User login
Best Practice Implementation and Clinical Inertia
From the Department of Medicine, Brigham and Women’s Hospital, and Harvard Medical School, Boston, MA.
Clinical inertia is defined as the failure of clinicians to initiate or escalate guideline-directed medical therapy to achieve treatment goals for well-defined clinical conditions.1,2 Evidence-based guidelines recommend optimal disease management with readily available medical therapies throughout the phases of clinical care. Unfortunately, the care provided to individual patients undergoes multiple modifications throughout the disease course, resulting in divergent pathways, significant deviations from treatment guidelines, and failure of “safeguard” checkpoints to reinstate, initiate, optimize, or stop treatments. Clinical inertia generally describes rigidity or resistance to change around implementing evidence-based guidelines. Furthermore, this term describes treatment behavior on the part of an individual clinician, not organizational inertia, which generally encompasses both internal (immediate clinical practice settings) and external factors (national and international guidelines and recommendations), eventually leading to resistance to optimizing disease treatment and therapeutic regimens. Individual clinicians’ clinical inertia in the form of resistance to guideline implementation and evidence-based principles can be one factor that drives organizational inertia. In turn, such individual behavior can be dictated by personal beliefs, knowledge, interpretation, skills, management principles, and biases. The terms therapeutic inertia or clinical inertia should not be confused with nonadherence from the patient’s standpoint when the clinician follows the best practice guidelines.3
Clinical inertia has been described in several clinical domains, including diabetes,4,5 hypertension,6,7 heart failure,8 depression,9 pulmonary medicine,10 and complex disease management.11 Clinicians can set suboptimal treatment goals due to specific beliefs and attitudes around optimal therapeutic goals. For example, when treating a patient with a chronic disease that is presently stable, a clinician could elect to initiate suboptimal treatment, as escalation of treatment might not be the priority in stable disease; they also may have concerns about overtreatment. Other factors that can contribute to clinical inertia (ie, undertreatment in the presence of indications for treatment) include those related to the patient, the clinical setting, and the organization, along with the importance of individualizing therapies in specific patients. Organizational inertia is the initial global resistance by the system to implementation, which can slow the dissemination and adaptation of best practices but eventually declines over time. Individual clinical inertia, on the other hand, will likely persist after the system-level rollout of guideline-based approaches.
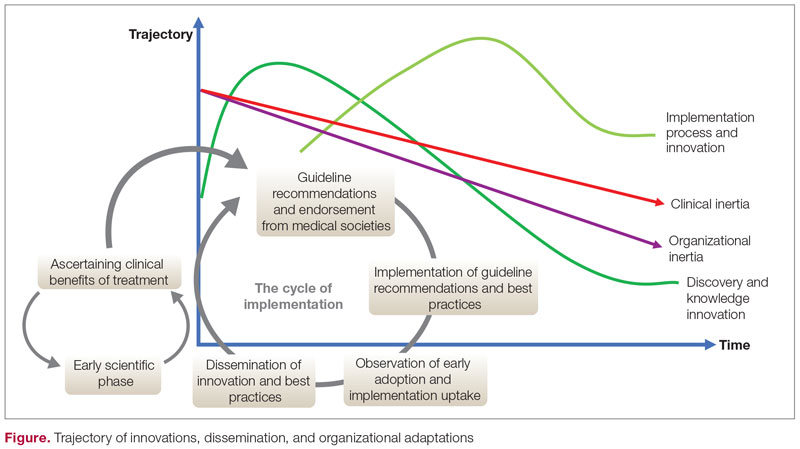
The trajectory of dissemination, implementation, and adaptation of innovations and best practices is illustrated in the Figure. When the guidelines and medical societies endorse the adaptation of innovations or practice change after the benefits of such innovations/change have been established by the regulatory bodies, uptake can be hindered by both organizational and clinical inertia. Overcoming inertia to system-level changes requires addressing individual clinicians, along with practice and organizational factors, in order to ensure systematic adaptations. From the clinicians’ view, training and cognitive interventions to improve the adaptation and coping skills can improve understanding of treatment options through standardized educational and behavioral modification tools, direct and indirect feedback around performance, and decision support through a continuous improvement approach on both individual and system levels.
Addressing inertia in clinical practice requires a deep understanding of the individual and organizational elements that foster resistance to adapting best practice models. Research that explores tools and approaches to overcome inertia in managing complex diseases is a key step in advancing clinical innovation and disseminating best practices.
Corresponding author: Ebrahim Barkoudah, MD, MPH; [email protected]
Disclosures: None reported.
1. Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135(9):825-834. doi:10.7326/0003-4819-135-9-200111060-00012
2. Allen JD, Curtiss FR, Fairman KA. Nonadherence, clinical inertia, or therapeutic inertia? J Manag Care Pharm. 2009;15(8):690-695. doi:10.18553/jmcp.2009.15.8.690
3. Zafar A, Davies M, Azhar A, Khunti K. Clinical inertia in management of T2DM. Prim Care Diabetes. 2010;4(4):203-207. doi:10.1016/j.pcd.2010.07.003
4. Khunti K, Davies MJ. Clinical inertia—time to reappraise the terminology? Prim Care Diabetes. 2017;11(2):105-106. doi:10.1016/j.pcd.2017.01.007
5. O’Connor PJ. Overcome clinical inertia to control systolic blood pressure. Arch Intern Med. 2003;163(22):2677-2678. doi:10.1001/archinte.163.22.2677
6. Faria C, Wenzel M, Lee KW, et al. A narrative review of clinical inertia: focus on hypertension. J Am Soc Hypertens. 2009;3(4):267-276. doi:10.1016/j.jash.2009.03.001
7. Jarjour M, Henri C, de Denus S, et al. Care gaps in adherence to heart failure guidelines: clinical inertia or physiological limitations? JACC Heart Fail. 2020;8(9):725-738. doi:10.1016/j.jchf.2020.04.019
8. Henke RM, Zaslavsky AM, McGuire TG, et al. Clinical inertia in depression treatment. Med Care. 2009;47(9):959-67. doi:10.1097/MLR.0b013e31819a5da0
9. Cooke CE, Sidel M, Belletti DA, Fuhlbrigge AL. Clinical inertia in the management of chronic obstructive pulmonary disease. COPD. 2012;9(1):73-80. doi:10.3109/15412555.2011.631957
10. Whitford DL, Al-Anjawi HA, Al-Baharna MM. Impact of clinical inertia on cardiovascular risk factors in patients with diabetes. Prim Care Diabetes. 2014;8(2):133-138. doi:10.1016/j.pcd.2013.10.007
From the Department of Medicine, Brigham and Women’s Hospital, and Harvard Medical School, Boston, MA.
Clinical inertia is defined as the failure of clinicians to initiate or escalate guideline-directed medical therapy to achieve treatment goals for well-defined clinical conditions.1,2 Evidence-based guidelines recommend optimal disease management with readily available medical therapies throughout the phases of clinical care. Unfortunately, the care provided to individual patients undergoes multiple modifications throughout the disease course, resulting in divergent pathways, significant deviations from treatment guidelines, and failure of “safeguard” checkpoints to reinstate, initiate, optimize, or stop treatments. Clinical inertia generally describes rigidity or resistance to change around implementing evidence-based guidelines. Furthermore, this term describes treatment behavior on the part of an individual clinician, not organizational inertia, which generally encompasses both internal (immediate clinical practice settings) and external factors (national and international guidelines and recommendations), eventually leading to resistance to optimizing disease treatment and therapeutic regimens. Individual clinicians’ clinical inertia in the form of resistance to guideline implementation and evidence-based principles can be one factor that drives organizational inertia. In turn, such individual behavior can be dictated by personal beliefs, knowledge, interpretation, skills, management principles, and biases. The terms therapeutic inertia or clinical inertia should not be confused with nonadherence from the patient’s standpoint when the clinician follows the best practice guidelines.3
Clinical inertia has been described in several clinical domains, including diabetes,4,5 hypertension,6,7 heart failure,8 depression,9 pulmonary medicine,10 and complex disease management.11 Clinicians can set suboptimal treatment goals due to specific beliefs and attitudes around optimal therapeutic goals. For example, when treating a patient with a chronic disease that is presently stable, a clinician could elect to initiate suboptimal treatment, as escalation of treatment might not be the priority in stable disease; they also may have concerns about overtreatment. Other factors that can contribute to clinical inertia (ie, undertreatment in the presence of indications for treatment) include those related to the patient, the clinical setting, and the organization, along with the importance of individualizing therapies in specific patients. Organizational inertia is the initial global resistance by the system to implementation, which can slow the dissemination and adaptation of best practices but eventually declines over time. Individual clinical inertia, on the other hand, will likely persist after the system-level rollout of guideline-based approaches.
The trajectory of dissemination, implementation, and adaptation of innovations and best practices is illustrated in the Figure. When the guidelines and medical societies endorse the adaptation of innovations or practice change after the benefits of such innovations/change have been established by the regulatory bodies, uptake can be hindered by both organizational and clinical inertia. Overcoming inertia to system-level changes requires addressing individual clinicians, along with practice and organizational factors, in order to ensure systematic adaptations. From the clinicians’ view, training and cognitive interventions to improve the adaptation and coping skills can improve understanding of treatment options through standardized educational and behavioral modification tools, direct and indirect feedback around performance, and decision support through a continuous improvement approach on both individual and system levels.

Addressing inertia in clinical practice requires a deep understanding of the individual and organizational elements that foster resistance to adapting best practice models. Research that explores tools and approaches to overcome inertia in managing complex diseases is a key step in advancing clinical innovation and disseminating best practices.
Corresponding author: Ebrahim Barkoudah, MD, MPH; [email protected]
Disclosures: None reported.
From the Department of Medicine, Brigham and Women’s Hospital, and Harvard Medical School, Boston, MA.
Clinical inertia is defined as the failure of clinicians to initiate or escalate guideline-directed medical therapy to achieve treatment goals for well-defined clinical conditions.1,2 Evidence-based guidelines recommend optimal disease management with readily available medical therapies throughout the phases of clinical care. Unfortunately, the care provided to individual patients undergoes multiple modifications throughout the disease course, resulting in divergent pathways, significant deviations from treatment guidelines, and failure of “safeguard” checkpoints to reinstate, initiate, optimize, or stop treatments. Clinical inertia generally describes rigidity or resistance to change around implementing evidence-based guidelines. Furthermore, this term describes treatment behavior on the part of an individual clinician, not organizational inertia, which generally encompasses both internal (immediate clinical practice settings) and external factors (national and international guidelines and recommendations), eventually leading to resistance to optimizing disease treatment and therapeutic regimens. Individual clinicians’ clinical inertia in the form of resistance to guideline implementation and evidence-based principles can be one factor that drives organizational inertia. In turn, such individual behavior can be dictated by personal beliefs, knowledge, interpretation, skills, management principles, and biases. The terms therapeutic inertia or clinical inertia should not be confused with nonadherence from the patient’s standpoint when the clinician follows the best practice guidelines.3
Clinical inertia has been described in several clinical domains, including diabetes,4,5 hypertension,6,7 heart failure,8 depression,9 pulmonary medicine,10 and complex disease management.11 Clinicians can set suboptimal treatment goals due to specific beliefs and attitudes around optimal therapeutic goals. For example, when treating a patient with a chronic disease that is presently stable, a clinician could elect to initiate suboptimal treatment, as escalation of treatment might not be the priority in stable disease; they also may have concerns about overtreatment. Other factors that can contribute to clinical inertia (ie, undertreatment in the presence of indications for treatment) include those related to the patient, the clinical setting, and the organization, along with the importance of individualizing therapies in specific patients. Organizational inertia is the initial global resistance by the system to implementation, which can slow the dissemination and adaptation of best practices but eventually declines over time. Individual clinical inertia, on the other hand, will likely persist after the system-level rollout of guideline-based approaches.
The trajectory of dissemination, implementation, and adaptation of innovations and best practices is illustrated in the Figure. When the guidelines and medical societies endorse the adaptation of innovations or practice change after the benefits of such innovations/change have been established by the regulatory bodies, uptake can be hindered by both organizational and clinical inertia. Overcoming inertia to system-level changes requires addressing individual clinicians, along with practice and organizational factors, in order to ensure systematic adaptations. From the clinicians’ view, training and cognitive interventions to improve the adaptation and coping skills can improve understanding of treatment options through standardized educational and behavioral modification tools, direct and indirect feedback around performance, and decision support through a continuous improvement approach on both individual and system levels.

Addressing inertia in clinical practice requires a deep understanding of the individual and organizational elements that foster resistance to adapting best practice models. Research that explores tools and approaches to overcome inertia in managing complex diseases is a key step in advancing clinical innovation and disseminating best practices.
Corresponding author: Ebrahim Barkoudah, MD, MPH; [email protected]
Disclosures: None reported.
1. Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135(9):825-834. doi:10.7326/0003-4819-135-9-200111060-00012
2. Allen JD, Curtiss FR, Fairman KA. Nonadherence, clinical inertia, or therapeutic inertia? J Manag Care Pharm. 2009;15(8):690-695. doi:10.18553/jmcp.2009.15.8.690
3. Zafar A, Davies M, Azhar A, Khunti K. Clinical inertia in management of T2DM. Prim Care Diabetes. 2010;4(4):203-207. doi:10.1016/j.pcd.2010.07.003
4. Khunti K, Davies MJ. Clinical inertia—time to reappraise the terminology? Prim Care Diabetes. 2017;11(2):105-106. doi:10.1016/j.pcd.2017.01.007
5. O’Connor PJ. Overcome clinical inertia to control systolic blood pressure. Arch Intern Med. 2003;163(22):2677-2678. doi:10.1001/archinte.163.22.2677
6. Faria C, Wenzel M, Lee KW, et al. A narrative review of clinical inertia: focus on hypertension. J Am Soc Hypertens. 2009;3(4):267-276. doi:10.1016/j.jash.2009.03.001
7. Jarjour M, Henri C, de Denus S, et al. Care gaps in adherence to heart failure guidelines: clinical inertia or physiological limitations? JACC Heart Fail. 2020;8(9):725-738. doi:10.1016/j.jchf.2020.04.019
8. Henke RM, Zaslavsky AM, McGuire TG, et al. Clinical inertia in depression treatment. Med Care. 2009;47(9):959-67. doi:10.1097/MLR.0b013e31819a5da0
9. Cooke CE, Sidel M, Belletti DA, Fuhlbrigge AL. Clinical inertia in the management of chronic obstructive pulmonary disease. COPD. 2012;9(1):73-80. doi:10.3109/15412555.2011.631957
10. Whitford DL, Al-Anjawi HA, Al-Baharna MM. Impact of clinical inertia on cardiovascular risk factors in patients with diabetes. Prim Care Diabetes. 2014;8(2):133-138. doi:10.1016/j.pcd.2013.10.007
1. Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135(9):825-834. doi:10.7326/0003-4819-135-9-200111060-00012
2. Allen JD, Curtiss FR, Fairman KA. Nonadherence, clinical inertia, or therapeutic inertia? J Manag Care Pharm. 2009;15(8):690-695. doi:10.18553/jmcp.2009.15.8.690
3. Zafar A, Davies M, Azhar A, Khunti K. Clinical inertia in management of T2DM. Prim Care Diabetes. 2010;4(4):203-207. doi:10.1016/j.pcd.2010.07.003
4. Khunti K, Davies MJ. Clinical inertia—time to reappraise the terminology? Prim Care Diabetes. 2017;11(2):105-106. doi:10.1016/j.pcd.2017.01.007
5. O’Connor PJ. Overcome clinical inertia to control systolic blood pressure. Arch Intern Med. 2003;163(22):2677-2678. doi:10.1001/archinte.163.22.2677
6. Faria C, Wenzel M, Lee KW, et al. A narrative review of clinical inertia: focus on hypertension. J Am Soc Hypertens. 2009;3(4):267-276. doi:10.1016/j.jash.2009.03.001
7. Jarjour M, Henri C, de Denus S, et al. Care gaps in adherence to heart failure guidelines: clinical inertia or physiological limitations? JACC Heart Fail. 2020;8(9):725-738. doi:10.1016/j.jchf.2020.04.019
8. Henke RM, Zaslavsky AM, McGuire TG, et al. Clinical inertia in depression treatment. Med Care. 2009;47(9):959-67. doi:10.1097/MLR.0b013e31819a5da0
9. Cooke CE, Sidel M, Belletti DA, Fuhlbrigge AL. Clinical inertia in the management of chronic obstructive pulmonary disease. COPD. 2012;9(1):73-80. doi:10.3109/15412555.2011.631957
10. Whitford DL, Al-Anjawi HA, Al-Baharna MM. Impact of clinical inertia on cardiovascular risk factors in patients with diabetes. Prim Care Diabetes. 2014;8(2):133-138. doi:10.1016/j.pcd.2013.10.007
Effectiveness of Colonoscopy for Colorectal Cancer Screening in Reducing Cancer-Related Mortality: Interpreting the Results From Two Ongoing Randomized Trials
Study 1 Overview (Bretthauer et al)
Objective: To evaluate the impact of screening colonoscopy on colon cancer–related death.
Design: Randomized trial conducted in 4 European countries.
Setting and participants: Presumptively healthy men and women between the ages of 55 and 64 years were selected from population registries in Poland, Norway, Sweden, and the Netherlands between 2009 and 2014. Eligible participants had not previously undergone screening. Patients with a diagnosis of colon cancer before trial entry were excluded.
Intervention: Participants were randomly assigned in a 1:2 ratio to undergo colonoscopy screening by invitation or to no invitation and no screening. Participants were randomized using a computer-generated allocation algorithm. Patients were stratified by age, sex, and municipality.
Main outcome measures: The primary endpoint of the study was risk of colorectal cancer and related death after a median follow-up of 10 to 15 years. The main secondary endpoint was death from any cause.
Main results: The study reported follow-up data from 84,585 participants (89.1% of all participants originally included in the trial). The remaining participants were either excluded or data could not be included due to lack of follow-up data from the usual-care group. Men (50.1%) and women (49.9%) were equally represented. The median age at entry was 59 years. The median follow-up was 10 years. Characteristics were otherwise balanced. Good bowel preparation was reported in 91% of all participants. Cecal intubation was achieved in 96.8% of all participants. The percentage of patients who underwent screening was 42% for the group, but screening rates varied by country (33%-60%). Colorectal cancer was diagnosed at screening in 62 participants (0.5% of screening group). Adenomas were detected in 30.7% of participants; 15 patients had polypectomy-related major bleeding. There were no perforations.
The risk of colorectal cancer at 10 years was 0.98% in the invited-to-screen group and 1.2% in the usual-care group (risk ratio, 0.82; 95% CI, 0.7-0.93). The reported number needed to invite to prevent 1 case of colon cancer in a 10-year period was 455. The risk of colorectal cancer–related death at 10 years was 0.28% in the invited-to-screen group and 0.31% in the usual-care group (risk ratio, 0.9; 95% CI, 0.64-1.16). An adjusted per-protocol analysis was performed to account for the estimated effect of screening if all participants assigned to the screening group underwent screening. In this analysis, the risk of colorectal cancer at 10 years was decreased from 1.22% to 0.84% (risk ratio, 0.69; 95% CI, 0.66-0.83).
Conclusion: Based on the results of this European randomized trial, the risk of colorectal cancer at 10 years was lower among those who were invited to undergo screening.
Study 2 Overview (Forsberg et al)
Objective: To investigate the effect of colorectal cancer screening with once-only colonoscopy or fecal immunochemical testing (FIT) on colorectal cancer mortality and incidence.
Design: Randomized controlled trial in Sweden utilizing a population registry.
Setting and participants: Patients aged 60 years at the time of entry were identified from a population-based registry from the Swedish Tax Agency.
Intervention: Individuals were assigned by an independent statistician to once-only colonoscopy, 2 rounds of FIT 2 years apart, or a control group in which no intervention was performed. Patients were assigned in a 1:6 ratio for colonoscopy vs control and a 1:2 ratio for FIT vs control.
Main outcome measures: The primary endpoint of the trial was colorectal cancer incidence and mortality.
Main results: A total of 278,280 participants were included in the study from March 1, 2014, through December 31, 2020 (31,140 in the colonoscopy group, 60,300 in the FIT group, and 186,840 in the control group). Of those in the colonoscopy group, 35% underwent colonoscopy, and 55% of those in the FIT group participated in testing. Colorectal cancer was detected in 0.16% (49) of people in the colonoscopy group and 0.2% (121) of people in the FIT test group (relative risk, 0.78; 95% CI, 0.56-1.09). The advanced adenoma detection rate was 2.05% in the colonoscopy group and 1.61% in the FIT group (relative risk, 1.27; 95% CI, 1.15-1.41). There were 2 perforations noted in the colonoscopy group and 15 major bleeding events. More right-sided adenomas were detected in the colonoscopy group.
Conclusion: The results of the current study highlight similar detection rates in the colonoscopy and FIT group. Should further follow-up show a benefit in disease-specific mortality, such screening strategies could be translated into population-based screening programs.
Commentary
The first colonoscopy screening recommendations were established in the mid 1990s in the United States, and over the subsequent 2 decades colonoscopy has been the recommended method and main modality for colorectal cancer screening in this country. The advantage of colonoscopy over other screening modalities (sigmoidoscopy and fecal-based testing) is that it can examine the entire large bowel and allow for removal of potential precancerous lesions. However, data to support colonoscopy as a screening modality for colorectal cancer are largely based on cohort studies.1,2 These studies have reported a significant reduction in the incidence of colon cancer. Additionally, colorectal cancer mortality was notably lower in the screened populations. For example, one study among health professionals found a nearly 70% reduction in colorectal cancer mortality in those who underwent at least 1 screening colonoscopy.3
There has been a lack of randomized clinical data to validate the efficacy of colonoscopy screening for reducing colorectal cancer–related deaths. The current study by Bretthauer et al addresses an important need and enhances our understanding of the efficacy of colorectal cancer screening with colonoscopy. In this randomized trial involving more than 84,000 participants from Poland, Norway, Sweden, and the Netherlands, there was a noted 18% decrease in the risk of colorectal cancer over a 10-year period in the intention-to-screen population. The reduction in the risk of death from colorectal cancer was not statistically significant (risk ratio, 0.90; 95% CI, 0.64-1.16). These results are surprising and certainly raise the question as to whether previous studies overestimated the effectiveness of colonoscopy in reducing the risk of colorectal cancer–related deaths. There are several limitations to the Bretthauer et al study, however.
Perhaps the most important limitation is the fact that only 42% of participants in the invited-to-screen cohort underwent screening colonoscopy. Therefore, this raises the question of whether the efficacy noted is simply due to a lack of participation in the screening protocol. In the adjusted per-protocol analysis, colonoscopy was estimated to reduce the risk of colorectal cancer by 31% and the risk of colorectal cancer–related death by around 50%. These findings are more in line with prior published studies regarding the efficacy of colorectal cancer screening. The authors plan to repeat this analysis at 15 years, and it is possible that the risk of colorectal cancer and colorectal cancer–related death can be reduced on subsequent follow-up.
While the results of the Bretthauer et al trial are important, randomized trials that directly compare the effectiveness of different colorectal cancer screening strategies are lacking. The Forsberg et al trial, also an ongoing study, seeks to address this vitally important gap in our current data. The SCREESCO trial is a study that compares the efficacy of colonoscopy with FIT every 2 years or no screening. The currently reported data are preliminary but show a similarly low rate of colonoscopy screening in those invited to do so (35%). This is a similar limitation to that noted in the Bretthauer et al study. Furthermore, there is some question regarding colonoscopy quality in this study, which had a very low reported adenoma detection rate.
While the current studies are important and provide quality randomized data on the effect of colorectal cancer screening, there remain many unanswered questions. Should the results presented by Bretthauer et al represent the current real-world scenario, then colonoscopy screening may not be viewed as an effective screening tool compared to simpler, less-invasive modalities (ie, FIT). Further follow-up from the SCREESCO trial will help shed light on this question. However, there are concerns with this study, including a very low participation rate, which could greatly underestimate the effectiveness of screening. Additional analysis and longer follow-up will be vital to fully understand the benefits of screening colonoscopy. In the meantime, screening remains an important tool for early detection of colorectal cancer and remains a category A recommendation by the United States Preventive Services Task Force.4
Applications for Clinical Practice and System Implementation
Current guidelines continue to strongly recommend screening for colorectal cancer for persons between 45 and 75 years of age (category B recommendation for those aged 45 to 49 years per the United States Preventive Services Task Force). Stool-based tests and direct visualization tests are both endorsed as screening options. Further follow-up from the presented studies is needed to help shed light on the magnitude of benefit of these modalities.
Practice Points
- Current guidelines continue to strongly recommend screening for colon cancer in those aged 45 to 75 years.
- The optimal modality for screening and the impact of screening on cancer-related mortality requires longer- term follow-up from these ongoing studies.
–Daniel Isaac, DO, MS
1. Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. Screening for Colorectal Cancer: An Evidence Update for the U.S. Preventive Services Task Force [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2021 May. Report No.: 20-05271-EF-1.
2. Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325(19):1978-1998. doi:10.1001/jama.2021.4417
3. Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369(12):1095-1105. doi:10.1056/NEJMoa1301969
4. U.S. Preventive Services Task Force. Colorectal cancer: screening. Published May 18, 2021. Accessed November 8, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening
Study 1 Overview (Bretthauer et al)
Objective: To evaluate the impact of screening colonoscopy on colon cancer–related death.
Design: Randomized trial conducted in 4 European countries.
Setting and participants: Presumptively healthy men and women between the ages of 55 and 64 years were selected from population registries in Poland, Norway, Sweden, and the Netherlands between 2009 and 2014. Eligible participants had not previously undergone screening. Patients with a diagnosis of colon cancer before trial entry were excluded.
Intervention: Participants were randomly assigned in a 1:2 ratio to undergo colonoscopy screening by invitation or to no invitation and no screening. Participants were randomized using a computer-generated allocation algorithm. Patients were stratified by age, sex, and municipality.
Main outcome measures: The primary endpoint of the study was risk of colorectal cancer and related death after a median follow-up of 10 to 15 years. The main secondary endpoint was death from any cause.
Main results: The study reported follow-up data from 84,585 participants (89.1% of all participants originally included in the trial). The remaining participants were either excluded or data could not be included due to lack of follow-up data from the usual-care group. Men (50.1%) and women (49.9%) were equally represented. The median age at entry was 59 years. The median follow-up was 10 years. Characteristics were otherwise balanced. Good bowel preparation was reported in 91% of all participants. Cecal intubation was achieved in 96.8% of all participants. The percentage of patients who underwent screening was 42% for the group, but screening rates varied by country (33%-60%). Colorectal cancer was diagnosed at screening in 62 participants (0.5% of screening group). Adenomas were detected in 30.7% of participants; 15 patients had polypectomy-related major bleeding. There were no perforations.
The risk of colorectal cancer at 10 years was 0.98% in the invited-to-screen group and 1.2% in the usual-care group (risk ratio, 0.82; 95% CI, 0.7-0.93). The reported number needed to invite to prevent 1 case of colon cancer in a 10-year period was 455. The risk of colorectal cancer–related death at 10 years was 0.28% in the invited-to-screen group and 0.31% in the usual-care group (risk ratio, 0.9; 95% CI, 0.64-1.16). An adjusted per-protocol analysis was performed to account for the estimated effect of screening if all participants assigned to the screening group underwent screening. In this analysis, the risk of colorectal cancer at 10 years was decreased from 1.22% to 0.84% (risk ratio, 0.69; 95% CI, 0.66-0.83).
Conclusion: Based on the results of this European randomized trial, the risk of colorectal cancer at 10 years was lower among those who were invited to undergo screening.
Study 2 Overview (Forsberg et al)
Objective: To investigate the effect of colorectal cancer screening with once-only colonoscopy or fecal immunochemical testing (FIT) on colorectal cancer mortality and incidence.
Design: Randomized controlled trial in Sweden utilizing a population registry.
Setting and participants: Patients aged 60 years at the time of entry were identified from a population-based registry from the Swedish Tax Agency.
Intervention: Individuals were assigned by an independent statistician to once-only colonoscopy, 2 rounds of FIT 2 years apart, or a control group in which no intervention was performed. Patients were assigned in a 1:6 ratio for colonoscopy vs control and a 1:2 ratio for FIT vs control.
Main outcome measures: The primary endpoint of the trial was colorectal cancer incidence and mortality.
Main results: A total of 278,280 participants were included in the study from March 1, 2014, through December 31, 2020 (31,140 in the colonoscopy group, 60,300 in the FIT group, and 186,840 in the control group). Of those in the colonoscopy group, 35% underwent colonoscopy, and 55% of those in the FIT group participated in testing. Colorectal cancer was detected in 0.16% (49) of people in the colonoscopy group and 0.2% (121) of people in the FIT test group (relative risk, 0.78; 95% CI, 0.56-1.09). The advanced adenoma detection rate was 2.05% in the colonoscopy group and 1.61% in the FIT group (relative risk, 1.27; 95% CI, 1.15-1.41). There were 2 perforations noted in the colonoscopy group and 15 major bleeding events. More right-sided adenomas were detected in the colonoscopy group.
Conclusion: The results of the current study highlight similar detection rates in the colonoscopy and FIT group. Should further follow-up show a benefit in disease-specific mortality, such screening strategies could be translated into population-based screening programs.
Commentary
The first colonoscopy screening recommendations were established in the mid 1990s in the United States, and over the subsequent 2 decades colonoscopy has been the recommended method and main modality for colorectal cancer screening in this country. The advantage of colonoscopy over other screening modalities (sigmoidoscopy and fecal-based testing) is that it can examine the entire large bowel and allow for removal of potential precancerous lesions. However, data to support colonoscopy as a screening modality for colorectal cancer are largely based on cohort studies.1,2 These studies have reported a significant reduction in the incidence of colon cancer. Additionally, colorectal cancer mortality was notably lower in the screened populations. For example, one study among health professionals found a nearly 70% reduction in colorectal cancer mortality in those who underwent at least 1 screening colonoscopy.3
There has been a lack of randomized clinical data to validate the efficacy of colonoscopy screening for reducing colorectal cancer–related deaths. The current study by Bretthauer et al addresses an important need and enhances our understanding of the efficacy of colorectal cancer screening with colonoscopy. In this randomized trial involving more than 84,000 participants from Poland, Norway, Sweden, and the Netherlands, there was a noted 18% decrease in the risk of colorectal cancer over a 10-year period in the intention-to-screen population. The reduction in the risk of death from colorectal cancer was not statistically significant (risk ratio, 0.90; 95% CI, 0.64-1.16). These results are surprising and certainly raise the question as to whether previous studies overestimated the effectiveness of colonoscopy in reducing the risk of colorectal cancer–related deaths. There are several limitations to the Bretthauer et al study, however.
Perhaps the most important limitation is the fact that only 42% of participants in the invited-to-screen cohort underwent screening colonoscopy. Therefore, this raises the question of whether the efficacy noted is simply due to a lack of participation in the screening protocol. In the adjusted per-protocol analysis, colonoscopy was estimated to reduce the risk of colorectal cancer by 31% and the risk of colorectal cancer–related death by around 50%. These findings are more in line with prior published studies regarding the efficacy of colorectal cancer screening. The authors plan to repeat this analysis at 15 years, and it is possible that the risk of colorectal cancer and colorectal cancer–related death can be reduced on subsequent follow-up.
While the results of the Bretthauer et al trial are important, randomized trials that directly compare the effectiveness of different colorectal cancer screening strategies are lacking. The Forsberg et al trial, also an ongoing study, seeks to address this vitally important gap in our current data. The SCREESCO trial is a study that compares the efficacy of colonoscopy with FIT every 2 years or no screening. The currently reported data are preliminary but show a similarly low rate of colonoscopy screening in those invited to do so (35%). This is a similar limitation to that noted in the Bretthauer et al study. Furthermore, there is some question regarding colonoscopy quality in this study, which had a very low reported adenoma detection rate.
While the current studies are important and provide quality randomized data on the effect of colorectal cancer screening, there remain many unanswered questions. Should the results presented by Bretthauer et al represent the current real-world scenario, then colonoscopy screening may not be viewed as an effective screening tool compared to simpler, less-invasive modalities (ie, FIT). Further follow-up from the SCREESCO trial will help shed light on this question. However, there are concerns with this study, including a very low participation rate, which could greatly underestimate the effectiveness of screening. Additional analysis and longer follow-up will be vital to fully understand the benefits of screening colonoscopy. In the meantime, screening remains an important tool for early detection of colorectal cancer and remains a category A recommendation by the United States Preventive Services Task Force.4
Applications for Clinical Practice and System Implementation
Current guidelines continue to strongly recommend screening for colorectal cancer for persons between 45 and 75 years of age (category B recommendation for those aged 45 to 49 years per the United States Preventive Services Task Force). Stool-based tests and direct visualization tests are both endorsed as screening options. Further follow-up from the presented studies is needed to help shed light on the magnitude of benefit of these modalities.
Practice Points
- Current guidelines continue to strongly recommend screening for colon cancer in those aged 45 to 75 years.
- The optimal modality for screening and the impact of screening on cancer-related mortality requires longer- term follow-up from these ongoing studies.
–Daniel Isaac, DO, MS
Study 1 Overview (Bretthauer et al)
Objective: To evaluate the impact of screening colonoscopy on colon cancer–related death.
Design: Randomized trial conducted in 4 European countries.
Setting and participants: Presumptively healthy men and women between the ages of 55 and 64 years were selected from population registries in Poland, Norway, Sweden, and the Netherlands between 2009 and 2014. Eligible participants had not previously undergone screening. Patients with a diagnosis of colon cancer before trial entry were excluded.
Intervention: Participants were randomly assigned in a 1:2 ratio to undergo colonoscopy screening by invitation or to no invitation and no screening. Participants were randomized using a computer-generated allocation algorithm. Patients were stratified by age, sex, and municipality.
Main outcome measures: The primary endpoint of the study was risk of colorectal cancer and related death after a median follow-up of 10 to 15 years. The main secondary endpoint was death from any cause.
Main results: The study reported follow-up data from 84,585 participants (89.1% of all participants originally included in the trial). The remaining participants were either excluded or data could not be included due to lack of follow-up data from the usual-care group. Men (50.1%) and women (49.9%) were equally represented. The median age at entry was 59 years. The median follow-up was 10 years. Characteristics were otherwise balanced. Good bowel preparation was reported in 91% of all participants. Cecal intubation was achieved in 96.8% of all participants. The percentage of patients who underwent screening was 42% for the group, but screening rates varied by country (33%-60%). Colorectal cancer was diagnosed at screening in 62 participants (0.5% of screening group). Adenomas were detected in 30.7% of participants; 15 patients had polypectomy-related major bleeding. There were no perforations.
The risk of colorectal cancer at 10 years was 0.98% in the invited-to-screen group and 1.2% in the usual-care group (risk ratio, 0.82; 95% CI, 0.7-0.93). The reported number needed to invite to prevent 1 case of colon cancer in a 10-year period was 455. The risk of colorectal cancer–related death at 10 years was 0.28% in the invited-to-screen group and 0.31% in the usual-care group (risk ratio, 0.9; 95% CI, 0.64-1.16). An adjusted per-protocol analysis was performed to account for the estimated effect of screening if all participants assigned to the screening group underwent screening. In this analysis, the risk of colorectal cancer at 10 years was decreased from 1.22% to 0.84% (risk ratio, 0.69; 95% CI, 0.66-0.83).
Conclusion: Based on the results of this European randomized trial, the risk of colorectal cancer at 10 years was lower among those who were invited to undergo screening.
Study 2 Overview (Forsberg et al)
Objective: To investigate the effect of colorectal cancer screening with once-only colonoscopy or fecal immunochemical testing (FIT) on colorectal cancer mortality and incidence.
Design: Randomized controlled trial in Sweden utilizing a population registry.
Setting and participants: Patients aged 60 years at the time of entry were identified from a population-based registry from the Swedish Tax Agency.
Intervention: Individuals were assigned by an independent statistician to once-only colonoscopy, 2 rounds of FIT 2 years apart, or a control group in which no intervention was performed. Patients were assigned in a 1:6 ratio for colonoscopy vs control and a 1:2 ratio for FIT vs control.
Main outcome measures: The primary endpoint of the trial was colorectal cancer incidence and mortality.
Main results: A total of 278,280 participants were included in the study from March 1, 2014, through December 31, 2020 (31,140 in the colonoscopy group, 60,300 in the FIT group, and 186,840 in the control group). Of those in the colonoscopy group, 35% underwent colonoscopy, and 55% of those in the FIT group participated in testing. Colorectal cancer was detected in 0.16% (49) of people in the colonoscopy group and 0.2% (121) of people in the FIT test group (relative risk, 0.78; 95% CI, 0.56-1.09). The advanced adenoma detection rate was 2.05% in the colonoscopy group and 1.61% in the FIT group (relative risk, 1.27; 95% CI, 1.15-1.41). There were 2 perforations noted in the colonoscopy group and 15 major bleeding events. More right-sided adenomas were detected in the colonoscopy group.
Conclusion: The results of the current study highlight similar detection rates in the colonoscopy and FIT group. Should further follow-up show a benefit in disease-specific mortality, such screening strategies could be translated into population-based screening programs.
Commentary
The first colonoscopy screening recommendations were established in the mid 1990s in the United States, and over the subsequent 2 decades colonoscopy has been the recommended method and main modality for colorectal cancer screening in this country. The advantage of colonoscopy over other screening modalities (sigmoidoscopy and fecal-based testing) is that it can examine the entire large bowel and allow for removal of potential precancerous lesions. However, data to support colonoscopy as a screening modality for colorectal cancer are largely based on cohort studies.1,2 These studies have reported a significant reduction in the incidence of colon cancer. Additionally, colorectal cancer mortality was notably lower in the screened populations. For example, one study among health professionals found a nearly 70% reduction in colorectal cancer mortality in those who underwent at least 1 screening colonoscopy.3
There has been a lack of randomized clinical data to validate the efficacy of colonoscopy screening for reducing colorectal cancer–related deaths. The current study by Bretthauer et al addresses an important need and enhances our understanding of the efficacy of colorectal cancer screening with colonoscopy. In this randomized trial involving more than 84,000 participants from Poland, Norway, Sweden, and the Netherlands, there was a noted 18% decrease in the risk of colorectal cancer over a 10-year period in the intention-to-screen population. The reduction in the risk of death from colorectal cancer was not statistically significant (risk ratio, 0.90; 95% CI, 0.64-1.16). These results are surprising and certainly raise the question as to whether previous studies overestimated the effectiveness of colonoscopy in reducing the risk of colorectal cancer–related deaths. There are several limitations to the Bretthauer et al study, however.
Perhaps the most important limitation is the fact that only 42% of participants in the invited-to-screen cohort underwent screening colonoscopy. Therefore, this raises the question of whether the efficacy noted is simply due to a lack of participation in the screening protocol. In the adjusted per-protocol analysis, colonoscopy was estimated to reduce the risk of colorectal cancer by 31% and the risk of colorectal cancer–related death by around 50%. These findings are more in line with prior published studies regarding the efficacy of colorectal cancer screening. The authors plan to repeat this analysis at 15 years, and it is possible that the risk of colorectal cancer and colorectal cancer–related death can be reduced on subsequent follow-up.
While the results of the Bretthauer et al trial are important, randomized trials that directly compare the effectiveness of different colorectal cancer screening strategies are lacking. The Forsberg et al trial, also an ongoing study, seeks to address this vitally important gap in our current data. The SCREESCO trial is a study that compares the efficacy of colonoscopy with FIT every 2 years or no screening. The currently reported data are preliminary but show a similarly low rate of colonoscopy screening in those invited to do so (35%). This is a similar limitation to that noted in the Bretthauer et al study. Furthermore, there is some question regarding colonoscopy quality in this study, which had a very low reported adenoma detection rate.
While the current studies are important and provide quality randomized data on the effect of colorectal cancer screening, there remain many unanswered questions. Should the results presented by Bretthauer et al represent the current real-world scenario, then colonoscopy screening may not be viewed as an effective screening tool compared to simpler, less-invasive modalities (ie, FIT). Further follow-up from the SCREESCO trial will help shed light on this question. However, there are concerns with this study, including a very low participation rate, which could greatly underestimate the effectiveness of screening. Additional analysis and longer follow-up will be vital to fully understand the benefits of screening colonoscopy. In the meantime, screening remains an important tool for early detection of colorectal cancer and remains a category A recommendation by the United States Preventive Services Task Force.4
Applications for Clinical Practice and System Implementation
Current guidelines continue to strongly recommend screening for colorectal cancer for persons between 45 and 75 years of age (category B recommendation for those aged 45 to 49 years per the United States Preventive Services Task Force). Stool-based tests and direct visualization tests are both endorsed as screening options. Further follow-up from the presented studies is needed to help shed light on the magnitude of benefit of these modalities.
Practice Points
- Current guidelines continue to strongly recommend screening for colon cancer in those aged 45 to 75 years.
- The optimal modality for screening and the impact of screening on cancer-related mortality requires longer- term follow-up from these ongoing studies.
–Daniel Isaac, DO, MS
1. Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. Screening for Colorectal Cancer: An Evidence Update for the U.S. Preventive Services Task Force [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2021 May. Report No.: 20-05271-EF-1.
2. Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325(19):1978-1998. doi:10.1001/jama.2021.4417
3. Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369(12):1095-1105. doi:10.1056/NEJMoa1301969
4. U.S. Preventive Services Task Force. Colorectal cancer: screening. Published May 18, 2021. Accessed November 8, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening
1. Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. Screening for Colorectal Cancer: An Evidence Update for the U.S. Preventive Services Task Force [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2021 May. Report No.: 20-05271-EF-1.
2. Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325(19):1978-1998. doi:10.1001/jama.2021.4417
3. Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369(12):1095-1105. doi:10.1056/NEJMoa1301969
4. U.S. Preventive Services Task Force. Colorectal cancer: screening. Published May 18, 2021. Accessed November 8, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening
The Long Arc of Justice for Veteran Benefits
This Veterans Day we honor the passing of the largest expansion of veterans benefits and services in history. On August 10, 2022, President Biden signed the Sergeant First Class Heath Robinson Honoring our Promise to Address Comprehensive Toxics (PACT) Act. This act was named for a combat medic who died of a rare form of lung cancer believed to be the result of a toxic military exposure. His widow was present during the President's State of the Union address that urged Congress to pass the legislation.2
Like all other congressional bills and government regulations, the PACT Act is complex in its details and still a work in progress. Simply put, the PACT Act expands and/or extends enrollment for a group of previously ineligible veterans. Eligibility will no longer require that veterans demonstrate a service-connected disability due to toxic exposure, including those from burn pits. This has long been a barrier for many veterans seeking benefits and not just related to toxic exposures. Logistical barriers and documentary losses have prevented many service members from establishing a clean chain of evidence for the injuries or illnesses they sustained while in uniform.
The new process is a massive step forward by the US Department of Veterans Affairs (VA) to establish high standards of procedural justice for settling beneficiary claims. The PACT Act removes the burden from the shoulders of the veteran and places it squarely on the VA to demonstrate that > 20 different medical conditions--primarily cancers and respiratory illnesses--are linked to toxic exposure. The VA must establish that exposure occurred to cohorts of service members in specific theaters and time frames. A veteran who served in that area and period and has one of the indexed illnesses is presumed to have been exposed in the line of duty.3,4
As a result, the VA instituted a new screening process to determine that toxic military exposures (a) led to illness; and (b) both exposure and illness are connected to service. According to the VA, the new process is evidence based, transparent, and allows the VA to fast-track policy decisions related to exposures. The PACT Act includes a provision intended to promote sustained implementation and prevent the program from succumbing as so many new initiatives have to inadequate adoption. VA is required to deploy its considerable internal research capacity to collaborate with external partners in and outside government to study military members with toxic exposures.4
Congress had initially proposed that the provisions of the PACT ACT would take effect in 2026, providing time to ramp up the process. The White House and VA telescoped that time line so veterans can begin now to apply for benefits that they could foreseeably receive in 2023. However, a long-standing problem for the VA has been unfunded agency or congressional mandates. These have often end in undermining the legislative intention or policy purpose of the program undermining their legislative intention or policy purpose through staffing shortages, leading to lack of or delayed access. The PACT Act promises to eschew the infamous Phoenix problem by providing increased personnel, training infrastructure, and technology resources for both the Veterans Benefit Administration and the Veterans Health Administration. Ironically, many seasoned VA observers expect the PACT expansion will lead to even larger backlogs of claims as hundreds of newly eligible veterans are added to the extant rolls of those seeking benefits.5
An estimated 1 in 5 veterans may be entitled to PACT benefits. The PACT Act is the latest of a long uneven movement toward distributive justice for veteran benefits and services. It is fitting in the month of Veterans Day 2022 to trace that trajectory. Congress first passed veteran benefits legislation in 1917, focused on soldiers with disabilities. This resulted in a massive investment in building hospitals. Ironically, part of the impetus for VA health care was an earlier toxic military exposure. World War I service members suffered from the detrimental effects of mustard gas among other chemical byproducts. In 1924, VA benefits and services underwent a momentous opening to include individuals with non-service-connected disabilities. Four years later, the VA tent became even bigger, welcoming women, National Guard, and militia members to receive care under its auspices.6
The PACT Act is a fitting memorial for Veterans Day as an increasingly divided country presents a unified response to veterans and their survivors exposed to a variety of toxins across multiple wars. The PACT Act was hard won with veterans and their advocates having to fight years of political bickering, government abdication of accountability, and scientific sparring before this bipartisan legislation passed.7 It covers Vietnam War veterans with several conditions due to Agent Orange exposure; Gulf War and post-9/11 veterans with cancer and respiratory conditions; and the service members deployed to Afghanistan and Iraq afflicted with illnesses due to the smoke of burn pits and other toxins.
As many areas of the country roll back LGBTQ+ rights to health care and social services, the VA has emerged as a leader in the movement for diversity and inclusion. VA Secretary McDonough provided a pathway to VA eligibility for other than honorably discharged veterans, including those LGBTQ+ persons discharged under Don't Ask, Don't Tell.8 Lest we take this new inclusivity for granted, we should never forget that this journey toward equity for the military and VA has been long, slow, and uneven. There are many difficult miles yet to travel if we are to achieve liberty and justice for veteran members of racial minorities, women, and other marginalized populations. Even the PACT Act does not cover all putative exposures to toxins.9 Yet it is a significant step closer to fulfilling the motto of the VA LGBTQ+ program: to serve all who served.10
- Parker T. Of justice and the conscience. In: Ten Sermons of Religion. Crosby, Nichols and Company; 1853:66-85.
- The White House. Fact sheet: President Biden signs the PACT Act and delivers on his promise to America's veterans. August 9, 2022. Accessed October 24, 2022. https://www.whitehouse.gov/briefing-room/statements-releases/2022/08/10/fact-sheet-president-biden-signs-the-pact-act-and-delivers-on-his-promise-to-americas-veterans
- Shane L. Vets can apply for all PACT benefits now after VA speeds up law. Military Times. September 1, 2022. Accessed October 24, 2022. https://www.militarytimes.com/news/burn-pits/2022/09/01/vets-can-apply-for-all-pact-act-benefits-now-after-va-speeds-up-law
- US Department of Veterans Affairs. The PACT Act and your VA benefits. Updated September 28, 2022. Accessed October 24, 2022. https://www.va.gov/resources/the-pact-act-and-your-va-benefits
- Wentling N. Discharged LGBTQ+ veterans now eligible for benefits under new guidance issued by VA. Stars & Stripes. September 20, 2021. Accessed October 24, 2022. https://www.stripes.com/veterans/2021-09-20/veterans-affairs-dont-ask-dont-tell-benefits-lgbt-discharges-2956761.html
- US Department of Veterans Affairs, VA History Office. History--Department of Veterans Affairs (VA). Updated May 27, 2021. Accessed October 24, 2022. https://www.va.gov/HISTORY/VA_History/Overview.asp
- Atkins D, Kilbourne A, Lipson L. Health equity research in the Veterans Health Administration: we've come far but aren't there yet. Am J Public Health. 2014;104(suppl 4):S525-S526. doi:10.2105/AJPH.2014.302216
- Stack MK. The soldiers came home sick. The government denied it was responsible. New York Times. Updated January 16, 2022. Accessed October 24, 2022. https://www.nytimes.com/2022/01/11/magazine/military-burn-pits.html
- Namaz A, Sagalyn D. VA secretary discusses health care overhaul helping veterans exposed to toxic burn pits. PBS NewsHour. September 1, 2022. Accessed October 24, 2022. https://www.pbs.org/newshour/show/va-secretary-discusses-health-care-overhaul-helping-veterans-exposed-to-toxic-burn-pits
- US Department of Veterans Affairs, Patient Care Services. VHA LGBTQ+ health program. Updated September 13, 2022. Accessed October 31, 2022. https://www.patientcare.va.gov/lgbt
This Veterans Day we honor the passing of the largest expansion of veterans benefits and services in history. On August 10, 2022, President Biden signed the Sergeant First Class Heath Robinson Honoring our Promise to Address Comprehensive Toxics (PACT) Act. This act was named for a combat medic who died of a rare form of lung cancer believed to be the result of a toxic military exposure. His widow was present during the President's State of the Union address that urged Congress to pass the legislation.2
Like all other congressional bills and government regulations, the PACT Act is complex in its details and still a work in progress. Simply put, the PACT Act expands and/or extends enrollment for a group of previously ineligible veterans. Eligibility will no longer require that veterans demonstrate a service-connected disability due to toxic exposure, including those from burn pits. This has long been a barrier for many veterans seeking benefits and not just related to toxic exposures. Logistical barriers and documentary losses have prevented many service members from establishing a clean chain of evidence for the injuries or illnesses they sustained while in uniform.
The new process is a massive step forward by the US Department of Veterans Affairs (VA) to establish high standards of procedural justice for settling beneficiary claims. The PACT Act removes the burden from the shoulders of the veteran and places it squarely on the VA to demonstrate that > 20 different medical conditions--primarily cancers and respiratory illnesses--are linked to toxic exposure. The VA must establish that exposure occurred to cohorts of service members in specific theaters and time frames. A veteran who served in that area and period and has one of the indexed illnesses is presumed to have been exposed in the line of duty.3,4
As a result, the VA instituted a new screening process to determine that toxic military exposures (a) led to illness; and (b) both exposure and illness are connected to service. According to the VA, the new process is evidence based, transparent, and allows the VA to fast-track policy decisions related to exposures. The PACT Act includes a provision intended to promote sustained implementation and prevent the program from succumbing as so many new initiatives have to inadequate adoption. VA is required to deploy its considerable internal research capacity to collaborate with external partners in and outside government to study military members with toxic exposures.4
Congress had initially proposed that the provisions of the PACT ACT would take effect in 2026, providing time to ramp up the process. The White House and VA telescoped that time line so veterans can begin now to apply for benefits that they could foreseeably receive in 2023. However, a long-standing problem for the VA has been unfunded agency or congressional mandates. These have often end in undermining the legislative intention or policy purpose of the program undermining their legislative intention or policy purpose through staffing shortages, leading to lack of or delayed access. The PACT Act promises to eschew the infamous Phoenix problem by providing increased personnel, training infrastructure, and technology resources for both the Veterans Benefit Administration and the Veterans Health Administration. Ironically, many seasoned VA observers expect the PACT expansion will lead to even larger backlogs of claims as hundreds of newly eligible veterans are added to the extant rolls of those seeking benefits.5
An estimated 1 in 5 veterans may be entitled to PACT benefits. The PACT Act is the latest of a long uneven movement toward distributive justice for veteran benefits and services. It is fitting in the month of Veterans Day 2022 to trace that trajectory. Congress first passed veteran benefits legislation in 1917, focused on soldiers with disabilities. This resulted in a massive investment in building hospitals. Ironically, part of the impetus for VA health care was an earlier toxic military exposure. World War I service members suffered from the detrimental effects of mustard gas among other chemical byproducts. In 1924, VA benefits and services underwent a momentous opening to include individuals with non-service-connected disabilities. Four years later, the VA tent became even bigger, welcoming women, National Guard, and militia members to receive care under its auspices.6
The PACT Act is a fitting memorial for Veterans Day as an increasingly divided country presents a unified response to veterans and their survivors exposed to a variety of toxins across multiple wars. The PACT Act was hard won with veterans and their advocates having to fight years of political bickering, government abdication of accountability, and scientific sparring before this bipartisan legislation passed.7 It covers Vietnam War veterans with several conditions due to Agent Orange exposure; Gulf War and post-9/11 veterans with cancer and respiratory conditions; and the service members deployed to Afghanistan and Iraq afflicted with illnesses due to the smoke of burn pits and other toxins.
As many areas of the country roll back LGBTQ+ rights to health care and social services, the VA has emerged as a leader in the movement for diversity and inclusion. VA Secretary McDonough provided a pathway to VA eligibility for other than honorably discharged veterans, including those LGBTQ+ persons discharged under Don't Ask, Don't Tell.8 Lest we take this new inclusivity for granted, we should never forget that this journey toward equity for the military and VA has been long, slow, and uneven. There are many difficult miles yet to travel if we are to achieve liberty and justice for veteran members of racial minorities, women, and other marginalized populations. Even the PACT Act does not cover all putative exposures to toxins.9 Yet it is a significant step closer to fulfilling the motto of the VA LGBTQ+ program: to serve all who served.10
This Veterans Day we honor the passing of the largest expansion of veterans benefits and services in history. On August 10, 2022, President Biden signed the Sergeant First Class Heath Robinson Honoring our Promise to Address Comprehensive Toxics (PACT) Act. This act was named for a combat medic who died of a rare form of lung cancer believed to be the result of a toxic military exposure. His widow was present during the President's State of the Union address that urged Congress to pass the legislation.2
Like all other congressional bills and government regulations, the PACT Act is complex in its details and still a work in progress. Simply put, the PACT Act expands and/or extends enrollment for a group of previously ineligible veterans. Eligibility will no longer require that veterans demonstrate a service-connected disability due to toxic exposure, including those from burn pits. This has long been a barrier for many veterans seeking benefits and not just related to toxic exposures. Logistical barriers and documentary losses have prevented many service members from establishing a clean chain of evidence for the injuries or illnesses they sustained while in uniform.
The new process is a massive step forward by the US Department of Veterans Affairs (VA) to establish high standards of procedural justice for settling beneficiary claims. The PACT Act removes the burden from the shoulders of the veteran and places it squarely on the VA to demonstrate that > 20 different medical conditions--primarily cancers and respiratory illnesses--are linked to toxic exposure. The VA must establish that exposure occurred to cohorts of service members in specific theaters and time frames. A veteran who served in that area and period and has one of the indexed illnesses is presumed to have been exposed in the line of duty.3,4
As a result, the VA instituted a new screening process to determine that toxic military exposures (a) led to illness; and (b) both exposure and illness are connected to service. According to the VA, the new process is evidence based, transparent, and allows the VA to fast-track policy decisions related to exposures. The PACT Act includes a provision intended to promote sustained implementation and prevent the program from succumbing as so many new initiatives have to inadequate adoption. VA is required to deploy its considerable internal research capacity to collaborate with external partners in and outside government to study military members with toxic exposures.4
Congress had initially proposed that the provisions of the PACT ACT would take effect in 2026, providing time to ramp up the process. The White House and VA telescoped that time line so veterans can begin now to apply for benefits that they could foreseeably receive in 2023. However, a long-standing problem for the VA has been unfunded agency or congressional mandates. These have often end in undermining the legislative intention or policy purpose of the program undermining their legislative intention or policy purpose through staffing shortages, leading to lack of or delayed access. The PACT Act promises to eschew the infamous Phoenix problem by providing increased personnel, training infrastructure, and technology resources for both the Veterans Benefit Administration and the Veterans Health Administration. Ironically, many seasoned VA observers expect the PACT expansion will lead to even larger backlogs of claims as hundreds of newly eligible veterans are added to the extant rolls of those seeking benefits.5
An estimated 1 in 5 veterans may be entitled to PACT benefits. The PACT Act is the latest of a long uneven movement toward distributive justice for veteran benefits and services. It is fitting in the month of Veterans Day 2022 to trace that trajectory. Congress first passed veteran benefits legislation in 1917, focused on soldiers with disabilities. This resulted in a massive investment in building hospitals. Ironically, part of the impetus for VA health care was an earlier toxic military exposure. World War I service members suffered from the detrimental effects of mustard gas among other chemical byproducts. In 1924, VA benefits and services underwent a momentous opening to include individuals with non-service-connected disabilities. Four years later, the VA tent became even bigger, welcoming women, National Guard, and militia members to receive care under its auspices.6
The PACT Act is a fitting memorial for Veterans Day as an increasingly divided country presents a unified response to veterans and their survivors exposed to a variety of toxins across multiple wars. The PACT Act was hard won with veterans and their advocates having to fight years of political bickering, government abdication of accountability, and scientific sparring before this bipartisan legislation passed.7 It covers Vietnam War veterans with several conditions due to Agent Orange exposure; Gulf War and post-9/11 veterans with cancer and respiratory conditions; and the service members deployed to Afghanistan and Iraq afflicted with illnesses due to the smoke of burn pits and other toxins.
As many areas of the country roll back LGBTQ+ rights to health care and social services, the VA has emerged as a leader in the movement for diversity and inclusion. VA Secretary McDonough provided a pathway to VA eligibility for other than honorably discharged veterans, including those LGBTQ+ persons discharged under Don't Ask, Don't Tell.8 Lest we take this new inclusivity for granted, we should never forget that this journey toward equity for the military and VA has been long, slow, and uneven. There are many difficult miles yet to travel if we are to achieve liberty and justice for veteran members of racial minorities, women, and other marginalized populations. Even the PACT Act does not cover all putative exposures to toxins.9 Yet it is a significant step closer to fulfilling the motto of the VA LGBTQ+ program: to serve all who served.10
- Parker T. Of justice and the conscience. In: Ten Sermons of Religion. Crosby, Nichols and Company; 1853:66-85.
- The White House. Fact sheet: President Biden signs the PACT Act and delivers on his promise to America's veterans. August 9, 2022. Accessed October 24, 2022. https://www.whitehouse.gov/briefing-room/statements-releases/2022/08/10/fact-sheet-president-biden-signs-the-pact-act-and-delivers-on-his-promise-to-americas-veterans
- Shane L. Vets can apply for all PACT benefits now after VA speeds up law. Military Times. September 1, 2022. Accessed October 24, 2022. https://www.militarytimes.com/news/burn-pits/2022/09/01/vets-can-apply-for-all-pact-act-benefits-now-after-va-speeds-up-law
- US Department of Veterans Affairs. The PACT Act and your VA benefits. Updated September 28, 2022. Accessed October 24, 2022. https://www.va.gov/resources/the-pact-act-and-your-va-benefits
- Wentling N. Discharged LGBTQ+ veterans now eligible for benefits under new guidance issued by VA. Stars & Stripes. September 20, 2021. Accessed October 24, 2022. https://www.stripes.com/veterans/2021-09-20/veterans-affairs-dont-ask-dont-tell-benefits-lgbt-discharges-2956761.html
- US Department of Veterans Affairs, VA History Office. History--Department of Veterans Affairs (VA). Updated May 27, 2021. Accessed October 24, 2022. https://www.va.gov/HISTORY/VA_History/Overview.asp
- Atkins D, Kilbourne A, Lipson L. Health equity research in the Veterans Health Administration: we've come far but aren't there yet. Am J Public Health. 2014;104(suppl 4):S525-S526. doi:10.2105/AJPH.2014.302216
- Stack MK. The soldiers came home sick. The government denied it was responsible. New York Times. Updated January 16, 2022. Accessed October 24, 2022. https://www.nytimes.com/2022/01/11/magazine/military-burn-pits.html
- Namaz A, Sagalyn D. VA secretary discusses health care overhaul helping veterans exposed to toxic burn pits. PBS NewsHour. September 1, 2022. Accessed October 24, 2022. https://www.pbs.org/newshour/show/va-secretary-discusses-health-care-overhaul-helping-veterans-exposed-to-toxic-burn-pits
- US Department of Veterans Affairs, Patient Care Services. VHA LGBTQ+ health program. Updated September 13, 2022. Accessed October 31, 2022. https://www.patientcare.va.gov/lgbt
- Parker T. Of justice and the conscience. In: Ten Sermons of Religion. Crosby, Nichols and Company; 1853:66-85.
- The White House. Fact sheet: President Biden signs the PACT Act and delivers on his promise to America's veterans. August 9, 2022. Accessed October 24, 2022. https://www.whitehouse.gov/briefing-room/statements-releases/2022/08/10/fact-sheet-president-biden-signs-the-pact-act-and-delivers-on-his-promise-to-americas-veterans
- Shane L. Vets can apply for all PACT benefits now after VA speeds up law. Military Times. September 1, 2022. Accessed October 24, 2022. https://www.militarytimes.com/news/burn-pits/2022/09/01/vets-can-apply-for-all-pact-act-benefits-now-after-va-speeds-up-law
- US Department of Veterans Affairs. The PACT Act and your VA benefits. Updated September 28, 2022. Accessed October 24, 2022. https://www.va.gov/resources/the-pact-act-and-your-va-benefits
- Wentling N. Discharged LGBTQ+ veterans now eligible for benefits under new guidance issued by VA. Stars & Stripes. September 20, 2021. Accessed October 24, 2022. https://www.stripes.com/veterans/2021-09-20/veterans-affairs-dont-ask-dont-tell-benefits-lgbt-discharges-2956761.html
- US Department of Veterans Affairs, VA History Office. History--Department of Veterans Affairs (VA). Updated May 27, 2021. Accessed October 24, 2022. https://www.va.gov/HISTORY/VA_History/Overview.asp
- Atkins D, Kilbourne A, Lipson L. Health equity research in the Veterans Health Administration: we've come far but aren't there yet. Am J Public Health. 2014;104(suppl 4):S525-S526. doi:10.2105/AJPH.2014.302216
- Stack MK. The soldiers came home sick. The government denied it was responsible. New York Times. Updated January 16, 2022. Accessed October 24, 2022. https://www.nytimes.com/2022/01/11/magazine/military-burn-pits.html
- Namaz A, Sagalyn D. VA secretary discusses health care overhaul helping veterans exposed to toxic burn pits. PBS NewsHour. September 1, 2022. Accessed October 24, 2022. https://www.pbs.org/newshour/show/va-secretary-discusses-health-care-overhaul-helping-veterans-exposed-to-toxic-burn-pits
- US Department of Veterans Affairs, Patient Care Services. VHA LGBTQ+ health program. Updated September 13, 2022. Accessed October 31, 2022. https://www.patientcare.va.gov/lgbt
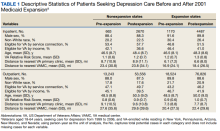
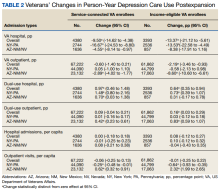
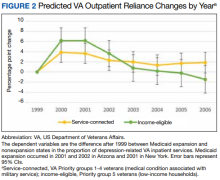
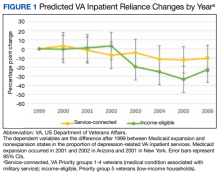
Medicaid Expansion and Veterans’ Reliance on the VA for Depression Care
The US Department of Veterans Affairs (VA) is the largest integrated health care system in the United States, providing care for more than 9 million veterans.1 With veterans experiencing mental health conditions like posttraumatic stress disorder (PTSD), substance use disorders, and other serious mental illnesses (SMI) at higher rates compared with the general population, the VA plays an important role in the provision of mental health services.2-5 Since the implementation of its Mental Health Strategic Plan in 2004, the VA has overseen the development of a wide array of mental health programs geared toward the complex needs of veterans. Research has demonstrated VA care outperforming Medicaid-reimbursed services in terms of the percentage of veterans filling antidepressants for at least 12 weeks after initiation of treatment for major depressive disorder (MDD), as well as posthospitalization follow-up.6
Eligible veterans enrolled in the VA often also seek non-VA care. Medicaid covers nearly 10% of all nonelderly veterans, and of these veterans, 39% rely solely on Medicaid for health care access.7 Today, Medicaid is the largest payer for mental health services in the US, providing coverage for approximately 27% of Americans who have SMI and helping fulfill unmet mental health needs.8,9 Understanding which of these systems veterans choose to use, and under which circumstances, is essential in guiding the allocation of limited health care resources.10
Beyond Medicaid, alternatives to VA care may include TRICARE, Medicare, Indian Health Services, and employer-based or self-purchased private insurance. While these options potentially increase convenience, choice, and access to health care practitioners (HCPs) and services not available at local VA systems, cross-system utilization with poor integration may cause care coordination and continuity problems, such as medication mismanagement and opioid overdose, unnecessary duplicate utilization, and possible increased mortality.11-15 As recent national legislative changes, such as the Patient Protection and Affordable Care Act (ACA), Veterans Access, Choice and Accountability Act, and the VA MISSION Act, continue to shift the health care landscape for veterans, questions surrounding how veterans are changing their health care use become significant.16,17
Here, we approach the impacts of Medicaid expansion on veterans’ reliance on the VA for mental health services with a unique lens. We leverage a difference-in-difference design to study 2 historical Medicaid expansions in Arizona (AZ) and New York (NY), which extended eligibility to childless adults in 2001. Prior Medicaid dual-eligible mental health research investigated reliance shifts during the immediate postenrollment year in a subset of veterans newly enrolled in Medicaid.18 However, this study took place in a period of relative policy stability. In contrast, we investigate the potential effects of a broad policy shift by analyzing state-level changes in veterans’ reliance over 6 years after a statewide Medicaid expansion. We match expansion states with demographically similar nonexpansion states to account for unobserved trends and confounding effects. Prior studies have used this method to evaluate post-Medicaid expansion mortality changes and changes in veteran dual enrollment and hospitalizations.10,19 While a study of ACA Medicaid expansion states would be ideal, Medicaid data from most states were only available through 2014 at the time of this analysis. Our study offers a quasi-experimental framework leveraging longitudinal data that can be applied as more post-ACA data become available.
Given the rising incidence of suicide among veterans, understanding care-seeking behaviors for depression among veterans is important as it is the most common psychiatric condition found in those who died by suicide.20,21 Furthermore, depression may be useful as a clinical proxy for mental health policy impacts, given that the Patient Health Questionnaire-9 (PHQ-9) screening tool is well validated and increasingly research accessible, and it is a chronic condition responsive to both well-managed pharmacologic treatment and psychotherapeutic interventions.22,23
In this study, we quantify the change in care-seeking behavior for depression among veterans after Medicaid expansion, using a quasi-experimental design. We hypothesize that new access to Medicaid would be associated with a shift away from using VA services for depression. Given the income-dependent eligibility requirements of Medicaid, we also hypothesize that veterans who qualified for VA coverage due to low income, determined by a regional means test (Priority group 5, “income-eligible”), would be more likely to shift care compared with those whose serviced-connected conditions related to their military service (Priority groups 1-4, “service-connected”) provide VA access.
Methods
To investigate the relative changes in veterans’ reliance on the VA for depression care after the 2001 NY and AZ Medicaid expansions We used a retrospective, difference-in-difference analysis. Our comparison pairings, based on prior demographic analyses were as follows: NY with Pennsylvania(PA); AZ with New Mexico and Nevada (NM/NV).19 The time frame of our analysis was 1999 to 2006, with pre- and postexpansion periods defined as 1999 to 2000 and 2001 to 2006, respectively.
Data
We included veterans aged 18 to 64 years, seeking care for depression from 1999 to 2006, who were also VA-enrolled and residing in our states of interest. We counted veterans as enrolled in Medicaid if they were enrolled at least 1 month in a given year.
Using similar methods like those used in prior studies, we selected patients with encounters documenting depression as the primary outpatient or inpatient diagnosis using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes: 296.2x for a single episode of major depressive disorder, 296.3x for a recurrent episode of MDD, 300.4 for dysthymia, and 311.0 for depression not otherwise specified.18,24 We used data from the Medicaid Analytic eXtract files (MAX) for Medicaid data and the VA Corporate Data Warehouse (CDW) for VA data. We chose 1999 as the first study year because it was the earliest year MAX data were available.
Our final sample included 1833 person-years pre-expansion and 7157 postexpansion in our inpatient analysis, as well as 31,767 person-years pre-expansion and 130,382 postexpansion in our outpatient analysis.
Outcomes and Variables
Our primary outcomes were comparative shifts in VA reliance between expansion and nonexpansion states after Medicaid expansion for both inpatient and outpatient depression care. For each year of study, we calculated a veteran’s VA reliance by aggregating the number of days with depression-related encounters at the VA and dividing by the total number of days with a VA or Medicaid depression-related encounters for the year. To provide context to these shifts in VA reliance, we further analyzed the changes in the proportion of annual VA-Medicaid dual users and annual per capita utilization of depression care across the VA and Medicaid.
We conducted subanalyses by income-eligible and service-connected veterans and adjusted our models for age, non-White race, sex, distances to the nearest inpatient and outpatient VA facilities, and VA Relative Risk Score, which is a measure of disease burden and clinical complexity validated specifically for veterans.25
Statistical Analysis
We used fractional logistic regression to model the adjusted effect of Medicaid expansion on VA reliance for depression care. In parallel, we leveraged ordered logit regression and negative binomial regression models to examine the proportion of VA-Medicaid dual users and the per capita utilization of Medicaid and VA depression care, respectively. To estimate the difference-in-difference effects, we used the interaction term of 2 categorical variables—expansion vs nonexpansion states and pre- vs postexpansion status—as the independent variable. We then calculated the average marginal effects with 95% CIs to estimate the differences in outcomes between expansion and nonexpansion states from pre- to postexpansion periods, as well as year-by-year shifts as a robustness check. We conducted these analyses using Stata MP, version 15.
Results
Baseline and postexpansion characteristics
VA Reliance
Overall, we observed postexpansion decreases in VA reliance for depression care
At the state level, reliance on the VA for inpatient depression care in NY decreased by 13.53 pp (95% CI, -22.58 to -4.49) for income-eligible veterans and 16.67 pp (95% CI, -24.53 to -8.80) for service-connected veterans. No relative differences were observed in the outpatient comparisons for both income-eligible (-0.58 pp; 95% CI, -2.13 to 0.98) and service-connected (0.05 pp; 95% CI, -1.00 to 1.10) veterans. In AZ, Medicaid expansion was associated with decreased VA reliance for outpatient depression care among income-eligible veterans (-8.60 pp; 95% CI, -10.60 to -6.61), greater than that for service-connected veterans (-2.89 pp; 95% CI, -4.02 to -1.77). This decrease in VA reliance was significant in the inpatient context only for service-connected veterans (-4.55 pp; 95% CI, -8.14 to -0.97), not income-eligible veterans (-8.38 pp; 95% CI, -17.91 to 1.16).
By applying the aggregate pp changes toward the postexpansion number of visits across both expansion and nonexpansion states, we found that expansion of Medicaid across all our study states would have resulted in 996 fewer hospitalizations and 10,109 fewer outpatient visits for depression at VA in the postexpansion period vs if no states had chosen to expand Medicaid.
Dual Use/Per Capita Utilization
Overall, Medicaid expansion was associated with greater dual use for inpatient depression care—a 0.97-pp (95% CI, 0.46 to 1.48) increase among service-connected veterans and a 0.64-pp (95% CI, 0.35 to 0.94) increase among income-eligible veterans.
At the state level, NY similarly showed increases in dual use among both service-connected (1.48 pp; 95% CI, 0.80 to 2.16) and income-eligible veterans (0.73 pp; 95% CI, 0.39 to 1.07) after Medicaid expansion. However, dual use in AZ increased significantly only among service-connected veterans (0.70 pp; 95% CI, 0.03 to 1.38), not income-eligible veterans (0.31 pp; 95% CI, -0.17 to 0.78).
Among outpatient visits, Medicaid expansion was associated with increased dual use only for income-eligible veterans (0.16 pp; 95% CI, 0.03-0.29), and not service-connected veterans (0.09 pp; 95% CI, -0.04 to 0.21). State-level analyses showed that Medicaid expansion in NY was not associated with changes in dual use for either service-connected (0.01 pp; 95% CI, -0.16 to 0.17) or income-eligible veterans (0.03 pp; 95% CI, -0.12 to 0.18), while expansion in AZ was associated with increases in dual use among both service-connected (0.42 pp; 95% CI, 0.23 to 0.61) and income-eligible veterans (0.83 pp; 95% CI, 0.59 to 1.07).
Concerning per capita utilization of depression care after Medicaid expansion, analyses showed no detectable changes for either inpatient or outpatient services, among both service-connected and income-eligible veterans. However, while this pattern held at the state level among hospitalizations, outpatient visit results showed divergent trends between AZ and NY. In NY, Medicaid expansion was associated with decreased per capita utilization of outpatient depression care among both service-connected (-0.25 visits annually; 95% CI, -0.48 to -0.01) and income-eligible veterans (-0.64 visits annually; 95% CI, -0.93 to -0.35). In AZ, Medicaid expansion was associated with increased per capita utilization of outpatient depression care among both service-connected (0.62 visits annually; 95% CI, 0.32-0.91) and income-eligible veterans (2.32 visits annually; 95% CI, 1.99-2.65).
Discussion
Our study quantified changes in depression-related health care utilization after Medicaid expansions in NY and AZ in 2001. Overall, the balance of evidence indicated that Medicaid expansion was associated with decreased reliance on the VA for depression-related services. There was an exception: income-eligible veterans in AZ did not shift their hospital care away from the VA in a statistically discernible way, although the point estimate was lower. More broadly, these findings concerning veterans’ reliance varied not only in inpatient vs outpatient services and income- vs service-connected eligibility, but also in the state-level contexts of veteran dual users and per capita utilization.
Given that the overall per capita utilization of depression care was unchanged from pre- to postexpansion periods, one might interpret the decreases in VA reliance and increases in Medicaid-VA dual users as a substitution effect from VA care to non-VA care. This could be plausible for hospitalizations where state-level analyses showed similarly stable levels of per capita utilization. However, state-level trends in our outpatient utilization analysis, especially with a substantial 2.32 pp increase in annual per capita visits among income-eligible veterans in AZ, leave open the possibility that in some cases veterans may be complementing VA care with Medicaid-reimbursed services.
The causes underlying these differences in reliance shifts between NY and AZ are likely also influenced by the policy contexts of their respective Medicaid expansions. For example, in 1999, NY passed Kendra’s Law, which established a procedure for obtaining court orders for assisted outpatient mental health treatment for individuals deemed unlikely to survive safely in the community.26 A reasonable inference is that there was less unfulfilled outpatient mental health need in NY under the existing accessibility provisioned by Kendra’s Law. In addition, while both states extended coverage to childless adults under 100% of the Federal Poverty level (FPL), the AZ Medicaid expansion was via a voters’ initiative and extended family coverage to 200% FPL vs 150% FPL for families in NY. Given that the AZ Medicaid expansion enjoyed both broader public participation and generosity in terms of eligibility, its uptake and therefore effect size may have been larger than in NY for nonacute outpatient care.
Our findings contribute to the growing body of literature surrounding the changes in health care utilization after Medicaid expansion, specifically for a newly dual-eligible population of veterans seeking mental health services for depression. While prior research concerning Medicare dual-enrolled veterans has shown high reliance on the VA for both mental health diagnoses and services, scholars have established the association of Medicaid enrollment with decreased VA reliance.27-29 Our analysis is the first to investigate state-level effects of Medicaid expansion on VA reliance for a single mental health condition using a natural experimental framework. We focus on a population that includes a large portion of veterans who are newly Medicaid-eligible due to a sweeping policy change and use demographically matched nonexpansion states to draw comparisons in VA reliance for depression care. Our findings of Medicaid expansion–associated decreases in VA reliance for depression care complement prior literature that describe Medicaid enrollment–associated decreases in VA reliance for overall mental health care.
Implications
From a systems-level perspective, the implications of shifting services away from the VA are complex and incompletely understood. The VA lacks interoperability with the electronic health records (EHRs) used by Medicaid clinicians. Consequently, significant issues of service duplication and incomplete clinical data exist for veterans seeking treatment outside of the VA system, posing health care quality and safety concerns.30 On one hand, Medicaid access is associated with increased health care utilization attributed to filling unmet needs for Medicare dual enrollees, as well as increased prescription filling for psychiatric medications.31,32 Furthermore, the only randomized control trial of Medicaid expansion to date was associated with a 9-pp decrease in positive screening rates for depression among those who received access at around 2 years postexpansion.33 On the other hand, the VA has developed a mental health system tailored to the particular needs of veterans, and health care practitioners at the VA have significantly greater rates of military cultural competency compared to those in nonmilitary settings (70% vs 24% in the TRICARE network and 8% among those with no military or TRICARE affiliation).34 Compared to individuals seeking mental health services with private insurance plans, veterans were about twice as likely to receive appropriate treatment for schizophrenia and depression at the VA.35 These documented strengths of VA mental health care may together help explain the small absolute number of visits that were associated with shifts away from VA overall after Medicaid expansion.
Finally, it is worth considering extrinsic factors that influence utilization among newly dual-eligible veterans. For example, hospitalizations are less likely to be planned than outpatient services, translating to a greater importance of proximity to a nearby medical facility than a veteran’s preference of where to seek care. In the same vein, major VA medical centers are fewer and more distant on average than VA outpatient clinics, therefore reducing the advantage of a Medicaid-reimbursed outpatient clinic in terms of distance.36 These realities may partially explain the proportionally larger shifts away from the VA for hospitalizations compared to outpatient care for depression.
Limitations and Future Directions
Our results should be interpreted within methodological and data limitations. With only 2 states in our sample, NY demonstrably skewed overall results, contributing 1.7 to 3 times more observations than AZ across subanalyses—a challenge also cited by Sommers and colleagues.19 Our veteran groupings were also unable to distinguish those veterans classified as service-connected who may also have qualified by income-eligible criteria (which would tend to understate the size of results) and those veterans who gained and then lost Medicaid coverage in a given year. Our study also faces limitations in generalizability and establishing causality. First, we included only 2 historical state Medicaid expansions, compared with the 38 states and Washington, DC, that have now expanded Medicaid to date under the ACA. Just in the 2 states from our study, we noted significant heterogeneity in the shifts associated with Medicaid expansion, which makes extrapolating specific trends difficult. Differences in underlying health care resources, legislation, and other external factors may limit the applicability of Medicaid expansion in the era of the ACA, as well as the Veterans Choice and MISSION acts. Second, while we leveraged a difference-in-difference analysis using demographically matched, neighboring comparison states, our findings are nevertheless drawn from observational data obviating causality. VA data for other sources of coverage such as private insurance are limited and not included in our study, and MAX datasets vary by quality across states, translating to potential gaps in our study cohort.28
Moving forward, our study demonstrates the potential for applying a natural experimental approach to studying dual-eligible veterans at the interface of Medicaid expansion. We focused on changes in VA reliance for the specific condition of depression and, in doing so, invite further inquiry into the impact of state mental health policy on outcomes more proximate to veterans’ outcomes. Clinical indicators, such as rates of antidepressant filling, utilization and duration of psychotherapy, and PHQ-9 scores, can similarly be investigated by natural experimental design. While current limits of administrative data and the siloing of EHRs may pose barriers to some of these avenues of research, multidisciplinary methodologies and data querying innovations such as natural language processing algorithms for clinical notes hold exciting opportunities to bridge the gap between policy and clinical efficacy.
Conclusions
This study applied a difference-in-difference analysis and found that Medicaid expansion is associated with decreases in VA reliance for both inpatient and outpatient services for depression. As additional data are generated from the Medicaid expansions of the ACA, similarly robust methods should be applied to further explore the impacts associated with such policy shifts and open the door to a better understanding of implications at the clinical level.
Acknowledgments
We acknowledge the efforts of Janine Wong, who proofread and formatted the manuscript.
1. US Department of Veterans Affairs, Veterans Health Administration. About VA. 2019. Updated September 27, 2022. Accessed September 29, 2022. https://www.va.gov/health/
2. Richardson LK, Frueh BC, Acierno R. Prevalence estimates of combat-related post-traumatic stress disorder: critical review. Aust N Z J Psychiatry. 2010;44(1):4-19. doi:10.3109/00048670903393597
3. Lan CW, Fiellin DA, Barry DT, et al. The epidemiology of substance use disorders in US veterans: a systematic review and analysis of assessment methods. Am J Addict. 2016;25(1):7-24. doi:10.1111/ajad.12319
4. Grant BF, Saha TD, June Ruan W, et al. Epidemiology of DSM-5 drug use disorder results from the national epidemiologic survey on alcohol and related conditions-III. JAMA Psychiat. 2016;73(1):39-47. doi:10.1001/jamapsychiatry.015.2132
5. Pemberton MR, Forman-Hoffman VL, Lipari RN, Ashley OS, Heller DC, Williams MR. Prevalence of past year substance use and mental illness by veteran status in a nationally representative sample. CBHSQ Data Review. Published November 9, 2016. Accessed October 6, 2022. https://www.samhsa.gov/data/report/prevalence-past-year-substance-use-and-mental-illness-veteran-status-nationally
6. Watkins KE, Pincus HA, Smith B, et al. Veterans Health Administration Mental Health Program Evaluation: Capstone Report. 2011. Accessed September 29, 2022. https://www.rand.org/pubs/technical_reports/TR956.html
7. Henry J. Kaiser Family Foundation. Medicaid’s role in covering veterans. June 29, 2017. Accessed September 29, 2022. https://www.kff.org/infographic/medicaids-role-in-covering-veterans
8. Substance Abuse and Mental Health Services Administration. Results from the 2016 National Survey on Drug Use and Health: detailed tables. September 7, 2017. Accessed September 29, 2022. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2016/NSDUH-DetTabs-2016.pdf
9. Wen H, Druss BG, Cummings JR. Effect of Medicaid expansions on health insurance coverage and access to care among low-income adults with behavioral health conditions. Health Serv Res. 2015;50:1787-1809. doi:10.1111/1475-6773.12411
10. O’Mahen PN, Petersen LA. Effects of state-level Medicaid expansion on Veterans Health Administration dual enrollment and utilization: potential implications for future coverage expansions. Med Care. 2020;58(6):526-533. doi:10.1097/MLR.0000000000001327
11. Ono SS, Dziak KM, Wittrock SM, et al. Treating dual-use patients across two health care systems: a qualitative study. Fed Pract. 2015;32(8):32-37.
12. Weeks WB, Mahar PJ, Wright SM. Utilization of VA and Medicare services by Medicare-eligible veterans: the impact of additional access points in a rural setting. J Healthc Manag. 2005;50(2):95-106.
13. Gellad WF, Thorpe JM, Zhao X, et al. Impact of dual use of Department of Veterans Affairs and Medicare part d drug benefits on potentially unsafe opioid use. Am J Public Health. 2018;108(2):248-255. doi:10.2105/AJPH.2017.304174
14. Coughlin SS, Young L. A review of dual health care system use by veterans with cardiometabolic disease. J Hosp Manag Health Policy. 2018;2:39. doi:10.21037/jhmhp.2018.07.05
15. Radomski TR, Zhao X, Thorpe CT, et al. The impact of medication-based risk adjustment on the association between veteran health outcomes and dual health system use. J Gen Intern Med. 2017;32(9):967-973. doi:10.1007/s11606-017-4064-4
16. Kullgren JT, Fagerlin A, Kerr EA. Completing the MISSION: a blueprint for helping veterans make the most of new choices. J Gen Intern Med. 2020;35(5):1567-1570. doi:10.1007/s11606-019-05404-w
17. VA MISSION Act of 2018, 38 USC §101 (2018). https://www.govinfo.gov/app/details/USCODE-2018-title38/USCODE-2018-title38-partI-chap1-sec101
18. Vanneman ME, Phibbs CS, Dally SK, Trivedi AN, Yoon J. The impact of Medicaid enrollment on Veterans Health Administration enrollees’ behavioral health services use. Health Serv Res. 2018;53(suppl 3):5238-5259. doi:10.1111/1475-6773.13062
19. Sommers BD, Baicker K, Epstein AM. Mortality and access to care among adults after state Medicaid expansions. N Engl J Med. 2012;367(11):1025-1034. doi:10.1056/NEJMsa1202099
20. US Department of Veterans Affairs Office of Mental Health. 2019 national veteran suicide prevention annual report. 2019. Accessed September 29, 2022. https://www.mentalhealth.va.gov/docs/data-sheets/2019/2019_National_Veteran_Suicide_Prevention_Annual_Report_508.pdf
21. Hawton K, Casañas I Comabella C, Haw C, Saunders K. Risk factors for suicide in individuals with depression: a systematic review. J Affect Disord. 2013;147(1-3):17-28. doi:10.1016/j.jad.2013.01.004
22. Adekkanattu P, Sholle ET, DeFerio J, Pathak J, Johnson SB, Campion TR Jr. Ascertaining depression severity by extracting Patient Health Questionnaire-9 (PHQ-9) scores from clinical notes. AMIA Annu Symp Proc. 2018;2018:147-156.
23. DeRubeis RJ, Siegle GJ, Hollon SD. Cognitive therapy versus medication for depression: treatment outcomes and neural mechanisms. Nat Rev Neurosci. 2008;9(10):788-796. doi:10.1038/nrn2345
24. Cully JA, Zimmer M, Khan MM, Petersen LA. Quality of depression care and its impact on health service use and mortality among veterans. Psychiatr Serv. 2008;59(12):1399-1405. doi:10.1176/ps.2008.59.12.1399
25. Byrne MM, Kuebeler M, Pietz K, Petersen LA. Effect of using information from only one system for dually eligible health care users. Med Care. 2006;44(8):768-773. doi:10.1097/01.mlr.0000218786.44722.14
26. Watkins KE, Smith B, Akincigil A, et al. The quality of medication treatment for mental disorders in the Department of Veterans Affairs and in private-sector plans. Psychiatr Serv. 2016;67(4):391-396. doi:10.1176/appi.ps.201400537
27. Petersen LA, Byrne MM, Daw CN, Hasche J, Reis B, Pietz K. Relationship between clinical conditions and use of Veterans Affairs health care among Medicare-enrolled veterans. Health Serv Res. 2010;45(3):762-791. doi:10.1111/j.1475-6773.2010.01107.x
28. Yoon J, Vanneman ME, Dally SK, Trivedi AN, Phibbs Ciaran S. Use of Veterans Affairs and Medicaid services for dually enrolled veterans. Health Serv Res. 2018;53(3):1539-1561. doi:10.1111/1475-6773.12727
29. Yoon J, Vanneman ME, Dally SK, Trivedi AN, Phibbs Ciaran S. Veterans’ reliance on VA care by type of service and distance to VA for nonelderly VA-Medicaid dual enrollees. Med Care. 2019;57(3):225-229. doi:10.1097/MLR.0000000000001066
30. Gaglioti A, Cozad A, Wittrock S, et al. Non-VA primary care providers’ perspectives on comanagement for rural veterans. Mil Med. 2014;179(11):1236-1243. doi:10.7205/MILMED-D-13-00342
31. Moon S, Shin J. Health care utilization among Medicare-Medicaid dual eligibles: a count data analysis. BMC Public Health. 2006;6(1):88. doi:10.1186/1471-2458-6-88
32. Henry J. Kaiser Family Foundation. Facilitating access to mental health services: a look at Medicaid, private insurance, and the uninsured. November 27, 2017. Accessed September 29, 2022. https://www.kff.org/medicaid/fact-sheet/facilitating-access-to-mental-health-services-a-look-at-medicaid-private-insurance-and-the-uninsured
33. Baicker K, Taubman SL, Allen HL, et al. The Oregon experiment - effects of Medicaid on clinical outcomes. N Engl J Med. 2013;368(18):1713-1722. doi:10.1056/NEJMsa1212321
34. Tanielian T, Farris C, Batka C, et al. Ready to serve: community-based provider capacity to deliver culturally competent, quality mental health care to veterans and their families. 2014. Accessed September 29, 2022. https://www.rand.org/content/dam/rand/pubs/research_reports/RR800/RR806/RAND_RR806.pdf
35. Kizer KW, Dudley RA. Extreme makeover: transformation of the Veterans Health Care System. Annu Rev Public Health. 2009;30(1):313-339. doi:10.1146/annurev.publhealth.29.020907.090940
36. Brennan KJ. Kendra’s Law: final report on the status of assisted outpatient treatment, appendix 2. 2002. Accessed September 29, 2022. https://omh.ny.gov/omhweb/kendra_web/finalreport/appendix2.htm
The US Department of Veterans Affairs (VA) is the largest integrated health care system in the United States, providing care for more than 9 million veterans.1 With veterans experiencing mental health conditions like posttraumatic stress disorder (PTSD), substance use disorders, and other serious mental illnesses (SMI) at higher rates compared with the general population, the VA plays an important role in the provision of mental health services.2-5 Since the implementation of its Mental Health Strategic Plan in 2004, the VA has overseen the development of a wide array of mental health programs geared toward the complex needs of veterans. Research has demonstrated VA care outperforming Medicaid-reimbursed services in terms of the percentage of veterans filling antidepressants for at least 12 weeks after initiation of treatment for major depressive disorder (MDD), as well as posthospitalization follow-up.6
Eligible veterans enrolled in the VA often also seek non-VA care. Medicaid covers nearly 10% of all nonelderly veterans, and of these veterans, 39% rely solely on Medicaid for health care access.7 Today, Medicaid is the largest payer for mental health services in the US, providing coverage for approximately 27% of Americans who have SMI and helping fulfill unmet mental health needs.8,9 Understanding which of these systems veterans choose to use, and under which circumstances, is essential in guiding the allocation of limited health care resources.10
Beyond Medicaid, alternatives to VA care may include TRICARE, Medicare, Indian Health Services, and employer-based or self-purchased private insurance. While these options potentially increase convenience, choice, and access to health care practitioners (HCPs) and services not available at local VA systems, cross-system utilization with poor integration may cause care coordination and continuity problems, such as medication mismanagement and opioid overdose, unnecessary duplicate utilization, and possible increased mortality.11-15 As recent national legislative changes, such as the Patient Protection and Affordable Care Act (ACA), Veterans Access, Choice and Accountability Act, and the VA MISSION Act, continue to shift the health care landscape for veterans, questions surrounding how veterans are changing their health care use become significant.16,17
Here, we approach the impacts of Medicaid expansion on veterans’ reliance on the VA for mental health services with a unique lens. We leverage a difference-in-difference design to study 2 historical Medicaid expansions in Arizona (AZ) and New York (NY), which extended eligibility to childless adults in 2001. Prior Medicaid dual-eligible mental health research investigated reliance shifts during the immediate postenrollment year in a subset of veterans newly enrolled in Medicaid.18 However, this study took place in a period of relative policy stability. In contrast, we investigate the potential effects of a broad policy shift by analyzing state-level changes in veterans’ reliance over 6 years after a statewide Medicaid expansion. We match expansion states with demographically similar nonexpansion states to account for unobserved trends and confounding effects. Prior studies have used this method to evaluate post-Medicaid expansion mortality changes and changes in veteran dual enrollment and hospitalizations.10,19 While a study of ACA Medicaid expansion states would be ideal, Medicaid data from most states were only available through 2014 at the time of this analysis. Our study offers a quasi-experimental framework leveraging longitudinal data that can be applied as more post-ACA data become available.
Given the rising incidence of suicide among veterans, understanding care-seeking behaviors for depression among veterans is important as it is the most common psychiatric condition found in those who died by suicide.20,21 Furthermore, depression may be useful as a clinical proxy for mental health policy impacts, given that the Patient Health Questionnaire-9 (PHQ-9) screening tool is well validated and increasingly research accessible, and it is a chronic condition responsive to both well-managed pharmacologic treatment and psychotherapeutic interventions.22,23
In this study, we quantify the change in care-seeking behavior for depression among veterans after Medicaid expansion, using a quasi-experimental design. We hypothesize that new access to Medicaid would be associated with a shift away from using VA services for depression. Given the income-dependent eligibility requirements of Medicaid, we also hypothesize that veterans who qualified for VA coverage due to low income, determined by a regional means test (Priority group 5, “income-eligible”), would be more likely to shift care compared with those whose serviced-connected conditions related to their military service (Priority groups 1-4, “service-connected”) provide VA access.
Methods
To investigate the relative changes in veterans’ reliance on the VA for depression care after the 2001 NY and AZ Medicaid expansions We used a retrospective, difference-in-difference analysis. Our comparison pairings, based on prior demographic analyses were as follows: NY with Pennsylvania(PA); AZ with New Mexico and Nevada (NM/NV).19 The time frame of our analysis was 1999 to 2006, with pre- and postexpansion periods defined as 1999 to 2000 and 2001 to 2006, respectively.
Data
We included veterans aged 18 to 64 years, seeking care for depression from 1999 to 2006, who were also VA-enrolled and residing in our states of interest. We counted veterans as enrolled in Medicaid if they were enrolled at least 1 month in a given year.
Using similar methods like those used in prior studies, we selected patients with encounters documenting depression as the primary outpatient or inpatient diagnosis using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes: 296.2x for a single episode of major depressive disorder, 296.3x for a recurrent episode of MDD, 300.4 for dysthymia, and 311.0 for depression not otherwise specified.18,24 We used data from the Medicaid Analytic eXtract files (MAX) for Medicaid data and the VA Corporate Data Warehouse (CDW) for VA data. We chose 1999 as the first study year because it was the earliest year MAX data were available.
Our final sample included 1833 person-years pre-expansion and 7157 postexpansion in our inpatient analysis, as well as 31,767 person-years pre-expansion and 130,382 postexpansion in our outpatient analysis.
Outcomes and Variables
Our primary outcomes were comparative shifts in VA reliance between expansion and nonexpansion states after Medicaid expansion for both inpatient and outpatient depression care. For each year of study, we calculated a veteran’s VA reliance by aggregating the number of days with depression-related encounters at the VA and dividing by the total number of days with a VA or Medicaid depression-related encounters for the year. To provide context to these shifts in VA reliance, we further analyzed the changes in the proportion of annual VA-Medicaid dual users and annual per capita utilization of depression care across the VA and Medicaid.
We conducted subanalyses by income-eligible and service-connected veterans and adjusted our models for age, non-White race, sex, distances to the nearest inpatient and outpatient VA facilities, and VA Relative Risk Score, which is a measure of disease burden and clinical complexity validated specifically for veterans.25
Statistical Analysis
We used fractional logistic regression to model the adjusted effect of Medicaid expansion on VA reliance for depression care. In parallel, we leveraged ordered logit regression and negative binomial regression models to examine the proportion of VA-Medicaid dual users and the per capita utilization of Medicaid and VA depression care, respectively. To estimate the difference-in-difference effects, we used the interaction term of 2 categorical variables—expansion vs nonexpansion states and pre- vs postexpansion status—as the independent variable. We then calculated the average marginal effects with 95% CIs to estimate the differences in outcomes between expansion and nonexpansion states from pre- to postexpansion periods, as well as year-by-year shifts as a robustness check. We conducted these analyses using Stata MP, version 15.
Results
Baseline and postexpansion characteristics
VA Reliance
Overall, we observed postexpansion decreases in VA reliance for depression care
At the state level, reliance on the VA for inpatient depression care in NY decreased by 13.53 pp (95% CI, -22.58 to -4.49) for income-eligible veterans and 16.67 pp (95% CI, -24.53 to -8.80) for service-connected veterans. No relative differences were observed in the outpatient comparisons for both income-eligible (-0.58 pp; 95% CI, -2.13 to 0.98) and service-connected (0.05 pp; 95% CI, -1.00 to 1.10) veterans. In AZ, Medicaid expansion was associated with decreased VA reliance for outpatient depression care among income-eligible veterans (-8.60 pp; 95% CI, -10.60 to -6.61), greater than that for service-connected veterans (-2.89 pp; 95% CI, -4.02 to -1.77). This decrease in VA reliance was significant in the inpatient context only for service-connected veterans (-4.55 pp; 95% CI, -8.14 to -0.97), not income-eligible veterans (-8.38 pp; 95% CI, -17.91 to 1.16).
By applying the aggregate pp changes toward the postexpansion number of visits across both expansion and nonexpansion states, we found that expansion of Medicaid across all our study states would have resulted in 996 fewer hospitalizations and 10,109 fewer outpatient visits for depression at VA in the postexpansion period vs if no states had chosen to expand Medicaid.
Dual Use/Per Capita Utilization
Overall, Medicaid expansion was associated with greater dual use for inpatient depression care—a 0.97-pp (95% CI, 0.46 to 1.48) increase among service-connected veterans and a 0.64-pp (95% CI, 0.35 to 0.94) increase among income-eligible veterans.
At the state level, NY similarly showed increases in dual use among both service-connected (1.48 pp; 95% CI, 0.80 to 2.16) and income-eligible veterans (0.73 pp; 95% CI, 0.39 to 1.07) after Medicaid expansion. However, dual use in AZ increased significantly only among service-connected veterans (0.70 pp; 95% CI, 0.03 to 1.38), not income-eligible veterans (0.31 pp; 95% CI, -0.17 to 0.78).
Among outpatient visits, Medicaid expansion was associated with increased dual use only for income-eligible veterans (0.16 pp; 95% CI, 0.03-0.29), and not service-connected veterans (0.09 pp; 95% CI, -0.04 to 0.21). State-level analyses showed that Medicaid expansion in NY was not associated with changes in dual use for either service-connected (0.01 pp; 95% CI, -0.16 to 0.17) or income-eligible veterans (0.03 pp; 95% CI, -0.12 to 0.18), while expansion in AZ was associated with increases in dual use among both service-connected (0.42 pp; 95% CI, 0.23 to 0.61) and income-eligible veterans (0.83 pp; 95% CI, 0.59 to 1.07).
Concerning per capita utilization of depression care after Medicaid expansion, analyses showed no detectable changes for either inpatient or outpatient services, among both service-connected and income-eligible veterans. However, while this pattern held at the state level among hospitalizations, outpatient visit results showed divergent trends between AZ and NY. In NY, Medicaid expansion was associated with decreased per capita utilization of outpatient depression care among both service-connected (-0.25 visits annually; 95% CI, -0.48 to -0.01) and income-eligible veterans (-0.64 visits annually; 95% CI, -0.93 to -0.35). In AZ, Medicaid expansion was associated with increased per capita utilization of outpatient depression care among both service-connected (0.62 visits annually; 95% CI, 0.32-0.91) and income-eligible veterans (2.32 visits annually; 95% CI, 1.99-2.65).
Discussion
Our study quantified changes in depression-related health care utilization after Medicaid expansions in NY and AZ in 2001. Overall, the balance of evidence indicated that Medicaid expansion was associated with decreased reliance on the VA for depression-related services. There was an exception: income-eligible veterans in AZ did not shift their hospital care away from the VA in a statistically discernible way, although the point estimate was lower. More broadly, these findings concerning veterans’ reliance varied not only in inpatient vs outpatient services and income- vs service-connected eligibility, but also in the state-level contexts of veteran dual users and per capita utilization.
Given that the overall per capita utilization of depression care was unchanged from pre- to postexpansion periods, one might interpret the decreases in VA reliance and increases in Medicaid-VA dual users as a substitution effect from VA care to non-VA care. This could be plausible for hospitalizations where state-level analyses showed similarly stable levels of per capita utilization. However, state-level trends in our outpatient utilization analysis, especially with a substantial 2.32 pp increase in annual per capita visits among income-eligible veterans in AZ, leave open the possibility that in some cases veterans may be complementing VA care with Medicaid-reimbursed services.
The causes underlying these differences in reliance shifts between NY and AZ are likely also influenced by the policy contexts of their respective Medicaid expansions. For example, in 1999, NY passed Kendra’s Law, which established a procedure for obtaining court orders for assisted outpatient mental health treatment for individuals deemed unlikely to survive safely in the community.26 A reasonable inference is that there was less unfulfilled outpatient mental health need in NY under the existing accessibility provisioned by Kendra’s Law. In addition, while both states extended coverage to childless adults under 100% of the Federal Poverty level (FPL), the AZ Medicaid expansion was via a voters’ initiative and extended family coverage to 200% FPL vs 150% FPL for families in NY. Given that the AZ Medicaid expansion enjoyed both broader public participation and generosity in terms of eligibility, its uptake and therefore effect size may have been larger than in NY for nonacute outpatient care.
Our findings contribute to the growing body of literature surrounding the changes in health care utilization after Medicaid expansion, specifically for a newly dual-eligible population of veterans seeking mental health services for depression. While prior research concerning Medicare dual-enrolled veterans has shown high reliance on the VA for both mental health diagnoses and services, scholars have established the association of Medicaid enrollment with decreased VA reliance.27-29 Our analysis is the first to investigate state-level effects of Medicaid expansion on VA reliance for a single mental health condition using a natural experimental framework. We focus on a population that includes a large portion of veterans who are newly Medicaid-eligible due to a sweeping policy change and use demographically matched nonexpansion states to draw comparisons in VA reliance for depression care. Our findings of Medicaid expansion–associated decreases in VA reliance for depression care complement prior literature that describe Medicaid enrollment–associated decreases in VA reliance for overall mental health care.
Implications
From a systems-level perspective, the implications of shifting services away from the VA are complex and incompletely understood. The VA lacks interoperability with the electronic health records (EHRs) used by Medicaid clinicians. Consequently, significant issues of service duplication and incomplete clinical data exist for veterans seeking treatment outside of the VA system, posing health care quality and safety concerns.30 On one hand, Medicaid access is associated with increased health care utilization attributed to filling unmet needs for Medicare dual enrollees, as well as increased prescription filling for psychiatric medications.31,32 Furthermore, the only randomized control trial of Medicaid expansion to date was associated with a 9-pp decrease in positive screening rates for depression among those who received access at around 2 years postexpansion.33 On the other hand, the VA has developed a mental health system tailored to the particular needs of veterans, and health care practitioners at the VA have significantly greater rates of military cultural competency compared to those in nonmilitary settings (70% vs 24% in the TRICARE network and 8% among those with no military or TRICARE affiliation).34 Compared to individuals seeking mental health services with private insurance plans, veterans were about twice as likely to receive appropriate treatment for schizophrenia and depression at the VA.35 These documented strengths of VA mental health care may together help explain the small absolute number of visits that were associated with shifts away from VA overall after Medicaid expansion.
Finally, it is worth considering extrinsic factors that influence utilization among newly dual-eligible veterans. For example, hospitalizations are less likely to be planned than outpatient services, translating to a greater importance of proximity to a nearby medical facility than a veteran’s preference of where to seek care. In the same vein, major VA medical centers are fewer and more distant on average than VA outpatient clinics, therefore reducing the advantage of a Medicaid-reimbursed outpatient clinic in terms of distance.36 These realities may partially explain the proportionally larger shifts away from the VA for hospitalizations compared to outpatient care for depression.
Limitations and Future Directions
Our results should be interpreted within methodological and data limitations. With only 2 states in our sample, NY demonstrably skewed overall results, contributing 1.7 to 3 times more observations than AZ across subanalyses—a challenge also cited by Sommers and colleagues.19 Our veteran groupings were also unable to distinguish those veterans classified as service-connected who may also have qualified by income-eligible criteria (which would tend to understate the size of results) and those veterans who gained and then lost Medicaid coverage in a given year. Our study also faces limitations in generalizability and establishing causality. First, we included only 2 historical state Medicaid expansions, compared with the 38 states and Washington, DC, that have now expanded Medicaid to date under the ACA. Just in the 2 states from our study, we noted significant heterogeneity in the shifts associated with Medicaid expansion, which makes extrapolating specific trends difficult. Differences in underlying health care resources, legislation, and other external factors may limit the applicability of Medicaid expansion in the era of the ACA, as well as the Veterans Choice and MISSION acts. Second, while we leveraged a difference-in-difference analysis using demographically matched, neighboring comparison states, our findings are nevertheless drawn from observational data obviating causality. VA data for other sources of coverage such as private insurance are limited and not included in our study, and MAX datasets vary by quality across states, translating to potential gaps in our study cohort.28
Moving forward, our study demonstrates the potential for applying a natural experimental approach to studying dual-eligible veterans at the interface of Medicaid expansion. We focused on changes in VA reliance for the specific condition of depression and, in doing so, invite further inquiry into the impact of state mental health policy on outcomes more proximate to veterans’ outcomes. Clinical indicators, such as rates of antidepressant filling, utilization and duration of psychotherapy, and PHQ-9 scores, can similarly be investigated by natural experimental design. While current limits of administrative data and the siloing of EHRs may pose barriers to some of these avenues of research, multidisciplinary methodologies and data querying innovations such as natural language processing algorithms for clinical notes hold exciting opportunities to bridge the gap between policy and clinical efficacy.
Conclusions
This study applied a difference-in-difference analysis and found that Medicaid expansion is associated with decreases in VA reliance for both inpatient and outpatient services for depression. As additional data are generated from the Medicaid expansions of the ACA, similarly robust methods should be applied to further explore the impacts associated with such policy shifts and open the door to a better understanding of implications at the clinical level.
Acknowledgments
We acknowledge the efforts of Janine Wong, who proofread and formatted the manuscript.
The US Department of Veterans Affairs (VA) is the largest integrated health care system in the United States, providing care for more than 9 million veterans.1 With veterans experiencing mental health conditions like posttraumatic stress disorder (PTSD), substance use disorders, and other serious mental illnesses (SMI) at higher rates compared with the general population, the VA plays an important role in the provision of mental health services.2-5 Since the implementation of its Mental Health Strategic Plan in 2004, the VA has overseen the development of a wide array of mental health programs geared toward the complex needs of veterans. Research has demonstrated VA care outperforming Medicaid-reimbursed services in terms of the percentage of veterans filling antidepressants for at least 12 weeks after initiation of treatment for major depressive disorder (MDD), as well as posthospitalization follow-up.6
Eligible veterans enrolled in the VA often also seek non-VA care. Medicaid covers nearly 10% of all nonelderly veterans, and of these veterans, 39% rely solely on Medicaid for health care access.7 Today, Medicaid is the largest payer for mental health services in the US, providing coverage for approximately 27% of Americans who have SMI and helping fulfill unmet mental health needs.8,9 Understanding which of these systems veterans choose to use, and under which circumstances, is essential in guiding the allocation of limited health care resources.10
Beyond Medicaid, alternatives to VA care may include TRICARE, Medicare, Indian Health Services, and employer-based or self-purchased private insurance. While these options potentially increase convenience, choice, and access to health care practitioners (HCPs) and services not available at local VA systems, cross-system utilization with poor integration may cause care coordination and continuity problems, such as medication mismanagement and opioid overdose, unnecessary duplicate utilization, and possible increased mortality.11-15 As recent national legislative changes, such as the Patient Protection and Affordable Care Act (ACA), Veterans Access, Choice and Accountability Act, and the VA MISSION Act, continue to shift the health care landscape for veterans, questions surrounding how veterans are changing their health care use become significant.16,17
Here, we approach the impacts of Medicaid expansion on veterans’ reliance on the VA for mental health services with a unique lens. We leverage a difference-in-difference design to study 2 historical Medicaid expansions in Arizona (AZ) and New York (NY), which extended eligibility to childless adults in 2001. Prior Medicaid dual-eligible mental health research investigated reliance shifts during the immediate postenrollment year in a subset of veterans newly enrolled in Medicaid.18 However, this study took place in a period of relative policy stability. In contrast, we investigate the potential effects of a broad policy shift by analyzing state-level changes in veterans’ reliance over 6 years after a statewide Medicaid expansion. We match expansion states with demographically similar nonexpansion states to account for unobserved trends and confounding effects. Prior studies have used this method to evaluate post-Medicaid expansion mortality changes and changes in veteran dual enrollment and hospitalizations.10,19 While a study of ACA Medicaid expansion states would be ideal, Medicaid data from most states were only available through 2014 at the time of this analysis. Our study offers a quasi-experimental framework leveraging longitudinal data that can be applied as more post-ACA data become available.
Given the rising incidence of suicide among veterans, understanding care-seeking behaviors for depression among veterans is important as it is the most common psychiatric condition found in those who died by suicide.20,21 Furthermore, depression may be useful as a clinical proxy for mental health policy impacts, given that the Patient Health Questionnaire-9 (PHQ-9) screening tool is well validated and increasingly research accessible, and it is a chronic condition responsive to both well-managed pharmacologic treatment and psychotherapeutic interventions.22,23
In this study, we quantify the change in care-seeking behavior for depression among veterans after Medicaid expansion, using a quasi-experimental design. We hypothesize that new access to Medicaid would be associated with a shift away from using VA services for depression. Given the income-dependent eligibility requirements of Medicaid, we also hypothesize that veterans who qualified for VA coverage due to low income, determined by a regional means test (Priority group 5, “income-eligible”), would be more likely to shift care compared with those whose serviced-connected conditions related to their military service (Priority groups 1-4, “service-connected”) provide VA access.
Methods
To investigate the relative changes in veterans’ reliance on the VA for depression care after the 2001 NY and AZ Medicaid expansions We used a retrospective, difference-in-difference analysis. Our comparison pairings, based on prior demographic analyses were as follows: NY with Pennsylvania(PA); AZ with New Mexico and Nevada (NM/NV).19 The time frame of our analysis was 1999 to 2006, with pre- and postexpansion periods defined as 1999 to 2000 and 2001 to 2006, respectively.
Data
We included veterans aged 18 to 64 years, seeking care for depression from 1999 to 2006, who were also VA-enrolled and residing in our states of interest. We counted veterans as enrolled in Medicaid if they were enrolled at least 1 month in a given year.
Using similar methods like those used in prior studies, we selected patients with encounters documenting depression as the primary outpatient or inpatient diagnosis using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes: 296.2x for a single episode of major depressive disorder, 296.3x for a recurrent episode of MDD, 300.4 for dysthymia, and 311.0 for depression not otherwise specified.18,24 We used data from the Medicaid Analytic eXtract files (MAX) for Medicaid data and the VA Corporate Data Warehouse (CDW) for VA data. We chose 1999 as the first study year because it was the earliest year MAX data were available.
Our final sample included 1833 person-years pre-expansion and 7157 postexpansion in our inpatient analysis, as well as 31,767 person-years pre-expansion and 130,382 postexpansion in our outpatient analysis.
Outcomes and Variables
Our primary outcomes were comparative shifts in VA reliance between expansion and nonexpansion states after Medicaid expansion for both inpatient and outpatient depression care. For each year of study, we calculated a veteran’s VA reliance by aggregating the number of days with depression-related encounters at the VA and dividing by the total number of days with a VA or Medicaid depression-related encounters for the year. To provide context to these shifts in VA reliance, we further analyzed the changes in the proportion of annual VA-Medicaid dual users and annual per capita utilization of depression care across the VA and Medicaid.
We conducted subanalyses by income-eligible and service-connected veterans and adjusted our models for age, non-White race, sex, distances to the nearest inpatient and outpatient VA facilities, and VA Relative Risk Score, which is a measure of disease burden and clinical complexity validated specifically for veterans.25
Statistical Analysis
We used fractional logistic regression to model the adjusted effect of Medicaid expansion on VA reliance for depression care. In parallel, we leveraged ordered logit regression and negative binomial regression models to examine the proportion of VA-Medicaid dual users and the per capita utilization of Medicaid and VA depression care, respectively. To estimate the difference-in-difference effects, we used the interaction term of 2 categorical variables—expansion vs nonexpansion states and pre- vs postexpansion status—as the independent variable. We then calculated the average marginal effects with 95% CIs to estimate the differences in outcomes between expansion and nonexpansion states from pre- to postexpansion periods, as well as year-by-year shifts as a robustness check. We conducted these analyses using Stata MP, version 15.
Results
Baseline and postexpansion characteristics
VA Reliance
Overall, we observed postexpansion decreases in VA reliance for depression care
At the state level, reliance on the VA for inpatient depression care in NY decreased by 13.53 pp (95% CI, -22.58 to -4.49) for income-eligible veterans and 16.67 pp (95% CI, -24.53 to -8.80) for service-connected veterans. No relative differences were observed in the outpatient comparisons for both income-eligible (-0.58 pp; 95% CI, -2.13 to 0.98) and service-connected (0.05 pp; 95% CI, -1.00 to 1.10) veterans. In AZ, Medicaid expansion was associated with decreased VA reliance for outpatient depression care among income-eligible veterans (-8.60 pp; 95% CI, -10.60 to -6.61), greater than that for service-connected veterans (-2.89 pp; 95% CI, -4.02 to -1.77). This decrease in VA reliance was significant in the inpatient context only for service-connected veterans (-4.55 pp; 95% CI, -8.14 to -0.97), not income-eligible veterans (-8.38 pp; 95% CI, -17.91 to 1.16).
By applying the aggregate pp changes toward the postexpansion number of visits across both expansion and nonexpansion states, we found that expansion of Medicaid across all our study states would have resulted in 996 fewer hospitalizations and 10,109 fewer outpatient visits for depression at VA in the postexpansion period vs if no states had chosen to expand Medicaid.
Dual Use/Per Capita Utilization
Overall, Medicaid expansion was associated with greater dual use for inpatient depression care—a 0.97-pp (95% CI, 0.46 to 1.48) increase among service-connected veterans and a 0.64-pp (95% CI, 0.35 to 0.94) increase among income-eligible veterans.
At the state level, NY similarly showed increases in dual use among both service-connected (1.48 pp; 95% CI, 0.80 to 2.16) and income-eligible veterans (0.73 pp; 95% CI, 0.39 to 1.07) after Medicaid expansion. However, dual use in AZ increased significantly only among service-connected veterans (0.70 pp; 95% CI, 0.03 to 1.38), not income-eligible veterans (0.31 pp; 95% CI, -0.17 to 0.78).
Among outpatient visits, Medicaid expansion was associated with increased dual use only for income-eligible veterans (0.16 pp; 95% CI, 0.03-0.29), and not service-connected veterans (0.09 pp; 95% CI, -0.04 to 0.21). State-level analyses showed that Medicaid expansion in NY was not associated with changes in dual use for either service-connected (0.01 pp; 95% CI, -0.16 to 0.17) or income-eligible veterans (0.03 pp; 95% CI, -0.12 to 0.18), while expansion in AZ was associated with increases in dual use among both service-connected (0.42 pp; 95% CI, 0.23 to 0.61) and income-eligible veterans (0.83 pp; 95% CI, 0.59 to 1.07).
Concerning per capita utilization of depression care after Medicaid expansion, analyses showed no detectable changes for either inpatient or outpatient services, among both service-connected and income-eligible veterans. However, while this pattern held at the state level among hospitalizations, outpatient visit results showed divergent trends between AZ and NY. In NY, Medicaid expansion was associated with decreased per capita utilization of outpatient depression care among both service-connected (-0.25 visits annually; 95% CI, -0.48 to -0.01) and income-eligible veterans (-0.64 visits annually; 95% CI, -0.93 to -0.35). In AZ, Medicaid expansion was associated with increased per capita utilization of outpatient depression care among both service-connected (0.62 visits annually; 95% CI, 0.32-0.91) and income-eligible veterans (2.32 visits annually; 95% CI, 1.99-2.65).
Discussion
Our study quantified changes in depression-related health care utilization after Medicaid expansions in NY and AZ in 2001. Overall, the balance of evidence indicated that Medicaid expansion was associated with decreased reliance on the VA for depression-related services. There was an exception: income-eligible veterans in AZ did not shift their hospital care away from the VA in a statistically discernible way, although the point estimate was lower. More broadly, these findings concerning veterans’ reliance varied not only in inpatient vs outpatient services and income- vs service-connected eligibility, but also in the state-level contexts of veteran dual users and per capita utilization.
Given that the overall per capita utilization of depression care was unchanged from pre- to postexpansion periods, one might interpret the decreases in VA reliance and increases in Medicaid-VA dual users as a substitution effect from VA care to non-VA care. This could be plausible for hospitalizations where state-level analyses showed similarly stable levels of per capita utilization. However, state-level trends in our outpatient utilization analysis, especially with a substantial 2.32 pp increase in annual per capita visits among income-eligible veterans in AZ, leave open the possibility that in some cases veterans may be complementing VA care with Medicaid-reimbursed services.
The causes underlying these differences in reliance shifts between NY and AZ are likely also influenced by the policy contexts of their respective Medicaid expansions. For example, in 1999, NY passed Kendra’s Law, which established a procedure for obtaining court orders for assisted outpatient mental health treatment for individuals deemed unlikely to survive safely in the community.26 A reasonable inference is that there was less unfulfilled outpatient mental health need in NY under the existing accessibility provisioned by Kendra’s Law. In addition, while both states extended coverage to childless adults under 100% of the Federal Poverty level (FPL), the AZ Medicaid expansion was via a voters’ initiative and extended family coverage to 200% FPL vs 150% FPL for families in NY. Given that the AZ Medicaid expansion enjoyed both broader public participation and generosity in terms of eligibility, its uptake and therefore effect size may have been larger than in NY for nonacute outpatient care.
Our findings contribute to the growing body of literature surrounding the changes in health care utilization after Medicaid expansion, specifically for a newly dual-eligible population of veterans seeking mental health services for depression. While prior research concerning Medicare dual-enrolled veterans has shown high reliance on the VA for both mental health diagnoses and services, scholars have established the association of Medicaid enrollment with decreased VA reliance.27-29 Our analysis is the first to investigate state-level effects of Medicaid expansion on VA reliance for a single mental health condition using a natural experimental framework. We focus on a population that includes a large portion of veterans who are newly Medicaid-eligible due to a sweeping policy change and use demographically matched nonexpansion states to draw comparisons in VA reliance for depression care. Our findings of Medicaid expansion–associated decreases in VA reliance for depression care complement prior literature that describe Medicaid enrollment–associated decreases in VA reliance for overall mental health care.
Implications
From a systems-level perspective, the implications of shifting services away from the VA are complex and incompletely understood. The VA lacks interoperability with the electronic health records (EHRs) used by Medicaid clinicians. Consequently, significant issues of service duplication and incomplete clinical data exist for veterans seeking treatment outside of the VA system, posing health care quality and safety concerns.30 On one hand, Medicaid access is associated with increased health care utilization attributed to filling unmet needs for Medicare dual enrollees, as well as increased prescription filling for psychiatric medications.31,32 Furthermore, the only randomized control trial of Medicaid expansion to date was associated with a 9-pp decrease in positive screening rates for depression among those who received access at around 2 years postexpansion.33 On the other hand, the VA has developed a mental health system tailored to the particular needs of veterans, and health care practitioners at the VA have significantly greater rates of military cultural competency compared to those in nonmilitary settings (70% vs 24% in the TRICARE network and 8% among those with no military or TRICARE affiliation).34 Compared to individuals seeking mental health services with private insurance plans, veterans were about twice as likely to receive appropriate treatment for schizophrenia and depression at the VA.35 These documented strengths of VA mental health care may together help explain the small absolute number of visits that were associated with shifts away from VA overall after Medicaid expansion.
Finally, it is worth considering extrinsic factors that influence utilization among newly dual-eligible veterans. For example, hospitalizations are less likely to be planned than outpatient services, translating to a greater importance of proximity to a nearby medical facility than a veteran’s preference of where to seek care. In the same vein, major VA medical centers are fewer and more distant on average than VA outpatient clinics, therefore reducing the advantage of a Medicaid-reimbursed outpatient clinic in terms of distance.36 These realities may partially explain the proportionally larger shifts away from the VA for hospitalizations compared to outpatient care for depression.
Limitations and Future Directions
Our results should be interpreted within methodological and data limitations. With only 2 states in our sample, NY demonstrably skewed overall results, contributing 1.7 to 3 times more observations than AZ across subanalyses—a challenge also cited by Sommers and colleagues.19 Our veteran groupings were also unable to distinguish those veterans classified as service-connected who may also have qualified by income-eligible criteria (which would tend to understate the size of results) and those veterans who gained and then lost Medicaid coverage in a given year. Our study also faces limitations in generalizability and establishing causality. First, we included only 2 historical state Medicaid expansions, compared with the 38 states and Washington, DC, that have now expanded Medicaid to date under the ACA. Just in the 2 states from our study, we noted significant heterogeneity in the shifts associated with Medicaid expansion, which makes extrapolating specific trends difficult. Differences in underlying health care resources, legislation, and other external factors may limit the applicability of Medicaid expansion in the era of the ACA, as well as the Veterans Choice and MISSION acts. Second, while we leveraged a difference-in-difference analysis using demographically matched, neighboring comparison states, our findings are nevertheless drawn from observational data obviating causality. VA data for other sources of coverage such as private insurance are limited and not included in our study, and MAX datasets vary by quality across states, translating to potential gaps in our study cohort.28
Moving forward, our study demonstrates the potential for applying a natural experimental approach to studying dual-eligible veterans at the interface of Medicaid expansion. We focused on changes in VA reliance for the specific condition of depression and, in doing so, invite further inquiry into the impact of state mental health policy on outcomes more proximate to veterans’ outcomes. Clinical indicators, such as rates of antidepressant filling, utilization and duration of psychotherapy, and PHQ-9 scores, can similarly be investigated by natural experimental design. While current limits of administrative data and the siloing of EHRs may pose barriers to some of these avenues of research, multidisciplinary methodologies and data querying innovations such as natural language processing algorithms for clinical notes hold exciting opportunities to bridge the gap between policy and clinical efficacy.
Conclusions
This study applied a difference-in-difference analysis and found that Medicaid expansion is associated with decreases in VA reliance for both inpatient and outpatient services for depression. As additional data are generated from the Medicaid expansions of the ACA, similarly robust methods should be applied to further explore the impacts associated with such policy shifts and open the door to a better understanding of implications at the clinical level.
Acknowledgments
We acknowledge the efforts of Janine Wong, who proofread and formatted the manuscript.
1. US Department of Veterans Affairs, Veterans Health Administration. About VA. 2019. Updated September 27, 2022. Accessed September 29, 2022. https://www.va.gov/health/
2. Richardson LK, Frueh BC, Acierno R. Prevalence estimates of combat-related post-traumatic stress disorder: critical review. Aust N Z J Psychiatry. 2010;44(1):4-19. doi:10.3109/00048670903393597
3. Lan CW, Fiellin DA, Barry DT, et al. The epidemiology of substance use disorders in US veterans: a systematic review and analysis of assessment methods. Am J Addict. 2016;25(1):7-24. doi:10.1111/ajad.12319
4. Grant BF, Saha TD, June Ruan W, et al. Epidemiology of DSM-5 drug use disorder results from the national epidemiologic survey on alcohol and related conditions-III. JAMA Psychiat. 2016;73(1):39-47. doi:10.1001/jamapsychiatry.015.2132
5. Pemberton MR, Forman-Hoffman VL, Lipari RN, Ashley OS, Heller DC, Williams MR. Prevalence of past year substance use and mental illness by veteran status in a nationally representative sample. CBHSQ Data Review. Published November 9, 2016. Accessed October 6, 2022. https://www.samhsa.gov/data/report/prevalence-past-year-substance-use-and-mental-illness-veteran-status-nationally
6. Watkins KE, Pincus HA, Smith B, et al. Veterans Health Administration Mental Health Program Evaluation: Capstone Report. 2011. Accessed September 29, 2022. https://www.rand.org/pubs/technical_reports/TR956.html
7. Henry J. Kaiser Family Foundation. Medicaid’s role in covering veterans. June 29, 2017. Accessed September 29, 2022. https://www.kff.org/infographic/medicaids-role-in-covering-veterans
8. Substance Abuse and Mental Health Services Administration. Results from the 2016 National Survey on Drug Use and Health: detailed tables. September 7, 2017. Accessed September 29, 2022. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2016/NSDUH-DetTabs-2016.pdf
9. Wen H, Druss BG, Cummings JR. Effect of Medicaid expansions on health insurance coverage and access to care among low-income adults with behavioral health conditions. Health Serv Res. 2015;50:1787-1809. doi:10.1111/1475-6773.12411
10. O’Mahen PN, Petersen LA. Effects of state-level Medicaid expansion on Veterans Health Administration dual enrollment and utilization: potential implications for future coverage expansions. Med Care. 2020;58(6):526-533. doi:10.1097/MLR.0000000000001327
11. Ono SS, Dziak KM, Wittrock SM, et al. Treating dual-use patients across two health care systems: a qualitative study. Fed Pract. 2015;32(8):32-37.
12. Weeks WB, Mahar PJ, Wright SM. Utilization of VA and Medicare services by Medicare-eligible veterans: the impact of additional access points in a rural setting. J Healthc Manag. 2005;50(2):95-106.
13. Gellad WF, Thorpe JM, Zhao X, et al. Impact of dual use of Department of Veterans Affairs and Medicare part d drug benefits on potentially unsafe opioid use. Am J Public Health. 2018;108(2):248-255. doi:10.2105/AJPH.2017.304174
14. Coughlin SS, Young L. A review of dual health care system use by veterans with cardiometabolic disease. J Hosp Manag Health Policy. 2018;2:39. doi:10.21037/jhmhp.2018.07.05
15. Radomski TR, Zhao X, Thorpe CT, et al. The impact of medication-based risk adjustment on the association between veteran health outcomes and dual health system use. J Gen Intern Med. 2017;32(9):967-973. doi:10.1007/s11606-017-4064-4
16. Kullgren JT, Fagerlin A, Kerr EA. Completing the MISSION: a blueprint for helping veterans make the most of new choices. J Gen Intern Med. 2020;35(5):1567-1570. doi:10.1007/s11606-019-05404-w
17. VA MISSION Act of 2018, 38 USC §101 (2018). https://www.govinfo.gov/app/details/USCODE-2018-title38/USCODE-2018-title38-partI-chap1-sec101
18. Vanneman ME, Phibbs CS, Dally SK, Trivedi AN, Yoon J. The impact of Medicaid enrollment on Veterans Health Administration enrollees’ behavioral health services use. Health Serv Res. 2018;53(suppl 3):5238-5259. doi:10.1111/1475-6773.13062
19. Sommers BD, Baicker K, Epstein AM. Mortality and access to care among adults after state Medicaid expansions. N Engl J Med. 2012;367(11):1025-1034. doi:10.1056/NEJMsa1202099
20. US Department of Veterans Affairs Office of Mental Health. 2019 national veteran suicide prevention annual report. 2019. Accessed September 29, 2022. https://www.mentalhealth.va.gov/docs/data-sheets/2019/2019_National_Veteran_Suicide_Prevention_Annual_Report_508.pdf
21. Hawton K, Casañas I Comabella C, Haw C, Saunders K. Risk factors for suicide in individuals with depression: a systematic review. J Affect Disord. 2013;147(1-3):17-28. doi:10.1016/j.jad.2013.01.004
22. Adekkanattu P, Sholle ET, DeFerio J, Pathak J, Johnson SB, Campion TR Jr. Ascertaining depression severity by extracting Patient Health Questionnaire-9 (PHQ-9) scores from clinical notes. AMIA Annu Symp Proc. 2018;2018:147-156.
23. DeRubeis RJ, Siegle GJ, Hollon SD. Cognitive therapy versus medication for depression: treatment outcomes and neural mechanisms. Nat Rev Neurosci. 2008;9(10):788-796. doi:10.1038/nrn2345
24. Cully JA, Zimmer M, Khan MM, Petersen LA. Quality of depression care and its impact on health service use and mortality among veterans. Psychiatr Serv. 2008;59(12):1399-1405. doi:10.1176/ps.2008.59.12.1399
25. Byrne MM, Kuebeler M, Pietz K, Petersen LA. Effect of using information from only one system for dually eligible health care users. Med Care. 2006;44(8):768-773. doi:10.1097/01.mlr.0000218786.44722.14
26. Watkins KE, Smith B, Akincigil A, et al. The quality of medication treatment for mental disorders in the Department of Veterans Affairs and in private-sector plans. Psychiatr Serv. 2016;67(4):391-396. doi:10.1176/appi.ps.201400537
27. Petersen LA, Byrne MM, Daw CN, Hasche J, Reis B, Pietz K. Relationship between clinical conditions and use of Veterans Affairs health care among Medicare-enrolled veterans. Health Serv Res. 2010;45(3):762-791. doi:10.1111/j.1475-6773.2010.01107.x
28. Yoon J, Vanneman ME, Dally SK, Trivedi AN, Phibbs Ciaran S. Use of Veterans Affairs and Medicaid services for dually enrolled veterans. Health Serv Res. 2018;53(3):1539-1561. doi:10.1111/1475-6773.12727
29. Yoon J, Vanneman ME, Dally SK, Trivedi AN, Phibbs Ciaran S. Veterans’ reliance on VA care by type of service and distance to VA for nonelderly VA-Medicaid dual enrollees. Med Care. 2019;57(3):225-229. doi:10.1097/MLR.0000000000001066
30. Gaglioti A, Cozad A, Wittrock S, et al. Non-VA primary care providers’ perspectives on comanagement for rural veterans. Mil Med. 2014;179(11):1236-1243. doi:10.7205/MILMED-D-13-00342
31. Moon S, Shin J. Health care utilization among Medicare-Medicaid dual eligibles: a count data analysis. BMC Public Health. 2006;6(1):88. doi:10.1186/1471-2458-6-88
32. Henry J. Kaiser Family Foundation. Facilitating access to mental health services: a look at Medicaid, private insurance, and the uninsured. November 27, 2017. Accessed September 29, 2022. https://www.kff.org/medicaid/fact-sheet/facilitating-access-to-mental-health-services-a-look-at-medicaid-private-insurance-and-the-uninsured
33. Baicker K, Taubman SL, Allen HL, et al. The Oregon experiment - effects of Medicaid on clinical outcomes. N Engl J Med. 2013;368(18):1713-1722. doi:10.1056/NEJMsa1212321
34. Tanielian T, Farris C, Batka C, et al. Ready to serve: community-based provider capacity to deliver culturally competent, quality mental health care to veterans and their families. 2014. Accessed September 29, 2022. https://www.rand.org/content/dam/rand/pubs/research_reports/RR800/RR806/RAND_RR806.pdf
35. Kizer KW, Dudley RA. Extreme makeover: transformation of the Veterans Health Care System. Annu Rev Public Health. 2009;30(1):313-339. doi:10.1146/annurev.publhealth.29.020907.090940
36. Brennan KJ. Kendra’s Law: final report on the status of assisted outpatient treatment, appendix 2. 2002. Accessed September 29, 2022. https://omh.ny.gov/omhweb/kendra_web/finalreport/appendix2.htm
1. US Department of Veterans Affairs, Veterans Health Administration. About VA. 2019. Updated September 27, 2022. Accessed September 29, 2022. https://www.va.gov/health/
2. Richardson LK, Frueh BC, Acierno R. Prevalence estimates of combat-related post-traumatic stress disorder: critical review. Aust N Z J Psychiatry. 2010;44(1):4-19. doi:10.3109/00048670903393597
3. Lan CW, Fiellin DA, Barry DT, et al. The epidemiology of substance use disorders in US veterans: a systematic review and analysis of assessment methods. Am J Addict. 2016;25(1):7-24. doi:10.1111/ajad.12319
4. Grant BF, Saha TD, June Ruan W, et al. Epidemiology of DSM-5 drug use disorder results from the national epidemiologic survey on alcohol and related conditions-III. JAMA Psychiat. 2016;73(1):39-47. doi:10.1001/jamapsychiatry.015.2132
5. Pemberton MR, Forman-Hoffman VL, Lipari RN, Ashley OS, Heller DC, Williams MR. Prevalence of past year substance use and mental illness by veteran status in a nationally representative sample. CBHSQ Data Review. Published November 9, 2016. Accessed October 6, 2022. https://www.samhsa.gov/data/report/prevalence-past-year-substance-use-and-mental-illness-veteran-status-nationally
6. Watkins KE, Pincus HA, Smith B, et al. Veterans Health Administration Mental Health Program Evaluation: Capstone Report. 2011. Accessed September 29, 2022. https://www.rand.org/pubs/technical_reports/TR956.html
7. Henry J. Kaiser Family Foundation. Medicaid’s role in covering veterans. June 29, 2017. Accessed September 29, 2022. https://www.kff.org/infographic/medicaids-role-in-covering-veterans
8. Substance Abuse and Mental Health Services Administration. Results from the 2016 National Survey on Drug Use and Health: detailed tables. September 7, 2017. Accessed September 29, 2022. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2016/NSDUH-DetTabs-2016.pdf
9. Wen H, Druss BG, Cummings JR. Effect of Medicaid expansions on health insurance coverage and access to care among low-income adults with behavioral health conditions. Health Serv Res. 2015;50:1787-1809. doi:10.1111/1475-6773.12411
10. O’Mahen PN, Petersen LA. Effects of state-level Medicaid expansion on Veterans Health Administration dual enrollment and utilization: potential implications for future coverage expansions. Med Care. 2020;58(6):526-533. doi:10.1097/MLR.0000000000001327
11. Ono SS, Dziak KM, Wittrock SM, et al. Treating dual-use patients across two health care systems: a qualitative study. Fed Pract. 2015;32(8):32-37.
12. Weeks WB, Mahar PJ, Wright SM. Utilization of VA and Medicare services by Medicare-eligible veterans: the impact of additional access points in a rural setting. J Healthc Manag. 2005;50(2):95-106.
13. Gellad WF, Thorpe JM, Zhao X, et al. Impact of dual use of Department of Veterans Affairs and Medicare part d drug benefits on potentially unsafe opioid use. Am J Public Health. 2018;108(2):248-255. doi:10.2105/AJPH.2017.304174
14. Coughlin SS, Young L. A review of dual health care system use by veterans with cardiometabolic disease. J Hosp Manag Health Policy. 2018;2:39. doi:10.21037/jhmhp.2018.07.05
15. Radomski TR, Zhao X, Thorpe CT, et al. The impact of medication-based risk adjustment on the association between veteran health outcomes and dual health system use. J Gen Intern Med. 2017;32(9):967-973. doi:10.1007/s11606-017-4064-4
16. Kullgren JT, Fagerlin A, Kerr EA. Completing the MISSION: a blueprint for helping veterans make the most of new choices. J Gen Intern Med. 2020;35(5):1567-1570. doi:10.1007/s11606-019-05404-w
17. VA MISSION Act of 2018, 38 USC §101 (2018). https://www.govinfo.gov/app/details/USCODE-2018-title38/USCODE-2018-title38-partI-chap1-sec101
18. Vanneman ME, Phibbs CS, Dally SK, Trivedi AN, Yoon J. The impact of Medicaid enrollment on Veterans Health Administration enrollees’ behavioral health services use. Health Serv Res. 2018;53(suppl 3):5238-5259. doi:10.1111/1475-6773.13062
19. Sommers BD, Baicker K, Epstein AM. Mortality and access to care among adults after state Medicaid expansions. N Engl J Med. 2012;367(11):1025-1034. doi:10.1056/NEJMsa1202099
20. US Department of Veterans Affairs Office of Mental Health. 2019 national veteran suicide prevention annual report. 2019. Accessed September 29, 2022. https://www.mentalhealth.va.gov/docs/data-sheets/2019/2019_National_Veteran_Suicide_Prevention_Annual_Report_508.pdf
21. Hawton K, Casañas I Comabella C, Haw C, Saunders K. Risk factors for suicide in individuals with depression: a systematic review. J Affect Disord. 2013;147(1-3):17-28. doi:10.1016/j.jad.2013.01.004
22. Adekkanattu P, Sholle ET, DeFerio J, Pathak J, Johnson SB, Campion TR Jr. Ascertaining depression severity by extracting Patient Health Questionnaire-9 (PHQ-9) scores from clinical notes. AMIA Annu Symp Proc. 2018;2018:147-156.
23. DeRubeis RJ, Siegle GJ, Hollon SD. Cognitive therapy versus medication for depression: treatment outcomes and neural mechanisms. Nat Rev Neurosci. 2008;9(10):788-796. doi:10.1038/nrn2345
24. Cully JA, Zimmer M, Khan MM, Petersen LA. Quality of depression care and its impact on health service use and mortality among veterans. Psychiatr Serv. 2008;59(12):1399-1405. doi:10.1176/ps.2008.59.12.1399
25. Byrne MM, Kuebeler M, Pietz K, Petersen LA. Effect of using information from only one system for dually eligible health care users. Med Care. 2006;44(8):768-773. doi:10.1097/01.mlr.0000218786.44722.14
26. Watkins KE, Smith B, Akincigil A, et al. The quality of medication treatment for mental disorders in the Department of Veterans Affairs and in private-sector plans. Psychiatr Serv. 2016;67(4):391-396. doi:10.1176/appi.ps.201400537
27. Petersen LA, Byrne MM, Daw CN, Hasche J, Reis B, Pietz K. Relationship between clinical conditions and use of Veterans Affairs health care among Medicare-enrolled veterans. Health Serv Res. 2010;45(3):762-791. doi:10.1111/j.1475-6773.2010.01107.x
28. Yoon J, Vanneman ME, Dally SK, Trivedi AN, Phibbs Ciaran S. Use of Veterans Affairs and Medicaid services for dually enrolled veterans. Health Serv Res. 2018;53(3):1539-1561. doi:10.1111/1475-6773.12727
29. Yoon J, Vanneman ME, Dally SK, Trivedi AN, Phibbs Ciaran S. Veterans’ reliance on VA care by type of service and distance to VA for nonelderly VA-Medicaid dual enrollees. Med Care. 2019;57(3):225-229. doi:10.1097/MLR.0000000000001066
30. Gaglioti A, Cozad A, Wittrock S, et al. Non-VA primary care providers’ perspectives on comanagement for rural veterans. Mil Med. 2014;179(11):1236-1243. doi:10.7205/MILMED-D-13-00342
31. Moon S, Shin J. Health care utilization among Medicare-Medicaid dual eligibles: a count data analysis. BMC Public Health. 2006;6(1):88. doi:10.1186/1471-2458-6-88
32. Henry J. Kaiser Family Foundation. Facilitating access to mental health services: a look at Medicaid, private insurance, and the uninsured. November 27, 2017. Accessed September 29, 2022. https://www.kff.org/medicaid/fact-sheet/facilitating-access-to-mental-health-services-a-look-at-medicaid-private-insurance-and-the-uninsured
33. Baicker K, Taubman SL, Allen HL, et al. The Oregon experiment - effects of Medicaid on clinical outcomes. N Engl J Med. 2013;368(18):1713-1722. doi:10.1056/NEJMsa1212321
34. Tanielian T, Farris C, Batka C, et al. Ready to serve: community-based provider capacity to deliver culturally competent, quality mental health care to veterans and their families. 2014. Accessed September 29, 2022. https://www.rand.org/content/dam/rand/pubs/research_reports/RR800/RR806/RAND_RR806.pdf
35. Kizer KW, Dudley RA. Extreme makeover: transformation of the Veterans Health Care System. Annu Rev Public Health. 2009;30(1):313-339. doi:10.1146/annurev.publhealth.29.020907.090940
36. Brennan KJ. Kendra’s Law: final report on the status of assisted outpatient treatment, appendix 2. 2002. Accessed September 29, 2022. https://omh.ny.gov/omhweb/kendra_web/finalreport/appendix2.htm
New CDC guidance on prescribing opioids for pain
The 2022 Clinical Practice Guideline provides guidance on determining whether to initiate opioids for pain; selecting opioids and determining opioid dosages; deciding duration of initial opioid prescription and conducting follow-up; and assessing risk and addressing potential harms of opioid use.
“Patients with pain should receive compassionate, safe, and effective pain care. We want clinicians and patients to have the information they need to weigh the benefits of different approaches to pain care, with the goal of helping people reduce their pain and improve their quality of life,” Christopher M. Jones, PharmD, DrPH, acting director for the CDC’s National Center for Injury Prevention and Control, said in a news release.
How to taper safely
The last guideline on the topic was released by CDC in 2016. Since then, new evidence has emerged regarding the benefits and risks of prescription opioids for acute and chronic pain, comparisons with nonopioid pain treatments, dosing strategies, opioid dose-dependent effects, risk mitigation strategies, and opioid tapering and discontinuation, the CDC says.
A “critical” addition to the 2022 guideline is advice on tapering opioids, Dr. Jones said during a press briefing.
“Practical tips on how to taper in an individualized patient-centered manner have been added to help clinicians if the decision is made to taper opioids, and the guideline explicitly advises against abrupt discontinuation or rapid dose reductions of opioids,” Dr. Jones said.
“That is based on lessons learned over the last several years as well as new science about how we approach tapering and the real harms that can result when patients are abruptly discontinued or rapidly tapered,” he added.
The updated guideline was published online Nov. 3 in the Morbidity and Mortality Weekly Report.
Key recommendations in the 100-page document include the following:
- In determining whether or not to initiate opioids, nonopioid therapies are at least as effective as opioids for many common types of acute pain. Use of nondrug and nonopioid drug therapies should be maximized as appropriate, and opioid therapy should only be considered for acute pain if it is anticipated that benefits outweigh risks to the patient.
- Before starting opioid therapy, providers should discuss with patients the realistic benefits and known risks of opioid therapy.
- Before starting ongoing opioid therapy for patients with subacute pain lasting 1 to 3 months or chronic pain lasting more than 3 months, providers should work with patients to establish treatment goals for pain and function, and consideration should be given as to how opioid therapy will be discontinued if benefits do not outweigh risks.
- Once opioids are started, the lowest effective dose of immediate-release opioids should be prescribed for no longer than needed for the expected duration of pain severe enough to require opioids.
- Within 1 to 4 weeks of starting opioid therapy for subacute or chronic pain, providers should work with patients to evaluate and carefully weigh benefits and risks of continuing opioid therapy; care should be exercised when increasing, continuing, or reducing opioid dosage.
- Before starting and periodically during ongoing opioid therapy, providers should evaluate risk for opioid-related harms and should work with patients to incorporate relevant strategies to mitigate risk, including offering naloxone and reviewing potential interactions with any other prescribed medications or substance used.
- Abrupt discontinuation of opioids should be avoided, especially for patients receiving high doses.
- For treating patients with opioid use disorder, treatment with evidence-based medications should be provided, or arrangements for such treatment should be made.
Dr. Jones emphasized that the guideline is “voluntary and meant to guide shared decision-making between a clinician and patient. It’s not meant to be implemented as absolute limits of policy or practice by clinicians, health systems, insurance companies, governmental entities.”
He also noted that the “current state of the overdose crisis, which is very much driven by illicit synthetic opioids, is not the aim of this guideline.
“The release of this guideline is really about advancing pain care and improving the lives of patients living with pain,” he said.
“We know that at least 1 in 5 people in the country have chronic pain. It’s one of the most common reasons why people present to their health care provider, and the goal here is to advance pain care, function, and quality of life for that patient population, while also reducing misuse, diversion, and consequences of prescription opioid misuse,” Dr. Jones added.
A version of this article first appeared on Medscape.com.
The 2022 Clinical Practice Guideline provides guidance on determining whether to initiate opioids for pain; selecting opioids and determining opioid dosages; deciding duration of initial opioid prescription and conducting follow-up; and assessing risk and addressing potential harms of opioid use.
“Patients with pain should receive compassionate, safe, and effective pain care. We want clinicians and patients to have the information they need to weigh the benefits of different approaches to pain care, with the goal of helping people reduce their pain and improve their quality of life,” Christopher M. Jones, PharmD, DrPH, acting director for the CDC’s National Center for Injury Prevention and Control, said in a news release.
How to taper safely
The last guideline on the topic was released by CDC in 2016. Since then, new evidence has emerged regarding the benefits and risks of prescription opioids for acute and chronic pain, comparisons with nonopioid pain treatments, dosing strategies, opioid dose-dependent effects, risk mitigation strategies, and opioid tapering and discontinuation, the CDC says.
A “critical” addition to the 2022 guideline is advice on tapering opioids, Dr. Jones said during a press briefing.
“Practical tips on how to taper in an individualized patient-centered manner have been added to help clinicians if the decision is made to taper opioids, and the guideline explicitly advises against abrupt discontinuation or rapid dose reductions of opioids,” Dr. Jones said.
“That is based on lessons learned over the last several years as well as new science about how we approach tapering and the real harms that can result when patients are abruptly discontinued or rapidly tapered,” he added.
The updated guideline was published online Nov. 3 in the Morbidity and Mortality Weekly Report.
Key recommendations in the 100-page document include the following:
- In determining whether or not to initiate opioids, nonopioid therapies are at least as effective as opioids for many common types of acute pain. Use of nondrug and nonopioid drug therapies should be maximized as appropriate, and opioid therapy should only be considered for acute pain if it is anticipated that benefits outweigh risks to the patient.
- Before starting opioid therapy, providers should discuss with patients the realistic benefits and known risks of opioid therapy.
- Before starting ongoing opioid therapy for patients with subacute pain lasting 1 to 3 months or chronic pain lasting more than 3 months, providers should work with patients to establish treatment goals for pain and function, and consideration should be given as to how opioid therapy will be discontinued if benefits do not outweigh risks.
- Once opioids are started, the lowest effective dose of immediate-release opioids should be prescribed for no longer than needed for the expected duration of pain severe enough to require opioids.
- Within 1 to 4 weeks of starting opioid therapy for subacute or chronic pain, providers should work with patients to evaluate and carefully weigh benefits and risks of continuing opioid therapy; care should be exercised when increasing, continuing, or reducing opioid dosage.
- Before starting and periodically during ongoing opioid therapy, providers should evaluate risk for opioid-related harms and should work with patients to incorporate relevant strategies to mitigate risk, including offering naloxone and reviewing potential interactions with any other prescribed medications or substance used.
- Abrupt discontinuation of opioids should be avoided, especially for patients receiving high doses.
- For treating patients with opioid use disorder, treatment with evidence-based medications should be provided, or arrangements for such treatment should be made.
Dr. Jones emphasized that the guideline is “voluntary and meant to guide shared decision-making between a clinician and patient. It’s not meant to be implemented as absolute limits of policy or practice by clinicians, health systems, insurance companies, governmental entities.”
He also noted that the “current state of the overdose crisis, which is very much driven by illicit synthetic opioids, is not the aim of this guideline.
“The release of this guideline is really about advancing pain care and improving the lives of patients living with pain,” he said.
“We know that at least 1 in 5 people in the country have chronic pain. It’s one of the most common reasons why people present to their health care provider, and the goal here is to advance pain care, function, and quality of life for that patient population, while also reducing misuse, diversion, and consequences of prescription opioid misuse,” Dr. Jones added.
A version of this article first appeared on Medscape.com.
The 2022 Clinical Practice Guideline provides guidance on determining whether to initiate opioids for pain; selecting opioids and determining opioid dosages; deciding duration of initial opioid prescription and conducting follow-up; and assessing risk and addressing potential harms of opioid use.
“Patients with pain should receive compassionate, safe, and effective pain care. We want clinicians and patients to have the information they need to weigh the benefits of different approaches to pain care, with the goal of helping people reduce their pain and improve their quality of life,” Christopher M. Jones, PharmD, DrPH, acting director for the CDC’s National Center for Injury Prevention and Control, said in a news release.
How to taper safely
The last guideline on the topic was released by CDC in 2016. Since then, new evidence has emerged regarding the benefits and risks of prescription opioids for acute and chronic pain, comparisons with nonopioid pain treatments, dosing strategies, opioid dose-dependent effects, risk mitigation strategies, and opioid tapering and discontinuation, the CDC says.
A “critical” addition to the 2022 guideline is advice on tapering opioids, Dr. Jones said during a press briefing.
“Practical tips on how to taper in an individualized patient-centered manner have been added to help clinicians if the decision is made to taper opioids, and the guideline explicitly advises against abrupt discontinuation or rapid dose reductions of opioids,” Dr. Jones said.
“That is based on lessons learned over the last several years as well as new science about how we approach tapering and the real harms that can result when patients are abruptly discontinued or rapidly tapered,” he added.
The updated guideline was published online Nov. 3 in the Morbidity and Mortality Weekly Report.
Key recommendations in the 100-page document include the following:
- In determining whether or not to initiate opioids, nonopioid therapies are at least as effective as opioids for many common types of acute pain. Use of nondrug and nonopioid drug therapies should be maximized as appropriate, and opioid therapy should only be considered for acute pain if it is anticipated that benefits outweigh risks to the patient.
- Before starting opioid therapy, providers should discuss with patients the realistic benefits and known risks of opioid therapy.
- Before starting ongoing opioid therapy for patients with subacute pain lasting 1 to 3 months or chronic pain lasting more than 3 months, providers should work with patients to establish treatment goals for pain and function, and consideration should be given as to how opioid therapy will be discontinued if benefits do not outweigh risks.
- Once opioids are started, the lowest effective dose of immediate-release opioids should be prescribed for no longer than needed for the expected duration of pain severe enough to require opioids.
- Within 1 to 4 weeks of starting opioid therapy for subacute or chronic pain, providers should work with patients to evaluate and carefully weigh benefits and risks of continuing opioid therapy; care should be exercised when increasing, continuing, or reducing opioid dosage.
- Before starting and periodically during ongoing opioid therapy, providers should evaluate risk for opioid-related harms and should work with patients to incorporate relevant strategies to mitigate risk, including offering naloxone and reviewing potential interactions with any other prescribed medications or substance used.
- Abrupt discontinuation of opioids should be avoided, especially for patients receiving high doses.
- For treating patients with opioid use disorder, treatment with evidence-based medications should be provided, or arrangements for such treatment should be made.
Dr. Jones emphasized that the guideline is “voluntary and meant to guide shared decision-making between a clinician and patient. It’s not meant to be implemented as absolute limits of policy or practice by clinicians, health systems, insurance companies, governmental entities.”
He also noted that the “current state of the overdose crisis, which is very much driven by illicit synthetic opioids, is not the aim of this guideline.
“The release of this guideline is really about advancing pain care and improving the lives of patients living with pain,” he said.
“We know that at least 1 in 5 people in the country have chronic pain. It’s one of the most common reasons why people present to their health care provider, and the goal here is to advance pain care, function, and quality of life for that patient population, while also reducing misuse, diversion, and consequences of prescription opioid misuse,” Dr. Jones added.
A version of this article first appeared on Medscape.com.
DoD will cover travel expenses for abortion care
Some 80,000 active-duty women are stationed in states with abortion restrictions or bans. That’s 40% of active-duty service women in the continental United States, according to research sponsored by the US Department of Defense (DoD) and released in September. Nearly all (95%) are of reproductive age. Annually, an estimated 2573 to 4126 women have an abortion, but just a handful of those are done at military treatment facilities. Moreover, roughly 275,000 DoD civilians also live in states with a full ban or extreme restrictions on access to abortion. Of those, more than 81,000 are women. Nearly 43% have no access to abortion or drastically abridged access.
The recent Supreme Court ruling in Dobbs v Jackson Women’s Health Organization has created uncertainty for those women and their families, and potential legal and financial risk for the health care practitioners who would provide reproductive care, Defense Secretary Lloyd Austin said in an October 20, 2022 memo.
Therefore, he has directed the DoD to take “all appropriate action… as soon as possible to ensure that our service members and their families can access reproductive health care and our health care providers can operate effectively.”
Among the actions he has approved: Paying for travel to reproductive health care—essentially, making it more feasible for members to cross state lines. Service members, he noted in the memo, are often required to travel or move to meet staffing, operational, and training requirements. The “practical effects,” he said, are that significant numbers of service members and their families “may be forced to travel greater distances, take more time off from work, and pay more out-of-pocket expenses to receive reproductive health care.”
Those effects, Austin said, “qualify as unusual, extraordinary, hardship, or emergency circumstances for service members and their dependents and will interfere with our ability to recruit, retain, and maintain the readiness of a highly qualified force.”
Women, who comprise 17% of the active-duty force, are the fastest-growing subpopulation in the military. For the past several years, according to the DoD research report, the military services have been “deliberately recruiting women”—who perform essential duties in every sector: health care and electrical and mechanical equipment repair, for example.
“The full effects of Dobbs on military readiness are yet to be known,” the report says, but it notes several potential problems: Women may not join the service knowing that they could end up in a state with restrictions. If already serving, they may leave. In some states, women face criminal prosecution.
The long arm of Dobbs reaches far into the future, too. For instance, if unintended pregnancies are carried to term, the DoD will need to provide care to women during pregnancy, delivery, and the postpartum period—and the family will need to care for the child. Looking only at women in states with restricted access or bans, the DoD estimates the number of unintended pregnancies annually would be 2800 among civilian employees and between 4400 and 4700 among active-duty service women.
Men are also directly affected: More than 40% of male service members are married to a civilian woman who is a TRICARE dependent, 20% of active-duty service women are married to a fellow service member, and active-duty service men might be responsible for pregnancies among women who are not DoD dependents but who might be unable to get an abortion, the DoD report notes.
Austin has directed the DoD to create a uniform policy that allows for appropriate administrative absence, to establish travel and transportation allowances, and to amend any applicable travel regulations to facilitate official travel to access noncovered reproductive health care that is unavailable within the local area of the service member’s permanent duty station.
So that health care practitioners do not have to face criminal or civil liability or risk losing their licenses, Austin directed the DoD to develop a program to reimburse applicable fees, as appropriate and consistent with applicable federal law, for DoD health care practitioners who wish to become licensed in a state other than that in which they are currently licensed. He also directed the DoD to develop a program to support DoD practitioners who are subject to adverse action, including indemnification of any verdict, judgment, or other monetary award consistent with applicable law.
“Our greatest strength is our people,” Austin wrote. “There is no higher priority than taking care of our people, and ensuring their health and well-being.” He directed that the actions outlined in the memorandum “be executed as soon as possible.”
Some 80,000 active-duty women are stationed in states with abortion restrictions or bans. That’s 40% of active-duty service women in the continental United States, according to research sponsored by the US Department of Defense (DoD) and released in September. Nearly all (95%) are of reproductive age. Annually, an estimated 2573 to 4126 women have an abortion, but just a handful of those are done at military treatment facilities. Moreover, roughly 275,000 DoD civilians also live in states with a full ban or extreme restrictions on access to abortion. Of those, more than 81,000 are women. Nearly 43% have no access to abortion or drastically abridged access.
The recent Supreme Court ruling in Dobbs v Jackson Women’s Health Organization has created uncertainty for those women and their families, and potential legal and financial risk for the health care practitioners who would provide reproductive care, Defense Secretary Lloyd Austin said in an October 20, 2022 memo.
Therefore, he has directed the DoD to take “all appropriate action… as soon as possible to ensure that our service members and their families can access reproductive health care and our health care providers can operate effectively.”
Among the actions he has approved: Paying for travel to reproductive health care—essentially, making it more feasible for members to cross state lines. Service members, he noted in the memo, are often required to travel or move to meet staffing, operational, and training requirements. The “practical effects,” he said, are that significant numbers of service members and their families “may be forced to travel greater distances, take more time off from work, and pay more out-of-pocket expenses to receive reproductive health care.”
Those effects, Austin said, “qualify as unusual, extraordinary, hardship, or emergency circumstances for service members and their dependents and will interfere with our ability to recruit, retain, and maintain the readiness of a highly qualified force.”
Women, who comprise 17% of the active-duty force, are the fastest-growing subpopulation in the military. For the past several years, according to the DoD research report, the military services have been “deliberately recruiting women”—who perform essential duties in every sector: health care and electrical and mechanical equipment repair, for example.
“The full effects of Dobbs on military readiness are yet to be known,” the report says, but it notes several potential problems: Women may not join the service knowing that they could end up in a state with restrictions. If already serving, they may leave. In some states, women face criminal prosecution.
The long arm of Dobbs reaches far into the future, too. For instance, if unintended pregnancies are carried to term, the DoD will need to provide care to women during pregnancy, delivery, and the postpartum period—and the family will need to care for the child. Looking only at women in states with restricted access or bans, the DoD estimates the number of unintended pregnancies annually would be 2800 among civilian employees and between 4400 and 4700 among active-duty service women.
Men are also directly affected: More than 40% of male service members are married to a civilian woman who is a TRICARE dependent, 20% of active-duty service women are married to a fellow service member, and active-duty service men might be responsible for pregnancies among women who are not DoD dependents but who might be unable to get an abortion, the DoD report notes.
Austin has directed the DoD to create a uniform policy that allows for appropriate administrative absence, to establish travel and transportation allowances, and to amend any applicable travel regulations to facilitate official travel to access noncovered reproductive health care that is unavailable within the local area of the service member’s permanent duty station.
So that health care practitioners do not have to face criminal or civil liability or risk losing their licenses, Austin directed the DoD to develop a program to reimburse applicable fees, as appropriate and consistent with applicable federal law, for DoD health care practitioners who wish to become licensed in a state other than that in which they are currently licensed. He also directed the DoD to develop a program to support DoD practitioners who are subject to adverse action, including indemnification of any verdict, judgment, or other monetary award consistent with applicable law.
“Our greatest strength is our people,” Austin wrote. “There is no higher priority than taking care of our people, and ensuring their health and well-being.” He directed that the actions outlined in the memorandum “be executed as soon as possible.”
Some 80,000 active-duty women are stationed in states with abortion restrictions or bans. That’s 40% of active-duty service women in the continental United States, according to research sponsored by the US Department of Defense (DoD) and released in September. Nearly all (95%) are of reproductive age. Annually, an estimated 2573 to 4126 women have an abortion, but just a handful of those are done at military treatment facilities. Moreover, roughly 275,000 DoD civilians also live in states with a full ban or extreme restrictions on access to abortion. Of those, more than 81,000 are women. Nearly 43% have no access to abortion or drastically abridged access.
The recent Supreme Court ruling in Dobbs v Jackson Women’s Health Organization has created uncertainty for those women and their families, and potential legal and financial risk for the health care practitioners who would provide reproductive care, Defense Secretary Lloyd Austin said in an October 20, 2022 memo.
Therefore, he has directed the DoD to take “all appropriate action… as soon as possible to ensure that our service members and their families can access reproductive health care and our health care providers can operate effectively.”
Among the actions he has approved: Paying for travel to reproductive health care—essentially, making it more feasible for members to cross state lines. Service members, he noted in the memo, are often required to travel or move to meet staffing, operational, and training requirements. The “practical effects,” he said, are that significant numbers of service members and their families “may be forced to travel greater distances, take more time off from work, and pay more out-of-pocket expenses to receive reproductive health care.”
Those effects, Austin said, “qualify as unusual, extraordinary, hardship, or emergency circumstances for service members and their dependents and will interfere with our ability to recruit, retain, and maintain the readiness of a highly qualified force.”
Women, who comprise 17% of the active-duty force, are the fastest-growing subpopulation in the military. For the past several years, according to the DoD research report, the military services have been “deliberately recruiting women”—who perform essential duties in every sector: health care and electrical and mechanical equipment repair, for example.
“The full effects of Dobbs on military readiness are yet to be known,” the report says, but it notes several potential problems: Women may not join the service knowing that they could end up in a state with restrictions. If already serving, they may leave. In some states, women face criminal prosecution.
The long arm of Dobbs reaches far into the future, too. For instance, if unintended pregnancies are carried to term, the DoD will need to provide care to women during pregnancy, delivery, and the postpartum period—and the family will need to care for the child. Looking only at women in states with restricted access or bans, the DoD estimates the number of unintended pregnancies annually would be 2800 among civilian employees and between 4400 and 4700 among active-duty service women.
Men are also directly affected: More than 40% of male service members are married to a civilian woman who is a TRICARE dependent, 20% of active-duty service women are married to a fellow service member, and active-duty service men might be responsible for pregnancies among women who are not DoD dependents but who might be unable to get an abortion, the DoD report notes.
Austin has directed the DoD to create a uniform policy that allows for appropriate administrative absence, to establish travel and transportation allowances, and to amend any applicable travel regulations to facilitate official travel to access noncovered reproductive health care that is unavailable within the local area of the service member’s permanent duty station.
So that health care practitioners do not have to face criminal or civil liability or risk losing their licenses, Austin directed the DoD to develop a program to reimburse applicable fees, as appropriate and consistent with applicable federal law, for DoD health care practitioners who wish to become licensed in a state other than that in which they are currently licensed. He also directed the DoD to develop a program to support DoD practitioners who are subject to adverse action, including indemnification of any verdict, judgment, or other monetary award consistent with applicable law.
“Our greatest strength is our people,” Austin wrote. “There is no higher priority than taking care of our people, and ensuring their health and well-being.” He directed that the actions outlined in the memorandum “be executed as soon as possible.”
VA Fast-Tracks Hiring to Address Critical Shortages
In an intensive push to fill acute workforce shortages, the US Department of Veterans Affairs (VA) is holding a “national onboarding surge event” the week of November 14. The goal is to get people who have already said yes to a job in the VA on that job more quickly. Every VA facility has been asked to submit a list of the highest-priority candidates, regardless of the position.
One of the most pressing reasons for getting more workers into the pipeline faster is that more and more veterans are entering VA care. As of October 1, tens of thousands of veterans will be eligible for VA health care, thanks to the Sergeant First Class Heath Robinson Honoring our Promise to Address Comprehensive Toxics Act of 2022 (PACT Act), passed in August, which expanded benefits for post-9/11 service members with illnesses due to toxic exposures.
Another reason is the need to fill the gaps left by attrition. In an October 19 press briefing, VA Undersecretary for Health Shereef Elnahal said the agency needs to hire about 52,000 employees per year just to keep up with the rate of health care professionals (HCPs) leaving the agency. At a September breakfast meeting with the Defense Writers Group, VA Secretary Denis McDonough said July 2022 marked the first month this year that the VA hired more nurses than it lost to retirement. He said the VA needs to hire 45,000 nurses over the next 3 years to keep up with attrition and growing demand for veteran care.
“We have to do a better job on hiring,” McDonough said. Streamlining the process is a major goal. Hiring rules loosened during the pandemic have since tightened back up. He pointed out that in many cases, the VA takes 90 to 100 days to onboard candidates and called the long-drawn-out process “being dragged through a bureaucratic morass.” During that time, he said, “They’re not being paid, they’re filling out paperwork… That’s disastrous.” In his press briefing, Elnahal said “we lose folks after we’ve made the selection” because the process is so long.
Moreover, the agency has a critical shortage not only of HCPs but the human resources professionals needed to fast-track the hirees’ progress. McDonough called it a “supply chain issue.” “We have the lowest ratio of human resource professionals per employee in the federal government by a long shot.” Partly, he said, because “a lot of our people end up hired away to other federal agencies.”
McDonough said the VA is also interested in transitioning more active-duty service members with in-demand skills, certifications, and talent into the VA workforce. “Cross-walking active duty into VA service much more aggressively,” he said, is another way to “grow that supply of ready, deployable, trained personnel.” The PACT Act gives the VA new incentives to entice workers, such as expanded recruitment, retention bonuses, and student loan repayment. The VA already provides training to about 1500 nurse and nurse residency programs across the VA, McDonough said but has plans for expanding to 5 times its current scope. He also addressed the question of a looming physician shortage: “Roughly 7 in 10 doctors in the United States will have had some portion of their training in a VA facility. We have to maintain that training function going forward.” The VA trains doctors, he added, “better than anybody else.”
The onboarding event will serve as a “national signal that we take this priority very seriously,” Elnahal said. “This will be not only a chance to have a step function improvement in the number of folks on board, which is an urgent priority, but to also set the groundwork for the more longitudinal work that we will need to do to improve the hiring process.”
Bulking up the workforce, he said, is “still far and away among our first priorities. Because if we don’t get our hospitals and facility staffed, it’s going to be a really hard effort to make process on the other priorities.”
In an intensive push to fill acute workforce shortages, the US Department of Veterans Affairs (VA) is holding a “national onboarding surge event” the week of November 14. The goal is to get people who have already said yes to a job in the VA on that job more quickly. Every VA facility has been asked to submit a list of the highest-priority candidates, regardless of the position.
One of the most pressing reasons for getting more workers into the pipeline faster is that more and more veterans are entering VA care. As of October 1, tens of thousands of veterans will be eligible for VA health care, thanks to the Sergeant First Class Heath Robinson Honoring our Promise to Address Comprehensive Toxics Act of 2022 (PACT Act), passed in August, which expanded benefits for post-9/11 service members with illnesses due to toxic exposures.
Another reason is the need to fill the gaps left by attrition. In an October 19 press briefing, VA Undersecretary for Health Shereef Elnahal said the agency needs to hire about 52,000 employees per year just to keep up with the rate of health care professionals (HCPs) leaving the agency. At a September breakfast meeting with the Defense Writers Group, VA Secretary Denis McDonough said July 2022 marked the first month this year that the VA hired more nurses than it lost to retirement. He said the VA needs to hire 45,000 nurses over the next 3 years to keep up with attrition and growing demand for veteran care.
“We have to do a better job on hiring,” McDonough said. Streamlining the process is a major goal. Hiring rules loosened during the pandemic have since tightened back up. He pointed out that in many cases, the VA takes 90 to 100 days to onboard candidates and called the long-drawn-out process “being dragged through a bureaucratic morass.” During that time, he said, “They’re not being paid, they’re filling out paperwork… That’s disastrous.” In his press briefing, Elnahal said “we lose folks after we’ve made the selection” because the process is so long.
Moreover, the agency has a critical shortage not only of HCPs but the human resources professionals needed to fast-track the hirees’ progress. McDonough called it a “supply chain issue.” “We have the lowest ratio of human resource professionals per employee in the federal government by a long shot.” Partly, he said, because “a lot of our people end up hired away to other federal agencies.”
McDonough said the VA is also interested in transitioning more active-duty service members with in-demand skills, certifications, and talent into the VA workforce. “Cross-walking active duty into VA service much more aggressively,” he said, is another way to “grow that supply of ready, deployable, trained personnel.” The PACT Act gives the VA new incentives to entice workers, such as expanded recruitment, retention bonuses, and student loan repayment. The VA already provides training to about 1500 nurse and nurse residency programs across the VA, McDonough said but has plans for expanding to 5 times its current scope. He also addressed the question of a looming physician shortage: “Roughly 7 in 10 doctors in the United States will have had some portion of their training in a VA facility. We have to maintain that training function going forward.” The VA trains doctors, he added, “better than anybody else.”
The onboarding event will serve as a “national signal that we take this priority very seriously,” Elnahal said. “This will be not only a chance to have a step function improvement in the number of folks on board, which is an urgent priority, but to also set the groundwork for the more longitudinal work that we will need to do to improve the hiring process.”
Bulking up the workforce, he said, is “still far and away among our first priorities. Because if we don’t get our hospitals and facility staffed, it’s going to be a really hard effort to make process on the other priorities.”
In an intensive push to fill acute workforce shortages, the US Department of Veterans Affairs (VA) is holding a “national onboarding surge event” the week of November 14. The goal is to get people who have already said yes to a job in the VA on that job more quickly. Every VA facility has been asked to submit a list of the highest-priority candidates, regardless of the position.
One of the most pressing reasons for getting more workers into the pipeline faster is that more and more veterans are entering VA care. As of October 1, tens of thousands of veterans will be eligible for VA health care, thanks to the Sergeant First Class Heath Robinson Honoring our Promise to Address Comprehensive Toxics Act of 2022 (PACT Act), passed in August, which expanded benefits for post-9/11 service members with illnesses due to toxic exposures.
Another reason is the need to fill the gaps left by attrition. In an October 19 press briefing, VA Undersecretary for Health Shereef Elnahal said the agency needs to hire about 52,000 employees per year just to keep up with the rate of health care professionals (HCPs) leaving the agency. At a September breakfast meeting with the Defense Writers Group, VA Secretary Denis McDonough said July 2022 marked the first month this year that the VA hired more nurses than it lost to retirement. He said the VA needs to hire 45,000 nurses over the next 3 years to keep up with attrition and growing demand for veteran care.
“We have to do a better job on hiring,” McDonough said. Streamlining the process is a major goal. Hiring rules loosened during the pandemic have since tightened back up. He pointed out that in many cases, the VA takes 90 to 100 days to onboard candidates and called the long-drawn-out process “being dragged through a bureaucratic morass.” During that time, he said, “They’re not being paid, they’re filling out paperwork… That’s disastrous.” In his press briefing, Elnahal said “we lose folks after we’ve made the selection” because the process is so long.
Moreover, the agency has a critical shortage not only of HCPs but the human resources professionals needed to fast-track the hirees’ progress. McDonough called it a “supply chain issue.” “We have the lowest ratio of human resource professionals per employee in the federal government by a long shot.” Partly, he said, because “a lot of our people end up hired away to other federal agencies.”
McDonough said the VA is also interested in transitioning more active-duty service members with in-demand skills, certifications, and talent into the VA workforce. “Cross-walking active duty into VA service much more aggressively,” he said, is another way to “grow that supply of ready, deployable, trained personnel.” The PACT Act gives the VA new incentives to entice workers, such as expanded recruitment, retention bonuses, and student loan repayment. The VA already provides training to about 1500 nurse and nurse residency programs across the VA, McDonough said but has plans for expanding to 5 times its current scope. He also addressed the question of a looming physician shortage: “Roughly 7 in 10 doctors in the United States will have had some portion of their training in a VA facility. We have to maintain that training function going forward.” The VA trains doctors, he added, “better than anybody else.”
The onboarding event will serve as a “national signal that we take this priority very seriously,” Elnahal said. “This will be not only a chance to have a step function improvement in the number of folks on board, which is an urgent priority, but to also set the groundwork for the more longitudinal work that we will need to do to improve the hiring process.”
Bulking up the workforce, he said, is “still far and away among our first priorities. Because if we don’t get our hospitals and facility staffed, it’s going to be a really hard effort to make process on the other priorities.”
VA Gets it Right on Suicide
For years, the US Department of Veterans Affairs (VA) has painstakingly labored to track, research, and address veteran suicide. Their exceptional work was dealt an unwarranted blow a month ago with the publication of an incomplete report entitled Operation Deep Dive (OpDD). The $3.9 million study from America’s Warrior Partnership (AWP) examined death data of former service members in 8 states between 2014 and 2018. The interim report criticized the VA for minimizing the extent of veteran suicide, asserting, “former service members take their own lives each year at a rate approximately 2.4 times greater than previously reported by the VA.”
The sensational results were accepted at face value and immediately garnered negative nationwide headlines, with lawmakers, media outlets, and veterans rushing to impugn the VA. Senate Committee on Veterans’ Affairs Ranking Republican Member Jerry Moran of Kansas opined, “The disparity between the numbers of veteran suicides reported by the VA and [OpDD] is concerning. We need an honest assessment of the scope of the problem.” A U.S. Medicine headline stated “VA undercounted thousands of veteran suicides. [OpDD] posited daily suicide rate is 240% higher.” Fox News declared, “Veterans committing suicide at rate 2 times higher than VA data show: study,” as did Military Times, “Veterans suicide rate may be double federal estimates, study suggests.”
Disturbingly, those who echoed AWP’s claims got the story backward. It’s AWP, not VA, whose suicide data and conclusions are faulty.
For starters, the VA data encompasses veterans across all 50 states, the District of Columbia, Puerto Rico, and the US Virgin Islands. In contrast, AWP inferred national veteran suicide figures based on partial, skewed data. As delineated by researchers in an in-press Military Medicine letter to the Editor, 7 of the 8 states sampled (Alabama, Florida, Maine, Massachusetts, Michigan, Minnesota, Montana, and Oregon) had suicide rates above the national average for the years under investigation. This factor alone overinflates AWP’s purported suicide numbers.
Additionally, AWP altered the definition of “taking one’s life” and then misapplied that designation. Conventionally, the term refers to suicide, but AWP used it to also include nonnatural deaths assessed by coroners and medical examiners as accidental or undetermined. Two examples of this self-injury mortality (SIM) are opioid overdoses and single-driver car crash deaths. AWP added suicides and SIMs to derive a total number of veterans who took their life and falsely contrasted that aggregate against the VA count of suicides. That’s like comparing the whole category of fruit to the subcategory of apples.
AWP should be applauded for drawing attention to and accounting for accidental and undetermined deaths. However, the standard protocol is to consider SIMs distinctly from suicides. Among the many reasons for precise labeling is so that grieving family members aren’t mistakenly informed that their loved one died by suicide. VA conveys the rate of veteran overdose deaths in separate reports, for example, the Veteran Drug Overdose Mortality, 2010-2019 publication. Those numbers were ignored in AWP’s calculations.
AWP was neglectful in another way. The second phase of the project—a deep examination of community-level factors preceding suicides and nonnatural deaths—began in 2019. This information was collected and analyzed through sociocultural death investigation (SDI) interviews of 3 to 4 family members, friends, and colleagues of the deceased. SDIs consisted of 19 factors, such as history of the veteran’s mental health problems, social connectedness, finances, group memberships, and access to firearms. However, the interim report omitted the preliminary analysis of these factors, which AWP stated would be made available this year.
OpDD conclusions were so unfounded that AWP’s analytic research partner, the University of Alabama, distanced itself from the interim report. “We were not consulted on the released figures,” Dr. Karl Hamner, the University of Alabama principal investigator on the study, told me. “We did not make any conclusions and we don’t endorse the reported findings about national rates or numbers per day. Nor did we make any statements about the VA’s data.”
As it happens, the VA’s 2022 National Veteran Suicide Prevention Annual Report was issued the same week as the OpDD report. VA found that veteran suicides decreased by 9.7% over the last 2 years, nearly twice the decrease for nonveterans. Yet, in a contemporaneous hearing of the House Committee on Veterans’ Affairs, AWP’s President and CEO Jim Lorraine testified that the progress preventing veteran suicide was “a disgrace” and “a failure.” He misattributed that it was VA (not AWP) that “must be more open and transparent about their data.”
Unsupported denigration of the VA tarnishes its reputation, undermining veterans’ trust in the health care system and increasing barriers to seeking needed services. More broadly, it fortifies those forces who wish to redirect allocations away from VA and towards non-VA veterans’ entities like AWP. The media and other stakeholders must take a lesson about getting the story straight before reflexively amplifying false accusations about the VA. Veterans deserve better.
For years, the US Department of Veterans Affairs (VA) has painstakingly labored to track, research, and address veteran suicide. Their exceptional work was dealt an unwarranted blow a month ago with the publication of an incomplete report entitled Operation Deep Dive (OpDD). The $3.9 million study from America’s Warrior Partnership (AWP) examined death data of former service members in 8 states between 2014 and 2018. The interim report criticized the VA for minimizing the extent of veteran suicide, asserting, “former service members take their own lives each year at a rate approximately 2.4 times greater than previously reported by the VA.”
The sensational results were accepted at face value and immediately garnered negative nationwide headlines, with lawmakers, media outlets, and veterans rushing to impugn the VA. Senate Committee on Veterans’ Affairs Ranking Republican Member Jerry Moran of Kansas opined, “The disparity between the numbers of veteran suicides reported by the VA and [OpDD] is concerning. We need an honest assessment of the scope of the problem.” A U.S. Medicine headline stated “VA undercounted thousands of veteran suicides. [OpDD] posited daily suicide rate is 240% higher.” Fox News declared, “Veterans committing suicide at rate 2 times higher than VA data show: study,” as did Military Times, “Veterans suicide rate may be double federal estimates, study suggests.”
Disturbingly, those who echoed AWP’s claims got the story backward. It’s AWP, not VA, whose suicide data and conclusions are faulty.
For starters, the VA data encompasses veterans across all 50 states, the District of Columbia, Puerto Rico, and the US Virgin Islands. In contrast, AWP inferred national veteran suicide figures based on partial, skewed data. As delineated by researchers in an in-press Military Medicine letter to the Editor, 7 of the 8 states sampled (Alabama, Florida, Maine, Massachusetts, Michigan, Minnesota, Montana, and Oregon) had suicide rates above the national average for the years under investigation. This factor alone overinflates AWP’s purported suicide numbers.
Additionally, AWP altered the definition of “taking one’s life” and then misapplied that designation. Conventionally, the term refers to suicide, but AWP used it to also include nonnatural deaths assessed by coroners and medical examiners as accidental or undetermined. Two examples of this self-injury mortality (SIM) are opioid overdoses and single-driver car crash deaths. AWP added suicides and SIMs to derive a total number of veterans who took their life and falsely contrasted that aggregate against the VA count of suicides. That’s like comparing the whole category of fruit to the subcategory of apples.
AWP should be applauded for drawing attention to and accounting for accidental and undetermined deaths. However, the standard protocol is to consider SIMs distinctly from suicides. Among the many reasons for precise labeling is so that grieving family members aren’t mistakenly informed that their loved one died by suicide. VA conveys the rate of veteran overdose deaths in separate reports, for example, the Veteran Drug Overdose Mortality, 2010-2019 publication. Those numbers were ignored in AWP’s calculations.
AWP was neglectful in another way. The second phase of the project—a deep examination of community-level factors preceding suicides and nonnatural deaths—began in 2019. This information was collected and analyzed through sociocultural death investigation (SDI) interviews of 3 to 4 family members, friends, and colleagues of the deceased. SDIs consisted of 19 factors, such as history of the veteran’s mental health problems, social connectedness, finances, group memberships, and access to firearms. However, the interim report omitted the preliminary analysis of these factors, which AWP stated would be made available this year.
OpDD conclusions were so unfounded that AWP’s analytic research partner, the University of Alabama, distanced itself from the interim report. “We were not consulted on the released figures,” Dr. Karl Hamner, the University of Alabama principal investigator on the study, told me. “We did not make any conclusions and we don’t endorse the reported findings about national rates or numbers per day. Nor did we make any statements about the VA’s data.”
As it happens, the VA’s 2022 National Veteran Suicide Prevention Annual Report was issued the same week as the OpDD report. VA found that veteran suicides decreased by 9.7% over the last 2 years, nearly twice the decrease for nonveterans. Yet, in a contemporaneous hearing of the House Committee on Veterans’ Affairs, AWP’s President and CEO Jim Lorraine testified that the progress preventing veteran suicide was “a disgrace” and “a failure.” He misattributed that it was VA (not AWP) that “must be more open and transparent about their data.”
Unsupported denigration of the VA tarnishes its reputation, undermining veterans’ trust in the health care system and increasing barriers to seeking needed services. More broadly, it fortifies those forces who wish to redirect allocations away from VA and towards non-VA veterans’ entities like AWP. The media and other stakeholders must take a lesson about getting the story straight before reflexively amplifying false accusations about the VA. Veterans deserve better.
For years, the US Department of Veterans Affairs (VA) has painstakingly labored to track, research, and address veteran suicide. Their exceptional work was dealt an unwarranted blow a month ago with the publication of an incomplete report entitled Operation Deep Dive (OpDD). The $3.9 million study from America’s Warrior Partnership (AWP) examined death data of former service members in 8 states between 2014 and 2018. The interim report criticized the VA for minimizing the extent of veteran suicide, asserting, “former service members take their own lives each year at a rate approximately 2.4 times greater than previously reported by the VA.”
The sensational results were accepted at face value and immediately garnered negative nationwide headlines, with lawmakers, media outlets, and veterans rushing to impugn the VA. Senate Committee on Veterans’ Affairs Ranking Republican Member Jerry Moran of Kansas opined, “The disparity between the numbers of veteran suicides reported by the VA and [OpDD] is concerning. We need an honest assessment of the scope of the problem.” A U.S. Medicine headline stated “VA undercounted thousands of veteran suicides. [OpDD] posited daily suicide rate is 240% higher.” Fox News declared, “Veterans committing suicide at rate 2 times higher than VA data show: study,” as did Military Times, “Veterans suicide rate may be double federal estimates, study suggests.”
Disturbingly, those who echoed AWP’s claims got the story backward. It’s AWP, not VA, whose suicide data and conclusions are faulty.
For starters, the VA data encompasses veterans across all 50 states, the District of Columbia, Puerto Rico, and the US Virgin Islands. In contrast, AWP inferred national veteran suicide figures based on partial, skewed data. As delineated by researchers in an in-press Military Medicine letter to the Editor, 7 of the 8 states sampled (Alabama, Florida, Maine, Massachusetts, Michigan, Minnesota, Montana, and Oregon) had suicide rates above the national average for the years under investigation. This factor alone overinflates AWP’s purported suicide numbers.
Additionally, AWP altered the definition of “taking one’s life” and then misapplied that designation. Conventionally, the term refers to suicide, but AWP used it to also include nonnatural deaths assessed by coroners and medical examiners as accidental or undetermined. Two examples of this self-injury mortality (SIM) are opioid overdoses and single-driver car crash deaths. AWP added suicides and SIMs to derive a total number of veterans who took their life and falsely contrasted that aggregate against the VA count of suicides. That’s like comparing the whole category of fruit to the subcategory of apples.
AWP should be applauded for drawing attention to and accounting for accidental and undetermined deaths. However, the standard protocol is to consider SIMs distinctly from suicides. Among the many reasons for precise labeling is so that grieving family members aren’t mistakenly informed that their loved one died by suicide. VA conveys the rate of veteran overdose deaths in separate reports, for example, the Veteran Drug Overdose Mortality, 2010-2019 publication. Those numbers were ignored in AWP’s calculations.
AWP was neglectful in another way. The second phase of the project—a deep examination of community-level factors preceding suicides and nonnatural deaths—began in 2019. This information was collected and analyzed through sociocultural death investigation (SDI) interviews of 3 to 4 family members, friends, and colleagues of the deceased. SDIs consisted of 19 factors, such as history of the veteran’s mental health problems, social connectedness, finances, group memberships, and access to firearms. However, the interim report omitted the preliminary analysis of these factors, which AWP stated would be made available this year.
OpDD conclusions were so unfounded that AWP’s analytic research partner, the University of Alabama, distanced itself from the interim report. “We were not consulted on the released figures,” Dr. Karl Hamner, the University of Alabama principal investigator on the study, told me. “We did not make any conclusions and we don’t endorse the reported findings about national rates or numbers per day. Nor did we make any statements about the VA’s data.”
As it happens, the VA’s 2022 National Veteran Suicide Prevention Annual Report was issued the same week as the OpDD report. VA found that veteran suicides decreased by 9.7% over the last 2 years, nearly twice the decrease for nonveterans. Yet, in a contemporaneous hearing of the House Committee on Veterans’ Affairs, AWP’s President and CEO Jim Lorraine testified that the progress preventing veteran suicide was “a disgrace” and “a failure.” He misattributed that it was VA (not AWP) that “must be more open and transparent about their data.”
Unsupported denigration of the VA tarnishes its reputation, undermining veterans’ trust in the health care system and increasing barriers to seeking needed services. More broadly, it fortifies those forces who wish to redirect allocations away from VA and towards non-VA veterans’ entities like AWP. The media and other stakeholders must take a lesson about getting the story straight before reflexively amplifying false accusations about the VA. Veterans deserve better.
Climate change: Commentary in four dermatology journals calls for emergency action
“moving beyond merely discussing skin-related impacts” and toward prioritizing both patient and planetary health.
Dermatologists must make emissions-saving changes in everyday practice, for instance, and the specialty must enlist key stakeholders in public health, nonprofits, and industry – that is, pharmaceutical and medical supply companies – in finding solutions to help mitigate and adapt to climate change, wrote Eva Rawlings Parker, MD, and Markus D. Boos, MD, PhD.
“We have an ethical imperative to act,” they wrote. “The time is now for dermatologists and our medical societies to collectively rise to meet this crisis.”
Their commentary was published online in the International Journal of Dermatology , Journal of the European Academy of Dermatology and Venereology, British Journal of Dermatology, and Pediatric Dermatology.
In an interview, Dr. Parker, assistant professor of dermatology at Vanderbilt University, Nashville, Tenn., said that she and Dr. Boos, associate professor in the division of dermatology and department of pediatrics at the University of Washington, Seattle, were motivated to write the editorial upon finding that dermatology was not represented among more than 230 medical journals that published an editorial in September 2021 calling for emergency action to limit global warming and protect health. In addition to the New England Journal of Medicine and The Lancet, the copublishing journals represented numerous specialties, from nursing and pediatrics, to cardiology, rheumatology, and gastroenterology.
The editorial was not published in any dermatology journals, Dr. Parker said. “It was incredibly disappointing for me along with many of my colleagues who advocate for climate action because we realized it was a missed opportunity for dermatology to align with other medical specialties and be on the forefront of leading climate action to protect health.”
‘A threat multiplier’
The impact of climate change on skin disease is “an incredibly important part of our conversation as dermatologists because many cutaneous diseases are climate sensitive and we’re often seeing the effects of climate change every day in our clinical practices,” Dr. Parker said.
In fact, the impact on skin disease needs to be explored much further through more robust research funding, so that dermatology can better understand not only the incidence and severity of climate-induced changes in skin diseases – including and beyond atopic dermatitis, acne, and psoriasis – but also the mechanisms and pathophysiology involved, she said.
However, the impacts are much broader, she and Dr. Boos, a pediatric dermatologist at Seattle Children’s Hospital, maintain in their commentary. “An essential concept to broker among dermatologists is that the impacts of climate change extend well beyond skin disease by also placing broad pressure” on infrastructure, the economy, financial markets, global supply chains, food and water insecurity, and more, they wrote, noting the deep inequities of climate change.
Climate change is a “threat multiplier for public health, equity, and health systems,” the commentary says. “The confluence of these climate-related pressures should sound alarm bells as they place enormous jeopardy on the practice of dermatology across all scales and regions.”
Health care is among the most carbon-intensive service sectors worldwide, contributing to almost 5% of greenhouse gas emissions globally, the commentary says. And nationally, of the estimated greenhouse gas emissions from the United States, the health care sector contributes 10%, Dr. Parker said in the interview, referring to a 2016 report.
In addition, according to a 2019 report, the United States is the top contributor to health care’s global climate footprint, contributing 27% of health care’s global emissions, Dr. Parker noted.
In their commentary, she and Dr. Boos wrote that individually and practice wide, dermatologists can impact decarbonization through measures such as virtual attendance at medical meetings and greater utilization of telehealth services. Reductions in carbon emissions were demonstrated for virtual isotretinoin follow-up visits in a recent study, and these savings could be extrapolated to other routine follow-up visits for conditions such as rosacea, monitoring of biologics in patients with well-controlled disease, and postoperative wound checks, they said.
But when it comes to measures such as significantly reducing packaging and waste and “curating supply chains to make them more sustainable,” it is medical societies that have the “larger voice and broader relationship with the pharmaceutical industry” and with medical supply manufacturers and distributors, Dr. Parker explained in the interview, noting the potential for reducing the extensive amount of packaging used for drug samples.
Dr. Parker cochairs the American Academy of Dermatology’s Expert Resource Group for Climate Change and Environmental Issues, which was established several years ago, and Dr. Boos is a member of the group’s executive committee.
AAD actions
In its 2018 Position Statement on Climate and Health, the American Academy of Dermatology resolved to raise awareness of the effects of climate change on the skin and educate patients about this, and to “work with other medical societies in ongoing and future efforts to educate the public and mitigate the effects of climate change on global health.”
Asked about the commentary’s call for more collaboration with industry and other stakeholders – and the impact that organized dermatology can have on planetary health – Mark D. Kaufmann, MD, president of the AAD, said in an email that the AAD is “first and foremost an organization focused on providing gold-standard educational resources for dermatologists.”
The academy recognizes that “there are many dermatologic consequences of climate change that will increasingly affect our patients and challenge our membership,” and it has provided education on climate change in forums such as articles, podcasts, and sessions at AAD meetings, said Dr. Kaufmann, clinical professor in the department of dermatology, Icahn School of Medicine at Mount Sinai, New York.
Regarding collaboration with other societies, he said that the AAD’s “focus to date has been on how to provide our members with educational resources to understand and prepare for how climate change may impact their practices and the dermatologic health of their patients,” he said.
The AAD has also sought to address its own carbon footprint and improve sustainability of its operations, including taking steps to reduce plastic and paper waste at its educational events, and to eliminate plastic waste associated with mailing resources like its member magazine, Dr. Kaufmann noted.
And in keeping with the Academy pledge – also articulated in the 2018 position statement – to support and facilitate dermatologists’ efforts to decrease their carbon footprint “in a cost effective (or cost-saving) manner,” Dr. Kaufmann said that the AAD has been offering a program called My Green Doctor as a free benefit of membership.
‘Be part of the solution’
In an interview, Mary E. Maloney, MD, professor of medicine and director of dermatologic surgery at the University of Massachusetts, Worcester, said her practice did an audit of their surgical area and found ways to increase the use of paper-packaged gauze – and decrease use of gauze in hard plastic containers – and otherwise decrease the amount of disposables, all of which take “huge amounts of resources” to create.
In the process, “we found significant savings,” she said. “Little things can turn out, in the long run, to be big things.”
Asked about the commentary, Dr. Maloney, who is involved in the AAD’s climate change resource group, said “the message is that yes, we need to be aware of the diseases affected by climate change. But our greater imperative is to be part of the solution and not part of the problem as far as doing things that affect climate change.”
Organized dermatology needs to broaden its advocacy, she said. “I don’t want us to stop advocating for things for our patients, but I do want us to start advocating for the world ... If we don’t try to [mitigate] climate change, we won’t have patients to advocate for.”
Dr. Parker, an associate editor of The Journal of Climate Change and Health, and Dr. Boos declared no conflicts of interest and no funding source for their commentary. Dr. Maloney said she has no conflicts of interest.
“moving beyond merely discussing skin-related impacts” and toward prioritizing both patient and planetary health.
Dermatologists must make emissions-saving changes in everyday practice, for instance, and the specialty must enlist key stakeholders in public health, nonprofits, and industry – that is, pharmaceutical and medical supply companies – in finding solutions to help mitigate and adapt to climate change, wrote Eva Rawlings Parker, MD, and Markus D. Boos, MD, PhD.
“We have an ethical imperative to act,” they wrote. “The time is now for dermatologists and our medical societies to collectively rise to meet this crisis.”
Their commentary was published online in the International Journal of Dermatology , Journal of the European Academy of Dermatology and Venereology, British Journal of Dermatology, and Pediatric Dermatology.
In an interview, Dr. Parker, assistant professor of dermatology at Vanderbilt University, Nashville, Tenn., said that she and Dr. Boos, associate professor in the division of dermatology and department of pediatrics at the University of Washington, Seattle, were motivated to write the editorial upon finding that dermatology was not represented among more than 230 medical journals that published an editorial in September 2021 calling for emergency action to limit global warming and protect health. In addition to the New England Journal of Medicine and The Lancet, the copublishing journals represented numerous specialties, from nursing and pediatrics, to cardiology, rheumatology, and gastroenterology.
The editorial was not published in any dermatology journals, Dr. Parker said. “It was incredibly disappointing for me along with many of my colleagues who advocate for climate action because we realized it was a missed opportunity for dermatology to align with other medical specialties and be on the forefront of leading climate action to protect health.”
‘A threat multiplier’
The impact of climate change on skin disease is “an incredibly important part of our conversation as dermatologists because many cutaneous diseases are climate sensitive and we’re often seeing the effects of climate change every day in our clinical practices,” Dr. Parker said.
In fact, the impact on skin disease needs to be explored much further through more robust research funding, so that dermatology can better understand not only the incidence and severity of climate-induced changes in skin diseases – including and beyond atopic dermatitis, acne, and psoriasis – but also the mechanisms and pathophysiology involved, she said.
However, the impacts are much broader, she and Dr. Boos, a pediatric dermatologist at Seattle Children’s Hospital, maintain in their commentary. “An essential concept to broker among dermatologists is that the impacts of climate change extend well beyond skin disease by also placing broad pressure” on infrastructure, the economy, financial markets, global supply chains, food and water insecurity, and more, they wrote, noting the deep inequities of climate change.
Climate change is a “threat multiplier for public health, equity, and health systems,” the commentary says. “The confluence of these climate-related pressures should sound alarm bells as they place enormous jeopardy on the practice of dermatology across all scales and regions.”
Health care is among the most carbon-intensive service sectors worldwide, contributing to almost 5% of greenhouse gas emissions globally, the commentary says. And nationally, of the estimated greenhouse gas emissions from the United States, the health care sector contributes 10%, Dr. Parker said in the interview, referring to a 2016 report.
In addition, according to a 2019 report, the United States is the top contributor to health care’s global climate footprint, contributing 27% of health care’s global emissions, Dr. Parker noted.
In their commentary, she and Dr. Boos wrote that individually and practice wide, dermatologists can impact decarbonization through measures such as virtual attendance at medical meetings and greater utilization of telehealth services. Reductions in carbon emissions were demonstrated for virtual isotretinoin follow-up visits in a recent study, and these savings could be extrapolated to other routine follow-up visits for conditions such as rosacea, monitoring of biologics in patients with well-controlled disease, and postoperative wound checks, they said.
But when it comes to measures such as significantly reducing packaging and waste and “curating supply chains to make them more sustainable,” it is medical societies that have the “larger voice and broader relationship with the pharmaceutical industry” and with medical supply manufacturers and distributors, Dr. Parker explained in the interview, noting the potential for reducing the extensive amount of packaging used for drug samples.
Dr. Parker cochairs the American Academy of Dermatology’s Expert Resource Group for Climate Change and Environmental Issues, which was established several years ago, and Dr. Boos is a member of the group’s executive committee.
AAD actions
In its 2018 Position Statement on Climate and Health, the American Academy of Dermatology resolved to raise awareness of the effects of climate change on the skin and educate patients about this, and to “work with other medical societies in ongoing and future efforts to educate the public and mitigate the effects of climate change on global health.”
Asked about the commentary’s call for more collaboration with industry and other stakeholders – and the impact that organized dermatology can have on planetary health – Mark D. Kaufmann, MD, president of the AAD, said in an email that the AAD is “first and foremost an organization focused on providing gold-standard educational resources for dermatologists.”
The academy recognizes that “there are many dermatologic consequences of climate change that will increasingly affect our patients and challenge our membership,” and it has provided education on climate change in forums such as articles, podcasts, and sessions at AAD meetings, said Dr. Kaufmann, clinical professor in the department of dermatology, Icahn School of Medicine at Mount Sinai, New York.
Regarding collaboration with other societies, he said that the AAD’s “focus to date has been on how to provide our members with educational resources to understand and prepare for how climate change may impact their practices and the dermatologic health of their patients,” he said.
The AAD has also sought to address its own carbon footprint and improve sustainability of its operations, including taking steps to reduce plastic and paper waste at its educational events, and to eliminate plastic waste associated with mailing resources like its member magazine, Dr. Kaufmann noted.
And in keeping with the Academy pledge – also articulated in the 2018 position statement – to support and facilitate dermatologists’ efforts to decrease their carbon footprint “in a cost effective (or cost-saving) manner,” Dr. Kaufmann said that the AAD has been offering a program called My Green Doctor as a free benefit of membership.
‘Be part of the solution’
In an interview, Mary E. Maloney, MD, professor of medicine and director of dermatologic surgery at the University of Massachusetts, Worcester, said her practice did an audit of their surgical area and found ways to increase the use of paper-packaged gauze – and decrease use of gauze in hard plastic containers – and otherwise decrease the amount of disposables, all of which take “huge amounts of resources” to create.
In the process, “we found significant savings,” she said. “Little things can turn out, in the long run, to be big things.”
Asked about the commentary, Dr. Maloney, who is involved in the AAD’s climate change resource group, said “the message is that yes, we need to be aware of the diseases affected by climate change. But our greater imperative is to be part of the solution and not part of the problem as far as doing things that affect climate change.”
Organized dermatology needs to broaden its advocacy, she said. “I don’t want us to stop advocating for things for our patients, but I do want us to start advocating for the world ... If we don’t try to [mitigate] climate change, we won’t have patients to advocate for.”
Dr. Parker, an associate editor of The Journal of Climate Change and Health, and Dr. Boos declared no conflicts of interest and no funding source for their commentary. Dr. Maloney said she has no conflicts of interest.
“moving beyond merely discussing skin-related impacts” and toward prioritizing both patient and planetary health.
Dermatologists must make emissions-saving changes in everyday practice, for instance, and the specialty must enlist key stakeholders in public health, nonprofits, and industry – that is, pharmaceutical and medical supply companies – in finding solutions to help mitigate and adapt to climate change, wrote Eva Rawlings Parker, MD, and Markus D. Boos, MD, PhD.
“We have an ethical imperative to act,” they wrote. “The time is now for dermatologists and our medical societies to collectively rise to meet this crisis.”
Their commentary was published online in the International Journal of Dermatology , Journal of the European Academy of Dermatology and Venereology, British Journal of Dermatology, and Pediatric Dermatology.
In an interview, Dr. Parker, assistant professor of dermatology at Vanderbilt University, Nashville, Tenn., said that she and Dr. Boos, associate professor in the division of dermatology and department of pediatrics at the University of Washington, Seattle, were motivated to write the editorial upon finding that dermatology was not represented among more than 230 medical journals that published an editorial in September 2021 calling for emergency action to limit global warming and protect health. In addition to the New England Journal of Medicine and The Lancet, the copublishing journals represented numerous specialties, from nursing and pediatrics, to cardiology, rheumatology, and gastroenterology.
The editorial was not published in any dermatology journals, Dr. Parker said. “It was incredibly disappointing for me along with many of my colleagues who advocate for climate action because we realized it was a missed opportunity for dermatology to align with other medical specialties and be on the forefront of leading climate action to protect health.”
‘A threat multiplier’
The impact of climate change on skin disease is “an incredibly important part of our conversation as dermatologists because many cutaneous diseases are climate sensitive and we’re often seeing the effects of climate change every day in our clinical practices,” Dr. Parker said.
In fact, the impact on skin disease needs to be explored much further through more robust research funding, so that dermatology can better understand not only the incidence and severity of climate-induced changes in skin diseases – including and beyond atopic dermatitis, acne, and psoriasis – but also the mechanisms and pathophysiology involved, she said.
However, the impacts are much broader, she and Dr. Boos, a pediatric dermatologist at Seattle Children’s Hospital, maintain in their commentary. “An essential concept to broker among dermatologists is that the impacts of climate change extend well beyond skin disease by also placing broad pressure” on infrastructure, the economy, financial markets, global supply chains, food and water insecurity, and more, they wrote, noting the deep inequities of climate change.
Climate change is a “threat multiplier for public health, equity, and health systems,” the commentary says. “The confluence of these climate-related pressures should sound alarm bells as they place enormous jeopardy on the practice of dermatology across all scales and regions.”
Health care is among the most carbon-intensive service sectors worldwide, contributing to almost 5% of greenhouse gas emissions globally, the commentary says. And nationally, of the estimated greenhouse gas emissions from the United States, the health care sector contributes 10%, Dr. Parker said in the interview, referring to a 2016 report.
In addition, according to a 2019 report, the United States is the top contributor to health care’s global climate footprint, contributing 27% of health care’s global emissions, Dr. Parker noted.
In their commentary, she and Dr. Boos wrote that individually and practice wide, dermatologists can impact decarbonization through measures such as virtual attendance at medical meetings and greater utilization of telehealth services. Reductions in carbon emissions were demonstrated for virtual isotretinoin follow-up visits in a recent study, and these savings could be extrapolated to other routine follow-up visits for conditions such as rosacea, monitoring of biologics in patients with well-controlled disease, and postoperative wound checks, they said.
But when it comes to measures such as significantly reducing packaging and waste and “curating supply chains to make them more sustainable,” it is medical societies that have the “larger voice and broader relationship with the pharmaceutical industry” and with medical supply manufacturers and distributors, Dr. Parker explained in the interview, noting the potential for reducing the extensive amount of packaging used for drug samples.
Dr. Parker cochairs the American Academy of Dermatology’s Expert Resource Group for Climate Change and Environmental Issues, which was established several years ago, and Dr. Boos is a member of the group’s executive committee.
AAD actions
In its 2018 Position Statement on Climate and Health, the American Academy of Dermatology resolved to raise awareness of the effects of climate change on the skin and educate patients about this, and to “work with other medical societies in ongoing and future efforts to educate the public and mitigate the effects of climate change on global health.”
Asked about the commentary’s call for more collaboration with industry and other stakeholders – and the impact that organized dermatology can have on planetary health – Mark D. Kaufmann, MD, president of the AAD, said in an email that the AAD is “first and foremost an organization focused on providing gold-standard educational resources for dermatologists.”
The academy recognizes that “there are many dermatologic consequences of climate change that will increasingly affect our patients and challenge our membership,” and it has provided education on climate change in forums such as articles, podcasts, and sessions at AAD meetings, said Dr. Kaufmann, clinical professor in the department of dermatology, Icahn School of Medicine at Mount Sinai, New York.
Regarding collaboration with other societies, he said that the AAD’s “focus to date has been on how to provide our members with educational resources to understand and prepare for how climate change may impact their practices and the dermatologic health of their patients,” he said.
The AAD has also sought to address its own carbon footprint and improve sustainability of its operations, including taking steps to reduce plastic and paper waste at its educational events, and to eliminate plastic waste associated with mailing resources like its member magazine, Dr. Kaufmann noted.
And in keeping with the Academy pledge – also articulated in the 2018 position statement – to support and facilitate dermatologists’ efforts to decrease their carbon footprint “in a cost effective (or cost-saving) manner,” Dr. Kaufmann said that the AAD has been offering a program called My Green Doctor as a free benefit of membership.
‘Be part of the solution’
In an interview, Mary E. Maloney, MD, professor of medicine and director of dermatologic surgery at the University of Massachusetts, Worcester, said her practice did an audit of their surgical area and found ways to increase the use of paper-packaged gauze – and decrease use of gauze in hard plastic containers – and otherwise decrease the amount of disposables, all of which take “huge amounts of resources” to create.
In the process, “we found significant savings,” she said. “Little things can turn out, in the long run, to be big things.”
Asked about the commentary, Dr. Maloney, who is involved in the AAD’s climate change resource group, said “the message is that yes, we need to be aware of the diseases affected by climate change. But our greater imperative is to be part of the solution and not part of the problem as far as doing things that affect climate change.”
Organized dermatology needs to broaden its advocacy, she said. “I don’t want us to stop advocating for things for our patients, but I do want us to start advocating for the world ... If we don’t try to [mitigate] climate change, we won’t have patients to advocate for.”
Dr. Parker, an associate editor of The Journal of Climate Change and Health, and Dr. Boos declared no conflicts of interest and no funding source for their commentary. Dr. Maloney said she has no conflicts of interest.
Dermatologists fear effects of Dobbs decision for patients on isotretinoin, methotrexate
More than 3 months after the Dobbs decision by the U.S. Supreme Court overturned Roe v. Wade and revoked the constitutional right to an abortion, Some have beefed up their already stringent instructions and lengthy conversations about avoiding pregnancy while on the medication.
The major fear is that a patient who is taking contraceptive precautions, in accordance with the isotretinoin risk-management program, iPLEDGE, but still becomes pregnant while on isotretinoin may find out about the pregnancy too late to undergo an abortion in her own state and may not be able to travel to another state – or the patient may live in a state where abortions are entirely prohibited and is unable to travel to another state.
Isotretinoin is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane; its former brand name was Accutane.
As of Oct. 7, a total of 14 states have banned most abortions, while 4 others have bans at 6, 15, 18, or 20 weeks. Attempts to restrict abortion on several other states are underway.
“To date, we don’t know of any specific effects of the Dobbs decision on isotretinoin prescribing, but with abortion access banned in many states, we anticipate that this could be a very real issue for individuals who accidentally become pregnant while taking isotretinoin,” said Ilona Frieden, MD, professor of dermatology and pediatrics at the University of California, San Francisco, and chair of the American Academy of Dermatology Association’s iPLEDGE Workgroup.
The iPLEDGE REMS (Risk Evaluation and Mitigation Strategy) is the Food and Drug Administration–required safety program that is in place to manage the risk of isotretinoin teratogenicity and minimize fetal exposure. The work group meets with the FDA and isotretinoin manufacturers to keep the program safe and operating smoothly. The iPLEDGE workgroup has not yet issued any specific statements on the implications of the Dobbs decision on prescribing isotretinoin.
But work on the issue is ongoing by the American Academy of Dermatology. In a statement issued in September, Mark D. Kaufmann, MD, president of the AAD, said that the academy “is continuing to work with its Patient Guidance for State Regulations Regarding Reproductive Health Task Force to help dermatologists best navigate state laws about how care should be implemented for patients who are or might become pregnant, and have been exposed to teratogenic medications.”
The task force, working with the academy, is “in the process of developing resources to help members better assist patients and have a productive and caring dialogue with them,” according to the statement. No specific timeline was given for when those resources might be available.
Methotrexate prescriptions
Also of concern are prescriptions for methotrexate, which is prescribed for psoriasis, atopic dermatitis, and other skin diseases. Soon after the Dobbs decision was announced on June 24, pharmacies began to require pharmacists in states that banned abortions to verify that a prescription for methotrexate was not intended for an abortion, since methotrexate is used in combination with misoprostol for termination of an early pregnancy.
The action was taken, spokespersons for several major pharmacies said, to comply with state laws. According to Kara Page, a CVS spokesperson: “Pharmacists are caught in the middle on this issue.” Laws in some states, she told this news organization, “restrict the dispensing of medications for the purpose of inducing an abortion. These laws, some of which include criminal penalties, have forced us to require pharmacists in these states to validate that the intended indication is not to terminate a pregnancy before they can fill a prescription for methotrexate.”
“New laws in various states require additional steps for dispensing certain prescriptions and apply to all pharmacies, including Walgreens,” Fraser Engerman, a spokesperson for Walgreens, told this news organization. “In these states, our pharmacists work closely with prescribers as needed, to fill lawful, clinically appropriate prescriptions. We provide ongoing training and information to help our pharmacists understand the latest requirements in their area, and with these supports, the expectation is they are empowered to fill these prescriptions.”
The iPLEDGE program has numerous requirements before a patient can begin isotretinoin treatment. Patients capable of becoming pregnant must agree to use two effective forms of birth control during the entire treatment period, which typically lasts 4 or 5 months, as well as 1 month before and 1 month after treatment, or commit to total abstinence during that time.
Perspective: A Georgia dermatologist
Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, who sees patients regularly, practices in Georgia, where abortion is now banned at about 6 weeks of pregnancy. Dr. Yeung worries that some dermatologists in Georgia and elsewhere may not even want to take the risk of prescribing isotretinoin, although the results in treating resistant acne are well documented.
That isn’t his only concern. “Some may not want to prescribe it to a patient who reports they are abstinent and instead require them to go on two forms [of contraception].” Or some women who are not sexually active with anyone who can get them pregnant may also be asked to go on contraception, he said. Abstinence is an alternative option in iPLEDGE.
In the past, he said, well before the Dobbs decision, some doctors have argued that iPLEDGE should not include abstinence as an option. That 2020 report was challenged by others who pointed out that removing the abstinence option would pose ethical issues and may disproportionately affect minorities and others.
Before the Dobbs decision, Dr. Yeung noted, dermatologists prescribing isotretinoin focused on pregnancy prevention but knew that if pregnancy accidentally occurred, abortion was available as an option. “The reality after the decision is, it may or may not be available to all our patients.”
Of the 14 states banning most abortions, 10 are clustered within the South and Southeast. A woman living in Arkansas, which bans most abortions, for example, is surrounded by 6 other states that do the same.
Perspective: An Arizona dermatologist
Christina Kranc, MD, is a general dermatologist in Phoenix and Scottsdale. Arizona now bans most abortions. However, this has not changed her practice much when prescribing isotretinoin, she told this news organization, because when selecting appropriate candidates for the medication, she is strict on the contraceptive requirement, and only very rarely agrees to a patient relying on abstinence.
And if a patient capable of becoming pregnant was only having sex with another patient capable of becoming pregnant? Dr. Kranc said she would still require contraception unless it was impossible for pregnancy to occur.
Among the many scenarios a dermatologist might have to consider are a lesbian cisgender woman who is having, or has only had, sexual activity with another cisgender women.
Perspective: A Connecticut dermatologist
The concern is not only about isotretinoin but all teratogenic drugs, according to Jane M. Grant-Kels, MD, vice chair of dermatology and professor of dermatology, pathology, and pediatrics at the University of Connecticut, Farmington. She often prescribes methotrexate, which is also teratogenic.
Her advice for colleagues: “Whether you believe in abortion or not is irrelevant; it’s something you discuss with your patients.” She, too, fears that doctors in states banning abortions will stop prescribing these medications, “and that is very sad.”
For those practicing in states limiting or banning abortions, Dr. Grant-Kels said, “They need to have an even longer discussion with their patients about how serious this is.” Those doctors need to talk about not only two or three types of birth control, but also discuss with the patient about the potential need for travel, should pregnancy occur and abortion be the chosen option.
Although the newer biologics are an option for psoriasis, they are expensive. And, she said, many insurers require a step-therapy approach, and “want you to start with cheaper medications,” such as methotrexate. As a result, “in some states you won’t have access to the targeted therapies unless a patient fails something like methotrexate.”
Dr. Grant-Kels worries in particular about low-income women who may not have the means to travel to get an abortion.
Need for EC education
In a recent survey of 57 pediatric dermatologists who prescribe isotretinoin, only a third said they felt confident in their understanding of emergency contraception.
The authors of the study noted that the most common reasons for pregnancies during isotretinoin therapy reported to the FDA from 2011 to 2017 “included ineffective or inconsistent use” of contraceptives and “unsuccessful abstinence,” and recommended that physicians who prescribe isotretinoin update and increase their understanding of emergency contraception.
Dr. Yeung, Dr. Kranc, Dr. Grant-Kels, and Dr. Frieden reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
More than 3 months after the Dobbs decision by the U.S. Supreme Court overturned Roe v. Wade and revoked the constitutional right to an abortion, Some have beefed up their already stringent instructions and lengthy conversations about avoiding pregnancy while on the medication.
The major fear is that a patient who is taking contraceptive precautions, in accordance with the isotretinoin risk-management program, iPLEDGE, but still becomes pregnant while on isotretinoin may find out about the pregnancy too late to undergo an abortion in her own state and may not be able to travel to another state – or the patient may live in a state where abortions are entirely prohibited and is unable to travel to another state.
Isotretinoin is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane; its former brand name was Accutane.
As of Oct. 7, a total of 14 states have banned most abortions, while 4 others have bans at 6, 15, 18, or 20 weeks. Attempts to restrict abortion on several other states are underway.
“To date, we don’t know of any specific effects of the Dobbs decision on isotretinoin prescribing, but with abortion access banned in many states, we anticipate that this could be a very real issue for individuals who accidentally become pregnant while taking isotretinoin,” said Ilona Frieden, MD, professor of dermatology and pediatrics at the University of California, San Francisco, and chair of the American Academy of Dermatology Association’s iPLEDGE Workgroup.
The iPLEDGE REMS (Risk Evaluation and Mitigation Strategy) is the Food and Drug Administration–required safety program that is in place to manage the risk of isotretinoin teratogenicity and minimize fetal exposure. The work group meets with the FDA and isotretinoin manufacturers to keep the program safe and operating smoothly. The iPLEDGE workgroup has not yet issued any specific statements on the implications of the Dobbs decision on prescribing isotretinoin.
But work on the issue is ongoing by the American Academy of Dermatology. In a statement issued in September, Mark D. Kaufmann, MD, president of the AAD, said that the academy “is continuing to work with its Patient Guidance for State Regulations Regarding Reproductive Health Task Force to help dermatologists best navigate state laws about how care should be implemented for patients who are or might become pregnant, and have been exposed to teratogenic medications.”
The task force, working with the academy, is “in the process of developing resources to help members better assist patients and have a productive and caring dialogue with them,” according to the statement. No specific timeline was given for when those resources might be available.
Methotrexate prescriptions
Also of concern are prescriptions for methotrexate, which is prescribed for psoriasis, atopic dermatitis, and other skin diseases. Soon after the Dobbs decision was announced on June 24, pharmacies began to require pharmacists in states that banned abortions to verify that a prescription for methotrexate was not intended for an abortion, since methotrexate is used in combination with misoprostol for termination of an early pregnancy.
The action was taken, spokespersons for several major pharmacies said, to comply with state laws. According to Kara Page, a CVS spokesperson: “Pharmacists are caught in the middle on this issue.” Laws in some states, she told this news organization, “restrict the dispensing of medications for the purpose of inducing an abortion. These laws, some of which include criminal penalties, have forced us to require pharmacists in these states to validate that the intended indication is not to terminate a pregnancy before they can fill a prescription for methotrexate.”
“New laws in various states require additional steps for dispensing certain prescriptions and apply to all pharmacies, including Walgreens,” Fraser Engerman, a spokesperson for Walgreens, told this news organization. “In these states, our pharmacists work closely with prescribers as needed, to fill lawful, clinically appropriate prescriptions. We provide ongoing training and information to help our pharmacists understand the latest requirements in their area, and with these supports, the expectation is they are empowered to fill these prescriptions.”
The iPLEDGE program has numerous requirements before a patient can begin isotretinoin treatment. Patients capable of becoming pregnant must agree to use two effective forms of birth control during the entire treatment period, which typically lasts 4 or 5 months, as well as 1 month before and 1 month after treatment, or commit to total abstinence during that time.
Perspective: A Georgia dermatologist
Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, who sees patients regularly, practices in Georgia, where abortion is now banned at about 6 weeks of pregnancy. Dr. Yeung worries that some dermatologists in Georgia and elsewhere may not even want to take the risk of prescribing isotretinoin, although the results in treating resistant acne are well documented.
That isn’t his only concern. “Some may not want to prescribe it to a patient who reports they are abstinent and instead require them to go on two forms [of contraception].” Or some women who are not sexually active with anyone who can get them pregnant may also be asked to go on contraception, he said. Abstinence is an alternative option in iPLEDGE.
In the past, he said, well before the Dobbs decision, some doctors have argued that iPLEDGE should not include abstinence as an option. That 2020 report was challenged by others who pointed out that removing the abstinence option would pose ethical issues and may disproportionately affect minorities and others.
Before the Dobbs decision, Dr. Yeung noted, dermatologists prescribing isotretinoin focused on pregnancy prevention but knew that if pregnancy accidentally occurred, abortion was available as an option. “The reality after the decision is, it may or may not be available to all our patients.”
Of the 14 states banning most abortions, 10 are clustered within the South and Southeast. A woman living in Arkansas, which bans most abortions, for example, is surrounded by 6 other states that do the same.
Perspective: An Arizona dermatologist
Christina Kranc, MD, is a general dermatologist in Phoenix and Scottsdale. Arizona now bans most abortions. However, this has not changed her practice much when prescribing isotretinoin, she told this news organization, because when selecting appropriate candidates for the medication, she is strict on the contraceptive requirement, and only very rarely agrees to a patient relying on abstinence.
And if a patient capable of becoming pregnant was only having sex with another patient capable of becoming pregnant? Dr. Kranc said she would still require contraception unless it was impossible for pregnancy to occur.
Among the many scenarios a dermatologist might have to consider are a lesbian cisgender woman who is having, or has only had, sexual activity with another cisgender women.
Perspective: A Connecticut dermatologist
The concern is not only about isotretinoin but all teratogenic drugs, according to Jane M. Grant-Kels, MD, vice chair of dermatology and professor of dermatology, pathology, and pediatrics at the University of Connecticut, Farmington. She often prescribes methotrexate, which is also teratogenic.
Her advice for colleagues: “Whether you believe in abortion or not is irrelevant; it’s something you discuss with your patients.” She, too, fears that doctors in states banning abortions will stop prescribing these medications, “and that is very sad.”
For those practicing in states limiting or banning abortions, Dr. Grant-Kels said, “They need to have an even longer discussion with their patients about how serious this is.” Those doctors need to talk about not only two or three types of birth control, but also discuss with the patient about the potential need for travel, should pregnancy occur and abortion be the chosen option.
Although the newer biologics are an option for psoriasis, they are expensive. And, she said, many insurers require a step-therapy approach, and “want you to start with cheaper medications,” such as methotrexate. As a result, “in some states you won’t have access to the targeted therapies unless a patient fails something like methotrexate.”
Dr. Grant-Kels worries in particular about low-income women who may not have the means to travel to get an abortion.
Need for EC education
In a recent survey of 57 pediatric dermatologists who prescribe isotretinoin, only a third said they felt confident in their understanding of emergency contraception.
The authors of the study noted that the most common reasons for pregnancies during isotretinoin therapy reported to the FDA from 2011 to 2017 “included ineffective or inconsistent use” of contraceptives and “unsuccessful abstinence,” and recommended that physicians who prescribe isotretinoin update and increase their understanding of emergency contraception.
Dr. Yeung, Dr. Kranc, Dr. Grant-Kels, and Dr. Frieden reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
More than 3 months after the Dobbs decision by the U.S. Supreme Court overturned Roe v. Wade and revoked the constitutional right to an abortion, Some have beefed up their already stringent instructions and lengthy conversations about avoiding pregnancy while on the medication.
The major fear is that a patient who is taking contraceptive precautions, in accordance with the isotretinoin risk-management program, iPLEDGE, but still becomes pregnant while on isotretinoin may find out about the pregnancy too late to undergo an abortion in her own state and may not be able to travel to another state – or the patient may live in a state where abortions are entirely prohibited and is unable to travel to another state.
Isotretinoin is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane; its former brand name was Accutane.
As of Oct. 7, a total of 14 states have banned most abortions, while 4 others have bans at 6, 15, 18, or 20 weeks. Attempts to restrict abortion on several other states are underway.
“To date, we don’t know of any specific effects of the Dobbs decision on isotretinoin prescribing, but with abortion access banned in many states, we anticipate that this could be a very real issue for individuals who accidentally become pregnant while taking isotretinoin,” said Ilona Frieden, MD, professor of dermatology and pediatrics at the University of California, San Francisco, and chair of the American Academy of Dermatology Association’s iPLEDGE Workgroup.
The iPLEDGE REMS (Risk Evaluation and Mitigation Strategy) is the Food and Drug Administration–required safety program that is in place to manage the risk of isotretinoin teratogenicity and minimize fetal exposure. The work group meets with the FDA and isotretinoin manufacturers to keep the program safe and operating smoothly. The iPLEDGE workgroup has not yet issued any specific statements on the implications of the Dobbs decision on prescribing isotretinoin.
But work on the issue is ongoing by the American Academy of Dermatology. In a statement issued in September, Mark D. Kaufmann, MD, president of the AAD, said that the academy “is continuing to work with its Patient Guidance for State Regulations Regarding Reproductive Health Task Force to help dermatologists best navigate state laws about how care should be implemented for patients who are or might become pregnant, and have been exposed to teratogenic medications.”
The task force, working with the academy, is “in the process of developing resources to help members better assist patients and have a productive and caring dialogue with them,” according to the statement. No specific timeline was given for when those resources might be available.
Methotrexate prescriptions
Also of concern are prescriptions for methotrexate, which is prescribed for psoriasis, atopic dermatitis, and other skin diseases. Soon after the Dobbs decision was announced on June 24, pharmacies began to require pharmacists in states that banned abortions to verify that a prescription for methotrexate was not intended for an abortion, since methotrexate is used in combination with misoprostol for termination of an early pregnancy.
The action was taken, spokespersons for several major pharmacies said, to comply with state laws. According to Kara Page, a CVS spokesperson: “Pharmacists are caught in the middle on this issue.” Laws in some states, she told this news organization, “restrict the dispensing of medications for the purpose of inducing an abortion. These laws, some of which include criminal penalties, have forced us to require pharmacists in these states to validate that the intended indication is not to terminate a pregnancy before they can fill a prescription for methotrexate.”
“New laws in various states require additional steps for dispensing certain prescriptions and apply to all pharmacies, including Walgreens,” Fraser Engerman, a spokesperson for Walgreens, told this news organization. “In these states, our pharmacists work closely with prescribers as needed, to fill lawful, clinically appropriate prescriptions. We provide ongoing training and information to help our pharmacists understand the latest requirements in their area, and with these supports, the expectation is they are empowered to fill these prescriptions.”
The iPLEDGE program has numerous requirements before a patient can begin isotretinoin treatment. Patients capable of becoming pregnant must agree to use two effective forms of birth control during the entire treatment period, which typically lasts 4 or 5 months, as well as 1 month before and 1 month after treatment, or commit to total abstinence during that time.
Perspective: A Georgia dermatologist
Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, who sees patients regularly, practices in Georgia, where abortion is now banned at about 6 weeks of pregnancy. Dr. Yeung worries that some dermatologists in Georgia and elsewhere may not even want to take the risk of prescribing isotretinoin, although the results in treating resistant acne are well documented.
That isn’t his only concern. “Some may not want to prescribe it to a patient who reports they are abstinent and instead require them to go on two forms [of contraception].” Or some women who are not sexually active with anyone who can get them pregnant may also be asked to go on contraception, he said. Abstinence is an alternative option in iPLEDGE.
In the past, he said, well before the Dobbs decision, some doctors have argued that iPLEDGE should not include abstinence as an option. That 2020 report was challenged by others who pointed out that removing the abstinence option would pose ethical issues and may disproportionately affect minorities and others.
Before the Dobbs decision, Dr. Yeung noted, dermatologists prescribing isotretinoin focused on pregnancy prevention but knew that if pregnancy accidentally occurred, abortion was available as an option. “The reality after the decision is, it may or may not be available to all our patients.”
Of the 14 states banning most abortions, 10 are clustered within the South and Southeast. A woman living in Arkansas, which bans most abortions, for example, is surrounded by 6 other states that do the same.
Perspective: An Arizona dermatologist
Christina Kranc, MD, is a general dermatologist in Phoenix and Scottsdale. Arizona now bans most abortions. However, this has not changed her practice much when prescribing isotretinoin, she told this news organization, because when selecting appropriate candidates for the medication, she is strict on the contraceptive requirement, and only very rarely agrees to a patient relying on abstinence.
And if a patient capable of becoming pregnant was only having sex with another patient capable of becoming pregnant? Dr. Kranc said she would still require contraception unless it was impossible for pregnancy to occur.
Among the many scenarios a dermatologist might have to consider are a lesbian cisgender woman who is having, or has only had, sexual activity with another cisgender women.
Perspective: A Connecticut dermatologist
The concern is not only about isotretinoin but all teratogenic drugs, according to Jane M. Grant-Kels, MD, vice chair of dermatology and professor of dermatology, pathology, and pediatrics at the University of Connecticut, Farmington. She often prescribes methotrexate, which is also teratogenic.
Her advice for colleagues: “Whether you believe in abortion or not is irrelevant; it’s something you discuss with your patients.” She, too, fears that doctors in states banning abortions will stop prescribing these medications, “and that is very sad.”
For those practicing in states limiting or banning abortions, Dr. Grant-Kels said, “They need to have an even longer discussion with their patients about how serious this is.” Those doctors need to talk about not only two or three types of birth control, but also discuss with the patient about the potential need for travel, should pregnancy occur and abortion be the chosen option.
Although the newer biologics are an option for psoriasis, they are expensive. And, she said, many insurers require a step-therapy approach, and “want you to start with cheaper medications,” such as methotrexate. As a result, “in some states you won’t have access to the targeted therapies unless a patient fails something like methotrexate.”
Dr. Grant-Kels worries in particular about low-income women who may not have the means to travel to get an abortion.
Need for EC education
In a recent survey of 57 pediatric dermatologists who prescribe isotretinoin, only a third said they felt confident in their understanding of emergency contraception.
The authors of the study noted that the most common reasons for pregnancies during isotretinoin therapy reported to the FDA from 2011 to 2017 “included ineffective or inconsistent use” of contraceptives and “unsuccessful abstinence,” and recommended that physicians who prescribe isotretinoin update and increase their understanding of emergency contraception.
Dr. Yeung, Dr. Kranc, Dr. Grant-Kels, and Dr. Frieden reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.