User login
Impact of Diagnostic Testing on Pediatric Patients With Pharyngitis: Evidence From a Large Health Plan
From the Department of Pharmaceutical and Health Economics, University of Southern California, Los Angeles, CA, (Drs. Sangha and McCombs), Department of Pediatrics, Keck School of Medicine, and Department of Clinical Pharmacy, School of Pharmacy, University of Southern California, Los Angeles, CA, (Dr. Steinberg), and Leonard Schaeffer Center for Health Policy and Economics, University of Southern California, Los Angeles, CA (Dr. McCombs).
Objective: The recommended treatment for children and adolescents under 18 years of age who have a positive test for group A Streptococcus (GAS) are antibiotics using the “test and treat” strategy to detect and treat GAS for pediatric pharyngitis. This study used paid claims data to document the extent to which real-world treatment patterns are consistent with these recommendations. We document the factors correlated with testing and treatment, then examine the effects of receiving a GAS test and being treated with an antibiotic impact the likelihood of a revisit for an acute respiratory tract infection within 28 days.
Methods: This retrospective cohort study used Optum Insight Clinformatics data for medical and pharmacy claims from 2011-2013 to identify episodes of care for children and adolescents with pharyngitis around their index visit (± 6 months). The sample population included children and adolescents under 18 years of age with a diagnosis of pharyngitis. Multivariable logistic regression analyses were used to document factors associated with receipt of GAS test and antibiotic treatment. Next, we used logistic regression models to estimate the impact of test and treat recommendation on revisit risk.
Results: There were 24 685 treatment episodes for children and adolescents diagnosed with pharyngitis. Nearly 47% of these episodes included a GAS test and 48% of tested patients were prescribed an antibiotic prescription. Failing to perform a GAS test increased the risk of a revisit within 28 days by 44%. The use of antibiotics by tested and untested patients had no impact on revisit risk.
Conclusion: While the judicious use of antibiotics is important in managing pharyngitis infections and managing complications, the use of rapid diagnostic tools was found to be the determining factor in reducing revisits for pediatric patients with pharyngitis.
Keywords: pediatrics; pharyngitis; respiratory infections; acute infections; diagnostic tests; group A Streptococcus; antibiotics; revisits.
Acute pharyngitis is a common acute respiratory tract infection (ARTI) in children. Group A β-hemolytic streptococci (GABHS) is the most common bacterial etiology for pediatric pharyngitis, accounting for 15% to 30% of cases.1
Beyond clinical assessment, laboratory diagnostic testing generally plays a limited role in guiding appropriate antibiotic prescribing for patients with an ARTI.2,3 Most diagnostic tests require 2 or 3 days to result, incur additional costs, and may delay treatment.4 While these tests do not provide clear and timely guidance on which specific antibiotic is appropriate for ARTI patients, this is not the case for patients with pharyngitis.5,6,7 A rapid diagnostic test exists to identify pharyngitis patients with GABHS which accounts for 1 in 4 children with acute sore throat.1,4,6 Both the American Academy of Pediatrics and the Infectious Diseases Society of America recommend antibiotic treatment for children and adolescents under 18 years of age who have a positive test for group A Streptococcus (GAS).8,9 This “test and treat” protocol has been consistently included in the Healthcare Effectiveness Data and Information Set (HEDIS) standards over time for pediatric pharyngitis patients aged 3 to 18 years before dispensing an antibiotic.10
Sinusitis, pneumonia, and acute otitis media are considered ARTIs where antibiotic treatment is justified. Therefore, pharyngitis of unclear etiology seen with these comorbid infections may not always undergo GAS testing but move directly to the patient being prescribed antibiotics. This analysis enumerates ARTI-related comorbidities present together with the initial coded pharyngitis diagnosis to evaluate their impact on the provider’s decision to test and treat, and on revisit risk.
Antibiotic treatment for GAS patients is likely to eradicate the acute GABHS infection within 10 days. Penicillin and amoxicillin are commonly recommended because of their narrow spectrum of activity, few adverse effects, established efficacy, and modest cost. Alternative antibiotics for patients with penicillin allergy, or with polymicrobial infection seen on culture results, include a first-generation cephalosporin, clindamycin, clarithromycin (Biaxin), or azithromycin (Zithromax).1,8,11 However, while compliance with these HEDIS guidelines has been evaluated, the outcome effects of following the HEDIS “test and treat” recommendations for children with pharyngitis have not been adequately evaluated.
These outcome evaluations have increasing importance as the latest HEDIS survey has shown testing rates in commercial Preferred Provider Organizations (PPO) falling from 86.4% in 2018 to 75.9% in 2019, the lowest rate of testing since 2009, with similar reductions under 80% for Health Maintenance Organizations (HMO).10 While health plans may execute cost-benefit analyses and algorithms to forge best practices for GAS testing in children and adolescents presenting with symptoms of pharyngitis, it is important to regard the wasteful resource utilization and additional cost of revisits that may offset any gains accrued by more focused GAS testing outside the existing clinical guidelines and HEDIS measures. This may be of particular importance in documenting infection and sparing antibiotic therapy in toddlers and younger.
The objective of this study was to investigate the correlation between testing and antibiotic use on the likelihood of a revisit for an acute respiratory tract infection within 28 days. To achieve this objective, this investigation consists of 3 sequential analyses. First, we document the factors associated with the decision to test the patient for a GABHS infection using the GAS test. Next, we document the factors associated with the decision to use an antibiotic to treat the patient as a function of having tested the patient. Finally, we investigate the impact of the testing and treatment decisions on the likelihood of a revisit within 28 days.
Methods
Study design
This was a retrospective cohort study of episodes of treatment for pediatric patients with pharyngitis. Episodes were identified using data derived from the Optum Insight Clinformatics claims database provided to the University of Southern California to facilitate the training of graduate students. These data cover commercially insured patients with both medical and pharmacy benefits. Data were retrieved from the 3-year period spanning 2011-2013. An episode of care was identified based on date of the first (index) outpatient visit for a pharyngitis diagnosis (International Classification of Diseases, Ninth Revision [ICD-9]: 462, 463, 034.0). Outpatient visits were defined by visit setting: ambulatory clinics, physician offices, emergency rooms, and urgent care facilities. Each pharyngitis treatment episode was then screened for at least a 6-month enrollment in a health insurance plan prior and subsequent to the index visit using Optum enrollment data. Finally, eligible treatment episodes were restricted to children and adolescents under 18 years of age, who had an index outpatient visit for a primary diagnosis of acute pharyngitis.
A diagnostic profile was created for each episode using the diagnoses recorded for the index visit. Up to 3 diagnoses may be recorded for any outpatient visit and the first recorded diagnosis was assumed to be the primary diagnosis for that episode. Any secondary diagnoses recorded on the index visit were used to define comorbidities present at the index visit. ARTI-related comorbidities included: acute otitis media (AOM), bronchitis, sinusitis, pneumonia, and upper respiratory infection (URI). Other comorbid medical diagnoses were documented using diagnostic data from the pre-index period. Dichotomous variables for the following categories were created: mental disorders, nervous system disorders, respiratory symptoms, fever, injury and poisoning, other, or no diseases.
Prior visits for other respiratory infections in the previous 90 days were also identified for patients based on their index visit for pharyngitis. Similarly, any subsequent visits, within 28 days of the index visit, were also recorded to measure the health outcome for analysis. Practice settings include physician offices and federally qualified health centers, state and local health clinics, outpatient hospitals facilities, emergency departments, and other outpatient settings such as walk-in retail health clinic or ambulatory centers. Providers include primary care physicians (family practice, pediatricians, internal medicine), specialty care physicians (emergency medicine, preventive medicine), nonphysician providers (nurse practitioners, physician assistants) and other providers (urgent care, acute outpatient care, ambulatory care centers). Seasons of the year were determined based on the index date of the episode to account for possible seasonality in pharyngitis treatment. Lastly, a previous visits variable was created to identify whether the child had nonpharyngitis ARTI visits in the 3 months prior to the index visit.
Demographic variables were created based on enrollment and the socioeconomic data available in the Optum socioeconomic status file. These variables include patient age, race, sex, household income, geographic location, practice setting type, provider specialty, and type of insurance. An estimate of patient household income was based on algorithms using census block groups. Income categories were informed by the federal guidelines for a family of 4. A low-income family was defined as earning less than $50 000; a middle-income family earned between $50 000 and $75 000, and a high-income family earned $75 000 and above.12 Patient insurance type was categorized as HMO, Exclusive Provider Organization (EPO), Point of Service (POS), and PPO. Race was identified as White, Black, Hispanic, and Asian. Patient location was defined according to national census regions.
Outcomes
GAS test
The HEDIS measures for pharyngitis recommend using the GAS test to identify the bacterial etiology of the pharyngitis infection. Patients who received the test were identified based on Current Procedural Terminology (CPT) codes 87070-71, 87081, 87430, 87650-52, and 87880.10
Antibiotic treatment
The pharmacy administrative claims dataset was used to identify study patients who filled a prescription for an antibiotic during their pharyngitis treatment episode. Optum pharmacy data identify the medications received, specifies the date of prescription filling, National Drug Codes, and American Hospital Formulary Service (AHFS) Classification System codes for each medication. We used the AHFS Pharmacologic-Therapeutic classification of antibiotics to create dichotomous variables documenting the antibacterial used by each patient.13 These are categorized under antibacterial including penicillins, cephalosporins (first, second, third, fourth generation cephalosporins), macrolides (first generation and others), tetracyclines, sulfonamides, fluoroquinolones (ciprofloxacin, levofloxacin, moxifloxacin), cephamycin, carbapenems, and β-lactam antibiotics (amoxicillin, amoxicillin/clavulanate, cephalexin, cefuroxime, cefdinir).
Revisits to physician or other provider
Revisits within 28 days were used as the measure of patient outcomes related to testing and filling of an antibiotic prescription for acute pharyngitis. Revisits may also be due to a patient returning for a follow-up, alternative treatment, worsening pharyngitis, or for another ARTI. An ARTI-related revisit also increases total resources used to treat pediatric pharyngitis patients.
Statistical analysis
Logistic regression was used for all 3 analyses conducted in this study. First, we determined the patient and treating physician characteristics that impact the decision to use GAS testing for pharyngitis. Second, we identified those factors that impact the decision to use antibiotic prescriptions among children who were diagnosed with pharyngitis adding in the dichotomous variable indicating if the patient had received a GAS test. Third, we used a logit regression analysis to document if receiving a GAS test and/or an antibiotic impacted the likelihood of a revisit by comparing revisit risk. To estimate the effect of testing and/or antibiotic use, we divided patients into 4 groups based on whether the patient received a GAS test and/or an antibiotic prescription. This specification of the analysis of revisits as an outcome focuses on adherence to HEDIS “test and treat” guidelines10:
- Patients who were not tested yet filled an antibiotic prescription. This decision was likely based on the clinician’s judgment of the patient’s signs and symptoms, and confirmational testing not performed.
- Patients who were not tested and did not fill an antibiotic prescription. Apparently, in the clinician’s judgment the patient’s signs and symptoms were such that the infection did not warrant treatment and the clinical presentation did not necessitate the GAS test to confirm the recorded diagnosis of pharyngitis.
- Patients who were tested and received antibiotic prescription, likely because the test was positive for GABHS.
- Patients who were tested and did not receive antibiotic prescription.
We tested for statistically significant differences in baseline characteristics across these 4 patient groups using t tests for continuous variables and χ2 tests for categorical variables. Odds ratios (OR) and CI were computed for the influential variables included the regression analyses.
We conducted a sensitivity analysis using a model specification which included the dichotomous variables for testing and for treatment, and the interaction term between these variables to assess if treatment effects varied in tested and untested patients. We also estimated this model of revisit risk using revisits within 7 days as the outcome variable.
All analyses were completed using STATA/IC 13 (StataCorp, College Station, TX).
Results
There were 24 685 treatment episodes for children diagnosed with pharyngitis. Nearly 47% of these episodes included GAS testing and 47% of the tested patients filled an antibiotic prescription. Similarly, 53% of patients were not tested and 49% of untested patients filled an antibiotic prescription. As a result, the 4 groups identified for analysis were evenly distributed: untested and no prescription (26.9%), untested and prescription (26.3%), tested and prescription (21.9%), and tested and no prescription (24.9%) (Figure).
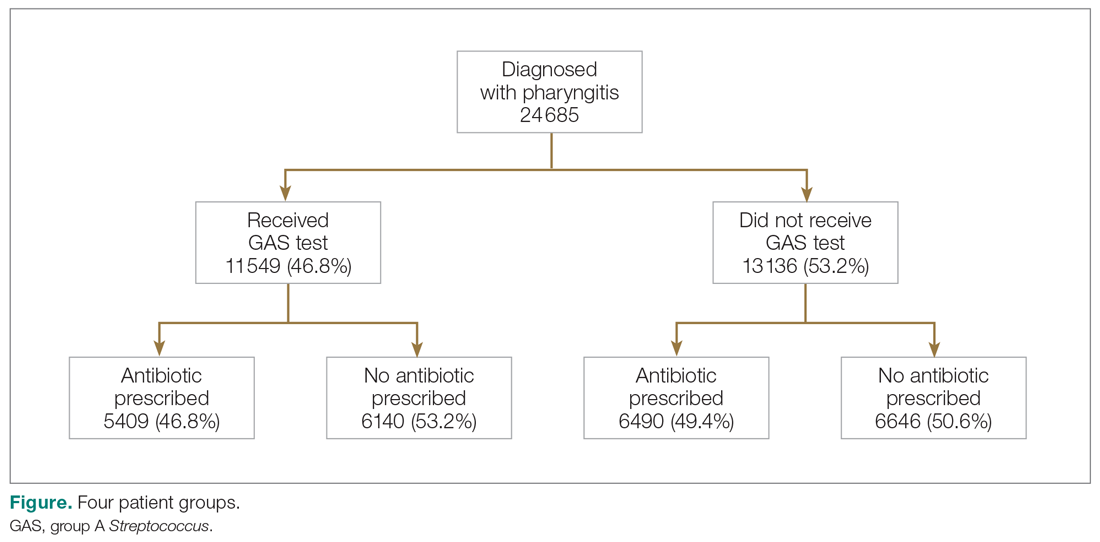
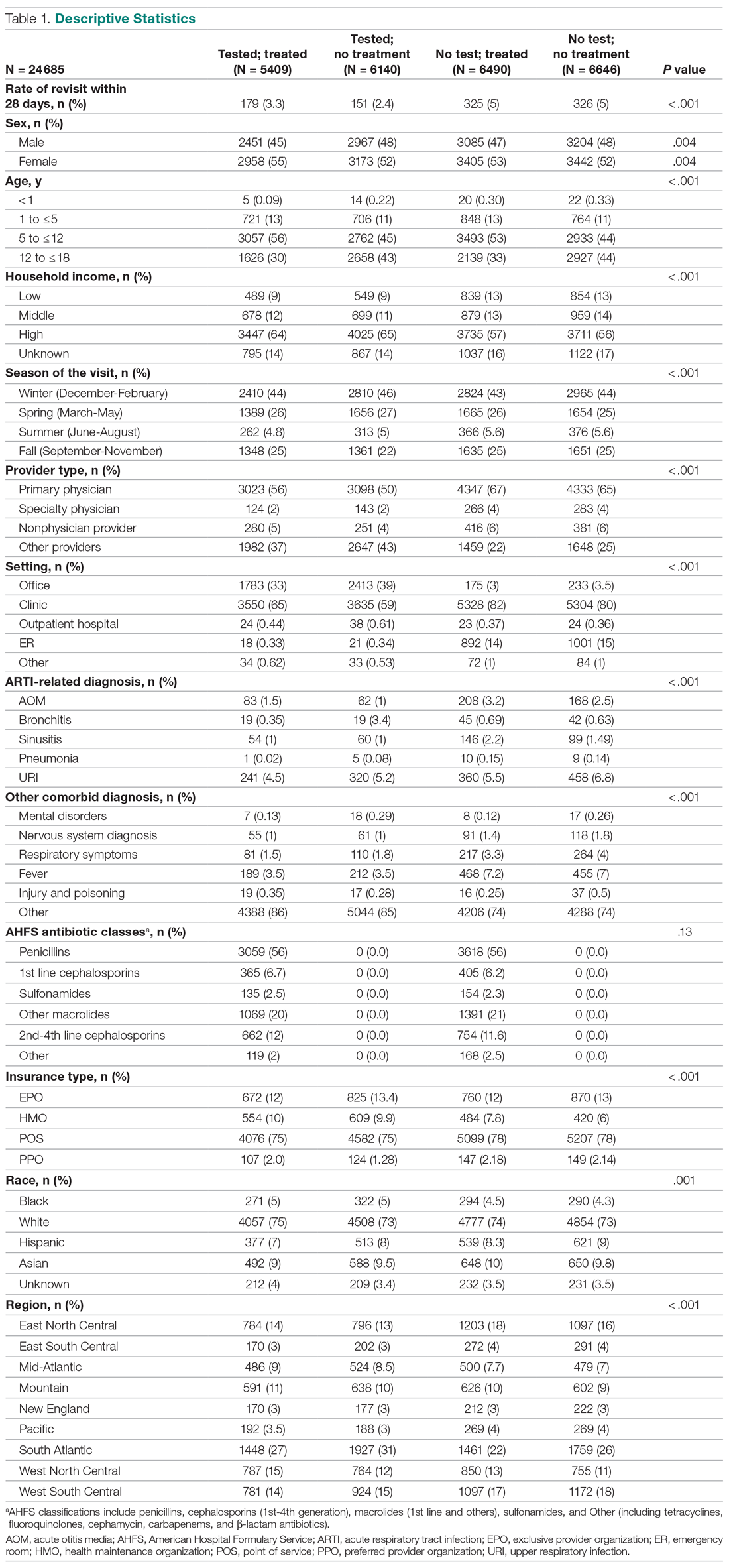
Table 1 presents the descriptive statistics for these 4 patient groups. Note first that the rate of revisits within 28 days is under 5% across all groups. Second, the 2 tested groups have a lower revisit rate than the untested groups: the tested and treated have a revisit rate of 3.3%, and the tested and untreated have a revisit rate of 2.4%, while both the untested groups have a revisit rate of nearly 5%. These small absolute differences in revisit rates across groups were statistically significant.
Factors associated with receiving GAS test
Several factors were found to impact the decision to test (Table 2). Only 9.7% of children were reported to have any ARTI coinfection. As expected, these comorbidities resulted in a significantly lower likelihood of receiving the GAS test: AOM, bronchitis, sinusitis, pneumonia, and URI as comorbid infections had a 48%, 41%, 37%, 63%, and 13% lower likelihood of receiving the GAS test, respectively, than those with no comorbidities. Similarly, children with fever and respiratory symptoms were 35% and 45% less likely to receiving the GAS test, respectively. This is consistent with our expectation that comorbid ARTI infections will lead many providers to forgo testing.
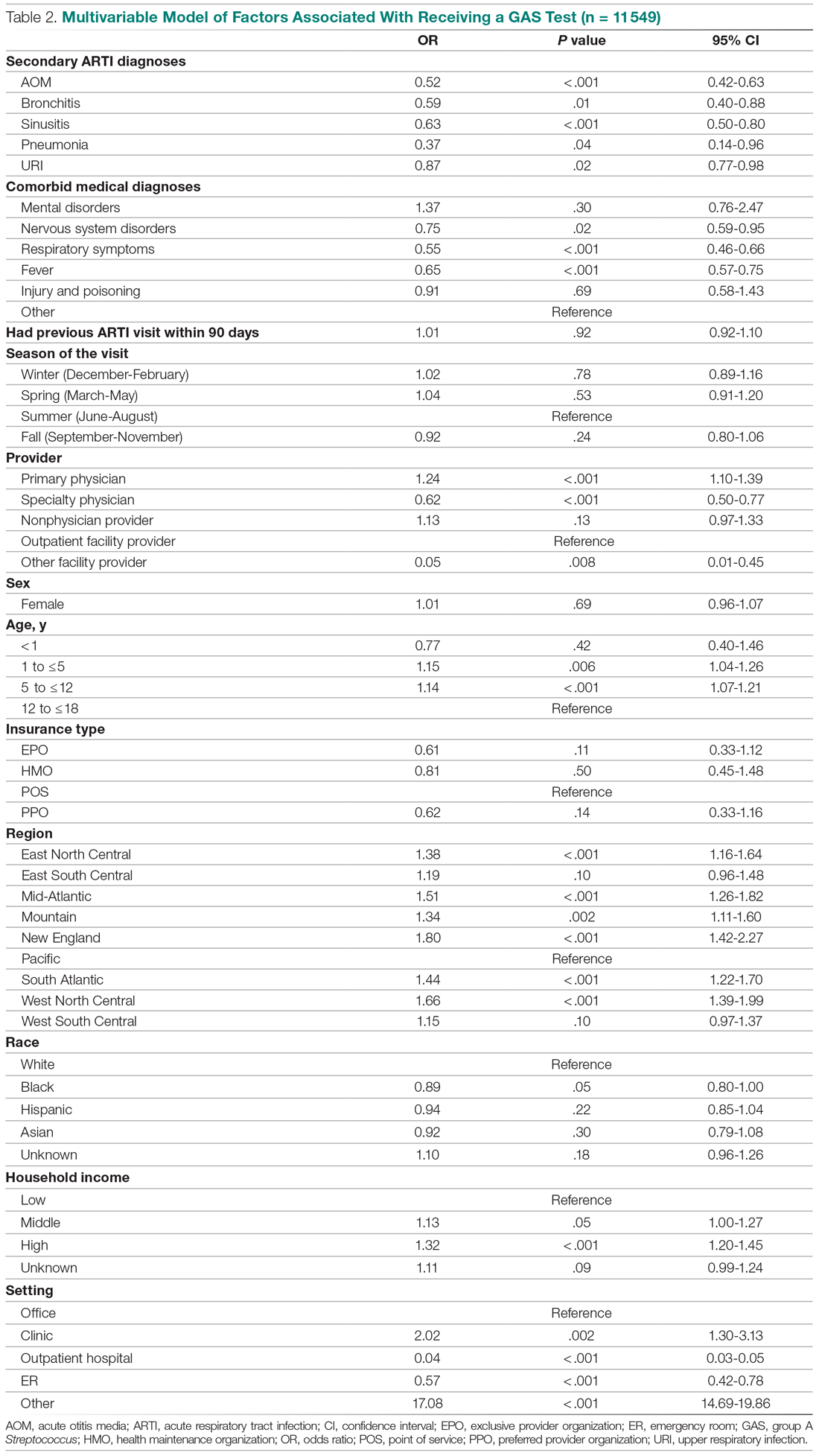
Provider type and patient age also plays a role in receipt of the GAS test. Relative to outpatient facility providers, primary care physicians were 24% more likely and specialty physicians were 38% less likely of employing the GAS test. The child’s age played a significant role in receipt of the GAS test. Children aged 1 to 5 years and 5 to 12 years were 15% and 14% more likely to receive the test compared to children older than 12 years.
Pharyngitis patients have disproportionately higher odds of receiving a GAS test in most regions of the country compared to the Pacific region. For instance, children in the Mid-Atlantic region have 51% higher odds of receiving a GAS test while children in New England have 80% higher odds of receiving the same test.
Black children have 11% lower odds of receiving the GAS test compared to White children. Both middle-income and high-income children have 12% and 32% higher odds of receiving the test compared to low-income children. Compared to office-based visits, children visiting a clinic were twice as likely to receive a GAS test while those seen in the emergency room have 43% lower odds of receiving a GAS test. Hospital outpatient departments, which account for less than 1% of all visits, rarely used a GAS test which could be a statistical artifact due to small sample size. Lastly, insurance and season of the year had no significant impact of receipt of a GAS test.
Factors associated with receiving antibiotic prescription
Surprisingly, receiving the GAS test has a small but insignificant impact on the likelihood that the patient will receive an antibiotic prescription (Table 3) (Adjusted OR = 1.055, P = .07). After controlling for receipt of a GAS test, children with AOM and sinusitis comorbidities have an increased likelihood of being prescribed an antibiotic. Children with URI have a lower likelihood of being prescribed an antibiotic. Additionally, relative to primary care physicians, children visiting nonphysician providers for pharyngitis were more likely to be prescribed an antibiotic.
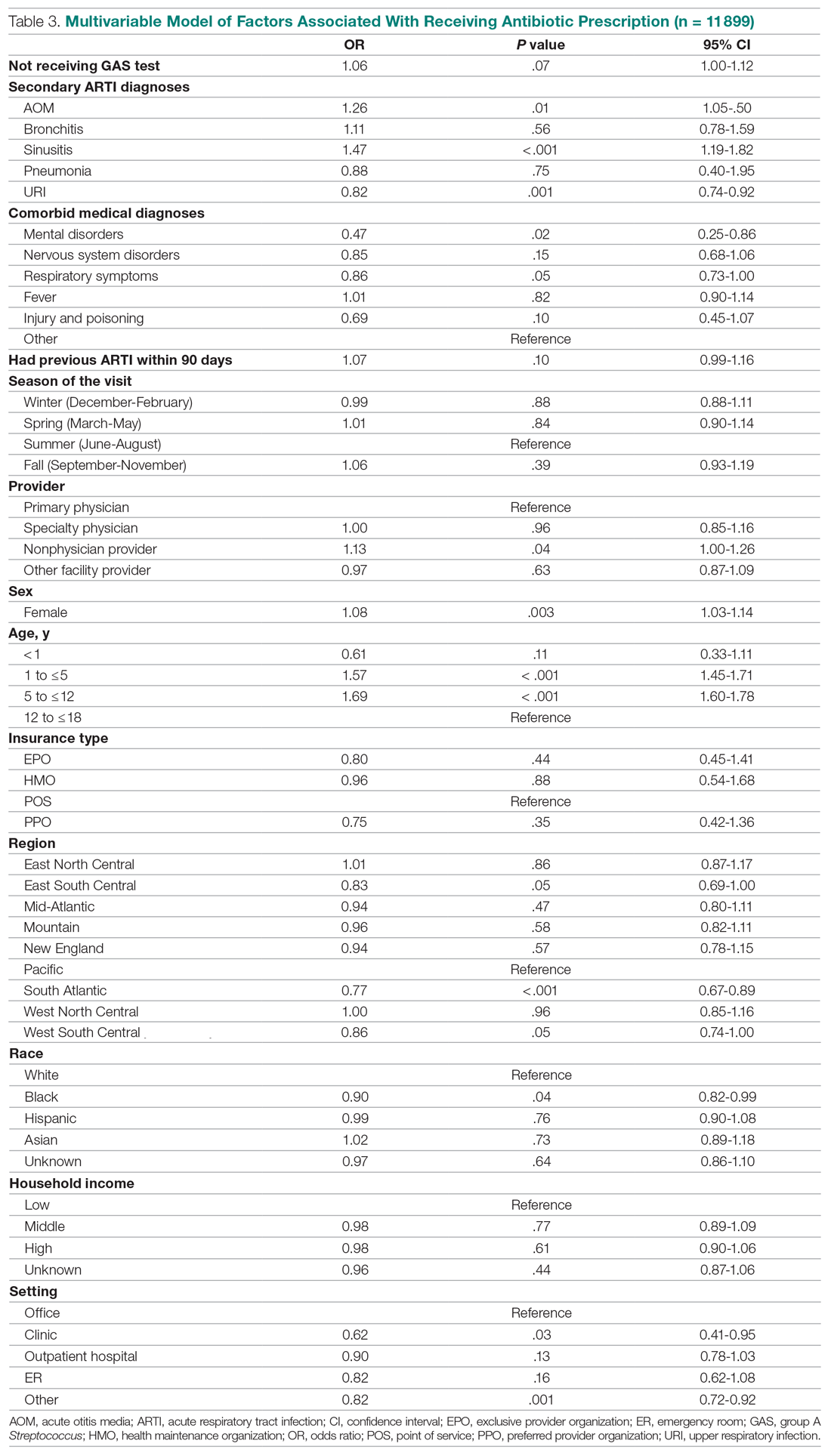
Children under 12 years of age were more likely to use an antibiotic compared to children 12 years and older. Geographically, there is some evidence of regional variation in antibiotic use as well. Children in the south Atlantic, west-south central, and southeast central regions had a significantly lower odds of being prescribed an antibiotic respectively than pharyngitis patients in the Pacific region. Black children had a 10% lower likelihood of being prescribed an antibiotic compared to White children, possibly related to their lower rate of GAS testing. Compared to office-based visits, children visiting a clinic were less likely to use an antibiotic. Household income, insurance type, and season had no significant impact on revisit risk.
Effects of GAS test and antibiotic prescriptions on likelihood of revisits
The multivariate analysis of the risk of a revisit within 28 days is presented in Table 4. Children with pharyngitis who tested and did not receive an antibiotic serve as the reference comparison group for this analysis to illustrate the impact of using the GAS test and treatment with an antibiotic. The results in Table 4 are quite clear: patients who receive the GAS test were significantly less likely to have a revisit within 28 days. Moreover, within the group of patients who were tested, those not receiving an antibiotic, presumedly because their GAS test was negative, experienced the lowest risk of a revisit. This result is consistent with the data in Table 1. Moreover, using an antibiotic had no impact on the likelihood of a revisit in patients not receiving the GAS test. This result is also consistent with Table 1.
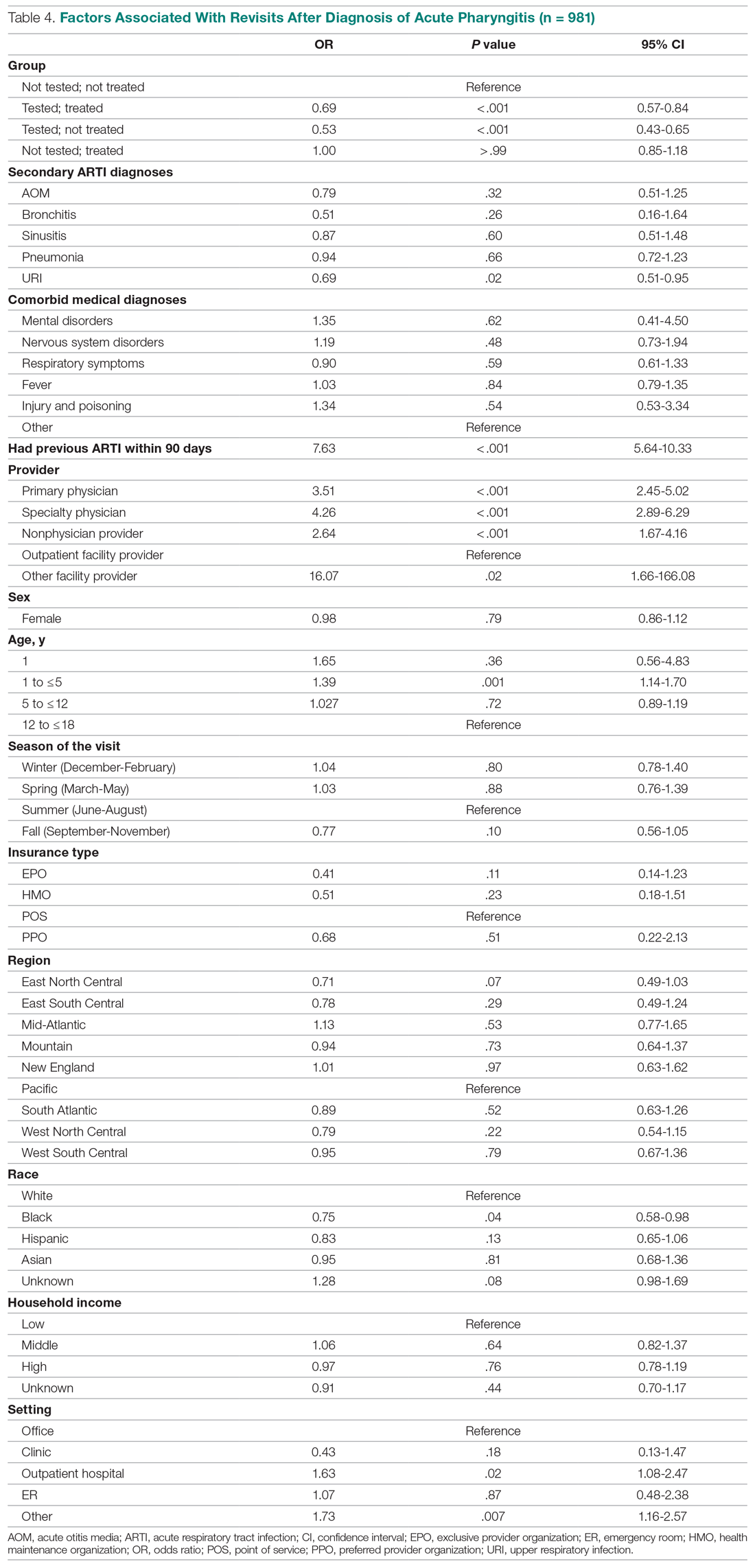
Other results from the analysis of revisit risk may be of interest to clinicians. Pharyngitis patients with a prior episode of treatment within 90 days for an acute respiratory tract infection were more than 7 times more likely to experience a revisit within 28 days of the pharyngitis diagnosis than patients without a history of recent ARTI infections. Age is also a risk factor in likelihood of initiating a revisit. Children under 1 year and children aged 1 to 5 years were more likely to have a revisit than children aged more than 12 years. Compared to White children, Black children were 25% (P = .04) less likely to have a revisit. The care setting also has a significant impact on revisit risk. Children visiting outpatient hospital and other care settings had a significantly higher revisit risk than those visiting a physician’s office. Lastly, household income, geographic region, season, medical comorbidities, gender, and insurance type have no significant impact on revisit risk.
Sensitivity analysis
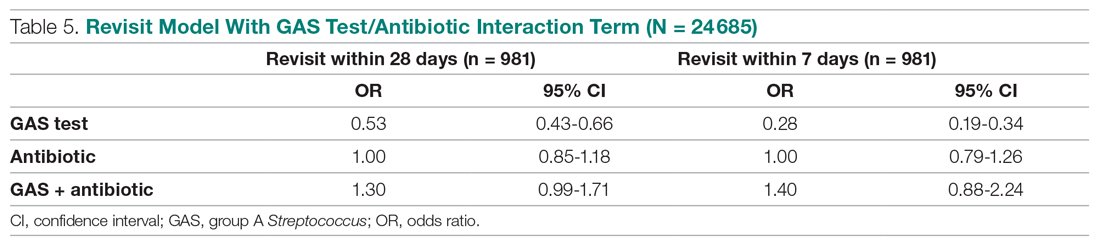
The results from the analysis of 7-day and 28-day revisit risk are summarized in Table 5. These results indicate that patients who were tested had a more significant decrease in revisit risk at 7 days (72%) than was evident at 28 days (47% reduction). Receiving an antibiotic, with or without the test, had no impact on revisit risk.
Discussion
Published data on revisits for pharyngitis are lacking with the concentration of prior research focused more on systemic complications of undertreated GABHS disease or on identifying carrier status. Our study results suggest that GAS testing is the most important factor in reducing revisit risk. Being prescribed an antibiotic, on its own, does not have a significant impact on the risk of a revisit. However, once the GAS test is used, the decision not to use an antibiotic was correlated with the lowest revisit rate, likely because the source of the pharyngitis infection was viral and more likely to resolve without a revisit. Prior studies have reported variable rates of testing among children with pharyngitis prescribed an antibiotic, ranging from 23% to 91%,14,15 with testing important toward more appropriate antibiotic use.16 More recently, among more than 67 000 patients aged 3 to 21 years presenting with sore throat and receiving a GAS test, 32.6% were positive.17
Our analysis found that more than 46% of pediatric pharyngitis patients were given the rapid GAS test. While this testing rate is substantially lower than HEDIS recommendations and lower than testing rates achieved by several health maintenance organizations,10 it is similar to the 53% of children receiving such testing in a recent National Ambulatory Medical Care Survey.18 Furthermore, we found that when antibiotics are prescribed following a GAS test, the revisit risk is not significantly reduced, possibly because antibiotics lower revisit risk when informed by diagnostic testing tools that determine the infectious organism. This is supported by a similar population analysis in which we observed reduced revisit rates in children with AOM managed with antibiotics within 3 days of index diagnosis.19
Several other factors also affect the likelihood of a child receiving the GAS test. Children aged 1 to 12 years were significantly more likely to receive the GAS test than children over the age of 12. This included children in the 1 to 5 years old bracket who had a 15% higher likelihood of undergoing a GAS test, despite children less than 3 years of age as not recommended targets for GAS testing.20 As expected, children with reported ARTI-associated comorbidities were also less likely to receive a GAS test. Additionally, specialty care physicians were less inclined to implement the GAS test, possibly because of diagnostic confidence without testing or referral after GAS was ruled out. Black and low-income children had statistically lower odds of receiving the test, even after controlling for other factors, and yet were less likely to consume a revisit. As the overall data suggested more revisits in those not tested, further study is needed to examine if race or income discrepancies are equity based. Finally, children in the Pacific region, compared to the rest of the nation, were the least likely to receive a GAS test and yet there were no significant differences in revisit rates by region. Regional differences in antibiotic use were also observed in our study, as has been seen by others.21
After statistically controlling for having received the diagnostic GAS test and filled a prescription for an antibiotic, there are multitude of factors that independently affect the revisit risk, the most important of which if which was a history of an ARTI infection in the prior 90 days. While prior visit history had no impact on the likelihood of being tested or filling an antibiotic, patients with prior visits were more than 7 times more likely to consume a revisit. This was not reflected in nor related to comorbid ARTIs as these patients did not have statistically higher revisits than those with pharyngitis as the sole-coded diagnosis. Moreover, speculation for bacterial etiology of primary or superinfection based on a recent history of ARTI accounting for revisits seems unlikely as it did not yield greater antibiotic use in that group. Further analysis is required to determine the clinical and behavioral factors that promote for prior ARTI history as a major factor in revisit risk after an index visit for pharyngitis.
Children aged between 1 and 5 years, though 15% more likely to be tested than those aged 12 through 17 years, were also 39% more likely to initiate a revisit compared to older children when statistically controlling for other covariates. This perhaps suggests longer illness, wrong diagnosis, delay in appropriate treatment, or more caution by parents and providers in this age group. Justification for testing children less than 3 years of age who are outside of the HEDIS suggested age group, when clinical judgement does not point to another infection source, can result in positivity rates between 22% and 30% as previously observed.22,23 Patients visiting nonphysician providers and outpatient facility providers were less likely to have a revisit than those visiting primary and specialty care physicians, though slightly higher propensity for antibiotic prescriptions was seen for nonphysician providers. Pediatricians have been noted to be less likely to prescribe antibiotics without GAS testing than nonpediatric providers, and more guidelines-compliant in prescribing.24
Recommendations to not test children under 3 years of age are based on the lack of acute rheumatic fever and other complications in this age group together with more frequent viral syndromes. Selectivity in applying clinical criteria to testing can be attempted to separate bacterial from viral illness. Postnasal drainage/rhinorrhea, hoarse voice, and cough have been used successfully to identify those with viral illness and less need for testing, with greater certainty of low risk for GABHS in those over 11 years of age without tonsillar exudates, cervical adenopathy, or fever.17 However, the marginal benefits of those who have all 3 features of viral illness versus none in identifying GAS positivity was 23.3% vs 37.6% - helpful, but certainly not diminishing the need for testing. These constitutional findings of viral URI also do not exclude the GAS carrier state that features these symptoms.25 Others have reinforced the doubt of pharyngeal exudates as the premier diagnostic finding for test-positive GAS.26
This study had several limitations. The Optum claims dataset only contains ICD-9 codes for diagnoses. It does not include data on infection severity and clinical findings related to symptoms, thus empiric treatment warranted based in clinical severity is not assessed. Antibiotics are commonly available as generics and very inexpensive. Patients may fill and pay for these prescriptions directly, in which case, a claim for payment may not be filed with Optum. This could result in an undercount of treated patients in our study.
There is no corresponding problem of missing medical claims for GAS testing which were obtained from the CPT codes within the Optum claims data set. However, we elected not to verify the test results due to these data being missing for 75% of the study population. Nevertheless, this study’s focus was less about justifying antibiotic treatment, but dealt with the outcomes generated by testing and treatment. Toward that end, we used CPT codes to identify a revisit, and while those can at times be affected by financial reimbursement incentives, differences related to revisits in the 4 patient groups should not be subject to bias.
Conclusion
This study used data from real world practices to document the patterns of GAS testing and antibiotic use in pediatric pharyngitis patients. Revisit rates were under 5% for all patient groups and the use of rapid diagnostic tools were found to be the determining factor in further reducing the risk of revisits. This supports the need for compliance with the HEDIS quality metric for pharyngitis to the recommended levels of rapid testing which have been falling in recent years. Use of more accurate antigen and newer molecular detection testing methods may help further delineate important factors in determining pediatric pharyngitis treatment and need for revisits.27
Corresponding author: Jeffrey McCombs, MD, University of Southern California School of Pharmacy, Department of Pharmaceutical and Health Economics, Leonard D. Schaeffer Center for Health Policy & Economics, 635 Downey Way, Verna & Peter Dauterive Hall 310, Los Angeles, CA 90089-3333; [email protected].
Financial disclosures: None.
1. Choby BA. Diagnosis and treatment of streptococcal pharyngitis. Am Fam Physician. 2009;79(5):383-390.
2. Briel M, Schuetz P, Mueller B, et al. Procalcitonin-guided antibiotic use vs a standard approach for acute respiratory tract infections in primary care. Arch of Intern Med. 2008;168(18):2000-2008. doi: 10.1001/archinte.168.18.2000
3. Maltezou HC, Tsagris V, Antoniadou A, et al. Evaluation of a rapid antigen detection test in the diagnosis of streptococcal pharyngitis in children and its impact on antibiotic prescription. J Antimicrob Chemother. 2008;62(6):1407-1412. doi: 10.1093/jac/dkn376
4. Neuner JM, Hamel MB, Phillips RS, et al. Diagnosis and management of adults with pharyngitis: a cost-effectiveness analysis. Ann Intern Med. 2003;139(2):113-122. doi:10.7326/0003-4819-139-2-200307150-00011
5. Gerber MA, Baltimore RS, Eaton CB, et al. Prevention of rheumatic fever and diagnosis and treatment of acute Streptococcal pharyngitis: a scientific statement from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young, the Interdisciplinary Council on Functional Genomics and Translational Biology, and the Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Academy of Pediatrics. Circulation. 2009;119(11):1541-1551. doi: 10.1161/CIRCULATIONAHA.109.191959
6. Gieseker KE, Roe MH, MacKenzie T, Todd JK. Evaluating the American Academy of Pediatrics diagnostic standard for Streptococcus pyogenes pharyngitis: backup culture versus repeat rapid antigen testing. Pediatrics. 2003;111(6):e666-e670. doi: 10.1542/peds.111.6.e666
7. Shapiro DJ, Lindgren CE, Neuman MI, Fine AM. Viral features and testing for Streptococcal pharyngitis. Pediatrics. 2017;139(5):e20163403. doi: 10.1542/peds.2016-3403
8. Shulman ST, Bisno AL, Clegg H, et al. Clinical practice guideline for the diagnosis and management of group A Streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55(10):e86–e102. doi: 10.1093/cid/cis629
9. Mangione-Smith R, McGlynn EA, Elliott MN, et al. Parent expectations for antibiotics, physician-parent communication, and satisfaction. Arch Pediatr Adolesc Med. 2001;155(7):800–806. doi: 10.1001/archpedi.155.7.800
10. Appropriate Testing for Children with Pharyngitis. HEDIS Measures and Technical Resources. National Committee for Quality Assurance. Accessed February 12, 2021. https://www.ncqa.org/hedis/measures/appropriate-testing-for-children-with-pharyngitis/
11. Linder JA, Bates DW, Lee GM, Finkelstein JA. Antibiotic treatment of children with sore throat. JAMA. 2005;294(18):2315-2322. doi: 10.1001/jama.294.18.2315
12. Crimmel BL. Health Insurance Coverage and Income Levels for the US Noninstitutionalized Population Under Age 65, 2001. Medical Expenditure Panel Survey, Agency for Healthcare Research and Quality. 2004. https://meps.ahrq.gov/data_files/publications/st40/stat40.pd
13. AHFS/ASHP. American Hospital Formulary Service Drug Information. 2012. AHFS drug information. 00--. Accessed January 4, 2021.
14. Mainous AG 3rd, Zoorob, RJ, Kohrs FP, Hagen MD. Streptococcal diagnostic testing and antibiotics prescribed for pediatric tonsillopharyngitis. Pediatr Infect Dis J. 1996;15(9):806-810. doi: 10.1097/00006454-199609000-00014
15. Benin AL, Vitkauskas G, Thornquist E, et al. Improving diagnostic testing and reducing overuse of antibiotics for children with pharyngitis: a useful role for the electronic medical record. Pediatr Infect Dis J. 2003;22(12):1043-1047. doi: 10.1097/01.inf.0000100577.76542.af
16. Luo R, Sickler J, Vahidnia F, et al. Diagnosis and Management of Group a Streptococcal Pharyngitis in the United States, 2011-2015. BMC Infect Dis. 2019;19(1):193-201. doi: 10.1186/s12879-019-3835-4
17. Shapiro DJ, Barak-Corren Y, Neuman MI, et al. Identifying Patients at Lowest Risk for Streptococcal Pharyngitis: A National Validation Study. J Pediatr. 2020;220:132-138.e2. doi: 10.1016/j.jpeds.2020.01.030. Epub 2020 Feb 14
18. Shapiro DJ, King LM, Fleming-Dutra KE, et al. Association between use of diagnostic tests and antibiotic prescribing for pharyngitis in the United States. Infect Control Hosp Epidemiol. 2020;41(4):479-481. doi: 10.1017/ice.2020.29
19. Sangha K, Steinberg I, McCombs JS. The impact of antibiotic treatment time and class of antibiotic for acute otitis media infections on the risk of revisits. Abs PDG4. Value in Health. 2019; 22:S163.
20. Ahluwalia T, Jain S, Norton L, Meade J, et al. Reducing Streptococcal Testing in Patients < 3 Years Old in an Emergency Department. Pediatrics. 2019;144(4):e20190174. doi: 10.1542/peds.2019-0174
21. McKay R, Mah A, Law MR, et al. Systematic Review of Factors Associated with Antibiotic Prescribing for Respiratory Tract Infections. Antimicrob Agents Chemother. 2016;60(7):4106-4118. doi: 10.1128/AAC.00209-16
22. Woods WA, Carter CT, Schlager TA. Detection of group A streptococci in children under 3 years of age with pharyngitis. Pediatr Emerg Care. 1999;15(5):338-340. doi: 10.1097/00006565-199910000-00011
23. Mendes N, Miguéis C, Lindo J, et al. Retrospective study of group A Streptococcus oropharyngeal infection diagnosis using a rapid antigenic detection test in a paediatric population from the central region of Portugal. Eur J Clin Microbiol Infect Dis. 2021;40(6):1235-1243. doi: 10.1007/s10096-021-04157-x
24. Frost HM, McLean HQ, Chow BDW. Variability in Antibiotic Prescribing for Upper Respiratory Illnesses by Provider Specialty. J Pediatr. 2018;203:76-85.e8. doi: 10.1016/j.jpeds.2018.07.044.
25. Rick AM, Zaheer HA, Martin JM. Clinical Features of Group A Streptococcus in Children With Pharyngitis: Carriers versus Acute Infection. Pediatr Infect Dis J. 2020;39(6):483-488. doi: 10.1097/INF.0000000000002602
26. Nadeau NL, Fine AM, Kimia A. Improving the prediction of streptococcal pharyngitis; time to move past exudate alone [published online ahead of print, 2020 Aug 16]. Am J Emerg Med. 2020;S0735-6757(20)30709-9. doi: 10.1016/j.ajem.2020.08.023
27. Mustafa Z, Ghaffari M. Diagnostic Methods, Clinical Guidelines, and Antibiotic Treatment for Group A Streptococcal Pharyngitis: A Narrative Review. Front Cell Infect Microbiol. 2020;10:563627. doi: 10.3389/fcimb.2020.563627
From the Department of Pharmaceutical and Health Economics, University of Southern California, Los Angeles, CA, (Drs. Sangha and McCombs), Department of Pediatrics, Keck School of Medicine, and Department of Clinical Pharmacy, School of Pharmacy, University of Southern California, Los Angeles, CA, (Dr. Steinberg), and Leonard Schaeffer Center for Health Policy and Economics, University of Southern California, Los Angeles, CA (Dr. McCombs).
Objective: The recommended treatment for children and adolescents under 18 years of age who have a positive test for group A Streptococcus (GAS) are antibiotics using the “test and treat” strategy to detect and treat GAS for pediatric pharyngitis. This study used paid claims data to document the extent to which real-world treatment patterns are consistent with these recommendations. We document the factors correlated with testing and treatment, then examine the effects of receiving a GAS test and being treated with an antibiotic impact the likelihood of a revisit for an acute respiratory tract infection within 28 days.
Methods: This retrospective cohort study used Optum Insight Clinformatics data for medical and pharmacy claims from 2011-2013 to identify episodes of care for children and adolescents with pharyngitis around their index visit (± 6 months). The sample population included children and adolescents under 18 years of age with a diagnosis of pharyngitis. Multivariable logistic regression analyses were used to document factors associated with receipt of GAS test and antibiotic treatment. Next, we used logistic regression models to estimate the impact of test and treat recommendation on revisit risk.
Results: There were 24 685 treatment episodes for children and adolescents diagnosed with pharyngitis. Nearly 47% of these episodes included a GAS test and 48% of tested patients were prescribed an antibiotic prescription. Failing to perform a GAS test increased the risk of a revisit within 28 days by 44%. The use of antibiotics by tested and untested patients had no impact on revisit risk.
Conclusion: While the judicious use of antibiotics is important in managing pharyngitis infections and managing complications, the use of rapid diagnostic tools was found to be the determining factor in reducing revisits for pediatric patients with pharyngitis.
Keywords: pediatrics; pharyngitis; respiratory infections; acute infections; diagnostic tests; group A Streptococcus; antibiotics; revisits.
Acute pharyngitis is a common acute respiratory tract infection (ARTI) in children. Group A β-hemolytic streptococci (GABHS) is the most common bacterial etiology for pediatric pharyngitis, accounting for 15% to 30% of cases.1
Beyond clinical assessment, laboratory diagnostic testing generally plays a limited role in guiding appropriate antibiotic prescribing for patients with an ARTI.2,3 Most diagnostic tests require 2 or 3 days to result, incur additional costs, and may delay treatment.4 While these tests do not provide clear and timely guidance on which specific antibiotic is appropriate for ARTI patients, this is not the case for patients with pharyngitis.5,6,7 A rapid diagnostic test exists to identify pharyngitis patients with GABHS which accounts for 1 in 4 children with acute sore throat.1,4,6 Both the American Academy of Pediatrics and the Infectious Diseases Society of America recommend antibiotic treatment for children and adolescents under 18 years of age who have a positive test for group A Streptococcus (GAS).8,9 This “test and treat” protocol has been consistently included in the Healthcare Effectiveness Data and Information Set (HEDIS) standards over time for pediatric pharyngitis patients aged 3 to 18 years before dispensing an antibiotic.10
Sinusitis, pneumonia, and acute otitis media are considered ARTIs where antibiotic treatment is justified. Therefore, pharyngitis of unclear etiology seen with these comorbid infections may not always undergo GAS testing but move directly to the patient being prescribed antibiotics. This analysis enumerates ARTI-related comorbidities present together with the initial coded pharyngitis diagnosis to evaluate their impact on the provider’s decision to test and treat, and on revisit risk.
Antibiotic treatment for GAS patients is likely to eradicate the acute GABHS infection within 10 days. Penicillin and amoxicillin are commonly recommended because of their narrow spectrum of activity, few adverse effects, established efficacy, and modest cost. Alternative antibiotics for patients with penicillin allergy, or with polymicrobial infection seen on culture results, include a first-generation cephalosporin, clindamycin, clarithromycin (Biaxin), or azithromycin (Zithromax).1,8,11 However, while compliance with these HEDIS guidelines has been evaluated, the outcome effects of following the HEDIS “test and treat” recommendations for children with pharyngitis have not been adequately evaluated.
These outcome evaluations have increasing importance as the latest HEDIS survey has shown testing rates in commercial Preferred Provider Organizations (PPO) falling from 86.4% in 2018 to 75.9% in 2019, the lowest rate of testing since 2009, with similar reductions under 80% for Health Maintenance Organizations (HMO).10 While health plans may execute cost-benefit analyses and algorithms to forge best practices for GAS testing in children and adolescents presenting with symptoms of pharyngitis, it is important to regard the wasteful resource utilization and additional cost of revisits that may offset any gains accrued by more focused GAS testing outside the existing clinical guidelines and HEDIS measures. This may be of particular importance in documenting infection and sparing antibiotic therapy in toddlers and younger.
The objective of this study was to investigate the correlation between testing and antibiotic use on the likelihood of a revisit for an acute respiratory tract infection within 28 days. To achieve this objective, this investigation consists of 3 sequential analyses. First, we document the factors associated with the decision to test the patient for a GABHS infection using the GAS test. Next, we document the factors associated with the decision to use an antibiotic to treat the patient as a function of having tested the patient. Finally, we investigate the impact of the testing and treatment decisions on the likelihood of a revisit within 28 days.
Methods
Study design
This was a retrospective cohort study of episodes of treatment for pediatric patients with pharyngitis. Episodes were identified using data derived from the Optum Insight Clinformatics claims database provided to the University of Southern California to facilitate the training of graduate students. These data cover commercially insured patients with both medical and pharmacy benefits. Data were retrieved from the 3-year period spanning 2011-2013. An episode of care was identified based on date of the first (index) outpatient visit for a pharyngitis diagnosis (International Classification of Diseases, Ninth Revision [ICD-9]: 462, 463, 034.0). Outpatient visits were defined by visit setting: ambulatory clinics, physician offices, emergency rooms, and urgent care facilities. Each pharyngitis treatment episode was then screened for at least a 6-month enrollment in a health insurance plan prior and subsequent to the index visit using Optum enrollment data. Finally, eligible treatment episodes were restricted to children and adolescents under 18 years of age, who had an index outpatient visit for a primary diagnosis of acute pharyngitis.
A diagnostic profile was created for each episode using the diagnoses recorded for the index visit. Up to 3 diagnoses may be recorded for any outpatient visit and the first recorded diagnosis was assumed to be the primary diagnosis for that episode. Any secondary diagnoses recorded on the index visit were used to define comorbidities present at the index visit. ARTI-related comorbidities included: acute otitis media (AOM), bronchitis, sinusitis, pneumonia, and upper respiratory infection (URI). Other comorbid medical diagnoses were documented using diagnostic data from the pre-index period. Dichotomous variables for the following categories were created: mental disorders, nervous system disorders, respiratory symptoms, fever, injury and poisoning, other, or no diseases.
Prior visits for other respiratory infections in the previous 90 days were also identified for patients based on their index visit for pharyngitis. Similarly, any subsequent visits, within 28 days of the index visit, were also recorded to measure the health outcome for analysis. Practice settings include physician offices and federally qualified health centers, state and local health clinics, outpatient hospitals facilities, emergency departments, and other outpatient settings such as walk-in retail health clinic or ambulatory centers. Providers include primary care physicians (family practice, pediatricians, internal medicine), specialty care physicians (emergency medicine, preventive medicine), nonphysician providers (nurse practitioners, physician assistants) and other providers (urgent care, acute outpatient care, ambulatory care centers). Seasons of the year were determined based on the index date of the episode to account for possible seasonality in pharyngitis treatment. Lastly, a previous visits variable was created to identify whether the child had nonpharyngitis ARTI visits in the 3 months prior to the index visit.
Demographic variables were created based on enrollment and the socioeconomic data available in the Optum socioeconomic status file. These variables include patient age, race, sex, household income, geographic location, practice setting type, provider specialty, and type of insurance. An estimate of patient household income was based on algorithms using census block groups. Income categories were informed by the federal guidelines for a family of 4. A low-income family was defined as earning less than $50 000; a middle-income family earned between $50 000 and $75 000, and a high-income family earned $75 000 and above.12 Patient insurance type was categorized as HMO, Exclusive Provider Organization (EPO), Point of Service (POS), and PPO. Race was identified as White, Black, Hispanic, and Asian. Patient location was defined according to national census regions.
Outcomes
GAS test
The HEDIS measures for pharyngitis recommend using the GAS test to identify the bacterial etiology of the pharyngitis infection. Patients who received the test were identified based on Current Procedural Terminology (CPT) codes 87070-71, 87081, 87430, 87650-52, and 87880.10
Antibiotic treatment
The pharmacy administrative claims dataset was used to identify study patients who filled a prescription for an antibiotic during their pharyngitis treatment episode. Optum pharmacy data identify the medications received, specifies the date of prescription filling, National Drug Codes, and American Hospital Formulary Service (AHFS) Classification System codes for each medication. We used the AHFS Pharmacologic-Therapeutic classification of antibiotics to create dichotomous variables documenting the antibacterial used by each patient.13 These are categorized under antibacterial including penicillins, cephalosporins (first, second, third, fourth generation cephalosporins), macrolides (first generation and others), tetracyclines, sulfonamides, fluoroquinolones (ciprofloxacin, levofloxacin, moxifloxacin), cephamycin, carbapenems, and β-lactam antibiotics (amoxicillin, amoxicillin/clavulanate, cephalexin, cefuroxime, cefdinir).
Revisits to physician or other provider
Revisits within 28 days were used as the measure of patient outcomes related to testing and filling of an antibiotic prescription for acute pharyngitis. Revisits may also be due to a patient returning for a follow-up, alternative treatment, worsening pharyngitis, or for another ARTI. An ARTI-related revisit also increases total resources used to treat pediatric pharyngitis patients.
Statistical analysis
Logistic regression was used for all 3 analyses conducted in this study. First, we determined the patient and treating physician characteristics that impact the decision to use GAS testing for pharyngitis. Second, we identified those factors that impact the decision to use antibiotic prescriptions among children who were diagnosed with pharyngitis adding in the dichotomous variable indicating if the patient had received a GAS test. Third, we used a logit regression analysis to document if receiving a GAS test and/or an antibiotic impacted the likelihood of a revisit by comparing revisit risk. To estimate the effect of testing and/or antibiotic use, we divided patients into 4 groups based on whether the patient received a GAS test and/or an antibiotic prescription. This specification of the analysis of revisits as an outcome focuses on adherence to HEDIS “test and treat” guidelines10:
- Patients who were not tested yet filled an antibiotic prescription. This decision was likely based on the clinician’s judgment of the patient’s signs and symptoms, and confirmational testing not performed.
- Patients who were not tested and did not fill an antibiotic prescription. Apparently, in the clinician’s judgment the patient’s signs and symptoms were such that the infection did not warrant treatment and the clinical presentation did not necessitate the GAS test to confirm the recorded diagnosis of pharyngitis.
- Patients who were tested and received antibiotic prescription, likely because the test was positive for GABHS.
- Patients who were tested and did not receive antibiotic prescription.
We tested for statistically significant differences in baseline characteristics across these 4 patient groups using t tests for continuous variables and χ2 tests for categorical variables. Odds ratios (OR) and CI were computed for the influential variables included the regression analyses.
We conducted a sensitivity analysis using a model specification which included the dichotomous variables for testing and for treatment, and the interaction term between these variables to assess if treatment effects varied in tested and untested patients. We also estimated this model of revisit risk using revisits within 7 days as the outcome variable.
All analyses were completed using STATA/IC 13 (StataCorp, College Station, TX).
Results
There were 24 685 treatment episodes for children diagnosed with pharyngitis. Nearly 47% of these episodes included GAS testing and 47% of the tested patients filled an antibiotic prescription. Similarly, 53% of patients were not tested and 49% of untested patients filled an antibiotic prescription. As a result, the 4 groups identified for analysis were evenly distributed: untested and no prescription (26.9%), untested and prescription (26.3%), tested and prescription (21.9%), and tested and no prescription (24.9%) (Figure).

Table 1 presents the descriptive statistics for these 4 patient groups. Note first that the rate of revisits within 28 days is under 5% across all groups. Second, the 2 tested groups have a lower revisit rate than the untested groups: the tested and treated have a revisit rate of 3.3%, and the tested and untreated have a revisit rate of 2.4%, while both the untested groups have a revisit rate of nearly 5%. These small absolute differences in revisit rates across groups were statistically significant.

Factors associated with receiving GAS test
Several factors were found to impact the decision to test (Table 2). Only 9.7% of children were reported to have any ARTI coinfection. As expected, these comorbidities resulted in a significantly lower likelihood of receiving the GAS test: AOM, bronchitis, sinusitis, pneumonia, and URI as comorbid infections had a 48%, 41%, 37%, 63%, and 13% lower likelihood of receiving the GAS test, respectively, than those with no comorbidities. Similarly, children with fever and respiratory symptoms were 35% and 45% less likely to receiving the GAS test, respectively. This is consistent with our expectation that comorbid ARTI infections will lead many providers to forgo testing.

Provider type and patient age also plays a role in receipt of the GAS test. Relative to outpatient facility providers, primary care physicians were 24% more likely and specialty physicians were 38% less likely of employing the GAS test. The child’s age played a significant role in receipt of the GAS test. Children aged 1 to 5 years and 5 to 12 years were 15% and 14% more likely to receive the test compared to children older than 12 years.
Pharyngitis patients have disproportionately higher odds of receiving a GAS test in most regions of the country compared to the Pacific region. For instance, children in the Mid-Atlantic region have 51% higher odds of receiving a GAS test while children in New England have 80% higher odds of receiving the same test.
Black children have 11% lower odds of receiving the GAS test compared to White children. Both middle-income and high-income children have 12% and 32% higher odds of receiving the test compared to low-income children. Compared to office-based visits, children visiting a clinic were twice as likely to receive a GAS test while those seen in the emergency room have 43% lower odds of receiving a GAS test. Hospital outpatient departments, which account for less than 1% of all visits, rarely used a GAS test which could be a statistical artifact due to small sample size. Lastly, insurance and season of the year had no significant impact of receipt of a GAS test.
Factors associated with receiving antibiotic prescription
Surprisingly, receiving the GAS test has a small but insignificant impact on the likelihood that the patient will receive an antibiotic prescription (Table 3) (Adjusted OR = 1.055, P = .07). After controlling for receipt of a GAS test, children with AOM and sinusitis comorbidities have an increased likelihood of being prescribed an antibiotic. Children with URI have a lower likelihood of being prescribed an antibiotic. Additionally, relative to primary care physicians, children visiting nonphysician providers for pharyngitis were more likely to be prescribed an antibiotic.

Children under 12 years of age were more likely to use an antibiotic compared to children 12 years and older. Geographically, there is some evidence of regional variation in antibiotic use as well. Children in the south Atlantic, west-south central, and southeast central regions had a significantly lower odds of being prescribed an antibiotic respectively than pharyngitis patients in the Pacific region. Black children had a 10% lower likelihood of being prescribed an antibiotic compared to White children, possibly related to their lower rate of GAS testing. Compared to office-based visits, children visiting a clinic were less likely to use an antibiotic. Household income, insurance type, and season had no significant impact on revisit risk.
Effects of GAS test and antibiotic prescriptions on likelihood of revisits
The multivariate analysis of the risk of a revisit within 28 days is presented in Table 4. Children with pharyngitis who tested and did not receive an antibiotic serve as the reference comparison group for this analysis to illustrate the impact of using the GAS test and treatment with an antibiotic. The results in Table 4 are quite clear: patients who receive the GAS test were significantly less likely to have a revisit within 28 days. Moreover, within the group of patients who were tested, those not receiving an antibiotic, presumedly because their GAS test was negative, experienced the lowest risk of a revisit. This result is consistent with the data in Table 1. Moreover, using an antibiotic had no impact on the likelihood of a revisit in patients not receiving the GAS test. This result is also consistent with Table 1.

Other results from the analysis of revisit risk may be of interest to clinicians. Pharyngitis patients with a prior episode of treatment within 90 days for an acute respiratory tract infection were more than 7 times more likely to experience a revisit within 28 days of the pharyngitis diagnosis than patients without a history of recent ARTI infections. Age is also a risk factor in likelihood of initiating a revisit. Children under 1 year and children aged 1 to 5 years were more likely to have a revisit than children aged more than 12 years. Compared to White children, Black children were 25% (P = .04) less likely to have a revisit. The care setting also has a significant impact on revisit risk. Children visiting outpatient hospital and other care settings had a significantly higher revisit risk than those visiting a physician’s office. Lastly, household income, geographic region, season, medical comorbidities, gender, and insurance type have no significant impact on revisit risk.
Sensitivity analysis
The results from the analysis of 7-day and 28-day revisit risk are summarized in Table 5. These results indicate that patients who were tested had a more significant decrease in revisit risk at 7 days (72%) than was evident at 28 days (47% reduction). Receiving an antibiotic, with or without the test, had no impact on revisit risk.

Discussion
Published data on revisits for pharyngitis are lacking with the concentration of prior research focused more on systemic complications of undertreated GABHS disease or on identifying carrier status. Our study results suggest that GAS testing is the most important factor in reducing revisit risk. Being prescribed an antibiotic, on its own, does not have a significant impact on the risk of a revisit. However, once the GAS test is used, the decision not to use an antibiotic was correlated with the lowest revisit rate, likely because the source of the pharyngitis infection was viral and more likely to resolve without a revisit. Prior studies have reported variable rates of testing among children with pharyngitis prescribed an antibiotic, ranging from 23% to 91%,14,15 with testing important toward more appropriate antibiotic use.16 More recently, among more than 67 000 patients aged 3 to 21 years presenting with sore throat and receiving a GAS test, 32.6% were positive.17
Our analysis found that more than 46% of pediatric pharyngitis patients were given the rapid GAS test. While this testing rate is substantially lower than HEDIS recommendations and lower than testing rates achieved by several health maintenance organizations,10 it is similar to the 53% of children receiving such testing in a recent National Ambulatory Medical Care Survey.18 Furthermore, we found that when antibiotics are prescribed following a GAS test, the revisit risk is not significantly reduced, possibly because antibiotics lower revisit risk when informed by diagnostic testing tools that determine the infectious organism. This is supported by a similar population analysis in which we observed reduced revisit rates in children with AOM managed with antibiotics within 3 days of index diagnosis.19
Several other factors also affect the likelihood of a child receiving the GAS test. Children aged 1 to 12 years were significantly more likely to receive the GAS test than children over the age of 12. This included children in the 1 to 5 years old bracket who had a 15% higher likelihood of undergoing a GAS test, despite children less than 3 years of age as not recommended targets for GAS testing.20 As expected, children with reported ARTI-associated comorbidities were also less likely to receive a GAS test. Additionally, specialty care physicians were less inclined to implement the GAS test, possibly because of diagnostic confidence without testing or referral after GAS was ruled out. Black and low-income children had statistically lower odds of receiving the test, even after controlling for other factors, and yet were less likely to consume a revisit. As the overall data suggested more revisits in those not tested, further study is needed to examine if race or income discrepancies are equity based. Finally, children in the Pacific region, compared to the rest of the nation, were the least likely to receive a GAS test and yet there were no significant differences in revisit rates by region. Regional differences in antibiotic use were also observed in our study, as has been seen by others.21
After statistically controlling for having received the diagnostic GAS test and filled a prescription for an antibiotic, there are multitude of factors that independently affect the revisit risk, the most important of which if which was a history of an ARTI infection in the prior 90 days. While prior visit history had no impact on the likelihood of being tested or filling an antibiotic, patients with prior visits were more than 7 times more likely to consume a revisit. This was not reflected in nor related to comorbid ARTIs as these patients did not have statistically higher revisits than those with pharyngitis as the sole-coded diagnosis. Moreover, speculation for bacterial etiology of primary or superinfection based on a recent history of ARTI accounting for revisits seems unlikely as it did not yield greater antibiotic use in that group. Further analysis is required to determine the clinical and behavioral factors that promote for prior ARTI history as a major factor in revisit risk after an index visit for pharyngitis.
Children aged between 1 and 5 years, though 15% more likely to be tested than those aged 12 through 17 years, were also 39% more likely to initiate a revisit compared to older children when statistically controlling for other covariates. This perhaps suggests longer illness, wrong diagnosis, delay in appropriate treatment, or more caution by parents and providers in this age group. Justification for testing children less than 3 years of age who are outside of the HEDIS suggested age group, when clinical judgement does not point to another infection source, can result in positivity rates between 22% and 30% as previously observed.22,23 Patients visiting nonphysician providers and outpatient facility providers were less likely to have a revisit than those visiting primary and specialty care physicians, though slightly higher propensity for antibiotic prescriptions was seen for nonphysician providers. Pediatricians have been noted to be less likely to prescribe antibiotics without GAS testing than nonpediatric providers, and more guidelines-compliant in prescribing.24
Recommendations to not test children under 3 years of age are based on the lack of acute rheumatic fever and other complications in this age group together with more frequent viral syndromes. Selectivity in applying clinical criteria to testing can be attempted to separate bacterial from viral illness. Postnasal drainage/rhinorrhea, hoarse voice, and cough have been used successfully to identify those with viral illness and less need for testing, with greater certainty of low risk for GABHS in those over 11 years of age without tonsillar exudates, cervical adenopathy, or fever.17 However, the marginal benefits of those who have all 3 features of viral illness versus none in identifying GAS positivity was 23.3% vs 37.6% - helpful, but certainly not diminishing the need for testing. These constitutional findings of viral URI also do not exclude the GAS carrier state that features these symptoms.25 Others have reinforced the doubt of pharyngeal exudates as the premier diagnostic finding for test-positive GAS.26
This study had several limitations. The Optum claims dataset only contains ICD-9 codes for diagnoses. It does not include data on infection severity and clinical findings related to symptoms, thus empiric treatment warranted based in clinical severity is not assessed. Antibiotics are commonly available as generics and very inexpensive. Patients may fill and pay for these prescriptions directly, in which case, a claim for payment may not be filed with Optum. This could result in an undercount of treated patients in our study.
There is no corresponding problem of missing medical claims for GAS testing which were obtained from the CPT codes within the Optum claims data set. However, we elected not to verify the test results due to these data being missing for 75% of the study population. Nevertheless, this study’s focus was less about justifying antibiotic treatment, but dealt with the outcomes generated by testing and treatment. Toward that end, we used CPT codes to identify a revisit, and while those can at times be affected by financial reimbursement incentives, differences related to revisits in the 4 patient groups should not be subject to bias.
Conclusion
This study used data from real world practices to document the patterns of GAS testing and antibiotic use in pediatric pharyngitis patients. Revisit rates were under 5% for all patient groups and the use of rapid diagnostic tools were found to be the determining factor in further reducing the risk of revisits. This supports the need for compliance with the HEDIS quality metric for pharyngitis to the recommended levels of rapid testing which have been falling in recent years. Use of more accurate antigen and newer molecular detection testing methods may help further delineate important factors in determining pediatric pharyngitis treatment and need for revisits.27
Corresponding author: Jeffrey McCombs, MD, University of Southern California School of Pharmacy, Department of Pharmaceutical and Health Economics, Leonard D. Schaeffer Center for Health Policy & Economics, 635 Downey Way, Verna & Peter Dauterive Hall 310, Los Angeles, CA 90089-3333; [email protected].
Financial disclosures: None.
From the Department of Pharmaceutical and Health Economics, University of Southern California, Los Angeles, CA, (Drs. Sangha and McCombs), Department of Pediatrics, Keck School of Medicine, and Department of Clinical Pharmacy, School of Pharmacy, University of Southern California, Los Angeles, CA, (Dr. Steinberg), and Leonard Schaeffer Center for Health Policy and Economics, University of Southern California, Los Angeles, CA (Dr. McCombs).
Objective: The recommended treatment for children and adolescents under 18 years of age who have a positive test for group A Streptococcus (GAS) are antibiotics using the “test and treat” strategy to detect and treat GAS for pediatric pharyngitis. This study used paid claims data to document the extent to which real-world treatment patterns are consistent with these recommendations. We document the factors correlated with testing and treatment, then examine the effects of receiving a GAS test and being treated with an antibiotic impact the likelihood of a revisit for an acute respiratory tract infection within 28 days.
Methods: This retrospective cohort study used Optum Insight Clinformatics data for medical and pharmacy claims from 2011-2013 to identify episodes of care for children and adolescents with pharyngitis around their index visit (± 6 months). The sample population included children and adolescents under 18 years of age with a diagnosis of pharyngitis. Multivariable logistic regression analyses were used to document factors associated with receipt of GAS test and antibiotic treatment. Next, we used logistic regression models to estimate the impact of test and treat recommendation on revisit risk.
Results: There were 24 685 treatment episodes for children and adolescents diagnosed with pharyngitis. Nearly 47% of these episodes included a GAS test and 48% of tested patients were prescribed an antibiotic prescription. Failing to perform a GAS test increased the risk of a revisit within 28 days by 44%. The use of antibiotics by tested and untested patients had no impact on revisit risk.
Conclusion: While the judicious use of antibiotics is important in managing pharyngitis infections and managing complications, the use of rapid diagnostic tools was found to be the determining factor in reducing revisits for pediatric patients with pharyngitis.
Keywords: pediatrics; pharyngitis; respiratory infections; acute infections; diagnostic tests; group A Streptococcus; antibiotics; revisits.
Acute pharyngitis is a common acute respiratory tract infection (ARTI) in children. Group A β-hemolytic streptococci (GABHS) is the most common bacterial etiology for pediatric pharyngitis, accounting for 15% to 30% of cases.1
Beyond clinical assessment, laboratory diagnostic testing generally plays a limited role in guiding appropriate antibiotic prescribing for patients with an ARTI.2,3 Most diagnostic tests require 2 or 3 days to result, incur additional costs, and may delay treatment.4 While these tests do not provide clear and timely guidance on which specific antibiotic is appropriate for ARTI patients, this is not the case for patients with pharyngitis.5,6,7 A rapid diagnostic test exists to identify pharyngitis patients with GABHS which accounts for 1 in 4 children with acute sore throat.1,4,6 Both the American Academy of Pediatrics and the Infectious Diseases Society of America recommend antibiotic treatment for children and adolescents under 18 years of age who have a positive test for group A Streptococcus (GAS).8,9 This “test and treat” protocol has been consistently included in the Healthcare Effectiveness Data and Information Set (HEDIS) standards over time for pediatric pharyngitis patients aged 3 to 18 years before dispensing an antibiotic.10
Sinusitis, pneumonia, and acute otitis media are considered ARTIs where antibiotic treatment is justified. Therefore, pharyngitis of unclear etiology seen with these comorbid infections may not always undergo GAS testing but move directly to the patient being prescribed antibiotics. This analysis enumerates ARTI-related comorbidities present together with the initial coded pharyngitis diagnosis to evaluate their impact on the provider’s decision to test and treat, and on revisit risk.
Antibiotic treatment for GAS patients is likely to eradicate the acute GABHS infection within 10 days. Penicillin and amoxicillin are commonly recommended because of their narrow spectrum of activity, few adverse effects, established efficacy, and modest cost. Alternative antibiotics for patients with penicillin allergy, or with polymicrobial infection seen on culture results, include a first-generation cephalosporin, clindamycin, clarithromycin (Biaxin), or azithromycin (Zithromax).1,8,11 However, while compliance with these HEDIS guidelines has been evaluated, the outcome effects of following the HEDIS “test and treat” recommendations for children with pharyngitis have not been adequately evaluated.
These outcome evaluations have increasing importance as the latest HEDIS survey has shown testing rates in commercial Preferred Provider Organizations (PPO) falling from 86.4% in 2018 to 75.9% in 2019, the lowest rate of testing since 2009, with similar reductions under 80% for Health Maintenance Organizations (HMO).10 While health plans may execute cost-benefit analyses and algorithms to forge best practices for GAS testing in children and adolescents presenting with symptoms of pharyngitis, it is important to regard the wasteful resource utilization and additional cost of revisits that may offset any gains accrued by more focused GAS testing outside the existing clinical guidelines and HEDIS measures. This may be of particular importance in documenting infection and sparing antibiotic therapy in toddlers and younger.
The objective of this study was to investigate the correlation between testing and antibiotic use on the likelihood of a revisit for an acute respiratory tract infection within 28 days. To achieve this objective, this investigation consists of 3 sequential analyses. First, we document the factors associated with the decision to test the patient for a GABHS infection using the GAS test. Next, we document the factors associated with the decision to use an antibiotic to treat the patient as a function of having tested the patient. Finally, we investigate the impact of the testing and treatment decisions on the likelihood of a revisit within 28 days.
Methods
Study design
This was a retrospective cohort study of episodes of treatment for pediatric patients with pharyngitis. Episodes were identified using data derived from the Optum Insight Clinformatics claims database provided to the University of Southern California to facilitate the training of graduate students. These data cover commercially insured patients with both medical and pharmacy benefits. Data were retrieved from the 3-year period spanning 2011-2013. An episode of care was identified based on date of the first (index) outpatient visit for a pharyngitis diagnosis (International Classification of Diseases, Ninth Revision [ICD-9]: 462, 463, 034.0). Outpatient visits were defined by visit setting: ambulatory clinics, physician offices, emergency rooms, and urgent care facilities. Each pharyngitis treatment episode was then screened for at least a 6-month enrollment in a health insurance plan prior and subsequent to the index visit using Optum enrollment data. Finally, eligible treatment episodes were restricted to children and adolescents under 18 years of age, who had an index outpatient visit for a primary diagnosis of acute pharyngitis.
A diagnostic profile was created for each episode using the diagnoses recorded for the index visit. Up to 3 diagnoses may be recorded for any outpatient visit and the first recorded diagnosis was assumed to be the primary diagnosis for that episode. Any secondary diagnoses recorded on the index visit were used to define comorbidities present at the index visit. ARTI-related comorbidities included: acute otitis media (AOM), bronchitis, sinusitis, pneumonia, and upper respiratory infection (URI). Other comorbid medical diagnoses were documented using diagnostic data from the pre-index period. Dichotomous variables for the following categories were created: mental disorders, nervous system disorders, respiratory symptoms, fever, injury and poisoning, other, or no diseases.
Prior visits for other respiratory infections in the previous 90 days were also identified for patients based on their index visit for pharyngitis. Similarly, any subsequent visits, within 28 days of the index visit, were also recorded to measure the health outcome for analysis. Practice settings include physician offices and federally qualified health centers, state and local health clinics, outpatient hospitals facilities, emergency departments, and other outpatient settings such as walk-in retail health clinic or ambulatory centers. Providers include primary care physicians (family practice, pediatricians, internal medicine), specialty care physicians (emergency medicine, preventive medicine), nonphysician providers (nurse practitioners, physician assistants) and other providers (urgent care, acute outpatient care, ambulatory care centers). Seasons of the year were determined based on the index date of the episode to account for possible seasonality in pharyngitis treatment. Lastly, a previous visits variable was created to identify whether the child had nonpharyngitis ARTI visits in the 3 months prior to the index visit.
Demographic variables were created based on enrollment and the socioeconomic data available in the Optum socioeconomic status file. These variables include patient age, race, sex, household income, geographic location, practice setting type, provider specialty, and type of insurance. An estimate of patient household income was based on algorithms using census block groups. Income categories were informed by the federal guidelines for a family of 4. A low-income family was defined as earning less than $50 000; a middle-income family earned between $50 000 and $75 000, and a high-income family earned $75 000 and above.12 Patient insurance type was categorized as HMO, Exclusive Provider Organization (EPO), Point of Service (POS), and PPO. Race was identified as White, Black, Hispanic, and Asian. Patient location was defined according to national census regions.
Outcomes
GAS test
The HEDIS measures for pharyngitis recommend using the GAS test to identify the bacterial etiology of the pharyngitis infection. Patients who received the test were identified based on Current Procedural Terminology (CPT) codes 87070-71, 87081, 87430, 87650-52, and 87880.10
Antibiotic treatment
The pharmacy administrative claims dataset was used to identify study patients who filled a prescription for an antibiotic during their pharyngitis treatment episode. Optum pharmacy data identify the medications received, specifies the date of prescription filling, National Drug Codes, and American Hospital Formulary Service (AHFS) Classification System codes for each medication. We used the AHFS Pharmacologic-Therapeutic classification of antibiotics to create dichotomous variables documenting the antibacterial used by each patient.13 These are categorized under antibacterial including penicillins, cephalosporins (first, second, third, fourth generation cephalosporins), macrolides (first generation and others), tetracyclines, sulfonamides, fluoroquinolones (ciprofloxacin, levofloxacin, moxifloxacin), cephamycin, carbapenems, and β-lactam antibiotics (amoxicillin, amoxicillin/clavulanate, cephalexin, cefuroxime, cefdinir).
Revisits to physician or other provider
Revisits within 28 days were used as the measure of patient outcomes related to testing and filling of an antibiotic prescription for acute pharyngitis. Revisits may also be due to a patient returning for a follow-up, alternative treatment, worsening pharyngitis, or for another ARTI. An ARTI-related revisit also increases total resources used to treat pediatric pharyngitis patients.
Statistical analysis
Logistic regression was used for all 3 analyses conducted in this study. First, we determined the patient and treating physician characteristics that impact the decision to use GAS testing for pharyngitis. Second, we identified those factors that impact the decision to use antibiotic prescriptions among children who were diagnosed with pharyngitis adding in the dichotomous variable indicating if the patient had received a GAS test. Third, we used a logit regression analysis to document if receiving a GAS test and/or an antibiotic impacted the likelihood of a revisit by comparing revisit risk. To estimate the effect of testing and/or antibiotic use, we divided patients into 4 groups based on whether the patient received a GAS test and/or an antibiotic prescription. This specification of the analysis of revisits as an outcome focuses on adherence to HEDIS “test and treat” guidelines10:
- Patients who were not tested yet filled an antibiotic prescription. This decision was likely based on the clinician’s judgment of the patient’s signs and symptoms, and confirmational testing not performed.
- Patients who were not tested and did not fill an antibiotic prescription. Apparently, in the clinician’s judgment the patient’s signs and symptoms were such that the infection did not warrant treatment and the clinical presentation did not necessitate the GAS test to confirm the recorded diagnosis of pharyngitis.
- Patients who were tested and received antibiotic prescription, likely because the test was positive for GABHS.
- Patients who were tested and did not receive antibiotic prescription.
We tested for statistically significant differences in baseline characteristics across these 4 patient groups using t tests for continuous variables and χ2 tests for categorical variables. Odds ratios (OR) and CI were computed for the influential variables included the regression analyses.
We conducted a sensitivity analysis using a model specification which included the dichotomous variables for testing and for treatment, and the interaction term between these variables to assess if treatment effects varied in tested and untested patients. We also estimated this model of revisit risk using revisits within 7 days as the outcome variable.
All analyses were completed using STATA/IC 13 (StataCorp, College Station, TX).
Results
There were 24 685 treatment episodes for children diagnosed with pharyngitis. Nearly 47% of these episodes included GAS testing and 47% of the tested patients filled an antibiotic prescription. Similarly, 53% of patients were not tested and 49% of untested patients filled an antibiotic prescription. As a result, the 4 groups identified for analysis were evenly distributed: untested and no prescription (26.9%), untested and prescription (26.3%), tested and prescription (21.9%), and tested and no prescription (24.9%) (Figure).

Table 1 presents the descriptive statistics for these 4 patient groups. Note first that the rate of revisits within 28 days is under 5% across all groups. Second, the 2 tested groups have a lower revisit rate than the untested groups: the tested and treated have a revisit rate of 3.3%, and the tested and untreated have a revisit rate of 2.4%, while both the untested groups have a revisit rate of nearly 5%. These small absolute differences in revisit rates across groups were statistically significant.

Factors associated with receiving GAS test
Several factors were found to impact the decision to test (Table 2). Only 9.7% of children were reported to have any ARTI coinfection. As expected, these comorbidities resulted in a significantly lower likelihood of receiving the GAS test: AOM, bronchitis, sinusitis, pneumonia, and URI as comorbid infections had a 48%, 41%, 37%, 63%, and 13% lower likelihood of receiving the GAS test, respectively, than those with no comorbidities. Similarly, children with fever and respiratory symptoms were 35% and 45% less likely to receiving the GAS test, respectively. This is consistent with our expectation that comorbid ARTI infections will lead many providers to forgo testing.

Provider type and patient age also plays a role in receipt of the GAS test. Relative to outpatient facility providers, primary care physicians were 24% more likely and specialty physicians were 38% less likely of employing the GAS test. The child’s age played a significant role in receipt of the GAS test. Children aged 1 to 5 years and 5 to 12 years were 15% and 14% more likely to receive the test compared to children older than 12 years.
Pharyngitis patients have disproportionately higher odds of receiving a GAS test in most regions of the country compared to the Pacific region. For instance, children in the Mid-Atlantic region have 51% higher odds of receiving a GAS test while children in New England have 80% higher odds of receiving the same test.
Black children have 11% lower odds of receiving the GAS test compared to White children. Both middle-income and high-income children have 12% and 32% higher odds of receiving the test compared to low-income children. Compared to office-based visits, children visiting a clinic were twice as likely to receive a GAS test while those seen in the emergency room have 43% lower odds of receiving a GAS test. Hospital outpatient departments, which account for less than 1% of all visits, rarely used a GAS test which could be a statistical artifact due to small sample size. Lastly, insurance and season of the year had no significant impact of receipt of a GAS test.
Factors associated with receiving antibiotic prescription
Surprisingly, receiving the GAS test has a small but insignificant impact on the likelihood that the patient will receive an antibiotic prescription (Table 3) (Adjusted OR = 1.055, P = .07). After controlling for receipt of a GAS test, children with AOM and sinusitis comorbidities have an increased likelihood of being prescribed an antibiotic. Children with URI have a lower likelihood of being prescribed an antibiotic. Additionally, relative to primary care physicians, children visiting nonphysician providers for pharyngitis were more likely to be prescribed an antibiotic.

Children under 12 years of age were more likely to use an antibiotic compared to children 12 years and older. Geographically, there is some evidence of regional variation in antibiotic use as well. Children in the south Atlantic, west-south central, and southeast central regions had a significantly lower odds of being prescribed an antibiotic respectively than pharyngitis patients in the Pacific region. Black children had a 10% lower likelihood of being prescribed an antibiotic compared to White children, possibly related to their lower rate of GAS testing. Compared to office-based visits, children visiting a clinic were less likely to use an antibiotic. Household income, insurance type, and season had no significant impact on revisit risk.
Effects of GAS test and antibiotic prescriptions on likelihood of revisits
The multivariate analysis of the risk of a revisit within 28 days is presented in Table 4. Children with pharyngitis who tested and did not receive an antibiotic serve as the reference comparison group for this analysis to illustrate the impact of using the GAS test and treatment with an antibiotic. The results in Table 4 are quite clear: patients who receive the GAS test were significantly less likely to have a revisit within 28 days. Moreover, within the group of patients who were tested, those not receiving an antibiotic, presumedly because their GAS test was negative, experienced the lowest risk of a revisit. This result is consistent with the data in Table 1. Moreover, using an antibiotic had no impact on the likelihood of a revisit in patients not receiving the GAS test. This result is also consistent with Table 1.

Other results from the analysis of revisit risk may be of interest to clinicians. Pharyngitis patients with a prior episode of treatment within 90 days for an acute respiratory tract infection were more than 7 times more likely to experience a revisit within 28 days of the pharyngitis diagnosis than patients without a history of recent ARTI infections. Age is also a risk factor in likelihood of initiating a revisit. Children under 1 year and children aged 1 to 5 years were more likely to have a revisit than children aged more than 12 years. Compared to White children, Black children were 25% (P = .04) less likely to have a revisit. The care setting also has a significant impact on revisit risk. Children visiting outpatient hospital and other care settings had a significantly higher revisit risk than those visiting a physician’s office. Lastly, household income, geographic region, season, medical comorbidities, gender, and insurance type have no significant impact on revisit risk.
Sensitivity analysis
The results from the analysis of 7-day and 28-day revisit risk are summarized in Table 5. These results indicate that patients who were tested had a more significant decrease in revisit risk at 7 days (72%) than was evident at 28 days (47% reduction). Receiving an antibiotic, with or without the test, had no impact on revisit risk.

Discussion
Published data on revisits for pharyngitis are lacking with the concentration of prior research focused more on systemic complications of undertreated GABHS disease or on identifying carrier status. Our study results suggest that GAS testing is the most important factor in reducing revisit risk. Being prescribed an antibiotic, on its own, does not have a significant impact on the risk of a revisit. However, once the GAS test is used, the decision not to use an antibiotic was correlated with the lowest revisit rate, likely because the source of the pharyngitis infection was viral and more likely to resolve without a revisit. Prior studies have reported variable rates of testing among children with pharyngitis prescribed an antibiotic, ranging from 23% to 91%,14,15 with testing important toward more appropriate antibiotic use.16 More recently, among more than 67 000 patients aged 3 to 21 years presenting with sore throat and receiving a GAS test, 32.6% were positive.17
Our analysis found that more than 46% of pediatric pharyngitis patients were given the rapid GAS test. While this testing rate is substantially lower than HEDIS recommendations and lower than testing rates achieved by several health maintenance organizations,10 it is similar to the 53% of children receiving such testing in a recent National Ambulatory Medical Care Survey.18 Furthermore, we found that when antibiotics are prescribed following a GAS test, the revisit risk is not significantly reduced, possibly because antibiotics lower revisit risk when informed by diagnostic testing tools that determine the infectious organism. This is supported by a similar population analysis in which we observed reduced revisit rates in children with AOM managed with antibiotics within 3 days of index diagnosis.19
Several other factors also affect the likelihood of a child receiving the GAS test. Children aged 1 to 12 years were significantly more likely to receive the GAS test than children over the age of 12. This included children in the 1 to 5 years old bracket who had a 15% higher likelihood of undergoing a GAS test, despite children less than 3 years of age as not recommended targets for GAS testing.20 As expected, children with reported ARTI-associated comorbidities were also less likely to receive a GAS test. Additionally, specialty care physicians were less inclined to implement the GAS test, possibly because of diagnostic confidence without testing or referral after GAS was ruled out. Black and low-income children had statistically lower odds of receiving the test, even after controlling for other factors, and yet were less likely to consume a revisit. As the overall data suggested more revisits in those not tested, further study is needed to examine if race or income discrepancies are equity based. Finally, children in the Pacific region, compared to the rest of the nation, were the least likely to receive a GAS test and yet there were no significant differences in revisit rates by region. Regional differences in antibiotic use were also observed in our study, as has been seen by others.21
After statistically controlling for having received the diagnostic GAS test and filled a prescription for an antibiotic, there are multitude of factors that independently affect the revisit risk, the most important of which if which was a history of an ARTI infection in the prior 90 days. While prior visit history had no impact on the likelihood of being tested or filling an antibiotic, patients with prior visits were more than 7 times more likely to consume a revisit. This was not reflected in nor related to comorbid ARTIs as these patients did not have statistically higher revisits than those with pharyngitis as the sole-coded diagnosis. Moreover, speculation for bacterial etiology of primary or superinfection based on a recent history of ARTI accounting for revisits seems unlikely as it did not yield greater antibiotic use in that group. Further analysis is required to determine the clinical and behavioral factors that promote for prior ARTI history as a major factor in revisit risk after an index visit for pharyngitis.
Children aged between 1 and 5 years, though 15% more likely to be tested than those aged 12 through 17 years, were also 39% more likely to initiate a revisit compared to older children when statistically controlling for other covariates. This perhaps suggests longer illness, wrong diagnosis, delay in appropriate treatment, or more caution by parents and providers in this age group. Justification for testing children less than 3 years of age who are outside of the HEDIS suggested age group, when clinical judgement does not point to another infection source, can result in positivity rates between 22% and 30% as previously observed.22,23 Patients visiting nonphysician providers and outpatient facility providers were less likely to have a revisit than those visiting primary and specialty care physicians, though slightly higher propensity for antibiotic prescriptions was seen for nonphysician providers. Pediatricians have been noted to be less likely to prescribe antibiotics without GAS testing than nonpediatric providers, and more guidelines-compliant in prescribing.24
Recommendations to not test children under 3 years of age are based on the lack of acute rheumatic fever and other complications in this age group together with more frequent viral syndromes. Selectivity in applying clinical criteria to testing can be attempted to separate bacterial from viral illness. Postnasal drainage/rhinorrhea, hoarse voice, and cough have been used successfully to identify those with viral illness and less need for testing, with greater certainty of low risk for GABHS in those over 11 years of age without tonsillar exudates, cervical adenopathy, or fever.17 However, the marginal benefits of those who have all 3 features of viral illness versus none in identifying GAS positivity was 23.3% vs 37.6% - helpful, but certainly not diminishing the need for testing. These constitutional findings of viral URI also do not exclude the GAS carrier state that features these symptoms.25 Others have reinforced the doubt of pharyngeal exudates as the premier diagnostic finding for test-positive GAS.26
This study had several limitations. The Optum claims dataset only contains ICD-9 codes for diagnoses. It does not include data on infection severity and clinical findings related to symptoms, thus empiric treatment warranted based in clinical severity is not assessed. Antibiotics are commonly available as generics and very inexpensive. Patients may fill and pay for these prescriptions directly, in which case, a claim for payment may not be filed with Optum. This could result in an undercount of treated patients in our study.
There is no corresponding problem of missing medical claims for GAS testing which were obtained from the CPT codes within the Optum claims data set. However, we elected not to verify the test results due to these data being missing for 75% of the study population. Nevertheless, this study’s focus was less about justifying antibiotic treatment, but dealt with the outcomes generated by testing and treatment. Toward that end, we used CPT codes to identify a revisit, and while those can at times be affected by financial reimbursement incentives, differences related to revisits in the 4 patient groups should not be subject to bias.
Conclusion
This study used data from real world practices to document the patterns of GAS testing and antibiotic use in pediatric pharyngitis patients. Revisit rates were under 5% for all patient groups and the use of rapid diagnostic tools were found to be the determining factor in further reducing the risk of revisits. This supports the need for compliance with the HEDIS quality metric for pharyngitis to the recommended levels of rapid testing which have been falling in recent years. Use of more accurate antigen and newer molecular detection testing methods may help further delineate important factors in determining pediatric pharyngitis treatment and need for revisits.27
Corresponding author: Jeffrey McCombs, MD, University of Southern California School of Pharmacy, Department of Pharmaceutical and Health Economics, Leonard D. Schaeffer Center for Health Policy & Economics, 635 Downey Way, Verna & Peter Dauterive Hall 310, Los Angeles, CA 90089-3333; [email protected].
Financial disclosures: None.
1. Choby BA. Diagnosis and treatment of streptococcal pharyngitis. Am Fam Physician. 2009;79(5):383-390.
2. Briel M, Schuetz P, Mueller B, et al. Procalcitonin-guided antibiotic use vs a standard approach for acute respiratory tract infections in primary care. Arch of Intern Med. 2008;168(18):2000-2008. doi: 10.1001/archinte.168.18.2000
3. Maltezou HC, Tsagris V, Antoniadou A, et al. Evaluation of a rapid antigen detection test in the diagnosis of streptococcal pharyngitis in children and its impact on antibiotic prescription. J Antimicrob Chemother. 2008;62(6):1407-1412. doi: 10.1093/jac/dkn376
4. Neuner JM, Hamel MB, Phillips RS, et al. Diagnosis and management of adults with pharyngitis: a cost-effectiveness analysis. Ann Intern Med. 2003;139(2):113-122. doi:10.7326/0003-4819-139-2-200307150-00011
5. Gerber MA, Baltimore RS, Eaton CB, et al. Prevention of rheumatic fever and diagnosis and treatment of acute Streptococcal pharyngitis: a scientific statement from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young, the Interdisciplinary Council on Functional Genomics and Translational Biology, and the Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Academy of Pediatrics. Circulation. 2009;119(11):1541-1551. doi: 10.1161/CIRCULATIONAHA.109.191959
6. Gieseker KE, Roe MH, MacKenzie T, Todd JK. Evaluating the American Academy of Pediatrics diagnostic standard for Streptococcus pyogenes pharyngitis: backup culture versus repeat rapid antigen testing. Pediatrics. 2003;111(6):e666-e670. doi: 10.1542/peds.111.6.e666
7. Shapiro DJ, Lindgren CE, Neuman MI, Fine AM. Viral features and testing for Streptococcal pharyngitis. Pediatrics. 2017;139(5):e20163403. doi: 10.1542/peds.2016-3403
8. Shulman ST, Bisno AL, Clegg H, et al. Clinical practice guideline for the diagnosis and management of group A Streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55(10):e86–e102. doi: 10.1093/cid/cis629
9. Mangione-Smith R, McGlynn EA, Elliott MN, et al. Parent expectations for antibiotics, physician-parent communication, and satisfaction. Arch Pediatr Adolesc Med. 2001;155(7):800–806. doi: 10.1001/archpedi.155.7.800
10. Appropriate Testing for Children with Pharyngitis. HEDIS Measures and Technical Resources. National Committee for Quality Assurance. Accessed February 12, 2021. https://www.ncqa.org/hedis/measures/appropriate-testing-for-children-with-pharyngitis/
11. Linder JA, Bates DW, Lee GM, Finkelstein JA. Antibiotic treatment of children with sore throat. JAMA. 2005;294(18):2315-2322. doi: 10.1001/jama.294.18.2315
12. Crimmel BL. Health Insurance Coverage and Income Levels for the US Noninstitutionalized Population Under Age 65, 2001. Medical Expenditure Panel Survey, Agency for Healthcare Research and Quality. 2004. https://meps.ahrq.gov/data_files/publications/st40/stat40.pd
13. AHFS/ASHP. American Hospital Formulary Service Drug Information. 2012. AHFS drug information. 00--. Accessed January 4, 2021.
14. Mainous AG 3rd, Zoorob, RJ, Kohrs FP, Hagen MD. Streptococcal diagnostic testing and antibiotics prescribed for pediatric tonsillopharyngitis. Pediatr Infect Dis J. 1996;15(9):806-810. doi: 10.1097/00006454-199609000-00014
15. Benin AL, Vitkauskas G, Thornquist E, et al. Improving diagnostic testing and reducing overuse of antibiotics for children with pharyngitis: a useful role for the electronic medical record. Pediatr Infect Dis J. 2003;22(12):1043-1047. doi: 10.1097/01.inf.0000100577.76542.af
16. Luo R, Sickler J, Vahidnia F, et al. Diagnosis and Management of Group a Streptococcal Pharyngitis in the United States, 2011-2015. BMC Infect Dis. 2019;19(1):193-201. doi: 10.1186/s12879-019-3835-4
17. Shapiro DJ, Barak-Corren Y, Neuman MI, et al. Identifying Patients at Lowest Risk for Streptococcal Pharyngitis: A National Validation Study. J Pediatr. 2020;220:132-138.e2. doi: 10.1016/j.jpeds.2020.01.030. Epub 2020 Feb 14
18. Shapiro DJ, King LM, Fleming-Dutra KE, et al. Association between use of diagnostic tests and antibiotic prescribing for pharyngitis in the United States. Infect Control Hosp Epidemiol. 2020;41(4):479-481. doi: 10.1017/ice.2020.29
19. Sangha K, Steinberg I, McCombs JS. The impact of antibiotic treatment time and class of antibiotic for acute otitis media infections on the risk of revisits. Abs PDG4. Value in Health. 2019; 22:S163.
20. Ahluwalia T, Jain S, Norton L, Meade J, et al. Reducing Streptococcal Testing in Patients < 3 Years Old in an Emergency Department. Pediatrics. 2019;144(4):e20190174. doi: 10.1542/peds.2019-0174
21. McKay R, Mah A, Law MR, et al. Systematic Review of Factors Associated with Antibiotic Prescribing for Respiratory Tract Infections. Antimicrob Agents Chemother. 2016;60(7):4106-4118. doi: 10.1128/AAC.00209-16
22. Woods WA, Carter CT, Schlager TA. Detection of group A streptococci in children under 3 years of age with pharyngitis. Pediatr Emerg Care. 1999;15(5):338-340. doi: 10.1097/00006565-199910000-00011
23. Mendes N, Miguéis C, Lindo J, et al. Retrospective study of group A Streptococcus oropharyngeal infection diagnosis using a rapid antigenic detection test in a paediatric population from the central region of Portugal. Eur J Clin Microbiol Infect Dis. 2021;40(6):1235-1243. doi: 10.1007/s10096-021-04157-x
24. Frost HM, McLean HQ, Chow BDW. Variability in Antibiotic Prescribing for Upper Respiratory Illnesses by Provider Specialty. J Pediatr. 2018;203:76-85.e8. doi: 10.1016/j.jpeds.2018.07.044.
25. Rick AM, Zaheer HA, Martin JM. Clinical Features of Group A Streptococcus in Children With Pharyngitis: Carriers versus Acute Infection. Pediatr Infect Dis J. 2020;39(6):483-488. doi: 10.1097/INF.0000000000002602
26. Nadeau NL, Fine AM, Kimia A. Improving the prediction of streptococcal pharyngitis; time to move past exudate alone [published online ahead of print, 2020 Aug 16]. Am J Emerg Med. 2020;S0735-6757(20)30709-9. doi: 10.1016/j.ajem.2020.08.023
27. Mustafa Z, Ghaffari M. Diagnostic Methods, Clinical Guidelines, and Antibiotic Treatment for Group A Streptococcal Pharyngitis: A Narrative Review. Front Cell Infect Microbiol. 2020;10:563627. doi: 10.3389/fcimb.2020.563627
1. Choby BA. Diagnosis and treatment of streptococcal pharyngitis. Am Fam Physician. 2009;79(5):383-390.
2. Briel M, Schuetz P, Mueller B, et al. Procalcitonin-guided antibiotic use vs a standard approach for acute respiratory tract infections in primary care. Arch of Intern Med. 2008;168(18):2000-2008. doi: 10.1001/archinte.168.18.2000
3. Maltezou HC, Tsagris V, Antoniadou A, et al. Evaluation of a rapid antigen detection test in the diagnosis of streptococcal pharyngitis in children and its impact on antibiotic prescription. J Antimicrob Chemother. 2008;62(6):1407-1412. doi: 10.1093/jac/dkn376
4. Neuner JM, Hamel MB, Phillips RS, et al. Diagnosis and management of adults with pharyngitis: a cost-effectiveness analysis. Ann Intern Med. 2003;139(2):113-122. doi:10.7326/0003-4819-139-2-200307150-00011
5. Gerber MA, Baltimore RS, Eaton CB, et al. Prevention of rheumatic fever and diagnosis and treatment of acute Streptococcal pharyngitis: a scientific statement from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young, the Interdisciplinary Council on Functional Genomics and Translational Biology, and the Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Academy of Pediatrics. Circulation. 2009;119(11):1541-1551. doi: 10.1161/CIRCULATIONAHA.109.191959
6. Gieseker KE, Roe MH, MacKenzie T, Todd JK. Evaluating the American Academy of Pediatrics diagnostic standard for Streptococcus pyogenes pharyngitis: backup culture versus repeat rapid antigen testing. Pediatrics. 2003;111(6):e666-e670. doi: 10.1542/peds.111.6.e666
7. Shapiro DJ, Lindgren CE, Neuman MI, Fine AM. Viral features and testing for Streptococcal pharyngitis. Pediatrics. 2017;139(5):e20163403. doi: 10.1542/peds.2016-3403
8. Shulman ST, Bisno AL, Clegg H, et al. Clinical practice guideline for the diagnosis and management of group A Streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55(10):e86–e102. doi: 10.1093/cid/cis629
9. Mangione-Smith R, McGlynn EA, Elliott MN, et al. Parent expectations for antibiotics, physician-parent communication, and satisfaction. Arch Pediatr Adolesc Med. 2001;155(7):800–806. doi: 10.1001/archpedi.155.7.800
10. Appropriate Testing for Children with Pharyngitis. HEDIS Measures and Technical Resources. National Committee for Quality Assurance. Accessed February 12, 2021. https://www.ncqa.org/hedis/measures/appropriate-testing-for-children-with-pharyngitis/
11. Linder JA, Bates DW, Lee GM, Finkelstein JA. Antibiotic treatment of children with sore throat. JAMA. 2005;294(18):2315-2322. doi: 10.1001/jama.294.18.2315
12. Crimmel BL. Health Insurance Coverage and Income Levels for the US Noninstitutionalized Population Under Age 65, 2001. Medical Expenditure Panel Survey, Agency for Healthcare Research and Quality. 2004. https://meps.ahrq.gov/data_files/publications/st40/stat40.pd
13. AHFS/ASHP. American Hospital Formulary Service Drug Information. 2012. AHFS drug information. 00--. Accessed January 4, 2021.
14. Mainous AG 3rd, Zoorob, RJ, Kohrs FP, Hagen MD. Streptococcal diagnostic testing and antibiotics prescribed for pediatric tonsillopharyngitis. Pediatr Infect Dis J. 1996;15(9):806-810. doi: 10.1097/00006454-199609000-00014
15. Benin AL, Vitkauskas G, Thornquist E, et al. Improving diagnostic testing and reducing overuse of antibiotics for children with pharyngitis: a useful role for the electronic medical record. Pediatr Infect Dis J. 2003;22(12):1043-1047. doi: 10.1097/01.inf.0000100577.76542.af
16. Luo R, Sickler J, Vahidnia F, et al. Diagnosis and Management of Group a Streptococcal Pharyngitis in the United States, 2011-2015. BMC Infect Dis. 2019;19(1):193-201. doi: 10.1186/s12879-019-3835-4
17. Shapiro DJ, Barak-Corren Y, Neuman MI, et al. Identifying Patients at Lowest Risk for Streptococcal Pharyngitis: A National Validation Study. J Pediatr. 2020;220:132-138.e2. doi: 10.1016/j.jpeds.2020.01.030. Epub 2020 Feb 14
18. Shapiro DJ, King LM, Fleming-Dutra KE, et al. Association between use of diagnostic tests and antibiotic prescribing for pharyngitis in the United States. Infect Control Hosp Epidemiol. 2020;41(4):479-481. doi: 10.1017/ice.2020.29
19. Sangha K, Steinberg I, McCombs JS. The impact of antibiotic treatment time and class of antibiotic for acute otitis media infections on the risk of revisits. Abs PDG4. Value in Health. 2019; 22:S163.
20. Ahluwalia T, Jain S, Norton L, Meade J, et al. Reducing Streptococcal Testing in Patients < 3 Years Old in an Emergency Department. Pediatrics. 2019;144(4):e20190174. doi: 10.1542/peds.2019-0174
21. McKay R, Mah A, Law MR, et al. Systematic Review of Factors Associated with Antibiotic Prescribing for Respiratory Tract Infections. Antimicrob Agents Chemother. 2016;60(7):4106-4118. doi: 10.1128/AAC.00209-16
22. Woods WA, Carter CT, Schlager TA. Detection of group A streptococci in children under 3 years of age with pharyngitis. Pediatr Emerg Care. 1999;15(5):338-340. doi: 10.1097/00006565-199910000-00011
23. Mendes N, Miguéis C, Lindo J, et al. Retrospective study of group A Streptococcus oropharyngeal infection diagnosis using a rapid antigenic detection test in a paediatric population from the central region of Portugal. Eur J Clin Microbiol Infect Dis. 2021;40(6):1235-1243. doi: 10.1007/s10096-021-04157-x
24. Frost HM, McLean HQ, Chow BDW. Variability in Antibiotic Prescribing for Upper Respiratory Illnesses by Provider Specialty. J Pediatr. 2018;203:76-85.e8. doi: 10.1016/j.jpeds.2018.07.044.
25. Rick AM, Zaheer HA, Martin JM. Clinical Features of Group A Streptococcus in Children With Pharyngitis: Carriers versus Acute Infection. Pediatr Infect Dis J. 2020;39(6):483-488. doi: 10.1097/INF.0000000000002602
26. Nadeau NL, Fine AM, Kimia A. Improving the prediction of streptococcal pharyngitis; time to move past exudate alone [published online ahead of print, 2020 Aug 16]. Am J Emerg Med. 2020;S0735-6757(20)30709-9. doi: 10.1016/j.ajem.2020.08.023
27. Mustafa Z, Ghaffari M. Diagnostic Methods, Clinical Guidelines, and Antibiotic Treatment for Group A Streptococcal Pharyngitis: A Narrative Review. Front Cell Infect Microbiol. 2020;10:563627. doi: 10.3389/fcimb.2020.563627
Cost Comparison of 2 Video Laryngoscopes in a Large Academic Center
From the Department of Anesthesiology, Thomas Jefferson University and Hospitals, Sidney Kimmel Medical College, Philadelphia, PA, and Sidney Kimmel Medical College at Thomas Jefferson University, Philadelphia, PA.
Objective: Retrospective study examining hospital cost information of patients requiring endotracheal intubation with video laryngoscopy. Provide a practical cost assessment on use of the McGRATH and GlideScope video laryngoscopes (VLs).
Methods: This study examined 52 hospital locations within a single, large university hospital, with most of those locations being hospital operating rooms. A total of 34 600 endotracheal intubations performed over 24 months, of which 11 345 were video laryngoscopies. Electronic medical records containing demographic data and information related to endotracheal intubation procedures, with monthly breakdowns between GlideScope and McGRATH intubations, were reviewed. Cost information calculated for equipment, blades, batteries, repairs, and subsequent analysis performed to determine cost differences between those 2 instruments during the COVID-19 period.
Results: A total of 5501 video laryngoscopy procedures were performed using the McGRATH VL and 5305 were performed using the GlideScope VL. Costs over 24 months were $181 093 lower (55.5%) for McGRATH compared to GlideScope. The mean (SD) monthly costs for GlideScope blades were $3837 ($1050) and $3236 ($538) for years 1 and 2, respectively, vs $1652 ($663) and $2933 ($585) for McGRATH blades (P < .001). Most total cost differences were attributed to equipment and blade purchases, which were $202 595 (65.0%) higher for GlideScope. During the COVID-19 period, the use of the McGRATH increased to 61% of all video laryngoscopy cases, compared to 37% for GlideScope (P < .001). Blade cost difference for the COVID-19 period was $128 higher for the McGRATH even though 293 more intubations were performed with that device.
Conclusions: Use of the McGRATH resulted in a cost savings of 55% compared to the GlideScope, and its use was highest during the COVID-19 period, which may be explained by its more portable and practical features.
Keywords: video laryngoscope; McGRATH; GlideScope; endotracheal intubation; hospital costs; COVID-19.
Hospitals have come to rely on video laryngoscopes (VLs) for tracheal intubation as necessary tools for better visualization of airways. Modern video laryngoscopy developed in the 2000s1 as a progression from direct laryngoscopy, which began in 1852 when Horace Green used a bent tongue spatula and sunlight to examine a child.2 VLs have seen many improvements and adaptations of their own, resulting in many different styles and types circulating around hospitals. The GlideScope (Verathon Inc, Bothell, WA) and the McGRATH (Medtronic, Minneapolis, MN) are examples of such instruments, which are now widely used in the US and are the 2 VLs of choice at our institution.
A few studies have compared VLs to direct laryngoscopes. In their systematic review, Lewis et al have shown the numerous benefits of using a VL over a direct laryngoscope. Some general conclusions were that the use of video laryngoscopy reduced the number of failed intubations, decreased laryngeal trauma, and provided improved visualizations.3 Other studies have compared the different types of VLs, including the McGRATH and the GlideScope, examining factors such as intubation time and display quality of the image. Two studies found that medical students were equally successful at using both the McGRATH and the GlideScope,4,5 while another study found that care providers using the GlideScope had quicker intubation times.6 Lastly, Savoldelli et al concluded that more providers preferred the McGRATH, which provided better laryngeal views,7 while their subsequent study showed more favorable learning curves of the Airtraq compared to the McGRATH and other VLs.8
Although there have been no reported differences in safety and effectiveness of the McGRATH and GlideScope devices, cost data on the use of these 2 popular laryngoscopes are lacking. Such information is important considering the increasing costs of medical technologies and the significant financial losses experienced by health care systems due to the COVID-19 crisis. The purpose of this retrospective cohort study was to compare the cost efficiency of the McGRATH MAC and GlideScope Core VLs at a large academic center.
Methods
This retrospective study was performed under exemption from the Thomas Jefferson University Institutional Review Board. The primary data sources consisted of hospital electronic patient records (EPIC) and cost information from the device manufacturers and hospital staff. The electronic patient data were provided by the EPIC Enterprise Analytics Business Intelligence group at Thomas Jefferson University Hospital (Center City Campus, Philadelphia, PA), while device costs were obtained from Verathon, Medtronic, and departmental staff responsible for purchasing equipment. Monthly data were obtained over a 24-month period (June 2018 through May 2020) when the McGRATH VL was placed into use in the department of anesthesiology. The 2 types of VLs were made available for use in a total of 52 locations, with the majority being hospital operating rooms.
The following variables were recorded: number of endotracheal intubations performed each month with breakdown between video laryngoscopy and flexible bronchoscopy airways, frequency of use for each type of laryngoscope, blades used, and equipment costs for use of each laryngoscope. Hospital cost estimates for both the McGRATH and GlideScope laryngoscopes included batteries, handles, blades, and the devices themselves. Cost data were also collected on frequency of device failure, maintenance, and replacement of parts and lost equipment.
Analysis
De-identified electronic medical records consisted of nominal and quantitative variables, with demographic data and information related to the endotracheal intubation procedure. All data were in chronological order and sorted by date after which coding was applied, to identify device type and allocate pertinent cost information. Descriptive statistics were reported as mean (SD) and sum for costs; frequency tables were generated for intubation procedures according to device type and time periods. Data were analyzed using the χ2 test, the student t test, and the Wilcoxon Mann-Whitney U test, with a P value set at .05 for statistical significance. SPSS version 26 and GraphPad Prism version 6 were used for all statistical analyses.
Results
A total of 34 600 endotracheal intubations were performed over the 24-month study period, and 11 345 (32.8%) were video laryngoscopy procedures. Out of all video laryngoscopy procedures, 5501 (48.5%) were performed using the McGRATH VL and 5305 (46.8%) were conducted using the GlideScope VL. The difference of 539 (4.8%) cases accounts for flexible bronchoscopy procedures and endotracheal intubations using other video laryngoscopy equipment. The mean (SD) monthly number of video laryngoscopy procedures for the 24 months was 221 (54) and 229 (89) for the GlideScope and McGRATH devices, respectively. Monthly endotracheal intubation distributions over 24 months trended upward for the McGRATH VL and downward for the GlideScope, but there was no statistically significant (P = .71) difference in overall use between the 2 instruments (Figure 1).
To examine the observed usage trends between the 2 VL during the first and last 12 months, a univariate ANOVA was conducted with the 2 time periods entered as predictors in the model. Video laryngoscopy intubations were performed (P = .001) more frequently with the GlideScope during the first 12 months; however, use of the McGRATH VL increased (P < .001) during the following 12 months compared to GlideScope. The GlideScope accounted for 54% of all VL intubations during the first 12 months, with the McGRATH accounting for 58% of all video laryngoscopy procedures for months 12 to 24. Additionally, the increase in video laryngoscopy procedures with the McGRATH during the last 3 months of the study period was despite an overall reduction in surgical volume due to the COVID-19 crisis, defined for this study as March 1, 2020, to May 31, 2020 (Figure 1). There was a statistically significant (P < .001) difference in the case distribution between use of the McGRATH and GlideScope VL for that period. The anesthesia personnel’s use of the McGRATH VL increased to 61% of all video laryngoscopy cases, compared to 37% for the GlideScope (Figure 2).
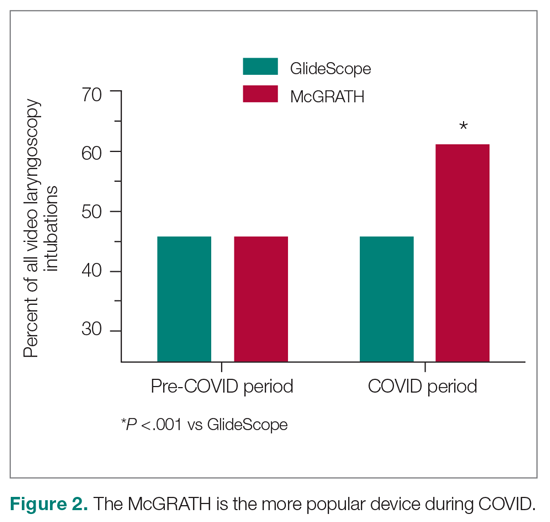
The total costs calculated for equipment, blades, and repairs are presented in Table 1 and yearly total costs are shown in Figure 3. Overall costs were $181 093 lower (55.5%) for the McGRATH VL compared to the GlideScope over the 24-month period. The mean (SD) monthly costs for GlideScope VL blades were $3837 ($1050) and $3236 ($538) for years 1 and 2, respectively, vs $1652 ($663) and $2933 ($585) for the McGRATH VL blades. Most of the total cost differences were attributed to equipment and blade purchases, which were $202 595 (65.0%) higher for the GlideScope compared to the McGRATH VL. The monthly blade costs alone were higher (P < .001) for the GlideScope over the 2-year period; however, the McGRATH VL required use of disposable stylets at a cost of $10 177 for all endotracheal intubations, compared to $700 for the GlideScope device.
An analysis was performed to determine whether costs differed between those 2 instruments during the COVID-19 period. There was a statistically significant (P < .001) difference in the case distribution between use of the McGRATH and GlideScope VLs during that period. The calculated blade cost difference for the COVID period was $128 higher for the McGRATH even though 293 more intubations were performed with that device (Table 2).
Discussion
We attempted to provide useful cost estimates by presenting pricing data reflecting the approximate cost that most large institutional anesthesia practices would incur for using those 2 specific devices and related peripherals. The main findings of our analysis showed that use of the McGRATH MAC VL resulted in a 55% cost savings compared to the GlideScope, with a similar number of cases performed with each device over the 24-month study period. We believe this represents a substantial savings to the department and institution, which has prompted internal review on the use of video laryngoscopy equipment. None of the McGRATH units failed; however, the GlideScope required 3 baton replacements.
Of note, use of the McGRATH MAC increased during the COVID-19 period, which may be explained by the fact that the operators found it to be a more portable device. Several physicians in the department commented that its smaller size made the McGRATH MAC more practical during the time when a plexiglass box was being used around the patient’s head to shield the intubator from aerosolized viral particles.
Although this study demonstrated the cost-saving value of the McGRATH over the GlideScope, a suggested next step would be to examine resource utilization related to video laryngoscopy use. The more dynamic tracking of the use of these devices should facilitate the assessment of existing related resources and decision making, to optimize the benefits of this initiative. We would anticipate reduced use of anesthesia personnel, such as technicians to assist with the management of this device which could be significant. As new respiratory viruses are appearing each year, video laryngoscopy will continue to gain increasing use in operating rooms and acute care locations. The adding of protective barriers between patients and providers calls for use of the most practical and effective VL devices, to protect personnel who are at high risk of contamination from airway secretions and aerosolized particles.9,10
The COVID-19 pandemic has demonstrated the value of anesthesiology in regards to analyzing and finding solutions to effectively manage infected patients or those suspected of infection in the perioperative environment. Inexpensive products are often avoided because cheaper devices are associated with being of lower quality. However, the association with cost and quality—and the assumption that a higher price is positively correlated with higher quality—is overall inconsistent in the medical literature.11 A more effective or higher quality treatment does not necessarily cost more and may actually end up costing less,12 as was the case in this study. We have been able to directly cut departmental expenses by using a more efficient and cost-effective device for intubations, without compromising safety and efficacy. Future studies should determine whether this significant reduction in costs from video laryngoscopy intubations with the McGRATH VL will be sustained across anesthesiology departments in the Jefferson Health Enterprise Hospitals, or other health systems, as well as its impact on workflow and personnel resources.
This analysis was restricted to one of the campuses of the Jefferson Health Enterprise. However, this is the largest anesthesia practice, encompassing several locations, which should reflect the general practice patterns across other anesthesiology departments in this large institution. The costs for the devices and peripherals may vary across anesthesia practices depending on volume and contracts negotiated with the suppliers. It was not possible to estimate this variability, which could change the total costs by a few percentage points. We recognize that there may be other costs associated with securing the McGRATH VL to prevent loss from theft or misplacement, which were not included in the study. Lastly, the inability to obtain randomized samples for the 2 groups treated with each device opens up the possibility of selection bias. There were, however, multiple intubators who were free to select 1 of the devices for endotracheal intubation, which may have reduced the effect of selection bias.
Conclusion
This study demonstrated that over a 24-month period use of the McGRATH MAC VL resulted in a cost reduction of around 55% compared to using the GlideScope for endotracheal intubation procedures performed at a major academic center. Over the first 3 months of the COVID-19 crisis, which our study included, use of the McGRATH VL increased while GlideScope use decreased. This was most likely related to the portability and smaller size of the McGRATH, which better facilitated intubations of COVID-19 patients.
Acknowledgements: The authors thank Craig Smith, Senior Anesthesia Technician, for his assistance with the cost information and excellent record-keeping related to the use of video laryngoscopes.
Corresponding author: Marc C. Torjman, PhD, Professor, Department of Anesthesiology, Sidney Kimmel Medical College at Thomas Jefferson University, 111 South 11th St, Suite G-8290, Philadelphia, PA 19107; [email protected].
Financial disclosures: Dr. Thaler has served as a consultant for Medtronic since September 2020. He has participated in 2 webinars on the routine use of video laryngoscopy.
Funding: This study was supported by the Department of Anesthesiology at Thomas Jefferson University.
1. Channa AB. Video laryngoscopes. Saudi J Anaesth. 2011;5(4):357-359.
2. Pieters BM, Eindhoven GB, Acott C, Van Zundert AAJ. Pioneers of laryngoscopy: indirect, direct and video laryngoscopy. Anaesth Intensive Care. 2015;43(suppl):4-11.
3. Lewis SR, Butler AR, Parker J, et al. Videolaryngoscopy versus direct laryngoscopy for adult patients requiring tracheal intubation. Cochrane Database Syst Rev. 2016;11(11):CD011136.
4. Kim W, Choi HJ, Lim T, Kang BS. Can the new McGrath laryngoscope rival the GlideScope Ranger portable video laryngoscope? A randomized manikin study. Am J Emerg Med. 2014;32(10):1225-1229.
5. Kim W, Choi HJ, Lim T, et al. Is McGrath MAC better than Glidescope Ranger for novice providers in the simulated difficult airway? A randomized manikin study. Resuscitation. 2014;85(suppl 1):S32.
6. Jeon WJ, Kim KH, Yeom JH, et al. A comparison of the Glidescope to the McGrath videolaryngoscope in patients. Korean J Anesthesiol. 2011;61(1):19-23.
7. Savoldelli GL, Schiffer E, Abegg C, et al. Comparison of the Glidescope, the McGrath, the Airtraq and the Macintosh laryngoscopes in simulated difficult airways. Anaesthesia. 2008;63(12):1358-1364.
8. Savoldelli GL, Schiffer E, Abegg C, et al. Learning curves of the Glidescope, the McGrath and the Airtraq laryngoscopes: a manikin study. Eur J Anaesthesiol. 2009;26(7):554-558.
9. Schumacher J, Arlidge J, Dudley D, et al. The impact of respiratory protective equipment on difficult airway management: a randomised, crossover, simulation study. Anaesthesia. 2020;75(10):1301-1306.
10. De Jong A, Pardo E, Rolle A, et al. Airway management for COVID-19: a move towards universal videolaryngoscope? Lancet Respir Med. 2020;8(6):555.
11. Hussey PS, Wertheimer S, Mehrotra A. The association between health care quality and cost: a systematic review. Ann Intern Med. 2013;158(1):27-34.
12. Mitton C, Dionne F, Peacock S, Sheps S. Quality and cost in healthcare: a relationship worth examining. Appl Health Econ Health Policy. 2006;5(4):201-208.
From the Department of Anesthesiology, Thomas Jefferson University and Hospitals, Sidney Kimmel Medical College, Philadelphia, PA, and Sidney Kimmel Medical College at Thomas Jefferson University, Philadelphia, PA.
Objective: Retrospective study examining hospital cost information of patients requiring endotracheal intubation with video laryngoscopy. Provide a practical cost assessment on use of the McGRATH and GlideScope video laryngoscopes (VLs).
Methods: This study examined 52 hospital locations within a single, large university hospital, with most of those locations being hospital operating rooms. A total of 34 600 endotracheal intubations performed over 24 months, of which 11 345 were video laryngoscopies. Electronic medical records containing demographic data and information related to endotracheal intubation procedures, with monthly breakdowns between GlideScope and McGRATH intubations, were reviewed. Cost information calculated for equipment, blades, batteries, repairs, and subsequent analysis performed to determine cost differences between those 2 instruments during the COVID-19 period.
Results: A total of 5501 video laryngoscopy procedures were performed using the McGRATH VL and 5305 were performed using the GlideScope VL. Costs over 24 months were $181 093 lower (55.5%) for McGRATH compared to GlideScope. The mean (SD) monthly costs for GlideScope blades were $3837 ($1050) and $3236 ($538) for years 1 and 2, respectively, vs $1652 ($663) and $2933 ($585) for McGRATH blades (P < .001). Most total cost differences were attributed to equipment and blade purchases, which were $202 595 (65.0%) higher for GlideScope. During the COVID-19 period, the use of the McGRATH increased to 61% of all video laryngoscopy cases, compared to 37% for GlideScope (P < .001). Blade cost difference for the COVID-19 period was $128 higher for the McGRATH even though 293 more intubations were performed with that device.
Conclusions: Use of the McGRATH resulted in a cost savings of 55% compared to the GlideScope, and its use was highest during the COVID-19 period, which may be explained by its more portable and practical features.
Keywords: video laryngoscope; McGRATH; GlideScope; endotracheal intubation; hospital costs; COVID-19.
Hospitals have come to rely on video laryngoscopes (VLs) for tracheal intubation as necessary tools for better visualization of airways. Modern video laryngoscopy developed in the 2000s1 as a progression from direct laryngoscopy, which began in 1852 when Horace Green used a bent tongue spatula and sunlight to examine a child.2 VLs have seen many improvements and adaptations of their own, resulting in many different styles and types circulating around hospitals. The GlideScope (Verathon Inc, Bothell, WA) and the McGRATH (Medtronic, Minneapolis, MN) are examples of such instruments, which are now widely used in the US and are the 2 VLs of choice at our institution.
A few studies have compared VLs to direct laryngoscopes. In their systematic review, Lewis et al have shown the numerous benefits of using a VL over a direct laryngoscope. Some general conclusions were that the use of video laryngoscopy reduced the number of failed intubations, decreased laryngeal trauma, and provided improved visualizations.3 Other studies have compared the different types of VLs, including the McGRATH and the GlideScope, examining factors such as intubation time and display quality of the image. Two studies found that medical students were equally successful at using both the McGRATH and the GlideScope,4,5 while another study found that care providers using the GlideScope had quicker intubation times.6 Lastly, Savoldelli et al concluded that more providers preferred the McGRATH, which provided better laryngeal views,7 while their subsequent study showed more favorable learning curves of the Airtraq compared to the McGRATH and other VLs.8
Although there have been no reported differences in safety and effectiveness of the McGRATH and GlideScope devices, cost data on the use of these 2 popular laryngoscopes are lacking. Such information is important considering the increasing costs of medical technologies and the significant financial losses experienced by health care systems due to the COVID-19 crisis. The purpose of this retrospective cohort study was to compare the cost efficiency of the McGRATH MAC and GlideScope Core VLs at a large academic center.
Methods
This retrospective study was performed under exemption from the Thomas Jefferson University Institutional Review Board. The primary data sources consisted of hospital electronic patient records (EPIC) and cost information from the device manufacturers and hospital staff. The electronic patient data were provided by the EPIC Enterprise Analytics Business Intelligence group at Thomas Jefferson University Hospital (Center City Campus, Philadelphia, PA), while device costs were obtained from Verathon, Medtronic, and departmental staff responsible for purchasing equipment. Monthly data were obtained over a 24-month period (June 2018 through May 2020) when the McGRATH VL was placed into use in the department of anesthesiology. The 2 types of VLs were made available for use in a total of 52 locations, with the majority being hospital operating rooms.
The following variables were recorded: number of endotracheal intubations performed each month with breakdown between video laryngoscopy and flexible bronchoscopy airways, frequency of use for each type of laryngoscope, blades used, and equipment costs for use of each laryngoscope. Hospital cost estimates for both the McGRATH and GlideScope laryngoscopes included batteries, handles, blades, and the devices themselves. Cost data were also collected on frequency of device failure, maintenance, and replacement of parts and lost equipment.
Analysis
De-identified electronic medical records consisted of nominal and quantitative variables, with demographic data and information related to the endotracheal intubation procedure. All data were in chronological order and sorted by date after which coding was applied, to identify device type and allocate pertinent cost information. Descriptive statistics were reported as mean (SD) and sum for costs; frequency tables were generated for intubation procedures according to device type and time periods. Data were analyzed using the χ2 test, the student t test, and the Wilcoxon Mann-Whitney U test, with a P value set at .05 for statistical significance. SPSS version 26 and GraphPad Prism version 6 were used for all statistical analyses.
Results
A total of 34 600 endotracheal intubations were performed over the 24-month study period, and 11 345 (32.8%) were video laryngoscopy procedures. Out of all video laryngoscopy procedures, 5501 (48.5%) were performed using the McGRATH VL and 5305 (46.8%) were conducted using the GlideScope VL. The difference of 539 (4.8%) cases accounts for flexible bronchoscopy procedures and endotracheal intubations using other video laryngoscopy equipment. The mean (SD) monthly number of video laryngoscopy procedures for the 24 months was 221 (54) and 229 (89) for the GlideScope and McGRATH devices, respectively. Monthly endotracheal intubation distributions over 24 months trended upward for the McGRATH VL and downward for the GlideScope, but there was no statistically significant (P = .71) difference in overall use between the 2 instruments (Figure 1).

To examine the observed usage trends between the 2 VL during the first and last 12 months, a univariate ANOVA was conducted with the 2 time periods entered as predictors in the model. Video laryngoscopy intubations were performed (P = .001) more frequently with the GlideScope during the first 12 months; however, use of the McGRATH VL increased (P < .001) during the following 12 months compared to GlideScope. The GlideScope accounted for 54% of all VL intubations during the first 12 months, with the McGRATH accounting for 58% of all video laryngoscopy procedures for months 12 to 24. Additionally, the increase in video laryngoscopy procedures with the McGRATH during the last 3 months of the study period was despite an overall reduction in surgical volume due to the COVID-19 crisis, defined for this study as March 1, 2020, to May 31, 2020 (Figure 1). There was a statistically significant (P < .001) difference in the case distribution between use of the McGRATH and GlideScope VL for that period. The anesthesia personnel’s use of the McGRATH VL increased to 61% of all video laryngoscopy cases, compared to 37% for the GlideScope (Figure 2).

The total costs calculated for equipment, blades, and repairs are presented in Table 1 and yearly total costs are shown in Figure 3. Overall costs were $181 093 lower (55.5%) for the McGRATH VL compared to the GlideScope over the 24-month period. The mean (SD) monthly costs for GlideScope VL blades were $3837 ($1050) and $3236 ($538) for years 1 and 2, respectively, vs $1652 ($663) and $2933 ($585) for the McGRATH VL blades. Most of the total cost differences were attributed to equipment and blade purchases, which were $202 595 (65.0%) higher for the GlideScope compared to the McGRATH VL. The monthly blade costs alone were higher (P < .001) for the GlideScope over the 2-year period; however, the McGRATH VL required use of disposable stylets at a cost of $10 177 for all endotracheal intubations, compared to $700 for the GlideScope device.

An analysis was performed to determine whether costs differed between those 2 instruments during the COVID-19 period. There was a statistically significant (P < .001) difference in the case distribution between use of the McGRATH and GlideScope VLs during that period. The calculated blade cost difference for the COVID period was $128 higher for the McGRATH even though 293 more intubations were performed with that device (Table 2).

Discussion
We attempted to provide useful cost estimates by presenting pricing data reflecting the approximate cost that most large institutional anesthesia practices would incur for using those 2 specific devices and related peripherals. The main findings of our analysis showed that use of the McGRATH MAC VL resulted in a 55% cost savings compared to the GlideScope, with a similar number of cases performed with each device over the 24-month study period. We believe this represents a substantial savings to the department and institution, which has prompted internal review on the use of video laryngoscopy equipment. None of the McGRATH units failed; however, the GlideScope required 3 baton replacements.

Of note, use of the McGRATH MAC increased during the COVID-19 period, which may be explained by the fact that the operators found it to be a more portable device. Several physicians in the department commented that its smaller size made the McGRATH MAC more practical during the time when a plexiglass box was being used around the patient’s head to shield the intubator from aerosolized viral particles.
Although this study demonstrated the cost-saving value of the McGRATH over the GlideScope, a suggested next step would be to examine resource utilization related to video laryngoscopy use. The more dynamic tracking of the use of these devices should facilitate the assessment of existing related resources and decision making, to optimize the benefits of this initiative. We would anticipate reduced use of anesthesia personnel, such as technicians to assist with the management of this device which could be significant. As new respiratory viruses are appearing each year, video laryngoscopy will continue to gain increasing use in operating rooms and acute care locations. The adding of protective barriers between patients and providers calls for use of the most practical and effective VL devices, to protect personnel who are at high risk of contamination from airway secretions and aerosolized particles.9,10
The COVID-19 pandemic has demonstrated the value of anesthesiology in regards to analyzing and finding solutions to effectively manage infected patients or those suspected of infection in the perioperative environment. Inexpensive products are often avoided because cheaper devices are associated with being of lower quality. However, the association with cost and quality—and the assumption that a higher price is positively correlated with higher quality—is overall inconsistent in the medical literature.11 A more effective or higher quality treatment does not necessarily cost more and may actually end up costing less,12 as was the case in this study. We have been able to directly cut departmental expenses by using a more efficient and cost-effective device for intubations, without compromising safety and efficacy. Future studies should determine whether this significant reduction in costs from video laryngoscopy intubations with the McGRATH VL will be sustained across anesthesiology departments in the Jefferson Health Enterprise Hospitals, or other health systems, as well as its impact on workflow and personnel resources.
This analysis was restricted to one of the campuses of the Jefferson Health Enterprise. However, this is the largest anesthesia practice, encompassing several locations, which should reflect the general practice patterns across other anesthesiology departments in this large institution. The costs for the devices and peripherals may vary across anesthesia practices depending on volume and contracts negotiated with the suppliers. It was not possible to estimate this variability, which could change the total costs by a few percentage points. We recognize that there may be other costs associated with securing the McGRATH VL to prevent loss from theft or misplacement, which were not included in the study. Lastly, the inability to obtain randomized samples for the 2 groups treated with each device opens up the possibility of selection bias. There were, however, multiple intubators who were free to select 1 of the devices for endotracheal intubation, which may have reduced the effect of selection bias.
Conclusion
This study demonstrated that over a 24-month period use of the McGRATH MAC VL resulted in a cost reduction of around 55% compared to using the GlideScope for endotracheal intubation procedures performed at a major academic center. Over the first 3 months of the COVID-19 crisis, which our study included, use of the McGRATH VL increased while GlideScope use decreased. This was most likely related to the portability and smaller size of the McGRATH, which better facilitated intubations of COVID-19 patients.
Acknowledgements: The authors thank Craig Smith, Senior Anesthesia Technician, for his assistance with the cost information and excellent record-keeping related to the use of video laryngoscopes.
Corresponding author: Marc C. Torjman, PhD, Professor, Department of Anesthesiology, Sidney Kimmel Medical College at Thomas Jefferson University, 111 South 11th St, Suite G-8290, Philadelphia, PA 19107; [email protected].
Financial disclosures: Dr. Thaler has served as a consultant for Medtronic since September 2020. He has participated in 2 webinars on the routine use of video laryngoscopy.
Funding: This study was supported by the Department of Anesthesiology at Thomas Jefferson University.
From the Department of Anesthesiology, Thomas Jefferson University and Hospitals, Sidney Kimmel Medical College, Philadelphia, PA, and Sidney Kimmel Medical College at Thomas Jefferson University, Philadelphia, PA.
Objective: Retrospective study examining hospital cost information of patients requiring endotracheal intubation with video laryngoscopy. Provide a practical cost assessment on use of the McGRATH and GlideScope video laryngoscopes (VLs).
Methods: This study examined 52 hospital locations within a single, large university hospital, with most of those locations being hospital operating rooms. A total of 34 600 endotracheal intubations performed over 24 months, of which 11 345 were video laryngoscopies. Electronic medical records containing demographic data and information related to endotracheal intubation procedures, with monthly breakdowns between GlideScope and McGRATH intubations, were reviewed. Cost information calculated for equipment, blades, batteries, repairs, and subsequent analysis performed to determine cost differences between those 2 instruments during the COVID-19 period.
Results: A total of 5501 video laryngoscopy procedures were performed using the McGRATH VL and 5305 were performed using the GlideScope VL. Costs over 24 months were $181 093 lower (55.5%) for McGRATH compared to GlideScope. The mean (SD) monthly costs for GlideScope blades were $3837 ($1050) and $3236 ($538) for years 1 and 2, respectively, vs $1652 ($663) and $2933 ($585) for McGRATH blades (P < .001). Most total cost differences were attributed to equipment and blade purchases, which were $202 595 (65.0%) higher for GlideScope. During the COVID-19 period, the use of the McGRATH increased to 61% of all video laryngoscopy cases, compared to 37% for GlideScope (P < .001). Blade cost difference for the COVID-19 period was $128 higher for the McGRATH even though 293 more intubations were performed with that device.
Conclusions: Use of the McGRATH resulted in a cost savings of 55% compared to the GlideScope, and its use was highest during the COVID-19 period, which may be explained by its more portable and practical features.
Keywords: video laryngoscope; McGRATH; GlideScope; endotracheal intubation; hospital costs; COVID-19.
Hospitals have come to rely on video laryngoscopes (VLs) for tracheal intubation as necessary tools for better visualization of airways. Modern video laryngoscopy developed in the 2000s1 as a progression from direct laryngoscopy, which began in 1852 when Horace Green used a bent tongue spatula and sunlight to examine a child.2 VLs have seen many improvements and adaptations of their own, resulting in many different styles and types circulating around hospitals. The GlideScope (Verathon Inc, Bothell, WA) and the McGRATH (Medtronic, Minneapolis, MN) are examples of such instruments, which are now widely used in the US and are the 2 VLs of choice at our institution.
A few studies have compared VLs to direct laryngoscopes. In their systematic review, Lewis et al have shown the numerous benefits of using a VL over a direct laryngoscope. Some general conclusions were that the use of video laryngoscopy reduced the number of failed intubations, decreased laryngeal trauma, and provided improved visualizations.3 Other studies have compared the different types of VLs, including the McGRATH and the GlideScope, examining factors such as intubation time and display quality of the image. Two studies found that medical students were equally successful at using both the McGRATH and the GlideScope,4,5 while another study found that care providers using the GlideScope had quicker intubation times.6 Lastly, Savoldelli et al concluded that more providers preferred the McGRATH, which provided better laryngeal views,7 while their subsequent study showed more favorable learning curves of the Airtraq compared to the McGRATH and other VLs.8
Although there have been no reported differences in safety and effectiveness of the McGRATH and GlideScope devices, cost data on the use of these 2 popular laryngoscopes are lacking. Such information is important considering the increasing costs of medical technologies and the significant financial losses experienced by health care systems due to the COVID-19 crisis. The purpose of this retrospective cohort study was to compare the cost efficiency of the McGRATH MAC and GlideScope Core VLs at a large academic center.
Methods
This retrospective study was performed under exemption from the Thomas Jefferson University Institutional Review Board. The primary data sources consisted of hospital electronic patient records (EPIC) and cost information from the device manufacturers and hospital staff. The electronic patient data were provided by the EPIC Enterprise Analytics Business Intelligence group at Thomas Jefferson University Hospital (Center City Campus, Philadelphia, PA), while device costs were obtained from Verathon, Medtronic, and departmental staff responsible for purchasing equipment. Monthly data were obtained over a 24-month period (June 2018 through May 2020) when the McGRATH VL was placed into use in the department of anesthesiology. The 2 types of VLs were made available for use in a total of 52 locations, with the majority being hospital operating rooms.
The following variables were recorded: number of endotracheal intubations performed each month with breakdown between video laryngoscopy and flexible bronchoscopy airways, frequency of use for each type of laryngoscope, blades used, and equipment costs for use of each laryngoscope. Hospital cost estimates for both the McGRATH and GlideScope laryngoscopes included batteries, handles, blades, and the devices themselves. Cost data were also collected on frequency of device failure, maintenance, and replacement of parts and lost equipment.
Analysis
De-identified electronic medical records consisted of nominal and quantitative variables, with demographic data and information related to the endotracheal intubation procedure. All data were in chronological order and sorted by date after which coding was applied, to identify device type and allocate pertinent cost information. Descriptive statistics were reported as mean (SD) and sum for costs; frequency tables were generated for intubation procedures according to device type and time periods. Data were analyzed using the χ2 test, the student t test, and the Wilcoxon Mann-Whitney U test, with a P value set at .05 for statistical significance. SPSS version 26 and GraphPad Prism version 6 were used for all statistical analyses.
Results
A total of 34 600 endotracheal intubations were performed over the 24-month study period, and 11 345 (32.8%) were video laryngoscopy procedures. Out of all video laryngoscopy procedures, 5501 (48.5%) were performed using the McGRATH VL and 5305 (46.8%) were conducted using the GlideScope VL. The difference of 539 (4.8%) cases accounts for flexible bronchoscopy procedures and endotracheal intubations using other video laryngoscopy equipment. The mean (SD) monthly number of video laryngoscopy procedures for the 24 months was 221 (54) and 229 (89) for the GlideScope and McGRATH devices, respectively. Monthly endotracheal intubation distributions over 24 months trended upward for the McGRATH VL and downward for the GlideScope, but there was no statistically significant (P = .71) difference in overall use between the 2 instruments (Figure 1).

To examine the observed usage trends between the 2 VL during the first and last 12 months, a univariate ANOVA was conducted with the 2 time periods entered as predictors in the model. Video laryngoscopy intubations were performed (P = .001) more frequently with the GlideScope during the first 12 months; however, use of the McGRATH VL increased (P < .001) during the following 12 months compared to GlideScope. The GlideScope accounted for 54% of all VL intubations during the first 12 months, with the McGRATH accounting for 58% of all video laryngoscopy procedures for months 12 to 24. Additionally, the increase in video laryngoscopy procedures with the McGRATH during the last 3 months of the study period was despite an overall reduction in surgical volume due to the COVID-19 crisis, defined for this study as March 1, 2020, to May 31, 2020 (Figure 1). There was a statistically significant (P < .001) difference in the case distribution between use of the McGRATH and GlideScope VL for that period. The anesthesia personnel’s use of the McGRATH VL increased to 61% of all video laryngoscopy cases, compared to 37% for the GlideScope (Figure 2).

The total costs calculated for equipment, blades, and repairs are presented in Table 1 and yearly total costs are shown in Figure 3. Overall costs were $181 093 lower (55.5%) for the McGRATH VL compared to the GlideScope over the 24-month period. The mean (SD) monthly costs for GlideScope VL blades were $3837 ($1050) and $3236 ($538) for years 1 and 2, respectively, vs $1652 ($663) and $2933 ($585) for the McGRATH VL blades. Most of the total cost differences were attributed to equipment and blade purchases, which were $202 595 (65.0%) higher for the GlideScope compared to the McGRATH VL. The monthly blade costs alone were higher (P < .001) for the GlideScope over the 2-year period; however, the McGRATH VL required use of disposable stylets at a cost of $10 177 for all endotracheal intubations, compared to $700 for the GlideScope device.

An analysis was performed to determine whether costs differed between those 2 instruments during the COVID-19 period. There was a statistically significant (P < .001) difference in the case distribution between use of the McGRATH and GlideScope VLs during that period. The calculated blade cost difference for the COVID period was $128 higher for the McGRATH even though 293 more intubations were performed with that device (Table 2).

Discussion
We attempted to provide useful cost estimates by presenting pricing data reflecting the approximate cost that most large institutional anesthesia practices would incur for using those 2 specific devices and related peripherals. The main findings of our analysis showed that use of the McGRATH MAC VL resulted in a 55% cost savings compared to the GlideScope, with a similar number of cases performed with each device over the 24-month study period. We believe this represents a substantial savings to the department and institution, which has prompted internal review on the use of video laryngoscopy equipment. None of the McGRATH units failed; however, the GlideScope required 3 baton replacements.

Of note, use of the McGRATH MAC increased during the COVID-19 period, which may be explained by the fact that the operators found it to be a more portable device. Several physicians in the department commented that its smaller size made the McGRATH MAC more practical during the time when a plexiglass box was being used around the patient’s head to shield the intubator from aerosolized viral particles.
Although this study demonstrated the cost-saving value of the McGRATH over the GlideScope, a suggested next step would be to examine resource utilization related to video laryngoscopy use. The more dynamic tracking of the use of these devices should facilitate the assessment of existing related resources and decision making, to optimize the benefits of this initiative. We would anticipate reduced use of anesthesia personnel, such as technicians to assist with the management of this device which could be significant. As new respiratory viruses are appearing each year, video laryngoscopy will continue to gain increasing use in operating rooms and acute care locations. The adding of protective barriers between patients and providers calls for use of the most practical and effective VL devices, to protect personnel who are at high risk of contamination from airway secretions and aerosolized particles.9,10
The COVID-19 pandemic has demonstrated the value of anesthesiology in regards to analyzing and finding solutions to effectively manage infected patients or those suspected of infection in the perioperative environment. Inexpensive products are often avoided because cheaper devices are associated with being of lower quality. However, the association with cost and quality—and the assumption that a higher price is positively correlated with higher quality—is overall inconsistent in the medical literature.11 A more effective or higher quality treatment does not necessarily cost more and may actually end up costing less,12 as was the case in this study. We have been able to directly cut departmental expenses by using a more efficient and cost-effective device for intubations, without compromising safety and efficacy. Future studies should determine whether this significant reduction in costs from video laryngoscopy intubations with the McGRATH VL will be sustained across anesthesiology departments in the Jefferson Health Enterprise Hospitals, or other health systems, as well as its impact on workflow and personnel resources.
This analysis was restricted to one of the campuses of the Jefferson Health Enterprise. However, this is the largest anesthesia practice, encompassing several locations, which should reflect the general practice patterns across other anesthesiology departments in this large institution. The costs for the devices and peripherals may vary across anesthesia practices depending on volume and contracts negotiated with the suppliers. It was not possible to estimate this variability, which could change the total costs by a few percentage points. We recognize that there may be other costs associated with securing the McGRATH VL to prevent loss from theft or misplacement, which were not included in the study. Lastly, the inability to obtain randomized samples for the 2 groups treated with each device opens up the possibility of selection bias. There were, however, multiple intubators who were free to select 1 of the devices for endotracheal intubation, which may have reduced the effect of selection bias.
Conclusion
This study demonstrated that over a 24-month period use of the McGRATH MAC VL resulted in a cost reduction of around 55% compared to using the GlideScope for endotracheal intubation procedures performed at a major academic center. Over the first 3 months of the COVID-19 crisis, which our study included, use of the McGRATH VL increased while GlideScope use decreased. This was most likely related to the portability and smaller size of the McGRATH, which better facilitated intubations of COVID-19 patients.
Acknowledgements: The authors thank Craig Smith, Senior Anesthesia Technician, for his assistance with the cost information and excellent record-keeping related to the use of video laryngoscopes.
Corresponding author: Marc C. Torjman, PhD, Professor, Department of Anesthesiology, Sidney Kimmel Medical College at Thomas Jefferson University, 111 South 11th St, Suite G-8290, Philadelphia, PA 19107; [email protected].
Financial disclosures: Dr. Thaler has served as a consultant for Medtronic since September 2020. He has participated in 2 webinars on the routine use of video laryngoscopy.
Funding: This study was supported by the Department of Anesthesiology at Thomas Jefferson University.
1. Channa AB. Video laryngoscopes. Saudi J Anaesth. 2011;5(4):357-359.
2. Pieters BM, Eindhoven GB, Acott C, Van Zundert AAJ. Pioneers of laryngoscopy: indirect, direct and video laryngoscopy. Anaesth Intensive Care. 2015;43(suppl):4-11.
3. Lewis SR, Butler AR, Parker J, et al. Videolaryngoscopy versus direct laryngoscopy for adult patients requiring tracheal intubation. Cochrane Database Syst Rev. 2016;11(11):CD011136.
4. Kim W, Choi HJ, Lim T, Kang BS. Can the new McGrath laryngoscope rival the GlideScope Ranger portable video laryngoscope? A randomized manikin study. Am J Emerg Med. 2014;32(10):1225-1229.
5. Kim W, Choi HJ, Lim T, et al. Is McGrath MAC better than Glidescope Ranger for novice providers in the simulated difficult airway? A randomized manikin study. Resuscitation. 2014;85(suppl 1):S32.
6. Jeon WJ, Kim KH, Yeom JH, et al. A comparison of the Glidescope to the McGrath videolaryngoscope in patients. Korean J Anesthesiol. 2011;61(1):19-23.
7. Savoldelli GL, Schiffer E, Abegg C, et al. Comparison of the Glidescope, the McGrath, the Airtraq and the Macintosh laryngoscopes in simulated difficult airways. Anaesthesia. 2008;63(12):1358-1364.
8. Savoldelli GL, Schiffer E, Abegg C, et al. Learning curves of the Glidescope, the McGrath and the Airtraq laryngoscopes: a manikin study. Eur J Anaesthesiol. 2009;26(7):554-558.
9. Schumacher J, Arlidge J, Dudley D, et al. The impact of respiratory protective equipment on difficult airway management: a randomised, crossover, simulation study. Anaesthesia. 2020;75(10):1301-1306.
10. De Jong A, Pardo E, Rolle A, et al. Airway management for COVID-19: a move towards universal videolaryngoscope? Lancet Respir Med. 2020;8(6):555.
11. Hussey PS, Wertheimer S, Mehrotra A. The association between health care quality and cost: a systematic review. Ann Intern Med. 2013;158(1):27-34.
12. Mitton C, Dionne F, Peacock S, Sheps S. Quality and cost in healthcare: a relationship worth examining. Appl Health Econ Health Policy. 2006;5(4):201-208.
1. Channa AB. Video laryngoscopes. Saudi J Anaesth. 2011;5(4):357-359.
2. Pieters BM, Eindhoven GB, Acott C, Van Zundert AAJ. Pioneers of laryngoscopy: indirect, direct and video laryngoscopy. Anaesth Intensive Care. 2015;43(suppl):4-11.
3. Lewis SR, Butler AR, Parker J, et al. Videolaryngoscopy versus direct laryngoscopy for adult patients requiring tracheal intubation. Cochrane Database Syst Rev. 2016;11(11):CD011136.
4. Kim W, Choi HJ, Lim T, Kang BS. Can the new McGrath laryngoscope rival the GlideScope Ranger portable video laryngoscope? A randomized manikin study. Am J Emerg Med. 2014;32(10):1225-1229.
5. Kim W, Choi HJ, Lim T, et al. Is McGrath MAC better than Glidescope Ranger for novice providers in the simulated difficult airway? A randomized manikin study. Resuscitation. 2014;85(suppl 1):S32.
6. Jeon WJ, Kim KH, Yeom JH, et al. A comparison of the Glidescope to the McGrath videolaryngoscope in patients. Korean J Anesthesiol. 2011;61(1):19-23.
7. Savoldelli GL, Schiffer E, Abegg C, et al. Comparison of the Glidescope, the McGrath, the Airtraq and the Macintosh laryngoscopes in simulated difficult airways. Anaesthesia. 2008;63(12):1358-1364.
8. Savoldelli GL, Schiffer E, Abegg C, et al. Learning curves of the Glidescope, the McGrath and the Airtraq laryngoscopes: a manikin study. Eur J Anaesthesiol. 2009;26(7):554-558.
9. Schumacher J, Arlidge J, Dudley D, et al. The impact of respiratory protective equipment on difficult airway management: a randomised, crossover, simulation study. Anaesthesia. 2020;75(10):1301-1306.
10. De Jong A, Pardo E, Rolle A, et al. Airway management for COVID-19: a move towards universal videolaryngoscope? Lancet Respir Med. 2020;8(6):555.
11. Hussey PS, Wertheimer S, Mehrotra A. The association between health care quality and cost: a systematic review. Ann Intern Med. 2013;158(1):27-34.
12. Mitton C, Dionne F, Peacock S, Sheps S. Quality and cost in healthcare: a relationship worth examining. Appl Health Econ Health Policy. 2006;5(4):201-208.
Practical Application of Self-Determination Theory to Achieve a Reduction in Postoperative Hypothermia Rate: A Quality Improvement Project
From Children’s Health System of Texas, Division of Pediatric Anesthesiology, Dallas, TX (Drs. Sakhai, Bocanegra, Chandran, Kimatian, and Kiss), UT Southwestern Medical Center, Department of Anesthesiology and Pain Management, Dallas, TX (Drs. Bocanegra, Chandran, Kimatian, and Kiss), and UT Southwestern Medical Center, Department of Population and Data Sciences, Dallas, TX (Dr. Reisch).
Objective: Policy-driven changes in medical practice have long been the norm. Seldom are changes in clinical practice sought to be brought about by a person’s tendency toward growth or self‐actualization. Many hospitals have instituted hypothermia bundles to help reduce the incidence of unanticipated postoperative hypothermia. Although successful in the short-term, sustained changes are difficult to maintain. We implemented a quality-improvement project focused on addressing the affective components of self-determination theory (SDT) to create sustainable behavioral change while satisfying providers’ basic psychological needs for autonomy, competence, and relatedness.
Methods: A total of 3 Plan-Do-Study-Act (PDSA) cycles were enacted over the span of 14 months at a major tertiary care pediatric hospital to recruit and motivate anesthesia providers and perioperative team members to reduce the percentage of hypothermic postsurgical patients by 50%. As an optional initial incentive for participation, anesthesiologists would qualify for American Board of Anesthesiology Maintenance of Certification in Anesthesiology (MOCA) Part 4 Quality Improvement credits for monitoring their own temperature data and participating in project-related meetings. Providers were given autonomy to develop a personal plan for achieving the desired goals.
Results: The median rate of hypothermia was reduced from 6.9% to 1.6% in July 2019 and was reduced again in July 2020 to 1.3%, an 81% reduction overall. A low hypothermia rate was successfully maintained for at least 21 subsequent months after participants received their MOCA credits in July 2019.
Conclusions: Using an approach that focused on the elements of competency, autonomy, and relatedness central to the principles of SDT, we observed the development of a new culture of vigilance for prevention of hypothermia that successfully endured beyond the project end date.
Keywords: postoperative hypothermia; self-determination theory; motivation; quality improvement.
Perioperative hypothermia, generally accepted as a core temperature less than 36 °C in clinical practice, is a common complication in the pediatric surgical population and is associated with poor postoperative outcomes.1 Hypothermic patients may develop respiratory depression, hypoglycemia, and metabolic acidosis that may lead to decreased oxygen delivery and end organ tissue hypoxia.2-4 Other potential detrimental effects of failing to maintain normal body temperature are impaired clotting factor enzyme function and platelet dysfunction, increasing the risk for postoperative bleeding.5,6 In addition, there are financial implications when hypothermic patients require care and resources postoperatively because of delayed emergence or shivering.7
The American Society of Anesthesiologists recommends intraoperative temperature monitoring for procedures when clinically significant changes in body temperature are anticipated.8 Maintenance of normothermia in the pediatric population is especially challenging owing to a larger skin-surface area compared with body mass ratio and less subcutaneous fat content than in adults. Preventing postoperative hypothermia starts preoperatively with parental education and can be as simple as covering the child with a blanket and setting the preoperative room to an acceptably warm temperature.9,10 Intraoperatively, maintaining operating room (OR) temperatures at or above 21.1 °C and using active warming devices and radiant warmers when appropriate are important techniques to preserve the child’s body temperature.11,12
Despite the knowledge of these risks and vigilant avoidance of hypothermia, unplanned perioperative hypothermia can occur in up to 70% of surgical patients.1 Beyond the clinical benefits, as health care marches toward a value-based payment methodology, quality indicators such as avoiding hypothermia may be linked directly to payment.
Self-determination theory (SDT) was first developed in 1980 by Deci and Ryan.13 The central premise of the theory states that people develop their full potential if circumstances allow them to satisfy their basic psychological needs: autonomy, competence, and relatedness. Under these conditions, people’s natural inclination toward growth can be realized, and they are more likely to internalize external goals. Under an extrinsic reward system, motivation can waver, as people may perceive rewards as controlling.
Many institutions have implemented hypothermia bundles to help decrease the rate of hypothermic patients, but while initially successful, the effectiveness of these interventions tends to fade over time as participants settle into old, comfortable routines.14 With SDT in mind, we designed our quality-improvement (QI) project with interventions to allow clinicians autonomy without instituting rigid guidelines or punitive actions. We aimed to directly address the affective components central to motivation and engagement so that we could bring about long-term meaningful changes in our practice.
Methods
Setting
The hypothermia QI intervention was instituted at a major tertiary care children’s hospital that performs more than 40 000 pediatric general anesthetics annually. Our division of pediatric anesthesiology consists of 66 fellowship-trained pediatric anesthesiologists, 15 or more rotating trainees per month, 13 anesthesiology assistants, 15 anesthesia technicians, and more than 50 perioperative nurses.
The most frequent pediatric surgeries include, but are not limited to, general surgery, otolaryngology, urology, gastroenterology, plastic surgery, neurosurgery, and dentistry. The surgeries are conducted in the hospital’s main operative floor, which consists of 15 ORs and 2 gastroenterology procedure rooms. Although the implementation of the QI project included several operating sites, we focused on collecting temperature data from surgical patients at our main campus recovery unit. We obtained the patients’ initial temperatures upon arrival to the recovery unit from a retrospective electronic health record review of all patients who underwent anesthesia from January 2016 through April 2021.
Postoperative hypothermia was identified as an area of potential improvement after several patients were reported to be hypothermic upon arrival to the recovery unit in the later part of 2018. Further review revealed significant heterogeneity of practices and lack of standardization of patient-warming methods. By comparing the temperatures pre- and postintervention, we could measure the effectiveness of the QI initiative. Prior to the start of our project, the hypothermia rate in our patient population was not actively tracked, and the effectiveness of our variable practice was not measured.
The cutoff for hypothermia for our QI project was defined as body temperature below 36 °C, since this value has been previously used in the literature and is commonly accepted in anesthesia practice as the delineation for hypothermia in patients undergoing general anesthesia.1
Interventions
This QI project was designed and modeled after the Institute for Healthcare Improvement Model for Improvement.15 Three cycles of Plan-Do-Study-Act (PDSA) were developed and instituted over a 14-month period until December 2019 (Table 1).
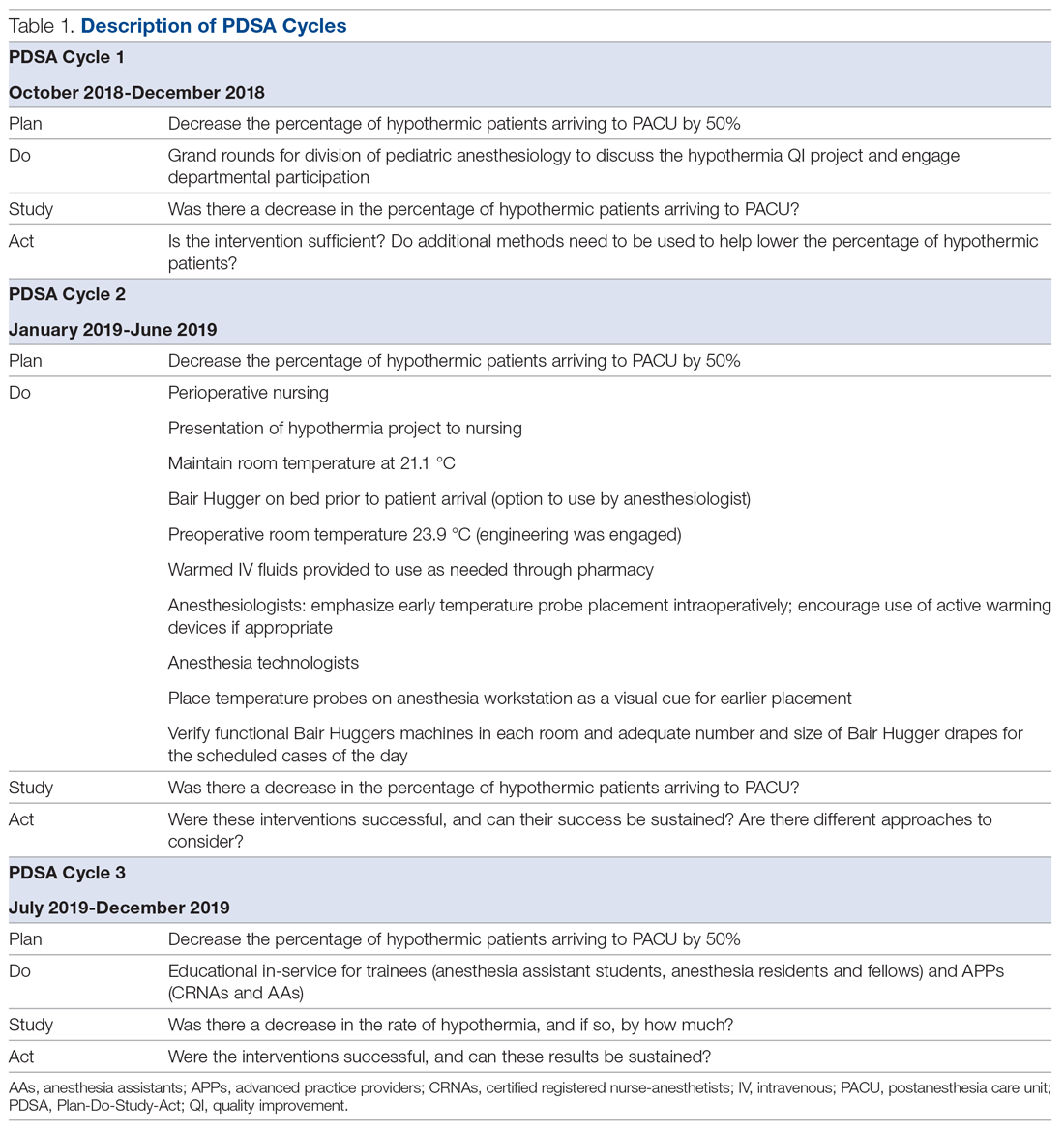
A retrospective review was conducted to determine the percentage of surgical patients arriving to our recovery units with an initial temperature reading of less than 36 °C. A project key driver diagram and smart aim were created and approved by the hospital’s continuing medical education (CME) committee for credit via the American Board of Medical Specialties (ABMS) Multi-Specialty Portfolio Program, Maintenance of Certification in Anesthesiology (MOCA) Part 4.
The first PDSA cycle involved introducing the QI project and sharing the aims of the project at a department grand rounds in the latter part of October 2018. Enrollment to participate in the project was open to all anesthesiologists in the division, and participants could earn up to 20 hours of MOCA Part 4 credits. A spreadsheet was developed and maintained to track each anesthesiologist’s monthly percentage of hypothermic patients. The de-identified patient data were shared with the division via monthly emails. In addition, individual providers with a hypothermic patient in the recovery room received a notification email.
The anesthesiologists participated in the QI project by reviewing their personal percentage of hypothermic patients on an ongoing basis to earn the credit. There was no explicit requirement to decrease their own rate of patients with body temperature less than 36 °C or expectation to achieve a predetermined goal, so the participants could not “fail.”
Because of the large interest in this project, a hypothermia committee was formed that consisted of 36 anesthesiologists. This group reviewed the data and exchanged ideas for improvement in November 2018 as part of the first PDSA cycle. The committee met monthly and was responsible for actively engaging other members of the department and perioperative staff to help in this multidisciplinary effort of combating hypothermia in our surgical pediatric population.
PDSA cycle 2 involved several major initiatives, including direct incorporation of the rest of the perioperative team. The perioperative nursing team was educated on the risks of hypothermia and engaged to take an active role by maintaining the operating suite temperature at 21.1 °C and turning on the Bair Hugger (3M) blanket to 43 °C on the OR bed prior to patient arrival to the OR. Additionally, anesthesia technicians (ATs) were tasked with ensuring an adequate supply of Bair Hugger drapes for all cases of the day. The facility’s engineering team was engaged to move the preoperative room temperature controls away from families (who frequently made the rooms cold) and instead set it at a consistent temperature of 23.9 °C. ATs were also asked to place axillary and nasal temperature probes on the anesthesia workstations as a visual reminder to facilitate temperature monitoring closer to the start of anesthesia (instead of the anesthesia provider having to remember to retrieve a temperature probe out of a drawer and place it on the patient). Furthermore, anesthesiologists were instructed via the aforementioned monthly emails and at monthly department meetings to place the temperature probes as early as possible in order to recognize and respond to intraoperative hypothermia in a timelier manner. Finally, supply chain leaders were informed of our expected increase in the use of the blankets and probes and proportionally increased ordering of these supplies to make sure availability would not present an obstacle.
In PDSA cycle 3, trainees (anesthesia assistant students, anesthesia residents and fellows) and advanced practice providers (APPs) (certified registered nurse-anesthetists [CRNAs] and certified anesthesia assistants [C-AAs]) were informed of the QI project. This initiative was guided toward improving vigilance for hypothermia in the rest of the anesthesia team members. The trainees and APPs usually set up the anesthesia area prior to patient arrival, so their recruitment in support of this effort would ensure appropriate OR temperature, active warming device deployment, and the availability and early placement of the correct temperature probe for the case. To facilitate personal accountability, the trainees and APPs were also emailed their own patients’ rate of hypothermia.
Along the course of the project, quarterly committee meetings and departmental monthly meetings served as venues to express concerns and look for areas of improvement, such as specific patterns or trends leading to hypothermic patients. One specific example was the identification of the gastrointestinal endoscopic patients having a rate of hypothermia that was 2% higher than average. Directed education on the importance of Bair Hugger blankets and using warm intravenous fluids worked well to decrease the rate of hypothermia in these patients. This collection of data was shared at regular intervals during monthly department meetings as well and more frequently using departmental emails. The hospital’s secure intranet SharePoint (Microsoft) site was used to share the data among providers.
Study of the interventions and measures
To study the effectiveness and impact of the project to motivate our anesthesiologists and other team members, we compared the first temperatures obtained in the recovery unit prior to the start of the intervention with those collected after the start of the QI project in November 2018. Because of the variability of temperature monitoring intraoperatively (nasal, axillary, rectal), we decided to use the temperature obtained by the nurse in the recovery room upon the patient’s arrival. Over the years analyzed, the nurse’s technique of measuring the temperature remained consistent. All patient temperature measurements were performed using the TAT-5000 (Exergen Corporation). This temporal artery thermometer has been previously shown to correlate well with bladder temperatures (70% of measurements differ by no more than 0.5 °C, as reported by Langham et al16).
Admittedly, we could not measure the degree of motivation or internalization of the project goals by our cohort, but we could measure the reduction in the rate of hypothermia and subjectively gauge engagement in the project by the various groups of participants and the sustainability of the results. In addition, all participating anesthesiologists received MOCA Part 4 credits in July 2019. We continued our data collection until April 2021 to determine if our project had brought about sustainable changes in practice that would continue past the initial motivator of obtaining CME credit.
Analysis
Data analysis was performed using Excel (Microsoft) and SAS, version 9.4 (SAS Institute).
The median of the monthly percentage of patients with a temperature of less than 36.0 °C was also determined for the preintervention time frame. This served as our baseline hypothermia rate, and we aimed to lower it by 50%. Run charts, a well-described methodology to gauge the effectiveness of the QI project, were constructed with the collected data.17
We performed additional analysis to adjust for different time periods throughout the year. The time period between January 2016 and October 2018 was considered preintervention. We considered November 2018 the start of our intervention, or more specifically, the start of our PDSA cycles. October 2018 was analyzed as part of the preintervention data. To account for seasonal temperature variations, the statistical analysis focused on the comparisons of the same calendar quarters for before and after starting intervention using Wilcoxon Mann-Whitney U tests. To reach an overall conclusion, the probabilities for the 4 quarters were combined for each criterion separately utilizing the Fisher χ2 combined probability method.
The hypothermia QI project was reviewed by the institutional review board and determined to be exempt.
Results
The temperatures of 40 875 patients were available for analysis for the preintervention period between January 2016 and October 2018. The median percentage of patients with temperatures less than 36.0 °C was 6.9% (interquartile range [IQR], 5.8%-8.4%). The highest percentage was in February 2016 (9.9%), and the lowest was in March 2018 (3.4%). Following the start of the first PDSA cycle, the next 6 consecutive rates of hypothermia were below the median preintervention value, and a new median for these percentages was calculated at 3.4% (IQR, 2.6%-4.3%). In July 2019, the proportion of hypothermic patients decreased once more for 6 consecutive months, yielding a new median of 1.6% (IQR, 1.2%-1.8%) and again in July 2020, to yield a median of 1.3% (IQR, 1.2%-1.5%) (Figure). In all, 33 799 patients were analyzed after the start of the project from November 2018 to the end of the data collection period through April 2021.
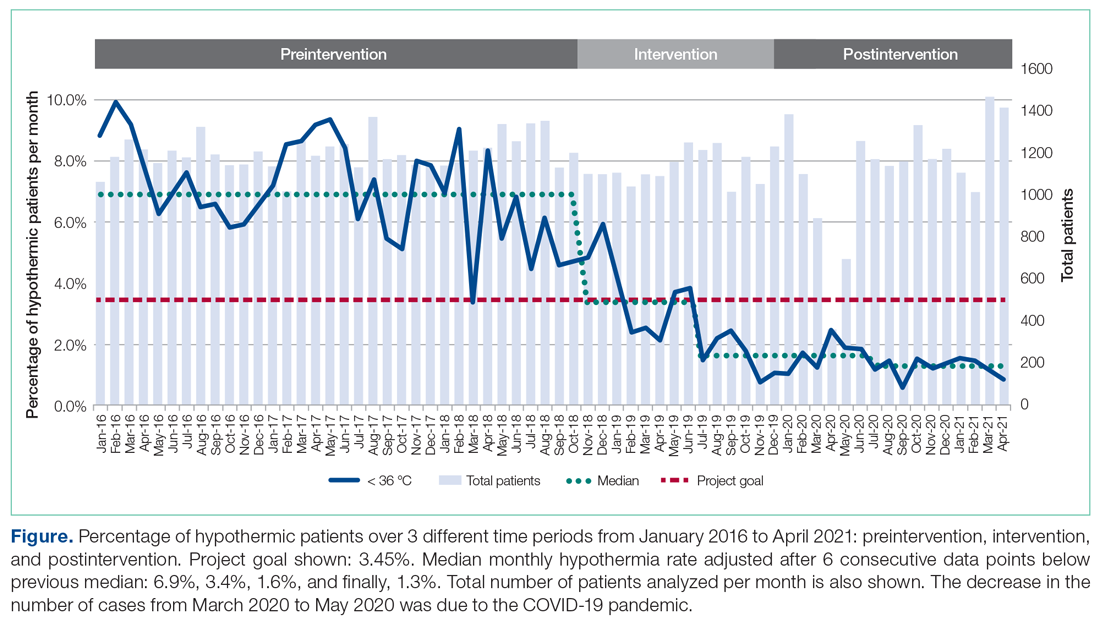
The preintervention monthly rates of hypothermia were compared, quarter to quarter, with those starting in November 2018 using the Wilcoxon Mann-Whitney U test. The decrease in proportion of hypothermic patients after the start of the intervention was statistically significant (P < .001). In addition, the percentage of patients with temperatures greater than 38 °C was not significantly different between the pre- and postintervention time periods (P < .25) (Table 2). The decrease in the number of patients available for analysis from March 2020 to May 2020 was due to the COVID-19 pandemic.
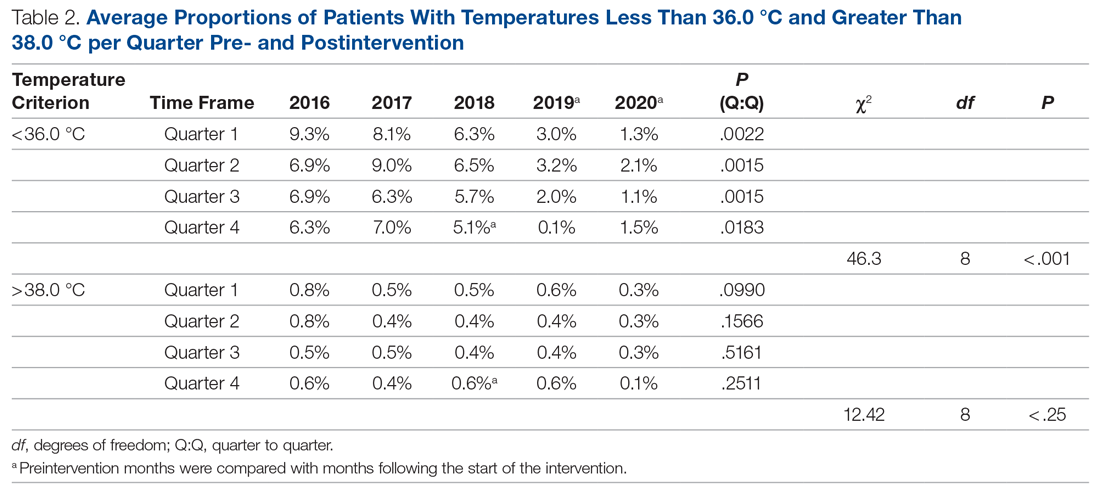
Subjectively, we did not experience any notable resistance to our efforts, and the experience was largely positive for everyone involved. Clinicians identified as having high monthly rates of hypothermia (5% or higher) corrected their numbers the following month after being notified via email or in person.
Discussion
To achieve changes in practice, the health care industry has relied on instituting guidelines, regulations, and policies, often with punitive consequences. We call into question this long-standing framework and propose a novel approach to help evolve the field of QI. Studies in human psychology have long demonstrated the demotivation power of a reward system and the negative response to attempts by authority to use incentives to control or coerce. In our QI project, we instituted 3 PDSA cycles and applied elements from SDT to motivate people’s behaviors. We demonstrate how a new culture focused on maintaining intraoperative normothermia was developed and brought about a measurable and significant decrease in the rate of hypothermia. The relevance of SDT, a widely accepted unifying theory that bridges and links social and personality psychology, should not be understated in health care. Authorities wishing to have long-standing influence should consider a person’s right to make their own decisions and, if possible, a unique way of doing things.
Positively reinforcing behavior has been shown to have a paradoxical effect by dampening an individual’s intrinsic motivation or desire to perform certain tasks.18 Deadlines, surveillance, and authoritative commands are also deterrents.19,20 We focused on providing the tools and information to the clinicians and relied on their innate need for autonomy, growth, and self-actualization to bring about change in clinical practice.21 Group meetings served as a construct for exchanging ideas and to encourage participation, but without the implementation of rigid guidelines or policies. Intraoperative active warming devices and temperature probes were made available, but their use was not mandated. The use of these devices was intentionally not audited to avoid any overbearing control. Providers were, however, given monthly temperature data to help individually assess the effectiveness of their interventions. We did not impose any negative or punitive actions for those clinicians who had high rates of hypothermic patients, and we did not reward those who had low rates of hypothermia. We wanted the participants to feel that the inner self was the source of their behavior, and this was in parallel with their own interests and values. If providers could feel their need for competency could be realized, we hoped they would continue to adhere to the measures we provided to maintain a low rate of hypothermia.
The effectiveness of our efforts was demonstrated by a decrease in the prevalence of postoperative hypothermia in our surgical patients. The initial decrease of the median rate of hypothermia from 6.9% to 3.4% occurred shortly into the start of the first PDSA cycle. The second PDSA cycle started in January 2019 with a multimodal approach and included almost all parties involved in the perioperative care of our surgical patients. Not only was this intervention responsible for a continued downward trend in the percentage of hypothermic patients, but it set the stage for the third and final PDSA cycle, which started in July 2019. The architecture was in place to integrate trainees and APPs to reinforce our initiative. Subsequently, the new median percentage of hypothermic patients was further decreased to an all-time low of 1.6% per month, satisfying and surpassing the goal of the QI project of decreasing the rate of hypothermia by only 50%. Our organization thereafter maintained a monthly hypothermia rate below 2%, except for April 2020, when it reached 2.5%. Our lowest median percentage was obtained after July 2020, reaching 1.3%.
To account for seasonal variations in temperatures and types of surgeries performed, we compared the percentage of hypothermic patients before and after the start of intervention, quarter by quarter. The decrease in the proportion of hypothermic patients after the start of intervention was statistically significant (P < .001). In addition, the data failed to prove any statistical difference for temperatures above 38 °C between the 2 periods, indicating that our interventions did not result in significant overwarming of patients. The clinical implications of decreasing the percentage of hypothermic patients from 6.9% to 1.3% is likely clinically important when considering the large number of patients who undergo surgery at large tertiary care pediatric centers. Even if simple interventions reduce hypothermia in only a handful of patients, routine applications of simple measures to keep patients normothermic is likely best clinical practice.
Anesthesiologists who participated in the hypothermia QI project by tracking the incidence of hypothermia in their patients were able to collect MOCA Part 4 credits in July 2019. There was no requirement for the individual anesthesiologist to reduce the rate of hypothermia or apply any of the encouraged strategies to obtain credit. As previously stated, there were also no rewards for obtaining low hypothermia rates for the providers. The temperature data continued to be collected through April 2021, 21 months after the credits were distributed, to demonstrate a continued, meaningful change, at least in the short-term. While the MOCA Part 4 credits likely served as an initial motivating factor to encourage participation in the QI project, they certainly were not responsible for the sustained low hypothermia rate after July 2019. We showed that the low rate of hypothermia was successfully maintained, indicating that the change in providers’ behavior was independent of the external motivator of obtaining the credit hours. Mere participation in the project by reviewing one’s temperature data was all that was required to obtain the credit. The Organismic Integration Theory, a mini-theory within SDT, best explains this phenomenon by describing any motivated behavior on a continuum ranging from controlled to autonomous.22 Do people perform the task resentfully, on their own volition because they believe it is the correct action, or somewhere in between? We explain the sustained low rates of hypothermia after the MOCA credits were distributed due to a shift to the autonomous end of the continuum with the clinician’s active willingness to meet the challenges and apply intrinsically motivated behaviors to lower the rate of hypothermia. The internalization of external motivators is difficult to prove, but the evidence supports that the methods we used to motivate individuals were effective and have resulted in a significant downward trend in our hypothermia rate.
There are several limitations to our QI project. The first involves the measuring of postoperative temperature in the recovery units. The temperatures were obtained using the same medical-grade infrared thermometer for all the patients, but other variables, such as timing and techniques, were not standardized. Secondly, overall surgical outcomes related to hypothermia were not tracked because we were unable to control for other confounding variables in our large cohort of patients, so we cannot say if the drop in the hypothermia rate had a clinically significant outcome. Thirdly, we propose that SDT offers a compellingly fitting explanation of the psychology of motivation in our efforts, but it may be possible that other theories may offer equally fitting explanations. The ability to measure the degree of motivation is lacking, and we did not explicitly ask participants what their specific source of motivation was. Aside from SDT, the reduction in hypothermia rate could also be attributed to the ease and availability of warming equipment that was made in each OR. This QI project was successfully applied to only 1 institution, so its ability to be widely applicable remains uncertain. In addition, data collection continued during the COVID-19 pandemic when case volumes decreased. However, by June 2020, the number of surgical cases at our institution had largely returned to prepandemic levels. Additional data collection beyond April 2021 would be helpful to determine if the reduction in hypothermia rates is truly sustained.
Conclusion
Overall, the importance of maintaining perioperative normothermia was well disseminated and agreed upon by all departments involved. Despite the limitations of the project, there was a significant reduction in rates of hypothermia, and sustainability of outcomes was consistently demonstrated in the poststudy period.
Using 3 cycles of the PDSA method, we successfully decreased the median rate of postoperative hypothermia in our pediatric surgical population from a preintervention value of 6.9% to 1.3%—a reduction of more than 81.2%. We provided motivation for members of our anesthesiology staff to participate by offering MOCA 2.0 Part 4 credits, but the lower rate of hypothermic patients was maintained for 15 months after the credits were distributed. Over the course of the project, there was a shift in culture, and extra vigilance was given to temperature monitoring and assessment. We attribute this sustained cultural change to the deliberate incorporation of the principles of competency, autonomy, and relatedness central to SDT to the structure of the interventions, avoiding rigid guidelines and pathways in favor of affective engagement to establish intrinsic motivation.
Acknowledgements: The authors thank Joan Reisch, PhD, for her assistance with the statistical analysis.
Corresponding author: Edgar Erold Kiss, MD, 1935 Medical District Dr, Dallas, TX 75235; [email protected].
Financial disclosures: None.
1. Leslie K, Sessler DI. Perioperative hypothermia in the high-risk surgical patient. Best Pract Res Clin Anaesthesiol. 2003;17(4):485-498.
2. Sessler DI. Forced-air warming in infants and children. Paediatr Anaesth. 2013;23(6):467-468.
3. Wetzel RC. Evaluation of children. In: Longnecker DE, Tinker JH, Morgan Jr GE, eds. Principles and Practice of Anesthesiology. 2nd ed. Mosby Publishers; 1999:445-447.
4. Witt L, Dennhardt N, Eich C, et al. Prevention of intraoperative hypothermia in neonates and infants: results of a prospective multicenter observational study with a new forced-air warming system with increased warm air flow. Paediatr Anaesth. 2013;23(6):469-474.
5. Blum R, Cote C. Pediatric equipment. In: Blum R, Cote C, eds. A Practice of Anaesthesia for Infants and Children. Saunders Elsevier; 2009:1099-1101.
6. Doufas AG. Consequences of inadvertent perioperative hypothermia. Best Pract Res Clin Anaesthesiol. 2003;17(4):535-549.
7. Mahoney CB, Odom J. Maintaining intraoperative normothermia: a meta-analysis of outcomes with costs. AANA J. 1999;67(2):155-163.
8. American Society of Anesthesiologists Committee on Standards and Practice Parameters. Standards for Basic Anesthetic Monitoring. Approved by the ASA House of Delegates October 21, 1986; last amended October 20, 2010; last affirmed October 28, 2015.
9. Horn E-P, Bein B, Böhm R, et al. The effect of short time periods of pre-operative warming in the prevention of peri-operative hypothermia. Anaesthesia. 2012;67(6):612-617.
10. Andrzejowski J, Hoyle J, Eapen G, Turnbull D. Effect of prewarming on post-induction core temperature and the incidence of inadvertent perioperative hypothermia in patients undergoing general anaesthesia. Br J Anaesth. 2008;101(5):627-631.
11. Sessler DI. Complications and treatment of mild hypothermia. Anesthesiology. 2001;95(2):531-543.
12. Bräuer A, English MJM, Steinmetz N, et al. Efficacy of forced-air warming systems with full body blankets. Can J Anaesth. 2007;54(1):34-41.
13. Deci EL, Ryan RM. The “what” and “why” of goal pursuits: human needs and the self‐determination of behavior. Psychol Inquiry. 2000;11(4):227-268.
14. Al-Shamari M, Puttha R, Yuen S, et al. G9 Can introduction of a hypothermia bundle reduce hypothermia in the newborns? Arch Dis Childhood. 2019;104(suppl 2):A4.1-A4.
15. Institute for Healthcare Improvement. How to improve. Accessed May 12, 2021. http://www.ihi.org/resources/Pages/HowtoImprove/default.aspx
16. Langham GE, Meheshwari A, You J, et al. Noninvasive temperature monitoring in postanesthesia care units. Anesthesiology. 2009;111(1):90-96.
17. Perla RJ, Provost LP, Murray SK. The run chart: a simple analytical tool for learning from variation in healthcare processes. BMJ Qual Saf. 2011;20(1):46-51.
18. Deci EL. Effects of externally mediated rewards on intrinsic motivation. J Pers Soc Psychol. 1971;18(1):105-115.
19. Deci EL, Koestner R, Ryan RM. A meta-analytic review of experiments examining the effects of extrinsic rewards on intrinsic motivation. Psychol Bull. 1999;125(6):627-668.
20. Deci EL, Koestner R, Ryan RM. The undermining effect is a reality after all—extrinsic rewards, task interest, and self-determination: Reply to Eisenberger, Pierce, and Cameron (1999) and Lepper, Henderlong, and Gingras (1999). Psychol Bull. 1999;125(6):692-700.
21. Maslow A. The Farther Reaches of Human Nature. Viking Press; 1971.
22. Sheldon KM, Prentice M. Self-determination theory as a foundation for personality researchers. J Pers. 2019;87(1):5-14.
From Children’s Health System of Texas, Division of Pediatric Anesthesiology, Dallas, TX (Drs. Sakhai, Bocanegra, Chandran, Kimatian, and Kiss), UT Southwestern Medical Center, Department of Anesthesiology and Pain Management, Dallas, TX (Drs. Bocanegra, Chandran, Kimatian, and Kiss), and UT Southwestern Medical Center, Department of Population and Data Sciences, Dallas, TX (Dr. Reisch).
Objective: Policy-driven changes in medical practice have long been the norm. Seldom are changes in clinical practice sought to be brought about by a person’s tendency toward growth or self‐actualization. Many hospitals have instituted hypothermia bundles to help reduce the incidence of unanticipated postoperative hypothermia. Although successful in the short-term, sustained changes are difficult to maintain. We implemented a quality-improvement project focused on addressing the affective components of self-determination theory (SDT) to create sustainable behavioral change while satisfying providers’ basic psychological needs for autonomy, competence, and relatedness.
Methods: A total of 3 Plan-Do-Study-Act (PDSA) cycles were enacted over the span of 14 months at a major tertiary care pediatric hospital to recruit and motivate anesthesia providers and perioperative team members to reduce the percentage of hypothermic postsurgical patients by 50%. As an optional initial incentive for participation, anesthesiologists would qualify for American Board of Anesthesiology Maintenance of Certification in Anesthesiology (MOCA) Part 4 Quality Improvement credits for monitoring their own temperature data and participating in project-related meetings. Providers were given autonomy to develop a personal plan for achieving the desired goals.
Results: The median rate of hypothermia was reduced from 6.9% to 1.6% in July 2019 and was reduced again in July 2020 to 1.3%, an 81% reduction overall. A low hypothermia rate was successfully maintained for at least 21 subsequent months after participants received their MOCA credits in July 2019.
Conclusions: Using an approach that focused on the elements of competency, autonomy, and relatedness central to the principles of SDT, we observed the development of a new culture of vigilance for prevention of hypothermia that successfully endured beyond the project end date.
Keywords: postoperative hypothermia; self-determination theory; motivation; quality improvement.
Perioperative hypothermia, generally accepted as a core temperature less than 36 °C in clinical practice, is a common complication in the pediatric surgical population and is associated with poor postoperative outcomes.1 Hypothermic patients may develop respiratory depression, hypoglycemia, and metabolic acidosis that may lead to decreased oxygen delivery and end organ tissue hypoxia.2-4 Other potential detrimental effects of failing to maintain normal body temperature are impaired clotting factor enzyme function and platelet dysfunction, increasing the risk for postoperative bleeding.5,6 In addition, there are financial implications when hypothermic patients require care and resources postoperatively because of delayed emergence or shivering.7
The American Society of Anesthesiologists recommends intraoperative temperature monitoring for procedures when clinically significant changes in body temperature are anticipated.8 Maintenance of normothermia in the pediatric population is especially challenging owing to a larger skin-surface area compared with body mass ratio and less subcutaneous fat content than in adults. Preventing postoperative hypothermia starts preoperatively with parental education and can be as simple as covering the child with a blanket and setting the preoperative room to an acceptably warm temperature.9,10 Intraoperatively, maintaining operating room (OR) temperatures at or above 21.1 °C and using active warming devices and radiant warmers when appropriate are important techniques to preserve the child’s body temperature.11,12
Despite the knowledge of these risks and vigilant avoidance of hypothermia, unplanned perioperative hypothermia can occur in up to 70% of surgical patients.1 Beyond the clinical benefits, as health care marches toward a value-based payment methodology, quality indicators such as avoiding hypothermia may be linked directly to payment.
Self-determination theory (SDT) was first developed in 1980 by Deci and Ryan.13 The central premise of the theory states that people develop their full potential if circumstances allow them to satisfy their basic psychological needs: autonomy, competence, and relatedness. Under these conditions, people’s natural inclination toward growth can be realized, and they are more likely to internalize external goals. Under an extrinsic reward system, motivation can waver, as people may perceive rewards as controlling.
Many institutions have implemented hypothermia bundles to help decrease the rate of hypothermic patients, but while initially successful, the effectiveness of these interventions tends to fade over time as participants settle into old, comfortable routines.14 With SDT in mind, we designed our quality-improvement (QI) project with interventions to allow clinicians autonomy without instituting rigid guidelines or punitive actions. We aimed to directly address the affective components central to motivation and engagement so that we could bring about long-term meaningful changes in our practice.
Methods
Setting
The hypothermia QI intervention was instituted at a major tertiary care children’s hospital that performs more than 40 000 pediatric general anesthetics annually. Our division of pediatric anesthesiology consists of 66 fellowship-trained pediatric anesthesiologists, 15 or more rotating trainees per month, 13 anesthesiology assistants, 15 anesthesia technicians, and more than 50 perioperative nurses.
The most frequent pediatric surgeries include, but are not limited to, general surgery, otolaryngology, urology, gastroenterology, plastic surgery, neurosurgery, and dentistry. The surgeries are conducted in the hospital’s main operative floor, which consists of 15 ORs and 2 gastroenterology procedure rooms. Although the implementation of the QI project included several operating sites, we focused on collecting temperature data from surgical patients at our main campus recovery unit. We obtained the patients’ initial temperatures upon arrival to the recovery unit from a retrospective electronic health record review of all patients who underwent anesthesia from January 2016 through April 2021.
Postoperative hypothermia was identified as an area of potential improvement after several patients were reported to be hypothermic upon arrival to the recovery unit in the later part of 2018. Further review revealed significant heterogeneity of practices and lack of standardization of patient-warming methods. By comparing the temperatures pre- and postintervention, we could measure the effectiveness of the QI initiative. Prior to the start of our project, the hypothermia rate in our patient population was not actively tracked, and the effectiveness of our variable practice was not measured.
The cutoff for hypothermia for our QI project was defined as body temperature below 36 °C, since this value has been previously used in the literature and is commonly accepted in anesthesia practice as the delineation for hypothermia in patients undergoing general anesthesia.1
Interventions
This QI project was designed and modeled after the Institute for Healthcare Improvement Model for Improvement.15 Three cycles of Plan-Do-Study-Act (PDSA) were developed and instituted over a 14-month period until December 2019 (Table 1).

A retrospective review was conducted to determine the percentage of surgical patients arriving to our recovery units with an initial temperature reading of less than 36 °C. A project key driver diagram and smart aim were created and approved by the hospital’s continuing medical education (CME) committee for credit via the American Board of Medical Specialties (ABMS) Multi-Specialty Portfolio Program, Maintenance of Certification in Anesthesiology (MOCA) Part 4.
The first PDSA cycle involved introducing the QI project and sharing the aims of the project at a department grand rounds in the latter part of October 2018. Enrollment to participate in the project was open to all anesthesiologists in the division, and participants could earn up to 20 hours of MOCA Part 4 credits. A spreadsheet was developed and maintained to track each anesthesiologist’s monthly percentage of hypothermic patients. The de-identified patient data were shared with the division via monthly emails. In addition, individual providers with a hypothermic patient in the recovery room received a notification email.
The anesthesiologists participated in the QI project by reviewing their personal percentage of hypothermic patients on an ongoing basis to earn the credit. There was no explicit requirement to decrease their own rate of patients with body temperature less than 36 °C or expectation to achieve a predetermined goal, so the participants could not “fail.”
Because of the large interest in this project, a hypothermia committee was formed that consisted of 36 anesthesiologists. This group reviewed the data and exchanged ideas for improvement in November 2018 as part of the first PDSA cycle. The committee met monthly and was responsible for actively engaging other members of the department and perioperative staff to help in this multidisciplinary effort of combating hypothermia in our surgical pediatric population.
PDSA cycle 2 involved several major initiatives, including direct incorporation of the rest of the perioperative team. The perioperative nursing team was educated on the risks of hypothermia and engaged to take an active role by maintaining the operating suite temperature at 21.1 °C and turning on the Bair Hugger (3M) blanket to 43 °C on the OR bed prior to patient arrival to the OR. Additionally, anesthesia technicians (ATs) were tasked with ensuring an adequate supply of Bair Hugger drapes for all cases of the day. The facility’s engineering team was engaged to move the preoperative room temperature controls away from families (who frequently made the rooms cold) and instead set it at a consistent temperature of 23.9 °C. ATs were also asked to place axillary and nasal temperature probes on the anesthesia workstations as a visual reminder to facilitate temperature monitoring closer to the start of anesthesia (instead of the anesthesia provider having to remember to retrieve a temperature probe out of a drawer and place it on the patient). Furthermore, anesthesiologists were instructed via the aforementioned monthly emails and at monthly department meetings to place the temperature probes as early as possible in order to recognize and respond to intraoperative hypothermia in a timelier manner. Finally, supply chain leaders were informed of our expected increase in the use of the blankets and probes and proportionally increased ordering of these supplies to make sure availability would not present an obstacle.
In PDSA cycle 3, trainees (anesthesia assistant students, anesthesia residents and fellows) and advanced practice providers (APPs) (certified registered nurse-anesthetists [CRNAs] and certified anesthesia assistants [C-AAs]) were informed of the QI project. This initiative was guided toward improving vigilance for hypothermia in the rest of the anesthesia team members. The trainees and APPs usually set up the anesthesia area prior to patient arrival, so their recruitment in support of this effort would ensure appropriate OR temperature, active warming device deployment, and the availability and early placement of the correct temperature probe for the case. To facilitate personal accountability, the trainees and APPs were also emailed their own patients’ rate of hypothermia.
Along the course of the project, quarterly committee meetings and departmental monthly meetings served as venues to express concerns and look for areas of improvement, such as specific patterns or trends leading to hypothermic patients. One specific example was the identification of the gastrointestinal endoscopic patients having a rate of hypothermia that was 2% higher than average. Directed education on the importance of Bair Hugger blankets and using warm intravenous fluids worked well to decrease the rate of hypothermia in these patients. This collection of data was shared at regular intervals during monthly department meetings as well and more frequently using departmental emails. The hospital’s secure intranet SharePoint (Microsoft) site was used to share the data among providers.
Study of the interventions and measures
To study the effectiveness and impact of the project to motivate our anesthesiologists and other team members, we compared the first temperatures obtained in the recovery unit prior to the start of the intervention with those collected after the start of the QI project in November 2018. Because of the variability of temperature monitoring intraoperatively (nasal, axillary, rectal), we decided to use the temperature obtained by the nurse in the recovery room upon the patient’s arrival. Over the years analyzed, the nurse’s technique of measuring the temperature remained consistent. All patient temperature measurements were performed using the TAT-5000 (Exergen Corporation). This temporal artery thermometer has been previously shown to correlate well with bladder temperatures (70% of measurements differ by no more than 0.5 °C, as reported by Langham et al16).
Admittedly, we could not measure the degree of motivation or internalization of the project goals by our cohort, but we could measure the reduction in the rate of hypothermia and subjectively gauge engagement in the project by the various groups of participants and the sustainability of the results. In addition, all participating anesthesiologists received MOCA Part 4 credits in July 2019. We continued our data collection until April 2021 to determine if our project had brought about sustainable changes in practice that would continue past the initial motivator of obtaining CME credit.
Analysis
Data analysis was performed using Excel (Microsoft) and SAS, version 9.4 (SAS Institute).
The median of the monthly percentage of patients with a temperature of less than 36.0 °C was also determined for the preintervention time frame. This served as our baseline hypothermia rate, and we aimed to lower it by 50%. Run charts, a well-described methodology to gauge the effectiveness of the QI project, were constructed with the collected data.17
We performed additional analysis to adjust for different time periods throughout the year. The time period between January 2016 and October 2018 was considered preintervention. We considered November 2018 the start of our intervention, or more specifically, the start of our PDSA cycles. October 2018 was analyzed as part of the preintervention data. To account for seasonal temperature variations, the statistical analysis focused on the comparisons of the same calendar quarters for before and after starting intervention using Wilcoxon Mann-Whitney U tests. To reach an overall conclusion, the probabilities for the 4 quarters were combined for each criterion separately utilizing the Fisher χ2 combined probability method.
The hypothermia QI project was reviewed by the institutional review board and determined to be exempt.
Results
The temperatures of 40 875 patients were available for analysis for the preintervention period between January 2016 and October 2018. The median percentage of patients with temperatures less than 36.0 °C was 6.9% (interquartile range [IQR], 5.8%-8.4%). The highest percentage was in February 2016 (9.9%), and the lowest was in March 2018 (3.4%). Following the start of the first PDSA cycle, the next 6 consecutive rates of hypothermia were below the median preintervention value, and a new median for these percentages was calculated at 3.4% (IQR, 2.6%-4.3%). In July 2019, the proportion of hypothermic patients decreased once more for 6 consecutive months, yielding a new median of 1.6% (IQR, 1.2%-1.8%) and again in July 2020, to yield a median of 1.3% (IQR, 1.2%-1.5%) (Figure). In all, 33 799 patients were analyzed after the start of the project from November 2018 to the end of the data collection period through April 2021.

The preintervention monthly rates of hypothermia were compared, quarter to quarter, with those starting in November 2018 using the Wilcoxon Mann-Whitney U test. The decrease in proportion of hypothermic patients after the start of the intervention was statistically significant (P < .001). In addition, the percentage of patients with temperatures greater than 38 °C was not significantly different between the pre- and postintervention time periods (P < .25) (Table 2). The decrease in the number of patients available for analysis from March 2020 to May 2020 was due to the COVID-19 pandemic.

Subjectively, we did not experience any notable resistance to our efforts, and the experience was largely positive for everyone involved. Clinicians identified as having high monthly rates of hypothermia (5% or higher) corrected their numbers the following month after being notified via email or in person.
Discussion
To achieve changes in practice, the health care industry has relied on instituting guidelines, regulations, and policies, often with punitive consequences. We call into question this long-standing framework and propose a novel approach to help evolve the field of QI. Studies in human psychology have long demonstrated the demotivation power of a reward system and the negative response to attempts by authority to use incentives to control or coerce. In our QI project, we instituted 3 PDSA cycles and applied elements from SDT to motivate people’s behaviors. We demonstrate how a new culture focused on maintaining intraoperative normothermia was developed and brought about a measurable and significant decrease in the rate of hypothermia. The relevance of SDT, a widely accepted unifying theory that bridges and links social and personality psychology, should not be understated in health care. Authorities wishing to have long-standing influence should consider a person’s right to make their own decisions and, if possible, a unique way of doing things.
Positively reinforcing behavior has been shown to have a paradoxical effect by dampening an individual’s intrinsic motivation or desire to perform certain tasks.18 Deadlines, surveillance, and authoritative commands are also deterrents.19,20 We focused on providing the tools and information to the clinicians and relied on their innate need for autonomy, growth, and self-actualization to bring about change in clinical practice.21 Group meetings served as a construct for exchanging ideas and to encourage participation, but without the implementation of rigid guidelines or policies. Intraoperative active warming devices and temperature probes were made available, but their use was not mandated. The use of these devices was intentionally not audited to avoid any overbearing control. Providers were, however, given monthly temperature data to help individually assess the effectiveness of their interventions. We did not impose any negative or punitive actions for those clinicians who had high rates of hypothermic patients, and we did not reward those who had low rates of hypothermia. We wanted the participants to feel that the inner self was the source of their behavior, and this was in parallel with their own interests and values. If providers could feel their need for competency could be realized, we hoped they would continue to adhere to the measures we provided to maintain a low rate of hypothermia.
The effectiveness of our efforts was demonstrated by a decrease in the prevalence of postoperative hypothermia in our surgical patients. The initial decrease of the median rate of hypothermia from 6.9% to 3.4% occurred shortly into the start of the first PDSA cycle. The second PDSA cycle started in January 2019 with a multimodal approach and included almost all parties involved in the perioperative care of our surgical patients. Not only was this intervention responsible for a continued downward trend in the percentage of hypothermic patients, but it set the stage for the third and final PDSA cycle, which started in July 2019. The architecture was in place to integrate trainees and APPs to reinforce our initiative. Subsequently, the new median percentage of hypothermic patients was further decreased to an all-time low of 1.6% per month, satisfying and surpassing the goal of the QI project of decreasing the rate of hypothermia by only 50%. Our organization thereafter maintained a monthly hypothermia rate below 2%, except for April 2020, when it reached 2.5%. Our lowest median percentage was obtained after July 2020, reaching 1.3%.
To account for seasonal variations in temperatures and types of surgeries performed, we compared the percentage of hypothermic patients before and after the start of intervention, quarter by quarter. The decrease in the proportion of hypothermic patients after the start of intervention was statistically significant (P < .001). In addition, the data failed to prove any statistical difference for temperatures above 38 °C between the 2 periods, indicating that our interventions did not result in significant overwarming of patients. The clinical implications of decreasing the percentage of hypothermic patients from 6.9% to 1.3% is likely clinically important when considering the large number of patients who undergo surgery at large tertiary care pediatric centers. Even if simple interventions reduce hypothermia in only a handful of patients, routine applications of simple measures to keep patients normothermic is likely best clinical practice.
Anesthesiologists who participated in the hypothermia QI project by tracking the incidence of hypothermia in their patients were able to collect MOCA Part 4 credits in July 2019. There was no requirement for the individual anesthesiologist to reduce the rate of hypothermia or apply any of the encouraged strategies to obtain credit. As previously stated, there were also no rewards for obtaining low hypothermia rates for the providers. The temperature data continued to be collected through April 2021, 21 months after the credits were distributed, to demonstrate a continued, meaningful change, at least in the short-term. While the MOCA Part 4 credits likely served as an initial motivating factor to encourage participation in the QI project, they certainly were not responsible for the sustained low hypothermia rate after July 2019. We showed that the low rate of hypothermia was successfully maintained, indicating that the change in providers’ behavior was independent of the external motivator of obtaining the credit hours. Mere participation in the project by reviewing one’s temperature data was all that was required to obtain the credit. The Organismic Integration Theory, a mini-theory within SDT, best explains this phenomenon by describing any motivated behavior on a continuum ranging from controlled to autonomous.22 Do people perform the task resentfully, on their own volition because they believe it is the correct action, or somewhere in between? We explain the sustained low rates of hypothermia after the MOCA credits were distributed due to a shift to the autonomous end of the continuum with the clinician’s active willingness to meet the challenges and apply intrinsically motivated behaviors to lower the rate of hypothermia. The internalization of external motivators is difficult to prove, but the evidence supports that the methods we used to motivate individuals were effective and have resulted in a significant downward trend in our hypothermia rate.
There are several limitations to our QI project. The first involves the measuring of postoperative temperature in the recovery units. The temperatures were obtained using the same medical-grade infrared thermometer for all the patients, but other variables, such as timing and techniques, were not standardized. Secondly, overall surgical outcomes related to hypothermia were not tracked because we were unable to control for other confounding variables in our large cohort of patients, so we cannot say if the drop in the hypothermia rate had a clinically significant outcome. Thirdly, we propose that SDT offers a compellingly fitting explanation of the psychology of motivation in our efforts, but it may be possible that other theories may offer equally fitting explanations. The ability to measure the degree of motivation is lacking, and we did not explicitly ask participants what their specific source of motivation was. Aside from SDT, the reduction in hypothermia rate could also be attributed to the ease and availability of warming equipment that was made in each OR. This QI project was successfully applied to only 1 institution, so its ability to be widely applicable remains uncertain. In addition, data collection continued during the COVID-19 pandemic when case volumes decreased. However, by June 2020, the number of surgical cases at our institution had largely returned to prepandemic levels. Additional data collection beyond April 2021 would be helpful to determine if the reduction in hypothermia rates is truly sustained.
Conclusion
Overall, the importance of maintaining perioperative normothermia was well disseminated and agreed upon by all departments involved. Despite the limitations of the project, there was a significant reduction in rates of hypothermia, and sustainability of outcomes was consistently demonstrated in the poststudy period.
Using 3 cycles of the PDSA method, we successfully decreased the median rate of postoperative hypothermia in our pediatric surgical population from a preintervention value of 6.9% to 1.3%—a reduction of more than 81.2%. We provided motivation for members of our anesthesiology staff to participate by offering MOCA 2.0 Part 4 credits, but the lower rate of hypothermic patients was maintained for 15 months after the credits were distributed. Over the course of the project, there was a shift in culture, and extra vigilance was given to temperature monitoring and assessment. We attribute this sustained cultural change to the deliberate incorporation of the principles of competency, autonomy, and relatedness central to SDT to the structure of the interventions, avoiding rigid guidelines and pathways in favor of affective engagement to establish intrinsic motivation.
Acknowledgements: The authors thank Joan Reisch, PhD, for her assistance with the statistical analysis.
Corresponding author: Edgar Erold Kiss, MD, 1935 Medical District Dr, Dallas, TX 75235; [email protected].
Financial disclosures: None.
From Children’s Health System of Texas, Division of Pediatric Anesthesiology, Dallas, TX (Drs. Sakhai, Bocanegra, Chandran, Kimatian, and Kiss), UT Southwestern Medical Center, Department of Anesthesiology and Pain Management, Dallas, TX (Drs. Bocanegra, Chandran, Kimatian, and Kiss), and UT Southwestern Medical Center, Department of Population and Data Sciences, Dallas, TX (Dr. Reisch).
Objective: Policy-driven changes in medical practice have long been the norm. Seldom are changes in clinical practice sought to be brought about by a person’s tendency toward growth or self‐actualization. Many hospitals have instituted hypothermia bundles to help reduce the incidence of unanticipated postoperative hypothermia. Although successful in the short-term, sustained changes are difficult to maintain. We implemented a quality-improvement project focused on addressing the affective components of self-determination theory (SDT) to create sustainable behavioral change while satisfying providers’ basic psychological needs for autonomy, competence, and relatedness.
Methods: A total of 3 Plan-Do-Study-Act (PDSA) cycles were enacted over the span of 14 months at a major tertiary care pediatric hospital to recruit and motivate anesthesia providers and perioperative team members to reduce the percentage of hypothermic postsurgical patients by 50%. As an optional initial incentive for participation, anesthesiologists would qualify for American Board of Anesthesiology Maintenance of Certification in Anesthesiology (MOCA) Part 4 Quality Improvement credits for monitoring their own temperature data and participating in project-related meetings. Providers were given autonomy to develop a personal plan for achieving the desired goals.
Results: The median rate of hypothermia was reduced from 6.9% to 1.6% in July 2019 and was reduced again in July 2020 to 1.3%, an 81% reduction overall. A low hypothermia rate was successfully maintained for at least 21 subsequent months after participants received their MOCA credits in July 2019.
Conclusions: Using an approach that focused on the elements of competency, autonomy, and relatedness central to the principles of SDT, we observed the development of a new culture of vigilance for prevention of hypothermia that successfully endured beyond the project end date.
Keywords: postoperative hypothermia; self-determination theory; motivation; quality improvement.
Perioperative hypothermia, generally accepted as a core temperature less than 36 °C in clinical practice, is a common complication in the pediatric surgical population and is associated with poor postoperative outcomes.1 Hypothermic patients may develop respiratory depression, hypoglycemia, and metabolic acidosis that may lead to decreased oxygen delivery and end organ tissue hypoxia.2-4 Other potential detrimental effects of failing to maintain normal body temperature are impaired clotting factor enzyme function and platelet dysfunction, increasing the risk for postoperative bleeding.5,6 In addition, there are financial implications when hypothermic patients require care and resources postoperatively because of delayed emergence or shivering.7
The American Society of Anesthesiologists recommends intraoperative temperature monitoring for procedures when clinically significant changes in body temperature are anticipated.8 Maintenance of normothermia in the pediatric population is especially challenging owing to a larger skin-surface area compared with body mass ratio and less subcutaneous fat content than in adults. Preventing postoperative hypothermia starts preoperatively with parental education and can be as simple as covering the child with a blanket and setting the preoperative room to an acceptably warm temperature.9,10 Intraoperatively, maintaining operating room (OR) temperatures at or above 21.1 °C and using active warming devices and radiant warmers when appropriate are important techniques to preserve the child’s body temperature.11,12
Despite the knowledge of these risks and vigilant avoidance of hypothermia, unplanned perioperative hypothermia can occur in up to 70% of surgical patients.1 Beyond the clinical benefits, as health care marches toward a value-based payment methodology, quality indicators such as avoiding hypothermia may be linked directly to payment.
Self-determination theory (SDT) was first developed in 1980 by Deci and Ryan.13 The central premise of the theory states that people develop their full potential if circumstances allow them to satisfy their basic psychological needs: autonomy, competence, and relatedness. Under these conditions, people’s natural inclination toward growth can be realized, and they are more likely to internalize external goals. Under an extrinsic reward system, motivation can waver, as people may perceive rewards as controlling.
Many institutions have implemented hypothermia bundles to help decrease the rate of hypothermic patients, but while initially successful, the effectiveness of these interventions tends to fade over time as participants settle into old, comfortable routines.14 With SDT in mind, we designed our quality-improvement (QI) project with interventions to allow clinicians autonomy without instituting rigid guidelines or punitive actions. We aimed to directly address the affective components central to motivation and engagement so that we could bring about long-term meaningful changes in our practice.
Methods
Setting
The hypothermia QI intervention was instituted at a major tertiary care children’s hospital that performs more than 40 000 pediatric general anesthetics annually. Our division of pediatric anesthesiology consists of 66 fellowship-trained pediatric anesthesiologists, 15 or more rotating trainees per month, 13 anesthesiology assistants, 15 anesthesia technicians, and more than 50 perioperative nurses.
The most frequent pediatric surgeries include, but are not limited to, general surgery, otolaryngology, urology, gastroenterology, plastic surgery, neurosurgery, and dentistry. The surgeries are conducted in the hospital’s main operative floor, which consists of 15 ORs and 2 gastroenterology procedure rooms. Although the implementation of the QI project included several operating sites, we focused on collecting temperature data from surgical patients at our main campus recovery unit. We obtained the patients’ initial temperatures upon arrival to the recovery unit from a retrospective electronic health record review of all patients who underwent anesthesia from January 2016 through April 2021.
Postoperative hypothermia was identified as an area of potential improvement after several patients were reported to be hypothermic upon arrival to the recovery unit in the later part of 2018. Further review revealed significant heterogeneity of practices and lack of standardization of patient-warming methods. By comparing the temperatures pre- and postintervention, we could measure the effectiveness of the QI initiative. Prior to the start of our project, the hypothermia rate in our patient population was not actively tracked, and the effectiveness of our variable practice was not measured.
The cutoff for hypothermia for our QI project was defined as body temperature below 36 °C, since this value has been previously used in the literature and is commonly accepted in anesthesia practice as the delineation for hypothermia in patients undergoing general anesthesia.1
Interventions
This QI project was designed and modeled after the Institute for Healthcare Improvement Model for Improvement.15 Three cycles of Plan-Do-Study-Act (PDSA) were developed and instituted over a 14-month period until December 2019 (Table 1).

A retrospective review was conducted to determine the percentage of surgical patients arriving to our recovery units with an initial temperature reading of less than 36 °C. A project key driver diagram and smart aim were created and approved by the hospital’s continuing medical education (CME) committee for credit via the American Board of Medical Specialties (ABMS) Multi-Specialty Portfolio Program, Maintenance of Certification in Anesthesiology (MOCA) Part 4.
The first PDSA cycle involved introducing the QI project and sharing the aims of the project at a department grand rounds in the latter part of October 2018. Enrollment to participate in the project was open to all anesthesiologists in the division, and participants could earn up to 20 hours of MOCA Part 4 credits. A spreadsheet was developed and maintained to track each anesthesiologist’s monthly percentage of hypothermic patients. The de-identified patient data were shared with the division via monthly emails. In addition, individual providers with a hypothermic patient in the recovery room received a notification email.
The anesthesiologists participated in the QI project by reviewing their personal percentage of hypothermic patients on an ongoing basis to earn the credit. There was no explicit requirement to decrease their own rate of patients with body temperature less than 36 °C or expectation to achieve a predetermined goal, so the participants could not “fail.”
Because of the large interest in this project, a hypothermia committee was formed that consisted of 36 anesthesiologists. This group reviewed the data and exchanged ideas for improvement in November 2018 as part of the first PDSA cycle. The committee met monthly and was responsible for actively engaging other members of the department and perioperative staff to help in this multidisciplinary effort of combating hypothermia in our surgical pediatric population.
PDSA cycle 2 involved several major initiatives, including direct incorporation of the rest of the perioperative team. The perioperative nursing team was educated on the risks of hypothermia and engaged to take an active role by maintaining the operating suite temperature at 21.1 °C and turning on the Bair Hugger (3M) blanket to 43 °C on the OR bed prior to patient arrival to the OR. Additionally, anesthesia technicians (ATs) were tasked with ensuring an adequate supply of Bair Hugger drapes for all cases of the day. The facility’s engineering team was engaged to move the preoperative room temperature controls away from families (who frequently made the rooms cold) and instead set it at a consistent temperature of 23.9 °C. ATs were also asked to place axillary and nasal temperature probes on the anesthesia workstations as a visual reminder to facilitate temperature monitoring closer to the start of anesthesia (instead of the anesthesia provider having to remember to retrieve a temperature probe out of a drawer and place it on the patient). Furthermore, anesthesiologists were instructed via the aforementioned monthly emails and at monthly department meetings to place the temperature probes as early as possible in order to recognize and respond to intraoperative hypothermia in a timelier manner. Finally, supply chain leaders were informed of our expected increase in the use of the blankets and probes and proportionally increased ordering of these supplies to make sure availability would not present an obstacle.
In PDSA cycle 3, trainees (anesthesia assistant students, anesthesia residents and fellows) and advanced practice providers (APPs) (certified registered nurse-anesthetists [CRNAs] and certified anesthesia assistants [C-AAs]) were informed of the QI project. This initiative was guided toward improving vigilance for hypothermia in the rest of the anesthesia team members. The trainees and APPs usually set up the anesthesia area prior to patient arrival, so their recruitment in support of this effort would ensure appropriate OR temperature, active warming device deployment, and the availability and early placement of the correct temperature probe for the case. To facilitate personal accountability, the trainees and APPs were also emailed their own patients’ rate of hypothermia.
Along the course of the project, quarterly committee meetings and departmental monthly meetings served as venues to express concerns and look for areas of improvement, such as specific patterns or trends leading to hypothermic patients. One specific example was the identification of the gastrointestinal endoscopic patients having a rate of hypothermia that was 2% higher than average. Directed education on the importance of Bair Hugger blankets and using warm intravenous fluids worked well to decrease the rate of hypothermia in these patients. This collection of data was shared at regular intervals during monthly department meetings as well and more frequently using departmental emails. The hospital’s secure intranet SharePoint (Microsoft) site was used to share the data among providers.
Study of the interventions and measures
To study the effectiveness and impact of the project to motivate our anesthesiologists and other team members, we compared the first temperatures obtained in the recovery unit prior to the start of the intervention with those collected after the start of the QI project in November 2018. Because of the variability of temperature monitoring intraoperatively (nasal, axillary, rectal), we decided to use the temperature obtained by the nurse in the recovery room upon the patient’s arrival. Over the years analyzed, the nurse’s technique of measuring the temperature remained consistent. All patient temperature measurements were performed using the TAT-5000 (Exergen Corporation). This temporal artery thermometer has been previously shown to correlate well with bladder temperatures (70% of measurements differ by no more than 0.5 °C, as reported by Langham et al16).
Admittedly, we could not measure the degree of motivation or internalization of the project goals by our cohort, but we could measure the reduction in the rate of hypothermia and subjectively gauge engagement in the project by the various groups of participants and the sustainability of the results. In addition, all participating anesthesiologists received MOCA Part 4 credits in July 2019. We continued our data collection until April 2021 to determine if our project had brought about sustainable changes in practice that would continue past the initial motivator of obtaining CME credit.
Analysis
Data analysis was performed using Excel (Microsoft) and SAS, version 9.4 (SAS Institute).
The median of the monthly percentage of patients with a temperature of less than 36.0 °C was also determined for the preintervention time frame. This served as our baseline hypothermia rate, and we aimed to lower it by 50%. Run charts, a well-described methodology to gauge the effectiveness of the QI project, were constructed with the collected data.17
We performed additional analysis to adjust for different time periods throughout the year. The time period between January 2016 and October 2018 was considered preintervention. We considered November 2018 the start of our intervention, or more specifically, the start of our PDSA cycles. October 2018 was analyzed as part of the preintervention data. To account for seasonal temperature variations, the statistical analysis focused on the comparisons of the same calendar quarters for before and after starting intervention using Wilcoxon Mann-Whitney U tests. To reach an overall conclusion, the probabilities for the 4 quarters were combined for each criterion separately utilizing the Fisher χ2 combined probability method.
The hypothermia QI project was reviewed by the institutional review board and determined to be exempt.
Results
The temperatures of 40 875 patients were available for analysis for the preintervention period between January 2016 and October 2018. The median percentage of patients with temperatures less than 36.0 °C was 6.9% (interquartile range [IQR], 5.8%-8.4%). The highest percentage was in February 2016 (9.9%), and the lowest was in March 2018 (3.4%). Following the start of the first PDSA cycle, the next 6 consecutive rates of hypothermia were below the median preintervention value, and a new median for these percentages was calculated at 3.4% (IQR, 2.6%-4.3%). In July 2019, the proportion of hypothermic patients decreased once more for 6 consecutive months, yielding a new median of 1.6% (IQR, 1.2%-1.8%) and again in July 2020, to yield a median of 1.3% (IQR, 1.2%-1.5%) (Figure). In all, 33 799 patients were analyzed after the start of the project from November 2018 to the end of the data collection period through April 2021.

The preintervention monthly rates of hypothermia were compared, quarter to quarter, with those starting in November 2018 using the Wilcoxon Mann-Whitney U test. The decrease in proportion of hypothermic patients after the start of the intervention was statistically significant (P < .001). In addition, the percentage of patients with temperatures greater than 38 °C was not significantly different between the pre- and postintervention time periods (P < .25) (Table 2). The decrease in the number of patients available for analysis from March 2020 to May 2020 was due to the COVID-19 pandemic.

Subjectively, we did not experience any notable resistance to our efforts, and the experience was largely positive for everyone involved. Clinicians identified as having high monthly rates of hypothermia (5% or higher) corrected their numbers the following month after being notified via email or in person.
Discussion
To achieve changes in practice, the health care industry has relied on instituting guidelines, regulations, and policies, often with punitive consequences. We call into question this long-standing framework and propose a novel approach to help evolve the field of QI. Studies in human psychology have long demonstrated the demotivation power of a reward system and the negative response to attempts by authority to use incentives to control or coerce. In our QI project, we instituted 3 PDSA cycles and applied elements from SDT to motivate people’s behaviors. We demonstrate how a new culture focused on maintaining intraoperative normothermia was developed and brought about a measurable and significant decrease in the rate of hypothermia. The relevance of SDT, a widely accepted unifying theory that bridges and links social and personality psychology, should not be understated in health care. Authorities wishing to have long-standing influence should consider a person’s right to make their own decisions and, if possible, a unique way of doing things.
Positively reinforcing behavior has been shown to have a paradoxical effect by dampening an individual’s intrinsic motivation or desire to perform certain tasks.18 Deadlines, surveillance, and authoritative commands are also deterrents.19,20 We focused on providing the tools and information to the clinicians and relied on their innate need for autonomy, growth, and self-actualization to bring about change in clinical practice.21 Group meetings served as a construct for exchanging ideas and to encourage participation, but without the implementation of rigid guidelines or policies. Intraoperative active warming devices and temperature probes were made available, but their use was not mandated. The use of these devices was intentionally not audited to avoid any overbearing control. Providers were, however, given monthly temperature data to help individually assess the effectiveness of their interventions. We did not impose any negative or punitive actions for those clinicians who had high rates of hypothermic patients, and we did not reward those who had low rates of hypothermia. We wanted the participants to feel that the inner self was the source of their behavior, and this was in parallel with their own interests and values. If providers could feel their need for competency could be realized, we hoped they would continue to adhere to the measures we provided to maintain a low rate of hypothermia.
The effectiveness of our efforts was demonstrated by a decrease in the prevalence of postoperative hypothermia in our surgical patients. The initial decrease of the median rate of hypothermia from 6.9% to 3.4% occurred shortly into the start of the first PDSA cycle. The second PDSA cycle started in January 2019 with a multimodal approach and included almost all parties involved in the perioperative care of our surgical patients. Not only was this intervention responsible for a continued downward trend in the percentage of hypothermic patients, but it set the stage for the third and final PDSA cycle, which started in July 2019. The architecture was in place to integrate trainees and APPs to reinforce our initiative. Subsequently, the new median percentage of hypothermic patients was further decreased to an all-time low of 1.6% per month, satisfying and surpassing the goal of the QI project of decreasing the rate of hypothermia by only 50%. Our organization thereafter maintained a monthly hypothermia rate below 2%, except for April 2020, when it reached 2.5%. Our lowest median percentage was obtained after July 2020, reaching 1.3%.
To account for seasonal variations in temperatures and types of surgeries performed, we compared the percentage of hypothermic patients before and after the start of intervention, quarter by quarter. The decrease in the proportion of hypothermic patients after the start of intervention was statistically significant (P < .001). In addition, the data failed to prove any statistical difference for temperatures above 38 °C between the 2 periods, indicating that our interventions did not result in significant overwarming of patients. The clinical implications of decreasing the percentage of hypothermic patients from 6.9% to 1.3% is likely clinically important when considering the large number of patients who undergo surgery at large tertiary care pediatric centers. Even if simple interventions reduce hypothermia in only a handful of patients, routine applications of simple measures to keep patients normothermic is likely best clinical practice.
Anesthesiologists who participated in the hypothermia QI project by tracking the incidence of hypothermia in their patients were able to collect MOCA Part 4 credits in July 2019. There was no requirement for the individual anesthesiologist to reduce the rate of hypothermia or apply any of the encouraged strategies to obtain credit. As previously stated, there were also no rewards for obtaining low hypothermia rates for the providers. The temperature data continued to be collected through April 2021, 21 months after the credits were distributed, to demonstrate a continued, meaningful change, at least in the short-term. While the MOCA Part 4 credits likely served as an initial motivating factor to encourage participation in the QI project, they certainly were not responsible for the sustained low hypothermia rate after July 2019. We showed that the low rate of hypothermia was successfully maintained, indicating that the change in providers’ behavior was independent of the external motivator of obtaining the credit hours. Mere participation in the project by reviewing one’s temperature data was all that was required to obtain the credit. The Organismic Integration Theory, a mini-theory within SDT, best explains this phenomenon by describing any motivated behavior on a continuum ranging from controlled to autonomous.22 Do people perform the task resentfully, on their own volition because they believe it is the correct action, or somewhere in between? We explain the sustained low rates of hypothermia after the MOCA credits were distributed due to a shift to the autonomous end of the continuum with the clinician’s active willingness to meet the challenges and apply intrinsically motivated behaviors to lower the rate of hypothermia. The internalization of external motivators is difficult to prove, but the evidence supports that the methods we used to motivate individuals were effective and have resulted in a significant downward trend in our hypothermia rate.
There are several limitations to our QI project. The first involves the measuring of postoperative temperature in the recovery units. The temperatures were obtained using the same medical-grade infrared thermometer for all the patients, but other variables, such as timing and techniques, were not standardized. Secondly, overall surgical outcomes related to hypothermia were not tracked because we were unable to control for other confounding variables in our large cohort of patients, so we cannot say if the drop in the hypothermia rate had a clinically significant outcome. Thirdly, we propose that SDT offers a compellingly fitting explanation of the psychology of motivation in our efforts, but it may be possible that other theories may offer equally fitting explanations. The ability to measure the degree of motivation is lacking, and we did not explicitly ask participants what their specific source of motivation was. Aside from SDT, the reduction in hypothermia rate could also be attributed to the ease and availability of warming equipment that was made in each OR. This QI project was successfully applied to only 1 institution, so its ability to be widely applicable remains uncertain. In addition, data collection continued during the COVID-19 pandemic when case volumes decreased. However, by June 2020, the number of surgical cases at our institution had largely returned to prepandemic levels. Additional data collection beyond April 2021 would be helpful to determine if the reduction in hypothermia rates is truly sustained.
Conclusion
Overall, the importance of maintaining perioperative normothermia was well disseminated and agreed upon by all departments involved. Despite the limitations of the project, there was a significant reduction in rates of hypothermia, and sustainability of outcomes was consistently demonstrated in the poststudy period.
Using 3 cycles of the PDSA method, we successfully decreased the median rate of postoperative hypothermia in our pediatric surgical population from a preintervention value of 6.9% to 1.3%—a reduction of more than 81.2%. We provided motivation for members of our anesthesiology staff to participate by offering MOCA 2.0 Part 4 credits, but the lower rate of hypothermic patients was maintained for 15 months after the credits were distributed. Over the course of the project, there was a shift in culture, and extra vigilance was given to temperature monitoring and assessment. We attribute this sustained cultural change to the deliberate incorporation of the principles of competency, autonomy, and relatedness central to SDT to the structure of the interventions, avoiding rigid guidelines and pathways in favor of affective engagement to establish intrinsic motivation.
Acknowledgements: The authors thank Joan Reisch, PhD, for her assistance with the statistical analysis.
Corresponding author: Edgar Erold Kiss, MD, 1935 Medical District Dr, Dallas, TX 75235; [email protected].
Financial disclosures: None.
1. Leslie K, Sessler DI. Perioperative hypothermia in the high-risk surgical patient. Best Pract Res Clin Anaesthesiol. 2003;17(4):485-498.
2. Sessler DI. Forced-air warming in infants and children. Paediatr Anaesth. 2013;23(6):467-468.
3. Wetzel RC. Evaluation of children. In: Longnecker DE, Tinker JH, Morgan Jr GE, eds. Principles and Practice of Anesthesiology. 2nd ed. Mosby Publishers; 1999:445-447.
4. Witt L, Dennhardt N, Eich C, et al. Prevention of intraoperative hypothermia in neonates and infants: results of a prospective multicenter observational study with a new forced-air warming system with increased warm air flow. Paediatr Anaesth. 2013;23(6):469-474.
5. Blum R, Cote C. Pediatric equipment. In: Blum R, Cote C, eds. A Practice of Anaesthesia for Infants and Children. Saunders Elsevier; 2009:1099-1101.
6. Doufas AG. Consequences of inadvertent perioperative hypothermia. Best Pract Res Clin Anaesthesiol. 2003;17(4):535-549.
7. Mahoney CB, Odom J. Maintaining intraoperative normothermia: a meta-analysis of outcomes with costs. AANA J. 1999;67(2):155-163.
8. American Society of Anesthesiologists Committee on Standards and Practice Parameters. Standards for Basic Anesthetic Monitoring. Approved by the ASA House of Delegates October 21, 1986; last amended October 20, 2010; last affirmed October 28, 2015.
9. Horn E-P, Bein B, Böhm R, et al. The effect of short time periods of pre-operative warming in the prevention of peri-operative hypothermia. Anaesthesia. 2012;67(6):612-617.
10. Andrzejowski J, Hoyle J, Eapen G, Turnbull D. Effect of prewarming on post-induction core temperature and the incidence of inadvertent perioperative hypothermia in patients undergoing general anaesthesia. Br J Anaesth. 2008;101(5):627-631.
11. Sessler DI. Complications and treatment of mild hypothermia. Anesthesiology. 2001;95(2):531-543.
12. Bräuer A, English MJM, Steinmetz N, et al. Efficacy of forced-air warming systems with full body blankets. Can J Anaesth. 2007;54(1):34-41.
13. Deci EL, Ryan RM. The “what” and “why” of goal pursuits: human needs and the self‐determination of behavior. Psychol Inquiry. 2000;11(4):227-268.
14. Al-Shamari M, Puttha R, Yuen S, et al. G9 Can introduction of a hypothermia bundle reduce hypothermia in the newborns? Arch Dis Childhood. 2019;104(suppl 2):A4.1-A4.
15. Institute for Healthcare Improvement. How to improve. Accessed May 12, 2021. http://www.ihi.org/resources/Pages/HowtoImprove/default.aspx
16. Langham GE, Meheshwari A, You J, et al. Noninvasive temperature monitoring in postanesthesia care units. Anesthesiology. 2009;111(1):90-96.
17. Perla RJ, Provost LP, Murray SK. The run chart: a simple analytical tool for learning from variation in healthcare processes. BMJ Qual Saf. 2011;20(1):46-51.
18. Deci EL. Effects of externally mediated rewards on intrinsic motivation. J Pers Soc Psychol. 1971;18(1):105-115.
19. Deci EL, Koestner R, Ryan RM. A meta-analytic review of experiments examining the effects of extrinsic rewards on intrinsic motivation. Psychol Bull. 1999;125(6):627-668.
20. Deci EL, Koestner R, Ryan RM. The undermining effect is a reality after all—extrinsic rewards, task interest, and self-determination: Reply to Eisenberger, Pierce, and Cameron (1999) and Lepper, Henderlong, and Gingras (1999). Psychol Bull. 1999;125(6):692-700.
21. Maslow A. The Farther Reaches of Human Nature. Viking Press; 1971.
22. Sheldon KM, Prentice M. Self-determination theory as a foundation for personality researchers. J Pers. 2019;87(1):5-14.
1. Leslie K, Sessler DI. Perioperative hypothermia in the high-risk surgical patient. Best Pract Res Clin Anaesthesiol. 2003;17(4):485-498.
2. Sessler DI. Forced-air warming in infants and children. Paediatr Anaesth. 2013;23(6):467-468.
3. Wetzel RC. Evaluation of children. In: Longnecker DE, Tinker JH, Morgan Jr GE, eds. Principles and Practice of Anesthesiology. 2nd ed. Mosby Publishers; 1999:445-447.
4. Witt L, Dennhardt N, Eich C, et al. Prevention of intraoperative hypothermia in neonates and infants: results of a prospective multicenter observational study with a new forced-air warming system with increased warm air flow. Paediatr Anaesth. 2013;23(6):469-474.
5. Blum R, Cote C. Pediatric equipment. In: Blum R, Cote C, eds. A Practice of Anaesthesia for Infants and Children. Saunders Elsevier; 2009:1099-1101.
6. Doufas AG. Consequences of inadvertent perioperative hypothermia. Best Pract Res Clin Anaesthesiol. 2003;17(4):535-549.
7. Mahoney CB, Odom J. Maintaining intraoperative normothermia: a meta-analysis of outcomes with costs. AANA J. 1999;67(2):155-163.
8. American Society of Anesthesiologists Committee on Standards and Practice Parameters. Standards for Basic Anesthetic Monitoring. Approved by the ASA House of Delegates October 21, 1986; last amended October 20, 2010; last affirmed October 28, 2015.
9. Horn E-P, Bein B, Böhm R, et al. The effect of short time periods of pre-operative warming in the prevention of peri-operative hypothermia. Anaesthesia. 2012;67(6):612-617.
10. Andrzejowski J, Hoyle J, Eapen G, Turnbull D. Effect of prewarming on post-induction core temperature and the incidence of inadvertent perioperative hypothermia in patients undergoing general anaesthesia. Br J Anaesth. 2008;101(5):627-631.
11. Sessler DI. Complications and treatment of mild hypothermia. Anesthesiology. 2001;95(2):531-543.
12. Bräuer A, English MJM, Steinmetz N, et al. Efficacy of forced-air warming systems with full body blankets. Can J Anaesth. 2007;54(1):34-41.
13. Deci EL, Ryan RM. The “what” and “why” of goal pursuits: human needs and the self‐determination of behavior. Psychol Inquiry. 2000;11(4):227-268.
14. Al-Shamari M, Puttha R, Yuen S, et al. G9 Can introduction of a hypothermia bundle reduce hypothermia in the newborns? Arch Dis Childhood. 2019;104(suppl 2):A4.1-A4.
15. Institute for Healthcare Improvement. How to improve. Accessed May 12, 2021. http://www.ihi.org/resources/Pages/HowtoImprove/default.aspx
16. Langham GE, Meheshwari A, You J, et al. Noninvasive temperature monitoring in postanesthesia care units. Anesthesiology. 2009;111(1):90-96.
17. Perla RJ, Provost LP, Murray SK. The run chart: a simple analytical tool for learning from variation in healthcare processes. BMJ Qual Saf. 2011;20(1):46-51.
18. Deci EL. Effects of externally mediated rewards on intrinsic motivation. J Pers Soc Psychol. 1971;18(1):105-115.
19. Deci EL, Koestner R, Ryan RM. A meta-analytic review of experiments examining the effects of extrinsic rewards on intrinsic motivation. Psychol Bull. 1999;125(6):627-668.
20. Deci EL, Koestner R, Ryan RM. The undermining effect is a reality after all—extrinsic rewards, task interest, and self-determination: Reply to Eisenberger, Pierce, and Cameron (1999) and Lepper, Henderlong, and Gingras (1999). Psychol Bull. 1999;125(6):692-700.
21. Maslow A. The Farther Reaches of Human Nature. Viking Press; 1971.
22. Sheldon KM, Prentice M. Self-determination theory as a foundation for personality researchers. J Pers. 2019;87(1):5-14.
Many pandemic-driven changes to cancer clinical trials should remain
Many of the changes to cancer clinical trials forced through by the COVID-19 pandemic should remain, as they have made trials “more patient centered and efficient,” according to a group of thought leaders in oncology.
Among the potential improvements were more efficient study enrollment through secure electronic platforms, direct shipment of oral drugs to patients, remote assessment of adverse events, and streamlined data collection.
These changes should be implemented on a permanent basis, the group argues in a commentary published online July 21, 2021, in Cancer Discovery, a journal of the American Association for Cancer Research.
“The ability to distribute oral investigational drugs by mail to patients at their home has probably been the single most impactful change to clinical trial conduct, linked with virtual visits with patients to assess side effects and symptoms,” commented lead author Keith Flaherty, MD, who is director of clinical research at Massachusetts General Hospital, a professor at Harvard Medical School, Boston, and a member of the AACR board of directors.
“This has made it more feasible for patients for whom participation in clinical trials poses a disruption of their ability to work or provide care for family members to participate in trials,” he added in a press statement issued by the AACR.
Pandemic halted many clinical trials
A survey of cancer programs in early 2020 showed that nearly 60% halted screening and/or enrollment for at least some trials because of COVID-19.
“In the spring of 2020, clinical trial conduct halted and then restarted focusing on the bare minimum procedures that first allowed patients continued access to their experimental therapies, and then allowed clinical trial sites and sponsors to collect information on the effects of the therapies,” the authors said.
“The COVID-19–induced changes to clinical trials were a big challenge, probably the largest change in clinical trial conduct since the start of modern oncology clinical testing,” they commented.
“But it also represents an opportunity to rethink the key aspects of clinical trial conduct that are strictly necessary to reach the goal of testing the effectiveness of cancer therapies, and which others are dispensable or provide only minor additional contributions,” they added.
As previously reported at the time by this news organization, efforts to find alternative approaches to conducting trials amid the pandemic led to the emergence of a few “silver linings.”
Key adaptations made to clinical trials and highlighted by the authors include:
- Uptake of remote consenting and telemedicine
- Use of alternative laboratories and imaging centers
- Delivery or administration of investigational drugs at patients’ homes or local clinics
- Commercial attainment of study drugs already approved for other indications
Indeed, the restrictions encountered during the pandemic underscore the importance of designing patient-centered trials versus study site–centered trials, added Antoni Ribas, MD, commentary coauthor and immediate past president of the AACR.
Many of the changes implemented during the pandemic could help increase access for patients living in underserved communities who are underrepresented in clinical trials, he explained.
Harnessing the lessons learned
The authors also recommended the following additional adaptations, which they believe will enhance efficiency and further expand access to clinical trials:
- Incorporating patient-reported outcomes and alternative endpoints in efficacy assessments
- Aiming for 100% remote drug infusions and monitoring
- Increasing funding for clinical trials conducted in underserved communities
- Expanding clinical trial eligibility to include patients with a wide range of comorbidities
- Reducing collection of low-grade adverse events and allowing minor protocol deviations
The group’s recommendations are based on discussions by the AACR COVID-19 and Cancer Task Force, in which they participated.
The American Society of Clinical Oncology is also working to leverage pandemic-related lessons to streamline care and trial planning.
ASCO’s “Road to Recovery” recommendations, published in December 2020, aim to “ensure lessons learned from the COVID-19 experience are used to craft a more equitable, accessible, and efficient clinical research system that protects patient safety, ensures scientific integrity, and maintains data quality,” the authors explained.
Dr. Flaherty and colleagues further underscore the importance of focusing on improvements going forward.
“Guided by lessons learned, many of the remote assessments and trial efficiencies deployed during the pandemic can be preserved and improved upon. We strongly encourage use of these streamlined procedures where appropriate in future prospectively designed cancer clinical trials,” they wrote.
Dr. Flaherty reported receiving personal fees from numerous pharmaceutical companies. Dr. Ribas reported receiving grants from Agilent and Bristol Myers Squibb.
A version of this article first appeared on Medscape.com.
Many of the changes to cancer clinical trials forced through by the COVID-19 pandemic should remain, as they have made trials “more patient centered and efficient,” according to a group of thought leaders in oncology.
Among the potential improvements were more efficient study enrollment through secure electronic platforms, direct shipment of oral drugs to patients, remote assessment of adverse events, and streamlined data collection.
These changes should be implemented on a permanent basis, the group argues in a commentary published online July 21, 2021, in Cancer Discovery, a journal of the American Association for Cancer Research.
“The ability to distribute oral investigational drugs by mail to patients at their home has probably been the single most impactful change to clinical trial conduct, linked with virtual visits with patients to assess side effects and symptoms,” commented lead author Keith Flaherty, MD, who is director of clinical research at Massachusetts General Hospital, a professor at Harvard Medical School, Boston, and a member of the AACR board of directors.
“This has made it more feasible for patients for whom participation in clinical trials poses a disruption of their ability to work or provide care for family members to participate in trials,” he added in a press statement issued by the AACR.
Pandemic halted many clinical trials
A survey of cancer programs in early 2020 showed that nearly 60% halted screening and/or enrollment for at least some trials because of COVID-19.
“In the spring of 2020, clinical trial conduct halted and then restarted focusing on the bare minimum procedures that first allowed patients continued access to their experimental therapies, and then allowed clinical trial sites and sponsors to collect information on the effects of the therapies,” the authors said.
“The COVID-19–induced changes to clinical trials were a big challenge, probably the largest change in clinical trial conduct since the start of modern oncology clinical testing,” they commented.
“But it also represents an opportunity to rethink the key aspects of clinical trial conduct that are strictly necessary to reach the goal of testing the effectiveness of cancer therapies, and which others are dispensable or provide only minor additional contributions,” they added.
As previously reported at the time by this news organization, efforts to find alternative approaches to conducting trials amid the pandemic led to the emergence of a few “silver linings.”
Key adaptations made to clinical trials and highlighted by the authors include:
- Uptake of remote consenting and telemedicine
- Use of alternative laboratories and imaging centers
- Delivery or administration of investigational drugs at patients’ homes or local clinics
- Commercial attainment of study drugs already approved for other indications
Indeed, the restrictions encountered during the pandemic underscore the importance of designing patient-centered trials versus study site–centered trials, added Antoni Ribas, MD, commentary coauthor and immediate past president of the AACR.
Many of the changes implemented during the pandemic could help increase access for patients living in underserved communities who are underrepresented in clinical trials, he explained.
Harnessing the lessons learned
The authors also recommended the following additional adaptations, which they believe will enhance efficiency and further expand access to clinical trials:
- Incorporating patient-reported outcomes and alternative endpoints in efficacy assessments
- Aiming for 100% remote drug infusions and monitoring
- Increasing funding for clinical trials conducted in underserved communities
- Expanding clinical trial eligibility to include patients with a wide range of comorbidities
- Reducing collection of low-grade adverse events and allowing minor protocol deviations
The group’s recommendations are based on discussions by the AACR COVID-19 and Cancer Task Force, in which they participated.
The American Society of Clinical Oncology is also working to leverage pandemic-related lessons to streamline care and trial planning.
ASCO’s “Road to Recovery” recommendations, published in December 2020, aim to “ensure lessons learned from the COVID-19 experience are used to craft a more equitable, accessible, and efficient clinical research system that protects patient safety, ensures scientific integrity, and maintains data quality,” the authors explained.
Dr. Flaherty and colleagues further underscore the importance of focusing on improvements going forward.
“Guided by lessons learned, many of the remote assessments and trial efficiencies deployed during the pandemic can be preserved and improved upon. We strongly encourage use of these streamlined procedures where appropriate in future prospectively designed cancer clinical trials,” they wrote.
Dr. Flaherty reported receiving personal fees from numerous pharmaceutical companies. Dr. Ribas reported receiving grants from Agilent and Bristol Myers Squibb.
A version of this article first appeared on Medscape.com.
Many of the changes to cancer clinical trials forced through by the COVID-19 pandemic should remain, as they have made trials “more patient centered and efficient,” according to a group of thought leaders in oncology.
Among the potential improvements were more efficient study enrollment through secure electronic platforms, direct shipment of oral drugs to patients, remote assessment of adverse events, and streamlined data collection.
These changes should be implemented on a permanent basis, the group argues in a commentary published online July 21, 2021, in Cancer Discovery, a journal of the American Association for Cancer Research.
“The ability to distribute oral investigational drugs by mail to patients at their home has probably been the single most impactful change to clinical trial conduct, linked with virtual visits with patients to assess side effects and symptoms,” commented lead author Keith Flaherty, MD, who is director of clinical research at Massachusetts General Hospital, a professor at Harvard Medical School, Boston, and a member of the AACR board of directors.
“This has made it more feasible for patients for whom participation in clinical trials poses a disruption of their ability to work or provide care for family members to participate in trials,” he added in a press statement issued by the AACR.
Pandemic halted many clinical trials
A survey of cancer programs in early 2020 showed that nearly 60% halted screening and/or enrollment for at least some trials because of COVID-19.
“In the spring of 2020, clinical trial conduct halted and then restarted focusing on the bare minimum procedures that first allowed patients continued access to their experimental therapies, and then allowed clinical trial sites and sponsors to collect information on the effects of the therapies,” the authors said.
“The COVID-19–induced changes to clinical trials were a big challenge, probably the largest change in clinical trial conduct since the start of modern oncology clinical testing,” they commented.
“But it also represents an opportunity to rethink the key aspects of clinical trial conduct that are strictly necessary to reach the goal of testing the effectiveness of cancer therapies, and which others are dispensable or provide only minor additional contributions,” they added.
As previously reported at the time by this news organization, efforts to find alternative approaches to conducting trials amid the pandemic led to the emergence of a few “silver linings.”
Key adaptations made to clinical trials and highlighted by the authors include:
- Uptake of remote consenting and telemedicine
- Use of alternative laboratories and imaging centers
- Delivery or administration of investigational drugs at patients’ homes or local clinics
- Commercial attainment of study drugs already approved for other indications
Indeed, the restrictions encountered during the pandemic underscore the importance of designing patient-centered trials versus study site–centered trials, added Antoni Ribas, MD, commentary coauthor and immediate past president of the AACR.
Many of the changes implemented during the pandemic could help increase access for patients living in underserved communities who are underrepresented in clinical trials, he explained.
Harnessing the lessons learned
The authors also recommended the following additional adaptations, which they believe will enhance efficiency and further expand access to clinical trials:
- Incorporating patient-reported outcomes and alternative endpoints in efficacy assessments
- Aiming for 100% remote drug infusions and monitoring
- Increasing funding for clinical trials conducted in underserved communities
- Expanding clinical trial eligibility to include patients with a wide range of comorbidities
- Reducing collection of low-grade adverse events and allowing minor protocol deviations
The group’s recommendations are based on discussions by the AACR COVID-19 and Cancer Task Force, in which they participated.
The American Society of Clinical Oncology is also working to leverage pandemic-related lessons to streamline care and trial planning.
ASCO’s “Road to Recovery” recommendations, published in December 2020, aim to “ensure lessons learned from the COVID-19 experience are used to craft a more equitable, accessible, and efficient clinical research system that protects patient safety, ensures scientific integrity, and maintains data quality,” the authors explained.
Dr. Flaherty and colleagues further underscore the importance of focusing on improvements going forward.
“Guided by lessons learned, many of the remote assessments and trial efficiencies deployed during the pandemic can be preserved and improved upon. We strongly encourage use of these streamlined procedures where appropriate in future prospectively designed cancer clinical trials,” they wrote.
Dr. Flaherty reported receiving personal fees from numerous pharmaceutical companies. Dr. Ribas reported receiving grants from Agilent and Bristol Myers Squibb.
A version of this article first appeared on Medscape.com.
CDC calls for masks in schools, hard-hit areas, even if vaccinated
The agency has called for masks in K-12 school settings and in areas of the United States experiencing high or substantial SARS-CoV-2 transmission, even for the fully vaccinated.
The move reverses a controversial announcement the agency made in May 2021 that fully vaccinated Americans could skip wearing a mask in most settings.
Unlike the increasing vaccination rates and decreasing case numbers reported in May, however, some regions of the United States are now reporting large jumps in COVID-19 case numbers. And the Delta variant as well as new evidence of transmission from breakthrough cases are largely driving these changes.
“Today we have new science related to the [D]elta variant that requires us to update the guidance on what you can do when you are fully vaccinated,” CDC Director Rochelle Walensky, MD, MPH, said during a media briefing July 27.
New evidence has emerged on breakthrough-case transmission risk, for example. “Information on the [D]elta variant from several states and other countries indicates that in rare cases, some people infected with the [D]elta variant after vaccination may be contagious and spread virus to others,” Dr. Walensky said, adding that the viral loads appear to be about the same in vaccinated and unvaccinated individuals.
“This new science is worrisome,” she said.
Even though unvaccinated people represent the vast majority of cases of transmission, Dr. Walensky said, “we thought it was important for [vaccinated] people to understand they have the potential to transmit the virus to others.”
As a result, in addition to continuing to strongly encourage everyone to get vaccinated, the CDC recommends that fully vaccinated people wear masks in public indoor settings to help prevent the spread of the Delta variant in areas with substantial or high transmission, Dr. Walensky said. “This includes schools.”
Masks in schools
The CDC is now recommending universal indoor masking for all teachers, staff, students, and visitors to K-12 schools, regardless of vaccination status. Their goal is to optimize safety and allow children to return to full-time in-person learning in the fall.
The CDC tracks substantial and high transmission rates through the agency’s COVID Data Tracker site. Substantial transmission means between 50 and 100 cases per 100,000 people reported over 7 days and high means more than 100 cases per 100,000 people.
The B.1.617.2, or Delta, variant is believed to be responsible for COVID-19 cases increasing more than 300% nationally from June 19 to July 23, 2021.
“A prudent move”
“I think it’s a prudent move. Given the dominance of the [D]elta variant and the caseloads that we are seeing rising in many locations across the United States, including in my backyard here in San Francisco,” Joe DeRisi, PhD, copresident of the Chan Zuckerberg Biohub and professor of biochemistry and biophysics at the University of California San Francisco, said in an interview.
Dr. DeRisi said he was not surprised that vaccinated people with breakthrough infections could be capable of transmitting the virus. He added that clinical testing done by the Biohub and UCSF produced a lot of data on viral load levels, “and they cover an enormous range.”
What was unexpected to him was the rapid rise of the dominant variant. “The rise of the [D]elta strain is astonishing. It’s happened so fast,” he said.
“I know it’s difficult”
Reacting to the news, Colleen Kraft, MD, said, “One of the things that we’re learning is that if we’re going to have low vaccine uptake or we have a number of people that can’t be vaccinated yet, such as children, that we really need to go back to stopping transmission, which involves mask wearing.”
“I know that it’s very difficult and people feel like we’re sliding backward,” Dr. Kraft said during a media briefing sponsored by Emory University held shortly after the CDC announcement.
She added that the CDC updated guidance seems appropriate. “I don’t think any of us really want to be in this position or want to go back to masking but…we’re finding ourselves in the same place we were a year ago, in July 2020.
“In general we just don’t want anybody to be infected even if there’s a small chance for you to be infected and there’s a small chance for you to transmit it,” said Dr. Kraft, who’s an assistant professor in the department of pathology and associate professor in the department of medicine, division of infectious diseases at Emory University School of Medicine in Atlanta.
Breakthrough transmissions
“The good news is you’re still unlikely to get critically ill if you’re vaccinated. But what has changed with the [D]elta variant is instead of being 90% plus protected from getting the virus at all, you’re probably more in the 70% to 80% range,” James T. McDeavitt, MD, told this news organization.
“So we’re seeing breakthrough infections,” said Dr. McDeavitt, executive vice president and dean of clinical affairs at Baylor College of Medicine in Houston. “We are starting to see [such people] are potentially infectious.” Even if a vaccinated person is individually much less likely to experience serious COVID-19 outcomes, “they can spread it to someone else who spreads it to someone else who is more vulnerable. It puts the more at-risk populations at further risk.”
It breaks down to individual and public health concerns. “I am fully vaccinated. I am very confident I am not going to end up in a hospital,” he said. “Now if I were unvaccinated, with the prevalence of the virus around the country, I’m probably in more danger than I’ve ever been in the course of the pandemic. The unvaccinated are really at risk right now.”
IDSA and AMA support mask change
The Infectious Diseases Society of America (IDSA) has released a statement supporting the new CDC recommendations. “To stay ahead of the spread of the highly transmissible Delta variant, IDSA also urges that in communities with moderate transmission rates, all individuals, even those who are vaccinated, wear masks in indoor public places,” stated IDSA President Barbara D. Alexander, MD, MHS.
“IDSA also supports CDC’s guidance recommending universal indoor masking for all teachers, staff, students, and visitors to K-12 schools, regardless of vaccination status, until vaccines are authorized and widely available to all children and vaccination rates are sufficient to control transmission.”
“Mask wearing will help reduce infections, prevent serious illnesses and death, limit strain on local hospitals and stave off the development of even more troubling variants,” she added.
The American Medical Association (AMA) also released a statement supporting the CDC’s policy changes.
“According to the CDC, emerging data indicates that vaccinated individuals infected with the Delta variant have similar viral loads as those who are unvaccinated and are capable of transmission,” AMA President Gerald E. Harmon, MD said in the statement.
“However, the science remains clear, the authorized vaccines remain safe and effective in preventing severe complications from COVID-19, including hospitalization and death,” he stated. “We strongly support the updated recommendations, which call for universal masking in areas of high or substantial COVID-19 transmission and in K-12 schools, to help reduce transmission of the virus. Wearing a mask is a small but important protective measure that can help us all stay safer.”
“The highest spread of cases and [most] severe outcomes are happening in places with low vaccination rates and among unvaccinated people,” Dr. Walensky said. “With the [D]elta variant, vaccinating more Americans now is more urgent than ever.”
“This moment, and the associated suffering, illness, and death, could have been avoided with higher vaccination coverage in this country,” she said.
A version of this article first appeared on Medscape.com.
The agency has called for masks in K-12 school settings and in areas of the United States experiencing high or substantial SARS-CoV-2 transmission, even for the fully vaccinated.
The move reverses a controversial announcement the agency made in May 2021 that fully vaccinated Americans could skip wearing a mask in most settings.
Unlike the increasing vaccination rates and decreasing case numbers reported in May, however, some regions of the United States are now reporting large jumps in COVID-19 case numbers. And the Delta variant as well as new evidence of transmission from breakthrough cases are largely driving these changes.
“Today we have new science related to the [D]elta variant that requires us to update the guidance on what you can do when you are fully vaccinated,” CDC Director Rochelle Walensky, MD, MPH, said during a media briefing July 27.
New evidence has emerged on breakthrough-case transmission risk, for example. “Information on the [D]elta variant from several states and other countries indicates that in rare cases, some people infected with the [D]elta variant after vaccination may be contagious and spread virus to others,” Dr. Walensky said, adding that the viral loads appear to be about the same in vaccinated and unvaccinated individuals.
“This new science is worrisome,” she said.
Even though unvaccinated people represent the vast majority of cases of transmission, Dr. Walensky said, “we thought it was important for [vaccinated] people to understand they have the potential to transmit the virus to others.”
As a result, in addition to continuing to strongly encourage everyone to get vaccinated, the CDC recommends that fully vaccinated people wear masks in public indoor settings to help prevent the spread of the Delta variant in areas with substantial or high transmission, Dr. Walensky said. “This includes schools.”
Masks in schools
The CDC is now recommending universal indoor masking for all teachers, staff, students, and visitors to K-12 schools, regardless of vaccination status. Their goal is to optimize safety and allow children to return to full-time in-person learning in the fall.
The CDC tracks substantial and high transmission rates through the agency’s COVID Data Tracker site. Substantial transmission means between 50 and 100 cases per 100,000 people reported over 7 days and high means more than 100 cases per 100,000 people.
The B.1.617.2, or Delta, variant is believed to be responsible for COVID-19 cases increasing more than 300% nationally from June 19 to July 23, 2021.
“A prudent move”
“I think it’s a prudent move. Given the dominance of the [D]elta variant and the caseloads that we are seeing rising in many locations across the United States, including in my backyard here in San Francisco,” Joe DeRisi, PhD, copresident of the Chan Zuckerberg Biohub and professor of biochemistry and biophysics at the University of California San Francisco, said in an interview.
Dr. DeRisi said he was not surprised that vaccinated people with breakthrough infections could be capable of transmitting the virus. He added that clinical testing done by the Biohub and UCSF produced a lot of data on viral load levels, “and they cover an enormous range.”
What was unexpected to him was the rapid rise of the dominant variant. “The rise of the [D]elta strain is astonishing. It’s happened so fast,” he said.
“I know it’s difficult”
Reacting to the news, Colleen Kraft, MD, said, “One of the things that we’re learning is that if we’re going to have low vaccine uptake or we have a number of people that can’t be vaccinated yet, such as children, that we really need to go back to stopping transmission, which involves mask wearing.”
“I know that it’s very difficult and people feel like we’re sliding backward,” Dr. Kraft said during a media briefing sponsored by Emory University held shortly after the CDC announcement.
She added that the CDC updated guidance seems appropriate. “I don’t think any of us really want to be in this position or want to go back to masking but…we’re finding ourselves in the same place we were a year ago, in July 2020.
“In general we just don’t want anybody to be infected even if there’s a small chance for you to be infected and there’s a small chance for you to transmit it,” said Dr. Kraft, who’s an assistant professor in the department of pathology and associate professor in the department of medicine, division of infectious diseases at Emory University School of Medicine in Atlanta.
Breakthrough transmissions
“The good news is you’re still unlikely to get critically ill if you’re vaccinated. But what has changed with the [D]elta variant is instead of being 90% plus protected from getting the virus at all, you’re probably more in the 70% to 80% range,” James T. McDeavitt, MD, told this news organization.
“So we’re seeing breakthrough infections,” said Dr. McDeavitt, executive vice president and dean of clinical affairs at Baylor College of Medicine in Houston. “We are starting to see [such people] are potentially infectious.” Even if a vaccinated person is individually much less likely to experience serious COVID-19 outcomes, “they can spread it to someone else who spreads it to someone else who is more vulnerable. It puts the more at-risk populations at further risk.”
It breaks down to individual and public health concerns. “I am fully vaccinated. I am very confident I am not going to end up in a hospital,” he said. “Now if I were unvaccinated, with the prevalence of the virus around the country, I’m probably in more danger than I’ve ever been in the course of the pandemic. The unvaccinated are really at risk right now.”
IDSA and AMA support mask change
The Infectious Diseases Society of America (IDSA) has released a statement supporting the new CDC recommendations. “To stay ahead of the spread of the highly transmissible Delta variant, IDSA also urges that in communities with moderate transmission rates, all individuals, even those who are vaccinated, wear masks in indoor public places,” stated IDSA President Barbara D. Alexander, MD, MHS.
“IDSA also supports CDC’s guidance recommending universal indoor masking for all teachers, staff, students, and visitors to K-12 schools, regardless of vaccination status, until vaccines are authorized and widely available to all children and vaccination rates are sufficient to control transmission.”
“Mask wearing will help reduce infections, prevent serious illnesses and death, limit strain on local hospitals and stave off the development of even more troubling variants,” she added.
The American Medical Association (AMA) also released a statement supporting the CDC’s policy changes.
“According to the CDC, emerging data indicates that vaccinated individuals infected with the Delta variant have similar viral loads as those who are unvaccinated and are capable of transmission,” AMA President Gerald E. Harmon, MD said in the statement.
“However, the science remains clear, the authorized vaccines remain safe and effective in preventing severe complications from COVID-19, including hospitalization and death,” he stated. “We strongly support the updated recommendations, which call for universal masking in areas of high or substantial COVID-19 transmission and in K-12 schools, to help reduce transmission of the virus. Wearing a mask is a small but important protective measure that can help us all stay safer.”
“The highest spread of cases and [most] severe outcomes are happening in places with low vaccination rates and among unvaccinated people,” Dr. Walensky said. “With the [D]elta variant, vaccinating more Americans now is more urgent than ever.”
“This moment, and the associated suffering, illness, and death, could have been avoided with higher vaccination coverage in this country,” she said.
A version of this article first appeared on Medscape.com.
The agency has called for masks in K-12 school settings and in areas of the United States experiencing high or substantial SARS-CoV-2 transmission, even for the fully vaccinated.
The move reverses a controversial announcement the agency made in May 2021 that fully vaccinated Americans could skip wearing a mask in most settings.
Unlike the increasing vaccination rates and decreasing case numbers reported in May, however, some regions of the United States are now reporting large jumps in COVID-19 case numbers. And the Delta variant as well as new evidence of transmission from breakthrough cases are largely driving these changes.
“Today we have new science related to the [D]elta variant that requires us to update the guidance on what you can do when you are fully vaccinated,” CDC Director Rochelle Walensky, MD, MPH, said during a media briefing July 27.
New evidence has emerged on breakthrough-case transmission risk, for example. “Information on the [D]elta variant from several states and other countries indicates that in rare cases, some people infected with the [D]elta variant after vaccination may be contagious and spread virus to others,” Dr. Walensky said, adding that the viral loads appear to be about the same in vaccinated and unvaccinated individuals.
“This new science is worrisome,” she said.
Even though unvaccinated people represent the vast majority of cases of transmission, Dr. Walensky said, “we thought it was important for [vaccinated] people to understand they have the potential to transmit the virus to others.”
As a result, in addition to continuing to strongly encourage everyone to get vaccinated, the CDC recommends that fully vaccinated people wear masks in public indoor settings to help prevent the spread of the Delta variant in areas with substantial or high transmission, Dr. Walensky said. “This includes schools.”
Masks in schools
The CDC is now recommending universal indoor masking for all teachers, staff, students, and visitors to K-12 schools, regardless of vaccination status. Their goal is to optimize safety and allow children to return to full-time in-person learning in the fall.
The CDC tracks substantial and high transmission rates through the agency’s COVID Data Tracker site. Substantial transmission means between 50 and 100 cases per 100,000 people reported over 7 days and high means more than 100 cases per 100,000 people.
The B.1.617.2, or Delta, variant is believed to be responsible for COVID-19 cases increasing more than 300% nationally from June 19 to July 23, 2021.
“A prudent move”
“I think it’s a prudent move. Given the dominance of the [D]elta variant and the caseloads that we are seeing rising in many locations across the United States, including in my backyard here in San Francisco,” Joe DeRisi, PhD, copresident of the Chan Zuckerberg Biohub and professor of biochemistry and biophysics at the University of California San Francisco, said in an interview.
Dr. DeRisi said he was not surprised that vaccinated people with breakthrough infections could be capable of transmitting the virus. He added that clinical testing done by the Biohub and UCSF produced a lot of data on viral load levels, “and they cover an enormous range.”
What was unexpected to him was the rapid rise of the dominant variant. “The rise of the [D]elta strain is astonishing. It’s happened so fast,” he said.
“I know it’s difficult”
Reacting to the news, Colleen Kraft, MD, said, “One of the things that we’re learning is that if we’re going to have low vaccine uptake or we have a number of people that can’t be vaccinated yet, such as children, that we really need to go back to stopping transmission, which involves mask wearing.”
“I know that it’s very difficult and people feel like we’re sliding backward,” Dr. Kraft said during a media briefing sponsored by Emory University held shortly after the CDC announcement.
She added that the CDC updated guidance seems appropriate. “I don’t think any of us really want to be in this position or want to go back to masking but…we’re finding ourselves in the same place we were a year ago, in July 2020.
“In general we just don’t want anybody to be infected even if there’s a small chance for you to be infected and there’s a small chance for you to transmit it,” said Dr. Kraft, who’s an assistant professor in the department of pathology and associate professor in the department of medicine, division of infectious diseases at Emory University School of Medicine in Atlanta.
Breakthrough transmissions
“The good news is you’re still unlikely to get critically ill if you’re vaccinated. But what has changed with the [D]elta variant is instead of being 90% plus protected from getting the virus at all, you’re probably more in the 70% to 80% range,” James T. McDeavitt, MD, told this news organization.
“So we’re seeing breakthrough infections,” said Dr. McDeavitt, executive vice president and dean of clinical affairs at Baylor College of Medicine in Houston. “We are starting to see [such people] are potentially infectious.” Even if a vaccinated person is individually much less likely to experience serious COVID-19 outcomes, “they can spread it to someone else who spreads it to someone else who is more vulnerable. It puts the more at-risk populations at further risk.”
It breaks down to individual and public health concerns. “I am fully vaccinated. I am very confident I am not going to end up in a hospital,” he said. “Now if I were unvaccinated, with the prevalence of the virus around the country, I’m probably in more danger than I’ve ever been in the course of the pandemic. The unvaccinated are really at risk right now.”
IDSA and AMA support mask change
The Infectious Diseases Society of America (IDSA) has released a statement supporting the new CDC recommendations. “To stay ahead of the spread of the highly transmissible Delta variant, IDSA also urges that in communities with moderate transmission rates, all individuals, even those who are vaccinated, wear masks in indoor public places,” stated IDSA President Barbara D. Alexander, MD, MHS.
“IDSA also supports CDC’s guidance recommending universal indoor masking for all teachers, staff, students, and visitors to K-12 schools, regardless of vaccination status, until vaccines are authorized and widely available to all children and vaccination rates are sufficient to control transmission.”
“Mask wearing will help reduce infections, prevent serious illnesses and death, limit strain on local hospitals and stave off the development of even more troubling variants,” she added.
The American Medical Association (AMA) also released a statement supporting the CDC’s policy changes.
“According to the CDC, emerging data indicates that vaccinated individuals infected with the Delta variant have similar viral loads as those who are unvaccinated and are capable of transmission,” AMA President Gerald E. Harmon, MD said in the statement.
“However, the science remains clear, the authorized vaccines remain safe and effective in preventing severe complications from COVID-19, including hospitalization and death,” he stated. “We strongly support the updated recommendations, which call for universal masking in areas of high or substantial COVID-19 transmission and in K-12 schools, to help reduce transmission of the virus. Wearing a mask is a small but important protective measure that can help us all stay safer.”
“The highest spread of cases and [most] severe outcomes are happening in places with low vaccination rates and among unvaccinated people,” Dr. Walensky said. “With the [D]elta variant, vaccinating more Americans now is more urgent than ever.”
“This moment, and the associated suffering, illness, and death, could have been avoided with higher vaccination coverage in this country,” she said.
A version of this article first appeared on Medscape.com.
The VA, California, and NYC requiring employee vaccinations
-- or, in the case of California and New York City, undergo regular testing.
The VA becomes the first federal agency to mandate COVID vaccinations for workers. In a news release, VA Secretary Denis McDonough said the mandate is “the best way to keep Veterans safe, especially as the Delta variant spreads across the country.”
VA health care personnel -- including doctors, dentists, podiatrists, optometrists, registered nurses, physician assistants, and chiropractors -- have 8 weeks to become fully vaccinated, the news release said. The New York Times reported that about 115,000 workers will be affected.
The trifecta of federal-state-municipal vaccine requirements arrived as the nation searches for ways to get more people vaccinated to tamp down the Delta variant.
Some organizations, including the military, have already said vaccinations will be required as soon as the Food and Drug Administration formally approves the vaccines, which are now given under emergency use authorizations. The FDA has said the Pfizer vaccine could receive full approval within months.
California Gov. Gavin Newsom said the requirements he announced July 27 were the first in the nation on the state level.
“As the state’s largest employer, we are leading by example and requiring all state and health care workers to show proof of vaccination or be tested regularly, and we are encouraging local governments and businesses to do the same,” he said in a news release.
California employees must provide proof of vaccination or get tested at least once a week. The policy starts Aug. 2 for state employees and Aug. 9 for state health care workers and employees of congregate facilities, such as jails or homeless shelters.
California, especially the southern part of the state, is grappling with a COVID-19 surge. The state’s daily case rate more than quadrupled, from a low of 1.9 cases per 100,000 in May to at least 9.5 cases per 100,000 today, the release said.
In New York City, Mayor Bill de Blasio had previously announced that city health and hospital employees and those working in Department of Health and Mental Hygiene clinical settings would be required to provide proof of vaccination or have regular testing.
On July 27 he expanded the rule to cover all city employees, with a Sept. 13 deadline for most of them, according to a news release.
“This is what it takes to continue our recovery for all of us while fighting back the Delta variant,” Mayor de Blasio said. “It’s going to take all of us to finally end the fight against COVID-19.”
“We have a moral responsibility to take every precaution possible to ensure we keep ourselves, our colleagues and loved ones safe,” NYC Health + Hospitals President and CEO Mitchell Katz, MD, said in the release. “Our city’s new testing requirement for city workers provides more [peace] of mind until more people get their safe and effective COVID-19 vaccine.”
NBC News reported the plan would affect about 340,000 employees.
A version of this article first appeared on WebMD.com.
-- or, in the case of California and New York City, undergo regular testing.
The VA becomes the first federal agency to mandate COVID vaccinations for workers. In a news release, VA Secretary Denis McDonough said the mandate is “the best way to keep Veterans safe, especially as the Delta variant spreads across the country.”
VA health care personnel -- including doctors, dentists, podiatrists, optometrists, registered nurses, physician assistants, and chiropractors -- have 8 weeks to become fully vaccinated, the news release said. The New York Times reported that about 115,000 workers will be affected.
The trifecta of federal-state-municipal vaccine requirements arrived as the nation searches for ways to get more people vaccinated to tamp down the Delta variant.
Some organizations, including the military, have already said vaccinations will be required as soon as the Food and Drug Administration formally approves the vaccines, which are now given under emergency use authorizations. The FDA has said the Pfizer vaccine could receive full approval within months.
California Gov. Gavin Newsom said the requirements he announced July 27 were the first in the nation on the state level.
“As the state’s largest employer, we are leading by example and requiring all state and health care workers to show proof of vaccination or be tested regularly, and we are encouraging local governments and businesses to do the same,” he said in a news release.
California employees must provide proof of vaccination or get tested at least once a week. The policy starts Aug. 2 for state employees and Aug. 9 for state health care workers and employees of congregate facilities, such as jails or homeless shelters.
California, especially the southern part of the state, is grappling with a COVID-19 surge. The state’s daily case rate more than quadrupled, from a low of 1.9 cases per 100,000 in May to at least 9.5 cases per 100,000 today, the release said.
In New York City, Mayor Bill de Blasio had previously announced that city health and hospital employees and those working in Department of Health and Mental Hygiene clinical settings would be required to provide proof of vaccination or have regular testing.
On July 27 he expanded the rule to cover all city employees, with a Sept. 13 deadline for most of them, according to a news release.
“This is what it takes to continue our recovery for all of us while fighting back the Delta variant,” Mayor de Blasio said. “It’s going to take all of us to finally end the fight against COVID-19.”
“We have a moral responsibility to take every precaution possible to ensure we keep ourselves, our colleagues and loved ones safe,” NYC Health + Hospitals President and CEO Mitchell Katz, MD, said in the release. “Our city’s new testing requirement for city workers provides more [peace] of mind until more people get their safe and effective COVID-19 vaccine.”
NBC News reported the plan would affect about 340,000 employees.
A version of this article first appeared on WebMD.com.
-- or, in the case of California and New York City, undergo regular testing.
The VA becomes the first federal agency to mandate COVID vaccinations for workers. In a news release, VA Secretary Denis McDonough said the mandate is “the best way to keep Veterans safe, especially as the Delta variant spreads across the country.”
VA health care personnel -- including doctors, dentists, podiatrists, optometrists, registered nurses, physician assistants, and chiropractors -- have 8 weeks to become fully vaccinated, the news release said. The New York Times reported that about 115,000 workers will be affected.
The trifecta of federal-state-municipal vaccine requirements arrived as the nation searches for ways to get more people vaccinated to tamp down the Delta variant.
Some organizations, including the military, have already said vaccinations will be required as soon as the Food and Drug Administration formally approves the vaccines, which are now given under emergency use authorizations. The FDA has said the Pfizer vaccine could receive full approval within months.
California Gov. Gavin Newsom said the requirements he announced July 27 were the first in the nation on the state level.
“As the state’s largest employer, we are leading by example and requiring all state and health care workers to show proof of vaccination or be tested regularly, and we are encouraging local governments and businesses to do the same,” he said in a news release.
California employees must provide proof of vaccination or get tested at least once a week. The policy starts Aug. 2 for state employees and Aug. 9 for state health care workers and employees of congregate facilities, such as jails or homeless shelters.
California, especially the southern part of the state, is grappling with a COVID-19 surge. The state’s daily case rate more than quadrupled, from a low of 1.9 cases per 100,000 in May to at least 9.5 cases per 100,000 today, the release said.
In New York City, Mayor Bill de Blasio had previously announced that city health and hospital employees and those working in Department of Health and Mental Hygiene clinical settings would be required to provide proof of vaccination or have regular testing.
On July 27 he expanded the rule to cover all city employees, with a Sept. 13 deadline for most of them, according to a news release.
“This is what it takes to continue our recovery for all of us while fighting back the Delta variant,” Mayor de Blasio said. “It’s going to take all of us to finally end the fight against COVID-19.”
“We have a moral responsibility to take every precaution possible to ensure we keep ourselves, our colleagues and loved ones safe,” NYC Health + Hospitals President and CEO Mitchell Katz, MD, said in the release. “Our city’s new testing requirement for city workers provides more [peace] of mind until more people get their safe and effective COVID-19 vaccine.”
NBC News reported the plan would affect about 340,000 employees.
A version of this article first appeared on WebMD.com.
Moving patients beyond injury and back to work
This month, JFP tackles a topic—work disability—that might, at first, seem a bit outside our usual wheelhouse of clinical review articles. Work disability is, however, a very important topic. The authors point out that “... primary care clinicians are asked to provide guidance about work activities in nearly 10% of their patient encounters; however, 25% of those clinicians thought they had little influence over work disability outcomes.” This statement suggests that we need to learn more about managing work-related disability and how to influence patients’ outcomes in a positive manner.
I suspect that we tend to be pessimistic about our ability to influence patient outcomes because we are uncertain about the best course of action. The authors of this article provide excellent information about how we can—and should—help ill and injured patients return to work.
As I read the article, I reflected on my own experience providing patients with advice about returning to work. Two points, in particular, struck a chord with me.
1. Many factors in the process are beyond our control. The physician’s role in helping patients return to work after an injury or illness is limited. The authors remind us that there are many patient and employer factors that are beyond our control and that influence patients’ successful return to work. Patient factors include motivation, mental health, and job satisfaction. Employer factors include job flexibility and disability benefits and policies. And of course, there are system factors that include laws governing work-related disability.
2. Our role, while limited, is important. By putting forth a positive attitude toward recovery and providing encouragement to patients, we can facilitate an earlier return to work.
I am cognizant of the pivotal role we can play with back injuries, a frequent cause of work disability. A great deal of excellent research over the past 20 years guides us regarding treatment and prognosis. Most back injuries are due to musculoskeletal injury and improve quickly during the first week, no matter what the therapy. By steering these patients clear of narcotics, telling them to remain as physically active as their pain will allow, and letting them know they will recover, we can pave the way for an early return to work.
Let us all take full advantage, then, of these important conversations with our patients. Armed with the strategies in this month’s article, we can increase the likelihood of our patients’ success.
This month, JFP tackles a topic—work disability—that might, at first, seem a bit outside our usual wheelhouse of clinical review articles. Work disability is, however, a very important topic. The authors point out that “... primary care clinicians are asked to provide guidance about work activities in nearly 10% of their patient encounters; however, 25% of those clinicians thought they had little influence over work disability outcomes.” This statement suggests that we need to learn more about managing work-related disability and how to influence patients’ outcomes in a positive manner.
I suspect that we tend to be pessimistic about our ability to influence patient outcomes because we are uncertain about the best course of action. The authors of this article provide excellent information about how we can—and should—help ill and injured patients return to work.
As I read the article, I reflected on my own experience providing patients with advice about returning to work. Two points, in particular, struck a chord with me.
1. Many factors in the process are beyond our control. The physician’s role in helping patients return to work after an injury or illness is limited. The authors remind us that there are many patient and employer factors that are beyond our control and that influence patients’ successful return to work. Patient factors include motivation, mental health, and job satisfaction. Employer factors include job flexibility and disability benefits and policies. And of course, there are system factors that include laws governing work-related disability.
2. Our role, while limited, is important. By putting forth a positive attitude toward recovery and providing encouragement to patients, we can facilitate an earlier return to work.
I am cognizant of the pivotal role we can play with back injuries, a frequent cause of work disability. A great deal of excellent research over the past 20 years guides us regarding treatment and prognosis. Most back injuries are due to musculoskeletal injury and improve quickly during the first week, no matter what the therapy. By steering these patients clear of narcotics, telling them to remain as physically active as their pain will allow, and letting them know they will recover, we can pave the way for an early return to work.
Let us all take full advantage, then, of these important conversations with our patients. Armed with the strategies in this month’s article, we can increase the likelihood of our patients’ success.
This month, JFP tackles a topic—work disability—that might, at first, seem a bit outside our usual wheelhouse of clinical review articles. Work disability is, however, a very important topic. The authors point out that “... primary care clinicians are asked to provide guidance about work activities in nearly 10% of their patient encounters; however, 25% of those clinicians thought they had little influence over work disability outcomes.” This statement suggests that we need to learn more about managing work-related disability and how to influence patients’ outcomes in a positive manner.
I suspect that we tend to be pessimistic about our ability to influence patient outcomes because we are uncertain about the best course of action. The authors of this article provide excellent information about how we can—and should—help ill and injured patients return to work.
As I read the article, I reflected on my own experience providing patients with advice about returning to work. Two points, in particular, struck a chord with me.
1. Many factors in the process are beyond our control. The physician’s role in helping patients return to work after an injury or illness is limited. The authors remind us that there are many patient and employer factors that are beyond our control and that influence patients’ successful return to work. Patient factors include motivation, mental health, and job satisfaction. Employer factors include job flexibility and disability benefits and policies. And of course, there are system factors that include laws governing work-related disability.
2. Our role, while limited, is important. By putting forth a positive attitude toward recovery and providing encouragement to patients, we can facilitate an earlier return to work.
I am cognizant of the pivotal role we can play with back injuries, a frequent cause of work disability. A great deal of excellent research over the past 20 years guides us regarding treatment and prognosis. Most back injuries are due to musculoskeletal injury and improve quickly during the first week, no matter what the therapy. By steering these patients clear of narcotics, telling them to remain as physically active as their pain will allow, and letting them know they will recover, we can pave the way for an early return to work.
Let us all take full advantage, then, of these important conversations with our patients. Armed with the strategies in this month’s article, we can increase the likelihood of our patients’ success.
Managing work disability to help patients return to the job
All clinicians who have patients who are employed play an essential role in work disability programs—whether or not those clinicians have received formal training in occupational health. A study found that primary care clinicians are asked to provide guidance about work activities in nearly 10% of their patient encounters; however, 25% of those clinicians thought they had little influence over work disability outcomes.1
In this article, we explain why it is important for family physicians to better manage work disability at the point of care, to help patients return to their pre-injury or pre-illness level of activity.
Why managing the duration of work disability matters
Each year, millions of American workers leave their jobs—temporarily or permanently—because of illness, injury, or the effects of a chronic condition.2 It is estimated that 893 million workdays are lost annually due to a new medical problem; an additional 527 million workdays are lost due to the impact of chronic health conditions on the ability to perform at work.3 The great majority of these lost workdays are the result of personal health conditions, not work-related problems; patients must therefore cope with the accompanying disruption of life and work.
Significant injury and illness can create a life crisis, especially when there is uncertainty about future livelihood, such as an income shortfall during a lengthy recovery. Only 40% of the US workforce is covered by a short-term disability insurance program; only 10% of low-wage and low-skill workers have this type of coverage.4 Benefits rarely replace loss of income entirely, and worker compensation insurance programs provide only partial wage replacement.
In short, work disability is destabilizing and can threaten overall well-being.5
Furthermore, the longer a person remains on temporary disability, the more likely that person is to move to a publicly funded disability program or leave the workforce entirely—thus, potentially losing future earnings and self-identity related to being a working member of society.6-8
Most of the annual cost of poor health for US employers derives from medical and wage benefits ($226 billion) and impaired or reduced employee performance ($223 billion).3 In addition, temporarily disabled workers likely account for a disproportionate share of health care costs: A study found that one-half of medical and pharmacy payments were paid out to the one-quarter of employees requiring disability benefits.9
Continue to: Benefits of staying on the job
Benefits of staying on the job. Research shows that there are physical and mental health benefits to remaining at, or returning to, work after an injury or illness.10,11 For example, in a longitudinal cohort of people with low back pain, immediate or early return to work (in 1-7 days) was associated with reduced pain and improved functioning at 3 months.12 Physicians who can guide patients safely back to normal activities, including work, minimize the physical and mental health impact of the injury or illness and avoid chronicity.13
Emphasizing the importance of health, not disease or injury
Health researchers have found that diagnosis, cause, and extent of morbidity do not adequately explain observed variability in the impact of health conditions, utilization of resources, or need for services. A wider view of the functional implications of an injury or illness is therefore required for physicians to effectively recommend disability duration.
The World Health Organization recommends a shift toward a more holistic view of health, impairment, and disability, including an emphasis on functional ability, intrinsic capacity, and environmental context.14 The American Medical Association, American College of Occupational and Environmental Medicine, and Canadian Medical Association emphasize that prolonged absence from one’s normal role can be detrimental to mental, physical, and social well-being.8 These advisory groups recommend that physicians encourage patients who are unable to work to (1) focus on restoring the rhythm of their everyday life in a stepwise fashion and (2) resume their usual responsibilities as soon as possible.
Advising a patient to focus on “what you can do,” not “what you can’t do,” might make all the difference in their return to productivity. Keeping the patient’s—as well as your own—attention focused on the positive process of recovery and documenting evidence of functional progress is an important addition to (or substitute for) detailed inquiries about pain and dysfunction.
Why does duration of disability vary so much from case to case?
Disability duration is influenced by the individual patient, employer, physician, jurisdiction, insurer or benefits structure, and access to care.15 For you to effectively manage a patient who is out of work for a medical reason, it is important to understand how nonmedical variables often influence the pace of recovery and the timing of return to work (FIGURE).
Continue to: Deficient communication
Deficient communication. Often, employers, insurers, third-party administrators, and clinicians—each a key stakeholder in disability care—are disconnected from one another, resulting in poor communication with the injured worker. Such fragmented communication can delay treatment and recovery.16 Data systems are not designed to measure the duration of disability or provide proactive notification for key stakeholders who might intervene to facilitate a patient’s recovery.
Alternatively, a collaborative approach to disability management has been shown to improve outcomes.17,18 Communication among the various professionals involved can be coordinated and expedited by a case manager or disability manager hired by the medical practice, the employer, or the insurance company.
Psychosocial and economic influences can radically affect the time it takes to return to pre-injury or pre-illness functional status. Demographic variables (age, sex, income, education, and support system) influence how a person responds to a debilitating injury or illness.19 Fear of re-injury, anxiety over the intensity of pain upon movement, worry over dependency on others, and resiliency play an important role when a patient is attempting to return to full activity.20,21
Job satisfaction has been identified as the most significant variable associated with prompt return to work.15 Work has many health-enhancing aspects, including socioeconomic status, psychosocial support, and self-identity22; however, not everyone wants, or feels ready, to go back to work even once they are physically able. Workplace variables, such as the patient–employee’s dislike of the position, coworkers, or manager, have been cited by physicians as leading barriers to returning to work at an appropriate time.23,24
Other external variables. Physicians should formulate activity prescriptions and medical restrictions based on the impact the medical condition has on the usual ability to function, as well as the anticipated impact of specific activities on the body’s natural healing process. However, Rainville and colleagues found that external variables—patient requests, employer characteristics, and jurisdiction issues—considerably influence physicians’ recommendations.20 For example, benefit structure might influence how long a patient wants to remain out of work—thus altering the requests they make to their physician. Jurisdictional characteristics, such as health care systems, state workers’ compensation departments, and payer systems, all influence a patient’s recovery timeline and time away from work.25
Continue to: What does your patient need so that they can recover?
What does your patient need so that they can recover? Individual and systemic factors must be appropriately addressed to minimize the impact that recovery from a disability has on a person’s life. Successful functional recovery enables the person to self-manage symptoms, reduce disruption-associated stress, preserve mental health, and maintain healthy relationships at home and work. An example is the patient who has successfully coped with the entire predicament that their medical condition posed and resumed their usual daily routine and responsibilities at home and at work—albeit sometimes with temporary or permanent modification necessitated by their specific condition.
Strategies that help patients stay at, or return to, their job
Physicians who anticipate, monitor, and actively manage the duration of a work disability can improve patient outcomes by minimizing life disruption, avoiding unnecessary medical care, and shortening the period of absence from work.
Key strategy: Set expectations for functional recovery early in the episode, including a forecast of how long it will take to get life and work back to normal.26,27 This is similar to discussing expectations about pain before surgery, which has been shown to decrease subsequent requests for opioids.28 It is crucial to educate the patient about timelines, define functional outcomes, and encourage them to set goals for recovery.29
Devise an evidence-based treatment plan. A fundamental way to reduce disability duration is to (1) devise a treatment plan that is evidence based and (2) take the most effective route to recovery. Given the pace with which medical research changes the understanding of diseases and treatments, it is essential to rely on up-to-date, comprehensive, independent, and authoritative resources to support your care decisions.
Aligning clinical practice with evidence-based medicine (EBM) is a good way to accomplish that goal. By definition, EBM practice guidelines recommend the safest and most effective treatments after unbiased assessment of the best available research. Increasingly, EBM is adopted to improve clinical and functional outcomes, establish national standards of care, and set criteria to evaluate clinical performance.30
Continue to: Utilize established guidelines
Utilize established guidelines. A tactic that can make it easier to discuss return to work with patients is to rely on an independent and authoritative reference set of codified disability duration guidelines, which, typically, can be searched by diagnosis, procedure, or presenting symptoms. Such guidelines provide a condition-specific expected duration of work disability in the form of number of days, with shortest, typical, and maximum durations for different levels of job demands. If necessary, you can then adjust the guideline’s estimated duration to account for the patient’s age, underlying state of health, comorbidities, and so forth.
The use of disability duration guidelines at the point of care can facilitate the process of setting early and appropriate expectations for a patient’s recovery. If a patient is confrontational in response to your recommendation on the duration of work disability, guidelines can be used to address specific objections and facilitate understanding of functional recovery.
Consider the employer’s needs. To support return-to-work efforts, your guidance about work should consider the employer’s business needs. Employers require that the patient’s abilities, restrictions, and limitations be described in concrete terms because they must decide which specific tasks are unsafe and which ones they can reasonably expect the recovering worker to perform. However, employers often fail to send information to the physician about the patient’s job tasks—such that the clinician must rely on patient self-reporting, which might be inaccurate, incomplete, or biased.15 When a patient needs protection against foreseeable harm, highlight specific activities that are currently unsafe on the recovery timeline.
Employers rely on the physician to (1) estimate what the patient can do and (2) describe work ability in clear, objective terms that both patient and employer can interpret (TABLE). For example, “no heavy lifting” might be hard for an employer to interpret; “may lift 10 pounds from the floor to the waist as many as 12 times an hour” might be applied in a more practical manner to help a patient return to work safely.31 Including specific numbers, rates, and metrics in activity restrictions can also help demonstrate improvement over the course of treatment.
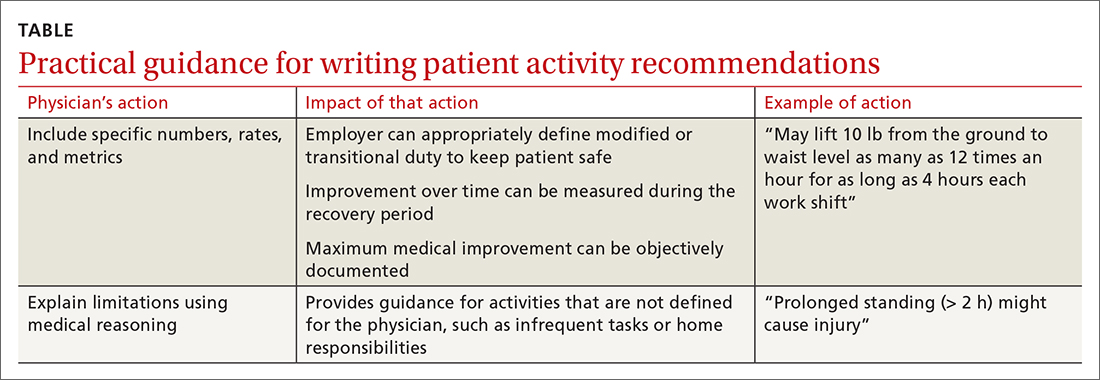
Be clear and specific on work restrictions. During recovery, it is important to tell the patient which temporary work restrictions are intended to prevent further injury or recurrence (prophylactic work restrictions) and which are an estimate of what they are able to do safely at work (capacity-based restrictions). Your written work restrictions form should be kept separate from private medical information because those restrictions will be the basis of subsequent conversations between patient and employer, who should be invited to give feedback if the guidance needs revision or clarification.
Continue to: Employer programs
Employer programs, such as modified duty, transitional duty, or early return to work programs, have been found to resolve claims faster and improve recovery outcomes.10,12 Such programs might also reduce occupational stress and improve productivity when an employee realizes that their functional abilities are matched to realistic job expectations during recovery.16 You can play an important role in empowering your patients to seek out these support programs.
What’s ahead for managing disability durations?
Work disability duration is influenced by the complex mix of biological, psychosocial, and economic variables that we have touched on here. All stakeholders involved in the recovery process should support the patient’s ability to live life with as few restrictions as possible; you play a key role in their recovery by focusing on ability, highlighting remaining capabilities, emphasizing activities that are safe to perform, and encouraging acceptance of, and adaptation to, any irrevocable losses.
This is a holistic approach that might help patients overcome the stress and anxiety associated with major life events arising from illness or injury that trigger disability benefits. Open communication and establishing a shared goal, among all involved, of the best possible outcome increases the likelihood that working patients will return to their familiar life or find another positive path forward.
Using EBM and disability duration guidelines can help decrease the length of life–work disruption by ensuring that patients are given a diagnosis, treated, and managed appropriately.32,33 Although these practices have been adopted by some physicians, health care systems, and insurers, they are not being implemented systematically and are unlikely to become ubiquitous unless they are mandated by payers or by law.
Family physicians are front-line providers for America’s workforce. They are distinctly situated to help patients achieve their best life at home and work. Improving the timeliness and quality of work guidance provided by the physician is an important way to minimize the impact of health problems on working people’s lives and livelihoods—and to help them stay employed.
CORRESPONDENCE
Kerri Wizner, MPH, 10355 Westmoor Drive, Westminster, CO 80021; [email protected].
1. Pransky G, Katz JN, Benjamin K, et al. Improving the physician role in evaluating work ability and managing disability: A survey of primary care practitioners. Disabil Rehabil. 2002;24:867-874. doi: 10.1080/09638280210142176
2. Hollenbeck K. Promoting Retention or Reemployment of Workers After a Significant Injury or Illness. Mathematica Policy Research; October 22, 2015. Accessed June 1, 2021. https://mathematica.org/publications/promoting-retention-or-reemployment-of-workers-after-a-significant-injury-or-illness
3. Poor health costs us employers $530 billion and 1.4 billion work days of absence and impaired performance according to Integrated Benefits Institute. Press release. November 15, 2018. Accessed June 1, 2021. www.ibiweb.org/poor-health-costs-us-employers-530-billion-and-1-4-billion-work-days-of-absence-and-impaired-performance
4. US Bureau of Labor Statistics. Life and disability insurance benefits: How extensive is the employer-provided safety net? BLS looks at life and disability benefits. Program Perspectives. 2010;2:7:1-4. Accessed June 8, 2021. www.bls.gov/opub/btn/archive/program-perspectives-on-life-and-disability-insurance-benefits.pdf
5. Kettlewell N, Morris RW, Ho N, et al. The differential impact of major life events on cognitive and affective wellbeing. SSM Popul Health. 2019;10:100533. doi: 10.1016/j.ssmph.2019.100533
6. Contreary K, Ben-Shalom Y, Gifford B. Using predictive analytics for early identification of short-term disability claimants who exhaust their benefits. J Occup Rehabil. 2018;28:584-596. doi: 10.1007/s10926-018-9815-5
7. Hultin H, Lindholm C, Möller J. Is there an association between long-term sick leave and disability pension and unemployment beyond the effect of health status? – A cohort study. PLoS One. 2012;7:e35614. doi: 10.1371/journal.pone.0035614
8. Canadian Medical Association. CMA policy: The treating physician’s role in helping patients return to work after an illness or injury (update 2013); 2013:1-6. Accessed June 1, 2021. https://policybase.cma.ca/documents/policypdf/PD13-05.pdf
9. Gifford B. Temporarily disabled workers account for a disproportionate share of health care payments. Health Aff (Millwood). 2017;36:245-249. doi:10.1377/hlthaff.2016.1013
10. Rueda S, Chambers L, Wilson M, et al. Association of returning to work with better health in working-aged adults: a systematic review. Am J Public Health. 2012;102:541-556. doi: 10.2105/AJPH.2011.300401
11. Modini M, Joyce S, Mykletun A, et al. The mental health benefits of employment: results of a systematic meta-review. Australas Psychiatry. 2016;24:331-336. doi: 10.1177/1039856215618523
12. Shaw WS, Nelson CC, Woiszwillo MJ, et al. Early return to work has benefits for relief of back pain and functional recovery after controlling for multiple confounds. J Occup Environ Med. 2018;60:901-910. doi: 10.1097/JOM.0000000000001380
13. Jurisic M, Bean M, Harbaugh J, et al. The personal physician’s role in helping patients with medical conditions stay at work or return to work. J Occup Environ Med. 2017;59:e125-e131. doi: 10.1097/JOM.0000000000001055
14. World Health Organization. Towards a common language for functioning, disability and health. ICF: The International Classification of Functioning, Disability and Health. 2002. Accessed June 2, 2021. www.who.int/classifications/icf/icfbeginnersguide.pdf
15. Talmage JB, Melhorn JM, Hyman MH. AMA Guides to the Evaluation of Work Ability and Return to Work. 2nd ed. American Medical Association; 2011.
16. Harrell M. Psychological factors and workforce health. In: Lee LP, Martin DW, Kancelbaum B. Occupational Medicine: A Basic Guide. American College of Occupational and Environmental Medicine; 2019. Accessed June 1, 2021. https://ohguides.acoem.org/07-psychological-factors-and-workforce-health-stress-management
17. Wickizer TM, Franklin GM, Fulton-Kehoe D. Innovations in occupational health care delivery can prevent entry into permanent disability: 8-year follow-up of the Washington State Centers for Occupational Health and Education. Med Care. 2018;56:1018-1023. doi: 10.1097/MLR.0000000000000991
18. Christian J, Wickizer T, Burton K. Implementing a community-focused health & work service. SSDI Solution Initiative, Fiscal Institute of the Committee for a Responsible Federal Budget. May 2019. Accessed June 2, 2021. www.crfb.org/sites/default/files/Implementing_a_Community-Focused_HWS.pdf
19. Macpherson RA, Koehoorn M, Fan J, et al. Do differences in work disability duration between men and women vary by province in Canada? J Occup Rehabil. 2018;29:560-568. doi: 10.1007/s10926-018-9819-1
20. Rainville J, Pransky G, Indahl A, et al. The physician as disability advisor for patients with musculoskeletal complaints. Spine (Phila Pa 1976). 2005;30:2579-2584. doi: 10.1097/01.brs.0000186589.69382.1d
21. Jay K, Thorsen SV, Sundstrup E, et al. Fear avoidance beliefs and risk of long-term sickness absence: prospective cohort study among workers with musculoskeletal pain. Pain Res Treat. 2018;2018:8347120. doi: 10.1155/2018/8347120
22. Burgard S, Lin KY. Bad jobs, bad health? How work and working conditions contribute to health disparities. Am Behav Sci. 2013;57:10.1177/0002764213487347. doi: 10.1177/0002764213487347
23. Soklaridis S, Tang G, Cartmill C, et al. “Can you go back to work?” Family physicians’ experiences with assessing patients’ functional ability to return to work. Can Fam Physician. 2011;57:202-209.
24. Peters SE, Truong AP, Johnston V. Stakeholders identify similar barriers but different strategies to facilitate return-to-work: a vignette of a worker with an upper extremity condition. Work. 2018;59:401-412. doi: 10.3233/WOR-182692
25. Shraim M, Cifuentes M, Willetts JL, et al. Regional socioeconomic disparities in outcomes for workers with low back pain in the United States. Am J Ind Med. 2017;60:472-483. doi: 10.1002/ajim.22712
26. Hill JC, Fritz JM. Psychosocial influences on low back pain, disability, and response to treatment. Phys Ther. 2011;91:712-721. doi: 10.2522/ptj.20100280
27. Aasdahl L, Pape K, Jensen C, et al. Associations between the readiness for return to work scale and return to work: a prospective study. J Occup Rehabil. 2018;28:97-106. doi: 10.1007/s10926-017-9705-2
28. Pino C, Covington M. Prescription of opioids for acute pain in opioid naïve patients. UpToDate Web site. February 9, 2021. Accessed June 2, 2021. www.uptodate.com/contents/prescription-of-opioids-for-acute-pain-in-opioid-naive-patients
29. Cancelliere C, Donovan J, Stochkendahl MJ, et al. Factors affecting return to work after injury or illness: best evidence synthesis of systematic reviews. Chiropr Man Therap. 2016;24:32. doi: 10.1186/s12998-016-0113-z
30. Lewis SJ, Orland BI. The importance and impact of evidence-based medicine. J Manag Care Pharm. 2004;10(5 suppl A):S3-S5. doi: 10.18553/jmcp.2004.10.S5-A.S3
31. Rupe KL. Work restrictions: documenting a patient’s return to work. Nurse Pract. 2010;35:49-53. doi: 10.1097/01.NPR.0000388901.49604.a8
32. Owens JD, Hegmann KT, Thiese MS, et al. Impacts of adherence to evidence-based medicine guidelines for the management of acute low back pain on costs of worker's compensation claims. J Occup Environ Med. 2019;61:445-452. doi: 10.1097/JOM.0000000000001593
33. Gaspar FW, Kownacki R, Zaidel CS, et al. Reducing disability durations and medical costs for patients with a carpal tunnel release surgery through the use of opioid prescribing guidelines. J Occup Environ Med. 2017;59:1180-1187. doi: 10.1097/JOM.0000000000001168
All clinicians who have patients who are employed play an essential role in work disability programs—whether or not those clinicians have received formal training in occupational health. A study found that primary care clinicians are asked to provide guidance about work activities in nearly 10% of their patient encounters; however, 25% of those clinicians thought they had little influence over work disability outcomes.1
In this article, we explain why it is important for family physicians to better manage work disability at the point of care, to help patients return to their pre-injury or pre-illness level of activity.
Why managing the duration of work disability matters
Each year, millions of American workers leave their jobs—temporarily or permanently—because of illness, injury, or the effects of a chronic condition.2 It is estimated that 893 million workdays are lost annually due to a new medical problem; an additional 527 million workdays are lost due to the impact of chronic health conditions on the ability to perform at work.3 The great majority of these lost workdays are the result of personal health conditions, not work-related problems; patients must therefore cope with the accompanying disruption of life and work.
Significant injury and illness can create a life crisis, especially when there is uncertainty about future livelihood, such as an income shortfall during a lengthy recovery. Only 40% of the US workforce is covered by a short-term disability insurance program; only 10% of low-wage and low-skill workers have this type of coverage.4 Benefits rarely replace loss of income entirely, and worker compensation insurance programs provide only partial wage replacement.
In short, work disability is destabilizing and can threaten overall well-being.5
Furthermore, the longer a person remains on temporary disability, the more likely that person is to move to a publicly funded disability program or leave the workforce entirely—thus, potentially losing future earnings and self-identity related to being a working member of society.6-8
Most of the annual cost of poor health for US employers derives from medical and wage benefits ($226 billion) and impaired or reduced employee performance ($223 billion).3 In addition, temporarily disabled workers likely account for a disproportionate share of health care costs: A study found that one-half of medical and pharmacy payments were paid out to the one-quarter of employees requiring disability benefits.9
Continue to: Benefits of staying on the job
Benefits of staying on the job. Research shows that there are physical and mental health benefits to remaining at, or returning to, work after an injury or illness.10,11 For example, in a longitudinal cohort of people with low back pain, immediate or early return to work (in 1-7 days) was associated with reduced pain and improved functioning at 3 months.12 Physicians who can guide patients safely back to normal activities, including work, minimize the physical and mental health impact of the injury or illness and avoid chronicity.13
Emphasizing the importance of health, not disease or injury
Health researchers have found that diagnosis, cause, and extent of morbidity do not adequately explain observed variability in the impact of health conditions, utilization of resources, or need for services. A wider view of the functional implications of an injury or illness is therefore required for physicians to effectively recommend disability duration.
The World Health Organization recommends a shift toward a more holistic view of health, impairment, and disability, including an emphasis on functional ability, intrinsic capacity, and environmental context.14 The American Medical Association, American College of Occupational and Environmental Medicine, and Canadian Medical Association emphasize that prolonged absence from one’s normal role can be detrimental to mental, physical, and social well-being.8 These advisory groups recommend that physicians encourage patients who are unable to work to (1) focus on restoring the rhythm of their everyday life in a stepwise fashion and (2) resume their usual responsibilities as soon as possible.
Advising a patient to focus on “what you can do,” not “what you can’t do,” might make all the difference in their return to productivity. Keeping the patient’s—as well as your own—attention focused on the positive process of recovery and documenting evidence of functional progress is an important addition to (or substitute for) detailed inquiries about pain and dysfunction.
Why does duration of disability vary so much from case to case?
Disability duration is influenced by the individual patient, employer, physician, jurisdiction, insurer or benefits structure, and access to care.15 For you to effectively manage a patient who is out of work for a medical reason, it is important to understand how nonmedical variables often influence the pace of recovery and the timing of return to work (FIGURE).

Continue to: Deficient communication
Deficient communication. Often, employers, insurers, third-party administrators, and clinicians—each a key stakeholder in disability care—are disconnected from one another, resulting in poor communication with the injured worker. Such fragmented communication can delay treatment and recovery.16 Data systems are not designed to measure the duration of disability or provide proactive notification for key stakeholders who might intervene to facilitate a patient’s recovery.
Alternatively, a collaborative approach to disability management has been shown to improve outcomes.17,18 Communication among the various professionals involved can be coordinated and expedited by a case manager or disability manager hired by the medical practice, the employer, or the insurance company.
Psychosocial and economic influences can radically affect the time it takes to return to pre-injury or pre-illness functional status. Demographic variables (age, sex, income, education, and support system) influence how a person responds to a debilitating injury or illness.19 Fear of re-injury, anxiety over the intensity of pain upon movement, worry over dependency on others, and resiliency play an important role when a patient is attempting to return to full activity.20,21
Job satisfaction has been identified as the most significant variable associated with prompt return to work.15 Work has many health-enhancing aspects, including socioeconomic status, psychosocial support, and self-identity22; however, not everyone wants, or feels ready, to go back to work even once they are physically able. Workplace variables, such as the patient–employee’s dislike of the position, coworkers, or manager, have been cited by physicians as leading barriers to returning to work at an appropriate time.23,24
Other external variables. Physicians should formulate activity prescriptions and medical restrictions based on the impact the medical condition has on the usual ability to function, as well as the anticipated impact of specific activities on the body’s natural healing process. However, Rainville and colleagues found that external variables—patient requests, employer characteristics, and jurisdiction issues—considerably influence physicians’ recommendations.20 For example, benefit structure might influence how long a patient wants to remain out of work—thus altering the requests they make to their physician. Jurisdictional characteristics, such as health care systems, state workers’ compensation departments, and payer systems, all influence a patient’s recovery timeline and time away from work.25
Continue to: What does your patient need so that they can recover?
What does your patient need so that they can recover? Individual and systemic factors must be appropriately addressed to minimize the impact that recovery from a disability has on a person’s life. Successful functional recovery enables the person to self-manage symptoms, reduce disruption-associated stress, preserve mental health, and maintain healthy relationships at home and work. An example is the patient who has successfully coped with the entire predicament that their medical condition posed and resumed their usual daily routine and responsibilities at home and at work—albeit sometimes with temporary or permanent modification necessitated by their specific condition.
Strategies that help patients stay at, or return to, their job
Physicians who anticipate, monitor, and actively manage the duration of a work disability can improve patient outcomes by minimizing life disruption, avoiding unnecessary medical care, and shortening the period of absence from work.
Key strategy: Set expectations for functional recovery early in the episode, including a forecast of how long it will take to get life and work back to normal.26,27 This is similar to discussing expectations about pain before surgery, which has been shown to decrease subsequent requests for opioids.28 It is crucial to educate the patient about timelines, define functional outcomes, and encourage them to set goals for recovery.29
Devise an evidence-based treatment plan. A fundamental way to reduce disability duration is to (1) devise a treatment plan that is evidence based and (2) take the most effective route to recovery. Given the pace with which medical research changes the understanding of diseases and treatments, it is essential to rely on up-to-date, comprehensive, independent, and authoritative resources to support your care decisions.
Aligning clinical practice with evidence-based medicine (EBM) is a good way to accomplish that goal. By definition, EBM practice guidelines recommend the safest and most effective treatments after unbiased assessment of the best available research. Increasingly, EBM is adopted to improve clinical and functional outcomes, establish national standards of care, and set criteria to evaluate clinical performance.30
Continue to: Utilize established guidelines
Utilize established guidelines. A tactic that can make it easier to discuss return to work with patients is to rely on an independent and authoritative reference set of codified disability duration guidelines, which, typically, can be searched by diagnosis, procedure, or presenting symptoms. Such guidelines provide a condition-specific expected duration of work disability in the form of number of days, with shortest, typical, and maximum durations for different levels of job demands. If necessary, you can then adjust the guideline’s estimated duration to account for the patient’s age, underlying state of health, comorbidities, and so forth.
The use of disability duration guidelines at the point of care can facilitate the process of setting early and appropriate expectations for a patient’s recovery. If a patient is confrontational in response to your recommendation on the duration of work disability, guidelines can be used to address specific objections and facilitate understanding of functional recovery.
Consider the employer’s needs. To support return-to-work efforts, your guidance about work should consider the employer’s business needs. Employers require that the patient’s abilities, restrictions, and limitations be described in concrete terms because they must decide which specific tasks are unsafe and which ones they can reasonably expect the recovering worker to perform. However, employers often fail to send information to the physician about the patient’s job tasks—such that the clinician must rely on patient self-reporting, which might be inaccurate, incomplete, or biased.15 When a patient needs protection against foreseeable harm, highlight specific activities that are currently unsafe on the recovery timeline.
Employers rely on the physician to (1) estimate what the patient can do and (2) describe work ability in clear, objective terms that both patient and employer can interpret (TABLE). For example, “no heavy lifting” might be hard for an employer to interpret; “may lift 10 pounds from the floor to the waist as many as 12 times an hour” might be applied in a more practical manner to help a patient return to work safely.31 Including specific numbers, rates, and metrics in activity restrictions can also help demonstrate improvement over the course of treatment.

Be clear and specific on work restrictions. During recovery, it is important to tell the patient which temporary work restrictions are intended to prevent further injury or recurrence (prophylactic work restrictions) and which are an estimate of what they are able to do safely at work (capacity-based restrictions). Your written work restrictions form should be kept separate from private medical information because those restrictions will be the basis of subsequent conversations between patient and employer, who should be invited to give feedback if the guidance needs revision or clarification.
Continue to: Employer programs
Employer programs, such as modified duty, transitional duty, or early return to work programs, have been found to resolve claims faster and improve recovery outcomes.10,12 Such programs might also reduce occupational stress and improve productivity when an employee realizes that their functional abilities are matched to realistic job expectations during recovery.16 You can play an important role in empowering your patients to seek out these support programs.
What’s ahead for managing disability durations?
Work disability duration is influenced by the complex mix of biological, psychosocial, and economic variables that we have touched on here. All stakeholders involved in the recovery process should support the patient’s ability to live life with as few restrictions as possible; you play a key role in their recovery by focusing on ability, highlighting remaining capabilities, emphasizing activities that are safe to perform, and encouraging acceptance of, and adaptation to, any irrevocable losses.
This is a holistic approach that might help patients overcome the stress and anxiety associated with major life events arising from illness or injury that trigger disability benefits. Open communication and establishing a shared goal, among all involved, of the best possible outcome increases the likelihood that working patients will return to their familiar life or find another positive path forward.
Using EBM and disability duration guidelines can help decrease the length of life–work disruption by ensuring that patients are given a diagnosis, treated, and managed appropriately.32,33 Although these practices have been adopted by some physicians, health care systems, and insurers, they are not being implemented systematically and are unlikely to become ubiquitous unless they are mandated by payers or by law.
Family physicians are front-line providers for America’s workforce. They are distinctly situated to help patients achieve their best life at home and work. Improving the timeliness and quality of work guidance provided by the physician is an important way to minimize the impact of health problems on working people’s lives and livelihoods—and to help them stay employed.
CORRESPONDENCE
Kerri Wizner, MPH, 10355 Westmoor Drive, Westminster, CO 80021; [email protected].
All clinicians who have patients who are employed play an essential role in work disability programs—whether or not those clinicians have received formal training in occupational health. A study found that primary care clinicians are asked to provide guidance about work activities in nearly 10% of their patient encounters; however, 25% of those clinicians thought they had little influence over work disability outcomes.1
In this article, we explain why it is important for family physicians to better manage work disability at the point of care, to help patients return to their pre-injury or pre-illness level of activity.
Why managing the duration of work disability matters
Each year, millions of American workers leave their jobs—temporarily or permanently—because of illness, injury, or the effects of a chronic condition.2 It is estimated that 893 million workdays are lost annually due to a new medical problem; an additional 527 million workdays are lost due to the impact of chronic health conditions on the ability to perform at work.3 The great majority of these lost workdays are the result of personal health conditions, not work-related problems; patients must therefore cope with the accompanying disruption of life and work.
Significant injury and illness can create a life crisis, especially when there is uncertainty about future livelihood, such as an income shortfall during a lengthy recovery. Only 40% of the US workforce is covered by a short-term disability insurance program; only 10% of low-wage and low-skill workers have this type of coverage.4 Benefits rarely replace loss of income entirely, and worker compensation insurance programs provide only partial wage replacement.
In short, work disability is destabilizing and can threaten overall well-being.5
Furthermore, the longer a person remains on temporary disability, the more likely that person is to move to a publicly funded disability program or leave the workforce entirely—thus, potentially losing future earnings and self-identity related to being a working member of society.6-8
Most of the annual cost of poor health for US employers derives from medical and wage benefits ($226 billion) and impaired or reduced employee performance ($223 billion).3 In addition, temporarily disabled workers likely account for a disproportionate share of health care costs: A study found that one-half of medical and pharmacy payments were paid out to the one-quarter of employees requiring disability benefits.9
Continue to: Benefits of staying on the job
Benefits of staying on the job. Research shows that there are physical and mental health benefits to remaining at, or returning to, work after an injury or illness.10,11 For example, in a longitudinal cohort of people with low back pain, immediate or early return to work (in 1-7 days) was associated with reduced pain and improved functioning at 3 months.12 Physicians who can guide patients safely back to normal activities, including work, minimize the physical and mental health impact of the injury or illness and avoid chronicity.13
Emphasizing the importance of health, not disease or injury
Health researchers have found that diagnosis, cause, and extent of morbidity do not adequately explain observed variability in the impact of health conditions, utilization of resources, or need for services. A wider view of the functional implications of an injury or illness is therefore required for physicians to effectively recommend disability duration.
The World Health Organization recommends a shift toward a more holistic view of health, impairment, and disability, including an emphasis on functional ability, intrinsic capacity, and environmental context.14 The American Medical Association, American College of Occupational and Environmental Medicine, and Canadian Medical Association emphasize that prolonged absence from one’s normal role can be detrimental to mental, physical, and social well-being.8 These advisory groups recommend that physicians encourage patients who are unable to work to (1) focus on restoring the rhythm of their everyday life in a stepwise fashion and (2) resume their usual responsibilities as soon as possible.
Advising a patient to focus on “what you can do,” not “what you can’t do,” might make all the difference in their return to productivity. Keeping the patient’s—as well as your own—attention focused on the positive process of recovery and documenting evidence of functional progress is an important addition to (or substitute for) detailed inquiries about pain and dysfunction.
Why does duration of disability vary so much from case to case?
Disability duration is influenced by the individual patient, employer, physician, jurisdiction, insurer or benefits structure, and access to care.15 For you to effectively manage a patient who is out of work for a medical reason, it is important to understand how nonmedical variables often influence the pace of recovery and the timing of return to work (FIGURE).

Continue to: Deficient communication
Deficient communication. Often, employers, insurers, third-party administrators, and clinicians—each a key stakeholder in disability care—are disconnected from one another, resulting in poor communication with the injured worker. Such fragmented communication can delay treatment and recovery.16 Data systems are not designed to measure the duration of disability or provide proactive notification for key stakeholders who might intervene to facilitate a patient’s recovery.
Alternatively, a collaborative approach to disability management has been shown to improve outcomes.17,18 Communication among the various professionals involved can be coordinated and expedited by a case manager or disability manager hired by the medical practice, the employer, or the insurance company.
Psychosocial and economic influences can radically affect the time it takes to return to pre-injury or pre-illness functional status. Demographic variables (age, sex, income, education, and support system) influence how a person responds to a debilitating injury or illness.19 Fear of re-injury, anxiety over the intensity of pain upon movement, worry over dependency on others, and resiliency play an important role when a patient is attempting to return to full activity.20,21
Job satisfaction has been identified as the most significant variable associated with prompt return to work.15 Work has many health-enhancing aspects, including socioeconomic status, psychosocial support, and self-identity22; however, not everyone wants, or feels ready, to go back to work even once they are physically able. Workplace variables, such as the patient–employee’s dislike of the position, coworkers, or manager, have been cited by physicians as leading barriers to returning to work at an appropriate time.23,24
Other external variables. Physicians should formulate activity prescriptions and medical restrictions based on the impact the medical condition has on the usual ability to function, as well as the anticipated impact of specific activities on the body’s natural healing process. However, Rainville and colleagues found that external variables—patient requests, employer characteristics, and jurisdiction issues—considerably influence physicians’ recommendations.20 For example, benefit structure might influence how long a patient wants to remain out of work—thus altering the requests they make to their physician. Jurisdictional characteristics, such as health care systems, state workers’ compensation departments, and payer systems, all influence a patient’s recovery timeline and time away from work.25
Continue to: What does your patient need so that they can recover?
What does your patient need so that they can recover? Individual and systemic factors must be appropriately addressed to minimize the impact that recovery from a disability has on a person’s life. Successful functional recovery enables the person to self-manage symptoms, reduce disruption-associated stress, preserve mental health, and maintain healthy relationships at home and work. An example is the patient who has successfully coped with the entire predicament that their medical condition posed and resumed their usual daily routine and responsibilities at home and at work—albeit sometimes with temporary or permanent modification necessitated by their specific condition.
Strategies that help patients stay at, or return to, their job
Physicians who anticipate, monitor, and actively manage the duration of a work disability can improve patient outcomes by minimizing life disruption, avoiding unnecessary medical care, and shortening the period of absence from work.
Key strategy: Set expectations for functional recovery early in the episode, including a forecast of how long it will take to get life and work back to normal.26,27 This is similar to discussing expectations about pain before surgery, which has been shown to decrease subsequent requests for opioids.28 It is crucial to educate the patient about timelines, define functional outcomes, and encourage them to set goals for recovery.29
Devise an evidence-based treatment plan. A fundamental way to reduce disability duration is to (1) devise a treatment plan that is evidence based and (2) take the most effective route to recovery. Given the pace with which medical research changes the understanding of diseases and treatments, it is essential to rely on up-to-date, comprehensive, independent, and authoritative resources to support your care decisions.
Aligning clinical practice with evidence-based medicine (EBM) is a good way to accomplish that goal. By definition, EBM practice guidelines recommend the safest and most effective treatments after unbiased assessment of the best available research. Increasingly, EBM is adopted to improve clinical and functional outcomes, establish national standards of care, and set criteria to evaluate clinical performance.30
Continue to: Utilize established guidelines
Utilize established guidelines. A tactic that can make it easier to discuss return to work with patients is to rely on an independent and authoritative reference set of codified disability duration guidelines, which, typically, can be searched by diagnosis, procedure, or presenting symptoms. Such guidelines provide a condition-specific expected duration of work disability in the form of number of days, with shortest, typical, and maximum durations for different levels of job demands. If necessary, you can then adjust the guideline’s estimated duration to account for the patient’s age, underlying state of health, comorbidities, and so forth.
The use of disability duration guidelines at the point of care can facilitate the process of setting early and appropriate expectations for a patient’s recovery. If a patient is confrontational in response to your recommendation on the duration of work disability, guidelines can be used to address specific objections and facilitate understanding of functional recovery.
Consider the employer’s needs. To support return-to-work efforts, your guidance about work should consider the employer’s business needs. Employers require that the patient’s abilities, restrictions, and limitations be described in concrete terms because they must decide which specific tasks are unsafe and which ones they can reasonably expect the recovering worker to perform. However, employers often fail to send information to the physician about the patient’s job tasks—such that the clinician must rely on patient self-reporting, which might be inaccurate, incomplete, or biased.15 When a patient needs protection against foreseeable harm, highlight specific activities that are currently unsafe on the recovery timeline.
Employers rely on the physician to (1) estimate what the patient can do and (2) describe work ability in clear, objective terms that both patient and employer can interpret (TABLE). For example, “no heavy lifting” might be hard for an employer to interpret; “may lift 10 pounds from the floor to the waist as many as 12 times an hour” might be applied in a more practical manner to help a patient return to work safely.31 Including specific numbers, rates, and metrics in activity restrictions can also help demonstrate improvement over the course of treatment.

Be clear and specific on work restrictions. During recovery, it is important to tell the patient which temporary work restrictions are intended to prevent further injury or recurrence (prophylactic work restrictions) and which are an estimate of what they are able to do safely at work (capacity-based restrictions). Your written work restrictions form should be kept separate from private medical information because those restrictions will be the basis of subsequent conversations between patient and employer, who should be invited to give feedback if the guidance needs revision or clarification.
Continue to: Employer programs
Employer programs, such as modified duty, transitional duty, or early return to work programs, have been found to resolve claims faster and improve recovery outcomes.10,12 Such programs might also reduce occupational stress and improve productivity when an employee realizes that their functional abilities are matched to realistic job expectations during recovery.16 You can play an important role in empowering your patients to seek out these support programs.
What’s ahead for managing disability durations?
Work disability duration is influenced by the complex mix of biological, psychosocial, and economic variables that we have touched on here. All stakeholders involved in the recovery process should support the patient’s ability to live life with as few restrictions as possible; you play a key role in their recovery by focusing on ability, highlighting remaining capabilities, emphasizing activities that are safe to perform, and encouraging acceptance of, and adaptation to, any irrevocable losses.
This is a holistic approach that might help patients overcome the stress and anxiety associated with major life events arising from illness or injury that trigger disability benefits. Open communication and establishing a shared goal, among all involved, of the best possible outcome increases the likelihood that working patients will return to their familiar life or find another positive path forward.
Using EBM and disability duration guidelines can help decrease the length of life–work disruption by ensuring that patients are given a diagnosis, treated, and managed appropriately.32,33 Although these practices have been adopted by some physicians, health care systems, and insurers, they are not being implemented systematically and are unlikely to become ubiquitous unless they are mandated by payers or by law.
Family physicians are front-line providers for America’s workforce. They are distinctly situated to help patients achieve their best life at home and work. Improving the timeliness and quality of work guidance provided by the physician is an important way to minimize the impact of health problems on working people’s lives and livelihoods—and to help them stay employed.
CORRESPONDENCE
Kerri Wizner, MPH, 10355 Westmoor Drive, Westminster, CO 80021; [email protected].
1. Pransky G, Katz JN, Benjamin K, et al. Improving the physician role in evaluating work ability and managing disability: A survey of primary care practitioners. Disabil Rehabil. 2002;24:867-874. doi: 10.1080/09638280210142176
2. Hollenbeck K. Promoting Retention or Reemployment of Workers After a Significant Injury or Illness. Mathematica Policy Research; October 22, 2015. Accessed June 1, 2021. https://mathematica.org/publications/promoting-retention-or-reemployment-of-workers-after-a-significant-injury-or-illness
3. Poor health costs us employers $530 billion and 1.4 billion work days of absence and impaired performance according to Integrated Benefits Institute. Press release. November 15, 2018. Accessed June 1, 2021. www.ibiweb.org/poor-health-costs-us-employers-530-billion-and-1-4-billion-work-days-of-absence-and-impaired-performance
4. US Bureau of Labor Statistics. Life and disability insurance benefits: How extensive is the employer-provided safety net? BLS looks at life and disability benefits. Program Perspectives. 2010;2:7:1-4. Accessed June 8, 2021. www.bls.gov/opub/btn/archive/program-perspectives-on-life-and-disability-insurance-benefits.pdf
5. Kettlewell N, Morris RW, Ho N, et al. The differential impact of major life events on cognitive and affective wellbeing. SSM Popul Health. 2019;10:100533. doi: 10.1016/j.ssmph.2019.100533
6. Contreary K, Ben-Shalom Y, Gifford B. Using predictive analytics for early identification of short-term disability claimants who exhaust their benefits. J Occup Rehabil. 2018;28:584-596. doi: 10.1007/s10926-018-9815-5
7. Hultin H, Lindholm C, Möller J. Is there an association between long-term sick leave and disability pension and unemployment beyond the effect of health status? – A cohort study. PLoS One. 2012;7:e35614. doi: 10.1371/journal.pone.0035614
8. Canadian Medical Association. CMA policy: The treating physician’s role in helping patients return to work after an illness or injury (update 2013); 2013:1-6. Accessed June 1, 2021. https://policybase.cma.ca/documents/policypdf/PD13-05.pdf
9. Gifford B. Temporarily disabled workers account for a disproportionate share of health care payments. Health Aff (Millwood). 2017;36:245-249. doi:10.1377/hlthaff.2016.1013
10. Rueda S, Chambers L, Wilson M, et al. Association of returning to work with better health in working-aged adults: a systematic review. Am J Public Health. 2012;102:541-556. doi: 10.2105/AJPH.2011.300401
11. Modini M, Joyce S, Mykletun A, et al. The mental health benefits of employment: results of a systematic meta-review. Australas Psychiatry. 2016;24:331-336. doi: 10.1177/1039856215618523
12. Shaw WS, Nelson CC, Woiszwillo MJ, et al. Early return to work has benefits for relief of back pain and functional recovery after controlling for multiple confounds. J Occup Environ Med. 2018;60:901-910. doi: 10.1097/JOM.0000000000001380
13. Jurisic M, Bean M, Harbaugh J, et al. The personal physician’s role in helping patients with medical conditions stay at work or return to work. J Occup Environ Med. 2017;59:e125-e131. doi: 10.1097/JOM.0000000000001055
14. World Health Organization. Towards a common language for functioning, disability and health. ICF: The International Classification of Functioning, Disability and Health. 2002. Accessed June 2, 2021. www.who.int/classifications/icf/icfbeginnersguide.pdf
15. Talmage JB, Melhorn JM, Hyman MH. AMA Guides to the Evaluation of Work Ability and Return to Work. 2nd ed. American Medical Association; 2011.
16. Harrell M. Psychological factors and workforce health. In: Lee LP, Martin DW, Kancelbaum B. Occupational Medicine: A Basic Guide. American College of Occupational and Environmental Medicine; 2019. Accessed June 1, 2021. https://ohguides.acoem.org/07-psychological-factors-and-workforce-health-stress-management
17. Wickizer TM, Franklin GM, Fulton-Kehoe D. Innovations in occupational health care delivery can prevent entry into permanent disability: 8-year follow-up of the Washington State Centers for Occupational Health and Education. Med Care. 2018;56:1018-1023. doi: 10.1097/MLR.0000000000000991
18. Christian J, Wickizer T, Burton K. Implementing a community-focused health & work service. SSDI Solution Initiative, Fiscal Institute of the Committee for a Responsible Federal Budget. May 2019. Accessed June 2, 2021. www.crfb.org/sites/default/files/Implementing_a_Community-Focused_HWS.pdf
19. Macpherson RA, Koehoorn M, Fan J, et al. Do differences in work disability duration between men and women vary by province in Canada? J Occup Rehabil. 2018;29:560-568. doi: 10.1007/s10926-018-9819-1
20. Rainville J, Pransky G, Indahl A, et al. The physician as disability advisor for patients with musculoskeletal complaints. Spine (Phila Pa 1976). 2005;30:2579-2584. doi: 10.1097/01.brs.0000186589.69382.1d
21. Jay K, Thorsen SV, Sundstrup E, et al. Fear avoidance beliefs and risk of long-term sickness absence: prospective cohort study among workers with musculoskeletal pain. Pain Res Treat. 2018;2018:8347120. doi: 10.1155/2018/8347120
22. Burgard S, Lin KY. Bad jobs, bad health? How work and working conditions contribute to health disparities. Am Behav Sci. 2013;57:10.1177/0002764213487347. doi: 10.1177/0002764213487347
23. Soklaridis S, Tang G, Cartmill C, et al. “Can you go back to work?” Family physicians’ experiences with assessing patients’ functional ability to return to work. Can Fam Physician. 2011;57:202-209.
24. Peters SE, Truong AP, Johnston V. Stakeholders identify similar barriers but different strategies to facilitate return-to-work: a vignette of a worker with an upper extremity condition. Work. 2018;59:401-412. doi: 10.3233/WOR-182692
25. Shraim M, Cifuentes M, Willetts JL, et al. Regional socioeconomic disparities in outcomes for workers with low back pain in the United States. Am J Ind Med. 2017;60:472-483. doi: 10.1002/ajim.22712
26. Hill JC, Fritz JM. Psychosocial influences on low back pain, disability, and response to treatment. Phys Ther. 2011;91:712-721. doi: 10.2522/ptj.20100280
27. Aasdahl L, Pape K, Jensen C, et al. Associations between the readiness for return to work scale and return to work: a prospective study. J Occup Rehabil. 2018;28:97-106. doi: 10.1007/s10926-017-9705-2
28. Pino C, Covington M. Prescription of opioids for acute pain in opioid naïve patients. UpToDate Web site. February 9, 2021. Accessed June 2, 2021. www.uptodate.com/contents/prescription-of-opioids-for-acute-pain-in-opioid-naive-patients
29. Cancelliere C, Donovan J, Stochkendahl MJ, et al. Factors affecting return to work after injury or illness: best evidence synthesis of systematic reviews. Chiropr Man Therap. 2016;24:32. doi: 10.1186/s12998-016-0113-z
30. Lewis SJ, Orland BI. The importance and impact of evidence-based medicine. J Manag Care Pharm. 2004;10(5 suppl A):S3-S5. doi: 10.18553/jmcp.2004.10.S5-A.S3
31. Rupe KL. Work restrictions: documenting a patient’s return to work. Nurse Pract. 2010;35:49-53. doi: 10.1097/01.NPR.0000388901.49604.a8
32. Owens JD, Hegmann KT, Thiese MS, et al. Impacts of adherence to evidence-based medicine guidelines for the management of acute low back pain on costs of worker's compensation claims. J Occup Environ Med. 2019;61:445-452. doi: 10.1097/JOM.0000000000001593
33. Gaspar FW, Kownacki R, Zaidel CS, et al. Reducing disability durations and medical costs for patients with a carpal tunnel release surgery through the use of opioid prescribing guidelines. J Occup Environ Med. 2017;59:1180-1187. doi: 10.1097/JOM.0000000000001168
1. Pransky G, Katz JN, Benjamin K, et al. Improving the physician role in evaluating work ability and managing disability: A survey of primary care practitioners. Disabil Rehabil. 2002;24:867-874. doi: 10.1080/09638280210142176
2. Hollenbeck K. Promoting Retention or Reemployment of Workers After a Significant Injury or Illness. Mathematica Policy Research; October 22, 2015. Accessed June 1, 2021. https://mathematica.org/publications/promoting-retention-or-reemployment-of-workers-after-a-significant-injury-or-illness
3. Poor health costs us employers $530 billion and 1.4 billion work days of absence and impaired performance according to Integrated Benefits Institute. Press release. November 15, 2018. Accessed June 1, 2021. www.ibiweb.org/poor-health-costs-us-employers-530-billion-and-1-4-billion-work-days-of-absence-and-impaired-performance
4. US Bureau of Labor Statistics. Life and disability insurance benefits: How extensive is the employer-provided safety net? BLS looks at life and disability benefits. Program Perspectives. 2010;2:7:1-4. Accessed June 8, 2021. www.bls.gov/opub/btn/archive/program-perspectives-on-life-and-disability-insurance-benefits.pdf
5. Kettlewell N, Morris RW, Ho N, et al. The differential impact of major life events on cognitive and affective wellbeing. SSM Popul Health. 2019;10:100533. doi: 10.1016/j.ssmph.2019.100533
6. Contreary K, Ben-Shalom Y, Gifford B. Using predictive analytics for early identification of short-term disability claimants who exhaust their benefits. J Occup Rehabil. 2018;28:584-596. doi: 10.1007/s10926-018-9815-5
7. Hultin H, Lindholm C, Möller J. Is there an association between long-term sick leave and disability pension and unemployment beyond the effect of health status? – A cohort study. PLoS One. 2012;7:e35614. doi: 10.1371/journal.pone.0035614
8. Canadian Medical Association. CMA policy: The treating physician’s role in helping patients return to work after an illness or injury (update 2013); 2013:1-6. Accessed June 1, 2021. https://policybase.cma.ca/documents/policypdf/PD13-05.pdf
9. Gifford B. Temporarily disabled workers account for a disproportionate share of health care payments. Health Aff (Millwood). 2017;36:245-249. doi:10.1377/hlthaff.2016.1013
10. Rueda S, Chambers L, Wilson M, et al. Association of returning to work with better health in working-aged adults: a systematic review. Am J Public Health. 2012;102:541-556. doi: 10.2105/AJPH.2011.300401
11. Modini M, Joyce S, Mykletun A, et al. The mental health benefits of employment: results of a systematic meta-review. Australas Psychiatry. 2016;24:331-336. doi: 10.1177/1039856215618523
12. Shaw WS, Nelson CC, Woiszwillo MJ, et al. Early return to work has benefits for relief of back pain and functional recovery after controlling for multiple confounds. J Occup Environ Med. 2018;60:901-910. doi: 10.1097/JOM.0000000000001380
13. Jurisic M, Bean M, Harbaugh J, et al. The personal physician’s role in helping patients with medical conditions stay at work or return to work. J Occup Environ Med. 2017;59:e125-e131. doi: 10.1097/JOM.0000000000001055
14. World Health Organization. Towards a common language for functioning, disability and health. ICF: The International Classification of Functioning, Disability and Health. 2002. Accessed June 2, 2021. www.who.int/classifications/icf/icfbeginnersguide.pdf
15. Talmage JB, Melhorn JM, Hyman MH. AMA Guides to the Evaluation of Work Ability and Return to Work. 2nd ed. American Medical Association; 2011.
16. Harrell M. Psychological factors and workforce health. In: Lee LP, Martin DW, Kancelbaum B. Occupational Medicine: A Basic Guide. American College of Occupational and Environmental Medicine; 2019. Accessed June 1, 2021. https://ohguides.acoem.org/07-psychological-factors-and-workforce-health-stress-management
17. Wickizer TM, Franklin GM, Fulton-Kehoe D. Innovations in occupational health care delivery can prevent entry into permanent disability: 8-year follow-up of the Washington State Centers for Occupational Health and Education. Med Care. 2018;56:1018-1023. doi: 10.1097/MLR.0000000000000991
18. Christian J, Wickizer T, Burton K. Implementing a community-focused health & work service. SSDI Solution Initiative, Fiscal Institute of the Committee for a Responsible Federal Budget. May 2019. Accessed June 2, 2021. www.crfb.org/sites/default/files/Implementing_a_Community-Focused_HWS.pdf
19. Macpherson RA, Koehoorn M, Fan J, et al. Do differences in work disability duration between men and women vary by province in Canada? J Occup Rehabil. 2018;29:560-568. doi: 10.1007/s10926-018-9819-1
20. Rainville J, Pransky G, Indahl A, et al. The physician as disability advisor for patients with musculoskeletal complaints. Spine (Phila Pa 1976). 2005;30:2579-2584. doi: 10.1097/01.brs.0000186589.69382.1d
21. Jay K, Thorsen SV, Sundstrup E, et al. Fear avoidance beliefs and risk of long-term sickness absence: prospective cohort study among workers with musculoskeletal pain. Pain Res Treat. 2018;2018:8347120. doi: 10.1155/2018/8347120
22. Burgard S, Lin KY. Bad jobs, bad health? How work and working conditions contribute to health disparities. Am Behav Sci. 2013;57:10.1177/0002764213487347. doi: 10.1177/0002764213487347
23. Soklaridis S, Tang G, Cartmill C, et al. “Can you go back to work?” Family physicians’ experiences with assessing patients’ functional ability to return to work. Can Fam Physician. 2011;57:202-209.
24. Peters SE, Truong AP, Johnston V. Stakeholders identify similar barriers but different strategies to facilitate return-to-work: a vignette of a worker with an upper extremity condition. Work. 2018;59:401-412. doi: 10.3233/WOR-182692
25. Shraim M, Cifuentes M, Willetts JL, et al. Regional socioeconomic disparities in outcomes for workers with low back pain in the United States. Am J Ind Med. 2017;60:472-483. doi: 10.1002/ajim.22712
26. Hill JC, Fritz JM. Psychosocial influences on low back pain, disability, and response to treatment. Phys Ther. 2011;91:712-721. doi: 10.2522/ptj.20100280
27. Aasdahl L, Pape K, Jensen C, et al. Associations between the readiness for return to work scale and return to work: a prospective study. J Occup Rehabil. 2018;28:97-106. doi: 10.1007/s10926-017-9705-2
28. Pino C, Covington M. Prescription of opioids for acute pain in opioid naïve patients. UpToDate Web site. February 9, 2021. Accessed June 2, 2021. www.uptodate.com/contents/prescription-of-opioids-for-acute-pain-in-opioid-naive-patients
29. Cancelliere C, Donovan J, Stochkendahl MJ, et al. Factors affecting return to work after injury or illness: best evidence synthesis of systematic reviews. Chiropr Man Therap. 2016;24:32. doi: 10.1186/s12998-016-0113-z
30. Lewis SJ, Orland BI. The importance and impact of evidence-based medicine. J Manag Care Pharm. 2004;10(5 suppl A):S3-S5. doi: 10.18553/jmcp.2004.10.S5-A.S3
31. Rupe KL. Work restrictions: documenting a patient’s return to work. Nurse Pract. 2010;35:49-53. doi: 10.1097/01.NPR.0000388901.49604.a8
32. Owens JD, Hegmann KT, Thiese MS, et al. Impacts of adherence to evidence-based medicine guidelines for the management of acute low back pain on costs of worker's compensation claims. J Occup Environ Med. 2019;61:445-452. doi: 10.1097/JOM.0000000000001593
33. Gaspar FW, Kownacki R, Zaidel CS, et al. Reducing disability durations and medical costs for patients with a carpal tunnel release surgery through the use of opioid prescribing guidelines. J Occup Environ Med. 2017;59:1180-1187. doi: 10.1097/JOM.0000000000001168
PRACTICE RECOMMENDATIONS
› Set appropriate expectations for the patient at the start of any episode of work disability: Estimate the course of functional recovery over time and the total duration of life–work disruption. A
› Include detailed activity prescriptions in the treatment plan, with stepwise progression over time toward full recovery. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Can family physicians accurately screen for AAA with point-of-care ultrasound?
EVIDENCE SUMMARY
Meta-analysis demonstrates accuracy of nonradiologist providers with POCUS
A systematic review and meta-analysis (11 studies; 946 exams) compared nonradiologist-performed AAA screening with POCUS vs radiologist-performed aortic imaging as a gold standard. Eight trials involved emergency medicine physicians (718 exams); 1 trial, surgical residents (104 exams); 1 trial, primary care internal medicine physicians (79 exams); and 1 trial, rural family physicians (45 exams). The majority of studies were conducted in Ireland, the United Kingdom, Australia, and Canada, with 4 trials performed in the United States.1
Researchers compared all POCUS exam findings with radiologist-performed imaging (using ultrasound, computed tomography, magnetic resonance imaging, or angiography) and with operative findings or pathology where available. There were 193 true positives, 8 false-positives, 740 true negatives, and 5 false-negatives. Primary care physicians identified 6 patients with AAA, with no false-positives or false-negatives. Overall, POCUS demonstrated a sensitivity of 0.975 (95% CI, 0.942 to 0.992) and a specificity of 0.989 (95% CI, 0.979 to 0.995).1
Nonradiologist providers received POCUS training as follows: emergency medicine residents, 5 hours to 3 days; emergency medicine physicians, 4 to 24 hours of didactics, 50 AAA scans, or American College of Emergency Medicine certification; and primary care physicians, 2.3 hours or 50 AAA scans. Information on training for surgical residents was not supplied. The authors rated the studies for quality (10-14 points on the 14-point QUADA quality score) and heterogeneity (I2 = 0 for sensitivity and I2 = .38 for specificity).1
European studies support FPs’ ability to diagnose AAA with POCUS
Two subsequent prospective diagnostic accuracy studies both found that POCUS performed by family physicians had 100% concordance with radiologist overread. The first study (in Spain) included 106 men (ages 50 and older; mean, 69 years) with chronic hypertension or a history of tobacco use. One family physician underwent training (duration not reported) by a radiologist, including experience measuring standard cross-sections of the aorta. Radiologists reviewed all POCUS images, which identified 6 patients with AAA (confirmed by CT scan). The concordance between the family physician and the radiologists was absolute (kappa = 1.0; sensitivity and specificity, 100%; positive and negative predictive values, both 1.0).2
The second study (in Denmark) compared 29 POCUS screenings for AAA performed by 5 family physicians vs a gold standard of a radiologist-performed abdominal ultrasound blinded to previous ultrasound findings. Four of the family physicians were board certified and 1 was a final-year resident in training. They all underwent a 3-day ultrasonography course that included initial e-learning followed by 2 days of hands-on training; all passed a final certification exam. The family physicians identified 1 patient with AAA. Radiologists overread all the scans and found 100% agreement with the 1 positive AAA and the 28 negative scans.3
Recommendations from others
In 2019, the US Preventive Services Task Force (USPSTF) offered a Grade “B” (moderate net benefit) recommendation for screening with ultrasonography for AAA in men ages 65 to 75 years who have ever smoked, and a Grade “C” recommendation (small net benefit) for screening men ages 65 to 75 years who have never smoked.4 In 2017, the Canadian Task Force on Preventive Health Care recommended screening all men ages 65 to 80 years with 1 ultrasound exam for AAA (weak recommendation; moderate-quality evidence). The Canadian Task Force also noted that, with adequate training, AAA screening could be performed in a family practice setting.5
Editor’s takeaway
While these studies evaluating POCUS performed by nonradiologists included a small number of family physicians, their finding that all participants (attending physicians and residents) demonstrate high sensitivity and specificity for AAA detection with relatively limited training bodes well for more widespread use of the technology. Offering POCUS to detect AAAs in family physician offices has the potential to dramatically improve access to USPSTF-recommended screening.
1. Concannon E, McHugh S, Healy DA, et al. Diagnostic accuracy of non-radiologist performed ultrasound for abdominal aortic aneurysm: systematic review and meta-analysis. Int J Clin Pract. 2014;9:1122-1129. doi: 10.1111/ijcp.12453
2. Sisó-Almirall A, Gilabert Solé R, Bru Saumell C, et al. Feasibility of hand-held-ultrasonography in the screening of abdominal aortic aneurysms and abdominal aortic atherosclerosis [article in Spanish]. Med Clin (Barc). 2013;141:417-422. doi: 10.1016/j.medcli.2013.02.038
3. Lindgaard K, Riisgaard L. ‘Validation of ultrasound examinations performed by general practitioners’. Scand J Prim Health Care. 2017;3:256-261. doi: 10.1080/02813432.2017.1358437
4. US Preventive Task Force. Screening for abdominal aortic aneurysm: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:2211-2218. doi:10.1001/jama.2019.18928
5. Canadian Task Force on Preventive Health Care. Recommendations on screening for abdominal aortic aneurysm in primary care. CMAJ. 2017;189:E1137-E1145. doi: 10.1503/cmaj.170118
EVIDENCE SUMMARY
Meta-analysis demonstrates accuracy of nonradiologist providers with POCUS
A systematic review and meta-analysis (11 studies; 946 exams) compared nonradiologist-performed AAA screening with POCUS vs radiologist-performed aortic imaging as a gold standard. Eight trials involved emergency medicine physicians (718 exams); 1 trial, surgical residents (104 exams); 1 trial, primary care internal medicine physicians (79 exams); and 1 trial, rural family physicians (45 exams). The majority of studies were conducted in Ireland, the United Kingdom, Australia, and Canada, with 4 trials performed in the United States.1
Researchers compared all POCUS exam findings with radiologist-performed imaging (using ultrasound, computed tomography, magnetic resonance imaging, or angiography) and with operative findings or pathology where available. There were 193 true positives, 8 false-positives, 740 true negatives, and 5 false-negatives. Primary care physicians identified 6 patients with AAA, with no false-positives or false-negatives. Overall, POCUS demonstrated a sensitivity of 0.975 (95% CI, 0.942 to 0.992) and a specificity of 0.989 (95% CI, 0.979 to 0.995).1
Nonradiologist providers received POCUS training as follows: emergency medicine residents, 5 hours to 3 days; emergency medicine physicians, 4 to 24 hours of didactics, 50 AAA scans, or American College of Emergency Medicine certification; and primary care physicians, 2.3 hours or 50 AAA scans. Information on training for surgical residents was not supplied. The authors rated the studies for quality (10-14 points on the 14-point QUADA quality score) and heterogeneity (I2 = 0 for sensitivity and I2 = .38 for specificity).1
European studies support FPs’ ability to diagnose AAA with POCUS
Two subsequent prospective diagnostic accuracy studies both found that POCUS performed by family physicians had 100% concordance with radiologist overread. The first study (in Spain) included 106 men (ages 50 and older; mean, 69 years) with chronic hypertension or a history of tobacco use. One family physician underwent training (duration not reported) by a radiologist, including experience measuring standard cross-sections of the aorta. Radiologists reviewed all POCUS images, which identified 6 patients with AAA (confirmed by CT scan). The concordance between the family physician and the radiologists was absolute (kappa = 1.0; sensitivity and specificity, 100%; positive and negative predictive values, both 1.0).2
The second study (in Denmark) compared 29 POCUS screenings for AAA performed by 5 family physicians vs a gold standard of a radiologist-performed abdominal ultrasound blinded to previous ultrasound findings. Four of the family physicians were board certified and 1 was a final-year resident in training. They all underwent a 3-day ultrasonography course that included initial e-learning followed by 2 days of hands-on training; all passed a final certification exam. The family physicians identified 1 patient with AAA. Radiologists overread all the scans and found 100% agreement with the 1 positive AAA and the 28 negative scans.3
Recommendations from others
In 2019, the US Preventive Services Task Force (USPSTF) offered a Grade “B” (moderate net benefit) recommendation for screening with ultrasonography for AAA in men ages 65 to 75 years who have ever smoked, and a Grade “C” recommendation (small net benefit) for screening men ages 65 to 75 years who have never smoked.4 In 2017, the Canadian Task Force on Preventive Health Care recommended screening all men ages 65 to 80 years with 1 ultrasound exam for AAA (weak recommendation; moderate-quality evidence). The Canadian Task Force also noted that, with adequate training, AAA screening could be performed in a family practice setting.5
Editor’s takeaway
While these studies evaluating POCUS performed by nonradiologists included a small number of family physicians, their finding that all participants (attending physicians and residents) demonstrate high sensitivity and specificity for AAA detection with relatively limited training bodes well for more widespread use of the technology. Offering POCUS to detect AAAs in family physician offices has the potential to dramatically improve access to USPSTF-recommended screening.
EVIDENCE SUMMARY
Meta-analysis demonstrates accuracy of nonradiologist providers with POCUS
A systematic review and meta-analysis (11 studies; 946 exams) compared nonradiologist-performed AAA screening with POCUS vs radiologist-performed aortic imaging as a gold standard. Eight trials involved emergency medicine physicians (718 exams); 1 trial, surgical residents (104 exams); 1 trial, primary care internal medicine physicians (79 exams); and 1 trial, rural family physicians (45 exams). The majority of studies were conducted in Ireland, the United Kingdom, Australia, and Canada, with 4 trials performed in the United States.1
Researchers compared all POCUS exam findings with radiologist-performed imaging (using ultrasound, computed tomography, magnetic resonance imaging, or angiography) and with operative findings or pathology where available. There were 193 true positives, 8 false-positives, 740 true negatives, and 5 false-negatives. Primary care physicians identified 6 patients with AAA, with no false-positives or false-negatives. Overall, POCUS demonstrated a sensitivity of 0.975 (95% CI, 0.942 to 0.992) and a specificity of 0.989 (95% CI, 0.979 to 0.995).1
Nonradiologist providers received POCUS training as follows: emergency medicine residents, 5 hours to 3 days; emergency medicine physicians, 4 to 24 hours of didactics, 50 AAA scans, or American College of Emergency Medicine certification; and primary care physicians, 2.3 hours or 50 AAA scans. Information on training for surgical residents was not supplied. The authors rated the studies for quality (10-14 points on the 14-point QUADA quality score) and heterogeneity (I2 = 0 for sensitivity and I2 = .38 for specificity).1
European studies support FPs’ ability to diagnose AAA with POCUS
Two subsequent prospective diagnostic accuracy studies both found that POCUS performed by family physicians had 100% concordance with radiologist overread. The first study (in Spain) included 106 men (ages 50 and older; mean, 69 years) with chronic hypertension or a history of tobacco use. One family physician underwent training (duration not reported) by a radiologist, including experience measuring standard cross-sections of the aorta. Radiologists reviewed all POCUS images, which identified 6 patients with AAA (confirmed by CT scan). The concordance between the family physician and the radiologists was absolute (kappa = 1.0; sensitivity and specificity, 100%; positive and negative predictive values, both 1.0).2
The second study (in Denmark) compared 29 POCUS screenings for AAA performed by 5 family physicians vs a gold standard of a radiologist-performed abdominal ultrasound blinded to previous ultrasound findings. Four of the family physicians were board certified and 1 was a final-year resident in training. They all underwent a 3-day ultrasonography course that included initial e-learning followed by 2 days of hands-on training; all passed a final certification exam. The family physicians identified 1 patient with AAA. Radiologists overread all the scans and found 100% agreement with the 1 positive AAA and the 28 negative scans.3
Recommendations from others
In 2019, the US Preventive Services Task Force (USPSTF) offered a Grade “B” (moderate net benefit) recommendation for screening with ultrasonography for AAA in men ages 65 to 75 years who have ever smoked, and a Grade “C” recommendation (small net benefit) for screening men ages 65 to 75 years who have never smoked.4 In 2017, the Canadian Task Force on Preventive Health Care recommended screening all men ages 65 to 80 years with 1 ultrasound exam for AAA (weak recommendation; moderate-quality evidence). The Canadian Task Force also noted that, with adequate training, AAA screening could be performed in a family practice setting.5
Editor’s takeaway
While these studies evaluating POCUS performed by nonradiologists included a small number of family physicians, their finding that all participants (attending physicians and residents) demonstrate high sensitivity and specificity for AAA detection with relatively limited training bodes well for more widespread use of the technology. Offering POCUS to detect AAAs in family physician offices has the potential to dramatically improve access to USPSTF-recommended screening.
1. Concannon E, McHugh S, Healy DA, et al. Diagnostic accuracy of non-radiologist performed ultrasound for abdominal aortic aneurysm: systematic review and meta-analysis. Int J Clin Pract. 2014;9:1122-1129. doi: 10.1111/ijcp.12453
2. Sisó-Almirall A, Gilabert Solé R, Bru Saumell C, et al. Feasibility of hand-held-ultrasonography in the screening of abdominal aortic aneurysms and abdominal aortic atherosclerosis [article in Spanish]. Med Clin (Barc). 2013;141:417-422. doi: 10.1016/j.medcli.2013.02.038
3. Lindgaard K, Riisgaard L. ‘Validation of ultrasound examinations performed by general practitioners’. Scand J Prim Health Care. 2017;3:256-261. doi: 10.1080/02813432.2017.1358437
4. US Preventive Task Force. Screening for abdominal aortic aneurysm: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:2211-2218. doi:10.1001/jama.2019.18928
5. Canadian Task Force on Preventive Health Care. Recommendations on screening for abdominal aortic aneurysm in primary care. CMAJ. 2017;189:E1137-E1145. doi: 10.1503/cmaj.170118
1. Concannon E, McHugh S, Healy DA, et al. Diagnostic accuracy of non-radiologist performed ultrasound for abdominal aortic aneurysm: systematic review and meta-analysis. Int J Clin Pract. 2014;9:1122-1129. doi: 10.1111/ijcp.12453
2. Sisó-Almirall A, Gilabert Solé R, Bru Saumell C, et al. Feasibility of hand-held-ultrasonography in the screening of abdominal aortic aneurysms and abdominal aortic atherosclerosis [article in Spanish]. Med Clin (Barc). 2013;141:417-422. doi: 10.1016/j.medcli.2013.02.038
3. Lindgaard K, Riisgaard L. ‘Validation of ultrasound examinations performed by general practitioners’. Scand J Prim Health Care. 2017;3:256-261. doi: 10.1080/02813432.2017.1358437
4. US Preventive Task Force. Screening for abdominal aortic aneurysm: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:2211-2218. doi:10.1001/jama.2019.18928
5. Canadian Task Force on Preventive Health Care. Recommendations on screening for abdominal aortic aneurysm in primary care. CMAJ. 2017;189:E1137-E1145. doi: 10.1503/cmaj.170118
EVIDENCE-BASED ANSWER:
Likely yes. Point-of-care ultrasound (POCUS) screening for abdominal aortic aneurysm (AAA) by nonradiologist physicians is 98% sensitive and 99% specific, compared with imaging performed by radiologists (strength of recommendation [SOR]: B, meta-analysis of diagnostic accuracy studies mostly involving emergency medicine physicians). European family physicians demonstrated 100% concordance with radiologist readings (SOR: C, very small subsequent diagnostic accuracy studies).
How to proceed when it comes to vitamin D
In April 2021, the US Preventive Services Task Force (USPSTF) published an updated recommendation on screening for vitamin D deficiency in adults. It reaffirmed an “I” statement first made in 2014: evidence is insufficient to balance the benefits and harms of screening.1 This recommendation applies to asymptomatic, community-dwelling, nonpregnant adults without conditions treatable with vitamin D. It’s important to remember that screening refers to testing asymptomatic individuals to detect a condition early before it causes illness. Testing performed to determine whether symptoms are evidence of an underlying condition is not screening but diagnostic testing.
The Task Force statement explains the problems they found with the current level of knowledge about screening for vitamin D deficiency. First, while 25-hydroxyvitamin D [25(OH)D] is considered the best test for vitamin D levels, it is hard to measure accurately and test results vary by the method used and laboratories doing the testing. There also is uncertainty about how best to measure vitamin D status in different racial and ethnic groups, especially those with dark skin pigmentation. In addition, 25(OH)D in the blood is predominantly the bound form, with only 10% to 15% being unbound and bioavailable. Current tests do not determine the amount of bound vs unbound 25(OH)D.1-3
There is no consensus about the optimal blood level of vitamin D or the level that defines deficiency. The Institute of Medicine (now the National Academy of Medicine—NAM) stated that serum 25(OH)D levels ≥ 20 ng/mL are adequate to meet the metabolic needs of 97.5% of people, and that levels of 12 to 20 ng/mL pose a risk of deficiency, with levels < 12 considered to be very low.4 The Endocrine Society defines deficiency as < 20 ng/mL and insufficiency as 21 to 29 ng/mL.5
The rate of testing for vitamin D deficiency in primary care in unknown, but there is evidence that since 2000, it has increased 80 fold at least among those with Medicare.6 Data from the 2011-2014 National Health and Nutrition Examination Survey showed that 5% of the population had 25(OH)D levels < 12 ng/mL and 18% had levels between 12 and 19 ng/mL.7 Some have estimated that as many as half of all adults would be considered vitamin D deficient or insufficient using current less conservative definitions, with higher rates in racial/ethnic minorities.2,8
There are no firm data on the frequency, or benefits, of screening for vitamin D levels in asymptomatic adults (and treating those found to have vitamin D deficiency). The Task Force looked for indirect evidence by examining the effect of treating vitamin D deficiency in a number of conditions and found that for some, there was adequate evidence of no benefit and for others there was inadequate evidence for possible benefits.9 No benefit was found for incidence of fractures, type 2 diabetes, and overall mortality.9 Inadequate evidence was found for incidence of cancer, cardiovascular disease, scores on measures of depression and physical functioning, and urinary tract infections in those with impaired fasting glucose.9
Known risk factors for low vitamin D levels include low vitamin D intake, older age, obesity, low UVB exposure or absorption due to long winter seasons in northern latitudes, sun avoidance, and dark skin pigmentation.1 In addition, certain medical conditions contribute to, or are caused by, low vitamin D levels—eg, osteoporosis, chronic kidney disease, malabsorption syndromes, and medication use (ie, glucocorticoids).1-3
The Task Force recommendation on screening for vitamin D deficiency differs from those of some other organizations. However, none recommend universal population-based screening. The Endocrine Society and the American Association of Clinical Endocrinologists recommend screening but only in those at risk for vitamin D deficiency.5,10 The American Academy of Family Physicians endorses the USPSTF recommendation.11
Continue to: Specific USPSTF topics related to vitamin D
Specific USPSTF topics related to vitamin D
The Task Force has specifically addressed 3 topics pertaining to vitamin D.

Prevention of falls in the elderly. In 2018 the Task Force recommended against the use of vitamin D to prevent falls in community-dwelling adults ≥ 65 years.12 This reversed its 2012 recommendation advising vitamin D supplementation to prevent falls. The Task Force re-examined the old evidence and looked at newer studies and concluded that their previous conclusion was wrong and that the evidence showed no benefit from vitamin D in preventing falls in the elderly. The reversal of a prior recommendation is rare for the USPSTF because of the rigor of its evidence reviews and its policy of not making a recommendation unless solid evidence for or against exists.
Prevention of cardiovascular disease and cancer. The Task Force concludes that current evidence is insufficient to assess the balance of benefits and harms in the use of single- or paired-nutrient supplements to prevent cardiovascular disease or cancer.13 (The exceptions are beta-carotene and vitamin E, which the Task Force recommends against.) This statement is consistent with the lack of evidence the Task Force found regarding prevention of these conditions by vitamin D supplementation in those who are vitamin D deficient.
Prevention of fractures in men and in premenopausal and postmenopausal women. For men and premenopausal women, the Task Force concludes that evidence is insufficient to assess the benefits and harms of vitamin D and calcium supplementation, alone or in combination, to prevent fractures.14 For prevention of fractures in postmenopausal women, there are 2 recommendations. The first one advises against the use of ≤ 400 IU of vitamin D and ≤ 1000 mg of calcium because the evidence indicates ineffectiveness. The second one is another “I” statement for the use of doses > 400 IU of vitamin D and > 1000 mg of calcium. These 3 recommendations apply to adults who live in the community and not in nursing homes or other institutional care facilities; they do not apply to those who have osteoporosis.
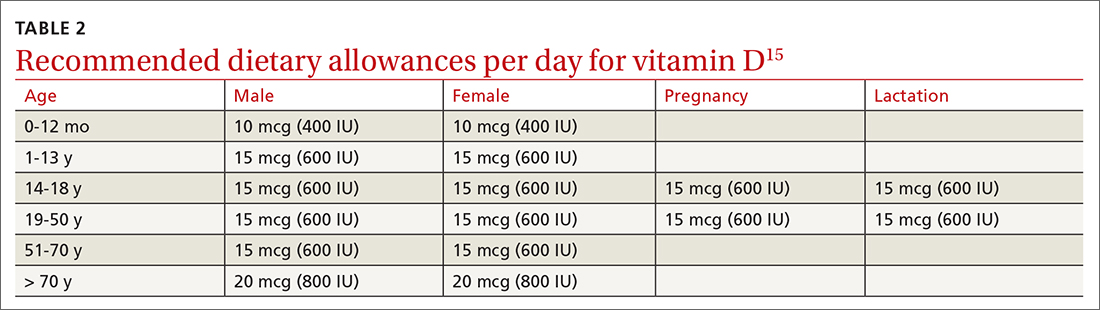
What should the family physician do?
Encourage all patients to take the recommended dietary allowances (RDA) of vitamin D. The RDA is the average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%-98%) healthy individuals. Most professional organizations recommend that adults ≥ 50 years consume 800 to 1000 IU of vitamin D daily. TABLE 2 lists the RDA for vitamin D by age and sex.15 The amount of vitamin D in selected food products is listed in TABLE 3.15 Some increase in levels of vitamin D can occur as a result of sun exposure, but current practices of sun avoidance make it difficult to achieve a significant contribution to vitamin D requirements.15
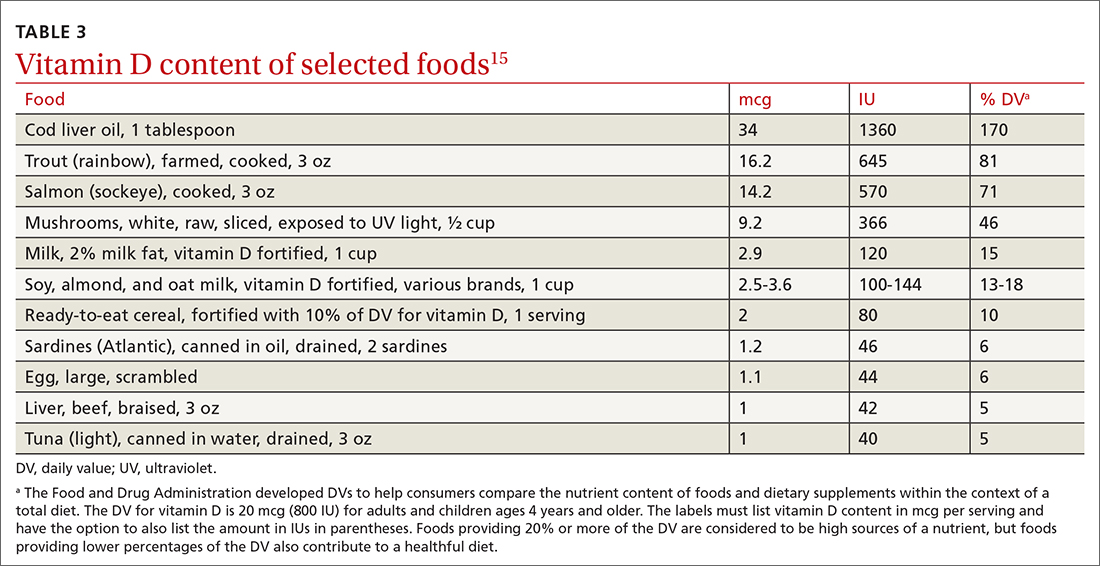
Continue to: Alternatives to universal screening
Alternatives to universal screening. Screening for vitamin D deficiency might benefit some patients, although there is no evidence to support it. Universal screening will likely lead to overdiagnosis and overtreatment based on what is essentially a poorly understood blood test. This was the concern expressed by the NAM.4,16 An editorial accompanying publication of the recent USPSTF recommendation suggested not measuring vitamin D levels but instead advising patients to consume the age-based RDA of vitamin D.3 For those at increased risk for vitamin D deficiency, advise a higher dose of vitamin D (eg, 2000 IU/d, which is still lower than the upper daily limit).3
Other options are to screen for vitamin D deficiency only in those at high risk for low vitamin D levels, and to test for vitamin D deficiency in those with symptoms associated with deficiency such as bone pain and muscle weakness. These options would be consistent with recommendations from the Endocrine Society.5 Some have recommended that if testing is ordered, it should be performed by a laboratory that uses liquid chromatography-mass spectrometry because it is the criterion standard.2
Treatment options. Vitamin D deficiency can be treated with either ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3). These treatments can also be recommended for those whose diets may not provide the RDA for vitamin D. Both are readily available over the counter and by prescription. The Task Force found that the harms of treating vitamin D deficiency with vitamin D at recommended doses are small to none.1 There is possibly a small increase in kidney stones with the combined use of 1000 mg/d calcium and 10 mcg (400 IU)/d vitamin D.17 Large doses of vitamin D can cause toxicity including marked hypercalcemia, nausea, vomiting, muscle weakness, neuropsychiatric disturbances, pain, loss of appetite, dehydration, polyuria, excessive thirst, and kidney stones.15A cautious evidence-based approach would be to selectively screen for vitamin D deficiency, conduct diagnostic testing when indicated, and advise vitamin D supplementation as needed.
1. USPSTF. Screening for vitamin D deficiency in adults: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1436-1442.
2. Michos ED, Kalyani RR, Segal JB. Why USPSTF still finds insufficient evidence to support screening for vitamin D deficiency. JAMA Netw Open. 2021;4:e213627.
3. Burnett-Bowie AAM, Cappola AR. The USPSTF 2021 recommendations on screening for asymptomatic vitamin D deficiency in adults: the challenge for clinicians continues. JAMA. 2021;325:1401-1402.
4. Institute of Medicine. Dietary reference intakes for calcium and vitamin D. National Academies Press; 2011. Accessed May 22, 2021. https://pubmed.ncbi.nlm.nih.gov/21796828/
5. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinolgy Metab. 2011;96:1911-1930.
6. Shahangian S, Alspach TD, Astles JR, et al. Trends in laboratory test volumes for Medicare part B reimbursements, 2000-2010. Arch Pathol Lab Med. 2014;138:189-203.
7. Herrick KA, Storandt RJ, Afful J, et al. Vitamin D status in the United States, 2011-2014. Am J Clin Nutr. 2019;110:150-157.
8. Forrest KYZ, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31:48-54.
9. Kahwati LC, LeBlanc E, Weber RP, et al. Screening for vitamin D deficiency in adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325:1443-1463.
10. Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2016. Endocr Pract. 2016;22(supp 4):1-42.
11. AAFP. Clinical preventive services. Accessed May 22, 2021. www.aafp.org/family-physician/patient-care/clinical-recommendations/aafp-cps.html
12. USPSTF. Falls prevention in community-dwelling older adults: interventions. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/falls-prevention-in-older-adults-interventions
13. USPSTF. Vitamin supplementation to prevent cancer and CVD: preventive medication. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-supplementation-to-prevent-cancer-and-cvd-counseling
14. USPSTF. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: preventive medication. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-d-calcium-or-combined-supplementation-for-the-primary-prevention-of-fractures-in-adults-preventive-medication
15. NIH. Vitamin D. Accessed May 22, 2021. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/
16. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53-58.
17. Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669-683.
In April 2021, the US Preventive Services Task Force (USPSTF) published an updated recommendation on screening for vitamin D deficiency in adults. It reaffirmed an “I” statement first made in 2014: evidence is insufficient to balance the benefits and harms of screening.1 This recommendation applies to asymptomatic, community-dwelling, nonpregnant adults without conditions treatable with vitamin D. It’s important to remember that screening refers to testing asymptomatic individuals to detect a condition early before it causes illness. Testing performed to determine whether symptoms are evidence of an underlying condition is not screening but diagnostic testing.
The Task Force statement explains the problems they found with the current level of knowledge about screening for vitamin D deficiency. First, while 25-hydroxyvitamin D [25(OH)D] is considered the best test for vitamin D levels, it is hard to measure accurately and test results vary by the method used and laboratories doing the testing. There also is uncertainty about how best to measure vitamin D status in different racial and ethnic groups, especially those with dark skin pigmentation. In addition, 25(OH)D in the blood is predominantly the bound form, with only 10% to 15% being unbound and bioavailable. Current tests do not determine the amount of bound vs unbound 25(OH)D.1-3
There is no consensus about the optimal blood level of vitamin D or the level that defines deficiency. The Institute of Medicine (now the National Academy of Medicine—NAM) stated that serum 25(OH)D levels ≥ 20 ng/mL are adequate to meet the metabolic needs of 97.5% of people, and that levels of 12 to 20 ng/mL pose a risk of deficiency, with levels < 12 considered to be very low.4 The Endocrine Society defines deficiency as < 20 ng/mL and insufficiency as 21 to 29 ng/mL.5
The rate of testing for vitamin D deficiency in primary care in unknown, but there is evidence that since 2000, it has increased 80 fold at least among those with Medicare.6 Data from the 2011-2014 National Health and Nutrition Examination Survey showed that 5% of the population had 25(OH)D levels < 12 ng/mL and 18% had levels between 12 and 19 ng/mL.7 Some have estimated that as many as half of all adults would be considered vitamin D deficient or insufficient using current less conservative definitions, with higher rates in racial/ethnic minorities.2,8
There are no firm data on the frequency, or benefits, of screening for vitamin D levels in asymptomatic adults (and treating those found to have vitamin D deficiency). The Task Force looked for indirect evidence by examining the effect of treating vitamin D deficiency in a number of conditions and found that for some, there was adequate evidence of no benefit and for others there was inadequate evidence for possible benefits.9 No benefit was found for incidence of fractures, type 2 diabetes, and overall mortality.9 Inadequate evidence was found for incidence of cancer, cardiovascular disease, scores on measures of depression and physical functioning, and urinary tract infections in those with impaired fasting glucose.9
Known risk factors for low vitamin D levels include low vitamin D intake, older age, obesity, low UVB exposure or absorption due to long winter seasons in northern latitudes, sun avoidance, and dark skin pigmentation.1 In addition, certain medical conditions contribute to, or are caused by, low vitamin D levels—eg, osteoporosis, chronic kidney disease, malabsorption syndromes, and medication use (ie, glucocorticoids).1-3
The Task Force recommendation on screening for vitamin D deficiency differs from those of some other organizations. However, none recommend universal population-based screening. The Endocrine Society and the American Association of Clinical Endocrinologists recommend screening but only in those at risk for vitamin D deficiency.5,10 The American Academy of Family Physicians endorses the USPSTF recommendation.11
Continue to: Specific USPSTF topics related to vitamin D
Specific USPSTF topics related to vitamin D
The Task Force has specifically addressed 3 topics pertaining to vitamin D.

Prevention of falls in the elderly. In 2018 the Task Force recommended against the use of vitamin D to prevent falls in community-dwelling adults ≥ 65 years.12 This reversed its 2012 recommendation advising vitamin D supplementation to prevent falls. The Task Force re-examined the old evidence and looked at newer studies and concluded that their previous conclusion was wrong and that the evidence showed no benefit from vitamin D in preventing falls in the elderly. The reversal of a prior recommendation is rare for the USPSTF because of the rigor of its evidence reviews and its policy of not making a recommendation unless solid evidence for or against exists.
Prevention of cardiovascular disease and cancer. The Task Force concludes that current evidence is insufficient to assess the balance of benefits and harms in the use of single- or paired-nutrient supplements to prevent cardiovascular disease or cancer.13 (The exceptions are beta-carotene and vitamin E, which the Task Force recommends against.) This statement is consistent with the lack of evidence the Task Force found regarding prevention of these conditions by vitamin D supplementation in those who are vitamin D deficient.
Prevention of fractures in men and in premenopausal and postmenopausal women. For men and premenopausal women, the Task Force concludes that evidence is insufficient to assess the benefits and harms of vitamin D and calcium supplementation, alone or in combination, to prevent fractures.14 For prevention of fractures in postmenopausal women, there are 2 recommendations. The first one advises against the use of ≤ 400 IU of vitamin D and ≤ 1000 mg of calcium because the evidence indicates ineffectiveness. The second one is another “I” statement for the use of doses > 400 IU of vitamin D and > 1000 mg of calcium. These 3 recommendations apply to adults who live in the community and not in nursing homes or other institutional care facilities; they do not apply to those who have osteoporosis.

What should the family physician do?
Encourage all patients to take the recommended dietary allowances (RDA) of vitamin D. The RDA is the average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%-98%) healthy individuals. Most professional organizations recommend that adults ≥ 50 years consume 800 to 1000 IU of vitamin D daily. TABLE 2 lists the RDA for vitamin D by age and sex.15 The amount of vitamin D in selected food products is listed in TABLE 3.15 Some increase in levels of vitamin D can occur as a result of sun exposure, but current practices of sun avoidance make it difficult to achieve a significant contribution to vitamin D requirements.15

Continue to: Alternatives to universal screening
Alternatives to universal screening. Screening for vitamin D deficiency might benefit some patients, although there is no evidence to support it. Universal screening will likely lead to overdiagnosis and overtreatment based on what is essentially a poorly understood blood test. This was the concern expressed by the NAM.4,16 An editorial accompanying publication of the recent USPSTF recommendation suggested not measuring vitamin D levels but instead advising patients to consume the age-based RDA of vitamin D.3 For those at increased risk for vitamin D deficiency, advise a higher dose of vitamin D (eg, 2000 IU/d, which is still lower than the upper daily limit).3
Other options are to screen for vitamin D deficiency only in those at high risk for low vitamin D levels, and to test for vitamin D deficiency in those with symptoms associated with deficiency such as bone pain and muscle weakness. These options would be consistent with recommendations from the Endocrine Society.5 Some have recommended that if testing is ordered, it should be performed by a laboratory that uses liquid chromatography-mass spectrometry because it is the criterion standard.2
Treatment options. Vitamin D deficiency can be treated with either ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3). These treatments can also be recommended for those whose diets may not provide the RDA for vitamin D. Both are readily available over the counter and by prescription. The Task Force found that the harms of treating vitamin D deficiency with vitamin D at recommended doses are small to none.1 There is possibly a small increase in kidney stones with the combined use of 1000 mg/d calcium and 10 mcg (400 IU)/d vitamin D.17 Large doses of vitamin D can cause toxicity including marked hypercalcemia, nausea, vomiting, muscle weakness, neuropsychiatric disturbances, pain, loss of appetite, dehydration, polyuria, excessive thirst, and kidney stones.15A cautious evidence-based approach would be to selectively screen for vitamin D deficiency, conduct diagnostic testing when indicated, and advise vitamin D supplementation as needed.
In April 2021, the US Preventive Services Task Force (USPSTF) published an updated recommendation on screening for vitamin D deficiency in adults. It reaffirmed an “I” statement first made in 2014: evidence is insufficient to balance the benefits and harms of screening.1 This recommendation applies to asymptomatic, community-dwelling, nonpregnant adults without conditions treatable with vitamin D. It’s important to remember that screening refers to testing asymptomatic individuals to detect a condition early before it causes illness. Testing performed to determine whether symptoms are evidence of an underlying condition is not screening but diagnostic testing.
The Task Force statement explains the problems they found with the current level of knowledge about screening for vitamin D deficiency. First, while 25-hydroxyvitamin D [25(OH)D] is considered the best test for vitamin D levels, it is hard to measure accurately and test results vary by the method used and laboratories doing the testing. There also is uncertainty about how best to measure vitamin D status in different racial and ethnic groups, especially those with dark skin pigmentation. In addition, 25(OH)D in the blood is predominantly the bound form, with only 10% to 15% being unbound and bioavailable. Current tests do not determine the amount of bound vs unbound 25(OH)D.1-3
There is no consensus about the optimal blood level of vitamin D or the level that defines deficiency. The Institute of Medicine (now the National Academy of Medicine—NAM) stated that serum 25(OH)D levels ≥ 20 ng/mL are adequate to meet the metabolic needs of 97.5% of people, and that levels of 12 to 20 ng/mL pose a risk of deficiency, with levels < 12 considered to be very low.4 The Endocrine Society defines deficiency as < 20 ng/mL and insufficiency as 21 to 29 ng/mL.5
The rate of testing for vitamin D deficiency in primary care in unknown, but there is evidence that since 2000, it has increased 80 fold at least among those with Medicare.6 Data from the 2011-2014 National Health and Nutrition Examination Survey showed that 5% of the population had 25(OH)D levels < 12 ng/mL and 18% had levels between 12 and 19 ng/mL.7 Some have estimated that as many as half of all adults would be considered vitamin D deficient or insufficient using current less conservative definitions, with higher rates in racial/ethnic minorities.2,8
There are no firm data on the frequency, or benefits, of screening for vitamin D levels in asymptomatic adults (and treating those found to have vitamin D deficiency). The Task Force looked for indirect evidence by examining the effect of treating vitamin D deficiency in a number of conditions and found that for some, there was adequate evidence of no benefit and for others there was inadequate evidence for possible benefits.9 No benefit was found for incidence of fractures, type 2 diabetes, and overall mortality.9 Inadequate evidence was found for incidence of cancer, cardiovascular disease, scores on measures of depression and physical functioning, and urinary tract infections in those with impaired fasting glucose.9
Known risk factors for low vitamin D levels include low vitamin D intake, older age, obesity, low UVB exposure or absorption due to long winter seasons in northern latitudes, sun avoidance, and dark skin pigmentation.1 In addition, certain medical conditions contribute to, or are caused by, low vitamin D levels—eg, osteoporosis, chronic kidney disease, malabsorption syndromes, and medication use (ie, glucocorticoids).1-3
The Task Force recommendation on screening for vitamin D deficiency differs from those of some other organizations. However, none recommend universal population-based screening. The Endocrine Society and the American Association of Clinical Endocrinologists recommend screening but only in those at risk for vitamin D deficiency.5,10 The American Academy of Family Physicians endorses the USPSTF recommendation.11
Continue to: Specific USPSTF topics related to vitamin D
Specific USPSTF topics related to vitamin D
The Task Force has specifically addressed 3 topics pertaining to vitamin D.

Prevention of falls in the elderly. In 2018 the Task Force recommended against the use of vitamin D to prevent falls in community-dwelling adults ≥ 65 years.12 This reversed its 2012 recommendation advising vitamin D supplementation to prevent falls. The Task Force re-examined the old evidence and looked at newer studies and concluded that their previous conclusion was wrong and that the evidence showed no benefit from vitamin D in preventing falls in the elderly. The reversal of a prior recommendation is rare for the USPSTF because of the rigor of its evidence reviews and its policy of not making a recommendation unless solid evidence for or against exists.
Prevention of cardiovascular disease and cancer. The Task Force concludes that current evidence is insufficient to assess the balance of benefits and harms in the use of single- or paired-nutrient supplements to prevent cardiovascular disease or cancer.13 (The exceptions are beta-carotene and vitamin E, which the Task Force recommends against.) This statement is consistent with the lack of evidence the Task Force found regarding prevention of these conditions by vitamin D supplementation in those who are vitamin D deficient.
Prevention of fractures in men and in premenopausal and postmenopausal women. For men and premenopausal women, the Task Force concludes that evidence is insufficient to assess the benefits and harms of vitamin D and calcium supplementation, alone or in combination, to prevent fractures.14 For prevention of fractures in postmenopausal women, there are 2 recommendations. The first one advises against the use of ≤ 400 IU of vitamin D and ≤ 1000 mg of calcium because the evidence indicates ineffectiveness. The second one is another “I” statement for the use of doses > 400 IU of vitamin D and > 1000 mg of calcium. These 3 recommendations apply to adults who live in the community and not in nursing homes or other institutional care facilities; they do not apply to those who have osteoporosis.

What should the family physician do?
Encourage all patients to take the recommended dietary allowances (RDA) of vitamin D. The RDA is the average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%-98%) healthy individuals. Most professional organizations recommend that adults ≥ 50 years consume 800 to 1000 IU of vitamin D daily. TABLE 2 lists the RDA for vitamin D by age and sex.15 The amount of vitamin D in selected food products is listed in TABLE 3.15 Some increase in levels of vitamin D can occur as a result of sun exposure, but current practices of sun avoidance make it difficult to achieve a significant contribution to vitamin D requirements.15

Continue to: Alternatives to universal screening
Alternatives to universal screening. Screening for vitamin D deficiency might benefit some patients, although there is no evidence to support it. Universal screening will likely lead to overdiagnosis and overtreatment based on what is essentially a poorly understood blood test. This was the concern expressed by the NAM.4,16 An editorial accompanying publication of the recent USPSTF recommendation suggested not measuring vitamin D levels but instead advising patients to consume the age-based RDA of vitamin D.3 For those at increased risk for vitamin D deficiency, advise a higher dose of vitamin D (eg, 2000 IU/d, which is still lower than the upper daily limit).3
Other options are to screen for vitamin D deficiency only in those at high risk for low vitamin D levels, and to test for vitamin D deficiency in those with symptoms associated with deficiency such as bone pain and muscle weakness. These options would be consistent with recommendations from the Endocrine Society.5 Some have recommended that if testing is ordered, it should be performed by a laboratory that uses liquid chromatography-mass spectrometry because it is the criterion standard.2
Treatment options. Vitamin D deficiency can be treated with either ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3). These treatments can also be recommended for those whose diets may not provide the RDA for vitamin D. Both are readily available over the counter and by prescription. The Task Force found that the harms of treating vitamin D deficiency with vitamin D at recommended doses are small to none.1 There is possibly a small increase in kidney stones with the combined use of 1000 mg/d calcium and 10 mcg (400 IU)/d vitamin D.17 Large doses of vitamin D can cause toxicity including marked hypercalcemia, nausea, vomiting, muscle weakness, neuropsychiatric disturbances, pain, loss of appetite, dehydration, polyuria, excessive thirst, and kidney stones.15A cautious evidence-based approach would be to selectively screen for vitamin D deficiency, conduct diagnostic testing when indicated, and advise vitamin D supplementation as needed.
1. USPSTF. Screening for vitamin D deficiency in adults: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1436-1442.
2. Michos ED, Kalyani RR, Segal JB. Why USPSTF still finds insufficient evidence to support screening for vitamin D deficiency. JAMA Netw Open. 2021;4:e213627.
3. Burnett-Bowie AAM, Cappola AR. The USPSTF 2021 recommendations on screening for asymptomatic vitamin D deficiency in adults: the challenge for clinicians continues. JAMA. 2021;325:1401-1402.
4. Institute of Medicine. Dietary reference intakes for calcium and vitamin D. National Academies Press; 2011. Accessed May 22, 2021. https://pubmed.ncbi.nlm.nih.gov/21796828/
5. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinolgy Metab. 2011;96:1911-1930.
6. Shahangian S, Alspach TD, Astles JR, et al. Trends in laboratory test volumes for Medicare part B reimbursements, 2000-2010. Arch Pathol Lab Med. 2014;138:189-203.
7. Herrick KA, Storandt RJ, Afful J, et al. Vitamin D status in the United States, 2011-2014. Am J Clin Nutr. 2019;110:150-157.
8. Forrest KYZ, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31:48-54.
9. Kahwati LC, LeBlanc E, Weber RP, et al. Screening for vitamin D deficiency in adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325:1443-1463.
10. Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2016. Endocr Pract. 2016;22(supp 4):1-42.
11. AAFP. Clinical preventive services. Accessed May 22, 2021. www.aafp.org/family-physician/patient-care/clinical-recommendations/aafp-cps.html
12. USPSTF. Falls prevention in community-dwelling older adults: interventions. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/falls-prevention-in-older-adults-interventions
13. USPSTF. Vitamin supplementation to prevent cancer and CVD: preventive medication. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-supplementation-to-prevent-cancer-and-cvd-counseling
14. USPSTF. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: preventive medication. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-d-calcium-or-combined-supplementation-for-the-primary-prevention-of-fractures-in-adults-preventive-medication
15. NIH. Vitamin D. Accessed May 22, 2021. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/
16. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53-58.
17. Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669-683.
1. USPSTF. Screening for vitamin D deficiency in adults: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1436-1442.
2. Michos ED, Kalyani RR, Segal JB. Why USPSTF still finds insufficient evidence to support screening for vitamin D deficiency. JAMA Netw Open. 2021;4:e213627.
3. Burnett-Bowie AAM, Cappola AR. The USPSTF 2021 recommendations on screening for asymptomatic vitamin D deficiency in adults: the challenge for clinicians continues. JAMA. 2021;325:1401-1402.
4. Institute of Medicine. Dietary reference intakes for calcium and vitamin D. National Academies Press; 2011. Accessed May 22, 2021. https://pubmed.ncbi.nlm.nih.gov/21796828/
5. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinolgy Metab. 2011;96:1911-1930.
6. Shahangian S, Alspach TD, Astles JR, et al. Trends in laboratory test volumes for Medicare part B reimbursements, 2000-2010. Arch Pathol Lab Med. 2014;138:189-203.
7. Herrick KA, Storandt RJ, Afful J, et al. Vitamin D status in the United States, 2011-2014. Am J Clin Nutr. 2019;110:150-157.
8. Forrest KYZ, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31:48-54.
9. Kahwati LC, LeBlanc E, Weber RP, et al. Screening for vitamin D deficiency in adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325:1443-1463.
10. Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2016. Endocr Pract. 2016;22(supp 4):1-42.
11. AAFP. Clinical preventive services. Accessed May 22, 2021. www.aafp.org/family-physician/patient-care/clinical-recommendations/aafp-cps.html
12. USPSTF. Falls prevention in community-dwelling older adults: interventions. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/falls-prevention-in-older-adults-interventions
13. USPSTF. Vitamin supplementation to prevent cancer and CVD: preventive medication. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-supplementation-to-prevent-cancer-and-cvd-counseling
14. USPSTF. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: preventive medication. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-d-calcium-or-combined-supplementation-for-the-primary-prevention-of-fractures-in-adults-preventive-medication
15. NIH. Vitamin D. Accessed May 22, 2021. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/
16. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53-58.
17. Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669-683.