User login
Strategies to treat food allergy with oral immunotherapy
according to a new review.
In OIT, a patient who is allergic to a specific food consumes increasing amounts of the allergen over time to reduce their risk for allergic reaction.
“OIT is an elective, usually noncurative procedure with inherent risks that require families to function as amateur medical professionals. Preparing them for this role is essential to protect patients and ensure the long-term success of this life-changing procedure,” lead author Douglas P. Mack, MD, MSc, a pediatric allergy, asthma, and immunology specialist at McMaster University in Hamilton, Ont., and colleagues write in Clinical & Experimental Allergy.
From strict avoidance to desensitization
Food allergy treatment has traditionally involved avoiding accidental exposure that may lead to anaphylaxis and providing rescue medication. In recent years, OIT “has been recommended by several guidelines as a primary option,” Dr. Mack and coauthors write. And with the “approval by European and USA regulators of [peanut allergen powder] Palforzia [Aimmune Therapeutics], there are now commercial and noncommercial forms of OIT available for use in several countries.”
They advise physicians to take a proactive, educational, supportive approach to patients and their families throughout the therapy.
“Ultimately, the decision to pursue OIT or continue avoidance strategies remains the responsibility of the family and the patients,” they write. “Some families may not be prepared for the role that they have to play in actively managing their child’s food allergy treatment.”
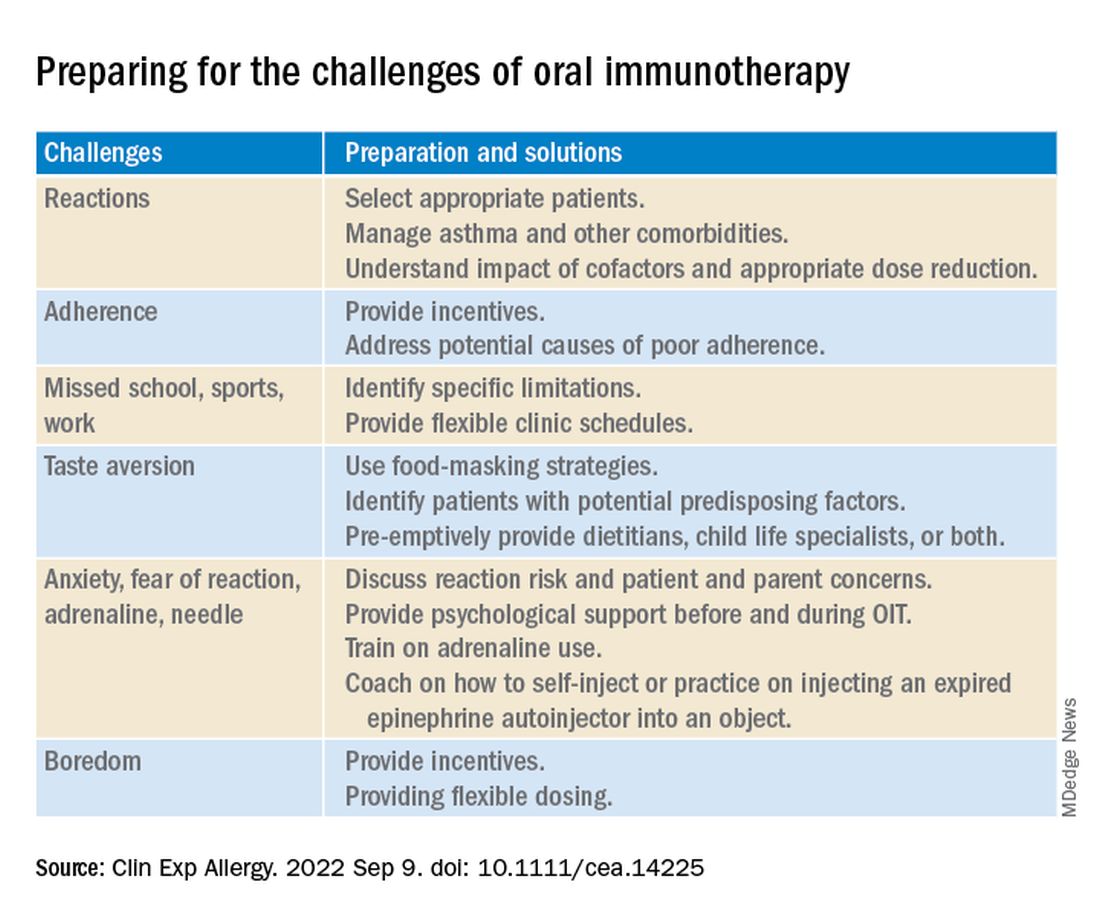
Strategies to overcome OIT challenges
Reviewing the literature about OIT for food allergy, the authors suggest various strategies physicians can use to help OIT patients and their families prepare for and overcome common treatment-related challenges.
Two experts welcome the report
Rita Kachru, MD, a specialist in allergy and immunology and a codirector of the food allergy program at UCLA Health in Los Angeles, called this “an excellent report about a wonderful, individualized option in food allergy management.
“The authors did an excellent job delineating OIT terminology, outlining the goals, risks, and benefits of OIT, noting that it’s not a cure, and emphasizing the crucial importance of discussions with each family throughout the process,” Dr. Kachru, who was not involved in developing the report, told this news organization.
“I thoroughly agree with their assessment,” she added. “The more you do OIT research and clinical care, the more you realize the pitfalls, the benefits, and the importance of patient goals and family dynamics. Discussing the goals, risks, benefits, and alternatives to OIT in detail with the family is crucial so they fully understand the process.”
Basil M. Kahwash, MD, an allergy, pulmonary, and critical care medicine specialist at Vanderbilt University Medical Center in Nashville, Tenn., said that providers in the immunology community have been discussing OIT for years and that he welcomes the well-written report that summarizes the evidence.
“It’s important to periodically summarize the evidence, as well as the consensus expert opinion about the evidence, so we may better inform our colleagues and patients,” said Dr. Kahwash, who was not involved in developing the report. “The authors are well-known experts in our field who have experience with OIT and with reviewing the evidence of food allergy.
“OIT can be a fantastic option for some patients, especially those who are very highly motivated and understand the process from start to finish. But OIT is not the best option for every child, and it’s not much of an option for adults,” he explained. “Patients need to be chosen carefully and understand the level of motivation required to safely follow through with the treatment.
“The report will hopefully affect patient care positively and allow patients to understand the limitations around OIT when they consider their candidacy for it,” he added. “In most cases, OIT patients will still need to avoid the allergen, but if a small amount accidentally gets into their food, they probably won’t have a very severe reaction to it.”
Dr. Kahwash would like to see data on patients who have seen long-term remission with OIT.
“Clearly, some patients benefit from OIT. What differentiates patients who benefit from OIT from those who do not?” he asked. “In the future, we need to consider possible biomarkers of patients who are and who aren’t good candidates for OIT.
“Regardless of OIT’s limitations, the potential for desensitization rather than strict avoidance represents a big step in the evolution of food allergy treatment,” Dr. Kahwash noted.
No funding details were provided. Dr. Mack and three coauthors report financial relationships with Aimmune. Aimmune Therapeutics is the manufacturer of Palforzia OIT (AR101 powder provided in capsules and sachets). Most coauthors also report financial relationships with other pharmaceutical companies. The full list can be found with the original article. Dr. Kachru was an investigator in the PALISADES clinical trial of AR101. Dr. Kahwash reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a new review.
In OIT, a patient who is allergic to a specific food consumes increasing amounts of the allergen over time to reduce their risk for allergic reaction.
“OIT is an elective, usually noncurative procedure with inherent risks that require families to function as amateur medical professionals. Preparing them for this role is essential to protect patients and ensure the long-term success of this life-changing procedure,” lead author Douglas P. Mack, MD, MSc, a pediatric allergy, asthma, and immunology specialist at McMaster University in Hamilton, Ont., and colleagues write in Clinical & Experimental Allergy.
From strict avoidance to desensitization
Food allergy treatment has traditionally involved avoiding accidental exposure that may lead to anaphylaxis and providing rescue medication. In recent years, OIT “has been recommended by several guidelines as a primary option,” Dr. Mack and coauthors write. And with the “approval by European and USA regulators of [peanut allergen powder] Palforzia [Aimmune Therapeutics], there are now commercial and noncommercial forms of OIT available for use in several countries.”
They advise physicians to take a proactive, educational, supportive approach to patients and their families throughout the therapy.
“Ultimately, the decision to pursue OIT or continue avoidance strategies remains the responsibility of the family and the patients,” they write. “Some families may not be prepared for the role that they have to play in actively managing their child’s food allergy treatment.”
Strategies to overcome OIT challenges
Reviewing the literature about OIT for food allergy, the authors suggest various strategies physicians can use to help OIT patients and their families prepare for and overcome common treatment-related challenges.

Two experts welcome the report
Rita Kachru, MD, a specialist in allergy and immunology and a codirector of the food allergy program at UCLA Health in Los Angeles, called this “an excellent report about a wonderful, individualized option in food allergy management.
“The authors did an excellent job delineating OIT terminology, outlining the goals, risks, and benefits of OIT, noting that it’s not a cure, and emphasizing the crucial importance of discussions with each family throughout the process,” Dr. Kachru, who was not involved in developing the report, told this news organization.
“I thoroughly agree with their assessment,” she added. “The more you do OIT research and clinical care, the more you realize the pitfalls, the benefits, and the importance of patient goals and family dynamics. Discussing the goals, risks, benefits, and alternatives to OIT in detail with the family is crucial so they fully understand the process.”
Basil M. Kahwash, MD, an allergy, pulmonary, and critical care medicine specialist at Vanderbilt University Medical Center in Nashville, Tenn., said that providers in the immunology community have been discussing OIT for years and that he welcomes the well-written report that summarizes the evidence.
“It’s important to periodically summarize the evidence, as well as the consensus expert opinion about the evidence, so we may better inform our colleagues and patients,” said Dr. Kahwash, who was not involved in developing the report. “The authors are well-known experts in our field who have experience with OIT and with reviewing the evidence of food allergy.
“OIT can be a fantastic option for some patients, especially those who are very highly motivated and understand the process from start to finish. But OIT is not the best option for every child, and it’s not much of an option for adults,” he explained. “Patients need to be chosen carefully and understand the level of motivation required to safely follow through with the treatment.
“The report will hopefully affect patient care positively and allow patients to understand the limitations around OIT when they consider their candidacy for it,” he added. “In most cases, OIT patients will still need to avoid the allergen, but if a small amount accidentally gets into their food, they probably won’t have a very severe reaction to it.”
Dr. Kahwash would like to see data on patients who have seen long-term remission with OIT.
“Clearly, some patients benefit from OIT. What differentiates patients who benefit from OIT from those who do not?” he asked. “In the future, we need to consider possible biomarkers of patients who are and who aren’t good candidates for OIT.
“Regardless of OIT’s limitations, the potential for desensitization rather than strict avoidance represents a big step in the evolution of food allergy treatment,” Dr. Kahwash noted.
No funding details were provided. Dr. Mack and three coauthors report financial relationships with Aimmune. Aimmune Therapeutics is the manufacturer of Palforzia OIT (AR101 powder provided in capsules and sachets). Most coauthors also report financial relationships with other pharmaceutical companies. The full list can be found with the original article. Dr. Kachru was an investigator in the PALISADES clinical trial of AR101. Dr. Kahwash reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a new review.
In OIT, a patient who is allergic to a specific food consumes increasing amounts of the allergen over time to reduce their risk for allergic reaction.
“OIT is an elective, usually noncurative procedure with inherent risks that require families to function as amateur medical professionals. Preparing them for this role is essential to protect patients and ensure the long-term success of this life-changing procedure,” lead author Douglas P. Mack, MD, MSc, a pediatric allergy, asthma, and immunology specialist at McMaster University in Hamilton, Ont., and colleagues write in Clinical & Experimental Allergy.
From strict avoidance to desensitization
Food allergy treatment has traditionally involved avoiding accidental exposure that may lead to anaphylaxis and providing rescue medication. In recent years, OIT “has been recommended by several guidelines as a primary option,” Dr. Mack and coauthors write. And with the “approval by European and USA regulators of [peanut allergen powder] Palforzia [Aimmune Therapeutics], there are now commercial and noncommercial forms of OIT available for use in several countries.”
They advise physicians to take a proactive, educational, supportive approach to patients and their families throughout the therapy.
“Ultimately, the decision to pursue OIT or continue avoidance strategies remains the responsibility of the family and the patients,” they write. “Some families may not be prepared for the role that they have to play in actively managing their child’s food allergy treatment.”
Strategies to overcome OIT challenges
Reviewing the literature about OIT for food allergy, the authors suggest various strategies physicians can use to help OIT patients and their families prepare for and overcome common treatment-related challenges.

Two experts welcome the report
Rita Kachru, MD, a specialist in allergy and immunology and a codirector of the food allergy program at UCLA Health in Los Angeles, called this “an excellent report about a wonderful, individualized option in food allergy management.
“The authors did an excellent job delineating OIT terminology, outlining the goals, risks, and benefits of OIT, noting that it’s not a cure, and emphasizing the crucial importance of discussions with each family throughout the process,” Dr. Kachru, who was not involved in developing the report, told this news organization.
“I thoroughly agree with their assessment,” she added. “The more you do OIT research and clinical care, the more you realize the pitfalls, the benefits, and the importance of patient goals and family dynamics. Discussing the goals, risks, benefits, and alternatives to OIT in detail with the family is crucial so they fully understand the process.”
Basil M. Kahwash, MD, an allergy, pulmonary, and critical care medicine specialist at Vanderbilt University Medical Center in Nashville, Tenn., said that providers in the immunology community have been discussing OIT for years and that he welcomes the well-written report that summarizes the evidence.
“It’s important to periodically summarize the evidence, as well as the consensus expert opinion about the evidence, so we may better inform our colleagues and patients,” said Dr. Kahwash, who was not involved in developing the report. “The authors are well-known experts in our field who have experience with OIT and with reviewing the evidence of food allergy.
“OIT can be a fantastic option for some patients, especially those who are very highly motivated and understand the process from start to finish. But OIT is not the best option for every child, and it’s not much of an option for adults,” he explained. “Patients need to be chosen carefully and understand the level of motivation required to safely follow through with the treatment.
“The report will hopefully affect patient care positively and allow patients to understand the limitations around OIT when they consider their candidacy for it,” he added. “In most cases, OIT patients will still need to avoid the allergen, but if a small amount accidentally gets into their food, they probably won’t have a very severe reaction to it.”
Dr. Kahwash would like to see data on patients who have seen long-term remission with OIT.
“Clearly, some patients benefit from OIT. What differentiates patients who benefit from OIT from those who do not?” he asked. “In the future, we need to consider possible biomarkers of patients who are and who aren’t good candidates for OIT.
“Regardless of OIT’s limitations, the potential for desensitization rather than strict avoidance represents a big step in the evolution of food allergy treatment,” Dr. Kahwash noted.
No funding details were provided. Dr. Mack and three coauthors report financial relationships with Aimmune. Aimmune Therapeutics is the manufacturer of Palforzia OIT (AR101 powder provided in capsules and sachets). Most coauthors also report financial relationships with other pharmaceutical companies. The full list can be found with the original article. Dr. Kachru was an investigator in the PALISADES clinical trial of AR101. Dr. Kahwash reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CLINICAL & EXPERIMENTAL ALLERGY
Gene ‘cut-and-paste’ treatment could offer hope for inherited immune system diseases
An “exciting” new gene-editing strategy means those born with a rare inherited disease of the immune system could be treated by repairing a fault in their cells.
CTLA-4 is a protein produced by T cells that helps to control the activity of the immune system. Most people carry two working copies of the gene responsible for producing CTLA-4, but those who have only one functional copy produce too little of the protein to sufficiently regulate the immune system.
For patients with the condition, CTLA-4 insufficiency causes regulatory T cells to function abnormally, leading to severe autoimmunity. The authors explained that the condition also affects effector T cells and thereby “hampers their immune system’s ‘memory,’ ” meaning patients can “struggle to fight off recurring infections by the same viruses and bacteria.” In some cases, it can also lead to lymphomas.
Gene editing to ‘cut’ out faulty genes and ‘paste’ in ‘corrected’ ones
The research, published in Science Translational Medicine, and led by scientists from University College London, demonstrated in human cells and in mice that the cell fault can be repaired.
The scientists used “cut-and-paste” gene-editing techniques. First, they used the CRISPR/Cas9 system to target the faulty gene in human T cells taken from patients with CTLA-4 insufficiency, and then snip the faulty CTLA-4 gene in two. Then, to repair the errors a corrected sequence of DNA – delivered to the cell using a modified virus – was pasted over the faulty part of the gene using a cellular DNA repair mechanism known as homology-directed repair.
The authors explained that this allowed them to “preserve” important sequences within the CTLA-4 gene – known as the intron – that allow it to be switched on and off by the cell only when needed.
The outcome was “restored levels of CTLA-4 in the cells to those seen in healthy T cells,” the authors said.
Claire Booth, PhD, Mahboubian professor of gene therapy and pediatric immunology, UCL Great Ormond Street Institute of Child Health, and co–senior author, said that it was “really exciting” to think about taking this treatment forward to patients. “If we can improve their symptoms and reduce their risk of getting lymphoproliferative disease this will be a major step forward.”
In addition, the researchers were also able to improve symptoms of the disease in mice with CTLA-4 insufficiency by giving them injections of gene-edited T cells.
Technique may help tackle many conditions
The current standard treatment for CTLA-4 insufficiency is a bone marrow transplant to replace the stem cells responsible for producing T cells. However, “transplants are risky” and require high doses of chemotherapy and many weeks in hospital, the authors explained. “Older patients with CTLA-4 insufficiency are typically not well enough to tolerate the transplant procedure.”
Dr. Booth highlighted that the approach has many “positive aspects”. By correcting the patient’s T cells, “we think it can improve many of the symptoms of the disease”, she said, and added that this new approach is much less toxic than a bone marrow transplant. “Collecting the T cells is easier and correcting the T cells is easier. With this approach the amount of time in hospital the patients would need would be far less.”
Emma Morris, PhD, professor of clinical cell and gene therapy and director of UCL’s division of infection and immunity, and co–senior author, said: “Genes that play critical roles in controlling immune responses are not switched on all the time and are very tightly regulated. The technique we have used allows us to leave the natural (endogenous) mechanisms controlling gene expression intact, at the same time as correcting the mistake in the gene itself.”
The researchers explained that, although CTLA-4 insufficiency is rare, the gene editing therapy could be a proof of principle of their approach that could be adapted to tackle other conditions.
“It’s a way of correcting genetic mutations that could potentially be applicable for other diseases,” suggested Dr. Morris. “The bigger picture is it allows us to correct genes that are dysregulated or overactive, but also allows us to understand much more about gene expression and gene regulation.”
The study was funded by the Wellcome Trust, the Association for Moleculary Pathology, the Medical Research Council, Alzheimer’s Research UK, and the UCLH/UCL NIHR Biomedical Research Centre. Dr. Morris is a founder sharehold of Quell Therapeutics and has received honoraria from Orchard Therapeutics, GlaxoSmithKline, and AstraZeneca. Dr. Booth has performed ad hoc consulting in the past 3 years for SOBI and Novartis and educational material production for SOBI and Chiesi. A patent on the intronic gene editing approach has been filed in the UK. The other authors declared that they have no completing interests.
A version of this article first appeared on Medscape UK.
An “exciting” new gene-editing strategy means those born with a rare inherited disease of the immune system could be treated by repairing a fault in their cells.
CTLA-4 is a protein produced by T cells that helps to control the activity of the immune system. Most people carry two working copies of the gene responsible for producing CTLA-4, but those who have only one functional copy produce too little of the protein to sufficiently regulate the immune system.
For patients with the condition, CTLA-4 insufficiency causes regulatory T cells to function abnormally, leading to severe autoimmunity. The authors explained that the condition also affects effector T cells and thereby “hampers their immune system’s ‘memory,’ ” meaning patients can “struggle to fight off recurring infections by the same viruses and bacteria.” In some cases, it can also lead to lymphomas.
Gene editing to ‘cut’ out faulty genes and ‘paste’ in ‘corrected’ ones
The research, published in Science Translational Medicine, and led by scientists from University College London, demonstrated in human cells and in mice that the cell fault can be repaired.
The scientists used “cut-and-paste” gene-editing techniques. First, they used the CRISPR/Cas9 system to target the faulty gene in human T cells taken from patients with CTLA-4 insufficiency, and then snip the faulty CTLA-4 gene in two. Then, to repair the errors a corrected sequence of DNA – delivered to the cell using a modified virus – was pasted over the faulty part of the gene using a cellular DNA repair mechanism known as homology-directed repair.
The authors explained that this allowed them to “preserve” important sequences within the CTLA-4 gene – known as the intron – that allow it to be switched on and off by the cell only when needed.
The outcome was “restored levels of CTLA-4 in the cells to those seen in healthy T cells,” the authors said.
Claire Booth, PhD, Mahboubian professor of gene therapy and pediatric immunology, UCL Great Ormond Street Institute of Child Health, and co–senior author, said that it was “really exciting” to think about taking this treatment forward to patients. “If we can improve their symptoms and reduce their risk of getting lymphoproliferative disease this will be a major step forward.”
In addition, the researchers were also able to improve symptoms of the disease in mice with CTLA-4 insufficiency by giving them injections of gene-edited T cells.
Technique may help tackle many conditions
The current standard treatment for CTLA-4 insufficiency is a bone marrow transplant to replace the stem cells responsible for producing T cells. However, “transplants are risky” and require high doses of chemotherapy and many weeks in hospital, the authors explained. “Older patients with CTLA-4 insufficiency are typically not well enough to tolerate the transplant procedure.”
Dr. Booth highlighted that the approach has many “positive aspects”. By correcting the patient’s T cells, “we think it can improve many of the symptoms of the disease”, she said, and added that this new approach is much less toxic than a bone marrow transplant. “Collecting the T cells is easier and correcting the T cells is easier. With this approach the amount of time in hospital the patients would need would be far less.”
Emma Morris, PhD, professor of clinical cell and gene therapy and director of UCL’s division of infection and immunity, and co–senior author, said: “Genes that play critical roles in controlling immune responses are not switched on all the time and are very tightly regulated. The technique we have used allows us to leave the natural (endogenous) mechanisms controlling gene expression intact, at the same time as correcting the mistake in the gene itself.”
The researchers explained that, although CTLA-4 insufficiency is rare, the gene editing therapy could be a proof of principle of their approach that could be adapted to tackle other conditions.
“It’s a way of correcting genetic mutations that could potentially be applicable for other diseases,” suggested Dr. Morris. “The bigger picture is it allows us to correct genes that are dysregulated or overactive, but also allows us to understand much more about gene expression and gene regulation.”
The study was funded by the Wellcome Trust, the Association for Moleculary Pathology, the Medical Research Council, Alzheimer’s Research UK, and the UCLH/UCL NIHR Biomedical Research Centre. Dr. Morris is a founder sharehold of Quell Therapeutics and has received honoraria from Orchard Therapeutics, GlaxoSmithKline, and AstraZeneca. Dr. Booth has performed ad hoc consulting in the past 3 years for SOBI and Novartis and educational material production for SOBI and Chiesi. A patent on the intronic gene editing approach has been filed in the UK. The other authors declared that they have no completing interests.
A version of this article first appeared on Medscape UK.
An “exciting” new gene-editing strategy means those born with a rare inherited disease of the immune system could be treated by repairing a fault in their cells.
CTLA-4 is a protein produced by T cells that helps to control the activity of the immune system. Most people carry two working copies of the gene responsible for producing CTLA-4, but those who have only one functional copy produce too little of the protein to sufficiently regulate the immune system.
For patients with the condition, CTLA-4 insufficiency causes regulatory T cells to function abnormally, leading to severe autoimmunity. The authors explained that the condition also affects effector T cells and thereby “hampers their immune system’s ‘memory,’ ” meaning patients can “struggle to fight off recurring infections by the same viruses and bacteria.” In some cases, it can also lead to lymphomas.
Gene editing to ‘cut’ out faulty genes and ‘paste’ in ‘corrected’ ones
The research, published in Science Translational Medicine, and led by scientists from University College London, demonstrated in human cells and in mice that the cell fault can be repaired.
The scientists used “cut-and-paste” gene-editing techniques. First, they used the CRISPR/Cas9 system to target the faulty gene in human T cells taken from patients with CTLA-4 insufficiency, and then snip the faulty CTLA-4 gene in two. Then, to repair the errors a corrected sequence of DNA – delivered to the cell using a modified virus – was pasted over the faulty part of the gene using a cellular DNA repair mechanism known as homology-directed repair.
The authors explained that this allowed them to “preserve” important sequences within the CTLA-4 gene – known as the intron – that allow it to be switched on and off by the cell only when needed.
The outcome was “restored levels of CTLA-4 in the cells to those seen in healthy T cells,” the authors said.
Claire Booth, PhD, Mahboubian professor of gene therapy and pediatric immunology, UCL Great Ormond Street Institute of Child Health, and co–senior author, said that it was “really exciting” to think about taking this treatment forward to patients. “If we can improve their symptoms and reduce their risk of getting lymphoproliferative disease this will be a major step forward.”
In addition, the researchers were also able to improve symptoms of the disease in mice with CTLA-4 insufficiency by giving them injections of gene-edited T cells.
Technique may help tackle many conditions
The current standard treatment for CTLA-4 insufficiency is a bone marrow transplant to replace the stem cells responsible for producing T cells. However, “transplants are risky” and require high doses of chemotherapy and many weeks in hospital, the authors explained. “Older patients with CTLA-4 insufficiency are typically not well enough to tolerate the transplant procedure.”
Dr. Booth highlighted that the approach has many “positive aspects”. By correcting the patient’s T cells, “we think it can improve many of the symptoms of the disease”, she said, and added that this new approach is much less toxic than a bone marrow transplant. “Collecting the T cells is easier and correcting the T cells is easier. With this approach the amount of time in hospital the patients would need would be far less.”
Emma Morris, PhD, professor of clinical cell and gene therapy and director of UCL’s division of infection and immunity, and co–senior author, said: “Genes that play critical roles in controlling immune responses are not switched on all the time and are very tightly regulated. The technique we have used allows us to leave the natural (endogenous) mechanisms controlling gene expression intact, at the same time as correcting the mistake in the gene itself.”
The researchers explained that, although CTLA-4 insufficiency is rare, the gene editing therapy could be a proof of principle of their approach that could be adapted to tackle other conditions.
“It’s a way of correcting genetic mutations that could potentially be applicable for other diseases,” suggested Dr. Morris. “The bigger picture is it allows us to correct genes that are dysregulated or overactive, but also allows us to understand much more about gene expression and gene regulation.”
The study was funded by the Wellcome Trust, the Association for Moleculary Pathology, the Medical Research Council, Alzheimer’s Research UK, and the UCLH/UCL NIHR Biomedical Research Centre. Dr. Morris is a founder sharehold of Quell Therapeutics and has received honoraria from Orchard Therapeutics, GlaxoSmithKline, and AstraZeneca. Dr. Booth has performed ad hoc consulting in the past 3 years for SOBI and Novartis and educational material production for SOBI and Chiesi. A patent on the intronic gene editing approach has been filed in the UK. The other authors declared that they have no completing interests.
A version of this article first appeared on Medscape UK.
FROM SCIENCE TRANSLATIONAL MEDICINE
Connected: Preterm infant program makes progress
Martha Welch, MD, spent the better part of three decades in private practice treating children with emotional, behavioral, and developmental disorders before accepting a job on the faculty of Columbia University, New York, in 1997.
She took the position, she said, with a mission: to find evidence to support what she’d observed in her practice – that parents could, by making stronger emotional connections, change the trajectory of development for preemie infants.
With that understanding, Dr. Welch created Family Nurture Intervention (FNI), which has been shown to improve the development of premature babies.
“We saw that no matter what happened to the baby, no matter how avoidant the baby might be, we’re able to overcome this with emotional expression,” Dr. Welch said.
Over the course of the intervention, families work with a specialist who helps bring mother and baby together – both physically and emotionally – until both are calm, which can initially take several hours and over time, minutes.
FNI appears to help families – especially mothers – re-establish an emotional connection often interrupted by their babies’ stressful and uncertain stay in a neonatal intensive care unit (NICU). In turn, both the infant and maternal nervous systems become better regulated, according to researchers.
Early challenges
Babies born preterm can face a range of short-term and long-term challenges, such as breathing problems due to an underdeveloped respiratory system, an increased risk of infection from an underdeveloped immune system, and learning difficulties, according to the Mayo Clinic.
Many aspects of FNI are not new: The neonatal intensive care unit has long incorporated activities such as scent cloth exchanges, talking to the baby, and skin-to-skin contact. But the approach Dr. Welch and her colleagues advocate emphasizes building a bond between the mother and the infant.
Mounting evidence shows that FNI can improve a wide range of outcomes for premature babies. In a 2021 study, for example, Dr. Welch’s group showed that FNI was associated with lower heart rates among preemies in the NICU. A 2016 study linked the intervention to reduced depression and anxiety symptoms in mothers of preterm infants. And a 2015 randomized controlled trial showed FNI improved development and behavioral outcomes in infants up to 18 months.
A new study published in Science Translational Medicine showed that the intervention led to a greater likelihood that babies had improved cognitive development later on, narrowing the developmental gap between healthy, full-term babies.
Dr. Welch and her colleagues tested to see if FNI measurably changed brain development in preterm infants who were born at 26-34 weeks of a pregnancy.
“We were blown away by the strength of the effect,” said Pauliina Yrjölä, MSc, a doctoral student and medical physicist at the University of Helsinki, who led the study on which Dr. Welch is a co-author.
Mothers in the intervention group made as much eye contact with the infants as possible and spoke with infants about their feelings.
Intimate sensory interactions between mothers and infants physically altered infants’ cortical networks in the brain and was later correlated to improved neurocognitive performance, according to the researchers.
“I was convinced there were physiological changes; I knew that from my clinical work,” Dr. Welch said. “I wanted to show it in this concrete, scientific way.”
Preterm babies face many hurdles
“If we can prevent problems in brain network organization to the extent that’s shown in this study and improve their outcomes, this is worth millions of dollars in terms of cost to society, schooling, health care, especially education, and families,” said Ruth Grunau, PhD, a professor in the Division of Neonatology in the department of pediatrics at the University of British Columbia, Vancouver, who was not involved with the most recent study but has worked with Dr. Welch previously.
Babies born too early, especially before 32 weeks, have higher rates of death and disability, according to the Centers for Disease Control and Prevention.
And preterm babies overall may experience breathing problems and feeding difficulties almost immediately following birth. They may also experience long-term problems such as developmental delays, vision problems, and hearing problems.
Dr. Grunau said that while many other programs and interventions have been used in the neonatal intensive care unit to help infants and mothers, the results from FNI stand out.
Ms. Yrjölä said she was surprised by the strength of the correlation as the infants continued to develop. The infants receiving the Family Nurture Intervention showed brain development close to the control group, which was infants born at full-term.
“Emotional connection is a state, not a trait – and a state can be changed,” said Dr. Welch. “And in this case, it can be changed by the parent through emotional expression.”
Steps clinicians can take
Dr. Welch said the approach is highly scalable, and two NICUs that participated in the FNI studies have implemented the program as standard care.
The approach is also gaining interest outside of the clinical setting, as preschool partners have expressed interest in implementing some of the methods to promote development.
Parents, family members, and teachers can use many of the FNI techniques – such as eye contact and emotional expression – to continue to develop and strengthen connection.
For clinicians who want to implement parts of the intervention on their own, Dr. Welch said doctors can observe if the baby looks at or turns toward their mother.
Clinicians can encourage mothers to express deep, emotional feelings toward the infant. Dr. Welch stressed that feelings don’t have to be positive, as many mothers with babies in the NICU have a hard time expressing positive emotions. Crying or talking about the difficulties of their childbirth experience count as expressing emotion. The important part is that the baby hears emotion, of any kind, in the mother’s voice, Dr. Welch said.
As the connection develops, it will eventually take less time for the mother and the baby to form a bond, and eventually the pair will become autonomically regulated.
“This is what gives us hope,” she said. “We affect each other in our autonomic nervous systems. It’s why this treatment works.”
The study was funded by the Finnish Pediatric Foundation, The Finnish Academy, the Juselius Foundation, Aivosäätiö, Neuroscience Center at University of Helsinki and Helsinki University Central Hospital, gifts from the Einhorn Family Charitable Trust, the Fleur Fairman Family, M. D. Stephenson, and The National Health and Medical Research Council of Australia. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Martha Welch, MD, spent the better part of three decades in private practice treating children with emotional, behavioral, and developmental disorders before accepting a job on the faculty of Columbia University, New York, in 1997.
She took the position, she said, with a mission: to find evidence to support what she’d observed in her practice – that parents could, by making stronger emotional connections, change the trajectory of development for preemie infants.
With that understanding, Dr. Welch created Family Nurture Intervention (FNI), which has been shown to improve the development of premature babies.
“We saw that no matter what happened to the baby, no matter how avoidant the baby might be, we’re able to overcome this with emotional expression,” Dr. Welch said.
Over the course of the intervention, families work with a specialist who helps bring mother and baby together – both physically and emotionally – until both are calm, which can initially take several hours and over time, minutes.
FNI appears to help families – especially mothers – re-establish an emotional connection often interrupted by their babies’ stressful and uncertain stay in a neonatal intensive care unit (NICU). In turn, both the infant and maternal nervous systems become better regulated, according to researchers.
Early challenges
Babies born preterm can face a range of short-term and long-term challenges, such as breathing problems due to an underdeveloped respiratory system, an increased risk of infection from an underdeveloped immune system, and learning difficulties, according to the Mayo Clinic.
Many aspects of FNI are not new: The neonatal intensive care unit has long incorporated activities such as scent cloth exchanges, talking to the baby, and skin-to-skin contact. But the approach Dr. Welch and her colleagues advocate emphasizes building a bond between the mother and the infant.
Mounting evidence shows that FNI can improve a wide range of outcomes for premature babies. In a 2021 study, for example, Dr. Welch’s group showed that FNI was associated with lower heart rates among preemies in the NICU. A 2016 study linked the intervention to reduced depression and anxiety symptoms in mothers of preterm infants. And a 2015 randomized controlled trial showed FNI improved development and behavioral outcomes in infants up to 18 months.
A new study published in Science Translational Medicine showed that the intervention led to a greater likelihood that babies had improved cognitive development later on, narrowing the developmental gap between healthy, full-term babies.
Dr. Welch and her colleagues tested to see if FNI measurably changed brain development in preterm infants who were born at 26-34 weeks of a pregnancy.
“We were blown away by the strength of the effect,” said Pauliina Yrjölä, MSc, a doctoral student and medical physicist at the University of Helsinki, who led the study on which Dr. Welch is a co-author.
Mothers in the intervention group made as much eye contact with the infants as possible and spoke with infants about their feelings.
Intimate sensory interactions between mothers and infants physically altered infants’ cortical networks in the brain and was later correlated to improved neurocognitive performance, according to the researchers.
“I was convinced there were physiological changes; I knew that from my clinical work,” Dr. Welch said. “I wanted to show it in this concrete, scientific way.”
Preterm babies face many hurdles
“If we can prevent problems in brain network organization to the extent that’s shown in this study and improve their outcomes, this is worth millions of dollars in terms of cost to society, schooling, health care, especially education, and families,” said Ruth Grunau, PhD, a professor in the Division of Neonatology in the department of pediatrics at the University of British Columbia, Vancouver, who was not involved with the most recent study but has worked with Dr. Welch previously.
Babies born too early, especially before 32 weeks, have higher rates of death and disability, according to the Centers for Disease Control and Prevention.
And preterm babies overall may experience breathing problems and feeding difficulties almost immediately following birth. They may also experience long-term problems such as developmental delays, vision problems, and hearing problems.
Dr. Grunau said that while many other programs and interventions have been used in the neonatal intensive care unit to help infants and mothers, the results from FNI stand out.
Ms. Yrjölä said she was surprised by the strength of the correlation as the infants continued to develop. The infants receiving the Family Nurture Intervention showed brain development close to the control group, which was infants born at full-term.
“Emotional connection is a state, not a trait – and a state can be changed,” said Dr. Welch. “And in this case, it can be changed by the parent through emotional expression.”
Steps clinicians can take
Dr. Welch said the approach is highly scalable, and two NICUs that participated in the FNI studies have implemented the program as standard care.
The approach is also gaining interest outside of the clinical setting, as preschool partners have expressed interest in implementing some of the methods to promote development.
Parents, family members, and teachers can use many of the FNI techniques – such as eye contact and emotional expression – to continue to develop and strengthen connection.
For clinicians who want to implement parts of the intervention on their own, Dr. Welch said doctors can observe if the baby looks at or turns toward their mother.
Clinicians can encourage mothers to express deep, emotional feelings toward the infant. Dr. Welch stressed that feelings don’t have to be positive, as many mothers with babies in the NICU have a hard time expressing positive emotions. Crying or talking about the difficulties of their childbirth experience count as expressing emotion. The important part is that the baby hears emotion, of any kind, in the mother’s voice, Dr. Welch said.
As the connection develops, it will eventually take less time for the mother and the baby to form a bond, and eventually the pair will become autonomically regulated.
“This is what gives us hope,” she said. “We affect each other in our autonomic nervous systems. It’s why this treatment works.”
The study was funded by the Finnish Pediatric Foundation, The Finnish Academy, the Juselius Foundation, Aivosäätiö, Neuroscience Center at University of Helsinki and Helsinki University Central Hospital, gifts from the Einhorn Family Charitable Trust, the Fleur Fairman Family, M. D. Stephenson, and The National Health and Medical Research Council of Australia. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Martha Welch, MD, spent the better part of three decades in private practice treating children with emotional, behavioral, and developmental disorders before accepting a job on the faculty of Columbia University, New York, in 1997.
She took the position, she said, with a mission: to find evidence to support what she’d observed in her practice – that parents could, by making stronger emotional connections, change the trajectory of development for preemie infants.
With that understanding, Dr. Welch created Family Nurture Intervention (FNI), which has been shown to improve the development of premature babies.
“We saw that no matter what happened to the baby, no matter how avoidant the baby might be, we’re able to overcome this with emotional expression,” Dr. Welch said.
Over the course of the intervention, families work with a specialist who helps bring mother and baby together – both physically and emotionally – until both are calm, which can initially take several hours and over time, minutes.
FNI appears to help families – especially mothers – re-establish an emotional connection often interrupted by their babies’ stressful and uncertain stay in a neonatal intensive care unit (NICU). In turn, both the infant and maternal nervous systems become better regulated, according to researchers.
Early challenges
Babies born preterm can face a range of short-term and long-term challenges, such as breathing problems due to an underdeveloped respiratory system, an increased risk of infection from an underdeveloped immune system, and learning difficulties, according to the Mayo Clinic.
Many aspects of FNI are not new: The neonatal intensive care unit has long incorporated activities such as scent cloth exchanges, talking to the baby, and skin-to-skin contact. But the approach Dr. Welch and her colleagues advocate emphasizes building a bond between the mother and the infant.
Mounting evidence shows that FNI can improve a wide range of outcomes for premature babies. In a 2021 study, for example, Dr. Welch’s group showed that FNI was associated with lower heart rates among preemies in the NICU. A 2016 study linked the intervention to reduced depression and anxiety symptoms in mothers of preterm infants. And a 2015 randomized controlled trial showed FNI improved development and behavioral outcomes in infants up to 18 months.
A new study published in Science Translational Medicine showed that the intervention led to a greater likelihood that babies had improved cognitive development later on, narrowing the developmental gap between healthy, full-term babies.
Dr. Welch and her colleagues tested to see if FNI measurably changed brain development in preterm infants who were born at 26-34 weeks of a pregnancy.
“We were blown away by the strength of the effect,” said Pauliina Yrjölä, MSc, a doctoral student and medical physicist at the University of Helsinki, who led the study on which Dr. Welch is a co-author.
Mothers in the intervention group made as much eye contact with the infants as possible and spoke with infants about their feelings.
Intimate sensory interactions between mothers and infants physically altered infants’ cortical networks in the brain and was later correlated to improved neurocognitive performance, according to the researchers.
“I was convinced there were physiological changes; I knew that from my clinical work,” Dr. Welch said. “I wanted to show it in this concrete, scientific way.”
Preterm babies face many hurdles
“If we can prevent problems in brain network organization to the extent that’s shown in this study and improve their outcomes, this is worth millions of dollars in terms of cost to society, schooling, health care, especially education, and families,” said Ruth Grunau, PhD, a professor in the Division of Neonatology in the department of pediatrics at the University of British Columbia, Vancouver, who was not involved with the most recent study but has worked with Dr. Welch previously.
Babies born too early, especially before 32 weeks, have higher rates of death and disability, according to the Centers for Disease Control and Prevention.
And preterm babies overall may experience breathing problems and feeding difficulties almost immediately following birth. They may also experience long-term problems such as developmental delays, vision problems, and hearing problems.
Dr. Grunau said that while many other programs and interventions have been used in the neonatal intensive care unit to help infants and mothers, the results from FNI stand out.
Ms. Yrjölä said she was surprised by the strength of the correlation as the infants continued to develop. The infants receiving the Family Nurture Intervention showed brain development close to the control group, which was infants born at full-term.
“Emotional connection is a state, not a trait – and a state can be changed,” said Dr. Welch. “And in this case, it can be changed by the parent through emotional expression.”
Steps clinicians can take
Dr. Welch said the approach is highly scalable, and two NICUs that participated in the FNI studies have implemented the program as standard care.
The approach is also gaining interest outside of the clinical setting, as preschool partners have expressed interest in implementing some of the methods to promote development.
Parents, family members, and teachers can use many of the FNI techniques – such as eye contact and emotional expression – to continue to develop and strengthen connection.
For clinicians who want to implement parts of the intervention on their own, Dr. Welch said doctors can observe if the baby looks at or turns toward their mother.
Clinicians can encourage mothers to express deep, emotional feelings toward the infant. Dr. Welch stressed that feelings don’t have to be positive, as many mothers with babies in the NICU have a hard time expressing positive emotions. Crying or talking about the difficulties of their childbirth experience count as expressing emotion. The important part is that the baby hears emotion, of any kind, in the mother’s voice, Dr. Welch said.
As the connection develops, it will eventually take less time for the mother and the baby to form a bond, and eventually the pair will become autonomically regulated.
“This is what gives us hope,” she said. “We affect each other in our autonomic nervous systems. It’s why this treatment works.”
The study was funded by the Finnish Pediatric Foundation, The Finnish Academy, the Juselius Foundation, Aivosäätiö, Neuroscience Center at University of Helsinki and Helsinki University Central Hospital, gifts from the Einhorn Family Charitable Trust, the Fleur Fairman Family, M. D. Stephenson, and The National Health and Medical Research Council of Australia. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 vaccine insights: The news beyond the headlines
Worldwide and across many diseases, vaccines have been transformative in reducing mortality—an effect that has been sustained with vaccines that protect against COVID-19.1 Since the first cases of SARS-CoV-2 infection were reported in late 2019, the pace of scientific investigation into the virus and the disease—made possible by unprecedented funding, infrastructure, and public and private partnerships—has been explosive. The result? A vast body of clinical and laboratory evidence about the safety and effectiveness of SARS-CoV-2 vaccines, which quickly became widely available.2-4
In this article, we review the basic underlying virology of SARS-CoV-2; the biotechnological basis of vaccines against COVID-19 that are available in the United States; and recommendations on how to provide those vaccines to your patients. Additional guidance for your practice appears in a select online bibliography, “COVID-19 vaccination resources.”
SIDEBAR
COVID-19 vaccination resources
Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States
Centers for Disease Control and Prevention
www.cdc.gov/vaccines/covid-19/clinical-considerations/interimconsiderations-us.html
COVID-19 ACIP vaccine recommendations
Advisory Committee on Immunization Practices (ACIP)
www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html
MMWR COVID-19 reports
Morbidity and Mortality Weekly Report
www.cdc.gov/mmwr/Novel_Coronavirus_Reports.html
A literature hub for tracking up-to-date scientific information about the 2019 novel coronavirus
National Center for Biotechnology Information of the National Library of Medicine
www.ncbi.nlm.nih.gov/research/coronavirus
Understanding COVID-19 vaccines
National Institutes of Health COVID-19 Research
https://covid19.nih.gov/treatments-and-vaccines/covid-19-vaccines
How COVID-19 affects pregnancy
National Institutes of Health COVID-19 Research
SARS-CoV-2 virology
As the SARS-CoV-2 virus approaches the host cell, normal cell proteases on the surface membrane cause a change in the shape of the SARS-CoV-2 spike protein. That spike protein conformation change allows the virus to avoid detection by the host’s immune system because its receptor-binding site is effectively hidden until just before entry into the cell.5,6 This process is analogous to a so-called lock-and-key method of entry, in which the key (ie, spike protein conformation) is hidden by the virus until the moment it is needed, thereby minimizing exposure of viral contents to the cell. As the virus spreads through the population, it adapts to improve infectivity and transmissibility and to evade developing immunity.7
After the spike protein changes shape, it attaches to an angiotensin-converting enzyme 2 (ACE-2) receptor on the host cell, allowing the virus to enter that cell. ACE-2 receptors are located in numerous human tissues: nasopharynx, lung, gastrointestinal tract, heart, thymus, lymph nodes, bone marrow, brain, arterial and venous endothelial cells, and testes.5 The variety of tissues that contain ACE-2 receptors explains the many sites of infection and location of symptoms with which SARS-CoV-2 infection can manifest, in addition to the respiratory system.
Basic mRNA vaccine immunology
Although messenger RNA (mRNA) vaccines seem novel, they have been in development for more than 30 years.8
mRNA encodes the protein for the antigen of interest and is delivered to the host muscle tissue. There, mRNA is translated into the antigen, which stimulates an immune response. Host enzymes then rapidly degrade the mRNA in the vaccine, and it is quickly eliminated from the host.
mRNA vaccines are attractive vaccine candidates, particularly in their application to emerging infectious diseases, for several reasons:
- They are nonreplicating.
- They do not integrate into the host genome.
- They are highly effective.
- They can produce antibody and cellular immunity.
- They can be produced (and modified) quickly on a large scale without having to grow the virus in eggs.
Continue to: Vaccines against SARS-CoV-2
Vaccines against SARS-CoV-2
Two vaccines (from Pfizer-BioNTech [Comirnaty] and from Moderna [Spikevax]) are US Food and Drug Administration (FDA)–approved for COVID-19; both utilize mRNA technology. Two other vaccines, which do not use mRNA technology, have an FDA emergency use authorization (from Janssen Biotech, of Johnson & Johnson [Janssen COVID-19 Vaccine] and from Novavax [Novavax COVID-19 Vaccine, Adjuvanted]).9
Pfizer-BioNTech and Moderna vaccines. The mRNA of these vaccines encodes the entire spike protein in its pre-fusion conformation, which is the antigen that is replicated in the host, inducing an immune response.10-12 (Recall the earlier lock-and-key analogy: This conformation structure ingeniously replicates the exposed 3-dimensional key to the host’s immune system.)
The Janssen vaccine utilizes a viral vector (a nonreplicating adenovirus that functions as carrier) to deliver its message to the host for antigen production (again, the spike protein) and an immune response.
The Novavax vaccine uses a recombinant nanoparticle protein composed of the full-length spike protein.13,14 In this review, we focus on the 2 available mRNA vaccines, (1) given their FDA-authorized status and (2) because Centers for Disease Control and Prevention (CDC) recommendations indicate a preference for mRNA vaccination over viral-vectored vaccination. However, we also address key points about the Janssen (Johnson & Johnson) vaccine.
Efficacy of COVID-19 vaccines
The first study to document the safety and efficacy of a SARS-CoV-2 vaccine (the Pfizer-BioNTech vaccine) was published just 12 months after the onset of the pandemic.10 This initial trial demonstrated a 95% efficacy in preventing symptomatic, laboratory-confirmed COVID-19 at 3-month follow-up.10 Clinical trial data on the efficacy of COVID-19 vaccines have continued to be published since that first landmark trial.
Continue to: Data from trials...
Data from trials in Israel that became available early in 2021 showed that, in mRNA-vaccinated adults, mechanical ventilation rates declined strikingly, particularly in patients > 70 years of age.15,16 This finding was corroborated by data from a surveillance study of multiple US hospitals, which showed that mRNA vaccines were > 90% effective in preventing hospitalization in adults > 65 years of age.17
Data published in May 2021 showed that the Pfizer-BioNTech and Moderna vaccines were 94% effective in preventing COVID-19-related hospitalization.18 During the end of the Delta wave of the pandemic and the emergence of the Omicron variant of SARS-CoV-2, unvaccinated people were 5 times as likely to be infected as vaccinated people.19
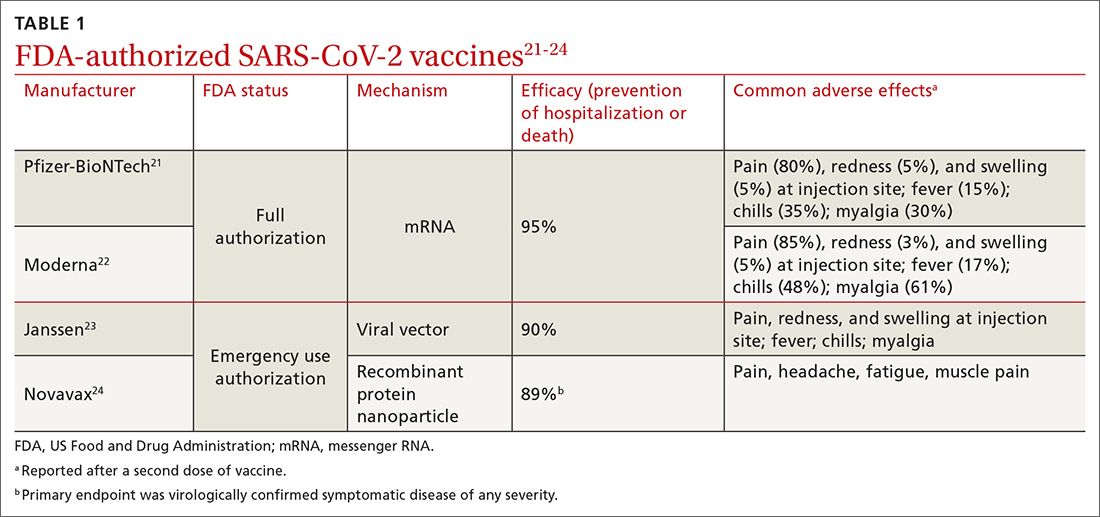
In March 2022, data from 21 US medical centers in 18 states demonstrated that adults who had received 3 doses of the vaccine were 94% less likely to be intubated or die than those who were unvaccinated.16 A July 2022 retrospective cohort study of 231,037 subjects showed that the risk of hospitalization for acute myocardial infarction or for stroke after COVID-19 infection was reduced by more than half in fully vaccinated (ie, 2 doses of an mRNA vaccine or the viral vector [Janssen/Johnson & Johnson] vaccine) subjects, compared to unvaccinated subjects.20 The efficacy of the vaccines is summarized in TABLE 1.21-24
Even in patients who have natural infection, several studies have shown that COVID-19 vaccination after natural infection increases the level and durability of immune response to infection and reinfection and improves clinical outcomes.9,20,25,26 In summary, published literature shows that (1) mRNA vaccines are highly effective at preventing infection and (2) they augment immunity achieved by infection with circulating virus.
Breakthrough infection. COVID-19 mRNA vaccines are associated with breakthrough infection (ie, infections in fully vaccinated people), a phenomenon influenced by the predominant viral variant circulating, the level of vaccine uptake in the studied population, and the timing of vaccination.27,28 Nevertheless, vaccinated people who experience breakthrough infection are much less likely to be hospitalized and die compared to those who are unvaccinated, and vaccination with an mRNA vaccine is more effective than immunity acquired from natural infection.29
Continue to: Vaccine adverse effects
Vaccine adverse effects: Common, rare, myths
Both early mRNA vaccine trials reported common minor adverse effects after vaccination (TABLE 121-24). These included redness and soreness at the injection site, fatigue, myalgias, fever, and nausea, and tended to be more common after the second dose. These adverse effects are similar to common adverse effects seen with other vaccines. Counseling information about adverse effects can be found on the CDC website.a
Two uncommon but serious adverse effects of COVID-19 vaccination are myocarditis or pericarditis after mRNA vaccination and thrombosis with thrombocytopenia syndrome (TTS), which occurs only with the Janssen vaccine.30,31
Myocarditis and pericarditis, particularly in young males (12 to 18 years), and mostly after a second dose of vaccine, was reported in May 2021. Since then, several studies have shown that the risk of myocarditis is slightly higher in males < 40 years of age, with a predicted case rate ranging from 1 to 10 excess cases for every 1 million patients vaccinated.30,32 This risk must be balanced against the rate of myocarditis associated with SARS-CoV-2 infection.
A large study in the United States demonstrated that the risk of myocarditis for those who contract COVID-19 is 16 times higher than it is for those who are disease free.33 Observational safety data from April 2022 showed that men ages 18 to 29 years had 7 to 8 times the risk of heart complications after natural infection, compared to men of those ages who had been vaccinated.34 In this study of 40 US health care systems, the incidence of myocarditis or pericarditis in that age group ranged from 55 to 100 cases for every 100,000 people after infection and from 6 to 15 cases for every 100,000 people after a second dose of an mRNA vaccine.34
A risk–benefit analysis conducted by the Advisory Committee on Immunization Practices (ACIP) ultimately supported the conclusions that (1) the risk of myocarditis secondary to vaccination is small and (2) clear benefits of preventing infection, hospitalization, death, and continued transmission outweigh that risk.35 Study of this question, utilizing vaccine safety and reporting systems around the world, has continued.
Continue to: There is emerging evidence...
There is emerging evidence that extending the interval between the 2 doses of vaccine decreases the risk of myocarditis, particularly in male adolescents.36 That evidence ultimately led the CDC to recommend that it might be optimal that an extended interval (ie, waiting 8 weeks between the first and second dose of vaccine), in particular for males ages 12 to 39 years, could be beneficial in decreasing the risk of myocarditis.
TTS. A population risk–benefit analysis of TTS was conducted by ACIP while use of the Janssen vaccine was paused in the United States in December 2021.36 The analysis determined that, although the risk of TTS was largely in younger women (18 to 49 years; 7 cases for every 1 million vaccine doses administered), benefits of the vaccine in preventing death, hospitalization, and a stay in the intensive care unit (ICU)—particularly if vaccination was delayed or there was a high rate of community infection—clearly outweighed risks. (The CDC estimated an incidence of 2 cases of TTS with more than 3 million doses of Janssen vaccine administered; assuming moderate transmission kinetics, more than 3500 hospitalizations and more than 350 deaths were prevented by vaccination.36) Ultimately, after the CDC analysis was released, vaccination utilizing the Janssen product resumed; however, the CDC offered the caveat that the Janssen vaccine should be used only in specific situations36 (eg, when there has been a severe reaction to mRNA vaccine or when access to mRNA or recombinant nanoparticle vaccine is limited).
Myths surrounding vaccination
Myth #1: SARS-CoV-2 vaccines contain tissue from aborted fetuses. This myth, which emerged during development of the vaccines, is often a conflation of the use of embryonic cell lines obtained decades ago to produce vaccines (a common practice—not only for vaccines but common pharmaceuticals and foods).37 There are no fetal cells or tissue in any SARS-CoV-2 vaccines, and the vaccines have been endorsed by several faith organizations.38
Myth #2: SARS-CoV-2 vaccines can cause sterility in men and women. This myth originated from a report in early December 2020 seeking to link a similarity in a protein involved in placental–uterine binding and a portion of the receptor-binding domain antigen produced by the vaccine.39 No studies support this myth; COVID-19 vaccines are recommended in pregnancy by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine.40,41
Myth #3: mRNA SARS-CoV-2 vaccines alter a recipient’s DNA. mRNA vaccines are broken down by cellular enzymes. They cannot be integrated into the host genome.8
Continue to: Boosters and vaccine mix-and-match
Boosters and vaccine mix-and-match
As the COVID-19 pandemic persists, with new variants of concern emerging, it has also become clear that immunity wanes. In July 2021, the first report was published after a cluster of breakthrough infections occurred in a town in Massachusetts.42 There was no recommendation, at the time, for a booster; the Delta variant was the predominant circulating strain. In this outbreak, there were 469 cases, 74% of which were in people who had received 2 doses of an mRNA vaccine.42 Five patients were hospitalized; none died.42 A key takeaway from this outbreak was that vaccination prevented death, even in the face of fairly wide breakthrough infection.
Newer data show that, although vaccine effectiveness against hospitalization was greater than 90% for the first 2 months after a third dose, it waned to 78% by 4 months.43 Published data, combined with real-world experience, show that boosters provide additional reduction in the risk of death and hospitalization. This has led to a recommendation that all patients ≥ 5 years of age receive a booster.19,26,43-48 The CDC now recommends that people who are ages 12 years and older receive a bivalent booster (containing both wild-type and Omicron-variant antigens) ≥ 2 months after their most recent booster or completed series.
Future booster recommendations will consider the durability of the immune response over time (measured against the original immunizing virus) and the mutation rate of the virus.49
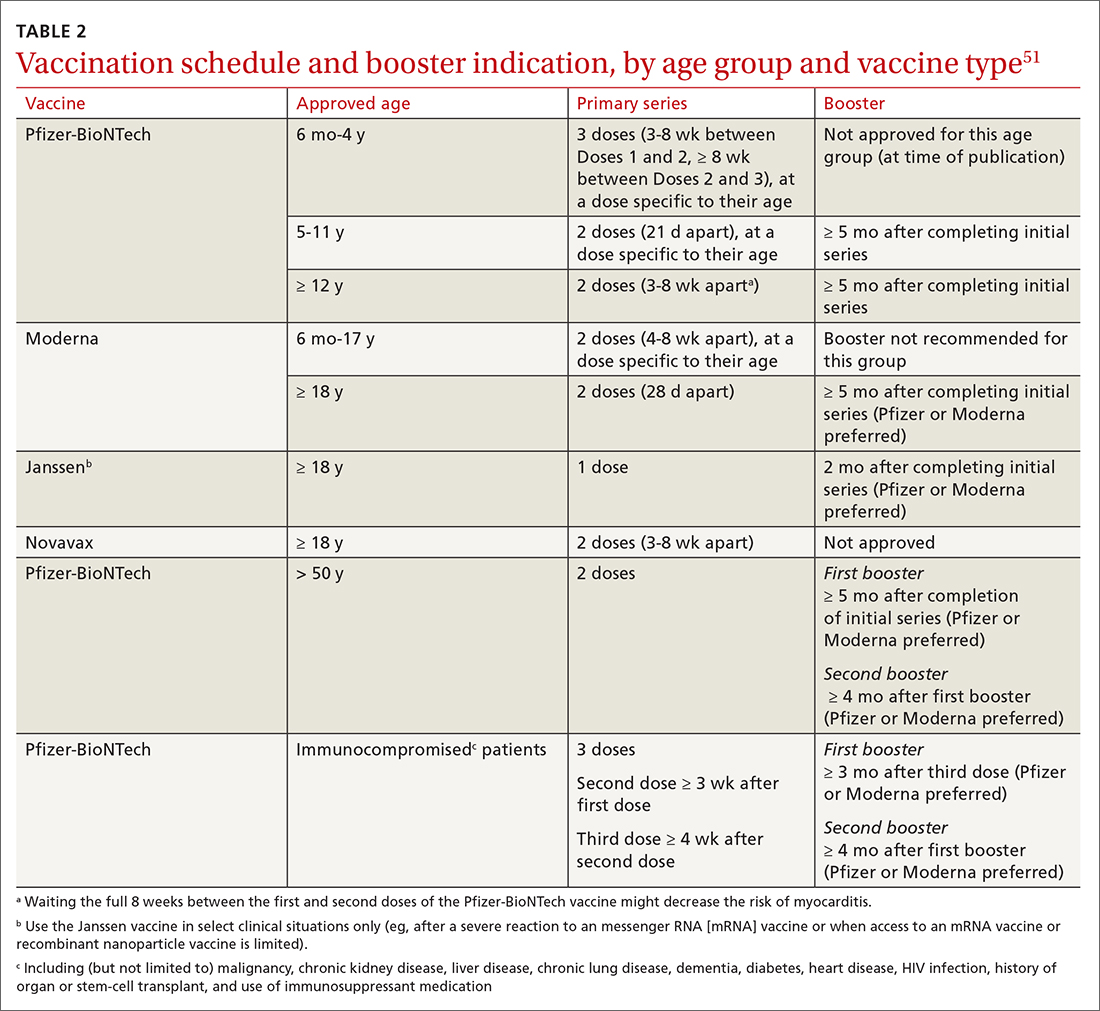
Given the limited supply of vaccine early in the pandemic, and the potential for future limitations, there was early interest in studying so-called mix-and-match SARS-CoV-2 vaccination—that is, receiving one product as a first series and then a different product as a booster, also known as heterologous booster vaccination. Although it is preferred that the 2 doses of the primary series be of the same vaccine product, studies that have examined this question support heterologous boosting as an acceptable approach to protective immunity50 (TABLE 251).
Vaccination in special populations
Three groups of patients have unique host characteristics that are important to consider when providing COVID-19 vaccination in your practice: pregnant patients, children, and patients in the broad category of “immunocompromised status.”
Continue to: Pregnant patients
Pregnant patients with SARS-CoV-2 infection are more likely to be hospitalized and have a higher risk of a stay in the ICU and need for mechanical ventilation. In a study of the course of illness in symptomatic pregnant patients who were hospitalized, 16.2% were admitted to an ICU and 8.5% were mechanically ventilated.52 CDC observational data have consistently supported the finding that (1) pregnant patients infected with SARS-CoV-2 are at increased risk of preterm labor and (2) their newborns are at increased risk of low birth weight and requiring admission to the neonatal ICU.53
A systematic review of 46 studies in pregnant and lactating patients showed no increased risk of adverse effects from COVID-19 vaccination.54 Furthermore, data from multiple studies demonstrate that immunoglobulin G antibodies cross the placenta to protect the infant at birth (ie, are found in umbilical cord blood and neonatal blood) and are found in breast milk. The precise kinetics and durability of these antibodies are unknown.
Pregnant patients were initially excluded from vaccine trials (although there were some patients ultimately found to be pregnant, or who became pregnant, during the trial). Careful examination of vaccine safety and efficacy data has supported the American College of Obstetricians and Gynecologists and European Board and College of Obstetrics and Gynaecology (EBCOG) recommendation that all pregnant patients be vaccinated. Furthermore, EBCOG recommends vaccination during the period of breastfeeding.55
Children. A major challenge during the pandemic has been to understand (1) the effect that infection with SARS-CoV-2 has on children and (2) the role of children in transmission of the virus. Although most children with COVID-19 have mild symptoms, a few require hospitalization and mechanical ventilation and some develop life-threatening multisystem inflammatory syndrome.56 In a large, retrospective study of more than 12,000 children with COVID-19, 5.3% required hospitalization and almost 20% of that subset were admitted to the ICU.57
Various hypotheses have been put forward to describe and explain the differences in disease expression between children and adults. These include:
- the absence of comorbidities often seen in adults
- evidence that pediatric patients might have reduced expression of ACE-2
- a more active T-cell response in infected children, due to an active thymus.56
Continue to: Although the number of children affected...
Although the number of children affected by severe SARS-CoV-2 infection is less than the number of adults, there have been important trends observed in infection and hospitalization as different variants have arisen.58 The Delta and Omicron variants have both been associated with a disturbing trend in the rate of hospitalization of pediatric patients, particularly from birth to 4 years—patients who were ineligible for vaccination at the time of the study.58 Ultimately, these data, combined with multiple studies of vaccine effectiveness in this age group, have led to an emergency use authorization for the Pfizer-BioNTech vaccination in pediatric populations and a recommendation from the American Academy of Pediatrics that all children ages 6 months and older be vaccinated.59,60
Immunocompromised patients. Patients broadly classified as immunocompromised have raised unique concerns. These patients have conditions such as malignancy, primary or secondary immunodeficiency, diabetes, and autoimmune disease; are taking certain classes of medication; or are of older age.61 Early in the pandemic, data showed that immunocompromised hosts could shed virus longer than hosts with an intact immune system—adding to their risk of transmitting SARS-CoV-2 and of viral adaptation for immune escape.62 Antibody response to vaccination was also less robust in this group.
There are limited data that demonstrate a short-lived reduction in risk of infection (in that study, Omicron was the prominent variant) with a fourth dose of an mRNA vaccine.63 Based on these data and FDA approval, the CDC recommends (1) an additional third primary dose and (2) a second booster for people who are moderately or severely immunocompromised. For those ages 50 years or older, a second booster is now required for their vaccination to be considered up to date.b
Predictions (or, why is a COVID-19 vaccine important?)
What does the future hold for our struggle with COVID-19? Perhaps we can learn lessons from the study of the 4 known seasonal coronaviruses, which cause the common cold and circulate annually.64 First, only relative immunity is produced after infection with a seasonal coronavirus.64 Studies of antibodies to seasonal coronaviruses seem to suggest that, although antibody titers remain high, correlation with decreased infection is lacking.65 Second, a dominant strain or 2 emerges each season, probably as a result of genetic variation and selective pressure for immune escape from neutralizing antibodies or cellular immunity.
The complex relationship among competing immune response duration, emergence of viral immune escape, increasing viral transmissibility, and societal viral source control (through vaccination, masking, distancing, testing, isolation, and treatment) widens the confidence bounds on our estimates of what the future holds. Late in 2020, the CDC began reporting wastewater surveillance data as a method for monitoring, and predicting changes in, community spread.66 During Spring 2022, the CDC reported an increase in detection of SARS-CoV-2 from a third of wastewater sampling sites around the United States. This observation coincided with (1) appearance of still more transmissible BA.2 and, later, BA.2.12.1 variants and (2) general relaxing of masking and social distancing guidelines, following the decline of the Omicron variant.
Continue to: At approximately that time...
At approximately that time, application to the FDA for a fourth shot (or a second booster) by Pfizer-BioNTech had been approved for adults > 50 years of age, at > 4 months after their previous vaccination.57 In view of warning signs from wastewater surveillance, priorities for vaccination should be to:
- increase uptake in the hesitant
- get boosters to the eligible
- prepare to tackle either seasonal or sporadic recurrence of COVID-19—whichever scenario the future brings.
As an example of how these priorities have been put into action, in September 2022, the FDA approved, and the CDC recommended, new bivalent boosters for everyone ≥ 12 years of age (Pfizer-BioNTech) or for all those ≥ 18 years of age (Moderna), to be administered ≥ 2 months after receipt of their most recent booster or primary series.
a www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html
b Visit www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html for more guidance on COVID-19 vaccination for immunocompromised patients.
CORRESPONDENCE
John L. Kiley, MD, 3551 Roger Brooke Drive, Fort Sam Houston, TX 78234; [email protected]
1. Orenstein W, Offitt P, Edwards KM, Plotkin S. Plotkin’s Vaccines. 7th ed. Elsevier; 2017:1-15.
2. Operation Warp Speed: implications for global vaccine security. Lancet Glob Health. 2021;9:e1017-e1021. doi: 10.1016/S2214-109X(21)00140-6
3. Lurie N, Saville M, Hatchett R, et al. Developing Covid-19 vaccines at pandemic speed. N Engl J Med. 2020;382:1969-1973. doi: 10.1056/NEJMp2005630
4. Slaoui M, Hepburn M. Developing safe and effective Covid vaccines—Operation Warp Speed’s strategy and approach. N Engl J Med. 2020;383:1701-1703. doi: 10.1056/NEJMp2027405
5. Hu B, Guo H, Zhou P, et al. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19:141-154. doi: 10.1038/s41579-020-00459-7
6. Hussain I, Pervaiz N, Khan A, et al. Evolutionary and structural analysis of SARS-CoV-2 specific evasion of host immunity. Genes Immun. 2020;21:409-419. doi: 10.1038/s41435-020-00120-6
7. Rando HM, Wellhausen N, Ghosh S, et al; COVID-19 Review Consortium. Identification and development of therapeutics for COVID-19. mSystems. 2021;6:e0023321. doi: 10.1128/mSystems.00233-21
8. Pardi N, Hogan MJ, Porter FW, et al. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261-279. doi: 10.1038/nrd.2017.243
9. National Center for Immunization and Respiratory Diseases. Use of COVID-19 vaccines in the United States: interim clinical considerations. Centers for Disease Control and Prevention. Updated August 22, 2022. Accessed August 27, 2022. www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html#references
10. Polack FP, Thomas SJ, Kitchin N, et al; . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603-2615. doi: 10.1056/NEJMoa2034577
11. Heinz FX, Stiasny K. Distinguishing features of current COVID-19 vaccines: knowns and unknowns of antigen presentation and modes of action. NPJ Vaccines. 2021;6:104. doi: 10.1038/s41541-021-00369-6
12. Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403-416. doi: 10.1056/NEJMoa2035389
13. Keech C, Albert G, Cho I, et al. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383:2320-2332. doi: 10.1056/NEJMoa2026920
14. Heath PT, Galiza EP, Baxter DN, et al; . Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med. 2021;385:1172-1183. doi: 10.1056/NEJMoa2107659
15. Rinott E, Youngster I, Lewis YE. Reduction in COVID-19 patients requiring mechanical ventilation following implementation of a national COVID-19 vaccination program—Israel, December 2020–February 2021. MMWR Morb Mortal Wkly Rep. 2021;70:326-328. doi: 10.15585/mmwr.mm7009e3
16. Tenforde MW, Self WH, Gaglani M, et al; IVY Network. Effectiveness of mRNA vaccination in preventing COVID-19-associated invasive mechanical ventilation and death—United States, March 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:459-465. doi: 10.15585/mmwr.mm7112e1
17. Moline HL, Whitaker M, Deng L, et al. Effectiveness of COVID-19 vaccines in preventing hospitalization among adults aged ≥ 65 years—COVID-NET, 13 States, February–April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1088-1093. doi: 10.15585/mmwr.mm7032e
18. Tenforde MW, Olson SM, Self WH, et al; ; . Effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 among hospitalized adults aged ≥ 65 years—United States, January–March 2021. MMWR Morb Mortal Wkly Rep. 2021;70:674-679. doi: 10.15585/mmwr.mm7018e1
19. Johnson AG, Amin AB, Ali AR, et al. COVID-19 incidence and death rates among unvaccinated and fully vaccinated adults with and without booster doses during periods of Delta and Omicron variant emergence—25 U.S. jurisdictions, April 4–December 25, 2021. MMWR Morb Mortal Wkly Rep. 2022;71:132-138. doi: 10.15585/mmwr.mm7104e2
20. Kim Y-E, Huh K, Park Y-J, et al. Association between vaccination and acute myocardial infarction and ischemic stroke after COVID-19 infection. JAMA. Published online July 22, 2022. doi: 10.1001/jama.2022.12992
21. Centers for Disease Control and Prevention. Pfizer-BioNTech COVID-19 vaccine reactions & adverse events. Updated June 21, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html
22. Centers for Disease Control and Prevention. The Moderna COVID-19 vaccine’s local reactions, systemic reactions, adverse events, and serious adverse events. Updated June 21, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html
23. Centers for Disease Control and Prevention. The Janssen COVID-19 vaccine’s local Reactions, Systemic reactions, adverse events, and serious adverse events. Updated August 12, 2021. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/janssen/reactogenicity.html
24. Centers for Disease Control and Prevention. Novavax COVID-19 vaccine local reactions, systemic reactions, adverse events, and serious adverse events. Updated August 31, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/novavax/reactogenicity.html
25. Greaney AJ, Loes AN, Gentles LE, et al. Antibodies elicited by mRNA-1273 vaccination bind more broadly to the receptor binding domain than do those from SARS-CoV-2 infection. Sci Transl Med. 2021;13:eabi9915. doi: 10.1126/scitranslmed.abi9915
26. Hall V, Foulkes S, Insalata F, et al. Protection against SARS-CoV-2 after Covid-19 vaccination and previous infection. N Engl J Med. 2022;386:1207-1220. doi: 10.1056/NEJMoa2118691
27. Klompas M. Understanding breakthrough infections following mRNA SARS-CoV-2 avccination. JAMA. 2021;326:2018-2020. doi: 10.1001/jama.2021.19063
28. Kustin T, Harel N, Finkel U, et al. Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals. Nat Med. 2021;27:1379-1384. doi: 10.1038/s41591-021-01413-7
29. Yu Y, Esposito D, Kang Z, et al. mRNA vaccine-induced antibodies more effective than natural immunity in neutralizing SARS-CoV-2 and its high affinity variants. Sci Rep. 2022;12:2628. doi: 10.1038/s41598-022-06629-2
30. Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:977-982. doi: 10.15585/mmwr.mm7027e2
31. MacNeil JR, Su JR, Broder KR, et al. Updated recommendations from the Advisory Committee on Immunization Practices for use of the Janssen (Johnson & Johnson) COVID-19 vaccine after reports of thrombosis with thrombocytopenia syndrome among vaccine recipients—United States, April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:651-656. doi: 10.15585/mmwr.mm7017e4
32. Patone M, Mei XW, Handunnetthi L, et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2022;28:410-422. doi: 10.1038/s41591-021-01630-0
33. Boehmer TK, Kompaniyets L, Lavery AM, et al. Association between COVID-19 and myocarditis using hospital-based administrative data—United States, March 2020–January 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1228-1232. doi: 10.15585/mmwr.mm7035e5
34. Block JP, Boehmer TK, Forrest CB, et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination—PCORnet, United States, January 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:517-523. doi: 10.15585/mmwr.mm7114e1
35. Rosemblum H. COVID-19 vaccines in adults: benefit–risk discussion. Centers for Disease Control and Prevention. July 22, 2021. Accessed September 21, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-07/05-COVID-Rosenblum-508.pdf
36. Buchan SA, Seo CY, Johnson C, et al. Epidemiology of myocarditis and pericarditis following mRNA vaccines in Ontario, Canada: by vaccine product, schedule and interval. medRxiv. 2021:12.02.21267156.
37. Wong A. The ethics of HEK 293. Natl Cathol Bioeth Q. 2006;6:473-495. doi: 10.5840/ncbq20066331
38. North Dakota Health. COVID-19 vaccines & fetal cell lines. Updated December 1, 2021. Accessed September 21, 2022. www.health.nd.gov/sites/www/files/documents/COVID%20Vaccine%20Page/COVID-19_Vaccine_Fetal_Cell_Handout.pdf
39. Abbasi J. Widespread misinformation about infertility continues to create COVID-19 vaccine hesitancy. JAMA. 2022;327:1013-1015. doi: 10.1001/jama.2022.2404
40. Halasa NB, Olson SM, Staat MA, et al; ; . Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19-associated hospitalization in infants aged < 6 months—17 States, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:264-270. doi: 10.15585/mmwr.mm7107e3
41. American College of Obstetricians and Gynecologists. ACOG and SMFM recommend COVID-19 vaccination for pregnant individuals. July 30, 2021. Accessed September 21, 2022. www.acog.org/news/news-releases/2021/07/acog-smfm-recommend-covid-19-vaccination-for-pregnant-individuals#:~:text=%E2%80%9CACOG%20is%20recommending%20vaccination%20of,complications%2C%20and%20because%20it%20isvaccines
42. Brown CM, Vostok J, Johnson H, et al. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings—Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1059-1062. doi: 10.15585/mmwr.mm7031e2
43. Ferdinands JM, Rao S, Dixon BE, et al. Waning 2-dose and 3-dose effectiveness of mRNA against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance—VISION Network, 10 states, August 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:255-263. doi: 10.15585/mmwr.mm7107e2
44. Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Effect of mRNA vaccine boosters against SARS-CoV-2 Omicron infection in Qatar. N Engl J Med. 2022;386:1804-1816. doi: 10.1056/NEJMoa2200797
45. Arbel R, Hammerman A, Sergienko R, et al. BNT162b2 vaccine booster and mortality due to Covid-19. N Engl J Med. 2021;385:2413-2420. doi: 10.1056/NEJMoa2115624
46. Bar-On YM, Goldberg Y, Mandel M, et al. Protection against Covid-19 by BNT162b2 booster across age groups. N Engl J Med. 2021;385:2421-2430. doi: 10.1056/NEJMoa2115926
47. Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385:1393-1400. doi: 10.1056/NEJMoa2114255
48. Mbaeyi S, Oliver SE, Collins JP, et al. The Advisory Committee on Immunization Practices’ interim recommendations for additional primary and booster doses of COVID-19 vaccines—United States, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1545-1552. doi: 10.15585/mmwr.mm7044e2
49. Chen X, Chen Z, Azman AS, et al. Neutralizing antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants induced by natural infection or vaccination: a systematic review and pooled analysis. Clin Infect Dis. 2022;74:734-742. doi: 10.1093/cid/ciab646
50. Atmar RL, Lyke KE, Deming ME, et al; . Homologous and heterologous Covid-19 booster vaccinations. N Engl J Med. 2022;386:1046-1057. doi: 10.1056/NEJMoa2116414
51. Centers for Disease Control and Prevention. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. Updated September 2, 2022. Accessed September 21, 2022. www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html
52. Ackerman CM, Nguyen JL, Ambati S, et al. Clinical and pregnancy outcomes of coronavirus disease 2019 among hospitalized pregnant women in the United States. Open Forum Infect Dis. 2022;9:ofab429. doi: 10.1093/ofid/ofab429
53. Osterman MJK, Valenzuela CP, Martin JA. Maternal and infant characteristics among women with confirmed or presumed cases of coronavirus disease (COVID-19) during pregnancy. National Center for Health Statistics. National Vital Statistics System. Updated August 11, 2022. Accessed September 21, 2022. www.cdc.gov/nchs/covid19/technical-linkage.htm
54. De Rose DU, Salvatori G, Dotta A, et al. SARS-CoV-2 vaccines during pregnancy and breastfeeding: a systematic review of maternal and neonatal outcomes. Viruses. 2022;14:539. doi: 10.3390/v14030539
55. Martins I, Louwen F, Ayres-de-Campos D, et al. EBCOG position statement on COVID-19 vaccination for pregnant and breastfeeding women. Eur J Obstet Gynecol Reprod Biol. 2021;262:256-258. doi: 10.1016/j.ejogrb.2021.05.021
56. Chou J, Thomas PG, Randolph AG. Immunology of SARS-CoV-2 infection in children. Nat Immunol. 2022;23:177-185. doi: 10.1038/s41590-021-01123-9
57. Parcha V, Booker KS, Kalra R, et al. A retrospective cohort study of 12,306 pediatric COVID-19 patients in the United States. Sci Rep. 2021;11:10231. doi: 10.1038/s41598-021-89553-1
58. Marks KJ, Whitaker M, Anglin O, et al; . Hospitalizations of children and adolescents with laboratory-confirmed COVID-19—COVID-NET, 14 states, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:271-278. doi: 10.15585/mmwr.mm7107e4
59. Price AM, Olson SM, Newhams MM, et al; . BNT162b2 protection against the Omicron variant in children and adolescents. N Engl J Med. 2022;386:1899-1909. doi: 10.1056/NEJMoa2202826
60. Maldonado YA, O’Leary ST, Banerjee R, et al; Committee on Infectious Diseases, American Academy of Pediatrics. COVID-19 vaccines in children and adolescents. Pediatrics. 2021;148:e2021052336. doi: 10.1542/peds.2021-052336
61. Lontok K. How effective are COVID-19 vaccines in immunocompromised people? American Society for Microbiology. August 12, 2021. Accessed September 21, 2022. https://asm.org/Articles/2021/August/How-Effective-Are-COVID-19-Vaccines-in-Immunocompr
62. Meiring S, Tempia S, Bhiman JN, et al; . Prolonged shedding of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) at high viral loads among hospitalized immunocompromised persons living with human immunodeficiency virus, South Africa. Clin Infect Dis. 2022;75:e144-e156. doi: 10.1093/cid/ciac077
63. Bar-On YM, Goldberg Y, Mandel M, et al. Protection by 4th dose of BNT162b2 against Omicron in Israel. medRxiv. 2022: 02.01.22270232. doi: 10.1101/2022.02.01.22270232
64. Monto AS, DeJonge PM, Callear AP, et al. Coronavirus occurrence and transmission over 8 years in the HIVE cohort of households in Michigan. J Infect Dis. 2020;222:9-16. doi: 10.1093/infdis/jiaa161
65. Petrie JG, Bazzi LA, McDermott AB, et al. Coronavirus occurrence in the Household Influenza Vaccine Evaluation (HIVE) cohort of Michigan households: reinfection frequency and serologic responses to seasonal and severe acute respiratory syndrome coronaviruses. J Infect Dis. 2021;224:49-59. doi: 10.1093/infdis/jiab161
66. Kirby AE, Walters MS, Jennings WC, et al. Using wastewater surveillance data to support the COVID-19 response—United States, 2020–2021. MMWR Morb Mortal Wkly Rep. 2021;70:1242-1244. doi: 10.15585/mmwr.mm7036a2
Worldwide and across many diseases, vaccines have been transformative in reducing mortality—an effect that has been sustained with vaccines that protect against COVID-19.1 Since the first cases of SARS-CoV-2 infection were reported in late 2019, the pace of scientific investigation into the virus and the disease—made possible by unprecedented funding, infrastructure, and public and private partnerships—has been explosive. The result? A vast body of clinical and laboratory evidence about the safety and effectiveness of SARS-CoV-2 vaccines, which quickly became widely available.2-4
In this article, we review the basic underlying virology of SARS-CoV-2; the biotechnological basis of vaccines against COVID-19 that are available in the United States; and recommendations on how to provide those vaccines to your patients. Additional guidance for your practice appears in a select online bibliography, “COVID-19 vaccination resources.”
SIDEBAR
COVID-19 vaccination resources
Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States
Centers for Disease Control and Prevention
www.cdc.gov/vaccines/covid-19/clinical-considerations/interimconsiderations-us.html
COVID-19 ACIP vaccine recommendations
Advisory Committee on Immunization Practices (ACIP)
www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html
MMWR COVID-19 reports
Morbidity and Mortality Weekly Report
www.cdc.gov/mmwr/Novel_Coronavirus_Reports.html
A literature hub for tracking up-to-date scientific information about the 2019 novel coronavirus
National Center for Biotechnology Information of the National Library of Medicine
www.ncbi.nlm.nih.gov/research/coronavirus
Understanding COVID-19 vaccines
National Institutes of Health COVID-19 Research
https://covid19.nih.gov/treatments-and-vaccines/covid-19-vaccines
How COVID-19 affects pregnancy
National Institutes of Health COVID-19 Research
SARS-CoV-2 virology
As the SARS-CoV-2 virus approaches the host cell, normal cell proteases on the surface membrane cause a change in the shape of the SARS-CoV-2 spike protein. That spike protein conformation change allows the virus to avoid detection by the host’s immune system because its receptor-binding site is effectively hidden until just before entry into the cell.5,6 This process is analogous to a so-called lock-and-key method of entry, in which the key (ie, spike protein conformation) is hidden by the virus until the moment it is needed, thereby minimizing exposure of viral contents to the cell. As the virus spreads through the population, it adapts to improve infectivity and transmissibility and to evade developing immunity.7
After the spike protein changes shape, it attaches to an angiotensin-converting enzyme 2 (ACE-2) receptor on the host cell, allowing the virus to enter that cell. ACE-2 receptors are located in numerous human tissues: nasopharynx, lung, gastrointestinal tract, heart, thymus, lymph nodes, bone marrow, brain, arterial and venous endothelial cells, and testes.5 The variety of tissues that contain ACE-2 receptors explains the many sites of infection and location of symptoms with which SARS-CoV-2 infection can manifest, in addition to the respiratory system.
Basic mRNA vaccine immunology
Although messenger RNA (mRNA) vaccines seem novel, they have been in development for more than 30 years.8
mRNA encodes the protein for the antigen of interest and is delivered to the host muscle tissue. There, mRNA is translated into the antigen, which stimulates an immune response. Host enzymes then rapidly degrade the mRNA in the vaccine, and it is quickly eliminated from the host.
mRNA vaccines are attractive vaccine candidates, particularly in their application to emerging infectious diseases, for several reasons:
- They are nonreplicating.
- They do not integrate into the host genome.
- They are highly effective.
- They can produce antibody and cellular immunity.
- They can be produced (and modified) quickly on a large scale without having to grow the virus in eggs.
Continue to: Vaccines against SARS-CoV-2
Vaccines against SARS-CoV-2
Two vaccines (from Pfizer-BioNTech [Comirnaty] and from Moderna [Spikevax]) are US Food and Drug Administration (FDA)–approved for COVID-19; both utilize mRNA technology. Two other vaccines, which do not use mRNA technology, have an FDA emergency use authorization (from Janssen Biotech, of Johnson & Johnson [Janssen COVID-19 Vaccine] and from Novavax [Novavax COVID-19 Vaccine, Adjuvanted]).9
Pfizer-BioNTech and Moderna vaccines. The mRNA of these vaccines encodes the entire spike protein in its pre-fusion conformation, which is the antigen that is replicated in the host, inducing an immune response.10-12 (Recall the earlier lock-and-key analogy: This conformation structure ingeniously replicates the exposed 3-dimensional key to the host’s immune system.)
The Janssen vaccine utilizes a viral vector (a nonreplicating adenovirus that functions as carrier) to deliver its message to the host for antigen production (again, the spike protein) and an immune response.
The Novavax vaccine uses a recombinant nanoparticle protein composed of the full-length spike protein.13,14 In this review, we focus on the 2 available mRNA vaccines, (1) given their FDA-authorized status and (2) because Centers for Disease Control and Prevention (CDC) recommendations indicate a preference for mRNA vaccination over viral-vectored vaccination. However, we also address key points about the Janssen (Johnson & Johnson) vaccine.
Efficacy of COVID-19 vaccines
The first study to document the safety and efficacy of a SARS-CoV-2 vaccine (the Pfizer-BioNTech vaccine) was published just 12 months after the onset of the pandemic.10 This initial trial demonstrated a 95% efficacy in preventing symptomatic, laboratory-confirmed COVID-19 at 3-month follow-up.10 Clinical trial data on the efficacy of COVID-19 vaccines have continued to be published since that first landmark trial.
Continue to: Data from trials...
Data from trials in Israel that became available early in 2021 showed that, in mRNA-vaccinated adults, mechanical ventilation rates declined strikingly, particularly in patients > 70 years of age.15,16 This finding was corroborated by data from a surveillance study of multiple US hospitals, which showed that mRNA vaccines were > 90% effective in preventing hospitalization in adults > 65 years of age.17
Data published in May 2021 showed that the Pfizer-BioNTech and Moderna vaccines were 94% effective in preventing COVID-19-related hospitalization.18 During the end of the Delta wave of the pandemic and the emergence of the Omicron variant of SARS-CoV-2, unvaccinated people were 5 times as likely to be infected as vaccinated people.19
In March 2022, data from 21 US medical centers in 18 states demonstrated that adults who had received 3 doses of the vaccine were 94% less likely to be intubated or die than those who were unvaccinated.16 A July 2022 retrospective cohort study of 231,037 subjects showed that the risk of hospitalization for acute myocardial infarction or for stroke after COVID-19 infection was reduced by more than half in fully vaccinated (ie, 2 doses of an mRNA vaccine or the viral vector [Janssen/Johnson & Johnson] vaccine) subjects, compared to unvaccinated subjects.20 The efficacy of the vaccines is summarized in TABLE 1.21-24

Even in patients who have natural infection, several studies have shown that COVID-19 vaccination after natural infection increases the level and durability of immune response to infection and reinfection and improves clinical outcomes.9,20,25,26 In summary, published literature shows that (1) mRNA vaccines are highly effective at preventing infection and (2) they augment immunity achieved by infection with circulating virus.
Breakthrough infection. COVID-19 mRNA vaccines are associated with breakthrough infection (ie, infections in fully vaccinated people), a phenomenon influenced by the predominant viral variant circulating, the level of vaccine uptake in the studied population, and the timing of vaccination.27,28 Nevertheless, vaccinated people who experience breakthrough infection are much less likely to be hospitalized and die compared to those who are unvaccinated, and vaccination with an mRNA vaccine is more effective than immunity acquired from natural infection.29
Continue to: Vaccine adverse effects
Vaccine adverse effects: Common, rare, myths
Both early mRNA vaccine trials reported common minor adverse effects after vaccination (TABLE 121-24). These included redness and soreness at the injection site, fatigue, myalgias, fever, and nausea, and tended to be more common after the second dose. These adverse effects are similar to common adverse effects seen with other vaccines. Counseling information about adverse effects can be found on the CDC website.a
Two uncommon but serious adverse effects of COVID-19 vaccination are myocarditis or pericarditis after mRNA vaccination and thrombosis with thrombocytopenia syndrome (TTS), which occurs only with the Janssen vaccine.30,31
Myocarditis and pericarditis, particularly in young males (12 to 18 years), and mostly after a second dose of vaccine, was reported in May 2021. Since then, several studies have shown that the risk of myocarditis is slightly higher in males < 40 years of age, with a predicted case rate ranging from 1 to 10 excess cases for every 1 million patients vaccinated.30,32 This risk must be balanced against the rate of myocarditis associated with SARS-CoV-2 infection.
A large study in the United States demonstrated that the risk of myocarditis for those who contract COVID-19 is 16 times higher than it is for those who are disease free.33 Observational safety data from April 2022 showed that men ages 18 to 29 years had 7 to 8 times the risk of heart complications after natural infection, compared to men of those ages who had been vaccinated.34 In this study of 40 US health care systems, the incidence of myocarditis or pericarditis in that age group ranged from 55 to 100 cases for every 100,000 people after infection and from 6 to 15 cases for every 100,000 people after a second dose of an mRNA vaccine.34
A risk–benefit analysis conducted by the Advisory Committee on Immunization Practices (ACIP) ultimately supported the conclusions that (1) the risk of myocarditis secondary to vaccination is small and (2) clear benefits of preventing infection, hospitalization, death, and continued transmission outweigh that risk.35 Study of this question, utilizing vaccine safety and reporting systems around the world, has continued.
Continue to: There is emerging evidence...
There is emerging evidence that extending the interval between the 2 doses of vaccine decreases the risk of myocarditis, particularly in male adolescents.36 That evidence ultimately led the CDC to recommend that it might be optimal that an extended interval (ie, waiting 8 weeks between the first and second dose of vaccine), in particular for males ages 12 to 39 years, could be beneficial in decreasing the risk of myocarditis.
TTS. A population risk–benefit analysis of TTS was conducted by ACIP while use of the Janssen vaccine was paused in the United States in December 2021.36 The analysis determined that, although the risk of TTS was largely in younger women (18 to 49 years; 7 cases for every 1 million vaccine doses administered), benefits of the vaccine in preventing death, hospitalization, and a stay in the intensive care unit (ICU)—particularly if vaccination was delayed or there was a high rate of community infection—clearly outweighed risks. (The CDC estimated an incidence of 2 cases of TTS with more than 3 million doses of Janssen vaccine administered; assuming moderate transmission kinetics, more than 3500 hospitalizations and more than 350 deaths were prevented by vaccination.36) Ultimately, after the CDC analysis was released, vaccination utilizing the Janssen product resumed; however, the CDC offered the caveat that the Janssen vaccine should be used only in specific situations36 (eg, when there has been a severe reaction to mRNA vaccine or when access to mRNA or recombinant nanoparticle vaccine is limited).
Myths surrounding vaccination
Myth #1: SARS-CoV-2 vaccines contain tissue from aborted fetuses. This myth, which emerged during development of the vaccines, is often a conflation of the use of embryonic cell lines obtained decades ago to produce vaccines (a common practice—not only for vaccines but common pharmaceuticals and foods).37 There are no fetal cells or tissue in any SARS-CoV-2 vaccines, and the vaccines have been endorsed by several faith organizations.38
Myth #2: SARS-CoV-2 vaccines can cause sterility in men and women. This myth originated from a report in early December 2020 seeking to link a similarity in a protein involved in placental–uterine binding and a portion of the receptor-binding domain antigen produced by the vaccine.39 No studies support this myth; COVID-19 vaccines are recommended in pregnancy by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine.40,41
Myth #3: mRNA SARS-CoV-2 vaccines alter a recipient’s DNA. mRNA vaccines are broken down by cellular enzymes. They cannot be integrated into the host genome.8
Continue to: Boosters and vaccine mix-and-match
Boosters and vaccine mix-and-match
As the COVID-19 pandemic persists, with new variants of concern emerging, it has also become clear that immunity wanes. In July 2021, the first report was published after a cluster of breakthrough infections occurred in a town in Massachusetts.42 There was no recommendation, at the time, for a booster; the Delta variant was the predominant circulating strain. In this outbreak, there were 469 cases, 74% of which were in people who had received 2 doses of an mRNA vaccine.42 Five patients were hospitalized; none died.42 A key takeaway from this outbreak was that vaccination prevented death, even in the face of fairly wide breakthrough infection.
Newer data show that, although vaccine effectiveness against hospitalization was greater than 90% for the first 2 months after a third dose, it waned to 78% by 4 months.43 Published data, combined with real-world experience, show that boosters provide additional reduction in the risk of death and hospitalization. This has led to a recommendation that all patients ≥ 5 years of age receive a booster.19,26,43-48 The CDC now recommends that people who are ages 12 years and older receive a bivalent booster (containing both wild-type and Omicron-variant antigens) ≥ 2 months after their most recent booster or completed series.
Future booster recommendations will consider the durability of the immune response over time (measured against the original immunizing virus) and the mutation rate of the virus.49
Given the limited supply of vaccine early in the pandemic, and the potential for future limitations, there was early interest in studying so-called mix-and-match SARS-CoV-2 vaccination—that is, receiving one product as a first series and then a different product as a booster, also known as heterologous booster vaccination. Although it is preferred that the 2 doses of the primary series be of the same vaccine product, studies that have examined this question support heterologous boosting as an acceptable approach to protective immunity50 (TABLE 251).

Vaccination in special populations
Three groups of patients have unique host characteristics that are important to consider when providing COVID-19 vaccination in your practice: pregnant patients, children, and patients in the broad category of “immunocompromised status.”
Continue to: Pregnant patients
Pregnant patients with SARS-CoV-2 infection are more likely to be hospitalized and have a higher risk of a stay in the ICU and need for mechanical ventilation. In a study of the course of illness in symptomatic pregnant patients who were hospitalized, 16.2% were admitted to an ICU and 8.5% were mechanically ventilated.52 CDC observational data have consistently supported the finding that (1) pregnant patients infected with SARS-CoV-2 are at increased risk of preterm labor and (2) their newborns are at increased risk of low birth weight and requiring admission to the neonatal ICU.53
A systematic review of 46 studies in pregnant and lactating patients showed no increased risk of adverse effects from COVID-19 vaccination.54 Furthermore, data from multiple studies demonstrate that immunoglobulin G antibodies cross the placenta to protect the infant at birth (ie, are found in umbilical cord blood and neonatal blood) and are found in breast milk. The precise kinetics and durability of these antibodies are unknown.
Pregnant patients were initially excluded from vaccine trials (although there were some patients ultimately found to be pregnant, or who became pregnant, during the trial). Careful examination of vaccine safety and efficacy data has supported the American College of Obstetricians and Gynecologists and European Board and College of Obstetrics and Gynaecology (EBCOG) recommendation that all pregnant patients be vaccinated. Furthermore, EBCOG recommends vaccination during the period of breastfeeding.55
Children. A major challenge during the pandemic has been to understand (1) the effect that infection with SARS-CoV-2 has on children and (2) the role of children in transmission of the virus. Although most children with COVID-19 have mild symptoms, a few require hospitalization and mechanical ventilation and some develop life-threatening multisystem inflammatory syndrome.56 In a large, retrospective study of more than 12,000 children with COVID-19, 5.3% required hospitalization and almost 20% of that subset were admitted to the ICU.57
Various hypotheses have been put forward to describe and explain the differences in disease expression between children and adults. These include:
- the absence of comorbidities often seen in adults
- evidence that pediatric patients might have reduced expression of ACE-2
- a more active T-cell response in infected children, due to an active thymus.56
Continue to: Although the number of children affected...
Although the number of children affected by severe SARS-CoV-2 infection is less than the number of adults, there have been important trends observed in infection and hospitalization as different variants have arisen.58 The Delta and Omicron variants have both been associated with a disturbing trend in the rate of hospitalization of pediatric patients, particularly from birth to 4 years—patients who were ineligible for vaccination at the time of the study.58 Ultimately, these data, combined with multiple studies of vaccine effectiveness in this age group, have led to an emergency use authorization for the Pfizer-BioNTech vaccination in pediatric populations and a recommendation from the American Academy of Pediatrics that all children ages 6 months and older be vaccinated.59,60
Immunocompromised patients. Patients broadly classified as immunocompromised have raised unique concerns. These patients have conditions such as malignancy, primary or secondary immunodeficiency, diabetes, and autoimmune disease; are taking certain classes of medication; or are of older age.61 Early in the pandemic, data showed that immunocompromised hosts could shed virus longer than hosts with an intact immune system—adding to their risk of transmitting SARS-CoV-2 and of viral adaptation for immune escape.62 Antibody response to vaccination was also less robust in this group.
There are limited data that demonstrate a short-lived reduction in risk of infection (in that study, Omicron was the prominent variant) with a fourth dose of an mRNA vaccine.63 Based on these data and FDA approval, the CDC recommends (1) an additional third primary dose and (2) a second booster for people who are moderately or severely immunocompromised. For those ages 50 years or older, a second booster is now required for their vaccination to be considered up to date.b
Predictions (or, why is a COVID-19 vaccine important?)
What does the future hold for our struggle with COVID-19? Perhaps we can learn lessons from the study of the 4 known seasonal coronaviruses, which cause the common cold and circulate annually.64 First, only relative immunity is produced after infection with a seasonal coronavirus.64 Studies of antibodies to seasonal coronaviruses seem to suggest that, although antibody titers remain high, correlation with decreased infection is lacking.65 Second, a dominant strain or 2 emerges each season, probably as a result of genetic variation and selective pressure for immune escape from neutralizing antibodies or cellular immunity.
The complex relationship among competing immune response duration, emergence of viral immune escape, increasing viral transmissibility, and societal viral source control (through vaccination, masking, distancing, testing, isolation, and treatment) widens the confidence bounds on our estimates of what the future holds. Late in 2020, the CDC began reporting wastewater surveillance data as a method for monitoring, and predicting changes in, community spread.66 During Spring 2022, the CDC reported an increase in detection of SARS-CoV-2 from a third of wastewater sampling sites around the United States. This observation coincided with (1) appearance of still more transmissible BA.2 and, later, BA.2.12.1 variants and (2) general relaxing of masking and social distancing guidelines, following the decline of the Omicron variant.
Continue to: At approximately that time...
At approximately that time, application to the FDA for a fourth shot (or a second booster) by Pfizer-BioNTech had been approved for adults > 50 years of age, at > 4 months after their previous vaccination.57 In view of warning signs from wastewater surveillance, priorities for vaccination should be to:
- increase uptake in the hesitant
- get boosters to the eligible
- prepare to tackle either seasonal or sporadic recurrence of COVID-19—whichever scenario the future brings.
As an example of how these priorities have been put into action, in September 2022, the FDA approved, and the CDC recommended, new bivalent boosters for everyone ≥ 12 years of age (Pfizer-BioNTech) or for all those ≥ 18 years of age (Moderna), to be administered ≥ 2 months after receipt of their most recent booster or primary series.
a www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html
b Visit www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html for more guidance on COVID-19 vaccination for immunocompromised patients.
CORRESPONDENCE
John L. Kiley, MD, 3551 Roger Brooke Drive, Fort Sam Houston, TX 78234; [email protected]
Worldwide and across many diseases, vaccines have been transformative in reducing mortality—an effect that has been sustained with vaccines that protect against COVID-19.1 Since the first cases of SARS-CoV-2 infection were reported in late 2019, the pace of scientific investigation into the virus and the disease—made possible by unprecedented funding, infrastructure, and public and private partnerships—has been explosive. The result? A vast body of clinical and laboratory evidence about the safety and effectiveness of SARS-CoV-2 vaccines, which quickly became widely available.2-4
In this article, we review the basic underlying virology of SARS-CoV-2; the biotechnological basis of vaccines against COVID-19 that are available in the United States; and recommendations on how to provide those vaccines to your patients. Additional guidance for your practice appears in a select online bibliography, “COVID-19 vaccination resources.”
SIDEBAR
COVID-19 vaccination resources
Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States
Centers for Disease Control and Prevention
www.cdc.gov/vaccines/covid-19/clinical-considerations/interimconsiderations-us.html
COVID-19 ACIP vaccine recommendations
Advisory Committee on Immunization Practices (ACIP)
www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html
MMWR COVID-19 reports
Morbidity and Mortality Weekly Report
www.cdc.gov/mmwr/Novel_Coronavirus_Reports.html
A literature hub for tracking up-to-date scientific information about the 2019 novel coronavirus
National Center for Biotechnology Information of the National Library of Medicine
www.ncbi.nlm.nih.gov/research/coronavirus
Understanding COVID-19 vaccines
National Institutes of Health COVID-19 Research
https://covid19.nih.gov/treatments-and-vaccines/covid-19-vaccines
How COVID-19 affects pregnancy
National Institutes of Health COVID-19 Research
SARS-CoV-2 virology
As the SARS-CoV-2 virus approaches the host cell, normal cell proteases on the surface membrane cause a change in the shape of the SARS-CoV-2 spike protein. That spike protein conformation change allows the virus to avoid detection by the host’s immune system because its receptor-binding site is effectively hidden until just before entry into the cell.5,6 This process is analogous to a so-called lock-and-key method of entry, in which the key (ie, spike protein conformation) is hidden by the virus until the moment it is needed, thereby minimizing exposure of viral contents to the cell. As the virus spreads through the population, it adapts to improve infectivity and transmissibility and to evade developing immunity.7
After the spike protein changes shape, it attaches to an angiotensin-converting enzyme 2 (ACE-2) receptor on the host cell, allowing the virus to enter that cell. ACE-2 receptors are located in numerous human tissues: nasopharynx, lung, gastrointestinal tract, heart, thymus, lymph nodes, bone marrow, brain, arterial and venous endothelial cells, and testes.5 The variety of tissues that contain ACE-2 receptors explains the many sites of infection and location of symptoms with which SARS-CoV-2 infection can manifest, in addition to the respiratory system.
Basic mRNA vaccine immunology
Although messenger RNA (mRNA) vaccines seem novel, they have been in development for more than 30 years.8
mRNA encodes the protein for the antigen of interest and is delivered to the host muscle tissue. There, mRNA is translated into the antigen, which stimulates an immune response. Host enzymes then rapidly degrade the mRNA in the vaccine, and it is quickly eliminated from the host.
mRNA vaccines are attractive vaccine candidates, particularly in their application to emerging infectious diseases, for several reasons:
- They are nonreplicating.
- They do not integrate into the host genome.
- They are highly effective.
- They can produce antibody and cellular immunity.
- They can be produced (and modified) quickly on a large scale without having to grow the virus in eggs.
Continue to: Vaccines against SARS-CoV-2
Vaccines against SARS-CoV-2
Two vaccines (from Pfizer-BioNTech [Comirnaty] and from Moderna [Spikevax]) are US Food and Drug Administration (FDA)–approved for COVID-19; both utilize mRNA technology. Two other vaccines, which do not use mRNA technology, have an FDA emergency use authorization (from Janssen Biotech, of Johnson & Johnson [Janssen COVID-19 Vaccine] and from Novavax [Novavax COVID-19 Vaccine, Adjuvanted]).9
Pfizer-BioNTech and Moderna vaccines. The mRNA of these vaccines encodes the entire spike protein in its pre-fusion conformation, which is the antigen that is replicated in the host, inducing an immune response.10-12 (Recall the earlier lock-and-key analogy: This conformation structure ingeniously replicates the exposed 3-dimensional key to the host’s immune system.)
The Janssen vaccine utilizes a viral vector (a nonreplicating adenovirus that functions as carrier) to deliver its message to the host for antigen production (again, the spike protein) and an immune response.
The Novavax vaccine uses a recombinant nanoparticle protein composed of the full-length spike protein.13,14 In this review, we focus on the 2 available mRNA vaccines, (1) given their FDA-authorized status and (2) because Centers for Disease Control and Prevention (CDC) recommendations indicate a preference for mRNA vaccination over viral-vectored vaccination. However, we also address key points about the Janssen (Johnson & Johnson) vaccine.
Efficacy of COVID-19 vaccines
The first study to document the safety and efficacy of a SARS-CoV-2 vaccine (the Pfizer-BioNTech vaccine) was published just 12 months after the onset of the pandemic.10 This initial trial demonstrated a 95% efficacy in preventing symptomatic, laboratory-confirmed COVID-19 at 3-month follow-up.10 Clinical trial data on the efficacy of COVID-19 vaccines have continued to be published since that first landmark trial.
Continue to: Data from trials...
Data from trials in Israel that became available early in 2021 showed that, in mRNA-vaccinated adults, mechanical ventilation rates declined strikingly, particularly in patients > 70 years of age.15,16 This finding was corroborated by data from a surveillance study of multiple US hospitals, which showed that mRNA vaccines were > 90% effective in preventing hospitalization in adults > 65 years of age.17
Data published in May 2021 showed that the Pfizer-BioNTech and Moderna vaccines were 94% effective in preventing COVID-19-related hospitalization.18 During the end of the Delta wave of the pandemic and the emergence of the Omicron variant of SARS-CoV-2, unvaccinated people were 5 times as likely to be infected as vaccinated people.19
In March 2022, data from 21 US medical centers in 18 states demonstrated that adults who had received 3 doses of the vaccine were 94% less likely to be intubated or die than those who were unvaccinated.16 A July 2022 retrospective cohort study of 231,037 subjects showed that the risk of hospitalization for acute myocardial infarction or for stroke after COVID-19 infection was reduced by more than half in fully vaccinated (ie, 2 doses of an mRNA vaccine or the viral vector [Janssen/Johnson & Johnson] vaccine) subjects, compared to unvaccinated subjects.20 The efficacy of the vaccines is summarized in TABLE 1.21-24

Even in patients who have natural infection, several studies have shown that COVID-19 vaccination after natural infection increases the level and durability of immune response to infection and reinfection and improves clinical outcomes.9,20,25,26 In summary, published literature shows that (1) mRNA vaccines are highly effective at preventing infection and (2) they augment immunity achieved by infection with circulating virus.
Breakthrough infection. COVID-19 mRNA vaccines are associated with breakthrough infection (ie, infections in fully vaccinated people), a phenomenon influenced by the predominant viral variant circulating, the level of vaccine uptake in the studied population, and the timing of vaccination.27,28 Nevertheless, vaccinated people who experience breakthrough infection are much less likely to be hospitalized and die compared to those who are unvaccinated, and vaccination with an mRNA vaccine is more effective than immunity acquired from natural infection.29
Continue to: Vaccine adverse effects
Vaccine adverse effects: Common, rare, myths
Both early mRNA vaccine trials reported common minor adverse effects after vaccination (TABLE 121-24). These included redness and soreness at the injection site, fatigue, myalgias, fever, and nausea, and tended to be more common after the second dose. These adverse effects are similar to common adverse effects seen with other vaccines. Counseling information about adverse effects can be found on the CDC website.a
Two uncommon but serious adverse effects of COVID-19 vaccination are myocarditis or pericarditis after mRNA vaccination and thrombosis with thrombocytopenia syndrome (TTS), which occurs only with the Janssen vaccine.30,31
Myocarditis and pericarditis, particularly in young males (12 to 18 years), and mostly after a second dose of vaccine, was reported in May 2021. Since then, several studies have shown that the risk of myocarditis is slightly higher in males < 40 years of age, with a predicted case rate ranging from 1 to 10 excess cases for every 1 million patients vaccinated.30,32 This risk must be balanced against the rate of myocarditis associated with SARS-CoV-2 infection.
A large study in the United States demonstrated that the risk of myocarditis for those who contract COVID-19 is 16 times higher than it is for those who are disease free.33 Observational safety data from April 2022 showed that men ages 18 to 29 years had 7 to 8 times the risk of heart complications after natural infection, compared to men of those ages who had been vaccinated.34 In this study of 40 US health care systems, the incidence of myocarditis or pericarditis in that age group ranged from 55 to 100 cases for every 100,000 people after infection and from 6 to 15 cases for every 100,000 people after a second dose of an mRNA vaccine.34
A risk–benefit analysis conducted by the Advisory Committee on Immunization Practices (ACIP) ultimately supported the conclusions that (1) the risk of myocarditis secondary to vaccination is small and (2) clear benefits of preventing infection, hospitalization, death, and continued transmission outweigh that risk.35 Study of this question, utilizing vaccine safety and reporting systems around the world, has continued.
Continue to: There is emerging evidence...
There is emerging evidence that extending the interval between the 2 doses of vaccine decreases the risk of myocarditis, particularly in male adolescents.36 That evidence ultimately led the CDC to recommend that it might be optimal that an extended interval (ie, waiting 8 weeks between the first and second dose of vaccine), in particular for males ages 12 to 39 years, could be beneficial in decreasing the risk of myocarditis.
TTS. A population risk–benefit analysis of TTS was conducted by ACIP while use of the Janssen vaccine was paused in the United States in December 2021.36 The analysis determined that, although the risk of TTS was largely in younger women (18 to 49 years; 7 cases for every 1 million vaccine doses administered), benefits of the vaccine in preventing death, hospitalization, and a stay in the intensive care unit (ICU)—particularly if vaccination was delayed or there was a high rate of community infection—clearly outweighed risks. (The CDC estimated an incidence of 2 cases of TTS with more than 3 million doses of Janssen vaccine administered; assuming moderate transmission kinetics, more than 3500 hospitalizations and more than 350 deaths were prevented by vaccination.36) Ultimately, after the CDC analysis was released, vaccination utilizing the Janssen product resumed; however, the CDC offered the caveat that the Janssen vaccine should be used only in specific situations36 (eg, when there has been a severe reaction to mRNA vaccine or when access to mRNA or recombinant nanoparticle vaccine is limited).
Myths surrounding vaccination
Myth #1: SARS-CoV-2 vaccines contain tissue from aborted fetuses. This myth, which emerged during development of the vaccines, is often a conflation of the use of embryonic cell lines obtained decades ago to produce vaccines (a common practice—not only for vaccines but common pharmaceuticals and foods).37 There are no fetal cells or tissue in any SARS-CoV-2 vaccines, and the vaccines have been endorsed by several faith organizations.38
Myth #2: SARS-CoV-2 vaccines can cause sterility in men and women. This myth originated from a report in early December 2020 seeking to link a similarity in a protein involved in placental–uterine binding and a portion of the receptor-binding domain antigen produced by the vaccine.39 No studies support this myth; COVID-19 vaccines are recommended in pregnancy by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine.40,41
Myth #3: mRNA SARS-CoV-2 vaccines alter a recipient’s DNA. mRNA vaccines are broken down by cellular enzymes. They cannot be integrated into the host genome.8
Continue to: Boosters and vaccine mix-and-match
Boosters and vaccine mix-and-match
As the COVID-19 pandemic persists, with new variants of concern emerging, it has also become clear that immunity wanes. In July 2021, the first report was published after a cluster of breakthrough infections occurred in a town in Massachusetts.42 There was no recommendation, at the time, for a booster; the Delta variant was the predominant circulating strain. In this outbreak, there were 469 cases, 74% of which were in people who had received 2 doses of an mRNA vaccine.42 Five patients were hospitalized; none died.42 A key takeaway from this outbreak was that vaccination prevented death, even in the face of fairly wide breakthrough infection.
Newer data show that, although vaccine effectiveness against hospitalization was greater than 90% for the first 2 months after a third dose, it waned to 78% by 4 months.43 Published data, combined with real-world experience, show that boosters provide additional reduction in the risk of death and hospitalization. This has led to a recommendation that all patients ≥ 5 years of age receive a booster.19,26,43-48 The CDC now recommends that people who are ages 12 years and older receive a bivalent booster (containing both wild-type and Omicron-variant antigens) ≥ 2 months after their most recent booster or completed series.
Future booster recommendations will consider the durability of the immune response over time (measured against the original immunizing virus) and the mutation rate of the virus.49
Given the limited supply of vaccine early in the pandemic, and the potential for future limitations, there was early interest in studying so-called mix-and-match SARS-CoV-2 vaccination—that is, receiving one product as a first series and then a different product as a booster, also known as heterologous booster vaccination. Although it is preferred that the 2 doses of the primary series be of the same vaccine product, studies that have examined this question support heterologous boosting as an acceptable approach to protective immunity50 (TABLE 251).

Vaccination in special populations
Three groups of patients have unique host characteristics that are important to consider when providing COVID-19 vaccination in your practice: pregnant patients, children, and patients in the broad category of “immunocompromised status.”
Continue to: Pregnant patients
Pregnant patients with SARS-CoV-2 infection are more likely to be hospitalized and have a higher risk of a stay in the ICU and need for mechanical ventilation. In a study of the course of illness in symptomatic pregnant patients who were hospitalized, 16.2% were admitted to an ICU and 8.5% were mechanically ventilated.52 CDC observational data have consistently supported the finding that (1) pregnant patients infected with SARS-CoV-2 are at increased risk of preterm labor and (2) their newborns are at increased risk of low birth weight and requiring admission to the neonatal ICU.53
A systematic review of 46 studies in pregnant and lactating patients showed no increased risk of adverse effects from COVID-19 vaccination.54 Furthermore, data from multiple studies demonstrate that immunoglobulin G antibodies cross the placenta to protect the infant at birth (ie, are found in umbilical cord blood and neonatal blood) and are found in breast milk. The precise kinetics and durability of these antibodies are unknown.
Pregnant patients were initially excluded from vaccine trials (although there were some patients ultimately found to be pregnant, or who became pregnant, during the trial). Careful examination of vaccine safety and efficacy data has supported the American College of Obstetricians and Gynecologists and European Board and College of Obstetrics and Gynaecology (EBCOG) recommendation that all pregnant patients be vaccinated. Furthermore, EBCOG recommends vaccination during the period of breastfeeding.55
Children. A major challenge during the pandemic has been to understand (1) the effect that infection with SARS-CoV-2 has on children and (2) the role of children in transmission of the virus. Although most children with COVID-19 have mild symptoms, a few require hospitalization and mechanical ventilation and some develop life-threatening multisystem inflammatory syndrome.56 In a large, retrospective study of more than 12,000 children with COVID-19, 5.3% required hospitalization and almost 20% of that subset were admitted to the ICU.57
Various hypotheses have been put forward to describe and explain the differences in disease expression between children and adults. These include:
- the absence of comorbidities often seen in adults
- evidence that pediatric patients might have reduced expression of ACE-2
- a more active T-cell response in infected children, due to an active thymus.56
Continue to: Although the number of children affected...
Although the number of children affected by severe SARS-CoV-2 infection is less than the number of adults, there have been important trends observed in infection and hospitalization as different variants have arisen.58 The Delta and Omicron variants have both been associated with a disturbing trend in the rate of hospitalization of pediatric patients, particularly from birth to 4 years—patients who were ineligible for vaccination at the time of the study.58 Ultimately, these data, combined with multiple studies of vaccine effectiveness in this age group, have led to an emergency use authorization for the Pfizer-BioNTech vaccination in pediatric populations and a recommendation from the American Academy of Pediatrics that all children ages 6 months and older be vaccinated.59,60
Immunocompromised patients. Patients broadly classified as immunocompromised have raised unique concerns. These patients have conditions such as malignancy, primary or secondary immunodeficiency, diabetes, and autoimmune disease; are taking certain classes of medication; or are of older age.61 Early in the pandemic, data showed that immunocompromised hosts could shed virus longer than hosts with an intact immune system—adding to their risk of transmitting SARS-CoV-2 and of viral adaptation for immune escape.62 Antibody response to vaccination was also less robust in this group.
There are limited data that demonstrate a short-lived reduction in risk of infection (in that study, Omicron was the prominent variant) with a fourth dose of an mRNA vaccine.63 Based on these data and FDA approval, the CDC recommends (1) an additional third primary dose and (2) a second booster for people who are moderately or severely immunocompromised. For those ages 50 years or older, a second booster is now required for their vaccination to be considered up to date.b
Predictions (or, why is a COVID-19 vaccine important?)
What does the future hold for our struggle with COVID-19? Perhaps we can learn lessons from the study of the 4 known seasonal coronaviruses, which cause the common cold and circulate annually.64 First, only relative immunity is produced after infection with a seasonal coronavirus.64 Studies of antibodies to seasonal coronaviruses seem to suggest that, although antibody titers remain high, correlation with decreased infection is lacking.65 Second, a dominant strain or 2 emerges each season, probably as a result of genetic variation and selective pressure for immune escape from neutralizing antibodies or cellular immunity.
The complex relationship among competing immune response duration, emergence of viral immune escape, increasing viral transmissibility, and societal viral source control (through vaccination, masking, distancing, testing, isolation, and treatment) widens the confidence bounds on our estimates of what the future holds. Late in 2020, the CDC began reporting wastewater surveillance data as a method for monitoring, and predicting changes in, community spread.66 During Spring 2022, the CDC reported an increase in detection of SARS-CoV-2 from a third of wastewater sampling sites around the United States. This observation coincided with (1) appearance of still more transmissible BA.2 and, later, BA.2.12.1 variants and (2) general relaxing of masking and social distancing guidelines, following the decline of the Omicron variant.
Continue to: At approximately that time...
At approximately that time, application to the FDA for a fourth shot (or a second booster) by Pfizer-BioNTech had been approved for adults > 50 years of age, at > 4 months after their previous vaccination.57 In view of warning signs from wastewater surveillance, priorities for vaccination should be to:
- increase uptake in the hesitant
- get boosters to the eligible
- prepare to tackle either seasonal or sporadic recurrence of COVID-19—whichever scenario the future brings.
As an example of how these priorities have been put into action, in September 2022, the FDA approved, and the CDC recommended, new bivalent boosters for everyone ≥ 12 years of age (Pfizer-BioNTech) or for all those ≥ 18 years of age (Moderna), to be administered ≥ 2 months after receipt of their most recent booster or primary series.
a www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html
b Visit www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html for more guidance on COVID-19 vaccination for immunocompromised patients.
CORRESPONDENCE
John L. Kiley, MD, 3551 Roger Brooke Drive, Fort Sam Houston, TX 78234; [email protected]
1. Orenstein W, Offitt P, Edwards KM, Plotkin S. Plotkin’s Vaccines. 7th ed. Elsevier; 2017:1-15.
2. Operation Warp Speed: implications for global vaccine security. Lancet Glob Health. 2021;9:e1017-e1021. doi: 10.1016/S2214-109X(21)00140-6
3. Lurie N, Saville M, Hatchett R, et al. Developing Covid-19 vaccines at pandemic speed. N Engl J Med. 2020;382:1969-1973. doi: 10.1056/NEJMp2005630
4. Slaoui M, Hepburn M. Developing safe and effective Covid vaccines—Operation Warp Speed’s strategy and approach. N Engl J Med. 2020;383:1701-1703. doi: 10.1056/NEJMp2027405
5. Hu B, Guo H, Zhou P, et al. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19:141-154. doi: 10.1038/s41579-020-00459-7
6. Hussain I, Pervaiz N, Khan A, et al. Evolutionary and structural analysis of SARS-CoV-2 specific evasion of host immunity. Genes Immun. 2020;21:409-419. doi: 10.1038/s41435-020-00120-6
7. Rando HM, Wellhausen N, Ghosh S, et al; COVID-19 Review Consortium. Identification and development of therapeutics for COVID-19. mSystems. 2021;6:e0023321. doi: 10.1128/mSystems.00233-21
8. Pardi N, Hogan MJ, Porter FW, et al. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261-279. doi: 10.1038/nrd.2017.243
9. National Center for Immunization and Respiratory Diseases. Use of COVID-19 vaccines in the United States: interim clinical considerations. Centers for Disease Control and Prevention. Updated August 22, 2022. Accessed August 27, 2022. www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html#references
10. Polack FP, Thomas SJ, Kitchin N, et al; . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603-2615. doi: 10.1056/NEJMoa2034577
11. Heinz FX, Stiasny K. Distinguishing features of current COVID-19 vaccines: knowns and unknowns of antigen presentation and modes of action. NPJ Vaccines. 2021;6:104. doi: 10.1038/s41541-021-00369-6
12. Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403-416. doi: 10.1056/NEJMoa2035389
13. Keech C, Albert G, Cho I, et al. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383:2320-2332. doi: 10.1056/NEJMoa2026920
14. Heath PT, Galiza EP, Baxter DN, et al; . Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med. 2021;385:1172-1183. doi: 10.1056/NEJMoa2107659
15. Rinott E, Youngster I, Lewis YE. Reduction in COVID-19 patients requiring mechanical ventilation following implementation of a national COVID-19 vaccination program—Israel, December 2020–February 2021. MMWR Morb Mortal Wkly Rep. 2021;70:326-328. doi: 10.15585/mmwr.mm7009e3
16. Tenforde MW, Self WH, Gaglani M, et al; IVY Network. Effectiveness of mRNA vaccination in preventing COVID-19-associated invasive mechanical ventilation and death—United States, March 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:459-465. doi: 10.15585/mmwr.mm7112e1
17. Moline HL, Whitaker M, Deng L, et al. Effectiveness of COVID-19 vaccines in preventing hospitalization among adults aged ≥ 65 years—COVID-NET, 13 States, February–April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1088-1093. doi: 10.15585/mmwr.mm7032e
18. Tenforde MW, Olson SM, Self WH, et al; ; . Effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 among hospitalized adults aged ≥ 65 years—United States, January–March 2021. MMWR Morb Mortal Wkly Rep. 2021;70:674-679. doi: 10.15585/mmwr.mm7018e1
19. Johnson AG, Amin AB, Ali AR, et al. COVID-19 incidence and death rates among unvaccinated and fully vaccinated adults with and without booster doses during periods of Delta and Omicron variant emergence—25 U.S. jurisdictions, April 4–December 25, 2021. MMWR Morb Mortal Wkly Rep. 2022;71:132-138. doi: 10.15585/mmwr.mm7104e2
20. Kim Y-E, Huh K, Park Y-J, et al. Association between vaccination and acute myocardial infarction and ischemic stroke after COVID-19 infection. JAMA. Published online July 22, 2022. doi: 10.1001/jama.2022.12992
21. Centers for Disease Control and Prevention. Pfizer-BioNTech COVID-19 vaccine reactions & adverse events. Updated June 21, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html
22. Centers for Disease Control and Prevention. The Moderna COVID-19 vaccine’s local reactions, systemic reactions, adverse events, and serious adverse events. Updated June 21, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html
23. Centers for Disease Control and Prevention. The Janssen COVID-19 vaccine’s local Reactions, Systemic reactions, adverse events, and serious adverse events. Updated August 12, 2021. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/janssen/reactogenicity.html
24. Centers for Disease Control and Prevention. Novavax COVID-19 vaccine local reactions, systemic reactions, adverse events, and serious adverse events. Updated August 31, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/novavax/reactogenicity.html
25. Greaney AJ, Loes AN, Gentles LE, et al. Antibodies elicited by mRNA-1273 vaccination bind more broadly to the receptor binding domain than do those from SARS-CoV-2 infection. Sci Transl Med. 2021;13:eabi9915. doi: 10.1126/scitranslmed.abi9915
26. Hall V, Foulkes S, Insalata F, et al. Protection against SARS-CoV-2 after Covid-19 vaccination and previous infection. N Engl J Med. 2022;386:1207-1220. doi: 10.1056/NEJMoa2118691
27. Klompas M. Understanding breakthrough infections following mRNA SARS-CoV-2 avccination. JAMA. 2021;326:2018-2020. doi: 10.1001/jama.2021.19063
28. Kustin T, Harel N, Finkel U, et al. Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals. Nat Med. 2021;27:1379-1384. doi: 10.1038/s41591-021-01413-7
29. Yu Y, Esposito D, Kang Z, et al. mRNA vaccine-induced antibodies more effective than natural immunity in neutralizing SARS-CoV-2 and its high affinity variants. Sci Rep. 2022;12:2628. doi: 10.1038/s41598-022-06629-2
30. Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:977-982. doi: 10.15585/mmwr.mm7027e2
31. MacNeil JR, Su JR, Broder KR, et al. Updated recommendations from the Advisory Committee on Immunization Practices for use of the Janssen (Johnson & Johnson) COVID-19 vaccine after reports of thrombosis with thrombocytopenia syndrome among vaccine recipients—United States, April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:651-656. doi: 10.15585/mmwr.mm7017e4
32. Patone M, Mei XW, Handunnetthi L, et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2022;28:410-422. doi: 10.1038/s41591-021-01630-0
33. Boehmer TK, Kompaniyets L, Lavery AM, et al. Association between COVID-19 and myocarditis using hospital-based administrative data—United States, March 2020–January 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1228-1232. doi: 10.15585/mmwr.mm7035e5
34. Block JP, Boehmer TK, Forrest CB, et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination—PCORnet, United States, January 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:517-523. doi: 10.15585/mmwr.mm7114e1
35. Rosemblum H. COVID-19 vaccines in adults: benefit–risk discussion. Centers for Disease Control and Prevention. July 22, 2021. Accessed September 21, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-07/05-COVID-Rosenblum-508.pdf
36. Buchan SA, Seo CY, Johnson C, et al. Epidemiology of myocarditis and pericarditis following mRNA vaccines in Ontario, Canada: by vaccine product, schedule and interval. medRxiv. 2021:12.02.21267156.
37. Wong A. The ethics of HEK 293. Natl Cathol Bioeth Q. 2006;6:473-495. doi: 10.5840/ncbq20066331
38. North Dakota Health. COVID-19 vaccines & fetal cell lines. Updated December 1, 2021. Accessed September 21, 2022. www.health.nd.gov/sites/www/files/documents/COVID%20Vaccine%20Page/COVID-19_Vaccine_Fetal_Cell_Handout.pdf
39. Abbasi J. Widespread misinformation about infertility continues to create COVID-19 vaccine hesitancy. JAMA. 2022;327:1013-1015. doi: 10.1001/jama.2022.2404
40. Halasa NB, Olson SM, Staat MA, et al; ; . Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19-associated hospitalization in infants aged < 6 months—17 States, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:264-270. doi: 10.15585/mmwr.mm7107e3
41. American College of Obstetricians and Gynecologists. ACOG and SMFM recommend COVID-19 vaccination for pregnant individuals. July 30, 2021. Accessed September 21, 2022. www.acog.org/news/news-releases/2021/07/acog-smfm-recommend-covid-19-vaccination-for-pregnant-individuals#:~:text=%E2%80%9CACOG%20is%20recommending%20vaccination%20of,complications%2C%20and%20because%20it%20isvaccines
42. Brown CM, Vostok J, Johnson H, et al. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings—Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1059-1062. doi: 10.15585/mmwr.mm7031e2
43. Ferdinands JM, Rao S, Dixon BE, et al. Waning 2-dose and 3-dose effectiveness of mRNA against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance—VISION Network, 10 states, August 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:255-263. doi: 10.15585/mmwr.mm7107e2
44. Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Effect of mRNA vaccine boosters against SARS-CoV-2 Omicron infection in Qatar. N Engl J Med. 2022;386:1804-1816. doi: 10.1056/NEJMoa2200797
45. Arbel R, Hammerman A, Sergienko R, et al. BNT162b2 vaccine booster and mortality due to Covid-19. N Engl J Med. 2021;385:2413-2420. doi: 10.1056/NEJMoa2115624
46. Bar-On YM, Goldberg Y, Mandel M, et al. Protection against Covid-19 by BNT162b2 booster across age groups. N Engl J Med. 2021;385:2421-2430. doi: 10.1056/NEJMoa2115926
47. Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385:1393-1400. doi: 10.1056/NEJMoa2114255
48. Mbaeyi S, Oliver SE, Collins JP, et al. The Advisory Committee on Immunization Practices’ interim recommendations for additional primary and booster doses of COVID-19 vaccines—United States, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1545-1552. doi: 10.15585/mmwr.mm7044e2
49. Chen X, Chen Z, Azman AS, et al. Neutralizing antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants induced by natural infection or vaccination: a systematic review and pooled analysis. Clin Infect Dis. 2022;74:734-742. doi: 10.1093/cid/ciab646
50. Atmar RL, Lyke KE, Deming ME, et al; . Homologous and heterologous Covid-19 booster vaccinations. N Engl J Med. 2022;386:1046-1057. doi: 10.1056/NEJMoa2116414
51. Centers for Disease Control and Prevention. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. Updated September 2, 2022. Accessed September 21, 2022. www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html
52. Ackerman CM, Nguyen JL, Ambati S, et al. Clinical and pregnancy outcomes of coronavirus disease 2019 among hospitalized pregnant women in the United States. Open Forum Infect Dis. 2022;9:ofab429. doi: 10.1093/ofid/ofab429
53. Osterman MJK, Valenzuela CP, Martin JA. Maternal and infant characteristics among women with confirmed or presumed cases of coronavirus disease (COVID-19) during pregnancy. National Center for Health Statistics. National Vital Statistics System. Updated August 11, 2022. Accessed September 21, 2022. www.cdc.gov/nchs/covid19/technical-linkage.htm
54. De Rose DU, Salvatori G, Dotta A, et al. SARS-CoV-2 vaccines during pregnancy and breastfeeding: a systematic review of maternal and neonatal outcomes. Viruses. 2022;14:539. doi: 10.3390/v14030539
55. Martins I, Louwen F, Ayres-de-Campos D, et al. EBCOG position statement on COVID-19 vaccination for pregnant and breastfeeding women. Eur J Obstet Gynecol Reprod Biol. 2021;262:256-258. doi: 10.1016/j.ejogrb.2021.05.021
56. Chou J, Thomas PG, Randolph AG. Immunology of SARS-CoV-2 infection in children. Nat Immunol. 2022;23:177-185. doi: 10.1038/s41590-021-01123-9
57. Parcha V, Booker KS, Kalra R, et al. A retrospective cohort study of 12,306 pediatric COVID-19 patients in the United States. Sci Rep. 2021;11:10231. doi: 10.1038/s41598-021-89553-1
58. Marks KJ, Whitaker M, Anglin O, et al; . Hospitalizations of children and adolescents with laboratory-confirmed COVID-19—COVID-NET, 14 states, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:271-278. doi: 10.15585/mmwr.mm7107e4
59. Price AM, Olson SM, Newhams MM, et al; . BNT162b2 protection against the Omicron variant in children and adolescents. N Engl J Med. 2022;386:1899-1909. doi: 10.1056/NEJMoa2202826
60. Maldonado YA, O’Leary ST, Banerjee R, et al; Committee on Infectious Diseases, American Academy of Pediatrics. COVID-19 vaccines in children and adolescents. Pediatrics. 2021;148:e2021052336. doi: 10.1542/peds.2021-052336
61. Lontok K. How effective are COVID-19 vaccines in immunocompromised people? American Society for Microbiology. August 12, 2021. Accessed September 21, 2022. https://asm.org/Articles/2021/August/How-Effective-Are-COVID-19-Vaccines-in-Immunocompr
62. Meiring S, Tempia S, Bhiman JN, et al; . Prolonged shedding of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) at high viral loads among hospitalized immunocompromised persons living with human immunodeficiency virus, South Africa. Clin Infect Dis. 2022;75:e144-e156. doi: 10.1093/cid/ciac077
63. Bar-On YM, Goldberg Y, Mandel M, et al. Protection by 4th dose of BNT162b2 against Omicron in Israel. medRxiv. 2022: 02.01.22270232. doi: 10.1101/2022.02.01.22270232
64. Monto AS, DeJonge PM, Callear AP, et al. Coronavirus occurrence and transmission over 8 years in the HIVE cohort of households in Michigan. J Infect Dis. 2020;222:9-16. doi: 10.1093/infdis/jiaa161
65. Petrie JG, Bazzi LA, McDermott AB, et al. Coronavirus occurrence in the Household Influenza Vaccine Evaluation (HIVE) cohort of Michigan households: reinfection frequency and serologic responses to seasonal and severe acute respiratory syndrome coronaviruses. J Infect Dis. 2021;224:49-59. doi: 10.1093/infdis/jiab161
66. Kirby AE, Walters MS, Jennings WC, et al. Using wastewater surveillance data to support the COVID-19 response—United States, 2020–2021. MMWR Morb Mortal Wkly Rep. 2021;70:1242-1244. doi: 10.15585/mmwr.mm7036a2
1. Orenstein W, Offitt P, Edwards KM, Plotkin S. Plotkin’s Vaccines. 7th ed. Elsevier; 2017:1-15.
2. Operation Warp Speed: implications for global vaccine security. Lancet Glob Health. 2021;9:e1017-e1021. doi: 10.1016/S2214-109X(21)00140-6
3. Lurie N, Saville M, Hatchett R, et al. Developing Covid-19 vaccines at pandemic speed. N Engl J Med. 2020;382:1969-1973. doi: 10.1056/NEJMp2005630
4. Slaoui M, Hepburn M. Developing safe and effective Covid vaccines—Operation Warp Speed’s strategy and approach. N Engl J Med. 2020;383:1701-1703. doi: 10.1056/NEJMp2027405
5. Hu B, Guo H, Zhou P, et al. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19:141-154. doi: 10.1038/s41579-020-00459-7
6. Hussain I, Pervaiz N, Khan A, et al. Evolutionary and structural analysis of SARS-CoV-2 specific evasion of host immunity. Genes Immun. 2020;21:409-419. doi: 10.1038/s41435-020-00120-6
7. Rando HM, Wellhausen N, Ghosh S, et al; COVID-19 Review Consortium. Identification and development of therapeutics for COVID-19. mSystems. 2021;6:e0023321. doi: 10.1128/mSystems.00233-21
8. Pardi N, Hogan MJ, Porter FW, et al. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261-279. doi: 10.1038/nrd.2017.243
9. National Center for Immunization and Respiratory Diseases. Use of COVID-19 vaccines in the United States: interim clinical considerations. Centers for Disease Control and Prevention. Updated August 22, 2022. Accessed August 27, 2022. www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html#references
10. Polack FP, Thomas SJ, Kitchin N, et al; . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603-2615. doi: 10.1056/NEJMoa2034577
11. Heinz FX, Stiasny K. Distinguishing features of current COVID-19 vaccines: knowns and unknowns of antigen presentation and modes of action. NPJ Vaccines. 2021;6:104. doi: 10.1038/s41541-021-00369-6
12. Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403-416. doi: 10.1056/NEJMoa2035389
13. Keech C, Albert G, Cho I, et al. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383:2320-2332. doi: 10.1056/NEJMoa2026920
14. Heath PT, Galiza EP, Baxter DN, et al; . Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med. 2021;385:1172-1183. doi: 10.1056/NEJMoa2107659
15. Rinott E, Youngster I, Lewis YE. Reduction in COVID-19 patients requiring mechanical ventilation following implementation of a national COVID-19 vaccination program—Israel, December 2020–February 2021. MMWR Morb Mortal Wkly Rep. 2021;70:326-328. doi: 10.15585/mmwr.mm7009e3
16. Tenforde MW, Self WH, Gaglani M, et al; IVY Network. Effectiveness of mRNA vaccination in preventing COVID-19-associated invasive mechanical ventilation and death—United States, March 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:459-465. doi: 10.15585/mmwr.mm7112e1
17. Moline HL, Whitaker M, Deng L, et al. Effectiveness of COVID-19 vaccines in preventing hospitalization among adults aged ≥ 65 years—COVID-NET, 13 States, February–April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1088-1093. doi: 10.15585/mmwr.mm7032e
18. Tenforde MW, Olson SM, Self WH, et al; ; . Effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 among hospitalized adults aged ≥ 65 years—United States, January–March 2021. MMWR Morb Mortal Wkly Rep. 2021;70:674-679. doi: 10.15585/mmwr.mm7018e1
19. Johnson AG, Amin AB, Ali AR, et al. COVID-19 incidence and death rates among unvaccinated and fully vaccinated adults with and without booster doses during periods of Delta and Omicron variant emergence—25 U.S. jurisdictions, April 4–December 25, 2021. MMWR Morb Mortal Wkly Rep. 2022;71:132-138. doi: 10.15585/mmwr.mm7104e2
20. Kim Y-E, Huh K, Park Y-J, et al. Association between vaccination and acute myocardial infarction and ischemic stroke after COVID-19 infection. JAMA. Published online July 22, 2022. doi: 10.1001/jama.2022.12992
21. Centers for Disease Control and Prevention. Pfizer-BioNTech COVID-19 vaccine reactions & adverse events. Updated June 21, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html
22. Centers for Disease Control and Prevention. The Moderna COVID-19 vaccine’s local reactions, systemic reactions, adverse events, and serious adverse events. Updated June 21, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html
23. Centers for Disease Control and Prevention. The Janssen COVID-19 vaccine’s local Reactions, Systemic reactions, adverse events, and serious adverse events. Updated August 12, 2021. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/janssen/reactogenicity.html
24. Centers for Disease Control and Prevention. Novavax COVID-19 vaccine local reactions, systemic reactions, adverse events, and serious adverse events. Updated August 31, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/novavax/reactogenicity.html
25. Greaney AJ, Loes AN, Gentles LE, et al. Antibodies elicited by mRNA-1273 vaccination bind more broadly to the receptor binding domain than do those from SARS-CoV-2 infection. Sci Transl Med. 2021;13:eabi9915. doi: 10.1126/scitranslmed.abi9915
26. Hall V, Foulkes S, Insalata F, et al. Protection against SARS-CoV-2 after Covid-19 vaccination and previous infection. N Engl J Med. 2022;386:1207-1220. doi: 10.1056/NEJMoa2118691
27. Klompas M. Understanding breakthrough infections following mRNA SARS-CoV-2 avccination. JAMA. 2021;326:2018-2020. doi: 10.1001/jama.2021.19063
28. Kustin T, Harel N, Finkel U, et al. Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals. Nat Med. 2021;27:1379-1384. doi: 10.1038/s41591-021-01413-7
29. Yu Y, Esposito D, Kang Z, et al. mRNA vaccine-induced antibodies more effective than natural immunity in neutralizing SARS-CoV-2 and its high affinity variants. Sci Rep. 2022;12:2628. doi: 10.1038/s41598-022-06629-2
30. Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:977-982. doi: 10.15585/mmwr.mm7027e2
31. MacNeil JR, Su JR, Broder KR, et al. Updated recommendations from the Advisory Committee on Immunization Practices for use of the Janssen (Johnson & Johnson) COVID-19 vaccine after reports of thrombosis with thrombocytopenia syndrome among vaccine recipients—United States, April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:651-656. doi: 10.15585/mmwr.mm7017e4
32. Patone M, Mei XW, Handunnetthi L, et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2022;28:410-422. doi: 10.1038/s41591-021-01630-0
33. Boehmer TK, Kompaniyets L, Lavery AM, et al. Association between COVID-19 and myocarditis using hospital-based administrative data—United States, March 2020–January 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1228-1232. doi: 10.15585/mmwr.mm7035e5
34. Block JP, Boehmer TK, Forrest CB, et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination—PCORnet, United States, January 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:517-523. doi: 10.15585/mmwr.mm7114e1
35. Rosemblum H. COVID-19 vaccines in adults: benefit–risk discussion. Centers for Disease Control and Prevention. July 22, 2021. Accessed September 21, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-07/05-COVID-Rosenblum-508.pdf
36. Buchan SA, Seo CY, Johnson C, et al. Epidemiology of myocarditis and pericarditis following mRNA vaccines in Ontario, Canada: by vaccine product, schedule and interval. medRxiv. 2021:12.02.21267156.
37. Wong A. The ethics of HEK 293. Natl Cathol Bioeth Q. 2006;6:473-495. doi: 10.5840/ncbq20066331
38. North Dakota Health. COVID-19 vaccines & fetal cell lines. Updated December 1, 2021. Accessed September 21, 2022. www.health.nd.gov/sites/www/files/documents/COVID%20Vaccine%20Page/COVID-19_Vaccine_Fetal_Cell_Handout.pdf
39. Abbasi J. Widespread misinformation about infertility continues to create COVID-19 vaccine hesitancy. JAMA. 2022;327:1013-1015. doi: 10.1001/jama.2022.2404
40. Halasa NB, Olson SM, Staat MA, et al; ; . Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19-associated hospitalization in infants aged < 6 months—17 States, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:264-270. doi: 10.15585/mmwr.mm7107e3
41. American College of Obstetricians and Gynecologists. ACOG and SMFM recommend COVID-19 vaccination for pregnant individuals. July 30, 2021. Accessed September 21, 2022. www.acog.org/news/news-releases/2021/07/acog-smfm-recommend-covid-19-vaccination-for-pregnant-individuals#:~:text=%E2%80%9CACOG%20is%20recommending%20vaccination%20of,complications%2C%20and%20because%20it%20isvaccines
42. Brown CM, Vostok J, Johnson H, et al. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings—Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1059-1062. doi: 10.15585/mmwr.mm7031e2
43. Ferdinands JM, Rao S, Dixon BE, et al. Waning 2-dose and 3-dose effectiveness of mRNA against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance—VISION Network, 10 states, August 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:255-263. doi: 10.15585/mmwr.mm7107e2
44. Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Effect of mRNA vaccine boosters against SARS-CoV-2 Omicron infection in Qatar. N Engl J Med. 2022;386:1804-1816. doi: 10.1056/NEJMoa2200797
45. Arbel R, Hammerman A, Sergienko R, et al. BNT162b2 vaccine booster and mortality due to Covid-19. N Engl J Med. 2021;385:2413-2420. doi: 10.1056/NEJMoa2115624
46. Bar-On YM, Goldberg Y, Mandel M, et al. Protection against Covid-19 by BNT162b2 booster across age groups. N Engl J Med. 2021;385:2421-2430. doi: 10.1056/NEJMoa2115926
47. Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385:1393-1400. doi: 10.1056/NEJMoa2114255
48. Mbaeyi S, Oliver SE, Collins JP, et al. The Advisory Committee on Immunization Practices’ interim recommendations for additional primary and booster doses of COVID-19 vaccines—United States, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1545-1552. doi: 10.15585/mmwr.mm7044e2
49. Chen X, Chen Z, Azman AS, et al. Neutralizing antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants induced by natural infection or vaccination: a systematic review and pooled analysis. Clin Infect Dis. 2022;74:734-742. doi: 10.1093/cid/ciab646
50. Atmar RL, Lyke KE, Deming ME, et al; . Homologous and heterologous Covid-19 booster vaccinations. N Engl J Med. 2022;386:1046-1057. doi: 10.1056/NEJMoa2116414
51. Centers for Disease Control and Prevention. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. Updated September 2, 2022. Accessed September 21, 2022. www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html
52. Ackerman CM, Nguyen JL, Ambati S, et al. Clinical and pregnancy outcomes of coronavirus disease 2019 among hospitalized pregnant women in the United States. Open Forum Infect Dis. 2022;9:ofab429. doi: 10.1093/ofid/ofab429
53. Osterman MJK, Valenzuela CP, Martin JA. Maternal and infant characteristics among women with confirmed or presumed cases of coronavirus disease (COVID-19) during pregnancy. National Center for Health Statistics. National Vital Statistics System. Updated August 11, 2022. Accessed September 21, 2022. www.cdc.gov/nchs/covid19/technical-linkage.htm
54. De Rose DU, Salvatori G, Dotta A, et al. SARS-CoV-2 vaccines during pregnancy and breastfeeding: a systematic review of maternal and neonatal outcomes. Viruses. 2022;14:539. doi: 10.3390/v14030539
55. Martins I, Louwen F, Ayres-de-Campos D, et al. EBCOG position statement on COVID-19 vaccination for pregnant and breastfeeding women. Eur J Obstet Gynecol Reprod Biol. 2021;262:256-258. doi: 10.1016/j.ejogrb.2021.05.021
56. Chou J, Thomas PG, Randolph AG. Immunology of SARS-CoV-2 infection in children. Nat Immunol. 2022;23:177-185. doi: 10.1038/s41590-021-01123-9
57. Parcha V, Booker KS, Kalra R, et al. A retrospective cohort study of 12,306 pediatric COVID-19 patients in the United States. Sci Rep. 2021;11:10231. doi: 10.1038/s41598-021-89553-1
58. Marks KJ, Whitaker M, Anglin O, et al; . Hospitalizations of children and adolescents with laboratory-confirmed COVID-19—COVID-NET, 14 states, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:271-278. doi: 10.15585/mmwr.mm7107e4
59. Price AM, Olson SM, Newhams MM, et al; . BNT162b2 protection against the Omicron variant in children and adolescents. N Engl J Med. 2022;386:1899-1909. doi: 10.1056/NEJMoa2202826
60. Maldonado YA, O’Leary ST, Banerjee R, et al; Committee on Infectious Diseases, American Academy of Pediatrics. COVID-19 vaccines in children and adolescents. Pediatrics. 2021;148:e2021052336. doi: 10.1542/peds.2021-052336
61. Lontok K. How effective are COVID-19 vaccines in immunocompromised people? American Society for Microbiology. August 12, 2021. Accessed September 21, 2022. https://asm.org/Articles/2021/August/How-Effective-Are-COVID-19-Vaccines-in-Immunocompr
62. Meiring S, Tempia S, Bhiman JN, et al; . Prolonged shedding of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) at high viral loads among hospitalized immunocompromised persons living with human immunodeficiency virus, South Africa. Clin Infect Dis. 2022;75:e144-e156. doi: 10.1093/cid/ciac077
63. Bar-On YM, Goldberg Y, Mandel M, et al. Protection by 4th dose of BNT162b2 against Omicron in Israel. medRxiv. 2022: 02.01.22270232. doi: 10.1101/2022.02.01.22270232
64. Monto AS, DeJonge PM, Callear AP, et al. Coronavirus occurrence and transmission over 8 years in the HIVE cohort of households in Michigan. J Infect Dis. 2020;222:9-16. doi: 10.1093/infdis/jiaa161
65. Petrie JG, Bazzi LA, McDermott AB, et al. Coronavirus occurrence in the Household Influenza Vaccine Evaluation (HIVE) cohort of Michigan households: reinfection frequency and serologic responses to seasonal and severe acute respiratory syndrome coronaviruses. J Infect Dis. 2021;224:49-59. doi: 10.1093/infdis/jiab161
66. Kirby AE, Walters MS, Jennings WC, et al. Using wastewater surveillance data to support the COVID-19 response—United States, 2020–2021. MMWR Morb Mortal Wkly Rep. 2021;70:1242-1244. doi: 10.15585/mmwr.mm7036a2
PRACTICE RECOMMENDATIONS
› Vaccinate all adults (≥ 18 years) against COVID-19, based on recommendations for the initial series and boosters. A
› Vaccinate patients against COVID-19 with evidence-based assurance that doing so reduces disease-related risk of hospitalization, myocardial infarction, stroke, need for mechanical ventilation, and death. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Vaccine update for the 2022-23 influenza season
In the 2020-2021 influenza season, there was practically no influenza circulating in the United States. This decline from seasonal expectations, described in a previous Practice Alert, was probably due to the interventions aimed at limiting the spread of COVID-19: masking, social distancing, working from home, and cancellation of large, crowded events.1 In 2021-2022 influenza returned, but only in moderation.
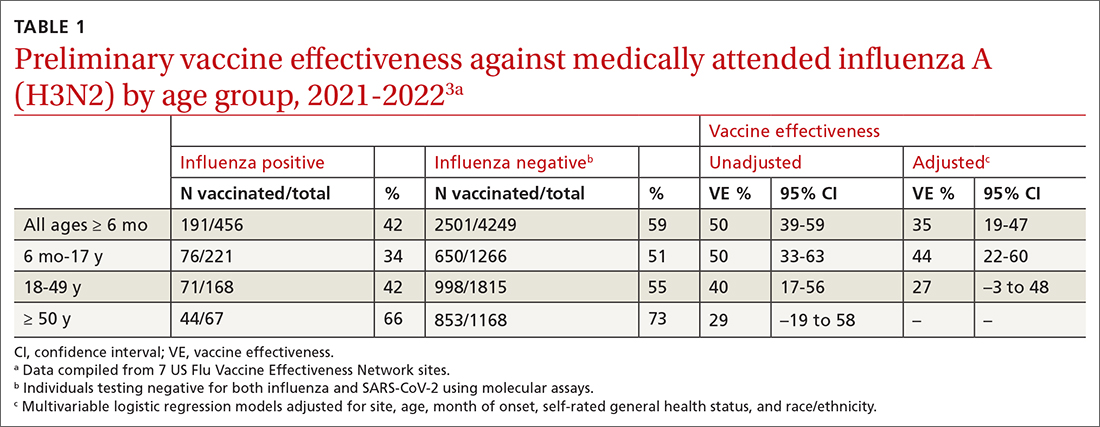
The Centers for Disease Control and Prevention (CDC) estimates there were between 82,000 to 170,000 hospitalizations and 5000 to 14,000 deaths attributed to influenza.2 In addition, US virologic surveillance indicates that 98.6% of specimens tested positive for influenza A.2 While the vaccine’s effectiveness in 2021-2022 was far below what was desired, it still prevented a great deal of flu morbidity and mortality and reduced acute respiratory illness due to influenza A(H3N2) virus by 35% (TABLE 1).3 All vaccines for the upcoming flu season are quadrivalent, containing 2 influenza A antigens and 2 influenza B antigens (TABLES 24 and 35).
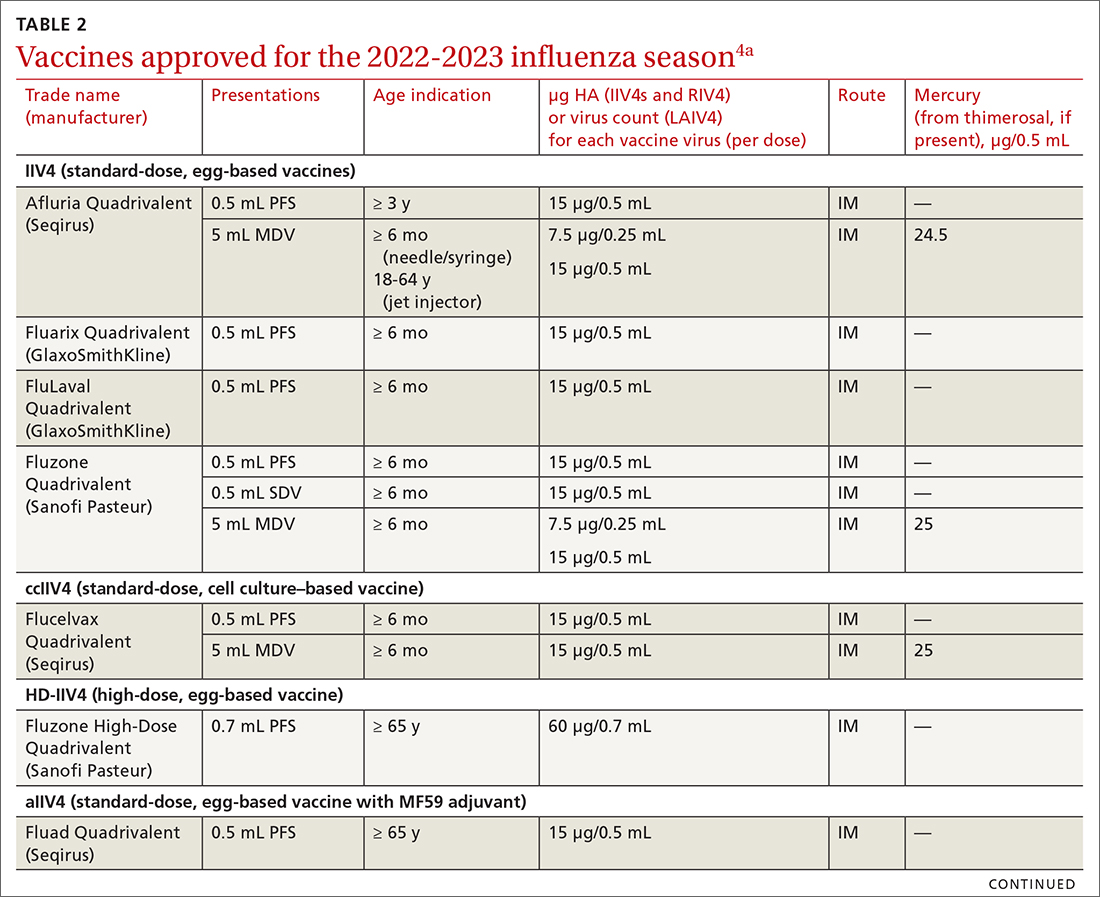
Vaccine effectiveness in older adults (≥ 65 years) has been very low. TABLE 46 shows vaccine effectiveness in the elderly for 10 influenza seasons between 2011 and 2020.6 In nearly half of those seasons, the estimated vaccine effectiveness was possibly nil. All influenza vaccines licensed for use in the United States are approved for use in those ≥ 65 years of age, except live attenuated influenza vaccine (LAIV).
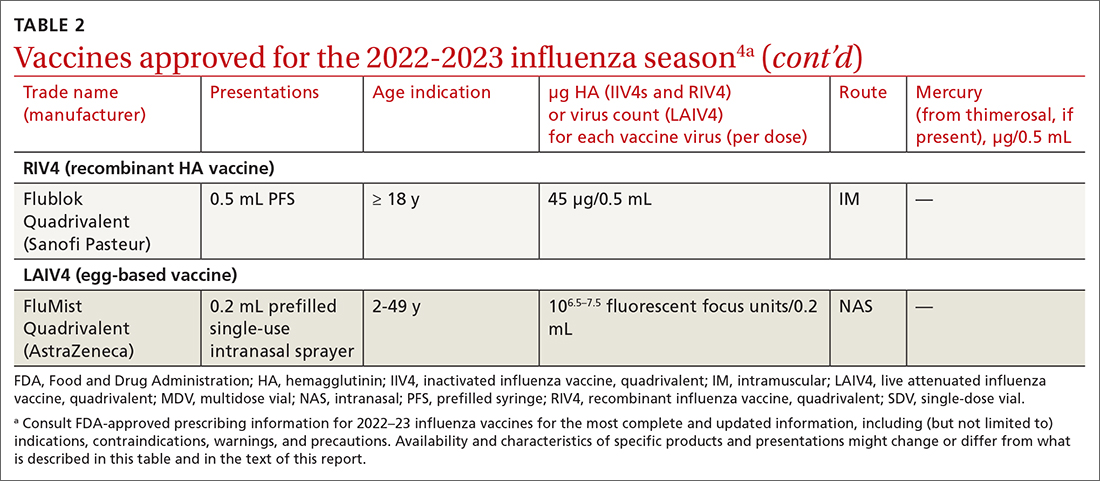
Three products were developed to address the issue of low vaccine effectiveness in the elderly. The Advisory Committee on Immunization Practices (ACIP) has not expressed a preference for any specific vaccine for this age group. The high-dose qudrivalent vaccine (HD-IIV4), Fluzone, contains 4 times the antigen level of the standard-dose vaccines (SD-IIV4)—60 μg vs 15 μg. Fluzone was initially approved in 2014 as a trivalent vaccine and was approved as a quadrivalent vaccine in 2019. The adjuvanted quadrivalent influenza vaccine (aIIV4), Fluad, was also inititally approved as a trivalent vaccine in 2015 and as quadrivalent in 2021. Both HD-IIV4 and aIIV4 are approved only for those ≥ 65 years of age. Recombinant quadrivalent influenza vaccine (RIV4), Flublok, is approved for ages ≥ 18 years and is produced by a process that does not involve eggs. It contains 3 times the antigen level as SD-IIV4 vaccines.
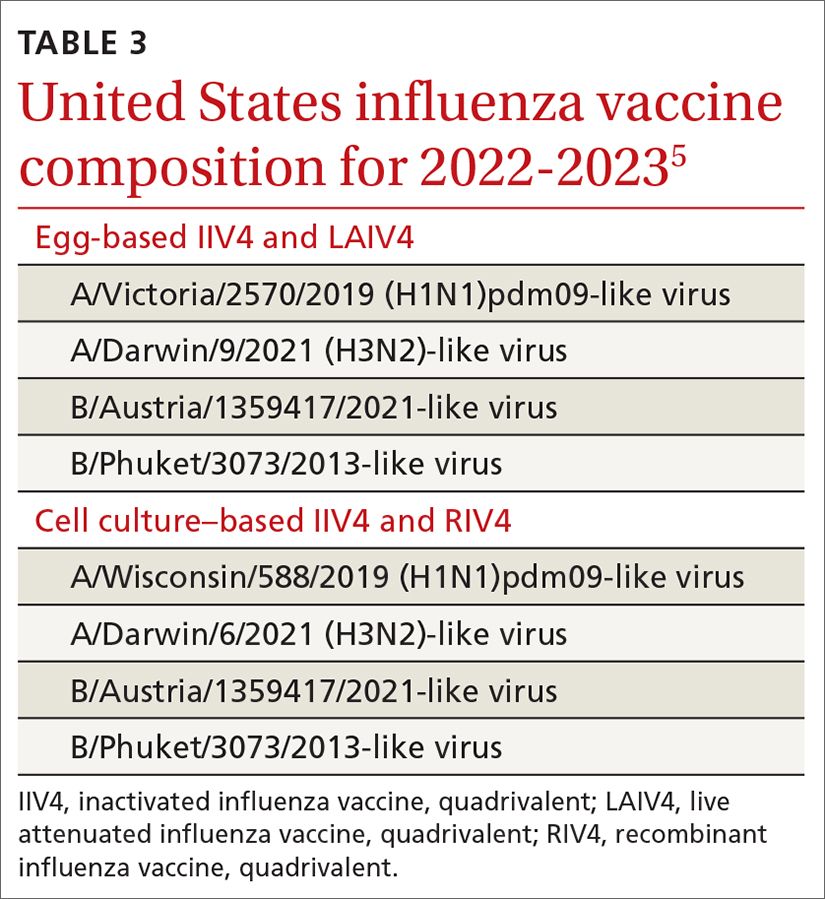
All 3 of these vaccines (HD-IIV4, aIIV4, and RIV4) have been compared with SD-IIV4 for effectiveness in the elderly and have yielded better outcomes. However, direct comparisons among the 3 vaccines have not shown robust evidence of superiority, and ACIP is unwilling to preferentially recommend one of them at this time. At its June 2022 meeting, ACIP voted to recommend any of these 3 options over the SD-IIV 4 options for those ≥ 65 years of age, with the caveat that if only an SD-IIV4 option is available it should be administered in preference to delaying vaccination.
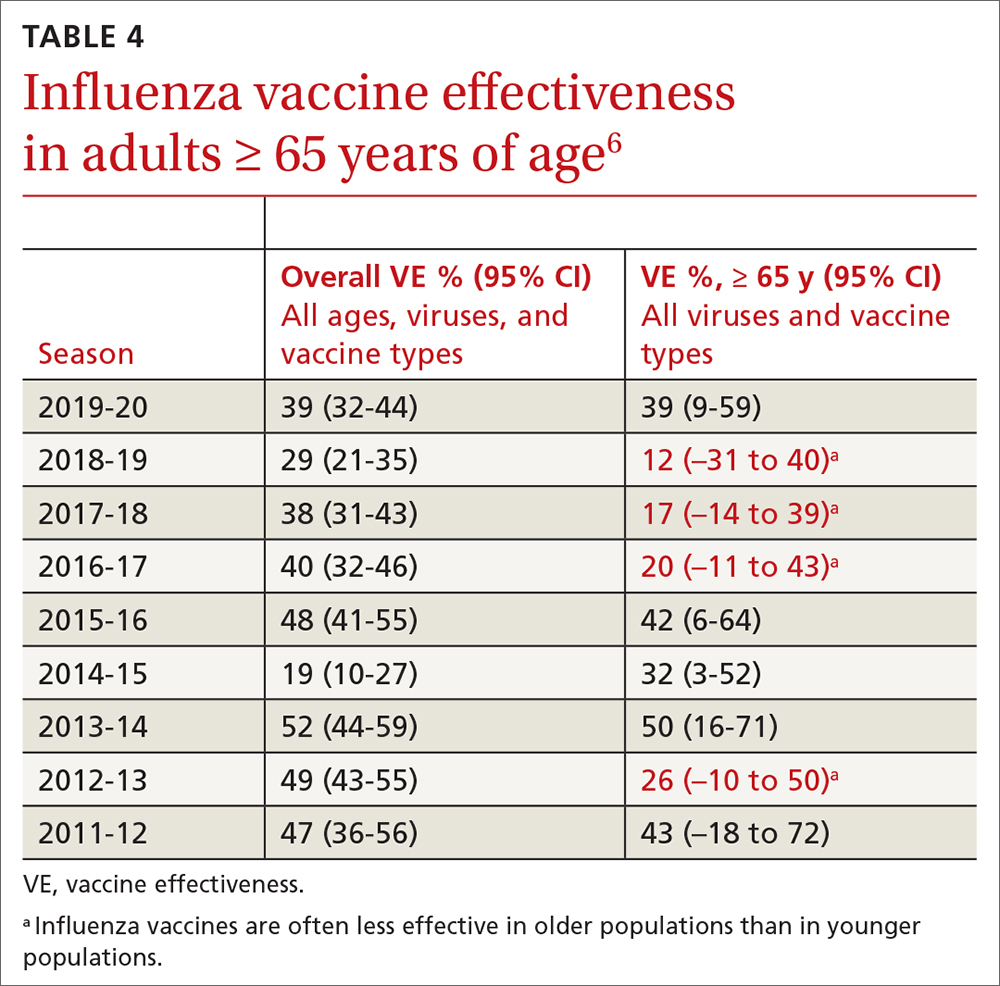
One other vaccine change for the upcoming season involves the cell culture–based quadrivalent inactivated influenza vaccine (ccIIV4), Flucelvax, which is now approved for those ages ≥ 6 months. It previously was approved only for ages ≥ 2 years. All unadjuvanted SD-IIV4 vaccines as well as ccIIV4 are now approved for everyone ≥ 6 months of age. LAIV continues to be approved for ages 2 through 49 years. The only influenza vaccine products that contain thimerosal are those in multidose vials (TABLE 24).
Promote vaccination and infection-control practices. ACIP continues to recommend influenza vaccine for all those ages ≥ 6 months, with 2 doses for those < 9 years old not previously vaccinated with an influenza vaccine. In addition to encouraging and offering influenza vaccine to patients and staff, we can minimize the spread of influenza in the community by robust infection-control practices in the clinical setting: masking and isolation of patients with respiratory symptoms, encouraging those with symptoms to stay at home and mask when around family members, advising frequent hand washing and respiratory hygiene, and using pre- and post-exposure chemoprophylaxis as appropriate. All recommendations regarding influenza for 2022-2023 can be found on the CDC website.4
1. Campos-Outcalt D. Influenza vaccine update, 2021-2022. J Fam Pract. 2021;70:399-402. doi: 10.12788/jfp.0277
2. Merced-Morales A, Daly P, Abd Elal AI, et al. Influenza activity and composition of the 2022-23 influenza vaccine—United States, 2021-22 season. MMWR Morb Mortal Wkly Rep. 2022;71;913-919. doi: 10.15585/mmwr.mm7129a1
3. CDC. National Center for Immunization and Respiratory Diseases. Preliminary Estimates of 2021–22 Seasonal Influenza Vaccine Effectiveness against Medically Attended Influenza. Accessed September 22, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-06-22-23/02-influenza-chung-508.pdf
4. Grohskopf LA, Blanton LH, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices – United States, 2022-23 influenza season. MMWR Recomm Rep. 2022;71:1-28. doi: http://dx.doi.org/10.15585/mmwr.rr7101a1
5. FDA. Influenza vaccine for the 2022-2023 season. Accessed September 22, 2022. www.fda.gov/vaccines-blood-biologics/lot-release/influenza-vaccine-2022-2023-season
6. Grohskopf L. Influenza vaccines for persons aged ≥ 65 years: evidence to recommendation (EtR) framework. Presented to the ACIP June 22, 2022. Accessed September 22, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-06-22-23/03-influenza-grohskopf-508.pdf
In the 2020-2021 influenza season, there was practically no influenza circulating in the United States. This decline from seasonal expectations, described in a previous Practice Alert, was probably due to the interventions aimed at limiting the spread of COVID-19: masking, social distancing, working from home, and cancellation of large, crowded events.1 In 2021-2022 influenza returned, but only in moderation.

The Centers for Disease Control and Prevention (CDC) estimates there were between 82,000 to 170,000 hospitalizations and 5000 to 14,000 deaths attributed to influenza.2 In addition, US virologic surveillance indicates that 98.6% of specimens tested positive for influenza A.2 While the vaccine’s effectiveness in 2021-2022 was far below what was desired, it still prevented a great deal of flu morbidity and mortality and reduced acute respiratory illness due to influenza A(H3N2) virus by 35% (TABLE 1).3 All vaccines for the upcoming flu season are quadrivalent, containing 2 influenza A antigens and 2 influenza B antigens (TABLES 24 and 35).

Vaccine effectiveness in older adults (≥ 65 years) has been very low. TABLE 46 shows vaccine effectiveness in the elderly for 10 influenza seasons between 2011 and 2020.6 In nearly half of those seasons, the estimated vaccine effectiveness was possibly nil. All influenza vaccines licensed for use in the United States are approved for use in those ≥ 65 years of age, except live attenuated influenza vaccine (LAIV).

Three products were developed to address the issue of low vaccine effectiveness in the elderly. The Advisory Committee on Immunization Practices (ACIP) has not expressed a preference for any specific vaccine for this age group. The high-dose qudrivalent vaccine (HD-IIV4), Fluzone, contains 4 times the antigen level of the standard-dose vaccines (SD-IIV4)—60 μg vs 15 μg. Fluzone was initially approved in 2014 as a trivalent vaccine and was approved as a quadrivalent vaccine in 2019. The adjuvanted quadrivalent influenza vaccine (aIIV4), Fluad, was also inititally approved as a trivalent vaccine in 2015 and as quadrivalent in 2021. Both HD-IIV4 and aIIV4 are approved only for those ≥ 65 years of age. Recombinant quadrivalent influenza vaccine (RIV4), Flublok, is approved for ages ≥ 18 years and is produced by a process that does not involve eggs. It contains 3 times the antigen level as SD-IIV4 vaccines.

All 3 of these vaccines (HD-IIV4, aIIV4, and RIV4) have been compared with SD-IIV4 for effectiveness in the elderly and have yielded better outcomes. However, direct comparisons among the 3 vaccines have not shown robust evidence of superiority, and ACIP is unwilling to preferentially recommend one of them at this time. At its June 2022 meeting, ACIP voted to recommend any of these 3 options over the SD-IIV 4 options for those ≥ 65 years of age, with the caveat that if only an SD-IIV4 option is available it should be administered in preference to delaying vaccination.

One other vaccine change for the upcoming season involves the cell culture–based quadrivalent inactivated influenza vaccine (ccIIV4), Flucelvax, which is now approved for those ages ≥ 6 months. It previously was approved only for ages ≥ 2 years. All unadjuvanted SD-IIV4 vaccines as well as ccIIV4 are now approved for everyone ≥ 6 months of age. LAIV continues to be approved for ages 2 through 49 years. The only influenza vaccine products that contain thimerosal are those in multidose vials (TABLE 24).
Promote vaccination and infection-control practices. ACIP continues to recommend influenza vaccine for all those ages ≥ 6 months, with 2 doses for those < 9 years old not previously vaccinated with an influenza vaccine. In addition to encouraging and offering influenza vaccine to patients and staff, we can minimize the spread of influenza in the community by robust infection-control practices in the clinical setting: masking and isolation of patients with respiratory symptoms, encouraging those with symptoms to stay at home and mask when around family members, advising frequent hand washing and respiratory hygiene, and using pre- and post-exposure chemoprophylaxis as appropriate. All recommendations regarding influenza for 2022-2023 can be found on the CDC website.4
In the 2020-2021 influenza season, there was practically no influenza circulating in the United States. This decline from seasonal expectations, described in a previous Practice Alert, was probably due to the interventions aimed at limiting the spread of COVID-19: masking, social distancing, working from home, and cancellation of large, crowded events.1 In 2021-2022 influenza returned, but only in moderation.

The Centers for Disease Control and Prevention (CDC) estimates there were between 82,000 to 170,000 hospitalizations and 5000 to 14,000 deaths attributed to influenza.2 In addition, US virologic surveillance indicates that 98.6% of specimens tested positive for influenza A.2 While the vaccine’s effectiveness in 2021-2022 was far below what was desired, it still prevented a great deal of flu morbidity and mortality and reduced acute respiratory illness due to influenza A(H3N2) virus by 35% (TABLE 1).3 All vaccines for the upcoming flu season are quadrivalent, containing 2 influenza A antigens and 2 influenza B antigens (TABLES 24 and 35).

Vaccine effectiveness in older adults (≥ 65 years) has been very low. TABLE 46 shows vaccine effectiveness in the elderly for 10 influenza seasons between 2011 and 2020.6 In nearly half of those seasons, the estimated vaccine effectiveness was possibly nil. All influenza vaccines licensed for use in the United States are approved for use in those ≥ 65 years of age, except live attenuated influenza vaccine (LAIV).

Three products were developed to address the issue of low vaccine effectiveness in the elderly. The Advisory Committee on Immunization Practices (ACIP) has not expressed a preference for any specific vaccine for this age group. The high-dose qudrivalent vaccine (HD-IIV4), Fluzone, contains 4 times the antigen level of the standard-dose vaccines (SD-IIV4)—60 μg vs 15 μg. Fluzone was initially approved in 2014 as a trivalent vaccine and was approved as a quadrivalent vaccine in 2019. The adjuvanted quadrivalent influenza vaccine (aIIV4), Fluad, was also inititally approved as a trivalent vaccine in 2015 and as quadrivalent in 2021. Both HD-IIV4 and aIIV4 are approved only for those ≥ 65 years of age. Recombinant quadrivalent influenza vaccine (RIV4), Flublok, is approved for ages ≥ 18 years and is produced by a process that does not involve eggs. It contains 3 times the antigen level as SD-IIV4 vaccines.

All 3 of these vaccines (HD-IIV4, aIIV4, and RIV4) have been compared with SD-IIV4 for effectiveness in the elderly and have yielded better outcomes. However, direct comparisons among the 3 vaccines have not shown robust evidence of superiority, and ACIP is unwilling to preferentially recommend one of them at this time. At its June 2022 meeting, ACIP voted to recommend any of these 3 options over the SD-IIV 4 options for those ≥ 65 years of age, with the caveat that if only an SD-IIV4 option is available it should be administered in preference to delaying vaccination.

One other vaccine change for the upcoming season involves the cell culture–based quadrivalent inactivated influenza vaccine (ccIIV4), Flucelvax, which is now approved for those ages ≥ 6 months. It previously was approved only for ages ≥ 2 years. All unadjuvanted SD-IIV4 vaccines as well as ccIIV4 are now approved for everyone ≥ 6 months of age. LAIV continues to be approved for ages 2 through 49 years. The only influenza vaccine products that contain thimerosal are those in multidose vials (TABLE 24).
Promote vaccination and infection-control practices. ACIP continues to recommend influenza vaccine for all those ages ≥ 6 months, with 2 doses for those < 9 years old not previously vaccinated with an influenza vaccine. In addition to encouraging and offering influenza vaccine to patients and staff, we can minimize the spread of influenza in the community by robust infection-control practices in the clinical setting: masking and isolation of patients with respiratory symptoms, encouraging those with symptoms to stay at home and mask when around family members, advising frequent hand washing and respiratory hygiene, and using pre- and post-exposure chemoprophylaxis as appropriate. All recommendations regarding influenza for 2022-2023 can be found on the CDC website.4
1. Campos-Outcalt D. Influenza vaccine update, 2021-2022. J Fam Pract. 2021;70:399-402. doi: 10.12788/jfp.0277
2. Merced-Morales A, Daly P, Abd Elal AI, et al. Influenza activity and composition of the 2022-23 influenza vaccine—United States, 2021-22 season. MMWR Morb Mortal Wkly Rep. 2022;71;913-919. doi: 10.15585/mmwr.mm7129a1
3. CDC. National Center for Immunization and Respiratory Diseases. Preliminary Estimates of 2021–22 Seasonal Influenza Vaccine Effectiveness against Medically Attended Influenza. Accessed September 22, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-06-22-23/02-influenza-chung-508.pdf
4. Grohskopf LA, Blanton LH, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices – United States, 2022-23 influenza season. MMWR Recomm Rep. 2022;71:1-28. doi: http://dx.doi.org/10.15585/mmwr.rr7101a1
5. FDA. Influenza vaccine for the 2022-2023 season. Accessed September 22, 2022. www.fda.gov/vaccines-blood-biologics/lot-release/influenza-vaccine-2022-2023-season
6. Grohskopf L. Influenza vaccines for persons aged ≥ 65 years: evidence to recommendation (EtR) framework. Presented to the ACIP June 22, 2022. Accessed September 22, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-06-22-23/03-influenza-grohskopf-508.pdf
1. Campos-Outcalt D. Influenza vaccine update, 2021-2022. J Fam Pract. 2021;70:399-402. doi: 10.12788/jfp.0277
2. Merced-Morales A, Daly P, Abd Elal AI, et al. Influenza activity and composition of the 2022-23 influenza vaccine—United States, 2021-22 season. MMWR Morb Mortal Wkly Rep. 2022;71;913-919. doi: 10.15585/mmwr.mm7129a1
3. CDC. National Center for Immunization and Respiratory Diseases. Preliminary Estimates of 2021–22 Seasonal Influenza Vaccine Effectiveness against Medically Attended Influenza. Accessed September 22, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-06-22-23/02-influenza-chung-508.pdf
4. Grohskopf LA, Blanton LH, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices – United States, 2022-23 influenza season. MMWR Recomm Rep. 2022;71:1-28. doi: http://dx.doi.org/10.15585/mmwr.rr7101a1
5. FDA. Influenza vaccine for the 2022-2023 season. Accessed September 22, 2022. www.fda.gov/vaccines-blood-biologics/lot-release/influenza-vaccine-2022-2023-season
6. Grohskopf L. Influenza vaccines for persons aged ≥ 65 years: evidence to recommendation (EtR) framework. Presented to the ACIP June 22, 2022. Accessed September 22, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-06-22-23/03-influenza-grohskopf-508.pdf
Evusheld PrEP may protect immunocompromised patients from severe COVID-19
Tixagevimab copackaged with cilgavimab (Evusheld) is a safe and effective preexposure prophylaxis (PrEP) in patients undergoing B-cell-depleting therapies who have poor immune response to COVID-19 vaccination and are at high risk for serious COVID-19 illness, a small, single-site study suggests.
Evusheld, the only COVID-19 PrEP option available, has Emergency Use Authorization (EUA) from the Food and Drug Administration for treatment of immunocompromised patients who may not respond sufficiently to COVID-19 vaccination and patients who’ve had a severe adverse reaction to COVID-19 vaccination.
“We report the largest real-world experience of Evusheld in this population, and our findings are encouraging,” lead study author Cassandra Calabrese, DO, rheumatologist and infectious disease specialist at Cleveland Clinic, said in an interview.
“Of 412 patients who received Evusheld, 12 [2.9%] developed breakthrough COVID-19, with 11 having mild courses and 1 who required hospitalization but recovered,” she added.
“Our data suggest that Evusheld PrEP, in combination with aggressive outpatient treatment of COVID-19, is likely effective in lowering risk of severe COVID in this vulnerable group.
“Practitioners who care for patients with immune-mediated inflammatory diseases should triage high-risk patients for Evusheld as well as rapid diagnosis and aggressive outpatient therapy if infected,” Dr. Calabrese advised.
For the study, Dr. Calabrese and colleagues at Cleveland Clinic searched the health care system pharmacy records for patients with immune‐mediated inflammatory diseases (IMIDs) or inborn errors of humoral immunity (IEI) who met the criteria to receive Evusheld. The researchers included patients on B-cell-depleting therapies or with humoral IEI who had received at least one dose of Evusheld and were later diagnosed with COVID-19, and they excluded those treated with B-cell-depleting therapies for cancer.
EVUSHELD was well tolerated
After extracting data on COVID-19 infection, vaccination status, and outcomes, they found that, between Jan. 18 and May 28, 2022, 412 patients with IMIDs or humoral IEI received Evusheld. No deaths occurred among these patients and, overall, they tolerated the medication well.
All 12 patients who experienced breakthrough COVID-19 infection were treated with B-cell-depleting therapies. Among the 12 patients:
- Six patients developed infection 13-84 (median 19) days after receiving 150 mg/150 mg tixagevimab/cilgavimab.
- Six patients developed infection 19-72 (median of 38.5) days after either a single dose of 300 mg/300 mg or a second dose of 150 mg/150 mg.
- Eleven patients had mild illness and recovered at home; one patient was hospitalized and treated with high-flow oxygen. All cases had been vaccinated against COVID-19 (five received two vaccinations, six received three, and one received four).
- One possible serious adverse event involved a patient with COVID-19 and immune-mediated thrombocytopenia (ITP) who was hospitalized soon after receiving Evusheld with ITP flare that resolved with intravenous immunoglobulin.
Dr. Calabrese acknowledged limitations to the study, including few patients, lack of a comparator group, and the study period falling during the Omicron wave.
“Also, nine of the breakthrough cases received additional COVID-19 therapy (oral antiviral or monoclonal antibody), which falls within standard of care for this high-risk group but prevents ascribing effectiveness to individual components of the regimen,” she added.
“Evusheld is authorized for PrEP against COVID-19 in patients at high risk for severe COVID due to suboptimal vaccine responses. This includes patients receiving B-cell-depleting drugs like rituximab, and patients with inborn errors of humoral immunity,” Dr. Calabrese explained.
“It is well known that this group of patients is at very high risk for severe COVID and death, even when fully vaccinated, and it has become clear that more strategies are needed to protect this vulnerable group, including use of Evusheld as well as aggressive treatment if infected,” she added.
Evusheld not always easy to obtain
Although the medication has been available in the United States since January 2022, Dr. Calabrese said, patients may not receive it because of barriers including lack of both awareness and access.
Davey Smith, MD, professor of medicine and head of infectious diseases and global public health at the University of California San Diego, in La Jolla, said in an interview that he was not surprised by the results, but added that the study was conducted in too few patients to draw any strong conclusions or affect patient care.
“This small study that showed that breakthrough infections occurred but were generally mild, provides a small glimpse of real-world use of tixagevimab/cilgavimab as PrEP for immunocompromised persons,” said Dr. Smith, who was not involved in the study.
“In the setting of Omicron and vaccination, I would expect the same outcomes reported even without the treatment,” he added.
Dr. Smith recommends larger related randomized, controlled trials to provide clinicians with sufficient data to guide them in their patient care.
Graham Snyder, MD, associate professor in the division of infectious diseases at the University of Pittsburgh and medical director of infection prevention and hospital epidemiology at the University of Pittsburgh Medical Center, noted that the study “adds to a quickly growing literature on the real-world benefits of tixagevimab/cilgavimab to protect vulnerable individuals with weakened immune systems from the complications of COVID-19.
“This study provides a modest addition to our understanding of the role and benefit of Evusheld,” Dr. Snyder said in an interview. “By characterizing only patients who have received Evusheld without an untreated comparison group, we can’t draw any inference about the extent of benefit the agent provided to these patients.
“Substantial data already show that this agent is effective in preventing complications of COVID-19 infection in immunocompromised individuals,” added Dr. Snyder, who was not involved in the study.
“ ‘Immunocompromised’ represents a very diverse set of clinical conditions,” he said. “The research agenda should therefore focus on a more refined description of the effect in specific populations and a continued understanding of the effect of Evusheld in the context of updated vaccination strategies and changing virus ecology.”
Dr. Calabrese and her colleagues wrote that larger, controlled trials are underway.
FDA: Evusheld may not neutralize certain SARS-CoV-2 variants
“The biggest unanswered question is how Evusheld will hold up against new variants,” Dr. Calabrese said.
In an Oct. 3, 2022, update, the Food and Drug Administration released a statement about the risk of developing COVID-19 from SARS-CoV-2 variants that are not neutralized by Evusheld. The statement mentions an updated fact sheet that describes reduced protection from Evusheld against the Omicron subvariant BA.4.6, which accounted for nearly 13% of all new COVID-19 cases in the United States in the week ending Oct. 1.
There was no outside funding for the study. Dr. Smith reported no relevant financial conflicts of interest. Dr. Snyder said he is an unpaid adviser to an AstraZeneca observational study that’s assessing the real-world effectiveness of Evusheld.
Tixagevimab copackaged with cilgavimab (Evusheld) is a safe and effective preexposure prophylaxis (PrEP) in patients undergoing B-cell-depleting therapies who have poor immune response to COVID-19 vaccination and are at high risk for serious COVID-19 illness, a small, single-site study suggests.
Evusheld, the only COVID-19 PrEP option available, has Emergency Use Authorization (EUA) from the Food and Drug Administration for treatment of immunocompromised patients who may not respond sufficiently to COVID-19 vaccination and patients who’ve had a severe adverse reaction to COVID-19 vaccination.
“We report the largest real-world experience of Evusheld in this population, and our findings are encouraging,” lead study author Cassandra Calabrese, DO, rheumatologist and infectious disease specialist at Cleveland Clinic, said in an interview.
“Of 412 patients who received Evusheld, 12 [2.9%] developed breakthrough COVID-19, with 11 having mild courses and 1 who required hospitalization but recovered,” she added.
“Our data suggest that Evusheld PrEP, in combination with aggressive outpatient treatment of COVID-19, is likely effective in lowering risk of severe COVID in this vulnerable group.
“Practitioners who care for patients with immune-mediated inflammatory diseases should triage high-risk patients for Evusheld as well as rapid diagnosis and aggressive outpatient therapy if infected,” Dr. Calabrese advised.
For the study, Dr. Calabrese and colleagues at Cleveland Clinic searched the health care system pharmacy records for patients with immune‐mediated inflammatory diseases (IMIDs) or inborn errors of humoral immunity (IEI) who met the criteria to receive Evusheld. The researchers included patients on B-cell-depleting therapies or with humoral IEI who had received at least one dose of Evusheld and were later diagnosed with COVID-19, and they excluded those treated with B-cell-depleting therapies for cancer.
EVUSHELD was well tolerated
After extracting data on COVID-19 infection, vaccination status, and outcomes, they found that, between Jan. 18 and May 28, 2022, 412 patients with IMIDs or humoral IEI received Evusheld. No deaths occurred among these patients and, overall, they tolerated the medication well.
All 12 patients who experienced breakthrough COVID-19 infection were treated with B-cell-depleting therapies. Among the 12 patients:
- Six patients developed infection 13-84 (median 19) days after receiving 150 mg/150 mg tixagevimab/cilgavimab.
- Six patients developed infection 19-72 (median of 38.5) days after either a single dose of 300 mg/300 mg or a second dose of 150 mg/150 mg.
- Eleven patients had mild illness and recovered at home; one patient was hospitalized and treated with high-flow oxygen. All cases had been vaccinated against COVID-19 (five received two vaccinations, six received three, and one received four).
- One possible serious adverse event involved a patient with COVID-19 and immune-mediated thrombocytopenia (ITP) who was hospitalized soon after receiving Evusheld with ITP flare that resolved with intravenous immunoglobulin.
Dr. Calabrese acknowledged limitations to the study, including few patients, lack of a comparator group, and the study period falling during the Omicron wave.
“Also, nine of the breakthrough cases received additional COVID-19 therapy (oral antiviral or monoclonal antibody), which falls within standard of care for this high-risk group but prevents ascribing effectiveness to individual components of the regimen,” she added.
“Evusheld is authorized for PrEP against COVID-19 in patients at high risk for severe COVID due to suboptimal vaccine responses. This includes patients receiving B-cell-depleting drugs like rituximab, and patients with inborn errors of humoral immunity,” Dr. Calabrese explained.
“It is well known that this group of patients is at very high risk for severe COVID and death, even when fully vaccinated, and it has become clear that more strategies are needed to protect this vulnerable group, including use of Evusheld as well as aggressive treatment if infected,” she added.
Evusheld not always easy to obtain
Although the medication has been available in the United States since January 2022, Dr. Calabrese said, patients may not receive it because of barriers including lack of both awareness and access.
Davey Smith, MD, professor of medicine and head of infectious diseases and global public health at the University of California San Diego, in La Jolla, said in an interview that he was not surprised by the results, but added that the study was conducted in too few patients to draw any strong conclusions or affect patient care.
“This small study that showed that breakthrough infections occurred but were generally mild, provides a small glimpse of real-world use of tixagevimab/cilgavimab as PrEP for immunocompromised persons,” said Dr. Smith, who was not involved in the study.
“In the setting of Omicron and vaccination, I would expect the same outcomes reported even without the treatment,” he added.
Dr. Smith recommends larger related randomized, controlled trials to provide clinicians with sufficient data to guide them in their patient care.
Graham Snyder, MD, associate professor in the division of infectious diseases at the University of Pittsburgh and medical director of infection prevention and hospital epidemiology at the University of Pittsburgh Medical Center, noted that the study “adds to a quickly growing literature on the real-world benefits of tixagevimab/cilgavimab to protect vulnerable individuals with weakened immune systems from the complications of COVID-19.
“This study provides a modest addition to our understanding of the role and benefit of Evusheld,” Dr. Snyder said in an interview. “By characterizing only patients who have received Evusheld without an untreated comparison group, we can’t draw any inference about the extent of benefit the agent provided to these patients.
“Substantial data already show that this agent is effective in preventing complications of COVID-19 infection in immunocompromised individuals,” added Dr. Snyder, who was not involved in the study.
“ ‘Immunocompromised’ represents a very diverse set of clinical conditions,” he said. “The research agenda should therefore focus on a more refined description of the effect in specific populations and a continued understanding of the effect of Evusheld in the context of updated vaccination strategies and changing virus ecology.”
Dr. Calabrese and her colleagues wrote that larger, controlled trials are underway.
FDA: Evusheld may not neutralize certain SARS-CoV-2 variants
“The biggest unanswered question is how Evusheld will hold up against new variants,” Dr. Calabrese said.
In an Oct. 3, 2022, update, the Food and Drug Administration released a statement about the risk of developing COVID-19 from SARS-CoV-2 variants that are not neutralized by Evusheld. The statement mentions an updated fact sheet that describes reduced protection from Evusheld against the Omicron subvariant BA.4.6, which accounted for nearly 13% of all new COVID-19 cases in the United States in the week ending Oct. 1.
There was no outside funding for the study. Dr. Smith reported no relevant financial conflicts of interest. Dr. Snyder said he is an unpaid adviser to an AstraZeneca observational study that’s assessing the real-world effectiveness of Evusheld.
Tixagevimab copackaged with cilgavimab (Evusheld) is a safe and effective preexposure prophylaxis (PrEP) in patients undergoing B-cell-depleting therapies who have poor immune response to COVID-19 vaccination and are at high risk for serious COVID-19 illness, a small, single-site study suggests.
Evusheld, the only COVID-19 PrEP option available, has Emergency Use Authorization (EUA) from the Food and Drug Administration for treatment of immunocompromised patients who may not respond sufficiently to COVID-19 vaccination and patients who’ve had a severe adverse reaction to COVID-19 vaccination.
“We report the largest real-world experience of Evusheld in this population, and our findings are encouraging,” lead study author Cassandra Calabrese, DO, rheumatologist and infectious disease specialist at Cleveland Clinic, said in an interview.
“Of 412 patients who received Evusheld, 12 [2.9%] developed breakthrough COVID-19, with 11 having mild courses and 1 who required hospitalization but recovered,” she added.
“Our data suggest that Evusheld PrEP, in combination with aggressive outpatient treatment of COVID-19, is likely effective in lowering risk of severe COVID in this vulnerable group.
“Practitioners who care for patients with immune-mediated inflammatory diseases should triage high-risk patients for Evusheld as well as rapid diagnosis and aggressive outpatient therapy if infected,” Dr. Calabrese advised.
For the study, Dr. Calabrese and colleagues at Cleveland Clinic searched the health care system pharmacy records for patients with immune‐mediated inflammatory diseases (IMIDs) or inborn errors of humoral immunity (IEI) who met the criteria to receive Evusheld. The researchers included patients on B-cell-depleting therapies or with humoral IEI who had received at least one dose of Evusheld and were later diagnosed with COVID-19, and they excluded those treated with B-cell-depleting therapies for cancer.
EVUSHELD was well tolerated
After extracting data on COVID-19 infection, vaccination status, and outcomes, they found that, between Jan. 18 and May 28, 2022, 412 patients with IMIDs or humoral IEI received Evusheld. No deaths occurred among these patients and, overall, they tolerated the medication well.
All 12 patients who experienced breakthrough COVID-19 infection were treated with B-cell-depleting therapies. Among the 12 patients:
- Six patients developed infection 13-84 (median 19) days after receiving 150 mg/150 mg tixagevimab/cilgavimab.
- Six patients developed infection 19-72 (median of 38.5) days after either a single dose of 300 mg/300 mg or a second dose of 150 mg/150 mg.
- Eleven patients had mild illness and recovered at home; one patient was hospitalized and treated with high-flow oxygen. All cases had been vaccinated against COVID-19 (five received two vaccinations, six received three, and one received four).
- One possible serious adverse event involved a patient with COVID-19 and immune-mediated thrombocytopenia (ITP) who was hospitalized soon after receiving Evusheld with ITP flare that resolved with intravenous immunoglobulin.
Dr. Calabrese acknowledged limitations to the study, including few patients, lack of a comparator group, and the study period falling during the Omicron wave.
“Also, nine of the breakthrough cases received additional COVID-19 therapy (oral antiviral or monoclonal antibody), which falls within standard of care for this high-risk group but prevents ascribing effectiveness to individual components of the regimen,” she added.
“Evusheld is authorized for PrEP against COVID-19 in patients at high risk for severe COVID due to suboptimal vaccine responses. This includes patients receiving B-cell-depleting drugs like rituximab, and patients with inborn errors of humoral immunity,” Dr. Calabrese explained.
“It is well known that this group of patients is at very high risk for severe COVID and death, even when fully vaccinated, and it has become clear that more strategies are needed to protect this vulnerable group, including use of Evusheld as well as aggressive treatment if infected,” she added.
Evusheld not always easy to obtain
Although the medication has been available in the United States since January 2022, Dr. Calabrese said, patients may not receive it because of barriers including lack of both awareness and access.
Davey Smith, MD, professor of medicine and head of infectious diseases and global public health at the University of California San Diego, in La Jolla, said in an interview that he was not surprised by the results, but added that the study was conducted in too few patients to draw any strong conclusions or affect patient care.
“This small study that showed that breakthrough infections occurred but were generally mild, provides a small glimpse of real-world use of tixagevimab/cilgavimab as PrEP for immunocompromised persons,” said Dr. Smith, who was not involved in the study.
“In the setting of Omicron and vaccination, I would expect the same outcomes reported even without the treatment,” he added.
Dr. Smith recommends larger related randomized, controlled trials to provide clinicians with sufficient data to guide them in their patient care.
Graham Snyder, MD, associate professor in the division of infectious diseases at the University of Pittsburgh and medical director of infection prevention and hospital epidemiology at the University of Pittsburgh Medical Center, noted that the study “adds to a quickly growing literature on the real-world benefits of tixagevimab/cilgavimab to protect vulnerable individuals with weakened immune systems from the complications of COVID-19.
“This study provides a modest addition to our understanding of the role and benefit of Evusheld,” Dr. Snyder said in an interview. “By characterizing only patients who have received Evusheld without an untreated comparison group, we can’t draw any inference about the extent of benefit the agent provided to these patients.
“Substantial data already show that this agent is effective in preventing complications of COVID-19 infection in immunocompromised individuals,” added Dr. Snyder, who was not involved in the study.
“ ‘Immunocompromised’ represents a very diverse set of clinical conditions,” he said. “The research agenda should therefore focus on a more refined description of the effect in specific populations and a continued understanding of the effect of Evusheld in the context of updated vaccination strategies and changing virus ecology.”
Dr. Calabrese and her colleagues wrote that larger, controlled trials are underway.
FDA: Evusheld may not neutralize certain SARS-CoV-2 variants
“The biggest unanswered question is how Evusheld will hold up against new variants,” Dr. Calabrese said.
In an Oct. 3, 2022, update, the Food and Drug Administration released a statement about the risk of developing COVID-19 from SARS-CoV-2 variants that are not neutralized by Evusheld. The statement mentions an updated fact sheet that describes reduced protection from Evusheld against the Omicron subvariant BA.4.6, which accounted for nearly 13% of all new COVID-19 cases in the United States in the week ending Oct. 1.
There was no outside funding for the study. Dr. Smith reported no relevant financial conflicts of interest. Dr. Snyder said he is an unpaid adviser to an AstraZeneca observational study that’s assessing the real-world effectiveness of Evusheld.
FROM RMD OPEN
Is another COVID-19 booster really needed?
Many countries around the globe are starting to roll out another booster of the COVID-19 vaccine but, with public interest waning and a sense of normalcy firmly installed in our minds, this may prove an ill-fated effort, unless authorities can provide a coherent answer to the question “Is another jab really needed?” (The short answer is a firm “yes,” of course.)
In what we could call the “chronic” phase of the pandemic, most countries have now settled for a certain number of daily cases and a (relatively low) number of complications and deaths. It’s the vaccines that have afforded us this peace of mind, lest we forget. But they are different to other vaccines that we are more familiar with, such as the MMR that we get as kids and then forget about for the rest of our lives. As good as the different COVID-19 vaccines are, they never came with the promise of generating lifelong antibodies. We knew early on that the immunity they provide slowly wanes with time. That doesn’t mean that those who have their vaccination records up to date (which included a booster probably earlier in 2022) are suddenly exposed. Data suggest that although people several months past their last booster would now be more prone to getting reinfected, the protection against severe disease still hangs around 85%. In other words, their chances of ending up in the hospital are low.
Why worry, then, about further boosting the immune system? The same studies show that an additional jab would increase this percentage up to 99%. Is this roughly 10% improvement really worth another worldwide vaccination campaign? Well, this is a numbers game, after all. The current form of the virus is extremely infectious, and the Northern Hemisphere is heading toward the cold months of the year, which we have seen in past years increases COVID-19 contagions, as you would expect from any airborne virus. Thus, it’s easy to expect a new peak in the number of cases, especially considering that we are not going to apply any of the usual restrictions to prevent this. In these conditions, extending the safety net to a further 10% of the population would substantially reduce the total number of victims. It seems like a good investment of resources.
We can be more surgical about it and direct this new vaccination campaign to the population most likely to end up in the hospital. People with concomitant pathologies are at the top of the list, but it’s also an age issue. On the basis of different studies of the most common ages of admission, the cutoff point for the booster varies from country to country, with the lowest being 50 and in other cases hovering around 65 years of age. Given the safety of these vaccines, if we can afford it, the wider we cast the net, the better, but at least we should make every effort to fully vaccinate the higher age brackets.
The final question is which vaccine to give. There are confounding studies about the importance of switching to Omicron-specific jabs, which are finally available. Although this seems like a good idea, since Omicron infections elicit a more effective range of antibodies and new variants seem to better escape our defenses, recent studies suggest that there actually may not be so much difference with the old formula.
The conclusion? This regimen of yearly boosters for some may be the scenario for the upcoming years, similar to what we already do for the flu, so we should get used to it.
Dr. Macip is associate professor, department of molecular and cellular biology, University of Leicester (England). He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Many countries around the globe are starting to roll out another booster of the COVID-19 vaccine but, with public interest waning and a sense of normalcy firmly installed in our minds, this may prove an ill-fated effort, unless authorities can provide a coherent answer to the question “Is another jab really needed?” (The short answer is a firm “yes,” of course.)
In what we could call the “chronic” phase of the pandemic, most countries have now settled for a certain number of daily cases and a (relatively low) number of complications and deaths. It’s the vaccines that have afforded us this peace of mind, lest we forget. But they are different to other vaccines that we are more familiar with, such as the MMR that we get as kids and then forget about for the rest of our lives. As good as the different COVID-19 vaccines are, they never came with the promise of generating lifelong antibodies. We knew early on that the immunity they provide slowly wanes with time. That doesn’t mean that those who have their vaccination records up to date (which included a booster probably earlier in 2022) are suddenly exposed. Data suggest that although people several months past their last booster would now be more prone to getting reinfected, the protection against severe disease still hangs around 85%. In other words, their chances of ending up in the hospital are low.
Why worry, then, about further boosting the immune system? The same studies show that an additional jab would increase this percentage up to 99%. Is this roughly 10% improvement really worth another worldwide vaccination campaign? Well, this is a numbers game, after all. The current form of the virus is extremely infectious, and the Northern Hemisphere is heading toward the cold months of the year, which we have seen in past years increases COVID-19 contagions, as you would expect from any airborne virus. Thus, it’s easy to expect a new peak in the number of cases, especially considering that we are not going to apply any of the usual restrictions to prevent this. In these conditions, extending the safety net to a further 10% of the population would substantially reduce the total number of victims. It seems like a good investment of resources.
We can be more surgical about it and direct this new vaccination campaign to the population most likely to end up in the hospital. People with concomitant pathologies are at the top of the list, but it’s also an age issue. On the basis of different studies of the most common ages of admission, the cutoff point for the booster varies from country to country, with the lowest being 50 and in other cases hovering around 65 years of age. Given the safety of these vaccines, if we can afford it, the wider we cast the net, the better, but at least we should make every effort to fully vaccinate the higher age brackets.
The final question is which vaccine to give. There are confounding studies about the importance of switching to Omicron-specific jabs, which are finally available. Although this seems like a good idea, since Omicron infections elicit a more effective range of antibodies and new variants seem to better escape our defenses, recent studies suggest that there actually may not be so much difference with the old formula.
The conclusion? This regimen of yearly boosters for some may be the scenario for the upcoming years, similar to what we already do for the flu, so we should get used to it.
Dr. Macip is associate professor, department of molecular and cellular biology, University of Leicester (England). He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Many countries around the globe are starting to roll out another booster of the COVID-19 vaccine but, with public interest waning and a sense of normalcy firmly installed in our minds, this may prove an ill-fated effort, unless authorities can provide a coherent answer to the question “Is another jab really needed?” (The short answer is a firm “yes,” of course.)
In what we could call the “chronic” phase of the pandemic, most countries have now settled for a certain number of daily cases and a (relatively low) number of complications and deaths. It’s the vaccines that have afforded us this peace of mind, lest we forget. But they are different to other vaccines that we are more familiar with, such as the MMR that we get as kids and then forget about for the rest of our lives. As good as the different COVID-19 vaccines are, they never came with the promise of generating lifelong antibodies. We knew early on that the immunity they provide slowly wanes with time. That doesn’t mean that those who have their vaccination records up to date (which included a booster probably earlier in 2022) are suddenly exposed. Data suggest that although people several months past their last booster would now be more prone to getting reinfected, the protection against severe disease still hangs around 85%. In other words, their chances of ending up in the hospital are low.
Why worry, then, about further boosting the immune system? The same studies show that an additional jab would increase this percentage up to 99%. Is this roughly 10% improvement really worth another worldwide vaccination campaign? Well, this is a numbers game, after all. The current form of the virus is extremely infectious, and the Northern Hemisphere is heading toward the cold months of the year, which we have seen in past years increases COVID-19 contagions, as you would expect from any airborne virus. Thus, it’s easy to expect a new peak in the number of cases, especially considering that we are not going to apply any of the usual restrictions to prevent this. In these conditions, extending the safety net to a further 10% of the population would substantially reduce the total number of victims. It seems like a good investment of resources.
We can be more surgical about it and direct this new vaccination campaign to the population most likely to end up in the hospital. People with concomitant pathologies are at the top of the list, but it’s also an age issue. On the basis of different studies of the most common ages of admission, the cutoff point for the booster varies from country to country, with the lowest being 50 and in other cases hovering around 65 years of age. Given the safety of these vaccines, if we can afford it, the wider we cast the net, the better, but at least we should make every effort to fully vaccinate the higher age brackets.
The final question is which vaccine to give. There are confounding studies about the importance of switching to Omicron-specific jabs, which are finally available. Although this seems like a good idea, since Omicron infections elicit a more effective range of antibodies and new variants seem to better escape our defenses, recent studies suggest that there actually may not be so much difference with the old formula.
The conclusion? This regimen of yearly boosters for some may be the scenario for the upcoming years, similar to what we already do for the flu, so we should get used to it.
Dr. Macip is associate professor, department of molecular and cellular biology, University of Leicester (England). He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Childhood peanut allergy linked with other legume allergies
, a retrospective study reports.
“Among children allergic to peanut, at least two-thirds were sensitized to one other legume, and legume allergy was diagnosed in one-quarter of the sensitized patients,” senior study author Amandine Divaret-Chauveau, MD, of the Centre Hospitalier Universitaire de Nancy in Vandoeuvre-les-Nancy, France, and colleagues reported in Pediatric Allergy and Immunology.
People worldwide are eating more legumes these days, the authors noted. High in protein, low in unsaturated fats, with low production costs, legumes are important components of increasingly vegetarian, healthy, sustainable diets.
Food allergens are the most common childhood triggers of allergic reactions. Among children in France, legumes cause 14.6% of food-related anaphylactic reactions, with peanut as the main allergen, they added.
Dr. Divaret-Chauveau and colleagues assessed the prevalence and relevance of sensitization to legumes among all children and adolescents aged 1-17 years who had peanut allergy and had been admitted to one academic pediatric allergy department over roughly 3 years, beginning in early 2017. For the 195 study participants, peanut allergy had been confirmed, and they had been documented to have consumed or to have sensitization to at least one nonpeanut legume; 69.7% were boys.
The researchers analyzed data on consumption history, skin-prick tests, specific immunoglobulin E status, prior allergic reactions, and oral food challenges for each legume. They found the following:
Among the 195 children with peanut allergy, 98.4% had at least one other atopic disease.
Of the 195 children with peanut allergy, 122 (63.9%) were sensitized to at least one other legume. Of these 122 children, 66.3% were sensitized to fenugreek, 42.2% to lentil, 39.9% to soy, and 34.2% to lupin.
Allergy to one or more legumes was confirmed for 27.9% of the 122 sensitized children, including 4.9% who had multiple legume allergies. Lentil, lupin, and pea were the main allergens.
Of the 118 children also having a nonlegume food allergy, the main food allergens were egg (57.6%), cow’s milk (33.0%), cashew (39.0%), pistachio (23.7%), and hazelnut (30.5%).
50% of allergic reactions to nonpeanut legumes were severe, often showing as asthma. Atopic comorbidities, including asthma, in most participants may have contributed to the severity of allergic reactions, the authors noted.
Allergy awareness needs to grow with plant-based diets
“The high prevalence of legume sensitization reported in our study highlights the need to explore legume consumption in children with PA [peanut allergy], and the need to investigate sensitization in the absence of consumption,” they added.
Jodi A. Shroba, MSN, APRN, CPNP, coordinator for the Food Allergy Program at Children’s Mercy Kansas City (Mo.), said in an interview that few data are available in the literature regarding allergies to legumes other than peanut.
“It was interesting that these authors found such a high legume sensitization in their peanut-allergic patients,” Dr. Shroba, who was not involved in the study, said by email. “As more people are starting to eat plant-based diets, it is important that we better understand their allergenicity and cross-reactivity so we can better help guide patient management and education.”
Deborah Albright, MD, assistant professor of pediatrics at the University of Pittsburgh, agreed.
“As plant-based protein consumption broadens worldwide, awareness of the potential for cross-reactivity and co-allergy amongst legumes will become increasingly important,” she said in a comment.
“However, positive allergy tests do not reliably correlate with true food allergy; therefore, the diagnosis of legume co-allergy should be confirmed by the individual patient’s history, a formal food challenge, or both,” advised Dr. Albright. She was not involved in the study.
“Cross-sensitization to other legumes in patients with a single legume allergy is common; however, true clinical reactivity is often not present,” she added. “Also, legume allergy test sensitization rates and objective reactivity on food challenge can vary by region, depending on diet and pollen aeroallergen exposure.
“Systematic exploration of tolerance versus co-allergy to other legumes should be considered in patients allergic to peanut or other legumes,” Dr. Albright said.
The authors recommend further research and registry data collection of legume anaphylaxis.
Details regarding funding for the study were not provided. The authors, Dr. Shroba, and Dr. Albright report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a retrospective study reports.
“Among children allergic to peanut, at least two-thirds were sensitized to one other legume, and legume allergy was diagnosed in one-quarter of the sensitized patients,” senior study author Amandine Divaret-Chauveau, MD, of the Centre Hospitalier Universitaire de Nancy in Vandoeuvre-les-Nancy, France, and colleagues reported in Pediatric Allergy and Immunology.
People worldwide are eating more legumes these days, the authors noted. High in protein, low in unsaturated fats, with low production costs, legumes are important components of increasingly vegetarian, healthy, sustainable diets.
Food allergens are the most common childhood triggers of allergic reactions. Among children in France, legumes cause 14.6% of food-related anaphylactic reactions, with peanut as the main allergen, they added.
Dr. Divaret-Chauveau and colleagues assessed the prevalence and relevance of sensitization to legumes among all children and adolescents aged 1-17 years who had peanut allergy and had been admitted to one academic pediatric allergy department over roughly 3 years, beginning in early 2017. For the 195 study participants, peanut allergy had been confirmed, and they had been documented to have consumed or to have sensitization to at least one nonpeanut legume; 69.7% were boys.
The researchers analyzed data on consumption history, skin-prick tests, specific immunoglobulin E status, prior allergic reactions, and oral food challenges for each legume. They found the following:
Among the 195 children with peanut allergy, 98.4% had at least one other atopic disease.
Of the 195 children with peanut allergy, 122 (63.9%) were sensitized to at least one other legume. Of these 122 children, 66.3% were sensitized to fenugreek, 42.2% to lentil, 39.9% to soy, and 34.2% to lupin.
Allergy to one or more legumes was confirmed for 27.9% of the 122 sensitized children, including 4.9% who had multiple legume allergies. Lentil, lupin, and pea were the main allergens.
Of the 118 children also having a nonlegume food allergy, the main food allergens were egg (57.6%), cow’s milk (33.0%), cashew (39.0%), pistachio (23.7%), and hazelnut (30.5%).
50% of allergic reactions to nonpeanut legumes were severe, often showing as asthma. Atopic comorbidities, including asthma, in most participants may have contributed to the severity of allergic reactions, the authors noted.
Allergy awareness needs to grow with plant-based diets
“The high prevalence of legume sensitization reported in our study highlights the need to explore legume consumption in children with PA [peanut allergy], and the need to investigate sensitization in the absence of consumption,” they added.
Jodi A. Shroba, MSN, APRN, CPNP, coordinator for the Food Allergy Program at Children’s Mercy Kansas City (Mo.), said in an interview that few data are available in the literature regarding allergies to legumes other than peanut.
“It was interesting that these authors found such a high legume sensitization in their peanut-allergic patients,” Dr. Shroba, who was not involved in the study, said by email. “As more people are starting to eat plant-based diets, it is important that we better understand their allergenicity and cross-reactivity so we can better help guide patient management and education.”
Deborah Albright, MD, assistant professor of pediatrics at the University of Pittsburgh, agreed.
“As plant-based protein consumption broadens worldwide, awareness of the potential for cross-reactivity and co-allergy amongst legumes will become increasingly important,” she said in a comment.
“However, positive allergy tests do not reliably correlate with true food allergy; therefore, the diagnosis of legume co-allergy should be confirmed by the individual patient’s history, a formal food challenge, or both,” advised Dr. Albright. She was not involved in the study.
“Cross-sensitization to other legumes in patients with a single legume allergy is common; however, true clinical reactivity is often not present,” she added. “Also, legume allergy test sensitization rates and objective reactivity on food challenge can vary by region, depending on diet and pollen aeroallergen exposure.
“Systematic exploration of tolerance versus co-allergy to other legumes should be considered in patients allergic to peanut or other legumes,” Dr. Albright said.
The authors recommend further research and registry data collection of legume anaphylaxis.
Details regarding funding for the study were not provided. The authors, Dr. Shroba, and Dr. Albright report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a retrospective study reports.
“Among children allergic to peanut, at least two-thirds were sensitized to one other legume, and legume allergy was diagnosed in one-quarter of the sensitized patients,” senior study author Amandine Divaret-Chauveau, MD, of the Centre Hospitalier Universitaire de Nancy in Vandoeuvre-les-Nancy, France, and colleagues reported in Pediatric Allergy and Immunology.
People worldwide are eating more legumes these days, the authors noted. High in protein, low in unsaturated fats, with low production costs, legumes are important components of increasingly vegetarian, healthy, sustainable diets.
Food allergens are the most common childhood triggers of allergic reactions. Among children in France, legumes cause 14.6% of food-related anaphylactic reactions, with peanut as the main allergen, they added.
Dr. Divaret-Chauveau and colleagues assessed the prevalence and relevance of sensitization to legumes among all children and adolescents aged 1-17 years who had peanut allergy and had been admitted to one academic pediatric allergy department over roughly 3 years, beginning in early 2017. For the 195 study participants, peanut allergy had been confirmed, and they had been documented to have consumed or to have sensitization to at least one nonpeanut legume; 69.7% were boys.
The researchers analyzed data on consumption history, skin-prick tests, specific immunoglobulin E status, prior allergic reactions, and oral food challenges for each legume. They found the following:
Among the 195 children with peanut allergy, 98.4% had at least one other atopic disease.
Of the 195 children with peanut allergy, 122 (63.9%) were sensitized to at least one other legume. Of these 122 children, 66.3% were sensitized to fenugreek, 42.2% to lentil, 39.9% to soy, and 34.2% to lupin.
Allergy to one or more legumes was confirmed for 27.9% of the 122 sensitized children, including 4.9% who had multiple legume allergies. Lentil, lupin, and pea were the main allergens.
Of the 118 children also having a nonlegume food allergy, the main food allergens were egg (57.6%), cow’s milk (33.0%), cashew (39.0%), pistachio (23.7%), and hazelnut (30.5%).
50% of allergic reactions to nonpeanut legumes were severe, often showing as asthma. Atopic comorbidities, including asthma, in most participants may have contributed to the severity of allergic reactions, the authors noted.
Allergy awareness needs to grow with plant-based diets
“The high prevalence of legume sensitization reported in our study highlights the need to explore legume consumption in children with PA [peanut allergy], and the need to investigate sensitization in the absence of consumption,” they added.
Jodi A. Shroba, MSN, APRN, CPNP, coordinator for the Food Allergy Program at Children’s Mercy Kansas City (Mo.), said in an interview that few data are available in the literature regarding allergies to legumes other than peanut.
“It was interesting that these authors found such a high legume sensitization in their peanut-allergic patients,” Dr. Shroba, who was not involved in the study, said by email. “As more people are starting to eat plant-based diets, it is important that we better understand their allergenicity and cross-reactivity so we can better help guide patient management and education.”
Deborah Albright, MD, assistant professor of pediatrics at the University of Pittsburgh, agreed.
“As plant-based protein consumption broadens worldwide, awareness of the potential for cross-reactivity and co-allergy amongst legumes will become increasingly important,” she said in a comment.
“However, positive allergy tests do not reliably correlate with true food allergy; therefore, the diagnosis of legume co-allergy should be confirmed by the individual patient’s history, a formal food challenge, or both,” advised Dr. Albright. She was not involved in the study.
“Cross-sensitization to other legumes in patients with a single legume allergy is common; however, true clinical reactivity is often not present,” she added. “Also, legume allergy test sensitization rates and objective reactivity on food challenge can vary by region, depending on diet and pollen aeroallergen exposure.
“Systematic exploration of tolerance versus co-allergy to other legumes should be considered in patients allergic to peanut or other legumes,” Dr. Albright said.
The authors recommend further research and registry data collection of legume anaphylaxis.
Details regarding funding for the study were not provided. The authors, Dr. Shroba, and Dr. Albright report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PEDIATRIC ALLERGY AND IMMUNOLOGY
Childhood peanut allergy linked with other legume allergies
French children with peanut allergy tend to have reactions to other legumes, including soy, lentil, pea, bean, lupin, and fenugreek, and those other allergies often lead to anaphylactic reactions, a retrospective study from France reports.
“Among children allergic to peanut, at least two-thirds were sensitized to one other legume, and legume allergy was diagnosed in one-quarter of the sensitized patients,” wrote senior study author Amandine Divaret-Chauveau, MD, of Centre Hospitalier Universitaire de Nancy, Vandoeuvre-les-Nancy, and colleagues. The report is in Pediatric Allergy and Immunology.
People worldwide are eating more legumes these days, the authors noted. High in protein, low in unsaturated fats, with low production costs, legumes are important components of increasingly vegetarian, healthy, sustainable diets.
Food allergens are the most common childhood triggers of allergic reactions. Among children in France, legumes cause 14.6% of food-related anaphylactic reactions, with peanut as the main allergen, they added.
Dr. Divaret-Chauveau and colleagues assessed the prevalence and relevance of sensitization to legumes among all children and adolescents aged 1-17 years who had peanut allergy and had been admitted to one academic pediatric allergy department over roughly 3 years, beginning in early 2017. For the 195 study participants, peanut allergy had been confirmed, and they had been documented to have consumed or to have sensitization to at least one non-peanut legume; 69.7% were boys.
The researchers analyzed data on consumption history, skin prick tests, specific immunoglobulin E status, prior allergic reactions, and oral food challenges for each legume. They found the following:
- Among the 195 children with peanut allergy, 98.4% had at least one other atopic disease.
- Of the 195 children with peanut allergy, 122 (63.9%) were sensitized to at least one other legume. Of these 122 children, 66.3% were sensitized to fenugreek, 42.2% to lentil, 39.9% to soy, and 34.2% to lupin.
- Allergy to one or more legumes was confirmed for 27.9% of the 122 sensitized children, including 4.9% who had multiple legume allergies. Lentil, lupin, and pea were the main allergens.
- Of the 118 children also having a non-legume food allergy, the main food allergens were egg (57.6%), cow’s milk (33.0%), cashew (39.0%), pistachio (23.7%), and hazelnut (30.5%).
- Fifty percent of allergic reactions to non-peanut legumes were severe, often showing as asthma. Atopic comorbidities, including asthma, in most participants may have contributed to the severity of allergic reactions, the authors noted.
Allergy awareness needs to grow with plant-based diets
“The high prevalence of legume sensitization reported in our study highlights the need to explore legume consumption in children with PA [peanut allergy], and the need to investigate sensitization in the absence of consumption,” they added.
Jodi A. Shroba, MSN, APRN, CPNP, coordinator for the Food Allergy Program at Children’s Mercy Kansas City, in Missouri, told this news organization that few data are available in the literature regarding allergies to legumes other than peanut.
“It was interesting that these authors found such a high legume sensitization in their peanut-allergic patients,” Ms. Shroba, who was not involved in the study, said by email. “As more people are starting to eat plant-based diets, it is important that we better understand their allergenicity and cross-reactivity so we can better help guide patient management and education.”
Deborah Albright, MD, assistant professor of pediatrics at the University of Pittsburgh, agreed.
“As plant-based protein consumption broadens worldwide, awareness of the potential for cross-reactivity and co-allergy amongst legumes will become increasingly important,” she said by email.
“However, positive allergy tests do not reliably correlate with true food allergy; therefore, the diagnosis of legume co-allergy should be confirmed by the individual patient’s history, a formal food challenge, or both,” advised Dr. Albright. She was not involved in the study.
“Cross-sensitization to other legumes in patients with a single legume allergy is common; however, true clinical reactivity is often not present,” she added. “Also, legume allergy test sensitization rates and objective reactivity on food challenge can vary by region, depending on diet and pollen aeroallergen exposure.
“Systematic exploration of tolerance versus co-allergy to other legumes should be considered in patients allergic to peanut or other legumes,” Dr. Albright said.
The authors recommend further research and registry data collection of legume anaphylaxis.
Details regarding funding for the study were not provided. The authors, Ms. Shroba, and Dr. Albright report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
French children with peanut allergy tend to have reactions to other legumes, including soy, lentil, pea, bean, lupin, and fenugreek, and those other allergies often lead to anaphylactic reactions, a retrospective study from France reports.
“Among children allergic to peanut, at least two-thirds were sensitized to one other legume, and legume allergy was diagnosed in one-quarter of the sensitized patients,” wrote senior study author Amandine Divaret-Chauveau, MD, of Centre Hospitalier Universitaire de Nancy, Vandoeuvre-les-Nancy, and colleagues. The report is in Pediatric Allergy and Immunology.
People worldwide are eating more legumes these days, the authors noted. High in protein, low in unsaturated fats, with low production costs, legumes are important components of increasingly vegetarian, healthy, sustainable diets.
Food allergens are the most common childhood triggers of allergic reactions. Among children in France, legumes cause 14.6% of food-related anaphylactic reactions, with peanut as the main allergen, they added.
Dr. Divaret-Chauveau and colleagues assessed the prevalence and relevance of sensitization to legumes among all children and adolescents aged 1-17 years who had peanut allergy and had been admitted to one academic pediatric allergy department over roughly 3 years, beginning in early 2017. For the 195 study participants, peanut allergy had been confirmed, and they had been documented to have consumed or to have sensitization to at least one non-peanut legume; 69.7% were boys.
The researchers analyzed data on consumption history, skin prick tests, specific immunoglobulin E status, prior allergic reactions, and oral food challenges for each legume. They found the following:
- Among the 195 children with peanut allergy, 98.4% had at least one other atopic disease.
- Of the 195 children with peanut allergy, 122 (63.9%) were sensitized to at least one other legume. Of these 122 children, 66.3% were sensitized to fenugreek, 42.2% to lentil, 39.9% to soy, and 34.2% to lupin.
- Allergy to one or more legumes was confirmed for 27.9% of the 122 sensitized children, including 4.9% who had multiple legume allergies. Lentil, lupin, and pea were the main allergens.
- Of the 118 children also having a non-legume food allergy, the main food allergens were egg (57.6%), cow’s milk (33.0%), cashew (39.0%), pistachio (23.7%), and hazelnut (30.5%).
- Fifty percent of allergic reactions to non-peanut legumes were severe, often showing as asthma. Atopic comorbidities, including asthma, in most participants may have contributed to the severity of allergic reactions, the authors noted.
Allergy awareness needs to grow with plant-based diets
“The high prevalence of legume sensitization reported in our study highlights the need to explore legume consumption in children with PA [peanut allergy], and the need to investigate sensitization in the absence of consumption,” they added.
Jodi A. Shroba, MSN, APRN, CPNP, coordinator for the Food Allergy Program at Children’s Mercy Kansas City, in Missouri, told this news organization that few data are available in the literature regarding allergies to legumes other than peanut.
“It was interesting that these authors found such a high legume sensitization in their peanut-allergic patients,” Ms. Shroba, who was not involved in the study, said by email. “As more people are starting to eat plant-based diets, it is important that we better understand their allergenicity and cross-reactivity so we can better help guide patient management and education.”
Deborah Albright, MD, assistant professor of pediatrics at the University of Pittsburgh, agreed.
“As plant-based protein consumption broadens worldwide, awareness of the potential for cross-reactivity and co-allergy amongst legumes will become increasingly important,” she said by email.
“However, positive allergy tests do not reliably correlate with true food allergy; therefore, the diagnosis of legume co-allergy should be confirmed by the individual patient’s history, a formal food challenge, or both,” advised Dr. Albright. She was not involved in the study.
“Cross-sensitization to other legumes in patients with a single legume allergy is common; however, true clinical reactivity is often not present,” she added. “Also, legume allergy test sensitization rates and objective reactivity on food challenge can vary by region, depending on diet and pollen aeroallergen exposure.
“Systematic exploration of tolerance versus co-allergy to other legumes should be considered in patients allergic to peanut or other legumes,” Dr. Albright said.
The authors recommend further research and registry data collection of legume anaphylaxis.
Details regarding funding for the study were not provided. The authors, Ms. Shroba, and Dr. Albright report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
French children with peanut allergy tend to have reactions to other legumes, including soy, lentil, pea, bean, lupin, and fenugreek, and those other allergies often lead to anaphylactic reactions, a retrospective study from France reports.
“Among children allergic to peanut, at least two-thirds were sensitized to one other legume, and legume allergy was diagnosed in one-quarter of the sensitized patients,” wrote senior study author Amandine Divaret-Chauveau, MD, of Centre Hospitalier Universitaire de Nancy, Vandoeuvre-les-Nancy, and colleagues. The report is in Pediatric Allergy and Immunology.
People worldwide are eating more legumes these days, the authors noted. High in protein, low in unsaturated fats, with low production costs, legumes are important components of increasingly vegetarian, healthy, sustainable diets.
Food allergens are the most common childhood triggers of allergic reactions. Among children in France, legumes cause 14.6% of food-related anaphylactic reactions, with peanut as the main allergen, they added.
Dr. Divaret-Chauveau and colleagues assessed the prevalence and relevance of sensitization to legumes among all children and adolescents aged 1-17 years who had peanut allergy and had been admitted to one academic pediatric allergy department over roughly 3 years, beginning in early 2017. For the 195 study participants, peanut allergy had been confirmed, and they had been documented to have consumed or to have sensitization to at least one non-peanut legume; 69.7% were boys.
The researchers analyzed data on consumption history, skin prick tests, specific immunoglobulin E status, prior allergic reactions, and oral food challenges for each legume. They found the following:
- Among the 195 children with peanut allergy, 98.4% had at least one other atopic disease.
- Of the 195 children with peanut allergy, 122 (63.9%) were sensitized to at least one other legume. Of these 122 children, 66.3% were sensitized to fenugreek, 42.2% to lentil, 39.9% to soy, and 34.2% to lupin.
- Allergy to one or more legumes was confirmed for 27.9% of the 122 sensitized children, including 4.9% who had multiple legume allergies. Lentil, lupin, and pea were the main allergens.
- Of the 118 children also having a non-legume food allergy, the main food allergens were egg (57.6%), cow’s milk (33.0%), cashew (39.0%), pistachio (23.7%), and hazelnut (30.5%).
- Fifty percent of allergic reactions to non-peanut legumes were severe, often showing as asthma. Atopic comorbidities, including asthma, in most participants may have contributed to the severity of allergic reactions, the authors noted.
Allergy awareness needs to grow with plant-based diets
“The high prevalence of legume sensitization reported in our study highlights the need to explore legume consumption in children with PA [peanut allergy], and the need to investigate sensitization in the absence of consumption,” they added.
Jodi A. Shroba, MSN, APRN, CPNP, coordinator for the Food Allergy Program at Children’s Mercy Kansas City, in Missouri, told this news organization that few data are available in the literature regarding allergies to legumes other than peanut.
“It was interesting that these authors found such a high legume sensitization in their peanut-allergic patients,” Ms. Shroba, who was not involved in the study, said by email. “As more people are starting to eat plant-based diets, it is important that we better understand their allergenicity and cross-reactivity so we can better help guide patient management and education.”
Deborah Albright, MD, assistant professor of pediatrics at the University of Pittsburgh, agreed.
“As plant-based protein consumption broadens worldwide, awareness of the potential for cross-reactivity and co-allergy amongst legumes will become increasingly important,” she said by email.
“However, positive allergy tests do not reliably correlate with true food allergy; therefore, the diagnosis of legume co-allergy should be confirmed by the individual patient’s history, a formal food challenge, or both,” advised Dr. Albright. She was not involved in the study.
“Cross-sensitization to other legumes in patients with a single legume allergy is common; however, true clinical reactivity is often not present,” she added. “Also, legume allergy test sensitization rates and objective reactivity on food challenge can vary by region, depending on diet and pollen aeroallergen exposure.
“Systematic exploration of tolerance versus co-allergy to other legumes should be considered in patients allergic to peanut or other legumes,” Dr. Albright said.
The authors recommend further research and registry data collection of legume anaphylaxis.
Details regarding funding for the study were not provided. The authors, Ms. Shroba, and Dr. Albright report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Childhood cow’s milk allergy raises health care costs
Managing children’s cow’s milk allergy is costly to families and to health care systems, largely owing to costs of prescriptions, according to an industry-sponsored study based on data from the United Kingdom.
“This large cohort study provides novel evidence of a significant health economic burden of cow’s milk allergy in children,” Abbie L. Cawood, PhD, RNutr, MICR, head of scientific affairs at Nutricia Ltd in Trowbridge, England, and colleagues wrote in Clinical and Translational Allergy.
“Management of cow’s milk allergy necessitates the exclusion of cow’s milk protein from the diet. Whilst breastmilk remains the ideal nutrient source in infants with cow’s milk allergy, infants who are not exclusively breastfed require a hypoallergenic formula,” added Dr. Cawood, a visiting research fellow at University of Southampton, and her coauthors.
Cow’s milk allergy, an immune‐mediated response to one or more proteins in cow’s milk, is one of the most common childhood food allergies and affects 2%-5% of infants in Europe. Management involves avoiding cow’s milk protein and treating possible related gastrointestinal, skin, respiratory, and other allergic conditions, the authors explained.
In their retrospective matched cohort study, Dr. Cawood and colleagues turned to The Health Improvement Network (THIN), a Cegedim Rx proprietary database of 2.9 million anonymized active patient records. They extracted data from nearly 7,000 case records covering 5 years (2015-2020).
They examined medication prescriptions and health care professional contacts based on diagnosis read-codes and hypoallergenic formula prescriptions and compared health care costs for children with cow’s milk allergy with the costs for those without.
They matched 3,499 children aged 1 year or younger who had confirmed or suspected cow’s milk allergy with the same number of children without cow’s milk allergy. Around half of the participants were boys, and the mean observation period was 4.2 years.
Children with cow’s milk allergy need more, costly health care
The researchers found:
- Medications were prescribed to significantly more children with cow’s milk allergy (CMA), at a higher rate, than to those without CMA. In particular, prescriptions for antireflux medication increased by almost 500%.
- Children with CMA needed significantly more health care contacts and at a higher rate than those without CMA.
- CMA was linked with additional potential health care costs of £1381.53 per person per year. Assuming a 2.5% prevalence from the estimated 2%-5% CMA prevalence range and extrapolating to the UK infant population, CMA may have added more than £25.7 million in annual health care costs nationwide.
“Several conditions in infancy necessitate the elimination of cow milk–based formulas and require extensively hydrolyzed or amino acid formulas or, if preferred or able, exclusive breast milk,” Kara E. Coffey, MD, assistant professor of pediatrics at the University of Pittsburgh, said by email.
“This study shows that, regardless of the reason for cow milk–based avoidance, these infants require more healthcare service utilizations (clinic visits, nutritional assessments, prescriptions) than [do] their peers, which is certainly a commitment of a lot of time and money for their families to ensure their ability to grow and thrive,” added Dr. Coffey, who was not involved in the study.
Jodi A. Shroba, MSN, APRN, CPNP, the coordinator for the Food Allergy Program at Children’s Mercy Kansas City, Mo., did not find these numbers surprising.
“Children with food allergies typically have other atopic comorbidities that require more visits to primary care physicians and specialists and more prescriptions,” Ms. Shroba, who was not involved in the study, said by email.
“An intriguing statement is that the U.K. guidelines recommend the involvement of a dietitian for children with cow’s milk allergy,” she noted. “In the United States, having a dietitian involved would be a wonderful addition to care, as avoidance of cow’s milk can cause nutritional and growth deficiencies. But not all healthcare practices have those resources available.
“The higher rate of antibiotic use and the almost 500% increase of antireflux prescriptions by the children with cow’s milk allergy warrant additional research,” she added.
Nutricia Ltd. funded the study. Dr. Cawood and one coauthor are employed by Nutricia, and all other coauthors have been employees of or have other financial relationships with Nutricia. One coauthor is employed by Cegedim Rx, which was funded for this research by Nutricia. Ms. Shroba and Dr. Coffey report no conflicts of interest with the study.
A version of this article first appeared on Medscape.com.
Managing children’s cow’s milk allergy is costly to families and to health care systems, largely owing to costs of prescriptions, according to an industry-sponsored study based on data from the United Kingdom.
“This large cohort study provides novel evidence of a significant health economic burden of cow’s milk allergy in children,” Abbie L. Cawood, PhD, RNutr, MICR, head of scientific affairs at Nutricia Ltd in Trowbridge, England, and colleagues wrote in Clinical and Translational Allergy.
“Management of cow’s milk allergy necessitates the exclusion of cow’s milk protein from the diet. Whilst breastmilk remains the ideal nutrient source in infants with cow’s milk allergy, infants who are not exclusively breastfed require a hypoallergenic formula,” added Dr. Cawood, a visiting research fellow at University of Southampton, and her coauthors.
Cow’s milk allergy, an immune‐mediated response to one or more proteins in cow’s milk, is one of the most common childhood food allergies and affects 2%-5% of infants in Europe. Management involves avoiding cow’s milk protein and treating possible related gastrointestinal, skin, respiratory, and other allergic conditions, the authors explained.
In their retrospective matched cohort study, Dr. Cawood and colleagues turned to The Health Improvement Network (THIN), a Cegedim Rx proprietary database of 2.9 million anonymized active patient records. They extracted data from nearly 7,000 case records covering 5 years (2015-2020).
They examined medication prescriptions and health care professional contacts based on diagnosis read-codes and hypoallergenic formula prescriptions and compared health care costs for children with cow’s milk allergy with the costs for those without.
They matched 3,499 children aged 1 year or younger who had confirmed or suspected cow’s milk allergy with the same number of children without cow’s milk allergy. Around half of the participants were boys, and the mean observation period was 4.2 years.
Children with cow’s milk allergy need more, costly health care
The researchers found:
- Medications were prescribed to significantly more children with cow’s milk allergy (CMA), at a higher rate, than to those without CMA. In particular, prescriptions for antireflux medication increased by almost 500%.
- Children with CMA needed significantly more health care contacts and at a higher rate than those without CMA.
- CMA was linked with additional potential health care costs of £1381.53 per person per year. Assuming a 2.5% prevalence from the estimated 2%-5% CMA prevalence range and extrapolating to the UK infant population, CMA may have added more than £25.7 million in annual health care costs nationwide.
“Several conditions in infancy necessitate the elimination of cow milk–based formulas and require extensively hydrolyzed or amino acid formulas or, if preferred or able, exclusive breast milk,” Kara E. Coffey, MD, assistant professor of pediatrics at the University of Pittsburgh, said by email.
“This study shows that, regardless of the reason for cow milk–based avoidance, these infants require more healthcare service utilizations (clinic visits, nutritional assessments, prescriptions) than [do] their peers, which is certainly a commitment of a lot of time and money for their families to ensure their ability to grow and thrive,” added Dr. Coffey, who was not involved in the study.
Jodi A. Shroba, MSN, APRN, CPNP, the coordinator for the Food Allergy Program at Children’s Mercy Kansas City, Mo., did not find these numbers surprising.
“Children with food allergies typically have other atopic comorbidities that require more visits to primary care physicians and specialists and more prescriptions,” Ms. Shroba, who was not involved in the study, said by email.
“An intriguing statement is that the U.K. guidelines recommend the involvement of a dietitian for children with cow’s milk allergy,” she noted. “In the United States, having a dietitian involved would be a wonderful addition to care, as avoidance of cow’s milk can cause nutritional and growth deficiencies. But not all healthcare practices have those resources available.
“The higher rate of antibiotic use and the almost 500% increase of antireflux prescriptions by the children with cow’s milk allergy warrant additional research,” she added.
Nutricia Ltd. funded the study. Dr. Cawood and one coauthor are employed by Nutricia, and all other coauthors have been employees of or have other financial relationships with Nutricia. One coauthor is employed by Cegedim Rx, which was funded for this research by Nutricia. Ms. Shroba and Dr. Coffey report no conflicts of interest with the study.
A version of this article first appeared on Medscape.com.
Managing children’s cow’s milk allergy is costly to families and to health care systems, largely owing to costs of prescriptions, according to an industry-sponsored study based on data from the United Kingdom.
“This large cohort study provides novel evidence of a significant health economic burden of cow’s milk allergy in children,” Abbie L. Cawood, PhD, RNutr, MICR, head of scientific affairs at Nutricia Ltd in Trowbridge, England, and colleagues wrote in Clinical and Translational Allergy.
“Management of cow’s milk allergy necessitates the exclusion of cow’s milk protein from the diet. Whilst breastmilk remains the ideal nutrient source in infants with cow’s milk allergy, infants who are not exclusively breastfed require a hypoallergenic formula,” added Dr. Cawood, a visiting research fellow at University of Southampton, and her coauthors.
Cow’s milk allergy, an immune‐mediated response to one or more proteins in cow’s milk, is one of the most common childhood food allergies and affects 2%-5% of infants in Europe. Management involves avoiding cow’s milk protein and treating possible related gastrointestinal, skin, respiratory, and other allergic conditions, the authors explained.
In their retrospective matched cohort study, Dr. Cawood and colleagues turned to The Health Improvement Network (THIN), a Cegedim Rx proprietary database of 2.9 million anonymized active patient records. They extracted data from nearly 7,000 case records covering 5 years (2015-2020).
They examined medication prescriptions and health care professional contacts based on diagnosis read-codes and hypoallergenic formula prescriptions and compared health care costs for children with cow’s milk allergy with the costs for those without.
They matched 3,499 children aged 1 year or younger who had confirmed or suspected cow’s milk allergy with the same number of children without cow’s milk allergy. Around half of the participants were boys, and the mean observation period was 4.2 years.
Children with cow’s milk allergy need more, costly health care
The researchers found:
- Medications were prescribed to significantly more children with cow’s milk allergy (CMA), at a higher rate, than to those without CMA. In particular, prescriptions for antireflux medication increased by almost 500%.
- Children with CMA needed significantly more health care contacts and at a higher rate than those without CMA.
- CMA was linked with additional potential health care costs of £1381.53 per person per year. Assuming a 2.5% prevalence from the estimated 2%-5% CMA prevalence range and extrapolating to the UK infant population, CMA may have added more than £25.7 million in annual health care costs nationwide.
“Several conditions in infancy necessitate the elimination of cow milk–based formulas and require extensively hydrolyzed or amino acid formulas or, if preferred or able, exclusive breast milk,” Kara E. Coffey, MD, assistant professor of pediatrics at the University of Pittsburgh, said by email.
“This study shows that, regardless of the reason for cow milk–based avoidance, these infants require more healthcare service utilizations (clinic visits, nutritional assessments, prescriptions) than [do] their peers, which is certainly a commitment of a lot of time and money for their families to ensure their ability to grow and thrive,” added Dr. Coffey, who was not involved in the study.
Jodi A. Shroba, MSN, APRN, CPNP, the coordinator for the Food Allergy Program at Children’s Mercy Kansas City, Mo., did not find these numbers surprising.
“Children with food allergies typically have other atopic comorbidities that require more visits to primary care physicians and specialists and more prescriptions,” Ms. Shroba, who was not involved in the study, said by email.
“An intriguing statement is that the U.K. guidelines recommend the involvement of a dietitian for children with cow’s milk allergy,” she noted. “In the United States, having a dietitian involved would be a wonderful addition to care, as avoidance of cow’s milk can cause nutritional and growth deficiencies. But not all healthcare practices have those resources available.
“The higher rate of antibiotic use and the almost 500% increase of antireflux prescriptions by the children with cow’s milk allergy warrant additional research,” she added.
Nutricia Ltd. funded the study. Dr. Cawood and one coauthor are employed by Nutricia, and all other coauthors have been employees of or have other financial relationships with Nutricia. One coauthor is employed by Cegedim Rx, which was funded for this research by Nutricia. Ms. Shroba and Dr. Coffey report no conflicts of interest with the study.
A version of this article first appeared on Medscape.com.
FROM CLINICAL AND TRANSLATIONAL ALLERGY