolizumab (Tecentriq) plus bevacizumab (Avastin) for up to 5 years until disease progression or unacceptable toxicity. Ipatasertib and cobimetinib are oral medications; all the others are administered intravenously on schedules that vary from once every 3 weeks (atezolizumab, trastuzumab) to weekly infusions 3 weeks out of 4 (paclitaxel). The study opened on Oct. 7 and hopes to enroll 200 participants at sites in Arizona, California, Minnesota, Missouri, Texas, Virginia, and worldwide. OS will be tracked, QoL will not.
Dr. Markman commented: “This is an interesting early study of the potential efficacy of a novel AKT inhibitor [ipatasertib] in rare gynecologic cancers.”
Platinum-resistant or refractory high-grade serous ovarian cancer. Adult women whose high-grade serous ovarian cancer is platinum resistant or refractory and who do not have germline BRCA mutations are sought for a phase 3 study comparing alpelisib (Piqray) plus olaparib (Lynparza) to investigator’s choice of chemotherapy. Alpelisib is approved for breast cancer in combination with fulvestrant; olaparib is approved for advanced ovarian cancer in platinum-responsive patients and/or those with BRCA- or HRD-positive tumors, so this study could lead to labeling changes for these drugs. Participants will either take daily oral doses of alpelisib plus olaparib or receive intravenous chemo on the appropriate schedules for approximately 2 years. Progression-free survival is the primary outcome measure; OS and QoL are secondary outcomes. The trial opened on July 2 and hopes to recruit 358 individuals in Singapore, Australia, Europe, and the United States (Arizona, Illinois, and Texas). .
All trial information is from the National Institutes of Health U.S. National Library of Medicine (online at clinicaltrials.gov).
Dr. Markman is not involved with any of these trials. He is a regular contributor to Medscape Oncology. He has received income in an amount equal to or greater than $250 from Genentech, AstraZeneca Celgene, Clovis, Amgen.
A version of this article first appeared on Medscape.com.
A number of clinical trials in gynecologic cancers have opened in recent months. Maybe one of your patients could benefit from being enrolled.
Uterine precancer (endometrial intraepithelial neoplasia). A phase 2 study sponsored by the National Cancer Institute is seeking adults with endometrial intraepithelial neoplasia (also called complex atypical hyperplasia or atypical hyperplasia) who are scheduled for hysterectomy within 3 months. Researchers are using the window of opportunity before an already-scheduled hysterectomy to see whether adding metformin to megestrol acetate, a treatment standard for nonsurgical patients, increases the effectiveness of megestrol in slowing this type of neoplasia. Participants will receive twice-daily oral medication for 4 weeks then undergo hysterectomy. The trial aims to enroll 50 participants. It began recruiting on Sept. 21 at its Northwestern University site in Evanston, Ill.; sites in California, Colorado, and North Carolina are also planned. The primary outcome is the change in endometrial cell proliferation. Overall survival (OS) and quality of life (QoL) will not be measured. .
Maurie Markman, MD, president of medicine and science at Cancer Treatment Centers of America, who is not involved in this trial, was approached for comment. “This is an interesting study concept, and patients with endometrial atypical hyperplasia may certainly wish to consider participation,” Dr. Markman said. He noted the “limited sample size” of the study.
Advanced, recurrent or refractory ovarian, fallopian, and endometrial cancers overexpressing folate receptor (FR)–alpha. Patients with these types of cancers are eligible for a phase 1/2 study of a new-concept targeted therapy called ELU-001. The molecular structure of ELU001 – called a C’Dot drug conjugate – consists of a drug “payload” riding with an FR-alpha–targeting molecule. For the first 28 days of the study, patients will receive escalating intravenous doses of ELU001 to determine the highest tolerated dose of the drug. All participants will then receive the selected dose for up to 12 months until lesions disappear (complete response) or there is a 30% decrease in the sum of the tumors’ longest diameter (partial response). This “basket” study – a trial involving a “basket” of different cancers with one genetic target – is hosted by New Experimental Therapeutics of San Antonio, which started recruitment for 166 patients on Sept. 13. Neither OS nor QoL will be tracked. .
Dr. Markman commented: “There is considerable interest in examining antineoplastic agents directed to tumor antigens overexpressed in ovarian cancer.”
Locally recurrent, unresectable, or metastatic cervical or endometrial cancer positive for PD-L1. Adults with these types of cancers are invited to join another basket trial, this time testing MK-7684A, a new coformulation of pembrolizumab (Keytruda) and the investigational drug vibostolimab. Most participants will receive intravenous infusions of either MK-7684A, MK-7684A plus chemotherapy, or pembrolizumab alone every 3 weeks for up to 3 years. One group will be given MK-7684A every 3 weeks plus paclitaxel weekly. People with endometrial cancer will also take a daily capsule of lenvatinib (Lenvima). The primary outcomes are response rate and progression-free survival; OS and QoL are secondary outcomes. The study opened on Sept. 16 and hopes to recruit 480 participants in eight countries with several different cancers, including cervical and endometrial cancers. U.S. patients can join at the City of Hope National Medical Center, Duarte, Calif. .
Persistent or recurrent rare epithelial tumors of the ovary, fallopian tube, or peritoneum. Adults with these cancers are sought for a phase 2 trial comparing four targeted-therapy regimens. Participants will receive either ipatasertib plus paclitaxel (Taxol); cobimetinib (Cotellic); trastuzumab emtansine (Kadcyla); or atezolizumab (Tecentriq) plus bevacizumab (Avastin) for up to 5 years until disease progression or unacceptable toxicity. Ipatasertib and cobimetinib are oral medications; all the others are administered intravenously on schedules that vary from once every 3 weeks (atezolizumab, trastuzumab) to weekly infusions 3 weeks out of 4 (paclitaxel). The study opened on Oct. 7 and hopes to enroll 200 participants at sites in Arizona, California, Minnesota, Missouri, Texas, Virginia, and worldwide. OS will be tracked, QoL will not.
Dr. Markman commented: “This is an interesting early study of the potential efficacy of a novel AKT inhibitor [ipatasertib] in rare gynecologic cancers.”
Platinum-resistant or refractory high-grade serous ovarian cancer. Adult women whose high-grade serous ovarian cancer is platinum resistant or refractory and who do not have germline BRCA mutations are sought for a phase 3 study comparing alpelisib (Piqray) plus olaparib (Lynparza) to investigator’s choice of chemotherapy. Alpelisib is approved for breast cancer in combination with fulvestrant; olaparib is approved for advanced ovarian cancer in platinum-responsive patients and/or those with BRCA- or HRD-positive tumors, so this study could lead to labeling changes for these drugs. Participants will either take daily oral doses of alpelisib plus olaparib or receive intravenous chemo on the appropriate schedules for approximately 2 years. Progression-free survival is the primary outcome measure; OS and QoL are secondary outcomes. The trial opened on July 2 and hopes to recruit 358 individuals in Singapore, Australia, Europe, and the United States (Arizona, Illinois, and Texas). .
All trial information is from the National Institutes of Health U.S. National Library of Medicine (online at clinicaltrials.gov).
Dr. Markman is not involved with any of these trials. He is a regular contributor to Medscape Oncology. He has received income in an amount equal to or greater than $250 from Genentech, AstraZeneca Celgene, Clovis, Amgen.
A version of this article first appeared on Medscape.com.