User login
Three-drug combo promising against high-risk CLL
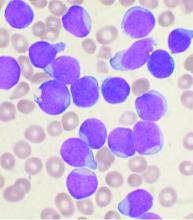
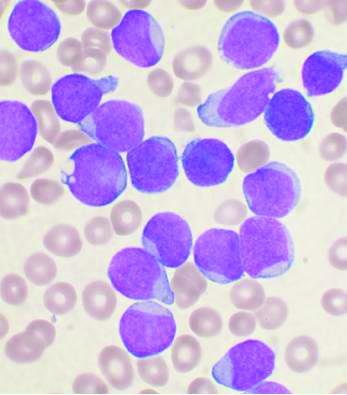
For patients with high-risk chronic lymphocytic leukemia (CLL), first-line therapy with a triple combination of targeted agents showed encouraging response rates in the phase 2 CLL2-GIVe trial.
Among 41 patients with untreated CLL bearing deleterious TP53 mutations and/or the 17p chromosomal deletion who received the GIVe regimen consisting of obinutuzumab (Gazyva), ibrutinib (Imbruvica), and venetoclax (Venclexta), the complete response rate at final restaging was 58.5%, and 33 patients with a confirmed response were negative for minimal residual disease after a median follow-up of 18.6 months, reported Henriette Huber, MD, of University Hospital Ulm, Germany.
“The GIVe regimen is promising first-line therapy for patients with high-risk CLL,” she said in a presentation during the virtual annual congress of the European Hematology Association.
The overall safety profile of the combination was acceptable, she said, but added that “some higher-grade infections are of concern.” The rate of grade 3 or greater infections/infestations in the study was 19.5%.
Sound rationale (with caveat)
Another adverse event of concern is the rate of atrial fibrillation in the comparatively young patient population (median age 62), noted Alexey Danilov, MD, PhD, of City of Hope in Duarte Calif., who commented on the study for MDedge.
He pointed out that second-generation Bruton’s tyrosine kinase (BTK) inhibitors such as acalabrutinib (Calquence) may pose a lower risk of atrial fibrillation than the BTK inhibitor ibrutinib used in the CLL2-GIVe study.
In general, however, the rationale for the combination is sound, Dr. Danilov said.
“Of all the patient populations that we deal with within CLL, this probably would be most appropriate for this type of therapy. Patients with deletion 17p or TP53 mutations still represent an unmet medical need compared to other patients who don’t have those mutations,” he said.
Patients with CLL bearing the mutations have lower clinical response rates to novel therapies and generally do not respond well to chemoimmunotherapy, he said.
“The question becomes whether using these all at the same time, versus sequential strategies – using one drug and then after that, at relapse, another – is better, and obviously this trial doesn’t address that,” he said.
Three targets
The investigators enrolled 24 men and 17 women with untreated CLL with del(17p) and/or TP53 mutations and adequate organ function (creatinine clearance rate of more than 50 mL/min). The median age was 62 (range 35-85 years); 78% of patients had Binet stage B or C disease. The median Cumulative Illness Rating Scale (CIRS) score was 3 (range 0 to 8).
All patients received treatment with the combination for 6 months. The CD20 inhibitor obinutuzumab was given in a dose of 1,000 mg on days 1, 8 and 15 of cycle 1 and day 1 of cycles 2-6. The BTK inhibitor ibrutinib was given continuously at a dose of 420 mg per day beginning on the first day of the first cycle. Venetoclax, a B-cell lymphoma 2 (BCL-2) inhibitor, was started on day 22 of cycle 1, and was increased to 400 mg per day over 5 weeks until the end of cycle 12.
If patients achieved a complete remission (CR) or CR with incomplete recovery of blood counts (CRi) according to International Workshop on CLL criteria at final restaging (performed with imaging at the end of cycle 12 followed by bone marrow biopsy 2 months later), ibrutinib would be stopped beginning at cycle 15. Patients who did not have a CR or CRi would continue on ibrutinib until cycle 36.
Encouraging results
All but 3 of the 41 patients reached final restaging. Analyses of efficacy and safety included all 41 patients.
The CR/CRi rate at final restaging, the primary endpoint, was accomplished in 24 patients (58.8%), and 14 patients (34.1%) had a partial response.
Of the three patients for whom responses could not be assessed, two died (one from ovarian cancer which was retrospectively determined to have been present at enrollment, and one at cycle 9 from cardiac failure), and the third patient withdrew consent at cycle 10.
In all, 33 patients (80.5%) were MRD-negative in peripheral blood, 4 remained MRD positive, and 4 were not assessed. Per protocol, 22 patients with undetectable MRD and a CR or CRi discontinued therapy at week 15. An additional 13 patients also discontinued therapy because of adverse events or other reasons, and 6 remained on therapy beyond cycle 15.
The most frequent adverse events of any grade through the end of cycle 14 were gastrointestinal disorders in 83%, none higher than grade 2; infections and infestations in 70.7%, of which 19.5% were grade 3 or greater in severity; and blood and lymphatic system disorders in 58.5%, most of which (53.7%) were grade 3 or greater.
Cardiac disorders were reported in 19.5% of all patients, including 12.2% with atrial fibrillation; grade 3 or greater atrial fibrillation occurred in 2.4% of patients.
There was one case each of cerebral aspergillosis, progressive multifocal leukoencephalopathy (without PCR testing), urosepsis, staphylococcal sepsis and febrile infection.
Laboratory confirmed tumor lysis syndrome, all grade 3 or greater, was reported in 9.8% of patients. Infusion-related reactions were reported in 29.3% of patients, with a total of 7.3% being grade 3 or greater.
The trial was supported by Janssen-Cilag and Roche. Dr. Huber disclosed travel reimbursement from Novartis. Dr. Danilov disclosed consulting for AbbVie, Janssen, and Genentech.
SOURCE: Huber H et al. EHA Congress. Abstract S157.
For patients with high-risk chronic lymphocytic leukemia (CLL), first-line therapy with a triple combination of targeted agents showed encouraging response rates in the phase 2 CLL2-GIVe trial.
Among 41 patients with untreated CLL bearing deleterious TP53 mutations and/or the 17p chromosomal deletion who received the GIVe regimen consisting of obinutuzumab (Gazyva), ibrutinib (Imbruvica), and venetoclax (Venclexta), the complete response rate at final restaging was 58.5%, and 33 patients with a confirmed response were negative for minimal residual disease after a median follow-up of 18.6 months, reported Henriette Huber, MD, of University Hospital Ulm, Germany.
“The GIVe regimen is promising first-line therapy for patients with high-risk CLL,” she said in a presentation during the virtual annual congress of the European Hematology Association.
The overall safety profile of the combination was acceptable, she said, but added that “some higher-grade infections are of concern.” The rate of grade 3 or greater infections/infestations in the study was 19.5%.
Sound rationale (with caveat)
Another adverse event of concern is the rate of atrial fibrillation in the comparatively young patient population (median age 62), noted Alexey Danilov, MD, PhD, of City of Hope in Duarte Calif., who commented on the study for MDedge.
He pointed out that second-generation Bruton’s tyrosine kinase (BTK) inhibitors such as acalabrutinib (Calquence) may pose a lower risk of atrial fibrillation than the BTK inhibitor ibrutinib used in the CLL2-GIVe study.
In general, however, the rationale for the combination is sound, Dr. Danilov said.
“Of all the patient populations that we deal with within CLL, this probably would be most appropriate for this type of therapy. Patients with deletion 17p or TP53 mutations still represent an unmet medical need compared to other patients who don’t have those mutations,” he said.
Patients with CLL bearing the mutations have lower clinical response rates to novel therapies and generally do not respond well to chemoimmunotherapy, he said.
“The question becomes whether using these all at the same time, versus sequential strategies – using one drug and then after that, at relapse, another – is better, and obviously this trial doesn’t address that,” he said.
Three targets
The investigators enrolled 24 men and 17 women with untreated CLL with del(17p) and/or TP53 mutations and adequate organ function (creatinine clearance rate of more than 50 mL/min). The median age was 62 (range 35-85 years); 78% of patients had Binet stage B or C disease. The median Cumulative Illness Rating Scale (CIRS) score was 3 (range 0 to 8).
All patients received treatment with the combination for 6 months. The CD20 inhibitor obinutuzumab was given in a dose of 1,000 mg on days 1, 8 and 15 of cycle 1 and day 1 of cycles 2-6. The BTK inhibitor ibrutinib was given continuously at a dose of 420 mg per day beginning on the first day of the first cycle. Venetoclax, a B-cell lymphoma 2 (BCL-2) inhibitor, was started on day 22 of cycle 1, and was increased to 400 mg per day over 5 weeks until the end of cycle 12.
If patients achieved a complete remission (CR) or CR with incomplete recovery of blood counts (CRi) according to International Workshop on CLL criteria at final restaging (performed with imaging at the end of cycle 12 followed by bone marrow biopsy 2 months later), ibrutinib would be stopped beginning at cycle 15. Patients who did not have a CR or CRi would continue on ibrutinib until cycle 36.
Encouraging results
All but 3 of the 41 patients reached final restaging. Analyses of efficacy and safety included all 41 patients.
The CR/CRi rate at final restaging, the primary endpoint, was accomplished in 24 patients (58.8%), and 14 patients (34.1%) had a partial response.
Of the three patients for whom responses could not be assessed, two died (one from ovarian cancer which was retrospectively determined to have been present at enrollment, and one at cycle 9 from cardiac failure), and the third patient withdrew consent at cycle 10.
In all, 33 patients (80.5%) were MRD-negative in peripheral blood, 4 remained MRD positive, and 4 were not assessed. Per protocol, 22 patients with undetectable MRD and a CR or CRi discontinued therapy at week 15. An additional 13 patients also discontinued therapy because of adverse events or other reasons, and 6 remained on therapy beyond cycle 15.
The most frequent adverse events of any grade through the end of cycle 14 were gastrointestinal disorders in 83%, none higher than grade 2; infections and infestations in 70.7%, of which 19.5% were grade 3 or greater in severity; and blood and lymphatic system disorders in 58.5%, most of which (53.7%) were grade 3 or greater.
Cardiac disorders were reported in 19.5% of all patients, including 12.2% with atrial fibrillation; grade 3 or greater atrial fibrillation occurred in 2.4% of patients.
There was one case each of cerebral aspergillosis, progressive multifocal leukoencephalopathy (without PCR testing), urosepsis, staphylococcal sepsis and febrile infection.
Laboratory confirmed tumor lysis syndrome, all grade 3 or greater, was reported in 9.8% of patients. Infusion-related reactions were reported in 29.3% of patients, with a total of 7.3% being grade 3 or greater.
The trial was supported by Janssen-Cilag and Roche. Dr. Huber disclosed travel reimbursement from Novartis. Dr. Danilov disclosed consulting for AbbVie, Janssen, and Genentech.
SOURCE: Huber H et al. EHA Congress. Abstract S157.
For patients with high-risk chronic lymphocytic leukemia (CLL), first-line therapy with a triple combination of targeted agents showed encouraging response rates in the phase 2 CLL2-GIVe trial.
Among 41 patients with untreated CLL bearing deleterious TP53 mutations and/or the 17p chromosomal deletion who received the GIVe regimen consisting of obinutuzumab (Gazyva), ibrutinib (Imbruvica), and venetoclax (Venclexta), the complete response rate at final restaging was 58.5%, and 33 patients with a confirmed response were negative for minimal residual disease after a median follow-up of 18.6 months, reported Henriette Huber, MD, of University Hospital Ulm, Germany.
“The GIVe regimen is promising first-line therapy for patients with high-risk CLL,” she said in a presentation during the virtual annual congress of the European Hematology Association.
The overall safety profile of the combination was acceptable, she said, but added that “some higher-grade infections are of concern.” The rate of grade 3 or greater infections/infestations in the study was 19.5%.
Sound rationale (with caveat)
Another adverse event of concern is the rate of atrial fibrillation in the comparatively young patient population (median age 62), noted Alexey Danilov, MD, PhD, of City of Hope in Duarte Calif., who commented on the study for MDedge.
He pointed out that second-generation Bruton’s tyrosine kinase (BTK) inhibitors such as acalabrutinib (Calquence) may pose a lower risk of atrial fibrillation than the BTK inhibitor ibrutinib used in the CLL2-GIVe study.
In general, however, the rationale for the combination is sound, Dr. Danilov said.
“Of all the patient populations that we deal with within CLL, this probably would be most appropriate for this type of therapy. Patients with deletion 17p or TP53 mutations still represent an unmet medical need compared to other patients who don’t have those mutations,” he said.
Patients with CLL bearing the mutations have lower clinical response rates to novel therapies and generally do not respond well to chemoimmunotherapy, he said.
“The question becomes whether using these all at the same time, versus sequential strategies – using one drug and then after that, at relapse, another – is better, and obviously this trial doesn’t address that,” he said.
Three targets
The investigators enrolled 24 men and 17 women with untreated CLL with del(17p) and/or TP53 mutations and adequate organ function (creatinine clearance rate of more than 50 mL/min). The median age was 62 (range 35-85 years); 78% of patients had Binet stage B or C disease. The median Cumulative Illness Rating Scale (CIRS) score was 3 (range 0 to 8).
All patients received treatment with the combination for 6 months. The CD20 inhibitor obinutuzumab was given in a dose of 1,000 mg on days 1, 8 and 15 of cycle 1 and day 1 of cycles 2-6. The BTK inhibitor ibrutinib was given continuously at a dose of 420 mg per day beginning on the first day of the first cycle. Venetoclax, a B-cell lymphoma 2 (BCL-2) inhibitor, was started on day 22 of cycle 1, and was increased to 400 mg per day over 5 weeks until the end of cycle 12.
If patients achieved a complete remission (CR) or CR with incomplete recovery of blood counts (CRi) according to International Workshop on CLL criteria at final restaging (performed with imaging at the end of cycle 12 followed by bone marrow biopsy 2 months later), ibrutinib would be stopped beginning at cycle 15. Patients who did not have a CR or CRi would continue on ibrutinib until cycle 36.
Encouraging results
All but 3 of the 41 patients reached final restaging. Analyses of efficacy and safety included all 41 patients.
The CR/CRi rate at final restaging, the primary endpoint, was accomplished in 24 patients (58.8%), and 14 patients (34.1%) had a partial response.
Of the three patients for whom responses could not be assessed, two died (one from ovarian cancer which was retrospectively determined to have been present at enrollment, and one at cycle 9 from cardiac failure), and the third patient withdrew consent at cycle 10.
In all, 33 patients (80.5%) were MRD-negative in peripheral blood, 4 remained MRD positive, and 4 were not assessed. Per protocol, 22 patients with undetectable MRD and a CR or CRi discontinued therapy at week 15. An additional 13 patients also discontinued therapy because of adverse events or other reasons, and 6 remained on therapy beyond cycle 15.
The most frequent adverse events of any grade through the end of cycle 14 were gastrointestinal disorders in 83%, none higher than grade 2; infections and infestations in 70.7%, of which 19.5% were grade 3 or greater in severity; and blood and lymphatic system disorders in 58.5%, most of which (53.7%) were grade 3 or greater.
Cardiac disorders were reported in 19.5% of all patients, including 12.2% with atrial fibrillation; grade 3 or greater atrial fibrillation occurred in 2.4% of patients.
There was one case each of cerebral aspergillosis, progressive multifocal leukoencephalopathy (without PCR testing), urosepsis, staphylococcal sepsis and febrile infection.
Laboratory confirmed tumor lysis syndrome, all grade 3 or greater, was reported in 9.8% of patients. Infusion-related reactions were reported in 29.3% of patients, with a total of 7.3% being grade 3 or greater.
The trial was supported by Janssen-Cilag and Roche. Dr. Huber disclosed travel reimbursement from Novartis. Dr. Danilov disclosed consulting for AbbVie, Janssen, and Genentech.
SOURCE: Huber H et al. EHA Congress. Abstract S157.
FROM EHA CONGRESS
Subcutaneous hep A vaccination as effective as intramuscular for bleeding disorder patients
Subcutaneous hepatitis A vaccination is as effective and may be safer for patients with bleeding disorders, according to a study by Mayumi Nakasone, MD, and colleagues.
The large number of donor exposures in bleeding disorder patients who require routine use of clotting factor concentrates remains a concern with regard to the risk of virus infection. Therefore, vaccinations for viruses such as hepatitis A are recommended. Although the intramuscular (IM) route is recommended for hepatitis A vaccination, patients with bleeding disorders have been advised to avoid IM injections because of the risk of bleeding and bruising of muscles, requiring infusion of clotting factor concentrates or other blood products for its treatment, according to Dr. Nakasone of the University of São Paulo and colleagues. They assessed 78 adult and pediatric patients with blood disorders randomized to vaccination for hepatitis A either subcutaneously (SC) or IM, according their study published on Vaccine.
The study was conducted at a single hemophilia center between May 2006 and February 2017.
Among the 78 patients, 58 (74.4%) presented hemophilia A (34 of the SC group and 24 of the IM group), 13 (16.7%) hemophilia B (4 of the SC group and 9 of the IM group) and 7 (8.9%) other bleeding disorders. There were no statistically significant differences between the SC and the IM groups in patients diagnosis or sex.
A total of 38 patients had serology performed after the first vaccine dose, determining seroconversion rates of 83.3% and 90.0% for the SC and the IM group, respectively, a nonsignificant difference. After the second vaccine dose, the seroconversion rate for the SC group was 97.5% and for the IM group was 97.4%, also a nonsignificant difference.
At a median of 9 years after a second vaccine dose, antibody titers for the SC group were slightly greater than the IM group (7.6 vs. 7.4), but this was also not a significant difference. There were no serious adverse events in both groups, according to Dr. Nakasone and colleagues. And although twice as many patients of the IM group required clotting factor concentrates for adverse events, compared with the SC group (15.8% vs. 7.5%), the difference was not significant.
“Hepatitis A vaccine administered subcutaneously is as immunogenic, long-term protective, and even safer as the intramuscular route for both children and adults not only with hemophilia, but also with other bleeding disorders,” the researchers concluded.
The authors declared that they had no disclosures.
SOURCE: Nakasone M et al. Vaccine 2020;38:4162-6.
Subcutaneous hepatitis A vaccination is as effective and may be safer for patients with bleeding disorders, according to a study by Mayumi Nakasone, MD, and colleagues.
The large number of donor exposures in bleeding disorder patients who require routine use of clotting factor concentrates remains a concern with regard to the risk of virus infection. Therefore, vaccinations for viruses such as hepatitis A are recommended. Although the intramuscular (IM) route is recommended for hepatitis A vaccination, patients with bleeding disorders have been advised to avoid IM injections because of the risk of bleeding and bruising of muscles, requiring infusion of clotting factor concentrates or other blood products for its treatment, according to Dr. Nakasone of the University of São Paulo and colleagues. They assessed 78 adult and pediatric patients with blood disorders randomized to vaccination for hepatitis A either subcutaneously (SC) or IM, according their study published on Vaccine.
The study was conducted at a single hemophilia center between May 2006 and February 2017.
Among the 78 patients, 58 (74.4%) presented hemophilia A (34 of the SC group and 24 of the IM group), 13 (16.7%) hemophilia B (4 of the SC group and 9 of the IM group) and 7 (8.9%) other bleeding disorders. There were no statistically significant differences between the SC and the IM groups in patients diagnosis or sex.
A total of 38 patients had serology performed after the first vaccine dose, determining seroconversion rates of 83.3% and 90.0% for the SC and the IM group, respectively, a nonsignificant difference. After the second vaccine dose, the seroconversion rate for the SC group was 97.5% and for the IM group was 97.4%, also a nonsignificant difference.
At a median of 9 years after a second vaccine dose, antibody titers for the SC group were slightly greater than the IM group (7.6 vs. 7.4), but this was also not a significant difference. There were no serious adverse events in both groups, according to Dr. Nakasone and colleagues. And although twice as many patients of the IM group required clotting factor concentrates for adverse events, compared with the SC group (15.8% vs. 7.5%), the difference was not significant.
“Hepatitis A vaccine administered subcutaneously is as immunogenic, long-term protective, and even safer as the intramuscular route for both children and adults not only with hemophilia, but also with other bleeding disorders,” the researchers concluded.
The authors declared that they had no disclosures.
SOURCE: Nakasone M et al. Vaccine 2020;38:4162-6.
Subcutaneous hepatitis A vaccination is as effective and may be safer for patients with bleeding disorders, according to a study by Mayumi Nakasone, MD, and colleagues.
The large number of donor exposures in bleeding disorder patients who require routine use of clotting factor concentrates remains a concern with regard to the risk of virus infection. Therefore, vaccinations for viruses such as hepatitis A are recommended. Although the intramuscular (IM) route is recommended for hepatitis A vaccination, patients with bleeding disorders have been advised to avoid IM injections because of the risk of bleeding and bruising of muscles, requiring infusion of clotting factor concentrates or other blood products for its treatment, according to Dr. Nakasone of the University of São Paulo and colleagues. They assessed 78 adult and pediatric patients with blood disorders randomized to vaccination for hepatitis A either subcutaneously (SC) or IM, according their study published on Vaccine.
The study was conducted at a single hemophilia center between May 2006 and February 2017.
Among the 78 patients, 58 (74.4%) presented hemophilia A (34 of the SC group and 24 of the IM group), 13 (16.7%) hemophilia B (4 of the SC group and 9 of the IM group) and 7 (8.9%) other bleeding disorders. There were no statistically significant differences between the SC and the IM groups in patients diagnosis or sex.
A total of 38 patients had serology performed after the first vaccine dose, determining seroconversion rates of 83.3% and 90.0% for the SC and the IM group, respectively, a nonsignificant difference. After the second vaccine dose, the seroconversion rate for the SC group was 97.5% and for the IM group was 97.4%, also a nonsignificant difference.
At a median of 9 years after a second vaccine dose, antibody titers for the SC group were slightly greater than the IM group (7.6 vs. 7.4), but this was also not a significant difference. There were no serious adverse events in both groups, according to Dr. Nakasone and colleagues. And although twice as many patients of the IM group required clotting factor concentrates for adverse events, compared with the SC group (15.8% vs. 7.5%), the difference was not significant.
“Hepatitis A vaccine administered subcutaneously is as immunogenic, long-term protective, and even safer as the intramuscular route for both children and adults not only with hemophilia, but also with other bleeding disorders,” the researchers concluded.
The authors declared that they had no disclosures.
SOURCE: Nakasone M et al. Vaccine 2020;38:4162-6.
FROM VACCINE
Key clinical point: Subcutaneous hepatitis A vaccination is as immunogenic, but appeared safer than intramuscular injections for bleeding disorder patients.
Major finding: A total of 38 patients assessed after first vaccine dose showed seroconversion rates of 83.3% and 90.0% for the subcutaneous versus the intramuscular group, respectively.
Study details: A comparison of hepatitis A vaccination administered subcutaneously or intramuscularly in 78 children and adults with hemophilia and other bleeding disorders.
Disclosures: The authors declared that they had no disclosures.
Source: Nakasone M et al. Vaccine. 2020;38:4162-6.
Tandem transplantation, long-term maintenance may extend MM remission
Tandem autologous hematopoietic stem cell transplantation (HSCT) could extend progression-free survival (PFS) for some patients with newly diagnosed multiple myeloma, based on long-term data from the phase 3 STaMINA trial.
While the intent-to-treat analysis showed no difference in 6-year PFS rate between single versus tandem HSCT, the as-treated analysis showed that patients who received two transplants had a 6-year PFS rate that was approximately 10% higher than those who received just one transplant, reported lead author Parameswaran Hari, MD, of the Medical College of Wisconsin, Milwaukee, who presented the findings as part of the American Society of Clinical Oncology virtual scientific program.
The STaMINA trial, also known as BMT CTN 0702, involved 758 patients who were randomized to receive one of three treatment regimens followed by 3 years of maintenance lenalidomide: tandem HSCT (auto/auto), single HSCT plus consolidation with lenalidomide/bortezomib/dexamethasone (auto/RVD), and single HSCT (auto/len).
“At the time, we intended the study to stop approximately 38 months from randomization, allowing for the time for transplant, and then 3 years of maintenance,” Dr. Hari said. However, as the results of lenalidomide maintenance in CALGB 00104 study were reported, they allowed for a follow-on protocol, which provided patients who are progression-free at the completion of the original STaMINA trial to go on to a second follow-on trial, which allowed lenalidomide maintenance on an indefinite basis, he added.
The present analysis looked at the long-term results of this follow-on trial, including the impact of discontinuing lenalidomide.
Aligning with the original study, the present intent-to-treat analysis showed no significant difference between treatment arms for 6-year PFS rate or overall survival. Respectively, PFS rates for auto/auto, auto/RVD, and auto/len were 43.9%, 39.7%, and 40.9% (P = .6).
But 32% of patients in the tandem group never underwent second HSCT, Dr. Hari noted, prompting the as-treated analysis. Although overall survival remained similar between groups, the 6-year PFS was significantly higher for patients who underwent tandem HSCT, at 49.4%, compared with 39.7% for auto/RVD and 38.6% for auto/len (P = .03).
Subgroup analysis showed the statistical benefit of tandem HSCT was driven by high-risk patients, who had a significantly better PFS after tandem transplant, compared with standard-risk patients, who showed no significant benefit.
Dr. Hari called the findings “provocative.”
“The tandem auto approach may still be relevant in high-risk multiple myeloma patients,” he said.
Dr. Hari and his colleagues also found that patients who stayed on maintenance lenalidomide after 38 months had a better 5-year PFS rate than those who discontinued maintenance therapy (79.5% vs. 61%; P = .0004). Subgroup analysis showed this benefit was statistically significant among patients with standard-risk disease (86.3% vs. 66%; P less than .001) but not among those in the high-risk subgroup (86.7% vs. 67.8%; P = .2).
However, Dr. Hari suggested that, based on the similarity of proportions between subgroups, the lack of significance in the high-risk subgroup was likely because of small sample size, suggesting the benefit of maintenance was actually shared across risk strata.
“Lenalidomide maintenance becomes a significant factor for preventing patients from progression,” Dr. Hari said, noting that the tandem transplant approach requires further study, and that he and his colleagues would soon publish minimal residual disease data.
He finished his presentation with a clear clinical recommendation. “Preplanned lenalidomide discontinuation at 3 years is not recommended based on inferior progression-free survival among those who stopped such therapy,” he said.
Invited discussant Joshua R. Richter, MD, of the Icahn School of Medicine at Mount Sinai, New York, said the findings encourage high-dose maintenance therapy, and for some, tandem HSCT.
“The STaMINA study presented today supports the notion that some patients with high-risk disease still may benefit and have further tumor burden reduction with the second transplant that leads to deeper remissions and hopefully abrogates diminished outcomes,” Dr. Richter said during a virtual presentation.
But improvements are needed to better identify such patients, Dr. Richter added. He highlighted a lack of standardization in risk modeling, with various factors currently employed, such as patient characteristics and genomic markers, among several others.
“Better definitions will allow us to cross compare and make true analyses about how to manage these patients,” Dr. Richter said. “Despite the improvements across the board that we’ve seen in myeloma patients, high-risk disease continues to represent a more complicated arena. And patients continue to suffer from worse outcomes, despite all of the other advances.”
The study was funded by the National Institutes of Health. The investigators disclosed additional relationships with Amgen, Celgene, Novartis, and others. Dr. Richter disclosed affiliations with Takeda, Sanofi, Janssen, and others.
SOURCE: Hari et al. ASCO 2020. Abstract 8506.
Tandem autologous hematopoietic stem cell transplantation (HSCT) could extend progression-free survival (PFS) for some patients with newly diagnosed multiple myeloma, based on long-term data from the phase 3 STaMINA trial.
While the intent-to-treat analysis showed no difference in 6-year PFS rate between single versus tandem HSCT, the as-treated analysis showed that patients who received two transplants had a 6-year PFS rate that was approximately 10% higher than those who received just one transplant, reported lead author Parameswaran Hari, MD, of the Medical College of Wisconsin, Milwaukee, who presented the findings as part of the American Society of Clinical Oncology virtual scientific program.
The STaMINA trial, also known as BMT CTN 0702, involved 758 patients who were randomized to receive one of three treatment regimens followed by 3 years of maintenance lenalidomide: tandem HSCT (auto/auto), single HSCT plus consolidation with lenalidomide/bortezomib/dexamethasone (auto/RVD), and single HSCT (auto/len).
“At the time, we intended the study to stop approximately 38 months from randomization, allowing for the time for transplant, and then 3 years of maintenance,” Dr. Hari said. However, as the results of lenalidomide maintenance in CALGB 00104 study were reported, they allowed for a follow-on protocol, which provided patients who are progression-free at the completion of the original STaMINA trial to go on to a second follow-on trial, which allowed lenalidomide maintenance on an indefinite basis, he added.
The present analysis looked at the long-term results of this follow-on trial, including the impact of discontinuing lenalidomide.
Aligning with the original study, the present intent-to-treat analysis showed no significant difference between treatment arms for 6-year PFS rate or overall survival. Respectively, PFS rates for auto/auto, auto/RVD, and auto/len were 43.9%, 39.7%, and 40.9% (P = .6).
But 32% of patients in the tandem group never underwent second HSCT, Dr. Hari noted, prompting the as-treated analysis. Although overall survival remained similar between groups, the 6-year PFS was significantly higher for patients who underwent tandem HSCT, at 49.4%, compared with 39.7% for auto/RVD and 38.6% for auto/len (P = .03).
Subgroup analysis showed the statistical benefit of tandem HSCT was driven by high-risk patients, who had a significantly better PFS after tandem transplant, compared with standard-risk patients, who showed no significant benefit.
Dr. Hari called the findings “provocative.”
“The tandem auto approach may still be relevant in high-risk multiple myeloma patients,” he said.
Dr. Hari and his colleagues also found that patients who stayed on maintenance lenalidomide after 38 months had a better 5-year PFS rate than those who discontinued maintenance therapy (79.5% vs. 61%; P = .0004). Subgroup analysis showed this benefit was statistically significant among patients with standard-risk disease (86.3% vs. 66%; P less than .001) but not among those in the high-risk subgroup (86.7% vs. 67.8%; P = .2).
However, Dr. Hari suggested that, based on the similarity of proportions between subgroups, the lack of significance in the high-risk subgroup was likely because of small sample size, suggesting the benefit of maintenance was actually shared across risk strata.
“Lenalidomide maintenance becomes a significant factor for preventing patients from progression,” Dr. Hari said, noting that the tandem transplant approach requires further study, and that he and his colleagues would soon publish minimal residual disease data.
He finished his presentation with a clear clinical recommendation. “Preplanned lenalidomide discontinuation at 3 years is not recommended based on inferior progression-free survival among those who stopped such therapy,” he said.
Invited discussant Joshua R. Richter, MD, of the Icahn School of Medicine at Mount Sinai, New York, said the findings encourage high-dose maintenance therapy, and for some, tandem HSCT.
“The STaMINA study presented today supports the notion that some patients with high-risk disease still may benefit and have further tumor burden reduction with the second transplant that leads to deeper remissions and hopefully abrogates diminished outcomes,” Dr. Richter said during a virtual presentation.
But improvements are needed to better identify such patients, Dr. Richter added. He highlighted a lack of standardization in risk modeling, with various factors currently employed, such as patient characteristics and genomic markers, among several others.
“Better definitions will allow us to cross compare and make true analyses about how to manage these patients,” Dr. Richter said. “Despite the improvements across the board that we’ve seen in myeloma patients, high-risk disease continues to represent a more complicated arena. And patients continue to suffer from worse outcomes, despite all of the other advances.”
The study was funded by the National Institutes of Health. The investigators disclosed additional relationships with Amgen, Celgene, Novartis, and others. Dr. Richter disclosed affiliations with Takeda, Sanofi, Janssen, and others.
SOURCE: Hari et al. ASCO 2020. Abstract 8506.
Tandem autologous hematopoietic stem cell transplantation (HSCT) could extend progression-free survival (PFS) for some patients with newly diagnosed multiple myeloma, based on long-term data from the phase 3 STaMINA trial.
While the intent-to-treat analysis showed no difference in 6-year PFS rate between single versus tandem HSCT, the as-treated analysis showed that patients who received two transplants had a 6-year PFS rate that was approximately 10% higher than those who received just one transplant, reported lead author Parameswaran Hari, MD, of the Medical College of Wisconsin, Milwaukee, who presented the findings as part of the American Society of Clinical Oncology virtual scientific program.
The STaMINA trial, also known as BMT CTN 0702, involved 758 patients who were randomized to receive one of three treatment regimens followed by 3 years of maintenance lenalidomide: tandem HSCT (auto/auto), single HSCT plus consolidation with lenalidomide/bortezomib/dexamethasone (auto/RVD), and single HSCT (auto/len).
“At the time, we intended the study to stop approximately 38 months from randomization, allowing for the time for transplant, and then 3 years of maintenance,” Dr. Hari said. However, as the results of lenalidomide maintenance in CALGB 00104 study were reported, they allowed for a follow-on protocol, which provided patients who are progression-free at the completion of the original STaMINA trial to go on to a second follow-on trial, which allowed lenalidomide maintenance on an indefinite basis, he added.
The present analysis looked at the long-term results of this follow-on trial, including the impact of discontinuing lenalidomide.
Aligning with the original study, the present intent-to-treat analysis showed no significant difference between treatment arms for 6-year PFS rate or overall survival. Respectively, PFS rates for auto/auto, auto/RVD, and auto/len were 43.9%, 39.7%, and 40.9% (P = .6).
But 32% of patients in the tandem group never underwent second HSCT, Dr. Hari noted, prompting the as-treated analysis. Although overall survival remained similar between groups, the 6-year PFS was significantly higher for patients who underwent tandem HSCT, at 49.4%, compared with 39.7% for auto/RVD and 38.6% for auto/len (P = .03).
Subgroup analysis showed the statistical benefit of tandem HSCT was driven by high-risk patients, who had a significantly better PFS after tandem transplant, compared with standard-risk patients, who showed no significant benefit.
Dr. Hari called the findings “provocative.”
“The tandem auto approach may still be relevant in high-risk multiple myeloma patients,” he said.
Dr. Hari and his colleagues also found that patients who stayed on maintenance lenalidomide after 38 months had a better 5-year PFS rate than those who discontinued maintenance therapy (79.5% vs. 61%; P = .0004). Subgroup analysis showed this benefit was statistically significant among patients with standard-risk disease (86.3% vs. 66%; P less than .001) but not among those in the high-risk subgroup (86.7% vs. 67.8%; P = .2).
However, Dr. Hari suggested that, based on the similarity of proportions between subgroups, the lack of significance in the high-risk subgroup was likely because of small sample size, suggesting the benefit of maintenance was actually shared across risk strata.
“Lenalidomide maintenance becomes a significant factor for preventing patients from progression,” Dr. Hari said, noting that the tandem transplant approach requires further study, and that he and his colleagues would soon publish minimal residual disease data.
He finished his presentation with a clear clinical recommendation. “Preplanned lenalidomide discontinuation at 3 years is not recommended based on inferior progression-free survival among those who stopped such therapy,” he said.
Invited discussant Joshua R. Richter, MD, of the Icahn School of Medicine at Mount Sinai, New York, said the findings encourage high-dose maintenance therapy, and for some, tandem HSCT.
“The STaMINA study presented today supports the notion that some patients with high-risk disease still may benefit and have further tumor burden reduction with the second transplant that leads to deeper remissions and hopefully abrogates diminished outcomes,” Dr. Richter said during a virtual presentation.
But improvements are needed to better identify such patients, Dr. Richter added. He highlighted a lack of standardization in risk modeling, with various factors currently employed, such as patient characteristics and genomic markers, among several others.
“Better definitions will allow us to cross compare and make true analyses about how to manage these patients,” Dr. Richter said. “Despite the improvements across the board that we’ve seen in myeloma patients, high-risk disease continues to represent a more complicated arena. And patients continue to suffer from worse outcomes, despite all of the other advances.”
The study was funded by the National Institutes of Health. The investigators disclosed additional relationships with Amgen, Celgene, Novartis, and others. Dr. Richter disclosed affiliations with Takeda, Sanofi, Janssen, and others.
SOURCE: Hari et al. ASCO 2020. Abstract 8506.
FROM ASCO 2020
One-fifth of stem cell transplantation patients develop PTSD
Approximately one-fifth of patients undergoing hematopoietic stem cell transplantation (HSCT) develop posttraumatic stress disorder (PTSD), based on a retrospective analysis.
Patient factors at time of transplantation, such as low quality of life and high anxiety, predicted PTSD 6 months later, reported lead author Sarah Griffith, MD, of Massachusetts General Hospital, Boston, who presented findings as part of the American Society of Clinical Oncology virtual scientific program.
“We know that patients admitted for HSCT are often isolated in the hospital for a prolonged period of time, usually about 3-4 weeks, and that they endure substantial toxicities that impact both their physical and psychological well-being,” Dr. Griffith said. “We also know from the literature that HSCT can be considered a traumatic event and that it may lead to clinically significant PTSD symptoms.” But studies evaluating the prevalence and characteristics of PTSD in this patient population have been lacking, she noted.
Dr. Griffith and her colleagues therefore conducted a retrospective analysis involving 250 adults with hematologic malignancies who underwent autologous or allogeneic HSCT during clinical trials conducted from 2014 to 2016. Median patient age was 56 years.
The first objective of the study was to measure the prevalence of PTSD. The second was to characterize features of PTSD such as intrusion, which entails reliving experiences in the form of nightmares or flashbacks, and hypervigilance, which encompasses insomnia, irritability, and hyperarousal for threat. The third objective was to determine risk factors at baseline.
At time of admission for HSCT, after 2 weeks of hospitalization, and again 6 months after transplantation, patients were evaluated using the Functional Assessment of Cancer Therapy–Bone Marrow Transplant (FACT-BMT), and the Hospital Anxiety and Depression Scale (HADS), which measured of quality of life, anxiety, and depression. Six months after HSCT, patients also underwent screening for PTSD with the Post-Traumatic Stress Checklist (PTSD-CL). Multivariate regression models were used to determine predictive risk factors.
Six months after HSCT, 18.9% of patients had clinically significant PTSD symptoms; most common were symptoms of avoidance (92.3%), hypervigilance (92.3%), and intrusion (76.9%). Among those who did not have clinically significant PTSD, almost one-quarter (24.5%) demonstrated significant hypervigilance, while 13.7% showed symptoms of avoidance.
“Clinically significant PTSD symptoms are common in the transplant population,” Dr. Griffith said.
Baseline predictors of PTSD included single status and lower quality of life. More severe PTSD was predicted by single status, younger age, higher baseline scores for anxiety or depression, and increased anxiety during hospitalization.
Concluding her presentation, Dr. Griffith said that the findings, while correlative and not causative, should prompt concern and intervention.
“It is very important to be aware of and to manage PTSD symptoms in these patients,” she said. “There are several baseline factors that can be identified prior to HSCT that may illuminate patients at risk for developing worse PTSD symptoms down the road, and these patients may benefit from tailored supportive care interventions.”
Specifically, Dr. Griffith recommended integrating palliative care into hospitalization, as this has been shown to reduce anxiety.
In a virtual presentation, invited discussant Nirali N. Shah, MD, of the National Cancer Institute, Bethesda, Md., highlighted the importance of the findings, while also noting that the impact of palliative care on risk of PTSD has yet to be demonstrated.
Dr. Shah suggested that future research may be improved through use of a formal diagnosis for PTSD, instead of a PTSD checklist, as was used in the present study.
“And certainly long-term follow-up would be important to evaluate the utility of this tool looking at symptoms beyond 6 months,” she said.
Dr. Shah went on to discuss the relevance of the findings for pediatric populations, as children may face unique risk factors and consequences related to PTSD.
“[PTSD in children] may be impacted by family dynamics and structure,” Dr. Shah said. “Children may also have significant neurocognitive implications as a result of their underlying disease or prior therapy. They may experience chronic pain as they go out into adulthood and long-term survivorship, and may also struggle with symptoms of anxiety and depression.”
According to Dr. Shah, one previous study involving more than 6,000 adult survivors of childhood cancer found that PTSD was relatively common, with prevalence rate of 9%, suggesting that interventional work is necessary.
“Applying the data in the study from Griffith et al. suggests that evaluation in the more proximal posttransplant period for children is needed to specifically evaluate PTSD and symptoms thereof, and to try to use this to identify an opportunity for intervention,” Dr. Shah said.
“Pediatric-specific assessments are essential to optimally capture disease and/or age-specific considerations,” she added.
The study was funded by the Lymphoma and Leukemia Society. The investigators disclosed additional relationships with Vector Oncology, Pfizer, AstraZeneca, and Gaido Health/BCG Digital Ventures.
SOURCE: Griffith et al. ASCO 2020. Abstract # 7505.
Approximately one-fifth of patients undergoing hematopoietic stem cell transplantation (HSCT) develop posttraumatic stress disorder (PTSD), based on a retrospective analysis.
Patient factors at time of transplantation, such as low quality of life and high anxiety, predicted PTSD 6 months later, reported lead author Sarah Griffith, MD, of Massachusetts General Hospital, Boston, who presented findings as part of the American Society of Clinical Oncology virtual scientific program.
“We know that patients admitted for HSCT are often isolated in the hospital for a prolonged period of time, usually about 3-4 weeks, and that they endure substantial toxicities that impact both their physical and psychological well-being,” Dr. Griffith said. “We also know from the literature that HSCT can be considered a traumatic event and that it may lead to clinically significant PTSD symptoms.” But studies evaluating the prevalence and characteristics of PTSD in this patient population have been lacking, she noted.
Dr. Griffith and her colleagues therefore conducted a retrospective analysis involving 250 adults with hematologic malignancies who underwent autologous or allogeneic HSCT during clinical trials conducted from 2014 to 2016. Median patient age was 56 years.
The first objective of the study was to measure the prevalence of PTSD. The second was to characterize features of PTSD such as intrusion, which entails reliving experiences in the form of nightmares or flashbacks, and hypervigilance, which encompasses insomnia, irritability, and hyperarousal for threat. The third objective was to determine risk factors at baseline.
At time of admission for HSCT, after 2 weeks of hospitalization, and again 6 months after transplantation, patients were evaluated using the Functional Assessment of Cancer Therapy–Bone Marrow Transplant (FACT-BMT), and the Hospital Anxiety and Depression Scale (HADS), which measured of quality of life, anxiety, and depression. Six months after HSCT, patients also underwent screening for PTSD with the Post-Traumatic Stress Checklist (PTSD-CL). Multivariate regression models were used to determine predictive risk factors.
Six months after HSCT, 18.9% of patients had clinically significant PTSD symptoms; most common were symptoms of avoidance (92.3%), hypervigilance (92.3%), and intrusion (76.9%). Among those who did not have clinically significant PTSD, almost one-quarter (24.5%) demonstrated significant hypervigilance, while 13.7% showed symptoms of avoidance.
“Clinically significant PTSD symptoms are common in the transplant population,” Dr. Griffith said.
Baseline predictors of PTSD included single status and lower quality of life. More severe PTSD was predicted by single status, younger age, higher baseline scores for anxiety or depression, and increased anxiety during hospitalization.
Concluding her presentation, Dr. Griffith said that the findings, while correlative and not causative, should prompt concern and intervention.
“It is very important to be aware of and to manage PTSD symptoms in these patients,” she said. “There are several baseline factors that can be identified prior to HSCT that may illuminate patients at risk for developing worse PTSD symptoms down the road, and these patients may benefit from tailored supportive care interventions.”
Specifically, Dr. Griffith recommended integrating palliative care into hospitalization, as this has been shown to reduce anxiety.
In a virtual presentation, invited discussant Nirali N. Shah, MD, of the National Cancer Institute, Bethesda, Md., highlighted the importance of the findings, while also noting that the impact of palliative care on risk of PTSD has yet to be demonstrated.
Dr. Shah suggested that future research may be improved through use of a formal diagnosis for PTSD, instead of a PTSD checklist, as was used in the present study.
“And certainly long-term follow-up would be important to evaluate the utility of this tool looking at symptoms beyond 6 months,” she said.
Dr. Shah went on to discuss the relevance of the findings for pediatric populations, as children may face unique risk factors and consequences related to PTSD.
“[PTSD in children] may be impacted by family dynamics and structure,” Dr. Shah said. “Children may also have significant neurocognitive implications as a result of their underlying disease or prior therapy. They may experience chronic pain as they go out into adulthood and long-term survivorship, and may also struggle with symptoms of anxiety and depression.”
According to Dr. Shah, one previous study involving more than 6,000 adult survivors of childhood cancer found that PTSD was relatively common, with prevalence rate of 9%, suggesting that interventional work is necessary.
“Applying the data in the study from Griffith et al. suggests that evaluation in the more proximal posttransplant period for children is needed to specifically evaluate PTSD and symptoms thereof, and to try to use this to identify an opportunity for intervention,” Dr. Shah said.
“Pediatric-specific assessments are essential to optimally capture disease and/or age-specific considerations,” she added.
The study was funded by the Lymphoma and Leukemia Society. The investigators disclosed additional relationships with Vector Oncology, Pfizer, AstraZeneca, and Gaido Health/BCG Digital Ventures.
SOURCE: Griffith et al. ASCO 2020. Abstract # 7505.
Approximately one-fifth of patients undergoing hematopoietic stem cell transplantation (HSCT) develop posttraumatic stress disorder (PTSD), based on a retrospective analysis.
Patient factors at time of transplantation, such as low quality of life and high anxiety, predicted PTSD 6 months later, reported lead author Sarah Griffith, MD, of Massachusetts General Hospital, Boston, who presented findings as part of the American Society of Clinical Oncology virtual scientific program.
“We know that patients admitted for HSCT are often isolated in the hospital for a prolonged period of time, usually about 3-4 weeks, and that they endure substantial toxicities that impact both their physical and psychological well-being,” Dr. Griffith said. “We also know from the literature that HSCT can be considered a traumatic event and that it may lead to clinically significant PTSD symptoms.” But studies evaluating the prevalence and characteristics of PTSD in this patient population have been lacking, she noted.
Dr. Griffith and her colleagues therefore conducted a retrospective analysis involving 250 adults with hematologic malignancies who underwent autologous or allogeneic HSCT during clinical trials conducted from 2014 to 2016. Median patient age was 56 years.
The first objective of the study was to measure the prevalence of PTSD. The second was to characterize features of PTSD such as intrusion, which entails reliving experiences in the form of nightmares or flashbacks, and hypervigilance, which encompasses insomnia, irritability, and hyperarousal for threat. The third objective was to determine risk factors at baseline.
At time of admission for HSCT, after 2 weeks of hospitalization, and again 6 months after transplantation, patients were evaluated using the Functional Assessment of Cancer Therapy–Bone Marrow Transplant (FACT-BMT), and the Hospital Anxiety and Depression Scale (HADS), which measured of quality of life, anxiety, and depression. Six months after HSCT, patients also underwent screening for PTSD with the Post-Traumatic Stress Checklist (PTSD-CL). Multivariate regression models were used to determine predictive risk factors.
Six months after HSCT, 18.9% of patients had clinically significant PTSD symptoms; most common were symptoms of avoidance (92.3%), hypervigilance (92.3%), and intrusion (76.9%). Among those who did not have clinically significant PTSD, almost one-quarter (24.5%) demonstrated significant hypervigilance, while 13.7% showed symptoms of avoidance.
“Clinically significant PTSD symptoms are common in the transplant population,” Dr. Griffith said.
Baseline predictors of PTSD included single status and lower quality of life. More severe PTSD was predicted by single status, younger age, higher baseline scores for anxiety or depression, and increased anxiety during hospitalization.
Concluding her presentation, Dr. Griffith said that the findings, while correlative and not causative, should prompt concern and intervention.
“It is very important to be aware of and to manage PTSD symptoms in these patients,” she said. “There are several baseline factors that can be identified prior to HSCT that may illuminate patients at risk for developing worse PTSD symptoms down the road, and these patients may benefit from tailored supportive care interventions.”
Specifically, Dr. Griffith recommended integrating palliative care into hospitalization, as this has been shown to reduce anxiety.
In a virtual presentation, invited discussant Nirali N. Shah, MD, of the National Cancer Institute, Bethesda, Md., highlighted the importance of the findings, while also noting that the impact of palliative care on risk of PTSD has yet to be demonstrated.
Dr. Shah suggested that future research may be improved through use of a formal diagnosis for PTSD, instead of a PTSD checklist, as was used in the present study.
“And certainly long-term follow-up would be important to evaluate the utility of this tool looking at symptoms beyond 6 months,” she said.
Dr. Shah went on to discuss the relevance of the findings for pediatric populations, as children may face unique risk factors and consequences related to PTSD.
“[PTSD in children] may be impacted by family dynamics and structure,” Dr. Shah said. “Children may also have significant neurocognitive implications as a result of their underlying disease or prior therapy. They may experience chronic pain as they go out into adulthood and long-term survivorship, and may also struggle with symptoms of anxiety and depression.”
According to Dr. Shah, one previous study involving more than 6,000 adult survivors of childhood cancer found that PTSD was relatively common, with prevalence rate of 9%, suggesting that interventional work is necessary.
“Applying the data in the study from Griffith et al. suggests that evaluation in the more proximal posttransplant period for children is needed to specifically evaluate PTSD and symptoms thereof, and to try to use this to identify an opportunity for intervention,” Dr. Shah said.
“Pediatric-specific assessments are essential to optimally capture disease and/or age-specific considerations,” she added.
The study was funded by the Lymphoma and Leukemia Society. The investigators disclosed additional relationships with Vector Oncology, Pfizer, AstraZeneca, and Gaido Health/BCG Digital Ventures.
SOURCE: Griffith et al. ASCO 2020. Abstract # 7505.
FROM ASCO 2020
Biologics may carry melanoma risk for patients with immune-mediated inflammatory diseases
The in a systematic review and meta-analysis published in JAMA Dermatology.
The studies included in the analysis, however, had limitations, including a lack of those comparing biologic and conventional systemic therapy in psoriasis and inflammatory bowel disease (IBD), according to Shamarke Esse, MRes, of the division of musculoskeletal and dermatological sciences at the University of Manchester (England) and colleagues. “We advocate for more large, well-designed studies of this issue to be performed to help improve certainty” regarding this association, they wrote.
Previous studies that have found an increased risk of melanoma in patients on biologics for psoriasis, rheumatoid arthritis, and IBD have “typically used the general population as the comparator,” they noted. There is a large amount of evidence that has established short-term efficacy and safety of biologics, compared with conventional systemic treatments, but concerns about longer-term cancer risk associated with biologics remains a concern. Moreover, they added, “melanoma is a highly immunogenic skin cancer and therefore of concern to patients treated with TNFIs [tumor necrosis factor inhibitors] because melanoma risk increases with suppression of the immune system and TNF-alpha plays an important role in the immune surveillance of tumors.12,13
In their review, the researchers identified seven cohort studies from MEDLINE, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL) databases published between January 1995 and February 2019 that evaluated melanoma risk in about 34,000 patients receiving biologics and 135,370 patients who had never been treated with biologics, and were receiving conventional systemic therapy for psoriasis, RA, or IBD. Of these, four studies were in patients with RA, two studies were in patients with IBD, and a single study was in patients with psoriasis. Six studies examined patients taking TNF inhibitors, but only one of six studies had information on specific TNF inhibitors (adalimumab, etanercept, and infliximab) in patients with RA. One study evaluated abatacept and rituximab in RA patients.
The researchers analyzed the pooled relative risk across all studies. Compared with patients who received conventional systemic therapy, there was a nonsignificant association with risk of melanoma in patients with psoriasis (hazard ratio, 1.57; 95% confidence interval, 0.61-4.09), RA (pooled relative risk, 1.20; 95% CI, 0.83-1.74), and IBD (pRR, 1.20; 95% CI, 0.60-2.40).
Among RA patients who received TNF inhibitors only, there was a slightly elevated nonsignificant risk of melanoma (pRR, 1.08; 95% CI, 0.81-1.43). Patients receiving rituximab had a pRR of 0.73 (95% CI, 0.38-1.39), and patients taking abatacept had a pRR of 1.43 (95% CI, 0.66-3.09), compared with RA patients receiving conventional systemic therapy. When excluding two major studies in the RA subgroup of patients in a sensitivity analysis, pooled risk estimates varied from 0.91 (95% CI, 0.69-1.18) to 1.95 (95% CI, 1.16- 3.30). There were no significant between-study heterogeneity or publication bias among the IBD and RA studies.
Mr. Esse and colleagues acknowledged the small number of IBD and psoriasis studies in the meta-analysis, which could affect pooled risk estimates. “Any future update of our study through the inclusion of newly published studies may produce significantly different pooled risk estimates than those reported in our meta-analysis,” they said. In addition, the use of health insurance databases, lack of risk factors for melanoma, and inconsistent information about treatment duration for patients receiving conventional systemic therapy were also limitations.
“Prospective cohort studies using an active comparator, new-user study design providing detailed information on treatment history, concomitant treatments, biologic and conventional systemic treatment duration, recreational and treatment-related UV exposure, skin color, and date of melanoma diagnosis are required to help improve certainty. These studies would also need to account for key risk factors and the latency period of melanoma,” the researchers said.
Mr. Esse disclosed being funded by a PhD studentship from the Psoriasis Association. One author disclosed receiving personal fees from Janssen, LEO Pharma, Lilly, and Novartis outside the study; another disclosed receiving grants and personal fees from those and several other pharmaceutical companies during the study, and personal fees from several pharmaceutical companies outside of the submitted work; the fourth author had no disclosures.
SOURCE: Esse S et al. JAMA Dermatol. 2020 May 20;e201300.
The in a systematic review and meta-analysis published in JAMA Dermatology.
The studies included in the analysis, however, had limitations, including a lack of those comparing biologic and conventional systemic therapy in psoriasis and inflammatory bowel disease (IBD), according to Shamarke Esse, MRes, of the division of musculoskeletal and dermatological sciences at the University of Manchester (England) and colleagues. “We advocate for more large, well-designed studies of this issue to be performed to help improve certainty” regarding this association, they wrote.
Previous studies that have found an increased risk of melanoma in patients on biologics for psoriasis, rheumatoid arthritis, and IBD have “typically used the general population as the comparator,” they noted. There is a large amount of evidence that has established short-term efficacy and safety of biologics, compared with conventional systemic treatments, but concerns about longer-term cancer risk associated with biologics remains a concern. Moreover, they added, “melanoma is a highly immunogenic skin cancer and therefore of concern to patients treated with TNFIs [tumor necrosis factor inhibitors] because melanoma risk increases with suppression of the immune system and TNF-alpha plays an important role in the immune surveillance of tumors.12,13
In their review, the researchers identified seven cohort studies from MEDLINE, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL) databases published between January 1995 and February 2019 that evaluated melanoma risk in about 34,000 patients receiving biologics and 135,370 patients who had never been treated with biologics, and were receiving conventional systemic therapy for psoriasis, RA, or IBD. Of these, four studies were in patients with RA, two studies were in patients with IBD, and a single study was in patients with psoriasis. Six studies examined patients taking TNF inhibitors, but only one of six studies had information on specific TNF inhibitors (adalimumab, etanercept, and infliximab) in patients with RA. One study evaluated abatacept and rituximab in RA patients.
The researchers analyzed the pooled relative risk across all studies. Compared with patients who received conventional systemic therapy, there was a nonsignificant association with risk of melanoma in patients with psoriasis (hazard ratio, 1.57; 95% confidence interval, 0.61-4.09), RA (pooled relative risk, 1.20; 95% CI, 0.83-1.74), and IBD (pRR, 1.20; 95% CI, 0.60-2.40).
Among RA patients who received TNF inhibitors only, there was a slightly elevated nonsignificant risk of melanoma (pRR, 1.08; 95% CI, 0.81-1.43). Patients receiving rituximab had a pRR of 0.73 (95% CI, 0.38-1.39), and patients taking abatacept had a pRR of 1.43 (95% CI, 0.66-3.09), compared with RA patients receiving conventional systemic therapy. When excluding two major studies in the RA subgroup of patients in a sensitivity analysis, pooled risk estimates varied from 0.91 (95% CI, 0.69-1.18) to 1.95 (95% CI, 1.16- 3.30). There were no significant between-study heterogeneity or publication bias among the IBD and RA studies.
Mr. Esse and colleagues acknowledged the small number of IBD and psoriasis studies in the meta-analysis, which could affect pooled risk estimates. “Any future update of our study through the inclusion of newly published studies may produce significantly different pooled risk estimates than those reported in our meta-analysis,” they said. In addition, the use of health insurance databases, lack of risk factors for melanoma, and inconsistent information about treatment duration for patients receiving conventional systemic therapy were also limitations.
“Prospective cohort studies using an active comparator, new-user study design providing detailed information on treatment history, concomitant treatments, biologic and conventional systemic treatment duration, recreational and treatment-related UV exposure, skin color, and date of melanoma diagnosis are required to help improve certainty. These studies would also need to account for key risk factors and the latency period of melanoma,” the researchers said.
Mr. Esse disclosed being funded by a PhD studentship from the Psoriasis Association. One author disclosed receiving personal fees from Janssen, LEO Pharma, Lilly, and Novartis outside the study; another disclosed receiving grants and personal fees from those and several other pharmaceutical companies during the study, and personal fees from several pharmaceutical companies outside of the submitted work; the fourth author had no disclosures.
SOURCE: Esse S et al. JAMA Dermatol. 2020 May 20;e201300.
The in a systematic review and meta-analysis published in JAMA Dermatology.
The studies included in the analysis, however, had limitations, including a lack of those comparing biologic and conventional systemic therapy in psoriasis and inflammatory bowel disease (IBD), according to Shamarke Esse, MRes, of the division of musculoskeletal and dermatological sciences at the University of Manchester (England) and colleagues. “We advocate for more large, well-designed studies of this issue to be performed to help improve certainty” regarding this association, they wrote.
Previous studies that have found an increased risk of melanoma in patients on biologics for psoriasis, rheumatoid arthritis, and IBD have “typically used the general population as the comparator,” they noted. There is a large amount of evidence that has established short-term efficacy and safety of biologics, compared with conventional systemic treatments, but concerns about longer-term cancer risk associated with biologics remains a concern. Moreover, they added, “melanoma is a highly immunogenic skin cancer and therefore of concern to patients treated with TNFIs [tumor necrosis factor inhibitors] because melanoma risk increases with suppression of the immune system and TNF-alpha plays an important role in the immune surveillance of tumors.12,13
In their review, the researchers identified seven cohort studies from MEDLINE, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL) databases published between January 1995 and February 2019 that evaluated melanoma risk in about 34,000 patients receiving biologics and 135,370 patients who had never been treated with biologics, and were receiving conventional systemic therapy for psoriasis, RA, or IBD. Of these, four studies were in patients with RA, two studies were in patients with IBD, and a single study was in patients with psoriasis. Six studies examined patients taking TNF inhibitors, but only one of six studies had information on specific TNF inhibitors (adalimumab, etanercept, and infliximab) in patients with RA. One study evaluated abatacept and rituximab in RA patients.
The researchers analyzed the pooled relative risk across all studies. Compared with patients who received conventional systemic therapy, there was a nonsignificant association with risk of melanoma in patients with psoriasis (hazard ratio, 1.57; 95% confidence interval, 0.61-4.09), RA (pooled relative risk, 1.20; 95% CI, 0.83-1.74), and IBD (pRR, 1.20; 95% CI, 0.60-2.40).
Among RA patients who received TNF inhibitors only, there was a slightly elevated nonsignificant risk of melanoma (pRR, 1.08; 95% CI, 0.81-1.43). Patients receiving rituximab had a pRR of 0.73 (95% CI, 0.38-1.39), and patients taking abatacept had a pRR of 1.43 (95% CI, 0.66-3.09), compared with RA patients receiving conventional systemic therapy. When excluding two major studies in the RA subgroup of patients in a sensitivity analysis, pooled risk estimates varied from 0.91 (95% CI, 0.69-1.18) to 1.95 (95% CI, 1.16- 3.30). There were no significant between-study heterogeneity or publication bias among the IBD and RA studies.
Mr. Esse and colleagues acknowledged the small number of IBD and psoriasis studies in the meta-analysis, which could affect pooled risk estimates. “Any future update of our study through the inclusion of newly published studies may produce significantly different pooled risk estimates than those reported in our meta-analysis,” they said. In addition, the use of health insurance databases, lack of risk factors for melanoma, and inconsistent information about treatment duration for patients receiving conventional systemic therapy were also limitations.
“Prospective cohort studies using an active comparator, new-user study design providing detailed information on treatment history, concomitant treatments, biologic and conventional systemic treatment duration, recreational and treatment-related UV exposure, skin color, and date of melanoma diagnosis are required to help improve certainty. These studies would also need to account for key risk factors and the latency period of melanoma,” the researchers said.
Mr. Esse disclosed being funded by a PhD studentship from the Psoriasis Association. One author disclosed receiving personal fees from Janssen, LEO Pharma, Lilly, and Novartis outside the study; another disclosed receiving grants and personal fees from those and several other pharmaceutical companies during the study, and personal fees from several pharmaceutical companies outside of the submitted work; the fourth author had no disclosures.
SOURCE: Esse S et al. JAMA Dermatol. 2020 May 20;e201300.
FROM JAMA DERMATOLOGY
Risk index stratifies pediatric leukemia patients undergoing HSCT
A disease risk index is now available for pediatric patients with acute myeloid leukemia or acute lymphoblastic leukemia who undergo allogeneic hematopoietic stem cell transplantation.
The model, which was developed and validated using data from more than 2,000 patients, stratifies probabilities of leukemia-free survival (LFS) into four risk groups for acute myeloid leukemia (AML) and three risk groups for acute lymphoblastic leukemia (ALL), reported lead author Muna Qayed, MD, of Emory University, Atlanta, who presented findings as part of the American Society of Clinical Oncology virtual scientific program.
“The outcome of stem cell transplantation for hematologic malignancy is influenced by disease type, cytogenetics, and disease status at transplantation,” Dr. Qayed said. “In adults, these attributes were used to develop the disease risk index, or DRI, that can stratify patients for overall survival for purposes such as prognostication or clinical trial entry.”
But no such model exists for pediatric patients, Dr. Qayed said, noting that the adult DRI was found to be inaccurate when applied to children.
“[T]he [adult] DRI did not differentiate [pediatric] patients by overall survival,” Dr. Qayed said. “Therefore, knowing that pediatric AML and ALL differ biologically from adult leukemia, and further, treatment strategies differ between adults and children, we aimed to develop a pediatric-specific DRI.”
This involved analysis of data from 1,135 children with AML and 1,228 children with ALL who underwent transplantation between 2008 and 2017. All patients had myeloablative conditioning, and 75% received an unrelated donor graft. Haploidentical transplants were excluded because of small sample size.
Analyses were conducted in AML and ALL cohorts, with patients in each population randomized to training and validation subgroups in a 1:1 ratio. The primary outcome was LFS. Cox regression models were used to identify significant characteristics, which were then integrated into a prognostic scoring system for the training groups. These scoring systems were then tested in the validation subgroups. Maximum likelihood was used to identify age cutoffs, which were 3 years for AML and 2 years for ALL.
In both cohorts, disease status at transplantation was characterized by complete remission and minimal residual disease status.
In the AML cohort, approximately one-third of patients were in first complete remission with negative minimal residual disease. Risk was stratified into four groups, including good, intermediate, high, and very high risk, with respective 5-year LFS probabilities of 81%, 56%, 44%, and 21%. Independent predictors of poorer outcome included unfavorable cytogenetics, first or second complete remission with minimal residual disease positivity, relapse at transplantation, and age less than 3 years.
In the ALL cohort, risk was stratified into three risk tiers: good, intermediate, and high, with 5-year LFS probabilities of 68%, 50%, and 15%, respectively. Independent predictors of poorer outcome included age less than 2 years, relapse at transplantation, and second complete remission regardless of minimal residual disease status.
The models for each disease also predicted overall survival.
For AML, hazard ratios, ascending from good to very-high-risk tiers, were 1.00, 3.52, 4.67, and 8.62. For ALL risk tiers, ascending hazard ratios were 1.00, 2.16, and 3.86.
“In summary, the pediatric disease risk index validated for leukemia-free survival and overall survival successfully stratifies children with acute leukemia at the time of transplantation,” Dr. Qayed said.
She concluded her presentation by highlighting the practicality and relevance of the new scoring system.
“The components included in the scoring system used information that is readily available pretransplantation, lending support to the deliverability of the prognostic scoring system,” Dr. Qayed said. “It can further be used for improved interpretation of multicenter data and in clinical trials for risk stratification.”
In a virtual presentation, invited discussant Nirali N. Shah, MD, of the National Cancer Institute, Bethesda, Md., first emphasized the clinical importance of an accurate disease risk index for pediatric patients.
“When going into transplant, the No. 1 question that all parents will ask is: ‘Will my child be cured?’ ” she said.
According to Dr. Shah, the risk model developed by Dr. Qayed and colleagues is built on a strong foundation, including adequate sample size, comprehensive disease characterization, exclusion of patients that did not undergo myeloablative conditioning, and use of minimal residual disease status.
Still, more work is needed, Dr. Shah said.
“This DRI will need to be prospectively tested and compared to other established risk factors. For instance, minimal residual disease alone can be further stratified and has a significant role in establishing risk for posttransplant relapse. And the development of acute graft-versus-host disease also plays an important role in posttransplant relapse.”
Dr. Shah went on to outline potential areas of improvement.
“[F]uture directions for this study could include incorporation of early posttransplant events like graft-versus-host disease, potential stratification of the minimal residual disease results among those patients in complete remission, and potential application of this DRI to the adolescent and young adult population, which may have slight variation even from the adult DRI.”The study was funded by the National Institutes of Health. The investigators disclosed no conflicts of interest
SOURCE: Qayed M et al. ASCO 2020, Abstract 7503.
A disease risk index is now available for pediatric patients with acute myeloid leukemia or acute lymphoblastic leukemia who undergo allogeneic hematopoietic stem cell transplantation.
The model, which was developed and validated using data from more than 2,000 patients, stratifies probabilities of leukemia-free survival (LFS) into four risk groups for acute myeloid leukemia (AML) and three risk groups for acute lymphoblastic leukemia (ALL), reported lead author Muna Qayed, MD, of Emory University, Atlanta, who presented findings as part of the American Society of Clinical Oncology virtual scientific program.
“The outcome of stem cell transplantation for hematologic malignancy is influenced by disease type, cytogenetics, and disease status at transplantation,” Dr. Qayed said. “In adults, these attributes were used to develop the disease risk index, or DRI, that can stratify patients for overall survival for purposes such as prognostication or clinical trial entry.”
But no such model exists for pediatric patients, Dr. Qayed said, noting that the adult DRI was found to be inaccurate when applied to children.
“[T]he [adult] DRI did not differentiate [pediatric] patients by overall survival,” Dr. Qayed said. “Therefore, knowing that pediatric AML and ALL differ biologically from adult leukemia, and further, treatment strategies differ between adults and children, we aimed to develop a pediatric-specific DRI.”
This involved analysis of data from 1,135 children with AML and 1,228 children with ALL who underwent transplantation between 2008 and 2017. All patients had myeloablative conditioning, and 75% received an unrelated donor graft. Haploidentical transplants were excluded because of small sample size.
Analyses were conducted in AML and ALL cohorts, with patients in each population randomized to training and validation subgroups in a 1:1 ratio. The primary outcome was LFS. Cox regression models were used to identify significant characteristics, which were then integrated into a prognostic scoring system for the training groups. These scoring systems were then tested in the validation subgroups. Maximum likelihood was used to identify age cutoffs, which were 3 years for AML and 2 years for ALL.
In both cohorts, disease status at transplantation was characterized by complete remission and minimal residual disease status.
In the AML cohort, approximately one-third of patients were in first complete remission with negative minimal residual disease. Risk was stratified into four groups, including good, intermediate, high, and very high risk, with respective 5-year LFS probabilities of 81%, 56%, 44%, and 21%. Independent predictors of poorer outcome included unfavorable cytogenetics, first or second complete remission with minimal residual disease positivity, relapse at transplantation, and age less than 3 years.
In the ALL cohort, risk was stratified into three risk tiers: good, intermediate, and high, with 5-year LFS probabilities of 68%, 50%, and 15%, respectively. Independent predictors of poorer outcome included age less than 2 years, relapse at transplantation, and second complete remission regardless of minimal residual disease status.
The models for each disease also predicted overall survival.
For AML, hazard ratios, ascending from good to very-high-risk tiers, were 1.00, 3.52, 4.67, and 8.62. For ALL risk tiers, ascending hazard ratios were 1.00, 2.16, and 3.86.
“In summary, the pediatric disease risk index validated for leukemia-free survival and overall survival successfully stratifies children with acute leukemia at the time of transplantation,” Dr. Qayed said.
She concluded her presentation by highlighting the practicality and relevance of the new scoring system.
“The components included in the scoring system used information that is readily available pretransplantation, lending support to the deliverability of the prognostic scoring system,” Dr. Qayed said. “It can further be used for improved interpretation of multicenter data and in clinical trials for risk stratification.”
In a virtual presentation, invited discussant Nirali N. Shah, MD, of the National Cancer Institute, Bethesda, Md., first emphasized the clinical importance of an accurate disease risk index for pediatric patients.
“When going into transplant, the No. 1 question that all parents will ask is: ‘Will my child be cured?’ ” she said.
According to Dr. Shah, the risk model developed by Dr. Qayed and colleagues is built on a strong foundation, including adequate sample size, comprehensive disease characterization, exclusion of patients that did not undergo myeloablative conditioning, and use of minimal residual disease status.
Still, more work is needed, Dr. Shah said.
“This DRI will need to be prospectively tested and compared to other established risk factors. For instance, minimal residual disease alone can be further stratified and has a significant role in establishing risk for posttransplant relapse. And the development of acute graft-versus-host disease also plays an important role in posttransplant relapse.”
Dr. Shah went on to outline potential areas of improvement.
“[F]uture directions for this study could include incorporation of early posttransplant events like graft-versus-host disease, potential stratification of the minimal residual disease results among those patients in complete remission, and potential application of this DRI to the adolescent and young adult population, which may have slight variation even from the adult DRI.”The study was funded by the National Institutes of Health. The investigators disclosed no conflicts of interest
SOURCE: Qayed M et al. ASCO 2020, Abstract 7503.
A disease risk index is now available for pediatric patients with acute myeloid leukemia or acute lymphoblastic leukemia who undergo allogeneic hematopoietic stem cell transplantation.
The model, which was developed and validated using data from more than 2,000 patients, stratifies probabilities of leukemia-free survival (LFS) into four risk groups for acute myeloid leukemia (AML) and three risk groups for acute lymphoblastic leukemia (ALL), reported lead author Muna Qayed, MD, of Emory University, Atlanta, who presented findings as part of the American Society of Clinical Oncology virtual scientific program.
“The outcome of stem cell transplantation for hematologic malignancy is influenced by disease type, cytogenetics, and disease status at transplantation,” Dr. Qayed said. “In adults, these attributes were used to develop the disease risk index, or DRI, that can stratify patients for overall survival for purposes such as prognostication or clinical trial entry.”
But no such model exists for pediatric patients, Dr. Qayed said, noting that the adult DRI was found to be inaccurate when applied to children.
“[T]he [adult] DRI did not differentiate [pediatric] patients by overall survival,” Dr. Qayed said. “Therefore, knowing that pediatric AML and ALL differ biologically from adult leukemia, and further, treatment strategies differ between adults and children, we aimed to develop a pediatric-specific DRI.”
This involved analysis of data from 1,135 children with AML and 1,228 children with ALL who underwent transplantation between 2008 and 2017. All patients had myeloablative conditioning, and 75% received an unrelated donor graft. Haploidentical transplants were excluded because of small sample size.
Analyses were conducted in AML and ALL cohorts, with patients in each population randomized to training and validation subgroups in a 1:1 ratio. The primary outcome was LFS. Cox regression models were used to identify significant characteristics, which were then integrated into a prognostic scoring system for the training groups. These scoring systems were then tested in the validation subgroups. Maximum likelihood was used to identify age cutoffs, which were 3 years for AML and 2 years for ALL.
In both cohorts, disease status at transplantation was characterized by complete remission and minimal residual disease status.
In the AML cohort, approximately one-third of patients were in first complete remission with negative minimal residual disease. Risk was stratified into four groups, including good, intermediate, high, and very high risk, with respective 5-year LFS probabilities of 81%, 56%, 44%, and 21%. Independent predictors of poorer outcome included unfavorable cytogenetics, first or second complete remission with minimal residual disease positivity, relapse at transplantation, and age less than 3 years.
In the ALL cohort, risk was stratified into three risk tiers: good, intermediate, and high, with 5-year LFS probabilities of 68%, 50%, and 15%, respectively. Independent predictors of poorer outcome included age less than 2 years, relapse at transplantation, and second complete remission regardless of minimal residual disease status.
The models for each disease also predicted overall survival.
For AML, hazard ratios, ascending from good to very-high-risk tiers, were 1.00, 3.52, 4.67, and 8.62. For ALL risk tiers, ascending hazard ratios were 1.00, 2.16, and 3.86.
“In summary, the pediatric disease risk index validated for leukemia-free survival and overall survival successfully stratifies children with acute leukemia at the time of transplantation,” Dr. Qayed said.
She concluded her presentation by highlighting the practicality and relevance of the new scoring system.
“The components included in the scoring system used information that is readily available pretransplantation, lending support to the deliverability of the prognostic scoring system,” Dr. Qayed said. “It can further be used for improved interpretation of multicenter data and in clinical trials for risk stratification.”
In a virtual presentation, invited discussant Nirali N. Shah, MD, of the National Cancer Institute, Bethesda, Md., first emphasized the clinical importance of an accurate disease risk index for pediatric patients.
“When going into transplant, the No. 1 question that all parents will ask is: ‘Will my child be cured?’ ” she said.
According to Dr. Shah, the risk model developed by Dr. Qayed and colleagues is built on a strong foundation, including adequate sample size, comprehensive disease characterization, exclusion of patients that did not undergo myeloablative conditioning, and use of minimal residual disease status.
Still, more work is needed, Dr. Shah said.
“This DRI will need to be prospectively tested and compared to other established risk factors. For instance, minimal residual disease alone can be further stratified and has a significant role in establishing risk for posttransplant relapse. And the development of acute graft-versus-host disease also plays an important role in posttransplant relapse.”
Dr. Shah went on to outline potential areas of improvement.
“[F]uture directions for this study could include incorporation of early posttransplant events like graft-versus-host disease, potential stratification of the minimal residual disease results among those patients in complete remission, and potential application of this DRI to the adolescent and young adult population, which may have slight variation even from the adult DRI.”The study was funded by the National Institutes of Health. The investigators disclosed no conflicts of interest
SOURCE: Qayed M et al. ASCO 2020, Abstract 7503.
FROM ASCO 2020
Adding avadomide shows promise for newly diagnosed DLBCL
For patients with newly diagnosed, high-risk diffuse large B-cell lymphoma (DLBCL), adding avadomide to standard therapy may reduce the likelihood of treatment failure, based on phase 1 results.
The regimen was well tolerated and had a complete response rate of 79%, reported lead author Neha Mehta-Shah, MD, of Washington University, St. Louis, who presented findings as part of the American Society of Clinical Oncology virtual scientific program.
Newly diagnosed DLBCL “remains a clinical challenge,” Dr. Mehta-Shah said, noting a treatment failure rate of 30%-50% for standard rituximab with cyclophosphamide, doxorubicin, vincristine, and prednisone every 21 days (R-CHOP-21), and worse outcomes among patients with high-risk disease.
In an effort to boost efficacy, the investigators turned to avadomide, a cereblon E3 ligase modulator, which previously demonstrated a complete response rate of 11% when used as monotherapy for patients with relapsed or refractory DLBCL.
The present phase 1 data included 35 adults with newly diagnosed DLBCL who had measurable lesions of at least 2.0 cm and International Prognostic Indices (IPI) between 3-5. Specifically, 51% of patients had an IPI of 3, while 49% had an IPI of 4-5.
All patients received standard R-CHOP with pegfilgrastim (to stimulate white blood cell growth), plus escalating doses of oral avadomide from 1-3 mg. Treatment was delivered, when tolerated, for up to six 21-day cycles. Depending on dose level, avadomide was given during either 2 or 3 out of 3 weeks. During treatment weeks, avadomide was given on 5 of 7 days.
The primary objectives were safety, tolerability, and complete response rate. Secondary objectives included biomarkers and additional efficacy measures, while exploratory analyses were also conducted to assess pharmacokinetics and pharmacodynamics.
Out of 34 patients evaluable for efficacy, the complete response rate was 79% and the objective response rate was 88%. After a median follow-up of 10 months, the 1-year progression-free survival rate was 80%.
“The combination showed promising efficacy,” Dr. Mehta-Shah said.
The regimen was also well tolerated and demonstrated an “acceptable safety profile consistent with either therapy alone,” Dr. Mehta-Shah added.
Most patients (91%) completed all six cycles of therapy. Median relative total dose intensities were 99% and 95% for avadomide and R-CHOP-21, respectively. Approximately two out of three patients (66%) required dose interruptions of avadomide, and 9% required dose reductions because of adverse events.
Dose-limiting toxicities occurred in six patients, including sepsis, febrile neutropenia caused by skin infections, febrile neutropenia with hypotension, febrile neutropenia, pneumonia, and neutropenia with bacterial hepatic infection. Based on these findings, the recommended phase 2 dose of avadomide was determined to be 3 mg given during 2 out of 3 weeks.
About 74% of patients had grade 3-4 treatment-emergent adverse events, the most common of which were neutropenia (54%), anemia (20%), leukopenia (20%), lymphopenia (14%), hypophosphatemia (14%), and febrile neutropenia (11%).
During the second cycle of therapy, one patient died because of concurrent pneumonia. After completion of therapy, two patients developed cardiac failure, and data gathered after each cycle showed that five patients had developed elevated levels of troponin or brain natriuretic peptide.
Flow cytometry showed that avadomide had proimmunomodulatory effects, including expansion of memory T-cell populations and enhanced proliferation of T cells and NK cells. The magnitude of this latter increase was greater than that observed in previous studies involving R-CHOP and durvalumab, Dr. Mehta-Shah noted.
“We look forward to following up on this data regarding the progression-free survival over a longer period of time,” Dr. Mehta-Shah said. “The results of this trial indicate that further evaluation of cereblon-modulating compounds combined with immunochemotherapy for patients with previously untreated diffuse large B-cell lymphoma is worthy of further exploration.”
Invited discussant Edward Yeh, MD, of the University of Missouri–Columbia, called cereblon modulators such as avadomide and thalidomide a “very exciting class of anticancer therapy.”
In a virtual presentation, Dr. Yeh noted that cereblon modulators have several advantages, including “good absorption, distribution, metabolism, [and] elimination,” plus relatively low susceptibility to mutation-directed drug resistance.
Despite some drawbacks, including difficulty to design and synthesize, Dr. Yeh suggested that this drug class may play a bigger role over time.
“We’re expecting more cancer therapy to come to fruition through the utilization of this posttranslational, protein-modification pathway,” he concluded.
The study was funded by Celgene. The investigators disclosed additional relationships with Kyowa Hakko Kirin, AstraZeneca, Roche, and others.
SOURCE: Mehta-Shah N et al. ASCO 2020, Abstract 3501.
For patients with newly diagnosed, high-risk diffuse large B-cell lymphoma (DLBCL), adding avadomide to standard therapy may reduce the likelihood of treatment failure, based on phase 1 results.
The regimen was well tolerated and had a complete response rate of 79%, reported lead author Neha Mehta-Shah, MD, of Washington University, St. Louis, who presented findings as part of the American Society of Clinical Oncology virtual scientific program.
Newly diagnosed DLBCL “remains a clinical challenge,” Dr. Mehta-Shah said, noting a treatment failure rate of 30%-50% for standard rituximab with cyclophosphamide, doxorubicin, vincristine, and prednisone every 21 days (R-CHOP-21), and worse outcomes among patients with high-risk disease.
In an effort to boost efficacy, the investigators turned to avadomide, a cereblon E3 ligase modulator, which previously demonstrated a complete response rate of 11% when used as monotherapy for patients with relapsed or refractory DLBCL.
The present phase 1 data included 35 adults with newly diagnosed DLBCL who had measurable lesions of at least 2.0 cm and International Prognostic Indices (IPI) between 3-5. Specifically, 51% of patients had an IPI of 3, while 49% had an IPI of 4-5.
All patients received standard R-CHOP with pegfilgrastim (to stimulate white blood cell growth), plus escalating doses of oral avadomide from 1-3 mg. Treatment was delivered, when tolerated, for up to six 21-day cycles. Depending on dose level, avadomide was given during either 2 or 3 out of 3 weeks. During treatment weeks, avadomide was given on 5 of 7 days.
The primary objectives were safety, tolerability, and complete response rate. Secondary objectives included biomarkers and additional efficacy measures, while exploratory analyses were also conducted to assess pharmacokinetics and pharmacodynamics.
Out of 34 patients evaluable for efficacy, the complete response rate was 79% and the objective response rate was 88%. After a median follow-up of 10 months, the 1-year progression-free survival rate was 80%.
“The combination showed promising efficacy,” Dr. Mehta-Shah said.
The regimen was also well tolerated and demonstrated an “acceptable safety profile consistent with either therapy alone,” Dr. Mehta-Shah added.
Most patients (91%) completed all six cycles of therapy. Median relative total dose intensities were 99% and 95% for avadomide and R-CHOP-21, respectively. Approximately two out of three patients (66%) required dose interruptions of avadomide, and 9% required dose reductions because of adverse events.
Dose-limiting toxicities occurred in six patients, including sepsis, febrile neutropenia caused by skin infections, febrile neutropenia with hypotension, febrile neutropenia, pneumonia, and neutropenia with bacterial hepatic infection. Based on these findings, the recommended phase 2 dose of avadomide was determined to be 3 mg given during 2 out of 3 weeks.
About 74% of patients had grade 3-4 treatment-emergent adverse events, the most common of which were neutropenia (54%), anemia (20%), leukopenia (20%), lymphopenia (14%), hypophosphatemia (14%), and febrile neutropenia (11%).
During the second cycle of therapy, one patient died because of concurrent pneumonia. After completion of therapy, two patients developed cardiac failure, and data gathered after each cycle showed that five patients had developed elevated levels of troponin or brain natriuretic peptide.
Flow cytometry showed that avadomide had proimmunomodulatory effects, including expansion of memory T-cell populations and enhanced proliferation of T cells and NK cells. The magnitude of this latter increase was greater than that observed in previous studies involving R-CHOP and durvalumab, Dr. Mehta-Shah noted.
“We look forward to following up on this data regarding the progression-free survival over a longer period of time,” Dr. Mehta-Shah said. “The results of this trial indicate that further evaluation of cereblon-modulating compounds combined with immunochemotherapy for patients with previously untreated diffuse large B-cell lymphoma is worthy of further exploration.”
Invited discussant Edward Yeh, MD, of the University of Missouri–Columbia, called cereblon modulators such as avadomide and thalidomide a “very exciting class of anticancer therapy.”
In a virtual presentation, Dr. Yeh noted that cereblon modulators have several advantages, including “good absorption, distribution, metabolism, [and] elimination,” plus relatively low susceptibility to mutation-directed drug resistance.
Despite some drawbacks, including difficulty to design and synthesize, Dr. Yeh suggested that this drug class may play a bigger role over time.
“We’re expecting more cancer therapy to come to fruition through the utilization of this posttranslational, protein-modification pathway,” he concluded.
The study was funded by Celgene. The investigators disclosed additional relationships with Kyowa Hakko Kirin, AstraZeneca, Roche, and others.
SOURCE: Mehta-Shah N et al. ASCO 2020, Abstract 3501.
For patients with newly diagnosed, high-risk diffuse large B-cell lymphoma (DLBCL), adding avadomide to standard therapy may reduce the likelihood of treatment failure, based on phase 1 results.
The regimen was well tolerated and had a complete response rate of 79%, reported lead author Neha Mehta-Shah, MD, of Washington University, St. Louis, who presented findings as part of the American Society of Clinical Oncology virtual scientific program.
Newly diagnosed DLBCL “remains a clinical challenge,” Dr. Mehta-Shah said, noting a treatment failure rate of 30%-50% for standard rituximab with cyclophosphamide, doxorubicin, vincristine, and prednisone every 21 days (R-CHOP-21), and worse outcomes among patients with high-risk disease.
In an effort to boost efficacy, the investigators turned to avadomide, a cereblon E3 ligase modulator, which previously demonstrated a complete response rate of 11% when used as monotherapy for patients with relapsed or refractory DLBCL.
The present phase 1 data included 35 adults with newly diagnosed DLBCL who had measurable lesions of at least 2.0 cm and International Prognostic Indices (IPI) between 3-5. Specifically, 51% of patients had an IPI of 3, while 49% had an IPI of 4-5.
All patients received standard R-CHOP with pegfilgrastim (to stimulate white blood cell growth), plus escalating doses of oral avadomide from 1-3 mg. Treatment was delivered, when tolerated, for up to six 21-day cycles. Depending on dose level, avadomide was given during either 2 or 3 out of 3 weeks. During treatment weeks, avadomide was given on 5 of 7 days.
The primary objectives were safety, tolerability, and complete response rate. Secondary objectives included biomarkers and additional efficacy measures, while exploratory analyses were also conducted to assess pharmacokinetics and pharmacodynamics.
Out of 34 patients evaluable for efficacy, the complete response rate was 79% and the objective response rate was 88%. After a median follow-up of 10 months, the 1-year progression-free survival rate was 80%.
“The combination showed promising efficacy,” Dr. Mehta-Shah said.
The regimen was also well tolerated and demonstrated an “acceptable safety profile consistent with either therapy alone,” Dr. Mehta-Shah added.
Most patients (91%) completed all six cycles of therapy. Median relative total dose intensities were 99% and 95% for avadomide and R-CHOP-21, respectively. Approximately two out of three patients (66%) required dose interruptions of avadomide, and 9% required dose reductions because of adverse events.
Dose-limiting toxicities occurred in six patients, including sepsis, febrile neutropenia caused by skin infections, febrile neutropenia with hypotension, febrile neutropenia, pneumonia, and neutropenia with bacterial hepatic infection. Based on these findings, the recommended phase 2 dose of avadomide was determined to be 3 mg given during 2 out of 3 weeks.
About 74% of patients had grade 3-4 treatment-emergent adverse events, the most common of which were neutropenia (54%), anemia (20%), leukopenia (20%), lymphopenia (14%), hypophosphatemia (14%), and febrile neutropenia (11%).
During the second cycle of therapy, one patient died because of concurrent pneumonia. After completion of therapy, two patients developed cardiac failure, and data gathered after each cycle showed that five patients had developed elevated levels of troponin or brain natriuretic peptide.
Flow cytometry showed that avadomide had proimmunomodulatory effects, including expansion of memory T-cell populations and enhanced proliferation of T cells and NK cells. The magnitude of this latter increase was greater than that observed in previous studies involving R-CHOP and durvalumab, Dr. Mehta-Shah noted.
“We look forward to following up on this data regarding the progression-free survival over a longer period of time,” Dr. Mehta-Shah said. “The results of this trial indicate that further evaluation of cereblon-modulating compounds combined with immunochemotherapy for patients with previously untreated diffuse large B-cell lymphoma is worthy of further exploration.”
Invited discussant Edward Yeh, MD, of the University of Missouri–Columbia, called cereblon modulators such as avadomide and thalidomide a “very exciting class of anticancer therapy.”
In a virtual presentation, Dr. Yeh noted that cereblon modulators have several advantages, including “good absorption, distribution, metabolism, [and] elimination,” plus relatively low susceptibility to mutation-directed drug resistance.
Despite some drawbacks, including difficulty to design and synthesize, Dr. Yeh suggested that this drug class may play a bigger role over time.
“We’re expecting more cancer therapy to come to fruition through the utilization of this posttranslational, protein-modification pathway,” he concluded.
The study was funded by Celgene. The investigators disclosed additional relationships with Kyowa Hakko Kirin, AstraZeneca, Roche, and others.
SOURCE: Mehta-Shah N et al. ASCO 2020, Abstract 3501.
FROM ASCO 2020
Teenage blood donors may need more time, supplements to replenish iron
and approximately 15% of males and one-third of females may be iron depleted 1 year after a red blood cell donation, according to a study published online in Pediatrics.
“Our findings ... support the strong encouragement of postdonation low-dose iron supplements, at least in teenagers with already low or borderline iron stores,” said Ralph R. Vassallo, MD and colleagues. An increased interdonation interval for teenage blood donors may need to be considered. Dr. Vassallo is executive vice president and chief medical and scientific officer at Vitalant, a community blood service provider based in Scottsdale, Ariz., that collects approximately 14% of the U.S. blood supply.
Data from a safety initiative
Iron deficiency may erode exercise performance, lead to pica, and have subtle cognitive effects. Iron that is lost during blood donation may be difficult to replace on an average Western diet, the researchers said. Knowledge of the time course of iron recovery is limited, and recent studies have found high rates of mild iron depletion or absent stores in teenage blood donors.
As a safety initiative, Vitalant began serum ferritin testing of predonation specimens from successful donations by 16- to 18-year-old donors to identify iron deficiency. The researchers defined inadequate iron stores as ferritin values less than 20 ng/mL in female donors and less than 30 ng/mL in male donors.
While whole-blood donors generally were deferred from red blood cell donation for 56 days and double red blood cell donors were deferred from any donation for 112 days, teenagers found to have low ferritin were deferred longer: 12 months for females and 6 months for males. Teenagers with low ferritin also were counseled by letter to take low-dose (18-28 mg) iron for 60 days.
Vitalant began conducting serum ferritin tests on Dec. 19, 2016, and the researchers analyzed data that were available through Dec. 31, 2018. The study included data from teen donors aged 16-18 years from 24 states.
In all, 125,384 teenagers donated whole blood or double red blood cells at least once, and 39% of females and 12% of males had a low-index ferritin value. The researchers focused on 30,806 teenagers (24.6%) who returned and successfully donated again within 25 months. In this cohort, 11% of female donors and 10% of male donors had a low-index ferritin value.
Twelve months after the first whole-blood donation, 80%-90% of males and 60%-70% of females had adequate iron stores. These proportions were about 10% lower for double red blood cell donors.
Among whole-blood donors, the percentage of donors with adequate iron stores at a follow-up donation “is highly dependent on index ferritin,” Dr. Vassallo and colleagues reported. For 90% of donors to have adequate iron stores within 3-9 months of index donation, index ferritin values had to be at least 47 ng/mL for males and 53 ng/mL for females, according to their analysis.
“At least 12 months are required for a sizeable proportion to reach next-donation ferritin values indicative of adequate iron stores,” the researchers said. “However, donor sex, donation type, first-time versus repeat status, and ferritin level at index were significant determinants of iron stores at the follow-up donation.”
“The enthusiasm of altruistic, highly motivated young donors makes large high school and college blood drives efficient and productive,” but these donors may be at increased risk for donation-related iron depletion, they said.
The estimated intervals needed to replenish iron stores represent best-case scenarios, the authors noted. The cohort did not include individuals deferred for low hemoglobin, likely due to iron deficiency, and these donors probably require more time to replenish iron stores.
The data suggest that health care providers should consider blood donation as a possible cause of low hemoglobin, Dr. Vassallo and colleagues said. Before prescribing higher doses of iron, however, physicians should assess a patient’s risk for other causes of iron deficiency such as gastrointestinal blood loss or celiac disease.
Shift collection efforts away from teenagers?
Vitalant’s findings “are of considerable value to other organizations collecting blood from teenagers,” Alan E. Mast, MD, PhD, said in an accompanying editorial. Dr. Mast is medical director and senior investigator at Versiti Blood Research Institute and associate professor of cell biology, neurobiology, and anatomy at Medical College of Wisconsin in Milwaukee. “The data presented confirm the high baseline level of iron deficiency in teenagers and its increase after blood donation. Pediatricians should be aware of the large number of teenagers donating blood in the United States, consider routinely asking about blood donation during routine physical examinations, and perform appropriate laboratory screening and treatment of iron deficiency.”
High school blood drives are a cost-effective way to collect blood, and stricter regulations on the collection of blood from teenagers may “have a detrimental effect on the blood supply,” Dr. Mast said. Nevertheless, “efforts should be made to reverse the trend of increasing collections from teenagers with increased emphasis on collection from adults.”
AABB (previously known as the American Association of Blood Banks), an organization of professionals who practice transfusion medicine, recommended in 2017 that blood collection agencies limit donors aged 16-18 years to one whole-blood donation per year, dispense iron supplements, or perform ferritin testing to advise donors about further steps, Dr. Mast noted.
“In the absence of other interventions, 1 year should be the minimum interdonation interval for teenagers,” said Dr. Mast. “If ferritin testing is performed, teenagers with ferritin levels [greater than] 50 ng/mL at index donation could perhaps donate twice per year.”
Dr. Vassallo has received payment from Fresenius Kabi and HemaStrat for scientific advisory board membership. Dr. Mast receives research funding from Novo Nordisk and has received honoraria for serving on Novo Nordisk advisory boards. He received no external funding for his commentary.
SOURCE: Vassallo RR et al. Pediatrics. 2020 Jun 5. doi: 10.1542/peds.2019-3316.
and approximately 15% of males and one-third of females may be iron depleted 1 year after a red blood cell donation, according to a study published online in Pediatrics.
“Our findings ... support the strong encouragement of postdonation low-dose iron supplements, at least in teenagers with already low or borderline iron stores,” said Ralph R. Vassallo, MD and colleagues. An increased interdonation interval for teenage blood donors may need to be considered. Dr. Vassallo is executive vice president and chief medical and scientific officer at Vitalant, a community blood service provider based in Scottsdale, Ariz., that collects approximately 14% of the U.S. blood supply.
Data from a safety initiative
Iron deficiency may erode exercise performance, lead to pica, and have subtle cognitive effects. Iron that is lost during blood donation may be difficult to replace on an average Western diet, the researchers said. Knowledge of the time course of iron recovery is limited, and recent studies have found high rates of mild iron depletion or absent stores in teenage blood donors.
As a safety initiative, Vitalant began serum ferritin testing of predonation specimens from successful donations by 16- to 18-year-old donors to identify iron deficiency. The researchers defined inadequate iron stores as ferritin values less than 20 ng/mL in female donors and less than 30 ng/mL in male donors.
While whole-blood donors generally were deferred from red blood cell donation for 56 days and double red blood cell donors were deferred from any donation for 112 days, teenagers found to have low ferritin were deferred longer: 12 months for females and 6 months for males. Teenagers with low ferritin also were counseled by letter to take low-dose (18-28 mg) iron for 60 days.
Vitalant began conducting serum ferritin tests on Dec. 19, 2016, and the researchers analyzed data that were available through Dec. 31, 2018. The study included data from teen donors aged 16-18 years from 24 states.
In all, 125,384 teenagers donated whole blood or double red blood cells at least once, and 39% of females and 12% of males had a low-index ferritin value. The researchers focused on 30,806 teenagers (24.6%) who returned and successfully donated again within 25 months. In this cohort, 11% of female donors and 10% of male donors had a low-index ferritin value.
Twelve months after the first whole-blood donation, 80%-90% of males and 60%-70% of females had adequate iron stores. These proportions were about 10% lower for double red blood cell donors.
Among whole-blood donors, the percentage of donors with adequate iron stores at a follow-up donation “is highly dependent on index ferritin,” Dr. Vassallo and colleagues reported. For 90% of donors to have adequate iron stores within 3-9 months of index donation, index ferritin values had to be at least 47 ng/mL for males and 53 ng/mL for females, according to their analysis.
“At least 12 months are required for a sizeable proportion to reach next-donation ferritin values indicative of adequate iron stores,” the researchers said. “However, donor sex, donation type, first-time versus repeat status, and ferritin level at index were significant determinants of iron stores at the follow-up donation.”
“The enthusiasm of altruistic, highly motivated young donors makes large high school and college blood drives efficient and productive,” but these donors may be at increased risk for donation-related iron depletion, they said.
The estimated intervals needed to replenish iron stores represent best-case scenarios, the authors noted. The cohort did not include individuals deferred for low hemoglobin, likely due to iron deficiency, and these donors probably require more time to replenish iron stores.
The data suggest that health care providers should consider blood donation as a possible cause of low hemoglobin, Dr. Vassallo and colleagues said. Before prescribing higher doses of iron, however, physicians should assess a patient’s risk for other causes of iron deficiency such as gastrointestinal blood loss or celiac disease.
Shift collection efforts away from teenagers?
Vitalant’s findings “are of considerable value to other organizations collecting blood from teenagers,” Alan E. Mast, MD, PhD, said in an accompanying editorial. Dr. Mast is medical director and senior investigator at Versiti Blood Research Institute and associate professor of cell biology, neurobiology, and anatomy at Medical College of Wisconsin in Milwaukee. “The data presented confirm the high baseline level of iron deficiency in teenagers and its increase after blood donation. Pediatricians should be aware of the large number of teenagers donating blood in the United States, consider routinely asking about blood donation during routine physical examinations, and perform appropriate laboratory screening and treatment of iron deficiency.”
High school blood drives are a cost-effective way to collect blood, and stricter regulations on the collection of blood from teenagers may “have a detrimental effect on the blood supply,” Dr. Mast said. Nevertheless, “efforts should be made to reverse the trend of increasing collections from teenagers with increased emphasis on collection from adults.”
AABB (previously known as the American Association of Blood Banks), an organization of professionals who practice transfusion medicine, recommended in 2017 that blood collection agencies limit donors aged 16-18 years to one whole-blood donation per year, dispense iron supplements, or perform ferritin testing to advise donors about further steps, Dr. Mast noted.
“In the absence of other interventions, 1 year should be the minimum interdonation interval for teenagers,” said Dr. Mast. “If ferritin testing is performed, teenagers with ferritin levels [greater than] 50 ng/mL at index donation could perhaps donate twice per year.”
Dr. Vassallo has received payment from Fresenius Kabi and HemaStrat for scientific advisory board membership. Dr. Mast receives research funding from Novo Nordisk and has received honoraria for serving on Novo Nordisk advisory boards. He received no external funding for his commentary.
SOURCE: Vassallo RR et al. Pediatrics. 2020 Jun 5. doi: 10.1542/peds.2019-3316.
and approximately 15% of males and one-third of females may be iron depleted 1 year after a red blood cell donation, according to a study published online in Pediatrics.
“Our findings ... support the strong encouragement of postdonation low-dose iron supplements, at least in teenagers with already low or borderline iron stores,” said Ralph R. Vassallo, MD and colleagues. An increased interdonation interval for teenage blood donors may need to be considered. Dr. Vassallo is executive vice president and chief medical and scientific officer at Vitalant, a community blood service provider based in Scottsdale, Ariz., that collects approximately 14% of the U.S. blood supply.
Data from a safety initiative
Iron deficiency may erode exercise performance, lead to pica, and have subtle cognitive effects. Iron that is lost during blood donation may be difficult to replace on an average Western diet, the researchers said. Knowledge of the time course of iron recovery is limited, and recent studies have found high rates of mild iron depletion or absent stores in teenage blood donors.
As a safety initiative, Vitalant began serum ferritin testing of predonation specimens from successful donations by 16- to 18-year-old donors to identify iron deficiency. The researchers defined inadequate iron stores as ferritin values less than 20 ng/mL in female donors and less than 30 ng/mL in male donors.
While whole-blood donors generally were deferred from red blood cell donation for 56 days and double red blood cell donors were deferred from any donation for 112 days, teenagers found to have low ferritin were deferred longer: 12 months for females and 6 months for males. Teenagers with low ferritin also were counseled by letter to take low-dose (18-28 mg) iron for 60 days.
Vitalant began conducting serum ferritin tests on Dec. 19, 2016, and the researchers analyzed data that were available through Dec. 31, 2018. The study included data from teen donors aged 16-18 years from 24 states.
In all, 125,384 teenagers donated whole blood or double red blood cells at least once, and 39% of females and 12% of males had a low-index ferritin value. The researchers focused on 30,806 teenagers (24.6%) who returned and successfully donated again within 25 months. In this cohort, 11% of female donors and 10% of male donors had a low-index ferritin value.
Twelve months after the first whole-blood donation, 80%-90% of males and 60%-70% of females had adequate iron stores. These proportions were about 10% lower for double red blood cell donors.
Among whole-blood donors, the percentage of donors with adequate iron stores at a follow-up donation “is highly dependent on index ferritin,” Dr. Vassallo and colleagues reported. For 90% of donors to have adequate iron stores within 3-9 months of index donation, index ferritin values had to be at least 47 ng/mL for males and 53 ng/mL for females, according to their analysis.
“At least 12 months are required for a sizeable proportion to reach next-donation ferritin values indicative of adequate iron stores,” the researchers said. “However, donor sex, donation type, first-time versus repeat status, and ferritin level at index were significant determinants of iron stores at the follow-up donation.”
“The enthusiasm of altruistic, highly motivated young donors makes large high school and college blood drives efficient and productive,” but these donors may be at increased risk for donation-related iron depletion, they said.
The estimated intervals needed to replenish iron stores represent best-case scenarios, the authors noted. The cohort did not include individuals deferred for low hemoglobin, likely due to iron deficiency, and these donors probably require more time to replenish iron stores.
The data suggest that health care providers should consider blood donation as a possible cause of low hemoglobin, Dr. Vassallo and colleagues said. Before prescribing higher doses of iron, however, physicians should assess a patient’s risk for other causes of iron deficiency such as gastrointestinal blood loss or celiac disease.
Shift collection efforts away from teenagers?
Vitalant’s findings “are of considerable value to other organizations collecting blood from teenagers,” Alan E. Mast, MD, PhD, said in an accompanying editorial. Dr. Mast is medical director and senior investigator at Versiti Blood Research Institute and associate professor of cell biology, neurobiology, and anatomy at Medical College of Wisconsin in Milwaukee. “The data presented confirm the high baseline level of iron deficiency in teenagers and its increase after blood donation. Pediatricians should be aware of the large number of teenagers donating blood in the United States, consider routinely asking about blood donation during routine physical examinations, and perform appropriate laboratory screening and treatment of iron deficiency.”
High school blood drives are a cost-effective way to collect blood, and stricter regulations on the collection of blood from teenagers may “have a detrimental effect on the blood supply,” Dr. Mast said. Nevertheless, “efforts should be made to reverse the trend of increasing collections from teenagers with increased emphasis on collection from adults.”
AABB (previously known as the American Association of Blood Banks), an organization of professionals who practice transfusion medicine, recommended in 2017 that blood collection agencies limit donors aged 16-18 years to one whole-blood donation per year, dispense iron supplements, or perform ferritin testing to advise donors about further steps, Dr. Mast noted.
“In the absence of other interventions, 1 year should be the minimum interdonation interval for teenagers,” said Dr. Mast. “If ferritin testing is performed, teenagers with ferritin levels [greater than] 50 ng/mL at index donation could perhaps donate twice per year.”
Dr. Vassallo has received payment from Fresenius Kabi and HemaStrat for scientific advisory board membership. Dr. Mast receives research funding from Novo Nordisk and has received honoraria for serving on Novo Nordisk advisory boards. He received no external funding for his commentary.
SOURCE: Vassallo RR et al. Pediatrics. 2020 Jun 5. doi: 10.1542/peds.2019-3316.
FROM PEDIATRICS
Key clinical point: Teenage blood donors may benefit for postdonation low-dose iron supplements, and an increase to the interdonation interval for teenage blood donors may need to be considered.
Major finding: A significant number of teenage blood donors already have low iron stores before they donate blood, and approximately 15% of males and one-third of females may be iron depleted 1 year after a red blood cell donation.
Study details: Analysis of serum ferritin values from 30,806 teens aged 16-18 years who donated blood twice within 25 months.
Disclosures: Dr. Vasallo disclosed remunerated scientific advisory board membership from Fresenius Kabi and HemaStrat.
Source: Vassallo RR et al. Pediatrics. 2020 Jun 5. doi: 10.1542/peds.2019-3316.
Acute lymphoblastic leukemia can be successfully treated in the frail elderly
A treatment schedule of very attenuated chemotherapy using standard drugs is feasible and effective in frail and elderly patients with acute lymphoblastic leukemia (ALL), according to a prospective study published in Clinical Lymphoma, Myeloma & Leukemia.
The study comprised 67 previously untreated patients with B- or T-lineage Philadelphia chromosome–negative ALL from 30 Spanish hospitals who were enrolled in the prospective, multicenter ALL-07FRAIL trial (NCT01358201) from the Spanish PETHEMA (Programa Español de Tratamientos en Hematologia) group from January 2008 to October 2019.
The median patient age in this analysis was 67 years and 51 patients (76%) were older than 70 years. The median Charlson Comorbidity Index was 5, with the main comorbidities being cardiovascular (47 patients), other neoplasia (24), diabetes (17), and very advanced age (>80 years; 12).
The attenuated treatment regimen consisted of a prephase with dexamethasone and intrathecal therapy with methotrexate was given for a maximum of 1 week. Then weekly induction therapy consisted of weekly vincristine (capped at 1 mg/week) and daily dexamethasone with a progressively decreasing dose along 4 weeks, as well as two additional doses of intrathecal methotrexate.
Those patients who achieved complete remission received maintenance therapy with mercaptopurine and methotrexate to complete 2 years of treatment. In addition, reinduction pulses with vincristine and dexamethasone were given every 3 months during the first year, according to Josep-Maria Ribera, MD, of the Universitat Autònoma de Barcelona, Badalona, Spain and colleagues on behalf of the PETHEMA group of the Spanish Society of Hematology.
The complete remission rate was 54% (36/67 patients). The median disease-free survival and overall survival were 6.9 months and 7.6 months, respectively.
Of the 32 patients who initiated maintenance therapy, 5 patients died of infection (2), hemorrhage (2), and acute cognitive impairment (1), and 23 relapsed, with a cumulative incidence of relapse of 74% and a median time to relapse of 12.3 months.
The most frequent toxic events reported were hematologic (neutropenia 77% and thrombocytopenia 54%, of grade III-IV in all cases) followed by infections, metabolic (mainly hyperglycemia), and neurologic, according to the researchers.
“The lack of similar trials specifically directed to this frail population is one of the major strengths of this study, and we consider that this minimal chemotherapy approach could be used as a backbone for addition of immuno/targeted therapy in this subset of infirm patients,” the researchers concluded.
The study was supported by the CERCA Program/Generalitat de Catalunya and the Josep Carreras Leukemia Research Institute. The authors reported having no disclosures.
SOURCE: Ribera J-M et al. Clin Lymphoma Myeloma Leuk. 2020 Apr 5. doi: 10.1016/j.clml.2020.03.011.
A treatment schedule of very attenuated chemotherapy using standard drugs is feasible and effective in frail and elderly patients with acute lymphoblastic leukemia (ALL), according to a prospective study published in Clinical Lymphoma, Myeloma & Leukemia.
The study comprised 67 previously untreated patients with B- or T-lineage Philadelphia chromosome–negative ALL from 30 Spanish hospitals who were enrolled in the prospective, multicenter ALL-07FRAIL trial (NCT01358201) from the Spanish PETHEMA (Programa Español de Tratamientos en Hematologia) group from January 2008 to October 2019.
The median patient age in this analysis was 67 years and 51 patients (76%) were older than 70 years. The median Charlson Comorbidity Index was 5, with the main comorbidities being cardiovascular (47 patients), other neoplasia (24), diabetes (17), and very advanced age (>80 years; 12).
The attenuated treatment regimen consisted of a prephase with dexamethasone and intrathecal therapy with methotrexate was given for a maximum of 1 week. Then weekly induction therapy consisted of weekly vincristine (capped at 1 mg/week) and daily dexamethasone with a progressively decreasing dose along 4 weeks, as well as two additional doses of intrathecal methotrexate.
Those patients who achieved complete remission received maintenance therapy with mercaptopurine and methotrexate to complete 2 years of treatment. In addition, reinduction pulses with vincristine and dexamethasone were given every 3 months during the first year, according to Josep-Maria Ribera, MD, of the Universitat Autònoma de Barcelona, Badalona, Spain and colleagues on behalf of the PETHEMA group of the Spanish Society of Hematology.
The complete remission rate was 54% (36/67 patients). The median disease-free survival and overall survival were 6.9 months and 7.6 months, respectively.
Of the 32 patients who initiated maintenance therapy, 5 patients died of infection (2), hemorrhage (2), and acute cognitive impairment (1), and 23 relapsed, with a cumulative incidence of relapse of 74% and a median time to relapse of 12.3 months.
The most frequent toxic events reported were hematologic (neutropenia 77% and thrombocytopenia 54%, of grade III-IV in all cases) followed by infections, metabolic (mainly hyperglycemia), and neurologic, according to the researchers.
“The lack of similar trials specifically directed to this frail population is one of the major strengths of this study, and we consider that this minimal chemotherapy approach could be used as a backbone for addition of immuno/targeted therapy in this subset of infirm patients,” the researchers concluded.
The study was supported by the CERCA Program/Generalitat de Catalunya and the Josep Carreras Leukemia Research Institute. The authors reported having no disclosures.
SOURCE: Ribera J-M et al. Clin Lymphoma Myeloma Leuk. 2020 Apr 5. doi: 10.1016/j.clml.2020.03.011.
A treatment schedule of very attenuated chemotherapy using standard drugs is feasible and effective in frail and elderly patients with acute lymphoblastic leukemia (ALL), according to a prospective study published in Clinical Lymphoma, Myeloma & Leukemia.
The study comprised 67 previously untreated patients with B- or T-lineage Philadelphia chromosome–negative ALL from 30 Spanish hospitals who were enrolled in the prospective, multicenter ALL-07FRAIL trial (NCT01358201) from the Spanish PETHEMA (Programa Español de Tratamientos en Hematologia) group from January 2008 to October 2019.
The median patient age in this analysis was 67 years and 51 patients (76%) were older than 70 years. The median Charlson Comorbidity Index was 5, with the main comorbidities being cardiovascular (47 patients), other neoplasia (24), diabetes (17), and very advanced age (>80 years; 12).
The attenuated treatment regimen consisted of a prephase with dexamethasone and intrathecal therapy with methotrexate was given for a maximum of 1 week. Then weekly induction therapy consisted of weekly vincristine (capped at 1 mg/week) and daily dexamethasone with a progressively decreasing dose along 4 weeks, as well as two additional doses of intrathecal methotrexate.
Those patients who achieved complete remission received maintenance therapy with mercaptopurine and methotrexate to complete 2 years of treatment. In addition, reinduction pulses with vincristine and dexamethasone were given every 3 months during the first year, according to Josep-Maria Ribera, MD, of the Universitat Autònoma de Barcelona, Badalona, Spain and colleagues on behalf of the PETHEMA group of the Spanish Society of Hematology.
The complete remission rate was 54% (36/67 patients). The median disease-free survival and overall survival were 6.9 months and 7.6 months, respectively.
Of the 32 patients who initiated maintenance therapy, 5 patients died of infection (2), hemorrhage (2), and acute cognitive impairment (1), and 23 relapsed, with a cumulative incidence of relapse of 74% and a median time to relapse of 12.3 months.
The most frequent toxic events reported were hematologic (neutropenia 77% and thrombocytopenia 54%, of grade III-IV in all cases) followed by infections, metabolic (mainly hyperglycemia), and neurologic, according to the researchers.
“The lack of similar trials specifically directed to this frail population is one of the major strengths of this study, and we consider that this minimal chemotherapy approach could be used as a backbone for addition of immuno/targeted therapy in this subset of infirm patients,” the researchers concluded.
The study was supported by the CERCA Program/Generalitat de Catalunya and the Josep Carreras Leukemia Research Institute. The authors reported having no disclosures.
SOURCE: Ribera J-M et al. Clin Lymphoma Myeloma Leuk. 2020 Apr 5. doi: 10.1016/j.clml.2020.03.011.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
MCC response varies based on immunosuppression type, especially CLL
Patients with Merkel cell carcinoma and chronic immunosuppression may fare better or worse on immunotherapy based on the reason for immunosuppression, according to recent research at the annual meeting of the Society for Investigative Dermatology, held virtually.
About 10% of patients with Merkel cell carcinoma (MCC) are immunosuppressed at diagnosis, and these patients tend to have a more aggressive disease course and worse disease-specific survival compared with immunocompetent patients, Lauren Zawacki, a research assistant in the Nghiem Lab at the University of Washington, Seattle, said in her presentation. Although patients are receiving immune checkpoint inhibitors such as anti-PD-1 and anti-PD-L1 as treatments, the efficacy and side effects on immunosuppressed patients have not been well studied because many of these patients are not eligible for clinical trials.
Ms. Zawacki and colleagues analyzed data from a prospective Seattle registry of 1,442 patients with MCC, identifying 179 patients with MCC who had chronic immunosuppression due to chronic lymphocytic leukemia (CLL), solid organ transplants, autoimmune disorders, other hematological malignancies, and HIV and AIDS. Non-Hodgkin lymphoma comprised 7 of 8 patients in the group with other hematological malignancies, and Crohn’s disease made up 5 of 6 patients in the autoimmune disorder group. Of the 179 patients with MCC and immunosuppression, 31 patients were treated with either anti-PD-1 or anti-PD-L1 therapy.
There was an objective response rate of 52%, with 14 patients having a complete response, 2 patients having a partial response, and 15 patients experiencing disease progression. Of the patients with disease progression, 11 died of MCC. The response rate in immunocompromised patients is similar to results seen by her group in immunocompetent patients (Nghiem P et al. N Engl J Med 2016; 374:2542-52), said Ms. Zawacki. “While the overall objective response rate is comparable between immunocompetent and immunosuppressed patients, the response rates vary greatly between the different types of immunosuppression,” she said.
When grouping response rates by immunosuppression type, they found 2 of 11 patients with CLL (18%) and 2 of 6 patients with autoimmune disease (33%) had an objective response, while 2 of 3 patients with HIV/AIDS (66%) and 7 of 7 patients with other hematologic malignancies (100%) had an objective response.
“While the numbers of the cohort are small, there still seems to be a considerable difference in the response rate between the different types of immune suppression, which is critical when we’re treating patients who typically have a more aggressive disease course,” said Ms. Zawacki.
In particular, the finding of no patients with MCC and CLL achieving a complete response interested Ms. Zawacki and her colleagues, since about one-fourth of patients in the Seattle registry have this combination of disease. “Not only did none of the CLL patients have a complete response, but 7 out of the 11 patients with CLL died from MCC,” she explained. When examining further, the researchers found 45% of patients in this group discontinued because of side effects of immunotherapy and had a median time to recurrence of 1.5 months. “This finding suggests that CLL in particular plays a large role in impairing the function of the immune system, leading to not only a more aggressive disease course, but a poorer response to immunotherapy,” she said.
“There is a significant need for improved interventions for patients with CLL and autoimmune disorders,” she added. “Research for immunosuppressed patients is critical given the associated aggressive disease course and their lack of inclusion in clinical trials.”
Ms. Zawacki acknowledged the small number of patients in the study as a limitation, and patients who received follow-up at outside facilities may have received slightly different care, which could impact adverse event reporting or reasons for study discontinuation.
“A multi-institutional study would be beneficial to expand the number of patients in that cohort and to help confirm the trend observed in this study. In addition, future studies should assess the role of combination systemic therapy, such as neutron radiation and immunotherapy together in order to see if the objective response can be approved among immunosuppressed patients,” she said.
This study was supported by funding from the MCC Patient Gift Fund, the National Cancer Institute, and a grant from NIH. Ms. Zawacki reports no relevant conflicts of interest.
SOURCE: Zawacki L. SID 2020, Abstract 497.
Patients with Merkel cell carcinoma and chronic immunosuppression may fare better or worse on immunotherapy based on the reason for immunosuppression, according to recent research at the annual meeting of the Society for Investigative Dermatology, held virtually.
About 10% of patients with Merkel cell carcinoma (MCC) are immunosuppressed at diagnosis, and these patients tend to have a more aggressive disease course and worse disease-specific survival compared with immunocompetent patients, Lauren Zawacki, a research assistant in the Nghiem Lab at the University of Washington, Seattle, said in her presentation. Although patients are receiving immune checkpoint inhibitors such as anti-PD-1 and anti-PD-L1 as treatments, the efficacy and side effects on immunosuppressed patients have not been well studied because many of these patients are not eligible for clinical trials.
Ms. Zawacki and colleagues analyzed data from a prospective Seattle registry of 1,442 patients with MCC, identifying 179 patients with MCC who had chronic immunosuppression due to chronic lymphocytic leukemia (CLL), solid organ transplants, autoimmune disorders, other hematological malignancies, and HIV and AIDS. Non-Hodgkin lymphoma comprised 7 of 8 patients in the group with other hematological malignancies, and Crohn’s disease made up 5 of 6 patients in the autoimmune disorder group. Of the 179 patients with MCC and immunosuppression, 31 patients were treated with either anti-PD-1 or anti-PD-L1 therapy.
There was an objective response rate of 52%, with 14 patients having a complete response, 2 patients having a partial response, and 15 patients experiencing disease progression. Of the patients with disease progression, 11 died of MCC. The response rate in immunocompromised patients is similar to results seen by her group in immunocompetent patients (Nghiem P et al. N Engl J Med 2016; 374:2542-52), said Ms. Zawacki. “While the overall objective response rate is comparable between immunocompetent and immunosuppressed patients, the response rates vary greatly between the different types of immunosuppression,” she said.
When grouping response rates by immunosuppression type, they found 2 of 11 patients with CLL (18%) and 2 of 6 patients with autoimmune disease (33%) had an objective response, while 2 of 3 patients with HIV/AIDS (66%) and 7 of 7 patients with other hematologic malignancies (100%) had an objective response.
“While the numbers of the cohort are small, there still seems to be a considerable difference in the response rate between the different types of immune suppression, which is critical when we’re treating patients who typically have a more aggressive disease course,” said Ms. Zawacki.
In particular, the finding of no patients with MCC and CLL achieving a complete response interested Ms. Zawacki and her colleagues, since about one-fourth of patients in the Seattle registry have this combination of disease. “Not only did none of the CLL patients have a complete response, but 7 out of the 11 patients with CLL died from MCC,” she explained. When examining further, the researchers found 45% of patients in this group discontinued because of side effects of immunotherapy and had a median time to recurrence of 1.5 months. “This finding suggests that CLL in particular plays a large role in impairing the function of the immune system, leading to not only a more aggressive disease course, but a poorer response to immunotherapy,” she said.
“There is a significant need for improved interventions for patients with CLL and autoimmune disorders,” she added. “Research for immunosuppressed patients is critical given the associated aggressive disease course and their lack of inclusion in clinical trials.”
Ms. Zawacki acknowledged the small number of patients in the study as a limitation, and patients who received follow-up at outside facilities may have received slightly different care, which could impact adverse event reporting or reasons for study discontinuation.
“A multi-institutional study would be beneficial to expand the number of patients in that cohort and to help confirm the trend observed in this study. In addition, future studies should assess the role of combination systemic therapy, such as neutron radiation and immunotherapy together in order to see if the objective response can be approved among immunosuppressed patients,” she said.
This study was supported by funding from the MCC Patient Gift Fund, the National Cancer Institute, and a grant from NIH. Ms. Zawacki reports no relevant conflicts of interest.
SOURCE: Zawacki L. SID 2020, Abstract 497.
Patients with Merkel cell carcinoma and chronic immunosuppression may fare better or worse on immunotherapy based on the reason for immunosuppression, according to recent research at the annual meeting of the Society for Investigative Dermatology, held virtually.
About 10% of patients with Merkel cell carcinoma (MCC) are immunosuppressed at diagnosis, and these patients tend to have a more aggressive disease course and worse disease-specific survival compared with immunocompetent patients, Lauren Zawacki, a research assistant in the Nghiem Lab at the University of Washington, Seattle, said in her presentation. Although patients are receiving immune checkpoint inhibitors such as anti-PD-1 and anti-PD-L1 as treatments, the efficacy and side effects on immunosuppressed patients have not been well studied because many of these patients are not eligible for clinical trials.
Ms. Zawacki and colleagues analyzed data from a prospective Seattle registry of 1,442 patients with MCC, identifying 179 patients with MCC who had chronic immunosuppression due to chronic lymphocytic leukemia (CLL), solid organ transplants, autoimmune disorders, other hematological malignancies, and HIV and AIDS. Non-Hodgkin lymphoma comprised 7 of 8 patients in the group with other hematological malignancies, and Crohn’s disease made up 5 of 6 patients in the autoimmune disorder group. Of the 179 patients with MCC and immunosuppression, 31 patients were treated with either anti-PD-1 or anti-PD-L1 therapy.
There was an objective response rate of 52%, with 14 patients having a complete response, 2 patients having a partial response, and 15 patients experiencing disease progression. Of the patients with disease progression, 11 died of MCC. The response rate in immunocompromised patients is similar to results seen by her group in immunocompetent patients (Nghiem P et al. N Engl J Med 2016; 374:2542-52), said Ms. Zawacki. “While the overall objective response rate is comparable between immunocompetent and immunosuppressed patients, the response rates vary greatly between the different types of immunosuppression,” she said.
When grouping response rates by immunosuppression type, they found 2 of 11 patients with CLL (18%) and 2 of 6 patients with autoimmune disease (33%) had an objective response, while 2 of 3 patients with HIV/AIDS (66%) and 7 of 7 patients with other hematologic malignancies (100%) had an objective response.
“While the numbers of the cohort are small, there still seems to be a considerable difference in the response rate between the different types of immune suppression, which is critical when we’re treating patients who typically have a more aggressive disease course,” said Ms. Zawacki.
In particular, the finding of no patients with MCC and CLL achieving a complete response interested Ms. Zawacki and her colleagues, since about one-fourth of patients in the Seattle registry have this combination of disease. “Not only did none of the CLL patients have a complete response, but 7 out of the 11 patients with CLL died from MCC,” she explained. When examining further, the researchers found 45% of patients in this group discontinued because of side effects of immunotherapy and had a median time to recurrence of 1.5 months. “This finding suggests that CLL in particular plays a large role in impairing the function of the immune system, leading to not only a more aggressive disease course, but a poorer response to immunotherapy,” she said.
“There is a significant need for improved interventions for patients with CLL and autoimmune disorders,” she added. “Research for immunosuppressed patients is critical given the associated aggressive disease course and their lack of inclusion in clinical trials.”
Ms. Zawacki acknowledged the small number of patients in the study as a limitation, and patients who received follow-up at outside facilities may have received slightly different care, which could impact adverse event reporting or reasons for study discontinuation.
“A multi-institutional study would be beneficial to expand the number of patients in that cohort and to help confirm the trend observed in this study. In addition, future studies should assess the role of combination systemic therapy, such as neutron radiation and immunotherapy together in order to see if the objective response can be approved among immunosuppressed patients,” she said.
This study was supported by funding from the MCC Patient Gift Fund, the National Cancer Institute, and a grant from NIH. Ms. Zawacki reports no relevant conflicts of interest.
SOURCE: Zawacki L. SID 2020, Abstract 497.
FROM SID 2020